User login
The truth about the ‘happy hormone’: Why we shouldn’t mess with dopamine
Google the word “dopamine” and you will learn that its nicknames are the “happy hormone” and the “pleasure molecule” and that it is among the most important chemicals in our brains. With The Guardian branding it “the Kim Kardashian of neurotransmitters,” dopamine has become a true pop-science darling – people across the globe have attempted to boost their mood with dopamine fasts and dopamine dressing.
A century ago, however, newly discovered dopamine was seen as an uninspiring chemical, nothing more than a precursor of noradrenaline. It took several stubborn and hardworking scientists to change that view.
Levodopa: An indifferent precursor
When Casimir Funk, PhD, a Polish biochemist and the discoverer of vitamins, first synthesized the dopamine precursor levodopa in 1911, he had no idea how important the molecule would prove to be in pharmacology and neurobiology. Nor did Markus Guggenheim, PhD, a Swiss biochemist, who isolated levodopa in 1913 from the seeds of a broad bean, Vicia faba. Dr. Guggenheim administered 1 g of levodopa to a rabbit, with no apparent negative consequences. He then prepared a larger dose (2.5 g) and tested it on himself. “Ten minutes after taking it, I felt very nauseous, I had to vomit twice,” he wrote in his paper. In the body, levodopa is converted into dopamine, which may act as an emetic – an effect Dr. Guggenheim didn’t understand. He simply abandoned his human study, erroneously concluding, on the basis of his animal research, that levodopa is “pharmacologically fairly indifferent.”
Around the same time, several scientists across Europe successfully synthesized dopamine, but those discoveries were shelved without much fanfare. For the next 3 decades, dopamine and levodopa were pushed into academic obscurity. Just before World War II, a group of German scientists showed that levodopa is metabolized to dopamine in the body, while another German researcher, Hermann Blaschko, MD, discovered that dopamine is an intermediary in the synthesis of noradrenaline. Even these findings, however, were not immediately accepted.
The dopamine story picked up pace in the post-war years with the observation that the hormone was present in various tissues and body fluids, although nowhere as abundantly as in the central nervous system. Intrigued, Dr. Blaschko, who (after escaping Nazi Germany, changing his name to Hugh, and starting work at Oxford [England] University) hypothesized that dopamine couldn’t be an unremarkable precursor of noradrenaline – it had to have some physiologic functions of its own. He asked his postdoctoral fellow, Oheh Hornykiewicz, MD, to test a few ideas. Dr. Hornykiewicz soon confirmed that dopamine lowered blood pressure in guinea pigs, proving that dopamine indeed had physiologic activity that was independent of other catecholamines.
Reserpine and rabbit ears
While Dr. Blaschko and Dr. Hornykiewicz were puzzling over dopamine’s physiologic role in the body, across the ocean at the National Heart Institute in Maryland, pharmacologist Bernard Brodie, PhD and colleagues were laying the groundwork for the discovery of dopamine’s starring role in the brain.
Spoiler alert: Dr. Brodie’s work showed that a new psychiatric drug known as reserpine was capable of fully depleting the brain’s stores of serotonin and – of greatest significance, as it turned out – mimicking the neuromuscular symptoms typical of Parkinson’s disease. The connection to dopamine would be made by new lab colleague Arvid Carlsson, MD, PhD, who would go on to win a Nobel Prize.
Derived from Rauwolfia serpentina (a plant that for centuries has been used in India for the treatment of mental illness, insomnia, and snake bites), reserpine was introduced in the West as a treatment for schizophrenia.
It worked marvels. In 1954, the press lauded the “dramatic” and seemingly “incredible”: results in treating “hopelessly insane patients.” Reserpine had a downside, however. Reports soon changed in tone regarding the drug’s severe side effects, including headaches, dizziness, vomiting, and, far more disturbingly, symptoms mimicking Parkinson’s disease, from muscular rigidity to tremors.
Dr. Brodie observed that, when reserpine was injected, animals became completely immobile. Serotonin nearly vanished from their brains, but bizarrely, drugs that spur serotonin production did not reverse the rabbits’ immobility.
Dr. Carlsson realized that other catecholamines must be involved in reserpine’s side effects, and he began to search for the culprits. He moved back to his native Sweden and ordered a spectrophotofluorimeter. In one of his experiments, Carlsson injected a pair of rabbits with reserpine, which caused the animals to become catatonic with flattened ears. After the researchers injected the animals with levodopa, within 15 minutes, the rabbits were hopping around, ears proudly vertical. “We were just as excited as the rabbits,” Dr. Carlsson later recalled in a 2016 interview. Dr. Carlsson realized that, because there was no noradrenaline in the rabbits’ brains, dopamine depletion must have been directly responsible for producing reserpine’s motor inhibitory effects.
Skeptics are silenced
In 1960, however, the medical community was not yet ready to accept that dopamine was anything but a boring intermediate between levodopa and noradrenaline. At a prestigious London symposium, Dr. Carlsson and his two colleagues presented their hypothesis that dopamine may be a neurotransmitter, thus implicating it in Parkinson’s disease. They were met with harsh criticism. Some of the experts said levodopa was nothing more than a poison. Dr. Carlsson later recalled facing “a profound and nearly unanimous skepticism regarding our points of view.”
That would soon change. Dr. Hornykiewicz, the biochemist who had earlier discovered dopamine’s BP-lowering effects, tested Dr. Carlsson’s ideas using the postmortem brains of Parkinson’s disease patients. It appeared Dr. Carlsson was right: Unlike in healthy brains, the striatum of patients with Parkinson’s disease contained almost no dopamine whatsoever. Beginning in 1961, in collaboration with neurologist Walther Birkmayer, MD, Hornykiewicz injected levodopa into 20 patients with Parkinson’s disease and observed a “miraculous” (albeit temporary) amelioration of rigidity, motionlessness, and speechlessness.
By the late 1960s, levodopa and dopamine were making headlines. A 1969 New York Times article described similar stunning improvements in patients with Parkinson’s disease who were treated with levodopa. A patient who had arrived at a hospital unable to speak, with hands clenched and rigid expression, was suddenly able to stride into his doctor’s office and even jog around. “I might say I’m a human being,” he told reporters. Although the treatment was expensive – equivalent to $210 in 2022 – physicians were deluged with requests for “dopa.” To this day, levodopa remains a gold standard in the treatment of Parkinson’s disease.
Still misunderstood
The history of dopamine, however, is not only about Parkinson’s disease but extends to the treatment of schizophrenia and addiction. When in the1940s a French military surgeon started giving a new antihistamine drug, promethazine, to prevent shock in soldiers undergoing surgery, he noticed a bizarre side effect: the soldiers would become euphoric yet oddly calm at the same time.
After the drug was modified by adding a chlorine atom and renamed chlorpromazine, it fast became a go-to treatment for psychosis. At the time, no one made the connection to dopamine. Contemporary doctors believed that it calmed people by lowering body temperature (common treatments for mental illness back in the day included swaddling patients in cold, wet sheets). Yet just like reserpine, chlorpromazine produced range of nasty side effects that closely mimicked Parkinson’s disease. This led a Dutch pharmacologist, Jacques van Rossum, to hypothesize that dopamine receptor blockade could explain chlorpromazine’s antipsychotic effects – an idea that remains widely accepted today.
In the 1970s, dopamine was linked with addiction through research on rodents, and this novel idea caught people’s imagination over the coming decades. A story on dopamine titled, “How We Get Addicted,” made the cover of Time in 1997.
Yet as the dopamine/addiction connection became widespread, it also became oversimplified. According to a 2015 article in Nature Reviews Neuroscience, a wave of low-quality research followed – nonreplicated, insufficient – which led the authors to conclude that we are “addicted to the dopamine theory of addiction.” Just about every pleasure under the sun was being attributed to dopamine, from eating delicious foods and playing computer games to sex, music, and hot showers. As recent science shows, however, dopamine is not simply about pleasure – it’s about reward prediction, response to stress, memory, learning, and even the functioning of the immune system. Since its first synthesis in the early 20th century, dopamine has often been misunderstood and oversimplified – and it seems the story is repeating itself now.
In one of his final interviews, Dr. Carlsson, who passed away in 2018 at the age of 95, warned about playing around with dopamine and, in particular, prescribing drugs that have an inhibitory action on this neurotransmitter. “Dopamine is involved in everything that happens in our brains – all its important functions,” he said.
We should be careful how we handle such a delicate and still little-known system.
A version of this article first appeared on Medscape.com.
Google the word “dopamine” and you will learn that its nicknames are the “happy hormone” and the “pleasure molecule” and that it is among the most important chemicals in our brains. With The Guardian branding it “the Kim Kardashian of neurotransmitters,” dopamine has become a true pop-science darling – people across the globe have attempted to boost their mood with dopamine fasts and dopamine dressing.
A century ago, however, newly discovered dopamine was seen as an uninspiring chemical, nothing more than a precursor of noradrenaline. It took several stubborn and hardworking scientists to change that view.
Levodopa: An indifferent precursor
When Casimir Funk, PhD, a Polish biochemist and the discoverer of vitamins, first synthesized the dopamine precursor levodopa in 1911, he had no idea how important the molecule would prove to be in pharmacology and neurobiology. Nor did Markus Guggenheim, PhD, a Swiss biochemist, who isolated levodopa in 1913 from the seeds of a broad bean, Vicia faba. Dr. Guggenheim administered 1 g of levodopa to a rabbit, with no apparent negative consequences. He then prepared a larger dose (2.5 g) and tested it on himself. “Ten minutes after taking it, I felt very nauseous, I had to vomit twice,” he wrote in his paper. In the body, levodopa is converted into dopamine, which may act as an emetic – an effect Dr. Guggenheim didn’t understand. He simply abandoned his human study, erroneously concluding, on the basis of his animal research, that levodopa is “pharmacologically fairly indifferent.”
Around the same time, several scientists across Europe successfully synthesized dopamine, but those discoveries were shelved without much fanfare. For the next 3 decades, dopamine and levodopa were pushed into academic obscurity. Just before World War II, a group of German scientists showed that levodopa is metabolized to dopamine in the body, while another German researcher, Hermann Blaschko, MD, discovered that dopamine is an intermediary in the synthesis of noradrenaline. Even these findings, however, were not immediately accepted.
The dopamine story picked up pace in the post-war years with the observation that the hormone was present in various tissues and body fluids, although nowhere as abundantly as in the central nervous system. Intrigued, Dr. Blaschko, who (after escaping Nazi Germany, changing his name to Hugh, and starting work at Oxford [England] University) hypothesized that dopamine couldn’t be an unremarkable precursor of noradrenaline – it had to have some physiologic functions of its own. He asked his postdoctoral fellow, Oheh Hornykiewicz, MD, to test a few ideas. Dr. Hornykiewicz soon confirmed that dopamine lowered blood pressure in guinea pigs, proving that dopamine indeed had physiologic activity that was independent of other catecholamines.
Reserpine and rabbit ears
While Dr. Blaschko and Dr. Hornykiewicz were puzzling over dopamine’s physiologic role in the body, across the ocean at the National Heart Institute in Maryland, pharmacologist Bernard Brodie, PhD and colleagues were laying the groundwork for the discovery of dopamine’s starring role in the brain.
Spoiler alert: Dr. Brodie’s work showed that a new psychiatric drug known as reserpine was capable of fully depleting the brain’s stores of serotonin and – of greatest significance, as it turned out – mimicking the neuromuscular symptoms typical of Parkinson’s disease. The connection to dopamine would be made by new lab colleague Arvid Carlsson, MD, PhD, who would go on to win a Nobel Prize.
Derived from Rauwolfia serpentina (a plant that for centuries has been used in India for the treatment of mental illness, insomnia, and snake bites), reserpine was introduced in the West as a treatment for schizophrenia.
It worked marvels. In 1954, the press lauded the “dramatic” and seemingly “incredible”: results in treating “hopelessly insane patients.” Reserpine had a downside, however. Reports soon changed in tone regarding the drug’s severe side effects, including headaches, dizziness, vomiting, and, far more disturbingly, symptoms mimicking Parkinson’s disease, from muscular rigidity to tremors.
Dr. Brodie observed that, when reserpine was injected, animals became completely immobile. Serotonin nearly vanished from their brains, but bizarrely, drugs that spur serotonin production did not reverse the rabbits’ immobility.
Dr. Carlsson realized that other catecholamines must be involved in reserpine’s side effects, and he began to search for the culprits. He moved back to his native Sweden and ordered a spectrophotofluorimeter. In one of his experiments, Carlsson injected a pair of rabbits with reserpine, which caused the animals to become catatonic with flattened ears. After the researchers injected the animals with levodopa, within 15 minutes, the rabbits were hopping around, ears proudly vertical. “We were just as excited as the rabbits,” Dr. Carlsson later recalled in a 2016 interview. Dr. Carlsson realized that, because there was no noradrenaline in the rabbits’ brains, dopamine depletion must have been directly responsible for producing reserpine’s motor inhibitory effects.
Skeptics are silenced
In 1960, however, the medical community was not yet ready to accept that dopamine was anything but a boring intermediate between levodopa and noradrenaline. At a prestigious London symposium, Dr. Carlsson and his two colleagues presented their hypothesis that dopamine may be a neurotransmitter, thus implicating it in Parkinson’s disease. They were met with harsh criticism. Some of the experts said levodopa was nothing more than a poison. Dr. Carlsson later recalled facing “a profound and nearly unanimous skepticism regarding our points of view.”
That would soon change. Dr. Hornykiewicz, the biochemist who had earlier discovered dopamine’s BP-lowering effects, tested Dr. Carlsson’s ideas using the postmortem brains of Parkinson’s disease patients. It appeared Dr. Carlsson was right: Unlike in healthy brains, the striatum of patients with Parkinson’s disease contained almost no dopamine whatsoever. Beginning in 1961, in collaboration with neurologist Walther Birkmayer, MD, Hornykiewicz injected levodopa into 20 patients with Parkinson’s disease and observed a “miraculous” (albeit temporary) amelioration of rigidity, motionlessness, and speechlessness.
By the late 1960s, levodopa and dopamine were making headlines. A 1969 New York Times article described similar stunning improvements in patients with Parkinson’s disease who were treated with levodopa. A patient who had arrived at a hospital unable to speak, with hands clenched and rigid expression, was suddenly able to stride into his doctor’s office and even jog around. “I might say I’m a human being,” he told reporters. Although the treatment was expensive – equivalent to $210 in 2022 – physicians were deluged with requests for “dopa.” To this day, levodopa remains a gold standard in the treatment of Parkinson’s disease.
Still misunderstood
The history of dopamine, however, is not only about Parkinson’s disease but extends to the treatment of schizophrenia and addiction. When in the1940s a French military surgeon started giving a new antihistamine drug, promethazine, to prevent shock in soldiers undergoing surgery, he noticed a bizarre side effect: the soldiers would become euphoric yet oddly calm at the same time.
After the drug was modified by adding a chlorine atom and renamed chlorpromazine, it fast became a go-to treatment for psychosis. At the time, no one made the connection to dopamine. Contemporary doctors believed that it calmed people by lowering body temperature (common treatments for mental illness back in the day included swaddling patients in cold, wet sheets). Yet just like reserpine, chlorpromazine produced range of nasty side effects that closely mimicked Parkinson’s disease. This led a Dutch pharmacologist, Jacques van Rossum, to hypothesize that dopamine receptor blockade could explain chlorpromazine’s antipsychotic effects – an idea that remains widely accepted today.
In the 1970s, dopamine was linked with addiction through research on rodents, and this novel idea caught people’s imagination over the coming decades. A story on dopamine titled, “How We Get Addicted,” made the cover of Time in 1997.
Yet as the dopamine/addiction connection became widespread, it also became oversimplified. According to a 2015 article in Nature Reviews Neuroscience, a wave of low-quality research followed – nonreplicated, insufficient – which led the authors to conclude that we are “addicted to the dopamine theory of addiction.” Just about every pleasure under the sun was being attributed to dopamine, from eating delicious foods and playing computer games to sex, music, and hot showers. As recent science shows, however, dopamine is not simply about pleasure – it’s about reward prediction, response to stress, memory, learning, and even the functioning of the immune system. Since its first synthesis in the early 20th century, dopamine has often been misunderstood and oversimplified – and it seems the story is repeating itself now.
In one of his final interviews, Dr. Carlsson, who passed away in 2018 at the age of 95, warned about playing around with dopamine and, in particular, prescribing drugs that have an inhibitory action on this neurotransmitter. “Dopamine is involved in everything that happens in our brains – all its important functions,” he said.
We should be careful how we handle such a delicate and still little-known system.
A version of this article first appeared on Medscape.com.
Google the word “dopamine” and you will learn that its nicknames are the “happy hormone” and the “pleasure molecule” and that it is among the most important chemicals in our brains. With The Guardian branding it “the Kim Kardashian of neurotransmitters,” dopamine has become a true pop-science darling – people across the globe have attempted to boost their mood with dopamine fasts and dopamine dressing.
A century ago, however, newly discovered dopamine was seen as an uninspiring chemical, nothing more than a precursor of noradrenaline. It took several stubborn and hardworking scientists to change that view.
Levodopa: An indifferent precursor
When Casimir Funk, PhD, a Polish biochemist and the discoverer of vitamins, first synthesized the dopamine precursor levodopa in 1911, he had no idea how important the molecule would prove to be in pharmacology and neurobiology. Nor did Markus Guggenheim, PhD, a Swiss biochemist, who isolated levodopa in 1913 from the seeds of a broad bean, Vicia faba. Dr. Guggenheim administered 1 g of levodopa to a rabbit, with no apparent negative consequences. He then prepared a larger dose (2.5 g) and tested it on himself. “Ten minutes after taking it, I felt very nauseous, I had to vomit twice,” he wrote in his paper. In the body, levodopa is converted into dopamine, which may act as an emetic – an effect Dr. Guggenheim didn’t understand. He simply abandoned his human study, erroneously concluding, on the basis of his animal research, that levodopa is “pharmacologically fairly indifferent.”
Around the same time, several scientists across Europe successfully synthesized dopamine, but those discoveries were shelved without much fanfare. For the next 3 decades, dopamine and levodopa were pushed into academic obscurity. Just before World War II, a group of German scientists showed that levodopa is metabolized to dopamine in the body, while another German researcher, Hermann Blaschko, MD, discovered that dopamine is an intermediary in the synthesis of noradrenaline. Even these findings, however, were not immediately accepted.
The dopamine story picked up pace in the post-war years with the observation that the hormone was present in various tissues and body fluids, although nowhere as abundantly as in the central nervous system. Intrigued, Dr. Blaschko, who (after escaping Nazi Germany, changing his name to Hugh, and starting work at Oxford [England] University) hypothesized that dopamine couldn’t be an unremarkable precursor of noradrenaline – it had to have some physiologic functions of its own. He asked his postdoctoral fellow, Oheh Hornykiewicz, MD, to test a few ideas. Dr. Hornykiewicz soon confirmed that dopamine lowered blood pressure in guinea pigs, proving that dopamine indeed had physiologic activity that was independent of other catecholamines.
Reserpine and rabbit ears
While Dr. Blaschko and Dr. Hornykiewicz were puzzling over dopamine’s physiologic role in the body, across the ocean at the National Heart Institute in Maryland, pharmacologist Bernard Brodie, PhD and colleagues were laying the groundwork for the discovery of dopamine’s starring role in the brain.
Spoiler alert: Dr. Brodie’s work showed that a new psychiatric drug known as reserpine was capable of fully depleting the brain’s stores of serotonin and – of greatest significance, as it turned out – mimicking the neuromuscular symptoms typical of Parkinson’s disease. The connection to dopamine would be made by new lab colleague Arvid Carlsson, MD, PhD, who would go on to win a Nobel Prize.
Derived from Rauwolfia serpentina (a plant that for centuries has been used in India for the treatment of mental illness, insomnia, and snake bites), reserpine was introduced in the West as a treatment for schizophrenia.
It worked marvels. In 1954, the press lauded the “dramatic” and seemingly “incredible”: results in treating “hopelessly insane patients.” Reserpine had a downside, however. Reports soon changed in tone regarding the drug’s severe side effects, including headaches, dizziness, vomiting, and, far more disturbingly, symptoms mimicking Parkinson’s disease, from muscular rigidity to tremors.
Dr. Brodie observed that, when reserpine was injected, animals became completely immobile. Serotonin nearly vanished from their brains, but bizarrely, drugs that spur serotonin production did not reverse the rabbits’ immobility.
Dr. Carlsson realized that other catecholamines must be involved in reserpine’s side effects, and he began to search for the culprits. He moved back to his native Sweden and ordered a spectrophotofluorimeter. In one of his experiments, Carlsson injected a pair of rabbits with reserpine, which caused the animals to become catatonic with flattened ears. After the researchers injected the animals with levodopa, within 15 minutes, the rabbits were hopping around, ears proudly vertical. “We were just as excited as the rabbits,” Dr. Carlsson later recalled in a 2016 interview. Dr. Carlsson realized that, because there was no noradrenaline in the rabbits’ brains, dopamine depletion must have been directly responsible for producing reserpine’s motor inhibitory effects.
Skeptics are silenced
In 1960, however, the medical community was not yet ready to accept that dopamine was anything but a boring intermediate between levodopa and noradrenaline. At a prestigious London symposium, Dr. Carlsson and his two colleagues presented their hypothesis that dopamine may be a neurotransmitter, thus implicating it in Parkinson’s disease. They were met with harsh criticism. Some of the experts said levodopa was nothing more than a poison. Dr. Carlsson later recalled facing “a profound and nearly unanimous skepticism regarding our points of view.”
That would soon change. Dr. Hornykiewicz, the biochemist who had earlier discovered dopamine’s BP-lowering effects, tested Dr. Carlsson’s ideas using the postmortem brains of Parkinson’s disease patients. It appeared Dr. Carlsson was right: Unlike in healthy brains, the striatum of patients with Parkinson’s disease contained almost no dopamine whatsoever. Beginning in 1961, in collaboration with neurologist Walther Birkmayer, MD, Hornykiewicz injected levodopa into 20 patients with Parkinson’s disease and observed a “miraculous” (albeit temporary) amelioration of rigidity, motionlessness, and speechlessness.
By the late 1960s, levodopa and dopamine were making headlines. A 1969 New York Times article described similar stunning improvements in patients with Parkinson’s disease who were treated with levodopa. A patient who had arrived at a hospital unable to speak, with hands clenched and rigid expression, was suddenly able to stride into his doctor’s office and even jog around. “I might say I’m a human being,” he told reporters. Although the treatment was expensive – equivalent to $210 in 2022 – physicians were deluged with requests for “dopa.” To this day, levodopa remains a gold standard in the treatment of Parkinson’s disease.
Still misunderstood
The history of dopamine, however, is not only about Parkinson’s disease but extends to the treatment of schizophrenia and addiction. When in the1940s a French military surgeon started giving a new antihistamine drug, promethazine, to prevent shock in soldiers undergoing surgery, he noticed a bizarre side effect: the soldiers would become euphoric yet oddly calm at the same time.
After the drug was modified by adding a chlorine atom and renamed chlorpromazine, it fast became a go-to treatment for psychosis. At the time, no one made the connection to dopamine. Contemporary doctors believed that it calmed people by lowering body temperature (common treatments for mental illness back in the day included swaddling patients in cold, wet sheets). Yet just like reserpine, chlorpromazine produced range of nasty side effects that closely mimicked Parkinson’s disease. This led a Dutch pharmacologist, Jacques van Rossum, to hypothesize that dopamine receptor blockade could explain chlorpromazine’s antipsychotic effects – an idea that remains widely accepted today.
In the 1970s, dopamine was linked with addiction through research on rodents, and this novel idea caught people’s imagination over the coming decades. A story on dopamine titled, “How We Get Addicted,” made the cover of Time in 1997.
Yet as the dopamine/addiction connection became widespread, it also became oversimplified. According to a 2015 article in Nature Reviews Neuroscience, a wave of low-quality research followed – nonreplicated, insufficient – which led the authors to conclude that we are “addicted to the dopamine theory of addiction.” Just about every pleasure under the sun was being attributed to dopamine, from eating delicious foods and playing computer games to sex, music, and hot showers. As recent science shows, however, dopamine is not simply about pleasure – it’s about reward prediction, response to stress, memory, learning, and even the functioning of the immune system. Since its first synthesis in the early 20th century, dopamine has often been misunderstood and oversimplified – and it seems the story is repeating itself now.
In one of his final interviews, Dr. Carlsson, who passed away in 2018 at the age of 95, warned about playing around with dopamine and, in particular, prescribing drugs that have an inhibitory action on this neurotransmitter. “Dopamine is involved in everything that happens in our brains – all its important functions,” he said.
We should be careful how we handle such a delicate and still little-known system.
A version of this article first appeared on Medscape.com.
Resistance training tied to improvements in Parkinson’s disease symptoms
, new research suggests.
A meta-analysis, which included 18 randomized controlled trials and more than 1,000 patients with Parkinson’s disease, showed that those who underwent resistance training had significantly greater improvement in motor impairment, muscle strength, and mobility/balance than their peers who underwent passive or placebo interventions.
However, there was no significant difference between patients who participated in resistance training and those who participated in other active physical interventions, including yoga.
Overall, the results highlight the importance that these patients should participate in some type of physical exercise, said the study’s lead author, Romina Gollan, MSc, an assistant researcher in the division of medical psychology, University of Cologne, Germany. “Patients should definitely be doing exercises, including resistance training, if they want to. But the type of exercise is of secondary interest,” she said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Positive but inconsistent
Previous reviews have suggested resistance training has positive effects on motor function in Parkinson’s disease. However, results from the included studies were inconsistent; and few reviews have examined nonmotor outcomes of resistance training in this population, the investigators noted.
After carrying out a literature search of studies that examined the effects of resistance training in Parkinson’s disease, the researchers included 18 randomized controlled trials in their current review. Among the 1,134 total participants, the mean age was 66 years, the mean Hoehn & Yahr stage was 2.3 (range 0-4), and the mean duration of Parkinson’s disease was 7.5 years.
The investigation was grouped into two meta-analysis groups: one examining resistance training versus a passive or placebo intervention and the other assessing resistance training versus active physical interventions, such as yoga.
During resistance training, participants use their full strength to do a repetition, working muscles to overcome a certain threshold, said Ms. Gollan. In contrast, a placebo intervention is “very low intensity” and involves a much lower threshold, she added.
Passive interventions include such things as stretching where the stimulus “is not high enough for muscles to adapt” and build strength, Ms. Gollan noted.
A passive intervention might also include “treatment as usual” or normal daily routines.
Patient preference important
The meta-analysis comparing resistance training groups with passive control groups showed significant large effects on muscle strength (standard mean difference, –0.84; 95% confidence interval, –1.29 to –0.39; P = .0003), motor impairment (SMD, –0.81; 95% CI, –1.34 to –0.27; P = .003), and mobility and balance (SMD, –1.80; 95% CI, –3.13 to –0.49; P = .007).
The review also showed significant but small effects on quality of life.
However, the meta-analysis that assessed resistance training versus other physical interventions showed no significant between-group differences.
Ms. Gollan noted that although there were some assessments of cognition and depression, the data were too limited to determine the impact of resistance training on these outcomes.
“We need more studies, especially randomized controlled trials, to investigate the effects of resistance training on nonmotor outcomes like depression and cognition,” she said.
Co-investigator Ann-Kristin Folkerts, PhD, who heads the University of Cologne medical psychology working group, noted that although exercise in general is beneficial for patients with Parkinson’s disease, the choice of activity should take patient preferences into consideration.
It is important that patients choose an exercise they enjoy “because otherwise they probably wouldn’t adhere to the treatment,” Dr. Folkerts said. “It’s important to have fun.”
Specific goals or objectives, such as improving quality of life or balance, should also be considered, she added.
Oversimplification?
Commenting on the research, Alice Nieuwboer, PhD, professor in the department of rehabilitation sciences and head of the neurorehabilitation research group at the University of Leuven, Belgium, disagreed that exercise type is of secondary importance in Parkinson’s disease.
“In my view, it’s of primary interest, especially at the mid- to later stages,” said Dr. Nieuwboer, who was not involved with the research.
She noted it is difficult to carry out meta-analyses of resistance training versus other interventions because studies comparing different exercise types “are rather scarce.”
“Another issue is that the dose may differ, so you’re comparing apples with pears,” said Dr. Nieuwboer.
She did agree that all patients should exercise, because it is “better than no exercise,” and they should be “free to choose a mode that interests them.”
However, she stressed that exercise requires significant effort on the part of patients with Parkinson’s disease, requires “sustained motivation,” and has to become habit-forming. This makes “exercise targeting” very important, with the target changing over the disease course, Dr. Nieuwboer said.
For example, for a patient at an early stage of the disease who can still move quite well, both resistance training and endurance training can improve fitness and health; but at a mid-stage, it is perhaps better for patients to work on balance and walking quality “to preempt the risk of falls and developing freezing,” she noted.
Later on, as movement becomes very difficult, “the exercise menu is even more restricted,” said Dr. Nieuwboer.
The bottom line is that a message saying “any movement counts” is an oversimplification, she added.
The study was funded by a grant from the German Federal Ministry of Education and Research. The investigators and Dr. Nieuwboer have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
A meta-analysis, which included 18 randomized controlled trials and more than 1,000 patients with Parkinson’s disease, showed that those who underwent resistance training had significantly greater improvement in motor impairment, muscle strength, and mobility/balance than their peers who underwent passive or placebo interventions.
However, there was no significant difference between patients who participated in resistance training and those who participated in other active physical interventions, including yoga.
Overall, the results highlight the importance that these patients should participate in some type of physical exercise, said the study’s lead author, Romina Gollan, MSc, an assistant researcher in the division of medical psychology, University of Cologne, Germany. “Patients should definitely be doing exercises, including resistance training, if they want to. But the type of exercise is of secondary interest,” she said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Positive but inconsistent
Previous reviews have suggested resistance training has positive effects on motor function in Parkinson’s disease. However, results from the included studies were inconsistent; and few reviews have examined nonmotor outcomes of resistance training in this population, the investigators noted.
After carrying out a literature search of studies that examined the effects of resistance training in Parkinson’s disease, the researchers included 18 randomized controlled trials in their current review. Among the 1,134 total participants, the mean age was 66 years, the mean Hoehn & Yahr stage was 2.3 (range 0-4), and the mean duration of Parkinson’s disease was 7.5 years.
The investigation was grouped into two meta-analysis groups: one examining resistance training versus a passive or placebo intervention and the other assessing resistance training versus active physical interventions, such as yoga.
During resistance training, participants use their full strength to do a repetition, working muscles to overcome a certain threshold, said Ms. Gollan. In contrast, a placebo intervention is “very low intensity” and involves a much lower threshold, she added.
Passive interventions include such things as stretching where the stimulus “is not high enough for muscles to adapt” and build strength, Ms. Gollan noted.
A passive intervention might also include “treatment as usual” or normal daily routines.
Patient preference important
The meta-analysis comparing resistance training groups with passive control groups showed significant large effects on muscle strength (standard mean difference, –0.84; 95% confidence interval, –1.29 to –0.39; P = .0003), motor impairment (SMD, –0.81; 95% CI, –1.34 to –0.27; P = .003), and mobility and balance (SMD, –1.80; 95% CI, –3.13 to –0.49; P = .007).
The review also showed significant but small effects on quality of life.
However, the meta-analysis that assessed resistance training versus other physical interventions showed no significant between-group differences.
Ms. Gollan noted that although there were some assessments of cognition and depression, the data were too limited to determine the impact of resistance training on these outcomes.
“We need more studies, especially randomized controlled trials, to investigate the effects of resistance training on nonmotor outcomes like depression and cognition,” she said.
Co-investigator Ann-Kristin Folkerts, PhD, who heads the University of Cologne medical psychology working group, noted that although exercise in general is beneficial for patients with Parkinson’s disease, the choice of activity should take patient preferences into consideration.
It is important that patients choose an exercise they enjoy “because otherwise they probably wouldn’t adhere to the treatment,” Dr. Folkerts said. “It’s important to have fun.”
Specific goals or objectives, such as improving quality of life or balance, should also be considered, she added.
Oversimplification?
Commenting on the research, Alice Nieuwboer, PhD, professor in the department of rehabilitation sciences and head of the neurorehabilitation research group at the University of Leuven, Belgium, disagreed that exercise type is of secondary importance in Parkinson’s disease.
“In my view, it’s of primary interest, especially at the mid- to later stages,” said Dr. Nieuwboer, who was not involved with the research.
She noted it is difficult to carry out meta-analyses of resistance training versus other interventions because studies comparing different exercise types “are rather scarce.”
“Another issue is that the dose may differ, so you’re comparing apples with pears,” said Dr. Nieuwboer.
She did agree that all patients should exercise, because it is “better than no exercise,” and they should be “free to choose a mode that interests them.”
However, she stressed that exercise requires significant effort on the part of patients with Parkinson’s disease, requires “sustained motivation,” and has to become habit-forming. This makes “exercise targeting” very important, with the target changing over the disease course, Dr. Nieuwboer said.
For example, for a patient at an early stage of the disease who can still move quite well, both resistance training and endurance training can improve fitness and health; but at a mid-stage, it is perhaps better for patients to work on balance and walking quality “to preempt the risk of falls and developing freezing,” she noted.
Later on, as movement becomes very difficult, “the exercise menu is even more restricted,” said Dr. Nieuwboer.
The bottom line is that a message saying “any movement counts” is an oversimplification, she added.
The study was funded by a grant from the German Federal Ministry of Education and Research. The investigators and Dr. Nieuwboer have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
A meta-analysis, which included 18 randomized controlled trials and more than 1,000 patients with Parkinson’s disease, showed that those who underwent resistance training had significantly greater improvement in motor impairment, muscle strength, and mobility/balance than their peers who underwent passive or placebo interventions.
However, there was no significant difference between patients who participated in resistance training and those who participated in other active physical interventions, including yoga.
Overall, the results highlight the importance that these patients should participate in some type of physical exercise, said the study’s lead author, Romina Gollan, MSc, an assistant researcher in the division of medical psychology, University of Cologne, Germany. “Patients should definitely be doing exercises, including resistance training, if they want to. But the type of exercise is of secondary interest,” she said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Positive but inconsistent
Previous reviews have suggested resistance training has positive effects on motor function in Parkinson’s disease. However, results from the included studies were inconsistent; and few reviews have examined nonmotor outcomes of resistance training in this population, the investigators noted.
After carrying out a literature search of studies that examined the effects of resistance training in Parkinson’s disease, the researchers included 18 randomized controlled trials in their current review. Among the 1,134 total participants, the mean age was 66 years, the mean Hoehn & Yahr stage was 2.3 (range 0-4), and the mean duration of Parkinson’s disease was 7.5 years.
The investigation was grouped into two meta-analysis groups: one examining resistance training versus a passive or placebo intervention and the other assessing resistance training versus active physical interventions, such as yoga.
During resistance training, participants use their full strength to do a repetition, working muscles to overcome a certain threshold, said Ms. Gollan. In contrast, a placebo intervention is “very low intensity” and involves a much lower threshold, she added.
Passive interventions include such things as stretching where the stimulus “is not high enough for muscles to adapt” and build strength, Ms. Gollan noted.
A passive intervention might also include “treatment as usual” or normal daily routines.
Patient preference important
The meta-analysis comparing resistance training groups with passive control groups showed significant large effects on muscle strength (standard mean difference, –0.84; 95% confidence interval, –1.29 to –0.39; P = .0003), motor impairment (SMD, –0.81; 95% CI, –1.34 to –0.27; P = .003), and mobility and balance (SMD, –1.80; 95% CI, –3.13 to –0.49; P = .007).
The review also showed significant but small effects on quality of life.
However, the meta-analysis that assessed resistance training versus other physical interventions showed no significant between-group differences.
Ms. Gollan noted that although there were some assessments of cognition and depression, the data were too limited to determine the impact of resistance training on these outcomes.
“We need more studies, especially randomized controlled trials, to investigate the effects of resistance training on nonmotor outcomes like depression and cognition,” she said.
Co-investigator Ann-Kristin Folkerts, PhD, who heads the University of Cologne medical psychology working group, noted that although exercise in general is beneficial for patients with Parkinson’s disease, the choice of activity should take patient preferences into consideration.
It is important that patients choose an exercise they enjoy “because otherwise they probably wouldn’t adhere to the treatment,” Dr. Folkerts said. “It’s important to have fun.”
Specific goals or objectives, such as improving quality of life or balance, should also be considered, she added.
Oversimplification?
Commenting on the research, Alice Nieuwboer, PhD, professor in the department of rehabilitation sciences and head of the neurorehabilitation research group at the University of Leuven, Belgium, disagreed that exercise type is of secondary importance in Parkinson’s disease.
“In my view, it’s of primary interest, especially at the mid- to later stages,” said Dr. Nieuwboer, who was not involved with the research.
She noted it is difficult to carry out meta-analyses of resistance training versus other interventions because studies comparing different exercise types “are rather scarce.”
“Another issue is that the dose may differ, so you’re comparing apples with pears,” said Dr. Nieuwboer.
She did agree that all patients should exercise, because it is “better than no exercise,” and they should be “free to choose a mode that interests them.”
However, she stressed that exercise requires significant effort on the part of patients with Parkinson’s disease, requires “sustained motivation,” and has to become habit-forming. This makes “exercise targeting” very important, with the target changing over the disease course, Dr. Nieuwboer said.
For example, for a patient at an early stage of the disease who can still move quite well, both resistance training and endurance training can improve fitness and health; but at a mid-stage, it is perhaps better for patients to work on balance and walking quality “to preempt the risk of falls and developing freezing,” she noted.
Later on, as movement becomes very difficult, “the exercise menu is even more restricted,” said Dr. Nieuwboer.
The bottom line is that a message saying “any movement counts” is an oversimplification, she added.
The study was funded by a grant from the German Federal Ministry of Education and Research. The investigators and Dr. Nieuwboer have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MDS 2022
Air pollution tied to stroke risk, subsequent CV events, and death
Results of a UK biobank study show high levels of air pollution were associated with an increased risk of transition from health to a first stroke and subsequent progression to cardiovascular (CV) events and death.
“These results indicate that understanding and reducing the effects of air pollutants on different transition stages in stroke will be beneficial in managing people’s health and preventing the occurrence and progression of stroke,” study investigator Hualiang Lin, PhD, of Sun Yat-sen University School of Public Health, Guangzhou, China, said in a news release.
The study was published online in the journal Neurology.
A way to stop stroke progression?
The researchers assessed air pollution exposure in 318,752 people (mean age, 56) from the UK biobank database. None had a history of stroke or heart disease at the start of the study. Annual concentrations of air pollution near where people lived were estimated through land-use regressions.
During an average follow-up of 12 years, 5,967 people had a stroke, 2,985 developed post-stroke CVD, and 1,020 died.
After adjusting for confounding factors, every 5 µg/m3 increase in exposure to fine particulate matter (PM2.5) was associated with a 24% increase in transition from healthy to first stroke (hazard ratio, 1.24; 95% confidence interval, 1.10-1.40) and a 30% increase in transition from being healthy to dying (HR, 1.30; 95% CI, 1.21-1.40).
PM2.5 is less than 2.5 microns in diameter and includes fly ash from coal combustion. The World Health Organization recommends that annual PM2.5 exposure should not exceed 5 µg/m3.
Those who had a stroke during the study had an average exposure of 10.03 µg/m3 of PM2.5, compared with 9.97 µg/m3 for those who did not have a stroke.
The air pollutants nitrogen oxide and nitrogen dioxide were also associated with an increased risk of stroke and death, but the associations were weaker.
“More research is needed, but it’s possible that decreasing exposure to heavy levels of air pollution could play a role in reducing the progression of stroke,” Dr. Lin said.
“People can reduce their exposure by staying indoors on heavy pollution days, reducing their outdoor exercise, wearing masks to filter out particulate matter, and using air purifiers,” Dr. Lin added.
Public policy implications
Reached for comment, Steffen E. Petersen, MD, MPH, professor of cardiovascular medicine, Barts Health NHS Trust, London, said the study “elegantly confirms the increased risk of stroke due to air pollution in the UK Biobank population study but interestingly suggests that the impact of air pollution may continue to adversely impact cardiovascular health even after the stroke occurred.”
“This is further evidence to inform policymakers to tackle air pollution and get levels below the recommended levels,” Dr. Petersen said.
“On a personal level, everyone, including stroke patients, may wish to consider personal measures to reduce exposure to air pollution, such as avoiding walking along polluted streets and rather take a less polluted route away from the main roads,” Dr. Petersen added.
The study had no targeted funding. Dr. Lin and Dr. Petersen report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a UK biobank study show high levels of air pollution were associated with an increased risk of transition from health to a first stroke and subsequent progression to cardiovascular (CV) events and death.
“These results indicate that understanding and reducing the effects of air pollutants on different transition stages in stroke will be beneficial in managing people’s health and preventing the occurrence and progression of stroke,” study investigator Hualiang Lin, PhD, of Sun Yat-sen University School of Public Health, Guangzhou, China, said in a news release.
The study was published online in the journal Neurology.
A way to stop stroke progression?
The researchers assessed air pollution exposure in 318,752 people (mean age, 56) from the UK biobank database. None had a history of stroke or heart disease at the start of the study. Annual concentrations of air pollution near where people lived were estimated through land-use regressions.
During an average follow-up of 12 years, 5,967 people had a stroke, 2,985 developed post-stroke CVD, and 1,020 died.
After adjusting for confounding factors, every 5 µg/m3 increase in exposure to fine particulate matter (PM2.5) was associated with a 24% increase in transition from healthy to first stroke (hazard ratio, 1.24; 95% confidence interval, 1.10-1.40) and a 30% increase in transition from being healthy to dying (HR, 1.30; 95% CI, 1.21-1.40).
PM2.5 is less than 2.5 microns in diameter and includes fly ash from coal combustion. The World Health Organization recommends that annual PM2.5 exposure should not exceed 5 µg/m3.
Those who had a stroke during the study had an average exposure of 10.03 µg/m3 of PM2.5, compared with 9.97 µg/m3 for those who did not have a stroke.
The air pollutants nitrogen oxide and nitrogen dioxide were also associated with an increased risk of stroke and death, but the associations were weaker.
“More research is needed, but it’s possible that decreasing exposure to heavy levels of air pollution could play a role in reducing the progression of stroke,” Dr. Lin said.
“People can reduce their exposure by staying indoors on heavy pollution days, reducing their outdoor exercise, wearing masks to filter out particulate matter, and using air purifiers,” Dr. Lin added.
Public policy implications
Reached for comment, Steffen E. Petersen, MD, MPH, professor of cardiovascular medicine, Barts Health NHS Trust, London, said the study “elegantly confirms the increased risk of stroke due to air pollution in the UK Biobank population study but interestingly suggests that the impact of air pollution may continue to adversely impact cardiovascular health even after the stroke occurred.”
“This is further evidence to inform policymakers to tackle air pollution and get levels below the recommended levels,” Dr. Petersen said.
“On a personal level, everyone, including stroke patients, may wish to consider personal measures to reduce exposure to air pollution, such as avoiding walking along polluted streets and rather take a less polluted route away from the main roads,” Dr. Petersen added.
The study had no targeted funding. Dr. Lin and Dr. Petersen report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a UK biobank study show high levels of air pollution were associated with an increased risk of transition from health to a first stroke and subsequent progression to cardiovascular (CV) events and death.
“These results indicate that understanding and reducing the effects of air pollutants on different transition stages in stroke will be beneficial in managing people’s health and preventing the occurrence and progression of stroke,” study investigator Hualiang Lin, PhD, of Sun Yat-sen University School of Public Health, Guangzhou, China, said in a news release.
The study was published online in the journal Neurology.
A way to stop stroke progression?
The researchers assessed air pollution exposure in 318,752 people (mean age, 56) from the UK biobank database. None had a history of stroke or heart disease at the start of the study. Annual concentrations of air pollution near where people lived were estimated through land-use regressions.
During an average follow-up of 12 years, 5,967 people had a stroke, 2,985 developed post-stroke CVD, and 1,020 died.
After adjusting for confounding factors, every 5 µg/m3 increase in exposure to fine particulate matter (PM2.5) was associated with a 24% increase in transition from healthy to first stroke (hazard ratio, 1.24; 95% confidence interval, 1.10-1.40) and a 30% increase in transition from being healthy to dying (HR, 1.30; 95% CI, 1.21-1.40).
PM2.5 is less than 2.5 microns in diameter and includes fly ash from coal combustion. The World Health Organization recommends that annual PM2.5 exposure should not exceed 5 µg/m3.
Those who had a stroke during the study had an average exposure of 10.03 µg/m3 of PM2.5, compared with 9.97 µg/m3 for those who did not have a stroke.
The air pollutants nitrogen oxide and nitrogen dioxide were also associated with an increased risk of stroke and death, but the associations were weaker.
“More research is needed, but it’s possible that decreasing exposure to heavy levels of air pollution could play a role in reducing the progression of stroke,” Dr. Lin said.
“People can reduce their exposure by staying indoors on heavy pollution days, reducing their outdoor exercise, wearing masks to filter out particulate matter, and using air purifiers,” Dr. Lin added.
Public policy implications
Reached for comment, Steffen E. Petersen, MD, MPH, professor of cardiovascular medicine, Barts Health NHS Trust, London, said the study “elegantly confirms the increased risk of stroke due to air pollution in the UK Biobank population study but interestingly suggests that the impact of air pollution may continue to adversely impact cardiovascular health even after the stroke occurred.”
“This is further evidence to inform policymakers to tackle air pollution and get levels below the recommended levels,” Dr. Petersen said.
“On a personal level, everyone, including stroke patients, may wish to consider personal measures to reduce exposure to air pollution, such as avoiding walking along polluted streets and rather take a less polluted route away from the main roads,” Dr. Petersen added.
The study had no targeted funding. Dr. Lin and Dr. Petersen report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New ICD-10-CM codes a ‘big switch-over’ for neurocognitive disorders
Revised ICD-10-CM codes for neurocognitive disorders are now in effect, the American Psychiatric Association has announced
The coding changes for major and mild neurocognitive disorders represent “the most consequential” coding changes for DSM-5 disorders since the Oct. 1, 2015, changeover from ICD-9-CM to ICD-10-CM,” Michael First, MD, professor of clinical psychiatry at Columbia University, in New York, wrote in a statement published in Psychiatric News.
The updated codes for neurocognitive disorders are “much more specific and indicate all the different types of behavioral problems that could occur with dementia,” First, who served as editor of the DSM-5-TR, added in an interview.
This year, coding changes that affect psychiatry are largely confined to major and mild neurocognitive disorders, but they represent “a big switch-over,” Dr. First said.
What’s new
The first three characters that make up the ICD-10-CM code for major neurocognitive disorder depend on the type of etiologic medical condition and are unchanged:
- F01 for major neurocognitive disorder caused by vascular disease
- F02 for major neurocognitive disorder caused by other medical conditions in which the specific etiologic medical condition is indicated by also listing the ICD-10-CM code for the medical condition
- F03 for major neurocognitive disorder when the medical etiology is unknown
However, DSM-5-TR diagnostic criteria for major neurocognitive disorder include severity specifiers (mild, moderate, severe), but there is no provision for indicating this “clinically important” information in the current ICD-10-CM code for major neurocognitive disorder, Dr. First explained.
The 2022 coding changes for major neurocognitive disorder include the provision of a fourth character code to indicate the severity of the major neurocognitive disorder – “A” indicates mild (difficulties with instrumental activities of daily living, such as housework and managing money); “B,” moderate (difficulties with basic activities of daily living, such as feeding and dressing); and “C,” severe (fully dependent) impairment.
The coding changes for major neurocognitive disorder also now include fifth and sixth characters to indicate the presence of an accompanying behavioral or psychological disturbance, such as agitation, psychotic disturbance, mood symptoms, and anxiety.
The update, which went into effect Oct. 1, also adds to ICD-10-CM two new mental disorder codes, F06.71 and F06.70 for mild neurocognitive disorder caused by a medical condition with or without a behavioral disturbance, respectively.
The coding changes affecting psychiatry are outlined in the APA’s 2022 DSM-5-TR Update: Supplement to the Diagnostic and Statistical Manual of Mental Disorders and DSM-5-TR Neurocognitive Disorders Supplement.
Annual event
Every Oct. 1, ICD-10-CM codes for all of medicine are updated, with new codes being added and others revised or deleted. Only a small fraction of the 68,000 codes is affected. Last year, 159 new codes were added, 25 codes were deleted, and 27 existing codes were revised.
All HIPAA-compliant health care entities are required to use the most up-to-date ICD-10-CM codes.
“I think there’s a grace period where you can still use the old codes, but there will be a point where if you use the old code, it’ll get rejected because it won’t be considered a valid code,” said Dr. First.
A version of this article first appeared on Medscape.com.
Revised ICD-10-CM codes for neurocognitive disorders are now in effect, the American Psychiatric Association has announced
The coding changes for major and mild neurocognitive disorders represent “the most consequential” coding changes for DSM-5 disorders since the Oct. 1, 2015, changeover from ICD-9-CM to ICD-10-CM,” Michael First, MD, professor of clinical psychiatry at Columbia University, in New York, wrote in a statement published in Psychiatric News.
The updated codes for neurocognitive disorders are “much more specific and indicate all the different types of behavioral problems that could occur with dementia,” First, who served as editor of the DSM-5-TR, added in an interview.
This year, coding changes that affect psychiatry are largely confined to major and mild neurocognitive disorders, but they represent “a big switch-over,” Dr. First said.
What’s new
The first three characters that make up the ICD-10-CM code for major neurocognitive disorder depend on the type of etiologic medical condition and are unchanged:
- F01 for major neurocognitive disorder caused by vascular disease
- F02 for major neurocognitive disorder caused by other medical conditions in which the specific etiologic medical condition is indicated by also listing the ICD-10-CM code for the medical condition
- F03 for major neurocognitive disorder when the medical etiology is unknown
However, DSM-5-TR diagnostic criteria for major neurocognitive disorder include severity specifiers (mild, moderate, severe), but there is no provision for indicating this “clinically important” information in the current ICD-10-CM code for major neurocognitive disorder, Dr. First explained.
The 2022 coding changes for major neurocognitive disorder include the provision of a fourth character code to indicate the severity of the major neurocognitive disorder – “A” indicates mild (difficulties with instrumental activities of daily living, such as housework and managing money); “B,” moderate (difficulties with basic activities of daily living, such as feeding and dressing); and “C,” severe (fully dependent) impairment.
The coding changes for major neurocognitive disorder also now include fifth and sixth characters to indicate the presence of an accompanying behavioral or psychological disturbance, such as agitation, psychotic disturbance, mood symptoms, and anxiety.
The update, which went into effect Oct. 1, also adds to ICD-10-CM two new mental disorder codes, F06.71 and F06.70 for mild neurocognitive disorder caused by a medical condition with or without a behavioral disturbance, respectively.
The coding changes affecting psychiatry are outlined in the APA’s 2022 DSM-5-TR Update: Supplement to the Diagnostic and Statistical Manual of Mental Disorders and DSM-5-TR Neurocognitive Disorders Supplement.
Annual event
Every Oct. 1, ICD-10-CM codes for all of medicine are updated, with new codes being added and others revised or deleted. Only a small fraction of the 68,000 codes is affected. Last year, 159 new codes were added, 25 codes were deleted, and 27 existing codes were revised.
All HIPAA-compliant health care entities are required to use the most up-to-date ICD-10-CM codes.
“I think there’s a grace period where you can still use the old codes, but there will be a point where if you use the old code, it’ll get rejected because it won’t be considered a valid code,” said Dr. First.
A version of this article first appeared on Medscape.com.
Revised ICD-10-CM codes for neurocognitive disorders are now in effect, the American Psychiatric Association has announced
The coding changes for major and mild neurocognitive disorders represent “the most consequential” coding changes for DSM-5 disorders since the Oct. 1, 2015, changeover from ICD-9-CM to ICD-10-CM,” Michael First, MD, professor of clinical psychiatry at Columbia University, in New York, wrote in a statement published in Psychiatric News.
The updated codes for neurocognitive disorders are “much more specific and indicate all the different types of behavioral problems that could occur with dementia,” First, who served as editor of the DSM-5-TR, added in an interview.
This year, coding changes that affect psychiatry are largely confined to major and mild neurocognitive disorders, but they represent “a big switch-over,” Dr. First said.
What’s new
The first three characters that make up the ICD-10-CM code for major neurocognitive disorder depend on the type of etiologic medical condition and are unchanged:
- F01 for major neurocognitive disorder caused by vascular disease
- F02 for major neurocognitive disorder caused by other medical conditions in which the specific etiologic medical condition is indicated by also listing the ICD-10-CM code for the medical condition
- F03 for major neurocognitive disorder when the medical etiology is unknown
However, DSM-5-TR diagnostic criteria for major neurocognitive disorder include severity specifiers (mild, moderate, severe), but there is no provision for indicating this “clinically important” information in the current ICD-10-CM code for major neurocognitive disorder, Dr. First explained.
The 2022 coding changes for major neurocognitive disorder include the provision of a fourth character code to indicate the severity of the major neurocognitive disorder – “A” indicates mild (difficulties with instrumental activities of daily living, such as housework and managing money); “B,” moderate (difficulties with basic activities of daily living, such as feeding and dressing); and “C,” severe (fully dependent) impairment.
The coding changes for major neurocognitive disorder also now include fifth and sixth characters to indicate the presence of an accompanying behavioral or psychological disturbance, such as agitation, psychotic disturbance, mood symptoms, and anxiety.
The update, which went into effect Oct. 1, also adds to ICD-10-CM two new mental disorder codes, F06.71 and F06.70 for mild neurocognitive disorder caused by a medical condition with or without a behavioral disturbance, respectively.
The coding changes affecting psychiatry are outlined in the APA’s 2022 DSM-5-TR Update: Supplement to the Diagnostic and Statistical Manual of Mental Disorders and DSM-5-TR Neurocognitive Disorders Supplement.
Annual event
Every Oct. 1, ICD-10-CM codes for all of medicine are updated, with new codes being added and others revised or deleted. Only a small fraction of the 68,000 codes is affected. Last year, 159 new codes were added, 25 codes were deleted, and 27 existing codes were revised.
All HIPAA-compliant health care entities are required to use the most up-to-date ICD-10-CM codes.
“I think there’s a grace period where you can still use the old codes, but there will be a point where if you use the old code, it’ll get rejected because it won’t be considered a valid code,” said Dr. First.
A version of this article first appeared on Medscape.com.
Bariatric surgery may up risk for epilepsy
Analyzing health records, investigators compared almost 17,000 patients who had undergone bariatric surgery with more than 620,000 individuals with obesity who had not undergone the surgery.
During a minimum 3-year follow-up period, the surgery group had a 45% higher risk of developing epilepsy than the nonsurgery group. Moreover, patients who had a stroke after their bariatric surgery were 14 times more likely to develop epilepsy than those who did not have a stroke.
“When considering having bariatric surgery, people should talk to their doctors about the benefits and risks,” senior investigator Jorge Burneo, MD, professor of neurology, biostatistics, and epidemiology and endowed chair in epilepsy at Western University, London, told this news organization.
“While there are many health benefits of weight loss, our findings suggest that epilepsy is a long-term risk of bariatric surgery for weight loss,” Dr. Burneo said.
The findings were published online in Neurology.
Unrecognized risk factor?
Bariatric surgery has become more common as global rates of obesity have increased. The surgery has been shown to reduce the risk for serious obesity-related conditions, the researchers note.
However, “in addition to the positive outcomes of bariatric surgery, several long-term neurological complications have also been identified,” they write.
One previous study reported increased epilepsy risk following gastric bypass. Those findings “suggest that bariatric surgery may be an unrecognized epilepsy risk factor; however, this possible association has not been thoroughly explored,” write the investigators.
Dr. Burneo said he conducted the study because he has seen patients with epilepsy in his clinic who were “without risk factors, with normal MRIs, who shared the history of having bariatric surgery before the development of epilepsy.”
The researchers’ primary objective was to “assess whether epilepsy risk is elevated following bariatric surgery for weight loss relative to a nonsurgical cohort of patients who are obese,” he noted.
The study used linked administrative health databases in Ontario, Canada. Patients were accrued from July 1, 2010, to Dec. 31, 2016, and were followed until Dec. 31, 2019. The analysis included 639,472 participants, 2.7% of whom had undergone bariatric surgery.
The “exposed” cohort consisted of all Ontario residents aged 18 years or older who had undergone bariatric surgery during the 6-year period (n = 16,958; 65.1% women; mean age, 47.4 years), while the “unexposed” cohort consisted of patients hospitalized with a diagnosis of obesity who had not undergone bariatric surgery (n = 622,514; 62.8% women; mean age, 47.6 years).
Patients with a history of seizures, epilepsy, epilepsy risk factors, prior brain surgery, psychiatric disorders, or drug or alcohol abuse/dependence were excluded from the analysis.
The researchers collected data on patients’ sociodemographic characteristics at the index date, as well as Charlson Comorbidity Index scores during the 2 years prior to index, and data regarding several specific comorbidities, such as diabetes mellitus, hypertension, sleep apnea, depression/anxiety, and cardiovascular factors.
The exposed and unexposed cohorts were followed for a median period of 5.8 and 5.9 person-years, respectively.
‘Unclear’ mechanisms
Before weighting, 0.4% of participants in the exposed cohort (n = 73) developed epilepsy, versus 0.2% of participants in the unexposed cohort (n = 1,260) by the end of the follow-up period.
In the weighted cohorts, there were 50.1 epilepsy diagnoses per 100,000 person-years, versus 34.1 per 100,000 person-years (rate difference, 16 per 100,000 person-years).
The multivariable analysis of the weighted cohort showed the hazard ratio for epilepsy cases that were associated with bariatric surgery was 1.45 (95% confidence interval, 1.35-1.56), after adjusting for sleep apnea and including stroke as a time-varying covariate.
Having a stroke during the follow-up period increased epilepsy 14-fold in the exposed cohort (HR, 14.03; 95% CI, 4.25-46.25).
The investigators note that they were unable to measure obesity status or body mass index throughout the study and that some obesity-related comorbidities “may affect epilepsy risk.”
In addition, Dr. Burneo reported that the study did not investigate potential causes and mechanisms of the association between bariatric surgery and epilepsy risk.
Hypotheses “include potential nutritional deficiencies, receipt of general anesthesia, or other unclear causes,” he said.
“Future research should investigate epilepsy as a potential long-term complication of bariatric surgery, exploring the possible effects of this procedure,” Dr. Burneo added.
Risk-benefit discussion
In a comment, Jacqueline French, MD, professor of neurology at NYU Grossman School of Medicine, and director of NYU’s Epilepsy Study Consortium, said she was “not 100% surprised by the findings” because she has seen in her clinical practice “a number of patients who developed epilepsy after bariatric surgery or had a history of bariatric surgery at the time they developed epilepsy.”
On the other hand, she has also seen patients who did not have a history of bariatric surgery and who developed epilepsy.
“I’m unable to tell if there is an association, although I’ve had it at the back of my head as a thought and wondered about it,” said Dr. French, who is also the chief medical and innovation officer at the Epilepsy Foundation. She was not involved with the study.
She noted that possible mechanisms underlying the association are that gastric bypass surgery leads to a “significant alteration” in nutrient absorption. Moreover, “we now know that the microbiome is associated with epilepsy” and that changes occur in the gut microbiome after bariatric surgery, Dr. French said.
There are two take-home messages for practicing clinicians, she added.
“Although the risk [of developing epilepsy] is very low, it should be presented as part of the risks and benefits to patients considering bariatric surgery,” she said.
“It’s equally important to follow up on the potential differences in these patients who go on to develop epilepsy following bariatric surgery,” said Dr. French. “Is there a certain metabolic profile or some nutrient previously absorbed that now is not absorbed that might predispose people to risk?”
This would be “enormously important to know because it might not just pertain to these people but to a whole other cohort of people who develop epilepsy,” Dr. French concluded.
The study was funded by the Ontario Ministry of Health and Ministry of Long-Term Care and by the Jack Cowin Endowed Chair in Epilepsy Research at Western University. Dr. Burneo holds the Jack Cowin Endowed Chair in Epilepsy Research at Western University. The other investigators and Dr. French have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Analyzing health records, investigators compared almost 17,000 patients who had undergone bariatric surgery with more than 620,000 individuals with obesity who had not undergone the surgery.
During a minimum 3-year follow-up period, the surgery group had a 45% higher risk of developing epilepsy than the nonsurgery group. Moreover, patients who had a stroke after their bariatric surgery were 14 times more likely to develop epilepsy than those who did not have a stroke.
“When considering having bariatric surgery, people should talk to their doctors about the benefits and risks,” senior investigator Jorge Burneo, MD, professor of neurology, biostatistics, and epidemiology and endowed chair in epilepsy at Western University, London, told this news organization.
“While there are many health benefits of weight loss, our findings suggest that epilepsy is a long-term risk of bariatric surgery for weight loss,” Dr. Burneo said.
The findings were published online in Neurology.
Unrecognized risk factor?
Bariatric surgery has become more common as global rates of obesity have increased. The surgery has been shown to reduce the risk for serious obesity-related conditions, the researchers note.
However, “in addition to the positive outcomes of bariatric surgery, several long-term neurological complications have also been identified,” they write.
One previous study reported increased epilepsy risk following gastric bypass. Those findings “suggest that bariatric surgery may be an unrecognized epilepsy risk factor; however, this possible association has not been thoroughly explored,” write the investigators.
Dr. Burneo said he conducted the study because he has seen patients with epilepsy in his clinic who were “without risk factors, with normal MRIs, who shared the history of having bariatric surgery before the development of epilepsy.”
The researchers’ primary objective was to “assess whether epilepsy risk is elevated following bariatric surgery for weight loss relative to a nonsurgical cohort of patients who are obese,” he noted.
The study used linked administrative health databases in Ontario, Canada. Patients were accrued from July 1, 2010, to Dec. 31, 2016, and were followed until Dec. 31, 2019. The analysis included 639,472 participants, 2.7% of whom had undergone bariatric surgery.
The “exposed” cohort consisted of all Ontario residents aged 18 years or older who had undergone bariatric surgery during the 6-year period (n = 16,958; 65.1% women; mean age, 47.4 years), while the “unexposed” cohort consisted of patients hospitalized with a diagnosis of obesity who had not undergone bariatric surgery (n = 622,514; 62.8% women; mean age, 47.6 years).
Patients with a history of seizures, epilepsy, epilepsy risk factors, prior brain surgery, psychiatric disorders, or drug or alcohol abuse/dependence were excluded from the analysis.
The researchers collected data on patients’ sociodemographic characteristics at the index date, as well as Charlson Comorbidity Index scores during the 2 years prior to index, and data regarding several specific comorbidities, such as diabetes mellitus, hypertension, sleep apnea, depression/anxiety, and cardiovascular factors.
The exposed and unexposed cohorts were followed for a median period of 5.8 and 5.9 person-years, respectively.
‘Unclear’ mechanisms
Before weighting, 0.4% of participants in the exposed cohort (n = 73) developed epilepsy, versus 0.2% of participants in the unexposed cohort (n = 1,260) by the end of the follow-up period.
In the weighted cohorts, there were 50.1 epilepsy diagnoses per 100,000 person-years, versus 34.1 per 100,000 person-years (rate difference, 16 per 100,000 person-years).
The multivariable analysis of the weighted cohort showed the hazard ratio for epilepsy cases that were associated with bariatric surgery was 1.45 (95% confidence interval, 1.35-1.56), after adjusting for sleep apnea and including stroke as a time-varying covariate.
Having a stroke during the follow-up period increased epilepsy 14-fold in the exposed cohort (HR, 14.03; 95% CI, 4.25-46.25).
The investigators note that they were unable to measure obesity status or body mass index throughout the study and that some obesity-related comorbidities “may affect epilepsy risk.”
In addition, Dr. Burneo reported that the study did not investigate potential causes and mechanisms of the association between bariatric surgery and epilepsy risk.
Hypotheses “include potential nutritional deficiencies, receipt of general anesthesia, or other unclear causes,” he said.
“Future research should investigate epilepsy as a potential long-term complication of bariatric surgery, exploring the possible effects of this procedure,” Dr. Burneo added.
Risk-benefit discussion
In a comment, Jacqueline French, MD, professor of neurology at NYU Grossman School of Medicine, and director of NYU’s Epilepsy Study Consortium, said she was “not 100% surprised by the findings” because she has seen in her clinical practice “a number of patients who developed epilepsy after bariatric surgery or had a history of bariatric surgery at the time they developed epilepsy.”
On the other hand, she has also seen patients who did not have a history of bariatric surgery and who developed epilepsy.
“I’m unable to tell if there is an association, although I’ve had it at the back of my head as a thought and wondered about it,” said Dr. French, who is also the chief medical and innovation officer at the Epilepsy Foundation. She was not involved with the study.
She noted that possible mechanisms underlying the association are that gastric bypass surgery leads to a “significant alteration” in nutrient absorption. Moreover, “we now know that the microbiome is associated with epilepsy” and that changes occur in the gut microbiome after bariatric surgery, Dr. French said.
There are two take-home messages for practicing clinicians, she added.
“Although the risk [of developing epilepsy] is very low, it should be presented as part of the risks and benefits to patients considering bariatric surgery,” she said.
“It’s equally important to follow up on the potential differences in these patients who go on to develop epilepsy following bariatric surgery,” said Dr. French. “Is there a certain metabolic profile or some nutrient previously absorbed that now is not absorbed that might predispose people to risk?”
This would be “enormously important to know because it might not just pertain to these people but to a whole other cohort of people who develop epilepsy,” Dr. French concluded.
The study was funded by the Ontario Ministry of Health and Ministry of Long-Term Care and by the Jack Cowin Endowed Chair in Epilepsy Research at Western University. Dr. Burneo holds the Jack Cowin Endowed Chair in Epilepsy Research at Western University. The other investigators and Dr. French have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Analyzing health records, investigators compared almost 17,000 patients who had undergone bariatric surgery with more than 620,000 individuals with obesity who had not undergone the surgery.
During a minimum 3-year follow-up period, the surgery group had a 45% higher risk of developing epilepsy than the nonsurgery group. Moreover, patients who had a stroke after their bariatric surgery were 14 times more likely to develop epilepsy than those who did not have a stroke.
“When considering having bariatric surgery, people should talk to their doctors about the benefits and risks,” senior investigator Jorge Burneo, MD, professor of neurology, biostatistics, and epidemiology and endowed chair in epilepsy at Western University, London, told this news organization.
“While there are many health benefits of weight loss, our findings suggest that epilepsy is a long-term risk of bariatric surgery for weight loss,” Dr. Burneo said.
The findings were published online in Neurology.
Unrecognized risk factor?
Bariatric surgery has become more common as global rates of obesity have increased. The surgery has been shown to reduce the risk for serious obesity-related conditions, the researchers note.
However, “in addition to the positive outcomes of bariatric surgery, several long-term neurological complications have also been identified,” they write.
One previous study reported increased epilepsy risk following gastric bypass. Those findings “suggest that bariatric surgery may be an unrecognized epilepsy risk factor; however, this possible association has not been thoroughly explored,” write the investigators.
Dr. Burneo said he conducted the study because he has seen patients with epilepsy in his clinic who were “without risk factors, with normal MRIs, who shared the history of having bariatric surgery before the development of epilepsy.”
The researchers’ primary objective was to “assess whether epilepsy risk is elevated following bariatric surgery for weight loss relative to a nonsurgical cohort of patients who are obese,” he noted.
The study used linked administrative health databases in Ontario, Canada. Patients were accrued from July 1, 2010, to Dec. 31, 2016, and were followed until Dec. 31, 2019. The analysis included 639,472 participants, 2.7% of whom had undergone bariatric surgery.
The “exposed” cohort consisted of all Ontario residents aged 18 years or older who had undergone bariatric surgery during the 6-year period (n = 16,958; 65.1% women; mean age, 47.4 years), while the “unexposed” cohort consisted of patients hospitalized with a diagnosis of obesity who had not undergone bariatric surgery (n = 622,514; 62.8% women; mean age, 47.6 years).
Patients with a history of seizures, epilepsy, epilepsy risk factors, prior brain surgery, psychiatric disorders, or drug or alcohol abuse/dependence were excluded from the analysis.
The researchers collected data on patients’ sociodemographic characteristics at the index date, as well as Charlson Comorbidity Index scores during the 2 years prior to index, and data regarding several specific comorbidities, such as diabetes mellitus, hypertension, sleep apnea, depression/anxiety, and cardiovascular factors.
The exposed and unexposed cohorts were followed for a median period of 5.8 and 5.9 person-years, respectively.
‘Unclear’ mechanisms
Before weighting, 0.4% of participants in the exposed cohort (n = 73) developed epilepsy, versus 0.2% of participants in the unexposed cohort (n = 1,260) by the end of the follow-up period.
In the weighted cohorts, there were 50.1 epilepsy diagnoses per 100,000 person-years, versus 34.1 per 100,000 person-years (rate difference, 16 per 100,000 person-years).
The multivariable analysis of the weighted cohort showed the hazard ratio for epilepsy cases that were associated with bariatric surgery was 1.45 (95% confidence interval, 1.35-1.56), after adjusting for sleep apnea and including stroke as a time-varying covariate.
Having a stroke during the follow-up period increased epilepsy 14-fold in the exposed cohort (HR, 14.03; 95% CI, 4.25-46.25).
The investigators note that they were unable to measure obesity status or body mass index throughout the study and that some obesity-related comorbidities “may affect epilepsy risk.”
In addition, Dr. Burneo reported that the study did not investigate potential causes and mechanisms of the association between bariatric surgery and epilepsy risk.
Hypotheses “include potential nutritional deficiencies, receipt of general anesthesia, or other unclear causes,” he said.
“Future research should investigate epilepsy as a potential long-term complication of bariatric surgery, exploring the possible effects of this procedure,” Dr. Burneo added.
Risk-benefit discussion
In a comment, Jacqueline French, MD, professor of neurology at NYU Grossman School of Medicine, and director of NYU’s Epilepsy Study Consortium, said she was “not 100% surprised by the findings” because she has seen in her clinical practice “a number of patients who developed epilepsy after bariatric surgery or had a history of bariatric surgery at the time they developed epilepsy.”
On the other hand, she has also seen patients who did not have a history of bariatric surgery and who developed epilepsy.
“I’m unable to tell if there is an association, although I’ve had it at the back of my head as a thought and wondered about it,” said Dr. French, who is also the chief medical and innovation officer at the Epilepsy Foundation. She was not involved with the study.
She noted that possible mechanisms underlying the association are that gastric bypass surgery leads to a “significant alteration” in nutrient absorption. Moreover, “we now know that the microbiome is associated with epilepsy” and that changes occur in the gut microbiome after bariatric surgery, Dr. French said.
There are two take-home messages for practicing clinicians, she added.
“Although the risk [of developing epilepsy] is very low, it should be presented as part of the risks and benefits to patients considering bariatric surgery,” she said.
“It’s equally important to follow up on the potential differences in these patients who go on to develop epilepsy following bariatric surgery,” said Dr. French. “Is there a certain metabolic profile or some nutrient previously absorbed that now is not absorbed that might predispose people to risk?”
This would be “enormously important to know because it might not just pertain to these people but to a whole other cohort of people who develop epilepsy,” Dr. French concluded.
The study was funded by the Ontario Ministry of Health and Ministry of Long-Term Care and by the Jack Cowin Endowed Chair in Epilepsy Research at Western University. Dr. Burneo holds the Jack Cowin Endowed Chair in Epilepsy Research at Western University. The other investigators and Dr. French have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
High Risk of Long COVID Neurologic Sequelae in Veterans
We now know that the effects of COVID-19 don’t always end when the infection seems over. Long COVID—the postacute sequelae—can encompass a wide range of extrapulmonary organ dysfunctions. Most studies on COVID-19 have had follow-ups of 6 months or less with a narrow selection of neurologic outcomes, say Evan Xu, Yan Xie, PhD, and Ziyad Al-Aly, MD of the US Department of Veterans Affairs (VA) St. Louis Health Care System in Missouri. The 12-month study of 11,652,484 people published in Nature Medicine sounds an alert: Get ready to care for more patients with long-term, even chronic, neurologic disorders from migraine to stroke.
The researchers “leveraged the breadth and depth” of the VA’s national health care databases to build 3 groups: 154,068 people who survived the first 30 days of COVID-19; 5,638,795 VA users with no evidence of COVID-19 infection; and 5,859,621 VA users during 2017 (ie, prepandemic). Altogether, the groups corresponded to 14,064,985 person-years of follow-up.
The findings, which the researchers termed robust, revealed substantial risks and burdens beyond the first 30 days of COVID-19 infection, including “an array of neurologic disorders spanning several disease categories.”
Patients were at greater risk for stroke (both ischemic and hemorrhagic), cognition and memory disorders, peripheral nervous system disorders, episodic disorders like migraine and seizures, extrapyramidal and movement disorders, mental health disorders, musculoskeletal disorders, sensory disorders, Guillain-Barré syndrome, and encephalitis or encephalopathy.
The researchers estimated the hazard ratio of any neurological sequelae as 1.42. The risks were elevated even in people who did not require hospitalization during acute COVID-19 and increased according to the care setting of the acute phase of the disease from nonhospitalized to hospitalized and admitted to intensive care.
“Given the colossal scale of the pandemic,” the researchers say, governments and health systems should consider these findings when devising policy for continued management and developing plans for a postpandemic world. Some of the disorders they report on, they note, “are serious chronic conditions that will impact some people for a lifetime.” They point to 2 key findings: first, regardless of age, people with COVID-19 had a higher risk of all the neurologic outcomes examined, and second, the analyses suggest that the effects on risk were stronger in younger adults.
“The effects of these disorders on younger lives are profound and cannot be overstated,” the researchers say. Equally troubling, they note, is the stronger effect of COVID-19 on mental health, musculoskeletal, and episodic disorders in older adults, “highlighting their vulnerability” to these disorders following COVID-19 infection.
“It is imperative,” the researchers conclude, “that we recognize the enormous challenges posed by long COVID and all its downstream long-term consequences” and design capacity planning and clinical care pathways to address the needs of people who make it past the acute phase of COVID-19
We now know that the effects of COVID-19 don’t always end when the infection seems over. Long COVID—the postacute sequelae—can encompass a wide range of extrapulmonary organ dysfunctions. Most studies on COVID-19 have had follow-ups of 6 months or less with a narrow selection of neurologic outcomes, say Evan Xu, Yan Xie, PhD, and Ziyad Al-Aly, MD of the US Department of Veterans Affairs (VA) St. Louis Health Care System in Missouri. The 12-month study of 11,652,484 people published in Nature Medicine sounds an alert: Get ready to care for more patients with long-term, even chronic, neurologic disorders from migraine to stroke.
The researchers “leveraged the breadth and depth” of the VA’s national health care databases to build 3 groups: 154,068 people who survived the first 30 days of COVID-19; 5,638,795 VA users with no evidence of COVID-19 infection; and 5,859,621 VA users during 2017 (ie, prepandemic). Altogether, the groups corresponded to 14,064,985 person-years of follow-up.
The findings, which the researchers termed robust, revealed substantial risks and burdens beyond the first 30 days of COVID-19 infection, including “an array of neurologic disorders spanning several disease categories.”
Patients were at greater risk for stroke (both ischemic and hemorrhagic), cognition and memory disorders, peripheral nervous system disorders, episodic disorders like migraine and seizures, extrapyramidal and movement disorders, mental health disorders, musculoskeletal disorders, sensory disorders, Guillain-Barré syndrome, and encephalitis or encephalopathy.
The researchers estimated the hazard ratio of any neurological sequelae as 1.42. The risks were elevated even in people who did not require hospitalization during acute COVID-19 and increased according to the care setting of the acute phase of the disease from nonhospitalized to hospitalized and admitted to intensive care.
“Given the colossal scale of the pandemic,” the researchers say, governments and health systems should consider these findings when devising policy for continued management and developing plans for a postpandemic world. Some of the disorders they report on, they note, “are serious chronic conditions that will impact some people for a lifetime.” They point to 2 key findings: first, regardless of age, people with COVID-19 had a higher risk of all the neurologic outcomes examined, and second, the analyses suggest that the effects on risk were stronger in younger adults.
“The effects of these disorders on younger lives are profound and cannot be overstated,” the researchers say. Equally troubling, they note, is the stronger effect of COVID-19 on mental health, musculoskeletal, and episodic disorders in older adults, “highlighting their vulnerability” to these disorders following COVID-19 infection.
“It is imperative,” the researchers conclude, “that we recognize the enormous challenges posed by long COVID and all its downstream long-term consequences” and design capacity planning and clinical care pathways to address the needs of people who make it past the acute phase of COVID-19
We now know that the effects of COVID-19 don’t always end when the infection seems over. Long COVID—the postacute sequelae—can encompass a wide range of extrapulmonary organ dysfunctions. Most studies on COVID-19 have had follow-ups of 6 months or less with a narrow selection of neurologic outcomes, say Evan Xu, Yan Xie, PhD, and Ziyad Al-Aly, MD of the US Department of Veterans Affairs (VA) St. Louis Health Care System in Missouri. The 12-month study of 11,652,484 people published in Nature Medicine sounds an alert: Get ready to care for more patients with long-term, even chronic, neurologic disorders from migraine to stroke.
The researchers “leveraged the breadth and depth” of the VA’s national health care databases to build 3 groups: 154,068 people who survived the first 30 days of COVID-19; 5,638,795 VA users with no evidence of COVID-19 infection; and 5,859,621 VA users during 2017 (ie, prepandemic). Altogether, the groups corresponded to 14,064,985 person-years of follow-up.
The findings, which the researchers termed robust, revealed substantial risks and burdens beyond the first 30 days of COVID-19 infection, including “an array of neurologic disorders spanning several disease categories.”
Patients were at greater risk for stroke (both ischemic and hemorrhagic), cognition and memory disorders, peripheral nervous system disorders, episodic disorders like migraine and seizures, extrapyramidal and movement disorders, mental health disorders, musculoskeletal disorders, sensory disorders, Guillain-Barré syndrome, and encephalitis or encephalopathy.
The researchers estimated the hazard ratio of any neurological sequelae as 1.42. The risks were elevated even in people who did not require hospitalization during acute COVID-19 and increased according to the care setting of the acute phase of the disease from nonhospitalized to hospitalized and admitted to intensive care.
“Given the colossal scale of the pandemic,” the researchers say, governments and health systems should consider these findings when devising policy for continued management and developing plans for a postpandemic world. Some of the disorders they report on, they note, “are serious chronic conditions that will impact some people for a lifetime.” They point to 2 key findings: first, regardless of age, people with COVID-19 had a higher risk of all the neurologic outcomes examined, and second, the analyses suggest that the effects on risk were stronger in younger adults.
“The effects of these disorders on younger lives are profound and cannot be overstated,” the researchers say. Equally troubling, they note, is the stronger effect of COVID-19 on mental health, musculoskeletal, and episodic disorders in older adults, “highlighting their vulnerability” to these disorders following COVID-19 infection.
“It is imperative,” the researchers conclude, “that we recognize the enormous challenges posed by long COVID and all its downstream long-term consequences” and design capacity planning and clinical care pathways to address the needs of people who make it past the acute phase of COVID-19
Using SNRIs to prevent migraines in patients with depression
Ms. D, age 45, has major depressive disorder (MDD), generalized anxiety disorder (GAD), migraines, and hypertension. At a follow-up visit, she says she has been under a lot of stress at work in the past several months and feels her antidepressant is not working well for her depression or anxiety. Ms. D notes that lately she has had more frequent migraines, occurring approximately 4 times per month during the past 3 months. She describes a severe throbbing frontal pain that occurs primarily on the left side of her head, but sometimes on the right side. Ms. D says she experiences nausea, vomiting, and photophobia during these migraine episodes. The migraines last up to 12 hours, but often resolve with sumatriptan 50 mg as needed.
Ms. D takes fluoxetine 60 mg/d for depression and anxiety, lisinopril 20 mg/d for hypertension, as well as a women’s multivitamin and vitamin D3 daily. She has not tried other antidepressants and misses doses of her medications about once every other week. Her blood pressure is 125/80 mm Hg; heart rate is 80 beats per minute; and temperature is 37° C. Ms. D’s treatment team is considering switching her to a medication that can act as preventative therapy for migraines while also treating her depression and anxiety.
Migraine is a chronic, disabling neurovascular disorder that affects approximately 15% of the United States population.1 It is the second-leading disabling condition worldwide and may negatively affect social, family, personal, academic, and occupational domains.2 Migraine is often characterized by throbbing pain, is frequently unilateral, and may last 24 to 72 hours.3 It may occur with or without aura and can be associated with nausea, vomiting, or sensitivity to light.3 Episodic migraines occur <15 days a month, while chronic migraines occur ≥15 days a month.4
Many psychiatric, neurologic, vascular, and cardiac comorbidities are more prevalent in individuals who experience migraine headaches compared to the general population. Common psychiatric comorbidities found in patients with migraines are depression, bipolar disorder, GAD, panic disorder, and posttraumatic stress disorder5; MDD is the most common.4 A person who experiences migraine headaches is 2 to 4 times more likely to develop MDD than one who does not experience migraine headaches.4
First-line treatments for preventing migraine including divalproex, topiramate, metoprolol, propranolol, and timolol.6 However, for some patients with migraines and comorbid depression or anxiety, an antidepressant may be an option. This article briefly reviews the evidence for using antidepressants that have been studied for their ability to decrease migraine frequency.
Antidepressants that can prevent migraine
Tricyclic antidepressants (TCAs) are second- or third-line options for migraine prevention.6 While TCAs have proven to be effective for preventing migraines, many patients are unable to tolerate their adverse effects (ie, anticholinergic effects, sedation).7 TCAs may be more appealing for younger patients, who may be less bothered by anticholinergic burden, or those who have difficulty sleeping.
Serotonin-norepinephrine reuptake inhibitors (SNRIs). There has been growing interest in understanding the potential utility of SNRIs as a preventative treatment for migraines. Research has found that SNRIs are as effective as TCAs for preventing migraines and also more tolerable in terms of adverse effects.7 SNRIs such as venlafaxine and duloxetine are currently prescribed off-label to prevent migraines despite a lack of FDA approval for this indication.8
Continue to: Understanding the safety and efficacy...
Understanding the safety and efficacy of SNRIs as preventative treatment for episodic migraines is useful, particularly for patients with comorbid depression. The Table8-17 details clinical information related to SNRI use.
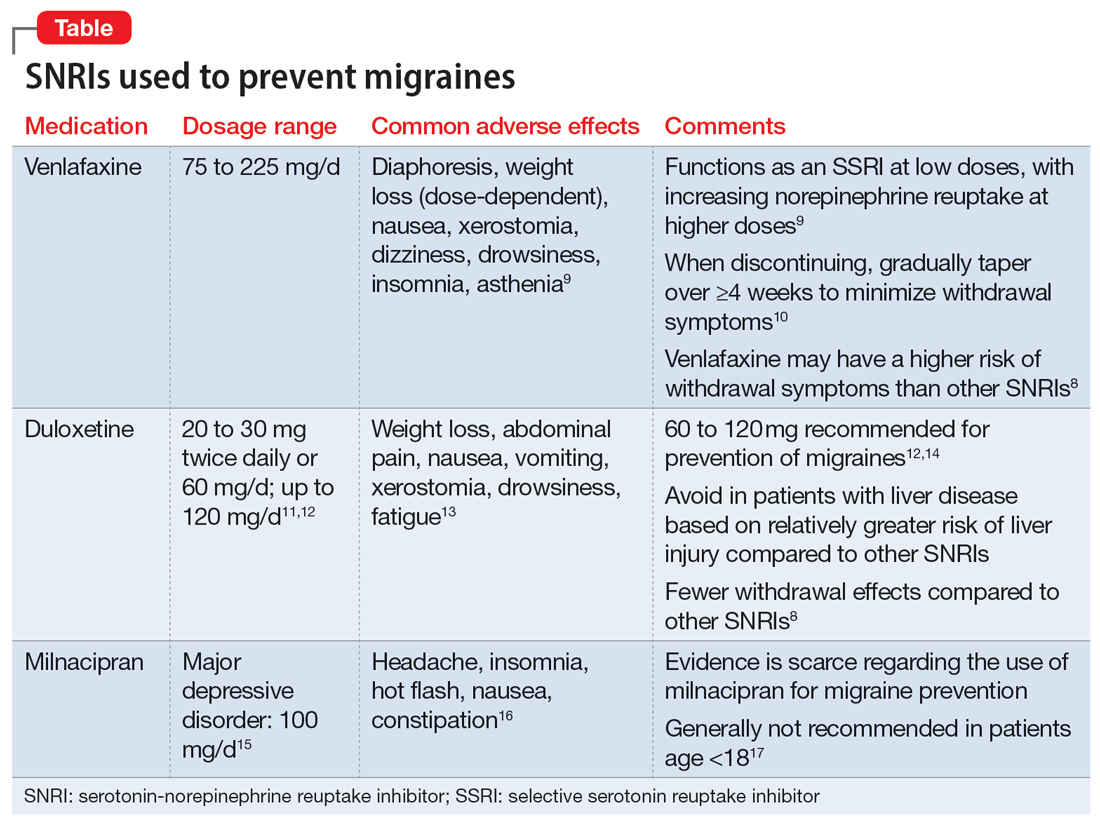
Duloxetine has demonstrated efficacy in preventing migraines in patients with comorbid depression.8 In a 2019 study, Kisler et al14 found that duloxetine 60 mg/d for 7 weeks was more effective for migraine prophylaxis than placebo as measured by the percentage of self-estimated migraine improvement by each patient compared to pretreatment levels (duloxetine: 52.3% ± 30.4%; placebo: 26.0% ± 27.3%; P = .001).
Venlafaxine has also demonstrated efficacy for preventing migraines in patients with comorbid depression.8 One study demonstrated a significant decrease in headaches per month with the use of venlafaxine 150 mg/d compared to placebo.18 Adelman et al19 found a reduction in migraine headaches per month (16.1 to 11.1, P < .0001) in patients who took venlafaxine for an average of 6 months with a mean dose of 150 mg/d. In a study of patients who did not have a mood disorder, Tarlaci20 found that venlafaxine reduced migraine headache independent of its antidepressant action.
Though milnacipran has not been studied as extensively as other SNRIs, evidence suggests it reduces the incidence of headaches and migraines, especially among episodic migraine patients. Although it has an equipotent effect on both serotonin and norepinephrine (NE) reuptake, milnacipran has a greater NE effect compared to other SNRIs approved for treating mood disorders. A prospective, single-arm study by Engel et al21 found a significant (P < .005) reduction from baseline in all headache and migraine days per month with the use of milnacipran 100 mg/d over the course of 3 months. The number of headache days per month was reduced by 4.2 compared to baseline. This same study reported improved functionality and reduced use of acute and symptomatic medications overall due to the decrease in headaches and migraines.21
In addition to demonstrating that certain SNRIs can effectively prevent migraine, some evidence suggests certain patients may benefit from the opportunity to decrease pill burden by using a single medication to treat both depression and migraine.22 Duloxetine may be preferred for patients who struggle with adherence (such as Ms. D) due to its relatively lower incidence of withdrawal symptoms compared to venlafaxine.8
CASE CONTINUED
Ms. D’s psychiatrist concludes she would be an appropriate candidate for treatment with an SNRI due to her history of MDD and chronic migraines. Because Ms. D expresses some difficulty remembering to take her medications, the psychiatrist recommends duloxetine because it is less likely to produce withdrawal symptoms compared to venlafaxine. To decrease pill burden, fluoxetine 60 mg is stopped with no taper due to its long half-life, and duloxetine is started at 30 mg/d, with a planned increase to 60 mg/d after 1 to 2 weeks as tolerated to target both mood and migraine prophylaxis. Duloxetine will not interact with Ms. D’s current medication regimen, including lisinopril, women’s multivitamin, or vitamin D3. The psychiatrist discusses the importance of medication adherence to improve her conditions effectively and safely. Ms. D’s heart rate and blood pressure will continue to be monitored.
Related Resources
- Leo RJ, Khalid K. Antidepressants for chronic pain. Current Psychiatry. 2019;18(2):8-16,21-22.
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
Drug Brand Names
Divalproex • Depakote
Duloxetine • Cymbalta
Fluoxetine • Prozac
Lisinopril • Zestril, Prinivil
Milnacipran • Savella
Sumatriptan • Imitrex
Topiramate • Topamax
Venlafaxine • Effexor
1. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496-505. doi:10.1111/head.13281
2. GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954-976. doi:10.1016/S1474-4422(18)30322-3
3. Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346(4):257-270. doi:10.1056/NEJMra010917
4. Amoozegar F. Depression comorbidity in migraine. Int Rev Psychiatry. 2017;29(5):504-515. doi:10.1080/09540261.2017.1326882
5. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631-649. doi:10.1016/j.ncl.2019.06.001
6. Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17-24.
7. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine (Baltimore). 2017;96(22):e6989. doi:10.1097/MD.0000000000006989
8. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
9. Venlafaxine. Lexicomp. 2021. http://online.lexi.com/
10. Ogle NR, Akkerman SR. Guidance for the discontinuation or switching of antidepressant therapies in adults. J Pharm Pract. 2013;26(4):389-396. doi:10.1177/0897190012467210
11. Duloxetine [package insert]. Indianapolis, IN: Eli Lilly and Company; 2004.
12. Young WB, Bradley KC, Anjum MW, et al. Duloxetine prophylaxis for episodic migraine in persons without depression: a prospective study. Headache. 2013;53(9):1430-1437.
13. Duloxetine. Lexicomp. 2021. http://online.lexi.com/
14. Kisler LB, Weissman-Fogel I, Coghill RC, et al. Individualization of migraine prevention: a randomized controlled trial of psychophysical-based prediction of duloxetine efficacy. Clin J Pain. 2019;35(9):753-765.
15. Mansuy L. Antidepressant therapy with milnacipran and venlafaxine. Neuropsychiatr Dis Treat. 2010;6 (Suppl I):17-22.
16. Milnacipran. Lexicomp. 2021. http://online.lexi.com/
17. Milnacipran. MedlinePlus. Updated January 22, 2022. Accessed August 19, 2022. https://medlineplus.gov/druginfo/meds/a609016.html
18. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152. doi:10.1111/j.1526-4610.2005.05029.x
19. Adelman LC, Adelman JU, Von Seggern R, et al. Venlafaxine extended release (XR) for the prophylaxis of migraine and tension-type headache: a retrospective study in a clinical setting. Headache. 2000;40(7):572-580. doi:10.1046/j.1526-4610.2000.00089.x
20. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258. doi:10.1097/WNF.0b013e3181a8c84f
21. Engel ER, Kudrow D, Rapoport AM. A prospective, open-label study of milnacipran in the prevention of headache in patients with episodic or chronic migraine. Neurol Sci. 2014;35(3):429-435. doi:10.1007/s10072-013-1536-0
22. Baumgartner A, Drame K, Geutjens S, et al. Does the polypill improve patient adherence compared to its individual formulations? A systematic review. Pharmaceutics. 2020;12(2):190.
Ms. D, age 45, has major depressive disorder (MDD), generalized anxiety disorder (GAD), migraines, and hypertension. At a follow-up visit, she says she has been under a lot of stress at work in the past several months and feels her antidepressant is not working well for her depression or anxiety. Ms. D notes that lately she has had more frequent migraines, occurring approximately 4 times per month during the past 3 months. She describes a severe throbbing frontal pain that occurs primarily on the left side of her head, but sometimes on the right side. Ms. D says she experiences nausea, vomiting, and photophobia during these migraine episodes. The migraines last up to 12 hours, but often resolve with sumatriptan 50 mg as needed.
Ms. D takes fluoxetine 60 mg/d for depression and anxiety, lisinopril 20 mg/d for hypertension, as well as a women’s multivitamin and vitamin D3 daily. She has not tried other antidepressants and misses doses of her medications about once every other week. Her blood pressure is 125/80 mm Hg; heart rate is 80 beats per minute; and temperature is 37° C. Ms. D’s treatment team is considering switching her to a medication that can act as preventative therapy for migraines while also treating her depression and anxiety.
Migraine is a chronic, disabling neurovascular disorder that affects approximately 15% of the United States population.1 It is the second-leading disabling condition worldwide and may negatively affect social, family, personal, academic, and occupational domains.2 Migraine is often characterized by throbbing pain, is frequently unilateral, and may last 24 to 72 hours.3 It may occur with or without aura and can be associated with nausea, vomiting, or sensitivity to light.3 Episodic migraines occur <15 days a month, while chronic migraines occur ≥15 days a month.4
Many psychiatric, neurologic, vascular, and cardiac comorbidities are more prevalent in individuals who experience migraine headaches compared to the general population. Common psychiatric comorbidities found in patients with migraines are depression, bipolar disorder, GAD, panic disorder, and posttraumatic stress disorder5; MDD is the most common.4 A person who experiences migraine headaches is 2 to 4 times more likely to develop MDD than one who does not experience migraine headaches.4
First-line treatments for preventing migraine including divalproex, topiramate, metoprolol, propranolol, and timolol.6 However, for some patients with migraines and comorbid depression or anxiety, an antidepressant may be an option. This article briefly reviews the evidence for using antidepressants that have been studied for their ability to decrease migraine frequency.
Antidepressants that can prevent migraine
Tricyclic antidepressants (TCAs) are second- or third-line options for migraine prevention.6 While TCAs have proven to be effective for preventing migraines, many patients are unable to tolerate their adverse effects (ie, anticholinergic effects, sedation).7 TCAs may be more appealing for younger patients, who may be less bothered by anticholinergic burden, or those who have difficulty sleeping.
Serotonin-norepinephrine reuptake inhibitors (SNRIs). There has been growing interest in understanding the potential utility of SNRIs as a preventative treatment for migraines. Research has found that SNRIs are as effective as TCAs for preventing migraines and also more tolerable in terms of adverse effects.7 SNRIs such as venlafaxine and duloxetine are currently prescribed off-label to prevent migraines despite a lack of FDA approval for this indication.8
Continue to: Understanding the safety and efficacy...
Understanding the safety and efficacy of SNRIs as preventative treatment for episodic migraines is useful, particularly for patients with comorbid depression. The Table8-17 details clinical information related to SNRI use.

Duloxetine has demonstrated efficacy in preventing migraines in patients with comorbid depression.8 In a 2019 study, Kisler et al14 found that duloxetine 60 mg/d for 7 weeks was more effective for migraine prophylaxis than placebo as measured by the percentage of self-estimated migraine improvement by each patient compared to pretreatment levels (duloxetine: 52.3% ± 30.4%; placebo: 26.0% ± 27.3%; P = .001).
Venlafaxine has also demonstrated efficacy for preventing migraines in patients with comorbid depression.8 One study demonstrated a significant decrease in headaches per month with the use of venlafaxine 150 mg/d compared to placebo.18 Adelman et al19 found a reduction in migraine headaches per month (16.1 to 11.1, P < .0001) in patients who took venlafaxine for an average of 6 months with a mean dose of 150 mg/d. In a study of patients who did not have a mood disorder, Tarlaci20 found that venlafaxine reduced migraine headache independent of its antidepressant action.
Though milnacipran has not been studied as extensively as other SNRIs, evidence suggests it reduces the incidence of headaches and migraines, especially among episodic migraine patients. Although it has an equipotent effect on both serotonin and norepinephrine (NE) reuptake, milnacipran has a greater NE effect compared to other SNRIs approved for treating mood disorders. A prospective, single-arm study by Engel et al21 found a significant (P < .005) reduction from baseline in all headache and migraine days per month with the use of milnacipran 100 mg/d over the course of 3 months. The number of headache days per month was reduced by 4.2 compared to baseline. This same study reported improved functionality and reduced use of acute and symptomatic medications overall due to the decrease in headaches and migraines.21
In addition to demonstrating that certain SNRIs can effectively prevent migraine, some evidence suggests certain patients may benefit from the opportunity to decrease pill burden by using a single medication to treat both depression and migraine.22 Duloxetine may be preferred for patients who struggle with adherence (such as Ms. D) due to its relatively lower incidence of withdrawal symptoms compared to venlafaxine.8
CASE CONTINUED
Ms. D’s psychiatrist concludes she would be an appropriate candidate for treatment with an SNRI due to her history of MDD and chronic migraines. Because Ms. D expresses some difficulty remembering to take her medications, the psychiatrist recommends duloxetine because it is less likely to produce withdrawal symptoms compared to venlafaxine. To decrease pill burden, fluoxetine 60 mg is stopped with no taper due to its long half-life, and duloxetine is started at 30 mg/d, with a planned increase to 60 mg/d after 1 to 2 weeks as tolerated to target both mood and migraine prophylaxis. Duloxetine will not interact with Ms. D’s current medication regimen, including lisinopril, women’s multivitamin, or vitamin D3. The psychiatrist discusses the importance of medication adherence to improve her conditions effectively and safely. Ms. D’s heart rate and blood pressure will continue to be monitored.
Related Resources
- Leo RJ, Khalid K. Antidepressants for chronic pain. Current Psychiatry. 2019;18(2):8-16,21-22.
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
Drug Brand Names
Divalproex • Depakote
Duloxetine • Cymbalta
Fluoxetine • Prozac
Lisinopril • Zestril, Prinivil
Milnacipran • Savella
Sumatriptan • Imitrex
Topiramate • Topamax
Venlafaxine • Effexor
Ms. D, age 45, has major depressive disorder (MDD), generalized anxiety disorder (GAD), migraines, and hypertension. At a follow-up visit, she says she has been under a lot of stress at work in the past several months and feels her antidepressant is not working well for her depression or anxiety. Ms. D notes that lately she has had more frequent migraines, occurring approximately 4 times per month during the past 3 months. She describes a severe throbbing frontal pain that occurs primarily on the left side of her head, but sometimes on the right side. Ms. D says she experiences nausea, vomiting, and photophobia during these migraine episodes. The migraines last up to 12 hours, but often resolve with sumatriptan 50 mg as needed.
Ms. D takes fluoxetine 60 mg/d for depression and anxiety, lisinopril 20 mg/d for hypertension, as well as a women’s multivitamin and vitamin D3 daily. She has not tried other antidepressants and misses doses of her medications about once every other week. Her blood pressure is 125/80 mm Hg; heart rate is 80 beats per minute; and temperature is 37° C. Ms. D’s treatment team is considering switching her to a medication that can act as preventative therapy for migraines while also treating her depression and anxiety.
Migraine is a chronic, disabling neurovascular disorder that affects approximately 15% of the United States population.1 It is the second-leading disabling condition worldwide and may negatively affect social, family, personal, academic, and occupational domains.2 Migraine is often characterized by throbbing pain, is frequently unilateral, and may last 24 to 72 hours.3 It may occur with or without aura and can be associated with nausea, vomiting, or sensitivity to light.3 Episodic migraines occur <15 days a month, while chronic migraines occur ≥15 days a month.4
Many psychiatric, neurologic, vascular, and cardiac comorbidities are more prevalent in individuals who experience migraine headaches compared to the general population. Common psychiatric comorbidities found in patients with migraines are depression, bipolar disorder, GAD, panic disorder, and posttraumatic stress disorder5; MDD is the most common.4 A person who experiences migraine headaches is 2 to 4 times more likely to develop MDD than one who does not experience migraine headaches.4
First-line treatments for preventing migraine including divalproex, topiramate, metoprolol, propranolol, and timolol.6 However, for some patients with migraines and comorbid depression or anxiety, an antidepressant may be an option. This article briefly reviews the evidence for using antidepressants that have been studied for their ability to decrease migraine frequency.
Antidepressants that can prevent migraine
Tricyclic antidepressants (TCAs) are second- or third-line options for migraine prevention.6 While TCAs have proven to be effective for preventing migraines, many patients are unable to tolerate their adverse effects (ie, anticholinergic effects, sedation).7 TCAs may be more appealing for younger patients, who may be less bothered by anticholinergic burden, or those who have difficulty sleeping.
Serotonin-norepinephrine reuptake inhibitors (SNRIs). There has been growing interest in understanding the potential utility of SNRIs as a preventative treatment for migraines. Research has found that SNRIs are as effective as TCAs for preventing migraines and also more tolerable in terms of adverse effects.7 SNRIs such as venlafaxine and duloxetine are currently prescribed off-label to prevent migraines despite a lack of FDA approval for this indication.8
Continue to: Understanding the safety and efficacy...
Understanding the safety and efficacy of SNRIs as preventative treatment for episodic migraines is useful, particularly for patients with comorbid depression. The Table8-17 details clinical information related to SNRI use.

Duloxetine has demonstrated efficacy in preventing migraines in patients with comorbid depression.8 In a 2019 study, Kisler et al14 found that duloxetine 60 mg/d for 7 weeks was more effective for migraine prophylaxis than placebo as measured by the percentage of self-estimated migraine improvement by each patient compared to pretreatment levels (duloxetine: 52.3% ± 30.4%; placebo: 26.0% ± 27.3%; P = .001).
Venlafaxine has also demonstrated efficacy for preventing migraines in patients with comorbid depression.8 One study demonstrated a significant decrease in headaches per month with the use of venlafaxine 150 mg/d compared to placebo.18 Adelman et al19 found a reduction in migraine headaches per month (16.1 to 11.1, P < .0001) in patients who took venlafaxine for an average of 6 months with a mean dose of 150 mg/d. In a study of patients who did not have a mood disorder, Tarlaci20 found that venlafaxine reduced migraine headache independent of its antidepressant action.
Though milnacipran has not been studied as extensively as other SNRIs, evidence suggests it reduces the incidence of headaches and migraines, especially among episodic migraine patients. Although it has an equipotent effect on both serotonin and norepinephrine (NE) reuptake, milnacipran has a greater NE effect compared to other SNRIs approved for treating mood disorders. A prospective, single-arm study by Engel et al21 found a significant (P < .005) reduction from baseline in all headache and migraine days per month with the use of milnacipran 100 mg/d over the course of 3 months. The number of headache days per month was reduced by 4.2 compared to baseline. This same study reported improved functionality and reduced use of acute and symptomatic medications overall due to the decrease in headaches and migraines.21
In addition to demonstrating that certain SNRIs can effectively prevent migraine, some evidence suggests certain patients may benefit from the opportunity to decrease pill burden by using a single medication to treat both depression and migraine.22 Duloxetine may be preferred for patients who struggle with adherence (such as Ms. D) due to its relatively lower incidence of withdrawal symptoms compared to venlafaxine.8
CASE CONTINUED
Ms. D’s psychiatrist concludes she would be an appropriate candidate for treatment with an SNRI due to her history of MDD and chronic migraines. Because Ms. D expresses some difficulty remembering to take her medications, the psychiatrist recommends duloxetine because it is less likely to produce withdrawal symptoms compared to venlafaxine. To decrease pill burden, fluoxetine 60 mg is stopped with no taper due to its long half-life, and duloxetine is started at 30 mg/d, with a planned increase to 60 mg/d after 1 to 2 weeks as tolerated to target both mood and migraine prophylaxis. Duloxetine will not interact with Ms. D’s current medication regimen, including lisinopril, women’s multivitamin, or vitamin D3. The psychiatrist discusses the importance of medication adherence to improve her conditions effectively and safely. Ms. D’s heart rate and blood pressure will continue to be monitored.
Related Resources
- Leo RJ, Khalid K. Antidepressants for chronic pain. Current Psychiatry. 2019;18(2):8-16,21-22.
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
Drug Brand Names
Divalproex • Depakote
Duloxetine • Cymbalta
Fluoxetine • Prozac
Lisinopril • Zestril, Prinivil
Milnacipran • Savella
Sumatriptan • Imitrex
Topiramate • Topamax
Venlafaxine • Effexor
1. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496-505. doi:10.1111/head.13281
2. GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954-976. doi:10.1016/S1474-4422(18)30322-3
3. Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346(4):257-270. doi:10.1056/NEJMra010917
4. Amoozegar F. Depression comorbidity in migraine. Int Rev Psychiatry. 2017;29(5):504-515. doi:10.1080/09540261.2017.1326882
5. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631-649. doi:10.1016/j.ncl.2019.06.001
6. Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17-24.
7. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine (Baltimore). 2017;96(22):e6989. doi:10.1097/MD.0000000000006989
8. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
9. Venlafaxine. Lexicomp. 2021. http://online.lexi.com/
10. Ogle NR, Akkerman SR. Guidance for the discontinuation or switching of antidepressant therapies in adults. J Pharm Pract. 2013;26(4):389-396. doi:10.1177/0897190012467210
11. Duloxetine [package insert]. Indianapolis, IN: Eli Lilly and Company; 2004.
12. Young WB, Bradley KC, Anjum MW, et al. Duloxetine prophylaxis for episodic migraine in persons without depression: a prospective study. Headache. 2013;53(9):1430-1437.
13. Duloxetine. Lexicomp. 2021. http://online.lexi.com/
14. Kisler LB, Weissman-Fogel I, Coghill RC, et al. Individualization of migraine prevention: a randomized controlled trial of psychophysical-based prediction of duloxetine efficacy. Clin J Pain. 2019;35(9):753-765.
15. Mansuy L. Antidepressant therapy with milnacipran and venlafaxine. Neuropsychiatr Dis Treat. 2010;6 (Suppl I):17-22.
16. Milnacipran. Lexicomp. 2021. http://online.lexi.com/
17. Milnacipran. MedlinePlus. Updated January 22, 2022. Accessed August 19, 2022. https://medlineplus.gov/druginfo/meds/a609016.html
18. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152. doi:10.1111/j.1526-4610.2005.05029.x
19. Adelman LC, Adelman JU, Von Seggern R, et al. Venlafaxine extended release (XR) for the prophylaxis of migraine and tension-type headache: a retrospective study in a clinical setting. Headache. 2000;40(7):572-580. doi:10.1046/j.1526-4610.2000.00089.x
20. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258. doi:10.1097/WNF.0b013e3181a8c84f
21. Engel ER, Kudrow D, Rapoport AM. A prospective, open-label study of milnacipran in the prevention of headache in patients with episodic or chronic migraine. Neurol Sci. 2014;35(3):429-435. doi:10.1007/s10072-013-1536-0
22. Baumgartner A, Drame K, Geutjens S, et al. Does the polypill improve patient adherence compared to its individual formulations? A systematic review. Pharmaceutics. 2020;12(2):190.
1. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496-505. doi:10.1111/head.13281
2. GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954-976. doi:10.1016/S1474-4422(18)30322-3
3. Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346(4):257-270. doi:10.1056/NEJMra010917
4. Amoozegar F. Depression comorbidity in migraine. Int Rev Psychiatry. 2017;29(5):504-515. doi:10.1080/09540261.2017.1326882
5. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631-649. doi:10.1016/j.ncl.2019.06.001
6. Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17-24.
7. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine (Baltimore). 2017;96(22):e6989. doi:10.1097/MD.0000000000006989
8. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
9. Venlafaxine. Lexicomp. 2021. http://online.lexi.com/
10. Ogle NR, Akkerman SR. Guidance for the discontinuation or switching of antidepressant therapies in adults. J Pharm Pract. 2013;26(4):389-396. doi:10.1177/0897190012467210
11. Duloxetine [package insert]. Indianapolis, IN: Eli Lilly and Company; 2004.
12. Young WB, Bradley KC, Anjum MW, et al. Duloxetine prophylaxis for episodic migraine in persons without depression: a prospective study. Headache. 2013;53(9):1430-1437.
13. Duloxetine. Lexicomp. 2021. http://online.lexi.com/
14. Kisler LB, Weissman-Fogel I, Coghill RC, et al. Individualization of migraine prevention: a randomized controlled trial of psychophysical-based prediction of duloxetine efficacy. Clin J Pain. 2019;35(9):753-765.
15. Mansuy L. Antidepressant therapy with milnacipran and venlafaxine. Neuropsychiatr Dis Treat. 2010;6 (Suppl I):17-22.
16. Milnacipran. Lexicomp. 2021. http://online.lexi.com/
17. Milnacipran. MedlinePlus. Updated January 22, 2022. Accessed August 19, 2022. https://medlineplus.gov/druginfo/meds/a609016.html
18. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152. doi:10.1111/j.1526-4610.2005.05029.x
19. Adelman LC, Adelman JU, Von Seggern R, et al. Venlafaxine extended release (XR) for the prophylaxis of migraine and tension-type headache: a retrospective study in a clinical setting. Headache. 2000;40(7):572-580. doi:10.1046/j.1526-4610.2000.00089.x
20. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258. doi:10.1097/WNF.0b013e3181a8c84f
21. Engel ER, Kudrow D, Rapoport AM. A prospective, open-label study of milnacipran in the prevention of headache in patients with episodic or chronic migraine. Neurol Sci. 2014;35(3):429-435. doi:10.1007/s10072-013-1536-0
22. Baumgartner A, Drame K, Geutjens S, et al. Does the polypill improve patient adherence compared to its individual formulations? A systematic review. Pharmaceutics. 2020;12(2):190.
Racial disparities in preventive services use seen among patients with spina bifida or cerebral palsy
Black adults also had lower odds of having a bone density screening, compared with White adults. Plus, comorbidities were highest among the Black patients, according to the paper, which was published in Annals of Family Medicine.
Elham Mahmoudi, PhD, and her coauthors examined private insurance claims from 11,635 patients with cerebral palsy (CP) or spina bifida over ten years from 2007 to 2017. The researchers analyzed comorbidities and compared the rates of different psychological, cardiometabolic, and musculoskeletal conditions among these patients.
Only 23% of Hispanic participants and 18% of Black participants attended an annual wellness visit, compared with 32% of the White participants.
Only 1% of Black and 2% of White participants received any bone density screening (odds ratio = 0.54, 95% confidence interval [CI], 0.31-0.95), a service that is essential for catching a patient’s potential risk for osteoporosis and fractures.
According to the researchers, patients accessed services such as bone density scans, cholesterol assessments, diabetes screenings, and annual wellness visits less than recommended for people with those chronic conditions.
“People with spina bifida and cerebral palsy have complex care needs. We know through our work that chronic conditions are much higher among them compared with adults without disabilities,” Dr. Mahmoudi, associate professor in the department of family medicine at University of Michigan, Ann Arbor, said in an interview. “I was surprised to see even with private insurance, the rate of using preventative services is so low among White people and minority populations.”
Comorbidities highest in Black participants
Black adults had the highest comorbidity score of 2.5, and Hispanic adults had the lowest comorbidity score of 1.8. For White adults in the study, the comorbidity score was 2.0.
Osteoporosis, a common concern for people with spina bifida or cerebral palsy, was detected in around 4% of all participants. Osteoarthritis was detected in 13.38% of Black participants, versus 8.53% of Hispanic participants and 11.09% of White participants.
Diabetes and hypertension were more common among Black participants than among Hispanic and White participants. The percentages of Black patients with hypertension and diabetes were 16.5% and 39.89%, respectively. Among the Hispanic and White adults, the percentages with hypertension were 22.3% and 28.2%, respectively, according to the paper.
Disparities in access
Jamil Paden, racial and health equity manager at the Christopher and Dana Reeve Foundation, said getting access to literature, transportation, tables, chairs, weigh scales, and imaging equipment that accommodate the needs of people with disabilities are some of the biggest challenges for people with disabilities who are trying to receive care.
“It’s not a one size fits all, we have to recognize that if someone doesn’t see themselves in a particular place, then it makes it more challenging for them to feel comfortable speaking up and saying things about their health, which would prevent a person from saying something early on,” Mr. Paden said in an interview. “That particular issue will continue to grow and become more of a health risk, or health challenge down the line.”
Mr. Paden emphasized intersections between class, race, and circumstances which can, together, make health care less equitable for people with disabilities, especially in underserved communities and communities of color. He urged health care providers to distance their practices from a “one size fits all” approach to treatment and engage in their patients’ individual lives and communities.
“It’s not enough to just say, Hey, you have a disability. So let me treat your disability ... You have to recognize that although a patient may have a dire diagnosis, they also are a person of color, and they have to navigate different aspects of life from their counterparts,” he said.
Dr. Mahmoudi said patient and provider understanding of the disability is often lacking. She recommended advocating for patients, noting that giving both patients and providers the tools to further educate themselves and apply that to their regular visits is a good first step.
“Just having access to a facility doesn’t mean they will get the services they need. Preventative services that are recommended for people with disabilities differ from the general population. Providers should be educated about that and the patient needs to be educated about that,” she added.
“Patients who do not approach clinicians get lost in the system. Maybe many facilities are not disability friendly, or they need health literacy. If they don’t know they are at risk for osteoporosis, for example, then they won’t ask,” Dr. Mahmoudi said.
The study was funded by The National Institute on Disability, Independent Living, and Rehabilitation Research. Dr. Mahmoudi and Mr. Paden report no relevant financial relationships.
Black adults also had lower odds of having a bone density screening, compared with White adults. Plus, comorbidities were highest among the Black patients, according to the paper, which was published in Annals of Family Medicine.
Elham Mahmoudi, PhD, and her coauthors examined private insurance claims from 11,635 patients with cerebral palsy (CP) or spina bifida over ten years from 2007 to 2017. The researchers analyzed comorbidities and compared the rates of different psychological, cardiometabolic, and musculoskeletal conditions among these patients.
Only 23% of Hispanic participants and 18% of Black participants attended an annual wellness visit, compared with 32% of the White participants.
Only 1% of Black and 2% of White participants received any bone density screening (odds ratio = 0.54, 95% confidence interval [CI], 0.31-0.95), a service that is essential for catching a patient’s potential risk for osteoporosis and fractures.
According to the researchers, patients accessed services such as bone density scans, cholesterol assessments, diabetes screenings, and annual wellness visits less than recommended for people with those chronic conditions.
“People with spina bifida and cerebral palsy have complex care needs. We know through our work that chronic conditions are much higher among them compared with adults without disabilities,” Dr. Mahmoudi, associate professor in the department of family medicine at University of Michigan, Ann Arbor, said in an interview. “I was surprised to see even with private insurance, the rate of using preventative services is so low among White people and minority populations.”
Comorbidities highest in Black participants
Black adults had the highest comorbidity score of 2.5, and Hispanic adults had the lowest comorbidity score of 1.8. For White adults in the study, the comorbidity score was 2.0.
Osteoporosis, a common concern for people with spina bifida or cerebral palsy, was detected in around 4% of all participants. Osteoarthritis was detected in 13.38% of Black participants, versus 8.53% of Hispanic participants and 11.09% of White participants.
Diabetes and hypertension were more common among Black participants than among Hispanic and White participants. The percentages of Black patients with hypertension and diabetes were 16.5% and 39.89%, respectively. Among the Hispanic and White adults, the percentages with hypertension were 22.3% and 28.2%, respectively, according to the paper.
Disparities in access
Jamil Paden, racial and health equity manager at the Christopher and Dana Reeve Foundation, said getting access to literature, transportation, tables, chairs, weigh scales, and imaging equipment that accommodate the needs of people with disabilities are some of the biggest challenges for people with disabilities who are trying to receive care.
“It’s not a one size fits all, we have to recognize that if someone doesn’t see themselves in a particular place, then it makes it more challenging for them to feel comfortable speaking up and saying things about their health, which would prevent a person from saying something early on,” Mr. Paden said in an interview. “That particular issue will continue to grow and become more of a health risk, or health challenge down the line.”
Mr. Paden emphasized intersections between class, race, and circumstances which can, together, make health care less equitable for people with disabilities, especially in underserved communities and communities of color. He urged health care providers to distance their practices from a “one size fits all” approach to treatment and engage in their patients’ individual lives and communities.
“It’s not enough to just say, Hey, you have a disability. So let me treat your disability ... You have to recognize that although a patient may have a dire diagnosis, they also are a person of color, and they have to navigate different aspects of life from their counterparts,” he said.
Dr. Mahmoudi said patient and provider understanding of the disability is often lacking. She recommended advocating for patients, noting that giving both patients and providers the tools to further educate themselves and apply that to their regular visits is a good first step.
“Just having access to a facility doesn’t mean they will get the services they need. Preventative services that are recommended for people with disabilities differ from the general population. Providers should be educated about that and the patient needs to be educated about that,” she added.
“Patients who do not approach clinicians get lost in the system. Maybe many facilities are not disability friendly, or they need health literacy. If they don’t know they are at risk for osteoporosis, for example, then they won’t ask,” Dr. Mahmoudi said.
The study was funded by The National Institute on Disability, Independent Living, and Rehabilitation Research. Dr. Mahmoudi and Mr. Paden report no relevant financial relationships.
Black adults also had lower odds of having a bone density screening, compared with White adults. Plus, comorbidities were highest among the Black patients, according to the paper, which was published in Annals of Family Medicine.
Elham Mahmoudi, PhD, and her coauthors examined private insurance claims from 11,635 patients with cerebral palsy (CP) or spina bifida over ten years from 2007 to 2017. The researchers analyzed comorbidities and compared the rates of different psychological, cardiometabolic, and musculoskeletal conditions among these patients.
Only 23% of Hispanic participants and 18% of Black participants attended an annual wellness visit, compared with 32% of the White participants.
Only 1% of Black and 2% of White participants received any bone density screening (odds ratio = 0.54, 95% confidence interval [CI], 0.31-0.95), a service that is essential for catching a patient’s potential risk for osteoporosis and fractures.
According to the researchers, patients accessed services such as bone density scans, cholesterol assessments, diabetes screenings, and annual wellness visits less than recommended for people with those chronic conditions.
“People with spina bifida and cerebral palsy have complex care needs. We know through our work that chronic conditions are much higher among them compared with adults without disabilities,” Dr. Mahmoudi, associate professor in the department of family medicine at University of Michigan, Ann Arbor, said in an interview. “I was surprised to see even with private insurance, the rate of using preventative services is so low among White people and minority populations.”
Comorbidities highest in Black participants
Black adults had the highest comorbidity score of 2.5, and Hispanic adults had the lowest comorbidity score of 1.8. For White adults in the study, the comorbidity score was 2.0.
Osteoporosis, a common concern for people with spina bifida or cerebral palsy, was detected in around 4% of all participants. Osteoarthritis was detected in 13.38% of Black participants, versus 8.53% of Hispanic participants and 11.09% of White participants.
Diabetes and hypertension were more common among Black participants than among Hispanic and White participants. The percentages of Black patients with hypertension and diabetes were 16.5% and 39.89%, respectively. Among the Hispanic and White adults, the percentages with hypertension were 22.3% and 28.2%, respectively, according to the paper.
Disparities in access
Jamil Paden, racial and health equity manager at the Christopher and Dana Reeve Foundation, said getting access to literature, transportation, tables, chairs, weigh scales, and imaging equipment that accommodate the needs of people with disabilities are some of the biggest challenges for people with disabilities who are trying to receive care.
“It’s not a one size fits all, we have to recognize that if someone doesn’t see themselves in a particular place, then it makes it more challenging for them to feel comfortable speaking up and saying things about their health, which would prevent a person from saying something early on,” Mr. Paden said in an interview. “That particular issue will continue to grow and become more of a health risk, or health challenge down the line.”
Mr. Paden emphasized intersections between class, race, and circumstances which can, together, make health care less equitable for people with disabilities, especially in underserved communities and communities of color. He urged health care providers to distance their practices from a “one size fits all” approach to treatment and engage in their patients’ individual lives and communities.
“It’s not enough to just say, Hey, you have a disability. So let me treat your disability ... You have to recognize that although a patient may have a dire diagnosis, they also are a person of color, and they have to navigate different aspects of life from their counterparts,” he said.
Dr. Mahmoudi said patient and provider understanding of the disability is often lacking. She recommended advocating for patients, noting that giving both patients and providers the tools to further educate themselves and apply that to their regular visits is a good first step.
“Just having access to a facility doesn’t mean they will get the services they need. Preventative services that are recommended for people with disabilities differ from the general population. Providers should be educated about that and the patient needs to be educated about that,” she added.
“Patients who do not approach clinicians get lost in the system. Maybe many facilities are not disability friendly, or they need health literacy. If they don’t know they are at risk for osteoporosis, for example, then they won’t ask,” Dr. Mahmoudi said.
The study was funded by The National Institute on Disability, Independent Living, and Rehabilitation Research. Dr. Mahmoudi and Mr. Paden report no relevant financial relationships.
FROM ANNALS OF FAMILY MEDICINE
ALS drug gets FDA panel thumbs-up after rare second look
In a rare second review of a new drug application,
By a vote of 7-2, the FDA Peripheral and Central Nervous System Drugs Advisory Committee reversed course on AMX0035 (Amylyx Pharmaceuticals), a combination of sodium phenylbutyrate and taurursodiol.
The panel previously voted 6-4 to reject the drug, ruling that data provided by Amylyx had failed to demonstrate that the survival benefit reported in the only clinical trial of AMX0035 so far was a direct result of the drug.
This time, two panelists who previously voted no were swayed by the drug maker’s new analysis of previously presented research, more than 1,300 public comments in support of the drug, supportive testimony from ALS patients and clinicians, and assurances from company executives that Amylyx would pull the drug from the market if results of an ongoing phase 3 clinical trial show the drug doesn’t work.
“As in March, today we have to have an internal dialogue between our scientific scrutiny and clinical compassion,” said Liana G. Apostolova, MD, from Indiana University, Indianapolis, who originally voted against the application.
“Today I also saw additional confirmatory evidence that was not unequivocally persuasive but was nonetheless reassuring,” Dr. Apostolova said. “Because of that I am voting in support of AMX0035.”
A rare second chance
ALS (Lou Gehrig’s disease) is a progressive, fatal neurodegenerative disease affecting nerve cells in the brain and spinal cord that causes loss of motor control. It is rare, affecting about 30,000 people in the United States with another 5,000 new cases diagnosed each year. Most people with the disease die within 2 years of diagnosis.
The FDA has approved two therapies for ALS, but both have limited efficacy.
Typically, FDA approval requires two large studies or one study with a “very persuasive” effect on survival.
Amylyx’s application is based on a single study, the multicenter, two-phase CENTAUR trial. In that trial, 137 people with ALS received AMX0035 or placebo for 24 weeks.
Researchers found that patients receiving AMX0035 had a 25% slower decline in function, compared with the those taking placebo. A change of 20% or more is considered clinically meaningful.
The investigators also found a statistically significant median difference of 4.8 months in time to death, first hospitalization, or tracheostomy/permanent assisted ventilation in the group originally assigned to receive AMX0035 compared with the group originally assigned to receive placebo (hazard ratio, 0.62; P = .023).
In the panel’s previous vote against the drug application, members cited several issues with the study, concluding that it did not offer persuasive or robust evidence of efficacy. They also cited missing data assumptions in the primary analysis, issues of randomization and imbalances in concomitant use of riluzole and edaravone, the two FDA-approved drugs for ALS.
The FDA later requested additional information from Amylyx, delayed its final ruling on the new drug application to Sept. 29, and called for a second review meeting – a virtually unheard-of move.
An FDA review posted in advance of the meeting Sept. 29 had hinted at a different outcome. In that report, regulators said new data from Amylyx were not “sufficiently independent or persuasive” to establish effectiveness.
However, FDA officials in the meeting stressed the importance of considering unmet medical need in ALS in the panel’s decision-making process.
“Recognizing the substantial unmet medical need in ALS, we feel that it is important that the committee is afforded the opportunity to consider this new information, along with the information presented at the prior meeting, in that context,” Billy Dunn, MD, director of the FDA Office of Neuroscience, said during the meeting.
Panelists heard additional data that Amylyx claims confirms the results of the CENTAUR study, including new analyses of the previously submitted survival data and new data from that study and an open-label extension.
They also provided new information on a biomarker data from a phase 2 study of AMX0035 to treat Alzheimer’s disease.
“I think we note the limitations of the analyses, but we still haven’t taken it off the table that they could be considered as confirmatory evidence and that’s why we’re here today,” said Teresa Buracchio, MD, director of the division of neurology for the FDA.
Two members of the panel who voted no in March stuck with that position at the Sept. 29 meeting.
“Unfortunately, I don’t believe the new evidence we’ve reviewed, while promising, combined with that prior evidence, constitutes substantial evidence of effectiveness,” said panelist Caleb Alexander, MD, a professor of epidemiology and medicine at the Johns Hopkins University Center for Drug Safety and Effectiveness, Baltimore.
Dr. Alexander, who also voted no in March, said that post hoc data presented at the meeting were not enough to assuage concerns that led him and others to reject the drug in March.
A challenging situation
Amylyx is currently leading the 48-week international, phase 3, placebo-controlled PHOENIX clinical trial of AMX0035. The study has enrolled about half of its 600-patient target.
“Undoubtedly, the results of the phase 3 study would be highly informative for a regulatory decision on the current ... review for AMX0035,” said Emily Freilich, MD, of the FDA.
However, results aren’t expected until late 2023 or early 2024, which “places the agency in a challenging situation of potentially making a regulatory decision that may not be subsequently confirmed by the results of the ongoing study.”
In June, Amylyx received conditional approval in Canada for the drug, but final approval depends on the outcome of the PHOENIX trial. The FDA does not offer a conditional approval track.
“If AMX0035 is not approved now, the FDA anticipated decision will likely happen in 2025, underscoring the critical importance of today’s outcome,” said Tammy Sarnelli, MPAHC, global head of Regulatory Affairs for Amylyx Pharmaceuticals.
If the FDA were to approve AMX0035 and results from the PHOENIX trial ultimately fail to prove efficacy, Justin Klee, co-CEO and cofounder of Amylyx Pharmaceuticals, said the company would withdraw the drug.
“To be clear, if PHOENIX is not successful, we will do what is right for patients, which includes voluntarily removing the product from the market,” Mr. Klee said.
Regardless of the company’s decision, FDA officials noted that the agency does have the ability to recall a drug from the market if studies show that it no longer meets requirements for approval.
“The FDA, with all due respect, significantly understates the complexity and likelihood of their pulling a product from the market,” Dr. Alexander said. “Whether or not they can ultimately pull a product from the market is no substitute for the evidentiary thresholds that are required for market access.”
A version of this article first appeared on Medscape.com.
In a rare second review of a new drug application,
By a vote of 7-2, the FDA Peripheral and Central Nervous System Drugs Advisory Committee reversed course on AMX0035 (Amylyx Pharmaceuticals), a combination of sodium phenylbutyrate and taurursodiol.
The panel previously voted 6-4 to reject the drug, ruling that data provided by Amylyx had failed to demonstrate that the survival benefit reported in the only clinical trial of AMX0035 so far was a direct result of the drug.
This time, two panelists who previously voted no were swayed by the drug maker’s new analysis of previously presented research, more than 1,300 public comments in support of the drug, supportive testimony from ALS patients and clinicians, and assurances from company executives that Amylyx would pull the drug from the market if results of an ongoing phase 3 clinical trial show the drug doesn’t work.
“As in March, today we have to have an internal dialogue between our scientific scrutiny and clinical compassion,” said Liana G. Apostolova, MD, from Indiana University, Indianapolis, who originally voted against the application.
“Today I also saw additional confirmatory evidence that was not unequivocally persuasive but was nonetheless reassuring,” Dr. Apostolova said. “Because of that I am voting in support of AMX0035.”
A rare second chance
ALS (Lou Gehrig’s disease) is a progressive, fatal neurodegenerative disease affecting nerve cells in the brain and spinal cord that causes loss of motor control. It is rare, affecting about 30,000 people in the United States with another 5,000 new cases diagnosed each year. Most people with the disease die within 2 years of diagnosis.
The FDA has approved two therapies for ALS, but both have limited efficacy.
Typically, FDA approval requires two large studies or one study with a “very persuasive” effect on survival.
Amylyx’s application is based on a single study, the multicenter, two-phase CENTAUR trial. In that trial, 137 people with ALS received AMX0035 or placebo for 24 weeks.
Researchers found that patients receiving AMX0035 had a 25% slower decline in function, compared with the those taking placebo. A change of 20% or more is considered clinically meaningful.
The investigators also found a statistically significant median difference of 4.8 months in time to death, first hospitalization, or tracheostomy/permanent assisted ventilation in the group originally assigned to receive AMX0035 compared with the group originally assigned to receive placebo (hazard ratio, 0.62; P = .023).
In the panel’s previous vote against the drug application, members cited several issues with the study, concluding that it did not offer persuasive or robust evidence of efficacy. They also cited missing data assumptions in the primary analysis, issues of randomization and imbalances in concomitant use of riluzole and edaravone, the two FDA-approved drugs for ALS.
The FDA later requested additional information from Amylyx, delayed its final ruling on the new drug application to Sept. 29, and called for a second review meeting – a virtually unheard-of move.
An FDA review posted in advance of the meeting Sept. 29 had hinted at a different outcome. In that report, regulators said new data from Amylyx were not “sufficiently independent or persuasive” to establish effectiveness.
However, FDA officials in the meeting stressed the importance of considering unmet medical need in ALS in the panel’s decision-making process.
“Recognizing the substantial unmet medical need in ALS, we feel that it is important that the committee is afforded the opportunity to consider this new information, along with the information presented at the prior meeting, in that context,” Billy Dunn, MD, director of the FDA Office of Neuroscience, said during the meeting.
Panelists heard additional data that Amylyx claims confirms the results of the CENTAUR study, including new analyses of the previously submitted survival data and new data from that study and an open-label extension.
They also provided new information on a biomarker data from a phase 2 study of AMX0035 to treat Alzheimer’s disease.
“I think we note the limitations of the analyses, but we still haven’t taken it off the table that they could be considered as confirmatory evidence and that’s why we’re here today,” said Teresa Buracchio, MD, director of the division of neurology for the FDA.
Two members of the panel who voted no in March stuck with that position at the Sept. 29 meeting.
“Unfortunately, I don’t believe the new evidence we’ve reviewed, while promising, combined with that prior evidence, constitutes substantial evidence of effectiveness,” said panelist Caleb Alexander, MD, a professor of epidemiology and medicine at the Johns Hopkins University Center for Drug Safety and Effectiveness, Baltimore.
Dr. Alexander, who also voted no in March, said that post hoc data presented at the meeting were not enough to assuage concerns that led him and others to reject the drug in March.
A challenging situation
Amylyx is currently leading the 48-week international, phase 3, placebo-controlled PHOENIX clinical trial of AMX0035. The study has enrolled about half of its 600-patient target.
“Undoubtedly, the results of the phase 3 study would be highly informative for a regulatory decision on the current ... review for AMX0035,” said Emily Freilich, MD, of the FDA.
However, results aren’t expected until late 2023 or early 2024, which “places the agency in a challenging situation of potentially making a regulatory decision that may not be subsequently confirmed by the results of the ongoing study.”
In June, Amylyx received conditional approval in Canada for the drug, but final approval depends on the outcome of the PHOENIX trial. The FDA does not offer a conditional approval track.
“If AMX0035 is not approved now, the FDA anticipated decision will likely happen in 2025, underscoring the critical importance of today’s outcome,” said Tammy Sarnelli, MPAHC, global head of Regulatory Affairs for Amylyx Pharmaceuticals.
If the FDA were to approve AMX0035 and results from the PHOENIX trial ultimately fail to prove efficacy, Justin Klee, co-CEO and cofounder of Amylyx Pharmaceuticals, said the company would withdraw the drug.
“To be clear, if PHOENIX is not successful, we will do what is right for patients, which includes voluntarily removing the product from the market,” Mr. Klee said.
Regardless of the company’s decision, FDA officials noted that the agency does have the ability to recall a drug from the market if studies show that it no longer meets requirements for approval.
“The FDA, with all due respect, significantly understates the complexity and likelihood of their pulling a product from the market,” Dr. Alexander said. “Whether or not they can ultimately pull a product from the market is no substitute for the evidentiary thresholds that are required for market access.”
A version of this article first appeared on Medscape.com.
In a rare second review of a new drug application,
By a vote of 7-2, the FDA Peripheral and Central Nervous System Drugs Advisory Committee reversed course on AMX0035 (Amylyx Pharmaceuticals), a combination of sodium phenylbutyrate and taurursodiol.
The panel previously voted 6-4 to reject the drug, ruling that data provided by Amylyx had failed to demonstrate that the survival benefit reported in the only clinical trial of AMX0035 so far was a direct result of the drug.
This time, two panelists who previously voted no were swayed by the drug maker’s new analysis of previously presented research, more than 1,300 public comments in support of the drug, supportive testimony from ALS patients and clinicians, and assurances from company executives that Amylyx would pull the drug from the market if results of an ongoing phase 3 clinical trial show the drug doesn’t work.
“As in March, today we have to have an internal dialogue between our scientific scrutiny and clinical compassion,” said Liana G. Apostolova, MD, from Indiana University, Indianapolis, who originally voted against the application.
“Today I also saw additional confirmatory evidence that was not unequivocally persuasive but was nonetheless reassuring,” Dr. Apostolova said. “Because of that I am voting in support of AMX0035.”
A rare second chance
ALS (Lou Gehrig’s disease) is a progressive, fatal neurodegenerative disease affecting nerve cells in the brain and spinal cord that causes loss of motor control. It is rare, affecting about 30,000 people in the United States with another 5,000 new cases diagnosed each year. Most people with the disease die within 2 years of diagnosis.
The FDA has approved two therapies for ALS, but both have limited efficacy.
Typically, FDA approval requires two large studies or one study with a “very persuasive” effect on survival.
Amylyx’s application is based on a single study, the multicenter, two-phase CENTAUR trial. In that trial, 137 people with ALS received AMX0035 or placebo for 24 weeks.
Researchers found that patients receiving AMX0035 had a 25% slower decline in function, compared with the those taking placebo. A change of 20% or more is considered clinically meaningful.
The investigators also found a statistically significant median difference of 4.8 months in time to death, first hospitalization, or tracheostomy/permanent assisted ventilation in the group originally assigned to receive AMX0035 compared with the group originally assigned to receive placebo (hazard ratio, 0.62; P = .023).
In the panel’s previous vote against the drug application, members cited several issues with the study, concluding that it did not offer persuasive or robust evidence of efficacy. They also cited missing data assumptions in the primary analysis, issues of randomization and imbalances in concomitant use of riluzole and edaravone, the two FDA-approved drugs for ALS.
The FDA later requested additional information from Amylyx, delayed its final ruling on the new drug application to Sept. 29, and called for a second review meeting – a virtually unheard-of move.
An FDA review posted in advance of the meeting Sept. 29 had hinted at a different outcome. In that report, regulators said new data from Amylyx were not “sufficiently independent or persuasive” to establish effectiveness.
However, FDA officials in the meeting stressed the importance of considering unmet medical need in ALS in the panel’s decision-making process.
“Recognizing the substantial unmet medical need in ALS, we feel that it is important that the committee is afforded the opportunity to consider this new information, along with the information presented at the prior meeting, in that context,” Billy Dunn, MD, director of the FDA Office of Neuroscience, said during the meeting.
Panelists heard additional data that Amylyx claims confirms the results of the CENTAUR study, including new analyses of the previously submitted survival data and new data from that study and an open-label extension.
They also provided new information on a biomarker data from a phase 2 study of AMX0035 to treat Alzheimer’s disease.
“I think we note the limitations of the analyses, but we still haven’t taken it off the table that they could be considered as confirmatory evidence and that’s why we’re here today,” said Teresa Buracchio, MD, director of the division of neurology for the FDA.
Two members of the panel who voted no in March stuck with that position at the Sept. 29 meeting.
“Unfortunately, I don’t believe the new evidence we’ve reviewed, while promising, combined with that prior evidence, constitutes substantial evidence of effectiveness,” said panelist Caleb Alexander, MD, a professor of epidemiology and medicine at the Johns Hopkins University Center for Drug Safety and Effectiveness, Baltimore.
Dr. Alexander, who also voted no in March, said that post hoc data presented at the meeting were not enough to assuage concerns that led him and others to reject the drug in March.
A challenging situation
Amylyx is currently leading the 48-week international, phase 3, placebo-controlled PHOENIX clinical trial of AMX0035. The study has enrolled about half of its 600-patient target.
“Undoubtedly, the results of the phase 3 study would be highly informative for a regulatory decision on the current ... review for AMX0035,” said Emily Freilich, MD, of the FDA.
However, results aren’t expected until late 2023 or early 2024, which “places the agency in a challenging situation of potentially making a regulatory decision that may not be subsequently confirmed by the results of the ongoing study.”
In June, Amylyx received conditional approval in Canada for the drug, but final approval depends on the outcome of the PHOENIX trial. The FDA does not offer a conditional approval track.
“If AMX0035 is not approved now, the FDA anticipated decision will likely happen in 2025, underscoring the critical importance of today’s outcome,” said Tammy Sarnelli, MPAHC, global head of Regulatory Affairs for Amylyx Pharmaceuticals.
If the FDA were to approve AMX0035 and results from the PHOENIX trial ultimately fail to prove efficacy, Justin Klee, co-CEO and cofounder of Amylyx Pharmaceuticals, said the company would withdraw the drug.
“To be clear, if PHOENIX is not successful, we will do what is right for patients, which includes voluntarily removing the product from the market,” Mr. Klee said.
Regardless of the company’s decision, FDA officials noted that the agency does have the ability to recall a drug from the market if studies show that it no longer meets requirements for approval.
“The FDA, with all due respect, significantly understates the complexity and likelihood of their pulling a product from the market,” Dr. Alexander said. “Whether or not they can ultimately pull a product from the market is no substitute for the evidentiary thresholds that are required for market access.”
A version of this article first appeared on Medscape.com.
Positive top-line phase 3 data for lecanemab in early Alzheimer’s
compared with placebo and decreased amyloid levels in the brain of adults enrolled in a phase 3 trial.
The Clarity AD trial included 1,795 adults with early AD and confirmed amyloid pathology in the brain. Treatment consisted of lecanemab 10 mg/kg biweekly or matching placebo.
Treatment with lecanemab met the primary endpoint, reducing clinical decline on the global cognitive and functional scale, the Clinical Dementia Rating–Sum of Boxes (CDR-SB), at 18 months by 27%, compared with placebo, with a treatment difference in the score change of –0.45 (P = .00005), the companies reported.
Starting as early as 6 months, across all time points, treatment with lecanemab yielded highly statistically significant changes in CDR-SB from baseline, compared with placebo (all P < .01).
The study also met all key secondary endpoints with highly statistically significant results, compared with placebo (P < .01).
Key secondary endpoints, in comparison with placebo, were change from baseline at 18 months in amyloid levels in the brain measured by amyloid PET, the AD Assessment Scale–cognitive subscale14 (ADAS-cog14), the AD Composite Score (ADCOMS), and the AD Cooperative Study–Activities of Daily Living Scale for Mild Cognitive Impairment (ADCS MCI-ADL).
Imaging abnormalities within expectations
Overall, rates of amyloid-related imaging abnormalities (ARIA) related to lecanemab were “within expectations,” the companies said.
The incidence of ARIA related to edema (ARIA-E) was 12.5% in the lecanemab group and 1.7% in the placebo group.
The incidence of symptomatic ARIA-E was 2.8% and 0.0%, respectively, and the rate of cerebral hemorrhage (ARIA-H) was 17.0% and 8.7%. The total incidence of ARIA (ARIA-E and/or ARIA-H) was 21.3% in the lecanemab group and 9.3% in the placebo group.
Full results of the Clarity AD trial will be presented in November at the Clinical Trials on Alzheimer’s Congress.
Incremental benefit
Responding to the findings, the Alzheimer’s Association said in a statement that it “enthusiastically welcomes” the positive findings. It noted that these are “the most encouraging results in clinical trials treating the underlying causes of Alzheimer’s to date.
“For people in the earliest stages of Alzheimer’s, this treatment has the potential to change the course of the disease in a clinically meaningful way. These results indicate lecanemab may give people more time at or near their full abilities to participate in daily life, remain independent and make future health care decisions,” the Alzheimer’s Association added.
Also weighing in, Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, said in a release that “the combination of the biomarker change – reduced amyloid – plus slowing of cognitive decline in this study is encouraging news for the 57 million patients around the world living with Alzheimer’s.
“However, amyloid-clearing drugs will provide an incremental benefit at best, and there is still a pressing need for the next generation of drugs focused on other targets based on our knowledge of the biology of aging,” Dr. Fillit cautioned.
“We are optimistic about the future as many of these drugs are in development, with 75% of drugs in the pipeline now targeting nonamyloid pathways of neurodegeneration,” he added.
In July 2022, the Food and Drug Administration accepted Eisai’s biologics license application for lecanemab under the accelerated approval pathway and granted priority review. Lecanemab has a prescription Drugs User Fee Act action date of Jan. 6, 2023.
A version of this article first appeared on Medscape.com.
compared with placebo and decreased amyloid levels in the brain of adults enrolled in a phase 3 trial.
The Clarity AD trial included 1,795 adults with early AD and confirmed amyloid pathology in the brain. Treatment consisted of lecanemab 10 mg/kg biweekly or matching placebo.
Treatment with lecanemab met the primary endpoint, reducing clinical decline on the global cognitive and functional scale, the Clinical Dementia Rating–Sum of Boxes (CDR-SB), at 18 months by 27%, compared with placebo, with a treatment difference in the score change of –0.45 (P = .00005), the companies reported.
Starting as early as 6 months, across all time points, treatment with lecanemab yielded highly statistically significant changes in CDR-SB from baseline, compared with placebo (all P < .01).
The study also met all key secondary endpoints with highly statistically significant results, compared with placebo (P < .01).
Key secondary endpoints, in comparison with placebo, were change from baseline at 18 months in amyloid levels in the brain measured by amyloid PET, the AD Assessment Scale–cognitive subscale14 (ADAS-cog14), the AD Composite Score (ADCOMS), and the AD Cooperative Study–Activities of Daily Living Scale for Mild Cognitive Impairment (ADCS MCI-ADL).
Imaging abnormalities within expectations
Overall, rates of amyloid-related imaging abnormalities (ARIA) related to lecanemab were “within expectations,” the companies said.
The incidence of ARIA related to edema (ARIA-E) was 12.5% in the lecanemab group and 1.7% in the placebo group.
The incidence of symptomatic ARIA-E was 2.8% and 0.0%, respectively, and the rate of cerebral hemorrhage (ARIA-H) was 17.0% and 8.7%. The total incidence of ARIA (ARIA-E and/or ARIA-H) was 21.3% in the lecanemab group and 9.3% in the placebo group.
Full results of the Clarity AD trial will be presented in November at the Clinical Trials on Alzheimer’s Congress.
Incremental benefit
Responding to the findings, the Alzheimer’s Association said in a statement that it “enthusiastically welcomes” the positive findings. It noted that these are “the most encouraging results in clinical trials treating the underlying causes of Alzheimer’s to date.
“For people in the earliest stages of Alzheimer’s, this treatment has the potential to change the course of the disease in a clinically meaningful way. These results indicate lecanemab may give people more time at or near their full abilities to participate in daily life, remain independent and make future health care decisions,” the Alzheimer’s Association added.
Also weighing in, Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, said in a release that “the combination of the biomarker change – reduced amyloid – plus slowing of cognitive decline in this study is encouraging news for the 57 million patients around the world living with Alzheimer’s.
“However, amyloid-clearing drugs will provide an incremental benefit at best, and there is still a pressing need for the next generation of drugs focused on other targets based on our knowledge of the biology of aging,” Dr. Fillit cautioned.
“We are optimistic about the future as many of these drugs are in development, with 75% of drugs in the pipeline now targeting nonamyloid pathways of neurodegeneration,” he added.
In July 2022, the Food and Drug Administration accepted Eisai’s biologics license application for lecanemab under the accelerated approval pathway and granted priority review. Lecanemab has a prescription Drugs User Fee Act action date of Jan. 6, 2023.
A version of this article first appeared on Medscape.com.
compared with placebo and decreased amyloid levels in the brain of adults enrolled in a phase 3 trial.
The Clarity AD trial included 1,795 adults with early AD and confirmed amyloid pathology in the brain. Treatment consisted of lecanemab 10 mg/kg biweekly or matching placebo.
Treatment with lecanemab met the primary endpoint, reducing clinical decline on the global cognitive and functional scale, the Clinical Dementia Rating–Sum of Boxes (CDR-SB), at 18 months by 27%, compared with placebo, with a treatment difference in the score change of –0.45 (P = .00005), the companies reported.
Starting as early as 6 months, across all time points, treatment with lecanemab yielded highly statistically significant changes in CDR-SB from baseline, compared with placebo (all P < .01).
The study also met all key secondary endpoints with highly statistically significant results, compared with placebo (P < .01).
Key secondary endpoints, in comparison with placebo, were change from baseline at 18 months in amyloid levels in the brain measured by amyloid PET, the AD Assessment Scale–cognitive subscale14 (ADAS-cog14), the AD Composite Score (ADCOMS), and the AD Cooperative Study–Activities of Daily Living Scale for Mild Cognitive Impairment (ADCS MCI-ADL).
Imaging abnormalities within expectations
Overall, rates of amyloid-related imaging abnormalities (ARIA) related to lecanemab were “within expectations,” the companies said.
The incidence of ARIA related to edema (ARIA-E) was 12.5% in the lecanemab group and 1.7% in the placebo group.
The incidence of symptomatic ARIA-E was 2.8% and 0.0%, respectively, and the rate of cerebral hemorrhage (ARIA-H) was 17.0% and 8.7%. The total incidence of ARIA (ARIA-E and/or ARIA-H) was 21.3% in the lecanemab group and 9.3% in the placebo group.
Full results of the Clarity AD trial will be presented in November at the Clinical Trials on Alzheimer’s Congress.
Incremental benefit
Responding to the findings, the Alzheimer’s Association said in a statement that it “enthusiastically welcomes” the positive findings. It noted that these are “the most encouraging results in clinical trials treating the underlying causes of Alzheimer’s to date.
“For people in the earliest stages of Alzheimer’s, this treatment has the potential to change the course of the disease in a clinically meaningful way. These results indicate lecanemab may give people more time at or near their full abilities to participate in daily life, remain independent and make future health care decisions,” the Alzheimer’s Association added.
Also weighing in, Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, said in a release that “the combination of the biomarker change – reduced amyloid – plus slowing of cognitive decline in this study is encouraging news for the 57 million patients around the world living with Alzheimer’s.
“However, amyloid-clearing drugs will provide an incremental benefit at best, and there is still a pressing need for the next generation of drugs focused on other targets based on our knowledge of the biology of aging,” Dr. Fillit cautioned.
“We are optimistic about the future as many of these drugs are in development, with 75% of drugs in the pipeline now targeting nonamyloid pathways of neurodegeneration,” he added.
In July 2022, the Food and Drug Administration accepted Eisai’s biologics license application for lecanemab under the accelerated approval pathway and granted priority review. Lecanemab has a prescription Drugs User Fee Act action date of Jan. 6, 2023.
A version of this article first appeared on Medscape.com.