User login
Adherence to oral contraceptive protocols prevents pregnancy
Combined oral contraceptives (COCs) remain a popular method of pregnancy prevention worldwide, but efficacy and failure rates can be difficult to determine, as real-word use does not always mirror clinical trials, wrote Mitchell D. Creinin, MD, of the University of California, Davis, and colleagues. Clinical trials include perfect use or method-failure rates, but data on pregnancy risk based on reported adherence alone are lacking, they said.
To assess the effects of missed pills on COC efficacy, the researchers reviewed data from a pair of parallel phase 3 trials, focusing only on adherence to the pill dosing regimen. The findings were published in Obstetrics & Gynecology.
The study population included 1,864 individuals from the United States and Canada, and 1,553 from Europe and Russia.
The participants were healthy, sexually active adults aged 16-50 years in monogamous relationships from 2016 through 2018 who agreed to used estetrol 15 mg and drospirenone 3 mg for up to 13 28-day cycles as their only contraceptive method. Condom use was permitted for protection against sexually transmitted infections if needed. The 28-day COCs included 24 hormonal tablets and 4 placebo tablets. Participants received written instructions for what to do it they missed pills.
The primary outcome was the relationship between missed pills and pregnancies.
A total of 31 pregnancies occurred across both studies; none of these occurred during cycles in which other contraception was used. Of 22 pregnancies in participants who reported taking all pills, 21 reported daily pill use during the cycle in which pregnancy occurred. One participant reported not taking one pill and one participant reported not taking two pills; neither correctly followed the instructions for missed pills.
Pregnancies occurred in .09% of cycles in which participants reported taking all pills, and in 0.25%, 0.83%, and 1.6% of cycles in which participants reported missing one pill, two pills, or more than two pills, respectively.
“Pregnancy rates exceeded 1% only in participants who did not correctly follow missed-pill instructions,” the researchers noted.
Pregnancy rates per cycle ranged from 0% to 0.21%, and 48.4% of the pregnancies occurred during the first four cycles of COC use. Approximately one-third (32.3%) of pregnancies occurred within the first week of a new pill pack.
“Fertilization does not appear to be related to the timing of missed pills within the cycle because pregnancy did not occur more frequently earlier in the cycle (after the placebo pills),” the researchers wrote in their discussion. This finding contradicts previous research suggesting that contraceptive failure rates decrease over the first year of use, they said. In addition, the formulation of the pill used may affect pregnancy rates when pills are missed, as some hormones have longer half-lives, they noted.
The study findings were limited by several factors, including the lack of adjustment for outcomes based on reported sexual activity per cycle, and by the reliance on self-reports.
However, the results were strengthened by the use of the clinical outcomes of pregnancy as the primary outcome, rather than characteristics and predictors of participants who missed pills, the researchers said.
The cycle-based methodology used in the current study may provide insight on the relationship between COC adherence and pregnancy risk that can inform future studies, they concluded.
Findings highlight the importance of options
“With increasing restrictions on abortion care, offering more contraceptive options for people is critical,” Lauren Owens, MD, associate professor of obstetrics and gynecology at the University of Washington, Seattle, said in an interview. “That’s not to say that having another pill option makes up for the harm people are experiencing as they navigate abortion bans and legal interference in their health care, but no one pill works for all people, and having more options is helpful,” she said.
Dr. Owens noted that the rates of pregnancy in the current study were lower than she traditionally associates with COCs, “although I usually discuss annual failure rates with patients, not failure rates per cycle, and the latter will clearly be lower.” In the current study, “The authors hypothesize some of this may be due to the longer half-life that estetrol has compared to ethinyl estradiol, the estrogen form more commonly found in oral contraceptive pills,” she said.
From a clinical standpoint, “I appreciated the linkage between number of missed pills and pregnancies occurring,” Dr. Owens said. “This is a good reminder to clinicians to talk to patients ahead of time about what to do when missed pills occur and to provide resources in advance that patients can reference when needed,” she said.
“The authors published other studies on this pill in the last year and it seems to work well and have a reasonable safety profile,” Dr. Owens told this news organization. However, “We still need to broaden the methods available to patients, particularly methods that people producing sperm can use. In the face of ongoing and escalating attacks on access to contraceptive care and abortion care, it’s more important than ever to do what we can to improve options for patients,” she said.
The study was supported by Estetra SRL, an affiliate company of Mithra Pharmaceuticals. Dr. Creinin disclosed relationships with multiple companies including Gedeon Richter, Mayne, and Organon. He disclosed serving on the advisory boards for Evofem, Fuji Pharma, Gedeon Richter, GlaxoSmithKline, Mayne, Merck, OLIC, Organon, and Searchlight, and serving as a consultant for Estetra SRL (including the current study), Libbs, Mayne, and Medicines360; his university department receives contraceptive research funding from Chemo Research SL, Evofem, HRA Pharma, Medicines360, Merck, and Sebela. Dr. Owens had no relevant financial conflicts to disclose.
Combined oral contraceptives (COCs) remain a popular method of pregnancy prevention worldwide, but efficacy and failure rates can be difficult to determine, as real-word use does not always mirror clinical trials, wrote Mitchell D. Creinin, MD, of the University of California, Davis, and colleagues. Clinical trials include perfect use or method-failure rates, but data on pregnancy risk based on reported adherence alone are lacking, they said.
To assess the effects of missed pills on COC efficacy, the researchers reviewed data from a pair of parallel phase 3 trials, focusing only on adherence to the pill dosing regimen. The findings were published in Obstetrics & Gynecology.
The study population included 1,864 individuals from the United States and Canada, and 1,553 from Europe and Russia.
The participants were healthy, sexually active adults aged 16-50 years in monogamous relationships from 2016 through 2018 who agreed to used estetrol 15 mg and drospirenone 3 mg for up to 13 28-day cycles as their only contraceptive method. Condom use was permitted for protection against sexually transmitted infections if needed. The 28-day COCs included 24 hormonal tablets and 4 placebo tablets. Participants received written instructions for what to do it they missed pills.
The primary outcome was the relationship between missed pills and pregnancies.
A total of 31 pregnancies occurred across both studies; none of these occurred during cycles in which other contraception was used. Of 22 pregnancies in participants who reported taking all pills, 21 reported daily pill use during the cycle in which pregnancy occurred. One participant reported not taking one pill and one participant reported not taking two pills; neither correctly followed the instructions for missed pills.
Pregnancies occurred in .09% of cycles in which participants reported taking all pills, and in 0.25%, 0.83%, and 1.6% of cycles in which participants reported missing one pill, two pills, or more than two pills, respectively.
“Pregnancy rates exceeded 1% only in participants who did not correctly follow missed-pill instructions,” the researchers noted.
Pregnancy rates per cycle ranged from 0% to 0.21%, and 48.4% of the pregnancies occurred during the first four cycles of COC use. Approximately one-third (32.3%) of pregnancies occurred within the first week of a new pill pack.
“Fertilization does not appear to be related to the timing of missed pills within the cycle because pregnancy did not occur more frequently earlier in the cycle (after the placebo pills),” the researchers wrote in their discussion. This finding contradicts previous research suggesting that contraceptive failure rates decrease over the first year of use, they said. In addition, the formulation of the pill used may affect pregnancy rates when pills are missed, as some hormones have longer half-lives, they noted.
The study findings were limited by several factors, including the lack of adjustment for outcomes based on reported sexual activity per cycle, and by the reliance on self-reports.
However, the results were strengthened by the use of the clinical outcomes of pregnancy as the primary outcome, rather than characteristics and predictors of participants who missed pills, the researchers said.
The cycle-based methodology used in the current study may provide insight on the relationship between COC adherence and pregnancy risk that can inform future studies, they concluded.
Findings highlight the importance of options
“With increasing restrictions on abortion care, offering more contraceptive options for people is critical,” Lauren Owens, MD, associate professor of obstetrics and gynecology at the University of Washington, Seattle, said in an interview. “That’s not to say that having another pill option makes up for the harm people are experiencing as they navigate abortion bans and legal interference in their health care, but no one pill works for all people, and having more options is helpful,” she said.
Dr. Owens noted that the rates of pregnancy in the current study were lower than she traditionally associates with COCs, “although I usually discuss annual failure rates with patients, not failure rates per cycle, and the latter will clearly be lower.” In the current study, “The authors hypothesize some of this may be due to the longer half-life that estetrol has compared to ethinyl estradiol, the estrogen form more commonly found in oral contraceptive pills,” she said.
From a clinical standpoint, “I appreciated the linkage between number of missed pills and pregnancies occurring,” Dr. Owens said. “This is a good reminder to clinicians to talk to patients ahead of time about what to do when missed pills occur and to provide resources in advance that patients can reference when needed,” she said.
“The authors published other studies on this pill in the last year and it seems to work well and have a reasonable safety profile,” Dr. Owens told this news organization. However, “We still need to broaden the methods available to patients, particularly methods that people producing sperm can use. In the face of ongoing and escalating attacks on access to contraceptive care and abortion care, it’s more important than ever to do what we can to improve options for patients,” she said.
The study was supported by Estetra SRL, an affiliate company of Mithra Pharmaceuticals. Dr. Creinin disclosed relationships with multiple companies including Gedeon Richter, Mayne, and Organon. He disclosed serving on the advisory boards for Evofem, Fuji Pharma, Gedeon Richter, GlaxoSmithKline, Mayne, Merck, OLIC, Organon, and Searchlight, and serving as a consultant for Estetra SRL (including the current study), Libbs, Mayne, and Medicines360; his university department receives contraceptive research funding from Chemo Research SL, Evofem, HRA Pharma, Medicines360, Merck, and Sebela. Dr. Owens had no relevant financial conflicts to disclose.
Combined oral contraceptives (COCs) remain a popular method of pregnancy prevention worldwide, but efficacy and failure rates can be difficult to determine, as real-word use does not always mirror clinical trials, wrote Mitchell D. Creinin, MD, of the University of California, Davis, and colleagues. Clinical trials include perfect use or method-failure rates, but data on pregnancy risk based on reported adherence alone are lacking, they said.
To assess the effects of missed pills on COC efficacy, the researchers reviewed data from a pair of parallel phase 3 trials, focusing only on adherence to the pill dosing regimen. The findings were published in Obstetrics & Gynecology.
The study population included 1,864 individuals from the United States and Canada, and 1,553 from Europe and Russia.
The participants were healthy, sexually active adults aged 16-50 years in monogamous relationships from 2016 through 2018 who agreed to used estetrol 15 mg and drospirenone 3 mg for up to 13 28-day cycles as their only contraceptive method. Condom use was permitted for protection against sexually transmitted infections if needed. The 28-day COCs included 24 hormonal tablets and 4 placebo tablets. Participants received written instructions for what to do it they missed pills.
The primary outcome was the relationship between missed pills and pregnancies.
A total of 31 pregnancies occurred across both studies; none of these occurred during cycles in which other contraception was used. Of 22 pregnancies in participants who reported taking all pills, 21 reported daily pill use during the cycle in which pregnancy occurred. One participant reported not taking one pill and one participant reported not taking two pills; neither correctly followed the instructions for missed pills.
Pregnancies occurred in .09% of cycles in which participants reported taking all pills, and in 0.25%, 0.83%, and 1.6% of cycles in which participants reported missing one pill, two pills, or more than two pills, respectively.
“Pregnancy rates exceeded 1% only in participants who did not correctly follow missed-pill instructions,” the researchers noted.
Pregnancy rates per cycle ranged from 0% to 0.21%, and 48.4% of the pregnancies occurred during the first four cycles of COC use. Approximately one-third (32.3%) of pregnancies occurred within the first week of a new pill pack.
“Fertilization does not appear to be related to the timing of missed pills within the cycle because pregnancy did not occur more frequently earlier in the cycle (after the placebo pills),” the researchers wrote in their discussion. This finding contradicts previous research suggesting that contraceptive failure rates decrease over the first year of use, they said. In addition, the formulation of the pill used may affect pregnancy rates when pills are missed, as some hormones have longer half-lives, they noted.
The study findings were limited by several factors, including the lack of adjustment for outcomes based on reported sexual activity per cycle, and by the reliance on self-reports.
However, the results were strengthened by the use of the clinical outcomes of pregnancy as the primary outcome, rather than characteristics and predictors of participants who missed pills, the researchers said.
The cycle-based methodology used in the current study may provide insight on the relationship between COC adherence and pregnancy risk that can inform future studies, they concluded.
Findings highlight the importance of options
“With increasing restrictions on abortion care, offering more contraceptive options for people is critical,” Lauren Owens, MD, associate professor of obstetrics and gynecology at the University of Washington, Seattle, said in an interview. “That’s not to say that having another pill option makes up for the harm people are experiencing as they navigate abortion bans and legal interference in their health care, but no one pill works for all people, and having more options is helpful,” she said.
Dr. Owens noted that the rates of pregnancy in the current study were lower than she traditionally associates with COCs, “although I usually discuss annual failure rates with patients, not failure rates per cycle, and the latter will clearly be lower.” In the current study, “The authors hypothesize some of this may be due to the longer half-life that estetrol has compared to ethinyl estradiol, the estrogen form more commonly found in oral contraceptive pills,” she said.
From a clinical standpoint, “I appreciated the linkage between number of missed pills and pregnancies occurring,” Dr. Owens said. “This is a good reminder to clinicians to talk to patients ahead of time about what to do when missed pills occur and to provide resources in advance that patients can reference when needed,” she said.
“The authors published other studies on this pill in the last year and it seems to work well and have a reasonable safety profile,” Dr. Owens told this news organization. However, “We still need to broaden the methods available to patients, particularly methods that people producing sperm can use. In the face of ongoing and escalating attacks on access to contraceptive care and abortion care, it’s more important than ever to do what we can to improve options for patients,” she said.
The study was supported by Estetra SRL, an affiliate company of Mithra Pharmaceuticals. Dr. Creinin disclosed relationships with multiple companies including Gedeon Richter, Mayne, and Organon. He disclosed serving on the advisory boards for Evofem, Fuji Pharma, Gedeon Richter, GlaxoSmithKline, Mayne, Merck, OLIC, Organon, and Searchlight, and serving as a consultant for Estetra SRL (including the current study), Libbs, Mayne, and Medicines360; his university department receives contraceptive research funding from Chemo Research SL, Evofem, HRA Pharma, Medicines360, Merck, and Sebela. Dr. Owens had no relevant financial conflicts to disclose.
FROM OBSTETRICS & GYNECOLOGY
Study: Prenatal supplements fail to meet nutrient needs
Although drugstore shelves might suggest otherwise,
In a new study published in the American Journal of Clinical Nutrition, investigators observed what many physicians have long suspected: Most prenatal vitamins and other supplements do not adequately make up the difference of what food-based intake of nutrients leave lacking. Despite patients believing they are getting everything they need with their product purchase, they fall short of guideline-recommended requirements.
“There is no magic pill,” said Katherine A. Sauder, PhD, an associate professor of pediatrics at the University of Colorado Anschutz Medical Campus, Aurora, and lead author of the study. “There is no easy answer here.”
The researchers analyzed 24-hour dietary intake data from 2,450 study participants across five states from 2007 to 2019. Dr. Sauder and colleagues focused on six of the more than 20 key nutrients recommended for pregnant people and determined the target dose for vitamin A, vitamin D, folate, calcium, iron, and omega-3 fatty acids.
The researchers tested more than 20,500 dietary supplements, of which 421 were prenatal products. Only 69 products – three prenatal – included all six nutrients. Just seven products – two prenatal – contained target doses for five nutrients. Only one product, which was not marketed as prenatal, contained target doses for all six nutrients but required seven tablets a serving and cost patients approximately $200 a month.
For many years, Dr. Sauder and her colleagues have struggled to identify the gold standard of vitamins for pregnant patients.
More than half of pregnant people in the United States are at risk of inadequate intake of vitamin D, folate, and iron from their diet alone, and one-third are at risk for insufficient intake of vitamin A and calcium.
Although more than 70% of pregnant women take dietary supplements, the products do not eliminate the risks for deficiencies.
The effects of inadequate nutrition during pregnancy may include neural tube defects, alterations in cardiovascular structure, and impaired neurocognitive development.
The researchers also looked at the challenges within the dietary supplement industry. The U.S. Food and Drug Administration regulates dietary supplements as foods rather than drugs and therefore does not require third-party verification that would ensure product ingredients match labels.
The researchers acknowledged the challenges in creating a one-size-fits-all nutritional supplement.
“The supplement industry is difficult, because you’re trying to create a product that works for a large, diverse group of people, but nutrition is very personal,” Dr. Sauder said.
Kendra Segura, MD, an ob.gyn. at the To Help Everyone Health and Wellness Center, Los Angeles, said she was unsurprised by the results.
“There’s no good prenatal vitamin out there,” Dr. Segura said. “There’s no ‘best.’ ”
Dr. Segura said she advises her patients to focus on increased nutritional intake with foods but added that that the lack of nutrients in diets and the need for supplements reflects the lack of availability of healthy food in some communities (known as “food deserts”), as well as poor dietary choices.
Diana Racusin, MD, an assistant professor of obstetrics, gynecology, and reproductive services at the University of Texas Health Science Center’s McGovern Medical School, Houston, also “wasn’t terribly surprised” by the findings. She stresses the importance of what patients eat more than the availability of supplements.
“What this is really showing us is we have work to do with our nutrition,” Dr. Racusin said.
Dr. Sauder’s biggest takeaway from her study is the need for more patient guidance for their nutrition beyond advising a supplement.
“We need better support for women to help them improve their diet during pregnancy so that they’re getting the nutrients they need from food,” she said, “and not having to rely on supplements as much.”
The study was supported by the Environmental Influences on Child Health Outcomes Program of the National Institutes of Health and by the nonprofit organization Autism Speaks. Dr. Sauder reports no relevant financial relationships. Two coauthors reported various conflicts of interest.
A version of this article first appeared on Medscape.com.
Although drugstore shelves might suggest otherwise,
In a new study published in the American Journal of Clinical Nutrition, investigators observed what many physicians have long suspected: Most prenatal vitamins and other supplements do not adequately make up the difference of what food-based intake of nutrients leave lacking. Despite patients believing they are getting everything they need with their product purchase, they fall short of guideline-recommended requirements.
“There is no magic pill,” said Katherine A. Sauder, PhD, an associate professor of pediatrics at the University of Colorado Anschutz Medical Campus, Aurora, and lead author of the study. “There is no easy answer here.”
The researchers analyzed 24-hour dietary intake data from 2,450 study participants across five states from 2007 to 2019. Dr. Sauder and colleagues focused on six of the more than 20 key nutrients recommended for pregnant people and determined the target dose for vitamin A, vitamin D, folate, calcium, iron, and omega-3 fatty acids.
The researchers tested more than 20,500 dietary supplements, of which 421 were prenatal products. Only 69 products – three prenatal – included all six nutrients. Just seven products – two prenatal – contained target doses for five nutrients. Only one product, which was not marketed as prenatal, contained target doses for all six nutrients but required seven tablets a serving and cost patients approximately $200 a month.
For many years, Dr. Sauder and her colleagues have struggled to identify the gold standard of vitamins for pregnant patients.
More than half of pregnant people in the United States are at risk of inadequate intake of vitamin D, folate, and iron from their diet alone, and one-third are at risk for insufficient intake of vitamin A and calcium.
Although more than 70% of pregnant women take dietary supplements, the products do not eliminate the risks for deficiencies.
The effects of inadequate nutrition during pregnancy may include neural tube defects, alterations in cardiovascular structure, and impaired neurocognitive development.
The researchers also looked at the challenges within the dietary supplement industry. The U.S. Food and Drug Administration regulates dietary supplements as foods rather than drugs and therefore does not require third-party verification that would ensure product ingredients match labels.
The researchers acknowledged the challenges in creating a one-size-fits-all nutritional supplement.
“The supplement industry is difficult, because you’re trying to create a product that works for a large, diverse group of people, but nutrition is very personal,” Dr. Sauder said.
Kendra Segura, MD, an ob.gyn. at the To Help Everyone Health and Wellness Center, Los Angeles, said she was unsurprised by the results.
“There’s no good prenatal vitamin out there,” Dr. Segura said. “There’s no ‘best.’ ”
Dr. Segura said she advises her patients to focus on increased nutritional intake with foods but added that that the lack of nutrients in diets and the need for supplements reflects the lack of availability of healthy food in some communities (known as “food deserts”), as well as poor dietary choices.
Diana Racusin, MD, an assistant professor of obstetrics, gynecology, and reproductive services at the University of Texas Health Science Center’s McGovern Medical School, Houston, also “wasn’t terribly surprised” by the findings. She stresses the importance of what patients eat more than the availability of supplements.
“What this is really showing us is we have work to do with our nutrition,” Dr. Racusin said.
Dr. Sauder’s biggest takeaway from her study is the need for more patient guidance for their nutrition beyond advising a supplement.
“We need better support for women to help them improve their diet during pregnancy so that they’re getting the nutrients they need from food,” she said, “and not having to rely on supplements as much.”
The study was supported by the Environmental Influences on Child Health Outcomes Program of the National Institutes of Health and by the nonprofit organization Autism Speaks. Dr. Sauder reports no relevant financial relationships. Two coauthors reported various conflicts of interest.
A version of this article first appeared on Medscape.com.
Although drugstore shelves might suggest otherwise,
In a new study published in the American Journal of Clinical Nutrition, investigators observed what many physicians have long suspected: Most prenatal vitamins and other supplements do not adequately make up the difference of what food-based intake of nutrients leave lacking. Despite patients believing they are getting everything they need with their product purchase, they fall short of guideline-recommended requirements.
“There is no magic pill,” said Katherine A. Sauder, PhD, an associate professor of pediatrics at the University of Colorado Anschutz Medical Campus, Aurora, and lead author of the study. “There is no easy answer here.”
The researchers analyzed 24-hour dietary intake data from 2,450 study participants across five states from 2007 to 2019. Dr. Sauder and colleagues focused on six of the more than 20 key nutrients recommended for pregnant people and determined the target dose for vitamin A, vitamin D, folate, calcium, iron, and omega-3 fatty acids.
The researchers tested more than 20,500 dietary supplements, of which 421 were prenatal products. Only 69 products – three prenatal – included all six nutrients. Just seven products – two prenatal – contained target doses for five nutrients. Only one product, which was not marketed as prenatal, contained target doses for all six nutrients but required seven tablets a serving and cost patients approximately $200 a month.
For many years, Dr. Sauder and her colleagues have struggled to identify the gold standard of vitamins for pregnant patients.
More than half of pregnant people in the United States are at risk of inadequate intake of vitamin D, folate, and iron from their diet alone, and one-third are at risk for insufficient intake of vitamin A and calcium.
Although more than 70% of pregnant women take dietary supplements, the products do not eliminate the risks for deficiencies.
The effects of inadequate nutrition during pregnancy may include neural tube defects, alterations in cardiovascular structure, and impaired neurocognitive development.
The researchers also looked at the challenges within the dietary supplement industry. The U.S. Food and Drug Administration regulates dietary supplements as foods rather than drugs and therefore does not require third-party verification that would ensure product ingredients match labels.
The researchers acknowledged the challenges in creating a one-size-fits-all nutritional supplement.
“The supplement industry is difficult, because you’re trying to create a product that works for a large, diverse group of people, but nutrition is very personal,” Dr. Sauder said.
Kendra Segura, MD, an ob.gyn. at the To Help Everyone Health and Wellness Center, Los Angeles, said she was unsurprised by the results.
“There’s no good prenatal vitamin out there,” Dr. Segura said. “There’s no ‘best.’ ”
Dr. Segura said she advises her patients to focus on increased nutritional intake with foods but added that that the lack of nutrients in diets and the need for supplements reflects the lack of availability of healthy food in some communities (known as “food deserts”), as well as poor dietary choices.
Diana Racusin, MD, an assistant professor of obstetrics, gynecology, and reproductive services at the University of Texas Health Science Center’s McGovern Medical School, Houston, also “wasn’t terribly surprised” by the findings. She stresses the importance of what patients eat more than the availability of supplements.
“What this is really showing us is we have work to do with our nutrition,” Dr. Racusin said.
Dr. Sauder’s biggest takeaway from her study is the need for more patient guidance for their nutrition beyond advising a supplement.
“We need better support for women to help them improve their diet during pregnancy so that they’re getting the nutrients they need from food,” she said, “and not having to rely on supplements as much.”
The study was supported by the Environmental Influences on Child Health Outcomes Program of the National Institutes of Health and by the nonprofit organization Autism Speaks. Dr. Sauder reports no relevant financial relationships. Two coauthors reported various conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF CLINICAL NUTRITION
Oophorectomies continue to dominate torsion treatment
Prompt surgical management is essential in cases of ovarian torsion in order to salvage ovarian function, and recent studies have shown that conservative management with detorsion does not increase postoperative complications, compared with oophorectomy, wrote Hannah Ryles, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
The American College of Obstetricians and Gynecologists issued practice guidelines in November 2016 that recommended ovarian conservation rather than oophorectomy to manage adnexal torsion in women wishing to preserve fertility. However, the impact of this guideline on clinical practice and surgical patterns remains unclear, the researchers said.
In a study published in Obstetrics and Gynecology, the researchers reviewed data from 402 patients who underwent surgeries before the updated ACOG guidelines (2008-2016) and 1,389 who underwent surgeries after the guidelines (2017-2020). Surgery data came from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database. The study population included women aged 18-50 years who underwent adnexal torsion surgery and were identified as having either oophorectomy or ovarian conservation surgery.
A total of 1,791 surgeries performed for adnexal torsion were included in the study; 542 (30.3%) involved ovarian conservation and 1,249 (69.7%) involved oophorectomy.
The proportion of oophorectomies was similar during the periods before and after the guidelines (71.9% vs. 69.1%; P = .16). However, the proportion of oophorectomies changed significantly across the entire study period, by approximately –1.6% each year.
Factors significantly associated with oophorectomy compared with ovarian conservation included older age (35 years vs. 28 years), higher body mass index (29.2 kg/m2 vs. 27.5 kg/m2), anemia (12.2% vs. 7.2%), hypertension (10.4% vs. 3.1%), and higher American Society of Anesthesiologists classification.
“There remains no defined acceptable rate of oophorectomy; this decision involves multiple factors, such as fertility and other patient desires after a risk and benefit discussion, menopausal status, concern for malignancy, and safety and feasibility of conservative procedures,” the researchers wrote in their discussion. However, in emergency situations, it may be difficult to determine a patient’s preferences, and a lack of desire for future fertility may be presumed, which may contribute to the relatively high oophorectomy rates over time, they said.
The findings were limited by several factors including the retrospective design and lack of data on surgical history, histopathology, and intraoperative appearance of the ovary, as well as lack of clinical data including the time from presentation to diagnosis or surgery, the researchers noted. “Although we were also unable to determine obstetric history and fertility desires, our median age of 32 years reflects a young cohort that was limited to women of reproductive age,” they added.
However, the results reflect studies suggesting that clinical practice often lags behind updated guidelines, and the findings were strengthened by the use of the NSQIP database and reflect a need for greater efforts to promote ovarian conservation in accordance with the current guidelines, the researchers concluded.
Consider unilateral oophorectomy
The current study highlights the discrepancy between the ACOG guidelines and clinical practice, with “disappointingly low” rates of ovarian preservation in the adult population, wrote Riley J. Young, MD, and Kimberly A. Kho, MD, both of the University of Texas Southwestern Medical Center, Dallas, in an accompanying editorial. The reasons for the discrepancy include clinical concerns for conserving a torsed ovary and the difficulty of assessing fertility desires in an emergency situation, they said.
However, consideration of unilateral oophorectomy as an option should be part of clinical decision-making, according to the editorialists. Previous studies suggest that retention of a single ovarian may still allow for a successful pregnancy, and the effects of unilateral oophorectomy have been studied in infertility and assisted reproductive technology settings.
Women with a single ovary have fewer eggs and require higher amounts of gonadotropins, but pregnancy is possible, the editorialists said. However, the long-term effects of unilateral oophorectomy are uncertain, and potential detrimental outcomes include increased mortality and cognitive impairment; therefore “we aim for premenopausal ovaries simply to be conserved, whether fertility is the stated goal or not,” they noted. This may include consideration of unilateral oophorectomy. “Each ovary conserved at midnight moves us closer to a more acceptable ovarian conservation rate,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Kho disclosed funding to her institution from Hologic for being on an investigator-initiated study, Dr. Young had no financial conflicts to disclose.
Prompt surgical management is essential in cases of ovarian torsion in order to salvage ovarian function, and recent studies have shown that conservative management with detorsion does not increase postoperative complications, compared with oophorectomy, wrote Hannah Ryles, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
The American College of Obstetricians and Gynecologists issued practice guidelines in November 2016 that recommended ovarian conservation rather than oophorectomy to manage adnexal torsion in women wishing to preserve fertility. However, the impact of this guideline on clinical practice and surgical patterns remains unclear, the researchers said.
In a study published in Obstetrics and Gynecology, the researchers reviewed data from 402 patients who underwent surgeries before the updated ACOG guidelines (2008-2016) and 1,389 who underwent surgeries after the guidelines (2017-2020). Surgery data came from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database. The study population included women aged 18-50 years who underwent adnexal torsion surgery and were identified as having either oophorectomy or ovarian conservation surgery.
A total of 1,791 surgeries performed for adnexal torsion were included in the study; 542 (30.3%) involved ovarian conservation and 1,249 (69.7%) involved oophorectomy.
The proportion of oophorectomies was similar during the periods before and after the guidelines (71.9% vs. 69.1%; P = .16). However, the proportion of oophorectomies changed significantly across the entire study period, by approximately –1.6% each year.
Factors significantly associated with oophorectomy compared with ovarian conservation included older age (35 years vs. 28 years), higher body mass index (29.2 kg/m2 vs. 27.5 kg/m2), anemia (12.2% vs. 7.2%), hypertension (10.4% vs. 3.1%), and higher American Society of Anesthesiologists classification.
“There remains no defined acceptable rate of oophorectomy; this decision involves multiple factors, such as fertility and other patient desires after a risk and benefit discussion, menopausal status, concern for malignancy, and safety and feasibility of conservative procedures,” the researchers wrote in their discussion. However, in emergency situations, it may be difficult to determine a patient’s preferences, and a lack of desire for future fertility may be presumed, which may contribute to the relatively high oophorectomy rates over time, they said.
The findings were limited by several factors including the retrospective design and lack of data on surgical history, histopathology, and intraoperative appearance of the ovary, as well as lack of clinical data including the time from presentation to diagnosis or surgery, the researchers noted. “Although we were also unable to determine obstetric history and fertility desires, our median age of 32 years reflects a young cohort that was limited to women of reproductive age,” they added.
However, the results reflect studies suggesting that clinical practice often lags behind updated guidelines, and the findings were strengthened by the use of the NSQIP database and reflect a need for greater efforts to promote ovarian conservation in accordance with the current guidelines, the researchers concluded.
Consider unilateral oophorectomy
The current study highlights the discrepancy between the ACOG guidelines and clinical practice, with “disappointingly low” rates of ovarian preservation in the adult population, wrote Riley J. Young, MD, and Kimberly A. Kho, MD, both of the University of Texas Southwestern Medical Center, Dallas, in an accompanying editorial. The reasons for the discrepancy include clinical concerns for conserving a torsed ovary and the difficulty of assessing fertility desires in an emergency situation, they said.
However, consideration of unilateral oophorectomy as an option should be part of clinical decision-making, according to the editorialists. Previous studies suggest that retention of a single ovarian may still allow for a successful pregnancy, and the effects of unilateral oophorectomy have been studied in infertility and assisted reproductive technology settings.
Women with a single ovary have fewer eggs and require higher amounts of gonadotropins, but pregnancy is possible, the editorialists said. However, the long-term effects of unilateral oophorectomy are uncertain, and potential detrimental outcomes include increased mortality and cognitive impairment; therefore “we aim for premenopausal ovaries simply to be conserved, whether fertility is the stated goal or not,” they noted. This may include consideration of unilateral oophorectomy. “Each ovary conserved at midnight moves us closer to a more acceptable ovarian conservation rate,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Kho disclosed funding to her institution from Hologic for being on an investigator-initiated study, Dr. Young had no financial conflicts to disclose.
Prompt surgical management is essential in cases of ovarian torsion in order to salvage ovarian function, and recent studies have shown that conservative management with detorsion does not increase postoperative complications, compared with oophorectomy, wrote Hannah Ryles, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
The American College of Obstetricians and Gynecologists issued practice guidelines in November 2016 that recommended ovarian conservation rather than oophorectomy to manage adnexal torsion in women wishing to preserve fertility. However, the impact of this guideline on clinical practice and surgical patterns remains unclear, the researchers said.
In a study published in Obstetrics and Gynecology, the researchers reviewed data from 402 patients who underwent surgeries before the updated ACOG guidelines (2008-2016) and 1,389 who underwent surgeries after the guidelines (2017-2020). Surgery data came from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database. The study population included women aged 18-50 years who underwent adnexal torsion surgery and were identified as having either oophorectomy or ovarian conservation surgery.
A total of 1,791 surgeries performed for adnexal torsion were included in the study; 542 (30.3%) involved ovarian conservation and 1,249 (69.7%) involved oophorectomy.
The proportion of oophorectomies was similar during the periods before and after the guidelines (71.9% vs. 69.1%; P = .16). However, the proportion of oophorectomies changed significantly across the entire study period, by approximately –1.6% each year.
Factors significantly associated with oophorectomy compared with ovarian conservation included older age (35 years vs. 28 years), higher body mass index (29.2 kg/m2 vs. 27.5 kg/m2), anemia (12.2% vs. 7.2%), hypertension (10.4% vs. 3.1%), and higher American Society of Anesthesiologists classification.
“There remains no defined acceptable rate of oophorectomy; this decision involves multiple factors, such as fertility and other patient desires after a risk and benefit discussion, menopausal status, concern for malignancy, and safety and feasibility of conservative procedures,” the researchers wrote in their discussion. However, in emergency situations, it may be difficult to determine a patient’s preferences, and a lack of desire for future fertility may be presumed, which may contribute to the relatively high oophorectomy rates over time, they said.
The findings were limited by several factors including the retrospective design and lack of data on surgical history, histopathology, and intraoperative appearance of the ovary, as well as lack of clinical data including the time from presentation to diagnosis or surgery, the researchers noted. “Although we were also unable to determine obstetric history and fertility desires, our median age of 32 years reflects a young cohort that was limited to women of reproductive age,” they added.
However, the results reflect studies suggesting that clinical practice often lags behind updated guidelines, and the findings were strengthened by the use of the NSQIP database and reflect a need for greater efforts to promote ovarian conservation in accordance with the current guidelines, the researchers concluded.
Consider unilateral oophorectomy
The current study highlights the discrepancy between the ACOG guidelines and clinical practice, with “disappointingly low” rates of ovarian preservation in the adult population, wrote Riley J. Young, MD, and Kimberly A. Kho, MD, both of the University of Texas Southwestern Medical Center, Dallas, in an accompanying editorial. The reasons for the discrepancy include clinical concerns for conserving a torsed ovary and the difficulty of assessing fertility desires in an emergency situation, they said.
However, consideration of unilateral oophorectomy as an option should be part of clinical decision-making, according to the editorialists. Previous studies suggest that retention of a single ovarian may still allow for a successful pregnancy, and the effects of unilateral oophorectomy have been studied in infertility and assisted reproductive technology settings.
Women with a single ovary have fewer eggs and require higher amounts of gonadotropins, but pregnancy is possible, the editorialists said. However, the long-term effects of unilateral oophorectomy are uncertain, and potential detrimental outcomes include increased mortality and cognitive impairment; therefore “we aim for premenopausal ovaries simply to be conserved, whether fertility is the stated goal or not,” they noted. This may include consideration of unilateral oophorectomy. “Each ovary conserved at midnight moves us closer to a more acceptable ovarian conservation rate,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Kho disclosed funding to her institution from Hologic for being on an investigator-initiated study, Dr. Young had no financial conflicts to disclose.
FROM OBSTETRICS & GYNECOLOGY
Routine third-trimester ultrasounds can detect likely breech births
Implementing universal ultrasound during the third trimester of pregnancy significantly reduced the number of undiagnosed breech presentations, according to a study published in PLOS Medicine. The effects held if sonographers used a traditional ultrasound machine or if midwives used a handheld ultrasound tool to perform what is known as a point-of-care ultrasound (POCUS) procedure.
“Giving pregnant women a third-trimester scan reduces the rate of undetected breech in labor by over two-thirds, which reduces the chances of harm to the baby,” said Asma Khalil, MBBCh, MD, professor of obstetrics and maternal-fetal medicine at the University of London’s St. George’s Hospital, and a coauthor of the new study.
Routine ultrasounds typically are performed from the 10th to the 13th week of pregnancy, not during the third trimester, when the risk for a breech birth would be most apparent. Breech births occur in 3%-4% of pregnancies, raising the risk that babies will experience broken bones or hemorrhage. Knowing that breech is possible before birth enables physicians to discuss options with the pregnant woman in advance, Dr. Khalil said. These steps include rotating the baby in the uterus or conducting a cesarean delivery. Such counseling is not possible if breech is undetected until spontaneous or induced labor.
“Breech presentation at term is not very common, but diagnosing it prior to the onset of labor or induction of labor offers patients much more flexibility in terms of options and planning,” said Cecilia B. Leggett, MD, a resident in obstetrics and gynecology at Cedars-Sinai in Los Angeles. Dr. Leggett, who was not involved in the study, has shown that handheld devices are as accurate at assessing fetal weight as are standard ultrasound machines.
Two tools, same result
Dr. Khalil and her colleagues compared the rates of undiagnosed breech presentations before and after implementing universal third-semester ultrasound at two hospitals in the United Kingdom. The requirement began in 2020; the study compared the rate of undiagnosed breeches from the period of 2016-2020 with that of 2020-2021.
St. George’s Hospital in London used a traditional ultrasound machine that is read by a sonographer, whereas the Norfolk and Norwich University Hospitals, in Norwich, England, employed midwives to use a handheld ultrasound device.
The rate of undiagnosed breech cases declined from 14.2% at St. George’s before the universal ultrasound requirement (82 missed cases of 578 breech births) to 2.8% after the requirement began (7 missed cases of 251 breech births). The story was similar at Norfolk and Norwich, where 16.2% missed breech cases occurred before the requirement (27 of 167) and 3.5% missed cases were reported after it (5 of 142).
The increased accuracy of breech diagnosis before labor probably led to fewer cases of impaired blood flow to a baby’s brain at birth, Dr. Khalil’s group reported, as well as a probable reduction in the number of stillborn babies or those who die extremely young.
Traditional ultrasound scans read by sonographers are expensive, Dr. Khalil noted, whereas the portable handheld devices are much cheaper and could be used widely to improve detection of breech births. That step would require robust training about how to properly use these devices, Dr. Leggett said.
“As we see more and more studies come out about technology for POCUS, I think it’s important to keep in mind that we need the education about the tools to be as accessible as the tools themselves,” she said.
Dr. Leggett had no relevant financial relationships. Dr. Khalil is a vice president of the Royal College of Obstetricians and Gynaecologists, is a trustee and the treasurer of the International Society of Ultrasound in Obstetrics and Gynecology, and has lectured at and consulted in several ultrasound-based projects, webinars, and educational events.
A version of this article first appeared on Medscape.com.
Implementing universal ultrasound during the third trimester of pregnancy significantly reduced the number of undiagnosed breech presentations, according to a study published in PLOS Medicine. The effects held if sonographers used a traditional ultrasound machine or if midwives used a handheld ultrasound tool to perform what is known as a point-of-care ultrasound (POCUS) procedure.
“Giving pregnant women a third-trimester scan reduces the rate of undetected breech in labor by over two-thirds, which reduces the chances of harm to the baby,” said Asma Khalil, MBBCh, MD, professor of obstetrics and maternal-fetal medicine at the University of London’s St. George’s Hospital, and a coauthor of the new study.
Routine ultrasounds typically are performed from the 10th to the 13th week of pregnancy, not during the third trimester, when the risk for a breech birth would be most apparent. Breech births occur in 3%-4% of pregnancies, raising the risk that babies will experience broken bones or hemorrhage. Knowing that breech is possible before birth enables physicians to discuss options with the pregnant woman in advance, Dr. Khalil said. These steps include rotating the baby in the uterus or conducting a cesarean delivery. Such counseling is not possible if breech is undetected until spontaneous or induced labor.
“Breech presentation at term is not very common, but diagnosing it prior to the onset of labor or induction of labor offers patients much more flexibility in terms of options and planning,” said Cecilia B. Leggett, MD, a resident in obstetrics and gynecology at Cedars-Sinai in Los Angeles. Dr. Leggett, who was not involved in the study, has shown that handheld devices are as accurate at assessing fetal weight as are standard ultrasound machines.
Two tools, same result
Dr. Khalil and her colleagues compared the rates of undiagnosed breech presentations before and after implementing universal third-semester ultrasound at two hospitals in the United Kingdom. The requirement began in 2020; the study compared the rate of undiagnosed breeches from the period of 2016-2020 with that of 2020-2021.
St. George’s Hospital in London used a traditional ultrasound machine that is read by a sonographer, whereas the Norfolk and Norwich University Hospitals, in Norwich, England, employed midwives to use a handheld ultrasound device.
The rate of undiagnosed breech cases declined from 14.2% at St. George’s before the universal ultrasound requirement (82 missed cases of 578 breech births) to 2.8% after the requirement began (7 missed cases of 251 breech births). The story was similar at Norfolk and Norwich, where 16.2% missed breech cases occurred before the requirement (27 of 167) and 3.5% missed cases were reported after it (5 of 142).
The increased accuracy of breech diagnosis before labor probably led to fewer cases of impaired blood flow to a baby’s brain at birth, Dr. Khalil’s group reported, as well as a probable reduction in the number of stillborn babies or those who die extremely young.
Traditional ultrasound scans read by sonographers are expensive, Dr. Khalil noted, whereas the portable handheld devices are much cheaper and could be used widely to improve detection of breech births. That step would require robust training about how to properly use these devices, Dr. Leggett said.
“As we see more and more studies come out about technology for POCUS, I think it’s important to keep in mind that we need the education about the tools to be as accessible as the tools themselves,” she said.
Dr. Leggett had no relevant financial relationships. Dr. Khalil is a vice president of the Royal College of Obstetricians and Gynaecologists, is a trustee and the treasurer of the International Society of Ultrasound in Obstetrics and Gynecology, and has lectured at and consulted in several ultrasound-based projects, webinars, and educational events.
A version of this article first appeared on Medscape.com.
Implementing universal ultrasound during the third trimester of pregnancy significantly reduced the number of undiagnosed breech presentations, according to a study published in PLOS Medicine. The effects held if sonographers used a traditional ultrasound machine or if midwives used a handheld ultrasound tool to perform what is known as a point-of-care ultrasound (POCUS) procedure.
“Giving pregnant women a third-trimester scan reduces the rate of undetected breech in labor by over two-thirds, which reduces the chances of harm to the baby,” said Asma Khalil, MBBCh, MD, professor of obstetrics and maternal-fetal medicine at the University of London’s St. George’s Hospital, and a coauthor of the new study.
Routine ultrasounds typically are performed from the 10th to the 13th week of pregnancy, not during the third trimester, when the risk for a breech birth would be most apparent. Breech births occur in 3%-4% of pregnancies, raising the risk that babies will experience broken bones or hemorrhage. Knowing that breech is possible before birth enables physicians to discuss options with the pregnant woman in advance, Dr. Khalil said. These steps include rotating the baby in the uterus or conducting a cesarean delivery. Such counseling is not possible if breech is undetected until spontaneous or induced labor.
“Breech presentation at term is not very common, but diagnosing it prior to the onset of labor or induction of labor offers patients much more flexibility in terms of options and planning,” said Cecilia B. Leggett, MD, a resident in obstetrics and gynecology at Cedars-Sinai in Los Angeles. Dr. Leggett, who was not involved in the study, has shown that handheld devices are as accurate at assessing fetal weight as are standard ultrasound machines.
Two tools, same result
Dr. Khalil and her colleagues compared the rates of undiagnosed breech presentations before and after implementing universal third-semester ultrasound at two hospitals in the United Kingdom. The requirement began in 2020; the study compared the rate of undiagnosed breeches from the period of 2016-2020 with that of 2020-2021.
St. George’s Hospital in London used a traditional ultrasound machine that is read by a sonographer, whereas the Norfolk and Norwich University Hospitals, in Norwich, England, employed midwives to use a handheld ultrasound device.
The rate of undiagnosed breech cases declined from 14.2% at St. George’s before the universal ultrasound requirement (82 missed cases of 578 breech births) to 2.8% after the requirement began (7 missed cases of 251 breech births). The story was similar at Norfolk and Norwich, where 16.2% missed breech cases occurred before the requirement (27 of 167) and 3.5% missed cases were reported after it (5 of 142).
The increased accuracy of breech diagnosis before labor probably led to fewer cases of impaired blood flow to a baby’s brain at birth, Dr. Khalil’s group reported, as well as a probable reduction in the number of stillborn babies or those who die extremely young.
Traditional ultrasound scans read by sonographers are expensive, Dr. Khalil noted, whereas the portable handheld devices are much cheaper and could be used widely to improve detection of breech births. That step would require robust training about how to properly use these devices, Dr. Leggett said.
“As we see more and more studies come out about technology for POCUS, I think it’s important to keep in mind that we need the education about the tools to be as accessible as the tools themselves,” she said.
Dr. Leggett had no relevant financial relationships. Dr. Khalil is a vice president of the Royal College of Obstetricians and Gynaecologists, is a trustee and the treasurer of the International Society of Ultrasound in Obstetrics and Gynecology, and has lectured at and consulted in several ultrasound-based projects, webinars, and educational events.
A version of this article first appeared on Medscape.com.
FROM PLOS MEDICINE
COVID-19 in pregnancy affects growth in child’s first year of life
in a new analysis.
This “exaggerated growth pattern observed among infants with COVID-19 exposure may in some cases be a catch-up response to a prenatal growth deficit,” Mollie W. Ockene and colleagues wrote in a report published recently in the Journal of Clinical Endocrinology & Metabolism.
But given that lower birth weight and accelerated postnatal weight gain are risk factors for cardiometabolic disease, the findings “raise concern” about whether children born to mothers with prenatal COVID-19 go on to develop obesity, diabetes, or cardiovascular disease, senior coauthors Andrea G. Edlow, MD, and Lindsay T. Fourman, MD, of Massachusetts General Hospital, Boston, told this news organization.
Further studies in larger numbers of patients with longer follow-up and detailed assessments are needed, the researchers said, but this points to “a potentially increased cardiometabolic disease risk for the large global population of children with in utero COVID-19 exposure.”
It will be “important for clinicians caring for children with in utero exposure to maternal COVID-19 to be aware of this history,” Dr. Edlow and Dr. Fourman added, “and to view the child’s growth trajectory and metabolic risk factors in a holistic context that includes this prenatal infection exposure.”
COVID-19 vaccination important during and prior to pregnancy
The study also underscores the importance of primary prevention of COVID-19 among women who are contemplating pregnancy or who are already pregnant, the researchers noted, “including the need for widespread implementation of protective measures such as indoor masking and COVID-19 vaccination and boosting during or prior to pregnancy.”
Dr. Edlow and Dr. Fourman added, “Given the disproportionate impact that COVID-19 has had on historically marginalized populations, adverse health outcomes following in utero exposure to maternal COVID-19 may threaten to widen existing disparities in child health.”
On the other hand, although “COVID-19 vaccination rates lagged behind in minority populations following the initial vaccine rollout,” they noted, “these differences have fortunately narrowed over time, particularly for Hispanic individuals, though they do still persist in the Black population,” according to a recent report.
BMI trajectories during first year of life
In utero exposure to COVID-19 has been linked to fetal/neonatal morbidity and mortality, including stillbirth, preterm birth, preeclampsia, and gestational hypertension, but less is known about infant outcomes during the first year of life.
The researchers aimed to compare weight, length, and BMI trajectories over the first year of life in infants with, versus without, in utero exposure to COVID-19.
They identified 149 infants with in utero exposure to COVID-19 and 127 unexposed infants; all were born between March 30, 2020, and May 30, 2021, to mothers who participated in the Mass General Brigham COVID-19 Perinatal Biorepository.
The study excluded infants whose mothers received the vaccine (n = 5) or who had unclear vaccination status during pregnancy (n = 4) to reduce sample heterogeneity.
At the time of the study, few women had received the COVID-19 vaccine because vaccines were approved by the Food and Drug Administration for emergency use in December 2020 and the CDC recommended them for all pregnant women much later, in August 2021.
The researchers examined the weight, length, and BMI of the infants at birth, and at 2, 6, and 12 months, standardized using World Health Organization (WHO) growth charts.
Compared with mothers who did not have COVID-19 during pregnancy, those who had COVID-19 were younger (mean age, 32 vs. 34 years) and had a higher earliest BMI during pregnancy (29 vs. 26 kg/m2) and greater parity (previous births, excluding the index pregnancy, 1.2 vs. 0.9), and they were more likely to be Hispanic or Black and less likely to have private insurance.
Compared with infants exposed to COVID-19 in utero, infants who were not exposed were more likely to be male (47% vs. 55%).
Both infant groups were equally likely to be breastfed (90%).
Compared with the unexposed infants, infants born to mothers with prenatal COVID-19 had lower BMI z-scores at birth (effect size, −0.35; P = .03) and greater gain in BMI z-scores from birth to 12 months (effect size, 0.53; P = .03), but they had similar length at birth and over 12 months, after adjustment for maternal age at delivery, ethnicity, parity, insurance status, and earliest BMI during pregnancy, as well as infant sex, date of birth, and if applicable, history of breastfeeding.
The study received funding from the National Institutes of Health, Harvard Nutrition Obesity Research Center, Boston Area Diabetes Endocrinology Research Centers, American Heart Association, and Simons Foundation. Ms. Ockene has reported no relevant financial relationships. Dr. Edlow has reported being a consultant for Mirvie and receiving research funding from Merck outside the study. Dr. Fourman has reported serving as a consultant and receiving grant funding to her institution from Amryt outside the study. Disclosures for the other authors are listed with the article.
in a new analysis.
This “exaggerated growth pattern observed among infants with COVID-19 exposure may in some cases be a catch-up response to a prenatal growth deficit,” Mollie W. Ockene and colleagues wrote in a report published recently in the Journal of Clinical Endocrinology & Metabolism.
But given that lower birth weight and accelerated postnatal weight gain are risk factors for cardiometabolic disease, the findings “raise concern” about whether children born to mothers with prenatal COVID-19 go on to develop obesity, diabetes, or cardiovascular disease, senior coauthors Andrea G. Edlow, MD, and Lindsay T. Fourman, MD, of Massachusetts General Hospital, Boston, told this news organization.
Further studies in larger numbers of patients with longer follow-up and detailed assessments are needed, the researchers said, but this points to “a potentially increased cardiometabolic disease risk for the large global population of children with in utero COVID-19 exposure.”
It will be “important for clinicians caring for children with in utero exposure to maternal COVID-19 to be aware of this history,” Dr. Edlow and Dr. Fourman added, “and to view the child’s growth trajectory and metabolic risk factors in a holistic context that includes this prenatal infection exposure.”
COVID-19 vaccination important during and prior to pregnancy
The study also underscores the importance of primary prevention of COVID-19 among women who are contemplating pregnancy or who are already pregnant, the researchers noted, “including the need for widespread implementation of protective measures such as indoor masking and COVID-19 vaccination and boosting during or prior to pregnancy.”
Dr. Edlow and Dr. Fourman added, “Given the disproportionate impact that COVID-19 has had on historically marginalized populations, adverse health outcomes following in utero exposure to maternal COVID-19 may threaten to widen existing disparities in child health.”
On the other hand, although “COVID-19 vaccination rates lagged behind in minority populations following the initial vaccine rollout,” they noted, “these differences have fortunately narrowed over time, particularly for Hispanic individuals, though they do still persist in the Black population,” according to a recent report.
BMI trajectories during first year of life
In utero exposure to COVID-19 has been linked to fetal/neonatal morbidity and mortality, including stillbirth, preterm birth, preeclampsia, and gestational hypertension, but less is known about infant outcomes during the first year of life.
The researchers aimed to compare weight, length, and BMI trajectories over the first year of life in infants with, versus without, in utero exposure to COVID-19.
They identified 149 infants with in utero exposure to COVID-19 and 127 unexposed infants; all were born between March 30, 2020, and May 30, 2021, to mothers who participated in the Mass General Brigham COVID-19 Perinatal Biorepository.
The study excluded infants whose mothers received the vaccine (n = 5) or who had unclear vaccination status during pregnancy (n = 4) to reduce sample heterogeneity.
At the time of the study, few women had received the COVID-19 vaccine because vaccines were approved by the Food and Drug Administration for emergency use in December 2020 and the CDC recommended them for all pregnant women much later, in August 2021.
The researchers examined the weight, length, and BMI of the infants at birth, and at 2, 6, and 12 months, standardized using World Health Organization (WHO) growth charts.
Compared with mothers who did not have COVID-19 during pregnancy, those who had COVID-19 were younger (mean age, 32 vs. 34 years) and had a higher earliest BMI during pregnancy (29 vs. 26 kg/m2) and greater parity (previous births, excluding the index pregnancy, 1.2 vs. 0.9), and they were more likely to be Hispanic or Black and less likely to have private insurance.
Compared with infants exposed to COVID-19 in utero, infants who were not exposed were more likely to be male (47% vs. 55%).
Both infant groups were equally likely to be breastfed (90%).
Compared with the unexposed infants, infants born to mothers with prenatal COVID-19 had lower BMI z-scores at birth (effect size, −0.35; P = .03) and greater gain in BMI z-scores from birth to 12 months (effect size, 0.53; P = .03), but they had similar length at birth and over 12 months, after adjustment for maternal age at delivery, ethnicity, parity, insurance status, and earliest BMI during pregnancy, as well as infant sex, date of birth, and if applicable, history of breastfeeding.
The study received funding from the National Institutes of Health, Harvard Nutrition Obesity Research Center, Boston Area Diabetes Endocrinology Research Centers, American Heart Association, and Simons Foundation. Ms. Ockene has reported no relevant financial relationships. Dr. Edlow has reported being a consultant for Mirvie and receiving research funding from Merck outside the study. Dr. Fourman has reported serving as a consultant and receiving grant funding to her institution from Amryt outside the study. Disclosures for the other authors are listed with the article.
in a new analysis.
This “exaggerated growth pattern observed among infants with COVID-19 exposure may in some cases be a catch-up response to a prenatal growth deficit,” Mollie W. Ockene and colleagues wrote in a report published recently in the Journal of Clinical Endocrinology & Metabolism.
But given that lower birth weight and accelerated postnatal weight gain are risk factors for cardiometabolic disease, the findings “raise concern” about whether children born to mothers with prenatal COVID-19 go on to develop obesity, diabetes, or cardiovascular disease, senior coauthors Andrea G. Edlow, MD, and Lindsay T. Fourman, MD, of Massachusetts General Hospital, Boston, told this news organization.
Further studies in larger numbers of patients with longer follow-up and detailed assessments are needed, the researchers said, but this points to “a potentially increased cardiometabolic disease risk for the large global population of children with in utero COVID-19 exposure.”
It will be “important for clinicians caring for children with in utero exposure to maternal COVID-19 to be aware of this history,” Dr. Edlow and Dr. Fourman added, “and to view the child’s growth trajectory and metabolic risk factors in a holistic context that includes this prenatal infection exposure.”
COVID-19 vaccination important during and prior to pregnancy
The study also underscores the importance of primary prevention of COVID-19 among women who are contemplating pregnancy or who are already pregnant, the researchers noted, “including the need for widespread implementation of protective measures such as indoor masking and COVID-19 vaccination and boosting during or prior to pregnancy.”
Dr. Edlow and Dr. Fourman added, “Given the disproportionate impact that COVID-19 has had on historically marginalized populations, adverse health outcomes following in utero exposure to maternal COVID-19 may threaten to widen existing disparities in child health.”
On the other hand, although “COVID-19 vaccination rates lagged behind in minority populations following the initial vaccine rollout,” they noted, “these differences have fortunately narrowed over time, particularly for Hispanic individuals, though they do still persist in the Black population,” according to a recent report.
BMI trajectories during first year of life
In utero exposure to COVID-19 has been linked to fetal/neonatal morbidity and mortality, including stillbirth, preterm birth, preeclampsia, and gestational hypertension, but less is known about infant outcomes during the first year of life.
The researchers aimed to compare weight, length, and BMI trajectories over the first year of life in infants with, versus without, in utero exposure to COVID-19.
They identified 149 infants with in utero exposure to COVID-19 and 127 unexposed infants; all were born between March 30, 2020, and May 30, 2021, to mothers who participated in the Mass General Brigham COVID-19 Perinatal Biorepository.
The study excluded infants whose mothers received the vaccine (n = 5) or who had unclear vaccination status during pregnancy (n = 4) to reduce sample heterogeneity.
At the time of the study, few women had received the COVID-19 vaccine because vaccines were approved by the Food and Drug Administration for emergency use in December 2020 and the CDC recommended them for all pregnant women much later, in August 2021.
The researchers examined the weight, length, and BMI of the infants at birth, and at 2, 6, and 12 months, standardized using World Health Organization (WHO) growth charts.
Compared with mothers who did not have COVID-19 during pregnancy, those who had COVID-19 were younger (mean age, 32 vs. 34 years) and had a higher earliest BMI during pregnancy (29 vs. 26 kg/m2) and greater parity (previous births, excluding the index pregnancy, 1.2 vs. 0.9), and they were more likely to be Hispanic or Black and less likely to have private insurance.
Compared with infants exposed to COVID-19 in utero, infants who were not exposed were more likely to be male (47% vs. 55%).
Both infant groups were equally likely to be breastfed (90%).
Compared with the unexposed infants, infants born to mothers with prenatal COVID-19 had lower BMI z-scores at birth (effect size, −0.35; P = .03) and greater gain in BMI z-scores from birth to 12 months (effect size, 0.53; P = .03), but they had similar length at birth and over 12 months, after adjustment for maternal age at delivery, ethnicity, parity, insurance status, and earliest BMI during pregnancy, as well as infant sex, date of birth, and if applicable, history of breastfeeding.
The study received funding from the National Institutes of Health, Harvard Nutrition Obesity Research Center, Boston Area Diabetes Endocrinology Research Centers, American Heart Association, and Simons Foundation. Ms. Ockene has reported no relevant financial relationships. Dr. Edlow has reported being a consultant for Mirvie and receiving research funding from Merck outside the study. Dr. Fourman has reported serving as a consultant and receiving grant funding to her institution from Amryt outside the study. Disclosures for the other authors are listed with the article.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Infant and maternal weight gain together amplify obesity risk
Rapid weight gain (RWG) in infants and the mother’s prepregnancy overweight have a synergistic effect in increasing the odds that a child will develop overweight or obesity, new research suggests.
Findings were published online in Pediatrics.
Each factor has independently been associated with higher risk of childhood obesity but whether the two factors together exacerbate the risk has not been well studied, according to the authors led by Stephanie Gilley, MD, PhD, department of pediatrics, section of nutrition, University of Colorado at Denver, Aurora.
“Pediatric providers should monitor infants for RWG, especially in the context of maternal obesity, to reduce future risk of obesity,” the authors conclude.
Dr. Gilley’s team studied mother-infant dyads (n = 414) from the Healthy Start Study, an observational prebirth cohort. RWG was defined as a weight-for-age z score increase of at least 0.67 from birth to 3-7 months.
They found that RWG boosted the link between prepregnancy body mass index (ppBMI) and BMI z score, especially in female infants. Females exposed to both maternal obesity with RWG had an average BMI at the 94th percentile (1.50 increase in childhood BMI z score) “nearly at the cutoff for classification of obesity,” compared with those exposed to normal ppBMI with no RWG, who had an average childhood BMI at the 51st percentile.
“Currently, our nutrition recommendations as pediatricians are that all children are fed the same, essentially, after they’re born. We don’t have different growth parameters or different trajectories or targets for children who may have had different in utero exposures,” Dr. Gilley said.
Do some children need more monitoring for RWG?
Though we can’t necessarily draw conclusions from this one study, she says, the findings raise the question of whether children who were exposed in utero to obesity should be monitored for RWG more closely.
Lydia Shook, MD, Mass General Brigham maternal-fetal specialist and codirector of the Diabetes in Pregnancy Program at Massachusetts General Hospital in Boston, said she was struck by the finding in this study that with female infants, but not males, RWG significantly modified the association between ppBMI and early childhood BMI z scores.
“It’s an interesting finding and should be followed up with larger cohorts,” she said, noting that some previous studies have shown males are more vulnerable to maternal obesity and RWG.
“[Often] when we stratify by sex, you really need larger groups to be able to see the differences well,” Dr. Shook said.
She said she also found it interesting that when the researchers adjusted for breastfeeding status or caloric intake in childhood, the findings did not substantially change.
“That’s something that would warrant further investigation in an observational study or controlled trial,” Dr. Shook said.
Preventing rapid weight gain
The authors note that they did not consider possible interventions for preventing RGW in the study, although there are many, Dr. Gilley said.
Dr. Gilley also noted that a limitation of this study is that the population studied was primarily White.
Recent studies have shown the benefits of responsive parenting (RP) interventions, including a large study in 2022 geared toward Black families to teach better infant sleep practices as a way to prevent rapid weight gain.
That study, which tested the SAAF intervention, (Strong African American Families) found that “RP infants were nearly half as likely to experience upward crossing of two major weight-for-age percentile lines (14.1%), compared with control infants (24.2%); P = .09; odds ratio, 0.52; 95% confidence interval, 0.24-1.12.”
Along with sleep interventions, Dr. Gilley said, some researchers are studying the effects on RWG of better paternal engagement, or more involvement with the Women, Infants, and Children program, particularly with lower-income families.
Other studies have looked at breastfeeding vs. formula feeding – “but there have been mixed results there” – and responsive feeding practices, such as teaching families to recognize when a baby is full.
Dr. Gilley said she hopes this work will help broaden the thinking when it comes to infant weight gain.
“We spend a lot of time thinking about babies who are not growing fast enough and very little time thinking about babies who are growing too fast,” she said, “especially in those first 4-6 months of life.”
Dr. Gilley points to a study that illustrates that point. Pesch et al. concluded in a 2021 study based on interviews that pediatricians “are uncertain about the concept, definition, management, and long-term risks of rapid infant weight gain.”
Authors and Dr. Gilley declare no relevant financial relationships.
Rapid weight gain (RWG) in infants and the mother’s prepregnancy overweight have a synergistic effect in increasing the odds that a child will develop overweight or obesity, new research suggests.
Findings were published online in Pediatrics.
Each factor has independently been associated with higher risk of childhood obesity but whether the two factors together exacerbate the risk has not been well studied, according to the authors led by Stephanie Gilley, MD, PhD, department of pediatrics, section of nutrition, University of Colorado at Denver, Aurora.
“Pediatric providers should monitor infants for RWG, especially in the context of maternal obesity, to reduce future risk of obesity,” the authors conclude.
Dr. Gilley’s team studied mother-infant dyads (n = 414) from the Healthy Start Study, an observational prebirth cohort. RWG was defined as a weight-for-age z score increase of at least 0.67 from birth to 3-7 months.
They found that RWG boosted the link between prepregnancy body mass index (ppBMI) and BMI z score, especially in female infants. Females exposed to both maternal obesity with RWG had an average BMI at the 94th percentile (1.50 increase in childhood BMI z score) “nearly at the cutoff for classification of obesity,” compared with those exposed to normal ppBMI with no RWG, who had an average childhood BMI at the 51st percentile.
“Currently, our nutrition recommendations as pediatricians are that all children are fed the same, essentially, after they’re born. We don’t have different growth parameters or different trajectories or targets for children who may have had different in utero exposures,” Dr. Gilley said.
Do some children need more monitoring for RWG?
Though we can’t necessarily draw conclusions from this one study, she says, the findings raise the question of whether children who were exposed in utero to obesity should be monitored for RWG more closely.
Lydia Shook, MD, Mass General Brigham maternal-fetal specialist and codirector of the Diabetes in Pregnancy Program at Massachusetts General Hospital in Boston, said she was struck by the finding in this study that with female infants, but not males, RWG significantly modified the association between ppBMI and early childhood BMI z scores.
“It’s an interesting finding and should be followed up with larger cohorts,” she said, noting that some previous studies have shown males are more vulnerable to maternal obesity and RWG.
“[Often] when we stratify by sex, you really need larger groups to be able to see the differences well,” Dr. Shook said.
She said she also found it interesting that when the researchers adjusted for breastfeeding status or caloric intake in childhood, the findings did not substantially change.
“That’s something that would warrant further investigation in an observational study or controlled trial,” Dr. Shook said.
Preventing rapid weight gain
The authors note that they did not consider possible interventions for preventing RGW in the study, although there are many, Dr. Gilley said.
Dr. Gilley also noted that a limitation of this study is that the population studied was primarily White.
Recent studies have shown the benefits of responsive parenting (RP) interventions, including a large study in 2022 geared toward Black families to teach better infant sleep practices as a way to prevent rapid weight gain.
That study, which tested the SAAF intervention, (Strong African American Families) found that “RP infants were nearly half as likely to experience upward crossing of two major weight-for-age percentile lines (14.1%), compared with control infants (24.2%); P = .09; odds ratio, 0.52; 95% confidence interval, 0.24-1.12.”
Along with sleep interventions, Dr. Gilley said, some researchers are studying the effects on RWG of better paternal engagement, or more involvement with the Women, Infants, and Children program, particularly with lower-income families.
Other studies have looked at breastfeeding vs. formula feeding – “but there have been mixed results there” – and responsive feeding practices, such as teaching families to recognize when a baby is full.
Dr. Gilley said she hopes this work will help broaden the thinking when it comes to infant weight gain.
“We spend a lot of time thinking about babies who are not growing fast enough and very little time thinking about babies who are growing too fast,” she said, “especially in those first 4-6 months of life.”
Dr. Gilley points to a study that illustrates that point. Pesch et al. concluded in a 2021 study based on interviews that pediatricians “are uncertain about the concept, definition, management, and long-term risks of rapid infant weight gain.”
Authors and Dr. Gilley declare no relevant financial relationships.
Rapid weight gain (RWG) in infants and the mother’s prepregnancy overweight have a synergistic effect in increasing the odds that a child will develop overweight or obesity, new research suggests.
Findings were published online in Pediatrics.
Each factor has independently been associated with higher risk of childhood obesity but whether the two factors together exacerbate the risk has not been well studied, according to the authors led by Stephanie Gilley, MD, PhD, department of pediatrics, section of nutrition, University of Colorado at Denver, Aurora.
“Pediatric providers should monitor infants for RWG, especially in the context of maternal obesity, to reduce future risk of obesity,” the authors conclude.
Dr. Gilley’s team studied mother-infant dyads (n = 414) from the Healthy Start Study, an observational prebirth cohort. RWG was defined as a weight-for-age z score increase of at least 0.67 from birth to 3-7 months.
They found that RWG boosted the link between prepregnancy body mass index (ppBMI) and BMI z score, especially in female infants. Females exposed to both maternal obesity with RWG had an average BMI at the 94th percentile (1.50 increase in childhood BMI z score) “nearly at the cutoff for classification of obesity,” compared with those exposed to normal ppBMI with no RWG, who had an average childhood BMI at the 51st percentile.
“Currently, our nutrition recommendations as pediatricians are that all children are fed the same, essentially, after they’re born. We don’t have different growth parameters or different trajectories or targets for children who may have had different in utero exposures,” Dr. Gilley said.
Do some children need more monitoring for RWG?
Though we can’t necessarily draw conclusions from this one study, she says, the findings raise the question of whether children who were exposed in utero to obesity should be monitored for RWG more closely.
Lydia Shook, MD, Mass General Brigham maternal-fetal specialist and codirector of the Diabetes in Pregnancy Program at Massachusetts General Hospital in Boston, said she was struck by the finding in this study that with female infants, but not males, RWG significantly modified the association between ppBMI and early childhood BMI z scores.
“It’s an interesting finding and should be followed up with larger cohorts,” she said, noting that some previous studies have shown males are more vulnerable to maternal obesity and RWG.
“[Often] when we stratify by sex, you really need larger groups to be able to see the differences well,” Dr. Shook said.
She said she also found it interesting that when the researchers adjusted for breastfeeding status or caloric intake in childhood, the findings did not substantially change.
“That’s something that would warrant further investigation in an observational study or controlled trial,” Dr. Shook said.
Preventing rapid weight gain
The authors note that they did not consider possible interventions for preventing RGW in the study, although there are many, Dr. Gilley said.
Dr. Gilley also noted that a limitation of this study is that the population studied was primarily White.
Recent studies have shown the benefits of responsive parenting (RP) interventions, including a large study in 2022 geared toward Black families to teach better infant sleep practices as a way to prevent rapid weight gain.
That study, which tested the SAAF intervention, (Strong African American Families) found that “RP infants were nearly half as likely to experience upward crossing of two major weight-for-age percentile lines (14.1%), compared with control infants (24.2%); P = .09; odds ratio, 0.52; 95% confidence interval, 0.24-1.12.”
Along with sleep interventions, Dr. Gilley said, some researchers are studying the effects on RWG of better paternal engagement, or more involvement with the Women, Infants, and Children program, particularly with lower-income families.
Other studies have looked at breastfeeding vs. formula feeding – “but there have been mixed results there” – and responsive feeding practices, such as teaching families to recognize when a baby is full.
Dr. Gilley said she hopes this work will help broaden the thinking when it comes to infant weight gain.
“We spend a lot of time thinking about babies who are not growing fast enough and very little time thinking about babies who are growing too fast,” she said, “especially in those first 4-6 months of life.”
Dr. Gilley points to a study that illustrates that point. Pesch et al. concluded in a 2021 study based on interviews that pediatricians “are uncertain about the concept, definition, management, and long-term risks of rapid infant weight gain.”
Authors and Dr. Gilley declare no relevant financial relationships.
FROM PEDIATRICS
Cesarean deliveries drop in women at low risk
Although clinically indicated cesarean deliveries may improve outcomes for mothers and infants, “when not clinically indicated, cesarean delivery is a major surgical intervention that increases risk for adverse outcomes,” wrote Anna M. Frappaolo of Columbia University College of Physicians and Surgeons, New York, and colleagues.
The Healthy People 2030 campaign includes the reduction of cesarean deliveries, but trends in these procedures, especially with regard to diagnoses of labor arrest, have not been well studied, the researchers said.
In an analysis published in JAMA Network Open, the researchers reviewed delivery hospitalizations using data from the National Inpatient Sample from 2000 to 2019.
Births deemed low risk for cesarean delivery were identified by using criteria of the Society for Maternal-Fetal Medicine and additional criteria, and joinpoint regression analysis was used to estimate changes.
The researchers examined overall trends in cesarean deliveries as well as trends for three specific diagnoses: nonreassuring fetal status, labor arrest, and obstructed labor.
The final analysis included 40,517,867 deliveries; of these, 4,885,716 (12.1%) were cesarean deliveries.
Overall, cesarean deliveries in patients deemed at low risk increased from 9.7% in 2000 to 13.9% in 2009, then plateaued and decreased from 13.0% in 2012 to 11.1% in 2019. The average annual percentage change (AAPC) for cesarean delivery was 6.4% for the years from 2000 to 2005, 1.2% from 2005 to 2009, and −2.2% from 2009 to 2019.
Cesarean delivery for nonreassuring fetal status increased over the entire study period, from 3.4% in 2000 to 5.1% in 2019. By contrast, overall cesarean delivery for labor arrest increased from 3.6% in 2000 to a high of 4.8% in 2009, then decreased to 2.7% in 2019. Cesarean deliveries with a diagnosis of obstructed labor decreased from 0.9% in 2008 to 0.3% in 2019.
More specifically, cesarean deliveries for labor arrest in the active phase, latent phase, and second stage of labor increased from 1.5% to 2.1%, 1.1% to 1.5%, and 0.9% to 1.3%, respectively, from 2000 to 2009, and decreased from 2.1% to 1.7% for the active phase, from 1.5% to 1.2% for the latent phase, and from 1.2% to 0.9% for the second stage between 2010 and 2019.
Patients with increased odds of cesarean delivery were older (aged 35-39 years vs. 25-29 years, adjusted odds ratio 1.27), delivered in a hospital in the South vs. the Northeast of the United States (aOR 1.11), and were more likely to be non-Hispanic Black vs. non-Hispanic White (OR 1.23).
Notably, changes in nomenclature and interpretation of intrapartum electronic fetal heart monitoring occurred during the study period, with recommendations for the adoption of a three-tiered system for fetal heart rate patterns in 2008. “It is possible that current evidence and nomenclature related to intrapartum FHR interpretation may result in identification of a larger number of fetuses deemed at indeterminate risk for abnormal acid-base status,” the researchers wrote in their discussion.
The study findings were limited by several factors including the use of administrative discharge data rather than clinical records, the exclusion of patients with chronic conditions associated with cesarean delivery, changes in billing codes during the study period, and the inability to account for the effect of health factors, maternal age, and use of assisted reproductive technology, the researchers noted.
However, the results were strengthened by the large sample size and 20-year study period, as well as the stratification of labor arrest by stage, and suggest uptake of newer recommendations, they said. “Future reductions in cesarean deliveries among patients at low risk for cesarean delivery may be dependent on improved assessment of intrapartum fetal status,” they concluded.
Consider populations and outcomes in cesarean risk assessment
The decreasing rates of cesarean deliveries in the current study can be seen as positive, but more research is needed to examine maternal and neonatal outcomes, and to consider other conditions that affect risk for cesarean delivery, Paolo Ivo Cavoretto, MD, and Massimo Candiani, MD, of IRCCS San Raffaele Scientific Institute, and Antonio Farina, MD, of the University of Bologna, Italy, wrote in an accompanying editorial.
Notably, the study authors identified a population aged 15-39 years as low risk, and an increased risk for cesarean delivery within this range increased with age. “Maternal age remains a major risk factor associated with the risk of cesarean delivery, both from results of this study and those of previous analyses assessing its independence from other related risk factors,” the editorialists said.
The study findings also reflect the changes in standards for labor duration during the study period, they noted. The longer duration of labor may reduce cesarean delivery rates, but it is not without maternal and fetal-neonatal risks, they wrote.
“To be sure that the described trend of cesarean delivery rate reduction can be considered positive, there would be the theoretical need to analyze other maternal-fetal-neonatal outcomes (e.g., rates of operative deliveries, neonatal acidemia, intensive care unit use, maternal hemorrhage, pelvic floor trauma and dysfunction, and psychological distress),” the editorialists concluded.
More research needed to explore clinical decisions
“Reducing the cesarean delivery rate is a top priority, but evidence is lacking on an optimal rate that improves maternal and neonatal outcomes,” Iris Krishna, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
“Hospital quality and safety committees have been working to decrease cesarean deliveries amongst low-risk women, and identifying contemporary trends gives us insight on whether some of these efforts have translated to a lower cesarean delivery rate,” she said.
Dr. Krishna said she was not surprised by the higher cesarean section rate in the South. “The decision for cesarean delivery is multifaceted, and although this study was not able to assess clinical indications for cesarean delivery or maternal and fetal outcomes, we cannot ignore that social determinants of health contribute greatly to overall health outcomes,” she said. The trends in the current study further underscore the geographic disparities in access to health care present in the South, she added.
“This study notes that cesarean delivery for nonreassuring fetal status increased; however, nonreassuring fetal status as an indication for cesarean delivery can be subjective,” Dr. Krishna said. “Hospital quality and safety committees should consider reviewing the clinical scenarios that led to this decision to identify opportunities for improvement and further education,” she said.
“Defining contemporary trends in cesarean delivery for low-risk patients has merit, but the study findings should be interpreted with caution,” said Dr. Krishna, who is a member of the Ob.Gyn. News advisory board. More research is needed to define an optimal cesarean section rate that promotes positive maternal and fetal outcomes, and to determine whether identifying an optimal rate should be based on patient risk profiles, she said.
The study received no outside funding. Lead author Ms. Frappaolo had no financial conflicts to disclose; nor did the editorial authors or Dr. Krishna.
Although clinically indicated cesarean deliveries may improve outcomes for mothers and infants, “when not clinically indicated, cesarean delivery is a major surgical intervention that increases risk for adverse outcomes,” wrote Anna M. Frappaolo of Columbia University College of Physicians and Surgeons, New York, and colleagues.
The Healthy People 2030 campaign includes the reduction of cesarean deliveries, but trends in these procedures, especially with regard to diagnoses of labor arrest, have not been well studied, the researchers said.
In an analysis published in JAMA Network Open, the researchers reviewed delivery hospitalizations using data from the National Inpatient Sample from 2000 to 2019.
Births deemed low risk for cesarean delivery were identified by using criteria of the Society for Maternal-Fetal Medicine and additional criteria, and joinpoint regression analysis was used to estimate changes.
The researchers examined overall trends in cesarean deliveries as well as trends for three specific diagnoses: nonreassuring fetal status, labor arrest, and obstructed labor.
The final analysis included 40,517,867 deliveries; of these, 4,885,716 (12.1%) were cesarean deliveries.
Overall, cesarean deliveries in patients deemed at low risk increased from 9.7% in 2000 to 13.9% in 2009, then plateaued and decreased from 13.0% in 2012 to 11.1% in 2019. The average annual percentage change (AAPC) for cesarean delivery was 6.4% for the years from 2000 to 2005, 1.2% from 2005 to 2009, and −2.2% from 2009 to 2019.
Cesarean delivery for nonreassuring fetal status increased over the entire study period, from 3.4% in 2000 to 5.1% in 2019. By contrast, overall cesarean delivery for labor arrest increased from 3.6% in 2000 to a high of 4.8% in 2009, then decreased to 2.7% in 2019. Cesarean deliveries with a diagnosis of obstructed labor decreased from 0.9% in 2008 to 0.3% in 2019.
More specifically, cesarean deliveries for labor arrest in the active phase, latent phase, and second stage of labor increased from 1.5% to 2.1%, 1.1% to 1.5%, and 0.9% to 1.3%, respectively, from 2000 to 2009, and decreased from 2.1% to 1.7% for the active phase, from 1.5% to 1.2% for the latent phase, and from 1.2% to 0.9% for the second stage between 2010 and 2019.
Patients with increased odds of cesarean delivery were older (aged 35-39 years vs. 25-29 years, adjusted odds ratio 1.27), delivered in a hospital in the South vs. the Northeast of the United States (aOR 1.11), and were more likely to be non-Hispanic Black vs. non-Hispanic White (OR 1.23).
Notably, changes in nomenclature and interpretation of intrapartum electronic fetal heart monitoring occurred during the study period, with recommendations for the adoption of a three-tiered system for fetal heart rate patterns in 2008. “It is possible that current evidence and nomenclature related to intrapartum FHR interpretation may result in identification of a larger number of fetuses deemed at indeterminate risk for abnormal acid-base status,” the researchers wrote in their discussion.
The study findings were limited by several factors including the use of administrative discharge data rather than clinical records, the exclusion of patients with chronic conditions associated with cesarean delivery, changes in billing codes during the study period, and the inability to account for the effect of health factors, maternal age, and use of assisted reproductive technology, the researchers noted.
However, the results were strengthened by the large sample size and 20-year study period, as well as the stratification of labor arrest by stage, and suggest uptake of newer recommendations, they said. “Future reductions in cesarean deliveries among patients at low risk for cesarean delivery may be dependent on improved assessment of intrapartum fetal status,” they concluded.
Consider populations and outcomes in cesarean risk assessment
The decreasing rates of cesarean deliveries in the current study can be seen as positive, but more research is needed to examine maternal and neonatal outcomes, and to consider other conditions that affect risk for cesarean delivery, Paolo Ivo Cavoretto, MD, and Massimo Candiani, MD, of IRCCS San Raffaele Scientific Institute, and Antonio Farina, MD, of the University of Bologna, Italy, wrote in an accompanying editorial.
Notably, the study authors identified a population aged 15-39 years as low risk, and an increased risk for cesarean delivery within this range increased with age. “Maternal age remains a major risk factor associated with the risk of cesarean delivery, both from results of this study and those of previous analyses assessing its independence from other related risk factors,” the editorialists said.
The study findings also reflect the changes in standards for labor duration during the study period, they noted. The longer duration of labor may reduce cesarean delivery rates, but it is not without maternal and fetal-neonatal risks, they wrote.
“To be sure that the described trend of cesarean delivery rate reduction can be considered positive, there would be the theoretical need to analyze other maternal-fetal-neonatal outcomes (e.g., rates of operative deliveries, neonatal acidemia, intensive care unit use, maternal hemorrhage, pelvic floor trauma and dysfunction, and psychological distress),” the editorialists concluded.
More research needed to explore clinical decisions
“Reducing the cesarean delivery rate is a top priority, but evidence is lacking on an optimal rate that improves maternal and neonatal outcomes,” Iris Krishna, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
“Hospital quality and safety committees have been working to decrease cesarean deliveries amongst low-risk women, and identifying contemporary trends gives us insight on whether some of these efforts have translated to a lower cesarean delivery rate,” she said.
Dr. Krishna said she was not surprised by the higher cesarean section rate in the South. “The decision for cesarean delivery is multifaceted, and although this study was not able to assess clinical indications for cesarean delivery or maternal and fetal outcomes, we cannot ignore that social determinants of health contribute greatly to overall health outcomes,” she said. The trends in the current study further underscore the geographic disparities in access to health care present in the South, she added.
“This study notes that cesarean delivery for nonreassuring fetal status increased; however, nonreassuring fetal status as an indication for cesarean delivery can be subjective,” Dr. Krishna said. “Hospital quality and safety committees should consider reviewing the clinical scenarios that led to this decision to identify opportunities for improvement and further education,” she said.
“Defining contemporary trends in cesarean delivery for low-risk patients has merit, but the study findings should be interpreted with caution,” said Dr. Krishna, who is a member of the Ob.Gyn. News advisory board. More research is needed to define an optimal cesarean section rate that promotes positive maternal and fetal outcomes, and to determine whether identifying an optimal rate should be based on patient risk profiles, she said.
The study received no outside funding. Lead author Ms. Frappaolo had no financial conflicts to disclose; nor did the editorial authors or Dr. Krishna.
Although clinically indicated cesarean deliveries may improve outcomes for mothers and infants, “when not clinically indicated, cesarean delivery is a major surgical intervention that increases risk for adverse outcomes,” wrote Anna M. Frappaolo of Columbia University College of Physicians and Surgeons, New York, and colleagues.
The Healthy People 2030 campaign includes the reduction of cesarean deliveries, but trends in these procedures, especially with regard to diagnoses of labor arrest, have not been well studied, the researchers said.
In an analysis published in JAMA Network Open, the researchers reviewed delivery hospitalizations using data from the National Inpatient Sample from 2000 to 2019.
Births deemed low risk for cesarean delivery were identified by using criteria of the Society for Maternal-Fetal Medicine and additional criteria, and joinpoint regression analysis was used to estimate changes.
The researchers examined overall trends in cesarean deliveries as well as trends for three specific diagnoses: nonreassuring fetal status, labor arrest, and obstructed labor.
The final analysis included 40,517,867 deliveries; of these, 4,885,716 (12.1%) were cesarean deliveries.
Overall, cesarean deliveries in patients deemed at low risk increased from 9.7% in 2000 to 13.9% in 2009, then plateaued and decreased from 13.0% in 2012 to 11.1% in 2019. The average annual percentage change (AAPC) for cesarean delivery was 6.4% for the years from 2000 to 2005, 1.2% from 2005 to 2009, and −2.2% from 2009 to 2019.
Cesarean delivery for nonreassuring fetal status increased over the entire study period, from 3.4% in 2000 to 5.1% in 2019. By contrast, overall cesarean delivery for labor arrest increased from 3.6% in 2000 to a high of 4.8% in 2009, then decreased to 2.7% in 2019. Cesarean deliveries with a diagnosis of obstructed labor decreased from 0.9% in 2008 to 0.3% in 2019.
More specifically, cesarean deliveries for labor arrest in the active phase, latent phase, and second stage of labor increased from 1.5% to 2.1%, 1.1% to 1.5%, and 0.9% to 1.3%, respectively, from 2000 to 2009, and decreased from 2.1% to 1.7% for the active phase, from 1.5% to 1.2% for the latent phase, and from 1.2% to 0.9% for the second stage between 2010 and 2019.
Patients with increased odds of cesarean delivery were older (aged 35-39 years vs. 25-29 years, adjusted odds ratio 1.27), delivered in a hospital in the South vs. the Northeast of the United States (aOR 1.11), and were more likely to be non-Hispanic Black vs. non-Hispanic White (OR 1.23).
Notably, changes in nomenclature and interpretation of intrapartum electronic fetal heart monitoring occurred during the study period, with recommendations for the adoption of a three-tiered system for fetal heart rate patterns in 2008. “It is possible that current evidence and nomenclature related to intrapartum FHR interpretation may result in identification of a larger number of fetuses deemed at indeterminate risk for abnormal acid-base status,” the researchers wrote in their discussion.
The study findings were limited by several factors including the use of administrative discharge data rather than clinical records, the exclusion of patients with chronic conditions associated with cesarean delivery, changes in billing codes during the study period, and the inability to account for the effect of health factors, maternal age, and use of assisted reproductive technology, the researchers noted.
However, the results were strengthened by the large sample size and 20-year study period, as well as the stratification of labor arrest by stage, and suggest uptake of newer recommendations, they said. “Future reductions in cesarean deliveries among patients at low risk for cesarean delivery may be dependent on improved assessment of intrapartum fetal status,” they concluded.
Consider populations and outcomes in cesarean risk assessment
The decreasing rates of cesarean deliveries in the current study can be seen as positive, but more research is needed to examine maternal and neonatal outcomes, and to consider other conditions that affect risk for cesarean delivery, Paolo Ivo Cavoretto, MD, and Massimo Candiani, MD, of IRCCS San Raffaele Scientific Institute, and Antonio Farina, MD, of the University of Bologna, Italy, wrote in an accompanying editorial.
Notably, the study authors identified a population aged 15-39 years as low risk, and an increased risk for cesarean delivery within this range increased with age. “Maternal age remains a major risk factor associated with the risk of cesarean delivery, both from results of this study and those of previous analyses assessing its independence from other related risk factors,” the editorialists said.
The study findings also reflect the changes in standards for labor duration during the study period, they noted. The longer duration of labor may reduce cesarean delivery rates, but it is not without maternal and fetal-neonatal risks, they wrote.
“To be sure that the described trend of cesarean delivery rate reduction can be considered positive, there would be the theoretical need to analyze other maternal-fetal-neonatal outcomes (e.g., rates of operative deliveries, neonatal acidemia, intensive care unit use, maternal hemorrhage, pelvic floor trauma and dysfunction, and psychological distress),” the editorialists concluded.
More research needed to explore clinical decisions
“Reducing the cesarean delivery rate is a top priority, but evidence is lacking on an optimal rate that improves maternal and neonatal outcomes,” Iris Krishna, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
“Hospital quality and safety committees have been working to decrease cesarean deliveries amongst low-risk women, and identifying contemporary trends gives us insight on whether some of these efforts have translated to a lower cesarean delivery rate,” she said.
Dr. Krishna said she was not surprised by the higher cesarean section rate in the South. “The decision for cesarean delivery is multifaceted, and although this study was not able to assess clinical indications for cesarean delivery or maternal and fetal outcomes, we cannot ignore that social determinants of health contribute greatly to overall health outcomes,” she said. The trends in the current study further underscore the geographic disparities in access to health care present in the South, she added.
“This study notes that cesarean delivery for nonreassuring fetal status increased; however, nonreassuring fetal status as an indication for cesarean delivery can be subjective,” Dr. Krishna said. “Hospital quality and safety committees should consider reviewing the clinical scenarios that led to this decision to identify opportunities for improvement and further education,” she said.
“Defining contemporary trends in cesarean delivery for low-risk patients has merit, but the study findings should be interpreted with caution,” said Dr. Krishna, who is a member of the Ob.Gyn. News advisory board. More research is needed to define an optimal cesarean section rate that promotes positive maternal and fetal outcomes, and to determine whether identifying an optimal rate should be based on patient risk profiles, she said.
The study received no outside funding. Lead author Ms. Frappaolo had no financial conflicts to disclose; nor did the editorial authors or Dr. Krishna.
FROM JAMA NETWORK OPEN
2023 Update on fertility
Total fertility rate and fertility care: Demographic shifts and changing demands
Vollset SE, Goren E, Yuan C-W, et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396:1285-1306.
The total fertility rate (TFR) globally is decreasing rapidly, and in the United States it is now 1.8 births per woman, well below the required replacement rate of 2.1 that maintains the population.1 These reduced TFRs result in significant demographic shifts that affect the economy, workforce, society, health care needs, environment, and geopolitical standing of every country. These changes also will shift demands for the volume and type of services delivered by women’s health care clinicians.
In addition to the TFR, mortality rates and migration rates play essential roles in determining a country’s population.2 Anticipation and planning for these population and health care service changes by each country’s government, business, professionals, and other stakeholders are imperative to manage their impact and optimize quality of life.

US standings in projected population and economic growth
The US population is predicted to peak at 364 million in 2062 and decrease to 336 million in 2100, at which time it will be the fourth largest country in the world, according to a forecasting analysis by Vollset and colleagues.1 China is expected to become the biggest economy in the world in 2035, but this is predicted to change because of its decreasing population so that by 2098 the United States will again be the country with the largest economy (FIGURE 1).1
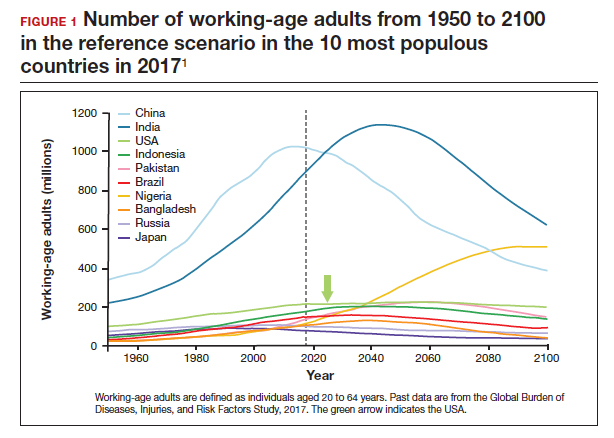
For the United States to maintain its economic and geopolitical standing, it is important to have policies that promote families. Other countries, especially in northern Europe, have implemented such policies. These include education of the population,economic incentives to create families, extended day care, and favorable tax policies.3 They also include increased access to family-forming fertility care. Such policies in Denmark have resulted in approximately 10% of all children being born from assisted reproductive technology (ART), compared with about 1.5% in the United States. Other countries have similar policies and success in increasing the number of children born from ART.
In the United States, the American Society for Reproductive Medicine (ASRM), RESOLVE: the National Infertility Association, the American Medical Women’s Association (AMWA), and others are promoting the need for increased access to fertility care and family-forming resources, primarily through family-forming benefits provided by companies.4 Such benefits are critical since the primary reason most people do not undergo fertility care is a lack of affordability. Only 1 person in 4 in the United States who needs fertility care receives treatment. Increased access would result in more babies being born to help address the reduced TFR.
Educational access, contraceptive goals, and access to fertility care
Continued trends in women’s educational attainment and access to contraception will hasten declines in the fertility rate and slow population growth (TABLE).1 These educational and contraceptive goals also must be pursued so that every person can achieve their individual reproductive life goals of having a family if and when they want to have a family. In addition to helping address the decreasing TFR, there is a fundamental right to found a family, as stated in the United Nations charter. It is a matter of social justice and equity that everyone who wants to have a family can access reproductive care on a nondiscriminatory basis when needed.
While the need for more and better insurance coverage for infertility has been well documented for many years, the decreasing TFR in the United States is an additional compelling reason that government, business, and other stakeholders should continue to increase access to fertility benefits and care. Women’s health care clinicians are encouraged to support these initiatives that also improve quality of life, equity, and social justice.
The decreasing global and US total fertility rate causes significant demographic changes, with major socioeconomic and health care consequences. The reduced TFR impacts women’s health care services, including the need for increased access to fertility care. Government and corporate policies, including those that improve access to fertility care, will help society adapt to these changes.
Continue to: A new comprehensive ovulatory disorders classification system developed by FIGO...
A new comprehensive ovulatory disorders classification system developed by FIGO
Munro MG, Balen AH, Cho S, et al; FIGO Committee on Menstrual Disorders and Related Health Impacts, and FIGO Committee on Reproductive Medicine, Endocrinology, and Infertility. The FIGO ovulatory disorders classification system. Fertil Steril. 2022;118:768-786.
Ovulatory disorders are well-recognized and common causes of infertility and abnormal uterine bleeding (AUB). Ovulatory disorders occur on a spectrum, with the most severe form being anovulation, and comprise a heterogeneous group that has been classically categorized based on an initial monograph published by the World Health Organization (WHO) in 1973. That classification was based on gonadotropin levels and categorized these disorders into 3 groups: 1) hypogonadotropic (such as hypothalamic amenorrhea), 2) eugonadotropic (such as polycystic ovary syndrome [PCOS]), and 3) hypergonadotropic (such as primary ovarian insufficiency). This initial classification was the subject of several subsequent iterations and modifications over the past 50 years; for example, at one point, ovulatory disorder caused by hyperprolactinemia was added as a separate fourth category. However, due to advances in endocrine assays, imaging technology, and genetics, our understanding of ovulatory disorders has expanded remarkably over the past several decades.
Previous FIGO classifications
Considering the emergent complexity of these disorders and the limitations of the original WHO classification to capture these subtleties adequately, the International Federation of Gynecology and Obstetrics (FIGO) recently developed and published a new classification system for ovulatory disorders.5 This new system was designed using a meticulously followed Delphi process with inputs from a diverse group of national and international professional organizations, subspecialty societies, specialty journals, recognized experts in the field, and lay individuals interested in the subject matter.
Of note, FIGO had previously published classification systems for nongestational normal and abnormal uterine bleeding in the reproductive years (FIGO AUB System 1),as well as a subsequent classification system that described potential causes of AUB symptoms (FIGO AUB System 2), with the 9 categories arranged under the acronym PALM-COEIN (Polyp, Adenomyosis, Leiomyoma, Malignancy–Coagulopathy, Ovulatory dysfunction, Endometrial disorders, Iatrogenic, and Not otherwise classified). This new FIGO classification of ovulatory disorders can be viewed as a continuation of the previous initiatives and aims to further categorize the subgroup of AUB-O (AUB with ovulatory disorders). However, it is important to recognize that while most ovulatory disorders manifest with the symptoms of AUB, the absence of AUB symptoms does not necessarily preclude ovulatory disorders.
New system uses a 3-tier approach
The new FIGO classification system for ovulatory disorders has adopted a 3-tier system.
The first tier is based on the anatomic components of the hypothalamic-pituitary-ovarian (HPO) axis and is referred to with the acronym HyPO, for Hypothalamic-Pituitary-Ovarian. Recognizing that PCOS refers to a distinct spectrum of conditions that share a variable combination of signs and symptoms caused to varying degrees by different pathophysiologic mechanisms that involve inherent ovarian follicular dysfunction, neuroendocrine dysfunction, insulin resistance, and androgen excess, it is categorized in a separate class of its own in the first tier, referred to with the letter P.
Adding PCOS to the anatomical categories referred to by HyPO, the first tier is overall referred to with the acronym HyPO-P (FIGURE 2).5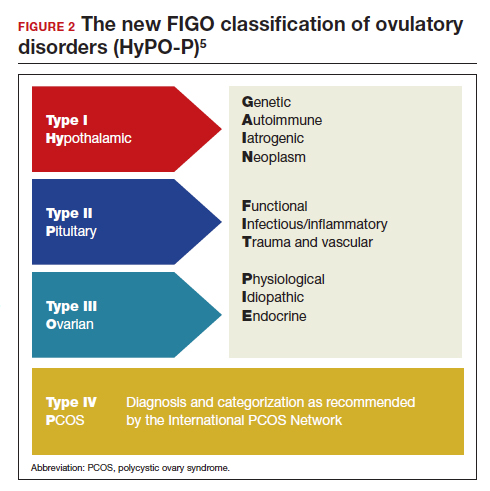
The second tier of stratification provides further etiologic details for any of the primary 3 anatomic classifications of hypothalamic, pituitary, and ovarian. These etiologies are arranged in 10 distinct groups under the mnemonic GAIN-FIT-PIE, which stands for Genetic, Autoimmune, Iatrogenic, Neoplasm; Functional, Infectious/inflammatory, Trauma and vascular; and Physiological, Idiopathic, Endocrine.
The third tier of the system refers to the specific clinical diagnosis. For example, an individual with Kallmann syndrome would be categorized as having type I (hypothalamic), Genetic, Kallmann syndrome, and an individual with PCOS would be categorized simply as having type IV, PCOS.
Our understanding of the etiology of ovulatory disorders has substantially increased over the past several decades. This progress has prompted the need to develop a more comprehensive classification system for these disorders. FIGO recently published a 3-tier classification system for ovulatory disorders that can be remembered with 2 mnemonics: HyPO-P and GAIN-FIT-PIE.
It is hoped that widespread adoption of this new classification system results in better and more concise communication between clinicians, researchers, and patients, ultimately leading to continued improvement in our understanding of the pathophysiology and management of ovulatory disorders.
Continue to: Live birth rate with conventional IVF shown noninferior to that with PGT-A...
Live birth rate with conventional IVF shown noninferior to that with PGT-A
Yan J, Qin Y, Zhao H, et al. Live birth with or without preimplantation genetic testing for aneuploidy. N Engl J Med. 2021;385:2047-2058.
Preimplantation genetic testing for aneuploidy (PGT-A) is increasingly used in many in vitro fertilization (IVF) cycles in the United States. Based on data from the Centers for Disease Control and Prevention, 43.8% of embryo transfers in the United States in 2019 included at least 1 PGT-A–tested embryo.6 Despite this widespread use, however, there are still no robust clinical data for PGT-A’s efficacy and safety, and the guidelines published by the ASRM do not recommend its routine use in all IVF cycles.7 In the past 2 to 3 years, several large studies have raised questions about the reported benefit of this technology.8,9
Details of the trial
In a multicenter, controlled, noninferiority trial conducted by Yan and colleagues, 1,212 subfertile women were randomly assigned to either conventional IVF with embryo selection based on morphology or embryo selection based on PGT-A with next-generation sequencing. Inclusion criteria were the diagnosis of subfertility, undergoing their first IVF cycle, female age of 20 to 37, and the availability of 3 or more good-quality blastocysts.
On day 5 of embryo culture, patients with 3 or more blastocysts were randomly assigned in a 1:1 ratio to either the PGT-A group or conventional IVF. All embryos were then frozen, and patients subsequently underwent frozen embryo transfer of a single blastocyst, selected based on either morphology or euploid result by PGT-A. If the initial transfer did not result in a live birth, and there were remaining transferable embryos (either a euploid embryo in the PGT-A group or a morphologically transferable embryo in the conventional IVF group), patients underwent successive frozen embryo transfers until either there was a live birth or no more embryos were available for transfer.
The study’s primary outcome was the cumulative live birth rate per randomly assigned patient that resulted from up to 3 frozen embryo transfer cycles within 1 year. There were 606 patients randomly assigned to the PGT-A group and 606 randomly assigned to the conventional IVF group.
In the PGT-A group, 468 women (77.2%) had live births; in the conventional IVF group, 496 women (81.8%) had live births. Women in the PGT-A group had a lower incidence of pregnancy loss compared with the conventional IVF group: 8.7% versus 12.6% (absolute difference of -3.9%; 95% confidence interval [CI], -7.5 to -0.2). There was no difference in obstetric and neonatal outcomes between the 2 groups. The authors concluded that among women with 3 or more good-quality blastocysts, conventional IVF resulted in a cumulative live birth rate that was noninferior to that of the PGT-A group.
Some benefit shown with PGT-A
Although the study by Yan and colleagues did not show any benefit, and even a possible reduction, with regard to cumulative live birth rate for PGT-A, it did show a 4% reduction in clinical pregnancy loss when PGT-A was used. Furthermore, the study design has been criticized for performing PGT-A on only 3 blastocysts in the PGT-A group. It is quite conceivable that the PGT-A group would have had more euploid embryos available for transfer if the study design had included all the available embryos instead of only 3. On the other hand, one could argue that if the authors had extended the study to include all the available embryos, the conventional group would have also had more embryos for transfer and, therefore, more chances for pregnancy and live birth.
It is also important to recognize that only patients who had at least 3 embryos available for biopsy were included in this study, and therefore the results of this study cannot be extended to patients with fewer embryos, such as those with diminished ovarian reserve.
In summary, based on this study’s results, we may conclude that for the good-prognosis patients in the age group of 20 to 37 who have at least 3 embryos available for biopsy, PGT-A may reduce the miscarriage rate by about 4%, but this benefit comes at the expense of about a 4% reduction in the cumulative live birth rate. ●
Despite the lack of robust evidence for efficacy, safety, and cost-effectiveness, PGT-A has been widely adopted into clinical IVF practice in the United States over the past several years. A large randomized controlled trial has suggested that, compared with conventional IVF, PGT-A application may actually result in a slightly lower cumulative live birth rate, while the miscarriage rate may be slightly higher with conventional IVF.
PGT-A is a novel and evolving technology with the potential to improve embryo selection in IVF; however, at this juncture, there is not enough clinical data for its universal and routine use in all IVF cycles. PGT-A can potentially be more helpful in older women (>38–40) with good ovarian reserve who are likely to have a larger cohort of embryos to select from. Patients must clearly understand this technology’s pros and cons before agreeing to incorporate it into their care plan.
- Vollset SE, Goren E, Yuan C-W, et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396:1285-1306.
- Dao TH, Docquier F, Maurel M, et al. Global migration in the twentieth and twenty-first centuries: the unstoppable force of demography. Rev World Econ. 2021;157:417-449.
- Atlas of fertility treatment policies in Europe. December 2021. Fertility Europe. Accessed December 29, 2022. https:// fertilityeurope.eu/atlas/#:~:text=Fertility%20Europe%20 in%20conjunction%20with%20the%20European%20 Parliamentary,The%20Atlas%20describes%20the%20 current%20situation%20in%202021
- AMWA’s physician fertility initiative. June 2021. American Medical Women’s Association. Accessed December 29, 2022. https://www.amwa-doc.org/our-work/initiatives/physician -infertility/
- Munro MG, Balen AH, Cho S, et al; FIGO Committee on Menstrual Disorders and Related Health Impacts, and FIGO Committee on Reproductive Medicine, Endocrinology, and Infertility. The FIGO ovulatory disorders classification system. Fertil Steril. 2022;118:768-786.
- Centers for Disease Control and Prevention. 2019 Assisted Reproductive Technology Fertility Clinic and National Summary Report. US Dept of Health and Human Services; 2021. Accessed February 24, 2023. https://www.cdc.gov/art /reports/2019/fertility-clinic.html
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109:429-436.
- Yan J, Qin Y, Zhao H, et al. Live birth with or without preimplantation genetic testing for aneuploidy. N Engl J Med. 2021;385:2047-2058.
- Kucherov A, Fazzari M, Lieman H, et al. PGT-A is associated with reduced cumulative live birth rate in first reported IVF stimulation cycles age ≤ 40: an analysis of 133,494 autologous cycles reported to SART CORS. J Assist Reprod Genet. 2023;40:137-149.
Total fertility rate and fertility care: Demographic shifts and changing demands
Vollset SE, Goren E, Yuan C-W, et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396:1285-1306.
The total fertility rate (TFR) globally is decreasing rapidly, and in the United States it is now 1.8 births per woman, well below the required replacement rate of 2.1 that maintains the population.1 These reduced TFRs result in significant demographic shifts that affect the economy, workforce, society, health care needs, environment, and geopolitical standing of every country. These changes also will shift demands for the volume and type of services delivered by women’s health care clinicians.
In addition to the TFR, mortality rates and migration rates play essential roles in determining a country’s population.2 Anticipation and planning for these population and health care service changes by each country’s government, business, professionals, and other stakeholders are imperative to manage their impact and optimize quality of life.

US standings in projected population and economic growth
The US population is predicted to peak at 364 million in 2062 and decrease to 336 million in 2100, at which time it will be the fourth largest country in the world, according to a forecasting analysis by Vollset and colleagues.1 China is expected to become the biggest economy in the world in 2035, but this is predicted to change because of its decreasing population so that by 2098 the United States will again be the country with the largest economy (FIGURE 1).1

For the United States to maintain its economic and geopolitical standing, it is important to have policies that promote families. Other countries, especially in northern Europe, have implemented such policies. These include education of the population,economic incentives to create families, extended day care, and favorable tax policies.3 They also include increased access to family-forming fertility care. Such policies in Denmark have resulted in approximately 10% of all children being born from assisted reproductive technology (ART), compared with about 1.5% in the United States. Other countries have similar policies and success in increasing the number of children born from ART.
In the United States, the American Society for Reproductive Medicine (ASRM), RESOLVE: the National Infertility Association, the American Medical Women’s Association (AMWA), and others are promoting the need for increased access to fertility care and family-forming resources, primarily through family-forming benefits provided by companies.4 Such benefits are critical since the primary reason most people do not undergo fertility care is a lack of affordability. Only 1 person in 4 in the United States who needs fertility care receives treatment. Increased access would result in more babies being born to help address the reduced TFR.
Educational access, contraceptive goals, and access to fertility care
Continued trends in women’s educational attainment and access to contraception will hasten declines in the fertility rate and slow population growth (TABLE).1 These educational and contraceptive goals also must be pursued so that every person can achieve their individual reproductive life goals of having a family if and when they want to have a family. In addition to helping address the decreasing TFR, there is a fundamental right to found a family, as stated in the United Nations charter. It is a matter of social justice and equity that everyone who wants to have a family can access reproductive care on a nondiscriminatory basis when needed.

While the need for more and better insurance coverage for infertility has been well documented for many years, the decreasing TFR in the United States is an additional compelling reason that government, business, and other stakeholders should continue to increase access to fertility benefits and care. Women’s health care clinicians are encouraged to support these initiatives that also improve quality of life, equity, and social justice.
The decreasing global and US total fertility rate causes significant demographic changes, with major socioeconomic and health care consequences. The reduced TFR impacts women’s health care services, including the need for increased access to fertility care. Government and corporate policies, including those that improve access to fertility care, will help society adapt to these changes.
Continue to: A new comprehensive ovulatory disorders classification system developed by FIGO...
A new comprehensive ovulatory disorders classification system developed by FIGO
Munro MG, Balen AH, Cho S, et al; FIGO Committee on Menstrual Disorders and Related Health Impacts, and FIGO Committee on Reproductive Medicine, Endocrinology, and Infertility. The FIGO ovulatory disorders classification system. Fertil Steril. 2022;118:768-786.
Ovulatory disorders are well-recognized and common causes of infertility and abnormal uterine bleeding (AUB). Ovulatory disorders occur on a spectrum, with the most severe form being anovulation, and comprise a heterogeneous group that has been classically categorized based on an initial monograph published by the World Health Organization (WHO) in 1973. That classification was based on gonadotropin levels and categorized these disorders into 3 groups: 1) hypogonadotropic (such as hypothalamic amenorrhea), 2) eugonadotropic (such as polycystic ovary syndrome [PCOS]), and 3) hypergonadotropic (such as primary ovarian insufficiency). This initial classification was the subject of several subsequent iterations and modifications over the past 50 years; for example, at one point, ovulatory disorder caused by hyperprolactinemia was added as a separate fourth category. However, due to advances in endocrine assays, imaging technology, and genetics, our understanding of ovulatory disorders has expanded remarkably over the past several decades.
Previous FIGO classifications
Considering the emergent complexity of these disorders and the limitations of the original WHO classification to capture these subtleties adequately, the International Federation of Gynecology and Obstetrics (FIGO) recently developed and published a new classification system for ovulatory disorders.5 This new system was designed using a meticulously followed Delphi process with inputs from a diverse group of national and international professional organizations, subspecialty societies, specialty journals, recognized experts in the field, and lay individuals interested in the subject matter.
Of note, FIGO had previously published classification systems for nongestational normal and abnormal uterine bleeding in the reproductive years (FIGO AUB System 1),as well as a subsequent classification system that described potential causes of AUB symptoms (FIGO AUB System 2), with the 9 categories arranged under the acronym PALM-COEIN (Polyp, Adenomyosis, Leiomyoma, Malignancy–Coagulopathy, Ovulatory dysfunction, Endometrial disorders, Iatrogenic, and Not otherwise classified). This new FIGO classification of ovulatory disorders can be viewed as a continuation of the previous initiatives and aims to further categorize the subgroup of AUB-O (AUB with ovulatory disorders). However, it is important to recognize that while most ovulatory disorders manifest with the symptoms of AUB, the absence of AUB symptoms does not necessarily preclude ovulatory disorders.
New system uses a 3-tier approach
The new FIGO classification system for ovulatory disorders has adopted a 3-tier system.
The first tier is based on the anatomic components of the hypothalamic-pituitary-ovarian (HPO) axis and is referred to with the acronym HyPO, for Hypothalamic-Pituitary-Ovarian. Recognizing that PCOS refers to a distinct spectrum of conditions that share a variable combination of signs and symptoms caused to varying degrees by different pathophysiologic mechanisms that involve inherent ovarian follicular dysfunction, neuroendocrine dysfunction, insulin resistance, and androgen excess, it is categorized in a separate class of its own in the first tier, referred to with the letter P.
Adding PCOS to the anatomical categories referred to by HyPO, the first tier is overall referred to with the acronym HyPO-P (FIGURE 2).5
The second tier of stratification provides further etiologic details for any of the primary 3 anatomic classifications of hypothalamic, pituitary, and ovarian. These etiologies are arranged in 10 distinct groups under the mnemonic GAIN-FIT-PIE, which stands for Genetic, Autoimmune, Iatrogenic, Neoplasm; Functional, Infectious/inflammatory, Trauma and vascular; and Physiological, Idiopathic, Endocrine.
The third tier of the system refers to the specific clinical diagnosis. For example, an individual with Kallmann syndrome would be categorized as having type I (hypothalamic), Genetic, Kallmann syndrome, and an individual with PCOS would be categorized simply as having type IV, PCOS.
Our understanding of the etiology of ovulatory disorders has substantially increased over the past several decades. This progress has prompted the need to develop a more comprehensive classification system for these disorders. FIGO recently published a 3-tier classification system for ovulatory disorders that can be remembered with 2 mnemonics: HyPO-P and GAIN-FIT-PIE.
It is hoped that widespread adoption of this new classification system results in better and more concise communication between clinicians, researchers, and patients, ultimately leading to continued improvement in our understanding of the pathophysiology and management of ovulatory disorders.
Continue to: Live birth rate with conventional IVF shown noninferior to that with PGT-A...
Live birth rate with conventional IVF shown noninferior to that with PGT-A
Yan J, Qin Y, Zhao H, et al. Live birth with or without preimplantation genetic testing for aneuploidy. N Engl J Med. 2021;385:2047-2058.
Preimplantation genetic testing for aneuploidy (PGT-A) is increasingly used in many in vitro fertilization (IVF) cycles in the United States. Based on data from the Centers for Disease Control and Prevention, 43.8% of embryo transfers in the United States in 2019 included at least 1 PGT-A–tested embryo.6 Despite this widespread use, however, there are still no robust clinical data for PGT-A’s efficacy and safety, and the guidelines published by the ASRM do not recommend its routine use in all IVF cycles.7 In the past 2 to 3 years, several large studies have raised questions about the reported benefit of this technology.8,9
Details of the trial
In a multicenter, controlled, noninferiority trial conducted by Yan and colleagues, 1,212 subfertile women were randomly assigned to either conventional IVF with embryo selection based on morphology or embryo selection based on PGT-A with next-generation sequencing. Inclusion criteria were the diagnosis of subfertility, undergoing their first IVF cycle, female age of 20 to 37, and the availability of 3 or more good-quality blastocysts.
On day 5 of embryo culture, patients with 3 or more blastocysts were randomly assigned in a 1:1 ratio to either the PGT-A group or conventional IVF. All embryos were then frozen, and patients subsequently underwent frozen embryo transfer of a single blastocyst, selected based on either morphology or euploid result by PGT-A. If the initial transfer did not result in a live birth, and there were remaining transferable embryos (either a euploid embryo in the PGT-A group or a morphologically transferable embryo in the conventional IVF group), patients underwent successive frozen embryo transfers until either there was a live birth or no more embryos were available for transfer.
The study’s primary outcome was the cumulative live birth rate per randomly assigned patient that resulted from up to 3 frozen embryo transfer cycles within 1 year. There were 606 patients randomly assigned to the PGT-A group and 606 randomly assigned to the conventional IVF group.
In the PGT-A group, 468 women (77.2%) had live births; in the conventional IVF group, 496 women (81.8%) had live births. Women in the PGT-A group had a lower incidence of pregnancy loss compared with the conventional IVF group: 8.7% versus 12.6% (absolute difference of -3.9%; 95% confidence interval [CI], -7.5 to -0.2). There was no difference in obstetric and neonatal outcomes between the 2 groups. The authors concluded that among women with 3 or more good-quality blastocysts, conventional IVF resulted in a cumulative live birth rate that was noninferior to that of the PGT-A group.
Some benefit shown with PGT-A
Although the study by Yan and colleagues did not show any benefit, and even a possible reduction, with regard to cumulative live birth rate for PGT-A, it did show a 4% reduction in clinical pregnancy loss when PGT-A was used. Furthermore, the study design has been criticized for performing PGT-A on only 3 blastocysts in the PGT-A group. It is quite conceivable that the PGT-A group would have had more euploid embryos available for transfer if the study design had included all the available embryos instead of only 3. On the other hand, one could argue that if the authors had extended the study to include all the available embryos, the conventional group would have also had more embryos for transfer and, therefore, more chances for pregnancy and live birth.
It is also important to recognize that only patients who had at least 3 embryos available for biopsy were included in this study, and therefore the results of this study cannot be extended to patients with fewer embryos, such as those with diminished ovarian reserve.
In summary, based on this study’s results, we may conclude that for the good-prognosis patients in the age group of 20 to 37 who have at least 3 embryos available for biopsy, PGT-A may reduce the miscarriage rate by about 4%, but this benefit comes at the expense of about a 4% reduction in the cumulative live birth rate. ●
Despite the lack of robust evidence for efficacy, safety, and cost-effectiveness, PGT-A has been widely adopted into clinical IVF practice in the United States over the past several years. A large randomized controlled trial has suggested that, compared with conventional IVF, PGT-A application may actually result in a slightly lower cumulative live birth rate, while the miscarriage rate may be slightly higher with conventional IVF.
PGT-A is a novel and evolving technology with the potential to improve embryo selection in IVF; however, at this juncture, there is not enough clinical data for its universal and routine use in all IVF cycles. PGT-A can potentially be more helpful in older women (>38–40) with good ovarian reserve who are likely to have a larger cohort of embryos to select from. Patients must clearly understand this technology’s pros and cons before agreeing to incorporate it into their care plan.
Total fertility rate and fertility care: Demographic shifts and changing demands
Vollset SE, Goren E, Yuan C-W, et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396:1285-1306.
The total fertility rate (TFR) globally is decreasing rapidly, and in the United States it is now 1.8 births per woman, well below the required replacement rate of 2.1 that maintains the population.1 These reduced TFRs result in significant demographic shifts that affect the economy, workforce, society, health care needs, environment, and geopolitical standing of every country. These changes also will shift demands for the volume and type of services delivered by women’s health care clinicians.
In addition to the TFR, mortality rates and migration rates play essential roles in determining a country’s population.2 Anticipation and planning for these population and health care service changes by each country’s government, business, professionals, and other stakeholders are imperative to manage their impact and optimize quality of life.

US standings in projected population and economic growth
The US population is predicted to peak at 364 million in 2062 and decrease to 336 million in 2100, at which time it will be the fourth largest country in the world, according to a forecasting analysis by Vollset and colleagues.1 China is expected to become the biggest economy in the world in 2035, but this is predicted to change because of its decreasing population so that by 2098 the United States will again be the country with the largest economy (FIGURE 1).1

For the United States to maintain its economic and geopolitical standing, it is important to have policies that promote families. Other countries, especially in northern Europe, have implemented such policies. These include education of the population,economic incentives to create families, extended day care, and favorable tax policies.3 They also include increased access to family-forming fertility care. Such policies in Denmark have resulted in approximately 10% of all children being born from assisted reproductive technology (ART), compared with about 1.5% in the United States. Other countries have similar policies and success in increasing the number of children born from ART.
In the United States, the American Society for Reproductive Medicine (ASRM), RESOLVE: the National Infertility Association, the American Medical Women’s Association (AMWA), and others are promoting the need for increased access to fertility care and family-forming resources, primarily through family-forming benefits provided by companies.4 Such benefits are critical since the primary reason most people do not undergo fertility care is a lack of affordability. Only 1 person in 4 in the United States who needs fertility care receives treatment. Increased access would result in more babies being born to help address the reduced TFR.
Educational access, contraceptive goals, and access to fertility care
Continued trends in women’s educational attainment and access to contraception will hasten declines in the fertility rate and slow population growth (TABLE).1 These educational and contraceptive goals also must be pursued so that every person can achieve their individual reproductive life goals of having a family if and when they want to have a family. In addition to helping address the decreasing TFR, there is a fundamental right to found a family, as stated in the United Nations charter. It is a matter of social justice and equity that everyone who wants to have a family can access reproductive care on a nondiscriminatory basis when needed.

While the need for more and better insurance coverage for infertility has been well documented for many years, the decreasing TFR in the United States is an additional compelling reason that government, business, and other stakeholders should continue to increase access to fertility benefits and care. Women’s health care clinicians are encouraged to support these initiatives that also improve quality of life, equity, and social justice.
The decreasing global and US total fertility rate causes significant demographic changes, with major socioeconomic and health care consequences. The reduced TFR impacts women’s health care services, including the need for increased access to fertility care. Government and corporate policies, including those that improve access to fertility care, will help society adapt to these changes.
Continue to: A new comprehensive ovulatory disorders classification system developed by FIGO...
A new comprehensive ovulatory disorders classification system developed by FIGO
Munro MG, Balen AH, Cho S, et al; FIGO Committee on Menstrual Disorders and Related Health Impacts, and FIGO Committee on Reproductive Medicine, Endocrinology, and Infertility. The FIGO ovulatory disorders classification system. Fertil Steril. 2022;118:768-786.
Ovulatory disorders are well-recognized and common causes of infertility and abnormal uterine bleeding (AUB). Ovulatory disorders occur on a spectrum, with the most severe form being anovulation, and comprise a heterogeneous group that has been classically categorized based on an initial monograph published by the World Health Organization (WHO) in 1973. That classification was based on gonadotropin levels and categorized these disorders into 3 groups: 1) hypogonadotropic (such as hypothalamic amenorrhea), 2) eugonadotropic (such as polycystic ovary syndrome [PCOS]), and 3) hypergonadotropic (such as primary ovarian insufficiency). This initial classification was the subject of several subsequent iterations and modifications over the past 50 years; for example, at one point, ovulatory disorder caused by hyperprolactinemia was added as a separate fourth category. However, due to advances in endocrine assays, imaging technology, and genetics, our understanding of ovulatory disorders has expanded remarkably over the past several decades.
Previous FIGO classifications
Considering the emergent complexity of these disorders and the limitations of the original WHO classification to capture these subtleties adequately, the International Federation of Gynecology and Obstetrics (FIGO) recently developed and published a new classification system for ovulatory disorders.5 This new system was designed using a meticulously followed Delphi process with inputs from a diverse group of national and international professional organizations, subspecialty societies, specialty journals, recognized experts in the field, and lay individuals interested in the subject matter.
Of note, FIGO had previously published classification systems for nongestational normal and abnormal uterine bleeding in the reproductive years (FIGO AUB System 1),as well as a subsequent classification system that described potential causes of AUB symptoms (FIGO AUB System 2), with the 9 categories arranged under the acronym PALM-COEIN (Polyp, Adenomyosis, Leiomyoma, Malignancy–Coagulopathy, Ovulatory dysfunction, Endometrial disorders, Iatrogenic, and Not otherwise classified). This new FIGO classification of ovulatory disorders can be viewed as a continuation of the previous initiatives and aims to further categorize the subgroup of AUB-O (AUB with ovulatory disorders). However, it is important to recognize that while most ovulatory disorders manifest with the symptoms of AUB, the absence of AUB symptoms does not necessarily preclude ovulatory disorders.
New system uses a 3-tier approach
The new FIGO classification system for ovulatory disorders has adopted a 3-tier system.
The first tier is based on the anatomic components of the hypothalamic-pituitary-ovarian (HPO) axis and is referred to with the acronym HyPO, for Hypothalamic-Pituitary-Ovarian. Recognizing that PCOS refers to a distinct spectrum of conditions that share a variable combination of signs and symptoms caused to varying degrees by different pathophysiologic mechanisms that involve inherent ovarian follicular dysfunction, neuroendocrine dysfunction, insulin resistance, and androgen excess, it is categorized in a separate class of its own in the first tier, referred to with the letter P.
Adding PCOS to the anatomical categories referred to by HyPO, the first tier is overall referred to with the acronym HyPO-P (FIGURE 2).5
The second tier of stratification provides further etiologic details for any of the primary 3 anatomic classifications of hypothalamic, pituitary, and ovarian. These etiologies are arranged in 10 distinct groups under the mnemonic GAIN-FIT-PIE, which stands for Genetic, Autoimmune, Iatrogenic, Neoplasm; Functional, Infectious/inflammatory, Trauma and vascular; and Physiological, Idiopathic, Endocrine.
The third tier of the system refers to the specific clinical diagnosis. For example, an individual with Kallmann syndrome would be categorized as having type I (hypothalamic), Genetic, Kallmann syndrome, and an individual with PCOS would be categorized simply as having type IV, PCOS.
Our understanding of the etiology of ovulatory disorders has substantially increased over the past several decades. This progress has prompted the need to develop a more comprehensive classification system for these disorders. FIGO recently published a 3-tier classification system for ovulatory disorders that can be remembered with 2 mnemonics: HyPO-P and GAIN-FIT-PIE.
It is hoped that widespread adoption of this new classification system results in better and more concise communication between clinicians, researchers, and patients, ultimately leading to continued improvement in our understanding of the pathophysiology and management of ovulatory disorders.
Continue to: Live birth rate with conventional IVF shown noninferior to that with PGT-A...
Live birth rate with conventional IVF shown noninferior to that with PGT-A
Yan J, Qin Y, Zhao H, et al. Live birth with or without preimplantation genetic testing for aneuploidy. N Engl J Med. 2021;385:2047-2058.
Preimplantation genetic testing for aneuploidy (PGT-A) is increasingly used in many in vitro fertilization (IVF) cycles in the United States. Based on data from the Centers for Disease Control and Prevention, 43.8% of embryo transfers in the United States in 2019 included at least 1 PGT-A–tested embryo.6 Despite this widespread use, however, there are still no robust clinical data for PGT-A’s efficacy and safety, and the guidelines published by the ASRM do not recommend its routine use in all IVF cycles.7 In the past 2 to 3 years, several large studies have raised questions about the reported benefit of this technology.8,9
Details of the trial
In a multicenter, controlled, noninferiority trial conducted by Yan and colleagues, 1,212 subfertile women were randomly assigned to either conventional IVF with embryo selection based on morphology or embryo selection based on PGT-A with next-generation sequencing. Inclusion criteria were the diagnosis of subfertility, undergoing their first IVF cycle, female age of 20 to 37, and the availability of 3 or more good-quality blastocysts.
On day 5 of embryo culture, patients with 3 or more blastocysts were randomly assigned in a 1:1 ratio to either the PGT-A group or conventional IVF. All embryos were then frozen, and patients subsequently underwent frozen embryo transfer of a single blastocyst, selected based on either morphology or euploid result by PGT-A. If the initial transfer did not result in a live birth, and there were remaining transferable embryos (either a euploid embryo in the PGT-A group or a morphologically transferable embryo in the conventional IVF group), patients underwent successive frozen embryo transfers until either there was a live birth or no more embryos were available for transfer.
The study’s primary outcome was the cumulative live birth rate per randomly assigned patient that resulted from up to 3 frozen embryo transfer cycles within 1 year. There were 606 patients randomly assigned to the PGT-A group and 606 randomly assigned to the conventional IVF group.
In the PGT-A group, 468 women (77.2%) had live births; in the conventional IVF group, 496 women (81.8%) had live births. Women in the PGT-A group had a lower incidence of pregnancy loss compared with the conventional IVF group: 8.7% versus 12.6% (absolute difference of -3.9%; 95% confidence interval [CI], -7.5 to -0.2). There was no difference in obstetric and neonatal outcomes between the 2 groups. The authors concluded that among women with 3 or more good-quality blastocysts, conventional IVF resulted in a cumulative live birth rate that was noninferior to that of the PGT-A group.
Some benefit shown with PGT-A
Although the study by Yan and colleagues did not show any benefit, and even a possible reduction, with regard to cumulative live birth rate for PGT-A, it did show a 4% reduction in clinical pregnancy loss when PGT-A was used. Furthermore, the study design has been criticized for performing PGT-A on only 3 blastocysts in the PGT-A group. It is quite conceivable that the PGT-A group would have had more euploid embryos available for transfer if the study design had included all the available embryos instead of only 3. On the other hand, one could argue that if the authors had extended the study to include all the available embryos, the conventional group would have also had more embryos for transfer and, therefore, more chances for pregnancy and live birth.
It is also important to recognize that only patients who had at least 3 embryos available for biopsy were included in this study, and therefore the results of this study cannot be extended to patients with fewer embryos, such as those with diminished ovarian reserve.
In summary, based on this study’s results, we may conclude that for the good-prognosis patients in the age group of 20 to 37 who have at least 3 embryos available for biopsy, PGT-A may reduce the miscarriage rate by about 4%, but this benefit comes at the expense of about a 4% reduction in the cumulative live birth rate. ●
Despite the lack of robust evidence for efficacy, safety, and cost-effectiveness, PGT-A has been widely adopted into clinical IVF practice in the United States over the past several years. A large randomized controlled trial has suggested that, compared with conventional IVF, PGT-A application may actually result in a slightly lower cumulative live birth rate, while the miscarriage rate may be slightly higher with conventional IVF.
PGT-A is a novel and evolving technology with the potential to improve embryo selection in IVF; however, at this juncture, there is not enough clinical data for its universal and routine use in all IVF cycles. PGT-A can potentially be more helpful in older women (>38–40) with good ovarian reserve who are likely to have a larger cohort of embryos to select from. Patients must clearly understand this technology’s pros and cons before agreeing to incorporate it into their care plan.
- Vollset SE, Goren E, Yuan C-W, et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396:1285-1306.
- Dao TH, Docquier F, Maurel M, et al. Global migration in the twentieth and twenty-first centuries: the unstoppable force of demography. Rev World Econ. 2021;157:417-449.
- Atlas of fertility treatment policies in Europe. December 2021. Fertility Europe. Accessed December 29, 2022. https:// fertilityeurope.eu/atlas/#:~:text=Fertility%20Europe%20 in%20conjunction%20with%20the%20European%20 Parliamentary,The%20Atlas%20describes%20the%20 current%20situation%20in%202021
- AMWA’s physician fertility initiative. June 2021. American Medical Women’s Association. Accessed December 29, 2022. https://www.amwa-doc.org/our-work/initiatives/physician -infertility/
- Munro MG, Balen AH, Cho S, et al; FIGO Committee on Menstrual Disorders and Related Health Impacts, and FIGO Committee on Reproductive Medicine, Endocrinology, and Infertility. The FIGO ovulatory disorders classification system. Fertil Steril. 2022;118:768-786.
- Centers for Disease Control and Prevention. 2019 Assisted Reproductive Technology Fertility Clinic and National Summary Report. US Dept of Health and Human Services; 2021. Accessed February 24, 2023. https://www.cdc.gov/art /reports/2019/fertility-clinic.html
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109:429-436.
- Yan J, Qin Y, Zhao H, et al. Live birth with or without preimplantation genetic testing for aneuploidy. N Engl J Med. 2021;385:2047-2058.
- Kucherov A, Fazzari M, Lieman H, et al. PGT-A is associated with reduced cumulative live birth rate in first reported IVF stimulation cycles age ≤ 40: an analysis of 133,494 autologous cycles reported to SART CORS. J Assist Reprod Genet. 2023;40:137-149.
- Vollset SE, Goren E, Yuan C-W, et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396:1285-1306.
- Dao TH, Docquier F, Maurel M, et al. Global migration in the twentieth and twenty-first centuries: the unstoppable force of demography. Rev World Econ. 2021;157:417-449.
- Atlas of fertility treatment policies in Europe. December 2021. Fertility Europe. Accessed December 29, 2022. https:// fertilityeurope.eu/atlas/#:~:text=Fertility%20Europe%20 in%20conjunction%20with%20the%20European%20 Parliamentary,The%20Atlas%20describes%20the%20 current%20situation%20in%202021
- AMWA’s physician fertility initiative. June 2021. American Medical Women’s Association. Accessed December 29, 2022. https://www.amwa-doc.org/our-work/initiatives/physician -infertility/
- Munro MG, Balen AH, Cho S, et al; FIGO Committee on Menstrual Disorders and Related Health Impacts, and FIGO Committee on Reproductive Medicine, Endocrinology, and Infertility. The FIGO ovulatory disorders classification system. Fertil Steril. 2022;118:768-786.
- Centers for Disease Control and Prevention. 2019 Assisted Reproductive Technology Fertility Clinic and National Summary Report. US Dept of Health and Human Services; 2021. Accessed February 24, 2023. https://www.cdc.gov/art /reports/2019/fertility-clinic.html
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109:429-436.
- Yan J, Qin Y, Zhao H, et al. Live birth with or without preimplantation genetic testing for aneuploidy. N Engl J Med. 2021;385:2047-2058.
- Kucherov A, Fazzari M, Lieman H, et al. PGT-A is associated with reduced cumulative live birth rate in first reported IVF stimulation cycles age ≤ 40: an analysis of 133,494 autologous cycles reported to SART CORS. J Assist Reprod Genet. 2023;40:137-149.
COMMENT & CONTROVERSY
Medical library access
During most of my clinical career I had an affiliation with a local medical school as a “Clinical Instructor” and then “Assistant Clinical Professor.” In addition to teaching medical students and residents from that institution that rotated through my hospital, it also gave me certain privileges, the most important of which was access to that institution’s electronic medical library. Using that access, even as an “LMD,” I have been able to contribute to the medical literature on subjects of interest to me and to others in my specialty.
Recently, now as an older clinician, I gave up my hospital privileges, although I continue my office practice. Giving up my hospital privileges meant that I no longer qualified as a faculty member—and therefore lost online access to the medical library. Still wishing to continue my medical writing, I have attempted to attain access to the medical literature by special request to that library, by contacting my state medical society, by contacting my national specialty organization, by contacting the department chair at the institution to which I had been affiliated, and by calling the Dean of the medical school to which my hospital was affiliated. Although meaning well, none was able to get me access to an online medical library. Thus, I am greatly hampered in my attempts to do research and to continue to write further papers on those areas in which I have previously published.
Is there no remedy for this? Should all clinicians who “age out” of institutional affiliations no longer be able to pursue research interests? And what about community physicians who have no academic affiliations? Can they not access the latest information they need to practice evidence-based, up-to-date medicine?
It makes no sense to me that access to the latest and most current aspects of medical care should be withheld from any clinician. For every clinician not to have access to such medical knowledge does a disservice to all those practicing medicine who wish to keep up to date and to all patients of American clinicians whose providers are prevented from practicing the best, evidence-based care.
Henry Lerner, MD
Boston, Massachusetts
Medical library access
During most of my clinical career I had an affiliation with a local medical school as a “Clinical Instructor” and then “Assistant Clinical Professor.” In addition to teaching medical students and residents from that institution that rotated through my hospital, it also gave me certain privileges, the most important of which was access to that institution’s electronic medical library. Using that access, even as an “LMD,” I have been able to contribute to the medical literature on subjects of interest to me and to others in my specialty.
Recently, now as an older clinician, I gave up my hospital privileges, although I continue my office practice. Giving up my hospital privileges meant that I no longer qualified as a faculty member—and therefore lost online access to the medical library. Still wishing to continue my medical writing, I have attempted to attain access to the medical literature by special request to that library, by contacting my state medical society, by contacting my national specialty organization, by contacting the department chair at the institution to which I had been affiliated, and by calling the Dean of the medical school to which my hospital was affiliated. Although meaning well, none was able to get me access to an online medical library. Thus, I am greatly hampered in my attempts to do research and to continue to write further papers on those areas in which I have previously published.
Is there no remedy for this? Should all clinicians who “age out” of institutional affiliations no longer be able to pursue research interests? And what about community physicians who have no academic affiliations? Can they not access the latest information they need to practice evidence-based, up-to-date medicine?
It makes no sense to me that access to the latest and most current aspects of medical care should be withheld from any clinician. For every clinician not to have access to such medical knowledge does a disservice to all those practicing medicine who wish to keep up to date and to all patients of American clinicians whose providers are prevented from practicing the best, evidence-based care.
Henry Lerner, MD
Boston, Massachusetts
Medical library access
During most of my clinical career I had an affiliation with a local medical school as a “Clinical Instructor” and then “Assistant Clinical Professor.” In addition to teaching medical students and residents from that institution that rotated through my hospital, it also gave me certain privileges, the most important of which was access to that institution’s electronic medical library. Using that access, even as an “LMD,” I have been able to contribute to the medical literature on subjects of interest to me and to others in my specialty.
Recently, now as an older clinician, I gave up my hospital privileges, although I continue my office practice. Giving up my hospital privileges meant that I no longer qualified as a faculty member—and therefore lost online access to the medical library. Still wishing to continue my medical writing, I have attempted to attain access to the medical literature by special request to that library, by contacting my state medical society, by contacting my national specialty organization, by contacting the department chair at the institution to which I had been affiliated, and by calling the Dean of the medical school to which my hospital was affiliated. Although meaning well, none was able to get me access to an online medical library. Thus, I am greatly hampered in my attempts to do research and to continue to write further papers on those areas in which I have previously published.
Is there no remedy for this? Should all clinicians who “age out” of institutional affiliations no longer be able to pursue research interests? And what about community physicians who have no academic affiliations? Can they not access the latest information they need to practice evidence-based, up-to-date medicine?
It makes no sense to me that access to the latest and most current aspects of medical care should be withheld from any clinician. For every clinician not to have access to such medical knowledge does a disservice to all those practicing medicine who wish to keep up to date and to all patients of American clinicians whose providers are prevented from practicing the best, evidence-based care.
Henry Lerner, MD
Boston, Massachusetts
Advances in the treatment of fetal demise in the second and third trimester
Clinical care for fetal demise is complex and multidimensional, including empathic emotional support for the patient and family members who are experiencing a tragedy, investigation of the cause of the demise, and a plan for emptying the uterus. This editorial narrowly focuses on the options for treatment of fetal demise with the goal of emptying the uterus while minimizing complications.
When planning treatment of fetal demise, focus on fetal size and gestational age
Most guidelines for the treatment of fetal demise use gestational age to guide selection of a treatment.1,2 I believe that fetal size is as important as gestational age for selecting a treatment plan. When considering treatment, there are 2 reasons why fetal size is as important as gestational age:
- The physiologic processes that caused fetal demise may have caused fetal growth restriction, resulting in a fetal size that is 2 or more weeks below expected fetal size for gestational age.
- Fetal demise may have occurred weeks before the diagnosis was made, resulting in gestational age being greater than fetal size. This editorial will use ultrasonography estimate of fetal size in gestational weeks to guide treatment recommendations. When discussing fetal size, we will use the convention of weeks-days (w-d). Twenty-five weeks and zero days gestation is represented as 25w0d.
Treatment in the second and third trimester is a 2-step process
Step 1: Cervical preparation
In most cases of first trimester fetal demise, no cervical preparation is necessary. Cervical dilation with metal dilators followed by uterine evacuation with an appropriately sized vacuum catheter is a highly successful treatment.3 However for second and third trimester fetal demise, it is best to use a 2-step process, beginning with cervical preparation followed by emptying the uterus. For example, at a fetal size of 13w0d to 16w0d, cervical preparation can be achieved by administering a single buccal dose of misoprostol 400 µg 3 to 4 hours prior to uterine evacuation or by inserting a Dilapan-S (Medicem Inc) osmotic cervical dilator 3 to 6 hours prior to uterine evacuation.4-7 At a fetal size of 16w0d to 19w6d, cervical preparation can be achieved by placing osmotic cervical dilators 4 to 6 hours before surgical evacuation and administering buccal misoprostol 400 µg 3 hours before surgical evacuation.8
Alternatively, from 16w0d to 25w0d osmotic cervical dilators can be placed on day 1 of a 2-day process, and the patient can return on day 2 to have the cervical dilators removed followed by surgical evacuation of the uterus. Mifepristone 200 mg oral dose can be administered on day 1 to facilitate cervical preparation. In my practice, I use mifepristone 200 mg on day 1 when the fetal size is ≥20w0d gestation. Options for cervical preparation include use of osmotic dilators, cervical balloons, misoprostol, and/or mifepristone. These options are discussed below. With fetal demise, natural physiologic processes often have caused sufficient cervical softening and dilation that no cervical preparation is necessary and immediate uterine surgical evacuation or induction of labor can be initiated.
Step 2: Emptying the uterus
In the second and third trimesters, the approach to uterine evacuation is based on fetal size. At fetal sizes <25w0d, options for emptying the uterus include surgical evacuation with a vacuum catheter and grasping forceps or induction of labor with misoprostol followed by vaginal birth and expulsion of the placenta. At fetal sizes ˃25w0d gestation, following completion of cervical preparation, the most common approaches to uterine evacuation are induction of labor with misoprostol or oxytocin. Rarely, with a stillbirth at term, some clinicians will select hysterotomy to empty the uterus, avoiding uterine rupture during labor induction for patients at the highest risk, including those with a prior classical cesarean birth or more than 2 prior cesarean births with a low-transverse uterine incision.
Osmotic cervical dilators
The 2 most used cervical dilators are Dilapan-S, a polyacrylate-based hydrogel rod, and laminaria, dried compressed seaweed stipe (stalk) from Laminaria japonica or Laminaria digitata. Dilapan-S rods are available in diameters of 3 mm and 4 mm and rod lengths of 55 mm and 65 mm. Laminaria dilators are available in diameters of 2, 3, 4, 5, 6, 8 and 10 mm and rod length of 60 and 70 mm. Dilapan-S dilators reach near-maximal dilation in approximately 4 to 6 hours but continue to expand over the following 18 hours to achieve a maximum dilation of 3.3 to 3.6 times their dry diameter.9 Laminaria dilators expand to 2.7 to 2.9 times their dry diameter over 24 hours.9
A general rule is that as many dilators as possible should be placed until significant resistance to the placement of additional dilators is encountered.10 In my practice, for fetal size ≥20 weeks’ gestation, I place 2 Dilapan-S rods, 4 mm in diameter, 55 mm in length, and then encircle the Dilapan-S with laminaria rods that are 4 mm in diameter and 60 mm in length. Once cervical resistance to the placement of the 4 mm laminaria rods is observed, I encircle those laminaria with laminaria 2 mm in diameter, filling in the interstices between the 4 mm laminaria. The next day, cervical dilation is routinely ≥3 cm.
In a retrospective study of 491 patients undergoing pregnancy termination after 14 weeks’ gestation, with a mean gestational age of 24 weeks, compared with no osmotic cervical dilators, inserting osmotic cervical dilators the day before initiating misoprostol for induction of labor resulted in a decrease in time to delivery (428 min vs 640 min; P<.001) and a decrease in total misoprostol dose (990 µg vs 1,449 µg; P<.0001).11
Cervical balloons
All clinicians know that a Foley catheter or a Cook cervical ripening balloon can be used for cervical preparation in the third trimester.12,13 The Foley catheter also has been reported to be useful for cervical preparation in the second trimester. In one study of 43 patients 17 to 24 weeks’ gestation scheduled for a second-trimester dilation and evacuation, an intracervical Foley catheter was placed the evening before evacuation, and the balloon was inflated with 30 mL to 50 mL of saline. At the same time, mifepristone 200 mg was administered to the patients.14 The following day, dilation and evacuation was performed. In 72% of cases no additional cervical dilation was required on the day of evacuation. The investigators concluded that if osmotic cervical dilators are not available, the placement of an intracervical Foley catheter plus administration of mifepristone facilitates performance of an evacuation on the following day. If the patient prefers a 1-day procedure, the Foley can be inserted in the morning to facilitate cervical preparation, and the uterus can be evacuated in the afternoon.
Continue to: Misoprostol...
Misoprostol
Misoprostol, a derivative of prostaglandin E1, is useful for both cervical preparation and induction of labor. The dose of misoprostol and the route of administration are major determinants of uterine response.15-19 When administered by an oral route, misoprostol has fast onset and offset of action and often does not cause sustained uterine contractions. Hence, oral misoprostol, at a low dose is useful for cervical ripening, but not as useful for stimulation of sustained uterine contractions for induction of labor. When administered by a buccal or vaginal route, misoprostol has prolonged activity and often results in sustained uterine contractions. At any given dose of misoprostol, buccal and vaginal misoprostol administration are more effective than oral administration in inducing sustained uterine contractions sufficient to empty the uterus.15-19
Mifepristone
Mifepristone, an anti-progestin, is useful for cervical preparation and sensitizing myocytes to the action of uterotonics. Progesterone reduces cell-to-cell communication among uterine myocytes, facilitating uterine quiescence by suppressing connexin 43 and other proteins. Mifepristone blocks the effect of progesterone, inducing the production of myocyte connexin 43, enhancing efficient cell-to-cell communication, permitting uterine myoctes to contract in unison, creating the potential for powerful and sustained contractions.20-23 Randomized clinical trials report that administration of mifepristone 200 mg prior to misoprostol induced labor results in more rapid emptying of the uterus.24-27
It takes time for mifepristone to have its full effect on uterine myocytes. Hence, most protocols recommend waiting 24 hours following mifepristone administration before initiating treatment with an agent to stimulate uterine contractions such as misoprostol or oxytocin. However, preliminary data suggest that partial benefit of mifepristone can be obtained when initiating misoprostol 3 to 5 hours after mifepristone administration.28 In a study of 481 patients undergoing induction of labor in the second or third trimester, the time from initiation of misoprostol to vaginal birth was 15 hours with no mifepristone pretreatment, 13.2 hours if mifepristone was administered 3 to 5 hours before initiating misoprostol, 9.3 hours if mifepristone was administered 24 hours before initiating misoprostol, and 10.5 hours if mifepristone was administered 48 hours before initiating misoprostol.28
Fetal size <25w0d gestation: Cervical preparation and surgical evacuation
For fetal demise at a fetal size less than 25w0d, if clinical experts are available, the best treatment option is cervical preparation followed by surgical evacuation of the uterus using a vacuum catheter and grasping forceps to empty the uterus.29,30 A disadvantage of surgical evacuation of the uterus is that an intact fetus is not available for the patient to hold and mourn, and pathologic examination of an intact fetus is not possible. An alternative approach is cervical preparation followed by induction of labor using misoprostol with the goal of delivering an intact fetus. Although no prospective clinical trials are available comparing these 2 options, retrospective studies have reported that, at fetal size <25w0d gestation, compared with induction of labor, surgical evacuation of the uterus results in fewer complications,30 including fewer cases of retained placenta requiring an unplanned procedure and fewer presumed uterine infections.29
For surgical evacuation of fetal demise with a fetal size of <25w0d gestation, the first step on day 1 is placement of osmotic cervical dilators. In addition to osmotic cervical dilators, if the gestational age or fetal size is ≥19 weeks’ gestation an oral dose of mifepristone 200 mg to facilitate cervical preparation may be considered. On day 2, the osmotic dilators are removed and surgical evacuation is performed. In one randomized study, for pregnancies at 19 to 24 weeks’ gestation, compared with osmotic dilators alone, administration of mifepristone 200 mg at the time of placement of osmotic dilators resulted in fewer procedures that were difficult to complete.31 In some cases, 2 consecutive days of cervical preparation with osmotic dilators may be needed to properly prepare the cervix for uterine evacuation. For example, the cervix of a nulliparous teenage patient may require 2 days of cervical preparation with osmotic dilators to facilitate uterine evacuation. In some cases of fetal demise, the cervix is already dilated to ≥3 cm and surgical evacuation of the uterus or induction of labor can be initiated without the need for cervical preparation.
Continue to: Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor...
Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor
Treatment of fetal demise at 14w0d to 28w6d gestation with the goal of the vaginal birth of an intact fetus is optimized by the administration of mifepristone for cervical preparation followed by induction of labor with misoprostol.26,27
In one clinical trial, 66 patients with fetal demise between 14w0d and 28w6d gestation were randomly assigned to receive mifepristone 200 mg or placebo followed 24 to 48 hours later with initiation of misoprostol induction of labor.26 Among the patients from 14w0d to 24 weeks’ gestation, the misoprostol dose was 400 µg vaginally every 6 hours. For patients from 24w0d to 28 weeks’ gestation, the misoprostol dose was 200 µg vaginally every 4 hours. At 24 hours, a consultant obstetrician determined if additional misoprostol should be given. The median time from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups was 6.8 hours and 10.5 hours (P=.002).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required fewer doses of misoprostol (2.1 vs 3.4; P= .002) and a lower total dose of misoprostol (768 µg vs 1,182 µg; P=.003). All patients in the mifepristone group delivered within 24 hours. By contrast, 13% of the patients in the placebo group delivered more than 24 hours after the initiation of misoprostol treatment. Five patients were readmitted with retained products of conception needing suction curettage, 4 in the placebo group and 1 in the mifepristone group.26
In a second clinical trial, 105 patients with fetal demise after 20 weeks of gestation were randomly assigned to receive mifepristone 200 mg or placebo.27 In this study, 86% of the patients were ≥26w0d gestation, with a mean gestational age of approximately 32w2d. Thirty-six to 48 hours later, misoprostol induction of labor was initiated. Among the patients from 20 to 25 completed weeks of gestation, the misoprostoldose was 100 µg vaginally every 6 hours for a maximum of 4 doses. For patients from ≥26 weeks’ gestation, the misoprostol dose was 50 µg vaginally every 4 hours for a maximum of 6 doses. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 9.8 hours and 16.3 hours, respectively (P=.001). Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required a lower total dose of misoprostol (110 µg vs 198 µg; P<.001). Delivery within 24 hours following initiation of misoprostol occurred in 93% and 73% of the patients in the mifepristone and placebo groups, respectively (P<.001). Compared with patients in the mifepristone group, shivering occurred more frequently among patients in the placebo group (7.5% vs 19.2%; P=.09), likely because they received greater doses of misoprostol.27
Fetal size ≥29w0d gestation
At a fetal size ≥29w0d gestation, if the cervix is ripe with a Bishop score of ≥7, oxytocin induction of labor is often used as a first-line treatment. If the cervix is not ripe, misoprostol induction of labor may be considered at doses less than those used in the second trimester of pregnancy.32 TABLES 1,1, 26, 33–36 2,37 and 337 summarize regimens proposed for fetal size ≥29w0d. One regimen begins with an initial misoprostol dose of 50 µg. If adequate uterine contractions occur, the 50 µg dose is repeated every 4 hours up to 6 total doses. If contractions are inadequate, the dose can be increased to 100 µg every 4 hours for 5 additional doses.
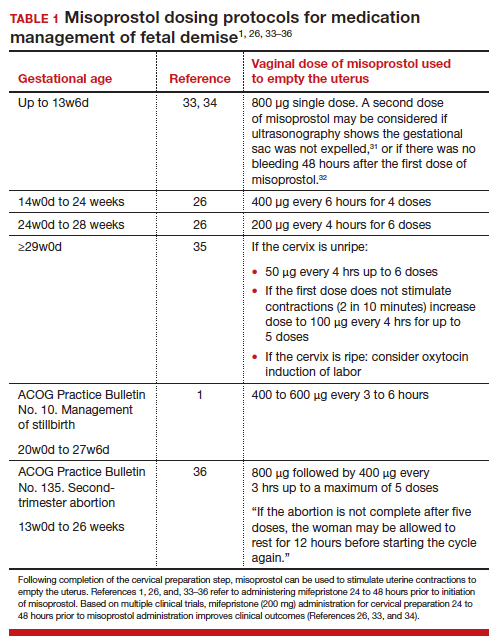

For fetal demise after 28w0d gestation, the
A multidisciplinary approach can optimize compassionate care
There are many gaps in the holistic care of patients and partners experiencing fetal demise. Patients with fetal demise often report that they did not receive sufficient information about the cause of the demise and wanted more opportunity to be involved in decision making about their care.39 The patient’s partner often reports feeling unacknowledged as a grieving parent.40 Fetal demise is experienced by many patients as a tragedy, triggering feelings of grief, anger, denial, anxiety and depression, sometimes resulting in isolation and substance misuse.
Using a 5-round Delphi process, experts identified 8 core goals in the care of patients with fetal demise:
- reduce stigma
- provide respectful care
- involve patients in care planning
- attempt to provide an explanation for the demise1
- acknowledge the depth of the grief response and provide emotional support
- offer information about ongoing psychological support
- provide information about future pregnancy planning
- provide opportunities for specialized training and support for care providers.41
Management of stillbirth is optimized by a multidisciplinary approach that includes the expert care of obstetrician-gynecologists, obstetric nurses, anesthesiologists, and expert consultation from social work, chaplaincy, and pathology. A heart-to-heart connection between clinician and patient is a key component of stillbirth care. ●
- American College of Obstetricians and Gynecologists. Management of stillbirth. ACOG Obstetric Care Consensus. No. 10. Obstet Gynecol. 2020;135:e110-132.
- Tsakiridis I, Giouleka S, Mamopoulos A, et al. Investigation and management of stillbirths: a descriptive review of major guidelines. J Perinat Med. 2022;50:796-813.
- Spingler T, Sonek J, Hoopman M, et al. Complication rate after termination of pregnancy due to fetal defects. Ultrasound Obstet Gynecol. 2023;Epub January 7.
- Goldberg AB, Drey EA, Whitaker AK, et al. Misoprostol compared with laminaria before early second-trimester surgical abortion: a randomized trial. Obstet Gynecol. 2005;106:234-241.
- Meirik O, My Huong NT, Piaggio G, et al. WHOR-GoP-MoF Regulation. Complications of first trimester abortion by vacuum aspiration after cervical preparation with and without misoprostol: a multicentre randomised trial. Lancet. 2012;379(9829):1817-1824.
- Bartz D, Maurer R, Allen RH, et al. Buccal misoprostol compared with synthetic osmotic cervical dilator before surgical abortion: a randomized controlled trial. Obstet Gynecol. 2013;122:57-63.
- Ngo LL, Mokashi M, Janiak E, et al. Acute complications with same-day versus overnight cervical preparation before dilation and evacuation at 14 to 16 weeks. Contraception. 2023;117:61-66.
- Kim CS, Dragoman M, Prosch L, et al. Same-day compared with overnight cervical preparation before dilation and evacuation between 16 and 19 6/7 weeks of gestation: a randomized controlled trial. Obstet Gynecol. 2022;139:1141-1144.
- Drunecky T, Reidingerova M, Plisova M, et al. Experimental comparison of properties of natural and synthetic osmotic dilators. Arch Gynecol Obstet. 2015;292:349-354.
- Hern WM. Laminaria versus Dilapan osmotic cervical dilators for outpatient dilation and evacuation abortion: randomized cohort comparison of 1001 patients. Am J Obstet Gynecol. 1994;171:1324-1328.
- Berthold C, Gomes David M, Gabriel P, et al. Effect of the addition of osmotic dilators to medical induction of labor abortion: a before-and-after study. Eur J Obstet Gynecol. 2020;244:185-189.
- Kemper JI, Li W, Goni S, et al. Foley catheter vs oral misoprostol for induction of labor: individual participant data meta-analysis. Ultrasound Obstet Gynecol. 2021;57:215-223.
- Attalli E, Kern Guy, Fouks Y, et al. Labor induction in third trimester non-viable fetus. J Matern Fetal Neonatal Med. 2022;Epub October 1.
- Fessehaye Sium A, Prager S, Wolderufael M, et al. Foley catheter for cervical preparation prior to second trimester dilation and evacuation: a supply-based alternative for surgical abortion: a case series. Contracept X. 2022;4:100085.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590.
- Barbieri RL. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022;34:8-10, 12.
- Andersen J, Grine E, Eng L, et al. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266-1276.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398-5407.
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284-290.
- Barbieri RL. Mifepristone for the treatment of miscarriage and fetal demise. OBG Manag. 2022;34:811, 15.
- Kapp N, Borgatta L, Stubblefield P, et al. Mifepristone in second-trimester medical abortion. Obstet Gynecol. 2007;110:1304-1310.
- Ngoc NTN, Shochet T, Raghavan S, et al. Mifepristone and misoprostol compared with misoprostol alone for second trimester abortion: a randomized controlled trial. Obstet Gynecol. 2011;118:601608.
- Allanson ER, Copson S, Spilsbury K, et al. Pretreatment with mifepristone compared with misoprostol alone for delivery after fetal death between 14 and 28 weeks of gestation. Obstet Gynecol. 2021;137:801-809.
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. J Obstet Gynaecol Res. 2015;41:1884-1890.
- Prodan N, Breisch J, Hoopman M, et al. Dosing interval between mifepristone and misoprostol in second and third trimester termination. Arch Gynecol Obstet. 2019;299:675-679.
- Edlow AG, Hou MY, Maurer R, et al. Uterine evacuation for second trimester fetal death and maternal morbidity. Obstet Gynecol. 2011;117:1-10.
- Bryan AG, Grimes DA, Garrett JM, et al. Second-trimester abortion for fetal anomalies or fetal death. Obstet Gynecol. 2011;117:788-792.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol or mifepristone compared with overnight osmotic dilators alone. Obstet Gynecol. 2015;126:599-609.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- American College of Obstetricians and Gynecologists. Second-trimester abortion. Practice Bulletin No. 135. Obstet Gynecol. 2013;121:1394-1406.
- Wingo E, Raifman S, Landau C, et al. Mifepristone-misoprostol versus misoprostol-alone regimen for medication abortion at ≥ 24 weeks gestation. Contraception. Appendix 1. 2020;102:99-103.
- American College of Obstetricians and Gynecologists. Vaginal birth after cesarean delivery. ACOG Practice Bulletin No. 205. Obstet Gynecol. 2019;133:e110-e127.
- Atkins B, Blencowe H, Boyle FM, et al. Is care of stillborn babies and their parents respectful? Results from an international online survey. BJOG. 2022;129:1731-1739.
- Haezell AEP, Siassakos D, Blencowe H, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387(10018):604-616.
- Shakespeare C, Merriel A, Bakhbakhi D, et al. The RESPECT Study for consensus on global bereavement care after stillbirth. Int J Gynaecol Obstet. 2020;149:137-147.
Clinical care for fetal demise is complex and multidimensional, including empathic emotional support for the patient and family members who are experiencing a tragedy, investigation of the cause of the demise, and a plan for emptying the uterus. This editorial narrowly focuses on the options for treatment of fetal demise with the goal of emptying the uterus while minimizing complications.
When planning treatment of fetal demise, focus on fetal size and gestational age
Most guidelines for the treatment of fetal demise use gestational age to guide selection of a treatment.1,2 I believe that fetal size is as important as gestational age for selecting a treatment plan. When considering treatment, there are 2 reasons why fetal size is as important as gestational age:
- The physiologic processes that caused fetal demise may have caused fetal growth restriction, resulting in a fetal size that is 2 or more weeks below expected fetal size for gestational age.
- Fetal demise may have occurred weeks before the diagnosis was made, resulting in gestational age being greater than fetal size. This editorial will use ultrasonography estimate of fetal size in gestational weeks to guide treatment recommendations. When discussing fetal size, we will use the convention of weeks-days (w-d). Twenty-five weeks and zero days gestation is represented as 25w0d.
Treatment in the second and third trimester is a 2-step process
Step 1: Cervical preparation
In most cases of first trimester fetal demise, no cervical preparation is necessary. Cervical dilation with metal dilators followed by uterine evacuation with an appropriately sized vacuum catheter is a highly successful treatment.3 However for second and third trimester fetal demise, it is best to use a 2-step process, beginning with cervical preparation followed by emptying the uterus. For example, at a fetal size of 13w0d to 16w0d, cervical preparation can be achieved by administering a single buccal dose of misoprostol 400 µg 3 to 4 hours prior to uterine evacuation or by inserting a Dilapan-S (Medicem Inc) osmotic cervical dilator 3 to 6 hours prior to uterine evacuation.4-7 At a fetal size of 16w0d to 19w6d, cervical preparation can be achieved by placing osmotic cervical dilators 4 to 6 hours before surgical evacuation and administering buccal misoprostol 400 µg 3 hours before surgical evacuation.8
Alternatively, from 16w0d to 25w0d osmotic cervical dilators can be placed on day 1 of a 2-day process, and the patient can return on day 2 to have the cervical dilators removed followed by surgical evacuation of the uterus. Mifepristone 200 mg oral dose can be administered on day 1 to facilitate cervical preparation. In my practice, I use mifepristone 200 mg on day 1 when the fetal size is ≥20w0d gestation. Options for cervical preparation include use of osmotic dilators, cervical balloons, misoprostol, and/or mifepristone. These options are discussed below. With fetal demise, natural physiologic processes often have caused sufficient cervical softening and dilation that no cervical preparation is necessary and immediate uterine surgical evacuation or induction of labor can be initiated.
Step 2: Emptying the uterus
In the second and third trimesters, the approach to uterine evacuation is based on fetal size. At fetal sizes <25w0d, options for emptying the uterus include surgical evacuation with a vacuum catheter and grasping forceps or induction of labor with misoprostol followed by vaginal birth and expulsion of the placenta. At fetal sizes ˃25w0d gestation, following completion of cervical preparation, the most common approaches to uterine evacuation are induction of labor with misoprostol or oxytocin. Rarely, with a stillbirth at term, some clinicians will select hysterotomy to empty the uterus, avoiding uterine rupture during labor induction for patients at the highest risk, including those with a prior classical cesarean birth or more than 2 prior cesarean births with a low-transverse uterine incision.
Osmotic cervical dilators
The 2 most used cervical dilators are Dilapan-S, a polyacrylate-based hydrogel rod, and laminaria, dried compressed seaweed stipe (stalk) from Laminaria japonica or Laminaria digitata. Dilapan-S rods are available in diameters of 3 mm and 4 mm and rod lengths of 55 mm and 65 mm. Laminaria dilators are available in diameters of 2, 3, 4, 5, 6, 8 and 10 mm and rod length of 60 and 70 mm. Dilapan-S dilators reach near-maximal dilation in approximately 4 to 6 hours but continue to expand over the following 18 hours to achieve a maximum dilation of 3.3 to 3.6 times their dry diameter.9 Laminaria dilators expand to 2.7 to 2.9 times their dry diameter over 24 hours.9
A general rule is that as many dilators as possible should be placed until significant resistance to the placement of additional dilators is encountered.10 In my practice, for fetal size ≥20 weeks’ gestation, I place 2 Dilapan-S rods, 4 mm in diameter, 55 mm in length, and then encircle the Dilapan-S with laminaria rods that are 4 mm in diameter and 60 mm in length. Once cervical resistance to the placement of the 4 mm laminaria rods is observed, I encircle those laminaria with laminaria 2 mm in diameter, filling in the interstices between the 4 mm laminaria. The next day, cervical dilation is routinely ≥3 cm.
In a retrospective study of 491 patients undergoing pregnancy termination after 14 weeks’ gestation, with a mean gestational age of 24 weeks, compared with no osmotic cervical dilators, inserting osmotic cervical dilators the day before initiating misoprostol for induction of labor resulted in a decrease in time to delivery (428 min vs 640 min; P<.001) and a decrease in total misoprostol dose (990 µg vs 1,449 µg; P<.0001).11
Cervical balloons
All clinicians know that a Foley catheter or a Cook cervical ripening balloon can be used for cervical preparation in the third trimester.12,13 The Foley catheter also has been reported to be useful for cervical preparation in the second trimester. In one study of 43 patients 17 to 24 weeks’ gestation scheduled for a second-trimester dilation and evacuation, an intracervical Foley catheter was placed the evening before evacuation, and the balloon was inflated with 30 mL to 50 mL of saline. At the same time, mifepristone 200 mg was administered to the patients.14 The following day, dilation and evacuation was performed. In 72% of cases no additional cervical dilation was required on the day of evacuation. The investigators concluded that if osmotic cervical dilators are not available, the placement of an intracervical Foley catheter plus administration of mifepristone facilitates performance of an evacuation on the following day. If the patient prefers a 1-day procedure, the Foley can be inserted in the morning to facilitate cervical preparation, and the uterus can be evacuated in the afternoon.
Continue to: Misoprostol...
Misoprostol
Misoprostol, a derivative of prostaglandin E1, is useful for both cervical preparation and induction of labor. The dose of misoprostol and the route of administration are major determinants of uterine response.15-19 When administered by an oral route, misoprostol has fast onset and offset of action and often does not cause sustained uterine contractions. Hence, oral misoprostol, at a low dose is useful for cervical ripening, but not as useful for stimulation of sustained uterine contractions for induction of labor. When administered by a buccal or vaginal route, misoprostol has prolonged activity and often results in sustained uterine contractions. At any given dose of misoprostol, buccal and vaginal misoprostol administration are more effective than oral administration in inducing sustained uterine contractions sufficient to empty the uterus.15-19
Mifepristone
Mifepristone, an anti-progestin, is useful for cervical preparation and sensitizing myocytes to the action of uterotonics. Progesterone reduces cell-to-cell communication among uterine myocytes, facilitating uterine quiescence by suppressing connexin 43 and other proteins. Mifepristone blocks the effect of progesterone, inducing the production of myocyte connexin 43, enhancing efficient cell-to-cell communication, permitting uterine myoctes to contract in unison, creating the potential for powerful and sustained contractions.20-23 Randomized clinical trials report that administration of mifepristone 200 mg prior to misoprostol induced labor results in more rapid emptying of the uterus.24-27
It takes time for mifepristone to have its full effect on uterine myocytes. Hence, most protocols recommend waiting 24 hours following mifepristone administration before initiating treatment with an agent to stimulate uterine contractions such as misoprostol or oxytocin. However, preliminary data suggest that partial benefit of mifepristone can be obtained when initiating misoprostol 3 to 5 hours after mifepristone administration.28 In a study of 481 patients undergoing induction of labor in the second or third trimester, the time from initiation of misoprostol to vaginal birth was 15 hours with no mifepristone pretreatment, 13.2 hours if mifepristone was administered 3 to 5 hours before initiating misoprostol, 9.3 hours if mifepristone was administered 24 hours before initiating misoprostol, and 10.5 hours if mifepristone was administered 48 hours before initiating misoprostol.28
Fetal size <25w0d gestation: Cervical preparation and surgical evacuation
For fetal demise at a fetal size less than 25w0d, if clinical experts are available, the best treatment option is cervical preparation followed by surgical evacuation of the uterus using a vacuum catheter and grasping forceps to empty the uterus.29,30 A disadvantage of surgical evacuation of the uterus is that an intact fetus is not available for the patient to hold and mourn, and pathologic examination of an intact fetus is not possible. An alternative approach is cervical preparation followed by induction of labor using misoprostol with the goal of delivering an intact fetus. Although no prospective clinical trials are available comparing these 2 options, retrospective studies have reported that, at fetal size <25w0d gestation, compared with induction of labor, surgical evacuation of the uterus results in fewer complications,30 including fewer cases of retained placenta requiring an unplanned procedure and fewer presumed uterine infections.29
For surgical evacuation of fetal demise with a fetal size of <25w0d gestation, the first step on day 1 is placement of osmotic cervical dilators. In addition to osmotic cervical dilators, if the gestational age or fetal size is ≥19 weeks’ gestation an oral dose of mifepristone 200 mg to facilitate cervical preparation may be considered. On day 2, the osmotic dilators are removed and surgical evacuation is performed. In one randomized study, for pregnancies at 19 to 24 weeks’ gestation, compared with osmotic dilators alone, administration of mifepristone 200 mg at the time of placement of osmotic dilators resulted in fewer procedures that were difficult to complete.31 In some cases, 2 consecutive days of cervical preparation with osmotic dilators may be needed to properly prepare the cervix for uterine evacuation. For example, the cervix of a nulliparous teenage patient may require 2 days of cervical preparation with osmotic dilators to facilitate uterine evacuation. In some cases of fetal demise, the cervix is already dilated to ≥3 cm and surgical evacuation of the uterus or induction of labor can be initiated without the need for cervical preparation.
Continue to: Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor...
Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor
Treatment of fetal demise at 14w0d to 28w6d gestation with the goal of the vaginal birth of an intact fetus is optimized by the administration of mifepristone for cervical preparation followed by induction of labor with misoprostol.26,27
In one clinical trial, 66 patients with fetal demise between 14w0d and 28w6d gestation were randomly assigned to receive mifepristone 200 mg or placebo followed 24 to 48 hours later with initiation of misoprostol induction of labor.26 Among the patients from 14w0d to 24 weeks’ gestation, the misoprostol dose was 400 µg vaginally every 6 hours. For patients from 24w0d to 28 weeks’ gestation, the misoprostol dose was 200 µg vaginally every 4 hours. At 24 hours, a consultant obstetrician determined if additional misoprostol should be given. The median time from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups was 6.8 hours and 10.5 hours (P=.002).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required fewer doses of misoprostol (2.1 vs 3.4; P= .002) and a lower total dose of misoprostol (768 µg vs 1,182 µg; P=.003). All patients in the mifepristone group delivered within 24 hours. By contrast, 13% of the patients in the placebo group delivered more than 24 hours after the initiation of misoprostol treatment. Five patients were readmitted with retained products of conception needing suction curettage, 4 in the placebo group and 1 in the mifepristone group.26
In a second clinical trial, 105 patients with fetal demise after 20 weeks of gestation were randomly assigned to receive mifepristone 200 mg or placebo.27 In this study, 86% of the patients were ≥26w0d gestation, with a mean gestational age of approximately 32w2d. Thirty-six to 48 hours later, misoprostol induction of labor was initiated. Among the patients from 20 to 25 completed weeks of gestation, the misoprostoldose was 100 µg vaginally every 6 hours for a maximum of 4 doses. For patients from ≥26 weeks’ gestation, the misoprostol dose was 50 µg vaginally every 4 hours for a maximum of 6 doses. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 9.8 hours and 16.3 hours, respectively (P=.001). Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required a lower total dose of misoprostol (110 µg vs 198 µg; P<.001). Delivery within 24 hours following initiation of misoprostol occurred in 93% and 73% of the patients in the mifepristone and placebo groups, respectively (P<.001). Compared with patients in the mifepristone group, shivering occurred more frequently among patients in the placebo group (7.5% vs 19.2%; P=.09), likely because they received greater doses of misoprostol.27
Fetal size ≥29w0d gestation
At a fetal size ≥29w0d gestation, if the cervix is ripe with a Bishop score of ≥7, oxytocin induction of labor is often used as a first-line treatment. If the cervix is not ripe, misoprostol induction of labor may be considered at doses less than those used in the second trimester of pregnancy.32 TABLES 1,1, 26, 33–36 2,37 and 337 summarize regimens proposed for fetal size ≥29w0d. One regimen begins with an initial misoprostol dose of 50 µg. If adequate uterine contractions occur, the 50 µg dose is repeated every 4 hours up to 6 total doses. If contractions are inadequate, the dose can be increased to 100 µg every 4 hours for 5 additional doses.



For fetal demise after 28w0d gestation, the
A multidisciplinary approach can optimize compassionate care
There are many gaps in the holistic care of patients and partners experiencing fetal demise. Patients with fetal demise often report that they did not receive sufficient information about the cause of the demise and wanted more opportunity to be involved in decision making about their care.39 The patient’s partner often reports feeling unacknowledged as a grieving parent.40 Fetal demise is experienced by many patients as a tragedy, triggering feelings of grief, anger, denial, anxiety and depression, sometimes resulting in isolation and substance misuse.
Using a 5-round Delphi process, experts identified 8 core goals in the care of patients with fetal demise:
- reduce stigma
- provide respectful care
- involve patients in care planning
- attempt to provide an explanation for the demise1
- acknowledge the depth of the grief response and provide emotional support
- offer information about ongoing psychological support
- provide information about future pregnancy planning
- provide opportunities for specialized training and support for care providers.41
Management of stillbirth is optimized by a multidisciplinary approach that includes the expert care of obstetrician-gynecologists, obstetric nurses, anesthesiologists, and expert consultation from social work, chaplaincy, and pathology. A heart-to-heart connection between clinician and patient is a key component of stillbirth care. ●
Clinical care for fetal demise is complex and multidimensional, including empathic emotional support for the patient and family members who are experiencing a tragedy, investigation of the cause of the demise, and a plan for emptying the uterus. This editorial narrowly focuses on the options for treatment of fetal demise with the goal of emptying the uterus while minimizing complications.
When planning treatment of fetal demise, focus on fetal size and gestational age
Most guidelines for the treatment of fetal demise use gestational age to guide selection of a treatment.1,2 I believe that fetal size is as important as gestational age for selecting a treatment plan. When considering treatment, there are 2 reasons why fetal size is as important as gestational age:
- The physiologic processes that caused fetal demise may have caused fetal growth restriction, resulting in a fetal size that is 2 or more weeks below expected fetal size for gestational age.
- Fetal demise may have occurred weeks before the diagnosis was made, resulting in gestational age being greater than fetal size. This editorial will use ultrasonography estimate of fetal size in gestational weeks to guide treatment recommendations. When discussing fetal size, we will use the convention of weeks-days (w-d). Twenty-five weeks and zero days gestation is represented as 25w0d.
Treatment in the second and third trimester is a 2-step process
Step 1: Cervical preparation
In most cases of first trimester fetal demise, no cervical preparation is necessary. Cervical dilation with metal dilators followed by uterine evacuation with an appropriately sized vacuum catheter is a highly successful treatment.3 However for second and third trimester fetal demise, it is best to use a 2-step process, beginning with cervical preparation followed by emptying the uterus. For example, at a fetal size of 13w0d to 16w0d, cervical preparation can be achieved by administering a single buccal dose of misoprostol 400 µg 3 to 4 hours prior to uterine evacuation or by inserting a Dilapan-S (Medicem Inc) osmotic cervical dilator 3 to 6 hours prior to uterine evacuation.4-7 At a fetal size of 16w0d to 19w6d, cervical preparation can be achieved by placing osmotic cervical dilators 4 to 6 hours before surgical evacuation and administering buccal misoprostol 400 µg 3 hours before surgical evacuation.8
Alternatively, from 16w0d to 25w0d osmotic cervical dilators can be placed on day 1 of a 2-day process, and the patient can return on day 2 to have the cervical dilators removed followed by surgical evacuation of the uterus. Mifepristone 200 mg oral dose can be administered on day 1 to facilitate cervical preparation. In my practice, I use mifepristone 200 mg on day 1 when the fetal size is ≥20w0d gestation. Options for cervical preparation include use of osmotic dilators, cervical balloons, misoprostol, and/or mifepristone. These options are discussed below. With fetal demise, natural physiologic processes often have caused sufficient cervical softening and dilation that no cervical preparation is necessary and immediate uterine surgical evacuation or induction of labor can be initiated.
Step 2: Emptying the uterus
In the second and third trimesters, the approach to uterine evacuation is based on fetal size. At fetal sizes <25w0d, options for emptying the uterus include surgical evacuation with a vacuum catheter and grasping forceps or induction of labor with misoprostol followed by vaginal birth and expulsion of the placenta. At fetal sizes ˃25w0d gestation, following completion of cervical preparation, the most common approaches to uterine evacuation are induction of labor with misoprostol or oxytocin. Rarely, with a stillbirth at term, some clinicians will select hysterotomy to empty the uterus, avoiding uterine rupture during labor induction for patients at the highest risk, including those with a prior classical cesarean birth or more than 2 prior cesarean births with a low-transverse uterine incision.
Osmotic cervical dilators
The 2 most used cervical dilators are Dilapan-S, a polyacrylate-based hydrogel rod, and laminaria, dried compressed seaweed stipe (stalk) from Laminaria japonica or Laminaria digitata. Dilapan-S rods are available in diameters of 3 mm and 4 mm and rod lengths of 55 mm and 65 mm. Laminaria dilators are available in diameters of 2, 3, 4, 5, 6, 8 and 10 mm and rod length of 60 and 70 mm. Dilapan-S dilators reach near-maximal dilation in approximately 4 to 6 hours but continue to expand over the following 18 hours to achieve a maximum dilation of 3.3 to 3.6 times their dry diameter.9 Laminaria dilators expand to 2.7 to 2.9 times their dry diameter over 24 hours.9
A general rule is that as many dilators as possible should be placed until significant resistance to the placement of additional dilators is encountered.10 In my practice, for fetal size ≥20 weeks’ gestation, I place 2 Dilapan-S rods, 4 mm in diameter, 55 mm in length, and then encircle the Dilapan-S with laminaria rods that are 4 mm in diameter and 60 mm in length. Once cervical resistance to the placement of the 4 mm laminaria rods is observed, I encircle those laminaria with laminaria 2 mm in diameter, filling in the interstices between the 4 mm laminaria. The next day, cervical dilation is routinely ≥3 cm.
In a retrospective study of 491 patients undergoing pregnancy termination after 14 weeks’ gestation, with a mean gestational age of 24 weeks, compared with no osmotic cervical dilators, inserting osmotic cervical dilators the day before initiating misoprostol for induction of labor resulted in a decrease in time to delivery (428 min vs 640 min; P<.001) and a decrease in total misoprostol dose (990 µg vs 1,449 µg; P<.0001).11
Cervical balloons
All clinicians know that a Foley catheter or a Cook cervical ripening balloon can be used for cervical preparation in the third trimester.12,13 The Foley catheter also has been reported to be useful for cervical preparation in the second trimester. In one study of 43 patients 17 to 24 weeks’ gestation scheduled for a second-trimester dilation and evacuation, an intracervical Foley catheter was placed the evening before evacuation, and the balloon was inflated with 30 mL to 50 mL of saline. At the same time, mifepristone 200 mg was administered to the patients.14 The following day, dilation and evacuation was performed. In 72% of cases no additional cervical dilation was required on the day of evacuation. The investigators concluded that if osmotic cervical dilators are not available, the placement of an intracervical Foley catheter plus administration of mifepristone facilitates performance of an evacuation on the following day. If the patient prefers a 1-day procedure, the Foley can be inserted in the morning to facilitate cervical preparation, and the uterus can be evacuated in the afternoon.
Continue to: Misoprostol...
Misoprostol
Misoprostol, a derivative of prostaglandin E1, is useful for both cervical preparation and induction of labor. The dose of misoprostol and the route of administration are major determinants of uterine response.15-19 When administered by an oral route, misoprostol has fast onset and offset of action and often does not cause sustained uterine contractions. Hence, oral misoprostol, at a low dose is useful for cervical ripening, but not as useful for stimulation of sustained uterine contractions for induction of labor. When administered by a buccal or vaginal route, misoprostol has prolonged activity and often results in sustained uterine contractions. At any given dose of misoprostol, buccal and vaginal misoprostol administration are more effective than oral administration in inducing sustained uterine contractions sufficient to empty the uterus.15-19
Mifepristone
Mifepristone, an anti-progestin, is useful for cervical preparation and sensitizing myocytes to the action of uterotonics. Progesterone reduces cell-to-cell communication among uterine myocytes, facilitating uterine quiescence by suppressing connexin 43 and other proteins. Mifepristone blocks the effect of progesterone, inducing the production of myocyte connexin 43, enhancing efficient cell-to-cell communication, permitting uterine myoctes to contract in unison, creating the potential for powerful and sustained contractions.20-23 Randomized clinical trials report that administration of mifepristone 200 mg prior to misoprostol induced labor results in more rapid emptying of the uterus.24-27
It takes time for mifepristone to have its full effect on uterine myocytes. Hence, most protocols recommend waiting 24 hours following mifepristone administration before initiating treatment with an agent to stimulate uterine contractions such as misoprostol or oxytocin. However, preliminary data suggest that partial benefit of mifepristone can be obtained when initiating misoprostol 3 to 5 hours after mifepristone administration.28 In a study of 481 patients undergoing induction of labor in the second or third trimester, the time from initiation of misoprostol to vaginal birth was 15 hours with no mifepristone pretreatment, 13.2 hours if mifepristone was administered 3 to 5 hours before initiating misoprostol, 9.3 hours if mifepristone was administered 24 hours before initiating misoprostol, and 10.5 hours if mifepristone was administered 48 hours before initiating misoprostol.28
Fetal size <25w0d gestation: Cervical preparation and surgical evacuation
For fetal demise at a fetal size less than 25w0d, if clinical experts are available, the best treatment option is cervical preparation followed by surgical evacuation of the uterus using a vacuum catheter and grasping forceps to empty the uterus.29,30 A disadvantage of surgical evacuation of the uterus is that an intact fetus is not available for the patient to hold and mourn, and pathologic examination of an intact fetus is not possible. An alternative approach is cervical preparation followed by induction of labor using misoprostol with the goal of delivering an intact fetus. Although no prospective clinical trials are available comparing these 2 options, retrospective studies have reported that, at fetal size <25w0d gestation, compared with induction of labor, surgical evacuation of the uterus results in fewer complications,30 including fewer cases of retained placenta requiring an unplanned procedure and fewer presumed uterine infections.29
For surgical evacuation of fetal demise with a fetal size of <25w0d gestation, the first step on day 1 is placement of osmotic cervical dilators. In addition to osmotic cervical dilators, if the gestational age or fetal size is ≥19 weeks’ gestation an oral dose of mifepristone 200 mg to facilitate cervical preparation may be considered. On day 2, the osmotic dilators are removed and surgical evacuation is performed. In one randomized study, for pregnancies at 19 to 24 weeks’ gestation, compared with osmotic dilators alone, administration of mifepristone 200 mg at the time of placement of osmotic dilators resulted in fewer procedures that were difficult to complete.31 In some cases, 2 consecutive days of cervical preparation with osmotic dilators may be needed to properly prepare the cervix for uterine evacuation. For example, the cervix of a nulliparous teenage patient may require 2 days of cervical preparation with osmotic dilators to facilitate uterine evacuation. In some cases of fetal demise, the cervix is already dilated to ≥3 cm and surgical evacuation of the uterus or induction of labor can be initiated without the need for cervical preparation.
Continue to: Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor...
Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor
Treatment of fetal demise at 14w0d to 28w6d gestation with the goal of the vaginal birth of an intact fetus is optimized by the administration of mifepristone for cervical preparation followed by induction of labor with misoprostol.26,27
In one clinical trial, 66 patients with fetal demise between 14w0d and 28w6d gestation were randomly assigned to receive mifepristone 200 mg or placebo followed 24 to 48 hours later with initiation of misoprostol induction of labor.26 Among the patients from 14w0d to 24 weeks’ gestation, the misoprostol dose was 400 µg vaginally every 6 hours. For patients from 24w0d to 28 weeks’ gestation, the misoprostol dose was 200 µg vaginally every 4 hours. At 24 hours, a consultant obstetrician determined if additional misoprostol should be given. The median time from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups was 6.8 hours and 10.5 hours (P=.002).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required fewer doses of misoprostol (2.1 vs 3.4; P= .002) and a lower total dose of misoprostol (768 µg vs 1,182 µg; P=.003). All patients in the mifepristone group delivered within 24 hours. By contrast, 13% of the patients in the placebo group delivered more than 24 hours after the initiation of misoprostol treatment. Five patients were readmitted with retained products of conception needing suction curettage, 4 in the placebo group and 1 in the mifepristone group.26
In a second clinical trial, 105 patients with fetal demise after 20 weeks of gestation were randomly assigned to receive mifepristone 200 mg or placebo.27 In this study, 86% of the patients were ≥26w0d gestation, with a mean gestational age of approximately 32w2d. Thirty-six to 48 hours later, misoprostol induction of labor was initiated. Among the patients from 20 to 25 completed weeks of gestation, the misoprostoldose was 100 µg vaginally every 6 hours for a maximum of 4 doses. For patients from ≥26 weeks’ gestation, the misoprostol dose was 50 µg vaginally every 4 hours for a maximum of 6 doses. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 9.8 hours and 16.3 hours, respectively (P=.001). Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required a lower total dose of misoprostol (110 µg vs 198 µg; P<.001). Delivery within 24 hours following initiation of misoprostol occurred in 93% and 73% of the patients in the mifepristone and placebo groups, respectively (P<.001). Compared with patients in the mifepristone group, shivering occurred more frequently among patients in the placebo group (7.5% vs 19.2%; P=.09), likely because they received greater doses of misoprostol.27
Fetal size ≥29w0d gestation
At a fetal size ≥29w0d gestation, if the cervix is ripe with a Bishop score of ≥7, oxytocin induction of labor is often used as a first-line treatment. If the cervix is not ripe, misoprostol induction of labor may be considered at doses less than those used in the second trimester of pregnancy.32 TABLES 1,1, 26, 33–36 2,37 and 337 summarize regimens proposed for fetal size ≥29w0d. One regimen begins with an initial misoprostol dose of 50 µg. If adequate uterine contractions occur, the 50 µg dose is repeated every 4 hours up to 6 total doses. If contractions are inadequate, the dose can be increased to 100 µg every 4 hours for 5 additional doses.



For fetal demise after 28w0d gestation, the
A multidisciplinary approach can optimize compassionate care
There are many gaps in the holistic care of patients and partners experiencing fetal demise. Patients with fetal demise often report that they did not receive sufficient information about the cause of the demise and wanted more opportunity to be involved in decision making about their care.39 The patient’s partner often reports feeling unacknowledged as a grieving parent.40 Fetal demise is experienced by many patients as a tragedy, triggering feelings of grief, anger, denial, anxiety and depression, sometimes resulting in isolation and substance misuse.
Using a 5-round Delphi process, experts identified 8 core goals in the care of patients with fetal demise:
- reduce stigma
- provide respectful care
- involve patients in care planning
- attempt to provide an explanation for the demise1
- acknowledge the depth of the grief response and provide emotional support
- offer information about ongoing psychological support
- provide information about future pregnancy planning
- provide opportunities for specialized training and support for care providers.41
Management of stillbirth is optimized by a multidisciplinary approach that includes the expert care of obstetrician-gynecologists, obstetric nurses, anesthesiologists, and expert consultation from social work, chaplaincy, and pathology. A heart-to-heart connection between clinician and patient is a key component of stillbirth care. ●
- American College of Obstetricians and Gynecologists. Management of stillbirth. ACOG Obstetric Care Consensus. No. 10. Obstet Gynecol. 2020;135:e110-132.
- Tsakiridis I, Giouleka S, Mamopoulos A, et al. Investigation and management of stillbirths: a descriptive review of major guidelines. J Perinat Med. 2022;50:796-813.
- Spingler T, Sonek J, Hoopman M, et al. Complication rate after termination of pregnancy due to fetal defects. Ultrasound Obstet Gynecol. 2023;Epub January 7.
- Goldberg AB, Drey EA, Whitaker AK, et al. Misoprostol compared with laminaria before early second-trimester surgical abortion: a randomized trial. Obstet Gynecol. 2005;106:234-241.
- Meirik O, My Huong NT, Piaggio G, et al. WHOR-GoP-MoF Regulation. Complications of first trimester abortion by vacuum aspiration after cervical preparation with and without misoprostol: a multicentre randomised trial. Lancet. 2012;379(9829):1817-1824.
- Bartz D, Maurer R, Allen RH, et al. Buccal misoprostol compared with synthetic osmotic cervical dilator before surgical abortion: a randomized controlled trial. Obstet Gynecol. 2013;122:57-63.
- Ngo LL, Mokashi M, Janiak E, et al. Acute complications with same-day versus overnight cervical preparation before dilation and evacuation at 14 to 16 weeks. Contraception. 2023;117:61-66.
- Kim CS, Dragoman M, Prosch L, et al. Same-day compared with overnight cervical preparation before dilation and evacuation between 16 and 19 6/7 weeks of gestation: a randomized controlled trial. Obstet Gynecol. 2022;139:1141-1144.
- Drunecky T, Reidingerova M, Plisova M, et al. Experimental comparison of properties of natural and synthetic osmotic dilators. Arch Gynecol Obstet. 2015;292:349-354.
- Hern WM. Laminaria versus Dilapan osmotic cervical dilators for outpatient dilation and evacuation abortion: randomized cohort comparison of 1001 patients. Am J Obstet Gynecol. 1994;171:1324-1328.
- Berthold C, Gomes David M, Gabriel P, et al. Effect of the addition of osmotic dilators to medical induction of labor abortion: a before-and-after study. Eur J Obstet Gynecol. 2020;244:185-189.
- Kemper JI, Li W, Goni S, et al. Foley catheter vs oral misoprostol for induction of labor: individual participant data meta-analysis. Ultrasound Obstet Gynecol. 2021;57:215-223.
- Attalli E, Kern Guy, Fouks Y, et al. Labor induction in third trimester non-viable fetus. J Matern Fetal Neonatal Med. 2022;Epub October 1.
- Fessehaye Sium A, Prager S, Wolderufael M, et al. Foley catheter for cervical preparation prior to second trimester dilation and evacuation: a supply-based alternative for surgical abortion: a case series. Contracept X. 2022;4:100085.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590.
- Barbieri RL. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022;34:8-10, 12.
- Andersen J, Grine E, Eng L, et al. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266-1276.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398-5407.
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284-290.
- Barbieri RL. Mifepristone for the treatment of miscarriage and fetal demise. OBG Manag. 2022;34:811, 15.
- Kapp N, Borgatta L, Stubblefield P, et al. Mifepristone in second-trimester medical abortion. Obstet Gynecol. 2007;110:1304-1310.
- Ngoc NTN, Shochet T, Raghavan S, et al. Mifepristone and misoprostol compared with misoprostol alone for second trimester abortion: a randomized controlled trial. Obstet Gynecol. 2011;118:601608.
- Allanson ER, Copson S, Spilsbury K, et al. Pretreatment with mifepristone compared with misoprostol alone for delivery after fetal death between 14 and 28 weeks of gestation. Obstet Gynecol. 2021;137:801-809.
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. J Obstet Gynaecol Res. 2015;41:1884-1890.
- Prodan N, Breisch J, Hoopman M, et al. Dosing interval between mifepristone and misoprostol in second and third trimester termination. Arch Gynecol Obstet. 2019;299:675-679.
- Edlow AG, Hou MY, Maurer R, et al. Uterine evacuation for second trimester fetal death and maternal morbidity. Obstet Gynecol. 2011;117:1-10.
- Bryan AG, Grimes DA, Garrett JM, et al. Second-trimester abortion for fetal anomalies or fetal death. Obstet Gynecol. 2011;117:788-792.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol or mifepristone compared with overnight osmotic dilators alone. Obstet Gynecol. 2015;126:599-609.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- American College of Obstetricians and Gynecologists. Second-trimester abortion. Practice Bulletin No. 135. Obstet Gynecol. 2013;121:1394-1406.
- Wingo E, Raifman S, Landau C, et al. Mifepristone-misoprostol versus misoprostol-alone regimen for medication abortion at ≥ 24 weeks gestation. Contraception. Appendix 1. 2020;102:99-103.
- American College of Obstetricians and Gynecologists. Vaginal birth after cesarean delivery. ACOG Practice Bulletin No. 205. Obstet Gynecol. 2019;133:e110-e127.
- Atkins B, Blencowe H, Boyle FM, et al. Is care of stillborn babies and their parents respectful? Results from an international online survey. BJOG. 2022;129:1731-1739.
- Haezell AEP, Siassakos D, Blencowe H, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387(10018):604-616.
- Shakespeare C, Merriel A, Bakhbakhi D, et al. The RESPECT Study for consensus on global bereavement care after stillbirth. Int J Gynaecol Obstet. 2020;149:137-147.
- American College of Obstetricians and Gynecologists. Management of stillbirth. ACOG Obstetric Care Consensus. No. 10. Obstet Gynecol. 2020;135:e110-132.
- Tsakiridis I, Giouleka S, Mamopoulos A, et al. Investigation and management of stillbirths: a descriptive review of major guidelines. J Perinat Med. 2022;50:796-813.
- Spingler T, Sonek J, Hoopman M, et al. Complication rate after termination of pregnancy due to fetal defects. Ultrasound Obstet Gynecol. 2023;Epub January 7.
- Goldberg AB, Drey EA, Whitaker AK, et al. Misoprostol compared with laminaria before early second-trimester surgical abortion: a randomized trial. Obstet Gynecol. 2005;106:234-241.
- Meirik O, My Huong NT, Piaggio G, et al. WHOR-GoP-MoF Regulation. Complications of first trimester abortion by vacuum aspiration after cervical preparation with and without misoprostol: a multicentre randomised trial. Lancet. 2012;379(9829):1817-1824.
- Bartz D, Maurer R, Allen RH, et al. Buccal misoprostol compared with synthetic osmotic cervical dilator before surgical abortion: a randomized controlled trial. Obstet Gynecol. 2013;122:57-63.
- Ngo LL, Mokashi M, Janiak E, et al. Acute complications with same-day versus overnight cervical preparation before dilation and evacuation at 14 to 16 weeks. Contraception. 2023;117:61-66.
- Kim CS, Dragoman M, Prosch L, et al. Same-day compared with overnight cervical preparation before dilation and evacuation between 16 and 19 6/7 weeks of gestation: a randomized controlled trial. Obstet Gynecol. 2022;139:1141-1144.
- Drunecky T, Reidingerova M, Plisova M, et al. Experimental comparison of properties of natural and synthetic osmotic dilators. Arch Gynecol Obstet. 2015;292:349-354.
- Hern WM. Laminaria versus Dilapan osmotic cervical dilators for outpatient dilation and evacuation abortion: randomized cohort comparison of 1001 patients. Am J Obstet Gynecol. 1994;171:1324-1328.
- Berthold C, Gomes David M, Gabriel P, et al. Effect of the addition of osmotic dilators to medical induction of labor abortion: a before-and-after study. Eur J Obstet Gynecol. 2020;244:185-189.
- Kemper JI, Li W, Goni S, et al. Foley catheter vs oral misoprostol for induction of labor: individual participant data meta-analysis. Ultrasound Obstet Gynecol. 2021;57:215-223.
- Attalli E, Kern Guy, Fouks Y, et al. Labor induction in third trimester non-viable fetus. J Matern Fetal Neonatal Med. 2022;Epub October 1.
- Fessehaye Sium A, Prager S, Wolderufael M, et al. Foley catheter for cervical preparation prior to second trimester dilation and evacuation: a supply-based alternative for surgical abortion: a case series. Contracept X. 2022;4:100085.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590.
- Barbieri RL. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022;34:8-10, 12.
- Andersen J, Grine E, Eng L, et al. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266-1276.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398-5407.
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284-290.
- Barbieri RL. Mifepristone for the treatment of miscarriage and fetal demise. OBG Manag. 2022;34:811, 15.
- Kapp N, Borgatta L, Stubblefield P, et al. Mifepristone in second-trimester medical abortion. Obstet Gynecol. 2007;110:1304-1310.
- Ngoc NTN, Shochet T, Raghavan S, et al. Mifepristone and misoprostol compared with misoprostol alone for second trimester abortion: a randomized controlled trial. Obstet Gynecol. 2011;118:601608.
- Allanson ER, Copson S, Spilsbury K, et al. Pretreatment with mifepristone compared with misoprostol alone for delivery after fetal death between 14 and 28 weeks of gestation. Obstet Gynecol. 2021;137:801-809.
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. J Obstet Gynaecol Res. 2015;41:1884-1890.
- Prodan N, Breisch J, Hoopman M, et al. Dosing interval between mifepristone and misoprostol in second and third trimester termination. Arch Gynecol Obstet. 2019;299:675-679.
- Edlow AG, Hou MY, Maurer R, et al. Uterine evacuation for second trimester fetal death and maternal morbidity. Obstet Gynecol. 2011;117:1-10.
- Bryan AG, Grimes DA, Garrett JM, et al. Second-trimester abortion for fetal anomalies or fetal death. Obstet Gynecol. 2011;117:788-792.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol or mifepristone compared with overnight osmotic dilators alone. Obstet Gynecol. 2015;126:599-609.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- American College of Obstetricians and Gynecologists. Second-trimester abortion. Practice Bulletin No. 135. Obstet Gynecol. 2013;121:1394-1406.
- Wingo E, Raifman S, Landau C, et al. Mifepristone-misoprostol versus misoprostol-alone regimen for medication abortion at ≥ 24 weeks gestation. Contraception. Appendix 1. 2020;102:99-103.
- American College of Obstetricians and Gynecologists. Vaginal birth after cesarean delivery. ACOG Practice Bulletin No. 205. Obstet Gynecol. 2019;133:e110-e127.
- Atkins B, Blencowe H, Boyle FM, et al. Is care of stillborn babies and their parents respectful? Results from an international online survey. BJOG. 2022;129:1731-1739.
- Haezell AEP, Siassakos D, Blencowe H, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387(10018):604-616.
- Shakespeare C, Merriel A, Bakhbakhi D, et al. The RESPECT Study for consensus on global bereavement care after stillbirth. Int J Gynaecol Obstet. 2020;149:137-147.






