User login
Spinal muscular atrophy: Patient care in the age of genetically targeted therapy
In 2016, the U.S. Food and Drug Administration approved nusinersen, the first treatment for spinal muscular atrophy (SMA). Until then, SMA had a mortality rate nearly double that of the general population.1 Two-thirds of patients were symptomatic within 6 months of birth and, in the absence of mechanical ventilation and other support, had a nearly 100% mortality rate by age 2.2
Five years later, there are three approved treatments for SMA, all of which have been shown to slow or even halt disease progression in many patients. Neurologists, whose SMA patient population once consisted almost entirely of children, are now treating more adults with the disease. Indeed, more than half of all people alive with SMA in the United States today are adults, according to Cure SMA.
“Managing SMA used to be clinic follow-ups where we were doing our best supportive care and watching people fall apart before our eyes,” said John Brandsema, MD, a physician and neuromuscular section head at the Children’s Hospital of Philadelphia. “Today, what we see in the vast majority of people is that they are either the same as they were before – which is completely against the natural history of this disease and something to be celebrated – or that people are really better with their function. It totally changes everything in the clinic.”
Among those changes are a more proactive approach to rehabilitation and an even greater emphasis on personalized medicine and multidisciplinary care. But there is also a need for updated treatment guidelines, a new classification system to measure disease severity, specific biomarkers to guide therapy choices, more data on long-term efficacy of existing therapeutics, new medications to complement those therapies, and a deeper understanding of a disease that may have treatment options but still has no cure.
Advances in early diagnosis
Patients with SMA lack a working copy of the survival motor neuron 1 (SMN1) gene, which provides instructions for producing a protein called SMN that is critical for the maintenance and function of motor neurons. Without this protein, motor neurons eventually die, causing debilitating and progressive muscle weakness that affects the ability to walk, eat, and breathe. SMA is rare, affecting about 1 in 10,000 newborns.
In approximately 96% of patients, SMA is caused by homozygous loss of the SMN1 gene. People with SMA have at least one copy of the SMN2 gene, sometimes called a “backup” gene, that also produces SMN protein. However, a single nucleotide difference between SMN2 and SMN1 causes about 90% of the protein produced by SMN2 to be truncated and less stable. Even with multiple copies of SMN2 present, as is the case with many infants with SMA, the amount of functional protein produced isn’t enough to compensate for the loss of SMN1.3
All three approved medications are SMN up-regulators and work to increase the amount of functional SMN protein. Starting these medications early, even before symptoms present, is critical to preserve motor function. Early treatment depends on early diagnosis, which became more widespread after 2018 when SMA was added to the federally Recommended Uniform Screening Panel for newborns. As of July 1, 2022, 47 states have incorporated SMA newborn screening into their state panel, ensuring that 97% of all infants born in the United States undergo SMA screening shortly after birth. Screening in the remaining states – Hawaii, Nevada, and South Carolina – and Washington, D.C. is expected by mid-2023.
SMA newborn screening is a PCR-based assay that detects homozygous SMN1 gene deletion found in about 95% of all people with SMA. The remaining 5% of cases are caused by various genetic mutations that can only be detected with gene sequencing. In these cases, and in children who don’t undergo SMA newborn screening, the disease is usually identified when symptoms are noticed by a parent, pediatrician, or primary care provider. But a study found that in 2018 only 52.7% of pediatricians correctly identified genetic testing as a requirement for a definitive diagnosis of SMA; in 2019, with a larger sample size, that number decreased to 45%.4 The lack of awareness of diagnostic requirements for SMA could contribute to delays in diagnosis, said Mary Schroth, MD, chief medical officer for Cure SMA and a coauthor of the study.
“In our world, suspicion of SMA in an infant is an emergency situation,” Dr. Schroth said. “These babies need to be referred immediately and have genetic testing so that treatment can begin as soon as possible.”
Based on the study findings, Dr. Schroth and others with Cure SMA launched a new tool in 2021 designed to help pediatricians, primary care physicians, and parents identify early signs of SMA, so that a referral to a pediatric neurologist happens quickly. Called SMArt Moves, the educational resource features videos and a checklist to help increase early detection in infants who had a negative SMA newborn screening result or did not receive SMA screening at birth.5
Who to treat, when, and with which treatment
For many patients, having multiple effective treatment options means that SMA is no longer a fatal disease in early childhood, but one that can be managed into adolescence and adulthood. The question for clinicians is, who do they treat, when, and with which treatment?
Studies have long shown that the number of copies of the backup gene that a patient has is inversely associated with disease severity.6 In 2018, a group of SMA experts published a treatment algorithm to help guide decision-making following a positive SMA newborn screening.7 The treatment guidelines were updated in 2020 based on clinical trial data for presymptomatic infants, and current recommendations include immediate treatment for infants with two to four copies of the SMN2 gene.8 For patients with only one copy of SMN2, most of whom will likely be symptomatic at birth, the guidelines recommend that treatment decisions be made jointly between the clinician and the family.7,8
Some suggest that the number of SMN2 copies a patient has should also be a factor in determining phenotype, which has started a conversation on the development of a new classification system.9 The original classification system for disease severity – Types 0-4 – was based on age of onset and degree of motor function achieved, with Type 0 developing prenatally and being the most severe and Type 4 developing in adulthood. Type 1 is the most common, affecting more than half of all people with SMA, followed by Types 2-4. In 2018, updated consensus care guidelines offered a revised classification system that better reflected disease progression in the age of therapy. The functional motor outcomes include nonsitters (historically Type I), sitters (historically Type 2/3), and walkers (historically Type 3/4).10,11 These guidelines are a start, but clinicians say more revision is needed.
“Types 1, 2, 3, 4 were based on function – getting to a certain point and then losing it, but now that we can treat this disease, people will shift categories based on therapeutic response or based on normal development that is possible now that the neurologic piece has been stabilized,” Dr. Brandsema said. “We need to completely change our thinking around all these different aspects of SMA management.”
While discussions of a new classification system for SMA are underway, another effort to update treatment recommendations is closer to completion. Led by Cure SMA, a group of about 50 physician experts in the United States and Europe who specialize in SMA are revising guidelines for diagnosis and treatment, the first time the recommendations have been updated since 2018. The updated recommendations, which should be published later this year, will focus on diagnosis and treatment considerations.
“We have three treatments that are available, and there are specific FDA indications for each of those, but it’s not totally clear just how those medications should be used or applied to different clinical situations,” said Dr. Schroth. “We’re in a rapid phase of learning right now in the SMA community, trying to understand how these treatments alter physiology and disease outcomes and how to best use the tools that we now have available to us. In parallel with clinical treatments, we have to be doing the best care we can to optimize the outcomes for those treatments.”
Research advances in 2021
Although all three drugs approved to treat SMA – nusinersen (Spinraza; Biogen), onasemnogene abeparvovec-xioi gene replacement therapy (Zolgensma; Novartis Gene Therapies), and risdiplam (Evrysdi, Genentech/Roche) – are highly effective, there are still unanswered questions and unmet needs. New research findings from 2021 focused on higher dosing, different drug-delivery methods, combination therapy, and complementary therapeutics to address SMA comorbidities.
Higher-dose nusinersen. The first drug approved to treat SMA, nusinersen is an antisense oligonucleotide approved for all ages and all SMA types. It works by altering splicing of the SMN2 gene pre-mRNA to make more complete SMN protein. Given as an intrathecal (IT) injection, four “loading doses” are administered within the first 2 months of treatment, followed by a maintenance dose every 4 months for the duration of the individual’s life.
Reports from patients of waning effects of nusinersen just prior to follow-up treatment have led some clinicians to ask if a higher dose may be needed. A study underway seeks to address that issue.
DEVOTE is a phase 2/3 trial to study the safety and efficacy of high-dose nusinersen in patients with SMA. Preliminary findings reported in 2021 found no adverse events among patients treated with 28 mg of nusinersen for 161-257 days.12 Another analysis from this trial found that higher doses are associated with greater decrease of plasma phosphorylated neurofilament heavy chain (pNF-H) levels in patients with SMA and may lead to clinically meaningful improvement in motor function beyond that observed with the approved 12 mg dose.13 The trial is ongoing.
Another trial, ASCEND, is a phase 3B study assessing higher dose nusinersen in patients previously treated with risdiplam. Recruitment for that trial began in October 2021.
Long-term efficacy and IT administration of SMA therapy. Several studies are looking at the long-term efficacy and alternate routes of administration of onasemnogene abeparvovec and other SMA therapies.
A one-time gene replacement therapy delivered via an IV infusion replaces the function of the missing or nonworking SMN1 gene with a new, working copy of the SMN1 gene. FDA approved in 2019, it is authorized for use in patients with SMA up to 2 years of age.
The latest data from an ongoing, long-term follow-up safety study of onasemnogene abeparvovec, published in May 2021, suggest that the treatment’s effects persist more than 5 years after treatment. Researchers followed 13 infants with symptomatic SMA type 1 since the beginning of the phase 1 clinical trial of the gene transfer therapy. All patients who received the therapeutic dose maintained their baseline motor function, and two of the patients actually improved without other SMN-targeted treatment. At a median 6.2 years after they received treatment, all were alive and none needed permanent ventilation.14
After a 2-year hold by the FDA, a study of IT administration of onasemnogene abeparvovec is now enrolling patients. Citing concerns from animal studies that IT administration might result in dorsal root ganglia injury, the FDA issued a partial hold on the STRONG trial in 2019. Following positive study results in nonhuman primates, the FDA announced the trial can continue. Novartis is launching a new phase 3 STEER trial to test the drug delivered intrathecally in patients aged 2-18 years with Type 2 SMA. IT administration could allow the gene therapy to be used safely and effectively in more patients with SMA.
Efficacy of risdiplam in more patients. The first oral treatment for SMA was approved by the FDA in 2020. It’s given once per day in patients with SMA of all ages and disease types. The drug increases functional SMN protein production by the SMN2 gene.
A July 2021 publication of the results of the FIREFISH study found that infants with Type I SMA treated with risdiplam for 12 months were significantly more likely to achieve motor milestones, such as sitting without support, compared with untreated infants with Type 1 SMA.15 Risdiplam is also effective in older patients with Type 2 or 3 SMA, according to results published in December from the SUNFISH clinical trial.16 Another study, RAINBOWFISH, is studying safety and efficacy at 24 months in presymptomatic infants started on treatment at up to 6 weeks of age.
The efficacy of risdiplam in previously treated patients is the subject of JEWELFISH, an ongoing study in patients 6 months to 60 years with SMA. Preliminary data presented at the 2020 Virtual SMA Research and Clinical Care Meeting suggest treatment with risdiplam led to a median two-fold increase in the amount of blood SMN protein levels after 4 weeks, which was sustained for at least 24 months.17
Combination therapy. Among the more eagerly awaited results are those from studies of combination therapies, including those that combine approved SMN up-regulators with new non–SMN-targeted therapeutics.
“We’re seeing that while these three approved therapies have dramatic results, especially for infants who are treated presymptomatically, there are still unmet medical needs in those patients, particularly for older teens and adults whose disease may have progressed before they were able to start therapy,” said Jackie Glascock, PhD, vice president of research for Cure SMA.
Of particular interest are studies of myostatin inhibitors, therapeutics that block the production of the protein myostatin. Myostatin acts on muscle cells to reduce muscle growth. Animal studies suggest that inhibiting myostatin increases muscle mass, which could be important in patients with muscle loss due to SMA.
Three experimental myostatin inhibitors are currently in clinical trials. MANATEE is a global phase 2-3 trial that aims to evaluate the safety and efficacy of the antimyostatin antibody GYM329 (RO7204239) in combination with risdiplam. SAPPHIRE is a phase 3 trial of apitegromab (SRK-015) in combination with nusinersen or risdiplam. RESILIANT is a phase 3 trial of tadefgrobep alfa in combination with other treatments.
A trial is underway to study the efficacy and safety of nusinersen in patients with persistent symptoms of SMA after treatment with the gene therapy. The phase 4 study, RESPOND, is enrolling children aged 2-36 months.
What’s needed next
Despite the advances in treatment and patient care, Dr. Brandsema, Dr. Schroth, and Dr. Glascock note that there remain unmet needs in the SMA community in a variety of areas.
Increased focus on adults with SMA. Before nusinersen, treatment of SMA mainly involved treating its symptoms. Many patients stopped seeing their neurologist, relying more heavily on pulmonary care specialists and/or primary care providers to address breathing, nutrition, and mobility problems. “Now with the approval of these treatments, they’re coming back to see their neurologists and are becoming more visible in the SMA community,” Dr. Schroth said.
Despite this re-emergence, a 2020 meta-analysis of studies on adults with SMA found a paucity of data on physical and occupational therapy, respiratory management, mental health care, and palliative care.18
“There is just so much work we need to do in the area of adult clinical care of SMA.”
Treatment algorithms. While the development of the newborn screening algorithm and revised patient care guidelines are helpful resources, clinicians still face uncertainty when choosing which therapy will work best for their patients. Treatment algorithms that help clinicians figure out what therapy or combination of therapies will offer the best outcomes for individual patients are desperately needed, Dr. Brandsema said.
“Each person’s experience of this disease is so unique to the individual based partly on their genetics and partly on the factors about what got them into care and how compliant they are with everything we’re trying to do to help them,” he said. “Biomarkers would help clinicians create personalized treatment plans for each patient.”
More basic science. While scientists have a good understanding of the SMN gene, there are many unanswered questions about the function of the SMN protein and its relationship to motor neuron loss. SMN is a ubiquitously expressed protein, and its function in other cell types is largely unknown. Despite all of the research advances, there is much basic science left to be done.
“We are strongly advocating to regulatory authorities that these aren’t cures and we need to continue to invest in the basic research,” Dr. Glascock said. “These biological questions that pertain to SMN and its function and expression really drive drug development. I really think that understanding those pathways better will lead us to more druggable targets.”
Two deaths from liver failure linked to spinal muscular atrophy drug
Two children taking the gene therapy drug onasemnogene abeparvovec (Zolgensma, Novartis) for spinal muscular atrophy (SMA) have died from acute liver failure, according to a statement issued by the drug’s manufacturer.
The patients were 4 months and 28 months of age and lived in Russia and Kazakhstan. They died 5-6 weeks after infusion with Zolgensma and approximately 1-10 days after the initiation of a corticosteroid taper.
These are the first known fatal cases of acute liver failure associated with the drug, which the company notes was a known side effect included in the product label and in a boxed warning in the United States.
“Following two recent patient fatalities, and in alignment with health authorities, we will be updating the labeling to specify that fatal acute liver failure has been reported,” the statement reads.
“While this is important safety information, it is not a new safety signal,” it adds.
Rare genetic disorder
SMA is a rare genetic disorder that affects about 1 in 10,000 newborns. Patients with SMA lack a working copy of the survival motor neuron 1 (SMN1) gene, which encodes a protein called SMN that is critical for the maintenance and function of motor neurons.
Without this protein, motor neurons eventually die, causing debilitating and progressive muscle weakness that affects the ability to walk, eat, and breathe.
Zolgensma, a one-time gene replacement therapy delivered via intravenous infusion, replaces the function of the missing or nonworking SMN1 gene with a new, working copy of the SMN1 gene.
The first gene therapy treatment for SMA, it was approved by the U.S. Food and Drug Administration in 2019 for patients with SMA up to 2 years of age. It is also the most expensive drug in the world, costing about $2.1 million for a one-time treatment.
“We have notified health authorities in all markets where Zolgensma is used, including the FDA, and are communicating to relevant healthcare professionals as an additional step in markets where this action is supported by health authorities,” the manufacturer’s statement says.
Studies have suggested that the treatment’s effects persist more than 5 years after infusion.
Clinical trials currently underway by Novartis are studying the drug’s long-term efficacy and safety and its potential use in older patients.
The company is also leading the phase 3 clinical trial STEER to test intrathecal (IT) administration of the drug in patients ages 2-18 years who have type 2 SMA.
That trial began late last year after the FDA lifted a 2-year partial hold on an earlier study. The FDA halted the STRONG trial in 2019, citing concerns from animal studies that IT administration may result in dorsal root ganglia injury. The partial hold was released last fall following positive study results in nonhuman primates.
None of the current trials will be affected by the two deaths reported, according to a Novartis spokesperson.
Kelli Whitlock Burton is a staff writer/reporter for Medscape Neurology and MDedge Neurology.
References
1. Viscidi E et al. Comparative all-cause mortality among a large population of patients with spinal muscular atrophy versus matched controls. Neurol Ther. 2022 Mar;11(1):449-457. doi: 10.1007/s40120-021-00307-7.
2. Finkel RS et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014 Aug 26;83(9):810-817. doi: 10.1212/WNL.0000000000000741.
3. Klotz J et al. Advances in the therapy of spinal muscular atrophy. J Pediatr. 2021 Sep;236:13-20.e1. doi: 10.1016/j.jpeds.2021.06.033.
4. Curry M et al. Awareness screening and referral patterns among pediatricians in the United States related to early clinical features of spinal muscular atrophy (SMA). BMC Pediatr. 2021 May;21(1):236. doi: 10.1186/s12887-021-02692-2.
5. SMArt Moves. https://smartmoves.curesma.org/
6. Swoboda KJ et al. Natural history of denervation in SMA: Relation to age, SMN2 copy number, and function. Ann Neurol. 2005 May;57(5):704-12. doi: 10.1002/ana.20473.
7. Glascock J et al. Treatment algorithm for infants diagnosed with spinal muscular atrophy through newborn screening. J Neuromuscul Dis. 2018;5(2):145-158. doi: 10.3233/JND-180304.
8. Glascock J et al. Revised recommendations for the treatment of infants diagnosed with spinal muscular atrophy via newborn screening who have 4 copies of SMN2. J Neuromuscul Dis. 2020;7(2):97-100. doi: 10.3233/JND-190468.
9. Talbot K, Tizzano EF. The clinical landscape for SMA in a new therapeutic era. Gene Ther. 2017 Sep;24(9):529-533. doi: 10.1038/gt.2017.52.
10. Mercuri E et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018 Feb;28(2):103-115. doi: 10.1016/j.nmd.2017.11.005.
11. Finkel RS et al. Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord. 2018 Mar;28(3):197-207. doi: 10.1016/j.nmd.2017.11.004.
12. Pascual SI et al. Ongoing phase 2/3 DEVOTE (232SM203) randomized, controlled study to explore high-dose nusinersen in SMA: Part A interim results and Part B enrollment update. Presented at MDA Clinical and Scientific Conference 2021, Mar 15-18.
13. Finkel RS et al. Scientific rationale for a higher dose of nusinersen. Presented at 2021 Cure SMA Annual Meeting, Jun 9-11. Abstract P46.
14. Mendell JR et al. Five-year extension results of the phase 1 START trial of onasemnogene abeparvovec in spinal muscular atrophy. JAMA Neurol. 2021 Jul;78(7):834-841. doi: 10.1001/jamaneurol.2021.1272.
15. Darras BT et al. Risdiplam-treated infants with type 1 spinal muscular atrophy versus historical controls. N Engl J Med. 2021 Jul 29;385(5):427-435. doi: 10.1056/NEJMoa2102047.
16. Mercuri E et al. Safety and efficacy of once-daily risdiplam in type 2 and non-ambulant type 3 spinal muscular atrophy (SUNFISH part 2): A phase 3, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2022 Jan;21(1):42-52. doi: 10.1016/S1474-4422(21)00367-7. Erratum in: Lancet Neurol. 2022 Feb;21(2):e2. doi: 10.1016/S1474-4422(22)00006-0. Correction in: Lancet Neurol. 2022 Mar;21(3):e3. doi: 10.1016/S1474-4422(22)00038-2.
17. Genentech announces 2-year risdiplam data from SUNFISH and new data from JEWELFISH in infants, children and adults with SMA. https://www.curesma.org/genentech-risdiplam-data-conference-2020/
18. Wan HWY et al. Health, wellbeing and lived experiences of adults with SMA: a scoping systematic review. Orphanet J Rare Dis. 2020;15(1):70. doi: 10.1186/s13023-020-1339-3.
In 2016, the U.S. Food and Drug Administration approved nusinersen, the first treatment for spinal muscular atrophy (SMA). Until then, SMA had a mortality rate nearly double that of the general population.1 Two-thirds of patients were symptomatic within 6 months of birth and, in the absence of mechanical ventilation and other support, had a nearly 100% mortality rate by age 2.2
Five years later, there are three approved treatments for SMA, all of which have been shown to slow or even halt disease progression in many patients. Neurologists, whose SMA patient population once consisted almost entirely of children, are now treating more adults with the disease. Indeed, more than half of all people alive with SMA in the United States today are adults, according to Cure SMA.
“Managing SMA used to be clinic follow-ups where we were doing our best supportive care and watching people fall apart before our eyes,” said John Brandsema, MD, a physician and neuromuscular section head at the Children’s Hospital of Philadelphia. “Today, what we see in the vast majority of people is that they are either the same as they were before – which is completely against the natural history of this disease and something to be celebrated – or that people are really better with their function. It totally changes everything in the clinic.”
Among those changes are a more proactive approach to rehabilitation and an even greater emphasis on personalized medicine and multidisciplinary care. But there is also a need for updated treatment guidelines, a new classification system to measure disease severity, specific biomarkers to guide therapy choices, more data on long-term efficacy of existing therapeutics, new medications to complement those therapies, and a deeper understanding of a disease that may have treatment options but still has no cure.
Advances in early diagnosis
Patients with SMA lack a working copy of the survival motor neuron 1 (SMN1) gene, which provides instructions for producing a protein called SMN that is critical for the maintenance and function of motor neurons. Without this protein, motor neurons eventually die, causing debilitating and progressive muscle weakness that affects the ability to walk, eat, and breathe. SMA is rare, affecting about 1 in 10,000 newborns.
In approximately 96% of patients, SMA is caused by homozygous loss of the SMN1 gene. People with SMA have at least one copy of the SMN2 gene, sometimes called a “backup” gene, that also produces SMN protein. However, a single nucleotide difference between SMN2 and SMN1 causes about 90% of the protein produced by SMN2 to be truncated and less stable. Even with multiple copies of SMN2 present, as is the case with many infants with SMA, the amount of functional protein produced isn’t enough to compensate for the loss of SMN1.3
All three approved medications are SMN up-regulators and work to increase the amount of functional SMN protein. Starting these medications early, even before symptoms present, is critical to preserve motor function. Early treatment depends on early diagnosis, which became more widespread after 2018 when SMA was added to the federally Recommended Uniform Screening Panel for newborns. As of July 1, 2022, 47 states have incorporated SMA newborn screening into their state panel, ensuring that 97% of all infants born in the United States undergo SMA screening shortly after birth. Screening in the remaining states – Hawaii, Nevada, and South Carolina – and Washington, D.C. is expected by mid-2023.
SMA newborn screening is a PCR-based assay that detects homozygous SMN1 gene deletion found in about 95% of all people with SMA. The remaining 5% of cases are caused by various genetic mutations that can only be detected with gene sequencing. In these cases, and in children who don’t undergo SMA newborn screening, the disease is usually identified when symptoms are noticed by a parent, pediatrician, or primary care provider. But a study found that in 2018 only 52.7% of pediatricians correctly identified genetic testing as a requirement for a definitive diagnosis of SMA; in 2019, with a larger sample size, that number decreased to 45%.4 The lack of awareness of diagnostic requirements for SMA could contribute to delays in diagnosis, said Mary Schroth, MD, chief medical officer for Cure SMA and a coauthor of the study.
“In our world, suspicion of SMA in an infant is an emergency situation,” Dr. Schroth said. “These babies need to be referred immediately and have genetic testing so that treatment can begin as soon as possible.”
Based on the study findings, Dr. Schroth and others with Cure SMA launched a new tool in 2021 designed to help pediatricians, primary care physicians, and parents identify early signs of SMA, so that a referral to a pediatric neurologist happens quickly. Called SMArt Moves, the educational resource features videos and a checklist to help increase early detection in infants who had a negative SMA newborn screening result or did not receive SMA screening at birth.5
Who to treat, when, and with which treatment
For many patients, having multiple effective treatment options means that SMA is no longer a fatal disease in early childhood, but one that can be managed into adolescence and adulthood. The question for clinicians is, who do they treat, when, and with which treatment?
Studies have long shown that the number of copies of the backup gene that a patient has is inversely associated with disease severity.6 In 2018, a group of SMA experts published a treatment algorithm to help guide decision-making following a positive SMA newborn screening.7 The treatment guidelines were updated in 2020 based on clinical trial data for presymptomatic infants, and current recommendations include immediate treatment for infants with two to four copies of the SMN2 gene.8 For patients with only one copy of SMN2, most of whom will likely be symptomatic at birth, the guidelines recommend that treatment decisions be made jointly between the clinician and the family.7,8
Some suggest that the number of SMN2 copies a patient has should also be a factor in determining phenotype, which has started a conversation on the development of a new classification system.9 The original classification system for disease severity – Types 0-4 – was based on age of onset and degree of motor function achieved, with Type 0 developing prenatally and being the most severe and Type 4 developing in adulthood. Type 1 is the most common, affecting more than half of all people with SMA, followed by Types 2-4. In 2018, updated consensus care guidelines offered a revised classification system that better reflected disease progression in the age of therapy. The functional motor outcomes include nonsitters (historically Type I), sitters (historically Type 2/3), and walkers (historically Type 3/4).10,11 These guidelines are a start, but clinicians say more revision is needed.
“Types 1, 2, 3, 4 were based on function – getting to a certain point and then losing it, but now that we can treat this disease, people will shift categories based on therapeutic response or based on normal development that is possible now that the neurologic piece has been stabilized,” Dr. Brandsema said. “We need to completely change our thinking around all these different aspects of SMA management.”
While discussions of a new classification system for SMA are underway, another effort to update treatment recommendations is closer to completion. Led by Cure SMA, a group of about 50 physician experts in the United States and Europe who specialize in SMA are revising guidelines for diagnosis and treatment, the first time the recommendations have been updated since 2018. The updated recommendations, which should be published later this year, will focus on diagnosis and treatment considerations.
“We have three treatments that are available, and there are specific FDA indications for each of those, but it’s not totally clear just how those medications should be used or applied to different clinical situations,” said Dr. Schroth. “We’re in a rapid phase of learning right now in the SMA community, trying to understand how these treatments alter physiology and disease outcomes and how to best use the tools that we now have available to us. In parallel with clinical treatments, we have to be doing the best care we can to optimize the outcomes for those treatments.”
Research advances in 2021
Although all three drugs approved to treat SMA – nusinersen (Spinraza; Biogen), onasemnogene abeparvovec-xioi gene replacement therapy (Zolgensma; Novartis Gene Therapies), and risdiplam (Evrysdi, Genentech/Roche) – are highly effective, there are still unanswered questions and unmet needs. New research findings from 2021 focused on higher dosing, different drug-delivery methods, combination therapy, and complementary therapeutics to address SMA comorbidities.
Higher-dose nusinersen. The first drug approved to treat SMA, nusinersen is an antisense oligonucleotide approved for all ages and all SMA types. It works by altering splicing of the SMN2 gene pre-mRNA to make more complete SMN protein. Given as an intrathecal (IT) injection, four “loading doses” are administered within the first 2 months of treatment, followed by a maintenance dose every 4 months for the duration of the individual’s life.
Reports from patients of waning effects of nusinersen just prior to follow-up treatment have led some clinicians to ask if a higher dose may be needed. A study underway seeks to address that issue.
DEVOTE is a phase 2/3 trial to study the safety and efficacy of high-dose nusinersen in patients with SMA. Preliminary findings reported in 2021 found no adverse events among patients treated with 28 mg of nusinersen for 161-257 days.12 Another analysis from this trial found that higher doses are associated with greater decrease of plasma phosphorylated neurofilament heavy chain (pNF-H) levels in patients with SMA and may lead to clinically meaningful improvement in motor function beyond that observed with the approved 12 mg dose.13 The trial is ongoing.
Another trial, ASCEND, is a phase 3B study assessing higher dose nusinersen in patients previously treated with risdiplam. Recruitment for that trial began in October 2021.
Long-term efficacy and IT administration of SMA therapy. Several studies are looking at the long-term efficacy and alternate routes of administration of onasemnogene abeparvovec and other SMA therapies.
A one-time gene replacement therapy delivered via an IV infusion replaces the function of the missing or nonworking SMN1 gene with a new, working copy of the SMN1 gene. FDA approved in 2019, it is authorized for use in patients with SMA up to 2 years of age.
The latest data from an ongoing, long-term follow-up safety study of onasemnogene abeparvovec, published in May 2021, suggest that the treatment’s effects persist more than 5 years after treatment. Researchers followed 13 infants with symptomatic SMA type 1 since the beginning of the phase 1 clinical trial of the gene transfer therapy. All patients who received the therapeutic dose maintained their baseline motor function, and two of the patients actually improved without other SMN-targeted treatment. At a median 6.2 years after they received treatment, all were alive and none needed permanent ventilation.14
After a 2-year hold by the FDA, a study of IT administration of onasemnogene abeparvovec is now enrolling patients. Citing concerns from animal studies that IT administration might result in dorsal root ganglia injury, the FDA issued a partial hold on the STRONG trial in 2019. Following positive study results in nonhuman primates, the FDA announced the trial can continue. Novartis is launching a new phase 3 STEER trial to test the drug delivered intrathecally in patients aged 2-18 years with Type 2 SMA. IT administration could allow the gene therapy to be used safely and effectively in more patients with SMA.
Efficacy of risdiplam in more patients. The first oral treatment for SMA was approved by the FDA in 2020. It’s given once per day in patients with SMA of all ages and disease types. The drug increases functional SMN protein production by the SMN2 gene.
A July 2021 publication of the results of the FIREFISH study found that infants with Type I SMA treated with risdiplam for 12 months were significantly more likely to achieve motor milestones, such as sitting without support, compared with untreated infants with Type 1 SMA.15 Risdiplam is also effective in older patients with Type 2 or 3 SMA, according to results published in December from the SUNFISH clinical trial.16 Another study, RAINBOWFISH, is studying safety and efficacy at 24 months in presymptomatic infants started on treatment at up to 6 weeks of age.
The efficacy of risdiplam in previously treated patients is the subject of JEWELFISH, an ongoing study in patients 6 months to 60 years with SMA. Preliminary data presented at the 2020 Virtual SMA Research and Clinical Care Meeting suggest treatment with risdiplam led to a median two-fold increase in the amount of blood SMN protein levels after 4 weeks, which was sustained for at least 24 months.17
Combination therapy. Among the more eagerly awaited results are those from studies of combination therapies, including those that combine approved SMN up-regulators with new non–SMN-targeted therapeutics.
“We’re seeing that while these three approved therapies have dramatic results, especially for infants who are treated presymptomatically, there are still unmet medical needs in those patients, particularly for older teens and adults whose disease may have progressed before they were able to start therapy,” said Jackie Glascock, PhD, vice president of research for Cure SMA.
Of particular interest are studies of myostatin inhibitors, therapeutics that block the production of the protein myostatin. Myostatin acts on muscle cells to reduce muscle growth. Animal studies suggest that inhibiting myostatin increases muscle mass, which could be important in patients with muscle loss due to SMA.
Three experimental myostatin inhibitors are currently in clinical trials. MANATEE is a global phase 2-3 trial that aims to evaluate the safety and efficacy of the antimyostatin antibody GYM329 (RO7204239) in combination with risdiplam. SAPPHIRE is a phase 3 trial of apitegromab (SRK-015) in combination with nusinersen or risdiplam. RESILIANT is a phase 3 trial of tadefgrobep alfa in combination with other treatments.
A trial is underway to study the efficacy and safety of nusinersen in patients with persistent symptoms of SMA after treatment with the gene therapy. The phase 4 study, RESPOND, is enrolling children aged 2-36 months.
What’s needed next
Despite the advances in treatment and patient care, Dr. Brandsema, Dr. Schroth, and Dr. Glascock note that there remain unmet needs in the SMA community in a variety of areas.
Increased focus on adults with SMA. Before nusinersen, treatment of SMA mainly involved treating its symptoms. Many patients stopped seeing their neurologist, relying more heavily on pulmonary care specialists and/or primary care providers to address breathing, nutrition, and mobility problems. “Now with the approval of these treatments, they’re coming back to see their neurologists and are becoming more visible in the SMA community,” Dr. Schroth said.
Despite this re-emergence, a 2020 meta-analysis of studies on adults with SMA found a paucity of data on physical and occupational therapy, respiratory management, mental health care, and palliative care.18
“There is just so much work we need to do in the area of adult clinical care of SMA.”
Treatment algorithms. While the development of the newborn screening algorithm and revised patient care guidelines are helpful resources, clinicians still face uncertainty when choosing which therapy will work best for their patients. Treatment algorithms that help clinicians figure out what therapy or combination of therapies will offer the best outcomes for individual patients are desperately needed, Dr. Brandsema said.
“Each person’s experience of this disease is so unique to the individual based partly on their genetics and partly on the factors about what got them into care and how compliant they are with everything we’re trying to do to help them,” he said. “Biomarkers would help clinicians create personalized treatment plans for each patient.”
More basic science. While scientists have a good understanding of the SMN gene, there are many unanswered questions about the function of the SMN protein and its relationship to motor neuron loss. SMN is a ubiquitously expressed protein, and its function in other cell types is largely unknown. Despite all of the research advances, there is much basic science left to be done.
“We are strongly advocating to regulatory authorities that these aren’t cures and we need to continue to invest in the basic research,” Dr. Glascock said. “These biological questions that pertain to SMN and its function and expression really drive drug development. I really think that understanding those pathways better will lead us to more druggable targets.”
Two deaths from liver failure linked to spinal muscular atrophy drug
Two children taking the gene therapy drug onasemnogene abeparvovec (Zolgensma, Novartis) for spinal muscular atrophy (SMA) have died from acute liver failure, according to a statement issued by the drug’s manufacturer.
The patients were 4 months and 28 months of age and lived in Russia and Kazakhstan. They died 5-6 weeks after infusion with Zolgensma and approximately 1-10 days after the initiation of a corticosteroid taper.
These are the first known fatal cases of acute liver failure associated with the drug, which the company notes was a known side effect included in the product label and in a boxed warning in the United States.
“Following two recent patient fatalities, and in alignment with health authorities, we will be updating the labeling to specify that fatal acute liver failure has been reported,” the statement reads.
“While this is important safety information, it is not a new safety signal,” it adds.
Rare genetic disorder
SMA is a rare genetic disorder that affects about 1 in 10,000 newborns. Patients with SMA lack a working copy of the survival motor neuron 1 (SMN1) gene, which encodes a protein called SMN that is critical for the maintenance and function of motor neurons.
Without this protein, motor neurons eventually die, causing debilitating and progressive muscle weakness that affects the ability to walk, eat, and breathe.
Zolgensma, a one-time gene replacement therapy delivered via intravenous infusion, replaces the function of the missing or nonworking SMN1 gene with a new, working copy of the SMN1 gene.
The first gene therapy treatment for SMA, it was approved by the U.S. Food and Drug Administration in 2019 for patients with SMA up to 2 years of age. It is also the most expensive drug in the world, costing about $2.1 million for a one-time treatment.
“We have notified health authorities in all markets where Zolgensma is used, including the FDA, and are communicating to relevant healthcare professionals as an additional step in markets where this action is supported by health authorities,” the manufacturer’s statement says.
Studies have suggested that the treatment’s effects persist more than 5 years after infusion.
Clinical trials currently underway by Novartis are studying the drug’s long-term efficacy and safety and its potential use in older patients.
The company is also leading the phase 3 clinical trial STEER to test intrathecal (IT) administration of the drug in patients ages 2-18 years who have type 2 SMA.
That trial began late last year after the FDA lifted a 2-year partial hold on an earlier study. The FDA halted the STRONG trial in 2019, citing concerns from animal studies that IT administration may result in dorsal root ganglia injury. The partial hold was released last fall following positive study results in nonhuman primates.
None of the current trials will be affected by the two deaths reported, according to a Novartis spokesperson.
Kelli Whitlock Burton is a staff writer/reporter for Medscape Neurology and MDedge Neurology.
References
1. Viscidi E et al. Comparative all-cause mortality among a large population of patients with spinal muscular atrophy versus matched controls. Neurol Ther. 2022 Mar;11(1):449-457. doi: 10.1007/s40120-021-00307-7.
2. Finkel RS et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014 Aug 26;83(9):810-817. doi: 10.1212/WNL.0000000000000741.
3. Klotz J et al. Advances in the therapy of spinal muscular atrophy. J Pediatr. 2021 Sep;236:13-20.e1. doi: 10.1016/j.jpeds.2021.06.033.
4. Curry M et al. Awareness screening and referral patterns among pediatricians in the United States related to early clinical features of spinal muscular atrophy (SMA). BMC Pediatr. 2021 May;21(1):236. doi: 10.1186/s12887-021-02692-2.
5. SMArt Moves. https://smartmoves.curesma.org/
6. Swoboda KJ et al. Natural history of denervation in SMA: Relation to age, SMN2 copy number, and function. Ann Neurol. 2005 May;57(5):704-12. doi: 10.1002/ana.20473.
7. Glascock J et al. Treatment algorithm for infants diagnosed with spinal muscular atrophy through newborn screening. J Neuromuscul Dis. 2018;5(2):145-158. doi: 10.3233/JND-180304.
8. Glascock J et al. Revised recommendations for the treatment of infants diagnosed with spinal muscular atrophy via newborn screening who have 4 copies of SMN2. J Neuromuscul Dis. 2020;7(2):97-100. doi: 10.3233/JND-190468.
9. Talbot K, Tizzano EF. The clinical landscape for SMA in a new therapeutic era. Gene Ther. 2017 Sep;24(9):529-533. doi: 10.1038/gt.2017.52.
10. Mercuri E et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018 Feb;28(2):103-115. doi: 10.1016/j.nmd.2017.11.005.
11. Finkel RS et al. Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord. 2018 Mar;28(3):197-207. doi: 10.1016/j.nmd.2017.11.004.
12. Pascual SI et al. Ongoing phase 2/3 DEVOTE (232SM203) randomized, controlled study to explore high-dose nusinersen in SMA: Part A interim results and Part B enrollment update. Presented at MDA Clinical and Scientific Conference 2021, Mar 15-18.
13. Finkel RS et al. Scientific rationale for a higher dose of nusinersen. Presented at 2021 Cure SMA Annual Meeting, Jun 9-11. Abstract P46.
14. Mendell JR et al. Five-year extension results of the phase 1 START trial of onasemnogene abeparvovec in spinal muscular atrophy. JAMA Neurol. 2021 Jul;78(7):834-841. doi: 10.1001/jamaneurol.2021.1272.
15. Darras BT et al. Risdiplam-treated infants with type 1 spinal muscular atrophy versus historical controls. N Engl J Med. 2021 Jul 29;385(5):427-435. doi: 10.1056/NEJMoa2102047.
16. Mercuri E et al. Safety and efficacy of once-daily risdiplam in type 2 and non-ambulant type 3 spinal muscular atrophy (SUNFISH part 2): A phase 3, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2022 Jan;21(1):42-52. doi: 10.1016/S1474-4422(21)00367-7. Erratum in: Lancet Neurol. 2022 Feb;21(2):e2. doi: 10.1016/S1474-4422(22)00006-0. Correction in: Lancet Neurol. 2022 Mar;21(3):e3. doi: 10.1016/S1474-4422(22)00038-2.
17. Genentech announces 2-year risdiplam data from SUNFISH and new data from JEWELFISH in infants, children and adults with SMA. https://www.curesma.org/genentech-risdiplam-data-conference-2020/
18. Wan HWY et al. Health, wellbeing and lived experiences of adults with SMA: a scoping systematic review. Orphanet J Rare Dis. 2020;15(1):70. doi: 10.1186/s13023-020-1339-3.
In 2016, the U.S. Food and Drug Administration approved nusinersen, the first treatment for spinal muscular atrophy (SMA). Until then, SMA had a mortality rate nearly double that of the general population.1 Two-thirds of patients were symptomatic within 6 months of birth and, in the absence of mechanical ventilation and other support, had a nearly 100% mortality rate by age 2.2
Five years later, there are three approved treatments for SMA, all of which have been shown to slow or even halt disease progression in many patients. Neurologists, whose SMA patient population once consisted almost entirely of children, are now treating more adults with the disease. Indeed, more than half of all people alive with SMA in the United States today are adults, according to Cure SMA.
“Managing SMA used to be clinic follow-ups where we were doing our best supportive care and watching people fall apart before our eyes,” said John Brandsema, MD, a physician and neuromuscular section head at the Children’s Hospital of Philadelphia. “Today, what we see in the vast majority of people is that they are either the same as they were before – which is completely against the natural history of this disease and something to be celebrated – or that people are really better with their function. It totally changes everything in the clinic.”
Among those changes are a more proactive approach to rehabilitation and an even greater emphasis on personalized medicine and multidisciplinary care. But there is also a need for updated treatment guidelines, a new classification system to measure disease severity, specific biomarkers to guide therapy choices, more data on long-term efficacy of existing therapeutics, new medications to complement those therapies, and a deeper understanding of a disease that may have treatment options but still has no cure.
Advances in early diagnosis
Patients with SMA lack a working copy of the survival motor neuron 1 (SMN1) gene, which provides instructions for producing a protein called SMN that is critical for the maintenance and function of motor neurons. Without this protein, motor neurons eventually die, causing debilitating and progressive muscle weakness that affects the ability to walk, eat, and breathe. SMA is rare, affecting about 1 in 10,000 newborns.
In approximately 96% of patients, SMA is caused by homozygous loss of the SMN1 gene. People with SMA have at least one copy of the SMN2 gene, sometimes called a “backup” gene, that also produces SMN protein. However, a single nucleotide difference between SMN2 and SMN1 causes about 90% of the protein produced by SMN2 to be truncated and less stable. Even with multiple copies of SMN2 present, as is the case with many infants with SMA, the amount of functional protein produced isn’t enough to compensate for the loss of SMN1.3
All three approved medications are SMN up-regulators and work to increase the amount of functional SMN protein. Starting these medications early, even before symptoms present, is critical to preserve motor function. Early treatment depends on early diagnosis, which became more widespread after 2018 when SMA was added to the federally Recommended Uniform Screening Panel for newborns. As of July 1, 2022, 47 states have incorporated SMA newborn screening into their state panel, ensuring that 97% of all infants born in the United States undergo SMA screening shortly after birth. Screening in the remaining states – Hawaii, Nevada, and South Carolina – and Washington, D.C. is expected by mid-2023.
SMA newborn screening is a PCR-based assay that detects homozygous SMN1 gene deletion found in about 95% of all people with SMA. The remaining 5% of cases are caused by various genetic mutations that can only be detected with gene sequencing. In these cases, and in children who don’t undergo SMA newborn screening, the disease is usually identified when symptoms are noticed by a parent, pediatrician, or primary care provider. But a study found that in 2018 only 52.7% of pediatricians correctly identified genetic testing as a requirement for a definitive diagnosis of SMA; in 2019, with a larger sample size, that number decreased to 45%.4 The lack of awareness of diagnostic requirements for SMA could contribute to delays in diagnosis, said Mary Schroth, MD, chief medical officer for Cure SMA and a coauthor of the study.
“In our world, suspicion of SMA in an infant is an emergency situation,” Dr. Schroth said. “These babies need to be referred immediately and have genetic testing so that treatment can begin as soon as possible.”
Based on the study findings, Dr. Schroth and others with Cure SMA launched a new tool in 2021 designed to help pediatricians, primary care physicians, and parents identify early signs of SMA, so that a referral to a pediatric neurologist happens quickly. Called SMArt Moves, the educational resource features videos and a checklist to help increase early detection in infants who had a negative SMA newborn screening result or did not receive SMA screening at birth.5
Who to treat, when, and with which treatment
For many patients, having multiple effective treatment options means that SMA is no longer a fatal disease in early childhood, but one that can be managed into adolescence and adulthood. The question for clinicians is, who do they treat, when, and with which treatment?
Studies have long shown that the number of copies of the backup gene that a patient has is inversely associated with disease severity.6 In 2018, a group of SMA experts published a treatment algorithm to help guide decision-making following a positive SMA newborn screening.7 The treatment guidelines were updated in 2020 based on clinical trial data for presymptomatic infants, and current recommendations include immediate treatment for infants with two to four copies of the SMN2 gene.8 For patients with only one copy of SMN2, most of whom will likely be symptomatic at birth, the guidelines recommend that treatment decisions be made jointly between the clinician and the family.7,8
Some suggest that the number of SMN2 copies a patient has should also be a factor in determining phenotype, which has started a conversation on the development of a new classification system.9 The original classification system for disease severity – Types 0-4 – was based on age of onset and degree of motor function achieved, with Type 0 developing prenatally and being the most severe and Type 4 developing in adulthood. Type 1 is the most common, affecting more than half of all people with SMA, followed by Types 2-4. In 2018, updated consensus care guidelines offered a revised classification system that better reflected disease progression in the age of therapy. The functional motor outcomes include nonsitters (historically Type I), sitters (historically Type 2/3), and walkers (historically Type 3/4).10,11 These guidelines are a start, but clinicians say more revision is needed.
“Types 1, 2, 3, 4 were based on function – getting to a certain point and then losing it, but now that we can treat this disease, people will shift categories based on therapeutic response or based on normal development that is possible now that the neurologic piece has been stabilized,” Dr. Brandsema said. “We need to completely change our thinking around all these different aspects of SMA management.”
While discussions of a new classification system for SMA are underway, another effort to update treatment recommendations is closer to completion. Led by Cure SMA, a group of about 50 physician experts in the United States and Europe who specialize in SMA are revising guidelines for diagnosis and treatment, the first time the recommendations have been updated since 2018. The updated recommendations, which should be published later this year, will focus on diagnosis and treatment considerations.
“We have three treatments that are available, and there are specific FDA indications for each of those, but it’s not totally clear just how those medications should be used or applied to different clinical situations,” said Dr. Schroth. “We’re in a rapid phase of learning right now in the SMA community, trying to understand how these treatments alter physiology and disease outcomes and how to best use the tools that we now have available to us. In parallel with clinical treatments, we have to be doing the best care we can to optimize the outcomes for those treatments.”
Research advances in 2021
Although all three drugs approved to treat SMA – nusinersen (Spinraza; Biogen), onasemnogene abeparvovec-xioi gene replacement therapy (Zolgensma; Novartis Gene Therapies), and risdiplam (Evrysdi, Genentech/Roche) – are highly effective, there are still unanswered questions and unmet needs. New research findings from 2021 focused on higher dosing, different drug-delivery methods, combination therapy, and complementary therapeutics to address SMA comorbidities.
Higher-dose nusinersen. The first drug approved to treat SMA, nusinersen is an antisense oligonucleotide approved for all ages and all SMA types. It works by altering splicing of the SMN2 gene pre-mRNA to make more complete SMN protein. Given as an intrathecal (IT) injection, four “loading doses” are administered within the first 2 months of treatment, followed by a maintenance dose every 4 months for the duration of the individual’s life.
Reports from patients of waning effects of nusinersen just prior to follow-up treatment have led some clinicians to ask if a higher dose may be needed. A study underway seeks to address that issue.
DEVOTE is a phase 2/3 trial to study the safety and efficacy of high-dose nusinersen in patients with SMA. Preliminary findings reported in 2021 found no adverse events among patients treated with 28 mg of nusinersen for 161-257 days.12 Another analysis from this trial found that higher doses are associated with greater decrease of plasma phosphorylated neurofilament heavy chain (pNF-H) levels in patients with SMA and may lead to clinically meaningful improvement in motor function beyond that observed with the approved 12 mg dose.13 The trial is ongoing.
Another trial, ASCEND, is a phase 3B study assessing higher dose nusinersen in patients previously treated with risdiplam. Recruitment for that trial began in October 2021.
Long-term efficacy and IT administration of SMA therapy. Several studies are looking at the long-term efficacy and alternate routes of administration of onasemnogene abeparvovec and other SMA therapies.
A one-time gene replacement therapy delivered via an IV infusion replaces the function of the missing or nonworking SMN1 gene with a new, working copy of the SMN1 gene. FDA approved in 2019, it is authorized for use in patients with SMA up to 2 years of age.
The latest data from an ongoing, long-term follow-up safety study of onasemnogene abeparvovec, published in May 2021, suggest that the treatment’s effects persist more than 5 years after treatment. Researchers followed 13 infants with symptomatic SMA type 1 since the beginning of the phase 1 clinical trial of the gene transfer therapy. All patients who received the therapeutic dose maintained their baseline motor function, and two of the patients actually improved without other SMN-targeted treatment. At a median 6.2 years after they received treatment, all were alive and none needed permanent ventilation.14
After a 2-year hold by the FDA, a study of IT administration of onasemnogene abeparvovec is now enrolling patients. Citing concerns from animal studies that IT administration might result in dorsal root ganglia injury, the FDA issued a partial hold on the STRONG trial in 2019. Following positive study results in nonhuman primates, the FDA announced the trial can continue. Novartis is launching a new phase 3 STEER trial to test the drug delivered intrathecally in patients aged 2-18 years with Type 2 SMA. IT administration could allow the gene therapy to be used safely and effectively in more patients with SMA.
Efficacy of risdiplam in more patients. The first oral treatment for SMA was approved by the FDA in 2020. It’s given once per day in patients with SMA of all ages and disease types. The drug increases functional SMN protein production by the SMN2 gene.
A July 2021 publication of the results of the FIREFISH study found that infants with Type I SMA treated with risdiplam for 12 months were significantly more likely to achieve motor milestones, such as sitting without support, compared with untreated infants with Type 1 SMA.15 Risdiplam is also effective in older patients with Type 2 or 3 SMA, according to results published in December from the SUNFISH clinical trial.16 Another study, RAINBOWFISH, is studying safety and efficacy at 24 months in presymptomatic infants started on treatment at up to 6 weeks of age.
The efficacy of risdiplam in previously treated patients is the subject of JEWELFISH, an ongoing study in patients 6 months to 60 years with SMA. Preliminary data presented at the 2020 Virtual SMA Research and Clinical Care Meeting suggest treatment with risdiplam led to a median two-fold increase in the amount of blood SMN protein levels after 4 weeks, which was sustained for at least 24 months.17
Combination therapy. Among the more eagerly awaited results are those from studies of combination therapies, including those that combine approved SMN up-regulators with new non–SMN-targeted therapeutics.
“We’re seeing that while these three approved therapies have dramatic results, especially for infants who are treated presymptomatically, there are still unmet medical needs in those patients, particularly for older teens and adults whose disease may have progressed before they were able to start therapy,” said Jackie Glascock, PhD, vice president of research for Cure SMA.
Of particular interest are studies of myostatin inhibitors, therapeutics that block the production of the protein myostatin. Myostatin acts on muscle cells to reduce muscle growth. Animal studies suggest that inhibiting myostatin increases muscle mass, which could be important in patients with muscle loss due to SMA.
Three experimental myostatin inhibitors are currently in clinical trials. MANATEE is a global phase 2-3 trial that aims to evaluate the safety and efficacy of the antimyostatin antibody GYM329 (RO7204239) in combination with risdiplam. SAPPHIRE is a phase 3 trial of apitegromab (SRK-015) in combination with nusinersen or risdiplam. RESILIANT is a phase 3 trial of tadefgrobep alfa in combination with other treatments.
A trial is underway to study the efficacy and safety of nusinersen in patients with persistent symptoms of SMA after treatment with the gene therapy. The phase 4 study, RESPOND, is enrolling children aged 2-36 months.
What’s needed next
Despite the advances in treatment and patient care, Dr. Brandsema, Dr. Schroth, and Dr. Glascock note that there remain unmet needs in the SMA community in a variety of areas.
Increased focus on adults with SMA. Before nusinersen, treatment of SMA mainly involved treating its symptoms. Many patients stopped seeing their neurologist, relying more heavily on pulmonary care specialists and/or primary care providers to address breathing, nutrition, and mobility problems. “Now with the approval of these treatments, they’re coming back to see their neurologists and are becoming more visible in the SMA community,” Dr. Schroth said.
Despite this re-emergence, a 2020 meta-analysis of studies on adults with SMA found a paucity of data on physical and occupational therapy, respiratory management, mental health care, and palliative care.18
“There is just so much work we need to do in the area of adult clinical care of SMA.”
Treatment algorithms. While the development of the newborn screening algorithm and revised patient care guidelines are helpful resources, clinicians still face uncertainty when choosing which therapy will work best for their patients. Treatment algorithms that help clinicians figure out what therapy or combination of therapies will offer the best outcomes for individual patients are desperately needed, Dr. Brandsema said.
“Each person’s experience of this disease is so unique to the individual based partly on their genetics and partly on the factors about what got them into care and how compliant they are with everything we’re trying to do to help them,” he said. “Biomarkers would help clinicians create personalized treatment plans for each patient.”
More basic science. While scientists have a good understanding of the SMN gene, there are many unanswered questions about the function of the SMN protein and its relationship to motor neuron loss. SMN is a ubiquitously expressed protein, and its function in other cell types is largely unknown. Despite all of the research advances, there is much basic science left to be done.
“We are strongly advocating to regulatory authorities that these aren’t cures and we need to continue to invest in the basic research,” Dr. Glascock said. “These biological questions that pertain to SMN and its function and expression really drive drug development. I really think that understanding those pathways better will lead us to more druggable targets.”
Two deaths from liver failure linked to spinal muscular atrophy drug
Two children taking the gene therapy drug onasemnogene abeparvovec (Zolgensma, Novartis) for spinal muscular atrophy (SMA) have died from acute liver failure, according to a statement issued by the drug’s manufacturer.
The patients were 4 months and 28 months of age and lived in Russia and Kazakhstan. They died 5-6 weeks after infusion with Zolgensma and approximately 1-10 days after the initiation of a corticosteroid taper.
These are the first known fatal cases of acute liver failure associated with the drug, which the company notes was a known side effect included in the product label and in a boxed warning in the United States.
“Following two recent patient fatalities, and in alignment with health authorities, we will be updating the labeling to specify that fatal acute liver failure has been reported,” the statement reads.
“While this is important safety information, it is not a new safety signal,” it adds.
Rare genetic disorder
SMA is a rare genetic disorder that affects about 1 in 10,000 newborns. Patients with SMA lack a working copy of the survival motor neuron 1 (SMN1) gene, which encodes a protein called SMN that is critical for the maintenance and function of motor neurons.
Without this protein, motor neurons eventually die, causing debilitating and progressive muscle weakness that affects the ability to walk, eat, and breathe.
Zolgensma, a one-time gene replacement therapy delivered via intravenous infusion, replaces the function of the missing or nonworking SMN1 gene with a new, working copy of the SMN1 gene.
The first gene therapy treatment for SMA, it was approved by the U.S. Food and Drug Administration in 2019 for patients with SMA up to 2 years of age. It is also the most expensive drug in the world, costing about $2.1 million for a one-time treatment.
“We have notified health authorities in all markets where Zolgensma is used, including the FDA, and are communicating to relevant healthcare professionals as an additional step in markets where this action is supported by health authorities,” the manufacturer’s statement says.
Studies have suggested that the treatment’s effects persist more than 5 years after infusion.
Clinical trials currently underway by Novartis are studying the drug’s long-term efficacy and safety and its potential use in older patients.
The company is also leading the phase 3 clinical trial STEER to test intrathecal (IT) administration of the drug in patients ages 2-18 years who have type 2 SMA.
That trial began late last year after the FDA lifted a 2-year partial hold on an earlier study. The FDA halted the STRONG trial in 2019, citing concerns from animal studies that IT administration may result in dorsal root ganglia injury. The partial hold was released last fall following positive study results in nonhuman primates.
None of the current trials will be affected by the two deaths reported, according to a Novartis spokesperson.
Kelli Whitlock Burton is a staff writer/reporter for Medscape Neurology and MDedge Neurology.
References
1. Viscidi E et al. Comparative all-cause mortality among a large population of patients with spinal muscular atrophy versus matched controls. Neurol Ther. 2022 Mar;11(1):449-457. doi: 10.1007/s40120-021-00307-7.
2. Finkel RS et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014 Aug 26;83(9):810-817. doi: 10.1212/WNL.0000000000000741.
3. Klotz J et al. Advances in the therapy of spinal muscular atrophy. J Pediatr. 2021 Sep;236:13-20.e1. doi: 10.1016/j.jpeds.2021.06.033.
4. Curry M et al. Awareness screening and referral patterns among pediatricians in the United States related to early clinical features of spinal muscular atrophy (SMA). BMC Pediatr. 2021 May;21(1):236. doi: 10.1186/s12887-021-02692-2.
5. SMArt Moves. https://smartmoves.curesma.org/
6. Swoboda KJ et al. Natural history of denervation in SMA: Relation to age, SMN2 copy number, and function. Ann Neurol. 2005 May;57(5):704-12. doi: 10.1002/ana.20473.
7. Glascock J et al. Treatment algorithm for infants diagnosed with spinal muscular atrophy through newborn screening. J Neuromuscul Dis. 2018;5(2):145-158. doi: 10.3233/JND-180304.
8. Glascock J et al. Revised recommendations for the treatment of infants diagnosed with spinal muscular atrophy via newborn screening who have 4 copies of SMN2. J Neuromuscul Dis. 2020;7(2):97-100. doi: 10.3233/JND-190468.
9. Talbot K, Tizzano EF. The clinical landscape for SMA in a new therapeutic era. Gene Ther. 2017 Sep;24(9):529-533. doi: 10.1038/gt.2017.52.
10. Mercuri E et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018 Feb;28(2):103-115. doi: 10.1016/j.nmd.2017.11.005.
11. Finkel RS et al. Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord. 2018 Mar;28(3):197-207. doi: 10.1016/j.nmd.2017.11.004.
12. Pascual SI et al. Ongoing phase 2/3 DEVOTE (232SM203) randomized, controlled study to explore high-dose nusinersen in SMA: Part A interim results and Part B enrollment update. Presented at MDA Clinical and Scientific Conference 2021, Mar 15-18.
13. Finkel RS et al. Scientific rationale for a higher dose of nusinersen. Presented at 2021 Cure SMA Annual Meeting, Jun 9-11. Abstract P46.
14. Mendell JR et al. Five-year extension results of the phase 1 START trial of onasemnogene abeparvovec in spinal muscular atrophy. JAMA Neurol. 2021 Jul;78(7):834-841. doi: 10.1001/jamaneurol.2021.1272.
15. Darras BT et al. Risdiplam-treated infants with type 1 spinal muscular atrophy versus historical controls. N Engl J Med. 2021 Jul 29;385(5):427-435. doi: 10.1056/NEJMoa2102047.
16. Mercuri E et al. Safety and efficacy of once-daily risdiplam in type 2 and non-ambulant type 3 spinal muscular atrophy (SUNFISH part 2): A phase 3, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2022 Jan;21(1):42-52. doi: 10.1016/S1474-4422(21)00367-7. Erratum in: Lancet Neurol. 2022 Feb;21(2):e2. doi: 10.1016/S1474-4422(22)00006-0. Correction in: Lancet Neurol. 2022 Mar;21(3):e3. doi: 10.1016/S1474-4422(22)00038-2.
17. Genentech announces 2-year risdiplam data from SUNFISH and new data from JEWELFISH in infants, children and adults with SMA. https://www.curesma.org/genentech-risdiplam-data-conference-2020/
18. Wan HWY et al. Health, wellbeing and lived experiences of adults with SMA: a scoping systematic review. Orphanet J Rare Dis. 2020;15(1):70. doi: 10.1186/s13023-020-1339-3.
Rett syndrome: Looking to the future and the promise of gene therapy
The dream of curing genetic disorders has been a persistent but elusive goal, even before the human genome was mapped. Once mapping of the human genome was complete in 2001, an entirely new avenue of potential treatments and cures for genetic diseases and disorders was opened.1,2
The disorders best suited for targeted gene therapy are monogenic; however, tools and delivery methods for editing the human genome were limited and difficult to apply, until the advent of the CRISPR system in 2012.3 CRISPR (an acronym of clustered regularly interspaced short palindromic repeats) has changed the way in which gene therapy strategies are pursued, with dozens of companies leveraging a variety of platforms to create potentially life-changing therapies for devastating rare diseases and disorders.
One of the rare monogenic disorders that is embracing multiple gene therapy approaches is Rett syndrome, a rare, debilitating neurodevelopmental disorder. In this review, we explore the molecular cause of Rett syndrome, disease presentation, current treatments, ongoing clinical trials, and therapies that are on the horizon.
Underlying molecular cause
Rett syndrome is caused by mutations in, or the absence of, the MECP2 gene, which produces methyl-CpG binding protein 2 (MECP2). The syndrome was first described clinically in 1954 by the Austrian physician Andreas Rett; it would take until 1982 before the disorder was officially named, eponymously, in a seminal paper by Hagberg.4 After Hagberg’s characterization, Rett syndrome became the predominant global clinical diagnosis identified among cognitively impaired females, with an incidence of 1 in every 10,000 to 15,000.4
In 1999, mutations in, and deletions of, MECP2 were identified as the cause of Rett syndrome.4,5 MECP2 is located on the X chromosome, in the Xq28 region, making Rett syndrome an X-linked dominant disorder.6 Rett syndrome is seen predominantly in females who are mosaic for mutant or deleted MECP2. Random X chromosome inactivation results in some cells expressing the mutant MECP2 allele and other cells expressing the normal functioning MECP2 allele; the percentage of cells expressing the normal allele correlates with the degree of syndrome severity.7-9
The incidence of Rett syndrome is much lower in males, in whom the syndrome was originally thought to be lethal; many observed male cases are either mosaic or occur in XXY males.10,11
Approximately 95% of cases of Rett syndrome are due to de novo mutations in MECP2, with a handful of specific mutations and large deletions accounting for more than 85% of cases.12 The fact that Rett syndrome is monogenic and most cases are caused by, in total, only a handful of mutations or deletions makes the syndrome a promising candidate for gene therapy.
At the molecular level, it has been observed that the MECP2 mutations of Rett syndrome lead to loss of gene function, thus disrupting the ability of the MECP2 nuclear protein to regulate global gene transcription through its binding to methylated DNA sites.12 A large percentage of these missense and nonsense mutations lead to a truncated or nonfunctional protein.12
One of the ways in which MECP2 regulates transcription is as a component of heterochromatin condensates and by separation of heterochromatin and euchromatin.13-15 It has been observed that the cells of Rett syndrome patients have an altered chromatin state, potentially contributing to transcriptional dysregulation.16,17 Several mutations observed in Rett syndrome patients occur in crucial domains for heterochromatin condensate formation, which helps explain this cellular phenotype.13 Introduction of a engineered “mini” MECP2 in a murine model of Rett syndrome has resulted in partial rescue of heterochromatin condensate formation and transcriptional regulation – fostering the hypothesis that correcting those genetic changes could lead to a potential therapy.18
Beyond the role of MECP2 in heterochromatin condensate formation, the gene interacts with more than 40 proteins that have diverse roles in cellular function, epigenetic modulation, and neuronal development. This volume of interactions contributes to MECP2 being a global gene regulatory protein that has far-reaching effects on transcriptional regulation across the genome.19-22
Epigenetic dysregulation has been associated with neurodevelopmental and neuropsychiatric disorders.23 Both insulin-like growth factor 1 (IGF-1) and brain-derived neurotrophic factor are transcriptional targets of MECP2, and are involved in neuronal differentiation, synaptic function, and neurite outgrowth.12 This helps explain the neurodevelopmental phenotypes observed in MECP2-mutated patients.
Notably, although Rett syndrome patients experience neurodevelopmental phenotypes at the cellular level, neuronal death is not readily observed. That observation provides hope that an interventional therapy after onset of symptoms might still be of benefit.
Presentation
Early neurotypical development. A hallmark of Rett syndrome is neurotypical physical and mental development until 6 to 24 months of age.
Stagnation is the first stage of the syndrome, involving a small but rapid decline in habitual milestones, such motor and language skills.12 Subtle signs, such as microcephaly and hypotonia, can also arise at this time but might be missed.24
Rapid regression follows stagnation. Speech and motor delays and impaired gait and breathing occur;12,25 purposeful hand skills are lost, replaced by repetitive hand-wringing movements that are a hallmark of the syndrome.12,24 Seizures are observed; they become more common during the next stage.12
Plateau. Language advances can be observed, but further deficits are seen in motor skills and hand coordination.12
Late motor deterioration stage. Late physical deficits develop, leading to lifelong impairments. The physical deficits observed are the result of severe muscle weakness, usually resulting in wheelchair dependency.12
Plateau. Patients then reach a second plateau. Regression stops; deficient physical and cognitive states stabilize and are maintained.25
At all stages of Rett syndrome, the following are observed:
- Gastrointestinal problems.
- Sleep disturbances.
- Abnormal cardiorespiratory coupling.
- Greater-than-expected mortality.12
Final regression. The patient is fully dependent for the rest of their lifespan, partially due to seizure activity.26,27
A life-changing diagnosis
A diagnosis of Rett syndrome is life-changing for a patient’s family; access to supportive groups of other patients and their families is extremely beneficial. Two helpful organizations – the Rett Syndrome Research Trust28 and International Rett Syndrome Foundation,29 – offer patient support and community and fund research.
Because X chromosome inactivation is random in Rett syndrome, the individual patient can present with a wide variety of phenotypic combinations – making the patient, and their needs, unique.12 During stages of regression, patients often experience emotional dysregulation and anxiety, which is attributable to their increasing physical difficulties.30 They often exhibit combinations of uncontrolled movements, including repetitive rocking, scratching, and self-injurious behavior.30 For most, regression subsides after the first 5 years of alternating development and regression; after that, their ultimate symptoms persist for life.25
As patients mature, they need to be monitored for proper nutrition and scoliosis.25 As adults, they are at risk of pneumonia, respiratory distress, status epilepticus, osteopenia, and lack of adequate food or water because of impaired ability to feed.25
The lifespan of Rett syndrome patients has increased, thanks to improvements in health care, advances in technology, and early genetic testing, which allows for earlier diagnosis, intervention, and management of symptoms.
Current treatments
When a female patient presents with regression and loss of milestones, sequencing of MECP2 is performed to verify whether Rett syndrome is the cause, by detecting any of the known mutations. Multiplex ligation-dependent probe amplification is also performed to detect major deletions.25
All available treatments for Rett syndrome are symptomatic; intensive early intervention is practiced.31 Multidisciplinary management – medical, psychiatric, and physical – is introduced almost immediately after diagnosis. Following diagnosis, patients are prescribed anti-seizure, sleep, and anxiety medications.31 Electroencephalography can be performed to identify seizure type. Neuromuscular blockage drugs can be prescribed to help with gait and stereotypic hand movements.25
Handguards or splints to the elbows can be prescribed by an occupational therapist to improve hand movement.25 Physical therapy can improve mobility; hydrotherapy and hippotherapy have been successful in helping to maintain mobility and muscle support.32,33 Nutritional management is implemented to control caloric intake and maintain the vitamin D level.31 Some patients experience constipation and urinary retention, putting them at risk of nephrolithiasis.
Once the signs and symptoms of Rett syndrome progress, and milestones regress to a certain point, patients need constant, full-time care for the rest of their lives.34 As symptomatic interventions have greatly improved patient outcomes and it has been shown that about 70% can reach adulthood with a potential lifespan of about 50 years.25
Although there is no cure for Rett syndrome and treatments are symptomatic, ongoing studies – both clinical and preclinical – offer promise that treatments will be developed that work at molecular and genetic levels.
Clinical trials
Advances in understanding of Rett syndrome have led to many therapies in clinical trials, several of which show promise.
Trofinetide. One of the most promising targets for downstream therapy, mentioned earlier, is IGF-1, which was the target of a successful phase 3 clinical trial, LAVENDER (sponsored by Acadia Pharmaceuticals).35,36 This trial studied trofinetide, a synthetic IGF-1 analog that inhibits neuroinflammation, restores glial function, corrects synaptic deficiencies, and regulates oxidative stress response.12,37,38 Initial results from phase 2 and phase 3 trials indicate improved scores for treated patients in the Rett syndrome Behaviour Questionnaire (RSBQ) and Clinical Global Impression–Improvement (CGI-I) scores, while also showing improvements in the Communication and Symbolic Behavior Scales Developmental Profile Infant–Toddler Checklist–Social composite score.36,39
The most common adverse events seen with trofinetide were diarrhea and vomiting.
Acadia Pharmaceuticals has filed for approval of a new drug application for trofinetide with the Food and Drug Administration, for which the company has been granted Fast Track Status and orphan drug designations. Most (95%) subjects in the phase 3 LAVENDER trial elected to continue taking trofinetide in the subsequent open-label Lilac and Lilac-2 extension studies.36 A current open-label phase 2/3 trial is recruiting patients 2 to 5 years of age to evaluate trofinetide.40 It is expected that, in the near future, this could be a drug given to Rett patients as an FDA-approved treatment.
Blarcamesine. Another small molecule drug, blarcamesine (also known as ANAVEX2-73), a sigma-1 receptor agonist, produced promising results in phase 2 clinical trials in adult Rett syndrome patients. The drug is in a phase 2/3 clinical trial for pediatric Rett syndrome patients (sponsored by Anavex Life Sciences).41-43
Phase 2 results indicated statistically significant and clinically meaningful improvement in RSBQ and CGI-I scores with blarcamesine. Improvement was initially observed within 4 weeks after the start of treatment and was sustained throughout the study. The drug was shown to be well tolerated, with minimal adverse effects; no serious adverse events were recorded. These results were observed in adult patients, demonstrating that improvements in Rett syndrome are possible even after regression.
Blarcamesine activates the sigma 1 receptor, which is pivotal to restoring cellular homeostasis and restoring neuroplasticity – deficiencies of which have been linked to autophagy and glutamate toxicity. The drug has also been explored as a potential treatment for other neurological disorders.44-47 Improvements in blarcamesine-treated patients further correlated with lower levels of glutamate in cerebrospinal fluid, which is a Rett syndrome biomarker, supporting the proposition that behavioral improvements were due to drug intervention.48,49 The phase 2 trial was modified into a phase 3 trial and additional endpoints were added.41-43
All patients in the phase 2 adult trial elected to continue in the extension study.
Based on these promising data, Anavex is pursuing an approval pathway for adult patients, while continuing dosage optimization phase 2/3 trials and recruitment for a pediatric trial.42,43
Is the future about gene therapy?
TSHA-102 (miniMECP2). Taysha Gene Therapies is developing a promising gene therapy, TSHA-102, for Rett syndrome, and is aiming to begin phase 1/2 clinical trials in 2022.50 The technology for this therapy relies on the delivery of a fragment of MECP2 (known as miniMECP2), which is regulated by a built-in microRNA regulator (miR-responsive auto-regulatory element, or miRARE) to help ameliorate MECP2 dosage toxicity. (Overexpression of MECP2 is toxic to neurons, which has made traditional [so to speak] gene replacement therapy difficult in Rett syndrome: Levels of MECP2 need to be tightly regulated, and the Taysha microRNA technology regulates levels of miniMECP2, thus reducing toxicity.)
The Taysha microRNA technology has yielded promising results in mouse studies for Rett syndrome; results indicate a lengthening of lifespan and delayed onset of gait abnormalities.51 TSHA-102 is in the preclinical stage but offers promise that it will be the first gene therapy for Rett syndrome to enter clinical trials.
As the field of gene therapy advances, several promising technologies are on the horizon that could potentially have disease-altering impacts on Rett syndrome. These therapies are divided into two broad categories: those at the gene level and those at the transcription and protein level. A few of these approaches are highlighted below.
Gene replacement involves adding a full or partial copy of MECP2 to neuronal cells. This type of therapy presents challenges, from delivery of the new gene to dosage concerns, because MECP2 can be toxic if overexpressed.52-54 Groundbreaking work was done in mouse models involving truncated MECP2, exhibiting phenotypic rescue and validating the gene-replacement approach.18 This strategy is being pursued by Neurogene, which has a uinique technology that allows for tuning of the gene’s expression to get the correct protein levels in the patient. Promising data was presented this year at the American Society of Gene and Cell Therapy conference.55
Early gene replacement therapy studies also laid the foundation for the minMECP2 and microRNA approach being used by Taysha Gene Therapies (discussed above).51
“Correcting” DNA mutations. A different approach at the genetic level involves “correcting” mutations in MECP2 at the DNA level. This is possible because, in a large subset of Rett syndrome patients who have the same missense or nonsense mutations, by using CRISPR, a gene editing tool (discussed above) a single base pair can be corrected.56,57 Previous research, in a Rett syndrome-model of induced pluripotent stem cells, showed that this type of editing is possible – and effective.52 An approach with particular promise involves use of a class of CRISPR proteins known as base editors that are able to specifically alter a single base of DNA.57 The technique has the potential to address many of the mutations seen in Rett syndrome; research on this type of technology is being pursued by Beam Therapeutics, and has the potential to impact Rett syndrome.58
Another promising “correction” approach is exonic editing, in which a much larger section of DNA – potentially, exons 3 and 4, which, taken together, comprise 97% of known MECP2 mutations seen in Rett syndrome – are replaced.59
In both CRISPR and exonic editing therapeutic approaches, endogenous levels of MECP2 expression would be maintained. Of note, both approaches are being pursued for use in treating other genetic disorders, which provides a boost in scaling-up work on addressing safety and efficacy concerns that accompany gene-editing approaches.58 One advantage to the DNA correction approach is that is has the potential to be a “one-and-done” treatment.
“Correcting” RNA. Beyond directly editing DNA, several therapeutic approaches are exploring the ability to edit RNA or to provide the protein directly to cells as enzyme replacement therapy. Such an RNA correction strategy leverages a technology that takes advantage of cells’ natural RNA editor, known as adenosine deaminase acting on RNA (ADAR), which corrects errors in cells’ RNA by providing specific guides to the cell. ADAR can be targeted to fix mutations in the MECP2 RNA transcript, resulting in a “corrected” MECP2 protein.60,61 This technology has delivered promising proof-of-concept evidence in cells and in murine models, and is in the therapeutic pipeline at VICO Therapeutics.62
Shape Therapeutics has also leveraged ADAR to “correct” mutated RNA; Rett syndrome is among the top priorities in the company’s pipeline.
Worth noting is that there are several advantages to the “correction” approach:
- Leveraging internal repair mechanisms minimizes the immune response.
- The flexibility of correction means that it can be used to address many of the mutations that cause Rett syndrome.63
Enzyme replacement therapy, in which the MECP2 protein produced by MECP2 would be directly replaced, is being explored in Rett syndrome patients. This technology has been used successfully in Pompe disease; however, Rett syndrome presents its own challenge because MECP2 needs to be delivered to the brain and neuronal cells.64
Where does this work stand? The technologies described in this section are in preclinical stages of study. Nonetheless, it is expected that several will enter human clinical trials during the next 5 years.
Conclusion
A diagnosis of Rett syndrome is a life-altering event for patients and their family. But there is more hope than ever for effective therapies and, eventually, a cure.
Multiple late-stage clinical trials in progress are demonstrating promising results from therapeutic products, with minimal adverse events. Remarkably, these interventions have delivered improvements to adult patients after regression has stabilized. With rapid progress being made in the field of gene therapy, several technologies for which are focused on Rett syndrome, a hopeful picture is emerging: that therapeutic intervention will be possible before regression, thus effectively treating and, potentially, even curing Rett syndrome.
The landscape is broadening. Add to this hope for approved therapies is the fact that Rett syndrome isn’t the only target being pursued with such strategies; in fact, researchers in the larger field of neurodevelopmental disorder study are working together to find common solutions to shared challenges – from how therapies are designed and delivered to how toxicity is minimized. Much of what is being explored in the Rett syndrome field is also under investigation in other neurodevelopmental syndromes, including Angelman, Prader-Willi, chromosome 15q11.2-13.1 duplication (dup15q), and Fragile X syndrome. This kind of parallel investigation benefits all parties and optimizes a treatment platform so that it can be applied across more than a single disorder.
Like many monogenic disorders, Rett syndrome is entering an exciting stage – at which the words “treatment” and “cure” can be spoken with intent and vision, not just wide-eyed optimism. These words portend real promise for patients who carry the weight of a diagnosis of Rett syndrome, and for their families.
Ms. Ambrose is a student in the master’s of science in human genetics and genomic data analytics program, Keck Graduate Institute, Claremont, Calif. Dr. Bailus is an assistant professor of genetics, Keck Graduate Institute. The authors report no conflict of interest related to this article.
References
1. Lander ES et al; . Initial sequencing and analysis of the human genome. Nature. 2001 Feb 15;409(6822):860-921. doi: 10.1038/35057062.
2. Venter JC et al. The sequence of the human genome. Science. 2001 Feb 16;291(5507):1304-51. doi: 10.1126/science.1058040.
3. Jinek M et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012 Aug 17;337(6096):816-21. doi: 10.1126/science.1225829.
4. Percy A. The American history of Rett syndrome. Pediatr Neurol. 2014 Jan;50(1):1-3. doi: 10.1016/j.pediatrneurol.2013.08.018.
5. Amir RE et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999 Oct;23(2):185-8. doi: 10.1038/13810.
6. Pitzianti MB et al. Rett syndrome in males: The different clinical course in two brothers with the same microduplication MECP2 Xq28. Int J Environ Res Public Health. 2019 Aug;16(17):3075. doi: 10.3390/ijerph16173075.
7. Ribeiro MC, MacDonald JL. Sex differences in Mecp2-mutant Rett syndrome model mice and the impact of cellular mosaicism in phenotype development. Brain Res. 2020 Feb 15;1729:146644. doi: 10.1016/j.brainres.2019.146644.
8. Bao X et al. X chromosome inactivation in Rett syndrome and its correlations with MECP2 mutations and phenotype. J Child Neurol. 2008 Jan;23(1):22-5. doi: 10.1177/0883073807307077.
9. Knudsen GPS et al. Increased skewing of X chromosome inactivation in Rett syndrome patients and their mothers. Eur J Hum Genet. 2006 Jul;14(11):1189-94. doi: 10.1038/sj.ejhg.5201682.
10. Chahil G et al. Rett syndrome in males: A case report and review of literature. Cureus. 2018;10(10):e3414. doi: 10.7759/cureus.3414.
11. Reichow B et al. Brief report: Systematic review of Rett syndrome in males. J Autism Dev Disord. 2015 Oct;45(10):3377-83. doi: 10.1007/s10803-015-2519-1.
12. Vashi N, Justice MJ. Treating Rett syndrome: From mouse models to human therapies. Mamm Genome. 2019 Jun;30(5-6):90-110. doi: 10.1007/s00335-019-09793-5.
13. Li CH et al. MeCP2 links heterochromatin condensates and neurodevelopmental disease. Nature. 2020 Oct;586(7829):440-4. doi: 10.1038/s41586-020-2574-4.
14. Schmidt A et al. MeCP2 and chromatin compartmentalization. Cells. 2020 Apr;9(4):878. doi: 10.3390/cells9040878.
15. Wang L et al. Rett syndrome-causing mutations compromise MeCP2-mediated liquid-liquid phase separation of chromatin. Cell Res. 2020 May;30(5):393-407. doi: 10.1038/s41422-020-0288-7.
16. Lin P et al. Transcriptome analysis of human brain tissue identifies reduced expression of complement complex C1Q genes in Rett syndrome. BMC Genomics. 2016;17:427. doi: 10.1186/s12864-016-2746-7.
17. Tudor M et al. Transcriptional profiling of a mouse model for Rett syndrome reveals subtle transcriptional changes in the brain. Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15536-41. doi: 10.1073/pnas.242566899.
18. Tillotson R et al. Radically truncated MeCP2 rescues Rett syndrome–like neurological defects. Nature. 2017 Oct 19;550(7676):398-401. doi: 10.1038/nature24058.
19. Connolly DR, Zhou Z. Genomic insights into MeCP2 function: A role for the maintenance of chromatin architecture. Curr Opin Neurobiol. 2019 Dec;59:174-9. doi: 10.1016/j.conb.2019.07.002.
20. Johnson BS et al. Biotin tagging of MeCP2 in mice reveals contextual insights into the Rett syndrome transcriptome. Nat Med. 2017 Oct;23(10):1203-14. doi: 10.1038/nm.4406.
21. Gabel HW et al. Disruption of DNA-methylation–dependent long gene repression in Rett syndrome. Nature. 2015 Jun 4;522(7554):89-93. doi: 10.1038/nature14319.
22. Lyst MJ, Bird A. Rett syndrome: A complex disorder with simple roots. Nat Rev Genet. 2015 May;16(5):261-75. doi: 10.1038/nrg3897.
23. Kuehner JN et al. Epigenetic regulations in neuropsychiatric disorders. Front Genet. 2019 Apr 4;10:268. doi: 10.3389/fgene.2019.00268.
24. Pejhan S, Rastegar M. Role of DNA methyl-CpG-binding protein MeCP2 in Rett syndrome pathobiology and mechanism of disease. Biomolecules. 2021 Jan;11(1):75. doi: 10.3390/biom11010075.
25. Fu C et al. Consensus guidelines on managing Rett syndrome across the lifespan. BMJ Paediatr Open. 2020;4(1):e000717. doi: 10.1136/bmjpo-2020-000717.
26. Operto FF et al. Epilepsy and genetic in Rett syndrome: A review. Brain Behav. 2019 May;9(5):e01250. doi: 10.1002/brb3.1250.
27. Nissenkorn A et al. Epilepsy in Rett syndrome – The experience of a National Rett Center. Epilepsia. 2010 Jul;51(7):1252-8. doi: 10.1111/j.1528-1167.2010.02597.x.
28. Welcome to the Rett cure community. Rett Syndrome Research Trust [Internet]. Updated Feb 8, 2022. Accessed Feb 23, 2022. https://reverserett.org.
29. About Rett syndrome. International Rett Syndrome Foundation [Internet]. Updated Jan 4, 2022. Accessed Feb 23, 2022. http://www.rettsyndrome.org.
30. Singh J, Santosh P. Key issues in Rett syndrome: Emotional, behavioural and autonomic dysregulation (EBAD) – A target for clinical trials. Orphanet J Rare Dis. 2018 Jul 31;13(1):128. doi: 10.1186/s13023-018-0873-8.
31. Banerjee A et al. Towards a better diagnosis and treatment of Rett syndrome: A model synaptic disorder. Brain. 2019 Feb 1;142(2):239-48. doi: 10.1093/brain/awy323.
32. Ager S et al. Parental experiences of scoliosis management in Rett syndrome. Disabil Rehabil. 2009 Sep 19;31(23):1917-24. doi: 10.1080/09638280902846392.
33. Budden SS. Management of Rett syndrome: A ten year experience. Neuropediatrics. 1995;26(2):75-7. doi: 10.1055/s-2007-979727.
34. Ip JPK et al. Rett syndrome: Insights into genetic, molecular and circuit mechanisms. Nat Rev Neurosci. 2018 Jun;19(6):368-82. doi: 10.1038/s41583-018-0006-3.
35. Acadia Pharmaceuticals Inc. Study of trofinetide for the treatment of girls and women with Rett syndrome (LAVENDER™). ClinicalTrials.gov identifier: NCT04181723. Updated Feb 17, 2022. Accessed Feb 23, 2022. https://clinicaltrials.gov/ct2/show/NCT04181723.
36. Acadia Pharmaceuticals announces positive top-line results from the pivotal phase 3 LAVENDER trial of trofinetide in Rett syndrome. Press release. Acadia Pharmaceuticals Inc. Dec 6, 2021. Accessed Feb 23, 2022. https://ir.acadia-pharm.com/news-releases/news-release-details/acadia-pharmaceuticals-announces-positive-top-line-results-1.
37. Copping NA et al. Emerging gene and small molecule therapies for the neurodevelopmental disorder Angelman syndrome. Neurotherapeutics. 2021 Jul;18(3):1535-47. doi: 10.1007/s13311-021-01082-x.
38. Riikonen R. Insulin-like growth factors in the pathogenesis of neurological diseases in children. Int J Mol Sci. 2017 Sep;18(10):2056. doi: 10.3390/ijms18102056.
39. Glaze DG et al; Rett 002 Study Group. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric Rett syndrome. Neurology. 2019 April 16;92(16):e1912-e1925. doi: 10.1212/WNL.0000000000007316.
40. Acadia Pharmaceuticals Inc. An open-label study of trofinetide for the treatment of girls two to five years of age who have Rett syndrome (DAFFODIL™). ClinicalTrials.gov Identifier: NCT04988867. Updated Jan 24, 2022. Accessed Feb 23, 2022. https://clinicaltrials.gov/ct2/show/NCT04988867.
41. Anavex Life Sciences announces ANAVEX®2-73 meets primary and secondary endpoints in clinical trial. Press release. Anavex Life Sciences Corp. Dec 15, 2020. Accessed Feb 23, 2022. http://www.anavex.com/post/anavex-life-sciences-announces-anavex-2-73-meets-primary-and-secondary-endpoints-in-clinical-trial.
42. Anavex Life Sciences Corp. ANAVEX2-73 study in patients with Rett syndrome (AVATAR). ClinicalTrials.gov Identifier: NCT03941444. Updated Jan 27, 2022. Accessed Feb 23, 2022. https://clinicaltrials.gov/ct2/show/NCT03941444.
43. Anavex Life Sciences Corp. ANAVEX2-73 study in pediatric patients with Rett syndrome (EXCELLENCE). ClinicalTrials.gov Identifier: NCT04304482. Updated Sep 28, 2021. Accessed Feb 23, 2022. http://www.clinicaltrials.gov/ct2/show/NCT04304482.
44.Christ MG et al. The Sigma-1 receptor at the crossroad of proteostasis, neurodegeneration, and autophagy. Trends Neurosci. 2020 Feb;43(2):79-81. doi: 10.1016/j.tins.2019.12.002.
45. Kaufmann WE et al. ANAVEX®2-73 (blarcamesine), a sigma-1 receptor agonist, ameliorates neurologic impairments in a mouse model of Rett syndrome. Pharmacol Biochem Behav. 2019 Dec;187:172796. doi: 10.1016/j.pbb.2019.172796.
46. Brimson JM et al. Dipentylammonium binds to the sigma-1 receptor and protects against glutamate toxicity, attenuates dopamine toxicity and potentiates neurite outgrowth in various cultured cell lines. Neurotox Res. 2018 Aug;34(2):263-72. doi: 10.1007/s12640-018-9883-5.
47. Kourrich S et al. The sigma-1 receptor: roles in neuronal plasticity and disease. Trends Neurosci. 2012 Dec;35(12):762-71. doi: 10.1016/j.tins.2012.09.007.
48. Lappalainen R, Riikonen RS. High levels of cerebrospinal fluid glutamate in Rett syndrome. Pediatr Neurol. 1996 Oct;15(3):213-6. doi: 10.1016/s0887-8994(96)00218-4.
49. Hamberger A et al. Elevated CSF glutamate in Rett syndrome. Neuropediatrics. 1992;23(4):212-3. doi: 10.1055/s-2008-1071344.
50. Inacio P. FDA acts to support development of potential gene therapy, TSHA-102. Rett Syndrome News [Internet]. Oct 16, 2020. Accessed Feb 23, 2022. https://rettsyndromenews.com/2020/10/16/fda-grants-orphan-drug-rare-pediatric-disease-status-to-tsha-102-potential-rett-gene-therapy.
51. Sinnett SE et al. Engineered microRNA-based regulatory element permits safe high-dose miniMECP2 gene therapy in Rett mice. Brain. 2021 Nov 29;144(10):3005-19. doi: 10.1093/brain/awab182.
52. Le TTH et al. Efficient and precise CRISPR/Cas9-mediated MECP2 modifications in human-induced pluripotent stem cells. Front Genet. 2019 Jul 2;10:625. doi: 10.3389/fgene.2019.00625.
53. Koerner MV et al. Toxicity of overexpressed MeCP2 is independent of HDAC3 activity. Genes Dev. 2018;32(23-24):1514-24. doi: 10.1101/gad.320325.118.
54. Heckman LD et al. Rett-causing mutations reveal two domains critical for MeCP2 function and for toxicity in MECP2 duplication syndrome mice. Elife. 2014;3:e02676. doi: 10.7554/eLife.02676.
55. Neurogene announces new development program in Rett syndrome utilizing novel EXACT technology platform [Internet]. Accessed Aug 12, 2022. https://www.neurogene.com/press-releases/neurogene-announces-new-development-program-in-rett-syndrome-utilizing-novel-exact-technology-platform/
56. Anzalone AV et al. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol. 2020 Jul;38(7):824-44. doi: 10.1038/s41587-020-0561-9.
57. Gaudelli NM, Komor AC, Rees HA, et al. Programmable base editing of A●T to G●C in genomic DNA without DNA cleavage. Nature. 2017 Nov 23;551(7681):464-71. doi: 10.1038/nature24644.
58. Coenraads M. How RSRT is driving the search for a Rett cure. Rett Syndrome Research Trust [Internet]. Dec 7, 2021. Accessed Feb 23, 2022. https://rettnews.org/articles/how-rsrt-is-driving-the-search-for-a-rett-cure.
59. Cutting-edge technologies to repair the underlying mutations that cause Rett. Rett Syndrome Research Trust [Internet]. Updated Nov 3, 2021. Accessed Feb 23, 2022. https://reverserett.org/research/cures/gene-editing.
60. Sinnamon JR et al. In vivo repair of a protein underlying a neurological disorder by programmable RNA editing. Cell Rep. 2020 Jul 14;32(2):107878. doi: 10.1016/j.celrep.2020.107878.
61. Sinnamon JR et al. Site-directed RNA repair of endogenous Mecp2 RNA in neurons. Proc Natl Acad Sci U S A. 2017 Oct 31;114(44):E9395-E9402. doi: 10.1073/pnas.1715320114.
62. Pipeline. VICO Therapeutics [Internet]. Updated Nov 5, 2021. Accessed Feb 23, 2022. https://vicotx.com/pipeline.
63. Therapeutics platform. Shape Therapeutics [Internet]. Updated Feb 20, 2021. Accessed Feb 23, 2022.
https://live-shapetx.pantheonsite.io/therapeutics-platform.
64. Koeberl DD et al. Glycogen storage disease types I and II: Treatment updates. J Inherit Metab Dis. 2007 Apr;30(2):159-64. doi: 10.1007/s10545-007-0519-9.
The dream of curing genetic disorders has been a persistent but elusive goal, even before the human genome was mapped. Once mapping of the human genome was complete in 2001, an entirely new avenue of potential treatments and cures for genetic diseases and disorders was opened.1,2
The disorders best suited for targeted gene therapy are monogenic; however, tools and delivery methods for editing the human genome were limited and difficult to apply, until the advent of the CRISPR system in 2012.3 CRISPR (an acronym of clustered regularly interspaced short palindromic repeats) has changed the way in which gene therapy strategies are pursued, with dozens of companies leveraging a variety of platforms to create potentially life-changing therapies for devastating rare diseases and disorders.
One of the rare monogenic disorders that is embracing multiple gene therapy approaches is Rett syndrome, a rare, debilitating neurodevelopmental disorder. In this review, we explore the molecular cause of Rett syndrome, disease presentation, current treatments, ongoing clinical trials, and therapies that are on the horizon.
Underlying molecular cause
Rett syndrome is caused by mutations in, or the absence of, the MECP2 gene, which produces methyl-CpG binding protein 2 (MECP2). The syndrome was first described clinically in 1954 by the Austrian physician Andreas Rett; it would take until 1982 before the disorder was officially named, eponymously, in a seminal paper by Hagberg.4 After Hagberg’s characterization, Rett syndrome became the predominant global clinical diagnosis identified among cognitively impaired females, with an incidence of 1 in every 10,000 to 15,000.4
In 1999, mutations in, and deletions of, MECP2 were identified as the cause of Rett syndrome.4,5 MECP2 is located on the X chromosome, in the Xq28 region, making Rett syndrome an X-linked dominant disorder.6 Rett syndrome is seen predominantly in females who are mosaic for mutant or deleted MECP2. Random X chromosome inactivation results in some cells expressing the mutant MECP2 allele and other cells expressing the normal functioning MECP2 allele; the percentage of cells expressing the normal allele correlates with the degree of syndrome severity.7-9
The incidence of Rett syndrome is much lower in males, in whom the syndrome was originally thought to be lethal; many observed male cases are either mosaic or occur in XXY males.10,11
Approximately 95% of cases of Rett syndrome are due to de novo mutations in MECP2, with a handful of specific mutations and large deletions accounting for more than 85% of cases.12 The fact that Rett syndrome is monogenic and most cases are caused by, in total, only a handful of mutations or deletions makes the syndrome a promising candidate for gene therapy.
At the molecular level, it has been observed that the MECP2 mutations of Rett syndrome lead to loss of gene function, thus disrupting the ability of the MECP2 nuclear protein to regulate global gene transcription through its binding to methylated DNA sites.12 A large percentage of these missense and nonsense mutations lead to a truncated or nonfunctional protein.12
One of the ways in which MECP2 regulates transcription is as a component of heterochromatin condensates and by separation of heterochromatin and euchromatin.13-15 It has been observed that the cells of Rett syndrome patients have an altered chromatin state, potentially contributing to transcriptional dysregulation.16,17 Several mutations observed in Rett syndrome patients occur in crucial domains for heterochromatin condensate formation, which helps explain this cellular phenotype.13 Introduction of a engineered “mini” MECP2 in a murine model of Rett syndrome has resulted in partial rescue of heterochromatin condensate formation and transcriptional regulation – fostering the hypothesis that correcting those genetic changes could lead to a potential therapy.18
Beyond the role of MECP2 in heterochromatin condensate formation, the gene interacts with more than 40 proteins that have diverse roles in cellular function, epigenetic modulation, and neuronal development. This volume of interactions contributes to MECP2 being a global gene regulatory protein that has far-reaching effects on transcriptional regulation across the genome.19-22
Epigenetic dysregulation has been associated with neurodevelopmental and neuropsychiatric disorders.23 Both insulin-like growth factor 1 (IGF-1) and brain-derived neurotrophic factor are transcriptional targets of MECP2, and are involved in neuronal differentiation, synaptic function, and neurite outgrowth.12 This helps explain the neurodevelopmental phenotypes observed in MECP2-mutated patients.
Notably, although Rett syndrome patients experience neurodevelopmental phenotypes at the cellular level, neuronal death is not readily observed. That observation provides hope that an interventional therapy after onset of symptoms might still be of benefit.
Presentation
Early neurotypical development. A hallmark of Rett syndrome is neurotypical physical and mental development until 6 to 24 months of age.
Stagnation is the first stage of the syndrome, involving a small but rapid decline in habitual milestones, such motor and language skills.12 Subtle signs, such as microcephaly and hypotonia, can also arise at this time but might be missed.24
Rapid regression follows stagnation. Speech and motor delays and impaired gait and breathing occur;12,25 purposeful hand skills are lost, replaced by repetitive hand-wringing movements that are a hallmark of the syndrome.12,24 Seizures are observed; they become more common during the next stage.12
Plateau. Language advances can be observed, but further deficits are seen in motor skills and hand coordination.12
Late motor deterioration stage. Late physical deficits develop, leading to lifelong impairments. The physical deficits observed are the result of severe muscle weakness, usually resulting in wheelchair dependency.12
Plateau. Patients then reach a second plateau. Regression stops; deficient physical and cognitive states stabilize and are maintained.25
At all stages of Rett syndrome, the following are observed:
- Gastrointestinal problems.
- Sleep disturbances.
- Abnormal cardiorespiratory coupling.
- Greater-than-expected mortality.12
Final regression. The patient is fully dependent for the rest of their lifespan, partially due to seizure activity.26,27
A life-changing diagnosis
A diagnosis of Rett syndrome is life-changing for a patient’s family; access to supportive groups of other patients and their families is extremely beneficial. Two helpful organizations – the Rett Syndrome Research Trust28 and International Rett Syndrome Foundation,29 – offer patient support and community and fund research.
Because X chromosome inactivation is random in Rett syndrome, the individual patient can present with a wide variety of phenotypic combinations – making the patient, and their needs, unique.12 During stages of regression, patients often experience emotional dysregulation and anxiety, which is attributable to their increasing physical difficulties.30 They often exhibit combinations of uncontrolled movements, including repetitive rocking, scratching, and self-injurious behavior.30 For most, regression subsides after the first 5 years of alternating development and regression; after that, their ultimate symptoms persist for life.25
As patients mature, they need to be monitored for proper nutrition and scoliosis.25 As adults, they are at risk of pneumonia, respiratory distress, status epilepticus, osteopenia, and lack of adequate food or water because of impaired ability to feed.25
The lifespan of Rett syndrome patients has increased, thanks to improvements in health care, advances in technology, and early genetic testing, which allows for earlier diagnosis, intervention, and management of symptoms.
Current treatments
When a female patient presents with regression and loss of milestones, sequencing of MECP2 is performed to verify whether Rett syndrome is the cause, by detecting any of the known mutations. Multiplex ligation-dependent probe amplification is also performed to detect major deletions.25
All available treatments for Rett syndrome are symptomatic; intensive early intervention is practiced.31 Multidisciplinary management – medical, psychiatric, and physical – is introduced almost immediately after diagnosis. Following diagnosis, patients are prescribed anti-seizure, sleep, and anxiety medications.31 Electroencephalography can be performed to identify seizure type. Neuromuscular blockage drugs can be prescribed to help with gait and stereotypic hand movements.25
Handguards or splints to the elbows can be prescribed by an occupational therapist to improve hand movement.25 Physical therapy can improve mobility; hydrotherapy and hippotherapy have been successful in helping to maintain mobility and muscle support.32,33 Nutritional management is implemented to control caloric intake and maintain the vitamin D level.31 Some patients experience constipation and urinary retention, putting them at risk of nephrolithiasis.
Once the signs and symptoms of Rett syndrome progress, and milestones regress to a certain point, patients need constant, full-time care for the rest of their lives.34 As symptomatic interventions have greatly improved patient outcomes and it has been shown that about 70% can reach adulthood with a potential lifespan of about 50 years.25
Although there is no cure for Rett syndrome and treatments are symptomatic, ongoing studies – both clinical and preclinical – offer promise that treatments will be developed that work at molecular and genetic levels.
Clinical trials
Advances in understanding of Rett syndrome have led to many therapies in clinical trials, several of which show promise.
Trofinetide. One of the most promising targets for downstream therapy, mentioned earlier, is IGF-1, which was the target of a successful phase 3 clinical trial, LAVENDER (sponsored by Acadia Pharmaceuticals).35,36 This trial studied trofinetide, a synthetic IGF-1 analog that inhibits neuroinflammation, restores glial function, corrects synaptic deficiencies, and regulates oxidative stress response.12,37,38 Initial results from phase 2 and phase 3 trials indicate improved scores for treated patients in the Rett syndrome Behaviour Questionnaire (RSBQ) and Clinical Global Impression–Improvement (CGI-I) scores, while also showing improvements in the Communication and Symbolic Behavior Scales Developmental Profile Infant–Toddler Checklist–Social composite score.36,39
The most common adverse events seen with trofinetide were diarrhea and vomiting.
Acadia Pharmaceuticals has filed for approval of a new drug application for trofinetide with the Food and Drug Administration, for which the company has been granted Fast Track Status and orphan drug designations. Most (95%) subjects in the phase 3 LAVENDER trial elected to continue taking trofinetide in the subsequent open-label Lilac and Lilac-2 extension studies.36 A current open-label phase 2/3 trial is recruiting patients 2 to 5 years of age to evaluate trofinetide.40 It is expected that, in the near future, this could be a drug given to Rett patients as an FDA-approved treatment.
Blarcamesine. Another small molecule drug, blarcamesine (also known as ANAVEX2-73), a sigma-1 receptor agonist, produced promising results in phase 2 clinical trials in adult Rett syndrome patients. The drug is in a phase 2/3 clinical trial for pediatric Rett syndrome patients (sponsored by Anavex Life Sciences).41-43
Phase 2 results indicated statistically significant and clinically meaningful improvement in RSBQ and CGI-I scores with blarcamesine. Improvement was initially observed within 4 weeks after the start of treatment and was sustained throughout the study. The drug was shown to be well tolerated, with minimal adverse effects; no serious adverse events were recorded. These results were observed in adult patients, demonstrating that improvements in Rett syndrome are possible even after regression.
Blarcamesine activates the sigma 1 receptor, which is pivotal to restoring cellular homeostasis and restoring neuroplasticity – deficiencies of which have been linked to autophagy and glutamate toxicity. The drug has also been explored as a potential treatment for other neurological disorders.44-47 Improvements in blarcamesine-treated patients further correlated with lower levels of glutamate in cerebrospinal fluid, which is a Rett syndrome biomarker, supporting the proposition that behavioral improvements were due to drug intervention.48,49 The phase 2 trial was modified into a phase 3 trial and additional endpoints were added.41-43
All patients in the phase 2 adult trial elected to continue in the extension study.
Based on these promising data, Anavex is pursuing an approval pathway for adult patients, while continuing dosage optimization phase 2/3 trials and recruitment for a pediatric trial.42,43
Is the future about gene therapy?
TSHA-102 (miniMECP2). Taysha Gene Therapies is developing a promising gene therapy, TSHA-102, for Rett syndrome, and is aiming to begin phase 1/2 clinical trials in 2022.50 The technology for this therapy relies on the delivery of a fragment of MECP2 (known as miniMECP2), which is regulated by a built-in microRNA regulator (miR-responsive auto-regulatory element, or miRARE) to help ameliorate MECP2 dosage toxicity. (Overexpression of MECP2 is toxic to neurons, which has made traditional [so to speak] gene replacement therapy difficult in Rett syndrome: Levels of MECP2 need to be tightly regulated, and the Taysha microRNA technology regulates levels of miniMECP2, thus reducing toxicity.)
The Taysha microRNA technology has yielded promising results in mouse studies for Rett syndrome; results indicate a lengthening of lifespan and delayed onset of gait abnormalities.51 TSHA-102 is in the preclinical stage but offers promise that it will be the first gene therapy for Rett syndrome to enter clinical trials.
As the field of gene therapy advances, several promising technologies are on the horizon that could potentially have disease-altering impacts on Rett syndrome. These therapies are divided into two broad categories: those at the gene level and those at the transcription and protein level. A few of these approaches are highlighted below.
Gene replacement involves adding a full or partial copy of MECP2 to neuronal cells. This type of therapy presents challenges, from delivery of the new gene to dosage concerns, because MECP2 can be toxic if overexpressed.52-54 Groundbreaking work was done in mouse models involving truncated MECP2, exhibiting phenotypic rescue and validating the gene-replacement approach.18 This strategy is being pursued by Neurogene, which has a uinique technology that allows for tuning of the gene’s expression to get the correct protein levels in the patient. Promising data was presented this year at the American Society of Gene and Cell Therapy conference.55
Early gene replacement therapy studies also laid the foundation for the minMECP2 and microRNA approach being used by Taysha Gene Therapies (discussed above).51
“Correcting” DNA mutations. A different approach at the genetic level involves “correcting” mutations in MECP2 at the DNA level. This is possible because, in a large subset of Rett syndrome patients who have the same missense or nonsense mutations, by using CRISPR, a gene editing tool (discussed above) a single base pair can be corrected.56,57 Previous research, in a Rett syndrome-model of induced pluripotent stem cells, showed that this type of editing is possible – and effective.52 An approach with particular promise involves use of a class of CRISPR proteins known as base editors that are able to specifically alter a single base of DNA.57 The technique has the potential to address many of the mutations seen in Rett syndrome; research on this type of technology is being pursued by Beam Therapeutics, and has the potential to impact Rett syndrome.58
Another promising “correction” approach is exonic editing, in which a much larger section of DNA – potentially, exons 3 and 4, which, taken together, comprise 97% of known MECP2 mutations seen in Rett syndrome – are replaced.59
In both CRISPR and exonic editing therapeutic approaches, endogenous levels of MECP2 expression would be maintained. Of note, both approaches are being pursued for use in treating other genetic disorders, which provides a boost in scaling-up work on addressing safety and efficacy concerns that accompany gene-editing approaches.58 One advantage to the DNA correction approach is that is has the potential to be a “one-and-done” treatment.
“Correcting” RNA. Beyond directly editing DNA, several therapeutic approaches are exploring the ability to edit RNA or to provide the protein directly to cells as enzyme replacement therapy. Such an RNA correction strategy leverages a technology that takes advantage of cells’ natural RNA editor, known as adenosine deaminase acting on RNA (ADAR), which corrects errors in cells’ RNA by providing specific guides to the cell. ADAR can be targeted to fix mutations in the MECP2 RNA transcript, resulting in a “corrected” MECP2 protein.60,61 This technology has delivered promising proof-of-concept evidence in cells and in murine models, and is in the therapeutic pipeline at VICO Therapeutics.62
Shape Therapeutics has also leveraged ADAR to “correct” mutated RNA; Rett syndrome is among the top priorities in the company’s pipeline.
Worth noting is that there are several advantages to the “correction” approach:
- Leveraging internal repair mechanisms minimizes the immune response.
- The flexibility of correction means that it can be used to address many of the mutations that cause Rett syndrome.63
Enzyme replacement therapy, in which the MECP2 protein produced by MECP2 would be directly replaced, is being explored in Rett syndrome patients. This technology has been used successfully in Pompe disease; however, Rett syndrome presents its own challenge because MECP2 needs to be delivered to the brain and neuronal cells.64
Where does this work stand? The technologies described in this section are in preclinical stages of study. Nonetheless, it is expected that several will enter human clinical trials during the next 5 years.
Conclusion
A diagnosis of Rett syndrome is a life-altering event for patients and their family. But there is more hope than ever for effective therapies and, eventually, a cure.
Multiple late-stage clinical trials in progress are demonstrating promising results from therapeutic products, with minimal adverse events. Remarkably, these interventions have delivered improvements to adult patients after regression has stabilized. With rapid progress being made in the field of gene therapy, several technologies for which are focused on Rett syndrome, a hopeful picture is emerging: that therapeutic intervention will be possible before regression, thus effectively treating and, potentially, even curing Rett syndrome.
The landscape is broadening. Add to this hope for approved therapies is the fact that Rett syndrome isn’t the only target being pursued with such strategies; in fact, researchers in the larger field of neurodevelopmental disorder study are working together to find common solutions to shared challenges – from how therapies are designed and delivered to how toxicity is minimized. Much of what is being explored in the Rett syndrome field is also under investigation in other neurodevelopmental syndromes, including Angelman, Prader-Willi, chromosome 15q11.2-13.1 duplication (dup15q), and Fragile X syndrome. This kind of parallel investigation benefits all parties and optimizes a treatment platform so that it can be applied across more than a single disorder.
Like many monogenic disorders, Rett syndrome is entering an exciting stage – at which the words “treatment” and “cure” can be spoken with intent and vision, not just wide-eyed optimism. These words portend real promise for patients who carry the weight of a diagnosis of Rett syndrome, and for their families.
Ms. Ambrose is a student in the master’s of science in human genetics and genomic data analytics program, Keck Graduate Institute, Claremont, Calif. Dr. Bailus is an assistant professor of genetics, Keck Graduate Institute. The authors report no conflict of interest related to this article.
References
1. Lander ES et al; . Initial sequencing and analysis of the human genome. Nature. 2001 Feb 15;409(6822):860-921. doi: 10.1038/35057062.
2. Venter JC et al. The sequence of the human genome. Science. 2001 Feb 16;291(5507):1304-51. doi: 10.1126/science.1058040.
3. Jinek M et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012 Aug 17;337(6096):816-21. doi: 10.1126/science.1225829.
4. Percy A. The American history of Rett syndrome. Pediatr Neurol. 2014 Jan;50(1):1-3. doi: 10.1016/j.pediatrneurol.2013.08.018.
5. Amir RE et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999 Oct;23(2):185-8. doi: 10.1038/13810.
6. Pitzianti MB et al. Rett syndrome in males: The different clinical course in two brothers with the same microduplication MECP2 Xq28. Int J Environ Res Public Health. 2019 Aug;16(17):3075. doi: 10.3390/ijerph16173075.
7. Ribeiro MC, MacDonald JL. Sex differences in Mecp2-mutant Rett syndrome model mice and the impact of cellular mosaicism in phenotype development. Brain Res. 2020 Feb 15;1729:146644. doi: 10.1016/j.brainres.2019.146644.
8. Bao X et al. X chromosome inactivation in Rett syndrome and its correlations with MECP2 mutations and phenotype. J Child Neurol. 2008 Jan;23(1):22-5. doi: 10.1177/0883073807307077.
9. Knudsen GPS et al. Increased skewing of X chromosome inactivation in Rett syndrome patients and their mothers. Eur J Hum Genet. 2006 Jul;14(11):1189-94. doi: 10.1038/sj.ejhg.5201682.
10. Chahil G et al. Rett syndrome in males: A case report and review of literature. Cureus. 2018;10(10):e3414. doi: 10.7759/cureus.3414.
11. Reichow B et al. Brief report: Systematic review of Rett syndrome in males. J Autism Dev Disord. 2015 Oct;45(10):3377-83. doi: 10.1007/s10803-015-2519-1.
12. Vashi N, Justice MJ. Treating Rett syndrome: From mouse models to human therapies. Mamm Genome. 2019 Jun;30(5-6):90-110. doi: 10.1007/s00335-019-09793-5.
13. Li CH et al. MeCP2 links heterochromatin condensates and neurodevelopmental disease. Nature. 2020 Oct;586(7829):440-4. doi: 10.1038/s41586-020-2574-4.
14. Schmidt A et al. MeCP2 and chromatin compartmentalization. Cells. 2020 Apr;9(4):878. doi: 10.3390/cells9040878.
15. Wang L et al. Rett syndrome-causing mutations compromise MeCP2-mediated liquid-liquid phase separation of chromatin. Cell Res. 2020 May;30(5):393-407. doi: 10.1038/s41422-020-0288-7.
16. Lin P et al. Transcriptome analysis of human brain tissue identifies reduced expression of complement complex C1Q genes in Rett syndrome. BMC Genomics. 2016;17:427. doi: 10.1186/s12864-016-2746-7.
17. Tudor M et al. Transcriptional profiling of a mouse model for Rett syndrome reveals subtle transcriptional changes in the brain. Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15536-41. doi: 10.1073/pnas.242566899.
18. Tillotson R et al. Radically truncated MeCP2 rescues Rett syndrome–like neurological defects. Nature. 2017 Oct 19;550(7676):398-401. doi: 10.1038/nature24058.
19. Connolly DR, Zhou Z. Genomic insights into MeCP2 function: A role for the maintenance of chromatin architecture. Curr Opin Neurobiol. 2019 Dec;59:174-9. doi: 10.1016/j.conb.2019.07.002.
20. Johnson BS et al. Biotin tagging of MeCP2 in mice reveals contextual insights into the Rett syndrome transcriptome. Nat Med. 2017 Oct;23(10):1203-14. doi: 10.1038/nm.4406.
21. Gabel HW et al. Disruption of DNA-methylation–dependent long gene repression in Rett syndrome. Nature. 2015 Jun 4;522(7554):89-93. doi: 10.1038/nature14319.
22. Lyst MJ, Bird A. Rett syndrome: A complex disorder with simple roots. Nat Rev Genet. 2015 May;16(5):261-75. doi: 10.1038/nrg3897.
23. Kuehner JN et al. Epigenetic regulations in neuropsychiatric disorders. Front Genet. 2019 Apr 4;10:268. doi: 10.3389/fgene.2019.00268.
24. Pejhan S, Rastegar M. Role of DNA methyl-CpG-binding protein MeCP2 in Rett syndrome pathobiology and mechanism of disease. Biomolecules. 2021 Jan;11(1):75. doi: 10.3390/biom11010075.
25. Fu C et al. Consensus guidelines on managing Rett syndrome across the lifespan. BMJ Paediatr Open. 2020;4(1):e000717. doi: 10.1136/bmjpo-2020-000717.
26. Operto FF et al. Epilepsy and genetic in Rett syndrome: A review. Brain Behav. 2019 May;9(5):e01250. doi: 10.1002/brb3.1250.
27. Nissenkorn A et al. Epilepsy in Rett syndrome – The experience of a National Rett Center. Epilepsia. 2010 Jul;51(7):1252-8. doi: 10.1111/j.1528-1167.2010.02597.x.
28. Welcome to the Rett cure community. Rett Syndrome Research Trust [Internet]. Updated Feb 8, 2022. Accessed Feb 23, 2022. https://reverserett.org.
29. About Rett syndrome. International Rett Syndrome Foundation [Internet]. Updated Jan 4, 2022. Accessed Feb 23, 2022. http://www.rettsyndrome.org.
30. Singh J, Santosh P. Key issues in Rett syndrome: Emotional, behavioural and autonomic dysregulation (EBAD) – A target for clinical trials. Orphanet J Rare Dis. 2018 Jul 31;13(1):128. doi: 10.1186/s13023-018-0873-8.
31. Banerjee A et al. Towards a better diagnosis and treatment of Rett syndrome: A model synaptic disorder. Brain. 2019 Feb 1;142(2):239-48. doi: 10.1093/brain/awy323.
32. Ager S et al. Parental experiences of scoliosis management in Rett syndrome. Disabil Rehabil. 2009 Sep 19;31(23):1917-24. doi: 10.1080/09638280902846392.
33. Budden SS. Management of Rett syndrome: A ten year experience. Neuropediatrics. 1995;26(2):75-7. doi: 10.1055/s-2007-979727.
34. Ip JPK et al. Rett syndrome: Insights into genetic, molecular and circuit mechanisms. Nat Rev Neurosci. 2018 Jun;19(6):368-82. doi: 10.1038/s41583-018-0006-3.
35. Acadia Pharmaceuticals Inc. Study of trofinetide for the treatment of girls and women with Rett syndrome (LAVENDER™). ClinicalTrials.gov identifier: NCT04181723. Updated Feb 17, 2022. Accessed Feb 23, 2022. https://clinicaltrials.gov/ct2/show/NCT04181723.
36. Acadia Pharmaceuticals announces positive top-line results from the pivotal phase 3 LAVENDER trial of trofinetide in Rett syndrome. Press release. Acadia Pharmaceuticals Inc. Dec 6, 2021. Accessed Feb 23, 2022. https://ir.acadia-pharm.com/news-releases/news-release-details/acadia-pharmaceuticals-announces-positive-top-line-results-1.
37. Copping NA et al. Emerging gene and small molecule therapies for the neurodevelopmental disorder Angelman syndrome. Neurotherapeutics. 2021 Jul;18(3):1535-47. doi: 10.1007/s13311-021-01082-x.
38. Riikonen R. Insulin-like growth factors in the pathogenesis of neurological diseases in children. Int J Mol Sci. 2017 Sep;18(10):2056. doi: 10.3390/ijms18102056.
39. Glaze DG et al; Rett 002 Study Group. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric Rett syndrome. Neurology. 2019 April 16;92(16):e1912-e1925. doi: 10.1212/WNL.0000000000007316.
40. Acadia Pharmaceuticals Inc. An open-label study of trofinetide for the treatment of girls two to five years of age who have Rett syndrome (DAFFODIL™). ClinicalTrials.gov Identifier: NCT04988867. Updated Jan 24, 2022. Accessed Feb 23, 2022. https://clinicaltrials.gov/ct2/show/NCT04988867.
41. Anavex Life Sciences announces ANAVEX®2-73 meets primary and secondary endpoints in clinical trial. Press release. Anavex Life Sciences Corp. Dec 15, 2020. Accessed Feb 23, 2022. http://www.anavex.com/post/anavex-life-sciences-announces-anavex-2-73-meets-primary-and-secondary-endpoints-in-clinical-trial.
42. Anavex Life Sciences Corp. ANAVEX2-73 study in patients with Rett syndrome (AVATAR). ClinicalTrials.gov Identifier: NCT03941444. Updated Jan 27, 2022. Accessed Feb 23, 2022. https://clinicaltrials.gov/ct2/show/NCT03941444.
43. Anavex Life Sciences Corp. ANAVEX2-73 study in pediatric patients with Rett syndrome (EXCELLENCE). ClinicalTrials.gov Identifier: NCT04304482. Updated Sep 28, 2021. Accessed Feb 23, 2022. http://www.clinicaltrials.gov/ct2/show/NCT04304482.
44.Christ MG et al. The Sigma-1 receptor at the crossroad of proteostasis, neurodegeneration, and autophagy. Trends Neurosci. 2020 Feb;43(2):79-81. doi: 10.1016/j.tins.2019.12.002.
45. Kaufmann WE et al. ANAVEX®2-73 (blarcamesine), a sigma-1 receptor agonist, ameliorates neurologic impairments in a mouse model of Rett syndrome. Pharmacol Biochem Behav. 2019 Dec;187:172796. doi: 10.1016/j.pbb.2019.172796.
46. Brimson JM et al. Dipentylammonium binds to the sigma-1 receptor and protects against glutamate toxicity, attenuates dopamine toxicity and potentiates neurite outgrowth in various cultured cell lines. Neurotox Res. 2018 Aug;34(2):263-72. doi: 10.1007/s12640-018-9883-5.
47. Kourrich S et al. The sigma-1 receptor: roles in neuronal plasticity and disease. Trends Neurosci. 2012 Dec;35(12):762-71. doi: 10.1016/j.tins.2012.09.007.
48. Lappalainen R, Riikonen RS. High levels of cerebrospinal fluid glutamate in Rett syndrome. Pediatr Neurol. 1996 Oct;15(3):213-6. doi: 10.1016/s0887-8994(96)00218-4.
49. Hamberger A et al. Elevated CSF glutamate in Rett syndrome. Neuropediatrics. 1992;23(4):212-3. doi: 10.1055/s-2008-1071344.
50. Inacio P. FDA acts to support development of potential gene therapy, TSHA-102. Rett Syndrome News [Internet]. Oct 16, 2020. Accessed Feb 23, 2022. https://rettsyndromenews.com/2020/10/16/fda-grants-orphan-drug-rare-pediatric-disease-status-to-tsha-102-potential-rett-gene-therapy.
51. Sinnett SE et al. Engineered microRNA-based regulatory element permits safe high-dose miniMECP2 gene therapy in Rett mice. Brain. 2021 Nov 29;144(10):3005-19. doi: 10.1093/brain/awab182.
52. Le TTH et al. Efficient and precise CRISPR/Cas9-mediated MECP2 modifications in human-induced pluripotent stem cells. Front Genet. 2019 Jul 2;10:625. doi: 10.3389/fgene.2019.00625.
53. Koerner MV et al. Toxicity of overexpressed MeCP2 is independent of HDAC3 activity. Genes Dev. 2018;32(23-24):1514-24. doi: 10.1101/gad.320325.118.
54. Heckman LD et al. Rett-causing mutations reveal two domains critical for MeCP2 function and for toxicity in MECP2 duplication syndrome mice. Elife. 2014;3:e02676. doi: 10.7554/eLife.02676.
55. Neurogene announces new development program in Rett syndrome utilizing novel EXACT technology platform [Internet]. Accessed Aug 12, 2022. https://www.neurogene.com/press-releases/neurogene-announces-new-development-program-in-rett-syndrome-utilizing-novel-exact-technology-platform/
56. Anzalone AV et al. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol. 2020 Jul;38(7):824-44. doi: 10.1038/s41587-020-0561-9.
57. Gaudelli NM, Komor AC, Rees HA, et al. Programmable base editing of A●T to G●C in genomic DNA without DNA cleavage. Nature. 2017 Nov 23;551(7681):464-71. doi: 10.1038/nature24644.
58. Coenraads M. How RSRT is driving the search for a Rett cure. Rett Syndrome Research Trust [Internet]. Dec 7, 2021. Accessed Feb 23, 2022. https://rettnews.org/articles/how-rsrt-is-driving-the-search-for-a-rett-cure.
59. Cutting-edge technologies to repair the underlying mutations that cause Rett. Rett Syndrome Research Trust [Internet]. Updated Nov 3, 2021. Accessed Feb 23, 2022. https://reverserett.org/research/cures/gene-editing.
60. Sinnamon JR et al. In vivo repair of a protein underlying a neurological disorder by programmable RNA editing. Cell Rep. 2020 Jul 14;32(2):107878. doi: 10.1016/j.celrep.2020.107878.
61. Sinnamon JR et al. Site-directed RNA repair of endogenous Mecp2 RNA in neurons. Proc Natl Acad Sci U S A. 2017 Oct 31;114(44):E9395-E9402. doi: 10.1073/pnas.1715320114.
62. Pipeline. VICO Therapeutics [Internet]. Updated Nov 5, 2021. Accessed Feb 23, 2022. https://vicotx.com/pipeline.
63. Therapeutics platform. Shape Therapeutics [Internet]. Updated Feb 20, 2021. Accessed Feb 23, 2022.
https://live-shapetx.pantheonsite.io/therapeutics-platform.
64. Koeberl DD et al. Glycogen storage disease types I and II: Treatment updates. J Inherit Metab Dis. 2007 Apr;30(2):159-64. doi: 10.1007/s10545-007-0519-9.
The dream of curing genetic disorders has been a persistent but elusive goal, even before the human genome was mapped. Once mapping of the human genome was complete in 2001, an entirely new avenue of potential treatments and cures for genetic diseases and disorders was opened.1,2
The disorders best suited for targeted gene therapy are monogenic; however, tools and delivery methods for editing the human genome were limited and difficult to apply, until the advent of the CRISPR system in 2012.3 CRISPR (an acronym of clustered regularly interspaced short palindromic repeats) has changed the way in which gene therapy strategies are pursued, with dozens of companies leveraging a variety of platforms to create potentially life-changing therapies for devastating rare diseases and disorders.
One of the rare monogenic disorders that is embracing multiple gene therapy approaches is Rett syndrome, a rare, debilitating neurodevelopmental disorder. In this review, we explore the molecular cause of Rett syndrome, disease presentation, current treatments, ongoing clinical trials, and therapies that are on the horizon.
Underlying molecular cause
Rett syndrome is caused by mutations in, or the absence of, the MECP2 gene, which produces methyl-CpG binding protein 2 (MECP2). The syndrome was first described clinically in 1954 by the Austrian physician Andreas Rett; it would take until 1982 before the disorder was officially named, eponymously, in a seminal paper by Hagberg.4 After Hagberg’s characterization, Rett syndrome became the predominant global clinical diagnosis identified among cognitively impaired females, with an incidence of 1 in every 10,000 to 15,000.4
In 1999, mutations in, and deletions of, MECP2 were identified as the cause of Rett syndrome.4,5 MECP2 is located on the X chromosome, in the Xq28 region, making Rett syndrome an X-linked dominant disorder.6 Rett syndrome is seen predominantly in females who are mosaic for mutant or deleted MECP2. Random X chromosome inactivation results in some cells expressing the mutant MECP2 allele and other cells expressing the normal functioning MECP2 allele; the percentage of cells expressing the normal allele correlates with the degree of syndrome severity.7-9
The incidence of Rett syndrome is much lower in males, in whom the syndrome was originally thought to be lethal; many observed male cases are either mosaic or occur in XXY males.10,11
Approximately 95% of cases of Rett syndrome are due to de novo mutations in MECP2, with a handful of specific mutations and large deletions accounting for more than 85% of cases.12 The fact that Rett syndrome is monogenic and most cases are caused by, in total, only a handful of mutations or deletions makes the syndrome a promising candidate for gene therapy.
At the molecular level, it has been observed that the MECP2 mutations of Rett syndrome lead to loss of gene function, thus disrupting the ability of the MECP2 nuclear protein to regulate global gene transcription through its binding to methylated DNA sites.12 A large percentage of these missense and nonsense mutations lead to a truncated or nonfunctional protein.12
One of the ways in which MECP2 regulates transcription is as a component of heterochromatin condensates and by separation of heterochromatin and euchromatin.13-15 It has been observed that the cells of Rett syndrome patients have an altered chromatin state, potentially contributing to transcriptional dysregulation.16,17 Several mutations observed in Rett syndrome patients occur in crucial domains for heterochromatin condensate formation, which helps explain this cellular phenotype.13 Introduction of a engineered “mini” MECP2 in a murine model of Rett syndrome has resulted in partial rescue of heterochromatin condensate formation and transcriptional regulation – fostering the hypothesis that correcting those genetic changes could lead to a potential therapy.18
Beyond the role of MECP2 in heterochromatin condensate formation, the gene interacts with more than 40 proteins that have diverse roles in cellular function, epigenetic modulation, and neuronal development. This volume of interactions contributes to MECP2 being a global gene regulatory protein that has far-reaching effects on transcriptional regulation across the genome.19-22
Epigenetic dysregulation has been associated with neurodevelopmental and neuropsychiatric disorders.23 Both insulin-like growth factor 1 (IGF-1) and brain-derived neurotrophic factor are transcriptional targets of MECP2, and are involved in neuronal differentiation, synaptic function, and neurite outgrowth.12 This helps explain the neurodevelopmental phenotypes observed in MECP2-mutated patients.
Notably, although Rett syndrome patients experience neurodevelopmental phenotypes at the cellular level, neuronal death is not readily observed. That observation provides hope that an interventional therapy after onset of symptoms might still be of benefit.
Presentation
Early neurotypical development. A hallmark of Rett syndrome is neurotypical physical and mental development until 6 to 24 months of age.
Stagnation is the first stage of the syndrome, involving a small but rapid decline in habitual milestones, such motor and language skills.12 Subtle signs, such as microcephaly and hypotonia, can also arise at this time but might be missed.24
Rapid regression follows stagnation. Speech and motor delays and impaired gait and breathing occur;12,25 purposeful hand skills are lost, replaced by repetitive hand-wringing movements that are a hallmark of the syndrome.12,24 Seizures are observed; they become more common during the next stage.12
Plateau. Language advances can be observed, but further deficits are seen in motor skills and hand coordination.12
Late motor deterioration stage. Late physical deficits develop, leading to lifelong impairments. The physical deficits observed are the result of severe muscle weakness, usually resulting in wheelchair dependency.12
Plateau. Patients then reach a second plateau. Regression stops; deficient physical and cognitive states stabilize and are maintained.25
At all stages of Rett syndrome, the following are observed:
- Gastrointestinal problems.
- Sleep disturbances.
- Abnormal cardiorespiratory coupling.
- Greater-than-expected mortality.12
Final regression. The patient is fully dependent for the rest of their lifespan, partially due to seizure activity.26,27
A life-changing diagnosis
A diagnosis of Rett syndrome is life-changing for a patient’s family; access to supportive groups of other patients and their families is extremely beneficial. Two helpful organizations – the Rett Syndrome Research Trust28 and International Rett Syndrome Foundation,29 – offer patient support and community and fund research.
Because X chromosome inactivation is random in Rett syndrome, the individual patient can present with a wide variety of phenotypic combinations – making the patient, and their needs, unique.12 During stages of regression, patients often experience emotional dysregulation and anxiety, which is attributable to their increasing physical difficulties.30 They often exhibit combinations of uncontrolled movements, including repetitive rocking, scratching, and self-injurious behavior.30 For most, regression subsides after the first 5 years of alternating development and regression; after that, their ultimate symptoms persist for life.25
As patients mature, they need to be monitored for proper nutrition and scoliosis.25 As adults, they are at risk of pneumonia, respiratory distress, status epilepticus, osteopenia, and lack of adequate food or water because of impaired ability to feed.25
The lifespan of Rett syndrome patients has increased, thanks to improvements in health care, advances in technology, and early genetic testing, which allows for earlier diagnosis, intervention, and management of symptoms.
Current treatments
When a female patient presents with regression and loss of milestones, sequencing of MECP2 is performed to verify whether Rett syndrome is the cause, by detecting any of the known mutations. Multiplex ligation-dependent probe amplification is also performed to detect major deletions.25
All available treatments for Rett syndrome are symptomatic; intensive early intervention is practiced.31 Multidisciplinary management – medical, psychiatric, and physical – is introduced almost immediately after diagnosis. Following diagnosis, patients are prescribed anti-seizure, sleep, and anxiety medications.31 Electroencephalography can be performed to identify seizure type. Neuromuscular blockage drugs can be prescribed to help with gait and stereotypic hand movements.25
Handguards or splints to the elbows can be prescribed by an occupational therapist to improve hand movement.25 Physical therapy can improve mobility; hydrotherapy and hippotherapy have been successful in helping to maintain mobility and muscle support.32,33 Nutritional management is implemented to control caloric intake and maintain the vitamin D level.31 Some patients experience constipation and urinary retention, putting them at risk of nephrolithiasis.
Once the signs and symptoms of Rett syndrome progress, and milestones regress to a certain point, patients need constant, full-time care for the rest of their lives.34 As symptomatic interventions have greatly improved patient outcomes and it has been shown that about 70% can reach adulthood with a potential lifespan of about 50 years.25
Although there is no cure for Rett syndrome and treatments are symptomatic, ongoing studies – both clinical and preclinical – offer promise that treatments will be developed that work at molecular and genetic levels.
Clinical trials
Advances in understanding of Rett syndrome have led to many therapies in clinical trials, several of which show promise.
Trofinetide. One of the most promising targets for downstream therapy, mentioned earlier, is IGF-1, which was the target of a successful phase 3 clinical trial, LAVENDER (sponsored by Acadia Pharmaceuticals).35,36 This trial studied trofinetide, a synthetic IGF-1 analog that inhibits neuroinflammation, restores glial function, corrects synaptic deficiencies, and regulates oxidative stress response.12,37,38 Initial results from phase 2 and phase 3 trials indicate improved scores for treated patients in the Rett syndrome Behaviour Questionnaire (RSBQ) and Clinical Global Impression–Improvement (CGI-I) scores, while also showing improvements in the Communication and Symbolic Behavior Scales Developmental Profile Infant–Toddler Checklist–Social composite score.36,39
The most common adverse events seen with trofinetide were diarrhea and vomiting.
Acadia Pharmaceuticals has filed for approval of a new drug application for trofinetide with the Food and Drug Administration, for which the company has been granted Fast Track Status and orphan drug designations. Most (95%) subjects in the phase 3 LAVENDER trial elected to continue taking trofinetide in the subsequent open-label Lilac and Lilac-2 extension studies.36 A current open-label phase 2/3 trial is recruiting patients 2 to 5 years of age to evaluate trofinetide.40 It is expected that, in the near future, this could be a drug given to Rett patients as an FDA-approved treatment.
Blarcamesine. Another small molecule drug, blarcamesine (also known as ANAVEX2-73), a sigma-1 receptor agonist, produced promising results in phase 2 clinical trials in adult Rett syndrome patients. The drug is in a phase 2/3 clinical trial for pediatric Rett syndrome patients (sponsored by Anavex Life Sciences).41-43
Phase 2 results indicated statistically significant and clinically meaningful improvement in RSBQ and CGI-I scores with blarcamesine. Improvement was initially observed within 4 weeks after the start of treatment and was sustained throughout the study. The drug was shown to be well tolerated, with minimal adverse effects; no serious adverse events were recorded. These results were observed in adult patients, demonstrating that improvements in Rett syndrome are possible even after regression.
Blarcamesine activates the sigma 1 receptor, which is pivotal to restoring cellular homeostasis and restoring neuroplasticity – deficiencies of which have been linked to autophagy and glutamate toxicity. The drug has also been explored as a potential treatment for other neurological disorders.44-47 Improvements in blarcamesine-treated patients further correlated with lower levels of glutamate in cerebrospinal fluid, which is a Rett syndrome biomarker, supporting the proposition that behavioral improvements were due to drug intervention.48,49 The phase 2 trial was modified into a phase 3 trial and additional endpoints were added.41-43
All patients in the phase 2 adult trial elected to continue in the extension study.
Based on these promising data, Anavex is pursuing an approval pathway for adult patients, while continuing dosage optimization phase 2/3 trials and recruitment for a pediatric trial.42,43
Is the future about gene therapy?
TSHA-102 (miniMECP2). Taysha Gene Therapies is developing a promising gene therapy, TSHA-102, for Rett syndrome, and is aiming to begin phase 1/2 clinical trials in 2022.50 The technology for this therapy relies on the delivery of a fragment of MECP2 (known as miniMECP2), which is regulated by a built-in microRNA regulator (miR-responsive auto-regulatory element, or miRARE) to help ameliorate MECP2 dosage toxicity. (Overexpression of MECP2 is toxic to neurons, which has made traditional [so to speak] gene replacement therapy difficult in Rett syndrome: Levels of MECP2 need to be tightly regulated, and the Taysha microRNA technology regulates levels of miniMECP2, thus reducing toxicity.)
The Taysha microRNA technology has yielded promising results in mouse studies for Rett syndrome; results indicate a lengthening of lifespan and delayed onset of gait abnormalities.51 TSHA-102 is in the preclinical stage but offers promise that it will be the first gene therapy for Rett syndrome to enter clinical trials.
As the field of gene therapy advances, several promising technologies are on the horizon that could potentially have disease-altering impacts on Rett syndrome. These therapies are divided into two broad categories: those at the gene level and those at the transcription and protein level. A few of these approaches are highlighted below.
Gene replacement involves adding a full or partial copy of MECP2 to neuronal cells. This type of therapy presents challenges, from delivery of the new gene to dosage concerns, because MECP2 can be toxic if overexpressed.52-54 Groundbreaking work was done in mouse models involving truncated MECP2, exhibiting phenotypic rescue and validating the gene-replacement approach.18 This strategy is being pursued by Neurogene, which has a uinique technology that allows for tuning of the gene’s expression to get the correct protein levels in the patient. Promising data was presented this year at the American Society of Gene and Cell Therapy conference.55
Early gene replacement therapy studies also laid the foundation for the minMECP2 and microRNA approach being used by Taysha Gene Therapies (discussed above).51
“Correcting” DNA mutations. A different approach at the genetic level involves “correcting” mutations in MECP2 at the DNA level. This is possible because, in a large subset of Rett syndrome patients who have the same missense or nonsense mutations, by using CRISPR, a gene editing tool (discussed above) a single base pair can be corrected.56,57 Previous research, in a Rett syndrome-model of induced pluripotent stem cells, showed that this type of editing is possible – and effective.52 An approach with particular promise involves use of a class of CRISPR proteins known as base editors that are able to specifically alter a single base of DNA.57 The technique has the potential to address many of the mutations seen in Rett syndrome; research on this type of technology is being pursued by Beam Therapeutics, and has the potential to impact Rett syndrome.58
Another promising “correction” approach is exonic editing, in which a much larger section of DNA – potentially, exons 3 and 4, which, taken together, comprise 97% of known MECP2 mutations seen in Rett syndrome – are replaced.59
In both CRISPR and exonic editing therapeutic approaches, endogenous levels of MECP2 expression would be maintained. Of note, both approaches are being pursued for use in treating other genetic disorders, which provides a boost in scaling-up work on addressing safety and efficacy concerns that accompany gene-editing approaches.58 One advantage to the DNA correction approach is that is has the potential to be a “one-and-done” treatment.
“Correcting” RNA. Beyond directly editing DNA, several therapeutic approaches are exploring the ability to edit RNA or to provide the protein directly to cells as enzyme replacement therapy. Such an RNA correction strategy leverages a technology that takes advantage of cells’ natural RNA editor, known as adenosine deaminase acting on RNA (ADAR), which corrects errors in cells’ RNA by providing specific guides to the cell. ADAR can be targeted to fix mutations in the MECP2 RNA transcript, resulting in a “corrected” MECP2 protein.60,61 This technology has delivered promising proof-of-concept evidence in cells and in murine models, and is in the therapeutic pipeline at VICO Therapeutics.62
Shape Therapeutics has also leveraged ADAR to “correct” mutated RNA; Rett syndrome is among the top priorities in the company’s pipeline.
Worth noting is that there are several advantages to the “correction” approach:
- Leveraging internal repair mechanisms minimizes the immune response.
- The flexibility of correction means that it can be used to address many of the mutations that cause Rett syndrome.63
Enzyme replacement therapy, in which the MECP2 protein produced by MECP2 would be directly replaced, is being explored in Rett syndrome patients. This technology has been used successfully in Pompe disease; however, Rett syndrome presents its own challenge because MECP2 needs to be delivered to the brain and neuronal cells.64
Where does this work stand? The technologies described in this section are in preclinical stages of study. Nonetheless, it is expected that several will enter human clinical trials during the next 5 years.
Conclusion
A diagnosis of Rett syndrome is a life-altering event for patients and their family. But there is more hope than ever for effective therapies and, eventually, a cure.
Multiple late-stage clinical trials in progress are demonstrating promising results from therapeutic products, with minimal adverse events. Remarkably, these interventions have delivered improvements to adult patients after regression has stabilized. With rapid progress being made in the field of gene therapy, several technologies for which are focused on Rett syndrome, a hopeful picture is emerging: that therapeutic intervention will be possible before regression, thus effectively treating and, potentially, even curing Rett syndrome.
The landscape is broadening. Add to this hope for approved therapies is the fact that Rett syndrome isn’t the only target being pursued with such strategies; in fact, researchers in the larger field of neurodevelopmental disorder study are working together to find common solutions to shared challenges – from how therapies are designed and delivered to how toxicity is minimized. Much of what is being explored in the Rett syndrome field is also under investigation in other neurodevelopmental syndromes, including Angelman, Prader-Willi, chromosome 15q11.2-13.1 duplication (dup15q), and Fragile X syndrome. This kind of parallel investigation benefits all parties and optimizes a treatment platform so that it can be applied across more than a single disorder.
Like many monogenic disorders, Rett syndrome is entering an exciting stage – at which the words “treatment” and “cure” can be spoken with intent and vision, not just wide-eyed optimism. These words portend real promise for patients who carry the weight of a diagnosis of Rett syndrome, and for their families.
Ms. Ambrose is a student in the master’s of science in human genetics and genomic data analytics program, Keck Graduate Institute, Claremont, Calif. Dr. Bailus is an assistant professor of genetics, Keck Graduate Institute. The authors report no conflict of interest related to this article.
References
1. Lander ES et al; . Initial sequencing and analysis of the human genome. Nature. 2001 Feb 15;409(6822):860-921. doi: 10.1038/35057062.
2. Venter JC et al. The sequence of the human genome. Science. 2001 Feb 16;291(5507):1304-51. doi: 10.1126/science.1058040.
3. Jinek M et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012 Aug 17;337(6096):816-21. doi: 10.1126/science.1225829.
4. Percy A. The American history of Rett syndrome. Pediatr Neurol. 2014 Jan;50(1):1-3. doi: 10.1016/j.pediatrneurol.2013.08.018.
5. Amir RE et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999 Oct;23(2):185-8. doi: 10.1038/13810.
6. Pitzianti MB et al. Rett syndrome in males: The different clinical course in two brothers with the same microduplication MECP2 Xq28. Int J Environ Res Public Health. 2019 Aug;16(17):3075. doi: 10.3390/ijerph16173075.
7. Ribeiro MC, MacDonald JL. Sex differences in Mecp2-mutant Rett syndrome model mice and the impact of cellular mosaicism in phenotype development. Brain Res. 2020 Feb 15;1729:146644. doi: 10.1016/j.brainres.2019.146644.
8. Bao X et al. X chromosome inactivation in Rett syndrome and its correlations with MECP2 mutations and phenotype. J Child Neurol. 2008 Jan;23(1):22-5. doi: 10.1177/0883073807307077.
9. Knudsen GPS et al. Increased skewing of X chromosome inactivation in Rett syndrome patients and their mothers. Eur J Hum Genet. 2006 Jul;14(11):1189-94. doi: 10.1038/sj.ejhg.5201682.
10. Chahil G et al. Rett syndrome in males: A case report and review of literature. Cureus. 2018;10(10):e3414. doi: 10.7759/cureus.3414.
11. Reichow B et al. Brief report: Systematic review of Rett syndrome in males. J Autism Dev Disord. 2015 Oct;45(10):3377-83. doi: 10.1007/s10803-015-2519-1.
12. Vashi N, Justice MJ. Treating Rett syndrome: From mouse models to human therapies. Mamm Genome. 2019 Jun;30(5-6):90-110. doi: 10.1007/s00335-019-09793-5.
13. Li CH et al. MeCP2 links heterochromatin condensates and neurodevelopmental disease. Nature. 2020 Oct;586(7829):440-4. doi: 10.1038/s41586-020-2574-4.
14. Schmidt A et al. MeCP2 and chromatin compartmentalization. Cells. 2020 Apr;9(4):878. doi: 10.3390/cells9040878.
15. Wang L et al. Rett syndrome-causing mutations compromise MeCP2-mediated liquid-liquid phase separation of chromatin. Cell Res. 2020 May;30(5):393-407. doi: 10.1038/s41422-020-0288-7.
16. Lin P et al. Transcriptome analysis of human brain tissue identifies reduced expression of complement complex C1Q genes in Rett syndrome. BMC Genomics. 2016;17:427. doi: 10.1186/s12864-016-2746-7.
17. Tudor M et al. Transcriptional profiling of a mouse model for Rett syndrome reveals subtle transcriptional changes in the brain. Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15536-41. doi: 10.1073/pnas.242566899.
18. Tillotson R et al. Radically truncated MeCP2 rescues Rett syndrome–like neurological defects. Nature. 2017 Oct 19;550(7676):398-401. doi: 10.1038/nature24058.
19. Connolly DR, Zhou Z. Genomic insights into MeCP2 function: A role for the maintenance of chromatin architecture. Curr Opin Neurobiol. 2019 Dec;59:174-9. doi: 10.1016/j.conb.2019.07.002.
20. Johnson BS et al. Biotin tagging of MeCP2 in mice reveals contextual insights into the Rett syndrome transcriptome. Nat Med. 2017 Oct;23(10):1203-14. doi: 10.1038/nm.4406.
21. Gabel HW et al. Disruption of DNA-methylation–dependent long gene repression in Rett syndrome. Nature. 2015 Jun 4;522(7554):89-93. doi: 10.1038/nature14319.
22. Lyst MJ, Bird A. Rett syndrome: A complex disorder with simple roots. Nat Rev Genet. 2015 May;16(5):261-75. doi: 10.1038/nrg3897.
23. Kuehner JN et al. Epigenetic regulations in neuropsychiatric disorders. Front Genet. 2019 Apr 4;10:268. doi: 10.3389/fgene.2019.00268.
24. Pejhan S, Rastegar M. Role of DNA methyl-CpG-binding protein MeCP2 in Rett syndrome pathobiology and mechanism of disease. Biomolecules. 2021 Jan;11(1):75. doi: 10.3390/biom11010075.
25. Fu C et al. Consensus guidelines on managing Rett syndrome across the lifespan. BMJ Paediatr Open. 2020;4(1):e000717. doi: 10.1136/bmjpo-2020-000717.
26. Operto FF et al. Epilepsy and genetic in Rett syndrome: A review. Brain Behav. 2019 May;9(5):e01250. doi: 10.1002/brb3.1250.
27. Nissenkorn A et al. Epilepsy in Rett syndrome – The experience of a National Rett Center. Epilepsia. 2010 Jul;51(7):1252-8. doi: 10.1111/j.1528-1167.2010.02597.x.
28. Welcome to the Rett cure community. Rett Syndrome Research Trust [Internet]. Updated Feb 8, 2022. Accessed Feb 23, 2022. https://reverserett.org.
29. About Rett syndrome. International Rett Syndrome Foundation [Internet]. Updated Jan 4, 2022. Accessed Feb 23, 2022. http://www.rettsyndrome.org.
30. Singh J, Santosh P. Key issues in Rett syndrome: Emotional, behavioural and autonomic dysregulation (EBAD) – A target for clinical trials. Orphanet J Rare Dis. 2018 Jul 31;13(1):128. doi: 10.1186/s13023-018-0873-8.
31. Banerjee A et al. Towards a better diagnosis and treatment of Rett syndrome: A model synaptic disorder. Brain. 2019 Feb 1;142(2):239-48. doi: 10.1093/brain/awy323.
32. Ager S et al. Parental experiences of scoliosis management in Rett syndrome. Disabil Rehabil. 2009 Sep 19;31(23):1917-24. doi: 10.1080/09638280902846392.
33. Budden SS. Management of Rett syndrome: A ten year experience. Neuropediatrics. 1995;26(2):75-7. doi: 10.1055/s-2007-979727.
34. Ip JPK et al. Rett syndrome: Insights into genetic, molecular and circuit mechanisms. Nat Rev Neurosci. 2018 Jun;19(6):368-82. doi: 10.1038/s41583-018-0006-3.
35. Acadia Pharmaceuticals Inc. Study of trofinetide for the treatment of girls and women with Rett syndrome (LAVENDER™). ClinicalTrials.gov identifier: NCT04181723. Updated Feb 17, 2022. Accessed Feb 23, 2022. https://clinicaltrials.gov/ct2/show/NCT04181723.
36. Acadia Pharmaceuticals announces positive top-line results from the pivotal phase 3 LAVENDER trial of trofinetide in Rett syndrome. Press release. Acadia Pharmaceuticals Inc. Dec 6, 2021. Accessed Feb 23, 2022. https://ir.acadia-pharm.com/news-releases/news-release-details/acadia-pharmaceuticals-announces-positive-top-line-results-1.
37. Copping NA et al. Emerging gene and small molecule therapies for the neurodevelopmental disorder Angelman syndrome. Neurotherapeutics. 2021 Jul;18(3):1535-47. doi: 10.1007/s13311-021-01082-x.
38. Riikonen R. Insulin-like growth factors in the pathogenesis of neurological diseases in children. Int J Mol Sci. 2017 Sep;18(10):2056. doi: 10.3390/ijms18102056.
39. Glaze DG et al; Rett 002 Study Group. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric Rett syndrome. Neurology. 2019 April 16;92(16):e1912-e1925. doi: 10.1212/WNL.0000000000007316.
40. Acadia Pharmaceuticals Inc. An open-label study of trofinetide for the treatment of girls two to five years of age who have Rett syndrome (DAFFODIL™). ClinicalTrials.gov Identifier: NCT04988867. Updated Jan 24, 2022. Accessed Feb 23, 2022. https://clinicaltrials.gov/ct2/show/NCT04988867.
41. Anavex Life Sciences announces ANAVEX®2-73 meets primary and secondary endpoints in clinical trial. Press release. Anavex Life Sciences Corp. Dec 15, 2020. Accessed Feb 23, 2022. http://www.anavex.com/post/anavex-life-sciences-announces-anavex-2-73-meets-primary-and-secondary-endpoints-in-clinical-trial.
42. Anavex Life Sciences Corp. ANAVEX2-73 study in patients with Rett syndrome (AVATAR). ClinicalTrials.gov Identifier: NCT03941444. Updated Jan 27, 2022. Accessed Feb 23, 2022. https://clinicaltrials.gov/ct2/show/NCT03941444.
43. Anavex Life Sciences Corp. ANAVEX2-73 study in pediatric patients with Rett syndrome (EXCELLENCE). ClinicalTrials.gov Identifier: NCT04304482. Updated Sep 28, 2021. Accessed Feb 23, 2022. http://www.clinicaltrials.gov/ct2/show/NCT04304482.
44.Christ MG et al. The Sigma-1 receptor at the crossroad of proteostasis, neurodegeneration, and autophagy. Trends Neurosci. 2020 Feb;43(2):79-81. doi: 10.1016/j.tins.2019.12.002.
45. Kaufmann WE et al. ANAVEX®2-73 (blarcamesine), a sigma-1 receptor agonist, ameliorates neurologic impairments in a mouse model of Rett syndrome. Pharmacol Biochem Behav. 2019 Dec;187:172796. doi: 10.1016/j.pbb.2019.172796.
46. Brimson JM et al. Dipentylammonium binds to the sigma-1 receptor and protects against glutamate toxicity, attenuates dopamine toxicity and potentiates neurite outgrowth in various cultured cell lines. Neurotox Res. 2018 Aug;34(2):263-72. doi: 10.1007/s12640-018-9883-5.
47. Kourrich S et al. The sigma-1 receptor: roles in neuronal plasticity and disease. Trends Neurosci. 2012 Dec;35(12):762-71. doi: 10.1016/j.tins.2012.09.007.
48. Lappalainen R, Riikonen RS. High levels of cerebrospinal fluid glutamate in Rett syndrome. Pediatr Neurol. 1996 Oct;15(3):213-6. doi: 10.1016/s0887-8994(96)00218-4.
49. Hamberger A et al. Elevated CSF glutamate in Rett syndrome. Neuropediatrics. 1992;23(4):212-3. doi: 10.1055/s-2008-1071344.
50. Inacio P. FDA acts to support development of potential gene therapy, TSHA-102. Rett Syndrome News [Internet]. Oct 16, 2020. Accessed Feb 23, 2022. https://rettsyndromenews.com/2020/10/16/fda-grants-orphan-drug-rare-pediatric-disease-status-to-tsha-102-potential-rett-gene-therapy.
51. Sinnett SE et al. Engineered microRNA-based regulatory element permits safe high-dose miniMECP2 gene therapy in Rett mice. Brain. 2021 Nov 29;144(10):3005-19. doi: 10.1093/brain/awab182.
52. Le TTH et al. Efficient and precise CRISPR/Cas9-mediated MECP2 modifications in human-induced pluripotent stem cells. Front Genet. 2019 Jul 2;10:625. doi: 10.3389/fgene.2019.00625.
53. Koerner MV et al. Toxicity of overexpressed MeCP2 is independent of HDAC3 activity. Genes Dev. 2018;32(23-24):1514-24. doi: 10.1101/gad.320325.118.
54. Heckman LD et al. Rett-causing mutations reveal two domains critical for MeCP2 function and for toxicity in MECP2 duplication syndrome mice. Elife. 2014;3:e02676. doi: 10.7554/eLife.02676.
55. Neurogene announces new development program in Rett syndrome utilizing novel EXACT technology platform [Internet]. Accessed Aug 12, 2022. https://www.neurogene.com/press-releases/neurogene-announces-new-development-program-in-rett-syndrome-utilizing-novel-exact-technology-platform/
56. Anzalone AV et al. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol. 2020 Jul;38(7):824-44. doi: 10.1038/s41587-020-0561-9.
57. Gaudelli NM, Komor AC, Rees HA, et al. Programmable base editing of A●T to G●C in genomic DNA without DNA cleavage. Nature. 2017 Nov 23;551(7681):464-71. doi: 10.1038/nature24644.
58. Coenraads M. How RSRT is driving the search for a Rett cure. Rett Syndrome Research Trust [Internet]. Dec 7, 2021. Accessed Feb 23, 2022. https://rettnews.org/articles/how-rsrt-is-driving-the-search-for-a-rett-cure.
59. Cutting-edge technologies to repair the underlying mutations that cause Rett. Rett Syndrome Research Trust [Internet]. Updated Nov 3, 2021. Accessed Feb 23, 2022. https://reverserett.org/research/cures/gene-editing.
60. Sinnamon JR et al. In vivo repair of a protein underlying a neurological disorder by programmable RNA editing. Cell Rep. 2020 Jul 14;32(2):107878. doi: 10.1016/j.celrep.2020.107878.
61. Sinnamon JR et al. Site-directed RNA repair of endogenous Mecp2 RNA in neurons. Proc Natl Acad Sci U S A. 2017 Oct 31;114(44):E9395-E9402. doi: 10.1073/pnas.1715320114.
62. Pipeline. VICO Therapeutics [Internet]. Updated Nov 5, 2021. Accessed Feb 23, 2022. https://vicotx.com/pipeline.
63. Therapeutics platform. Shape Therapeutics [Internet]. Updated Feb 20, 2021. Accessed Feb 23, 2022.
https://live-shapetx.pantheonsite.io/therapeutics-platform.
64. Koeberl DD et al. Glycogen storage disease types I and II: Treatment updates. J Inherit Metab Dis. 2007 Apr;30(2):159-64. doi: 10.1007/s10545-007-0519-9.
FDA confirms nationwide Adderall shortage
The U.S. Food and Drug Administration which are approved for treating attention deficit hyperactivity disorder and narcolepsy.
The FDA announcement follows weeks of reports of a shortage of the drug by pharmacy chains and Adderall users.
The agency said it is in “frequent” contact with all manufacturers of Adderall – and reported that one of those companies, Teva, is experiencing ongoing intermittent manufacturing delays.
Other manufacturers continue to produce amphetamine mixed salts, but there is not enough supply to continue to meet U.S. market demand through those producers, the FDA noted.
“Until supply is restored, there are alternative therapies, including the extended-release version of amphetamine mixed salts, available to health care professionals and their patients for amphetamine mixed salts’ approved indications,” the agency said.
Patients should work with their health care provider to determine their best treatment option, it added.
The organization is continuing to monitor the supply of Adderall and to help manufacturers resolve the shortage.
Its Drug Shortage webpage has additional information about the situation and is updated regularly.
“We continue to use all the tools we have available to help keep supply available for patients and will provide public updates regarding the Adderall shortage,” the FDA said.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration which are approved for treating attention deficit hyperactivity disorder and narcolepsy.
The FDA announcement follows weeks of reports of a shortage of the drug by pharmacy chains and Adderall users.
The agency said it is in “frequent” contact with all manufacturers of Adderall – and reported that one of those companies, Teva, is experiencing ongoing intermittent manufacturing delays.
Other manufacturers continue to produce amphetamine mixed salts, but there is not enough supply to continue to meet U.S. market demand through those producers, the FDA noted.
“Until supply is restored, there are alternative therapies, including the extended-release version of amphetamine mixed salts, available to health care professionals and their patients for amphetamine mixed salts’ approved indications,” the agency said.
Patients should work with their health care provider to determine their best treatment option, it added.
The organization is continuing to monitor the supply of Adderall and to help manufacturers resolve the shortage.
Its Drug Shortage webpage has additional information about the situation and is updated regularly.
“We continue to use all the tools we have available to help keep supply available for patients and will provide public updates regarding the Adderall shortage,” the FDA said.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration which are approved for treating attention deficit hyperactivity disorder and narcolepsy.
The FDA announcement follows weeks of reports of a shortage of the drug by pharmacy chains and Adderall users.
The agency said it is in “frequent” contact with all manufacturers of Adderall – and reported that one of those companies, Teva, is experiencing ongoing intermittent manufacturing delays.
Other manufacturers continue to produce amphetamine mixed salts, but there is not enough supply to continue to meet U.S. market demand through those producers, the FDA noted.
“Until supply is restored, there are alternative therapies, including the extended-release version of amphetamine mixed salts, available to health care professionals and their patients for amphetamine mixed salts’ approved indications,” the agency said.
Patients should work with their health care provider to determine their best treatment option, it added.
The organization is continuing to monitor the supply of Adderall and to help manufacturers resolve the shortage.
Its Drug Shortage webpage has additional information about the situation and is updated regularly.
“We continue to use all the tools we have available to help keep supply available for patients and will provide public updates regarding the Adderall shortage,” the FDA said.
A version of this article first appeared on Medscape.com.
Birth weight below 25th percentile linked to child development problems
Babies born from the 37th week of pregnancy who are mild to moderately small for gestational age (SGA) could benefit from monitoring to check for developmental problems, a study suggested.
A team of researchers at Coventry (England) University found that birth weight below the 25th percentile was associated with more developmental concerns in early childhood than a weight between the 25th and 74th percentile.
Those difficulties were apparent at percentiles higher than the conventional threshold defining SGA, they noted.
Low and high extremes of birth weight have been associated with adverse pregnancy and neonatal health outcomes, but little is known about the effects on motor skills, socialization, language, and other developmental markers for the entire range of birth weights for nonpremature babies.
Study linked health databases to child assessment results
To find out more, researchers conducted a population-based cohort study of 686,284 singleton infants born from 37 weeks of gestation, linking pregnancy and birth records from health databases covering all of Scotland to child development assessments carried out between the ages of 2 and 3.5 years.
The researchers looked for associations between birth weight and early childhood developmental concerns, taking into account confounders, such as maternal age, the mother’s medical history during pregnancy, early pregnancy body mass index, deprivation, ethnicity, alcohol use, and smoking history.
The study, published in the open access journal PLOS Medicine, found that babies born below the 25th percentile for birth weight had a higher risk of developmental concerns, compared with babies born between the 25th and 74th percentiles, with the infants who had the lowest birth weight most at risk of later developmental difficulties.
Those born between the 10th and 24th percentile had a relative risk of 1.07 (95% confidence interval, 1.03-1.12; P < .001); between the 3rd and 9th percentile, the RR was 1.18 (95% CI, 1.12-1.25, P < .001), and below the 3rd percentile the RR was 1.37 (95% CI, 1.24-1.50; P < .001).
No substantial increase in the risk of early childhood developmental concerns was identified for larger birth weight categories in the 75th-89th percentile range, the researchers reported.
Monitoring and support
The researchers concluded that having mild to moderate SGA “is an unrecognized, potentially important contributor to the prevalence of developmental concerns.”
Before this study, babies below the 10th percentile were usually considered at risk for developmental concerns. However, the investigation found a greater number of babies within the 10th-24th percentile range of birth weights with these issues, simply because there were a larger number of babies within that population.
Abiodun Adanikin, MBBS, PhD, MPH, of Coventry University’s Centre for Healthcare Research, and study first author, said: “Though it is mostly unrecognized, babies who are mild to moderately small at birth are key contributors to the burden of childhood developmental concerns. They may need closer monitoring and increased support to reduce the risk of developmental concerns.”
The study also involved colleagues from the University of Bristol (England), the University of Glasgow, the University of Cambridge (England), and Queen Mary University of London.
This work was supported by a Wellbeing of Women Research Grant. One author has received research support from Roche Diagnostics, GSK, Illumina, and Sera Prognostics (fetal growth restriction, preeclampsia and preterm birth). He has been a paid consultant to GSK (preterm birth) and is a member of a Data Monitoring Committee for GSK trials of RSV vaccination in pregnancy. He is one of three named inventors on a patent application filed by Cambridge Enterprise for novel predictive test for fetal growth disorder. He is an academic editor on PLOS Medicine’s editorial board. The authors declare no other competing interest.
A version of this article first appeared on Medscape UK.
Babies born from the 37th week of pregnancy who are mild to moderately small for gestational age (SGA) could benefit from monitoring to check for developmental problems, a study suggested.
A team of researchers at Coventry (England) University found that birth weight below the 25th percentile was associated with more developmental concerns in early childhood than a weight between the 25th and 74th percentile.
Those difficulties were apparent at percentiles higher than the conventional threshold defining SGA, they noted.
Low and high extremes of birth weight have been associated with adverse pregnancy and neonatal health outcomes, but little is known about the effects on motor skills, socialization, language, and other developmental markers for the entire range of birth weights for nonpremature babies.
Study linked health databases to child assessment results
To find out more, researchers conducted a population-based cohort study of 686,284 singleton infants born from 37 weeks of gestation, linking pregnancy and birth records from health databases covering all of Scotland to child development assessments carried out between the ages of 2 and 3.5 years.
The researchers looked for associations between birth weight and early childhood developmental concerns, taking into account confounders, such as maternal age, the mother’s medical history during pregnancy, early pregnancy body mass index, deprivation, ethnicity, alcohol use, and smoking history.
The study, published in the open access journal PLOS Medicine, found that babies born below the 25th percentile for birth weight had a higher risk of developmental concerns, compared with babies born between the 25th and 74th percentiles, with the infants who had the lowest birth weight most at risk of later developmental difficulties.
Those born between the 10th and 24th percentile had a relative risk of 1.07 (95% confidence interval, 1.03-1.12; P < .001); between the 3rd and 9th percentile, the RR was 1.18 (95% CI, 1.12-1.25, P < .001), and below the 3rd percentile the RR was 1.37 (95% CI, 1.24-1.50; P < .001).
No substantial increase in the risk of early childhood developmental concerns was identified for larger birth weight categories in the 75th-89th percentile range, the researchers reported.
Monitoring and support
The researchers concluded that having mild to moderate SGA “is an unrecognized, potentially important contributor to the prevalence of developmental concerns.”
Before this study, babies below the 10th percentile were usually considered at risk for developmental concerns. However, the investigation found a greater number of babies within the 10th-24th percentile range of birth weights with these issues, simply because there were a larger number of babies within that population.
Abiodun Adanikin, MBBS, PhD, MPH, of Coventry University’s Centre for Healthcare Research, and study first author, said: “Though it is mostly unrecognized, babies who are mild to moderately small at birth are key contributors to the burden of childhood developmental concerns. They may need closer monitoring and increased support to reduce the risk of developmental concerns.”
The study also involved colleagues from the University of Bristol (England), the University of Glasgow, the University of Cambridge (England), and Queen Mary University of London.
This work was supported by a Wellbeing of Women Research Grant. One author has received research support from Roche Diagnostics, GSK, Illumina, and Sera Prognostics (fetal growth restriction, preeclampsia and preterm birth). He has been a paid consultant to GSK (preterm birth) and is a member of a Data Monitoring Committee for GSK trials of RSV vaccination in pregnancy. He is one of three named inventors on a patent application filed by Cambridge Enterprise for novel predictive test for fetal growth disorder. He is an academic editor on PLOS Medicine’s editorial board. The authors declare no other competing interest.
A version of this article first appeared on Medscape UK.
Babies born from the 37th week of pregnancy who are mild to moderately small for gestational age (SGA) could benefit from monitoring to check for developmental problems, a study suggested.
A team of researchers at Coventry (England) University found that birth weight below the 25th percentile was associated with more developmental concerns in early childhood than a weight between the 25th and 74th percentile.
Those difficulties were apparent at percentiles higher than the conventional threshold defining SGA, they noted.
Low and high extremes of birth weight have been associated with adverse pregnancy and neonatal health outcomes, but little is known about the effects on motor skills, socialization, language, and other developmental markers for the entire range of birth weights for nonpremature babies.
Study linked health databases to child assessment results
To find out more, researchers conducted a population-based cohort study of 686,284 singleton infants born from 37 weeks of gestation, linking pregnancy and birth records from health databases covering all of Scotland to child development assessments carried out between the ages of 2 and 3.5 years.
The researchers looked for associations between birth weight and early childhood developmental concerns, taking into account confounders, such as maternal age, the mother’s medical history during pregnancy, early pregnancy body mass index, deprivation, ethnicity, alcohol use, and smoking history.
The study, published in the open access journal PLOS Medicine, found that babies born below the 25th percentile for birth weight had a higher risk of developmental concerns, compared with babies born between the 25th and 74th percentiles, with the infants who had the lowest birth weight most at risk of later developmental difficulties.
Those born between the 10th and 24th percentile had a relative risk of 1.07 (95% confidence interval, 1.03-1.12; P < .001); between the 3rd and 9th percentile, the RR was 1.18 (95% CI, 1.12-1.25, P < .001), and below the 3rd percentile the RR was 1.37 (95% CI, 1.24-1.50; P < .001).
No substantial increase in the risk of early childhood developmental concerns was identified for larger birth weight categories in the 75th-89th percentile range, the researchers reported.
Monitoring and support
The researchers concluded that having mild to moderate SGA “is an unrecognized, potentially important contributor to the prevalence of developmental concerns.”
Before this study, babies below the 10th percentile were usually considered at risk for developmental concerns. However, the investigation found a greater number of babies within the 10th-24th percentile range of birth weights with these issues, simply because there were a larger number of babies within that population.
Abiodun Adanikin, MBBS, PhD, MPH, of Coventry University’s Centre for Healthcare Research, and study first author, said: “Though it is mostly unrecognized, babies who are mild to moderately small at birth are key contributors to the burden of childhood developmental concerns. They may need closer monitoring and increased support to reduce the risk of developmental concerns.”
The study also involved colleagues from the University of Bristol (England), the University of Glasgow, the University of Cambridge (England), and Queen Mary University of London.
This work was supported by a Wellbeing of Women Research Grant. One author has received research support from Roche Diagnostics, GSK, Illumina, and Sera Prognostics (fetal growth restriction, preeclampsia and preterm birth). He has been a paid consultant to GSK (preterm birth) and is a member of a Data Monitoring Committee for GSK trials of RSV vaccination in pregnancy. He is one of three named inventors on a patent application filed by Cambridge Enterprise for novel predictive test for fetal growth disorder. He is an academic editor on PLOS Medicine’s editorial board. The authors declare no other competing interest.
A version of this article first appeared on Medscape UK.
FROM PLOS MEDICINE
Home-based transcranial stimulation succeeds for MDD
Major depressive disorder (MDD) remains a leading cause of disability and a significant predictor of suicide worldwide, Rachel D. Woodham, PhD, of the University of East London and colleagues wrote.
Transcranial direct current stimulation (tDCS) has demonstrated effectiveness as a noninvasive therapy for MDD, but requires frequent sessions, and repeat visits to treatment centers are a barrier for many patients, they noted. The tDCS procedure involves delivery of a weak direct electric current via placement of electrodes, usually with the anode over the left dorsolateral prefrontal cortex and the cathode over the right dorsolateral prefrontal cortex, suborbital, or frontotemporal region.
“The current changes neuronal membrane potential and facilitates discharge,” but “in contrast to rTMS and ECT, tDCS does not directly trigger an action potential,” the researchers wrote. The most common side effects reported with tDCS are tingling, itching, burning sensation, skin redness or headache.
The researchers proposed that tDCS could be provided at home under real-time remote supervision.
In an open-label feasibility study published in the Journal of Psychiatric Research, they recruited 26 adults with MDD in current depressive episodes of moderate to severe severity. In addition to maintaining their current treatment regimens of medication, psychotherapy, or cognitive behavioral therapy, participants used tDCS at home in 30-minute sessions, for a total of 21 sessions over 6 weeks. A researcher was present in person or on a real-time video call for each at-home session.
The primary outcome of Hamilton Rating Scale for Depression (HAMD) score improved significantly, from a mean of 19.12 at baseline to 5.33 after 6 weeks. At 3 months, the mean HAMD score was 5.65, and 78.2% of patients met the criteria for clinical remission (HAMD score less than 9). At 6 months, patients maintained this improvement, with a mean HAMD score of 5.43 and 73.9% of the participants in clinical remission. The majority of participants (24 of 26) completed the full 6-week treatment.
Clinical assessments were conducted at baseline, at the end of the 6-week treatment period, at 3 months, and at 6 months, and included not only the HAMD, but also the Hamilton Anxiety Rating Scale (HAMA), Sheehan Disability Scale (SDS), Patient Health Questionnaire–9 (PHQ-9), and Young Mania Rating Scale. All participants showed significant improvements in HAMA, SDS, and PHQ-9 scores from baseline that endured from the end of the treatment period to the 6 months’ follow-up.
The tDCS involved a bilateral frontal montage, F3 anode, F4 cathode, 2mA, and two different devices were used.
All participants reported the acceptability of at-home tDCS as either “very acceptable” or “quite acceptable.”
The results were limited by the open-label feasibility design and lack of a sham control treatment; therefore, the findings of efficacy are preliminary, the researchers emphasized. “Having real-time supervision for each session likely contributed to symptom improvement.”
However, the results support the feasibility of at-home tDCS to improve outcomes both short- and long-term in patients with moderate to severe MDD, the researchers said. Larger, sham-controlled trials are needed to show efficacy, and additional assessment of feasibility should include the use of app-based devices, which may be more feasible for individuals with lower socioeconomic status.
The study received no outside funding. The study was supported by the Rosetrees Trust. The researchers had no financial conflicts to disclose.
Major depressive disorder (MDD) remains a leading cause of disability and a significant predictor of suicide worldwide, Rachel D. Woodham, PhD, of the University of East London and colleagues wrote.
Transcranial direct current stimulation (tDCS) has demonstrated effectiveness as a noninvasive therapy for MDD, but requires frequent sessions, and repeat visits to treatment centers are a barrier for many patients, they noted. The tDCS procedure involves delivery of a weak direct electric current via placement of electrodes, usually with the anode over the left dorsolateral prefrontal cortex and the cathode over the right dorsolateral prefrontal cortex, suborbital, or frontotemporal region.
“The current changes neuronal membrane potential and facilitates discharge,” but “in contrast to rTMS and ECT, tDCS does not directly trigger an action potential,” the researchers wrote. The most common side effects reported with tDCS are tingling, itching, burning sensation, skin redness or headache.
The researchers proposed that tDCS could be provided at home under real-time remote supervision.
In an open-label feasibility study published in the Journal of Psychiatric Research, they recruited 26 adults with MDD in current depressive episodes of moderate to severe severity. In addition to maintaining their current treatment regimens of medication, psychotherapy, or cognitive behavioral therapy, participants used tDCS at home in 30-minute sessions, for a total of 21 sessions over 6 weeks. A researcher was present in person or on a real-time video call for each at-home session.
The primary outcome of Hamilton Rating Scale for Depression (HAMD) score improved significantly, from a mean of 19.12 at baseline to 5.33 after 6 weeks. At 3 months, the mean HAMD score was 5.65, and 78.2% of patients met the criteria for clinical remission (HAMD score less than 9). At 6 months, patients maintained this improvement, with a mean HAMD score of 5.43 and 73.9% of the participants in clinical remission. The majority of participants (24 of 26) completed the full 6-week treatment.
Clinical assessments were conducted at baseline, at the end of the 6-week treatment period, at 3 months, and at 6 months, and included not only the HAMD, but also the Hamilton Anxiety Rating Scale (HAMA), Sheehan Disability Scale (SDS), Patient Health Questionnaire–9 (PHQ-9), and Young Mania Rating Scale. All participants showed significant improvements in HAMA, SDS, and PHQ-9 scores from baseline that endured from the end of the treatment period to the 6 months’ follow-up.
The tDCS involved a bilateral frontal montage, F3 anode, F4 cathode, 2mA, and two different devices were used.
All participants reported the acceptability of at-home tDCS as either “very acceptable” or “quite acceptable.”
The results were limited by the open-label feasibility design and lack of a sham control treatment; therefore, the findings of efficacy are preliminary, the researchers emphasized. “Having real-time supervision for each session likely contributed to symptom improvement.”
However, the results support the feasibility of at-home tDCS to improve outcomes both short- and long-term in patients with moderate to severe MDD, the researchers said. Larger, sham-controlled trials are needed to show efficacy, and additional assessment of feasibility should include the use of app-based devices, which may be more feasible for individuals with lower socioeconomic status.
The study received no outside funding. The study was supported by the Rosetrees Trust. The researchers had no financial conflicts to disclose.
Major depressive disorder (MDD) remains a leading cause of disability and a significant predictor of suicide worldwide, Rachel D. Woodham, PhD, of the University of East London and colleagues wrote.
Transcranial direct current stimulation (tDCS) has demonstrated effectiveness as a noninvasive therapy for MDD, but requires frequent sessions, and repeat visits to treatment centers are a barrier for many patients, they noted. The tDCS procedure involves delivery of a weak direct electric current via placement of electrodes, usually with the anode over the left dorsolateral prefrontal cortex and the cathode over the right dorsolateral prefrontal cortex, suborbital, or frontotemporal region.
“The current changes neuronal membrane potential and facilitates discharge,” but “in contrast to rTMS and ECT, tDCS does not directly trigger an action potential,” the researchers wrote. The most common side effects reported with tDCS are tingling, itching, burning sensation, skin redness or headache.
The researchers proposed that tDCS could be provided at home under real-time remote supervision.
In an open-label feasibility study published in the Journal of Psychiatric Research, they recruited 26 adults with MDD in current depressive episodes of moderate to severe severity. In addition to maintaining their current treatment regimens of medication, psychotherapy, or cognitive behavioral therapy, participants used tDCS at home in 30-minute sessions, for a total of 21 sessions over 6 weeks. A researcher was present in person or on a real-time video call for each at-home session.
The primary outcome of Hamilton Rating Scale for Depression (HAMD) score improved significantly, from a mean of 19.12 at baseline to 5.33 after 6 weeks. At 3 months, the mean HAMD score was 5.65, and 78.2% of patients met the criteria for clinical remission (HAMD score less than 9). At 6 months, patients maintained this improvement, with a mean HAMD score of 5.43 and 73.9% of the participants in clinical remission. The majority of participants (24 of 26) completed the full 6-week treatment.
Clinical assessments were conducted at baseline, at the end of the 6-week treatment period, at 3 months, and at 6 months, and included not only the HAMD, but also the Hamilton Anxiety Rating Scale (HAMA), Sheehan Disability Scale (SDS), Patient Health Questionnaire–9 (PHQ-9), and Young Mania Rating Scale. All participants showed significant improvements in HAMA, SDS, and PHQ-9 scores from baseline that endured from the end of the treatment period to the 6 months’ follow-up.
The tDCS involved a bilateral frontal montage, F3 anode, F4 cathode, 2mA, and two different devices were used.
All participants reported the acceptability of at-home tDCS as either “very acceptable” or “quite acceptable.”
The results were limited by the open-label feasibility design and lack of a sham control treatment; therefore, the findings of efficacy are preliminary, the researchers emphasized. “Having real-time supervision for each session likely contributed to symptom improvement.”
However, the results support the feasibility of at-home tDCS to improve outcomes both short- and long-term in patients with moderate to severe MDD, the researchers said. Larger, sham-controlled trials are needed to show efficacy, and additional assessment of feasibility should include the use of app-based devices, which may be more feasible for individuals with lower socioeconomic status.
The study received no outside funding. The study was supported by the Rosetrees Trust. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
Poor visual acuity linked to depression, changes in brain structure
After multiple adjustments, analysis of data from more than 114,000 participants in the UK Biobank Study showed that visual impairment was linked to a 19% higher risk for depression.
In addition, imaging results showed a significant link between deteriorating brain structures and depression in those with poor visual acuity.
“Our findings highlight the value of visual health in association with mental health,” Xiayin Zhang, PhD, Guangdong Eye Institute, department of ophthalmology, Guangdong Provincial People’s Hospital, Guangzhou, China, and colleagues write.
“Screening of vision at an early stage should be embedded in the middle-aged and older population to stratify the vulnerable population at risk for depression,” the investigators add.
The findings were published online in JAMA Network Open.
UK biobank analyses
The analysis included 114,583 participants (54.5% women; mean age, 56.8 years) from the UK Biobank who completed standardized questionnaires and underwent ocular examinations.
To test distance visual acuity, all were asked to read letters on lines from the top to the bottom of a chart while wearing prescribed optical correction. Visual impairment was defined as visual acuity worse than 0.3 logarithm of the minimum angle of resolution (LogMAR) units.
Depressive symptoms were self-reported using the two-item Patient Health Questionnaire (PHQ-2), in which a score of 3 or more indicates depression. As well, a medical practitioner conducted an assessment of depression at baseline.
Among the participants, 87.2% had no visual impairment or depression and acted as the healthy control group. In addition, 3.2% showed visual impairment, 10% reported a diagnosis of depression, and 0.4% had both.
Researchers adjusted for age, sex, race, ethnicity, education, smoking, alcohol consumption, physical activity, family history of severe depression, obesity, hypertension, diabetes, hyperlipidemia, and deprivation on the Townsend index.
Among those with visual impairment, 12.4% had depression, compared with 9.9% without visual impairment.
Structure deterioration
After adjusting for potential confounders, visual impairment was associated with a 19% higher risk for depression (odds ratio, 1.19; 95% confidence interval, 1.05-1.34; P = .003). In addition, 1-line–worse visual acuity was associated with 5% higher odds of depression (OR, 1.05; 95% CI, 1.04-1.07; P < .001).
The association between visual acuity and depression was found in both younger (39-58 years) and older (59-72 years) groups, as well as in both men and women.
The researchers also explored the association between depressive symptoms and brain structure using MRI scans from a subset of 7,844 individuals (51% women; 2% with visual impairment).
Results showed linear associations between PHQ-2 scores and the left volume of gray matter in the supracalcarine cortex (coefficient, 7.61; 95% CI, 3.9-11.3; adjusted P = .006).
The investigators note that the supracalcarine cortex is spatially connected to the primary visual cortex, suggesting the visual cortex may be involved in the pathogenesis of depression.
PHQ-2 scores were also associated with mean isotropic volume fraction (ISOVF) in the right fornix (cres) and/or stria terminalis (coefficient, .003; 95% CI, 0.001-0.004; adjusted P = .01).
The links “could be moderated by visual acuity, whereby increased PHQ score was associated with higher ISOVF levels only among those with poorer visual acuity (P = .02 for interaction),” the investigators report.
These results “suggest that poorer visual acuity was associated with greater depressive symptoms and may have contributed to the related deterioration of the fornix and stria terminalis,” they add.
They note that previous studies have supported the hypothesis that the fornix and stria terminalis are involved in the pathophysiology of other brain-related conditions, including schizophrenia, bipolar disorder, and autism spectrum disorder.
However, the investigators did not have information on how long the participants had experienced visual impairment, so they couldn’t investigate whether results were affected by time. Additional study limitations cited were that depression may affect vision and that a large proportion of the participants (89.3%) were White.
Study ‘adds nuance’
Commenting on the study, Ipsit V. Vahia, MD, of the department of psychiatry at Harvard Medical School, Boston, and associate chief of geriatric psychiatry at McLean Hospital, Belmont, Mass., said the study “adds nuance to our understanding” of the well-established relationship between vision deficits and depression.
“It indicates that even mild visual deficits may be associated with depression,” said Dr. Vahia, who was not involved with the research.
The investigators validated this association by showing that visual acuity was also associated with neuroimaging markers of depression, he added.
Although the study was not designed to demonstrate causal relationships between mood and vision and its findings do not confirm that correcting visual acuity deficits will resolve depressive symptoms, “the large study sample and high quality of data should give clinicians confidence in the study’s findings,” Dr. Vahia said.
“Correcting visual acuity deficits can be considered standard care for older adults worldwide, and this study suggests that providing this standard care could also benefit mental health,” he concluded.
The study was supported by the National Natural Science Foundation of China, the China Postdoctoral Science Foundation, the Outstanding Young Talent Trainee Program of Guangdong Provincial People’s Hospital, the Guangdong Provincial People’s Hospital Scientific Research Funds for Leading Medical Talents and Distinguished Young Scholars in Guangdong Province, the Talent Introduction Fund of Guangdong Provincial People’s Hospital, the Science and Technology Program of Guangzhou, China, the Project of Special Research on Cardiovascular Diseases, the Research Foundation of Medical Science and Technology of Guangdong Province, the University of Melbourne at Research Accelerator Program, and the CERA (Centre for Eye Research Australia) Foundation and Victorian State Government for the Centre for Eye Research Australia. The investigators and Dr. Vahia have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After multiple adjustments, analysis of data from more than 114,000 participants in the UK Biobank Study showed that visual impairment was linked to a 19% higher risk for depression.
In addition, imaging results showed a significant link between deteriorating brain structures and depression in those with poor visual acuity.
“Our findings highlight the value of visual health in association with mental health,” Xiayin Zhang, PhD, Guangdong Eye Institute, department of ophthalmology, Guangdong Provincial People’s Hospital, Guangzhou, China, and colleagues write.
“Screening of vision at an early stage should be embedded in the middle-aged and older population to stratify the vulnerable population at risk for depression,” the investigators add.
The findings were published online in JAMA Network Open.
UK biobank analyses
The analysis included 114,583 participants (54.5% women; mean age, 56.8 years) from the UK Biobank who completed standardized questionnaires and underwent ocular examinations.
To test distance visual acuity, all were asked to read letters on lines from the top to the bottom of a chart while wearing prescribed optical correction. Visual impairment was defined as visual acuity worse than 0.3 logarithm of the minimum angle of resolution (LogMAR) units.
Depressive symptoms were self-reported using the two-item Patient Health Questionnaire (PHQ-2), in which a score of 3 or more indicates depression. As well, a medical practitioner conducted an assessment of depression at baseline.
Among the participants, 87.2% had no visual impairment or depression and acted as the healthy control group. In addition, 3.2% showed visual impairment, 10% reported a diagnosis of depression, and 0.4% had both.
Researchers adjusted for age, sex, race, ethnicity, education, smoking, alcohol consumption, physical activity, family history of severe depression, obesity, hypertension, diabetes, hyperlipidemia, and deprivation on the Townsend index.
Among those with visual impairment, 12.4% had depression, compared with 9.9% without visual impairment.
Structure deterioration
After adjusting for potential confounders, visual impairment was associated with a 19% higher risk for depression (odds ratio, 1.19; 95% confidence interval, 1.05-1.34; P = .003). In addition, 1-line–worse visual acuity was associated with 5% higher odds of depression (OR, 1.05; 95% CI, 1.04-1.07; P < .001).
The association between visual acuity and depression was found in both younger (39-58 years) and older (59-72 years) groups, as well as in both men and women.
The researchers also explored the association between depressive symptoms and brain structure using MRI scans from a subset of 7,844 individuals (51% women; 2% with visual impairment).
Results showed linear associations between PHQ-2 scores and the left volume of gray matter in the supracalcarine cortex (coefficient, 7.61; 95% CI, 3.9-11.3; adjusted P = .006).
The investigators note that the supracalcarine cortex is spatially connected to the primary visual cortex, suggesting the visual cortex may be involved in the pathogenesis of depression.
PHQ-2 scores were also associated with mean isotropic volume fraction (ISOVF) in the right fornix (cres) and/or stria terminalis (coefficient, .003; 95% CI, 0.001-0.004; adjusted P = .01).
The links “could be moderated by visual acuity, whereby increased PHQ score was associated with higher ISOVF levels only among those with poorer visual acuity (P = .02 for interaction),” the investigators report.
These results “suggest that poorer visual acuity was associated with greater depressive symptoms and may have contributed to the related deterioration of the fornix and stria terminalis,” they add.
They note that previous studies have supported the hypothesis that the fornix and stria terminalis are involved in the pathophysiology of other brain-related conditions, including schizophrenia, bipolar disorder, and autism spectrum disorder.
However, the investigators did not have information on how long the participants had experienced visual impairment, so they couldn’t investigate whether results were affected by time. Additional study limitations cited were that depression may affect vision and that a large proportion of the participants (89.3%) were White.
Study ‘adds nuance’
Commenting on the study, Ipsit V. Vahia, MD, of the department of psychiatry at Harvard Medical School, Boston, and associate chief of geriatric psychiatry at McLean Hospital, Belmont, Mass., said the study “adds nuance to our understanding” of the well-established relationship between vision deficits and depression.
“It indicates that even mild visual deficits may be associated with depression,” said Dr. Vahia, who was not involved with the research.
The investigators validated this association by showing that visual acuity was also associated with neuroimaging markers of depression, he added.
Although the study was not designed to demonstrate causal relationships between mood and vision and its findings do not confirm that correcting visual acuity deficits will resolve depressive symptoms, “the large study sample and high quality of data should give clinicians confidence in the study’s findings,” Dr. Vahia said.
“Correcting visual acuity deficits can be considered standard care for older adults worldwide, and this study suggests that providing this standard care could also benefit mental health,” he concluded.
The study was supported by the National Natural Science Foundation of China, the China Postdoctoral Science Foundation, the Outstanding Young Talent Trainee Program of Guangdong Provincial People’s Hospital, the Guangdong Provincial People’s Hospital Scientific Research Funds for Leading Medical Talents and Distinguished Young Scholars in Guangdong Province, the Talent Introduction Fund of Guangdong Provincial People’s Hospital, the Science and Technology Program of Guangzhou, China, the Project of Special Research on Cardiovascular Diseases, the Research Foundation of Medical Science and Technology of Guangdong Province, the University of Melbourne at Research Accelerator Program, and the CERA (Centre for Eye Research Australia) Foundation and Victorian State Government for the Centre for Eye Research Australia. The investigators and Dr. Vahia have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After multiple adjustments, analysis of data from more than 114,000 participants in the UK Biobank Study showed that visual impairment was linked to a 19% higher risk for depression.
In addition, imaging results showed a significant link between deteriorating brain structures and depression in those with poor visual acuity.
“Our findings highlight the value of visual health in association with mental health,” Xiayin Zhang, PhD, Guangdong Eye Institute, department of ophthalmology, Guangdong Provincial People’s Hospital, Guangzhou, China, and colleagues write.
“Screening of vision at an early stage should be embedded in the middle-aged and older population to stratify the vulnerable population at risk for depression,” the investigators add.
The findings were published online in JAMA Network Open.
UK biobank analyses
The analysis included 114,583 participants (54.5% women; mean age, 56.8 years) from the UK Biobank who completed standardized questionnaires and underwent ocular examinations.
To test distance visual acuity, all were asked to read letters on lines from the top to the bottom of a chart while wearing prescribed optical correction. Visual impairment was defined as visual acuity worse than 0.3 logarithm of the minimum angle of resolution (LogMAR) units.
Depressive symptoms were self-reported using the two-item Patient Health Questionnaire (PHQ-2), in which a score of 3 or more indicates depression. As well, a medical practitioner conducted an assessment of depression at baseline.
Among the participants, 87.2% had no visual impairment or depression and acted as the healthy control group. In addition, 3.2% showed visual impairment, 10% reported a diagnosis of depression, and 0.4% had both.
Researchers adjusted for age, sex, race, ethnicity, education, smoking, alcohol consumption, physical activity, family history of severe depression, obesity, hypertension, diabetes, hyperlipidemia, and deprivation on the Townsend index.
Among those with visual impairment, 12.4% had depression, compared with 9.9% without visual impairment.
Structure deterioration
After adjusting for potential confounders, visual impairment was associated with a 19% higher risk for depression (odds ratio, 1.19; 95% confidence interval, 1.05-1.34; P = .003). In addition, 1-line–worse visual acuity was associated with 5% higher odds of depression (OR, 1.05; 95% CI, 1.04-1.07; P < .001).
The association between visual acuity and depression was found in both younger (39-58 years) and older (59-72 years) groups, as well as in both men and women.
The researchers also explored the association between depressive symptoms and brain structure using MRI scans from a subset of 7,844 individuals (51% women; 2% with visual impairment).
Results showed linear associations between PHQ-2 scores and the left volume of gray matter in the supracalcarine cortex (coefficient, 7.61; 95% CI, 3.9-11.3; adjusted P = .006).
The investigators note that the supracalcarine cortex is spatially connected to the primary visual cortex, suggesting the visual cortex may be involved in the pathogenesis of depression.
PHQ-2 scores were also associated with mean isotropic volume fraction (ISOVF) in the right fornix (cres) and/or stria terminalis (coefficient, .003; 95% CI, 0.001-0.004; adjusted P = .01).
The links “could be moderated by visual acuity, whereby increased PHQ score was associated with higher ISOVF levels only among those with poorer visual acuity (P = .02 for interaction),” the investigators report.
These results “suggest that poorer visual acuity was associated with greater depressive symptoms and may have contributed to the related deterioration of the fornix and stria terminalis,” they add.
They note that previous studies have supported the hypothesis that the fornix and stria terminalis are involved in the pathophysiology of other brain-related conditions, including schizophrenia, bipolar disorder, and autism spectrum disorder.
However, the investigators did not have information on how long the participants had experienced visual impairment, so they couldn’t investigate whether results were affected by time. Additional study limitations cited were that depression may affect vision and that a large proportion of the participants (89.3%) were White.
Study ‘adds nuance’
Commenting on the study, Ipsit V. Vahia, MD, of the department of psychiatry at Harvard Medical School, Boston, and associate chief of geriatric psychiatry at McLean Hospital, Belmont, Mass., said the study “adds nuance to our understanding” of the well-established relationship between vision deficits and depression.
“It indicates that even mild visual deficits may be associated with depression,” said Dr. Vahia, who was not involved with the research.
The investigators validated this association by showing that visual acuity was also associated with neuroimaging markers of depression, he added.
Although the study was not designed to demonstrate causal relationships between mood and vision and its findings do not confirm that correcting visual acuity deficits will resolve depressive symptoms, “the large study sample and high quality of data should give clinicians confidence in the study’s findings,” Dr. Vahia said.
“Correcting visual acuity deficits can be considered standard care for older adults worldwide, and this study suggests that providing this standard care could also benefit mental health,” he concluded.
The study was supported by the National Natural Science Foundation of China, the China Postdoctoral Science Foundation, the Outstanding Young Talent Trainee Program of Guangdong Provincial People’s Hospital, the Guangdong Provincial People’s Hospital Scientific Research Funds for Leading Medical Talents and Distinguished Young Scholars in Guangdong Province, the Talent Introduction Fund of Guangdong Provincial People’s Hospital, the Science and Technology Program of Guangzhou, China, the Project of Special Research on Cardiovascular Diseases, the Research Foundation of Medical Science and Technology of Guangdong Province, the University of Melbourne at Research Accelerator Program, and the CERA (Centre for Eye Research Australia) Foundation and Victorian State Government for the Centre for Eye Research Australia. The investigators and Dr. Vahia have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Pediatricians urged to check for vision problems after concussion
Pediatricians should consider screening children suspected of having a concussion for resulting vision problems that are often overlooked, according to the American Academy of Pediatrics.
Christina Master, MD, a pediatrician and sports medicine specialist at the Children’s Hospital of Philadelphia, said many doctors don’t think of vision problems when examining children who’ve experienced a head injury. But the issues are common and can significantly affect a child’s performance in school and sports, and disrupt daily life.
Dr. Master led a team of sports medicine and vision specialists who wrote an AAP policy statement on vision and concussion. She summarized the new recommendations during a plenary session Oct. 9 at the American Academy of Pediatrics National Conference.
Dr. Master told this news organization that the vast majority of the estimated 1.4 million U.S. children and adolescents who have concussions annually are treated in pediatricians’ offices.
Up to 40% of young patients experience symptoms such as blurred vision, light sensitivity, and double vision following a concussion, the panel said. In addition, children with vision problems are more likely to have prolonged recoveries and delays in returning to school than children who have concussions but don’t have similar eyesight issues.
Concussions affect neurologic pathways of the visual system and disturb basic functions such as the ability of the eyes to change focus from a distant object to a near one.
Dr. Master said most pediatricians do not routinely check for vision problems following a concussion, and children themselves may not recognize that they have vision deficits “unless you ask them very specifically.”
In addition to asking children about their vision, the policy statement recommends pediatricians conduct a thorough exam to assess ocular alignment, the ability to track a moving object, and the ability to maintain focus on an image while moving.
Dr. Master said that an assessment of vision and balance, which is described in an accompanying clinical report, lasts about 5 minutes and is easy for pediatricians to learn.
Managing vision problems
Pediatricians can guide parents in talking to their child’s school about accommodations such as extra time on classroom tasks, creating materials with enlarged fonts, and using preprinted or audio notes, the statement said.
At school, vision deficits can interfere with reading by causing children to skip words, lose their place, become fatigued, or lose interest, according to the statement.
Children can also take breaks from visual stressors such as bright lights and screens, and use prescription glasses temporarily to correct blurred vision, the panel noted.
Although most children will recover from a concussion on their own within 4 weeks, up to one-third will have persistent symptoms and may benefit from seeing a specialist who can provide treatment such as rehabilitative exercises. While evidence suggests that referring some children to specialty care within a week of a concussion improves outcomes, the signs of who would benefit are not always clear, according to the panel.
Specialties such as sports medicine, neurology, physiatry, otorhinolaryngology, and occupational therapy may provide care for prolonged symptoms, Dr. Master said.
The panel noted that more study is needed on treatment options such as rehabilitation exercises, which have been shown to help with balance and dizziness.
Dr. Master said the panel did not recommend that pediatricians provide a home exercise program to treat concussion, as she does in her practice, explaining that “it’s not clear that it’s necessary for all kids.”
One author of the policy statement, Ankoor Shah, MD, PhD, reported an intellectual property relationship with Rebion involving a patent application for a pediatric vision screener. Others, including Dr. Master, reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pediatricians should consider screening children suspected of having a concussion for resulting vision problems that are often overlooked, according to the American Academy of Pediatrics.
Christina Master, MD, a pediatrician and sports medicine specialist at the Children’s Hospital of Philadelphia, said many doctors don’t think of vision problems when examining children who’ve experienced a head injury. But the issues are common and can significantly affect a child’s performance in school and sports, and disrupt daily life.
Dr. Master led a team of sports medicine and vision specialists who wrote an AAP policy statement on vision and concussion. She summarized the new recommendations during a plenary session Oct. 9 at the American Academy of Pediatrics National Conference.
Dr. Master told this news organization that the vast majority of the estimated 1.4 million U.S. children and adolescents who have concussions annually are treated in pediatricians’ offices.
Up to 40% of young patients experience symptoms such as blurred vision, light sensitivity, and double vision following a concussion, the panel said. In addition, children with vision problems are more likely to have prolonged recoveries and delays in returning to school than children who have concussions but don’t have similar eyesight issues.
Concussions affect neurologic pathways of the visual system and disturb basic functions such as the ability of the eyes to change focus from a distant object to a near one.
Dr. Master said most pediatricians do not routinely check for vision problems following a concussion, and children themselves may not recognize that they have vision deficits “unless you ask them very specifically.”
In addition to asking children about their vision, the policy statement recommends pediatricians conduct a thorough exam to assess ocular alignment, the ability to track a moving object, and the ability to maintain focus on an image while moving.
Dr. Master said that an assessment of vision and balance, which is described in an accompanying clinical report, lasts about 5 minutes and is easy for pediatricians to learn.
Managing vision problems
Pediatricians can guide parents in talking to their child’s school about accommodations such as extra time on classroom tasks, creating materials with enlarged fonts, and using preprinted or audio notes, the statement said.
At school, vision deficits can interfere with reading by causing children to skip words, lose their place, become fatigued, or lose interest, according to the statement.
Children can also take breaks from visual stressors such as bright lights and screens, and use prescription glasses temporarily to correct blurred vision, the panel noted.
Although most children will recover from a concussion on their own within 4 weeks, up to one-third will have persistent symptoms and may benefit from seeing a specialist who can provide treatment such as rehabilitative exercises. While evidence suggests that referring some children to specialty care within a week of a concussion improves outcomes, the signs of who would benefit are not always clear, according to the panel.
Specialties such as sports medicine, neurology, physiatry, otorhinolaryngology, and occupational therapy may provide care for prolonged symptoms, Dr. Master said.
The panel noted that more study is needed on treatment options such as rehabilitation exercises, which have been shown to help with balance and dizziness.
Dr. Master said the panel did not recommend that pediatricians provide a home exercise program to treat concussion, as she does in her practice, explaining that “it’s not clear that it’s necessary for all kids.”
One author of the policy statement, Ankoor Shah, MD, PhD, reported an intellectual property relationship with Rebion involving a patent application for a pediatric vision screener. Others, including Dr. Master, reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pediatricians should consider screening children suspected of having a concussion for resulting vision problems that are often overlooked, according to the American Academy of Pediatrics.
Christina Master, MD, a pediatrician and sports medicine specialist at the Children’s Hospital of Philadelphia, said many doctors don’t think of vision problems when examining children who’ve experienced a head injury. But the issues are common and can significantly affect a child’s performance in school and sports, and disrupt daily life.
Dr. Master led a team of sports medicine and vision specialists who wrote an AAP policy statement on vision and concussion. She summarized the new recommendations during a plenary session Oct. 9 at the American Academy of Pediatrics National Conference.
Dr. Master told this news organization that the vast majority of the estimated 1.4 million U.S. children and adolescents who have concussions annually are treated in pediatricians’ offices.
Up to 40% of young patients experience symptoms such as blurred vision, light sensitivity, and double vision following a concussion, the panel said. In addition, children with vision problems are more likely to have prolonged recoveries and delays in returning to school than children who have concussions but don’t have similar eyesight issues.
Concussions affect neurologic pathways of the visual system and disturb basic functions such as the ability of the eyes to change focus from a distant object to a near one.
Dr. Master said most pediatricians do not routinely check for vision problems following a concussion, and children themselves may not recognize that they have vision deficits “unless you ask them very specifically.”
In addition to asking children about their vision, the policy statement recommends pediatricians conduct a thorough exam to assess ocular alignment, the ability to track a moving object, and the ability to maintain focus on an image while moving.
Dr. Master said that an assessment of vision and balance, which is described in an accompanying clinical report, lasts about 5 minutes and is easy for pediatricians to learn.
Managing vision problems
Pediatricians can guide parents in talking to their child’s school about accommodations such as extra time on classroom tasks, creating materials with enlarged fonts, and using preprinted or audio notes, the statement said.
At school, vision deficits can interfere with reading by causing children to skip words, lose their place, become fatigued, or lose interest, according to the statement.
Children can also take breaks from visual stressors such as bright lights and screens, and use prescription glasses temporarily to correct blurred vision, the panel noted.
Although most children will recover from a concussion on their own within 4 weeks, up to one-third will have persistent symptoms and may benefit from seeing a specialist who can provide treatment such as rehabilitative exercises. While evidence suggests that referring some children to specialty care within a week of a concussion improves outcomes, the signs of who would benefit are not always clear, according to the panel.
Specialties such as sports medicine, neurology, physiatry, otorhinolaryngology, and occupational therapy may provide care for prolonged symptoms, Dr. Master said.
The panel noted that more study is needed on treatment options such as rehabilitation exercises, which have been shown to help with balance and dizziness.
Dr. Master said the panel did not recommend that pediatricians provide a home exercise program to treat concussion, as she does in her practice, explaining that “it’s not clear that it’s necessary for all kids.”
One author of the policy statement, Ankoor Shah, MD, PhD, reported an intellectual property relationship with Rebion involving a patent application for a pediatric vision screener. Others, including Dr. Master, reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAP 2022
Older diabetes drugs linked to dementia risk -- one lower, one higher
a new observational study in patients with type 2 diabetes suggests.
The data, obtained from nationwide electronic medical records from the Department of Veterans Affairs, yielded a 22% lower risk of dementia with TZD monotherapy and a 12% elevated risk with sulfonylurea monotherapy, compared with metformin monotherapy. The apparent protective effects of TZDs were greater among individuals with overweight or obesity.
“Our findings provide additional information to aid clinicians’ selection of [glucose-lowering medications] for patients with mild or moderate type 2 diabetes and [who] are at high risk of dementia,” Xin Tang and colleagues wrote in their article, published online in BMJ Open Diabetes Research & Care.
The results “add substantially to the literature concerning the effects of [glucose-lowering medications] on dementia where previous findings have been inconsistent. Studies with a follow-up time of less than 3 years have mainly reported null associations, while studies with longer a follow-up time typically yielded protective findings. With a mean follow-up time of 6.8 years, we had a sufficient duration to detect treatment differences,” the investigators wrote.
“Supplementing [a] sulfonylurea with either metformin or [a] TZD may partially offset its prodementia effects. These findings may help inform medication selection for elderly patients with T2D at high risk of dementia,” they added.
Randomized trials needed to determine cause and effect
Ivan Koychev, PhD, a senior clinical researcher in the department of psychiatry at the University of Oxford (England), told the UK Science Media Centre: “This is a large, well-conducted real-world data study that highlights the importance of checking whether already prescribed medications may be useful for preventing dementia.”
The findings regarding TZDs, also known as glitazones, are in line with existing literature suggesting dementia protection with other drugs prescribed for type 2 diabetes that weren’t examined in the current study, such as newer agents like glucagonlike peptide–1 (GLP-1) agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors, Dr. Koychev said.
“The main limitations of this study is that following the initial 2-year period the authors were interested in, the participants may have been prescribed one of the other type 2 diabetes drugs [GLP-1 agonists or SGLT2 inhibitors] that have been found to reduce dementia risk, thus potentially making the direct glitazone [TZD] effect more difficult to discern,” Dr. Koychev noted.
And, he pointed out that the study design limits attribution of causality. “It is also important to note that people with type 2 diabetes do run a higher risk of both dementia and cognitive deficits and that these medications are only prescribed in these patients, so all this data is from this patient group rather than the general population.”
James Connell, PhD, head of translational science at Alzheimer’s Research UK, agreed. “While this observational study found that those with type 2 diabetes taking thiazolidinedione had a lower dementia risk than those on the most common medication for type 2 diabetes, it only shows an association between taking the drug and dementia risk and not a causal relationship.
“Double-blind and placebo-controlled clinical trials are needed to see whether the drug [TDZ] could help lower dementia risk in people with and without diabetes. Anyone with any questions about what treatments they are receiving should speak to their doctor,” he told the UK Science Media Centre.
Opposite effects of sulfonylureas, TZDs versus metformin
The study authors analyzed 559,106 VA patients with type 2 diabetes who initiated glucose-lowering medication during 2001-2017 and took it for at least a year. They were aged 60 years or older and did not have dementia at baseline. Most were White (76.8%) and male (96.9%), two-thirds (63.1%) had obesity, and mean hemoglobin A1c was 6.8%.
Overall, 31,125 developed all-cause dementia. The incidence rate was 8.2 cases per 1,000 person-years, ranging from 6.2 cases per 1,000 person-years among those taking metformin monotherapy to 13.4 cases per 1,000 person-years in those taking both sulfonylurea and a TZD.
Compared with metformin monotherapy, the hazard ratio for all-cause dementia for sulfonylurea monotherapy was a significant 1.12. The increased risk was also seen for vascular dementia, with an HR of 1.14.
In contrast, TZD monotherapy was associated with a significantly lower risk for all-cause dementia (HR, 0.78), as well as for Alzheimer’s disease (HR, 0.89) and vascular dementia (HR, 0.43), compared with metformin monotherapy.
The combination of metformin and TZD also lowered the risk of all-cause dementia, while regimens including sulfonylureas raised the risks for all-cause and vascular dementia.
Most of the results didn’t change significantly when the drug exposure window was extended to 2 years.
Effects more pronounced in those with obesity
The protective 1-year effects of TZD monotherapy and of metformin plus TZD, compared with metformin alone, were more significant among participants aged 75 or younger and with a body mass index above 25 kg/m2, compared with those who were older than 75 years and with normal BMIs, respectively.
On the other hand, the greater risk for dementia incurred with sulfonylureas was further increased among those with higher BMI.
This research was partially funded by grants from the National Human Genome Research Institute, the National Science Foundation, the National Institute of Diabetes and Digestive and Kidney Disease, and the National Heart, Lung, and Blood Institute. Dr. Koychev is chief investigator for a trial, sponsored by Oxford University and funded by Novo Nordisk, testing whether the GLP-1 agonist semaglutide reduces the risk for dementia in aging adults.
A version of this article first appeared on Medscape.com.
a new observational study in patients with type 2 diabetes suggests.
The data, obtained from nationwide electronic medical records from the Department of Veterans Affairs, yielded a 22% lower risk of dementia with TZD monotherapy and a 12% elevated risk with sulfonylurea monotherapy, compared with metformin monotherapy. The apparent protective effects of TZDs were greater among individuals with overweight or obesity.
“Our findings provide additional information to aid clinicians’ selection of [glucose-lowering medications] for patients with mild or moderate type 2 diabetes and [who] are at high risk of dementia,” Xin Tang and colleagues wrote in their article, published online in BMJ Open Diabetes Research & Care.
The results “add substantially to the literature concerning the effects of [glucose-lowering medications] on dementia where previous findings have been inconsistent. Studies with a follow-up time of less than 3 years have mainly reported null associations, while studies with longer a follow-up time typically yielded protective findings. With a mean follow-up time of 6.8 years, we had a sufficient duration to detect treatment differences,” the investigators wrote.
“Supplementing [a] sulfonylurea with either metformin or [a] TZD may partially offset its prodementia effects. These findings may help inform medication selection for elderly patients with T2D at high risk of dementia,” they added.
Randomized trials needed to determine cause and effect
Ivan Koychev, PhD, a senior clinical researcher in the department of psychiatry at the University of Oxford (England), told the UK Science Media Centre: “This is a large, well-conducted real-world data study that highlights the importance of checking whether already prescribed medications may be useful for preventing dementia.”
The findings regarding TZDs, also known as glitazones, are in line with existing literature suggesting dementia protection with other drugs prescribed for type 2 diabetes that weren’t examined in the current study, such as newer agents like glucagonlike peptide–1 (GLP-1) agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors, Dr. Koychev said.
“The main limitations of this study is that following the initial 2-year period the authors were interested in, the participants may have been prescribed one of the other type 2 diabetes drugs [GLP-1 agonists or SGLT2 inhibitors] that have been found to reduce dementia risk, thus potentially making the direct glitazone [TZD] effect more difficult to discern,” Dr. Koychev noted.
And, he pointed out that the study design limits attribution of causality. “It is also important to note that people with type 2 diabetes do run a higher risk of both dementia and cognitive deficits and that these medications are only prescribed in these patients, so all this data is from this patient group rather than the general population.”
James Connell, PhD, head of translational science at Alzheimer’s Research UK, agreed. “While this observational study found that those with type 2 diabetes taking thiazolidinedione had a lower dementia risk than those on the most common medication for type 2 diabetes, it only shows an association between taking the drug and dementia risk and not a causal relationship.
“Double-blind and placebo-controlled clinical trials are needed to see whether the drug [TDZ] could help lower dementia risk in people with and without diabetes. Anyone with any questions about what treatments they are receiving should speak to their doctor,” he told the UK Science Media Centre.
Opposite effects of sulfonylureas, TZDs versus metformin
The study authors analyzed 559,106 VA patients with type 2 diabetes who initiated glucose-lowering medication during 2001-2017 and took it for at least a year. They were aged 60 years or older and did not have dementia at baseline. Most were White (76.8%) and male (96.9%), two-thirds (63.1%) had obesity, and mean hemoglobin A1c was 6.8%.
Overall, 31,125 developed all-cause dementia. The incidence rate was 8.2 cases per 1,000 person-years, ranging from 6.2 cases per 1,000 person-years among those taking metformin monotherapy to 13.4 cases per 1,000 person-years in those taking both sulfonylurea and a TZD.
Compared with metformin monotherapy, the hazard ratio for all-cause dementia for sulfonylurea monotherapy was a significant 1.12. The increased risk was also seen for vascular dementia, with an HR of 1.14.
In contrast, TZD monotherapy was associated with a significantly lower risk for all-cause dementia (HR, 0.78), as well as for Alzheimer’s disease (HR, 0.89) and vascular dementia (HR, 0.43), compared with metformin monotherapy.
The combination of metformin and TZD also lowered the risk of all-cause dementia, while regimens including sulfonylureas raised the risks for all-cause and vascular dementia.
Most of the results didn’t change significantly when the drug exposure window was extended to 2 years.
Effects more pronounced in those with obesity
The protective 1-year effects of TZD monotherapy and of metformin plus TZD, compared with metformin alone, were more significant among participants aged 75 or younger and with a body mass index above 25 kg/m2, compared with those who were older than 75 years and with normal BMIs, respectively.
On the other hand, the greater risk for dementia incurred with sulfonylureas was further increased among those with higher BMI.
This research was partially funded by grants from the National Human Genome Research Institute, the National Science Foundation, the National Institute of Diabetes and Digestive and Kidney Disease, and the National Heart, Lung, and Blood Institute. Dr. Koychev is chief investigator for a trial, sponsored by Oxford University and funded by Novo Nordisk, testing whether the GLP-1 agonist semaglutide reduces the risk for dementia in aging adults.
A version of this article first appeared on Medscape.com.
a new observational study in patients with type 2 diabetes suggests.
The data, obtained from nationwide electronic medical records from the Department of Veterans Affairs, yielded a 22% lower risk of dementia with TZD monotherapy and a 12% elevated risk with sulfonylurea monotherapy, compared with metformin monotherapy. The apparent protective effects of TZDs were greater among individuals with overweight or obesity.
“Our findings provide additional information to aid clinicians’ selection of [glucose-lowering medications] for patients with mild or moderate type 2 diabetes and [who] are at high risk of dementia,” Xin Tang and colleagues wrote in their article, published online in BMJ Open Diabetes Research & Care.
The results “add substantially to the literature concerning the effects of [glucose-lowering medications] on dementia where previous findings have been inconsistent. Studies with a follow-up time of less than 3 years have mainly reported null associations, while studies with longer a follow-up time typically yielded protective findings. With a mean follow-up time of 6.8 years, we had a sufficient duration to detect treatment differences,” the investigators wrote.
“Supplementing [a] sulfonylurea with either metformin or [a] TZD may partially offset its prodementia effects. These findings may help inform medication selection for elderly patients with T2D at high risk of dementia,” they added.
Randomized trials needed to determine cause and effect
Ivan Koychev, PhD, a senior clinical researcher in the department of psychiatry at the University of Oxford (England), told the UK Science Media Centre: “This is a large, well-conducted real-world data study that highlights the importance of checking whether already prescribed medications may be useful for preventing dementia.”
The findings regarding TZDs, also known as glitazones, are in line with existing literature suggesting dementia protection with other drugs prescribed for type 2 diabetes that weren’t examined in the current study, such as newer agents like glucagonlike peptide–1 (GLP-1) agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors, Dr. Koychev said.
“The main limitations of this study is that following the initial 2-year period the authors were interested in, the participants may have been prescribed one of the other type 2 diabetes drugs [GLP-1 agonists or SGLT2 inhibitors] that have been found to reduce dementia risk, thus potentially making the direct glitazone [TZD] effect more difficult to discern,” Dr. Koychev noted.
And, he pointed out that the study design limits attribution of causality. “It is also important to note that people with type 2 diabetes do run a higher risk of both dementia and cognitive deficits and that these medications are only prescribed in these patients, so all this data is from this patient group rather than the general population.”
James Connell, PhD, head of translational science at Alzheimer’s Research UK, agreed. “While this observational study found that those with type 2 diabetes taking thiazolidinedione had a lower dementia risk than those on the most common medication for type 2 diabetes, it only shows an association between taking the drug and dementia risk and not a causal relationship.
“Double-blind and placebo-controlled clinical trials are needed to see whether the drug [TDZ] could help lower dementia risk in people with and without diabetes. Anyone with any questions about what treatments they are receiving should speak to their doctor,” he told the UK Science Media Centre.
Opposite effects of sulfonylureas, TZDs versus metformin
The study authors analyzed 559,106 VA patients with type 2 diabetes who initiated glucose-lowering medication during 2001-2017 and took it for at least a year. They were aged 60 years or older and did not have dementia at baseline. Most were White (76.8%) and male (96.9%), two-thirds (63.1%) had obesity, and mean hemoglobin A1c was 6.8%.
Overall, 31,125 developed all-cause dementia. The incidence rate was 8.2 cases per 1,000 person-years, ranging from 6.2 cases per 1,000 person-years among those taking metformin monotherapy to 13.4 cases per 1,000 person-years in those taking both sulfonylurea and a TZD.
Compared with metformin monotherapy, the hazard ratio for all-cause dementia for sulfonylurea monotherapy was a significant 1.12. The increased risk was also seen for vascular dementia, with an HR of 1.14.
In contrast, TZD monotherapy was associated with a significantly lower risk for all-cause dementia (HR, 0.78), as well as for Alzheimer’s disease (HR, 0.89) and vascular dementia (HR, 0.43), compared with metformin monotherapy.
The combination of metformin and TZD also lowered the risk of all-cause dementia, while regimens including sulfonylureas raised the risks for all-cause and vascular dementia.
Most of the results didn’t change significantly when the drug exposure window was extended to 2 years.
Effects more pronounced in those with obesity
The protective 1-year effects of TZD monotherapy and of metformin plus TZD, compared with metformin alone, were more significant among participants aged 75 or younger and with a body mass index above 25 kg/m2, compared with those who were older than 75 years and with normal BMIs, respectively.
On the other hand, the greater risk for dementia incurred with sulfonylureas was further increased among those with higher BMI.
This research was partially funded by grants from the National Human Genome Research Institute, the National Science Foundation, the National Institute of Diabetes and Digestive and Kidney Disease, and the National Heart, Lung, and Blood Institute. Dr. Koychev is chief investigator for a trial, sponsored by Oxford University and funded by Novo Nordisk, testing whether the GLP-1 agonist semaglutide reduces the risk for dementia in aging adults.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN DIABETES RESEARCH & CARE
Epidemic of brain fog? Long COVID’s effects worry experts
Weeks after Jeannie Volpe caught COVID-19 in November 2020, she could no longer do her job running sexual assault support groups in Anniston, Ala., because she kept forgetting the details that survivors had shared with her. “People were telling me they were having to revisit their traumatic memories, which isn’t fair to anybody,” the 47-year-old says.
Ms. Volpe has been diagnosed with long-COVID autonomic dysfunction, which includes severe muscle pain, depression, anxiety, and a loss of thinking skills. Some of her symptoms are more commonly known as brain fog, and they’re among the most frequent problems reported by people who have long-term issues after a bout of COVID-19.
Many experts and medical professionals say they haven’t even begun to scratch the surface of what impact this will have in years to come.
“I’m very worried that we have an epidemic of neurologic dysfunction coming down the pike,” says Pamela Davis, MD, PhD, a research professor at Case Western Reserve University, Cleveland.
In the 2 years Ms. Volpe has been living with long COVID, her executive function – the mental processes that enable people to focus attention, retain information, and multitask – has been so diminished that she had to relearn to drive. One of the various doctors assessing her has suggested speech therapy to help Ms. Volpe relearn how to form words. “I can see the words I want to say in my mind, but I can’t make them come out of my mouth,” she says in a sluggish voice that gives away her condition.
All of those symptoms make it difficult for her to care for herself. Without a job and health insurance, Ms. Volpe says she’s researched assisted suicide in the states that allow it but has ultimately decided she wants to live.
“People tell you things like you should be grateful you survived it, and you should; but you shouldn’t expect somebody to not grieve after losing their autonomy, their career, their finances.”
The findings of researchers studying the brain effects of COVID-19 reinforce what people with long COVID have been dealing with from the start. Their experiences aren’t imaginary; they’re consistent with neurological disorders – including myalgic encephalomyelitis, also known as chronic fatigue syndrome, or ME/CFS – which carry much more weight in the public imagination than the term brain fog, which can often be used dismissively.
Studies have found that COVID-19 is linked to conditions such as strokes; seizures; and mood, memory, and movement disorders.
While there are still a lot of unanswered questions about exactly how COVID-19 affects the brain and what the long-term effects are, there’s enough reason to suggest people should be trying to avoid both infection and reinfection until researchers get more answers.
Worldwide, it’s estimated that COVID-19 has contributed to more than 40 million new cases of neurological disorders, says Ziyad Al-Aly, MD, a clinical epidemiologist and long COVID researcher at Washington University in St. Louis. In his latest study of 14 million medical records of the U.S. Department of Veterans Affairs, the country’s largest integrated health care system, researchers found that regardless of age, gender, race, and lifestyle,
He noted that some of the conditions, such as headaches and mild decline in memory and sharpness, may improve and go away over time. But others that showed up, such as stroke, encephalitis (inflammation of the brain), and Guillain-Barré syndrome (a rare disorder in which the body’s immune system attacks the nerves), often lead to lasting damage. Dr. Al-Aly’s team found that neurological conditions were 7% more likely in those who had COVID-19 than in those who had never been infected.
What’s more, researchers noticed that compared with control groups, the risk of post-COVID thinking problems was more pronounced in people in their 30s, 40s, and 50s – a group that usually would be very unlikely to have these problems. For those over the age of 60, the risks stood out less because at that stage of life, such thinking problems aren’t as rare.
Another study of the veterans system last year showed that COVID-19 survivors were at a 46% higher risk of considering suicide after 1 year.
“We need to be paying attention to this,” says Dr. Al-Aly. “What we’ve seen is really the tip of the iceberg.” He worries that millions of people, including youths, will lose out on employment and education while dealing with long-term disabilities – and the economic and societal implications of such a fallout. “What we will all be left with is the aftermath of sheer devastation in some people’s lives,” he says.
Igor Koralnik, MD, chief of neuro-infectious disease and global neurology at Northwestern University, Chicago, has been running a specialized long COVID clinic. His team published a paper in March 2021 detailing what they saw in their first 100 patients. “About half the population in the study missed at least 10 days of work. This is going to have persistent impact on the workforce,” Dr. Koralnik said in a podcast posted on the Northwestern website. “We have seen that not only [do] patients have symptoms, but they have decreased quality of life.”
For older people and their caregivers, the risk of potential neurodegenerative diseases that the virus has shown to accelerate, such as dementia, is also a big concern. Alzheimer’s is already the fifth leading cause of death for people 65 and older.
In a recent study of more than 6 million people over the age of 65, Dr. Davis and her team at Case Western found the risk of Alzheimer’s in the year after COVID-19 increased by 50%-80%. The chances were especially high for women older than 85.
To date, there are no good treatments for Alzheimer’s, yet total health care costs for long-term care and hospice services for people with dementia topped $300 billion in 2020. That doesn’t even include the related costs to families.
“The downstream effect of having someone with Alzheimer’s being taken care of by a family member can be devastating on everyone,” she says. “Sometimes the caregivers don’t weather that very well.”
When Dr. Davis’s own father got Alzheimer’s at age 86, her mother took care of him until she had a stroke one morning while making breakfast. Dr. Davis attributes the stroke to the stress of caregiving. That left Dr. Davis no choice but to seek housing where both her parents could get care.
Looking at the broader picture, Dr. Davis believes widespread isolation, loneliness, and grief during the pandemic, and the disease of COVID-19 itself, will continue to have a profound impact on psychiatric diagnoses. This in turn could trigger a wave of new substance abuse as a result of unchecked mental health problems.
Still, not all brain experts are jumping to worst-case scenarios, with a lot yet to be understood before sounding the alarm. Joanna Hellmuth, MD, a neurologist and researcher at the University of California, San Francisco, cautions against reading too much into early data, including any assumptions that COVID-19 causes neurodegeneration or irreversible damage in the brain.
Even with before-and-after brain scans by University of Oxford, England, researchers that show structural changes to the brain after infection, she points out that they didn’t actually study the clinical symptoms of the people in the study, so it’s too soon to reach conclusions about associated cognitive problems.
“It’s an important piece of the puzzle, but we don’t know how that fits together with everything else,” says Dr. Hellmuth. “Some of my patients get better. … I haven’t seen a single person get worse since the pandemic started, and so I’m hopeful.”
A version of this article first appeared on WebMD.com.
Weeks after Jeannie Volpe caught COVID-19 in November 2020, she could no longer do her job running sexual assault support groups in Anniston, Ala., because she kept forgetting the details that survivors had shared with her. “People were telling me they were having to revisit their traumatic memories, which isn’t fair to anybody,” the 47-year-old says.
Ms. Volpe has been diagnosed with long-COVID autonomic dysfunction, which includes severe muscle pain, depression, anxiety, and a loss of thinking skills. Some of her symptoms are more commonly known as brain fog, and they’re among the most frequent problems reported by people who have long-term issues after a bout of COVID-19.
Many experts and medical professionals say they haven’t even begun to scratch the surface of what impact this will have in years to come.
“I’m very worried that we have an epidemic of neurologic dysfunction coming down the pike,” says Pamela Davis, MD, PhD, a research professor at Case Western Reserve University, Cleveland.
In the 2 years Ms. Volpe has been living with long COVID, her executive function – the mental processes that enable people to focus attention, retain information, and multitask – has been so diminished that she had to relearn to drive. One of the various doctors assessing her has suggested speech therapy to help Ms. Volpe relearn how to form words. “I can see the words I want to say in my mind, but I can’t make them come out of my mouth,” she says in a sluggish voice that gives away her condition.
All of those symptoms make it difficult for her to care for herself. Without a job and health insurance, Ms. Volpe says she’s researched assisted suicide in the states that allow it but has ultimately decided she wants to live.
“People tell you things like you should be grateful you survived it, and you should; but you shouldn’t expect somebody to not grieve after losing their autonomy, their career, their finances.”
The findings of researchers studying the brain effects of COVID-19 reinforce what people with long COVID have been dealing with from the start. Their experiences aren’t imaginary; they’re consistent with neurological disorders – including myalgic encephalomyelitis, also known as chronic fatigue syndrome, or ME/CFS – which carry much more weight in the public imagination than the term brain fog, which can often be used dismissively.
Studies have found that COVID-19 is linked to conditions such as strokes; seizures; and mood, memory, and movement disorders.
While there are still a lot of unanswered questions about exactly how COVID-19 affects the brain and what the long-term effects are, there’s enough reason to suggest people should be trying to avoid both infection and reinfection until researchers get more answers.
Worldwide, it’s estimated that COVID-19 has contributed to more than 40 million new cases of neurological disorders, says Ziyad Al-Aly, MD, a clinical epidemiologist and long COVID researcher at Washington University in St. Louis. In his latest study of 14 million medical records of the U.S. Department of Veterans Affairs, the country’s largest integrated health care system, researchers found that regardless of age, gender, race, and lifestyle,
He noted that some of the conditions, such as headaches and mild decline in memory and sharpness, may improve and go away over time. But others that showed up, such as stroke, encephalitis (inflammation of the brain), and Guillain-Barré syndrome (a rare disorder in which the body’s immune system attacks the nerves), often lead to lasting damage. Dr. Al-Aly’s team found that neurological conditions were 7% more likely in those who had COVID-19 than in those who had never been infected.
What’s more, researchers noticed that compared with control groups, the risk of post-COVID thinking problems was more pronounced in people in their 30s, 40s, and 50s – a group that usually would be very unlikely to have these problems. For those over the age of 60, the risks stood out less because at that stage of life, such thinking problems aren’t as rare.
Another study of the veterans system last year showed that COVID-19 survivors were at a 46% higher risk of considering suicide after 1 year.
“We need to be paying attention to this,” says Dr. Al-Aly. “What we’ve seen is really the tip of the iceberg.” He worries that millions of people, including youths, will lose out on employment and education while dealing with long-term disabilities – and the economic and societal implications of such a fallout. “What we will all be left with is the aftermath of sheer devastation in some people’s lives,” he says.
Igor Koralnik, MD, chief of neuro-infectious disease and global neurology at Northwestern University, Chicago, has been running a specialized long COVID clinic. His team published a paper in March 2021 detailing what they saw in their first 100 patients. “About half the population in the study missed at least 10 days of work. This is going to have persistent impact on the workforce,” Dr. Koralnik said in a podcast posted on the Northwestern website. “We have seen that not only [do] patients have symptoms, but they have decreased quality of life.”
For older people and their caregivers, the risk of potential neurodegenerative diseases that the virus has shown to accelerate, such as dementia, is also a big concern. Alzheimer’s is already the fifth leading cause of death for people 65 and older.
In a recent study of more than 6 million people over the age of 65, Dr. Davis and her team at Case Western found the risk of Alzheimer’s in the year after COVID-19 increased by 50%-80%. The chances were especially high for women older than 85.
To date, there are no good treatments for Alzheimer’s, yet total health care costs for long-term care and hospice services for people with dementia topped $300 billion in 2020. That doesn’t even include the related costs to families.
“The downstream effect of having someone with Alzheimer’s being taken care of by a family member can be devastating on everyone,” she says. “Sometimes the caregivers don’t weather that very well.”
When Dr. Davis’s own father got Alzheimer’s at age 86, her mother took care of him until she had a stroke one morning while making breakfast. Dr. Davis attributes the stroke to the stress of caregiving. That left Dr. Davis no choice but to seek housing where both her parents could get care.
Looking at the broader picture, Dr. Davis believes widespread isolation, loneliness, and grief during the pandemic, and the disease of COVID-19 itself, will continue to have a profound impact on psychiatric diagnoses. This in turn could trigger a wave of new substance abuse as a result of unchecked mental health problems.
Still, not all brain experts are jumping to worst-case scenarios, with a lot yet to be understood before sounding the alarm. Joanna Hellmuth, MD, a neurologist and researcher at the University of California, San Francisco, cautions against reading too much into early data, including any assumptions that COVID-19 causes neurodegeneration or irreversible damage in the brain.
Even with before-and-after brain scans by University of Oxford, England, researchers that show structural changes to the brain after infection, she points out that they didn’t actually study the clinical symptoms of the people in the study, so it’s too soon to reach conclusions about associated cognitive problems.
“It’s an important piece of the puzzle, but we don’t know how that fits together with everything else,” says Dr. Hellmuth. “Some of my patients get better. … I haven’t seen a single person get worse since the pandemic started, and so I’m hopeful.”
A version of this article first appeared on WebMD.com.
Weeks after Jeannie Volpe caught COVID-19 in November 2020, she could no longer do her job running sexual assault support groups in Anniston, Ala., because she kept forgetting the details that survivors had shared with her. “People were telling me they were having to revisit their traumatic memories, which isn’t fair to anybody,” the 47-year-old says.
Ms. Volpe has been diagnosed with long-COVID autonomic dysfunction, which includes severe muscle pain, depression, anxiety, and a loss of thinking skills. Some of her symptoms are more commonly known as brain fog, and they’re among the most frequent problems reported by people who have long-term issues after a bout of COVID-19.
Many experts and medical professionals say they haven’t even begun to scratch the surface of what impact this will have in years to come.
“I’m very worried that we have an epidemic of neurologic dysfunction coming down the pike,” says Pamela Davis, MD, PhD, a research professor at Case Western Reserve University, Cleveland.
In the 2 years Ms. Volpe has been living with long COVID, her executive function – the mental processes that enable people to focus attention, retain information, and multitask – has been so diminished that she had to relearn to drive. One of the various doctors assessing her has suggested speech therapy to help Ms. Volpe relearn how to form words. “I can see the words I want to say in my mind, but I can’t make them come out of my mouth,” she says in a sluggish voice that gives away her condition.
All of those symptoms make it difficult for her to care for herself. Without a job and health insurance, Ms. Volpe says she’s researched assisted suicide in the states that allow it but has ultimately decided she wants to live.
“People tell you things like you should be grateful you survived it, and you should; but you shouldn’t expect somebody to not grieve after losing their autonomy, their career, their finances.”
The findings of researchers studying the brain effects of COVID-19 reinforce what people with long COVID have been dealing with from the start. Their experiences aren’t imaginary; they’re consistent with neurological disorders – including myalgic encephalomyelitis, also known as chronic fatigue syndrome, or ME/CFS – which carry much more weight in the public imagination than the term brain fog, which can often be used dismissively.
Studies have found that COVID-19 is linked to conditions such as strokes; seizures; and mood, memory, and movement disorders.
While there are still a lot of unanswered questions about exactly how COVID-19 affects the brain and what the long-term effects are, there’s enough reason to suggest people should be trying to avoid both infection and reinfection until researchers get more answers.
Worldwide, it’s estimated that COVID-19 has contributed to more than 40 million new cases of neurological disorders, says Ziyad Al-Aly, MD, a clinical epidemiologist and long COVID researcher at Washington University in St. Louis. In his latest study of 14 million medical records of the U.S. Department of Veterans Affairs, the country’s largest integrated health care system, researchers found that regardless of age, gender, race, and lifestyle,
He noted that some of the conditions, such as headaches and mild decline in memory and sharpness, may improve and go away over time. But others that showed up, such as stroke, encephalitis (inflammation of the brain), and Guillain-Barré syndrome (a rare disorder in which the body’s immune system attacks the nerves), often lead to lasting damage. Dr. Al-Aly’s team found that neurological conditions were 7% more likely in those who had COVID-19 than in those who had never been infected.
What’s more, researchers noticed that compared with control groups, the risk of post-COVID thinking problems was more pronounced in people in their 30s, 40s, and 50s – a group that usually would be very unlikely to have these problems. For those over the age of 60, the risks stood out less because at that stage of life, such thinking problems aren’t as rare.
Another study of the veterans system last year showed that COVID-19 survivors were at a 46% higher risk of considering suicide after 1 year.
“We need to be paying attention to this,” says Dr. Al-Aly. “What we’ve seen is really the tip of the iceberg.” He worries that millions of people, including youths, will lose out on employment and education while dealing with long-term disabilities – and the economic and societal implications of such a fallout. “What we will all be left with is the aftermath of sheer devastation in some people’s lives,” he says.
Igor Koralnik, MD, chief of neuro-infectious disease and global neurology at Northwestern University, Chicago, has been running a specialized long COVID clinic. His team published a paper in March 2021 detailing what they saw in their first 100 patients. “About half the population in the study missed at least 10 days of work. This is going to have persistent impact on the workforce,” Dr. Koralnik said in a podcast posted on the Northwestern website. “We have seen that not only [do] patients have symptoms, but they have decreased quality of life.”
For older people and their caregivers, the risk of potential neurodegenerative diseases that the virus has shown to accelerate, such as dementia, is also a big concern. Alzheimer’s is already the fifth leading cause of death for people 65 and older.
In a recent study of more than 6 million people over the age of 65, Dr. Davis and her team at Case Western found the risk of Alzheimer’s in the year after COVID-19 increased by 50%-80%. The chances were especially high for women older than 85.
To date, there are no good treatments for Alzheimer’s, yet total health care costs for long-term care and hospice services for people with dementia topped $300 billion in 2020. That doesn’t even include the related costs to families.
“The downstream effect of having someone with Alzheimer’s being taken care of by a family member can be devastating on everyone,” she says. “Sometimes the caregivers don’t weather that very well.”
When Dr. Davis’s own father got Alzheimer’s at age 86, her mother took care of him until she had a stroke one morning while making breakfast. Dr. Davis attributes the stroke to the stress of caregiving. That left Dr. Davis no choice but to seek housing where both her parents could get care.
Looking at the broader picture, Dr. Davis believes widespread isolation, loneliness, and grief during the pandemic, and the disease of COVID-19 itself, will continue to have a profound impact on psychiatric diagnoses. This in turn could trigger a wave of new substance abuse as a result of unchecked mental health problems.
Still, not all brain experts are jumping to worst-case scenarios, with a lot yet to be understood before sounding the alarm. Joanna Hellmuth, MD, a neurologist and researcher at the University of California, San Francisco, cautions against reading too much into early data, including any assumptions that COVID-19 causes neurodegeneration or irreversible damage in the brain.
Even with before-and-after brain scans by University of Oxford, England, researchers that show structural changes to the brain after infection, she points out that they didn’t actually study the clinical symptoms of the people in the study, so it’s too soon to reach conclusions about associated cognitive problems.
“It’s an important piece of the puzzle, but we don’t know how that fits together with everything else,” says Dr. Hellmuth. “Some of my patients get better. … I haven’t seen a single person get worse since the pandemic started, and so I’m hopeful.”
A version of this article first appeared on WebMD.com.
Would your patient benefit from a monoclonal antibody?
Small-molecule drugs such as aspirin, albuterol, atorvastatin, and lisinopril are the backbone of disease management in family medicine.1 However, large-molecule biological drugs such as monoclonal antibodies (MAbs) are increasingly prescribed to treat common conditions. In the past decade, MAbs comprised 20% of all drug approvals by the US Food and Drug Administration (FDA), and today they represent more than half of drugs currently in development.2 Fifteen MAbs have been approved by the FDA over the past decade for asthma, atopic dermatitis (AD), hyperlipidemia, osteoporosis, and migraine prevention.3 This review details what makes MAbs unique and what you should know about them.
The uniqueness of monoclonal antibodies
MAbs are biologics, but not all biologics are MAbs—eg, adalimumab (Humira) is a MAb, but etanercept (Enbrel) is not. MAbs are therapeutic proteins made possible by hybridoma technology used to create an antibody with single specificity.4-6 Monoclonal antibodies differ from small-molecule drugs in structure, dosing, route of administration, manufacturing, metabolism, drug interactions, and elimination (TABLE 17-9).
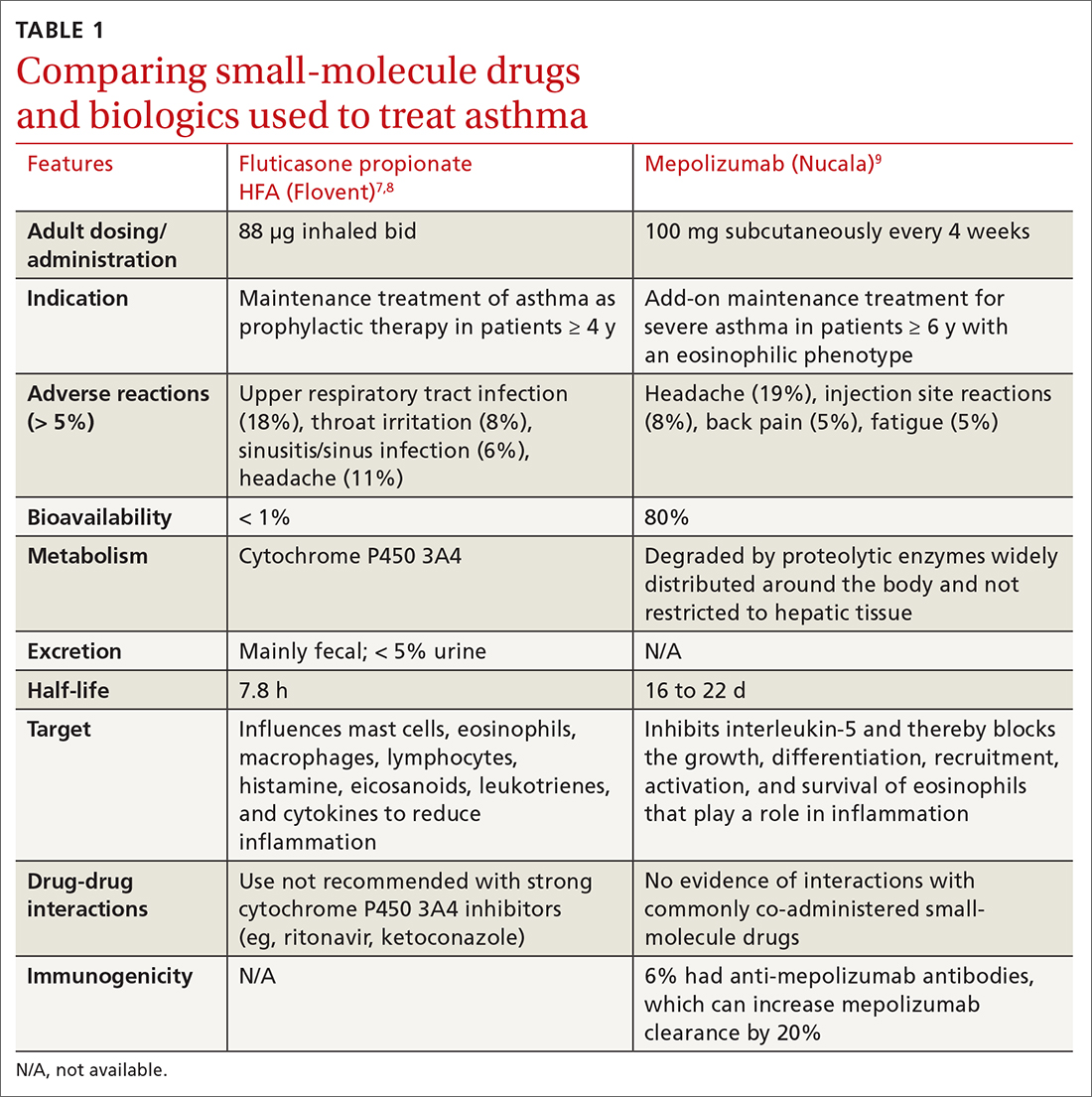
MAbs can be classified as naked, “without any drug or radioactive material attached to them,” or conjugated, “joined to a chemotherapy drug, radioactive isotope, or toxin.”10 MAbs work in several ways, including competitively inhibiting ligand-receptor binding, receptor blockade, or cell elimination from indirect immune system activities such as antibody-dependent cell-mediated cytotoxicity.11,12
Monoclonal antibody uses in family medicine
Asthma
Several MAbs have been approved for use in severe asthma, including but not limited to: omalizumab (Xolair),13 mepolizumab (Nucala),9,14 and dupilumab (Dupixent).15
Omalizumab is a humanized MAb that prevents IgE antibodies from binding to mast cells and basophils, thereby reducing inflammatory mediators.13 A systematic review found that, compared with placebo, omalizumab used in patients with inadequately controlled moderate-to-severe asthma led to significantly fewer asthma exacerbations (absolute risk reduction [ARR], 16% vs 26%; odds ratio [OR] = 0.55; 95% CI, 0.42-0.60; number needed to treat [NNT] = 10) and fewer hospitalizations (ARR, 0.5% vs 3%; OR = 0.16; 95% CI, 0.06-0.42; NNT = 40).13
Significantly more patients in the omalizumab group were able to withdraw from, or reduce, the dose of ICS. GINA recommends omalizumab for patients with positive skin sensitization, total serum IgE ≥ 30 IU/mL, weight within 30 kg to 150 kg, history of childhood asthma and recent exacerbations, and blood eosinophils ≥ 260/mcL.16 Omalizumab is also approved for use in chronic spontaneous urticaria and nasal polyps.
Mepolizumab
Continue to: Another trial found that...
Another trial found that mepolizumab reduced total OCS doses in patients with severe asthma by 50% without increasing exacerbations or worsening asthma control.18 All 3 anti-IL-5 drugs—including not only mepolizumab, but also benralizumab (Fasenra) and reslizumab (Cinqair)—appear to yield similar improvements. A 2017 systematic review found all anti-IL-5 treatments reduced rates of clinically significant asthma exacerbations (treatment with OCS for ≥ 3 days) by roughly 50% in patients with severe eosinophilic asthma and a history of ≥ 2 exacerbations in the past year.
Dupilumab is a humanized MAb that inhibits IL-4 and IL-13, which influence multiple cell types involved in inflammation (eg, mast cells, eosinophils) and inflammatory mediators (histamine, leukotrienes, cytokines).15 In a recent study of patients with uncontrolled asthma, dupilumab 200 mg every 2 weeks compared with placebo showed a modest reduction in the annualized rate of severe asthma exacerbations (0.46 exacerbations vs 0.87, respectively). Dupilumab was effective in patients with blood eosinophil counts ≥ 150/μL but was ineffective in patients with eosinophil counts < 150/μL.15
For patients ≥ 12 years old with severe eosinophilic asthma, GINA recommends using dupilumab as add-on therapy for an initial trial of 4 months at doses of 200 or 300 mg SC every 2 weeks, with preference for 300 mg SC every 2 weeks for OCS-dependent asthma. Dupilumab is approved for use in AD and chronic rhinosinusitis with nasal polyposis. If a biologic agent is not successful after a 4-month trial, consider a 6- to 12-month trial. If efficacy is still minimal, consider switching to an alternative biologic therapy approved for asthma.16
❯ Asthma: Test your skills
Subjective findings: A 19-year-old man presents to your clinic. He has a history of nasal polyps and allergic asthma. At age 18, he was given a diagnosis of severe persistent asthma. He has shortness of breath during waking hours 4 times per week, and treats each of these episodes with albuterol. He also wakes up about twice a week with shortness of breath and has some limitations in normal activities. He reports missing his prescribed fluticasone/salmeterol 500/50 μg, 1 inhalation bid, only once each month. In the last year, he has had 2 exacerbations requiring oral steroids.
Medications: Albuterol 90 μg, 1-2 inhalations, q6h prn; fluticasone/salmeterol 500/50 μg, 1 inhalation bid; tiotropium 1.25 μg, 2 puffs/d; montelukast 10 mg every morning; prednisone 10 mg/d.
Continue to: Objective data
Objective data: Patient is in no apparent distress and afebrile, and oxygen saturation on room air is 97%. Ht, 70 inches; wt, 75 kg. Labs: IgE, 15 IU/mL; serum eosinophils, 315/μL.
Which MAb would be appropriate for this patient? Given that the patient has a blood eosinophil level ≥ 300/μL and severe exacerbations, adult-onset asthma, nasal polyposis, and maintenance OCS at baseline, it would be reasonable to initiate mepolizumab 100 mg SC every 4 weeks, or dupilumab 600 mg once, then 300 mg SC every 2 weeks. Both agents can be self-administered.
Atopic dermatitis
Two MAbs—dupilumab and tralokinumab (Adbry; inhibits IL-13)—are approved for treatment of AD in adults that is uncontrolled with conventional therapy.15,19 Dupilumab is also approved for children ≥ 6 months old.20 Both MAbs are dosed at 600 mg SC, followed by 300 mg every 2 weeks. Dupilumab was compared with placebo in adult patients who had moderate-to-severe AD inadequately controlled on topical corticosteroids (TCSs), to determine the proportion of patients in each group achieving improvement of either 0 or 1 points or ≥ 2 points in the 5-point Investigator Global Assessment (IGA) score from baseline to 16 weeks.21 Thirty-seven percent of patients receiving dupilumab 300 mg SC weekly and 38% of patients receiving dupilumab 300 mg SC every 2 weeks achieved the primary outcome, compared with 10% of those receiving placebo (P < .001).21 Similar IGA scores were reported when dupilumab was combined with TCS, compared with placebo.22
It would be reasonable to consider dupilumab or tralokinumab in patients with: cutaneous atrophy or hypothalamic-pituitary-adrenal axis suppression with TCS, concerns of malignancy with topical calcineurin inhibitors, or problems with the alternative systemic therapies (cyclosporine-induced hypertension, nephrotoxicity, or immunosuppression; azathioprine-induced malignancy; or methotrexate-induced bone marrow suppression, renal impairment, hepatotoxicity, pneumonitis, or gastrointestinal toxicity).23
A distinct advantage of MAbs over other systemic agents in the management of AD is that MAbs do not require frequent monitoring of blood pressure, renal or liver function, complete blood count with differential, electrolytes, or uric acid. Additionally, MAbs have fewer black box warnings and adverse reactions when compared with other systemic agents.
Continue to: Hyperlipidemia
Hyperlipidemia
Three MAbs are approved for use in hyperlipidemia: the angiopoietin-like protein 3 (ANGPTL3) inhibitor evinacumab (Evkeeza)24 and 2 proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, evolocumab (Repatha)25 and alirocumab (Praluent).26
ANGPTL3 inhibitors block ANGPTL3 and reduce endothelial lipase and lipoprotein lipase activity, which in turn decreases low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride formation. PCSK9 inhibitors prevent PCSK9 from binding to LDL receptors, thereby maintaining the number of active LDL receptors and increasing LDL-C removal.
Evinacumab is indicated for homozygous familial hypercholesterolemia and is administered intravenously every 4 weeks. Evinacumab has not been evaluated for effects on cardiovascular morbidity and mortality.
Evolocumab 140 mg SC every 2 weeks or 420 mg SC monthly has been studied in patients on statin therapy with LDL-C ≥ 70 mg/dL. Patients on evolocumab experienced significantly less of the composite endpoint of cardiovascular death, myocardial infarction (MI), stroke, hospitalization for unstable angina, or coronary revascularization compared with placebo (9.8% vs 11.3%; hazard ratio [HR] = 0.85; 95% CI, 0.79-0.92; NNT = 67.27
Alirocumab 75 mg SC every 2 weeks has also been studied in patients receiving statin therapy with LDL-C ≥ 70 mg/dL. Patients taking alirocumab experienced significantly less of the composite endpoint of death from coronary heart disease, nonfatal MI, ischemic stroke, or hospitalization for unstable angina compared with placebo (9.5% vs 11.1%; HR = 0.85; 95% CI, 0.78-0.93; NNT = 63).
Continue to: According to the 2018...
According to the 2018 AHA Cholesterol Guidelines, PCSK9 inhibitors are indicated for patients receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe) with LDL-C ≥ 70 mg/dL, if they have had multiple atherosclerotic cardiovascular disease (ASCVD) events or 1 major ASCVD event with multiple high-risk conditions (eg, heterozygous familial hypercholesterolemia, history of coronary artery bypass grafting or percutaneous coronary intervention, hypertension, estimated glomerular filtration rate of 15 to 59 mL/min/1.73m2).29 For patients without prior ASCVD events or high-risk conditions who are receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe), PCSK9 inhibitors are indicated if the LDL-C remains ≥ 100 mg/dL.
Osteoporosis
The 2 MAbs approved for use in osteoporosis are the receptor activator of nuclear factor kB ligand (RANKL) inhibitor denosumab (Prolia)30 and the sclerostin inhibitor romosozumab (Evenity).31
Denosumab prevents RANKL from binding to the RANK receptor, thereby inhibiting osteoclast formation and decreasing bone resorption. Denosumab is approved for use in women and men who are at high risk of osteoporotic fracture, including those taking OCSs, men receiving androgen deprivation therapy for prostate cancer, and women receiving adjuvant aromatase inhibitor therapy for breast cancer.
In a 3-year randomized trial, denosumab 60 mg SC every 6 months was compared with placebo in postmenopausal women with T-scores < –2.5, but not < –4.0 at the lumbar spine or total hip. Denosumab significantly reduced new radiographic vertebral fractures (2.3% vs 7.2%; risk ratio [RR] = 0.32; 95% CI, 0.26-0.41; NNT = 21), hip fracture (0.7% vs 1.2%), and nonvertebral fracture (6.5% vs 8.0%).32 Denosumab carries an increased risk of multiple vertebral fractures following discontinuation, skin infections, dermatologic reactions, and severe bone, joint, and muscle pain.
Romosozumab inhibits sclerostin, thereby increasing bone formation and, to a lesser degree, decreasing bone resorption. Romosozumab is approved for use in postmenopausal women at high risk for fracture (ie, those with a history of osteoporotic fracture or multiple risk factors for fracture) or in patients who have not benefited from or are intolerant of other therapies. In one study, postmenopausal women with a T-score of –2.5 to –3.5 at the total hip or femoral neck were randomly assigned to receive either romosozumab 210 mg SC or placebo for 12 months, then each group was switched to denosumab 60 mg SC for 12 months. After the first year, prior to initiating denosumab, patients taking romosozumab experienced significantly fewer new vertebral fractures than patients taking placebo (0.5% vs 1.8%; RR = 0.27; 95% CI, 0.16-0.47; NNT = 77); however, there was no significant difference between the 2 groups with nonvertebral fractures (HR = 0.75; 95% CI, 0.53-1.05).33
Continue to: In another study...
In another study, romosozumab 210 mg SC was compared with alendronate 70 mg weekly, followed by alendronate 70 mg weekly in both groups. Over the first 12 months, patients treated with romosozumab saw a significant reduction in the incidence of new vertebral fractures (4% vs 6.3%; RR = 0.63, P < .003; NNT = 44). Patients treated with romosozumab with alendronate added for another 12 months also saw a significant reduction in new incidence of vertebral fractures (6.2% vs 11.9%; RR = 0.52; P < .001; NNT = 18).34 There was a higher risk of cardiovascular events among patients receiving romosozumab compared with those treated with alendronate, so romosozumab should not be used in individuals who have had an MI or stroke within the previous year.34 Denosumab and romosozumab offer an advantage over some bisphosphonates in that they require less frequent dosing and can be used in patients with renal impairment (creatinine clearance < 35 mL/min, in which zoledronic acid is contraindicated and alendronate is not recommended; < 30 mL/min, in which risedronate and ibandronate are not recommended).
Migraine prevention
Four
Erenumab, fremanezumab, and galcanezumab are all available in subcutaneous autoinjectors (or syringe with fremanezumab). Eptinezumab is an intravenous (IV) infusion given every 3 months.
Erenumab is available in both 70-mg and 140-mg dosing options. Fremanezumab can be given as 225 mg monthly or 675 mg quarterly. Galcanezumab has an initial loading dose of 240 mg followed by 120 mg given monthly. Erenumab targets the CGRP receptor; the others target the CGRP ligand. Eptinezumab has 100% bioavailability and reaches maximum serum concentration sooner than the other antagonists (due to its route of administration), but it must be given in an infusion center. Few insurers approve the use of eptinezumab unless a trial of least 1 of the monthly injectables has failed.
There are no head-to-head studies of the medications in this class. Additionally, differing study designs, definitions, statistical analyses, endpoints, and responder-rate calculations make it challenging to compare them directly against one another. At the very least, all of the CGRP MAbs have efficacy comparable to conventional preventive migraine medications such as propranolol, amitriptyline, and topiramate.40
Continue to: The most commonly reported adverse...
The most commonly reported adverse effect for all 4 CGRPs is injection site reaction, which was highest with the quarterly fremanezumab dose (45%).37 Constipation was most notable with the 140-mg dose of erenumab (3%)35; with the other CGRP MAbs it is comparable to that seen with placebo (< 1%).
Erenumab-induced hypertension has been identified in 61 cases reported through the FDA Adverse Event Reporting System (FAERS) as of 2021.41 This was not reported during MAb development programs, nor was it noted during clinical trials. Blood pressure elevation was seen within 1 week of injection in nearly 50% of the cases, and nearly one-third had pre-existing hypertension.41 Due to these findings, the erenumab prescribing information was updated to include hypertension in its warnings and precautions. It is possible that hypertension could be a class effect, although trial data and posthoc studies have yet to bear that out. Since erenumab was the first CGRP antagonist brought to market (May 2018 vs September 2018 for fremanezumab and galcanezumab), it may have accumulated more FAERS reports. Nearly all studies exclude patients with older age, uncontrolled hypertension, and unstable cardiovascular disease, which could impact data.41
Overall, this class of medications is very well tolerated, easy to use (again, excluding eptinezumab), and maintains a low adverse effect profile, giving added value compared with conventional preventive migraine medications.
The American Headache Society recommends a preventive oral therapy for at least 3 months before trying an alternative medication. After treatment failure with at least 2 oral agents, CGRP MAbs are recommended.42 CGRP antagonists offer convenient dosing, bypass gastrointestinal metabolism (which is useful in patients with nausea/vomiting), and have fewer adverse effects than traditional oral medications.
Worth noting. Several newer oral agents have been recently approved for migraine prevention, including atogepant (Qulipta) and rimegepant (Nurtec), which are also CGRP antagonists. Rimegepant is approved for both acute migraine treatment and prevention.
Continue to: Migraine
❯ Migraine: Test your skills
Subjective findings: A 25-year-old woman presents to your clinic for management of episodic migraines with aura. Her baseline average migraine frequency is 9 headache days/month. Her migraines are becoming more frequent despite treatment. She fears IV medication use and avoids hospitals.
History: Hypertension, irritable bowel syndrome with constipation (IBS-C), and depression. The patient is not pregnant or trying to get pregnant.
Medications: Current medications (for previous 4 months) include propranolol 40 mg at bedtime, linaclotide 145 μg/d, citalopram 20 mg/d, and sumatriptan 50 mg prn. Past medications include venlafaxine 150 mg po bid for 5 months.
What would be appropriate for this patient? This patient meets the criteria for using a CGRP antagonist because she has tried 2 preventive treatments for more than 60 to 90 days. Erenumab is not the best option, given the patient’s history of hypertension and IBS-C. The patient fears hospitals and IV medications, making eptinezumab a less-than-ideal choice. Depending on her insurance, fremanezumab or galcanezumab would be good options at this time.
CGRP antagonists have not been studied or evaluated in pregnancy, but if this patient becomes pregnant, a first-line agent for prevention would be propranolol, and a second-line agent would be a tricyclic antidepressant, memantine, or verapamil. Avoid ergotamines and antiepileptics (topiramate or valproate) in pregnancy.43,44
Continue to: The challenges associated with MAbs
The challenges associated with MAbs
MAbs can be expensive (TABLE 2),45 some prohibitively so. On a population scale, biologics account for around 40% of prescription drug spending and may cost 22 times more than small-molecule drugs.46 Estimates in 2016 showed that MAbs comprise $90.2 billion (43%) of the biologic market.46
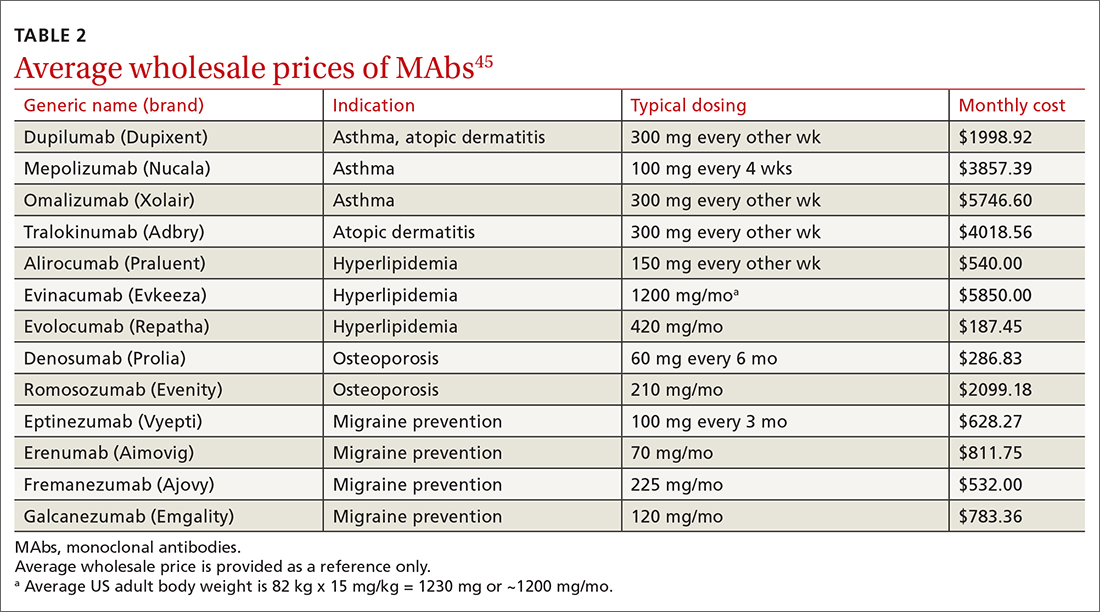
MAbs also require prior authorization forms to be submitted. Prior authorization criteria vary by state and by insurance plan. In my (ES) experience, submitting letters of medical necessity justifying the need for therapy or expertise in the disease states for which the MAb is being prescribed help your patient get the medication they need.
Expect to see additional MAbs approved in the future. If the costs come down, adoption of these agents into practice will likely increase.
CORRESPONDENCE
Evelyn Sbar, MD, Texas Tech University Health Sciences Center, 1400 South Coulter Street, Suite 5100, Amarillo, TX 79106; [email protected]
1. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. National Center for Health Statistics. Accessed June 15, 2022. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
2. IDBS. The future of biologics drug development is today. June 27, 2018. Accessed June 15, 2022. www.idbs.com/blog/2018/06/the-future-of-biologics-drug-development-is-today/
3. Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society. Accessed June 15, 2022. www.antibodysociety.org/resources/approved-antibodies/
4. FDA. Code of Federal Regulations, Title 21, Chapter I, Subchapter F biologics. March 29, 2022. Accessed June 15, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.3
5. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495-497. doi: 10.1038/256495a0
6. Raejewsky K. The advent and rise of monoclonal antibodies. Nature. November 4, 2019. Accessed June 15, 2022. www.nature.com/articles/d41586-019-02840-w
7. Flovent. Prescribing information. GlaxoSmithKline; 2010. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2010/021433s015lbl.pdf
8. NLM. National Center for Biotechnology Information. PubChem. Method for the preparation of fluticasone and related 17beta-carbothioic esters using a novel carbothioic acid synthesis and novel purification methods. Accessed June 15, 2022. pubchem.ncbi.nlm.nih.gov/patent/WO-0162722-A2
9. Nucala. Prescribing information. GlaxoSmithKline; 2019. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761122s000lbl.pdf
10. Argyriou AA, Kalofonos HP. Recent advances relating to the clinical application of naked monoclonal antibodies in solid tumors. Mol Med. 2009;15:183-191. doi: 10.2119/molmed.2009.00007
11. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548-558. doi: 10.1038/clpt.2008.170
12. Zahavi D, AlDeghaither D, O’Connell A, et al. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antib Ther. 2018;1:7-12. doi: 10.1093/abt/tby002
13. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014:CD003559. doi: 10.1002/14651858.CD003559.pub4
14. Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834. doi: 10.1002/14651858.CD010834.pub3
15. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486-2496. doi: 10.1056/NEJMoa1804092
16. GINA. Global strategy for asthma management and prevention. 2022 Difficult-to-treat and severe asthma guide—slide set. Accessed June 23, 2022. https://ginasthma.org/severeasthma/
17. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207. doi: 10.1056/NEJMoa1403290
18. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197. doi: 10.1056/NEJMoa1403291
19. Adbry. Prescribing information. Leo Pharma Inc; 2021. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf
20. Dupixent. Prescribing information. Regeneron Pharmaceuticals; 2022. Accessed October 5, 2022. https://www.regeneron.com/downloads/dupixent_fpi.pdf
21. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi: 10.1056/NEJMoa1610020
22. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. doi: 10.1016/s0140-6736(17)31191-1
23. Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi: 10.1016/j.jaad.2014.03.030
24. Evkeeza. Prescribing information. Regeneron Pharmaceuticals; 2021. Accessed June 24, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761181s000lbl.pdf
25. Repatha. Prescribing information. Amgen; 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf
26. Praluent. Prescribing information. Sanofi Aventis and Regeneron Pharmaceuticals. 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s002lbl.pdf
27. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722. doi: 10.1056/NEJMoa1615664
28. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097-2107. doi:10.1056/NEJMoa1801174
29. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
30. Prolia. Prescribing information. Amgen; 2010. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf
31. Evenity. Prescribing information. Amgen; 2019. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761062s000lbl.pdf
32. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
33. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543. doi: 10.1056/NEJMoa1607948
34. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427. doi: 10.1056/NEJMoa1708322
35. Aimovig. Prescribing information. Amgen; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf
36. Vyepti. Prescribing information. Lundbeck Seattle BioPharmaceuticals; 2020. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf
37. Ajovy. Prescribing information. Teva Pharmaceuticals; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf
38. Emgality. Prescribing information. Eli Lilly and Co.; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf
39. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350. doi: 10.1038/s41582-018-0003-1
40. Vandervorst F. Van Deun L, Van Dycke A, et al. CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain. 2021;22:128. doi: 10.1186/s10194-021-01335-2
41. Saely S, Croteau D, Jawidzik L, et al. Hypertension: a new safety risk for patients treated with erenumab. Headache. 2021;61:202-208. doi: 10.1111/head.14051
42. American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1-18. doi: 10.1111/head.13456
43. Burch R. Headache in pregnancy and the puerperium. Neurol Clin. 2019;37:31-51. doi: 10.1016/j.ncl.2018.09.004
44. Burch R. Epidemiology and treatment of menstrual migraine and migraine during pregnancy and lactation: a narrative review. Headache. 2020;60:200-216. doi: 10.1111/head.13665
45. Lexi-Comp. Lexi-drug database. Accessed April 4, 2022. https://online.lexi.com/lco/action/login
46. Walker N. Biologics: driving force in pharma. Pharma’s Almanac. June 5, 2017. Accessed June 15, 2020. www.pharmasalmanac.com/articles/biologics-driving-force-in-pharma
Small-molecule drugs such as aspirin, albuterol, atorvastatin, and lisinopril are the backbone of disease management in family medicine.1 However, large-molecule biological drugs such as monoclonal antibodies (MAbs) are increasingly prescribed to treat common conditions. In the past decade, MAbs comprised 20% of all drug approvals by the US Food and Drug Administration (FDA), and today they represent more than half of drugs currently in development.2 Fifteen MAbs have been approved by the FDA over the past decade for asthma, atopic dermatitis (AD), hyperlipidemia, osteoporosis, and migraine prevention.3 This review details what makes MAbs unique and what you should know about them.
The uniqueness of monoclonal antibodies
MAbs are biologics, but not all biologics are MAbs—eg, adalimumab (Humira) is a MAb, but etanercept (Enbrel) is not. MAbs are therapeutic proteins made possible by hybridoma technology used to create an antibody with single specificity.4-6 Monoclonal antibodies differ from small-molecule drugs in structure, dosing, route of administration, manufacturing, metabolism, drug interactions, and elimination (TABLE 17-9).

MAbs can be classified as naked, “without any drug or radioactive material attached to them,” or conjugated, “joined to a chemotherapy drug, radioactive isotope, or toxin.”10 MAbs work in several ways, including competitively inhibiting ligand-receptor binding, receptor blockade, or cell elimination from indirect immune system activities such as antibody-dependent cell-mediated cytotoxicity.11,12
Monoclonal antibody uses in family medicine
Asthma
Several MAbs have been approved for use in severe asthma, including but not limited to: omalizumab (Xolair),13 mepolizumab (Nucala),9,14 and dupilumab (Dupixent).15
Omalizumab is a humanized MAb that prevents IgE antibodies from binding to mast cells and basophils, thereby reducing inflammatory mediators.13 A systematic review found that, compared with placebo, omalizumab used in patients with inadequately controlled moderate-to-severe asthma led to significantly fewer asthma exacerbations (absolute risk reduction [ARR], 16% vs 26%; odds ratio [OR] = 0.55; 95% CI, 0.42-0.60; number needed to treat [NNT] = 10) and fewer hospitalizations (ARR, 0.5% vs 3%; OR = 0.16; 95% CI, 0.06-0.42; NNT = 40).13
Significantly more patients in the omalizumab group were able to withdraw from, or reduce, the dose of ICS. GINA recommends omalizumab for patients with positive skin sensitization, total serum IgE ≥ 30 IU/mL, weight within 30 kg to 150 kg, history of childhood asthma and recent exacerbations, and blood eosinophils ≥ 260/mcL.16 Omalizumab is also approved for use in chronic spontaneous urticaria and nasal polyps.
Mepolizumab
Continue to: Another trial found that...
Another trial found that mepolizumab reduced total OCS doses in patients with severe asthma by 50% without increasing exacerbations or worsening asthma control.18 All 3 anti-IL-5 drugs—including not only mepolizumab, but also benralizumab (Fasenra) and reslizumab (Cinqair)—appear to yield similar improvements. A 2017 systematic review found all anti-IL-5 treatments reduced rates of clinically significant asthma exacerbations (treatment with OCS for ≥ 3 days) by roughly 50% in patients with severe eosinophilic asthma and a history of ≥ 2 exacerbations in the past year.
Dupilumab is a humanized MAb that inhibits IL-4 and IL-13, which influence multiple cell types involved in inflammation (eg, mast cells, eosinophils) and inflammatory mediators (histamine, leukotrienes, cytokines).15 In a recent study of patients with uncontrolled asthma, dupilumab 200 mg every 2 weeks compared with placebo showed a modest reduction in the annualized rate of severe asthma exacerbations (0.46 exacerbations vs 0.87, respectively). Dupilumab was effective in patients with blood eosinophil counts ≥ 150/μL but was ineffective in patients with eosinophil counts < 150/μL.15
For patients ≥ 12 years old with severe eosinophilic asthma, GINA recommends using dupilumab as add-on therapy for an initial trial of 4 months at doses of 200 or 300 mg SC every 2 weeks, with preference for 300 mg SC every 2 weeks for OCS-dependent asthma. Dupilumab is approved for use in AD and chronic rhinosinusitis with nasal polyposis. If a biologic agent is not successful after a 4-month trial, consider a 6- to 12-month trial. If efficacy is still minimal, consider switching to an alternative biologic therapy approved for asthma.16
❯ Asthma: Test your skills
Subjective findings: A 19-year-old man presents to your clinic. He has a history of nasal polyps and allergic asthma. At age 18, he was given a diagnosis of severe persistent asthma. He has shortness of breath during waking hours 4 times per week, and treats each of these episodes with albuterol. He also wakes up about twice a week with shortness of breath and has some limitations in normal activities. He reports missing his prescribed fluticasone/salmeterol 500/50 μg, 1 inhalation bid, only once each month. In the last year, he has had 2 exacerbations requiring oral steroids.
Medications: Albuterol 90 μg, 1-2 inhalations, q6h prn; fluticasone/salmeterol 500/50 μg, 1 inhalation bid; tiotropium 1.25 μg, 2 puffs/d; montelukast 10 mg every morning; prednisone 10 mg/d.
Continue to: Objective data
Objective data: Patient is in no apparent distress and afebrile, and oxygen saturation on room air is 97%. Ht, 70 inches; wt, 75 kg. Labs: IgE, 15 IU/mL; serum eosinophils, 315/μL.
Which MAb would be appropriate for this patient? Given that the patient has a blood eosinophil level ≥ 300/μL and severe exacerbations, adult-onset asthma, nasal polyposis, and maintenance OCS at baseline, it would be reasonable to initiate mepolizumab 100 mg SC every 4 weeks, or dupilumab 600 mg once, then 300 mg SC every 2 weeks. Both agents can be self-administered.
Atopic dermatitis
Two MAbs—dupilumab and tralokinumab (Adbry; inhibits IL-13)—are approved for treatment of AD in adults that is uncontrolled with conventional therapy.15,19 Dupilumab is also approved for children ≥ 6 months old.20 Both MAbs are dosed at 600 mg SC, followed by 300 mg every 2 weeks. Dupilumab was compared with placebo in adult patients who had moderate-to-severe AD inadequately controlled on topical corticosteroids (TCSs), to determine the proportion of patients in each group achieving improvement of either 0 or 1 points or ≥ 2 points in the 5-point Investigator Global Assessment (IGA) score from baseline to 16 weeks.21 Thirty-seven percent of patients receiving dupilumab 300 mg SC weekly and 38% of patients receiving dupilumab 300 mg SC every 2 weeks achieved the primary outcome, compared with 10% of those receiving placebo (P < .001).21 Similar IGA scores were reported when dupilumab was combined with TCS, compared with placebo.22
It would be reasonable to consider dupilumab or tralokinumab in patients with: cutaneous atrophy or hypothalamic-pituitary-adrenal axis suppression with TCS, concerns of malignancy with topical calcineurin inhibitors, or problems with the alternative systemic therapies (cyclosporine-induced hypertension, nephrotoxicity, or immunosuppression; azathioprine-induced malignancy; or methotrexate-induced bone marrow suppression, renal impairment, hepatotoxicity, pneumonitis, or gastrointestinal toxicity).23
A distinct advantage of MAbs over other systemic agents in the management of AD is that MAbs do not require frequent monitoring of blood pressure, renal or liver function, complete blood count with differential, electrolytes, or uric acid. Additionally, MAbs have fewer black box warnings and adverse reactions when compared with other systemic agents.
Continue to: Hyperlipidemia
Hyperlipidemia
Three MAbs are approved for use in hyperlipidemia: the angiopoietin-like protein 3 (ANGPTL3) inhibitor evinacumab (Evkeeza)24 and 2 proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, evolocumab (Repatha)25 and alirocumab (Praluent).26
ANGPTL3 inhibitors block ANGPTL3 and reduce endothelial lipase and lipoprotein lipase activity, which in turn decreases low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride formation. PCSK9 inhibitors prevent PCSK9 from binding to LDL receptors, thereby maintaining the number of active LDL receptors and increasing LDL-C removal.
Evinacumab is indicated for homozygous familial hypercholesterolemia and is administered intravenously every 4 weeks. Evinacumab has not been evaluated for effects on cardiovascular morbidity and mortality.
Evolocumab 140 mg SC every 2 weeks or 420 mg SC monthly has been studied in patients on statin therapy with LDL-C ≥ 70 mg/dL. Patients on evolocumab experienced significantly less of the composite endpoint of cardiovascular death, myocardial infarction (MI), stroke, hospitalization for unstable angina, or coronary revascularization compared with placebo (9.8% vs 11.3%; hazard ratio [HR] = 0.85; 95% CI, 0.79-0.92; NNT = 67.27
Alirocumab 75 mg SC every 2 weeks has also been studied in patients receiving statin therapy with LDL-C ≥ 70 mg/dL. Patients taking alirocumab experienced significantly less of the composite endpoint of death from coronary heart disease, nonfatal MI, ischemic stroke, or hospitalization for unstable angina compared with placebo (9.5% vs 11.1%; HR = 0.85; 95% CI, 0.78-0.93; NNT = 63).
Continue to: According to the 2018...
According to the 2018 AHA Cholesterol Guidelines, PCSK9 inhibitors are indicated for patients receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe) with LDL-C ≥ 70 mg/dL, if they have had multiple atherosclerotic cardiovascular disease (ASCVD) events or 1 major ASCVD event with multiple high-risk conditions (eg, heterozygous familial hypercholesterolemia, history of coronary artery bypass grafting or percutaneous coronary intervention, hypertension, estimated glomerular filtration rate of 15 to 59 mL/min/1.73m2).29 For patients without prior ASCVD events or high-risk conditions who are receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe), PCSK9 inhibitors are indicated if the LDL-C remains ≥ 100 mg/dL.
Osteoporosis
The 2 MAbs approved for use in osteoporosis are the receptor activator of nuclear factor kB ligand (RANKL) inhibitor denosumab (Prolia)30 and the sclerostin inhibitor romosozumab (Evenity).31
Denosumab prevents RANKL from binding to the RANK receptor, thereby inhibiting osteoclast formation and decreasing bone resorption. Denosumab is approved for use in women and men who are at high risk of osteoporotic fracture, including those taking OCSs, men receiving androgen deprivation therapy for prostate cancer, and women receiving adjuvant aromatase inhibitor therapy for breast cancer.
In a 3-year randomized trial, denosumab 60 mg SC every 6 months was compared with placebo in postmenopausal women with T-scores < –2.5, but not < –4.0 at the lumbar spine or total hip. Denosumab significantly reduced new radiographic vertebral fractures (2.3% vs 7.2%; risk ratio [RR] = 0.32; 95% CI, 0.26-0.41; NNT = 21), hip fracture (0.7% vs 1.2%), and nonvertebral fracture (6.5% vs 8.0%).32 Denosumab carries an increased risk of multiple vertebral fractures following discontinuation, skin infections, dermatologic reactions, and severe bone, joint, and muscle pain.
Romosozumab inhibits sclerostin, thereby increasing bone formation and, to a lesser degree, decreasing bone resorption. Romosozumab is approved for use in postmenopausal women at high risk for fracture (ie, those with a history of osteoporotic fracture or multiple risk factors for fracture) or in patients who have not benefited from or are intolerant of other therapies. In one study, postmenopausal women with a T-score of –2.5 to –3.5 at the total hip or femoral neck were randomly assigned to receive either romosozumab 210 mg SC or placebo for 12 months, then each group was switched to denosumab 60 mg SC for 12 months. After the first year, prior to initiating denosumab, patients taking romosozumab experienced significantly fewer new vertebral fractures than patients taking placebo (0.5% vs 1.8%; RR = 0.27; 95% CI, 0.16-0.47; NNT = 77); however, there was no significant difference between the 2 groups with nonvertebral fractures (HR = 0.75; 95% CI, 0.53-1.05).33
Continue to: In another study...
In another study, romosozumab 210 mg SC was compared with alendronate 70 mg weekly, followed by alendronate 70 mg weekly in both groups. Over the first 12 months, patients treated with romosozumab saw a significant reduction in the incidence of new vertebral fractures (4% vs 6.3%; RR = 0.63, P < .003; NNT = 44). Patients treated with romosozumab with alendronate added for another 12 months also saw a significant reduction in new incidence of vertebral fractures (6.2% vs 11.9%; RR = 0.52; P < .001; NNT = 18).34 There was a higher risk of cardiovascular events among patients receiving romosozumab compared with those treated with alendronate, so romosozumab should not be used in individuals who have had an MI or stroke within the previous year.34 Denosumab and romosozumab offer an advantage over some bisphosphonates in that they require less frequent dosing and can be used in patients with renal impairment (creatinine clearance < 35 mL/min, in which zoledronic acid is contraindicated and alendronate is not recommended; < 30 mL/min, in which risedronate and ibandronate are not recommended).
Migraine prevention
Four
Erenumab, fremanezumab, and galcanezumab are all available in subcutaneous autoinjectors (or syringe with fremanezumab). Eptinezumab is an intravenous (IV) infusion given every 3 months.
Erenumab is available in both 70-mg and 140-mg dosing options. Fremanezumab can be given as 225 mg monthly or 675 mg quarterly. Galcanezumab has an initial loading dose of 240 mg followed by 120 mg given monthly. Erenumab targets the CGRP receptor; the others target the CGRP ligand. Eptinezumab has 100% bioavailability and reaches maximum serum concentration sooner than the other antagonists (due to its route of administration), but it must be given in an infusion center. Few insurers approve the use of eptinezumab unless a trial of least 1 of the monthly injectables has failed.
There are no head-to-head studies of the medications in this class. Additionally, differing study designs, definitions, statistical analyses, endpoints, and responder-rate calculations make it challenging to compare them directly against one another. At the very least, all of the CGRP MAbs have efficacy comparable to conventional preventive migraine medications such as propranolol, amitriptyline, and topiramate.40
Continue to: The most commonly reported adverse...
The most commonly reported adverse effect for all 4 CGRPs is injection site reaction, which was highest with the quarterly fremanezumab dose (45%).37 Constipation was most notable with the 140-mg dose of erenumab (3%)35; with the other CGRP MAbs it is comparable to that seen with placebo (< 1%).
Erenumab-induced hypertension has been identified in 61 cases reported through the FDA Adverse Event Reporting System (FAERS) as of 2021.41 This was not reported during MAb development programs, nor was it noted during clinical trials. Blood pressure elevation was seen within 1 week of injection in nearly 50% of the cases, and nearly one-third had pre-existing hypertension.41 Due to these findings, the erenumab prescribing information was updated to include hypertension in its warnings and precautions. It is possible that hypertension could be a class effect, although trial data and posthoc studies have yet to bear that out. Since erenumab was the first CGRP antagonist brought to market (May 2018 vs September 2018 for fremanezumab and galcanezumab), it may have accumulated more FAERS reports. Nearly all studies exclude patients with older age, uncontrolled hypertension, and unstable cardiovascular disease, which could impact data.41
Overall, this class of medications is very well tolerated, easy to use (again, excluding eptinezumab), and maintains a low adverse effect profile, giving added value compared with conventional preventive migraine medications.
The American Headache Society recommends a preventive oral therapy for at least 3 months before trying an alternative medication. After treatment failure with at least 2 oral agents, CGRP MAbs are recommended.42 CGRP antagonists offer convenient dosing, bypass gastrointestinal metabolism (which is useful in patients with nausea/vomiting), and have fewer adverse effects than traditional oral medications.
Worth noting. Several newer oral agents have been recently approved for migraine prevention, including atogepant (Qulipta) and rimegepant (Nurtec), which are also CGRP antagonists. Rimegepant is approved for both acute migraine treatment and prevention.
Continue to: Migraine
❯ Migraine: Test your skills
Subjective findings: A 25-year-old woman presents to your clinic for management of episodic migraines with aura. Her baseline average migraine frequency is 9 headache days/month. Her migraines are becoming more frequent despite treatment. She fears IV medication use and avoids hospitals.
History: Hypertension, irritable bowel syndrome with constipation (IBS-C), and depression. The patient is not pregnant or trying to get pregnant.
Medications: Current medications (for previous 4 months) include propranolol 40 mg at bedtime, linaclotide 145 μg/d, citalopram 20 mg/d, and sumatriptan 50 mg prn. Past medications include venlafaxine 150 mg po bid for 5 months.
What would be appropriate for this patient? This patient meets the criteria for using a CGRP antagonist because she has tried 2 preventive treatments for more than 60 to 90 days. Erenumab is not the best option, given the patient’s history of hypertension and IBS-C. The patient fears hospitals and IV medications, making eptinezumab a less-than-ideal choice. Depending on her insurance, fremanezumab or galcanezumab would be good options at this time.
CGRP antagonists have not been studied or evaluated in pregnancy, but if this patient becomes pregnant, a first-line agent for prevention would be propranolol, and a second-line agent would be a tricyclic antidepressant, memantine, or verapamil. Avoid ergotamines and antiepileptics (topiramate or valproate) in pregnancy.43,44
Continue to: The challenges associated with MAbs
The challenges associated with MAbs
MAbs can be expensive (TABLE 2),45 some prohibitively so. On a population scale, biologics account for around 40% of prescription drug spending and may cost 22 times more than small-molecule drugs.46 Estimates in 2016 showed that MAbs comprise $90.2 billion (43%) of the biologic market.46

MAbs also require prior authorization forms to be submitted. Prior authorization criteria vary by state and by insurance plan. In my (ES) experience, submitting letters of medical necessity justifying the need for therapy or expertise in the disease states for which the MAb is being prescribed help your patient get the medication they need.
Expect to see additional MAbs approved in the future. If the costs come down, adoption of these agents into practice will likely increase.
CORRESPONDENCE
Evelyn Sbar, MD, Texas Tech University Health Sciences Center, 1400 South Coulter Street, Suite 5100, Amarillo, TX 79106; [email protected]
Small-molecule drugs such as aspirin, albuterol, atorvastatin, and lisinopril are the backbone of disease management in family medicine.1 However, large-molecule biological drugs such as monoclonal antibodies (MAbs) are increasingly prescribed to treat common conditions. In the past decade, MAbs comprised 20% of all drug approvals by the US Food and Drug Administration (FDA), and today they represent more than half of drugs currently in development.2 Fifteen MAbs have been approved by the FDA over the past decade for asthma, atopic dermatitis (AD), hyperlipidemia, osteoporosis, and migraine prevention.3 This review details what makes MAbs unique and what you should know about them.
The uniqueness of monoclonal antibodies
MAbs are biologics, but not all biologics are MAbs—eg, adalimumab (Humira) is a MAb, but etanercept (Enbrel) is not. MAbs are therapeutic proteins made possible by hybridoma technology used to create an antibody with single specificity.4-6 Monoclonal antibodies differ from small-molecule drugs in structure, dosing, route of administration, manufacturing, metabolism, drug interactions, and elimination (TABLE 17-9).

MAbs can be classified as naked, “without any drug or radioactive material attached to them,” or conjugated, “joined to a chemotherapy drug, radioactive isotope, or toxin.”10 MAbs work in several ways, including competitively inhibiting ligand-receptor binding, receptor blockade, or cell elimination from indirect immune system activities such as antibody-dependent cell-mediated cytotoxicity.11,12
Monoclonal antibody uses in family medicine
Asthma
Several MAbs have been approved for use in severe asthma, including but not limited to: omalizumab (Xolair),13 mepolizumab (Nucala),9,14 and dupilumab (Dupixent).15
Omalizumab is a humanized MAb that prevents IgE antibodies from binding to mast cells and basophils, thereby reducing inflammatory mediators.13 A systematic review found that, compared with placebo, omalizumab used in patients with inadequately controlled moderate-to-severe asthma led to significantly fewer asthma exacerbations (absolute risk reduction [ARR], 16% vs 26%; odds ratio [OR] = 0.55; 95% CI, 0.42-0.60; number needed to treat [NNT] = 10) and fewer hospitalizations (ARR, 0.5% vs 3%; OR = 0.16; 95% CI, 0.06-0.42; NNT = 40).13
Significantly more patients in the omalizumab group were able to withdraw from, or reduce, the dose of ICS. GINA recommends omalizumab for patients with positive skin sensitization, total serum IgE ≥ 30 IU/mL, weight within 30 kg to 150 kg, history of childhood asthma and recent exacerbations, and blood eosinophils ≥ 260/mcL.16 Omalizumab is also approved for use in chronic spontaneous urticaria and nasal polyps.
Mepolizumab
Continue to: Another trial found that...
Another trial found that mepolizumab reduced total OCS doses in patients with severe asthma by 50% without increasing exacerbations or worsening asthma control.18 All 3 anti-IL-5 drugs—including not only mepolizumab, but also benralizumab (Fasenra) and reslizumab (Cinqair)—appear to yield similar improvements. A 2017 systematic review found all anti-IL-5 treatments reduced rates of clinically significant asthma exacerbations (treatment with OCS for ≥ 3 days) by roughly 50% in patients with severe eosinophilic asthma and a history of ≥ 2 exacerbations in the past year.
Dupilumab is a humanized MAb that inhibits IL-4 and IL-13, which influence multiple cell types involved in inflammation (eg, mast cells, eosinophils) and inflammatory mediators (histamine, leukotrienes, cytokines).15 In a recent study of patients with uncontrolled asthma, dupilumab 200 mg every 2 weeks compared with placebo showed a modest reduction in the annualized rate of severe asthma exacerbations (0.46 exacerbations vs 0.87, respectively). Dupilumab was effective in patients with blood eosinophil counts ≥ 150/μL but was ineffective in patients with eosinophil counts < 150/μL.15
For patients ≥ 12 years old with severe eosinophilic asthma, GINA recommends using dupilumab as add-on therapy for an initial trial of 4 months at doses of 200 or 300 mg SC every 2 weeks, with preference for 300 mg SC every 2 weeks for OCS-dependent asthma. Dupilumab is approved for use in AD and chronic rhinosinusitis with nasal polyposis. If a biologic agent is not successful after a 4-month trial, consider a 6- to 12-month trial. If efficacy is still minimal, consider switching to an alternative biologic therapy approved for asthma.16
❯ Asthma: Test your skills
Subjective findings: A 19-year-old man presents to your clinic. He has a history of nasal polyps and allergic asthma. At age 18, he was given a diagnosis of severe persistent asthma. He has shortness of breath during waking hours 4 times per week, and treats each of these episodes with albuterol. He also wakes up about twice a week with shortness of breath and has some limitations in normal activities. He reports missing his prescribed fluticasone/salmeterol 500/50 μg, 1 inhalation bid, only once each month. In the last year, he has had 2 exacerbations requiring oral steroids.
Medications: Albuterol 90 μg, 1-2 inhalations, q6h prn; fluticasone/salmeterol 500/50 μg, 1 inhalation bid; tiotropium 1.25 μg, 2 puffs/d; montelukast 10 mg every morning; prednisone 10 mg/d.
Continue to: Objective data
Objective data: Patient is in no apparent distress and afebrile, and oxygen saturation on room air is 97%. Ht, 70 inches; wt, 75 kg. Labs: IgE, 15 IU/mL; serum eosinophils, 315/μL.
Which MAb would be appropriate for this patient? Given that the patient has a blood eosinophil level ≥ 300/μL and severe exacerbations, adult-onset asthma, nasal polyposis, and maintenance OCS at baseline, it would be reasonable to initiate mepolizumab 100 mg SC every 4 weeks, or dupilumab 600 mg once, then 300 mg SC every 2 weeks. Both agents can be self-administered.
Atopic dermatitis
Two MAbs—dupilumab and tralokinumab (Adbry; inhibits IL-13)—are approved for treatment of AD in adults that is uncontrolled with conventional therapy.15,19 Dupilumab is also approved for children ≥ 6 months old.20 Both MAbs are dosed at 600 mg SC, followed by 300 mg every 2 weeks. Dupilumab was compared with placebo in adult patients who had moderate-to-severe AD inadequately controlled on topical corticosteroids (TCSs), to determine the proportion of patients in each group achieving improvement of either 0 or 1 points or ≥ 2 points in the 5-point Investigator Global Assessment (IGA) score from baseline to 16 weeks.21 Thirty-seven percent of patients receiving dupilumab 300 mg SC weekly and 38% of patients receiving dupilumab 300 mg SC every 2 weeks achieved the primary outcome, compared with 10% of those receiving placebo (P < .001).21 Similar IGA scores were reported when dupilumab was combined with TCS, compared with placebo.22
It would be reasonable to consider dupilumab or tralokinumab in patients with: cutaneous atrophy or hypothalamic-pituitary-adrenal axis suppression with TCS, concerns of malignancy with topical calcineurin inhibitors, or problems with the alternative systemic therapies (cyclosporine-induced hypertension, nephrotoxicity, or immunosuppression; azathioprine-induced malignancy; or methotrexate-induced bone marrow suppression, renal impairment, hepatotoxicity, pneumonitis, or gastrointestinal toxicity).23
A distinct advantage of MAbs over other systemic agents in the management of AD is that MAbs do not require frequent monitoring of blood pressure, renal or liver function, complete blood count with differential, electrolytes, or uric acid. Additionally, MAbs have fewer black box warnings and adverse reactions when compared with other systemic agents.
Continue to: Hyperlipidemia
Hyperlipidemia
Three MAbs are approved for use in hyperlipidemia: the angiopoietin-like protein 3 (ANGPTL3) inhibitor evinacumab (Evkeeza)24 and 2 proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, evolocumab (Repatha)25 and alirocumab (Praluent).26
ANGPTL3 inhibitors block ANGPTL3 and reduce endothelial lipase and lipoprotein lipase activity, which in turn decreases low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride formation. PCSK9 inhibitors prevent PCSK9 from binding to LDL receptors, thereby maintaining the number of active LDL receptors and increasing LDL-C removal.
Evinacumab is indicated for homozygous familial hypercholesterolemia and is administered intravenously every 4 weeks. Evinacumab has not been evaluated for effects on cardiovascular morbidity and mortality.
Evolocumab 140 mg SC every 2 weeks or 420 mg SC monthly has been studied in patients on statin therapy with LDL-C ≥ 70 mg/dL. Patients on evolocumab experienced significantly less of the composite endpoint of cardiovascular death, myocardial infarction (MI), stroke, hospitalization for unstable angina, or coronary revascularization compared with placebo (9.8% vs 11.3%; hazard ratio [HR] = 0.85; 95% CI, 0.79-0.92; NNT = 67.27
Alirocumab 75 mg SC every 2 weeks has also been studied in patients receiving statin therapy with LDL-C ≥ 70 mg/dL. Patients taking alirocumab experienced significantly less of the composite endpoint of death from coronary heart disease, nonfatal MI, ischemic stroke, or hospitalization for unstable angina compared with placebo (9.5% vs 11.1%; HR = 0.85; 95% CI, 0.78-0.93; NNT = 63).
Continue to: According to the 2018...
According to the 2018 AHA Cholesterol Guidelines, PCSK9 inhibitors are indicated for patients receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe) with LDL-C ≥ 70 mg/dL, if they have had multiple atherosclerotic cardiovascular disease (ASCVD) events or 1 major ASCVD event with multiple high-risk conditions (eg, heterozygous familial hypercholesterolemia, history of coronary artery bypass grafting or percutaneous coronary intervention, hypertension, estimated glomerular filtration rate of 15 to 59 mL/min/1.73m2).29 For patients without prior ASCVD events or high-risk conditions who are receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe), PCSK9 inhibitors are indicated if the LDL-C remains ≥ 100 mg/dL.
Osteoporosis
The 2 MAbs approved for use in osteoporosis are the receptor activator of nuclear factor kB ligand (RANKL) inhibitor denosumab (Prolia)30 and the sclerostin inhibitor romosozumab (Evenity).31
Denosumab prevents RANKL from binding to the RANK receptor, thereby inhibiting osteoclast formation and decreasing bone resorption. Denosumab is approved for use in women and men who are at high risk of osteoporotic fracture, including those taking OCSs, men receiving androgen deprivation therapy for prostate cancer, and women receiving adjuvant aromatase inhibitor therapy for breast cancer.
In a 3-year randomized trial, denosumab 60 mg SC every 6 months was compared with placebo in postmenopausal women with T-scores < –2.5, but not < –4.0 at the lumbar spine or total hip. Denosumab significantly reduced new radiographic vertebral fractures (2.3% vs 7.2%; risk ratio [RR] = 0.32; 95% CI, 0.26-0.41; NNT = 21), hip fracture (0.7% vs 1.2%), and nonvertebral fracture (6.5% vs 8.0%).32 Denosumab carries an increased risk of multiple vertebral fractures following discontinuation, skin infections, dermatologic reactions, and severe bone, joint, and muscle pain.
Romosozumab inhibits sclerostin, thereby increasing bone formation and, to a lesser degree, decreasing bone resorption. Romosozumab is approved for use in postmenopausal women at high risk for fracture (ie, those with a history of osteoporotic fracture or multiple risk factors for fracture) or in patients who have not benefited from or are intolerant of other therapies. In one study, postmenopausal women with a T-score of –2.5 to –3.5 at the total hip or femoral neck were randomly assigned to receive either romosozumab 210 mg SC or placebo for 12 months, then each group was switched to denosumab 60 mg SC for 12 months. After the first year, prior to initiating denosumab, patients taking romosozumab experienced significantly fewer new vertebral fractures than patients taking placebo (0.5% vs 1.8%; RR = 0.27; 95% CI, 0.16-0.47; NNT = 77); however, there was no significant difference between the 2 groups with nonvertebral fractures (HR = 0.75; 95% CI, 0.53-1.05).33
Continue to: In another study...
In another study, romosozumab 210 mg SC was compared with alendronate 70 mg weekly, followed by alendronate 70 mg weekly in both groups. Over the first 12 months, patients treated with romosozumab saw a significant reduction in the incidence of new vertebral fractures (4% vs 6.3%; RR = 0.63, P < .003; NNT = 44). Patients treated with romosozumab with alendronate added for another 12 months also saw a significant reduction in new incidence of vertebral fractures (6.2% vs 11.9%; RR = 0.52; P < .001; NNT = 18).34 There was a higher risk of cardiovascular events among patients receiving romosozumab compared with those treated with alendronate, so romosozumab should not be used in individuals who have had an MI or stroke within the previous year.34 Denosumab and romosozumab offer an advantage over some bisphosphonates in that they require less frequent dosing and can be used in patients with renal impairment (creatinine clearance < 35 mL/min, in which zoledronic acid is contraindicated and alendronate is not recommended; < 30 mL/min, in which risedronate and ibandronate are not recommended).
Migraine prevention
Four
Erenumab, fremanezumab, and galcanezumab are all available in subcutaneous autoinjectors (or syringe with fremanezumab). Eptinezumab is an intravenous (IV) infusion given every 3 months.
Erenumab is available in both 70-mg and 140-mg dosing options. Fremanezumab can be given as 225 mg monthly or 675 mg quarterly. Galcanezumab has an initial loading dose of 240 mg followed by 120 mg given monthly. Erenumab targets the CGRP receptor; the others target the CGRP ligand. Eptinezumab has 100% bioavailability and reaches maximum serum concentration sooner than the other antagonists (due to its route of administration), but it must be given in an infusion center. Few insurers approve the use of eptinezumab unless a trial of least 1 of the monthly injectables has failed.
There are no head-to-head studies of the medications in this class. Additionally, differing study designs, definitions, statistical analyses, endpoints, and responder-rate calculations make it challenging to compare them directly against one another. At the very least, all of the CGRP MAbs have efficacy comparable to conventional preventive migraine medications such as propranolol, amitriptyline, and topiramate.40
Continue to: The most commonly reported adverse...
The most commonly reported adverse effect for all 4 CGRPs is injection site reaction, which was highest with the quarterly fremanezumab dose (45%).37 Constipation was most notable with the 140-mg dose of erenumab (3%)35; with the other CGRP MAbs it is comparable to that seen with placebo (< 1%).
Erenumab-induced hypertension has been identified in 61 cases reported through the FDA Adverse Event Reporting System (FAERS) as of 2021.41 This was not reported during MAb development programs, nor was it noted during clinical trials. Blood pressure elevation was seen within 1 week of injection in nearly 50% of the cases, and nearly one-third had pre-existing hypertension.41 Due to these findings, the erenumab prescribing information was updated to include hypertension in its warnings and precautions. It is possible that hypertension could be a class effect, although trial data and posthoc studies have yet to bear that out. Since erenumab was the first CGRP antagonist brought to market (May 2018 vs September 2018 for fremanezumab and galcanezumab), it may have accumulated more FAERS reports. Nearly all studies exclude patients with older age, uncontrolled hypertension, and unstable cardiovascular disease, which could impact data.41
Overall, this class of medications is very well tolerated, easy to use (again, excluding eptinezumab), and maintains a low adverse effect profile, giving added value compared with conventional preventive migraine medications.
The American Headache Society recommends a preventive oral therapy for at least 3 months before trying an alternative medication. After treatment failure with at least 2 oral agents, CGRP MAbs are recommended.42 CGRP antagonists offer convenient dosing, bypass gastrointestinal metabolism (which is useful in patients with nausea/vomiting), and have fewer adverse effects than traditional oral medications.
Worth noting. Several newer oral agents have been recently approved for migraine prevention, including atogepant (Qulipta) and rimegepant (Nurtec), which are also CGRP antagonists. Rimegepant is approved for both acute migraine treatment and prevention.
Continue to: Migraine
❯ Migraine: Test your skills
Subjective findings: A 25-year-old woman presents to your clinic for management of episodic migraines with aura. Her baseline average migraine frequency is 9 headache days/month. Her migraines are becoming more frequent despite treatment. She fears IV medication use and avoids hospitals.
History: Hypertension, irritable bowel syndrome with constipation (IBS-C), and depression. The patient is not pregnant or trying to get pregnant.
Medications: Current medications (for previous 4 months) include propranolol 40 mg at bedtime, linaclotide 145 μg/d, citalopram 20 mg/d, and sumatriptan 50 mg prn. Past medications include venlafaxine 150 mg po bid for 5 months.
What would be appropriate for this patient? This patient meets the criteria for using a CGRP antagonist because she has tried 2 preventive treatments for more than 60 to 90 days. Erenumab is not the best option, given the patient’s history of hypertension and IBS-C. The patient fears hospitals and IV medications, making eptinezumab a less-than-ideal choice. Depending on her insurance, fremanezumab or galcanezumab would be good options at this time.
CGRP antagonists have not been studied or evaluated in pregnancy, but if this patient becomes pregnant, a first-line agent for prevention would be propranolol, and a second-line agent would be a tricyclic antidepressant, memantine, or verapamil. Avoid ergotamines and antiepileptics (topiramate or valproate) in pregnancy.43,44
Continue to: The challenges associated with MAbs
The challenges associated with MAbs
MAbs can be expensive (TABLE 2),45 some prohibitively so. On a population scale, biologics account for around 40% of prescription drug spending and may cost 22 times more than small-molecule drugs.46 Estimates in 2016 showed that MAbs comprise $90.2 billion (43%) of the biologic market.46

MAbs also require prior authorization forms to be submitted. Prior authorization criteria vary by state and by insurance plan. In my (ES) experience, submitting letters of medical necessity justifying the need for therapy or expertise in the disease states for which the MAb is being prescribed help your patient get the medication they need.
Expect to see additional MAbs approved in the future. If the costs come down, adoption of these agents into practice will likely increase.
CORRESPONDENCE
Evelyn Sbar, MD, Texas Tech University Health Sciences Center, 1400 South Coulter Street, Suite 5100, Amarillo, TX 79106; [email protected]
1. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. National Center for Health Statistics. Accessed June 15, 2022. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
2. IDBS. The future of biologics drug development is today. June 27, 2018. Accessed June 15, 2022. www.idbs.com/blog/2018/06/the-future-of-biologics-drug-development-is-today/
3. Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society. Accessed June 15, 2022. www.antibodysociety.org/resources/approved-antibodies/
4. FDA. Code of Federal Regulations, Title 21, Chapter I, Subchapter F biologics. March 29, 2022. Accessed June 15, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.3
5. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495-497. doi: 10.1038/256495a0
6. Raejewsky K. The advent and rise of monoclonal antibodies. Nature. November 4, 2019. Accessed June 15, 2022. www.nature.com/articles/d41586-019-02840-w
7. Flovent. Prescribing information. GlaxoSmithKline; 2010. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2010/021433s015lbl.pdf
8. NLM. National Center for Biotechnology Information. PubChem. Method for the preparation of fluticasone and related 17beta-carbothioic esters using a novel carbothioic acid synthesis and novel purification methods. Accessed June 15, 2022. pubchem.ncbi.nlm.nih.gov/patent/WO-0162722-A2
9. Nucala. Prescribing information. GlaxoSmithKline; 2019. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761122s000lbl.pdf
10. Argyriou AA, Kalofonos HP. Recent advances relating to the clinical application of naked monoclonal antibodies in solid tumors. Mol Med. 2009;15:183-191. doi: 10.2119/molmed.2009.00007
11. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548-558. doi: 10.1038/clpt.2008.170
12. Zahavi D, AlDeghaither D, O’Connell A, et al. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antib Ther. 2018;1:7-12. doi: 10.1093/abt/tby002
13. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014:CD003559. doi: 10.1002/14651858.CD003559.pub4
14. Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834. doi: 10.1002/14651858.CD010834.pub3
15. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486-2496. doi: 10.1056/NEJMoa1804092
16. GINA. Global strategy for asthma management and prevention. 2022 Difficult-to-treat and severe asthma guide—slide set. Accessed June 23, 2022. https://ginasthma.org/severeasthma/
17. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207. doi: 10.1056/NEJMoa1403290
18. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197. doi: 10.1056/NEJMoa1403291
19. Adbry. Prescribing information. Leo Pharma Inc; 2021. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf
20. Dupixent. Prescribing information. Regeneron Pharmaceuticals; 2022. Accessed October 5, 2022. https://www.regeneron.com/downloads/dupixent_fpi.pdf
21. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi: 10.1056/NEJMoa1610020
22. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. doi: 10.1016/s0140-6736(17)31191-1
23. Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi: 10.1016/j.jaad.2014.03.030
24. Evkeeza. Prescribing information. Regeneron Pharmaceuticals; 2021. Accessed June 24, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761181s000lbl.pdf
25. Repatha. Prescribing information. Amgen; 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf
26. Praluent. Prescribing information. Sanofi Aventis and Regeneron Pharmaceuticals. 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s002lbl.pdf
27. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722. doi: 10.1056/NEJMoa1615664
28. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097-2107. doi:10.1056/NEJMoa1801174
29. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
30. Prolia. Prescribing information. Amgen; 2010. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf
31. Evenity. Prescribing information. Amgen; 2019. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761062s000lbl.pdf
32. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
33. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543. doi: 10.1056/NEJMoa1607948
34. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427. doi: 10.1056/NEJMoa1708322
35. Aimovig. Prescribing information. Amgen; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf
36. Vyepti. Prescribing information. Lundbeck Seattle BioPharmaceuticals; 2020. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf
37. Ajovy. Prescribing information. Teva Pharmaceuticals; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf
38. Emgality. Prescribing information. Eli Lilly and Co.; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf
39. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350. doi: 10.1038/s41582-018-0003-1
40. Vandervorst F. Van Deun L, Van Dycke A, et al. CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain. 2021;22:128. doi: 10.1186/s10194-021-01335-2
41. Saely S, Croteau D, Jawidzik L, et al. Hypertension: a new safety risk for patients treated with erenumab. Headache. 2021;61:202-208. doi: 10.1111/head.14051
42. American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1-18. doi: 10.1111/head.13456
43. Burch R. Headache in pregnancy and the puerperium. Neurol Clin. 2019;37:31-51. doi: 10.1016/j.ncl.2018.09.004
44. Burch R. Epidemiology and treatment of menstrual migraine and migraine during pregnancy and lactation: a narrative review. Headache. 2020;60:200-216. doi: 10.1111/head.13665
45. Lexi-Comp. Lexi-drug database. Accessed April 4, 2022. https://online.lexi.com/lco/action/login
46. Walker N. Biologics: driving force in pharma. Pharma’s Almanac. June 5, 2017. Accessed June 15, 2020. www.pharmasalmanac.com/articles/biologics-driving-force-in-pharma
1. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. National Center for Health Statistics. Accessed June 15, 2022. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
2. IDBS. The future of biologics drug development is today. June 27, 2018. Accessed June 15, 2022. www.idbs.com/blog/2018/06/the-future-of-biologics-drug-development-is-today/
3. Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society. Accessed June 15, 2022. www.antibodysociety.org/resources/approved-antibodies/
4. FDA. Code of Federal Regulations, Title 21, Chapter I, Subchapter F biologics. March 29, 2022. Accessed June 15, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.3
5. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495-497. doi: 10.1038/256495a0
6. Raejewsky K. The advent and rise of monoclonal antibodies. Nature. November 4, 2019. Accessed June 15, 2022. www.nature.com/articles/d41586-019-02840-w
7. Flovent. Prescribing information. GlaxoSmithKline; 2010. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2010/021433s015lbl.pdf
8. NLM. National Center for Biotechnology Information. PubChem. Method for the preparation of fluticasone and related 17beta-carbothioic esters using a novel carbothioic acid synthesis and novel purification methods. Accessed June 15, 2022. pubchem.ncbi.nlm.nih.gov/patent/WO-0162722-A2
9. Nucala. Prescribing information. GlaxoSmithKline; 2019. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761122s000lbl.pdf
10. Argyriou AA, Kalofonos HP. Recent advances relating to the clinical application of naked monoclonal antibodies in solid tumors. Mol Med. 2009;15:183-191. doi: 10.2119/molmed.2009.00007
11. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548-558. doi: 10.1038/clpt.2008.170
12. Zahavi D, AlDeghaither D, O’Connell A, et al. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antib Ther. 2018;1:7-12. doi: 10.1093/abt/tby002
13. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014:CD003559. doi: 10.1002/14651858.CD003559.pub4
14. Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834. doi: 10.1002/14651858.CD010834.pub3
15. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486-2496. doi: 10.1056/NEJMoa1804092
16. GINA. Global strategy for asthma management and prevention. 2022 Difficult-to-treat and severe asthma guide—slide set. Accessed June 23, 2022. https://ginasthma.org/severeasthma/
17. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207. doi: 10.1056/NEJMoa1403290
18. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197. doi: 10.1056/NEJMoa1403291
19. Adbry. Prescribing information. Leo Pharma Inc; 2021. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf
20. Dupixent. Prescribing information. Regeneron Pharmaceuticals; 2022. Accessed October 5, 2022. https://www.regeneron.com/downloads/dupixent_fpi.pdf
21. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi: 10.1056/NEJMoa1610020
22. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. doi: 10.1016/s0140-6736(17)31191-1
23. Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi: 10.1016/j.jaad.2014.03.030
24. Evkeeza. Prescribing information. Regeneron Pharmaceuticals; 2021. Accessed June 24, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761181s000lbl.pdf
25. Repatha. Prescribing information. Amgen; 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf
26. Praluent. Prescribing information. Sanofi Aventis and Regeneron Pharmaceuticals. 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s002lbl.pdf
27. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722. doi: 10.1056/NEJMoa1615664
28. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097-2107. doi:10.1056/NEJMoa1801174
29. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
30. Prolia. Prescribing information. Amgen; 2010. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf
31. Evenity. Prescribing information. Amgen; 2019. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761062s000lbl.pdf
32. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
33. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543. doi: 10.1056/NEJMoa1607948
34. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427. doi: 10.1056/NEJMoa1708322
35. Aimovig. Prescribing information. Amgen; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf
36. Vyepti. Prescribing information. Lundbeck Seattle BioPharmaceuticals; 2020. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf
37. Ajovy. Prescribing information. Teva Pharmaceuticals; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf
38. Emgality. Prescribing information. Eli Lilly and Co.; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf
39. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350. doi: 10.1038/s41582-018-0003-1
40. Vandervorst F. Van Deun L, Van Dycke A, et al. CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain. 2021;22:128. doi: 10.1186/s10194-021-01335-2
41. Saely S, Croteau D, Jawidzik L, et al. Hypertension: a new safety risk for patients treated with erenumab. Headache. 2021;61:202-208. doi: 10.1111/head.14051
42. American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1-18. doi: 10.1111/head.13456
43. Burch R. Headache in pregnancy and the puerperium. Neurol Clin. 2019;37:31-51. doi: 10.1016/j.ncl.2018.09.004
44. Burch R. Epidemiology and treatment of menstrual migraine and migraine during pregnancy and lactation: a narrative review. Headache. 2020;60:200-216. doi: 10.1111/head.13665
45. Lexi-Comp. Lexi-drug database. Accessed April 4, 2022. https://online.lexi.com/lco/action/login
46. Walker N. Biologics: driving force in pharma. Pharma’s Almanac. June 5, 2017. Accessed June 15, 2020. www.pharmasalmanac.com/articles/biologics-driving-force-in-pharma
PRACTICE RECOMMENDATIONS
› Consider anti-immunoglobulin E, anti-interleukin 5, or anti-interleukin 4/interleukin 13 for patients with moderate-to-severe asthma and type 2 airway inflammation. B
› Consider dupilumab for patients with moderate-to-severe atopic dermatitis (with or without topical corticosteroids), or when traditional oral therapies are inadequate or contraindicated. B
› Consider proprotein convertase subtilisin/kexin type 9 inhibitors for patients with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease when maximally tolerated statins or ezetimibe have not lowered low-density lipoprotein cholesterol levels far enough. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series