User login
Getting hypertension under control in the youngest of patients
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
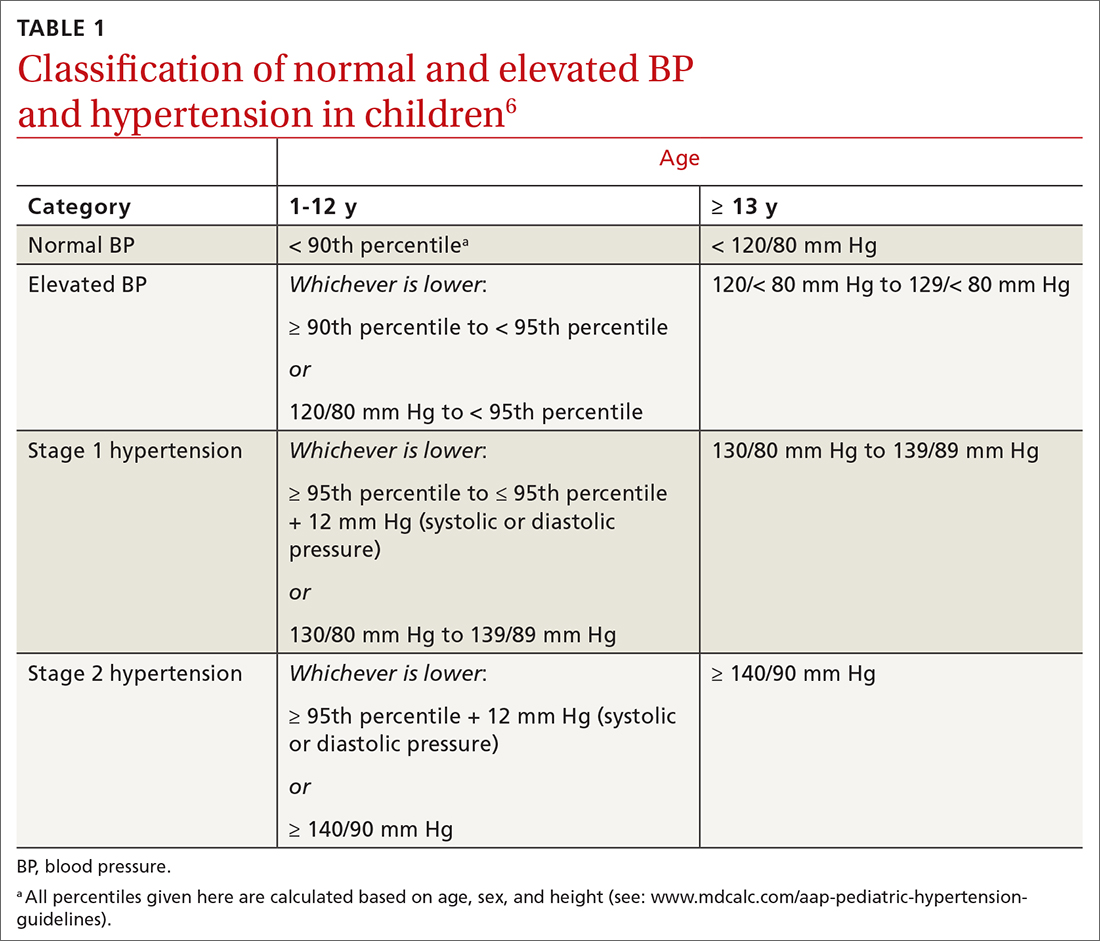
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7
As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16

Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
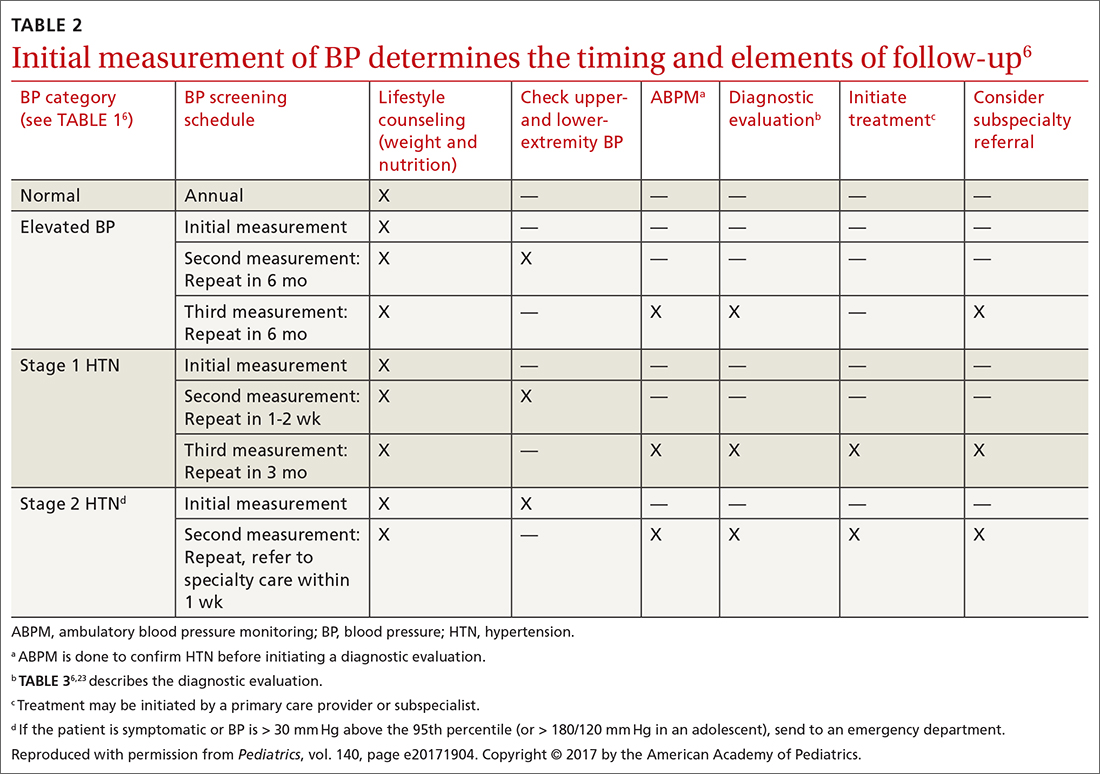
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.
ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.
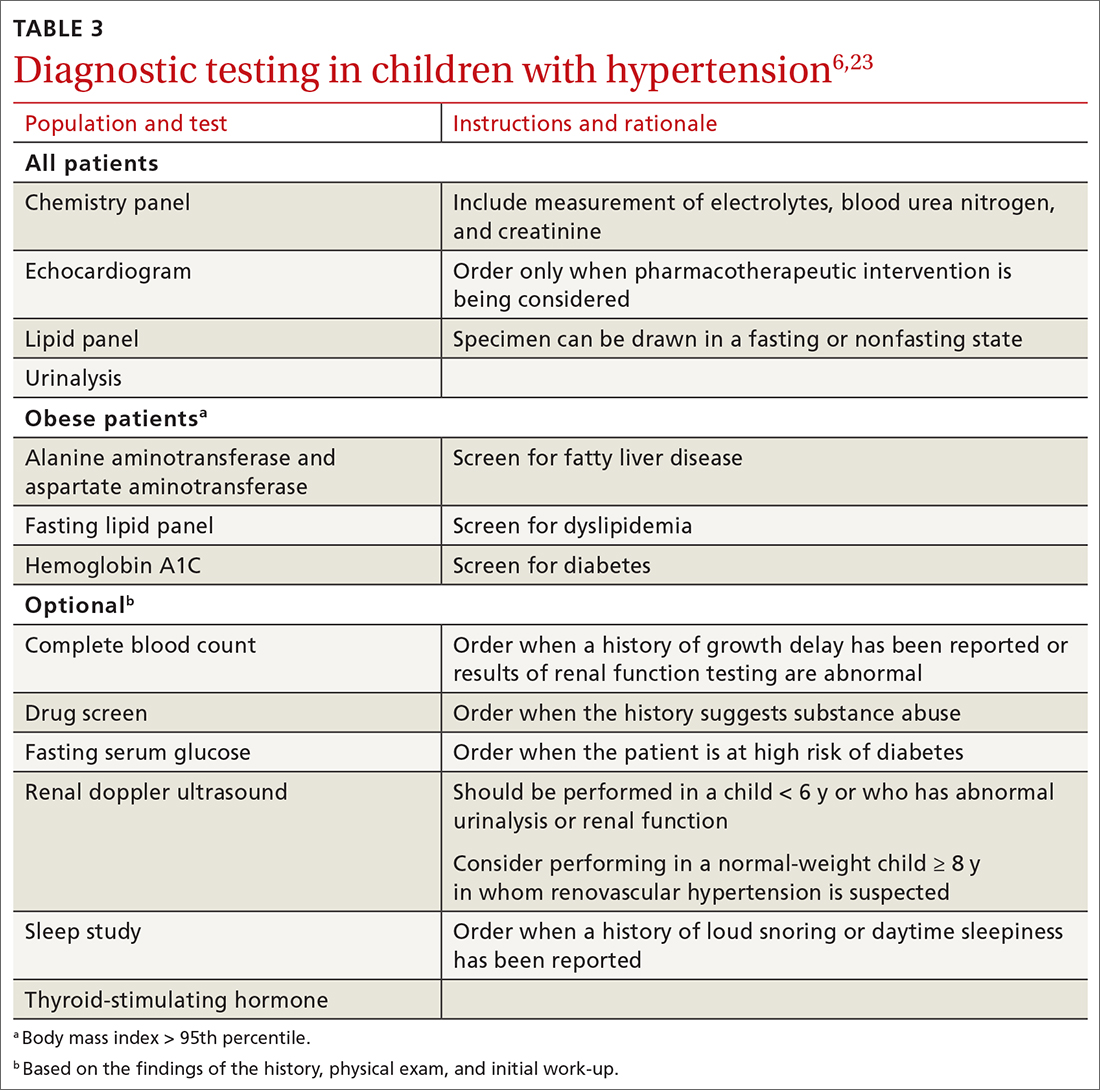
TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.

Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
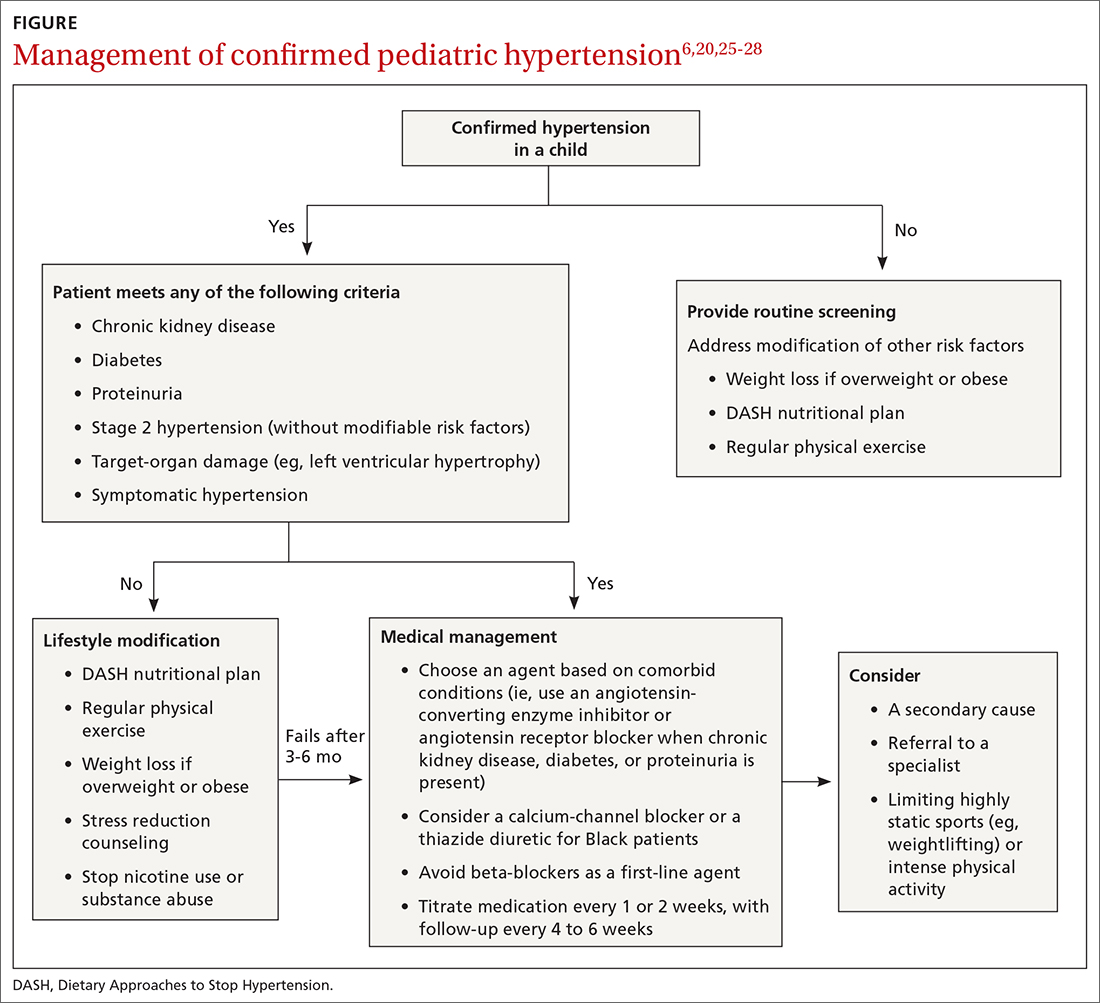
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.
Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; [email protected]
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7

As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16

Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.

ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.

TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.

Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.

Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; [email protected]
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7

As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16

Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.

ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.

TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.

Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.

Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; [email protected]
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
PRACTICE RECOMMENDATIONS
› Measure the blood pressure (BP) of all children 3 years and older annually; those who have a specific comorbid condition (eg, obesity, diabetes, renal disease, or an aortic-arch abnormality) or who are taking medication known to elevate BP should have their BP checked at every health care visit. C
› Encourage lifestyle modification as the initial treatment for elevated BP or hypertension in children. A
› Utilize pharmacotherapy for (1) children with stage 1 hypertension who have failed to meet BP goals after 3 to 6 months of lifestyle modification and (2) children with stage 2 hypertension who do not have a modifiable risk factor, such as obesity. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Prediction rule identifies low infection risk in febrile infants
A clinical prediction rule combining procalcitonin, absolute neutrophil count, and urinalysis effectively identified most febrile infants at low risk for serious bacterial infections, based on data from 702 individuals
The clinical prediction rule (CPR) described in 2019 in JAMA Pediatrics was developed by the Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN) to identify febrile infants at low risk for serious bacterial infections in order to reduce unnecessary procedures, antibiotics use, and hospitalization, according to April Clawson, MD, of Arkansas Children’s Hospital, Little Rock, and colleagues.
In a poster presented at the Pediatric Academic Societies annual meeting, the researchers conducted an external validation of the rule via a retrospective, observational study of febrile infants aged 60 days and younger who presented to an urban pediatric ED between October 2014 and June 2019. The study population included 702 infants with an average age of 36 days. Approximately 45% were female, and 60% were White. Fever was defined as 38° C or greater. Exclusion criteria were prematurity, receipt of antibiotics in the past 48 hours, presence of an indwelling medical device, and evidence of focal infection (not including otitis media); those who were critically ill at presentation or had a previous medical condition were excluded as well, the researchers said. A serious bacterial infection (SBI) was defined as a urinary tract infection (UTI), bacteremia, or bacterial meningitis.
Based on the CPR, a patient is considered low risk for an SBI if all the following criteria are met: normal urinalysis (defined as absence of leukocyte esterase, nitrite, and 5 or less white blood cells per high power field); an absolute neutrophil count of 4,090/mL or less; and procalcitonin of 1.71 ng/mL or less.
Overall, 62 infants (8.8%) were diagnosed with an SBI, similar to the 9.3% seen in the parent study of the CPR, Dr. Clawson said.
Of these, 42 had a UTI only (6%), 10 had bacteremia only (1.4%), and 1 had meningitis only (0.1%). Another five infants had UTI with bacteremia (0.7%), and four had bacteremia and meningitis (0.6%).
According to the CPR, 432 infants met criteria for low risk and 270 were considered high risk. A total of five infants who were classified as low risk had SBIs, including two with UTIs, two with bacteremia, and one with meningitis.
“The CPR derived and validated by Kupperman et al. had a decreased sensitivity for the patients in our study and missed some SBIs,” Dr. Clawson noted. “However, it had a strong negative predictive value, so it may still be a useful CPR.”
The sensitivity for the CPR in the parent study and the current study was 97.7 and 91.9, respectively; specificity was 60 and 66.7, respectively. The negative predictive values for the parent and current studies were 99.6 and 98.8, respectively, and the positive predictive values were 20.7 and 21.1.
The results support the potential of the CPR, but more external validation is needed, they said.
PECARN rule keeps it simple
“It has always been a challenge to identify infants with fever with serious bacterial infections when they are well-appearing,” Yashas Nathani, MD, of Oklahoma University, Oklahoma City, said in an interview. “The clinical prediction rule offers a simple, step-by-step approach for pediatricians and emergency medicine physicians to stratify infants in high or low risk categories for SBIs. However, as with everything, validation of protocols, guidelines and decision-making algorithms is extremely important, especially as more clinicians start to employ this CPR to their daily practice. This study objectively puts the CPR to the test and offers an independent external validation.
“Although this study had a lower sensitivity in identifying infants with SBI using the clinical prediction rule as compared to the original study, the robust validation of negative predictive value is extremely important and not surprising,” said Dr. Nathani. “The goal of this CPR is to identify infants with low-risk for SBI and the stated NPV helps clinicians in doing just that.”
Overall, “the clinical prediction rule is a fantastic resource for physicians to identify potentially sick infants with fever, especially the ones that appear well on initial evaluation,” said Dr. Nathani. However, “it is important to acknowledge that this is merely a guideline, and not an absolute rule. Clinicians also must remain cautious, as this rule does not incorporate the presence of viral pathogens as a factor.
“It is important to continue the scientific quest to refine our approach in identifying infants with serious bacterial infections when fever is the only presentation,” Dr. Nathani noted. “Additional research is needed to continue fine-tuning this CPR and the thresholds for procalcitonin and absolute neutrophil counts to improve the sensitivity and specificity.” Research also is needed to explore whether this CPR can be extended to incorporate viral testing, “as a large number of infants with fever have viral pathogens as the primary etiology,” he concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Nathani had no financial conflicts to disclose.
A clinical prediction rule combining procalcitonin, absolute neutrophil count, and urinalysis effectively identified most febrile infants at low risk for serious bacterial infections, based on data from 702 individuals
The clinical prediction rule (CPR) described in 2019 in JAMA Pediatrics was developed by the Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN) to identify febrile infants at low risk for serious bacterial infections in order to reduce unnecessary procedures, antibiotics use, and hospitalization, according to April Clawson, MD, of Arkansas Children’s Hospital, Little Rock, and colleagues.
In a poster presented at the Pediatric Academic Societies annual meeting, the researchers conducted an external validation of the rule via a retrospective, observational study of febrile infants aged 60 days and younger who presented to an urban pediatric ED between October 2014 and June 2019. The study population included 702 infants with an average age of 36 days. Approximately 45% were female, and 60% were White. Fever was defined as 38° C or greater. Exclusion criteria were prematurity, receipt of antibiotics in the past 48 hours, presence of an indwelling medical device, and evidence of focal infection (not including otitis media); those who were critically ill at presentation or had a previous medical condition were excluded as well, the researchers said. A serious bacterial infection (SBI) was defined as a urinary tract infection (UTI), bacteremia, or bacterial meningitis.
Based on the CPR, a patient is considered low risk for an SBI if all the following criteria are met: normal urinalysis (defined as absence of leukocyte esterase, nitrite, and 5 or less white blood cells per high power field); an absolute neutrophil count of 4,090/mL or less; and procalcitonin of 1.71 ng/mL or less.
Overall, 62 infants (8.8%) were diagnosed with an SBI, similar to the 9.3% seen in the parent study of the CPR, Dr. Clawson said.
Of these, 42 had a UTI only (6%), 10 had bacteremia only (1.4%), and 1 had meningitis only (0.1%). Another five infants had UTI with bacteremia (0.7%), and four had bacteremia and meningitis (0.6%).
According to the CPR, 432 infants met criteria for low risk and 270 were considered high risk. A total of five infants who were classified as low risk had SBIs, including two with UTIs, two with bacteremia, and one with meningitis.
“The CPR derived and validated by Kupperman et al. had a decreased sensitivity for the patients in our study and missed some SBIs,” Dr. Clawson noted. “However, it had a strong negative predictive value, so it may still be a useful CPR.”
The sensitivity for the CPR in the parent study and the current study was 97.7 and 91.9, respectively; specificity was 60 and 66.7, respectively. The negative predictive values for the parent and current studies were 99.6 and 98.8, respectively, and the positive predictive values were 20.7 and 21.1.
The results support the potential of the CPR, but more external validation is needed, they said.
PECARN rule keeps it simple
“It has always been a challenge to identify infants with fever with serious bacterial infections when they are well-appearing,” Yashas Nathani, MD, of Oklahoma University, Oklahoma City, said in an interview. “The clinical prediction rule offers a simple, step-by-step approach for pediatricians and emergency medicine physicians to stratify infants in high or low risk categories for SBIs. However, as with everything, validation of protocols, guidelines and decision-making algorithms is extremely important, especially as more clinicians start to employ this CPR to their daily practice. This study objectively puts the CPR to the test and offers an independent external validation.
“Although this study had a lower sensitivity in identifying infants with SBI using the clinical prediction rule as compared to the original study, the robust validation of negative predictive value is extremely important and not surprising,” said Dr. Nathani. “The goal of this CPR is to identify infants with low-risk for SBI and the stated NPV helps clinicians in doing just that.”
Overall, “the clinical prediction rule is a fantastic resource for physicians to identify potentially sick infants with fever, especially the ones that appear well on initial evaluation,” said Dr. Nathani. However, “it is important to acknowledge that this is merely a guideline, and not an absolute rule. Clinicians also must remain cautious, as this rule does not incorporate the presence of viral pathogens as a factor.
“It is important to continue the scientific quest to refine our approach in identifying infants with serious bacterial infections when fever is the only presentation,” Dr. Nathani noted. “Additional research is needed to continue fine-tuning this CPR and the thresholds for procalcitonin and absolute neutrophil counts to improve the sensitivity and specificity.” Research also is needed to explore whether this CPR can be extended to incorporate viral testing, “as a large number of infants with fever have viral pathogens as the primary etiology,” he concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Nathani had no financial conflicts to disclose.
A clinical prediction rule combining procalcitonin, absolute neutrophil count, and urinalysis effectively identified most febrile infants at low risk for serious bacterial infections, based on data from 702 individuals
The clinical prediction rule (CPR) described in 2019 in JAMA Pediatrics was developed by the Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN) to identify febrile infants at low risk for serious bacterial infections in order to reduce unnecessary procedures, antibiotics use, and hospitalization, according to April Clawson, MD, of Arkansas Children’s Hospital, Little Rock, and colleagues.
In a poster presented at the Pediatric Academic Societies annual meeting, the researchers conducted an external validation of the rule via a retrospective, observational study of febrile infants aged 60 days and younger who presented to an urban pediatric ED between October 2014 and June 2019. The study population included 702 infants with an average age of 36 days. Approximately 45% were female, and 60% were White. Fever was defined as 38° C or greater. Exclusion criteria were prematurity, receipt of antibiotics in the past 48 hours, presence of an indwelling medical device, and evidence of focal infection (not including otitis media); those who were critically ill at presentation or had a previous medical condition were excluded as well, the researchers said. A serious bacterial infection (SBI) was defined as a urinary tract infection (UTI), bacteremia, or bacterial meningitis.
Based on the CPR, a patient is considered low risk for an SBI if all the following criteria are met: normal urinalysis (defined as absence of leukocyte esterase, nitrite, and 5 or less white blood cells per high power field); an absolute neutrophil count of 4,090/mL or less; and procalcitonin of 1.71 ng/mL or less.
Overall, 62 infants (8.8%) were diagnosed with an SBI, similar to the 9.3% seen in the parent study of the CPR, Dr. Clawson said.
Of these, 42 had a UTI only (6%), 10 had bacteremia only (1.4%), and 1 had meningitis only (0.1%). Another five infants had UTI with bacteremia (0.7%), and four had bacteremia and meningitis (0.6%).
According to the CPR, 432 infants met criteria for low risk and 270 were considered high risk. A total of five infants who were classified as low risk had SBIs, including two with UTIs, two with bacteremia, and one with meningitis.
“The CPR derived and validated by Kupperman et al. had a decreased sensitivity for the patients in our study and missed some SBIs,” Dr. Clawson noted. “However, it had a strong negative predictive value, so it may still be a useful CPR.”
The sensitivity for the CPR in the parent study and the current study was 97.7 and 91.9, respectively; specificity was 60 and 66.7, respectively. The negative predictive values for the parent and current studies were 99.6 and 98.8, respectively, and the positive predictive values were 20.7 and 21.1.
The results support the potential of the CPR, but more external validation is needed, they said.
PECARN rule keeps it simple
“It has always been a challenge to identify infants with fever with serious bacterial infections when they are well-appearing,” Yashas Nathani, MD, of Oklahoma University, Oklahoma City, said in an interview. “The clinical prediction rule offers a simple, step-by-step approach for pediatricians and emergency medicine physicians to stratify infants in high or low risk categories for SBIs. However, as with everything, validation of protocols, guidelines and decision-making algorithms is extremely important, especially as more clinicians start to employ this CPR to their daily practice. This study objectively puts the CPR to the test and offers an independent external validation.
“Although this study had a lower sensitivity in identifying infants with SBI using the clinical prediction rule as compared to the original study, the robust validation of negative predictive value is extremely important and not surprising,” said Dr. Nathani. “The goal of this CPR is to identify infants with low-risk for SBI and the stated NPV helps clinicians in doing just that.”
Overall, “the clinical prediction rule is a fantastic resource for physicians to identify potentially sick infants with fever, especially the ones that appear well on initial evaluation,” said Dr. Nathani. However, “it is important to acknowledge that this is merely a guideline, and not an absolute rule. Clinicians also must remain cautious, as this rule does not incorporate the presence of viral pathogens as a factor.
“It is important to continue the scientific quest to refine our approach in identifying infants with serious bacterial infections when fever is the only presentation,” Dr. Nathani noted. “Additional research is needed to continue fine-tuning this CPR and the thresholds for procalcitonin and absolute neutrophil counts to improve the sensitivity and specificity.” Research also is needed to explore whether this CPR can be extended to incorporate viral testing, “as a large number of infants with fever have viral pathogens as the primary etiology,” he concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Nathani had no financial conflicts to disclose.
FROM PAS 2021
Secukinumab provides clinical benefit in phase 3 juvenile arthritis trial
Favorable safety sustained at 104 weeks
Secukinumab (Cosentyx), an interleukin-17A inhibitor, is effective and reasonably well tolerated for treatment of enthesitis-related arthritis (ERA) and juvenile psoriatic arthritis (JPsA) in children and adolescents, according to a phase 3 trial presented at a late breaking abstracts session of the annual European Congress of Rheumatology.
On the primary outcome of time to flare, the curves for secukinumab and placebo separated almost immediately, with fewer than half the number of flares occurring in the experimental arm over the course of the study, according to Nicolino Ruperto, MD, senior research scientist at IRCCS Istituto Giannina Gaslini in Genoa, Italy.
The trial, called JUNIPERA, was conducted over 2 years and included an open-label treatment period (TP1) and then a randomized, placebo-controlled comparison (TP2). In TP1, 86 children were initiated on open-label secukinumab administered subcutaneously on weeks 1, 2, 3, 4, 8, and 12. The dose was 75 mg for children less than 50 kg and 150 kg for those heavier.
Average patient age was 13.1 years
Of these 86 children, 52 had ERA and 34 had JPsA. Disease duration of at least 6 months was required for entry. Patients up to the age of 18 years were permitted to enroll. The average age was 13.1 years. Most patients, two-thirds of whom were male, had received an immunomodulator prior to study entry.
At the end of TP1, 69.9% of patients had achieved 70% improvement in the Juvenile Idiopathic Arthritis American College of Rheumatology joint score (JIA ACR70). The 90.4% of patients who achieved JIA ACR30 were invited to enroll in TP2. A total of 75 patients did so.
At the end of TP2, response rates strongly favored secukinumab over placebo for JIA ACR30 (89.2% vs. 64.9%; P = .014) and JIA ACR70 (67.7% vs. 43.2%; P = .042). Higher but not statistically significant differences in response rates were seen for secukinumab over placebo for JIA ACR50 (78.4% vs. 62.2%; P = .152), JIA ACR90 (51.4% vs. 40.5%; P = .431) and JIA ACR100 (43.2% vs. 37.8%; P = .755).
During TP2, there were 10 flares in the group randomized to secukinumab versus 21 flares in the placebo group, translating by hazard ratio (HR) into a 72% risk reduction (HR, 0.28; P < .001).
Side effects similar to those in adults
The types and rates of serious adverse events were similar to those reported previously in adult patients, according to Dr. Ruperto. Although the rate of serious adverse events (14.6% vs. 10.6%) was only moderately higher in the experimental arm, more patients randomized to secukinumab than placebo discontinued therapy (13.2% vs. 6.3%) before the end of follow-up.
The side effects that occurred more commonly on secukinumab included gastrointestinal complaints, such as diarrhea (22.9% vs. 15.8%). Other adverse events occurring in more than 10% of patients included headache and nasopharyngitis, but most side effects were mild and resolved.
Although the proportion of patients with flare increased over time in both groups, Dr. Ruperto reported that protection against flares and relative improvement in clinical markers of disease activity relative to placebo “were sustained out to 2 years of follow-up.”
The submission of these data to regulatory agencies is anticipated. If secukinumab is given an indication for these forms of arthritis, it will join an indication for plaque psoriasis in children that was granted just a few days before these data were presented. The psoriasis indication is the only current use approved for children in the United States.
More biologics needed for JPsA
Additional biologics will be helpful for children with arthritis who are poorly controlled on available treatments, according to Natasha M. Ruth, MD, director of the division of pediatric rheumatology at the Medical University of South Carolina, Charleston. Dr. Ruth was senior author of a case study published 2 years ago in which secukinumab was used to control psoriatic arthritis and nail manifestations of psoriasis.
“It was a girl who had already failed to improve adequately to TNF inhibitors,” reported Dr. Ruth, who had said the child and her parent were very concerned about the nail appearance.
“The nail involvement completely resolved, so it was a very good result in a difficult situation,” Dr. Ruth explained. She said that the decision to try secukinumab was made collaboratively in a clinic in which dermatologists and rheumatologists at her institution work together on difficult cases.
“There is a need for more biologics with different mechanisms of action,” Dr. Ruth said. Based on her experience, secukinumab could be an important addition to treatment options.
Dr. Ruperto reported having financial relationships with more than 20 pharmaceutical companies, including Novartis, which provided financial support for this trial. Many coauthors had financial relationships with multiple companies, including Novartis, and some were employees of the company. Dr. Ruth reported having no potential conflicts of interest.
Favorable safety sustained at 104 weeks
Favorable safety sustained at 104 weeks
Secukinumab (Cosentyx), an interleukin-17A inhibitor, is effective and reasonably well tolerated for treatment of enthesitis-related arthritis (ERA) and juvenile psoriatic arthritis (JPsA) in children and adolescents, according to a phase 3 trial presented at a late breaking abstracts session of the annual European Congress of Rheumatology.
On the primary outcome of time to flare, the curves for secukinumab and placebo separated almost immediately, with fewer than half the number of flares occurring in the experimental arm over the course of the study, according to Nicolino Ruperto, MD, senior research scientist at IRCCS Istituto Giannina Gaslini in Genoa, Italy.
The trial, called JUNIPERA, was conducted over 2 years and included an open-label treatment period (TP1) and then a randomized, placebo-controlled comparison (TP2). In TP1, 86 children were initiated on open-label secukinumab administered subcutaneously on weeks 1, 2, 3, 4, 8, and 12. The dose was 75 mg for children less than 50 kg and 150 kg for those heavier.
Average patient age was 13.1 years
Of these 86 children, 52 had ERA and 34 had JPsA. Disease duration of at least 6 months was required for entry. Patients up to the age of 18 years were permitted to enroll. The average age was 13.1 years. Most patients, two-thirds of whom were male, had received an immunomodulator prior to study entry.
At the end of TP1, 69.9% of patients had achieved 70% improvement in the Juvenile Idiopathic Arthritis American College of Rheumatology joint score (JIA ACR70). The 90.4% of patients who achieved JIA ACR30 were invited to enroll in TP2. A total of 75 patients did so.
At the end of TP2, response rates strongly favored secukinumab over placebo for JIA ACR30 (89.2% vs. 64.9%; P = .014) and JIA ACR70 (67.7% vs. 43.2%; P = .042). Higher but not statistically significant differences in response rates were seen for secukinumab over placebo for JIA ACR50 (78.4% vs. 62.2%; P = .152), JIA ACR90 (51.4% vs. 40.5%; P = .431) and JIA ACR100 (43.2% vs. 37.8%; P = .755).
During TP2, there were 10 flares in the group randomized to secukinumab versus 21 flares in the placebo group, translating by hazard ratio (HR) into a 72% risk reduction (HR, 0.28; P < .001).
Side effects similar to those in adults
The types and rates of serious adverse events were similar to those reported previously in adult patients, according to Dr. Ruperto. Although the rate of serious adverse events (14.6% vs. 10.6%) was only moderately higher in the experimental arm, more patients randomized to secukinumab than placebo discontinued therapy (13.2% vs. 6.3%) before the end of follow-up.
The side effects that occurred more commonly on secukinumab included gastrointestinal complaints, such as diarrhea (22.9% vs. 15.8%). Other adverse events occurring in more than 10% of patients included headache and nasopharyngitis, but most side effects were mild and resolved.
Although the proportion of patients with flare increased over time in both groups, Dr. Ruperto reported that protection against flares and relative improvement in clinical markers of disease activity relative to placebo “were sustained out to 2 years of follow-up.”
The submission of these data to regulatory agencies is anticipated. If secukinumab is given an indication for these forms of arthritis, it will join an indication for plaque psoriasis in children that was granted just a few days before these data were presented. The psoriasis indication is the only current use approved for children in the United States.
More biologics needed for JPsA
Additional biologics will be helpful for children with arthritis who are poorly controlled on available treatments, according to Natasha M. Ruth, MD, director of the division of pediatric rheumatology at the Medical University of South Carolina, Charleston. Dr. Ruth was senior author of a case study published 2 years ago in which secukinumab was used to control psoriatic arthritis and nail manifestations of psoriasis.
“It was a girl who had already failed to improve adequately to TNF inhibitors,” reported Dr. Ruth, who had said the child and her parent were very concerned about the nail appearance.
“The nail involvement completely resolved, so it was a very good result in a difficult situation,” Dr. Ruth explained. She said that the decision to try secukinumab was made collaboratively in a clinic in which dermatologists and rheumatologists at her institution work together on difficult cases.
“There is a need for more biologics with different mechanisms of action,” Dr. Ruth said. Based on her experience, secukinumab could be an important addition to treatment options.
Dr. Ruperto reported having financial relationships with more than 20 pharmaceutical companies, including Novartis, which provided financial support for this trial. Many coauthors had financial relationships with multiple companies, including Novartis, and some were employees of the company. Dr. Ruth reported having no potential conflicts of interest.
Secukinumab (Cosentyx), an interleukin-17A inhibitor, is effective and reasonably well tolerated for treatment of enthesitis-related arthritis (ERA) and juvenile psoriatic arthritis (JPsA) in children and adolescents, according to a phase 3 trial presented at a late breaking abstracts session of the annual European Congress of Rheumatology.
On the primary outcome of time to flare, the curves for secukinumab and placebo separated almost immediately, with fewer than half the number of flares occurring in the experimental arm over the course of the study, according to Nicolino Ruperto, MD, senior research scientist at IRCCS Istituto Giannina Gaslini in Genoa, Italy.
The trial, called JUNIPERA, was conducted over 2 years and included an open-label treatment period (TP1) and then a randomized, placebo-controlled comparison (TP2). In TP1, 86 children were initiated on open-label secukinumab administered subcutaneously on weeks 1, 2, 3, 4, 8, and 12. The dose was 75 mg for children less than 50 kg and 150 kg for those heavier.
Average patient age was 13.1 years
Of these 86 children, 52 had ERA and 34 had JPsA. Disease duration of at least 6 months was required for entry. Patients up to the age of 18 years were permitted to enroll. The average age was 13.1 years. Most patients, two-thirds of whom were male, had received an immunomodulator prior to study entry.
At the end of TP1, 69.9% of patients had achieved 70% improvement in the Juvenile Idiopathic Arthritis American College of Rheumatology joint score (JIA ACR70). The 90.4% of patients who achieved JIA ACR30 were invited to enroll in TP2. A total of 75 patients did so.
At the end of TP2, response rates strongly favored secukinumab over placebo for JIA ACR30 (89.2% vs. 64.9%; P = .014) and JIA ACR70 (67.7% vs. 43.2%; P = .042). Higher but not statistically significant differences in response rates were seen for secukinumab over placebo for JIA ACR50 (78.4% vs. 62.2%; P = .152), JIA ACR90 (51.4% vs. 40.5%; P = .431) and JIA ACR100 (43.2% vs. 37.8%; P = .755).
During TP2, there were 10 flares in the group randomized to secukinumab versus 21 flares in the placebo group, translating by hazard ratio (HR) into a 72% risk reduction (HR, 0.28; P < .001).
Side effects similar to those in adults
The types and rates of serious adverse events were similar to those reported previously in adult patients, according to Dr. Ruperto. Although the rate of serious adverse events (14.6% vs. 10.6%) was only moderately higher in the experimental arm, more patients randomized to secukinumab than placebo discontinued therapy (13.2% vs. 6.3%) before the end of follow-up.
The side effects that occurred more commonly on secukinumab included gastrointestinal complaints, such as diarrhea (22.9% vs. 15.8%). Other adverse events occurring in more than 10% of patients included headache and nasopharyngitis, but most side effects were mild and resolved.
Although the proportion of patients with flare increased over time in both groups, Dr. Ruperto reported that protection against flares and relative improvement in clinical markers of disease activity relative to placebo “were sustained out to 2 years of follow-up.”
The submission of these data to regulatory agencies is anticipated. If secukinumab is given an indication for these forms of arthritis, it will join an indication for plaque psoriasis in children that was granted just a few days before these data were presented. The psoriasis indication is the only current use approved for children in the United States.
More biologics needed for JPsA
Additional biologics will be helpful for children with arthritis who are poorly controlled on available treatments, according to Natasha M. Ruth, MD, director of the division of pediatric rheumatology at the Medical University of South Carolina, Charleston. Dr. Ruth was senior author of a case study published 2 years ago in which secukinumab was used to control psoriatic arthritis and nail manifestations of psoriasis.
“It was a girl who had already failed to improve adequately to TNF inhibitors,” reported Dr. Ruth, who had said the child and her parent were very concerned about the nail appearance.
“The nail involvement completely resolved, so it was a very good result in a difficult situation,” Dr. Ruth explained. She said that the decision to try secukinumab was made collaboratively in a clinic in which dermatologists and rheumatologists at her institution work together on difficult cases.
“There is a need for more biologics with different mechanisms of action,” Dr. Ruth said. Based on her experience, secukinumab could be an important addition to treatment options.
Dr. Ruperto reported having financial relationships with more than 20 pharmaceutical companies, including Novartis, which provided financial support for this trial. Many coauthors had financial relationships with multiple companies, including Novartis, and some were employees of the company. Dr. Ruth reported having no potential conflicts of interest.
FROM THE EULAR 2021 CONGRESS
As new cases fall, U.S. passes 4 million children with COVID-19
Even as the number of new COVID-19 cases continues to drop, the United States reached the 4-million mark for infected children, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
. That weekly total, the lowest since June of 2020, comes from 49 states (excluding N.Y.), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
Children represent 14.1% of all COVID-19 cases since the beginning of the pandemic, while the corresponding figure for the week ending June 10 was 19.0%. That weekly proportion of cases among children had been rising pretty steadily through the winter and early spring, but the situation has become much more volatile over the last month, the AAP/CHA data show.
Use of the Pfizer-BioNTech vaccine in children aged 16-17 years, of course, didn’t begin until April, and the vaccine wasn’t authorized for children aged 12-15 years until mid-May. The Moderna and Johnson & Johnson vaccines have not received such authorization yet, but Moderna is in the process of seeking an emergency-use recommendation from the Food and Drug Administration.
In the younger group of children who are currently eligible, completion of the vaccine regimen took a big jump in the week ending June 14, according to the Centers for Disease Control and Prevention. The cumulative share of those aged 12-15 years who had received a second dose jumped from 4.1% on June 7 to 11.4% on June 14, with comparable numbers for 16- and 17-year-olds coming in at 26.4% and 29.1%.
Activity over just the last 14 days, however, shows a slight decrease in children aged 12-15 getting a first dose: For just the 2 weeks ending June 7, 17.9% of all children in the age group initiated a first dose, but for the 14 days ending June 14, only 17.1% of the age group did so, the CDC said on its COVID Data Tracker site.
For children aged 16-17 years – of whom less than 30% have reached full vaccination – activity seems to have stagnated: 4.8% of all 16- to 17-year-olds initiated a first vaccination during the 14 days ending June 7, compared with 4.7% who did so during the 14 days ending June 14, the CDC reported.
Older age groups with higher completion rates are still producing greater vaccine initiation. As of June 14, those aged 25-39 years had a completion rate of 41.9% and 24.0% of the age group had received a first dose in the previous 2 weeks, while 61.4% of those aged 50-64 were fully vaccinated, and 18.0% had gotten their first dose, the CDC data indicate.
Even as the number of new COVID-19 cases continues to drop, the United States reached the 4-million mark for infected children, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
. That weekly total, the lowest since June of 2020, comes from 49 states (excluding N.Y.), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
Children represent 14.1% of all COVID-19 cases since the beginning of the pandemic, while the corresponding figure for the week ending June 10 was 19.0%. That weekly proportion of cases among children had been rising pretty steadily through the winter and early spring, but the situation has become much more volatile over the last month, the AAP/CHA data show.
Use of the Pfizer-BioNTech vaccine in children aged 16-17 years, of course, didn’t begin until April, and the vaccine wasn’t authorized for children aged 12-15 years until mid-May. The Moderna and Johnson & Johnson vaccines have not received such authorization yet, but Moderna is in the process of seeking an emergency-use recommendation from the Food and Drug Administration.
In the younger group of children who are currently eligible, completion of the vaccine regimen took a big jump in the week ending June 14, according to the Centers for Disease Control and Prevention. The cumulative share of those aged 12-15 years who had received a second dose jumped from 4.1% on June 7 to 11.4% on June 14, with comparable numbers for 16- and 17-year-olds coming in at 26.4% and 29.1%.
Activity over just the last 14 days, however, shows a slight decrease in children aged 12-15 getting a first dose: For just the 2 weeks ending June 7, 17.9% of all children in the age group initiated a first dose, but for the 14 days ending June 14, only 17.1% of the age group did so, the CDC said on its COVID Data Tracker site.
For children aged 16-17 years – of whom less than 30% have reached full vaccination – activity seems to have stagnated: 4.8% of all 16- to 17-year-olds initiated a first vaccination during the 14 days ending June 7, compared with 4.7% who did so during the 14 days ending June 14, the CDC reported.
Older age groups with higher completion rates are still producing greater vaccine initiation. As of June 14, those aged 25-39 years had a completion rate of 41.9% and 24.0% of the age group had received a first dose in the previous 2 weeks, while 61.4% of those aged 50-64 were fully vaccinated, and 18.0% had gotten their first dose, the CDC data indicate.
Even as the number of new COVID-19 cases continues to drop, the United States reached the 4-million mark for infected children, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
. That weekly total, the lowest since June of 2020, comes from 49 states (excluding N.Y.), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
Children represent 14.1% of all COVID-19 cases since the beginning of the pandemic, while the corresponding figure for the week ending June 10 was 19.0%. That weekly proportion of cases among children had been rising pretty steadily through the winter and early spring, but the situation has become much more volatile over the last month, the AAP/CHA data show.
Use of the Pfizer-BioNTech vaccine in children aged 16-17 years, of course, didn’t begin until April, and the vaccine wasn’t authorized for children aged 12-15 years until mid-May. The Moderna and Johnson & Johnson vaccines have not received such authorization yet, but Moderna is in the process of seeking an emergency-use recommendation from the Food and Drug Administration.
In the younger group of children who are currently eligible, completion of the vaccine regimen took a big jump in the week ending June 14, according to the Centers for Disease Control and Prevention. The cumulative share of those aged 12-15 years who had received a second dose jumped from 4.1% on June 7 to 11.4% on June 14, with comparable numbers for 16- and 17-year-olds coming in at 26.4% and 29.1%.
Activity over just the last 14 days, however, shows a slight decrease in children aged 12-15 getting a first dose: For just the 2 weeks ending June 7, 17.9% of all children in the age group initiated a first dose, but for the 14 days ending June 14, only 17.1% of the age group did so, the CDC said on its COVID Data Tracker site.
For children aged 16-17 years – of whom less than 30% have reached full vaccination – activity seems to have stagnated: 4.8% of all 16- to 17-year-olds initiated a first vaccination during the 14 days ending June 7, compared with 4.7% who did so during the 14 days ending June 14, the CDC reported.
Older age groups with higher completion rates are still producing greater vaccine initiation. As of June 14, those aged 25-39 years had a completion rate of 41.9% and 24.0% of the age group had received a first dose in the previous 2 weeks, while 61.4% of those aged 50-64 were fully vaccinated, and 18.0% had gotten their first dose, the CDC data indicate.
U.S. News releases Best Children’s Hospitals list, with changes
Released June 15, the 2021-2022 rankings, which acknowledge 50 U.S. centers for delivering exceptional care in several specialties, also give the Massachusetts hospital the top spot in 4 of 10 pediatric specialties assessed: nephrology, neurology and neurosurgery, pulmonology and lung surgery, and urology.
Children’s Hospital of Philadelphia retains second spot in the annually updated list, and Texas Children’s Hospital, in Houston, moves up a rung to third place, bumping Cincinnati Children’s Hospital Medical Center from third to fourth place. Children’s Hospital Los Angeles comes in at no. 5.
The remaining top 10 placements, in descending order, are as follows:
Children’s Hospital Colorado in Aurora; Children’s National Hospital in Washington; Nationwide Children’s Hospital in Columbus, Ohio; UPMS Children’s Hospital of Pittsburgh; and Lucile Packard Children’s Hospital Stanford (Calif.).
New regional rankings
This year’s edition offers something new, adding rankings within states and multiple-state rankings within seven regions to facilitate choice. “The Best Children’s Hospitals rankings have always highlighted hospitals that excel in specialized care,” said Ben Harder, chief of health analysis and managing editor at U.S. News, in a press release. “Now, this year’s new state and regional rankings can help families identify conveniently located hospitals capable of meeting their child’s needs. As the pandemic continues to affect travel, finding high-quality care close to home has never been more important.”
Across the seven regions, the top-ranked institutions are as follows:
- Mid-Atlantic – Children’s Hospital of Philadelphia.
- Midwest – Cincinnati Children’s Hospital Medical Center.
- New England – Boston Children’s Hospital.
- Pacific – Children’s Hospital Los Angeles.
- Rocky Mountains – Children’s Hospital Colorado.
- Southeast – Children’s Healthcare of Atlanta and Monroe Carell Jr. Children’s Hospital of Vanderbilt, in Nashville, Tenn.
- Southwest – Texas Children’s Hospital.
Specialties
Boston Children’s not only topped the overall list but also led in four specialties. For the other six specialties that were ranked, the top hospitals on the honor roll are as follows:
- Cancer – Children’s Hospital of Philadelphia.
- Cardiology and heart surgery – Texas Children’s Hospital.
- Diabetes and endocrinology – Children’s Hospital of Philadelphia.
- Gastroenterology and gastrointestinal surgery – Children’s Hospital Colorado.
- Neonatology – Children’s National Hospital.
- Orthopedics – Children’s Hospital of Philadelphia.
For the past 15 years, the objective of the rankings has been to offer a starting point for parents in making decisions about the best place to take very sick children for high-quality care. The editors of the rankings acknowledge that considerations of travel costs and insurance coverage are other factors to consider.
Helpful for families
The rankings are helpful for families, according to Joe W. St. Geme, III, MD, Children’s Hospital of Philadelphia’s physician-in-chief and chair of its department of pediatrics. “Some parents, especially those coming from outside an area, find them useful when deciding on care away from home,” he told this news organization. “Most types of pediatric care are available in the community, but sometimes a child has an unusual disease or complex disease for which local care is not available.”
Dr. St. Geme said the new regional rankings may be useful in helping parents decide where to bring a child for care that is closer to where they live.
A top ranking from U.S. News is just one indication of a hospital›s overall performance, according to Angela Lorts, MD, MBA, director of the Ventricular Assist Device Program, at Cincinnati Children’s Hospital Medical Center.
“Parents seeking care for their child should use the data to ask questions and understand the limitations,” she told this news organization. “Rankings are only based on a small subset of the children we care for. Many of the metrics may not pertain to their child and may not reflect the care they will receive.”
In her view, ranking will not give parents all the information they need about medical care and outcomes for specific conditions.
Hospital reaction
Hospitals can use the rankings to target improvements, says Dr. St. Geme. “These rankings can provide an opportunity for some benchmarking, to see what other institutions are doing and how they’re able to deliver care. They can serve as a source of ideas and can influence planning,” he said.
He cautioned that the data are not as complete as they could be. “A number of services are not included, and we try to keep that in mind,” he said.
Rankings may also affect recruitment, Dr. St. Geme added, because higher-ranked institutions may find it easier to attract sought-after clinicians and investigators in needed areas.
Another sphere of influence is philanthropy and fund raising. “People are much more likely to consider making both small and large donations to a high-ranked institution,” said J. Howard Smart, MD, chair of pediatrics at Sharp Rees-Stealy Medical Group and chair-elect of the physician leadership council at Sharp Mary Birch Hospital for Women and Newborns in San Diego.
Dr. St. Geme agrees. “Philanthropists are interested in making investments where they feel they’re a sure bet, and rankings may indicate a sure bet. But their impact on government funding and grant support is probably less.”
Ultimately, however, some families may not have lot of choice in where to go when their children are sick, Dr. Smart said. “And people probably don’t choose a location to live in based on nearby children’s hospitals the way they do for schools,” he said.
What about hospitals that continue to rank much lower on the 50-institution list – excellent though they must be to make it onto the honor roll. “To be on the list but not to have risen in rank in recent years might be a disappointment,” said Dr. St. Geme. “But it might also motivate a hospital to think about making internal investments in order to strengthen a particular service. And it may motivate nonranked hospitals to improve care in order to break into the list.”
Dr. Lorts points out that the annual survey process requires hospitals to track the clinical outcomes of a subset of patients, which may lead to improvement in these areas. It also requires data collection on structure and process, which drives needs assessments of select hospital areas. “But ideally, all hospitals would be tracking important outcomes, benchmarking to peer hospitals, and improving where needed without the U.S. News incentive,” she said.
This year’s data, compiled by research and consulting firm RTI International, derive from feedback on more than 1,200 questions provided by 118 responding institutions. Details on each hospital on the list and the methodology used in the analysis are available on U.S. News & World Report’s website.
A version of this article first appeared on Medscape.com.
Released June 15, the 2021-2022 rankings, which acknowledge 50 U.S. centers for delivering exceptional care in several specialties, also give the Massachusetts hospital the top spot in 4 of 10 pediatric specialties assessed: nephrology, neurology and neurosurgery, pulmonology and lung surgery, and urology.
Children’s Hospital of Philadelphia retains second spot in the annually updated list, and Texas Children’s Hospital, in Houston, moves up a rung to third place, bumping Cincinnati Children’s Hospital Medical Center from third to fourth place. Children’s Hospital Los Angeles comes in at no. 5.
The remaining top 10 placements, in descending order, are as follows:
Children’s Hospital Colorado in Aurora; Children’s National Hospital in Washington; Nationwide Children’s Hospital in Columbus, Ohio; UPMS Children’s Hospital of Pittsburgh; and Lucile Packard Children’s Hospital Stanford (Calif.).
New regional rankings
This year’s edition offers something new, adding rankings within states and multiple-state rankings within seven regions to facilitate choice. “The Best Children’s Hospitals rankings have always highlighted hospitals that excel in specialized care,” said Ben Harder, chief of health analysis and managing editor at U.S. News, in a press release. “Now, this year’s new state and regional rankings can help families identify conveniently located hospitals capable of meeting their child’s needs. As the pandemic continues to affect travel, finding high-quality care close to home has never been more important.”
Across the seven regions, the top-ranked institutions are as follows:
- Mid-Atlantic – Children’s Hospital of Philadelphia.
- Midwest – Cincinnati Children’s Hospital Medical Center.
- New England – Boston Children’s Hospital.
- Pacific – Children’s Hospital Los Angeles.
- Rocky Mountains – Children’s Hospital Colorado.
- Southeast – Children’s Healthcare of Atlanta and Monroe Carell Jr. Children’s Hospital of Vanderbilt, in Nashville, Tenn.
- Southwest – Texas Children’s Hospital.
Specialties
Boston Children’s not only topped the overall list but also led in four specialties. For the other six specialties that were ranked, the top hospitals on the honor roll are as follows:
- Cancer – Children’s Hospital of Philadelphia.
- Cardiology and heart surgery – Texas Children’s Hospital.
- Diabetes and endocrinology – Children’s Hospital of Philadelphia.
- Gastroenterology and gastrointestinal surgery – Children’s Hospital Colorado.
- Neonatology – Children’s National Hospital.
- Orthopedics – Children’s Hospital of Philadelphia.
For the past 15 years, the objective of the rankings has been to offer a starting point for parents in making decisions about the best place to take very sick children for high-quality care. The editors of the rankings acknowledge that considerations of travel costs and insurance coverage are other factors to consider.
Helpful for families
The rankings are helpful for families, according to Joe W. St. Geme, III, MD, Children’s Hospital of Philadelphia’s physician-in-chief and chair of its department of pediatrics. “Some parents, especially those coming from outside an area, find them useful when deciding on care away from home,” he told this news organization. “Most types of pediatric care are available in the community, but sometimes a child has an unusual disease or complex disease for which local care is not available.”
Dr. St. Geme said the new regional rankings may be useful in helping parents decide where to bring a child for care that is closer to where they live.
A top ranking from U.S. News is just one indication of a hospital›s overall performance, according to Angela Lorts, MD, MBA, director of the Ventricular Assist Device Program, at Cincinnati Children’s Hospital Medical Center.
“Parents seeking care for their child should use the data to ask questions and understand the limitations,” she told this news organization. “Rankings are only based on a small subset of the children we care for. Many of the metrics may not pertain to their child and may not reflect the care they will receive.”
In her view, ranking will not give parents all the information they need about medical care and outcomes for specific conditions.
Hospital reaction
Hospitals can use the rankings to target improvements, says Dr. St. Geme. “These rankings can provide an opportunity for some benchmarking, to see what other institutions are doing and how they’re able to deliver care. They can serve as a source of ideas and can influence planning,” he said.
He cautioned that the data are not as complete as they could be. “A number of services are not included, and we try to keep that in mind,” he said.
Rankings may also affect recruitment, Dr. St. Geme added, because higher-ranked institutions may find it easier to attract sought-after clinicians and investigators in needed areas.
Another sphere of influence is philanthropy and fund raising. “People are much more likely to consider making both small and large donations to a high-ranked institution,” said J. Howard Smart, MD, chair of pediatrics at Sharp Rees-Stealy Medical Group and chair-elect of the physician leadership council at Sharp Mary Birch Hospital for Women and Newborns in San Diego.
Dr. St. Geme agrees. “Philanthropists are interested in making investments where they feel they’re a sure bet, and rankings may indicate a sure bet. But their impact on government funding and grant support is probably less.”
Ultimately, however, some families may not have lot of choice in where to go when their children are sick, Dr. Smart said. “And people probably don’t choose a location to live in based on nearby children’s hospitals the way they do for schools,” he said.
What about hospitals that continue to rank much lower on the 50-institution list – excellent though they must be to make it onto the honor roll. “To be on the list but not to have risen in rank in recent years might be a disappointment,” said Dr. St. Geme. “But it might also motivate a hospital to think about making internal investments in order to strengthen a particular service. And it may motivate nonranked hospitals to improve care in order to break into the list.”
Dr. Lorts points out that the annual survey process requires hospitals to track the clinical outcomes of a subset of patients, which may lead to improvement in these areas. It also requires data collection on structure and process, which drives needs assessments of select hospital areas. “But ideally, all hospitals would be tracking important outcomes, benchmarking to peer hospitals, and improving where needed without the U.S. News incentive,” she said.
This year’s data, compiled by research and consulting firm RTI International, derive from feedback on more than 1,200 questions provided by 118 responding institutions. Details on each hospital on the list and the methodology used in the analysis are available on U.S. News & World Report’s website.
A version of this article first appeared on Medscape.com.
Released June 15, the 2021-2022 rankings, which acknowledge 50 U.S. centers for delivering exceptional care in several specialties, also give the Massachusetts hospital the top spot in 4 of 10 pediatric specialties assessed: nephrology, neurology and neurosurgery, pulmonology and lung surgery, and urology.
Children’s Hospital of Philadelphia retains second spot in the annually updated list, and Texas Children’s Hospital, in Houston, moves up a rung to third place, bumping Cincinnati Children’s Hospital Medical Center from third to fourth place. Children’s Hospital Los Angeles comes in at no. 5.
The remaining top 10 placements, in descending order, are as follows:
Children’s Hospital Colorado in Aurora; Children’s National Hospital in Washington; Nationwide Children’s Hospital in Columbus, Ohio; UPMS Children’s Hospital of Pittsburgh; and Lucile Packard Children’s Hospital Stanford (Calif.).
New regional rankings
This year’s edition offers something new, adding rankings within states and multiple-state rankings within seven regions to facilitate choice. “The Best Children’s Hospitals rankings have always highlighted hospitals that excel in specialized care,” said Ben Harder, chief of health analysis and managing editor at U.S. News, in a press release. “Now, this year’s new state and regional rankings can help families identify conveniently located hospitals capable of meeting their child’s needs. As the pandemic continues to affect travel, finding high-quality care close to home has never been more important.”
Across the seven regions, the top-ranked institutions are as follows:
- Mid-Atlantic – Children’s Hospital of Philadelphia.
- Midwest – Cincinnati Children’s Hospital Medical Center.
- New England – Boston Children’s Hospital.
- Pacific – Children’s Hospital Los Angeles.
- Rocky Mountains – Children’s Hospital Colorado.
- Southeast – Children’s Healthcare of Atlanta and Monroe Carell Jr. Children’s Hospital of Vanderbilt, in Nashville, Tenn.
- Southwest – Texas Children’s Hospital.
Specialties
Boston Children’s not only topped the overall list but also led in four specialties. For the other six specialties that were ranked, the top hospitals on the honor roll are as follows:
- Cancer – Children’s Hospital of Philadelphia.
- Cardiology and heart surgery – Texas Children’s Hospital.
- Diabetes and endocrinology – Children’s Hospital of Philadelphia.
- Gastroenterology and gastrointestinal surgery – Children’s Hospital Colorado.
- Neonatology – Children’s National Hospital.
- Orthopedics – Children’s Hospital of Philadelphia.
For the past 15 years, the objective of the rankings has been to offer a starting point for parents in making decisions about the best place to take very sick children for high-quality care. The editors of the rankings acknowledge that considerations of travel costs and insurance coverage are other factors to consider.
Helpful for families
The rankings are helpful for families, according to Joe W. St. Geme, III, MD, Children’s Hospital of Philadelphia’s physician-in-chief and chair of its department of pediatrics. “Some parents, especially those coming from outside an area, find them useful when deciding on care away from home,” he told this news organization. “Most types of pediatric care are available in the community, but sometimes a child has an unusual disease or complex disease for which local care is not available.”
Dr. St. Geme said the new regional rankings may be useful in helping parents decide where to bring a child for care that is closer to where they live.
A top ranking from U.S. News is just one indication of a hospital›s overall performance, according to Angela Lorts, MD, MBA, director of the Ventricular Assist Device Program, at Cincinnati Children’s Hospital Medical Center.
“Parents seeking care for their child should use the data to ask questions and understand the limitations,” she told this news organization. “Rankings are only based on a small subset of the children we care for. Many of the metrics may not pertain to their child and may not reflect the care they will receive.”
In her view, ranking will not give parents all the information they need about medical care and outcomes for specific conditions.
Hospital reaction
Hospitals can use the rankings to target improvements, says Dr. St. Geme. “These rankings can provide an opportunity for some benchmarking, to see what other institutions are doing and how they’re able to deliver care. They can serve as a source of ideas and can influence planning,” he said.
He cautioned that the data are not as complete as they could be. “A number of services are not included, and we try to keep that in mind,” he said.
Rankings may also affect recruitment, Dr. St. Geme added, because higher-ranked institutions may find it easier to attract sought-after clinicians and investigators in needed areas.
Another sphere of influence is philanthropy and fund raising. “People are much more likely to consider making both small and large donations to a high-ranked institution,” said J. Howard Smart, MD, chair of pediatrics at Sharp Rees-Stealy Medical Group and chair-elect of the physician leadership council at Sharp Mary Birch Hospital for Women and Newborns in San Diego.
Dr. St. Geme agrees. “Philanthropists are interested in making investments where they feel they’re a sure bet, and rankings may indicate a sure bet. But their impact on government funding and grant support is probably less.”
Ultimately, however, some families may not have lot of choice in where to go when their children are sick, Dr. Smart said. “And people probably don’t choose a location to live in based on nearby children’s hospitals the way they do for schools,” he said.
What about hospitals that continue to rank much lower on the 50-institution list – excellent though they must be to make it onto the honor roll. “To be on the list but not to have risen in rank in recent years might be a disappointment,” said Dr. St. Geme. “But it might also motivate a hospital to think about making internal investments in order to strengthen a particular service. And it may motivate nonranked hospitals to improve care in order to break into the list.”
Dr. Lorts points out that the annual survey process requires hospitals to track the clinical outcomes of a subset of patients, which may lead to improvement in these areas. It also requires data collection on structure and process, which drives needs assessments of select hospital areas. “But ideally, all hospitals would be tracking important outcomes, benchmarking to peer hospitals, and improving where needed without the U.S. News incentive,” she said.
This year’s data, compiled by research and consulting firm RTI International, derive from feedback on more than 1,200 questions provided by 118 responding institutions. Details on each hospital on the list and the methodology used in the analysis are available on U.S. News & World Report’s website.
A version of this article first appeared on Medscape.com.
An otherwise healthy 1-month-old female presents with lesions on the face, scalp, and chest
A potassium hydroxide preparation (KOH) from skin scrapings from the scalp lesions demonstrated no fungal elements. Further laboratory work up revealed a normal blood cell count, normal liver enzymes, an antinuclear antibody (ANA) titer of less than 1:80, a positive anti–Sjögren’s syndrome type B (SSB) antibody but negative anti–Sjögren’s syndrome type A (SSA) antibody and anti-U1RNP antibody. An electrocardiogram revealed no abnormalities. Liver function tests were normal. The complete blood count showed mild thrombocytopenia. Given the typical skin lesions and the positive SSB test and associated thrombocytopenia, the baby was diagnosed with neonatal lupus erythematosus.
Because of the diagnosis of neonatal lupus the mother was also tested and was found to have an elevated ANA of 1:640, positive SSB and antiphospholipid antibodies. The mother was healthy and her review of systems was negative for any collagen vascular disease–related symptoms.
Discussion
Neonatal lupus erythematosus (NLE) is a rare form of systemic lupus erythematosus (SLE) believed to be caused by transplacental transfer of anti-Ro (Sjögren’s syndrome antigen A, SSA), or, less commonly, anti-La (Sjögren’s syndrome antigen B, SSB) from mothers who are positive for these antibodies. Approximately 95% of NLE is associated with maternal anti-SSA; of these cases, 40% are also associated with maternal anti-SSB.1 Only about 2% of children of mothers who have anti-SSA or anti-SSB develop NLE, a finding that has led some researchers to postulate that maternal factors, fetal genetic factors, and environmental factors determine which children of anti-SSA or SSB positive mothers develop NLE.
A recent review found no association between the development of NLE and fetal birth weight, prematurity, or age.3 Over half of mothers of children who develop NLE are asymptomatic at the time of diagnosis of the neonate,3 though many become symptomatic in following years. Of mothers who are symptomatic, SLE and undifferentiated autoimmune syndrome are the most common diagnoses, though NLE has been rarely reported in the offspring of mothers with Sjögren’s syndrome, rheumatoid arthritis, and psoriasis.4,5
Fetal genetics are not an absolute determinant of development of NLE, as discordance in the development of NLE in twins has been reported. However, certain genetic relationships have been established. Fetal mutations in tumor necrosis factor–alpha appear to increase the likelihood of cutaneous manifestations. Mutations in transforming growth factor beta appear to increase the likelihood of cardiac manifestations, and experiments in cultured mouse cardiocytes have shown anti-SSB antibodies to impair macrophage phagocytosis of apoptotic cells in the developing fetal heart. These observations taken together suggest a fibroblast-mediated response to unphagocytosed cardiocyte debris may account for conduction abnormalities in neonates with NLE-induced heart block.6
Cutaneous disease in NLE is possible at birth, but more skin findings develop upon exposure to the sun. Nearly 80% of neonates affected by NLE develop cutaneous manifestations in the first few months of life. The head, neck, and extensor surfaces of the arms are most commonly affected, presumably because they are most likely to be exposed to the sun. Erythematous, annular, or discoid lesions are most common, and periorbital erythema with or without scale (“raccoon eyes”) should prompt consideration of NLE. However, annular, or discoid lesions are sometimes not present in NLE; telangiectasias, bullae, atrophic divots (“ice-pick scars”) or ulcerations may be seen instead. Lesions in the genital area have been described in fewer than 5% of patients with NLE.
The differential diagnosis of annular, scaly lesions in neonates includes annular erythema of infancy, tinea corporis, and seborrheic dermatitis. Annular erythema of infancy is a rare skin condition characterized by a cyclical eruption of erythematous annular lesions with minimal scaling which resolve spontaneously within a few weeks to months without leaving scaring or pigment changes. There is no treatment needed as the lesions self-resolve.7 Acute urticaria can sometimes appear similar to NLE but these are not scaly and also the lesions will disappear within 24-36 hours, compared with NLE lesions, which may take weeks to months to go away. Seborrheic dermatitis is a common skin condition seen in newborns with in the first few weeks of life and can present as scaly annular erythematous plaques on the face, scalp, torso, and the diaper area. Seborrheic dermatitis usually responds well to a combination of an antiyeast cream and a low-potency topical corticosteroid medication.
When NLE is suspected, diagnostic testing for lupus antibodies (anti-SSA, anti-SSB, and anti-U1RNP) in both maternal and neonatal serum should be undertaken. The presence of a characteristic rash plus maternal or neonatal antibodies is sufficient to make the diagnosis. If the rash is less characteristic, a biopsy showing an interface dermatitis can help solidify the diagnosis. Neonates with cutaneous manifestations of lupus may also have systemic disease. The most common and serious complication is heart block, whose pathophysiology is described above. Neonates with evidence of first-, second-, or third-degree heart block should be referred to a pediatric cardiologist for careful monitoring and management. Hepatic involvement has been reported, but is usually mild. Hematologic abnormalities have also been described that include anemia, neutropenia, and thrombocytopenia, which resolve by 9 months of age. Central nervous system involvement may rarely occur. The mainstay of treatment for the rash in NLE is diligent sun avoidance and sun protection. Topical corticosteroids may be used, but are not needed as the rash typically resolves by 9 months to 1 year without treatment. Mothers who have one child with NLE should be advised that they are more likely to have another with NLE – the risk is as high as 30%-40% in the second child. Hydroxychloroquine taken during subsequent pregnancies can reduce the incidence of cardiac complications,8 as can the so-called “triple therapy” of plasmapheresis, steroids, and IVIg.9
The cutaneous manifestations of NLE are usually self-limiting. However, they can serve as important clues that can prompt diagnosis of SLE in the mother, investigation of cardiac complications in the infant, and appropriate preventative care in future pregnancies.
Dr. Matiz is with the department of dermatology, Southern California Permanente Medical Group, San Diego. Mr. Kusari is with the department of dermatology, University of California, San Francisco.
References
1. Moretti D et al. Int J Dermatol. 2014;53(12):1508-12.
2. Buyon JP et al. Nature Clin Prac Rheum. 2009;5(3):139-48.
3. Li Y-Q et al. Int J Rheum Dis. 2015;18(7):761-7.
4. Rivera TL et al. Annals Rheum Dis. 2009;68(6):828-35.
5. Li L et al. Zhonghua er ke za zhi 2011;49(2):146-50.
6. Izmirly PM et al. Clin Rheumatol. 2011;30(12):1641-5.
7. Toledo-Alberola F and Betlloch-Mas I. Actas Dermosifiliogr. 2010 Jul;101(6):473-84.
8. Izmirly PM et al. Circulation. 2012;126(1):76-82.
9. Martinez-Sanchez N et al. Autoimmun Rev. 2015;14(5):423-8.
A potassium hydroxide preparation (KOH) from skin scrapings from the scalp lesions demonstrated no fungal elements. Further laboratory work up revealed a normal blood cell count, normal liver enzymes, an antinuclear antibody (ANA) titer of less than 1:80, a positive anti–Sjögren’s syndrome type B (SSB) antibody but negative anti–Sjögren’s syndrome type A (SSA) antibody and anti-U1RNP antibody. An electrocardiogram revealed no abnormalities. Liver function tests were normal. The complete blood count showed mild thrombocytopenia. Given the typical skin lesions and the positive SSB test and associated thrombocytopenia, the baby was diagnosed with neonatal lupus erythematosus.
Because of the diagnosis of neonatal lupus the mother was also tested and was found to have an elevated ANA of 1:640, positive SSB and antiphospholipid antibodies. The mother was healthy and her review of systems was negative for any collagen vascular disease–related symptoms.
Discussion
Neonatal lupus erythematosus (NLE) is a rare form of systemic lupus erythematosus (SLE) believed to be caused by transplacental transfer of anti-Ro (Sjögren’s syndrome antigen A, SSA), or, less commonly, anti-La (Sjögren’s syndrome antigen B, SSB) from mothers who are positive for these antibodies. Approximately 95% of NLE is associated with maternal anti-SSA; of these cases, 40% are also associated with maternal anti-SSB.1 Only about 2% of children of mothers who have anti-SSA or anti-SSB develop NLE, a finding that has led some researchers to postulate that maternal factors, fetal genetic factors, and environmental factors determine which children of anti-SSA or SSB positive mothers develop NLE.
A recent review found no association between the development of NLE and fetal birth weight, prematurity, or age.3 Over half of mothers of children who develop NLE are asymptomatic at the time of diagnosis of the neonate,3 though many become symptomatic in following years. Of mothers who are symptomatic, SLE and undifferentiated autoimmune syndrome are the most common diagnoses, though NLE has been rarely reported in the offspring of mothers with Sjögren’s syndrome, rheumatoid arthritis, and psoriasis.4,5
Fetal genetics are not an absolute determinant of development of NLE, as discordance in the development of NLE in twins has been reported. However, certain genetic relationships have been established. Fetal mutations in tumor necrosis factor–alpha appear to increase the likelihood of cutaneous manifestations. Mutations in transforming growth factor beta appear to increase the likelihood of cardiac manifestations, and experiments in cultured mouse cardiocytes have shown anti-SSB antibodies to impair macrophage phagocytosis of apoptotic cells in the developing fetal heart. These observations taken together suggest a fibroblast-mediated response to unphagocytosed cardiocyte debris may account for conduction abnormalities in neonates with NLE-induced heart block.6
Cutaneous disease in NLE is possible at birth, but more skin findings develop upon exposure to the sun. Nearly 80% of neonates affected by NLE develop cutaneous manifestations in the first few months of life. The head, neck, and extensor surfaces of the arms are most commonly affected, presumably because they are most likely to be exposed to the sun. Erythematous, annular, or discoid lesions are most common, and periorbital erythema with or without scale (“raccoon eyes”) should prompt consideration of NLE. However, annular, or discoid lesions are sometimes not present in NLE; telangiectasias, bullae, atrophic divots (“ice-pick scars”) or ulcerations may be seen instead. Lesions in the genital area have been described in fewer than 5% of patients with NLE.
The differential diagnosis of annular, scaly lesions in neonates includes annular erythema of infancy, tinea corporis, and seborrheic dermatitis. Annular erythema of infancy is a rare skin condition characterized by a cyclical eruption of erythematous annular lesions with minimal scaling which resolve spontaneously within a few weeks to months without leaving scaring or pigment changes. There is no treatment needed as the lesions self-resolve.7 Acute urticaria can sometimes appear similar to NLE but these are not scaly and also the lesions will disappear within 24-36 hours, compared with NLE lesions, which may take weeks to months to go away. Seborrheic dermatitis is a common skin condition seen in newborns with in the first few weeks of life and can present as scaly annular erythematous plaques on the face, scalp, torso, and the diaper area. Seborrheic dermatitis usually responds well to a combination of an antiyeast cream and a low-potency topical corticosteroid medication.
When NLE is suspected, diagnostic testing for lupus antibodies (anti-SSA, anti-SSB, and anti-U1RNP) in both maternal and neonatal serum should be undertaken. The presence of a characteristic rash plus maternal or neonatal antibodies is sufficient to make the diagnosis. If the rash is less characteristic, a biopsy showing an interface dermatitis can help solidify the diagnosis. Neonates with cutaneous manifestations of lupus may also have systemic disease. The most common and serious complication is heart block, whose pathophysiology is described above. Neonates with evidence of first-, second-, or third-degree heart block should be referred to a pediatric cardiologist for careful monitoring and management. Hepatic involvement has been reported, but is usually mild. Hematologic abnormalities have also been described that include anemia, neutropenia, and thrombocytopenia, which resolve by 9 months of age. Central nervous system involvement may rarely occur. The mainstay of treatment for the rash in NLE is diligent sun avoidance and sun protection. Topical corticosteroids may be used, but are not needed as the rash typically resolves by 9 months to 1 year without treatment. Mothers who have one child with NLE should be advised that they are more likely to have another with NLE – the risk is as high as 30%-40% in the second child. Hydroxychloroquine taken during subsequent pregnancies can reduce the incidence of cardiac complications,8 as can the so-called “triple therapy” of plasmapheresis, steroids, and IVIg.9
The cutaneous manifestations of NLE are usually self-limiting. However, they can serve as important clues that can prompt diagnosis of SLE in the mother, investigation of cardiac complications in the infant, and appropriate preventative care in future pregnancies.
Dr. Matiz is with the department of dermatology, Southern California Permanente Medical Group, San Diego. Mr. Kusari is with the department of dermatology, University of California, San Francisco.
References
1. Moretti D et al. Int J Dermatol. 2014;53(12):1508-12.
2. Buyon JP et al. Nature Clin Prac Rheum. 2009;5(3):139-48.
3. Li Y-Q et al. Int J Rheum Dis. 2015;18(7):761-7.
4. Rivera TL et al. Annals Rheum Dis. 2009;68(6):828-35.
5. Li L et al. Zhonghua er ke za zhi 2011;49(2):146-50.
6. Izmirly PM et al. Clin Rheumatol. 2011;30(12):1641-5.
7. Toledo-Alberola F and Betlloch-Mas I. Actas Dermosifiliogr. 2010 Jul;101(6):473-84.
8. Izmirly PM et al. Circulation. 2012;126(1):76-82.
9. Martinez-Sanchez N et al. Autoimmun Rev. 2015;14(5):423-8.
A potassium hydroxide preparation (KOH) from skin scrapings from the scalp lesions demonstrated no fungal elements. Further laboratory work up revealed a normal blood cell count, normal liver enzymes, an antinuclear antibody (ANA) titer of less than 1:80, a positive anti–Sjögren’s syndrome type B (SSB) antibody but negative anti–Sjögren’s syndrome type A (SSA) antibody and anti-U1RNP antibody. An electrocardiogram revealed no abnormalities. Liver function tests were normal. The complete blood count showed mild thrombocytopenia. Given the typical skin lesions and the positive SSB test and associated thrombocytopenia, the baby was diagnosed with neonatal lupus erythematosus.
Because of the diagnosis of neonatal lupus the mother was also tested and was found to have an elevated ANA of 1:640, positive SSB and antiphospholipid antibodies. The mother was healthy and her review of systems was negative for any collagen vascular disease–related symptoms.
Discussion
Neonatal lupus erythematosus (NLE) is a rare form of systemic lupus erythematosus (SLE) believed to be caused by transplacental transfer of anti-Ro (Sjögren’s syndrome antigen A, SSA), or, less commonly, anti-La (Sjögren’s syndrome antigen B, SSB) from mothers who are positive for these antibodies. Approximately 95% of NLE is associated with maternal anti-SSA; of these cases, 40% are also associated with maternal anti-SSB.1 Only about 2% of children of mothers who have anti-SSA or anti-SSB develop NLE, a finding that has led some researchers to postulate that maternal factors, fetal genetic factors, and environmental factors determine which children of anti-SSA or SSB positive mothers develop NLE.
A recent review found no association between the development of NLE and fetal birth weight, prematurity, or age.3 Over half of mothers of children who develop NLE are asymptomatic at the time of diagnosis of the neonate,3 though many become symptomatic in following years. Of mothers who are symptomatic, SLE and undifferentiated autoimmune syndrome are the most common diagnoses, though NLE has been rarely reported in the offspring of mothers with Sjögren’s syndrome, rheumatoid arthritis, and psoriasis.4,5
Fetal genetics are not an absolute determinant of development of NLE, as discordance in the development of NLE in twins has been reported. However, certain genetic relationships have been established. Fetal mutations in tumor necrosis factor–alpha appear to increase the likelihood of cutaneous manifestations. Mutations in transforming growth factor beta appear to increase the likelihood of cardiac manifestations, and experiments in cultured mouse cardiocytes have shown anti-SSB antibodies to impair macrophage phagocytosis of apoptotic cells in the developing fetal heart. These observations taken together suggest a fibroblast-mediated response to unphagocytosed cardiocyte debris may account for conduction abnormalities in neonates with NLE-induced heart block.6
Cutaneous disease in NLE is possible at birth, but more skin findings develop upon exposure to the sun. Nearly 80% of neonates affected by NLE develop cutaneous manifestations in the first few months of life. The head, neck, and extensor surfaces of the arms are most commonly affected, presumably because they are most likely to be exposed to the sun. Erythematous, annular, or discoid lesions are most common, and periorbital erythema with or without scale (“raccoon eyes”) should prompt consideration of NLE. However, annular, or discoid lesions are sometimes not present in NLE; telangiectasias, bullae, atrophic divots (“ice-pick scars”) or ulcerations may be seen instead. Lesions in the genital area have been described in fewer than 5% of patients with NLE.
The differential diagnosis of annular, scaly lesions in neonates includes annular erythema of infancy, tinea corporis, and seborrheic dermatitis. Annular erythema of infancy is a rare skin condition characterized by a cyclical eruption of erythematous annular lesions with minimal scaling which resolve spontaneously within a few weeks to months without leaving scaring or pigment changes. There is no treatment needed as the lesions self-resolve.7 Acute urticaria can sometimes appear similar to NLE but these are not scaly and also the lesions will disappear within 24-36 hours, compared with NLE lesions, which may take weeks to months to go away. Seborrheic dermatitis is a common skin condition seen in newborns with in the first few weeks of life and can present as scaly annular erythematous plaques on the face, scalp, torso, and the diaper area. Seborrheic dermatitis usually responds well to a combination of an antiyeast cream and a low-potency topical corticosteroid medication.
When NLE is suspected, diagnostic testing for lupus antibodies (anti-SSA, anti-SSB, and anti-U1RNP) in both maternal and neonatal serum should be undertaken. The presence of a characteristic rash plus maternal or neonatal antibodies is sufficient to make the diagnosis. If the rash is less characteristic, a biopsy showing an interface dermatitis can help solidify the diagnosis. Neonates with cutaneous manifestations of lupus may also have systemic disease. The most common and serious complication is heart block, whose pathophysiology is described above. Neonates with evidence of first-, second-, or third-degree heart block should be referred to a pediatric cardiologist for careful monitoring and management. Hepatic involvement has been reported, but is usually mild. Hematologic abnormalities have also been described that include anemia, neutropenia, and thrombocytopenia, which resolve by 9 months of age. Central nervous system involvement may rarely occur. The mainstay of treatment for the rash in NLE is diligent sun avoidance and sun protection. Topical corticosteroids may be used, but are not needed as the rash typically resolves by 9 months to 1 year without treatment. Mothers who have one child with NLE should be advised that they are more likely to have another with NLE – the risk is as high as 30%-40% in the second child. Hydroxychloroquine taken during subsequent pregnancies can reduce the incidence of cardiac complications,8 as can the so-called “triple therapy” of plasmapheresis, steroids, and IVIg.9
The cutaneous manifestations of NLE are usually self-limiting. However, they can serve as important clues that can prompt diagnosis of SLE in the mother, investigation of cardiac complications in the infant, and appropriate preventative care in future pregnancies.
Dr. Matiz is with the department of dermatology, Southern California Permanente Medical Group, San Diego. Mr. Kusari is with the department of dermatology, University of California, San Francisco.
References
1. Moretti D et al. Int J Dermatol. 2014;53(12):1508-12.
2. Buyon JP et al. Nature Clin Prac Rheum. 2009;5(3):139-48.
3. Li Y-Q et al. Int J Rheum Dis. 2015;18(7):761-7.
4. Rivera TL et al. Annals Rheum Dis. 2009;68(6):828-35.
5. Li L et al. Zhonghua er ke za zhi 2011;49(2):146-50.
6. Izmirly PM et al. Clin Rheumatol. 2011;30(12):1641-5.
7. Toledo-Alberola F and Betlloch-Mas I. Actas Dermosifiliogr. 2010 Jul;101(6):473-84.
8. Izmirly PM et al. Circulation. 2012;126(1):76-82.
9. Martinez-Sanchez N et al. Autoimmun Rev. 2015;14(5):423-8.
A 1-month-old, full-term female, born via normal vaginal delivery, presented to the dermatology clinic with a 3-week history of recurrent skin lesions on the scalp, face, and chest. The mother has been treating the lesions with breast milk and most recently with clotrimazole cream without resolution.
The mother of the baby is a healthy 32-year-old female with no past medical history. She had adequate prenatal care, and all the prenatal infectious and genetic tests were normal. The baby has been healthy and growing well. There is no history of associated fevers, chills, or any other symptoms. The family took no recent trips, and the parents are not affected. There are no other children at home and they have a cat and a dog. The family history is noncontributory.
On physical examination the baby was not in acute distress and her vital signs were normal. On skin examination she had several erythematous annular plaques and patches on the face, scalp, and upper chest (Fig. 1). There was no liver or spleen enlargement and no lymphadenopathy was palpated on exam.
FDA: More metformin extended-release tablets recalled
Two lots of metformin HCl extended-release tablets have been recalled by Viona Pharmaceuticals because unacceptable levels of nitrosodimethylamine (NDMA), a likely carcinogen, were found in the 750-mg tablets.
According to a June 11 alert from the Food and Drug Administration, the affected lot numbers are M915601 and M915602.
This generic product was made by Cadila Healthcare, Ahmedabad, India, in November 2019 with an expiration date of October 2021, and distributed throughout the United States. The pill is white to off-white, capsule-shaped, uncoated tablets, debossed with “Z”, “C” on one side and “20” on the other side.
No adverse events related to the lots involved in the recall have been reported, the FDA said. It also recommends that clinicians continue to prescribe metformin when clinically appropriate.
In late 2019, the FDA announced it had become aware of NDMA in some metformin products in other countries. The agency immediately began testing to determine whether the metformin in the U.S. supply was at risk, as part of the ongoing investigation into nitrosamine impurities across medication types, which included recalls of hypertension and heartburn medications within the past 3 years.
In February 2020, the FDA reported that they hadn’t found NDMA levels that exceeded the acceptable daily intake. But starting in May 2020, voluntary recalls by, numerous manufacturers have been announced as levels of the compound exceeded that cutoff.
Two lots of metformin HCl extended-release tablets have been recalled by Viona Pharmaceuticals because unacceptable levels of nitrosodimethylamine (NDMA), a likely carcinogen, were found in the 750-mg tablets.
According to a June 11 alert from the Food and Drug Administration, the affected lot numbers are M915601 and M915602.
This generic product was made by Cadila Healthcare, Ahmedabad, India, in November 2019 with an expiration date of October 2021, and distributed throughout the United States. The pill is white to off-white, capsule-shaped, uncoated tablets, debossed with “Z”, “C” on one side and “20” on the other side.
No adverse events related to the lots involved in the recall have been reported, the FDA said. It also recommends that clinicians continue to prescribe metformin when clinically appropriate.
In late 2019, the FDA announced it had become aware of NDMA in some metformin products in other countries. The agency immediately began testing to determine whether the metformin in the U.S. supply was at risk, as part of the ongoing investigation into nitrosamine impurities across medication types, which included recalls of hypertension and heartburn medications within the past 3 years.
In February 2020, the FDA reported that they hadn’t found NDMA levels that exceeded the acceptable daily intake. But starting in May 2020, voluntary recalls by, numerous manufacturers have been announced as levels of the compound exceeded that cutoff.
Two lots of metformin HCl extended-release tablets have been recalled by Viona Pharmaceuticals because unacceptable levels of nitrosodimethylamine (NDMA), a likely carcinogen, were found in the 750-mg tablets.
According to a June 11 alert from the Food and Drug Administration, the affected lot numbers are M915601 and M915602.
This generic product was made by Cadila Healthcare, Ahmedabad, India, in November 2019 with an expiration date of October 2021, and distributed throughout the United States. The pill is white to off-white, capsule-shaped, uncoated tablets, debossed with “Z”, “C” on one side and “20” on the other side.
No adverse events related to the lots involved in the recall have been reported, the FDA said. It also recommends that clinicians continue to prescribe metformin when clinically appropriate.
In late 2019, the FDA announced it had become aware of NDMA in some metformin products in other countries. The agency immediately began testing to determine whether the metformin in the U.S. supply was at risk, as part of the ongoing investigation into nitrosamine impurities across medication types, which included recalls of hypertension and heartburn medications within the past 3 years.
In February 2020, the FDA reported that they hadn’t found NDMA levels that exceeded the acceptable daily intake. But starting in May 2020, voluntary recalls by, numerous manufacturers have been announced as levels of the compound exceeded that cutoff.
FROM THE FOOD AND DRUG ADMINISTRATION
Screaming for screens: Digital well-being in the 2020s
Charlie is a 15-year-old male whose medical history includes overweight and autism spectrum disorder. While his autism symptoms are stable and he is doing fairly well in school, your sense is that he is underperforming and unhappy. His screening for anxiety and depression is not outstanding and you wonder whether to leave well enough alone.
Historically, pediatrician queries about media use happen in a minority of visits,1 overcrowded by the multitude of screening and acute care needs, let alone the pressures of electronic health record prompts, billing, and documentation. Yet the COVID-19 pandemic has emphasized what was already getting louder: screen life is becoming a ubiquitous, increasing, and normative function of child development. Digital well-being exhibits bidirectional interactions with most of the core indicators of child health: sleep, nutrition, safety, mood, relationships, and many other aspects of physical and mental health.1
The pandemic unveiled the blessings and curses of digital life by shifting many into remote work and school situations where screen time became both necessary and uncontrollable. Reeling with changes in employment, health, finances, and more, families struggled to forge a new screen-life balance that could bridge academic, professional, and recreational use.
Research has wavered in producing a verdict on the effects of screen time, in part because of limitations in methodology and follow-up time,2 and exacerbated by the quickly changing nature of screen use. Screen time may put youth at risk for obesity and behavior problems,3 but the latter may be mediated in part by loss of sleep because of late-night digital activity.4 While survey data at the population level show little link between screen time and well-being impairments,5 zooming in on individuals may tell a different story. Twenge and Campbell show light use of digital media (compared with nonuse) is associated with greater well-being while heavy use is associated with lower well-being and a higher risk for depression and suicidal behavior – especially in girls.6,7 Largely cross-sectional data show a small detriment to psychological well-being associated with digital technology, though this may be bidirectional and does not clearly differentiate types of technology.2
Recent neuroscience suggests that, compared with active play, sedentary screen time after school reduced impulse control and increased brain activity in regions associated with craving.8 This may explain some of the link between screen time and obesity. Brain imaging of preschoolers showed that greater screen time correlated with lower reading readiness as well as less integrity of white-matter tracts involved in language and executive function,9 whereas nurturing home reading practices were protective for language development and white matter integrity.10
Returning to the care of Charlie, providers may benefit from taking time to reflect on their own digital environment. What does the patient-side view of your office look like? Many offices use telephone reminders and patient portals, fill prescriptions electronically, and have waiting rooms with WiFi or devices for children’s use. Office visits share space with providers’ desktops, laptops, and smartphones, with EMRs guiding the visit. EMRs may come home for evening documentation. How does this affect provider digital well-being? How do you start the conversation with families about digital well-being?
The American Academy of Pediatrics recommends media screening be incorporated into routine pediatric care, with several tools available to support this. Adapting the HEADSSS model for psychosocial check-ins, Clark and colleagues propose an additional “S” to capture screen time.11 Their model queries which apps and social media are used, quantity of use, effects on self-confidence, and whether cyberbullying or sexting are occurring. Smartphones themselves provide an eye-opening and accessible dataset, with built-in features (for example, Screen Time for iOS) tracking not just daily duration of use, but also how frequently the phone is picked up and which apps get more use. Screening may be followed by motivational coaching, emphasizing nonjudgment, curiosity, empathy, and flexibility — for patient and provider.12
In Charlie’s case, screening reveals heavy use of social Internet games that connect him with like-minded peers. While he describes an inclusiveness and level of socialization that he has not found outside the home, the quantity of use is interfering with sleep, schoolwork, and physical activity.
Significant problematic Internet use may lead to intervention or referral – addictive behaviors and mental health symptoms may warrant connection with mental health providers. Cyberbullying or unsafe behaviors may additionally benefit from parental and school-based support. There is early and limited evidence that psychological and educational interventions may be of benefit for problematic Internet use.13
When digital life is not so dramatically affecting well-being, providers may begin by working with families on a media use plan. The AAP offers its own website to support this. Other well-researched and well-designed sites include Digital Wellness Lab For Parents, with developmentally staged information and plentiful research, and Common Sense Media, which reviews apps, movies, and more; plus they have a knowledge/advice section under “Parents Need to Know.” Keep in mind that digital media can also support youth in managing psychiatric problems, e.g., a digital intervention promoting positive psychology practices looked very helpful for young people with psychosis.14
For Charlie, a health coaching approach is adopted. Using Gabrielli’s TECH parenting rubric,15 Charlie’s parents are coached to make space to talk about and coview media and apps, as well as creating a Family Media Use Plan for everyone – parents included. Alongside setting limits on screen time; health promotion activities like exercise, reading, and schoolwork are also rewarded with extra screen time. When Charlie returns 3 months later, the family reports that, in recognition of their collective digital overload, they preserved dinnertime and after 10 p.m. as screen-free downtime. While they still have concerns about Charlie’s gaming and social life, his sleep is somewhat improved and family tension is lower.
Attention to digital well-being stands to benefit provider and patient alike, and over time may gain from the scaffolding of handouts, standardized assessments, and health coaching providers that may be in place to support other important domains like sleep hygiene, food security, and parenting.
Dr. Rosenfeld is assistant professor, University of Vermont, Vermont Center for Children, Youth, and Families, Burlington. He has no relevant disclosures.
References
1. Chassiakos YR et al. Pediatrics. 2016;138(5)e20162593.
2. Orben A. Soc Psychiatry Psych Epi. 2020;55(4):407.
3. Fang K et al. Child Care Health Dev. 2019;45(5):744-53.
4. Janssen X et al. Sleep Med Rev. 2020;49:101226.
5. George MJ et al. J Ped. 2020;219:180.
6. Twenge JM and Campbell WK. Psychiatry Q. 2019;90(2):311-31.
7. Twenge JM and Martin GN. J Adolesc. 2020;79:91.
8. Efraim M et al. Brain Imaging Behav. 2021;15(1):177-89.
9. Hutton JS et al. JAMA Pediatr. 2020;174(1):e193869.
10. Hutton JS et al. Acta Paediatr. 2020;109(7):1376-86.
11. Clark DL et al. Pediatrics. 2018;141(6).
12. Jericho M and Elliot A. Clin Child Psychol Psychiatry. 2020;25(3):662.
13. Malinauskas R and Malinauskine V. J Behav Addict. 2019;8(4):613.
14. Lim MH et al. Soc Psychiatry Psychiatr Epi. 2020;55(7):877-89.
15. Gabrielli J et al. Pediatrics. 2018;142(1)e20173718.
Charlie is a 15-year-old male whose medical history includes overweight and autism spectrum disorder. While his autism symptoms are stable and he is doing fairly well in school, your sense is that he is underperforming and unhappy. His screening for anxiety and depression is not outstanding and you wonder whether to leave well enough alone.
Historically, pediatrician queries about media use happen in a minority of visits,1 overcrowded by the multitude of screening and acute care needs, let alone the pressures of electronic health record prompts, billing, and documentation. Yet the COVID-19 pandemic has emphasized what was already getting louder: screen life is becoming a ubiquitous, increasing, and normative function of child development. Digital well-being exhibits bidirectional interactions with most of the core indicators of child health: sleep, nutrition, safety, mood, relationships, and many other aspects of physical and mental health.1
The pandemic unveiled the blessings and curses of digital life by shifting many into remote work and school situations where screen time became both necessary and uncontrollable. Reeling with changes in employment, health, finances, and more, families struggled to forge a new screen-life balance that could bridge academic, professional, and recreational use.
Research has wavered in producing a verdict on the effects of screen time, in part because of limitations in methodology and follow-up time,2 and exacerbated by the quickly changing nature of screen use. Screen time may put youth at risk for obesity and behavior problems,3 but the latter may be mediated in part by loss of sleep because of late-night digital activity.4 While survey data at the population level show little link between screen time and well-being impairments,5 zooming in on individuals may tell a different story. Twenge and Campbell show light use of digital media (compared with nonuse) is associated with greater well-being while heavy use is associated with lower well-being and a higher risk for depression and suicidal behavior – especially in girls.6,7 Largely cross-sectional data show a small detriment to psychological well-being associated with digital technology, though this may be bidirectional and does not clearly differentiate types of technology.2
Recent neuroscience suggests that, compared with active play, sedentary screen time after school reduced impulse control and increased brain activity in regions associated with craving.8 This may explain some of the link between screen time and obesity. Brain imaging of preschoolers showed that greater screen time correlated with lower reading readiness as well as less integrity of white-matter tracts involved in language and executive function,9 whereas nurturing home reading practices were protective for language development and white matter integrity.10
Returning to the care of Charlie, providers may benefit from taking time to reflect on their own digital environment. What does the patient-side view of your office look like? Many offices use telephone reminders and patient portals, fill prescriptions electronically, and have waiting rooms with WiFi or devices for children’s use. Office visits share space with providers’ desktops, laptops, and smartphones, with EMRs guiding the visit. EMRs may come home for evening documentation. How does this affect provider digital well-being? How do you start the conversation with families about digital well-being?
The American Academy of Pediatrics recommends media screening be incorporated into routine pediatric care, with several tools available to support this. Adapting the HEADSSS model for psychosocial check-ins, Clark and colleagues propose an additional “S” to capture screen time.11 Their model queries which apps and social media are used, quantity of use, effects on self-confidence, and whether cyberbullying or sexting are occurring. Smartphones themselves provide an eye-opening and accessible dataset, with built-in features (for example, Screen Time for iOS) tracking not just daily duration of use, but also how frequently the phone is picked up and which apps get more use. Screening may be followed by motivational coaching, emphasizing nonjudgment, curiosity, empathy, and flexibility — for patient and provider.12
In Charlie’s case, screening reveals heavy use of social Internet games that connect him with like-minded peers. While he describes an inclusiveness and level of socialization that he has not found outside the home, the quantity of use is interfering with sleep, schoolwork, and physical activity.
Significant problematic Internet use may lead to intervention or referral – addictive behaviors and mental health symptoms may warrant connection with mental health providers. Cyberbullying or unsafe behaviors may additionally benefit from parental and school-based support. There is early and limited evidence that psychological and educational interventions may be of benefit for problematic Internet use.13
When digital life is not so dramatically affecting well-being, providers may begin by working with families on a media use plan. The AAP offers its own website to support this. Other well-researched and well-designed sites include Digital Wellness Lab For Parents, with developmentally staged information and plentiful research, and Common Sense Media, which reviews apps, movies, and more; plus they have a knowledge/advice section under “Parents Need to Know.” Keep in mind that digital media can also support youth in managing psychiatric problems, e.g., a digital intervention promoting positive psychology practices looked very helpful for young people with psychosis.14
For Charlie, a health coaching approach is adopted. Using Gabrielli’s TECH parenting rubric,15 Charlie’s parents are coached to make space to talk about and coview media and apps, as well as creating a Family Media Use Plan for everyone – parents included. Alongside setting limits on screen time; health promotion activities like exercise, reading, and schoolwork are also rewarded with extra screen time. When Charlie returns 3 months later, the family reports that, in recognition of their collective digital overload, they preserved dinnertime and after 10 p.m. as screen-free downtime. While they still have concerns about Charlie’s gaming and social life, his sleep is somewhat improved and family tension is lower.
Attention to digital well-being stands to benefit provider and patient alike, and over time may gain from the scaffolding of handouts, standardized assessments, and health coaching providers that may be in place to support other important domains like sleep hygiene, food security, and parenting.
Dr. Rosenfeld is assistant professor, University of Vermont, Vermont Center for Children, Youth, and Families, Burlington. He has no relevant disclosures.
References
1. Chassiakos YR et al. Pediatrics. 2016;138(5)e20162593.
2. Orben A. Soc Psychiatry Psych Epi. 2020;55(4):407.
3. Fang K et al. Child Care Health Dev. 2019;45(5):744-53.
4. Janssen X et al. Sleep Med Rev. 2020;49:101226.
5. George MJ et al. J Ped. 2020;219:180.
6. Twenge JM and Campbell WK. Psychiatry Q. 2019;90(2):311-31.
7. Twenge JM and Martin GN. J Adolesc. 2020;79:91.
8. Efraim M et al. Brain Imaging Behav. 2021;15(1):177-89.
9. Hutton JS et al. JAMA Pediatr. 2020;174(1):e193869.
10. Hutton JS et al. Acta Paediatr. 2020;109(7):1376-86.
11. Clark DL et al. Pediatrics. 2018;141(6).
12. Jericho M and Elliot A. Clin Child Psychol Psychiatry. 2020;25(3):662.
13. Malinauskas R and Malinauskine V. J Behav Addict. 2019;8(4):613.
14. Lim MH et al. Soc Psychiatry Psychiatr Epi. 2020;55(7):877-89.
15. Gabrielli J et al. Pediatrics. 2018;142(1)e20173718.
Charlie is a 15-year-old male whose medical history includes overweight and autism spectrum disorder. While his autism symptoms are stable and he is doing fairly well in school, your sense is that he is underperforming and unhappy. His screening for anxiety and depression is not outstanding and you wonder whether to leave well enough alone.
Historically, pediatrician queries about media use happen in a minority of visits,1 overcrowded by the multitude of screening and acute care needs, let alone the pressures of electronic health record prompts, billing, and documentation. Yet the COVID-19 pandemic has emphasized what was already getting louder: screen life is becoming a ubiquitous, increasing, and normative function of child development. Digital well-being exhibits bidirectional interactions with most of the core indicators of child health: sleep, nutrition, safety, mood, relationships, and many other aspects of physical and mental health.1
The pandemic unveiled the blessings and curses of digital life by shifting many into remote work and school situations where screen time became both necessary and uncontrollable. Reeling with changes in employment, health, finances, and more, families struggled to forge a new screen-life balance that could bridge academic, professional, and recreational use.
Research has wavered in producing a verdict on the effects of screen time, in part because of limitations in methodology and follow-up time,2 and exacerbated by the quickly changing nature of screen use. Screen time may put youth at risk for obesity and behavior problems,3 but the latter may be mediated in part by loss of sleep because of late-night digital activity.4 While survey data at the population level show little link between screen time and well-being impairments,5 zooming in on individuals may tell a different story. Twenge and Campbell show light use of digital media (compared with nonuse) is associated with greater well-being while heavy use is associated with lower well-being and a higher risk for depression and suicidal behavior – especially in girls.6,7 Largely cross-sectional data show a small detriment to psychological well-being associated with digital technology, though this may be bidirectional and does not clearly differentiate types of technology.2
Recent neuroscience suggests that, compared with active play, sedentary screen time after school reduced impulse control and increased brain activity in regions associated with craving.8 This may explain some of the link between screen time and obesity. Brain imaging of preschoolers showed that greater screen time correlated with lower reading readiness as well as less integrity of white-matter tracts involved in language and executive function,9 whereas nurturing home reading practices were protective for language development and white matter integrity.10
Returning to the care of Charlie, providers may benefit from taking time to reflect on their own digital environment. What does the patient-side view of your office look like? Many offices use telephone reminders and patient portals, fill prescriptions electronically, and have waiting rooms with WiFi or devices for children’s use. Office visits share space with providers’ desktops, laptops, and smartphones, with EMRs guiding the visit. EMRs may come home for evening documentation. How does this affect provider digital well-being? How do you start the conversation with families about digital well-being?
The American Academy of Pediatrics recommends media screening be incorporated into routine pediatric care, with several tools available to support this. Adapting the HEADSSS model for psychosocial check-ins, Clark and colleagues propose an additional “S” to capture screen time.11 Their model queries which apps and social media are used, quantity of use, effects on self-confidence, and whether cyberbullying or sexting are occurring. Smartphones themselves provide an eye-opening and accessible dataset, with built-in features (for example, Screen Time for iOS) tracking not just daily duration of use, but also how frequently the phone is picked up and which apps get more use. Screening may be followed by motivational coaching, emphasizing nonjudgment, curiosity, empathy, and flexibility — for patient and provider.12
In Charlie’s case, screening reveals heavy use of social Internet games that connect him with like-minded peers. While he describes an inclusiveness and level of socialization that he has not found outside the home, the quantity of use is interfering with sleep, schoolwork, and physical activity.
Significant problematic Internet use may lead to intervention or referral – addictive behaviors and mental health symptoms may warrant connection with mental health providers. Cyberbullying or unsafe behaviors may additionally benefit from parental and school-based support. There is early and limited evidence that psychological and educational interventions may be of benefit for problematic Internet use.13
When digital life is not so dramatically affecting well-being, providers may begin by working with families on a media use plan. The AAP offers its own website to support this. Other well-researched and well-designed sites include Digital Wellness Lab For Parents, with developmentally staged information and plentiful research, and Common Sense Media, which reviews apps, movies, and more; plus they have a knowledge/advice section under “Parents Need to Know.” Keep in mind that digital media can also support youth in managing psychiatric problems, e.g., a digital intervention promoting positive psychology practices looked very helpful for young people with psychosis.14
For Charlie, a health coaching approach is adopted. Using Gabrielli’s TECH parenting rubric,15 Charlie’s parents are coached to make space to talk about and coview media and apps, as well as creating a Family Media Use Plan for everyone – parents included. Alongside setting limits on screen time; health promotion activities like exercise, reading, and schoolwork are also rewarded with extra screen time. When Charlie returns 3 months later, the family reports that, in recognition of their collective digital overload, they preserved dinnertime and after 10 p.m. as screen-free downtime. While they still have concerns about Charlie’s gaming and social life, his sleep is somewhat improved and family tension is lower.
Attention to digital well-being stands to benefit provider and patient alike, and over time may gain from the scaffolding of handouts, standardized assessments, and health coaching providers that may be in place to support other important domains like sleep hygiene, food security, and parenting.
Dr. Rosenfeld is assistant professor, University of Vermont, Vermont Center for Children, Youth, and Families, Burlington. He has no relevant disclosures.
References
1. Chassiakos YR et al. Pediatrics. 2016;138(5)e20162593.
2. Orben A. Soc Psychiatry Psych Epi. 2020;55(4):407.
3. Fang K et al. Child Care Health Dev. 2019;45(5):744-53.
4. Janssen X et al. Sleep Med Rev. 2020;49:101226.
5. George MJ et al. J Ped. 2020;219:180.
6. Twenge JM and Campbell WK. Psychiatry Q. 2019;90(2):311-31.
7. Twenge JM and Martin GN. J Adolesc. 2020;79:91.
8. Efraim M et al. Brain Imaging Behav. 2021;15(1):177-89.
9. Hutton JS et al. JAMA Pediatr. 2020;174(1):e193869.
10. Hutton JS et al. Acta Paediatr. 2020;109(7):1376-86.
11. Clark DL et al. Pediatrics. 2018;141(6).
12. Jericho M and Elliot A. Clin Child Psychol Psychiatry. 2020;25(3):662.
13. Malinauskas R and Malinauskine V. J Behav Addict. 2019;8(4):613.
14. Lim MH et al. Soc Psychiatry Psychiatr Epi. 2020;55(7):877-89.
15. Gabrielli J et al. Pediatrics. 2018;142(1)e20173718.
Is your patient having an existential crisis?
The news is portraying our modern time as an existential crisis as though our very existence is threatened. An existential crisis is a profound feeling of lack of meaning, choice, or freedom in one’s life that makes even existing seem worthless. It can emerge as early as 5 years old, especially in introspective, gifted children, when they realize that death is permanent and universal, after a real loss or a story of a loss or failure, or from a sense of guilt.
The past 18 months of COVID-19 have been a perfect storm for developing an existential crisis. One of the main sources of life meaning for children is friendships. COVID-19 has reduced or blocked access to old and new friends. Younger children, when asked what makes a friend, will say “we like to do the same things.” Virtual play dates help but don’t replace shared experiences.
School provides meaning for children not only from socializing but also from accomplishing academic tasks – fulfilling Erickson’s stages of “mastery” and “productivity.” Teachers were better able to carry out hands-on activities, group assignments, and field trips in person so that all children and learning styles were engaged and successful. Not having in-person school has also meant loss of extracurricular activities, sports, and clubs as sources of mastery.
Loss of the structure of daily life, common during COVID-19, for waking, dressing, meals, chores, homework time, bathing, or bedtime can be profoundly disorienting.
For adolescents, opportunities to contribute to society and become productive by volunteering or being employed have been stunted by quarantine and social distancing. Some teens have had to care for relatives at home so that parents can earn a living, which, while meaningful, blocks age-essential socializing.
Meaning can also be created at any age by community structures and agreed upon beliefs such as religion. While religious membership is low in the United States, members have been largely unable to attend services. Following sports teams, an alternate “religion” and source of identity, was on hold for many months.
Existential despair can also come from major life losses. COVID-19 has taken a terrible toll of lives, homes, and jobs for millions. As short-term thinkers, when children see so many of their plans and dreams for making the team, having a girlfriend, going to prom, attending summer camp, or graduating, it feels like the end of the world they had imagined. Even the most important source of meaning – connection to family – has been disrupted by lockdown, illness, or loss.
The loss of choice and freedom goes beyond being stuck indoors. Advanced classes and exams, as well as resume-building jobs or volunteering, which teens saw as essential to college, disappeared; sometimes also the money needed was exhausted by COVID-19 unemployment. Work-at-home parents supervising virtual school see their children’s malaise or panic and pressure them to work harder, which is impossible for despairing children. Observing a parent losing his or her job makes a teen’s own career aspirations uncertain. Teen depression and suicidal ideation/acts have shot up from hopelessness, with loss of meaning at the core.
A profound sense of powerlessness has taken over. COVID-19, an invisible threat, has taken down lives. Even with amazingly effective vaccines available, fear and helplessness have burned into our brains. Helplessness to stop structural racism and the arbitrary killings of our own Black citizens by police has finally registered. And climate change is now reported as an impending disaster that may not be stoppable.
So this must be the worst time in history, right? Actually, no. The past 60 years have been a period of historically remarkable stability of government, economy, and natural forces. Perhaps knowing no other world has made these problems appear unsolvable to the parents of our patients. Their own sense of meaning has been challenged in a way similar to that of their children. Perhaps from lack of privacy or peers, parents have been sharing their own sense of powerlessness with their children directly or indirectly, making it harder to reassure them.
With COVID-19 waning in the United States, many of the sources of meaning just discussed can be reinstated by way of in-person play dates, school, sports, socializing, practicing religion, volunteering, and getting jobs. Although there is “existential therapy,” what our children need most is adult leadership showing confidence in life’s meaning, even if we have to hide our own worries. Parents can point out that, even if it takes years, people have made it through difficult times in the past, and there are many positive alternatives for education and employment.
Children need to repeatedly hear about ways they are valued that are not dependent on accomplishments. Thanking them for and telling others about their effort, ideas, curiosity, integrity, love, and kindness point out meaning for their existence independent of world events. Parents need to establish routines and rules for children to demonstrate that life goes on as usual. Chores helpful to the family are a practical contribution. Family activities that are challenging and unpredictable set up for discussing, modeling, and building resilience; for example, visiting new places, camping, hiking, trying a new sport, or adopting a pet give opportunities to say: “Oh, well, we’ll find another way.”
Parents can share stories or books about people who made it through tougher times, such as Abraham Lincoln, or better, personal, or family experiences overcoming challenges. Recalling and nicknaming instances of the child’s own resilience is valuable. Books such as “The Little Engine That Could,” “Chicken Little,” and fairy tales of overcoming doubts when facing challenges can be helpful. “Stay calm and carry on,” a saying from the British when they were being bombed during World War II, has become a meme.
As clinicians we need to sort out significant complicated grief, anxiety, obsessive compulsive disorder, depression, or suicidal ideation, and provide assessment and treatment. But when children get stuck in existential futility, in addition to engaging them in meaningful activities, we can advise parents to coach them to distract themselves, “put the thoughts in a box in your head” to consider later, and/or write down or photograph things that make them grateful. Good lessons for us all to reinvent meaning in our lives.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. Email her at [email protected].
The news is portraying our modern time as an existential crisis as though our very existence is threatened. An existential crisis is a profound feeling of lack of meaning, choice, or freedom in one’s life that makes even existing seem worthless. It can emerge as early as 5 years old, especially in introspective, gifted children, when they realize that death is permanent and universal, after a real loss or a story of a loss or failure, or from a sense of guilt.
The past 18 months of COVID-19 have been a perfect storm for developing an existential crisis. One of the main sources of life meaning for children is friendships. COVID-19 has reduced or blocked access to old and new friends. Younger children, when asked what makes a friend, will say “we like to do the same things.” Virtual play dates help but don’t replace shared experiences.
School provides meaning for children not only from socializing but also from accomplishing academic tasks – fulfilling Erickson’s stages of “mastery” and “productivity.” Teachers were better able to carry out hands-on activities, group assignments, and field trips in person so that all children and learning styles were engaged and successful. Not having in-person school has also meant loss of extracurricular activities, sports, and clubs as sources of mastery.
Loss of the structure of daily life, common during COVID-19, for waking, dressing, meals, chores, homework time, bathing, or bedtime can be profoundly disorienting.
For adolescents, opportunities to contribute to society and become productive by volunteering or being employed have been stunted by quarantine and social distancing. Some teens have had to care for relatives at home so that parents can earn a living, which, while meaningful, blocks age-essential socializing.
Meaning can also be created at any age by community structures and agreed upon beliefs such as religion. While religious membership is low in the United States, members have been largely unable to attend services. Following sports teams, an alternate “religion” and source of identity, was on hold for many months.
Existential despair can also come from major life losses. COVID-19 has taken a terrible toll of lives, homes, and jobs for millions. As short-term thinkers, when children see so many of their plans and dreams for making the team, having a girlfriend, going to prom, attending summer camp, or graduating, it feels like the end of the world they had imagined. Even the most important source of meaning – connection to family – has been disrupted by lockdown, illness, or loss.
The loss of choice and freedom goes beyond being stuck indoors. Advanced classes and exams, as well as resume-building jobs or volunteering, which teens saw as essential to college, disappeared; sometimes also the money needed was exhausted by COVID-19 unemployment. Work-at-home parents supervising virtual school see their children’s malaise or panic and pressure them to work harder, which is impossible for despairing children. Observing a parent losing his or her job makes a teen’s own career aspirations uncertain. Teen depression and suicidal ideation/acts have shot up from hopelessness, with loss of meaning at the core.
A profound sense of powerlessness has taken over. COVID-19, an invisible threat, has taken down lives. Even with amazingly effective vaccines available, fear and helplessness have burned into our brains. Helplessness to stop structural racism and the arbitrary killings of our own Black citizens by police has finally registered. And climate change is now reported as an impending disaster that may not be stoppable.
So this must be the worst time in history, right? Actually, no. The past 60 years have been a period of historically remarkable stability of government, economy, and natural forces. Perhaps knowing no other world has made these problems appear unsolvable to the parents of our patients. Their own sense of meaning has been challenged in a way similar to that of their children. Perhaps from lack of privacy or peers, parents have been sharing their own sense of powerlessness with their children directly or indirectly, making it harder to reassure them.
With COVID-19 waning in the United States, many of the sources of meaning just discussed can be reinstated by way of in-person play dates, school, sports, socializing, practicing religion, volunteering, and getting jobs. Although there is “existential therapy,” what our children need most is adult leadership showing confidence in life’s meaning, even if we have to hide our own worries. Parents can point out that, even if it takes years, people have made it through difficult times in the past, and there are many positive alternatives for education and employment.
Children need to repeatedly hear about ways they are valued that are not dependent on accomplishments. Thanking them for and telling others about their effort, ideas, curiosity, integrity, love, and kindness point out meaning for their existence independent of world events. Parents need to establish routines and rules for children to demonstrate that life goes on as usual. Chores helpful to the family are a practical contribution. Family activities that are challenging and unpredictable set up for discussing, modeling, and building resilience; for example, visiting new places, camping, hiking, trying a new sport, or adopting a pet give opportunities to say: “Oh, well, we’ll find another way.”
Parents can share stories or books about people who made it through tougher times, such as Abraham Lincoln, or better, personal, or family experiences overcoming challenges. Recalling and nicknaming instances of the child’s own resilience is valuable. Books such as “The Little Engine That Could,” “Chicken Little,” and fairy tales of overcoming doubts when facing challenges can be helpful. “Stay calm and carry on,” a saying from the British when they were being bombed during World War II, has become a meme.
As clinicians we need to sort out significant complicated grief, anxiety, obsessive compulsive disorder, depression, or suicidal ideation, and provide assessment and treatment. But when children get stuck in existential futility, in addition to engaging them in meaningful activities, we can advise parents to coach them to distract themselves, “put the thoughts in a box in your head” to consider later, and/or write down or photograph things that make them grateful. Good lessons for us all to reinvent meaning in our lives.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. Email her at [email protected].
The news is portraying our modern time as an existential crisis as though our very existence is threatened. An existential crisis is a profound feeling of lack of meaning, choice, or freedom in one’s life that makes even existing seem worthless. It can emerge as early as 5 years old, especially in introspective, gifted children, when they realize that death is permanent and universal, after a real loss or a story of a loss or failure, or from a sense of guilt.
The past 18 months of COVID-19 have been a perfect storm for developing an existential crisis. One of the main sources of life meaning for children is friendships. COVID-19 has reduced or blocked access to old and new friends. Younger children, when asked what makes a friend, will say “we like to do the same things.” Virtual play dates help but don’t replace shared experiences.
School provides meaning for children not only from socializing but also from accomplishing academic tasks – fulfilling Erickson’s stages of “mastery” and “productivity.” Teachers were better able to carry out hands-on activities, group assignments, and field trips in person so that all children and learning styles were engaged and successful. Not having in-person school has also meant loss of extracurricular activities, sports, and clubs as sources of mastery.
Loss of the structure of daily life, common during COVID-19, for waking, dressing, meals, chores, homework time, bathing, or bedtime can be profoundly disorienting.
For adolescents, opportunities to contribute to society and become productive by volunteering or being employed have been stunted by quarantine and social distancing. Some teens have had to care for relatives at home so that parents can earn a living, which, while meaningful, blocks age-essential socializing.
Meaning can also be created at any age by community structures and agreed upon beliefs such as religion. While religious membership is low in the United States, members have been largely unable to attend services. Following sports teams, an alternate “religion” and source of identity, was on hold for many months.
Existential despair can also come from major life losses. COVID-19 has taken a terrible toll of lives, homes, and jobs for millions. As short-term thinkers, when children see so many of their plans and dreams for making the team, having a girlfriend, going to prom, attending summer camp, or graduating, it feels like the end of the world they had imagined. Even the most important source of meaning – connection to family – has been disrupted by lockdown, illness, or loss.
The loss of choice and freedom goes beyond being stuck indoors. Advanced classes and exams, as well as resume-building jobs or volunteering, which teens saw as essential to college, disappeared; sometimes also the money needed was exhausted by COVID-19 unemployment. Work-at-home parents supervising virtual school see their children’s malaise or panic and pressure them to work harder, which is impossible for despairing children. Observing a parent losing his or her job makes a teen’s own career aspirations uncertain. Teen depression and suicidal ideation/acts have shot up from hopelessness, with loss of meaning at the core.
A profound sense of powerlessness has taken over. COVID-19, an invisible threat, has taken down lives. Even with amazingly effective vaccines available, fear and helplessness have burned into our brains. Helplessness to stop structural racism and the arbitrary killings of our own Black citizens by police has finally registered. And climate change is now reported as an impending disaster that may not be stoppable.
So this must be the worst time in history, right? Actually, no. The past 60 years have been a period of historically remarkable stability of government, economy, and natural forces. Perhaps knowing no other world has made these problems appear unsolvable to the parents of our patients. Their own sense of meaning has been challenged in a way similar to that of their children. Perhaps from lack of privacy or peers, parents have been sharing their own sense of powerlessness with their children directly or indirectly, making it harder to reassure them.
With COVID-19 waning in the United States, many of the sources of meaning just discussed can be reinstated by way of in-person play dates, school, sports, socializing, practicing religion, volunteering, and getting jobs. Although there is “existential therapy,” what our children need most is adult leadership showing confidence in life’s meaning, even if we have to hide our own worries. Parents can point out that, even if it takes years, people have made it through difficult times in the past, and there are many positive alternatives for education and employment.
Children need to repeatedly hear about ways they are valued that are not dependent on accomplishments. Thanking them for and telling others about their effort, ideas, curiosity, integrity, love, and kindness point out meaning for their existence independent of world events. Parents need to establish routines and rules for children to demonstrate that life goes on as usual. Chores helpful to the family are a practical contribution. Family activities that are challenging and unpredictable set up for discussing, modeling, and building resilience; for example, visiting new places, camping, hiking, trying a new sport, or adopting a pet give opportunities to say: “Oh, well, we’ll find another way.”
Parents can share stories or books about people who made it through tougher times, such as Abraham Lincoln, or better, personal, or family experiences overcoming challenges. Recalling and nicknaming instances of the child’s own resilience is valuable. Books such as “The Little Engine That Could,” “Chicken Little,” and fairy tales of overcoming doubts when facing challenges can be helpful. “Stay calm and carry on,” a saying from the British when they were being bombed during World War II, has become a meme.
As clinicians we need to sort out significant complicated grief, anxiety, obsessive compulsive disorder, depression, or suicidal ideation, and provide assessment and treatment. But when children get stuck in existential futility, in addition to engaging them in meaningful activities, we can advise parents to coach them to distract themselves, “put the thoughts in a box in your head” to consider later, and/or write down or photograph things that make them grateful. Good lessons for us all to reinvent meaning in our lives.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. Email her at [email protected].
Infections in infants: An update
Converge 2021 session
Febrile Infant Update
Presenter
Russell J. McCulloh, MD
Session summary
Infections in infants aged younger than 90 days have been the subject of intense study in pediatric hospital medicine for many years. With the guidance of our talented presenter Dr. Russell McCulloh of Children’s Hospital & Medical Center in Omaha, Neb., the audience explored the historical perspective and evolution of this scientific question, including successes, special situations, newer screening tests, and description of cutting-edge scoring tools and platforms.
The challenge – Tens of thousands of infants present for care in the setting of fever each year. We know that our physical exam and history-taking skills are unlikely to be helpful in risk stratification. We have been guided by the desire to separate serious bacterial infection (SBI: bone infection, meningitis, pneumonia, urinary tract infection, bacteremia, enteritis) from invasive bacterial infection (IBI: meningitis and bacteremia). Data has shown that no test is 100% sensitive or specific, therefore we have to balance risk of disease to cost and invasiveness of tests. Important questions include whether to test and how to stratify by age, who to admit, and who to provide antibiotics.
The wins and exceptions – Fortunately, the early Boston, Philadelphia, and Rochester criteria set the stage for safely reducing testing. The current American College of Emergency Physicians guidelines for infants aged 29-90 days allows for lumbar puncture to be optional knowing that a look back using prior criteria identified no cases of meningitis in the low risk group. Similarly, in low-risk infants aged less that 29 days in nearly 4,000 cases there were just 2 infants with meningitis. Universal screening of moms for Group B Streptococcus with delivery of antibiotics in appropriate cases has dramatically decreased incidence of SBI. The Hib and pneumovax vaccines have likewise decreased incidence of SBI. Exceptions persist, including knowledge that infants with herpes simplex virus disease will not have fever in 50% of cases and that risk of HSV transmission is highest (25%-60% transmission) in mothers with primary disease. Given risk of HSV CNS disease after 1 week of age, in any high-risk infant less than 21 days, the mantra remains to test and treat.
The cutting edge – Thanks to ongoing research, we now have the PECARN and REVISE study groups to further aid decision-making. With PECARN we know that SBI in infants is extremely unlikely (negative predictive value, 99.7%) with a negative urinalysis , absolute neutrophil count less than 4,090, and procalcitonin less than 1.71. REVISE has revealed that infants with positive viral testing are unlikely to have SBI (7%-12%), particularly with influenza and RSV disease. Procalcitonin has also recently been shown to be an effective tool to rule out disease with the highest negative predictive value among available inflammatory markers. The just-published Aronson rule identifies a scoring system for IBI (using age less than 21 days/1pt; temp 38-38.4° C/2pt; >38.5° C/4pt; abnormal urinalysis/3pt; and absolute neutrophil count >5185/2pt) where any score greater than2 provides a sensitivity of 98.8% and NPV in validation studies of 99.4%. Likewise, multiplex polymerase chain reaction testing of spinal fluid has allowed for additional insight in pretreated cases and has helped us to remove antibiotic treatment from cases where parechovirus and enterovirus are positive because of the low risk for concomitant bacterial meningitis. As we await the release of revised national American Academy of Pediatrics guidelines, it is safe to say great progress has been made in the care of young febrile infants with shorter length of stay and fewer tests for all.
Key takeaways
- Numerous screening tests, rules, and scoring tools have been created to improve identification of infants with IBI, a low-frequency, high-morbidity event. The most recent with negative predictive values of 99.7% and 99.4% are the PECARN and Aronson scoring tools.
- Recent studies of the febrile infant population indicate that the odds of UTI or bacteremia in infants with respiratory symptoms is low, particularly for RSV and influenza.
- Among newer tests developed, a negative procalcitonin has the highest negative predictive value.
- Viral pathogens identified on cerebrospinal fluid molecular testing can be helpful in pretreated cases and indicative of low likelihood of bacterial meningitis allowing for observation off of antibiotics.
Dr. King is a hospitalist, associate director for medical education and associate program director for the pediatrics residency program at Children’s Minnesota in Minneapolis. She has shared some of her resident teaching, presentation skills, and peer-coaching work on a national level.
Converge 2021 session
Febrile Infant Update
Presenter
Russell J. McCulloh, MD
Session summary
Infections in infants aged younger than 90 days have been the subject of intense study in pediatric hospital medicine for many years. With the guidance of our talented presenter Dr. Russell McCulloh of Children’s Hospital & Medical Center in Omaha, Neb., the audience explored the historical perspective and evolution of this scientific question, including successes, special situations, newer screening tests, and description of cutting-edge scoring tools and platforms.
The challenge – Tens of thousands of infants present for care in the setting of fever each year. We know that our physical exam and history-taking skills are unlikely to be helpful in risk stratification. We have been guided by the desire to separate serious bacterial infection (SBI: bone infection, meningitis, pneumonia, urinary tract infection, bacteremia, enteritis) from invasive bacterial infection (IBI: meningitis and bacteremia). Data has shown that no test is 100% sensitive or specific, therefore we have to balance risk of disease to cost and invasiveness of tests. Important questions include whether to test and how to stratify by age, who to admit, and who to provide antibiotics.
The wins and exceptions – Fortunately, the early Boston, Philadelphia, and Rochester criteria set the stage for safely reducing testing. The current American College of Emergency Physicians guidelines for infants aged 29-90 days allows for lumbar puncture to be optional knowing that a look back using prior criteria identified no cases of meningitis in the low risk group. Similarly, in low-risk infants aged less that 29 days in nearly 4,000 cases there were just 2 infants with meningitis. Universal screening of moms for Group B Streptococcus with delivery of antibiotics in appropriate cases has dramatically decreased incidence of SBI. The Hib and pneumovax vaccines have likewise decreased incidence of SBI. Exceptions persist, including knowledge that infants with herpes simplex virus disease will not have fever in 50% of cases and that risk of HSV transmission is highest (25%-60% transmission) in mothers with primary disease. Given risk of HSV CNS disease after 1 week of age, in any high-risk infant less than 21 days, the mantra remains to test and treat.
The cutting edge – Thanks to ongoing research, we now have the PECARN and REVISE study groups to further aid decision-making. With PECARN we know that SBI in infants is extremely unlikely (negative predictive value, 99.7%) with a negative urinalysis , absolute neutrophil count less than 4,090, and procalcitonin less than 1.71. REVISE has revealed that infants with positive viral testing are unlikely to have SBI (7%-12%), particularly with influenza and RSV disease. Procalcitonin has also recently been shown to be an effective tool to rule out disease with the highest negative predictive value among available inflammatory markers. The just-published Aronson rule identifies a scoring system for IBI (using age less than 21 days/1pt; temp 38-38.4° C/2pt; >38.5° C/4pt; abnormal urinalysis/3pt; and absolute neutrophil count >5185/2pt) where any score greater than2 provides a sensitivity of 98.8% and NPV in validation studies of 99.4%. Likewise, multiplex polymerase chain reaction testing of spinal fluid has allowed for additional insight in pretreated cases and has helped us to remove antibiotic treatment from cases where parechovirus and enterovirus are positive because of the low risk for concomitant bacterial meningitis. As we await the release of revised national American Academy of Pediatrics guidelines, it is safe to say great progress has been made in the care of young febrile infants with shorter length of stay and fewer tests for all.
Key takeaways
- Numerous screening tests, rules, and scoring tools have been created to improve identification of infants with IBI, a low-frequency, high-morbidity event. The most recent with negative predictive values of 99.7% and 99.4% are the PECARN and Aronson scoring tools.
- Recent studies of the febrile infant population indicate that the odds of UTI or bacteremia in infants with respiratory symptoms is low, particularly for RSV and influenza.
- Among newer tests developed, a negative procalcitonin has the highest negative predictive value.
- Viral pathogens identified on cerebrospinal fluid molecular testing can be helpful in pretreated cases and indicative of low likelihood of bacterial meningitis allowing for observation off of antibiotics.
Dr. King is a hospitalist, associate director for medical education and associate program director for the pediatrics residency program at Children’s Minnesota in Minneapolis. She has shared some of her resident teaching, presentation skills, and peer-coaching work on a national level.
Converge 2021 session
Febrile Infant Update
Presenter
Russell J. McCulloh, MD
Session summary
Infections in infants aged younger than 90 days have been the subject of intense study in pediatric hospital medicine for many years. With the guidance of our talented presenter Dr. Russell McCulloh of Children’s Hospital & Medical Center in Omaha, Neb., the audience explored the historical perspective and evolution of this scientific question, including successes, special situations, newer screening tests, and description of cutting-edge scoring tools and platforms.
The challenge – Tens of thousands of infants present for care in the setting of fever each year. We know that our physical exam and history-taking skills are unlikely to be helpful in risk stratification. We have been guided by the desire to separate serious bacterial infection (SBI: bone infection, meningitis, pneumonia, urinary tract infection, bacteremia, enteritis) from invasive bacterial infection (IBI: meningitis and bacteremia). Data has shown that no test is 100% sensitive or specific, therefore we have to balance risk of disease to cost and invasiveness of tests. Important questions include whether to test and how to stratify by age, who to admit, and who to provide antibiotics.
The wins and exceptions – Fortunately, the early Boston, Philadelphia, and Rochester criteria set the stage for safely reducing testing. The current American College of Emergency Physicians guidelines for infants aged 29-90 days allows for lumbar puncture to be optional knowing that a look back using prior criteria identified no cases of meningitis in the low risk group. Similarly, in low-risk infants aged less that 29 days in nearly 4,000 cases there were just 2 infants with meningitis. Universal screening of moms for Group B Streptococcus with delivery of antibiotics in appropriate cases has dramatically decreased incidence of SBI. The Hib and pneumovax vaccines have likewise decreased incidence of SBI. Exceptions persist, including knowledge that infants with herpes simplex virus disease will not have fever in 50% of cases and that risk of HSV transmission is highest (25%-60% transmission) in mothers with primary disease. Given risk of HSV CNS disease after 1 week of age, in any high-risk infant less than 21 days, the mantra remains to test and treat.
The cutting edge – Thanks to ongoing research, we now have the PECARN and REVISE study groups to further aid decision-making. With PECARN we know that SBI in infants is extremely unlikely (negative predictive value, 99.7%) with a negative urinalysis , absolute neutrophil count less than 4,090, and procalcitonin less than 1.71. REVISE has revealed that infants with positive viral testing are unlikely to have SBI (7%-12%), particularly with influenza and RSV disease. Procalcitonin has also recently been shown to be an effective tool to rule out disease with the highest negative predictive value among available inflammatory markers. The just-published Aronson rule identifies a scoring system for IBI (using age less than 21 days/1pt; temp 38-38.4° C/2pt; >38.5° C/4pt; abnormal urinalysis/3pt; and absolute neutrophil count >5185/2pt) where any score greater than2 provides a sensitivity of 98.8% and NPV in validation studies of 99.4%. Likewise, multiplex polymerase chain reaction testing of spinal fluid has allowed for additional insight in pretreated cases and has helped us to remove antibiotic treatment from cases where parechovirus and enterovirus are positive because of the low risk for concomitant bacterial meningitis. As we await the release of revised national American Academy of Pediatrics guidelines, it is safe to say great progress has been made in the care of young febrile infants with shorter length of stay and fewer tests for all.
Key takeaways
- Numerous screening tests, rules, and scoring tools have been created to improve identification of infants with IBI, a low-frequency, high-morbidity event. The most recent with negative predictive values of 99.7% and 99.4% are the PECARN and Aronson scoring tools.
- Recent studies of the febrile infant population indicate that the odds of UTI or bacteremia in infants with respiratory symptoms is low, particularly for RSV and influenza.
- Among newer tests developed, a negative procalcitonin has the highest negative predictive value.
- Viral pathogens identified on cerebrospinal fluid molecular testing can be helpful in pretreated cases and indicative of low likelihood of bacterial meningitis allowing for observation off of antibiotics.
Dr. King is a hospitalist, associate director for medical education and associate program director for the pediatrics residency program at Children’s Minnesota in Minneapolis. She has shared some of her resident teaching, presentation skills, and peer-coaching work on a national level.
FROM SHM CONVERGE 2021