User login
‘Lopioid protocol’ – low-dose opioids – proposed for fracture surgery
In a paper presented at the annual meeting of the American Academy of Orthopaedic Surgeons, researchers from NYU reported on the implementation of their multimodal strategy, dubbed the “lopioid protocol.”
According to the 2019 National Survey on Drug Use and Health, orthopedic surgeons are the third-highest opioid prescribers in the United States.
Kennneth A. Egol, MD, vice chair of the department of orthopedic surgery at NYU, who is the first author of the study, was motivated to help create the protocol following misconceptions that orthopedic surgeons were helping to fuel the opioid epidemic.
Dr. Egol pointed to the year 1995, when pain became the fifth vital sign after body temperature, pulse rate, respiratory rate, and blood pressure.
Since then, in light of the opioid epidemic, the focus of physicians has shifted away from prescribing strong pain medication and reducing pain scores to zero to instead reducing pain to a manageable level.
Reducing opioid prescriptions can be challenging when patients are prescribed an anti-inflammatory and they subsequently ask their physician for a “pain pill.” Patients sometimes don’t understand that inflammation is what causes pain.
It can also be difficult to convince patients that medications that they can buy over the counter can adequately control their pain, as confirmed in numerous studies.
Multimodal pain therapy aims to reduce the need for opioids by supplementing their use with other oral medications and, at times, long-lasting regional nerve blocks.
Anti-inflammatories act at the site of injury or surgery where inflammation is occurring. Nerves then carry the pain signal to the brain. These signals can be dampened by medications such as gabapentin that act on the nerves themselves. The pain signal is received in the brain, where opioids act by binding to receptors in the brain.
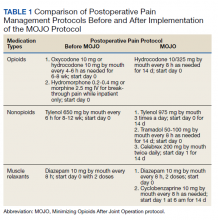
The so-called lopioid protocol does not eliminate opioids completely but rather uses “safer” opioids, such as tramadol, in lieu of stronger narcotics.
The protocol began at NYU on Jan. 1, 2019. It consists in the prescribing of tramadol, meloxicam, gabapentin, and acetaminophen.
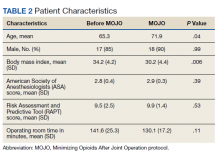
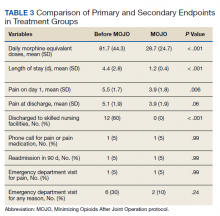
The study presented at the AAOS meeting demonstrated statistically significant reductions in visual analogue pain scores at discharge and subsequent medication refills for the 931 patients in the lopioid group, compared with a group of 848 patients who received narcotic prescriptions containing oxycodone from the year prior to the protocol initiation.
Educating patients on the rationale for the prescription combination can help to allay their fears. Dr. Egol thinks it’s important for physicians to explain the dangers of opioids to patients. He said in an interview that he also believes surgeons need to “give [patients] an understanding of why we are pursuing these protocols. They also need to know we will not ignore their pain and concerns.”
Brannon Orton, MD, is an orthopedic surgeon at Confluence Health, in Moses Lake, Wash. He sees a large number of trauma patients and thinks NYU is doing a good job of addressing a difficult problem in orthopedics – especially in the field of trauma.
He said in an interview: “Managing narcotics postoperatively can be challenging due to the fact that many people come into these fractures with a history of narcotic use.” Not only are they used to turning to opioids for pain relief, but they also may have built up a tolerance to them.
Although he hasn’t been using the lopioid protocol specifically, he has been following a multimodal approach regarding the postoperative use of narcotics. Of the study by Dr. Egol and colleagues, he said, “I think their paper presents an effective way of decreasing use of oral narcotics and still adequately managing patients’ pain postoperatively.” Dr. Orton’s own practice utilizes tramadol, acetaminophen, and ibuprofen after fracture surgery.
From Dr. Orton’s perspective, a significant challenge in implementing the lopioid protocol in practice is simply sticking to the plan. “It can become difficult when patients are pressuring staff or physicians for more narcotics. However, I feel that if everybody is on the same page with the plan, then it can be very doable.”
Dr. Egol and NYU try to limit narcotic prescriptions beginning with the patient’s initial visit to the ED. The ED physicians at his institution only “prescribe small amounts of narcotics. Our ED people really limit the amount of opioids prescribed.”
Dr. Egol recommends that all practitioners begin with nonnarcotic medication, even if treating a fracture nonoperatively. “Start low and go higher. I always try to start with NSAIDs and Tylenol,” he said.
Dr. Egol and Dr. Orton reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
In a paper presented at the annual meeting of the American Academy of Orthopaedic Surgeons, researchers from NYU reported on the implementation of their multimodal strategy, dubbed the “lopioid protocol.”
According to the 2019 National Survey on Drug Use and Health, orthopedic surgeons are the third-highest opioid prescribers in the United States.
Kennneth A. Egol, MD, vice chair of the department of orthopedic surgery at NYU, who is the first author of the study, was motivated to help create the protocol following misconceptions that orthopedic surgeons were helping to fuel the opioid epidemic.
Dr. Egol pointed to the year 1995, when pain became the fifth vital sign after body temperature, pulse rate, respiratory rate, and blood pressure.
Since then, in light of the opioid epidemic, the focus of physicians has shifted away from prescribing strong pain medication and reducing pain scores to zero to instead reducing pain to a manageable level.
Reducing opioid prescriptions can be challenging when patients are prescribed an anti-inflammatory and they subsequently ask their physician for a “pain pill.” Patients sometimes don’t understand that inflammation is what causes pain.
It can also be difficult to convince patients that medications that they can buy over the counter can adequately control their pain, as confirmed in numerous studies.
Multimodal pain therapy aims to reduce the need for opioids by supplementing their use with other oral medications and, at times, long-lasting regional nerve blocks.
Anti-inflammatories act at the site of injury or surgery where inflammation is occurring. Nerves then carry the pain signal to the brain. These signals can be dampened by medications such as gabapentin that act on the nerves themselves. The pain signal is received in the brain, where opioids act by binding to receptors in the brain.
The so-called lopioid protocol does not eliminate opioids completely but rather uses “safer” opioids, such as tramadol, in lieu of stronger narcotics.
The protocol began at NYU on Jan. 1, 2019. It consists in the prescribing of tramadol, meloxicam, gabapentin, and acetaminophen.
The study presented at the AAOS meeting demonstrated statistically significant reductions in visual analogue pain scores at discharge and subsequent medication refills for the 931 patients in the lopioid group, compared with a group of 848 patients who received narcotic prescriptions containing oxycodone from the year prior to the protocol initiation.
Educating patients on the rationale for the prescription combination can help to allay their fears. Dr. Egol thinks it’s important for physicians to explain the dangers of opioids to patients. He said in an interview that he also believes surgeons need to “give [patients] an understanding of why we are pursuing these protocols. They also need to know we will not ignore their pain and concerns.”
Brannon Orton, MD, is an orthopedic surgeon at Confluence Health, in Moses Lake, Wash. He sees a large number of trauma patients and thinks NYU is doing a good job of addressing a difficult problem in orthopedics – especially in the field of trauma.
He said in an interview: “Managing narcotics postoperatively can be challenging due to the fact that many people come into these fractures with a history of narcotic use.” Not only are they used to turning to opioids for pain relief, but they also may have built up a tolerance to them.
Although he hasn’t been using the lopioid protocol specifically, he has been following a multimodal approach regarding the postoperative use of narcotics. Of the study by Dr. Egol and colleagues, he said, “I think their paper presents an effective way of decreasing use of oral narcotics and still adequately managing patients’ pain postoperatively.” Dr. Orton’s own practice utilizes tramadol, acetaminophen, and ibuprofen after fracture surgery.
From Dr. Orton’s perspective, a significant challenge in implementing the lopioid protocol in practice is simply sticking to the plan. “It can become difficult when patients are pressuring staff or physicians for more narcotics. However, I feel that if everybody is on the same page with the plan, then it can be very doable.”
Dr. Egol and NYU try to limit narcotic prescriptions beginning with the patient’s initial visit to the ED. The ED physicians at his institution only “prescribe small amounts of narcotics. Our ED people really limit the amount of opioids prescribed.”
Dr. Egol recommends that all practitioners begin with nonnarcotic medication, even if treating a fracture nonoperatively. “Start low and go higher. I always try to start with NSAIDs and Tylenol,” he said.
Dr. Egol and Dr. Orton reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
In a paper presented at the annual meeting of the American Academy of Orthopaedic Surgeons, researchers from NYU reported on the implementation of their multimodal strategy, dubbed the “lopioid protocol.”
According to the 2019 National Survey on Drug Use and Health, orthopedic surgeons are the third-highest opioid prescribers in the United States.
Kennneth A. Egol, MD, vice chair of the department of orthopedic surgery at NYU, who is the first author of the study, was motivated to help create the protocol following misconceptions that orthopedic surgeons were helping to fuel the opioid epidemic.
Dr. Egol pointed to the year 1995, when pain became the fifth vital sign after body temperature, pulse rate, respiratory rate, and blood pressure.
Since then, in light of the opioid epidemic, the focus of physicians has shifted away from prescribing strong pain medication and reducing pain scores to zero to instead reducing pain to a manageable level.
Reducing opioid prescriptions can be challenging when patients are prescribed an anti-inflammatory and they subsequently ask their physician for a “pain pill.” Patients sometimes don’t understand that inflammation is what causes pain.
It can also be difficult to convince patients that medications that they can buy over the counter can adequately control their pain, as confirmed in numerous studies.
Multimodal pain therapy aims to reduce the need for opioids by supplementing their use with other oral medications and, at times, long-lasting regional nerve blocks.
Anti-inflammatories act at the site of injury or surgery where inflammation is occurring. Nerves then carry the pain signal to the brain. These signals can be dampened by medications such as gabapentin that act on the nerves themselves. The pain signal is received in the brain, where opioids act by binding to receptors in the brain.
The so-called lopioid protocol does not eliminate opioids completely but rather uses “safer” opioids, such as tramadol, in lieu of stronger narcotics.
The protocol began at NYU on Jan. 1, 2019. It consists in the prescribing of tramadol, meloxicam, gabapentin, and acetaminophen.
The study presented at the AAOS meeting demonstrated statistically significant reductions in visual analogue pain scores at discharge and subsequent medication refills for the 931 patients in the lopioid group, compared with a group of 848 patients who received narcotic prescriptions containing oxycodone from the year prior to the protocol initiation.
Educating patients on the rationale for the prescription combination can help to allay their fears. Dr. Egol thinks it’s important for physicians to explain the dangers of opioids to patients. He said in an interview that he also believes surgeons need to “give [patients] an understanding of why we are pursuing these protocols. They also need to know we will not ignore their pain and concerns.”
Brannon Orton, MD, is an orthopedic surgeon at Confluence Health, in Moses Lake, Wash. He sees a large number of trauma patients and thinks NYU is doing a good job of addressing a difficult problem in orthopedics – especially in the field of trauma.
He said in an interview: “Managing narcotics postoperatively can be challenging due to the fact that many people come into these fractures with a history of narcotic use.” Not only are they used to turning to opioids for pain relief, but they also may have built up a tolerance to them.
Although he hasn’t been using the lopioid protocol specifically, he has been following a multimodal approach regarding the postoperative use of narcotics. Of the study by Dr. Egol and colleagues, he said, “I think their paper presents an effective way of decreasing use of oral narcotics and still adequately managing patients’ pain postoperatively.” Dr. Orton’s own practice utilizes tramadol, acetaminophen, and ibuprofen after fracture surgery.
From Dr. Orton’s perspective, a significant challenge in implementing the lopioid protocol in practice is simply sticking to the plan. “It can become difficult when patients are pressuring staff or physicians for more narcotics. However, I feel that if everybody is on the same page with the plan, then it can be very doable.”
Dr. Egol and NYU try to limit narcotic prescriptions beginning with the patient’s initial visit to the ED. The ED physicians at his institution only “prescribe small amounts of narcotics. Our ED people really limit the amount of opioids prescribed.”
Dr. Egol recommends that all practitioners begin with nonnarcotic medication, even if treating a fracture nonoperatively. “Start low and go higher. I always try to start with NSAIDs and Tylenol,” he said.
Dr. Egol and Dr. Orton reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
High tibial osteotomy achieves sustained improvements in knee OA
A study of long-term outcomes after medial opening wedge high tibial osteotomy for knee osteoarthritis suggests the procedure is associated with significant and sustained improvements in pain, function, quality of life, and gait biomechanics.
At the OARSI 2021 World Congress, PhD candidate Codie Primeau, MSc, of the Fowler Kennedy Sport Medicine Clinic at the University of Western Ontario, London, presented the findings from a 10-year prospective cohort study of 102 patients with symptomatic medial compartment knee osteoarthritis who underwent medial opening wedge high tibial osteotomy but did not get a total knee replacement during the study.
The surgical procedure aims to correct malalignment by redistributing knee joint loads away from the affected compartment of the knee, with the ultimate goal of slowing disease progression and improving pain and function, Mr. Primeau told the conference, which was sponsored by the Osteoarthritis Research Society International.
At 10 years, the procedure was associated with a mean 14.3-point improvement in the 0-100 Knee Injury and Osteoarthritis Outcome Score (KOOS) for pain, a mean 12-point improvement in the score for function in daily living, a 15.5-point improvement in the score for function in sport and recreation, and a 24.5-point improvement in knee-related quality of life score. Researchers also saw a 35%-45% reduction in the magnitude of the external knee adduction moment from baseline, and a gradual reduction in the knee flexion moment over the course of the study.
While the improvements did decline somewhat over the 10 years, 53% of patients still met the criteria for responder at the end of the follow-up period, meaning that they had a relative change of at least 20% in both KOOS pain and function scores, and an absolute change of at least 10 points.
Mr. Primeau noted that the patient population represented those who were the best candidates for high tibial osteotomy, in that they were keen to avoid total knee replacement.
“While these types of patients may have the best outcomes, our studies suggest patients traditionally not considered ideal candidates for HTO [high tibial osteotomy] – such as females, and patients with limited disease in other knee compartments – also have large improvements in pain and function after HTO, and around 70% of those patients do not get a total knee replacement within 10 years,” he said in an interview.
Mr. Primeau suggested that the improvements achieved with high tibial osteotomy might extend the time before a knee replacement is required, or even help some patients avoid it altogether.
“Importantly, recent studies show HTO does not complicate future joint replacement surgery,” he said. “One can get a knee replacement after HTO; the reverse is not possible.”
The ideal patient for a high tibial osteotomy would be one whose osteoarthritis was confined to the medial compartment of the knee, was younger – in their 40s or 50s – and with relatively high activity levels, he said. Some studies also suggest better outcomes in men than women.
In response to an audience question about the rehabilitation requirements after high tibial osteotomy, Mr. Primeau commented that the design of the plates used in the procedure have changed over time, and this has influenced rehabilitation needs. When the study began, patients needed anywhere from 8 to 12 weeks of no weight bearing, using crutches, to allow for bone consolidation to occur.
“Since then, plate designs have changed a lot, and patients are able to start ambulating as early as 2 weeks after the surgery now,” he said. The rehabilitation is similar to what is required for knee osteoarthritis in general, focusing on range of motion, strengthening, proprioception, and muscle training.
No conflicts of interest were declared.
A study of long-term outcomes after medial opening wedge high tibial osteotomy for knee osteoarthritis suggests the procedure is associated with significant and sustained improvements in pain, function, quality of life, and gait biomechanics.
At the OARSI 2021 World Congress, PhD candidate Codie Primeau, MSc, of the Fowler Kennedy Sport Medicine Clinic at the University of Western Ontario, London, presented the findings from a 10-year prospective cohort study of 102 patients with symptomatic medial compartment knee osteoarthritis who underwent medial opening wedge high tibial osteotomy but did not get a total knee replacement during the study.
The surgical procedure aims to correct malalignment by redistributing knee joint loads away from the affected compartment of the knee, with the ultimate goal of slowing disease progression and improving pain and function, Mr. Primeau told the conference, which was sponsored by the Osteoarthritis Research Society International.
At 10 years, the procedure was associated with a mean 14.3-point improvement in the 0-100 Knee Injury and Osteoarthritis Outcome Score (KOOS) for pain, a mean 12-point improvement in the score for function in daily living, a 15.5-point improvement in the score for function in sport and recreation, and a 24.5-point improvement in knee-related quality of life score. Researchers also saw a 35%-45% reduction in the magnitude of the external knee adduction moment from baseline, and a gradual reduction in the knee flexion moment over the course of the study.
While the improvements did decline somewhat over the 10 years, 53% of patients still met the criteria for responder at the end of the follow-up period, meaning that they had a relative change of at least 20% in both KOOS pain and function scores, and an absolute change of at least 10 points.
Mr. Primeau noted that the patient population represented those who were the best candidates for high tibial osteotomy, in that they were keen to avoid total knee replacement.
“While these types of patients may have the best outcomes, our studies suggest patients traditionally not considered ideal candidates for HTO [high tibial osteotomy] – such as females, and patients with limited disease in other knee compartments – also have large improvements in pain and function after HTO, and around 70% of those patients do not get a total knee replacement within 10 years,” he said in an interview.
Mr. Primeau suggested that the improvements achieved with high tibial osteotomy might extend the time before a knee replacement is required, or even help some patients avoid it altogether.
“Importantly, recent studies show HTO does not complicate future joint replacement surgery,” he said. “One can get a knee replacement after HTO; the reverse is not possible.”
The ideal patient for a high tibial osteotomy would be one whose osteoarthritis was confined to the medial compartment of the knee, was younger – in their 40s or 50s – and with relatively high activity levels, he said. Some studies also suggest better outcomes in men than women.
In response to an audience question about the rehabilitation requirements after high tibial osteotomy, Mr. Primeau commented that the design of the plates used in the procedure have changed over time, and this has influenced rehabilitation needs. When the study began, patients needed anywhere from 8 to 12 weeks of no weight bearing, using crutches, to allow for bone consolidation to occur.
“Since then, plate designs have changed a lot, and patients are able to start ambulating as early as 2 weeks after the surgery now,” he said. The rehabilitation is similar to what is required for knee osteoarthritis in general, focusing on range of motion, strengthening, proprioception, and muscle training.
No conflicts of interest were declared.
A study of long-term outcomes after medial opening wedge high tibial osteotomy for knee osteoarthritis suggests the procedure is associated with significant and sustained improvements in pain, function, quality of life, and gait biomechanics.
At the OARSI 2021 World Congress, PhD candidate Codie Primeau, MSc, of the Fowler Kennedy Sport Medicine Clinic at the University of Western Ontario, London, presented the findings from a 10-year prospective cohort study of 102 patients with symptomatic medial compartment knee osteoarthritis who underwent medial opening wedge high tibial osteotomy but did not get a total knee replacement during the study.
The surgical procedure aims to correct malalignment by redistributing knee joint loads away from the affected compartment of the knee, with the ultimate goal of slowing disease progression and improving pain and function, Mr. Primeau told the conference, which was sponsored by the Osteoarthritis Research Society International.
At 10 years, the procedure was associated with a mean 14.3-point improvement in the 0-100 Knee Injury and Osteoarthritis Outcome Score (KOOS) for pain, a mean 12-point improvement in the score for function in daily living, a 15.5-point improvement in the score for function in sport and recreation, and a 24.5-point improvement in knee-related quality of life score. Researchers also saw a 35%-45% reduction in the magnitude of the external knee adduction moment from baseline, and a gradual reduction in the knee flexion moment over the course of the study.
While the improvements did decline somewhat over the 10 years, 53% of patients still met the criteria for responder at the end of the follow-up period, meaning that they had a relative change of at least 20% in both KOOS pain and function scores, and an absolute change of at least 10 points.
Mr. Primeau noted that the patient population represented those who were the best candidates for high tibial osteotomy, in that they were keen to avoid total knee replacement.
“While these types of patients may have the best outcomes, our studies suggest patients traditionally not considered ideal candidates for HTO [high tibial osteotomy] – such as females, and patients with limited disease in other knee compartments – also have large improvements in pain and function after HTO, and around 70% of those patients do not get a total knee replacement within 10 years,” he said in an interview.
Mr. Primeau suggested that the improvements achieved with high tibial osteotomy might extend the time before a knee replacement is required, or even help some patients avoid it altogether.
“Importantly, recent studies show HTO does not complicate future joint replacement surgery,” he said. “One can get a knee replacement after HTO; the reverse is not possible.”
The ideal patient for a high tibial osteotomy would be one whose osteoarthritis was confined to the medial compartment of the knee, was younger – in their 40s or 50s – and with relatively high activity levels, he said. Some studies also suggest better outcomes in men than women.
In response to an audience question about the rehabilitation requirements after high tibial osteotomy, Mr. Primeau commented that the design of the plates used in the procedure have changed over time, and this has influenced rehabilitation needs. When the study began, patients needed anywhere from 8 to 12 weeks of no weight bearing, using crutches, to allow for bone consolidation to occur.
“Since then, plate designs have changed a lot, and patients are able to start ambulating as early as 2 weeks after the surgery now,” he said. The rehabilitation is similar to what is required for knee osteoarthritis in general, focusing on range of motion, strengthening, proprioception, and muscle training.
No conflicts of interest were declared.
FROM OARSI 2021
Intramuscular glucocorticoid injections seen as noninferior to intra-articular in knee OA
Intramuscular injections of glucocorticoids have efficacy similar to that of intra-articular injections in reducing pain in knee osteoarthritis but without the concerns about joint infection and the challenges of administration, according to results from a randomized, controlled trial reported at the OARSI 2021 World Congress.
Intra-articular injections of glucocorticoids are commonly used to relieve OA pain, but some general practitioners have difficulty administering them to patients, said Qiuke Wang, a PhD candidate at Erasmus University Medical Center in Rotterdam, the Netherlands. There are also concerns about whether intra-articular injections may cause damage to knee cartilage, Mr. Wang said at the conference, which is sponsored by the Osteoarthritis Research Society International.
Mr. Wang and colleagues conducted a randomized, controlled trial in which 145 patients with symptomatic knee OA received either an intramuscular or intra-articular injection of 40 mg triamcinolone acetonide, and then followed up at regular intervals for 24 weeks.
The study showed that Knee Injury and Osteoarthritis Outcome Scores for pain improved in both the intra-articular and intramuscular groups. Improvements in pain scores peaked in the intra-articular injection group at the 4-week mark, when the difference with intramuscular injections was statistically significant. However, the two groups showed no significant differences in pain improvement at the 8-, 12-, and 24-week follow-up points.
“Intra-articular injection can act immediately on inhibiting joint inflammation after injection,” Mr. Wang said in an interview. “In contrast, for intramuscular injection, glucocorticoid needs firstly to be absorbed by muscle into blood and then travel into the knee via the circulatory system.”
The study also showed no significant differences between the two groups in the secondary outcomes of patient symptoms, stiffness, function, and sport and quality of life scores. There were more adverse events in the intra-articular injection group: 42% of patients reported an adverse event, compared to 33% in the intramuscular group, and the adverse events reported in the intramuscular group were nonserious events, such as headache and flushing.
Mr. Wang told the conference that while the intramuscular injection was inferior to intra-articular injections at 4 weeks, it was noninferior at 8 and 24 weeks and should be considered an effective way to reduce pain in patients with knee OA.
“This trial provides evidence for shared decision making because in some cases a patient may have a preference for specific injection and the GP may feel incompetent to administer the intra-articular injection,” he said.
An audience member pointed out that there was now a growing body of evidence suggesting that intra-articular injections may contribute to faster progression of knee OA because of effects on knee cartilage.
Mr. Wang acknowledged that their own research had shown these side effects of intra-articular injections, which was why the trial was intended to examine whether intramuscular injections might achieve the same pain relief.
“In the real practice, I would say that both injections are effective, but the intra-articular injection may provide a slightly [better] effect in the short term,” he said.
Commenting on the findings, Martin van der Esch, PhD, of Amsterdam University of Applied Sciences, said there were no guidelines as to whether intra-articular or intramuscular injections were the best option, so it really came down to the clinician’s decision.
“Therefore this is really an interesting study, because it gives some light – not the answer – but some light in what direction it could go for specific groups of patients,” Dr. van der Esch said in an interview.
Dr. van der Esch suggested that intramuscular injections might be more appropriate for patients with more systemic disease affecting multiple joints, but intra-articular injections might offer greater benefits in a patient with severe and long-lasting disease in a single joint.
No conflicts of interest were declared.
Intramuscular injections of glucocorticoids have efficacy similar to that of intra-articular injections in reducing pain in knee osteoarthritis but without the concerns about joint infection and the challenges of administration, according to results from a randomized, controlled trial reported at the OARSI 2021 World Congress.
Intra-articular injections of glucocorticoids are commonly used to relieve OA pain, but some general practitioners have difficulty administering them to patients, said Qiuke Wang, a PhD candidate at Erasmus University Medical Center in Rotterdam, the Netherlands. There are also concerns about whether intra-articular injections may cause damage to knee cartilage, Mr. Wang said at the conference, which is sponsored by the Osteoarthritis Research Society International.
Mr. Wang and colleagues conducted a randomized, controlled trial in which 145 patients with symptomatic knee OA received either an intramuscular or intra-articular injection of 40 mg triamcinolone acetonide, and then followed up at regular intervals for 24 weeks.
The study showed that Knee Injury and Osteoarthritis Outcome Scores for pain improved in both the intra-articular and intramuscular groups. Improvements in pain scores peaked in the intra-articular injection group at the 4-week mark, when the difference with intramuscular injections was statistically significant. However, the two groups showed no significant differences in pain improvement at the 8-, 12-, and 24-week follow-up points.
“Intra-articular injection can act immediately on inhibiting joint inflammation after injection,” Mr. Wang said in an interview. “In contrast, for intramuscular injection, glucocorticoid needs firstly to be absorbed by muscle into blood and then travel into the knee via the circulatory system.”
The study also showed no significant differences between the two groups in the secondary outcomes of patient symptoms, stiffness, function, and sport and quality of life scores. There were more adverse events in the intra-articular injection group: 42% of patients reported an adverse event, compared to 33% in the intramuscular group, and the adverse events reported in the intramuscular group were nonserious events, such as headache and flushing.
Mr. Wang told the conference that while the intramuscular injection was inferior to intra-articular injections at 4 weeks, it was noninferior at 8 and 24 weeks and should be considered an effective way to reduce pain in patients with knee OA.
“This trial provides evidence for shared decision making because in some cases a patient may have a preference for specific injection and the GP may feel incompetent to administer the intra-articular injection,” he said.
An audience member pointed out that there was now a growing body of evidence suggesting that intra-articular injections may contribute to faster progression of knee OA because of effects on knee cartilage.
Mr. Wang acknowledged that their own research had shown these side effects of intra-articular injections, which was why the trial was intended to examine whether intramuscular injections might achieve the same pain relief.
“In the real practice, I would say that both injections are effective, but the intra-articular injection may provide a slightly [better] effect in the short term,” he said.
Commenting on the findings, Martin van der Esch, PhD, of Amsterdam University of Applied Sciences, said there were no guidelines as to whether intra-articular or intramuscular injections were the best option, so it really came down to the clinician’s decision.
“Therefore this is really an interesting study, because it gives some light – not the answer – but some light in what direction it could go for specific groups of patients,” Dr. van der Esch said in an interview.
Dr. van der Esch suggested that intramuscular injections might be more appropriate for patients with more systemic disease affecting multiple joints, but intra-articular injections might offer greater benefits in a patient with severe and long-lasting disease in a single joint.
No conflicts of interest were declared.
Intramuscular injections of glucocorticoids have efficacy similar to that of intra-articular injections in reducing pain in knee osteoarthritis but without the concerns about joint infection and the challenges of administration, according to results from a randomized, controlled trial reported at the OARSI 2021 World Congress.
Intra-articular injections of glucocorticoids are commonly used to relieve OA pain, but some general practitioners have difficulty administering them to patients, said Qiuke Wang, a PhD candidate at Erasmus University Medical Center in Rotterdam, the Netherlands. There are also concerns about whether intra-articular injections may cause damage to knee cartilage, Mr. Wang said at the conference, which is sponsored by the Osteoarthritis Research Society International.
Mr. Wang and colleagues conducted a randomized, controlled trial in which 145 patients with symptomatic knee OA received either an intramuscular or intra-articular injection of 40 mg triamcinolone acetonide, and then followed up at regular intervals for 24 weeks.
The study showed that Knee Injury and Osteoarthritis Outcome Scores for pain improved in both the intra-articular and intramuscular groups. Improvements in pain scores peaked in the intra-articular injection group at the 4-week mark, when the difference with intramuscular injections was statistically significant. However, the two groups showed no significant differences in pain improvement at the 8-, 12-, and 24-week follow-up points.
“Intra-articular injection can act immediately on inhibiting joint inflammation after injection,” Mr. Wang said in an interview. “In contrast, for intramuscular injection, glucocorticoid needs firstly to be absorbed by muscle into blood and then travel into the knee via the circulatory system.”
The study also showed no significant differences between the two groups in the secondary outcomes of patient symptoms, stiffness, function, and sport and quality of life scores. There were more adverse events in the intra-articular injection group: 42% of patients reported an adverse event, compared to 33% in the intramuscular group, and the adverse events reported in the intramuscular group were nonserious events, such as headache and flushing.
Mr. Wang told the conference that while the intramuscular injection was inferior to intra-articular injections at 4 weeks, it was noninferior at 8 and 24 weeks and should be considered an effective way to reduce pain in patients with knee OA.
“This trial provides evidence for shared decision making because in some cases a patient may have a preference for specific injection and the GP may feel incompetent to administer the intra-articular injection,” he said.
An audience member pointed out that there was now a growing body of evidence suggesting that intra-articular injections may contribute to faster progression of knee OA because of effects on knee cartilage.
Mr. Wang acknowledged that their own research had shown these side effects of intra-articular injections, which was why the trial was intended to examine whether intramuscular injections might achieve the same pain relief.
“In the real practice, I would say that both injections are effective, but the intra-articular injection may provide a slightly [better] effect in the short term,” he said.
Commenting on the findings, Martin van der Esch, PhD, of Amsterdam University of Applied Sciences, said there were no guidelines as to whether intra-articular or intramuscular injections were the best option, so it really came down to the clinician’s decision.
“Therefore this is really an interesting study, because it gives some light – not the answer – but some light in what direction it could go for specific groups of patients,” Dr. van der Esch said in an interview.
Dr. van der Esch suggested that intramuscular injections might be more appropriate for patients with more systemic disease affecting multiple joints, but intra-articular injections might offer greater benefits in a patient with severe and long-lasting disease in a single joint.
No conflicts of interest were declared.
FROM OARSI 2021
Weight cycling linked to cartilage degeneration in knee OA
Repetitive weight loss and gain in overweight or obese patients with knee osteoarthritis is associated with significantly greater cartilage and bone marrow edema degeneration than stable weight or steady weight loss, research suggests.
A presentation at the OARSI 2021 World Congress outlined the results of a study using Osteoarthritis Initiative data from 2,271 individuals with knee osteoarthritis and a body mass index (BMI) of 25 kg/m2 or above, which examined the effects of “weight cycling” on OA outcomes.
Gabby Joseph, PhD, of the University of California, San Francisco, told the conference – which was sponsored by the Osteoarthritis Research Society International – that previous studies had shown weight loss improves OA symptoms and slow progression, and weight gain increases OA risk. However no studies had yet examined the effects of weight cycling.
The study compared 4 years of MRI data for those who showed less than 3% loss or gain in weight over that time – the control group – versus those who lost more than 5% over that time and those who gained more than 5%. Among these were 249 individuals in the top 10% of annual weight change over that period, who were designated as weight cyclers. They tended to be younger, female, and with slightly higher average BMI than noncyclers.
Weight cyclers had significantly greater progression of cartilage degeneration and bone marrow edema degeneration – as measured by whole-organ magnetic resonance score – than did noncyclers, regardless of their overall weight gain or loss by the end of the study period.
However, the study did not see any significant differences in meniscus progression between cyclers and noncyclers, and cartilage thickness decreased in all groups over the 4 years with no significant effects associated with weight gain, loss, or cycling. Dr. Joseph commented that future studies could use voxel-based relaxometry to more closely study localized cartilage abnormalities.
Researchers also examined the effect of weight cycling on changes to walking speed, and found weight cyclers had significantly lower walking speeds by the end of the 4 years, regardless of overall weight change.
“What we’ve seen is that fluctuations are not beneficial for your joints,” Dr. Joseph told the conference. “When we advise patients that they want to lose weight, we want to do this in a very steady fashion; we don’t want yo-yo dieting.” She gave the example of one patient who started the study with a BMI of 36, went up to 40 then went down to 32.
Commenting on the study, Lisa Carlesso, PhD, of McMaster University, Hamilton, Ont., said it addresses an important issue because weight cycling is common as people struggle to maintain weight loss.
While it is difficult to speculate on the physiological mechanisms that might explain the effect, Dr. Carlesso noted that there were significantly more women than men among the weight cyclers.
“We know, for example, that obese women with knee OA have significantly higher levels of the adipokine leptin, compared to men, and leptin is involved in cartilage degeneration,” Dr. Carlesso said. “Similarly, we don’t have any information about joint alignment or measures of joint load, two things that could factor into the structural changes found.”
She suggested both these possibilities could be explored in future studies of weight cycling and its effects.
“It has opened up new lines of inquiry to be examined to mechanistically explain the relationship between cycling and worse cartilage and bone marrow degeneration,” Dr. Carlesso said.
The study was supported by the National Institutes of Health. No conflicts of interest were declared.
Repetitive weight loss and gain in overweight or obese patients with knee osteoarthritis is associated with significantly greater cartilage and bone marrow edema degeneration than stable weight or steady weight loss, research suggests.
A presentation at the OARSI 2021 World Congress outlined the results of a study using Osteoarthritis Initiative data from 2,271 individuals with knee osteoarthritis and a body mass index (BMI) of 25 kg/m2 or above, which examined the effects of “weight cycling” on OA outcomes.
Gabby Joseph, PhD, of the University of California, San Francisco, told the conference – which was sponsored by the Osteoarthritis Research Society International – that previous studies had shown weight loss improves OA symptoms and slow progression, and weight gain increases OA risk. However no studies had yet examined the effects of weight cycling.
The study compared 4 years of MRI data for those who showed less than 3% loss or gain in weight over that time – the control group – versus those who lost more than 5% over that time and those who gained more than 5%. Among these were 249 individuals in the top 10% of annual weight change over that period, who were designated as weight cyclers. They tended to be younger, female, and with slightly higher average BMI than noncyclers.
Weight cyclers had significantly greater progression of cartilage degeneration and bone marrow edema degeneration – as measured by whole-organ magnetic resonance score – than did noncyclers, regardless of their overall weight gain or loss by the end of the study period.
However, the study did not see any significant differences in meniscus progression between cyclers and noncyclers, and cartilage thickness decreased in all groups over the 4 years with no significant effects associated with weight gain, loss, or cycling. Dr. Joseph commented that future studies could use voxel-based relaxometry to more closely study localized cartilage abnormalities.
Researchers also examined the effect of weight cycling on changes to walking speed, and found weight cyclers had significantly lower walking speeds by the end of the 4 years, regardless of overall weight change.
“What we’ve seen is that fluctuations are not beneficial for your joints,” Dr. Joseph told the conference. “When we advise patients that they want to lose weight, we want to do this in a very steady fashion; we don’t want yo-yo dieting.” She gave the example of one patient who started the study with a BMI of 36, went up to 40 then went down to 32.
Commenting on the study, Lisa Carlesso, PhD, of McMaster University, Hamilton, Ont., said it addresses an important issue because weight cycling is common as people struggle to maintain weight loss.
While it is difficult to speculate on the physiological mechanisms that might explain the effect, Dr. Carlesso noted that there were significantly more women than men among the weight cyclers.
“We know, for example, that obese women with knee OA have significantly higher levels of the adipokine leptin, compared to men, and leptin is involved in cartilage degeneration,” Dr. Carlesso said. “Similarly, we don’t have any information about joint alignment or measures of joint load, two things that could factor into the structural changes found.”
She suggested both these possibilities could be explored in future studies of weight cycling and its effects.
“It has opened up new lines of inquiry to be examined to mechanistically explain the relationship between cycling and worse cartilage and bone marrow degeneration,” Dr. Carlesso said.
The study was supported by the National Institutes of Health. No conflicts of interest were declared.
Repetitive weight loss and gain in overweight or obese patients with knee osteoarthritis is associated with significantly greater cartilage and bone marrow edema degeneration than stable weight or steady weight loss, research suggests.
A presentation at the OARSI 2021 World Congress outlined the results of a study using Osteoarthritis Initiative data from 2,271 individuals with knee osteoarthritis and a body mass index (BMI) of 25 kg/m2 or above, which examined the effects of “weight cycling” on OA outcomes.
Gabby Joseph, PhD, of the University of California, San Francisco, told the conference – which was sponsored by the Osteoarthritis Research Society International – that previous studies had shown weight loss improves OA symptoms and slow progression, and weight gain increases OA risk. However no studies had yet examined the effects of weight cycling.
The study compared 4 years of MRI data for those who showed less than 3% loss or gain in weight over that time – the control group – versus those who lost more than 5% over that time and those who gained more than 5%. Among these were 249 individuals in the top 10% of annual weight change over that period, who were designated as weight cyclers. They tended to be younger, female, and with slightly higher average BMI than noncyclers.
Weight cyclers had significantly greater progression of cartilage degeneration and bone marrow edema degeneration – as measured by whole-organ magnetic resonance score – than did noncyclers, regardless of their overall weight gain or loss by the end of the study period.
However, the study did not see any significant differences in meniscus progression between cyclers and noncyclers, and cartilage thickness decreased in all groups over the 4 years with no significant effects associated with weight gain, loss, or cycling. Dr. Joseph commented that future studies could use voxel-based relaxometry to more closely study localized cartilage abnormalities.
Researchers also examined the effect of weight cycling on changes to walking speed, and found weight cyclers had significantly lower walking speeds by the end of the 4 years, regardless of overall weight change.
“What we’ve seen is that fluctuations are not beneficial for your joints,” Dr. Joseph told the conference. “When we advise patients that they want to lose weight, we want to do this in a very steady fashion; we don’t want yo-yo dieting.” She gave the example of one patient who started the study with a BMI of 36, went up to 40 then went down to 32.
Commenting on the study, Lisa Carlesso, PhD, of McMaster University, Hamilton, Ont., said it addresses an important issue because weight cycling is common as people struggle to maintain weight loss.
While it is difficult to speculate on the physiological mechanisms that might explain the effect, Dr. Carlesso noted that there were significantly more women than men among the weight cyclers.
“We know, for example, that obese women with knee OA have significantly higher levels of the adipokine leptin, compared to men, and leptin is involved in cartilage degeneration,” Dr. Carlesso said. “Similarly, we don’t have any information about joint alignment or measures of joint load, two things that could factor into the structural changes found.”
She suggested both these possibilities could be explored in future studies of weight cycling and its effects.
“It has opened up new lines of inquiry to be examined to mechanistically explain the relationship between cycling and worse cartilage and bone marrow degeneration,” Dr. Carlesso said.
The study was supported by the National Institutes of Health. No conflicts of interest were declared.
FROM OARSI 2021
COVID-19 vaccination in RMD patients: Safety data “reassuring”
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
FROM ANNALS OF THE RHEUMATIC DISEASES
Success in achondroplasia spurs testing vosoritide in more growth disorders
On the basis of the quality of sustained bone growth achieved with vosoritide in dwarfism, studies are underway or being considered for more diseases that impair bone growth, according to discussion that followed the presentation of a phase 3 trial extension study at the annual meeting of the Endocrine Society.
After 1 year on active therapy in the randomized trial and another year in the extension study, patients in the vosoritide group had sustained growth velocity while placebo group patients who crossed over to active therapy caught up, reported Ravi Savarirayan, MD, Murdoch Children’s Research Institute, University of Melbourne, Australia.
Moreover, the quality and type of bone growth, such as the improvement in body segment ratios over the second year of the study, support a durable benefit. Dr. Savarirayan said that improvements in activities of daily living are expected from this improvement in upper-to-lower body segment ratios, as well as the growth seen in the limbs.
Currently there is no approved pharmacologic therapy for achondroplasia in the United States. Growth hormone has been approved in Japan, but Dr. Savarirayan said its effects have been limited. Surgery such as limb lengthening is another option, but this approach is not uniformly effective and carries risks.
The 52-week results from the multinational phase 3 trial with vosoritide, which stimulates bone growth, were published last year in The Lancet. In that trial, 121 patients between the ages of 5 and 18 years with achondroplasia were randomized to vosoritide at a dose of 15 μg/kg once daily or placebo.
Relative to those in the placebo arm, which did not experience any change in growth, the median growth at the end of 52 weeks was 1.75 cm/year greater (6.71 vs. 3.99 cm).
After crossover, placebo patients catch up
In the extension study, the placebo patients were crossed over to the active therapy and both groups were followed for an additional 52 weeks. Over this period, velocity declined modestly in those in the group initially randomized to vosoritide but climbed steeply in the placebo group so that rates after 1 year were nearly identical (5.57 vs. 5.65 cm, respectively).
“The results suggest this medication may well have a durable effect,” said Dr. Savarirayan, who believes that the benefit is derived from stimulation of the growth plates. Based on the very similar efficacy observed in the placebo group once switched to active therapy, the response to vosoritide appears to be predictable.
Of the 60 patients initially randomized to vosoritide, 58 entered the extension. Of the patients who did not remain in the study, two left due to discomfort from injection-site reactions. All 61 patients initially assigned to placebo crossed over.
“We did not see any evidence of tachyphylaxis in the randomized study or in the extension,” Dr. Savarirayan said.
Although two more patients initiated on vosoritide discontinued treatment before the end of 2 years, there were no new adverse events observed. Rather, injection-site pain, which self-resolved in all patients, appears to be the most significant side effect.
“In children, the daily subcutaneous injections can be an issue,” Dr. Savarirayan acknowledged.
Injection site reactions most common adverse event
In a detailed evaluation of safety in a previously published dose-finding phase 2 study, injection-site reactions were also the most common of treatment-related adverse events, but there were no episodes of anaphylaxis or other grade 3 or higher hypersensitivity reactions (N Engl J Med. 2019 Jul 4;381:25-35).
Prior to clinical trials, continuous infusion of endogenous C-type natriuretic peptide demonstrated an ability to stimulate long-bone growth in experimental studies. Vosoritide, a recombinant analogue of C-type natriuretic peptide, appears to provide the same activity but offers a longer half-life.
Based on the benefits observed in achondroplasia, other applications are now being explored.
“When you evaluate the quality of the bone growth associated with vosoritide, it is normal,” said Melita Irving, MD, a consultant in clinical genetics at the Guy’s and St .Thomas’ NHS Trust, London. Dr. Irving has been involved in other research initiatives with this therapy and she cited a variety of evidence that has supported healthy bone development, including favorable changes in markers of bone growth such as type 10 collagen.
As a result, vosoritide, which is now under review by the U.S. Food and Drug Administration for treatment of dwarfism, is being pursued for several other diseases that result in abnormal bone growth, such as hypochondroplasia. Not least, clinical studies in idiopathic short stature have reached “early stages,” Dr. Irving said.
Dr. Savarirayan and Dr. Irving report no relevant conflicts of interest.
On the basis of the quality of sustained bone growth achieved with vosoritide in dwarfism, studies are underway or being considered for more diseases that impair bone growth, according to discussion that followed the presentation of a phase 3 trial extension study at the annual meeting of the Endocrine Society.
After 1 year on active therapy in the randomized trial and another year in the extension study, patients in the vosoritide group had sustained growth velocity while placebo group patients who crossed over to active therapy caught up, reported Ravi Savarirayan, MD, Murdoch Children’s Research Institute, University of Melbourne, Australia.
Moreover, the quality and type of bone growth, such as the improvement in body segment ratios over the second year of the study, support a durable benefit. Dr. Savarirayan said that improvements in activities of daily living are expected from this improvement in upper-to-lower body segment ratios, as well as the growth seen in the limbs.
Currently there is no approved pharmacologic therapy for achondroplasia in the United States. Growth hormone has been approved in Japan, but Dr. Savarirayan said its effects have been limited. Surgery such as limb lengthening is another option, but this approach is not uniformly effective and carries risks.
The 52-week results from the multinational phase 3 trial with vosoritide, which stimulates bone growth, were published last year in The Lancet. In that trial, 121 patients between the ages of 5 and 18 years with achondroplasia were randomized to vosoritide at a dose of 15 μg/kg once daily or placebo.
Relative to those in the placebo arm, which did not experience any change in growth, the median growth at the end of 52 weeks was 1.75 cm/year greater (6.71 vs. 3.99 cm).
After crossover, placebo patients catch up
In the extension study, the placebo patients were crossed over to the active therapy and both groups were followed for an additional 52 weeks. Over this period, velocity declined modestly in those in the group initially randomized to vosoritide but climbed steeply in the placebo group so that rates after 1 year were nearly identical (5.57 vs. 5.65 cm, respectively).
“The results suggest this medication may well have a durable effect,” said Dr. Savarirayan, who believes that the benefit is derived from stimulation of the growth plates. Based on the very similar efficacy observed in the placebo group once switched to active therapy, the response to vosoritide appears to be predictable.
Of the 60 patients initially randomized to vosoritide, 58 entered the extension. Of the patients who did not remain in the study, two left due to discomfort from injection-site reactions. All 61 patients initially assigned to placebo crossed over.
“We did not see any evidence of tachyphylaxis in the randomized study or in the extension,” Dr. Savarirayan said.
Although two more patients initiated on vosoritide discontinued treatment before the end of 2 years, there were no new adverse events observed. Rather, injection-site pain, which self-resolved in all patients, appears to be the most significant side effect.
“In children, the daily subcutaneous injections can be an issue,” Dr. Savarirayan acknowledged.
Injection site reactions most common adverse event
In a detailed evaluation of safety in a previously published dose-finding phase 2 study, injection-site reactions were also the most common of treatment-related adverse events, but there were no episodes of anaphylaxis or other grade 3 or higher hypersensitivity reactions (N Engl J Med. 2019 Jul 4;381:25-35).
Prior to clinical trials, continuous infusion of endogenous C-type natriuretic peptide demonstrated an ability to stimulate long-bone growth in experimental studies. Vosoritide, a recombinant analogue of C-type natriuretic peptide, appears to provide the same activity but offers a longer half-life.
Based on the benefits observed in achondroplasia, other applications are now being explored.
“When you evaluate the quality of the bone growth associated with vosoritide, it is normal,” said Melita Irving, MD, a consultant in clinical genetics at the Guy’s and St .Thomas’ NHS Trust, London. Dr. Irving has been involved in other research initiatives with this therapy and she cited a variety of evidence that has supported healthy bone development, including favorable changes in markers of bone growth such as type 10 collagen.
As a result, vosoritide, which is now under review by the U.S. Food and Drug Administration for treatment of dwarfism, is being pursued for several other diseases that result in abnormal bone growth, such as hypochondroplasia. Not least, clinical studies in idiopathic short stature have reached “early stages,” Dr. Irving said.
Dr. Savarirayan and Dr. Irving report no relevant conflicts of interest.
On the basis of the quality of sustained bone growth achieved with vosoritide in dwarfism, studies are underway or being considered for more diseases that impair bone growth, according to discussion that followed the presentation of a phase 3 trial extension study at the annual meeting of the Endocrine Society.
After 1 year on active therapy in the randomized trial and another year in the extension study, patients in the vosoritide group had sustained growth velocity while placebo group patients who crossed over to active therapy caught up, reported Ravi Savarirayan, MD, Murdoch Children’s Research Institute, University of Melbourne, Australia.
Moreover, the quality and type of bone growth, such as the improvement in body segment ratios over the second year of the study, support a durable benefit. Dr. Savarirayan said that improvements in activities of daily living are expected from this improvement in upper-to-lower body segment ratios, as well as the growth seen in the limbs.
Currently there is no approved pharmacologic therapy for achondroplasia in the United States. Growth hormone has been approved in Japan, but Dr. Savarirayan said its effects have been limited. Surgery such as limb lengthening is another option, but this approach is not uniformly effective and carries risks.
The 52-week results from the multinational phase 3 trial with vosoritide, which stimulates bone growth, were published last year in The Lancet. In that trial, 121 patients between the ages of 5 and 18 years with achondroplasia were randomized to vosoritide at a dose of 15 μg/kg once daily or placebo.
Relative to those in the placebo arm, which did not experience any change in growth, the median growth at the end of 52 weeks was 1.75 cm/year greater (6.71 vs. 3.99 cm).
After crossover, placebo patients catch up
In the extension study, the placebo patients were crossed over to the active therapy and both groups were followed for an additional 52 weeks. Over this period, velocity declined modestly in those in the group initially randomized to vosoritide but climbed steeply in the placebo group so that rates after 1 year were nearly identical (5.57 vs. 5.65 cm, respectively).
“The results suggest this medication may well have a durable effect,” said Dr. Savarirayan, who believes that the benefit is derived from stimulation of the growth plates. Based on the very similar efficacy observed in the placebo group once switched to active therapy, the response to vosoritide appears to be predictable.
Of the 60 patients initially randomized to vosoritide, 58 entered the extension. Of the patients who did not remain in the study, two left due to discomfort from injection-site reactions. All 61 patients initially assigned to placebo crossed over.
“We did not see any evidence of tachyphylaxis in the randomized study or in the extension,” Dr. Savarirayan said.
Although two more patients initiated on vosoritide discontinued treatment before the end of 2 years, there were no new adverse events observed. Rather, injection-site pain, which self-resolved in all patients, appears to be the most significant side effect.
“In children, the daily subcutaneous injections can be an issue,” Dr. Savarirayan acknowledged.
Injection site reactions most common adverse event
In a detailed evaluation of safety in a previously published dose-finding phase 2 study, injection-site reactions were also the most common of treatment-related adverse events, but there were no episodes of anaphylaxis or other grade 3 or higher hypersensitivity reactions (N Engl J Med. 2019 Jul 4;381:25-35).
Prior to clinical trials, continuous infusion of endogenous C-type natriuretic peptide demonstrated an ability to stimulate long-bone growth in experimental studies. Vosoritide, a recombinant analogue of C-type natriuretic peptide, appears to provide the same activity but offers a longer half-life.
Based on the benefits observed in achondroplasia, other applications are now being explored.
“When you evaluate the quality of the bone growth associated with vosoritide, it is normal,” said Melita Irving, MD, a consultant in clinical genetics at the Guy’s and St .Thomas’ NHS Trust, London. Dr. Irving has been involved in other research initiatives with this therapy and she cited a variety of evidence that has supported healthy bone development, including favorable changes in markers of bone growth such as type 10 collagen.
As a result, vosoritide, which is now under review by the U.S. Food and Drug Administration for treatment of dwarfism, is being pursued for several other diseases that result in abnormal bone growth, such as hypochondroplasia. Not least, clinical studies in idiopathic short stature have reached “early stages,” Dr. Irving said.
Dr. Savarirayan and Dr. Irving report no relevant conflicts of interest.
FROM ENDO 2021
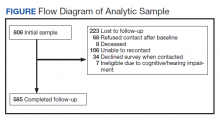
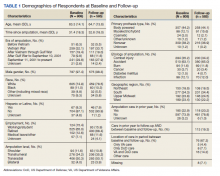
Amputation Care Quality and Satisfaction With Prosthetic Limb Services: A Longitudinal Study of Veterans With Upper Limb Amputation
Veterans with upper limb amputation (ULA) are a small, but important population, who have received more attention in the past decade due to the increased growth of the population of veterans with conflict-related amputation from recent military engagements. Among the 808 veterans with ULA receiving any care in the US Department of Veterans Affairs (VA) from 2010 to 2015 who participated in our national study, an estimated 28 to 35% had a conflict-related amputation.1 The care of these individuals with ULA is highly specialized, and there is a recognized shortage of experienced professionals in this area.2,3 The provision of high-quality prosthetic care is increasingly complex with advances in technology, such as externally powered devices with multiple functions.
The VA is a comprehensive, integrated health care system that serves more than 8.9 million veterans each year. Interdisciplinary amputation care is provided within the VA through a traditional clinic setting or by using one of several currently available virtual care modalities.4,5 In consultation with the veteran, VA health care providers (HCPs) prescribe prostheses and services based on the clinical needs and furnish authorized items and services to eligible veterans. Prescribed items and/or services are furnished either by internal VA resources or through a community-based prosthetist who is an authorized vendor or contractor. Although several studies have reported that the majority of veterans with ULA utilize VA services for at least some aspects of their health care, little is known about: (1) prosthetic limb care satisfaction or the quality of care that veterans receive; or (2) how care within the VA or Department of Defense (DoD) compares with care provided in the civilian sector.6-8
Earlier studies that examined the amputation rehabilitation services received by veterans with ULA pointed to quality gaps in care and differences in satisfaction in the VA and DoD when compared with the civilian sector but were limited in their scope and methodology.7,8 A 2008 study of veterans of the Vietnam War, Operation Iraqi Freedom (OIF), and Operation Enduring Freedom (OEF) compared satisfaction by location of care receipt (DoD only, VA only, private only, and multiple sources). That study dichotomized response categories for items related to satisfaction with care (satisfied/not), but did not estimate degree of satisfaction, calculate summary scores of the items, or utilize validated care satisfaction metrics. That study found that a greater proportion of Vietnam War veterans (compared with OIF/OEF veterans receiving care in the private sector) agreed that they “had a role in choosing prosthesis” and disagreed that they wanted to change their current prosthesis to another type.7 The assumption made by the authors is that much of this private sector care was actually VA-sponsored care prescribed and procured by the VA but delivered in the community. However, no data were collected to confirm or refute this assumption, and it is possible that some care was both VA sponsored and delivered, some was VA sponsored but commercially delivered, and in some cases, care was sponsored by other sources and delivered in still other facilities.
A 2012 VA Office of the Inspector General study of OIF, OEF, and Operation New Dawn (OND) veterans reported lower prosthetic satisfaction for veterans with upper limb when compared with lower limb amputation and described respondents concerns about lack of VA prosthetic expertise, difficulty with accessing VA services, and dissatisfaction with the sometimes lengthy approval process for obtaining fee-basis or VA contract care.8 Although this report suggested that there were quality gaps and areas for improvement, it did not employ validated metrics of prosthesis or care satisfaction and instead relied on qualitative data collected through telephone interviews.
Given the VA effort to enhance the quality and consistency of its amputation care services through the formal establishment of the Amputation System of Care, which began in 2008, further evaluation of care satisfaction and quality of care is warranted. In 2014 the VA and DoD released the first evidence-based clinical practice guidelines (CPGs) for the rehabilitation of persons with ULA.2 The CPG describes care paths to improve outcomes and basic tenets of amputation rehabilitation care and can be used to identify process activities that are essential aspects of quality care. However, the extent to which the CPG has impacted the quality and timeliness of care for veterans with ULA are presently unclear.
Thus, the purposes of this study were to: (1) measure veteran satisfaction with prosthetic limb care and identify factors associated with service satisfaction; (2) assess quality indicators that potentially reflect CPG) adoption; (3) compare care satisfaction and quality for those who received care in or outside of the VA or DoD; and (4) evaluate change in satisfaction over time.
Methods
The study was approved by the VA Central Institutional Review Board (IRB) (Study #16-20) and Human Research Protection Office, U.S. Army Medical Research and Development Command. The sampling frame consisted of veterans with major ULA who received care in the VA between 2010 and 2015 identified in VA Corporate Data Warehouse. We sent recruitment packages to nondeceased veterans who had current addresses and phone numbers. Those who did not opt out or inform us that they did not meet eligibility criteria were contacted by study interviewers. A waiver of documentation of written informed consent was obtained from the VA Central IRB. When reached by the study interviewer, Veterans provided oral informed consent. At baseline, 808 veterans were interviewed for a response rate of 47.7% as calculated by the American Association for Public Opinion Research (AAPOR) methodology.9 Follow-up interviews approximately 1 year later (mean [SD] 367 [16.8] days), were conducted with 585 respondents for a 72.4% response rate (Figure).
Survey Content
Development and pilot testing of the survey instrument previously was reported.1 The content of the survey drew from existing survey items and metrics, and included new items specifically designed to address patterns of amputation care, based on care goals within the CPG. All new and modified items were tested and refined through cognitive interviews with 10 participants, and tested with an additional 13 participants.
The survey collected data on demographics, amputation characteristics (year of amputation, level, laterality, and etiology), current prosthesis use, and type of prosthesis. This article focused on the sections of the survey pertaining to satisfaction with prosthetic care and indicators of quality of care. A description of the content of the full survey and a synopsis of overall findings are reported in a prior publication.1 The key independent, dependent, and other variables utilized in the analyses reported in this manuscript are described below.
Primary Independent Variables
In the follow-up survey, we asked respondents whether they had any amputation care in the prior 12 months, and if so to indicate where they had gone for care. We categorized respondents as having received VA/DoD care if they reported any care at the VA or DoD, and as having received non-VA/DoD care if they did not report care at the VA or DoD but indicated that they had received amputation care between baseline and follow-up.
Two primary outcomes were utilized; the Orthotics and Prosthetics User’s Survey (OPUS), client satisfaction with services scale (CSS), and a measure of care quality specifically developed for this study. The CSS is a measure developed specifically for orthotic and prosthesis users.10 This 11-item scale measures satisfaction with prosthetic limb services and contains items evaluating facets of care such as courtesy received from prosthetists and clinical staff, care coordination, appointment wait time, willingness of the prosthetist to listen to participant concerns, and satisfaction with prosthesis training. Items are rated on a 4-point scale (strongly agree [1] to strongly disagree [4]), thus higher CSS scores indicate worse satisfaction with services. The CSS was administered only to prosthesis users.
The Quality of Care assessment developed for this study contained items pertaining to amputation related care receipt and care quality. These items were generated by the study team in consultation with representatives from the VA/DoD Extremity Amputation Center of Excellence after review of the ULA rehabilitation CPG. Survey questions asked respondents about the clinicians visited for amputation related care in the past 12 months, whether they had an annual amputation-related checkup, whether any clinician had assessed their function, worked with them to identify goals, and/or to develop an amputation-related care plan. Respondents were also asked whether there had been family/caregiver involvement in their care and care coordination, whether a peer visitor was involved in their initial care, whether they had received information about amputation management in the prior year, and whether they had amputation-related pain. Those that indicated that they had amputation-related pain were subsequently asked whether their pain was well managed, whether they used medication for pain management, and whether they used any nonpharmaceutical strategies.
Quality of Care Index
We initially developed 15 indicator items of quality of care. We selected 7 of the items to create a summary index. We omitted 3 items about pain management, since these items were completed only by participants who indicated that they had experienced pain; however, we examined the 3 pain items individually given the importance of this topic. We omitted an additional 2 items from the summary index because they would not be sensitive to change because they pertained to the care in the year after initial amputation. One of these items asked whether caregivers were involved in initial amputation management and the other asked whether a peer visit occurred after amputation. Another 3 items were omitted because they only were completed by small subsamples due to intentional skip patterns in the survey. These items addressed whether clinical HCPs discussed amputation care goals in the prior year, worked to develop a care plan in the prior year, or helped to coordinate care after a move. Completion rates for all items considered for the index are shown in eAppendix 1 (Available at doi:10.12788/fp.0096). After item selection, we generated an index score, which was the number of reported “yes” responses to the seven relevant items.
Other Variables
We created a single variable called level/laterality which categorized ULA as unilateral or bilateral. We further categorized respondents with unilateral amputation by their amputation level. We categorized respondents as transradial for wrist joint or below the elbow amputations; transhumeral for at or above the elbow amputations; and shoulder for shoulder joint or forequarter amputations. Participants indicated the amputation etiology using 7 yes/no variables: combat injury, accident, burn, cancer, diabetes mellitus, and infection. Participants could select ≥ 1 etiology.
Primary prosthesis type was categorized as body powered, myoelectric/hybrid, cosmetic, other/unknown, or nonuser. The service era was classified based on amputation date as Before Vietnam, Vietnam War, After Vietnam to Gulf War, After Gulf War to September 10, 2001, and September 11, 2001 to present. For race, individuals with > 1 race were classified as other. We classified participants by region, using the station identification of the most recent VA medical center that they had visited between January 1, 2010 and December 30, 2015.
The survey also employed 2 measures of satisfaction with the prosthesis, the Trinity Amputation and Prosthetic Experience Scale (TAPES) satisfaction scale and the OPUS Client Satisfaction with Devices (CSD). TAPES consists of 10 items addressing color, shape, noise, appearance, weight, usefulness, reliability, fit, comfort and overall satisfaction.11 Items are rated on a 5-point Likert scale from very dissatisfied (1) to very satisfied (5). An 8-item version of the CSD scale was created for this study, after conducting a Rasch analysis (using Winsteps version 4.4.2) of the original 11-item CSD. The 8 items assess satisfaction with prosthesis fit, weight, comfort, donning ease, appearance, durability, skin contact, and pain. Items are rated on a 4-point scale from strongly agree (1) to strongly disagree (4); higher CSD scores indicate less satisfaction with devices. Psychometric analysis of the revised CSD score was reported in a prior publication.12 We also reported on the CSS using the original 10-item measure.
Data Analyses
We described characteristics of respondents at baseline and follow-up. We used baseline data to calculate CSS scores and described scores for all participants, for subgroups of unilateral and bilateral amputees, and for unilateral amputees stratified by amputation level. Wilcoxon rank sum tests were used to compare the CSS item and total scores of 461 prosthesis users with unilateral amputation and 29 with bilateral amputation. To identify factors that we hypothesized might be associated with CSS scores at baseline, we developed separate bivariate linear regression models. We added those factors that were associated with CSS scores at P ≤ .1 in bivariate analyses to a multivariable linear regression model of factors associated with CSS score. The P ≤ .1 threshold was used to ensure that relevant confounders were controlled for in regression models. We excluded 309 participants with no reported prosthesis use (who were not asked to complete the CSS), 20 participants with other/unknown prosthesis types, and 106 with missing data on amputation care in the prior year or on satisfaction metrics. We used baseline data for this analysis to maximize the sample size.
We compared CSS scores for those who reported receiving care within or outside of the VA or DoD in the prior year, using Wilcoxon Mann-Whitney rank tests. We also compared scores of individual quality of care items for these groups using Fisher exact tests. We chose to examine individual items rather than the full Index because several items specified care receipt within the VA and thus would be inappropriate to utilize in comparisons by site location; however, we described responses to all items. In these analyses, we excluded 2 respondents who had conflicting information regarding location of care. We used follow-up data for this analysis because it allowed us to identify location of care received in the prior year.
We also described the CSS scores, the 7-item Quality of Care Index and responses to other items related to quality of care at baseline and follow-up. To examine whether satisfaction with prosthetic care or aspects of care quality had changed over time, we compared baseline and follow-up CSS and quality of care scores for respondents who had measures at both times using Wilcoxon signed ranks tests. Individual items were compared using McNemar tests.
Results
Respondents were 97.4% male and included 776 unilateral amputees and 32 bilateral amputees with a mean (SD) age of 63.3 (14.1) years (Table 1). Respondents had lost their limbs a mean (SD) 31.4 (14.1) years prior, and half were transradial, 34.2% transhumeral, and 11.6% shoulder amputation. At baseline 185 (22.9%) participants received amputation-related care in the prior year and 118 (20.2%) participants received amputation-related care within 1 year of follow-up. Of respondents, 113 (19.3%) stated that their care was between baseline and follow-up and 89 (78.8%) of these received care at either the VA, the DoD or both; just 16 (14.2%) received care elsewhere.
Mean (SD) CSS scores were highest (lower satisfaction) for those with amputation at the shoulder and lowest for those with transhumeral amputation: 42.2 (20.0) vs 33.4 (20.8). Persons with bilateral amputation were less satisfied in almost every category when compared with those with unilateral amputation, although the total CSS score was not substantially different. Wilcoxon rank sum analyses revealed statistically significant differences in wait time satisfaction: bilateral amputees were less satisfied than unilateral amputees. Factors associated with overall CSS score in bivariate analyses were CSD score, TAPES score, amputation care receipt, prosthesis type, race, and region of care (eAppendix 2, available at doi:10.12788/fp.0096).
In the multivariate regression model of baseline CSS scores, only 2 variables were independently associated with CSS scores: CSD score and recent amputation care (Table 3). For each 1-point increase in CSD score there was a 0.7 point increase in CSS score. Those with amputation care in the prior year had higher satisfaction when compared with those who had not received care (P = .003).
For participants who indicated that they received amputation care between baseline and follow-up, CSS mean (SD) scores were better, but not statistically different, for those who reported care in the VA or DoD vs private care, 31.6 (22.6) vs 38.0 (17.7) (Table 4). When compared with community-based care, more participants who received care in the VA or DoD in the prior year had a functional assessment in that time period (33.7% vs 7.1%, P = .06), were contacted by HCPs outside of appointments (42.7% vs 18.8%, P = .07), and received information about amputation care in the prior year (41.6% vs 0%, P =.002). There was no difference in the proportion whose family/caregivers were involved in care in the prior year.
No statistically significant differences were observed in paired comparisons of the CSS and Quality of Care Index at baseline or follow-up for all participants with data at both time points (Table 5; eAppendix 3 available at doi:10.12788/fp.0096). Individual pain measures did not differ significantly between timepoints. Quality Index mean (SD) scores were 1.3 (1.5) and 1.2 (1.5) at baseline and follow-up, respectively (P = .07). For the 214 prosthesis users with longitudinal data, baseline CSS mean (SD) scores were generally worse at baseline than at follow-up: 34.4 (19.8) vs 32.5 (21.0) (P = .23). Family/caregiver involvement in amputation care was significantly higher in the year before baseline when compared with the year prior to follow-up (24.4% vs 17.7%, P = .001). There were no other statistically significant differences in Quality of Care items between baseline and follow-up.
Discussion
Our longitudinal study provides insights into the experiences of veterans with major ULA related to satisfaction with prosthetic limb care services and receipt of amputation-related care. We reported on the development and use of a new summary measure of amputation care quality, which we designed to capture some of the key elements of care quality as provided in the VA/DoD CPG.2
We used baseline data to identify factors independently associated with prosthetic limb care satisfaction as measured by a previously validated measure, the OPUS CSS. The CSS addresses satisfaction with prosthetic limb services and does not reflect satisfaction with other amputation care services. We found that persons who received amputation care in the prior year had CSS scores that were a mean 5.1 points better than those who had not received recent care. Although causality cannot be determined with this investigation, this finding highlights an important relationship between frequency of care and satisfaction, which can be leveraged by the VA in future care initiatives. Care satisfaction was also better by 0.7 points for every 1-point decrease (indicating higher satisfaction) in the OPUS CSD prosthetic satisfaction scale. This finding isn’t surprising, given that a major purpose of prosthetic limb care services is to procure and fit a satisfactory device. To determine whether these same relationships were observed in the smaller, longitudinal cohort data at follow-up, we repeated these models and found similar relationships between recent care receipt and prosthesis satisfaction and satisfaction with services. We believe that these findings are meaningful and emphasize the importance of both service and device satisfaction to the veteran with an ULA. Lower service satisfaction scores among those with amputations at the shoulder and those with bilateral limb loss suggest that these individuals may benefit from different service delivery approaches.
We did observe a difference in satisfaction scores by geographic region in the follow-up (but not the baseline) data with satisfaction higher in the Western vs the Southern region (data not shown). This finding suggests a need for continued monitoring of care satisfaction over time to determine whether differences by region persist. We grouped respondents into geographic region based on the location where they had received their most recent VA care of any type. Many veterans receive care at multiple VA locations. Thus, it is possible that some veterans received their amputation care at a non-VA facility or a VA facility in a different region.
Our findings related to prosthetic limb care services satisfaction are generalizable to veteran prosthesis users. Findings may not be generalizable to nonusers, because in our study, the CSS only was administered to prosthesis users. Thus, we were unable to identify factors associated with care satisfaction for persons who were not current users of an upper limb prosthesis.
The study findings confirmed that most veterans with ULA receive amputation-related care in the VA or DoD. We compared CSS and Quality of Care item scores for those who reported receiving care at the VA or DoD vs elsewhere. Amputation care within the VA is complex. Some services are provided at VA facilities and some are ordered by VA clinicians but provided by community-based HCPs. However, we found that better (though not statistically significantly different) CSS scores and several Quality of Care items were endorsed by a significantly more of those reporting care in the VA or DoD as compared to elsewhere. Given the dissemination of a rehabilitation of upper limb amputees CPG, we hypothesized that VA and DoD HCPs would be more aware of care guidelines and would provide better care. Overall, our findings supported this hypothesis while also suggesting that areas such as caregiver involvement and peer visitation may benefit from additional attention and program improvement.
We used longitudinal data to describe and compare CSS and Quality of Care Index scores. Our analyses did not detect any statistically significant differences between baseline and follow-up. This finding may reflect that this was a relatively stable population with regard to amputation experiences given the mean time since amputation was 31.4 years. However, we also recognize that our measures may not have captured all aspects of care satisfaction or quality. It is possible that there were other changes that had occurred over the course of the year that were not captured by the CSS or by the Quality of Care Index. It is also possible that some implementation and adoption of the CPG had happened prior to our baseline survey. Finally, it is possible that some elements of the CPG have not yet been fully integrated into clinical care. We believe that the latter is likely, given that nearly 80% of respondents did not report receiving any amputation care within the past year at follow-up, though the CPGs recommend an annual visit.
Aside from recall bias, 2 explanations must be considered relative to the low rate of adherence to the CPG recommendation for an annual follow-up. The first is that the CPG simply may not be widely adopted. The second is that the majority of patients with ULA who use prostheses use a body-powered system. These tend to be low maintenance, long-lasting systems and may ultimately not need annual maintenance and repair. Further, if the veteran’s body-powered system is functioning properly and health status has not changed, they may simply be opting out of an annual visit despite the CPG recommendation. Nonetheless, this apparent low rate of annual follow-up emphasizes the need for additional process improvement measures for the VA.
Strengths and Limitations
The VA provides a unique setting for a nationally representative study of amputation rehabilitation because it has centralized data sources that can be used to identify veterans with ULA. Our study had a strong response rate, and its prosthetic limb care satisfaction findings are generalizable to all veterans with major ULA who received VA care from 2010 to 2015. However, there are limits to generalizability outside of this population to civilians or to veterans who do not receive VA care. To examine possible nonresponse bias, which could limit generalizability, we compared the baseline characteristics of respondents and nonrespondents to the follow-up study (eAppendix 4 available at doi:10.12788/fp.0096). There were no significant differences in satisfaction, quality of care, or receipt of amputation-related care between those lost to follow-up and those with follow-up data. Although, we did find small differences in gender, race, and service era (defined by amputation date). We do not believe that these differences threaten the interpretation of findings at follow-up, but there may be limits to generalizability of these findings to the full baseline sample. The data were from a telephone survey of veterans. It is possible that some veterans did not recall their care receipt or did not understand some of the questions and thus may not have accurately answered questions related to type of care received or the timing of that care.
Our interpretation of findings comparing care received within the VA and DoD or elsewhere is also limited because we cannot say with certainty whether those who indicated no care in the VA or DoD actually had care that was sponsored by the VA or DoD as contract or fee-basis care. Just 8 respondents indicated that they had received care only outside of the VA or DoD in the prior year. There were also some limitations in the collection of data about care location. We asked about receipt of amputation care in the prior year and about location of any amputation care received between baseline and follow-up, and there were differences in responses. Thus, we used a combination of these items to identify location of care received in the prior year.
Despite these limitations, we believe that our study provides novel, important findings about the satisfaction with prosthetic limb care services and quality of amputation rehabilitation care for veterans. Findings from this study can be used to address amputation and prosthetic limb care satisfaction and quality weaknesses highlighted and to benchmark care satisfaction and CPG compliance. Other studies evaluating care guideline compliance have used indicators obtained from clinical records or data repositories.13-15 Future work could combine self-reported satisfaction and care quality measures with indicators obtained from clinical or repository sources to provide a more complete snapshot of care delivery.
Conclusions
We reported on a national survey of veterans with major upper limb loss that assessed satisfaction with prosthetic limb care services and quality of amputation care. Satisfaction with prosthetic limb care was independently associated with satisfaction with the prosthesis, and receipt of care within the prior year. Most of the veterans surveyed received care within the VA or DoD and reported receiving higher quality of care, when compared with those who received care outside of the VA or DoD. Satisfaction with care and quality of care were stable over the year of this study. Data presented in this study can serve to direct VA amputation care process improvement initiatives as benchmarks for future work. Future studies are needed to track satisfaction with and quality of care for veterans with ULA.
1. Resnik L, Ekerholm S, Borgia M, Clark MA. A national study of veterans with major upper limb amputation: Survey methods, participants, and summary findings. PLoS One. 2019;14(3):e0213578. Published 2019 Mar 14. doi:10.1371/journal.pone.0213578
2. US Department of Defense, US Department of Veterans Affairs, Management of Upper Extremity Amputation Rehabilitation Working Group. VA/DoD clinical practice guideline for the management of upper extremity amputation rehabilitation.Published 2014. Accessed February 18, 2021. https://www.healthquality.va.gov/guidelines/Rehab/UEAR/VADoDCPGManagementofUEAR121614Corrected508.pdf
3. Jette AM. The Promise of Assistive Technology to Enhance Work Participation. Phys Ther. 2017;97(7):691-692. doi:10.1093/ptj/pzx054
4. Webster JB, Poorman CE, Cifu DX. Guest editorial: Department of Veterans Affairs amputations system of care: 5 years of accomplishments and outcomes. J Rehabil Res Dev. 2014;51(4):vii-xvi. doi:10.1682/JRRD.2014.01.0024
5. Scholten J, Poorman C, Culver L, Webster JB. Department of Veterans Affairs polytrauma telerehabilitation: twenty-first century care. Phys Med Rehabil Clin N Am. 2019;30(1):207-215. doi:10.1016/j.pmr.2018.08.003
6. Melcer T, Walker J, Bhatnagar V, Richard E. Clinic use at the Departments of Defense and Veterans Affairs following combat related amputations. Mil Med. 2020;185(1-2):e244-e253. doi:10.1093/milmed/usz149
7. Berke GM, Fergason J, Milani JR, et al. Comparison of satisfaction with current prosthetic care in veterans and servicemembers from Vietnam and OIF/OEF conflicts with major traumatic limb loss. J Rehabil Res Dev. 2010;47(4):361-371. doi:10.1682/jrrd.2009.12.0193
8. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection prosthetic limb care in VA facilities. Published March 8, 2012. Accessed February 18, 2021. https://www.va.gov/oig/pubs/VAOIG-11-02138-116.pdf 9. American Association for Public Opinion Research. Response rates - an overview. Accessed February 18, 2021. https://www.aapor.org/Education-Resources/For-Researchers/Poll-Survey-FAQ/Response-Rates-An-Overview.aspx
10. Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the Orthotics and Prosthetics Users’ Survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191-206. doi:10.1080/03093640308726682
11. Desmond DM, MacLachlan M. Factor structure of the Trinity Amputation and Prosthesis Experience Scales (TAPES) with individuals with acquired upper limb amputations. Am J Phys Med Rehabil. 2005;84(7):506-513. doi:10.1097/01.phm.0000166885.16180.63
12. Resnik L, Borgia M, Heinemann AW, Clark MA. Prosthesis satisfaction in a national sample of veterans with upper limb amputation. Prosthet Orthot Int. 2020;44(2):81-91. doi:10.1177/0309364619895201
13. Ho TH, Caughey GE, Shakib S. Guideline compliance in chronic heart failure patients with multiple comorbid diseases: evaluation of an individualised multidisciplinary model of care. PLoS One. 2014;9(4):e93129. Published 2014 Apr 8. doi:10.1371/journal.pone.0093129
14. Mitchell KB, Lin H, Shen Y, et al. DCIS and axillary nodal evaluation: compliance with national guidelines. BMC Surg. 2017;17(1):12. Published 2017 Feb 7. doi:10.1186/s12893-017-0210-5
15. Moesker MJ, de Groot JF, Damen NL, et al. Guideline compliance for bridging anticoagulation use in vitamin-K antagonist patients; practice variation and factors associated with non-compliance. Thromb J. 2019;17:15. Published 2019 Aug 5. doi:10.1186/s12959-019-0204-x
Veterans with upper limb amputation (ULA) are a small, but important population, who have received more attention in the past decade due to the increased growth of the population of veterans with conflict-related amputation from recent military engagements. Among the 808 veterans with ULA receiving any care in the US Department of Veterans Affairs (VA) from 2010 to 2015 who participated in our national study, an estimated 28 to 35% had a conflict-related amputation.1 The care of these individuals with ULA is highly specialized, and there is a recognized shortage of experienced professionals in this area.2,3 The provision of high-quality prosthetic care is increasingly complex with advances in technology, such as externally powered devices with multiple functions.
The VA is a comprehensive, integrated health care system that serves more than 8.9 million veterans each year. Interdisciplinary amputation care is provided within the VA through a traditional clinic setting or by using one of several currently available virtual care modalities.4,5 In consultation with the veteran, VA health care providers (HCPs) prescribe prostheses and services based on the clinical needs and furnish authorized items and services to eligible veterans. Prescribed items and/or services are furnished either by internal VA resources or through a community-based prosthetist who is an authorized vendor or contractor. Although several studies have reported that the majority of veterans with ULA utilize VA services for at least some aspects of their health care, little is known about: (1) prosthetic limb care satisfaction or the quality of care that veterans receive; or (2) how care within the VA or Department of Defense (DoD) compares with care provided in the civilian sector.6-8
Earlier studies that examined the amputation rehabilitation services received by veterans with ULA pointed to quality gaps in care and differences in satisfaction in the VA and DoD when compared with the civilian sector but were limited in their scope and methodology.7,8 A 2008 study of veterans of the Vietnam War, Operation Iraqi Freedom (OIF), and Operation Enduring Freedom (OEF) compared satisfaction by location of care receipt (DoD only, VA only, private only, and multiple sources). That study dichotomized response categories for items related to satisfaction with care (satisfied/not), but did not estimate degree of satisfaction, calculate summary scores of the items, or utilize validated care satisfaction metrics. That study found that a greater proportion of Vietnam War veterans (compared with OIF/OEF veterans receiving care in the private sector) agreed that they “had a role in choosing prosthesis” and disagreed that they wanted to change their current prosthesis to another type.7 The assumption made by the authors is that much of this private sector care was actually VA-sponsored care prescribed and procured by the VA but delivered in the community. However, no data were collected to confirm or refute this assumption, and it is possible that some care was both VA sponsored and delivered, some was VA sponsored but commercially delivered, and in some cases, care was sponsored by other sources and delivered in still other facilities.
A 2012 VA Office of the Inspector General study of OIF, OEF, and Operation New Dawn (OND) veterans reported lower prosthetic satisfaction for veterans with upper limb when compared with lower limb amputation and described respondents concerns about lack of VA prosthetic expertise, difficulty with accessing VA services, and dissatisfaction with the sometimes lengthy approval process for obtaining fee-basis or VA contract care.8 Although this report suggested that there were quality gaps and areas for improvement, it did not employ validated metrics of prosthesis or care satisfaction and instead relied on qualitative data collected through telephone interviews.
Given the VA effort to enhance the quality and consistency of its amputation care services through the formal establishment of the Amputation System of Care, which began in 2008, further evaluation of care satisfaction and quality of care is warranted. In 2014 the VA and DoD released the first evidence-based clinical practice guidelines (CPGs) for the rehabilitation of persons with ULA.2 The CPG describes care paths to improve outcomes and basic tenets of amputation rehabilitation care and can be used to identify process activities that are essential aspects of quality care. However, the extent to which the CPG has impacted the quality and timeliness of care for veterans with ULA are presently unclear.
Thus, the purposes of this study were to: (1) measure veteran satisfaction with prosthetic limb care and identify factors associated with service satisfaction; (2) assess quality indicators that potentially reflect CPG) adoption; (3) compare care satisfaction and quality for those who received care in or outside of the VA or DoD; and (4) evaluate change in satisfaction over time.
Methods
The study was approved by the VA Central Institutional Review Board (IRB) (Study #16-20) and Human Research Protection Office, U.S. Army Medical Research and Development Command. The sampling frame consisted of veterans with major ULA who received care in the VA between 2010 and 2015 identified in VA Corporate Data Warehouse. We sent recruitment packages to nondeceased veterans who had current addresses and phone numbers. Those who did not opt out or inform us that they did not meet eligibility criteria were contacted by study interviewers. A waiver of documentation of written informed consent was obtained from the VA Central IRB. When reached by the study interviewer, Veterans provided oral informed consent. At baseline, 808 veterans were interviewed for a response rate of 47.7% as calculated by the American Association for Public Opinion Research (AAPOR) methodology.9 Follow-up interviews approximately 1 year later (mean [SD] 367 [16.8] days), were conducted with 585 respondents for a 72.4% response rate (Figure).
Survey Content
Development and pilot testing of the survey instrument previously was reported.1 The content of the survey drew from existing survey items and metrics, and included new items specifically designed to address patterns of amputation care, based on care goals within the CPG. All new and modified items were tested and refined through cognitive interviews with 10 participants, and tested with an additional 13 participants.
The survey collected data on demographics, amputation characteristics (year of amputation, level, laterality, and etiology), current prosthesis use, and type of prosthesis. This article focused on the sections of the survey pertaining to satisfaction with prosthetic care and indicators of quality of care. A description of the content of the full survey and a synopsis of overall findings are reported in a prior publication.1 The key independent, dependent, and other variables utilized in the analyses reported in this manuscript are described below.
Primary Independent Variables
In the follow-up survey, we asked respondents whether they had any amputation care in the prior 12 months, and if so to indicate where they had gone for care. We categorized respondents as having received VA/DoD care if they reported any care at the VA or DoD, and as having received non-VA/DoD care if they did not report care at the VA or DoD but indicated that they had received amputation care between baseline and follow-up.
Two primary outcomes were utilized; the Orthotics and Prosthetics User’s Survey (OPUS), client satisfaction with services scale (CSS), and a measure of care quality specifically developed for this study. The CSS is a measure developed specifically for orthotic and prosthesis users.10 This 11-item scale measures satisfaction with prosthetic limb services and contains items evaluating facets of care such as courtesy received from prosthetists and clinical staff, care coordination, appointment wait time, willingness of the prosthetist to listen to participant concerns, and satisfaction with prosthesis training. Items are rated on a 4-point scale (strongly agree [1] to strongly disagree [4]), thus higher CSS scores indicate worse satisfaction with services. The CSS was administered only to prosthesis users.
The Quality of Care assessment developed for this study contained items pertaining to amputation related care receipt and care quality. These items were generated by the study team in consultation with representatives from the VA/DoD Extremity Amputation Center of Excellence after review of the ULA rehabilitation CPG. Survey questions asked respondents about the clinicians visited for amputation related care in the past 12 months, whether they had an annual amputation-related checkup, whether any clinician had assessed their function, worked with them to identify goals, and/or to develop an amputation-related care plan. Respondents were also asked whether there had been family/caregiver involvement in their care and care coordination, whether a peer visitor was involved in their initial care, whether they had received information about amputation management in the prior year, and whether they had amputation-related pain. Those that indicated that they had amputation-related pain were subsequently asked whether their pain was well managed, whether they used medication for pain management, and whether they used any nonpharmaceutical strategies.
Quality of Care Index
We initially developed 15 indicator items of quality of care. We selected 7 of the items to create a summary index. We omitted 3 items about pain management, since these items were completed only by participants who indicated that they had experienced pain; however, we examined the 3 pain items individually given the importance of this topic. We omitted an additional 2 items from the summary index because they would not be sensitive to change because they pertained to the care in the year after initial amputation. One of these items asked whether caregivers were involved in initial amputation management and the other asked whether a peer visit occurred after amputation. Another 3 items were omitted because they only were completed by small subsamples due to intentional skip patterns in the survey. These items addressed whether clinical HCPs discussed amputation care goals in the prior year, worked to develop a care plan in the prior year, or helped to coordinate care after a move. Completion rates for all items considered for the index are shown in eAppendix 1 (Available at doi:10.12788/fp.0096). After item selection, we generated an index score, which was the number of reported “yes” responses to the seven relevant items.
Other Variables
We created a single variable called level/laterality which categorized ULA as unilateral or bilateral. We further categorized respondents with unilateral amputation by their amputation level. We categorized respondents as transradial for wrist joint or below the elbow amputations; transhumeral for at or above the elbow amputations; and shoulder for shoulder joint or forequarter amputations. Participants indicated the amputation etiology using 7 yes/no variables: combat injury, accident, burn, cancer, diabetes mellitus, and infection. Participants could select ≥ 1 etiology.
Primary prosthesis type was categorized as body powered, myoelectric/hybrid, cosmetic, other/unknown, or nonuser. The service era was classified based on amputation date as Before Vietnam, Vietnam War, After Vietnam to Gulf War, After Gulf War to September 10, 2001, and September 11, 2001 to present. For race, individuals with > 1 race were classified as other. We classified participants by region, using the station identification of the most recent VA medical center that they had visited between January 1, 2010 and December 30, 2015.
The survey also employed 2 measures of satisfaction with the prosthesis, the Trinity Amputation and Prosthetic Experience Scale (TAPES) satisfaction scale and the OPUS Client Satisfaction with Devices (CSD). TAPES consists of 10 items addressing color, shape, noise, appearance, weight, usefulness, reliability, fit, comfort and overall satisfaction.11 Items are rated on a 5-point Likert scale from very dissatisfied (1) to very satisfied (5). An 8-item version of the CSD scale was created for this study, after conducting a Rasch analysis (using Winsteps version 4.4.2) of the original 11-item CSD. The 8 items assess satisfaction with prosthesis fit, weight, comfort, donning ease, appearance, durability, skin contact, and pain. Items are rated on a 4-point scale from strongly agree (1) to strongly disagree (4); higher CSD scores indicate less satisfaction with devices. Psychometric analysis of the revised CSD score was reported in a prior publication.12 We also reported on the CSS using the original 10-item measure.
Data Analyses
We described characteristics of respondents at baseline and follow-up. We used baseline data to calculate CSS scores and described scores for all participants, for subgroups of unilateral and bilateral amputees, and for unilateral amputees stratified by amputation level. Wilcoxon rank sum tests were used to compare the CSS item and total scores of 461 prosthesis users with unilateral amputation and 29 with bilateral amputation. To identify factors that we hypothesized might be associated with CSS scores at baseline, we developed separate bivariate linear regression models. We added those factors that were associated with CSS scores at P ≤ .1 in bivariate analyses to a multivariable linear regression model of factors associated with CSS score. The P ≤ .1 threshold was used to ensure that relevant confounders were controlled for in regression models. We excluded 309 participants with no reported prosthesis use (who were not asked to complete the CSS), 20 participants with other/unknown prosthesis types, and 106 with missing data on amputation care in the prior year or on satisfaction metrics. We used baseline data for this analysis to maximize the sample size.
We compared CSS scores for those who reported receiving care within or outside of the VA or DoD in the prior year, using Wilcoxon Mann-Whitney rank tests. We also compared scores of individual quality of care items for these groups using Fisher exact tests. We chose to examine individual items rather than the full Index because several items specified care receipt within the VA and thus would be inappropriate to utilize in comparisons by site location; however, we described responses to all items. In these analyses, we excluded 2 respondents who had conflicting information regarding location of care. We used follow-up data for this analysis because it allowed us to identify location of care received in the prior year.
We also described the CSS scores, the 7-item Quality of Care Index and responses to other items related to quality of care at baseline and follow-up. To examine whether satisfaction with prosthetic care or aspects of care quality had changed over time, we compared baseline and follow-up CSS and quality of care scores for respondents who had measures at both times using Wilcoxon signed ranks tests. Individual items were compared using McNemar tests.
Results
Respondents were 97.4% male and included 776 unilateral amputees and 32 bilateral amputees with a mean (SD) age of 63.3 (14.1) years (Table 1). Respondents had lost their limbs a mean (SD) 31.4 (14.1) years prior, and half were transradial, 34.2% transhumeral, and 11.6% shoulder amputation. At baseline 185 (22.9%) participants received amputation-related care in the prior year and 118 (20.2%) participants received amputation-related care within 1 year of follow-up. Of respondents, 113 (19.3%) stated that their care was between baseline and follow-up and 89 (78.8%) of these received care at either the VA, the DoD or both; just 16 (14.2%) received care elsewhere.
Mean (SD) CSS scores were highest (lower satisfaction) for those with amputation at the shoulder and lowest for those with transhumeral amputation: 42.2 (20.0) vs 33.4 (20.8). Persons with bilateral amputation were less satisfied in almost every category when compared with those with unilateral amputation, although the total CSS score was not substantially different. Wilcoxon rank sum analyses revealed statistically significant differences in wait time satisfaction: bilateral amputees were less satisfied than unilateral amputees. Factors associated with overall CSS score in bivariate analyses were CSD score, TAPES score, amputation care receipt, prosthesis type, race, and region of care (eAppendix 2, available at doi:10.12788/fp.0096).
In the multivariate regression model of baseline CSS scores, only 2 variables were independently associated with CSS scores: CSD score and recent amputation care (Table 3). For each 1-point increase in CSD score there was a 0.7 point increase in CSS score. Those with amputation care in the prior year had higher satisfaction when compared with those who had not received care (P = .003).
For participants who indicated that they received amputation care between baseline and follow-up, CSS mean (SD) scores were better, but not statistically different, for those who reported care in the VA or DoD vs private care, 31.6 (22.6) vs 38.0 (17.7) (Table 4). When compared with community-based care, more participants who received care in the VA or DoD in the prior year had a functional assessment in that time period (33.7% vs 7.1%, P = .06), were contacted by HCPs outside of appointments (42.7% vs 18.8%, P = .07), and received information about amputation care in the prior year (41.6% vs 0%, P =.002). There was no difference in the proportion whose family/caregivers were involved in care in the prior year.
No statistically significant differences were observed in paired comparisons of the CSS and Quality of Care Index at baseline or follow-up for all participants with data at both time points (Table 5; eAppendix 3 available at doi:10.12788/fp.0096). Individual pain measures did not differ significantly between timepoints. Quality Index mean (SD) scores were 1.3 (1.5) and 1.2 (1.5) at baseline and follow-up, respectively (P = .07). For the 214 prosthesis users with longitudinal data, baseline CSS mean (SD) scores were generally worse at baseline than at follow-up: 34.4 (19.8) vs 32.5 (21.0) (P = .23). Family/caregiver involvement in amputation care was significantly higher in the year before baseline when compared with the year prior to follow-up (24.4% vs 17.7%, P = .001). There were no other statistically significant differences in Quality of Care items between baseline and follow-up.
Discussion
Our longitudinal study provides insights into the experiences of veterans with major ULA related to satisfaction with prosthetic limb care services and receipt of amputation-related care. We reported on the development and use of a new summary measure of amputation care quality, which we designed to capture some of the key elements of care quality as provided in the VA/DoD CPG.2
We used baseline data to identify factors independently associated with prosthetic limb care satisfaction as measured by a previously validated measure, the OPUS CSS. The CSS addresses satisfaction with prosthetic limb services and does not reflect satisfaction with other amputation care services. We found that persons who received amputation care in the prior year had CSS scores that were a mean 5.1 points better than those who had not received recent care. Although causality cannot be determined with this investigation, this finding highlights an important relationship between frequency of care and satisfaction, which can be leveraged by the VA in future care initiatives. Care satisfaction was also better by 0.7 points for every 1-point decrease (indicating higher satisfaction) in the OPUS CSD prosthetic satisfaction scale. This finding isn’t surprising, given that a major purpose of prosthetic limb care services is to procure and fit a satisfactory device. To determine whether these same relationships were observed in the smaller, longitudinal cohort data at follow-up, we repeated these models and found similar relationships between recent care receipt and prosthesis satisfaction and satisfaction with services. We believe that these findings are meaningful and emphasize the importance of both service and device satisfaction to the veteran with an ULA. Lower service satisfaction scores among those with amputations at the shoulder and those with bilateral limb loss suggest that these individuals may benefit from different service delivery approaches.
We did observe a difference in satisfaction scores by geographic region in the follow-up (but not the baseline) data with satisfaction higher in the Western vs the Southern region (data not shown). This finding suggests a need for continued monitoring of care satisfaction over time to determine whether differences by region persist. We grouped respondents into geographic region based on the location where they had received their most recent VA care of any type. Many veterans receive care at multiple VA locations. Thus, it is possible that some veterans received their amputation care at a non-VA facility or a VA facility in a different region.
Our findings related to prosthetic limb care services satisfaction are generalizable to veteran prosthesis users. Findings may not be generalizable to nonusers, because in our study, the CSS only was administered to prosthesis users. Thus, we were unable to identify factors associated with care satisfaction for persons who were not current users of an upper limb prosthesis.
The study findings confirmed that most veterans with ULA receive amputation-related care in the VA or DoD. We compared CSS and Quality of Care item scores for those who reported receiving care at the VA or DoD vs elsewhere. Amputation care within the VA is complex. Some services are provided at VA facilities and some are ordered by VA clinicians but provided by community-based HCPs. However, we found that better (though not statistically significantly different) CSS scores and several Quality of Care items were endorsed by a significantly more of those reporting care in the VA or DoD as compared to elsewhere. Given the dissemination of a rehabilitation of upper limb amputees CPG, we hypothesized that VA and DoD HCPs would be more aware of care guidelines and would provide better care. Overall, our findings supported this hypothesis while also suggesting that areas such as caregiver involvement and peer visitation may benefit from additional attention and program improvement.
We used longitudinal data to describe and compare CSS and Quality of Care Index scores. Our analyses did not detect any statistically significant differences between baseline and follow-up. This finding may reflect that this was a relatively stable population with regard to amputation experiences given the mean time since amputation was 31.4 years. However, we also recognize that our measures may not have captured all aspects of care satisfaction or quality. It is possible that there were other changes that had occurred over the course of the year that were not captured by the CSS or by the Quality of Care Index. It is also possible that some implementation and adoption of the CPG had happened prior to our baseline survey. Finally, it is possible that some elements of the CPG have not yet been fully integrated into clinical care. We believe that the latter is likely, given that nearly 80% of respondents did not report receiving any amputation care within the past year at follow-up, though the CPGs recommend an annual visit.
Aside from recall bias, 2 explanations must be considered relative to the low rate of adherence to the CPG recommendation for an annual follow-up. The first is that the CPG simply may not be widely adopted. The second is that the majority of patients with ULA who use prostheses use a body-powered system. These tend to be low maintenance, long-lasting systems and may ultimately not need annual maintenance and repair. Further, if the veteran’s body-powered system is functioning properly and health status has not changed, they may simply be opting out of an annual visit despite the CPG recommendation. Nonetheless, this apparent low rate of annual follow-up emphasizes the need for additional process improvement measures for the VA.
Strengths and Limitations
The VA provides a unique setting for a nationally representative study of amputation rehabilitation because it has centralized data sources that can be used to identify veterans with ULA. Our study had a strong response rate, and its prosthetic limb care satisfaction findings are generalizable to all veterans with major ULA who received VA care from 2010 to 2015. However, there are limits to generalizability outside of this population to civilians or to veterans who do not receive VA care. To examine possible nonresponse bias, which could limit generalizability, we compared the baseline characteristics of respondents and nonrespondents to the follow-up study (eAppendix 4 available at doi:10.12788/fp.0096). There were no significant differences in satisfaction, quality of care, or receipt of amputation-related care between those lost to follow-up and those with follow-up data. Although, we did find small differences in gender, race, and service era (defined by amputation date). We do not believe that these differences threaten the interpretation of findings at follow-up, but there may be limits to generalizability of these findings to the full baseline sample. The data were from a telephone survey of veterans. It is possible that some veterans did not recall their care receipt or did not understand some of the questions and thus may not have accurately answered questions related to type of care received or the timing of that care.
Our interpretation of findings comparing care received within the VA and DoD or elsewhere is also limited because we cannot say with certainty whether those who indicated no care in the VA or DoD actually had care that was sponsored by the VA or DoD as contract or fee-basis care. Just 8 respondents indicated that they had received care only outside of the VA or DoD in the prior year. There were also some limitations in the collection of data about care location. We asked about receipt of amputation care in the prior year and about location of any amputation care received between baseline and follow-up, and there were differences in responses. Thus, we used a combination of these items to identify location of care received in the prior year.
Despite these limitations, we believe that our study provides novel, important findings about the satisfaction with prosthetic limb care services and quality of amputation rehabilitation care for veterans. Findings from this study can be used to address amputation and prosthetic limb care satisfaction and quality weaknesses highlighted and to benchmark care satisfaction and CPG compliance. Other studies evaluating care guideline compliance have used indicators obtained from clinical records or data repositories.13-15 Future work could combine self-reported satisfaction and care quality measures with indicators obtained from clinical or repository sources to provide a more complete snapshot of care delivery.
Conclusions
We reported on a national survey of veterans with major upper limb loss that assessed satisfaction with prosthetic limb care services and quality of amputation care. Satisfaction with prosthetic limb care was independently associated with satisfaction with the prosthesis, and receipt of care within the prior year. Most of the veterans surveyed received care within the VA or DoD and reported receiving higher quality of care, when compared with those who received care outside of the VA or DoD. Satisfaction with care and quality of care were stable over the year of this study. Data presented in this study can serve to direct VA amputation care process improvement initiatives as benchmarks for future work. Future studies are needed to track satisfaction with and quality of care for veterans with ULA.
Veterans with upper limb amputation (ULA) are a small, but important population, who have received more attention in the past decade due to the increased growth of the population of veterans with conflict-related amputation from recent military engagements. Among the 808 veterans with ULA receiving any care in the US Department of Veterans Affairs (VA) from 2010 to 2015 who participated in our national study, an estimated 28 to 35% had a conflict-related amputation.1 The care of these individuals with ULA is highly specialized, and there is a recognized shortage of experienced professionals in this area.2,3 The provision of high-quality prosthetic care is increasingly complex with advances in technology, such as externally powered devices with multiple functions.
The VA is a comprehensive, integrated health care system that serves more than 8.9 million veterans each year. Interdisciplinary amputation care is provided within the VA through a traditional clinic setting or by using one of several currently available virtual care modalities.4,5 In consultation with the veteran, VA health care providers (HCPs) prescribe prostheses and services based on the clinical needs and furnish authorized items and services to eligible veterans. Prescribed items and/or services are furnished either by internal VA resources or through a community-based prosthetist who is an authorized vendor or contractor. Although several studies have reported that the majority of veterans with ULA utilize VA services for at least some aspects of their health care, little is known about: (1) prosthetic limb care satisfaction or the quality of care that veterans receive; or (2) how care within the VA or Department of Defense (DoD) compares with care provided in the civilian sector.6-8
Earlier studies that examined the amputation rehabilitation services received by veterans with ULA pointed to quality gaps in care and differences in satisfaction in the VA and DoD when compared with the civilian sector but were limited in their scope and methodology.7,8 A 2008 study of veterans of the Vietnam War, Operation Iraqi Freedom (OIF), and Operation Enduring Freedom (OEF) compared satisfaction by location of care receipt (DoD only, VA only, private only, and multiple sources). That study dichotomized response categories for items related to satisfaction with care (satisfied/not), but did not estimate degree of satisfaction, calculate summary scores of the items, or utilize validated care satisfaction metrics. That study found that a greater proportion of Vietnam War veterans (compared with OIF/OEF veterans receiving care in the private sector) agreed that they “had a role in choosing prosthesis” and disagreed that they wanted to change their current prosthesis to another type.7 The assumption made by the authors is that much of this private sector care was actually VA-sponsored care prescribed and procured by the VA but delivered in the community. However, no data were collected to confirm or refute this assumption, and it is possible that some care was both VA sponsored and delivered, some was VA sponsored but commercially delivered, and in some cases, care was sponsored by other sources and delivered in still other facilities.
A 2012 VA Office of the Inspector General study of OIF, OEF, and Operation New Dawn (OND) veterans reported lower prosthetic satisfaction for veterans with upper limb when compared with lower limb amputation and described respondents concerns about lack of VA prosthetic expertise, difficulty with accessing VA services, and dissatisfaction with the sometimes lengthy approval process for obtaining fee-basis or VA contract care.8 Although this report suggested that there were quality gaps and areas for improvement, it did not employ validated metrics of prosthesis or care satisfaction and instead relied on qualitative data collected through telephone interviews.
Given the VA effort to enhance the quality and consistency of its amputation care services through the formal establishment of the Amputation System of Care, which began in 2008, further evaluation of care satisfaction and quality of care is warranted. In 2014 the VA and DoD released the first evidence-based clinical practice guidelines (CPGs) for the rehabilitation of persons with ULA.2 The CPG describes care paths to improve outcomes and basic tenets of amputation rehabilitation care and can be used to identify process activities that are essential aspects of quality care. However, the extent to which the CPG has impacted the quality and timeliness of care for veterans with ULA are presently unclear.
Thus, the purposes of this study were to: (1) measure veteran satisfaction with prosthetic limb care and identify factors associated with service satisfaction; (2) assess quality indicators that potentially reflect CPG) adoption; (3) compare care satisfaction and quality for those who received care in or outside of the VA or DoD; and (4) evaluate change in satisfaction over time.
Methods
The study was approved by the VA Central Institutional Review Board (IRB) (Study #16-20) and Human Research Protection Office, U.S. Army Medical Research and Development Command. The sampling frame consisted of veterans with major ULA who received care in the VA between 2010 and 2015 identified in VA Corporate Data Warehouse. We sent recruitment packages to nondeceased veterans who had current addresses and phone numbers. Those who did not opt out or inform us that they did not meet eligibility criteria were contacted by study interviewers. A waiver of documentation of written informed consent was obtained from the VA Central IRB. When reached by the study interviewer, Veterans provided oral informed consent. At baseline, 808 veterans were interviewed for a response rate of 47.7% as calculated by the American Association for Public Opinion Research (AAPOR) methodology.9 Follow-up interviews approximately 1 year later (mean [SD] 367 [16.8] days), were conducted with 585 respondents for a 72.4% response rate (Figure).
Survey Content
Development and pilot testing of the survey instrument previously was reported.1 The content of the survey drew from existing survey items and metrics, and included new items specifically designed to address patterns of amputation care, based on care goals within the CPG. All new and modified items were tested and refined through cognitive interviews with 10 participants, and tested with an additional 13 participants.
The survey collected data on demographics, amputation characteristics (year of amputation, level, laterality, and etiology), current prosthesis use, and type of prosthesis. This article focused on the sections of the survey pertaining to satisfaction with prosthetic care and indicators of quality of care. A description of the content of the full survey and a synopsis of overall findings are reported in a prior publication.1 The key independent, dependent, and other variables utilized in the analyses reported in this manuscript are described below.
Primary Independent Variables
In the follow-up survey, we asked respondents whether they had any amputation care in the prior 12 months, and if so to indicate where they had gone for care. We categorized respondents as having received VA/DoD care if they reported any care at the VA or DoD, and as having received non-VA/DoD care if they did not report care at the VA or DoD but indicated that they had received amputation care between baseline and follow-up.
Two primary outcomes were utilized; the Orthotics and Prosthetics User’s Survey (OPUS), client satisfaction with services scale (CSS), and a measure of care quality specifically developed for this study. The CSS is a measure developed specifically for orthotic and prosthesis users.10 This 11-item scale measures satisfaction with prosthetic limb services and contains items evaluating facets of care such as courtesy received from prosthetists and clinical staff, care coordination, appointment wait time, willingness of the prosthetist to listen to participant concerns, and satisfaction with prosthesis training. Items are rated on a 4-point scale (strongly agree [1] to strongly disagree [4]), thus higher CSS scores indicate worse satisfaction with services. The CSS was administered only to prosthesis users.
The Quality of Care assessment developed for this study contained items pertaining to amputation related care receipt and care quality. These items were generated by the study team in consultation with representatives from the VA/DoD Extremity Amputation Center of Excellence after review of the ULA rehabilitation CPG. Survey questions asked respondents about the clinicians visited for amputation related care in the past 12 months, whether they had an annual amputation-related checkup, whether any clinician had assessed their function, worked with them to identify goals, and/or to develop an amputation-related care plan. Respondents were also asked whether there had been family/caregiver involvement in their care and care coordination, whether a peer visitor was involved in their initial care, whether they had received information about amputation management in the prior year, and whether they had amputation-related pain. Those that indicated that they had amputation-related pain were subsequently asked whether their pain was well managed, whether they used medication for pain management, and whether they used any nonpharmaceutical strategies.
Quality of Care Index
We initially developed 15 indicator items of quality of care. We selected 7 of the items to create a summary index. We omitted 3 items about pain management, since these items were completed only by participants who indicated that they had experienced pain; however, we examined the 3 pain items individually given the importance of this topic. We omitted an additional 2 items from the summary index because they would not be sensitive to change because they pertained to the care in the year after initial amputation. One of these items asked whether caregivers were involved in initial amputation management and the other asked whether a peer visit occurred after amputation. Another 3 items were omitted because they only were completed by small subsamples due to intentional skip patterns in the survey. These items addressed whether clinical HCPs discussed amputation care goals in the prior year, worked to develop a care plan in the prior year, or helped to coordinate care after a move. Completion rates for all items considered for the index are shown in eAppendix 1 (Available at doi:10.12788/fp.0096). After item selection, we generated an index score, which was the number of reported “yes” responses to the seven relevant items.
Other Variables
We created a single variable called level/laterality which categorized ULA as unilateral or bilateral. We further categorized respondents with unilateral amputation by their amputation level. We categorized respondents as transradial for wrist joint or below the elbow amputations; transhumeral for at or above the elbow amputations; and shoulder for shoulder joint or forequarter amputations. Participants indicated the amputation etiology using 7 yes/no variables: combat injury, accident, burn, cancer, diabetes mellitus, and infection. Participants could select ≥ 1 etiology.
Primary prosthesis type was categorized as body powered, myoelectric/hybrid, cosmetic, other/unknown, or nonuser. The service era was classified based on amputation date as Before Vietnam, Vietnam War, After Vietnam to Gulf War, After Gulf War to September 10, 2001, and September 11, 2001 to present. For race, individuals with > 1 race were classified as other. We classified participants by region, using the station identification of the most recent VA medical center that they had visited between January 1, 2010 and December 30, 2015.
The survey also employed 2 measures of satisfaction with the prosthesis, the Trinity Amputation and Prosthetic Experience Scale (TAPES) satisfaction scale and the OPUS Client Satisfaction with Devices (CSD). TAPES consists of 10 items addressing color, shape, noise, appearance, weight, usefulness, reliability, fit, comfort and overall satisfaction.11 Items are rated on a 5-point Likert scale from very dissatisfied (1) to very satisfied (5). An 8-item version of the CSD scale was created for this study, after conducting a Rasch analysis (using Winsteps version 4.4.2) of the original 11-item CSD. The 8 items assess satisfaction with prosthesis fit, weight, comfort, donning ease, appearance, durability, skin contact, and pain. Items are rated on a 4-point scale from strongly agree (1) to strongly disagree (4); higher CSD scores indicate less satisfaction with devices. Psychometric analysis of the revised CSD score was reported in a prior publication.12 We also reported on the CSS using the original 10-item measure.
Data Analyses
We described characteristics of respondents at baseline and follow-up. We used baseline data to calculate CSS scores and described scores for all participants, for subgroups of unilateral and bilateral amputees, and for unilateral amputees stratified by amputation level. Wilcoxon rank sum tests were used to compare the CSS item and total scores of 461 prosthesis users with unilateral amputation and 29 with bilateral amputation. To identify factors that we hypothesized might be associated with CSS scores at baseline, we developed separate bivariate linear regression models. We added those factors that were associated with CSS scores at P ≤ .1 in bivariate analyses to a multivariable linear regression model of factors associated with CSS score. The P ≤ .1 threshold was used to ensure that relevant confounders were controlled for in regression models. We excluded 309 participants with no reported prosthesis use (who were not asked to complete the CSS), 20 participants with other/unknown prosthesis types, and 106 with missing data on amputation care in the prior year or on satisfaction metrics. We used baseline data for this analysis to maximize the sample size.
We compared CSS scores for those who reported receiving care within or outside of the VA or DoD in the prior year, using Wilcoxon Mann-Whitney rank tests. We also compared scores of individual quality of care items for these groups using Fisher exact tests. We chose to examine individual items rather than the full Index because several items specified care receipt within the VA and thus would be inappropriate to utilize in comparisons by site location; however, we described responses to all items. In these analyses, we excluded 2 respondents who had conflicting information regarding location of care. We used follow-up data for this analysis because it allowed us to identify location of care received in the prior year.
We also described the CSS scores, the 7-item Quality of Care Index and responses to other items related to quality of care at baseline and follow-up. To examine whether satisfaction with prosthetic care or aspects of care quality had changed over time, we compared baseline and follow-up CSS and quality of care scores for respondents who had measures at both times using Wilcoxon signed ranks tests. Individual items were compared using McNemar tests.
Results
Respondents were 97.4% male and included 776 unilateral amputees and 32 bilateral amputees with a mean (SD) age of 63.3 (14.1) years (Table 1). Respondents had lost their limbs a mean (SD) 31.4 (14.1) years prior, and half were transradial, 34.2% transhumeral, and 11.6% shoulder amputation. At baseline 185 (22.9%) participants received amputation-related care in the prior year and 118 (20.2%) participants received amputation-related care within 1 year of follow-up. Of respondents, 113 (19.3%) stated that their care was between baseline and follow-up and 89 (78.8%) of these received care at either the VA, the DoD or both; just 16 (14.2%) received care elsewhere.
Mean (SD) CSS scores were highest (lower satisfaction) for those with amputation at the shoulder and lowest for those with transhumeral amputation: 42.2 (20.0) vs 33.4 (20.8). Persons with bilateral amputation were less satisfied in almost every category when compared with those with unilateral amputation, although the total CSS score was not substantially different. Wilcoxon rank sum analyses revealed statistically significant differences in wait time satisfaction: bilateral amputees were less satisfied than unilateral amputees. Factors associated with overall CSS score in bivariate analyses were CSD score, TAPES score, amputation care receipt, prosthesis type, race, and region of care (eAppendix 2, available at doi:10.12788/fp.0096).
In the multivariate regression model of baseline CSS scores, only 2 variables were independently associated with CSS scores: CSD score and recent amputation care (Table 3). For each 1-point increase in CSD score there was a 0.7 point increase in CSS score. Those with amputation care in the prior year had higher satisfaction when compared with those who had not received care (P = .003).
For participants who indicated that they received amputation care between baseline and follow-up, CSS mean (SD) scores were better, but not statistically different, for those who reported care in the VA or DoD vs private care, 31.6 (22.6) vs 38.0 (17.7) (Table 4). When compared with community-based care, more participants who received care in the VA or DoD in the prior year had a functional assessment in that time period (33.7% vs 7.1%, P = .06), were contacted by HCPs outside of appointments (42.7% vs 18.8%, P = .07), and received information about amputation care in the prior year (41.6% vs 0%, P =.002). There was no difference in the proportion whose family/caregivers were involved in care in the prior year.
No statistically significant differences were observed in paired comparisons of the CSS and Quality of Care Index at baseline or follow-up for all participants with data at both time points (Table 5; eAppendix 3 available at doi:10.12788/fp.0096). Individual pain measures did not differ significantly between timepoints. Quality Index mean (SD) scores were 1.3 (1.5) and 1.2 (1.5) at baseline and follow-up, respectively (P = .07). For the 214 prosthesis users with longitudinal data, baseline CSS mean (SD) scores were generally worse at baseline than at follow-up: 34.4 (19.8) vs 32.5 (21.0) (P = .23). Family/caregiver involvement in amputation care was significantly higher in the year before baseline when compared with the year prior to follow-up (24.4% vs 17.7%, P = .001). There were no other statistically significant differences in Quality of Care items between baseline and follow-up.
Discussion
Our longitudinal study provides insights into the experiences of veterans with major ULA related to satisfaction with prosthetic limb care services and receipt of amputation-related care. We reported on the development and use of a new summary measure of amputation care quality, which we designed to capture some of the key elements of care quality as provided in the VA/DoD CPG.2
We used baseline data to identify factors independently associated with prosthetic limb care satisfaction as measured by a previously validated measure, the OPUS CSS. The CSS addresses satisfaction with prosthetic limb services and does not reflect satisfaction with other amputation care services. We found that persons who received amputation care in the prior year had CSS scores that were a mean 5.1 points better than those who had not received recent care. Although causality cannot be determined with this investigation, this finding highlights an important relationship between frequency of care and satisfaction, which can be leveraged by the VA in future care initiatives. Care satisfaction was also better by 0.7 points for every 1-point decrease (indicating higher satisfaction) in the OPUS CSD prosthetic satisfaction scale. This finding isn’t surprising, given that a major purpose of prosthetic limb care services is to procure and fit a satisfactory device. To determine whether these same relationships were observed in the smaller, longitudinal cohort data at follow-up, we repeated these models and found similar relationships between recent care receipt and prosthesis satisfaction and satisfaction with services. We believe that these findings are meaningful and emphasize the importance of both service and device satisfaction to the veteran with an ULA. Lower service satisfaction scores among those with amputations at the shoulder and those with bilateral limb loss suggest that these individuals may benefit from different service delivery approaches.
We did observe a difference in satisfaction scores by geographic region in the follow-up (but not the baseline) data with satisfaction higher in the Western vs the Southern region (data not shown). This finding suggests a need for continued monitoring of care satisfaction over time to determine whether differences by region persist. We grouped respondents into geographic region based on the location where they had received their most recent VA care of any type. Many veterans receive care at multiple VA locations. Thus, it is possible that some veterans received their amputation care at a non-VA facility or a VA facility in a different region.
Our findings related to prosthetic limb care services satisfaction are generalizable to veteran prosthesis users. Findings may not be generalizable to nonusers, because in our study, the CSS only was administered to prosthesis users. Thus, we were unable to identify factors associated with care satisfaction for persons who were not current users of an upper limb prosthesis.
The study findings confirmed that most veterans with ULA receive amputation-related care in the VA or DoD. We compared CSS and Quality of Care item scores for those who reported receiving care at the VA or DoD vs elsewhere. Amputation care within the VA is complex. Some services are provided at VA facilities and some are ordered by VA clinicians but provided by community-based HCPs. However, we found that better (though not statistically significantly different) CSS scores and several Quality of Care items were endorsed by a significantly more of those reporting care in the VA or DoD as compared to elsewhere. Given the dissemination of a rehabilitation of upper limb amputees CPG, we hypothesized that VA and DoD HCPs would be more aware of care guidelines and would provide better care. Overall, our findings supported this hypothesis while also suggesting that areas such as caregiver involvement and peer visitation may benefit from additional attention and program improvement.
We used longitudinal data to describe and compare CSS and Quality of Care Index scores. Our analyses did not detect any statistically significant differences between baseline and follow-up. This finding may reflect that this was a relatively stable population with regard to amputation experiences given the mean time since amputation was 31.4 years. However, we also recognize that our measures may not have captured all aspects of care satisfaction or quality. It is possible that there were other changes that had occurred over the course of the year that were not captured by the CSS or by the Quality of Care Index. It is also possible that some implementation and adoption of the CPG had happened prior to our baseline survey. Finally, it is possible that some elements of the CPG have not yet been fully integrated into clinical care. We believe that the latter is likely, given that nearly 80% of respondents did not report receiving any amputation care within the past year at follow-up, though the CPGs recommend an annual visit.
Aside from recall bias, 2 explanations must be considered relative to the low rate of adherence to the CPG recommendation for an annual follow-up. The first is that the CPG simply may not be widely adopted. The second is that the majority of patients with ULA who use prostheses use a body-powered system. These tend to be low maintenance, long-lasting systems and may ultimately not need annual maintenance and repair. Further, if the veteran’s body-powered system is functioning properly and health status has not changed, they may simply be opting out of an annual visit despite the CPG recommendation. Nonetheless, this apparent low rate of annual follow-up emphasizes the need for additional process improvement measures for the VA.
Strengths and Limitations
The VA provides a unique setting for a nationally representative study of amputation rehabilitation because it has centralized data sources that can be used to identify veterans with ULA. Our study had a strong response rate, and its prosthetic limb care satisfaction findings are generalizable to all veterans with major ULA who received VA care from 2010 to 2015. However, there are limits to generalizability outside of this population to civilians or to veterans who do not receive VA care. To examine possible nonresponse bias, which could limit generalizability, we compared the baseline characteristics of respondents and nonrespondents to the follow-up study (eAppendix 4 available at doi:10.12788/fp.0096). There were no significant differences in satisfaction, quality of care, or receipt of amputation-related care between those lost to follow-up and those with follow-up data. Although, we did find small differences in gender, race, and service era (defined by amputation date). We do not believe that these differences threaten the interpretation of findings at follow-up, but there may be limits to generalizability of these findings to the full baseline sample. The data were from a telephone survey of veterans. It is possible that some veterans did not recall their care receipt or did not understand some of the questions and thus may not have accurately answered questions related to type of care received or the timing of that care.
Our interpretation of findings comparing care received within the VA and DoD or elsewhere is also limited because we cannot say with certainty whether those who indicated no care in the VA or DoD actually had care that was sponsored by the VA or DoD as contract or fee-basis care. Just 8 respondents indicated that they had received care only outside of the VA or DoD in the prior year. There were also some limitations in the collection of data about care location. We asked about receipt of amputation care in the prior year and about location of any amputation care received between baseline and follow-up, and there were differences in responses. Thus, we used a combination of these items to identify location of care received in the prior year.
Despite these limitations, we believe that our study provides novel, important findings about the satisfaction with prosthetic limb care services and quality of amputation rehabilitation care for veterans. Findings from this study can be used to address amputation and prosthetic limb care satisfaction and quality weaknesses highlighted and to benchmark care satisfaction and CPG compliance. Other studies evaluating care guideline compliance have used indicators obtained from clinical records or data repositories.13-15 Future work could combine self-reported satisfaction and care quality measures with indicators obtained from clinical or repository sources to provide a more complete snapshot of care delivery.
Conclusions
We reported on a national survey of veterans with major upper limb loss that assessed satisfaction with prosthetic limb care services and quality of amputation care. Satisfaction with prosthetic limb care was independently associated with satisfaction with the prosthesis, and receipt of care within the prior year. Most of the veterans surveyed received care within the VA or DoD and reported receiving higher quality of care, when compared with those who received care outside of the VA or DoD. Satisfaction with care and quality of care were stable over the year of this study. Data presented in this study can serve to direct VA amputation care process improvement initiatives as benchmarks for future work. Future studies are needed to track satisfaction with and quality of care for veterans with ULA.
1. Resnik L, Ekerholm S, Borgia M, Clark MA. A national study of veterans with major upper limb amputation: Survey methods, participants, and summary findings. PLoS One. 2019;14(3):e0213578. Published 2019 Mar 14. doi:10.1371/journal.pone.0213578
2. US Department of Defense, US Department of Veterans Affairs, Management of Upper Extremity Amputation Rehabilitation Working Group. VA/DoD clinical practice guideline for the management of upper extremity amputation rehabilitation.Published 2014. Accessed February 18, 2021. https://www.healthquality.va.gov/guidelines/Rehab/UEAR/VADoDCPGManagementofUEAR121614Corrected508.pdf
3. Jette AM. The Promise of Assistive Technology to Enhance Work Participation. Phys Ther. 2017;97(7):691-692. doi:10.1093/ptj/pzx054
4. Webster JB, Poorman CE, Cifu DX. Guest editorial: Department of Veterans Affairs amputations system of care: 5 years of accomplishments and outcomes. J Rehabil Res Dev. 2014;51(4):vii-xvi. doi:10.1682/JRRD.2014.01.0024
5. Scholten J, Poorman C, Culver L, Webster JB. Department of Veterans Affairs polytrauma telerehabilitation: twenty-first century care. Phys Med Rehabil Clin N Am. 2019;30(1):207-215. doi:10.1016/j.pmr.2018.08.003
6. Melcer T, Walker J, Bhatnagar V, Richard E. Clinic use at the Departments of Defense and Veterans Affairs following combat related amputations. Mil Med. 2020;185(1-2):e244-e253. doi:10.1093/milmed/usz149
7. Berke GM, Fergason J, Milani JR, et al. Comparison of satisfaction with current prosthetic care in veterans and servicemembers from Vietnam and OIF/OEF conflicts with major traumatic limb loss. J Rehabil Res Dev. 2010;47(4):361-371. doi:10.1682/jrrd.2009.12.0193
8. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection prosthetic limb care in VA facilities. Published March 8, 2012. Accessed February 18, 2021. https://www.va.gov/oig/pubs/VAOIG-11-02138-116.pdf 9. American Association for Public Opinion Research. Response rates - an overview. Accessed February 18, 2021. https://www.aapor.org/Education-Resources/For-Researchers/Poll-Survey-FAQ/Response-Rates-An-Overview.aspx
10. Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the Orthotics and Prosthetics Users’ Survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191-206. doi:10.1080/03093640308726682
11. Desmond DM, MacLachlan M. Factor structure of the Trinity Amputation and Prosthesis Experience Scales (TAPES) with individuals with acquired upper limb amputations. Am J Phys Med Rehabil. 2005;84(7):506-513. doi:10.1097/01.phm.0000166885.16180.63
12. Resnik L, Borgia M, Heinemann AW, Clark MA. Prosthesis satisfaction in a national sample of veterans with upper limb amputation. Prosthet Orthot Int. 2020;44(2):81-91. doi:10.1177/0309364619895201
13. Ho TH, Caughey GE, Shakib S. Guideline compliance in chronic heart failure patients with multiple comorbid diseases: evaluation of an individualised multidisciplinary model of care. PLoS One. 2014;9(4):e93129. Published 2014 Apr 8. doi:10.1371/journal.pone.0093129
14. Mitchell KB, Lin H, Shen Y, et al. DCIS and axillary nodal evaluation: compliance with national guidelines. BMC Surg. 2017;17(1):12. Published 2017 Feb 7. doi:10.1186/s12893-017-0210-5
15. Moesker MJ, de Groot JF, Damen NL, et al. Guideline compliance for bridging anticoagulation use in vitamin-K antagonist patients; practice variation and factors associated with non-compliance. Thromb J. 2019;17:15. Published 2019 Aug 5. doi:10.1186/s12959-019-0204-x
1. Resnik L, Ekerholm S, Borgia M, Clark MA. A national study of veterans with major upper limb amputation: Survey methods, participants, and summary findings. PLoS One. 2019;14(3):e0213578. Published 2019 Mar 14. doi:10.1371/journal.pone.0213578
2. US Department of Defense, US Department of Veterans Affairs, Management of Upper Extremity Amputation Rehabilitation Working Group. VA/DoD clinical practice guideline for the management of upper extremity amputation rehabilitation.Published 2014. Accessed February 18, 2021. https://www.healthquality.va.gov/guidelines/Rehab/UEAR/VADoDCPGManagementofUEAR121614Corrected508.pdf
3. Jette AM. The Promise of Assistive Technology to Enhance Work Participation. Phys Ther. 2017;97(7):691-692. doi:10.1093/ptj/pzx054
4. Webster JB, Poorman CE, Cifu DX. Guest editorial: Department of Veterans Affairs amputations system of care: 5 years of accomplishments and outcomes. J Rehabil Res Dev. 2014;51(4):vii-xvi. doi:10.1682/JRRD.2014.01.0024
5. Scholten J, Poorman C, Culver L, Webster JB. Department of Veterans Affairs polytrauma telerehabilitation: twenty-first century care. Phys Med Rehabil Clin N Am. 2019;30(1):207-215. doi:10.1016/j.pmr.2018.08.003
6. Melcer T, Walker J, Bhatnagar V, Richard E. Clinic use at the Departments of Defense and Veterans Affairs following combat related amputations. Mil Med. 2020;185(1-2):e244-e253. doi:10.1093/milmed/usz149
7. Berke GM, Fergason J, Milani JR, et al. Comparison of satisfaction with current prosthetic care in veterans and servicemembers from Vietnam and OIF/OEF conflicts with major traumatic limb loss. J Rehabil Res Dev. 2010;47(4):361-371. doi:10.1682/jrrd.2009.12.0193
8. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection prosthetic limb care in VA facilities. Published March 8, 2012. Accessed February 18, 2021. https://www.va.gov/oig/pubs/VAOIG-11-02138-116.pdf 9. American Association for Public Opinion Research. Response rates - an overview. Accessed February 18, 2021. https://www.aapor.org/Education-Resources/For-Researchers/Poll-Survey-FAQ/Response-Rates-An-Overview.aspx
10. Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the Orthotics and Prosthetics Users’ Survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191-206. doi:10.1080/03093640308726682
11. Desmond DM, MacLachlan M. Factor structure of the Trinity Amputation and Prosthesis Experience Scales (TAPES) with individuals with acquired upper limb amputations. Am J Phys Med Rehabil. 2005;84(7):506-513. doi:10.1097/01.phm.0000166885.16180.63
12. Resnik L, Borgia M, Heinemann AW, Clark MA. Prosthesis satisfaction in a national sample of veterans with upper limb amputation. Prosthet Orthot Int. 2020;44(2):81-91. doi:10.1177/0309364619895201
13. Ho TH, Caughey GE, Shakib S. Guideline compliance in chronic heart failure patients with multiple comorbid diseases: evaluation of an individualised multidisciplinary model of care. PLoS One. 2014;9(4):e93129. Published 2014 Apr 8. doi:10.1371/journal.pone.0093129
14. Mitchell KB, Lin H, Shen Y, et al. DCIS and axillary nodal evaluation: compliance with national guidelines. BMC Surg. 2017;17(1):12. Published 2017 Feb 7. doi:10.1186/s12893-017-0210-5
15. Moesker MJ, de Groot JF, Damen NL, et al. Guideline compliance for bridging anticoagulation use in vitamin-K antagonist patients; practice variation and factors associated with non-compliance. Thromb J. 2019;17:15. Published 2019 Aug 5. doi:10.1186/s12959-019-0204-x
OA risk-reduction program targets injured knees
A novel educational and personalized physical therapy program is showing signs that it may help people to mitigate their risk of developing knee osteoarthritis after an injury.
Speaking at the Canadian Arthritis Research Conference: Research with Impact, Jackie Whittaker, PhD, observed that initial work from the Stop Osteoarthritis (SOAR) program showed that meaningful improvements in knee-related quality of life and improvement in participants’ perceived self-management could be achieved.
Further feasibility work is ongoing and a proof-of-concept and phase 3 study need to follow, but the research suggests the approach could potentially help to reduce the substantial burden of managing people who develop posttraumatic OA (PTOA) of the knee.
Understanding the post–knee injury period
“Despite the progress that we’ve made in preventing injuries, and reducing disability in people with osteoarthritis, we lack good evidence about what should be done in the period between joint injury and the onset of osteoarthritis to delay or halt that onset,” Dr. Whittaker said at the virtual meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis.
That’s where the SOAR program comes in. For the past 8 years, Dr. Whittaker, an assistant professor in the department of physical therapy at the University of British Columbia in Vancouver and affiliated to Arthritis Research Canada, and collaborators have been looking into the post–knee injury period with the aim of developing an intervention that could potentially reduce the risk of OA further down the line.
Much work has gone into understanding the burden and risk factors for PTOA of the knee in order to know who exactly to target with the intervention and what the risk factors may be for the subsequent development of OA .
This research suggests that knee injuries are most commonly seen in people aged between 15 and 35 years who participated in sporting or other physical activities, so this is the target population for the SOAR intervention.
Broadly speaking, sustaining any knee injury is associated with a sixfold increased risk for subsequent PTOA, Dr. Whittaker observed.
“Despite the fact that ACL [anterior cruciate ligament] and meniscal tears get all the press, collateral ligament injury are still associated with about a fivefold increased risk of osteoarthritis, and therefore maybe shouldn’t be so easily dismissed as an important target,” Dr. Whittaker said.
Postinjury risk factors for OA
“Basically, what all prevention comes down to is our understanding of risk factors and our ability to be able to modify them,” she said.
Previous joint injury is one of the strongest and most established modifiable risk factors for developing knee OA, and Dr. Whittaker and associates have performed two small but “mighty” cohort studies comparing people who have and have not had a knee injury. These two studies have looked at different time periods following injury to see if they could first identify the risk factors for developing OA some 3-10 years later, and then to look more closely at some of those risk factors in first 2 years after injury with a view to targeting these with an intervention.
Data analysis of the latter study is still ongoing but have shown that, among injured subjects, there is a fear of movement and reinjury, knee strength is weaker in both injured and uninjured knees, and they are perhaps less physically active than those who have not been injured.
“Going into those two studies, we knew that this group of people already [had an] increased risk for osteoarthritis because they had an injury. However, what we found is that it looks like this risk may be compounded through adiposity [and] deficits in muscle strength and physical inactivity, which are associated with pain, stiffness, lack of confidence, and at times, unrealistic expectations and poor pacing,” Dr. Whittaker said.
She added: “It also looks like some of these additional factors and particular adiposity or fat gain may develop after injury, which would then give us a concrete target for delaying or halting the onset of osteoarthritis in the segment of the population.”
SOAR program components
The SOAR program intervention is an 8-week, physiotherapist-led program that targets people aged 15-35 years who have had a sport-related knee injury and received formal care. All of this is conducted via videoconferencing software and starts off with a 2-hour group education session or “knee camp.” This is followed by a one-on-one assessment with a physiotherapist and setting exercise and physical activity goals for the week.
Participants then undertake their personalized exercise and physical activity programs at home and track their progress using an activity monitor. They can participate in an optional weekly group exercise class and receive weekly one-on-one physiotherapy counseling where goals can be modified and any issues participants might be experiencing solved.
According to Dr. Whittaker, “this program really aims to increase participants capacity to manage their elevated risk for osteoarthritis, and we’re doing this by also optimizing their knee muscle function and their physical activity participation.”
While the knee camp enables a therapeutic alliance to be formed between participants and their physiotherapists, the weekly group classes provide social support and an opportunity to interact with others.
“Brief action planning builds self-efficacy [and] promotes autonomous health behaviors, while goal setting and tracking provide accountability, feedback about progress, and facilitated adherence,” she said.
And finally, regular communication with a physiotherapist in the program ensures timely support to learn how to navigate obstacles and helps participants to learn how to deal with their own knee health.
Testing the feasibility of the SOAR program intervention
“Currently we are smack in the middle of our feasibility study,” Dr. Whittaker said. So far, four physiotherapists have been trained to deliver an abridged, 4-week version of the program, and 25 of a planned 30 participants have been enrolled.
Results seem promising so far. No participants have dropped out of the program to date and attendance is at 100%.
“Based on data from the first 12 participants who completed the program, we are meeting all of our ‘a priori’ program benchmarks,” Dr. Whittaker said.
“It is very early days,” she emphasized, but “we are excited to see clinically important improvements in both knee-related quality of life and perceived self-management.
“This gives us some confidence that maybe all this time that we’ve put into developing our intervention is paying off, but obviously time will tell if we’re headed in the right direction,” she said. “Perhaps in time, we may be able to look at whether or not the individuals that participated in that program have fewer symptoms of OA disease. But that will obviously take us a few years before we’ll be able to get to that point.”
Dr. Whittaker acknowledged receiving funding for the SOAR program from the Arthritis Society, the Michael Smith Foundation for Health Research, BC SUPPORT Unit, and the Canadian Musculoskeletal Rehab Network.
A novel educational and personalized physical therapy program is showing signs that it may help people to mitigate their risk of developing knee osteoarthritis after an injury.
Speaking at the Canadian Arthritis Research Conference: Research with Impact, Jackie Whittaker, PhD, observed that initial work from the Stop Osteoarthritis (SOAR) program showed that meaningful improvements in knee-related quality of life and improvement in participants’ perceived self-management could be achieved.
Further feasibility work is ongoing and a proof-of-concept and phase 3 study need to follow, but the research suggests the approach could potentially help to reduce the substantial burden of managing people who develop posttraumatic OA (PTOA) of the knee.
Understanding the post–knee injury period
“Despite the progress that we’ve made in preventing injuries, and reducing disability in people with osteoarthritis, we lack good evidence about what should be done in the period between joint injury and the onset of osteoarthritis to delay or halt that onset,” Dr. Whittaker said at the virtual meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis.
That’s where the SOAR program comes in. For the past 8 years, Dr. Whittaker, an assistant professor in the department of physical therapy at the University of British Columbia in Vancouver and affiliated to Arthritis Research Canada, and collaborators have been looking into the post–knee injury period with the aim of developing an intervention that could potentially reduce the risk of OA further down the line.
Much work has gone into understanding the burden and risk factors for PTOA of the knee in order to know who exactly to target with the intervention and what the risk factors may be for the subsequent development of OA .
This research suggests that knee injuries are most commonly seen in people aged between 15 and 35 years who participated in sporting or other physical activities, so this is the target population for the SOAR intervention.
Broadly speaking, sustaining any knee injury is associated with a sixfold increased risk for subsequent PTOA, Dr. Whittaker observed.
“Despite the fact that ACL [anterior cruciate ligament] and meniscal tears get all the press, collateral ligament injury are still associated with about a fivefold increased risk of osteoarthritis, and therefore maybe shouldn’t be so easily dismissed as an important target,” Dr. Whittaker said.
Postinjury risk factors for OA
“Basically, what all prevention comes down to is our understanding of risk factors and our ability to be able to modify them,” she said.
Previous joint injury is one of the strongest and most established modifiable risk factors for developing knee OA, and Dr. Whittaker and associates have performed two small but “mighty” cohort studies comparing people who have and have not had a knee injury. These two studies have looked at different time periods following injury to see if they could first identify the risk factors for developing OA some 3-10 years later, and then to look more closely at some of those risk factors in first 2 years after injury with a view to targeting these with an intervention.
Data analysis of the latter study is still ongoing but have shown that, among injured subjects, there is a fear of movement and reinjury, knee strength is weaker in both injured and uninjured knees, and they are perhaps less physically active than those who have not been injured.
“Going into those two studies, we knew that this group of people already [had an] increased risk for osteoarthritis because they had an injury. However, what we found is that it looks like this risk may be compounded through adiposity [and] deficits in muscle strength and physical inactivity, which are associated with pain, stiffness, lack of confidence, and at times, unrealistic expectations and poor pacing,” Dr. Whittaker said.
She added: “It also looks like some of these additional factors and particular adiposity or fat gain may develop after injury, which would then give us a concrete target for delaying or halting the onset of osteoarthritis in the segment of the population.”
SOAR program components
The SOAR program intervention is an 8-week, physiotherapist-led program that targets people aged 15-35 years who have had a sport-related knee injury and received formal care. All of this is conducted via videoconferencing software and starts off with a 2-hour group education session or “knee camp.” This is followed by a one-on-one assessment with a physiotherapist and setting exercise and physical activity goals for the week.
Participants then undertake their personalized exercise and physical activity programs at home and track their progress using an activity monitor. They can participate in an optional weekly group exercise class and receive weekly one-on-one physiotherapy counseling where goals can be modified and any issues participants might be experiencing solved.
According to Dr. Whittaker, “this program really aims to increase participants capacity to manage their elevated risk for osteoarthritis, and we’re doing this by also optimizing their knee muscle function and their physical activity participation.”
While the knee camp enables a therapeutic alliance to be formed between participants and their physiotherapists, the weekly group classes provide social support and an opportunity to interact with others.
“Brief action planning builds self-efficacy [and] promotes autonomous health behaviors, while goal setting and tracking provide accountability, feedback about progress, and facilitated adherence,” she said.
And finally, regular communication with a physiotherapist in the program ensures timely support to learn how to navigate obstacles and helps participants to learn how to deal with their own knee health.
Testing the feasibility of the SOAR program intervention
“Currently we are smack in the middle of our feasibility study,” Dr. Whittaker said. So far, four physiotherapists have been trained to deliver an abridged, 4-week version of the program, and 25 of a planned 30 participants have been enrolled.
Results seem promising so far. No participants have dropped out of the program to date and attendance is at 100%.
“Based on data from the first 12 participants who completed the program, we are meeting all of our ‘a priori’ program benchmarks,” Dr. Whittaker said.
“It is very early days,” she emphasized, but “we are excited to see clinically important improvements in both knee-related quality of life and perceived self-management.
“This gives us some confidence that maybe all this time that we’ve put into developing our intervention is paying off, but obviously time will tell if we’re headed in the right direction,” she said. “Perhaps in time, we may be able to look at whether or not the individuals that participated in that program have fewer symptoms of OA disease. But that will obviously take us a few years before we’ll be able to get to that point.”
Dr. Whittaker acknowledged receiving funding for the SOAR program from the Arthritis Society, the Michael Smith Foundation for Health Research, BC SUPPORT Unit, and the Canadian Musculoskeletal Rehab Network.
A novel educational and personalized physical therapy program is showing signs that it may help people to mitigate their risk of developing knee osteoarthritis after an injury.
Speaking at the Canadian Arthritis Research Conference: Research with Impact, Jackie Whittaker, PhD, observed that initial work from the Stop Osteoarthritis (SOAR) program showed that meaningful improvements in knee-related quality of life and improvement in participants’ perceived self-management could be achieved.
Further feasibility work is ongoing and a proof-of-concept and phase 3 study need to follow, but the research suggests the approach could potentially help to reduce the substantial burden of managing people who develop posttraumatic OA (PTOA) of the knee.
Understanding the post–knee injury period
“Despite the progress that we’ve made in preventing injuries, and reducing disability in people with osteoarthritis, we lack good evidence about what should be done in the period between joint injury and the onset of osteoarthritis to delay or halt that onset,” Dr. Whittaker said at the virtual meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis.
That’s where the SOAR program comes in. For the past 8 years, Dr. Whittaker, an assistant professor in the department of physical therapy at the University of British Columbia in Vancouver and affiliated to Arthritis Research Canada, and collaborators have been looking into the post–knee injury period with the aim of developing an intervention that could potentially reduce the risk of OA further down the line.
Much work has gone into understanding the burden and risk factors for PTOA of the knee in order to know who exactly to target with the intervention and what the risk factors may be for the subsequent development of OA .
This research suggests that knee injuries are most commonly seen in people aged between 15 and 35 years who participated in sporting or other physical activities, so this is the target population for the SOAR intervention.
Broadly speaking, sustaining any knee injury is associated with a sixfold increased risk for subsequent PTOA, Dr. Whittaker observed.
“Despite the fact that ACL [anterior cruciate ligament] and meniscal tears get all the press, collateral ligament injury are still associated with about a fivefold increased risk of osteoarthritis, and therefore maybe shouldn’t be so easily dismissed as an important target,” Dr. Whittaker said.
Postinjury risk factors for OA
“Basically, what all prevention comes down to is our understanding of risk factors and our ability to be able to modify them,” she said.
Previous joint injury is one of the strongest and most established modifiable risk factors for developing knee OA, and Dr. Whittaker and associates have performed two small but “mighty” cohort studies comparing people who have and have not had a knee injury. These two studies have looked at different time periods following injury to see if they could first identify the risk factors for developing OA some 3-10 years later, and then to look more closely at some of those risk factors in first 2 years after injury with a view to targeting these with an intervention.
Data analysis of the latter study is still ongoing but have shown that, among injured subjects, there is a fear of movement and reinjury, knee strength is weaker in both injured and uninjured knees, and they are perhaps less physically active than those who have not been injured.
“Going into those two studies, we knew that this group of people already [had an] increased risk for osteoarthritis because they had an injury. However, what we found is that it looks like this risk may be compounded through adiposity [and] deficits in muscle strength and physical inactivity, which are associated with pain, stiffness, lack of confidence, and at times, unrealistic expectations and poor pacing,” Dr. Whittaker said.
She added: “It also looks like some of these additional factors and particular adiposity or fat gain may develop after injury, which would then give us a concrete target for delaying or halting the onset of osteoarthritis in the segment of the population.”
SOAR program components
The SOAR program intervention is an 8-week, physiotherapist-led program that targets people aged 15-35 years who have had a sport-related knee injury and received formal care. All of this is conducted via videoconferencing software and starts off with a 2-hour group education session or “knee camp.” This is followed by a one-on-one assessment with a physiotherapist and setting exercise and physical activity goals for the week.
Participants then undertake their personalized exercise and physical activity programs at home and track their progress using an activity monitor. They can participate in an optional weekly group exercise class and receive weekly one-on-one physiotherapy counseling where goals can be modified and any issues participants might be experiencing solved.
According to Dr. Whittaker, “this program really aims to increase participants capacity to manage their elevated risk for osteoarthritis, and we’re doing this by also optimizing their knee muscle function and their physical activity participation.”
While the knee camp enables a therapeutic alliance to be formed between participants and their physiotherapists, the weekly group classes provide social support and an opportunity to interact with others.
“Brief action planning builds self-efficacy [and] promotes autonomous health behaviors, while goal setting and tracking provide accountability, feedback about progress, and facilitated adherence,” she said.
And finally, regular communication with a physiotherapist in the program ensures timely support to learn how to navigate obstacles and helps participants to learn how to deal with their own knee health.
Testing the feasibility of the SOAR program intervention
“Currently we are smack in the middle of our feasibility study,” Dr. Whittaker said. So far, four physiotherapists have been trained to deliver an abridged, 4-week version of the program, and 25 of a planned 30 participants have been enrolled.
Results seem promising so far. No participants have dropped out of the program to date and attendance is at 100%.
“Based on data from the first 12 participants who completed the program, we are meeting all of our ‘a priori’ program benchmarks,” Dr. Whittaker said.
“It is very early days,” she emphasized, but “we are excited to see clinically important improvements in both knee-related quality of life and perceived self-management.
“This gives us some confidence that maybe all this time that we’ve put into developing our intervention is paying off, but obviously time will tell if we’re headed in the right direction,” she said. “Perhaps in time, we may be able to look at whether or not the individuals that participated in that program have fewer symptoms of OA disease. But that will obviously take us a few years before we’ll be able to get to that point.”
Dr. Whittaker acknowledged receiving funding for the SOAR program from the Arthritis Society, the Michael Smith Foundation for Health Research, BC SUPPORT Unit, and the Canadian Musculoskeletal Rehab Network.
FROM CARC 2021
Minimizing Opioids After Joint Operation: Protocol to Decrease Postoperative Opioid Use After Primary Total Knee Arthroplasty
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
Meta-analysis: No evidence that SNRIs relieve back pain
While some guidelines support serotonin norepinephrine reuptake inhibitors (SNRIs) as treatments for back pain, a new systematic review and meta-analysis of existing research found no firm evidence of a benefit. Adverse effects, however, are common.
“Our review shows that, although these medicines are effective, the effect is small and unlikely to be considered clinically important by most patients,” wrote the authors of the review, which appeared Jan. 20 in the BMJ. “Our review also showed that about two-thirds of patients using SNRIs experience adverse events.”
However, the report hinted that certain classes of antidepressants may provide significant relief in knee OA and sciatica.
According to a 2018 review, 10 of 15 clinical guidelines from around the world – including those of the American College of Physicians – recommended antidepressants as treatments for low back pain, and 2 advised against them. “Evidence supporting the use of antidepressants is, however, uncertain,” wrote the authors of the new review, led by Giovanni E. Ferreira, PhD, of the University of Sydney. “Systematic reviews of antidepressants for back pain and osteoarthritis have either not included several published trials, considered only one type of antidepressant (e.g., duloxetine), or failed to assess the certainty of evidence.”
For the new review, the authors analyzed 33 randomized, controlled trials with a total of 5,318 subjects. Both published data and unpublished data from clinical trial registries were included.
Back pain trials
A total of 19 trials examined back pain, mostly lower back pain (16 trials), and none lasted more than 1 year. Fifteen examined SNRIs while others looked at other kinds of antidepressants.
The researchers found that “the effect of SNRIs was small [on back pain] and below this review’s predetermined threshold of clinical importance. ... Evidence ranging from low to very low certainty showed no benefit of a range of antidepressant classes, including SSRIs [selective serotonin reuptake inhibitors], tetracyclic antidepressants, SARIs [serotonin antagonist and reuptake inhibitors], and NDRIs [norepinephrine and dopamine reuptake inhibitors] for pain and disability across follow-ups of 2 weeks or less, 3-13 weeks, and 3-12 months.”
Sciatica trials
Six trials examined antidepressants as treatments for sciatica. Very-low-certainty evidence suggested that SNRIs reduced pain at up to 2 weeks (1 trial, n = 50) but not at 3-13 weeks (3 trials, n = 96). The results of trials of tricyclic antidepressants (TCAs) were the opposite: low- to very-low-certainty evidence suggested the drugs didn’t reduce pain at up to 2 weeks (2 trials, n = 94) but did at 3-13 weeks (2 trials, n = 114) and 3-12 months (1 trial, n = 60).
“All sciatica trials were small, had imprecise estimates, and were at high risk of bias, which reduced the certainty of evidence to low and very low,” the authors cautioned. “This level of uncertainty indicates that the true estimate of effect of TCAs and SNRIs for sciatica is likely to be substantially different from what we estimated in our review.”
Knee OA trials
Eight trials examined SNRIs in knee OA. Moderate-certainty evidence linked the drugs to less pain at up to 2 weeks (four trials, n = 1,328) and low-certainty evidence linked them to less pain at 3-13 weeks (eight trials, n = 1,941). Low-certainty evidence also linked the drugs to less disability at 2 weeks or less (one trial, n = 353) and 3-13 weeks (seven trials, n = 1,810).
In knee OA, “the effect of SNRIs was small and below this review’s predetermined threshold of clinical importance,” the researchers wrote. “However, the lower limit of the confidence interval did contain clinically important effects for pain, but not for disability.”
Antidepressant side effects in trials
A total of 21 trials (n = 4,107) looked at side effects when antidepressants were studied as treatments for back pain and OA. Low-certainty evidence in 13 SNRI trials (n = 3,447) suggested a higher risk of any adverse events in antidepressant versus placebo (62.5% vs. 49.7%; relative risk, 1.23, 95% confidence interval, 1.16-1.30), but there was no significantly higher risk of serious adverse events in 10 SNRI trials with 3,309 subjects (1.6% vs. 1.3%; RR, 1.12, 95% CI, 0.61-2.07).
As for adverse effects of non-SNRIs, “the number of studies evaluating the safety of other antidepressant classes was small, trials were underpowered to detect harm, and the certainty of evidence ranged from low to very low,” the researchers wrote.
Going forward, the authors said that “large, definitive randomized trials that are free of industry ties are urgently needed to resolve uncertainties about the efficacy of antidepressants for sciatica and osteoarthritis highlighted by this review.”
‘Largely ineffective’ drug treatments
In an accompanying commentary, Martin Underwood, of the University of Warwick in Coventry, England, and Colin Tysall, of the University Hospitals of Coventry and Warwickshire, also in Coventry, noted that “drug treatments are largely ineffective for back pain and osteoarthritis and have the potential for serious harm. We need to work harder to help people with these disorders to live better with their pain without recourse to the prescription pad.”
However, they noted that SNRIs may still be helpful for patients with back pain or OA. “Absolute effect sizes for physical treatments for low-back pain are of similar magnitudes to those reported here and translate into numbers needed to treat of between five and nine. If the same were true for SNRIs, some people might choose to a try that option for a 1 in 10 chance of a worthwhile reduction in pain after 3 months. They can easily stop if treatment is ineffective or does not suit them.”
The research received no specific funding. The review authors disclosed relationships with GlaxoSmithKline (postgraduate scholarship), Pfizer (investigational product for two trials), and Flexeze (provision of heat wraps for a trial). Mr. Underwood reported being a director and shareholder of Clinvivo. Mr. Tysall reported no disclosures.
While some guidelines support serotonin norepinephrine reuptake inhibitors (SNRIs) as treatments for back pain, a new systematic review and meta-analysis of existing research found no firm evidence of a benefit. Adverse effects, however, are common.
“Our review shows that, although these medicines are effective, the effect is small and unlikely to be considered clinically important by most patients,” wrote the authors of the review, which appeared Jan. 20 in the BMJ. “Our review also showed that about two-thirds of patients using SNRIs experience adverse events.”
However, the report hinted that certain classes of antidepressants may provide significant relief in knee OA and sciatica.
According to a 2018 review, 10 of 15 clinical guidelines from around the world – including those of the American College of Physicians – recommended antidepressants as treatments for low back pain, and 2 advised against them. “Evidence supporting the use of antidepressants is, however, uncertain,” wrote the authors of the new review, led by Giovanni E. Ferreira, PhD, of the University of Sydney. “Systematic reviews of antidepressants for back pain and osteoarthritis have either not included several published trials, considered only one type of antidepressant (e.g., duloxetine), or failed to assess the certainty of evidence.”
For the new review, the authors analyzed 33 randomized, controlled trials with a total of 5,318 subjects. Both published data and unpublished data from clinical trial registries were included.
Back pain trials
A total of 19 trials examined back pain, mostly lower back pain (16 trials), and none lasted more than 1 year. Fifteen examined SNRIs while others looked at other kinds of antidepressants.
The researchers found that “the effect of SNRIs was small [on back pain] and below this review’s predetermined threshold of clinical importance. ... Evidence ranging from low to very low certainty showed no benefit of a range of antidepressant classes, including SSRIs [selective serotonin reuptake inhibitors], tetracyclic antidepressants, SARIs [serotonin antagonist and reuptake inhibitors], and NDRIs [norepinephrine and dopamine reuptake inhibitors] for pain and disability across follow-ups of 2 weeks or less, 3-13 weeks, and 3-12 months.”
Sciatica trials
Six trials examined antidepressants as treatments for sciatica. Very-low-certainty evidence suggested that SNRIs reduced pain at up to 2 weeks (1 trial, n = 50) but not at 3-13 weeks (3 trials, n = 96). The results of trials of tricyclic antidepressants (TCAs) were the opposite: low- to very-low-certainty evidence suggested the drugs didn’t reduce pain at up to 2 weeks (2 trials, n = 94) but did at 3-13 weeks (2 trials, n = 114) and 3-12 months (1 trial, n = 60).
“All sciatica trials were small, had imprecise estimates, and were at high risk of bias, which reduced the certainty of evidence to low and very low,” the authors cautioned. “This level of uncertainty indicates that the true estimate of effect of TCAs and SNRIs for sciatica is likely to be substantially different from what we estimated in our review.”
Knee OA trials
Eight trials examined SNRIs in knee OA. Moderate-certainty evidence linked the drugs to less pain at up to 2 weeks (four trials, n = 1,328) and low-certainty evidence linked them to less pain at 3-13 weeks (eight trials, n = 1,941). Low-certainty evidence also linked the drugs to less disability at 2 weeks or less (one trial, n = 353) and 3-13 weeks (seven trials, n = 1,810).
In knee OA, “the effect of SNRIs was small and below this review’s predetermined threshold of clinical importance,” the researchers wrote. “However, the lower limit of the confidence interval did contain clinically important effects for pain, but not for disability.”
Antidepressant side effects in trials
A total of 21 trials (n = 4,107) looked at side effects when antidepressants were studied as treatments for back pain and OA. Low-certainty evidence in 13 SNRI trials (n = 3,447) suggested a higher risk of any adverse events in antidepressant versus placebo (62.5% vs. 49.7%; relative risk, 1.23, 95% confidence interval, 1.16-1.30), but there was no significantly higher risk of serious adverse events in 10 SNRI trials with 3,309 subjects (1.6% vs. 1.3%; RR, 1.12, 95% CI, 0.61-2.07).
As for adverse effects of non-SNRIs, “the number of studies evaluating the safety of other antidepressant classes was small, trials were underpowered to detect harm, and the certainty of evidence ranged from low to very low,” the researchers wrote.
Going forward, the authors said that “large, definitive randomized trials that are free of industry ties are urgently needed to resolve uncertainties about the efficacy of antidepressants for sciatica and osteoarthritis highlighted by this review.”
‘Largely ineffective’ drug treatments
In an accompanying commentary, Martin Underwood, of the University of Warwick in Coventry, England, and Colin Tysall, of the University Hospitals of Coventry and Warwickshire, also in Coventry, noted that “drug treatments are largely ineffective for back pain and osteoarthritis and have the potential for serious harm. We need to work harder to help people with these disorders to live better with their pain without recourse to the prescription pad.”
However, they noted that SNRIs may still be helpful for patients with back pain or OA. “Absolute effect sizes for physical treatments for low-back pain are of similar magnitudes to those reported here and translate into numbers needed to treat of between five and nine. If the same were true for SNRIs, some people might choose to a try that option for a 1 in 10 chance of a worthwhile reduction in pain after 3 months. They can easily stop if treatment is ineffective or does not suit them.”
The research received no specific funding. The review authors disclosed relationships with GlaxoSmithKline (postgraduate scholarship), Pfizer (investigational product for two trials), and Flexeze (provision of heat wraps for a trial). Mr. Underwood reported being a director and shareholder of Clinvivo. Mr. Tysall reported no disclosures.
While some guidelines support serotonin norepinephrine reuptake inhibitors (SNRIs) as treatments for back pain, a new systematic review and meta-analysis of existing research found no firm evidence of a benefit. Adverse effects, however, are common.
“Our review shows that, although these medicines are effective, the effect is small and unlikely to be considered clinically important by most patients,” wrote the authors of the review, which appeared Jan. 20 in the BMJ. “Our review also showed that about two-thirds of patients using SNRIs experience adverse events.”
However, the report hinted that certain classes of antidepressants may provide significant relief in knee OA and sciatica.
According to a 2018 review, 10 of 15 clinical guidelines from around the world – including those of the American College of Physicians – recommended antidepressants as treatments for low back pain, and 2 advised against them. “Evidence supporting the use of antidepressants is, however, uncertain,” wrote the authors of the new review, led by Giovanni E. Ferreira, PhD, of the University of Sydney. “Systematic reviews of antidepressants for back pain and osteoarthritis have either not included several published trials, considered only one type of antidepressant (e.g., duloxetine), or failed to assess the certainty of evidence.”
For the new review, the authors analyzed 33 randomized, controlled trials with a total of 5,318 subjects. Both published data and unpublished data from clinical trial registries were included.
Back pain trials
A total of 19 trials examined back pain, mostly lower back pain (16 trials), and none lasted more than 1 year. Fifteen examined SNRIs while others looked at other kinds of antidepressants.
The researchers found that “the effect of SNRIs was small [on back pain] and below this review’s predetermined threshold of clinical importance. ... Evidence ranging from low to very low certainty showed no benefit of a range of antidepressant classes, including SSRIs [selective serotonin reuptake inhibitors], tetracyclic antidepressants, SARIs [serotonin antagonist and reuptake inhibitors], and NDRIs [norepinephrine and dopamine reuptake inhibitors] for pain and disability across follow-ups of 2 weeks or less, 3-13 weeks, and 3-12 months.”
Sciatica trials
Six trials examined antidepressants as treatments for sciatica. Very-low-certainty evidence suggested that SNRIs reduced pain at up to 2 weeks (1 trial, n = 50) but not at 3-13 weeks (3 trials, n = 96). The results of trials of tricyclic antidepressants (TCAs) were the opposite: low- to very-low-certainty evidence suggested the drugs didn’t reduce pain at up to 2 weeks (2 trials, n = 94) but did at 3-13 weeks (2 trials, n = 114) and 3-12 months (1 trial, n = 60).
“All sciatica trials were small, had imprecise estimates, and were at high risk of bias, which reduced the certainty of evidence to low and very low,” the authors cautioned. “This level of uncertainty indicates that the true estimate of effect of TCAs and SNRIs for sciatica is likely to be substantially different from what we estimated in our review.”
Knee OA trials
Eight trials examined SNRIs in knee OA. Moderate-certainty evidence linked the drugs to less pain at up to 2 weeks (four trials, n = 1,328) and low-certainty evidence linked them to less pain at 3-13 weeks (eight trials, n = 1,941). Low-certainty evidence also linked the drugs to less disability at 2 weeks or less (one trial, n = 353) and 3-13 weeks (seven trials, n = 1,810).
In knee OA, “the effect of SNRIs was small and below this review’s predetermined threshold of clinical importance,” the researchers wrote. “However, the lower limit of the confidence interval did contain clinically important effects for pain, but not for disability.”
Antidepressant side effects in trials
A total of 21 trials (n = 4,107) looked at side effects when antidepressants were studied as treatments for back pain and OA. Low-certainty evidence in 13 SNRI trials (n = 3,447) suggested a higher risk of any adverse events in antidepressant versus placebo (62.5% vs. 49.7%; relative risk, 1.23, 95% confidence interval, 1.16-1.30), but there was no significantly higher risk of serious adverse events in 10 SNRI trials with 3,309 subjects (1.6% vs. 1.3%; RR, 1.12, 95% CI, 0.61-2.07).
As for adverse effects of non-SNRIs, “the number of studies evaluating the safety of other antidepressant classes was small, trials were underpowered to detect harm, and the certainty of evidence ranged from low to very low,” the researchers wrote.
Going forward, the authors said that “large, definitive randomized trials that are free of industry ties are urgently needed to resolve uncertainties about the efficacy of antidepressants for sciatica and osteoarthritis highlighted by this review.”
‘Largely ineffective’ drug treatments
In an accompanying commentary, Martin Underwood, of the University of Warwick in Coventry, England, and Colin Tysall, of the University Hospitals of Coventry and Warwickshire, also in Coventry, noted that “drug treatments are largely ineffective for back pain and osteoarthritis and have the potential for serious harm. We need to work harder to help people with these disorders to live better with their pain without recourse to the prescription pad.”
However, they noted that SNRIs may still be helpful for patients with back pain or OA. “Absolute effect sizes for physical treatments for low-back pain are of similar magnitudes to those reported here and translate into numbers needed to treat of between five and nine. If the same were true for SNRIs, some people might choose to a try that option for a 1 in 10 chance of a worthwhile reduction in pain after 3 months. They can easily stop if treatment is ineffective or does not suit them.”
The research received no specific funding. The review authors disclosed relationships with GlaxoSmithKline (postgraduate scholarship), Pfizer (investigational product for two trials), and Flexeze (provision of heat wraps for a trial). Mr. Underwood reported being a director and shareholder of Clinvivo. Mr. Tysall reported no disclosures.
FROM THE BMJ