User login
Opicapone increased on-time without dyskinesia in patients with Parkinson’s disease
PHILADELPHIA -
The 2-hour improvement was considered clinically meaningful, although the average patient in the studies had about 6 hours of off-time, said investigator Peter LeWitt, MD, of Henry Ford Hospital in West Bloomfield, Mich., and the department of neurology at Wayne State University, Detroit. Dr. LeWitt and colleagues will present the data at the annual meeting of the American Academy of Neurology.
“While this is a substantial improvement, it is 2 hours improvement over a total of 6 hours of off-time, which is not perfect,” Dr. LeWitt said in an interview. “So how could we do better is the challenge for all of us who are doing research.”
Opicapone is under development in the United States; it is currently approved in the European Union as adjunctive therapy to preparations of levodopa/DOPA decarboxylase inhibitors for patients with Parkinson’s disease and end-of-dose motor fluctuations.
The ability of opicapone to prolong the clinical actions of levodopa has been evaluated in BIPARK-1 and BIPARK-2. These two international phase 3 studies evaluated the third-generation COMT inhibitor against placebo and, in the case of BIPARK-1, against the COMT inhibitor entacapone as an active control. Each study was 14-15 weeks in duration and included a 1-year open-label phase.
In BIPARK-1, on-time without troublesome dyskinesia was significantly increased for opicapone 50 mg versus placebo, with an absolute increase of 1.9 versus 0.9 hours, respectively, from baseline to week 14 or 15 (P = .002), investigators said. Similarly, BIPARK-2 data showed an increase in this endpoint, at 1.7 versus 0.9 hours for opicapone and placebo, respectively (P = .025).
The 50-mg dose of opicapone was received by 115 patients in BIPARK-1 and 147 patients in BIPARK-2, while placebo was received by 120 and 135 patients in those two studies, respectively.
In the long-term extension studies, the mean change in on-time without dyskinesia from baseline to the end of the open-label endpoint was 2.0 hours for all 494 opicapone-treated patients in BIPARK-1 and 1.8 hours for all 339 opicapone-treated patients in BIPARK-2.
Dyskinesia was reported as a treatment-emergent adverse effect for 17.4% of opicapone-treated patients and 6.2% of placebo-treated patients, according to results of a pooled safety analysis of BIPARK-1 and BIPARK-2. However, only 1.9% of opicapone-treated patients and 0.4% of placebo-treated patients had treatment-emergent dyskinesia leading to discontinuation, and the dyskinesia was considered serious in 0.3% of the opicapone group and 0.0% of the placebo group, investigators added.
Neurocrine Biosciences has announced plans to file a New Drug Application for opicapone for Parkinson’s disease in the United States. That filing is expected to take place in the second quarter of 2019, according to an April 29 press release.
Dr. LeWitt disclosed that he has served as an advisor to Neurocrine Biosciences. He also provided disclosures related to Acadia, Acorda, Adamas, BioElectron Technology, Biotie, Britannia, Intec, Jazz Pharmaceuticals, Lundbeck, the Michael J. Fox Foundation for Parkinson’s Research, Merz, NeuroDerm, the Parkinson Study Group, Pfizer, Prexton, Sage, Scion, Sunovion, SynAgile, and US WorldMeds.
SOURCE: LeWitt P et al. AAN 2019, Abstract S4.003.
PHILADELPHIA -
The 2-hour improvement was considered clinically meaningful, although the average patient in the studies had about 6 hours of off-time, said investigator Peter LeWitt, MD, of Henry Ford Hospital in West Bloomfield, Mich., and the department of neurology at Wayne State University, Detroit. Dr. LeWitt and colleagues will present the data at the annual meeting of the American Academy of Neurology.
“While this is a substantial improvement, it is 2 hours improvement over a total of 6 hours of off-time, which is not perfect,” Dr. LeWitt said in an interview. “So how could we do better is the challenge for all of us who are doing research.”
Opicapone is under development in the United States; it is currently approved in the European Union as adjunctive therapy to preparations of levodopa/DOPA decarboxylase inhibitors for patients with Parkinson’s disease and end-of-dose motor fluctuations.
The ability of opicapone to prolong the clinical actions of levodopa has been evaluated in BIPARK-1 and BIPARK-2. These two international phase 3 studies evaluated the third-generation COMT inhibitor against placebo and, in the case of BIPARK-1, against the COMT inhibitor entacapone as an active control. Each study was 14-15 weeks in duration and included a 1-year open-label phase.
In BIPARK-1, on-time without troublesome dyskinesia was significantly increased for opicapone 50 mg versus placebo, with an absolute increase of 1.9 versus 0.9 hours, respectively, from baseline to week 14 or 15 (P = .002), investigators said. Similarly, BIPARK-2 data showed an increase in this endpoint, at 1.7 versus 0.9 hours for opicapone and placebo, respectively (P = .025).
The 50-mg dose of opicapone was received by 115 patients in BIPARK-1 and 147 patients in BIPARK-2, while placebo was received by 120 and 135 patients in those two studies, respectively.
In the long-term extension studies, the mean change in on-time without dyskinesia from baseline to the end of the open-label endpoint was 2.0 hours for all 494 opicapone-treated patients in BIPARK-1 and 1.8 hours for all 339 opicapone-treated patients in BIPARK-2.
Dyskinesia was reported as a treatment-emergent adverse effect for 17.4% of opicapone-treated patients and 6.2% of placebo-treated patients, according to results of a pooled safety analysis of BIPARK-1 and BIPARK-2. However, only 1.9% of opicapone-treated patients and 0.4% of placebo-treated patients had treatment-emergent dyskinesia leading to discontinuation, and the dyskinesia was considered serious in 0.3% of the opicapone group and 0.0% of the placebo group, investigators added.
Neurocrine Biosciences has announced plans to file a New Drug Application for opicapone for Parkinson’s disease in the United States. That filing is expected to take place in the second quarter of 2019, according to an April 29 press release.
Dr. LeWitt disclosed that he has served as an advisor to Neurocrine Biosciences. He also provided disclosures related to Acadia, Acorda, Adamas, BioElectron Technology, Biotie, Britannia, Intec, Jazz Pharmaceuticals, Lundbeck, the Michael J. Fox Foundation for Parkinson’s Research, Merz, NeuroDerm, the Parkinson Study Group, Pfizer, Prexton, Sage, Scion, Sunovion, SynAgile, and US WorldMeds.
SOURCE: LeWitt P et al. AAN 2019, Abstract S4.003.
PHILADELPHIA -
The 2-hour improvement was considered clinically meaningful, although the average patient in the studies had about 6 hours of off-time, said investigator Peter LeWitt, MD, of Henry Ford Hospital in West Bloomfield, Mich., and the department of neurology at Wayne State University, Detroit. Dr. LeWitt and colleagues will present the data at the annual meeting of the American Academy of Neurology.
“While this is a substantial improvement, it is 2 hours improvement over a total of 6 hours of off-time, which is not perfect,” Dr. LeWitt said in an interview. “So how could we do better is the challenge for all of us who are doing research.”
Opicapone is under development in the United States; it is currently approved in the European Union as adjunctive therapy to preparations of levodopa/DOPA decarboxylase inhibitors for patients with Parkinson’s disease and end-of-dose motor fluctuations.
The ability of opicapone to prolong the clinical actions of levodopa has been evaluated in BIPARK-1 and BIPARK-2. These two international phase 3 studies evaluated the third-generation COMT inhibitor against placebo and, in the case of BIPARK-1, against the COMT inhibitor entacapone as an active control. Each study was 14-15 weeks in duration and included a 1-year open-label phase.
In BIPARK-1, on-time without troublesome dyskinesia was significantly increased for opicapone 50 mg versus placebo, with an absolute increase of 1.9 versus 0.9 hours, respectively, from baseline to week 14 or 15 (P = .002), investigators said. Similarly, BIPARK-2 data showed an increase in this endpoint, at 1.7 versus 0.9 hours for opicapone and placebo, respectively (P = .025).
The 50-mg dose of opicapone was received by 115 patients in BIPARK-1 and 147 patients in BIPARK-2, while placebo was received by 120 and 135 patients in those two studies, respectively.
In the long-term extension studies, the mean change in on-time without dyskinesia from baseline to the end of the open-label endpoint was 2.0 hours for all 494 opicapone-treated patients in BIPARK-1 and 1.8 hours for all 339 opicapone-treated patients in BIPARK-2.
Dyskinesia was reported as a treatment-emergent adverse effect for 17.4% of opicapone-treated patients and 6.2% of placebo-treated patients, according to results of a pooled safety analysis of BIPARK-1 and BIPARK-2. However, only 1.9% of opicapone-treated patients and 0.4% of placebo-treated patients had treatment-emergent dyskinesia leading to discontinuation, and the dyskinesia was considered serious in 0.3% of the opicapone group and 0.0% of the placebo group, investigators added.
Neurocrine Biosciences has announced plans to file a New Drug Application for opicapone for Parkinson’s disease in the United States. That filing is expected to take place in the second quarter of 2019, according to an April 29 press release.
Dr. LeWitt disclosed that he has served as an advisor to Neurocrine Biosciences. He also provided disclosures related to Acadia, Acorda, Adamas, BioElectron Technology, Biotie, Britannia, Intec, Jazz Pharmaceuticals, Lundbeck, the Michael J. Fox Foundation for Parkinson’s Research, Merz, NeuroDerm, the Parkinson Study Group, Pfizer, Prexton, Sage, Scion, Sunovion, SynAgile, and US WorldMeds.
SOURCE: LeWitt P et al. AAN 2019, Abstract S4.003.
FROM AAN 2019
Evaluating and managing postural tachycardia syndrome
Some people, most of them relatively young women, experience lightheadedness, a racing heart, and other symptoms (but not hypotension) when they stand up, in a condition known as postural tachycardia syndrome (POTS).1 Although not known to shorten life,1 it can be physically and mentally debilitating.2,3 Therapy rarely cures it, but a multifaceted approach can substantially improve quality of life.
This review outlines the evaluation and diagnosis of POTS and provides guidance for a therapy regimen.
HOW IS POTS DEFINED?
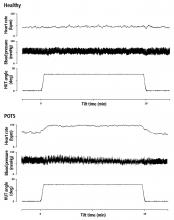
POTS is a multifactorial syndrome rather than a specific disease. It is characterized by all of the following1,4–6:
- An increase in heart rate of ≥ 30 bpm, or ≥ 40 bpm for those under age 19, within 10 minutes of standing from a supine position
- Sustained tachycardia (> 30 seconds)
- Absence of orthostatic hypotension (a fall in blood pressure of ≥ 20/10 mm Hg)
- Frequent and chronic duration (≥ 6 months).
These features are critical to diagnosis. Hemodynamic criteria in isolation may describe postural tachycardia but are not sufficient to diagnose POTS.
The prevalence of POTS is estimated to be between 0.2% and 1.0%,7 affecting up to 3 million people in the United States. Most cases arise between ages 13 and 50, with a female-to-male ratio of 5:1.8
MANY NAMES, SAME CONDITION
In 1871, Da Costa9 described a condition he called “irritable heart syndrome” that had characteristics similar to those of POTS, including extreme fatigue and exercise intolerance. Decades later, Lewis10 and Wood11 provided more detailed descriptions of the disorder, renaming it “soldier’s heart” or “Da Costa syndrome.” As other cases were documented, more terms arose, including “effort syndrome” and “mitral valve prolapse syndrome.”
In 1982, Rosen and Cryer12 were the first to use the term “postural tachycardia syndrome” for patients with disabling tachycardia upon standing without orthostatic hypotension. In 1986, Fouad et al13 described patients with postural tachycardia, orthostatic intolerance, and a small degree of hypotension as having “idiopathic hypovolemia.”
In 1993, Schondorf and Low14 established the current definition of POTS, leading to increased awareness and research efforts to understand its pathophysiology.
MULTIFACTORIAL PATHOPHYSIOLOGY
During the last 2 decades, several often-overlapping forms of POTS have been recognized, all of which share a final common pathway of sustained orthostatic tachycardia.15–19 In addition, a number of common comorbidities were identified through review of large clinic populations of POTS.20,21
Hypovolemic POTS
Up to 70% of patients with POTS have hypovolemia. The average plasma volume deficit is about 13%, which typically causes only insignificant changes in heart rate and norepinephrine levels while a patient is supine. However, blood pooling associated with upright posture further compromises cardiac output and consequently increases sympathetic nerve activity. Abnormalities in the renin-angiotensin-aldosterone volume regulation system are also suspected to impair sodium retention, contributing to hypovolemia.1,22
Neuropathic POTS
About half of patients with POTS have partial sympathetic denervation (particularly in the lower limbs) and inadequate vasoconstriction upon standing, leading to reduced venous return and stroke volume.17,23 A compensatory increase in sympathetic tone results in tachycardia to maintain cardiac output and blood pressure.
Hyperadrenergic POTS
Up to 50% of patients with POTS have high norepinephrine levels (≥ 600 pg/mL) when upright. This subtype, hyperadrenergic POTS, is characterized by an increase in systolic blood pressure of at least 10 mm Hg within 10 minutes of standing, with concomitant tachycardia that can be similar to or greater than that seen in nonhyperadrenergic POTS. Patients with hyperadrenergic POTS tend to report more prominent symptoms of sympathetic activation, such as palpitations, anxiety, and tremulousness.24,25
Norepinephrine transporter deficiency
The norepinephrine transporter (NET) is on the presynaptic cleft of sympathetic neurons and serves to clear synaptic norepinephrine. NET deficiency leads to a hyperadrenergic state and elevated sympathetic nerve activation.18 NET deficiency may be induced by common antidepressants (eg, tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors) and attention-deficit disorder medications.4
Mast cell activation syndrome
The relationship between mast cell activation syndrome and POTS is poorly understood.4,26 Mast cell activation syndrome has been described in a subset of patients with POTS who have sinus tachycardia accompanied by severe episodic flushing. Patients with this subtype have a hyperadrenergic response to postural change and elevated urine methylhistamine during flushing episodes.
Patients with mast cell activation syndrome tend to have strong allergic symptoms and may also have severe gastrointestinal problems, food sensitivities, dermatographism, and neuropathy. Diagnosis can be difficult, as the condition is associated with numerous markers with varying sensitivity and specificity.
Autoimmune origin
A significant minority of patients report a viral-like illness before the onset of POTS symptoms, suggesting a possible autoimmune-mediated or inflammatory cause. Also, some autoimmune disorders (eg, Sjögren syndrome) can present with a POTS-like manifestation.
Research into the role of autoantibodies in the pathophysiology of POTS offers the potential to develop novel therapeutic targets. Autoantibodies that have been reported in POTS include those against M1 to M3 muscarinic receptors (present in over 87% of patients with POTS),27 cardiac lipid raft-associated proteins,28 adrenergic G-protein coupled receptors, alpha-1-adrenergic receptors, and beta-1- and beta-2-adrenergic receptors.29 Although commercial enzyme-linked immunosorbent assays can assess for these antibody fragments, it is not known whether targeting the antibodies improves outcomes. At this time, antibody testing for POTS should be confined to the research setting.
LINKS TO OTHER SYNDROMES
POTS is often associated with other conditions whose symptoms cannot be explained by postural intolerance or tachycardia.
Ehlers-Danlos syndromes are a group of inherited heterogeneous disorders involving joint hypermobility, skin hyperextensibility, and tissue fragility.30 The hypermobile subtype is most commonly associated with POTS, with patients often having symptoms of autonomic dysregulation and autonomic test abnormalities.31–33 Patients with POTS may have a history of joint subluxations, joint pain, cervical instability, and spontaneous epidural leaks. The reason for the overlap between the two syndromes is not clear.
Chronic fatigue syndrome is characterized by persistent fatigue that does not resolve with rest and is not necessarily associated with orthostatic changes. More than 75% of patients with POTS report general fatigue as a major complaint, and up to 23% meet the full criteria for chronic fatigue syndrome.34
DIAGNOSTIC STRATEGY
A patient presenting with symptoms suggestive of POTS should first undergo a detailed history and physical examination. Other causes of sinus tachycardia should be considered.
Detailed history, symptom review
The history should focus on determining symptom burden, including tachycardia onset, frequency, severity, and triggers; the presence of syncope; and the impact of symptoms on daily function and quality of life.
Presyncope and its associated symptoms occur in less than one-third of patients with POTS, and syncope is not a principal feature.4 If syncope is the predominant complaint, alternative causes should be investigated. The usual cause of syncope in the general population is thought to be vasovagal.
In addition to orthostatic intolerance, gastrointestinal disturbances are common in POTS, presenting as abdominal pain, heartburn, irregular bowel movements, diarrhea, or constipation. Symptoms of gastroparesis are less common. Gastrointestinal symptoms tend to be prolonged, lasting hours and occurring multiple times a week. They tend not to improve in the supine position.35
POTS-associated symptoms may develop insidiously, but patients often report onset after an acute stressor such as pregnancy, major surgery, or a presumed viral illness.4 Whether these putative triggers are causative or coincidental is unknown. Symptoms of orthostatic intolerance tend to be exacerbated by dehydration, heat, alcohol, exercise, and menstruation.36,37
Consider the family history: 1 in 8 patients with POTS reports familial orthostatic intolerance,38 suggesting a genetic role in some patients. Inquire about symptoms or a previous diagnosis of Ehlers-Danlos syndrome and mast cell activation syndrome.
Consider other conditions
Pheochromocytoma causes hyperadrenergic symptoms (eg, palpitations, lightheadedness) like those in POTS, but patients with pheochromocytoma typically have these symptoms while supine. Pheochromocytoma is also characterized by plasma norepinephrine levels much higher than in POTS.4 Plasma metanephrine testing helps diagnose or rule out pheochromocytoma.5
Inappropriate sinus tachycardia, like pheochromocytoma, also has clinical features similar to those of POTS, as well as tachycardia present when supine. It involves higher sympathetic tone and lower parasympathetic tone compared with POTS; patients commonly have a daytime resting heart rate of at least 100 bpm or a 24-hour mean heart rate of at least 90 bpm.1,42 While the intrinsic heart rate is heightened in inappropriate sinus tachycardia, it is not different between POTS patients and healthy individuals.42,43 Distinguishing POTS from inappropriate sinus tachycardia is further complicated by the broad inclusion criteria of most studies of inappropriate sinus tachycardia, which failed to exclude patients with POTS.44 The Heart Rhythm Society recently adopted distinct definitions for the 2 conditions.1
Physical examination: Focus on vital signs
Dependent acrocyanosis—dark red-blue discoloration of the lower legs that is cold to the touch—occurs in about half of patients with POTS upon standing.4 Dependent acrocyanosis is associated with joint hypermobility and Ehlers-Danlos syndrome, so these conditions should also be considered if findings are positive.
Laboratory testing for other causes
Laboratory testing is used mainly to detect primary causes of sinus tachycardia. Tests should include:
- Complete blood cell count with hematocrit (for severe anemia)
- Thyroid-stimulating hormone level (for hyperthyroidism)
- Electrolyte panel (for significant electrolyte disturbances).
Evidence is insufficient to support routinely measuring the vitamin B12 level, iron indices, and serum markers for celiac disease, although these may be done if the history or physical examination suggests related problems.4 Sicca symptoms (severe dry eye or dry mouth) should trigger evaluation for Sjögren syndrome.
Electrocardiography needed
Electrocardiography should be performed to investigate for cardiac conduction abnormalities as well as for resting markers of a supraventricular tachyarrhythmia. Extended ambulatory (Holter) monitoring may be useful to evaluate for a transient reentrant tachyarrhythmia4; however, it does not record body position, so it can be difficult to determine if detected episodes of tachycardia are related to posture.
Additional testing for select cases
Further investigation is usually not needed to diagnose POTS but should be considered in some cases. Advanced tests are typically performed at a tertiary care referral center and include:
- Quantitative sensory testing to evaluate for small-fiber neuropathy (ie, Quantitative Sudomotor Axon Reflex Test, or QSART), which occurs in the neuropathic POTS subtype
- Formal autonomic function testing to characterize neurovascular responsiveness
- Supine and standing plasma norepinephrine levels (fractionated catecholamines) to characterize the net activation of the sympathetic nervous system
- Blood volume assessments to assess hypovolemia
- Formal exercise testing to objectively quantify exercise capacity.
GRADED MANAGEMENT
No single universal gold-standard therapy exists for POTS, and management should be individually determined with the primary goals of treating symptoms and restoring function. A graded approach should be used, starting with conservative nonpharmacologic therapies and adding medications as needed.
While the disease course varies substantially from patient to patient, proper management is strongly associated with eventual symptom improvement.1
NONPHARMACOLOGIC STEPS FIRST
Education
Patients should be informed of the nature of their condition and referred to appropriate healthcare personnel. POTS is a chronic illness requiring individualized coping strategies, intensive physician interaction, and support of a multidisciplinary team. Patients and family members can be reassured that most symptoms improve over time with appropriate diagnosis and treatment.1 Patients should be advised to avoid aggravating triggers and activities.
Exercise
Exercise programs are encouraged but should be introduced gradually, as physical activity can exacerbate symptoms, especially at the outset. Several studies have reported benefits from a short-term (3-month) program, in which the patient gradually progresses from non-upright exercise (eg, rowing machine, recumbent cycle, swimming) to upright endurance exercises. At the end of these programs, significant cardiac remodeling, improved quality of life, and reduced heart rate responses to standing have been reported, and benefits have been reported to persist in patients who continued exercising after the 3-month study period.46,47
Despite the benefits of exercise interventions, compliance is low.46,47 To prevent early discouragement, patients should be advised that it can take 4 to 6 weeks of continued exercise before benefits appear. Patients are encouraged to exercise every other day for 30 minutes or more. Regimens should primarily focus on aerobic conditioning, but resistance training, concentrating on thigh muscles, can also help. Exercise is a treatment and not a cure, and benefits can rapidly disappear if regular activity (at least 3 times per week) is stopped.48
Compression stockings
Compression stockings help reduce peripheral venous pooling and enhance venous return to the heart. Waist-high stockings with compression of at least 30 to 40 mm Hg offer the best results.
Diet
Increased fluid and salt intake is advisable for patients with suspected hypovolemia. At least 2 to 3 L of water accompanied by 10 to 12 g of daily sodium intake is recommended.1 This can usually be accomplished with diet and salt added to food, but salt tablets can be used if the patient prefers. The resultant plasma volume expansion may help reduce the reflex tachycardia upon standing.49
Check medications
Rescue therapy with saline infusion
Intravenous saline infusion can augment blood volume in patients who are clinically decompensated and present with severe symptoms.1 Intermittent infusion of 1 L of normal saline has been found to significantly reduce orthostatic tachycardia and related symptoms in patients with POTS, contributing to improved quality of life.51,52
Chronic saline infusions are not recommended for long-term care because of the risk of access complications and infection.1 Moak et al53 reported a high rate of bacteremia in a cohort of children with POTS with regular saline infusions, most of whom had a central line. On the other hand, Ruzieh et al54 reported significantly improved symptoms with regular saline infusions without a high rate of complications, but patients in this study received infusions for only a few months and through a peripheral intravenous catheter.
DRUG THERAPY
No medications are approved by the US Food and Drug Administration (FDA) or Health Canada specifically for treating POTS, making all pharmacologic recommendations off-label. Although the drugs discussed below have been evaluated for POTS in controlled laboratory settings, they have yet to be tested in robust clinical trials.
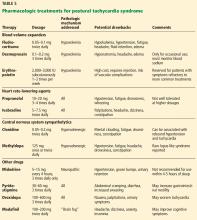
Blood volume expansion
Several drugs expand blood volume, which may reduce orthostatic tachycardia.
Fludrocortisone is a synthetic aldosterone analogue that enhances sodium and water retention. Although one observational study found that it normalizes hemodynamic changes in response to orthostatic stress, no high-level evidence exists for its effectiveness for POTS.55 It is generally well tolerated, although possible adverse effects include hyperkalemia, hypertension, fatigue, nausea, headache, and edema.5,56
Desmopressin is a synthetic version of a natural antidiuretic hormone that increases kidney-mediated free-water reabsorption without sodium retention. It significantly reduces upright heart rate in patients with POTS and improves symptom burden. Although potential adverse effects include edema and headache, hyponatremia is the primary concern with daily use, especially with the increased water intake advised for POTS.57 Patients should be advised to use desmopressin no more than once a week for the acute improvement of symptoms. Intermittent monitoring of serum sodium levels is recommended for safety.
Erythropoietin replacement has been suggested for treating POTS to address the significant deficit in red blood cell volume. Although erythropoietin therapy has a direct vasoconstrictive effect and largely improves red blood cell volume in patients with POTS, it does not expand plasma volume, so orthostatic tachycardia is not itself reduced.22 Nevertheless, it may significantly improve POTS symptoms refractory to more common methods of treatment, and it should be reserved for such cases. In addition to the lack of effect on orthostatic tachycardia, drawbacks to using erythropoietin include its high cost, the need for subcutaneous administration, and the risk of life-threatening complications such as myocardial infarction and stroke.58,59
Heart rate-lowering agents
Propranolol, a nonselective beta-adrenergic antagonist, can significantly reduce standing heart rate and improve symptoms at low dosages (10–20 mg). Higher dosages can further restrain orthostatic tachycardia but are not as well tolerated, mainly due to hypotension and worsening of existing symptoms such as fatigue.60 Regular-acting propranolol works for about 4 to 5 hours per dose, so full-day coverage often requires dosing 4 times per day.
Ivabradine is a selective blocker of the “funny” (If) channel that reduces the sinus node firing rate without affecting blood pressure, so it slows heart rate without causing supine hypertension or orthostatic hypotension.
A retrospective case series found that 60% of patients with POTS treated with ivabradine reported symptomatic improvement, and all patients experienced reduced tachycardia with continued use.61 Ivabradine has not been compared with placebo or propranolol in a randomized controlled trial, and it has not been well studied in pregnancy and so should be avoided because of potential teratogenic effects.
When prescribing ivabradine for women of childbearing age, a negative pregnancy test may be documented prior to initiation of therapy, and the use of highly effective methods of contraception is recommended. Ivabradine should be avoided in women contemplating pregnancy. Insurance coverage can limit access to ivabradine in the United States.
Central nervous system sympatholytics
Patients with prominent hyperadrenergic features may benefit from central sympatholytic agents. However, these drugs may not be well tolerated in patients with neuropathic POTS because of the effects of reduced systemic vascular resistance5 and the possible exacerbation of drowsiness, fatigue, and mental clouding.4 Patients can be extremely sensitive to these medications, so they should initially be prescribed at the lowest dose, then gradually increased as tolerated.
Clonidine, an alpha-2-adrenergic agonist, decreases central sympathetic tone. In hyperadrenergic patients, clonidine can stabilize heart rate and blood pressure, thereby reducing orthostatic symptoms.62
Methyldopa has effects similar to those of clonidine but is easier to titrate owing to its longer half-life.63 Methyldopa is typically started at 125 mg at bedtime and increased to 125 mg twice daily, if tolerated.
Other agents
Midodrine is a prodrug. The active form, an alpha-1-adrenergic agonist, constricts peripheral veins and arteries to increase vascular resistance and venous return, thereby reducing orthostatic tachycardia.52 It is most useful in patients with impaired peripheral vasoconstriction (eg, neuropathic POTS) and may be less effective in those with hyperadrenergic POTS.64 Major limitations of midodrine include worsening supine hypertension and possible urinary retention.39
Because of midodrine’s short half-life, frequent dosing is required during daytime hours (eg, 8 AM, noon, and 4 PM), but it should not be taken within 4 to 5 hours of sleep because of the risk of supine hypertension. Midodrine is typically started at 2.5 to 5 mg per dose and can be titrated up to 15 mg per dose.
Midodrine is an FDA pregnancy category C drug (adverse effects in pregnancy seen in animal models, but evidence lacking in humans). While ideally it should be avoided, we have used it safely in pregnant women with disabling POTS symptoms.
Pyridostigmine, an acetylcholinesterase inhibitor, increases cardiovagal tone and possibly sympathetic tone. It has been reported to significantly reduce standing heart rate and improve symptom burden in patients with POTS.65 However, pyridostigmine increases gastrointestinal mobility, leading to severe adverse effects in over 20% of patients, including abdominal cramps, nausea, and diarrhea.66
Droxidopa, a synthetic amino acid precursor of norepinephrine, improves dizziness and fatigue in POTS with minimal effects on blood pressure.67
Modafinil, a psychostimulant, may improve POTS-associated cognitive symptoms.4 It also raises upright blood pressure without significantly worsening standing heart rate or acute orthostatic symptoms.68
EFFECTS OF COMORBID DISORDERS ON MANAGEMENT
Ehlers-Danlos syndrome
Pharmacologic approaches to POTS should not be altered based on the presence of Ehlers-Danlos syndrome, but because many of these patients are prone to joint dislocation, exercise prescriptions may need adjusting.
A medical genetics consult is recommended for patients with Ehlers-Danlos syndrome. Although the hypermobile type (the form most commonly associated with POTS) is not associated with aortopathy, it can be confused with classical and vascular Ehlers-Danlos syndromes, which require serial aortic screening.30
Mast cell activation syndrome
Consultation with an allergist or immunologist may help patients with severe symptoms.
Autoantibodies and autoimmunity
Treatment of the underlying disorder is recommended and can result in significantly improved POTS symptoms.
SPECIALTY CARE REFERRAL
POTS can be challenging to manage. Given the range of physiologic, emotional, and functional distress patients experience, it often requires significant physician time and multidisciplinary care. Patients with continued severe or debilitating symptoms may benefit from referral to a tertiary-care center with experience in autonomic nervous system disorders.
PROGNOSIS
Limited data are available on the long-term prognosis of POTS, and more studies are needed in pediatric and adult populations. No deaths have been reported in the handful of published cases of POTS in patients older than 50.1 Some pediatric studies suggest that some teenagers “outgrow” their POTS. However, these data are not robust, and an alternative explanation is that as they get older, they see adult physicians for their POTS symptoms and so are lost to study follow-up.6,44,69
We have not often seen POTS simply resolve without ongoing treatment. However, in our experience, most patients have improved symptoms and function with multimodal treatment (ie, exercise, salt, water, stockings, and some medications) and time.
- Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015; 12(6):e41–e63. doi:10.1016/j.hrthm.2015.03.029
- Bagai K, Song Y, Ling JF, et al. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med 2011; 7(2):204–210. pmid:21509337
- Benrud-Larson LM, Dewar MS, Sandroni P, Rummans TA, Haythornthwaite JA, Low PA. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc 2002; 77(6):531–537. doi:10.4065/77.6.531
- Raj SR. Postural tachycardia syndrome (POTS). Circulation 2013; 127(23):2336–2342. doi:10.1161/CIRCULATIONAHA.112.144501
- Raj SR. The postural tachycardia syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J 2006; 6(2):84–99. pmid:16943900
- Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: what is abnormal? J Pediatr 2012; 160(2):222–226. doi:10.1016/j.jpeds.2011.08.054
- Mar PL, Raj SR. Neuronal and hormonal perturbations in postural tachycardia syndrome. Front Physiol 2014; 5:220. doi:10.3389/fphys.2014.00220
- Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology 2007; 69(8):790–798. doi:10.1212/01.wnl.0000267663.05398.40
- Da Costa JM. On irritable heart: a clinical study of a form of functional cardiac disorder and its consequences. Am J Med Sci 1871; 61(121):2–52.
- Lewis T. The tolerance of physical exertion, as shown by soldiers suffering from so-called “irritable heart.” Br Med J 1918; 1(2987):363–365. pmid:20768980
- Wood P. Da Costa’s syndrome (or effort syndrome): lecture I. Br Med J 1941; 1(4194):767–772. pmid:20783672
- Rosen SG, Cryer PE. Postural tachycardia syndrome. Reversal of sympathetic hyperresponsiveness and clinical improvement during sodium loading. Am J Med 1982; 72(5):847–850.
- Fouad FM, Tadena-Thome L, Bravo EL, Tarazi RC. Idiopathic hypovolemia. Ann Intern Med 1986; 104(3):298–303. pmid:3511818
- Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 1993; 43(1):132–137. pmid:8423877
- Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med 2000; 343(12):847–855. doi:10.1056/NEJM200009213431204
- Raj SR, Robertson D. Blood volume perturbations in the postural tachycardia syndrome. Am J Med Sci 2007; 334(1):57–60. doi:10.1097/MAJ.0b013e318063c6c0
- Jacob G, Costa F, Shannon JR, et al. The neuropathic postural tachycardia syndrome. N Engl J Med 2000; 343(14):1008–1014. doi:10.1056/NEJM200010053431404
- Shannon JR, Flattem NL, Jordan J, et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med 2000; 342(8):541–549. doi:10.1056/NEJM200002243420803
- Jones PK, Shaw BH, Raj SR. Clinical challenges in the diagnosis and management of postural tachycardia syndrome. Pract Neurol 2016; 16(6):431–438. doi:10.1136/practneurol-2016-001405
- Gunning WT, Karabin BL, Blomquist TM, Grubb BP. Postural orthostatic tachycardia syndrome is associated with platelet storage pool deficiency. Medicine (Baltimore) 2016; 95(37):e4849. doi:10.1097/MD.0000000000004849
- Kanjwal K, Sheikh M, Karabin B, Kanjwal Y, Grubb BP. Neurocardiogenic syncope coexisting with postural orthostatic tachycardia syndrome in patients suffering from orthostatic intolerance: a combined form of autonomic dysfunction. Pacing Clin Electrophysiol 2011; 34(5):549–554. doi:10.1111/j.1540-8159.2010.02994.x
- Raj SR, Biaggioni I, Yamhure PC, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation 2005; 111(13):1574–1582. doi:10.1161/01.CIR.0000160356.97313.5D
- Gibbons CH, Bonyhay I, Benson A, Wang N, Freeman R. Structural and functional small fiber abnormalities in the neuropathic postural tachycardia syndrome. PLoS One 2013; 8(12):e84716. doi:10.1371/journal.pone.0084716
- Low PA, Sandroni P, Joyner M, Shen WK. Postural tachycardia syndrome (POTS). J Cardiovasc Electrophysiol 2009; 20(3):352–358. doi:10.1111/j.1540-8167.2008.01407.x
- Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Grubb BP. Clinical presentation and management of patients with hyperadrenergic postural orthostatic tachycardia syndrome. A single center experience. Cardiol J 2011; 18(5):527–531. pmid:21947988
- Shibao C, Arzubiaga C, Roberts J, et al. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension 2005; 45(3):385–390. doi:10.1161/01.HYP.0000158259.68614.40
- Dubey D, Hopkins S, Vernino S. M1 and M2 muscarinic receptor antibodies among patients with postural orthostatic tachycardia syndrome: potential disease biomarker [abstract]. J Clin Neuromuscul Dis 2016; 17(3):179S.
- Wang XL, Ling TY, Charlesworth MC, et al. Autoimmunoreactive IgGs against cardiac lipid raft-associated proteins in patients with postural orthostatic tachycardia syndrome. Transl Res 2013; 162(1):34–44. doi:10.1016/j.trsl.2013.03.002
- Li H, Yu X, Liles C, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc 2014; 3(1):e000755. doi:10.1161/JAHA.113.000755
- Malfait F, Francomano C, Byers P, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet 2017; 175(1):8–26. doi:10.1002/ajmg.c.31552
- Wallman D, Weinberg J, Hohler AD. Ehlers-Danlos syndrome and postural tachycardia syndrome: a relationship study. J Neurol Sci 2014; 340(1-2):99–102. doi:10.1016/j.jns.2014.03.002
- De Wandele I, Calders P, Peersman W, et al. Autonomic symptom burden in the hypermobility type of Ehlers-Danlos syndrome: a comparative study with two other EDS types, fibromyalgia, and healthy controls. Semin Arthritis Rheum 2014; 44(3):353–361. doi:10.1016/j.semarthrit.2014.05.013
- Gazit Y, Nahir AM, Grahame R, Jacob G. Dysautonomia in the joint hypermobility syndrome. Am J Med 2003; 115(1):33–40. pmid:12867232
- Okamoto LE, Raj SR, Peltier A, et al. Neurohumoral and haemodynamic profile in postural tachycardia and chronic fatigue syndromes. Clin Sci (Lond) 2012; 122(4):183–192. doi:10.1042/CS20110200
- Wang LB, Culbertson CJ, Deb A, Morgenshtern K, Huang H, Hohler AD. Gastrointestinal dysfunction in postural tachycardia syndrome. J Neurol Sci 2015; 359(1-2):193–196. doi:10.1016/j.jns.2015.10.052
- Raj S, Sheldon R. Management of postural tachycardia syndrome, inappropriate sinus tachycardia and vasovagal syncope. Arrhythm Electrophysiol Rev 2016; 5(2):122–129. doi:10.15420/AER.2016.7.2
- Peggs KJ, Nguyen H, Enayat D, Keller NR, Al-Hendy A, Raj SR. Gynecologic disorders and menstrual cycle lightheadedness in postural tachycardia syndrome. Int J Gynaecol Obstet 2012; 118(3):242–246. doi:10.1016/j.ijgo.2012.04.014
- Thieben MJ, Sandroni P, Sletten DM, et al. Postural orthostatic tachycardia syndrome: the Mayo Clinic experience. Mayo Clin Proc 2007; 82(3):308–313. doi:10.4065/82.3.308
- Deb A, Morgenshtern K, Culbertson CJ, Wang LB, Hohler AD. A survey-based analysis of symptoms in patients with postural orthostatic tachycardia syndrome. J Am Coll Cardiol 2015; 28(7):157–159. pmid:25829642
- Ertek S, Cicero AF. Hyperthyroidism and cardiovascular complications: a narrative review on the basis of pathophysiology. Arch Med Sci 2013; 9(5):944–952. doi:10.5114/aoms.2013.38685
- Rangno RE, Langlois S. Comparison of withdrawal phenomena after propranolol, metoprolol and pindolol. Br J Clin Pharmacol 1982; 13(suppl 2):345S–351S. pmid:6125187
- Nwazue VC, Paranjape SY, Black BK, et al. Postural tachycardia syndrome and inappropriate sinus tachycardia: role of autonomic modulation and sinus node automaticity. J Am Heart Assoc 2014; 3(2):e000700. doi:10.1161/JAHA.113.000700
- Morillo CA, Klein GJ, Thakur RK, Li H, Zardini M, Yee R. Mechanism of “inappropriate” sinus tachycardia. Role of sympathovagal balance. Circulation 1994; 90(2):873–877. pmid:7913886
- Grubb BP. Postural tachycardia syndrome. Circulation 2008; 117(21):2814–2817. doi:10.1161/CIRCULATIONAHA.107.761643
- Bhatia R, Kizilbash SJ, Ahrens SP, et al. Outcomes of adolescent-onset postural orthostatic tachycardia syndrome. J Pediatr 2016; 173:149–153. doi:10.1016/j.jpeds.2016.02.035
- George SA, Bivens TB, Howden EJ, et al. The international POTS registry: evaluating the efficacy of an exercise training intervention in a community setting. Heart Rhythm 2016; 13(4):943–950. doi:10.1016/j.hrthm.2015.12.012
- Fu Q, VanGundy TB, Galbreath MM, et al. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol 2010; 55(25):2858–2868. doi:10.1016/j.jacc.2010.02.043
- Raj SR. Row, row, row your way to treating postural tachycardia syndrome. Heart Rhythm 2016; 13(4):951–952. doi:10.1016/j.hrthm.2015.12.039
- Celedonio JE, Garland EM, Nwazue VC, et al. Effects of high sodium intake on blood volume and catecholamines in patients with postural tachycardia syndrome and healthy females [abstract]. Clin Auton Res 2014; 24:211.
- Garland EM, Celedonio JE, Raj SR. Postural tachycardia syndrome: beyond orthostatic intolerance. Curr Neurol Neurosci Rep 2015; 15(9):60. doi:10.1007/s11910-015-0583-8
- Gordon VM, Opfer-Gehrking TL, Novak V, Low PA. Hemodynamic and symptomatic effects of acute interventions on tilt in patients with postural tachycardia syndrome. Clin Auton Res 2000; 10:29–33. pmid:10750641
- Jacob G, Shannon JR, Black B, et al. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation 1997; 96(2):575–580. pmid:9244228
- Moak JP, Leong D, Fabian R, et al. Intravenous hydration for management of medication-resistant orthostatic intolerance in the adolescent and young adult. Pediatr Cardiol 2016; 37(2):278–282. doi:10.1007/s00246-015-1274-6
- Ruzieh M, Baugh A, Dasa O, et al. Effects of intermittent intravenous saline infusions in patients with medication-refractory postural tachycardia syndrome. J Interv Card Electrophysiol 2017; 48(3):255–260. doi:10.1007/s10840-017-0225-y
- Freitas J, Santos R, Azevedo E, Costa O, Carvalho M, de Freitas AF. Clinical improvement in patients with orthostatic intolerance after treatment with bisoprolol and fludrocortisone. Clin Auton Res 2000; 10(5):293–299. pmid:11198485
- Lee AK, Krahn AD. Evaluation of syncope: focus on diagnosis and treatment of neurally mediated syncope. Expert Rev Cardiovasc Ther 2016; 14(6):725–736. doi:10.1586/14779072.2016.1164034
- Coffin ST, Black BK, Biaggioni I, et al. Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Heart Rhythm 2012; 9(9):1484–1490. doi:10.1016/j.hrthm.2012.05.002
- Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Sheikh M, Grubb BP. Erythropoietin in the treatment of postural orthostatic tachycardia syndrome. Am J Ther 2012; 19(2):92–95. doi:10.1097/MJT.0b013e3181ef621a
- Hoeldtke RD, Horvath GG, Bryner KD. Treatment of orthostatic tachycardia with erythropoietin. Am J Med 1995; 99(5):525–529. pmid:7485211
- Raj SR, Black BK, Biaggioni I, et al. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation 2009; 120(9):725–734. doi:10.1161/CIRCULATIONAHA.108.846501
- McDonald C, Frith J, Newton JL. Single centre experience of ivabradine in postural orthostatic tachycardia syndrome. Europace 2011; 13(3):427–430. doi:10.1093/europace/euq390
- Gaffney FA, Lane LB, Pettinger W, Blomqvist G. Effects of long-term clonidine administration on the hemodynamic and neuroendocrine postural responses of patients with dysautonomia. Chest 1983; 83(suppl 2):436–438. pmid:6295714
- Jacob G, Biaggioni I. Idiopathic orthostatic intolerance and postural tachycardia syndromes. Am J Med Sci 1999; 317(2):88–101. pmid:10037112
- Ross AJ, Ocon AJ, Medow MS, Stewart JM. A double-blind placebo-controlled cross-over study of the vascular effects of midodrine in neuropathic compared with hyperadrenergic postural tachycardia syndrome. Clin Sci (Lond) 2014; 126(4):289–296. doi:10.1042/CS20130222
- Raj SR, Black BK, Biaggioni I, Harris PA, Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation 2005; 111(21):2734–2340. doi:10.1161/CIRCULATIONAHA.104.497594
- Kanjwal K, Karabin B, Sheikh M, et al. Pyridostigmine in the treatment of postural orthostatic tachycardia: A single-center experience. Pacing Clin Electrophysiol 2011; 34(6):750–755. doi:10.1111/j.1540-8159.2011.03047.x
- Ruzieh M, Dasa O, Pacenta A, Karabin B, Grubb B. Droxidopa in the treatment of postural orthostatic tachycardia syndrome. Am J Ther 2017; 24(2):e157–e161. doi:10.1097/MJT.0000000000000468
- Kpaeyeh AG Jr, Mar PL, Raj V, et al. Hemodynamic profiles and tolerability of modafinil in the treatment of POTS: a randomized placebo-controlled trial. J Clin Psychopharmacol 2014; 34(6):738–741. doi:10.1097/JCP.0000000000000221
- Lai CC, Fischer PR, Brands CK, et al. Outcomes in adolescents with postural orthostatic tachycardia syndrome treated with midodrine and beta-blockers. Pacing Clin Electrophysiol 2009; 32(2):234–238. doi:10.1111/j.1540-8159.2008.02207.x
Some people, most of them relatively young women, experience lightheadedness, a racing heart, and other symptoms (but not hypotension) when they stand up, in a condition known as postural tachycardia syndrome (POTS).1 Although not known to shorten life,1 it can be physically and mentally debilitating.2,3 Therapy rarely cures it, but a multifaceted approach can substantially improve quality of life.
This review outlines the evaluation and diagnosis of POTS and provides guidance for a therapy regimen.
HOW IS POTS DEFINED?
POTS is a multifactorial syndrome rather than a specific disease. It is characterized by all of the following1,4–6:
- An increase in heart rate of ≥ 30 bpm, or ≥ 40 bpm for those under age 19, within 10 minutes of standing from a supine position
- Sustained tachycardia (> 30 seconds)
- Absence of orthostatic hypotension (a fall in blood pressure of ≥ 20/10 mm Hg)
- Frequent and chronic duration (≥ 6 months).
These features are critical to diagnosis. Hemodynamic criteria in isolation may describe postural tachycardia but are not sufficient to diagnose POTS.
The prevalence of POTS is estimated to be between 0.2% and 1.0%,7 affecting up to 3 million people in the United States. Most cases arise between ages 13 and 50, with a female-to-male ratio of 5:1.8
MANY NAMES, SAME CONDITION
In 1871, Da Costa9 described a condition he called “irritable heart syndrome” that had characteristics similar to those of POTS, including extreme fatigue and exercise intolerance. Decades later, Lewis10 and Wood11 provided more detailed descriptions of the disorder, renaming it “soldier’s heart” or “Da Costa syndrome.” As other cases were documented, more terms arose, including “effort syndrome” and “mitral valve prolapse syndrome.”
In 1982, Rosen and Cryer12 were the first to use the term “postural tachycardia syndrome” for patients with disabling tachycardia upon standing without orthostatic hypotension. In 1986, Fouad et al13 described patients with postural tachycardia, orthostatic intolerance, and a small degree of hypotension as having “idiopathic hypovolemia.”
In 1993, Schondorf and Low14 established the current definition of POTS, leading to increased awareness and research efforts to understand its pathophysiology.
MULTIFACTORIAL PATHOPHYSIOLOGY
During the last 2 decades, several often-overlapping forms of POTS have been recognized, all of which share a final common pathway of sustained orthostatic tachycardia.15–19 In addition, a number of common comorbidities were identified through review of large clinic populations of POTS.20,21
Hypovolemic POTS
Up to 70% of patients with POTS have hypovolemia. The average plasma volume deficit is about 13%, which typically causes only insignificant changes in heart rate and norepinephrine levels while a patient is supine. However, blood pooling associated with upright posture further compromises cardiac output and consequently increases sympathetic nerve activity. Abnormalities in the renin-angiotensin-aldosterone volume regulation system are also suspected to impair sodium retention, contributing to hypovolemia.1,22
Neuropathic POTS
About half of patients with POTS have partial sympathetic denervation (particularly in the lower limbs) and inadequate vasoconstriction upon standing, leading to reduced venous return and stroke volume.17,23 A compensatory increase in sympathetic tone results in tachycardia to maintain cardiac output and blood pressure.
Hyperadrenergic POTS
Up to 50% of patients with POTS have high norepinephrine levels (≥ 600 pg/mL) when upright. This subtype, hyperadrenergic POTS, is characterized by an increase in systolic blood pressure of at least 10 mm Hg within 10 minutes of standing, with concomitant tachycardia that can be similar to or greater than that seen in nonhyperadrenergic POTS. Patients with hyperadrenergic POTS tend to report more prominent symptoms of sympathetic activation, such as palpitations, anxiety, and tremulousness.24,25
Norepinephrine transporter deficiency
The norepinephrine transporter (NET) is on the presynaptic cleft of sympathetic neurons and serves to clear synaptic norepinephrine. NET deficiency leads to a hyperadrenergic state and elevated sympathetic nerve activation.18 NET deficiency may be induced by common antidepressants (eg, tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors) and attention-deficit disorder medications.4
Mast cell activation syndrome
The relationship between mast cell activation syndrome and POTS is poorly understood.4,26 Mast cell activation syndrome has been described in a subset of patients with POTS who have sinus tachycardia accompanied by severe episodic flushing. Patients with this subtype have a hyperadrenergic response to postural change and elevated urine methylhistamine during flushing episodes.
Patients with mast cell activation syndrome tend to have strong allergic symptoms and may also have severe gastrointestinal problems, food sensitivities, dermatographism, and neuropathy. Diagnosis can be difficult, as the condition is associated with numerous markers with varying sensitivity and specificity.
Autoimmune origin
A significant minority of patients report a viral-like illness before the onset of POTS symptoms, suggesting a possible autoimmune-mediated or inflammatory cause. Also, some autoimmune disorders (eg, Sjögren syndrome) can present with a POTS-like manifestation.
Research into the role of autoantibodies in the pathophysiology of POTS offers the potential to develop novel therapeutic targets. Autoantibodies that have been reported in POTS include those against M1 to M3 muscarinic receptors (present in over 87% of patients with POTS),27 cardiac lipid raft-associated proteins,28 adrenergic G-protein coupled receptors, alpha-1-adrenergic receptors, and beta-1- and beta-2-adrenergic receptors.29 Although commercial enzyme-linked immunosorbent assays can assess for these antibody fragments, it is not known whether targeting the antibodies improves outcomes. At this time, antibody testing for POTS should be confined to the research setting.
LINKS TO OTHER SYNDROMES
POTS is often associated with other conditions whose symptoms cannot be explained by postural intolerance or tachycardia.
Ehlers-Danlos syndromes are a group of inherited heterogeneous disorders involving joint hypermobility, skin hyperextensibility, and tissue fragility.30 The hypermobile subtype is most commonly associated with POTS, with patients often having symptoms of autonomic dysregulation and autonomic test abnormalities.31–33 Patients with POTS may have a history of joint subluxations, joint pain, cervical instability, and spontaneous epidural leaks. The reason for the overlap between the two syndromes is not clear.
Chronic fatigue syndrome is characterized by persistent fatigue that does not resolve with rest and is not necessarily associated with orthostatic changes. More than 75% of patients with POTS report general fatigue as a major complaint, and up to 23% meet the full criteria for chronic fatigue syndrome.34
DIAGNOSTIC STRATEGY
A patient presenting with symptoms suggestive of POTS should first undergo a detailed history and physical examination. Other causes of sinus tachycardia should be considered.
Detailed history, symptom review
The history should focus on determining symptom burden, including tachycardia onset, frequency, severity, and triggers; the presence of syncope; and the impact of symptoms on daily function and quality of life.
Presyncope and its associated symptoms occur in less than one-third of patients with POTS, and syncope is not a principal feature.4 If syncope is the predominant complaint, alternative causes should be investigated. The usual cause of syncope in the general population is thought to be vasovagal.
In addition to orthostatic intolerance, gastrointestinal disturbances are common in POTS, presenting as abdominal pain, heartburn, irregular bowel movements, diarrhea, or constipation. Symptoms of gastroparesis are less common. Gastrointestinal symptoms tend to be prolonged, lasting hours and occurring multiple times a week. They tend not to improve in the supine position.35
POTS-associated symptoms may develop insidiously, but patients often report onset after an acute stressor such as pregnancy, major surgery, or a presumed viral illness.4 Whether these putative triggers are causative or coincidental is unknown. Symptoms of orthostatic intolerance tend to be exacerbated by dehydration, heat, alcohol, exercise, and menstruation.36,37
Consider the family history: 1 in 8 patients with POTS reports familial orthostatic intolerance,38 suggesting a genetic role in some patients. Inquire about symptoms or a previous diagnosis of Ehlers-Danlos syndrome and mast cell activation syndrome.
Consider other conditions
Pheochromocytoma causes hyperadrenergic symptoms (eg, palpitations, lightheadedness) like those in POTS, but patients with pheochromocytoma typically have these symptoms while supine. Pheochromocytoma is also characterized by plasma norepinephrine levels much higher than in POTS.4 Plasma metanephrine testing helps diagnose or rule out pheochromocytoma.5
Inappropriate sinus tachycardia, like pheochromocytoma, also has clinical features similar to those of POTS, as well as tachycardia present when supine. It involves higher sympathetic tone and lower parasympathetic tone compared with POTS; patients commonly have a daytime resting heart rate of at least 100 bpm or a 24-hour mean heart rate of at least 90 bpm.1,42 While the intrinsic heart rate is heightened in inappropriate sinus tachycardia, it is not different between POTS patients and healthy individuals.42,43 Distinguishing POTS from inappropriate sinus tachycardia is further complicated by the broad inclusion criteria of most studies of inappropriate sinus tachycardia, which failed to exclude patients with POTS.44 The Heart Rhythm Society recently adopted distinct definitions for the 2 conditions.1
Physical examination: Focus on vital signs
Dependent acrocyanosis—dark red-blue discoloration of the lower legs that is cold to the touch—occurs in about half of patients with POTS upon standing.4 Dependent acrocyanosis is associated with joint hypermobility and Ehlers-Danlos syndrome, so these conditions should also be considered if findings are positive.
Laboratory testing for other causes
Laboratory testing is used mainly to detect primary causes of sinus tachycardia. Tests should include:
- Complete blood cell count with hematocrit (for severe anemia)
- Thyroid-stimulating hormone level (for hyperthyroidism)
- Electrolyte panel (for significant electrolyte disturbances).
Evidence is insufficient to support routinely measuring the vitamin B12 level, iron indices, and serum markers for celiac disease, although these may be done if the history or physical examination suggests related problems.4 Sicca symptoms (severe dry eye or dry mouth) should trigger evaluation for Sjögren syndrome.
Electrocardiography needed
Electrocardiography should be performed to investigate for cardiac conduction abnormalities as well as for resting markers of a supraventricular tachyarrhythmia. Extended ambulatory (Holter) monitoring may be useful to evaluate for a transient reentrant tachyarrhythmia4; however, it does not record body position, so it can be difficult to determine if detected episodes of tachycardia are related to posture.
Additional testing for select cases
Further investigation is usually not needed to diagnose POTS but should be considered in some cases. Advanced tests are typically performed at a tertiary care referral center and include:
- Quantitative sensory testing to evaluate for small-fiber neuropathy (ie, Quantitative Sudomotor Axon Reflex Test, or QSART), which occurs in the neuropathic POTS subtype
- Formal autonomic function testing to characterize neurovascular responsiveness
- Supine and standing plasma norepinephrine levels (fractionated catecholamines) to characterize the net activation of the sympathetic nervous system
- Blood volume assessments to assess hypovolemia
- Formal exercise testing to objectively quantify exercise capacity.
GRADED MANAGEMENT
No single universal gold-standard therapy exists for POTS, and management should be individually determined with the primary goals of treating symptoms and restoring function. A graded approach should be used, starting with conservative nonpharmacologic therapies and adding medications as needed.
While the disease course varies substantially from patient to patient, proper management is strongly associated with eventual symptom improvement.1
NONPHARMACOLOGIC STEPS FIRST
Education
Patients should be informed of the nature of their condition and referred to appropriate healthcare personnel. POTS is a chronic illness requiring individualized coping strategies, intensive physician interaction, and support of a multidisciplinary team. Patients and family members can be reassured that most symptoms improve over time with appropriate diagnosis and treatment.1 Patients should be advised to avoid aggravating triggers and activities.
Exercise
Exercise programs are encouraged but should be introduced gradually, as physical activity can exacerbate symptoms, especially at the outset. Several studies have reported benefits from a short-term (3-month) program, in which the patient gradually progresses from non-upright exercise (eg, rowing machine, recumbent cycle, swimming) to upright endurance exercises. At the end of these programs, significant cardiac remodeling, improved quality of life, and reduced heart rate responses to standing have been reported, and benefits have been reported to persist in patients who continued exercising after the 3-month study period.46,47
Despite the benefits of exercise interventions, compliance is low.46,47 To prevent early discouragement, patients should be advised that it can take 4 to 6 weeks of continued exercise before benefits appear. Patients are encouraged to exercise every other day for 30 minutes or more. Regimens should primarily focus on aerobic conditioning, but resistance training, concentrating on thigh muscles, can also help. Exercise is a treatment and not a cure, and benefits can rapidly disappear if regular activity (at least 3 times per week) is stopped.48
Compression stockings
Compression stockings help reduce peripheral venous pooling and enhance venous return to the heart. Waist-high stockings with compression of at least 30 to 40 mm Hg offer the best results.
Diet
Increased fluid and salt intake is advisable for patients with suspected hypovolemia. At least 2 to 3 L of water accompanied by 10 to 12 g of daily sodium intake is recommended.1 This can usually be accomplished with diet and salt added to food, but salt tablets can be used if the patient prefers. The resultant plasma volume expansion may help reduce the reflex tachycardia upon standing.49
Check medications
Rescue therapy with saline infusion
Intravenous saline infusion can augment blood volume in patients who are clinically decompensated and present with severe symptoms.1 Intermittent infusion of 1 L of normal saline has been found to significantly reduce orthostatic tachycardia and related symptoms in patients with POTS, contributing to improved quality of life.51,52
Chronic saline infusions are not recommended for long-term care because of the risk of access complications and infection.1 Moak et al53 reported a high rate of bacteremia in a cohort of children with POTS with regular saline infusions, most of whom had a central line. On the other hand, Ruzieh et al54 reported significantly improved symptoms with regular saline infusions without a high rate of complications, but patients in this study received infusions for only a few months and through a peripheral intravenous catheter.
DRUG THERAPY
No medications are approved by the US Food and Drug Administration (FDA) or Health Canada specifically for treating POTS, making all pharmacologic recommendations off-label. Although the drugs discussed below have been evaluated for POTS in controlled laboratory settings, they have yet to be tested in robust clinical trials.
Blood volume expansion
Several drugs expand blood volume, which may reduce orthostatic tachycardia.
Fludrocortisone is a synthetic aldosterone analogue that enhances sodium and water retention. Although one observational study found that it normalizes hemodynamic changes in response to orthostatic stress, no high-level evidence exists for its effectiveness for POTS.55 It is generally well tolerated, although possible adverse effects include hyperkalemia, hypertension, fatigue, nausea, headache, and edema.5,56
Desmopressin is a synthetic version of a natural antidiuretic hormone that increases kidney-mediated free-water reabsorption without sodium retention. It significantly reduces upright heart rate in patients with POTS and improves symptom burden. Although potential adverse effects include edema and headache, hyponatremia is the primary concern with daily use, especially with the increased water intake advised for POTS.57 Patients should be advised to use desmopressin no more than once a week for the acute improvement of symptoms. Intermittent monitoring of serum sodium levels is recommended for safety.
Erythropoietin replacement has been suggested for treating POTS to address the significant deficit in red blood cell volume. Although erythropoietin therapy has a direct vasoconstrictive effect and largely improves red blood cell volume in patients with POTS, it does not expand plasma volume, so orthostatic tachycardia is not itself reduced.22 Nevertheless, it may significantly improve POTS symptoms refractory to more common methods of treatment, and it should be reserved for such cases. In addition to the lack of effect on orthostatic tachycardia, drawbacks to using erythropoietin include its high cost, the need for subcutaneous administration, and the risk of life-threatening complications such as myocardial infarction and stroke.58,59
Heart rate-lowering agents
Propranolol, a nonselective beta-adrenergic antagonist, can significantly reduce standing heart rate and improve symptoms at low dosages (10–20 mg). Higher dosages can further restrain orthostatic tachycardia but are not as well tolerated, mainly due to hypotension and worsening of existing symptoms such as fatigue.60 Regular-acting propranolol works for about 4 to 5 hours per dose, so full-day coverage often requires dosing 4 times per day.
Ivabradine is a selective blocker of the “funny” (If) channel that reduces the sinus node firing rate without affecting blood pressure, so it slows heart rate without causing supine hypertension or orthostatic hypotension.
A retrospective case series found that 60% of patients with POTS treated with ivabradine reported symptomatic improvement, and all patients experienced reduced tachycardia with continued use.61 Ivabradine has not been compared with placebo or propranolol in a randomized controlled trial, and it has not been well studied in pregnancy and so should be avoided because of potential teratogenic effects.
When prescribing ivabradine for women of childbearing age, a negative pregnancy test may be documented prior to initiation of therapy, and the use of highly effective methods of contraception is recommended. Ivabradine should be avoided in women contemplating pregnancy. Insurance coverage can limit access to ivabradine in the United States.
Central nervous system sympatholytics
Patients with prominent hyperadrenergic features may benefit from central sympatholytic agents. However, these drugs may not be well tolerated in patients with neuropathic POTS because of the effects of reduced systemic vascular resistance5 and the possible exacerbation of drowsiness, fatigue, and mental clouding.4 Patients can be extremely sensitive to these medications, so they should initially be prescribed at the lowest dose, then gradually increased as tolerated.
Clonidine, an alpha-2-adrenergic agonist, decreases central sympathetic tone. In hyperadrenergic patients, clonidine can stabilize heart rate and blood pressure, thereby reducing orthostatic symptoms.62
Methyldopa has effects similar to those of clonidine but is easier to titrate owing to its longer half-life.63 Methyldopa is typically started at 125 mg at bedtime and increased to 125 mg twice daily, if tolerated.
Other agents
Midodrine is a prodrug. The active form, an alpha-1-adrenergic agonist, constricts peripheral veins and arteries to increase vascular resistance and venous return, thereby reducing orthostatic tachycardia.52 It is most useful in patients with impaired peripheral vasoconstriction (eg, neuropathic POTS) and may be less effective in those with hyperadrenergic POTS.64 Major limitations of midodrine include worsening supine hypertension and possible urinary retention.39
Because of midodrine’s short half-life, frequent dosing is required during daytime hours (eg, 8 AM, noon, and 4 PM), but it should not be taken within 4 to 5 hours of sleep because of the risk of supine hypertension. Midodrine is typically started at 2.5 to 5 mg per dose and can be titrated up to 15 mg per dose.
Midodrine is an FDA pregnancy category C drug (adverse effects in pregnancy seen in animal models, but evidence lacking in humans). While ideally it should be avoided, we have used it safely in pregnant women with disabling POTS symptoms.
Pyridostigmine, an acetylcholinesterase inhibitor, increases cardiovagal tone and possibly sympathetic tone. It has been reported to significantly reduce standing heart rate and improve symptom burden in patients with POTS.65 However, pyridostigmine increases gastrointestinal mobility, leading to severe adverse effects in over 20% of patients, including abdominal cramps, nausea, and diarrhea.66
Droxidopa, a synthetic amino acid precursor of norepinephrine, improves dizziness and fatigue in POTS with minimal effects on blood pressure.67
Modafinil, a psychostimulant, may improve POTS-associated cognitive symptoms.4 It also raises upright blood pressure without significantly worsening standing heart rate or acute orthostatic symptoms.68
EFFECTS OF COMORBID DISORDERS ON MANAGEMENT
Ehlers-Danlos syndrome
Pharmacologic approaches to POTS should not be altered based on the presence of Ehlers-Danlos syndrome, but because many of these patients are prone to joint dislocation, exercise prescriptions may need adjusting.
A medical genetics consult is recommended for patients with Ehlers-Danlos syndrome. Although the hypermobile type (the form most commonly associated with POTS) is not associated with aortopathy, it can be confused with classical and vascular Ehlers-Danlos syndromes, which require serial aortic screening.30
Mast cell activation syndrome
Consultation with an allergist or immunologist may help patients with severe symptoms.
Autoantibodies and autoimmunity
Treatment of the underlying disorder is recommended and can result in significantly improved POTS symptoms.
SPECIALTY CARE REFERRAL
POTS can be challenging to manage. Given the range of physiologic, emotional, and functional distress patients experience, it often requires significant physician time and multidisciplinary care. Patients with continued severe or debilitating symptoms may benefit from referral to a tertiary-care center with experience in autonomic nervous system disorders.
PROGNOSIS
Limited data are available on the long-term prognosis of POTS, and more studies are needed in pediatric and adult populations. No deaths have been reported in the handful of published cases of POTS in patients older than 50.1 Some pediatric studies suggest that some teenagers “outgrow” their POTS. However, these data are not robust, and an alternative explanation is that as they get older, they see adult physicians for their POTS symptoms and so are lost to study follow-up.6,44,69
We have not often seen POTS simply resolve without ongoing treatment. However, in our experience, most patients have improved symptoms and function with multimodal treatment (ie, exercise, salt, water, stockings, and some medications) and time.
Some people, most of them relatively young women, experience lightheadedness, a racing heart, and other symptoms (but not hypotension) when they stand up, in a condition known as postural tachycardia syndrome (POTS).1 Although not known to shorten life,1 it can be physically and mentally debilitating.2,3 Therapy rarely cures it, but a multifaceted approach can substantially improve quality of life.
This review outlines the evaluation and diagnosis of POTS and provides guidance for a therapy regimen.
HOW IS POTS DEFINED?
POTS is a multifactorial syndrome rather than a specific disease. It is characterized by all of the following1,4–6:
- An increase in heart rate of ≥ 30 bpm, or ≥ 40 bpm for those under age 19, within 10 minutes of standing from a supine position
- Sustained tachycardia (> 30 seconds)
- Absence of orthostatic hypotension (a fall in blood pressure of ≥ 20/10 mm Hg)
- Frequent and chronic duration (≥ 6 months).
These features are critical to diagnosis. Hemodynamic criteria in isolation may describe postural tachycardia but are not sufficient to diagnose POTS.
The prevalence of POTS is estimated to be between 0.2% and 1.0%,7 affecting up to 3 million people in the United States. Most cases arise between ages 13 and 50, with a female-to-male ratio of 5:1.8
MANY NAMES, SAME CONDITION
In 1871, Da Costa9 described a condition he called “irritable heart syndrome” that had characteristics similar to those of POTS, including extreme fatigue and exercise intolerance. Decades later, Lewis10 and Wood11 provided more detailed descriptions of the disorder, renaming it “soldier’s heart” or “Da Costa syndrome.” As other cases were documented, more terms arose, including “effort syndrome” and “mitral valve prolapse syndrome.”
In 1982, Rosen and Cryer12 were the first to use the term “postural tachycardia syndrome” for patients with disabling tachycardia upon standing without orthostatic hypotension. In 1986, Fouad et al13 described patients with postural tachycardia, orthostatic intolerance, and a small degree of hypotension as having “idiopathic hypovolemia.”
In 1993, Schondorf and Low14 established the current definition of POTS, leading to increased awareness and research efforts to understand its pathophysiology.
MULTIFACTORIAL PATHOPHYSIOLOGY
During the last 2 decades, several often-overlapping forms of POTS have been recognized, all of which share a final common pathway of sustained orthostatic tachycardia.15–19 In addition, a number of common comorbidities were identified through review of large clinic populations of POTS.20,21
Hypovolemic POTS
Up to 70% of patients with POTS have hypovolemia. The average plasma volume deficit is about 13%, which typically causes only insignificant changes in heart rate and norepinephrine levels while a patient is supine. However, blood pooling associated with upright posture further compromises cardiac output and consequently increases sympathetic nerve activity. Abnormalities in the renin-angiotensin-aldosterone volume regulation system are also suspected to impair sodium retention, contributing to hypovolemia.1,22
Neuropathic POTS
About half of patients with POTS have partial sympathetic denervation (particularly in the lower limbs) and inadequate vasoconstriction upon standing, leading to reduced venous return and stroke volume.17,23 A compensatory increase in sympathetic tone results in tachycardia to maintain cardiac output and blood pressure.
Hyperadrenergic POTS
Up to 50% of patients with POTS have high norepinephrine levels (≥ 600 pg/mL) when upright. This subtype, hyperadrenergic POTS, is characterized by an increase in systolic blood pressure of at least 10 mm Hg within 10 minutes of standing, with concomitant tachycardia that can be similar to or greater than that seen in nonhyperadrenergic POTS. Patients with hyperadrenergic POTS tend to report more prominent symptoms of sympathetic activation, such as palpitations, anxiety, and tremulousness.24,25
Norepinephrine transporter deficiency
The norepinephrine transporter (NET) is on the presynaptic cleft of sympathetic neurons and serves to clear synaptic norepinephrine. NET deficiency leads to a hyperadrenergic state and elevated sympathetic nerve activation.18 NET deficiency may be induced by common antidepressants (eg, tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors) and attention-deficit disorder medications.4
Mast cell activation syndrome
The relationship between mast cell activation syndrome and POTS is poorly understood.4,26 Mast cell activation syndrome has been described in a subset of patients with POTS who have sinus tachycardia accompanied by severe episodic flushing. Patients with this subtype have a hyperadrenergic response to postural change and elevated urine methylhistamine during flushing episodes.
Patients with mast cell activation syndrome tend to have strong allergic symptoms and may also have severe gastrointestinal problems, food sensitivities, dermatographism, and neuropathy. Diagnosis can be difficult, as the condition is associated with numerous markers with varying sensitivity and specificity.
Autoimmune origin
A significant minority of patients report a viral-like illness before the onset of POTS symptoms, suggesting a possible autoimmune-mediated or inflammatory cause. Also, some autoimmune disorders (eg, Sjögren syndrome) can present with a POTS-like manifestation.
Research into the role of autoantibodies in the pathophysiology of POTS offers the potential to develop novel therapeutic targets. Autoantibodies that have been reported in POTS include those against M1 to M3 muscarinic receptors (present in over 87% of patients with POTS),27 cardiac lipid raft-associated proteins,28 adrenergic G-protein coupled receptors, alpha-1-adrenergic receptors, and beta-1- and beta-2-adrenergic receptors.29 Although commercial enzyme-linked immunosorbent assays can assess for these antibody fragments, it is not known whether targeting the antibodies improves outcomes. At this time, antibody testing for POTS should be confined to the research setting.
LINKS TO OTHER SYNDROMES
POTS is often associated with other conditions whose symptoms cannot be explained by postural intolerance or tachycardia.
Ehlers-Danlos syndromes are a group of inherited heterogeneous disorders involving joint hypermobility, skin hyperextensibility, and tissue fragility.30 The hypermobile subtype is most commonly associated with POTS, with patients often having symptoms of autonomic dysregulation and autonomic test abnormalities.31–33 Patients with POTS may have a history of joint subluxations, joint pain, cervical instability, and spontaneous epidural leaks. The reason for the overlap between the two syndromes is not clear.
Chronic fatigue syndrome is characterized by persistent fatigue that does not resolve with rest and is not necessarily associated with orthostatic changes. More than 75% of patients with POTS report general fatigue as a major complaint, and up to 23% meet the full criteria for chronic fatigue syndrome.34
DIAGNOSTIC STRATEGY
A patient presenting with symptoms suggestive of POTS should first undergo a detailed history and physical examination. Other causes of sinus tachycardia should be considered.
Detailed history, symptom review
The history should focus on determining symptom burden, including tachycardia onset, frequency, severity, and triggers; the presence of syncope; and the impact of symptoms on daily function and quality of life.
Presyncope and its associated symptoms occur in less than one-third of patients with POTS, and syncope is not a principal feature.4 If syncope is the predominant complaint, alternative causes should be investigated. The usual cause of syncope in the general population is thought to be vasovagal.
In addition to orthostatic intolerance, gastrointestinal disturbances are common in POTS, presenting as abdominal pain, heartburn, irregular bowel movements, diarrhea, or constipation. Symptoms of gastroparesis are less common. Gastrointestinal symptoms tend to be prolonged, lasting hours and occurring multiple times a week. They tend not to improve in the supine position.35
POTS-associated symptoms may develop insidiously, but patients often report onset after an acute stressor such as pregnancy, major surgery, or a presumed viral illness.4 Whether these putative triggers are causative or coincidental is unknown. Symptoms of orthostatic intolerance tend to be exacerbated by dehydration, heat, alcohol, exercise, and menstruation.36,37
Consider the family history: 1 in 8 patients with POTS reports familial orthostatic intolerance,38 suggesting a genetic role in some patients. Inquire about symptoms or a previous diagnosis of Ehlers-Danlos syndrome and mast cell activation syndrome.
Consider other conditions
Pheochromocytoma causes hyperadrenergic symptoms (eg, palpitations, lightheadedness) like those in POTS, but patients with pheochromocytoma typically have these symptoms while supine. Pheochromocytoma is also characterized by plasma norepinephrine levels much higher than in POTS.4 Plasma metanephrine testing helps diagnose or rule out pheochromocytoma.5
Inappropriate sinus tachycardia, like pheochromocytoma, also has clinical features similar to those of POTS, as well as tachycardia present when supine. It involves higher sympathetic tone and lower parasympathetic tone compared with POTS; patients commonly have a daytime resting heart rate of at least 100 bpm or a 24-hour mean heart rate of at least 90 bpm.1,42 While the intrinsic heart rate is heightened in inappropriate sinus tachycardia, it is not different between POTS patients and healthy individuals.42,43 Distinguishing POTS from inappropriate sinus tachycardia is further complicated by the broad inclusion criteria of most studies of inappropriate sinus tachycardia, which failed to exclude patients with POTS.44 The Heart Rhythm Society recently adopted distinct definitions for the 2 conditions.1
Physical examination: Focus on vital signs
Dependent acrocyanosis—dark red-blue discoloration of the lower legs that is cold to the touch—occurs in about half of patients with POTS upon standing.4 Dependent acrocyanosis is associated with joint hypermobility and Ehlers-Danlos syndrome, so these conditions should also be considered if findings are positive.
Laboratory testing for other causes
Laboratory testing is used mainly to detect primary causes of sinus tachycardia. Tests should include:
- Complete blood cell count with hematocrit (for severe anemia)
- Thyroid-stimulating hormone level (for hyperthyroidism)
- Electrolyte panel (for significant electrolyte disturbances).
Evidence is insufficient to support routinely measuring the vitamin B12 level, iron indices, and serum markers for celiac disease, although these may be done if the history or physical examination suggests related problems.4 Sicca symptoms (severe dry eye or dry mouth) should trigger evaluation for Sjögren syndrome.
Electrocardiography needed
Electrocardiography should be performed to investigate for cardiac conduction abnormalities as well as for resting markers of a supraventricular tachyarrhythmia. Extended ambulatory (Holter) monitoring may be useful to evaluate for a transient reentrant tachyarrhythmia4; however, it does not record body position, so it can be difficult to determine if detected episodes of tachycardia are related to posture.
Additional testing for select cases
Further investigation is usually not needed to diagnose POTS but should be considered in some cases. Advanced tests are typically performed at a tertiary care referral center and include:
- Quantitative sensory testing to evaluate for small-fiber neuropathy (ie, Quantitative Sudomotor Axon Reflex Test, or QSART), which occurs in the neuropathic POTS subtype
- Formal autonomic function testing to characterize neurovascular responsiveness
- Supine and standing plasma norepinephrine levels (fractionated catecholamines) to characterize the net activation of the sympathetic nervous system
- Blood volume assessments to assess hypovolemia
- Formal exercise testing to objectively quantify exercise capacity.
GRADED MANAGEMENT
No single universal gold-standard therapy exists for POTS, and management should be individually determined with the primary goals of treating symptoms and restoring function. A graded approach should be used, starting with conservative nonpharmacologic therapies and adding medications as needed.
While the disease course varies substantially from patient to patient, proper management is strongly associated with eventual symptom improvement.1
NONPHARMACOLOGIC STEPS FIRST
Education
Patients should be informed of the nature of their condition and referred to appropriate healthcare personnel. POTS is a chronic illness requiring individualized coping strategies, intensive physician interaction, and support of a multidisciplinary team. Patients and family members can be reassured that most symptoms improve over time with appropriate diagnosis and treatment.1 Patients should be advised to avoid aggravating triggers and activities.
Exercise
Exercise programs are encouraged but should be introduced gradually, as physical activity can exacerbate symptoms, especially at the outset. Several studies have reported benefits from a short-term (3-month) program, in which the patient gradually progresses from non-upright exercise (eg, rowing machine, recumbent cycle, swimming) to upright endurance exercises. At the end of these programs, significant cardiac remodeling, improved quality of life, and reduced heart rate responses to standing have been reported, and benefits have been reported to persist in patients who continued exercising after the 3-month study period.46,47
Despite the benefits of exercise interventions, compliance is low.46,47 To prevent early discouragement, patients should be advised that it can take 4 to 6 weeks of continued exercise before benefits appear. Patients are encouraged to exercise every other day for 30 minutes or more. Regimens should primarily focus on aerobic conditioning, but resistance training, concentrating on thigh muscles, can also help. Exercise is a treatment and not a cure, and benefits can rapidly disappear if regular activity (at least 3 times per week) is stopped.48
Compression stockings
Compression stockings help reduce peripheral venous pooling and enhance venous return to the heart. Waist-high stockings with compression of at least 30 to 40 mm Hg offer the best results.
Diet
Increased fluid and salt intake is advisable for patients with suspected hypovolemia. At least 2 to 3 L of water accompanied by 10 to 12 g of daily sodium intake is recommended.1 This can usually be accomplished with diet and salt added to food, but salt tablets can be used if the patient prefers. The resultant plasma volume expansion may help reduce the reflex tachycardia upon standing.49
Check medications
Rescue therapy with saline infusion
Intravenous saline infusion can augment blood volume in patients who are clinically decompensated and present with severe symptoms.1 Intermittent infusion of 1 L of normal saline has been found to significantly reduce orthostatic tachycardia and related symptoms in patients with POTS, contributing to improved quality of life.51,52
Chronic saline infusions are not recommended for long-term care because of the risk of access complications and infection.1 Moak et al53 reported a high rate of bacteremia in a cohort of children with POTS with regular saline infusions, most of whom had a central line. On the other hand, Ruzieh et al54 reported significantly improved symptoms with regular saline infusions without a high rate of complications, but patients in this study received infusions for only a few months and through a peripheral intravenous catheter.
DRUG THERAPY
No medications are approved by the US Food and Drug Administration (FDA) or Health Canada specifically for treating POTS, making all pharmacologic recommendations off-label. Although the drugs discussed below have been evaluated for POTS in controlled laboratory settings, they have yet to be tested in robust clinical trials.
Blood volume expansion
Several drugs expand blood volume, which may reduce orthostatic tachycardia.
Fludrocortisone is a synthetic aldosterone analogue that enhances sodium and water retention. Although one observational study found that it normalizes hemodynamic changes in response to orthostatic stress, no high-level evidence exists for its effectiveness for POTS.55 It is generally well tolerated, although possible adverse effects include hyperkalemia, hypertension, fatigue, nausea, headache, and edema.5,56
Desmopressin is a synthetic version of a natural antidiuretic hormone that increases kidney-mediated free-water reabsorption without sodium retention. It significantly reduces upright heart rate in patients with POTS and improves symptom burden. Although potential adverse effects include edema and headache, hyponatremia is the primary concern with daily use, especially with the increased water intake advised for POTS.57 Patients should be advised to use desmopressin no more than once a week for the acute improvement of symptoms. Intermittent monitoring of serum sodium levels is recommended for safety.
Erythropoietin replacement has been suggested for treating POTS to address the significant deficit in red blood cell volume. Although erythropoietin therapy has a direct vasoconstrictive effect and largely improves red blood cell volume in patients with POTS, it does not expand plasma volume, so orthostatic tachycardia is not itself reduced.22 Nevertheless, it may significantly improve POTS symptoms refractory to more common methods of treatment, and it should be reserved for such cases. In addition to the lack of effect on orthostatic tachycardia, drawbacks to using erythropoietin include its high cost, the need for subcutaneous administration, and the risk of life-threatening complications such as myocardial infarction and stroke.58,59
Heart rate-lowering agents
Propranolol, a nonselective beta-adrenergic antagonist, can significantly reduce standing heart rate and improve symptoms at low dosages (10–20 mg). Higher dosages can further restrain orthostatic tachycardia but are not as well tolerated, mainly due to hypotension and worsening of existing symptoms such as fatigue.60 Regular-acting propranolol works for about 4 to 5 hours per dose, so full-day coverage often requires dosing 4 times per day.
Ivabradine is a selective blocker of the “funny” (If) channel that reduces the sinus node firing rate without affecting blood pressure, so it slows heart rate without causing supine hypertension or orthostatic hypotension.
A retrospective case series found that 60% of patients with POTS treated with ivabradine reported symptomatic improvement, and all patients experienced reduced tachycardia with continued use.61 Ivabradine has not been compared with placebo or propranolol in a randomized controlled trial, and it has not been well studied in pregnancy and so should be avoided because of potential teratogenic effects.
When prescribing ivabradine for women of childbearing age, a negative pregnancy test may be documented prior to initiation of therapy, and the use of highly effective methods of contraception is recommended. Ivabradine should be avoided in women contemplating pregnancy. Insurance coverage can limit access to ivabradine in the United States.
Central nervous system sympatholytics
Patients with prominent hyperadrenergic features may benefit from central sympatholytic agents. However, these drugs may not be well tolerated in patients with neuropathic POTS because of the effects of reduced systemic vascular resistance5 and the possible exacerbation of drowsiness, fatigue, and mental clouding.4 Patients can be extremely sensitive to these medications, so they should initially be prescribed at the lowest dose, then gradually increased as tolerated.
Clonidine, an alpha-2-adrenergic agonist, decreases central sympathetic tone. In hyperadrenergic patients, clonidine can stabilize heart rate and blood pressure, thereby reducing orthostatic symptoms.62
Methyldopa has effects similar to those of clonidine but is easier to titrate owing to its longer half-life.63 Methyldopa is typically started at 125 mg at bedtime and increased to 125 mg twice daily, if tolerated.
Other agents
Midodrine is a prodrug. The active form, an alpha-1-adrenergic agonist, constricts peripheral veins and arteries to increase vascular resistance and venous return, thereby reducing orthostatic tachycardia.52 It is most useful in patients with impaired peripheral vasoconstriction (eg, neuropathic POTS) and may be less effective in those with hyperadrenergic POTS.64 Major limitations of midodrine include worsening supine hypertension and possible urinary retention.39
Because of midodrine’s short half-life, frequent dosing is required during daytime hours (eg, 8 AM, noon, and 4 PM), but it should not be taken within 4 to 5 hours of sleep because of the risk of supine hypertension. Midodrine is typically started at 2.5 to 5 mg per dose and can be titrated up to 15 mg per dose.
Midodrine is an FDA pregnancy category C drug (adverse effects in pregnancy seen in animal models, but evidence lacking in humans). While ideally it should be avoided, we have used it safely in pregnant women with disabling POTS symptoms.
Pyridostigmine, an acetylcholinesterase inhibitor, increases cardiovagal tone and possibly sympathetic tone. It has been reported to significantly reduce standing heart rate and improve symptom burden in patients with POTS.65 However, pyridostigmine increases gastrointestinal mobility, leading to severe adverse effects in over 20% of patients, including abdominal cramps, nausea, and diarrhea.66
Droxidopa, a synthetic amino acid precursor of norepinephrine, improves dizziness and fatigue in POTS with minimal effects on blood pressure.67
Modafinil, a psychostimulant, may improve POTS-associated cognitive symptoms.4 It also raises upright blood pressure without significantly worsening standing heart rate or acute orthostatic symptoms.68
EFFECTS OF COMORBID DISORDERS ON MANAGEMENT
Ehlers-Danlos syndrome
Pharmacologic approaches to POTS should not be altered based on the presence of Ehlers-Danlos syndrome, but because many of these patients are prone to joint dislocation, exercise prescriptions may need adjusting.
A medical genetics consult is recommended for patients with Ehlers-Danlos syndrome. Although the hypermobile type (the form most commonly associated with POTS) is not associated with aortopathy, it can be confused with classical and vascular Ehlers-Danlos syndromes, which require serial aortic screening.30
Mast cell activation syndrome
Consultation with an allergist or immunologist may help patients with severe symptoms.
Autoantibodies and autoimmunity
Treatment of the underlying disorder is recommended and can result in significantly improved POTS symptoms.
SPECIALTY CARE REFERRAL
POTS can be challenging to manage. Given the range of physiologic, emotional, and functional distress patients experience, it often requires significant physician time and multidisciplinary care. Patients with continued severe or debilitating symptoms may benefit from referral to a tertiary-care center with experience in autonomic nervous system disorders.
PROGNOSIS
Limited data are available on the long-term prognosis of POTS, and more studies are needed in pediatric and adult populations. No deaths have been reported in the handful of published cases of POTS in patients older than 50.1 Some pediatric studies suggest that some teenagers “outgrow” their POTS. However, these data are not robust, and an alternative explanation is that as they get older, they see adult physicians for their POTS symptoms and so are lost to study follow-up.6,44,69
We have not often seen POTS simply resolve without ongoing treatment. However, in our experience, most patients have improved symptoms and function with multimodal treatment (ie, exercise, salt, water, stockings, and some medications) and time.
- Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015; 12(6):e41–e63. doi:10.1016/j.hrthm.2015.03.029
- Bagai K, Song Y, Ling JF, et al. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med 2011; 7(2):204–210. pmid:21509337
- Benrud-Larson LM, Dewar MS, Sandroni P, Rummans TA, Haythornthwaite JA, Low PA. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc 2002; 77(6):531–537. doi:10.4065/77.6.531
- Raj SR. Postural tachycardia syndrome (POTS). Circulation 2013; 127(23):2336–2342. doi:10.1161/CIRCULATIONAHA.112.144501
- Raj SR. The postural tachycardia syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J 2006; 6(2):84–99. pmid:16943900
- Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: what is abnormal? J Pediatr 2012; 160(2):222–226. doi:10.1016/j.jpeds.2011.08.054
- Mar PL, Raj SR. Neuronal and hormonal perturbations in postural tachycardia syndrome. Front Physiol 2014; 5:220. doi:10.3389/fphys.2014.00220
- Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology 2007; 69(8):790–798. doi:10.1212/01.wnl.0000267663.05398.40
- Da Costa JM. On irritable heart: a clinical study of a form of functional cardiac disorder and its consequences. Am J Med Sci 1871; 61(121):2–52.
- Lewis T. The tolerance of physical exertion, as shown by soldiers suffering from so-called “irritable heart.” Br Med J 1918; 1(2987):363–365. pmid:20768980
- Wood P. Da Costa’s syndrome (or effort syndrome): lecture I. Br Med J 1941; 1(4194):767–772. pmid:20783672
- Rosen SG, Cryer PE. Postural tachycardia syndrome. Reversal of sympathetic hyperresponsiveness and clinical improvement during sodium loading. Am J Med 1982; 72(5):847–850.
- Fouad FM, Tadena-Thome L, Bravo EL, Tarazi RC. Idiopathic hypovolemia. Ann Intern Med 1986; 104(3):298–303. pmid:3511818
- Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 1993; 43(1):132–137. pmid:8423877
- Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med 2000; 343(12):847–855. doi:10.1056/NEJM200009213431204
- Raj SR, Robertson D. Blood volume perturbations in the postural tachycardia syndrome. Am J Med Sci 2007; 334(1):57–60. doi:10.1097/MAJ.0b013e318063c6c0
- Jacob G, Costa F, Shannon JR, et al. The neuropathic postural tachycardia syndrome. N Engl J Med 2000; 343(14):1008–1014. doi:10.1056/NEJM200010053431404
- Shannon JR, Flattem NL, Jordan J, et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med 2000; 342(8):541–549. doi:10.1056/NEJM200002243420803
- Jones PK, Shaw BH, Raj SR. Clinical challenges in the diagnosis and management of postural tachycardia syndrome. Pract Neurol 2016; 16(6):431–438. doi:10.1136/practneurol-2016-001405
- Gunning WT, Karabin BL, Blomquist TM, Grubb BP. Postural orthostatic tachycardia syndrome is associated with platelet storage pool deficiency. Medicine (Baltimore) 2016; 95(37):e4849. doi:10.1097/MD.0000000000004849
- Kanjwal K, Sheikh M, Karabin B, Kanjwal Y, Grubb BP. Neurocardiogenic syncope coexisting with postural orthostatic tachycardia syndrome in patients suffering from orthostatic intolerance: a combined form of autonomic dysfunction. Pacing Clin Electrophysiol 2011; 34(5):549–554. doi:10.1111/j.1540-8159.2010.02994.x
- Raj SR, Biaggioni I, Yamhure PC, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation 2005; 111(13):1574–1582. doi:10.1161/01.CIR.0000160356.97313.5D
- Gibbons CH, Bonyhay I, Benson A, Wang N, Freeman R. Structural and functional small fiber abnormalities in the neuropathic postural tachycardia syndrome. PLoS One 2013; 8(12):e84716. doi:10.1371/journal.pone.0084716
- Low PA, Sandroni P, Joyner M, Shen WK. Postural tachycardia syndrome (POTS). J Cardiovasc Electrophysiol 2009; 20(3):352–358. doi:10.1111/j.1540-8167.2008.01407.x
- Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Grubb BP. Clinical presentation and management of patients with hyperadrenergic postural orthostatic tachycardia syndrome. A single center experience. Cardiol J 2011; 18(5):527–531. pmid:21947988
- Shibao C, Arzubiaga C, Roberts J, et al. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension 2005; 45(3):385–390. doi:10.1161/01.HYP.0000158259.68614.40
- Dubey D, Hopkins S, Vernino S. M1 and M2 muscarinic receptor antibodies among patients with postural orthostatic tachycardia syndrome: potential disease biomarker [abstract]. J Clin Neuromuscul Dis 2016; 17(3):179S.
- Wang XL, Ling TY, Charlesworth MC, et al. Autoimmunoreactive IgGs against cardiac lipid raft-associated proteins in patients with postural orthostatic tachycardia syndrome. Transl Res 2013; 162(1):34–44. doi:10.1016/j.trsl.2013.03.002
- Li H, Yu X, Liles C, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc 2014; 3(1):e000755. doi:10.1161/JAHA.113.000755
- Malfait F, Francomano C, Byers P, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet 2017; 175(1):8–26. doi:10.1002/ajmg.c.31552
- Wallman D, Weinberg J, Hohler AD. Ehlers-Danlos syndrome and postural tachycardia syndrome: a relationship study. J Neurol Sci 2014; 340(1-2):99–102. doi:10.1016/j.jns.2014.03.002
- De Wandele I, Calders P, Peersman W, et al. Autonomic symptom burden in the hypermobility type of Ehlers-Danlos syndrome: a comparative study with two other EDS types, fibromyalgia, and healthy controls. Semin Arthritis Rheum 2014; 44(3):353–361. doi:10.1016/j.semarthrit.2014.05.013
- Gazit Y, Nahir AM, Grahame R, Jacob G. Dysautonomia in the joint hypermobility syndrome. Am J Med 2003; 115(1):33–40. pmid:12867232
- Okamoto LE, Raj SR, Peltier A, et al. Neurohumoral and haemodynamic profile in postural tachycardia and chronic fatigue syndromes. Clin Sci (Lond) 2012; 122(4):183–192. doi:10.1042/CS20110200
- Wang LB, Culbertson CJ, Deb A, Morgenshtern K, Huang H, Hohler AD. Gastrointestinal dysfunction in postural tachycardia syndrome. J Neurol Sci 2015; 359(1-2):193–196. doi:10.1016/j.jns.2015.10.052
- Raj S, Sheldon R. Management of postural tachycardia syndrome, inappropriate sinus tachycardia and vasovagal syncope. Arrhythm Electrophysiol Rev 2016; 5(2):122–129. doi:10.15420/AER.2016.7.2
- Peggs KJ, Nguyen H, Enayat D, Keller NR, Al-Hendy A, Raj SR. Gynecologic disorders and menstrual cycle lightheadedness in postural tachycardia syndrome. Int J Gynaecol Obstet 2012; 118(3):242–246. doi:10.1016/j.ijgo.2012.04.014
- Thieben MJ, Sandroni P, Sletten DM, et al. Postural orthostatic tachycardia syndrome: the Mayo Clinic experience. Mayo Clin Proc 2007; 82(3):308–313. doi:10.4065/82.3.308
- Deb A, Morgenshtern K, Culbertson CJ, Wang LB, Hohler AD. A survey-based analysis of symptoms in patients with postural orthostatic tachycardia syndrome. J Am Coll Cardiol 2015; 28(7):157–159. pmid:25829642
- Ertek S, Cicero AF. Hyperthyroidism and cardiovascular complications: a narrative review on the basis of pathophysiology. Arch Med Sci 2013; 9(5):944–952. doi:10.5114/aoms.2013.38685
- Rangno RE, Langlois S. Comparison of withdrawal phenomena after propranolol, metoprolol and pindolol. Br J Clin Pharmacol 1982; 13(suppl 2):345S–351S. pmid:6125187
- Nwazue VC, Paranjape SY, Black BK, et al. Postural tachycardia syndrome and inappropriate sinus tachycardia: role of autonomic modulation and sinus node automaticity. J Am Heart Assoc 2014; 3(2):e000700. doi:10.1161/JAHA.113.000700
- Morillo CA, Klein GJ, Thakur RK, Li H, Zardini M, Yee R. Mechanism of “inappropriate” sinus tachycardia. Role of sympathovagal balance. Circulation 1994; 90(2):873–877. pmid:7913886
- Grubb BP. Postural tachycardia syndrome. Circulation 2008; 117(21):2814–2817. doi:10.1161/CIRCULATIONAHA.107.761643
- Bhatia R, Kizilbash SJ, Ahrens SP, et al. Outcomes of adolescent-onset postural orthostatic tachycardia syndrome. J Pediatr 2016; 173:149–153. doi:10.1016/j.jpeds.2016.02.035
- George SA, Bivens TB, Howden EJ, et al. The international POTS registry: evaluating the efficacy of an exercise training intervention in a community setting. Heart Rhythm 2016; 13(4):943–950. doi:10.1016/j.hrthm.2015.12.012
- Fu Q, VanGundy TB, Galbreath MM, et al. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol 2010; 55(25):2858–2868. doi:10.1016/j.jacc.2010.02.043
- Raj SR. Row, row, row your way to treating postural tachycardia syndrome. Heart Rhythm 2016; 13(4):951–952. doi:10.1016/j.hrthm.2015.12.039
- Celedonio JE, Garland EM, Nwazue VC, et al. Effects of high sodium intake on blood volume and catecholamines in patients with postural tachycardia syndrome and healthy females [abstract]. Clin Auton Res 2014; 24:211.
- Garland EM, Celedonio JE, Raj SR. Postural tachycardia syndrome: beyond orthostatic intolerance. Curr Neurol Neurosci Rep 2015; 15(9):60. doi:10.1007/s11910-015-0583-8
- Gordon VM, Opfer-Gehrking TL, Novak V, Low PA. Hemodynamic and symptomatic effects of acute interventions on tilt in patients with postural tachycardia syndrome. Clin Auton Res 2000; 10:29–33. pmid:10750641
- Jacob G, Shannon JR, Black B, et al. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation 1997; 96(2):575–580. pmid:9244228
- Moak JP, Leong D, Fabian R, et al. Intravenous hydration for management of medication-resistant orthostatic intolerance in the adolescent and young adult. Pediatr Cardiol 2016; 37(2):278–282. doi:10.1007/s00246-015-1274-6
- Ruzieh M, Baugh A, Dasa O, et al. Effects of intermittent intravenous saline infusions in patients with medication-refractory postural tachycardia syndrome. J Interv Card Electrophysiol 2017; 48(3):255–260. doi:10.1007/s10840-017-0225-y
- Freitas J, Santos R, Azevedo E, Costa O, Carvalho M, de Freitas AF. Clinical improvement in patients with orthostatic intolerance after treatment with bisoprolol and fludrocortisone. Clin Auton Res 2000; 10(5):293–299. pmid:11198485
- Lee AK, Krahn AD. Evaluation of syncope: focus on diagnosis and treatment of neurally mediated syncope. Expert Rev Cardiovasc Ther 2016; 14(6):725–736. doi:10.1586/14779072.2016.1164034
- Coffin ST, Black BK, Biaggioni I, et al. Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Heart Rhythm 2012; 9(9):1484–1490. doi:10.1016/j.hrthm.2012.05.002
- Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Sheikh M, Grubb BP. Erythropoietin in the treatment of postural orthostatic tachycardia syndrome. Am J Ther 2012; 19(2):92–95. doi:10.1097/MJT.0b013e3181ef621a
- Hoeldtke RD, Horvath GG, Bryner KD. Treatment of orthostatic tachycardia with erythropoietin. Am J Med 1995; 99(5):525–529. pmid:7485211
- Raj SR, Black BK, Biaggioni I, et al. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation 2009; 120(9):725–734. doi:10.1161/CIRCULATIONAHA.108.846501
- McDonald C, Frith J, Newton JL. Single centre experience of ivabradine in postural orthostatic tachycardia syndrome. Europace 2011; 13(3):427–430. doi:10.1093/europace/euq390
- Gaffney FA, Lane LB, Pettinger W, Blomqvist G. Effects of long-term clonidine administration on the hemodynamic and neuroendocrine postural responses of patients with dysautonomia. Chest 1983; 83(suppl 2):436–438. pmid:6295714
- Jacob G, Biaggioni I. Idiopathic orthostatic intolerance and postural tachycardia syndromes. Am J Med Sci 1999; 317(2):88–101. pmid:10037112
- Ross AJ, Ocon AJ, Medow MS, Stewart JM. A double-blind placebo-controlled cross-over study of the vascular effects of midodrine in neuropathic compared with hyperadrenergic postural tachycardia syndrome. Clin Sci (Lond) 2014; 126(4):289–296. doi:10.1042/CS20130222
- Raj SR, Black BK, Biaggioni I, Harris PA, Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation 2005; 111(21):2734–2340. doi:10.1161/CIRCULATIONAHA.104.497594
- Kanjwal K, Karabin B, Sheikh M, et al. Pyridostigmine in the treatment of postural orthostatic tachycardia: A single-center experience. Pacing Clin Electrophysiol 2011; 34(6):750–755. doi:10.1111/j.1540-8159.2011.03047.x
- Ruzieh M, Dasa O, Pacenta A, Karabin B, Grubb B. Droxidopa in the treatment of postural orthostatic tachycardia syndrome. Am J Ther 2017; 24(2):e157–e161. doi:10.1097/MJT.0000000000000468
- Kpaeyeh AG Jr, Mar PL, Raj V, et al. Hemodynamic profiles and tolerability of modafinil in the treatment of POTS: a randomized placebo-controlled trial. J Clin Psychopharmacol 2014; 34(6):738–741. doi:10.1097/JCP.0000000000000221
- Lai CC, Fischer PR, Brands CK, et al. Outcomes in adolescents with postural orthostatic tachycardia syndrome treated with midodrine and beta-blockers. Pacing Clin Electrophysiol 2009; 32(2):234–238. doi:10.1111/j.1540-8159.2008.02207.x
- Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015; 12(6):e41–e63. doi:10.1016/j.hrthm.2015.03.029
- Bagai K, Song Y, Ling JF, et al. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med 2011; 7(2):204–210. pmid:21509337
- Benrud-Larson LM, Dewar MS, Sandroni P, Rummans TA, Haythornthwaite JA, Low PA. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc 2002; 77(6):531–537. doi:10.4065/77.6.531
- Raj SR. Postural tachycardia syndrome (POTS). Circulation 2013; 127(23):2336–2342. doi:10.1161/CIRCULATIONAHA.112.144501
- Raj SR. The postural tachycardia syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J 2006; 6(2):84–99. pmid:16943900
- Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: what is abnormal? J Pediatr 2012; 160(2):222–226. doi:10.1016/j.jpeds.2011.08.054
- Mar PL, Raj SR. Neuronal and hormonal perturbations in postural tachycardia syndrome. Front Physiol 2014; 5:220. doi:10.3389/fphys.2014.00220
- Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology 2007; 69(8):790–798. doi:10.1212/01.wnl.0000267663.05398.40
- Da Costa JM. On irritable heart: a clinical study of a form of functional cardiac disorder and its consequences. Am J Med Sci 1871; 61(121):2–52.
- Lewis T. The tolerance of physical exertion, as shown by soldiers suffering from so-called “irritable heart.” Br Med J 1918; 1(2987):363–365. pmid:20768980
- Wood P. Da Costa’s syndrome (or effort syndrome): lecture I. Br Med J 1941; 1(4194):767–772. pmid:20783672
- Rosen SG, Cryer PE. Postural tachycardia syndrome. Reversal of sympathetic hyperresponsiveness and clinical improvement during sodium loading. Am J Med 1982; 72(5):847–850.
- Fouad FM, Tadena-Thome L, Bravo EL, Tarazi RC. Idiopathic hypovolemia. Ann Intern Med 1986; 104(3):298–303. pmid:3511818
- Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 1993; 43(1):132–137. pmid:8423877
- Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med 2000; 343(12):847–855. doi:10.1056/NEJM200009213431204
- Raj SR, Robertson D. Blood volume perturbations in the postural tachycardia syndrome. Am J Med Sci 2007; 334(1):57–60. doi:10.1097/MAJ.0b013e318063c6c0
- Jacob G, Costa F, Shannon JR, et al. The neuropathic postural tachycardia syndrome. N Engl J Med 2000; 343(14):1008–1014. doi:10.1056/NEJM200010053431404
- Shannon JR, Flattem NL, Jordan J, et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med 2000; 342(8):541–549. doi:10.1056/NEJM200002243420803
- Jones PK, Shaw BH, Raj SR. Clinical challenges in the diagnosis and management of postural tachycardia syndrome. Pract Neurol 2016; 16(6):431–438. doi:10.1136/practneurol-2016-001405
- Gunning WT, Karabin BL, Blomquist TM, Grubb BP. Postural orthostatic tachycardia syndrome is associated with platelet storage pool deficiency. Medicine (Baltimore) 2016; 95(37):e4849. doi:10.1097/MD.0000000000004849
- Kanjwal K, Sheikh M, Karabin B, Kanjwal Y, Grubb BP. Neurocardiogenic syncope coexisting with postural orthostatic tachycardia syndrome in patients suffering from orthostatic intolerance: a combined form of autonomic dysfunction. Pacing Clin Electrophysiol 2011; 34(5):549–554. doi:10.1111/j.1540-8159.2010.02994.x
- Raj SR, Biaggioni I, Yamhure PC, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation 2005; 111(13):1574–1582. doi:10.1161/01.CIR.0000160356.97313.5D
- Gibbons CH, Bonyhay I, Benson A, Wang N, Freeman R. Structural and functional small fiber abnormalities in the neuropathic postural tachycardia syndrome. PLoS One 2013; 8(12):e84716. doi:10.1371/journal.pone.0084716
- Low PA, Sandroni P, Joyner M, Shen WK. Postural tachycardia syndrome (POTS). J Cardiovasc Electrophysiol 2009; 20(3):352–358. doi:10.1111/j.1540-8167.2008.01407.x
- Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Grubb BP. Clinical presentation and management of patients with hyperadrenergic postural orthostatic tachycardia syndrome. A single center experience. Cardiol J 2011; 18(5):527–531. pmid:21947988
- Shibao C, Arzubiaga C, Roberts J, et al. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension 2005; 45(3):385–390. doi:10.1161/01.HYP.0000158259.68614.40
- Dubey D, Hopkins S, Vernino S. M1 and M2 muscarinic receptor antibodies among patients with postural orthostatic tachycardia syndrome: potential disease biomarker [abstract]. J Clin Neuromuscul Dis 2016; 17(3):179S.
- Wang XL, Ling TY, Charlesworth MC, et al. Autoimmunoreactive IgGs against cardiac lipid raft-associated proteins in patients with postural orthostatic tachycardia syndrome. Transl Res 2013; 162(1):34–44. doi:10.1016/j.trsl.2013.03.002
- Li H, Yu X, Liles C, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc 2014; 3(1):e000755. doi:10.1161/JAHA.113.000755
- Malfait F, Francomano C, Byers P, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet 2017; 175(1):8–26. doi:10.1002/ajmg.c.31552
- Wallman D, Weinberg J, Hohler AD. Ehlers-Danlos syndrome and postural tachycardia syndrome: a relationship study. J Neurol Sci 2014; 340(1-2):99–102. doi:10.1016/j.jns.2014.03.002
- De Wandele I, Calders P, Peersman W, et al. Autonomic symptom burden in the hypermobility type of Ehlers-Danlos syndrome: a comparative study with two other EDS types, fibromyalgia, and healthy controls. Semin Arthritis Rheum 2014; 44(3):353–361. doi:10.1016/j.semarthrit.2014.05.013
- Gazit Y, Nahir AM, Grahame R, Jacob G. Dysautonomia in the joint hypermobility syndrome. Am J Med 2003; 115(1):33–40. pmid:12867232
- Okamoto LE, Raj SR, Peltier A, et al. Neurohumoral and haemodynamic profile in postural tachycardia and chronic fatigue syndromes. Clin Sci (Lond) 2012; 122(4):183–192. doi:10.1042/CS20110200
- Wang LB, Culbertson CJ, Deb A, Morgenshtern K, Huang H, Hohler AD. Gastrointestinal dysfunction in postural tachycardia syndrome. J Neurol Sci 2015; 359(1-2):193–196. doi:10.1016/j.jns.2015.10.052
- Raj S, Sheldon R. Management of postural tachycardia syndrome, inappropriate sinus tachycardia and vasovagal syncope. Arrhythm Electrophysiol Rev 2016; 5(2):122–129. doi:10.15420/AER.2016.7.2
- Peggs KJ, Nguyen H, Enayat D, Keller NR, Al-Hendy A, Raj SR. Gynecologic disorders and menstrual cycle lightheadedness in postural tachycardia syndrome. Int J Gynaecol Obstet 2012; 118(3):242–246. doi:10.1016/j.ijgo.2012.04.014
- Thieben MJ, Sandroni P, Sletten DM, et al. Postural orthostatic tachycardia syndrome: the Mayo Clinic experience. Mayo Clin Proc 2007; 82(3):308–313. doi:10.4065/82.3.308
- Deb A, Morgenshtern K, Culbertson CJ, Wang LB, Hohler AD. A survey-based analysis of symptoms in patients with postural orthostatic tachycardia syndrome. J Am Coll Cardiol 2015; 28(7):157–159. pmid:25829642
- Ertek S, Cicero AF. Hyperthyroidism and cardiovascular complications: a narrative review on the basis of pathophysiology. Arch Med Sci 2013; 9(5):944–952. doi:10.5114/aoms.2013.38685
- Rangno RE, Langlois S. Comparison of withdrawal phenomena after propranolol, metoprolol and pindolol. Br J Clin Pharmacol 1982; 13(suppl 2):345S–351S. pmid:6125187
- Nwazue VC, Paranjape SY, Black BK, et al. Postural tachycardia syndrome and inappropriate sinus tachycardia: role of autonomic modulation and sinus node automaticity. J Am Heart Assoc 2014; 3(2):e000700. doi:10.1161/JAHA.113.000700
- Morillo CA, Klein GJ, Thakur RK, Li H, Zardini M, Yee R. Mechanism of “inappropriate” sinus tachycardia. Role of sympathovagal balance. Circulation 1994; 90(2):873–877. pmid:7913886
- Grubb BP. Postural tachycardia syndrome. Circulation 2008; 117(21):2814–2817. doi:10.1161/CIRCULATIONAHA.107.761643
- Bhatia R, Kizilbash SJ, Ahrens SP, et al. Outcomes of adolescent-onset postural orthostatic tachycardia syndrome. J Pediatr 2016; 173:149–153. doi:10.1016/j.jpeds.2016.02.035
- George SA, Bivens TB, Howden EJ, et al. The international POTS registry: evaluating the efficacy of an exercise training intervention in a community setting. Heart Rhythm 2016; 13(4):943–950. doi:10.1016/j.hrthm.2015.12.012
- Fu Q, VanGundy TB, Galbreath MM, et al. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol 2010; 55(25):2858–2868. doi:10.1016/j.jacc.2010.02.043
- Raj SR. Row, row, row your way to treating postural tachycardia syndrome. Heart Rhythm 2016; 13(4):951–952. doi:10.1016/j.hrthm.2015.12.039
- Celedonio JE, Garland EM, Nwazue VC, et al. Effects of high sodium intake on blood volume and catecholamines in patients with postural tachycardia syndrome and healthy females [abstract]. Clin Auton Res 2014; 24:211.
- Garland EM, Celedonio JE, Raj SR. Postural tachycardia syndrome: beyond orthostatic intolerance. Curr Neurol Neurosci Rep 2015; 15(9):60. doi:10.1007/s11910-015-0583-8
- Gordon VM, Opfer-Gehrking TL, Novak V, Low PA. Hemodynamic and symptomatic effects of acute interventions on tilt in patients with postural tachycardia syndrome. Clin Auton Res 2000; 10:29–33. pmid:10750641
- Jacob G, Shannon JR, Black B, et al. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation 1997; 96(2):575–580. pmid:9244228
- Moak JP, Leong D, Fabian R, et al. Intravenous hydration for management of medication-resistant orthostatic intolerance in the adolescent and young adult. Pediatr Cardiol 2016; 37(2):278–282. doi:10.1007/s00246-015-1274-6
- Ruzieh M, Baugh A, Dasa O, et al. Effects of intermittent intravenous saline infusions in patients with medication-refractory postural tachycardia syndrome. J Interv Card Electrophysiol 2017; 48(3):255–260. doi:10.1007/s10840-017-0225-y
- Freitas J, Santos R, Azevedo E, Costa O, Carvalho M, de Freitas AF. Clinical improvement in patients with orthostatic intolerance after treatment with bisoprolol and fludrocortisone. Clin Auton Res 2000; 10(5):293–299. pmid:11198485
- Lee AK, Krahn AD. Evaluation of syncope: focus on diagnosis and treatment of neurally mediated syncope. Expert Rev Cardiovasc Ther 2016; 14(6):725–736. doi:10.1586/14779072.2016.1164034
- Coffin ST, Black BK, Biaggioni I, et al. Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Heart Rhythm 2012; 9(9):1484–1490. doi:10.1016/j.hrthm.2012.05.002
- Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Sheikh M, Grubb BP. Erythropoietin in the treatment of postural orthostatic tachycardia syndrome. Am J Ther 2012; 19(2):92–95. doi:10.1097/MJT.0b013e3181ef621a
- Hoeldtke RD, Horvath GG, Bryner KD. Treatment of orthostatic tachycardia with erythropoietin. Am J Med 1995; 99(5):525–529. pmid:7485211
- Raj SR, Black BK, Biaggioni I, et al. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation 2009; 120(9):725–734. doi:10.1161/CIRCULATIONAHA.108.846501
- McDonald C, Frith J, Newton JL. Single centre experience of ivabradine in postural orthostatic tachycardia syndrome. Europace 2011; 13(3):427–430. doi:10.1093/europace/euq390
- Gaffney FA, Lane LB, Pettinger W, Blomqvist G. Effects of long-term clonidine administration on the hemodynamic and neuroendocrine postural responses of patients with dysautonomia. Chest 1983; 83(suppl 2):436–438. pmid:6295714
- Jacob G, Biaggioni I. Idiopathic orthostatic intolerance and postural tachycardia syndromes. Am J Med Sci 1999; 317(2):88–101. pmid:10037112
- Ross AJ, Ocon AJ, Medow MS, Stewart JM. A double-blind placebo-controlled cross-over study of the vascular effects of midodrine in neuropathic compared with hyperadrenergic postural tachycardia syndrome. Clin Sci (Lond) 2014; 126(4):289–296. doi:10.1042/CS20130222
- Raj SR, Black BK, Biaggioni I, Harris PA, Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation 2005; 111(21):2734–2340. doi:10.1161/CIRCULATIONAHA.104.497594
- Kanjwal K, Karabin B, Sheikh M, et al. Pyridostigmine in the treatment of postural orthostatic tachycardia: A single-center experience. Pacing Clin Electrophysiol 2011; 34(6):750–755. doi:10.1111/j.1540-8159.2011.03047.x
- Ruzieh M, Dasa O, Pacenta A, Karabin B, Grubb B. Droxidopa in the treatment of postural orthostatic tachycardia syndrome. Am J Ther 2017; 24(2):e157–e161. doi:10.1097/MJT.0000000000000468
- Kpaeyeh AG Jr, Mar PL, Raj V, et al. Hemodynamic profiles and tolerability of modafinil in the treatment of POTS: a randomized placebo-controlled trial. J Clin Psychopharmacol 2014; 34(6):738–741. doi:10.1097/JCP.0000000000000221
- Lai CC, Fischer PR, Brands CK, et al. Outcomes in adolescents with postural orthostatic tachycardia syndrome treated with midodrine and beta-blockers. Pacing Clin Electrophysiol 2009; 32(2):234–238. doi:10.1111/j.1540-8159.2008.02207.x
KEY POINTS
- Several POTS subtypes have been recognized, including hypovolemic, neuropathic, and hyperadrenergic forms, overlapping with Ehlers-Danlos syndrome, mast cell activation, and autoimmune syndromes.
- Treatment should take a graded approach, beginning with increasing salt and water intake, exercise, and compression stockings.
- If needed, consider medications to expand blood volume, slow heart rate, or reduce central sympathetic tone.
- Certain medications, including venodilators, diuretics, and serotonin-norepinephrine reuptake inhibitors, can exacerbate symptoms and should be avoided.
Dabigatran-induced esophagitis
A 74-year-old man presented to the gastroenterology clinic with a 2-day history of retrosternal discomfort. His vital signs were normal, and laboratory testing showed a normal leukocyte count.
Esophagogastroduodenoscopy (EGD) revealed longitudinal sloughing mucosal casts in the middle and lower esophagus (Figure 1).
The patient had been taking dabigatran 110 mg twice daily for 2 years because of nonvalvular atrial fibrillation. He was also taking amlodipine 2.5 mg/day for hypertension.
DABIGATRAN-INDUCED ESOPHAGITIS
Although no study has investigated the overall prevalence of dabigatran-induced esophagitis, a retrospective database review of 91 patients taking dabigatran and undergoing upper-gastrointestinal endoscopy reported that 19 (20.9%) had endoscopic signs of dabigatran-induced esophagitis.2
Typical symptoms are the acute onset of chest pain, epigastralgia, odynophagia, and dysphagia. But patients can also have no symptoms or only mild symptoms.2,3
Despite dabigatran’s anticoagulant activity, there have been few reports of bleeding, perhaps because the lesions tend to be superficial on the surface of the esophageal mucosa.
Symptoms usually resolve within 1 week after stopping dabigatran and starting a proton pump inhibitor. To prevent mucosal injury, patients should be instructed to take dabigatran with sufficient water and to remain in an upright position for at least 30 minutes afterward.4
- Baehr PH, McDonald GB. Esophageal infections: risk factors, presentation, diagnosis, and treatment. Gastroenterology 1994; 106(2):509–532. pmid:7980741
- Toya Y, Nakamura S, Tomita K, et al. Dabigatran-induced esophagitis: the prevalence and endoscopic characteristics. J Gastroenterol Hepatol 2016; 31(3):610–614. doi:10.1111/jgh.13024
- Ueta E, Fujikawa T, Imagawa A. A case of a slightly symptomatic exfoliative oesophagitis. BMJ Case Rep 2015; pii:bcr2015211925. doi:10.1136/bcr-2015-211925
- Ootani A, Hayashi Y, Miyagi Y. Dabigatran-induced esophagitis. Clin Gastroenterol Hepatol 2014; 12(7):e55–e56. doi:10.1016/j.cgh.2013.09.010
A 74-year-old man presented to the gastroenterology clinic with a 2-day history of retrosternal discomfort. His vital signs were normal, and laboratory testing showed a normal leukocyte count.
Esophagogastroduodenoscopy (EGD) revealed longitudinal sloughing mucosal casts in the middle and lower esophagus (Figure 1).
The patient had been taking dabigatran 110 mg twice daily for 2 years because of nonvalvular atrial fibrillation. He was also taking amlodipine 2.5 mg/day for hypertension.
DABIGATRAN-INDUCED ESOPHAGITIS
Although no study has investigated the overall prevalence of dabigatran-induced esophagitis, a retrospective database review of 91 patients taking dabigatran and undergoing upper-gastrointestinal endoscopy reported that 19 (20.9%) had endoscopic signs of dabigatran-induced esophagitis.2
Typical symptoms are the acute onset of chest pain, epigastralgia, odynophagia, and dysphagia. But patients can also have no symptoms or only mild symptoms.2,3
Despite dabigatran’s anticoagulant activity, there have been few reports of bleeding, perhaps because the lesions tend to be superficial on the surface of the esophageal mucosa.
Symptoms usually resolve within 1 week after stopping dabigatran and starting a proton pump inhibitor. To prevent mucosal injury, patients should be instructed to take dabigatran with sufficient water and to remain in an upright position for at least 30 minutes afterward.4
A 74-year-old man presented to the gastroenterology clinic with a 2-day history of retrosternal discomfort. His vital signs were normal, and laboratory testing showed a normal leukocyte count.
Esophagogastroduodenoscopy (EGD) revealed longitudinal sloughing mucosal casts in the middle and lower esophagus (Figure 1).
The patient had been taking dabigatran 110 mg twice daily for 2 years because of nonvalvular atrial fibrillation. He was also taking amlodipine 2.5 mg/day for hypertension.
DABIGATRAN-INDUCED ESOPHAGITIS
Although no study has investigated the overall prevalence of dabigatran-induced esophagitis, a retrospective database review of 91 patients taking dabigatran and undergoing upper-gastrointestinal endoscopy reported that 19 (20.9%) had endoscopic signs of dabigatran-induced esophagitis.2
Typical symptoms are the acute onset of chest pain, epigastralgia, odynophagia, and dysphagia. But patients can also have no symptoms or only mild symptoms.2,3
Despite dabigatran’s anticoagulant activity, there have been few reports of bleeding, perhaps because the lesions tend to be superficial on the surface of the esophageal mucosa.
Symptoms usually resolve within 1 week after stopping dabigatran and starting a proton pump inhibitor. To prevent mucosal injury, patients should be instructed to take dabigatran with sufficient water and to remain in an upright position for at least 30 minutes afterward.4
- Baehr PH, McDonald GB. Esophageal infections: risk factors, presentation, diagnosis, and treatment. Gastroenterology 1994; 106(2):509–532. pmid:7980741
- Toya Y, Nakamura S, Tomita K, et al. Dabigatran-induced esophagitis: the prevalence and endoscopic characteristics. J Gastroenterol Hepatol 2016; 31(3):610–614. doi:10.1111/jgh.13024
- Ueta E, Fujikawa T, Imagawa A. A case of a slightly symptomatic exfoliative oesophagitis. BMJ Case Rep 2015; pii:bcr2015211925. doi:10.1136/bcr-2015-211925
- Ootani A, Hayashi Y, Miyagi Y. Dabigatran-induced esophagitis. Clin Gastroenterol Hepatol 2014; 12(7):e55–e56. doi:10.1016/j.cgh.2013.09.010
- Baehr PH, McDonald GB. Esophageal infections: risk factors, presentation, diagnosis, and treatment. Gastroenterology 1994; 106(2):509–532. pmid:7980741
- Toya Y, Nakamura S, Tomita K, et al. Dabigatran-induced esophagitis: the prevalence and endoscopic characteristics. J Gastroenterol Hepatol 2016; 31(3):610–614. doi:10.1111/jgh.13024
- Ueta E, Fujikawa T, Imagawa A. A case of a slightly symptomatic exfoliative oesophagitis. BMJ Case Rep 2015; pii:bcr2015211925. doi:10.1136/bcr-2015-211925
- Ootani A, Hayashi Y, Miyagi Y. Dabigatran-induced esophagitis. Clin Gastroenterol Hepatol 2014; 12(7):e55–e56. doi:10.1016/j.cgh.2013.09.010
Click for Credit: Migraine & stroke risk; Aspirin for CV events; more
Here are 5 articles from the May issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Subclinical hypothyroidism boosts immediate risk of heart failure
To take the posttest, go to: https://bit.ly/2IK0YiL
Expires January 24, 2020
2. Meta-analysis supports aspirin to reduce cardiovascular events
To take the posttest, go to: https://bit.ly/2GJLgSB
Expires January 24, 2020
3. Age of migraine onset may affect stroke risk
To take the posttest, go to: https://bit.ly/2ZAJ5YR
Expires January 24, 2020
4. Women with RA have reduced chance of live birth after assisted reproduction treatment
To take the posttest, go to: https://bit.ly/2VvKRLF
Expires January 27, 2020
5. New SLE disease activity measure beats SLEDAI-2K
To take the posttest, go to: https://bit.ly/2W8SVPA
Expires January 31, 2020
Here are 5 articles from the May issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Subclinical hypothyroidism boosts immediate risk of heart failure
To take the posttest, go to: https://bit.ly/2IK0YiL
Expires January 24, 2020
2. Meta-analysis supports aspirin to reduce cardiovascular events
To take the posttest, go to: https://bit.ly/2GJLgSB
Expires January 24, 2020
3. Age of migraine onset may affect stroke risk
To take the posttest, go to: https://bit.ly/2ZAJ5YR
Expires January 24, 2020
4. Women with RA have reduced chance of live birth after assisted reproduction treatment
To take the posttest, go to: https://bit.ly/2VvKRLF
Expires January 27, 2020
5. New SLE disease activity measure beats SLEDAI-2K
To take the posttest, go to: https://bit.ly/2W8SVPA
Expires January 31, 2020
Here are 5 articles from the May issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Subclinical hypothyroidism boosts immediate risk of heart failure
To take the posttest, go to: https://bit.ly/2IK0YiL
Expires January 24, 2020
2. Meta-analysis supports aspirin to reduce cardiovascular events
To take the posttest, go to: https://bit.ly/2GJLgSB
Expires January 24, 2020
3. Age of migraine onset may affect stroke risk
To take the posttest, go to: https://bit.ly/2ZAJ5YR
Expires January 24, 2020
4. Women with RA have reduced chance of live birth after assisted reproduction treatment
To take the posttest, go to: https://bit.ly/2VvKRLF
Expires January 27, 2020
5. New SLE disease activity measure beats SLEDAI-2K
To take the posttest, go to: https://bit.ly/2W8SVPA
Expires January 31, 2020
Your patient’s brain is different at every visit
Unlike other organs in the human body, the brain is constantly changing. The main driver for this ongoing re-engineering across various neural circuits is “experiential neuroplasticity,” which creates billions of new synapses and dendrite spines as well as new connections. And as the brain reinvents itself from day to day, the mind evolves as well.
The neurobiologic re-sculpting of the brain’s complex innards continuously encodes memories of what we learn and experience during waking hours, including all that we see, hear, feel, think, contemplate, plan, and decide. However, in addition to the ongoing intrinsic neuroplasticity that records life’s experiences within neural circuits, there are many extrinsic factors that can further modify the brain and the “psyche” it generates via electrical, neurochemical, and physiological mechanisms. That’s why every patient a psychiatrist sees at follow-up visits will have a brain that will be different from the previous encounter.
Consider the following factors that can modify a patient’s brain (for better or worse) between sessions:
- Psychotherapy that the patient received at the last session will biologically modify his or her brain. Creating new insights and understanding of one’s behavior and “connecting the dots” of the past and present emotions and reactions are all associated with neuroplastic changes within the brain.
- Mood or psychotic episodes. Depressive, manic, or psychotic episodes are associated with neuroinflammation, oxidative stress, and apoptotic effects, which can disrupt the brain’s cytoarchitecture. That’s why psychiatrists must inquire about such episodes between visits and document the possible effects on the patient’s mental status.
- Psychotropic medications all bind to one or more brain receptors to exert therapeutic or adverse effects, both of which are associated with changes in neurotransmitter pathways. A key component of every follow-up visit is to gauge the risks and benefits of the pharmacotherapy prescribed at the prior visit.
- Nonpsychiatric prescription medications are often associated with iatrogenic effects on the brain apart from their intended target organs. These iatrogenic effects include anxiety, depression, mania, psychosis, and cognitive changes. That’s why during each visit, the physician or nurse practitioner must review all prescription medications and consider their potential effects on the patient’s mental status.
- Over-the-counter drugs and supplements may exert neurologic effects via histaminergic, muscarinic, glutamatergic, adrenergic, or serotonergic effects—all of which can alter brain chemistry and contribute to mental status changes. They can also inhibit or induce cytochrome enzymes and induce adverse effects or loss of efficacy of the primary psychotropic medication the patient takes.
- Medical illness, even as simple as an upper respiratory viral infection, can alter brain function due to illness-induced physiological aberrations, including pain and peripheral inflammation, with neurologic consequences. Common metabolic disorders such as diabetes, hyperlipidemia, and hypertension can exert mental status changes.
- Alcohol and drugs of abuse alter brain structure and function and can induce psychological and cognitive changes. Inquiring about the amount and frequency of alcohol and recreational drug use must be done in detail at every visit.
- Stressful events. It is almost impossible for a psychiatric patient not to encounter stressful life events between visits. Coping with any mental disorder can be quite stressful and challenging due to its social, vocational, or personal consequences. Stress increases cortisol, which is associated with deleterious inflammatory effects on the brain. Persistent stress can lead to hippocampal atrophy because of the abundance of glucocorticoid receptors in the hippocampus. Inquiry about stressors must be part of every psychiatric follow-up visit. Multiple psychological, physiological, and behavioral effects are well known to be generated by stress, especially in individuals already impaired by mental illness.
- Diet. What a patient eats (or avoids eating) can affect the brain. High-fat diets can be inflammatory, while a diet rich in fruits, vegetables, and nuts can be neuroprotective. The microbiota and the enteric brain—both in the gastrointestinal tract—have been reported to influence mood and behavior. (For more on this, see “Gut microbiota and its implications for psychiatry: A review of 3 studies” on page 40 and “It takes guts to be mentally ill: Microbiota and psychopathology,” From the Editor,
Current Psychiatry , September 2018, p. 4-6.) - Obesity is associated with brain atrophy as well as depression. Weight should be assessed at every visit and coupled with counseling about diet and exercise.
- Exercise, or the lack of it, can alter the brain in good or bad ways. Many studies have shown that regular exercise can induce hippocampal neurogenesis and sharpen memory and cognition. On the other hand, a sedentary lifestyle can be detrimental to the heart, bones, and brain, with an elevation in cerebrovascular and cardiovascular risks, both of which can progressively alter brain structure and function.
- Concussion, contusions, and traumatic brain injury obviously can activate the microglia and trigger neurologic sequelae and mental repercussions. At every visit, patients should be asked if they have experienced a mild or severe head injury, whether it is accidental or sports-related.
- Dehydration, especially on the day of the visit, can alter mental status in subtle ways. Cerebral ventricular volume has been shown to change with dehydration. Asking a patient about daily fluid intake should be a standard question, especially for older patients, who may experience hypotension and mental status changes due to hypovolemia.
- Sleep, whether too much or too little, is associated with brain effects and can impact cognition and behavior. Asking patients about sleep is important because it can affect the brain, and also can be a symptom of unresolved psychiatric disorders. Chronic sleep disorders are associated with neuroinflammation.
- Menstrual cycle. Various neurotransmitters fluctuate during a woman’s menstrual cycle. Her cognition becomes sharper around ovulation, and that may influence her mental status and perhaps the neuroplasticity of her brain.
- Pregnancy and its major hormone changes can change brain structure and function. Estrogen, progesterone, and prolactin have different structural effects on the brain that can help the future mother care for her dependent baby. Asking about missed periods and pregnancy during childbearing years can be useful during psychiatric encounters.
Continue to: In summary...
In summary, numerous variables can affect the patient’s brain between visits, influencing his or her mental status. The ever-changing brain can be challenging to assess, especially in brief 15- to 20-minute follow-up sessions that have become more common in psychiatry. Perhaps patients should help their psychiatrists or nurse practitioners by completing a checklist with all the above variables, either online on the day of their appointment or on a form in the waiting room immediately prior to the visit. This might also increase patients’ awareness of the importance of participating in monitoring themselves.
And finally, let’s not forget that the psychiatrist’s brain also changes continuously due to his or her own daily experiences, stresses, diet, lifestyle, medical illness, or medications. Thus, at every psychiatric session, the brains of both patient and psychiatrist are very different from the previous encounter!
To comment on this editorial or other topics of interest: [email protected].
Unlike other organs in the human body, the brain is constantly changing. The main driver for this ongoing re-engineering across various neural circuits is “experiential neuroplasticity,” which creates billions of new synapses and dendrite spines as well as new connections. And as the brain reinvents itself from day to day, the mind evolves as well.
The neurobiologic re-sculpting of the brain’s complex innards continuously encodes memories of what we learn and experience during waking hours, including all that we see, hear, feel, think, contemplate, plan, and decide. However, in addition to the ongoing intrinsic neuroplasticity that records life’s experiences within neural circuits, there are many extrinsic factors that can further modify the brain and the “psyche” it generates via electrical, neurochemical, and physiological mechanisms. That’s why every patient a psychiatrist sees at follow-up visits will have a brain that will be different from the previous encounter.
Consider the following factors that can modify a patient’s brain (for better or worse) between sessions:
- Psychotherapy that the patient received at the last session will biologically modify his or her brain. Creating new insights and understanding of one’s behavior and “connecting the dots” of the past and present emotions and reactions are all associated with neuroplastic changes within the brain.
- Mood or psychotic episodes. Depressive, manic, or psychotic episodes are associated with neuroinflammation, oxidative stress, and apoptotic effects, which can disrupt the brain’s cytoarchitecture. That’s why psychiatrists must inquire about such episodes between visits and document the possible effects on the patient’s mental status.
- Psychotropic medications all bind to one or more brain receptors to exert therapeutic or adverse effects, both of which are associated with changes in neurotransmitter pathways. A key component of every follow-up visit is to gauge the risks and benefits of the pharmacotherapy prescribed at the prior visit.
- Nonpsychiatric prescription medications are often associated with iatrogenic effects on the brain apart from their intended target organs. These iatrogenic effects include anxiety, depression, mania, psychosis, and cognitive changes. That’s why during each visit, the physician or nurse practitioner must review all prescription medications and consider their potential effects on the patient’s mental status.
- Over-the-counter drugs and supplements may exert neurologic effects via histaminergic, muscarinic, glutamatergic, adrenergic, or serotonergic effects—all of which can alter brain chemistry and contribute to mental status changes. They can also inhibit or induce cytochrome enzymes and induce adverse effects or loss of efficacy of the primary psychotropic medication the patient takes.
- Medical illness, even as simple as an upper respiratory viral infection, can alter brain function due to illness-induced physiological aberrations, including pain and peripheral inflammation, with neurologic consequences. Common metabolic disorders such as diabetes, hyperlipidemia, and hypertension can exert mental status changes.
- Alcohol and drugs of abuse alter brain structure and function and can induce psychological and cognitive changes. Inquiring about the amount and frequency of alcohol and recreational drug use must be done in detail at every visit.
- Stressful events. It is almost impossible for a psychiatric patient not to encounter stressful life events between visits. Coping with any mental disorder can be quite stressful and challenging due to its social, vocational, or personal consequences. Stress increases cortisol, which is associated with deleterious inflammatory effects on the brain. Persistent stress can lead to hippocampal atrophy because of the abundance of glucocorticoid receptors in the hippocampus. Inquiry about stressors must be part of every psychiatric follow-up visit. Multiple psychological, physiological, and behavioral effects are well known to be generated by stress, especially in individuals already impaired by mental illness.
- Diet. What a patient eats (or avoids eating) can affect the brain. High-fat diets can be inflammatory, while a diet rich in fruits, vegetables, and nuts can be neuroprotective. The microbiota and the enteric brain—both in the gastrointestinal tract—have been reported to influence mood and behavior. (For more on this, see “Gut microbiota and its implications for psychiatry: A review of 3 studies” on page 40 and “It takes guts to be mentally ill: Microbiota and psychopathology,” From the Editor,
Current Psychiatry , September 2018, p. 4-6.) - Obesity is associated with brain atrophy as well as depression. Weight should be assessed at every visit and coupled with counseling about diet and exercise.
- Exercise, or the lack of it, can alter the brain in good or bad ways. Many studies have shown that regular exercise can induce hippocampal neurogenesis and sharpen memory and cognition. On the other hand, a sedentary lifestyle can be detrimental to the heart, bones, and brain, with an elevation in cerebrovascular and cardiovascular risks, both of which can progressively alter brain structure and function.
- Concussion, contusions, and traumatic brain injury obviously can activate the microglia and trigger neurologic sequelae and mental repercussions. At every visit, patients should be asked if they have experienced a mild or severe head injury, whether it is accidental or sports-related.
- Dehydration, especially on the day of the visit, can alter mental status in subtle ways. Cerebral ventricular volume has been shown to change with dehydration. Asking a patient about daily fluid intake should be a standard question, especially for older patients, who may experience hypotension and mental status changes due to hypovolemia.
- Sleep, whether too much or too little, is associated with brain effects and can impact cognition and behavior. Asking patients about sleep is important because it can affect the brain, and also can be a symptom of unresolved psychiatric disorders. Chronic sleep disorders are associated with neuroinflammation.
- Menstrual cycle. Various neurotransmitters fluctuate during a woman’s menstrual cycle. Her cognition becomes sharper around ovulation, and that may influence her mental status and perhaps the neuroplasticity of her brain.
- Pregnancy and its major hormone changes can change brain structure and function. Estrogen, progesterone, and prolactin have different structural effects on the brain that can help the future mother care for her dependent baby. Asking about missed periods and pregnancy during childbearing years can be useful during psychiatric encounters.
Continue to: In summary...
In summary, numerous variables can affect the patient’s brain between visits, influencing his or her mental status. The ever-changing brain can be challenging to assess, especially in brief 15- to 20-minute follow-up sessions that have become more common in psychiatry. Perhaps patients should help their psychiatrists or nurse practitioners by completing a checklist with all the above variables, either online on the day of their appointment or on a form in the waiting room immediately prior to the visit. This might also increase patients’ awareness of the importance of participating in monitoring themselves.
And finally, let’s not forget that the psychiatrist’s brain also changes continuously due to his or her own daily experiences, stresses, diet, lifestyle, medical illness, or medications. Thus, at every psychiatric session, the brains of both patient and psychiatrist are very different from the previous encounter!
To comment on this editorial or other topics of interest: [email protected].
Unlike other organs in the human body, the brain is constantly changing. The main driver for this ongoing re-engineering across various neural circuits is “experiential neuroplasticity,” which creates billions of new synapses and dendrite spines as well as new connections. And as the brain reinvents itself from day to day, the mind evolves as well.
The neurobiologic re-sculpting of the brain’s complex innards continuously encodes memories of what we learn and experience during waking hours, including all that we see, hear, feel, think, contemplate, plan, and decide. However, in addition to the ongoing intrinsic neuroplasticity that records life’s experiences within neural circuits, there are many extrinsic factors that can further modify the brain and the “psyche” it generates via electrical, neurochemical, and physiological mechanisms. That’s why every patient a psychiatrist sees at follow-up visits will have a brain that will be different from the previous encounter.
Consider the following factors that can modify a patient’s brain (for better or worse) between sessions:
- Psychotherapy that the patient received at the last session will biologically modify his or her brain. Creating new insights and understanding of one’s behavior and “connecting the dots” of the past and present emotions and reactions are all associated with neuroplastic changes within the brain.
- Mood or psychotic episodes. Depressive, manic, or psychotic episodes are associated with neuroinflammation, oxidative stress, and apoptotic effects, which can disrupt the brain’s cytoarchitecture. That’s why psychiatrists must inquire about such episodes between visits and document the possible effects on the patient’s mental status.
- Psychotropic medications all bind to one or more brain receptors to exert therapeutic or adverse effects, both of which are associated with changes in neurotransmitter pathways. A key component of every follow-up visit is to gauge the risks and benefits of the pharmacotherapy prescribed at the prior visit.
- Nonpsychiatric prescription medications are often associated with iatrogenic effects on the brain apart from their intended target organs. These iatrogenic effects include anxiety, depression, mania, psychosis, and cognitive changes. That’s why during each visit, the physician or nurse practitioner must review all prescription medications and consider their potential effects on the patient’s mental status.
- Over-the-counter drugs and supplements may exert neurologic effects via histaminergic, muscarinic, glutamatergic, adrenergic, or serotonergic effects—all of which can alter brain chemistry and contribute to mental status changes. They can also inhibit or induce cytochrome enzymes and induce adverse effects or loss of efficacy of the primary psychotropic medication the patient takes.
- Medical illness, even as simple as an upper respiratory viral infection, can alter brain function due to illness-induced physiological aberrations, including pain and peripheral inflammation, with neurologic consequences. Common metabolic disorders such as diabetes, hyperlipidemia, and hypertension can exert mental status changes.
- Alcohol and drugs of abuse alter brain structure and function and can induce psychological and cognitive changes. Inquiring about the amount and frequency of alcohol and recreational drug use must be done in detail at every visit.
- Stressful events. It is almost impossible for a psychiatric patient not to encounter stressful life events between visits. Coping with any mental disorder can be quite stressful and challenging due to its social, vocational, or personal consequences. Stress increases cortisol, which is associated with deleterious inflammatory effects on the brain. Persistent stress can lead to hippocampal atrophy because of the abundance of glucocorticoid receptors in the hippocampus. Inquiry about stressors must be part of every psychiatric follow-up visit. Multiple psychological, physiological, and behavioral effects are well known to be generated by stress, especially in individuals already impaired by mental illness.
- Diet. What a patient eats (or avoids eating) can affect the brain. High-fat diets can be inflammatory, while a diet rich in fruits, vegetables, and nuts can be neuroprotective. The microbiota and the enteric brain—both in the gastrointestinal tract—have been reported to influence mood and behavior. (For more on this, see “Gut microbiota and its implications for psychiatry: A review of 3 studies” on page 40 and “It takes guts to be mentally ill: Microbiota and psychopathology,” From the Editor,
Current Psychiatry , September 2018, p. 4-6.) - Obesity is associated with brain atrophy as well as depression. Weight should be assessed at every visit and coupled with counseling about diet and exercise.
- Exercise, or the lack of it, can alter the brain in good or bad ways. Many studies have shown that regular exercise can induce hippocampal neurogenesis and sharpen memory and cognition. On the other hand, a sedentary lifestyle can be detrimental to the heart, bones, and brain, with an elevation in cerebrovascular and cardiovascular risks, both of which can progressively alter brain structure and function.
- Concussion, contusions, and traumatic brain injury obviously can activate the microglia and trigger neurologic sequelae and mental repercussions. At every visit, patients should be asked if they have experienced a mild or severe head injury, whether it is accidental or sports-related.
- Dehydration, especially on the day of the visit, can alter mental status in subtle ways. Cerebral ventricular volume has been shown to change with dehydration. Asking a patient about daily fluid intake should be a standard question, especially for older patients, who may experience hypotension and mental status changes due to hypovolemia.
- Sleep, whether too much or too little, is associated with brain effects and can impact cognition and behavior. Asking patients about sleep is important because it can affect the brain, and also can be a symptom of unresolved psychiatric disorders. Chronic sleep disorders are associated with neuroinflammation.
- Menstrual cycle. Various neurotransmitters fluctuate during a woman’s menstrual cycle. Her cognition becomes sharper around ovulation, and that may influence her mental status and perhaps the neuroplasticity of her brain.
- Pregnancy and its major hormone changes can change brain structure and function. Estrogen, progesterone, and prolactin have different structural effects on the brain that can help the future mother care for her dependent baby. Asking about missed periods and pregnancy during childbearing years can be useful during psychiatric encounters.
Continue to: In summary...
In summary, numerous variables can affect the patient’s brain between visits, influencing his or her mental status. The ever-changing brain can be challenging to assess, especially in brief 15- to 20-minute follow-up sessions that have become more common in psychiatry. Perhaps patients should help their psychiatrists or nurse practitioners by completing a checklist with all the above variables, either online on the day of their appointment or on a form in the waiting room immediately prior to the visit. This might also increase patients’ awareness of the importance of participating in monitoring themselves.
And finally, let’s not forget that the psychiatrist’s brain also changes continuously due to his or her own daily experiences, stresses, diet, lifestyle, medical illness, or medications. Thus, at every psychiatric session, the brains of both patient and psychiatrist are very different from the previous encounter!
To comment on this editorial or other topics of interest: [email protected].
Sleep apnea is linked with tau accumulation in the brain
PHILADELPHIA – according to data that will be presented at the annual meeting of the American Academy of Neurology. Tau accumulation is a biomarker of Alzheimer’s disease, and the finding suggests a possible explanation for the apparent association between sleep disruption and dementia.
“Our research results raise the possibility that sleep apnea affects tau accumulation,” said Diego Z. Carvalho, MD, of the Mayo Clinic in Rochester, Minn., in a press release. “But it is also possible that higher levels of tau in other regions may predispose a person to sleep apnea, so longer studies are now needed to solve this chicken-and-egg problem.”
Previous research had suggested an association between sleep disruption and increased risk of dementia. Obstructive sleep apnea in particular has been associated with this increased risk. The pathological processes that account for this association are unknown, however.
Dr. Carvalho and colleagues decided to evaluate whether apneas during sleep, reported by the patient or an informant, were associated with high levels of tau in cognitively normal elderly individuals. The investigators identified 288 participants in the Mayo Clinic Study of Aging for their analysis. Eligible participants were aged 65 years or older, had no cognitive impairment, had undergone tau PET and amyloid PET scans, and had completed a questionnaire that solicited information about witnessed apneas during sleep (either from patients or bed partners). Dr. Carvalho’s group took the entorhinal cortex as its region of interest because it is highly susceptible to tau accumulation. The entorhinal cortex is involved in memory, navigation, and the perception of time. They chose the cerebellum crus as their reference region.
The investigators created a linear model to evaluate the association between tau in the entorhinal cortex and witnessed apneas. They controlled the data for age, sex, years of education, body mass index, hypertension, hyperlipidemia, diabetes, reduced sleep, excessive daytime sleepiness, and global amyloid.
In all, 43 participants (15%) had witnessed apneas during sleep. Witnessed apneas were significantly associated with tau in the entorhinal cortex. After controlling for potential confounders, Dr. Carvalho and colleagues estimated a 0.049 elevation in the entorhinal cortex tau standardized uptake value ratio (95% confidence interval, 0.011–0.087; P = 0.012).
The study had a relatively small sample size, and its results require validation. Other important limitations include the absence of sleep studies to confirm the presence and severity of sleep apnea and a lack of information about whether participants already were receiving treatment for sleep apnea.
The National Institutes of Health supported the study.
SOURCE: Carvalho D et al. AAN 2019, Abstract P3.6-021.
PHILADELPHIA – according to data that will be presented at the annual meeting of the American Academy of Neurology. Tau accumulation is a biomarker of Alzheimer’s disease, and the finding suggests a possible explanation for the apparent association between sleep disruption and dementia.
“Our research results raise the possibility that sleep apnea affects tau accumulation,” said Diego Z. Carvalho, MD, of the Mayo Clinic in Rochester, Minn., in a press release. “But it is also possible that higher levels of tau in other regions may predispose a person to sleep apnea, so longer studies are now needed to solve this chicken-and-egg problem.”
Previous research had suggested an association between sleep disruption and increased risk of dementia. Obstructive sleep apnea in particular has been associated with this increased risk. The pathological processes that account for this association are unknown, however.
Dr. Carvalho and colleagues decided to evaluate whether apneas during sleep, reported by the patient or an informant, were associated with high levels of tau in cognitively normal elderly individuals. The investigators identified 288 participants in the Mayo Clinic Study of Aging for their analysis. Eligible participants were aged 65 years or older, had no cognitive impairment, had undergone tau PET and amyloid PET scans, and had completed a questionnaire that solicited information about witnessed apneas during sleep (either from patients or bed partners). Dr. Carvalho’s group took the entorhinal cortex as its region of interest because it is highly susceptible to tau accumulation. The entorhinal cortex is involved in memory, navigation, and the perception of time. They chose the cerebellum crus as their reference region.
The investigators created a linear model to evaluate the association between tau in the entorhinal cortex and witnessed apneas. They controlled the data for age, sex, years of education, body mass index, hypertension, hyperlipidemia, diabetes, reduced sleep, excessive daytime sleepiness, and global amyloid.
In all, 43 participants (15%) had witnessed apneas during sleep. Witnessed apneas were significantly associated with tau in the entorhinal cortex. After controlling for potential confounders, Dr. Carvalho and colleagues estimated a 0.049 elevation in the entorhinal cortex tau standardized uptake value ratio (95% confidence interval, 0.011–0.087; P = 0.012).
The study had a relatively small sample size, and its results require validation. Other important limitations include the absence of sleep studies to confirm the presence and severity of sleep apnea and a lack of information about whether participants already were receiving treatment for sleep apnea.
The National Institutes of Health supported the study.
SOURCE: Carvalho D et al. AAN 2019, Abstract P3.6-021.
PHILADELPHIA – according to data that will be presented at the annual meeting of the American Academy of Neurology. Tau accumulation is a biomarker of Alzheimer’s disease, and the finding suggests a possible explanation for the apparent association between sleep disruption and dementia.
“Our research results raise the possibility that sleep apnea affects tau accumulation,” said Diego Z. Carvalho, MD, of the Mayo Clinic in Rochester, Minn., in a press release. “But it is also possible that higher levels of tau in other regions may predispose a person to sleep apnea, so longer studies are now needed to solve this chicken-and-egg problem.”
Previous research had suggested an association between sleep disruption and increased risk of dementia. Obstructive sleep apnea in particular has been associated with this increased risk. The pathological processes that account for this association are unknown, however.
Dr. Carvalho and colleagues decided to evaluate whether apneas during sleep, reported by the patient or an informant, were associated with high levels of tau in cognitively normal elderly individuals. The investigators identified 288 participants in the Mayo Clinic Study of Aging for their analysis. Eligible participants were aged 65 years or older, had no cognitive impairment, had undergone tau PET and amyloid PET scans, and had completed a questionnaire that solicited information about witnessed apneas during sleep (either from patients or bed partners). Dr. Carvalho’s group took the entorhinal cortex as its region of interest because it is highly susceptible to tau accumulation. The entorhinal cortex is involved in memory, navigation, and the perception of time. They chose the cerebellum crus as their reference region.
The investigators created a linear model to evaluate the association between tau in the entorhinal cortex and witnessed apneas. They controlled the data for age, sex, years of education, body mass index, hypertension, hyperlipidemia, diabetes, reduced sleep, excessive daytime sleepiness, and global amyloid.
In all, 43 participants (15%) had witnessed apneas during sleep. Witnessed apneas were significantly associated with tau in the entorhinal cortex. After controlling for potential confounders, Dr. Carvalho and colleagues estimated a 0.049 elevation in the entorhinal cortex tau standardized uptake value ratio (95% confidence interval, 0.011–0.087; P = 0.012).
The study had a relatively small sample size, and its results require validation. Other important limitations include the absence of sleep studies to confirm the presence and severity of sleep apnea and a lack of information about whether participants already were receiving treatment for sleep apnea.
The National Institutes of Health supported the study.
SOURCE: Carvalho D et al. AAN 2019, Abstract P3.6-021.
FROM AAN 2019
Neurodevelopmental concerns may emerge later in Zika-exposed infants
BALTIMORE – Most infants prenatally exposed to Zika showed relatively normal neurodevelopment if their fetal MRI and birth head circumference were normal, but others with similarly initial normal measures appeared to struggle with social cognition and mobility as they got older, according to a new study.
“I think we need to be cautious with saying that these children are normal when these normal-appearing children may not be doing as well as we think,” lead author Sarah Mulkey, MD, of Children’s National Health System and George Washington University, Washington, said in an interview. “While most children are showing fairly normal development, there are some children who are … becoming more abnormal over time.”
Dr. Mulkey shared her findings at the Pediatric Academic Societies annual meeting. She and her colleagues had previously published a prospective study of 82 Zika-exposed infants’ fetal brain MRIs. In their new study, they followed up with the 78 Colombian infants from that study whose fetal neuroimaging and birth head circumstance had been normal.
The researchers used the Alberta Infant Motor Scale (AIMS) and the Warner Initial Developmental Evaluation of Adaptive and Functional Skills (WIDEA) to evaluate 72 of the children, 34 of whom underwent assessment twice. Forty of the children were an average 5.7 months old when evaluated, and 66 were an average 13.5 months old.
As the children got older, their overall WIDEA z-score and their subscores in the social cognition domain and especially in the mobility domain trended downward. Three of the children had AIMS scores two standard deviations below normal, but the rest fell within the normal range.
Their WIDEA communication z-score hovered relatively close to the norm, but self-care also showed a very slight slope downward, albeit not as substantially as in the social cognition and mobility domains.
The younger a child is, the fewer skills they generally show related to neurocognitive development, Dr. Mulkey explained. But as they grow older and are expected to show more skills, it becomes more apparent where gaps and delays might exist.
“We can see that there are a lot of kids doing well, but some of these kids certainly are not,” she said. “Until children have a long time to develop, you really can’t see these changes unless you follow them long-term.”
The researchers also looked separately at a subgroup of 19 children (26%) whose cranial ultrasounds showed mild nonspecific findings. These findings – such as lenticulostriate vasculopathy, choroid plexus cysts, subependymal cysts and calcifications – do not usually indicate any problems, but they appeared in a quarter of this population, considerably more than the approximately 5% typically seen in the general population, Dr. Mulkey said.
Though the findings did not reach significance, infants in this subgroup tended to have a lower WIDEA mobility z-scores (P = .054) and lower AIMS scores (P = .26) than the Zika-exposed infants with normal cranial ultrasounds.
“Mild nonspecific cranial ultrasound findings may represent a mild injury” related to exposure to their mother’s Zika infection during pregnancy, the researchers suggested. “It may be a risk factor for the lower mobility outcome,” Dr. Mulkey said.
The researchers hope to continue later follow-ups as the children age.
The research was funded by the Thrasher Research Fund. Dr. Mulkey had no conflicts of interest.
BALTIMORE – Most infants prenatally exposed to Zika showed relatively normal neurodevelopment if their fetal MRI and birth head circumference were normal, but others with similarly initial normal measures appeared to struggle with social cognition and mobility as they got older, according to a new study.
“I think we need to be cautious with saying that these children are normal when these normal-appearing children may not be doing as well as we think,” lead author Sarah Mulkey, MD, of Children’s National Health System and George Washington University, Washington, said in an interview. “While most children are showing fairly normal development, there are some children who are … becoming more abnormal over time.”
Dr. Mulkey shared her findings at the Pediatric Academic Societies annual meeting. She and her colleagues had previously published a prospective study of 82 Zika-exposed infants’ fetal brain MRIs. In their new study, they followed up with the 78 Colombian infants from that study whose fetal neuroimaging and birth head circumstance had been normal.
The researchers used the Alberta Infant Motor Scale (AIMS) and the Warner Initial Developmental Evaluation of Adaptive and Functional Skills (WIDEA) to evaluate 72 of the children, 34 of whom underwent assessment twice. Forty of the children were an average 5.7 months old when evaluated, and 66 were an average 13.5 months old.
As the children got older, their overall WIDEA z-score and their subscores in the social cognition domain and especially in the mobility domain trended downward. Three of the children had AIMS scores two standard deviations below normal, but the rest fell within the normal range.
Their WIDEA communication z-score hovered relatively close to the norm, but self-care also showed a very slight slope downward, albeit not as substantially as in the social cognition and mobility domains.
The younger a child is, the fewer skills they generally show related to neurocognitive development, Dr. Mulkey explained. But as they grow older and are expected to show more skills, it becomes more apparent where gaps and delays might exist.
“We can see that there are a lot of kids doing well, but some of these kids certainly are not,” she said. “Until children have a long time to develop, you really can’t see these changes unless you follow them long-term.”
The researchers also looked separately at a subgroup of 19 children (26%) whose cranial ultrasounds showed mild nonspecific findings. These findings – such as lenticulostriate vasculopathy, choroid plexus cysts, subependymal cysts and calcifications – do not usually indicate any problems, but they appeared in a quarter of this population, considerably more than the approximately 5% typically seen in the general population, Dr. Mulkey said.
Though the findings did not reach significance, infants in this subgroup tended to have a lower WIDEA mobility z-scores (P = .054) and lower AIMS scores (P = .26) than the Zika-exposed infants with normal cranial ultrasounds.
“Mild nonspecific cranial ultrasound findings may represent a mild injury” related to exposure to their mother’s Zika infection during pregnancy, the researchers suggested. “It may be a risk factor for the lower mobility outcome,” Dr. Mulkey said.
The researchers hope to continue later follow-ups as the children age.
The research was funded by the Thrasher Research Fund. Dr. Mulkey had no conflicts of interest.
BALTIMORE – Most infants prenatally exposed to Zika showed relatively normal neurodevelopment if their fetal MRI and birth head circumference were normal, but others with similarly initial normal measures appeared to struggle with social cognition and mobility as they got older, according to a new study.
“I think we need to be cautious with saying that these children are normal when these normal-appearing children may not be doing as well as we think,” lead author Sarah Mulkey, MD, of Children’s National Health System and George Washington University, Washington, said in an interview. “While most children are showing fairly normal development, there are some children who are … becoming more abnormal over time.”
Dr. Mulkey shared her findings at the Pediatric Academic Societies annual meeting. She and her colleagues had previously published a prospective study of 82 Zika-exposed infants’ fetal brain MRIs. In their new study, they followed up with the 78 Colombian infants from that study whose fetal neuroimaging and birth head circumstance had been normal.
The researchers used the Alberta Infant Motor Scale (AIMS) and the Warner Initial Developmental Evaluation of Adaptive and Functional Skills (WIDEA) to evaluate 72 of the children, 34 of whom underwent assessment twice. Forty of the children were an average 5.7 months old when evaluated, and 66 were an average 13.5 months old.
As the children got older, their overall WIDEA z-score and their subscores in the social cognition domain and especially in the mobility domain trended downward. Three of the children had AIMS scores two standard deviations below normal, but the rest fell within the normal range.
Their WIDEA communication z-score hovered relatively close to the norm, but self-care also showed a very slight slope downward, albeit not as substantially as in the social cognition and mobility domains.
The younger a child is, the fewer skills they generally show related to neurocognitive development, Dr. Mulkey explained. But as they grow older and are expected to show more skills, it becomes more apparent where gaps and delays might exist.
“We can see that there are a lot of kids doing well, but some of these kids certainly are not,” she said. “Until children have a long time to develop, you really can’t see these changes unless you follow them long-term.”
The researchers also looked separately at a subgroup of 19 children (26%) whose cranial ultrasounds showed mild nonspecific findings. These findings – such as lenticulostriate vasculopathy, choroid plexus cysts, subependymal cysts and calcifications – do not usually indicate any problems, but they appeared in a quarter of this population, considerably more than the approximately 5% typically seen in the general population, Dr. Mulkey said.
Though the findings did not reach significance, infants in this subgroup tended to have a lower WIDEA mobility z-scores (P = .054) and lower AIMS scores (P = .26) than the Zika-exposed infants with normal cranial ultrasounds.
“Mild nonspecific cranial ultrasound findings may represent a mild injury” related to exposure to their mother’s Zika infection during pregnancy, the researchers suggested. “It may be a risk factor for the lower mobility outcome,” Dr. Mulkey said.
The researchers hope to continue later follow-ups as the children age.
The research was funded by the Thrasher Research Fund. Dr. Mulkey had no conflicts of interest.
REPORTING FROM PAS 2019
Key clinical point: .
Major finding: Zika-exposed infants with normal fetal MRI neuroimaging showed increasingly lower mobility and social cognition skills as they approached their first birthday.
Study details: The findings are based on neurodevelopmental assessments of 72 Zika-exposed Colombian children at 4-18 months old.
Disclosures: The research was funded by the Thrasher Research Fund. Dr. Mulkey had no conflicts of interest.
Medical cannabis relieved pain, decreased opioid use in elderly
results of a recent retrospective chart review suggest. Treatment with medical cannabis improved pain, sleep, anxiety, and neuropathy in patients aged 75 years of age and older, and was associated with reduced use of opioids in about one-third of cases, according to authors of the study, which will be presented at the annual meeting of the American Academy of Neurology.
“Our findings are promising and can help fuel further research into medical marijuana as an additional option for this group of people who often have chronic conditions,” said lead investigator Laszlo Mechtler, MD, of Dent Neurologic Institute in Buffalo, N.Y., in a news release. However, additional randomized, placebo-controlled studies are needed to confirm results of this study, Dr. Mechtler added.
The chart review focused on 204 elderly patients who participated in New York State’s medical marijuana program and were followed in a neurologic outpatient setting. The cohort included 129 female and 75 male patients, ranging in age from 75 to 102 years, with a mean age of 81 years. The medical marijuana was taken by mouth as a liquid extract tincture, capsule, or in an electronic vaporizer.
With an average exposure time of 16.8 weeks, 69% of patients experienced symptomatic benefit, according to patient self-report. The most commonly reported benefit was relief of chronic pain in 49%, while improvements in sleep, neuropathy, and anxiety were reported in 18%, 15%, and 10%, respectively. Reductions in opioid pain medication were noted in about one-third of cases, they found.
While 34% of patients had adverse effects on medical marijuana, only 21% reported adverse effects after cannabinoid doses were adjusted, investigators said. Adverse effects led to discontinuation of medical cannabis in seven patients, or 3.4% of the overall cohort. Somnolence, disequilibrium, and gastrointestinal disturbance were the most common adverse effects, occurring in 13%, 7%, and 7% of patients, respectively. Euphoria was reported in 3% of patients.
Among patients who had no reported adverse effects, the most commonly used formulation was a balanced 1:1 tincture of tetrahydrocannabinol to cannabidiol, investigators said.
Further trials could explore optimal dosing of medical cannabis in elderly patients and shed more light on adverse effects such as somnolence and disequilibrium, according to Dr. Mechtler and colleagues.
The study was supported by the Dent Family Foundation.
SOURCE: Bargnes V et al. AAN 2019, Abstract P4.1-014.
results of a recent retrospective chart review suggest. Treatment with medical cannabis improved pain, sleep, anxiety, and neuropathy in patients aged 75 years of age and older, and was associated with reduced use of opioids in about one-third of cases, according to authors of the study, which will be presented at the annual meeting of the American Academy of Neurology.
“Our findings are promising and can help fuel further research into medical marijuana as an additional option for this group of people who often have chronic conditions,” said lead investigator Laszlo Mechtler, MD, of Dent Neurologic Institute in Buffalo, N.Y., in a news release. However, additional randomized, placebo-controlled studies are needed to confirm results of this study, Dr. Mechtler added.
The chart review focused on 204 elderly patients who participated in New York State’s medical marijuana program and were followed in a neurologic outpatient setting. The cohort included 129 female and 75 male patients, ranging in age from 75 to 102 years, with a mean age of 81 years. The medical marijuana was taken by mouth as a liquid extract tincture, capsule, or in an electronic vaporizer.
With an average exposure time of 16.8 weeks, 69% of patients experienced symptomatic benefit, according to patient self-report. The most commonly reported benefit was relief of chronic pain in 49%, while improvements in sleep, neuropathy, and anxiety were reported in 18%, 15%, and 10%, respectively. Reductions in opioid pain medication were noted in about one-third of cases, they found.
While 34% of patients had adverse effects on medical marijuana, only 21% reported adverse effects after cannabinoid doses were adjusted, investigators said. Adverse effects led to discontinuation of medical cannabis in seven patients, or 3.4% of the overall cohort. Somnolence, disequilibrium, and gastrointestinal disturbance were the most common adverse effects, occurring in 13%, 7%, and 7% of patients, respectively. Euphoria was reported in 3% of patients.
Among patients who had no reported adverse effects, the most commonly used formulation was a balanced 1:1 tincture of tetrahydrocannabinol to cannabidiol, investigators said.
Further trials could explore optimal dosing of medical cannabis in elderly patients and shed more light on adverse effects such as somnolence and disequilibrium, according to Dr. Mechtler and colleagues.
The study was supported by the Dent Family Foundation.
SOURCE: Bargnes V et al. AAN 2019, Abstract P4.1-014.
results of a recent retrospective chart review suggest. Treatment with medical cannabis improved pain, sleep, anxiety, and neuropathy in patients aged 75 years of age and older, and was associated with reduced use of opioids in about one-third of cases, according to authors of the study, which will be presented at the annual meeting of the American Academy of Neurology.
“Our findings are promising and can help fuel further research into medical marijuana as an additional option for this group of people who often have chronic conditions,” said lead investigator Laszlo Mechtler, MD, of Dent Neurologic Institute in Buffalo, N.Y., in a news release. However, additional randomized, placebo-controlled studies are needed to confirm results of this study, Dr. Mechtler added.
The chart review focused on 204 elderly patients who participated in New York State’s medical marijuana program and were followed in a neurologic outpatient setting. The cohort included 129 female and 75 male patients, ranging in age from 75 to 102 years, with a mean age of 81 years. The medical marijuana was taken by mouth as a liquid extract tincture, capsule, or in an electronic vaporizer.
With an average exposure time of 16.8 weeks, 69% of patients experienced symptomatic benefit, according to patient self-report. The most commonly reported benefit was relief of chronic pain in 49%, while improvements in sleep, neuropathy, and anxiety were reported in 18%, 15%, and 10%, respectively. Reductions in opioid pain medication were noted in about one-third of cases, they found.
While 34% of patients had adverse effects on medical marijuana, only 21% reported adverse effects after cannabinoid doses were adjusted, investigators said. Adverse effects led to discontinuation of medical cannabis in seven patients, or 3.4% of the overall cohort. Somnolence, disequilibrium, and gastrointestinal disturbance were the most common adverse effects, occurring in 13%, 7%, and 7% of patients, respectively. Euphoria was reported in 3% of patients.
Among patients who had no reported adverse effects, the most commonly used formulation was a balanced 1:1 tincture of tetrahydrocannabinol to cannabidiol, investigators said.
Further trials could explore optimal dosing of medical cannabis in elderly patients and shed more light on adverse effects such as somnolence and disequilibrium, according to Dr. Mechtler and colleagues.
The study was supported by the Dent Family Foundation.
SOURCE: Bargnes V et al. AAN 2019, Abstract P4.1-014.
FROM AAN 2019
Is pro soccer a risk factor for ALS?
according to Italian researchers who reviewed trading cards of about 25,000 male professional soccer players who played in Italy. The researchers then scanned news reports to find which of those players developed the rare neurologic disease. Players who developed ALS were a much younger age at diagnosis when compared with the general population, according to the researchers, who will present their findings at the annual meeting of the American Academy of Neurology.
While the findings might implicate professional-level soccer in the development of ALS, there could be other factors at work, said lead author Ettore Beghi, MD, of the Mario Negri Institute for Pharmacological Research, Milan. “Repeated traumatic events, heavy physical exercise, and substance use could also be factors in the increased ALS risk among soccer players,” Dr. Beghi said in a news release. “In addition, genetics may play a role.”
The ALS-related deaths of several Italian pro soccer players sparked suggestions that the disease and the sport could be somehow linked, according to Dr. Beghi and colleagues. To determine whether professional soccer players are at increased ALS risk, they reviewed the archives of the country’s major soccer card publisher from the years 1959 to 2000, recording the name, date, and place of birth; field position; and team history for the tens of thousands of players they identified.
News reports revealed that 33 players in that cohort developed ALS, compared with 17.6 cases that would be expected based on Italian general population estimates, according to Dr. Beghi and colleagues.
The number of cases per 100,000 person-years was 1.9 for all the soccer players, and 4.7 for those who were younger than 45 years at diagnosis, researchers said. In general, soccer players were younger at diagnosis, with a median age of ALS onset of 43.3 years, versus 62.5 years in the general population, they added.
These findings cannot be applied to those who play soccer below the professional level, since only professional athletes were studied, Dr. Beghi said. Moreover, the results should not be construed to suggest that people avoid playing soccer, he said, adding that the researchers had few specific details on the players’ ALS diagnoses.
Patients with ALS more often report head injuries, compared with the general population, while links between exercise and ALS have been found in some studies, but not others, according to the researchers.
“Clinical and experimental observations suggest an association between ALS and use of nonsteroidal anti-inflammatory agents and dietary supplements, including branched chain amino acids,” researchers added in the abstract for their report.
The study by Dr. Beghi and colleagues was supported by the Mario Negri Institute in Milan.
Source: Beghi E et al. AAN 2019, Abstract S1.001.
according to Italian researchers who reviewed trading cards of about 25,000 male professional soccer players who played in Italy. The researchers then scanned news reports to find which of those players developed the rare neurologic disease. Players who developed ALS were a much younger age at diagnosis when compared with the general population, according to the researchers, who will present their findings at the annual meeting of the American Academy of Neurology.
While the findings might implicate professional-level soccer in the development of ALS, there could be other factors at work, said lead author Ettore Beghi, MD, of the Mario Negri Institute for Pharmacological Research, Milan. “Repeated traumatic events, heavy physical exercise, and substance use could also be factors in the increased ALS risk among soccer players,” Dr. Beghi said in a news release. “In addition, genetics may play a role.”
The ALS-related deaths of several Italian pro soccer players sparked suggestions that the disease and the sport could be somehow linked, according to Dr. Beghi and colleagues. To determine whether professional soccer players are at increased ALS risk, they reviewed the archives of the country’s major soccer card publisher from the years 1959 to 2000, recording the name, date, and place of birth; field position; and team history for the tens of thousands of players they identified.
News reports revealed that 33 players in that cohort developed ALS, compared with 17.6 cases that would be expected based on Italian general population estimates, according to Dr. Beghi and colleagues.
The number of cases per 100,000 person-years was 1.9 for all the soccer players, and 4.7 for those who were younger than 45 years at diagnosis, researchers said. In general, soccer players were younger at diagnosis, with a median age of ALS onset of 43.3 years, versus 62.5 years in the general population, they added.
These findings cannot be applied to those who play soccer below the professional level, since only professional athletes were studied, Dr. Beghi said. Moreover, the results should not be construed to suggest that people avoid playing soccer, he said, adding that the researchers had few specific details on the players’ ALS diagnoses.
Patients with ALS more often report head injuries, compared with the general population, while links between exercise and ALS have been found in some studies, but not others, according to the researchers.
“Clinical and experimental observations suggest an association between ALS and use of nonsteroidal anti-inflammatory agents and dietary supplements, including branched chain amino acids,” researchers added in the abstract for their report.
The study by Dr. Beghi and colleagues was supported by the Mario Negri Institute in Milan.
Source: Beghi E et al. AAN 2019, Abstract S1.001.
according to Italian researchers who reviewed trading cards of about 25,000 male professional soccer players who played in Italy. The researchers then scanned news reports to find which of those players developed the rare neurologic disease. Players who developed ALS were a much younger age at diagnosis when compared with the general population, according to the researchers, who will present their findings at the annual meeting of the American Academy of Neurology.
While the findings might implicate professional-level soccer in the development of ALS, there could be other factors at work, said lead author Ettore Beghi, MD, of the Mario Negri Institute for Pharmacological Research, Milan. “Repeated traumatic events, heavy physical exercise, and substance use could also be factors in the increased ALS risk among soccer players,” Dr. Beghi said in a news release. “In addition, genetics may play a role.”
The ALS-related deaths of several Italian pro soccer players sparked suggestions that the disease and the sport could be somehow linked, according to Dr. Beghi and colleagues. To determine whether professional soccer players are at increased ALS risk, they reviewed the archives of the country’s major soccer card publisher from the years 1959 to 2000, recording the name, date, and place of birth; field position; and team history for the tens of thousands of players they identified.
News reports revealed that 33 players in that cohort developed ALS, compared with 17.6 cases that would be expected based on Italian general population estimates, according to Dr. Beghi and colleagues.
The number of cases per 100,000 person-years was 1.9 for all the soccer players, and 4.7 for those who were younger than 45 years at diagnosis, researchers said. In general, soccer players were younger at diagnosis, with a median age of ALS onset of 43.3 years, versus 62.5 years in the general population, they added.
These findings cannot be applied to those who play soccer below the professional level, since only professional athletes were studied, Dr. Beghi said. Moreover, the results should not be construed to suggest that people avoid playing soccer, he said, adding that the researchers had few specific details on the players’ ALS diagnoses.
Patients with ALS more often report head injuries, compared with the general population, while links between exercise and ALS have been found in some studies, but not others, according to the researchers.
“Clinical and experimental observations suggest an association between ALS and use of nonsteroidal anti-inflammatory agents and dietary supplements, including branched chain amino acids,” researchers added in the abstract for their report.
The study by Dr. Beghi and colleagues was supported by the Mario Negri Institute in Milan.
Source: Beghi E et al. AAN 2019, Abstract S1.001.
FROM AAN 2019
Does BMI affect outcomes after ischemic stroke?
according to research that will be presented at the annual meeting of the American Academy of Neurology.
“One possible explanation is that people who are overweight or obese may have a nutritional reserve that may help them survive during prolonged illness,” said Zuolu Liu, MD, of the University of California, Los Angeles, in a press release. “More research is needed to investigate the relationship between BMI and stroke.”
The obesity paradox was first noted when studies suggested that being overweight improved survival in patients with kidney disease or heart disease. Investigators previously examined whether the obesity paradox is observed in stroke, but their studies were underpowered and produced ambiguous results.
Dr. Liu and colleagues sought to evaluate the relationship between BMI and 90-day outcomes of acute ischemic stroke. They examined data for all participants in the FAST-MAG trial, which studied whether prehospital treatment with magnesium improved disability outcomes of acute ischemic stroke. Dr. Liu and colleagues focused on the outcomes of death, disability or death (that is, modified Rankin Scale score of 2-6), and low stroke-related quality of life (that is, Stroke Impact Scale score less than 70). They analyzed potential relationships with BMI univariately and in multivariate models that adjusted for 12 prognostic variables, such as high blood pressure, high cholesterol, and smoking.
Dr. Liu’s group included 1,033 participants in its study. The population’s mean age was 71 years, and 45.1% of the population was female. Mean National Institutes of Health Stroke Scale (NIHSS) score was 10.6, and mean BMI was 27.5 kg/m2.
The investigators found an inverse association between the risk of death and BMI. Adjusted odds ratios for mortality were 1.67 for underweight participants, 0.85 for overweight participants, 0.54 for obese participants, and 0.38 for severely obese participants, compared with participants of normal weight. Similarly, the risk of disability had a U-shaped relationship with BMI. Odds ratios for disability or death were 1.19 for underweight participants, 0.78 for overweight participants, 0.72 for obese participants, and 0.96 for severely obese participants, compared with participants of normal weight. This relationship was attenuated after adjustment for other prognostic factors, however. Dr. Liu’s group did not find a significant association between BMI and low stroke-related quality of life.
The study was limited by the fact that all participants were from Southern California, which potentially reduced the generalizability of the results. The racial and ethnic composition of the study population, however, is similar to that of the national population, said the researchers.
No study sponsor was reported.
SOURCE: Liu Z et al. AAN 2019, Abstract P3.3-01.
according to research that will be presented at the annual meeting of the American Academy of Neurology.
“One possible explanation is that people who are overweight or obese may have a nutritional reserve that may help them survive during prolonged illness,” said Zuolu Liu, MD, of the University of California, Los Angeles, in a press release. “More research is needed to investigate the relationship between BMI and stroke.”
The obesity paradox was first noted when studies suggested that being overweight improved survival in patients with kidney disease or heart disease. Investigators previously examined whether the obesity paradox is observed in stroke, but their studies were underpowered and produced ambiguous results.
Dr. Liu and colleagues sought to evaluate the relationship between BMI and 90-day outcomes of acute ischemic stroke. They examined data for all participants in the FAST-MAG trial, which studied whether prehospital treatment with magnesium improved disability outcomes of acute ischemic stroke. Dr. Liu and colleagues focused on the outcomes of death, disability or death (that is, modified Rankin Scale score of 2-6), and low stroke-related quality of life (that is, Stroke Impact Scale score less than 70). They analyzed potential relationships with BMI univariately and in multivariate models that adjusted for 12 prognostic variables, such as high blood pressure, high cholesterol, and smoking.
Dr. Liu’s group included 1,033 participants in its study. The population’s mean age was 71 years, and 45.1% of the population was female. Mean National Institutes of Health Stroke Scale (NIHSS) score was 10.6, and mean BMI was 27.5 kg/m2.
The investigators found an inverse association between the risk of death and BMI. Adjusted odds ratios for mortality were 1.67 for underweight participants, 0.85 for overweight participants, 0.54 for obese participants, and 0.38 for severely obese participants, compared with participants of normal weight. Similarly, the risk of disability had a U-shaped relationship with BMI. Odds ratios for disability or death were 1.19 for underweight participants, 0.78 for overweight participants, 0.72 for obese participants, and 0.96 for severely obese participants, compared with participants of normal weight. This relationship was attenuated after adjustment for other prognostic factors, however. Dr. Liu’s group did not find a significant association between BMI and low stroke-related quality of life.
The study was limited by the fact that all participants were from Southern California, which potentially reduced the generalizability of the results. The racial and ethnic composition of the study population, however, is similar to that of the national population, said the researchers.
No study sponsor was reported.
SOURCE: Liu Z et al. AAN 2019, Abstract P3.3-01.
according to research that will be presented at the annual meeting of the American Academy of Neurology.
“One possible explanation is that people who are overweight or obese may have a nutritional reserve that may help them survive during prolonged illness,” said Zuolu Liu, MD, of the University of California, Los Angeles, in a press release. “More research is needed to investigate the relationship between BMI and stroke.”
The obesity paradox was first noted when studies suggested that being overweight improved survival in patients with kidney disease or heart disease. Investigators previously examined whether the obesity paradox is observed in stroke, but their studies were underpowered and produced ambiguous results.
Dr. Liu and colleagues sought to evaluate the relationship between BMI and 90-day outcomes of acute ischemic stroke. They examined data for all participants in the FAST-MAG trial, which studied whether prehospital treatment with magnesium improved disability outcomes of acute ischemic stroke. Dr. Liu and colleagues focused on the outcomes of death, disability or death (that is, modified Rankin Scale score of 2-6), and low stroke-related quality of life (that is, Stroke Impact Scale score less than 70). They analyzed potential relationships with BMI univariately and in multivariate models that adjusted for 12 prognostic variables, such as high blood pressure, high cholesterol, and smoking.
Dr. Liu’s group included 1,033 participants in its study. The population’s mean age was 71 years, and 45.1% of the population was female. Mean National Institutes of Health Stroke Scale (NIHSS) score was 10.6, and mean BMI was 27.5 kg/m2.
The investigators found an inverse association between the risk of death and BMI. Adjusted odds ratios for mortality were 1.67 for underweight participants, 0.85 for overweight participants, 0.54 for obese participants, and 0.38 for severely obese participants, compared with participants of normal weight. Similarly, the risk of disability had a U-shaped relationship with BMI. Odds ratios for disability or death were 1.19 for underweight participants, 0.78 for overweight participants, 0.72 for obese participants, and 0.96 for severely obese participants, compared with participants of normal weight. This relationship was attenuated after adjustment for other prognostic factors, however. Dr. Liu’s group did not find a significant association between BMI and low stroke-related quality of life.
The study was limited by the fact that all participants were from Southern California, which potentially reduced the generalizability of the results. The racial and ethnic composition of the study population, however, is similar to that of the national population, said the researchers.
No study sponsor was reported.
SOURCE: Liu Z et al. AAN 2019, Abstract P3.3-01.
FROM AAN 2019