User login
A saliva test for diagnosing endometriosis?
A French research team has developed a microRNA (miRNA) signature for diagnosing endometriosis through a simple saliva test. Its validation in a larger cohort could soon allow doctors to have a cheap, noninvasive, and accurate tool to use in diagnosing a disease that, for the time being, is difficult to identify with any certainty. The researchers suggest that their methodology could be used as a blueprint to investigate other pathologies, both benign and malignant.
ENDO-miRNA study
miRNAs regulate as much as 60% of gene expression at the posttranscriptional level. In the setting of endometriosis, several authors have evaluated the relevance of a blood-based miRNA signature, but the results are discordant because of methodological and control group issues. Other researchers have also sought to develop a miRNA saliva test. A French team wanted to determine whether it was possible to define a saliva-based diagnostic miRNome signature that would allow patients with and without endometriosis to be differentiated and, from there, develop the first specific diagnostic test for the disease.
The prospective ENDO-miRNA study included saliva samples obtained from women with chronic pelvic pain suggestive of endometriosis. Exploratory procedures were performed to look for lesions. All the patients underwent either a laparoscopic procedure (therapeutic or diagnostic laparoscopy) and/or MRI imaging. For the patients who underwent laparoscopy, diagnosis was confirmed by histology. For the patients diagnosed with endometriosis without laparoscopic evaluation, all had MRI imaging with features of deep endometriosis.
One part of the study involved the identification of a biomarker based on genomewide miRNA expression profiling by small RNA sequencing using next-generation sequencing. The second part involved the development of a saliva-based miRNA diagnostic signature according to expression and accuracy profiling using a random forest algorithm.
High sensitivity, specificity
Among the 200 patients (mean age, 31 years) enrolled in the study, 76.5% (n = 153) were diagnosed with endometriosis. On average, their pain was statistically more severe than that of the women in the control group. The Visual Analogue Scale (VAS) scores were, respectively: dysmenorrhea 6 versus 5.0 (P < .001), dyspareunia 5.28 versus 4.95 (P < .001), and urinary pain during menstruation 4.35 versus 2.84 (P < .001).
Next-generation sequencing identified an average of 2,561 expressed miRNAs in the saliva samples. The feature selection method generated a subset of 109 miRNAs composing the endometriosis diagnostic signature. Among those miRNAs, 29 were associated with the main signaling pathways of endometriosis: PI3K/AKT, PTEN, Wnt/beta-catenin, HIF1-alpha/NF kappa B, and YAP/TAZ/EGFR.
The accuracy and reproducibility of the signature were tested on several data sets randomly composed of the same proportion of controls and patients with endometriosis. The respective sensitivity, specificity, and area under the curve for the diagnostic miRNA signature were 96.7%, 100%, and 98.3%, respectively.
The study’s results support the use of a saliva-based miRNA signature for diagnosing whether a patient is discordant/complex (chronic pelvic pain suggestive of endometriosis and both negative clinical examination and imaging findings) or has early-stage or advanced-stage endometriosis.
A version of this article first appeared on Medscape.com.
A French research team has developed a microRNA (miRNA) signature for diagnosing endometriosis through a simple saliva test. Its validation in a larger cohort could soon allow doctors to have a cheap, noninvasive, and accurate tool to use in diagnosing a disease that, for the time being, is difficult to identify with any certainty. The researchers suggest that their methodology could be used as a blueprint to investigate other pathologies, both benign and malignant.
ENDO-miRNA study
miRNAs regulate as much as 60% of gene expression at the posttranscriptional level. In the setting of endometriosis, several authors have evaluated the relevance of a blood-based miRNA signature, but the results are discordant because of methodological and control group issues. Other researchers have also sought to develop a miRNA saliva test. A French team wanted to determine whether it was possible to define a saliva-based diagnostic miRNome signature that would allow patients with and without endometriosis to be differentiated and, from there, develop the first specific diagnostic test for the disease.
The prospective ENDO-miRNA study included saliva samples obtained from women with chronic pelvic pain suggestive of endometriosis. Exploratory procedures were performed to look for lesions. All the patients underwent either a laparoscopic procedure (therapeutic or diagnostic laparoscopy) and/or MRI imaging. For the patients who underwent laparoscopy, diagnosis was confirmed by histology. For the patients diagnosed with endometriosis without laparoscopic evaluation, all had MRI imaging with features of deep endometriosis.
One part of the study involved the identification of a biomarker based on genomewide miRNA expression profiling by small RNA sequencing using next-generation sequencing. The second part involved the development of a saliva-based miRNA diagnostic signature according to expression and accuracy profiling using a random forest algorithm.
High sensitivity, specificity
Among the 200 patients (mean age, 31 years) enrolled in the study, 76.5% (n = 153) were diagnosed with endometriosis. On average, their pain was statistically more severe than that of the women in the control group. The Visual Analogue Scale (VAS) scores were, respectively: dysmenorrhea 6 versus 5.0 (P < .001), dyspareunia 5.28 versus 4.95 (P < .001), and urinary pain during menstruation 4.35 versus 2.84 (P < .001).
Next-generation sequencing identified an average of 2,561 expressed miRNAs in the saliva samples. The feature selection method generated a subset of 109 miRNAs composing the endometriosis diagnostic signature. Among those miRNAs, 29 were associated with the main signaling pathways of endometriosis: PI3K/AKT, PTEN, Wnt/beta-catenin, HIF1-alpha/NF kappa B, and YAP/TAZ/EGFR.
The accuracy and reproducibility of the signature were tested on several data sets randomly composed of the same proportion of controls and patients with endometriosis. The respective sensitivity, specificity, and area under the curve for the diagnostic miRNA signature were 96.7%, 100%, and 98.3%, respectively.
The study’s results support the use of a saliva-based miRNA signature for diagnosing whether a patient is discordant/complex (chronic pelvic pain suggestive of endometriosis and both negative clinical examination and imaging findings) or has early-stage or advanced-stage endometriosis.
A version of this article first appeared on Medscape.com.
A French research team has developed a microRNA (miRNA) signature for diagnosing endometriosis through a simple saliva test. Its validation in a larger cohort could soon allow doctors to have a cheap, noninvasive, and accurate tool to use in diagnosing a disease that, for the time being, is difficult to identify with any certainty. The researchers suggest that their methodology could be used as a blueprint to investigate other pathologies, both benign and malignant.
ENDO-miRNA study
miRNAs regulate as much as 60% of gene expression at the posttranscriptional level. In the setting of endometriosis, several authors have evaluated the relevance of a blood-based miRNA signature, but the results are discordant because of methodological and control group issues. Other researchers have also sought to develop a miRNA saliva test. A French team wanted to determine whether it was possible to define a saliva-based diagnostic miRNome signature that would allow patients with and without endometriosis to be differentiated and, from there, develop the first specific diagnostic test for the disease.
The prospective ENDO-miRNA study included saliva samples obtained from women with chronic pelvic pain suggestive of endometriosis. Exploratory procedures were performed to look for lesions. All the patients underwent either a laparoscopic procedure (therapeutic or diagnostic laparoscopy) and/or MRI imaging. For the patients who underwent laparoscopy, diagnosis was confirmed by histology. For the patients diagnosed with endometriosis without laparoscopic evaluation, all had MRI imaging with features of deep endometriosis.
One part of the study involved the identification of a biomarker based on genomewide miRNA expression profiling by small RNA sequencing using next-generation sequencing. The second part involved the development of a saliva-based miRNA diagnostic signature according to expression and accuracy profiling using a random forest algorithm.
High sensitivity, specificity
Among the 200 patients (mean age, 31 years) enrolled in the study, 76.5% (n = 153) were diagnosed with endometriosis. On average, their pain was statistically more severe than that of the women in the control group. The Visual Analogue Scale (VAS) scores were, respectively: dysmenorrhea 6 versus 5.0 (P < .001), dyspareunia 5.28 versus 4.95 (P < .001), and urinary pain during menstruation 4.35 versus 2.84 (P < .001).
Next-generation sequencing identified an average of 2,561 expressed miRNAs in the saliva samples. The feature selection method generated a subset of 109 miRNAs composing the endometriosis diagnostic signature. Among those miRNAs, 29 were associated with the main signaling pathways of endometriosis: PI3K/AKT, PTEN, Wnt/beta-catenin, HIF1-alpha/NF kappa B, and YAP/TAZ/EGFR.
The accuracy and reproducibility of the signature were tested on several data sets randomly composed of the same proportion of controls and patients with endometriosis. The respective sensitivity, specificity, and area under the curve for the diagnostic miRNA signature were 96.7%, 100%, and 98.3%, respectively.
The study’s results support the use of a saliva-based miRNA signature for diagnosing whether a patient is discordant/complex (chronic pelvic pain suggestive of endometriosis and both negative clinical examination and imaging findings) or has early-stage or advanced-stage endometriosis.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL MEDICINE
Orphenadrine recalled due to possible nitrosamine impurity
Recent tests of 13 lots of the skeletal muscle relaxant Orphenadrine Citrate 100 mg Extended Release (ER) found unacceptably high levels of a nitrosamine impurity in the tablets, leading manufacturer Sandoz (Princeton, N.J.) to announce a voluntary recall of the lots on March 21.
The nitrosamine impurity detected (N-methyl-N-nitroso-2-[(2-methylphenyl)phenylmethoxy]ethanamine [NMOA or Nitroso-Orphenadrine]) may potentially be consumed at a level higher than the Food and Drug Administration’s Acceptable Daily Intake of 26.5 ng/day. Nitrosamines have carcinogenic potency when present above the allowable exposure limits, according to Sandoz, but the company said it “has not received any reports of adverse events related to the presence of a nitrosamine impurity in the lot.”
The Orphenadrine Citrate 100 mg ER Tablets were shipped to customers from August 2019 to April 2021 and have lot numbers of JX6411, JX6413, KC0723, KC3303, KE4348, KE7169, KE4349, KL3199, KM0072, KS3939, LA7704, LA7703, and LA9243.
The lots contain 100- and 1,000-count bottles of Orphenadrine Citrate ER Tablets, which are used as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute painful musculoskeletal conditions.
The recall does not apply to any other strengths of Sandoz’s Orphenadrine Citrate ER Tablets or to other lot numbers of the product.
Sandoz advises that wholesalers and distributors should “immediately stop distribution of the recalled product and quarantine and return all recalled product in their inventory.” The company advises consumers to stop taking the recalled product and immediately consult with their physicians to obtain another prescription, notifying them of any problems that may be related to taking or using the tablets.
Sandoz says that retailers and consumers should contact Sedgwick directly by phone at 844-491-7869 or email at [email protected] to return the recalled product, and report adverse reactions to Sandoz by phone at (800) 525-8747 or by email at [email protected]. Adverse reactions and quality problems can be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail, or by fax to 1-800-FDA-0178.
A version of this article first appeared on Medscape.com.
Recent tests of 13 lots of the skeletal muscle relaxant Orphenadrine Citrate 100 mg Extended Release (ER) found unacceptably high levels of a nitrosamine impurity in the tablets, leading manufacturer Sandoz (Princeton, N.J.) to announce a voluntary recall of the lots on March 21.
The nitrosamine impurity detected (N-methyl-N-nitroso-2-[(2-methylphenyl)phenylmethoxy]ethanamine [NMOA or Nitroso-Orphenadrine]) may potentially be consumed at a level higher than the Food and Drug Administration’s Acceptable Daily Intake of 26.5 ng/day. Nitrosamines have carcinogenic potency when present above the allowable exposure limits, according to Sandoz, but the company said it “has not received any reports of adverse events related to the presence of a nitrosamine impurity in the lot.”
The Orphenadrine Citrate 100 mg ER Tablets were shipped to customers from August 2019 to April 2021 and have lot numbers of JX6411, JX6413, KC0723, KC3303, KE4348, KE7169, KE4349, KL3199, KM0072, KS3939, LA7704, LA7703, and LA9243.
The lots contain 100- and 1,000-count bottles of Orphenadrine Citrate ER Tablets, which are used as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute painful musculoskeletal conditions.
The recall does not apply to any other strengths of Sandoz’s Orphenadrine Citrate ER Tablets or to other lot numbers of the product.
Sandoz advises that wholesalers and distributors should “immediately stop distribution of the recalled product and quarantine and return all recalled product in their inventory.” The company advises consumers to stop taking the recalled product and immediately consult with their physicians to obtain another prescription, notifying them of any problems that may be related to taking or using the tablets.
Sandoz says that retailers and consumers should contact Sedgwick directly by phone at 844-491-7869 or email at [email protected] to return the recalled product, and report adverse reactions to Sandoz by phone at (800) 525-8747 or by email at [email protected]. Adverse reactions and quality problems can be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail, or by fax to 1-800-FDA-0178.
A version of this article first appeared on Medscape.com.
Recent tests of 13 lots of the skeletal muscle relaxant Orphenadrine Citrate 100 mg Extended Release (ER) found unacceptably high levels of a nitrosamine impurity in the tablets, leading manufacturer Sandoz (Princeton, N.J.) to announce a voluntary recall of the lots on March 21.
The nitrosamine impurity detected (N-methyl-N-nitroso-2-[(2-methylphenyl)phenylmethoxy]ethanamine [NMOA or Nitroso-Orphenadrine]) may potentially be consumed at a level higher than the Food and Drug Administration’s Acceptable Daily Intake of 26.5 ng/day. Nitrosamines have carcinogenic potency when present above the allowable exposure limits, according to Sandoz, but the company said it “has not received any reports of adverse events related to the presence of a nitrosamine impurity in the lot.”
The Orphenadrine Citrate 100 mg ER Tablets were shipped to customers from August 2019 to April 2021 and have lot numbers of JX6411, JX6413, KC0723, KC3303, KE4348, KE7169, KE4349, KL3199, KM0072, KS3939, LA7704, LA7703, and LA9243.
The lots contain 100- and 1,000-count bottles of Orphenadrine Citrate ER Tablets, which are used as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute painful musculoskeletal conditions.
The recall does not apply to any other strengths of Sandoz’s Orphenadrine Citrate ER Tablets or to other lot numbers of the product.
Sandoz advises that wholesalers and distributors should “immediately stop distribution of the recalled product and quarantine and return all recalled product in their inventory.” The company advises consumers to stop taking the recalled product and immediately consult with their physicians to obtain another prescription, notifying them of any problems that may be related to taking or using the tablets.
Sandoz says that retailers and consumers should contact Sedgwick directly by phone at 844-491-7869 or email at [email protected] to return the recalled product, and report adverse reactions to Sandoz by phone at (800) 525-8747 or by email at [email protected]. Adverse reactions and quality problems can be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail, or by fax to 1-800-FDA-0178.
A version of this article first appeared on Medscape.com.
Evaluation of the Empower Veterans Program for Military Veterans With Chronic Pain
From Neurology/Chronic Pain Management Services, Department of Veterans Affairs (VA) Maryland Health Care System, Baltimore VA Medical Center, Baltimore, MD (Dr. Uche), and School of Nursing, Washburn University, Topeka, KS (Drs. Jamison and Waugh).
Abstract
Objective: The purpose of this quality improvement project was to abstract and analyze previously collected data from veterans with high-impact chronic pain who attended the Empower Veterans Program (EVP) offered by a Veterans Administration facility in the northeastern United States.
Methods: This quality improvement project used data collected from veterans with chronic pain who completed the veterans health care facility’s EVP between August 2017 and August 2019. Pre- and post-intervention data on pain intensity, pain interference, quality of life, and pain catastrophizing were compared using paired t-tests.
Results: Although data were abstracted from 115 patients, the final sample included
Conclusion: Veterans with chronic high-impact noncancer pain who completed the EVP had reduced pain intensity, pain interference, pain catastrophizing as well as improved quality of life and satisfaction with their health.
Keywords: musculoskeletal pain, Veterans Affairs, complementary and integrative health, acceptance and commitment therapy, mind-body therapies, whole health, multidisciplinary pain management.
More than 100 million American adults suffer from chronic pain; costs associated with managing chronic pain are approximately $635 billion
One such program is the Empower Veterans Program (EVP). Originally developed at the Atlanta Veterans Affairs Health Care System, the EVP is a CIH modality based on the biopsychosocial model of pain developed by psychiatrist George Engel in 1977.3 The biopsychosocial model of pain recognizes that pain is a complex, multidimensional, biopsychosocial experience. Under this model, the mind and body work in unison as interconnected entities. Because the model acknowledges biological, psychological, and social components of pain and illness,4 treatment focuses on all aspects of a person’s health, life, and relationships.
The EVP fits into the VHA Pain Management Stepped Care Model and is an adjunctive complement for that model.5-7 The EVP complements care at the first step, where patient/family provide self-care and where care is provided by patient-aligned primary care teams, at the second step, which includes secondary consultation with multidisciplinary pain medicine specialty teams and other specialists, and at the third step, with the addition of tertiary interdisciplinary pain centers.
The VA Maryland Health Care System (VAMHCS) implemented the EVP as part of a quality improvement project for the management of chronic pain. The objectives of the program were to reduce pain intensity, pain catastrophizing, and pain interference, as well as improve functionality and quality of life among veterans with chronic high-impact noncancer pain. More than 2 years after the program was implemented, collected data had not been analyzed. The purpose of this quality improvement project was to abstract and analyze the previously collected data from veterans with high-impact chronic pain who attended an EVP offered by the VAMHCS. The results of the data analysis were used to inform decisions regarding the future of the program.
Methods
This quality improvement project used the Plan-Do-Study-Act (PDSA) process.8 The first 2 phases of the PDSA cycle (Plan and Do) were completed by a team of VA employees from the VAMHCS, who donated their time to establish and implement the program at the project site. This team consisted of psychologists, a physical therapist, a social worker, and a chaplain, and included support from medical administrative staff. This team planned and implemented the EVP at the VA facility based on the model developed at the Atlanta VA Health Care System. During the “Do” phase, the team collected data on pain intensity, pain interference, quality of life, and pain negative cognition (catastrophizing) before the intervention and post intervention. They also collected data on program outcome (patient treatment satisfaction) post intervention. Because these employees did not have time to retrieve and analyze the data, they welcomed the opportunity to have the data analyzed by the investigators during the Study phase of the PDSA cycle. Based on the results of the analysis, recommendations for program changes were made during the Act phase of the cycle.
Intervention
The EVP was developed as a 10-week (30 hours) interdisciplinary CIH approach that coached veterans with chronic pain to live fuller lives based on their individual values and what matters to them. EVP is the “What Else” management modality for the 5% of veterans with high-impact chronic pain.9 The EVP provided functional restoration through its components of whole health, mindfulness training, coaching calls, acceptance and commitment therapy, and mindful movement. It used the Wheel of Health with the 4 key components of me, self-care, professional care, and community.10,11
Veterans who had a diagnosis of chronic nonmalignant pain for 3 months or more and who agreed to participate in the EVP at this facility attended 3-hour classes every Tuesday with a cohort of 8 to 12 peers and engaged in one-on-one coaching with interdisciplinary team members. During the class sessions, veterans were coached to understand and accept their pain and commit to maintaining function despite their pain. Mindful movement by the physical therapist emphasized the pivotal place of exercise in pain management. The therapist used the mantra “Motion is Lotion.”9 The guiding principle of the EVP was that small incremental changes can have a big impact on the individual’s whole life. Emphasis was placed on increasing self-efficacy and mindful awareness for veterans with high-impact pain by giving them “Skills before Pills.”9
Outcome Measures
Outcome measures included the Numerical Pain Rating Scale (NPRS), the Multidimensional Pain Inventory (MPI), the World Health Organization Quality of Life assessment (WHOQOL-BREF), the Pain Catastrophizing Scale (PCS), and the Pain Treatment Satisfaction Scale (PTSS). Cronbach alpha coefficients were calculated to assess internal consistency reliability of these measures in the sample of veterans who completed the EVP.
NPRS. The NPRS is ubiquitous as a screening tool in many health care environments and its use is mandated by the VA health care system.12 The choice of the NPRS as the tool for pain screening in the VA health care system was based on a large body of research that supports the reliability and validity of the NPRS as a single index of pain intensity or severity. Studies suggest that the NPRS is valid for use in the assessment of acute, cancer, or chronic nonmalignant pain and in varied clinical settings.13 The NPRS has 4 items, each on a scale of 0 to 10. For the purpose of this project, only 3 items were used. The 3 items assessed the worst pain, usual pain, and the current pain (right now). The higher the score, the higher the pain intensity. Cronbach alpha coefficients on the NPRS obtained from the current sample of veterans were 0.85 on both pre- and postintervention assessments.
MPI. The MPI is an easily accessible, reliable, and valid self-report questionnaire that measures the impact of pain on an individual’s life, quality of social support, and general activity.14 This instrument is a short version of the West Haven-Yale MPI.15 The MPI contains 9 items rated on a scale from 0 to 6. The higher the score, the greater pain interference a person is experiencing. The MPI produces reliable, valid information for diagnostic purposes and for therapy outcome studies.16 The MPI had a Cronbach alpha of 0.90 on pre-intervention and 0.92 on postintervention assessments in the current sample.
WHOQOL-BREF. The WHOQOL-BREF is a measure of quality of life and is an abbreviated version of the WHOQOL-100. Quality of life is defined by the World Health Organization17 “as an individuals’ perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns.” The WHOQOL-BREF contains 26 items. The first 2 items were examined separately; the first item asks individuals to rate their overall quality of life and the second asks individuals how satisfied they are with their health. The remaining 24 items were used to calculate the following 4 domain scores: physical health, psychological health, social relationship, and environment.18 Each item is measured on a scale of 1 to 5. Higher scores denote higher or better quality of life. Domain scores have demonstrated good reliability and validity.19-21 Cronbach alpha coefficients for the domain subscales ranged from 0.63 to 0.84 in the current sample, with the lowest alphas for the 3-item Social Relationships Domain.
PCS. The PCS is a widely used measure of catastrophic thinking related to pain. Catastrophizing has been conceived by Sullivan and colleagues as “an exaggerated negative mental set brought to bear during actual or anticipated painful experience.”22 The PCS provides a total score and scores for the following subscales: rumination, magnification, and helplessness.23 It has been used in a variety of chronic pain populations and has demonstrated good reliability and validity in clinical as well as nonclinical samples.24-26 The PCS has 13 items rated on a scale of 0 to 4. Higher scores mean greater negative pain cognition (catastrophizing). In the current sample, the PCS total scale had a Cronbach alpha coefficient of 0.95 and 0.94 on the 2 assessments. The coefficients for the subscales ranged from 0.81 to 0.90.
PTSS. The PTSS is a 5-item tool that measures patient satisfaction with pain treatment. It includes items that address overall satisfaction, staff warmth, staff skill level, ease of scheduling appointments, and recommendation of the program to other veterans. It was derived from the post-treatment version of The Pain Outcome Questionnaire-VA and has demonstrated reliability and validity.27 The questions are scaled from 0 to 10. High scores on the PTSS denote high patient satisfaction with the EVP. The Cronbach alpha coefficient on the PTSS obtained from the current sample was 0.80.
Data Gathering and Analysis
Prior to starting the Study phase, Washburn University’s Institutional Review Board (IRB) and the VA IRB approved the project. The VA IRB, through its affiliate, gave a Not Human Research Determination and granted a waiver of informed consent and the Health Insurance Portability and Accountability Act authorization. The VA facility’s Research and Development department also approved the quality improvement project.
Once these approvals were obtained, the Study phase began with the abstraction of retrospective data obtained from veterans who participated in the VA health care facility’s EVP between
Veterans who completed the program were compared to veterans who did not complete the program on age, gender, and baseline measures. The investigators used independent samples t-tests to compare completers and noncompleters on age, pain intensity, pain interference, quality of life, and pain catastrophizing. They used the chi-square test of independence to analyze the association between gender and program completion.
Data were included in the pre- and postintervention analysis if the veteran completed the NPRS, MPI, WHOQOL-BREF, and PCS pre and post intervention. This became an important eligibility requirement as some of the tools/measures were changed towards the end of the review period in 2019. Pre- and postintervention data on pain intensity, pain interference, quality of life, pain catastrophizing, and patient satisfaction were compared using paired samples t-test at .004 level of significance based on the Bonferroni correction.28 Data on patient satisfaction with pain treatment were collected at program completion (week 8 or 10) and were analyzed using descriptive statistics.
Effect sizes (Cohen’s d) were calculated to determine the substantive significance or magnitude of the mean differences in scores. Effect sizes (expressed as absolute values of Cohen’s d) were calculated as the mean difference divided by the standard deviation. Values of 0.2 were considered a small effect size, 0.5 a medium effect size, and 0.8 a large effect size.29
Results
Data were abstracted for 115 veterans who started the EVP. Of these, 48 left the program, leaving 67 veterans (58%) who completed the program. Completers and noncompleters were similar in age, gender, and baseline measures (Table 1). Fifty-three (79%) completers and 35 (73%) noncompleters were male. A chi-square test of independence showed no significant association between gender and program completion (χ21 [N = 115] = .595, P = .440).

Comparison of pre-and postintervention mean scale scores resulted in statistically significant

Analysis of data obtained using the PTSS yielded high mean scores for items that focused on patient satisfaction with treatment (Table 3). Scaled statistics yielded a mean (SD) of 46.95 (4.40). These results denoted overall patient satisfaction with the EVP.

Discussion
The purpose of this quality improvement project was to abstract and analyze previously collected data from veterans with high-impact chronic pain who attended the EVP. Comparison of pre-intervention and postintervention data obtained from 67 veterans who completed the program revealed improvements in pain intensity, pain interference, negative cognition (catastrophizing), and quality of life. The differences were statistically significant and clinically meaningful, with medium and large effect sizes. In addition, veterans reported high satisfaction with the EVP.
The EVP includes CIH approaches that have demonstrated effectiveness among veterans and other populations with chronic pain. A wealth of studies, for example, support the effectiveness of CIH approaches among veterans.30-34 Other studies focus on specific CIH approaches that are components of the EVP. Evidence supports, for example, the efficacy of mindfulness-based stress reduction,35-39 acceptance and commitment therapy,40-43 brief peer support intervention,44 and interdisciplinary biopsychosocial rehabilitation.45,46
While empirical evidence supports components of the EVP, only one study focused on the outcomes of the Atlanta VA EVP among veterans with chronic pain. Results of a qualitative study conducted by Penney and Haro47 described the experience of veterans with the EVP. Those veterans reported adopting new self-care or lifestyle practices for pain management and health, accepting pain, being better able to adjust and set boundaries, feeling more in control, participating in life, and changing their medication use.
The mean baseline scores from the current sample were similar to samples of patients with chronic pain in other studies (NPRS,48 MPI,48 and PCS48-51). After converting scores on the WHOQOL-BREF from those that ranged from 4 to 20 to those that ranged from 0 to 100,18 the scores from the current sample were similar to those of other studies of patients with chronic pain.48,52,53Several strengths of the project should be noted. Data were collected using well established measurement tools that had previously demonstrated reliability and validity. All the tools used in data collection demonstrated good internal consistency reliabilities in the current sample of veterans. Weaknesses of the project include the use of a convenience sample of veterans and small sample size. Data were not available on the number of veterans who were offered participation or on how many veterans declined enrollment. The sample of veterans who chose to participate in the EVP may or may not have been representative of the population of veterans with high-impact chronic pain. As a pre- and postintervention design with no comparison group, the results are subject to multiple threats to internal validity, including the Hawthorne effect, maturation in the form of healing, and attrition. Reasons for leaving the program had not been recorded, so the investigators had no way of knowing factors that may have contributed to attrition. Also, data on when veterans left the program were unavailable. Research is needed with a control group to reduce the effect of confounding variables on the outcome measures. This project used data collected at a single VA facility, which limits its generalizability.
While completers and noncompleters of the EVP were similar on age, gender, and baseline measures, there may have been unidentified characteristics that influenced program completion. The investigators noticed the presence of more missing data among noncompleters compared to completers on the pre-intervention PCS; thus, noncompleters may have scored lower than completers on this instrument simply because there were more individual items that were unanswered/missing among this group of noncompleters.
Data were analyzed using a limited number of outcome measures that had previously been collected. Other outcome measures might include whether EVP participants reduced their use of medications, clinical resources, and personnel. Future projects, for example, could determine whether the EVP is effective in reducing opioid analgesic medication use and decreasing primary care and emergency department visits. Cost-benefit analyses could be completed to determine whether EVP is associated with financial savings.
Because no follow-up assessments were made to determine whether improvements were maintained over time, the project focus was limited to an evaluation of the short-term changes in the outcome measures. Future projects could include a follow-up assessment of the veterans 1- or 2-years post completion of the EVP.
Data for the project were collected prior to the COVID-19 pandemic, when the EVP was implemented through face-to-face meetings with participants and their peers. It is not clear how changes to the delivery of the program (such as offering it through telehealth) might impact veterans’ satisfaction with the program, willingness to complete it, and other variables of interest.
The results of this project were made available to stakeholders with recommendations for program expansion both at the current location and at other VA facilities, including the recommendation to hire additional personnel that would implement the program. As the VA network of facilities expand the EVP program and adapt it for telehealth delivery, the investigators recommended a similar analysis of data be performed following telehealth delivery. If delivery through telehealth is shown to improve outcome measures, the EVP could provide pain management treatment options for patients challenged by transportation barriers, including rural veterans.
Conclusion
This quality improvement project provided evidence of improvement in measures of pain severity, pain interference, negative cognition (catastrophizing), quality of life, and patient treatment satisfaction among veterans with chronic high-impact pain. Findings have been well received by the northeastern VA as well as the Veterans Integrated Systems Network 5. The results of the analyses were used to inform decisions regarding the future of the program.
Disclaimer: This material is the result of work supported with resources and the use of facilities at the VA Maryland Health Care System, Baltimore, Maryland. The views expressed are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the United States Government.
Acknowledgments: The authors thank Dr. Arianna Perra, the recent past coordinator of the Empower Veterans Program (EVP), who provided initial insights and support that motivated the decision to evaluate the program. We also thank the veterans and VA EVP clinicians who contributed data for the evaluation, and Dr. Michael Saenger (Director, TelePain-EVP: EVP) and Dr. Robert Lavin for their ongoing support, care, and concern for veteran patients. We also thank Dr. Beverly Bradley and the neurology service administrative team for their guidance in the process of obtaining necessary VA approvals for this project.
Corresponding author: Jessica U. Uche, DNP, CRNP-Family; [email protected]
doi:10.12788/jcom.0089
1. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press (US); 2011.
2. Bastian LA, Heapy A, Becker WC, et al. Understanding pain and pain treatment for veterans: responding to the federal pain research strategy. Pain Med. 2018;19(suppl_1); S1-S4. doi:10.1093/pm/pny1433
3. Engle GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129-136. doi:10.1126/science.847460
4. Bevers K, Watts L, Kishino ND, et al. The biopsychosocial model of the assessment, prevention, and treatment of chronic pain. US Neurology. 2016;12(2):98-104. doi:10.17925/USN.2016.12.02.98
5. Bair MJ, Ang D, Wu J, et al. Evaluation of stepped care for chronic pain (ESCAPE) in veterans of the Iraq and Afghanistan conflicts: A randomized clinical trial. JAMA Intern Med. 2015;175(5):682-689. doi:10.1001/jamainternmed.2015.97
6. Veterans Health Administration. Pain Management. VHA Directive 2009-053. Washington, DC: Department of Veterans Affairs; 2009.https://www.va.gov/painmanagement/docs/vha09paindirective.pdf
7. Moore BA, Anderson D, Dorflinger L, et al. Stepped care model for pain management and quality of pain care in long-term opioid therapy. J Rehabil Res Dev. 2016;53(1):137-146. doi:10.1682/JRRD.2014.10.0254
8. Institute for Healthcare Improvement. How to improve. Accessed March 14, 2022. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx
9. Saenger M. Empower Veterans Program. APA PCSS-O Webinars. Evidence CAM LBP 2016.
10. Gaudet T, Kligler B. Whole health in the whole system of the Veterans Administration: How will we know we have reached this future state? J Altern Complement Med. 2019;25(S1):S7-S11. doi:10.1089/acm.2018.29061.gau
11. Veterans Health Administration. Whole health: Circle of health. Updated April 1, 2021. Accessed March 14, 2022. https://www.va.gov/WHOLEHEALTH/circle-of-health/index.asp
12. Krebs EE, Carey TS, Weinberger M. Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med. 2007;22(10):1453-1458. doi:10.1007/s11606-007-0321-2
13. Veterans Health Administration. Pain as the 5th vital sign toolkit. October 2000, revised edition. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee. https://www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf
14. McKillop JM, Nielson WR. Improving the usefulness of the Multidimensional Pain Inventory. Pain Res Manag. 2011;16(4):239-244. doi:10.1155/2011/873424
15. Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain.1985;23(4):345-356. doi:10.1016/0304-3959(85)90004-1
16. Verra ML, Angst F, Staal JB, et al. Reliability of the multidimensional pain inventory and stability of the MPI classification system in chronic back pain. BMC Musculoskelet Disord. 2012;13:155. doi:10.1186/1471-2474-13-155
17. Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol Med. 1998;28(3):551-558. doi:10.1017/s0033291798006667
18. World Health Organization. Division of Mental Health. WHOQOL-BREF: introduction, administration, scoring and generic version of the assessment: field trial version, December 1996. Accessed March 14, 2022. https://apps.who.int/iris/handle/10665/63529
19. Guay S, Fortin C, Fikretoglu D, et al. Validation of the WHOQOL-BREF in a sample of male treatment-seeking veterans. Mil Psychol. 2015;27(2):85-92. doi:10.1037/mil0000065
20. Skevington S, Lotfy M, O’Connell K, WHOQOL Group. The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A Report from the WHOQOL Group. Qual Life Res. 2004;13(2):299-310. doi:10.1023/B:QURE.0000018486.91360.00
21. Stratton KJ, Bender MC, Cameron JJ, Pickett TC. Development and evaluation of a behavioral pain management treatment program in a Veterans Affairs Medical Center. Mil Med. 2015;180(3):263-268. doi:10.7205/MILMED-D-14-00281.
22. Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17(1):52-64. doi:10.1097/00002508-200103000-00008
23. Sullivan JL. The Pain Catastrophizing Scale: User manual. Accessed March 14, 2022. https://studylib.net/doc/8330191/the-pain-catastrophizing-scale---dr.-michael-sullivan
24. Darnall BD, Sturgeon JA, Cook KF, et al. Development and validation of a daily pain catastrophizing scale. J Pain. 2017;18(9):1139-1149. doi:10.1016/j.jpain.2017.05.003
25. Osman A, Barrios FX, Kopper BA, et al. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med. 1997;20(6):589-605. doi:10.1023/a:1025570508954
26. Sullivan MJL, Bishop S, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assessment. 1995;7(4):524-532. doi:10.1037/1040-3590.7.4.524
27. Walker R, Clark M, Gironda R. Psychometric characteristics of the Pain Treatment Satisfaction Scale. J Pain. 2015;6(3Suppl.):S76.
28. Emerson RW. Bonferroni correction and type I error. J Vis Impair Blind. 2020;114(1):77-78. doi:10.1177/0145482X20901378
29. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Routledge; 1988. doi:10.4324/9780203771587
30. Craner JR, Lake ES, Bancroft KA, George LL. Treatment outcomes and mechanisms for an ACT-based 10-week interdisciplinary chronic pain rehabilitation program. Pain Pract. 2020;20(1):44-54. doi:10.1111/papr.12824
31. Han L, Goulet JL, Skanderson M, et al. Evaluation of complementary and integrative health approaches among US veterans with musculoskeletal pain using propensity score methods. Pain Med. 2019;20(1):90-102. doi:10.1093/pm/pny027
32. Herman PM, Yuan AH, Cefalu MS, et al. The use of complementary and integrative health approaches for chronic musculoskeletal pain in younger US veterans: an economic evaluation. PLoS One. 2019;14(6):e0217831. doi:10.1371/journal.pone.0217831
33. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Global Health; Board on Health Sciences Policy; Global Forum on Innovation in Health Professional Education; Forum on Neuroscience and Nervous System Disorders; Stroud C, Posey Norris SM, Bain L, eds. The Role of Nonpharmacological Approaches to Pain Management: Proceedings of a Workshop. National Academies Press (US); April 12, 2019.
34. Richmond H, Hall AM, Copsey B, et al. The effectiveness of cognitive behavioural treatment for non-specific low back pain: a systematic review and meta-analysis. PLoS One. 2015;10(8):e0134192. doi:10.1371/journal.pone.0134192

35. Kearney DJ, Simpson TL, Malte CA, et al. Mindfulness-based stress reduction in addition to usual care is associated with improvements in pain, fatigue, and cognitive failures among veterans with Gulf War illness. Am J Med. 2016;129(2):204-214. doi:10.1016/j.amjmed.2015.09.015
36. Khoo E, Small R, Cheng W, et al. Comparative evaluation of group-based mindfulness-based stress reduction and cognitive behavioral therapy for the treatment and management of chronic pain: a systematic review and network meta-analysis. Evid Based Ment Health. 2019;22(1):26-35. doi:10.1136/ebmental-2018-300062
37. Khusid MA, Vythilingam M. The emerging role of mindfulness meditation as effective self-management strategy, Part 2: clinical implications for chronic pain, substance misuse, and insomnia. Mil Med. 2016;181(9):969-975. doi:10.7205/MILMED-D-14-00678
38. la Cour P, Petersen M. Effects of mindfulness meditation on chronic pain: A randomized controlled trial. Pain Med. 2015;16(4):641-652. doi:10.1111/pme.12605
39. Zou L, Zhang Y, Yang L, et al. Are mindful exercises safe and beneficial for treating chronic lower back pain? A systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2019;8(5):628. doi:10.3390/jcm8050628
40. Hughes LS, Clark J, Colclough JA, et al. Acceptance and commitment therapy (ACT) for chronic pain: a systematic review and meta-analyses. Clin J Pain. 2017;33(6):552-568. doi:10.1097/AJP.0000000000000425
41. Kemani MK, Olsson GL, Lekander M, et al. Efficacy and cost-effectiveness of acceptance and commitment therapy and applied relaxation for longstanding pain: a randomized controlled trial. Clin J Pain. 2015;31(11):1004-1016. doi:10.1097/AJP.0000000000000203
42. Scott W, Daly A, Yu L, McCracken LM. Treatment of chronic pain for adults 65 and over: analyses of outcomes and changes in psychological flexibility following interdisciplinary acceptance and commitment therapy (ACT). Pain Med. 2017;18(2):252. doi:10.1093/pm/pnw073
43. Veehof MM, Trompetter HR, Bohlmeijer ET, Schreurs KMG. Acceptance- and mindfulness-based interventions for the treatment of chronic pain: a meta-analytic review. Cogn Behav Ther. 2016;45(1):5-31. doi:10.1080/16506073.2015.1098724
44. Matthias MS, McGuire AB, Kukla M, et al. A brief peer support intervention for veterans with chronic musculoskeletal pain: a pilot study of feasibility and effectiveness. Pain Med. 2015;16(1):81-87. doi:10.1111/pme.12571
45. Anamkath NS, Palyo SA, Jacobs SC, et al. An interdisciplinary pain rehabilitation program for veterans with chronic pain: description and initial evaluation of outcomes. Pain Res Manag. 2018;2018(3941682):1-9. doi:10.1155/2018/3941682
46. Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain. Cochrane Database Syst Rev. 2014;9: CD000963. doi:10.1002/14651858.CD000963.pub3
47. Penney LS, Haro E. Qualitative evaluation of an interdisciplinary chronic pain intervention: Outcomes and barriers and facilitators to ongoing pain management. J Pain Res. 2019;12:865-878. doi:10.2147/JPR.S185652
48. Murphy JL, Cordova MJ, Dedert EA. Cognitive behavioral therapy for chronic pain in veterans; Evidence for clinical effectiveness in a model program. Psychol Serv. 2022;19(1):95-102. doi:10.1037/ser0000506
49. Katz L, Patterson L, Zacharias R. Evaluation of an interdisciplinary chronic pain program and predictors of readiness for change. Can J Pain. 2019;3(1):70-78. doi:10.1080/24740527.2019.1582296
50. Majumder SMM, Ahmed S, Shazzad N, et al. Translation, cross-cultural adaptation and validation of the Pain Catastrophizing Scale (PCS) into Bengali I patients with chronic non-malignant musculoskeletal pain. Int J Rheum Dis. 2020;23:1481-1487. doi:10.1111/1756-185X.13954
51. Margiotta F, Hannigan A, Imran A, et al. Pain, perceived injustice, and pain catastrophizing in chronic pain patients in Ireland. Pain Pract. 2016;17(5):663-668. doi:10.1111/papr.12
52. Bras M, Milunovic V, Boban M, et al. Quality of live in Croatian Homeland war (1991-1995) veterans who suffer from post-traumatic stress disorder and chronic pain. Health Qual Life Out. 2011;9:56. doi:10.1186/1477-7525-9-56
53. Liu C-H, Kung Y-Y, Lin C-L, et al. Therapeutic efficacy and the impact of the “dose” effect of acupuncture to treat sciatica: A randomized controlled pilot study. J Pain Res. 2019;12:3511-3520. doi:10.2147/JPR.S210672
From Neurology/Chronic Pain Management Services, Department of Veterans Affairs (VA) Maryland Health Care System, Baltimore VA Medical Center, Baltimore, MD (Dr. Uche), and School of Nursing, Washburn University, Topeka, KS (Drs. Jamison and Waugh).
Abstract
Objective: The purpose of this quality improvement project was to abstract and analyze previously collected data from veterans with high-impact chronic pain who attended the Empower Veterans Program (EVP) offered by a Veterans Administration facility in the northeastern United States.
Methods: This quality improvement project used data collected from veterans with chronic pain who completed the veterans health care facility’s EVP between August 2017 and August 2019. Pre- and post-intervention data on pain intensity, pain interference, quality of life, and pain catastrophizing were compared using paired t-tests.
Results: Although data were abstracted from 115 patients, the final sample included
Conclusion: Veterans with chronic high-impact noncancer pain who completed the EVP had reduced pain intensity, pain interference, pain catastrophizing as well as improved quality of life and satisfaction with their health.
Keywords: musculoskeletal pain, Veterans Affairs, complementary and integrative health, acceptance and commitment therapy, mind-body therapies, whole health, multidisciplinary pain management.
More than 100 million American adults suffer from chronic pain; costs associated with managing chronic pain are approximately $635 billion
One such program is the Empower Veterans Program (EVP). Originally developed at the Atlanta Veterans Affairs Health Care System, the EVP is a CIH modality based on the biopsychosocial model of pain developed by psychiatrist George Engel in 1977.3 The biopsychosocial model of pain recognizes that pain is a complex, multidimensional, biopsychosocial experience. Under this model, the mind and body work in unison as interconnected entities. Because the model acknowledges biological, psychological, and social components of pain and illness,4 treatment focuses on all aspects of a person’s health, life, and relationships.
The EVP fits into the VHA Pain Management Stepped Care Model and is an adjunctive complement for that model.5-7 The EVP complements care at the first step, where patient/family provide self-care and where care is provided by patient-aligned primary care teams, at the second step, which includes secondary consultation with multidisciplinary pain medicine specialty teams and other specialists, and at the third step, with the addition of tertiary interdisciplinary pain centers.
The VA Maryland Health Care System (VAMHCS) implemented the EVP as part of a quality improvement project for the management of chronic pain. The objectives of the program were to reduce pain intensity, pain catastrophizing, and pain interference, as well as improve functionality and quality of life among veterans with chronic high-impact noncancer pain. More than 2 years after the program was implemented, collected data had not been analyzed. The purpose of this quality improvement project was to abstract and analyze the previously collected data from veterans with high-impact chronic pain who attended an EVP offered by the VAMHCS. The results of the data analysis were used to inform decisions regarding the future of the program.
Methods
This quality improvement project used the Plan-Do-Study-Act (PDSA) process.8 The first 2 phases of the PDSA cycle (Plan and Do) were completed by a team of VA employees from the VAMHCS, who donated their time to establish and implement the program at the project site. This team consisted of psychologists, a physical therapist, a social worker, and a chaplain, and included support from medical administrative staff. This team planned and implemented the EVP at the VA facility based on the model developed at the Atlanta VA Health Care System. During the “Do” phase, the team collected data on pain intensity, pain interference, quality of life, and pain negative cognition (catastrophizing) before the intervention and post intervention. They also collected data on program outcome (patient treatment satisfaction) post intervention. Because these employees did not have time to retrieve and analyze the data, they welcomed the opportunity to have the data analyzed by the investigators during the Study phase of the PDSA cycle. Based on the results of the analysis, recommendations for program changes were made during the Act phase of the cycle.
Intervention
The EVP was developed as a 10-week (30 hours) interdisciplinary CIH approach that coached veterans with chronic pain to live fuller lives based on their individual values and what matters to them. EVP is the “What Else” management modality for the 5% of veterans with high-impact chronic pain.9 The EVP provided functional restoration through its components of whole health, mindfulness training, coaching calls, acceptance and commitment therapy, and mindful movement. It used the Wheel of Health with the 4 key components of me, self-care, professional care, and community.10,11
Veterans who had a diagnosis of chronic nonmalignant pain for 3 months or more and who agreed to participate in the EVP at this facility attended 3-hour classes every Tuesday with a cohort of 8 to 12 peers and engaged in one-on-one coaching with interdisciplinary team members. During the class sessions, veterans were coached to understand and accept their pain and commit to maintaining function despite their pain. Mindful movement by the physical therapist emphasized the pivotal place of exercise in pain management. The therapist used the mantra “Motion is Lotion.”9 The guiding principle of the EVP was that small incremental changes can have a big impact on the individual’s whole life. Emphasis was placed on increasing self-efficacy and mindful awareness for veterans with high-impact pain by giving them “Skills before Pills.”9
Outcome Measures
Outcome measures included the Numerical Pain Rating Scale (NPRS), the Multidimensional Pain Inventory (MPI), the World Health Organization Quality of Life assessment (WHOQOL-BREF), the Pain Catastrophizing Scale (PCS), and the Pain Treatment Satisfaction Scale (PTSS). Cronbach alpha coefficients were calculated to assess internal consistency reliability of these measures in the sample of veterans who completed the EVP.
NPRS. The NPRS is ubiquitous as a screening tool in many health care environments and its use is mandated by the VA health care system.12 The choice of the NPRS as the tool for pain screening in the VA health care system was based on a large body of research that supports the reliability and validity of the NPRS as a single index of pain intensity or severity. Studies suggest that the NPRS is valid for use in the assessment of acute, cancer, or chronic nonmalignant pain and in varied clinical settings.13 The NPRS has 4 items, each on a scale of 0 to 10. For the purpose of this project, only 3 items were used. The 3 items assessed the worst pain, usual pain, and the current pain (right now). The higher the score, the higher the pain intensity. Cronbach alpha coefficients on the NPRS obtained from the current sample of veterans were 0.85 on both pre- and postintervention assessments.
MPI. The MPI is an easily accessible, reliable, and valid self-report questionnaire that measures the impact of pain on an individual’s life, quality of social support, and general activity.14 This instrument is a short version of the West Haven-Yale MPI.15 The MPI contains 9 items rated on a scale from 0 to 6. The higher the score, the greater pain interference a person is experiencing. The MPI produces reliable, valid information for diagnostic purposes and for therapy outcome studies.16 The MPI had a Cronbach alpha of 0.90 on pre-intervention and 0.92 on postintervention assessments in the current sample.
WHOQOL-BREF. The WHOQOL-BREF is a measure of quality of life and is an abbreviated version of the WHOQOL-100. Quality of life is defined by the World Health Organization17 “as an individuals’ perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns.” The WHOQOL-BREF contains 26 items. The first 2 items were examined separately; the first item asks individuals to rate their overall quality of life and the second asks individuals how satisfied they are with their health. The remaining 24 items were used to calculate the following 4 domain scores: physical health, psychological health, social relationship, and environment.18 Each item is measured on a scale of 1 to 5. Higher scores denote higher or better quality of life. Domain scores have demonstrated good reliability and validity.19-21 Cronbach alpha coefficients for the domain subscales ranged from 0.63 to 0.84 in the current sample, with the lowest alphas for the 3-item Social Relationships Domain.
PCS. The PCS is a widely used measure of catastrophic thinking related to pain. Catastrophizing has been conceived by Sullivan and colleagues as “an exaggerated negative mental set brought to bear during actual or anticipated painful experience.”22 The PCS provides a total score and scores for the following subscales: rumination, magnification, and helplessness.23 It has been used in a variety of chronic pain populations and has demonstrated good reliability and validity in clinical as well as nonclinical samples.24-26 The PCS has 13 items rated on a scale of 0 to 4. Higher scores mean greater negative pain cognition (catastrophizing). In the current sample, the PCS total scale had a Cronbach alpha coefficient of 0.95 and 0.94 on the 2 assessments. The coefficients for the subscales ranged from 0.81 to 0.90.
PTSS. The PTSS is a 5-item tool that measures patient satisfaction with pain treatment. It includes items that address overall satisfaction, staff warmth, staff skill level, ease of scheduling appointments, and recommendation of the program to other veterans. It was derived from the post-treatment version of The Pain Outcome Questionnaire-VA and has demonstrated reliability and validity.27 The questions are scaled from 0 to 10. High scores on the PTSS denote high patient satisfaction with the EVP. The Cronbach alpha coefficient on the PTSS obtained from the current sample was 0.80.
Data Gathering and Analysis
Prior to starting the Study phase, Washburn University’s Institutional Review Board (IRB) and the VA IRB approved the project. The VA IRB, through its affiliate, gave a Not Human Research Determination and granted a waiver of informed consent and the Health Insurance Portability and Accountability Act authorization. The VA facility’s Research and Development department also approved the quality improvement project.
Once these approvals were obtained, the Study phase began with the abstraction of retrospective data obtained from veterans who participated in the VA health care facility’s EVP between
Veterans who completed the program were compared to veterans who did not complete the program on age, gender, and baseline measures. The investigators used independent samples t-tests to compare completers and noncompleters on age, pain intensity, pain interference, quality of life, and pain catastrophizing. They used the chi-square test of independence to analyze the association between gender and program completion.
Data were included in the pre- and postintervention analysis if the veteran completed the NPRS, MPI, WHOQOL-BREF, and PCS pre and post intervention. This became an important eligibility requirement as some of the tools/measures were changed towards the end of the review period in 2019. Pre- and postintervention data on pain intensity, pain interference, quality of life, pain catastrophizing, and patient satisfaction were compared using paired samples t-test at .004 level of significance based on the Bonferroni correction.28 Data on patient satisfaction with pain treatment were collected at program completion (week 8 or 10) and were analyzed using descriptive statistics.
Effect sizes (Cohen’s d) were calculated to determine the substantive significance or magnitude of the mean differences in scores. Effect sizes (expressed as absolute values of Cohen’s d) were calculated as the mean difference divided by the standard deviation. Values of 0.2 were considered a small effect size, 0.5 a medium effect size, and 0.8 a large effect size.29
Results
Data were abstracted for 115 veterans who started the EVP. Of these, 48 left the program, leaving 67 veterans (58%) who completed the program. Completers and noncompleters were similar in age, gender, and baseline measures (Table 1). Fifty-three (79%) completers and 35 (73%) noncompleters were male. A chi-square test of independence showed no significant association between gender and program completion (χ21 [N = 115] = .595, P = .440).

Comparison of pre-and postintervention mean scale scores resulted in statistically significant

Analysis of data obtained using the PTSS yielded high mean scores for items that focused on patient satisfaction with treatment (Table 3). Scaled statistics yielded a mean (SD) of 46.95 (4.40). These results denoted overall patient satisfaction with the EVP.

Discussion
The purpose of this quality improvement project was to abstract and analyze previously collected data from veterans with high-impact chronic pain who attended the EVP. Comparison of pre-intervention and postintervention data obtained from 67 veterans who completed the program revealed improvements in pain intensity, pain interference, negative cognition (catastrophizing), and quality of life. The differences were statistically significant and clinically meaningful, with medium and large effect sizes. In addition, veterans reported high satisfaction with the EVP.
The EVP includes CIH approaches that have demonstrated effectiveness among veterans and other populations with chronic pain. A wealth of studies, for example, support the effectiveness of CIH approaches among veterans.30-34 Other studies focus on specific CIH approaches that are components of the EVP. Evidence supports, for example, the efficacy of mindfulness-based stress reduction,35-39 acceptance and commitment therapy,40-43 brief peer support intervention,44 and interdisciplinary biopsychosocial rehabilitation.45,46
While empirical evidence supports components of the EVP, only one study focused on the outcomes of the Atlanta VA EVP among veterans with chronic pain. Results of a qualitative study conducted by Penney and Haro47 described the experience of veterans with the EVP. Those veterans reported adopting new self-care or lifestyle practices for pain management and health, accepting pain, being better able to adjust and set boundaries, feeling more in control, participating in life, and changing their medication use.
The mean baseline scores from the current sample were similar to samples of patients with chronic pain in other studies (NPRS,48 MPI,48 and PCS48-51). After converting scores on the WHOQOL-BREF from those that ranged from 4 to 20 to those that ranged from 0 to 100,18 the scores from the current sample were similar to those of other studies of patients with chronic pain.48,52,53Several strengths of the project should be noted. Data were collected using well established measurement tools that had previously demonstrated reliability and validity. All the tools used in data collection demonstrated good internal consistency reliabilities in the current sample of veterans. Weaknesses of the project include the use of a convenience sample of veterans and small sample size. Data were not available on the number of veterans who were offered participation or on how many veterans declined enrollment. The sample of veterans who chose to participate in the EVP may or may not have been representative of the population of veterans with high-impact chronic pain. As a pre- and postintervention design with no comparison group, the results are subject to multiple threats to internal validity, including the Hawthorne effect, maturation in the form of healing, and attrition. Reasons for leaving the program had not been recorded, so the investigators had no way of knowing factors that may have contributed to attrition. Also, data on when veterans left the program were unavailable. Research is needed with a control group to reduce the effect of confounding variables on the outcome measures. This project used data collected at a single VA facility, which limits its generalizability.
While completers and noncompleters of the EVP were similar on age, gender, and baseline measures, there may have been unidentified characteristics that influenced program completion. The investigators noticed the presence of more missing data among noncompleters compared to completers on the pre-intervention PCS; thus, noncompleters may have scored lower than completers on this instrument simply because there were more individual items that were unanswered/missing among this group of noncompleters.
Data were analyzed using a limited number of outcome measures that had previously been collected. Other outcome measures might include whether EVP participants reduced their use of medications, clinical resources, and personnel. Future projects, for example, could determine whether the EVP is effective in reducing opioid analgesic medication use and decreasing primary care and emergency department visits. Cost-benefit analyses could be completed to determine whether EVP is associated with financial savings.
Because no follow-up assessments were made to determine whether improvements were maintained over time, the project focus was limited to an evaluation of the short-term changes in the outcome measures. Future projects could include a follow-up assessment of the veterans 1- or 2-years post completion of the EVP.
Data for the project were collected prior to the COVID-19 pandemic, when the EVP was implemented through face-to-face meetings with participants and their peers. It is not clear how changes to the delivery of the program (such as offering it through telehealth) might impact veterans’ satisfaction with the program, willingness to complete it, and other variables of interest.
The results of this project were made available to stakeholders with recommendations for program expansion both at the current location and at other VA facilities, including the recommendation to hire additional personnel that would implement the program. As the VA network of facilities expand the EVP program and adapt it for telehealth delivery, the investigators recommended a similar analysis of data be performed following telehealth delivery. If delivery through telehealth is shown to improve outcome measures, the EVP could provide pain management treatment options for patients challenged by transportation barriers, including rural veterans.
Conclusion
This quality improvement project provided evidence of improvement in measures of pain severity, pain interference, negative cognition (catastrophizing), quality of life, and patient treatment satisfaction among veterans with chronic high-impact pain. Findings have been well received by the northeastern VA as well as the Veterans Integrated Systems Network 5. The results of the analyses were used to inform decisions regarding the future of the program.
Disclaimer: This material is the result of work supported with resources and the use of facilities at the VA Maryland Health Care System, Baltimore, Maryland. The views expressed are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the United States Government.
Acknowledgments: The authors thank Dr. Arianna Perra, the recent past coordinator of the Empower Veterans Program (EVP), who provided initial insights and support that motivated the decision to evaluate the program. We also thank the veterans and VA EVP clinicians who contributed data for the evaluation, and Dr. Michael Saenger (Director, TelePain-EVP: EVP) and Dr. Robert Lavin for their ongoing support, care, and concern for veteran patients. We also thank Dr. Beverly Bradley and the neurology service administrative team for their guidance in the process of obtaining necessary VA approvals for this project.
Corresponding author: Jessica U. Uche, DNP, CRNP-Family; [email protected]
doi:10.12788/jcom.0089
From Neurology/Chronic Pain Management Services, Department of Veterans Affairs (VA) Maryland Health Care System, Baltimore VA Medical Center, Baltimore, MD (Dr. Uche), and School of Nursing, Washburn University, Topeka, KS (Drs. Jamison and Waugh).
Abstract
Objective: The purpose of this quality improvement project was to abstract and analyze previously collected data from veterans with high-impact chronic pain who attended the Empower Veterans Program (EVP) offered by a Veterans Administration facility in the northeastern United States.
Methods: This quality improvement project used data collected from veterans with chronic pain who completed the veterans health care facility’s EVP between August 2017 and August 2019. Pre- and post-intervention data on pain intensity, pain interference, quality of life, and pain catastrophizing were compared using paired t-tests.
Results: Although data were abstracted from 115 patients, the final sample included
Conclusion: Veterans with chronic high-impact noncancer pain who completed the EVP had reduced pain intensity, pain interference, pain catastrophizing as well as improved quality of life and satisfaction with their health.
Keywords: musculoskeletal pain, Veterans Affairs, complementary and integrative health, acceptance and commitment therapy, mind-body therapies, whole health, multidisciplinary pain management.
More than 100 million American adults suffer from chronic pain; costs associated with managing chronic pain are approximately $635 billion
One such program is the Empower Veterans Program (EVP). Originally developed at the Atlanta Veterans Affairs Health Care System, the EVP is a CIH modality based on the biopsychosocial model of pain developed by psychiatrist George Engel in 1977.3 The biopsychosocial model of pain recognizes that pain is a complex, multidimensional, biopsychosocial experience. Under this model, the mind and body work in unison as interconnected entities. Because the model acknowledges biological, psychological, and social components of pain and illness,4 treatment focuses on all aspects of a person’s health, life, and relationships.
The EVP fits into the VHA Pain Management Stepped Care Model and is an adjunctive complement for that model.5-7 The EVP complements care at the first step, where patient/family provide self-care and where care is provided by patient-aligned primary care teams, at the second step, which includes secondary consultation with multidisciplinary pain medicine specialty teams and other specialists, and at the third step, with the addition of tertiary interdisciplinary pain centers.
The VA Maryland Health Care System (VAMHCS) implemented the EVP as part of a quality improvement project for the management of chronic pain. The objectives of the program were to reduce pain intensity, pain catastrophizing, and pain interference, as well as improve functionality and quality of life among veterans with chronic high-impact noncancer pain. More than 2 years after the program was implemented, collected data had not been analyzed. The purpose of this quality improvement project was to abstract and analyze the previously collected data from veterans with high-impact chronic pain who attended an EVP offered by the VAMHCS. The results of the data analysis were used to inform decisions regarding the future of the program.
Methods
This quality improvement project used the Plan-Do-Study-Act (PDSA) process.8 The first 2 phases of the PDSA cycle (Plan and Do) were completed by a team of VA employees from the VAMHCS, who donated their time to establish and implement the program at the project site. This team consisted of psychologists, a physical therapist, a social worker, and a chaplain, and included support from medical administrative staff. This team planned and implemented the EVP at the VA facility based on the model developed at the Atlanta VA Health Care System. During the “Do” phase, the team collected data on pain intensity, pain interference, quality of life, and pain negative cognition (catastrophizing) before the intervention and post intervention. They also collected data on program outcome (patient treatment satisfaction) post intervention. Because these employees did not have time to retrieve and analyze the data, they welcomed the opportunity to have the data analyzed by the investigators during the Study phase of the PDSA cycle. Based on the results of the analysis, recommendations for program changes were made during the Act phase of the cycle.
Intervention
The EVP was developed as a 10-week (30 hours) interdisciplinary CIH approach that coached veterans with chronic pain to live fuller lives based on their individual values and what matters to them. EVP is the “What Else” management modality for the 5% of veterans with high-impact chronic pain.9 The EVP provided functional restoration through its components of whole health, mindfulness training, coaching calls, acceptance and commitment therapy, and mindful movement. It used the Wheel of Health with the 4 key components of me, self-care, professional care, and community.10,11
Veterans who had a diagnosis of chronic nonmalignant pain for 3 months or more and who agreed to participate in the EVP at this facility attended 3-hour classes every Tuesday with a cohort of 8 to 12 peers and engaged in one-on-one coaching with interdisciplinary team members. During the class sessions, veterans were coached to understand and accept their pain and commit to maintaining function despite their pain. Mindful movement by the physical therapist emphasized the pivotal place of exercise in pain management. The therapist used the mantra “Motion is Lotion.”9 The guiding principle of the EVP was that small incremental changes can have a big impact on the individual’s whole life. Emphasis was placed on increasing self-efficacy and mindful awareness for veterans with high-impact pain by giving them “Skills before Pills.”9
Outcome Measures
Outcome measures included the Numerical Pain Rating Scale (NPRS), the Multidimensional Pain Inventory (MPI), the World Health Organization Quality of Life assessment (WHOQOL-BREF), the Pain Catastrophizing Scale (PCS), and the Pain Treatment Satisfaction Scale (PTSS). Cronbach alpha coefficients were calculated to assess internal consistency reliability of these measures in the sample of veterans who completed the EVP.
NPRS. The NPRS is ubiquitous as a screening tool in many health care environments and its use is mandated by the VA health care system.12 The choice of the NPRS as the tool for pain screening in the VA health care system was based on a large body of research that supports the reliability and validity of the NPRS as a single index of pain intensity or severity. Studies suggest that the NPRS is valid for use in the assessment of acute, cancer, or chronic nonmalignant pain and in varied clinical settings.13 The NPRS has 4 items, each on a scale of 0 to 10. For the purpose of this project, only 3 items were used. The 3 items assessed the worst pain, usual pain, and the current pain (right now). The higher the score, the higher the pain intensity. Cronbach alpha coefficients on the NPRS obtained from the current sample of veterans were 0.85 on both pre- and postintervention assessments.
MPI. The MPI is an easily accessible, reliable, and valid self-report questionnaire that measures the impact of pain on an individual’s life, quality of social support, and general activity.14 This instrument is a short version of the West Haven-Yale MPI.15 The MPI contains 9 items rated on a scale from 0 to 6. The higher the score, the greater pain interference a person is experiencing. The MPI produces reliable, valid information for diagnostic purposes and for therapy outcome studies.16 The MPI had a Cronbach alpha of 0.90 on pre-intervention and 0.92 on postintervention assessments in the current sample.
WHOQOL-BREF. The WHOQOL-BREF is a measure of quality of life and is an abbreviated version of the WHOQOL-100. Quality of life is defined by the World Health Organization17 “as an individuals’ perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns.” The WHOQOL-BREF contains 26 items. The first 2 items were examined separately; the first item asks individuals to rate their overall quality of life and the second asks individuals how satisfied they are with their health. The remaining 24 items were used to calculate the following 4 domain scores: physical health, psychological health, social relationship, and environment.18 Each item is measured on a scale of 1 to 5. Higher scores denote higher or better quality of life. Domain scores have demonstrated good reliability and validity.19-21 Cronbach alpha coefficients for the domain subscales ranged from 0.63 to 0.84 in the current sample, with the lowest alphas for the 3-item Social Relationships Domain.
PCS. The PCS is a widely used measure of catastrophic thinking related to pain. Catastrophizing has been conceived by Sullivan and colleagues as “an exaggerated negative mental set brought to bear during actual or anticipated painful experience.”22 The PCS provides a total score and scores for the following subscales: rumination, magnification, and helplessness.23 It has been used in a variety of chronic pain populations and has demonstrated good reliability and validity in clinical as well as nonclinical samples.24-26 The PCS has 13 items rated on a scale of 0 to 4. Higher scores mean greater negative pain cognition (catastrophizing). In the current sample, the PCS total scale had a Cronbach alpha coefficient of 0.95 and 0.94 on the 2 assessments. The coefficients for the subscales ranged from 0.81 to 0.90.
PTSS. The PTSS is a 5-item tool that measures patient satisfaction with pain treatment. It includes items that address overall satisfaction, staff warmth, staff skill level, ease of scheduling appointments, and recommendation of the program to other veterans. It was derived from the post-treatment version of The Pain Outcome Questionnaire-VA and has demonstrated reliability and validity.27 The questions are scaled from 0 to 10. High scores on the PTSS denote high patient satisfaction with the EVP. The Cronbach alpha coefficient on the PTSS obtained from the current sample was 0.80.
Data Gathering and Analysis
Prior to starting the Study phase, Washburn University’s Institutional Review Board (IRB) and the VA IRB approved the project. The VA IRB, through its affiliate, gave a Not Human Research Determination and granted a waiver of informed consent and the Health Insurance Portability and Accountability Act authorization. The VA facility’s Research and Development department also approved the quality improvement project.
Once these approvals were obtained, the Study phase began with the abstraction of retrospective data obtained from veterans who participated in the VA health care facility’s EVP between
Veterans who completed the program were compared to veterans who did not complete the program on age, gender, and baseline measures. The investigators used independent samples t-tests to compare completers and noncompleters on age, pain intensity, pain interference, quality of life, and pain catastrophizing. They used the chi-square test of independence to analyze the association between gender and program completion.
Data were included in the pre- and postintervention analysis if the veteran completed the NPRS, MPI, WHOQOL-BREF, and PCS pre and post intervention. This became an important eligibility requirement as some of the tools/measures were changed towards the end of the review period in 2019. Pre- and postintervention data on pain intensity, pain interference, quality of life, pain catastrophizing, and patient satisfaction were compared using paired samples t-test at .004 level of significance based on the Bonferroni correction.28 Data on patient satisfaction with pain treatment were collected at program completion (week 8 or 10) and were analyzed using descriptive statistics.
Effect sizes (Cohen’s d) were calculated to determine the substantive significance or magnitude of the mean differences in scores. Effect sizes (expressed as absolute values of Cohen’s d) were calculated as the mean difference divided by the standard deviation. Values of 0.2 were considered a small effect size, 0.5 a medium effect size, and 0.8 a large effect size.29
Results
Data were abstracted for 115 veterans who started the EVP. Of these, 48 left the program, leaving 67 veterans (58%) who completed the program. Completers and noncompleters were similar in age, gender, and baseline measures (Table 1). Fifty-three (79%) completers and 35 (73%) noncompleters were male. A chi-square test of independence showed no significant association between gender and program completion (χ21 [N = 115] = .595, P = .440).

Comparison of pre-and postintervention mean scale scores resulted in statistically significant

Analysis of data obtained using the PTSS yielded high mean scores for items that focused on patient satisfaction with treatment (Table 3). Scaled statistics yielded a mean (SD) of 46.95 (4.40). These results denoted overall patient satisfaction with the EVP.

Discussion
The purpose of this quality improvement project was to abstract and analyze previously collected data from veterans with high-impact chronic pain who attended the EVP. Comparison of pre-intervention and postintervention data obtained from 67 veterans who completed the program revealed improvements in pain intensity, pain interference, negative cognition (catastrophizing), and quality of life. The differences were statistically significant and clinically meaningful, with medium and large effect sizes. In addition, veterans reported high satisfaction with the EVP.
The EVP includes CIH approaches that have demonstrated effectiveness among veterans and other populations with chronic pain. A wealth of studies, for example, support the effectiveness of CIH approaches among veterans.30-34 Other studies focus on specific CIH approaches that are components of the EVP. Evidence supports, for example, the efficacy of mindfulness-based stress reduction,35-39 acceptance and commitment therapy,40-43 brief peer support intervention,44 and interdisciplinary biopsychosocial rehabilitation.45,46
While empirical evidence supports components of the EVP, only one study focused on the outcomes of the Atlanta VA EVP among veterans with chronic pain. Results of a qualitative study conducted by Penney and Haro47 described the experience of veterans with the EVP. Those veterans reported adopting new self-care or lifestyle practices for pain management and health, accepting pain, being better able to adjust and set boundaries, feeling more in control, participating in life, and changing their medication use.
The mean baseline scores from the current sample were similar to samples of patients with chronic pain in other studies (NPRS,48 MPI,48 and PCS48-51). After converting scores on the WHOQOL-BREF from those that ranged from 4 to 20 to those that ranged from 0 to 100,18 the scores from the current sample were similar to those of other studies of patients with chronic pain.48,52,53Several strengths of the project should be noted. Data were collected using well established measurement tools that had previously demonstrated reliability and validity. All the tools used in data collection demonstrated good internal consistency reliabilities in the current sample of veterans. Weaknesses of the project include the use of a convenience sample of veterans and small sample size. Data were not available on the number of veterans who were offered participation or on how many veterans declined enrollment. The sample of veterans who chose to participate in the EVP may or may not have been representative of the population of veterans with high-impact chronic pain. As a pre- and postintervention design with no comparison group, the results are subject to multiple threats to internal validity, including the Hawthorne effect, maturation in the form of healing, and attrition. Reasons for leaving the program had not been recorded, so the investigators had no way of knowing factors that may have contributed to attrition. Also, data on when veterans left the program were unavailable. Research is needed with a control group to reduce the effect of confounding variables on the outcome measures. This project used data collected at a single VA facility, which limits its generalizability.
While completers and noncompleters of the EVP were similar on age, gender, and baseline measures, there may have been unidentified characteristics that influenced program completion. The investigators noticed the presence of more missing data among noncompleters compared to completers on the pre-intervention PCS; thus, noncompleters may have scored lower than completers on this instrument simply because there were more individual items that were unanswered/missing among this group of noncompleters.
Data were analyzed using a limited number of outcome measures that had previously been collected. Other outcome measures might include whether EVP participants reduced their use of medications, clinical resources, and personnel. Future projects, for example, could determine whether the EVP is effective in reducing opioid analgesic medication use and decreasing primary care and emergency department visits. Cost-benefit analyses could be completed to determine whether EVP is associated with financial savings.
Because no follow-up assessments were made to determine whether improvements were maintained over time, the project focus was limited to an evaluation of the short-term changes in the outcome measures. Future projects could include a follow-up assessment of the veterans 1- or 2-years post completion of the EVP.
Data for the project were collected prior to the COVID-19 pandemic, when the EVP was implemented through face-to-face meetings with participants and their peers. It is not clear how changes to the delivery of the program (such as offering it through telehealth) might impact veterans’ satisfaction with the program, willingness to complete it, and other variables of interest.
The results of this project were made available to stakeholders with recommendations for program expansion both at the current location and at other VA facilities, including the recommendation to hire additional personnel that would implement the program. As the VA network of facilities expand the EVP program and adapt it for telehealth delivery, the investigators recommended a similar analysis of data be performed following telehealth delivery. If delivery through telehealth is shown to improve outcome measures, the EVP could provide pain management treatment options for patients challenged by transportation barriers, including rural veterans.
Conclusion
This quality improvement project provided evidence of improvement in measures of pain severity, pain interference, negative cognition (catastrophizing), quality of life, and patient treatment satisfaction among veterans with chronic high-impact pain. Findings have been well received by the northeastern VA as well as the Veterans Integrated Systems Network 5. The results of the analyses were used to inform decisions regarding the future of the program.
Disclaimer: This material is the result of work supported with resources and the use of facilities at the VA Maryland Health Care System, Baltimore, Maryland. The views expressed are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the United States Government.
Acknowledgments: The authors thank Dr. Arianna Perra, the recent past coordinator of the Empower Veterans Program (EVP), who provided initial insights and support that motivated the decision to evaluate the program. We also thank the veterans and VA EVP clinicians who contributed data for the evaluation, and Dr. Michael Saenger (Director, TelePain-EVP: EVP) and Dr. Robert Lavin for their ongoing support, care, and concern for veteran patients. We also thank Dr. Beverly Bradley and the neurology service administrative team for their guidance in the process of obtaining necessary VA approvals for this project.
Corresponding author: Jessica U. Uche, DNP, CRNP-Family; [email protected]
doi:10.12788/jcom.0089
1. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press (US); 2011.
2. Bastian LA, Heapy A, Becker WC, et al. Understanding pain and pain treatment for veterans: responding to the federal pain research strategy. Pain Med. 2018;19(suppl_1); S1-S4. doi:10.1093/pm/pny1433
3. Engle GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129-136. doi:10.1126/science.847460
4. Bevers K, Watts L, Kishino ND, et al. The biopsychosocial model of the assessment, prevention, and treatment of chronic pain. US Neurology. 2016;12(2):98-104. doi:10.17925/USN.2016.12.02.98
5. Bair MJ, Ang D, Wu J, et al. Evaluation of stepped care for chronic pain (ESCAPE) in veterans of the Iraq and Afghanistan conflicts: A randomized clinical trial. JAMA Intern Med. 2015;175(5):682-689. doi:10.1001/jamainternmed.2015.97
6. Veterans Health Administration. Pain Management. VHA Directive 2009-053. Washington, DC: Department of Veterans Affairs; 2009.https://www.va.gov/painmanagement/docs/vha09paindirective.pdf
7. Moore BA, Anderson D, Dorflinger L, et al. Stepped care model for pain management and quality of pain care in long-term opioid therapy. J Rehabil Res Dev. 2016;53(1):137-146. doi:10.1682/JRRD.2014.10.0254
8. Institute for Healthcare Improvement. How to improve. Accessed March 14, 2022. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx
9. Saenger M. Empower Veterans Program. APA PCSS-O Webinars. Evidence CAM LBP 2016.
10. Gaudet T, Kligler B. Whole health in the whole system of the Veterans Administration: How will we know we have reached this future state? J Altern Complement Med. 2019;25(S1):S7-S11. doi:10.1089/acm.2018.29061.gau
11. Veterans Health Administration. Whole health: Circle of health. Updated April 1, 2021. Accessed March 14, 2022. https://www.va.gov/WHOLEHEALTH/circle-of-health/index.asp
12. Krebs EE, Carey TS, Weinberger M. Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med. 2007;22(10):1453-1458. doi:10.1007/s11606-007-0321-2
13. Veterans Health Administration. Pain as the 5th vital sign toolkit. October 2000, revised edition. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee. https://www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf
14. McKillop JM, Nielson WR. Improving the usefulness of the Multidimensional Pain Inventory. Pain Res Manag. 2011;16(4):239-244. doi:10.1155/2011/873424
15. Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain.1985;23(4):345-356. doi:10.1016/0304-3959(85)90004-1
16. Verra ML, Angst F, Staal JB, et al. Reliability of the multidimensional pain inventory and stability of the MPI classification system in chronic back pain. BMC Musculoskelet Disord. 2012;13:155. doi:10.1186/1471-2474-13-155
17. Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol Med. 1998;28(3):551-558. doi:10.1017/s0033291798006667
18. World Health Organization. Division of Mental Health. WHOQOL-BREF: introduction, administration, scoring and generic version of the assessment: field trial version, December 1996. Accessed March 14, 2022. https://apps.who.int/iris/handle/10665/63529
19. Guay S, Fortin C, Fikretoglu D, et al. Validation of the WHOQOL-BREF in a sample of male treatment-seeking veterans. Mil Psychol. 2015;27(2):85-92. doi:10.1037/mil0000065
20. Skevington S, Lotfy M, O’Connell K, WHOQOL Group. The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A Report from the WHOQOL Group. Qual Life Res. 2004;13(2):299-310. doi:10.1023/B:QURE.0000018486.91360.00
21. Stratton KJ, Bender MC, Cameron JJ, Pickett TC. Development and evaluation of a behavioral pain management treatment program in a Veterans Affairs Medical Center. Mil Med. 2015;180(3):263-268. doi:10.7205/MILMED-D-14-00281.
22. Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17(1):52-64. doi:10.1097/00002508-200103000-00008
23. Sullivan JL. The Pain Catastrophizing Scale: User manual. Accessed March 14, 2022. https://studylib.net/doc/8330191/the-pain-catastrophizing-scale---dr.-michael-sullivan
24. Darnall BD, Sturgeon JA, Cook KF, et al. Development and validation of a daily pain catastrophizing scale. J Pain. 2017;18(9):1139-1149. doi:10.1016/j.jpain.2017.05.003
25. Osman A, Barrios FX, Kopper BA, et al. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med. 1997;20(6):589-605. doi:10.1023/a:1025570508954
26. Sullivan MJL, Bishop S, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assessment. 1995;7(4):524-532. doi:10.1037/1040-3590.7.4.524
27. Walker R, Clark M, Gironda R. Psychometric characteristics of the Pain Treatment Satisfaction Scale. J Pain. 2015;6(3Suppl.):S76.
28. Emerson RW. Bonferroni correction and type I error. J Vis Impair Blind. 2020;114(1):77-78. doi:10.1177/0145482X20901378
29. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Routledge; 1988. doi:10.4324/9780203771587
30. Craner JR, Lake ES, Bancroft KA, George LL. Treatment outcomes and mechanisms for an ACT-based 10-week interdisciplinary chronic pain rehabilitation program. Pain Pract. 2020;20(1):44-54. doi:10.1111/papr.12824
31. Han L, Goulet JL, Skanderson M, et al. Evaluation of complementary and integrative health approaches among US veterans with musculoskeletal pain using propensity score methods. Pain Med. 2019;20(1):90-102. doi:10.1093/pm/pny027
32. Herman PM, Yuan AH, Cefalu MS, et al. The use of complementary and integrative health approaches for chronic musculoskeletal pain in younger US veterans: an economic evaluation. PLoS One. 2019;14(6):e0217831. doi:10.1371/journal.pone.0217831
33. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Global Health; Board on Health Sciences Policy; Global Forum on Innovation in Health Professional Education; Forum on Neuroscience and Nervous System Disorders; Stroud C, Posey Norris SM, Bain L, eds. The Role of Nonpharmacological Approaches to Pain Management: Proceedings of a Workshop. National Academies Press (US); April 12, 2019.
34. Richmond H, Hall AM, Copsey B, et al. The effectiveness of cognitive behavioural treatment for non-specific low back pain: a systematic review and meta-analysis. PLoS One. 2015;10(8):e0134192. doi:10.1371/journal.pone.0134192

35. Kearney DJ, Simpson TL, Malte CA, et al. Mindfulness-based stress reduction in addition to usual care is associated with improvements in pain, fatigue, and cognitive failures among veterans with Gulf War illness. Am J Med. 2016;129(2):204-214. doi:10.1016/j.amjmed.2015.09.015
36. Khoo E, Small R, Cheng W, et al. Comparative evaluation of group-based mindfulness-based stress reduction and cognitive behavioral therapy for the treatment and management of chronic pain: a systematic review and network meta-analysis. Evid Based Ment Health. 2019;22(1):26-35. doi:10.1136/ebmental-2018-300062
37. Khusid MA, Vythilingam M. The emerging role of mindfulness meditation as effective self-management strategy, Part 2: clinical implications for chronic pain, substance misuse, and insomnia. Mil Med. 2016;181(9):969-975. doi:10.7205/MILMED-D-14-00678
38. la Cour P, Petersen M. Effects of mindfulness meditation on chronic pain: A randomized controlled trial. Pain Med. 2015;16(4):641-652. doi:10.1111/pme.12605
39. Zou L, Zhang Y, Yang L, et al. Are mindful exercises safe and beneficial for treating chronic lower back pain? A systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2019;8(5):628. doi:10.3390/jcm8050628
40. Hughes LS, Clark J, Colclough JA, et al. Acceptance and commitment therapy (ACT) for chronic pain: a systematic review and meta-analyses. Clin J Pain. 2017;33(6):552-568. doi:10.1097/AJP.0000000000000425
41. Kemani MK, Olsson GL, Lekander M, et al. Efficacy and cost-effectiveness of acceptance and commitment therapy and applied relaxation for longstanding pain: a randomized controlled trial. Clin J Pain. 2015;31(11):1004-1016. doi:10.1097/AJP.0000000000000203
42. Scott W, Daly A, Yu L, McCracken LM. Treatment of chronic pain for adults 65 and over: analyses of outcomes and changes in psychological flexibility following interdisciplinary acceptance and commitment therapy (ACT). Pain Med. 2017;18(2):252. doi:10.1093/pm/pnw073
43. Veehof MM, Trompetter HR, Bohlmeijer ET, Schreurs KMG. Acceptance- and mindfulness-based interventions for the treatment of chronic pain: a meta-analytic review. Cogn Behav Ther. 2016;45(1):5-31. doi:10.1080/16506073.2015.1098724
44. Matthias MS, McGuire AB, Kukla M, et al. A brief peer support intervention for veterans with chronic musculoskeletal pain: a pilot study of feasibility and effectiveness. Pain Med. 2015;16(1):81-87. doi:10.1111/pme.12571
45. Anamkath NS, Palyo SA, Jacobs SC, et al. An interdisciplinary pain rehabilitation program for veterans with chronic pain: description and initial evaluation of outcomes. Pain Res Manag. 2018;2018(3941682):1-9. doi:10.1155/2018/3941682
46. Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain. Cochrane Database Syst Rev. 2014;9: CD000963. doi:10.1002/14651858.CD000963.pub3
47. Penney LS, Haro E. Qualitative evaluation of an interdisciplinary chronic pain intervention: Outcomes and barriers and facilitators to ongoing pain management. J Pain Res. 2019;12:865-878. doi:10.2147/JPR.S185652
48. Murphy JL, Cordova MJ, Dedert EA. Cognitive behavioral therapy for chronic pain in veterans; Evidence for clinical effectiveness in a model program. Psychol Serv. 2022;19(1):95-102. doi:10.1037/ser0000506
49. Katz L, Patterson L, Zacharias R. Evaluation of an interdisciplinary chronic pain program and predictors of readiness for change. Can J Pain. 2019;3(1):70-78. doi:10.1080/24740527.2019.1582296
50. Majumder SMM, Ahmed S, Shazzad N, et al. Translation, cross-cultural adaptation and validation of the Pain Catastrophizing Scale (PCS) into Bengali I patients with chronic non-malignant musculoskeletal pain. Int J Rheum Dis. 2020;23:1481-1487. doi:10.1111/1756-185X.13954
51. Margiotta F, Hannigan A, Imran A, et al. Pain, perceived injustice, and pain catastrophizing in chronic pain patients in Ireland. Pain Pract. 2016;17(5):663-668. doi:10.1111/papr.12
52. Bras M, Milunovic V, Boban M, et al. Quality of live in Croatian Homeland war (1991-1995) veterans who suffer from post-traumatic stress disorder and chronic pain. Health Qual Life Out. 2011;9:56. doi:10.1186/1477-7525-9-56
53. Liu C-H, Kung Y-Y, Lin C-L, et al. Therapeutic efficacy and the impact of the “dose” effect of acupuncture to treat sciatica: A randomized controlled pilot study. J Pain Res. 2019;12:3511-3520. doi:10.2147/JPR.S210672
1. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press (US); 2011.
2. Bastian LA, Heapy A, Becker WC, et al. Understanding pain and pain treatment for veterans: responding to the federal pain research strategy. Pain Med. 2018;19(suppl_1); S1-S4. doi:10.1093/pm/pny1433
3. Engle GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129-136. doi:10.1126/science.847460
4. Bevers K, Watts L, Kishino ND, et al. The biopsychosocial model of the assessment, prevention, and treatment of chronic pain. US Neurology. 2016;12(2):98-104. doi:10.17925/USN.2016.12.02.98
5. Bair MJ, Ang D, Wu J, et al. Evaluation of stepped care for chronic pain (ESCAPE) in veterans of the Iraq and Afghanistan conflicts: A randomized clinical trial. JAMA Intern Med. 2015;175(5):682-689. doi:10.1001/jamainternmed.2015.97
6. Veterans Health Administration. Pain Management. VHA Directive 2009-053. Washington, DC: Department of Veterans Affairs; 2009.https://www.va.gov/painmanagement/docs/vha09paindirective.pdf
7. Moore BA, Anderson D, Dorflinger L, et al. Stepped care model for pain management and quality of pain care in long-term opioid therapy. J Rehabil Res Dev. 2016;53(1):137-146. doi:10.1682/JRRD.2014.10.0254
8. Institute for Healthcare Improvement. How to improve. Accessed March 14, 2022. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx
9. Saenger M. Empower Veterans Program. APA PCSS-O Webinars. Evidence CAM LBP 2016.
10. Gaudet T, Kligler B. Whole health in the whole system of the Veterans Administration: How will we know we have reached this future state? J Altern Complement Med. 2019;25(S1):S7-S11. doi:10.1089/acm.2018.29061.gau
11. Veterans Health Administration. Whole health: Circle of health. Updated April 1, 2021. Accessed March 14, 2022. https://www.va.gov/WHOLEHEALTH/circle-of-health/index.asp
12. Krebs EE, Carey TS, Weinberger M. Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med. 2007;22(10):1453-1458. doi:10.1007/s11606-007-0321-2
13. Veterans Health Administration. Pain as the 5th vital sign toolkit. October 2000, revised edition. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee. https://www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf
14. McKillop JM, Nielson WR. Improving the usefulness of the Multidimensional Pain Inventory. Pain Res Manag. 2011;16(4):239-244. doi:10.1155/2011/873424
15. Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain.1985;23(4):345-356. doi:10.1016/0304-3959(85)90004-1
16. Verra ML, Angst F, Staal JB, et al. Reliability of the multidimensional pain inventory and stability of the MPI classification system in chronic back pain. BMC Musculoskelet Disord. 2012;13:155. doi:10.1186/1471-2474-13-155
17. Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol Med. 1998;28(3):551-558. doi:10.1017/s0033291798006667
18. World Health Organization. Division of Mental Health. WHOQOL-BREF: introduction, administration, scoring and generic version of the assessment: field trial version, December 1996. Accessed March 14, 2022. https://apps.who.int/iris/handle/10665/63529
19. Guay S, Fortin C, Fikretoglu D, et al. Validation of the WHOQOL-BREF in a sample of male treatment-seeking veterans. Mil Psychol. 2015;27(2):85-92. doi:10.1037/mil0000065
20. Skevington S, Lotfy M, O’Connell K, WHOQOL Group. The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A Report from the WHOQOL Group. Qual Life Res. 2004;13(2):299-310. doi:10.1023/B:QURE.0000018486.91360.00
21. Stratton KJ, Bender MC, Cameron JJ, Pickett TC. Development and evaluation of a behavioral pain management treatment program in a Veterans Affairs Medical Center. Mil Med. 2015;180(3):263-268. doi:10.7205/MILMED-D-14-00281.
22. Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17(1):52-64. doi:10.1097/00002508-200103000-00008
23. Sullivan JL. The Pain Catastrophizing Scale: User manual. Accessed March 14, 2022. https://studylib.net/doc/8330191/the-pain-catastrophizing-scale---dr.-michael-sullivan
24. Darnall BD, Sturgeon JA, Cook KF, et al. Development and validation of a daily pain catastrophizing scale. J Pain. 2017;18(9):1139-1149. doi:10.1016/j.jpain.2017.05.003
25. Osman A, Barrios FX, Kopper BA, et al. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med. 1997;20(6):589-605. doi:10.1023/a:1025570508954
26. Sullivan MJL, Bishop S, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assessment. 1995;7(4):524-532. doi:10.1037/1040-3590.7.4.524
27. Walker R, Clark M, Gironda R. Psychometric characteristics of the Pain Treatment Satisfaction Scale. J Pain. 2015;6(3Suppl.):S76.
28. Emerson RW. Bonferroni correction and type I error. J Vis Impair Blind. 2020;114(1):77-78. doi:10.1177/0145482X20901378
29. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Routledge; 1988. doi:10.4324/9780203771587
30. Craner JR, Lake ES, Bancroft KA, George LL. Treatment outcomes and mechanisms for an ACT-based 10-week interdisciplinary chronic pain rehabilitation program. Pain Pract. 2020;20(1):44-54. doi:10.1111/papr.12824
31. Han L, Goulet JL, Skanderson M, et al. Evaluation of complementary and integrative health approaches among US veterans with musculoskeletal pain using propensity score methods. Pain Med. 2019;20(1):90-102. doi:10.1093/pm/pny027
32. Herman PM, Yuan AH, Cefalu MS, et al. The use of complementary and integrative health approaches for chronic musculoskeletal pain in younger US veterans: an economic evaluation. PLoS One. 2019;14(6):e0217831. doi:10.1371/journal.pone.0217831
33. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Global Health; Board on Health Sciences Policy; Global Forum on Innovation in Health Professional Education; Forum on Neuroscience and Nervous System Disorders; Stroud C, Posey Norris SM, Bain L, eds. The Role of Nonpharmacological Approaches to Pain Management: Proceedings of a Workshop. National Academies Press (US); April 12, 2019.
34. Richmond H, Hall AM, Copsey B, et al. The effectiveness of cognitive behavioural treatment for non-specific low back pain: a systematic review and meta-analysis. PLoS One. 2015;10(8):e0134192. doi:10.1371/journal.pone.0134192

35. Kearney DJ, Simpson TL, Malte CA, et al. Mindfulness-based stress reduction in addition to usual care is associated with improvements in pain, fatigue, and cognitive failures among veterans with Gulf War illness. Am J Med. 2016;129(2):204-214. doi:10.1016/j.amjmed.2015.09.015
36. Khoo E, Small R, Cheng W, et al. Comparative evaluation of group-based mindfulness-based stress reduction and cognitive behavioral therapy for the treatment and management of chronic pain: a systematic review and network meta-analysis. Evid Based Ment Health. 2019;22(1):26-35. doi:10.1136/ebmental-2018-300062
37. Khusid MA, Vythilingam M. The emerging role of mindfulness meditation as effective self-management strategy, Part 2: clinical implications for chronic pain, substance misuse, and insomnia. Mil Med. 2016;181(9):969-975. doi:10.7205/MILMED-D-14-00678
38. la Cour P, Petersen M. Effects of mindfulness meditation on chronic pain: A randomized controlled trial. Pain Med. 2015;16(4):641-652. doi:10.1111/pme.12605
39. Zou L, Zhang Y, Yang L, et al. Are mindful exercises safe and beneficial for treating chronic lower back pain? A systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2019;8(5):628. doi:10.3390/jcm8050628
40. Hughes LS, Clark J, Colclough JA, et al. Acceptance and commitment therapy (ACT) for chronic pain: a systematic review and meta-analyses. Clin J Pain. 2017;33(6):552-568. doi:10.1097/AJP.0000000000000425
41. Kemani MK, Olsson GL, Lekander M, et al. Efficacy and cost-effectiveness of acceptance and commitment therapy and applied relaxation for longstanding pain: a randomized controlled trial. Clin J Pain. 2015;31(11):1004-1016. doi:10.1097/AJP.0000000000000203
42. Scott W, Daly A, Yu L, McCracken LM. Treatment of chronic pain for adults 65 and over: analyses of outcomes and changes in psychological flexibility following interdisciplinary acceptance and commitment therapy (ACT). Pain Med. 2017;18(2):252. doi:10.1093/pm/pnw073
43. Veehof MM, Trompetter HR, Bohlmeijer ET, Schreurs KMG. Acceptance- and mindfulness-based interventions for the treatment of chronic pain: a meta-analytic review. Cogn Behav Ther. 2016;45(1):5-31. doi:10.1080/16506073.2015.1098724
44. Matthias MS, McGuire AB, Kukla M, et al. A brief peer support intervention for veterans with chronic musculoskeletal pain: a pilot study of feasibility and effectiveness. Pain Med. 2015;16(1):81-87. doi:10.1111/pme.12571
45. Anamkath NS, Palyo SA, Jacobs SC, et al. An interdisciplinary pain rehabilitation program for veterans with chronic pain: description and initial evaluation of outcomes. Pain Res Manag. 2018;2018(3941682):1-9. doi:10.1155/2018/3941682
46. Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain. Cochrane Database Syst Rev. 2014;9: CD000963. doi:10.1002/14651858.CD000963.pub3
47. Penney LS, Haro E. Qualitative evaluation of an interdisciplinary chronic pain intervention: Outcomes and barriers and facilitators to ongoing pain management. J Pain Res. 2019;12:865-878. doi:10.2147/JPR.S185652
48. Murphy JL, Cordova MJ, Dedert EA. Cognitive behavioral therapy for chronic pain in veterans; Evidence for clinical effectiveness in a model program. Psychol Serv. 2022;19(1):95-102. doi:10.1037/ser0000506
49. Katz L, Patterson L, Zacharias R. Evaluation of an interdisciplinary chronic pain program and predictors of readiness for change. Can J Pain. 2019;3(1):70-78. doi:10.1080/24740527.2019.1582296
50. Majumder SMM, Ahmed S, Shazzad N, et al. Translation, cross-cultural adaptation and validation of the Pain Catastrophizing Scale (PCS) into Bengali I patients with chronic non-malignant musculoskeletal pain. Int J Rheum Dis. 2020;23:1481-1487. doi:10.1111/1756-185X.13954
51. Margiotta F, Hannigan A, Imran A, et al. Pain, perceived injustice, and pain catastrophizing in chronic pain patients in Ireland. Pain Pract. 2016;17(5):663-668. doi:10.1111/papr.12
52. Bras M, Milunovic V, Boban M, et al. Quality of live in Croatian Homeland war (1991-1995) veterans who suffer from post-traumatic stress disorder and chronic pain. Health Qual Life Out. 2011;9:56. doi:10.1186/1477-7525-9-56
53. Liu C-H, Kung Y-Y, Lin C-L, et al. Therapeutic efficacy and the impact of the “dose” effect of acupuncture to treat sciatica: A randomized controlled pilot study. J Pain Res. 2019;12:3511-3520. doi:10.2147/JPR.S210672
Characterizing Opioid Response in Older Veterans in the Post-Acute Setting
Older adults admitted to post-acute settings frequently have complex rehabilitation needs and multimorbidity, which predisposes them to pain management challenges.1,2 The prevalence of pain in post-acute and long-term care is as high as 65%, and opioid use is common among this population with 1 in 7 residents receiving long-term opioids.3,4
Opioids that do not adequately control pain represent a missed opportunity for deprescribing. There is limited evidence regarding efficacy of long-term opioid use (> 90 days) for improving pain and physical functioning.5 In addition, long-term opioid use carries significant risks, including overdose-related death, dependence, and increased emergency department visits.5 These risks are likely to be pronounced among veterans receiving post-acute care (PAC) who are older, have comorbid psychiatric disorders, are prescribed several centrally acting medications, and experience substance use disorder (SUD).6
Older adults are at increased risk for opioid toxicity because of reduced drug clearance and smaller therapeutic window.5 Centers for Disease Control and Prevention (CDC) guidelines recommend frequently assessing patients for benefit in terms of sustained improvement in pain as well as physical function.5 If pain and functional improvements are minimal, opioid use and nonopioid pain management strategies should be considered. Some patients will struggle with this approach. Directly asking patients about the effectiveness of opioids is challenging. Opioid users with chronic pain frequently report problems with opioids even as they describe them as indispensable for pain management.7,8
Earlier studies have assessed patient perspectives regarding opioid difficulties as well as their helpfulness, which could introduce recall bias. Patient-level factors that contribute to a global sense of distress, in addition to the presence of painful physical conditions, also could contribute to patients requesting opioids without experiencing adequate pain relief. One study in veterans residing in PAC facilities found that individuals with depression, posttraumatic stress disorder (PTSD), and SUD were more likely to report pain and receive scheduled analgesics; this effect persisted in individuals with PTSD even after adjusting for demographic and functional status variables.9 The study looked only at analgesics as a class and did not examine opioids specifically. It is possible that distressed individuals, such as those with uncontrolled depression, PTSD, and SUD, might be more likely to report high pain levels and receive opioids with inadequate benefit and increased risk. Identifying the primary condition causing distress and targeting treatment to that condition (ie, depression) is preferable to escalating opioids in an attempt to treat pain in the context of nonresponse. Assessing an individual’s aggregate response to opioids rather than relying on a single self-report is a useful addition to current pain management strategies.
The goal of this study was to pilot a method of identifying opioid-nonresponsive pain using administrative data, measure its prevalence in a PAC population of veterans, and explore clinical and demographic correlates with particular attention to variates that could indicate high levels of psychological and physical distress. Identifying pain that is poorly responsive to opioids would give clinicians the opportunity to avoid or minimize opioid use and prioritize treatments that are likely to improve the resident’s pain, quality of life, and physical function while minimizing recall bias. We hypothesized that pain that responds poorly to opioids would be prevalent among veterans residing in a PAC unit. We considered that veterans with pain poorly responsive to opioids would be more likely to have factors that would place them at increased risk of adverse effects, such as comorbid psychiatric conditions, history of SUD, and multimorbidity, providing further rationale for clinical equipoise in that population.6
Methods
This was a small, retrospective cross-sectional study using administrative data and chart review. The study included veterans who were administered opioids while residing in a single US Department of Veterans Affairs (VA) community living center PAC (CLC-PAC) unit during at least 1 of 4 nonconsecutive, random days in 2016 and 2017. The study was approved by the institutional review board of the Ann Arbor VA Health System (#2017-1034) as part of a larger project involving models of care in vulnerable older veterans.
Inclusion criteria were the presence of at least moderate pain (≥ 4 on a 0 to 10 scale); receiving ≥ 2 opioids ordered as needed over the prespecified 24-hour observation period; and having ≥ 2 pre-and postopioid administration pain scores during the observation period. Veterans who did not meet these criteria were excluded. At the time of initial sample selection, we did not capture information related to coprescribed analgesics, including a standing order of opioids. To obtain the sample, we initially characterized all veterans on the 4 days residing in the CLC-PAC unit as those reporting at least moderate pain (≥ 4) and those who reported no or mild pain (< 4). The cut point of 4 of 10 is consistent with moderate pain based on earlier work showing higher likelihood of pain that interferes with physical function.10 We then restricted the sample to veterans who received ≥ 2 opioids ordered as needed for pain and had ≥ 2 pre- and postopioid administration numeric pain rating scores during the 24-hour observation period. This methodology was chosen to enrich our sample for those who received opioids regularly for ongoing pain. Opioids were defined as full µ-opioid receptor agonists and included hydrocodone, oxycodone, morphine, hydromorphone, fentanyl, tramadol, and methadone.
Medication administration data were obtained from the VA corporate data warehouse, which houses all barcode medication administration data collected at the point of care. The dataset includes pain scores gathered by nursing staff before and after administering an as-needed analgesic. The corporate data warehouse records data/time of pain scores and the analgesic name, dosage, formulation, and date/time of administration. Using a standardized assessment form developed iteratively, we calculated opioid dosage in oral morphine equivalents (OME) for comparison.11,12 All abstracted data were reexamined for accuracy. Data initially were collected in an anonymized, blinded fashion. Participants were then unblinded for chart review. Initial data was captured in resident-days instead of unique residents because an individual resident might have been admitted on several observation days. We were primarily interested in how pain responded to opioids administered in response to resident request; therefore, we did not examine response to opioids that were continuously ordered (ie, scheduled). We did consider scheduled opioids when calculating total daily opioid dosage during the chart review.
Outcome of Interest
The primary outcome of interest was an individual’s response to as-needed opioids, which we defined as change in the pain score after opioid administration. The pre-opioid pain score was the score that immediately preceded administration of an as-needed opioid. The postopioid administration pain score was the first score after opioid administration if obtained within 3 hours of administration. Scores collected > 3 hours after opioid administration were excluded because they no longer accurately reflected the impact of the opioid due to the short half-lives. Observations were excluded if an opioid was administered without a recorded pain score; this occurred once for 6 individuals. Observations also were excluded if an opioid was administered but the data were captured on the following day (outside of the 24-hour window); this occurred once for 3 individuals.
We calculated a ∆ score by subtracting the postopioid pain rating score from the pre-opioid score. Individual ∆ scores were then averaged over the 24-hour period (range, 2-5 opioid doses). For example, if an individual reported a pre-opioid pain score of 10, and a postopioid pain score of 2, the ∆ was recorded as 8. If the individual’s next pre-opioid score was 10, and post-opioid score was 6, the ∆ was recorded as 4. ∆ scores over the 24-hour period were averaged together to determine that individual’s response to as-needed opioids. In the previous example, the mean ∆ score is 6. Lower mean ∆ scores reflect decreased responsiveness to opioids’ analgesic effect.
Demographic and clinical data were obtained from electronic health record review using a standardized assessment form. These data included information about medical and psychiatric comorbidities, specialist consultations, and CLC-PAC unit admission indications and diagnoses. Medications of interest were categorized as antidepressants, antipsychotics, benzodiazepines, muscle relaxants, hypnotics, stimulants, antiepileptic drugs/mood stabilizers (including gabapentin and pregabalin), and all adjuvant analgesics. Adjuvant analgesics were defined as medications administered for pain as documented by chart notes or those ordered as needed for pain, and analyzed as a composite variable. Antidepressants with analgesic properties (serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants) were considered adjuvant analgesics. Psychiatric information collected included presence of mood, anxiety, and psychotic disorders, and PTSD. SUD information was collected separately from other psychiatric disorders.
Analyses
The study population was described using tabulations for categorical data and means and standard deviations for continuous data. Responsiveness to opioids was analyzed as a continuous variable. Those with higher mean ∆ scores were considered to have pain relatively more responsive to opioids, while lower mean ∆ scores indicated pain less responsive to opioids. We constructed linear regression models controlling for average pre-opioid pain rating scores to explore associations between opioid responsiveness and variables of interest. All analyses were completed using Stata version 15. This study was not adequately powered to detect differences across the spectrum of opioid responsiveness, although the authors have reported differences in this article.
Results
Over the 4-day observational period there were 146 resident-days. Of these, 88 (60.3%) reported at least 1 pain score of ≥ 4. Of those, 61 (41.8%) received ≥ 1 as-needed opioid for pain. We identified 46 resident-days meeting study criteria of ≥ 2 pre- and postanalgesic scores. We identified 41 unique individuals (Figure 1). Two individuals were admitted to the CLC-PAC unit on 2 of the 4 observation days, and 1 individual was admitted to the CLC-PAC unit on 3 of the 4 observation days. For individuals admitted several days, we included data only from the initial observation day.
Response to opioids varied greatly in this sample. The mean (SD) ∆ pain score was 3.4 (1.6) and ranged from 0.5 to 6.3. Using linear regression, we found no relationship between admission indication, medical comorbidities (including active cancer), and opioid responsiveness (Table).
Psychiatric disorders were highly prevalent, with 25 individuals (61.0%) having ≥ 1 any psychiatric diagnosis identified on chart review. The presence of any psychiatric diagnosis was significantly associated with reduced responsiveness to opioids (β = −1.08; 95% CI, −2.04 to −0.13; P = .03). SUDs also were common, with 17 individuals (41.5%) having an active SUD; most were tobacco/nicotine. Twenty-six veterans (63.4%) had documentation of SUD in remission with 19 (46.3%) for substances other than tobacco/nicotine. There was no indication that any veteran in the sample was prescribed medication for opioid use disorder (OUD) at the time of observation. There was no relationship between opioid responsiveness and SUDs, neither active or in remission. Consults to other services that suggested distress or difficult-to-control symptoms also were frequent. Consults to the pain service were significantly associated with reduced responsiveness to opioids (β = −1.75; 95% CI, −3.33 to −0.17; P = .03). Association between psychiatry consultation and reduced opioid responsiveness trended toward significance (β = −0.95; 95% CI, −2.06 to 0.17; P = .09) (Figures 2 and 3). There was no significant association with palliative medicine consultation and opioid responsiveness.
A poorer response to opioids was associated with a significantly higher as-needed opioid dosage (β = −0.02; 95% CI, −0.04 to −0.01; P = .002) as well as a trend toward higher total opioid dosage (β = −0.005; 95% CI, −0.01 to 0.0003; P = .06) (Figure 4). Thirty-eight (92.7%) participants received nonopioid adjuvant analgesics for pain. More than half (56.1%) received antidepressants or gabapentinoids (51.2%), although we did not assess whether they were prescribed for pain or another indication. We did not identify a relationship between any specific psychoactive drug class and opioid responsiveness in this sample.
Discussion
This exploratory study used readily available administrative data in a CLC-PAC unit to assess responsiveness to opioids via a numeric mean ∆ score, with higher values indicating more pain relief in response to opioids. We then constructed linear regression models to characterize the relationship between the mean ∆ score and factors known to be associated with difficult-to-control pain and psychosocial distress. As expected, opioid responsiveness was highly variable among residents; some residents experienced essentially no reduction in pain, on average, despite receiving opioids. Psychiatric comorbidity, higher dosage in OMEs, and the presence of a pain service consult significantly correlated with poorer response to opioids. To our knowledge, this is the first study to quantify opioid responsiveness and describe the relationship with clinical correlates in the understudied PAC population.
Earlier research has demonstrated a relationship between the presence of psychiatric disorders and increased likelihood of receiving any analgesics among veterans residing in PAC.9 Our study adds to the literature by quantifying opioid response using readily available administrative data and examining associations with psychiatric diagnoses. These findings highlight the possibility that attempting to treat high levels of pain by escalating the opioid dosage in patients with a comorbid psychiatric diagnosis should be re-addressed, particularly if there is no meaningful pain reduction at lower opioid dosages. Our sample had a variety of admission diagnoses and medical comorbidities, however, we did not identify a relationship with opioid responsiveness, including an active cancer diagnosis. Although SUDs were highly prevalent in our sample, there was no relationship with opioid responsiveness. This suggests that lack of response to opioids is not merely a matter of drug tolerance or an indication of drug-seeking behavior.
Factors Impacting Response
Many factors could affect whether an individual obtains an adequate analgesic response to opioids or other pain medications, including variations in genes encoding opioid receptors and hepatic enzymes involved in drug metabolism and an individual’s opioid exposure history.13 The phenomenon of requiring more drug to produce the same relief after repeated exposures (ie, tolerance) is well known.14 Opioid-induced hyperalgesia is a phenomenon whereby a patient’s overall pain increases while receiving opioids, but each opioid dose might be perceived as beneficial.15 Increasingly, psychosocial distress is an important factor in opioid response. Adverse selection is the process culminating in those with psychosocial distress and/or SUDs being prescribed more opioids for longer durations.16 Our data suggests that this process could play a role in PAC settings. In addition, exaggerating pain to obtain additional opioids for nonmedical purposes, such as euphoria or relaxation, also is possible.17
When clinically assessing an individual whose pain is not well controlled despite escalating opioid dosages, prescribers must consider which of these factors likely is predominant. However, the first step of determining who has a poor opioid response is not straightforward. Directly asking patients is challenging; many individuals perceive opioids to be helpful while simultaneously reporting inadequately controlled pain.7,8 The primary value of this study is the possibility of providing prescribers a quick, simple method of assessing a patient’s response to opioids. Using this method, individuals who are responding poorly to opioids, including those who might exaggerate pain for secondary gain, could be identified. Health care professionals could consider revisiting pain management strategies, assess for the presence of OUD, or evaluate other contributors to inadequately controlled pain. Although we only collected data regarding response to opioids in this study, any pain medication administered as needed (ie, nonsteroidal anti-inflammatory drugs, acetaminophen) could be analyzed using this methodology, allowing identification of other helpful pain management strategies. We began the validation process with extensive chart review, but further validation is required before this method can be applied to routine clinical practice.
Patients who report uncontrolled pain despite receiving opioids are a clinically challenging population. The traditional strategy has been to escalate opioids, which is recommended by the World Health Organization stepladder approach for patients with cancer pain and limited life expectancy.18 Applying this approach to a general population of patients with chronic pain is ineffective and dangerous.19 The CDC and the VA/US Department of Defense (VA/DoD) guidelines both recommend carefully reassessing risks and benefits at total daily dosages > 50 OME and avoid increasing dosages to > 90 OME daily in most circumstances.5,20 Our finding that participants taking higher dosages of opioids were not more likely to have better control over their pain supports this recommendation.
Limitations
This study has several limitations, the most significant is its small sample size because of the exploratory nature of the project. Results are based on a small pilot sample enriched to include individuals with at least moderate pain who receive opioids frequently at 1 VA CLC-PAC unit; therefore, the results might not be representative of all veterans or a more general population. Our small sample size limits power to detect small differences. Data collected should be used to inform formal power calculations before subsequent larger studies to select adequate sample size. Validation studies, including samples from the same population using different dates, which reproduce findings are an important step. Moreover, we only had data on a single dimension of pain (intensity/severity), as measured by the pain scale, which nursing staff used to make a real-time clinical decision of whether to administer an as-needed opioid. Future studies should consider using pain measures that provide multidimensional assessment (ie, severity, functional interference) and/or were developed specifically for veterans, such as the Defense and Veterans Pain Rating Scale.21
Our study was cross-sectional in nature and addressed a single 24-hour period of data per participant. The years of data collection (2016 and 2017) followed a decline in overall opioid prescribing that has continued, likely influenced by CDC and VA/DoD guidelines.22 It is unclear whether our observations are an accurate reflection of individuals’ response over time or whether prescribing practices in PAC have shifted.
We did not consider the type of pain being treated or explore clinicians’ reasons for prescribing opioids, therefore limiting our ability to know whether opioids were indicated. Information regarding OUD and other SUDs was limited to what was documented in the chart during the CLC-PAC unit admission. We did not have information on length of exposure to opioids. It is possible that opioid tolerance could play a role in reducing opioid responsiveness. However, simple tolerance would not be expected to explain robust correlations with psychiatric comorbidities. Also, simple tolerance would be expected to be overcome with higher opioid dosages, whereas our study demonstrates less responsiveness. These data suggests that some individuals’ pain might be poorly opioid responsive, and psychiatric factors could increase this risk. We used a novel data source in combination with chart review; to our knowledge, barcode medication administration data have not been used in this manner previously. Future work needs to validate this method, using larger sample sizes and several clinical sites. Finally, we used regression models that controlled for average pre-opioid pain rating scores, which is only 1 covariate important for examining effects. Larger studies with adequate power should control for multiple covariates known to be associated with pain and opioid response.
Conclusions
Opioid responsiveness is important clinically yet challenging to assess. This pilot study identifies a way of classifying pain as relatively opioid nonresponsive using administrative data but requires further validation before considering scaling for more general use. The possibility that a substantial percentage of residents in a CLC-PAC unit could be receiving increasing dosages of opioids without adequate benefit justifies the need for more research and underscores the need for prescribers to assess individuals frequently for ongoing benefit of opioids regardless of diagnosis or mechanism of pain.
Acknowledgments
The authors thank Andrzej Galecki, Corey Powell, and the University of Michigan Consulting for Statistics, Computing and Analytics Research Center for assistance with statistical analysis.
1. Marshall TL, Reinhardt JP. Pain management in the last 6 months of life: predictors of opioid and non-opioid use. J Am Med Dir Assoc. 2019;20(6):789-790. doi:10.1016/j.jamda.2019.02.026
2. Tait RC, Chibnall JT. Pain in older subacute care patients: associations with clinical status and treatment. Pain Med. 2002;3(3):231-239. doi:10.1046/j.1526-4637.2002.02031.x
3. Pimentel CB, Briesacher BA, Gurwitz JH, Rosen AB, Pimentel MT, Lapane KL. Pain management in nursing home residents with cancer. J Am Geriatr Soc. 2015;63(4):633-641. doi:10.1111/jgs.13345
4. Hunnicutt JN, Tjia J, Lapane KL. Hospice use and pain management in elderly nursing home residents with cancer. J Pain Symptom Manage. 2017;53(3):561-570. doi:10.1016/j.jpainsymman.2016.10.369
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65(No. RR-1):1-49. doi:10.15585/mmwr.rr6501e1
6. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
7. Goesling J, Moser SE, Lin LA, Hassett AL, Wasserman RA, Brummett CM. Discrepancies between perceived benefit of opioids and self-reported patient outcomes. Pain Med. 2018;19(2):297-306. doi:10.1093/pm/pnw263
8. Sullivan M, Von Korff M, Banta-Green C. Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain. Pain. 2010;149(2):345-353. doi:10.1016/j.pain.2010.02.037
9. Brennan PL, Greenbaum MA, Lemke S, Schutte KK. Mental health disorder, pain, and pain treatment among long-term care residents: evidence from the Minimum Data Set 3.0. Aging Ment Health. 2019;23(9):1146-1155. doi:10.1080/13607863.2018.1481922
10. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176-183. doi:10.3978/j.issn.2224-5820.2015.09.04
11. Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. 2017. Accessed December 15, 2021. https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf
12. Nielsen S, Degenhardt L, Hoban B, Gisev N. Comparing opioids: a guide to estimating oral morphine equivalents (OME) in research. NDARC Technical Report No. 329. National Drug and Alcohol Research Centre; 2014. Accessed December 15, 2021. http://www.drugsandalcohol.ie/22703/1/NDARC Comparing opioids.pdf
13. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11(2):237-248.
14. Collin E, Cesselin F. Neurobiological mechanisms of opioid tolerance and dependence. Clin Neuropharmacol. 1991;14(6):465-488. doi:10.1097/00002826-199112000-00001
15. Higgins C, Smith BH, Matthews K. Evidence of opioid-induced hyperalgesia in clinical populations after chronic opioid exposure: a systematic review and meta-analysis. Br J Anaesth. 2019;122(6):e114-e126. doi:10.1016/j.bja.2018.09.019
16. Howe CQ, Sullivan MD. The missing ‘P’ in pain management: how the current opioid epidemic highlights the need for psychiatric services in chronic pain care. Gen Hosp Psychiatry. 2014;36(1):99-104. doi:10.1016/j.genhosppsych.2013.10.003
17. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publ No PEP19-5068, NSDUH Ser H-54. 2019;170:51-58. Accessed December 15, 2021. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf
18. World Health Organization. WHO’s cancer pain ladder for adults. Accessed September 21, 2018. www.who.int/ncds/management/palliative-care/Infographic-cancer-pain-lowres.pdf
19. Ballantyne JC, Kalso E, Stannard C. WHO analgesic ladder: a good concept gone astray. BMJ. 2016;352:i20. doi:10.1136/bmj.i20
20. The Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. US Dept of Veterans Affairs and Dept of Defense; 2017. Accessed December 15, 2021. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf
21. Defense & Veterans Pain Rating Scale (DVPRS). Defense & Veterans Center for Integrative Pain Management. Accessed July 21, 2021. https://www.dvcipm.org/clinical-resources/defense-veterans-pain-rating-scale-dvprs/
22. Guy GP Jr, Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. doi:10.15585/mmwr.mm6626a4
Older adults admitted to post-acute settings frequently have complex rehabilitation needs and multimorbidity, which predisposes them to pain management challenges.1,2 The prevalence of pain in post-acute and long-term care is as high as 65%, and opioid use is common among this population with 1 in 7 residents receiving long-term opioids.3,4
Opioids that do not adequately control pain represent a missed opportunity for deprescribing. There is limited evidence regarding efficacy of long-term opioid use (> 90 days) for improving pain and physical functioning.5 In addition, long-term opioid use carries significant risks, including overdose-related death, dependence, and increased emergency department visits.5 These risks are likely to be pronounced among veterans receiving post-acute care (PAC) who are older, have comorbid psychiatric disorders, are prescribed several centrally acting medications, and experience substance use disorder (SUD).6
Older adults are at increased risk for opioid toxicity because of reduced drug clearance and smaller therapeutic window.5 Centers for Disease Control and Prevention (CDC) guidelines recommend frequently assessing patients for benefit in terms of sustained improvement in pain as well as physical function.5 If pain and functional improvements are minimal, opioid use and nonopioid pain management strategies should be considered. Some patients will struggle with this approach. Directly asking patients about the effectiveness of opioids is challenging. Opioid users with chronic pain frequently report problems with opioids even as they describe them as indispensable for pain management.7,8
Earlier studies have assessed patient perspectives regarding opioid difficulties as well as their helpfulness, which could introduce recall bias. Patient-level factors that contribute to a global sense of distress, in addition to the presence of painful physical conditions, also could contribute to patients requesting opioids without experiencing adequate pain relief. One study in veterans residing in PAC facilities found that individuals with depression, posttraumatic stress disorder (PTSD), and SUD were more likely to report pain and receive scheduled analgesics; this effect persisted in individuals with PTSD even after adjusting for demographic and functional status variables.9 The study looked only at analgesics as a class and did not examine opioids specifically. It is possible that distressed individuals, such as those with uncontrolled depression, PTSD, and SUD, might be more likely to report high pain levels and receive opioids with inadequate benefit and increased risk. Identifying the primary condition causing distress and targeting treatment to that condition (ie, depression) is preferable to escalating opioids in an attempt to treat pain in the context of nonresponse. Assessing an individual’s aggregate response to opioids rather than relying on a single self-report is a useful addition to current pain management strategies.
The goal of this study was to pilot a method of identifying opioid-nonresponsive pain using administrative data, measure its prevalence in a PAC population of veterans, and explore clinical and demographic correlates with particular attention to variates that could indicate high levels of psychological and physical distress. Identifying pain that is poorly responsive to opioids would give clinicians the opportunity to avoid or minimize opioid use and prioritize treatments that are likely to improve the resident’s pain, quality of life, and physical function while minimizing recall bias. We hypothesized that pain that responds poorly to opioids would be prevalent among veterans residing in a PAC unit. We considered that veterans with pain poorly responsive to opioids would be more likely to have factors that would place them at increased risk of adverse effects, such as comorbid psychiatric conditions, history of SUD, and multimorbidity, providing further rationale for clinical equipoise in that population.6
Methods
This was a small, retrospective cross-sectional study using administrative data and chart review. The study included veterans who were administered opioids while residing in a single US Department of Veterans Affairs (VA) community living center PAC (CLC-PAC) unit during at least 1 of 4 nonconsecutive, random days in 2016 and 2017. The study was approved by the institutional review board of the Ann Arbor VA Health System (#2017-1034) as part of a larger project involving models of care in vulnerable older veterans.
Inclusion criteria were the presence of at least moderate pain (≥ 4 on a 0 to 10 scale); receiving ≥ 2 opioids ordered as needed over the prespecified 24-hour observation period; and having ≥ 2 pre-and postopioid administration pain scores during the observation period. Veterans who did not meet these criteria were excluded. At the time of initial sample selection, we did not capture information related to coprescribed analgesics, including a standing order of opioids. To obtain the sample, we initially characterized all veterans on the 4 days residing in the CLC-PAC unit as those reporting at least moderate pain (≥ 4) and those who reported no or mild pain (< 4). The cut point of 4 of 10 is consistent with moderate pain based on earlier work showing higher likelihood of pain that interferes with physical function.10 We then restricted the sample to veterans who received ≥ 2 opioids ordered as needed for pain and had ≥ 2 pre- and postopioid administration numeric pain rating scores during the 24-hour observation period. This methodology was chosen to enrich our sample for those who received opioids regularly for ongoing pain. Opioids were defined as full µ-opioid receptor agonists and included hydrocodone, oxycodone, morphine, hydromorphone, fentanyl, tramadol, and methadone.
Medication administration data were obtained from the VA corporate data warehouse, which houses all barcode medication administration data collected at the point of care. The dataset includes pain scores gathered by nursing staff before and after administering an as-needed analgesic. The corporate data warehouse records data/time of pain scores and the analgesic name, dosage, formulation, and date/time of administration. Using a standardized assessment form developed iteratively, we calculated opioid dosage in oral morphine equivalents (OME) for comparison.11,12 All abstracted data were reexamined for accuracy. Data initially were collected in an anonymized, blinded fashion. Participants were then unblinded for chart review. Initial data was captured in resident-days instead of unique residents because an individual resident might have been admitted on several observation days. We were primarily interested in how pain responded to opioids administered in response to resident request; therefore, we did not examine response to opioids that were continuously ordered (ie, scheduled). We did consider scheduled opioids when calculating total daily opioid dosage during the chart review.
Outcome of Interest
The primary outcome of interest was an individual’s response to as-needed opioids, which we defined as change in the pain score after opioid administration. The pre-opioid pain score was the score that immediately preceded administration of an as-needed opioid. The postopioid administration pain score was the first score after opioid administration if obtained within 3 hours of administration. Scores collected > 3 hours after opioid administration were excluded because they no longer accurately reflected the impact of the opioid due to the short half-lives. Observations were excluded if an opioid was administered without a recorded pain score; this occurred once for 6 individuals. Observations also were excluded if an opioid was administered but the data were captured on the following day (outside of the 24-hour window); this occurred once for 3 individuals.
We calculated a ∆ score by subtracting the postopioid pain rating score from the pre-opioid score. Individual ∆ scores were then averaged over the 24-hour period (range, 2-5 opioid doses). For example, if an individual reported a pre-opioid pain score of 10, and a postopioid pain score of 2, the ∆ was recorded as 8. If the individual’s next pre-opioid score was 10, and post-opioid score was 6, the ∆ was recorded as 4. ∆ scores over the 24-hour period were averaged together to determine that individual’s response to as-needed opioids. In the previous example, the mean ∆ score is 6. Lower mean ∆ scores reflect decreased responsiveness to opioids’ analgesic effect.
Demographic and clinical data were obtained from electronic health record review using a standardized assessment form. These data included information about medical and psychiatric comorbidities, specialist consultations, and CLC-PAC unit admission indications and diagnoses. Medications of interest were categorized as antidepressants, antipsychotics, benzodiazepines, muscle relaxants, hypnotics, stimulants, antiepileptic drugs/mood stabilizers (including gabapentin and pregabalin), and all adjuvant analgesics. Adjuvant analgesics were defined as medications administered for pain as documented by chart notes or those ordered as needed for pain, and analyzed as a composite variable. Antidepressants with analgesic properties (serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants) were considered adjuvant analgesics. Psychiatric information collected included presence of mood, anxiety, and psychotic disorders, and PTSD. SUD information was collected separately from other psychiatric disorders.
Analyses
The study population was described using tabulations for categorical data and means and standard deviations for continuous data. Responsiveness to opioids was analyzed as a continuous variable. Those with higher mean ∆ scores were considered to have pain relatively more responsive to opioids, while lower mean ∆ scores indicated pain less responsive to opioids. We constructed linear regression models controlling for average pre-opioid pain rating scores to explore associations between opioid responsiveness and variables of interest. All analyses were completed using Stata version 15. This study was not adequately powered to detect differences across the spectrum of opioid responsiveness, although the authors have reported differences in this article.
Results
Over the 4-day observational period there were 146 resident-days. Of these, 88 (60.3%) reported at least 1 pain score of ≥ 4. Of those, 61 (41.8%) received ≥ 1 as-needed opioid for pain. We identified 46 resident-days meeting study criteria of ≥ 2 pre- and postanalgesic scores. We identified 41 unique individuals (Figure 1). Two individuals were admitted to the CLC-PAC unit on 2 of the 4 observation days, and 1 individual was admitted to the CLC-PAC unit on 3 of the 4 observation days. For individuals admitted several days, we included data only from the initial observation day.
Response to opioids varied greatly in this sample. The mean (SD) ∆ pain score was 3.4 (1.6) and ranged from 0.5 to 6.3. Using linear regression, we found no relationship between admission indication, medical comorbidities (including active cancer), and opioid responsiveness (Table).
Psychiatric disorders were highly prevalent, with 25 individuals (61.0%) having ≥ 1 any psychiatric diagnosis identified on chart review. The presence of any psychiatric diagnosis was significantly associated with reduced responsiveness to opioids (β = −1.08; 95% CI, −2.04 to −0.13; P = .03). SUDs also were common, with 17 individuals (41.5%) having an active SUD; most were tobacco/nicotine. Twenty-six veterans (63.4%) had documentation of SUD in remission with 19 (46.3%) for substances other than tobacco/nicotine. There was no indication that any veteran in the sample was prescribed medication for opioid use disorder (OUD) at the time of observation. There was no relationship between opioid responsiveness and SUDs, neither active or in remission. Consults to other services that suggested distress or difficult-to-control symptoms also were frequent. Consults to the pain service were significantly associated with reduced responsiveness to opioids (β = −1.75; 95% CI, −3.33 to −0.17; P = .03). Association between psychiatry consultation and reduced opioid responsiveness trended toward significance (β = −0.95; 95% CI, −2.06 to 0.17; P = .09) (Figures 2 and 3). There was no significant association with palliative medicine consultation and opioid responsiveness.
A poorer response to opioids was associated with a significantly higher as-needed opioid dosage (β = −0.02; 95% CI, −0.04 to −0.01; P = .002) as well as a trend toward higher total opioid dosage (β = −0.005; 95% CI, −0.01 to 0.0003; P = .06) (Figure 4). Thirty-eight (92.7%) participants received nonopioid adjuvant analgesics for pain. More than half (56.1%) received antidepressants or gabapentinoids (51.2%), although we did not assess whether they were prescribed for pain or another indication. We did not identify a relationship between any specific psychoactive drug class and opioid responsiveness in this sample.
Discussion
This exploratory study used readily available administrative data in a CLC-PAC unit to assess responsiveness to opioids via a numeric mean ∆ score, with higher values indicating more pain relief in response to opioids. We then constructed linear regression models to characterize the relationship between the mean ∆ score and factors known to be associated with difficult-to-control pain and psychosocial distress. As expected, opioid responsiveness was highly variable among residents; some residents experienced essentially no reduction in pain, on average, despite receiving opioids. Psychiatric comorbidity, higher dosage in OMEs, and the presence of a pain service consult significantly correlated with poorer response to opioids. To our knowledge, this is the first study to quantify opioid responsiveness and describe the relationship with clinical correlates in the understudied PAC population.
Earlier research has demonstrated a relationship between the presence of psychiatric disorders and increased likelihood of receiving any analgesics among veterans residing in PAC.9 Our study adds to the literature by quantifying opioid response using readily available administrative data and examining associations with psychiatric diagnoses. These findings highlight the possibility that attempting to treat high levels of pain by escalating the opioid dosage in patients with a comorbid psychiatric diagnosis should be re-addressed, particularly if there is no meaningful pain reduction at lower opioid dosages. Our sample had a variety of admission diagnoses and medical comorbidities, however, we did not identify a relationship with opioid responsiveness, including an active cancer diagnosis. Although SUDs were highly prevalent in our sample, there was no relationship with opioid responsiveness. This suggests that lack of response to opioids is not merely a matter of drug tolerance or an indication of drug-seeking behavior.
Factors Impacting Response
Many factors could affect whether an individual obtains an adequate analgesic response to opioids or other pain medications, including variations in genes encoding opioid receptors and hepatic enzymes involved in drug metabolism and an individual’s opioid exposure history.13 The phenomenon of requiring more drug to produce the same relief after repeated exposures (ie, tolerance) is well known.14 Opioid-induced hyperalgesia is a phenomenon whereby a patient’s overall pain increases while receiving opioids, but each opioid dose might be perceived as beneficial.15 Increasingly, psychosocial distress is an important factor in opioid response. Adverse selection is the process culminating in those with psychosocial distress and/or SUDs being prescribed more opioids for longer durations.16 Our data suggests that this process could play a role in PAC settings. In addition, exaggerating pain to obtain additional opioids for nonmedical purposes, such as euphoria or relaxation, also is possible.17
When clinically assessing an individual whose pain is not well controlled despite escalating opioid dosages, prescribers must consider which of these factors likely is predominant. However, the first step of determining who has a poor opioid response is not straightforward. Directly asking patients is challenging; many individuals perceive opioids to be helpful while simultaneously reporting inadequately controlled pain.7,8 The primary value of this study is the possibility of providing prescribers a quick, simple method of assessing a patient’s response to opioids. Using this method, individuals who are responding poorly to opioids, including those who might exaggerate pain for secondary gain, could be identified. Health care professionals could consider revisiting pain management strategies, assess for the presence of OUD, or evaluate other contributors to inadequately controlled pain. Although we only collected data regarding response to opioids in this study, any pain medication administered as needed (ie, nonsteroidal anti-inflammatory drugs, acetaminophen) could be analyzed using this methodology, allowing identification of other helpful pain management strategies. We began the validation process with extensive chart review, but further validation is required before this method can be applied to routine clinical practice.
Patients who report uncontrolled pain despite receiving opioids are a clinically challenging population. The traditional strategy has been to escalate opioids, which is recommended by the World Health Organization stepladder approach for patients with cancer pain and limited life expectancy.18 Applying this approach to a general population of patients with chronic pain is ineffective and dangerous.19 The CDC and the VA/US Department of Defense (VA/DoD) guidelines both recommend carefully reassessing risks and benefits at total daily dosages > 50 OME and avoid increasing dosages to > 90 OME daily in most circumstances.5,20 Our finding that participants taking higher dosages of opioids were not more likely to have better control over their pain supports this recommendation.
Limitations
This study has several limitations, the most significant is its small sample size because of the exploratory nature of the project. Results are based on a small pilot sample enriched to include individuals with at least moderate pain who receive opioids frequently at 1 VA CLC-PAC unit; therefore, the results might not be representative of all veterans or a more general population. Our small sample size limits power to detect small differences. Data collected should be used to inform formal power calculations before subsequent larger studies to select adequate sample size. Validation studies, including samples from the same population using different dates, which reproduce findings are an important step. Moreover, we only had data on a single dimension of pain (intensity/severity), as measured by the pain scale, which nursing staff used to make a real-time clinical decision of whether to administer an as-needed opioid. Future studies should consider using pain measures that provide multidimensional assessment (ie, severity, functional interference) and/or were developed specifically for veterans, such as the Defense and Veterans Pain Rating Scale.21
Our study was cross-sectional in nature and addressed a single 24-hour period of data per participant. The years of data collection (2016 and 2017) followed a decline in overall opioid prescribing that has continued, likely influenced by CDC and VA/DoD guidelines.22 It is unclear whether our observations are an accurate reflection of individuals’ response over time or whether prescribing practices in PAC have shifted.
We did not consider the type of pain being treated or explore clinicians’ reasons for prescribing opioids, therefore limiting our ability to know whether opioids were indicated. Information regarding OUD and other SUDs was limited to what was documented in the chart during the CLC-PAC unit admission. We did not have information on length of exposure to opioids. It is possible that opioid tolerance could play a role in reducing opioid responsiveness. However, simple tolerance would not be expected to explain robust correlations with psychiatric comorbidities. Also, simple tolerance would be expected to be overcome with higher opioid dosages, whereas our study demonstrates less responsiveness. These data suggests that some individuals’ pain might be poorly opioid responsive, and psychiatric factors could increase this risk. We used a novel data source in combination with chart review; to our knowledge, barcode medication administration data have not been used in this manner previously. Future work needs to validate this method, using larger sample sizes and several clinical sites. Finally, we used regression models that controlled for average pre-opioid pain rating scores, which is only 1 covariate important for examining effects. Larger studies with adequate power should control for multiple covariates known to be associated with pain and opioid response.
Conclusions
Opioid responsiveness is important clinically yet challenging to assess. This pilot study identifies a way of classifying pain as relatively opioid nonresponsive using administrative data but requires further validation before considering scaling for more general use. The possibility that a substantial percentage of residents in a CLC-PAC unit could be receiving increasing dosages of opioids without adequate benefit justifies the need for more research and underscores the need for prescribers to assess individuals frequently for ongoing benefit of opioids regardless of diagnosis or mechanism of pain.
Acknowledgments
The authors thank Andrzej Galecki, Corey Powell, and the University of Michigan Consulting for Statistics, Computing and Analytics Research Center for assistance with statistical analysis.
Older adults admitted to post-acute settings frequently have complex rehabilitation needs and multimorbidity, which predisposes them to pain management challenges.1,2 The prevalence of pain in post-acute and long-term care is as high as 65%, and opioid use is common among this population with 1 in 7 residents receiving long-term opioids.3,4
Opioids that do not adequately control pain represent a missed opportunity for deprescribing. There is limited evidence regarding efficacy of long-term opioid use (> 90 days) for improving pain and physical functioning.5 In addition, long-term opioid use carries significant risks, including overdose-related death, dependence, and increased emergency department visits.5 These risks are likely to be pronounced among veterans receiving post-acute care (PAC) who are older, have comorbid psychiatric disorders, are prescribed several centrally acting medications, and experience substance use disorder (SUD).6
Older adults are at increased risk for opioid toxicity because of reduced drug clearance and smaller therapeutic window.5 Centers for Disease Control and Prevention (CDC) guidelines recommend frequently assessing patients for benefit in terms of sustained improvement in pain as well as physical function.5 If pain and functional improvements are minimal, opioid use and nonopioid pain management strategies should be considered. Some patients will struggle with this approach. Directly asking patients about the effectiveness of opioids is challenging. Opioid users with chronic pain frequently report problems with opioids even as they describe them as indispensable for pain management.7,8
Earlier studies have assessed patient perspectives regarding opioid difficulties as well as their helpfulness, which could introduce recall bias. Patient-level factors that contribute to a global sense of distress, in addition to the presence of painful physical conditions, also could contribute to patients requesting opioids without experiencing adequate pain relief. One study in veterans residing in PAC facilities found that individuals with depression, posttraumatic stress disorder (PTSD), and SUD were more likely to report pain and receive scheduled analgesics; this effect persisted in individuals with PTSD even after adjusting for demographic and functional status variables.9 The study looked only at analgesics as a class and did not examine opioids specifically. It is possible that distressed individuals, such as those with uncontrolled depression, PTSD, and SUD, might be more likely to report high pain levels and receive opioids with inadequate benefit and increased risk. Identifying the primary condition causing distress and targeting treatment to that condition (ie, depression) is preferable to escalating opioids in an attempt to treat pain in the context of nonresponse. Assessing an individual’s aggregate response to opioids rather than relying on a single self-report is a useful addition to current pain management strategies.
The goal of this study was to pilot a method of identifying opioid-nonresponsive pain using administrative data, measure its prevalence in a PAC population of veterans, and explore clinical and demographic correlates with particular attention to variates that could indicate high levels of psychological and physical distress. Identifying pain that is poorly responsive to opioids would give clinicians the opportunity to avoid or minimize opioid use and prioritize treatments that are likely to improve the resident’s pain, quality of life, and physical function while minimizing recall bias. We hypothesized that pain that responds poorly to opioids would be prevalent among veterans residing in a PAC unit. We considered that veterans with pain poorly responsive to opioids would be more likely to have factors that would place them at increased risk of adverse effects, such as comorbid psychiatric conditions, history of SUD, and multimorbidity, providing further rationale for clinical equipoise in that population.6
Methods
This was a small, retrospective cross-sectional study using administrative data and chart review. The study included veterans who were administered opioids while residing in a single US Department of Veterans Affairs (VA) community living center PAC (CLC-PAC) unit during at least 1 of 4 nonconsecutive, random days in 2016 and 2017. The study was approved by the institutional review board of the Ann Arbor VA Health System (#2017-1034) as part of a larger project involving models of care in vulnerable older veterans.
Inclusion criteria were the presence of at least moderate pain (≥ 4 on a 0 to 10 scale); receiving ≥ 2 opioids ordered as needed over the prespecified 24-hour observation period; and having ≥ 2 pre-and postopioid administration pain scores during the observation period. Veterans who did not meet these criteria were excluded. At the time of initial sample selection, we did not capture information related to coprescribed analgesics, including a standing order of opioids. To obtain the sample, we initially characterized all veterans on the 4 days residing in the CLC-PAC unit as those reporting at least moderate pain (≥ 4) and those who reported no or mild pain (< 4). The cut point of 4 of 10 is consistent with moderate pain based on earlier work showing higher likelihood of pain that interferes with physical function.10 We then restricted the sample to veterans who received ≥ 2 opioids ordered as needed for pain and had ≥ 2 pre- and postopioid administration numeric pain rating scores during the 24-hour observation period. This methodology was chosen to enrich our sample for those who received opioids regularly for ongoing pain. Opioids were defined as full µ-opioid receptor agonists and included hydrocodone, oxycodone, morphine, hydromorphone, fentanyl, tramadol, and methadone.
Medication administration data were obtained from the VA corporate data warehouse, which houses all barcode medication administration data collected at the point of care. The dataset includes pain scores gathered by nursing staff before and after administering an as-needed analgesic. The corporate data warehouse records data/time of pain scores and the analgesic name, dosage, formulation, and date/time of administration. Using a standardized assessment form developed iteratively, we calculated opioid dosage in oral morphine equivalents (OME) for comparison.11,12 All abstracted data were reexamined for accuracy. Data initially were collected in an anonymized, blinded fashion. Participants were then unblinded for chart review. Initial data was captured in resident-days instead of unique residents because an individual resident might have been admitted on several observation days. We were primarily interested in how pain responded to opioids administered in response to resident request; therefore, we did not examine response to opioids that were continuously ordered (ie, scheduled). We did consider scheduled opioids when calculating total daily opioid dosage during the chart review.
Outcome of Interest
The primary outcome of interest was an individual’s response to as-needed opioids, which we defined as change in the pain score after opioid administration. The pre-opioid pain score was the score that immediately preceded administration of an as-needed opioid. The postopioid administration pain score was the first score after opioid administration if obtained within 3 hours of administration. Scores collected > 3 hours after opioid administration were excluded because they no longer accurately reflected the impact of the opioid due to the short half-lives. Observations were excluded if an opioid was administered without a recorded pain score; this occurred once for 6 individuals. Observations also were excluded if an opioid was administered but the data were captured on the following day (outside of the 24-hour window); this occurred once for 3 individuals.
We calculated a ∆ score by subtracting the postopioid pain rating score from the pre-opioid score. Individual ∆ scores were then averaged over the 24-hour period (range, 2-5 opioid doses). For example, if an individual reported a pre-opioid pain score of 10, and a postopioid pain score of 2, the ∆ was recorded as 8. If the individual’s next pre-opioid score was 10, and post-opioid score was 6, the ∆ was recorded as 4. ∆ scores over the 24-hour period were averaged together to determine that individual’s response to as-needed opioids. In the previous example, the mean ∆ score is 6. Lower mean ∆ scores reflect decreased responsiveness to opioids’ analgesic effect.
Demographic and clinical data were obtained from electronic health record review using a standardized assessment form. These data included information about medical and psychiatric comorbidities, specialist consultations, and CLC-PAC unit admission indications and diagnoses. Medications of interest were categorized as antidepressants, antipsychotics, benzodiazepines, muscle relaxants, hypnotics, stimulants, antiepileptic drugs/mood stabilizers (including gabapentin and pregabalin), and all adjuvant analgesics. Adjuvant analgesics were defined as medications administered for pain as documented by chart notes or those ordered as needed for pain, and analyzed as a composite variable. Antidepressants with analgesic properties (serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants) were considered adjuvant analgesics. Psychiatric information collected included presence of mood, anxiety, and psychotic disorders, and PTSD. SUD information was collected separately from other psychiatric disorders.
Analyses
The study population was described using tabulations for categorical data and means and standard deviations for continuous data. Responsiveness to opioids was analyzed as a continuous variable. Those with higher mean ∆ scores were considered to have pain relatively more responsive to opioids, while lower mean ∆ scores indicated pain less responsive to opioids. We constructed linear regression models controlling for average pre-opioid pain rating scores to explore associations between opioid responsiveness and variables of interest. All analyses were completed using Stata version 15. This study was not adequately powered to detect differences across the spectrum of opioid responsiveness, although the authors have reported differences in this article.
Results
Over the 4-day observational period there were 146 resident-days. Of these, 88 (60.3%) reported at least 1 pain score of ≥ 4. Of those, 61 (41.8%) received ≥ 1 as-needed opioid for pain. We identified 46 resident-days meeting study criteria of ≥ 2 pre- and postanalgesic scores. We identified 41 unique individuals (Figure 1). Two individuals were admitted to the CLC-PAC unit on 2 of the 4 observation days, and 1 individual was admitted to the CLC-PAC unit on 3 of the 4 observation days. For individuals admitted several days, we included data only from the initial observation day.
Response to opioids varied greatly in this sample. The mean (SD) ∆ pain score was 3.4 (1.6) and ranged from 0.5 to 6.3. Using linear regression, we found no relationship between admission indication, medical comorbidities (including active cancer), and opioid responsiveness (Table).
Psychiatric disorders were highly prevalent, with 25 individuals (61.0%) having ≥ 1 any psychiatric diagnosis identified on chart review. The presence of any psychiatric diagnosis was significantly associated with reduced responsiveness to opioids (β = −1.08; 95% CI, −2.04 to −0.13; P = .03). SUDs also were common, with 17 individuals (41.5%) having an active SUD; most were tobacco/nicotine. Twenty-six veterans (63.4%) had documentation of SUD in remission with 19 (46.3%) for substances other than tobacco/nicotine. There was no indication that any veteran in the sample was prescribed medication for opioid use disorder (OUD) at the time of observation. There was no relationship between opioid responsiveness and SUDs, neither active or in remission. Consults to other services that suggested distress or difficult-to-control symptoms also were frequent. Consults to the pain service were significantly associated with reduced responsiveness to opioids (β = −1.75; 95% CI, −3.33 to −0.17; P = .03). Association between psychiatry consultation and reduced opioid responsiveness trended toward significance (β = −0.95; 95% CI, −2.06 to 0.17; P = .09) (Figures 2 and 3). There was no significant association with palliative medicine consultation and opioid responsiveness.
A poorer response to opioids was associated with a significantly higher as-needed opioid dosage (β = −0.02; 95% CI, −0.04 to −0.01; P = .002) as well as a trend toward higher total opioid dosage (β = −0.005; 95% CI, −0.01 to 0.0003; P = .06) (Figure 4). Thirty-eight (92.7%) participants received nonopioid adjuvant analgesics for pain. More than half (56.1%) received antidepressants or gabapentinoids (51.2%), although we did not assess whether they were prescribed for pain or another indication. We did not identify a relationship between any specific psychoactive drug class and opioid responsiveness in this sample.
Discussion
This exploratory study used readily available administrative data in a CLC-PAC unit to assess responsiveness to opioids via a numeric mean ∆ score, with higher values indicating more pain relief in response to opioids. We then constructed linear regression models to characterize the relationship between the mean ∆ score and factors known to be associated with difficult-to-control pain and psychosocial distress. As expected, opioid responsiveness was highly variable among residents; some residents experienced essentially no reduction in pain, on average, despite receiving opioids. Psychiatric comorbidity, higher dosage in OMEs, and the presence of a pain service consult significantly correlated with poorer response to opioids. To our knowledge, this is the first study to quantify opioid responsiveness and describe the relationship with clinical correlates in the understudied PAC population.
Earlier research has demonstrated a relationship between the presence of psychiatric disorders and increased likelihood of receiving any analgesics among veterans residing in PAC.9 Our study adds to the literature by quantifying opioid response using readily available administrative data and examining associations with psychiatric diagnoses. These findings highlight the possibility that attempting to treat high levels of pain by escalating the opioid dosage in patients with a comorbid psychiatric diagnosis should be re-addressed, particularly if there is no meaningful pain reduction at lower opioid dosages. Our sample had a variety of admission diagnoses and medical comorbidities, however, we did not identify a relationship with opioid responsiveness, including an active cancer diagnosis. Although SUDs were highly prevalent in our sample, there was no relationship with opioid responsiveness. This suggests that lack of response to opioids is not merely a matter of drug tolerance or an indication of drug-seeking behavior.
Factors Impacting Response
Many factors could affect whether an individual obtains an adequate analgesic response to opioids or other pain medications, including variations in genes encoding opioid receptors and hepatic enzymes involved in drug metabolism and an individual’s opioid exposure history.13 The phenomenon of requiring more drug to produce the same relief after repeated exposures (ie, tolerance) is well known.14 Opioid-induced hyperalgesia is a phenomenon whereby a patient’s overall pain increases while receiving opioids, but each opioid dose might be perceived as beneficial.15 Increasingly, psychosocial distress is an important factor in opioid response. Adverse selection is the process culminating in those with psychosocial distress and/or SUDs being prescribed more opioids for longer durations.16 Our data suggests that this process could play a role in PAC settings. In addition, exaggerating pain to obtain additional opioids for nonmedical purposes, such as euphoria or relaxation, also is possible.17
When clinically assessing an individual whose pain is not well controlled despite escalating opioid dosages, prescribers must consider which of these factors likely is predominant. However, the first step of determining who has a poor opioid response is not straightforward. Directly asking patients is challenging; many individuals perceive opioids to be helpful while simultaneously reporting inadequately controlled pain.7,8 The primary value of this study is the possibility of providing prescribers a quick, simple method of assessing a patient’s response to opioids. Using this method, individuals who are responding poorly to opioids, including those who might exaggerate pain for secondary gain, could be identified. Health care professionals could consider revisiting pain management strategies, assess for the presence of OUD, or evaluate other contributors to inadequately controlled pain. Although we only collected data regarding response to opioids in this study, any pain medication administered as needed (ie, nonsteroidal anti-inflammatory drugs, acetaminophen) could be analyzed using this methodology, allowing identification of other helpful pain management strategies. We began the validation process with extensive chart review, but further validation is required before this method can be applied to routine clinical practice.
Patients who report uncontrolled pain despite receiving opioids are a clinically challenging population. The traditional strategy has been to escalate opioids, which is recommended by the World Health Organization stepladder approach for patients with cancer pain and limited life expectancy.18 Applying this approach to a general population of patients with chronic pain is ineffective and dangerous.19 The CDC and the VA/US Department of Defense (VA/DoD) guidelines both recommend carefully reassessing risks and benefits at total daily dosages > 50 OME and avoid increasing dosages to > 90 OME daily in most circumstances.5,20 Our finding that participants taking higher dosages of opioids were not more likely to have better control over their pain supports this recommendation.
Limitations
This study has several limitations, the most significant is its small sample size because of the exploratory nature of the project. Results are based on a small pilot sample enriched to include individuals with at least moderate pain who receive opioids frequently at 1 VA CLC-PAC unit; therefore, the results might not be representative of all veterans or a more general population. Our small sample size limits power to detect small differences. Data collected should be used to inform formal power calculations before subsequent larger studies to select adequate sample size. Validation studies, including samples from the same population using different dates, which reproduce findings are an important step. Moreover, we only had data on a single dimension of pain (intensity/severity), as measured by the pain scale, which nursing staff used to make a real-time clinical decision of whether to administer an as-needed opioid. Future studies should consider using pain measures that provide multidimensional assessment (ie, severity, functional interference) and/or were developed specifically for veterans, such as the Defense and Veterans Pain Rating Scale.21
Our study was cross-sectional in nature and addressed a single 24-hour period of data per participant. The years of data collection (2016 and 2017) followed a decline in overall opioid prescribing that has continued, likely influenced by CDC and VA/DoD guidelines.22 It is unclear whether our observations are an accurate reflection of individuals’ response over time or whether prescribing practices in PAC have shifted.
We did not consider the type of pain being treated or explore clinicians’ reasons for prescribing opioids, therefore limiting our ability to know whether opioids were indicated. Information regarding OUD and other SUDs was limited to what was documented in the chart during the CLC-PAC unit admission. We did not have information on length of exposure to opioids. It is possible that opioid tolerance could play a role in reducing opioid responsiveness. However, simple tolerance would not be expected to explain robust correlations with psychiatric comorbidities. Also, simple tolerance would be expected to be overcome with higher opioid dosages, whereas our study demonstrates less responsiveness. These data suggests that some individuals’ pain might be poorly opioid responsive, and psychiatric factors could increase this risk. We used a novel data source in combination with chart review; to our knowledge, barcode medication administration data have not been used in this manner previously. Future work needs to validate this method, using larger sample sizes and several clinical sites. Finally, we used regression models that controlled for average pre-opioid pain rating scores, which is only 1 covariate important for examining effects. Larger studies with adequate power should control for multiple covariates known to be associated with pain and opioid response.
Conclusions
Opioid responsiveness is important clinically yet challenging to assess. This pilot study identifies a way of classifying pain as relatively opioid nonresponsive using administrative data but requires further validation before considering scaling for more general use. The possibility that a substantial percentage of residents in a CLC-PAC unit could be receiving increasing dosages of opioids without adequate benefit justifies the need for more research and underscores the need for prescribers to assess individuals frequently for ongoing benefit of opioids regardless of diagnosis or mechanism of pain.
Acknowledgments
The authors thank Andrzej Galecki, Corey Powell, and the University of Michigan Consulting for Statistics, Computing and Analytics Research Center for assistance with statistical analysis.
1. Marshall TL, Reinhardt JP. Pain management in the last 6 months of life: predictors of opioid and non-opioid use. J Am Med Dir Assoc. 2019;20(6):789-790. doi:10.1016/j.jamda.2019.02.026
2. Tait RC, Chibnall JT. Pain in older subacute care patients: associations with clinical status and treatment. Pain Med. 2002;3(3):231-239. doi:10.1046/j.1526-4637.2002.02031.x
3. Pimentel CB, Briesacher BA, Gurwitz JH, Rosen AB, Pimentel MT, Lapane KL. Pain management in nursing home residents with cancer. J Am Geriatr Soc. 2015;63(4):633-641. doi:10.1111/jgs.13345
4. Hunnicutt JN, Tjia J, Lapane KL. Hospice use and pain management in elderly nursing home residents with cancer. J Pain Symptom Manage. 2017;53(3):561-570. doi:10.1016/j.jpainsymman.2016.10.369
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65(No. RR-1):1-49. doi:10.15585/mmwr.rr6501e1
6. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
7. Goesling J, Moser SE, Lin LA, Hassett AL, Wasserman RA, Brummett CM. Discrepancies between perceived benefit of opioids and self-reported patient outcomes. Pain Med. 2018;19(2):297-306. doi:10.1093/pm/pnw263
8. Sullivan M, Von Korff M, Banta-Green C. Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain. Pain. 2010;149(2):345-353. doi:10.1016/j.pain.2010.02.037
9. Brennan PL, Greenbaum MA, Lemke S, Schutte KK. Mental health disorder, pain, and pain treatment among long-term care residents: evidence from the Minimum Data Set 3.0. Aging Ment Health. 2019;23(9):1146-1155. doi:10.1080/13607863.2018.1481922
10. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176-183. doi:10.3978/j.issn.2224-5820.2015.09.04
11. Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. 2017. Accessed December 15, 2021. https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf
12. Nielsen S, Degenhardt L, Hoban B, Gisev N. Comparing opioids: a guide to estimating oral morphine equivalents (OME) in research. NDARC Technical Report No. 329. National Drug and Alcohol Research Centre; 2014. Accessed December 15, 2021. http://www.drugsandalcohol.ie/22703/1/NDARC Comparing opioids.pdf
13. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11(2):237-248.
14. Collin E, Cesselin F. Neurobiological mechanisms of opioid tolerance and dependence. Clin Neuropharmacol. 1991;14(6):465-488. doi:10.1097/00002826-199112000-00001
15. Higgins C, Smith BH, Matthews K. Evidence of opioid-induced hyperalgesia in clinical populations after chronic opioid exposure: a systematic review and meta-analysis. Br J Anaesth. 2019;122(6):e114-e126. doi:10.1016/j.bja.2018.09.019
16. Howe CQ, Sullivan MD. The missing ‘P’ in pain management: how the current opioid epidemic highlights the need for psychiatric services in chronic pain care. Gen Hosp Psychiatry. 2014;36(1):99-104. doi:10.1016/j.genhosppsych.2013.10.003
17. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publ No PEP19-5068, NSDUH Ser H-54. 2019;170:51-58. Accessed December 15, 2021. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf
18. World Health Organization. WHO’s cancer pain ladder for adults. Accessed September 21, 2018. www.who.int/ncds/management/palliative-care/Infographic-cancer-pain-lowres.pdf
19. Ballantyne JC, Kalso E, Stannard C. WHO analgesic ladder: a good concept gone astray. BMJ. 2016;352:i20. doi:10.1136/bmj.i20
20. The Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. US Dept of Veterans Affairs and Dept of Defense; 2017. Accessed December 15, 2021. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf
21. Defense & Veterans Pain Rating Scale (DVPRS). Defense & Veterans Center for Integrative Pain Management. Accessed July 21, 2021. https://www.dvcipm.org/clinical-resources/defense-veterans-pain-rating-scale-dvprs/
22. Guy GP Jr, Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. doi:10.15585/mmwr.mm6626a4
1. Marshall TL, Reinhardt JP. Pain management in the last 6 months of life: predictors of opioid and non-opioid use. J Am Med Dir Assoc. 2019;20(6):789-790. doi:10.1016/j.jamda.2019.02.026
2. Tait RC, Chibnall JT. Pain in older subacute care patients: associations with clinical status and treatment. Pain Med. 2002;3(3):231-239. doi:10.1046/j.1526-4637.2002.02031.x
3. Pimentel CB, Briesacher BA, Gurwitz JH, Rosen AB, Pimentel MT, Lapane KL. Pain management in nursing home residents with cancer. J Am Geriatr Soc. 2015;63(4):633-641. doi:10.1111/jgs.13345
4. Hunnicutt JN, Tjia J, Lapane KL. Hospice use and pain management in elderly nursing home residents with cancer. J Pain Symptom Manage. 2017;53(3):561-570. doi:10.1016/j.jpainsymman.2016.10.369
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65(No. RR-1):1-49. doi:10.15585/mmwr.rr6501e1
6. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
7. Goesling J, Moser SE, Lin LA, Hassett AL, Wasserman RA, Brummett CM. Discrepancies between perceived benefit of opioids and self-reported patient outcomes. Pain Med. 2018;19(2):297-306. doi:10.1093/pm/pnw263
8. Sullivan M, Von Korff M, Banta-Green C. Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain. Pain. 2010;149(2):345-353. doi:10.1016/j.pain.2010.02.037
9. Brennan PL, Greenbaum MA, Lemke S, Schutte KK. Mental health disorder, pain, and pain treatment among long-term care residents: evidence from the Minimum Data Set 3.0. Aging Ment Health. 2019;23(9):1146-1155. doi:10.1080/13607863.2018.1481922
10. Woo A, Lechner B, Fu T, et al. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med. 2015;4(4):176-183. doi:10.3978/j.issn.2224-5820.2015.09.04
11. Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. 2017. Accessed December 15, 2021. https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf
12. Nielsen S, Degenhardt L, Hoban B, Gisev N. Comparing opioids: a guide to estimating oral morphine equivalents (OME) in research. NDARC Technical Report No. 329. National Drug and Alcohol Research Centre; 2014. Accessed December 15, 2021. http://www.drugsandalcohol.ie/22703/1/NDARC Comparing opioids.pdf
13. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11(2):237-248.
14. Collin E, Cesselin F. Neurobiological mechanisms of opioid tolerance and dependence. Clin Neuropharmacol. 1991;14(6):465-488. doi:10.1097/00002826-199112000-00001
15. Higgins C, Smith BH, Matthews K. Evidence of opioid-induced hyperalgesia in clinical populations after chronic opioid exposure: a systematic review and meta-analysis. Br J Anaesth. 2019;122(6):e114-e126. doi:10.1016/j.bja.2018.09.019
16. Howe CQ, Sullivan MD. The missing ‘P’ in pain management: how the current opioid epidemic highlights the need for psychiatric services in chronic pain care. Gen Hosp Psychiatry. 2014;36(1):99-104. doi:10.1016/j.genhosppsych.2013.10.003
17. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publ No PEP19-5068, NSDUH Ser H-54. 2019;170:51-58. Accessed December 15, 2021. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf
18. World Health Organization. WHO’s cancer pain ladder for adults. Accessed September 21, 2018. www.who.int/ncds/management/palliative-care/Infographic-cancer-pain-lowres.pdf
19. Ballantyne JC, Kalso E, Stannard C. WHO analgesic ladder: a good concept gone astray. BMJ. 2016;352:i20. doi:10.1136/bmj.i20
20. The Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. US Dept of Veterans Affairs and Dept of Defense; 2017. Accessed December 15, 2021. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf
21. Defense & Veterans Pain Rating Scale (DVPRS). Defense & Veterans Center for Integrative Pain Management. Accessed July 21, 2021. https://www.dvcipm.org/clinical-resources/defense-veterans-pain-rating-scale-dvprs/
22. Guy GP Jr, Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. doi:10.15585/mmwr.mm6626a4
Relax. The dog-tor will see you now
Patients in the emergency room who spent just 10 minutes with a trained therapy dog reported less pain, anxiety, and depression and improved well-being, researchers from the University of Saskatchewan in Canada found.
“The ER is an important community resource but also a scary place for most people,” James Stempien, MD, provincial department head of emergency medicine with the Saskatchewan Health Authority, who worked on the study, said in an interview.
“People tend to visit the ER on the worst day of their life, either for them or a loved one. Interacting with a therapy dog can make the ER visit a little calmer. We have also seen benefit for the staff that get to interact with the dogs as well,” he says.
“Thanks to our wonderful therapy dog volunteer teams, the cost is minimal and the result is priceless,” Dr. Stempien says.
The study, published in the journal PLOS One, builds on earlier “uncontrolled” studies by the Saskatchewan team.
Those studies showed that most ER patients wanted to visit with the therapy dog, if given a chance. After the encounter, patients reported feeling more comfortable, happier, and less distressed while waiting in the ER.
“A controlled trial was the natural next step,” says study investigator Colleen Dell, PhD, of One Health and Wellness at the University of Saskatchewan.
The study was done at the Royal University Hospital (RUH) in Saskatoon, Saskatchewan -- the first emergency department in Canada to introduce therapy dogs to improve the experience of waiting patients.
Nearly 200 adults visiting the ER received either a 10-minute visit with a therapy dog and its handler in addition to usual care or just usual care.
“This did not occur in patients in the ER who did not visit with a therapy dog.
“This gives us confidence in the intervention,” Dr. Dell says.
Pain is a major reason that patients come to the ER, and interactions with a therapy dog may distract from that pain, the researchers believe.
The study results lend more evidence to research that shows animals can help in medical settings, says Kara Rauscher, a licensed social worker and interim director of behavioral health for Nashville CARES in Tennessee, who wasn’t involved in the study.
“There are clearly opportunities to replicate this study in other emergency departments to strengthen our understanding of the potential benefits of these programs,” she says.
Part of her work at Nashville CARES, an AIDS service organization, has been supporting care that moves away from questions like, “What’s wrong with you?” to patient-focused questions like, “What happened to you?” It is a practice known as trauma-informed care.
“This includes bringing in therapy dogs for staff to spend time with during the workday; anecdotally, our staff reported a reduction in stress and improvements in mood,” Ms. Rauscher says.
A version of this article first appeared on WebMD.com.
Patients in the emergency room who spent just 10 minutes with a trained therapy dog reported less pain, anxiety, and depression and improved well-being, researchers from the University of Saskatchewan in Canada found.
“The ER is an important community resource but also a scary place for most people,” James Stempien, MD, provincial department head of emergency medicine with the Saskatchewan Health Authority, who worked on the study, said in an interview.
“People tend to visit the ER on the worst day of their life, either for them or a loved one. Interacting with a therapy dog can make the ER visit a little calmer. We have also seen benefit for the staff that get to interact with the dogs as well,” he says.
“Thanks to our wonderful therapy dog volunteer teams, the cost is minimal and the result is priceless,” Dr. Stempien says.
The study, published in the journal PLOS One, builds on earlier “uncontrolled” studies by the Saskatchewan team.
Those studies showed that most ER patients wanted to visit with the therapy dog, if given a chance. After the encounter, patients reported feeling more comfortable, happier, and less distressed while waiting in the ER.
“A controlled trial was the natural next step,” says study investigator Colleen Dell, PhD, of One Health and Wellness at the University of Saskatchewan.
The study was done at the Royal University Hospital (RUH) in Saskatoon, Saskatchewan -- the first emergency department in Canada to introduce therapy dogs to improve the experience of waiting patients.
Nearly 200 adults visiting the ER received either a 10-minute visit with a therapy dog and its handler in addition to usual care or just usual care.
“This did not occur in patients in the ER who did not visit with a therapy dog.
“This gives us confidence in the intervention,” Dr. Dell says.
Pain is a major reason that patients come to the ER, and interactions with a therapy dog may distract from that pain, the researchers believe.
The study results lend more evidence to research that shows animals can help in medical settings, says Kara Rauscher, a licensed social worker and interim director of behavioral health for Nashville CARES in Tennessee, who wasn’t involved in the study.
“There are clearly opportunities to replicate this study in other emergency departments to strengthen our understanding of the potential benefits of these programs,” she says.
Part of her work at Nashville CARES, an AIDS service organization, has been supporting care that moves away from questions like, “What’s wrong with you?” to patient-focused questions like, “What happened to you?” It is a practice known as trauma-informed care.
“This includes bringing in therapy dogs for staff to spend time with during the workday; anecdotally, our staff reported a reduction in stress and improvements in mood,” Ms. Rauscher says.
A version of this article first appeared on WebMD.com.
Patients in the emergency room who spent just 10 minutes with a trained therapy dog reported less pain, anxiety, and depression and improved well-being, researchers from the University of Saskatchewan in Canada found.
“The ER is an important community resource but also a scary place for most people,” James Stempien, MD, provincial department head of emergency medicine with the Saskatchewan Health Authority, who worked on the study, said in an interview.
“People tend to visit the ER on the worst day of their life, either for them or a loved one. Interacting with a therapy dog can make the ER visit a little calmer. We have also seen benefit for the staff that get to interact with the dogs as well,” he says.
“Thanks to our wonderful therapy dog volunteer teams, the cost is minimal and the result is priceless,” Dr. Stempien says.
The study, published in the journal PLOS One, builds on earlier “uncontrolled” studies by the Saskatchewan team.
Those studies showed that most ER patients wanted to visit with the therapy dog, if given a chance. After the encounter, patients reported feeling more comfortable, happier, and less distressed while waiting in the ER.
“A controlled trial was the natural next step,” says study investigator Colleen Dell, PhD, of One Health and Wellness at the University of Saskatchewan.
The study was done at the Royal University Hospital (RUH) in Saskatoon, Saskatchewan -- the first emergency department in Canada to introduce therapy dogs to improve the experience of waiting patients.
Nearly 200 adults visiting the ER received either a 10-minute visit with a therapy dog and its handler in addition to usual care or just usual care.
“This did not occur in patients in the ER who did not visit with a therapy dog.
“This gives us confidence in the intervention,” Dr. Dell says.
Pain is a major reason that patients come to the ER, and interactions with a therapy dog may distract from that pain, the researchers believe.
The study results lend more evidence to research that shows animals can help in medical settings, says Kara Rauscher, a licensed social worker and interim director of behavioral health for Nashville CARES in Tennessee, who wasn’t involved in the study.
“There are clearly opportunities to replicate this study in other emergency departments to strengthen our understanding of the potential benefits of these programs,” she says.
Part of her work at Nashville CARES, an AIDS service organization, has been supporting care that moves away from questions like, “What’s wrong with you?” to patient-focused questions like, “What happened to you?” It is a practice known as trauma-informed care.
“This includes bringing in therapy dogs for staff to spend time with during the workday; anecdotally, our staff reported a reduction in stress and improvements in mood,” Ms. Rauscher says.
A version of this article first appeared on WebMD.com.
FROM PLOS ONE
Twelve physicians sentenced in illegal opioid, billing fraud scheme
according to the U.S. Department of Justice (DOJ). The defendants’s activities also resulted in more than $250 million in false billings.
“It is unconscionable that doctors and health care professionals would violate their oath to do no harm and exploit vulnerable patients struggling with addiction,” said Assistant Attorney General Kenneth Polite Jr., of the Justice Department’s Criminal Division, in the announcement. “These are not just crimes of greed, these are crimes that make this country’s opioid crisis even worse – and that is why the department will continue to relentlessly pursue these cases.”
Francisco Patino, MD, 66, a Wayne County, Michigan-based emergency medicine physician and part owner of one of the clinics involved, purchased cars, jewelry, and vacations as a result of the fraudulent activity, according to federal officials. He will face sentencing at a later date on a variety of counts related to defrauding the United States, health care fraud, money laundering, and wire fraud after his conviction in a 2021 trial.
Prosecutors also allege that he laundered money through a diet program and spent funds on sponsoring mixed martial arts fighters, the Detroit News reported.
Mashiyat Rashid, Dr. Patino’s business partner and part owner of the Tri-County Wellness Group, was sentenced to 15 years in prison and ordered to pay more than $51 million in restitution in connection with his guilty plea to one count of conspiracy to commit health care fraud and wire fraud, in addition to one count of money laundering. As a result of the scheme, Mr. Rashid bought courtside tickets to the NBA Finals, expensive real estate, and private jet flights, according to the DOJ.
Gold bars, indoor basketball courts, luxury cars, and swimming pools were purchases secured by other defendants who were involved in the fraudulent scheme.
Court documents and evidence show that the physicians required that patients receive unnecessary and costly back injections in return for opioids, per the agency charged with enforcing federal law. The scheme, which took place from 2007 to 2018, involved a network of pain clinics across multiple states. Referred to as “pill mills” by the DOJ, the pain clinics dispensed the high-dosage prescriptions, such as oxycodone, to drug dealers and patients with opioid use disorder.
The procedures, billed to insurance, were for facet joint injections. According to the DOJ, the injections were chosen because they generated high reimbursements rather than being medically necessary.
The Detroit News reported in September that some of the medically unnecessary drugs prescribed by Dr. Patino, which included fentanyl, oxycodone, and oxymorphone, per an indictment, were resold “on the street.” Dr. Patino wrote prescriptions for more than 2.2 million pills between 2016 and 2017.
Dr. Patino and Mr. Rashid join physicians and others in the health care field, who are charged or sentenced for their involvement in the scheme. In total, five physicians were convicted in two separate trials and 18 defendants pleaded guilty, per the DOJ. Meanwhile, seven defendants await sentencing.
Included in this group were:
- Spilios Pappas, MD, 63, an emergency medicine specialist in Lucas County, Ohio, sentenced to nine years in prison and ordered to pay $32.2 million in restitution
- Joseph Betro, DO, 60, an emergency medicine physician from Oakland County, Mich., sentenced to nine years in prison and ordered to pay $27.4 million in restitution
- Tariq Omar, MD, 63, a pulmonologist from Oakland County, Mich., sentenced to eight years in prison and ordered to pay $24.2 million in restitution
- Mohammed Zahoor, MD, 53, a neurologist from Oakland County, Mich., sentenced to eight years in prison and ordered to pay $36.6 million in restitution
The four physicians worked at various clinics under the Tri-County Wellness Group, operated by Mr. Rashid, according to federal officials. During their employment with the clinics, they defrauded Medicare of more than $150 million through the scheme that involved opioids for medically unnecessary services, the DOJ noted.
Shortly after being indicted, Dr. Pappas posted a fundraising page for his legal services, claiming he and the other doctors had no idea what was going on and that he was “sickened and nauseous” when learning of the details of the case.
More than $16 million was forfeited by the defendants to the United States, according to the DOJ.
A version of this article first appeared on Medscape.com.
according to the U.S. Department of Justice (DOJ). The defendants’s activities also resulted in more than $250 million in false billings.
“It is unconscionable that doctors and health care professionals would violate their oath to do no harm and exploit vulnerable patients struggling with addiction,” said Assistant Attorney General Kenneth Polite Jr., of the Justice Department’s Criminal Division, in the announcement. “These are not just crimes of greed, these are crimes that make this country’s opioid crisis even worse – and that is why the department will continue to relentlessly pursue these cases.”
Francisco Patino, MD, 66, a Wayne County, Michigan-based emergency medicine physician and part owner of one of the clinics involved, purchased cars, jewelry, and vacations as a result of the fraudulent activity, according to federal officials. He will face sentencing at a later date on a variety of counts related to defrauding the United States, health care fraud, money laundering, and wire fraud after his conviction in a 2021 trial.
Prosecutors also allege that he laundered money through a diet program and spent funds on sponsoring mixed martial arts fighters, the Detroit News reported.
Mashiyat Rashid, Dr. Patino’s business partner and part owner of the Tri-County Wellness Group, was sentenced to 15 years in prison and ordered to pay more than $51 million in restitution in connection with his guilty plea to one count of conspiracy to commit health care fraud and wire fraud, in addition to one count of money laundering. As a result of the scheme, Mr. Rashid bought courtside tickets to the NBA Finals, expensive real estate, and private jet flights, according to the DOJ.
Gold bars, indoor basketball courts, luxury cars, and swimming pools were purchases secured by other defendants who were involved in the fraudulent scheme.
Court documents and evidence show that the physicians required that patients receive unnecessary and costly back injections in return for opioids, per the agency charged with enforcing federal law. The scheme, which took place from 2007 to 2018, involved a network of pain clinics across multiple states. Referred to as “pill mills” by the DOJ, the pain clinics dispensed the high-dosage prescriptions, such as oxycodone, to drug dealers and patients with opioid use disorder.
The procedures, billed to insurance, were for facet joint injections. According to the DOJ, the injections were chosen because they generated high reimbursements rather than being medically necessary.
The Detroit News reported in September that some of the medically unnecessary drugs prescribed by Dr. Patino, which included fentanyl, oxycodone, and oxymorphone, per an indictment, were resold “on the street.” Dr. Patino wrote prescriptions for more than 2.2 million pills between 2016 and 2017.
Dr. Patino and Mr. Rashid join physicians and others in the health care field, who are charged or sentenced for their involvement in the scheme. In total, five physicians were convicted in two separate trials and 18 defendants pleaded guilty, per the DOJ. Meanwhile, seven defendants await sentencing.
Included in this group were:
- Spilios Pappas, MD, 63, an emergency medicine specialist in Lucas County, Ohio, sentenced to nine years in prison and ordered to pay $32.2 million in restitution
- Joseph Betro, DO, 60, an emergency medicine physician from Oakland County, Mich., sentenced to nine years in prison and ordered to pay $27.4 million in restitution
- Tariq Omar, MD, 63, a pulmonologist from Oakland County, Mich., sentenced to eight years in prison and ordered to pay $24.2 million in restitution
- Mohammed Zahoor, MD, 53, a neurologist from Oakland County, Mich., sentenced to eight years in prison and ordered to pay $36.6 million in restitution
The four physicians worked at various clinics under the Tri-County Wellness Group, operated by Mr. Rashid, according to federal officials. During their employment with the clinics, they defrauded Medicare of more than $150 million through the scheme that involved opioids for medically unnecessary services, the DOJ noted.
Shortly after being indicted, Dr. Pappas posted a fundraising page for his legal services, claiming he and the other doctors had no idea what was going on and that he was “sickened and nauseous” when learning of the details of the case.
More than $16 million was forfeited by the defendants to the United States, according to the DOJ.
A version of this article first appeared on Medscape.com.
according to the U.S. Department of Justice (DOJ). The defendants’s activities also resulted in more than $250 million in false billings.
“It is unconscionable that doctors and health care professionals would violate their oath to do no harm and exploit vulnerable patients struggling with addiction,” said Assistant Attorney General Kenneth Polite Jr., of the Justice Department’s Criminal Division, in the announcement. “These are not just crimes of greed, these are crimes that make this country’s opioid crisis even worse – and that is why the department will continue to relentlessly pursue these cases.”
Francisco Patino, MD, 66, a Wayne County, Michigan-based emergency medicine physician and part owner of one of the clinics involved, purchased cars, jewelry, and vacations as a result of the fraudulent activity, according to federal officials. He will face sentencing at a later date on a variety of counts related to defrauding the United States, health care fraud, money laundering, and wire fraud after his conviction in a 2021 trial.
Prosecutors also allege that he laundered money through a diet program and spent funds on sponsoring mixed martial arts fighters, the Detroit News reported.
Mashiyat Rashid, Dr. Patino’s business partner and part owner of the Tri-County Wellness Group, was sentenced to 15 years in prison and ordered to pay more than $51 million in restitution in connection with his guilty plea to one count of conspiracy to commit health care fraud and wire fraud, in addition to one count of money laundering. As a result of the scheme, Mr. Rashid bought courtside tickets to the NBA Finals, expensive real estate, and private jet flights, according to the DOJ.
Gold bars, indoor basketball courts, luxury cars, and swimming pools were purchases secured by other defendants who were involved in the fraudulent scheme.
Court documents and evidence show that the physicians required that patients receive unnecessary and costly back injections in return for opioids, per the agency charged with enforcing federal law. The scheme, which took place from 2007 to 2018, involved a network of pain clinics across multiple states. Referred to as “pill mills” by the DOJ, the pain clinics dispensed the high-dosage prescriptions, such as oxycodone, to drug dealers and patients with opioid use disorder.
The procedures, billed to insurance, were for facet joint injections. According to the DOJ, the injections were chosen because they generated high reimbursements rather than being medically necessary.
The Detroit News reported in September that some of the medically unnecessary drugs prescribed by Dr. Patino, which included fentanyl, oxycodone, and oxymorphone, per an indictment, were resold “on the street.” Dr. Patino wrote prescriptions for more than 2.2 million pills between 2016 and 2017.
Dr. Patino and Mr. Rashid join physicians and others in the health care field, who are charged or sentenced for their involvement in the scheme. In total, five physicians were convicted in two separate trials and 18 defendants pleaded guilty, per the DOJ. Meanwhile, seven defendants await sentencing.
Included in this group were:
- Spilios Pappas, MD, 63, an emergency medicine specialist in Lucas County, Ohio, sentenced to nine years in prison and ordered to pay $32.2 million in restitution
- Joseph Betro, DO, 60, an emergency medicine physician from Oakland County, Mich., sentenced to nine years in prison and ordered to pay $27.4 million in restitution
- Tariq Omar, MD, 63, a pulmonologist from Oakland County, Mich., sentenced to eight years in prison and ordered to pay $24.2 million in restitution
- Mohammed Zahoor, MD, 53, a neurologist from Oakland County, Mich., sentenced to eight years in prison and ordered to pay $36.6 million in restitution
The four physicians worked at various clinics under the Tri-County Wellness Group, operated by Mr. Rashid, according to federal officials. During their employment with the clinics, they defrauded Medicare of more than $150 million through the scheme that involved opioids for medically unnecessary services, the DOJ noted.
Shortly after being indicted, Dr. Pappas posted a fundraising page for his legal services, claiming he and the other doctors had no idea what was going on and that he was “sickened and nauseous” when learning of the details of the case.
More than $16 million was forfeited by the defendants to the United States, according to the DOJ.
A version of this article first appeared on Medscape.com.
Addiction expert says CBD may help people cut cannabis use
PARIS – Following the suspension of the decree that banned the sale of cannabidiol (CBD) flowers, raw cannabis is again available in France for over-the-counter sales. The “feel-good” plant is praised for its relaxing properties.
The suspension of the ban, which lasted for 3 weeks, is a mixed blessing for businesses that sell CBD-based products in France. Professional organizations in this booming sector filed a petition with France’s highest administrative court, the Council of State. At the end of January, the court suspended the government decree that banned the sale of cannabis-derived CBD flowers and leaves; however, it has yet to hand down a final decision as to the legality of the decree.
In just a few years, numerous shops have opened across France. They no longer have to settle for selling processed CBD products such as chocolate, oils, cookies – even wine. They can resume the sale of CBD hemp, which mainly comes in clusters of flower buds and can be smoked or used as an infusion.
Cannabis-derived CBD must have less than 0.2% tetrahydrocannabinol (THC) to be considered “feel-good hemp,” which is used in various consumer goods (such as food, cosmetics, and e-cigarette liquids) and is praised for its calming effects. But not all hemp is the same. Medical hemp, which is currently in clinical trials, combines varying doses of CBD and THC. And then there is THC-rich psychotropic hemp, which is illegal to sell.
The government’s decree cites health concerns as a justification for the ban. While uncertainties remain, “research studies have shown that CBD acts on dopamine and serotonin receptors in the brain. ... Therefore, using CBD can produce psychoactive, sedative, and sleep-inducing effects.” In addition to a preventive approach, the authorities cite the difficulties in distinguishing cannabis-derived CBD from THC-rich illegal cannabis – difficulties that complicate efforts in the war on drugs.
The government’s position sows confusion among consumers, who are attracted by the arguments in favor of CBD and intrigued by the promise of the substance’s calming effects. This confusion is heightened by the fact that there are not enough scientific data either to declare that CBD poses a real risk or, alternatively, to confirm that it has beneficial effects. While some studies have suggested that CBD has a potential benefit for treating anxiety, pain, and sleep problems, others suggest that it may instead be a placebo effect.
What actual benefit can be expected from CBD-derived products, in particular from using the plant’s raw extracts? We asked Dan Velea, MD, an addiction psychiatrist in Paris, to give us his thoughts.
What do you think of the government’s position of banning the sale of cannabis-derived CBD flowers and leaves?
Dr. Velea: I don’t understand the reasoning behind this ban. Unlike THC, CBD is not an addictive substance. We’ve suspected that CBD had beneficial effects ever since noting that, just like THC, it could bind to the two types of cannabinoid receptors found in our bodies, CB1 and CB2, but without inducing a psychotropic effect or giving rise to dependence.
The CBD-derived products that are available on the market have infinitesimal amounts of THC – the threshold is 0.2% – which pose no risk. These products seem to be a particularly good alternative for certain at-risk users who are looking for a way that will help them cut down on their use of “traditional” cannabis, which has THC. However, due to a lack of research, the benefits of CBD cannot be confirmed.
Now that the decree’s been suspended, we can leave behind the ideological debate that has been built around cannabis in France. It’s time to focus only on discussions based on science. On that note, we also have to encourage people to do more research into cannabis’s therapeutic value.
You believe that CBD can help people cut down on their cannabis use. Is that based on what you see in your practice as an addiction specialist?
Dr. Velea: Of course, they don’t get the same dazzling effect that’s produced by THC, very high concentrations of which are often found in cannabis. But for them, the sensation they get from CBD is still pleasant. They describe it as having a soothing and fun effect; they quite like it. Given that the vast majority of people use cannabis recreationally, we can consider that this effect is no small benefit.
Even those who are highly dependent prefer to alternate, using CBD during the day and having just one THC joint in the evening. This makes them feel a lot better. In addition, it clearly reduces the health risks. In my opinion, CBD can be viewed as an alternative for people whose cannabis use is problematic. If a patient asks me about it, I give them an unequivocal answer: There are fewer risks associated with CBD than with regular cannabis.
Isn’t there still a risk for abuse? A dose of cannabidiol that shouldn’t be exceeded?
Dr. Velea: Honestly, apart from the harmful effects of smoking CBD, I don’t see any health risks associated with its use. I’ve never had a patient present with complaints after using these products. No one has ever told me that they became addicted or experienced psychotropic effects. There are no changes in behavior, even at high doses. It should be mentioned that World Health Organization experts hold that there’s no abuse or dependence potential associated with the use of pure CBD. Furthermore, they say that the product is generally well tolerated.
What other actual benefits does CBD have? People mention its relaxing, even anxiolytic, effects.
Dr. Velea: CBD-derived products are praised for their relaxing properties, which particularly help improve one’s sleep. It’s a question of knowing whether these are actual benefits or whether a placebo effect is involved here – something that would be enhanced in a person who firmly believes that these products bring about a sense of well-being. Even when CBD is used for pain relief, we can’t rule out the placebo effect as playing an important role in the outcome.
Some patients with serious diseases have been able to find comfort by using CBD. However, because there haven’t been any well-designed randomized studies, we’ve never been able to show clearly that the beneficial effect comes from the product itself. It’s also possible that the soothing, muscle-relaxing effect induced by CBD’s stimulation of cannabinoid receptors is actually what’s helping to relieve the pain. But this has yet to be proved.
So, what position can a doctor take toward patients who express their desire to use CBD-derived products?
Dr. Velea: Without reliable studies to back them up, it’s difficult to say. Also, at the moment, there are legal gray areas that don’t allow doctors to take a position. As a result, users are put at a disadvantage and not given the opportunity to make the choice to use CBD-derived products in an informed manner. Even so, I think that as long as we don’t have scientific data, the use of these products must be limited to recreational use with the aim of bringing about relaxation. In the case of a sleep disorder, for example, doctors can’t replace standard therapeutic management aimed at improving the patient’s sleep cycles. For now, the only genuinely interesting aspect of CBD that I can see is that it makes it possible to cut down on the use of THC-containing cannabis.
A version of this article first appeared on Medscape.com.
PARIS – Following the suspension of the decree that banned the sale of cannabidiol (CBD) flowers, raw cannabis is again available in France for over-the-counter sales. The “feel-good” plant is praised for its relaxing properties.
The suspension of the ban, which lasted for 3 weeks, is a mixed blessing for businesses that sell CBD-based products in France. Professional organizations in this booming sector filed a petition with France’s highest administrative court, the Council of State. At the end of January, the court suspended the government decree that banned the sale of cannabis-derived CBD flowers and leaves; however, it has yet to hand down a final decision as to the legality of the decree.
In just a few years, numerous shops have opened across France. They no longer have to settle for selling processed CBD products such as chocolate, oils, cookies – even wine. They can resume the sale of CBD hemp, which mainly comes in clusters of flower buds and can be smoked or used as an infusion.
Cannabis-derived CBD must have less than 0.2% tetrahydrocannabinol (THC) to be considered “feel-good hemp,” which is used in various consumer goods (such as food, cosmetics, and e-cigarette liquids) and is praised for its calming effects. But not all hemp is the same. Medical hemp, which is currently in clinical trials, combines varying doses of CBD and THC. And then there is THC-rich psychotropic hemp, which is illegal to sell.
The government’s decree cites health concerns as a justification for the ban. While uncertainties remain, “research studies have shown that CBD acts on dopamine and serotonin receptors in the brain. ... Therefore, using CBD can produce psychoactive, sedative, and sleep-inducing effects.” In addition to a preventive approach, the authorities cite the difficulties in distinguishing cannabis-derived CBD from THC-rich illegal cannabis – difficulties that complicate efforts in the war on drugs.
The government’s position sows confusion among consumers, who are attracted by the arguments in favor of CBD and intrigued by the promise of the substance’s calming effects. This confusion is heightened by the fact that there are not enough scientific data either to declare that CBD poses a real risk or, alternatively, to confirm that it has beneficial effects. While some studies have suggested that CBD has a potential benefit for treating anxiety, pain, and sleep problems, others suggest that it may instead be a placebo effect.
What actual benefit can be expected from CBD-derived products, in particular from using the plant’s raw extracts? We asked Dan Velea, MD, an addiction psychiatrist in Paris, to give us his thoughts.
What do you think of the government’s position of banning the sale of cannabis-derived CBD flowers and leaves?
Dr. Velea: I don’t understand the reasoning behind this ban. Unlike THC, CBD is not an addictive substance. We’ve suspected that CBD had beneficial effects ever since noting that, just like THC, it could bind to the two types of cannabinoid receptors found in our bodies, CB1 and CB2, but without inducing a psychotropic effect or giving rise to dependence.
The CBD-derived products that are available on the market have infinitesimal amounts of THC – the threshold is 0.2% – which pose no risk. These products seem to be a particularly good alternative for certain at-risk users who are looking for a way that will help them cut down on their use of “traditional” cannabis, which has THC. However, due to a lack of research, the benefits of CBD cannot be confirmed.
Now that the decree’s been suspended, we can leave behind the ideological debate that has been built around cannabis in France. It’s time to focus only on discussions based on science. On that note, we also have to encourage people to do more research into cannabis’s therapeutic value.
You believe that CBD can help people cut down on their cannabis use. Is that based on what you see in your practice as an addiction specialist?
Dr. Velea: Of course, they don’t get the same dazzling effect that’s produced by THC, very high concentrations of which are often found in cannabis. But for them, the sensation they get from CBD is still pleasant. They describe it as having a soothing and fun effect; they quite like it. Given that the vast majority of people use cannabis recreationally, we can consider that this effect is no small benefit.
Even those who are highly dependent prefer to alternate, using CBD during the day and having just one THC joint in the evening. This makes them feel a lot better. In addition, it clearly reduces the health risks. In my opinion, CBD can be viewed as an alternative for people whose cannabis use is problematic. If a patient asks me about it, I give them an unequivocal answer: There are fewer risks associated with CBD than with regular cannabis.
Isn’t there still a risk for abuse? A dose of cannabidiol that shouldn’t be exceeded?
Dr. Velea: Honestly, apart from the harmful effects of smoking CBD, I don’t see any health risks associated with its use. I’ve never had a patient present with complaints after using these products. No one has ever told me that they became addicted or experienced psychotropic effects. There are no changes in behavior, even at high doses. It should be mentioned that World Health Organization experts hold that there’s no abuse or dependence potential associated with the use of pure CBD. Furthermore, they say that the product is generally well tolerated.
What other actual benefits does CBD have? People mention its relaxing, even anxiolytic, effects.
Dr. Velea: CBD-derived products are praised for their relaxing properties, which particularly help improve one’s sleep. It’s a question of knowing whether these are actual benefits or whether a placebo effect is involved here – something that would be enhanced in a person who firmly believes that these products bring about a sense of well-being. Even when CBD is used for pain relief, we can’t rule out the placebo effect as playing an important role in the outcome.
Some patients with serious diseases have been able to find comfort by using CBD. However, because there haven’t been any well-designed randomized studies, we’ve never been able to show clearly that the beneficial effect comes from the product itself. It’s also possible that the soothing, muscle-relaxing effect induced by CBD’s stimulation of cannabinoid receptors is actually what’s helping to relieve the pain. But this has yet to be proved.
So, what position can a doctor take toward patients who express their desire to use CBD-derived products?
Dr. Velea: Without reliable studies to back them up, it’s difficult to say. Also, at the moment, there are legal gray areas that don’t allow doctors to take a position. As a result, users are put at a disadvantage and not given the opportunity to make the choice to use CBD-derived products in an informed manner. Even so, I think that as long as we don’t have scientific data, the use of these products must be limited to recreational use with the aim of bringing about relaxation. In the case of a sleep disorder, for example, doctors can’t replace standard therapeutic management aimed at improving the patient’s sleep cycles. For now, the only genuinely interesting aspect of CBD that I can see is that it makes it possible to cut down on the use of THC-containing cannabis.
A version of this article first appeared on Medscape.com.
PARIS – Following the suspension of the decree that banned the sale of cannabidiol (CBD) flowers, raw cannabis is again available in France for over-the-counter sales. The “feel-good” plant is praised for its relaxing properties.
The suspension of the ban, which lasted for 3 weeks, is a mixed blessing for businesses that sell CBD-based products in France. Professional organizations in this booming sector filed a petition with France’s highest administrative court, the Council of State. At the end of January, the court suspended the government decree that banned the sale of cannabis-derived CBD flowers and leaves; however, it has yet to hand down a final decision as to the legality of the decree.
In just a few years, numerous shops have opened across France. They no longer have to settle for selling processed CBD products such as chocolate, oils, cookies – even wine. They can resume the sale of CBD hemp, which mainly comes in clusters of flower buds and can be smoked or used as an infusion.
Cannabis-derived CBD must have less than 0.2% tetrahydrocannabinol (THC) to be considered “feel-good hemp,” which is used in various consumer goods (such as food, cosmetics, and e-cigarette liquids) and is praised for its calming effects. But not all hemp is the same. Medical hemp, which is currently in clinical trials, combines varying doses of CBD and THC. And then there is THC-rich psychotropic hemp, which is illegal to sell.
The government’s decree cites health concerns as a justification for the ban. While uncertainties remain, “research studies have shown that CBD acts on dopamine and serotonin receptors in the brain. ... Therefore, using CBD can produce psychoactive, sedative, and sleep-inducing effects.” In addition to a preventive approach, the authorities cite the difficulties in distinguishing cannabis-derived CBD from THC-rich illegal cannabis – difficulties that complicate efforts in the war on drugs.
The government’s position sows confusion among consumers, who are attracted by the arguments in favor of CBD and intrigued by the promise of the substance’s calming effects. This confusion is heightened by the fact that there are not enough scientific data either to declare that CBD poses a real risk or, alternatively, to confirm that it has beneficial effects. While some studies have suggested that CBD has a potential benefit for treating anxiety, pain, and sleep problems, others suggest that it may instead be a placebo effect.
What actual benefit can be expected from CBD-derived products, in particular from using the plant’s raw extracts? We asked Dan Velea, MD, an addiction psychiatrist in Paris, to give us his thoughts.
What do you think of the government’s position of banning the sale of cannabis-derived CBD flowers and leaves?
Dr. Velea: I don’t understand the reasoning behind this ban. Unlike THC, CBD is not an addictive substance. We’ve suspected that CBD had beneficial effects ever since noting that, just like THC, it could bind to the two types of cannabinoid receptors found in our bodies, CB1 and CB2, but without inducing a psychotropic effect or giving rise to dependence.
The CBD-derived products that are available on the market have infinitesimal amounts of THC – the threshold is 0.2% – which pose no risk. These products seem to be a particularly good alternative for certain at-risk users who are looking for a way that will help them cut down on their use of “traditional” cannabis, which has THC. However, due to a lack of research, the benefits of CBD cannot be confirmed.
Now that the decree’s been suspended, we can leave behind the ideological debate that has been built around cannabis in France. It’s time to focus only on discussions based on science. On that note, we also have to encourage people to do more research into cannabis’s therapeutic value.
You believe that CBD can help people cut down on their cannabis use. Is that based on what you see in your practice as an addiction specialist?
Dr. Velea: Of course, they don’t get the same dazzling effect that’s produced by THC, very high concentrations of which are often found in cannabis. But for them, the sensation they get from CBD is still pleasant. They describe it as having a soothing and fun effect; they quite like it. Given that the vast majority of people use cannabis recreationally, we can consider that this effect is no small benefit.
Even those who are highly dependent prefer to alternate, using CBD during the day and having just one THC joint in the evening. This makes them feel a lot better. In addition, it clearly reduces the health risks. In my opinion, CBD can be viewed as an alternative for people whose cannabis use is problematic. If a patient asks me about it, I give them an unequivocal answer: There are fewer risks associated with CBD than with regular cannabis.
Isn’t there still a risk for abuse? A dose of cannabidiol that shouldn’t be exceeded?
Dr. Velea: Honestly, apart from the harmful effects of smoking CBD, I don’t see any health risks associated with its use. I’ve never had a patient present with complaints after using these products. No one has ever told me that they became addicted or experienced psychotropic effects. There are no changes in behavior, even at high doses. It should be mentioned that World Health Organization experts hold that there’s no abuse or dependence potential associated with the use of pure CBD. Furthermore, they say that the product is generally well tolerated.
What other actual benefits does CBD have? People mention its relaxing, even anxiolytic, effects.
Dr. Velea: CBD-derived products are praised for their relaxing properties, which particularly help improve one’s sleep. It’s a question of knowing whether these are actual benefits or whether a placebo effect is involved here – something that would be enhanced in a person who firmly believes that these products bring about a sense of well-being. Even when CBD is used for pain relief, we can’t rule out the placebo effect as playing an important role in the outcome.
Some patients with serious diseases have been able to find comfort by using CBD. However, because there haven’t been any well-designed randomized studies, we’ve never been able to show clearly that the beneficial effect comes from the product itself. It’s also possible that the soothing, muscle-relaxing effect induced by CBD’s stimulation of cannabinoid receptors is actually what’s helping to relieve the pain. But this has yet to be proved.
So, what position can a doctor take toward patients who express their desire to use CBD-derived products?
Dr. Velea: Without reliable studies to back them up, it’s difficult to say. Also, at the moment, there are legal gray areas that don’t allow doctors to take a position. As a result, users are put at a disadvantage and not given the opportunity to make the choice to use CBD-derived products in an informed manner. Even so, I think that as long as we don’t have scientific data, the use of these products must be limited to recreational use with the aim of bringing about relaxation. In the case of a sleep disorder, for example, doctors can’t replace standard therapeutic management aimed at improving the patient’s sleep cycles. For now, the only genuinely interesting aspect of CBD that I can see is that it makes it possible to cut down on the use of THC-containing cannabis.
A version of this article first appeared on Medscape.com.
FDA, DEA pushed to make gabapentin a controlled substance to stop ‘widespread misuse’
In a bid to stop abuse and diversion of the anticonvulsant gabapentin, a watchdog group is petitioning federal regulators to make the drug a controlled substance.
Gabapentin is a generic drug, best known under the brand name Neurontin. The petition also covers the related drug gabapentin enacarbil (Horizant).
Public Citizen requested that gabapentin come under the DEA’s Schedule V category, which already includes the similar drug pregabalin (Lyrica). Schedule V is the lowest rung on the DEA’s drug schedule, meaning it has lower potential for abuse then Schedule I through IV drugs. This tier also includes cough preparations with less than 200 milligrams of codeine.
Classifying gabapentin as a Schedule V drug would facilitate better tracking of the drug’s use and misuse and put in place educational and limitation requirements to mitigate the risk of addiction, overdose, and death, Michael Abrams, MPH, PhD, senior health researcher with Public Citizen’s Health Research Group, and colleagues write in the petition.
‘Widespread misuse’
There is “substantial evidence of widespread misuse” of gabapentin, plausibly helped by “extraordinary levels of off-label prescribing,” Public Citizen said in the petition.
Some estimates have pegged off-label use at more than 90%, with gabapentin prescribed for indications such as chronic cough, hiccups, postoperative pain, and postmenopausal hot flashes, the group said.
“Moreover, there are numerous reports indicating that gabapentin is widely used and diverted on the street to induce ‘highs’ or otherwise self-medicate,” Public Citizen said. “Both gabapentin and pregabalin have been empirically linked to the opioid overdose epidemic as drugs that potentiate the activity of these oftentimes deadly analgesics.”
This news organization tried several times to reach Azurity for comment but did not receive a response. Pfizer included gabapentin in the portfolio of drugs used to create the Viatris spin-off, which took place in 2020. Pfizer referred this news organization to Viatris for comment, but it also did not respond.
It is unclear how the FDA and DEA will respond to the petition. Public Citizen has received a reply from the FDA, in which the agency acknowledged receipt of the petition. However, the “acceptance of the petition for filing is a procedural matter and in no way reflects the agency’s decision on the substantive merits of the petition,” the FDA said in a letter.
As is common practice, the agency assigned a docket number for the petition, FDA-2022-P-0149. The docket’s website allows interested parties to track the issue.
‘Unnoticed’ abuse
There have been rising concerns about risks and abuse of gabapentin in recent years. In its petition, Public Citizen noted that the United Kingdom and several U.S. states have already sought tighter control of gabapentin prescriptions.
In 2019, the United Kingdom announced it would reclassify both pregabalin and gabapentin as class C controlled substances because of the rising numbers of deaths linked to the drugs.
As of November 2020, seven states – Alabama, Kentucky, Michigan, North Dakota, Tennessee, Virginia, and West Virginia – had classified gabapentin as a schedule V drug, while another 12 states required prescription monitoring of the drug, Public Citizen noted.
In 2018, researchers at the University of Louisville, Kentucky, a state that has been hit particularly hard by the opioid crisis, tried to draw more attention to the risks of gabapentin.
“Amid the opioid epidemic, abuse of a different prescription painkiller has widely gone unnoticed,” the University said in a press release at the time.
The release highlighted a study led by Rachel Vickers Smith, PhD, assistant professor in the University of Louisville School of Nursing that was published in Psychology of Addictive Behaviors.
It included 33 individuals who reported recent recreational use of gabapentin. Use of the drug was combined with buprenorphine, other opioids, cocaine, and caffeine to produce effects such as muscle relaxation, pain reduction, sleep induction, feeling drunk, and feeling “high.”
In the press release, Dr. Vickers Smith said individuals who abuse gabapentin often mix it with opioids, marijuana, cocaine, and opioid treatment medication, compounding side effects to the central nervous system that include euphoria and sedation.
In addition, some individuals who primarily abused opioid pain medication have turned to gabapentin after law-enforcement actions made it more difficult to obtain prescription opioids, she noted.
“People are looking for other drugs to substitute for opioids, and gabapentin has filled that place for some,” Dr. Vickers Smith said. “Some have said it gives them a high similar to opioids.”
FDA 2019 warning
In 2019, the FDA issued a warning about serious breathing difficulties associated with gabapentin and pregabalin in patients with respiratory risk factors.
These factors include opioid use and other drugs that depress the central nervous system, as well as conditions such as chronic obstructive pulmonary disease that reduce lung function. Older patients are also at higher risk, the FDA said.
The agency noted that gabapentinoids are often co-prescribed with opioids for for medical conditions and abused in combination with opioids. Data collected in 2016 from an office-based physician survey showed 14% of patient encounters involving gabapentin also involved opioids, the FDA said.
“Our evaluation shows that the use of these medicines, often referred to as gabapentinoids, has been growing for prescribed medical use, as well as misuse and abuse,” the agency said in its 2019 alert.
A version of this article first appeared on Medscape.com.
In a bid to stop abuse and diversion of the anticonvulsant gabapentin, a watchdog group is petitioning federal regulators to make the drug a controlled substance.
Gabapentin is a generic drug, best known under the brand name Neurontin. The petition also covers the related drug gabapentin enacarbil (Horizant).
Public Citizen requested that gabapentin come under the DEA’s Schedule V category, which already includes the similar drug pregabalin (Lyrica). Schedule V is the lowest rung on the DEA’s drug schedule, meaning it has lower potential for abuse then Schedule I through IV drugs. This tier also includes cough preparations with less than 200 milligrams of codeine.
Classifying gabapentin as a Schedule V drug would facilitate better tracking of the drug’s use and misuse and put in place educational and limitation requirements to mitigate the risk of addiction, overdose, and death, Michael Abrams, MPH, PhD, senior health researcher with Public Citizen’s Health Research Group, and colleagues write in the petition.
‘Widespread misuse’
There is “substantial evidence of widespread misuse” of gabapentin, plausibly helped by “extraordinary levels of off-label prescribing,” Public Citizen said in the petition.
Some estimates have pegged off-label use at more than 90%, with gabapentin prescribed for indications such as chronic cough, hiccups, postoperative pain, and postmenopausal hot flashes, the group said.
“Moreover, there are numerous reports indicating that gabapentin is widely used and diverted on the street to induce ‘highs’ or otherwise self-medicate,” Public Citizen said. “Both gabapentin and pregabalin have been empirically linked to the opioid overdose epidemic as drugs that potentiate the activity of these oftentimes deadly analgesics.”
This news organization tried several times to reach Azurity for comment but did not receive a response. Pfizer included gabapentin in the portfolio of drugs used to create the Viatris spin-off, which took place in 2020. Pfizer referred this news organization to Viatris for comment, but it also did not respond.
It is unclear how the FDA and DEA will respond to the petition. Public Citizen has received a reply from the FDA, in which the agency acknowledged receipt of the petition. However, the “acceptance of the petition for filing is a procedural matter and in no way reflects the agency’s decision on the substantive merits of the petition,” the FDA said in a letter.
As is common practice, the agency assigned a docket number for the petition, FDA-2022-P-0149. The docket’s website allows interested parties to track the issue.
‘Unnoticed’ abuse
There have been rising concerns about risks and abuse of gabapentin in recent years. In its petition, Public Citizen noted that the United Kingdom and several U.S. states have already sought tighter control of gabapentin prescriptions.
In 2019, the United Kingdom announced it would reclassify both pregabalin and gabapentin as class C controlled substances because of the rising numbers of deaths linked to the drugs.
As of November 2020, seven states – Alabama, Kentucky, Michigan, North Dakota, Tennessee, Virginia, and West Virginia – had classified gabapentin as a schedule V drug, while another 12 states required prescription monitoring of the drug, Public Citizen noted.
In 2018, researchers at the University of Louisville, Kentucky, a state that has been hit particularly hard by the opioid crisis, tried to draw more attention to the risks of gabapentin.
“Amid the opioid epidemic, abuse of a different prescription painkiller has widely gone unnoticed,” the University said in a press release at the time.
The release highlighted a study led by Rachel Vickers Smith, PhD, assistant professor in the University of Louisville School of Nursing that was published in Psychology of Addictive Behaviors.
It included 33 individuals who reported recent recreational use of gabapentin. Use of the drug was combined with buprenorphine, other opioids, cocaine, and caffeine to produce effects such as muscle relaxation, pain reduction, sleep induction, feeling drunk, and feeling “high.”
In the press release, Dr. Vickers Smith said individuals who abuse gabapentin often mix it with opioids, marijuana, cocaine, and opioid treatment medication, compounding side effects to the central nervous system that include euphoria and sedation.
In addition, some individuals who primarily abused opioid pain medication have turned to gabapentin after law-enforcement actions made it more difficult to obtain prescription opioids, she noted.
“People are looking for other drugs to substitute for opioids, and gabapentin has filled that place for some,” Dr. Vickers Smith said. “Some have said it gives them a high similar to opioids.”
FDA 2019 warning
In 2019, the FDA issued a warning about serious breathing difficulties associated with gabapentin and pregabalin in patients with respiratory risk factors.
These factors include opioid use and other drugs that depress the central nervous system, as well as conditions such as chronic obstructive pulmonary disease that reduce lung function. Older patients are also at higher risk, the FDA said.
The agency noted that gabapentinoids are often co-prescribed with opioids for for medical conditions and abused in combination with opioids. Data collected in 2016 from an office-based physician survey showed 14% of patient encounters involving gabapentin also involved opioids, the FDA said.
“Our evaluation shows that the use of these medicines, often referred to as gabapentinoids, has been growing for prescribed medical use, as well as misuse and abuse,” the agency said in its 2019 alert.
A version of this article first appeared on Medscape.com.
In a bid to stop abuse and diversion of the anticonvulsant gabapentin, a watchdog group is petitioning federal regulators to make the drug a controlled substance.
Gabapentin is a generic drug, best known under the brand name Neurontin. The petition also covers the related drug gabapentin enacarbil (Horizant).
Public Citizen requested that gabapentin come under the DEA’s Schedule V category, which already includes the similar drug pregabalin (Lyrica). Schedule V is the lowest rung on the DEA’s drug schedule, meaning it has lower potential for abuse then Schedule I through IV drugs. This tier also includes cough preparations with less than 200 milligrams of codeine.
Classifying gabapentin as a Schedule V drug would facilitate better tracking of the drug’s use and misuse and put in place educational and limitation requirements to mitigate the risk of addiction, overdose, and death, Michael Abrams, MPH, PhD, senior health researcher with Public Citizen’s Health Research Group, and colleagues write in the petition.
‘Widespread misuse’
There is “substantial evidence of widespread misuse” of gabapentin, plausibly helped by “extraordinary levels of off-label prescribing,” Public Citizen said in the petition.
Some estimates have pegged off-label use at more than 90%, with gabapentin prescribed for indications such as chronic cough, hiccups, postoperative pain, and postmenopausal hot flashes, the group said.
“Moreover, there are numerous reports indicating that gabapentin is widely used and diverted on the street to induce ‘highs’ or otherwise self-medicate,” Public Citizen said. “Both gabapentin and pregabalin have been empirically linked to the opioid overdose epidemic as drugs that potentiate the activity of these oftentimes deadly analgesics.”
This news organization tried several times to reach Azurity for comment but did not receive a response. Pfizer included gabapentin in the portfolio of drugs used to create the Viatris spin-off, which took place in 2020. Pfizer referred this news organization to Viatris for comment, but it also did not respond.
It is unclear how the FDA and DEA will respond to the petition. Public Citizen has received a reply from the FDA, in which the agency acknowledged receipt of the petition. However, the “acceptance of the petition for filing is a procedural matter and in no way reflects the agency’s decision on the substantive merits of the petition,” the FDA said in a letter.
As is common practice, the agency assigned a docket number for the petition, FDA-2022-P-0149. The docket’s website allows interested parties to track the issue.
‘Unnoticed’ abuse
There have been rising concerns about risks and abuse of gabapentin in recent years. In its petition, Public Citizen noted that the United Kingdom and several U.S. states have already sought tighter control of gabapentin prescriptions.
In 2019, the United Kingdom announced it would reclassify both pregabalin and gabapentin as class C controlled substances because of the rising numbers of deaths linked to the drugs.
As of November 2020, seven states – Alabama, Kentucky, Michigan, North Dakota, Tennessee, Virginia, and West Virginia – had classified gabapentin as a schedule V drug, while another 12 states required prescription monitoring of the drug, Public Citizen noted.
In 2018, researchers at the University of Louisville, Kentucky, a state that has been hit particularly hard by the opioid crisis, tried to draw more attention to the risks of gabapentin.
“Amid the opioid epidemic, abuse of a different prescription painkiller has widely gone unnoticed,” the University said in a press release at the time.
The release highlighted a study led by Rachel Vickers Smith, PhD, assistant professor in the University of Louisville School of Nursing that was published in Psychology of Addictive Behaviors.
It included 33 individuals who reported recent recreational use of gabapentin. Use of the drug was combined with buprenorphine, other opioids, cocaine, and caffeine to produce effects such as muscle relaxation, pain reduction, sleep induction, feeling drunk, and feeling “high.”
In the press release, Dr. Vickers Smith said individuals who abuse gabapentin often mix it with opioids, marijuana, cocaine, and opioid treatment medication, compounding side effects to the central nervous system that include euphoria and sedation.
In addition, some individuals who primarily abused opioid pain medication have turned to gabapentin after law-enforcement actions made it more difficult to obtain prescription opioids, she noted.
“People are looking for other drugs to substitute for opioids, and gabapentin has filled that place for some,” Dr. Vickers Smith said. “Some have said it gives them a high similar to opioids.”
FDA 2019 warning
In 2019, the FDA issued a warning about serious breathing difficulties associated with gabapentin and pregabalin in patients with respiratory risk factors.
These factors include opioid use and other drugs that depress the central nervous system, as well as conditions such as chronic obstructive pulmonary disease that reduce lung function. Older patients are also at higher risk, the FDA said.
The agency noted that gabapentinoids are often co-prescribed with opioids for for medical conditions and abused in combination with opioids. Data collected in 2016 from an office-based physician survey showed 14% of patient encounters involving gabapentin also involved opioids, the FDA said.
“Our evaluation shows that the use of these medicines, often referred to as gabapentinoids, has been growing for prescribed medical use, as well as misuse and abuse,” the agency said in its 2019 alert.
A version of this article first appeared on Medscape.com.
Dyspareunia: Keys to biopsychosocial evaluation and treatment planning
Dyspareunia is persistent or recurrent pain before, during, or after sexual contact and is not limited to cisgender individuals or vaginal intercourse.1-3 With a prevalence as high as 45% in the United States,2-5 it is one of the most common complaints in gynecologic practices.5,6
Causes and contributing factors
There are many possible causes of dyspareunia.2,4,6 While some patients have a single cause, most cases are complex, with multiple overlapping causes and maintaining factors.4,6 Identifying each contributing factor can help you appropriately address all components.
Physical conditions. The range of physical contributors to dyspareunia includes inflammatory processes, structural abnormalities, musculoskeletal dysfunctions, pelvic organ disorders, injuries, iatrogenic effects, infections, allergic reactions, sensitization, hormonal changes, medication effects, adhesions, autoimmune disorders, and other pain syndromes (TABLE 12-4,6-11).

Inadequate arousal. One of the primary causes of pain during vaginal penetration is inadequate arousal and lubrication.1,2,9-11 Arousal is the phase of the sexual response cycle that leads to genital tumescence and prepares the genitals for sexual contact through penile/clitoral erection, vaginal engorgement, and lubrication, which prevents pain and enhances pleasurable sensation.9-11
While some physical conditions can lead to an inability to lubricate, the most common causes of inadequate lubrication are psychosocial-behavioral, wherein patients have the same physical ability to lubricate as patients without genital pain but do not progress through the arousal phase.9-11 Behavioral factors such as inadequate or ineffective foreplay can fail to produce engorgement and lubrication, while psychosocial factors such as low attraction to partner, relationship stressors, anxiety, or low self-esteem can have an inhibitory effect on sexual arousal.1,2,9-11 Psychosocial and behavioral factors may also be maintaining factors or consequences of dyspareunia, and need to be assessed and treated.1,2,9-11
Psychological trauma. Exposure to psychological traumas and the development of posttraumatic stress disorder (PTSD) have been linked with the development of pain disorders in general and dyspareunia specifically. Most patients seeking treatment for chronic pain disorders have a history of physical or sexual abuse.12 Changes in physiologic processes (eg, neurochemical, endocrine) that occur with PTSD interfere with the sexual response cycle, and sexual traumas specifically have been linked with pelvic floor dysfunction.13,14 Additionally, when PTSD is caused by a sexual trauma, even consensual sexual encounters can trigger flashbacks, intrusive memories, hyperarousal, and muscle tension that interfere with the sexual response cycle and contribute to genital pain.13
Vaginismus is both a physiologic and psychological contributor to dyspareunia.1,2,4 Patients experiencing pain can develop anxiety about repeated pain and involuntarily contract their pelvic muscles, thereby creating more pain, increasing anxiety, decreasing lubrication, and causing pelvic floor dysfunction.1-4,6 Consequently, all patients with dyspareunia should be assessed and continually monitored for symptoms of vaginismus.
Continue to: Anxiety
Anxiety. As with other pain disorders, anxiety develops around pain triggers.10,15 When expecting sexual activity, patients can experience extreme worry and panic attacks.10,15,16 The distress of sexual encounters can interfere with physiologic arousal and sexual desire, impacting all phases of the sexual response cycle.1,2
Relationship issues. Difficulty engaging in or avoidance of sexual activity can interfere with romantic relationships.2,10,16 Severe pain or vaginismus contractions can prevent penetration, leading to unconsummated marriages and an inability to conceive through intercourse.10 The distress surrounding sexual encounters can precipitate erectile dysfunction in male partners, or partners may continue to demand sexual encounters despite the patient’s pain, further impacting the relationship and heightening sexual distress.10 These stressors have led to relationships ending, patients reluctantly agreeing to nonmonogamy to appease their partners, and patients avoiding relationships altogether.10,16
Devalued self-image. Difficulties with sexuality and relationships impact the self-image of patients with dyspareunia. Diminished self-image may include feeling “inadequate” as a woman and as a sexual partner, or feeling like a “failure.”16 Women with dyspareunia often have more distress related to their body image, physical appearance, and genital self-image than do women without genital pain.17 Feeling resentment toward their body, or feeling “ugly,” embarrassed, shamed, “broken,” and “useless” also contribute to increased depressive symptoms found in patients with dyspareunia.16,18
Making the diagnosis
Most patients do not report symptoms unless directly asked2,7; therefore, it is recommended that all patients be screened as a part of an initial intake and before any genital exam (TABLE 22-4,6,7,9,11,19,20).4,7,21 If this screen is positive, a separate appointment may be needed for a thorough evaluation and before any attempt is made at a genital exam.4,7
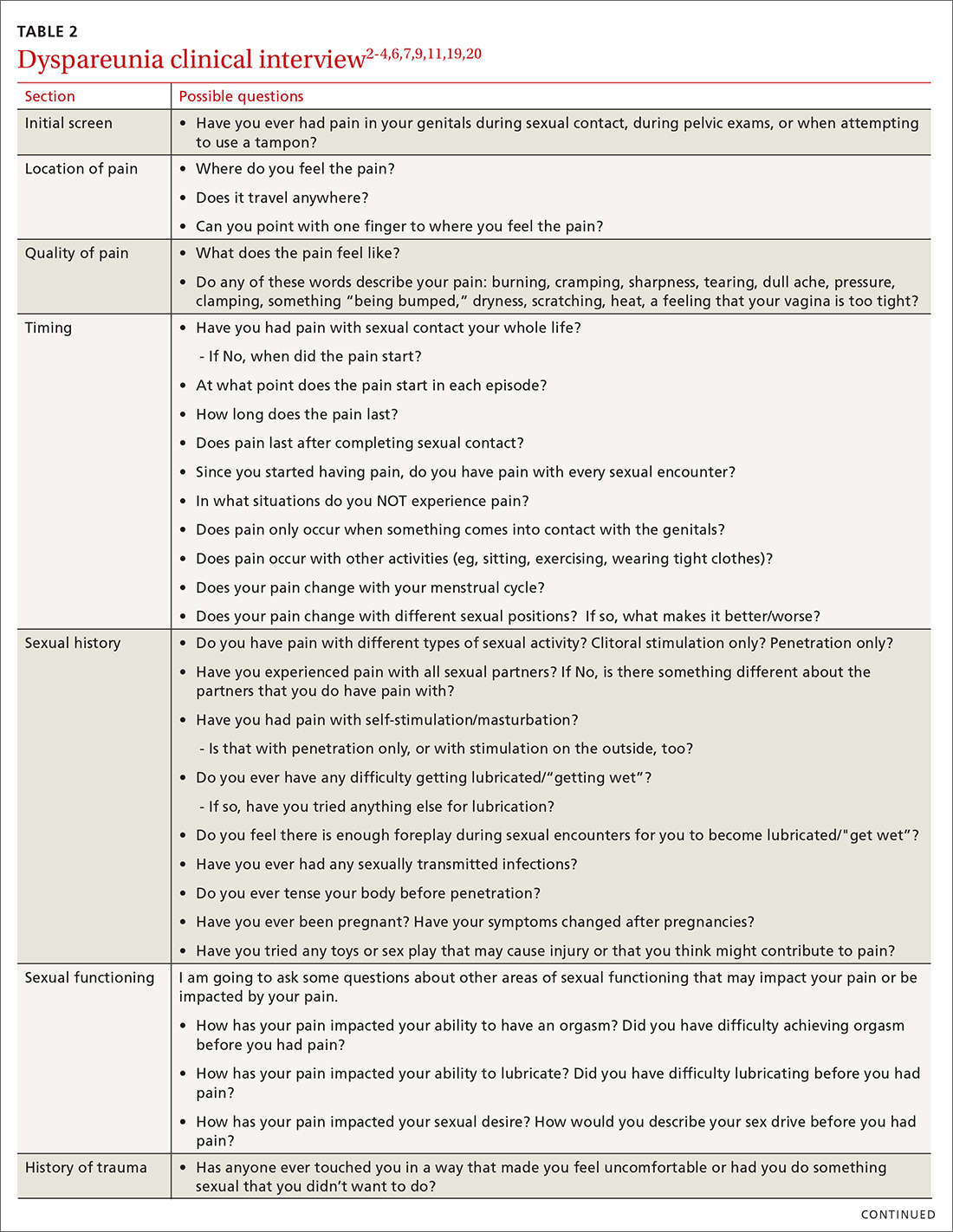
Items to include in the clinical interview
Given the range of possible causes of dyspareunia and its contributing factors and symptoms, a thorough clinical interview is essential. Begin with a review of the patient’s complete medical and surgical history to identify possible known contributors to genital pain.4 Pregnancy history is of particular importance as the prevalence of postpartum dyspareunia is 35%, with risk being greater for patients who experienced dyspareunia symptoms before pregnancy.22

Knowing the location and quality of pain is important for differentiating between possible diagnoses, as is specifying dyspareunia as lifelong or acquired, superficial or deep, and primary or secondary.1-4,6 Confirm the specific location(s) of pain—eg, at the introitus, in the vestibule, on the labia, in the perineum, or near the clitoris.2,4,6 A diagram or model may be needed to help patients to localize pain.4
To help narrow the differential, include the following elements in your assessment: pain quality, timing (eg, initial onset, episode onset, episode duration, situational triggers), alleviating factors, symptoms in surrounding structures (eg, bladder, bowel, muscles, bones), sexual history, other areas of sexual functioning, history of psychological trauma, relationship effects, and mental health (TABLE 22-4,6,7,9,11,19,20 and Table 323-28). Screening for a history of sexual trauma is particularly important, as a recent systematic review and meta-analysis found that women with a history of sexual assault had a 42% higher risk of gynecologic problems overall, a 74% higher risk of dyspareunia, and a 71% higher risk of vaginismus than women without a history of sexual assault.29 Using measures such as the Female Sexual Function Index or the McGill Pain Questionnaire can help patients more thoroughly describe their symptoms (TABLE 323-28).3
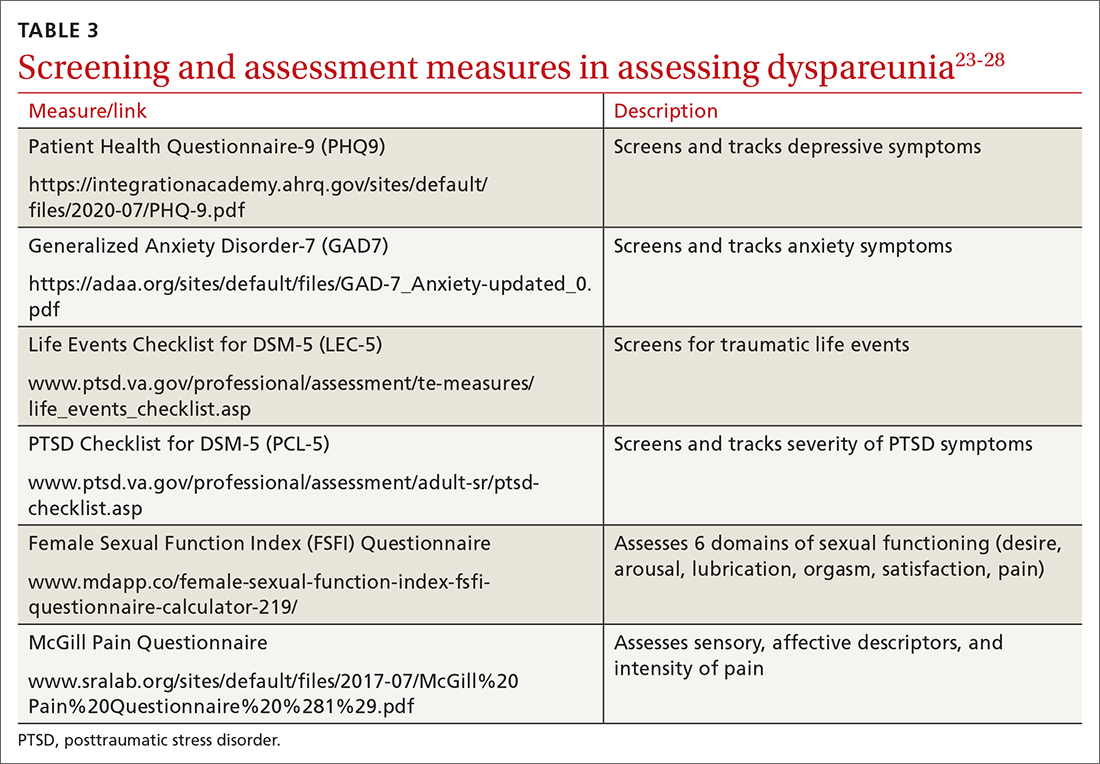
Continue to: Guidelines for the physical exam
Guidelines for the physical exam
Before the exam, ensure the patient has not used any topical genital treatment in the past 2 weeks that may interfere with sensitivity to the exam.4 To decrease patients’ anxiety about the exam, remind them that they can stop the exam at any time.7 Also consider offering the use of a mirror to better pinpoint the location of pain, and to possibly help the patient learn more about her anatomy.2,7
Begin the exam by palpating surrounding areas that may be involved in pain, including the abdomen and musculoskeletal features.3,6,19 Next visually inspect the external genitalia for lesions, abrasions, discoloration, erythema, or other abnormal findings.2,3,6 Ask the patient for permission before contacting the genitals. Because the labia may be a site of pain, apply gentle pressure in retracting it to fully examine the vestibule.6,7 Contraction of the pelvic floor muscles during approach or initial palpation could signal possible vaginismus.4
After visual inspection of external genitalia, use a cotton swab to map the vulva and vestibule in a clockwise fashion to precisely identify any painful locations.2-4,6 If the patient’s history of pain has been intermittent, it’s possible that the cotton swab will not elicit pain on the day of the initial exam, but it may on other days.4
Begin the internal exam by inserting a single finger into the first inch of the vagina and have the patient squeeze and release to assess tenderness, muscle tightness, and control.2,6 Advance the finger further into the vagina and palpate clockwise, examining the levator muscles, obturator muscles, rectum, urethra, and bladder for abnormal tightness or reproduction of pain.2,4,6 Complete a bimanual exam to evaluate the pelvic organs and adnexa.2,4 If indicated, a more thorough evaluation of pelvic floor musculature can be performed by a physical therapist or gynecologist who specializes in pelvic pain.2-4
If the patient consents to further evaluation, consider using a small speculum, advanced slowly, for further internal examination, noting any lesions, abrasions, discharge, ectropion, or tenderness.2-4,7 A rectal exam may also be needed in cases of deep dyspareunia.6 Initial work-up may include a potassium hydroxide wet prep, sexually transmitted infection testing, and pelvic ultrasound.2,4 In some cases, laparoscopy or biopsy may be needed.2,4
Treatments for common causes
Treatment often begins with education about anatomy, to help patients communicate about symptoms and engage more fully in their care.3 Additional education may be needed on genital functioning and the necessity of adequate stimulation and lubrication prior to penetration.1,2,9-11 A discussion of treatments for the wide range of possible causes of dyspareunia is outside the scope of this article. However, some basic behavioral changes may help patients address some of the more common contributing factors.
For example, if vaginal infection is suspected, advise patients to discontinue the use of harsh soaps, known vaginal irritants (eg, perfumed products, bath additives), and douches.3 Recommend using only preservative- and alcohol-free lubricants for sexual contact, and avoiding lubricants with added functions (eg, warming).3 It’s worth noting that avoidance of tight clothing and thong underwear due to possible risk for infections may not be necessary. A recent study found that women who frequently wore thong underwear (more than half of the time) were no more likely to develop urinary tract infections, yeast vaginitis, or bacterial vaginosis than those who avoid such items.30 However, noncotton underwear fabric, rather than tightness, was associated with yeast vaginitis30; therefore, patients may want to consider using only breathable underwear.3
Continue to: Medication
Medication. Medication may be used to treat the underlying contributing conditions or the symptom of pain directly. Some common options are particularly important for patients whose dyspareunia does not have an identifiable cause. These medications include anti-inflammatory agents, topical anesthetics, tricyclic antidepressants, and hormonal treatments.2-4 Since effectiveness varies based on subtypes of pain, select a medication according to the location, timing, and hypothesized mechanism of pain.3,31,32
Medication for deep pain. A meta-analysis and systematic review found that patients with some types of chronic pelvic pain with pain deep in the vagina or pelvis experienced greater than 50% reduction in pain using medroxyprogesterone acetate compared with placebo.33 Other treatments for deep pain depend on physical exam findings.
Medication for superficial pain. Many remedies have been tried, with at least 26 different treatments for vulvodynia pain alone.16 Only some of these treatments have supporting evidence. For patients with vulvar pain, an intent-to-treat RCT found that patients using a topical steroid experienced a 23% reduction in pain from pre-treatment to 6-month follow-up.32
Surgery is also effective for vulvar pain.34,35 For provoked vestibulodynia (in which pain is localized to the vestibule and triggered by contact with the vulva), or vulvar vestibulitis, RCTs have found that vestibulectomy has stronger effects on pain than other treatments,31,35 with a 53% reduction in pain during intercourse and a 70% reduction in vestibular pain overall.35 However, while vestibulectomy is effective for provoked vestibulodynia, it is not recommended for generalized vulvodynia, in which pain is diffuse across the vulva and occurs without vulvar contact.34
Unsupported treatments. A number of other treatments have not yet been found effective. Although lidocaine for vulvar pain is often used, RCTs have not found any significant reduction in symptoms, and a double-blind RCT found that lidocaine ointment actually performed worse than placebo.31,34 Similarly, oral tricyclics have not been found to decrease vulvar pain more than placebo in double-blind studies.31,34 Furthermore, a meta-analysis of RCTs comparing treatments with placebo for vestibular pain found no significant decrease in dyspareunia for topical conjugated estrogen, topical lidocaine, oral desipramine, oral desipramine with topical lidocaine, laser therapy, or transcranial direct current.32
Tx risks to consider. Risks and benefits of dyspareunia treatment options should be thoroughly weighed and discussed with the patient.2-4 Vestibulectomy, despite reducing pain for many patients, has led to increased pain for 9% of patients who underwent the procedure.35 Topical treatments may lead to allergic reactions, inflammation, and worsening of symptoms,4 and hormonal treatments have been found to increase the risk of weight gain and bloating and are not appropriate for patients trying to conceive.33
Coordinate care with other providers
While medications and surgery can reduce pain, they have not been shown to improve other aspects of sexual functioning such as sexual satisfaction, frequency of sexual intercourse, or overall sense of sexual functioning.35 Additionally, pain reduction does not address muscle tension, anxiety, self-esteem, and relationship problems. As a result, a multidisciplinary approach is generally needed.3,4,32,33
Continue to: Physical therapists
Physical therapists. Pelvic floor physical therapists are often members of the dyspareunia treatment team and can provide a thorough evaluation and treatment of pelvic floor disorders.2-4 An RCT with intent-to-treat analysis found that pain was reduced by 71% following pelvic floor physical therapy.36 Another RCT found that 90% of patients reported a clinically meaningful decrease in pain with pelvic floor physical therapy.37 In addition to addressing pain, pelvic floor physical therapy has also been found to improve sexual functioning, sexual satisfaction, distress, and patient perception of improvement.34,36,37
Behavioral health specialists. Psychotherapists, especially those trained in sex therapy, couples therapy, or cognitive behavioral therapy (CBT), are also typically on the treatment team. Multiple RCTs have found evidence of CBT’s effectiveness in the direct treatment of dyspareunia pain. Bergeron et al35 found a 37.5% reduction in vulvar vestibulitis pain intensity during intercourse after patients completed group CBT. Another intent-to-treat RCT found that patients receiving CBT experienced more pain reduction (~ 30%) than patients who were treated with a topical steroid.38
In addition to having a direct impact on pain, CBT has also been found to have a clinically and statistically significant positive impact on other aspects of sexual experience, such as overall sexuality, self-efficacy, overall sexual functioning, frequency of intercourse, and catastrophizing.34,38 A recent meta-analysis of RCTs found that about 80% of vaginismus patients were able to achieve penetrative intercourse after treatment with behavioral sex therapy or CBT.39 This success rate was not exceeded by physical or surgical treatments.39
When PTSD is thought to be a contributing factor, trauma therapy will likely be needed in addition to treatments for dyspareunia. First-line treatments for PTSD include cognitive processing therapy, prolonged exposure, trauma-focused CBT, and cognitive therapy.40
Psychotherapists can also help patients reduce anxiety, reintroduce sexual contact without triggering pain or anxiety, address emotional and self-esteem effects of dyspareunia, address relationship issues, and refocus sexual encounters on pleasure rather than pain avoidance.2-4 Despite patient reports of high treatment satisfaction following therapy,38 many patients may initially lack confidence in psychotherapy as a treatment for pain35 and may need to be educated on its effectiveness and multidimensional benefits.
Gynecologists. Often a gynecologist with specialization in pelvic pain is an essential member of the team for diagnostic clarification, recommendation of treatment options, and performance of more advanced treatments.2,3 If pain has become chronic, the patient may also benefit from a pain management team and support groups.2,3
Follow-up steps
Patients who screen negative for dyspareunia should be re-screened periodically. Continue to assess patients diagnosed with dyspareunia for vaginismus symptoms (if they are not initially present) to ensure that the treatment plan is appropriately adjusted. Once treatment has begun, ask about adverse effects and confidence in the treatment plan to minimize negative impacts on treatment adherence and to anticipate a need for a change in the treatment approach.31,35 In addition to tracking treatment effects on pain, continue to assess for patient-centered outcomes such as emotional functioning, self-esteem, and sexual and relationship satisfaction.34 The Female Sexual Function Index can be a useful tool to track symptoms.27,34
Finally, patients who do not experience sufficient improvement in symptoms and functioning with initial treatment may need continued support and encouragement. Given the broad range of contributing factors and the high number of potential treatments, patients may find hope in learning that multiple other treatment options may be available.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family and Community Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC 663, Chicago, IL 60612; [email protected]
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. American Psychiatric Publishing; 2013.
2. Seehusen DA, Baird DC, Bode DV. Dyspareunia in women. Am Fam Phys. 2014;90:465-470.
3. Sorensen J, Bautista KE, Lamvu G, et al. Evaluation and treatment of female sexual pain: a clinical review. Cureus. 2018;10:e2379.
4. MacNeill C. Dyspareunia. Obstet Gynecol Clin North Am. 2006;33:565-77.
5. Latthe P, Latthe M, Say L, et al. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6:177.
6. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
7. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
8. Ünlü Z, Yentur A, Çakil N. Pudendal nerve neuropathy: An unknown-rare cause of pelvic pain. Arch Rheumatol. 2016;31:102-103.
9. Dewitte M, Borg C, Lowenstein L. A psychosocial approach to female genital pain. Nat Rev Urol. 2018;15:25-41.
10. Masters WH, Johnson VE. Human Sexual Inadequacy. 1st ed. Little, Brown; 1970.
11. Rathus SA, Nevid JS, Fichner-Rathus L. Human Sexuality in a World of Diversity. 5th ed. Allyn and Bacon; 2002.
12. Bailey BE, Freedenfeld RN, Kiser RS, et al. Lifetime physical and sexual abuse in chronic pain patients: psychosocial correlates and treatment outcomes. Disabil Rehabil. 2003;25:331-342.
13. Yehuda R, Lehrner A, Rosenbaum TY. PTSD and sexual dysfunction in men and women. J Sex Med. 2015;12:1107-1119.
14. Postma R, Bicanic I, van der Vaart H, et al. Pelvic floor muscle problems mediate sexual problems in young adult rape victims. J Sex Med. 2013;10:1978-1987.
15. Binik YM, Bergeron S, Khalifé S. Dyspareunia and vaginismus: so-called sexual pain. In: Leiblum SR, ed. 4th ed. Principles and Practice of Sex Therapy. The Guilford Press; 2007:124-156.
16. Ayling K, Ussher JM. “If sex hurts, am I still a woman?” The subjective experience of vulvodynia in hetero-sexual women. Arch Sex Behav. 2008;37:294-304.
17. Pazmany E, Bergeron S, Van Oudenhove L, et al. Body image and genital self-image in pre-menopausal women with dyspareunia. Arch Sex Behav. 2013;42:999-1010.
18. Maillé DL, Bergeron S, Lambert B. Body image in women with primary and secondary provoked vestibulodynia: a controlled study. J Sex Med. 2015;12:505-515.
19. Ryan L, Hawton K. Female dyspareunia. BMJ. 2004;328:1357.
20. Waldura JF, Arora I, Randall AM, et al. Fifty shades of stigma: exploring the health care experiences of kink-oriented patients. J Sex Med. 2016;13:1918-1929.
21. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
22. Banaei M, Kariman N, Ozgoli G, et al. Prevalence of postpartum dyspareunia: a systematic review and meta-analysis. Int J Gynaecol Obstet. 2021;153:14-24.
23. Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509-515.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. U.S. Department of Veterans Affairs. PTSD: National Center for PTSD. Life events checklist for DSM-5 (LEC-5). Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp
26. Weathers FW, Litz BT, Keane TM, et al. The PTSD checklist for DSM-5 (PCL-5). 2013. Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
27. Rosen R, Brown C, Heiman J, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
28. Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191-197.
29. Hassam T, Kelso E, Chowdary P, et al. Sexual assault as a risk factor for gynaecological morbidity: an exploratory systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;255:222-230.
30. Hamlin AA, Sheeder J, Muffly TM. Brief versus thong hygiene in obstetrics and gynecology (B-THONG): a survey study. J Obstet Gynaecol Res. 2019;45:1190-1196.
31. Foster DC, Kotok MB, Huang LS, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116:583-593.
32. Pérez-López FR, Bueno-Notivol J, Hernandez AV, et al. Systematic review and meta-analysis of the effects of treatment modalities for vestibulodynia in women. Eur J Contracept Reprod Health Care. 2019;24:337-346.
33. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
34. Goldstein AT, Pukall CF, Brown C, et al. Vulvodynia: assessment and treatment. J Sex Med. 2016;13:572-590.
35. Bergeron S, Binik YM, Khalifé S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297-306.
36. Schvartzman R, Schvartzman L, Ferreira CF, et al. Physical therapy intervention for women with dyspareunia: a randomized clinical trial. J Sex Marital Ther. 2019;45:378-394.
37. Morin M, Dumoulin C, Bergeron S, et al. Multimodal physical therapy versus topical lidocaine for provoked vestibulodynia: a multicenter, randomized trial. Am J Obstet Gynecol. 2021;224:189.e1-189.e12.
38. Bergeron S, Khalifé S, Dupuis M-J, et al. A randomized clinical trial comparing group cognitive-behavioral therapy and a topical steroid for women with dyspareunia. J Consult Clin Psychol. 2016;84:259-268.
39. Maseroli E, Scavello I, Rastrelli G, et al. Outcome of medical and psychosexual interventions for vaginismus: a systematic review and meta-analysis. J Sex Med. 2018;15:1752-1764.
40. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. 2017. Accessed February 3, 2022. www.apa.org/ptsd-guideline/ptsd.pdf
Dyspareunia is persistent or recurrent pain before, during, or after sexual contact and is not limited to cisgender individuals or vaginal intercourse.1-3 With a prevalence as high as 45% in the United States,2-5 it is one of the most common complaints in gynecologic practices.5,6
Causes and contributing factors
There are many possible causes of dyspareunia.2,4,6 While some patients have a single cause, most cases are complex, with multiple overlapping causes and maintaining factors.4,6 Identifying each contributing factor can help you appropriately address all components.
Physical conditions. The range of physical contributors to dyspareunia includes inflammatory processes, structural abnormalities, musculoskeletal dysfunctions, pelvic organ disorders, injuries, iatrogenic effects, infections, allergic reactions, sensitization, hormonal changes, medication effects, adhesions, autoimmune disorders, and other pain syndromes (TABLE 12-4,6-11).

Inadequate arousal. One of the primary causes of pain during vaginal penetration is inadequate arousal and lubrication.1,2,9-11 Arousal is the phase of the sexual response cycle that leads to genital tumescence and prepares the genitals for sexual contact through penile/clitoral erection, vaginal engorgement, and lubrication, which prevents pain and enhances pleasurable sensation.9-11
While some physical conditions can lead to an inability to lubricate, the most common causes of inadequate lubrication are psychosocial-behavioral, wherein patients have the same physical ability to lubricate as patients without genital pain but do not progress through the arousal phase.9-11 Behavioral factors such as inadequate or ineffective foreplay can fail to produce engorgement and lubrication, while psychosocial factors such as low attraction to partner, relationship stressors, anxiety, or low self-esteem can have an inhibitory effect on sexual arousal.1,2,9-11 Psychosocial and behavioral factors may also be maintaining factors or consequences of dyspareunia, and need to be assessed and treated.1,2,9-11
Psychological trauma. Exposure to psychological traumas and the development of posttraumatic stress disorder (PTSD) have been linked with the development of pain disorders in general and dyspareunia specifically. Most patients seeking treatment for chronic pain disorders have a history of physical or sexual abuse.12 Changes in physiologic processes (eg, neurochemical, endocrine) that occur with PTSD interfere with the sexual response cycle, and sexual traumas specifically have been linked with pelvic floor dysfunction.13,14 Additionally, when PTSD is caused by a sexual trauma, even consensual sexual encounters can trigger flashbacks, intrusive memories, hyperarousal, and muscle tension that interfere with the sexual response cycle and contribute to genital pain.13
Vaginismus is both a physiologic and psychological contributor to dyspareunia.1,2,4 Patients experiencing pain can develop anxiety about repeated pain and involuntarily contract their pelvic muscles, thereby creating more pain, increasing anxiety, decreasing lubrication, and causing pelvic floor dysfunction.1-4,6 Consequently, all patients with dyspareunia should be assessed and continually monitored for symptoms of vaginismus.
Continue to: Anxiety
Anxiety. As with other pain disorders, anxiety develops around pain triggers.10,15 When expecting sexual activity, patients can experience extreme worry and panic attacks.10,15,16 The distress of sexual encounters can interfere with physiologic arousal and sexual desire, impacting all phases of the sexual response cycle.1,2
Relationship issues. Difficulty engaging in or avoidance of sexual activity can interfere with romantic relationships.2,10,16 Severe pain or vaginismus contractions can prevent penetration, leading to unconsummated marriages and an inability to conceive through intercourse.10 The distress surrounding sexual encounters can precipitate erectile dysfunction in male partners, or partners may continue to demand sexual encounters despite the patient’s pain, further impacting the relationship and heightening sexual distress.10 These stressors have led to relationships ending, patients reluctantly agreeing to nonmonogamy to appease their partners, and patients avoiding relationships altogether.10,16
Devalued self-image. Difficulties with sexuality and relationships impact the self-image of patients with dyspareunia. Diminished self-image may include feeling “inadequate” as a woman and as a sexual partner, or feeling like a “failure.”16 Women with dyspareunia often have more distress related to their body image, physical appearance, and genital self-image than do women without genital pain.17 Feeling resentment toward their body, or feeling “ugly,” embarrassed, shamed, “broken,” and “useless” also contribute to increased depressive symptoms found in patients with dyspareunia.16,18
Making the diagnosis
Most patients do not report symptoms unless directly asked2,7; therefore, it is recommended that all patients be screened as a part of an initial intake and before any genital exam (TABLE 22-4,6,7,9,11,19,20).4,7,21 If this screen is positive, a separate appointment may be needed for a thorough evaluation and before any attempt is made at a genital exam.4,7

Items to include in the clinical interview
Given the range of possible causes of dyspareunia and its contributing factors and symptoms, a thorough clinical interview is essential. Begin with a review of the patient’s complete medical and surgical history to identify possible known contributors to genital pain.4 Pregnancy history is of particular importance as the prevalence of postpartum dyspareunia is 35%, with risk being greater for patients who experienced dyspareunia symptoms before pregnancy.22

Knowing the location and quality of pain is important for differentiating between possible diagnoses, as is specifying dyspareunia as lifelong or acquired, superficial or deep, and primary or secondary.1-4,6 Confirm the specific location(s) of pain—eg, at the introitus, in the vestibule, on the labia, in the perineum, or near the clitoris.2,4,6 A diagram or model may be needed to help patients to localize pain.4
To help narrow the differential, include the following elements in your assessment: pain quality, timing (eg, initial onset, episode onset, episode duration, situational triggers), alleviating factors, symptoms in surrounding structures (eg, bladder, bowel, muscles, bones), sexual history, other areas of sexual functioning, history of psychological trauma, relationship effects, and mental health (TABLE 22-4,6,7,9,11,19,20 and Table 323-28). Screening for a history of sexual trauma is particularly important, as a recent systematic review and meta-analysis found that women with a history of sexual assault had a 42% higher risk of gynecologic problems overall, a 74% higher risk of dyspareunia, and a 71% higher risk of vaginismus than women without a history of sexual assault.29 Using measures such as the Female Sexual Function Index or the McGill Pain Questionnaire can help patients more thoroughly describe their symptoms (TABLE 323-28).3

Continue to: Guidelines for the physical exam
Guidelines for the physical exam
Before the exam, ensure the patient has not used any topical genital treatment in the past 2 weeks that may interfere with sensitivity to the exam.4 To decrease patients’ anxiety about the exam, remind them that they can stop the exam at any time.7 Also consider offering the use of a mirror to better pinpoint the location of pain, and to possibly help the patient learn more about her anatomy.2,7
Begin the exam by palpating surrounding areas that may be involved in pain, including the abdomen and musculoskeletal features.3,6,19 Next visually inspect the external genitalia for lesions, abrasions, discoloration, erythema, or other abnormal findings.2,3,6 Ask the patient for permission before contacting the genitals. Because the labia may be a site of pain, apply gentle pressure in retracting it to fully examine the vestibule.6,7 Contraction of the pelvic floor muscles during approach or initial palpation could signal possible vaginismus.4
After visual inspection of external genitalia, use a cotton swab to map the vulva and vestibule in a clockwise fashion to precisely identify any painful locations.2-4,6 If the patient’s history of pain has been intermittent, it’s possible that the cotton swab will not elicit pain on the day of the initial exam, but it may on other days.4
Begin the internal exam by inserting a single finger into the first inch of the vagina and have the patient squeeze and release to assess tenderness, muscle tightness, and control.2,6 Advance the finger further into the vagina and palpate clockwise, examining the levator muscles, obturator muscles, rectum, urethra, and bladder for abnormal tightness or reproduction of pain.2,4,6 Complete a bimanual exam to evaluate the pelvic organs and adnexa.2,4 If indicated, a more thorough evaluation of pelvic floor musculature can be performed by a physical therapist or gynecologist who specializes in pelvic pain.2-4
If the patient consents to further evaluation, consider using a small speculum, advanced slowly, for further internal examination, noting any lesions, abrasions, discharge, ectropion, or tenderness.2-4,7 A rectal exam may also be needed in cases of deep dyspareunia.6 Initial work-up may include a potassium hydroxide wet prep, sexually transmitted infection testing, and pelvic ultrasound.2,4 In some cases, laparoscopy or biopsy may be needed.2,4
Treatments for common causes
Treatment often begins with education about anatomy, to help patients communicate about symptoms and engage more fully in their care.3 Additional education may be needed on genital functioning and the necessity of adequate stimulation and lubrication prior to penetration.1,2,9-11 A discussion of treatments for the wide range of possible causes of dyspareunia is outside the scope of this article. However, some basic behavioral changes may help patients address some of the more common contributing factors.
For example, if vaginal infection is suspected, advise patients to discontinue the use of harsh soaps, known vaginal irritants (eg, perfumed products, bath additives), and douches.3 Recommend using only preservative- and alcohol-free lubricants for sexual contact, and avoiding lubricants with added functions (eg, warming).3 It’s worth noting that avoidance of tight clothing and thong underwear due to possible risk for infections may not be necessary. A recent study found that women who frequently wore thong underwear (more than half of the time) were no more likely to develop urinary tract infections, yeast vaginitis, or bacterial vaginosis than those who avoid such items.30 However, noncotton underwear fabric, rather than tightness, was associated with yeast vaginitis30; therefore, patients may want to consider using only breathable underwear.3
Continue to: Medication
Medication. Medication may be used to treat the underlying contributing conditions or the symptom of pain directly. Some common options are particularly important for patients whose dyspareunia does not have an identifiable cause. These medications include anti-inflammatory agents, topical anesthetics, tricyclic antidepressants, and hormonal treatments.2-4 Since effectiveness varies based on subtypes of pain, select a medication according to the location, timing, and hypothesized mechanism of pain.3,31,32
Medication for deep pain. A meta-analysis and systematic review found that patients with some types of chronic pelvic pain with pain deep in the vagina or pelvis experienced greater than 50% reduction in pain using medroxyprogesterone acetate compared with placebo.33 Other treatments for deep pain depend on physical exam findings.
Medication for superficial pain. Many remedies have been tried, with at least 26 different treatments for vulvodynia pain alone.16 Only some of these treatments have supporting evidence. For patients with vulvar pain, an intent-to-treat RCT found that patients using a topical steroid experienced a 23% reduction in pain from pre-treatment to 6-month follow-up.32
Surgery is also effective for vulvar pain.34,35 For provoked vestibulodynia (in which pain is localized to the vestibule and triggered by contact with the vulva), or vulvar vestibulitis, RCTs have found that vestibulectomy has stronger effects on pain than other treatments,31,35 with a 53% reduction in pain during intercourse and a 70% reduction in vestibular pain overall.35 However, while vestibulectomy is effective for provoked vestibulodynia, it is not recommended for generalized vulvodynia, in which pain is diffuse across the vulva and occurs without vulvar contact.34
Unsupported treatments. A number of other treatments have not yet been found effective. Although lidocaine for vulvar pain is often used, RCTs have not found any significant reduction in symptoms, and a double-blind RCT found that lidocaine ointment actually performed worse than placebo.31,34 Similarly, oral tricyclics have not been found to decrease vulvar pain more than placebo in double-blind studies.31,34 Furthermore, a meta-analysis of RCTs comparing treatments with placebo for vestibular pain found no significant decrease in dyspareunia for topical conjugated estrogen, topical lidocaine, oral desipramine, oral desipramine with topical lidocaine, laser therapy, or transcranial direct current.32
Tx risks to consider. Risks and benefits of dyspareunia treatment options should be thoroughly weighed and discussed with the patient.2-4 Vestibulectomy, despite reducing pain for many patients, has led to increased pain for 9% of patients who underwent the procedure.35 Topical treatments may lead to allergic reactions, inflammation, and worsening of symptoms,4 and hormonal treatments have been found to increase the risk of weight gain and bloating and are not appropriate for patients trying to conceive.33
Coordinate care with other providers
While medications and surgery can reduce pain, they have not been shown to improve other aspects of sexual functioning such as sexual satisfaction, frequency of sexual intercourse, or overall sense of sexual functioning.35 Additionally, pain reduction does not address muscle tension, anxiety, self-esteem, and relationship problems. As a result, a multidisciplinary approach is generally needed.3,4,32,33
Continue to: Physical therapists
Physical therapists. Pelvic floor physical therapists are often members of the dyspareunia treatment team and can provide a thorough evaluation and treatment of pelvic floor disorders.2-4 An RCT with intent-to-treat analysis found that pain was reduced by 71% following pelvic floor physical therapy.36 Another RCT found that 90% of patients reported a clinically meaningful decrease in pain with pelvic floor physical therapy.37 In addition to addressing pain, pelvic floor physical therapy has also been found to improve sexual functioning, sexual satisfaction, distress, and patient perception of improvement.34,36,37
Behavioral health specialists. Psychotherapists, especially those trained in sex therapy, couples therapy, or cognitive behavioral therapy (CBT), are also typically on the treatment team. Multiple RCTs have found evidence of CBT’s effectiveness in the direct treatment of dyspareunia pain. Bergeron et al35 found a 37.5% reduction in vulvar vestibulitis pain intensity during intercourse after patients completed group CBT. Another intent-to-treat RCT found that patients receiving CBT experienced more pain reduction (~ 30%) than patients who were treated with a topical steroid.38
In addition to having a direct impact on pain, CBT has also been found to have a clinically and statistically significant positive impact on other aspects of sexual experience, such as overall sexuality, self-efficacy, overall sexual functioning, frequency of intercourse, and catastrophizing.34,38 A recent meta-analysis of RCTs found that about 80% of vaginismus patients were able to achieve penetrative intercourse after treatment with behavioral sex therapy or CBT.39 This success rate was not exceeded by physical or surgical treatments.39
When PTSD is thought to be a contributing factor, trauma therapy will likely be needed in addition to treatments for dyspareunia. First-line treatments for PTSD include cognitive processing therapy, prolonged exposure, trauma-focused CBT, and cognitive therapy.40
Psychotherapists can also help patients reduce anxiety, reintroduce sexual contact without triggering pain or anxiety, address emotional and self-esteem effects of dyspareunia, address relationship issues, and refocus sexual encounters on pleasure rather than pain avoidance.2-4 Despite patient reports of high treatment satisfaction following therapy,38 many patients may initially lack confidence in psychotherapy as a treatment for pain35 and may need to be educated on its effectiveness and multidimensional benefits.
Gynecologists. Often a gynecologist with specialization in pelvic pain is an essential member of the team for diagnostic clarification, recommendation of treatment options, and performance of more advanced treatments.2,3 If pain has become chronic, the patient may also benefit from a pain management team and support groups.2,3
Follow-up steps
Patients who screen negative for dyspareunia should be re-screened periodically. Continue to assess patients diagnosed with dyspareunia for vaginismus symptoms (if they are not initially present) to ensure that the treatment plan is appropriately adjusted. Once treatment has begun, ask about adverse effects and confidence in the treatment plan to minimize negative impacts on treatment adherence and to anticipate a need for a change in the treatment approach.31,35 In addition to tracking treatment effects on pain, continue to assess for patient-centered outcomes such as emotional functioning, self-esteem, and sexual and relationship satisfaction.34 The Female Sexual Function Index can be a useful tool to track symptoms.27,34
Finally, patients who do not experience sufficient improvement in symptoms and functioning with initial treatment may need continued support and encouragement. Given the broad range of contributing factors and the high number of potential treatments, patients may find hope in learning that multiple other treatment options may be available.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family and Community Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC 663, Chicago, IL 60612; [email protected]
Dyspareunia is persistent or recurrent pain before, during, or after sexual contact and is not limited to cisgender individuals or vaginal intercourse.1-3 With a prevalence as high as 45% in the United States,2-5 it is one of the most common complaints in gynecologic practices.5,6
Causes and contributing factors
There are many possible causes of dyspareunia.2,4,6 While some patients have a single cause, most cases are complex, with multiple overlapping causes and maintaining factors.4,6 Identifying each contributing factor can help you appropriately address all components.
Physical conditions. The range of physical contributors to dyspareunia includes inflammatory processes, structural abnormalities, musculoskeletal dysfunctions, pelvic organ disorders, injuries, iatrogenic effects, infections, allergic reactions, sensitization, hormonal changes, medication effects, adhesions, autoimmune disorders, and other pain syndromes (TABLE 12-4,6-11).

Inadequate arousal. One of the primary causes of pain during vaginal penetration is inadequate arousal and lubrication.1,2,9-11 Arousal is the phase of the sexual response cycle that leads to genital tumescence and prepares the genitals for sexual contact through penile/clitoral erection, vaginal engorgement, and lubrication, which prevents pain and enhances pleasurable sensation.9-11
While some physical conditions can lead to an inability to lubricate, the most common causes of inadequate lubrication are psychosocial-behavioral, wherein patients have the same physical ability to lubricate as patients without genital pain but do not progress through the arousal phase.9-11 Behavioral factors such as inadequate or ineffective foreplay can fail to produce engorgement and lubrication, while psychosocial factors such as low attraction to partner, relationship stressors, anxiety, or low self-esteem can have an inhibitory effect on sexual arousal.1,2,9-11 Psychosocial and behavioral factors may also be maintaining factors or consequences of dyspareunia, and need to be assessed and treated.1,2,9-11
Psychological trauma. Exposure to psychological traumas and the development of posttraumatic stress disorder (PTSD) have been linked with the development of pain disorders in general and dyspareunia specifically. Most patients seeking treatment for chronic pain disorders have a history of physical or sexual abuse.12 Changes in physiologic processes (eg, neurochemical, endocrine) that occur with PTSD interfere with the sexual response cycle, and sexual traumas specifically have been linked with pelvic floor dysfunction.13,14 Additionally, when PTSD is caused by a sexual trauma, even consensual sexual encounters can trigger flashbacks, intrusive memories, hyperarousal, and muscle tension that interfere with the sexual response cycle and contribute to genital pain.13
Vaginismus is both a physiologic and psychological contributor to dyspareunia.1,2,4 Patients experiencing pain can develop anxiety about repeated pain and involuntarily contract their pelvic muscles, thereby creating more pain, increasing anxiety, decreasing lubrication, and causing pelvic floor dysfunction.1-4,6 Consequently, all patients with dyspareunia should be assessed and continually monitored for symptoms of vaginismus.
Continue to: Anxiety
Anxiety. As with other pain disorders, anxiety develops around pain triggers.10,15 When expecting sexual activity, patients can experience extreme worry and panic attacks.10,15,16 The distress of sexual encounters can interfere with physiologic arousal and sexual desire, impacting all phases of the sexual response cycle.1,2
Relationship issues. Difficulty engaging in or avoidance of sexual activity can interfere with romantic relationships.2,10,16 Severe pain or vaginismus contractions can prevent penetration, leading to unconsummated marriages and an inability to conceive through intercourse.10 The distress surrounding sexual encounters can precipitate erectile dysfunction in male partners, or partners may continue to demand sexual encounters despite the patient’s pain, further impacting the relationship and heightening sexual distress.10 These stressors have led to relationships ending, patients reluctantly agreeing to nonmonogamy to appease their partners, and patients avoiding relationships altogether.10,16
Devalued self-image. Difficulties with sexuality and relationships impact the self-image of patients with dyspareunia. Diminished self-image may include feeling “inadequate” as a woman and as a sexual partner, or feeling like a “failure.”16 Women with dyspareunia often have more distress related to their body image, physical appearance, and genital self-image than do women without genital pain.17 Feeling resentment toward their body, or feeling “ugly,” embarrassed, shamed, “broken,” and “useless” also contribute to increased depressive symptoms found in patients with dyspareunia.16,18
Making the diagnosis
Most patients do not report symptoms unless directly asked2,7; therefore, it is recommended that all patients be screened as a part of an initial intake and before any genital exam (TABLE 22-4,6,7,9,11,19,20).4,7,21 If this screen is positive, a separate appointment may be needed for a thorough evaluation and before any attempt is made at a genital exam.4,7

Items to include in the clinical interview
Given the range of possible causes of dyspareunia and its contributing factors and symptoms, a thorough clinical interview is essential. Begin with a review of the patient’s complete medical and surgical history to identify possible known contributors to genital pain.4 Pregnancy history is of particular importance as the prevalence of postpartum dyspareunia is 35%, with risk being greater for patients who experienced dyspareunia symptoms before pregnancy.22

Knowing the location and quality of pain is important for differentiating between possible diagnoses, as is specifying dyspareunia as lifelong or acquired, superficial or deep, and primary or secondary.1-4,6 Confirm the specific location(s) of pain—eg, at the introitus, in the vestibule, on the labia, in the perineum, or near the clitoris.2,4,6 A diagram or model may be needed to help patients to localize pain.4
To help narrow the differential, include the following elements in your assessment: pain quality, timing (eg, initial onset, episode onset, episode duration, situational triggers), alleviating factors, symptoms in surrounding structures (eg, bladder, bowel, muscles, bones), sexual history, other areas of sexual functioning, history of psychological trauma, relationship effects, and mental health (TABLE 22-4,6,7,9,11,19,20 and Table 323-28). Screening for a history of sexual trauma is particularly important, as a recent systematic review and meta-analysis found that women with a history of sexual assault had a 42% higher risk of gynecologic problems overall, a 74% higher risk of dyspareunia, and a 71% higher risk of vaginismus than women without a history of sexual assault.29 Using measures such as the Female Sexual Function Index or the McGill Pain Questionnaire can help patients more thoroughly describe their symptoms (TABLE 323-28).3

Continue to: Guidelines for the physical exam
Guidelines for the physical exam
Before the exam, ensure the patient has not used any topical genital treatment in the past 2 weeks that may interfere with sensitivity to the exam.4 To decrease patients’ anxiety about the exam, remind them that they can stop the exam at any time.7 Also consider offering the use of a mirror to better pinpoint the location of pain, and to possibly help the patient learn more about her anatomy.2,7
Begin the exam by palpating surrounding areas that may be involved in pain, including the abdomen and musculoskeletal features.3,6,19 Next visually inspect the external genitalia for lesions, abrasions, discoloration, erythema, or other abnormal findings.2,3,6 Ask the patient for permission before contacting the genitals. Because the labia may be a site of pain, apply gentle pressure in retracting it to fully examine the vestibule.6,7 Contraction of the pelvic floor muscles during approach or initial palpation could signal possible vaginismus.4
After visual inspection of external genitalia, use a cotton swab to map the vulva and vestibule in a clockwise fashion to precisely identify any painful locations.2-4,6 If the patient’s history of pain has been intermittent, it’s possible that the cotton swab will not elicit pain on the day of the initial exam, but it may on other days.4
Begin the internal exam by inserting a single finger into the first inch of the vagina and have the patient squeeze and release to assess tenderness, muscle tightness, and control.2,6 Advance the finger further into the vagina and palpate clockwise, examining the levator muscles, obturator muscles, rectum, urethra, and bladder for abnormal tightness or reproduction of pain.2,4,6 Complete a bimanual exam to evaluate the pelvic organs and adnexa.2,4 If indicated, a more thorough evaluation of pelvic floor musculature can be performed by a physical therapist or gynecologist who specializes in pelvic pain.2-4
If the patient consents to further evaluation, consider using a small speculum, advanced slowly, for further internal examination, noting any lesions, abrasions, discharge, ectropion, or tenderness.2-4,7 A rectal exam may also be needed in cases of deep dyspareunia.6 Initial work-up may include a potassium hydroxide wet prep, sexually transmitted infection testing, and pelvic ultrasound.2,4 In some cases, laparoscopy or biopsy may be needed.2,4
Treatments for common causes
Treatment often begins with education about anatomy, to help patients communicate about symptoms and engage more fully in their care.3 Additional education may be needed on genital functioning and the necessity of adequate stimulation and lubrication prior to penetration.1,2,9-11 A discussion of treatments for the wide range of possible causes of dyspareunia is outside the scope of this article. However, some basic behavioral changes may help patients address some of the more common contributing factors.
For example, if vaginal infection is suspected, advise patients to discontinue the use of harsh soaps, known vaginal irritants (eg, perfumed products, bath additives), and douches.3 Recommend using only preservative- and alcohol-free lubricants for sexual contact, and avoiding lubricants with added functions (eg, warming).3 It’s worth noting that avoidance of tight clothing and thong underwear due to possible risk for infections may not be necessary. A recent study found that women who frequently wore thong underwear (more than half of the time) were no more likely to develop urinary tract infections, yeast vaginitis, or bacterial vaginosis than those who avoid such items.30 However, noncotton underwear fabric, rather than tightness, was associated with yeast vaginitis30; therefore, patients may want to consider using only breathable underwear.3
Continue to: Medication
Medication. Medication may be used to treat the underlying contributing conditions or the symptom of pain directly. Some common options are particularly important for patients whose dyspareunia does not have an identifiable cause. These medications include anti-inflammatory agents, topical anesthetics, tricyclic antidepressants, and hormonal treatments.2-4 Since effectiveness varies based on subtypes of pain, select a medication according to the location, timing, and hypothesized mechanism of pain.3,31,32
Medication for deep pain. A meta-analysis and systematic review found that patients with some types of chronic pelvic pain with pain deep in the vagina or pelvis experienced greater than 50% reduction in pain using medroxyprogesterone acetate compared with placebo.33 Other treatments for deep pain depend on physical exam findings.
Medication for superficial pain. Many remedies have been tried, with at least 26 different treatments for vulvodynia pain alone.16 Only some of these treatments have supporting evidence. For patients with vulvar pain, an intent-to-treat RCT found that patients using a topical steroid experienced a 23% reduction in pain from pre-treatment to 6-month follow-up.32
Surgery is also effective for vulvar pain.34,35 For provoked vestibulodynia (in which pain is localized to the vestibule and triggered by contact with the vulva), or vulvar vestibulitis, RCTs have found that vestibulectomy has stronger effects on pain than other treatments,31,35 with a 53% reduction in pain during intercourse and a 70% reduction in vestibular pain overall.35 However, while vestibulectomy is effective for provoked vestibulodynia, it is not recommended for generalized vulvodynia, in which pain is diffuse across the vulva and occurs without vulvar contact.34
Unsupported treatments. A number of other treatments have not yet been found effective. Although lidocaine for vulvar pain is often used, RCTs have not found any significant reduction in symptoms, and a double-blind RCT found that lidocaine ointment actually performed worse than placebo.31,34 Similarly, oral tricyclics have not been found to decrease vulvar pain more than placebo in double-blind studies.31,34 Furthermore, a meta-analysis of RCTs comparing treatments with placebo for vestibular pain found no significant decrease in dyspareunia for topical conjugated estrogen, topical lidocaine, oral desipramine, oral desipramine with topical lidocaine, laser therapy, or transcranial direct current.32
Tx risks to consider. Risks and benefits of dyspareunia treatment options should be thoroughly weighed and discussed with the patient.2-4 Vestibulectomy, despite reducing pain for many patients, has led to increased pain for 9% of patients who underwent the procedure.35 Topical treatments may lead to allergic reactions, inflammation, and worsening of symptoms,4 and hormonal treatments have been found to increase the risk of weight gain and bloating and are not appropriate for patients trying to conceive.33
Coordinate care with other providers
While medications and surgery can reduce pain, they have not been shown to improve other aspects of sexual functioning such as sexual satisfaction, frequency of sexual intercourse, or overall sense of sexual functioning.35 Additionally, pain reduction does not address muscle tension, anxiety, self-esteem, and relationship problems. As a result, a multidisciplinary approach is generally needed.3,4,32,33
Continue to: Physical therapists
Physical therapists. Pelvic floor physical therapists are often members of the dyspareunia treatment team and can provide a thorough evaluation and treatment of pelvic floor disorders.2-4 An RCT with intent-to-treat analysis found that pain was reduced by 71% following pelvic floor physical therapy.36 Another RCT found that 90% of patients reported a clinically meaningful decrease in pain with pelvic floor physical therapy.37 In addition to addressing pain, pelvic floor physical therapy has also been found to improve sexual functioning, sexual satisfaction, distress, and patient perception of improvement.34,36,37
Behavioral health specialists. Psychotherapists, especially those trained in sex therapy, couples therapy, or cognitive behavioral therapy (CBT), are also typically on the treatment team. Multiple RCTs have found evidence of CBT’s effectiveness in the direct treatment of dyspareunia pain. Bergeron et al35 found a 37.5% reduction in vulvar vestibulitis pain intensity during intercourse after patients completed group CBT. Another intent-to-treat RCT found that patients receiving CBT experienced more pain reduction (~ 30%) than patients who were treated with a topical steroid.38
In addition to having a direct impact on pain, CBT has also been found to have a clinically and statistically significant positive impact on other aspects of sexual experience, such as overall sexuality, self-efficacy, overall sexual functioning, frequency of intercourse, and catastrophizing.34,38 A recent meta-analysis of RCTs found that about 80% of vaginismus patients were able to achieve penetrative intercourse after treatment with behavioral sex therapy or CBT.39 This success rate was not exceeded by physical or surgical treatments.39
When PTSD is thought to be a contributing factor, trauma therapy will likely be needed in addition to treatments for dyspareunia. First-line treatments for PTSD include cognitive processing therapy, prolonged exposure, trauma-focused CBT, and cognitive therapy.40
Psychotherapists can also help patients reduce anxiety, reintroduce sexual contact without triggering pain or anxiety, address emotional and self-esteem effects of dyspareunia, address relationship issues, and refocus sexual encounters on pleasure rather than pain avoidance.2-4 Despite patient reports of high treatment satisfaction following therapy,38 many patients may initially lack confidence in psychotherapy as a treatment for pain35 and may need to be educated on its effectiveness and multidimensional benefits.
Gynecologists. Often a gynecologist with specialization in pelvic pain is an essential member of the team for diagnostic clarification, recommendation of treatment options, and performance of more advanced treatments.2,3 If pain has become chronic, the patient may also benefit from a pain management team and support groups.2,3
Follow-up steps
Patients who screen negative for dyspareunia should be re-screened periodically. Continue to assess patients diagnosed with dyspareunia for vaginismus symptoms (if they are not initially present) to ensure that the treatment plan is appropriately adjusted. Once treatment has begun, ask about adverse effects and confidence in the treatment plan to minimize negative impacts on treatment adherence and to anticipate a need for a change in the treatment approach.31,35 In addition to tracking treatment effects on pain, continue to assess for patient-centered outcomes such as emotional functioning, self-esteem, and sexual and relationship satisfaction.34 The Female Sexual Function Index can be a useful tool to track symptoms.27,34
Finally, patients who do not experience sufficient improvement in symptoms and functioning with initial treatment may need continued support and encouragement. Given the broad range of contributing factors and the high number of potential treatments, patients may find hope in learning that multiple other treatment options may be available.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family and Community Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC 663, Chicago, IL 60612; [email protected]
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. American Psychiatric Publishing; 2013.
2. Seehusen DA, Baird DC, Bode DV. Dyspareunia in women. Am Fam Phys. 2014;90:465-470.
3. Sorensen J, Bautista KE, Lamvu G, et al. Evaluation and treatment of female sexual pain: a clinical review. Cureus. 2018;10:e2379.
4. MacNeill C. Dyspareunia. Obstet Gynecol Clin North Am. 2006;33:565-77.
5. Latthe P, Latthe M, Say L, et al. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6:177.
6. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
7. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
8. Ünlü Z, Yentur A, Çakil N. Pudendal nerve neuropathy: An unknown-rare cause of pelvic pain. Arch Rheumatol. 2016;31:102-103.
9. Dewitte M, Borg C, Lowenstein L. A psychosocial approach to female genital pain. Nat Rev Urol. 2018;15:25-41.
10. Masters WH, Johnson VE. Human Sexual Inadequacy. 1st ed. Little, Brown; 1970.
11. Rathus SA, Nevid JS, Fichner-Rathus L. Human Sexuality in a World of Diversity. 5th ed. Allyn and Bacon; 2002.
12. Bailey BE, Freedenfeld RN, Kiser RS, et al. Lifetime physical and sexual abuse in chronic pain patients: psychosocial correlates and treatment outcomes. Disabil Rehabil. 2003;25:331-342.
13. Yehuda R, Lehrner A, Rosenbaum TY. PTSD and sexual dysfunction in men and women. J Sex Med. 2015;12:1107-1119.
14. Postma R, Bicanic I, van der Vaart H, et al. Pelvic floor muscle problems mediate sexual problems in young adult rape victims. J Sex Med. 2013;10:1978-1987.
15. Binik YM, Bergeron S, Khalifé S. Dyspareunia and vaginismus: so-called sexual pain. In: Leiblum SR, ed. 4th ed. Principles and Practice of Sex Therapy. The Guilford Press; 2007:124-156.
16. Ayling K, Ussher JM. “If sex hurts, am I still a woman?” The subjective experience of vulvodynia in hetero-sexual women. Arch Sex Behav. 2008;37:294-304.
17. Pazmany E, Bergeron S, Van Oudenhove L, et al. Body image and genital self-image in pre-menopausal women with dyspareunia. Arch Sex Behav. 2013;42:999-1010.
18. Maillé DL, Bergeron S, Lambert B. Body image in women with primary and secondary provoked vestibulodynia: a controlled study. J Sex Med. 2015;12:505-515.
19. Ryan L, Hawton K. Female dyspareunia. BMJ. 2004;328:1357.
20. Waldura JF, Arora I, Randall AM, et al. Fifty shades of stigma: exploring the health care experiences of kink-oriented patients. J Sex Med. 2016;13:1918-1929.
21. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
22. Banaei M, Kariman N, Ozgoli G, et al. Prevalence of postpartum dyspareunia: a systematic review and meta-analysis. Int J Gynaecol Obstet. 2021;153:14-24.
23. Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509-515.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. U.S. Department of Veterans Affairs. PTSD: National Center for PTSD. Life events checklist for DSM-5 (LEC-5). Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp
26. Weathers FW, Litz BT, Keane TM, et al. The PTSD checklist for DSM-5 (PCL-5). 2013. Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
27. Rosen R, Brown C, Heiman J, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
28. Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191-197.
29. Hassam T, Kelso E, Chowdary P, et al. Sexual assault as a risk factor for gynaecological morbidity: an exploratory systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;255:222-230.
30. Hamlin AA, Sheeder J, Muffly TM. Brief versus thong hygiene in obstetrics and gynecology (B-THONG): a survey study. J Obstet Gynaecol Res. 2019;45:1190-1196.
31. Foster DC, Kotok MB, Huang LS, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116:583-593.
32. Pérez-López FR, Bueno-Notivol J, Hernandez AV, et al. Systematic review and meta-analysis of the effects of treatment modalities for vestibulodynia in women. Eur J Contracept Reprod Health Care. 2019;24:337-346.
33. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
34. Goldstein AT, Pukall CF, Brown C, et al. Vulvodynia: assessment and treatment. J Sex Med. 2016;13:572-590.
35. Bergeron S, Binik YM, Khalifé S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297-306.
36. Schvartzman R, Schvartzman L, Ferreira CF, et al. Physical therapy intervention for women with dyspareunia: a randomized clinical trial. J Sex Marital Ther. 2019;45:378-394.
37. Morin M, Dumoulin C, Bergeron S, et al. Multimodal physical therapy versus topical lidocaine for provoked vestibulodynia: a multicenter, randomized trial. Am J Obstet Gynecol. 2021;224:189.e1-189.e12.
38. Bergeron S, Khalifé S, Dupuis M-J, et al. A randomized clinical trial comparing group cognitive-behavioral therapy and a topical steroid for women with dyspareunia. J Consult Clin Psychol. 2016;84:259-268.
39. Maseroli E, Scavello I, Rastrelli G, et al. Outcome of medical and psychosexual interventions for vaginismus: a systematic review and meta-analysis. J Sex Med. 2018;15:1752-1764.
40. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. 2017. Accessed February 3, 2022. www.apa.org/ptsd-guideline/ptsd.pdf
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. American Psychiatric Publishing; 2013.
2. Seehusen DA, Baird DC, Bode DV. Dyspareunia in women. Am Fam Phys. 2014;90:465-470.
3. Sorensen J, Bautista KE, Lamvu G, et al. Evaluation and treatment of female sexual pain: a clinical review. Cureus. 2018;10:e2379.
4. MacNeill C. Dyspareunia. Obstet Gynecol Clin North Am. 2006;33:565-77.
5. Latthe P, Latthe M, Say L, et al. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6:177.
6. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
7. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
8. Ünlü Z, Yentur A, Çakil N. Pudendal nerve neuropathy: An unknown-rare cause of pelvic pain. Arch Rheumatol. 2016;31:102-103.
9. Dewitte M, Borg C, Lowenstein L. A psychosocial approach to female genital pain. Nat Rev Urol. 2018;15:25-41.
10. Masters WH, Johnson VE. Human Sexual Inadequacy. 1st ed. Little, Brown; 1970.
11. Rathus SA, Nevid JS, Fichner-Rathus L. Human Sexuality in a World of Diversity. 5th ed. Allyn and Bacon; 2002.
12. Bailey BE, Freedenfeld RN, Kiser RS, et al. Lifetime physical and sexual abuse in chronic pain patients: psychosocial correlates and treatment outcomes. Disabil Rehabil. 2003;25:331-342.
13. Yehuda R, Lehrner A, Rosenbaum TY. PTSD and sexual dysfunction in men and women. J Sex Med. 2015;12:1107-1119.
14. Postma R, Bicanic I, van der Vaart H, et al. Pelvic floor muscle problems mediate sexual problems in young adult rape victims. J Sex Med. 2013;10:1978-1987.
15. Binik YM, Bergeron S, Khalifé S. Dyspareunia and vaginismus: so-called sexual pain. In: Leiblum SR, ed. 4th ed. Principles and Practice of Sex Therapy. The Guilford Press; 2007:124-156.
16. Ayling K, Ussher JM. “If sex hurts, am I still a woman?” The subjective experience of vulvodynia in hetero-sexual women. Arch Sex Behav. 2008;37:294-304.
17. Pazmany E, Bergeron S, Van Oudenhove L, et al. Body image and genital self-image in pre-menopausal women with dyspareunia. Arch Sex Behav. 2013;42:999-1010.
18. Maillé DL, Bergeron S, Lambert B. Body image in women with primary and secondary provoked vestibulodynia: a controlled study. J Sex Med. 2015;12:505-515.
19. Ryan L, Hawton K. Female dyspareunia. BMJ. 2004;328:1357.
20. Waldura JF, Arora I, Randall AM, et al. Fifty shades of stigma: exploring the health care experiences of kink-oriented patients. J Sex Med. 2016;13:1918-1929.
21. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
22. Banaei M, Kariman N, Ozgoli G, et al. Prevalence of postpartum dyspareunia: a systematic review and meta-analysis. Int J Gynaecol Obstet. 2021;153:14-24.
23. Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509-515.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. U.S. Department of Veterans Affairs. PTSD: National Center for PTSD. Life events checklist for DSM-5 (LEC-5). Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp
26. Weathers FW, Litz BT, Keane TM, et al. The PTSD checklist for DSM-5 (PCL-5). 2013. Accessed February 3, 2022. www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
27. Rosen R, Brown C, Heiman J, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
28. Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191-197.
29. Hassam T, Kelso E, Chowdary P, et al. Sexual assault as a risk factor for gynaecological morbidity: an exploratory systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;255:222-230.
30. Hamlin AA, Sheeder J, Muffly TM. Brief versus thong hygiene in obstetrics and gynecology (B-THONG): a survey study. J Obstet Gynaecol Res. 2019;45:1190-1196.
31. Foster DC, Kotok MB, Huang LS, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet Gynecol. 2010;116:583-593.
32. Pérez-López FR, Bueno-Notivol J, Hernandez AV, et al. Systematic review and meta-analysis of the effects of treatment modalities for vestibulodynia in women. Eur J Contracept Reprod Health Care. 2019;24:337-346.
33. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
34. Goldstein AT, Pukall CF, Brown C, et al. Vulvodynia: assessment and treatment. J Sex Med. 2016;13:572-590.
35. Bergeron S, Binik YM, Khalifé S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297-306.
36. Schvartzman R, Schvartzman L, Ferreira CF, et al. Physical therapy intervention for women with dyspareunia: a randomized clinical trial. J Sex Marital Ther. 2019;45:378-394.
37. Morin M, Dumoulin C, Bergeron S, et al. Multimodal physical therapy versus topical lidocaine for provoked vestibulodynia: a multicenter, randomized trial. Am J Obstet Gynecol. 2021;224:189.e1-189.e12.
38. Bergeron S, Khalifé S, Dupuis M-J, et al. A randomized clinical trial comparing group cognitive-behavioral therapy and a topical steroid for women with dyspareunia. J Consult Clin Psychol. 2016;84:259-268.
39. Maseroli E, Scavello I, Rastrelli G, et al. Outcome of medical and psychosexual interventions for vaginismus: a systematic review and meta-analysis. J Sex Med. 2018;15:1752-1764.
40. American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. 2017. Accessed February 3, 2022. www.apa.org/ptsd-guideline/ptsd.pdf
PRACTICE RECOMMENDATIONS
› Screen all patients for sexual dysfunctions, as patients often do not report symptoms on their own. B
› Refer patients with dyspareunia for psychotherapy to address both pain and psychosocial causes and sequela of dyspareunia. A
› Refer patients with dyspareunia for pelvic floor physical therapy to address pain and sexual functioning. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Former physician sentenced to 20 years in pill mill case
A former pain medicine physician received a sentence of 20 years in prison for selling opioids and writing prescriptions for patients who were abusing or diverting the medications.
Patrick Titus, 58, operated Lighthouse Internal Medicine in Milford, Delaware, from 2005-2014.
Federal prosecutors said Mr. Titus unlawfully distributed or dispensed opioids including fentanyl, morphine, methadone, OxyContin, and oxycodone outside the scope of practice and often prescribed them in combination with each other or in other dangerous combinations. Mr. Titus distributed over 1 million pills, said the government.
In a 2018 indictment, the government said that Mr. Titus would, “at the first and nearly every follow-up visit” prescribe opioids in high dosages, often without conducting an exam or reviewing any urine test results. He would also write prescriptions for opioids without getting patients’s prior medical records or reviewing test results and rarely referred patients to alternative pain treatments such as physical therapy, psychotherapy, or massage.
According to the indictment, he ignored “red flags,” including that patients would come from long distances, sometimes from out of state, and would pay cash, despite having Medicaid coverage.
“Today’s sentencing makes clear that medical professionals who recklessly prescribe opioids and endanger the safety and health of patients will be held accountable,” said Anne Milgram, a Drug Enforcement Administration administrator.
“This sentence is a reminder that the Department of Justice will hold accountable those doctors who are illegitimately prescribing opioids and fueling the country’s opioid crisis,” said Assistant Attorney General Kenneth A. Polite Jr., of the Justice Department’s Criminal Division, in the same statement. “Doctors who commit these unlawful acts exploit their roles as stewards of their patients’s care for their own profit,” he added.
The sentence follows Mr. Titus’s 2-week jury trial in 2021, when he was convicted of 13 counts of unlawful distribution and dispensing of controlled substances and one count of maintaining his practice primarily as a location to sell drugs. Mr. Titus faced a maximum of 20 years per count.
At the time of his conviction, Mr. Titus’s attorney said he planned to appeal, according to Delaware Online.
Delaware suspended Mr. Titus’s registration to prescribe controlled substances for 1 year in 2011. At the time, the state said it had determined that his continued prescribing “poses [an] imminent danger to the public health or safety.”
The state found that from January to November 2011, Mr. Titus issued 3,941 prescriptions for almost 750,000 pills for 17 different controlled substances, all sent to a single pharmacy.
The state also alleged that he wrote prescriptions for controlled substances to patients with felony convictions for drug trafficking and to at least one patient who his staff told him was selling the opioid that Mr. Titus had prescribed. It later determined that Mr. Titus continued prescribing even after it had suspended his DEA registration.
According to a 2014 consent agreement, the state subsequently ordered another 1-year suspension of his DEA registration, to be followed by a 3-year probation period.
Meanwhile, the same year, the state Board of Medical Licensure put Mr. Titus’s medical license on probation for 2 years and ordered him to complete 15 continuing medical education credits in medical recordkeeping, ethics, how to detect diversion and abuse, and in some other areas, and to pay a $7,500 fine.
In 2016, the medical board revoked Mr. Titus’s license, after finding that he continued to prescribe pain medications to patients he did not screen or monitor and for a multitude of other infractions.
A version of this article first appeared on Medscape.com.
A former pain medicine physician received a sentence of 20 years in prison for selling opioids and writing prescriptions for patients who were abusing or diverting the medications.
Patrick Titus, 58, operated Lighthouse Internal Medicine in Milford, Delaware, from 2005-2014.
Federal prosecutors said Mr. Titus unlawfully distributed or dispensed opioids including fentanyl, morphine, methadone, OxyContin, and oxycodone outside the scope of practice and often prescribed them in combination with each other or in other dangerous combinations. Mr. Titus distributed over 1 million pills, said the government.
In a 2018 indictment, the government said that Mr. Titus would, “at the first and nearly every follow-up visit” prescribe opioids in high dosages, often without conducting an exam or reviewing any urine test results. He would also write prescriptions for opioids without getting patients’s prior medical records or reviewing test results and rarely referred patients to alternative pain treatments such as physical therapy, psychotherapy, or massage.
According to the indictment, he ignored “red flags,” including that patients would come from long distances, sometimes from out of state, and would pay cash, despite having Medicaid coverage.
“Today’s sentencing makes clear that medical professionals who recklessly prescribe opioids and endanger the safety and health of patients will be held accountable,” said Anne Milgram, a Drug Enforcement Administration administrator.
“This sentence is a reminder that the Department of Justice will hold accountable those doctors who are illegitimately prescribing opioids and fueling the country’s opioid crisis,” said Assistant Attorney General Kenneth A. Polite Jr., of the Justice Department’s Criminal Division, in the same statement. “Doctors who commit these unlawful acts exploit their roles as stewards of their patients’s care for their own profit,” he added.
The sentence follows Mr. Titus’s 2-week jury trial in 2021, when he was convicted of 13 counts of unlawful distribution and dispensing of controlled substances and one count of maintaining his practice primarily as a location to sell drugs. Mr. Titus faced a maximum of 20 years per count.
At the time of his conviction, Mr. Titus’s attorney said he planned to appeal, according to Delaware Online.
Delaware suspended Mr. Titus’s registration to prescribe controlled substances for 1 year in 2011. At the time, the state said it had determined that his continued prescribing “poses [an] imminent danger to the public health or safety.”
The state found that from January to November 2011, Mr. Titus issued 3,941 prescriptions for almost 750,000 pills for 17 different controlled substances, all sent to a single pharmacy.
The state also alleged that he wrote prescriptions for controlled substances to patients with felony convictions for drug trafficking and to at least one patient who his staff told him was selling the opioid that Mr. Titus had prescribed. It later determined that Mr. Titus continued prescribing even after it had suspended his DEA registration.
According to a 2014 consent agreement, the state subsequently ordered another 1-year suspension of his DEA registration, to be followed by a 3-year probation period.
Meanwhile, the same year, the state Board of Medical Licensure put Mr. Titus’s medical license on probation for 2 years and ordered him to complete 15 continuing medical education credits in medical recordkeeping, ethics, how to detect diversion and abuse, and in some other areas, and to pay a $7,500 fine.
In 2016, the medical board revoked Mr. Titus’s license, after finding that he continued to prescribe pain medications to patients he did not screen or monitor and for a multitude of other infractions.
A version of this article first appeared on Medscape.com.
A former pain medicine physician received a sentence of 20 years in prison for selling opioids and writing prescriptions for patients who were abusing or diverting the medications.
Patrick Titus, 58, operated Lighthouse Internal Medicine in Milford, Delaware, from 2005-2014.
Federal prosecutors said Mr. Titus unlawfully distributed or dispensed opioids including fentanyl, morphine, methadone, OxyContin, and oxycodone outside the scope of practice and often prescribed them in combination with each other or in other dangerous combinations. Mr. Titus distributed over 1 million pills, said the government.
In a 2018 indictment, the government said that Mr. Titus would, “at the first and nearly every follow-up visit” prescribe opioids in high dosages, often without conducting an exam or reviewing any urine test results. He would also write prescriptions for opioids without getting patients’s prior medical records or reviewing test results and rarely referred patients to alternative pain treatments such as physical therapy, psychotherapy, or massage.
According to the indictment, he ignored “red flags,” including that patients would come from long distances, sometimes from out of state, and would pay cash, despite having Medicaid coverage.
“Today’s sentencing makes clear that medical professionals who recklessly prescribe opioids and endanger the safety and health of patients will be held accountable,” said Anne Milgram, a Drug Enforcement Administration administrator.
“This sentence is a reminder that the Department of Justice will hold accountable those doctors who are illegitimately prescribing opioids and fueling the country’s opioid crisis,” said Assistant Attorney General Kenneth A. Polite Jr., of the Justice Department’s Criminal Division, in the same statement. “Doctors who commit these unlawful acts exploit their roles as stewards of their patients’s care for their own profit,” he added.
The sentence follows Mr. Titus’s 2-week jury trial in 2021, when he was convicted of 13 counts of unlawful distribution and dispensing of controlled substances and one count of maintaining his practice primarily as a location to sell drugs. Mr. Titus faced a maximum of 20 years per count.
At the time of his conviction, Mr. Titus’s attorney said he planned to appeal, according to Delaware Online.
Delaware suspended Mr. Titus’s registration to prescribe controlled substances for 1 year in 2011. At the time, the state said it had determined that his continued prescribing “poses [an] imminent danger to the public health or safety.”
The state found that from January to November 2011, Mr. Titus issued 3,941 prescriptions for almost 750,000 pills for 17 different controlled substances, all sent to a single pharmacy.
The state also alleged that he wrote prescriptions for controlled substances to patients with felony convictions for drug trafficking and to at least one patient who his staff told him was selling the opioid that Mr. Titus had prescribed. It later determined that Mr. Titus continued prescribing even after it had suspended his DEA registration.
According to a 2014 consent agreement, the state subsequently ordered another 1-year suspension of his DEA registration, to be followed by a 3-year probation period.
Meanwhile, the same year, the state Board of Medical Licensure put Mr. Titus’s medical license on probation for 2 years and ordered him to complete 15 continuing medical education credits in medical recordkeeping, ethics, how to detect diversion and abuse, and in some other areas, and to pay a $7,500 fine.
In 2016, the medical board revoked Mr. Titus’s license, after finding that he continued to prescribe pain medications to patients he did not screen or monitor and for a multitude of other infractions.
A version of this article first appeared on Medscape.com.