User login
High-intensity statins may cut risk of joint replacement
TORONTO – comparing nearly 180,000 statin users with an equal number of propensity-matched nonusers, Jie Wei, PhD, reported at the OARSI 2019 World Congress.
Less intensive statin therapy was associated with significantly less need for joint replacement surgery in rheumatoid arthritis patients, but not in those with osteoarthritis, she said at the meeting, sponsored by the Osteoarthritis Research Society International.
“In summary, statins may reduce the risk of joint replacement, especially when given at high strength and in people with rheumatoid arthritis,” said Dr. Wei, an epidemiologist at Massachusetts General Hospital, Boston, and Central South University in Changsha, Hunan, China.
She was quick to note that this study can’t be considered the final, definitive word on the topic, since other investigators’ studies of the relationship between statin usage and joint replacement surgery for arthritis have yielded conflicting results. However, given the thoroughly established super-favorable risk/benefit ratio of statins for the prevention of cardiovascular morbidity and mortality, the possibility of a prospective, randomized, controlled trial addressing the joint surgery issue is for ethical reasons a train that’s left the station.
Dr. Wei presented an analysis drawn from the U.K. Clinical Practice Research Datalink for the years 1989 through mid-2017. The initial sample included the medical records of 17.1 million patients, or 26% of the total U.K. population. From that massive pool, she and her coinvestigators zeroed in on 178,467 statin users and an equal number of non–statin-user controls under the care of 718 primary care physicians, with the pairs propensity score-matched on the basis of age, gender, locality, comorbid conditions, nonstatin medications, lifestyle factors, and duration of rheumatoid arthritis or osteoarthritis. The mean age of the matched pairs was 62 years, 52% were women, and the mean prospective follow-up was 6.5 years.
The use of high-intensity statin therapy – for example, atorvastatin at 40-80 mg/day or rosuvastatin (Crestor) at 20-40 mg/day – was independently associated with a 21% reduction in the risk of knee or hip replacement surgery for osteoarthritis and a 90% reduction for rheumatoid arthritis, compared with statin nonusers. Notably, joint replacement surgery for osteoarthritis was roughly 25-fold more common than for rheumatoid arthritis.
Statin therapy overall, including the more widely prescribed low- and intermediate-intensity regimens, was associated with a 23% reduction in joint replacement surgery for rheumatoid arthritis, compared with statin nonusers, but had no significant impact on surgery for the osteoarthritis population.
A couple of distinguished American rheumatologists in the audience rose to voice reluctance about drawing broad conclusions from this study.
“Bias, as you’ve said yourself, is a bit of a concern,” said David T. Felson, MD, professor of medicine and public health and director of clinical epidemiology at Boston University.
He was troubled that the study design was such that anyone who filled as few as two statin prescriptions during the more than 6-year study period was categorized as a statin user. That, he said, muddies the waters. Does the database contain information on duration of statin therapy, and whether joint replacement surgery was more likely to occur when patients were on or off statin therapy? he asked.
It does, Dr. Wei replied, adding that she will take that suggestion for additional analysis back to her international team of coinvestigators.
“It seems to me,” said Jeffrey N. Katz, MD, “that the major risk of potential bias is that people who were provided high-intensity statins were prescribed that because they were at risk for or had cardiac disease.”
That high cardiovascular risk might have curbed orthopedic surgeons’ enthusiasm to operate. Thus, it would be helpful to learn whether patients who underwent joint replacement were less likely to have undergone coronary revascularization or other cardiac interventions than were those without joint replacement, according to Dr. Katz, professor of medicine and orthopedic surgery at Harvard Medical School, Boston.
Dr. Wei agreed that confounding by indication is always a possibility in an observational study such as this. Identification of a plausible mechanism by which statins might reduce the risk of joint replacement surgery in rheumatoid arthritis – something that hasn’t happened yet – would help counter such concerns.
She noted that a separate recent analysis of the U.K. Clinical Practice Research Datalink by other investigators concluded that statin therapy started up to 5 years following total hip or knee replacement was associated with a significantly reduced risk of revision arthroplasty. Moreover, the benefit was treatment duration-dependent: Patients on statin therapy for more than 5 years were 26% less likely to undergo revision arthroplasty than were those on a statin for less than 1 year (J Rheumatol. 2019 Mar 15. doi: 10.3899/jrheum.180574).
On the other hand, Swedish investigators found that statin use wasn’t associated with a reduced risk of consultation or surgery for osteoarthritis in a pooled analysis of four cohort studies totaling more than 132,000 Swedes followed for 7.5 years (Osteoarthritis Cartilage. 2017 Nov;25[11]:1804-13).
Dr. Wei reported having no financial conflicts regarding the study, which was supported by the National Clinical Research Center of Geriatric Disorders in Hunan, China, and several British universities.
SOURCE: Sarmanova A et al. Osteoarthritis cartilage. 2019 Apr;27[suppl 1]:S78-S79. Abstract 77.
TORONTO – comparing nearly 180,000 statin users with an equal number of propensity-matched nonusers, Jie Wei, PhD, reported at the OARSI 2019 World Congress.
Less intensive statin therapy was associated with significantly less need for joint replacement surgery in rheumatoid arthritis patients, but not in those with osteoarthritis, she said at the meeting, sponsored by the Osteoarthritis Research Society International.
“In summary, statins may reduce the risk of joint replacement, especially when given at high strength and in people with rheumatoid arthritis,” said Dr. Wei, an epidemiologist at Massachusetts General Hospital, Boston, and Central South University in Changsha, Hunan, China.
She was quick to note that this study can’t be considered the final, definitive word on the topic, since other investigators’ studies of the relationship between statin usage and joint replacement surgery for arthritis have yielded conflicting results. However, given the thoroughly established super-favorable risk/benefit ratio of statins for the prevention of cardiovascular morbidity and mortality, the possibility of a prospective, randomized, controlled trial addressing the joint surgery issue is for ethical reasons a train that’s left the station.
Dr. Wei presented an analysis drawn from the U.K. Clinical Practice Research Datalink for the years 1989 through mid-2017. The initial sample included the medical records of 17.1 million patients, or 26% of the total U.K. population. From that massive pool, she and her coinvestigators zeroed in on 178,467 statin users and an equal number of non–statin-user controls under the care of 718 primary care physicians, with the pairs propensity score-matched on the basis of age, gender, locality, comorbid conditions, nonstatin medications, lifestyle factors, and duration of rheumatoid arthritis or osteoarthritis. The mean age of the matched pairs was 62 years, 52% were women, and the mean prospective follow-up was 6.5 years.
The use of high-intensity statin therapy – for example, atorvastatin at 40-80 mg/day or rosuvastatin (Crestor) at 20-40 mg/day – was independently associated with a 21% reduction in the risk of knee or hip replacement surgery for osteoarthritis and a 90% reduction for rheumatoid arthritis, compared with statin nonusers. Notably, joint replacement surgery for osteoarthritis was roughly 25-fold more common than for rheumatoid arthritis.
Statin therapy overall, including the more widely prescribed low- and intermediate-intensity regimens, was associated with a 23% reduction in joint replacement surgery for rheumatoid arthritis, compared with statin nonusers, but had no significant impact on surgery for the osteoarthritis population.
A couple of distinguished American rheumatologists in the audience rose to voice reluctance about drawing broad conclusions from this study.
“Bias, as you’ve said yourself, is a bit of a concern,” said David T. Felson, MD, professor of medicine and public health and director of clinical epidemiology at Boston University.
He was troubled that the study design was such that anyone who filled as few as two statin prescriptions during the more than 6-year study period was categorized as a statin user. That, he said, muddies the waters. Does the database contain information on duration of statin therapy, and whether joint replacement surgery was more likely to occur when patients were on or off statin therapy? he asked.
It does, Dr. Wei replied, adding that she will take that suggestion for additional analysis back to her international team of coinvestigators.
“It seems to me,” said Jeffrey N. Katz, MD, “that the major risk of potential bias is that people who were provided high-intensity statins were prescribed that because they were at risk for or had cardiac disease.”
That high cardiovascular risk might have curbed orthopedic surgeons’ enthusiasm to operate. Thus, it would be helpful to learn whether patients who underwent joint replacement were less likely to have undergone coronary revascularization or other cardiac interventions than were those without joint replacement, according to Dr. Katz, professor of medicine and orthopedic surgery at Harvard Medical School, Boston.
Dr. Wei agreed that confounding by indication is always a possibility in an observational study such as this. Identification of a plausible mechanism by which statins might reduce the risk of joint replacement surgery in rheumatoid arthritis – something that hasn’t happened yet – would help counter such concerns.
She noted that a separate recent analysis of the U.K. Clinical Practice Research Datalink by other investigators concluded that statin therapy started up to 5 years following total hip or knee replacement was associated with a significantly reduced risk of revision arthroplasty. Moreover, the benefit was treatment duration-dependent: Patients on statin therapy for more than 5 years were 26% less likely to undergo revision arthroplasty than were those on a statin for less than 1 year (J Rheumatol. 2019 Mar 15. doi: 10.3899/jrheum.180574).
On the other hand, Swedish investigators found that statin use wasn’t associated with a reduced risk of consultation or surgery for osteoarthritis in a pooled analysis of four cohort studies totaling more than 132,000 Swedes followed for 7.5 years (Osteoarthritis Cartilage. 2017 Nov;25[11]:1804-13).
Dr. Wei reported having no financial conflicts regarding the study, which was supported by the National Clinical Research Center of Geriatric Disorders in Hunan, China, and several British universities.
SOURCE: Sarmanova A et al. Osteoarthritis cartilage. 2019 Apr;27[suppl 1]:S78-S79. Abstract 77.
TORONTO – comparing nearly 180,000 statin users with an equal number of propensity-matched nonusers, Jie Wei, PhD, reported at the OARSI 2019 World Congress.
Less intensive statin therapy was associated with significantly less need for joint replacement surgery in rheumatoid arthritis patients, but not in those with osteoarthritis, she said at the meeting, sponsored by the Osteoarthritis Research Society International.
“In summary, statins may reduce the risk of joint replacement, especially when given at high strength and in people with rheumatoid arthritis,” said Dr. Wei, an epidemiologist at Massachusetts General Hospital, Boston, and Central South University in Changsha, Hunan, China.
She was quick to note that this study can’t be considered the final, definitive word on the topic, since other investigators’ studies of the relationship between statin usage and joint replacement surgery for arthritis have yielded conflicting results. However, given the thoroughly established super-favorable risk/benefit ratio of statins for the prevention of cardiovascular morbidity and mortality, the possibility of a prospective, randomized, controlled trial addressing the joint surgery issue is for ethical reasons a train that’s left the station.
Dr. Wei presented an analysis drawn from the U.K. Clinical Practice Research Datalink for the years 1989 through mid-2017. The initial sample included the medical records of 17.1 million patients, or 26% of the total U.K. population. From that massive pool, she and her coinvestigators zeroed in on 178,467 statin users and an equal number of non–statin-user controls under the care of 718 primary care physicians, with the pairs propensity score-matched on the basis of age, gender, locality, comorbid conditions, nonstatin medications, lifestyle factors, and duration of rheumatoid arthritis or osteoarthritis. The mean age of the matched pairs was 62 years, 52% were women, and the mean prospective follow-up was 6.5 years.
The use of high-intensity statin therapy – for example, atorvastatin at 40-80 mg/day or rosuvastatin (Crestor) at 20-40 mg/day – was independently associated with a 21% reduction in the risk of knee or hip replacement surgery for osteoarthritis and a 90% reduction for rheumatoid arthritis, compared with statin nonusers. Notably, joint replacement surgery for osteoarthritis was roughly 25-fold more common than for rheumatoid arthritis.
Statin therapy overall, including the more widely prescribed low- and intermediate-intensity regimens, was associated with a 23% reduction in joint replacement surgery for rheumatoid arthritis, compared with statin nonusers, but had no significant impact on surgery for the osteoarthritis population.
A couple of distinguished American rheumatologists in the audience rose to voice reluctance about drawing broad conclusions from this study.
“Bias, as you’ve said yourself, is a bit of a concern,” said David T. Felson, MD, professor of medicine and public health and director of clinical epidemiology at Boston University.
He was troubled that the study design was such that anyone who filled as few as two statin prescriptions during the more than 6-year study period was categorized as a statin user. That, he said, muddies the waters. Does the database contain information on duration of statin therapy, and whether joint replacement surgery was more likely to occur when patients were on or off statin therapy? he asked.
It does, Dr. Wei replied, adding that she will take that suggestion for additional analysis back to her international team of coinvestigators.
“It seems to me,” said Jeffrey N. Katz, MD, “that the major risk of potential bias is that people who were provided high-intensity statins were prescribed that because they were at risk for or had cardiac disease.”
That high cardiovascular risk might have curbed orthopedic surgeons’ enthusiasm to operate. Thus, it would be helpful to learn whether patients who underwent joint replacement were less likely to have undergone coronary revascularization or other cardiac interventions than were those without joint replacement, according to Dr. Katz, professor of medicine and orthopedic surgery at Harvard Medical School, Boston.
Dr. Wei agreed that confounding by indication is always a possibility in an observational study such as this. Identification of a plausible mechanism by which statins might reduce the risk of joint replacement surgery in rheumatoid arthritis – something that hasn’t happened yet – would help counter such concerns.
She noted that a separate recent analysis of the U.K. Clinical Practice Research Datalink by other investigators concluded that statin therapy started up to 5 years following total hip or knee replacement was associated with a significantly reduced risk of revision arthroplasty. Moreover, the benefit was treatment duration-dependent: Patients on statin therapy for more than 5 years were 26% less likely to undergo revision arthroplasty than were those on a statin for less than 1 year (J Rheumatol. 2019 Mar 15. doi: 10.3899/jrheum.180574).
On the other hand, Swedish investigators found that statin use wasn’t associated with a reduced risk of consultation or surgery for osteoarthritis in a pooled analysis of four cohort studies totaling more than 132,000 Swedes followed for 7.5 years (Osteoarthritis Cartilage. 2017 Nov;25[11]:1804-13).
Dr. Wei reported having no financial conflicts regarding the study, which was supported by the National Clinical Research Center of Geriatric Disorders in Hunan, China, and several British universities.
SOURCE: Sarmanova A et al. Osteoarthritis cartilage. 2019 Apr;27[suppl 1]:S78-S79. Abstract 77.
REPORTING FROM OARSI 2019
Key clinical point: High-intensity statin therapy may reduce need for joint replacement in arthritis.
Major finding: The risk of knee or hip replacement surgery for rheumatoid arthritis was slashed by 90%, and by 21% for osteoarthritis.
Study details: This study included nearly 180,000 statin users propensity score-matched to an equal number of nonusers and prospectively followed for a mean of 6.5 years.
Disclosures: The study was supported by the National Clinical Research Center of Geriatric Disorders at Central South University in Hunan, China, and by several British universities. The presenter reported having no financial conflicts of interest.
Source: Sarmanova A et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S78-S79. Abstract 77.
Give Her a Shoulder to Cry on
ANSWER
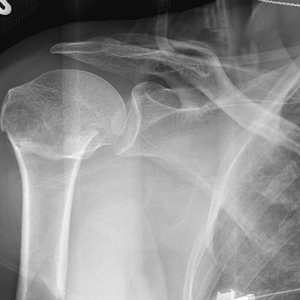
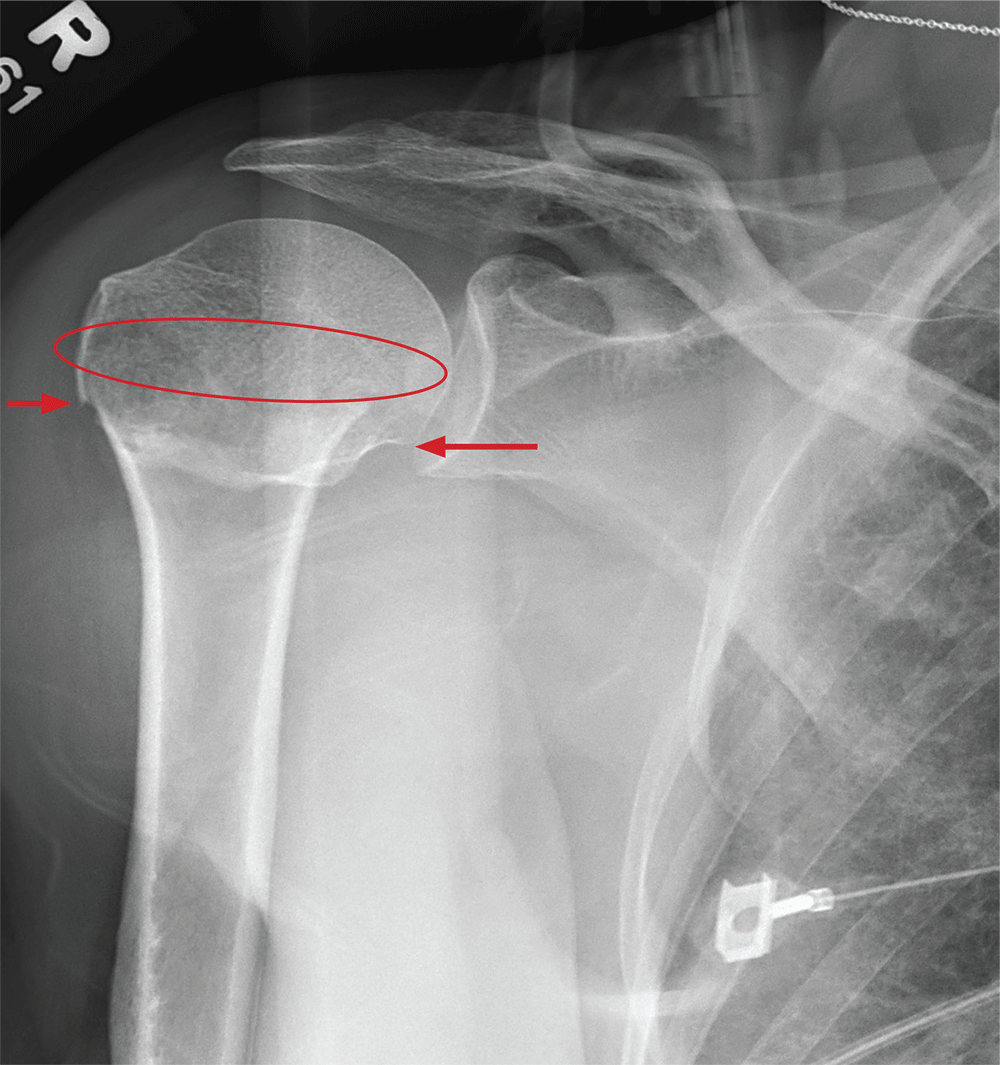
The radiograph demonstrates an acute horizontal fracture through the humeral neck. There is some slight lateral displacement of the fracture fragment.
The patient’s right arm was placed in a sling. Prompt orthopedic consultation was then obtained.

ANSWER
The radiograph demonstrates an acute horizontal fracture through the humeral neck. There is some slight lateral displacement of the fracture fragment.
The patient’s right arm was placed in a sling. Prompt orthopedic consultation was then obtained.

ANSWER
The radiograph demonstrates an acute horizontal fracture through the humeral neck. There is some slight lateral displacement of the fracture fragment.
The patient’s right arm was placed in a sling. Prompt orthopedic consultation was then obtained.
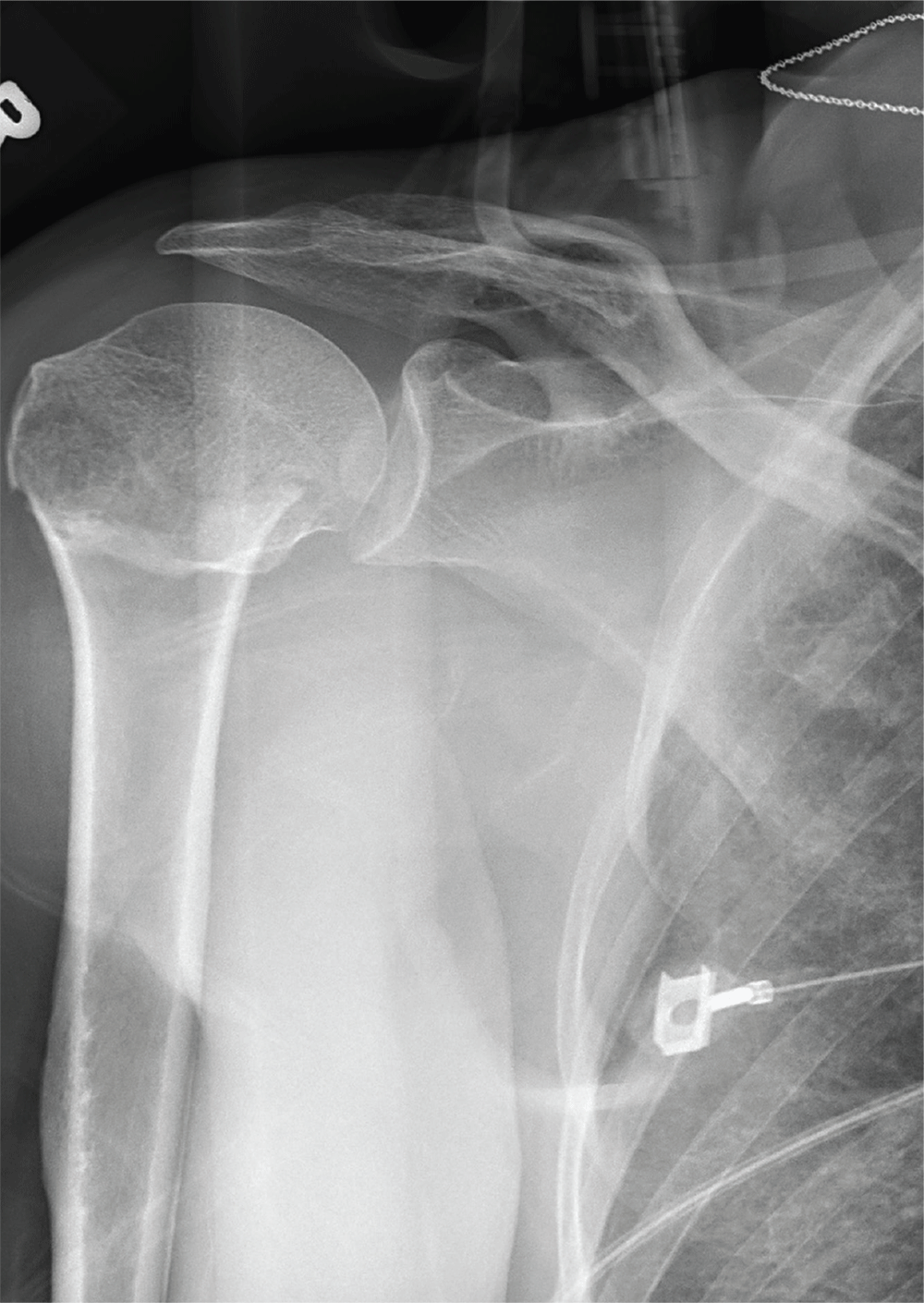
After a motor vehicle collision, a 70-year-old woman is brought to your emergency department by EMS personnel. She was a restrained driver in a vehicle crossing an intersection when she was broadsided by a tractor trailer traveling at high speed. Her airbags deployed, and she believes she briefly lost consciousness. Her biggest complaint is pain in her right shoulder.
Her medical history is significant for hypertension and hypothyroidism. On primary survey, you note an elderly woman who is in full cervical spine immobilization on a long backboard. Her Glasgow Coma Scale score is 15. She is in mild distress but has normal vital signs.
The patient has scattered abrasions and bruises on her body. Her right shoulder has mild to moderate tenderness to palpation and a decreased range of motion. Distally in that arm, she has good pulses and is neurovascularly intact.
You obtain a portable radiograph of the right shoulder (shown). What is your impression?
Good Notes Can Deter Litigation
At 11:15
While in the ED, the patient was examined and treated by a PA. At approximately 12:13
Given the lack of any positive pertinent findings, the PA irrigated the patient’s wounds and applied 1% lidocaine to all affected fingers so that pain would not mask any potential physical exam findings. He also used single-layer absorbable sutures to repair the injured digits. In addition, the PA tested the plaintiff for both distal interphalangeal (DIP) and proximal interphalangeal (PIP) flexion function and recorded normal results.
The PA discharged the patient from the ED at 5:56
The PA provided no further care or treatment to the patient following the visit to the hospital’s ED. However, the patient contended that he suffered an injury to the tendons of his right hand, which ultimately required several surgical procedures. He sued the hospital, the PA, the PA’s medical office, his supervising physician, and the physician who performed the later surgical procedures. The supervising physician and the surgeon were ultimately let out of the case by summary judgment motions. The hospital, which was named as a defendant under a respondeat superior theory, was also dismissed from the case when it was established that the PA was employed by his medical office and not by the hospital directly. The PA stipulated that he was within his course and scope of employment at the time he treated the plaintiff.
Continue to: Plaintiff's counsel contended...
Plaintiff’s counsel contended that the defendant PA was negligent in his examination and evaluation of the plaintiff’s digit lacerations and that he was negligent for failing to splint the plaintiff’s hand. Counsel also contended that the defendant was negligent for failing to refer the plaintiff to a hand surgeon (either directly or through the plaintiff’s primary care provider) and/or for failing to seek the assistance of his supervising physician, who was on site at the hospital’s ED and available for consultation.
Defense counsel argued that the defendant met the applicable standard of care at all times, in all aspects of his visit with the plaintiff in the early morning hours of September 1, 2014, and that there was nothing that he either did or did not do that was a substantial factor in causing the plaintiff’s alleged injuries and damages. The defendant claimed that upon his arrival at the patient’s bedside, the plaintiff verbally indicated to him that he could move his fingers (extension and flexion). He also claimed that he visualized the plaintiff moving his fingers while they were wrapped in the dressing that the plaintiff had placed on himself after the injury-producing event. However, the plaintiff disputed the defendant’s claim, denying ever being asked to extend and flex his fingers. The plaintiff also claimed that he never was able to make a full fist with his fingers on the night in question while in the ED, either by way of passive or active flexion.
Defense counsel noted that the defendant’s dictated ED note stated that the range of motion of all the plaintiff’s phalanges were normal, with no deficits, at all times while in the ED. The defendant testified about how he tested and evaluated the plaintiff’s DIP function. He also testified that he had the plaintiff lay his hand on the table, palm side up, and then laid his own hand across the plaintiff’s hand so as to isolate the DIP joint on each finger. He explained that he then had the plaintiff flex his fingers, which allowed him to determine whether there had been any kind of injury to the flexor digitorum profundus tendon (responsible for DIP function in the hand). The defendant claimed that he did the test for all the lacerated fingers and characterized them as active (as opposed to passive) flexion. Thus, he claimed that his physical exam findings were that the plaintiff had full range of motion (ROM) intact following the DIP function testing, which helped him conclude that the plaintiff did not have completely lacerated tendons as of that visit.
The defendant further explained that if the tendons were completely lacerated, the plaintiff would have had nonexistent DIP functioning on examination. The defendant testified that if he suspected a tendon laceration in a patient such as the plaintiff, his practice would be to notify his supervising physician in the ED and then either refer the patient to a primary care provider for an orthopedic hand surgeon referral or directly refer the patient to an orthopedic hand surgeon. He claimed that he took no such actions because there was no indication, from his perspective, that the plaintiff had suffered any tendon damage based on his physical exam findings, the plaintiff’s ability to make a fist, and the x-ray results.
Continue to: VERDICT
VERDICT
After a 5-day trial and 7 hours of deliberation, the jury found in favor of the defendants.
COMMENTARY
As human beings, we do a lot with our hands. They are vulnerable to injury, and misdiagnosis may result in life-altering debility. The impact is even greater when one’s livelihood requires fine dexterity. Thus, tendon lacerations are relatively common and must be managed properly.
In this case, we are told that the PA documented in his notes that the plaintiff had range of motion in all phalanges and no deficits. We are also told the defendant testified regarding his procedure for hand examination. But we are not told that his note included the details of his exam—and by inference, we have reason to suspect it did not.
You might think, “The jury found in favor of the defense, so why does this matter?” Because a well-documented chart may prevent liability.
If you wish to avoid lawsuits, it is helpful to understand how they originate: An aggrieved patient contacts a plaintiff’s lawyer, insists he or she has been wronged, and asks the lawyer to take the case. Often faced with the ticking clock of statute of limitations (the absolute deadline to file), plaintiff’s counsel will review whatever records are available (which may not be all of them), looking for perceived deficiencies of care. The case may also be reviewed by a medical professional (generally a physician) prior to filing; some states require an affidavit of merit—an attestation that there is just cause to bring the action.
Whether reviewed only by plaintiff’s counsel or with the aid of an expert, a well-documented medical record may prevent a case from being filed. Medical malpractice cases are a huge gamble for plaintiff firms: They are expensive, time consuming, difficult to litigate, document heavy, and technically complex—falling outside the experience of most lawyers. They are also less likely than other cases to be settled, thanks to National Practitioner Data Bank recording requirements and (in several states) automatic medical board inquiry for potential adverse action against a medical or nursing professional following settlement. Clinicians will often fight tooth and nail to avoid an adverse recording, hospital credentialing woes, and state investigation. A medical malpractice case can be a trap for both the clinician and the plaintiff’s attorney stuck with a bad case.
Continue to: In the early stages...
In the early stages of potential litigation, before a case is filed in court, do yourself a favor: Help plaintiff’s counsel realize it will be a losing case. You actually start the process much earlier, by conducting the proper exam and documenting lavishly. This is particularly important with specialty exams, such as the hand exam in this case.
Here, simply noting “positive ROM and distal CSM [circulation, sensation, and motion] intact” is inadequate. Why? Because it is a conclusion, not evidence of the specialty examination that was diligently performed. The mechanism of injury and initial presentation roused the clinician’s suspicions sufficiently to conduct a thorough hand examination—but the mechanics of the exam were not included, only conclusions. The trouble is, those conclusions may have been based on sound medical evidence or they may have been hastily and improvidently drawn. A plaintiff’s firm deciding whether to take this case doesn’t know but will bet on the latter.
The clinician testified he performed a detailed and thorough examination of the plaintiff’s hand. Had plaintiff’s counsel been confronted with the full details of the exam—which showed the defendant PA tested all the PIPs and DIPs by isolating each finger—early on, this case may never have been filed. Thus, conduct and document specialty exams fully. If you need a cheat sheet for exams you don’t do often, use one—that is still solid practice. If you don’t do many pelvic exams or mental status exams, make sure you aren’t missing anything. Practicing medicine is an open-book exam; if you need materials, use them.
Good documentation leads to good defense, and any good defense lawyer will recommend the Jerry Maguire rule: “Help me help you.” Solid records make a case easier to defend and win at all phases of litigation. Of course, this is not a universal cure that will prevent all lawsuits. But even if the case is filed, the strength of your records may have convinced stronger, more capable medical malpractice firms to turn it down. This is something of value: It is “you helping you” and potent proof that your human head weighs more than 8 lb.
IN SUMMARY
A well-documented chart may prevent liability by showcasing the strength of your care and preventing no-win lawsuits from being filed. Help the plaintiff’s attorney realize, early on, that he or she is facing a costly uphill battle. The key word is early, when the medical records are first reviewed—not 18 months later, when the attorney hears your testimony at deposition and realizes that he or she has invested time and sweat in a case only to learn that your care was fabulous. Showcase that fabulous care early and short circuit the whole process by detailing the substance of a key exam (not just conclusions) in the record. Detailed notes may spare you from a visit by a sheriff you don’t know holding papers you don’t want.
At 11:15
While in the ED, the patient was examined and treated by a PA. At approximately 12:13
Given the lack of any positive pertinent findings, the PA irrigated the patient’s wounds and applied 1% lidocaine to all affected fingers so that pain would not mask any potential physical exam findings. He also used single-layer absorbable sutures to repair the injured digits. In addition, the PA tested the plaintiff for both distal interphalangeal (DIP) and proximal interphalangeal (PIP) flexion function and recorded normal results.
The PA discharged the patient from the ED at 5:56
The PA provided no further care or treatment to the patient following the visit to the hospital’s ED. However, the patient contended that he suffered an injury to the tendons of his right hand, which ultimately required several surgical procedures. He sued the hospital, the PA, the PA’s medical office, his supervising physician, and the physician who performed the later surgical procedures. The supervising physician and the surgeon were ultimately let out of the case by summary judgment motions. The hospital, which was named as a defendant under a respondeat superior theory, was also dismissed from the case when it was established that the PA was employed by his medical office and not by the hospital directly. The PA stipulated that he was within his course and scope of employment at the time he treated the plaintiff.
Continue to: Plaintiff's counsel contended...
Plaintiff’s counsel contended that the defendant PA was negligent in his examination and evaluation of the plaintiff’s digit lacerations and that he was negligent for failing to splint the plaintiff’s hand. Counsel also contended that the defendant was negligent for failing to refer the plaintiff to a hand surgeon (either directly or through the plaintiff’s primary care provider) and/or for failing to seek the assistance of his supervising physician, who was on site at the hospital’s ED and available for consultation.
Defense counsel argued that the defendant met the applicable standard of care at all times, in all aspects of his visit with the plaintiff in the early morning hours of September 1, 2014, and that there was nothing that he either did or did not do that was a substantial factor in causing the plaintiff’s alleged injuries and damages. The defendant claimed that upon his arrival at the patient’s bedside, the plaintiff verbally indicated to him that he could move his fingers (extension and flexion). He also claimed that he visualized the plaintiff moving his fingers while they were wrapped in the dressing that the plaintiff had placed on himself after the injury-producing event. However, the plaintiff disputed the defendant’s claim, denying ever being asked to extend and flex his fingers. The plaintiff also claimed that he never was able to make a full fist with his fingers on the night in question while in the ED, either by way of passive or active flexion.
Defense counsel noted that the defendant’s dictated ED note stated that the range of motion of all the plaintiff’s phalanges were normal, with no deficits, at all times while in the ED. The defendant testified about how he tested and evaluated the plaintiff’s DIP function. He also testified that he had the plaintiff lay his hand on the table, palm side up, and then laid his own hand across the plaintiff’s hand so as to isolate the DIP joint on each finger. He explained that he then had the plaintiff flex his fingers, which allowed him to determine whether there had been any kind of injury to the flexor digitorum profundus tendon (responsible for DIP function in the hand). The defendant claimed that he did the test for all the lacerated fingers and characterized them as active (as opposed to passive) flexion. Thus, he claimed that his physical exam findings were that the plaintiff had full range of motion (ROM) intact following the DIP function testing, which helped him conclude that the plaintiff did not have completely lacerated tendons as of that visit.
The defendant further explained that if the tendons were completely lacerated, the plaintiff would have had nonexistent DIP functioning on examination. The defendant testified that if he suspected a tendon laceration in a patient such as the plaintiff, his practice would be to notify his supervising physician in the ED and then either refer the patient to a primary care provider for an orthopedic hand surgeon referral or directly refer the patient to an orthopedic hand surgeon. He claimed that he took no such actions because there was no indication, from his perspective, that the plaintiff had suffered any tendon damage based on his physical exam findings, the plaintiff’s ability to make a fist, and the x-ray results.
Continue to: VERDICT
VERDICT
After a 5-day trial and 7 hours of deliberation, the jury found in favor of the defendants.
COMMENTARY
As human beings, we do a lot with our hands. They are vulnerable to injury, and misdiagnosis may result in life-altering debility. The impact is even greater when one’s livelihood requires fine dexterity. Thus, tendon lacerations are relatively common and must be managed properly.
In this case, we are told that the PA documented in his notes that the plaintiff had range of motion in all phalanges and no deficits. We are also told the defendant testified regarding his procedure for hand examination. But we are not told that his note included the details of his exam—and by inference, we have reason to suspect it did not.
You might think, “The jury found in favor of the defense, so why does this matter?” Because a well-documented chart may prevent liability.
If you wish to avoid lawsuits, it is helpful to understand how they originate: An aggrieved patient contacts a plaintiff’s lawyer, insists he or she has been wronged, and asks the lawyer to take the case. Often faced with the ticking clock of statute of limitations (the absolute deadline to file), plaintiff’s counsel will review whatever records are available (which may not be all of them), looking for perceived deficiencies of care. The case may also be reviewed by a medical professional (generally a physician) prior to filing; some states require an affidavit of merit—an attestation that there is just cause to bring the action.
Whether reviewed only by plaintiff’s counsel or with the aid of an expert, a well-documented medical record may prevent a case from being filed. Medical malpractice cases are a huge gamble for plaintiff firms: They are expensive, time consuming, difficult to litigate, document heavy, and technically complex—falling outside the experience of most lawyers. They are also less likely than other cases to be settled, thanks to National Practitioner Data Bank recording requirements and (in several states) automatic medical board inquiry for potential adverse action against a medical or nursing professional following settlement. Clinicians will often fight tooth and nail to avoid an adverse recording, hospital credentialing woes, and state investigation. A medical malpractice case can be a trap for both the clinician and the plaintiff’s attorney stuck with a bad case.
Continue to: In the early stages...
In the early stages of potential litigation, before a case is filed in court, do yourself a favor: Help plaintiff’s counsel realize it will be a losing case. You actually start the process much earlier, by conducting the proper exam and documenting lavishly. This is particularly important with specialty exams, such as the hand exam in this case.
Here, simply noting “positive ROM and distal CSM [circulation, sensation, and motion] intact” is inadequate. Why? Because it is a conclusion, not evidence of the specialty examination that was diligently performed. The mechanism of injury and initial presentation roused the clinician’s suspicions sufficiently to conduct a thorough hand examination—but the mechanics of the exam were not included, only conclusions. The trouble is, those conclusions may have been based on sound medical evidence or they may have been hastily and improvidently drawn. A plaintiff’s firm deciding whether to take this case doesn’t know but will bet on the latter.
The clinician testified he performed a detailed and thorough examination of the plaintiff’s hand. Had plaintiff’s counsel been confronted with the full details of the exam—which showed the defendant PA tested all the PIPs and DIPs by isolating each finger—early on, this case may never have been filed. Thus, conduct and document specialty exams fully. If you need a cheat sheet for exams you don’t do often, use one—that is still solid practice. If you don’t do many pelvic exams or mental status exams, make sure you aren’t missing anything. Practicing medicine is an open-book exam; if you need materials, use them.
Good documentation leads to good defense, and any good defense lawyer will recommend the Jerry Maguire rule: “Help me help you.” Solid records make a case easier to defend and win at all phases of litigation. Of course, this is not a universal cure that will prevent all lawsuits. But even if the case is filed, the strength of your records may have convinced stronger, more capable medical malpractice firms to turn it down. This is something of value: It is “you helping you” and potent proof that your human head weighs more than 8 lb.
IN SUMMARY
A well-documented chart may prevent liability by showcasing the strength of your care and preventing no-win lawsuits from being filed. Help the plaintiff’s attorney realize, early on, that he or she is facing a costly uphill battle. The key word is early, when the medical records are first reviewed—not 18 months later, when the attorney hears your testimony at deposition and realizes that he or she has invested time and sweat in a case only to learn that your care was fabulous. Showcase that fabulous care early and short circuit the whole process by detailing the substance of a key exam (not just conclusions) in the record. Detailed notes may spare you from a visit by a sheriff you don’t know holding papers you don’t want.
At 11:15
While in the ED, the patient was examined and treated by a PA. At approximately 12:13
Given the lack of any positive pertinent findings, the PA irrigated the patient’s wounds and applied 1% lidocaine to all affected fingers so that pain would not mask any potential physical exam findings. He also used single-layer absorbable sutures to repair the injured digits. In addition, the PA tested the plaintiff for both distal interphalangeal (DIP) and proximal interphalangeal (PIP) flexion function and recorded normal results.
The PA discharged the patient from the ED at 5:56
The PA provided no further care or treatment to the patient following the visit to the hospital’s ED. However, the patient contended that he suffered an injury to the tendons of his right hand, which ultimately required several surgical procedures. He sued the hospital, the PA, the PA’s medical office, his supervising physician, and the physician who performed the later surgical procedures. The supervising physician and the surgeon were ultimately let out of the case by summary judgment motions. The hospital, which was named as a defendant under a respondeat superior theory, was also dismissed from the case when it was established that the PA was employed by his medical office and not by the hospital directly. The PA stipulated that he was within his course and scope of employment at the time he treated the plaintiff.
Continue to: Plaintiff's counsel contended...
Plaintiff’s counsel contended that the defendant PA was negligent in his examination and evaluation of the plaintiff’s digit lacerations and that he was negligent for failing to splint the plaintiff’s hand. Counsel also contended that the defendant was negligent for failing to refer the plaintiff to a hand surgeon (either directly or through the plaintiff’s primary care provider) and/or for failing to seek the assistance of his supervising physician, who was on site at the hospital’s ED and available for consultation.
Defense counsel argued that the defendant met the applicable standard of care at all times, in all aspects of his visit with the plaintiff in the early morning hours of September 1, 2014, and that there was nothing that he either did or did not do that was a substantial factor in causing the plaintiff’s alleged injuries and damages. The defendant claimed that upon his arrival at the patient’s bedside, the plaintiff verbally indicated to him that he could move his fingers (extension and flexion). He also claimed that he visualized the plaintiff moving his fingers while they were wrapped in the dressing that the plaintiff had placed on himself after the injury-producing event. However, the plaintiff disputed the defendant’s claim, denying ever being asked to extend and flex his fingers. The plaintiff also claimed that he never was able to make a full fist with his fingers on the night in question while in the ED, either by way of passive or active flexion.
Defense counsel noted that the defendant’s dictated ED note stated that the range of motion of all the plaintiff’s phalanges were normal, with no deficits, at all times while in the ED. The defendant testified about how he tested and evaluated the plaintiff’s DIP function. He also testified that he had the plaintiff lay his hand on the table, palm side up, and then laid his own hand across the plaintiff’s hand so as to isolate the DIP joint on each finger. He explained that he then had the plaintiff flex his fingers, which allowed him to determine whether there had been any kind of injury to the flexor digitorum profundus tendon (responsible for DIP function in the hand). The defendant claimed that he did the test for all the lacerated fingers and characterized them as active (as opposed to passive) flexion. Thus, he claimed that his physical exam findings were that the plaintiff had full range of motion (ROM) intact following the DIP function testing, which helped him conclude that the plaintiff did not have completely lacerated tendons as of that visit.
The defendant further explained that if the tendons were completely lacerated, the plaintiff would have had nonexistent DIP functioning on examination. The defendant testified that if he suspected a tendon laceration in a patient such as the plaintiff, his practice would be to notify his supervising physician in the ED and then either refer the patient to a primary care provider for an orthopedic hand surgeon referral or directly refer the patient to an orthopedic hand surgeon. He claimed that he took no such actions because there was no indication, from his perspective, that the plaintiff had suffered any tendon damage based on his physical exam findings, the plaintiff’s ability to make a fist, and the x-ray results.
Continue to: VERDICT
VERDICT
After a 5-day trial and 7 hours of deliberation, the jury found in favor of the defendants.
COMMENTARY
As human beings, we do a lot with our hands. They are vulnerable to injury, and misdiagnosis may result in life-altering debility. The impact is even greater when one’s livelihood requires fine dexterity. Thus, tendon lacerations are relatively common and must be managed properly.
In this case, we are told that the PA documented in his notes that the plaintiff had range of motion in all phalanges and no deficits. We are also told the defendant testified regarding his procedure for hand examination. But we are not told that his note included the details of his exam—and by inference, we have reason to suspect it did not.
You might think, “The jury found in favor of the defense, so why does this matter?” Because a well-documented chart may prevent liability.
If you wish to avoid lawsuits, it is helpful to understand how they originate: An aggrieved patient contacts a plaintiff’s lawyer, insists he or she has been wronged, and asks the lawyer to take the case. Often faced with the ticking clock of statute of limitations (the absolute deadline to file), plaintiff’s counsel will review whatever records are available (which may not be all of them), looking for perceived deficiencies of care. The case may also be reviewed by a medical professional (generally a physician) prior to filing; some states require an affidavit of merit—an attestation that there is just cause to bring the action.
Whether reviewed only by plaintiff’s counsel or with the aid of an expert, a well-documented medical record may prevent a case from being filed. Medical malpractice cases are a huge gamble for plaintiff firms: They are expensive, time consuming, difficult to litigate, document heavy, and technically complex—falling outside the experience of most lawyers. They are also less likely than other cases to be settled, thanks to National Practitioner Data Bank recording requirements and (in several states) automatic medical board inquiry for potential adverse action against a medical or nursing professional following settlement. Clinicians will often fight tooth and nail to avoid an adverse recording, hospital credentialing woes, and state investigation. A medical malpractice case can be a trap for both the clinician and the plaintiff’s attorney stuck with a bad case.
Continue to: In the early stages...
In the early stages of potential litigation, before a case is filed in court, do yourself a favor: Help plaintiff’s counsel realize it will be a losing case. You actually start the process much earlier, by conducting the proper exam and documenting lavishly. This is particularly important with specialty exams, such as the hand exam in this case.
Here, simply noting “positive ROM and distal CSM [circulation, sensation, and motion] intact” is inadequate. Why? Because it is a conclusion, not evidence of the specialty examination that was diligently performed. The mechanism of injury and initial presentation roused the clinician’s suspicions sufficiently to conduct a thorough hand examination—but the mechanics of the exam were not included, only conclusions. The trouble is, those conclusions may have been based on sound medical evidence or they may have been hastily and improvidently drawn. A plaintiff’s firm deciding whether to take this case doesn’t know but will bet on the latter.
The clinician testified he performed a detailed and thorough examination of the plaintiff’s hand. Had plaintiff’s counsel been confronted with the full details of the exam—which showed the defendant PA tested all the PIPs and DIPs by isolating each finger—early on, this case may never have been filed. Thus, conduct and document specialty exams fully. If you need a cheat sheet for exams you don’t do often, use one—that is still solid practice. If you don’t do many pelvic exams or mental status exams, make sure you aren’t missing anything. Practicing medicine is an open-book exam; if you need materials, use them.
Good documentation leads to good defense, and any good defense lawyer will recommend the Jerry Maguire rule: “Help me help you.” Solid records make a case easier to defend and win at all phases of litigation. Of course, this is not a universal cure that will prevent all lawsuits. But even if the case is filed, the strength of your records may have convinced stronger, more capable medical malpractice firms to turn it down. This is something of value: It is “you helping you” and potent proof that your human head weighs more than 8 lb.
IN SUMMARY
A well-documented chart may prevent liability by showcasing the strength of your care and preventing no-win lawsuits from being filed. Help the plaintiff’s attorney realize, early on, that he or she is facing a costly uphill battle. The key word is early, when the medical records are first reviewed—not 18 months later, when the attorney hears your testimony at deposition and realizes that he or she has invested time and sweat in a case only to learn that your care was fabulous. Showcase that fabulous care early and short circuit the whole process by detailing the substance of a key exam (not just conclusions) in the record. Detailed notes may spare you from a visit by a sheriff you don’t know holding papers you don’t want.
A Robotic Hand Device Safety Study for People With Cervical Spinal Cord Injury (FULL)
An estimated 282,000 people in the US are living with spinal cord injury (SCI).1 Damage to the cervical spinal cord is the most prevalent. Among cervical spinal cord trauma, injury to levels C4, C5, and C6 have the highest occurrence.1 Damage to these levels has significant implications for functional status. Depending on pathology, patients’ functional status can range from requiring assistance for all activities of daily living (ADL) to potentially living independently.
Improving upper-limb function is vital to achieving independence. About half of people with tetraplegia judge hand and arm function to be the top factor that would improve quality of life (QOL).2 Persons with traumatic cervical SCI may lose the ability to use their hands from motor deficits, sensory dysfunction, proprioception problem, and/or loss of coordination. In addition, they may develop joint contracture, spasticity, pain, and other complications. Thus, their independence and ADL are affected significantly by multiple mechanisms of pathology.
Upper-extremity rehabilitation that emphasizes strengthening and maintaining functional range of motion (ROM) is fundamental to SCI rehabilitation. Rehabilitation to restore partial hand function has included ROM exercises, splinting, surgical procedures in the form of tendon transfers and various electrical stimulation devices, such as implantable neuroprostheses.2-7 These interventions improve the ability to grasp, hold, and release objects in selected individuals; however, they have not been universally accepted. Traditional modalities, such as active ROM (AROM) and passive ROM (PROM) and electrical stimulation remain highly used in upper-extremity rehabilitation. Devices have been developed to provide either PROM or electrical stimulation to improve hand function and to prevent muscle atrophy. Therapist- and caregiver-directed PROM exercises are time consuming and labor intensive. An innovative therapeutic approach that can provide all these modalities more efficiently is needed in SCI rehabilitation.
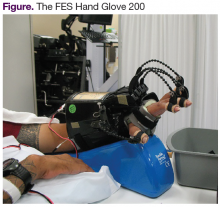
Until now, a single device that combines AROM and PROM simultaneously has not been available. A robotic system, the FES Hand Glove 200 (Robotix Hand Therapy Inc, Colorado Springs, CO), was developed to improve hand function (Figure).
Methods
This prospective safety study evaluated the occurrence of adverse effects (AEs) associated with the use of the FES Hand Glove 200. The study was performed in the Occupational Therapy Section of the Spinal Cord Injury Center at the James A. Haley Veterans’ Hospital (JAHVH) and approved by the JAHVH Research and Development Committee as well as the University of South Florida Investigational Review Board. For recruitment, the goals of the study as well as the inclusion and exclusion criteria were presented to the Spinal Cord Injury Center health care providers. Potential candidates of the study were referred to the study team from these providers.
Screening of the referred candidates was conducted by physicians during inpatient evaluations. All subjects signed a consent form. Participants included active-duty military or veterans with traumatic SCI at levels C4 to C8 and American Spinal Injury Association Impairment Scale (AIS) grades A, B, C, and D. Participants were aged 18 to 60 years, at least 1-month post-SCI, medically stable, and had impairments in upper-extremities strength and ROM or function, including hand.
Subjects were excluded if any of the following were present: seizure within 3 months of study; active cancer; heterotopic ossification below the shoulder; new acute hand injuries of the study limb; unhealed fractures of the study limb; myocardial infarction within 12 months; severe cognitive impairment determined by a Modified Rancho Score below VI8; severe aphasia; pregnancy; skin irritations or open wounds in the study limb; fixed contractures of > 40° of the metacarpophalangeal (MP) or proximal interphalangeal (PIP) joints of the study hand; unwillingness to perform all of the therapies and assessments required for the study; active implant device (eg, pacemaker, implanted cardiac defibrillator, neurostimulator or drug infusion device); major psychological disorder; severe residual spasticity despite maximal medical therapy; muscle power grade of more than 3+ on wrist and finger extensors and flexors of the study limb; recent or current participation in research that could influence study response; pain that prevents participation in the study; or concurrent use of transcutaneous electrical stimulation on the study arm.
The following data were documented: level of SCI, AIS-score; complete medical history; physical examination (including skin integrity); and vital signs of bilateral upper extremities. A nurse practitioner (NP) certified in Functional Independent Measure (FIM) conducted chart reviews and/or in-person interviews of each subject to establish a FIM score before and after 6 weeks of research treatment. Two experienced occupational therapists (OTs) conducted detailed hand evaluations before the research treatment interventions. An OT provided subjects with education on the use, care, and precautions of the FES Hand Glove 200. The OT adjusted the device on the subject’s hand for proper fitting, including initial available PROM, and optimal muscle stimulation.
The OT then implemented the treatment protocol using the FES Hand Glove 200 in 1 hand per the subjects’ preference. The subjects received 30 minutes of PROM only on the FES Hand Glove 200, followed by an additional 30 minutes of PROM with FES for 1 hour of therapy per session. The study participants were treated 4 times per week for 6 weeks. Before and after each session, OTs evaluated and documented any loss of skin integrity and pain. Autonomic dysreflexia occurred when systolic BP increased > 20 to 30 mm Hg with symptoms such as headache, profuse sweating, or blurred vision was reported.9 The FES Hand Glove 200 was set up for PROM to the thumb and to digits 2 to 5 and for electrical muscle stimulation of the finger extensors and flexors. No other therapeutic exercise was performed during the study period on the other extremity. Primary and secondary outcomes were collected at the end of the 6-week intervention.
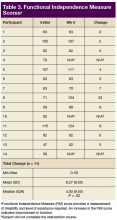
Primary outcomes included complications from the use of FES Hand Glove 200, including skin integrity and any joint deformity as drawn on a figure, changes of pain level by visual analog scale (VAS), and total number of autonomic dyreflexia episodes. Secondary measured outcomes included changes in PROM and AROM of wrist, metacarpal joint and interphalangeal joints of thumbs and digits 2 to 5 ≥ 10°; hand and pinch strength decline of > 1 lb; decline in manual muscle test, and FIM score, which is a validated measurement of disability and the level of assistance required for ADL.10
Statistical analyses were performed using SAS version 4 (Cary, NC) to assess the degree of change in the improvement score, which was defined as the postintervention score minus the preintervention score. However, because of the large standard error due to small sample sizes, the normality assumption was not satisfied for all the outcomes considered.
Results
Of the 20 participants screened, 14 men aged between 19 and 66 years with cervical SCI level of C4 to C6 AIS grades A to D were enrolled in the study. Three did not complete the 6-week trial due to SCI-related medical complications, which were unrelated to the use of the FES Hand Glove 200. They continued with regular OT treatment or self-directed home exercises after they were seen by the treating physician. (Table 1)
Skin integrity of all subjects was maintained throughout the study. One subject had a right-elbow wound before the intervention, which was unchanged at the end of the study. After 6 weeks of experimental intervention, there was no wrist or finger joint deformity noted and no increase in pain level except for 1 subject who reported increased pain that was unrelated to use of the device. No occurrence of autonomic dysreflexia was recorded during the use of FES Hand Glove 200 (Table 2).
For the secondary outcomes, there was no significant decrease in AROM or PROM ≥ 10° in forearm, wrist, or finger joints in any participants. There was no loss of strength > 1 lb as measured by gross grasp, pinch tip, 3-point, or lateral grip. There was no decline in motor strength per manual muscle testing. No worsening of FIM score was noted (Table 3).
Although this was not an efficacy study primarily, participants improved in several areas. Improvements included active and passive movements in the forearm, wrist, and hand. There also was significant improvement in strength of the extensor digitorum communis (EDC) muscle. Data are available on request to the authors.
Discussion
Passive ROM and AROM exercises and FES are common strategies to improve certain hand functions in people with cervical SCI. Many people, however, may experience limited duration or efficiency of rehabilitation secondary to lack of resources. Technologic advancement allowed the combination of PROM exercise and FES using the FES Hand Glove 200 device. The eventual goal of using this device is to enhance QOL by improving upper-extremity function. Because this device is not commercially available, its safety and tolerability are being tested prior to clinical use. Although 3 subjects withdrew from the study due to nondevice-related medical reasons, 11 subjects completed the study. Potential AEs included skin wounds, burns, tendon sprain or rupture, edema, and pain. At the end of the 6-week study period, there was no loss of skin integrity, no joint deformity, and no increase in hand or finger edema in all subjects. Increase in pain level at 6 weeks was noted in only 1 subject.
One concern was that overuse of such devices could potentially cause muscle fatigue, leading to decreased strength. Pinch grasp and manual muscle testing were evaluated, and no decrease in any of these parameters was noted at the end of study. Although this was not an efficacy study, there was some evidence of improved ROM of multiple wrist and finger joints as well as the EDC muscle strength.
Limitations
Limitations of the study included the duration of treatment of eight 30-minute sessions per week over a 6-week period. A longer treatment duration could result in repetition-related injuries and should be tested in future trials. Finally, the sample size of this study was relatively small. Future studies of different treatment frequency, longer duration of use and monitoring, and using a larger sample size are suggested. An efficacy study of this device using a randomized controlled design is indicated. As people with cervical SCI rank upper-extremity dysfunction as one of the top impairments that negatively impacts QOL, rehabilitation strategy to improve such functions should continue to be a research priority.2
Conclusion
This study supports the safety and tolerability of a 6-week course using FES Hand Glove 200 in traumatic SCI tetraplegic subjects. Additionally, data from this study suggest possible efficacy in enhancing ROM of various wrist and finger joints as well as certain muscle group. Further studies of efficacy with larger numbers of subjects are warranted.
Click here to read the digital edition.
1. NSCISC National Spinal Cord Injury Statistic Center. 2016 annual report—public version. https://www.nscisc.uab.edu/public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf. Published 2016. Accessed March 19, 2018.
2. Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med. 2005;37(1):32-36.
3. O’Driscoll SW, Giori NJ. Continuous passive motion (CPM): theory and principles of clinical application. J Rehabil Res Dev. 2000;37(2):179-188.
4. Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: a pilot study. Neurorehabil Neural Repair. 2007;21(3):207-215.
5. de Kroon JR, Ijzerman MJ, Lankhorst GJ, Zilvold G. Electrical stimulation of the upper limb in stroke stimulation of the extensors of the hand vs. alternate stimulation of flexors and extensors. Am J Phys Med Rehabil. 2004;83(8):592-600.
6. Alon G, McBride K, Levitt AF. Feasibility of randomised clinical trial of early initiation and prolonged, home-base FES training to enhance upper limb functional recovery following stroke. https://www.researchgate.net /publication/237724608_Feasibility_of_randomised_clinical_trial_of_early _initiation_and_prolonged_home-based_FES_training_to_enhance_upper_limb _functional_recovery_following_stroke. Published 2004. Accessed March 21, 2018.
7. Alon G, McBride K. Persons with C5-C6 tetraplegia achieve selected functional gains using a neuroprosthesis. Arch Phys Med Rehabil. 2003;84(1):119-124.
8. Hagen C, Malkmus D, Durham P. Rancho Los Amigos Cognitive Scale. http://file .lacounty.gov/SDSInter/dhs/218118_RLOCFProfessionalReferenceCard-English .pdf. Published 1979. Accessed March 19, 2018.
9. Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81(4):506-516.
10. Grey N, Kennedy P. The Functional Independence Measure: a comparative study of clinician and self rating. Paraplegia. 1993;31(7):457-461.
An estimated 282,000 people in the US are living with spinal cord injury (SCI).1 Damage to the cervical spinal cord is the most prevalent. Among cervical spinal cord trauma, injury to levels C4, C5, and C6 have the highest occurrence.1 Damage to these levels has significant implications for functional status. Depending on pathology, patients’ functional status can range from requiring assistance for all activities of daily living (ADL) to potentially living independently.
Improving upper-limb function is vital to achieving independence. About half of people with tetraplegia judge hand and arm function to be the top factor that would improve quality of life (QOL).2 Persons with traumatic cervical SCI may lose the ability to use their hands from motor deficits, sensory dysfunction, proprioception problem, and/or loss of coordination. In addition, they may develop joint contracture, spasticity, pain, and other complications. Thus, their independence and ADL are affected significantly by multiple mechanisms of pathology.
Upper-extremity rehabilitation that emphasizes strengthening and maintaining functional range of motion (ROM) is fundamental to SCI rehabilitation. Rehabilitation to restore partial hand function has included ROM exercises, splinting, surgical procedures in the form of tendon transfers and various electrical stimulation devices, such as implantable neuroprostheses.2-7 These interventions improve the ability to grasp, hold, and release objects in selected individuals; however, they have not been universally accepted. Traditional modalities, such as active ROM (AROM) and passive ROM (PROM) and electrical stimulation remain highly used in upper-extremity rehabilitation. Devices have been developed to provide either PROM or electrical stimulation to improve hand function and to prevent muscle atrophy. Therapist- and caregiver-directed PROM exercises are time consuming and labor intensive. An innovative therapeutic approach that can provide all these modalities more efficiently is needed in SCI rehabilitation.
Until now, a single device that combines AROM and PROM simultaneously has not been available. A robotic system, the FES Hand Glove 200 (Robotix Hand Therapy Inc, Colorado Springs, CO), was developed to improve hand function (Figure).
Methods
This prospective safety study evaluated the occurrence of adverse effects (AEs) associated with the use of the FES Hand Glove 200. The study was performed in the Occupational Therapy Section of the Spinal Cord Injury Center at the James A. Haley Veterans’ Hospital (JAHVH) and approved by the JAHVH Research and Development Committee as well as the University of South Florida Investigational Review Board. For recruitment, the goals of the study as well as the inclusion and exclusion criteria were presented to the Spinal Cord Injury Center health care providers. Potential candidates of the study were referred to the study team from these providers.
Screening of the referred candidates was conducted by physicians during inpatient evaluations. All subjects signed a consent form. Participants included active-duty military or veterans with traumatic SCI at levels C4 to C8 and American Spinal Injury Association Impairment Scale (AIS) grades A, B, C, and D. Participants were aged 18 to 60 years, at least 1-month post-SCI, medically stable, and had impairments in upper-extremities strength and ROM or function, including hand.
Subjects were excluded if any of the following were present: seizure within 3 months of study; active cancer; heterotopic ossification below the shoulder; new acute hand injuries of the study limb; unhealed fractures of the study limb; myocardial infarction within 12 months; severe cognitive impairment determined by a Modified Rancho Score below VI8; severe aphasia; pregnancy; skin irritations or open wounds in the study limb; fixed contractures of > 40° of the metacarpophalangeal (MP) or proximal interphalangeal (PIP) joints of the study hand; unwillingness to perform all of the therapies and assessments required for the study; active implant device (eg, pacemaker, implanted cardiac defibrillator, neurostimulator or drug infusion device); major psychological disorder; severe residual spasticity despite maximal medical therapy; muscle power grade of more than 3+ on wrist and finger extensors and flexors of the study limb; recent or current participation in research that could influence study response; pain that prevents participation in the study; or concurrent use of transcutaneous electrical stimulation on the study arm.
The following data were documented: level of SCI, AIS-score; complete medical history; physical examination (including skin integrity); and vital signs of bilateral upper extremities. A nurse practitioner (NP) certified in Functional Independent Measure (FIM) conducted chart reviews and/or in-person interviews of each subject to establish a FIM score before and after 6 weeks of research treatment. Two experienced occupational therapists (OTs) conducted detailed hand evaluations before the research treatment interventions. An OT provided subjects with education on the use, care, and precautions of the FES Hand Glove 200. The OT adjusted the device on the subject’s hand for proper fitting, including initial available PROM, and optimal muscle stimulation.
The OT then implemented the treatment protocol using the FES Hand Glove 200 in 1 hand per the subjects’ preference. The subjects received 30 minutes of PROM only on the FES Hand Glove 200, followed by an additional 30 minutes of PROM with FES for 1 hour of therapy per session. The study participants were treated 4 times per week for 6 weeks. Before and after each session, OTs evaluated and documented any loss of skin integrity and pain. Autonomic dysreflexia occurred when systolic BP increased > 20 to 30 mm Hg with symptoms such as headache, profuse sweating, or blurred vision was reported.9 The FES Hand Glove 200 was set up for PROM to the thumb and to digits 2 to 5 and for electrical muscle stimulation of the finger extensors and flexors. No other therapeutic exercise was performed during the study period on the other extremity. Primary and secondary outcomes were collected at the end of the 6-week intervention.
Primary outcomes included complications from the use of FES Hand Glove 200, including skin integrity and any joint deformity as drawn on a figure, changes of pain level by visual analog scale (VAS), and total number of autonomic dyreflexia episodes. Secondary measured outcomes included changes in PROM and AROM of wrist, metacarpal joint and interphalangeal joints of thumbs and digits 2 to 5 ≥ 10°; hand and pinch strength decline of > 1 lb; decline in manual muscle test, and FIM score, which is a validated measurement of disability and the level of assistance required for ADL.10
Statistical analyses were performed using SAS version 4 (Cary, NC) to assess the degree of change in the improvement score, which was defined as the postintervention score minus the preintervention score. However, because of the large standard error due to small sample sizes, the normality assumption was not satisfied for all the outcomes considered.
Results
Of the 20 participants screened, 14 men aged between 19 and 66 years with cervical SCI level of C4 to C6 AIS grades A to D were enrolled in the study. Three did not complete the 6-week trial due to SCI-related medical complications, which were unrelated to the use of the FES Hand Glove 200. They continued with regular OT treatment or self-directed home exercises after they were seen by the treating physician. (Table 1)
Skin integrity of all subjects was maintained throughout the study. One subject had a right-elbow wound before the intervention, which was unchanged at the end of the study. After 6 weeks of experimental intervention, there was no wrist or finger joint deformity noted and no increase in pain level except for 1 subject who reported increased pain that was unrelated to use of the device. No occurrence of autonomic dysreflexia was recorded during the use of FES Hand Glove 200 (Table 2).
For the secondary outcomes, there was no significant decrease in AROM or PROM ≥ 10° in forearm, wrist, or finger joints in any participants. There was no loss of strength > 1 lb as measured by gross grasp, pinch tip, 3-point, or lateral grip. There was no decline in motor strength per manual muscle testing. No worsening of FIM score was noted (Table 3).
Although this was not an efficacy study primarily, participants improved in several areas. Improvements included active and passive movements in the forearm, wrist, and hand. There also was significant improvement in strength of the extensor digitorum communis (EDC) muscle. Data are available on request to the authors.
Discussion
Passive ROM and AROM exercises and FES are common strategies to improve certain hand functions in people with cervical SCI. Many people, however, may experience limited duration or efficiency of rehabilitation secondary to lack of resources. Technologic advancement allowed the combination of PROM exercise and FES using the FES Hand Glove 200 device. The eventual goal of using this device is to enhance QOL by improving upper-extremity function. Because this device is not commercially available, its safety and tolerability are being tested prior to clinical use. Although 3 subjects withdrew from the study due to nondevice-related medical reasons, 11 subjects completed the study. Potential AEs included skin wounds, burns, tendon sprain or rupture, edema, and pain. At the end of the 6-week study period, there was no loss of skin integrity, no joint deformity, and no increase in hand or finger edema in all subjects. Increase in pain level at 6 weeks was noted in only 1 subject.
One concern was that overuse of such devices could potentially cause muscle fatigue, leading to decreased strength. Pinch grasp and manual muscle testing were evaluated, and no decrease in any of these parameters was noted at the end of study. Although this was not an efficacy study, there was some evidence of improved ROM of multiple wrist and finger joints as well as the EDC muscle strength.
Limitations
Limitations of the study included the duration of treatment of eight 30-minute sessions per week over a 6-week period. A longer treatment duration could result in repetition-related injuries and should be tested in future trials. Finally, the sample size of this study was relatively small. Future studies of different treatment frequency, longer duration of use and monitoring, and using a larger sample size are suggested. An efficacy study of this device using a randomized controlled design is indicated. As people with cervical SCI rank upper-extremity dysfunction as one of the top impairments that negatively impacts QOL, rehabilitation strategy to improve such functions should continue to be a research priority.2
Conclusion
This study supports the safety and tolerability of a 6-week course using FES Hand Glove 200 in traumatic SCI tetraplegic subjects. Additionally, data from this study suggest possible efficacy in enhancing ROM of various wrist and finger joints as well as certain muscle group. Further studies of efficacy with larger numbers of subjects are warranted.
Click here to read the digital edition.
An estimated 282,000 people in the US are living with spinal cord injury (SCI).1 Damage to the cervical spinal cord is the most prevalent. Among cervical spinal cord trauma, injury to levels C4, C5, and C6 have the highest occurrence.1 Damage to these levels has significant implications for functional status. Depending on pathology, patients’ functional status can range from requiring assistance for all activities of daily living (ADL) to potentially living independently.
Improving upper-limb function is vital to achieving independence. About half of people with tetraplegia judge hand and arm function to be the top factor that would improve quality of life (QOL).2 Persons with traumatic cervical SCI may lose the ability to use their hands from motor deficits, sensory dysfunction, proprioception problem, and/or loss of coordination. In addition, they may develop joint contracture, spasticity, pain, and other complications. Thus, their independence and ADL are affected significantly by multiple mechanisms of pathology.
Upper-extremity rehabilitation that emphasizes strengthening and maintaining functional range of motion (ROM) is fundamental to SCI rehabilitation. Rehabilitation to restore partial hand function has included ROM exercises, splinting, surgical procedures in the form of tendon transfers and various electrical stimulation devices, such as implantable neuroprostheses.2-7 These interventions improve the ability to grasp, hold, and release objects in selected individuals; however, they have not been universally accepted. Traditional modalities, such as active ROM (AROM) and passive ROM (PROM) and electrical stimulation remain highly used in upper-extremity rehabilitation. Devices have been developed to provide either PROM or electrical stimulation to improve hand function and to prevent muscle atrophy. Therapist- and caregiver-directed PROM exercises are time consuming and labor intensive. An innovative therapeutic approach that can provide all these modalities more efficiently is needed in SCI rehabilitation.
Until now, a single device that combines AROM and PROM simultaneously has not been available. A robotic system, the FES Hand Glove 200 (Robotix Hand Therapy Inc, Colorado Springs, CO), was developed to improve hand function (Figure).
Methods
This prospective safety study evaluated the occurrence of adverse effects (AEs) associated with the use of the FES Hand Glove 200. The study was performed in the Occupational Therapy Section of the Spinal Cord Injury Center at the James A. Haley Veterans’ Hospital (JAHVH) and approved by the JAHVH Research and Development Committee as well as the University of South Florida Investigational Review Board. For recruitment, the goals of the study as well as the inclusion and exclusion criteria were presented to the Spinal Cord Injury Center health care providers. Potential candidates of the study were referred to the study team from these providers.
Screening of the referred candidates was conducted by physicians during inpatient evaluations. All subjects signed a consent form. Participants included active-duty military or veterans with traumatic SCI at levels C4 to C8 and American Spinal Injury Association Impairment Scale (AIS) grades A, B, C, and D. Participants were aged 18 to 60 years, at least 1-month post-SCI, medically stable, and had impairments in upper-extremities strength and ROM or function, including hand.
Subjects were excluded if any of the following were present: seizure within 3 months of study; active cancer; heterotopic ossification below the shoulder; new acute hand injuries of the study limb; unhealed fractures of the study limb; myocardial infarction within 12 months; severe cognitive impairment determined by a Modified Rancho Score below VI8; severe aphasia; pregnancy; skin irritations or open wounds in the study limb; fixed contractures of > 40° of the metacarpophalangeal (MP) or proximal interphalangeal (PIP) joints of the study hand; unwillingness to perform all of the therapies and assessments required for the study; active implant device (eg, pacemaker, implanted cardiac defibrillator, neurostimulator or drug infusion device); major psychological disorder; severe residual spasticity despite maximal medical therapy; muscle power grade of more than 3+ on wrist and finger extensors and flexors of the study limb; recent or current participation in research that could influence study response; pain that prevents participation in the study; or concurrent use of transcutaneous electrical stimulation on the study arm.
The following data were documented: level of SCI, AIS-score; complete medical history; physical examination (including skin integrity); and vital signs of bilateral upper extremities. A nurse practitioner (NP) certified in Functional Independent Measure (FIM) conducted chart reviews and/or in-person interviews of each subject to establish a FIM score before and after 6 weeks of research treatment. Two experienced occupational therapists (OTs) conducted detailed hand evaluations before the research treatment interventions. An OT provided subjects with education on the use, care, and precautions of the FES Hand Glove 200. The OT adjusted the device on the subject’s hand for proper fitting, including initial available PROM, and optimal muscle stimulation.
The OT then implemented the treatment protocol using the FES Hand Glove 200 in 1 hand per the subjects’ preference. The subjects received 30 minutes of PROM only on the FES Hand Glove 200, followed by an additional 30 minutes of PROM with FES for 1 hour of therapy per session. The study participants were treated 4 times per week for 6 weeks. Before and after each session, OTs evaluated and documented any loss of skin integrity and pain. Autonomic dysreflexia occurred when systolic BP increased > 20 to 30 mm Hg with symptoms such as headache, profuse sweating, or blurred vision was reported.9 The FES Hand Glove 200 was set up for PROM to the thumb and to digits 2 to 5 and for electrical muscle stimulation of the finger extensors and flexors. No other therapeutic exercise was performed during the study period on the other extremity. Primary and secondary outcomes were collected at the end of the 6-week intervention.
Primary outcomes included complications from the use of FES Hand Glove 200, including skin integrity and any joint deformity as drawn on a figure, changes of pain level by visual analog scale (VAS), and total number of autonomic dyreflexia episodes. Secondary measured outcomes included changes in PROM and AROM of wrist, metacarpal joint and interphalangeal joints of thumbs and digits 2 to 5 ≥ 10°; hand and pinch strength decline of > 1 lb; decline in manual muscle test, and FIM score, which is a validated measurement of disability and the level of assistance required for ADL.10
Statistical analyses were performed using SAS version 4 (Cary, NC) to assess the degree of change in the improvement score, which was defined as the postintervention score minus the preintervention score. However, because of the large standard error due to small sample sizes, the normality assumption was not satisfied for all the outcomes considered.
Results
Of the 20 participants screened, 14 men aged between 19 and 66 years with cervical SCI level of C4 to C6 AIS grades A to D were enrolled in the study. Three did not complete the 6-week trial due to SCI-related medical complications, which were unrelated to the use of the FES Hand Glove 200. They continued with regular OT treatment or self-directed home exercises after they were seen by the treating physician. (Table 1)
Skin integrity of all subjects was maintained throughout the study. One subject had a right-elbow wound before the intervention, which was unchanged at the end of the study. After 6 weeks of experimental intervention, there was no wrist or finger joint deformity noted and no increase in pain level except for 1 subject who reported increased pain that was unrelated to use of the device. No occurrence of autonomic dysreflexia was recorded during the use of FES Hand Glove 200 (Table 2).
For the secondary outcomes, there was no significant decrease in AROM or PROM ≥ 10° in forearm, wrist, or finger joints in any participants. There was no loss of strength > 1 lb as measured by gross grasp, pinch tip, 3-point, or lateral grip. There was no decline in motor strength per manual muscle testing. No worsening of FIM score was noted (Table 3).
Although this was not an efficacy study primarily, participants improved in several areas. Improvements included active and passive movements in the forearm, wrist, and hand. There also was significant improvement in strength of the extensor digitorum communis (EDC) muscle. Data are available on request to the authors.
Discussion
Passive ROM and AROM exercises and FES are common strategies to improve certain hand functions in people with cervical SCI. Many people, however, may experience limited duration or efficiency of rehabilitation secondary to lack of resources. Technologic advancement allowed the combination of PROM exercise and FES using the FES Hand Glove 200 device. The eventual goal of using this device is to enhance QOL by improving upper-extremity function. Because this device is not commercially available, its safety and tolerability are being tested prior to clinical use. Although 3 subjects withdrew from the study due to nondevice-related medical reasons, 11 subjects completed the study. Potential AEs included skin wounds, burns, tendon sprain or rupture, edema, and pain. At the end of the 6-week study period, there was no loss of skin integrity, no joint deformity, and no increase in hand or finger edema in all subjects. Increase in pain level at 6 weeks was noted in only 1 subject.
One concern was that overuse of such devices could potentially cause muscle fatigue, leading to decreased strength. Pinch grasp and manual muscle testing were evaluated, and no decrease in any of these parameters was noted at the end of study. Although this was not an efficacy study, there was some evidence of improved ROM of multiple wrist and finger joints as well as the EDC muscle strength.
Limitations
Limitations of the study included the duration of treatment of eight 30-minute sessions per week over a 6-week period. A longer treatment duration could result in repetition-related injuries and should be tested in future trials. Finally, the sample size of this study was relatively small. Future studies of different treatment frequency, longer duration of use and monitoring, and using a larger sample size are suggested. An efficacy study of this device using a randomized controlled design is indicated. As people with cervical SCI rank upper-extremity dysfunction as one of the top impairments that negatively impacts QOL, rehabilitation strategy to improve such functions should continue to be a research priority.2
Conclusion
This study supports the safety and tolerability of a 6-week course using FES Hand Glove 200 in traumatic SCI tetraplegic subjects. Additionally, data from this study suggest possible efficacy in enhancing ROM of various wrist and finger joints as well as certain muscle group. Further studies of efficacy with larger numbers of subjects are warranted.
Click here to read the digital edition.
1. NSCISC National Spinal Cord Injury Statistic Center. 2016 annual report—public version. https://www.nscisc.uab.edu/public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf. Published 2016. Accessed March 19, 2018.
2. Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med. 2005;37(1):32-36.
3. O’Driscoll SW, Giori NJ. Continuous passive motion (CPM): theory and principles of clinical application. J Rehabil Res Dev. 2000;37(2):179-188.
4. Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: a pilot study. Neurorehabil Neural Repair. 2007;21(3):207-215.
5. de Kroon JR, Ijzerman MJ, Lankhorst GJ, Zilvold G. Electrical stimulation of the upper limb in stroke stimulation of the extensors of the hand vs. alternate stimulation of flexors and extensors. Am J Phys Med Rehabil. 2004;83(8):592-600.
6. Alon G, McBride K, Levitt AF. Feasibility of randomised clinical trial of early initiation and prolonged, home-base FES training to enhance upper limb functional recovery following stroke. https://www.researchgate.net /publication/237724608_Feasibility_of_randomised_clinical_trial_of_early _initiation_and_prolonged_home-based_FES_training_to_enhance_upper_limb _functional_recovery_following_stroke. Published 2004. Accessed March 21, 2018.
7. Alon G, McBride K. Persons with C5-C6 tetraplegia achieve selected functional gains using a neuroprosthesis. Arch Phys Med Rehabil. 2003;84(1):119-124.
8. Hagen C, Malkmus D, Durham P. Rancho Los Amigos Cognitive Scale. http://file .lacounty.gov/SDSInter/dhs/218118_RLOCFProfessionalReferenceCard-English .pdf. Published 1979. Accessed March 19, 2018.
9. Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81(4):506-516.
10. Grey N, Kennedy P. The Functional Independence Measure: a comparative study of clinician and self rating. Paraplegia. 1993;31(7):457-461.
1. NSCISC National Spinal Cord Injury Statistic Center. 2016 annual report—public version. https://www.nscisc.uab.edu/public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf. Published 2016. Accessed March 19, 2018.
2. Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med. 2005;37(1):32-36.
3. O’Driscoll SW, Giori NJ. Continuous passive motion (CPM): theory and principles of clinical application. J Rehabil Res Dev. 2000;37(2):179-188.
4. Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: a pilot study. Neurorehabil Neural Repair. 2007;21(3):207-215.
5. de Kroon JR, Ijzerman MJ, Lankhorst GJ, Zilvold G. Electrical stimulation of the upper limb in stroke stimulation of the extensors of the hand vs. alternate stimulation of flexors and extensors. Am J Phys Med Rehabil. 2004;83(8):592-600.
6. Alon G, McBride K, Levitt AF. Feasibility of randomised clinical trial of early initiation and prolonged, home-base FES training to enhance upper limb functional recovery following stroke. https://www.researchgate.net /publication/237724608_Feasibility_of_randomised_clinical_trial_of_early _initiation_and_prolonged_home-based_FES_training_to_enhance_upper_limb _functional_recovery_following_stroke. Published 2004. Accessed March 21, 2018.
7. Alon G, McBride K. Persons with C5-C6 tetraplegia achieve selected functional gains using a neuroprosthesis. Arch Phys Med Rehabil. 2003;84(1):119-124.
8. Hagen C, Malkmus D, Durham P. Rancho Los Amigos Cognitive Scale. http://file .lacounty.gov/SDSInter/dhs/218118_RLOCFProfessionalReferenceCard-English .pdf. Published 1979. Accessed March 19, 2018.
9. Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81(4):506-516.
10. Grey N, Kennedy P. The Functional Independence Measure: a comparative study of clinician and self rating. Paraplegia. 1993;31(7):457-461.
Restricting opioids after knee surgery did not increase refills
according to a study in the Journal of Arthroplasty.
Contrary to concerns that restrictive opioid prescribing might increase the number of patient call-ins and refill requests, one academic institution had significantly fewer call-ins and refills after it implemented a strict postoperative opioid prescribing protocol on Jan. 1, 2018.
“Orthopedic surgeons might be reluctant to change practice without evidence that new, more-restrictive practice will not impede patient care,” the researchers wrote. “As the current study demonstrates, there is room to significantly decrease postoperative opioid prescriptions in total joint arthroplasty. This places patients at lower risk of opioid abuse and diversion without significantly altering the risk of postoperative complications or compromising postoperative pain control.”
Opioid overuse is a major public health concern, and orthopedic surgeons may overprescribe opioids after surgery. The University of Iowa Hospitals and Clinics in Iowa City implemented strict postoperative opioid prescription guidelines that are based on the American Academy of Orthopedic Surgeons Clinical Practice Guidelines. As part of the protocol, patients receive a preoperative education session that emphasizes risks associated with opioid use. Before initiating this protocol, postoperative drug choice and quantity had not been standardized.
To examine changes in opioid prescriptions and the number of call-ins, postoperative complications, and prescription refill requests after the implementation of the restrictive opioid prescribing protocol, investigators at the institution conducted a retrospective study.
Andrew J. Holte, a researcher in the department of orthopedics and rehabilitation, and his colleagues reviewed cases from June 2017 to February 2018. Their analysis included 399 patients who underwent total hip arthroplasty or total knee arthroplasty.
In all, 282 patients underwent surgery before the restrictive protocol (the historical cohort) and 117 after (the restrictive cohort). In the historical cohort, about 48% of the patients underwent total knee arthroplasty. In the restrictive cohort, about 44% underwent total knee arthroplasty. Patients had an average age of about 61 years, and approximately 52% were women.
According to comparisons of morphine mg equivalents (MME), the historical cohort received significantly larger mean initial opioid prescriptions (752 MME vs. 387 MME), significantly more refills per patient (0.5 vs. 0.3), and significantly more medication through refills (253 MME vs. 84 MME).
“For reference, 50 pills of 5 mg oxycodone is equivalent to 300 MMEs,” the authors noted.
A multivariable model found that younger age and total knee arthroplasty, compared with total hip arthroplasty, were associated with increased likelihood of requests for refills and patient call-ins.
“Surprisingly, there were significantly more patient call-ins and requests for refills of opioids in the historical cohort,” Mr. Holte and his colleagues said. “Although this study did not collect direct data on patient pain scores, we believe that call-ins and requests for refills are sufficient surrogate markers for inadequate pain control.”
The study does not account for prescriptions from other providers or whether patients took none, some, or all of their filled prescriptions. Future studies are needed to assess how reduced opioid prescriptions affect pain and functional outcomes in the long term, the researchers said.
One or more study authors disclosed potential conflicts of interest. The disclosures can be found in Appendix A, Supplementary Data, at the end of the journal article.
SOURCE: Holte AJ et al. J Arthroplasty. 2019 Feb 20. doi: 10.1016/j.arth.2019.02.022.
according to a study in the Journal of Arthroplasty.
Contrary to concerns that restrictive opioid prescribing might increase the number of patient call-ins and refill requests, one academic institution had significantly fewer call-ins and refills after it implemented a strict postoperative opioid prescribing protocol on Jan. 1, 2018.
“Orthopedic surgeons might be reluctant to change practice without evidence that new, more-restrictive practice will not impede patient care,” the researchers wrote. “As the current study demonstrates, there is room to significantly decrease postoperative opioid prescriptions in total joint arthroplasty. This places patients at lower risk of opioid abuse and diversion without significantly altering the risk of postoperative complications or compromising postoperative pain control.”
Opioid overuse is a major public health concern, and orthopedic surgeons may overprescribe opioids after surgery. The University of Iowa Hospitals and Clinics in Iowa City implemented strict postoperative opioid prescription guidelines that are based on the American Academy of Orthopedic Surgeons Clinical Practice Guidelines. As part of the protocol, patients receive a preoperative education session that emphasizes risks associated with opioid use. Before initiating this protocol, postoperative drug choice and quantity had not been standardized.
To examine changes in opioid prescriptions and the number of call-ins, postoperative complications, and prescription refill requests after the implementation of the restrictive opioid prescribing protocol, investigators at the institution conducted a retrospective study.
Andrew J. Holte, a researcher in the department of orthopedics and rehabilitation, and his colleagues reviewed cases from June 2017 to February 2018. Their analysis included 399 patients who underwent total hip arthroplasty or total knee arthroplasty.
In all, 282 patients underwent surgery before the restrictive protocol (the historical cohort) and 117 after (the restrictive cohort). In the historical cohort, about 48% of the patients underwent total knee arthroplasty. In the restrictive cohort, about 44% underwent total knee arthroplasty. Patients had an average age of about 61 years, and approximately 52% were women.
According to comparisons of morphine mg equivalents (MME), the historical cohort received significantly larger mean initial opioid prescriptions (752 MME vs. 387 MME), significantly more refills per patient (0.5 vs. 0.3), and significantly more medication through refills (253 MME vs. 84 MME).
“For reference, 50 pills of 5 mg oxycodone is equivalent to 300 MMEs,” the authors noted.
A multivariable model found that younger age and total knee arthroplasty, compared with total hip arthroplasty, were associated with increased likelihood of requests for refills and patient call-ins.
“Surprisingly, there were significantly more patient call-ins and requests for refills of opioids in the historical cohort,” Mr. Holte and his colleagues said. “Although this study did not collect direct data on patient pain scores, we believe that call-ins and requests for refills are sufficient surrogate markers for inadequate pain control.”
The study does not account for prescriptions from other providers or whether patients took none, some, or all of their filled prescriptions. Future studies are needed to assess how reduced opioid prescriptions affect pain and functional outcomes in the long term, the researchers said.
One or more study authors disclosed potential conflicts of interest. The disclosures can be found in Appendix A, Supplementary Data, at the end of the journal article.
SOURCE: Holte AJ et al. J Arthroplasty. 2019 Feb 20. doi: 10.1016/j.arth.2019.02.022.
according to a study in the Journal of Arthroplasty.
Contrary to concerns that restrictive opioid prescribing might increase the number of patient call-ins and refill requests, one academic institution had significantly fewer call-ins and refills after it implemented a strict postoperative opioid prescribing protocol on Jan. 1, 2018.
“Orthopedic surgeons might be reluctant to change practice without evidence that new, more-restrictive practice will not impede patient care,” the researchers wrote. “As the current study demonstrates, there is room to significantly decrease postoperative opioid prescriptions in total joint arthroplasty. This places patients at lower risk of opioid abuse and diversion without significantly altering the risk of postoperative complications or compromising postoperative pain control.”
Opioid overuse is a major public health concern, and orthopedic surgeons may overprescribe opioids after surgery. The University of Iowa Hospitals and Clinics in Iowa City implemented strict postoperative opioid prescription guidelines that are based on the American Academy of Orthopedic Surgeons Clinical Practice Guidelines. As part of the protocol, patients receive a preoperative education session that emphasizes risks associated with opioid use. Before initiating this protocol, postoperative drug choice and quantity had not been standardized.
To examine changes in opioid prescriptions and the number of call-ins, postoperative complications, and prescription refill requests after the implementation of the restrictive opioid prescribing protocol, investigators at the institution conducted a retrospective study.
Andrew J. Holte, a researcher in the department of orthopedics and rehabilitation, and his colleagues reviewed cases from June 2017 to February 2018. Their analysis included 399 patients who underwent total hip arthroplasty or total knee arthroplasty.
In all, 282 patients underwent surgery before the restrictive protocol (the historical cohort) and 117 after (the restrictive cohort). In the historical cohort, about 48% of the patients underwent total knee arthroplasty. In the restrictive cohort, about 44% underwent total knee arthroplasty. Patients had an average age of about 61 years, and approximately 52% were women.
According to comparisons of morphine mg equivalents (MME), the historical cohort received significantly larger mean initial opioid prescriptions (752 MME vs. 387 MME), significantly more refills per patient (0.5 vs. 0.3), and significantly more medication through refills (253 MME vs. 84 MME).
“For reference, 50 pills of 5 mg oxycodone is equivalent to 300 MMEs,” the authors noted.
A multivariable model found that younger age and total knee arthroplasty, compared with total hip arthroplasty, were associated with increased likelihood of requests for refills and patient call-ins.
“Surprisingly, there were significantly more patient call-ins and requests for refills of opioids in the historical cohort,” Mr. Holte and his colleagues said. “Although this study did not collect direct data on patient pain scores, we believe that call-ins and requests for refills are sufficient surrogate markers for inadequate pain control.”
The study does not account for prescriptions from other providers or whether patients took none, some, or all of their filled prescriptions. Future studies are needed to assess how reduced opioid prescriptions affect pain and functional outcomes in the long term, the researchers said.
One or more study authors disclosed potential conflicts of interest. The disclosures can be found in Appendix A, Supplementary Data, at the end of the journal article.
SOURCE: Holte AJ et al. J Arthroplasty. 2019 Feb 20. doi: 10.1016/j.arth.2019.02.022.
FROM THE JOURNAL OF ARTHROPLASTY
Malpractice: Diagnostic errors top allegation involving children
Diagnostic error is the most common allegation against pediatricians when sued by patients and their families, a study finds.
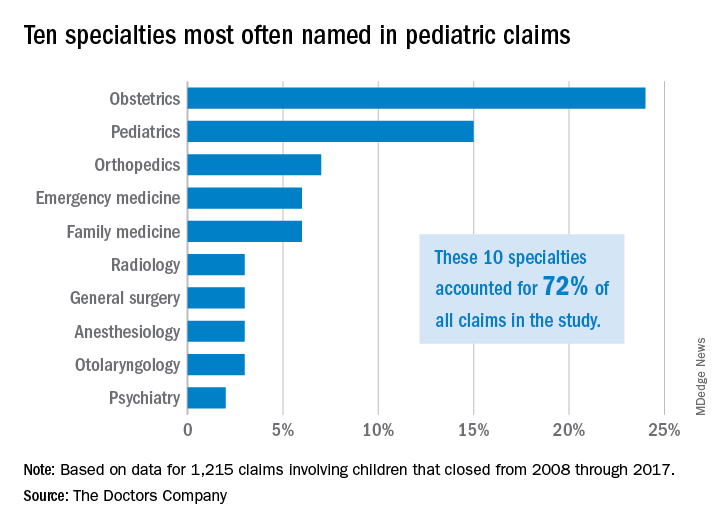
Investigators with The Doctors Company, a national medical liability insurer, examined 1,215 closed claims involving children from the company’s database between 2008 and 2017. Results showed that diagnostic mistakes, including delayed diagnosis, incorrect diagnosis, and failure to diagnose, were the most common accusations among claims that involved children ages 1 through 17. Poor medical treatment was the second most common allegation for claims that involved children aged 1-9, while surgical treatment-related error was the second most frequent accusation for children ages 10-17.
Pediatricians, orthopedic surgeons, and emergency medicine physicians were the most frequently named specialists in claims associated with children older than 1 month. Obstetricians were most frequently defendants in claims involving neonates. For these cases, errors during labor and delivery care were the most common complaints.
Of the 1,215 claims, obstetricians were named in 24% of the cases and pediatricians were named in 15% of the cases. The majority of claims were filed against physicians in the first 3 years following the medical incident alleged, according to the study, published by The Doctors Company.
The average patient payment in each case was $630,456, and the average expense to defend each claim was $157,502, according to the analysis. Claims that involved neonates had the highest average payment ($936,843) and the highest defense costs ($187,117), while claims involving children aged 10-17 years had the lowest average payment ($386,849) and cost the least to defend ($129,816).
For cases involving neonates, the type of therapy selected during labor and delivery and how it was managed were the most common factors contributing to the alleged injury, according to the analysis.
The most frequent factors contributing to patient harm for other age groups involved patient assessment issues and communication problems between the patient/family and the physician. Inadequate patient assessments were closely linked to incorrect diagnoses, while incomplete communication between patients/family members and providers impacted clinicians’ ability to make correct diagnoses, according to the study.
This analysis “shows that pediatric malpractice lawsuits impact nearly every area of medicine,” William F. Getman, MD, a pediatrician in Austin, Tex., said in an interview. “I was surprised to see that the most common age of a patient in a malpractice lawsuit was less than 1 month old. This age group also sustained the most severe injuries and had the highest indemnity paid.”
The study offers several key takeaways, including the importance of identifying system weaknesses in your medical practice and evaluating if improvements are needed, according to Darrell Ranum, vice president for patient safety and risk management for The Doctors Company.
Simple improvements, such as implementing tracking mechanisms for test results and referrals, can reduce the chance that important information falls through the cracks and delays diagnosis or treatment, Mr. Ranum said in an interview.
“When parents raise questions about their child’s complaints, this is the best opportunity to identify illnesses and conditions that represent a serious threat to children,” he said. “Prepare office staff members to know what complaints need to be evaluated by a clinician or require immediate care.”
In addition, the study findings point to the need to improve communication in all areas of the practice spectrum, Dr. Getman said.
“Many of the lawsuits could have been avoided by improvements in communication – doctor to patient, patient to doctor, doctor to nurse, doctor to doctor, nurse to patient, etc.,” he said. “Finding more effective and accurate ways to communicate will avoid mistakes, improve care, and improve outcomes. Examples of ways to improve communication include use of an interpreter when indicated, verbal and written explanations of instructions, and system improvements in tracking messages/labs/data. There are innumerable other ways to improve communication in health care.”
SOURCE: Ranum, D. The Doctor’s Advocate. First Quarter 2019.
Diagnostic error is the most common allegation against pediatricians when sued by patients and their families, a study finds.

Investigators with The Doctors Company, a national medical liability insurer, examined 1,215 closed claims involving children from the company’s database between 2008 and 2017. Results showed that diagnostic mistakes, including delayed diagnosis, incorrect diagnosis, and failure to diagnose, were the most common accusations among claims that involved children ages 1 through 17. Poor medical treatment was the second most common allegation for claims that involved children aged 1-9, while surgical treatment-related error was the second most frequent accusation for children ages 10-17.
Pediatricians, orthopedic surgeons, and emergency medicine physicians were the most frequently named specialists in claims associated with children older than 1 month. Obstetricians were most frequently defendants in claims involving neonates. For these cases, errors during labor and delivery care were the most common complaints.
Of the 1,215 claims, obstetricians were named in 24% of the cases and pediatricians were named in 15% of the cases. The majority of claims were filed against physicians in the first 3 years following the medical incident alleged, according to the study, published by The Doctors Company.
The average patient payment in each case was $630,456, and the average expense to defend each claim was $157,502, according to the analysis. Claims that involved neonates had the highest average payment ($936,843) and the highest defense costs ($187,117), while claims involving children aged 10-17 years had the lowest average payment ($386,849) and cost the least to defend ($129,816).
For cases involving neonates, the type of therapy selected during labor and delivery and how it was managed were the most common factors contributing to the alleged injury, according to the analysis.
The most frequent factors contributing to patient harm for other age groups involved patient assessment issues and communication problems between the patient/family and the physician. Inadequate patient assessments were closely linked to incorrect diagnoses, while incomplete communication between patients/family members and providers impacted clinicians’ ability to make correct diagnoses, according to the study.
This analysis “shows that pediatric malpractice lawsuits impact nearly every area of medicine,” William F. Getman, MD, a pediatrician in Austin, Tex., said in an interview. “I was surprised to see that the most common age of a patient in a malpractice lawsuit was less than 1 month old. This age group also sustained the most severe injuries and had the highest indemnity paid.”
The study offers several key takeaways, including the importance of identifying system weaknesses in your medical practice and evaluating if improvements are needed, according to Darrell Ranum, vice president for patient safety and risk management for The Doctors Company.
Simple improvements, such as implementing tracking mechanisms for test results and referrals, can reduce the chance that important information falls through the cracks and delays diagnosis or treatment, Mr. Ranum said in an interview.
“When parents raise questions about their child’s complaints, this is the best opportunity to identify illnesses and conditions that represent a serious threat to children,” he said. “Prepare office staff members to know what complaints need to be evaluated by a clinician or require immediate care.”
In addition, the study findings point to the need to improve communication in all areas of the practice spectrum, Dr. Getman said.
“Many of the lawsuits could have been avoided by improvements in communication – doctor to patient, patient to doctor, doctor to nurse, doctor to doctor, nurse to patient, etc.,” he said. “Finding more effective and accurate ways to communicate will avoid mistakes, improve care, and improve outcomes. Examples of ways to improve communication include use of an interpreter when indicated, verbal and written explanations of instructions, and system improvements in tracking messages/labs/data. There are innumerable other ways to improve communication in health care.”
SOURCE: Ranum, D. The Doctor’s Advocate. First Quarter 2019.
Diagnostic error is the most common allegation against pediatricians when sued by patients and their families, a study finds.

Investigators with The Doctors Company, a national medical liability insurer, examined 1,215 closed claims involving children from the company’s database between 2008 and 2017. Results showed that diagnostic mistakes, including delayed diagnosis, incorrect diagnosis, and failure to diagnose, were the most common accusations among claims that involved children ages 1 through 17. Poor medical treatment was the second most common allegation for claims that involved children aged 1-9, while surgical treatment-related error was the second most frequent accusation for children ages 10-17.
Pediatricians, orthopedic surgeons, and emergency medicine physicians were the most frequently named specialists in claims associated with children older than 1 month. Obstetricians were most frequently defendants in claims involving neonates. For these cases, errors during labor and delivery care were the most common complaints.
Of the 1,215 claims, obstetricians were named in 24% of the cases and pediatricians were named in 15% of the cases. The majority of claims were filed against physicians in the first 3 years following the medical incident alleged, according to the study, published by The Doctors Company.
The average patient payment in each case was $630,456, and the average expense to defend each claim was $157,502, according to the analysis. Claims that involved neonates had the highest average payment ($936,843) and the highest defense costs ($187,117), while claims involving children aged 10-17 years had the lowest average payment ($386,849) and cost the least to defend ($129,816).
For cases involving neonates, the type of therapy selected during labor and delivery and how it was managed were the most common factors contributing to the alleged injury, according to the analysis.
The most frequent factors contributing to patient harm for other age groups involved patient assessment issues and communication problems between the patient/family and the physician. Inadequate patient assessments were closely linked to incorrect diagnoses, while incomplete communication between patients/family members and providers impacted clinicians’ ability to make correct diagnoses, according to the study.
This analysis “shows that pediatric malpractice lawsuits impact nearly every area of medicine,” William F. Getman, MD, a pediatrician in Austin, Tex., said in an interview. “I was surprised to see that the most common age of a patient in a malpractice lawsuit was less than 1 month old. This age group also sustained the most severe injuries and had the highest indemnity paid.”
The study offers several key takeaways, including the importance of identifying system weaknesses in your medical practice and evaluating if improvements are needed, according to Darrell Ranum, vice president for patient safety and risk management for The Doctors Company.
Simple improvements, such as implementing tracking mechanisms for test results and referrals, can reduce the chance that important information falls through the cracks and delays diagnosis or treatment, Mr. Ranum said in an interview.
“When parents raise questions about their child’s complaints, this is the best opportunity to identify illnesses and conditions that represent a serious threat to children,” he said. “Prepare office staff members to know what complaints need to be evaluated by a clinician or require immediate care.”
In addition, the study findings point to the need to improve communication in all areas of the practice spectrum, Dr. Getman said.
“Many of the lawsuits could have been avoided by improvements in communication – doctor to patient, patient to doctor, doctor to nurse, doctor to doctor, nurse to patient, etc.,” he said. “Finding more effective and accurate ways to communicate will avoid mistakes, improve care, and improve outcomes. Examples of ways to improve communication include use of an interpreter when indicated, verbal and written explanations of instructions, and system improvements in tracking messages/labs/data. There are innumerable other ways to improve communication in health care.”
SOURCE: Ranum, D. The Doctor’s Advocate. First Quarter 2019.
Restless Legs Syndrome Among Veterans With Spinal Cord Lesions (FULL)
Spinal cord injuries (SCI) are common in veteran populations.1 Veterans with spinal cord injuries and disorders (SCI/D) also may have concurrent sleep disturbances. Spinal cord injury typically causes spasticity.2,3 Hypersensitivity of the flexor reflex pathways is believed to cause painful muscle spasms in patients with SCI.4 Neuropathic pain at or below the level of the lesion also is common.
Restless legs syndrome (RLS) is a common sleep disorder that affects sleep quality and can occur concomitantly with spinal cord lesions.5 In about 80% of RLS cases, involuntary movements of legs across hip, knee, and ankle joints during sleep, known as periodic limb movement during sleep (PLMS), occurs.6 Several studies showed increased prevalence of PLMS in patients with SCI, and some case reports suggest an increased prevalence of RLS in this population.7,8 One small study showed that 100% of patients with SCI had symptoms of RLS.6 Another study found that SCI could trigger PLMS.8
The pathophysiology of RLS and PLMS in patients with SCI is not fully understood, but case reports describing PLM in SCI patients points to a possible role of central pattern generators and the flexor reflex afferents in the pathophysiology of PLMS.9,10 Changes of the tissue microstructure in the midbrain and upper cervical spinal cord have been described in patients with RLS.11The objective of this study was to assess the prevalence of RLS in a veteran population with SCI/D and
Methods
The institutional review and ethical approval boards of the Minneapolis VA Health Care System approved the study. Within the VA system, 666 patients with SCI/D were identified using a national database. Of the 666 people, 316 were excluded, 199 were included, and 151 were deceased.
Patients aged between 18 and 65 years were included in the study. Charts of patients who had been discharged with the diagnosis of SCI from 2002 to 2008 were studied. All patients met the inclusion criteria of the International Restless Legs Syndrome Study Group diagnosis.
Exclusion criteria were as follows: Patients with evidence of brain pathology (eg, stroke), concurrent neurologic condition associated with RLS (Parkinson disease, spinocerebellar ataxia, peripheral neuropathy), concurrent psychiatric condition within the setting of treatment with dopamine antagonists, secondary causes of RLS (renal failure/uremia, iron deficiency, rheumatoid arthritis, and pregnancy) and a recent history of alcohol or drug misuse or current evidence of substance use of < 1 year.
A patient list was compiled that included the etiology of the SCI (vascular injury, multiple sclerosis [MS], trauma, unknown, and other), the level(s) and completeness of the SCI per radiology report, RLS pharmacotherapies, and pertinent medical history.
Axial T2-weighted images on magnetic resonance imaging (MRI) scans were retrospectively reviewed. Sagittal T1/T2-weighted and axial T2-weighted sequences were performed routinely on all patients with spinal cord lesions. The analysis included the extension of the lesion on both sagittal and axial distributions. The anatomic location of the cord lesion was categorized by the following: (1) pure gray matter (central cord); (2) white matter (dorsal [D], dorsolateral [DL], ventral [V], ventrolateral areas [VL]).
A questionnaire using standard diagnostic criteria for RLS was mailed to the 199 patients who met the inclusion criteria (Appendix A).
All analyses were carried out using StataCorp STATA 13 (College Station, TX). Descriptive statistics were used. The analyses were carried out using chi-square and Fisher exact tests. Differences between the groups were considered statistically significant at P < .05. The data were analyzed to obtain point prevalence among patients with SCI, and comparisons were made among the different subgroups.
Results
Of the 162 patients who chose to participate in the study, the sleep specialists confirmed 31 (19%) to have RLS, 112 (69%) were confirmed negative for RLS, and an additional 19 (12%) screened positive for RLS but were not confirmed to have RLS by the sleep specialists (Figure 1).
The etiology of SCI was subdivided into 4 groups: MS, trauma, vascular, and other/unknown. Within each group (– RLS vs + RLS), MS and trauma were the most common etiologies with 55% MS and 36% trauma in the + RLS group.
When comparing RLS among the spinal cord levels (cervical, thoracic, lumbar and cervical + thoracic), only the cervical + thoracic subgroup (18% + RLS vs 5% – RLS) showed a significant difference (Figure 2).
There was no significant difference found with the prevalence of RLS in the axial plane of the spinal cord lesions (ventral/ventro-lateral/central cord vs dorsal/dorsolateral) or by the completeness of spinal cord lesions, P = .76. There was a higher prevalence of incomplete cord injury, however, within each subgroup of RLS.
The Mann-Whitney test was used to analyze the burden of disease in both groups (+ RLS vs – RLS). Moderate level of burden was most frequently reported with a higher prevalence within the + RLS group. Of those receiving treatment for RLS, 71% were + RLS vs 46% – RLS with a P value of .01. Symptoms of RLS after cord injury were 89% + RLS vs 55% – RLS with a P value of .03.
Discussion
This study represents one of the first studies to determine the prevalence of RLS in veterans with spinal cord disease. Research in this area is important to raise awareness of RLS among the veteran population with and without SCI and disorders. Restless legs syndrome often escapes diagnosis because of difficulty understanding the patient’s descriptions of their sensations. In addition, RLS may cause debilitating symptoms of sleep deprivation, daytime sleepiness, discomfort, and fatigue, which often results in decreased quality of life (QOL). Proper screening and treatment may improve QOL.
A study by Kumru and colleagues showed a similar rate of RLS in patients with SCI and RLS symptoms presented in the first year after SCI as did this study (18% vs 19%, respectively).4 In that study, RLS was more common in patients with lesions in lumbosacral area. Kumru and colleagues also showed that a dopaminergic medication improved symptoms of RLS in this population, whereas this study did not explore treatment outcomes.4
The pathogenesis of RLS is not fully known, but hereditary factors, iron metabolism, and the brain dopaminergic system are thought to be involved.11 It is hypothesized that spinal cord lesions allow the appearance of RLS symptoms and spinal leg movement generator by blocking descending inhibitory spinal pathways.12 One hypothesis is that damage to A11 nuclei (the main source of dopamine in the spinal cord or its diencephalospinal tract in animals) causes hyperexcitability of the spinal cord and leads to PLM and RLS symptoms.13 As the axons of A11 nuclei are present along the whole span of the spinal cord, SCI/D in patients with RLS might interrupt this dopaminergic tract and produce the RLS symptoms.
Limitations
This study included only veterans, so the prevalence may not apply to the nonveteran SCI population. Also, the population mainly was male, and there was no accurate information on race. Ferritin levels of the patients were not checked and is a major factor in RLS. The reported onset of RLS after the SCI could be due to recall bias.
Conclusion
The prevalence of RLS in veterans with SCI is above that reported in the general population (19% vs 10%, respectively). Furthermore, those with RLS have symptoms that often started after the SCI (suggesting causality) and required therapy due to their level of RLS symptom burden. A spectrum of severity of symptoms is present among those with RLS, with 83% having moderate-to-severe RLS affecting their QOL.
Although there was not a statistically significant relationship between RLS and spinal cord lesion level, there was a slightly higher prevalence of RLS at the cervical and thoracic levels, which may be relevant for future studies. There was no difference found between the RLS subgroups with respect to the location of the lesion within the spinal cord; however, a larger sample size may be needed to determine whether this would reach statistical significance. Prompt search for symptoms of RLS in veterans with SCI is warranted to provide adequate treatment to improve sleep health and QOL in this population.
1. Lasfargues JE, Custis D, Morrone F, Carswell J, Nguyen T. A model for estimating spinal cord injury prevalence in the United States. Paraplegia. 1995;33(2):62-68.
2. Sjölund BH. Pain and rehabilitation after spinal cord injury: the case of sensory spasticity? Brain Res Brain Res Rev. 2002;40(1-3):250-256.
3. Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord. 2005;43(10):577-586.
4. Kumru H, Vidal J, Benito J, et al. Restless leg syndrome in patients with spinal cord injury. Parkinsonism Relat Disord. 2015;21(12):1461-1464.
5. Wilt TJ, MacDonald R, Ouellette J, et al. Pharmacologic therapy for primary restless legs syndrome: a systematic review and meta-analysis. JAMA Intern Med. 2013;173(7):496-505.
6. American Academy of Sleep Medicine. The International Classification of Sleep Disorders: Diagnostic and Coding Manual. (AASM ICSD-3). 3rd ed. Westchester, IL: American Academy of Sleep Medicine; 2014.
7. Telles SC, Alves RC, Chadi G. Periodic limb movements during sleep and restless legs syndrome in patients with ASIA A spinal cord injury. J Neurol Sci. 2011;303(1-2):119-123.
8. Telles SC, Alves RS, Chadi G. Spinal cord injury as a trigger to develop periodic leg movements during sleep: an evolutionary perspective. Arq Neuropsiquiatr. 2012;70(11):880-884.
9. Tings T, Baier PC, Paulus W, Trenkwalder C. Restless legs syndrome induced by impairment of sensory spinal pathways. J Neurol. 2003;250(4):499-500.
10. Paulus W, Trenkwalder C. Less is more: pathophysiology of dopaminergic-therapy-related augmentation in restless legs syndrome. Lancet Neurol. 2006;5(10):878-886.
11. Silber MH, Ehrenberg BL, Allen RP, et al; Medical Advisory Board of the Restless Legs Syndrome Foundation. An algorithm for the management of restless legs syndrome. Mayo Clin Proc. 2004;79(7):916-922.
12. Hartmann M, Pfister R, Pfadenhauer K. Restless legs syndrome associated with spinal cord lesions. J Neurol Neurosurg Psychiatry. 1999;66(5):688-689.
13. Clemens S, Rye D, Hochman S. Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67(1):125-130.
Spinal cord injuries (SCI) are common in veteran populations.1 Veterans with spinal cord injuries and disorders (SCI/D) also may have concurrent sleep disturbances. Spinal cord injury typically causes spasticity.2,3 Hypersensitivity of the flexor reflex pathways is believed to cause painful muscle spasms in patients with SCI.4 Neuropathic pain at or below the level of the lesion also is common.
Restless legs syndrome (RLS) is a common sleep disorder that affects sleep quality and can occur concomitantly with spinal cord lesions.5 In about 80% of RLS cases, involuntary movements of legs across hip, knee, and ankle joints during sleep, known as periodic limb movement during sleep (PLMS), occurs.6 Several studies showed increased prevalence of PLMS in patients with SCI, and some case reports suggest an increased prevalence of RLS in this population.7,8 One small study showed that 100% of patients with SCI had symptoms of RLS.6 Another study found that SCI could trigger PLMS.8
The pathophysiology of RLS and PLMS in patients with SCI is not fully understood, but case reports describing PLM in SCI patients points to a possible role of central pattern generators and the flexor reflex afferents in the pathophysiology of PLMS.9,10 Changes of the tissue microstructure in the midbrain and upper cervical spinal cord have been described in patients with RLS.11The objective of this study was to assess the prevalence of RLS in a veteran population with SCI/D and
Methods
The institutional review and ethical approval boards of the Minneapolis VA Health Care System approved the study. Within the VA system, 666 patients with SCI/D were identified using a national database. Of the 666 people, 316 were excluded, 199 were included, and 151 were deceased.
Patients aged between 18 and 65 years were included in the study. Charts of patients who had been discharged with the diagnosis of SCI from 2002 to 2008 were studied. All patients met the inclusion criteria of the International Restless Legs Syndrome Study Group diagnosis.
Exclusion criteria were as follows: Patients with evidence of brain pathology (eg, stroke), concurrent neurologic condition associated with RLS (Parkinson disease, spinocerebellar ataxia, peripheral neuropathy), concurrent psychiatric condition within the setting of treatment with dopamine antagonists, secondary causes of RLS (renal failure/uremia, iron deficiency, rheumatoid arthritis, and pregnancy) and a recent history of alcohol or drug misuse or current evidence of substance use of < 1 year.
A patient list was compiled that included the etiology of the SCI (vascular injury, multiple sclerosis [MS], trauma, unknown, and other), the level(s) and completeness of the SCI per radiology report, RLS pharmacotherapies, and pertinent medical history.
Axial T2-weighted images on magnetic resonance imaging (MRI) scans were retrospectively reviewed. Sagittal T1/T2-weighted and axial T2-weighted sequences were performed routinely on all patients with spinal cord lesions. The analysis included the extension of the lesion on both sagittal and axial distributions. The anatomic location of the cord lesion was categorized by the following: (1) pure gray matter (central cord); (2) white matter (dorsal [D], dorsolateral [DL], ventral [V], ventrolateral areas [VL]).
A questionnaire using standard diagnostic criteria for RLS was mailed to the 199 patients who met the inclusion criteria (Appendix A).
All analyses were carried out using StataCorp STATA 13 (College Station, TX). Descriptive statistics were used. The analyses were carried out using chi-square and Fisher exact tests. Differences between the groups were considered statistically significant at P < .05. The data were analyzed to obtain point prevalence among patients with SCI, and comparisons were made among the different subgroups.
Results
Of the 162 patients who chose to participate in the study, the sleep specialists confirmed 31 (19%) to have RLS, 112 (69%) were confirmed negative for RLS, and an additional 19 (12%) screened positive for RLS but were not confirmed to have RLS by the sleep specialists (Figure 1).
The etiology of SCI was subdivided into 4 groups: MS, trauma, vascular, and other/unknown. Within each group (– RLS vs + RLS), MS and trauma were the most common etiologies with 55% MS and 36% trauma in the + RLS group.
When comparing RLS among the spinal cord levels (cervical, thoracic, lumbar and cervical + thoracic), only the cervical + thoracic subgroup (18% + RLS vs 5% – RLS) showed a significant difference (Figure 2).
There was no significant difference found with the prevalence of RLS in the axial plane of the spinal cord lesions (ventral/ventro-lateral/central cord vs dorsal/dorsolateral) or by the completeness of spinal cord lesions, P = .76. There was a higher prevalence of incomplete cord injury, however, within each subgroup of RLS.
The Mann-Whitney test was used to analyze the burden of disease in both groups (+ RLS vs – RLS). Moderate level of burden was most frequently reported with a higher prevalence within the + RLS group. Of those receiving treatment for RLS, 71% were + RLS vs 46% – RLS with a P value of .01. Symptoms of RLS after cord injury were 89% + RLS vs 55% – RLS with a P value of .03.
Discussion
This study represents one of the first studies to determine the prevalence of RLS in veterans with spinal cord disease. Research in this area is important to raise awareness of RLS among the veteran population with and without SCI and disorders. Restless legs syndrome often escapes diagnosis because of difficulty understanding the patient’s descriptions of their sensations. In addition, RLS may cause debilitating symptoms of sleep deprivation, daytime sleepiness, discomfort, and fatigue, which often results in decreased quality of life (QOL). Proper screening and treatment may improve QOL.
A study by Kumru and colleagues showed a similar rate of RLS in patients with SCI and RLS symptoms presented in the first year after SCI as did this study (18% vs 19%, respectively).4 In that study, RLS was more common in patients with lesions in lumbosacral area. Kumru and colleagues also showed that a dopaminergic medication improved symptoms of RLS in this population, whereas this study did not explore treatment outcomes.4
The pathogenesis of RLS is not fully known, but hereditary factors, iron metabolism, and the brain dopaminergic system are thought to be involved.11 It is hypothesized that spinal cord lesions allow the appearance of RLS symptoms and spinal leg movement generator by blocking descending inhibitory spinal pathways.12 One hypothesis is that damage to A11 nuclei (the main source of dopamine in the spinal cord or its diencephalospinal tract in animals) causes hyperexcitability of the spinal cord and leads to PLM and RLS symptoms.13 As the axons of A11 nuclei are present along the whole span of the spinal cord, SCI/D in patients with RLS might interrupt this dopaminergic tract and produce the RLS symptoms.
Limitations
This study included only veterans, so the prevalence may not apply to the nonveteran SCI population. Also, the population mainly was male, and there was no accurate information on race. Ferritin levels of the patients were not checked and is a major factor in RLS. The reported onset of RLS after the SCI could be due to recall bias.
Conclusion
The prevalence of RLS in veterans with SCI is above that reported in the general population (19% vs 10%, respectively). Furthermore, those with RLS have symptoms that often started after the SCI (suggesting causality) and required therapy due to their level of RLS symptom burden. A spectrum of severity of symptoms is present among those with RLS, with 83% having moderate-to-severe RLS affecting their QOL.
Although there was not a statistically significant relationship between RLS and spinal cord lesion level, there was a slightly higher prevalence of RLS at the cervical and thoracic levels, which may be relevant for future studies. There was no difference found between the RLS subgroups with respect to the location of the lesion within the spinal cord; however, a larger sample size may be needed to determine whether this would reach statistical significance. Prompt search for symptoms of RLS in veterans with SCI is warranted to provide adequate treatment to improve sleep health and QOL in this population.
Spinal cord injuries (SCI) are common in veteran populations.1 Veterans with spinal cord injuries and disorders (SCI/D) also may have concurrent sleep disturbances. Spinal cord injury typically causes spasticity.2,3 Hypersensitivity of the flexor reflex pathways is believed to cause painful muscle spasms in patients with SCI.4 Neuropathic pain at or below the level of the lesion also is common.
Restless legs syndrome (RLS) is a common sleep disorder that affects sleep quality and can occur concomitantly with spinal cord lesions.5 In about 80% of RLS cases, involuntary movements of legs across hip, knee, and ankle joints during sleep, known as periodic limb movement during sleep (PLMS), occurs.6 Several studies showed increased prevalence of PLMS in patients with SCI, and some case reports suggest an increased prevalence of RLS in this population.7,8 One small study showed that 100% of patients with SCI had symptoms of RLS.6 Another study found that SCI could trigger PLMS.8
The pathophysiology of RLS and PLMS in patients with SCI is not fully understood, but case reports describing PLM in SCI patients points to a possible role of central pattern generators and the flexor reflex afferents in the pathophysiology of PLMS.9,10 Changes of the tissue microstructure in the midbrain and upper cervical spinal cord have been described in patients with RLS.11The objective of this study was to assess the prevalence of RLS in a veteran population with SCI/D and
Methods
The institutional review and ethical approval boards of the Minneapolis VA Health Care System approved the study. Within the VA system, 666 patients with SCI/D were identified using a national database. Of the 666 people, 316 were excluded, 199 were included, and 151 were deceased.
Patients aged between 18 and 65 years were included in the study. Charts of patients who had been discharged with the diagnosis of SCI from 2002 to 2008 were studied. All patients met the inclusion criteria of the International Restless Legs Syndrome Study Group diagnosis.
Exclusion criteria were as follows: Patients with evidence of brain pathology (eg, stroke), concurrent neurologic condition associated with RLS (Parkinson disease, spinocerebellar ataxia, peripheral neuropathy), concurrent psychiatric condition within the setting of treatment with dopamine antagonists, secondary causes of RLS (renal failure/uremia, iron deficiency, rheumatoid arthritis, and pregnancy) and a recent history of alcohol or drug misuse or current evidence of substance use of < 1 year.
A patient list was compiled that included the etiology of the SCI (vascular injury, multiple sclerosis [MS], trauma, unknown, and other), the level(s) and completeness of the SCI per radiology report, RLS pharmacotherapies, and pertinent medical history.
Axial T2-weighted images on magnetic resonance imaging (MRI) scans were retrospectively reviewed. Sagittal T1/T2-weighted and axial T2-weighted sequences were performed routinely on all patients with spinal cord lesions. The analysis included the extension of the lesion on both sagittal and axial distributions. The anatomic location of the cord lesion was categorized by the following: (1) pure gray matter (central cord); (2) white matter (dorsal [D], dorsolateral [DL], ventral [V], ventrolateral areas [VL]).
A questionnaire using standard diagnostic criteria for RLS was mailed to the 199 patients who met the inclusion criteria (Appendix A).
All analyses were carried out using StataCorp STATA 13 (College Station, TX). Descriptive statistics were used. The analyses were carried out using chi-square and Fisher exact tests. Differences between the groups were considered statistically significant at P < .05. The data were analyzed to obtain point prevalence among patients with SCI, and comparisons were made among the different subgroups.
Results
Of the 162 patients who chose to participate in the study, the sleep specialists confirmed 31 (19%) to have RLS, 112 (69%) were confirmed negative for RLS, and an additional 19 (12%) screened positive for RLS but were not confirmed to have RLS by the sleep specialists (Figure 1).
The etiology of SCI was subdivided into 4 groups: MS, trauma, vascular, and other/unknown. Within each group (– RLS vs + RLS), MS and trauma were the most common etiologies with 55% MS and 36% trauma in the + RLS group.
When comparing RLS among the spinal cord levels (cervical, thoracic, lumbar and cervical + thoracic), only the cervical + thoracic subgroup (18% + RLS vs 5% – RLS) showed a significant difference (Figure 2).
There was no significant difference found with the prevalence of RLS in the axial plane of the spinal cord lesions (ventral/ventro-lateral/central cord vs dorsal/dorsolateral) or by the completeness of spinal cord lesions, P = .76. There was a higher prevalence of incomplete cord injury, however, within each subgroup of RLS.
The Mann-Whitney test was used to analyze the burden of disease in both groups (+ RLS vs – RLS). Moderate level of burden was most frequently reported with a higher prevalence within the + RLS group. Of those receiving treatment for RLS, 71% were + RLS vs 46% – RLS with a P value of .01. Symptoms of RLS after cord injury were 89% + RLS vs 55% – RLS with a P value of .03.
Discussion
This study represents one of the first studies to determine the prevalence of RLS in veterans with spinal cord disease. Research in this area is important to raise awareness of RLS among the veteran population with and without SCI and disorders. Restless legs syndrome often escapes diagnosis because of difficulty understanding the patient’s descriptions of their sensations. In addition, RLS may cause debilitating symptoms of sleep deprivation, daytime sleepiness, discomfort, and fatigue, which often results in decreased quality of life (QOL). Proper screening and treatment may improve QOL.
A study by Kumru and colleagues showed a similar rate of RLS in patients with SCI and RLS symptoms presented in the first year after SCI as did this study (18% vs 19%, respectively).4 In that study, RLS was more common in patients with lesions in lumbosacral area. Kumru and colleagues also showed that a dopaminergic medication improved symptoms of RLS in this population, whereas this study did not explore treatment outcomes.4
The pathogenesis of RLS is not fully known, but hereditary factors, iron metabolism, and the brain dopaminergic system are thought to be involved.11 It is hypothesized that spinal cord lesions allow the appearance of RLS symptoms and spinal leg movement generator by blocking descending inhibitory spinal pathways.12 One hypothesis is that damage to A11 nuclei (the main source of dopamine in the spinal cord or its diencephalospinal tract in animals) causes hyperexcitability of the spinal cord and leads to PLM and RLS symptoms.13 As the axons of A11 nuclei are present along the whole span of the spinal cord, SCI/D in patients with RLS might interrupt this dopaminergic tract and produce the RLS symptoms.
Limitations
This study included only veterans, so the prevalence may not apply to the nonveteran SCI population. Also, the population mainly was male, and there was no accurate information on race. Ferritin levels of the patients were not checked and is a major factor in RLS. The reported onset of RLS after the SCI could be due to recall bias.
Conclusion
The prevalence of RLS in veterans with SCI is above that reported in the general population (19% vs 10%, respectively). Furthermore, those with RLS have symptoms that often started after the SCI (suggesting causality) and required therapy due to their level of RLS symptom burden. A spectrum of severity of symptoms is present among those with RLS, with 83% having moderate-to-severe RLS affecting their QOL.
Although there was not a statistically significant relationship between RLS and spinal cord lesion level, there was a slightly higher prevalence of RLS at the cervical and thoracic levels, which may be relevant for future studies. There was no difference found between the RLS subgroups with respect to the location of the lesion within the spinal cord; however, a larger sample size may be needed to determine whether this would reach statistical significance. Prompt search for symptoms of RLS in veterans with SCI is warranted to provide adequate treatment to improve sleep health and QOL in this population.
1. Lasfargues JE, Custis D, Morrone F, Carswell J, Nguyen T. A model for estimating spinal cord injury prevalence in the United States. Paraplegia. 1995;33(2):62-68.
2. Sjölund BH. Pain and rehabilitation after spinal cord injury: the case of sensory spasticity? Brain Res Brain Res Rev. 2002;40(1-3):250-256.
3. Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord. 2005;43(10):577-586.
4. Kumru H, Vidal J, Benito J, et al. Restless leg syndrome in patients with spinal cord injury. Parkinsonism Relat Disord. 2015;21(12):1461-1464.
5. Wilt TJ, MacDonald R, Ouellette J, et al. Pharmacologic therapy for primary restless legs syndrome: a systematic review and meta-analysis. JAMA Intern Med. 2013;173(7):496-505.
6. American Academy of Sleep Medicine. The International Classification of Sleep Disorders: Diagnostic and Coding Manual. (AASM ICSD-3). 3rd ed. Westchester, IL: American Academy of Sleep Medicine; 2014.
7. Telles SC, Alves RC, Chadi G. Periodic limb movements during sleep and restless legs syndrome in patients with ASIA A spinal cord injury. J Neurol Sci. 2011;303(1-2):119-123.
8. Telles SC, Alves RS, Chadi G. Spinal cord injury as a trigger to develop periodic leg movements during sleep: an evolutionary perspective. Arq Neuropsiquiatr. 2012;70(11):880-884.
9. Tings T, Baier PC, Paulus W, Trenkwalder C. Restless legs syndrome induced by impairment of sensory spinal pathways. J Neurol. 2003;250(4):499-500.
10. Paulus W, Trenkwalder C. Less is more: pathophysiology of dopaminergic-therapy-related augmentation in restless legs syndrome. Lancet Neurol. 2006;5(10):878-886.
11. Silber MH, Ehrenberg BL, Allen RP, et al; Medical Advisory Board of the Restless Legs Syndrome Foundation. An algorithm for the management of restless legs syndrome. Mayo Clin Proc. 2004;79(7):916-922.
12. Hartmann M, Pfister R, Pfadenhauer K. Restless legs syndrome associated with spinal cord lesions. J Neurol Neurosurg Psychiatry. 1999;66(5):688-689.
13. Clemens S, Rye D, Hochman S. Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67(1):125-130.
1. Lasfargues JE, Custis D, Morrone F, Carswell J, Nguyen T. A model for estimating spinal cord injury prevalence in the United States. Paraplegia. 1995;33(2):62-68.
2. Sjölund BH. Pain and rehabilitation after spinal cord injury: the case of sensory spasticity? Brain Res Brain Res Rev. 2002;40(1-3):250-256.
3. Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord. 2005;43(10):577-586.
4. Kumru H, Vidal J, Benito J, et al. Restless leg syndrome in patients with spinal cord injury. Parkinsonism Relat Disord. 2015;21(12):1461-1464.
5. Wilt TJ, MacDonald R, Ouellette J, et al. Pharmacologic therapy for primary restless legs syndrome: a systematic review and meta-analysis. JAMA Intern Med. 2013;173(7):496-505.
6. American Academy of Sleep Medicine. The International Classification of Sleep Disorders: Diagnostic and Coding Manual. (AASM ICSD-3). 3rd ed. Westchester, IL: American Academy of Sleep Medicine; 2014.
7. Telles SC, Alves RC, Chadi G. Periodic limb movements during sleep and restless legs syndrome in patients with ASIA A spinal cord injury. J Neurol Sci. 2011;303(1-2):119-123.
8. Telles SC, Alves RS, Chadi G. Spinal cord injury as a trigger to develop periodic leg movements during sleep: an evolutionary perspective. Arq Neuropsiquiatr. 2012;70(11):880-884.
9. Tings T, Baier PC, Paulus W, Trenkwalder C. Restless legs syndrome induced by impairment of sensory spinal pathways. J Neurol. 2003;250(4):499-500.
10. Paulus W, Trenkwalder C. Less is more: pathophysiology of dopaminergic-therapy-related augmentation in restless legs syndrome. Lancet Neurol. 2006;5(10):878-886.
11. Silber MH, Ehrenberg BL, Allen RP, et al; Medical Advisory Board of the Restless Legs Syndrome Foundation. An algorithm for the management of restless legs syndrome. Mayo Clin Proc. 2004;79(7):916-922.
12. Hartmann M, Pfister R, Pfadenhauer K. Restless legs syndrome associated with spinal cord lesions. J Neurol Neurosurg Psychiatry. 1999;66(5):688-689.
13. Clemens S, Rye D, Hochman S. Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67(1):125-130.
More Than His Car Is Bent Out of Shape
ANSWER
The radiograph demonstrates bilateral hip dislocations. On the right, the femoral head appears to be posteriorly dislocated and slightly internally rotated. On the left, the femoral head appears to be anteriorly and superiorly dislocated (although evaluation is limited by a single view). Neither side appears to have any obvious fractures.
The patient’s dislocations were promptly reduced in the trauma bay by the orthopedic service before he was sent for CT.

ANSWER
The radiograph demonstrates bilateral hip dislocations. On the right, the femoral head appears to be posteriorly dislocated and slightly internally rotated. On the left, the femoral head appears to be anteriorly and superiorly dislocated (although evaluation is limited by a single view). Neither side appears to have any obvious fractures.
The patient’s dislocations were promptly reduced in the trauma bay by the orthopedic service before he was sent for CT.

ANSWER
The radiograph demonstrates bilateral hip dislocations. On the right, the femoral head appears to be posteriorly dislocated and slightly internally rotated. On the left, the femoral head appears to be anteriorly and superiorly dislocated (although evaluation is limited by a single view). Neither side appears to have any obvious fractures.
The patient’s dislocations were promptly reduced in the trauma bay by the orthopedic service before he was sent for CT.

A 30-year-old man is broug
Upon arrival, he is immediately intubated because emergency personnel had difficulty intubating him in the field. He has a Glasgow Coma Scale score of 3T. The patient’s blood pressure is 90/40 mm Hg and his heart rate, 150 beats/min. He appears to have deformities in his lower extremities.
You obtain portable radiographs of the chest and pelvis. The latter is shown. What is your impression?
Bariatric surgery may be appropriate for class 1 obesity
LAS VEGAS – Once reserved for the most obese patients, bariatric surgery is on the road to becoming an option for millions of Americans who are just a step beyond overweight, even those with a body mass index as low as 30 kg/m2.
In regard to patients with lower levels of obesity, “we should be intervening in this chronic disease earlier rather than later,” said Stacy A. Brethauer, MD, professor of surgery at the Ohio State University, Columbus, in a presentation about new standards for bariatric surgery at the 2019 Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
Bariatric treatment “should be offered after nonsurgical [weight-loss] therapy has failed,” he said. “That’s not where you stop. You continue to escalate as you would for heart disease or cancer.”
As Dr. Brethauer noted, research suggests that all categories of obesity – including so-called class 1 obesity (defined as a BMI from 30.0 to 34.9 kg/m2) – boost the risk of multiple diseases, including hypertension, coronary artery disease, congestive heart failure, stroke, asthma, pulmonary embolism, gallbladder disease, several types of cancer, osteoarthritis, knee pain and chronic back pain.
“There is no question that class 1 obesity is clearly putting people at risk,” he said. “Ultimately, you can conclude from all this evidence that class 1 is a chronic disease, and it deserves to be treated effectively.”
There are, of course, various nonsurgical treatments for obesity, including diet and exercise and pharmacotherapy. However, systematic reviews have found that people find it extremely difficult to keep the weight off after 1 year regardless of the strategy they adopt.
Beyond a year, Dr. Brethauer said, “you get poor maintenance of weight control, and you get poor control of metabolic burden. You don’t have a durable efficacy.”
In the past, bariatric surgery wasn’t considered an option for patients with class 1 obesity. It’s traditionally been reserved for patients with BMIs at or above 35 kg/m2. But this standard has evolved in recent years.
In 2018, Dr. Brethauer coauthored an updated position statement by the American Society for Metabolic and Bariatric Surgery that encouraged bariatric surgery in certain mildly obese patients.
“For most people with class I obesity,” the statement on bariatric surgery states, “it is clear that the nonsurgical group of therapies will not provide a durable solution to their disease of obesity.”
The statement went on to say that “surgical intervention should be considered after failure of nonsurgical treatments” in the class 1 population.
Bariatric surgery in the class 1 population does more than reduce obesity, Dr. Brethauer said. “Over the last 5 years or so, a large body of literature has emerged,” he said, and both systematic reviews and randomized trails have shown significant postsurgery improvements in comorbidities such as diabetes.
“It’s important to emphasize that these patients don’t become underweight,” he said. “The body finds a healthy set point. They don’t become underweight or malnourished because you’re operating on a lower-weight group.”
Are weight-loss operations safe in class 1 patients? The American Society for Metabolic and Bariatric Surgery statement says that research has found “bariatric surgery is associated with modest morbidity and very low mortality in patients with class I obesity.”
In fact, Dr. Brethauer said, the mortality rate in this population is “less than gallbladder surgery, less than hip surgery, less than hysterectomy, less than knee surgery – operations people are being referred for and undergoing all the time.”
He added: “The case can be made very clearly based on this data that these operations are safe in this patient population. Not only are they safe, they have durable and significant impact on comorbidities.”
Global Academy for Medical Education and this news organization are owned by the same parent company. Dr. Brethauer discloses relationships with Medtronic (speaker) and GI Windows (consultant).
LAS VEGAS – Once reserved for the most obese patients, bariatric surgery is on the road to becoming an option for millions of Americans who are just a step beyond overweight, even those with a body mass index as low as 30 kg/m2.
In regard to patients with lower levels of obesity, “we should be intervening in this chronic disease earlier rather than later,” said Stacy A. Brethauer, MD, professor of surgery at the Ohio State University, Columbus, in a presentation about new standards for bariatric surgery at the 2019 Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
Bariatric treatment “should be offered after nonsurgical [weight-loss] therapy has failed,” he said. “That’s not where you stop. You continue to escalate as you would for heart disease or cancer.”
As Dr. Brethauer noted, research suggests that all categories of obesity – including so-called class 1 obesity (defined as a BMI from 30.0 to 34.9 kg/m2) – boost the risk of multiple diseases, including hypertension, coronary artery disease, congestive heart failure, stroke, asthma, pulmonary embolism, gallbladder disease, several types of cancer, osteoarthritis, knee pain and chronic back pain.
“There is no question that class 1 obesity is clearly putting people at risk,” he said. “Ultimately, you can conclude from all this evidence that class 1 is a chronic disease, and it deserves to be treated effectively.”
There are, of course, various nonsurgical treatments for obesity, including diet and exercise and pharmacotherapy. However, systematic reviews have found that people find it extremely difficult to keep the weight off after 1 year regardless of the strategy they adopt.
Beyond a year, Dr. Brethauer said, “you get poor maintenance of weight control, and you get poor control of metabolic burden. You don’t have a durable efficacy.”
In the past, bariatric surgery wasn’t considered an option for patients with class 1 obesity. It’s traditionally been reserved for patients with BMIs at or above 35 kg/m2. But this standard has evolved in recent years.
In 2018, Dr. Brethauer coauthored an updated position statement by the American Society for Metabolic and Bariatric Surgery that encouraged bariatric surgery in certain mildly obese patients.
“For most people with class I obesity,” the statement on bariatric surgery states, “it is clear that the nonsurgical group of therapies will not provide a durable solution to their disease of obesity.”
The statement went on to say that “surgical intervention should be considered after failure of nonsurgical treatments” in the class 1 population.
Bariatric surgery in the class 1 population does more than reduce obesity, Dr. Brethauer said. “Over the last 5 years or so, a large body of literature has emerged,” he said, and both systematic reviews and randomized trails have shown significant postsurgery improvements in comorbidities such as diabetes.
“It’s important to emphasize that these patients don’t become underweight,” he said. “The body finds a healthy set point. They don’t become underweight or malnourished because you’re operating on a lower-weight group.”
Are weight-loss operations safe in class 1 patients? The American Society for Metabolic and Bariatric Surgery statement says that research has found “bariatric surgery is associated with modest morbidity and very low mortality in patients with class I obesity.”
In fact, Dr. Brethauer said, the mortality rate in this population is “less than gallbladder surgery, less than hip surgery, less than hysterectomy, less than knee surgery – operations people are being referred for and undergoing all the time.”
He added: “The case can be made very clearly based on this data that these operations are safe in this patient population. Not only are they safe, they have durable and significant impact on comorbidities.”
Global Academy for Medical Education and this news organization are owned by the same parent company. Dr. Brethauer discloses relationships with Medtronic (speaker) and GI Windows (consultant).
LAS VEGAS – Once reserved for the most obese patients, bariatric surgery is on the road to becoming an option for millions of Americans who are just a step beyond overweight, even those with a body mass index as low as 30 kg/m2.
In regard to patients with lower levels of obesity, “we should be intervening in this chronic disease earlier rather than later,” said Stacy A. Brethauer, MD, professor of surgery at the Ohio State University, Columbus, in a presentation about new standards for bariatric surgery at the 2019 Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
Bariatric treatment “should be offered after nonsurgical [weight-loss] therapy has failed,” he said. “That’s not where you stop. You continue to escalate as you would for heart disease or cancer.”
As Dr. Brethauer noted, research suggests that all categories of obesity – including so-called class 1 obesity (defined as a BMI from 30.0 to 34.9 kg/m2) – boost the risk of multiple diseases, including hypertension, coronary artery disease, congestive heart failure, stroke, asthma, pulmonary embolism, gallbladder disease, several types of cancer, osteoarthritis, knee pain and chronic back pain.
“There is no question that class 1 obesity is clearly putting people at risk,” he said. “Ultimately, you can conclude from all this evidence that class 1 is a chronic disease, and it deserves to be treated effectively.”
There are, of course, various nonsurgical treatments for obesity, including diet and exercise and pharmacotherapy. However, systematic reviews have found that people find it extremely difficult to keep the weight off after 1 year regardless of the strategy they adopt.
Beyond a year, Dr. Brethauer said, “you get poor maintenance of weight control, and you get poor control of metabolic burden. You don’t have a durable efficacy.”
In the past, bariatric surgery wasn’t considered an option for patients with class 1 obesity. It’s traditionally been reserved for patients with BMIs at or above 35 kg/m2. But this standard has evolved in recent years.
In 2018, Dr. Brethauer coauthored an updated position statement by the American Society for Metabolic and Bariatric Surgery that encouraged bariatric surgery in certain mildly obese patients.
“For most people with class I obesity,” the statement on bariatric surgery states, “it is clear that the nonsurgical group of therapies will not provide a durable solution to their disease of obesity.”
The statement went on to say that “surgical intervention should be considered after failure of nonsurgical treatments” in the class 1 population.
Bariatric surgery in the class 1 population does more than reduce obesity, Dr. Brethauer said. “Over the last 5 years or so, a large body of literature has emerged,” he said, and both systematic reviews and randomized trails have shown significant postsurgery improvements in comorbidities such as diabetes.
“It’s important to emphasize that these patients don’t become underweight,” he said. “The body finds a healthy set point. They don’t become underweight or malnourished because you’re operating on a lower-weight group.”
Are weight-loss operations safe in class 1 patients? The American Society for Metabolic and Bariatric Surgery statement says that research has found “bariatric surgery is associated with modest morbidity and very low mortality in patients with class I obesity.”
In fact, Dr. Brethauer said, the mortality rate in this population is “less than gallbladder surgery, less than hip surgery, less than hysterectomy, less than knee surgery – operations people are being referred for and undergoing all the time.”
He added: “The case can be made very clearly based on this data that these operations are safe in this patient population. Not only are they safe, they have durable and significant impact on comorbidities.”
Global Academy for Medical Education and this news organization are owned by the same parent company. Dr. Brethauer discloses relationships with Medtronic (speaker) and GI Windows (consultant).
REPORTING FROM MISS
After a Need for Speed, a Spine Entwined?
ANSWER
The radiograph demonstrates a comminuted fracture of the superior end plate of L1. There is a loss of height of about 20% to 25%.
Of note, there is a lucency that appears to extend horizontally through the pedicles and into the spinous process. If this observation is accurate, then the patient would have a three-column injury. Such fractures are known as Chance fractures, and they are typically unstable and require operative intervention for stabilization.
Chance fractures are better visualized on CT. Subsequent testing

ANSWER
The radiograph demonstrates a comminuted fracture of the superior end plate of L1. There is a loss of height of about 20% to 25%.
Of note, there is a lucency that appears to extend horizontally through the pedicles and into the spinous process. If this observation is accurate, then the patient would have a three-column injury. Such fractures are known as Chance fractures, and they are typically unstable and require operative intervention for stabilization.
Chance fractures are better visualized on CT. Subsequent testing

ANSWER
The radiograph demonstrates a comminuted fracture of the superior end plate of L1. There is a loss of height of about 20% to 25%.
Of note, there is a lucency that appears to extend horizontally through the pedicles and into the spinous process. If this observation is accurate, then the patient would have a three-column injury. Such fractures are known as Chance fractures, and they are typically unstable and require operative intervention for stabilization.
Chance fractures are better visualized on CT. Subsequent testing
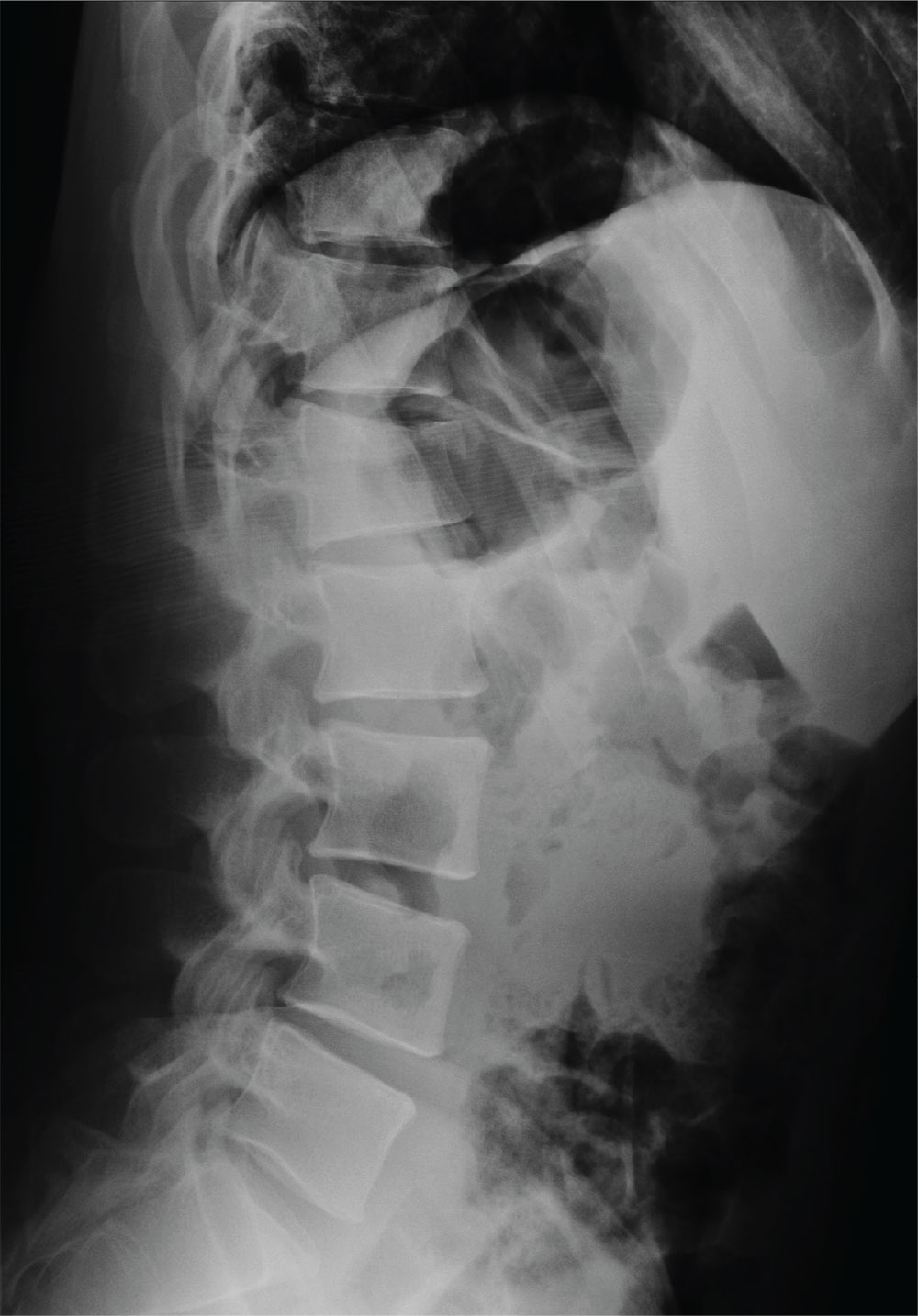
A 28-year-old man is brought to your emergency department via ambulance after a motor vehicle accident. The patient was a restrained driver who was driving too fast, lost control, and ended up in a ditch. His vehicle then rolled over several times. He reportedly self-extricated and was ambulatory at the scene.
His primary complaints include left shoulder, chest wall, and back pain. He denies any significant medical history.
On physical examination, you note a man with normal vital signs who, although slightly anxious, is in no obvious distress. He has moderate tenderness over the left shoulder and sternum and at the thoracolumbar juncture. No step-offs are appreciated. He is able to move all extremities and is neurovascularly intact.
As you await lab results, you obtain a lateral radiograph of the lumbar spine (shown). What is your impression?