User login
Shoulder Injury Related to Vaccine Administration: A Rare Reaction
Localized reactions and transient pain at the site of vaccine administration are frequent and well-described occurrences that are typically short-lived and mild in nature. The most common findings at the injection site are soreness, erythema, and edema.1 Although less common, generalized shoulder dysfunction after vaccine administration also has been reported. Bodor and colleagues described a peri-articular inflammatory response that led to shoulder pain and weakness.2 A single case report by Kuether and colleagues described atraumatic osteonecrosis of the humeral head after H1N1 vaccine administration in the deltoid.3 In 2010, shoulder injury related to vaccine administration (SIRVA) was described by Atanasoff and colleagues as the rapid onset of shoulder pain and dysfunction persisting as a complication of deltoid muscle vaccination in a case series of 13 patients.4 In our report, we present a case of an active-duty male eventually diagnosed with SIRVA after influenza vaccination and discuss factors that may prevent vaccine-related shoulder injuries.
Case Presentation
A 31-year-old active-duty male presented to the Allergy clinic for evaluation of persistent left shoulder pain and decreased range of motion (ROM) following influenza vaccination 4 months prior. He reported a history of chronic low back and right shoulder pain. Although the patient had a traumatic injury to his right shoulder, which was corrected with surgery, he had no surgeries on the left shoulder. He reported no prior pain or known trauma to his left shoulder. He had no personal or family history of atopy or vaccine reactions.
The patient weighed 91 kg and received an intramuscular (IM) quadrivalent influenza vaccine with a 25-gauge, 1-inch needle during a mass influenza immunization. He recalled that the site of vaccination was slightly more than 3 cm below the top of the shoulder in a region correlating to the left deltoid. The vaccine was administered while he was standing with his arm extended, adducted, and internally rotated. The patient experienced intense pain immediately after the vaccination and noted decreased ROM. Initially, he dismissed the pain and decreased ROM as routine but sought medical attention when there was no improvement after 3 weeks.
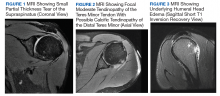
Six weeks after the onset of symptoms, a magnetic resonance image (MRI) revealed tendinopathy of the left distal subscapularis, infraspinatus, supraspinatus, and teres minor tendon. These findings were suggestive of a small partial thickness tear of the supraspinatus (Figure 1), possible calcific tendinopathy of the distal teres minor (Figure 2), and underlying humeral head edema (Figure 3). The patient was evaluated by Orthopedics and experienced no relief from ibuprofen, celecoxib, and a steroid/lidocaine intra-articular injection. Laboratory studies included an unremarkable complete blood count and erythrocyte sedimentation rate. He was diagnosed with SIRVA and continued in physical therapy with incomplete resolution of symptoms 6 months postvaccination.
Discussion
According to a 2018 report issued by the Centers for Disease Control and Prevention, local reactions following immunizations are seen in up to 80% of administered vaccine doses.1 While most of these reactions are mild, transient, cutaneous reactions, rarely these also may persist and impact quality of life significantly. SIRVA is one such process that can lead to persistent musculoskeletal dysfunction. SIRVA presents as shoulder pain and limited ROM that occurs after the administration of an injectable vaccine. In 2011, the Institute of Medicine determined that evidence supported a causal relationship between vaccine administration and deltoid bursitis.5
In 2017, SIRVA was included in the Vaccine Injury Compensation Program (VICP), a federal program that can provide compensation to individuals injured by certain vaccines.6 A diagnosis of SIRVA can be considered in patients who experience pain within 48 hours of vaccination, have no prior history of pain or dysfunction of the affected shoulder prior to vaccine administration, and have symptoms limited to the shoulder in which the vaccine was administered where no other abnormality is present to explain these symptoms (eg, brachial neuritis, other neuropathy). Currently, patients with back pain or musculoskeletal complaints that do not include the shoulder following deltoid vaccination do not meet the reporting criteria for SIRVA in the VICP.6
The exact prevalence or incidence of SIRVA is unknown. In a 2017 systematic review of the literature and the Spanish Pharmacovigilance System database, Martín Arias and colleagues found 45 cases of new onset, unilateral shoulder dysfunction without associated neuropathy or autoimmune conditions following vaccine administration. They noted a female to male predominance (71.1% vs 28.9%) with a mean age of 53.6 years (range 22-89 y). Most of the cases occurred following influenza vaccine (62%); pneumococcal vaccine was the next most common (13%).7 Shoulder injury also has been reported after tetanus-diphtheria toxoids, human papilloma virus, and hepatitis A virus vaccines.4,7 The review noted that all patients had onset of pain within the first week following vaccination with the majority (81%) having pain in the first 24 hours. Two cases found in the Spanish database had pain onset 2 months postvaccination.7 Atanasoff and colleagues found that 93% of patients had pain onset within 24 hours of vaccination with 54% reporting immediate pain.4
The Vaccine Adverse Event Reporting System (VAERS) tracks reports of shoulder dysfunction following certain vaccinations, but the system is unable to establish causality. According to VAERS reporting, between 2010 and 2016, there were 1006 possible reports of shoulder dysfunction following inactivated influenza vaccination (IIV) compared with an estimated 130 million doses of IIV given each influenza season in the US.8
Bodor and Montalvo postulated that vaccine antigen was being over penetrated into the synovial space of the shoulder, as the subdeltoid/subacromial bursa is located a mere 0.8 to 1.6 cm below the skin surface in patients with healthy body mass index.2 Atanasoff and colleagues expounded that antibodies from previous vaccination or natural infection may then form antigen-antibody complexes, creating prolonged local immune and inflammatory responses leading to bursitis or tendonitis.4 Martín Arias and colleagues hypothesized that improper injection technique, including wrong insertion angle, incorrect needle type/size, and failure to account for the patient’s physical characteristics were the most likely causes of SIRVA.7
Proper vaccine administration ensures that vaccinations are delivered in a safe and efficacious manner. Safe vaccination practices include the use of trained personnel who receive comprehensive, competency-based training regarding vaccine administration.1 Aspiration prior to an injection is a practice that has not been evaluated fully. Given that the 2 routinely recommended locations for IM vaccines (deltoid muscle in adults or vastus lateralis muscle in infants) lack large blood vessels, the practice of aspiration prior to an IM vaccine is not currently deemed necessary.1 Additional safe vaccine practices include the selection of appropriate needle length for muscle penetration and that anatomic landmarks determine the location of vaccination.1 Despite this, in a survey of 100 medical professionals, half could not name any structure at risk from improper deltoid vaccination technique.9
Cook and colleagues used anthropomorphic data to evaluate the potential for injury to the subdeltoid/subacromial bursa and/or the axillary nerve.10 Based on these data, they recommended safe IM vaccine administration can be assured by using the midpoint of the deltoid muscle located midway between the acromion and deltoid tuberosity with the arm abducted to 60°.10,11 In 46% of SIRVA cases described by Atanasoff and colleagues, patients reported that the vaccine was administered “too high.”4 The study also recommended that the clinician and the patient be in the seated position to ensure proper needle angle and location of administration.4 For most adults, a 1-inch needle is appropriate for vaccine administration in the deltoid; however, in females weighing < 70 kg and males < 75 kg, a 5/8-inch needle is recommended to avoid injury.7
Our 91-kg patient was appropriately administered his vaccine with a 1-inch needle. As he experienced immediate pain, it is unlikely that his symptoms were due to an immune-mediated process, as this would not be expected to occur immediately. Improper location of vaccine administration is a proposed mechanism of injury for our patient, though this cannot be confirmed by history alone. His prior history of traumatic injury to the opposite shoulder could represent a confounding factor as no prior imaging was available for the vaccine-affected shoulder. A preexisting shoulder abnormality or injury cannot be completely excluded, and it is possible that an underlying prior shoulder injury was aggravated postvaccination.
Evaluation and Treatment
There is no standardized approach for the evaluation of SIRVA to date. Awareness of SIRVA and a high index of suspicion are necessary to evaluate patients with shoulder concerns postvaccination. Laboratory evaluation should be considered to evaluate for other potential diagnoses (eg, infection, rheumatologic concerns). Routine X-rays are not helpful in cases of SIRVA. Ultrasound may be considered as it can show bursa abnormalities consistent with bursitis.2 MRI of the affected shoulder may provide improved diagnostic capability if SIRVA is suspected. MRI findings vary but include intraosseous edema, bursitis, tendonitis, and rotator cuff tears.4,12 Complete rotator cuff tears were found in 15% of cases reviewed by Atanasoff and colleagues.4 While there is no recommended timing for MRI, 63% of MRIs were performed within 3 months of symptom onset.4 As SIRVA is not a neurologic injury, nerve conduction, electromyographic studies, and neurologic evaluation or testing are expected to be normal.
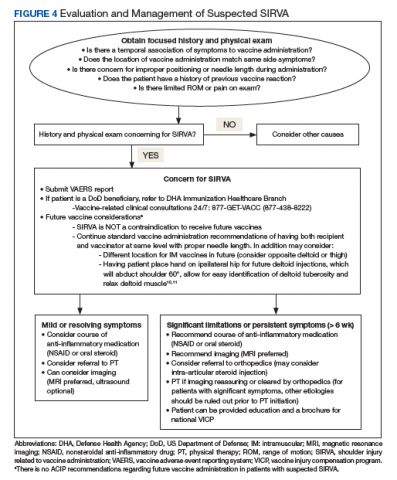
Treatment of SIRVA and other vaccine-related shoulder injuries typically have involved pain management (eg, nonsteroidal anti-inflammatory agents), intra-articular steroid injections, and physical therapy, though some patients never experience complete resolution of symptoms.2,4,7 Both patients with vaccination-related shoulder dysfunction described by Bodor and colleagues improved after intra-articular triamcinolone injections, with up to 3 injections before complete resolution of pain in one patient.2 Orthopedics evaluation may need to be considered for persistent symptoms. According to Atanasoff and colleagues, most patients were symptomatic for at least 6 months, and complete recovery was seen in less than one-third of patients.4 Although the development of SIRVA is not a contraindication to future doses of the presumed causative vaccine, subsequent vaccination should include careful consideration of other administration sites if possible (eg, vastus lateralis may be used for IM injections in adults) (Figure 4).
Reporting
A diagnosis or concern for SIRVA also should be reported to the VAERS, the national database established in order to detect possible safety problems with US-licensed vaccines. VAERS reports can be submitted by anyone with concerns for vaccine adverse reactions, including patients, caregivers, and health care professionals at vaers.hhs.gov/reportevent.html. Additional information regarding VICP can be obtained at www.hrsa.gov/vaccine-compensation/index.html.
Military-Specific Issues
The military values readiness, which includes ensuring that active-duty members remain up-to-date on life-saving vaccinations. Immunization is of critical importance to mobility and success of the overall mission. Mobility processing lines where immunizations can be provided to multiple active-duty members can be a successful strategy for mass immunizations. Although the quick administration of immunizations maintains readiness and provides a medically necessary service, it also may increase the chances of incorrect vaccine placement in the deltoid, causing long-term shoulder immobility that may impact a service member’s retainability. The benefits of mobility processing lines can continue to outweigh the risks of immunization administration by ensuring proper staff training, seating both the administrator and recipient of vaccination, and selecting a proper needle length and site of administration specific to each recipient.
Conclusion
Correct administration of vaccines is of utmost importance in preventing SIRVA and other vaccine-related shoulder dysfunctions. Proper staff training and refresher training can help prevent vaccine-related shoulder injuries. Additionally, clinicians should be aware of this potential complication and maintain a high index of suspicion when evaluating patients with postvaccination shoulder complaints.
1. Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. https://www.cdc.gov/vaccines/pubs/pinkbook/vac-admin.html. Published 2015. Accessed June 3, 2019.
2. Bodor M, Montalvo E. Vaccination-related shoulder dysfunction. Vaccine. 2007;25(4):585-587.
3. Kuether G, Dietrich B, Smith T, Peter C, Gruessner S. Atraumatic osteonecrosis of the humeral head after influenza A-(H1N1) v-2009 vaccination. Vaccine. 2011;29(40):6830-6833.
4. Atanasoff S, Ryan T, Lightfoot R, Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28(51):8049-8052.
5. Institute of Medicine. Adverse effects of vaccines: evidence and causality. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2011/Adverse-Effects-of-Vaccines-Evidence-and-Causality/Vaccine-report-brief-FINAL.pdf. Published August 2011. Accessed June 3, 2019.
6. Health Resources and Services Administration, Health and Human Services Administration. National vaccine injury compensation program: revisions to the vaccine injury table. https://www.federalregister.gov/documents/2017/01/19/2017-00701/national-vaccine-injury-compensation-program-revisions-to-the-vaccine-injury-table. Published January 19, 2017. Accessed June 3, 2019.
7. Martín Arias LH, Sanz Fadrique R, Sáinz Gil M, Salgueiro-Vazquez ME. Risk of bursitis and other injuries and dysfunctions of the shoulder following vaccinations. Vaccine. 2017;35(37):4870-4876.
8. Centers for Disease Control and Prevention. Reports of shoulder dysfunction following inactivated influenza vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2010-2016. https://stacks.cdc.gov/view/cdc/57624. Published January 4, 2018. Accessed June 3, 2019.
9. McGarvey MA, Hooper AC. The deltoid intramuscular injection site in the adult. Current practice among general practitioners and practice nurses. Ir Med J. 2005;98(4):105-107.
10. Cook IF. An evidence based protocol for the prevention of upper arm injury related to vaccine administration (UAIRVA). Hum Vaccin. 2011;7(8):845-848.
11. Cook IF. Best vaccination practice and medically attended injection site events following deltoid intramuscular injection. Hum Vaccin Immunother. 2015;11(5):1184-1191.
12. Okur G, Chaney KA, Lomasney LM. Magnetic resonance imaging of abnormal shoulder pain following influenza vaccination. Skeletal Radiol. 2014;43(9):1325-1331.
Localized reactions and transient pain at the site of vaccine administration are frequent and well-described occurrences that are typically short-lived and mild in nature. The most common findings at the injection site are soreness, erythema, and edema.1 Although less common, generalized shoulder dysfunction after vaccine administration also has been reported. Bodor and colleagues described a peri-articular inflammatory response that led to shoulder pain and weakness.2 A single case report by Kuether and colleagues described atraumatic osteonecrosis of the humeral head after H1N1 vaccine administration in the deltoid.3 In 2010, shoulder injury related to vaccine administration (SIRVA) was described by Atanasoff and colleagues as the rapid onset of shoulder pain and dysfunction persisting as a complication of deltoid muscle vaccination in a case series of 13 patients.4 In our report, we present a case of an active-duty male eventually diagnosed with SIRVA after influenza vaccination and discuss factors that may prevent vaccine-related shoulder injuries.
Case Presentation
A 31-year-old active-duty male presented to the Allergy clinic for evaluation of persistent left shoulder pain and decreased range of motion (ROM) following influenza vaccination 4 months prior. He reported a history of chronic low back and right shoulder pain. Although the patient had a traumatic injury to his right shoulder, which was corrected with surgery, he had no surgeries on the left shoulder. He reported no prior pain or known trauma to his left shoulder. He had no personal or family history of atopy or vaccine reactions.
The patient weighed 91 kg and received an intramuscular (IM) quadrivalent influenza vaccine with a 25-gauge, 1-inch needle during a mass influenza immunization. He recalled that the site of vaccination was slightly more than 3 cm below the top of the shoulder in a region correlating to the left deltoid. The vaccine was administered while he was standing with his arm extended, adducted, and internally rotated. The patient experienced intense pain immediately after the vaccination and noted decreased ROM. Initially, he dismissed the pain and decreased ROM as routine but sought medical attention when there was no improvement after 3 weeks.
Six weeks after the onset of symptoms, a magnetic resonance image (MRI) revealed tendinopathy of the left distal subscapularis, infraspinatus, supraspinatus, and teres minor tendon. These findings were suggestive of a small partial thickness tear of the supraspinatus (Figure 1), possible calcific tendinopathy of the distal teres minor (Figure 2), and underlying humeral head edema (Figure 3). The patient was evaluated by Orthopedics and experienced no relief from ibuprofen, celecoxib, and a steroid/lidocaine intra-articular injection. Laboratory studies included an unremarkable complete blood count and erythrocyte sedimentation rate. He was diagnosed with SIRVA and continued in physical therapy with incomplete resolution of symptoms 6 months postvaccination.
Discussion
According to a 2018 report issued by the Centers for Disease Control and Prevention, local reactions following immunizations are seen in up to 80% of administered vaccine doses.1 While most of these reactions are mild, transient, cutaneous reactions, rarely these also may persist and impact quality of life significantly. SIRVA is one such process that can lead to persistent musculoskeletal dysfunction. SIRVA presents as shoulder pain and limited ROM that occurs after the administration of an injectable vaccine. In 2011, the Institute of Medicine determined that evidence supported a causal relationship between vaccine administration and deltoid bursitis.5
In 2017, SIRVA was included in the Vaccine Injury Compensation Program (VICP), a federal program that can provide compensation to individuals injured by certain vaccines.6 A diagnosis of SIRVA can be considered in patients who experience pain within 48 hours of vaccination, have no prior history of pain or dysfunction of the affected shoulder prior to vaccine administration, and have symptoms limited to the shoulder in which the vaccine was administered where no other abnormality is present to explain these symptoms (eg, brachial neuritis, other neuropathy). Currently, patients with back pain or musculoskeletal complaints that do not include the shoulder following deltoid vaccination do not meet the reporting criteria for SIRVA in the VICP.6
The exact prevalence or incidence of SIRVA is unknown. In a 2017 systematic review of the literature and the Spanish Pharmacovigilance System database, Martín Arias and colleagues found 45 cases of new onset, unilateral shoulder dysfunction without associated neuropathy or autoimmune conditions following vaccine administration. They noted a female to male predominance (71.1% vs 28.9%) with a mean age of 53.6 years (range 22-89 y). Most of the cases occurred following influenza vaccine (62%); pneumococcal vaccine was the next most common (13%).7 Shoulder injury also has been reported after tetanus-diphtheria toxoids, human papilloma virus, and hepatitis A virus vaccines.4,7 The review noted that all patients had onset of pain within the first week following vaccination with the majority (81%) having pain in the first 24 hours. Two cases found in the Spanish database had pain onset 2 months postvaccination.7 Atanasoff and colleagues found that 93% of patients had pain onset within 24 hours of vaccination with 54% reporting immediate pain.4
The Vaccine Adverse Event Reporting System (VAERS) tracks reports of shoulder dysfunction following certain vaccinations, but the system is unable to establish causality. According to VAERS reporting, between 2010 and 2016, there were 1006 possible reports of shoulder dysfunction following inactivated influenza vaccination (IIV) compared with an estimated 130 million doses of IIV given each influenza season in the US.8
Bodor and Montalvo postulated that vaccine antigen was being over penetrated into the synovial space of the shoulder, as the subdeltoid/subacromial bursa is located a mere 0.8 to 1.6 cm below the skin surface in patients with healthy body mass index.2 Atanasoff and colleagues expounded that antibodies from previous vaccination or natural infection may then form antigen-antibody complexes, creating prolonged local immune and inflammatory responses leading to bursitis or tendonitis.4 Martín Arias and colleagues hypothesized that improper injection technique, including wrong insertion angle, incorrect needle type/size, and failure to account for the patient’s physical characteristics were the most likely causes of SIRVA.7
Proper vaccine administration ensures that vaccinations are delivered in a safe and efficacious manner. Safe vaccination practices include the use of trained personnel who receive comprehensive, competency-based training regarding vaccine administration.1 Aspiration prior to an injection is a practice that has not been evaluated fully. Given that the 2 routinely recommended locations for IM vaccines (deltoid muscle in adults or vastus lateralis muscle in infants) lack large blood vessels, the practice of aspiration prior to an IM vaccine is not currently deemed necessary.1 Additional safe vaccine practices include the selection of appropriate needle length for muscle penetration and that anatomic landmarks determine the location of vaccination.1 Despite this, in a survey of 100 medical professionals, half could not name any structure at risk from improper deltoid vaccination technique.9
Cook and colleagues used anthropomorphic data to evaluate the potential for injury to the subdeltoid/subacromial bursa and/or the axillary nerve.10 Based on these data, they recommended safe IM vaccine administration can be assured by using the midpoint of the deltoid muscle located midway between the acromion and deltoid tuberosity with the arm abducted to 60°.10,11 In 46% of SIRVA cases described by Atanasoff and colleagues, patients reported that the vaccine was administered “too high.”4 The study also recommended that the clinician and the patient be in the seated position to ensure proper needle angle and location of administration.4 For most adults, a 1-inch needle is appropriate for vaccine administration in the deltoid; however, in females weighing < 70 kg and males < 75 kg, a 5/8-inch needle is recommended to avoid injury.7
Our 91-kg patient was appropriately administered his vaccine with a 1-inch needle. As he experienced immediate pain, it is unlikely that his symptoms were due to an immune-mediated process, as this would not be expected to occur immediately. Improper location of vaccine administration is a proposed mechanism of injury for our patient, though this cannot be confirmed by history alone. His prior history of traumatic injury to the opposite shoulder could represent a confounding factor as no prior imaging was available for the vaccine-affected shoulder. A preexisting shoulder abnormality or injury cannot be completely excluded, and it is possible that an underlying prior shoulder injury was aggravated postvaccination.
Evaluation and Treatment
There is no standardized approach for the evaluation of SIRVA to date. Awareness of SIRVA and a high index of suspicion are necessary to evaluate patients with shoulder concerns postvaccination. Laboratory evaluation should be considered to evaluate for other potential diagnoses (eg, infection, rheumatologic concerns). Routine X-rays are not helpful in cases of SIRVA. Ultrasound may be considered as it can show bursa abnormalities consistent with bursitis.2 MRI of the affected shoulder may provide improved diagnostic capability if SIRVA is suspected. MRI findings vary but include intraosseous edema, bursitis, tendonitis, and rotator cuff tears.4,12 Complete rotator cuff tears were found in 15% of cases reviewed by Atanasoff and colleagues.4 While there is no recommended timing for MRI, 63% of MRIs were performed within 3 months of symptom onset.4 As SIRVA is not a neurologic injury, nerve conduction, electromyographic studies, and neurologic evaluation or testing are expected to be normal.
Treatment of SIRVA and other vaccine-related shoulder injuries typically have involved pain management (eg, nonsteroidal anti-inflammatory agents), intra-articular steroid injections, and physical therapy, though some patients never experience complete resolution of symptoms.2,4,7 Both patients with vaccination-related shoulder dysfunction described by Bodor and colleagues improved after intra-articular triamcinolone injections, with up to 3 injections before complete resolution of pain in one patient.2 Orthopedics evaluation may need to be considered for persistent symptoms. According to Atanasoff and colleagues, most patients were symptomatic for at least 6 months, and complete recovery was seen in less than one-third of patients.4 Although the development of SIRVA is not a contraindication to future doses of the presumed causative vaccine, subsequent vaccination should include careful consideration of other administration sites if possible (eg, vastus lateralis may be used for IM injections in adults) (Figure 4).
Reporting
A diagnosis or concern for SIRVA also should be reported to the VAERS, the national database established in order to detect possible safety problems with US-licensed vaccines. VAERS reports can be submitted by anyone with concerns for vaccine adverse reactions, including patients, caregivers, and health care professionals at vaers.hhs.gov/reportevent.html. Additional information regarding VICP can be obtained at www.hrsa.gov/vaccine-compensation/index.html.
Military-Specific Issues
The military values readiness, which includes ensuring that active-duty members remain up-to-date on life-saving vaccinations. Immunization is of critical importance to mobility and success of the overall mission. Mobility processing lines where immunizations can be provided to multiple active-duty members can be a successful strategy for mass immunizations. Although the quick administration of immunizations maintains readiness and provides a medically necessary service, it also may increase the chances of incorrect vaccine placement in the deltoid, causing long-term shoulder immobility that may impact a service member’s retainability. The benefits of mobility processing lines can continue to outweigh the risks of immunization administration by ensuring proper staff training, seating both the administrator and recipient of vaccination, and selecting a proper needle length and site of administration specific to each recipient.
Conclusion
Correct administration of vaccines is of utmost importance in preventing SIRVA and other vaccine-related shoulder dysfunctions. Proper staff training and refresher training can help prevent vaccine-related shoulder injuries. Additionally, clinicians should be aware of this potential complication and maintain a high index of suspicion when evaluating patients with postvaccination shoulder complaints.
Localized reactions and transient pain at the site of vaccine administration are frequent and well-described occurrences that are typically short-lived and mild in nature. The most common findings at the injection site are soreness, erythema, and edema.1 Although less common, generalized shoulder dysfunction after vaccine administration also has been reported. Bodor and colleagues described a peri-articular inflammatory response that led to shoulder pain and weakness.2 A single case report by Kuether and colleagues described atraumatic osteonecrosis of the humeral head after H1N1 vaccine administration in the deltoid.3 In 2010, shoulder injury related to vaccine administration (SIRVA) was described by Atanasoff and colleagues as the rapid onset of shoulder pain and dysfunction persisting as a complication of deltoid muscle vaccination in a case series of 13 patients.4 In our report, we present a case of an active-duty male eventually diagnosed with SIRVA after influenza vaccination and discuss factors that may prevent vaccine-related shoulder injuries.
Case Presentation
A 31-year-old active-duty male presented to the Allergy clinic for evaluation of persistent left shoulder pain and decreased range of motion (ROM) following influenza vaccination 4 months prior. He reported a history of chronic low back and right shoulder pain. Although the patient had a traumatic injury to his right shoulder, which was corrected with surgery, he had no surgeries on the left shoulder. He reported no prior pain or known trauma to his left shoulder. He had no personal or family history of atopy or vaccine reactions.
The patient weighed 91 kg and received an intramuscular (IM) quadrivalent influenza vaccine with a 25-gauge, 1-inch needle during a mass influenza immunization. He recalled that the site of vaccination was slightly more than 3 cm below the top of the shoulder in a region correlating to the left deltoid. The vaccine was administered while he was standing with his arm extended, adducted, and internally rotated. The patient experienced intense pain immediately after the vaccination and noted decreased ROM. Initially, he dismissed the pain and decreased ROM as routine but sought medical attention when there was no improvement after 3 weeks.
Six weeks after the onset of symptoms, a magnetic resonance image (MRI) revealed tendinopathy of the left distal subscapularis, infraspinatus, supraspinatus, and teres minor tendon. These findings were suggestive of a small partial thickness tear of the supraspinatus (Figure 1), possible calcific tendinopathy of the distal teres minor (Figure 2), and underlying humeral head edema (Figure 3). The patient was evaluated by Orthopedics and experienced no relief from ibuprofen, celecoxib, and a steroid/lidocaine intra-articular injection. Laboratory studies included an unremarkable complete blood count and erythrocyte sedimentation rate. He was diagnosed with SIRVA and continued in physical therapy with incomplete resolution of symptoms 6 months postvaccination.
Discussion
According to a 2018 report issued by the Centers for Disease Control and Prevention, local reactions following immunizations are seen in up to 80% of administered vaccine doses.1 While most of these reactions are mild, transient, cutaneous reactions, rarely these also may persist and impact quality of life significantly. SIRVA is one such process that can lead to persistent musculoskeletal dysfunction. SIRVA presents as shoulder pain and limited ROM that occurs after the administration of an injectable vaccine. In 2011, the Institute of Medicine determined that evidence supported a causal relationship between vaccine administration and deltoid bursitis.5
In 2017, SIRVA was included in the Vaccine Injury Compensation Program (VICP), a federal program that can provide compensation to individuals injured by certain vaccines.6 A diagnosis of SIRVA can be considered in patients who experience pain within 48 hours of vaccination, have no prior history of pain or dysfunction of the affected shoulder prior to vaccine administration, and have symptoms limited to the shoulder in which the vaccine was administered where no other abnormality is present to explain these symptoms (eg, brachial neuritis, other neuropathy). Currently, patients with back pain or musculoskeletal complaints that do not include the shoulder following deltoid vaccination do not meet the reporting criteria for SIRVA in the VICP.6
The exact prevalence or incidence of SIRVA is unknown. In a 2017 systematic review of the literature and the Spanish Pharmacovigilance System database, Martín Arias and colleagues found 45 cases of new onset, unilateral shoulder dysfunction without associated neuropathy or autoimmune conditions following vaccine administration. They noted a female to male predominance (71.1% vs 28.9%) with a mean age of 53.6 years (range 22-89 y). Most of the cases occurred following influenza vaccine (62%); pneumococcal vaccine was the next most common (13%).7 Shoulder injury also has been reported after tetanus-diphtheria toxoids, human papilloma virus, and hepatitis A virus vaccines.4,7 The review noted that all patients had onset of pain within the first week following vaccination with the majority (81%) having pain in the first 24 hours. Two cases found in the Spanish database had pain onset 2 months postvaccination.7 Atanasoff and colleagues found that 93% of patients had pain onset within 24 hours of vaccination with 54% reporting immediate pain.4
The Vaccine Adverse Event Reporting System (VAERS) tracks reports of shoulder dysfunction following certain vaccinations, but the system is unable to establish causality. According to VAERS reporting, between 2010 and 2016, there were 1006 possible reports of shoulder dysfunction following inactivated influenza vaccination (IIV) compared with an estimated 130 million doses of IIV given each influenza season in the US.8
Bodor and Montalvo postulated that vaccine antigen was being over penetrated into the synovial space of the shoulder, as the subdeltoid/subacromial bursa is located a mere 0.8 to 1.6 cm below the skin surface in patients with healthy body mass index.2 Atanasoff and colleagues expounded that antibodies from previous vaccination or natural infection may then form antigen-antibody complexes, creating prolonged local immune and inflammatory responses leading to bursitis or tendonitis.4 Martín Arias and colleagues hypothesized that improper injection technique, including wrong insertion angle, incorrect needle type/size, and failure to account for the patient’s physical characteristics were the most likely causes of SIRVA.7
Proper vaccine administration ensures that vaccinations are delivered in a safe and efficacious manner. Safe vaccination practices include the use of trained personnel who receive comprehensive, competency-based training regarding vaccine administration.1 Aspiration prior to an injection is a practice that has not been evaluated fully. Given that the 2 routinely recommended locations for IM vaccines (deltoid muscle in adults or vastus lateralis muscle in infants) lack large blood vessels, the practice of aspiration prior to an IM vaccine is not currently deemed necessary.1 Additional safe vaccine practices include the selection of appropriate needle length for muscle penetration and that anatomic landmarks determine the location of vaccination.1 Despite this, in a survey of 100 medical professionals, half could not name any structure at risk from improper deltoid vaccination technique.9
Cook and colleagues used anthropomorphic data to evaluate the potential for injury to the subdeltoid/subacromial bursa and/or the axillary nerve.10 Based on these data, they recommended safe IM vaccine administration can be assured by using the midpoint of the deltoid muscle located midway between the acromion and deltoid tuberosity with the arm abducted to 60°.10,11 In 46% of SIRVA cases described by Atanasoff and colleagues, patients reported that the vaccine was administered “too high.”4 The study also recommended that the clinician and the patient be in the seated position to ensure proper needle angle and location of administration.4 For most adults, a 1-inch needle is appropriate for vaccine administration in the deltoid; however, in females weighing < 70 kg and males < 75 kg, a 5/8-inch needle is recommended to avoid injury.7
Our 91-kg patient was appropriately administered his vaccine with a 1-inch needle. As he experienced immediate pain, it is unlikely that his symptoms were due to an immune-mediated process, as this would not be expected to occur immediately. Improper location of vaccine administration is a proposed mechanism of injury for our patient, though this cannot be confirmed by history alone. His prior history of traumatic injury to the opposite shoulder could represent a confounding factor as no prior imaging was available for the vaccine-affected shoulder. A preexisting shoulder abnormality or injury cannot be completely excluded, and it is possible that an underlying prior shoulder injury was aggravated postvaccination.
Evaluation and Treatment
There is no standardized approach for the evaluation of SIRVA to date. Awareness of SIRVA and a high index of suspicion are necessary to evaluate patients with shoulder concerns postvaccination. Laboratory evaluation should be considered to evaluate for other potential diagnoses (eg, infection, rheumatologic concerns). Routine X-rays are not helpful in cases of SIRVA. Ultrasound may be considered as it can show bursa abnormalities consistent with bursitis.2 MRI of the affected shoulder may provide improved diagnostic capability if SIRVA is suspected. MRI findings vary but include intraosseous edema, bursitis, tendonitis, and rotator cuff tears.4,12 Complete rotator cuff tears were found in 15% of cases reviewed by Atanasoff and colleagues.4 While there is no recommended timing for MRI, 63% of MRIs were performed within 3 months of symptom onset.4 As SIRVA is not a neurologic injury, nerve conduction, electromyographic studies, and neurologic evaluation or testing are expected to be normal.
Treatment of SIRVA and other vaccine-related shoulder injuries typically have involved pain management (eg, nonsteroidal anti-inflammatory agents), intra-articular steroid injections, and physical therapy, though some patients never experience complete resolution of symptoms.2,4,7 Both patients with vaccination-related shoulder dysfunction described by Bodor and colleagues improved after intra-articular triamcinolone injections, with up to 3 injections before complete resolution of pain in one patient.2 Orthopedics evaluation may need to be considered for persistent symptoms. According to Atanasoff and colleagues, most patients were symptomatic for at least 6 months, and complete recovery was seen in less than one-third of patients.4 Although the development of SIRVA is not a contraindication to future doses of the presumed causative vaccine, subsequent vaccination should include careful consideration of other administration sites if possible (eg, vastus lateralis may be used for IM injections in adults) (Figure 4).
Reporting
A diagnosis or concern for SIRVA also should be reported to the VAERS, the national database established in order to detect possible safety problems with US-licensed vaccines. VAERS reports can be submitted by anyone with concerns for vaccine adverse reactions, including patients, caregivers, and health care professionals at vaers.hhs.gov/reportevent.html. Additional information regarding VICP can be obtained at www.hrsa.gov/vaccine-compensation/index.html.
Military-Specific Issues
The military values readiness, which includes ensuring that active-duty members remain up-to-date on life-saving vaccinations. Immunization is of critical importance to mobility and success of the overall mission. Mobility processing lines where immunizations can be provided to multiple active-duty members can be a successful strategy for mass immunizations. Although the quick administration of immunizations maintains readiness and provides a medically necessary service, it also may increase the chances of incorrect vaccine placement in the deltoid, causing long-term shoulder immobility that may impact a service member’s retainability. The benefits of mobility processing lines can continue to outweigh the risks of immunization administration by ensuring proper staff training, seating both the administrator and recipient of vaccination, and selecting a proper needle length and site of administration specific to each recipient.
Conclusion
Correct administration of vaccines is of utmost importance in preventing SIRVA and other vaccine-related shoulder dysfunctions. Proper staff training and refresher training can help prevent vaccine-related shoulder injuries. Additionally, clinicians should be aware of this potential complication and maintain a high index of suspicion when evaluating patients with postvaccination shoulder complaints.
1. Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. https://www.cdc.gov/vaccines/pubs/pinkbook/vac-admin.html. Published 2015. Accessed June 3, 2019.
2. Bodor M, Montalvo E. Vaccination-related shoulder dysfunction. Vaccine. 2007;25(4):585-587.
3. Kuether G, Dietrich B, Smith T, Peter C, Gruessner S. Atraumatic osteonecrosis of the humeral head after influenza A-(H1N1) v-2009 vaccination. Vaccine. 2011;29(40):6830-6833.
4. Atanasoff S, Ryan T, Lightfoot R, Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28(51):8049-8052.
5. Institute of Medicine. Adverse effects of vaccines: evidence and causality. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2011/Adverse-Effects-of-Vaccines-Evidence-and-Causality/Vaccine-report-brief-FINAL.pdf. Published August 2011. Accessed June 3, 2019.
6. Health Resources and Services Administration, Health and Human Services Administration. National vaccine injury compensation program: revisions to the vaccine injury table. https://www.federalregister.gov/documents/2017/01/19/2017-00701/national-vaccine-injury-compensation-program-revisions-to-the-vaccine-injury-table. Published January 19, 2017. Accessed June 3, 2019.
7. Martín Arias LH, Sanz Fadrique R, Sáinz Gil M, Salgueiro-Vazquez ME. Risk of bursitis and other injuries and dysfunctions of the shoulder following vaccinations. Vaccine. 2017;35(37):4870-4876.
8. Centers for Disease Control and Prevention. Reports of shoulder dysfunction following inactivated influenza vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2010-2016. https://stacks.cdc.gov/view/cdc/57624. Published January 4, 2018. Accessed June 3, 2019.
9. McGarvey MA, Hooper AC. The deltoid intramuscular injection site in the adult. Current practice among general practitioners and practice nurses. Ir Med J. 2005;98(4):105-107.
10. Cook IF. An evidence based protocol for the prevention of upper arm injury related to vaccine administration (UAIRVA). Hum Vaccin. 2011;7(8):845-848.
11. Cook IF. Best vaccination practice and medically attended injection site events following deltoid intramuscular injection. Hum Vaccin Immunother. 2015;11(5):1184-1191.
12. Okur G, Chaney KA, Lomasney LM. Magnetic resonance imaging of abnormal shoulder pain following influenza vaccination. Skeletal Radiol. 2014;43(9):1325-1331.
1. Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. https://www.cdc.gov/vaccines/pubs/pinkbook/vac-admin.html. Published 2015. Accessed June 3, 2019.
2. Bodor M, Montalvo E. Vaccination-related shoulder dysfunction. Vaccine. 2007;25(4):585-587.
3. Kuether G, Dietrich B, Smith T, Peter C, Gruessner S. Atraumatic osteonecrosis of the humeral head after influenza A-(H1N1) v-2009 vaccination. Vaccine. 2011;29(40):6830-6833.
4. Atanasoff S, Ryan T, Lightfoot R, Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28(51):8049-8052.
5. Institute of Medicine. Adverse effects of vaccines: evidence and causality. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2011/Adverse-Effects-of-Vaccines-Evidence-and-Causality/Vaccine-report-brief-FINAL.pdf. Published August 2011. Accessed June 3, 2019.
6. Health Resources and Services Administration, Health and Human Services Administration. National vaccine injury compensation program: revisions to the vaccine injury table. https://www.federalregister.gov/documents/2017/01/19/2017-00701/national-vaccine-injury-compensation-program-revisions-to-the-vaccine-injury-table. Published January 19, 2017. Accessed June 3, 2019.
7. Martín Arias LH, Sanz Fadrique R, Sáinz Gil M, Salgueiro-Vazquez ME. Risk of bursitis and other injuries and dysfunctions of the shoulder following vaccinations. Vaccine. 2017;35(37):4870-4876.
8. Centers for Disease Control and Prevention. Reports of shoulder dysfunction following inactivated influenza vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2010-2016. https://stacks.cdc.gov/view/cdc/57624. Published January 4, 2018. Accessed June 3, 2019.
9. McGarvey MA, Hooper AC. The deltoid intramuscular injection site in the adult. Current practice among general practitioners and practice nurses. Ir Med J. 2005;98(4):105-107.
10. Cook IF. An evidence based protocol for the prevention of upper arm injury related to vaccine administration (UAIRVA). Hum Vaccin. 2011;7(8):845-848.
11. Cook IF. Best vaccination practice and medically attended injection site events following deltoid intramuscular injection. Hum Vaccin Immunother. 2015;11(5):1184-1191.
12. Okur G, Chaney KA, Lomasney LM. Magnetic resonance imaging of abnormal shoulder pain following influenza vaccination. Skeletal Radiol. 2014;43(9):1325-1331.
Sacroiliac Joint Dysfunction in Patients With Low Back Pain
Patients experiencing sacroiliac joint (SIJ) dysfunction might show symptoms that overlap with those seen in lumbar spine pathology. This article reviews diagnostic tools that assist practitioners to discern the true pain generator in patients with low back pain (LBP) and therapeutic approaches when the cause is SIJ dysfunction.
Prevalence
Most of the US population will experience LBP at some point in their lives. A 2002 National Health Interview survey found that more than one-quarter (26.4%) of 31 044 respondents had complained of LBP in the previous 3 months.1 About 74 million individuals in the US experienced LBP in the past 3 months.1 A full 10% of the US population is expected to suffer from chronic LBP, and it is estimated that 2.3% of all visits to physicians are related to LBP.1
The etiology of LBP often is unclear even after thorough clinical and radiographic evaluation because of the myriad possible mechanisms. Degenerative disc disease, facet arthropathy, ligamentous hypertrophy, muscle spasm, hip arthropathy, and SIJ dysfunction are potential pain generators and exact clinical and radiographic correlation is not always possible. Compounding this difficulty is the lack of specificity with current diagnostic techniques. For example, many patients will have disc desiccation or herniation without any LBP or radicular symptoms on radiographic studies, such as X-rays, computed tomography (CT), and magnetic resonance imaging (MRI). As such, providers of patients with diffuse radiographic abnormalities often have to identify a specific pain generator, which might not have any role in the patient’s pain.
Other tests, such as electromyographic studies, positron emission tomography (PET) scans, discography, and epidural steroid injections, can help pinpoint a specific pain generator. These tests might help determine whether the patient has a surgically treatable condition and could help predict whether a patient’s symptoms will respond to surgery.
However, the standard spine surgery workup often fails to identify an obvious pain generator in many individuals. The significant number of patients that fall into this category has prompted spine surgeons to consider other potential etiologies for LBP, and SIJ dysfunction has become a rapidly developing field of research.
Sacroiliac Joint Dysfunction
The SIJ is a bilateral, C-shaped synovial joint surrounded by a fibrous capsule and affixes the sacrum to the ilia. Several sacral ligaments and pelvic muscles support the SIJ. The L5 nerve ventral ramus and lumbosacral trunk pass anteriorly and the S1 nerve ventral ramus passes inferiorly to the joint capsule. The SIJ is innervated by the dorsal rami of L4-S3 nerve roots, transmitting nociception and temperature. Mechanisms of injury to the SIJ could arise from intra- and extra-articular etiologies, including capsular disruption, ligamentous tension, muscular inflammation, shearing, fractures, arthritis, and infection.2 Patients could develop SIJ pain spontaneously or after a traumatic event or repetitive shear.3 Risk factors for developing SIJ dysfunction include a history of lumbar fusion, scoliosis, leg length discrepancies, sustained athletic activity, pregnancy, seronegative HLA-B27 spondyloarthropathies, or gait abnormalities. Inflammation of the SIJ and surrounding structures secondary to an environmental insult in susceptible individuals is a common theme among these etiologies.2
Pain from the SIJ is localized to an area of approximately 3 cm × 10 cm that is inferior to the ipsilateral posterior superior iliac spine.4 Referred pain maps from SIJ dysfunction extend in the L5-S1 nerve distributions, commonly seen in the buttocks, groin, posterior thigh, and lower leg with radicular symptoms. However, this pain distribution demonstrates extensive variability among patients and bears strong similarities to discogenic or facet joint sources of LBP.5-7 Direct communication has been shown between the SIJ and adjacent neural structures, namely the L5 nerve, sacral foramina, and the lumbosacral plexus. These direct pathways could explain an inflammatory mechanism for lower extremity symptoms seen in SIJ dysfunction.8
The prevalence of SIJ dysfunction among patients with LBP is estimated to be 15% to 30%, an extraordinary number given the total number of patients presenting with LBP every year.9 These patients might represent a significant segment of patients with an unrevealing standard spine evaluation. Despite the large number of patients who experience SIJ dysfunction, there is disagreement about optimal methods for diagnosis and treatment.
Diagnosis
The International Association for the Study of Pain has proposed criteria for evaluating patients who have suspected SIJ dysfunction: Pain must be in the SIJ area, should be reproducible by performing specific provocative maneuvers, and must be relieved by injection of local anesthetic into the SIJ.10 These criteria provide a sound foundation, but in clinical practice, patients often defy categorization.
The presence of pain in the area inferior to the posterior superior iliac spine and lateral to the gluteal fold with pain referral patterns in the L5-S1 nerve distributions is highly sensitive for identifying patients with SIJ dysfunction. Furthermore, pain arising from the SIJ will not be above the level of the L5 nerve sensory distribution. However, this diagnostic finding alone is not specific and might represent other etiologies known to produce similar pain, such as intervertebral discs and facet joints. Patients with SIJ dysfunction often describe their pain as sciatica-like, recurrent, and triggered with bending or twisting motions. It is worsened with any activity loading the SIJ, such as walking, climbing stairs, standing, or sitting upright. SIJ pain might be accompanied by dyspareunia and changes in bladder function because of the nerves involved.11
The use of provocative maneuvers for testing SIJ dysfunction is controversial because of the high rate of false positives and the inability to distinguish whether the SIJ or an adjacent structure is affected. However, the diagnostic utility of specific stress tests has been studied, and clusters of tests are recommended if a health care provider (HCP) suspects SIJ dysfunction. A diagnostic algorithm should first focus on using the distraction test and the thigh thrust test. Distraction is done by applying vertically oriented pressure to the anterior superior iliac spine while aiming posteriorly, therefore distracting the SIJ. During the thigh thrust test the examiner fixates the patient’s sacrum against the table with the left hand and applies a vertical force through the line of the femur aiming posteriorly, producing a posterior shearing force at the SIJ. Studies show that the thigh thrust test is the most sensitive, and the distraction test is the most specific. If both tests are positive, there is reasonable evidence to suggest SIJ dysfunction as the source of LBP.
If there are not 2 positive results, the addition of the compression test, followed by the sacral thrust test also can point to the diagnosis. The compression test is performed with vertical downward force applied to the iliac crest with the patient lying on each side, compressing the SIJ by transverse pressure across the pelvis. The sacral thrust test is performed with vertical force applied to the midline posterior sacrum at its apex directed anteriorly with the patient lying prone, producing a shearing force at the SIJs. The Gaenslen test uses a torsion force by applying a superior and posterior force to the right knee and posteriorly directed force to the left knee. Omitting the Gaenslen test has not been shown to compromise diagnostic efficacy of the other tests and can be safely excluded.12
A HCP can rule out SIJ dysfunction if these provocation tests are negative. However, the diagnostic predictive value of these tests is subject to variability among HCPs, and their reliability is increased when used in clusters.9,13
Imaging for the SIJ should begin with anterior/posterior, oblique, and lateral view plain X-rays of the pelvis (Figures 1 and 2), which will rule out other pathologies by identifying other sources of LBP, such as spondylolisthesis or hip osteoarthritis. HCPs should obtain lumbar and pelvis CT images to identify inflammatory or degenerative changes within the SIJ. CT images provide the high resolution that is needed to identify pathologies, such as fractures and tumors within the pelvic ring that could cause similar pain. MRI does not reliably depict a dysfunctional ligamentous apparatus within the SIJ; however, it can help identify inflammatory sacroiliitis, such as is seen in the spondyloarthropathies.11,14 Recent studies show combined single photon emission tomography and CT (SPECT-CT) might be the most promising imaging modality to reveal mechanical failure of load transfer with increased scintigraphic uptake in the posterior and superior SIJ ligamentous attachments. The joint loses its characteristic “dumbbell” shape in affected patients with about 50% higher uptake than unaffected joints. These findings were evident in patients who experienced pelvic trauma or during the peripartum period.15,16
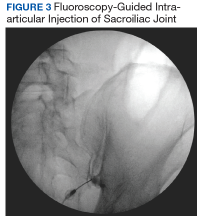
Fluoroscopy-guided intra-articular injection of a local anesthetic (lidocaine) and/or a corticosteroid (triamcinolone) has the dual functionality of diagnosis and treatment (Figure 3). It often is considered the most reliable method to diagnose SIJ dysfunction and has the benefit of pain relief for up to 1 year. However, intra-articular injections lack diagnostic validity because the solution often extravasates to extracapsular structures. This confounds the source of the pain and makes it difficult to interpret these diagnostic injections. In addition, the injection might not reach the entire SIJ capsule and could result in a false-negative diagnosis.17,18 Periarticular injections have been shown to result in better pain relief in patients diagnosed with SIJ dysfunction than intra-articular injections. Periarticular injections also are easier to perform and could be a first-step option for these patients.19
Treatment
Nonoperative management of SIJ dysfunction includes exercise programs, physical therapy, manual manipulation therapy, sacroiliac belts, and periodic articular injections. Efficacy of these methods is variable, and analgesics often do not significantly benefit this type of pain. Another nonoperative approach is radiofrequency ablation (RFA) of the lumbar dorsal rami and lateral sacral branches, which can vary based on the number of rami treated as well as the technique used. About two-thirds of patients report pain relief after RFA.2 When successful, pain is relieved for 6 to 12 months, which is a temporary yet effective option for patients experiencing SIJ dysfunction.14,20
Fusion Surgery
Cadaver studies show that biomechanical stabilization of the SIJ leads to decreased range of motion in flexion/extension, lateral bending, and axial rotation. This results in a decreased need for periarticular muscular and ligamentous support, therefore facilitating load transfer across the SIJ.21,22 Patients undergoing minimally invasive surgery report better pain relief compared with those receiving open surgery at 12 months postoperatively.23 The 2 main SIJ fusion approaches used are the lateral transarticular and the dorsal approaches. In the dorsal approach, the SIJ is distracted and allograft dowels or titanium cages with graft are inserted into the joint space posteriorly through the back. When approaching laterally, hollow screw implants filled with graft or triangular titanium implants are placed across the joint, accessing the SIJ through the iliac bones using imaging guidance. This lateral transiliac approach using porous titanium triangular rods currently is the most studied technique.24
A recent prospective, multicenter trial included 423 patients with SIJ dysfunction who were randomized to receive SIJ fusion with triangular titanium implants vs a control group who received nonoperative management. Patients in the SIJ fusion group showed substantially greater improvement in pain (81.4%) compared with that of the nonoperative group (26.1%) 6 months after surgery. Pain relief in the SIJ fusion group was maintained at > 80% at 1 and 2 year follow-up, while the nonoperative group’s pain relief decreased to < 10% at the follow-ups. Measures of quality of life and disability also improved for the SIJ fusion group compared with that of the nonoperative group. Patients who were crossed over from conservative management to SIJ fusion after 6 months demonstrated improvements that were similar to those in the SIJ fusion group by the end of the study. Only 3% of patients required surgical revision. The strongest predictor of pain relief after surgery was a diagnostic SIJ anesthetic block of 30 to 60 minutes, which resulted in > 75% pain reduction.21,25 Additional predictors of successful SIJ fusion include nonsmokers, nonopioid users, and older patients who have a longer time course of SIJ pain.26
Another study investigating the outcomes of SIJ fusion, RFA, and conservative management with a 6-year follow-up demonstrated similar results.27 This further confirms the durability of the surgical group’s outcome, which sustained significant improvement compared with RFA and conservative management group in pain relief, daily function, and opioid use.
HCPs should consider SIJ fusion for patients who have at least 6 months of unsuccessful nonoperative management, significant SIJ pain (> 5 in a 10-point scale), ≥ 3 positive provocation tests, and at least 50% pain relief (> 75% preferred) with diagnostic intra-articular anesthetic injection.14 It is reasonable for primary care providers to refer these patients to a neurosurgeon or orthopedic spine surgeon for possible fusion. Patients with earlier lumbar/lumbosacral spinal fusions and persistent LBP should be evaluated for potential SIJ dysfunction. SIJ dysfunction after lumbosacral fusion could be considered a form of distal pseudarthrosis resulting from increased motion at the joint. One study found its incidence correlated with the number of segments fused in the lumbar spine.28 Another study found that about one-third of patients with persistent LBP after lumbosacral fusion could be attributed to SIJ dysfunction.29
Case Presentation
A 27-year-old female army veteran presented with bilateral buttock pain, which she described as a dull, aching pain across her sacral region, 8 out of 10 in severity. The pain was in a L5-S1 pattern. The pain was bilateral, with the right side worse than the left, and worsened with lateral bending and load transferring. She reported no numbness, tingling, or weakness.
On physical examination, she had full strength in her lower extremities and intact sensation. She reported tenderness to palpation of the sacrum and SIJ. Her gait was normal. The patient had positive thigh thrust and distraction tests. Lumbar spine X-ray, CT, MRI, and electromyographic studies did not show any pathology. She described little or no relief with analgesics or physical therapy. Previous L4-L5 and L5-S1 facet anesthetic injections and transforaminal epidural steroid injections provided minimal pain relief immediately after the procedures. Bilateral SIJ anesthetic injections under fluoroscopic guidance decreased her pain severity from a 7 to 3 out of 10 for 2 to 3 months before returning to her baseline. Radiofrequency ablation of the right SIJ under fluoroscopy provided moderate relief for about 4 months.
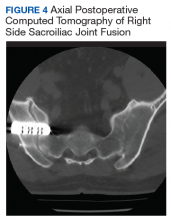
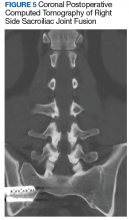
After exhausting nonoperative management for SIJ dysfunction without adequate pain control, the patient was referred to neurosurgery for surgical fusion. The patient was deemed an appropriate surgical candidate and underwent a right-sided SIJ fusion (Figures 4 and 5). At her 6-month and 1-year follow-up appointments, she had lasting pain relief, 2 out of 10.
Conclusion
SIJ dysfunction is widely overlooked because of the difficulty in distinguishing it from other similarly presenting syndromes. However, with a detailed history, appropriate physical maneuvers, imaging, and adequate response to intra-articular anesthetic, providers can reach an accurate diagnosis that will inform subsequent treatments. After failure of nonsurgical methods, patients with SIJ dysfunction should be considered for minimally invasive fusion techniques, which have proven to be a safe, effective, and viable treatment option.
1. Zaidi HA, Montoure AJ, Dickman CA. Surgical and clinical efficacy of sacroiliac joint fusion: a systematic review of the literature. J Neurosurg Spine. 2015;23(1):59-66.
2. Cohen SP. Sacroiliac joint pain: a comprehensive review of anatomy, diagnosis, and treatment. Anesth Analg. 2005;101(5):1440-1453.
3. Chou LH, Slipman CW, Bhagia SM, et al. Inciting events initiating injection‐proven sacroiliac joint syndrome. Pain Med. 2004;5(1):26-32.
4. Dreyfuss P, Dreyer SJ, Cole A, Mayo K. Sacroiliac joint pain. J Am Acad Orthop Surg. 2004;12(4):255-265.
5. Buijs E, Visser L, Groen G. Sciatica and the sacroiliac joint: a forgotten concept. Br J Anaesth. 2007;99(5):713-716.
6. Fortin JD, Dwyer AP, West S, Pier J. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I: asymptomatic volunteers. Spine (Phila Pa 1976). 1994;19(13):1475-1482.
7. Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976). 1995;20(1):31-37.
8. Fortin JD, Washington WJ, Falco FJ. Three pathways between the sacroiliac joint and neural structures. ANJR Am J Neuroradiol. 1999;20(8):1429-1434.
9. Szadek KM, van der Wurff P, van Tulder MW, Zuurmond WW, Perez RS. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain. 2009;10(4):354-368.
10. Merskey H, Bogduk N, eds. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed. Seattle, WA: IASP Press; 1994.
11. Cusi MF. Paradigm for assessment and treatment of SIJ mechanical dysfunction. J Bodyw Mov Ther. 2010;14(2):152-161.
12. Laslett M, Aprill CN, McDonald B, Young SB. Diagnosis of sacroiliac joint pain: validity of individual provocation tests and composites of tests. Man Ther. 2005;10(3):207-218.
13. Laslett M. Evidence-based diagnosis and treatment of the painful sacroiliac joint. J Man Manip Ther. 2008;16(3):142-152.
14. Polly DW Jr. The sacroiliac joint. Neurosurg Clin N Am. 2017;28(3):301-312.
15. Cusi M, Van Der Wall H, Saunders J, Fogelman I. Metabolic disturbances identified by SPECT-CT in patients with a clinical diagnosis of sacroiliac joint incompetence. Eur Spine J. 2013;22(7):1674-1682.
16. Tofuku K, Koga H, Komiya S. The diagnostic value of single-photon emission computed tomography/computed tomography for severe sacroiliac joint dysfunction. Eur Spine J. 2015;24(4):859-863.
17. Kennedy DJ, Engel A, Kreiner DS, Nampiaparampil D, Duszynski B, MacVicar J. Fluoroscopically guided diagnostic and therapeutic intra‐articular sacroiliac joint injections: a systematic review. Pain Med. 2015;16(8):1500-1518.
18. Schneider BJ, Huynh L, Levin J, Rinkaekan P, Kordi R, Kennedy DJ. Does immediate pain relief after an injection into the sacroiliac joint with anesthetic and corticosteroid predict subsequent pain relief? Pain Med. 2018;19(2):244-251.
19. Murakami E, Tanaka Y, Aizawa T, Ishizuka M, Kokubun S. Effect of periarticular and intraarticular lidocaine injections for sacroiliac joint pain: prospective comparative study. J Orthop Sci. 2007;12(3):274-280.
20. Cohen SP, Hurley RW, Buckenmaier CC 3rd, Kurihara C, Morlando B, Dragovich A. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279-288.
21. Polly DW, Cher DJ, Wine KD, et al; INSITE Study Group. Randomized controlled trial of minimally invasive sacroiliac joint fusion using triangular titanium implants vs nonsurgical management for sacroiliac joint dysfunction: 12-month outcomes. Neurosurgery. 2015;77(5):674-690.
22. Soriano-Baron H, Lindsey DP, Rodriguez-Martinez N, et al. The effect of implant placement on sacroiliac joint range of motion: posterior versus transarticular. Spine. 2015;40(9):E525-E530.
23. Smith AG, Capobianco R, Cher D, et al. Open versus minimally invasive sacroiliac joint fusion: a multi-center comparison of perioperative measures and clinical outcomes. Ann Surg Innov Res. 2013;7(1):14.
24. Rashbaum RF, Ohnmeiss DD, Lindley EM, Kitchel SH, Patel VV. Sacroiliac joint pain and its treatment. Clin Spine Surg. 2016;29(2):42-48.
25. Polly DW, Swofford J, Whang PG, et al. Two-year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion vs. non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg. 2016;10:28.
26. Dengler J, Duhon B, Whang P, et al. Predictors of outcome in conservative and minimally invasive surgical management of pain originating from the sacroiliac joint: a pooled analysis. Spine (Phila Pa 1976). 2017;42(21):1664-1673.
27. Vanaclocha V, Herrera JM, Sáiz-Sapena N, Rivera-Paz M, Verdú-López F. Minimally invasive sacroiliac joint fusion, radiofrequency denervation, and conservative management for sacroiliac joint pain: 6-year comparative case series. Neurosurgery. 2018;82(1):48-55.
28. Unoki E, Abe E, Murai H, Kobayashi T, Abe T. Fusion of multiple segments can increase the incidence of sacroiliac joint pain after lumbar or lumbosacral fusion. Spine (Phila Pa 1976). 2016;41(12):999-1005.
29. Katz V, Schofferman J, Reynolds J. The sacroiliac joint: a potential cause of pain after lumbar fusion to the sacrum. J Spinal Disord Tech. 2003;16(1):96-99.
Patients experiencing sacroiliac joint (SIJ) dysfunction might show symptoms that overlap with those seen in lumbar spine pathology. This article reviews diagnostic tools that assist practitioners to discern the true pain generator in patients with low back pain (LBP) and therapeutic approaches when the cause is SIJ dysfunction.
Prevalence
Most of the US population will experience LBP at some point in their lives. A 2002 National Health Interview survey found that more than one-quarter (26.4%) of 31 044 respondents had complained of LBP in the previous 3 months.1 About 74 million individuals in the US experienced LBP in the past 3 months.1 A full 10% of the US population is expected to suffer from chronic LBP, and it is estimated that 2.3% of all visits to physicians are related to LBP.1
The etiology of LBP often is unclear even after thorough clinical and radiographic evaluation because of the myriad possible mechanisms. Degenerative disc disease, facet arthropathy, ligamentous hypertrophy, muscle spasm, hip arthropathy, and SIJ dysfunction are potential pain generators and exact clinical and radiographic correlation is not always possible. Compounding this difficulty is the lack of specificity with current diagnostic techniques. For example, many patients will have disc desiccation or herniation without any LBP or radicular symptoms on radiographic studies, such as X-rays, computed tomography (CT), and magnetic resonance imaging (MRI). As such, providers of patients with diffuse radiographic abnormalities often have to identify a specific pain generator, which might not have any role in the patient’s pain.
Other tests, such as electromyographic studies, positron emission tomography (PET) scans, discography, and epidural steroid injections, can help pinpoint a specific pain generator. These tests might help determine whether the patient has a surgically treatable condition and could help predict whether a patient’s symptoms will respond to surgery.
However, the standard spine surgery workup often fails to identify an obvious pain generator in many individuals. The significant number of patients that fall into this category has prompted spine surgeons to consider other potential etiologies for LBP, and SIJ dysfunction has become a rapidly developing field of research.
Sacroiliac Joint Dysfunction
The SIJ is a bilateral, C-shaped synovial joint surrounded by a fibrous capsule and affixes the sacrum to the ilia. Several sacral ligaments and pelvic muscles support the SIJ. The L5 nerve ventral ramus and lumbosacral trunk pass anteriorly and the S1 nerve ventral ramus passes inferiorly to the joint capsule. The SIJ is innervated by the dorsal rami of L4-S3 nerve roots, transmitting nociception and temperature. Mechanisms of injury to the SIJ could arise from intra- and extra-articular etiologies, including capsular disruption, ligamentous tension, muscular inflammation, shearing, fractures, arthritis, and infection.2 Patients could develop SIJ pain spontaneously or after a traumatic event or repetitive shear.3 Risk factors for developing SIJ dysfunction include a history of lumbar fusion, scoliosis, leg length discrepancies, sustained athletic activity, pregnancy, seronegative HLA-B27 spondyloarthropathies, or gait abnormalities. Inflammation of the SIJ and surrounding structures secondary to an environmental insult in susceptible individuals is a common theme among these etiologies.2
Pain from the SIJ is localized to an area of approximately 3 cm × 10 cm that is inferior to the ipsilateral posterior superior iliac spine.4 Referred pain maps from SIJ dysfunction extend in the L5-S1 nerve distributions, commonly seen in the buttocks, groin, posterior thigh, and lower leg with radicular symptoms. However, this pain distribution demonstrates extensive variability among patients and bears strong similarities to discogenic or facet joint sources of LBP.5-7 Direct communication has been shown between the SIJ and adjacent neural structures, namely the L5 nerve, sacral foramina, and the lumbosacral plexus. These direct pathways could explain an inflammatory mechanism for lower extremity symptoms seen in SIJ dysfunction.8
The prevalence of SIJ dysfunction among patients with LBP is estimated to be 15% to 30%, an extraordinary number given the total number of patients presenting with LBP every year.9 These patients might represent a significant segment of patients with an unrevealing standard spine evaluation. Despite the large number of patients who experience SIJ dysfunction, there is disagreement about optimal methods for diagnosis and treatment.
Diagnosis
The International Association for the Study of Pain has proposed criteria for evaluating patients who have suspected SIJ dysfunction: Pain must be in the SIJ area, should be reproducible by performing specific provocative maneuvers, and must be relieved by injection of local anesthetic into the SIJ.10 These criteria provide a sound foundation, but in clinical practice, patients often defy categorization.
The presence of pain in the area inferior to the posterior superior iliac spine and lateral to the gluteal fold with pain referral patterns in the L5-S1 nerve distributions is highly sensitive for identifying patients with SIJ dysfunction. Furthermore, pain arising from the SIJ will not be above the level of the L5 nerve sensory distribution. However, this diagnostic finding alone is not specific and might represent other etiologies known to produce similar pain, such as intervertebral discs and facet joints. Patients with SIJ dysfunction often describe their pain as sciatica-like, recurrent, and triggered with bending or twisting motions. It is worsened with any activity loading the SIJ, such as walking, climbing stairs, standing, or sitting upright. SIJ pain might be accompanied by dyspareunia and changes in bladder function because of the nerves involved.11
The use of provocative maneuvers for testing SIJ dysfunction is controversial because of the high rate of false positives and the inability to distinguish whether the SIJ or an adjacent structure is affected. However, the diagnostic utility of specific stress tests has been studied, and clusters of tests are recommended if a health care provider (HCP) suspects SIJ dysfunction. A diagnostic algorithm should first focus on using the distraction test and the thigh thrust test. Distraction is done by applying vertically oriented pressure to the anterior superior iliac spine while aiming posteriorly, therefore distracting the SIJ. During the thigh thrust test the examiner fixates the patient’s sacrum against the table with the left hand and applies a vertical force through the line of the femur aiming posteriorly, producing a posterior shearing force at the SIJ. Studies show that the thigh thrust test is the most sensitive, and the distraction test is the most specific. If both tests are positive, there is reasonable evidence to suggest SIJ dysfunction as the source of LBP.
If there are not 2 positive results, the addition of the compression test, followed by the sacral thrust test also can point to the diagnosis. The compression test is performed with vertical downward force applied to the iliac crest with the patient lying on each side, compressing the SIJ by transverse pressure across the pelvis. The sacral thrust test is performed with vertical force applied to the midline posterior sacrum at its apex directed anteriorly with the patient lying prone, producing a shearing force at the SIJs. The Gaenslen test uses a torsion force by applying a superior and posterior force to the right knee and posteriorly directed force to the left knee. Omitting the Gaenslen test has not been shown to compromise diagnostic efficacy of the other tests and can be safely excluded.12
A HCP can rule out SIJ dysfunction if these provocation tests are negative. However, the diagnostic predictive value of these tests is subject to variability among HCPs, and their reliability is increased when used in clusters.9,13
Imaging for the SIJ should begin with anterior/posterior, oblique, and lateral view plain X-rays of the pelvis (Figures 1 and 2), which will rule out other pathologies by identifying other sources of LBP, such as spondylolisthesis or hip osteoarthritis. HCPs should obtain lumbar and pelvis CT images to identify inflammatory or degenerative changes within the SIJ. CT images provide the high resolution that is needed to identify pathologies, such as fractures and tumors within the pelvic ring that could cause similar pain. MRI does not reliably depict a dysfunctional ligamentous apparatus within the SIJ; however, it can help identify inflammatory sacroiliitis, such as is seen in the spondyloarthropathies.11,14 Recent studies show combined single photon emission tomography and CT (SPECT-CT) might be the most promising imaging modality to reveal mechanical failure of load transfer with increased scintigraphic uptake in the posterior and superior SIJ ligamentous attachments. The joint loses its characteristic “dumbbell” shape in affected patients with about 50% higher uptake than unaffected joints. These findings were evident in patients who experienced pelvic trauma or during the peripartum period.15,16
Fluoroscopy-guided intra-articular injection of a local anesthetic (lidocaine) and/or a corticosteroid (triamcinolone) has the dual functionality of diagnosis and treatment (Figure 3). It often is considered the most reliable method to diagnose SIJ dysfunction and has the benefit of pain relief for up to 1 year. However, intra-articular injections lack diagnostic validity because the solution often extravasates to extracapsular structures. This confounds the source of the pain and makes it difficult to interpret these diagnostic injections. In addition, the injection might not reach the entire SIJ capsule and could result in a false-negative diagnosis.17,18 Periarticular injections have been shown to result in better pain relief in patients diagnosed with SIJ dysfunction than intra-articular injections. Periarticular injections also are easier to perform and could be a first-step option for these patients.19
Treatment
Nonoperative management of SIJ dysfunction includes exercise programs, physical therapy, manual manipulation therapy, sacroiliac belts, and periodic articular injections. Efficacy of these methods is variable, and analgesics often do not significantly benefit this type of pain. Another nonoperative approach is radiofrequency ablation (RFA) of the lumbar dorsal rami and lateral sacral branches, which can vary based on the number of rami treated as well as the technique used. About two-thirds of patients report pain relief after RFA.2 When successful, pain is relieved for 6 to 12 months, which is a temporary yet effective option for patients experiencing SIJ dysfunction.14,20
Fusion Surgery
Cadaver studies show that biomechanical stabilization of the SIJ leads to decreased range of motion in flexion/extension, lateral bending, and axial rotation. This results in a decreased need for periarticular muscular and ligamentous support, therefore facilitating load transfer across the SIJ.21,22 Patients undergoing minimally invasive surgery report better pain relief compared with those receiving open surgery at 12 months postoperatively.23 The 2 main SIJ fusion approaches used are the lateral transarticular and the dorsal approaches. In the dorsal approach, the SIJ is distracted and allograft dowels or titanium cages with graft are inserted into the joint space posteriorly through the back. When approaching laterally, hollow screw implants filled with graft or triangular titanium implants are placed across the joint, accessing the SIJ through the iliac bones using imaging guidance. This lateral transiliac approach using porous titanium triangular rods currently is the most studied technique.24
A recent prospective, multicenter trial included 423 patients with SIJ dysfunction who were randomized to receive SIJ fusion with triangular titanium implants vs a control group who received nonoperative management. Patients in the SIJ fusion group showed substantially greater improvement in pain (81.4%) compared with that of the nonoperative group (26.1%) 6 months after surgery. Pain relief in the SIJ fusion group was maintained at > 80% at 1 and 2 year follow-up, while the nonoperative group’s pain relief decreased to < 10% at the follow-ups. Measures of quality of life and disability also improved for the SIJ fusion group compared with that of the nonoperative group. Patients who were crossed over from conservative management to SIJ fusion after 6 months demonstrated improvements that were similar to those in the SIJ fusion group by the end of the study. Only 3% of patients required surgical revision. The strongest predictor of pain relief after surgery was a diagnostic SIJ anesthetic block of 30 to 60 minutes, which resulted in > 75% pain reduction.21,25 Additional predictors of successful SIJ fusion include nonsmokers, nonopioid users, and older patients who have a longer time course of SIJ pain.26
Another study investigating the outcomes of SIJ fusion, RFA, and conservative management with a 6-year follow-up demonstrated similar results.27 This further confirms the durability of the surgical group’s outcome, which sustained significant improvement compared with RFA and conservative management group in pain relief, daily function, and opioid use.
HCPs should consider SIJ fusion for patients who have at least 6 months of unsuccessful nonoperative management, significant SIJ pain (> 5 in a 10-point scale), ≥ 3 positive provocation tests, and at least 50% pain relief (> 75% preferred) with diagnostic intra-articular anesthetic injection.14 It is reasonable for primary care providers to refer these patients to a neurosurgeon or orthopedic spine surgeon for possible fusion. Patients with earlier lumbar/lumbosacral spinal fusions and persistent LBP should be evaluated for potential SIJ dysfunction. SIJ dysfunction after lumbosacral fusion could be considered a form of distal pseudarthrosis resulting from increased motion at the joint. One study found its incidence correlated with the number of segments fused in the lumbar spine.28 Another study found that about one-third of patients with persistent LBP after lumbosacral fusion could be attributed to SIJ dysfunction.29
Case Presentation
A 27-year-old female army veteran presented with bilateral buttock pain, which she described as a dull, aching pain across her sacral region, 8 out of 10 in severity. The pain was in a L5-S1 pattern. The pain was bilateral, with the right side worse than the left, and worsened with lateral bending and load transferring. She reported no numbness, tingling, or weakness.
On physical examination, she had full strength in her lower extremities and intact sensation. She reported tenderness to palpation of the sacrum and SIJ. Her gait was normal. The patient had positive thigh thrust and distraction tests. Lumbar spine X-ray, CT, MRI, and electromyographic studies did not show any pathology. She described little or no relief with analgesics or physical therapy. Previous L4-L5 and L5-S1 facet anesthetic injections and transforaminal epidural steroid injections provided minimal pain relief immediately after the procedures. Bilateral SIJ anesthetic injections under fluoroscopic guidance decreased her pain severity from a 7 to 3 out of 10 for 2 to 3 months before returning to her baseline. Radiofrequency ablation of the right SIJ under fluoroscopy provided moderate relief for about 4 months.
After exhausting nonoperative management for SIJ dysfunction without adequate pain control, the patient was referred to neurosurgery for surgical fusion. The patient was deemed an appropriate surgical candidate and underwent a right-sided SIJ fusion (Figures 4 and 5). At her 6-month and 1-year follow-up appointments, she had lasting pain relief, 2 out of 10.
Conclusion
SIJ dysfunction is widely overlooked because of the difficulty in distinguishing it from other similarly presenting syndromes. However, with a detailed history, appropriate physical maneuvers, imaging, and adequate response to intra-articular anesthetic, providers can reach an accurate diagnosis that will inform subsequent treatments. After failure of nonsurgical methods, patients with SIJ dysfunction should be considered for minimally invasive fusion techniques, which have proven to be a safe, effective, and viable treatment option.
Patients experiencing sacroiliac joint (SIJ) dysfunction might show symptoms that overlap with those seen in lumbar spine pathology. This article reviews diagnostic tools that assist practitioners to discern the true pain generator in patients with low back pain (LBP) and therapeutic approaches when the cause is SIJ dysfunction.
Prevalence
Most of the US population will experience LBP at some point in their lives. A 2002 National Health Interview survey found that more than one-quarter (26.4%) of 31 044 respondents had complained of LBP in the previous 3 months.1 About 74 million individuals in the US experienced LBP in the past 3 months.1 A full 10% of the US population is expected to suffer from chronic LBP, and it is estimated that 2.3% of all visits to physicians are related to LBP.1
The etiology of LBP often is unclear even after thorough clinical and radiographic evaluation because of the myriad possible mechanisms. Degenerative disc disease, facet arthropathy, ligamentous hypertrophy, muscle spasm, hip arthropathy, and SIJ dysfunction are potential pain generators and exact clinical and radiographic correlation is not always possible. Compounding this difficulty is the lack of specificity with current diagnostic techniques. For example, many patients will have disc desiccation or herniation without any LBP or radicular symptoms on radiographic studies, such as X-rays, computed tomography (CT), and magnetic resonance imaging (MRI). As such, providers of patients with diffuse radiographic abnormalities often have to identify a specific pain generator, which might not have any role in the patient’s pain.
Other tests, such as electromyographic studies, positron emission tomography (PET) scans, discography, and epidural steroid injections, can help pinpoint a specific pain generator. These tests might help determine whether the patient has a surgically treatable condition and could help predict whether a patient’s symptoms will respond to surgery.
However, the standard spine surgery workup often fails to identify an obvious pain generator in many individuals. The significant number of patients that fall into this category has prompted spine surgeons to consider other potential etiologies for LBP, and SIJ dysfunction has become a rapidly developing field of research.
Sacroiliac Joint Dysfunction
The SIJ is a bilateral, C-shaped synovial joint surrounded by a fibrous capsule and affixes the sacrum to the ilia. Several sacral ligaments and pelvic muscles support the SIJ. The L5 nerve ventral ramus and lumbosacral trunk pass anteriorly and the S1 nerve ventral ramus passes inferiorly to the joint capsule. The SIJ is innervated by the dorsal rami of L4-S3 nerve roots, transmitting nociception and temperature. Mechanisms of injury to the SIJ could arise from intra- and extra-articular etiologies, including capsular disruption, ligamentous tension, muscular inflammation, shearing, fractures, arthritis, and infection.2 Patients could develop SIJ pain spontaneously or after a traumatic event or repetitive shear.3 Risk factors for developing SIJ dysfunction include a history of lumbar fusion, scoliosis, leg length discrepancies, sustained athletic activity, pregnancy, seronegative HLA-B27 spondyloarthropathies, or gait abnormalities. Inflammation of the SIJ and surrounding structures secondary to an environmental insult in susceptible individuals is a common theme among these etiologies.2
Pain from the SIJ is localized to an area of approximately 3 cm × 10 cm that is inferior to the ipsilateral posterior superior iliac spine.4 Referred pain maps from SIJ dysfunction extend in the L5-S1 nerve distributions, commonly seen in the buttocks, groin, posterior thigh, and lower leg with radicular symptoms. However, this pain distribution demonstrates extensive variability among patients and bears strong similarities to discogenic or facet joint sources of LBP.5-7 Direct communication has been shown between the SIJ and adjacent neural structures, namely the L5 nerve, sacral foramina, and the lumbosacral plexus. These direct pathways could explain an inflammatory mechanism for lower extremity symptoms seen in SIJ dysfunction.8
The prevalence of SIJ dysfunction among patients with LBP is estimated to be 15% to 30%, an extraordinary number given the total number of patients presenting with LBP every year.9 These patients might represent a significant segment of patients with an unrevealing standard spine evaluation. Despite the large number of patients who experience SIJ dysfunction, there is disagreement about optimal methods for diagnosis and treatment.
Diagnosis
The International Association for the Study of Pain has proposed criteria for evaluating patients who have suspected SIJ dysfunction: Pain must be in the SIJ area, should be reproducible by performing specific provocative maneuvers, and must be relieved by injection of local anesthetic into the SIJ.10 These criteria provide a sound foundation, but in clinical practice, patients often defy categorization.
The presence of pain in the area inferior to the posterior superior iliac spine and lateral to the gluteal fold with pain referral patterns in the L5-S1 nerve distributions is highly sensitive for identifying patients with SIJ dysfunction. Furthermore, pain arising from the SIJ will not be above the level of the L5 nerve sensory distribution. However, this diagnostic finding alone is not specific and might represent other etiologies known to produce similar pain, such as intervertebral discs and facet joints. Patients with SIJ dysfunction often describe their pain as sciatica-like, recurrent, and triggered with bending or twisting motions. It is worsened with any activity loading the SIJ, such as walking, climbing stairs, standing, or sitting upright. SIJ pain might be accompanied by dyspareunia and changes in bladder function because of the nerves involved.11
The use of provocative maneuvers for testing SIJ dysfunction is controversial because of the high rate of false positives and the inability to distinguish whether the SIJ or an adjacent structure is affected. However, the diagnostic utility of specific stress tests has been studied, and clusters of tests are recommended if a health care provider (HCP) suspects SIJ dysfunction. A diagnostic algorithm should first focus on using the distraction test and the thigh thrust test. Distraction is done by applying vertically oriented pressure to the anterior superior iliac spine while aiming posteriorly, therefore distracting the SIJ. During the thigh thrust test the examiner fixates the patient’s sacrum against the table with the left hand and applies a vertical force through the line of the femur aiming posteriorly, producing a posterior shearing force at the SIJ. Studies show that the thigh thrust test is the most sensitive, and the distraction test is the most specific. If both tests are positive, there is reasonable evidence to suggest SIJ dysfunction as the source of LBP.
If there are not 2 positive results, the addition of the compression test, followed by the sacral thrust test also can point to the diagnosis. The compression test is performed with vertical downward force applied to the iliac crest with the patient lying on each side, compressing the SIJ by transverse pressure across the pelvis. The sacral thrust test is performed with vertical force applied to the midline posterior sacrum at its apex directed anteriorly with the patient lying prone, producing a shearing force at the SIJs. The Gaenslen test uses a torsion force by applying a superior and posterior force to the right knee and posteriorly directed force to the left knee. Omitting the Gaenslen test has not been shown to compromise diagnostic efficacy of the other tests and can be safely excluded.12
A HCP can rule out SIJ dysfunction if these provocation tests are negative. However, the diagnostic predictive value of these tests is subject to variability among HCPs, and their reliability is increased when used in clusters.9,13
Imaging for the SIJ should begin with anterior/posterior, oblique, and lateral view plain X-rays of the pelvis (Figures 1 and 2), which will rule out other pathologies by identifying other sources of LBP, such as spondylolisthesis or hip osteoarthritis. HCPs should obtain lumbar and pelvis CT images to identify inflammatory or degenerative changes within the SIJ. CT images provide the high resolution that is needed to identify pathologies, such as fractures and tumors within the pelvic ring that could cause similar pain. MRI does not reliably depict a dysfunctional ligamentous apparatus within the SIJ; however, it can help identify inflammatory sacroiliitis, such as is seen in the spondyloarthropathies.11,14 Recent studies show combined single photon emission tomography and CT (SPECT-CT) might be the most promising imaging modality to reveal mechanical failure of load transfer with increased scintigraphic uptake in the posterior and superior SIJ ligamentous attachments. The joint loses its characteristic “dumbbell” shape in affected patients with about 50% higher uptake than unaffected joints. These findings were evident in patients who experienced pelvic trauma or during the peripartum period.15,16
Fluoroscopy-guided intra-articular injection of a local anesthetic (lidocaine) and/or a corticosteroid (triamcinolone) has the dual functionality of diagnosis and treatment (Figure 3). It often is considered the most reliable method to diagnose SIJ dysfunction and has the benefit of pain relief for up to 1 year. However, intra-articular injections lack diagnostic validity because the solution often extravasates to extracapsular structures. This confounds the source of the pain and makes it difficult to interpret these diagnostic injections. In addition, the injection might not reach the entire SIJ capsule and could result in a false-negative diagnosis.17,18 Periarticular injections have been shown to result in better pain relief in patients diagnosed with SIJ dysfunction than intra-articular injections. Periarticular injections also are easier to perform and could be a first-step option for these patients.19
Treatment
Nonoperative management of SIJ dysfunction includes exercise programs, physical therapy, manual manipulation therapy, sacroiliac belts, and periodic articular injections. Efficacy of these methods is variable, and analgesics often do not significantly benefit this type of pain. Another nonoperative approach is radiofrequency ablation (RFA) of the lumbar dorsal rami and lateral sacral branches, which can vary based on the number of rami treated as well as the technique used. About two-thirds of patients report pain relief after RFA.2 When successful, pain is relieved for 6 to 12 months, which is a temporary yet effective option for patients experiencing SIJ dysfunction.14,20
Fusion Surgery
Cadaver studies show that biomechanical stabilization of the SIJ leads to decreased range of motion in flexion/extension, lateral bending, and axial rotation. This results in a decreased need for periarticular muscular and ligamentous support, therefore facilitating load transfer across the SIJ.21,22 Patients undergoing minimally invasive surgery report better pain relief compared with those receiving open surgery at 12 months postoperatively.23 The 2 main SIJ fusion approaches used are the lateral transarticular and the dorsal approaches. In the dorsal approach, the SIJ is distracted and allograft dowels or titanium cages with graft are inserted into the joint space posteriorly through the back. When approaching laterally, hollow screw implants filled with graft or triangular titanium implants are placed across the joint, accessing the SIJ through the iliac bones using imaging guidance. This lateral transiliac approach using porous titanium triangular rods currently is the most studied technique.24
A recent prospective, multicenter trial included 423 patients with SIJ dysfunction who were randomized to receive SIJ fusion with triangular titanium implants vs a control group who received nonoperative management. Patients in the SIJ fusion group showed substantially greater improvement in pain (81.4%) compared with that of the nonoperative group (26.1%) 6 months after surgery. Pain relief in the SIJ fusion group was maintained at > 80% at 1 and 2 year follow-up, while the nonoperative group’s pain relief decreased to < 10% at the follow-ups. Measures of quality of life and disability also improved for the SIJ fusion group compared with that of the nonoperative group. Patients who were crossed over from conservative management to SIJ fusion after 6 months demonstrated improvements that were similar to those in the SIJ fusion group by the end of the study. Only 3% of patients required surgical revision. The strongest predictor of pain relief after surgery was a diagnostic SIJ anesthetic block of 30 to 60 minutes, which resulted in > 75% pain reduction.21,25 Additional predictors of successful SIJ fusion include nonsmokers, nonopioid users, and older patients who have a longer time course of SIJ pain.26
Another study investigating the outcomes of SIJ fusion, RFA, and conservative management with a 6-year follow-up demonstrated similar results.27 This further confirms the durability of the surgical group’s outcome, which sustained significant improvement compared with RFA and conservative management group in pain relief, daily function, and opioid use.
HCPs should consider SIJ fusion for patients who have at least 6 months of unsuccessful nonoperative management, significant SIJ pain (> 5 in a 10-point scale), ≥ 3 positive provocation tests, and at least 50% pain relief (> 75% preferred) with diagnostic intra-articular anesthetic injection.14 It is reasonable for primary care providers to refer these patients to a neurosurgeon or orthopedic spine surgeon for possible fusion. Patients with earlier lumbar/lumbosacral spinal fusions and persistent LBP should be evaluated for potential SIJ dysfunction. SIJ dysfunction after lumbosacral fusion could be considered a form of distal pseudarthrosis resulting from increased motion at the joint. One study found its incidence correlated with the number of segments fused in the lumbar spine.28 Another study found that about one-third of patients with persistent LBP after lumbosacral fusion could be attributed to SIJ dysfunction.29
Case Presentation
A 27-year-old female army veteran presented with bilateral buttock pain, which she described as a dull, aching pain across her sacral region, 8 out of 10 in severity. The pain was in a L5-S1 pattern. The pain was bilateral, with the right side worse than the left, and worsened with lateral bending and load transferring. She reported no numbness, tingling, or weakness.
On physical examination, she had full strength in her lower extremities and intact sensation. She reported tenderness to palpation of the sacrum and SIJ. Her gait was normal. The patient had positive thigh thrust and distraction tests. Lumbar spine X-ray, CT, MRI, and electromyographic studies did not show any pathology. She described little or no relief with analgesics or physical therapy. Previous L4-L5 and L5-S1 facet anesthetic injections and transforaminal epidural steroid injections provided minimal pain relief immediately after the procedures. Bilateral SIJ anesthetic injections under fluoroscopic guidance decreased her pain severity from a 7 to 3 out of 10 for 2 to 3 months before returning to her baseline. Radiofrequency ablation of the right SIJ under fluoroscopy provided moderate relief for about 4 months.
After exhausting nonoperative management for SIJ dysfunction without adequate pain control, the patient was referred to neurosurgery for surgical fusion. The patient was deemed an appropriate surgical candidate and underwent a right-sided SIJ fusion (Figures 4 and 5). At her 6-month and 1-year follow-up appointments, she had lasting pain relief, 2 out of 10.
Conclusion
SIJ dysfunction is widely overlooked because of the difficulty in distinguishing it from other similarly presenting syndromes. However, with a detailed history, appropriate physical maneuvers, imaging, and adequate response to intra-articular anesthetic, providers can reach an accurate diagnosis that will inform subsequent treatments. After failure of nonsurgical methods, patients with SIJ dysfunction should be considered for minimally invasive fusion techniques, which have proven to be a safe, effective, and viable treatment option.
1. Zaidi HA, Montoure AJ, Dickman CA. Surgical and clinical efficacy of sacroiliac joint fusion: a systematic review of the literature. J Neurosurg Spine. 2015;23(1):59-66.
2. Cohen SP. Sacroiliac joint pain: a comprehensive review of anatomy, diagnosis, and treatment. Anesth Analg. 2005;101(5):1440-1453.
3. Chou LH, Slipman CW, Bhagia SM, et al. Inciting events initiating injection‐proven sacroiliac joint syndrome. Pain Med. 2004;5(1):26-32.
4. Dreyfuss P, Dreyer SJ, Cole A, Mayo K. Sacroiliac joint pain. J Am Acad Orthop Surg. 2004;12(4):255-265.
5. Buijs E, Visser L, Groen G. Sciatica and the sacroiliac joint: a forgotten concept. Br J Anaesth. 2007;99(5):713-716.
6. Fortin JD, Dwyer AP, West S, Pier J. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I: asymptomatic volunteers. Spine (Phila Pa 1976). 1994;19(13):1475-1482.
7. Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976). 1995;20(1):31-37.
8. Fortin JD, Washington WJ, Falco FJ. Three pathways between the sacroiliac joint and neural structures. ANJR Am J Neuroradiol. 1999;20(8):1429-1434.
9. Szadek KM, van der Wurff P, van Tulder MW, Zuurmond WW, Perez RS. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain. 2009;10(4):354-368.
10. Merskey H, Bogduk N, eds. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed. Seattle, WA: IASP Press; 1994.
11. Cusi MF. Paradigm for assessment and treatment of SIJ mechanical dysfunction. J Bodyw Mov Ther. 2010;14(2):152-161.
12. Laslett M, Aprill CN, McDonald B, Young SB. Diagnosis of sacroiliac joint pain: validity of individual provocation tests and composites of tests. Man Ther. 2005;10(3):207-218.
13. Laslett M. Evidence-based diagnosis and treatment of the painful sacroiliac joint. J Man Manip Ther. 2008;16(3):142-152.
14. Polly DW Jr. The sacroiliac joint. Neurosurg Clin N Am. 2017;28(3):301-312.
15. Cusi M, Van Der Wall H, Saunders J, Fogelman I. Metabolic disturbances identified by SPECT-CT in patients with a clinical diagnosis of sacroiliac joint incompetence. Eur Spine J. 2013;22(7):1674-1682.
16. Tofuku K, Koga H, Komiya S. The diagnostic value of single-photon emission computed tomography/computed tomography for severe sacroiliac joint dysfunction. Eur Spine J. 2015;24(4):859-863.
17. Kennedy DJ, Engel A, Kreiner DS, Nampiaparampil D, Duszynski B, MacVicar J. Fluoroscopically guided diagnostic and therapeutic intra‐articular sacroiliac joint injections: a systematic review. Pain Med. 2015;16(8):1500-1518.
18. Schneider BJ, Huynh L, Levin J, Rinkaekan P, Kordi R, Kennedy DJ. Does immediate pain relief after an injection into the sacroiliac joint with anesthetic and corticosteroid predict subsequent pain relief? Pain Med. 2018;19(2):244-251.
19. Murakami E, Tanaka Y, Aizawa T, Ishizuka M, Kokubun S. Effect of periarticular and intraarticular lidocaine injections for sacroiliac joint pain: prospective comparative study. J Orthop Sci. 2007;12(3):274-280.
20. Cohen SP, Hurley RW, Buckenmaier CC 3rd, Kurihara C, Morlando B, Dragovich A. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279-288.
21. Polly DW, Cher DJ, Wine KD, et al; INSITE Study Group. Randomized controlled trial of minimally invasive sacroiliac joint fusion using triangular titanium implants vs nonsurgical management for sacroiliac joint dysfunction: 12-month outcomes. Neurosurgery. 2015;77(5):674-690.
22. Soriano-Baron H, Lindsey DP, Rodriguez-Martinez N, et al. The effect of implant placement on sacroiliac joint range of motion: posterior versus transarticular. Spine. 2015;40(9):E525-E530.
23. Smith AG, Capobianco R, Cher D, et al. Open versus minimally invasive sacroiliac joint fusion: a multi-center comparison of perioperative measures and clinical outcomes. Ann Surg Innov Res. 2013;7(1):14.
24. Rashbaum RF, Ohnmeiss DD, Lindley EM, Kitchel SH, Patel VV. Sacroiliac joint pain and its treatment. Clin Spine Surg. 2016;29(2):42-48.
25. Polly DW, Swofford J, Whang PG, et al. Two-year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion vs. non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg. 2016;10:28.
26. Dengler J, Duhon B, Whang P, et al. Predictors of outcome in conservative and minimally invasive surgical management of pain originating from the sacroiliac joint: a pooled analysis. Spine (Phila Pa 1976). 2017;42(21):1664-1673.
27. Vanaclocha V, Herrera JM, Sáiz-Sapena N, Rivera-Paz M, Verdú-López F. Minimally invasive sacroiliac joint fusion, radiofrequency denervation, and conservative management for sacroiliac joint pain: 6-year comparative case series. Neurosurgery. 2018;82(1):48-55.
28. Unoki E, Abe E, Murai H, Kobayashi T, Abe T. Fusion of multiple segments can increase the incidence of sacroiliac joint pain after lumbar or lumbosacral fusion. Spine (Phila Pa 1976). 2016;41(12):999-1005.
29. Katz V, Schofferman J, Reynolds J. The sacroiliac joint: a potential cause of pain after lumbar fusion to the sacrum. J Spinal Disord Tech. 2003;16(1):96-99.
1. Zaidi HA, Montoure AJ, Dickman CA. Surgical and clinical efficacy of sacroiliac joint fusion: a systematic review of the literature. J Neurosurg Spine. 2015;23(1):59-66.
2. Cohen SP. Sacroiliac joint pain: a comprehensive review of anatomy, diagnosis, and treatment. Anesth Analg. 2005;101(5):1440-1453.
3. Chou LH, Slipman CW, Bhagia SM, et al. Inciting events initiating injection‐proven sacroiliac joint syndrome. Pain Med. 2004;5(1):26-32.
4. Dreyfuss P, Dreyer SJ, Cole A, Mayo K. Sacroiliac joint pain. J Am Acad Orthop Surg. 2004;12(4):255-265.
5. Buijs E, Visser L, Groen G. Sciatica and the sacroiliac joint: a forgotten concept. Br J Anaesth. 2007;99(5):713-716.
6. Fortin JD, Dwyer AP, West S, Pier J. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I: asymptomatic volunteers. Spine (Phila Pa 1976). 1994;19(13):1475-1482.
7. Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976). 1995;20(1):31-37.
8. Fortin JD, Washington WJ, Falco FJ. Three pathways between the sacroiliac joint and neural structures. ANJR Am J Neuroradiol. 1999;20(8):1429-1434.
9. Szadek KM, van der Wurff P, van Tulder MW, Zuurmond WW, Perez RS. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain. 2009;10(4):354-368.
10. Merskey H, Bogduk N, eds. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed. Seattle, WA: IASP Press; 1994.
11. Cusi MF. Paradigm for assessment and treatment of SIJ mechanical dysfunction. J Bodyw Mov Ther. 2010;14(2):152-161.
12. Laslett M, Aprill CN, McDonald B, Young SB. Diagnosis of sacroiliac joint pain: validity of individual provocation tests and composites of tests. Man Ther. 2005;10(3):207-218.
13. Laslett M. Evidence-based diagnosis and treatment of the painful sacroiliac joint. J Man Manip Ther. 2008;16(3):142-152.
14. Polly DW Jr. The sacroiliac joint. Neurosurg Clin N Am. 2017;28(3):301-312.
15. Cusi M, Van Der Wall H, Saunders J, Fogelman I. Metabolic disturbances identified by SPECT-CT in patients with a clinical diagnosis of sacroiliac joint incompetence. Eur Spine J. 2013;22(7):1674-1682.
16. Tofuku K, Koga H, Komiya S. The diagnostic value of single-photon emission computed tomography/computed tomography for severe sacroiliac joint dysfunction. Eur Spine J. 2015;24(4):859-863.
17. Kennedy DJ, Engel A, Kreiner DS, Nampiaparampil D, Duszynski B, MacVicar J. Fluoroscopically guided diagnostic and therapeutic intra‐articular sacroiliac joint injections: a systematic review. Pain Med. 2015;16(8):1500-1518.
18. Schneider BJ, Huynh L, Levin J, Rinkaekan P, Kordi R, Kennedy DJ. Does immediate pain relief after an injection into the sacroiliac joint with anesthetic and corticosteroid predict subsequent pain relief? Pain Med. 2018;19(2):244-251.
19. Murakami E, Tanaka Y, Aizawa T, Ishizuka M, Kokubun S. Effect of periarticular and intraarticular lidocaine injections for sacroiliac joint pain: prospective comparative study. J Orthop Sci. 2007;12(3):274-280.
20. Cohen SP, Hurley RW, Buckenmaier CC 3rd, Kurihara C, Morlando B, Dragovich A. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279-288.
21. Polly DW, Cher DJ, Wine KD, et al; INSITE Study Group. Randomized controlled trial of minimally invasive sacroiliac joint fusion using triangular titanium implants vs nonsurgical management for sacroiliac joint dysfunction: 12-month outcomes. Neurosurgery. 2015;77(5):674-690.
22. Soriano-Baron H, Lindsey DP, Rodriguez-Martinez N, et al. The effect of implant placement on sacroiliac joint range of motion: posterior versus transarticular. Spine. 2015;40(9):E525-E530.
23. Smith AG, Capobianco R, Cher D, et al. Open versus minimally invasive sacroiliac joint fusion: a multi-center comparison of perioperative measures and clinical outcomes. Ann Surg Innov Res. 2013;7(1):14.
24. Rashbaum RF, Ohnmeiss DD, Lindley EM, Kitchel SH, Patel VV. Sacroiliac joint pain and its treatment. Clin Spine Surg. 2016;29(2):42-48.
25. Polly DW, Swofford J, Whang PG, et al. Two-year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion vs. non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg. 2016;10:28.
26. Dengler J, Duhon B, Whang P, et al. Predictors of outcome in conservative and minimally invasive surgical management of pain originating from the sacroiliac joint: a pooled analysis. Spine (Phila Pa 1976). 2017;42(21):1664-1673.
27. Vanaclocha V, Herrera JM, Sáiz-Sapena N, Rivera-Paz M, Verdú-López F. Minimally invasive sacroiliac joint fusion, radiofrequency denervation, and conservative management for sacroiliac joint pain: 6-year comparative case series. Neurosurgery. 2018;82(1):48-55.
28. Unoki E, Abe E, Murai H, Kobayashi T, Abe T. Fusion of multiple segments can increase the incidence of sacroiliac joint pain after lumbar or lumbosacral fusion. Spine (Phila Pa 1976). 2016;41(12):999-1005.
29. Katz V, Schofferman J, Reynolds J. The sacroiliac joint: a potential cause of pain after lumbar fusion to the sacrum. J Spinal Disord Tech. 2003;16(1):96-99.
Dermatitis associated with surgical implants merits conservative approach
NEW YORK – In patients who develop dermatitis after implantation of a device containing materials to which they have a contact allergy, explantation is not necessarily a cure for their symptoms.
“It can be difficult to predict who will or will not clear when the device is removed. In addition, in some cases device explantation could lead to other issues,” cautioned Ari M. Goldminz, MD, a dermatologist at Brigham and Women’s Hospital, Boston, MA. “Offering non-surgical options and a thorough investigation of other potential causes unrelated to the implant may provide a path to avoid explantation.” However, for other patients removal of the device might be the preferred option.
During his presentation at the American Academy of Dermatology summer meeting, he described illustrative cases. These patients developed dermatitis within weeks or months after receiving a surgical implant, and tested positive for a material that was in the implanted device.
In one of these cases, the suspected problem was a metal plate containing chromium that was placed during an orthopedic repair. Subsequent patch testing revealed a reaction to chromium and the implant was eventually removed.
However, there was no improvement in dermatitis following removal of the metal plate.
“The symptoms ultimately improved after starting on a low-chromium diet and avoiding other allergens identified on patch testing, such as those found in skin care products,” Dr. Goldminz explained. He does not discount the role that the implant may have played in the onset of dermatitis,, but improvement required avoidance strategies other than device explantation.
There are studies suggesting that patch testing prior to surgery can help certain patients and surgeons select implant materials, such as when patients have a clinical history of metal sensitivity (Arch Dermatol. 2012 Jun;148[6]:687-93). However, other studies have also found that positive patch test results do not necessarily predict outcomes following surgery (J Arthroplasty. 2016 Aug;31[8]1717-21).
Although it might make sense to consider pre-operative patch testing in patients with a clinical history of rashes from metallic objects, Dr. Goldminz indicated that some patients might still need to weigh the benefits of the implant against the risks of a hypersensitivity reaction when no devices free of the allergen are available.
“In certain cases, patients might decide the risk-to-benefit ratio favors the device depending on factors such as the indication for the surgery, alternative options available, and what device removal might involve,” Dr. Goldminz said. Additionally, when patients develop rashes thought to be related to materials present in an implanted device, there are also medical treatments that can be considered if device explantation is not preferred or if it is an impractical approach.
NEW YORK – In patients who develop dermatitis after implantation of a device containing materials to which they have a contact allergy, explantation is not necessarily a cure for their symptoms.
“It can be difficult to predict who will or will not clear when the device is removed. In addition, in some cases device explantation could lead to other issues,” cautioned Ari M. Goldminz, MD, a dermatologist at Brigham and Women’s Hospital, Boston, MA. “Offering non-surgical options and a thorough investigation of other potential causes unrelated to the implant may provide a path to avoid explantation.” However, for other patients removal of the device might be the preferred option.
During his presentation at the American Academy of Dermatology summer meeting, he described illustrative cases. These patients developed dermatitis within weeks or months after receiving a surgical implant, and tested positive for a material that was in the implanted device.
In one of these cases, the suspected problem was a metal plate containing chromium that was placed during an orthopedic repair. Subsequent patch testing revealed a reaction to chromium and the implant was eventually removed.
However, there was no improvement in dermatitis following removal of the metal plate.
“The symptoms ultimately improved after starting on a low-chromium diet and avoiding other allergens identified on patch testing, such as those found in skin care products,” Dr. Goldminz explained. He does not discount the role that the implant may have played in the onset of dermatitis,, but improvement required avoidance strategies other than device explantation.
There are studies suggesting that patch testing prior to surgery can help certain patients and surgeons select implant materials, such as when patients have a clinical history of metal sensitivity (Arch Dermatol. 2012 Jun;148[6]:687-93). However, other studies have also found that positive patch test results do not necessarily predict outcomes following surgery (J Arthroplasty. 2016 Aug;31[8]1717-21).
Although it might make sense to consider pre-operative patch testing in patients with a clinical history of rashes from metallic objects, Dr. Goldminz indicated that some patients might still need to weigh the benefits of the implant against the risks of a hypersensitivity reaction when no devices free of the allergen are available.
“In certain cases, patients might decide the risk-to-benefit ratio favors the device depending on factors such as the indication for the surgery, alternative options available, and what device removal might involve,” Dr. Goldminz said. Additionally, when patients develop rashes thought to be related to materials present in an implanted device, there are also medical treatments that can be considered if device explantation is not preferred or if it is an impractical approach.
NEW YORK – In patients who develop dermatitis after implantation of a device containing materials to which they have a contact allergy, explantation is not necessarily a cure for their symptoms.
“It can be difficult to predict who will or will not clear when the device is removed. In addition, in some cases device explantation could lead to other issues,” cautioned Ari M. Goldminz, MD, a dermatologist at Brigham and Women’s Hospital, Boston, MA. “Offering non-surgical options and a thorough investigation of other potential causes unrelated to the implant may provide a path to avoid explantation.” However, for other patients removal of the device might be the preferred option.
During his presentation at the American Academy of Dermatology summer meeting, he described illustrative cases. These patients developed dermatitis within weeks or months after receiving a surgical implant, and tested positive for a material that was in the implanted device.
In one of these cases, the suspected problem was a metal plate containing chromium that was placed during an orthopedic repair. Subsequent patch testing revealed a reaction to chromium and the implant was eventually removed.
However, there was no improvement in dermatitis following removal of the metal plate.
“The symptoms ultimately improved after starting on a low-chromium diet and avoiding other allergens identified on patch testing, such as those found in skin care products,” Dr. Goldminz explained. He does not discount the role that the implant may have played in the onset of dermatitis,, but improvement required avoidance strategies other than device explantation.
There are studies suggesting that patch testing prior to surgery can help certain patients and surgeons select implant materials, such as when patients have a clinical history of metal sensitivity (Arch Dermatol. 2012 Jun;148[6]:687-93). However, other studies have also found that positive patch test results do not necessarily predict outcomes following surgery (J Arthroplasty. 2016 Aug;31[8]1717-21).
Although it might make sense to consider pre-operative patch testing in patients with a clinical history of rashes from metallic objects, Dr. Goldminz indicated that some patients might still need to weigh the benefits of the implant against the risks of a hypersensitivity reaction when no devices free of the allergen are available.
“In certain cases, patients might decide the risk-to-benefit ratio favors the device depending on factors such as the indication for the surgery, alternative options available, and what device removal might involve,” Dr. Goldminz said. Additionally, when patients develop rashes thought to be related to materials present in an implanted device, there are also medical treatments that can be considered if device explantation is not preferred or if it is an impractical approach.
REPORTING FROM SUMMER AAD 2019
There’s Mischief Afoot

ANSWER
The radiograph demonstrates no evidence of an acute fracture or soft-tissue gas to suggest an abscess. Of note, though, within the tibiotalar joint, the patient has bony destruction and settling of the articular surfaces of both the distal tibia and fibula into the talus and calcaneus.
This finding is typically associated with neuropathic arthropathy (also known as a Charcot joint). This pathologic process is typically seen in a weight-bearing joint that develops progressive degeneration from chronic loss of sensation.

ANSWER
The radiograph demonstrates no evidence of an acute fracture or soft-tissue gas to suggest an abscess. Of note, though, within the tibiotalar joint, the patient has bony destruction and settling of the articular surfaces of both the distal tibia and fibula into the talus and calcaneus.
This finding is typically associated with neuropathic arthropathy (also known as a Charcot joint). This pathologic process is typically seen in a weight-bearing joint that develops progressive degeneration from chronic loss of sensation.

ANSWER
The radiograph demonstrates no evidence of an acute fracture or soft-tissue gas to suggest an abscess. Of note, though, within the tibiotalar joint, the patient has bony destruction and settling of the articular surfaces of both the distal tibia and fibula into the talus and calcaneus.
This finding is typically associated with neuropathic arthropathy (also known as a Charcot joint). This pathologic process is typically seen in a weight-bearing joint that develops progressive degeneration from chronic loss of sensation.

A 70-year-old man presents for evaluation of left foot pain, redness, and swelling. He reports injuring the foot a week ago; he went to the emergency department for evaluation of the cut he had sustained, which required stapling.
The patient has a chronic foot ulcer for which a home health aide provides wound care and dressing changes. His medical history is significant for hypertension, stroke with chronic left-sided weakness, congestive heart failure, and chronic renal insufficiency. He admits to daily tobacco use, and his medical record reflects a history of drug use.
On physical exam, you note an elderly, chronically ill male in no obvious distress. His vital signs are stable, and he is afebrile. Inspection of his left foot shows generalized swelling and redness. Good distal pulses are appreciated. On the dorsal aspect, there is a healing wound with a single staple present. On the heel is a 2-cm stage 2 ulcer with some scant purulent drainage.
Bloodwork and a radiograph of the left foot are ordered; lateral view is shown. What is your impression?
Fluoroscopically Guided Lateral Approach Hip Injection
Hip injections are performed as diagnostic and therapeutic interventions across a variety of medical subspecialties, including but not limited to those practicing physical medicine and rehabilitation, pain medicine, sports medicine, orthopedic surgery, and radiology. Traditional image-guided intra-articular hip injection commonly uses an anterior-oblique approach from a starting point on the anterior groin traversing soft tissue anterior to the femoral neck to the target needle placement at the femoral head-neck junction.
In fluoroscopic procedures, a coaxial technique for needles placement is used for safe and precise insertion of needles. An X-ray beam is angled in line with the projected path of the needle from skin entry point to injection target. Coaxial, en face technique (also called EF, parallel, hub view, down the barrel, or barrel view) appears as a single radiopaque dot over the target injection site.1 This technique minimizes needle redirection for correction of the injection path and minimal disturbance of surrounding tissue on the approach to the intended target.
Noncoaxial technique, as used in the anterior-oblique approach, intentionally directs the needle away from a skin entry point, the needle barrel traversing the X-ray beam toward an injection target. Clinical challenges to injection with the anterior-oblique approach include using a noncoaxial technique. Additional challenges to the anterior-oblique (also referred to as anterior) approach are body habitus and pannus, proximity to neurovascular structures, and patient positioning. By understanding the risks and benefits of varied technical approaches to accomplish a clinical goal and outcome, trainees are better able to select the technique most appropriate for a varied patient population.
Common risks to patients for all intra-articular interventions include bleeding, infection, and pain. Risk of damage to nearby structures is often mentioned as part of a standard informed consent process as it relates to the femoral vein, artery, and nerve that are in close anatomical proximity to the target injection site. When prior studies have examined the risk of complications resulting from intra-articular hip injections, a common conclusion is that despite a relatively low-risk profile for skilled interventionalists, efforts to avoid needle placement in the medial 50% of the femoral head on antero-posterior imaging is recommended.2
The anterior technique is a commonly described approach, and the same can be used for both ultrasound-guided and fluoroscopically guided hip injections.3 Using ultrasound guidance, the anterior technique can be performed with in-plane direct visualization of the needle throughout the procedure. With fluoroscopic guidance, the anterior approach is performed out-of-plane, using the noncoaxial technique. This requires the interventionalist to use tactile and anatomic guidance to the target injection site. The anterior approach for hip injection is one of few interventions where coaxial technique is not used for the procedure, making the instruction for a learner less concrete and potentially more challenging related to the needle path not under direct visualization in plane with the X-ray beam.
Technical guidance and detailed instruction for the lateral approach is infrequently described in fluoroscopic interventional texts. Reference to a lateral approach hip injection was made as early as the 1970s, without detail provided on the technique, with respect to the advantage of visualization of the hip joint for needle placement when hardware is in place.4 A more recent article described a lateral approach technique involving the patient in a decubitus (lateral) supine position, which presents limitations in consistent fluoroscopic imaging and can be a challenging static position for the patient to maintain.5
The retrospective review of anterior-oblique and lateral approach procedures in this study aims to demonstrate that there is no significant difference in radiation exposure, rate of successful intra-articular injection, or complication rate. If proven as a noninferior technique, the lateral approach may be a valuable interventional skill to those performing hip injections. Potential benefits to the patient and provider include options for the provider to access the joint using either technique. Additionally, the approach can be added to the instructional plan for those practitioners providing technical instruction to trainees within their health care system.
Methods
The institutional review board at the VA Ann Arbor Healthcare System reviewed and granted approval for this study. One of 5 interventional pain physician staff members at the VA Ann Arbor Healthcare System performed fluoroscopically guided hip injections. Interventional pain fellows under the direct supervision of board-certified physicians performed the procedures for the study cases. Supervising physicians included both physiatrists and anesthesiologists. Images were reviewed and evaluated without corresponding patient biographic data.
For cases using the lateral approach, the patients were positioned supine on the fluoroscopy table. In anterior-posterior and lateral views, trajectory lines are drawn using a long metal marking rod held adjacent to the patient. With pulsed low-dose fluoroscopy, transverse lines are drawn to identify midpoint of the femoral head in lateral view (Figure 1A, x-axis) and the most direct line from skin to lateral femoral head neck junction joint target (Figure 1B, z-axis). Also confirmed in lateral view, the z-axis marked line drawn on the skin is used to confirm that this transverse plane crosses the overlapping femoral heads (Figure 1A, y-axis).
The cross-section of these transverse and coronal plane lines identifies the starting point for the most direct approach from skin to injection target at femoral head-neck junction. Using the coaxial technique in the lateral view, the needle is introduced and advanced using intermittent fluoroscopic images to the lateral joint target. Continuing in this view, the interventionalist can ensure that advancing the needle to the osseous endpoint will place the tip at the midpoint of the femoral head at the target on the lateral surface, avoiding inadvertent advance of the needle anterior or posterior the femoral head. Final needle placement confirmation is then completed in antero-posterior view (Figure 2A). Contrast enhancement is used to confirm intra-articular spread (Figure 2B).
Cases included in the study were performed over an 8-month period in 2017. Case images recorded in IntelliSpace PACS Radiology software (Andover, MA) were included by creating a list of all cases performed and documented using the major joint injection procedure code. The cases reviewed began with the most recent cases. Two research team members (1 radiologist and 1 interventional pain physician) reviewed the series of saved images for each patient and the associated procedure report. The research team members documented and recorded de-identified study data in Microsoft Excel (Redmond, WA).
Imaging reports, using the saved images and the associated procedure report, were classified for technical approach (anterior, lateral, or inconclusive), success of joint injection as evidenced by appropriate contrast enhancement within the joint space (successful, unsuccessful, or incomplete images), documented use of sedation (yes, no), patient positioning (supine, prone), radiation exposure dose, radiation exposure time, and additional comments, such as “notable pannus” or “hardware present” to annotate significant findings on imaging review.
Statistical Analysis
The distribution of 2 outcomes used to compare rates of complication, radiation dose, and exposure time was checked using the Shapiro-Wilk test. Power analysis determined that inclusion of 30 anterior and 30 lateral cases results in adequate power to detect a 1-point mean difference, assuming a standard deviation of 1.5 in each group. Both radiation dose and exposure time were found to be nonnormally distributed (W = 0.65, P < .001; W = 0.86, P < .001; respectively). Median and interquartile range (IQR) of dose and time in seconds for anterior and lateral approaches were computed. Median differences in radiation dose and exposure time between anterior and lateral approaches were assessed with the k-sample test of equality of medians. All analyses were conducted using Stata Version 14.1 (College Station, TX).
Results
Between June 2017 and January 2018, 88 cases were reviewed as performed, with 30 anterior and 30 lateral approach cases included in this retrospective comparison study. A total of 28 cases were excluded from the study for using an inconclusive approach, multiple or bilateral procedures, cases without recorded dose and time data, and inadequately saved images to provide meaningful data (Figure 3).
Rate of successful intervention with needle placement confirmed within the articular space on contrast enhancement was not significantly different in the study groups with 96.7% (29 of 30) anterior approach cases reported as successful, 100% (30 of 30) lateral approach cases reported as successful. Overhanging pannus in the viewing area was reported in 5 anterior approach cases and 4 lateral cases. Hardware was noted in 2 lateral approach cases, none in anterior approach cases. Sedation was used for 3 of the anterior approach cases and none of the lateral approach cases.
Patients undergoing the lateral approach received a higher median radiation dose than did those undergoing the anterior approach, but this was not statistically significant (P = .07) (Table). Those undergoing the lateral approach also had a longer median exposure time than did those undergoing the anterior approach, but this also was not statistically significant (P = .3). With no immediate complications reported in any of the studied interventions, there was no difference in complication rates between anterior and lateral approach cases.
Discussion
Pain medicine fellows who have previously completed residency in a variety of disciplines, often either anesthesiology or physical medicine and rehabilitation, perform fluoroscopically guided procedures and benefit from increased experience with coaxial technique as this improves needle depth and location awareness. Once mastered, this skill set can be applied to and useful for multiple interventional pain procedures. Similar technical instruction with an emphasis on coaxial technique for hip injections as performed in the anterior or anterolateral approach can be used in both fluoroscopic and ultrasound-guided procedures, including facet injection, transforaminal epidural steroid injection, and myriad other procedures performed to ameliorate pain. There are advantages to pursuing a similar approach with all image-guided procedures. Evaluated in this comparison study is an alternative technique that has potential for risk reduction benefit with reduced proximity to neurovascular structures, which ultimately leads to a safer procedure profile.
Using a lateral approach, the interventionalist determines a starting point, entering the skin at a greater distance from any overlying pannus and the elevated concentration of gram-negative and gram-positive bacteria contained within the inguinal skin.6 A previous study demonstrated improved success of intra-articular needle tip placement without image guidance in patients with body mass index (BMI) < 30.7 A prior study of anterior approach using anatomic landmarks as compared to lateral approach demonstrated the anterior approach pierced or contacted the femoral nerve in 27% of anterior cases and came within 5 mm of 60% of anterior cases.2 Use of image guidance, whether ultrasound, fluoroscopy, or computed tomography (CT) is preferred related to reduced risk of contact with adjacent neurovascular structures. Anatomic surface landmarks have been described as an alternative injection technique, without the use of fluoroscopy for confirmatory initial, intraprocedure, and final placement.8 Palpation of anatomic structures is required for this nonimage-guided technique, and although similar to the described technique in this study, the anatomically guided injection starting point is more lateral than the anterior approach but not in the most lateral position in the transverse plane that is used for this fluoroscopically guided lateral approach study.
Physiologic characteristics of subjects and technical aspects of fluoroscopy both can be factors in radiation dose and exposure times for hip injections. Patient BMI was not included in the data collection, but further study would seek to determine whether BMI is a significant risk for any increased radiation dose and exposure times using lateral approach injections. Use of lateral images for fluoroscopy requires penetration of X-ray beam through more tissue compared with that of anterior-posterior images. Further study of these techniques would benefit from comparing the pulse rate of fluoroscopic images and collimation (or focusing of the radiation beam over a smaller area of tissue) as factors in any observed increase in total radiation dose and exposure times.
Improving the safety profile of this procedure could have a positive impact on the patient population receiving fluoroscopic hip injections, both within the VA Ann Arbor Health System and elsewhere. While the study population was limited to the VA patient population seeking subspecialty nonsurgical joint care at a single tertiary care center, this technique is generalizable and can be used in most patients, as hip pain is a common condition necessitating nonoperative evaluation and treatment.
Radiation Exposures
As our analysis demonstrates, mean radiation dose exposure for each group was consistent with low (≤ 3 mSv) to moderate (> 3-20 mSv) annual effective doses in the general population.7 Both anterior and lateral median radiation dose of 1 mGy and 3 mGy, respectively, are within the standard exposure for radiographs of the pelvis (1.31 mGy).9 It is therefore reasonable to consider a lateral approach for hip injection, given the benefits of direct coaxial approach and avoiding needle entry through higher bacteria-concentrated skin.
The lateral approach did have increased radiation dose and exposure time, although it was not statistically significantly greater than the anterior approach. The difference between radiation dose and time to perform either technique was not clinically significant. One potential explanation for this is that the lateral technique has increased tissue to penetrate, which can be reduced with collimation and other fluoroscopic image adjustments. Additionally, as trainees progress in competency, fewer images should need to be obtained.7 We hypothesize that as familiarity and comfort with this technique increase, the number of images necessary for successful injection would decrease, leading to decreased radiation dose and exposure time. We would expect that in the hands of a board-certified interventionalist, radiation dose and exposure time would be significantly decreased as compared to our current dataset, and this is an area of planned further study. With our existing dataset, the majority of procedures were performed with trainees, with inadequate information documented for comparison of dose over time and procedural experience under individual physicians.
Notable strengths of this study are the direct comparison of the anterior approach when compared to the lateral approach with regard to radiation dose and exposure time, which we have not seen described in the literature. A detailed description of the technique may result in increased utilization by other providers. Data were collected from multiple providers, as board-certified pain physicians and board-eligible interventional pain fellows performed the procedures. This variability in providers increases the generalizability of the findings, with a variety of providers, disciplines, years of experiences, and type of training represented.
Limitations
Limitations include the retrospective nature of the study and the relatively small sample size. However, even with this limitation, it is notable that no statistically significant differences were observed in mean radiation dose or fluoroscopy exposure time, making the lateral approach, at minimum, a noninferior technique. Combined with the improved safety profile, this technique is a viable alternative to the traditional anterior-oblique approach. Further study should be performed, such as a prospective, randomized control trial investigating the 2 techniques and following pain scores and functional ability after the procedure.
Conclusion
Given the decreased procedural risk related to proximity of neurovascular structures and coaxial technique for needle advancement, lateral approach for hip injection should be considered by those in any discipline performing fluoroscopically guided procedures. Lateral technique may be particularly useful in technically challenging cases and when skin entry at the anterior groin is suboptimal, as a noninferior alternative to traditional anterior method.
1. Cianfoni A, Boulter DJ, Rumboldt Z, Sapton T, Bonaldi G. Guidelines to imaging landmarks for interventional spine procedures: fluoroscopy and CT anatomy. Neurographics. 2011;1(1):39-48.
2. Leopold SS, Battista V, Oliverio JA. Safety and efficacy of intraarticular hip injection using anatomic landmarks. Clin Orthop Relat Res. 2001;(391):192-197.
3. Dodré E, Lefebvre G, Cockenpot E, Chastanet P, Cotten A. Interventional MSK procedures: the hip. Br J Radiol. 2016;89(1057):20150408.
4. Hankey S, McCall IW, Park WM, O’Connor BT. Technical problems in arthrography of the painful hip arthroplasty. Clin Radiol. 1979;30(6):653-656.
5. Yasar E, Singh JR, Hill J, Akuthota V. Image-guided injections of the hip. J Nov Physiother Phys Rehabil. 2014;1(2):39-48.
6. Aly R, Maibach HI. Aerobic microbial flora of intertrigenous skin. Appl Environ Microbiol. 1977;33(1):97-100.
7. Fazel R, Krumholz HM, Wang W, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361(9):849-857.
8. Masoud MA, Said HG. Intra-articular hip injection using anatomic surface landmarks. Arthosc Tech. 2013;2(2):e147-e149.
9. Ofori K, Gordon SW, Akrobortu E, Ampene AA, Darko EO. Estimation of adult patient doses for selected x-ray diagnostic examinations. J Radiat Res Appl Sci. 2014;7(4):459-462.
Hip injections are performed as diagnostic and therapeutic interventions across a variety of medical subspecialties, including but not limited to those practicing physical medicine and rehabilitation, pain medicine, sports medicine, orthopedic surgery, and radiology. Traditional image-guided intra-articular hip injection commonly uses an anterior-oblique approach from a starting point on the anterior groin traversing soft tissue anterior to the femoral neck to the target needle placement at the femoral head-neck junction.
In fluoroscopic procedures, a coaxial technique for needles placement is used for safe and precise insertion of needles. An X-ray beam is angled in line with the projected path of the needle from skin entry point to injection target. Coaxial, en face technique (also called EF, parallel, hub view, down the barrel, or barrel view) appears as a single radiopaque dot over the target injection site.1 This technique minimizes needle redirection for correction of the injection path and minimal disturbance of surrounding tissue on the approach to the intended target.
Noncoaxial technique, as used in the anterior-oblique approach, intentionally directs the needle away from a skin entry point, the needle barrel traversing the X-ray beam toward an injection target. Clinical challenges to injection with the anterior-oblique approach include using a noncoaxial technique. Additional challenges to the anterior-oblique (also referred to as anterior) approach are body habitus and pannus, proximity to neurovascular structures, and patient positioning. By understanding the risks and benefits of varied technical approaches to accomplish a clinical goal and outcome, trainees are better able to select the technique most appropriate for a varied patient population.
Common risks to patients for all intra-articular interventions include bleeding, infection, and pain. Risk of damage to nearby structures is often mentioned as part of a standard informed consent process as it relates to the femoral vein, artery, and nerve that are in close anatomical proximity to the target injection site. When prior studies have examined the risk of complications resulting from intra-articular hip injections, a common conclusion is that despite a relatively low-risk profile for skilled interventionalists, efforts to avoid needle placement in the medial 50% of the femoral head on antero-posterior imaging is recommended.2
The anterior technique is a commonly described approach, and the same can be used for both ultrasound-guided and fluoroscopically guided hip injections.3 Using ultrasound guidance, the anterior technique can be performed with in-plane direct visualization of the needle throughout the procedure. With fluoroscopic guidance, the anterior approach is performed out-of-plane, using the noncoaxial technique. This requires the interventionalist to use tactile and anatomic guidance to the target injection site. The anterior approach for hip injection is one of few interventions where coaxial technique is not used for the procedure, making the instruction for a learner less concrete and potentially more challenging related to the needle path not under direct visualization in plane with the X-ray beam.
Technical guidance and detailed instruction for the lateral approach is infrequently described in fluoroscopic interventional texts. Reference to a lateral approach hip injection was made as early as the 1970s, without detail provided on the technique, with respect to the advantage of visualization of the hip joint for needle placement when hardware is in place.4 A more recent article described a lateral approach technique involving the patient in a decubitus (lateral) supine position, which presents limitations in consistent fluoroscopic imaging and can be a challenging static position for the patient to maintain.5
The retrospective review of anterior-oblique and lateral approach procedures in this study aims to demonstrate that there is no significant difference in radiation exposure, rate of successful intra-articular injection, or complication rate. If proven as a noninferior technique, the lateral approach may be a valuable interventional skill to those performing hip injections. Potential benefits to the patient and provider include options for the provider to access the joint using either technique. Additionally, the approach can be added to the instructional plan for those practitioners providing technical instruction to trainees within their health care system.
Methods
The institutional review board at the VA Ann Arbor Healthcare System reviewed and granted approval for this study. One of 5 interventional pain physician staff members at the VA Ann Arbor Healthcare System performed fluoroscopically guided hip injections. Interventional pain fellows under the direct supervision of board-certified physicians performed the procedures for the study cases. Supervising physicians included both physiatrists and anesthesiologists. Images were reviewed and evaluated without corresponding patient biographic data.
For cases using the lateral approach, the patients were positioned supine on the fluoroscopy table. In anterior-posterior and lateral views, trajectory lines are drawn using a long metal marking rod held adjacent to the patient. With pulsed low-dose fluoroscopy, transverse lines are drawn to identify midpoint of the femoral head in lateral view (Figure 1A, x-axis) and the most direct line from skin to lateral femoral head neck junction joint target (Figure 1B, z-axis). Also confirmed in lateral view, the z-axis marked line drawn on the skin is used to confirm that this transverse plane crosses the overlapping femoral heads (Figure 1A, y-axis).
The cross-section of these transverse and coronal plane lines identifies the starting point for the most direct approach from skin to injection target at femoral head-neck junction. Using the coaxial technique in the lateral view, the needle is introduced and advanced using intermittent fluoroscopic images to the lateral joint target. Continuing in this view, the interventionalist can ensure that advancing the needle to the osseous endpoint will place the tip at the midpoint of the femoral head at the target on the lateral surface, avoiding inadvertent advance of the needle anterior or posterior the femoral head. Final needle placement confirmation is then completed in antero-posterior view (Figure 2A). Contrast enhancement is used to confirm intra-articular spread (Figure 2B).
Cases included in the study were performed over an 8-month period in 2017. Case images recorded in IntelliSpace PACS Radiology software (Andover, MA) were included by creating a list of all cases performed and documented using the major joint injection procedure code. The cases reviewed began with the most recent cases. Two research team members (1 radiologist and 1 interventional pain physician) reviewed the series of saved images for each patient and the associated procedure report. The research team members documented and recorded de-identified study data in Microsoft Excel (Redmond, WA).
Imaging reports, using the saved images and the associated procedure report, were classified for technical approach (anterior, lateral, or inconclusive), success of joint injection as evidenced by appropriate contrast enhancement within the joint space (successful, unsuccessful, or incomplete images), documented use of sedation (yes, no), patient positioning (supine, prone), radiation exposure dose, radiation exposure time, and additional comments, such as “notable pannus” or “hardware present” to annotate significant findings on imaging review.
Statistical Analysis
The distribution of 2 outcomes used to compare rates of complication, radiation dose, and exposure time was checked using the Shapiro-Wilk test. Power analysis determined that inclusion of 30 anterior and 30 lateral cases results in adequate power to detect a 1-point mean difference, assuming a standard deviation of 1.5 in each group. Both radiation dose and exposure time were found to be nonnormally distributed (W = 0.65, P < .001; W = 0.86, P < .001; respectively). Median and interquartile range (IQR) of dose and time in seconds for anterior and lateral approaches were computed. Median differences in radiation dose and exposure time between anterior and lateral approaches were assessed with the k-sample test of equality of medians. All analyses were conducted using Stata Version 14.1 (College Station, TX).
Results
Between June 2017 and January 2018, 88 cases were reviewed as performed, with 30 anterior and 30 lateral approach cases included in this retrospective comparison study. A total of 28 cases were excluded from the study for using an inconclusive approach, multiple or bilateral procedures, cases without recorded dose and time data, and inadequately saved images to provide meaningful data (Figure 3).
Rate of successful intervention with needle placement confirmed within the articular space on contrast enhancement was not significantly different in the study groups with 96.7% (29 of 30) anterior approach cases reported as successful, 100% (30 of 30) lateral approach cases reported as successful. Overhanging pannus in the viewing area was reported in 5 anterior approach cases and 4 lateral cases. Hardware was noted in 2 lateral approach cases, none in anterior approach cases. Sedation was used for 3 of the anterior approach cases and none of the lateral approach cases.
Patients undergoing the lateral approach received a higher median radiation dose than did those undergoing the anterior approach, but this was not statistically significant (P = .07) (Table). Those undergoing the lateral approach also had a longer median exposure time than did those undergoing the anterior approach, but this also was not statistically significant (P = .3). With no immediate complications reported in any of the studied interventions, there was no difference in complication rates between anterior and lateral approach cases.
Discussion
Pain medicine fellows who have previously completed residency in a variety of disciplines, often either anesthesiology or physical medicine and rehabilitation, perform fluoroscopically guided procedures and benefit from increased experience with coaxial technique as this improves needle depth and location awareness. Once mastered, this skill set can be applied to and useful for multiple interventional pain procedures. Similar technical instruction with an emphasis on coaxial technique for hip injections as performed in the anterior or anterolateral approach can be used in both fluoroscopic and ultrasound-guided procedures, including facet injection, transforaminal epidural steroid injection, and myriad other procedures performed to ameliorate pain. There are advantages to pursuing a similar approach with all image-guided procedures. Evaluated in this comparison study is an alternative technique that has potential for risk reduction benefit with reduced proximity to neurovascular structures, which ultimately leads to a safer procedure profile.
Using a lateral approach, the interventionalist determines a starting point, entering the skin at a greater distance from any overlying pannus and the elevated concentration of gram-negative and gram-positive bacteria contained within the inguinal skin.6 A previous study demonstrated improved success of intra-articular needle tip placement without image guidance in patients with body mass index (BMI) < 30.7 A prior study of anterior approach using anatomic landmarks as compared to lateral approach demonstrated the anterior approach pierced or contacted the femoral nerve in 27% of anterior cases and came within 5 mm of 60% of anterior cases.2 Use of image guidance, whether ultrasound, fluoroscopy, or computed tomography (CT) is preferred related to reduced risk of contact with adjacent neurovascular structures. Anatomic surface landmarks have been described as an alternative injection technique, without the use of fluoroscopy for confirmatory initial, intraprocedure, and final placement.8 Palpation of anatomic structures is required for this nonimage-guided technique, and although similar to the described technique in this study, the anatomically guided injection starting point is more lateral than the anterior approach but not in the most lateral position in the transverse plane that is used for this fluoroscopically guided lateral approach study.
Physiologic characteristics of subjects and technical aspects of fluoroscopy both can be factors in radiation dose and exposure times for hip injections. Patient BMI was not included in the data collection, but further study would seek to determine whether BMI is a significant risk for any increased radiation dose and exposure times using lateral approach injections. Use of lateral images for fluoroscopy requires penetration of X-ray beam through more tissue compared with that of anterior-posterior images. Further study of these techniques would benefit from comparing the pulse rate of fluoroscopic images and collimation (or focusing of the radiation beam over a smaller area of tissue) as factors in any observed increase in total radiation dose and exposure times.
Improving the safety profile of this procedure could have a positive impact on the patient population receiving fluoroscopic hip injections, both within the VA Ann Arbor Health System and elsewhere. While the study population was limited to the VA patient population seeking subspecialty nonsurgical joint care at a single tertiary care center, this technique is generalizable and can be used in most patients, as hip pain is a common condition necessitating nonoperative evaluation and treatment.
Radiation Exposures
As our analysis demonstrates, mean radiation dose exposure for each group was consistent with low (≤ 3 mSv) to moderate (> 3-20 mSv) annual effective doses in the general population.7 Both anterior and lateral median radiation dose of 1 mGy and 3 mGy, respectively, are within the standard exposure for radiographs of the pelvis (1.31 mGy).9 It is therefore reasonable to consider a lateral approach for hip injection, given the benefits of direct coaxial approach and avoiding needle entry through higher bacteria-concentrated skin.
The lateral approach did have increased radiation dose and exposure time, although it was not statistically significantly greater than the anterior approach. The difference between radiation dose and time to perform either technique was not clinically significant. One potential explanation for this is that the lateral technique has increased tissue to penetrate, which can be reduced with collimation and other fluoroscopic image adjustments. Additionally, as trainees progress in competency, fewer images should need to be obtained.7 We hypothesize that as familiarity and comfort with this technique increase, the number of images necessary for successful injection would decrease, leading to decreased radiation dose and exposure time. We would expect that in the hands of a board-certified interventionalist, radiation dose and exposure time would be significantly decreased as compared to our current dataset, and this is an area of planned further study. With our existing dataset, the majority of procedures were performed with trainees, with inadequate information documented for comparison of dose over time and procedural experience under individual physicians.
Notable strengths of this study are the direct comparison of the anterior approach when compared to the lateral approach with regard to radiation dose and exposure time, which we have not seen described in the literature. A detailed description of the technique may result in increased utilization by other providers. Data were collected from multiple providers, as board-certified pain physicians and board-eligible interventional pain fellows performed the procedures. This variability in providers increases the generalizability of the findings, with a variety of providers, disciplines, years of experiences, and type of training represented.
Limitations
Limitations include the retrospective nature of the study and the relatively small sample size. However, even with this limitation, it is notable that no statistically significant differences were observed in mean radiation dose or fluoroscopy exposure time, making the lateral approach, at minimum, a noninferior technique. Combined with the improved safety profile, this technique is a viable alternative to the traditional anterior-oblique approach. Further study should be performed, such as a prospective, randomized control trial investigating the 2 techniques and following pain scores and functional ability after the procedure.
Conclusion
Given the decreased procedural risk related to proximity of neurovascular structures and coaxial technique for needle advancement, lateral approach for hip injection should be considered by those in any discipline performing fluoroscopically guided procedures. Lateral technique may be particularly useful in technically challenging cases and when skin entry at the anterior groin is suboptimal, as a noninferior alternative to traditional anterior method.
Hip injections are performed as diagnostic and therapeutic interventions across a variety of medical subspecialties, including but not limited to those practicing physical medicine and rehabilitation, pain medicine, sports medicine, orthopedic surgery, and radiology. Traditional image-guided intra-articular hip injection commonly uses an anterior-oblique approach from a starting point on the anterior groin traversing soft tissue anterior to the femoral neck to the target needle placement at the femoral head-neck junction.
In fluoroscopic procedures, a coaxial technique for needles placement is used for safe and precise insertion of needles. An X-ray beam is angled in line with the projected path of the needle from skin entry point to injection target. Coaxial, en face technique (also called EF, parallel, hub view, down the barrel, or barrel view) appears as a single radiopaque dot over the target injection site.1 This technique minimizes needle redirection for correction of the injection path and minimal disturbance of surrounding tissue on the approach to the intended target.
Noncoaxial technique, as used in the anterior-oblique approach, intentionally directs the needle away from a skin entry point, the needle barrel traversing the X-ray beam toward an injection target. Clinical challenges to injection with the anterior-oblique approach include using a noncoaxial technique. Additional challenges to the anterior-oblique (also referred to as anterior) approach are body habitus and pannus, proximity to neurovascular structures, and patient positioning. By understanding the risks and benefits of varied technical approaches to accomplish a clinical goal and outcome, trainees are better able to select the technique most appropriate for a varied patient population.
Common risks to patients for all intra-articular interventions include bleeding, infection, and pain. Risk of damage to nearby structures is often mentioned as part of a standard informed consent process as it relates to the femoral vein, artery, and nerve that are in close anatomical proximity to the target injection site. When prior studies have examined the risk of complications resulting from intra-articular hip injections, a common conclusion is that despite a relatively low-risk profile for skilled interventionalists, efforts to avoid needle placement in the medial 50% of the femoral head on antero-posterior imaging is recommended.2
The anterior technique is a commonly described approach, and the same can be used for both ultrasound-guided and fluoroscopically guided hip injections.3 Using ultrasound guidance, the anterior technique can be performed with in-plane direct visualization of the needle throughout the procedure. With fluoroscopic guidance, the anterior approach is performed out-of-plane, using the noncoaxial technique. This requires the interventionalist to use tactile and anatomic guidance to the target injection site. The anterior approach for hip injection is one of few interventions where coaxial technique is not used for the procedure, making the instruction for a learner less concrete and potentially more challenging related to the needle path not under direct visualization in plane with the X-ray beam.
Technical guidance and detailed instruction for the lateral approach is infrequently described in fluoroscopic interventional texts. Reference to a lateral approach hip injection was made as early as the 1970s, without detail provided on the technique, with respect to the advantage of visualization of the hip joint for needle placement when hardware is in place.4 A more recent article described a lateral approach technique involving the patient in a decubitus (lateral) supine position, which presents limitations in consistent fluoroscopic imaging and can be a challenging static position for the patient to maintain.5
The retrospective review of anterior-oblique and lateral approach procedures in this study aims to demonstrate that there is no significant difference in radiation exposure, rate of successful intra-articular injection, or complication rate. If proven as a noninferior technique, the lateral approach may be a valuable interventional skill to those performing hip injections. Potential benefits to the patient and provider include options for the provider to access the joint using either technique. Additionally, the approach can be added to the instructional plan for those practitioners providing technical instruction to trainees within their health care system.
Methods
The institutional review board at the VA Ann Arbor Healthcare System reviewed and granted approval for this study. One of 5 interventional pain physician staff members at the VA Ann Arbor Healthcare System performed fluoroscopically guided hip injections. Interventional pain fellows under the direct supervision of board-certified physicians performed the procedures for the study cases. Supervising physicians included both physiatrists and anesthesiologists. Images were reviewed and evaluated without corresponding patient biographic data.
For cases using the lateral approach, the patients were positioned supine on the fluoroscopy table. In anterior-posterior and lateral views, trajectory lines are drawn using a long metal marking rod held adjacent to the patient. With pulsed low-dose fluoroscopy, transverse lines are drawn to identify midpoint of the femoral head in lateral view (Figure 1A, x-axis) and the most direct line from skin to lateral femoral head neck junction joint target (Figure 1B, z-axis). Also confirmed in lateral view, the z-axis marked line drawn on the skin is used to confirm that this transverse plane crosses the overlapping femoral heads (Figure 1A, y-axis).
The cross-section of these transverse and coronal plane lines identifies the starting point for the most direct approach from skin to injection target at femoral head-neck junction. Using the coaxial technique in the lateral view, the needle is introduced and advanced using intermittent fluoroscopic images to the lateral joint target. Continuing in this view, the interventionalist can ensure that advancing the needle to the osseous endpoint will place the tip at the midpoint of the femoral head at the target on the lateral surface, avoiding inadvertent advance of the needle anterior or posterior the femoral head. Final needle placement confirmation is then completed in antero-posterior view (Figure 2A). Contrast enhancement is used to confirm intra-articular spread (Figure 2B).
Cases included in the study were performed over an 8-month period in 2017. Case images recorded in IntelliSpace PACS Radiology software (Andover, MA) were included by creating a list of all cases performed and documented using the major joint injection procedure code. The cases reviewed began with the most recent cases. Two research team members (1 radiologist and 1 interventional pain physician) reviewed the series of saved images for each patient and the associated procedure report. The research team members documented and recorded de-identified study data in Microsoft Excel (Redmond, WA).
Imaging reports, using the saved images and the associated procedure report, were classified for technical approach (anterior, lateral, or inconclusive), success of joint injection as evidenced by appropriate contrast enhancement within the joint space (successful, unsuccessful, or incomplete images), documented use of sedation (yes, no), patient positioning (supine, prone), radiation exposure dose, radiation exposure time, and additional comments, such as “notable pannus” or “hardware present” to annotate significant findings on imaging review.
Statistical Analysis
The distribution of 2 outcomes used to compare rates of complication, radiation dose, and exposure time was checked using the Shapiro-Wilk test. Power analysis determined that inclusion of 30 anterior and 30 lateral cases results in adequate power to detect a 1-point mean difference, assuming a standard deviation of 1.5 in each group. Both radiation dose and exposure time were found to be nonnormally distributed (W = 0.65, P < .001; W = 0.86, P < .001; respectively). Median and interquartile range (IQR) of dose and time in seconds for anterior and lateral approaches were computed. Median differences in radiation dose and exposure time between anterior and lateral approaches were assessed with the k-sample test of equality of medians. All analyses were conducted using Stata Version 14.1 (College Station, TX).
Results
Between June 2017 and January 2018, 88 cases were reviewed as performed, with 30 anterior and 30 lateral approach cases included in this retrospective comparison study. A total of 28 cases were excluded from the study for using an inconclusive approach, multiple or bilateral procedures, cases without recorded dose and time data, and inadequately saved images to provide meaningful data (Figure 3).
Rate of successful intervention with needle placement confirmed within the articular space on contrast enhancement was not significantly different in the study groups with 96.7% (29 of 30) anterior approach cases reported as successful, 100% (30 of 30) lateral approach cases reported as successful. Overhanging pannus in the viewing area was reported in 5 anterior approach cases and 4 lateral cases. Hardware was noted in 2 lateral approach cases, none in anterior approach cases. Sedation was used for 3 of the anterior approach cases and none of the lateral approach cases.
Patients undergoing the lateral approach received a higher median radiation dose than did those undergoing the anterior approach, but this was not statistically significant (P = .07) (Table). Those undergoing the lateral approach also had a longer median exposure time than did those undergoing the anterior approach, but this also was not statistically significant (P = .3). With no immediate complications reported in any of the studied interventions, there was no difference in complication rates between anterior and lateral approach cases.
Discussion
Pain medicine fellows who have previously completed residency in a variety of disciplines, often either anesthesiology or physical medicine and rehabilitation, perform fluoroscopically guided procedures and benefit from increased experience with coaxial technique as this improves needle depth and location awareness. Once mastered, this skill set can be applied to and useful for multiple interventional pain procedures. Similar technical instruction with an emphasis on coaxial technique for hip injections as performed in the anterior or anterolateral approach can be used in both fluoroscopic and ultrasound-guided procedures, including facet injection, transforaminal epidural steroid injection, and myriad other procedures performed to ameliorate pain. There are advantages to pursuing a similar approach with all image-guided procedures. Evaluated in this comparison study is an alternative technique that has potential for risk reduction benefit with reduced proximity to neurovascular structures, which ultimately leads to a safer procedure profile.
Using a lateral approach, the interventionalist determines a starting point, entering the skin at a greater distance from any overlying pannus and the elevated concentration of gram-negative and gram-positive bacteria contained within the inguinal skin.6 A previous study demonstrated improved success of intra-articular needle tip placement without image guidance in patients with body mass index (BMI) < 30.7 A prior study of anterior approach using anatomic landmarks as compared to lateral approach demonstrated the anterior approach pierced or contacted the femoral nerve in 27% of anterior cases and came within 5 mm of 60% of anterior cases.2 Use of image guidance, whether ultrasound, fluoroscopy, or computed tomography (CT) is preferred related to reduced risk of contact with adjacent neurovascular structures. Anatomic surface landmarks have been described as an alternative injection technique, without the use of fluoroscopy for confirmatory initial, intraprocedure, and final placement.8 Palpation of anatomic structures is required for this nonimage-guided technique, and although similar to the described technique in this study, the anatomically guided injection starting point is more lateral than the anterior approach but not in the most lateral position in the transverse plane that is used for this fluoroscopically guided lateral approach study.
Physiologic characteristics of subjects and technical aspects of fluoroscopy both can be factors in radiation dose and exposure times for hip injections. Patient BMI was not included in the data collection, but further study would seek to determine whether BMI is a significant risk for any increased radiation dose and exposure times using lateral approach injections. Use of lateral images for fluoroscopy requires penetration of X-ray beam through more tissue compared with that of anterior-posterior images. Further study of these techniques would benefit from comparing the pulse rate of fluoroscopic images and collimation (or focusing of the radiation beam over a smaller area of tissue) as factors in any observed increase in total radiation dose and exposure times.
Improving the safety profile of this procedure could have a positive impact on the patient population receiving fluoroscopic hip injections, both within the VA Ann Arbor Health System and elsewhere. While the study population was limited to the VA patient population seeking subspecialty nonsurgical joint care at a single tertiary care center, this technique is generalizable and can be used in most patients, as hip pain is a common condition necessitating nonoperative evaluation and treatment.
Radiation Exposures
As our analysis demonstrates, mean radiation dose exposure for each group was consistent with low (≤ 3 mSv) to moderate (> 3-20 mSv) annual effective doses in the general population.7 Both anterior and lateral median radiation dose of 1 mGy and 3 mGy, respectively, are within the standard exposure for radiographs of the pelvis (1.31 mGy).9 It is therefore reasonable to consider a lateral approach for hip injection, given the benefits of direct coaxial approach and avoiding needle entry through higher bacteria-concentrated skin.
The lateral approach did have increased radiation dose and exposure time, although it was not statistically significantly greater than the anterior approach. The difference between radiation dose and time to perform either technique was not clinically significant. One potential explanation for this is that the lateral technique has increased tissue to penetrate, which can be reduced with collimation and other fluoroscopic image adjustments. Additionally, as trainees progress in competency, fewer images should need to be obtained.7 We hypothesize that as familiarity and comfort with this technique increase, the number of images necessary for successful injection would decrease, leading to decreased radiation dose and exposure time. We would expect that in the hands of a board-certified interventionalist, radiation dose and exposure time would be significantly decreased as compared to our current dataset, and this is an area of planned further study. With our existing dataset, the majority of procedures were performed with trainees, with inadequate information documented for comparison of dose over time and procedural experience under individual physicians.
Notable strengths of this study are the direct comparison of the anterior approach when compared to the lateral approach with regard to radiation dose and exposure time, which we have not seen described in the literature. A detailed description of the technique may result in increased utilization by other providers. Data were collected from multiple providers, as board-certified pain physicians and board-eligible interventional pain fellows performed the procedures. This variability in providers increases the generalizability of the findings, with a variety of providers, disciplines, years of experiences, and type of training represented.
Limitations
Limitations include the retrospective nature of the study and the relatively small sample size. However, even with this limitation, it is notable that no statistically significant differences were observed in mean radiation dose or fluoroscopy exposure time, making the lateral approach, at minimum, a noninferior technique. Combined with the improved safety profile, this technique is a viable alternative to the traditional anterior-oblique approach. Further study should be performed, such as a prospective, randomized control trial investigating the 2 techniques and following pain scores and functional ability after the procedure.
Conclusion
Given the decreased procedural risk related to proximity of neurovascular structures and coaxial technique for needle advancement, lateral approach for hip injection should be considered by those in any discipline performing fluoroscopically guided procedures. Lateral technique may be particularly useful in technically challenging cases and when skin entry at the anterior groin is suboptimal, as a noninferior alternative to traditional anterior method.
1. Cianfoni A, Boulter DJ, Rumboldt Z, Sapton T, Bonaldi G. Guidelines to imaging landmarks for interventional spine procedures: fluoroscopy and CT anatomy. Neurographics. 2011;1(1):39-48.
2. Leopold SS, Battista V, Oliverio JA. Safety and efficacy of intraarticular hip injection using anatomic landmarks. Clin Orthop Relat Res. 2001;(391):192-197.
3. Dodré E, Lefebvre G, Cockenpot E, Chastanet P, Cotten A. Interventional MSK procedures: the hip. Br J Radiol. 2016;89(1057):20150408.
4. Hankey S, McCall IW, Park WM, O’Connor BT. Technical problems in arthrography of the painful hip arthroplasty. Clin Radiol. 1979;30(6):653-656.
5. Yasar E, Singh JR, Hill J, Akuthota V. Image-guided injections of the hip. J Nov Physiother Phys Rehabil. 2014;1(2):39-48.
6. Aly R, Maibach HI. Aerobic microbial flora of intertrigenous skin. Appl Environ Microbiol. 1977;33(1):97-100.
7. Fazel R, Krumholz HM, Wang W, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361(9):849-857.
8. Masoud MA, Said HG. Intra-articular hip injection using anatomic surface landmarks. Arthosc Tech. 2013;2(2):e147-e149.
9. Ofori K, Gordon SW, Akrobortu E, Ampene AA, Darko EO. Estimation of adult patient doses for selected x-ray diagnostic examinations. J Radiat Res Appl Sci. 2014;7(4):459-462.
1. Cianfoni A, Boulter DJ, Rumboldt Z, Sapton T, Bonaldi G. Guidelines to imaging landmarks for interventional spine procedures: fluoroscopy and CT anatomy. Neurographics. 2011;1(1):39-48.
2. Leopold SS, Battista V, Oliverio JA. Safety and efficacy of intraarticular hip injection using anatomic landmarks. Clin Orthop Relat Res. 2001;(391):192-197.
3. Dodré E, Lefebvre G, Cockenpot E, Chastanet P, Cotten A. Interventional MSK procedures: the hip. Br J Radiol. 2016;89(1057):20150408.
4. Hankey S, McCall IW, Park WM, O’Connor BT. Technical problems in arthrography of the painful hip arthroplasty. Clin Radiol. 1979;30(6):653-656.
5. Yasar E, Singh JR, Hill J, Akuthota V. Image-guided injections of the hip. J Nov Physiother Phys Rehabil. 2014;1(2):39-48.
6. Aly R, Maibach HI. Aerobic microbial flora of intertrigenous skin. Appl Environ Microbiol. 1977;33(1):97-100.
7. Fazel R, Krumholz HM, Wang W, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361(9):849-857.
8. Masoud MA, Said HG. Intra-articular hip injection using anatomic surface landmarks. Arthosc Tech. 2013;2(2):e147-e149.
9. Ofori K, Gordon SW, Akrobortu E, Ampene AA, Darko EO. Estimation of adult patient doses for selected x-ray diagnostic examinations. J Radiat Res Appl Sci. 2014;7(4):459-462.
Opioid use curbed with patient education and lower prescription quantities
Patients given lower prescription quantities of opioid tablets with and without opioid education used significantly less of the medication compared with those given more tablets and no education, according to data from 264 adults and adolescents who underwent anterior cruciate ligament (ACL) surgery.
Although lower default prescription programs have been shown to reduce the number of tablets prescribed, “the effect of reduced prescription quantities on actual patient opioid consumption remains undetermined,” wrote Kevin X. Farley, BS, of Emory University, Atlanta, and colleagues.
In a study published in JAMA, the researchers examined whether patients took fewer tablets if given fewer, and whether patient education about opioids further reduced the number of tablets taken.
The study population included adults and adolescents who underwent ACL surgery at a single center. The patients were divided into three groups: 109 patients received 50 opioid tablets after surgery, 78 received 30 tablets plus education prior to surgery about appropriate opioid use and alternative pain management, and 77 received 30 tablets but no education on opioid use.
Patients given 50 tablets consumed an average of 25 tablets for an average of 5.8 days. By contrast, patients given 30 tablets but no opioid education consumed an average of 16 tablets for an average of 4.5 days, and those given 30 tablets and preoperative education consumed an average of 12 tablets for an average of 3.5 days.
In addition, patients given 30 tablets reported lower levels of constipation and fatigue compared with patients given 50 tablets. No differences were seen in medication refills among the groups.
The findings were limited by several factors including the use of data from a single center, the lack of randomization, and the potential for recall bias, the researchers noted. However, the results suggest that prescribing fewer tablets may further reduce use, as each group consumed approximately half of the tablets given, the researchers added.
“Further investigation should evaluate whether similar opioid stewardship and education protocols would be successful in other patient populations,” they said.
Corresponding author John Xerogeanes, MD, disclosed personal fees from Arthrex and stock options from Trice. The other researchers had no financial conflicts to disclose.
Patients given lower prescription quantities of opioid tablets with and without opioid education used significantly less of the medication compared with those given more tablets and no education, according to data from 264 adults and adolescents who underwent anterior cruciate ligament (ACL) surgery.
Although lower default prescription programs have been shown to reduce the number of tablets prescribed, “the effect of reduced prescription quantities on actual patient opioid consumption remains undetermined,” wrote Kevin X. Farley, BS, of Emory University, Atlanta, and colleagues.
In a study published in JAMA, the researchers examined whether patients took fewer tablets if given fewer, and whether patient education about opioids further reduced the number of tablets taken.
The study population included adults and adolescents who underwent ACL surgery at a single center. The patients were divided into three groups: 109 patients received 50 opioid tablets after surgery, 78 received 30 tablets plus education prior to surgery about appropriate opioid use and alternative pain management, and 77 received 30 tablets but no education on opioid use.
Patients given 50 tablets consumed an average of 25 tablets for an average of 5.8 days. By contrast, patients given 30 tablets but no opioid education consumed an average of 16 tablets for an average of 4.5 days, and those given 30 tablets and preoperative education consumed an average of 12 tablets for an average of 3.5 days.
In addition, patients given 30 tablets reported lower levels of constipation and fatigue compared with patients given 50 tablets. No differences were seen in medication refills among the groups.
The findings were limited by several factors including the use of data from a single center, the lack of randomization, and the potential for recall bias, the researchers noted. However, the results suggest that prescribing fewer tablets may further reduce use, as each group consumed approximately half of the tablets given, the researchers added.
“Further investigation should evaluate whether similar opioid stewardship and education protocols would be successful in other patient populations,” they said.
Corresponding author John Xerogeanes, MD, disclosed personal fees from Arthrex and stock options from Trice. The other researchers had no financial conflicts to disclose.
Patients given lower prescription quantities of opioid tablets with and without opioid education used significantly less of the medication compared with those given more tablets and no education, according to data from 264 adults and adolescents who underwent anterior cruciate ligament (ACL) surgery.
Although lower default prescription programs have been shown to reduce the number of tablets prescribed, “the effect of reduced prescription quantities on actual patient opioid consumption remains undetermined,” wrote Kevin X. Farley, BS, of Emory University, Atlanta, and colleagues.
In a study published in JAMA, the researchers examined whether patients took fewer tablets if given fewer, and whether patient education about opioids further reduced the number of tablets taken.
The study population included adults and adolescents who underwent ACL surgery at a single center. The patients were divided into three groups: 109 patients received 50 opioid tablets after surgery, 78 received 30 tablets plus education prior to surgery about appropriate opioid use and alternative pain management, and 77 received 30 tablets but no education on opioid use.
Patients given 50 tablets consumed an average of 25 tablets for an average of 5.8 days. By contrast, patients given 30 tablets but no opioid education consumed an average of 16 tablets for an average of 4.5 days, and those given 30 tablets and preoperative education consumed an average of 12 tablets for an average of 3.5 days.
In addition, patients given 30 tablets reported lower levels of constipation and fatigue compared with patients given 50 tablets. No differences were seen in medication refills among the groups.
The findings were limited by several factors including the use of data from a single center, the lack of randomization, and the potential for recall bias, the researchers noted. However, the results suggest that prescribing fewer tablets may further reduce use, as each group consumed approximately half of the tablets given, the researchers added.
“Further investigation should evaluate whether similar opioid stewardship and education protocols would be successful in other patient populations,” they said.
Corresponding author John Xerogeanes, MD, disclosed personal fees from Arthrex and stock options from Trice. The other researchers had no financial conflicts to disclose.
FROM JAMA
Key clinical point: Patient education and fewer tablets prescribed significantly reduced the amount of opioid tablets taken compared with no education and more tablets prescribed.
Major finding: Patients given 50 tablets and no patient education, 30 tablets and no patient education, and 30 tablets plus education consumed an average of 25, 16, and 12 tablets, respectively.
Study details: The data come from 264 adolescents and adults who underwent ACL surgery at a single center.
Disclosures: Corresponding author John Xerogeanes, MD, disclosed personal fees from Arthrex and stock options from Trice. The other researchers had no financial conflicts to disclose.
Source: Farley KX et al. JAMA. 2019 June 25.321(24):2465-7.
These Hips Don’t Lie
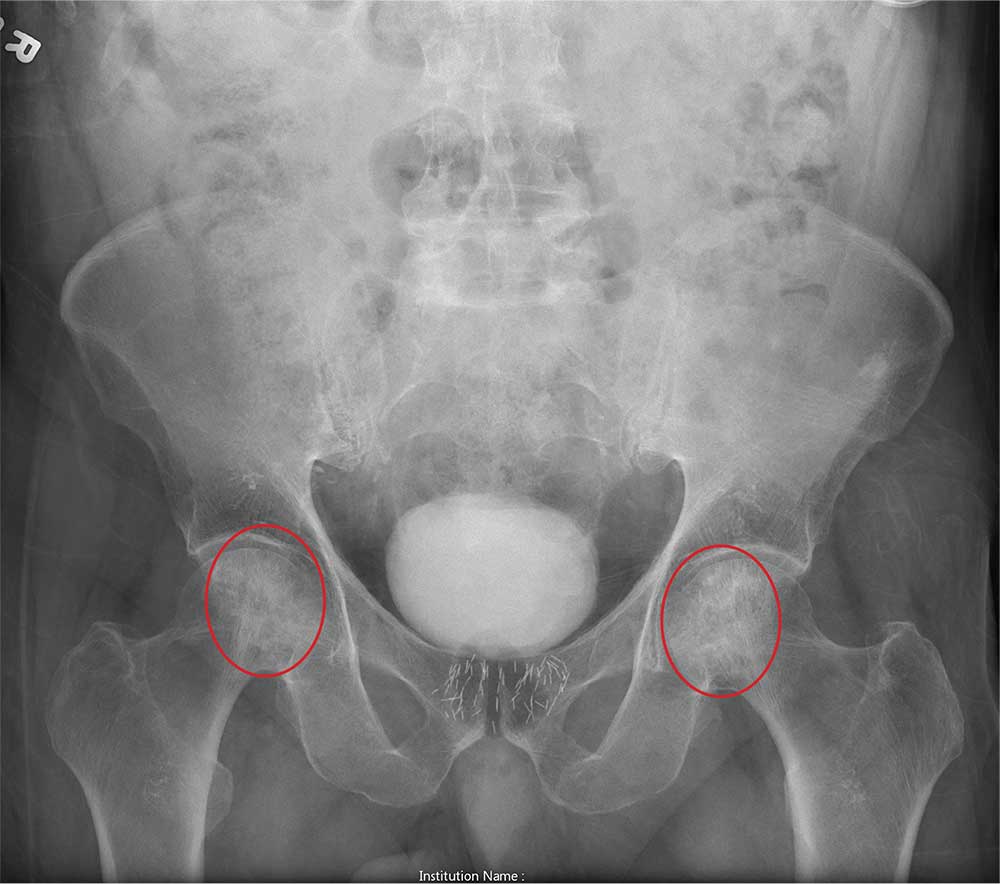
ANSWER
The radiograph shows no evidence of an acute fracture. Incidental findings include excreted contrast within the bladder and radiopaque markers from prostatic seed implants.
Fairly extensive sclerosis is noted within both femoral heads, which is suggestive of osteonecrosis (also known as avascular necrosis). Orthopedic consult was requested for further workup of this specific problem.

ANSWER
The radiograph shows no evidence of an acute fracture. Incidental findings include excreted contrast within the bladder and radiopaque markers from prostatic seed implants.
Fairly extensive sclerosis is noted within both femoral heads, which is suggestive of osteonecrosis (also known as avascular necrosis). Orthopedic consult was requested for further workup of this specific problem.

ANSWER
The radiograph shows no evidence of an acute fracture. Incidental findings include excreted contrast within the bladder and radiopaque markers from prostatic seed implants.
Fairly extensive sclerosis is noted within both femoral heads, which is suggestive of osteonecrosis (also known as avascular necrosis). Orthopedic consult was requested for further workup of this specific problem.
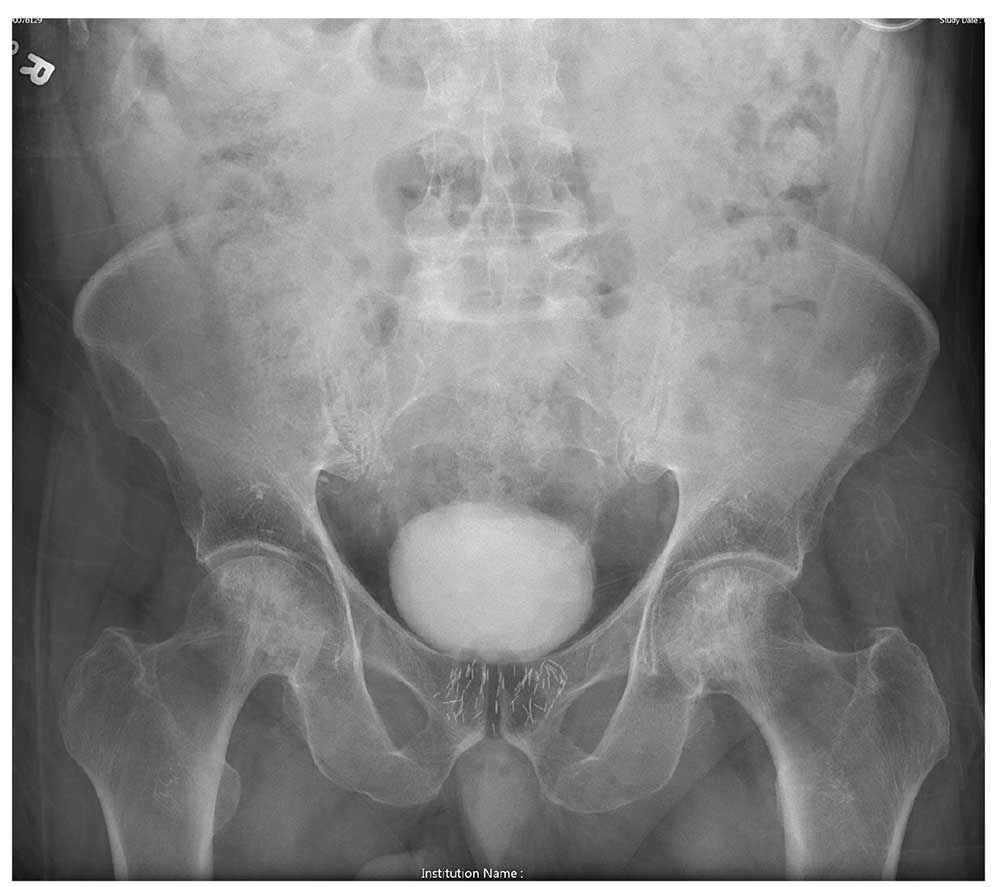
An 80-year-old man is transferred to your facility for evaluation of a lumbar compression fracture he sustained from a motor vehicle collision. The patient was a restrained driver in a vehicle that was broadsided at an unknown speed. His airbags deployed. In addition to mild back discomfort, he complains of severe right hip pain.
His medical history is significant for prostate cancer and coronary artery disease. Surgical history includes remote cardiac bypass surgery and recent revascularization with stents.
On examination, you note an elderly male who is awake and alert. His vital signs are stable. He is able to move all extremities
A portable pelvis radiograph is obtained (shown). What is your impression?
Pain coping skills training doesn’t improve knee arthroplasty outcomes
TORONTO – A high level of pain catastrophizing prior to scheduled knee arthroplasty is not, as previously thought, a harbinger of poor outcomes, and affected patients don’t benefit from cognitive-behavioral therapy–based training in pain coping skills, Daniel L. Riddle, PhD, reported at the OARSI 2019 World Congress.
“The take-home message for us is knee arthroplasty is incredibly effective and there really is no reason to do pain coping skills training in these high–pain catastrophizing patients because the great majority of them have such good outcomes,” said Dr. Riddle, professor of physical therapy at Virginia Commonwealth University, Richmond.
“The other clear message from our trial is that, when you have pain-catastrophizing patients and you lower their pain, their catastrophizing is also lowered. So pain catastrophizing is clearly a response to pain and not a personality trait per se,” he said at the meeting sponsored by the Osteoarthritis Research Society International.
He presented the results of a 402-patient, randomized, three-arm, single-blind trial conducted at five U.S. medical centers. All participants were scheduled for knee arthroplasty for osteoarthritis, and all had moderate- to high-level pain catastrophizing as reflected in the group’s average Pain Catastrophizing Score of 30. They were assigned to an arthritis education active control group, usual care, or an intervention developed specifically for this study: a cognitive-behavioral therapy–based training program for pain coping skills. Similar pain coping skills training interventions have been shown to be beneficial in patients with medically treated knee OA but hadn’t previously been evaluated in surgically treated patients. The primary study endpoint was change in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain Scale at 2, 6, and 12 months after surgery.
The improvement in WOMAC pain in the three study arms was virtually superimposable, going from an average pain score of about 12 preoperatively to 2 postoperatively.
“This was a clear no-effect trial,” Dr. Riddle observed. “These are patients we thought to be at increased risk for poor outcome, but indeed they’re not.”
Pain Catastrophizing Scores improved from 30 preoperatively to roughly 7 at 1 year. “We’ve never seen pain catastrophizing improvements of this magnitude,” the researcher commented.
The study participants typically had a large number of chronically painful areas, but only minimal change in pain scores occurred except in the surgically treated knee.
Of note, even with the impressively large improvements in knee pain, function, and other secondary endpoints in the study group as a whole, roughly 20% of study participants experienced essentially no improvement in their function-limiting knee pain during the first year after arthroplasty. These nonresponders were spread equally across all three study arms. Further research will be needed to develop interventions to help this challenging patient subgroup.
The pain coping skills training consisted of 8 weekly sessions, each an hour long, which began prior to surgery and continued afterward. The intervention was delivered by physical therapists who had been trained by pain psychologists with expertise in cognitive-behavioral therapy. The intervention was delivered by telephone and in face-to-face sessions. The trainers were tracked over the course of the study to make sure that the structured intervention was delivered as planned.
Dr. Riddle reported having no financial conflicts regarding the National Institutes of Health-funded study, the full details of which have been published (J Bone Joint Surg Am. 2019 Feb 6;101[3]:218-227).
TORONTO – A high level of pain catastrophizing prior to scheduled knee arthroplasty is not, as previously thought, a harbinger of poor outcomes, and affected patients don’t benefit from cognitive-behavioral therapy–based training in pain coping skills, Daniel L. Riddle, PhD, reported at the OARSI 2019 World Congress.
“The take-home message for us is knee arthroplasty is incredibly effective and there really is no reason to do pain coping skills training in these high–pain catastrophizing patients because the great majority of them have such good outcomes,” said Dr. Riddle, professor of physical therapy at Virginia Commonwealth University, Richmond.
“The other clear message from our trial is that, when you have pain-catastrophizing patients and you lower their pain, their catastrophizing is also lowered. So pain catastrophizing is clearly a response to pain and not a personality trait per se,” he said at the meeting sponsored by the Osteoarthritis Research Society International.
He presented the results of a 402-patient, randomized, three-arm, single-blind trial conducted at five U.S. medical centers. All participants were scheduled for knee arthroplasty for osteoarthritis, and all had moderate- to high-level pain catastrophizing as reflected in the group’s average Pain Catastrophizing Score of 30. They were assigned to an arthritis education active control group, usual care, or an intervention developed specifically for this study: a cognitive-behavioral therapy–based training program for pain coping skills. Similar pain coping skills training interventions have been shown to be beneficial in patients with medically treated knee OA but hadn’t previously been evaluated in surgically treated patients. The primary study endpoint was change in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain Scale at 2, 6, and 12 months after surgery.
The improvement in WOMAC pain in the three study arms was virtually superimposable, going from an average pain score of about 12 preoperatively to 2 postoperatively.
“This was a clear no-effect trial,” Dr. Riddle observed. “These are patients we thought to be at increased risk for poor outcome, but indeed they’re not.”
Pain Catastrophizing Scores improved from 30 preoperatively to roughly 7 at 1 year. “We’ve never seen pain catastrophizing improvements of this magnitude,” the researcher commented.
The study participants typically had a large number of chronically painful areas, but only minimal change in pain scores occurred except in the surgically treated knee.
Of note, even with the impressively large improvements in knee pain, function, and other secondary endpoints in the study group as a whole, roughly 20% of study participants experienced essentially no improvement in their function-limiting knee pain during the first year after arthroplasty. These nonresponders were spread equally across all three study arms. Further research will be needed to develop interventions to help this challenging patient subgroup.
The pain coping skills training consisted of 8 weekly sessions, each an hour long, which began prior to surgery and continued afterward. The intervention was delivered by physical therapists who had been trained by pain psychologists with expertise in cognitive-behavioral therapy. The intervention was delivered by telephone and in face-to-face sessions. The trainers were tracked over the course of the study to make sure that the structured intervention was delivered as planned.
Dr. Riddle reported having no financial conflicts regarding the National Institutes of Health-funded study, the full details of which have been published (J Bone Joint Surg Am. 2019 Feb 6;101[3]:218-227).
TORONTO – A high level of pain catastrophizing prior to scheduled knee arthroplasty is not, as previously thought, a harbinger of poor outcomes, and affected patients don’t benefit from cognitive-behavioral therapy–based training in pain coping skills, Daniel L. Riddle, PhD, reported at the OARSI 2019 World Congress.
“The take-home message for us is knee arthroplasty is incredibly effective and there really is no reason to do pain coping skills training in these high–pain catastrophizing patients because the great majority of them have such good outcomes,” said Dr. Riddle, professor of physical therapy at Virginia Commonwealth University, Richmond.
“The other clear message from our trial is that, when you have pain-catastrophizing patients and you lower their pain, their catastrophizing is also lowered. So pain catastrophizing is clearly a response to pain and not a personality trait per se,” he said at the meeting sponsored by the Osteoarthritis Research Society International.
He presented the results of a 402-patient, randomized, three-arm, single-blind trial conducted at five U.S. medical centers. All participants were scheduled for knee arthroplasty for osteoarthritis, and all had moderate- to high-level pain catastrophizing as reflected in the group’s average Pain Catastrophizing Score of 30. They were assigned to an arthritis education active control group, usual care, or an intervention developed specifically for this study: a cognitive-behavioral therapy–based training program for pain coping skills. Similar pain coping skills training interventions have been shown to be beneficial in patients with medically treated knee OA but hadn’t previously been evaluated in surgically treated patients. The primary study endpoint was change in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain Scale at 2, 6, and 12 months after surgery.
The improvement in WOMAC pain in the three study arms was virtually superimposable, going from an average pain score of about 12 preoperatively to 2 postoperatively.
“This was a clear no-effect trial,” Dr. Riddle observed. “These are patients we thought to be at increased risk for poor outcome, but indeed they’re not.”
Pain Catastrophizing Scores improved from 30 preoperatively to roughly 7 at 1 year. “We’ve never seen pain catastrophizing improvements of this magnitude,” the researcher commented.
The study participants typically had a large number of chronically painful areas, but only minimal change in pain scores occurred except in the surgically treated knee.
Of note, even with the impressively large improvements in knee pain, function, and other secondary endpoints in the study group as a whole, roughly 20% of study participants experienced essentially no improvement in their function-limiting knee pain during the first year after arthroplasty. These nonresponders were spread equally across all three study arms. Further research will be needed to develop interventions to help this challenging patient subgroup.
The pain coping skills training consisted of 8 weekly sessions, each an hour long, which began prior to surgery and continued afterward. The intervention was delivered by physical therapists who had been trained by pain psychologists with expertise in cognitive-behavioral therapy. The intervention was delivered by telephone and in face-to-face sessions. The trainers were tracked over the course of the study to make sure that the structured intervention was delivered as planned.
Dr. Riddle reported having no financial conflicts regarding the National Institutes of Health-funded study, the full details of which have been published (J Bone Joint Surg Am. 2019 Feb 6;101[3]:218-227).
REPORTING FROM OARSI 2019
Revision total joint replacements linked to higher infection risk
, according to the findings of a large cohort study.
In absolute terms, these rates were 15.6% after revision total knee replacement and 8.6% after revision total hip replacement, vs. 2.1% and 2.1% after the respective primary surgeries, wrote Charles E. Edmiston Jr., PhD, and associates. “This result is consistent with results from several other studies that have reported an increased risk after revision procedures,” they added. Such information is essential for developing care bundles based on presenting risk factors, they wrote in a report published in the American Journal of Infection Control.
Population aging and comorbidities are fueling the need for primary and revision joint replacement surgery. “Such trends highlight the need for solutions to cost-effectively prevent and treat surgical site infections, one of the common complications after total joint replacement procedures,” the researchers wrote. Using IBM MarketScan and Medicare data, they studied 335,134 total knee replacements and 163,547 total hip replacements performed in the United States between 2009 and 2015.
After adjustment for potential confounders, the comorbidities that were most strongly linked to surgical site infections during the 90 days after primary or revision joint replacement were AIDS, paralysis, coagulopathy, metastatic cancer, heart failure, alcohol use disorder, obesity, fluid electrolyte disorders, and chronic pulmonary disorders. The estimated odds ratios for these correlates ranged from 1.33 to 1.58, and all confidence intervals reached statistical significance.
In general, these findings reflect those of smaller studies, the researchers noted. “The challenge for the future will be the development of evidence-based surgical care bundles that focus on the peri-, intra-, and postoperative components of orthopedic patient care, especially in patients undergoing periprosthetic revision.”
Johnson & Johnson Medical Device Companies provided funding. Three coinvestigators reported employment and stockholder ties to the company. In addition, Dr. Edmiston and one coinvestigator reported speaker fees for Ethicon, a Johnson & Johnson company.
SOURCE: Edmiston CE et al. Am J Infect Control. 2019 May 6. doi: 10.1016/j.ajic.2019.03.030.
, according to the findings of a large cohort study.
In absolute terms, these rates were 15.6% after revision total knee replacement and 8.6% after revision total hip replacement, vs. 2.1% and 2.1% after the respective primary surgeries, wrote Charles E. Edmiston Jr., PhD, and associates. “This result is consistent with results from several other studies that have reported an increased risk after revision procedures,” they added. Such information is essential for developing care bundles based on presenting risk factors, they wrote in a report published in the American Journal of Infection Control.
Population aging and comorbidities are fueling the need for primary and revision joint replacement surgery. “Such trends highlight the need for solutions to cost-effectively prevent and treat surgical site infections, one of the common complications after total joint replacement procedures,” the researchers wrote. Using IBM MarketScan and Medicare data, they studied 335,134 total knee replacements and 163,547 total hip replacements performed in the United States between 2009 and 2015.
After adjustment for potential confounders, the comorbidities that were most strongly linked to surgical site infections during the 90 days after primary or revision joint replacement were AIDS, paralysis, coagulopathy, metastatic cancer, heart failure, alcohol use disorder, obesity, fluid electrolyte disorders, and chronic pulmonary disorders. The estimated odds ratios for these correlates ranged from 1.33 to 1.58, and all confidence intervals reached statistical significance.
In general, these findings reflect those of smaller studies, the researchers noted. “The challenge for the future will be the development of evidence-based surgical care bundles that focus on the peri-, intra-, and postoperative components of orthopedic patient care, especially in patients undergoing periprosthetic revision.”
Johnson & Johnson Medical Device Companies provided funding. Three coinvestigators reported employment and stockholder ties to the company. In addition, Dr. Edmiston and one coinvestigator reported speaker fees for Ethicon, a Johnson & Johnson company.
SOURCE: Edmiston CE et al. Am J Infect Control. 2019 May 6. doi: 10.1016/j.ajic.2019.03.030.
, according to the findings of a large cohort study.
In absolute terms, these rates were 15.6% after revision total knee replacement and 8.6% after revision total hip replacement, vs. 2.1% and 2.1% after the respective primary surgeries, wrote Charles E. Edmiston Jr., PhD, and associates. “This result is consistent with results from several other studies that have reported an increased risk after revision procedures,” they added. Such information is essential for developing care bundles based on presenting risk factors, they wrote in a report published in the American Journal of Infection Control.
Population aging and comorbidities are fueling the need for primary and revision joint replacement surgery. “Such trends highlight the need for solutions to cost-effectively prevent and treat surgical site infections, one of the common complications after total joint replacement procedures,” the researchers wrote. Using IBM MarketScan and Medicare data, they studied 335,134 total knee replacements and 163,547 total hip replacements performed in the United States between 2009 and 2015.
After adjustment for potential confounders, the comorbidities that were most strongly linked to surgical site infections during the 90 days after primary or revision joint replacement were AIDS, paralysis, coagulopathy, metastatic cancer, heart failure, alcohol use disorder, obesity, fluid electrolyte disorders, and chronic pulmonary disorders. The estimated odds ratios for these correlates ranged from 1.33 to 1.58, and all confidence intervals reached statistical significance.
In general, these findings reflect those of smaller studies, the researchers noted. “The challenge for the future will be the development of evidence-based surgical care bundles that focus on the peri-, intra-, and postoperative components of orthopedic patient care, especially in patients undergoing periprosthetic revision.”
Johnson & Johnson Medical Device Companies provided funding. Three coinvestigators reported employment and stockholder ties to the company. In addition, Dr. Edmiston and one coinvestigator reported speaker fees for Ethicon, a Johnson & Johnson company.
SOURCE: Edmiston CE et al. Am J Infect Control. 2019 May 6. doi: 10.1016/j.ajic.2019.03.030.
FROM THE AMERICAN JOURNAL OF INFECTION CONTROL
Bundled payment for OA surgery linked to more emergency department visits
And therein lies a key lesson for health policy makers who have embraced bundled payments to reduce rising health care costs, Mayilee Canizares, PhD, observed at the OARSI 2019 World Congress.
In Ontario, with patients discharged sooner and directly to home, there was the negative impact of increased emergency department visits after surgery, Dr. Canizares, of the University Health Network in Toronto, said at OARSI 2019 World Congress, sponsored by the Osteoarthritis Research Society International. “Our findings highlight the importance of coordinating the appropriate support services as well as the need to continue assessing the optimal discharge care plan for osteoarthritis patients undergoing surgery.”
Dr. Canizares’ study of the Ontario-wide experience with orthopedic surgery for osteoarthritis during 2004-2016 received the OARSI 2019 award for the meeting’s top-rated study in clinical epidemiology/health services research.
Using administrative data from Canada’s national health care system, Dr. Canizares and her coinvestigators found that the number of individuals undergoing elective orthopedic surgery for osteoarthritis ballooned from 22,700 in 2004 to 41,900 in 2016, representing an increase from 246 to 381 procedures per 100,000 people. During this time, the mean length of stay declined from about 5 days to just under 3 days, the 30-day readmission rate dropped from 4.2% to 3.4%, and the rate of emergency department visits within 30 days post discharge rose steadily from 8.7% in 2004 to 14.1% in 2016.
Roughly half of the operations were total knee replacements and one-third were hip replacements. The profile of patients undergoing surgery changed little over the course of the 12-year study with the exception that in more recent years patients presented with more comorbidities: Indeed, three or more comorbid conditions were present in 2.9% of the surgical patients in 2004 compared to 4.2% in 2016.
In multivariate logistic regression analyses, patient characteristics didn’t explain the change over time in early readmission or unplanned emergency department visit rates. However, discharge disposition did: By 2014, more patients were being discharged home, and in nearly half of cases that was being done without support.
Dr. Canizares reported having no financial conflicts regarding her study, funded by the Toronto General and Western Hospital Foundation.
SOURCE: Canizares M. OARSI, Abstract 16.
And therein lies a key lesson for health policy makers who have embraced bundled payments to reduce rising health care costs, Mayilee Canizares, PhD, observed at the OARSI 2019 World Congress.
In Ontario, with patients discharged sooner and directly to home, there was the negative impact of increased emergency department visits after surgery, Dr. Canizares, of the University Health Network in Toronto, said at OARSI 2019 World Congress, sponsored by the Osteoarthritis Research Society International. “Our findings highlight the importance of coordinating the appropriate support services as well as the need to continue assessing the optimal discharge care plan for osteoarthritis patients undergoing surgery.”
Dr. Canizares’ study of the Ontario-wide experience with orthopedic surgery for osteoarthritis during 2004-2016 received the OARSI 2019 award for the meeting’s top-rated study in clinical epidemiology/health services research.
Using administrative data from Canada’s national health care system, Dr. Canizares and her coinvestigators found that the number of individuals undergoing elective orthopedic surgery for osteoarthritis ballooned from 22,700 in 2004 to 41,900 in 2016, representing an increase from 246 to 381 procedures per 100,000 people. During this time, the mean length of stay declined from about 5 days to just under 3 days, the 30-day readmission rate dropped from 4.2% to 3.4%, and the rate of emergency department visits within 30 days post discharge rose steadily from 8.7% in 2004 to 14.1% in 2016.
Roughly half of the operations were total knee replacements and one-third were hip replacements. The profile of patients undergoing surgery changed little over the course of the 12-year study with the exception that in more recent years patients presented with more comorbidities: Indeed, three or more comorbid conditions were present in 2.9% of the surgical patients in 2004 compared to 4.2% in 2016.
In multivariate logistic regression analyses, patient characteristics didn’t explain the change over time in early readmission or unplanned emergency department visit rates. However, discharge disposition did: By 2014, more patients were being discharged home, and in nearly half of cases that was being done without support.
Dr. Canizares reported having no financial conflicts regarding her study, funded by the Toronto General and Western Hospital Foundation.
SOURCE: Canizares M. OARSI, Abstract 16.
And therein lies a key lesson for health policy makers who have embraced bundled payments to reduce rising health care costs, Mayilee Canizares, PhD, observed at the OARSI 2019 World Congress.
In Ontario, with patients discharged sooner and directly to home, there was the negative impact of increased emergency department visits after surgery, Dr. Canizares, of the University Health Network in Toronto, said at OARSI 2019 World Congress, sponsored by the Osteoarthritis Research Society International. “Our findings highlight the importance of coordinating the appropriate support services as well as the need to continue assessing the optimal discharge care plan for osteoarthritis patients undergoing surgery.”
Dr. Canizares’ study of the Ontario-wide experience with orthopedic surgery for osteoarthritis during 2004-2016 received the OARSI 2019 award for the meeting’s top-rated study in clinical epidemiology/health services research.
Using administrative data from Canada’s national health care system, Dr. Canizares and her coinvestigators found that the number of individuals undergoing elective orthopedic surgery for osteoarthritis ballooned from 22,700 in 2004 to 41,900 in 2016, representing an increase from 246 to 381 procedures per 100,000 people. During this time, the mean length of stay declined from about 5 days to just under 3 days, the 30-day readmission rate dropped from 4.2% to 3.4%, and the rate of emergency department visits within 30 days post discharge rose steadily from 8.7% in 2004 to 14.1% in 2016.
Roughly half of the operations were total knee replacements and one-third were hip replacements. The profile of patients undergoing surgery changed little over the course of the 12-year study with the exception that in more recent years patients presented with more comorbidities: Indeed, three or more comorbid conditions were present in 2.9% of the surgical patients in 2004 compared to 4.2% in 2016.
In multivariate logistic regression analyses, patient characteristics didn’t explain the change over time in early readmission or unplanned emergency department visit rates. However, discharge disposition did: By 2014, more patients were being discharged home, and in nearly half of cases that was being done without support.
Dr. Canizares reported having no financial conflicts regarding her study, funded by the Toronto General and Western Hospital Foundation.
SOURCE: Canizares M. OARSI, Abstract 16.
REPORTING FROM OARSI 2019