User login
Few stroke patients undergo osteoporosis screening, treatment
Although stroke is a risk factor for osteoporosis, falls, and fractures, very few people who have experienced a recent stroke are either screened for osteoporosis or treated, research suggests.
Writing in Stroke, researchers presented an analysis of Ontario registry data from 16,581 patients who were aged 65 years or older and presented with stroke between 2003 and 2013.
Overall, just 5.1% of patients underwent bone mineral density testing. Of the 1,577 patients who had experienced a prior fracture, 71 (4.7%) had bone mineral density testing, and only 2.9% of those who had not had prior bone mineral density testing were tested after their stroke. Bone mineral density testing was more likely in patients who were younger, who were female, and who experienced a low-trauma fracture in the year after their stroke.
In total, 15.5% of patients were prescribed osteoporosis drugs in the first year after their stroke. However, only 7.8% of those who had fractures before the stroke and 14.8% of those with fractures after the stroke received osteoporosis treatment after the stroke. Patients who were female, had prior osteoporosis, had experienced prior fracture, had previously undergone bone mineral density testing, or had experienced a fracture or fall after their stroke were more likely to receive osteoporosis pharmacotherapy.
The authors found that the neither the severity of stroke nor the presence of other comorbidities was associated with an increased likelihood of screening or treatment of osteoporosis after the stroke.
Stroke is associated with up to a fourfold increased risk of osteoporosis and fracture, compared with healthy controls, most probably because of reduced mobility and an increased risk of falls, wrote Eshita Kapoor of the department of medicine at the University of Toronto and her coauthors.
“Screening and treatment may be particularly low poststroke because of under-recognition of osteoporosis as a consequence of stroke, a selective focus on the management of cardiovascular risk and stroke recovery, or factors such as dysphagia precluding use of oral bisphosphonates,” the authors wrote.
While the association is noted in U.S. stroke guidelines, there are few recommendations for treatment aside from fall prevention strategies, which the authors noted was a missed opportunity for prevention.
“Use of a risk prediction score to identify those at particularly high short-term risk of fractures after stroke may help to prioritize patients for osteoporosis testing and treatment,” they suggested.
The study was funded by the Heart and Stroke Foundation of Canada and was supported by ICES (Institute for Clinical Evaluative Sciences) and the Ontario Ministry of Health and Long-Term Care. One author declared consultancies for the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Kapoor E et al. Stroke. 2019 April 25. doi: 10.1161/STROKEAHA.118.024685
Although stroke is a risk factor for osteoporosis, falls, and fractures, very few people who have experienced a recent stroke are either screened for osteoporosis or treated, research suggests.
Writing in Stroke, researchers presented an analysis of Ontario registry data from 16,581 patients who were aged 65 years or older and presented with stroke between 2003 and 2013.
Overall, just 5.1% of patients underwent bone mineral density testing. Of the 1,577 patients who had experienced a prior fracture, 71 (4.7%) had bone mineral density testing, and only 2.9% of those who had not had prior bone mineral density testing were tested after their stroke. Bone mineral density testing was more likely in patients who were younger, who were female, and who experienced a low-trauma fracture in the year after their stroke.
In total, 15.5% of patients were prescribed osteoporosis drugs in the first year after their stroke. However, only 7.8% of those who had fractures before the stroke and 14.8% of those with fractures after the stroke received osteoporosis treatment after the stroke. Patients who were female, had prior osteoporosis, had experienced prior fracture, had previously undergone bone mineral density testing, or had experienced a fracture or fall after their stroke were more likely to receive osteoporosis pharmacotherapy.
The authors found that the neither the severity of stroke nor the presence of other comorbidities was associated with an increased likelihood of screening or treatment of osteoporosis after the stroke.
Stroke is associated with up to a fourfold increased risk of osteoporosis and fracture, compared with healthy controls, most probably because of reduced mobility and an increased risk of falls, wrote Eshita Kapoor of the department of medicine at the University of Toronto and her coauthors.
“Screening and treatment may be particularly low poststroke because of under-recognition of osteoporosis as a consequence of stroke, a selective focus on the management of cardiovascular risk and stroke recovery, or factors such as dysphagia precluding use of oral bisphosphonates,” the authors wrote.
While the association is noted in U.S. stroke guidelines, there are few recommendations for treatment aside from fall prevention strategies, which the authors noted was a missed opportunity for prevention.
“Use of a risk prediction score to identify those at particularly high short-term risk of fractures after stroke may help to prioritize patients for osteoporosis testing and treatment,” they suggested.
The study was funded by the Heart and Stroke Foundation of Canada and was supported by ICES (Institute for Clinical Evaluative Sciences) and the Ontario Ministry of Health and Long-Term Care. One author declared consultancies for the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Kapoor E et al. Stroke. 2019 April 25. doi: 10.1161/STROKEAHA.118.024685
Although stroke is a risk factor for osteoporosis, falls, and fractures, very few people who have experienced a recent stroke are either screened for osteoporosis or treated, research suggests.
Writing in Stroke, researchers presented an analysis of Ontario registry data from 16,581 patients who were aged 65 years or older and presented with stroke between 2003 and 2013.
Overall, just 5.1% of patients underwent bone mineral density testing. Of the 1,577 patients who had experienced a prior fracture, 71 (4.7%) had bone mineral density testing, and only 2.9% of those who had not had prior bone mineral density testing were tested after their stroke. Bone mineral density testing was more likely in patients who were younger, who were female, and who experienced a low-trauma fracture in the year after their stroke.
In total, 15.5% of patients were prescribed osteoporosis drugs in the first year after their stroke. However, only 7.8% of those who had fractures before the stroke and 14.8% of those with fractures after the stroke received osteoporosis treatment after the stroke. Patients who were female, had prior osteoporosis, had experienced prior fracture, had previously undergone bone mineral density testing, or had experienced a fracture or fall after their stroke were more likely to receive osteoporosis pharmacotherapy.
The authors found that the neither the severity of stroke nor the presence of other comorbidities was associated with an increased likelihood of screening or treatment of osteoporosis after the stroke.
Stroke is associated with up to a fourfold increased risk of osteoporosis and fracture, compared with healthy controls, most probably because of reduced mobility and an increased risk of falls, wrote Eshita Kapoor of the department of medicine at the University of Toronto and her coauthors.
“Screening and treatment may be particularly low poststroke because of under-recognition of osteoporosis as a consequence of stroke, a selective focus on the management of cardiovascular risk and stroke recovery, or factors such as dysphagia precluding use of oral bisphosphonates,” the authors wrote.
While the association is noted in U.S. stroke guidelines, there are few recommendations for treatment aside from fall prevention strategies, which the authors noted was a missed opportunity for prevention.
“Use of a risk prediction score to identify those at particularly high short-term risk of fractures after stroke may help to prioritize patients for osteoporosis testing and treatment,” they suggested.
The study was funded by the Heart and Stroke Foundation of Canada and was supported by ICES (Institute for Clinical Evaluative Sciences) and the Ontario Ministry of Health and Long-Term Care. One author declared consultancies for the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Kapoor E et al. Stroke. 2019 April 25. doi: 10.1161/STROKEAHA.118.024685
FROM STROKE
CDC warns against misuse of opioid-prescribing guideline
Officials at the Centers for Disease Control and Prevention are warning against the misapplication of the agency’s 2016 guidelines on opioid prescribing, as well as clarifying dosage recommendations for patients starting or stopping pain medications.
In a perspective published in the New England Journal of Medicine on April 24, lead author Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control, conveyed concern that some policies and practices derived from the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain are inconsistent with the recommendations and often go beyond their scope.
Misapplication examples include inappropriately applying the guideline to patients in active cancer treatment, patients experiencing acute sickle cell crises, or patients experiencing postsurgical pain, Dr. Dowell wrote.
The guideline offers guidance to clinicians treating chronic pain in adults who are already receiving opioids long-term at high dosages, she noted. It includes advice on maximizing nonopioid treatment, reviewing risks associated with continuing high-dose opioids, and collaborating with patients who agree to taper dosage, among other guidance.
Any application of the guideline’s dosage recommendation that results in hard limits or “cutting off” opioids is also an incorrect use of the recommendations, according to Dr. Dowell.
While the guideline advises clinicians to start opioids at the lowest effective dosage and avoid increasing dosage to 90 morphine milligram equivalents per day or more, that statement does not suggest discontinuation of opioids already prescribed at high dosages, according to the CDC’s clarification.
The guidance also does not apply to patients receiving or starting medication-assisted treatment for opioid use disorder.
The commentary comes after a trio of organizations raised concerns that insurers are inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology, raised the issue in a letter to the CDC in February. In response, Dr. Dowell clarified that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
In the perspective, Dr. Dowell wrote that the CDC is evaluating the intended and unintended impact of the 2016 opioid-prescribing guideline on clinician and patient outcomes and that the agency is committed to updating the recommendations when new evidence is available.
Officials at the Centers for Disease Control and Prevention are warning against the misapplication of the agency’s 2016 guidelines on opioid prescribing, as well as clarifying dosage recommendations for patients starting or stopping pain medications.
In a perspective published in the New England Journal of Medicine on April 24, lead author Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control, conveyed concern that some policies and practices derived from the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain are inconsistent with the recommendations and often go beyond their scope.
Misapplication examples include inappropriately applying the guideline to patients in active cancer treatment, patients experiencing acute sickle cell crises, or patients experiencing postsurgical pain, Dr. Dowell wrote.
The guideline offers guidance to clinicians treating chronic pain in adults who are already receiving opioids long-term at high dosages, she noted. It includes advice on maximizing nonopioid treatment, reviewing risks associated with continuing high-dose opioids, and collaborating with patients who agree to taper dosage, among other guidance.
Any application of the guideline’s dosage recommendation that results in hard limits or “cutting off” opioids is also an incorrect use of the recommendations, according to Dr. Dowell.
While the guideline advises clinicians to start opioids at the lowest effective dosage and avoid increasing dosage to 90 morphine milligram equivalents per day or more, that statement does not suggest discontinuation of opioids already prescribed at high dosages, according to the CDC’s clarification.
The guidance also does not apply to patients receiving or starting medication-assisted treatment for opioid use disorder.
The commentary comes after a trio of organizations raised concerns that insurers are inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology, raised the issue in a letter to the CDC in February. In response, Dr. Dowell clarified that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
In the perspective, Dr. Dowell wrote that the CDC is evaluating the intended and unintended impact of the 2016 opioid-prescribing guideline on clinician and patient outcomes and that the agency is committed to updating the recommendations when new evidence is available.
Officials at the Centers for Disease Control and Prevention are warning against the misapplication of the agency’s 2016 guidelines on opioid prescribing, as well as clarifying dosage recommendations for patients starting or stopping pain medications.
In a perspective published in the New England Journal of Medicine on April 24, lead author Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control, conveyed concern that some policies and practices derived from the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain are inconsistent with the recommendations and often go beyond their scope.
Misapplication examples include inappropriately applying the guideline to patients in active cancer treatment, patients experiencing acute sickle cell crises, or patients experiencing postsurgical pain, Dr. Dowell wrote.
The guideline offers guidance to clinicians treating chronic pain in adults who are already receiving opioids long-term at high dosages, she noted. It includes advice on maximizing nonopioid treatment, reviewing risks associated with continuing high-dose opioids, and collaborating with patients who agree to taper dosage, among other guidance.
Any application of the guideline’s dosage recommendation that results in hard limits or “cutting off” opioids is also an incorrect use of the recommendations, according to Dr. Dowell.
While the guideline advises clinicians to start opioids at the lowest effective dosage and avoid increasing dosage to 90 morphine milligram equivalents per day or more, that statement does not suggest discontinuation of opioids already prescribed at high dosages, according to the CDC’s clarification.
The guidance also does not apply to patients receiving or starting medication-assisted treatment for opioid use disorder.
The commentary comes after a trio of organizations raised concerns that insurers are inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology, raised the issue in a letter to the CDC in February. In response, Dr. Dowell clarified that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
In the perspective, Dr. Dowell wrote that the CDC is evaluating the intended and unintended impact of the 2016 opioid-prescribing guideline on clinician and patient outcomes and that the agency is committed to updating the recommendations when new evidence is available.
In pain treatment, racial bias common among physician trainees
MILWAUKEE – More than 40% of white physician trainees demonstrated racial bias in medical decision making about treatment of low back pain, as did 31% of nonwhite trainees. However, just 6% of white residents and fellows, and 10% of the nonwhite residents and fellows, reported that patient race had factored into their treatment decisions in a virtual patient task.
The 444 medical residents and fellows who participated viewed video vignettes presenting 12 virtual patients who presented with low back pain, wrote Alexis Grant of Indiana University–Purdue University Indianapolis and her colleagues. In a poster presentation at the scientific meeting of the American Pain Society, Ms. Grant, a doctoral student in clinical psychology, and her collaborators explained that participants agreed to view a series of 12 videos of virtual patients.
The videos presented male and female virtual patients who were black or white and who had jobs associated with low or high socioeconomic status (SES). Information in text vignettes accompanying the videos included occupation, pain etiology, physical exam findings, and pain intensity by self-report.
After viewing the videos and reading the vignettes, participating clinicians were asked to use a 0-100 visual analog scale to report their likelihood of referring patients to a pain specialist or to physical therapy and of recommending opioid or nonopioid analgesia.
“Next, they rated the degree to which they considered different sources of patient information when making treatment decision,” Ms. Grant and her coauthors wrote. Statistical analysis “examined the extent to which providers demonstrated statistically reliable treatment differences across patient race and SES.” These findings were compared with how clinicians reported they used patient race and SES in decision making.
Demonstrated race-based decision making occurred for 41% of white and 31% of nonwhite clinicians. About two-thirds of providers (67.3%) were white, and of the remainder, 26.3% were Asian, 4.4% were classified as “other,” and 2.1% were black. The respondents were aged a mean 29.7 years, and were 42.3% female.
In addition, Ms. Grant and her coauthors estimated provider SES by asking about parental SES, dividing respondents into low (less than $38,000), medium ($38,000-$75,000), and high (greater than $75,000) SES categories.
and similar across levels of provider SES, at 41%, 43%, and 38% for low, medium, and high SES residents and fellows, respectively. However, the disconnect between reported and demonstrated bias that was seen with race was not seen with SES bias, with 43%-48% of providers in each SES group reporting that they had factored patient SES into their treatment decision making.
“These results suggest that providers have low awareness of making different pain treatment decisions” for black patients, compared with decision making for white patients, Ms. Grant and her colleagues wrote. “Decision-making awareness did not substantially differ across provider race or SES.” She and her collaborators called for more research into whether raising awareness about demonstrated racial bias in decision making can improve both racial and socioeconomic gaps in pain care.
The authors reported funding from the National Institutes of Health. They reported no conflicts of interest.
MILWAUKEE – More than 40% of white physician trainees demonstrated racial bias in medical decision making about treatment of low back pain, as did 31% of nonwhite trainees. However, just 6% of white residents and fellows, and 10% of the nonwhite residents and fellows, reported that patient race had factored into their treatment decisions in a virtual patient task.
The 444 medical residents and fellows who participated viewed video vignettes presenting 12 virtual patients who presented with low back pain, wrote Alexis Grant of Indiana University–Purdue University Indianapolis and her colleagues. In a poster presentation at the scientific meeting of the American Pain Society, Ms. Grant, a doctoral student in clinical psychology, and her collaborators explained that participants agreed to view a series of 12 videos of virtual patients.
The videos presented male and female virtual patients who were black or white and who had jobs associated with low or high socioeconomic status (SES). Information in text vignettes accompanying the videos included occupation, pain etiology, physical exam findings, and pain intensity by self-report.
After viewing the videos and reading the vignettes, participating clinicians were asked to use a 0-100 visual analog scale to report their likelihood of referring patients to a pain specialist or to physical therapy and of recommending opioid or nonopioid analgesia.
“Next, they rated the degree to which they considered different sources of patient information when making treatment decision,” Ms. Grant and her coauthors wrote. Statistical analysis “examined the extent to which providers demonstrated statistically reliable treatment differences across patient race and SES.” These findings were compared with how clinicians reported they used patient race and SES in decision making.
Demonstrated race-based decision making occurred for 41% of white and 31% of nonwhite clinicians. About two-thirds of providers (67.3%) were white, and of the remainder, 26.3% were Asian, 4.4% were classified as “other,” and 2.1% were black. The respondents were aged a mean 29.7 years, and were 42.3% female.
In addition, Ms. Grant and her coauthors estimated provider SES by asking about parental SES, dividing respondents into low (less than $38,000), medium ($38,000-$75,000), and high (greater than $75,000) SES categories.
and similar across levels of provider SES, at 41%, 43%, and 38% for low, medium, and high SES residents and fellows, respectively. However, the disconnect between reported and demonstrated bias that was seen with race was not seen with SES bias, with 43%-48% of providers in each SES group reporting that they had factored patient SES into their treatment decision making.
“These results suggest that providers have low awareness of making different pain treatment decisions” for black patients, compared with decision making for white patients, Ms. Grant and her colleagues wrote. “Decision-making awareness did not substantially differ across provider race or SES.” She and her collaborators called for more research into whether raising awareness about demonstrated racial bias in decision making can improve both racial and socioeconomic gaps in pain care.
The authors reported funding from the National Institutes of Health. They reported no conflicts of interest.
MILWAUKEE – More than 40% of white physician trainees demonstrated racial bias in medical decision making about treatment of low back pain, as did 31% of nonwhite trainees. However, just 6% of white residents and fellows, and 10% of the nonwhite residents and fellows, reported that patient race had factored into their treatment decisions in a virtual patient task.
The 444 medical residents and fellows who participated viewed video vignettes presenting 12 virtual patients who presented with low back pain, wrote Alexis Grant of Indiana University–Purdue University Indianapolis and her colleagues. In a poster presentation at the scientific meeting of the American Pain Society, Ms. Grant, a doctoral student in clinical psychology, and her collaborators explained that participants agreed to view a series of 12 videos of virtual patients.
The videos presented male and female virtual patients who were black or white and who had jobs associated with low or high socioeconomic status (SES). Information in text vignettes accompanying the videos included occupation, pain etiology, physical exam findings, and pain intensity by self-report.
After viewing the videos and reading the vignettes, participating clinicians were asked to use a 0-100 visual analog scale to report their likelihood of referring patients to a pain specialist or to physical therapy and of recommending opioid or nonopioid analgesia.
“Next, they rated the degree to which they considered different sources of patient information when making treatment decision,” Ms. Grant and her coauthors wrote. Statistical analysis “examined the extent to which providers demonstrated statistically reliable treatment differences across patient race and SES.” These findings were compared with how clinicians reported they used patient race and SES in decision making.
Demonstrated race-based decision making occurred for 41% of white and 31% of nonwhite clinicians. About two-thirds of providers (67.3%) were white, and of the remainder, 26.3% were Asian, 4.4% were classified as “other,” and 2.1% were black. The respondents were aged a mean 29.7 years, and were 42.3% female.
In addition, Ms. Grant and her coauthors estimated provider SES by asking about parental SES, dividing respondents into low (less than $38,000), medium ($38,000-$75,000), and high (greater than $75,000) SES categories.
and similar across levels of provider SES, at 41%, 43%, and 38% for low, medium, and high SES residents and fellows, respectively. However, the disconnect between reported and demonstrated bias that was seen with race was not seen with SES bias, with 43%-48% of providers in each SES group reporting that they had factored patient SES into their treatment decision making.
“These results suggest that providers have low awareness of making different pain treatment decisions” for black patients, compared with decision making for white patients, Ms. Grant and her colleagues wrote. “Decision-making awareness did not substantially differ across provider race or SES.” She and her collaborators called for more research into whether raising awareness about demonstrated racial bias in decision making can improve both racial and socioeconomic gaps in pain care.
The authors reported funding from the National Institutes of Health. They reported no conflicts of interest.
REPORTING FROM APS 2019
MS: Partnering With Patients to Improve Health
Sharon, a 19-year-old woman, has a history of right optic neuritis and paraparesis that occurred 2 years ago. At that time, the diagnosis of multiple sclerosis (MS) was confirmed by a brain MRI and lumbar puncture. She has been taking disease-modifying therapy for 2 years and rarely misses a dose. Lately, however, she has experienced worsening symptoms and feels that her MS is progressing. Her neurologist doesn’t agree; he informs her that a recent MRI shows no changes, and her neurologic examination is within normal limits. At his suggestion, she presents to her primary care provider for an annual check-up.
HISTORY & PHYSICAL EXAM
Sharon’s height is 5 ft 2 in and her weight, 170 lb. Her blood pressure is 140/88 mm Hg and pulse, 80 beats/min and regular. Review of systems is remarkable for fatigue, visual changes when she is overheated, and weight gain of about 50 lb during the past year. Her lungs are clear to percussion and auscultation.
Her current medications include oral disease-modifying therapy, which she takes daily; an oral contraceptive (for regulation of her menstrual cycle; she says she is not sexually active); and an occasional pain reliever for headache.
CLINICAL IMPRESSION
Following history-taking and examination, the clinician notes the following impressions about Sharon’s health status:
Obesity: Examination reveals an overweight female with a BMI of 31.1.
Physical inactivity: As a legal secretary, Sharon sits at her desk most of the day. Her exercise is limited to walking to and from the bus to get to work. She has limited time for social activities due to fatigue. She spends most of her time watching television or visiting her parents.
Heat intolerance: While describing her lifestyle, Sharon notes that she does not participate in outdoor activity due to heat intolerance.
Continue to: Ambulation difficulty
Ambulation difficulty: Sharon’s walking and balance are worse than they were 6 months ago—a problem she relates to her MS, not her increased weight. She walks with a wide-based ataxic gait and transfers with difficulty, using the arms of her chair to stand up.
Poor nutritional habits: Sharon reports an irregular diet with an occasional breakfast, a sandwich for lunch, and a microwavable meal for dinner. Between meals, she snacks on nutrition bars, chocolate, and hot and cold coffee.
Smoking: Sharon smokes 1 pack of cigarettes daily.
Headache: As noted, Sharon reports occasional analgesic use for relief of headache pain.
The clinician’s impression is as follows: relapsing MS treated with disease-modifying therapy; obesity; ambulation difficulty; heat intolerance; sedentary lifestyle; and headache. In addition, the patient has the following risk factors: smoking; suboptimal activity and exercise; and poor nutritional habits.
Continue to: DISCUSSION
DISCUSSION
Sharon has relapsing MS treated with disease-modifying therapy. But she also demonstrates or reports several independent risk factors, including borderline hypertension; obesity; inadequate diet; lack of activity and exercise; and possible lack of insight into her disease.1
The plan of care for Sharon should include a review of her MS disease course. As this is explained, it is important to emphasize how adherence to the care plan will yield positive outcomes from the treatment. For example, the patient should understand that the underlying cause of damage in MS is related to the immune system. Providing this education might involve 1 or 2 sessions with written material, simple graphics, and explanation on how disease-modifying therapies work. Even a simple statement such as
The next step is to review Sharon’s risk factors for worsening MS, along with the impact these have on her general health. This might entail a long discussion focusing on the patient’s diet, minimal activity and exercise, and smoking. Sharon’s provider explained how all 3 factors can contribute to poor general health and have been shown to negatively affect MS. There is a general impression that wellness and neurologic diseases such as MS are disconnected. The clinician must “reconnect” the 2 through encouragement, education, and coaching.
By working closely with the patient and providing the education to help her make informed decisions about her health, the clinician can develop a plan to implement that has the patient’s full support. For a patient like Sharon, this includes
- Dietary modifications to improve nutrition and promote healthy weight loss
- A program of daily walking to improve stamina and support the patient’s weight loss program2
- Smoking cessation, including participation in a local support group of former smokers.3
Continue to: In Sharon's case...
In Sharon’s case, both she and her clinician agreed that it was important to meet regularly to assess progress toward their mutually agreed-upon goals. It is not enough to devise a plan—providers need to support patients in their efforts to improve their health. Meeting regularly can motivate patients to stay on track, and it gives providers an opportunity to address problems or concerns that might interfere with the patient’s progress.
1. Dalgas U, Stenager E. Exercise and disease progression in multiple sclerosis: can exercise slow down the progression of multiple sclerosis? Ther Adv Neurol Disord. 2012;5(2):81-95.
2. Gianfrancesco MA, Barcellos LF. Obesity and multiple sclerosis susceptibility: a review. J Neurol Neuromedicine. 2016:1(7):1-5.
3. Healy BC, Eman A, Guttmann CRG, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66(7):858-864.
Sharon, a 19-year-old woman, has a history of right optic neuritis and paraparesis that occurred 2 years ago. At that time, the diagnosis of multiple sclerosis (MS) was confirmed by a brain MRI and lumbar puncture. She has been taking disease-modifying therapy for 2 years and rarely misses a dose. Lately, however, she has experienced worsening symptoms and feels that her MS is progressing. Her neurologist doesn’t agree; he informs her that a recent MRI shows no changes, and her neurologic examination is within normal limits. At his suggestion, she presents to her primary care provider for an annual check-up.
HISTORY & PHYSICAL EXAM
Sharon’s height is 5 ft 2 in and her weight, 170 lb. Her blood pressure is 140/88 mm Hg and pulse, 80 beats/min and regular. Review of systems is remarkable for fatigue, visual changes when she is overheated, and weight gain of about 50 lb during the past year. Her lungs are clear to percussion and auscultation.
Her current medications include oral disease-modifying therapy, which she takes daily; an oral contraceptive (for regulation of her menstrual cycle; she says she is not sexually active); and an occasional pain reliever for headache.
CLINICAL IMPRESSION
Following history-taking and examination, the clinician notes the following impressions about Sharon’s health status:
Obesity: Examination reveals an overweight female with a BMI of 31.1.
Physical inactivity: As a legal secretary, Sharon sits at her desk most of the day. Her exercise is limited to walking to and from the bus to get to work. She has limited time for social activities due to fatigue. She spends most of her time watching television or visiting her parents.
Heat intolerance: While describing her lifestyle, Sharon notes that she does not participate in outdoor activity due to heat intolerance.
Continue to: Ambulation difficulty
Ambulation difficulty: Sharon’s walking and balance are worse than they were 6 months ago—a problem she relates to her MS, not her increased weight. She walks with a wide-based ataxic gait and transfers with difficulty, using the arms of her chair to stand up.
Poor nutritional habits: Sharon reports an irregular diet with an occasional breakfast, a sandwich for lunch, and a microwavable meal for dinner. Between meals, she snacks on nutrition bars, chocolate, and hot and cold coffee.
Smoking: Sharon smokes 1 pack of cigarettes daily.
Headache: As noted, Sharon reports occasional analgesic use for relief of headache pain.
The clinician’s impression is as follows: relapsing MS treated with disease-modifying therapy; obesity; ambulation difficulty; heat intolerance; sedentary lifestyle; and headache. In addition, the patient has the following risk factors: smoking; suboptimal activity and exercise; and poor nutritional habits.
Continue to: DISCUSSION
DISCUSSION
Sharon has relapsing MS treated with disease-modifying therapy. But she also demonstrates or reports several independent risk factors, including borderline hypertension; obesity; inadequate diet; lack of activity and exercise; and possible lack of insight into her disease.1
The plan of care for Sharon should include a review of her MS disease course. As this is explained, it is important to emphasize how adherence to the care plan will yield positive outcomes from the treatment. For example, the patient should understand that the underlying cause of damage in MS is related to the immune system. Providing this education might involve 1 or 2 sessions with written material, simple graphics, and explanation on how disease-modifying therapies work. Even a simple statement such as
The next step is to review Sharon’s risk factors for worsening MS, along with the impact these have on her general health. This might entail a long discussion focusing on the patient’s diet, minimal activity and exercise, and smoking. Sharon’s provider explained how all 3 factors can contribute to poor general health and have been shown to negatively affect MS. There is a general impression that wellness and neurologic diseases such as MS are disconnected. The clinician must “reconnect” the 2 through encouragement, education, and coaching.
By working closely with the patient and providing the education to help her make informed decisions about her health, the clinician can develop a plan to implement that has the patient’s full support. For a patient like Sharon, this includes
- Dietary modifications to improve nutrition and promote healthy weight loss
- A program of daily walking to improve stamina and support the patient’s weight loss program2
- Smoking cessation, including participation in a local support group of former smokers.3
Continue to: In Sharon's case...
In Sharon’s case, both she and her clinician agreed that it was important to meet regularly to assess progress toward their mutually agreed-upon goals. It is not enough to devise a plan—providers need to support patients in their efforts to improve their health. Meeting regularly can motivate patients to stay on track, and it gives providers an opportunity to address problems or concerns that might interfere with the patient’s progress.
Sharon, a 19-year-old woman, has a history of right optic neuritis and paraparesis that occurred 2 years ago. At that time, the diagnosis of multiple sclerosis (MS) was confirmed by a brain MRI and lumbar puncture. She has been taking disease-modifying therapy for 2 years and rarely misses a dose. Lately, however, she has experienced worsening symptoms and feels that her MS is progressing. Her neurologist doesn’t agree; he informs her that a recent MRI shows no changes, and her neurologic examination is within normal limits. At his suggestion, she presents to her primary care provider for an annual check-up.
HISTORY & PHYSICAL EXAM
Sharon’s height is 5 ft 2 in and her weight, 170 lb. Her blood pressure is 140/88 mm Hg and pulse, 80 beats/min and regular. Review of systems is remarkable for fatigue, visual changes when she is overheated, and weight gain of about 50 lb during the past year. Her lungs are clear to percussion and auscultation.
Her current medications include oral disease-modifying therapy, which she takes daily; an oral contraceptive (for regulation of her menstrual cycle; she says she is not sexually active); and an occasional pain reliever for headache.
CLINICAL IMPRESSION
Following history-taking and examination, the clinician notes the following impressions about Sharon’s health status:
Obesity: Examination reveals an overweight female with a BMI of 31.1.
Physical inactivity: As a legal secretary, Sharon sits at her desk most of the day. Her exercise is limited to walking to and from the bus to get to work. She has limited time for social activities due to fatigue. She spends most of her time watching television or visiting her parents.
Heat intolerance: While describing her lifestyle, Sharon notes that she does not participate in outdoor activity due to heat intolerance.
Continue to: Ambulation difficulty
Ambulation difficulty: Sharon’s walking and balance are worse than they were 6 months ago—a problem she relates to her MS, not her increased weight. She walks with a wide-based ataxic gait and transfers with difficulty, using the arms of her chair to stand up.
Poor nutritional habits: Sharon reports an irregular diet with an occasional breakfast, a sandwich for lunch, and a microwavable meal for dinner. Between meals, she snacks on nutrition bars, chocolate, and hot and cold coffee.
Smoking: Sharon smokes 1 pack of cigarettes daily.
Headache: As noted, Sharon reports occasional analgesic use for relief of headache pain.
The clinician’s impression is as follows: relapsing MS treated with disease-modifying therapy; obesity; ambulation difficulty; heat intolerance; sedentary lifestyle; and headache. In addition, the patient has the following risk factors: smoking; suboptimal activity and exercise; and poor nutritional habits.
Continue to: DISCUSSION
DISCUSSION
Sharon has relapsing MS treated with disease-modifying therapy. But she also demonstrates or reports several independent risk factors, including borderline hypertension; obesity; inadequate diet; lack of activity and exercise; and possible lack of insight into her disease.1
The plan of care for Sharon should include a review of her MS disease course. As this is explained, it is important to emphasize how adherence to the care plan will yield positive outcomes from the treatment. For example, the patient should understand that the underlying cause of damage in MS is related to the immune system. Providing this education might involve 1 or 2 sessions with written material, simple graphics, and explanation on how disease-modifying therapies work. Even a simple statement such as
The next step is to review Sharon’s risk factors for worsening MS, along with the impact these have on her general health. This might entail a long discussion focusing on the patient’s diet, minimal activity and exercise, and smoking. Sharon’s provider explained how all 3 factors can contribute to poor general health and have been shown to negatively affect MS. There is a general impression that wellness and neurologic diseases such as MS are disconnected. The clinician must “reconnect” the 2 through encouragement, education, and coaching.
By working closely with the patient and providing the education to help her make informed decisions about her health, the clinician can develop a plan to implement that has the patient’s full support. For a patient like Sharon, this includes
- Dietary modifications to improve nutrition and promote healthy weight loss
- A program of daily walking to improve stamina and support the patient’s weight loss program2
- Smoking cessation, including participation in a local support group of former smokers.3
Continue to: In Sharon's case...
In Sharon’s case, both she and her clinician agreed that it was important to meet regularly to assess progress toward their mutually agreed-upon goals. It is not enough to devise a plan—providers need to support patients in their efforts to improve their health. Meeting regularly can motivate patients to stay on track, and it gives providers an opportunity to address problems or concerns that might interfere with the patient’s progress.
1. Dalgas U, Stenager E. Exercise and disease progression in multiple sclerosis: can exercise slow down the progression of multiple sclerosis? Ther Adv Neurol Disord. 2012;5(2):81-95.
2. Gianfrancesco MA, Barcellos LF. Obesity and multiple sclerosis susceptibility: a review. J Neurol Neuromedicine. 2016:1(7):1-5.
3. Healy BC, Eman A, Guttmann CRG, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66(7):858-864.
1. Dalgas U, Stenager E. Exercise and disease progression in multiple sclerosis: can exercise slow down the progression of multiple sclerosis? Ther Adv Neurol Disord. 2012;5(2):81-95.
2. Gianfrancesco MA, Barcellos LF. Obesity and multiple sclerosis susceptibility: a review. J Neurol Neuromedicine. 2016:1(7):1-5.
3. Healy BC, Eman A, Guttmann CRG, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66(7):858-864.
A Robotic Hand Device Safety Study for People With Cervical Spinal Cord Injury (FULL)
An estimated 282,000 people in the US are living with spinal cord injury (SCI).1 Damage to the cervical spinal cord is the most prevalent. Among cervical spinal cord trauma, injury to levels C4, C5, and C6 have the highest occurrence.1 Damage to these levels has significant implications for functional status. Depending on pathology, patients’ functional status can range from requiring assistance for all activities of daily living (ADL) to potentially living independently.
Improving upper-limb function is vital to achieving independence. About half of people with tetraplegia judge hand and arm function to be the top factor that would improve quality of life (QOL).2 Persons with traumatic cervical SCI may lose the ability to use their hands from motor deficits, sensory dysfunction, proprioception problem, and/or loss of coordination. In addition, they may develop joint contracture, spasticity, pain, and other complications. Thus, their independence and ADL are affected significantly by multiple mechanisms of pathology.
Upper-extremity rehabilitation that emphasizes strengthening and maintaining functional range of motion (ROM) is fundamental to SCI rehabilitation. Rehabilitation to restore partial hand function has included ROM exercises, splinting, surgical procedures in the form of tendon transfers and various electrical stimulation devices, such as implantable neuroprostheses.2-7 These interventions improve the ability to grasp, hold, and release objects in selected individuals; however, they have not been universally accepted. Traditional modalities, such as active ROM (AROM) and passive ROM (PROM) and electrical stimulation remain highly used in upper-extremity rehabilitation. Devices have been developed to provide either PROM or electrical stimulation to improve hand function and to prevent muscle atrophy. Therapist- and caregiver-directed PROM exercises are time consuming and labor intensive. An innovative therapeutic approach that can provide all these modalities more efficiently is needed in SCI rehabilitation.
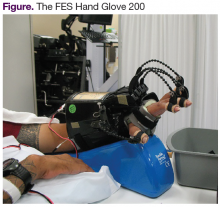
Until now, a single device that combines AROM and PROM simultaneously has not been available. A robotic system, the FES Hand Glove 200 (Robotix Hand Therapy Inc, Colorado Springs, CO), was developed to improve hand function (Figure).
Methods
This prospective safety study evaluated the occurrence of adverse effects (AEs) associated with the use of the FES Hand Glove 200. The study was performed in the Occupational Therapy Section of the Spinal Cord Injury Center at the James A. Haley Veterans’ Hospital (JAHVH) and approved by the JAHVH Research and Development Committee as well as the University of South Florida Investigational Review Board. For recruitment, the goals of the study as well as the inclusion and exclusion criteria were presented to the Spinal Cord Injury Center health care providers. Potential candidates of the study were referred to the study team from these providers.
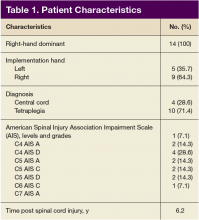
Screening of the referred candidates was conducted by physicians during inpatient evaluations. All subjects signed a consent form. Participants included active-duty military or veterans with traumatic SCI at levels C4 to C8 and American Spinal Injury Association Impairment Scale (AIS) grades A, B, C, and D. Participants were aged 18 to 60 years, at least 1-month post-SCI, medically stable, and had impairments in upper-extremities strength and ROM or function, including hand.
Subjects were excluded if any of the following were present: seizure within 3 months of study; active cancer; heterotopic ossification below the shoulder; new acute hand injuries of the study limb; unhealed fractures of the study limb; myocardial infarction within 12 months; severe cognitive impairment determined by a Modified Rancho Score below VI8; severe aphasia; pregnancy; skin irritations or open wounds in the study limb; fixed contractures of > 40° of the metacarpophalangeal (MP) or proximal interphalangeal (PIP) joints of the study hand; unwillingness to perform all of the therapies and assessments required for the study; active implant device (eg, pacemaker, implanted cardiac defibrillator, neurostimulator or drug infusion device); major psychological disorder; severe residual spasticity despite maximal medical therapy; muscle power grade of more than 3+ on wrist and finger extensors and flexors of the study limb; recent or current participation in research that could influence study response; pain that prevents participation in the study; or concurrent use of transcutaneous electrical stimulation on the study arm.
The following data were documented: level of SCI, AIS-score; complete medical history; physical examination (including skin integrity); and vital signs of bilateral upper extremities. A nurse practitioner (NP) certified in Functional Independent Measure (FIM) conducted chart reviews and/or in-person interviews of each subject to establish a FIM score before and after 6 weeks of research treatment. Two experienced occupational therapists (OTs) conducted detailed hand evaluations before the research treatment interventions. An OT provided subjects with education on the use, care, and precautions of the FES Hand Glove 200. The OT adjusted the device on the subject’s hand for proper fitting, including initial available PROM, and optimal muscle stimulation.
The OT then implemented the treatment protocol using the FES Hand Glove 200 in 1 hand per the subjects’ preference. The subjects received 30 minutes of PROM only on the FES Hand Glove 200, followed by an additional 30 minutes of PROM with FES for 1 hour of therapy per session. The study participants were treated 4 times per week for 6 weeks. Before and after each session, OTs evaluated and documented any loss of skin integrity and pain. Autonomic dysreflexia occurred when systolic BP increased > 20 to 30 mm Hg with symptoms such as headache, profuse sweating, or blurred vision was reported.9 The FES Hand Glove 200 was set up for PROM to the thumb and to digits 2 to 5 and for electrical muscle stimulation of the finger extensors and flexors. No other therapeutic exercise was performed during the study period on the other extremity. Primary and secondary outcomes were collected at the end of the 6-week intervention.
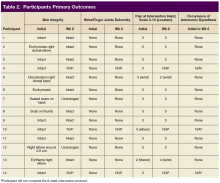
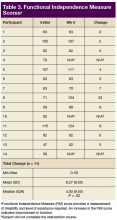
Primary outcomes included complications from the use of FES Hand Glove 200, including skin integrity and any joint deformity as drawn on a figure, changes of pain level by visual analog scale (VAS), and total number of autonomic dyreflexia episodes. Secondary measured outcomes included changes in PROM and AROM of wrist, metacarpal joint and interphalangeal joints of thumbs and digits 2 to 5 ≥ 10°; hand and pinch strength decline of > 1 lb; decline in manual muscle test, and FIM score, which is a validated measurement of disability and the level of assistance required for ADL.10
Statistical analyses were performed using SAS version 4 (Cary, NC) to assess the degree of change in the improvement score, which was defined as the postintervention score minus the preintervention score. However, because of the large standard error due to small sample sizes, the normality assumption was not satisfied for all the outcomes considered.
Results
Of the 20 participants screened, 14 men aged between 19 and 66 years with cervical SCI level of C4 to C6 AIS grades A to D were enrolled in the study. Three did not complete the 6-week trial due to SCI-related medical complications, which were unrelated to the use of the FES Hand Glove 200. They continued with regular OT treatment or self-directed home exercises after they were seen by the treating physician. (Table 1)
Skin integrity of all subjects was maintained throughout the study. One subject had a right-elbow wound before the intervention, which was unchanged at the end of the study. After 6 weeks of experimental intervention, there was no wrist or finger joint deformity noted and no increase in pain level except for 1 subject who reported increased pain that was unrelated to use of the device. No occurrence of autonomic dysreflexia was recorded during the use of FES Hand Glove 200 (Table 2).
For the secondary outcomes, there was no significant decrease in AROM or PROM ≥ 10° in forearm, wrist, or finger joints in any participants. There was no loss of strength > 1 lb as measured by gross grasp, pinch tip, 3-point, or lateral grip. There was no decline in motor strength per manual muscle testing. No worsening of FIM score was noted (Table 3).
Although this was not an efficacy study primarily, participants improved in several areas. Improvements included active and passive movements in the forearm, wrist, and hand. There also was significant improvement in strength of the extensor digitorum communis (EDC) muscle. Data are available on request to the authors.
Discussion
Passive ROM and AROM exercises and FES are common strategies to improve certain hand functions in people with cervical SCI. Many people, however, may experience limited duration or efficiency of rehabilitation secondary to lack of resources. Technologic advancement allowed the combination of PROM exercise and FES using the FES Hand Glove 200 device. The eventual goal of using this device is to enhance QOL by improving upper-extremity function. Because this device is not commercially available, its safety and tolerability are being tested prior to clinical use. Although 3 subjects withdrew from the study due to nondevice-related medical reasons, 11 subjects completed the study. Potential AEs included skin wounds, burns, tendon sprain or rupture, edema, and pain. At the end of the 6-week study period, there was no loss of skin integrity, no joint deformity, and no increase in hand or finger edema in all subjects. Increase in pain level at 6 weeks was noted in only 1 subject.
One concern was that overuse of such devices could potentially cause muscle fatigue, leading to decreased strength. Pinch grasp and manual muscle testing were evaluated, and no decrease in any of these parameters was noted at the end of study. Although this was not an efficacy study, there was some evidence of improved ROM of multiple wrist and finger joints as well as the EDC muscle strength.
Limitations
Limitations of the study included the duration of treatment of eight 30-minute sessions per week over a 6-week period. A longer treatment duration could result in repetition-related injuries and should be tested in future trials. Finally, the sample size of this study was relatively small. Future studies of different treatment frequency, longer duration of use and monitoring, and using a larger sample size are suggested. An efficacy study of this device using a randomized controlled design is indicated. As people with cervical SCI rank upper-extremity dysfunction as one of the top impairments that negatively impacts QOL, rehabilitation strategy to improve such functions should continue to be a research priority.2
Conclusion
This study supports the safety and tolerability of a 6-week course using FES Hand Glove 200 in traumatic SCI tetraplegic subjects. Additionally, data from this study suggest possible efficacy in enhancing ROM of various wrist and finger joints as well as certain muscle group. Further studies of efficacy with larger numbers of subjects are warranted.
Click here to read the digital edition.
1. NSCISC National Spinal Cord Injury Statistic Center. 2016 annual report—public version. https://www.nscisc.uab.edu/public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf. Published 2016. Accessed March 19, 2018.
2. Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med. 2005;37(1):32-36.
3. O’Driscoll SW, Giori NJ. Continuous passive motion (CPM): theory and principles of clinical application. J Rehabil Res Dev. 2000;37(2):179-188.
4. Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: a pilot study. Neurorehabil Neural Repair. 2007;21(3):207-215.
5. de Kroon JR, Ijzerman MJ, Lankhorst GJ, Zilvold G. Electrical stimulation of the upper limb in stroke stimulation of the extensors of the hand vs. alternate stimulation of flexors and extensors. Am J Phys Med Rehabil. 2004;83(8):592-600.
6. Alon G, McBride K, Levitt AF. Feasibility of randomised clinical trial of early initiation and prolonged, home-base FES training to enhance upper limb functional recovery following stroke. https://www.researchgate.net /publication/237724608_Feasibility_of_randomised_clinical_trial_of_early _initiation_and_prolonged_home-based_FES_training_to_enhance_upper_limb _functional_recovery_following_stroke. Published 2004. Accessed March 21, 2018.
7. Alon G, McBride K. Persons with C5-C6 tetraplegia achieve selected functional gains using a neuroprosthesis. Arch Phys Med Rehabil. 2003;84(1):119-124.
8. Hagen C, Malkmus D, Durham P. Rancho Los Amigos Cognitive Scale. http://file .lacounty.gov/SDSInter/dhs/218118_RLOCFProfessionalReferenceCard-English .pdf. Published 1979. Accessed March 19, 2018.
9. Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81(4):506-516.
10. Grey N, Kennedy P. The Functional Independence Measure: a comparative study of clinician and self rating. Paraplegia. 1993;31(7):457-461.
An estimated 282,000 people in the US are living with spinal cord injury (SCI).1 Damage to the cervical spinal cord is the most prevalent. Among cervical spinal cord trauma, injury to levels C4, C5, and C6 have the highest occurrence.1 Damage to these levels has significant implications for functional status. Depending on pathology, patients’ functional status can range from requiring assistance for all activities of daily living (ADL) to potentially living independently.
Improving upper-limb function is vital to achieving independence. About half of people with tetraplegia judge hand and arm function to be the top factor that would improve quality of life (QOL).2 Persons with traumatic cervical SCI may lose the ability to use their hands from motor deficits, sensory dysfunction, proprioception problem, and/or loss of coordination. In addition, they may develop joint contracture, spasticity, pain, and other complications. Thus, their independence and ADL are affected significantly by multiple mechanisms of pathology.
Upper-extremity rehabilitation that emphasizes strengthening and maintaining functional range of motion (ROM) is fundamental to SCI rehabilitation. Rehabilitation to restore partial hand function has included ROM exercises, splinting, surgical procedures in the form of tendon transfers and various electrical stimulation devices, such as implantable neuroprostheses.2-7 These interventions improve the ability to grasp, hold, and release objects in selected individuals; however, they have not been universally accepted. Traditional modalities, such as active ROM (AROM) and passive ROM (PROM) and electrical stimulation remain highly used in upper-extremity rehabilitation. Devices have been developed to provide either PROM or electrical stimulation to improve hand function and to prevent muscle atrophy. Therapist- and caregiver-directed PROM exercises are time consuming and labor intensive. An innovative therapeutic approach that can provide all these modalities more efficiently is needed in SCI rehabilitation.
Until now, a single device that combines AROM and PROM simultaneously has not been available. A robotic system, the FES Hand Glove 200 (Robotix Hand Therapy Inc, Colorado Springs, CO), was developed to improve hand function (Figure).
Methods
This prospective safety study evaluated the occurrence of adverse effects (AEs) associated with the use of the FES Hand Glove 200. The study was performed in the Occupational Therapy Section of the Spinal Cord Injury Center at the James A. Haley Veterans’ Hospital (JAHVH) and approved by the JAHVH Research and Development Committee as well as the University of South Florida Investigational Review Board. For recruitment, the goals of the study as well as the inclusion and exclusion criteria were presented to the Spinal Cord Injury Center health care providers. Potential candidates of the study were referred to the study team from these providers.
Screening of the referred candidates was conducted by physicians during inpatient evaluations. All subjects signed a consent form. Participants included active-duty military or veterans with traumatic SCI at levels C4 to C8 and American Spinal Injury Association Impairment Scale (AIS) grades A, B, C, and D. Participants were aged 18 to 60 years, at least 1-month post-SCI, medically stable, and had impairments in upper-extremities strength and ROM or function, including hand.
Subjects were excluded if any of the following were present: seizure within 3 months of study; active cancer; heterotopic ossification below the shoulder; new acute hand injuries of the study limb; unhealed fractures of the study limb; myocardial infarction within 12 months; severe cognitive impairment determined by a Modified Rancho Score below VI8; severe aphasia; pregnancy; skin irritations or open wounds in the study limb; fixed contractures of > 40° of the metacarpophalangeal (MP) or proximal interphalangeal (PIP) joints of the study hand; unwillingness to perform all of the therapies and assessments required for the study; active implant device (eg, pacemaker, implanted cardiac defibrillator, neurostimulator or drug infusion device); major psychological disorder; severe residual spasticity despite maximal medical therapy; muscle power grade of more than 3+ on wrist and finger extensors and flexors of the study limb; recent or current participation in research that could influence study response; pain that prevents participation in the study; or concurrent use of transcutaneous electrical stimulation on the study arm.
The following data were documented: level of SCI, AIS-score; complete medical history; physical examination (including skin integrity); and vital signs of bilateral upper extremities. A nurse practitioner (NP) certified in Functional Independent Measure (FIM) conducted chart reviews and/or in-person interviews of each subject to establish a FIM score before and after 6 weeks of research treatment. Two experienced occupational therapists (OTs) conducted detailed hand evaluations before the research treatment interventions. An OT provided subjects with education on the use, care, and precautions of the FES Hand Glove 200. The OT adjusted the device on the subject’s hand for proper fitting, including initial available PROM, and optimal muscle stimulation.
The OT then implemented the treatment protocol using the FES Hand Glove 200 in 1 hand per the subjects’ preference. The subjects received 30 minutes of PROM only on the FES Hand Glove 200, followed by an additional 30 minutes of PROM with FES for 1 hour of therapy per session. The study participants were treated 4 times per week for 6 weeks. Before and after each session, OTs evaluated and documented any loss of skin integrity and pain. Autonomic dysreflexia occurred when systolic BP increased > 20 to 30 mm Hg with symptoms such as headache, profuse sweating, or blurred vision was reported.9 The FES Hand Glove 200 was set up for PROM to the thumb and to digits 2 to 5 and for electrical muscle stimulation of the finger extensors and flexors. No other therapeutic exercise was performed during the study period on the other extremity. Primary and secondary outcomes were collected at the end of the 6-week intervention.
Primary outcomes included complications from the use of FES Hand Glove 200, including skin integrity and any joint deformity as drawn on a figure, changes of pain level by visual analog scale (VAS), and total number of autonomic dyreflexia episodes. Secondary measured outcomes included changes in PROM and AROM of wrist, metacarpal joint and interphalangeal joints of thumbs and digits 2 to 5 ≥ 10°; hand and pinch strength decline of > 1 lb; decline in manual muscle test, and FIM score, which is a validated measurement of disability and the level of assistance required for ADL.10
Statistical analyses were performed using SAS version 4 (Cary, NC) to assess the degree of change in the improvement score, which was defined as the postintervention score minus the preintervention score. However, because of the large standard error due to small sample sizes, the normality assumption was not satisfied for all the outcomes considered.
Results
Of the 20 participants screened, 14 men aged between 19 and 66 years with cervical SCI level of C4 to C6 AIS grades A to D were enrolled in the study. Three did not complete the 6-week trial due to SCI-related medical complications, which were unrelated to the use of the FES Hand Glove 200. They continued with regular OT treatment or self-directed home exercises after they were seen by the treating physician. (Table 1)
Skin integrity of all subjects was maintained throughout the study. One subject had a right-elbow wound before the intervention, which was unchanged at the end of the study. After 6 weeks of experimental intervention, there was no wrist or finger joint deformity noted and no increase in pain level except for 1 subject who reported increased pain that was unrelated to use of the device. No occurrence of autonomic dysreflexia was recorded during the use of FES Hand Glove 200 (Table 2).
For the secondary outcomes, there was no significant decrease in AROM or PROM ≥ 10° in forearm, wrist, or finger joints in any participants. There was no loss of strength > 1 lb as measured by gross grasp, pinch tip, 3-point, or lateral grip. There was no decline in motor strength per manual muscle testing. No worsening of FIM score was noted (Table 3).
Although this was not an efficacy study primarily, participants improved in several areas. Improvements included active and passive movements in the forearm, wrist, and hand. There also was significant improvement in strength of the extensor digitorum communis (EDC) muscle. Data are available on request to the authors.
Discussion
Passive ROM and AROM exercises and FES are common strategies to improve certain hand functions in people with cervical SCI. Many people, however, may experience limited duration or efficiency of rehabilitation secondary to lack of resources. Technologic advancement allowed the combination of PROM exercise and FES using the FES Hand Glove 200 device. The eventual goal of using this device is to enhance QOL by improving upper-extremity function. Because this device is not commercially available, its safety and tolerability are being tested prior to clinical use. Although 3 subjects withdrew from the study due to nondevice-related medical reasons, 11 subjects completed the study. Potential AEs included skin wounds, burns, tendon sprain or rupture, edema, and pain. At the end of the 6-week study period, there was no loss of skin integrity, no joint deformity, and no increase in hand or finger edema in all subjects. Increase in pain level at 6 weeks was noted in only 1 subject.
One concern was that overuse of such devices could potentially cause muscle fatigue, leading to decreased strength. Pinch grasp and manual muscle testing were evaluated, and no decrease in any of these parameters was noted at the end of study. Although this was not an efficacy study, there was some evidence of improved ROM of multiple wrist and finger joints as well as the EDC muscle strength.
Limitations
Limitations of the study included the duration of treatment of eight 30-minute sessions per week over a 6-week period. A longer treatment duration could result in repetition-related injuries and should be tested in future trials. Finally, the sample size of this study was relatively small. Future studies of different treatment frequency, longer duration of use and monitoring, and using a larger sample size are suggested. An efficacy study of this device using a randomized controlled design is indicated. As people with cervical SCI rank upper-extremity dysfunction as one of the top impairments that negatively impacts QOL, rehabilitation strategy to improve such functions should continue to be a research priority.2
Conclusion
This study supports the safety and tolerability of a 6-week course using FES Hand Glove 200 in traumatic SCI tetraplegic subjects. Additionally, data from this study suggest possible efficacy in enhancing ROM of various wrist and finger joints as well as certain muscle group. Further studies of efficacy with larger numbers of subjects are warranted.
Click here to read the digital edition.
An estimated 282,000 people in the US are living with spinal cord injury (SCI).1 Damage to the cervical spinal cord is the most prevalent. Among cervical spinal cord trauma, injury to levels C4, C5, and C6 have the highest occurrence.1 Damage to these levels has significant implications for functional status. Depending on pathology, patients’ functional status can range from requiring assistance for all activities of daily living (ADL) to potentially living independently.
Improving upper-limb function is vital to achieving independence. About half of people with tetraplegia judge hand and arm function to be the top factor that would improve quality of life (QOL).2 Persons with traumatic cervical SCI may lose the ability to use their hands from motor deficits, sensory dysfunction, proprioception problem, and/or loss of coordination. In addition, they may develop joint contracture, spasticity, pain, and other complications. Thus, their independence and ADL are affected significantly by multiple mechanisms of pathology.
Upper-extremity rehabilitation that emphasizes strengthening and maintaining functional range of motion (ROM) is fundamental to SCI rehabilitation. Rehabilitation to restore partial hand function has included ROM exercises, splinting, surgical procedures in the form of tendon transfers and various electrical stimulation devices, such as implantable neuroprostheses.2-7 These interventions improve the ability to grasp, hold, and release objects in selected individuals; however, they have not been universally accepted. Traditional modalities, such as active ROM (AROM) and passive ROM (PROM) and electrical stimulation remain highly used in upper-extremity rehabilitation. Devices have been developed to provide either PROM or electrical stimulation to improve hand function and to prevent muscle atrophy. Therapist- and caregiver-directed PROM exercises are time consuming and labor intensive. An innovative therapeutic approach that can provide all these modalities more efficiently is needed in SCI rehabilitation.
Until now, a single device that combines AROM and PROM simultaneously has not been available. A robotic system, the FES Hand Glove 200 (Robotix Hand Therapy Inc, Colorado Springs, CO), was developed to improve hand function (Figure).
Methods
This prospective safety study evaluated the occurrence of adverse effects (AEs) associated with the use of the FES Hand Glove 200. The study was performed in the Occupational Therapy Section of the Spinal Cord Injury Center at the James A. Haley Veterans’ Hospital (JAHVH) and approved by the JAHVH Research and Development Committee as well as the University of South Florida Investigational Review Board. For recruitment, the goals of the study as well as the inclusion and exclusion criteria were presented to the Spinal Cord Injury Center health care providers. Potential candidates of the study were referred to the study team from these providers.
Screening of the referred candidates was conducted by physicians during inpatient evaluations. All subjects signed a consent form. Participants included active-duty military or veterans with traumatic SCI at levels C4 to C8 and American Spinal Injury Association Impairment Scale (AIS) grades A, B, C, and D. Participants were aged 18 to 60 years, at least 1-month post-SCI, medically stable, and had impairments in upper-extremities strength and ROM or function, including hand.
Subjects were excluded if any of the following were present: seizure within 3 months of study; active cancer; heterotopic ossification below the shoulder; new acute hand injuries of the study limb; unhealed fractures of the study limb; myocardial infarction within 12 months; severe cognitive impairment determined by a Modified Rancho Score below VI8; severe aphasia; pregnancy; skin irritations or open wounds in the study limb; fixed contractures of > 40° of the metacarpophalangeal (MP) or proximal interphalangeal (PIP) joints of the study hand; unwillingness to perform all of the therapies and assessments required for the study; active implant device (eg, pacemaker, implanted cardiac defibrillator, neurostimulator or drug infusion device); major psychological disorder; severe residual spasticity despite maximal medical therapy; muscle power grade of more than 3+ on wrist and finger extensors and flexors of the study limb; recent or current participation in research that could influence study response; pain that prevents participation in the study; or concurrent use of transcutaneous electrical stimulation on the study arm.
The following data were documented: level of SCI, AIS-score; complete medical history; physical examination (including skin integrity); and vital signs of bilateral upper extremities. A nurse practitioner (NP) certified in Functional Independent Measure (FIM) conducted chart reviews and/or in-person interviews of each subject to establish a FIM score before and after 6 weeks of research treatment. Two experienced occupational therapists (OTs) conducted detailed hand evaluations before the research treatment interventions. An OT provided subjects with education on the use, care, and precautions of the FES Hand Glove 200. The OT adjusted the device on the subject’s hand for proper fitting, including initial available PROM, and optimal muscle stimulation.
The OT then implemented the treatment protocol using the FES Hand Glove 200 in 1 hand per the subjects’ preference. The subjects received 30 minutes of PROM only on the FES Hand Glove 200, followed by an additional 30 minutes of PROM with FES for 1 hour of therapy per session. The study participants were treated 4 times per week for 6 weeks. Before and after each session, OTs evaluated and documented any loss of skin integrity and pain. Autonomic dysreflexia occurred when systolic BP increased > 20 to 30 mm Hg with symptoms such as headache, profuse sweating, or blurred vision was reported.9 The FES Hand Glove 200 was set up for PROM to the thumb and to digits 2 to 5 and for electrical muscle stimulation of the finger extensors and flexors. No other therapeutic exercise was performed during the study period on the other extremity. Primary and secondary outcomes were collected at the end of the 6-week intervention.
Primary outcomes included complications from the use of FES Hand Glove 200, including skin integrity and any joint deformity as drawn on a figure, changes of pain level by visual analog scale (VAS), and total number of autonomic dyreflexia episodes. Secondary measured outcomes included changes in PROM and AROM of wrist, metacarpal joint and interphalangeal joints of thumbs and digits 2 to 5 ≥ 10°; hand and pinch strength decline of > 1 lb; decline in manual muscle test, and FIM score, which is a validated measurement of disability and the level of assistance required for ADL.10
Statistical analyses were performed using SAS version 4 (Cary, NC) to assess the degree of change in the improvement score, which was defined as the postintervention score minus the preintervention score. However, because of the large standard error due to small sample sizes, the normality assumption was not satisfied for all the outcomes considered.
Results
Of the 20 participants screened, 14 men aged between 19 and 66 years with cervical SCI level of C4 to C6 AIS grades A to D were enrolled in the study. Three did not complete the 6-week trial due to SCI-related medical complications, which were unrelated to the use of the FES Hand Glove 200. They continued with regular OT treatment or self-directed home exercises after they were seen by the treating physician. (Table 1)
Skin integrity of all subjects was maintained throughout the study. One subject had a right-elbow wound before the intervention, which was unchanged at the end of the study. After 6 weeks of experimental intervention, there was no wrist or finger joint deformity noted and no increase in pain level except for 1 subject who reported increased pain that was unrelated to use of the device. No occurrence of autonomic dysreflexia was recorded during the use of FES Hand Glove 200 (Table 2).
For the secondary outcomes, there was no significant decrease in AROM or PROM ≥ 10° in forearm, wrist, or finger joints in any participants. There was no loss of strength > 1 lb as measured by gross grasp, pinch tip, 3-point, or lateral grip. There was no decline in motor strength per manual muscle testing. No worsening of FIM score was noted (Table 3).
Although this was not an efficacy study primarily, participants improved in several areas. Improvements included active and passive movements in the forearm, wrist, and hand. There also was significant improvement in strength of the extensor digitorum communis (EDC) muscle. Data are available on request to the authors.
Discussion
Passive ROM and AROM exercises and FES are common strategies to improve certain hand functions in people with cervical SCI. Many people, however, may experience limited duration or efficiency of rehabilitation secondary to lack of resources. Technologic advancement allowed the combination of PROM exercise and FES using the FES Hand Glove 200 device. The eventual goal of using this device is to enhance QOL by improving upper-extremity function. Because this device is not commercially available, its safety and tolerability are being tested prior to clinical use. Although 3 subjects withdrew from the study due to nondevice-related medical reasons, 11 subjects completed the study. Potential AEs included skin wounds, burns, tendon sprain or rupture, edema, and pain. At the end of the 6-week study period, there was no loss of skin integrity, no joint deformity, and no increase in hand or finger edema in all subjects. Increase in pain level at 6 weeks was noted in only 1 subject.
One concern was that overuse of such devices could potentially cause muscle fatigue, leading to decreased strength. Pinch grasp and manual muscle testing were evaluated, and no decrease in any of these parameters was noted at the end of study. Although this was not an efficacy study, there was some evidence of improved ROM of multiple wrist and finger joints as well as the EDC muscle strength.
Limitations
Limitations of the study included the duration of treatment of eight 30-minute sessions per week over a 6-week period. A longer treatment duration could result in repetition-related injuries and should be tested in future trials. Finally, the sample size of this study was relatively small. Future studies of different treatment frequency, longer duration of use and monitoring, and using a larger sample size are suggested. An efficacy study of this device using a randomized controlled design is indicated. As people with cervical SCI rank upper-extremity dysfunction as one of the top impairments that negatively impacts QOL, rehabilitation strategy to improve such functions should continue to be a research priority.2
Conclusion
This study supports the safety and tolerability of a 6-week course using FES Hand Glove 200 in traumatic SCI tetraplegic subjects. Additionally, data from this study suggest possible efficacy in enhancing ROM of various wrist and finger joints as well as certain muscle group. Further studies of efficacy with larger numbers of subjects are warranted.
Click here to read the digital edition.
1. NSCISC National Spinal Cord Injury Statistic Center. 2016 annual report—public version. https://www.nscisc.uab.edu/public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf. Published 2016. Accessed March 19, 2018.
2. Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med. 2005;37(1):32-36.
3. O’Driscoll SW, Giori NJ. Continuous passive motion (CPM): theory and principles of clinical application. J Rehabil Res Dev. 2000;37(2):179-188.
4. Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: a pilot study. Neurorehabil Neural Repair. 2007;21(3):207-215.
5. de Kroon JR, Ijzerman MJ, Lankhorst GJ, Zilvold G. Electrical stimulation of the upper limb in stroke stimulation of the extensors of the hand vs. alternate stimulation of flexors and extensors. Am J Phys Med Rehabil. 2004;83(8):592-600.
6. Alon G, McBride K, Levitt AF. Feasibility of randomised clinical trial of early initiation and prolonged, home-base FES training to enhance upper limb functional recovery following stroke. https://www.researchgate.net /publication/237724608_Feasibility_of_randomised_clinical_trial_of_early _initiation_and_prolonged_home-based_FES_training_to_enhance_upper_limb _functional_recovery_following_stroke. Published 2004. Accessed March 21, 2018.
7. Alon G, McBride K. Persons with C5-C6 tetraplegia achieve selected functional gains using a neuroprosthesis. Arch Phys Med Rehabil. 2003;84(1):119-124.
8. Hagen C, Malkmus D, Durham P. Rancho Los Amigos Cognitive Scale. http://file .lacounty.gov/SDSInter/dhs/218118_RLOCFProfessionalReferenceCard-English .pdf. Published 1979. Accessed March 19, 2018.
9. Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81(4):506-516.
10. Grey N, Kennedy P. The Functional Independence Measure: a comparative study of clinician and self rating. Paraplegia. 1993;31(7):457-461.
1. NSCISC National Spinal Cord Injury Statistic Center. 2016 annual report—public version. https://www.nscisc.uab.edu/public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf. Published 2016. Accessed March 19, 2018.
2. Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med. 2005;37(1):32-36.
3. O’Driscoll SW, Giori NJ. Continuous passive motion (CPM): theory and principles of clinical application. J Rehabil Res Dev. 2000;37(2):179-188.
4. Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: a pilot study. Neurorehabil Neural Repair. 2007;21(3):207-215.
5. de Kroon JR, Ijzerman MJ, Lankhorst GJ, Zilvold G. Electrical stimulation of the upper limb in stroke stimulation of the extensors of the hand vs. alternate stimulation of flexors and extensors. Am J Phys Med Rehabil. 2004;83(8):592-600.
6. Alon G, McBride K, Levitt AF. Feasibility of randomised clinical trial of early initiation and prolonged, home-base FES training to enhance upper limb functional recovery following stroke. https://www.researchgate.net /publication/237724608_Feasibility_of_randomised_clinical_trial_of_early _initiation_and_prolonged_home-based_FES_training_to_enhance_upper_limb _functional_recovery_following_stroke. Published 2004. Accessed March 21, 2018.
7. Alon G, McBride K. Persons with C5-C6 tetraplegia achieve selected functional gains using a neuroprosthesis. Arch Phys Med Rehabil. 2003;84(1):119-124.
8. Hagen C, Malkmus D, Durham P. Rancho Los Amigos Cognitive Scale. http://file .lacounty.gov/SDSInter/dhs/218118_RLOCFProfessionalReferenceCard-English .pdf. Published 1979. Accessed March 19, 2018.
9. Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81(4):506-516.
10. Grey N, Kennedy P. The Functional Independence Measure: a comparative study of clinician and self rating. Paraplegia. 1993;31(7):457-461.
VA Weighs Improvements to Disability Determination Process
The severity of traumatic brain injury (TBI) is typically defined at the time of the initial injury, but a diagnosis may not come for months or even years later. Given the complexities of diagnosing what might be a slowly revealed condition, with signs and symptoms that may manifest over time; the need for self-report of symptoms; and the time that might have elapsed since the original injury, a diagnostician needs not only to have experience with TBI but to stay abreast of the state of the science.
As of now, only health care professionals in 4 specialties—neurologist, neurosurgeon, physiatrist, or psychiatrist—are allowed to diagnose TBI in the VA’s disability compensation process. A new congressionally mandated report by the National Academies of Sciences, Engineering, and Medicine, though, is advising that it’s training and experience that count, not necessarily the specialty.
In Evaluation of the Disability Determination Process for Traumatic Brain Injury in Veterans, a committee of experts in emergency medicine, neurology, neurosurgery, psychiatry, psychology, physical medicine and rehabilitation, and epidemiology and biostatistics review the process and current literature on TBI. The committee advises that any health care professional with “pertinent and ongoing brain injury training and experience” and up-to-date knowledge about TBI should be included in the diagnostic process.
The disability compensation is a tax-free benefit paid to veterans with disabilities resulting from disease or injury incurred or aggravated during active military service. The amount is determined in a 6-step process beginning when the veteran (or a proxy) files a claim. An approved clinician typically must diagnose and evaluate the degree of impairment, functional limitation, and disability.
Between 2000 and 2018, an estimated 384,000 incidents of TBI occurred in the military. That increasing prevalence means more medical specialties now include TBI training in their curriculum. The committee notes that at least 18 brain injury programs are accredited by the Accreditation Council for Graduate Medical Education to train physicians in many specialties to diagnose, treat, and rehabilitate patients with brain injury.
Among other recommendations, the committee advised that the VA take specific actions to increase transparency at both individual and systemwide levels, such as providing veterans full access to the details of their examinations, allowing veterans to rate the quality of their evaluations, and providing public access to detailed systemwide data on the outcomes of evaluations and outcome quality. Those changes will represent a “fundamental enhancement” in the quality of disability evaluations, the committee says, which added that shifting from a focus on the consistency of the process and practitioner qualifications to a focus on the accuracy of the outcome of the evaluation will help identify steps or components in the process that warrant improvement.
It also suggested regularly updating the Veteran Affairs Schedule for Rating Disabilities and the Disability Benefits Questionnaires (DBQs) for residuals of TBI to “better reflect the current state of medical knowledge.” The committee found that 3 important residuals of TBI are not adequately covered by any of the existing DBQs: insomnia, vestibular dysfunction, and near-vision dysfunction. Although 4 DBQs (mental disorder, chronic fatigue syndrome, PTSD, and sleep apnea) contain isolated questions related to insomnia and sleep disruption, no single DBQ, the committee says, combines them all “in a way that captures the full extent of disability associated with post-TBI sleep disruption.” Similarly, no single DBQ captures the full extent of disability associated with post-TBI vestibular dysfunction or the disability associated with near-vision dysfunction.
The committee sums up: “[B]y adopting an explicit learning structure in which the reliability and validity of disability determinations are directly assessed, the VA will be able to devote its resources to those modifications and enhancements … that will have the greatest impact in improving the service provided to injured veterans.”
The severity of traumatic brain injury (TBI) is typically defined at the time of the initial injury, but a diagnosis may not come for months or even years later. Given the complexities of diagnosing what might be a slowly revealed condition, with signs and symptoms that may manifest over time; the need for self-report of symptoms; and the time that might have elapsed since the original injury, a diagnostician needs not only to have experience with TBI but to stay abreast of the state of the science.
As of now, only health care professionals in 4 specialties—neurologist, neurosurgeon, physiatrist, or psychiatrist—are allowed to diagnose TBI in the VA’s disability compensation process. A new congressionally mandated report by the National Academies of Sciences, Engineering, and Medicine, though, is advising that it’s training and experience that count, not necessarily the specialty.
In Evaluation of the Disability Determination Process for Traumatic Brain Injury in Veterans, a committee of experts in emergency medicine, neurology, neurosurgery, psychiatry, psychology, physical medicine and rehabilitation, and epidemiology and biostatistics review the process and current literature on TBI. The committee advises that any health care professional with “pertinent and ongoing brain injury training and experience” and up-to-date knowledge about TBI should be included in the diagnostic process.
The disability compensation is a tax-free benefit paid to veterans with disabilities resulting from disease or injury incurred or aggravated during active military service. The amount is determined in a 6-step process beginning when the veteran (or a proxy) files a claim. An approved clinician typically must diagnose and evaluate the degree of impairment, functional limitation, and disability.
Between 2000 and 2018, an estimated 384,000 incidents of TBI occurred in the military. That increasing prevalence means more medical specialties now include TBI training in their curriculum. The committee notes that at least 18 brain injury programs are accredited by the Accreditation Council for Graduate Medical Education to train physicians in many specialties to diagnose, treat, and rehabilitate patients with brain injury.
Among other recommendations, the committee advised that the VA take specific actions to increase transparency at both individual and systemwide levels, such as providing veterans full access to the details of their examinations, allowing veterans to rate the quality of their evaluations, and providing public access to detailed systemwide data on the outcomes of evaluations and outcome quality. Those changes will represent a “fundamental enhancement” in the quality of disability evaluations, the committee says, which added that shifting from a focus on the consistency of the process and practitioner qualifications to a focus on the accuracy of the outcome of the evaluation will help identify steps or components in the process that warrant improvement.
It also suggested regularly updating the Veteran Affairs Schedule for Rating Disabilities and the Disability Benefits Questionnaires (DBQs) for residuals of TBI to “better reflect the current state of medical knowledge.” The committee found that 3 important residuals of TBI are not adequately covered by any of the existing DBQs: insomnia, vestibular dysfunction, and near-vision dysfunction. Although 4 DBQs (mental disorder, chronic fatigue syndrome, PTSD, and sleep apnea) contain isolated questions related to insomnia and sleep disruption, no single DBQ, the committee says, combines them all “in a way that captures the full extent of disability associated with post-TBI sleep disruption.” Similarly, no single DBQ captures the full extent of disability associated with post-TBI vestibular dysfunction or the disability associated with near-vision dysfunction.
The committee sums up: “[B]y adopting an explicit learning structure in which the reliability and validity of disability determinations are directly assessed, the VA will be able to devote its resources to those modifications and enhancements … that will have the greatest impact in improving the service provided to injured veterans.”
The severity of traumatic brain injury (TBI) is typically defined at the time of the initial injury, but a diagnosis may not come for months or even years later. Given the complexities of diagnosing what might be a slowly revealed condition, with signs and symptoms that may manifest over time; the need for self-report of symptoms; and the time that might have elapsed since the original injury, a diagnostician needs not only to have experience with TBI but to stay abreast of the state of the science.
As of now, only health care professionals in 4 specialties—neurologist, neurosurgeon, physiatrist, or psychiatrist—are allowed to diagnose TBI in the VA’s disability compensation process. A new congressionally mandated report by the National Academies of Sciences, Engineering, and Medicine, though, is advising that it’s training and experience that count, not necessarily the specialty.
In Evaluation of the Disability Determination Process for Traumatic Brain Injury in Veterans, a committee of experts in emergency medicine, neurology, neurosurgery, psychiatry, psychology, physical medicine and rehabilitation, and epidemiology and biostatistics review the process and current literature on TBI. The committee advises that any health care professional with “pertinent and ongoing brain injury training and experience” and up-to-date knowledge about TBI should be included in the diagnostic process.
The disability compensation is a tax-free benefit paid to veterans with disabilities resulting from disease or injury incurred or aggravated during active military service. The amount is determined in a 6-step process beginning when the veteran (or a proxy) files a claim. An approved clinician typically must diagnose and evaluate the degree of impairment, functional limitation, and disability.
Between 2000 and 2018, an estimated 384,000 incidents of TBI occurred in the military. That increasing prevalence means more medical specialties now include TBI training in their curriculum. The committee notes that at least 18 brain injury programs are accredited by the Accreditation Council for Graduate Medical Education to train physicians in many specialties to diagnose, treat, and rehabilitate patients with brain injury.
Among other recommendations, the committee advised that the VA take specific actions to increase transparency at both individual and systemwide levels, such as providing veterans full access to the details of their examinations, allowing veterans to rate the quality of their evaluations, and providing public access to detailed systemwide data on the outcomes of evaluations and outcome quality. Those changes will represent a “fundamental enhancement” in the quality of disability evaluations, the committee says, which added that shifting from a focus on the consistency of the process and practitioner qualifications to a focus on the accuracy of the outcome of the evaluation will help identify steps or components in the process that warrant improvement.
It also suggested regularly updating the Veteran Affairs Schedule for Rating Disabilities and the Disability Benefits Questionnaires (DBQs) for residuals of TBI to “better reflect the current state of medical knowledge.” The committee found that 3 important residuals of TBI are not adequately covered by any of the existing DBQs: insomnia, vestibular dysfunction, and near-vision dysfunction. Although 4 DBQs (mental disorder, chronic fatigue syndrome, PTSD, and sleep apnea) contain isolated questions related to insomnia and sleep disruption, no single DBQ, the committee says, combines them all “in a way that captures the full extent of disability associated with post-TBI sleep disruption.” Similarly, no single DBQ captures the full extent of disability associated with post-TBI vestibular dysfunction or the disability associated with near-vision dysfunction.
The committee sums up: “[B]y adopting an explicit learning structure in which the reliability and validity of disability determinations are directly assessed, the VA will be able to devote its resources to those modifications and enhancements … that will have the greatest impact in improving the service provided to injured veterans.”
Ventricular Arrhythmia Due to MS Treatment
Fingolimod, a sphingosine-1-phosphate receptor modulator, has been used to treat > 55,000 patients in the US, according to the manufacturer (Gilenya/Novartis). It is believed to work by keeping lymphocytes from migrating into the CNS, sequestering them in the lymph nodes.
Although it has been found effective in randomized controlled trials, fingolimod is also known to have a wide range of adverse effects (AEs), including some that are serious and even life-threatening, such as bradycardia and atrioventricular block. The drug is contraindicated for patients who have had myocardial infarction, unstable angina, or heart failure, among other conditions. Ventricular tachycardia has been reported only once, but clinicians from Hurley Medical Center in Flint, Michigan, suggest that it may actually be an underrecognized cause of sudden death.
They describe the case of their patient, a 63-year-old woman with relapsing-remitting multiple sclerosis and hypertension who was about to start fingolomod. She underwent a basal ECG to be cleared before starting treatment. She received her first dose of fingolimod at the cardiology office, was monitored for 6 hours, and went home with a surface-mounted Holter monitor.
Two weeks later, she was in the emergency department because the monitor had captured ventricular tachycardia, and she was reporting palpitations.
Lab work was normal; the echocardiogram was normal. Cardiac monitoring showed no other evidence of cardiac arrhythmias. Her only other medication was amlodipine. The fingolimod was held back. She was observed for4 days then discharged in a stable condition. Her clinicians followed her for 2 months but the arrhythmia did not return.
Although this patient had no further arrhythmias, the authors warn that serious outcomes are possible. They urge health care practitioners to let patients know of this potential AE and advise them to report symptoms such as palpitations immediately.
Fingolimod, a sphingosine-1-phosphate receptor modulator, has been used to treat > 55,000 patients in the US, according to the manufacturer (Gilenya/Novartis). It is believed to work by keeping lymphocytes from migrating into the CNS, sequestering them in the lymph nodes.
Although it has been found effective in randomized controlled trials, fingolimod is also known to have a wide range of adverse effects (AEs), including some that are serious and even life-threatening, such as bradycardia and atrioventricular block. The drug is contraindicated for patients who have had myocardial infarction, unstable angina, or heart failure, among other conditions. Ventricular tachycardia has been reported only once, but clinicians from Hurley Medical Center in Flint, Michigan, suggest that it may actually be an underrecognized cause of sudden death.
They describe the case of their patient, a 63-year-old woman with relapsing-remitting multiple sclerosis and hypertension who was about to start fingolomod. She underwent a basal ECG to be cleared before starting treatment. She received her first dose of fingolimod at the cardiology office, was monitored for 6 hours, and went home with a surface-mounted Holter monitor.
Two weeks later, she was in the emergency department because the monitor had captured ventricular tachycardia, and she was reporting palpitations.
Lab work was normal; the echocardiogram was normal. Cardiac monitoring showed no other evidence of cardiac arrhythmias. Her only other medication was amlodipine. The fingolimod was held back. She was observed for4 days then discharged in a stable condition. Her clinicians followed her for 2 months but the arrhythmia did not return.
Although this patient had no further arrhythmias, the authors warn that serious outcomes are possible. They urge health care practitioners to let patients know of this potential AE and advise them to report symptoms such as palpitations immediately.
Fingolimod, a sphingosine-1-phosphate receptor modulator, has been used to treat > 55,000 patients in the US, according to the manufacturer (Gilenya/Novartis). It is believed to work by keeping lymphocytes from migrating into the CNS, sequestering them in the lymph nodes.
Although it has been found effective in randomized controlled trials, fingolimod is also known to have a wide range of adverse effects (AEs), including some that are serious and even life-threatening, such as bradycardia and atrioventricular block. The drug is contraindicated for patients who have had myocardial infarction, unstable angina, or heart failure, among other conditions. Ventricular tachycardia has been reported only once, but clinicians from Hurley Medical Center in Flint, Michigan, suggest that it may actually be an underrecognized cause of sudden death.
They describe the case of their patient, a 63-year-old woman with relapsing-remitting multiple sclerosis and hypertension who was about to start fingolomod. She underwent a basal ECG to be cleared before starting treatment. She received her first dose of fingolimod at the cardiology office, was monitored for 6 hours, and went home with a surface-mounted Holter monitor.
Two weeks later, she was in the emergency department because the monitor had captured ventricular tachycardia, and she was reporting palpitations.
Lab work was normal; the echocardiogram was normal. Cardiac monitoring showed no other evidence of cardiac arrhythmias. Her only other medication was amlodipine. The fingolimod was held back. She was observed for4 days then discharged in a stable condition. Her clinicians followed her for 2 months but the arrhythmia did not return.
Although this patient had no further arrhythmias, the authors warn that serious outcomes are possible. They urge health care practitioners to let patients know of this potential AE and advise them to report symptoms such as palpitations immediately.
FDA approves generic naloxone spray for opioid overdose treatment
The Food and Drug Administration on April 19 approved the first generic naloxone hydrochloride nasal spray (Narcan) as treatment for stopping or reversing an opioid overdose.
“In the wake of the opioid crisis, a number of efforts are underway to make this emergency overdose reversal treatment more readily available and more accessible,” said Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, in a press release. “In addition to this approval of the first generic naloxone nasal spray, moving forward, we will prioritize our review of generic drug applications for naloxone.”
The agency said the naloxone nasal spray does not need assembly and can be used by anyone, regardless of medical training. If the spray is administered quickly after the overdose begins, the effect of the opioid will be countered, often within minutes. However, patients should still seek immediate medical attention.
The FDA cautioned that, when used on a patient with an opioid dependence, naloxone can cause severe opioid withdrawal, characterized by symptoms such as body aches, diarrhea, tachycardia, fever, runny nose, sneezing, goose bumps, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure.
Find the full press release on the FDA website.
The Food and Drug Administration on April 19 approved the first generic naloxone hydrochloride nasal spray (Narcan) as treatment for stopping or reversing an opioid overdose.
“In the wake of the opioid crisis, a number of efforts are underway to make this emergency overdose reversal treatment more readily available and more accessible,” said Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, in a press release. “In addition to this approval of the first generic naloxone nasal spray, moving forward, we will prioritize our review of generic drug applications for naloxone.”
The agency said the naloxone nasal spray does not need assembly and can be used by anyone, regardless of medical training. If the spray is administered quickly after the overdose begins, the effect of the opioid will be countered, often within minutes. However, patients should still seek immediate medical attention.
The FDA cautioned that, when used on a patient with an opioid dependence, naloxone can cause severe opioid withdrawal, characterized by symptoms such as body aches, diarrhea, tachycardia, fever, runny nose, sneezing, goose bumps, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure.
Find the full press release on the FDA website.
The Food and Drug Administration on April 19 approved the first generic naloxone hydrochloride nasal spray (Narcan) as treatment for stopping or reversing an opioid overdose.
“In the wake of the opioid crisis, a number of efforts are underway to make this emergency overdose reversal treatment more readily available and more accessible,” said Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, in a press release. “In addition to this approval of the first generic naloxone nasal spray, moving forward, we will prioritize our review of generic drug applications for naloxone.”
The agency said the naloxone nasal spray does not need assembly and can be used by anyone, regardless of medical training. If the spray is administered quickly after the overdose begins, the effect of the opioid will be countered, often within minutes. However, patients should still seek immediate medical attention.
The FDA cautioned that, when used on a patient with an opioid dependence, naloxone can cause severe opioid withdrawal, characterized by symptoms such as body aches, diarrhea, tachycardia, fever, runny nose, sneezing, goose bumps, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure.
Find the full press release on the FDA website.
Postvaccination febrile seizures are no more severe than other febrile seizures
according to a study in Pediatrics.
Lucy Deng, MBBS, of the University of Sydney and her colleagues investigated 1,022 index febrile seizures in children aged 6 years or less, of which 6% (n = 67) were VP-FSs and 94% (n = 955) were NVP-FSs. Both univariate and multivariate analyses showed no increased risk of severe seizure associated with VP-FSs, compared with NVP-FS. Most of the febrile seizures of either type were brief (15 minutes or less) and had a length of stay of 1 day or less; there also were no differences in 24-hour recurrence. The most common symptom was respiratory, and the rates were similar in each group (62.7% with VP-FS vs. 62.8% with NVP-FS). In keeping with a known 100% increased risk associated with measles vaccination, 84% of VP-FSs were associated with measles-containing vaccines. The majority of the remaining VP-FSs occurred after combination vaccines.
One limitation is that, because these cases were documented in sentinel tertiary pediatric hospitals, the case ascertainment may not be representative. Also, the small proportion of VP-FSs and limited cohort size means the study may not have been powered to detect true differences in prolonged seizures between the groups, Dr. Deng and her colleagues wrote.
“This study confirms that VP-FSs are clinically not any different from NVP-FSs and should be managed the same way,” the researchers concluded.
The authors reported no relevant financial disclosures, although Dr. Deng is supported by the University of Sydney Training Program scholarship, and two other study authors are supported by Australian National Health and Medical Research Council Career Development Fellowships. The study was funded by a grant from the Australian Government Department of Health and the National Health and Medical Research Council.
SOURCE: Deng L et al. Pediatrics. 2019 Apr 19. doi: 10.1542/peds.2018-2120.
according to a study in Pediatrics.
Lucy Deng, MBBS, of the University of Sydney and her colleagues investigated 1,022 index febrile seizures in children aged 6 years or less, of which 6% (n = 67) were VP-FSs and 94% (n = 955) were NVP-FSs. Both univariate and multivariate analyses showed no increased risk of severe seizure associated with VP-FSs, compared with NVP-FS. Most of the febrile seizures of either type were brief (15 minutes or less) and had a length of stay of 1 day or less; there also were no differences in 24-hour recurrence. The most common symptom was respiratory, and the rates were similar in each group (62.7% with VP-FS vs. 62.8% with NVP-FS). In keeping with a known 100% increased risk associated with measles vaccination, 84% of VP-FSs were associated with measles-containing vaccines. The majority of the remaining VP-FSs occurred after combination vaccines.
One limitation is that, because these cases were documented in sentinel tertiary pediatric hospitals, the case ascertainment may not be representative. Also, the small proportion of VP-FSs and limited cohort size means the study may not have been powered to detect true differences in prolonged seizures between the groups, Dr. Deng and her colleagues wrote.
“This study confirms that VP-FSs are clinically not any different from NVP-FSs and should be managed the same way,” the researchers concluded.
The authors reported no relevant financial disclosures, although Dr. Deng is supported by the University of Sydney Training Program scholarship, and two other study authors are supported by Australian National Health and Medical Research Council Career Development Fellowships. The study was funded by a grant from the Australian Government Department of Health and the National Health and Medical Research Council.
SOURCE: Deng L et al. Pediatrics. 2019 Apr 19. doi: 10.1542/peds.2018-2120.
according to a study in Pediatrics.
Lucy Deng, MBBS, of the University of Sydney and her colleagues investigated 1,022 index febrile seizures in children aged 6 years or less, of which 6% (n = 67) were VP-FSs and 94% (n = 955) were NVP-FSs. Both univariate and multivariate analyses showed no increased risk of severe seizure associated with VP-FSs, compared with NVP-FS. Most of the febrile seizures of either type were brief (15 minutes or less) and had a length of stay of 1 day or less; there also were no differences in 24-hour recurrence. The most common symptom was respiratory, and the rates were similar in each group (62.7% with VP-FS vs. 62.8% with NVP-FS). In keeping with a known 100% increased risk associated with measles vaccination, 84% of VP-FSs were associated with measles-containing vaccines. The majority of the remaining VP-FSs occurred after combination vaccines.
One limitation is that, because these cases were documented in sentinel tertiary pediatric hospitals, the case ascertainment may not be representative. Also, the small proportion of VP-FSs and limited cohort size means the study may not have been powered to detect true differences in prolonged seizures between the groups, Dr. Deng and her colleagues wrote.
“This study confirms that VP-FSs are clinically not any different from NVP-FSs and should be managed the same way,” the researchers concluded.
The authors reported no relevant financial disclosures, although Dr. Deng is supported by the University of Sydney Training Program scholarship, and two other study authors are supported by Australian National Health and Medical Research Council Career Development Fellowships. The study was funded by a grant from the Australian Government Department of Health and the National Health and Medical Research Council.
SOURCE: Deng L et al. Pediatrics. 2019 Apr 19. doi: 10.1542/peds.2018-2120.
FROM PEDIATRICS
Can immune checkpoint inhibitors treat PML?
investigators reported in the New England Journal of Medicine.
Three research teams described 10 cases in which patients with PML received pembrolizumab or nivolumab.
In one study, researchers administered pembrolizumab to eight adults with PML. Five patients had clinical improvement or stabilization, whereas 3 patients did not. Among the patients with clinical improvement, treatment led to reduced JC viral load in cerebrospinal fluid (CSF) and increased CD4+ and CD8+ anti–JC virus activity in vitro. Among patients without clinical improvement, treatment did not meaningfully change viral load or antiviral cellular immune response.
In a separate letter, researchers in Germany described an additional patient with PML who had clinical stabilization and no disease progression on MRI after treatment with pembrolizumab.
In another letter, researchers in France described a patient with PML whose condition improved after treatment with nivolumab.
“Do pembrolizumab and nivolumab fit the bill for treatment of PML? The current reports are encouraging but suggest that the presence of JC virus–specific T cells in the blood is a prerequisite for their use,” said Igor J. Koralnik, MD, of the department of neurological sciences at Rush University Medical Center in Chicago, in an accompanying editorial. “A controlled trial may be needed to determine whether immune checkpoint inhibitors are indeed able to keep JC virus in check in patients with PML.”
Reinvigorating T cells
Both monoclonal antibodies target programmed cell death protein 1 (PD-1), which inhibits T-cell proliferation and cytokine production when it binds its associated ligand, Dr. Koralnik said. Pembrolizumab and nivolumab block this inhibition and have been used to spur T-cell activity against tumors in patients with cancer.
PML, an often fatal brain infection caused by the JC virus in patients with immunosuppression, has no specific treatment. Management hinges on “recovery of the immune system, either by treating the underlying cause of immunosuppression or by discontinuing the use of immunosuppressive medications,” said Dr. Koralnik.
Pembrolizumab
Prior studies have found that PD-1 expression is elevated on T lymphocytes of patients with PML. To determine whether PD-1 blockade with pembrolizumab reinvigorates anti–JC virus immune activity in patients with PML, Irene Cortese, MD, of the National Institutes of Health’s Neuroimmunology Clinic and her research colleagues administered pembrolizumab at a dose of 2 mg/kg of body weight every 4-6 weeks to eight adults with PML. The patients received 1-3 doses, and each patient had a different underlying condition.
In all patients, treatment induced down-regulation of PD-1 expression on lymphocytes in CSF and peripheral blood, and five of the eight patients had clinical stabilization or improvement. Of the other three patients who did not improve, one had stabilized prior to treatment and remained stable. The other two patients died from PML.
Additional reports
Separately, Sebastian Rauer, MD, of Albert Ludwigs University in Freiburg, Germany, and his colleagues reported that a patient with PML whose symptoms culminated in mutism in February 2018 began speaking again after receiving five infusions of pembrolizumab over 10 weeks. “In addition, the size and number of lesions on MRI decreased, and JCV was no longer detectable in CSF,” Dr. Rauer and his colleagues wrote. “The patient has remained stable as of the end of March 2019, with persistent but abating psychomotor slowing, aphasia, and disorientation.”
Finally, Ondine Walter, of Toulouse (France) University Hospital and colleagues described the case of a 60-year-old woman with PML who received nivolumab on a compassionate-use basis. Two weeks after treatment, JC viral load in CSF and blood had decreased. “Starting 8 weeks after the initiation of nivolumab therapy, the patient’s neurologic symptoms and signs stabilized, and subsequently she showed improved alertness, and the ptosis and hemiplegia abated.”
Reason for caution
Prior studies, however, give reasons for caution when considering the potential use of immune checkpoint inhibitors to treat PML, Dr. Koralnik noted. In one case, a patient developed an inflammatory form of PML known as immune reconstitution inflammatory syndrome after receiving nivolumab (J Neurovirol. 2019 March 12. doi: 10.1007/s13365-019-00738-x). In addition, researchers have reported a case of PML that occurred after 1 year of nivolumab treatment, and four cases of PML related to nivolumab have been reported in pharmacovigilance databases (Emerg Infect Dis. 2018;24:1594-6). The cost and safety profiles of the medications also may be considerations, Dr. Koralnik said.
The study by Dr. Cortese and colleagues was funded by the National Institutes of Health, and the authors had no relevant disclosures. Some of the research letter authors disclosed grants and personal fees from pharmaceutical companies.
SOURCES: Cortese I et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMoa1815039; Rauer S et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1817193; Walter O et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1816198; Koralnik IJ. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMe1904140.
investigators reported in the New England Journal of Medicine.
Three research teams described 10 cases in which patients with PML received pembrolizumab or nivolumab.
In one study, researchers administered pembrolizumab to eight adults with PML. Five patients had clinical improvement or stabilization, whereas 3 patients did not. Among the patients with clinical improvement, treatment led to reduced JC viral load in cerebrospinal fluid (CSF) and increased CD4+ and CD8+ anti–JC virus activity in vitro. Among patients without clinical improvement, treatment did not meaningfully change viral load or antiviral cellular immune response.
In a separate letter, researchers in Germany described an additional patient with PML who had clinical stabilization and no disease progression on MRI after treatment with pembrolizumab.
In another letter, researchers in France described a patient with PML whose condition improved after treatment with nivolumab.
“Do pembrolizumab and nivolumab fit the bill for treatment of PML? The current reports are encouraging but suggest that the presence of JC virus–specific T cells in the blood is a prerequisite for their use,” said Igor J. Koralnik, MD, of the department of neurological sciences at Rush University Medical Center in Chicago, in an accompanying editorial. “A controlled trial may be needed to determine whether immune checkpoint inhibitors are indeed able to keep JC virus in check in patients with PML.”
Reinvigorating T cells
Both monoclonal antibodies target programmed cell death protein 1 (PD-1), which inhibits T-cell proliferation and cytokine production when it binds its associated ligand, Dr. Koralnik said. Pembrolizumab and nivolumab block this inhibition and have been used to spur T-cell activity against tumors in patients with cancer.
PML, an often fatal brain infection caused by the JC virus in patients with immunosuppression, has no specific treatment. Management hinges on “recovery of the immune system, either by treating the underlying cause of immunosuppression or by discontinuing the use of immunosuppressive medications,” said Dr. Koralnik.
Pembrolizumab
Prior studies have found that PD-1 expression is elevated on T lymphocytes of patients with PML. To determine whether PD-1 blockade with pembrolizumab reinvigorates anti–JC virus immune activity in patients with PML, Irene Cortese, MD, of the National Institutes of Health’s Neuroimmunology Clinic and her research colleagues administered pembrolizumab at a dose of 2 mg/kg of body weight every 4-6 weeks to eight adults with PML. The patients received 1-3 doses, and each patient had a different underlying condition.
In all patients, treatment induced down-regulation of PD-1 expression on lymphocytes in CSF and peripheral blood, and five of the eight patients had clinical stabilization or improvement. Of the other three patients who did not improve, one had stabilized prior to treatment and remained stable. The other two patients died from PML.
Additional reports
Separately, Sebastian Rauer, MD, of Albert Ludwigs University in Freiburg, Germany, and his colleagues reported that a patient with PML whose symptoms culminated in mutism in February 2018 began speaking again after receiving five infusions of pembrolizumab over 10 weeks. “In addition, the size and number of lesions on MRI decreased, and JCV was no longer detectable in CSF,” Dr. Rauer and his colleagues wrote. “The patient has remained stable as of the end of March 2019, with persistent but abating psychomotor slowing, aphasia, and disorientation.”
Finally, Ondine Walter, of Toulouse (France) University Hospital and colleagues described the case of a 60-year-old woman with PML who received nivolumab on a compassionate-use basis. Two weeks after treatment, JC viral load in CSF and blood had decreased. “Starting 8 weeks after the initiation of nivolumab therapy, the patient’s neurologic symptoms and signs stabilized, and subsequently she showed improved alertness, and the ptosis and hemiplegia abated.”
Reason for caution
Prior studies, however, give reasons for caution when considering the potential use of immune checkpoint inhibitors to treat PML, Dr. Koralnik noted. In one case, a patient developed an inflammatory form of PML known as immune reconstitution inflammatory syndrome after receiving nivolumab (J Neurovirol. 2019 March 12. doi: 10.1007/s13365-019-00738-x). In addition, researchers have reported a case of PML that occurred after 1 year of nivolumab treatment, and four cases of PML related to nivolumab have been reported in pharmacovigilance databases (Emerg Infect Dis. 2018;24:1594-6). The cost and safety profiles of the medications also may be considerations, Dr. Koralnik said.
The study by Dr. Cortese and colleagues was funded by the National Institutes of Health, and the authors had no relevant disclosures. Some of the research letter authors disclosed grants and personal fees from pharmaceutical companies.
SOURCES: Cortese I et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMoa1815039; Rauer S et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1817193; Walter O et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1816198; Koralnik IJ. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMe1904140.
investigators reported in the New England Journal of Medicine.
Three research teams described 10 cases in which patients with PML received pembrolizumab or nivolumab.
In one study, researchers administered pembrolizumab to eight adults with PML. Five patients had clinical improvement or stabilization, whereas 3 patients did not. Among the patients with clinical improvement, treatment led to reduced JC viral load in cerebrospinal fluid (CSF) and increased CD4+ and CD8+ anti–JC virus activity in vitro. Among patients without clinical improvement, treatment did not meaningfully change viral load or antiviral cellular immune response.
In a separate letter, researchers in Germany described an additional patient with PML who had clinical stabilization and no disease progression on MRI after treatment with pembrolizumab.
In another letter, researchers in France described a patient with PML whose condition improved after treatment with nivolumab.
“Do pembrolizumab and nivolumab fit the bill for treatment of PML? The current reports are encouraging but suggest that the presence of JC virus–specific T cells in the blood is a prerequisite for their use,” said Igor J. Koralnik, MD, of the department of neurological sciences at Rush University Medical Center in Chicago, in an accompanying editorial. “A controlled trial may be needed to determine whether immune checkpoint inhibitors are indeed able to keep JC virus in check in patients with PML.”
Reinvigorating T cells
Both monoclonal antibodies target programmed cell death protein 1 (PD-1), which inhibits T-cell proliferation and cytokine production when it binds its associated ligand, Dr. Koralnik said. Pembrolizumab and nivolumab block this inhibition and have been used to spur T-cell activity against tumors in patients with cancer.
PML, an often fatal brain infection caused by the JC virus in patients with immunosuppression, has no specific treatment. Management hinges on “recovery of the immune system, either by treating the underlying cause of immunosuppression or by discontinuing the use of immunosuppressive medications,” said Dr. Koralnik.
Pembrolizumab
Prior studies have found that PD-1 expression is elevated on T lymphocytes of patients with PML. To determine whether PD-1 blockade with pembrolizumab reinvigorates anti–JC virus immune activity in patients with PML, Irene Cortese, MD, of the National Institutes of Health’s Neuroimmunology Clinic and her research colleagues administered pembrolizumab at a dose of 2 mg/kg of body weight every 4-6 weeks to eight adults with PML. The patients received 1-3 doses, and each patient had a different underlying condition.
In all patients, treatment induced down-regulation of PD-1 expression on lymphocytes in CSF and peripheral blood, and five of the eight patients had clinical stabilization or improvement. Of the other three patients who did not improve, one had stabilized prior to treatment and remained stable. The other two patients died from PML.
Additional reports
Separately, Sebastian Rauer, MD, of Albert Ludwigs University in Freiburg, Germany, and his colleagues reported that a patient with PML whose symptoms culminated in mutism in February 2018 began speaking again after receiving five infusions of pembrolizumab over 10 weeks. “In addition, the size and number of lesions on MRI decreased, and JCV was no longer detectable in CSF,” Dr. Rauer and his colleagues wrote. “The patient has remained stable as of the end of March 2019, with persistent but abating psychomotor slowing, aphasia, and disorientation.”
Finally, Ondine Walter, of Toulouse (France) University Hospital and colleagues described the case of a 60-year-old woman with PML who received nivolumab on a compassionate-use basis. Two weeks after treatment, JC viral load in CSF and blood had decreased. “Starting 8 weeks after the initiation of nivolumab therapy, the patient’s neurologic symptoms and signs stabilized, and subsequently she showed improved alertness, and the ptosis and hemiplegia abated.”
Reason for caution
Prior studies, however, give reasons for caution when considering the potential use of immune checkpoint inhibitors to treat PML, Dr. Koralnik noted. In one case, a patient developed an inflammatory form of PML known as immune reconstitution inflammatory syndrome after receiving nivolumab (J Neurovirol. 2019 March 12. doi: 10.1007/s13365-019-00738-x). In addition, researchers have reported a case of PML that occurred after 1 year of nivolumab treatment, and four cases of PML related to nivolumab have been reported in pharmacovigilance databases (Emerg Infect Dis. 2018;24:1594-6). The cost and safety profiles of the medications also may be considerations, Dr. Koralnik said.
The study by Dr. Cortese and colleagues was funded by the National Institutes of Health, and the authors had no relevant disclosures. Some of the research letter authors disclosed grants and personal fees from pharmaceutical companies.
SOURCES: Cortese I et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMoa1815039; Rauer S et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1817193; Walter O et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1816198; Koralnik IJ. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMe1904140.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE