User login
Extended Holter screening finds A-fib faster after ischemic strokes
MONTREAL – Enhanced and prolonged rhythm monitoring for atrial fibrillation in patients with a recent acute ischemic stroke did not find more arrhythmias, it just found them faster, in a randomized study with 398 German patients.
“Prolonged and enhanced monitoring identified atrial fibrillation cases that otherwise were detected years later,” Rolf Wachter, MD, said at the World Stroke Congress. Enhanced and prolonged monitoring (EPM) “should be considered for all stroke patients, regardless of the suspected stroke etiology, if detection of atrial fibrillation is of clinical relevance,” said Dr. Wachter, a cardiologist and professor at the University Clinic in Leipzig, Germany.
Based on 3-year follow-up of patients enrolled in the FIND-AF study, which randomized patients within 7 days of an acute ischemic stroke to either EPM for atrial fibrillation (AF) or standard work-up and follow-up, Dr. Wachter calculated that every six such patients who underwent EMP for AF yielded one added patient who could receive anticoagulant prophylaxis for 1 year, an effect that should result in fewer incident strokes and deaths. The data he reported showed after 3 years a “favorable trend” toward fewer strokes and deaths among patients who underwent EPM.
FIND-AF RANDOMISED (A Prospective, Randomised, Controlled Study to Determine the Detection of Atrial Fibrillation by Prolonged and Enhanced Holter Monitoring as Compared to Usual Care in Stroke Patients) ran at four German centers during May 2013–August 2014. It enrolled 398 patients aged 60 years or older within 7 days of an acute ischemic stroke who were in sinus rhythm and had no AF history. Enrolled patients could have any type of suspected stroke etiology, but the study excluded patients with severe stenosis in their ipsilateral carotid or intracranial arteries. The study randomized patients to received EPM or a standard work-up. The “enhanced” part of EPM meant review of Holter monitor recordings by a single, dedicated core laboratory. The “prolonged” part meant routinely screening patients for an atrial arrhythmia using a Holter monitor worn for 10 consecutive days on three occasions: at entry into the study, 3 months later, and 6 months later.
The study’s primary endpoint was the number of patients diagnosed with AF after 6 months, which was 27 of 200 patients (13.5%) in the EPM arm and 9 of 198 patients (4.5%) in the control arm, a statistically significant difference, Dr. Wachter and his associates reported in Lancet Neurology (2017 Apr 1;16[4]:282-90).
The additional 30 months of follow-up included in the new report by Dr. Wachter resulted in identification of 3 more patients with AF in the EPM group and 13 more patients in the control arm, which brought the total number of patients with AF identified over 3 years to 30 in the EPM group (15%), and 22 in the control group (11%), a difference that was not statistically significant. In other words, both approaches found roughly the same percentage of patients with AF, but the EPM method found them quicker.
During the extended 36-month follow-up, 12 patients in the EPM group had an ischemic stroke and 9 patients died, with a combined stroke and death rate of about 8%. In the control group, 19 patients had a second ischemic stroke and 13 died, with a combined rate of about 15%. A statistical test of the difference between the combined stroke and death rates in these two groups produced a P value of .08.
FIND-AF was funded by Boehringer Ingelheim. Dr. Wachter has been a speaker on behalf of and has received research funding from Boehringer Ingelheim, and he has also been a speaker on behalf of Bayer, BMS/Pfizer, and Daiichi Sankyo.
SOURCE: Wachter R et al. World Stroke Congress, Abstract.
MONTREAL – Enhanced and prolonged rhythm monitoring for atrial fibrillation in patients with a recent acute ischemic stroke did not find more arrhythmias, it just found them faster, in a randomized study with 398 German patients.
“Prolonged and enhanced monitoring identified atrial fibrillation cases that otherwise were detected years later,” Rolf Wachter, MD, said at the World Stroke Congress. Enhanced and prolonged monitoring (EPM) “should be considered for all stroke patients, regardless of the suspected stroke etiology, if detection of atrial fibrillation is of clinical relevance,” said Dr. Wachter, a cardiologist and professor at the University Clinic in Leipzig, Germany.
Based on 3-year follow-up of patients enrolled in the FIND-AF study, which randomized patients within 7 days of an acute ischemic stroke to either EPM for atrial fibrillation (AF) or standard work-up and follow-up, Dr. Wachter calculated that every six such patients who underwent EMP for AF yielded one added patient who could receive anticoagulant prophylaxis for 1 year, an effect that should result in fewer incident strokes and deaths. The data he reported showed after 3 years a “favorable trend” toward fewer strokes and deaths among patients who underwent EPM.
FIND-AF RANDOMISED (A Prospective, Randomised, Controlled Study to Determine the Detection of Atrial Fibrillation by Prolonged and Enhanced Holter Monitoring as Compared to Usual Care in Stroke Patients) ran at four German centers during May 2013–August 2014. It enrolled 398 patients aged 60 years or older within 7 days of an acute ischemic stroke who were in sinus rhythm and had no AF history. Enrolled patients could have any type of suspected stroke etiology, but the study excluded patients with severe stenosis in their ipsilateral carotid or intracranial arteries. The study randomized patients to received EPM or a standard work-up. The “enhanced” part of EPM meant review of Holter monitor recordings by a single, dedicated core laboratory. The “prolonged” part meant routinely screening patients for an atrial arrhythmia using a Holter monitor worn for 10 consecutive days on three occasions: at entry into the study, 3 months later, and 6 months later.
The study’s primary endpoint was the number of patients diagnosed with AF after 6 months, which was 27 of 200 patients (13.5%) in the EPM arm and 9 of 198 patients (4.5%) in the control arm, a statistically significant difference, Dr. Wachter and his associates reported in Lancet Neurology (2017 Apr 1;16[4]:282-90).
The additional 30 months of follow-up included in the new report by Dr. Wachter resulted in identification of 3 more patients with AF in the EPM group and 13 more patients in the control arm, which brought the total number of patients with AF identified over 3 years to 30 in the EPM group (15%), and 22 in the control group (11%), a difference that was not statistically significant. In other words, both approaches found roughly the same percentage of patients with AF, but the EPM method found them quicker.
During the extended 36-month follow-up, 12 patients in the EPM group had an ischemic stroke and 9 patients died, with a combined stroke and death rate of about 8%. In the control group, 19 patients had a second ischemic stroke and 13 died, with a combined rate of about 15%. A statistical test of the difference between the combined stroke and death rates in these two groups produced a P value of .08.
FIND-AF was funded by Boehringer Ingelheim. Dr. Wachter has been a speaker on behalf of and has received research funding from Boehringer Ingelheim, and he has also been a speaker on behalf of Bayer, BMS/Pfizer, and Daiichi Sankyo.
SOURCE: Wachter R et al. World Stroke Congress, Abstract.
MONTREAL – Enhanced and prolonged rhythm monitoring for atrial fibrillation in patients with a recent acute ischemic stroke did not find more arrhythmias, it just found them faster, in a randomized study with 398 German patients.
“Prolonged and enhanced monitoring identified atrial fibrillation cases that otherwise were detected years later,” Rolf Wachter, MD, said at the World Stroke Congress. Enhanced and prolonged monitoring (EPM) “should be considered for all stroke patients, regardless of the suspected stroke etiology, if detection of atrial fibrillation is of clinical relevance,” said Dr. Wachter, a cardiologist and professor at the University Clinic in Leipzig, Germany.
Based on 3-year follow-up of patients enrolled in the FIND-AF study, which randomized patients within 7 days of an acute ischemic stroke to either EPM for atrial fibrillation (AF) or standard work-up and follow-up, Dr. Wachter calculated that every six such patients who underwent EMP for AF yielded one added patient who could receive anticoagulant prophylaxis for 1 year, an effect that should result in fewer incident strokes and deaths. The data he reported showed after 3 years a “favorable trend” toward fewer strokes and deaths among patients who underwent EPM.
FIND-AF RANDOMISED (A Prospective, Randomised, Controlled Study to Determine the Detection of Atrial Fibrillation by Prolonged and Enhanced Holter Monitoring as Compared to Usual Care in Stroke Patients) ran at four German centers during May 2013–August 2014. It enrolled 398 patients aged 60 years or older within 7 days of an acute ischemic stroke who were in sinus rhythm and had no AF history. Enrolled patients could have any type of suspected stroke etiology, but the study excluded patients with severe stenosis in their ipsilateral carotid or intracranial arteries. The study randomized patients to received EPM or a standard work-up. The “enhanced” part of EPM meant review of Holter monitor recordings by a single, dedicated core laboratory. The “prolonged” part meant routinely screening patients for an atrial arrhythmia using a Holter monitor worn for 10 consecutive days on three occasions: at entry into the study, 3 months later, and 6 months later.
The study’s primary endpoint was the number of patients diagnosed with AF after 6 months, which was 27 of 200 patients (13.5%) in the EPM arm and 9 of 198 patients (4.5%) in the control arm, a statistically significant difference, Dr. Wachter and his associates reported in Lancet Neurology (2017 Apr 1;16[4]:282-90).
The additional 30 months of follow-up included in the new report by Dr. Wachter resulted in identification of 3 more patients with AF in the EPM group and 13 more patients in the control arm, which brought the total number of patients with AF identified over 3 years to 30 in the EPM group (15%), and 22 in the control group (11%), a difference that was not statistically significant. In other words, both approaches found roughly the same percentage of patients with AF, but the EPM method found them quicker.
During the extended 36-month follow-up, 12 patients in the EPM group had an ischemic stroke and 9 patients died, with a combined stroke and death rate of about 8%. In the control group, 19 patients had a second ischemic stroke and 13 died, with a combined rate of about 15%. A statistical test of the difference between the combined stroke and death rates in these two groups produced a P value of .08.
FIND-AF was funded by Boehringer Ingelheim. Dr. Wachter has been a speaker on behalf of and has received research funding from Boehringer Ingelheim, and he has also been a speaker on behalf of Bayer, BMS/Pfizer, and Daiichi Sankyo.
SOURCE: Wachter R et al. World Stroke Congress, Abstract.
REPORTING FROM THE WORLD STROKE CONGRESS
Key clinical point: Enhanced and prolonged monitoring for atrial fibrillation did not find more arrhythmias, but it did find them faster.
Major finding: Every six patients who underwent extended arrhythmia screening produced one additional patient eligible for a year of anticoagulant prophylaxis.
Study details: Three-year follow-up of FIND-AF, a multicenter, German study with 398 patients.
Disclosures: FIND-AF was funded by Boehringer Ingelheim. Dr. Wachter has been a speaker on behalf of and has received research funding from Boehringer Ingelheim, and he has also been a speaker on behalf of Bayer, BMS/Pfizer, and Daiichi Sankyo.
Source: Wachter R et al. World Stroke Congress.
How often is AED treatment delayed for patients with epilepsy?
NEW ORLEANS – , according to an Australian study presented at the annual meeting of the American Epilepsy Society. Most untreated patients begin an AED after experiencing subsequent seizures, however.
“The decision to start or withhold treatment reflects the complex interplay between factors perceived to influence the predicted risk of seizure recurrence, which remain imprecise, and personal factors,” said lead study author Zhibin Chen, PhD, a biostatistician at the University of Melbourne and colleagues.
Many patients with epilepsy in resource-poor countries may not receive AED therapy for socioeconomic reasons, but little is known about untreated epilepsy in high-income countries. To assess the extent of and reasons for patients not receiving AEDs when treatment is accessible and affordable, Dr. Chen and colleagues prospectively recruited adult patients who attended the first-seizure clinics of publicly funded hospitals in Western Australia between May 1, 1999, and May 31, 2016. The patients had new-onset seizures and were referred by primary care or emergency department physicians. The health care system provided universal coverage for patients’ hospital admissions, outpatient visits, investigations, and treatment.
The researchers identified patients with newly diagnosed epilepsy and reviewed medical records to determine the proportion of untreated patients and the reasons for not starting treatment at each follow-up visit. The investigators compared the sociodemographic factors, neuroimaging, and EEG findings of treated and untreated patients.
In all, 1,317 people attended the clinics during the study period, and 610 patients (61% male; median age, 40) received a diagnosis of epilepsy and met 2014 International League Against Epilepsy (ILAE) diagnostic criteria for epilepsy. Patients were followed for a median of 5.7 years.
Of the 610 patients with epilepsy, 31% did not start AED treatment at the time of diagnosis – 16.4% because the neurologist did not recommend treatment and 14.6% because the patient declined treatment despite a neurologist’s recommendation to start therapy.
Patients’ reasons for not starting treatment included doubts about the need for treatment or about the epilepsy diagnosis, as well as concerns about medication side effects. Neurologists’ reasons for not beginning treatment included a patient having only one seizure and awaiting further results. The presence of seizure-precipitating factors (e.g., flashing lights, sleep deprivation, stress, or alcohol use) was another reason that patients and neurologists commonly cited for not initiating treatment.
Among the 189 initially untreated patients, 62.4% started treatment after a median delay of 95 days, “mainly after further seizures,” the investigators said. Patients with epilepsy who were older, from lower socioeconomic areas, had experienced more seizures, or had epileptogenic lesions on neuroimaging were more likely to initiate AED treatment at diagnosis.
“The percentage of people who were not initially prescribed AEDs was much higher than expected and suggests that untreated epilepsy exists not just in resource-poor, but also in wealthy countries,” said Dr. Chen.
More research is needed to assess the long-term outcomes of patient with seizure-precipitating factors who initiate AEDs immediately, compared with those who try avoidance of precipitating factors alone, said Dr. Chen.
This study was supported by a grant from UCB Pharma.
SOURCE: Chen Z et al. AES 2018, Abstract 3.421.
NEW ORLEANS – , according to an Australian study presented at the annual meeting of the American Epilepsy Society. Most untreated patients begin an AED after experiencing subsequent seizures, however.
“The decision to start or withhold treatment reflects the complex interplay between factors perceived to influence the predicted risk of seizure recurrence, which remain imprecise, and personal factors,” said lead study author Zhibin Chen, PhD, a biostatistician at the University of Melbourne and colleagues.
Many patients with epilepsy in resource-poor countries may not receive AED therapy for socioeconomic reasons, but little is known about untreated epilepsy in high-income countries. To assess the extent of and reasons for patients not receiving AEDs when treatment is accessible and affordable, Dr. Chen and colleagues prospectively recruited adult patients who attended the first-seizure clinics of publicly funded hospitals in Western Australia between May 1, 1999, and May 31, 2016. The patients had new-onset seizures and were referred by primary care or emergency department physicians. The health care system provided universal coverage for patients’ hospital admissions, outpatient visits, investigations, and treatment.
The researchers identified patients with newly diagnosed epilepsy and reviewed medical records to determine the proportion of untreated patients and the reasons for not starting treatment at each follow-up visit. The investigators compared the sociodemographic factors, neuroimaging, and EEG findings of treated and untreated patients.
In all, 1,317 people attended the clinics during the study period, and 610 patients (61% male; median age, 40) received a diagnosis of epilepsy and met 2014 International League Against Epilepsy (ILAE) diagnostic criteria for epilepsy. Patients were followed for a median of 5.7 years.
Of the 610 patients with epilepsy, 31% did not start AED treatment at the time of diagnosis – 16.4% because the neurologist did not recommend treatment and 14.6% because the patient declined treatment despite a neurologist’s recommendation to start therapy.
Patients’ reasons for not starting treatment included doubts about the need for treatment or about the epilepsy diagnosis, as well as concerns about medication side effects. Neurologists’ reasons for not beginning treatment included a patient having only one seizure and awaiting further results. The presence of seizure-precipitating factors (e.g., flashing lights, sleep deprivation, stress, or alcohol use) was another reason that patients and neurologists commonly cited for not initiating treatment.
Among the 189 initially untreated patients, 62.4% started treatment after a median delay of 95 days, “mainly after further seizures,” the investigators said. Patients with epilepsy who were older, from lower socioeconomic areas, had experienced more seizures, or had epileptogenic lesions on neuroimaging were more likely to initiate AED treatment at diagnosis.
“The percentage of people who were not initially prescribed AEDs was much higher than expected and suggests that untreated epilepsy exists not just in resource-poor, but also in wealthy countries,” said Dr. Chen.
More research is needed to assess the long-term outcomes of patient with seizure-precipitating factors who initiate AEDs immediately, compared with those who try avoidance of precipitating factors alone, said Dr. Chen.
This study was supported by a grant from UCB Pharma.
SOURCE: Chen Z et al. AES 2018, Abstract 3.421.
NEW ORLEANS – , according to an Australian study presented at the annual meeting of the American Epilepsy Society. Most untreated patients begin an AED after experiencing subsequent seizures, however.
“The decision to start or withhold treatment reflects the complex interplay between factors perceived to influence the predicted risk of seizure recurrence, which remain imprecise, and personal factors,” said lead study author Zhibin Chen, PhD, a biostatistician at the University of Melbourne and colleagues.
Many patients with epilepsy in resource-poor countries may not receive AED therapy for socioeconomic reasons, but little is known about untreated epilepsy in high-income countries. To assess the extent of and reasons for patients not receiving AEDs when treatment is accessible and affordable, Dr. Chen and colleagues prospectively recruited adult patients who attended the first-seizure clinics of publicly funded hospitals in Western Australia between May 1, 1999, and May 31, 2016. The patients had new-onset seizures and were referred by primary care or emergency department physicians. The health care system provided universal coverage for patients’ hospital admissions, outpatient visits, investigations, and treatment.
The researchers identified patients with newly diagnosed epilepsy and reviewed medical records to determine the proportion of untreated patients and the reasons for not starting treatment at each follow-up visit. The investigators compared the sociodemographic factors, neuroimaging, and EEG findings of treated and untreated patients.
In all, 1,317 people attended the clinics during the study period, and 610 patients (61% male; median age, 40) received a diagnosis of epilepsy and met 2014 International League Against Epilepsy (ILAE) diagnostic criteria for epilepsy. Patients were followed for a median of 5.7 years.
Of the 610 patients with epilepsy, 31% did not start AED treatment at the time of diagnosis – 16.4% because the neurologist did not recommend treatment and 14.6% because the patient declined treatment despite a neurologist’s recommendation to start therapy.
Patients’ reasons for not starting treatment included doubts about the need for treatment or about the epilepsy diagnosis, as well as concerns about medication side effects. Neurologists’ reasons for not beginning treatment included a patient having only one seizure and awaiting further results. The presence of seizure-precipitating factors (e.g., flashing lights, sleep deprivation, stress, or alcohol use) was another reason that patients and neurologists commonly cited for not initiating treatment.
Among the 189 initially untreated patients, 62.4% started treatment after a median delay of 95 days, “mainly after further seizures,” the investigators said. Patients with epilepsy who were older, from lower socioeconomic areas, had experienced more seizures, or had epileptogenic lesions on neuroimaging were more likely to initiate AED treatment at diagnosis.
“The percentage of people who were not initially prescribed AEDs was much higher than expected and suggests that untreated epilepsy exists not just in resource-poor, but also in wealthy countries,” said Dr. Chen.
More research is needed to assess the long-term outcomes of patient with seizure-precipitating factors who initiate AEDs immediately, compared with those who try avoidance of precipitating factors alone, said Dr. Chen.
This study was supported by a grant from UCB Pharma.
SOURCE: Chen Z et al. AES 2018, Abstract 3.421.
REPORTING FROM AES 2018
Key clinical point: Delayed initiation of antiepileptic drug treatment may be more common than thought.
Major finding: More than 30% of patients with epilepsy do not initiate treatment at diagnosis.
Study details: Review of 610 patients with newly diagnosed epilepsy who were seen at clinics in Western Australia.
Disclosures: The study was supported by a grant from UCB Pharma.
Source: Chen Z et al. AES 2018, Abstract 3.421.
Understanding properties of fentanyl, other opioids key to treatment
Naloxone increasingly coming up short, expert says
BONITA SPRINGS, FLA. – Treating disorders tied to the use of highly potent synthetic opioids (HPSO), such as fentanyl, is challenging at best, an expert said at the annual meeting of the American Academy of Addiction Psychiatry.
“We have essentially no data to guide pharmacotherapy management decisions for the leading cause of fatal overdose deaths in the U.S.,” said John J. Mariani, MD, associate professor of clinical psychiatry at Columbia University, New York. “In the absence of data to make evidence-based recommendations, we still need to treat patients.”
That means taking what is known about the properties of those drugs into account when making treatment decisions, he said. Fentanyl quickly crosses the blood-brain barrier and is rapidly distributed to peripheral tissue. It has a short duration of action, but its duration can be extended with multiple injections or an infusion, he said. Research suggests that it has opioid receptor affinity similar to that of morphine, and it’s not known why it is up to 100 times more potent than morphine.
From his own experience, he offered some suggestions on treating patients who use HPSOs:
- Buprenorphine: Clinicians using buprenorphine as induction treatment have to wait longer from the last use to the first dose because of its longer effective half-life, and other medications might be needed to manage withdrawal. For maintenance, higher doses possibly should be considered to prevent HPSO override and to maintain opioid tolerance.
- Extended-release naltrexone: This involves a more difficult induction, and there is a question of whether inpatient treatment is better than outpatient, he said. For maintenance, more frequent administration should be considered, with closer monitoring for the risk of override and more urine toxicology testing.
- Methadone: For induction, methadone could offer an advantage over buprenorphine, because there is no risk of precipitated withdrawal. For maintenance, Dr. Mariani said, it’s not known whether standard doses protect against raising tolerance out of the reach of HPSOs’ effects.
- Naloxone: He said there have been increasing reports of multiple doses being needed to reverse an overdose. Because of the shorter time between substance use and death with fentanyl, more reports have been filed on unsuccessful attempts to revive people with naloxone despite multiple doses or stronger doses. Some naloxone programs have been giving more than two standard doses or using devices that give higher doses, he said. Also, , he said.
Addiction psychiatrists “need to educate patients, families, and other clinicians of this new risk of using opioids,” he said.
Meanwhile, in another talk, Thomas Kosten, MD, described progress in the efforts to develop a vaccine against fentanyl addiction, in the hopes of preventing overdoses. Researchers are taking cues from the failed attempt to develop a cocaine vaccine a few years ago, in which not enough antibodies were produced in about half the patients.
This time, researchers are using toll-like receptor agonists to boost the effects of the main vaccine component, known as norcocaine. Those agonists can more than double the antibody increase that is seen without them, said Dr. Kosten, professor of psychiatry at Baylor College of Medicine, Houston.
So far, researchers have found that the vaccine produces blockade of fentanyl analgesia and respiratory depression in rats. Dr. Kosten said his lab is looking for funding to continue the research. “It looks like we’re going to have some money in February to start making the vaccine,” he said.
Dr. Mariani and Dr. Kosten reported no relevant disclosures.
Naloxone increasingly coming up short, expert says
Naloxone increasingly coming up short, expert says
BONITA SPRINGS, FLA. – Treating disorders tied to the use of highly potent synthetic opioids (HPSO), such as fentanyl, is challenging at best, an expert said at the annual meeting of the American Academy of Addiction Psychiatry.
“We have essentially no data to guide pharmacotherapy management decisions for the leading cause of fatal overdose deaths in the U.S.,” said John J. Mariani, MD, associate professor of clinical psychiatry at Columbia University, New York. “In the absence of data to make evidence-based recommendations, we still need to treat patients.”
That means taking what is known about the properties of those drugs into account when making treatment decisions, he said. Fentanyl quickly crosses the blood-brain barrier and is rapidly distributed to peripheral tissue. It has a short duration of action, but its duration can be extended with multiple injections or an infusion, he said. Research suggests that it has opioid receptor affinity similar to that of morphine, and it’s not known why it is up to 100 times more potent than morphine.
From his own experience, he offered some suggestions on treating patients who use HPSOs:
- Buprenorphine: Clinicians using buprenorphine as induction treatment have to wait longer from the last use to the first dose because of its longer effective half-life, and other medications might be needed to manage withdrawal. For maintenance, higher doses possibly should be considered to prevent HPSO override and to maintain opioid tolerance.
- Extended-release naltrexone: This involves a more difficult induction, and there is a question of whether inpatient treatment is better than outpatient, he said. For maintenance, more frequent administration should be considered, with closer monitoring for the risk of override and more urine toxicology testing.
- Methadone: For induction, methadone could offer an advantage over buprenorphine, because there is no risk of precipitated withdrawal. For maintenance, Dr. Mariani said, it’s not known whether standard doses protect against raising tolerance out of the reach of HPSOs’ effects.
- Naloxone: He said there have been increasing reports of multiple doses being needed to reverse an overdose. Because of the shorter time between substance use and death with fentanyl, more reports have been filed on unsuccessful attempts to revive people with naloxone despite multiple doses or stronger doses. Some naloxone programs have been giving more than two standard doses or using devices that give higher doses, he said. Also, , he said.
Addiction psychiatrists “need to educate patients, families, and other clinicians of this new risk of using opioids,” he said.
Meanwhile, in another talk, Thomas Kosten, MD, described progress in the efforts to develop a vaccine against fentanyl addiction, in the hopes of preventing overdoses. Researchers are taking cues from the failed attempt to develop a cocaine vaccine a few years ago, in which not enough antibodies were produced in about half the patients.
This time, researchers are using toll-like receptor agonists to boost the effects of the main vaccine component, known as norcocaine. Those agonists can more than double the antibody increase that is seen without them, said Dr. Kosten, professor of psychiatry at Baylor College of Medicine, Houston.
So far, researchers have found that the vaccine produces blockade of fentanyl analgesia and respiratory depression in rats. Dr. Kosten said his lab is looking for funding to continue the research. “It looks like we’re going to have some money in February to start making the vaccine,” he said.
Dr. Mariani and Dr. Kosten reported no relevant disclosures.
BONITA SPRINGS, FLA. – Treating disorders tied to the use of highly potent synthetic opioids (HPSO), such as fentanyl, is challenging at best, an expert said at the annual meeting of the American Academy of Addiction Psychiatry.
“We have essentially no data to guide pharmacotherapy management decisions for the leading cause of fatal overdose deaths in the U.S.,” said John J. Mariani, MD, associate professor of clinical psychiatry at Columbia University, New York. “In the absence of data to make evidence-based recommendations, we still need to treat patients.”
That means taking what is known about the properties of those drugs into account when making treatment decisions, he said. Fentanyl quickly crosses the blood-brain barrier and is rapidly distributed to peripheral tissue. It has a short duration of action, but its duration can be extended with multiple injections or an infusion, he said. Research suggests that it has opioid receptor affinity similar to that of morphine, and it’s not known why it is up to 100 times more potent than morphine.
From his own experience, he offered some suggestions on treating patients who use HPSOs:
- Buprenorphine: Clinicians using buprenorphine as induction treatment have to wait longer from the last use to the first dose because of its longer effective half-life, and other medications might be needed to manage withdrawal. For maintenance, higher doses possibly should be considered to prevent HPSO override and to maintain opioid tolerance.
- Extended-release naltrexone: This involves a more difficult induction, and there is a question of whether inpatient treatment is better than outpatient, he said. For maintenance, more frequent administration should be considered, with closer monitoring for the risk of override and more urine toxicology testing.
- Methadone: For induction, methadone could offer an advantage over buprenorphine, because there is no risk of precipitated withdrawal. For maintenance, Dr. Mariani said, it’s not known whether standard doses protect against raising tolerance out of the reach of HPSOs’ effects.
- Naloxone: He said there have been increasing reports of multiple doses being needed to reverse an overdose. Because of the shorter time between substance use and death with fentanyl, more reports have been filed on unsuccessful attempts to revive people with naloxone despite multiple doses or stronger doses. Some naloxone programs have been giving more than two standard doses or using devices that give higher doses, he said. Also, , he said.
Addiction psychiatrists “need to educate patients, families, and other clinicians of this new risk of using opioids,” he said.
Meanwhile, in another talk, Thomas Kosten, MD, described progress in the efforts to develop a vaccine against fentanyl addiction, in the hopes of preventing overdoses. Researchers are taking cues from the failed attempt to develop a cocaine vaccine a few years ago, in which not enough antibodies were produced in about half the patients.
This time, researchers are using toll-like receptor agonists to boost the effects of the main vaccine component, known as norcocaine. Those agonists can more than double the antibody increase that is seen without them, said Dr. Kosten, professor of psychiatry at Baylor College of Medicine, Houston.
So far, researchers have found that the vaccine produces blockade of fentanyl analgesia and respiratory depression in rats. Dr. Kosten said his lab is looking for funding to continue the research. “It looks like we’re going to have some money in February to start making the vaccine,” he said.
Dr. Mariani and Dr. Kosten reported no relevant disclosures.
REPORTING FROM AAAP 2018
Bioequivalents lamotrigine, levetiracetam control new-onset focal seizures equally well
NEW ORLEANS – Bioequivalent generic formulations of levetiracetam and lamotrigine reduced seizures by a similar extent over 2 years in a retrospective study of patients with newly diagnosed focal epilepsy.
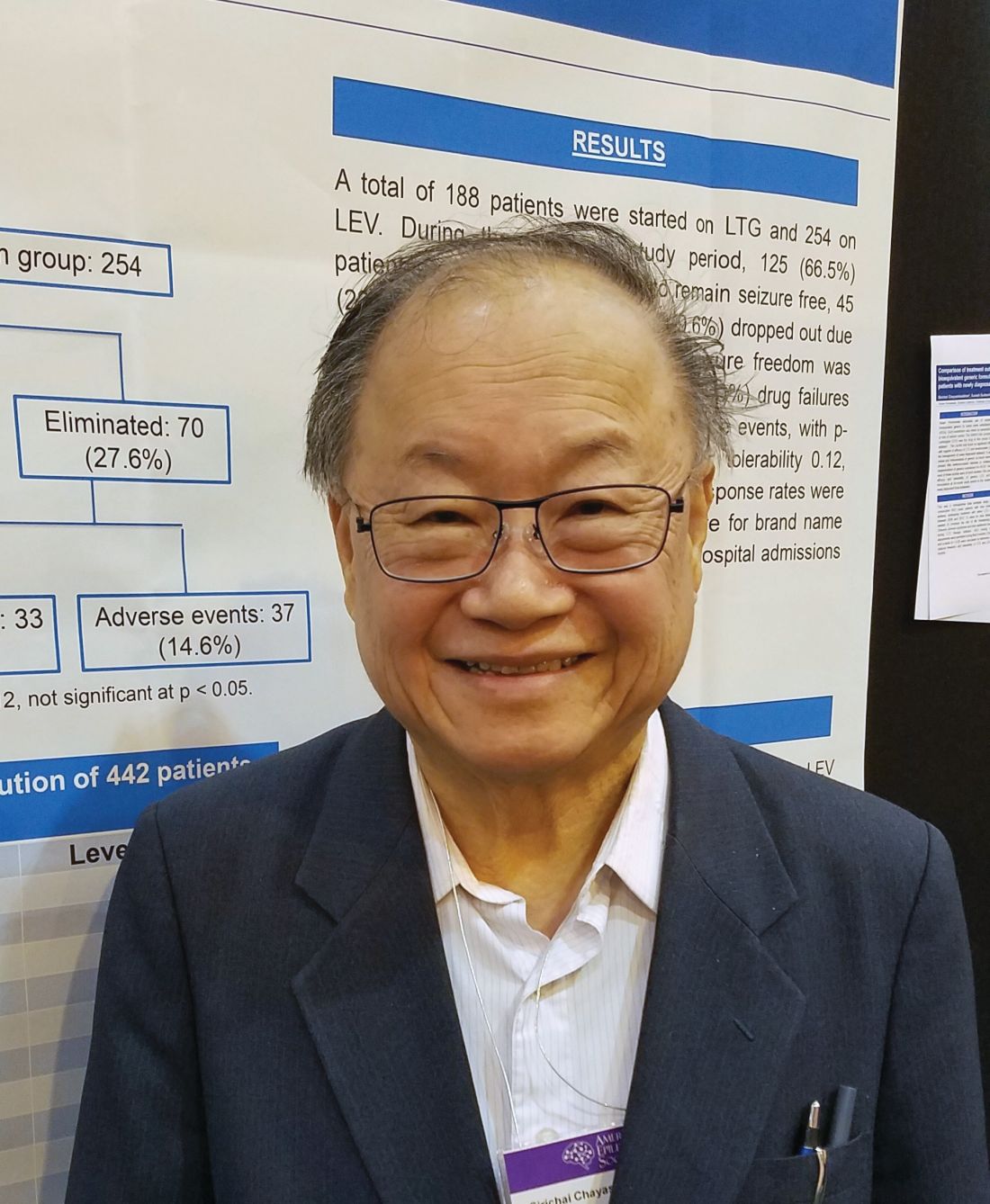
Each drug had a specific adverse event profile, with lamotrigine associated with rash and levetiracetam with mood disorders, Sirichai Chayasirisobhon, MD, said at the annual meeting of the American Epilepsy Society. This finding can play into the initial therapeutic decision, said Dr. Chayasirisobhon of Kaiser Permanente Southern California. “If someone comes in with depression or mood disorder, I will start on lamotrigine, not levetiracetam. And we can decrease the chance of rash with a very slow titration, as we did here, starting with just 5 mg/kg and working up over 6 months.”
Although the drugs have a somewhat similar teratogenic profile, Dr. Chayasirisobhon added that he favors lamotrigine for women of childbearing years. “It’s a little bit better choice for them I think.”
His retrospective analysis followed 442 patients from first seizure and medical therapy for 2 years. The generic medications came from Kaiser Permanente’s central pharmacy. They were single-source, with a proven 95% bioequivalence. The main outcome was the percentage of patients who became seizure free and remained so. Any seizure, whether febrile, breakthroughs, or from titration, was considered a failure. These patients were dropped from the study. Any patient who developed a drug-related rash was dropped from the study and started on another medication.
More women than men took lamotrigine (113 vs. 75), whereas more men took levetiracetam (148 vs. 106). Those taking lamotrigine were younger than were those taking levetiracetam (30 vs. 40 years).
At the end of 2 years, there was no statistically significant difference in the primary outcome of being free from seizures (66.5% with lamotrigine vs. 72.4% with levetiracetam). In the lamotrigine group, 33.5% were eliminated from the study, 24% because they had a seizure, and the rest due to an adverse event. In the levetiracetam group, 27.6% were eliminated, 13% because they had a seizure and the rest because of an adverse event.
Adverse events in the lamotrigine group included rash (12), dizziness (3), lethargy (1), and mood changes (2). Among the levetiracetam group, adverse events included dizziness (3), lethargy (7), mood changes (20), slowed thinking (4), depression (2) and headache (1).
“Rash was the main event we saw in this group, and this was even when we did a very slow titration of 5 mg/kg per week,” Dr. Chayasirisobhon said. “Any sign of rash or itching at all, we told them to stop immediately and call us. Fortunately, we had no cases of Steven-Johnson syndrome and all our cases of rash were transient. But in the levetiracetam group, the mood changes are the major thing. Some of the patients became very agitated and aggressive. Whenever we see a patient for the first time, we always ask about mood changes, and we instruct the family to call and report any changes in mood immediately.”
Aside from reproductive age, however, Dr. Chayasirisobhon generally prefers to start new patients on levetiracetam. Its safety profile is remarkable, he said, recounting a case report he published in 2010 (Acta Neurol Taiwan. 2010;19:292-5).
The paper describes a male patient who decided to commit suicide after an argument with his wife. He took his levetiracetam and walked to his father’s grave, swallowing pills the entire time. When he arrived at the grave, he had taken around 65 grams of the medication. “The amazing thing was, he’s still walking, just a little unsteady. Then he decided he’s not ready to die,” Dr. Chayasirisobhon said. “He was able to call 911, so he’s still talking fine. When they checked his level it was so high, but he remained unimpaired except for the unsteady gait and some nystagmus.”
The study did not receive outside funding. Dr. Chayasirisobhon had no financial disclosures.
NEW ORLEANS – Bioequivalent generic formulations of levetiracetam and lamotrigine reduced seizures by a similar extent over 2 years in a retrospective study of patients with newly diagnosed focal epilepsy.
Each drug had a specific adverse event profile, with lamotrigine associated with rash and levetiracetam with mood disorders, Sirichai Chayasirisobhon, MD, said at the annual meeting of the American Epilepsy Society. This finding can play into the initial therapeutic decision, said Dr. Chayasirisobhon of Kaiser Permanente Southern California. “If someone comes in with depression or mood disorder, I will start on lamotrigine, not levetiracetam. And we can decrease the chance of rash with a very slow titration, as we did here, starting with just 5 mg/kg and working up over 6 months.”
Although the drugs have a somewhat similar teratogenic profile, Dr. Chayasirisobhon added that he favors lamotrigine for women of childbearing years. “It’s a little bit better choice for them I think.”
His retrospective analysis followed 442 patients from first seizure and medical therapy for 2 years. The generic medications came from Kaiser Permanente’s central pharmacy. They were single-source, with a proven 95% bioequivalence. The main outcome was the percentage of patients who became seizure free and remained so. Any seizure, whether febrile, breakthroughs, or from titration, was considered a failure. These patients were dropped from the study. Any patient who developed a drug-related rash was dropped from the study and started on another medication.
More women than men took lamotrigine (113 vs. 75), whereas more men took levetiracetam (148 vs. 106). Those taking lamotrigine were younger than were those taking levetiracetam (30 vs. 40 years).
At the end of 2 years, there was no statistically significant difference in the primary outcome of being free from seizures (66.5% with lamotrigine vs. 72.4% with levetiracetam). In the lamotrigine group, 33.5% were eliminated from the study, 24% because they had a seizure, and the rest due to an adverse event. In the levetiracetam group, 27.6% were eliminated, 13% because they had a seizure and the rest because of an adverse event.
Adverse events in the lamotrigine group included rash (12), dizziness (3), lethargy (1), and mood changes (2). Among the levetiracetam group, adverse events included dizziness (3), lethargy (7), mood changes (20), slowed thinking (4), depression (2) and headache (1).
“Rash was the main event we saw in this group, and this was even when we did a very slow titration of 5 mg/kg per week,” Dr. Chayasirisobhon said. “Any sign of rash or itching at all, we told them to stop immediately and call us. Fortunately, we had no cases of Steven-Johnson syndrome and all our cases of rash were transient. But in the levetiracetam group, the mood changes are the major thing. Some of the patients became very agitated and aggressive. Whenever we see a patient for the first time, we always ask about mood changes, and we instruct the family to call and report any changes in mood immediately.”
Aside from reproductive age, however, Dr. Chayasirisobhon generally prefers to start new patients on levetiracetam. Its safety profile is remarkable, he said, recounting a case report he published in 2010 (Acta Neurol Taiwan. 2010;19:292-5).
The paper describes a male patient who decided to commit suicide after an argument with his wife. He took his levetiracetam and walked to his father’s grave, swallowing pills the entire time. When he arrived at the grave, he had taken around 65 grams of the medication. “The amazing thing was, he’s still walking, just a little unsteady. Then he decided he’s not ready to die,” Dr. Chayasirisobhon said. “He was able to call 911, so he’s still talking fine. When they checked his level it was so high, but he remained unimpaired except for the unsteady gait and some nystagmus.”
The study did not receive outside funding. Dr. Chayasirisobhon had no financial disclosures.
NEW ORLEANS – Bioequivalent generic formulations of levetiracetam and lamotrigine reduced seizures by a similar extent over 2 years in a retrospective study of patients with newly diagnosed focal epilepsy.
Each drug had a specific adverse event profile, with lamotrigine associated with rash and levetiracetam with mood disorders, Sirichai Chayasirisobhon, MD, said at the annual meeting of the American Epilepsy Society. This finding can play into the initial therapeutic decision, said Dr. Chayasirisobhon of Kaiser Permanente Southern California. “If someone comes in with depression or mood disorder, I will start on lamotrigine, not levetiracetam. And we can decrease the chance of rash with a very slow titration, as we did here, starting with just 5 mg/kg and working up over 6 months.”
Although the drugs have a somewhat similar teratogenic profile, Dr. Chayasirisobhon added that he favors lamotrigine for women of childbearing years. “It’s a little bit better choice for them I think.”
His retrospective analysis followed 442 patients from first seizure and medical therapy for 2 years. The generic medications came from Kaiser Permanente’s central pharmacy. They were single-source, with a proven 95% bioequivalence. The main outcome was the percentage of patients who became seizure free and remained so. Any seizure, whether febrile, breakthroughs, or from titration, was considered a failure. These patients were dropped from the study. Any patient who developed a drug-related rash was dropped from the study and started on another medication.
More women than men took lamotrigine (113 vs. 75), whereas more men took levetiracetam (148 vs. 106). Those taking lamotrigine were younger than were those taking levetiracetam (30 vs. 40 years).
At the end of 2 years, there was no statistically significant difference in the primary outcome of being free from seizures (66.5% with lamotrigine vs. 72.4% with levetiracetam). In the lamotrigine group, 33.5% were eliminated from the study, 24% because they had a seizure, and the rest due to an adverse event. In the levetiracetam group, 27.6% were eliminated, 13% because they had a seizure and the rest because of an adverse event.
Adverse events in the lamotrigine group included rash (12), dizziness (3), lethargy (1), and mood changes (2). Among the levetiracetam group, adverse events included dizziness (3), lethargy (7), mood changes (20), slowed thinking (4), depression (2) and headache (1).
“Rash was the main event we saw in this group, and this was even when we did a very slow titration of 5 mg/kg per week,” Dr. Chayasirisobhon said. “Any sign of rash or itching at all, we told them to stop immediately and call us. Fortunately, we had no cases of Steven-Johnson syndrome and all our cases of rash were transient. But in the levetiracetam group, the mood changes are the major thing. Some of the patients became very agitated and aggressive. Whenever we see a patient for the first time, we always ask about mood changes, and we instruct the family to call and report any changes in mood immediately.”
Aside from reproductive age, however, Dr. Chayasirisobhon generally prefers to start new patients on levetiracetam. Its safety profile is remarkable, he said, recounting a case report he published in 2010 (Acta Neurol Taiwan. 2010;19:292-5).
The paper describes a male patient who decided to commit suicide after an argument with his wife. He took his levetiracetam and walked to his father’s grave, swallowing pills the entire time. When he arrived at the grave, he had taken around 65 grams of the medication. “The amazing thing was, he’s still walking, just a little unsteady. Then he decided he’s not ready to die,” Dr. Chayasirisobhon said. “He was able to call 911, so he’s still talking fine. When they checked his level it was so high, but he remained unimpaired except for the unsteady gait and some nystagmus.”
The study did not receive outside funding. Dr. Chayasirisobhon had no financial disclosures.
REPORTING FROM AES 2018
Key clinical point:
Major finding: At 2 years, 66.5% of the lamotrigine group and 72.4% of the levetiracetam group were seizure free.
Study details: The retrospective study comprised 442 patients.
Disclosures: The study did not receive outside funding. Dr. Chayasirisobhon had no financial disclosures.
Source: Chayasirisobhon S et al. AES 2018, Abstract 2.147.
Neurologic Disease Eventually Affects Half of Women and One-Third of Men
Findings strengthen the call for prioritizing the focus on preventive interventions at the population level.
Around one-half of women and one-third of men will develop dementia, stroke, or parkinsonism during their lifetime, according to a study published online ahead of print October 2 in the Journal of Neurology, Neurosurgery & Psychiatry.
The population-based Rotterdam study involved 12,102 individuals (57.7% women) who were ages 45 or older and free of neurologic disease at baseline. This cohort was followed for 26 years. Silvan Licher, a PhD student in the Department of Epidemiology at Erasmus MC University Medical Center Rotterdam in the Netherlands, and colleagues found that a 45-year-old woman had a 48.2% overall remaining lifetime risk of developing dementia, stroke, or parkinsonism, while a 45-year-old man had a 36.3% lifetime risk.
“There are currently no disease-modifying drugs available for dementia and most causes of parkinsonism, and prevention of stroke is hampered by suboptimal adherence to effective preventive strategies or unmet guideline thresholds,” the authors wrote. “Yet a delay in onset of these common neurologic diseases by merely a few years could reduce the population burden of these diseases substantially.”
Women age 45 had a significantly higher lifetime risk than men of developing dementia (31.4% vs 18.6%, respectively) and stroke (21.6% vs 19.3%), but the risk of parkinsonism was similar between the sexes. Women also had a significantly greater lifetime risk of developing more than one neurologic disease, compared with men (4% vs 3.1%), largely because of the overlap between dementia and stroke.
At age 45, women had the greatest risk of dementia, but as men and women aged, their remaining lifetime risk of dementia increased relative to other neurologic diseases. After age 85, 66.6% of first diagnoses in women and 55.6% in men were dementia. By comparison, first manifestation of stroke was the greatest threat to men age 45. Men also were at a significantly higher risk for stroke at a younger age—before age 75—than were women (8.4% vs 5.8%, respectively). In the case of parkinsonism, the lifetime risk peaked earlier than it did for dementia and stroke and was relatively low after age 85, with no significant differences in risk between men and women.
The authors considered what effect a delay in disease onset and occurrence might have on remaining lifetime risk for neurologic disease. They found that a one-, two-, or three-year delay in the onset of all neurologic disease was associated with a 20% reduction in lifetime risk in individuals age 45 or older, and a greater than 50% reduction in risk in the oldest. A three-year delay in the onset of dementia reduced the lifetime risk by 15% for men and women age 45 and conveyed a 30% reduction in risk to those age 45 or older.
The Rotterdam study is supported by Erasmus MC and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research; the Netherlands Organization for Health Research and Development; the Research Institute for Diseases in the Elderly; the Netherlands Genomics Initiative; the Ministry of Education, Culture, and Science; the Ministry of Health, Welfare, and Sports; the European Commission and the Municipality of Rotterdam; the Netherlands Consortium for Healthy Aging; and the Dutch Heart Foundation.
—Bianca Nogrady
Suggested Reading
Licher S, Darweesh SKL, Wolters FJ, et al. Lifetime risk of common neurological diseases in the elderly population. J Neurol Neurosurg Psychiatry. 2018 Oct 2 [Epub ahead of print].
Findings strengthen the call for prioritizing the focus on preventive interventions at the population level.
Findings strengthen the call for prioritizing the focus on preventive interventions at the population level.
Around one-half of women and one-third of men will develop dementia, stroke, or parkinsonism during their lifetime, according to a study published online ahead of print October 2 in the Journal of Neurology, Neurosurgery & Psychiatry.
The population-based Rotterdam study involved 12,102 individuals (57.7% women) who were ages 45 or older and free of neurologic disease at baseline. This cohort was followed for 26 years. Silvan Licher, a PhD student in the Department of Epidemiology at Erasmus MC University Medical Center Rotterdam in the Netherlands, and colleagues found that a 45-year-old woman had a 48.2% overall remaining lifetime risk of developing dementia, stroke, or parkinsonism, while a 45-year-old man had a 36.3% lifetime risk.
“There are currently no disease-modifying drugs available for dementia and most causes of parkinsonism, and prevention of stroke is hampered by suboptimal adherence to effective preventive strategies or unmet guideline thresholds,” the authors wrote. “Yet a delay in onset of these common neurologic diseases by merely a few years could reduce the population burden of these diseases substantially.”
Women age 45 had a significantly higher lifetime risk than men of developing dementia (31.4% vs 18.6%, respectively) and stroke (21.6% vs 19.3%), but the risk of parkinsonism was similar between the sexes. Women also had a significantly greater lifetime risk of developing more than one neurologic disease, compared with men (4% vs 3.1%), largely because of the overlap between dementia and stroke.
At age 45, women had the greatest risk of dementia, but as men and women aged, their remaining lifetime risk of dementia increased relative to other neurologic diseases. After age 85, 66.6% of first diagnoses in women and 55.6% in men were dementia. By comparison, first manifestation of stroke was the greatest threat to men age 45. Men also were at a significantly higher risk for stroke at a younger age—before age 75—than were women (8.4% vs 5.8%, respectively). In the case of parkinsonism, the lifetime risk peaked earlier than it did for dementia and stroke and was relatively low after age 85, with no significant differences in risk between men and women.
The authors considered what effect a delay in disease onset and occurrence might have on remaining lifetime risk for neurologic disease. They found that a one-, two-, or three-year delay in the onset of all neurologic disease was associated with a 20% reduction in lifetime risk in individuals age 45 or older, and a greater than 50% reduction in risk in the oldest. A three-year delay in the onset of dementia reduced the lifetime risk by 15% for men and women age 45 and conveyed a 30% reduction in risk to those age 45 or older.
The Rotterdam study is supported by Erasmus MC and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research; the Netherlands Organization for Health Research and Development; the Research Institute for Diseases in the Elderly; the Netherlands Genomics Initiative; the Ministry of Education, Culture, and Science; the Ministry of Health, Welfare, and Sports; the European Commission and the Municipality of Rotterdam; the Netherlands Consortium for Healthy Aging; and the Dutch Heart Foundation.
—Bianca Nogrady
Suggested Reading
Licher S, Darweesh SKL, Wolters FJ, et al. Lifetime risk of common neurological diseases in the elderly population. J Neurol Neurosurg Psychiatry. 2018 Oct 2 [Epub ahead of print].
Around one-half of women and one-third of men will develop dementia, stroke, or parkinsonism during their lifetime, according to a study published online ahead of print October 2 in the Journal of Neurology, Neurosurgery & Psychiatry.
The population-based Rotterdam study involved 12,102 individuals (57.7% women) who were ages 45 or older and free of neurologic disease at baseline. This cohort was followed for 26 years. Silvan Licher, a PhD student in the Department of Epidemiology at Erasmus MC University Medical Center Rotterdam in the Netherlands, and colleagues found that a 45-year-old woman had a 48.2% overall remaining lifetime risk of developing dementia, stroke, or parkinsonism, while a 45-year-old man had a 36.3% lifetime risk.
“There are currently no disease-modifying drugs available for dementia and most causes of parkinsonism, and prevention of stroke is hampered by suboptimal adherence to effective preventive strategies or unmet guideline thresholds,” the authors wrote. “Yet a delay in onset of these common neurologic diseases by merely a few years could reduce the population burden of these diseases substantially.”
Women age 45 had a significantly higher lifetime risk than men of developing dementia (31.4% vs 18.6%, respectively) and stroke (21.6% vs 19.3%), but the risk of parkinsonism was similar between the sexes. Women also had a significantly greater lifetime risk of developing more than one neurologic disease, compared with men (4% vs 3.1%), largely because of the overlap between dementia and stroke.
At age 45, women had the greatest risk of dementia, but as men and women aged, their remaining lifetime risk of dementia increased relative to other neurologic diseases. After age 85, 66.6% of first diagnoses in women and 55.6% in men were dementia. By comparison, first manifestation of stroke was the greatest threat to men age 45. Men also were at a significantly higher risk for stroke at a younger age—before age 75—than were women (8.4% vs 5.8%, respectively). In the case of parkinsonism, the lifetime risk peaked earlier than it did for dementia and stroke and was relatively low after age 85, with no significant differences in risk between men and women.
The authors considered what effect a delay in disease onset and occurrence might have on remaining lifetime risk for neurologic disease. They found that a one-, two-, or three-year delay in the onset of all neurologic disease was associated with a 20% reduction in lifetime risk in individuals age 45 or older, and a greater than 50% reduction in risk in the oldest. A three-year delay in the onset of dementia reduced the lifetime risk by 15% for men and women age 45 and conveyed a 30% reduction in risk to those age 45 or older.
The Rotterdam study is supported by Erasmus MC and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research; the Netherlands Organization for Health Research and Development; the Research Institute for Diseases in the Elderly; the Netherlands Genomics Initiative; the Ministry of Education, Culture, and Science; the Ministry of Health, Welfare, and Sports; the European Commission and the Municipality of Rotterdam; the Netherlands Consortium for Healthy Aging; and the Dutch Heart Foundation.
—Bianca Nogrady
Suggested Reading
Licher S, Darweesh SKL, Wolters FJ, et al. Lifetime risk of common neurological diseases in the elderly population. J Neurol Neurosurg Psychiatry. 2018 Oct 2 [Epub ahead of print].
‘Error neuron’ EEG findings could open up future clinical applications
, and this activity can be tracked through a scalp EEG pattern called error-related negativity, according to findings from experiments carried out during intracranial EEG recordings of candidates for surgical treatment of epilepsy.
“Our results suggest that coordinated neural activity can serve as a substrate for information routing that enables the performance-monitoring system to communicate the need for behavioral control to other brain regions, including those that maintain flexible goal information, such as the lateral prefrontal cortex and the frontal polar cortex,” first author Zhongzheng Fu, a PhD student at the California Institute of Technology in Pasadena, Calif., and Cedars-Sinai Medical Center, Los Angeles, and his colleagues reported in Neuron.
The findings offer insights that could lead to treatments for conditions in which the important executive function task of error self-monitoring is unbalanced, such as obsessive-compulsive disorder and schizophrenia, the authors noted in a press release.
“We discovered that the activity of error neurons correlates with the size of the ERN [error-related negativity],” Mr. Fu said. “This identifies the brain area that causes the ERN and helps explain what it signifies. This new insight might allow doctors to use the ERN as a standard tool to diagnose mental diseases and monitor responses to treatment.”
Error neuron firing and intracranial ERN occurred first in pre-supplementary motor area (pre-SMA), then in the dorsal anterior cingulate cortex (dACC) about 50 ms later, with significant correlations between firing and intracranial ERN in both locations. In dACC, this activity, with error-integrating neuron responses, correlated with magnitude of post-error slowing (PES).
Previous research suggested a link between “the detection of self-generated errors, as reflected in the ERN, with changes in cognitive control, as exhibited behaviorally in PES,” the investigators wrote. “However, several electroencephalogram (EEG) studies have failed to find a significant relationship between PES and ERN.”
The present study involved intracranial EEG of 29 candidates for surgical treatment of epilepsy and scalp EEG of 12 control participants, with each modality measuring activity in the frontal cortex. Both cohorts performed a rapid version of the color-word Stroop task, in which the words “red,” “green,” or “blue” were printed either in corresponding or noncorresponding colors of red, green, or blue. Subjects were presented various color-word combinations while being asked to click one of three buttons indicating the color of the word as quickly as possible. The investigators monitored neuronal activity throughout, discarding responses that were too slow.
As found in previous trials, the subjects demonstrated the “Stroop effect,” which refers to a slower response when word and color are incongruent (224.9 ms difference; P less than .001). As anticipated, correct responses following correct responses were faster than were correct responses following erroneous responses, which defines PES.
In the intracranial EEG group, the investigators isolated 1,171 neurons, of which 618 were located in dACC and 553 in pre-SMA. Using a Poisson regression model and correlations with erroneous responses, the investigators identified 99 “type I” error neurons in dACC and 118 in pre-SMA, based on higher frequency of firing during erroneous responses than during correct responses. At a single-cell level, error neuron mean spike rates were highest when intracranial ERN amplitude was greatest, such that error neuron firing in dACC and pre-SMA had maximal likelihood ratios of 7.9 (P = .01) and 15.1 (P less than .001), respectively. The strength of correlation between intracranial ERN and error neuron firing rate was directly related to PES magnitude exclusively in the dACC (maximum likelihood ratio of 13.9; P = .015). In post-error trials, faster error-integrating neuron firing rates in dACC predicted greater PES (maximal likelihood ratio of 18.3; P less than .001).
The study was funded by the National Institutes of Health, the McKnight Endowment for Neuroscience, and the National Science Foundation. The authors declared no conflicts of interest.
SOURCE: Fu Z et al. Neuron. 2018 Dec 4. doi: 10.1016/j.neuron.2018.11.016
, and this activity can be tracked through a scalp EEG pattern called error-related negativity, according to findings from experiments carried out during intracranial EEG recordings of candidates for surgical treatment of epilepsy.
“Our results suggest that coordinated neural activity can serve as a substrate for information routing that enables the performance-monitoring system to communicate the need for behavioral control to other brain regions, including those that maintain flexible goal information, such as the lateral prefrontal cortex and the frontal polar cortex,” first author Zhongzheng Fu, a PhD student at the California Institute of Technology in Pasadena, Calif., and Cedars-Sinai Medical Center, Los Angeles, and his colleagues reported in Neuron.
The findings offer insights that could lead to treatments for conditions in which the important executive function task of error self-monitoring is unbalanced, such as obsessive-compulsive disorder and schizophrenia, the authors noted in a press release.
“We discovered that the activity of error neurons correlates with the size of the ERN [error-related negativity],” Mr. Fu said. “This identifies the brain area that causes the ERN and helps explain what it signifies. This new insight might allow doctors to use the ERN as a standard tool to diagnose mental diseases and monitor responses to treatment.”
Error neuron firing and intracranial ERN occurred first in pre-supplementary motor area (pre-SMA), then in the dorsal anterior cingulate cortex (dACC) about 50 ms later, with significant correlations between firing and intracranial ERN in both locations. In dACC, this activity, with error-integrating neuron responses, correlated with magnitude of post-error slowing (PES).
Previous research suggested a link between “the detection of self-generated errors, as reflected in the ERN, with changes in cognitive control, as exhibited behaviorally in PES,” the investigators wrote. “However, several electroencephalogram (EEG) studies have failed to find a significant relationship between PES and ERN.”
The present study involved intracranial EEG of 29 candidates for surgical treatment of epilepsy and scalp EEG of 12 control participants, with each modality measuring activity in the frontal cortex. Both cohorts performed a rapid version of the color-word Stroop task, in which the words “red,” “green,” or “blue” were printed either in corresponding or noncorresponding colors of red, green, or blue. Subjects were presented various color-word combinations while being asked to click one of three buttons indicating the color of the word as quickly as possible. The investigators monitored neuronal activity throughout, discarding responses that were too slow.
As found in previous trials, the subjects demonstrated the “Stroop effect,” which refers to a slower response when word and color are incongruent (224.9 ms difference; P less than .001). As anticipated, correct responses following correct responses were faster than were correct responses following erroneous responses, which defines PES.
In the intracranial EEG group, the investigators isolated 1,171 neurons, of which 618 were located in dACC and 553 in pre-SMA. Using a Poisson regression model and correlations with erroneous responses, the investigators identified 99 “type I” error neurons in dACC and 118 in pre-SMA, based on higher frequency of firing during erroneous responses than during correct responses. At a single-cell level, error neuron mean spike rates were highest when intracranial ERN amplitude was greatest, such that error neuron firing in dACC and pre-SMA had maximal likelihood ratios of 7.9 (P = .01) and 15.1 (P less than .001), respectively. The strength of correlation between intracranial ERN and error neuron firing rate was directly related to PES magnitude exclusively in the dACC (maximum likelihood ratio of 13.9; P = .015). In post-error trials, faster error-integrating neuron firing rates in dACC predicted greater PES (maximal likelihood ratio of 18.3; P less than .001).
The study was funded by the National Institutes of Health, the McKnight Endowment for Neuroscience, and the National Science Foundation. The authors declared no conflicts of interest.
SOURCE: Fu Z et al. Neuron. 2018 Dec 4. doi: 10.1016/j.neuron.2018.11.016
, and this activity can be tracked through a scalp EEG pattern called error-related negativity, according to findings from experiments carried out during intracranial EEG recordings of candidates for surgical treatment of epilepsy.
“Our results suggest that coordinated neural activity can serve as a substrate for information routing that enables the performance-monitoring system to communicate the need for behavioral control to other brain regions, including those that maintain flexible goal information, such as the lateral prefrontal cortex and the frontal polar cortex,” first author Zhongzheng Fu, a PhD student at the California Institute of Technology in Pasadena, Calif., and Cedars-Sinai Medical Center, Los Angeles, and his colleagues reported in Neuron.
The findings offer insights that could lead to treatments for conditions in which the important executive function task of error self-monitoring is unbalanced, such as obsessive-compulsive disorder and schizophrenia, the authors noted in a press release.
“We discovered that the activity of error neurons correlates with the size of the ERN [error-related negativity],” Mr. Fu said. “This identifies the brain area that causes the ERN and helps explain what it signifies. This new insight might allow doctors to use the ERN as a standard tool to diagnose mental diseases and monitor responses to treatment.”
Error neuron firing and intracranial ERN occurred first in pre-supplementary motor area (pre-SMA), then in the dorsal anterior cingulate cortex (dACC) about 50 ms later, with significant correlations between firing and intracranial ERN in both locations. In dACC, this activity, with error-integrating neuron responses, correlated with magnitude of post-error slowing (PES).
Previous research suggested a link between “the detection of self-generated errors, as reflected in the ERN, with changes in cognitive control, as exhibited behaviorally in PES,” the investigators wrote. “However, several electroencephalogram (EEG) studies have failed to find a significant relationship between PES and ERN.”
The present study involved intracranial EEG of 29 candidates for surgical treatment of epilepsy and scalp EEG of 12 control participants, with each modality measuring activity in the frontal cortex. Both cohorts performed a rapid version of the color-word Stroop task, in which the words “red,” “green,” or “blue” were printed either in corresponding or noncorresponding colors of red, green, or blue. Subjects were presented various color-word combinations while being asked to click one of three buttons indicating the color of the word as quickly as possible. The investigators monitored neuronal activity throughout, discarding responses that were too slow.
As found in previous trials, the subjects demonstrated the “Stroop effect,” which refers to a slower response when word and color are incongruent (224.9 ms difference; P less than .001). As anticipated, correct responses following correct responses were faster than were correct responses following erroneous responses, which defines PES.
In the intracranial EEG group, the investigators isolated 1,171 neurons, of which 618 were located in dACC and 553 in pre-SMA. Using a Poisson regression model and correlations with erroneous responses, the investigators identified 99 “type I” error neurons in dACC and 118 in pre-SMA, based on higher frequency of firing during erroneous responses than during correct responses. At a single-cell level, error neuron mean spike rates were highest when intracranial ERN amplitude was greatest, such that error neuron firing in dACC and pre-SMA had maximal likelihood ratios of 7.9 (P = .01) and 15.1 (P less than .001), respectively. The strength of correlation between intracranial ERN and error neuron firing rate was directly related to PES magnitude exclusively in the dACC (maximum likelihood ratio of 13.9; P = .015). In post-error trials, faster error-integrating neuron firing rates in dACC predicted greater PES (maximal likelihood ratio of 18.3; P less than .001).
The study was funded by the National Institutes of Health, the McKnight Endowment for Neuroscience, and the National Science Foundation. The authors declared no conflicts of interest.
SOURCE: Fu Z et al. Neuron. 2018 Dec 4. doi: 10.1016/j.neuron.2018.11.016
FROM NEURON
Study elicits patients’ most disturbing epilepsy symptoms
NEW ORLEANS – according to a study presented at the annual meeting of the American Epilepsy Society. The most prominent symptoms and effects on daily life may differ in the early, middle, and late stages of the disease, the results suggest.
Lead study author Jacqueline A. French, MD, professor of neurology at New York University, and her colleagues interviewed 62 patients with focal-onset epilepsy to examine patients’ experiences living with epilepsy. The investigators focused on salient symptoms and functional impacts – those that were reported by at least 50% of patients and were associated with a high degree of disturbance (patients rated them 5 or greater on a scale from 0 [no disturbance] to 10 [high disturbance]).
Of 51 symptoms that patients described during the interviews, the following 8 met the salience criteria for the total cohort: twitching or tremors, confusion, difficulty in talking, loss of awareness of others’ presence, stiffening, impaired consciousness or loss of consciousness, difficulty in remembering, and dizziness or lightheadedness. Patients reported salient functional impacts on driving and transportation, work and school, and leisure and social activities. Some symptoms met salience criteria among patients in certain stages of the disease (for example, tongue biting in patients with early-stage epilepsy and anxiety, fear, or panic in late-stage epilepsy) but not among patients in the other cohorts.
“These findings underscore the need to consider all these experiences when developing patient-reported outcome measures for use in clinical trials,” said Dr. French and her colleagues. “It may be useful to tailor measures of patient experiences to the patient’s stage of disease.”
Previous qualitative studies of epilepsy symptoms and burdens were based on small numbers of patients and interviews at a single center. For the present study, the researchers conducted qualitative, semistructured, in-person interviews with adults with focal epilepsy in different areas of the United States (such as California, Minnesota, New York, Ohio, and Pennsylvania). Patients were grouped by early, middle, or late disease stage. Patients in the early cohort (n = 19) had at least two seizures in the past year, a diagnosis of focal epilepsy in the past year, and had not yet received antiepileptic drug (AED) treatment or had received treatment with only one AED and had not failed treatment. Patients in the middle cohort (n = 17) had at least one seizure in the past year, a diagnosis of focal epilepsy within the past 5 years, and had failed one AED because of lack of efficacy or had received their first add-on AED. Patients in the late cohort (n = 26) had at least one seizure every 3 months during the past year, a diagnosis of focal epilepsy at age 12 years or older, and inadequate response to treatment of at least 3 months with two AEDs that were tolerated and appropriately chosen.
Patients’ mean age was 37 years (range, 19-60 years), 73% were female, 79% were white, 69% had a college degree as their highest level of education, and 65% were employed. Patients’ seizure types included simple partial without motor signs (52%), simple partial with motor signs (16%), complex partial (68%), or secondarily generalized (65%).
While driving or transportation was a salient impact for all three groups, memory loss was a salient impact in the early and middle cohorts only. Headaches and sadness or depression were salient impacts for the late cohort only.
This study was funded by Eisai and two of the authors are former or current employees of Eisai.
SOURCE: French JA et al. AES 2018, Abstract 1.196.
NEW ORLEANS – according to a study presented at the annual meeting of the American Epilepsy Society. The most prominent symptoms and effects on daily life may differ in the early, middle, and late stages of the disease, the results suggest.
Lead study author Jacqueline A. French, MD, professor of neurology at New York University, and her colleagues interviewed 62 patients with focal-onset epilepsy to examine patients’ experiences living with epilepsy. The investigators focused on salient symptoms and functional impacts – those that were reported by at least 50% of patients and were associated with a high degree of disturbance (patients rated them 5 or greater on a scale from 0 [no disturbance] to 10 [high disturbance]).
Of 51 symptoms that patients described during the interviews, the following 8 met the salience criteria for the total cohort: twitching or tremors, confusion, difficulty in talking, loss of awareness of others’ presence, stiffening, impaired consciousness or loss of consciousness, difficulty in remembering, and dizziness or lightheadedness. Patients reported salient functional impacts on driving and transportation, work and school, and leisure and social activities. Some symptoms met salience criteria among patients in certain stages of the disease (for example, tongue biting in patients with early-stage epilepsy and anxiety, fear, or panic in late-stage epilepsy) but not among patients in the other cohorts.
“These findings underscore the need to consider all these experiences when developing patient-reported outcome measures for use in clinical trials,” said Dr. French and her colleagues. “It may be useful to tailor measures of patient experiences to the patient’s stage of disease.”
Previous qualitative studies of epilepsy symptoms and burdens were based on small numbers of patients and interviews at a single center. For the present study, the researchers conducted qualitative, semistructured, in-person interviews with adults with focal epilepsy in different areas of the United States (such as California, Minnesota, New York, Ohio, and Pennsylvania). Patients were grouped by early, middle, or late disease stage. Patients in the early cohort (n = 19) had at least two seizures in the past year, a diagnosis of focal epilepsy in the past year, and had not yet received antiepileptic drug (AED) treatment or had received treatment with only one AED and had not failed treatment. Patients in the middle cohort (n = 17) had at least one seizure in the past year, a diagnosis of focal epilepsy within the past 5 years, and had failed one AED because of lack of efficacy or had received their first add-on AED. Patients in the late cohort (n = 26) had at least one seizure every 3 months during the past year, a diagnosis of focal epilepsy at age 12 years or older, and inadequate response to treatment of at least 3 months with two AEDs that were tolerated and appropriately chosen.
Patients’ mean age was 37 years (range, 19-60 years), 73% were female, 79% were white, 69% had a college degree as their highest level of education, and 65% were employed. Patients’ seizure types included simple partial without motor signs (52%), simple partial with motor signs (16%), complex partial (68%), or secondarily generalized (65%).
While driving or transportation was a salient impact for all three groups, memory loss was a salient impact in the early and middle cohorts only. Headaches and sadness or depression were salient impacts for the late cohort only.
This study was funded by Eisai and two of the authors are former or current employees of Eisai.
SOURCE: French JA et al. AES 2018, Abstract 1.196.
NEW ORLEANS – according to a study presented at the annual meeting of the American Epilepsy Society. The most prominent symptoms and effects on daily life may differ in the early, middle, and late stages of the disease, the results suggest.
Lead study author Jacqueline A. French, MD, professor of neurology at New York University, and her colleagues interviewed 62 patients with focal-onset epilepsy to examine patients’ experiences living with epilepsy. The investigators focused on salient symptoms and functional impacts – those that were reported by at least 50% of patients and were associated with a high degree of disturbance (patients rated them 5 or greater on a scale from 0 [no disturbance] to 10 [high disturbance]).
Of 51 symptoms that patients described during the interviews, the following 8 met the salience criteria for the total cohort: twitching or tremors, confusion, difficulty in talking, loss of awareness of others’ presence, stiffening, impaired consciousness or loss of consciousness, difficulty in remembering, and dizziness or lightheadedness. Patients reported salient functional impacts on driving and transportation, work and school, and leisure and social activities. Some symptoms met salience criteria among patients in certain stages of the disease (for example, tongue biting in patients with early-stage epilepsy and anxiety, fear, or panic in late-stage epilepsy) but not among patients in the other cohorts.
“These findings underscore the need to consider all these experiences when developing patient-reported outcome measures for use in clinical trials,” said Dr. French and her colleagues. “It may be useful to tailor measures of patient experiences to the patient’s stage of disease.”
Previous qualitative studies of epilepsy symptoms and burdens were based on small numbers of patients and interviews at a single center. For the present study, the researchers conducted qualitative, semistructured, in-person interviews with adults with focal epilepsy in different areas of the United States (such as California, Minnesota, New York, Ohio, and Pennsylvania). Patients were grouped by early, middle, or late disease stage. Patients in the early cohort (n = 19) had at least two seizures in the past year, a diagnosis of focal epilepsy in the past year, and had not yet received antiepileptic drug (AED) treatment or had received treatment with only one AED and had not failed treatment. Patients in the middle cohort (n = 17) had at least one seizure in the past year, a diagnosis of focal epilepsy within the past 5 years, and had failed one AED because of lack of efficacy or had received their first add-on AED. Patients in the late cohort (n = 26) had at least one seizure every 3 months during the past year, a diagnosis of focal epilepsy at age 12 years or older, and inadequate response to treatment of at least 3 months with two AEDs that were tolerated and appropriately chosen.
Patients’ mean age was 37 years (range, 19-60 years), 73% were female, 79% were white, 69% had a college degree as their highest level of education, and 65% were employed. Patients’ seizure types included simple partial without motor signs (52%), simple partial with motor signs (16%), complex partial (68%), or secondarily generalized (65%).
While driving or transportation was a salient impact for all three groups, memory loss was a salient impact in the early and middle cohorts only. Headaches and sadness or depression were salient impacts for the late cohort only.
This study was funded by Eisai and two of the authors are former or current employees of Eisai.
SOURCE: French JA et al. AES 2018, Abstract 1.196.
REPORTING FROM AES 2018
Key clinical point: The most prominent symptoms and functional impacts of epilepsy may differ in the early, middle, and late stages of the disease.
Major finding: More than 50% of patients reported functional impacts on driving and transportation, work and school, and leisure and social activities.
Study details: An analysis of data from semistructured interviews with 62 adults with focal epilepsy.
Disclosures: This study was funded by Eisai and two of the authors are former or current employees of Eisai.
Source: French JA et al. AES 2018, Abstract 1.196.
Corpora callosa of young football players could be at risk
Younger players are not immune to the brain damage that can come with playing football, National Public Radio says, quoting a grim report from the annual meeting of the Radiological Society of North America.
The technique of magnetic resonance imaging, which essentially records a video of brain structure and function in real time, was used to scan the brains of 26 boys aged an average of 12 years before and after a season of football. The findings were compared with the brain scans of 26 other boys of similar age who were not football players, according to NPR.
Damage to the region that connects the two halves of the brain was evident in a majority of the football players but not in their control counterparts. in the shape of the corpus callosum. When these changes come at a time in life when the brain is developing, the results can be lifetime consequences on thought, behavior, and emotion.
“You have to understand that the NFL players were also most likely once collegiate players; they were also high school players and they were also probably youth players,” says radiologist Christopher T. Whitlow, MD, PhD, of Wake Forest Baptist Health in Winston-Salem, N.C., and a coauthor of the new findings, in the interview. “To us, it’s more than a question about concussions, it’s a question about long-term cumulative exposure.
“We don’t know what [the results] mean … do these changes persist over time? Do they accumulate with multiple seasons? And then No. 3, probably the most important: Do they have any relevance to long-term health?”
Average can be just fine
The need to excel is drilled into many people from childhood. Hard work is a virtue, but the pressure to shine can have disastrous consequences. In South Korea, for example, academic pressure is a major cause of suicide in youth.
A recent TED Women conference held in Palm Springs, Calif., provided some reassurance for those in the “forgotten middle” – those who were adequate but not stellar students, and who do their work diligently but not outstandingly.
“Those at the top get noticed and those at the bottom get extra help but no one really thinks about the kids in the middle who make up the majority,” says Danielle R. Moss Lee, EdD, a social activist and chief of the New York Civil Liberties Union, who spoke at the conference. These folks can be valuable contributors but are often overlooked. As a result, when it comes to excelling, they “check out.”
“We have to create different ways to harness their potential. I didn’t appreciate how average I was until I was a college student and I bumped into a science teacher and he couldn’t believe what college I was attending,” Dr. Lee says.
Sometimes it takes a push from a loved one to spur action. In Dr. Lee’s case, she says she was happy being an average student. Her mother’s search for extracurricular activities led her to discover writing and set her on a path to personal and professional accomplishment.
Dr. Lee’s message was that “the middle isn’t a permanent location.”
Others experts see the situation differently. “Most psychological traits are evenly distributed, meaning that a significant proportion of the population will have average intelligence and leadership potential,” says Tomas Chamorro-Premuzic, PhD, of University College London, in an interview with BBC News.
“The world’s progress depends on those who stand out via their exceptional and innovative contributions, but these individuals are part of the top 1% in their field, combining truly unconventional levels of talent, work ethic, and focus,” Dr. Chamorro-Premuzic says. “For the remaining 99% of us, the acceptance that our talents and motivation are much more conventional, and unlikely to result in world-changing accomplishments, would reflect a healthier, more rational self-concept than illusions of grandiosity or fantasized talent.”
Shared sorrow mark club members
A recent article in Time described the experiences of some who have lost their children and whose worlds have been forever altered. From all walks of life and diverse backgrounds, these folks become tethered together. “It’s a club you spend your whole life hoping you won’t ever become a part of,” says Nicole Hockley, whose son Dylan, 6, was killed in the December 2012 shooting at Sandy Hook Elementary School in Connecticut. “But once you’re in, you’re in.”
Mitchell Dworet and Melissa Wiley are connected by death of their children. Mr. Dworet’s 17-year-old son Nicholas was killed during the shooting at Marjory Stoneman Douglas High School in Parkland, Fla., in February 2018. A month later, Ms. Willey’s daughter Jaelynn, 16, was shot to death by a fellow student at Great Mills High School in Maryland. They connected through Facebook. “I felt like I should reach out. I wanted to pay it forward,” explains Mr. Dworet.
“When you’ve gone through this kind of tragedy with other people, you see their humanity, where they’re coming from,” says Darrell Scott, whose 17-year-old daughter Rachel was killed at Columbine. Politics can differ – as can views on the painful issue of gun control measures – and friendships might not develop. Still, however, they share one enduring bond.
The connection with others can help in the immediate aftermath, and can continue to be important over time. “When you lose a child violently and publicly, there’s an outpouring of support at first,” said Sandy Phillips, whose 24-year-old daughter Jessi was shot with 11 others at a cinema in Aurora, Colo., in 2012. “Once the vigils are over and the media is gone, that’s when things get really bad. The world moves on, and you don’t. You can’t. It’s a pain you can’t outrun.”
“A huge emotional jolt”
In the aftermath of the magnitude 7.0 earthquake that shook Anchorage, Alaska, on Nov. 30, and the many aftershocks, residents are scrambling to cope with their changed lives. For those who lost possessions, the pain is real. But there comes the realization for many that they survived and that material possessions can, for the most part, be replaced.
Psychological changes, meanwhile, can prove profound and lasting. Researchers have found that large earthquakes can produce PTSD and anxiety. Some survivors can come away from earthquakes with difficulty concentrating and hypervigilance.
As one resident explains to Anchorage Daily News, “I felt yesterday like I had one final nerve and every aftershock was playing on that nerve.”
K.J. Worbey, a mental health counselor for Southcentral Foundation – an Alaska Native health care organization – describes the experience as a “huge emotional jolt.” She adds there is “lots of uncertainly about our own safety. Safety of our families and our homes. ... When we are faced with that kind of an emotional crisis, it takes a whole lot of energy to navigate it.”
Ms. Worbey recommends limiting alcohol, eating a healthy diet, and exercising appropriately. “Try to get some energy out. Try and get that excess emotional stuff out,” she said. Other prudent measures include sticking to a normal routine as much as is possible, including mealtimes and sleep, and talking with neighbors and friends.
Drug diversions can cost lives
A recent article in the Dallas Morning News has highlighted the humanity of health caregivers. Within the past several years, two nurses at University of Texas Southwestern Medical Center’s Williams P. Clements Jr. Hospital in Dallas have died of self-inflicted drug overdoses during a work shift.
It’s unusual for one hospital to have two caregivers die of overdoses in such a short time, experts say.
“This is an extreme example,” says Kimberly New, a nurse and lawyer in Tennessee who consults with hospitals nationwide on how to prevent diversions. “That type of alarming situation would be the reason to bring someone in and look at their controls.”
For addicted health care staff, access to their drug of need can be as near as the hospital’s drug supply room. In the past 4 years, hospitals in Texas have reported more than 200 thefts by employees. The tally is likely much higher, as thefts go undetected. The consequences of the thefts in terms of overdoses and deaths are unknown, as those details are not tracked.
Other consequences also hit home for those tasked with providing care: While focusing on their addictions, a nurse or other caregiver can dangerously comprise their duties. This can, in turn, compromise patient care – and can threaten survival if an oversight or mistake is egregiously wrong.
The response by hospitals like the Clements facility is typically a hard-line approach to institute procedures to safeguard the drugs from diversion. This tact is necessary but completely overlooks the reasons for the drug addiction. As with such measures, the effect can be to drive the abuser underground. Hiding the addiction and raiding the hospital’s drug supply can be preferred over admitting the problem and risking the health care workers’ careers – and ultimately, their lives.
Younger players are not immune to the brain damage that can come with playing football, National Public Radio says, quoting a grim report from the annual meeting of the Radiological Society of North America.
The technique of magnetic resonance imaging, which essentially records a video of brain structure and function in real time, was used to scan the brains of 26 boys aged an average of 12 years before and after a season of football. The findings were compared with the brain scans of 26 other boys of similar age who were not football players, according to NPR.
Damage to the region that connects the two halves of the brain was evident in a majority of the football players but not in their control counterparts. in the shape of the corpus callosum. When these changes come at a time in life when the brain is developing, the results can be lifetime consequences on thought, behavior, and emotion.
“You have to understand that the NFL players were also most likely once collegiate players; they were also high school players and they were also probably youth players,” says radiologist Christopher T. Whitlow, MD, PhD, of Wake Forest Baptist Health in Winston-Salem, N.C., and a coauthor of the new findings, in the interview. “To us, it’s more than a question about concussions, it’s a question about long-term cumulative exposure.
“We don’t know what [the results] mean … do these changes persist over time? Do they accumulate with multiple seasons? And then No. 3, probably the most important: Do they have any relevance to long-term health?”
Average can be just fine
The need to excel is drilled into many people from childhood. Hard work is a virtue, but the pressure to shine can have disastrous consequences. In South Korea, for example, academic pressure is a major cause of suicide in youth.
A recent TED Women conference held in Palm Springs, Calif., provided some reassurance for those in the “forgotten middle” – those who were adequate but not stellar students, and who do their work diligently but not outstandingly.
“Those at the top get noticed and those at the bottom get extra help but no one really thinks about the kids in the middle who make up the majority,” says Danielle R. Moss Lee, EdD, a social activist and chief of the New York Civil Liberties Union, who spoke at the conference. These folks can be valuable contributors but are often overlooked. As a result, when it comes to excelling, they “check out.”
“We have to create different ways to harness their potential. I didn’t appreciate how average I was until I was a college student and I bumped into a science teacher and he couldn’t believe what college I was attending,” Dr. Lee says.
Sometimes it takes a push from a loved one to spur action. In Dr. Lee’s case, she says she was happy being an average student. Her mother’s search for extracurricular activities led her to discover writing and set her on a path to personal and professional accomplishment.
Dr. Lee’s message was that “the middle isn’t a permanent location.”
Others experts see the situation differently. “Most psychological traits are evenly distributed, meaning that a significant proportion of the population will have average intelligence and leadership potential,” says Tomas Chamorro-Premuzic, PhD, of University College London, in an interview with BBC News.
“The world’s progress depends on those who stand out via their exceptional and innovative contributions, but these individuals are part of the top 1% in their field, combining truly unconventional levels of talent, work ethic, and focus,” Dr. Chamorro-Premuzic says. “For the remaining 99% of us, the acceptance that our talents and motivation are much more conventional, and unlikely to result in world-changing accomplishments, would reflect a healthier, more rational self-concept than illusions of grandiosity or fantasized talent.”
Shared sorrow mark club members
A recent article in Time described the experiences of some who have lost their children and whose worlds have been forever altered. From all walks of life and diverse backgrounds, these folks become tethered together. “It’s a club you spend your whole life hoping you won’t ever become a part of,” says Nicole Hockley, whose son Dylan, 6, was killed in the December 2012 shooting at Sandy Hook Elementary School in Connecticut. “But once you’re in, you’re in.”
Mitchell Dworet and Melissa Wiley are connected by death of their children. Mr. Dworet’s 17-year-old son Nicholas was killed during the shooting at Marjory Stoneman Douglas High School in Parkland, Fla., in February 2018. A month later, Ms. Willey’s daughter Jaelynn, 16, was shot to death by a fellow student at Great Mills High School in Maryland. They connected through Facebook. “I felt like I should reach out. I wanted to pay it forward,” explains Mr. Dworet.
“When you’ve gone through this kind of tragedy with other people, you see their humanity, where they’re coming from,” says Darrell Scott, whose 17-year-old daughter Rachel was killed at Columbine. Politics can differ – as can views on the painful issue of gun control measures – and friendships might not develop. Still, however, they share one enduring bond.
The connection with others can help in the immediate aftermath, and can continue to be important over time. “When you lose a child violently and publicly, there’s an outpouring of support at first,” said Sandy Phillips, whose 24-year-old daughter Jessi was shot with 11 others at a cinema in Aurora, Colo., in 2012. “Once the vigils are over and the media is gone, that’s when things get really bad. The world moves on, and you don’t. You can’t. It’s a pain you can’t outrun.”
“A huge emotional jolt”
In the aftermath of the magnitude 7.0 earthquake that shook Anchorage, Alaska, on Nov. 30, and the many aftershocks, residents are scrambling to cope with their changed lives. For those who lost possessions, the pain is real. But there comes the realization for many that they survived and that material possessions can, for the most part, be replaced.
Psychological changes, meanwhile, can prove profound and lasting. Researchers have found that large earthquakes can produce PTSD and anxiety. Some survivors can come away from earthquakes with difficulty concentrating and hypervigilance.
As one resident explains to Anchorage Daily News, “I felt yesterday like I had one final nerve and every aftershock was playing on that nerve.”
K.J. Worbey, a mental health counselor for Southcentral Foundation – an Alaska Native health care organization – describes the experience as a “huge emotional jolt.” She adds there is “lots of uncertainly about our own safety. Safety of our families and our homes. ... When we are faced with that kind of an emotional crisis, it takes a whole lot of energy to navigate it.”
Ms. Worbey recommends limiting alcohol, eating a healthy diet, and exercising appropriately. “Try to get some energy out. Try and get that excess emotional stuff out,” she said. Other prudent measures include sticking to a normal routine as much as is possible, including mealtimes and sleep, and talking with neighbors and friends.
Drug diversions can cost lives
A recent article in the Dallas Morning News has highlighted the humanity of health caregivers. Within the past several years, two nurses at University of Texas Southwestern Medical Center’s Williams P. Clements Jr. Hospital in Dallas have died of self-inflicted drug overdoses during a work shift.
It’s unusual for one hospital to have two caregivers die of overdoses in such a short time, experts say.
“This is an extreme example,” says Kimberly New, a nurse and lawyer in Tennessee who consults with hospitals nationwide on how to prevent diversions. “That type of alarming situation would be the reason to bring someone in and look at their controls.”
For addicted health care staff, access to their drug of need can be as near as the hospital’s drug supply room. In the past 4 years, hospitals in Texas have reported more than 200 thefts by employees. The tally is likely much higher, as thefts go undetected. The consequences of the thefts in terms of overdoses and deaths are unknown, as those details are not tracked.
Other consequences also hit home for those tasked with providing care: While focusing on their addictions, a nurse or other caregiver can dangerously comprise their duties. This can, in turn, compromise patient care – and can threaten survival if an oversight or mistake is egregiously wrong.
The response by hospitals like the Clements facility is typically a hard-line approach to institute procedures to safeguard the drugs from diversion. This tact is necessary but completely overlooks the reasons for the drug addiction. As with such measures, the effect can be to drive the abuser underground. Hiding the addiction and raiding the hospital’s drug supply can be preferred over admitting the problem and risking the health care workers’ careers – and ultimately, their lives.
Younger players are not immune to the brain damage that can come with playing football, National Public Radio says, quoting a grim report from the annual meeting of the Radiological Society of North America.
The technique of magnetic resonance imaging, which essentially records a video of brain structure and function in real time, was used to scan the brains of 26 boys aged an average of 12 years before and after a season of football. The findings were compared with the brain scans of 26 other boys of similar age who were not football players, according to NPR.
Damage to the region that connects the two halves of the brain was evident in a majority of the football players but not in their control counterparts. in the shape of the corpus callosum. When these changes come at a time in life when the brain is developing, the results can be lifetime consequences on thought, behavior, and emotion.
“You have to understand that the NFL players were also most likely once collegiate players; they were also high school players and they were also probably youth players,” says radiologist Christopher T. Whitlow, MD, PhD, of Wake Forest Baptist Health in Winston-Salem, N.C., and a coauthor of the new findings, in the interview. “To us, it’s more than a question about concussions, it’s a question about long-term cumulative exposure.
“We don’t know what [the results] mean … do these changes persist over time? Do they accumulate with multiple seasons? And then No. 3, probably the most important: Do they have any relevance to long-term health?”
Average can be just fine
The need to excel is drilled into many people from childhood. Hard work is a virtue, but the pressure to shine can have disastrous consequences. In South Korea, for example, academic pressure is a major cause of suicide in youth.
A recent TED Women conference held in Palm Springs, Calif., provided some reassurance for those in the “forgotten middle” – those who were adequate but not stellar students, and who do their work diligently but not outstandingly.
“Those at the top get noticed and those at the bottom get extra help but no one really thinks about the kids in the middle who make up the majority,” says Danielle R. Moss Lee, EdD, a social activist and chief of the New York Civil Liberties Union, who spoke at the conference. These folks can be valuable contributors but are often overlooked. As a result, when it comes to excelling, they “check out.”
“We have to create different ways to harness their potential. I didn’t appreciate how average I was until I was a college student and I bumped into a science teacher and he couldn’t believe what college I was attending,” Dr. Lee says.
Sometimes it takes a push from a loved one to spur action. In Dr. Lee’s case, she says she was happy being an average student. Her mother’s search for extracurricular activities led her to discover writing and set her on a path to personal and professional accomplishment.
Dr. Lee’s message was that “the middle isn’t a permanent location.”
Others experts see the situation differently. “Most psychological traits are evenly distributed, meaning that a significant proportion of the population will have average intelligence and leadership potential,” says Tomas Chamorro-Premuzic, PhD, of University College London, in an interview with BBC News.
“The world’s progress depends on those who stand out via their exceptional and innovative contributions, but these individuals are part of the top 1% in their field, combining truly unconventional levels of talent, work ethic, and focus,” Dr. Chamorro-Premuzic says. “For the remaining 99% of us, the acceptance that our talents and motivation are much more conventional, and unlikely to result in world-changing accomplishments, would reflect a healthier, more rational self-concept than illusions of grandiosity or fantasized talent.”
Shared sorrow mark club members
A recent article in Time described the experiences of some who have lost their children and whose worlds have been forever altered. From all walks of life and diverse backgrounds, these folks become tethered together. “It’s a club you spend your whole life hoping you won’t ever become a part of,” says Nicole Hockley, whose son Dylan, 6, was killed in the December 2012 shooting at Sandy Hook Elementary School in Connecticut. “But once you’re in, you’re in.”
Mitchell Dworet and Melissa Wiley are connected by death of their children. Mr. Dworet’s 17-year-old son Nicholas was killed during the shooting at Marjory Stoneman Douglas High School in Parkland, Fla., in February 2018. A month later, Ms. Willey’s daughter Jaelynn, 16, was shot to death by a fellow student at Great Mills High School in Maryland. They connected through Facebook. “I felt like I should reach out. I wanted to pay it forward,” explains Mr. Dworet.
“When you’ve gone through this kind of tragedy with other people, you see their humanity, where they’re coming from,” says Darrell Scott, whose 17-year-old daughter Rachel was killed at Columbine. Politics can differ – as can views on the painful issue of gun control measures – and friendships might not develop. Still, however, they share one enduring bond.
The connection with others can help in the immediate aftermath, and can continue to be important over time. “When you lose a child violently and publicly, there’s an outpouring of support at first,” said Sandy Phillips, whose 24-year-old daughter Jessi was shot with 11 others at a cinema in Aurora, Colo., in 2012. “Once the vigils are over and the media is gone, that’s when things get really bad. The world moves on, and you don’t. You can’t. It’s a pain you can’t outrun.”
“A huge emotional jolt”
In the aftermath of the magnitude 7.0 earthquake that shook Anchorage, Alaska, on Nov. 30, and the many aftershocks, residents are scrambling to cope with their changed lives. For those who lost possessions, the pain is real. But there comes the realization for many that they survived and that material possessions can, for the most part, be replaced.
Psychological changes, meanwhile, can prove profound and lasting. Researchers have found that large earthquakes can produce PTSD and anxiety. Some survivors can come away from earthquakes with difficulty concentrating and hypervigilance.
As one resident explains to Anchorage Daily News, “I felt yesterday like I had one final nerve and every aftershock was playing on that nerve.”
K.J. Worbey, a mental health counselor for Southcentral Foundation – an Alaska Native health care organization – describes the experience as a “huge emotional jolt.” She adds there is “lots of uncertainly about our own safety. Safety of our families and our homes. ... When we are faced with that kind of an emotional crisis, it takes a whole lot of energy to navigate it.”
Ms. Worbey recommends limiting alcohol, eating a healthy diet, and exercising appropriately. “Try to get some energy out. Try and get that excess emotional stuff out,” she said. Other prudent measures include sticking to a normal routine as much as is possible, including mealtimes and sleep, and talking with neighbors and friends.
Drug diversions can cost lives
A recent article in the Dallas Morning News has highlighted the humanity of health caregivers. Within the past several years, two nurses at University of Texas Southwestern Medical Center’s Williams P. Clements Jr. Hospital in Dallas have died of self-inflicted drug overdoses during a work shift.
It’s unusual for one hospital to have two caregivers die of overdoses in such a short time, experts say.
“This is an extreme example,” says Kimberly New, a nurse and lawyer in Tennessee who consults with hospitals nationwide on how to prevent diversions. “That type of alarming situation would be the reason to bring someone in and look at their controls.”
For addicted health care staff, access to their drug of need can be as near as the hospital’s drug supply room. In the past 4 years, hospitals in Texas have reported more than 200 thefts by employees. The tally is likely much higher, as thefts go undetected. The consequences of the thefts in terms of overdoses and deaths are unknown, as those details are not tracked.
Other consequences also hit home for those tasked with providing care: While focusing on their addictions, a nurse or other caregiver can dangerously comprise their duties. This can, in turn, compromise patient care – and can threaten survival if an oversight or mistake is egregiously wrong.
The response by hospitals like the Clements facility is typically a hard-line approach to institute procedures to safeguard the drugs from diversion. This tact is necessary but completely overlooks the reasons for the drug addiction. As with such measures, the effect can be to drive the abuser underground. Hiding the addiction and raiding the hospital’s drug supply can be preferred over admitting the problem and risking the health care workers’ careers – and ultimately, their lives.
Nicotine patch may be an effective precision therapy for select epilepsies
NEW ORLEANS – according to research presented at the annual meeting of the American Epilepsy Society. Of four epilepsy patients at one center who received nicotine-patch treatment, three had a good clinical response, one of whom became seizure free.
“We confirm that, in select patients, treatment with a nicotine patch ... can be an effective precision therapy for epilepsy. We propose consideration of nicotine-patch treatment in refractory patients with known cholinergic nicotine receptor subunit variants, especially those with a clinical history consistent with autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE),” said Jordana Fox, DO, and Alison Dolce, MD, both with the University of Texas Southwestern Medical Center in Dallas.
Gene variants in CHRAn4,CHRNA2, and CHRNB2 can cause ADNFLE. Preclinical and n-of-1 studies have suggested that nicotine may be a precision treatment for ADNFLE.
Dr. Fox and Dr. Dolce reviewed next-generation sequencing epilepsy panels from patients seen at Children’s Medical Center, Dallas, during 2011-2015 to identify patients with nAChR gene variants (CHNRA4, CHRNA2, CHRNB2, and CHRNA7). They reviewed patients’ medical and laboratory records, including genetic variant details and treatment history, and focused on patients who underwent a trial of nicotine-patch treatment.
Of the 21 patients who had nAChR gene variants, 4 tried treatment with a nicotine patch, either 7 mg or 14 mg. The patients who received nicotine-patch treatment had genetic variants in CHRNA4, CHRNB2, and CHRNA2. Three of the patients who tried nicotine-patch treatment had a greater than 50% reduction in seizures, whereas one had no treatment response.
“One patient became seizure free and is now treated with the nicotine patch as monotherapy,” Dr. Fox said.
The patient with complete resolution of seizures had a heterozygous disease–causing mutation in CHRNB2. This patient had nocturnal focal seizures, normal neuroimaging, and had been receiving treatment with oxcarbazepine and zonisamide.
The review identified four patients with nAChR gene variants and the ADNFLE phenotype who have not been treated with nicotine. Further phenotype-genotype characterizations and preclinical studies will help neurologists understand the mechanisms of these complex gene variants.
The researchers received no funding for the study and had no relevant financial disclosures.
SOURCE: Fox J et al. AES 2018, Abstract 1.230.
NEW ORLEANS – according to research presented at the annual meeting of the American Epilepsy Society. Of four epilepsy patients at one center who received nicotine-patch treatment, three had a good clinical response, one of whom became seizure free.
“We confirm that, in select patients, treatment with a nicotine patch ... can be an effective precision therapy for epilepsy. We propose consideration of nicotine-patch treatment in refractory patients with known cholinergic nicotine receptor subunit variants, especially those with a clinical history consistent with autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE),” said Jordana Fox, DO, and Alison Dolce, MD, both with the University of Texas Southwestern Medical Center in Dallas.
Gene variants in CHRAn4,CHRNA2, and CHRNB2 can cause ADNFLE. Preclinical and n-of-1 studies have suggested that nicotine may be a precision treatment for ADNFLE.
Dr. Fox and Dr. Dolce reviewed next-generation sequencing epilepsy panels from patients seen at Children’s Medical Center, Dallas, during 2011-2015 to identify patients with nAChR gene variants (CHNRA4, CHRNA2, CHRNB2, and CHRNA7). They reviewed patients’ medical and laboratory records, including genetic variant details and treatment history, and focused on patients who underwent a trial of nicotine-patch treatment.
Of the 21 patients who had nAChR gene variants, 4 tried treatment with a nicotine patch, either 7 mg or 14 mg. The patients who received nicotine-patch treatment had genetic variants in CHRNA4, CHRNB2, and CHRNA2. Three of the patients who tried nicotine-patch treatment had a greater than 50% reduction in seizures, whereas one had no treatment response.
“One patient became seizure free and is now treated with the nicotine patch as monotherapy,” Dr. Fox said.
The patient with complete resolution of seizures had a heterozygous disease–causing mutation in CHRNB2. This patient had nocturnal focal seizures, normal neuroimaging, and had been receiving treatment with oxcarbazepine and zonisamide.
The review identified four patients with nAChR gene variants and the ADNFLE phenotype who have not been treated with nicotine. Further phenotype-genotype characterizations and preclinical studies will help neurologists understand the mechanisms of these complex gene variants.
The researchers received no funding for the study and had no relevant financial disclosures.
SOURCE: Fox J et al. AES 2018, Abstract 1.230.
NEW ORLEANS – according to research presented at the annual meeting of the American Epilepsy Society. Of four epilepsy patients at one center who received nicotine-patch treatment, three had a good clinical response, one of whom became seizure free.
“We confirm that, in select patients, treatment with a nicotine patch ... can be an effective precision therapy for epilepsy. We propose consideration of nicotine-patch treatment in refractory patients with known cholinergic nicotine receptor subunit variants, especially those with a clinical history consistent with autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE),” said Jordana Fox, DO, and Alison Dolce, MD, both with the University of Texas Southwestern Medical Center in Dallas.
Gene variants in CHRAn4,CHRNA2, and CHRNB2 can cause ADNFLE. Preclinical and n-of-1 studies have suggested that nicotine may be a precision treatment for ADNFLE.
Dr. Fox and Dr. Dolce reviewed next-generation sequencing epilepsy panels from patients seen at Children’s Medical Center, Dallas, during 2011-2015 to identify patients with nAChR gene variants (CHNRA4, CHRNA2, CHRNB2, and CHRNA7). They reviewed patients’ medical and laboratory records, including genetic variant details and treatment history, and focused on patients who underwent a trial of nicotine-patch treatment.
Of the 21 patients who had nAChR gene variants, 4 tried treatment with a nicotine patch, either 7 mg or 14 mg. The patients who received nicotine-patch treatment had genetic variants in CHRNA4, CHRNB2, and CHRNA2. Three of the patients who tried nicotine-patch treatment had a greater than 50% reduction in seizures, whereas one had no treatment response.
“One patient became seizure free and is now treated with the nicotine patch as monotherapy,” Dr. Fox said.
The patient with complete resolution of seizures had a heterozygous disease–causing mutation in CHRNB2. This patient had nocturnal focal seizures, normal neuroimaging, and had been receiving treatment with oxcarbazepine and zonisamide.
The review identified four patients with nAChR gene variants and the ADNFLE phenotype who have not been treated with nicotine. Further phenotype-genotype characterizations and preclinical studies will help neurologists understand the mechanisms of these complex gene variants.
The researchers received no funding for the study and had no relevant financial disclosures.
SOURCE: Fox J et al. AES 2018, Abstract 1.230.
REPORTING FROM AES 2018
Key clinical point: In select patients with epilepsy, nicotine may be an effective precision therapy.
Major finding: Of four patients who received nicotine-patch treatment at one center, three had a good clinical response, one of whom became seizure free.
Study details: Single-center chart review of 21 patients with gene variants in subunits of the nicotinic acetylcholine receptor.
Disclosures: The researchers received no funding for the study and had no relevant financial disclosures.
Source: Fox J et al. AES 2018, Abstract 1.230.
Common AEDs confer modestly increased risk of major congenital malformations
NEW ORLEANS – The most commonly used antiepileptic drugs modestly increased the risk of major congenital malformations among prenatally exposed infants in the MONEAD study.
Malformations occurred among 5% of pregnancies exposed to the medications – higher than the 2% background rate – but this was still much lower than the 9%-10% rate associated with valproate.
Overall, however, the message of the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic (MONEAD) study is quite reassuring, Kimford J. Meador, MD, said at the annual meeting of the American Epilepsy Society. MONEAD is an ongoing, prospective study to determine both maternal outcomes and long-term childhood neurodevelopmental outcomes associated with the use of antiepileptic drugs (AEDs) during pregnancy.
“The rate of malformations was higher than I thought it would be, and higher than the 2% background rate, but it’s still a modest increase and most babies are born completely normal,” Dr. Meador, professor of neurology and neurosciences at Stanford (Calif.) University, said in an interview. “I think the news here is good, and it’s especially reassuring when you put it in the context that, 60 years ago, there were laws that women with epilepsy couldn’t get married, and some states even had laws to sterilize women. I think that’s absurd when most infants born to these women are without malformations and the risk of miscarriage is very low.”
Another positive finding, he said, is that valproate use among pregnant women is now practically nonexistent. Only 1 of 351 pregnant women with epilepsy and just 2 of a comparator group of 109 nonpregnant women with epilepsy were taking it. That’s great news, said Dr. Meador, who also initiated the NEAD (Neurodevelopmental Effects of Antiepileptic Drugs) study in the early 2000s. NEAD determined the drug’s serious teratogenic potential.
In addition to the cohorts of pregnant and nonpregnant women with epilepsy, 105 healthy pregnant women enrolled in the MONEAD study. Women will be monitored during pregnancy and postpartum to measure maternal outcomes and their children will be monitored from birth through age 6 years to measure their health and developmental outcomes.
The study has six primary outcomes, three for the women and three for their children.
- Determine if women with epilepsy have increased seizures during pregnancy and delineate the contributing factors.
- Determine if C-section rate is increased in women with epilepsy and delineate contributing factors.
- Determine if women with epilepsy have an increased risk for depression during pregnancy and the postpartum period and characterize risk factors.
- Determine the long-term effects of in utero AED exposure on verbal intellectual abilities and other neurobehavioral outcomes.
- Determine if small-for-gestational age and other adverse neonatal outcomes are increased.
- Determine if breastfeeding when taking AEDs impairs the child’s ultimate verbal and other cognitive outcomes.
Rates of miscarriage and neonatal malformations were not primary study outcomes, but the descriptive data were collected and are of high interest, Dr. Meador said.
At baseline, all the women had a mean age of about 30 years. Most (75%) were on monotherapy, 20% were on polytherapy, and the rest were not taking an AED. About 60% had focal epilepsy, 31% had generalized epilepsy, and the remainder had an unclassified seizure disorder. Three subjects had multiple seizure types. The most commonly used AEDs were lamotrigine and levetiracetam (both about 30%); 4% were taking zonisamide, 4% carbamazepine, and 4% oxcarbazepine. Topiramate was being used for 2% of the pregnant woman and 5% of the nonpregnant woman. The combination of lamotrigine and levetiracetam was used for 9.0% of pregnant and 5.5% of nonpregnant women, and other polytherapies in 12.0% of the pregnant and 14.0% of the nonpregnant woman. About 4% of the pregnant and 1% of the nonpregnant women were not taking any AED.
There were 10 (2.8%) spontaneous miscarriages among the pregnant women with epilepsy and none among the healthy pregnant women. Spontaneous miscarriages weren’t associated with acute seizures, and there were no major congenital malformations reported among them. There were also two elective abortions among the pregnant women with epilepsy.
There were 18 major congenital malformations among the pregnant woman with epilepsy (5%). A total of 14 were among pregnancies exposed to monotherapy, 3 were in polytherapy-exposed pregnancies, and 1 was in the group not taking any AEDs.
The malformations were:
- Carbamazepine (one case) – hydronephrosis.
- Gabapentin (one case) – inguinal hernia.
- Lamotrigine (five cases) – aortic coarctation, cryptorchidism, hydronephrosis, pectus excavatum, and morning glory syndrome (a funnel-shaped optic nerve disc associated with impaired visual acuity).
- Levetiracetam (five cases) – atrial septal defect, buried penis syndrome, cryptorchidism, hypoplastic aortic valve, ventricular septal defect.
- Topiramate (one case) – ventricular septal defect.
- Zonisamide (one case) – inguinal hernia, absent pinna.
- Lamotrigine plus clonazepam (one case) – cardiomyopathy.
- Lamotrigine plus levetiracetam (one case) – microcephaly, myelomeningocele, Chiari II malformation.
- Levetiracetam plus phenobarbital (one case) – bilateral inguinal hernia.
MONEAD is funded by the National Institutes of Health; Dr. Meador reported no financial disclosures.
SOURCE: Meador KJ et al. AES 2018, Abstract 3.231.
NEW ORLEANS – The most commonly used antiepileptic drugs modestly increased the risk of major congenital malformations among prenatally exposed infants in the MONEAD study.
Malformations occurred among 5% of pregnancies exposed to the medications – higher than the 2% background rate – but this was still much lower than the 9%-10% rate associated with valproate.
Overall, however, the message of the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic (MONEAD) study is quite reassuring, Kimford J. Meador, MD, said at the annual meeting of the American Epilepsy Society. MONEAD is an ongoing, prospective study to determine both maternal outcomes and long-term childhood neurodevelopmental outcomes associated with the use of antiepileptic drugs (AEDs) during pregnancy.
“The rate of malformations was higher than I thought it would be, and higher than the 2% background rate, but it’s still a modest increase and most babies are born completely normal,” Dr. Meador, professor of neurology and neurosciences at Stanford (Calif.) University, said in an interview. “I think the news here is good, and it’s especially reassuring when you put it in the context that, 60 years ago, there were laws that women with epilepsy couldn’t get married, and some states even had laws to sterilize women. I think that’s absurd when most infants born to these women are without malformations and the risk of miscarriage is very low.”
Another positive finding, he said, is that valproate use among pregnant women is now practically nonexistent. Only 1 of 351 pregnant women with epilepsy and just 2 of a comparator group of 109 nonpregnant women with epilepsy were taking it. That’s great news, said Dr. Meador, who also initiated the NEAD (Neurodevelopmental Effects of Antiepileptic Drugs) study in the early 2000s. NEAD determined the drug’s serious teratogenic potential.
In addition to the cohorts of pregnant and nonpregnant women with epilepsy, 105 healthy pregnant women enrolled in the MONEAD study. Women will be monitored during pregnancy and postpartum to measure maternal outcomes and their children will be monitored from birth through age 6 years to measure their health and developmental outcomes.
The study has six primary outcomes, three for the women and three for their children.
- Determine if women with epilepsy have increased seizures during pregnancy and delineate the contributing factors.
- Determine if C-section rate is increased in women with epilepsy and delineate contributing factors.
- Determine if women with epilepsy have an increased risk for depression during pregnancy and the postpartum period and characterize risk factors.
- Determine the long-term effects of in utero AED exposure on verbal intellectual abilities and other neurobehavioral outcomes.
- Determine if small-for-gestational age and other adverse neonatal outcomes are increased.
- Determine if breastfeeding when taking AEDs impairs the child’s ultimate verbal and other cognitive outcomes.
Rates of miscarriage and neonatal malformations were not primary study outcomes, but the descriptive data were collected and are of high interest, Dr. Meador said.
At baseline, all the women had a mean age of about 30 years. Most (75%) were on monotherapy, 20% were on polytherapy, and the rest were not taking an AED. About 60% had focal epilepsy, 31% had generalized epilepsy, and the remainder had an unclassified seizure disorder. Three subjects had multiple seizure types. The most commonly used AEDs were lamotrigine and levetiracetam (both about 30%); 4% were taking zonisamide, 4% carbamazepine, and 4% oxcarbazepine. Topiramate was being used for 2% of the pregnant woman and 5% of the nonpregnant woman. The combination of lamotrigine and levetiracetam was used for 9.0% of pregnant and 5.5% of nonpregnant women, and other polytherapies in 12.0% of the pregnant and 14.0% of the nonpregnant woman. About 4% of the pregnant and 1% of the nonpregnant women were not taking any AED.
There were 10 (2.8%) spontaneous miscarriages among the pregnant women with epilepsy and none among the healthy pregnant women. Spontaneous miscarriages weren’t associated with acute seizures, and there were no major congenital malformations reported among them. There were also two elective abortions among the pregnant women with epilepsy.
There were 18 major congenital malformations among the pregnant woman with epilepsy (5%). A total of 14 were among pregnancies exposed to monotherapy, 3 were in polytherapy-exposed pregnancies, and 1 was in the group not taking any AEDs.
The malformations were:
- Carbamazepine (one case) – hydronephrosis.
- Gabapentin (one case) – inguinal hernia.
- Lamotrigine (five cases) – aortic coarctation, cryptorchidism, hydronephrosis, pectus excavatum, and morning glory syndrome (a funnel-shaped optic nerve disc associated with impaired visual acuity).
- Levetiracetam (five cases) – atrial septal defect, buried penis syndrome, cryptorchidism, hypoplastic aortic valve, ventricular septal defect.
- Topiramate (one case) – ventricular septal defect.
- Zonisamide (one case) – inguinal hernia, absent pinna.
- Lamotrigine plus clonazepam (one case) – cardiomyopathy.
- Lamotrigine plus levetiracetam (one case) – microcephaly, myelomeningocele, Chiari II malformation.
- Levetiracetam plus phenobarbital (one case) – bilateral inguinal hernia.
MONEAD is funded by the National Institutes of Health; Dr. Meador reported no financial disclosures.
SOURCE: Meador KJ et al. AES 2018, Abstract 3.231.
NEW ORLEANS – The most commonly used antiepileptic drugs modestly increased the risk of major congenital malformations among prenatally exposed infants in the MONEAD study.
Malformations occurred among 5% of pregnancies exposed to the medications – higher than the 2% background rate – but this was still much lower than the 9%-10% rate associated with valproate.
Overall, however, the message of the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic (MONEAD) study is quite reassuring, Kimford J. Meador, MD, said at the annual meeting of the American Epilepsy Society. MONEAD is an ongoing, prospective study to determine both maternal outcomes and long-term childhood neurodevelopmental outcomes associated with the use of antiepileptic drugs (AEDs) during pregnancy.
“The rate of malformations was higher than I thought it would be, and higher than the 2% background rate, but it’s still a modest increase and most babies are born completely normal,” Dr. Meador, professor of neurology and neurosciences at Stanford (Calif.) University, said in an interview. “I think the news here is good, and it’s especially reassuring when you put it in the context that, 60 years ago, there were laws that women with epilepsy couldn’t get married, and some states even had laws to sterilize women. I think that’s absurd when most infants born to these women are without malformations and the risk of miscarriage is very low.”
Another positive finding, he said, is that valproate use among pregnant women is now practically nonexistent. Only 1 of 351 pregnant women with epilepsy and just 2 of a comparator group of 109 nonpregnant women with epilepsy were taking it. That’s great news, said Dr. Meador, who also initiated the NEAD (Neurodevelopmental Effects of Antiepileptic Drugs) study in the early 2000s. NEAD determined the drug’s serious teratogenic potential.
In addition to the cohorts of pregnant and nonpregnant women with epilepsy, 105 healthy pregnant women enrolled in the MONEAD study. Women will be monitored during pregnancy and postpartum to measure maternal outcomes and their children will be monitored from birth through age 6 years to measure their health and developmental outcomes.
The study has six primary outcomes, three for the women and three for their children.
- Determine if women with epilepsy have increased seizures during pregnancy and delineate the contributing factors.
- Determine if C-section rate is increased in women with epilepsy and delineate contributing factors.
- Determine if women with epilepsy have an increased risk for depression during pregnancy and the postpartum period and characterize risk factors.
- Determine the long-term effects of in utero AED exposure on verbal intellectual abilities and other neurobehavioral outcomes.
- Determine if small-for-gestational age and other adverse neonatal outcomes are increased.
- Determine if breastfeeding when taking AEDs impairs the child’s ultimate verbal and other cognitive outcomes.
Rates of miscarriage and neonatal malformations were not primary study outcomes, but the descriptive data were collected and are of high interest, Dr. Meador said.
At baseline, all the women had a mean age of about 30 years. Most (75%) were on monotherapy, 20% were on polytherapy, and the rest were not taking an AED. About 60% had focal epilepsy, 31% had generalized epilepsy, and the remainder had an unclassified seizure disorder. Three subjects had multiple seizure types. The most commonly used AEDs were lamotrigine and levetiracetam (both about 30%); 4% were taking zonisamide, 4% carbamazepine, and 4% oxcarbazepine. Topiramate was being used for 2% of the pregnant woman and 5% of the nonpregnant woman. The combination of lamotrigine and levetiracetam was used for 9.0% of pregnant and 5.5% of nonpregnant women, and other polytherapies in 12.0% of the pregnant and 14.0% of the nonpregnant woman. About 4% of the pregnant and 1% of the nonpregnant women were not taking any AED.
There were 10 (2.8%) spontaneous miscarriages among the pregnant women with epilepsy and none among the healthy pregnant women. Spontaneous miscarriages weren’t associated with acute seizures, and there were no major congenital malformations reported among them. There were also two elective abortions among the pregnant women with epilepsy.
There were 18 major congenital malformations among the pregnant woman with epilepsy (5%). A total of 14 were among pregnancies exposed to monotherapy, 3 were in polytherapy-exposed pregnancies, and 1 was in the group not taking any AEDs.
The malformations were:
- Carbamazepine (one case) – hydronephrosis.
- Gabapentin (one case) – inguinal hernia.
- Lamotrigine (five cases) – aortic coarctation, cryptorchidism, hydronephrosis, pectus excavatum, and morning glory syndrome (a funnel-shaped optic nerve disc associated with impaired visual acuity).
- Levetiracetam (five cases) – atrial septal defect, buried penis syndrome, cryptorchidism, hypoplastic aortic valve, ventricular septal defect.
- Topiramate (one case) – ventricular septal defect.
- Zonisamide (one case) – inguinal hernia, absent pinna.
- Lamotrigine plus clonazepam (one case) – cardiomyopathy.
- Lamotrigine plus levetiracetam (one case) – microcephaly, myelomeningocele, Chiari II malformation.
- Levetiracetam plus phenobarbital (one case) – bilateral inguinal hernia.
MONEAD is funded by the National Institutes of Health; Dr. Meador reported no financial disclosures.
SOURCE: Meador KJ et al. AES 2018, Abstract 3.231.
REPORTING FROM AES 2018
Key clinical point:
Major finding: The malformation rate was 5% in exposed pregnancies.
Study details: The MONEAD study comprised 351 pregnant women with epilepsy, 109 nonpregnant women with epilepsy, and 105 healthy pregnant women.
Disclosures: The National Institutes of Health funded the study; Dr. Meador reported no financial disclosures.
Source: Meador KJ et al. AES 2018, Abstract 3.231.