User login
Intent to vaccinate kids against COVID higher among vaccinated parents
“Parental vaccine hesitancy is a major issue for schools resuming in-person instruction, potentially requiring regular testing, strict mask wearing, and physical distancing for safe operation,” wrote lead author Madhura S. Rane, PhD, from the City University of New York in New York City, and colleagues in their paper, published online in JAMA Pediatrics.
The survey was conducted in June 2021 of 1,162 parents with children ranging in age from 2 to 17 years. The majority of parents (74.4%) were already vaccinated/vaccine-willing ,while 25.6% were vaccine hesitant. The study cohort, including both 1,652 children and their parents, was part of the nationwide CHASING COVID.
Vaccinated parents overall were more willing to vaccinate or had already vaccinated their eligible children when compared with vaccine-hesitant parents: 64.9% vs. 8.3% for children 2-4 years of age; 77.6% vs. 12.1% for children 5-11 years of age; 81.3% vs. 13.9% for children 12-15 years of age; and 86.4% vs. 12.7% for children 16-17 years of age; P < .001.
The researchers found greater hesitancy among Black and Hispanic parents, compared with parents who were non-Hispanic White, women, younger, and did not have a college education. Parents of children who were currently attending school remotely or only partially, were found to be more willing to vaccinate their children when compared to parents of children who were attending school fully in person.
The authors also found that parents who knew someone who had died of COVID-19 or had experienced a prior COVID-19 infection, were more willing to vaccinate their children.
Hesitance in vaccinated parents
Interestingly, 10% of COVID-vaccinated parents said they were still hesitant to vaccinate their kids because of concern for long-term adverse effects of the vaccine.
“These data point out that vaccine concerns may exist even among vaccinated or vaccine-favorable parents, so we should ask any parent who has not vaccinated their child whether we can discuss their concerns and perhaps move their opinions,” said William T. Basco Jr, MD, MS, a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics.
In an interview, when asked whether recent approval of the vaccine for children aged 5-11 will likely aid in overcoming parental hesitancy, Dr. Basco replied: “Absolutely. As more children get the vaccine and people know a neighbor or nephew or cousin, etc., who received the vaccine and did fine, it will engender greater comfort and allow parents to feel better about having their own child receive the vaccine.”
Advice for clinicians from outside expert
“We can always start by asking parents if we can help them understand the vaccine and the need for it. The tidal wave of disinformation is huge, but we can, on a daily basis, offer to help families navigate this decision,” concluded Dr. Basco, who was not involved with the new paper.
Funding for this study was provided through grants from the National Institute of Allergy and Infectious Diseases, the CUNY Institute of Implementation Science in Population Health, and the COVID-19 Grant Program of the CUNY Graduate School of Public Health and Health Policy. The authors and Dr. Basco have disclosed no relevant financial relationships.
“Parental vaccine hesitancy is a major issue for schools resuming in-person instruction, potentially requiring regular testing, strict mask wearing, and physical distancing for safe operation,” wrote lead author Madhura S. Rane, PhD, from the City University of New York in New York City, and colleagues in their paper, published online in JAMA Pediatrics.
The survey was conducted in June 2021 of 1,162 parents with children ranging in age from 2 to 17 years. The majority of parents (74.4%) were already vaccinated/vaccine-willing ,while 25.6% were vaccine hesitant. The study cohort, including both 1,652 children and their parents, was part of the nationwide CHASING COVID.
Vaccinated parents overall were more willing to vaccinate or had already vaccinated their eligible children when compared with vaccine-hesitant parents: 64.9% vs. 8.3% for children 2-4 years of age; 77.6% vs. 12.1% for children 5-11 years of age; 81.3% vs. 13.9% for children 12-15 years of age; and 86.4% vs. 12.7% for children 16-17 years of age; P < .001.
The researchers found greater hesitancy among Black and Hispanic parents, compared with parents who were non-Hispanic White, women, younger, and did not have a college education. Parents of children who were currently attending school remotely or only partially, were found to be more willing to vaccinate their children when compared to parents of children who were attending school fully in person.
The authors also found that parents who knew someone who had died of COVID-19 or had experienced a prior COVID-19 infection, were more willing to vaccinate their children.
Hesitance in vaccinated parents
Interestingly, 10% of COVID-vaccinated parents said they were still hesitant to vaccinate their kids because of concern for long-term adverse effects of the vaccine.
“These data point out that vaccine concerns may exist even among vaccinated or vaccine-favorable parents, so we should ask any parent who has not vaccinated their child whether we can discuss their concerns and perhaps move their opinions,” said William T. Basco Jr, MD, MS, a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics.
In an interview, when asked whether recent approval of the vaccine for children aged 5-11 will likely aid in overcoming parental hesitancy, Dr. Basco replied: “Absolutely. As more children get the vaccine and people know a neighbor or nephew or cousin, etc., who received the vaccine and did fine, it will engender greater comfort and allow parents to feel better about having their own child receive the vaccine.”
Advice for clinicians from outside expert
“We can always start by asking parents if we can help them understand the vaccine and the need for it. The tidal wave of disinformation is huge, but we can, on a daily basis, offer to help families navigate this decision,” concluded Dr. Basco, who was not involved with the new paper.
Funding for this study was provided through grants from the National Institute of Allergy and Infectious Diseases, the CUNY Institute of Implementation Science in Population Health, and the COVID-19 Grant Program of the CUNY Graduate School of Public Health and Health Policy. The authors and Dr. Basco have disclosed no relevant financial relationships.
“Parental vaccine hesitancy is a major issue for schools resuming in-person instruction, potentially requiring regular testing, strict mask wearing, and physical distancing for safe operation,” wrote lead author Madhura S. Rane, PhD, from the City University of New York in New York City, and colleagues in their paper, published online in JAMA Pediatrics.
The survey was conducted in June 2021 of 1,162 parents with children ranging in age from 2 to 17 years. The majority of parents (74.4%) were already vaccinated/vaccine-willing ,while 25.6% were vaccine hesitant. The study cohort, including both 1,652 children and their parents, was part of the nationwide CHASING COVID.
Vaccinated parents overall were more willing to vaccinate or had already vaccinated their eligible children when compared with vaccine-hesitant parents: 64.9% vs. 8.3% for children 2-4 years of age; 77.6% vs. 12.1% for children 5-11 years of age; 81.3% vs. 13.9% for children 12-15 years of age; and 86.4% vs. 12.7% for children 16-17 years of age; P < .001.
The researchers found greater hesitancy among Black and Hispanic parents, compared with parents who were non-Hispanic White, women, younger, and did not have a college education. Parents of children who were currently attending school remotely or only partially, were found to be more willing to vaccinate their children when compared to parents of children who were attending school fully in person.
The authors also found that parents who knew someone who had died of COVID-19 or had experienced a prior COVID-19 infection, were more willing to vaccinate their children.
Hesitance in vaccinated parents
Interestingly, 10% of COVID-vaccinated parents said they were still hesitant to vaccinate their kids because of concern for long-term adverse effects of the vaccine.
“These data point out that vaccine concerns may exist even among vaccinated or vaccine-favorable parents, so we should ask any parent who has not vaccinated their child whether we can discuss their concerns and perhaps move their opinions,” said William T. Basco Jr, MD, MS, a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics.
In an interview, when asked whether recent approval of the vaccine for children aged 5-11 will likely aid in overcoming parental hesitancy, Dr. Basco replied: “Absolutely. As more children get the vaccine and people know a neighbor or nephew or cousin, etc., who received the vaccine and did fine, it will engender greater comfort and allow parents to feel better about having their own child receive the vaccine.”
Advice for clinicians from outside expert
“We can always start by asking parents if we can help them understand the vaccine and the need for it. The tidal wave of disinformation is huge, but we can, on a daily basis, offer to help families navigate this decision,” concluded Dr. Basco, who was not involved with the new paper.
Funding for this study was provided through grants from the National Institute of Allergy and Infectious Diseases, the CUNY Institute of Implementation Science in Population Health, and the COVID-19 Grant Program of the CUNY Graduate School of Public Health and Health Policy. The authors and Dr. Basco have disclosed no relevant financial relationships.
FROM JAMA PEDIATRICS
Children and COVID-19: 7 million cases and still counting
Total COVID-19 cases in children surpassed the 7-million mark as new cases rose slightly after the previous week’s decline, according to the American Academy of Pediatrics and the Children’s Hospital Association.
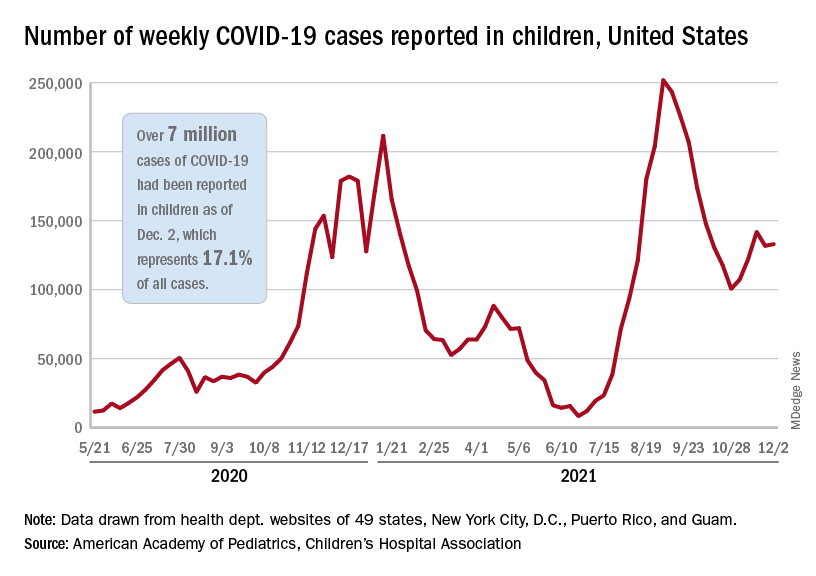
, the AAP and CHA said in their weekly COVID-19 report. New cases had dropped the previous week after 3 straight weeks of increases since late October.
The Centers for Disease Control and Prevention puts the total number of child COVID-19 cases at 6.2 million, but both estimates are based on all-age totals – 40 million for the CDC and 41 million for the AAP/CHA – that are well short of the CDC’s latest cumulative figure, which is now just over 49 million, so the actual figures are undoubtedly higher.
Meanwhile, the 1-month anniversary of 5- to 11-year-olds’ vaccine eligibility brought many completions: 923,000 received their second dose during the week ending Dec. 6, compared with 405,000 the previous week. About 16.9% (4.9 million) of children aged 5-11 have gotten at least one dose of the COVID-19 vaccine thus far, of whom almost 1.5 million children (5.1% of the age group) are now fully vaccinated, the CDC said on its COVID-19 Data Tracker.
The pace of vaccinations, however, is much lower for older children. Weekly numbers for all COVID-19 vaccinations, both first and second doses, dropped from 84,000 (Nov. 23-29) to 70,000 (Nov. 30 to Dec. 6), for those aged 12-17 years. In that group, 61.6% have received at least one dose and 51.8% are fully vaccinated, the CDC said.
The pace of vaccinations varies for younger children as well, when geography is considered. The AAP analyzed the CDC’s data and found that 42% of all 5- to 11-year-olds in Vermont had received at least one dose as of Dec. 1, followed by Massachusetts (33%), Maine (30%), and Rhode Island (28%). At the other end of the vaccination scale are Alabama, Louisiana, Mississippi, and West Virginia, all with 4%, the AAP reported.
As the United States puts 7 million children infected with COVID-19 in its rear view mirror, another milestone is looming ahead: The CDC’s current count of deaths in children is 974.
Total COVID-19 cases in children surpassed the 7-million mark as new cases rose slightly after the previous week’s decline, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, the AAP and CHA said in their weekly COVID-19 report. New cases had dropped the previous week after 3 straight weeks of increases since late October.
The Centers for Disease Control and Prevention puts the total number of child COVID-19 cases at 6.2 million, but both estimates are based on all-age totals – 40 million for the CDC and 41 million for the AAP/CHA – that are well short of the CDC’s latest cumulative figure, which is now just over 49 million, so the actual figures are undoubtedly higher.
Meanwhile, the 1-month anniversary of 5- to 11-year-olds’ vaccine eligibility brought many completions: 923,000 received their second dose during the week ending Dec. 6, compared with 405,000 the previous week. About 16.9% (4.9 million) of children aged 5-11 have gotten at least one dose of the COVID-19 vaccine thus far, of whom almost 1.5 million children (5.1% of the age group) are now fully vaccinated, the CDC said on its COVID-19 Data Tracker.
The pace of vaccinations, however, is much lower for older children. Weekly numbers for all COVID-19 vaccinations, both first and second doses, dropped from 84,000 (Nov. 23-29) to 70,000 (Nov. 30 to Dec. 6), for those aged 12-17 years. In that group, 61.6% have received at least one dose and 51.8% are fully vaccinated, the CDC said.
The pace of vaccinations varies for younger children as well, when geography is considered. The AAP analyzed the CDC’s data and found that 42% of all 5- to 11-year-olds in Vermont had received at least one dose as of Dec. 1, followed by Massachusetts (33%), Maine (30%), and Rhode Island (28%). At the other end of the vaccination scale are Alabama, Louisiana, Mississippi, and West Virginia, all with 4%, the AAP reported.
As the United States puts 7 million children infected with COVID-19 in its rear view mirror, another milestone is looming ahead: The CDC’s current count of deaths in children is 974.
Total COVID-19 cases in children surpassed the 7-million mark as new cases rose slightly after the previous week’s decline, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, the AAP and CHA said in their weekly COVID-19 report. New cases had dropped the previous week after 3 straight weeks of increases since late October.
The Centers for Disease Control and Prevention puts the total number of child COVID-19 cases at 6.2 million, but both estimates are based on all-age totals – 40 million for the CDC and 41 million for the AAP/CHA – that are well short of the CDC’s latest cumulative figure, which is now just over 49 million, so the actual figures are undoubtedly higher.
Meanwhile, the 1-month anniversary of 5- to 11-year-olds’ vaccine eligibility brought many completions: 923,000 received their second dose during the week ending Dec. 6, compared with 405,000 the previous week. About 16.9% (4.9 million) of children aged 5-11 have gotten at least one dose of the COVID-19 vaccine thus far, of whom almost 1.5 million children (5.1% of the age group) are now fully vaccinated, the CDC said on its COVID-19 Data Tracker.
The pace of vaccinations, however, is much lower for older children. Weekly numbers for all COVID-19 vaccinations, both first and second doses, dropped from 84,000 (Nov. 23-29) to 70,000 (Nov. 30 to Dec. 6), for those aged 12-17 years. In that group, 61.6% have received at least one dose and 51.8% are fully vaccinated, the CDC said.
The pace of vaccinations varies for younger children as well, when geography is considered. The AAP analyzed the CDC’s data and found that 42% of all 5- to 11-year-olds in Vermont had received at least one dose as of Dec. 1, followed by Massachusetts (33%), Maine (30%), and Rhode Island (28%). At the other end of the vaccination scale are Alabama, Louisiana, Mississippi, and West Virginia, all with 4%, the AAP reported.
As the United States puts 7 million children infected with COVID-19 in its rear view mirror, another milestone is looming ahead: The CDC’s current count of deaths in children is 974.
Does inadequate sleep increase obesity risk in children?
Evidence summary
Multiple analyses suggest short sleep increases obesity risk
Three recent, large systematic reviews of prospective cohort studies with meta-analyses in infants, children, and adolescents all found associations between short sleep at intake and later excessive weight.
The largest meta-analysis included 42 prospective studies with 75,499 patients ranging in age from infancy to adolescence and with follow-up ranging from 1 to 27 years. In a pooled analysis, short sleep—variously defined across trials and mostly assessed by parental report—was associated with an increased risk of obesity or overweight (relative risk [RR] = 1.58; 95% CI, 1.35-1.85; I2= 92%), compared to normal and long sleep. When the authors adjusted for suspected publication bias using a “trim and fill” method, short sleep remained associated with later overweight or obesity (RR = 1.42; 95% CI, 1.12-1.81). Short sleep was associated with later unhealthy weight status in all age groups: 0 to < 3 years (RR = 1.4; 95% CI, 1.19-1.65); 3 to < 9 years (RR = 1.57; 95% CI, 1.4-1.76);9 to < 12 years (RR = 2.23; 95% CI, 2.18-2.27); and 12 to 18 years (RR = 1.3; 95% CI, 1.11-1.53). In addition to high heterogeneity, limitations of the review included variability in the definition of short sleep, use of parent- or self-reported sleep duration, and variability in classification of overweight and obesity in primary studies.1
A second systematic review and meta-analysis included 25 longitudinal studies (20 of which overlapped with the previously discussed meta-analysis) of children and adolescents (N = 56,584). Patients ranged in age from infancy to 16 years, and follow-up ranged from 6 months to 10 years (mean, 3.4 years). Children and adolescents with the shortest sleep duration were more likely to be overweight or obese at follow-up (pooled odds ratio [OR] = 1.76; 95% CI, 1.39-2.23; I2 = 70.5%) than those with the longest sleep duration. Due to the overlap in studies, the limitations of this analysis were similar to those already mentioned. Lack of a linear association between sleep duration and weight was cited as evidence of possible publication bias; the authors did not attempt to correct for it.2
The third systematic review and meta-analysis included 22 longitudinal studies (18 overlapped with first meta-analysis and 17 with the second) of children and adolescents (N = 24,821) ages 6 months to 18 years. Follow-up ranged from 1 to 27 years. This meta-analysis standardized the categories of sleep duration using recommendations from the Sleep Health Foundation. Patients with short sleep duration had an increased risk of overweight or obesity compared with patients sleeping “normal” or “longer than normal” durations (pooled OR = 2.15; 95% CI, 1.64-2.81; I2 = 67%). The authors indicated that their analysis could have been more robust if information about daytime sleep (ie, napping) had been available, but it was not collected in many of the included studies.3
Accelerometer data quantify the sleep/obesity association
A subsequent cohort study (N = 202) sought to better examine the association between sleep characteristics and adiposity by measuring sleep duration using accelerometers. Toddlers (ages 12 to 26 months) without previous medical history were recruited from early childhood education centers. Patients wore accelerometers for 7 consecutive days and then returned to the clinic after 12 months for collection of biometric information. Researchers measured body morphology with the BMI z-score (ie, the number of standard deviations from the mean). Every additional hour of total sleep time was associated with a 0.12-unit lower BMI z-score (95% CI, –0.23 to –0.01) at 1 year. However, every hour increase in nap duration was associated with a 0.41-unit higher BMI z-score (95% CI, 0.14-0.68).4
Recommendations from others
In 2016, the American Academy of Sleep Medicine (AASM) recommended the following sleep durations (per 24 hours): infants ages 4 to 12 months, 12-16 hours; children 1 to 2 years, 11-14 hours; children 3 to 5 years, 10-13 hours; children 6 to 12 years, 9-12 hours; and teenagers 13 to 18 years, 8-10 hours. The AASM further stated that sleeping the recommended number of hours was associated with better health outcomes, and that sleeping too few hours increased the risk of various health conditions, including obesity.5 In 2015, the American Academy of Pediatrics Committee on Nutrition acknowledged the association between obesity and short sleep duration and recommended that health care professionals counsel parents about age-appropriate sleep guidelines.6
Editor’s takeaway
Studies demonstrate that short sleep duration in pediatric patients is associated with later weight gain. However, associations do not prove a causal link, and other factors may contribute to both weight gain and poor sleep.
1. Miller MA, Kruisbrink M, Wallace J, et al. Sleep duration and incidence of obesity in infants, children, and adolescents: a systematic review and meta-analysis of prospective studies. Sleep. 2018;41:1-19. doi: 10.1093/sleep/zsy018
2. Ruan H, Xun P, Cai W, et al. Habitual sleep duration and risk of childhood obesity: systematic review and dose-response meta-analysis of prospective cohort studies. Sci Rep. 2015;5:16160. doi: 10.1038/srep16160
3. Fatima Y, Doi SA, Mamun AA. Longitudinal impact of sleep on overweight and obesity in children and adolescents: a systematic review and bias-adjusted meta-analysis. Obes Rev. 2015;16:137-149. doi: 10.1111/obr.12245
4. Zhang Z, Pereira JR, Sousa-Sá E, et al. The cross‐sectional and prospective associations between sleep characteristics and adiposity in toddlers: results from the GET UP! study. Pediatr Obes. 2019;14:e1255. doi: 10.1111/ijpo.12557
5. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785-786. doi: 10.5664/jcsm.5866
6. Daniels SR, Hassink SG; American Academy of Pediatrics Committee on Nutrition. The role of the pediatrician in primary prevention of obesity. Pediatrics 2015;136:e275-e292. doi: 10.1542/peds.2015-1558
Evidence summary
Multiple analyses suggest short sleep increases obesity risk
Three recent, large systematic reviews of prospective cohort studies with meta-analyses in infants, children, and adolescents all found associations between short sleep at intake and later excessive weight.
The largest meta-analysis included 42 prospective studies with 75,499 patients ranging in age from infancy to adolescence and with follow-up ranging from 1 to 27 years. In a pooled analysis, short sleep—variously defined across trials and mostly assessed by parental report—was associated with an increased risk of obesity or overweight (relative risk [RR] = 1.58; 95% CI, 1.35-1.85; I2= 92%), compared to normal and long sleep. When the authors adjusted for suspected publication bias using a “trim and fill” method, short sleep remained associated with later overweight or obesity (RR = 1.42; 95% CI, 1.12-1.81). Short sleep was associated with later unhealthy weight status in all age groups: 0 to < 3 years (RR = 1.4; 95% CI, 1.19-1.65); 3 to < 9 years (RR = 1.57; 95% CI, 1.4-1.76);9 to < 12 years (RR = 2.23; 95% CI, 2.18-2.27); and 12 to 18 years (RR = 1.3; 95% CI, 1.11-1.53). In addition to high heterogeneity, limitations of the review included variability in the definition of short sleep, use of parent- or self-reported sleep duration, and variability in classification of overweight and obesity in primary studies.1
A second systematic review and meta-analysis included 25 longitudinal studies (20 of which overlapped with the previously discussed meta-analysis) of children and adolescents (N = 56,584). Patients ranged in age from infancy to 16 years, and follow-up ranged from 6 months to 10 years (mean, 3.4 years). Children and adolescents with the shortest sleep duration were more likely to be overweight or obese at follow-up (pooled odds ratio [OR] = 1.76; 95% CI, 1.39-2.23; I2 = 70.5%) than those with the longest sleep duration. Due to the overlap in studies, the limitations of this analysis were similar to those already mentioned. Lack of a linear association between sleep duration and weight was cited as evidence of possible publication bias; the authors did not attempt to correct for it.2
The third systematic review and meta-analysis included 22 longitudinal studies (18 overlapped with first meta-analysis and 17 with the second) of children and adolescents (N = 24,821) ages 6 months to 18 years. Follow-up ranged from 1 to 27 years. This meta-analysis standardized the categories of sleep duration using recommendations from the Sleep Health Foundation. Patients with short sleep duration had an increased risk of overweight or obesity compared with patients sleeping “normal” or “longer than normal” durations (pooled OR = 2.15; 95% CI, 1.64-2.81; I2 = 67%). The authors indicated that their analysis could have been more robust if information about daytime sleep (ie, napping) had been available, but it was not collected in many of the included studies.3
Accelerometer data quantify the sleep/obesity association
A subsequent cohort study (N = 202) sought to better examine the association between sleep characteristics and adiposity by measuring sleep duration using accelerometers. Toddlers (ages 12 to 26 months) without previous medical history were recruited from early childhood education centers. Patients wore accelerometers for 7 consecutive days and then returned to the clinic after 12 months for collection of biometric information. Researchers measured body morphology with the BMI z-score (ie, the number of standard deviations from the mean). Every additional hour of total sleep time was associated with a 0.12-unit lower BMI z-score (95% CI, –0.23 to –0.01) at 1 year. However, every hour increase in nap duration was associated with a 0.41-unit higher BMI z-score (95% CI, 0.14-0.68).4
Recommendations from others
In 2016, the American Academy of Sleep Medicine (AASM) recommended the following sleep durations (per 24 hours): infants ages 4 to 12 months, 12-16 hours; children 1 to 2 years, 11-14 hours; children 3 to 5 years, 10-13 hours; children 6 to 12 years, 9-12 hours; and teenagers 13 to 18 years, 8-10 hours. The AASM further stated that sleeping the recommended number of hours was associated with better health outcomes, and that sleeping too few hours increased the risk of various health conditions, including obesity.5 In 2015, the American Academy of Pediatrics Committee on Nutrition acknowledged the association between obesity and short sleep duration and recommended that health care professionals counsel parents about age-appropriate sleep guidelines.6
Editor’s takeaway
Studies demonstrate that short sleep duration in pediatric patients is associated with later weight gain. However, associations do not prove a causal link, and other factors may contribute to both weight gain and poor sleep.
Evidence summary
Multiple analyses suggest short sleep increases obesity risk
Three recent, large systematic reviews of prospective cohort studies with meta-analyses in infants, children, and adolescents all found associations between short sleep at intake and later excessive weight.
The largest meta-analysis included 42 prospective studies with 75,499 patients ranging in age from infancy to adolescence and with follow-up ranging from 1 to 27 years. In a pooled analysis, short sleep—variously defined across trials and mostly assessed by parental report—was associated with an increased risk of obesity or overweight (relative risk [RR] = 1.58; 95% CI, 1.35-1.85; I2= 92%), compared to normal and long sleep. When the authors adjusted for suspected publication bias using a “trim and fill” method, short sleep remained associated with later overweight or obesity (RR = 1.42; 95% CI, 1.12-1.81). Short sleep was associated with later unhealthy weight status in all age groups: 0 to < 3 years (RR = 1.4; 95% CI, 1.19-1.65); 3 to < 9 years (RR = 1.57; 95% CI, 1.4-1.76);9 to < 12 years (RR = 2.23; 95% CI, 2.18-2.27); and 12 to 18 years (RR = 1.3; 95% CI, 1.11-1.53). In addition to high heterogeneity, limitations of the review included variability in the definition of short sleep, use of parent- or self-reported sleep duration, and variability in classification of overweight and obesity in primary studies.1
A second systematic review and meta-analysis included 25 longitudinal studies (20 of which overlapped with the previously discussed meta-analysis) of children and adolescents (N = 56,584). Patients ranged in age from infancy to 16 years, and follow-up ranged from 6 months to 10 years (mean, 3.4 years). Children and adolescents with the shortest sleep duration were more likely to be overweight or obese at follow-up (pooled odds ratio [OR] = 1.76; 95% CI, 1.39-2.23; I2 = 70.5%) than those with the longest sleep duration. Due to the overlap in studies, the limitations of this analysis were similar to those already mentioned. Lack of a linear association between sleep duration and weight was cited as evidence of possible publication bias; the authors did not attempt to correct for it.2
The third systematic review and meta-analysis included 22 longitudinal studies (18 overlapped with first meta-analysis and 17 with the second) of children and adolescents (N = 24,821) ages 6 months to 18 years. Follow-up ranged from 1 to 27 years. This meta-analysis standardized the categories of sleep duration using recommendations from the Sleep Health Foundation. Patients with short sleep duration had an increased risk of overweight or obesity compared with patients sleeping “normal” or “longer than normal” durations (pooled OR = 2.15; 95% CI, 1.64-2.81; I2 = 67%). The authors indicated that their analysis could have been more robust if information about daytime sleep (ie, napping) had been available, but it was not collected in many of the included studies.3
Accelerometer data quantify the sleep/obesity association
A subsequent cohort study (N = 202) sought to better examine the association between sleep characteristics and adiposity by measuring sleep duration using accelerometers. Toddlers (ages 12 to 26 months) without previous medical history were recruited from early childhood education centers. Patients wore accelerometers for 7 consecutive days and then returned to the clinic after 12 months for collection of biometric information. Researchers measured body morphology with the BMI z-score (ie, the number of standard deviations from the mean). Every additional hour of total sleep time was associated with a 0.12-unit lower BMI z-score (95% CI, –0.23 to –0.01) at 1 year. However, every hour increase in nap duration was associated with a 0.41-unit higher BMI z-score (95% CI, 0.14-0.68).4
Recommendations from others
In 2016, the American Academy of Sleep Medicine (AASM) recommended the following sleep durations (per 24 hours): infants ages 4 to 12 months, 12-16 hours; children 1 to 2 years, 11-14 hours; children 3 to 5 years, 10-13 hours; children 6 to 12 years, 9-12 hours; and teenagers 13 to 18 years, 8-10 hours. The AASM further stated that sleeping the recommended number of hours was associated with better health outcomes, and that sleeping too few hours increased the risk of various health conditions, including obesity.5 In 2015, the American Academy of Pediatrics Committee on Nutrition acknowledged the association between obesity and short sleep duration and recommended that health care professionals counsel parents about age-appropriate sleep guidelines.6
Editor’s takeaway
Studies demonstrate that short sleep duration in pediatric patients is associated with later weight gain. However, associations do not prove a causal link, and other factors may contribute to both weight gain and poor sleep.
1. Miller MA, Kruisbrink M, Wallace J, et al. Sleep duration and incidence of obesity in infants, children, and adolescents: a systematic review and meta-analysis of prospective studies. Sleep. 2018;41:1-19. doi: 10.1093/sleep/zsy018
2. Ruan H, Xun P, Cai W, et al. Habitual sleep duration and risk of childhood obesity: systematic review and dose-response meta-analysis of prospective cohort studies. Sci Rep. 2015;5:16160. doi: 10.1038/srep16160
3. Fatima Y, Doi SA, Mamun AA. Longitudinal impact of sleep on overweight and obesity in children and adolescents: a systematic review and bias-adjusted meta-analysis. Obes Rev. 2015;16:137-149. doi: 10.1111/obr.12245
4. Zhang Z, Pereira JR, Sousa-Sá E, et al. The cross‐sectional and prospective associations between sleep characteristics and adiposity in toddlers: results from the GET UP! study. Pediatr Obes. 2019;14:e1255. doi: 10.1111/ijpo.12557
5. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785-786. doi: 10.5664/jcsm.5866
6. Daniels SR, Hassink SG; American Academy of Pediatrics Committee on Nutrition. The role of the pediatrician in primary prevention of obesity. Pediatrics 2015;136:e275-e292. doi: 10.1542/peds.2015-1558
1. Miller MA, Kruisbrink M, Wallace J, et al. Sleep duration and incidence of obesity in infants, children, and adolescents: a systematic review and meta-analysis of prospective studies. Sleep. 2018;41:1-19. doi: 10.1093/sleep/zsy018
2. Ruan H, Xun P, Cai W, et al. Habitual sleep duration and risk of childhood obesity: systematic review and dose-response meta-analysis of prospective cohort studies. Sci Rep. 2015;5:16160. doi: 10.1038/srep16160
3. Fatima Y, Doi SA, Mamun AA. Longitudinal impact of sleep on overweight and obesity in children and adolescents: a systematic review and bias-adjusted meta-analysis. Obes Rev. 2015;16:137-149. doi: 10.1111/obr.12245
4. Zhang Z, Pereira JR, Sousa-Sá E, et al. The cross‐sectional and prospective associations between sleep characteristics and adiposity in toddlers: results from the GET UP! study. Pediatr Obes. 2019;14:e1255. doi: 10.1111/ijpo.12557
5. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785-786. doi: 10.5664/jcsm.5866
6. Daniels SR, Hassink SG; American Academy of Pediatrics Committee on Nutrition. The role of the pediatrician in primary prevention of obesity. Pediatrics 2015;136:e275-e292. doi: 10.1542/peds.2015-1558
EVIDENCE-BASED ANSWER:
Yes, a link has been established but not a cause-effect relationship. Shorter reported sleep duration in childhood is associated with an increased risk of overweight or obesity years later (strength of recommendation [SOR]: B, meta-analyses of prospective cohort trials with high heterogeneity). In toddlers, accelerometer documentation of short sleep duration is associated with elevation of body mass index (BMI) at 1-year follow-up (SOR: B, prospective cohort). Adequate sleep is recommended to help prevent excessive weight gain in children (SOR: C, expert opinion).
Dust mite immunotherapy may help some with eczema
, but improvement in the primary outcome was not significant, new data show.
Results of the small, randomized, double-blind, placebo-controlled trial were published recently in The Journal of Allergy and Clinical Immunology: In Practice.
Lead author Sarah Sella Langer, MD, of the department of medicine, Ribeirão Preto (Brazil) Medical School, University of São Paulo, and colleagues said their results suggest HDM SLIT is safe and effective as an add-on treatment.
The dust mite extract therapy had no major side effects after 18 months of treatment, the authors reported.
The researchers included data from 66 patients who completed the study. The participants were at least 3 years old, registered at least 15 on the SCORing Atopic Dermatitis (SCORAD) measure, and had a skin prick test and/or immunoglobulin E (IgE) test for sensitization to dust mites.
Patients were grouped by age (younger than 12 years or 12 years and older) to receive HDM SLIT (n = 35) or placebo (n = 31) 3 days a week for the study period – between May 2018 and June 2020 – at the Clinical Research Unit of Ribeirão Preto Medical School Hospital.
At baseline, the mean SCORAD was 46.9 (range, 17-87).
After 18 months, 74.2% and 58% of patients in HDM SLIT and placebo groups, respectively, showed at least a15-point decrease in SCORAD (relative risk, 1.28; 95% confidence interval, 0.89-1.83). However, those primary outcome results did not reach statistical significance.
On the other hand, some secondary outcomes did show significant results.
At 95% CI, the researchers reported significant objective-SCORAD decreases of 56.8% and 34.9% in HDM SLIT and placebo groups (average difference, 21.3). Significantly more patients had a score of 0 or 1 on the 5-point Investigator’s Global Assessment scale in the intervention group than in the placebo group (14/35 vs. 5/31; RR, 2.63).
There were no significant changes in the Eczema Area and Severity Index, the visual analogue scale for symptoms, the pruritus scale, or the Dermatology Life Quality Index.
Patients in the trial, most of whom had moderate to severe disease, continued to be treated with usual, individualized therapy for AD, in accordance with current guidelines and experts’ recommendations.
Tina Sindher, MD, an allergist with the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University, , told this news organization that the results are not robust enough to recommend the immunotherapy widely.
She pointed out that even in the placebo group, more than half the patients met the primary endpoint.
However, she did say HDM SLIT could be considered as an add-on treatment for the right patients, especially since risk for an allergic reaction or other adverse condition is small. The most common adverse effects were headache and abdominal pain, and they were reported in both the treatment and placebo groups.
With AD, she said, “there is no one drug that’s right for everyone,” because genetics and environment make the kind of symptoms and severity and duration different for each patient.
It all comes down to risk and benefits, she said.
She said if she had a patient with an environmental allergy who’s trying to manage nasal congestion and also happened to have eczema, “I think they’re a great candidate for sublingual dust mite therapy because then not only am I treating their nasal congestions, their other symptoms, it may also help their eczema,” Dr. Sindher said.
Without those concurrent conditions, she said, the benefits of dust mite immunotherapy would not outweigh the risks or the potential burden on the patient of having to take the SLIT.
She said she would present the choice to the patient, and if other treatments haven’t been successful and the patient wants to try it, she would be open to a trial period.
The study was supported by the Brazilian National Council for Scientific and Technological Development, the Institute of Investigation in Immunology, the National Institutes of Science and Technology, the Brazilian National Council for Scientific and Technological Development, and the São Paulo Research Foundation. The mite extract for immunotherapy was provided by the laboratory IPI-ASAC Brasil/ASAC Pharma Brasil. Dr. Langer received a doctoral scholarship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES). Dr. Sindher reported no relevant financial relationships.
Commentary by Lawrence F. Eichenfield, MD
Environmental triggers of atopic dermatitis (AD) may be difficult to assess, especially as children with AD commonly develop “overlap” conditions of allergic rhinitis, food allergy, and asthma. The place of immunotherapy in treatment of AD has been controversial over the years, with mixed results from studies on its effect on eczema in different subpopulations. However, a holistic view of allergy care makes consideration of environmental allergies reasonable. The study by Dr. Langer and colleagues was a well-designed double-blind placebo-controlled trial of house dust mite sublingual immunotherapy in mite-sensitized AD patients aged 3 and older with at least mild AD, though the mean eczema severity was severe. After 18 months, there was an impressive 74% decrease in eczema score (SCORAD), but also a 58% decrease in the placebo group. While the primary outcome measure wasn’t statistically significant, some secondary ones were. I agree with the commentary in the article that the data doesn’t support immunotherapy being advised to everyone, while its use as an add-on treatment for certain patients in whom the eczema may overlap with other allergic manifestations is reasonable. For several years at Rady Children’s Hospital, San Diego, we have run a multidisciplinary atopic dermatitis program where patients are comanaged by dermatology and allergy. We have learned to appreciate that a broad perspective on managing comorbid conditions in children with AD really helps the patients and families to understand the many effects of inflammatory and allergic conditions, with improved outcomes and quality of life.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
, but improvement in the primary outcome was not significant, new data show.
Results of the small, randomized, double-blind, placebo-controlled trial were published recently in The Journal of Allergy and Clinical Immunology: In Practice.
Lead author Sarah Sella Langer, MD, of the department of medicine, Ribeirão Preto (Brazil) Medical School, University of São Paulo, and colleagues said their results suggest HDM SLIT is safe and effective as an add-on treatment.
The dust mite extract therapy had no major side effects after 18 months of treatment, the authors reported.
The researchers included data from 66 patients who completed the study. The participants were at least 3 years old, registered at least 15 on the SCORing Atopic Dermatitis (SCORAD) measure, and had a skin prick test and/or immunoglobulin E (IgE) test for sensitization to dust mites.
Patients were grouped by age (younger than 12 years or 12 years and older) to receive HDM SLIT (n = 35) or placebo (n = 31) 3 days a week for the study period – between May 2018 and June 2020 – at the Clinical Research Unit of Ribeirão Preto Medical School Hospital.
At baseline, the mean SCORAD was 46.9 (range, 17-87).
After 18 months, 74.2% and 58% of patients in HDM SLIT and placebo groups, respectively, showed at least a15-point decrease in SCORAD (relative risk, 1.28; 95% confidence interval, 0.89-1.83). However, those primary outcome results did not reach statistical significance.
On the other hand, some secondary outcomes did show significant results.
At 95% CI, the researchers reported significant objective-SCORAD decreases of 56.8% and 34.9% in HDM SLIT and placebo groups (average difference, 21.3). Significantly more patients had a score of 0 or 1 on the 5-point Investigator’s Global Assessment scale in the intervention group than in the placebo group (14/35 vs. 5/31; RR, 2.63).
There were no significant changes in the Eczema Area and Severity Index, the visual analogue scale for symptoms, the pruritus scale, or the Dermatology Life Quality Index.
Patients in the trial, most of whom had moderate to severe disease, continued to be treated with usual, individualized therapy for AD, in accordance with current guidelines and experts’ recommendations.
Tina Sindher, MD, an allergist with the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University, , told this news organization that the results are not robust enough to recommend the immunotherapy widely.
She pointed out that even in the placebo group, more than half the patients met the primary endpoint.
However, she did say HDM SLIT could be considered as an add-on treatment for the right patients, especially since risk for an allergic reaction or other adverse condition is small. The most common adverse effects were headache and abdominal pain, and they were reported in both the treatment and placebo groups.
With AD, she said, “there is no one drug that’s right for everyone,” because genetics and environment make the kind of symptoms and severity and duration different for each patient.
It all comes down to risk and benefits, she said.
She said if she had a patient with an environmental allergy who’s trying to manage nasal congestion and also happened to have eczema, “I think they’re a great candidate for sublingual dust mite therapy because then not only am I treating their nasal congestions, their other symptoms, it may also help their eczema,” Dr. Sindher said.
Without those concurrent conditions, she said, the benefits of dust mite immunotherapy would not outweigh the risks or the potential burden on the patient of having to take the SLIT.
She said she would present the choice to the patient, and if other treatments haven’t been successful and the patient wants to try it, she would be open to a trial period.
The study was supported by the Brazilian National Council for Scientific and Technological Development, the Institute of Investigation in Immunology, the National Institutes of Science and Technology, the Brazilian National Council for Scientific and Technological Development, and the São Paulo Research Foundation. The mite extract for immunotherapy was provided by the laboratory IPI-ASAC Brasil/ASAC Pharma Brasil. Dr. Langer received a doctoral scholarship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES). Dr. Sindher reported no relevant financial relationships.
Commentary by Lawrence F. Eichenfield, MD
Environmental triggers of atopic dermatitis (AD) may be difficult to assess, especially as children with AD commonly develop “overlap” conditions of allergic rhinitis, food allergy, and asthma. The place of immunotherapy in treatment of AD has been controversial over the years, with mixed results from studies on its effect on eczema in different subpopulations. However, a holistic view of allergy care makes consideration of environmental allergies reasonable. The study by Dr. Langer and colleagues was a well-designed double-blind placebo-controlled trial of house dust mite sublingual immunotherapy in mite-sensitized AD patients aged 3 and older with at least mild AD, though the mean eczema severity was severe. After 18 months, there was an impressive 74% decrease in eczema score (SCORAD), but also a 58% decrease in the placebo group. While the primary outcome measure wasn’t statistically significant, some secondary ones were. I agree with the commentary in the article that the data doesn’t support immunotherapy being advised to everyone, while its use as an add-on treatment for certain patients in whom the eczema may overlap with other allergic manifestations is reasonable. For several years at Rady Children’s Hospital, San Diego, we have run a multidisciplinary atopic dermatitis program where patients are comanaged by dermatology and allergy. We have learned to appreciate that a broad perspective on managing comorbid conditions in children with AD really helps the patients and families to understand the many effects of inflammatory and allergic conditions, with improved outcomes and quality of life.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
, but improvement in the primary outcome was not significant, new data show.
Results of the small, randomized, double-blind, placebo-controlled trial were published recently in The Journal of Allergy and Clinical Immunology: In Practice.
Lead author Sarah Sella Langer, MD, of the department of medicine, Ribeirão Preto (Brazil) Medical School, University of São Paulo, and colleagues said their results suggest HDM SLIT is safe and effective as an add-on treatment.
The dust mite extract therapy had no major side effects after 18 months of treatment, the authors reported.
The researchers included data from 66 patients who completed the study. The participants were at least 3 years old, registered at least 15 on the SCORing Atopic Dermatitis (SCORAD) measure, and had a skin prick test and/or immunoglobulin E (IgE) test for sensitization to dust mites.
Patients were grouped by age (younger than 12 years or 12 years and older) to receive HDM SLIT (n = 35) or placebo (n = 31) 3 days a week for the study period – between May 2018 and June 2020 – at the Clinical Research Unit of Ribeirão Preto Medical School Hospital.
At baseline, the mean SCORAD was 46.9 (range, 17-87).
After 18 months, 74.2% and 58% of patients in HDM SLIT and placebo groups, respectively, showed at least a15-point decrease in SCORAD (relative risk, 1.28; 95% confidence interval, 0.89-1.83). However, those primary outcome results did not reach statistical significance.
On the other hand, some secondary outcomes did show significant results.
At 95% CI, the researchers reported significant objective-SCORAD decreases of 56.8% and 34.9% in HDM SLIT and placebo groups (average difference, 21.3). Significantly more patients had a score of 0 or 1 on the 5-point Investigator’s Global Assessment scale in the intervention group than in the placebo group (14/35 vs. 5/31; RR, 2.63).
There were no significant changes in the Eczema Area and Severity Index, the visual analogue scale for symptoms, the pruritus scale, or the Dermatology Life Quality Index.
Patients in the trial, most of whom had moderate to severe disease, continued to be treated with usual, individualized therapy for AD, in accordance with current guidelines and experts’ recommendations.
Tina Sindher, MD, an allergist with the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University, , told this news organization that the results are not robust enough to recommend the immunotherapy widely.
She pointed out that even in the placebo group, more than half the patients met the primary endpoint.
However, she did say HDM SLIT could be considered as an add-on treatment for the right patients, especially since risk for an allergic reaction or other adverse condition is small. The most common adverse effects were headache and abdominal pain, and they were reported in both the treatment and placebo groups.
With AD, she said, “there is no one drug that’s right for everyone,” because genetics and environment make the kind of symptoms and severity and duration different for each patient.
It all comes down to risk and benefits, she said.
She said if she had a patient with an environmental allergy who’s trying to manage nasal congestion and also happened to have eczema, “I think they’re a great candidate for sublingual dust mite therapy because then not only am I treating their nasal congestions, their other symptoms, it may also help their eczema,” Dr. Sindher said.
Without those concurrent conditions, she said, the benefits of dust mite immunotherapy would not outweigh the risks or the potential burden on the patient of having to take the SLIT.
She said she would present the choice to the patient, and if other treatments haven’t been successful and the patient wants to try it, she would be open to a trial period.
The study was supported by the Brazilian National Council for Scientific and Technological Development, the Institute of Investigation in Immunology, the National Institutes of Science and Technology, the Brazilian National Council for Scientific and Technological Development, and the São Paulo Research Foundation. The mite extract for immunotherapy was provided by the laboratory IPI-ASAC Brasil/ASAC Pharma Brasil. Dr. Langer received a doctoral scholarship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES). Dr. Sindher reported no relevant financial relationships.
Commentary by Lawrence F. Eichenfield, MD
Environmental triggers of atopic dermatitis (AD) may be difficult to assess, especially as children with AD commonly develop “overlap” conditions of allergic rhinitis, food allergy, and asthma. The place of immunotherapy in treatment of AD has been controversial over the years, with mixed results from studies on its effect on eczema in different subpopulations. However, a holistic view of allergy care makes consideration of environmental allergies reasonable. The study by Dr. Langer and colleagues was a well-designed double-blind placebo-controlled trial of house dust mite sublingual immunotherapy in mite-sensitized AD patients aged 3 and older with at least mild AD, though the mean eczema severity was severe. After 18 months, there was an impressive 74% decrease in eczema score (SCORAD), but also a 58% decrease in the placebo group. While the primary outcome measure wasn’t statistically significant, some secondary ones were. I agree with the commentary in the article that the data doesn’t support immunotherapy being advised to everyone, while its use as an add-on treatment for certain patients in whom the eczema may overlap with other allergic manifestations is reasonable. For several years at Rady Children’s Hospital, San Diego, we have run a multidisciplinary atopic dermatitis program where patients are comanaged by dermatology and allergy. We have learned to appreciate that a broad perspective on managing comorbid conditions in children with AD really helps the patients and families to understand the many effects of inflammatory and allergic conditions, with improved outcomes and quality of life.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
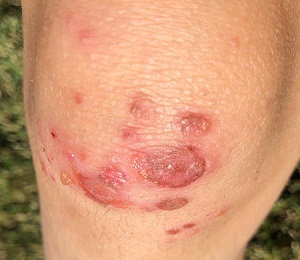
Tender Subcutaneous Nodule in a Prepubescent Boy
The Diagnosis: Dermatomyofibroma
Dermatomyofibroma is an uncommon, benign, cutaneous mesenchymal neoplasm composed of fibroblasts and myofibroblasts.1-3 This skin tumor was first described in 1991 by Hugel4 in the German literature as plaquelike fibromatosis. Pediatric dermatomyofibromas are exceedingly rare, with pediatric patients ranging in age from infants to teenagers.1
Clinically, dermatomyofibromas appear as long-standing, isolated, ill-demarcated, flesh-colored, slightly hyperpigmented or erythematous nodules or plaques that may be raised or indurated.1 Dermatomyofibromas may present with constant mild pain or pruritus, though in most cases the lesions are asymptomatic.1,3 The clinical presentation of dermatomyofibroma has a few distinct differences in children compared to adults. In adulthood, dermatomyofibroma has a strong female predominance and most commonly is located on the shoulder and adjacent upper body regions, including the axilla, neck, upper arm, and upper trunk.1-3 In childhood, the majority of dermatomyofibromas occur in young boys and usually are located on the neck with other upper body regions occurring less frequently.1,2 A shared characteristic includes the tendency for dermatomyofibromas to have an initial period of enlargement followed by stabilization or slow growth.1 Reported pediatric lesions have ranged in size from 4 to 60 mm with an average size of 14.9 mm (median, 12 mm).2
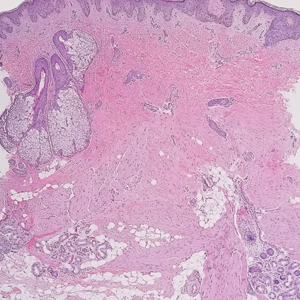
The diagnosis of dermatomyofibroma is based on histopathologic features in addition to clinical presentation. Histology from punch biopsy usually reveals a noninvasive dermal proliferation of bland, uniform, slender spindle cells oriented parallel to the overlying epidermis with increased and fragmented elastic fibers.1,3 Infiltration into the mid or deep dermis is common. The adnexal structures usually are spared; the stroma contains collagen and increased small blood vessels; and there typically is no inflammatory infiltrate, except for occasional scattered mast cells.2 Cytologically, the monomorphic spindleshaped tumor cells have an ill-defined, pale, eosinophilic cytoplasm and nuclei that are elongated with tapered edges.3 Dermatomyofibroma has a variable immunohistochemical profile, as it may stain focally positive for CD34 or smooth muscle actin, with occasional staining of factor XIIIa, desmin, calponin, or vimentin.1-3 Normal to increased levels of often fragmented elastic fibers is a helpful clue in distinguishing dermatomyofibroma from dermatofibroma, hypertrophic scar, dermatofibrosarcoma protuberans, and pilar leiomyoma, in which elastic fibers typically are reduced.3 Differential diagnoses of mesenchymal tumors in children include desmoid fibromatosis, connective tissue nevus, myofibromatosis, and smooth muscle hamartoma.1
A punch biopsy with clinical observation and followup is recommended for the management of lesions in cosmetically sensitive areas or in very young children who may not tolerate surgery. In symptomatic or cosmetically unappealing cases of dermatomyofibroma, simple surgical excision remains a viable treatment option. Recurrence is uncommon, even if only partially excised, and no instances of metastasis have been reported.1-5
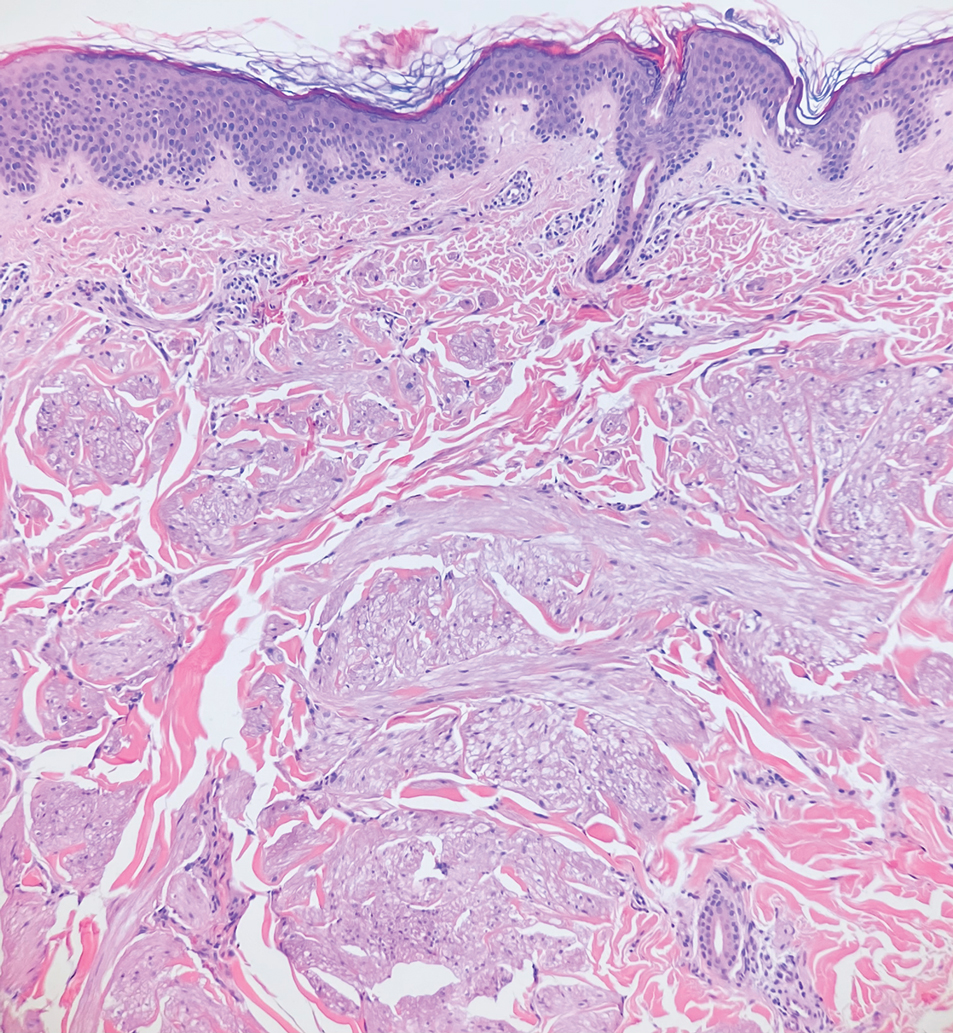
Dermatomyofibromas may be mistaken for several other entities both benign and malignant. For example, the benign dermatofibroma is the second most common fibrohistiocytic tumor of the skin and presents as a firm, nontender, minimally elevated to dome-shaped papule that usually measures less than or equal to 1 cm in diameter with or without overlying skin changes.5,6 It primarily is seen in adults with a slight female predominance and favors the lower extremities.5 Patients usually are asymptomatic but often report a history of local trauma at the lesion site.6 Histologically, dermatofibroma is characterized by a nodular dermal proliferation of spindleshaped fibrous cells and histiocytes in a storiform pattern (Figure 1).6 Epidermal induction with acanthosis overlying the tumor often is found with occasional basilar hyperpigmentation.5 Dermatofibroma also characteristically has trapped collagen (“collagen balls”) seen at the periphery.5,6
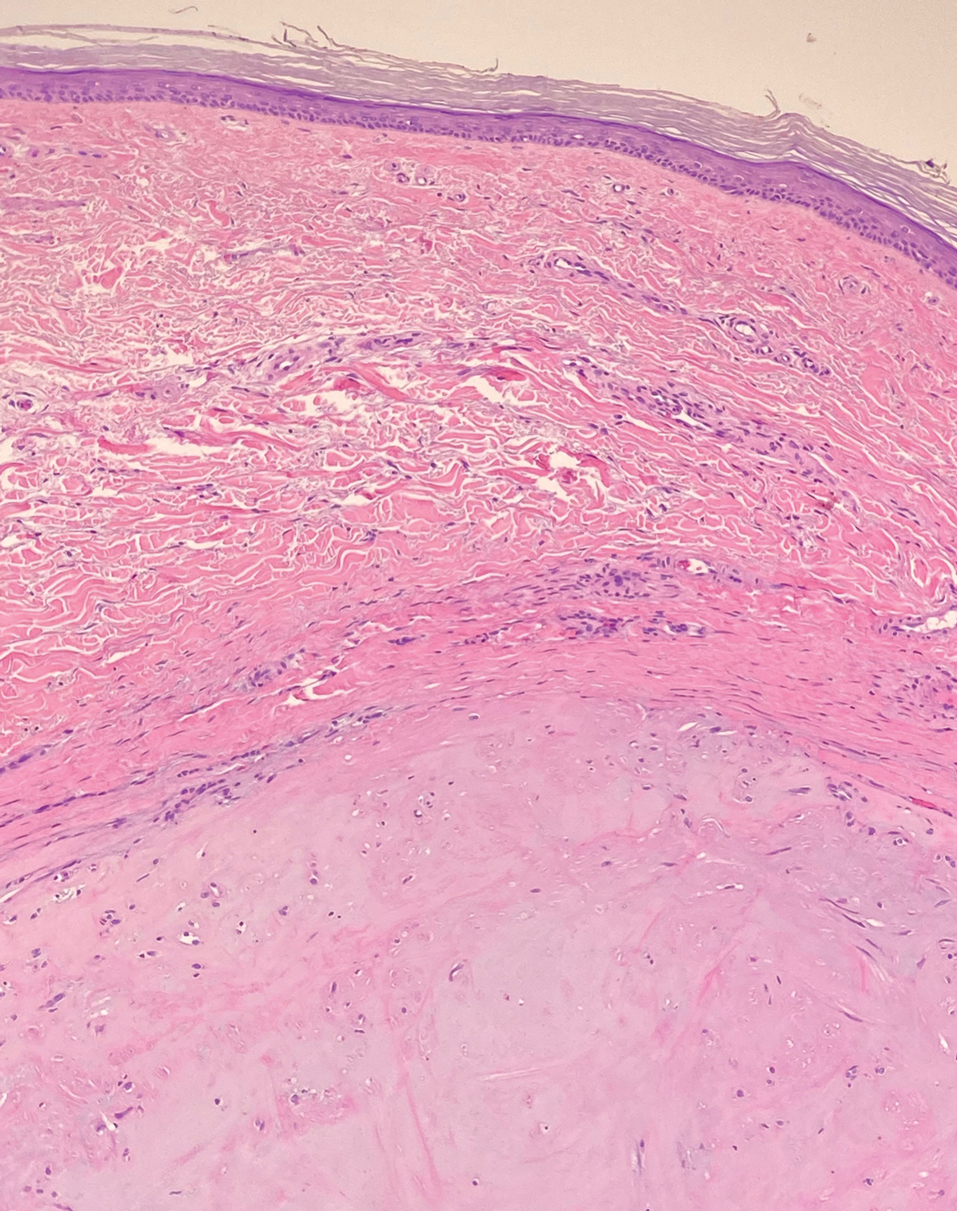
Piloleiomyomas are benign smooth muscle tumors arising from arrector pili muscles that may be solitary or multiple.5 Clinically, they typically present as firm, reddish-brown to flesh-colored papules or nodules that develop more commonly in adulthood.5,7 Piloleiomyomas favor the extremities and trunk, particularly the shoulder, and can be associated with spontaneous or induced pain. Histologically, piloleiomyomas are well circumscribed and centered within the reticular dermis situated closely to hair follicles (Figure 2).5 They are composed of numerous interlacing fascicles or whorls of smooth muscle cells with abundant eosinophilic cytoplasm and blunt-ended, cigar-shaped nuclei.5,7
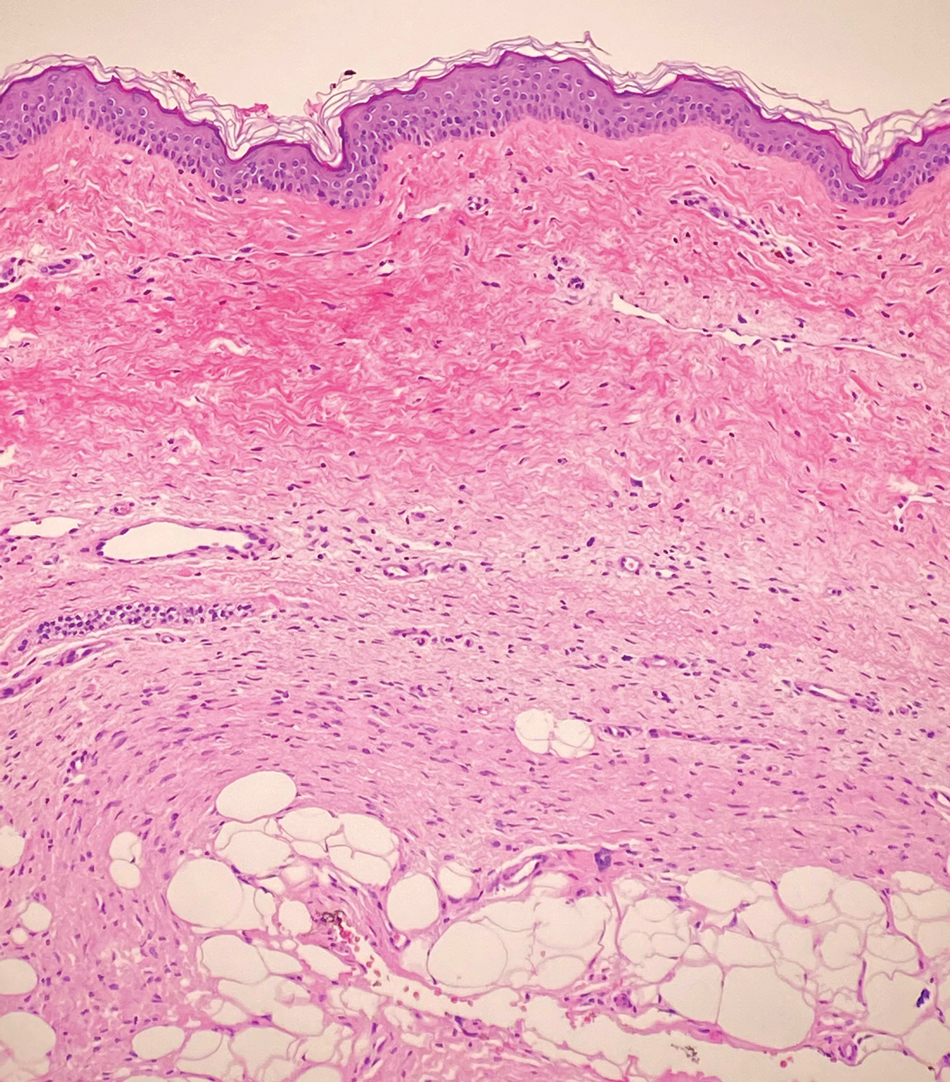
Solitary cutaneous myofibroma is a benign fibrous tumor found in adolescents and adults and is the counterpart to infantile myofibromatosis.8 Clinically, myofibromas typically present as painless, slow-growing, firm nodules with an occasional bluish hue. Histologically, solitary cutaneous myofibromas appear in a biphasic pattern, with hemangiopericytomatous components as well as spindle cells arranged in short bundles and fascicles resembling leiomyoma (Figure 3). The spindle cells also have abundant eosinophilic cytoplasm with short plump nuclei; the random, irregularly intersecting angles can be used to help differentiate myofibromas from smooth muscle lesions.8 Solitary cutaneous myofibroma is in the differential diagnosis for dermatomyofibroma because of their shared myofibroblastic nature.9
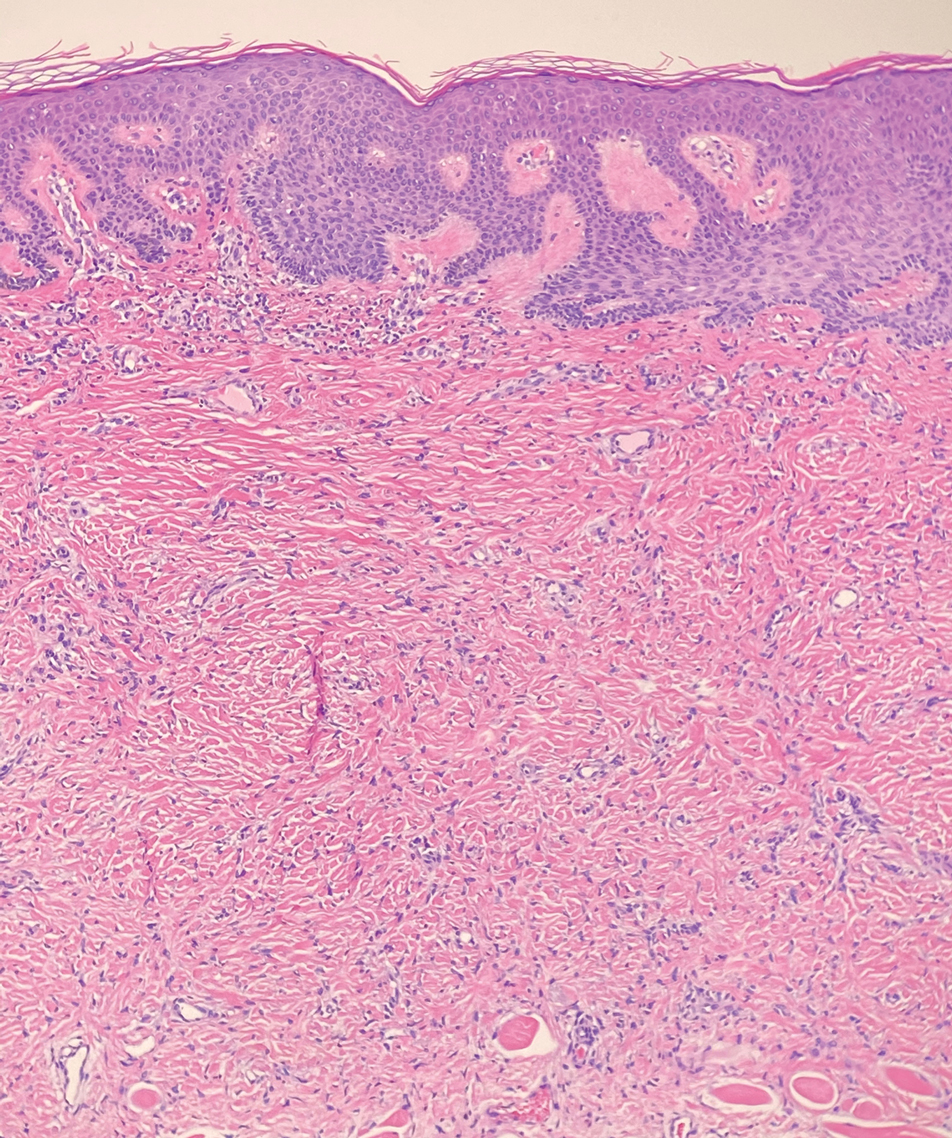
Dermatofibrosarcoma protuberans (DFSP) is an uncommon, locally invasive sarcoma with a high recurrence rate that favors young to middle-aged adults, with rare childhood onset reported.5,10,11 Clinically, DFSP typically presents as an asymptomatic, slow-growing, firm, flesh-colored, indurated plaque that develops into a violaceous to reddish-brown nodule.5 The atrophic variant of DFSP is characterized by a nonprotuberant lesion and can be especially difficult to distinguish from other entities such as dermatomyofibroma.11 The majority of DFSP lesions occur on the trunk, particularly in the shoulder or pelvic region.5 Histologically, early plaque lesions are comprised of monomorphic spindle cells arranged in long fascicles (parallel to the skin surface), infiltrating adnexal structures, and subcutaneous adipocytes in a multilayered honeycomb pattern; the spindle cells of late nodular lesions are arranged in short fascicles in a matted or storiform pattern (Figure 4).5,10 Early stages of DFSP as well as variations in childhood-onset DFSP can easily be misdiagnosed and incompletely excised.5
- Ma JE, Wieland CN, Tollefson MM. Dermatomyofibromas arising in children: report of two new cases and review of the literature. Pediatr Dermatol. 2017;34:347-351.
- Tardio JC, Azorin D, Hernandez-Nunez A, et al. Dermatomyofibromas presenting in pediatric patients: clinicopathologic characteristics and differential diagnosis. J Cutan Pathol. 2011;38:967-972.
- Mentzel T, Kutzner H. Dermatomyofibroma: clinicopathologic and immunohistochemical analysis of 56 cases and reappraisal of a rare and distinct cutaneous neoplasm. Am J Dermatopathol. 2009;31:44-49.
- Hugel H. Plaque-like dermal fibromatosis. Hautarzt. 1991;42:223-226.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. WB Saunders Co; 2012.
- Myers DJ, Fillman EP. Dermatofibroma. StatPearls [Internet]. StatPearls Publishing; 2020.
- Dilek N, Yuksel D, Sehitoglu I, et al. Cutaneous leiomyoma in a child: a case report. Oncol Lett. 2013;5:1163-1164.
- Roh HS, Paek JO, Yu HJ, et al. Solitary cutaneous myofibroma on the sole: an unusual localization. Ann Dermatol. 2012;24:220-222.
- Weedon D, Strutton G, Rubin AI, et al. Weedon’s Skin Pathology. Churchill Livingstone/Elsevier; 2010.
- Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004;101:2503-2508.
- Akay BN, Unlu E, Erdem C, et al. Dermatoscopic findings of atrophic dermatofibrosarcoma protuberans. Dermatol Pract Concept. 2015;5:71-73.
The Diagnosis: Dermatomyofibroma
Dermatomyofibroma is an uncommon, benign, cutaneous mesenchymal neoplasm composed of fibroblasts and myofibroblasts.1-3 This skin tumor was first described in 1991 by Hugel4 in the German literature as plaquelike fibromatosis. Pediatric dermatomyofibromas are exceedingly rare, with pediatric patients ranging in age from infants to teenagers.1
Clinically, dermatomyofibromas appear as long-standing, isolated, ill-demarcated, flesh-colored, slightly hyperpigmented or erythematous nodules or plaques that may be raised or indurated.1 Dermatomyofibromas may present with constant mild pain or pruritus, though in most cases the lesions are asymptomatic.1,3 The clinical presentation of dermatomyofibroma has a few distinct differences in children compared to adults. In adulthood, dermatomyofibroma has a strong female predominance and most commonly is located on the shoulder and adjacent upper body regions, including the axilla, neck, upper arm, and upper trunk.1-3 In childhood, the majority of dermatomyofibromas occur in young boys and usually are located on the neck with other upper body regions occurring less frequently.1,2 A shared characteristic includes the tendency for dermatomyofibromas to have an initial period of enlargement followed by stabilization or slow growth.1 Reported pediatric lesions have ranged in size from 4 to 60 mm with an average size of 14.9 mm (median, 12 mm).2
The diagnosis of dermatomyofibroma is based on histopathologic features in addition to clinical presentation. Histology from punch biopsy usually reveals a noninvasive dermal proliferation of bland, uniform, slender spindle cells oriented parallel to the overlying epidermis with increased and fragmented elastic fibers.1,3 Infiltration into the mid or deep dermis is common. The adnexal structures usually are spared; the stroma contains collagen and increased small blood vessels; and there typically is no inflammatory infiltrate, except for occasional scattered mast cells.2 Cytologically, the monomorphic spindleshaped tumor cells have an ill-defined, pale, eosinophilic cytoplasm and nuclei that are elongated with tapered edges.3 Dermatomyofibroma has a variable immunohistochemical profile, as it may stain focally positive for CD34 or smooth muscle actin, with occasional staining of factor XIIIa, desmin, calponin, or vimentin.1-3 Normal to increased levels of often fragmented elastic fibers is a helpful clue in distinguishing dermatomyofibroma from dermatofibroma, hypertrophic scar, dermatofibrosarcoma protuberans, and pilar leiomyoma, in which elastic fibers typically are reduced.3 Differential diagnoses of mesenchymal tumors in children include desmoid fibromatosis, connective tissue nevus, myofibromatosis, and smooth muscle hamartoma.1
A punch biopsy with clinical observation and followup is recommended for the management of lesions in cosmetically sensitive areas or in very young children who may not tolerate surgery. In symptomatic or cosmetically unappealing cases of dermatomyofibroma, simple surgical excision remains a viable treatment option. Recurrence is uncommon, even if only partially excised, and no instances of metastasis have been reported.1-5
Dermatomyofibromas may be mistaken for several other entities both benign and malignant. For example, the benign dermatofibroma is the second most common fibrohistiocytic tumor of the skin and presents as a firm, nontender, minimally elevated to dome-shaped papule that usually measures less than or equal to 1 cm in diameter with or without overlying skin changes.5,6 It primarily is seen in adults with a slight female predominance and favors the lower extremities.5 Patients usually are asymptomatic but often report a history of local trauma at the lesion site.6 Histologically, dermatofibroma is characterized by a nodular dermal proliferation of spindleshaped fibrous cells and histiocytes in a storiform pattern (Figure 1).6 Epidermal induction with acanthosis overlying the tumor often is found with occasional basilar hyperpigmentation.5 Dermatofibroma also characteristically has trapped collagen (“collagen balls”) seen at the periphery.5,6

Piloleiomyomas are benign smooth muscle tumors arising from arrector pili muscles that may be solitary or multiple.5 Clinically, they typically present as firm, reddish-brown to flesh-colored papules or nodules that develop more commonly in adulthood.5,7 Piloleiomyomas favor the extremities and trunk, particularly the shoulder, and can be associated with spontaneous or induced pain. Histologically, piloleiomyomas are well circumscribed and centered within the reticular dermis situated closely to hair follicles (Figure 2).5 They are composed of numerous interlacing fascicles or whorls of smooth muscle cells with abundant eosinophilic cytoplasm and blunt-ended, cigar-shaped nuclei.5,7

Solitary cutaneous myofibroma is a benign fibrous tumor found in adolescents and adults and is the counterpart to infantile myofibromatosis.8 Clinically, myofibromas typically present as painless, slow-growing, firm nodules with an occasional bluish hue. Histologically, solitary cutaneous myofibromas appear in a biphasic pattern, with hemangiopericytomatous components as well as spindle cells arranged in short bundles and fascicles resembling leiomyoma (Figure 3). The spindle cells also have abundant eosinophilic cytoplasm with short plump nuclei; the random, irregularly intersecting angles can be used to help differentiate myofibromas from smooth muscle lesions.8 Solitary cutaneous myofibroma is in the differential diagnosis for dermatomyofibroma because of their shared myofibroblastic nature.9

Dermatofibrosarcoma protuberans (DFSP) is an uncommon, locally invasive sarcoma with a high recurrence rate that favors young to middle-aged adults, with rare childhood onset reported.5,10,11 Clinically, DFSP typically presents as an asymptomatic, slow-growing, firm, flesh-colored, indurated plaque that develops into a violaceous to reddish-brown nodule.5 The atrophic variant of DFSP is characterized by a nonprotuberant lesion and can be especially difficult to distinguish from other entities such as dermatomyofibroma.11 The majority of DFSP lesions occur on the trunk, particularly in the shoulder or pelvic region.5 Histologically, early plaque lesions are comprised of monomorphic spindle cells arranged in long fascicles (parallel to the skin surface), infiltrating adnexal structures, and subcutaneous adipocytes in a multilayered honeycomb pattern; the spindle cells of late nodular lesions are arranged in short fascicles in a matted or storiform pattern (Figure 4).5,10 Early stages of DFSP as well as variations in childhood-onset DFSP can easily be misdiagnosed and incompletely excised.5

The Diagnosis: Dermatomyofibroma
Dermatomyofibroma is an uncommon, benign, cutaneous mesenchymal neoplasm composed of fibroblasts and myofibroblasts.1-3 This skin tumor was first described in 1991 by Hugel4 in the German literature as plaquelike fibromatosis. Pediatric dermatomyofibromas are exceedingly rare, with pediatric patients ranging in age from infants to teenagers.1
Clinically, dermatomyofibromas appear as long-standing, isolated, ill-demarcated, flesh-colored, slightly hyperpigmented or erythematous nodules or plaques that may be raised or indurated.1 Dermatomyofibromas may present with constant mild pain or pruritus, though in most cases the lesions are asymptomatic.1,3 The clinical presentation of dermatomyofibroma has a few distinct differences in children compared to adults. In adulthood, dermatomyofibroma has a strong female predominance and most commonly is located on the shoulder and adjacent upper body regions, including the axilla, neck, upper arm, and upper trunk.1-3 In childhood, the majority of dermatomyofibromas occur in young boys and usually are located on the neck with other upper body regions occurring less frequently.1,2 A shared characteristic includes the tendency for dermatomyofibromas to have an initial period of enlargement followed by stabilization or slow growth.1 Reported pediatric lesions have ranged in size from 4 to 60 mm with an average size of 14.9 mm (median, 12 mm).2
The diagnosis of dermatomyofibroma is based on histopathologic features in addition to clinical presentation. Histology from punch biopsy usually reveals a noninvasive dermal proliferation of bland, uniform, slender spindle cells oriented parallel to the overlying epidermis with increased and fragmented elastic fibers.1,3 Infiltration into the mid or deep dermis is common. The adnexal structures usually are spared; the stroma contains collagen and increased small blood vessels; and there typically is no inflammatory infiltrate, except for occasional scattered mast cells.2 Cytologically, the monomorphic spindleshaped tumor cells have an ill-defined, pale, eosinophilic cytoplasm and nuclei that are elongated with tapered edges.3 Dermatomyofibroma has a variable immunohistochemical profile, as it may stain focally positive for CD34 or smooth muscle actin, with occasional staining of factor XIIIa, desmin, calponin, or vimentin.1-3 Normal to increased levels of often fragmented elastic fibers is a helpful clue in distinguishing dermatomyofibroma from dermatofibroma, hypertrophic scar, dermatofibrosarcoma protuberans, and pilar leiomyoma, in which elastic fibers typically are reduced.3 Differential diagnoses of mesenchymal tumors in children include desmoid fibromatosis, connective tissue nevus, myofibromatosis, and smooth muscle hamartoma.1
A punch biopsy with clinical observation and followup is recommended for the management of lesions in cosmetically sensitive areas or in very young children who may not tolerate surgery. In symptomatic or cosmetically unappealing cases of dermatomyofibroma, simple surgical excision remains a viable treatment option. Recurrence is uncommon, even if only partially excised, and no instances of metastasis have been reported.1-5
Dermatomyofibromas may be mistaken for several other entities both benign and malignant. For example, the benign dermatofibroma is the second most common fibrohistiocytic tumor of the skin and presents as a firm, nontender, minimally elevated to dome-shaped papule that usually measures less than or equal to 1 cm in diameter with or without overlying skin changes.5,6 It primarily is seen in adults with a slight female predominance and favors the lower extremities.5 Patients usually are asymptomatic but often report a history of local trauma at the lesion site.6 Histologically, dermatofibroma is characterized by a nodular dermal proliferation of spindleshaped fibrous cells and histiocytes in a storiform pattern (Figure 1).6 Epidermal induction with acanthosis overlying the tumor often is found with occasional basilar hyperpigmentation.5 Dermatofibroma also characteristically has trapped collagen (“collagen balls”) seen at the periphery.5,6

Piloleiomyomas are benign smooth muscle tumors arising from arrector pili muscles that may be solitary or multiple.5 Clinically, they typically present as firm, reddish-brown to flesh-colored papules or nodules that develop more commonly in adulthood.5,7 Piloleiomyomas favor the extremities and trunk, particularly the shoulder, and can be associated with spontaneous or induced pain. Histologically, piloleiomyomas are well circumscribed and centered within the reticular dermis situated closely to hair follicles (Figure 2).5 They are composed of numerous interlacing fascicles or whorls of smooth muscle cells with abundant eosinophilic cytoplasm and blunt-ended, cigar-shaped nuclei.5,7

Solitary cutaneous myofibroma is a benign fibrous tumor found in adolescents and adults and is the counterpart to infantile myofibromatosis.8 Clinically, myofibromas typically present as painless, slow-growing, firm nodules with an occasional bluish hue. Histologically, solitary cutaneous myofibromas appear in a biphasic pattern, with hemangiopericytomatous components as well as spindle cells arranged in short bundles and fascicles resembling leiomyoma (Figure 3). The spindle cells also have abundant eosinophilic cytoplasm with short plump nuclei; the random, irregularly intersecting angles can be used to help differentiate myofibromas from smooth muscle lesions.8 Solitary cutaneous myofibroma is in the differential diagnosis for dermatomyofibroma because of their shared myofibroblastic nature.9

Dermatofibrosarcoma protuberans (DFSP) is an uncommon, locally invasive sarcoma with a high recurrence rate that favors young to middle-aged adults, with rare childhood onset reported.5,10,11 Clinically, DFSP typically presents as an asymptomatic, slow-growing, firm, flesh-colored, indurated plaque that develops into a violaceous to reddish-brown nodule.5 The atrophic variant of DFSP is characterized by a nonprotuberant lesion and can be especially difficult to distinguish from other entities such as dermatomyofibroma.11 The majority of DFSP lesions occur on the trunk, particularly in the shoulder or pelvic region.5 Histologically, early plaque lesions are comprised of monomorphic spindle cells arranged in long fascicles (parallel to the skin surface), infiltrating adnexal structures, and subcutaneous adipocytes in a multilayered honeycomb pattern; the spindle cells of late nodular lesions are arranged in short fascicles in a matted or storiform pattern (Figure 4).5,10 Early stages of DFSP as well as variations in childhood-onset DFSP can easily be misdiagnosed and incompletely excised.5

- Ma JE, Wieland CN, Tollefson MM. Dermatomyofibromas arising in children: report of two new cases and review of the literature. Pediatr Dermatol. 2017;34:347-351.
- Tardio JC, Azorin D, Hernandez-Nunez A, et al. Dermatomyofibromas presenting in pediatric patients: clinicopathologic characteristics and differential diagnosis. J Cutan Pathol. 2011;38:967-972.
- Mentzel T, Kutzner H. Dermatomyofibroma: clinicopathologic and immunohistochemical analysis of 56 cases and reappraisal of a rare and distinct cutaneous neoplasm. Am J Dermatopathol. 2009;31:44-49.
- Hugel H. Plaque-like dermal fibromatosis. Hautarzt. 1991;42:223-226.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. WB Saunders Co; 2012.
- Myers DJ, Fillman EP. Dermatofibroma. StatPearls [Internet]. StatPearls Publishing; 2020.
- Dilek N, Yuksel D, Sehitoglu I, et al. Cutaneous leiomyoma in a child: a case report. Oncol Lett. 2013;5:1163-1164.
- Roh HS, Paek JO, Yu HJ, et al. Solitary cutaneous myofibroma on the sole: an unusual localization. Ann Dermatol. 2012;24:220-222.
- Weedon D, Strutton G, Rubin AI, et al. Weedon’s Skin Pathology. Churchill Livingstone/Elsevier; 2010.
- Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004;101:2503-2508.
- Akay BN, Unlu E, Erdem C, et al. Dermatoscopic findings of atrophic dermatofibrosarcoma protuberans. Dermatol Pract Concept. 2015;5:71-73.
- Ma JE, Wieland CN, Tollefson MM. Dermatomyofibromas arising in children: report of two new cases and review of the literature. Pediatr Dermatol. 2017;34:347-351.
- Tardio JC, Azorin D, Hernandez-Nunez A, et al. Dermatomyofibromas presenting in pediatric patients: clinicopathologic characteristics and differential diagnosis. J Cutan Pathol. 2011;38:967-972.
- Mentzel T, Kutzner H. Dermatomyofibroma: clinicopathologic and immunohistochemical analysis of 56 cases and reappraisal of a rare and distinct cutaneous neoplasm. Am J Dermatopathol. 2009;31:44-49.
- Hugel H. Plaque-like dermal fibromatosis. Hautarzt. 1991;42:223-226.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. WB Saunders Co; 2012.
- Myers DJ, Fillman EP. Dermatofibroma. StatPearls [Internet]. StatPearls Publishing; 2020.
- Dilek N, Yuksel D, Sehitoglu I, et al. Cutaneous leiomyoma in a child: a case report. Oncol Lett. 2013;5:1163-1164.
- Roh HS, Paek JO, Yu HJ, et al. Solitary cutaneous myofibroma on the sole: an unusual localization. Ann Dermatol. 2012;24:220-222.
- Weedon D, Strutton G, Rubin AI, et al. Weedon’s Skin Pathology. Churchill Livingstone/Elsevier; 2010.
- Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004;101:2503-2508.
- Akay BN, Unlu E, Erdem C, et al. Dermatoscopic findings of atrophic dermatofibrosarcoma protuberans. Dermatol Pract Concept. 2015;5:71-73.
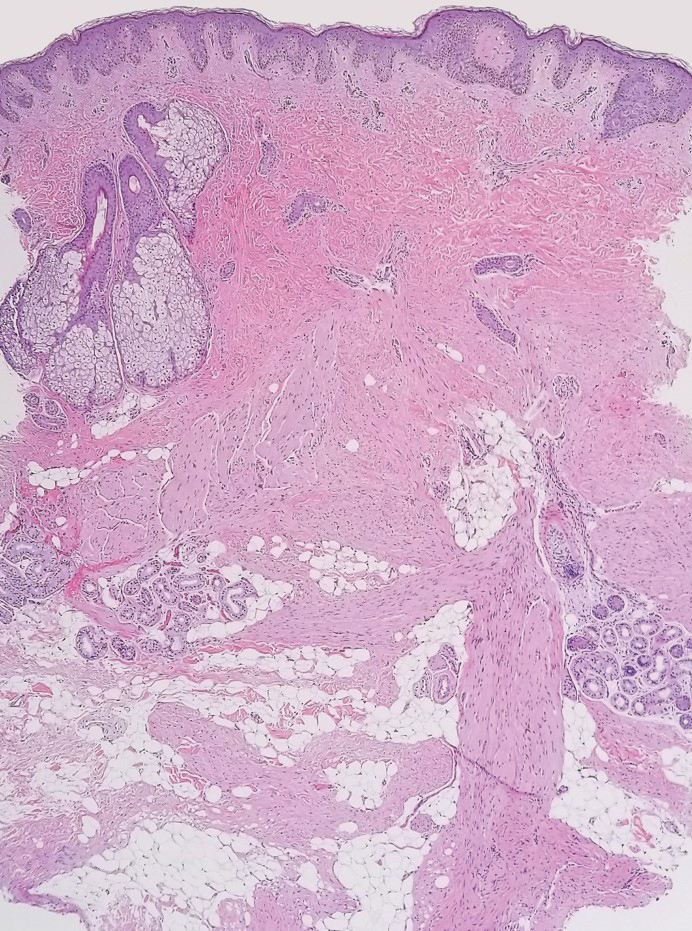

A 12-year-old boy with olive skin presented with a tender subcutaneous nodule on the back of 6 months’ duration. He reported the lesion initially grew rapidly with increasing pain for approximately 3 months with subsequent stabilization in size and modest resolution of his symptoms. Physical examination revealed a solitary, 15-mm, ill-defined, indurated, tender, subcutaneous nodule with subtle overlying hyperpigmentation on the left side of the upper back. Hematoxylin and eosin staining of a 4-mm punch biopsy revealed a nonencapsulated mass of monomorphic eosinophilic spindle cells organized into fascicles arranged predominantly parallel to the skin surface. The mass extended from the mid reticular dermis to the upper subcutis, sparing adnexal structures.
An expensive lesson
In mid-July my son strained his neck working out at the gym.
It was an obvious generic muscle pull. I told him to take some ibuprofen and use a heating pad. My wife, a nurse, told him the same thing.
Regrettably, while my medical training (hopefully) counts for something with my patients, it doesn’t mean much to my kids. The unqualified opinions of their friends and Google are far more worthwhile, convincing him he had any number of serious injuries.
As a result, while we were at work he went to the emergency department to get checked out. He was evaluated by one of my colleagues who did x-rays and a cervical spine CT. (I figure the last one was because my son kept reminding them I was a doctor). After all the results were in, the ED physician told him he had a muscle strain, and to take ibuprofen and use a heating pad.
Big surprise, huh? I’m sure he was shocked to find out that his old man knew what he was doing. Of course, I didn’t order any tests so the ED doc tops me for that in my son’s mind.
But kids not believing their parents is nothing new, and I can’t claim innocence either from what I remember of being a teenager.
Fast-forward to today. From what I can see, the total bills for his little adventure in modern medicine were around $4,000-$5,000. Granted, I’m well aware that what gets charged has no relationship to what’s actually going to be collected but I’m not going to write about modern medical charges or collections or even defensive medicine. I understand all those, and certainly don’t fault my ED colleague for how he handled it.
Reassurance isn’t cheap, though. When it’s all over, our out-of-pocket share will be roughly $1,000, which we certainly hadn’t planned for in the usually money-tight months of December and January.
That’s a lot of money for ibuprofen and a heating pad (we had both at home, and they’re around $20 total at Target, anyway).
There’s certainly no shortage of research on unnecessary ED visits for minor things, but to me this is a classic example of it. Beyond just the financial cost (which, admittedly, I’m pretty irritated with him about) he tied up a bed and ED staff that someone in more dire circumstances may have needed.
His injury could have been handled at an urgent care, or, even better, just by staying home, listening to us, and using ibuprofen and a heating pad.
, and clarify what constitutes an emergency in the first place. There’s no shortage of urgent cares and other walk-in clinics that are there specifically to handle such things during daylight hours, if they need to be seen at all.
Of course, I can’t change the results of Google searches, or the age-old teenage belief that parents are morons.
But he is going to learn about what constitutes an emergency, and what else that $1,000 could have been used for.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In mid-July my son strained his neck working out at the gym.
It was an obvious generic muscle pull. I told him to take some ibuprofen and use a heating pad. My wife, a nurse, told him the same thing.
Regrettably, while my medical training (hopefully) counts for something with my patients, it doesn’t mean much to my kids. The unqualified opinions of their friends and Google are far more worthwhile, convincing him he had any number of serious injuries.
As a result, while we were at work he went to the emergency department to get checked out. He was evaluated by one of my colleagues who did x-rays and a cervical spine CT. (I figure the last one was because my son kept reminding them I was a doctor). After all the results were in, the ED physician told him he had a muscle strain, and to take ibuprofen and use a heating pad.
Big surprise, huh? I’m sure he was shocked to find out that his old man knew what he was doing. Of course, I didn’t order any tests so the ED doc tops me for that in my son’s mind.
But kids not believing their parents is nothing new, and I can’t claim innocence either from what I remember of being a teenager.
Fast-forward to today. From what I can see, the total bills for his little adventure in modern medicine were around $4,000-$5,000. Granted, I’m well aware that what gets charged has no relationship to what’s actually going to be collected but I’m not going to write about modern medical charges or collections or even defensive medicine. I understand all those, and certainly don’t fault my ED colleague for how he handled it.
Reassurance isn’t cheap, though. When it’s all over, our out-of-pocket share will be roughly $1,000, which we certainly hadn’t planned for in the usually money-tight months of December and January.
That’s a lot of money for ibuprofen and a heating pad (we had both at home, and they’re around $20 total at Target, anyway).
There’s certainly no shortage of research on unnecessary ED visits for minor things, but to me this is a classic example of it. Beyond just the financial cost (which, admittedly, I’m pretty irritated with him about) he tied up a bed and ED staff that someone in more dire circumstances may have needed.
His injury could have been handled at an urgent care, or, even better, just by staying home, listening to us, and using ibuprofen and a heating pad.
, and clarify what constitutes an emergency in the first place. There’s no shortage of urgent cares and other walk-in clinics that are there specifically to handle such things during daylight hours, if they need to be seen at all.
Of course, I can’t change the results of Google searches, or the age-old teenage belief that parents are morons.
But he is going to learn about what constitutes an emergency, and what else that $1,000 could have been used for.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In mid-July my son strained his neck working out at the gym.
It was an obvious generic muscle pull. I told him to take some ibuprofen and use a heating pad. My wife, a nurse, told him the same thing.
Regrettably, while my medical training (hopefully) counts for something with my patients, it doesn’t mean much to my kids. The unqualified opinions of their friends and Google are far more worthwhile, convincing him he had any number of serious injuries.
As a result, while we were at work he went to the emergency department to get checked out. He was evaluated by one of my colleagues who did x-rays and a cervical spine CT. (I figure the last one was because my son kept reminding them I was a doctor). After all the results were in, the ED physician told him he had a muscle strain, and to take ibuprofen and use a heating pad.
Big surprise, huh? I’m sure he was shocked to find out that his old man knew what he was doing. Of course, I didn’t order any tests so the ED doc tops me for that in my son’s mind.
But kids not believing their parents is nothing new, and I can’t claim innocence either from what I remember of being a teenager.
Fast-forward to today. From what I can see, the total bills for his little adventure in modern medicine were around $4,000-$5,000. Granted, I’m well aware that what gets charged has no relationship to what’s actually going to be collected but I’m not going to write about modern medical charges or collections or even defensive medicine. I understand all those, and certainly don’t fault my ED colleague for how he handled it.
Reassurance isn’t cheap, though. When it’s all over, our out-of-pocket share will be roughly $1,000, which we certainly hadn’t planned for in the usually money-tight months of December and January.
That’s a lot of money for ibuprofen and a heating pad (we had both at home, and they’re around $20 total at Target, anyway).
There’s certainly no shortage of research on unnecessary ED visits for minor things, but to me this is a classic example of it. Beyond just the financial cost (which, admittedly, I’m pretty irritated with him about) he tied up a bed and ED staff that someone in more dire circumstances may have needed.
His injury could have been handled at an urgent care, or, even better, just by staying home, listening to us, and using ibuprofen and a heating pad.
, and clarify what constitutes an emergency in the first place. There’s no shortage of urgent cares and other walk-in clinics that are there specifically to handle such things during daylight hours, if they need to be seen at all.
Of course, I can’t change the results of Google searches, or the age-old teenage belief that parents are morons.
But he is going to learn about what constitutes an emergency, and what else that $1,000 could have been used for.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In (K)need of Help
ANSWER
The correct answer is bullous impetigo (choice “a”).
Had the source of the problem been MRSA (choice “b”), there would have been marked tenderness and swelling. Psoriasis vulgaris (choice “c”) was a possibility; however, it almost never manifests with blisters, it rarely comes on as quickly as this patient’s problem did, and it produces scale that is far thicker and more tenacious than that seen in bullous impetigo. Nummular eczema (choice “d”) does not manifest with blisters and would likely have caused itching.
DISCUSSION
Impetigo is one of many common skin diseases—other examples include granuloma annulare and lichen planus—that have bullous variants, which can make diagnosis challenging. Impetigo is easy to diagnose in its more common papulosquamous form. But it also can manifest with flaccid blisters that last only a short time, leaving the round, scaly lesions seen in this case.
Staphylococcus aureus, the organism responsible for bullous impetigo, elaborates serine proteases, which bind to and cleave desmoglein-1. This effectively destroys the connections between skin layers, creating a space that is quickly filled with serum. The level of this separation is typically subcorneal, which allows the formation of a very thin, friable roof for the bulla.
This modern version of impetigo is not considered dangerous, despite its association with staph aureus. In pre-antibiotic times, the predominant organism was streptococcal, some types of which could be nephritogenic—that is, capable of causing Bright disease or, as it is now known, acute post-streptococcal glomerulonephritis. Fortunately, this potentially fatal condition is only rarely seen in modern times.
Two items of note about this case: Bullous impetigo, contrary to what was seen in this patient, typically favors intertriginous areas. And a key factor is the history of atopy, which renders the patient more susceptible to skin infections of all kinds.
Treatment
The patient responded well to topical mupirocin ointment, applied three times a day. In rare instances, impetigo can require an oral antibiotic, such as cephalexin.
ANSWER
The correct answer is bullous impetigo (choice “a”).
Had the source of the problem been MRSA (choice “b”), there would have been marked tenderness and swelling. Psoriasis vulgaris (choice “c”) was a possibility; however, it almost never manifests with blisters, it rarely comes on as quickly as this patient’s problem did, and it produces scale that is far thicker and more tenacious than that seen in bullous impetigo. Nummular eczema (choice “d”) does not manifest with blisters and would likely have caused itching.
DISCUSSION
Impetigo is one of many common skin diseases—other examples include granuloma annulare and lichen planus—that have bullous variants, which can make diagnosis challenging. Impetigo is easy to diagnose in its more common papulosquamous form. But it also can manifest with flaccid blisters that last only a short time, leaving the round, scaly lesions seen in this case.
Staphylococcus aureus, the organism responsible for bullous impetigo, elaborates serine proteases, which bind to and cleave desmoglein-1. This effectively destroys the connections between skin layers, creating a space that is quickly filled with serum. The level of this separation is typically subcorneal, which allows the formation of a very thin, friable roof for the bulla.
This modern version of impetigo is not considered dangerous, despite its association with staph aureus. In pre-antibiotic times, the predominant organism was streptococcal, some types of which could be nephritogenic—that is, capable of causing Bright disease or, as it is now known, acute post-streptococcal glomerulonephritis. Fortunately, this potentially fatal condition is only rarely seen in modern times.
Two items of note about this case: Bullous impetigo, contrary to what was seen in this patient, typically favors intertriginous areas. And a key factor is the history of atopy, which renders the patient more susceptible to skin infections of all kinds.
Treatment
The patient responded well to topical mupirocin ointment, applied three times a day. In rare instances, impetigo can require an oral antibiotic, such as cephalexin.
ANSWER
The correct answer is bullous impetigo (choice “a”).
Had the source of the problem been MRSA (choice “b”), there would have been marked tenderness and swelling. Psoriasis vulgaris (choice “c”) was a possibility; however, it almost never manifests with blisters, it rarely comes on as quickly as this patient’s problem did, and it produces scale that is far thicker and more tenacious than that seen in bullous impetigo. Nummular eczema (choice “d”) does not manifest with blisters and would likely have caused itching.
DISCUSSION
Impetigo is one of many common skin diseases—other examples include granuloma annulare and lichen planus—that have bullous variants, which can make diagnosis challenging. Impetigo is easy to diagnose in its more common papulosquamous form. But it also can manifest with flaccid blisters that last only a short time, leaving the round, scaly lesions seen in this case.
Staphylococcus aureus, the organism responsible for bullous impetigo, elaborates serine proteases, which bind to and cleave desmoglein-1. This effectively destroys the connections between skin layers, creating a space that is quickly filled with serum. The level of this separation is typically subcorneal, which allows the formation of a very thin, friable roof for the bulla.
This modern version of impetigo is not considered dangerous, despite its association with staph aureus. In pre-antibiotic times, the predominant organism was streptococcal, some types of which could be nephritogenic—that is, capable of causing Bright disease or, as it is now known, acute post-streptococcal glomerulonephritis. Fortunately, this potentially fatal condition is only rarely seen in modern times.
Two items of note about this case: Bullous impetigo, contrary to what was seen in this patient, typically favors intertriginous areas. And a key factor is the history of atopy, which renders the patient more susceptible to skin infections of all kinds.
Treatment
The patient responded well to topical mupirocin ointment, applied three times a day. In rare instances, impetigo can require an oral antibiotic, such as cephalexin.
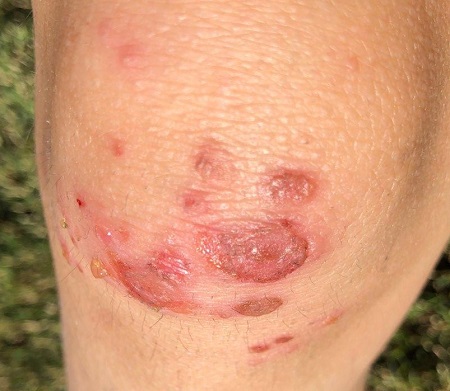
The parents of a 6-year-old boy were quite concerned about several lesions on the child’s left knee. The asymptomatic blisters had first appeared about 2 weeks prior. A topical steroid cream prescribed by the family’s primary care provider had not helped.
No one else in the family was similarly affected. They all were reportedly quite healthy, although all were atopic—prone to seasonal allergies and eczema.
Examination revealed at least 6 round, scaly, red lesions on the patient’s knee, ranging from 3 mm to 3 cm in diameter. According to the parents, these had first appeared as intact blisters. There was little to no tenderness or redness around the lesions, and there was no palpable adenopathy in the groin.
The child was in no distress and was afebrile.
Baked milk immunotherapy may help children with cow’s milk allergy
, new research suggests.
The small, ongoing clinical trial has enabled some participants – all of whom reacted to less than a tablespoon of baked milk at baseline – to begin incorporating baked milk products into everyday diets and to eat in restaurants with less fear of allergic reactions, reported study author Jennifer Dantzer, MD, MHS, assistant professor of pediatrics in the division of pediatric allergy, immunology, and rheumatology at Johns Hopkins University in Baltimore.
Cow’s milk is the most common food allergy in young children, and “for many, it’s a constant stressor that’s always there,” Dr. Dantzer said in an interview. “For a lot of families, this impacts where they eat out, if they eat out, and sometimes where they vacation, or a lot of the social activities they do.
“This was a unique group of kids with a very severe milk phenotype who were reactive to teeny doses and may not have qualified or done well with other types of oral immunotherapy,” she added. “Using a modified allergen – baked milk – seems to work. But for now, we think this is something that still needs further research before it’s ready for a clinical setting.”
The study, for which 24-month unblinded results are being tallied, was recently published in the Journal of Allergy and Clinical Immunology .
About 2%-3% of preschool-age children are affected by cow’s milk allergy. Children often outgrow it, but for about 20% of children, it persists into adolescence and adulthood. The only current management approaches are avoidance and emergency medications to treat reactions.
But for those with severe milk allergy who react to even trace amounts of milk in any form, the now-routine clinical practice of introducing baked milk isn’t an option, Dr. Dantzer said. The new trial stood out from prior research by using lower starting doses and a more gradual dose escalation of extensively heated milk to determine if oral immunotherapy could be safer but still effective.
Dr. Dantzer and her team randomly assigned 30 participants (aged 3-18 years) into two blinded groups. For 12 months, one group received baked milk oral immunotherapy (BMOIT), and the other a placebo consisting of tapioca flour. At baseline, for all participants, the milk skin prick test wheal diameter was ≥ 3 mm, and the cow’s milk immunoglobulin E (IgE) level was > 5 kU/L. All the children experienced positive dose-limiting reactions to < 1 tablespoon of baked milk protein but could tolerate at least 3 mg on initial dose escalation.
Measured doses of baked milk and placebo powders were supplied to participants for all doses consumed at home. Participants were given instructions on how to prepare it in cupcake or muffin batter. Over 12 months, doses were gradually increased to a maximum cumulative dose of 4,044 mg baked milk protein, or approximately a half tablespoon.
Researchers collected blood samples for immune studies, and participants or their parents completed quality-of-life questionnaires that asked about food anxiety, social and dietary limitations, emotional impact, risk for accidental ingestion, and allergen avoidance.
Fourteen of 15 participants (93%) in the BMOIT group reached the goal-maintenance dose of 2,000 mg of baked milk protein (about a quarter tablespoon). Of those who completed the 12-month challenge, 11 of 14 (79%) in the BMOIT group tolerated 4,000 mg of baked milk protein, compared to none in the placebo group.
“We anticipated that by starting with really small amounts, we would be able to build up the amount of baked milk these kids could tolerate,” Dr. Dantzer said. “We were very pleased by how many could reach the maximal dose at the end of the first year. Once we get the results of the second year, that will provide a lot of additional detail about how this translates into unheated milk amounts they can tolerate and introduce into their diet at home.”
No significant changes were found in IgE levels over time in either study group. Most in the BMOIT group reported improvement in at least one quality-of-life domain, while more in the placebo group reported improvements in only the emotional impact domain.
Adverse events such as gastrointestinal side effects occurred in both groups of participants, but the vast majority of events were mild, Dr. Dantzer said. Fewer than 1% of dosing-related reactions were severe. Four participants required epinephrine.
“This highlights how this needs to be done by someone comfortable and trained, and not by a family at home on their own,” Dr. Dantzer said. “But potentially in the future, this concept of using a modified allergen could be applied to more kids with milk allergy.”
A Montreal-based pediatric allergy specialist who was not involved in the study said the results weren’t surprising. “We’ve known for a good while that the allergenic proteins found in certain foods, or caused by milk in this context, are influenced by the way in which food is processed,” said Christine McCusker, MD, associate professor of pediatrics and director of the division of pediatric allergy, immunology, and dermatology at Montreal Children’s Hospital at McGill University Health Center.
But “having this relatively definitive data that supports what you’re suggesting to patients is obviously the way to optimize your management,” Dr. McCusker said in an interview. “These types of studies are important steps, especially in this age of increased food allergies where many of these things can be dealt with in very young children before their immune systems are fixed.”
Dr. Dantzer and Dr. McCusker agreed that the small size of the study was a limitation, though “waiting for more participants means you don’t always get information out there in a timely manner,” Dr. McCusker said.
She said additional research should focus on preidentifying which children may be prone to severe, lasting food allergies. “If you have a milk allergy that will stay with you the rest of your life and we could maybe modify that outcome with early, targeted intervention, that would be the nirvana of the field,” Dr. McCusker said.
Dr. Dantzer said her research “showed us that oral immunotherapy is an option, but not a perfect option.
“We still need to keep working on other alternatives that can be even safer and potentially work better,” she added.
The study was supported by the Myra Reinhard Family Foundation. Dr. Dantzer and Dr. McCusker report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
The small, ongoing clinical trial has enabled some participants – all of whom reacted to less than a tablespoon of baked milk at baseline – to begin incorporating baked milk products into everyday diets and to eat in restaurants with less fear of allergic reactions, reported study author Jennifer Dantzer, MD, MHS, assistant professor of pediatrics in the division of pediatric allergy, immunology, and rheumatology at Johns Hopkins University in Baltimore.
Cow’s milk is the most common food allergy in young children, and “for many, it’s a constant stressor that’s always there,” Dr. Dantzer said in an interview. “For a lot of families, this impacts where they eat out, if they eat out, and sometimes where they vacation, or a lot of the social activities they do.
“This was a unique group of kids with a very severe milk phenotype who were reactive to teeny doses and may not have qualified or done well with other types of oral immunotherapy,” she added. “Using a modified allergen – baked milk – seems to work. But for now, we think this is something that still needs further research before it’s ready for a clinical setting.”
The study, for which 24-month unblinded results are being tallied, was recently published in the Journal of Allergy and Clinical Immunology .
About 2%-3% of preschool-age children are affected by cow’s milk allergy. Children often outgrow it, but for about 20% of children, it persists into adolescence and adulthood. The only current management approaches are avoidance and emergency medications to treat reactions.
But for those with severe milk allergy who react to even trace amounts of milk in any form, the now-routine clinical practice of introducing baked milk isn’t an option, Dr. Dantzer said. The new trial stood out from prior research by using lower starting doses and a more gradual dose escalation of extensively heated milk to determine if oral immunotherapy could be safer but still effective.
Dr. Dantzer and her team randomly assigned 30 participants (aged 3-18 years) into two blinded groups. For 12 months, one group received baked milk oral immunotherapy (BMOIT), and the other a placebo consisting of tapioca flour. At baseline, for all participants, the milk skin prick test wheal diameter was ≥ 3 mm, and the cow’s milk immunoglobulin E (IgE) level was > 5 kU/L. All the children experienced positive dose-limiting reactions to < 1 tablespoon of baked milk protein but could tolerate at least 3 mg on initial dose escalation.
Measured doses of baked milk and placebo powders were supplied to participants for all doses consumed at home. Participants were given instructions on how to prepare it in cupcake or muffin batter. Over 12 months, doses were gradually increased to a maximum cumulative dose of 4,044 mg baked milk protein, or approximately a half tablespoon.
Researchers collected blood samples for immune studies, and participants or their parents completed quality-of-life questionnaires that asked about food anxiety, social and dietary limitations, emotional impact, risk for accidental ingestion, and allergen avoidance.
Fourteen of 15 participants (93%) in the BMOIT group reached the goal-maintenance dose of 2,000 mg of baked milk protein (about a quarter tablespoon). Of those who completed the 12-month challenge, 11 of 14 (79%) in the BMOIT group tolerated 4,000 mg of baked milk protein, compared to none in the placebo group.
“We anticipated that by starting with really small amounts, we would be able to build up the amount of baked milk these kids could tolerate,” Dr. Dantzer said. “We were very pleased by how many could reach the maximal dose at the end of the first year. Once we get the results of the second year, that will provide a lot of additional detail about how this translates into unheated milk amounts they can tolerate and introduce into their diet at home.”
No significant changes were found in IgE levels over time in either study group. Most in the BMOIT group reported improvement in at least one quality-of-life domain, while more in the placebo group reported improvements in only the emotional impact domain.
Adverse events such as gastrointestinal side effects occurred in both groups of participants, but the vast majority of events were mild, Dr. Dantzer said. Fewer than 1% of dosing-related reactions were severe. Four participants required epinephrine.
“This highlights how this needs to be done by someone comfortable and trained, and not by a family at home on their own,” Dr. Dantzer said. “But potentially in the future, this concept of using a modified allergen could be applied to more kids with milk allergy.”
A Montreal-based pediatric allergy specialist who was not involved in the study said the results weren’t surprising. “We’ve known for a good while that the allergenic proteins found in certain foods, or caused by milk in this context, are influenced by the way in which food is processed,” said Christine McCusker, MD, associate professor of pediatrics and director of the division of pediatric allergy, immunology, and dermatology at Montreal Children’s Hospital at McGill University Health Center.
But “having this relatively definitive data that supports what you’re suggesting to patients is obviously the way to optimize your management,” Dr. McCusker said in an interview. “These types of studies are important steps, especially in this age of increased food allergies where many of these things can be dealt with in very young children before their immune systems are fixed.”
Dr. Dantzer and Dr. McCusker agreed that the small size of the study was a limitation, though “waiting for more participants means you don’t always get information out there in a timely manner,” Dr. McCusker said.
She said additional research should focus on preidentifying which children may be prone to severe, lasting food allergies. “If you have a milk allergy that will stay with you the rest of your life and we could maybe modify that outcome with early, targeted intervention, that would be the nirvana of the field,” Dr. McCusker said.
Dr. Dantzer said her research “showed us that oral immunotherapy is an option, but not a perfect option.
“We still need to keep working on other alternatives that can be even safer and potentially work better,” she added.
The study was supported by the Myra Reinhard Family Foundation. Dr. Dantzer and Dr. McCusker report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
The small, ongoing clinical trial has enabled some participants – all of whom reacted to less than a tablespoon of baked milk at baseline – to begin incorporating baked milk products into everyday diets and to eat in restaurants with less fear of allergic reactions, reported study author Jennifer Dantzer, MD, MHS, assistant professor of pediatrics in the division of pediatric allergy, immunology, and rheumatology at Johns Hopkins University in Baltimore.
Cow’s milk is the most common food allergy in young children, and “for many, it’s a constant stressor that’s always there,” Dr. Dantzer said in an interview. “For a lot of families, this impacts where they eat out, if they eat out, and sometimes where they vacation, or a lot of the social activities they do.
“This was a unique group of kids with a very severe milk phenotype who were reactive to teeny doses and may not have qualified or done well with other types of oral immunotherapy,” she added. “Using a modified allergen – baked milk – seems to work. But for now, we think this is something that still needs further research before it’s ready for a clinical setting.”
The study, for which 24-month unblinded results are being tallied, was recently published in the Journal of Allergy and Clinical Immunology .
About 2%-3% of preschool-age children are affected by cow’s milk allergy. Children often outgrow it, but for about 20% of children, it persists into adolescence and adulthood. The only current management approaches are avoidance and emergency medications to treat reactions.
But for those with severe milk allergy who react to even trace amounts of milk in any form, the now-routine clinical practice of introducing baked milk isn’t an option, Dr. Dantzer said. The new trial stood out from prior research by using lower starting doses and a more gradual dose escalation of extensively heated milk to determine if oral immunotherapy could be safer but still effective.
Dr. Dantzer and her team randomly assigned 30 participants (aged 3-18 years) into two blinded groups. For 12 months, one group received baked milk oral immunotherapy (BMOIT), and the other a placebo consisting of tapioca flour. At baseline, for all participants, the milk skin prick test wheal diameter was ≥ 3 mm, and the cow’s milk immunoglobulin E (IgE) level was > 5 kU/L. All the children experienced positive dose-limiting reactions to < 1 tablespoon of baked milk protein but could tolerate at least 3 mg on initial dose escalation.
Measured doses of baked milk and placebo powders were supplied to participants for all doses consumed at home. Participants were given instructions on how to prepare it in cupcake or muffin batter. Over 12 months, doses were gradually increased to a maximum cumulative dose of 4,044 mg baked milk protein, or approximately a half tablespoon.
Researchers collected blood samples for immune studies, and participants or their parents completed quality-of-life questionnaires that asked about food anxiety, social and dietary limitations, emotional impact, risk for accidental ingestion, and allergen avoidance.
Fourteen of 15 participants (93%) in the BMOIT group reached the goal-maintenance dose of 2,000 mg of baked milk protein (about a quarter tablespoon). Of those who completed the 12-month challenge, 11 of 14 (79%) in the BMOIT group tolerated 4,000 mg of baked milk protein, compared to none in the placebo group.
“We anticipated that by starting with really small amounts, we would be able to build up the amount of baked milk these kids could tolerate,” Dr. Dantzer said. “We were very pleased by how many could reach the maximal dose at the end of the first year. Once we get the results of the second year, that will provide a lot of additional detail about how this translates into unheated milk amounts they can tolerate and introduce into their diet at home.”
No significant changes were found in IgE levels over time in either study group. Most in the BMOIT group reported improvement in at least one quality-of-life domain, while more in the placebo group reported improvements in only the emotional impact domain.
Adverse events such as gastrointestinal side effects occurred in both groups of participants, but the vast majority of events were mild, Dr. Dantzer said. Fewer than 1% of dosing-related reactions were severe. Four participants required epinephrine.
“This highlights how this needs to be done by someone comfortable and trained, and not by a family at home on their own,” Dr. Dantzer said. “But potentially in the future, this concept of using a modified allergen could be applied to more kids with milk allergy.”
A Montreal-based pediatric allergy specialist who was not involved in the study said the results weren’t surprising. “We’ve known for a good while that the allergenic proteins found in certain foods, or caused by milk in this context, are influenced by the way in which food is processed,” said Christine McCusker, MD, associate professor of pediatrics and director of the division of pediatric allergy, immunology, and dermatology at Montreal Children’s Hospital at McGill University Health Center.
But “having this relatively definitive data that supports what you’re suggesting to patients is obviously the way to optimize your management,” Dr. McCusker said in an interview. “These types of studies are important steps, especially in this age of increased food allergies where many of these things can be dealt with in very young children before their immune systems are fixed.”
Dr. Dantzer and Dr. McCusker agreed that the small size of the study was a limitation, though “waiting for more participants means you don’t always get information out there in a timely manner,” Dr. McCusker said.
She said additional research should focus on preidentifying which children may be prone to severe, lasting food allergies. “If you have a milk allergy that will stay with you the rest of your life and we could maybe modify that outcome with early, targeted intervention, that would be the nirvana of the field,” Dr. McCusker said.
Dr. Dantzer said her research “showed us that oral immunotherapy is an option, but not a perfect option.
“We still need to keep working on other alternatives that can be even safer and potentially work better,” she added.
The study was supported by the Myra Reinhard Family Foundation. Dr. Dantzer and Dr. McCusker report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Record-breaking autism rates reported with new CDC criteria
Childhood autism rates are at the highest level since the Centers for Disease Control and Prevention began tracking the disorder in 2000, new data released Dec. 2 show.
The increase likely reflects improvements in diagnosis and identification of autism spectrum disorder (ASD), not an increase in incidence, study authors with the CDC’s Autism and Developmental Disabilities Monitoring (ADDM) Network told this news organization.
Using a new surveillance methodology, researchers found that 2.3% of 8-year-olds in communities in 11 states across the United States had an autism diagnosis in 2018, up from 1.9% in 2016.
A separate report on early identification in 4-year-olds shows that children born in 2014 were 50% more likely to receive an autism diagnosis or ASD special education classification by 48 months of age than those born in 2010, signaling improved early diagnosis.
Taken together, the data suggest efforts to raise awareness about autism are working, though researchers were quick to say much work remains.
“It was not surprising to me and in fact it was reassuring that the number of children diagnosed with autism is higher and is actually approaching prevalence of autism that has been noted in some national surveys of parents,” Stuart Shapira, MD, PhD, associate director for science in CDC’s National Center on Birth Defects and Developmental Disability, told this news organization.
“It means we’re doing a better job of identifying children, which helps to get them into services earlier so they can achieve their best developmental outcome.”
The studies, published online in Morbidity and Mortality Weekly Report, are the first to use a new ASD surveillance protocol that relies on ASD diagnosis or special education classification and billing codes and eliminates comprehensive records analysis by trained clinician reviewers.
Racial disparities
The updated methodology was less labor intensive and reduced the time it took to produce the report, but it is not without its critics, who claim the new protocol will undercount the number of children with ASD.
Created in 2000 and funded by the CDC, the ADDM Network is the only surveillance program in the United States that tracks the number and characteristics of children with ASD in multiple communities in the U.S.
When ADDM released its first report in 2007 from six states and based on data from the year 2000, ASD prevalence was 6.7 per 1,000 children, or 1 in 150 children.
In the latest report, which includes data from 2018, the autism prevalence rate across 11 states was 23.0 per 1,000 children, or 1 in 44 children.
That rate is closer to reported autism prevalence from the National Survey of Children’s Health and the National Health Interview Survey, both of which rely on parent-reported ASD diagnoses.
in Arizona, Arkansas, California, Georgia, Maryland, Minnesota, Missouri, New Jersey, Tennessee, Utah, and Wisconsin.
Children were counted as having autism if their records included an ASD diagnosis, a special education classification of ASD, or an ASD International Classification of Diseases (ICD) code. A total of 5,058 children met those criteria.
Rates of ASD ranged from a low of 1.7% in Missouri to 3.9% in California and were 4.2 times higher in boys than in girls. Just under half of the children with ASD were evaluated by age 36 months.
Although the overall ASD prevalence was similar among White, Black, Hispanic, and Asian/Pacific Islander children, the report highlighted a number of other racial disparities overall and in individual states.
For example, among those with ASD and data on cognitive ability, 35.2% had an intelligence quotient score of 70 or lower. Black children with ASD were far more likely to have an IQ of 70 or less (49.8%) than Hispanic (33.1%) or White (29.7%) children.
“The persistent disparities in co-occurring intellectual disabilities in children with autism is something that we continue to see and suggests that we need to better understand exactly what’s happening,” Matthew Maenner, PhD, an epidemiologist and autism surveillance team lead with the CDC’s National Center on Birth Defects and Developmental Disabilities, told this news organization.
Another long-standing trend observed again in the new report on prevalence among 8-year-olds is low ASD prevalence among Hispanic children. While the overall estimate showed similar autism rates, a closer review of state-level data reveals a different picture.
“In almost half of the sites, Hispanic children were less likely to be identified as having ASD,” he said. “This gets lost if you look only at the overall estimate.”
New methodology
When ADDM released its first report in 2007, autism diagnosis was widely inadequate in the United States. Relying on only confirmed ASD diagnoses would significantly underestimate the number of children with the disorder, so the CDC added “active case finding” to the protocol.
Trained clinician reviewers analyzed individual notes from medical and educational records for every 8-year-old in ADDM Network sites, looking for evidence of characteristics and behaviors associated with autism. The process was labor- and time-intensive and took up to 4 years to complete.
In 2018, the CDC began investigating ways to speed the process and came up with the strategy used in the latest report. The new protocol was faster, easier, and less expensive. Although he says cost was never the deciding factor, Dr. Maenner acknowledges that had they stuck with the original protocol, they would have been forced to reduce the number of ADDM Network sites.
Dr. Maenner argues that a comparison of the two protocols shows the new method doesn’t compromise accuracy and may actually capture children who lacked the medical or educational records the previous protocol required for a count. But not everyone agrees.
“I thought the point was to be as accurate and complete as possible in doing the surveillance,” Walter Zahorodny, PhD, associate professor of pediatrics at Rutgers University, New Brunswick, N.J., and principal investigator of the New Jersey ADDM Network site, told this news organization. “In states where there’s a high detail of information in records, like New Jersey, it’s going to underestimate the count.”
Dr. Zahorodny says the latest data prove his point. In 2016, under the old methodology, ASD prevalence was 3.1% in the state. In 2018, under the new protocol, prevalence was 2.84%, a decrease of about 20% that Dr. Zahorodny pins squarely on the elimination of ADDM clinical reviewers.
But New Jersey is the only state that participated in both the 2016 and 2018 surveillance periods to report a decrease in ASD prevalence. The other eight states all found autism rates in their states went up.
Sydney Pettygrove, PhD, associate professor of public health and pediatrics at the University of Arizona, Tucson, and a principal investigator for the ADDM site in Arizona, told this news organization that when she first learned the CDC was rolling out a new methodology, she and other investigators were concerned.
“People were really upset. I was really upset,” she said. “I had formed an opinion based on the earlier data that this would not be a good idea.”
In 2000, when ASD surveillance began in Arizona, nearly 30% of children identified by ADDM clinical reviewers as having autism had no mention of the disorder in their records. Today, that percentage is closer to 5%.
“In 2000 it would have been catastrophic to try to estimate the prevalence of autism with the new protocol,” said Dr. Pettygrove. As it turns out, under the new protocol, prevalence rates in Arizona increased from 16.0 per 1,000 children in 2016 to 24.9 in 2018.
Built-in bias eliminated?
In addition to speeding up the process, the new methodology might have other benefits as well. Under the old ADDM surveillance protocol, children who lacked certain medical or educational records did not meet the ASD case definition and weren’t counted.
A 2019 study showed that this disproportionately affected Black and Hispanic children, who had significantly less access to health care professionals than White children.
As a result, “the old methodology had a bias built into it,” Maureen Durkin, PhD, DrPH, coauthor of that study and chair of population health sciences at the University of Wisconsin–Madison and principal investigator for the ADDM site in Wisconsin, told this news organization.
“Clinician reviewers ended up putting these children in the ‘suspected ASD’ category because they couldn’t call it a case under the case definition,” Dr. Durkin said. “There was a fairly large percentage of suspected cases and a disproportionate number of those kids were children of color.”
Although she can’t say for sure, Dr. Durkin said it’s possible the new protocol could eliminate some of that bias.
CDC researchers also attribute the new method to an expanded study of early diagnosis among 4-year-olds. In previous years, only a handful of the ADDM Network sites participating in the 8-year-old surveillance project also studied early diagnosis in 4-year-olds.
This year, all 11 sites took part in the early diagnosis analysis, tripling the number of children included in the analysis. That made it possible to include, for the first time, Asian/Pacific Islander children in this analysis.
In the past, ASD prevalence has trended higher in White children, compared with other racial groups. The new data found that ASD prevalence among 4-year-olds was significantly lower in White children (12.9 per 1,000 children) than in Black, Hispanic, or Asian/Pacific Islander children (16.6, 21.1, and 22.7 per 1,000, respectively). Prevalence in American Indian/Alaska Native children was the lowest among all racial groups (11.5 per 1,000).
It’s the first time researchers have seen this pattern in any ADDM report, Kelly Shaw, PhD, lead author of that study and an epidemiologist with the National Center on Birth Defects and Developmental Disability at the CDC, told this news organization.
These data don’t provide clues about the potential cause of that disparity, Dr. Shaw said. It’s likely an indication of better identification of ASD in those communities, she said, and not a sign of increased incidence of autism among Black, Hispanic, or Asian/Pacific Islander children.
“We don’t have any evidence to suggest or expect that autism would be increasing differentially among groups,” Dr. Shaw said.
The data suggest “we are making some progress but there certainly is still room for improvement,” Dr. Shaw said.
Study authors report no conflicts of interest.
A version of this article first appeared on Medscape.com.
Childhood autism rates are at the highest level since the Centers for Disease Control and Prevention began tracking the disorder in 2000, new data released Dec. 2 show.
The increase likely reflects improvements in diagnosis and identification of autism spectrum disorder (ASD), not an increase in incidence, study authors with the CDC’s Autism and Developmental Disabilities Monitoring (ADDM) Network told this news organization.
Using a new surveillance methodology, researchers found that 2.3% of 8-year-olds in communities in 11 states across the United States had an autism diagnosis in 2018, up from 1.9% in 2016.
A separate report on early identification in 4-year-olds shows that children born in 2014 were 50% more likely to receive an autism diagnosis or ASD special education classification by 48 months of age than those born in 2010, signaling improved early diagnosis.
Taken together, the data suggest efforts to raise awareness about autism are working, though researchers were quick to say much work remains.
“It was not surprising to me and in fact it was reassuring that the number of children diagnosed with autism is higher and is actually approaching prevalence of autism that has been noted in some national surveys of parents,” Stuart Shapira, MD, PhD, associate director for science in CDC’s National Center on Birth Defects and Developmental Disability, told this news organization.
“It means we’re doing a better job of identifying children, which helps to get them into services earlier so they can achieve their best developmental outcome.”
The studies, published online in Morbidity and Mortality Weekly Report, are the first to use a new ASD surveillance protocol that relies on ASD diagnosis or special education classification and billing codes and eliminates comprehensive records analysis by trained clinician reviewers.
Racial disparities
The updated methodology was less labor intensive and reduced the time it took to produce the report, but it is not without its critics, who claim the new protocol will undercount the number of children with ASD.
Created in 2000 and funded by the CDC, the ADDM Network is the only surveillance program in the United States that tracks the number and characteristics of children with ASD in multiple communities in the U.S.
When ADDM released its first report in 2007 from six states and based on data from the year 2000, ASD prevalence was 6.7 per 1,000 children, or 1 in 150 children.
In the latest report, which includes data from 2018, the autism prevalence rate across 11 states was 23.0 per 1,000 children, or 1 in 44 children.
That rate is closer to reported autism prevalence from the National Survey of Children’s Health and the National Health Interview Survey, both of which rely on parent-reported ASD diagnoses.
in Arizona, Arkansas, California, Georgia, Maryland, Minnesota, Missouri, New Jersey, Tennessee, Utah, and Wisconsin.
Children were counted as having autism if their records included an ASD diagnosis, a special education classification of ASD, or an ASD International Classification of Diseases (ICD) code. A total of 5,058 children met those criteria.
Rates of ASD ranged from a low of 1.7% in Missouri to 3.9% in California and were 4.2 times higher in boys than in girls. Just under half of the children with ASD were evaluated by age 36 months.
Although the overall ASD prevalence was similar among White, Black, Hispanic, and Asian/Pacific Islander children, the report highlighted a number of other racial disparities overall and in individual states.
For example, among those with ASD and data on cognitive ability, 35.2% had an intelligence quotient score of 70 or lower. Black children with ASD were far more likely to have an IQ of 70 or less (49.8%) than Hispanic (33.1%) or White (29.7%) children.
“The persistent disparities in co-occurring intellectual disabilities in children with autism is something that we continue to see and suggests that we need to better understand exactly what’s happening,” Matthew Maenner, PhD, an epidemiologist and autism surveillance team lead with the CDC’s National Center on Birth Defects and Developmental Disabilities, told this news organization.
Another long-standing trend observed again in the new report on prevalence among 8-year-olds is low ASD prevalence among Hispanic children. While the overall estimate showed similar autism rates, a closer review of state-level data reveals a different picture.
“In almost half of the sites, Hispanic children were less likely to be identified as having ASD,” he said. “This gets lost if you look only at the overall estimate.”
New methodology
When ADDM released its first report in 2007, autism diagnosis was widely inadequate in the United States. Relying on only confirmed ASD diagnoses would significantly underestimate the number of children with the disorder, so the CDC added “active case finding” to the protocol.
Trained clinician reviewers analyzed individual notes from medical and educational records for every 8-year-old in ADDM Network sites, looking for evidence of characteristics and behaviors associated with autism. The process was labor- and time-intensive and took up to 4 years to complete.
In 2018, the CDC began investigating ways to speed the process and came up with the strategy used in the latest report. The new protocol was faster, easier, and less expensive. Although he says cost was never the deciding factor, Dr. Maenner acknowledges that had they stuck with the original protocol, they would have been forced to reduce the number of ADDM Network sites.
Dr. Maenner argues that a comparison of the two protocols shows the new method doesn’t compromise accuracy and may actually capture children who lacked the medical or educational records the previous protocol required for a count. But not everyone agrees.
“I thought the point was to be as accurate and complete as possible in doing the surveillance,” Walter Zahorodny, PhD, associate professor of pediatrics at Rutgers University, New Brunswick, N.J., and principal investigator of the New Jersey ADDM Network site, told this news organization. “In states where there’s a high detail of information in records, like New Jersey, it’s going to underestimate the count.”
Dr. Zahorodny says the latest data prove his point. In 2016, under the old methodology, ASD prevalence was 3.1% in the state. In 2018, under the new protocol, prevalence was 2.84%, a decrease of about 20% that Dr. Zahorodny pins squarely on the elimination of ADDM clinical reviewers.
But New Jersey is the only state that participated in both the 2016 and 2018 surveillance periods to report a decrease in ASD prevalence. The other eight states all found autism rates in their states went up.
Sydney Pettygrove, PhD, associate professor of public health and pediatrics at the University of Arizona, Tucson, and a principal investigator for the ADDM site in Arizona, told this news organization that when she first learned the CDC was rolling out a new methodology, she and other investigators were concerned.
“People were really upset. I was really upset,” she said. “I had formed an opinion based on the earlier data that this would not be a good idea.”
In 2000, when ASD surveillance began in Arizona, nearly 30% of children identified by ADDM clinical reviewers as having autism had no mention of the disorder in their records. Today, that percentage is closer to 5%.
“In 2000 it would have been catastrophic to try to estimate the prevalence of autism with the new protocol,” said Dr. Pettygrove. As it turns out, under the new protocol, prevalence rates in Arizona increased from 16.0 per 1,000 children in 2016 to 24.9 in 2018.
Built-in bias eliminated?
In addition to speeding up the process, the new methodology might have other benefits as well. Under the old ADDM surveillance protocol, children who lacked certain medical or educational records did not meet the ASD case definition and weren’t counted.
A 2019 study showed that this disproportionately affected Black and Hispanic children, who had significantly less access to health care professionals than White children.
As a result, “the old methodology had a bias built into it,” Maureen Durkin, PhD, DrPH, coauthor of that study and chair of population health sciences at the University of Wisconsin–Madison and principal investigator for the ADDM site in Wisconsin, told this news organization.
“Clinician reviewers ended up putting these children in the ‘suspected ASD’ category because they couldn’t call it a case under the case definition,” Dr. Durkin said. “There was a fairly large percentage of suspected cases and a disproportionate number of those kids were children of color.”
Although she can’t say for sure, Dr. Durkin said it’s possible the new protocol could eliminate some of that bias.
CDC researchers also attribute the new method to an expanded study of early diagnosis among 4-year-olds. In previous years, only a handful of the ADDM Network sites participating in the 8-year-old surveillance project also studied early diagnosis in 4-year-olds.
This year, all 11 sites took part in the early diagnosis analysis, tripling the number of children included in the analysis. That made it possible to include, for the first time, Asian/Pacific Islander children in this analysis.
In the past, ASD prevalence has trended higher in White children, compared with other racial groups. The new data found that ASD prevalence among 4-year-olds was significantly lower in White children (12.9 per 1,000 children) than in Black, Hispanic, or Asian/Pacific Islander children (16.6, 21.1, and 22.7 per 1,000, respectively). Prevalence in American Indian/Alaska Native children was the lowest among all racial groups (11.5 per 1,000).
It’s the first time researchers have seen this pattern in any ADDM report, Kelly Shaw, PhD, lead author of that study and an epidemiologist with the National Center on Birth Defects and Developmental Disability at the CDC, told this news organization.
These data don’t provide clues about the potential cause of that disparity, Dr. Shaw said. It’s likely an indication of better identification of ASD in those communities, she said, and not a sign of increased incidence of autism among Black, Hispanic, or Asian/Pacific Islander children.
“We don’t have any evidence to suggest or expect that autism would be increasing differentially among groups,” Dr. Shaw said.
The data suggest “we are making some progress but there certainly is still room for improvement,” Dr. Shaw said.
Study authors report no conflicts of interest.
A version of this article first appeared on Medscape.com.
Childhood autism rates are at the highest level since the Centers for Disease Control and Prevention began tracking the disorder in 2000, new data released Dec. 2 show.
The increase likely reflects improvements in diagnosis and identification of autism spectrum disorder (ASD), not an increase in incidence, study authors with the CDC’s Autism and Developmental Disabilities Monitoring (ADDM) Network told this news organization.
Using a new surveillance methodology, researchers found that 2.3% of 8-year-olds in communities in 11 states across the United States had an autism diagnosis in 2018, up from 1.9% in 2016.
A separate report on early identification in 4-year-olds shows that children born in 2014 were 50% more likely to receive an autism diagnosis or ASD special education classification by 48 months of age than those born in 2010, signaling improved early diagnosis.
Taken together, the data suggest efforts to raise awareness about autism are working, though researchers were quick to say much work remains.
“It was not surprising to me and in fact it was reassuring that the number of children diagnosed with autism is higher and is actually approaching prevalence of autism that has been noted in some national surveys of parents,” Stuart Shapira, MD, PhD, associate director for science in CDC’s National Center on Birth Defects and Developmental Disability, told this news organization.
“It means we’re doing a better job of identifying children, which helps to get them into services earlier so they can achieve their best developmental outcome.”
The studies, published online in Morbidity and Mortality Weekly Report, are the first to use a new ASD surveillance protocol that relies on ASD diagnosis or special education classification and billing codes and eliminates comprehensive records analysis by trained clinician reviewers.
Racial disparities
The updated methodology was less labor intensive and reduced the time it took to produce the report, but it is not without its critics, who claim the new protocol will undercount the number of children with ASD.
Created in 2000 and funded by the CDC, the ADDM Network is the only surveillance program in the United States that tracks the number and characteristics of children with ASD in multiple communities in the U.S.
When ADDM released its first report in 2007 from six states and based on data from the year 2000, ASD prevalence was 6.7 per 1,000 children, or 1 in 150 children.
In the latest report, which includes data from 2018, the autism prevalence rate across 11 states was 23.0 per 1,000 children, or 1 in 44 children.
That rate is closer to reported autism prevalence from the National Survey of Children’s Health and the National Health Interview Survey, both of which rely on parent-reported ASD diagnoses.
in Arizona, Arkansas, California, Georgia, Maryland, Minnesota, Missouri, New Jersey, Tennessee, Utah, and Wisconsin.
Children were counted as having autism if their records included an ASD diagnosis, a special education classification of ASD, or an ASD International Classification of Diseases (ICD) code. A total of 5,058 children met those criteria.
Rates of ASD ranged from a low of 1.7% in Missouri to 3.9% in California and were 4.2 times higher in boys than in girls. Just under half of the children with ASD were evaluated by age 36 months.
Although the overall ASD prevalence was similar among White, Black, Hispanic, and Asian/Pacific Islander children, the report highlighted a number of other racial disparities overall and in individual states.
For example, among those with ASD and data on cognitive ability, 35.2% had an intelligence quotient score of 70 or lower. Black children with ASD were far more likely to have an IQ of 70 or less (49.8%) than Hispanic (33.1%) or White (29.7%) children.
“The persistent disparities in co-occurring intellectual disabilities in children with autism is something that we continue to see and suggests that we need to better understand exactly what’s happening,” Matthew Maenner, PhD, an epidemiologist and autism surveillance team lead with the CDC’s National Center on Birth Defects and Developmental Disabilities, told this news organization.
Another long-standing trend observed again in the new report on prevalence among 8-year-olds is low ASD prevalence among Hispanic children. While the overall estimate showed similar autism rates, a closer review of state-level data reveals a different picture.
“In almost half of the sites, Hispanic children were less likely to be identified as having ASD,” he said. “This gets lost if you look only at the overall estimate.”
New methodology
When ADDM released its first report in 2007, autism diagnosis was widely inadequate in the United States. Relying on only confirmed ASD diagnoses would significantly underestimate the number of children with the disorder, so the CDC added “active case finding” to the protocol.
Trained clinician reviewers analyzed individual notes from medical and educational records for every 8-year-old in ADDM Network sites, looking for evidence of characteristics and behaviors associated with autism. The process was labor- and time-intensive and took up to 4 years to complete.
In 2018, the CDC began investigating ways to speed the process and came up with the strategy used in the latest report. The new protocol was faster, easier, and less expensive. Although he says cost was never the deciding factor, Dr. Maenner acknowledges that had they stuck with the original protocol, they would have been forced to reduce the number of ADDM Network sites.
Dr. Maenner argues that a comparison of the two protocols shows the new method doesn’t compromise accuracy and may actually capture children who lacked the medical or educational records the previous protocol required for a count. But not everyone agrees.
“I thought the point was to be as accurate and complete as possible in doing the surveillance,” Walter Zahorodny, PhD, associate professor of pediatrics at Rutgers University, New Brunswick, N.J., and principal investigator of the New Jersey ADDM Network site, told this news organization. “In states where there’s a high detail of information in records, like New Jersey, it’s going to underestimate the count.”
Dr. Zahorodny says the latest data prove his point. In 2016, under the old methodology, ASD prevalence was 3.1% in the state. In 2018, under the new protocol, prevalence was 2.84%, a decrease of about 20% that Dr. Zahorodny pins squarely on the elimination of ADDM clinical reviewers.
But New Jersey is the only state that participated in both the 2016 and 2018 surveillance periods to report a decrease in ASD prevalence. The other eight states all found autism rates in their states went up.
Sydney Pettygrove, PhD, associate professor of public health and pediatrics at the University of Arizona, Tucson, and a principal investigator for the ADDM site in Arizona, told this news organization that when she first learned the CDC was rolling out a new methodology, she and other investigators were concerned.
“People were really upset. I was really upset,” she said. “I had formed an opinion based on the earlier data that this would not be a good idea.”
In 2000, when ASD surveillance began in Arizona, nearly 30% of children identified by ADDM clinical reviewers as having autism had no mention of the disorder in their records. Today, that percentage is closer to 5%.
“In 2000 it would have been catastrophic to try to estimate the prevalence of autism with the new protocol,” said Dr. Pettygrove. As it turns out, under the new protocol, prevalence rates in Arizona increased from 16.0 per 1,000 children in 2016 to 24.9 in 2018.
Built-in bias eliminated?
In addition to speeding up the process, the new methodology might have other benefits as well. Under the old ADDM surveillance protocol, children who lacked certain medical or educational records did not meet the ASD case definition and weren’t counted.
A 2019 study showed that this disproportionately affected Black and Hispanic children, who had significantly less access to health care professionals than White children.
As a result, “the old methodology had a bias built into it,” Maureen Durkin, PhD, DrPH, coauthor of that study and chair of population health sciences at the University of Wisconsin–Madison and principal investigator for the ADDM site in Wisconsin, told this news organization.
“Clinician reviewers ended up putting these children in the ‘suspected ASD’ category because they couldn’t call it a case under the case definition,” Dr. Durkin said. “There was a fairly large percentage of suspected cases and a disproportionate number of those kids were children of color.”
Although she can’t say for sure, Dr. Durkin said it’s possible the new protocol could eliminate some of that bias.
CDC researchers also attribute the new method to an expanded study of early diagnosis among 4-year-olds. In previous years, only a handful of the ADDM Network sites participating in the 8-year-old surveillance project also studied early diagnosis in 4-year-olds.
This year, all 11 sites took part in the early diagnosis analysis, tripling the number of children included in the analysis. That made it possible to include, for the first time, Asian/Pacific Islander children in this analysis.
In the past, ASD prevalence has trended higher in White children, compared with other racial groups. The new data found that ASD prevalence among 4-year-olds was significantly lower in White children (12.9 per 1,000 children) than in Black, Hispanic, or Asian/Pacific Islander children (16.6, 21.1, and 22.7 per 1,000, respectively). Prevalence in American Indian/Alaska Native children was the lowest among all racial groups (11.5 per 1,000).
It’s the first time researchers have seen this pattern in any ADDM report, Kelly Shaw, PhD, lead author of that study and an epidemiologist with the National Center on Birth Defects and Developmental Disability at the CDC, told this news organization.
These data don’t provide clues about the potential cause of that disparity, Dr. Shaw said. It’s likely an indication of better identification of ASD in those communities, she said, and not a sign of increased incidence of autism among Black, Hispanic, or Asian/Pacific Islander children.
“We don’t have any evidence to suggest or expect that autism would be increasing differentially among groups,” Dr. Shaw said.
The data suggest “we are making some progress but there certainly is still room for improvement,” Dr. Shaw said.
Study authors report no conflicts of interest.
A version of this article first appeared on Medscape.com.
Study shows wider gaps, broader inequities in U.S. sex education than 25 years ago
American teenagers receive less formal sex education today than they did 25 years ago, with “troubling” racial inequities that leave youth of color and queer youth at greater risk than other teens for sexually transmitted diseases and unintended pregnancy, according to a new study.
“Many adolescents do not receive any instruction on essential topics or do not receive this instruction until after the first sex,” wrote Laura D. Lindberg, PhD, and Leslie M. Kantor, PhD, MPH, from the Guttmacher Institute, New York, and the department of urban-global public health at Rutgers University, Piscataway, N.J., respectively. “These gaps in sex education in the U.S. are uneven, and gender, racial, and other disparities are widespread,” they added, calling for “robust efforts ... to ensure equity and reduce health disparities.”
The study used cross-sectional data from the 2011-2015 and 2015-2019 National Surveys of Family Growth (NSFG) to examine content, timing, and location of formal sex education among 15- to 19-year-olds in the United States. The data came from samples of 2,047 females and 2,087 males in 2011-2015, and 1,894 females and 1,918 males in 2015-2019. The majority of respondents were aged 15-17 years and non-Hispanic White, with another quarter being Hispanic, and 14% Black.
The survey asked respondents whether, before they turned 18, they had ever received formal instruction at school, church, a community center, “or some other place” about how to say no to sex, methods of birth control, STDs, how to prevent HIV/AIDS, abstaining until marriage to have sex, where to get birth control, and how to use a condom.
Follow-up questions asked about what grade instruction was first received and whether it had occurred before first penile-vaginal intercourse. The 2015-2019 survey also asked about the location of instruction, but only concerning methods of birth control and abstinence until marriage.
The results showed that HIV and STD prevention was the most commonly reported area of instruction, received by more than 90% of both males and females. However, beyond this there were imbalances, with only about half (49%-55%) of respondents receiving instruction meeting the Surgeon General’s Healthy People 2030 composite sex education goal. Lack of instruction on birth control drove this result for 80% of respondents. Specifically, there was a strong slant emphasizing abstinence over birth control instruction. Over both survey periods and both genders, more respondents reported instruction on how to say no to sex (79%-84%) and abstaining until marriage (58%-73%), compared with where to obtain birth control (40%-53%) or how to use a condom (54%-60%). “Overall, about 20% of adolescents received instruction from multiple sources about waiting until marriage, but only 5%-8% received birth control information from multiple settings,” they reported.
There were racial/ethnic and sexual orientation differences in the scope and balance of instruction reported by teens. Less than half of Black (45%) and Hispanic (47%) males received instruction on the combined Healthy People topics, compared with 57% of White males. Black females were less likely (30%) than White females (45%) to receive information on where to get birth control before the first sex. Nonstraight males were less likely than straight males to receive instruction about STIs or HIV/AIDS (83% vs. 93%).
In addition, religious attendance emerged as a key factor in the receipt of sex education, “with more frequent religious attendance associated with a greater likelihood of instruction about delaying sex and less likelihood of instruction about contraception,” the authors noted.
Comparing their findings to previous NSFG surveys, the researchers commented that “the share of adolescents receiving instruction about birth control was higher in 1995 than in 2015-2019 for both the genders; in 1995, 87% of females and 81% of males reported sex education about birth control methods, compared with 64% and 63% in 2015-2019, respectively.” The findings “should spur policy makers at the national, state, and local levels to ensure the broader provision of sex education and that school districts serving young people of color are the focus of additional efforts and funding.”
Asked for comment, John Santelli, MD, MPH, professor of population and family health and pediatrics at Columbia University, New York, who was not involved with the study, said the findings fit into a series of studies by Lindberg going back to 1988 showing that receipt of formal sex education before age 18 has declined over time.
“We, the adults, in America can do better by our young people,” he said in an interview. “Adolescents need sex education that is science based, medically accurate, and developmentally appropriate. Many adolescents are not receiving education that the CDC and health professionals recommend including information about where to get birth control, condom skills, and even, how to say no to sex. The neglect of young Black and Hispanic men is very concerning. However, we are not doing a great job in educating most of our adolescents. Health care providers can be influential in speaking with parents about their children’s education about sex. We need to activate parents, health care providers, and members of the faith community to investigate what is happening about sex education in their own communities.”
Dr. Santelli noted that there are multiple ways to strengthen the provision of sex education in the United States. In a recent commentary, he and his coauthors highlighted the National Sex Education Standards (NSES), which, “developed in partnership between sex education organizations and health professionals, provide clear, consistent, and straightforward guidance on the essential content for students in grades K-12.” The NSES were also used in the development of the CDC’s recently released Health Education Curriculum Analysis Tool.
The commentary takes a strong stand against the recently released revised Medical Institute for Sexual Heath K-12 Standards for Optimal Sexual Development, which, compared with the NSES, are “seriously flawed from both scientific and human rights’ perspectives,” they wrote. “States and local communities aiming to improve adolescent sexual and reproductive health and looking for national standards on sex education should adopt the NSES.”
Dr. Lindberg and Dr. Kantor disclosed no conflicts of interest. Dr. Santelli teaches public health students about adolescent health and chairs the board of directors of the Sexuality Information and Education Council of the United States. He disclosed no financial conflicts.
American teenagers receive less formal sex education today than they did 25 years ago, with “troubling” racial inequities that leave youth of color and queer youth at greater risk than other teens for sexually transmitted diseases and unintended pregnancy, according to a new study.
“Many adolescents do not receive any instruction on essential topics or do not receive this instruction until after the first sex,” wrote Laura D. Lindberg, PhD, and Leslie M. Kantor, PhD, MPH, from the Guttmacher Institute, New York, and the department of urban-global public health at Rutgers University, Piscataway, N.J., respectively. “These gaps in sex education in the U.S. are uneven, and gender, racial, and other disparities are widespread,” they added, calling for “robust efforts ... to ensure equity and reduce health disparities.”
The study used cross-sectional data from the 2011-2015 and 2015-2019 National Surveys of Family Growth (NSFG) to examine content, timing, and location of formal sex education among 15- to 19-year-olds in the United States. The data came from samples of 2,047 females and 2,087 males in 2011-2015, and 1,894 females and 1,918 males in 2015-2019. The majority of respondents were aged 15-17 years and non-Hispanic White, with another quarter being Hispanic, and 14% Black.
The survey asked respondents whether, before they turned 18, they had ever received formal instruction at school, church, a community center, “or some other place” about how to say no to sex, methods of birth control, STDs, how to prevent HIV/AIDS, abstaining until marriage to have sex, where to get birth control, and how to use a condom.
Follow-up questions asked about what grade instruction was first received and whether it had occurred before first penile-vaginal intercourse. The 2015-2019 survey also asked about the location of instruction, but only concerning methods of birth control and abstinence until marriage.
The results showed that HIV and STD prevention was the most commonly reported area of instruction, received by more than 90% of both males and females. However, beyond this there were imbalances, with only about half (49%-55%) of respondents receiving instruction meeting the Surgeon General’s Healthy People 2030 composite sex education goal. Lack of instruction on birth control drove this result for 80% of respondents. Specifically, there was a strong slant emphasizing abstinence over birth control instruction. Over both survey periods and both genders, more respondents reported instruction on how to say no to sex (79%-84%) and abstaining until marriage (58%-73%), compared with where to obtain birth control (40%-53%) or how to use a condom (54%-60%). “Overall, about 20% of adolescents received instruction from multiple sources about waiting until marriage, but only 5%-8% received birth control information from multiple settings,” they reported.
There were racial/ethnic and sexual orientation differences in the scope and balance of instruction reported by teens. Less than half of Black (45%) and Hispanic (47%) males received instruction on the combined Healthy People topics, compared with 57% of White males. Black females were less likely (30%) than White females (45%) to receive information on where to get birth control before the first sex. Nonstraight males were less likely than straight males to receive instruction about STIs or HIV/AIDS (83% vs. 93%).
In addition, religious attendance emerged as a key factor in the receipt of sex education, “with more frequent religious attendance associated with a greater likelihood of instruction about delaying sex and less likelihood of instruction about contraception,” the authors noted.
Comparing their findings to previous NSFG surveys, the researchers commented that “the share of adolescents receiving instruction about birth control was higher in 1995 than in 2015-2019 for both the genders; in 1995, 87% of females and 81% of males reported sex education about birth control methods, compared with 64% and 63% in 2015-2019, respectively.” The findings “should spur policy makers at the national, state, and local levels to ensure the broader provision of sex education and that school districts serving young people of color are the focus of additional efforts and funding.”
Asked for comment, John Santelli, MD, MPH, professor of population and family health and pediatrics at Columbia University, New York, who was not involved with the study, said the findings fit into a series of studies by Lindberg going back to 1988 showing that receipt of formal sex education before age 18 has declined over time.
“We, the adults, in America can do better by our young people,” he said in an interview. “Adolescents need sex education that is science based, medically accurate, and developmentally appropriate. Many adolescents are not receiving education that the CDC and health professionals recommend including information about where to get birth control, condom skills, and even, how to say no to sex. The neglect of young Black and Hispanic men is very concerning. However, we are not doing a great job in educating most of our adolescents. Health care providers can be influential in speaking with parents about their children’s education about sex. We need to activate parents, health care providers, and members of the faith community to investigate what is happening about sex education in their own communities.”
Dr. Santelli noted that there are multiple ways to strengthen the provision of sex education in the United States. In a recent commentary, he and his coauthors highlighted the National Sex Education Standards (NSES), which, “developed in partnership between sex education organizations and health professionals, provide clear, consistent, and straightforward guidance on the essential content for students in grades K-12.” The NSES were also used in the development of the CDC’s recently released Health Education Curriculum Analysis Tool.
The commentary takes a strong stand against the recently released revised Medical Institute for Sexual Heath K-12 Standards for Optimal Sexual Development, which, compared with the NSES, are “seriously flawed from both scientific and human rights’ perspectives,” they wrote. “States and local communities aiming to improve adolescent sexual and reproductive health and looking for national standards on sex education should adopt the NSES.”
Dr. Lindberg and Dr. Kantor disclosed no conflicts of interest. Dr. Santelli teaches public health students about adolescent health and chairs the board of directors of the Sexuality Information and Education Council of the United States. He disclosed no financial conflicts.
American teenagers receive less formal sex education today than they did 25 years ago, with “troubling” racial inequities that leave youth of color and queer youth at greater risk than other teens for sexually transmitted diseases and unintended pregnancy, according to a new study.
“Many adolescents do not receive any instruction on essential topics or do not receive this instruction until after the first sex,” wrote Laura D. Lindberg, PhD, and Leslie M. Kantor, PhD, MPH, from the Guttmacher Institute, New York, and the department of urban-global public health at Rutgers University, Piscataway, N.J., respectively. “These gaps in sex education in the U.S. are uneven, and gender, racial, and other disparities are widespread,” they added, calling for “robust efforts ... to ensure equity and reduce health disparities.”
The study used cross-sectional data from the 2011-2015 and 2015-2019 National Surveys of Family Growth (NSFG) to examine content, timing, and location of formal sex education among 15- to 19-year-olds in the United States. The data came from samples of 2,047 females and 2,087 males in 2011-2015, and 1,894 females and 1,918 males in 2015-2019. The majority of respondents were aged 15-17 years and non-Hispanic White, with another quarter being Hispanic, and 14% Black.
The survey asked respondents whether, before they turned 18, they had ever received formal instruction at school, church, a community center, “or some other place” about how to say no to sex, methods of birth control, STDs, how to prevent HIV/AIDS, abstaining until marriage to have sex, where to get birth control, and how to use a condom.
Follow-up questions asked about what grade instruction was first received and whether it had occurred before first penile-vaginal intercourse. The 2015-2019 survey also asked about the location of instruction, but only concerning methods of birth control and abstinence until marriage.
The results showed that HIV and STD prevention was the most commonly reported area of instruction, received by more than 90% of both males and females. However, beyond this there were imbalances, with only about half (49%-55%) of respondents receiving instruction meeting the Surgeon General’s Healthy People 2030 composite sex education goal. Lack of instruction on birth control drove this result for 80% of respondents. Specifically, there was a strong slant emphasizing abstinence over birth control instruction. Over both survey periods and both genders, more respondents reported instruction on how to say no to sex (79%-84%) and abstaining until marriage (58%-73%), compared with where to obtain birth control (40%-53%) or how to use a condom (54%-60%). “Overall, about 20% of adolescents received instruction from multiple sources about waiting until marriage, but only 5%-8% received birth control information from multiple settings,” they reported.
There were racial/ethnic and sexual orientation differences in the scope and balance of instruction reported by teens. Less than half of Black (45%) and Hispanic (47%) males received instruction on the combined Healthy People topics, compared with 57% of White males. Black females were less likely (30%) than White females (45%) to receive information on where to get birth control before the first sex. Nonstraight males were less likely than straight males to receive instruction about STIs or HIV/AIDS (83% vs. 93%).
In addition, religious attendance emerged as a key factor in the receipt of sex education, “with more frequent religious attendance associated with a greater likelihood of instruction about delaying sex and less likelihood of instruction about contraception,” the authors noted.
Comparing their findings to previous NSFG surveys, the researchers commented that “the share of adolescents receiving instruction about birth control was higher in 1995 than in 2015-2019 for both the genders; in 1995, 87% of females and 81% of males reported sex education about birth control methods, compared with 64% and 63% in 2015-2019, respectively.” The findings “should spur policy makers at the national, state, and local levels to ensure the broader provision of sex education and that school districts serving young people of color are the focus of additional efforts and funding.”
Asked for comment, John Santelli, MD, MPH, professor of population and family health and pediatrics at Columbia University, New York, who was not involved with the study, said the findings fit into a series of studies by Lindberg going back to 1988 showing that receipt of formal sex education before age 18 has declined over time.
“We, the adults, in America can do better by our young people,” he said in an interview. “Adolescents need sex education that is science based, medically accurate, and developmentally appropriate. Many adolescents are not receiving education that the CDC and health professionals recommend including information about where to get birth control, condom skills, and even, how to say no to sex. The neglect of young Black and Hispanic men is very concerning. However, we are not doing a great job in educating most of our adolescents. Health care providers can be influential in speaking with parents about their children’s education about sex. We need to activate parents, health care providers, and members of the faith community to investigate what is happening about sex education in their own communities.”
Dr. Santelli noted that there are multiple ways to strengthen the provision of sex education in the United States. In a recent commentary, he and his coauthors highlighted the National Sex Education Standards (NSES), which, “developed in partnership between sex education organizations and health professionals, provide clear, consistent, and straightforward guidance on the essential content for students in grades K-12.” The NSES were also used in the development of the CDC’s recently released Health Education Curriculum Analysis Tool.
The commentary takes a strong stand against the recently released revised Medical Institute for Sexual Heath K-12 Standards for Optimal Sexual Development, which, compared with the NSES, are “seriously flawed from both scientific and human rights’ perspectives,” they wrote. “States and local communities aiming to improve adolescent sexual and reproductive health and looking for national standards on sex education should adopt the NSES.”
Dr. Lindberg and Dr. Kantor disclosed no conflicts of interest. Dr. Santelli teaches public health students about adolescent health and chairs the board of directors of the Sexuality Information and Education Council of the United States. He disclosed no financial conflicts.
FROM THE JOURNAL OF ADOLESCENT HEALTH