User login
Ages and Stages Questionnaire a first step to find developmental delays
The commonly used but sometimes debated Ages and Stages Questionnaire (ASQ), has modest utility for identifying developmental delays in young children, an Australian review and meta-analysis found.
On this easily administered parent-completed screening tool, scores of more than 2 standard deviations below the mean in more than one of five domains had moderate sensitivity and specificity to predict any delay, severe delay, motor delay, and cognitive delay, according to neonatologist Shripada Rao, PhD, a clinical associate professor in the neonatal intensive care unit at Perth Hospital and the University of Western Australia, also in Perth, and colleagues.
If a child of 12-60 months passes all ASQ domains, there is a moderate probability that child does not have severe developmental delay, the researchers concluded. If a child in that age range fails the motor or cognitive domain, there is a moderate probability that some motor or cognitive delay is present. The authors say the tool may work best as a screening test to identify children in need of more formal assessment.
“Our meta-analysis found that ASQ was somewhat more predictive in older children (older than 24 months), compared with younger age groups of 12-24 months,” Dr. Rao said in an interview. “However, the sample size for these comparisons was too small to reach definite conclusions, and we have called for future studies to evaluate ASQ separately for different age groups.”
Early identification of developmental delay in children is essential to enable timely intervention,” Dr. Rao and associates wrote in JAMA Pediatrics.
While formal assessments such as the Bayley Scales of Infant and Toddler Development are the gold standard, they are time-consuming and expensive, need the physical attendance of both the child and caregivers, and “thus may not be feasible in resource-limited settings or in pandemic conditions.”
According to Barbara J. Howard, MD, commenting on a recent update to the Center for Disease Control and Prevention’s developmental milestones guide, Learn the Signs. Act Early, fewer than 25% of children with delays or disabilities receive intervention before age 3 and most with emotional, behavioral, and developmental condition, other than autism spectrum disorder receive no intervention before age 5.
The ASQ
As an accessible alternative, the ASQ consists of questions on communication (language), gross-motor, fine-motor, problem-solving (cognitive), and personal-adaptive skills. The survey requires only 10-15 minutes, is relatively inexpensive, and also establishes a sense of parental involvement, the authors noted.
“Based on the generally accepted interpretation of LR [likelihood ratio] values, if a child passes ASQ-2SD, there is a moderate probability that the child does not have severe delay,” the investigators concluded.
The analysis
The final meta-analysis reviewed 36 eligible ASQ studies published from 1997 to 2022. Looking at the four indicators of pooled sensitivity, specificity, and positive and negative likelihood ratios, the following respective predictive values emerged for scores of more than 2 SDs below the mean across several domains: sensitivity of 0.77 (95% confidence interval, 0.64-0.86), specificity of 0.81 (95% CI 0.75-0.86), positive likelihood ratio of 4.10 (95% CI 3.17-5.30), and a negative likelihood ratio of 0.28 (95% CI, 0.18-0.44)
They cautioned, however, that the certainty of evidence from the reviewed studies was low or very low and given the small sample sizes for comparing domains, clinicians should be circumspect in interpreting the results.
An initial step
Commenting on the paper but not involved in it, David G. Fagan, MD, vice chairman of pediatric ambulatory administration in the department of pediatrics at Cohen Children’s Medical Center, New York, agreed that screening tools such as the ASQ have a place in clinical practice. “However, the purpose of a screening tool is not to make the diagnosis but to identify children at risk for developmental delays,” he said in an interview. “The meta-analysis highlights the fact that no screening is 100% accurate and that results need to be interpreted in context.
“Before screening tools were widely used, pediatricians trusted their gut,” Dr. Fagan continued. “‘I know it when I see it,’ which obviously resulted in tremendous variability based on experience.”
He added that, even if a child passes this validated questionnaire, any concern on the part of a parent or pediatrician about developmental delay should be addressed with further assessment.
The future
According to Dr. Rao, clinicians should continue to screen for developmental delays in young children using the ASQ. “Given the long wait times to see a developmental pediatrician or a clinical psychologist, a screening tool such as ASQ will enable appropriate triaging.”
Going forward, however, studies should evaluate this questionnaire separately for different age groups such as less than 12 months, 12-23 months, and at least 24 months. They should also be prospective in design and entail a low risk of bias, as well as report raw numbers for true and false positives and negatives. “Even if they use their own cutoff ASQ scores, they should also give results for the conventional cutoff scores to enable comparison with other studies,” the authors wrote.
The authors disclosed no specific funding for this study and no competing interests. Dr. Fagan disclosed no competing interests with regard to his comments.
The commonly used but sometimes debated Ages and Stages Questionnaire (ASQ), has modest utility for identifying developmental delays in young children, an Australian review and meta-analysis found.
On this easily administered parent-completed screening tool, scores of more than 2 standard deviations below the mean in more than one of five domains had moderate sensitivity and specificity to predict any delay, severe delay, motor delay, and cognitive delay, according to neonatologist Shripada Rao, PhD, a clinical associate professor in the neonatal intensive care unit at Perth Hospital and the University of Western Australia, also in Perth, and colleagues.
If a child of 12-60 months passes all ASQ domains, there is a moderate probability that child does not have severe developmental delay, the researchers concluded. If a child in that age range fails the motor or cognitive domain, there is a moderate probability that some motor or cognitive delay is present. The authors say the tool may work best as a screening test to identify children in need of more formal assessment.
“Our meta-analysis found that ASQ was somewhat more predictive in older children (older than 24 months), compared with younger age groups of 12-24 months,” Dr. Rao said in an interview. “However, the sample size for these comparisons was too small to reach definite conclusions, and we have called for future studies to evaluate ASQ separately for different age groups.”
Early identification of developmental delay in children is essential to enable timely intervention,” Dr. Rao and associates wrote in JAMA Pediatrics.
While formal assessments such as the Bayley Scales of Infant and Toddler Development are the gold standard, they are time-consuming and expensive, need the physical attendance of both the child and caregivers, and “thus may not be feasible in resource-limited settings or in pandemic conditions.”
According to Barbara J. Howard, MD, commenting on a recent update to the Center for Disease Control and Prevention’s developmental milestones guide, Learn the Signs. Act Early, fewer than 25% of children with delays or disabilities receive intervention before age 3 and most with emotional, behavioral, and developmental condition, other than autism spectrum disorder receive no intervention before age 5.
The ASQ
As an accessible alternative, the ASQ consists of questions on communication (language), gross-motor, fine-motor, problem-solving (cognitive), and personal-adaptive skills. The survey requires only 10-15 minutes, is relatively inexpensive, and also establishes a sense of parental involvement, the authors noted.
“Based on the generally accepted interpretation of LR [likelihood ratio] values, if a child passes ASQ-2SD, there is a moderate probability that the child does not have severe delay,” the investigators concluded.
The analysis
The final meta-analysis reviewed 36 eligible ASQ studies published from 1997 to 2022. Looking at the four indicators of pooled sensitivity, specificity, and positive and negative likelihood ratios, the following respective predictive values emerged for scores of more than 2 SDs below the mean across several domains: sensitivity of 0.77 (95% confidence interval, 0.64-0.86), specificity of 0.81 (95% CI 0.75-0.86), positive likelihood ratio of 4.10 (95% CI 3.17-5.30), and a negative likelihood ratio of 0.28 (95% CI, 0.18-0.44)
They cautioned, however, that the certainty of evidence from the reviewed studies was low or very low and given the small sample sizes for comparing domains, clinicians should be circumspect in interpreting the results.
An initial step
Commenting on the paper but not involved in it, David G. Fagan, MD, vice chairman of pediatric ambulatory administration in the department of pediatrics at Cohen Children’s Medical Center, New York, agreed that screening tools such as the ASQ have a place in clinical practice. “However, the purpose of a screening tool is not to make the diagnosis but to identify children at risk for developmental delays,” he said in an interview. “The meta-analysis highlights the fact that no screening is 100% accurate and that results need to be interpreted in context.
“Before screening tools were widely used, pediatricians trusted their gut,” Dr. Fagan continued. “‘I know it when I see it,’ which obviously resulted in tremendous variability based on experience.”
He added that, even if a child passes this validated questionnaire, any concern on the part of a parent or pediatrician about developmental delay should be addressed with further assessment.
The future
According to Dr. Rao, clinicians should continue to screen for developmental delays in young children using the ASQ. “Given the long wait times to see a developmental pediatrician or a clinical psychologist, a screening tool such as ASQ will enable appropriate triaging.”
Going forward, however, studies should evaluate this questionnaire separately for different age groups such as less than 12 months, 12-23 months, and at least 24 months. They should also be prospective in design and entail a low risk of bias, as well as report raw numbers for true and false positives and negatives. “Even if they use their own cutoff ASQ scores, they should also give results for the conventional cutoff scores to enable comparison with other studies,” the authors wrote.
The authors disclosed no specific funding for this study and no competing interests. Dr. Fagan disclosed no competing interests with regard to his comments.
The commonly used but sometimes debated Ages and Stages Questionnaire (ASQ), has modest utility for identifying developmental delays in young children, an Australian review and meta-analysis found.
On this easily administered parent-completed screening tool, scores of more than 2 standard deviations below the mean in more than one of five domains had moderate sensitivity and specificity to predict any delay, severe delay, motor delay, and cognitive delay, according to neonatologist Shripada Rao, PhD, a clinical associate professor in the neonatal intensive care unit at Perth Hospital and the University of Western Australia, also in Perth, and colleagues.
If a child of 12-60 months passes all ASQ domains, there is a moderate probability that child does not have severe developmental delay, the researchers concluded. If a child in that age range fails the motor or cognitive domain, there is a moderate probability that some motor or cognitive delay is present. The authors say the tool may work best as a screening test to identify children in need of more formal assessment.
“Our meta-analysis found that ASQ was somewhat more predictive in older children (older than 24 months), compared with younger age groups of 12-24 months,” Dr. Rao said in an interview. “However, the sample size for these comparisons was too small to reach definite conclusions, and we have called for future studies to evaluate ASQ separately for different age groups.”
Early identification of developmental delay in children is essential to enable timely intervention,” Dr. Rao and associates wrote in JAMA Pediatrics.
While formal assessments such as the Bayley Scales of Infant and Toddler Development are the gold standard, they are time-consuming and expensive, need the physical attendance of both the child and caregivers, and “thus may not be feasible in resource-limited settings or in pandemic conditions.”
According to Barbara J. Howard, MD, commenting on a recent update to the Center for Disease Control and Prevention’s developmental milestones guide, Learn the Signs. Act Early, fewer than 25% of children with delays or disabilities receive intervention before age 3 and most with emotional, behavioral, and developmental condition, other than autism spectrum disorder receive no intervention before age 5.
The ASQ
As an accessible alternative, the ASQ consists of questions on communication (language), gross-motor, fine-motor, problem-solving (cognitive), and personal-adaptive skills. The survey requires only 10-15 minutes, is relatively inexpensive, and also establishes a sense of parental involvement, the authors noted.
“Based on the generally accepted interpretation of LR [likelihood ratio] values, if a child passes ASQ-2SD, there is a moderate probability that the child does not have severe delay,” the investigators concluded.
The analysis
The final meta-analysis reviewed 36 eligible ASQ studies published from 1997 to 2022. Looking at the four indicators of pooled sensitivity, specificity, and positive and negative likelihood ratios, the following respective predictive values emerged for scores of more than 2 SDs below the mean across several domains: sensitivity of 0.77 (95% confidence interval, 0.64-0.86), specificity of 0.81 (95% CI 0.75-0.86), positive likelihood ratio of 4.10 (95% CI 3.17-5.30), and a negative likelihood ratio of 0.28 (95% CI, 0.18-0.44)
They cautioned, however, that the certainty of evidence from the reviewed studies was low or very low and given the small sample sizes for comparing domains, clinicians should be circumspect in interpreting the results.
An initial step
Commenting on the paper but not involved in it, David G. Fagan, MD, vice chairman of pediatric ambulatory administration in the department of pediatrics at Cohen Children’s Medical Center, New York, agreed that screening tools such as the ASQ have a place in clinical practice. “However, the purpose of a screening tool is not to make the diagnosis but to identify children at risk for developmental delays,” he said in an interview. “The meta-analysis highlights the fact that no screening is 100% accurate and that results need to be interpreted in context.
“Before screening tools were widely used, pediatricians trusted their gut,” Dr. Fagan continued. “‘I know it when I see it,’ which obviously resulted in tremendous variability based on experience.”
He added that, even if a child passes this validated questionnaire, any concern on the part of a parent or pediatrician about developmental delay should be addressed with further assessment.
The future
According to Dr. Rao, clinicians should continue to screen for developmental delays in young children using the ASQ. “Given the long wait times to see a developmental pediatrician or a clinical psychologist, a screening tool such as ASQ will enable appropriate triaging.”
Going forward, however, studies should evaluate this questionnaire separately for different age groups such as less than 12 months, 12-23 months, and at least 24 months. They should also be prospective in design and entail a low risk of bias, as well as report raw numbers for true and false positives and negatives. “Even if they use their own cutoff ASQ scores, they should also give results for the conventional cutoff scores to enable comparison with other studies,” the authors wrote.
The authors disclosed no specific funding for this study and no competing interests. Dr. Fagan disclosed no competing interests with regard to his comments.
FROM JAMA PEDIATRICS
Ketamine promising for rare condition linked to autism
Also known as Helsmoortel–Van Der Aa syndrome, ADNP syndrome is caused by mutations in the ADNP gene. Studies in animal models suggest that low-dose ketamine increases expression of ADNP and is neuroprotective.
Intrigued by the preclinical evidence, Alexander Kolevzon, MD, clinical director of the Seaver Autism Center at Mount Sinai, New York, and colleagues treated 10 children with ADNP syndrome with a single low dose of ketamine (0.5mg/kg) infused intravenously over 40 minutes. The children ranged in ages 6-12 years.
Using parent-report instruments to assess treatment effects, ketamine was associated with “nominally significant” improvement in a variety of domains, including social behavior, attention-deficit and hyperactivity, restricted and repetitive behaviors, and sensory sensitivities.
Parent reports of improvement in these domains aligned with clinician-rated assessments based on the Clinical Global Impressions–Improvement scale.
The results also highlight the potential utility of electrophysiological measurement of auditory steady-state response and eye-tracking to track change with ketamine treatment, the researchers say.
The study was published online in Human Genetics and Genomic (HGG) Advances.
Hypothesis-generating
Ketamine was generally well tolerated. There were no clinically significant abnormalities in laboratory or cardiac monitoring, and there were no serious adverse events (AEs).
Treatment emergent AEs were all mild to moderate and no child required any interventions.
The most common AEs were elation/silliness in five children (50%), all of whom had a history of similar symptoms. Drowsiness and fatigue occurred in four children (40%) and two of them had a history of drowsiness. Aggression was likewise relatively common, reported in four children (40%), all of whom had aggression at baseline.
Decreased appetite emerged as a new AE in three children (30%), increased anxiety occurred in three children (30%), and irritability, nausea/vomiting, and restlessness each occurred in two children (20%).
The researchers caution that the findings are intended to be “hypothesis generating.”
“We are encouraged by these findings, which provide preliminary support for ketamine to help reduce negative effects of this devastating syndrome,” Dr. Kolevzon said in a news release from Mount Sinai.
Ketamine might help ease symptoms of ADNP syndrome “by increasing expression of the ADNP gene or by promoting synaptic plasticity through glutamatergic pathways,” Dr. Kolevzon told this news organization.
The next step, he said, is to get “a larger, placebo-controlled study approved for funding using repeated dosing over a longer duration of time. We are working with the FDA to get the design approved for an investigational new drug application.”
Support for the study was provided by the ADNP Kids Foundation and the Foundation for Mood Disorders. Support for mediKanren was provided by the National Center for Advancing Translational Sciences, and National Institutes of Health through the Biomedical Data Translator Program. Dr. Kolevzon is on the scientific advisory board of Ovid Therapeutics, Ritrova Therapeutics, and Jaguar Therapeutics and consults to Acadia, Alkermes, GW Pharmaceuticals, Neuren Pharmaceuticals, Clinilabs Drug Development Corporation, and Scioto Biosciences.
A version of this article first appeared on Medscape.com.
Also known as Helsmoortel–Van Der Aa syndrome, ADNP syndrome is caused by mutations in the ADNP gene. Studies in animal models suggest that low-dose ketamine increases expression of ADNP and is neuroprotective.
Intrigued by the preclinical evidence, Alexander Kolevzon, MD, clinical director of the Seaver Autism Center at Mount Sinai, New York, and colleagues treated 10 children with ADNP syndrome with a single low dose of ketamine (0.5mg/kg) infused intravenously over 40 minutes. The children ranged in ages 6-12 years.
Using parent-report instruments to assess treatment effects, ketamine was associated with “nominally significant” improvement in a variety of domains, including social behavior, attention-deficit and hyperactivity, restricted and repetitive behaviors, and sensory sensitivities.
Parent reports of improvement in these domains aligned with clinician-rated assessments based on the Clinical Global Impressions–Improvement scale.
The results also highlight the potential utility of electrophysiological measurement of auditory steady-state response and eye-tracking to track change with ketamine treatment, the researchers say.
The study was published online in Human Genetics and Genomic (HGG) Advances.
Hypothesis-generating
Ketamine was generally well tolerated. There were no clinically significant abnormalities in laboratory or cardiac monitoring, and there were no serious adverse events (AEs).
Treatment emergent AEs were all mild to moderate and no child required any interventions.
The most common AEs were elation/silliness in five children (50%), all of whom had a history of similar symptoms. Drowsiness and fatigue occurred in four children (40%) and two of them had a history of drowsiness. Aggression was likewise relatively common, reported in four children (40%), all of whom had aggression at baseline.
Decreased appetite emerged as a new AE in three children (30%), increased anxiety occurred in three children (30%), and irritability, nausea/vomiting, and restlessness each occurred in two children (20%).
The researchers caution that the findings are intended to be “hypothesis generating.”
“We are encouraged by these findings, which provide preliminary support for ketamine to help reduce negative effects of this devastating syndrome,” Dr. Kolevzon said in a news release from Mount Sinai.
Ketamine might help ease symptoms of ADNP syndrome “by increasing expression of the ADNP gene or by promoting synaptic plasticity through glutamatergic pathways,” Dr. Kolevzon told this news organization.
The next step, he said, is to get “a larger, placebo-controlled study approved for funding using repeated dosing over a longer duration of time. We are working with the FDA to get the design approved for an investigational new drug application.”
Support for the study was provided by the ADNP Kids Foundation and the Foundation for Mood Disorders. Support for mediKanren was provided by the National Center for Advancing Translational Sciences, and National Institutes of Health through the Biomedical Data Translator Program. Dr. Kolevzon is on the scientific advisory board of Ovid Therapeutics, Ritrova Therapeutics, and Jaguar Therapeutics and consults to Acadia, Alkermes, GW Pharmaceuticals, Neuren Pharmaceuticals, Clinilabs Drug Development Corporation, and Scioto Biosciences.
A version of this article first appeared on Medscape.com.
Also known as Helsmoortel–Van Der Aa syndrome, ADNP syndrome is caused by mutations in the ADNP gene. Studies in animal models suggest that low-dose ketamine increases expression of ADNP and is neuroprotective.
Intrigued by the preclinical evidence, Alexander Kolevzon, MD, clinical director of the Seaver Autism Center at Mount Sinai, New York, and colleagues treated 10 children with ADNP syndrome with a single low dose of ketamine (0.5mg/kg) infused intravenously over 40 minutes. The children ranged in ages 6-12 years.
Using parent-report instruments to assess treatment effects, ketamine was associated with “nominally significant” improvement in a variety of domains, including social behavior, attention-deficit and hyperactivity, restricted and repetitive behaviors, and sensory sensitivities.
Parent reports of improvement in these domains aligned with clinician-rated assessments based on the Clinical Global Impressions–Improvement scale.
The results also highlight the potential utility of electrophysiological measurement of auditory steady-state response and eye-tracking to track change with ketamine treatment, the researchers say.
The study was published online in Human Genetics and Genomic (HGG) Advances.
Hypothesis-generating
Ketamine was generally well tolerated. There were no clinically significant abnormalities in laboratory or cardiac monitoring, and there were no serious adverse events (AEs).
Treatment emergent AEs were all mild to moderate and no child required any interventions.
The most common AEs were elation/silliness in five children (50%), all of whom had a history of similar symptoms. Drowsiness and fatigue occurred in four children (40%) and two of them had a history of drowsiness. Aggression was likewise relatively common, reported in four children (40%), all of whom had aggression at baseline.
Decreased appetite emerged as a new AE in three children (30%), increased anxiety occurred in three children (30%), and irritability, nausea/vomiting, and restlessness each occurred in two children (20%).
The researchers caution that the findings are intended to be “hypothesis generating.”
“We are encouraged by these findings, which provide preliminary support for ketamine to help reduce negative effects of this devastating syndrome,” Dr. Kolevzon said in a news release from Mount Sinai.
Ketamine might help ease symptoms of ADNP syndrome “by increasing expression of the ADNP gene or by promoting synaptic plasticity through glutamatergic pathways,” Dr. Kolevzon told this news organization.
The next step, he said, is to get “a larger, placebo-controlled study approved for funding using repeated dosing over a longer duration of time. We are working with the FDA to get the design approved for an investigational new drug application.”
Support for the study was provided by the ADNP Kids Foundation and the Foundation for Mood Disorders. Support for mediKanren was provided by the National Center for Advancing Translational Sciences, and National Institutes of Health through the Biomedical Data Translator Program. Dr. Kolevzon is on the scientific advisory board of Ovid Therapeutics, Ritrova Therapeutics, and Jaguar Therapeutics and consults to Acadia, Alkermes, GW Pharmaceuticals, Neuren Pharmaceuticals, Clinilabs Drug Development Corporation, and Scioto Biosciences.
A version of this article first appeared on Medscape.com.
From Human Genetics and Genomic Advances
Parent training pays off for children with autism
“Referrals for parent training should now be considered the expected standard for medical practice,” said a member of the research team, Timothy B. Smith, PhD, a professor of psychology at Brigham Young University, Provo, Utah.
Programs that show parents how to teach functional skills and address maladaptive behaviors, also known as parent-mediated or parent-implemented interventions, offer an alternative to one-on-one professional services, which are in short supply, according to the paper, which was published in the Journal of Autism and Developmental Disorders.
Methods and results
The meta-analysis included 54 papers based on randomized clinical trials involving 2,895 children, which compared the effects of various parent interventions with professional treatment, treatment as usual, or being on a wait-list to receive an intervention.
Overall the research team reported “moderately strong” average benefits from the parent-mediated interventions (Hedges’ g, 0.553), indicating a medium effect size. Parent interventions had the greatest effect on outcomes involving positive behavior and social skills (0.603), followed by language and communication (0.545), maladaptive behavior (0.519), and life skills (0.239).
Similar benefits were observed regardless of a child’s age or sex or which parent or parents implemented an intervention. The effects also appeared to be consistent regardless of intervention characteristics, such as the number of training sessions parents received, although the researchers noted that many studies did not provide data on such details.
Paul Carbone, MD, a professor of pediatrics at the University of Utah, Salt Lake City, who was not involved in the review, said it demonstrates that such parental engagement is “vitally important” and pediatricians “should not hesitate to refer interested families.”
Dr. Carbone, who is the medical director of an assessment program for children with suspected developmental disabilities, said many training programs for parents have adopted telehealth, adding to their convenience. To make appropriate referrals, primary care clinicians should become acquainted with local programs and learn which outcomes they target, he said.
Dr. Smith noted that primary care physicians are “better trained now than ever” to identify autism spectrum disorder and therefore are among the first to identify those conditions and help parents understand “that their actions at home absolutely make a difference in the child’s development.”
Overcoming limitations, future research needs
The research team attempted to overcome limitations with previous reviews by using comprehensive search terms and other methods to identify relevant studies, including some that had not been published. They included only studies that reflect common practice of training multiple parents simultaneously, they wrote.
Dr. Smith noted that long-term outcomes data and further study to compare effects on children with mild, moderate, and severe autism are needed.
Although logic would suggest greater benefits for children with severe disease, there are no data to demonstrate that, he said.
The authors of the study and Dr. Carbone reported no relevant competing interests.
“Referrals for parent training should now be considered the expected standard for medical practice,” said a member of the research team, Timothy B. Smith, PhD, a professor of psychology at Brigham Young University, Provo, Utah.
Programs that show parents how to teach functional skills and address maladaptive behaviors, also known as parent-mediated or parent-implemented interventions, offer an alternative to one-on-one professional services, which are in short supply, according to the paper, which was published in the Journal of Autism and Developmental Disorders.
Methods and results
The meta-analysis included 54 papers based on randomized clinical trials involving 2,895 children, which compared the effects of various parent interventions with professional treatment, treatment as usual, or being on a wait-list to receive an intervention.
Overall the research team reported “moderately strong” average benefits from the parent-mediated interventions (Hedges’ g, 0.553), indicating a medium effect size. Parent interventions had the greatest effect on outcomes involving positive behavior and social skills (0.603), followed by language and communication (0.545), maladaptive behavior (0.519), and life skills (0.239).
Similar benefits were observed regardless of a child’s age or sex or which parent or parents implemented an intervention. The effects also appeared to be consistent regardless of intervention characteristics, such as the number of training sessions parents received, although the researchers noted that many studies did not provide data on such details.
Paul Carbone, MD, a professor of pediatrics at the University of Utah, Salt Lake City, who was not involved in the review, said it demonstrates that such parental engagement is “vitally important” and pediatricians “should not hesitate to refer interested families.”
Dr. Carbone, who is the medical director of an assessment program for children with suspected developmental disabilities, said many training programs for parents have adopted telehealth, adding to their convenience. To make appropriate referrals, primary care clinicians should become acquainted with local programs and learn which outcomes they target, he said.
Dr. Smith noted that primary care physicians are “better trained now than ever” to identify autism spectrum disorder and therefore are among the first to identify those conditions and help parents understand “that their actions at home absolutely make a difference in the child’s development.”
Overcoming limitations, future research needs
The research team attempted to overcome limitations with previous reviews by using comprehensive search terms and other methods to identify relevant studies, including some that had not been published. They included only studies that reflect common practice of training multiple parents simultaneously, they wrote.
Dr. Smith noted that long-term outcomes data and further study to compare effects on children with mild, moderate, and severe autism are needed.
Although logic would suggest greater benefits for children with severe disease, there are no data to demonstrate that, he said.
The authors of the study and Dr. Carbone reported no relevant competing interests.
“Referrals for parent training should now be considered the expected standard for medical practice,” said a member of the research team, Timothy B. Smith, PhD, a professor of psychology at Brigham Young University, Provo, Utah.
Programs that show parents how to teach functional skills and address maladaptive behaviors, also known as parent-mediated or parent-implemented interventions, offer an alternative to one-on-one professional services, which are in short supply, according to the paper, which was published in the Journal of Autism and Developmental Disorders.
Methods and results
The meta-analysis included 54 papers based on randomized clinical trials involving 2,895 children, which compared the effects of various parent interventions with professional treatment, treatment as usual, or being on a wait-list to receive an intervention.
Overall the research team reported “moderately strong” average benefits from the parent-mediated interventions (Hedges’ g, 0.553), indicating a medium effect size. Parent interventions had the greatest effect on outcomes involving positive behavior and social skills (0.603), followed by language and communication (0.545), maladaptive behavior (0.519), and life skills (0.239).
Similar benefits were observed regardless of a child’s age or sex or which parent or parents implemented an intervention. The effects also appeared to be consistent regardless of intervention characteristics, such as the number of training sessions parents received, although the researchers noted that many studies did not provide data on such details.
Paul Carbone, MD, a professor of pediatrics at the University of Utah, Salt Lake City, who was not involved in the review, said it demonstrates that such parental engagement is “vitally important” and pediatricians “should not hesitate to refer interested families.”
Dr. Carbone, who is the medical director of an assessment program for children with suspected developmental disabilities, said many training programs for parents have adopted telehealth, adding to their convenience. To make appropriate referrals, primary care clinicians should become acquainted with local programs and learn which outcomes they target, he said.
Dr. Smith noted that primary care physicians are “better trained now than ever” to identify autism spectrum disorder and therefore are among the first to identify those conditions and help parents understand “that their actions at home absolutely make a difference in the child’s development.”
Overcoming limitations, future research needs
The research team attempted to overcome limitations with previous reviews by using comprehensive search terms and other methods to identify relevant studies, including some that had not been published. They included only studies that reflect common practice of training multiple parents simultaneously, they wrote.
Dr. Smith noted that long-term outcomes data and further study to compare effects on children with mild, moderate, and severe autism are needed.
Although logic would suggest greater benefits for children with severe disease, there are no data to demonstrate that, he said.
The authors of the study and Dr. Carbone reported no relevant competing interests.
FROM JOURNAL OF AUTISM AND DEVELOPMENTAL DISORDERS
Largest-ever study into the effects of cannabis on the brain
The largest-ever independent study into the effects of cannabis on the brain is being carried out in the United Kingdom.
Even though cannabis is the most commonly used illegal drug in the United Kingdom and medicinal cannabis has been legal there since 2018 little is known about why some people react badly to it and others seem to benefit from it.
According to Home Office figures on drug use from 2019, 7.6% of adults aged 16-59 used cannabis in the previous year.
Medicinal cannabis in the United Kingdom can only be prescribed if no other licensed medicine could help the patient. At the moment, GPs can’t prescribe it, only specialist hospital doctors can. The National Health Service says it can only be used in three circumstances: in rare, severe epilepsy; to deal with chemotherapy side effects such as nausea; or to help with multiple sclerosis.
As part of the Cannabis&Me study, KCL needs to get 3,000 current cannabis users and 3,000 non–cannabis users to take part in an online survey, with a third of those survey respondents then taking part in a face-to-face assessment that includes virtual reality (VR) and psychological analysis. The study also aims to determine how the DNA of cannabis users and their endocannabinoid system impacts their experiences, both negative and positive, with the drug.
The study is spearheaded by Marta Di Forti, MD, PhD, and has been allocated over £2.5 million in funding by the Medical Research Council.
This news organization asked Dr. Di Forti about the study.
Question: How do you describe the study?
Answer: “It’s a really unique study. We are aiming to see what’s happening to people using cannabis in the privacy of their homes for medicinal, recreational reasons, or whatever other reason.
“The debate on cannabis has always been quite polarized. There have been people who experience adversities with cannabis use, especially psychosis, whose families may perhaps like cannabis to be abolished if possible. Then there are other people who are saying they get positive benefits from using cannabis.”
Q: So where does the study come in?
A: “The study wants to bring the two sides of the argument together and understand what’s really happening. The group I see as a clinician comes to severe harm when they use cannabis regularly. We want to find out who they are and whether we can identify them. While we need to make sure they never come to harm when using cannabis, we need to consider others who won’t come to harm from using cannabis and give them a chance to use it in a way that’s beneficial.”
Q: How does the study work?
A: “The first step of the study is to use an online questionnaire that can be filled in by anyone aged 18-45 who lives in the London area or can travel here if selected. The first set of questions are a general idea of their cannabis use: ‘Why do they use it?’ ‘What are its benefits?’ Then, general questions on what their life has been like up to that point: ‘Did they have any adversities in childhood?’ ‘How is their mood and anxiety levels?’ ‘Do they experience any paranoid responses in everyday life?’ It probably takes between 30 and 40 minutes to fill out the questionnaire.”
Q: Can you explain about paranoid responses?
A: “We go through the questionnaires looking at people’s paranoid response to everyday life, not in a clinical disorder term, just in terms of the differences in how we respond to certain circumstances. For example: ‘How do you feel if someone’s staring at you on the Tube?’ Some people are afraid, some feel uncomfortable, some people don’t notice, and others think a person is staring at them as they look good or another such positive feeling. So, we give people a paranoia score and will invite some at the top and some at the bottom of that score for a face-to-face assessment. We want to select those people who are using cannabis daily and they are getting either no paranoia or high paranoia.”
Q: What happens at the face-to-face assessments?
A: “We do two things which are very novel. We ask them to take part in a virtual reality experience. They are in a lovely shop and within this experience they come across challenges, which may or may not induce a benign paranoia response. We will ask them to donate a sample of blood before they go into the VR set. We will test for tetrahydrocannabinol (THC) and cannabidiol (CBD). We will also look at the metabolites of the two. People don’t take into account how differently individuals metabolize cannabis, which could be one of the reasons why some people can tolerate it and others can’t.”
Q: There’s also a genetic aspect of the study?
A: “From the same sample, we will extract DNA to look at the genetics across the genome and compare genetic variations between high and low paranoia in the context of cannabis use. Also, we will look at the epigenetics, as we have learned from neuroscience, and also cancer, that sometimes a substance we ingest has an effect on our health. It’s perhaps an interaction with the way our DNA is written but also with the changes to the way our DNA is read and translated into biology if exposed to that substance. We know that smoking tobacco does have an impact at an epigenetic level on the DNA. We do know that in people who stop smoking, these impacts on the epigenetics are partially reversed. This work hasn’t been done properly for cannabis.
“There have been four published studies that have looked at the effect of cannabis use on epigenetics but they have been quite inconclusive, and they haven’t looked at large numbers of current users taking into account how much they are using. Moreover, we do know that when THC and CBD get into our bodies, they interact with something that is already embedded in our biology which is the endocannabinoid system. Therefore, in the blood samples we also aim to measures the levels of the endocannabinoids we naturally produce.
“All of this data will then be analyzed to see if we can get close to understanding what makes some cannabis users susceptible to paranoia while others who are using cannabis get some benefits, even in the domain of mental health.”
Q: Who are you looking for to take part in your study?
A: “What we don’t want is to get only people who are the classic friends and family of academics to do the study. We want a representative sample of people out there who are using cannabis. My ideal candidate would be someone who hates me and usually sends me abusive emails saying I’m against cannabis, which is wrong. All I want to find out is who is susceptible to harm which will keep everybody else safe. We are not trying to demonize cannabis; it’s exactly the opposite. We would like people from all ethnic and socioeconomic backgrounds to join to give voice to everyone out there using cannabis, the reasons why, and the effects they experience.”
Q: Will this study perhaps give more information of when it’s appropriate to prescribe medicinal cannabis, as it’s still quite unusual for it to be prescribed in the United Kingdom isn’t it?
A: “Absolutely spot on. That’s exactly the point. We want to hear from people who are receiving medicinal cannabis as a prescription, as they are likely to take it on a daily basis and daily use is what epidemiological studies have linked to the highest risk of psychosis. There will be people taking THC everyday for pain, nausea, for Crohn’s disease, and more.
“Normally when you receive a prescription for a medication the physician in charge will tell you the potential side effects which will be monitored to make sure it’s safe, and you may have to swap to a different medication. Now this isn’t really happening with medicinal cannabis, which is one of the reasons clinicians are anxious about prescribing it, and they have been criticized for not prescribing it very much. There’s much less structure and guidance about ‘psychosis-related’ side effects monitoring. If we can really identify those people who are likely to develop psychosis or disabling paranoia when they use cannabis, physicians might be more prepared to prescribe more widely when indicated.
“You could even have a virtual reality scenario available as a screening tool when you get prescribed medicinal cannabis, to see if there are changes in your perception of the world, which is ultimately what psychosis is about. Could this be a way of implementing safe prescribing which will encourage physicians to use safe cannabis compounds and make some people less anxious about it?
“This study is not here to highlight the negativity of cannabis, on the contrary it’s to understand how it can be used recreationally, but even more important, medicinally in a safe way so people that are coming to no harm can continue to do so and people who are at risk can be kept safe, or at least monitored adequately.”
A version of this article first appeared on Medscape UK.
The largest-ever independent study into the effects of cannabis on the brain is being carried out in the United Kingdom.
Even though cannabis is the most commonly used illegal drug in the United Kingdom and medicinal cannabis has been legal there since 2018 little is known about why some people react badly to it and others seem to benefit from it.
According to Home Office figures on drug use from 2019, 7.6% of adults aged 16-59 used cannabis in the previous year.
Medicinal cannabis in the United Kingdom can only be prescribed if no other licensed medicine could help the patient. At the moment, GPs can’t prescribe it, only specialist hospital doctors can. The National Health Service says it can only be used in three circumstances: in rare, severe epilepsy; to deal with chemotherapy side effects such as nausea; or to help with multiple sclerosis.
As part of the Cannabis&Me study, KCL needs to get 3,000 current cannabis users and 3,000 non–cannabis users to take part in an online survey, with a third of those survey respondents then taking part in a face-to-face assessment that includes virtual reality (VR) and psychological analysis. The study also aims to determine how the DNA of cannabis users and their endocannabinoid system impacts their experiences, both negative and positive, with the drug.
The study is spearheaded by Marta Di Forti, MD, PhD, and has been allocated over £2.5 million in funding by the Medical Research Council.
This news organization asked Dr. Di Forti about the study.
Question: How do you describe the study?
Answer: “It’s a really unique study. We are aiming to see what’s happening to people using cannabis in the privacy of their homes for medicinal, recreational reasons, or whatever other reason.
“The debate on cannabis has always been quite polarized. There have been people who experience adversities with cannabis use, especially psychosis, whose families may perhaps like cannabis to be abolished if possible. Then there are other people who are saying they get positive benefits from using cannabis.”
Q: So where does the study come in?
A: “The study wants to bring the two sides of the argument together and understand what’s really happening. The group I see as a clinician comes to severe harm when they use cannabis regularly. We want to find out who they are and whether we can identify them. While we need to make sure they never come to harm when using cannabis, we need to consider others who won’t come to harm from using cannabis and give them a chance to use it in a way that’s beneficial.”
Q: How does the study work?
A: “The first step of the study is to use an online questionnaire that can be filled in by anyone aged 18-45 who lives in the London area or can travel here if selected. The first set of questions are a general idea of their cannabis use: ‘Why do they use it?’ ‘What are its benefits?’ Then, general questions on what their life has been like up to that point: ‘Did they have any adversities in childhood?’ ‘How is their mood and anxiety levels?’ ‘Do they experience any paranoid responses in everyday life?’ It probably takes between 30 and 40 minutes to fill out the questionnaire.”
Q: Can you explain about paranoid responses?
A: “We go through the questionnaires looking at people’s paranoid response to everyday life, not in a clinical disorder term, just in terms of the differences in how we respond to certain circumstances. For example: ‘How do you feel if someone’s staring at you on the Tube?’ Some people are afraid, some feel uncomfortable, some people don’t notice, and others think a person is staring at them as they look good or another such positive feeling. So, we give people a paranoia score and will invite some at the top and some at the bottom of that score for a face-to-face assessment. We want to select those people who are using cannabis daily and they are getting either no paranoia or high paranoia.”
Q: What happens at the face-to-face assessments?
A: “We do two things which are very novel. We ask them to take part in a virtual reality experience. They are in a lovely shop and within this experience they come across challenges, which may or may not induce a benign paranoia response. We will ask them to donate a sample of blood before they go into the VR set. We will test for tetrahydrocannabinol (THC) and cannabidiol (CBD). We will also look at the metabolites of the two. People don’t take into account how differently individuals metabolize cannabis, which could be one of the reasons why some people can tolerate it and others can’t.”
Q: There’s also a genetic aspect of the study?
A: “From the same sample, we will extract DNA to look at the genetics across the genome and compare genetic variations between high and low paranoia in the context of cannabis use. Also, we will look at the epigenetics, as we have learned from neuroscience, and also cancer, that sometimes a substance we ingest has an effect on our health. It’s perhaps an interaction with the way our DNA is written but also with the changes to the way our DNA is read and translated into biology if exposed to that substance. We know that smoking tobacco does have an impact at an epigenetic level on the DNA. We do know that in people who stop smoking, these impacts on the epigenetics are partially reversed. This work hasn’t been done properly for cannabis.
“There have been four published studies that have looked at the effect of cannabis use on epigenetics but they have been quite inconclusive, and they haven’t looked at large numbers of current users taking into account how much they are using. Moreover, we do know that when THC and CBD get into our bodies, they interact with something that is already embedded in our biology which is the endocannabinoid system. Therefore, in the blood samples we also aim to measures the levels of the endocannabinoids we naturally produce.
“All of this data will then be analyzed to see if we can get close to understanding what makes some cannabis users susceptible to paranoia while others who are using cannabis get some benefits, even in the domain of mental health.”
Q: Who are you looking for to take part in your study?
A: “What we don’t want is to get only people who are the classic friends and family of academics to do the study. We want a representative sample of people out there who are using cannabis. My ideal candidate would be someone who hates me and usually sends me abusive emails saying I’m against cannabis, which is wrong. All I want to find out is who is susceptible to harm which will keep everybody else safe. We are not trying to demonize cannabis; it’s exactly the opposite. We would like people from all ethnic and socioeconomic backgrounds to join to give voice to everyone out there using cannabis, the reasons why, and the effects they experience.”
Q: Will this study perhaps give more information of when it’s appropriate to prescribe medicinal cannabis, as it’s still quite unusual for it to be prescribed in the United Kingdom isn’t it?
A: “Absolutely spot on. That’s exactly the point. We want to hear from people who are receiving medicinal cannabis as a prescription, as they are likely to take it on a daily basis and daily use is what epidemiological studies have linked to the highest risk of psychosis. There will be people taking THC everyday for pain, nausea, for Crohn’s disease, and more.
“Normally when you receive a prescription for a medication the physician in charge will tell you the potential side effects which will be monitored to make sure it’s safe, and you may have to swap to a different medication. Now this isn’t really happening with medicinal cannabis, which is one of the reasons clinicians are anxious about prescribing it, and they have been criticized for not prescribing it very much. There’s much less structure and guidance about ‘psychosis-related’ side effects monitoring. If we can really identify those people who are likely to develop psychosis or disabling paranoia when they use cannabis, physicians might be more prepared to prescribe more widely when indicated.
“You could even have a virtual reality scenario available as a screening tool when you get prescribed medicinal cannabis, to see if there are changes in your perception of the world, which is ultimately what psychosis is about. Could this be a way of implementing safe prescribing which will encourage physicians to use safe cannabis compounds and make some people less anxious about it?
“This study is not here to highlight the negativity of cannabis, on the contrary it’s to understand how it can be used recreationally, but even more important, medicinally in a safe way so people that are coming to no harm can continue to do so and people who are at risk can be kept safe, or at least monitored adequately.”
A version of this article first appeared on Medscape UK.
The largest-ever independent study into the effects of cannabis on the brain is being carried out in the United Kingdom.
Even though cannabis is the most commonly used illegal drug in the United Kingdom and medicinal cannabis has been legal there since 2018 little is known about why some people react badly to it and others seem to benefit from it.
According to Home Office figures on drug use from 2019, 7.6% of adults aged 16-59 used cannabis in the previous year.
Medicinal cannabis in the United Kingdom can only be prescribed if no other licensed medicine could help the patient. At the moment, GPs can’t prescribe it, only specialist hospital doctors can. The National Health Service says it can only be used in three circumstances: in rare, severe epilepsy; to deal with chemotherapy side effects such as nausea; or to help with multiple sclerosis.
As part of the Cannabis&Me study, KCL needs to get 3,000 current cannabis users and 3,000 non–cannabis users to take part in an online survey, with a third of those survey respondents then taking part in a face-to-face assessment that includes virtual reality (VR) and psychological analysis. The study also aims to determine how the DNA of cannabis users and their endocannabinoid system impacts their experiences, both negative and positive, with the drug.
The study is spearheaded by Marta Di Forti, MD, PhD, and has been allocated over £2.5 million in funding by the Medical Research Council.
This news organization asked Dr. Di Forti about the study.
Question: How do you describe the study?
Answer: “It’s a really unique study. We are aiming to see what’s happening to people using cannabis in the privacy of their homes for medicinal, recreational reasons, or whatever other reason.
“The debate on cannabis has always been quite polarized. There have been people who experience adversities with cannabis use, especially psychosis, whose families may perhaps like cannabis to be abolished if possible. Then there are other people who are saying they get positive benefits from using cannabis.”
Q: So where does the study come in?
A: “The study wants to bring the two sides of the argument together and understand what’s really happening. The group I see as a clinician comes to severe harm when they use cannabis regularly. We want to find out who they are and whether we can identify them. While we need to make sure they never come to harm when using cannabis, we need to consider others who won’t come to harm from using cannabis and give them a chance to use it in a way that’s beneficial.”
Q: How does the study work?
A: “The first step of the study is to use an online questionnaire that can be filled in by anyone aged 18-45 who lives in the London area or can travel here if selected. The first set of questions are a general idea of their cannabis use: ‘Why do they use it?’ ‘What are its benefits?’ Then, general questions on what their life has been like up to that point: ‘Did they have any adversities in childhood?’ ‘How is their mood and anxiety levels?’ ‘Do they experience any paranoid responses in everyday life?’ It probably takes between 30 and 40 minutes to fill out the questionnaire.”
Q: Can you explain about paranoid responses?
A: “We go through the questionnaires looking at people’s paranoid response to everyday life, not in a clinical disorder term, just in terms of the differences in how we respond to certain circumstances. For example: ‘How do you feel if someone’s staring at you on the Tube?’ Some people are afraid, some feel uncomfortable, some people don’t notice, and others think a person is staring at them as they look good or another such positive feeling. So, we give people a paranoia score and will invite some at the top and some at the bottom of that score for a face-to-face assessment. We want to select those people who are using cannabis daily and they are getting either no paranoia or high paranoia.”
Q: What happens at the face-to-face assessments?
A: “We do two things which are very novel. We ask them to take part in a virtual reality experience. They are in a lovely shop and within this experience they come across challenges, which may or may not induce a benign paranoia response. We will ask them to donate a sample of blood before they go into the VR set. We will test for tetrahydrocannabinol (THC) and cannabidiol (CBD). We will also look at the metabolites of the two. People don’t take into account how differently individuals metabolize cannabis, which could be one of the reasons why some people can tolerate it and others can’t.”
Q: There’s also a genetic aspect of the study?
A: “From the same sample, we will extract DNA to look at the genetics across the genome and compare genetic variations between high and low paranoia in the context of cannabis use. Also, we will look at the epigenetics, as we have learned from neuroscience, and also cancer, that sometimes a substance we ingest has an effect on our health. It’s perhaps an interaction with the way our DNA is written but also with the changes to the way our DNA is read and translated into biology if exposed to that substance. We know that smoking tobacco does have an impact at an epigenetic level on the DNA. We do know that in people who stop smoking, these impacts on the epigenetics are partially reversed. This work hasn’t been done properly for cannabis.
“There have been four published studies that have looked at the effect of cannabis use on epigenetics but they have been quite inconclusive, and they haven’t looked at large numbers of current users taking into account how much they are using. Moreover, we do know that when THC and CBD get into our bodies, they interact with something that is already embedded in our biology which is the endocannabinoid system. Therefore, in the blood samples we also aim to measures the levels of the endocannabinoids we naturally produce.
“All of this data will then be analyzed to see if we can get close to understanding what makes some cannabis users susceptible to paranoia while others who are using cannabis get some benefits, even in the domain of mental health.”
Q: Who are you looking for to take part in your study?
A: “What we don’t want is to get only people who are the classic friends and family of academics to do the study. We want a representative sample of people out there who are using cannabis. My ideal candidate would be someone who hates me and usually sends me abusive emails saying I’m against cannabis, which is wrong. All I want to find out is who is susceptible to harm which will keep everybody else safe. We are not trying to demonize cannabis; it’s exactly the opposite. We would like people from all ethnic and socioeconomic backgrounds to join to give voice to everyone out there using cannabis, the reasons why, and the effects they experience.”
Q: Will this study perhaps give more information of when it’s appropriate to prescribe medicinal cannabis, as it’s still quite unusual for it to be prescribed in the United Kingdom isn’t it?
A: “Absolutely spot on. That’s exactly the point. We want to hear from people who are receiving medicinal cannabis as a prescription, as they are likely to take it on a daily basis and daily use is what epidemiological studies have linked to the highest risk of psychosis. There will be people taking THC everyday for pain, nausea, for Crohn’s disease, and more.
“Normally when you receive a prescription for a medication the physician in charge will tell you the potential side effects which will be monitored to make sure it’s safe, and you may have to swap to a different medication. Now this isn’t really happening with medicinal cannabis, which is one of the reasons clinicians are anxious about prescribing it, and they have been criticized for not prescribing it very much. There’s much less structure and guidance about ‘psychosis-related’ side effects monitoring. If we can really identify those people who are likely to develop psychosis or disabling paranoia when they use cannabis, physicians might be more prepared to prescribe more widely when indicated.
“You could even have a virtual reality scenario available as a screening tool when you get prescribed medicinal cannabis, to see if there are changes in your perception of the world, which is ultimately what psychosis is about. Could this be a way of implementing safe prescribing which will encourage physicians to use safe cannabis compounds and make some people less anxious about it?
“This study is not here to highlight the negativity of cannabis, on the contrary it’s to understand how it can be used recreationally, but even more important, medicinally in a safe way so people that are coming to no harm can continue to do so and people who are at risk can be kept safe, or at least monitored adequately.”
A version of this article first appeared on Medscape UK.
Fish in pregnancy not dangerous after all, says new study
A new study has called into question the decades-long official guidance advising pregnant women to limit consumption of certain fish because of their potentially high mercury content. That advice was based particularly on one 1997 study suggesting a correlation between fetal exposure to methylmercury and cognitive dysfunction at age 7.
The U.K’s National Health Service currently advises not only pregnant women but also all those who are potentially fertile (those “who are planning a pregnancy or may have a child one day”) to limit oily fish consumption to no more than two portions per week. During pregnancy and while trying to get pregnant, women are advised to avoid shark, swordfish, and marlin altogether.
Suspicions arose from study involving consumption of pilot whale
However, researchers from the University of Bristol (England) now suggest that assumptions generated by the original 1997 study – of a cohort of women in the Faroe Islands – were unwarranted. “It was clearly stated that the methylmercury levels were associated with consumption of pilot whale (a sea mammal, not a fish),” they said.
The pilot whale is a species known to concentrate cadmium and mercury, and indeed in 1989 Faroe Islanders themselves had been advised to limit consumption of both whale meat and blubber, and to abstain completely from liver and kidneys.
Yet, as the authors pointed out, following the 1997 study, “the subsequent assumptions were that seafood in general was responsible for increased mercury levels in the mother.”
New study shows ‘no evidence of harm’
Their new research, published in NeuroToxicology, has now shown that “there is no evidence of harm from these fish,” they said. They recommend that advice for pregnant women should now be revised.
The study drew together analyses on over 4,131 pregnant mothers from the Avon Longitudinal Study of Parents and Children (ALSPAC), also known as the ‘Children of the 90s’ study, with similar detailed studies conducted in the Seychelles. The two populations differ considerably in their frequency of fish consumption: fish is a major component of the diet in the Seychelles, but eaten less frequently in the Avon study area, centered on Bristol.
The team looked for studies using the data from these two contrasting cohorts where mercury levels had been measured during pregnancy and the children followed up at frequent intervals during their childhood. Longitudinal studies in the Seychelles “have not demonstrated harmful cognitive effects in children with increasing maternal mercury levels”, they reported.
The same proved true in the United Kingdom, a more-developed country where fish is eaten less frequently, they found. They summarized the results from various papers that used ALSPAC data and found no adverse associations between total mercury levels measured in maternal whole blood and umbilical cord tissue with children’s cognitive development, in terms of either IQ or scholastic abilities.
In addition, extensive dietary questionnaires during pregnancy had allowed estimates of total fish intake to be calculated, as well as variations in the amount of each type of seafood consumed. “Although seafood is a source of dietary mercury, it appeared to explain a relatively small proportion (9%) of the variation in total blood mercury in our U.K. study population,” they said – actually less than the variance attributable to socio-demographic characteristics of the mother (10.4%).
Positive benefits of eating fish irrespective of type
What mattered was not which types of fish were eaten but whether the woman ate fish or not, which emerged as the most important factor. The mother’s prenatal mercury level was positively associated with her child’s IQ if she had eaten fish in pregnancy, but not if she had not.
“Significantly beneficial associations with prenatal mercury levels were shown for total and performance IQ, mathematical/scientific reasoning, and birth weight, in fish-consuming versus non–fish-consuming mothers,” the authors said. “These beneficial findings are similar to those observed in the Seychelles, where fish consumption is high and prenatal mercury levels are 10 times higher than U.S. levels.”
Caroline Taylor, PhD, senior research fellow and coauthor of the study, said: “We found that the mother’s mercury level during pregnancy is likely to have no adverse effect on the development of the child provided that the mother eats fish. If she did not eat fish, then there was some evidence that her mercury level could have a harmful effect on the child.”
The team said that this was because the essential nutrients in the fish could be protective against the mercury content of the fish. “This could be because of the benefits from the mix of essential nutrients that fish provides, including long-chain fatty acids, iodine, vitamin D and selenium,” said Dr. Taylor.
Women stopped eating any fish ‘to be on the safe side’
The authors called for a change in official guidance. “Health advice to pregnant women concerning consumption of mercury-containing foods has resulted in anxiety, with subsequent avoidance of fish consumption during pregnancy.” Seafood contains many nutrients crucial for children’s growth and development, but “there is the possibility that some women will stop eating any fish ‘to be on the safe side.’ ”
The authors said: “Although advice to pregnant women was generally that fish was good, the accompanying caveat was to avoid fish with high levels of mercury. Psychologically, the latter was the message that women remembered, and the general reaction has been for women to reduce their intake of all seafood.”
Coauthor Jean Golding, emeritus professor of pediatric and perinatal epidemiology at the University of Bristol, said: “It is important that advisories from health professionals revise their advice warning against eating certain species of fish. There is no evidence of harm from these fish, but there is evidence from different countries that such advice can cause confusion in pregnant women. The guidance for pregnancy should highlight ‘Eat at least two portions of fish a week, one of which should be oily’ – and omit all warnings that certain fish should not be eaten.”
The study was funded via core support for ALSPAC by the UK Medical Research Council and the UK Wellcome Trust.
A version of this article first appeared on Medscape UK.
A new study has called into question the decades-long official guidance advising pregnant women to limit consumption of certain fish because of their potentially high mercury content. That advice was based particularly on one 1997 study suggesting a correlation between fetal exposure to methylmercury and cognitive dysfunction at age 7.
The U.K’s National Health Service currently advises not only pregnant women but also all those who are potentially fertile (those “who are planning a pregnancy or may have a child one day”) to limit oily fish consumption to no more than two portions per week. During pregnancy and while trying to get pregnant, women are advised to avoid shark, swordfish, and marlin altogether.
Suspicions arose from study involving consumption of pilot whale
However, researchers from the University of Bristol (England) now suggest that assumptions generated by the original 1997 study – of a cohort of women in the Faroe Islands – were unwarranted. “It was clearly stated that the methylmercury levels were associated with consumption of pilot whale (a sea mammal, not a fish),” they said.
The pilot whale is a species known to concentrate cadmium and mercury, and indeed in 1989 Faroe Islanders themselves had been advised to limit consumption of both whale meat and blubber, and to abstain completely from liver and kidneys.
Yet, as the authors pointed out, following the 1997 study, “the subsequent assumptions were that seafood in general was responsible for increased mercury levels in the mother.”
New study shows ‘no evidence of harm’
Their new research, published in NeuroToxicology, has now shown that “there is no evidence of harm from these fish,” they said. They recommend that advice for pregnant women should now be revised.
The study drew together analyses on over 4,131 pregnant mothers from the Avon Longitudinal Study of Parents and Children (ALSPAC), also known as the ‘Children of the 90s’ study, with similar detailed studies conducted in the Seychelles. The two populations differ considerably in their frequency of fish consumption: fish is a major component of the diet in the Seychelles, but eaten less frequently in the Avon study area, centered on Bristol.
The team looked for studies using the data from these two contrasting cohorts where mercury levels had been measured during pregnancy and the children followed up at frequent intervals during their childhood. Longitudinal studies in the Seychelles “have not demonstrated harmful cognitive effects in children with increasing maternal mercury levels”, they reported.
The same proved true in the United Kingdom, a more-developed country where fish is eaten less frequently, they found. They summarized the results from various papers that used ALSPAC data and found no adverse associations between total mercury levels measured in maternal whole blood and umbilical cord tissue with children’s cognitive development, in terms of either IQ or scholastic abilities.
In addition, extensive dietary questionnaires during pregnancy had allowed estimates of total fish intake to be calculated, as well as variations in the amount of each type of seafood consumed. “Although seafood is a source of dietary mercury, it appeared to explain a relatively small proportion (9%) of the variation in total blood mercury in our U.K. study population,” they said – actually less than the variance attributable to socio-demographic characteristics of the mother (10.4%).
Positive benefits of eating fish irrespective of type
What mattered was not which types of fish were eaten but whether the woman ate fish or not, which emerged as the most important factor. The mother’s prenatal mercury level was positively associated with her child’s IQ if she had eaten fish in pregnancy, but not if she had not.
“Significantly beneficial associations with prenatal mercury levels were shown for total and performance IQ, mathematical/scientific reasoning, and birth weight, in fish-consuming versus non–fish-consuming mothers,” the authors said. “These beneficial findings are similar to those observed in the Seychelles, where fish consumption is high and prenatal mercury levels are 10 times higher than U.S. levels.”
Caroline Taylor, PhD, senior research fellow and coauthor of the study, said: “We found that the mother’s mercury level during pregnancy is likely to have no adverse effect on the development of the child provided that the mother eats fish. If she did not eat fish, then there was some evidence that her mercury level could have a harmful effect on the child.”
The team said that this was because the essential nutrients in the fish could be protective against the mercury content of the fish. “This could be because of the benefits from the mix of essential nutrients that fish provides, including long-chain fatty acids, iodine, vitamin D and selenium,” said Dr. Taylor.
Women stopped eating any fish ‘to be on the safe side’
The authors called for a change in official guidance. “Health advice to pregnant women concerning consumption of mercury-containing foods has resulted in anxiety, with subsequent avoidance of fish consumption during pregnancy.” Seafood contains many nutrients crucial for children’s growth and development, but “there is the possibility that some women will stop eating any fish ‘to be on the safe side.’ ”
The authors said: “Although advice to pregnant women was generally that fish was good, the accompanying caveat was to avoid fish with high levels of mercury. Psychologically, the latter was the message that women remembered, and the general reaction has been for women to reduce their intake of all seafood.”
Coauthor Jean Golding, emeritus professor of pediatric and perinatal epidemiology at the University of Bristol, said: “It is important that advisories from health professionals revise their advice warning against eating certain species of fish. There is no evidence of harm from these fish, but there is evidence from different countries that such advice can cause confusion in pregnant women. The guidance for pregnancy should highlight ‘Eat at least two portions of fish a week, one of which should be oily’ – and omit all warnings that certain fish should not be eaten.”
The study was funded via core support for ALSPAC by the UK Medical Research Council and the UK Wellcome Trust.
A version of this article first appeared on Medscape UK.
A new study has called into question the decades-long official guidance advising pregnant women to limit consumption of certain fish because of their potentially high mercury content. That advice was based particularly on one 1997 study suggesting a correlation between fetal exposure to methylmercury and cognitive dysfunction at age 7.
The U.K’s National Health Service currently advises not only pregnant women but also all those who are potentially fertile (those “who are planning a pregnancy or may have a child one day”) to limit oily fish consumption to no more than two portions per week. During pregnancy and while trying to get pregnant, women are advised to avoid shark, swordfish, and marlin altogether.
Suspicions arose from study involving consumption of pilot whale
However, researchers from the University of Bristol (England) now suggest that assumptions generated by the original 1997 study – of a cohort of women in the Faroe Islands – were unwarranted. “It was clearly stated that the methylmercury levels were associated with consumption of pilot whale (a sea mammal, not a fish),” they said.
The pilot whale is a species known to concentrate cadmium and mercury, and indeed in 1989 Faroe Islanders themselves had been advised to limit consumption of both whale meat and blubber, and to abstain completely from liver and kidneys.
Yet, as the authors pointed out, following the 1997 study, “the subsequent assumptions were that seafood in general was responsible for increased mercury levels in the mother.”
New study shows ‘no evidence of harm’
Their new research, published in NeuroToxicology, has now shown that “there is no evidence of harm from these fish,” they said. They recommend that advice for pregnant women should now be revised.
The study drew together analyses on over 4,131 pregnant mothers from the Avon Longitudinal Study of Parents and Children (ALSPAC), also known as the ‘Children of the 90s’ study, with similar detailed studies conducted in the Seychelles. The two populations differ considerably in their frequency of fish consumption: fish is a major component of the diet in the Seychelles, but eaten less frequently in the Avon study area, centered on Bristol.
The team looked for studies using the data from these two contrasting cohorts where mercury levels had been measured during pregnancy and the children followed up at frequent intervals during their childhood. Longitudinal studies in the Seychelles “have not demonstrated harmful cognitive effects in children with increasing maternal mercury levels”, they reported.
The same proved true in the United Kingdom, a more-developed country where fish is eaten less frequently, they found. They summarized the results from various papers that used ALSPAC data and found no adverse associations between total mercury levels measured in maternal whole blood and umbilical cord tissue with children’s cognitive development, in terms of either IQ or scholastic abilities.
In addition, extensive dietary questionnaires during pregnancy had allowed estimates of total fish intake to be calculated, as well as variations in the amount of each type of seafood consumed. “Although seafood is a source of dietary mercury, it appeared to explain a relatively small proportion (9%) of the variation in total blood mercury in our U.K. study population,” they said – actually less than the variance attributable to socio-demographic characteristics of the mother (10.4%).
Positive benefits of eating fish irrespective of type
What mattered was not which types of fish were eaten but whether the woman ate fish or not, which emerged as the most important factor. The mother’s prenatal mercury level was positively associated with her child’s IQ if she had eaten fish in pregnancy, but not if she had not.
“Significantly beneficial associations with prenatal mercury levels were shown for total and performance IQ, mathematical/scientific reasoning, and birth weight, in fish-consuming versus non–fish-consuming mothers,” the authors said. “These beneficial findings are similar to those observed in the Seychelles, where fish consumption is high and prenatal mercury levels are 10 times higher than U.S. levels.”
Caroline Taylor, PhD, senior research fellow and coauthor of the study, said: “We found that the mother’s mercury level during pregnancy is likely to have no adverse effect on the development of the child provided that the mother eats fish. If she did not eat fish, then there was some evidence that her mercury level could have a harmful effect on the child.”
The team said that this was because the essential nutrients in the fish could be protective against the mercury content of the fish. “This could be because of the benefits from the mix of essential nutrients that fish provides, including long-chain fatty acids, iodine, vitamin D and selenium,” said Dr. Taylor.
Women stopped eating any fish ‘to be on the safe side’
The authors called for a change in official guidance. “Health advice to pregnant women concerning consumption of mercury-containing foods has resulted in anxiety, with subsequent avoidance of fish consumption during pregnancy.” Seafood contains many nutrients crucial for children’s growth and development, but “there is the possibility that some women will stop eating any fish ‘to be on the safe side.’ ”
The authors said: “Although advice to pregnant women was generally that fish was good, the accompanying caveat was to avoid fish with high levels of mercury. Psychologically, the latter was the message that women remembered, and the general reaction has been for women to reduce their intake of all seafood.”
Coauthor Jean Golding, emeritus professor of pediatric and perinatal epidemiology at the University of Bristol, said: “It is important that advisories from health professionals revise their advice warning against eating certain species of fish. There is no evidence of harm from these fish, but there is evidence from different countries that such advice can cause confusion in pregnant women. The guidance for pregnancy should highlight ‘Eat at least two portions of fish a week, one of which should be oily’ – and omit all warnings that certain fish should not be eaten.”
The study was funded via core support for ALSPAC by the UK Medical Research Council and the UK Wellcome Trust.
A version of this article first appeared on Medscape UK.
FROM NEUROTOXICOLOGY
Baseline neuromotor abnormalities persist in schizophrenia
Neuromotor abnormalities in psychotic disorders have long been ignored as side effects of antipsychotic drugs, but they are gaining new attention as a component of the disease process, with implications for outcomes and management, wrote Victor Peralta, MD, PhD, of Servicio Navarro de Salud, Pamplona, Spain, and colleagues.
Previous research has suggested links between increased levels of parkinsonism, dyskinesia, and NSS and poor symptomatic and functional outcomes, but “the impact of primary neuromotor dysfunction on the long-term course and outcome of psychotic disorders remains largely unknown,” they said.
In a study published in Schizophrenia Research , the investigators identified 243 consecutive schizophrenia patients admitted to a psychiatric ward at a single center.
Patients were assessed at baseline for variables including parkinsonism, dyskinesia, NSS, and catatonia, and were reassessed 21 years later for the same variables, along with psychopathology, functioning, personal recovery, cognitive performance, and comorbidity.
Overall, baseline dyskinesia and NSS measures were stable over time, with Intraclass Correlation Coefficients (ICC) of 0.92 and 0.86, respectively, while rating stability was low for parkinsonism and catatonia (ICC = 0.42 and 0.31, respectively).
Baseline dyskinesia and NSS each were independent predictors of more positive and negative symptoms, poor functioning, and less personal recovery at 21 years. In a multivariate model, neuromotor dysfunction at follow-up was significantly associated with family history of schizophrenia, obstetric complications, neurodevelopmental delay, and premorbid IQ, as well as baseline dyskinesia and NSS; “these variables explained 51% of the variance in the neuromotor outcome, 35% of which corresponded to baseline dyskinesia and NSS,” the researchers said. As for other outcomes, baseline neuromotor ratings predicted a range from 4% for medical comorbidity to 15% for cognitive impairment.
“The distinction between primary and drug-induced neuromotor dysfunction is a very complex issue, mainly because antipsychotic drugs may cause de novo motor dysfunction, such as improve or worsen the disease-based motor dysfunction,” the researchers explained in their discussion.
Baseline parkinsonism, dyskinesia, and NSS were significantly related to increased risk of antipsychotic exposure over the illness course, possibly because primary neuromotor dysfunction was predictive of greater severity of illness in general, which confounds differentiation between primary and drug-induced motor symptoms, they noted.
The study findings were limited by several factors including potential selection bias because of the selection of first-admission psychosis, which may limit generalizability, the researchers noted. Other limitations include the use of standard clinical rating scales rather than instrumental procedures to measuring neuromotor abnormalities.
However, “our findings confirm the significance of baseline and follow-up neuromotor abnormalities as a core dimension of psychosis,” and future studies “should complement clinical rating scales with instrumental assessment to capture neuromotor dysfunction more comprehensively,” they said.
The results highlight the clinical relevance of examining neuromotor abnormalities as a routine part of practice prior to starting antipsychotics because of their potential as predictors of long-term outcomes “and to disentangle the primary versus drug-induced character of neuromotor impairment in treated patients,” they concluded.
The study was supported by the Spanish Ministry of Economy, Industry, and Competitiveness, and the Regional Government of Navarra. The researchers had no financial conflicts to disclose.
Neuromotor abnormalities in psychotic disorders have long been ignored as side effects of antipsychotic drugs, but they are gaining new attention as a component of the disease process, with implications for outcomes and management, wrote Victor Peralta, MD, PhD, of Servicio Navarro de Salud, Pamplona, Spain, and colleagues.
Previous research has suggested links between increased levels of parkinsonism, dyskinesia, and NSS and poor symptomatic and functional outcomes, but “the impact of primary neuromotor dysfunction on the long-term course and outcome of psychotic disorders remains largely unknown,” they said.
In a study published in Schizophrenia Research , the investigators identified 243 consecutive schizophrenia patients admitted to a psychiatric ward at a single center.
Patients were assessed at baseline for variables including parkinsonism, dyskinesia, NSS, and catatonia, and were reassessed 21 years later for the same variables, along with psychopathology, functioning, personal recovery, cognitive performance, and comorbidity.
Overall, baseline dyskinesia and NSS measures were stable over time, with Intraclass Correlation Coefficients (ICC) of 0.92 and 0.86, respectively, while rating stability was low for parkinsonism and catatonia (ICC = 0.42 and 0.31, respectively).
Baseline dyskinesia and NSS each were independent predictors of more positive and negative symptoms, poor functioning, and less personal recovery at 21 years. In a multivariate model, neuromotor dysfunction at follow-up was significantly associated with family history of schizophrenia, obstetric complications, neurodevelopmental delay, and premorbid IQ, as well as baseline dyskinesia and NSS; “these variables explained 51% of the variance in the neuromotor outcome, 35% of which corresponded to baseline dyskinesia and NSS,” the researchers said. As for other outcomes, baseline neuromotor ratings predicted a range from 4% for medical comorbidity to 15% for cognitive impairment.
“The distinction between primary and drug-induced neuromotor dysfunction is a very complex issue, mainly because antipsychotic drugs may cause de novo motor dysfunction, such as improve or worsen the disease-based motor dysfunction,” the researchers explained in their discussion.
Baseline parkinsonism, dyskinesia, and NSS were significantly related to increased risk of antipsychotic exposure over the illness course, possibly because primary neuromotor dysfunction was predictive of greater severity of illness in general, which confounds differentiation between primary and drug-induced motor symptoms, they noted.
The study findings were limited by several factors including potential selection bias because of the selection of first-admission psychosis, which may limit generalizability, the researchers noted. Other limitations include the use of standard clinical rating scales rather than instrumental procedures to measuring neuromotor abnormalities.
However, “our findings confirm the significance of baseline and follow-up neuromotor abnormalities as a core dimension of psychosis,” and future studies “should complement clinical rating scales with instrumental assessment to capture neuromotor dysfunction more comprehensively,” they said.
The results highlight the clinical relevance of examining neuromotor abnormalities as a routine part of practice prior to starting antipsychotics because of their potential as predictors of long-term outcomes “and to disentangle the primary versus drug-induced character of neuromotor impairment in treated patients,” they concluded.
The study was supported by the Spanish Ministry of Economy, Industry, and Competitiveness, and the Regional Government of Navarra. The researchers had no financial conflicts to disclose.
Neuromotor abnormalities in psychotic disorders have long been ignored as side effects of antipsychotic drugs, but they are gaining new attention as a component of the disease process, with implications for outcomes and management, wrote Victor Peralta, MD, PhD, of Servicio Navarro de Salud, Pamplona, Spain, and colleagues.
Previous research has suggested links between increased levels of parkinsonism, dyskinesia, and NSS and poor symptomatic and functional outcomes, but “the impact of primary neuromotor dysfunction on the long-term course and outcome of psychotic disorders remains largely unknown,” they said.
In a study published in Schizophrenia Research , the investigators identified 243 consecutive schizophrenia patients admitted to a psychiatric ward at a single center.
Patients were assessed at baseline for variables including parkinsonism, dyskinesia, NSS, and catatonia, and were reassessed 21 years later for the same variables, along with psychopathology, functioning, personal recovery, cognitive performance, and comorbidity.
Overall, baseline dyskinesia and NSS measures were stable over time, with Intraclass Correlation Coefficients (ICC) of 0.92 and 0.86, respectively, while rating stability was low for parkinsonism and catatonia (ICC = 0.42 and 0.31, respectively).
Baseline dyskinesia and NSS each were independent predictors of more positive and negative symptoms, poor functioning, and less personal recovery at 21 years. In a multivariate model, neuromotor dysfunction at follow-up was significantly associated with family history of schizophrenia, obstetric complications, neurodevelopmental delay, and premorbid IQ, as well as baseline dyskinesia and NSS; “these variables explained 51% of the variance in the neuromotor outcome, 35% of which corresponded to baseline dyskinesia and NSS,” the researchers said. As for other outcomes, baseline neuromotor ratings predicted a range from 4% for medical comorbidity to 15% for cognitive impairment.
“The distinction between primary and drug-induced neuromotor dysfunction is a very complex issue, mainly because antipsychotic drugs may cause de novo motor dysfunction, such as improve or worsen the disease-based motor dysfunction,” the researchers explained in their discussion.
Baseline parkinsonism, dyskinesia, and NSS were significantly related to increased risk of antipsychotic exposure over the illness course, possibly because primary neuromotor dysfunction was predictive of greater severity of illness in general, which confounds differentiation between primary and drug-induced motor symptoms, they noted.
The study findings were limited by several factors including potential selection bias because of the selection of first-admission psychosis, which may limit generalizability, the researchers noted. Other limitations include the use of standard clinical rating scales rather than instrumental procedures to measuring neuromotor abnormalities.
However, “our findings confirm the significance of baseline and follow-up neuromotor abnormalities as a core dimension of psychosis,” and future studies “should complement clinical rating scales with instrumental assessment to capture neuromotor dysfunction more comprehensively,” they said.
The results highlight the clinical relevance of examining neuromotor abnormalities as a routine part of practice prior to starting antipsychotics because of their potential as predictors of long-term outcomes “and to disentangle the primary versus drug-induced character of neuromotor impairment in treated patients,” they concluded.
The study was supported by the Spanish Ministry of Economy, Industry, and Competitiveness, and the Regional Government of Navarra. The researchers had no financial conflicts to disclose.
FROM SCHIZOPHRENIA RESEARCH
New Parkinson’s test developed thanks to woman who could smell the disease
The test has been years in the making after academics realized that Joy Milne could smell the condition.
The 72-year-old from Perth, Scotland, has a rare condition that gives her a heightened sense of smell.
She noticed that her late husband Les developed a different odor when he was 33 – some 12 years before he was diagnosed with the disease, which leads to parts of the brain become progressively damaged over many years.
Mrs. Milne, dubbed ‘the woman who can smell Parkinson’s, described a “musky” aroma, different from his normal scent.
Her observation piqued the interest of scientists who decided to research what she could smell, and whether this could be harnessed to help identify people with the neurological condition.
‘Early phases of research’
Years later, academics at the University of Manchester (England) have made a breakthrough by developing a test that can identify people with Parkinson’s disease using a simple cotton bud run along the back of the neck.
Researchers can examine the sample to identify molecules linked to the disease to help diagnose whether someone has the disease.
While still in the early phases of research, scientists are excited about the prospect of the NHS being able to deploy a simple test for the disease.
There is currently no definitive test for Parkinson’s disease, with diagnosis based on a patient’s symptoms and medical history.
If the new skin swab is successful outside laboratory conditions it could be rolled out to achieve faster diagnosis.
Mrs. Milne told the PA news agency that it was “not acceptable” that people with Parkinson’s had such high degrees of neurologic damage at the time of diagnosis, adding: “I think it has to be detected far earlier – the same as cancer and diabetes, earlier diagnosis means far more efficient treatment and a better lifestyle for people.
“It has been found that exercise and change of diet can make a phenomenal difference.”
She said her husband, a former doctor, was “determined” to find the right researcher to examine the link between odor and Parkinson’s and they sought out Tilo Kunath, PhD, at the University of Edinburgh in 2012.
Chemical change in sebum
Dr. Kunath paired up with Perdita Barran, PhD, to examine Mrs. Milne’s sense of smell.
The scientists believed that the scent may be caused by a chemical change in skin oil, known as sebum, that is triggered by the disease.
In their preliminary work they asked Mrs. Milne to smell t-shirts worn by people who have Parkinson’s and those who did not.
Mrs. Milne correctly identified the t-shirts worn by Parkinson’s patients but she also said that one from the group of people without Parkinson’s smelled like the disease – 8 months later the individual who wore the t-shirt was diagnosed with Parkinson’s.
Researchers hoped the finding could lead to a test being developed to detect Parkinson’s, working under the assumption that if they were able to identify a unique chemical signature in the skin linked to Parkinson’s, they may eventually be able to diagnose the condition from simple skin swabs.
In 2019 researchers at the University of Manchester, led by Dr. Barran, announced that they had identified molecules linked to the disease found in skin swabs.
And now the scientists have developed a test using this information.
The tests have been successfully conducted in research labs and now scientists are assessing whether they can be used in hospital settings.
If successful, the test could potentially be used in the NHS so GPs can refer patients for Parkinson’s tests.
The findings, which have been published in the Journal of the American Chemical Society, detail how sebum can be analyzed with mass spectrometry – a method which weighs molecules – to identify the disease.
Some molecules are present only in people who have Parkinson’s disease.
Researchers compared swabs from 79 people with Parkinson’s with a healthy control group of 71 people.
Dr. Barran told the PA news agency: “At the moment, there are no cures for Parkinson’s, but a confirmatory diagnostic would allow them to get the right treatment and get the drugs that will help to alleviate their symptoms.
“There would also be nonpharmaceutical interventions, including movement and also nutritional classes, which can really help.
“And I think most critically, it will allow them to have a confirmed diagnosis to actually know what’s wrong with them.”
She added: “What we are now doing is seeing if [hospital laboratories] can do what we’ve done in a research lab in a hospital lab. Once that’s happened then we want to see if we can make this a confirmatory diagnostic that could be used along with the referral process from a GP to a consultant. At the moment in Greater Manchester there are about 18,000 people waiting for a neurological consult and just to clear that list, without any new people joining it, will take up to 2 years. Of those 10%-15% are suspect Parkinson’s. Our test would be able to tell them whether they did or whether they didn’t [have Parkinson’s] and allow them to be referred to the right specialist. So at the moment, we’re talking about being able to refer people in a timely manner to the right specialism and that will be transformative.”
Mrs. Milne may be able to smell other diseases
Mrs. Milne is now working with scientists around the world to see if she can smell other diseases like cancer and tuberculosis.
“I have to go shopping very early or very late because of people’s perfumes, I can’t go into the chemical aisle in the supermarket,” she told the PA news agency. “So yes, a curse sometimes but I have also been out to Tanzania and have done research on TB, and research on cancer in the U.S. – just preliminary work. So it is a curse and a benefit.”
She said that she can sometimes smell people who have Parkinson’s while in the supermarket or walking down the street but has been told by medical ethicists she cannot tell them. “Which GP would accept a man or a woman walking in saying ‘the woman who smells Parkinson’s has told me I have it?’ Maybe in the future but not now.”
Mrs. Milne said that her husband, who died 7 years ago, was like a “changed man” after researchers found the link between Parkinson’s and odor.
A version of this article first appeared on Medscape UK.
The test has been years in the making after academics realized that Joy Milne could smell the condition.
The 72-year-old from Perth, Scotland, has a rare condition that gives her a heightened sense of smell.
She noticed that her late husband Les developed a different odor when he was 33 – some 12 years before he was diagnosed with the disease, which leads to parts of the brain become progressively damaged over many years.
Mrs. Milne, dubbed ‘the woman who can smell Parkinson’s, described a “musky” aroma, different from his normal scent.
Her observation piqued the interest of scientists who decided to research what she could smell, and whether this could be harnessed to help identify people with the neurological condition.
‘Early phases of research’
Years later, academics at the University of Manchester (England) have made a breakthrough by developing a test that can identify people with Parkinson’s disease using a simple cotton bud run along the back of the neck.
Researchers can examine the sample to identify molecules linked to the disease to help diagnose whether someone has the disease.
While still in the early phases of research, scientists are excited about the prospect of the NHS being able to deploy a simple test for the disease.
There is currently no definitive test for Parkinson’s disease, with diagnosis based on a patient’s symptoms and medical history.
If the new skin swab is successful outside laboratory conditions it could be rolled out to achieve faster diagnosis.
Mrs. Milne told the PA news agency that it was “not acceptable” that people with Parkinson’s had such high degrees of neurologic damage at the time of diagnosis, adding: “I think it has to be detected far earlier – the same as cancer and diabetes, earlier diagnosis means far more efficient treatment and a better lifestyle for people.
“It has been found that exercise and change of diet can make a phenomenal difference.”
She said her husband, a former doctor, was “determined” to find the right researcher to examine the link between odor and Parkinson’s and they sought out Tilo Kunath, PhD, at the University of Edinburgh in 2012.
Chemical change in sebum
Dr. Kunath paired up with Perdita Barran, PhD, to examine Mrs. Milne’s sense of smell.
The scientists believed that the scent may be caused by a chemical change in skin oil, known as sebum, that is triggered by the disease.
In their preliminary work they asked Mrs. Milne to smell t-shirts worn by people who have Parkinson’s and those who did not.
Mrs. Milne correctly identified the t-shirts worn by Parkinson’s patients but she also said that one from the group of people without Parkinson’s smelled like the disease – 8 months later the individual who wore the t-shirt was diagnosed with Parkinson’s.
Researchers hoped the finding could lead to a test being developed to detect Parkinson’s, working under the assumption that if they were able to identify a unique chemical signature in the skin linked to Parkinson’s, they may eventually be able to diagnose the condition from simple skin swabs.
In 2019 researchers at the University of Manchester, led by Dr. Barran, announced that they had identified molecules linked to the disease found in skin swabs.
And now the scientists have developed a test using this information.
The tests have been successfully conducted in research labs and now scientists are assessing whether they can be used in hospital settings.
If successful, the test could potentially be used in the NHS so GPs can refer patients for Parkinson’s tests.
The findings, which have been published in the Journal of the American Chemical Society, detail how sebum can be analyzed with mass spectrometry – a method which weighs molecules – to identify the disease.
Some molecules are present only in people who have Parkinson’s disease.
Researchers compared swabs from 79 people with Parkinson’s with a healthy control group of 71 people.
Dr. Barran told the PA news agency: “At the moment, there are no cures for Parkinson’s, but a confirmatory diagnostic would allow them to get the right treatment and get the drugs that will help to alleviate their symptoms.
“There would also be nonpharmaceutical interventions, including movement and also nutritional classes, which can really help.
“And I think most critically, it will allow them to have a confirmed diagnosis to actually know what’s wrong with them.”
She added: “What we are now doing is seeing if [hospital laboratories] can do what we’ve done in a research lab in a hospital lab. Once that’s happened then we want to see if we can make this a confirmatory diagnostic that could be used along with the referral process from a GP to a consultant. At the moment in Greater Manchester there are about 18,000 people waiting for a neurological consult and just to clear that list, without any new people joining it, will take up to 2 years. Of those 10%-15% are suspect Parkinson’s. Our test would be able to tell them whether they did or whether they didn’t [have Parkinson’s] and allow them to be referred to the right specialist. So at the moment, we’re talking about being able to refer people in a timely manner to the right specialism and that will be transformative.”
Mrs. Milne may be able to smell other diseases
Mrs. Milne is now working with scientists around the world to see if she can smell other diseases like cancer and tuberculosis.
“I have to go shopping very early or very late because of people’s perfumes, I can’t go into the chemical aisle in the supermarket,” she told the PA news agency. “So yes, a curse sometimes but I have also been out to Tanzania and have done research on TB, and research on cancer in the U.S. – just preliminary work. So it is a curse and a benefit.”
She said that she can sometimes smell people who have Parkinson’s while in the supermarket or walking down the street but has been told by medical ethicists she cannot tell them. “Which GP would accept a man or a woman walking in saying ‘the woman who smells Parkinson’s has told me I have it?’ Maybe in the future but not now.”
Mrs. Milne said that her husband, who died 7 years ago, was like a “changed man” after researchers found the link between Parkinson’s and odor.
A version of this article first appeared on Medscape UK.
The test has been years in the making after academics realized that Joy Milne could smell the condition.
The 72-year-old from Perth, Scotland, has a rare condition that gives her a heightened sense of smell.
She noticed that her late husband Les developed a different odor when he was 33 – some 12 years before he was diagnosed with the disease, which leads to parts of the brain become progressively damaged over many years.
Mrs. Milne, dubbed ‘the woman who can smell Parkinson’s, described a “musky” aroma, different from his normal scent.
Her observation piqued the interest of scientists who decided to research what she could smell, and whether this could be harnessed to help identify people with the neurological condition.
‘Early phases of research’
Years later, academics at the University of Manchester (England) have made a breakthrough by developing a test that can identify people with Parkinson’s disease using a simple cotton bud run along the back of the neck.
Researchers can examine the sample to identify molecules linked to the disease to help diagnose whether someone has the disease.
While still in the early phases of research, scientists are excited about the prospect of the NHS being able to deploy a simple test for the disease.
There is currently no definitive test for Parkinson’s disease, with diagnosis based on a patient’s symptoms and medical history.
If the new skin swab is successful outside laboratory conditions it could be rolled out to achieve faster diagnosis.
Mrs. Milne told the PA news agency that it was “not acceptable” that people with Parkinson’s had such high degrees of neurologic damage at the time of diagnosis, adding: “I think it has to be detected far earlier – the same as cancer and diabetes, earlier diagnosis means far more efficient treatment and a better lifestyle for people.
“It has been found that exercise and change of diet can make a phenomenal difference.”
She said her husband, a former doctor, was “determined” to find the right researcher to examine the link between odor and Parkinson’s and they sought out Tilo Kunath, PhD, at the University of Edinburgh in 2012.
Chemical change in sebum
Dr. Kunath paired up with Perdita Barran, PhD, to examine Mrs. Milne’s sense of smell.
The scientists believed that the scent may be caused by a chemical change in skin oil, known as sebum, that is triggered by the disease.
In their preliminary work they asked Mrs. Milne to smell t-shirts worn by people who have Parkinson’s and those who did not.
Mrs. Milne correctly identified the t-shirts worn by Parkinson’s patients but she also said that one from the group of people without Parkinson’s smelled like the disease – 8 months later the individual who wore the t-shirt was diagnosed with Parkinson’s.
Researchers hoped the finding could lead to a test being developed to detect Parkinson’s, working under the assumption that if they were able to identify a unique chemical signature in the skin linked to Parkinson’s, they may eventually be able to diagnose the condition from simple skin swabs.
In 2019 researchers at the University of Manchester, led by Dr. Barran, announced that they had identified molecules linked to the disease found in skin swabs.
And now the scientists have developed a test using this information.
The tests have been successfully conducted in research labs and now scientists are assessing whether they can be used in hospital settings.
If successful, the test could potentially be used in the NHS so GPs can refer patients for Parkinson’s tests.
The findings, which have been published in the Journal of the American Chemical Society, detail how sebum can be analyzed with mass spectrometry – a method which weighs molecules – to identify the disease.
Some molecules are present only in people who have Parkinson’s disease.
Researchers compared swabs from 79 people with Parkinson’s with a healthy control group of 71 people.
Dr. Barran told the PA news agency: “At the moment, there are no cures for Parkinson’s, but a confirmatory diagnostic would allow them to get the right treatment and get the drugs that will help to alleviate their symptoms.
“There would also be nonpharmaceutical interventions, including movement and also nutritional classes, which can really help.
“And I think most critically, it will allow them to have a confirmed diagnosis to actually know what’s wrong with them.”
She added: “What we are now doing is seeing if [hospital laboratories] can do what we’ve done in a research lab in a hospital lab. Once that’s happened then we want to see if we can make this a confirmatory diagnostic that could be used along with the referral process from a GP to a consultant. At the moment in Greater Manchester there are about 18,000 people waiting for a neurological consult and just to clear that list, without any new people joining it, will take up to 2 years. Of those 10%-15% are suspect Parkinson’s. Our test would be able to tell them whether they did or whether they didn’t [have Parkinson’s] and allow them to be referred to the right specialist. So at the moment, we’re talking about being able to refer people in a timely manner to the right specialism and that will be transformative.”
Mrs. Milne may be able to smell other diseases
Mrs. Milne is now working with scientists around the world to see if she can smell other diseases like cancer and tuberculosis.
“I have to go shopping very early or very late because of people’s perfumes, I can’t go into the chemical aisle in the supermarket,” she told the PA news agency. “So yes, a curse sometimes but I have also been out to Tanzania and have done research on TB, and research on cancer in the U.S. – just preliminary work. So it is a curse and a benefit.”
She said that she can sometimes smell people who have Parkinson’s while in the supermarket or walking down the street but has been told by medical ethicists she cannot tell them. “Which GP would accept a man or a woman walking in saying ‘the woman who smells Parkinson’s has told me I have it?’ Maybe in the future but not now.”
Mrs. Milne said that her husband, who died 7 years ago, was like a “changed man” after researchers found the link between Parkinson’s and odor.
A version of this article first appeared on Medscape UK.
Subtle visual dysfunctions often precede early-stage psychosis
A multinational group of investigators found that VisDys were reported considerably more often by patients with recent-onset psychosis and CHR than by those with recent-onset depression or a group acting as healthy control participants.
In addition, vision problems of higher severity were associated with less functional remission both for patients at CHR and those with recent-onset psychosis. Among patients with CHR, VisDys was also linked to lower quality of life (QOL), higher depressiveness, and more severe impairment of visuospatial constructability.
The researchers used fMRI imaging to compare resting-state functional brain connectivity in participants with recent-onset psychosis, CHR, and recent-onset depression. They found that the occipital (ON) and frontoparietal (FPN) subnetworks were particularly implicated in VisDys.
“Subtle VisDys should be regarded as a frequent phenomenon across the psychosis spectrum, impinging negatively on patients’ current ability to function in several settings of their daily and social life, their QOL, and visuospatial abilities,” write investigators led by Johanna Schwarzer, Institute for Translational Psychiatry, University of Muenster (Germany).
“These large-sample study findings suggest that VisDys are clinically highly relevant not only in [recent-onset psychosis] but especially in CHR,” they stated.
The findings were published online in Neuropsychopharmacology.
Subtle, underrecognized
Unlike patients with nonpsychotic disorders, approximately 50%-60% of patients diagnosed with schizophrenia report VisDys involving brightness, motion, form, color perception, or distorted perception of their own face, the researchers reported.
These “subtle” VisDys are “often underrecognized during clinical examination, despite their clinical relevance related to suicidal ideation, cognitive impairment, or poorer treatment response,” they wrote.
Most research into these vision problems in patients with schizophrenia has focused on patients in which the illness is in a stable, chronic state – although VisDys often appear years before the diagnosis of a psychotic disorder.
Moreover, there has been little research into the neurobiological underpinnings of VisDys, specifically in early states of psychosis and/or in comparison to other disorders, such as depression.
The Personalised Prognostic Indicators for Early Psychosis Management (PRONIA) Consortium studied the psychophysiological phenomenon of VisDys in a large sample of adolescents and young adults. The sample consisted of three diagnostic groups: those with recent-onset psychosis, those with CHR, and those with recent-onset depression.
VisDys in daily life were measured using the Schizophrenia Proneness Instrument–Adult Scale (SPI-A), which assesses basic symptoms that indicate increased risk for psychosis.
Visual information processing
Resting-state imaging data on intrinsic brain networks were also assessed in the PRONIA sample and were analyzed across 12,720 functional connectivities between 160 regions of interest across the whole brain.
In particular, the researchers were interested in the primary networks involved in visual information processing, especially the dorsal visual stream, with further focus on the ON and FPN intrinsic subnetworks.
The ON was chosen because it comprises “primary visual processing pathways,” while the FPN is “widely suggested to modulate attention related to visual information processing at higher cognitive levels.”
The investigators used a machine-learning multivariate pattern analysis approach that “enables the consideration of multiple interactions within brain systems.”
The current study involved 721 participants from the PRONIA database, including 147 participants with recent-onset psychosis (mean age, 28.45 years; 60.5% men), 143 with CHR (mean age, 26.97 years; about 50% men), 151 with recent-onset depression (mean age, 29.13 years; 47% men), and 280 in the healthy-controls group (mean age, 28.54 years; 39.4% men).
The researchers selected 14 items to assess from the SPI-A that represented different aspects of VisDys. Severity was defined by the maximum frequency within the past 3 months – from 1 (never) to 6 (daily).
The 14 items were as follows: oversensitivity to light and/or certain visual perception objects, photopsia, micropsia/macropsia, near and tele-vision, metamorphopsia, changes in color vision, altered perception of a patient’s own face, pseudomovements of optic stimuli, diplopia or oblique vision, disturbances of the estimation of distances or sizes, disturbances of the perception of straight lines/contours, maintenance of optic stimuli “visual echoes,” partial seeing (including tubular vision), and captivation of attention by details of the visual field.
Participants also completed the Beck Depression Inventory–II scale (BDI-II), the Positive and Negative Syndrome Scale (PANSS), the Functional Remission in General Schizophrenia, and several other scales that measure global and social functioning.
Other assessments included QOL and the Rey-Osterrieth Complex Figure Test, which is a neuropsychological measurement of visuospatial constructability.
Specific to early-stage psychosis?
Results showed that VisDys were reported more frequently in both recent-onset psychosis and CHR groups compared with the recent-onset depression and healthy control groups (50.34% and 55.94% vs. 16.56% and 4.28%, respectively).
The investigators noted that VisDys sum scores “showed high internal consistency” (Cronbachs alpha, 0.78 over all participants).
Among those with recent-onset psychosis, a higher VisDys sum score was correlated with lower scores for functional remission (P = .036) and social functioning (P = .014).
In CHR, higher VisDys sum scores were associated with lower scores for health-related functional remission (P = .024), lower physical and psychological QOL (P = .004 and P = .015, respectively), more severe depression on the BDI-II (P = .021), and more impaired visuospatial constructability (P = .027).
Among those with recent-onset depression and their healthy peers, “no relevant correlations were found between VisDys sum scores and any parameters representing functional remission, QOL, depressiveness, or visuospatial constructability,” the researchers wrote.
A total of 135 participants with recent-onset psychosis, 128 with CHR, and 134 with recent-onset depression also underwent resting-state fMRI.
ON functional connectivity predicted presence of VisDys in patients with recent-onset psychosis and those with CHR, with a balanced accuracy of 60.17% (P = .0001) and 67.38% (P = .029), respectively. In the combined recent-onset psychosis plus CHR sample, VisDys were predicted by FPN functional connectivity (balanced accuracy, 61.1%; P = .006).
“Findings from multivariate pattern analysis support a model of functional integrity within ON and FPN driving the VisDys phenomenon and being implicated in core disease mechanisms of early psychosis states,” the investigators noted.
“The main findings from this large sample study support the idea of VisDys being specific to the psychosis spectrum already at early stages,” while being less frequently reported in recent-onset depression, they wrote. VisDys also “appeared negligible” among those without psychiatric disorders.
Regular assessment needed
Steven Silverstein, PhD, professor of biopsychosocial medicine and professor of psychiatry, neuroscience, and ophthalmology, Center for Visual Science, University of Rochester (N.Y.) Medical Center, called the findings “important” because “they will increase appreciation in the field of mental health for the frequency and disabling nature of visual symptoms and the need for regular assessment in routine clinical practice with people at risk for or with psychotic disorders.”
In addition, “the brain imaging findings are providing needed information that could lead to treatments that target the brain networks generating the visual symptoms,” such as neurofeedback or brain stimulation, said Dr. Silverstein, who was not involved with the research.
The study was funded by a grant for the PRONIA Consortium. Individual researchers received funding from NARSAD Young Investigator Award of the Brain and Behavior Research Foundation, the Koeln Fortune Program/Faculty of Medicine, the University of Cologne, and the European Union’s Horizon 2020 research and innovation program. Open Access funding was enabled and organized by Projekt DEAL. Ms. Schwarzer and Dr. Silverstein reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A multinational group of investigators found that VisDys were reported considerably more often by patients with recent-onset psychosis and CHR than by those with recent-onset depression or a group acting as healthy control participants.
In addition, vision problems of higher severity were associated with less functional remission both for patients at CHR and those with recent-onset psychosis. Among patients with CHR, VisDys was also linked to lower quality of life (QOL), higher depressiveness, and more severe impairment of visuospatial constructability.
The researchers used fMRI imaging to compare resting-state functional brain connectivity in participants with recent-onset psychosis, CHR, and recent-onset depression. They found that the occipital (ON) and frontoparietal (FPN) subnetworks were particularly implicated in VisDys.
“Subtle VisDys should be regarded as a frequent phenomenon across the psychosis spectrum, impinging negatively on patients’ current ability to function in several settings of their daily and social life, their QOL, and visuospatial abilities,” write investigators led by Johanna Schwarzer, Institute for Translational Psychiatry, University of Muenster (Germany).
“These large-sample study findings suggest that VisDys are clinically highly relevant not only in [recent-onset psychosis] but especially in CHR,” they stated.
The findings were published online in Neuropsychopharmacology.
Subtle, underrecognized
Unlike patients with nonpsychotic disorders, approximately 50%-60% of patients diagnosed with schizophrenia report VisDys involving brightness, motion, form, color perception, or distorted perception of their own face, the researchers reported.
These “subtle” VisDys are “often underrecognized during clinical examination, despite their clinical relevance related to suicidal ideation, cognitive impairment, or poorer treatment response,” they wrote.
Most research into these vision problems in patients with schizophrenia has focused on patients in which the illness is in a stable, chronic state – although VisDys often appear years before the diagnosis of a psychotic disorder.
Moreover, there has been little research into the neurobiological underpinnings of VisDys, specifically in early states of psychosis and/or in comparison to other disorders, such as depression.
The Personalised Prognostic Indicators for Early Psychosis Management (PRONIA) Consortium studied the psychophysiological phenomenon of VisDys in a large sample of adolescents and young adults. The sample consisted of three diagnostic groups: those with recent-onset psychosis, those with CHR, and those with recent-onset depression.
VisDys in daily life were measured using the Schizophrenia Proneness Instrument–Adult Scale (SPI-A), which assesses basic symptoms that indicate increased risk for psychosis.
Visual information processing
Resting-state imaging data on intrinsic brain networks were also assessed in the PRONIA sample and were analyzed across 12,720 functional connectivities between 160 regions of interest across the whole brain.
In particular, the researchers were interested in the primary networks involved in visual information processing, especially the dorsal visual stream, with further focus on the ON and FPN intrinsic subnetworks.
The ON was chosen because it comprises “primary visual processing pathways,” while the FPN is “widely suggested to modulate attention related to visual information processing at higher cognitive levels.”
The investigators used a machine-learning multivariate pattern analysis approach that “enables the consideration of multiple interactions within brain systems.”
The current study involved 721 participants from the PRONIA database, including 147 participants with recent-onset psychosis (mean age, 28.45 years; 60.5% men), 143 with CHR (mean age, 26.97 years; about 50% men), 151 with recent-onset depression (mean age, 29.13 years; 47% men), and 280 in the healthy-controls group (mean age, 28.54 years; 39.4% men).
The researchers selected 14 items to assess from the SPI-A that represented different aspects of VisDys. Severity was defined by the maximum frequency within the past 3 months – from 1 (never) to 6 (daily).
The 14 items were as follows: oversensitivity to light and/or certain visual perception objects, photopsia, micropsia/macropsia, near and tele-vision, metamorphopsia, changes in color vision, altered perception of a patient’s own face, pseudomovements of optic stimuli, diplopia or oblique vision, disturbances of the estimation of distances or sizes, disturbances of the perception of straight lines/contours, maintenance of optic stimuli “visual echoes,” partial seeing (including tubular vision), and captivation of attention by details of the visual field.
Participants also completed the Beck Depression Inventory–II scale (BDI-II), the Positive and Negative Syndrome Scale (PANSS), the Functional Remission in General Schizophrenia, and several other scales that measure global and social functioning.
Other assessments included QOL and the Rey-Osterrieth Complex Figure Test, which is a neuropsychological measurement of visuospatial constructability.
Specific to early-stage psychosis?
Results showed that VisDys were reported more frequently in both recent-onset psychosis and CHR groups compared with the recent-onset depression and healthy control groups (50.34% and 55.94% vs. 16.56% and 4.28%, respectively).
The investigators noted that VisDys sum scores “showed high internal consistency” (Cronbachs alpha, 0.78 over all participants).
Among those with recent-onset psychosis, a higher VisDys sum score was correlated with lower scores for functional remission (P = .036) and social functioning (P = .014).
In CHR, higher VisDys sum scores were associated with lower scores for health-related functional remission (P = .024), lower physical and psychological QOL (P = .004 and P = .015, respectively), more severe depression on the BDI-II (P = .021), and more impaired visuospatial constructability (P = .027).
Among those with recent-onset depression and their healthy peers, “no relevant correlations were found between VisDys sum scores and any parameters representing functional remission, QOL, depressiveness, or visuospatial constructability,” the researchers wrote.
A total of 135 participants with recent-onset psychosis, 128 with CHR, and 134 with recent-onset depression also underwent resting-state fMRI.
ON functional connectivity predicted presence of VisDys in patients with recent-onset psychosis and those with CHR, with a balanced accuracy of 60.17% (P = .0001) and 67.38% (P = .029), respectively. In the combined recent-onset psychosis plus CHR sample, VisDys were predicted by FPN functional connectivity (balanced accuracy, 61.1%; P = .006).
“Findings from multivariate pattern analysis support a model of functional integrity within ON and FPN driving the VisDys phenomenon and being implicated in core disease mechanisms of early psychosis states,” the investigators noted.
“The main findings from this large sample study support the idea of VisDys being specific to the psychosis spectrum already at early stages,” while being less frequently reported in recent-onset depression, they wrote. VisDys also “appeared negligible” among those without psychiatric disorders.
Regular assessment needed
Steven Silverstein, PhD, professor of biopsychosocial medicine and professor of psychiatry, neuroscience, and ophthalmology, Center for Visual Science, University of Rochester (N.Y.) Medical Center, called the findings “important” because “they will increase appreciation in the field of mental health for the frequency and disabling nature of visual symptoms and the need for regular assessment in routine clinical practice with people at risk for or with psychotic disorders.”
In addition, “the brain imaging findings are providing needed information that could lead to treatments that target the brain networks generating the visual symptoms,” such as neurofeedback or brain stimulation, said Dr. Silverstein, who was not involved with the research.
The study was funded by a grant for the PRONIA Consortium. Individual researchers received funding from NARSAD Young Investigator Award of the Brain and Behavior Research Foundation, the Koeln Fortune Program/Faculty of Medicine, the University of Cologne, and the European Union’s Horizon 2020 research and innovation program. Open Access funding was enabled and organized by Projekt DEAL. Ms. Schwarzer and Dr. Silverstein reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A multinational group of investigators found that VisDys were reported considerably more often by patients with recent-onset psychosis and CHR than by those with recent-onset depression or a group acting as healthy control participants.
In addition, vision problems of higher severity were associated with less functional remission both for patients at CHR and those with recent-onset psychosis. Among patients with CHR, VisDys was also linked to lower quality of life (QOL), higher depressiveness, and more severe impairment of visuospatial constructability.
The researchers used fMRI imaging to compare resting-state functional brain connectivity in participants with recent-onset psychosis, CHR, and recent-onset depression. They found that the occipital (ON) and frontoparietal (FPN) subnetworks were particularly implicated in VisDys.
“Subtle VisDys should be regarded as a frequent phenomenon across the psychosis spectrum, impinging negatively on patients’ current ability to function in several settings of their daily and social life, their QOL, and visuospatial abilities,” write investigators led by Johanna Schwarzer, Institute for Translational Psychiatry, University of Muenster (Germany).
“These large-sample study findings suggest that VisDys are clinically highly relevant not only in [recent-onset psychosis] but especially in CHR,” they stated.
The findings were published online in Neuropsychopharmacology.
Subtle, underrecognized
Unlike patients with nonpsychotic disorders, approximately 50%-60% of patients diagnosed with schizophrenia report VisDys involving brightness, motion, form, color perception, or distorted perception of their own face, the researchers reported.
These “subtle” VisDys are “often underrecognized during clinical examination, despite their clinical relevance related to suicidal ideation, cognitive impairment, or poorer treatment response,” they wrote.
Most research into these vision problems in patients with schizophrenia has focused on patients in which the illness is in a stable, chronic state – although VisDys often appear years before the diagnosis of a psychotic disorder.
Moreover, there has been little research into the neurobiological underpinnings of VisDys, specifically in early states of psychosis and/or in comparison to other disorders, such as depression.
The Personalised Prognostic Indicators for Early Psychosis Management (PRONIA) Consortium studied the psychophysiological phenomenon of VisDys in a large sample of adolescents and young adults. The sample consisted of three diagnostic groups: those with recent-onset psychosis, those with CHR, and those with recent-onset depression.
VisDys in daily life were measured using the Schizophrenia Proneness Instrument–Adult Scale (SPI-A), which assesses basic symptoms that indicate increased risk for psychosis.
Visual information processing
Resting-state imaging data on intrinsic brain networks were also assessed in the PRONIA sample and were analyzed across 12,720 functional connectivities between 160 regions of interest across the whole brain.
In particular, the researchers were interested in the primary networks involved in visual information processing, especially the dorsal visual stream, with further focus on the ON and FPN intrinsic subnetworks.
The ON was chosen because it comprises “primary visual processing pathways,” while the FPN is “widely suggested to modulate attention related to visual information processing at higher cognitive levels.”
The investigators used a machine-learning multivariate pattern analysis approach that “enables the consideration of multiple interactions within brain systems.”
The current study involved 721 participants from the PRONIA database, including 147 participants with recent-onset psychosis (mean age, 28.45 years; 60.5% men), 143 with CHR (mean age, 26.97 years; about 50% men), 151 with recent-onset depression (mean age, 29.13 years; 47% men), and 280 in the healthy-controls group (mean age, 28.54 years; 39.4% men).
The researchers selected 14 items to assess from the SPI-A that represented different aspects of VisDys. Severity was defined by the maximum frequency within the past 3 months – from 1 (never) to 6 (daily).
The 14 items were as follows: oversensitivity to light and/or certain visual perception objects, photopsia, micropsia/macropsia, near and tele-vision, metamorphopsia, changes in color vision, altered perception of a patient’s own face, pseudomovements of optic stimuli, diplopia or oblique vision, disturbances of the estimation of distances or sizes, disturbances of the perception of straight lines/contours, maintenance of optic stimuli “visual echoes,” partial seeing (including tubular vision), and captivation of attention by details of the visual field.
Participants also completed the Beck Depression Inventory–II scale (BDI-II), the Positive and Negative Syndrome Scale (PANSS), the Functional Remission in General Schizophrenia, and several other scales that measure global and social functioning.
Other assessments included QOL and the Rey-Osterrieth Complex Figure Test, which is a neuropsychological measurement of visuospatial constructability.
Specific to early-stage psychosis?
Results showed that VisDys were reported more frequently in both recent-onset psychosis and CHR groups compared with the recent-onset depression and healthy control groups (50.34% and 55.94% vs. 16.56% and 4.28%, respectively).
The investigators noted that VisDys sum scores “showed high internal consistency” (Cronbachs alpha, 0.78 over all participants).
Among those with recent-onset psychosis, a higher VisDys sum score was correlated with lower scores for functional remission (P = .036) and social functioning (P = .014).
In CHR, higher VisDys sum scores were associated with lower scores for health-related functional remission (P = .024), lower physical and psychological QOL (P = .004 and P = .015, respectively), more severe depression on the BDI-II (P = .021), and more impaired visuospatial constructability (P = .027).
Among those with recent-onset depression and their healthy peers, “no relevant correlations were found between VisDys sum scores and any parameters representing functional remission, QOL, depressiveness, or visuospatial constructability,” the researchers wrote.
A total of 135 participants with recent-onset psychosis, 128 with CHR, and 134 with recent-onset depression also underwent resting-state fMRI.
ON functional connectivity predicted presence of VisDys in patients with recent-onset psychosis and those with CHR, with a balanced accuracy of 60.17% (P = .0001) and 67.38% (P = .029), respectively. In the combined recent-onset psychosis plus CHR sample, VisDys were predicted by FPN functional connectivity (balanced accuracy, 61.1%; P = .006).
“Findings from multivariate pattern analysis support a model of functional integrity within ON and FPN driving the VisDys phenomenon and being implicated in core disease mechanisms of early psychosis states,” the investigators noted.
“The main findings from this large sample study support the idea of VisDys being specific to the psychosis spectrum already at early stages,” while being less frequently reported in recent-onset depression, they wrote. VisDys also “appeared negligible” among those without psychiatric disorders.
Regular assessment needed
Steven Silverstein, PhD, professor of biopsychosocial medicine and professor of psychiatry, neuroscience, and ophthalmology, Center for Visual Science, University of Rochester (N.Y.) Medical Center, called the findings “important” because “they will increase appreciation in the field of mental health for the frequency and disabling nature of visual symptoms and the need for regular assessment in routine clinical practice with people at risk for or with psychotic disorders.”
In addition, “the brain imaging findings are providing needed information that could lead to treatments that target the brain networks generating the visual symptoms,” such as neurofeedback or brain stimulation, said Dr. Silverstein, who was not involved with the research.
The study was funded by a grant for the PRONIA Consortium. Individual researchers received funding from NARSAD Young Investigator Award of the Brain and Behavior Research Foundation, the Koeln Fortune Program/Faculty of Medicine, the University of Cologne, and the European Union’s Horizon 2020 research and innovation program. Open Access funding was enabled and organized by Projekt DEAL. Ms. Schwarzer and Dr. Silverstein reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROPSYCHOPHARMACOLOGY
Blood type linked to higher risk for early onset stroke
Conversely, results from a meta-analysis of nearly 17,000 cases of ischemic stroke in adults younger than 60 years showed that having type O blood reduced the risk for EOS by 12%.
In addition, the associations with risk were significantly stronger in EOS than in those with late-onset stroke (LOS), pointing to a stronger role for prothrombotic factors in younger patients, the researchers noted.
“What this is telling us is that maybe what makes you susceptible to stroke as a young adult is the blood type, which is really giving you a much higher risk of clotting and stroke compared to later onset,” coinvestigator Braxton Mitchell, PhD, professor of medicine and epidemiology and public health at the University of Maryland, Baltimore, said in an interview.
The findings were published online in Neurology.
Strong association
The genome-wide association study (GWAS) was done as part of the Genetics of Early Onset Ischemic Stroke Consortium, a collaboration of 48 different studies across North America, Europe, Japan, Pakistan, and Australia. It assessed early onset ischemic stroke in patients aged 18-59 years.
Researchers included data from 16,927 patients with stroke. Of these, 5,825 had a stroke before age 60, defined as early onset. GWAS results were also examined for nearly 600,000 individuals without stroke.
Results showed two genetic variants tied to blood types A and O emerged as highly associated with risk for early stroke.
Researchers found that the protective effects of type O were significantly stronger with EOS vs. LOS (odds ratio [OR], 0.88 vs. 0.96, respectively; P = .001). Likewise, the association between type A and increased EOS risk was significantly stronger than that found in LOS (OR, 1.16 vs. 1.05; P = .005).
Using polygenic risk scores, the investigators also found that the greater genetic risk for venous thromboembolism, another prothrombotic condition, was more strongly associated with EOS compared with LOS (P = .008).
Previous studies have shown a link between stroke risk and variants of the ABO gene, which determines blood type. The new analysis suggests that type A and O gene variants represent nearly all of those genetically linked with early stroke, the researchers noted.
While the findings point to blood type as a risk factor for stroke in younger people, Dr. Mitchell cautions that “at the moment, blood group does not have implications for preventive care.”
“The risk of stroke due to blood type is smaller than other risk factors that we know about, like smoking and hypertension,” he said. “I would be much more worried about these other risk factors, especially because those may be modifiable.”
He noted the next step in the study is to assess how blood type interacts with other known risk factors to raise stroke risk.
“There may be a subset of people where, if you have blood type A and you have some of these other risk factors, it’s possible that you may be at particularly high risk,” Dr. Mitchell said.
More research needed on younger patients
In an accompanying editorial, Jennifer Juhl Majersik, MD, associate professor of neurology at the University of Utah, Salt Lake City, and Paul Lacaze, PhD, associate professor and head of the public health genomics program at Monash University, Australia, noted that the study fills a gap in stroke research, which often focuses mostly on older individuals.
“In approximately 40% of people with EOS, the stroke is cryptogenic, and there is scant data from clinical trials to guide the selection of preventative strategies in this population, as people with EOS are often excluded from trials,” Dr. Majersik and Dr. Lacaze wrote.
“This work has deepened our understanding of EOS pathophysiology,” they added.
The editorialists noted that future research can build on the results from this analysis, “with the goal of a more precise understanding of stroke pathophysiology, leading to targeted preventative treatments for EOS and a reduction in disability in patients’ most productive years.”
Dr. Mitchell echoed the call for greater inclusion of young patients with stroke in clinical trials.
“As we’re learning, stroke in older folks isn’t the same as stroke in younger people,” he said. “There are many shared risk factors but there are also some that are different ... so there really is a need to include younger people.”
A version of this article first appeared on Medscape.com.
Conversely, results from a meta-analysis of nearly 17,000 cases of ischemic stroke in adults younger than 60 years showed that having type O blood reduced the risk for EOS by 12%.
In addition, the associations with risk were significantly stronger in EOS than in those with late-onset stroke (LOS), pointing to a stronger role for prothrombotic factors in younger patients, the researchers noted.
“What this is telling us is that maybe what makes you susceptible to stroke as a young adult is the blood type, which is really giving you a much higher risk of clotting and stroke compared to later onset,” coinvestigator Braxton Mitchell, PhD, professor of medicine and epidemiology and public health at the University of Maryland, Baltimore, said in an interview.
The findings were published online in Neurology.
Strong association
The genome-wide association study (GWAS) was done as part of the Genetics of Early Onset Ischemic Stroke Consortium, a collaboration of 48 different studies across North America, Europe, Japan, Pakistan, and Australia. It assessed early onset ischemic stroke in patients aged 18-59 years.
Researchers included data from 16,927 patients with stroke. Of these, 5,825 had a stroke before age 60, defined as early onset. GWAS results were also examined for nearly 600,000 individuals without stroke.
Results showed two genetic variants tied to blood types A and O emerged as highly associated with risk for early stroke.
Researchers found that the protective effects of type O were significantly stronger with EOS vs. LOS (odds ratio [OR], 0.88 vs. 0.96, respectively; P = .001). Likewise, the association between type A and increased EOS risk was significantly stronger than that found in LOS (OR, 1.16 vs. 1.05; P = .005).
Using polygenic risk scores, the investigators also found that the greater genetic risk for venous thromboembolism, another prothrombotic condition, was more strongly associated with EOS compared with LOS (P = .008).
Previous studies have shown a link between stroke risk and variants of the ABO gene, which determines blood type. The new analysis suggests that type A and O gene variants represent nearly all of those genetically linked with early stroke, the researchers noted.
While the findings point to blood type as a risk factor for stroke in younger people, Dr. Mitchell cautions that “at the moment, blood group does not have implications for preventive care.”
“The risk of stroke due to blood type is smaller than other risk factors that we know about, like smoking and hypertension,” he said. “I would be much more worried about these other risk factors, especially because those may be modifiable.”
He noted the next step in the study is to assess how blood type interacts with other known risk factors to raise stroke risk.
“There may be a subset of people where, if you have blood type A and you have some of these other risk factors, it’s possible that you may be at particularly high risk,” Dr. Mitchell said.
More research needed on younger patients
In an accompanying editorial, Jennifer Juhl Majersik, MD, associate professor of neurology at the University of Utah, Salt Lake City, and Paul Lacaze, PhD, associate professor and head of the public health genomics program at Monash University, Australia, noted that the study fills a gap in stroke research, which often focuses mostly on older individuals.
“In approximately 40% of people with EOS, the stroke is cryptogenic, and there is scant data from clinical trials to guide the selection of preventative strategies in this population, as people with EOS are often excluded from trials,” Dr. Majersik and Dr. Lacaze wrote.
“This work has deepened our understanding of EOS pathophysiology,” they added.
The editorialists noted that future research can build on the results from this analysis, “with the goal of a more precise understanding of stroke pathophysiology, leading to targeted preventative treatments for EOS and a reduction in disability in patients’ most productive years.”
Dr. Mitchell echoed the call for greater inclusion of young patients with stroke in clinical trials.
“As we’re learning, stroke in older folks isn’t the same as stroke in younger people,” he said. “There are many shared risk factors but there are also some that are different ... so there really is a need to include younger people.”
A version of this article first appeared on Medscape.com.
Conversely, results from a meta-analysis of nearly 17,000 cases of ischemic stroke in adults younger than 60 years showed that having type O blood reduced the risk for EOS by 12%.
In addition, the associations with risk were significantly stronger in EOS than in those with late-onset stroke (LOS), pointing to a stronger role for prothrombotic factors in younger patients, the researchers noted.
“What this is telling us is that maybe what makes you susceptible to stroke as a young adult is the blood type, which is really giving you a much higher risk of clotting and stroke compared to later onset,” coinvestigator Braxton Mitchell, PhD, professor of medicine and epidemiology and public health at the University of Maryland, Baltimore, said in an interview.
The findings were published online in Neurology.
Strong association
The genome-wide association study (GWAS) was done as part of the Genetics of Early Onset Ischemic Stroke Consortium, a collaboration of 48 different studies across North America, Europe, Japan, Pakistan, and Australia. It assessed early onset ischemic stroke in patients aged 18-59 years.
Researchers included data from 16,927 patients with stroke. Of these, 5,825 had a stroke before age 60, defined as early onset. GWAS results were also examined for nearly 600,000 individuals without stroke.
Results showed two genetic variants tied to blood types A and O emerged as highly associated with risk for early stroke.
Researchers found that the protective effects of type O were significantly stronger with EOS vs. LOS (odds ratio [OR], 0.88 vs. 0.96, respectively; P = .001). Likewise, the association between type A and increased EOS risk was significantly stronger than that found in LOS (OR, 1.16 vs. 1.05; P = .005).
Using polygenic risk scores, the investigators also found that the greater genetic risk for venous thromboembolism, another prothrombotic condition, was more strongly associated with EOS compared with LOS (P = .008).
Previous studies have shown a link between stroke risk and variants of the ABO gene, which determines blood type. The new analysis suggests that type A and O gene variants represent nearly all of those genetically linked with early stroke, the researchers noted.
While the findings point to blood type as a risk factor for stroke in younger people, Dr. Mitchell cautions that “at the moment, blood group does not have implications for preventive care.”
“The risk of stroke due to blood type is smaller than other risk factors that we know about, like smoking and hypertension,” he said. “I would be much more worried about these other risk factors, especially because those may be modifiable.”
He noted the next step in the study is to assess how blood type interacts with other known risk factors to raise stroke risk.
“There may be a subset of people where, if you have blood type A and you have some of these other risk factors, it’s possible that you may be at particularly high risk,” Dr. Mitchell said.
More research needed on younger patients
In an accompanying editorial, Jennifer Juhl Majersik, MD, associate professor of neurology at the University of Utah, Salt Lake City, and Paul Lacaze, PhD, associate professor and head of the public health genomics program at Monash University, Australia, noted that the study fills a gap in stroke research, which often focuses mostly on older individuals.
“In approximately 40% of people with EOS, the stroke is cryptogenic, and there is scant data from clinical trials to guide the selection of preventative strategies in this population, as people with EOS are often excluded from trials,” Dr. Majersik and Dr. Lacaze wrote.
“This work has deepened our understanding of EOS pathophysiology,” they added.
The editorialists noted that future research can build on the results from this analysis, “with the goal of a more precise understanding of stroke pathophysiology, leading to targeted preventative treatments for EOS and a reduction in disability in patients’ most productive years.”
Dr. Mitchell echoed the call for greater inclusion of young patients with stroke in clinical trials.
“As we’re learning, stroke in older folks isn’t the same as stroke in younger people,” he said. “There are many shared risk factors but there are also some that are different ... so there really is a need to include younger people.”
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Neuropsychiatric symptoms after stroke
Many patients experience neuropsychiatric symptoms following stroke. There is tremendous variation in the type, severity, and timeline of these symptoms, which have the potential to significantly impact patients’ quality of life. Some symptoms occur as a direct result of ischemic injury to brain structures regulating behavior, executive function, perception, or affect. Other symptoms occur indirectly due to the patient’s often-difficult experiences with the health care system, disrupted routines, or altered poststroke functional abilities. Psychiatric symptoms are not as easily recognized as classic stroke symptoms (such as hemiparesis) and are frequently overlooked, especially in the acute phase. However, these symptoms can negatively influence patients’ interpersonal relationships, rehabilitation, and employment.
Patients and families may not realize certain symptoms are stroke-related and may not discuss them with their clinicians. It is important to ask about and recognize psychiatric symptoms in patients who have experienced a stroke so you can provide optimal education and treatment. In this article, we review the types of psychiatric symptoms associated with strokes in specific brain regions (Table1-10). We also describe symptoms that do not appear directly related to the anatomical structures affected by the infarct, including delirium, psychosis, depression, anxiety, and posttraumatic stress.
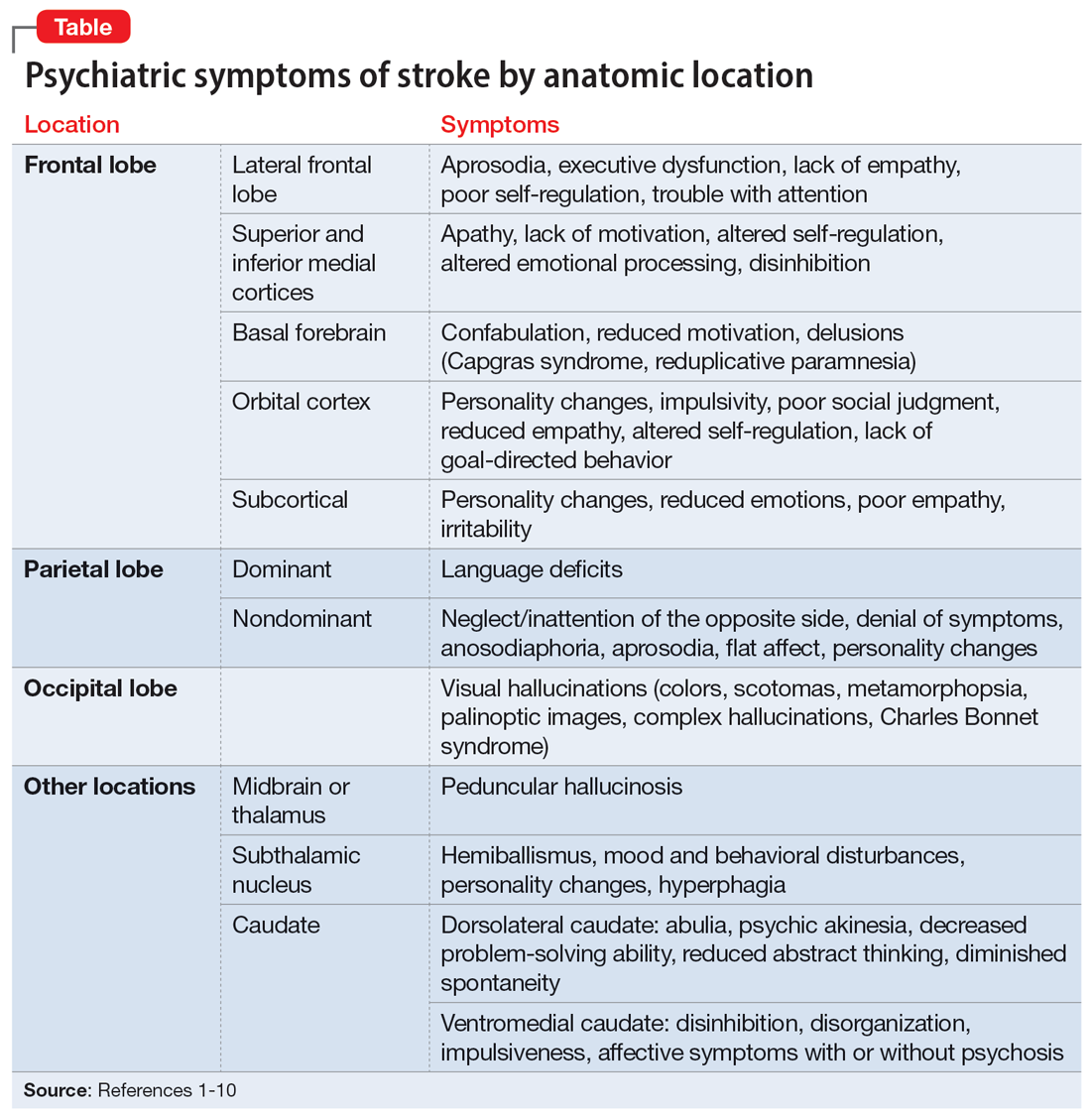
Symptoms associated with stroke in specific regions
Frontal lobe strokes
The frontal lobes are the largest lobes in the brain, and damage to areas within these lobes can cause behavioral and personality changes. Lesions in the lateral frontal cortex can cause aprosodia (difficulty expressing or comprehending variations in tone of voice), which can lead to communication errors. Lateral frontal cortex injury can cause executive dysfunction and a lack of empathy1 as well as trouble with attention, planning, and self-regulation that may affect daily functioning. Strokes affecting the superior and inferior mesial cortices may result in apathy, lack of motivation, altered self-regulation, altered emotional processing, and disinhibition. Patients who experience a basal forebrain stroke may exhibit confabulation, reduced motivation, and delusions such as Capgras syndrome (the belief that a person or place has been replaced by an exact copy) and reduplicative paramnesia (the belief that a place has been either moved, duplicated, or exists in 2 places simultaneously). Strokes involving the orbital cortex can be associated with personality changes, impulsivity, poor social judgment, reduced empathy, altered self-regulation, lack of goal-directed behavior, and environmental dependency.
Some strokes may occur primarily in the subcortical white matter within the frontal lobes. Symptoms may be due to a single stroke with sudden onset, or due to repeated ischemic events that accumulate over time, as seen with microvascular disease. In the case of microvascular disease, the onset of symptoms may be insidious and the course progressive. Infarcts in the subcortical area can also cause personality changes (though typically more subtle when compared to orbitofrontal strokes), reduced emotions, poor empathy, and irritability.1 Patients may lack insight into some of or all these symptoms following a frontal lobe infarct, which makes it critical to gather collateral information from the patient’s friends or family.
Parietal lobe strokes
Symptomatology from parietal strokes depends on whether the stroke affects the dominant or nondominant hemisphere. Dominant parietal lesions cause language deficits, and psychiatric symptoms may be difficult to elucidate due to the patient’s inability to communicate.2 On the other hand, patients with nondominant parietal stroke may have neglect of, or inattention to, the opposite (typically left) side.3 This often manifests as a reluctance to use the affected limb or limbs, in some cases despite a lack of true weakness or motor dysfunction. In addition, patients may also have visual and/or tactile inattention towards the affected side, despite a lack of gross visual or sensory impairment.2 In rare cases, a patient’s stroke may be misdiagnosed as a functional disorder due to the perceived unwillingness to use a neurologically intact limb. In severe cases, patients may not recognize an affected extremity as their own. Patients are also frequently unaware of deficits affecting their nondominant side and may argue with those attempting to explain their deficit. Anosodiaphoria—an abnormal lack of concern regarding their deficits—may also be observed. Additionally, aprosodia, flat affect, and personality changes may result from strokes affecting the nondominant hemisphere, which can impact the patient’s relationships and social functioning.3
Occipital lobe strokes
While negative or loss-of-function symptomatology is one of the hallmarks of stroke, occipital lobe infarcts can pose an exception. Although vision loss is the most common symptom with occipital lobe strokes, some patients experience visual hallucinations that may occur acutely or subacutely. In the acute phase, patients may report hallucinations of varied description,4 including poorly formed areas of color, scotomas, metamorphopsia (visual distortion in which straight lines appear curved), more complex and formed hallucinations and/or palinoptic images (images or brief scenes that continue to be perceived after looking away). These hallucinations, often referred to as release phenomena or release hallucinations, are thought to result from disinhibition of the visual cortex, which then fires spontaneously.
Hallucinations are associated with either infarction or hemorrhage in the posterior cerebral artery territory. In some cases, the hallucinations may take on a formed, complex appearance, and Charles Bonnet syndrome (visual hallucinations in the setting of vision loss, with insight into the hallucinations) has been identified in a small portion of patients.5
Continue to: The duration of these...
The duration of these hallucinations varies. Some patients describe very short periods of the disturbance, lasting minutes to hours and corresponding with the onset of their stroke. Others experience prolonged hallucinations, which frequently evolve into formed, complex images, lasting from days to months.6 In the setting of cortical stroke, patients may be at risk for seizures, which could manifest as visual hallucinations. It is essential to ensure that epileptic causes of hallucinations have been ruled out, because seizures may require treatment and other precautions.
Other stroke locations
Strokes in other locations also can result in psychiatric or behavioral symptoms. Acute stroke in the subcortical midbrain or thalamus may result in peduncular hallucinosis, a syndrome of vivid visual hallucinations.7 The midbrain (most commonly the reticular formation) is usually affected; however, certain lesions of the thalamus may also cause peduncular hallucinosis. This phenomenon is theorized to be due to an increase in serotonin activity relative to acetylcholine and is often accompanied by drowsiness.
The subthalamic nucleus is most frequently associated with disordered movement such as hemiballismus, but also causes disturbances in mood and behavior, including hyperphagia and personality changes.8 Irritability, aggressiveness, disinhibition, anxiety, and obscene speech may also be seen with lesions of the subthalamic nucleus.
Finally, the caudate nucleus may cause alterations in executive functioning and behavior.9 A stroke in the dorsolateral caudate may cause abulia and psychic akinesia, decreased problem-solving ability, reduced abstract thinking, and/or diminished spontaneity, whereas an infarct in the ventromedial region of the nucleus may cause disinhibition, disorganization, impulsiveness, and, in severe cases, affective symptoms with psychosis.10 Strokes in any of these areas are at risk for being misdiagnosed because patients may not have a hemiparesis, and isolated positive or psychiatric symptoms may not be recognized as stroke.
Symptoms not related to stroke location
Delirium and psychosis
Following a stroke, a patient may exhibit neuropsychiatric symptoms that do not appear to relate directly to the anatomical structures affected by the infarct. In the acute phase, factors such as older age and medical complications (including infection, metabolic derangement, and lack of sleep due to frequent neurologic checks) create a high risk of delirium.11 Differentiating delirium from alterations in mental status due to seizure, cerebral edema, or other medical complications is essential, and delirium precautions should be exercised to the greatest extent possible. Other neuropsychiatric symptoms may manifest following hospitalization.
Continue to: Poststroke psychosis...
Poststroke psychosis often presents subacutely. Among these patients, the most common psychosis is delusion disorder, followed by schizophrenia-like psychosis and mood disorder with psychotic features.12 Some evidence suggests antipsychotics may be highly effective for many of these patients.12 Poststroke psychosis does appear to correlate somewhat with nondominant hemisphere lesions, including the frontal lobe, parietal lobe, temporal lobe, and/or caudate nucleus. Because high mortality and poor functional outcomes have been associated with poststroke psychosis, early intervention is essential.
Depression
Depression is a common problem following stroke, affecting approximately 35% of stroke patients.13 In addition to impairing quality of life, depression negatively impacts rehabilitation and increases caregiver burden. There is significant variability regarding risk factors that increases the likelihood of poststroke depression; however, psychiatric history, dysphagia, and poor social support consistently correlate with a higher risk.14,15 Characteristics of a patient’s stroke, such as lesion volume and the ability to perform activities of daily living, are also risk factors. Identifying depression among patients who recently had a stroke is sometimes difficult due to a plethora of confounding factors. Patients may not communicate well due to aphasia, while strokes in other locations may result in an altered affect. Depending on the stroke location, patients may also suffer anosognosia (a lack of awareness of their deficits), which may impair their ability to learn and use adaptive strategies and equipment. An additional confounder is the significant overlap between depressive symptoms and those seen in the setting of a major medical event or hospitalization (decreased appetite, fatigue, etc). The prevalence of depression peaks approximately 3 to 6 months after stroke, with symptoms lasting 9 to 12 months on average, although many patients experience symptoms significantly longer.14 Because symptoms can begin within hours to days following a stroke, it is essential that both hospital and outpatient clinicians assess for depression when indicated. Patients with poststroke depression should receive prompt treatment because appropriate treatment correlates with improved rehabilitation, and most patients respond well to antidepressants.16 Early treatment reduces mortality and improves compliance with secondary stroke prevention measures, including pharmacotherapy.17
Anxiety and posttraumatic stress
Anxiety and anxiety-related disorders are additional potential complications following stroke that significantly influence patient outcomes and well-being. The abrupt, unexpected onset of stroke is often frightening to patients and families. The potential for life-altering deficits as well as intense, often invasive, interactions with the health care system does little to assuage patients’ fear. Stroke patients must contend with a change in neurologic function while processing their difficult experiences, and may develop profound fear of a recurrent stroke. As many as 22% of patients have an anxiety disorder 3 months after they have a stroke.18 Phobic disorder is the most prevalent subtype, followed by generalized anxiety disorder. Younger age and previous anxiety or depression place patients at greater risk of developing poststroke anxiety. Patients suffering from poststroke anxiety have a reduced quality of life, are more dependent, and show restricted participation in rehabilitation, all of which culminate in poorer outcomes.
Many patients describe their experiences surrounding their stroke as traumatic, and posttraumatic stress disorder (PTSD) is increasingly acknowledged as a potential complication for patients with recent stroke.19 PTSD profoundly impacts patient quality of life. Interestingly, most patients who develop poststroke PTSD do not have a history of other psychiatric illness, and it is difficult to predict who may develop PTSD. Relatively little is known regarding optimal treatment strategies for poststroke PTSD, or the efficacy of pharmacotherapy and psychotherapeutic strategies to treat it.
Goals: Improve recovery and quality of life
Neuropsychiatric symptoms are common following a stroke and may manifest in a variety of ways. While some symptoms are a direct consequence of injury to a specific brain region, other symptoms may be a response to loss of independence, disability, experience with the medical system, or fear of recurrent stroke. The onset of psychiatric symptoms can be acute, beginning during hospitalization, or delayed. Understanding the association of psychiatric symptoms with the anatomical location of stroke may assist clinicians in identifying such symptoms. This knowledge informs conversations with patients and their caregivers, who may benefit from understanding that such symptoms are common after stroke. Furthermore, identifying psychiatric complications following stroke may affect rehabilitation. Additional investigation is necessary to find more effective treatment modalities and improve early intervention.
Continue to: Bottom Line
Bottom Line
Neuropsychiatric symptoms are frequently overlooked in patients with recent stroke. These symptoms include delirium, psychosis, depression, anxiety, and posttraumatic stress disorder, and can be the direct result of injury to neuroanatomical structures or a consequence of the patient’s experience. Prompt treatment can maximize stroke recovery and quality of life.
Related Resources
- Zhang S, Xu M, Liu ZJ, et al. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. 2020;10(6):125-138. doi:10.5498/wjp. v10.i6.125
- Saha G, Chakraborty K, Pattojoshi A. Management of psychiatric disorders in patients with stroke and traumatic brain injury. Indian J Psychiatry. 2022;64(Suppl 2): S344-S354.
1. Eslinger PJ, Reichwein RK. Frontal lobe stroke syndromes. In: Caplan LR, van Gijn J, eds. Stroke Syndromes. 3rd ed. Cambridge University Press; 2012:232-241.
2. Critchley M, Russell WR, Zangwill OL. Discussion on parietal lobe syndromes. Proc R Soc Med. 1951;44(4):337-346.
3. Hier DB, Mondlock J, Caplan LR. Behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33(3):337-344.
4. Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977;2(5):432-436.
5. Kumral E, Uluakay A, Donmez A. Complex visual hallucinations following stroke: epileptic origin or a deafferentiation phenomenon? Austin J Cerebrovasc Dis & Stroke. 2014;1(1):1005.
6. Lee JS, Ko KH, Oh JH, et al. Charles Bonnet syndrome after occipital infarction. J Neurosonol Neuroimag. 2018;10(2):154-157.
7. Young JB. Peduncular hallucinosis. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Elsevier; 2014:848.
8. Etemadifar M, Abtahi SH, Abtahi SM, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. 2012;2012:768580. doi:10.1155/2012/768580
9. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108.
10. Wang PY. Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei). 1991;47(3):199-203.
11. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-33.
12. Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89(8):879-885.
13. Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris). 2008;164(10):837-840.
14. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253-264.
15. Pritchard KT, Hreha KP, Hong I. Dysphagia associated with risk of depressive symptoms among stroke survivors after discharge from a cluster of inpatient rehabilitation facilities. Swallowing Rehabil. 2020;3(1):33-44.
16. Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829-1832.
17. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
18. Chun HY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556-564.
19. Garton AL, Sisti JA, Gupta VP, et al. Poststroke post-traumatic stress disorder: a review. Stroke. 2017;48(2):507-512.
Many patients experience neuropsychiatric symptoms following stroke. There is tremendous variation in the type, severity, and timeline of these symptoms, which have the potential to significantly impact patients’ quality of life. Some symptoms occur as a direct result of ischemic injury to brain structures regulating behavior, executive function, perception, or affect. Other symptoms occur indirectly due to the patient’s often-difficult experiences with the health care system, disrupted routines, or altered poststroke functional abilities. Psychiatric symptoms are not as easily recognized as classic stroke symptoms (such as hemiparesis) and are frequently overlooked, especially in the acute phase. However, these symptoms can negatively influence patients’ interpersonal relationships, rehabilitation, and employment.
Patients and families may not realize certain symptoms are stroke-related and may not discuss them with their clinicians. It is important to ask about and recognize psychiatric symptoms in patients who have experienced a stroke so you can provide optimal education and treatment. In this article, we review the types of psychiatric symptoms associated with strokes in specific brain regions (Table1-10). We also describe symptoms that do not appear directly related to the anatomical structures affected by the infarct, including delirium, psychosis, depression, anxiety, and posttraumatic stress.

Symptoms associated with stroke in specific regions
Frontal lobe strokes
The frontal lobes are the largest lobes in the brain, and damage to areas within these lobes can cause behavioral and personality changes. Lesions in the lateral frontal cortex can cause aprosodia (difficulty expressing or comprehending variations in tone of voice), which can lead to communication errors. Lateral frontal cortex injury can cause executive dysfunction and a lack of empathy1 as well as trouble with attention, planning, and self-regulation that may affect daily functioning. Strokes affecting the superior and inferior mesial cortices may result in apathy, lack of motivation, altered self-regulation, altered emotional processing, and disinhibition. Patients who experience a basal forebrain stroke may exhibit confabulation, reduced motivation, and delusions such as Capgras syndrome (the belief that a person or place has been replaced by an exact copy) and reduplicative paramnesia (the belief that a place has been either moved, duplicated, or exists in 2 places simultaneously). Strokes involving the orbital cortex can be associated with personality changes, impulsivity, poor social judgment, reduced empathy, altered self-regulation, lack of goal-directed behavior, and environmental dependency.
Some strokes may occur primarily in the subcortical white matter within the frontal lobes. Symptoms may be due to a single stroke with sudden onset, or due to repeated ischemic events that accumulate over time, as seen with microvascular disease. In the case of microvascular disease, the onset of symptoms may be insidious and the course progressive. Infarcts in the subcortical area can also cause personality changes (though typically more subtle when compared to orbitofrontal strokes), reduced emotions, poor empathy, and irritability.1 Patients may lack insight into some of or all these symptoms following a frontal lobe infarct, which makes it critical to gather collateral information from the patient’s friends or family.
Parietal lobe strokes
Symptomatology from parietal strokes depends on whether the stroke affects the dominant or nondominant hemisphere. Dominant parietal lesions cause language deficits, and psychiatric symptoms may be difficult to elucidate due to the patient’s inability to communicate.2 On the other hand, patients with nondominant parietal stroke may have neglect of, or inattention to, the opposite (typically left) side.3 This often manifests as a reluctance to use the affected limb or limbs, in some cases despite a lack of true weakness or motor dysfunction. In addition, patients may also have visual and/or tactile inattention towards the affected side, despite a lack of gross visual or sensory impairment.2 In rare cases, a patient’s stroke may be misdiagnosed as a functional disorder due to the perceived unwillingness to use a neurologically intact limb. In severe cases, patients may not recognize an affected extremity as their own. Patients are also frequently unaware of deficits affecting their nondominant side and may argue with those attempting to explain their deficit. Anosodiaphoria—an abnormal lack of concern regarding their deficits—may also be observed. Additionally, aprosodia, flat affect, and personality changes may result from strokes affecting the nondominant hemisphere, which can impact the patient’s relationships and social functioning.3
Occipital lobe strokes
While negative or loss-of-function symptomatology is one of the hallmarks of stroke, occipital lobe infarcts can pose an exception. Although vision loss is the most common symptom with occipital lobe strokes, some patients experience visual hallucinations that may occur acutely or subacutely. In the acute phase, patients may report hallucinations of varied description,4 including poorly formed areas of color, scotomas, metamorphopsia (visual distortion in which straight lines appear curved), more complex and formed hallucinations and/or palinoptic images (images or brief scenes that continue to be perceived after looking away). These hallucinations, often referred to as release phenomena or release hallucinations, are thought to result from disinhibition of the visual cortex, which then fires spontaneously.
Hallucinations are associated with either infarction or hemorrhage in the posterior cerebral artery territory. In some cases, the hallucinations may take on a formed, complex appearance, and Charles Bonnet syndrome (visual hallucinations in the setting of vision loss, with insight into the hallucinations) has been identified in a small portion of patients.5
Continue to: The duration of these...
The duration of these hallucinations varies. Some patients describe very short periods of the disturbance, lasting minutes to hours and corresponding with the onset of their stroke. Others experience prolonged hallucinations, which frequently evolve into formed, complex images, lasting from days to months.6 In the setting of cortical stroke, patients may be at risk for seizures, which could manifest as visual hallucinations. It is essential to ensure that epileptic causes of hallucinations have been ruled out, because seizures may require treatment and other precautions.
Other stroke locations
Strokes in other locations also can result in psychiatric or behavioral symptoms. Acute stroke in the subcortical midbrain or thalamus may result in peduncular hallucinosis, a syndrome of vivid visual hallucinations.7 The midbrain (most commonly the reticular formation) is usually affected; however, certain lesions of the thalamus may also cause peduncular hallucinosis. This phenomenon is theorized to be due to an increase in serotonin activity relative to acetylcholine and is often accompanied by drowsiness.
The subthalamic nucleus is most frequently associated with disordered movement such as hemiballismus, but also causes disturbances in mood and behavior, including hyperphagia and personality changes.8 Irritability, aggressiveness, disinhibition, anxiety, and obscene speech may also be seen with lesions of the subthalamic nucleus.
Finally, the caudate nucleus may cause alterations in executive functioning and behavior.9 A stroke in the dorsolateral caudate may cause abulia and psychic akinesia, decreased problem-solving ability, reduced abstract thinking, and/or diminished spontaneity, whereas an infarct in the ventromedial region of the nucleus may cause disinhibition, disorganization, impulsiveness, and, in severe cases, affective symptoms with psychosis.10 Strokes in any of these areas are at risk for being misdiagnosed because patients may not have a hemiparesis, and isolated positive or psychiatric symptoms may not be recognized as stroke.
Symptoms not related to stroke location
Delirium and psychosis
Following a stroke, a patient may exhibit neuropsychiatric symptoms that do not appear to relate directly to the anatomical structures affected by the infarct. In the acute phase, factors such as older age and medical complications (including infection, metabolic derangement, and lack of sleep due to frequent neurologic checks) create a high risk of delirium.11 Differentiating delirium from alterations in mental status due to seizure, cerebral edema, or other medical complications is essential, and delirium precautions should be exercised to the greatest extent possible. Other neuropsychiatric symptoms may manifest following hospitalization.
Continue to: Poststroke psychosis...
Poststroke psychosis often presents subacutely. Among these patients, the most common psychosis is delusion disorder, followed by schizophrenia-like psychosis and mood disorder with psychotic features.12 Some evidence suggests antipsychotics may be highly effective for many of these patients.12 Poststroke psychosis does appear to correlate somewhat with nondominant hemisphere lesions, including the frontal lobe, parietal lobe, temporal lobe, and/or caudate nucleus. Because high mortality and poor functional outcomes have been associated with poststroke psychosis, early intervention is essential.
Depression
Depression is a common problem following stroke, affecting approximately 35% of stroke patients.13 In addition to impairing quality of life, depression negatively impacts rehabilitation and increases caregiver burden. There is significant variability regarding risk factors that increases the likelihood of poststroke depression; however, psychiatric history, dysphagia, and poor social support consistently correlate with a higher risk.14,15 Characteristics of a patient’s stroke, such as lesion volume and the ability to perform activities of daily living, are also risk factors. Identifying depression among patients who recently had a stroke is sometimes difficult due to a plethora of confounding factors. Patients may not communicate well due to aphasia, while strokes in other locations may result in an altered affect. Depending on the stroke location, patients may also suffer anosognosia (a lack of awareness of their deficits), which may impair their ability to learn and use adaptive strategies and equipment. An additional confounder is the significant overlap between depressive symptoms and those seen in the setting of a major medical event or hospitalization (decreased appetite, fatigue, etc). The prevalence of depression peaks approximately 3 to 6 months after stroke, with symptoms lasting 9 to 12 months on average, although many patients experience symptoms significantly longer.14 Because symptoms can begin within hours to days following a stroke, it is essential that both hospital and outpatient clinicians assess for depression when indicated. Patients with poststroke depression should receive prompt treatment because appropriate treatment correlates with improved rehabilitation, and most patients respond well to antidepressants.16 Early treatment reduces mortality and improves compliance with secondary stroke prevention measures, including pharmacotherapy.17
Anxiety and posttraumatic stress
Anxiety and anxiety-related disorders are additional potential complications following stroke that significantly influence patient outcomes and well-being. The abrupt, unexpected onset of stroke is often frightening to patients and families. The potential for life-altering deficits as well as intense, often invasive, interactions with the health care system does little to assuage patients’ fear. Stroke patients must contend with a change in neurologic function while processing their difficult experiences, and may develop profound fear of a recurrent stroke. As many as 22% of patients have an anxiety disorder 3 months after they have a stroke.18 Phobic disorder is the most prevalent subtype, followed by generalized anxiety disorder. Younger age and previous anxiety or depression place patients at greater risk of developing poststroke anxiety. Patients suffering from poststroke anxiety have a reduced quality of life, are more dependent, and show restricted participation in rehabilitation, all of which culminate in poorer outcomes.
Many patients describe their experiences surrounding their stroke as traumatic, and posttraumatic stress disorder (PTSD) is increasingly acknowledged as a potential complication for patients with recent stroke.19 PTSD profoundly impacts patient quality of life. Interestingly, most patients who develop poststroke PTSD do not have a history of other psychiatric illness, and it is difficult to predict who may develop PTSD. Relatively little is known regarding optimal treatment strategies for poststroke PTSD, or the efficacy of pharmacotherapy and psychotherapeutic strategies to treat it.
Goals: Improve recovery and quality of life
Neuropsychiatric symptoms are common following a stroke and may manifest in a variety of ways. While some symptoms are a direct consequence of injury to a specific brain region, other symptoms may be a response to loss of independence, disability, experience with the medical system, or fear of recurrent stroke. The onset of psychiatric symptoms can be acute, beginning during hospitalization, or delayed. Understanding the association of psychiatric symptoms with the anatomical location of stroke may assist clinicians in identifying such symptoms. This knowledge informs conversations with patients and their caregivers, who may benefit from understanding that such symptoms are common after stroke. Furthermore, identifying psychiatric complications following stroke may affect rehabilitation. Additional investigation is necessary to find more effective treatment modalities and improve early intervention.
Continue to: Bottom Line
Bottom Line
Neuropsychiatric symptoms are frequently overlooked in patients with recent stroke. These symptoms include delirium, psychosis, depression, anxiety, and posttraumatic stress disorder, and can be the direct result of injury to neuroanatomical structures or a consequence of the patient’s experience. Prompt treatment can maximize stroke recovery and quality of life.
Related Resources
- Zhang S, Xu M, Liu ZJ, et al. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. 2020;10(6):125-138. doi:10.5498/wjp. v10.i6.125
- Saha G, Chakraborty K, Pattojoshi A. Management of psychiatric disorders in patients with stroke and traumatic brain injury. Indian J Psychiatry. 2022;64(Suppl 2): S344-S354.
Many patients experience neuropsychiatric symptoms following stroke. There is tremendous variation in the type, severity, and timeline of these symptoms, which have the potential to significantly impact patients’ quality of life. Some symptoms occur as a direct result of ischemic injury to brain structures regulating behavior, executive function, perception, or affect. Other symptoms occur indirectly due to the patient’s often-difficult experiences with the health care system, disrupted routines, or altered poststroke functional abilities. Psychiatric symptoms are not as easily recognized as classic stroke symptoms (such as hemiparesis) and are frequently overlooked, especially in the acute phase. However, these symptoms can negatively influence patients’ interpersonal relationships, rehabilitation, and employment.
Patients and families may not realize certain symptoms are stroke-related and may not discuss them with their clinicians. It is important to ask about and recognize psychiatric symptoms in patients who have experienced a stroke so you can provide optimal education and treatment. In this article, we review the types of psychiatric symptoms associated with strokes in specific brain regions (Table1-10). We also describe symptoms that do not appear directly related to the anatomical structures affected by the infarct, including delirium, psychosis, depression, anxiety, and posttraumatic stress.

Symptoms associated with stroke in specific regions
Frontal lobe strokes
The frontal lobes are the largest lobes in the brain, and damage to areas within these lobes can cause behavioral and personality changes. Lesions in the lateral frontal cortex can cause aprosodia (difficulty expressing or comprehending variations in tone of voice), which can lead to communication errors. Lateral frontal cortex injury can cause executive dysfunction and a lack of empathy1 as well as trouble with attention, planning, and self-regulation that may affect daily functioning. Strokes affecting the superior and inferior mesial cortices may result in apathy, lack of motivation, altered self-regulation, altered emotional processing, and disinhibition. Patients who experience a basal forebrain stroke may exhibit confabulation, reduced motivation, and delusions such as Capgras syndrome (the belief that a person or place has been replaced by an exact copy) and reduplicative paramnesia (the belief that a place has been either moved, duplicated, or exists in 2 places simultaneously). Strokes involving the orbital cortex can be associated with personality changes, impulsivity, poor social judgment, reduced empathy, altered self-regulation, lack of goal-directed behavior, and environmental dependency.
Some strokes may occur primarily in the subcortical white matter within the frontal lobes. Symptoms may be due to a single stroke with sudden onset, or due to repeated ischemic events that accumulate over time, as seen with microvascular disease. In the case of microvascular disease, the onset of symptoms may be insidious and the course progressive. Infarcts in the subcortical area can also cause personality changes (though typically more subtle when compared to orbitofrontal strokes), reduced emotions, poor empathy, and irritability.1 Patients may lack insight into some of or all these symptoms following a frontal lobe infarct, which makes it critical to gather collateral information from the patient’s friends or family.
Parietal lobe strokes
Symptomatology from parietal strokes depends on whether the stroke affects the dominant or nondominant hemisphere. Dominant parietal lesions cause language deficits, and psychiatric symptoms may be difficult to elucidate due to the patient’s inability to communicate.2 On the other hand, patients with nondominant parietal stroke may have neglect of, or inattention to, the opposite (typically left) side.3 This often manifests as a reluctance to use the affected limb or limbs, in some cases despite a lack of true weakness or motor dysfunction. In addition, patients may also have visual and/or tactile inattention towards the affected side, despite a lack of gross visual or sensory impairment.2 In rare cases, a patient’s stroke may be misdiagnosed as a functional disorder due to the perceived unwillingness to use a neurologically intact limb. In severe cases, patients may not recognize an affected extremity as their own. Patients are also frequently unaware of deficits affecting their nondominant side and may argue with those attempting to explain their deficit. Anosodiaphoria—an abnormal lack of concern regarding their deficits—may also be observed. Additionally, aprosodia, flat affect, and personality changes may result from strokes affecting the nondominant hemisphere, which can impact the patient’s relationships and social functioning.3
Occipital lobe strokes
While negative or loss-of-function symptomatology is one of the hallmarks of stroke, occipital lobe infarcts can pose an exception. Although vision loss is the most common symptom with occipital lobe strokes, some patients experience visual hallucinations that may occur acutely or subacutely. In the acute phase, patients may report hallucinations of varied description,4 including poorly formed areas of color, scotomas, metamorphopsia (visual distortion in which straight lines appear curved), more complex and formed hallucinations and/or palinoptic images (images or brief scenes that continue to be perceived after looking away). These hallucinations, often referred to as release phenomena or release hallucinations, are thought to result from disinhibition of the visual cortex, which then fires spontaneously.
Hallucinations are associated with either infarction or hemorrhage in the posterior cerebral artery territory. In some cases, the hallucinations may take on a formed, complex appearance, and Charles Bonnet syndrome (visual hallucinations in the setting of vision loss, with insight into the hallucinations) has been identified in a small portion of patients.5
Continue to: The duration of these...
The duration of these hallucinations varies. Some patients describe very short periods of the disturbance, lasting minutes to hours and corresponding with the onset of their stroke. Others experience prolonged hallucinations, which frequently evolve into formed, complex images, lasting from days to months.6 In the setting of cortical stroke, patients may be at risk for seizures, which could manifest as visual hallucinations. It is essential to ensure that epileptic causes of hallucinations have been ruled out, because seizures may require treatment and other precautions.
Other stroke locations
Strokes in other locations also can result in psychiatric or behavioral symptoms. Acute stroke in the subcortical midbrain or thalamus may result in peduncular hallucinosis, a syndrome of vivid visual hallucinations.7 The midbrain (most commonly the reticular formation) is usually affected; however, certain lesions of the thalamus may also cause peduncular hallucinosis. This phenomenon is theorized to be due to an increase in serotonin activity relative to acetylcholine and is often accompanied by drowsiness.
The subthalamic nucleus is most frequently associated with disordered movement such as hemiballismus, but also causes disturbances in mood and behavior, including hyperphagia and personality changes.8 Irritability, aggressiveness, disinhibition, anxiety, and obscene speech may also be seen with lesions of the subthalamic nucleus.
Finally, the caudate nucleus may cause alterations in executive functioning and behavior.9 A stroke in the dorsolateral caudate may cause abulia and psychic akinesia, decreased problem-solving ability, reduced abstract thinking, and/or diminished spontaneity, whereas an infarct in the ventromedial region of the nucleus may cause disinhibition, disorganization, impulsiveness, and, in severe cases, affective symptoms with psychosis.10 Strokes in any of these areas are at risk for being misdiagnosed because patients may not have a hemiparesis, and isolated positive or psychiatric symptoms may not be recognized as stroke.
Symptoms not related to stroke location
Delirium and psychosis
Following a stroke, a patient may exhibit neuropsychiatric symptoms that do not appear to relate directly to the anatomical structures affected by the infarct. In the acute phase, factors such as older age and medical complications (including infection, metabolic derangement, and lack of sleep due to frequent neurologic checks) create a high risk of delirium.11 Differentiating delirium from alterations in mental status due to seizure, cerebral edema, or other medical complications is essential, and delirium precautions should be exercised to the greatest extent possible. Other neuropsychiatric symptoms may manifest following hospitalization.
Continue to: Poststroke psychosis...
Poststroke psychosis often presents subacutely. Among these patients, the most common psychosis is delusion disorder, followed by schizophrenia-like psychosis and mood disorder with psychotic features.12 Some evidence suggests antipsychotics may be highly effective for many of these patients.12 Poststroke psychosis does appear to correlate somewhat with nondominant hemisphere lesions, including the frontal lobe, parietal lobe, temporal lobe, and/or caudate nucleus. Because high mortality and poor functional outcomes have been associated with poststroke psychosis, early intervention is essential.
Depression
Depression is a common problem following stroke, affecting approximately 35% of stroke patients.13 In addition to impairing quality of life, depression negatively impacts rehabilitation and increases caregiver burden. There is significant variability regarding risk factors that increases the likelihood of poststroke depression; however, psychiatric history, dysphagia, and poor social support consistently correlate with a higher risk.14,15 Characteristics of a patient’s stroke, such as lesion volume and the ability to perform activities of daily living, are also risk factors. Identifying depression among patients who recently had a stroke is sometimes difficult due to a plethora of confounding factors. Patients may not communicate well due to aphasia, while strokes in other locations may result in an altered affect. Depending on the stroke location, patients may also suffer anosognosia (a lack of awareness of their deficits), which may impair their ability to learn and use adaptive strategies and equipment. An additional confounder is the significant overlap between depressive symptoms and those seen in the setting of a major medical event or hospitalization (decreased appetite, fatigue, etc). The prevalence of depression peaks approximately 3 to 6 months after stroke, with symptoms lasting 9 to 12 months on average, although many patients experience symptoms significantly longer.14 Because symptoms can begin within hours to days following a stroke, it is essential that both hospital and outpatient clinicians assess for depression when indicated. Patients with poststroke depression should receive prompt treatment because appropriate treatment correlates with improved rehabilitation, and most patients respond well to antidepressants.16 Early treatment reduces mortality and improves compliance with secondary stroke prevention measures, including pharmacotherapy.17
Anxiety and posttraumatic stress
Anxiety and anxiety-related disorders are additional potential complications following stroke that significantly influence patient outcomes and well-being. The abrupt, unexpected onset of stroke is often frightening to patients and families. The potential for life-altering deficits as well as intense, often invasive, interactions with the health care system does little to assuage patients’ fear. Stroke patients must contend with a change in neurologic function while processing their difficult experiences, and may develop profound fear of a recurrent stroke. As many as 22% of patients have an anxiety disorder 3 months after they have a stroke.18 Phobic disorder is the most prevalent subtype, followed by generalized anxiety disorder. Younger age and previous anxiety or depression place patients at greater risk of developing poststroke anxiety. Patients suffering from poststroke anxiety have a reduced quality of life, are more dependent, and show restricted participation in rehabilitation, all of which culminate in poorer outcomes.
Many patients describe their experiences surrounding their stroke as traumatic, and posttraumatic stress disorder (PTSD) is increasingly acknowledged as a potential complication for patients with recent stroke.19 PTSD profoundly impacts patient quality of life. Interestingly, most patients who develop poststroke PTSD do not have a history of other psychiatric illness, and it is difficult to predict who may develop PTSD. Relatively little is known regarding optimal treatment strategies for poststroke PTSD, or the efficacy of pharmacotherapy and psychotherapeutic strategies to treat it.
Goals: Improve recovery and quality of life
Neuropsychiatric symptoms are common following a stroke and may manifest in a variety of ways. While some symptoms are a direct consequence of injury to a specific brain region, other symptoms may be a response to loss of independence, disability, experience with the medical system, or fear of recurrent stroke. The onset of psychiatric symptoms can be acute, beginning during hospitalization, or delayed. Understanding the association of psychiatric symptoms with the anatomical location of stroke may assist clinicians in identifying such symptoms. This knowledge informs conversations with patients and their caregivers, who may benefit from understanding that such symptoms are common after stroke. Furthermore, identifying psychiatric complications following stroke may affect rehabilitation. Additional investigation is necessary to find more effective treatment modalities and improve early intervention.
Continue to: Bottom Line
Bottom Line
Neuropsychiatric symptoms are frequently overlooked in patients with recent stroke. These symptoms include delirium, psychosis, depression, anxiety, and posttraumatic stress disorder, and can be the direct result of injury to neuroanatomical structures or a consequence of the patient’s experience. Prompt treatment can maximize stroke recovery and quality of life.
Related Resources
- Zhang S, Xu M, Liu ZJ, et al. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. 2020;10(6):125-138. doi:10.5498/wjp. v10.i6.125
- Saha G, Chakraborty K, Pattojoshi A. Management of psychiatric disorders in patients with stroke and traumatic brain injury. Indian J Psychiatry. 2022;64(Suppl 2): S344-S354.
1. Eslinger PJ, Reichwein RK. Frontal lobe stroke syndromes. In: Caplan LR, van Gijn J, eds. Stroke Syndromes. 3rd ed. Cambridge University Press; 2012:232-241.
2. Critchley M, Russell WR, Zangwill OL. Discussion on parietal lobe syndromes. Proc R Soc Med. 1951;44(4):337-346.
3. Hier DB, Mondlock J, Caplan LR. Behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33(3):337-344.
4. Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977;2(5):432-436.
5. Kumral E, Uluakay A, Donmez A. Complex visual hallucinations following stroke: epileptic origin or a deafferentiation phenomenon? Austin J Cerebrovasc Dis & Stroke. 2014;1(1):1005.
6. Lee JS, Ko KH, Oh JH, et al. Charles Bonnet syndrome after occipital infarction. J Neurosonol Neuroimag. 2018;10(2):154-157.
7. Young JB. Peduncular hallucinosis. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Elsevier; 2014:848.
8. Etemadifar M, Abtahi SH, Abtahi SM, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. 2012;2012:768580. doi:10.1155/2012/768580
9. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108.
10. Wang PY. Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei). 1991;47(3):199-203.
11. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-33.
12. Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89(8):879-885.
13. Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris). 2008;164(10):837-840.
14. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253-264.
15. Pritchard KT, Hreha KP, Hong I. Dysphagia associated with risk of depressive symptoms among stroke survivors after discharge from a cluster of inpatient rehabilitation facilities. Swallowing Rehabil. 2020;3(1):33-44.
16. Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829-1832.
17. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
18. Chun HY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556-564.
19. Garton AL, Sisti JA, Gupta VP, et al. Poststroke post-traumatic stress disorder: a review. Stroke. 2017;48(2):507-512.
1. Eslinger PJ, Reichwein RK. Frontal lobe stroke syndromes. In: Caplan LR, van Gijn J, eds. Stroke Syndromes. 3rd ed. Cambridge University Press; 2012:232-241.
2. Critchley M, Russell WR, Zangwill OL. Discussion on parietal lobe syndromes. Proc R Soc Med. 1951;44(4):337-346.
3. Hier DB, Mondlock J, Caplan LR. Behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33(3):337-344.
4. Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977;2(5):432-436.
5. Kumral E, Uluakay A, Donmez A. Complex visual hallucinations following stroke: epileptic origin or a deafferentiation phenomenon? Austin J Cerebrovasc Dis & Stroke. 2014;1(1):1005.
6. Lee JS, Ko KH, Oh JH, et al. Charles Bonnet syndrome after occipital infarction. J Neurosonol Neuroimag. 2018;10(2):154-157.
7. Young JB. Peduncular hallucinosis. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Elsevier; 2014:848.
8. Etemadifar M, Abtahi SH, Abtahi SM, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. 2012;2012:768580. doi:10.1155/2012/768580
9. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108.
10. Wang PY. Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei). 1991;47(3):199-203.
11. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-33.
12. Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89(8):879-885.
13. Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris). 2008;164(10):837-840.
14. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253-264.
15. Pritchard KT, Hreha KP, Hong I. Dysphagia associated with risk of depressive symptoms among stroke survivors after discharge from a cluster of inpatient rehabilitation facilities. Swallowing Rehabil. 2020;3(1):33-44.
16. Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829-1832.
17. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
18. Chun HY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556-564.
19. Garton AL, Sisti JA, Gupta VP, et al. Poststroke post-traumatic stress disorder: a review. Stroke. 2017;48(2):507-512.










