User login
Research Highlights From ESMO Breast Cancer
Among the topics the speakers addressed were breast cancer prevention, early breast cancer, advanced breast cancer, and supportive care.
In recent years, the way clinicians look at carcinogenesis in breast cancer has changed, and many new targets for potential early detection and prevention have emerged, said Suzette Delaloge, MD, of Gustave Roussy, Paris, France, in her presentation at the meeting.
Instant risk assessment at different time points could potentially intercept cancer among high-risk individuals, she said.
A study by Mikael Eriksson, PhD, and colleagues focused on external validation of the Profound AI tool to identify breast cancer risk in the general population. The researchers showed an area under the curve of 0.72 in their AI risk model, which has the potential to be clinically meaningful, although it must be prospectively validated, Dr. Delaloge said in her presentation.
She also reviewed two studies on the use of genes to further refine breast cancer risk among carriers. One of these, a prospective study presented in a session by Kelly-Anne Phillips, MD, of Peter MacCallum Cancer Center, Melbourne, Australia, used the CANRISK online risk assessment tool and validated increased breast cancer risk in BRCA1 and BRCA2 carriers, with AUCs of 0.79 and 0.78, respectively. The other study, which was by Maria Rezqallah Aron, MD, and colleagues examined polygenic scores as a way to refine breast cancer risk stratification among carriers of the ALM and PALB2 genes as well. These genes might be useful in identifying individuals who could benefit from early intervention, including surgery, Dr. Delaloge said.
Translational Research
“Preparing my talk, I felt like a kid in a candy store,” because of the amount of new translational research presented, including several studies of endocrine treatment–based approaches to therapy, said Marleen Kok, MD, of the Netherlands Cancer Institute, Amsterdam.
In her presentation, Dr. Kok highlighted findings from an analysis of patients in the monarchE study (a trial of high-risk patients) showing a consistent improvement in invasive disease-free survival for the subset of patients with germline BRCA1 and BRCA2 mutations who received abemaciclib plus endocrine therapy.
The value of tumor-infiltrating lymphocytes (TILs) on patients who are not receiving chemotherapy is important because of the focus on prognosis, and prospective trials are underway, she said.
A poster on the impact of chemotherapy and stromal tumor-infiltrating lymphocytes (sTILs) in stage I triple-negative breast cancer showed no association between chemotherapy and better outcomes regardless of sTILs in patients who did and did not receive chemotherapy, which has implications for potential treatment sparing in this population, Dr. Kok noted.
Artificial Intelligence (AI) was the subject of several posters at the meeting, and Dr. Kok identified a multisite European study of an automated HER2 scoring system as notable for its size and accuracy. In the study, the accuracy among pathologists was much higher with the assistance of AI, she said. Using AI for more complex analysis has shown success, she said.
Dr. Kok ended her talk with a poster that surveyed breast cancer patients about their understanding of their disease. The results showed that less than half (44%) of patients reported that their healthcare providers had given them enough information to learn about their breast cancer type, and less than one third could recall terminology about biomarkers; the study is important because it shows that clinicians need to do better in explaining these terms to patients, Dr. Kok said.
Early Breast Cancer
Right-sizing therapy, meaning identifying the right treatment for every patient, is a key element of new research in early breast cancer, said Erika Hamilton, MD, of the Sarah Cannon Research Institute, Nashville, Tenn.
She highlighted safety and treatment duration updates from the NATALEE study, which compared adjuvant ribociclib plus nonsteroidal aromatase inhibitor (NSAI) to NSAI alone for ER+/HER2- breast cancer. The current analysis presented at the meeting showed significant benefits with the addition of ribociclib and no evidence of new safety signals or adverse event exacerbations at 3 years, she said. Dose modifications had no significant impact on efficacy, she added.
The findings of no impact of dose reduction on efficacy in both the NATALEE and monarchE studies provide important information on whether dosage can be reduced in patients, which will increase the odds that patients will tolerate extended therapy with good outcomes and stay on their prescribed therapies, Dr. Hamilton emphasized.
The CARABELA study, a phase 2 trial of neoadjuvant letrozole plus abemaciclib vs adriamycin and cyclophosphamide (AC), showed clinically similar response rates but did not meet its endpoint for residual cancer burden (RCB) scores. These data add to results from other studies and show that it is too soon to universally replace neoadjuvant chemotherapy as first-line treatment for highly proliferative ER+ breast cancer, Dr. Hamilton said in her presentation.
Advanced Breast Cancer
Take-home messages about advanced breast cancer include growing evidence for the potential benefits of antibody drug conjugates (ADCs), said Eva Ciruelos, MD, of University Hospital, Madrid, Spain. The TROPION-BREAST01 study, a phase 3 randomized trial, showed significant and clinically meaningful improvement in progression-free survival in patients with previously treated, inoperable, or metastatic HR+/HER2- breast cancer who received datopotamab deruxtecan (Dato-DXd) compared with those who received chemotherapy.
Data from an additional safety analysis were presented at the meeting; although Dato-DXd, a trophoblast cell-surface antigen 2 (TROP2)–directed antibody-drug conjugate, was well-tolerated, it is important to remain aware of toxicities, notably oral mucositis, which occurred in 55.6% of the patients in the study across all grades, and ocular surface toxicity, which occurred in 40% of patients across all grades, Dr. Ciruelos emphasized.
Key research in the area of advanced triple-negative breast cancer included data from the IMPASSION 132 study. This study is “specifically centered on early relapsers,” a population often excluded from other trials, Dr. Ciruelos said. In this study, patients with advanced triple-negative breast cancer were randomized to chemotherapy with or without atezolizumab, and the study showed no benefits with atezolizumab for overall survival, progression-free survival, or overall response rate, she said. “This is something to work with, because this is a very refractory population,” Dr. Ciruelos noted.
New immunotherapy combinations are needed to improve survival in advanced breast cancer patients, Dr. Ciruelos said. At the meeting, researchers presented interim data from a subset of patients in the MORPHEUS-pan breast cancer trial, a phase 1B/2 study involving multiple treatment combinations in locally advanced/metastatic breast cancer patients.
The interim analysis included 18-week data from triple-negative breast cancer patients and compared outcomes for patients randomized to atezolizumab with or without sacituzumab govitecan (SG).
The study was small, with only 31 patients in the combination arm and 11 controls, but the results were promising, with an overall response rate of 76.7% in the combination arm vs 66.7% in the control arm, Dr. Ciruelos said.
Supportive Care
Key supportive care takeaways included data on pregnancy in young breast cancer survivors and the safety of vaginal estrogen therapy in breast cancer patients with genitourinary symptoms, said Anne May, MD, of the University Medical Center Utrecht, Utrecht, Netherlands.
A study previously published in JAMA including nearly 5000 BRCA carriers who were diagnosed with invasive breast cancer at age 40 years or younger showed no association between pregnancy after breast cancer and adverse maternal or fetal outcomes, and pregnancy had no significant impact on overall survival. The authors presented new data on the safety of assisted reproductive techniques (ART) based on the 543 pregnancies in the original study, at the meeting. Of these, 436 conceived naturally, and 107 used ART. After a median of 9.1 years, ART had no effect on disease-free survival compared to natural conception (hazard ratio [HR], 0.64). Based on these findings, fertility preservation should be offered to all women who receive a breast cancer diagnosis and are interested in future fertility, Dr. May said.
Conceiving after breast cancer treatment and follow-up should not be contraindicated for young BRCA carriers, she added.No trial data are available for the effects of vaginal estrogen therapy (VET) on disease-free survival in breast cancer survivors with genitourinary symptoms caused by declining estrogen levels, Dr. May said. However, researchers in France and Switzerland conducted an emulation of a hypothetical target trial using data from the French National social security system for more than 130,000 individuals. Although VET therapy had no impact on disease-free survival in most breast cancer survivors overall, it did have a negative impact in a subset of patients with HR-positive and HR-negative tumors who were treated with aromatase inhibitors. The study was hypothetical, but important because the results suggest that clinicians can safely propose VTE to patients who report genitourinary symptoms after treatment for early-stage breast cancer with tamoxifen, but VTE should be avoided in patients treated with aromatase inhibitors, Dr. May said.
Dr. Delaloge disclosed research support to her institution from AstraZeneca, MSD, Bristol Myers Squibb, Sanofi, Taiho, Novartis, European Commission, INCa, Banque des Territoires, and Fondation Philanthropia. She also disclosed honoraria to her institution from AstraZeneca, Gilead, Novartis, Elsan, Besins, Sanofi, Exact Sciences, and Lilly, as well as travel support from Novartis.
Dr. Kok disclosed research funding from AstraZeneca, Bristol Myers Squibb, Daichi, and Roche, and advisory board membership/speaker’s fees from Alderaan Biotechnology, BIONTECH, Domain Therapeutics, AstraZeneca, Daichi, Bristol Myers Squibb, Gilead, Medscape, MSD, and Roche.
Dr. Hamilton disclosed a consulting advisory role (to her institution) for Accutar Biotechology, AstraZeneca, Daiichi Sankyo, Ellipses Pharma, Entos, Forsum Pharma, Gilead Sciences, Greenwich LifeSciences, Jazz Pharmaceuticals, Lilly, Medical Pharma Services, Mersana, Novartis, Olema Pharmaceuticals, Orum Therapeutics, Roche/Genentech, Stemline Therapeutics, ands others. She also disclosed contracted research/grant support to her institution only from Abbvie, Acerta Pharma, Accutar Biotechnology , ADC Therapeutics, AKESOBIO Australia , Amgen, Aravive, ArQule, Artios, Arvinas, AstraZeneca, AtlasMedx, BeiGene, Black Diamond and others.
Dr. Ciruelos disclosed serving as an external advisor for Roche, MSD, Gilead, AstraZeneca, Daichii Sankyo, Reveal Genomics, Pfizer, Novartis, and Lilly, as well as serving as a speaker for Roche, MSD, Gilead, AstraZeneca, Daichii Sankyo, Reveal Genomics, Pfizer, Novartis, Lilly, and Pierre Fabre. She also disclosed travel grants from Roche, Pfizer, and AstraZeneca, and research grants from Seagen and Roche.
Dr. May had no financial conflicts to disclose.
Among the topics the speakers addressed were breast cancer prevention, early breast cancer, advanced breast cancer, and supportive care.
In recent years, the way clinicians look at carcinogenesis in breast cancer has changed, and many new targets for potential early detection and prevention have emerged, said Suzette Delaloge, MD, of Gustave Roussy, Paris, France, in her presentation at the meeting.
Instant risk assessment at different time points could potentially intercept cancer among high-risk individuals, she said.
A study by Mikael Eriksson, PhD, and colleagues focused on external validation of the Profound AI tool to identify breast cancer risk in the general population. The researchers showed an area under the curve of 0.72 in their AI risk model, which has the potential to be clinically meaningful, although it must be prospectively validated, Dr. Delaloge said in her presentation.
She also reviewed two studies on the use of genes to further refine breast cancer risk among carriers. One of these, a prospective study presented in a session by Kelly-Anne Phillips, MD, of Peter MacCallum Cancer Center, Melbourne, Australia, used the CANRISK online risk assessment tool and validated increased breast cancer risk in BRCA1 and BRCA2 carriers, with AUCs of 0.79 and 0.78, respectively. The other study, which was by Maria Rezqallah Aron, MD, and colleagues examined polygenic scores as a way to refine breast cancer risk stratification among carriers of the ALM and PALB2 genes as well. These genes might be useful in identifying individuals who could benefit from early intervention, including surgery, Dr. Delaloge said.
Translational Research
“Preparing my talk, I felt like a kid in a candy store,” because of the amount of new translational research presented, including several studies of endocrine treatment–based approaches to therapy, said Marleen Kok, MD, of the Netherlands Cancer Institute, Amsterdam.
In her presentation, Dr. Kok highlighted findings from an analysis of patients in the monarchE study (a trial of high-risk patients) showing a consistent improvement in invasive disease-free survival for the subset of patients with germline BRCA1 and BRCA2 mutations who received abemaciclib plus endocrine therapy.
The value of tumor-infiltrating lymphocytes (TILs) on patients who are not receiving chemotherapy is important because of the focus on prognosis, and prospective trials are underway, she said.
A poster on the impact of chemotherapy and stromal tumor-infiltrating lymphocytes (sTILs) in stage I triple-negative breast cancer showed no association between chemotherapy and better outcomes regardless of sTILs in patients who did and did not receive chemotherapy, which has implications for potential treatment sparing in this population, Dr. Kok noted.
Artificial Intelligence (AI) was the subject of several posters at the meeting, and Dr. Kok identified a multisite European study of an automated HER2 scoring system as notable for its size and accuracy. In the study, the accuracy among pathologists was much higher with the assistance of AI, she said. Using AI for more complex analysis has shown success, she said.
Dr. Kok ended her talk with a poster that surveyed breast cancer patients about their understanding of their disease. The results showed that less than half (44%) of patients reported that their healthcare providers had given them enough information to learn about their breast cancer type, and less than one third could recall terminology about biomarkers; the study is important because it shows that clinicians need to do better in explaining these terms to patients, Dr. Kok said.
Early Breast Cancer
Right-sizing therapy, meaning identifying the right treatment for every patient, is a key element of new research in early breast cancer, said Erika Hamilton, MD, of the Sarah Cannon Research Institute, Nashville, Tenn.
She highlighted safety and treatment duration updates from the NATALEE study, which compared adjuvant ribociclib plus nonsteroidal aromatase inhibitor (NSAI) to NSAI alone for ER+/HER2- breast cancer. The current analysis presented at the meeting showed significant benefits with the addition of ribociclib and no evidence of new safety signals or adverse event exacerbations at 3 years, she said. Dose modifications had no significant impact on efficacy, she added.
The findings of no impact of dose reduction on efficacy in both the NATALEE and monarchE studies provide important information on whether dosage can be reduced in patients, which will increase the odds that patients will tolerate extended therapy with good outcomes and stay on their prescribed therapies, Dr. Hamilton emphasized.
The CARABELA study, a phase 2 trial of neoadjuvant letrozole plus abemaciclib vs adriamycin and cyclophosphamide (AC), showed clinically similar response rates but did not meet its endpoint for residual cancer burden (RCB) scores. These data add to results from other studies and show that it is too soon to universally replace neoadjuvant chemotherapy as first-line treatment for highly proliferative ER+ breast cancer, Dr. Hamilton said in her presentation.
Advanced Breast Cancer
Take-home messages about advanced breast cancer include growing evidence for the potential benefits of antibody drug conjugates (ADCs), said Eva Ciruelos, MD, of University Hospital, Madrid, Spain. The TROPION-BREAST01 study, a phase 3 randomized trial, showed significant and clinically meaningful improvement in progression-free survival in patients with previously treated, inoperable, or metastatic HR+/HER2- breast cancer who received datopotamab deruxtecan (Dato-DXd) compared with those who received chemotherapy.
Data from an additional safety analysis were presented at the meeting; although Dato-DXd, a trophoblast cell-surface antigen 2 (TROP2)–directed antibody-drug conjugate, was well-tolerated, it is important to remain aware of toxicities, notably oral mucositis, which occurred in 55.6% of the patients in the study across all grades, and ocular surface toxicity, which occurred in 40% of patients across all grades, Dr. Ciruelos emphasized.
Key research in the area of advanced triple-negative breast cancer included data from the IMPASSION 132 study. This study is “specifically centered on early relapsers,” a population often excluded from other trials, Dr. Ciruelos said. In this study, patients with advanced triple-negative breast cancer were randomized to chemotherapy with or without atezolizumab, and the study showed no benefits with atezolizumab for overall survival, progression-free survival, or overall response rate, she said. “This is something to work with, because this is a very refractory population,” Dr. Ciruelos noted.
New immunotherapy combinations are needed to improve survival in advanced breast cancer patients, Dr. Ciruelos said. At the meeting, researchers presented interim data from a subset of patients in the MORPHEUS-pan breast cancer trial, a phase 1B/2 study involving multiple treatment combinations in locally advanced/metastatic breast cancer patients.
The interim analysis included 18-week data from triple-negative breast cancer patients and compared outcomes for patients randomized to atezolizumab with or without sacituzumab govitecan (SG).
The study was small, with only 31 patients in the combination arm and 11 controls, but the results were promising, with an overall response rate of 76.7% in the combination arm vs 66.7% in the control arm, Dr. Ciruelos said.
Supportive Care
Key supportive care takeaways included data on pregnancy in young breast cancer survivors and the safety of vaginal estrogen therapy in breast cancer patients with genitourinary symptoms, said Anne May, MD, of the University Medical Center Utrecht, Utrecht, Netherlands.
A study previously published in JAMA including nearly 5000 BRCA carriers who were diagnosed with invasive breast cancer at age 40 years or younger showed no association between pregnancy after breast cancer and adverse maternal or fetal outcomes, and pregnancy had no significant impact on overall survival. The authors presented new data on the safety of assisted reproductive techniques (ART) based on the 543 pregnancies in the original study, at the meeting. Of these, 436 conceived naturally, and 107 used ART. After a median of 9.1 years, ART had no effect on disease-free survival compared to natural conception (hazard ratio [HR], 0.64). Based on these findings, fertility preservation should be offered to all women who receive a breast cancer diagnosis and are interested in future fertility, Dr. May said.
Conceiving after breast cancer treatment and follow-up should not be contraindicated for young BRCA carriers, she added.No trial data are available for the effects of vaginal estrogen therapy (VET) on disease-free survival in breast cancer survivors with genitourinary symptoms caused by declining estrogen levels, Dr. May said. However, researchers in France and Switzerland conducted an emulation of a hypothetical target trial using data from the French National social security system for more than 130,000 individuals. Although VET therapy had no impact on disease-free survival in most breast cancer survivors overall, it did have a negative impact in a subset of patients with HR-positive and HR-negative tumors who were treated with aromatase inhibitors. The study was hypothetical, but important because the results suggest that clinicians can safely propose VTE to patients who report genitourinary symptoms after treatment for early-stage breast cancer with tamoxifen, but VTE should be avoided in patients treated with aromatase inhibitors, Dr. May said.
Dr. Delaloge disclosed research support to her institution from AstraZeneca, MSD, Bristol Myers Squibb, Sanofi, Taiho, Novartis, European Commission, INCa, Banque des Territoires, and Fondation Philanthropia. She also disclosed honoraria to her institution from AstraZeneca, Gilead, Novartis, Elsan, Besins, Sanofi, Exact Sciences, and Lilly, as well as travel support from Novartis.
Dr. Kok disclosed research funding from AstraZeneca, Bristol Myers Squibb, Daichi, and Roche, and advisory board membership/speaker’s fees from Alderaan Biotechnology, BIONTECH, Domain Therapeutics, AstraZeneca, Daichi, Bristol Myers Squibb, Gilead, Medscape, MSD, and Roche.
Dr. Hamilton disclosed a consulting advisory role (to her institution) for Accutar Biotechology, AstraZeneca, Daiichi Sankyo, Ellipses Pharma, Entos, Forsum Pharma, Gilead Sciences, Greenwich LifeSciences, Jazz Pharmaceuticals, Lilly, Medical Pharma Services, Mersana, Novartis, Olema Pharmaceuticals, Orum Therapeutics, Roche/Genentech, Stemline Therapeutics, ands others. She also disclosed contracted research/grant support to her institution only from Abbvie, Acerta Pharma, Accutar Biotechnology , ADC Therapeutics, AKESOBIO Australia , Amgen, Aravive, ArQule, Artios, Arvinas, AstraZeneca, AtlasMedx, BeiGene, Black Diamond and others.
Dr. Ciruelos disclosed serving as an external advisor for Roche, MSD, Gilead, AstraZeneca, Daichii Sankyo, Reveal Genomics, Pfizer, Novartis, and Lilly, as well as serving as a speaker for Roche, MSD, Gilead, AstraZeneca, Daichii Sankyo, Reveal Genomics, Pfizer, Novartis, Lilly, and Pierre Fabre. She also disclosed travel grants from Roche, Pfizer, and AstraZeneca, and research grants from Seagen and Roche.
Dr. May had no financial conflicts to disclose.
Among the topics the speakers addressed were breast cancer prevention, early breast cancer, advanced breast cancer, and supportive care.
In recent years, the way clinicians look at carcinogenesis in breast cancer has changed, and many new targets for potential early detection and prevention have emerged, said Suzette Delaloge, MD, of Gustave Roussy, Paris, France, in her presentation at the meeting.
Instant risk assessment at different time points could potentially intercept cancer among high-risk individuals, she said.
A study by Mikael Eriksson, PhD, and colleagues focused on external validation of the Profound AI tool to identify breast cancer risk in the general population. The researchers showed an area under the curve of 0.72 in their AI risk model, which has the potential to be clinically meaningful, although it must be prospectively validated, Dr. Delaloge said in her presentation.
She also reviewed two studies on the use of genes to further refine breast cancer risk among carriers. One of these, a prospective study presented in a session by Kelly-Anne Phillips, MD, of Peter MacCallum Cancer Center, Melbourne, Australia, used the CANRISK online risk assessment tool and validated increased breast cancer risk in BRCA1 and BRCA2 carriers, with AUCs of 0.79 and 0.78, respectively. The other study, which was by Maria Rezqallah Aron, MD, and colleagues examined polygenic scores as a way to refine breast cancer risk stratification among carriers of the ALM and PALB2 genes as well. These genes might be useful in identifying individuals who could benefit from early intervention, including surgery, Dr. Delaloge said.
Translational Research
“Preparing my talk, I felt like a kid in a candy store,” because of the amount of new translational research presented, including several studies of endocrine treatment–based approaches to therapy, said Marleen Kok, MD, of the Netherlands Cancer Institute, Amsterdam.
In her presentation, Dr. Kok highlighted findings from an analysis of patients in the monarchE study (a trial of high-risk patients) showing a consistent improvement in invasive disease-free survival for the subset of patients with germline BRCA1 and BRCA2 mutations who received abemaciclib plus endocrine therapy.
The value of tumor-infiltrating lymphocytes (TILs) on patients who are not receiving chemotherapy is important because of the focus on prognosis, and prospective trials are underway, she said.
A poster on the impact of chemotherapy and stromal tumor-infiltrating lymphocytes (sTILs) in stage I triple-negative breast cancer showed no association between chemotherapy and better outcomes regardless of sTILs in patients who did and did not receive chemotherapy, which has implications for potential treatment sparing in this population, Dr. Kok noted.
Artificial Intelligence (AI) was the subject of several posters at the meeting, and Dr. Kok identified a multisite European study of an automated HER2 scoring system as notable for its size and accuracy. In the study, the accuracy among pathologists was much higher with the assistance of AI, she said. Using AI for more complex analysis has shown success, she said.
Dr. Kok ended her talk with a poster that surveyed breast cancer patients about their understanding of their disease. The results showed that less than half (44%) of patients reported that their healthcare providers had given them enough information to learn about their breast cancer type, and less than one third could recall terminology about biomarkers; the study is important because it shows that clinicians need to do better in explaining these terms to patients, Dr. Kok said.
Early Breast Cancer
Right-sizing therapy, meaning identifying the right treatment for every patient, is a key element of new research in early breast cancer, said Erika Hamilton, MD, of the Sarah Cannon Research Institute, Nashville, Tenn.
She highlighted safety and treatment duration updates from the NATALEE study, which compared adjuvant ribociclib plus nonsteroidal aromatase inhibitor (NSAI) to NSAI alone for ER+/HER2- breast cancer. The current analysis presented at the meeting showed significant benefits with the addition of ribociclib and no evidence of new safety signals or adverse event exacerbations at 3 years, she said. Dose modifications had no significant impact on efficacy, she added.
The findings of no impact of dose reduction on efficacy in both the NATALEE and monarchE studies provide important information on whether dosage can be reduced in patients, which will increase the odds that patients will tolerate extended therapy with good outcomes and stay on their prescribed therapies, Dr. Hamilton emphasized.
The CARABELA study, a phase 2 trial of neoadjuvant letrozole plus abemaciclib vs adriamycin and cyclophosphamide (AC), showed clinically similar response rates but did not meet its endpoint for residual cancer burden (RCB) scores. These data add to results from other studies and show that it is too soon to universally replace neoadjuvant chemotherapy as first-line treatment for highly proliferative ER+ breast cancer, Dr. Hamilton said in her presentation.
Advanced Breast Cancer
Take-home messages about advanced breast cancer include growing evidence for the potential benefits of antibody drug conjugates (ADCs), said Eva Ciruelos, MD, of University Hospital, Madrid, Spain. The TROPION-BREAST01 study, a phase 3 randomized trial, showed significant and clinically meaningful improvement in progression-free survival in patients with previously treated, inoperable, or metastatic HR+/HER2- breast cancer who received datopotamab deruxtecan (Dato-DXd) compared with those who received chemotherapy.
Data from an additional safety analysis were presented at the meeting; although Dato-DXd, a trophoblast cell-surface antigen 2 (TROP2)–directed antibody-drug conjugate, was well-tolerated, it is important to remain aware of toxicities, notably oral mucositis, which occurred in 55.6% of the patients in the study across all grades, and ocular surface toxicity, which occurred in 40% of patients across all grades, Dr. Ciruelos emphasized.
Key research in the area of advanced triple-negative breast cancer included data from the IMPASSION 132 study. This study is “specifically centered on early relapsers,” a population often excluded from other trials, Dr. Ciruelos said. In this study, patients with advanced triple-negative breast cancer were randomized to chemotherapy with or without atezolizumab, and the study showed no benefits with atezolizumab for overall survival, progression-free survival, or overall response rate, she said. “This is something to work with, because this is a very refractory population,” Dr. Ciruelos noted.
New immunotherapy combinations are needed to improve survival in advanced breast cancer patients, Dr. Ciruelos said. At the meeting, researchers presented interim data from a subset of patients in the MORPHEUS-pan breast cancer trial, a phase 1B/2 study involving multiple treatment combinations in locally advanced/metastatic breast cancer patients.
The interim analysis included 18-week data from triple-negative breast cancer patients and compared outcomes for patients randomized to atezolizumab with or without sacituzumab govitecan (SG).
The study was small, with only 31 patients in the combination arm and 11 controls, but the results were promising, with an overall response rate of 76.7% in the combination arm vs 66.7% in the control arm, Dr. Ciruelos said.
Supportive Care
Key supportive care takeaways included data on pregnancy in young breast cancer survivors and the safety of vaginal estrogen therapy in breast cancer patients with genitourinary symptoms, said Anne May, MD, of the University Medical Center Utrecht, Utrecht, Netherlands.
A study previously published in JAMA including nearly 5000 BRCA carriers who were diagnosed with invasive breast cancer at age 40 years or younger showed no association between pregnancy after breast cancer and adverse maternal or fetal outcomes, and pregnancy had no significant impact on overall survival. The authors presented new data on the safety of assisted reproductive techniques (ART) based on the 543 pregnancies in the original study, at the meeting. Of these, 436 conceived naturally, and 107 used ART. After a median of 9.1 years, ART had no effect on disease-free survival compared to natural conception (hazard ratio [HR], 0.64). Based on these findings, fertility preservation should be offered to all women who receive a breast cancer diagnosis and are interested in future fertility, Dr. May said.
Conceiving after breast cancer treatment and follow-up should not be contraindicated for young BRCA carriers, she added.No trial data are available for the effects of vaginal estrogen therapy (VET) on disease-free survival in breast cancer survivors with genitourinary symptoms caused by declining estrogen levels, Dr. May said. However, researchers in France and Switzerland conducted an emulation of a hypothetical target trial using data from the French National social security system for more than 130,000 individuals. Although VET therapy had no impact on disease-free survival in most breast cancer survivors overall, it did have a negative impact in a subset of patients with HR-positive and HR-negative tumors who were treated with aromatase inhibitors. The study was hypothetical, but important because the results suggest that clinicians can safely propose VTE to patients who report genitourinary symptoms after treatment for early-stage breast cancer with tamoxifen, but VTE should be avoided in patients treated with aromatase inhibitors, Dr. May said.
Dr. Delaloge disclosed research support to her institution from AstraZeneca, MSD, Bristol Myers Squibb, Sanofi, Taiho, Novartis, European Commission, INCa, Banque des Territoires, and Fondation Philanthropia. She also disclosed honoraria to her institution from AstraZeneca, Gilead, Novartis, Elsan, Besins, Sanofi, Exact Sciences, and Lilly, as well as travel support from Novartis.
Dr. Kok disclosed research funding from AstraZeneca, Bristol Myers Squibb, Daichi, and Roche, and advisory board membership/speaker’s fees from Alderaan Biotechnology, BIONTECH, Domain Therapeutics, AstraZeneca, Daichi, Bristol Myers Squibb, Gilead, Medscape, MSD, and Roche.
Dr. Hamilton disclosed a consulting advisory role (to her institution) for Accutar Biotechology, AstraZeneca, Daiichi Sankyo, Ellipses Pharma, Entos, Forsum Pharma, Gilead Sciences, Greenwich LifeSciences, Jazz Pharmaceuticals, Lilly, Medical Pharma Services, Mersana, Novartis, Olema Pharmaceuticals, Orum Therapeutics, Roche/Genentech, Stemline Therapeutics, ands others. She also disclosed contracted research/grant support to her institution only from Abbvie, Acerta Pharma, Accutar Biotechnology , ADC Therapeutics, AKESOBIO Australia , Amgen, Aravive, ArQule, Artios, Arvinas, AstraZeneca, AtlasMedx, BeiGene, Black Diamond and others.
Dr. Ciruelos disclosed serving as an external advisor for Roche, MSD, Gilead, AstraZeneca, Daichii Sankyo, Reveal Genomics, Pfizer, Novartis, and Lilly, as well as serving as a speaker for Roche, MSD, Gilead, AstraZeneca, Daichii Sankyo, Reveal Genomics, Pfizer, Novartis, Lilly, and Pierre Fabre. She also disclosed travel grants from Roche, Pfizer, and AstraZeneca, and research grants from Seagen and Roche.
Dr. May had no financial conflicts to disclose.
FROM ESMO BREAST CANCER 2024
Urine Tests Could Be ‘Enormous Step’ in Diagnosing Cancer
Emerging science suggests that the body’s “liquid gold” could be particularly useful for liquid biopsies, offering a convenient, pain-free, and cost-effective way to spot otherwise hard-to-detect cancers.
“The search for cancer biomarkers that can be detected in urine could provide an enormous step forward to decrease cancer patient mortality,” said Kenneth R. Shroyer, MD, PhD, a pathologist at Stony Brook University, Stony Brook, New York, who studies cancer biomarkers.
Physicians have long known that urine can reveal a lot about our health — that’s why urinalysis has been part of medicine for 6000 years. Urine tests can detect diabetes, pregnancy, drug use, and urinary or kidney conditions.
But other conditions leave clues in urine, too, and cancer may be one of the most promising. “Urine testing could detect biomarkers of early-stage cancers, not only from local but also distant sites,” Dr. Shroyer said. It could also help flag recurrence in cancer survivors who have undergone treatment.
Granted, cancer biomarkers in urine are not nearly as widely studied as those in the blood, Dr. Shroyer noted. But a new wave of urine tests suggests research is gaining pace.
“The recent availability of high-throughput screening technologies has enabled researchers to investigate cancer from a top-down, comprehensive approach,” said Pak Kin Wong, PhD, professor of mechanical engineering, biomedical engineering, and surgery at The Pennsylvania State University. “We are starting to understand the rich information that can be obtained from urine.”
Urine is mostly water (about 95%) and urea, a metabolic byproduct that imparts that signature yellow color (about 2%). The other 3% is a mix of waste products, minerals, and other compounds the kidneys removed from the blood. Even in trace amounts, these substances say a lot.
Among them are “exfoliated cancer cells, cell-free DNA, hormones, and the urine microbiota — the collection of microbes in our urinary tract system,” Dr. Wong said.
“It is highly promising to be one of the major biological fluids used for screening, diagnosis, prognosis, and monitoring treatment efficiency in the era of precision medicine,” Dr. Wong said.
How Urine Testing Could Reveal Cancer
Still, as exciting as the prospect is, there’s a lot to consider in the hunt for cancer biomarkers in urine. These biomarkers must be able to pass through the renal nephrons (filtering units), remain stable in urine, and have high-level sensitivity, Dr. Shroyer said. They should also have high specificity for cancer vs benign conditions and be expressed at early stages, before the primary tumor has spread.
“At this stage, few circulating biomarkers have been found that are both sensitive and specific for early-stage disease,” said Dr. Shroyer.
But there are a few promising examples under investigation in humans:
Prostate cancer. Researchers at the University of Michigan have developed a urine test that detects high-grade prostate cancer more accurately than existing tests, including PHI, SelectMDx, 4Kscore, EPI, MPS, and IsoPSA.
The MyProstateScore 2.0 (MPS2) test, which looks for 18 genes associated with high-grade tumors, could reduce unnecessary biopsies in men with elevated prostate-specific antigen levels, according to a paper published in JAMA Oncology.
It makes sense. The prostate gland secretes fluid that becomes part of the semen, traces of which enter urine. After a digital rectal exam, even more prostate fluid enters the urine. If a patient has prostate cancer, genetic material from the cancer cells will infiltrate the urine.
In the MPS2 test, researchers used polymerase chain reaction (PCR) testing in urine. “The technology used for COVID PCR is essentially the same as the PCR used to detect transcripts associated with high-grade prostate cancer in urine,” said study author Arul Chinnaiyan, MD, PhD, director of the Michigan Center for Translational Pathology at the University of Michigan, Ann Arbor. “In the case of the MPS2 test, we are doing PCR on 18 genes simultaneously on urine samples.”
A statistical model uses levels of that genetic material to predict the risk for high-grade disease, helping doctors decide what to do next. At 95% sensitivity, the MPS2 model could eliminate 35%-45% of unnecessary biopsies, compared with 15%-30% for the other tests, and reduce repeat biopsies by 46%-51%, compared with 9%-21% for the other tests.
Head and neck cancer. In a paper published in JCI Insight, researchers described a test that finds ultra-short fragments of DNA in urine to enable early detection of head and neck cancers caused by human papillomavirus.
“Our data show that a relatively small volume of urine (30-60 mL) gives overall detection results comparable to a tube of blood,” said study author Muneesh Tewari, MD, PhD, professor of hematology and oncology at the University of Michigan .
A larger volume of urine could potentially “make cancer detection even more sensitive than blood,” Dr. Tewari said, “allowing cancers to be detected at the earliest stages when they are more curable.”
The team used a technique called droplet digital PCR to detect DNA fragments that are “ultra-short” (less than 50 base pairs long) and usually missed by conventional PCR testing. This transrenal cell-free tumor DNA, which travels from the tumor into the bloodstream, is broken down small enough to pass through the kidneys and into the urine. But the fragments are still long enough to carry information about the tumor’s genetic signature.
This test could spot cancer before a tumor grows big enough — about a centimeter wide and carrying a billion cells — to spot on a CT scan or other imaging test. “When we are instead detecting fragments of DNA released from a tumor,” said Dr. Tewari, “our testing methods are very sensitive and can detect DNA in urine that came from just 5-10 cells in a tumor that died and released their DNA into the blood, which then made its way into the urine.”
Pancreatic cancer. Pancreatic ductal adenocarcinoma is one of the deadliest cancers, largely because it is diagnosed so late. A urine panel now in clinical trials could help doctors diagnose the cancer before it has spread so more people can have the tumor surgically removed, improving prognosis.
Using enzyme-linked immunosorbent assay test, a common lab method that detects antibodies and other proteins, the team measured expression levels for three genes (LYVE1, REG1B, and TFF1) in urine samples collected from people up to 5 years before they were diagnosed with pancreatic cancer. The researchers combined this result with patients’ urinary creatinine levels, a common component of existing urinalysis, and their age to develop a risk score.
This score performed similarly to an existing blood test, CA19-9, in predicting patients’ risk for pancreatic cancer up to 1 year before diagnosis. When combined with CA19-9, the urinary panel helped spot cancer up to 2 years before diagnosis.
According to a paper in the International Journal of Cancer, “the urine panel and affiliated PancRISK are currently being validated in a prospective clinical study (UroPanc).” If all goes well, they could be implemented in clinical practice in a few years as a “noninvasive stratification tool” to identify patients for further testing, speeding up diagnosis, and saving lives.
Limitations and Promises
Each cancer type is different, and more research is needed to map out which substances in urine predict which cancers and to develop tests for mass adoption. “There are medical and technological hurdles to the large-scale implementation of urine analysis for complex diseases such as cancer,” said Dr. Wong.
One possibility: Scientists and clinicians could collaborate and use artificial intelligence techniques to combine urine test results with other data.
“It is likely that future diagnostics may combine urine with other biological samples such as feces and saliva, among others,” said Dr. Wong. “This is especially true when novel data science and machine learning techniques can integrate comprehensive data from patients that span genetic, proteomic, metabolic, microbiomic, and even behavioral data to evaluate a patient’s condition.”
One thing that excites Dr. Tewari about urine-based cancer testing: “We think it could be especially impactful for patients living in rural areas or other areas with less access to healthcare services,” he said.
A version of this article appeared on Medscape.com.
Emerging science suggests that the body’s “liquid gold” could be particularly useful for liquid biopsies, offering a convenient, pain-free, and cost-effective way to spot otherwise hard-to-detect cancers.
“The search for cancer biomarkers that can be detected in urine could provide an enormous step forward to decrease cancer patient mortality,” said Kenneth R. Shroyer, MD, PhD, a pathologist at Stony Brook University, Stony Brook, New York, who studies cancer biomarkers.
Physicians have long known that urine can reveal a lot about our health — that’s why urinalysis has been part of medicine for 6000 years. Urine tests can detect diabetes, pregnancy, drug use, and urinary or kidney conditions.
But other conditions leave clues in urine, too, and cancer may be one of the most promising. “Urine testing could detect biomarkers of early-stage cancers, not only from local but also distant sites,” Dr. Shroyer said. It could also help flag recurrence in cancer survivors who have undergone treatment.
Granted, cancer biomarkers in urine are not nearly as widely studied as those in the blood, Dr. Shroyer noted. But a new wave of urine tests suggests research is gaining pace.
“The recent availability of high-throughput screening technologies has enabled researchers to investigate cancer from a top-down, comprehensive approach,” said Pak Kin Wong, PhD, professor of mechanical engineering, biomedical engineering, and surgery at The Pennsylvania State University. “We are starting to understand the rich information that can be obtained from urine.”
Urine is mostly water (about 95%) and urea, a metabolic byproduct that imparts that signature yellow color (about 2%). The other 3% is a mix of waste products, minerals, and other compounds the kidneys removed from the blood. Even in trace amounts, these substances say a lot.
Among them are “exfoliated cancer cells, cell-free DNA, hormones, and the urine microbiota — the collection of microbes in our urinary tract system,” Dr. Wong said.
“It is highly promising to be one of the major biological fluids used for screening, diagnosis, prognosis, and monitoring treatment efficiency in the era of precision medicine,” Dr. Wong said.
How Urine Testing Could Reveal Cancer
Still, as exciting as the prospect is, there’s a lot to consider in the hunt for cancer biomarkers in urine. These biomarkers must be able to pass through the renal nephrons (filtering units), remain stable in urine, and have high-level sensitivity, Dr. Shroyer said. They should also have high specificity for cancer vs benign conditions and be expressed at early stages, before the primary tumor has spread.
“At this stage, few circulating biomarkers have been found that are both sensitive and specific for early-stage disease,” said Dr. Shroyer.
But there are a few promising examples under investigation in humans:
Prostate cancer. Researchers at the University of Michigan have developed a urine test that detects high-grade prostate cancer more accurately than existing tests, including PHI, SelectMDx, 4Kscore, EPI, MPS, and IsoPSA.
The MyProstateScore 2.0 (MPS2) test, which looks for 18 genes associated with high-grade tumors, could reduce unnecessary biopsies in men with elevated prostate-specific antigen levels, according to a paper published in JAMA Oncology.
It makes sense. The prostate gland secretes fluid that becomes part of the semen, traces of which enter urine. After a digital rectal exam, even more prostate fluid enters the urine. If a patient has prostate cancer, genetic material from the cancer cells will infiltrate the urine.
In the MPS2 test, researchers used polymerase chain reaction (PCR) testing in urine. “The technology used for COVID PCR is essentially the same as the PCR used to detect transcripts associated with high-grade prostate cancer in urine,” said study author Arul Chinnaiyan, MD, PhD, director of the Michigan Center for Translational Pathology at the University of Michigan, Ann Arbor. “In the case of the MPS2 test, we are doing PCR on 18 genes simultaneously on urine samples.”
A statistical model uses levels of that genetic material to predict the risk for high-grade disease, helping doctors decide what to do next. At 95% sensitivity, the MPS2 model could eliminate 35%-45% of unnecessary biopsies, compared with 15%-30% for the other tests, and reduce repeat biopsies by 46%-51%, compared with 9%-21% for the other tests.
Head and neck cancer. In a paper published in JCI Insight, researchers described a test that finds ultra-short fragments of DNA in urine to enable early detection of head and neck cancers caused by human papillomavirus.
“Our data show that a relatively small volume of urine (30-60 mL) gives overall detection results comparable to a tube of blood,” said study author Muneesh Tewari, MD, PhD, professor of hematology and oncology at the University of Michigan .
A larger volume of urine could potentially “make cancer detection even more sensitive than blood,” Dr. Tewari said, “allowing cancers to be detected at the earliest stages when they are more curable.”
The team used a technique called droplet digital PCR to detect DNA fragments that are “ultra-short” (less than 50 base pairs long) and usually missed by conventional PCR testing. This transrenal cell-free tumor DNA, which travels from the tumor into the bloodstream, is broken down small enough to pass through the kidneys and into the urine. But the fragments are still long enough to carry information about the tumor’s genetic signature.
This test could spot cancer before a tumor grows big enough — about a centimeter wide and carrying a billion cells — to spot on a CT scan or other imaging test. “When we are instead detecting fragments of DNA released from a tumor,” said Dr. Tewari, “our testing methods are very sensitive and can detect DNA in urine that came from just 5-10 cells in a tumor that died and released their DNA into the blood, which then made its way into the urine.”
Pancreatic cancer. Pancreatic ductal adenocarcinoma is one of the deadliest cancers, largely because it is diagnosed so late. A urine panel now in clinical trials could help doctors diagnose the cancer before it has spread so more people can have the tumor surgically removed, improving prognosis.
Using enzyme-linked immunosorbent assay test, a common lab method that detects antibodies and other proteins, the team measured expression levels for three genes (LYVE1, REG1B, and TFF1) in urine samples collected from people up to 5 years before they were diagnosed with pancreatic cancer. The researchers combined this result with patients’ urinary creatinine levels, a common component of existing urinalysis, and their age to develop a risk score.
This score performed similarly to an existing blood test, CA19-9, in predicting patients’ risk for pancreatic cancer up to 1 year before diagnosis. When combined with CA19-9, the urinary panel helped spot cancer up to 2 years before diagnosis.
According to a paper in the International Journal of Cancer, “the urine panel and affiliated PancRISK are currently being validated in a prospective clinical study (UroPanc).” If all goes well, they could be implemented in clinical practice in a few years as a “noninvasive stratification tool” to identify patients for further testing, speeding up diagnosis, and saving lives.
Limitations and Promises
Each cancer type is different, and more research is needed to map out which substances in urine predict which cancers and to develop tests for mass adoption. “There are medical and technological hurdles to the large-scale implementation of urine analysis for complex diseases such as cancer,” said Dr. Wong.
One possibility: Scientists and clinicians could collaborate and use artificial intelligence techniques to combine urine test results with other data.
“It is likely that future diagnostics may combine urine with other biological samples such as feces and saliva, among others,” said Dr. Wong. “This is especially true when novel data science and machine learning techniques can integrate comprehensive data from patients that span genetic, proteomic, metabolic, microbiomic, and even behavioral data to evaluate a patient’s condition.”
One thing that excites Dr. Tewari about urine-based cancer testing: “We think it could be especially impactful for patients living in rural areas or other areas with less access to healthcare services,” he said.
A version of this article appeared on Medscape.com.
Emerging science suggests that the body’s “liquid gold” could be particularly useful for liquid biopsies, offering a convenient, pain-free, and cost-effective way to spot otherwise hard-to-detect cancers.
“The search for cancer biomarkers that can be detected in urine could provide an enormous step forward to decrease cancer patient mortality,” said Kenneth R. Shroyer, MD, PhD, a pathologist at Stony Brook University, Stony Brook, New York, who studies cancer biomarkers.
Physicians have long known that urine can reveal a lot about our health — that’s why urinalysis has been part of medicine for 6000 years. Urine tests can detect diabetes, pregnancy, drug use, and urinary or kidney conditions.
But other conditions leave clues in urine, too, and cancer may be one of the most promising. “Urine testing could detect biomarkers of early-stage cancers, not only from local but also distant sites,” Dr. Shroyer said. It could also help flag recurrence in cancer survivors who have undergone treatment.
Granted, cancer biomarkers in urine are not nearly as widely studied as those in the blood, Dr. Shroyer noted. But a new wave of urine tests suggests research is gaining pace.
“The recent availability of high-throughput screening technologies has enabled researchers to investigate cancer from a top-down, comprehensive approach,” said Pak Kin Wong, PhD, professor of mechanical engineering, biomedical engineering, and surgery at The Pennsylvania State University. “We are starting to understand the rich information that can be obtained from urine.”
Urine is mostly water (about 95%) and urea, a metabolic byproduct that imparts that signature yellow color (about 2%). The other 3% is a mix of waste products, minerals, and other compounds the kidneys removed from the blood. Even in trace amounts, these substances say a lot.
Among them are “exfoliated cancer cells, cell-free DNA, hormones, and the urine microbiota — the collection of microbes in our urinary tract system,” Dr. Wong said.
“It is highly promising to be one of the major biological fluids used for screening, diagnosis, prognosis, and monitoring treatment efficiency in the era of precision medicine,” Dr. Wong said.
How Urine Testing Could Reveal Cancer
Still, as exciting as the prospect is, there’s a lot to consider in the hunt for cancer biomarkers in urine. These biomarkers must be able to pass through the renal nephrons (filtering units), remain stable in urine, and have high-level sensitivity, Dr. Shroyer said. They should also have high specificity for cancer vs benign conditions and be expressed at early stages, before the primary tumor has spread.
“At this stage, few circulating biomarkers have been found that are both sensitive and specific for early-stage disease,” said Dr. Shroyer.
But there are a few promising examples under investigation in humans:
Prostate cancer. Researchers at the University of Michigan have developed a urine test that detects high-grade prostate cancer more accurately than existing tests, including PHI, SelectMDx, 4Kscore, EPI, MPS, and IsoPSA.
The MyProstateScore 2.0 (MPS2) test, which looks for 18 genes associated with high-grade tumors, could reduce unnecessary biopsies in men with elevated prostate-specific antigen levels, according to a paper published in JAMA Oncology.
It makes sense. The prostate gland secretes fluid that becomes part of the semen, traces of which enter urine. After a digital rectal exam, even more prostate fluid enters the urine. If a patient has prostate cancer, genetic material from the cancer cells will infiltrate the urine.
In the MPS2 test, researchers used polymerase chain reaction (PCR) testing in urine. “The technology used for COVID PCR is essentially the same as the PCR used to detect transcripts associated with high-grade prostate cancer in urine,” said study author Arul Chinnaiyan, MD, PhD, director of the Michigan Center for Translational Pathology at the University of Michigan, Ann Arbor. “In the case of the MPS2 test, we are doing PCR on 18 genes simultaneously on urine samples.”
A statistical model uses levels of that genetic material to predict the risk for high-grade disease, helping doctors decide what to do next. At 95% sensitivity, the MPS2 model could eliminate 35%-45% of unnecessary biopsies, compared with 15%-30% for the other tests, and reduce repeat biopsies by 46%-51%, compared with 9%-21% for the other tests.
Head and neck cancer. In a paper published in JCI Insight, researchers described a test that finds ultra-short fragments of DNA in urine to enable early detection of head and neck cancers caused by human papillomavirus.
“Our data show that a relatively small volume of urine (30-60 mL) gives overall detection results comparable to a tube of blood,” said study author Muneesh Tewari, MD, PhD, professor of hematology and oncology at the University of Michigan .
A larger volume of urine could potentially “make cancer detection even more sensitive than blood,” Dr. Tewari said, “allowing cancers to be detected at the earliest stages when they are more curable.”
The team used a technique called droplet digital PCR to detect DNA fragments that are “ultra-short” (less than 50 base pairs long) and usually missed by conventional PCR testing. This transrenal cell-free tumor DNA, which travels from the tumor into the bloodstream, is broken down small enough to pass through the kidneys and into the urine. But the fragments are still long enough to carry information about the tumor’s genetic signature.
This test could spot cancer before a tumor grows big enough — about a centimeter wide and carrying a billion cells — to spot on a CT scan or other imaging test. “When we are instead detecting fragments of DNA released from a tumor,” said Dr. Tewari, “our testing methods are very sensitive and can detect DNA in urine that came from just 5-10 cells in a tumor that died and released their DNA into the blood, which then made its way into the urine.”
Pancreatic cancer. Pancreatic ductal adenocarcinoma is one of the deadliest cancers, largely because it is diagnosed so late. A urine panel now in clinical trials could help doctors diagnose the cancer before it has spread so more people can have the tumor surgically removed, improving prognosis.
Using enzyme-linked immunosorbent assay test, a common lab method that detects antibodies and other proteins, the team measured expression levels for three genes (LYVE1, REG1B, and TFF1) in urine samples collected from people up to 5 years before they were diagnosed with pancreatic cancer. The researchers combined this result with patients’ urinary creatinine levels, a common component of existing urinalysis, and their age to develop a risk score.
This score performed similarly to an existing blood test, CA19-9, in predicting patients’ risk for pancreatic cancer up to 1 year before diagnosis. When combined with CA19-9, the urinary panel helped spot cancer up to 2 years before diagnosis.
According to a paper in the International Journal of Cancer, “the urine panel and affiliated PancRISK are currently being validated in a prospective clinical study (UroPanc).” If all goes well, they could be implemented in clinical practice in a few years as a “noninvasive stratification tool” to identify patients for further testing, speeding up diagnosis, and saving lives.
Limitations and Promises
Each cancer type is different, and more research is needed to map out which substances in urine predict which cancers and to develop tests for mass adoption. “There are medical and technological hurdles to the large-scale implementation of urine analysis for complex diseases such as cancer,” said Dr. Wong.
One possibility: Scientists and clinicians could collaborate and use artificial intelligence techniques to combine urine test results with other data.
“It is likely that future diagnostics may combine urine with other biological samples such as feces and saliva, among others,” said Dr. Wong. “This is especially true when novel data science and machine learning techniques can integrate comprehensive data from patients that span genetic, proteomic, metabolic, microbiomic, and even behavioral data to evaluate a patient’s condition.”
One thing that excites Dr. Tewari about urine-based cancer testing: “We think it could be especially impactful for patients living in rural areas or other areas with less access to healthcare services,” he said.
A version of this article appeared on Medscape.com.
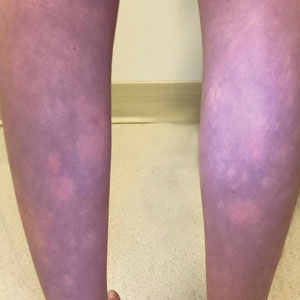
Transient Symmetric Blanching Macules on a Background of Reticulate Erythema
The Diagnosis: BASCULE Syndrome
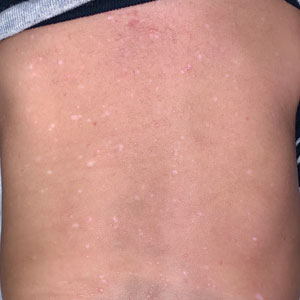
The patient had previously been thought to have livedo reticularis by primary care. Repeat antinuclear antibody (ANA) testing was positive (1:1280 homogeneous [reflexive titers all negative]). However, upon dermatologic evaluation, the manifestation of the rash in addition to onset occurring with postural changes challenged the livedo reticularis diagnosis. Extensive research and consultation with dermatologic colleagues led to the diagnosis of the rare entity BASCULE syndrome. BASCULE (Bier anemic spots, cyanosis, and urticarialike eruption) syndrome was described by Bessis et al1 in 2016. It is a rare condition but may be underreported.2 It is a benign pediatric disorder in the vascular acrosyndrome family that is characterized by underlying vasomotor dysfunction in distal regions of the body. Raynaud phenomenon is a widely known member of this family. As seen in our patient, it typically presents on the distal legs and feet with numerous irregular hypopigmented macules on a cyanotic background. Red-orange papules may appear on the hypopigmented macules and often are pruritic. Lesions on the distal upper extremities are less common, and a case involving the trunk has been reported.3 Onset generally begins within a couple of minutes of standing or mechanical compression of the lower legs, with full reversal of symptoms occurring within minutes of laying down or walking. Commonly reported associated symptoms include tenderness, pruritus, edema, and pain; however, the cutaneous lesions may be asymptomatic. The condition tends to affect adolescents, as seen in our patient; however, there have been reports in infants as young as 3 months to adults aged 19 years.2
The pathophysiology behind BASCULE syndrome remains unclear but is believed to be centered around the role of physiologic venous stasis that occurs when standing. The hypoxia secondary to stasis is thought to induce amplified vasoconstriction of arterioles. These responses are further exaggerated due to absence of venoarteriolar reflexes in dermal ascending arterioles, leading to Bier spots.2 The role of mast cells and eosinophils remains unclear. It is a clinical diagnosis without clear histologic findings; therefore, biopsy was not pursued in our patient.
Although BASCULE syndrome is a benign entity, it is imperative that it be recognized to avoid a time consuming, expensive, and anxiety-producing diagnostic workup, as occurred in our patient. Although not a manifestation of systemic disease, BASCULE syndrome may be associated with orthostatic hypotension in up to 20% of cases.2,4 Therefore, these patients should undergo orthostatic testing, including the tilt table test. In our patient, these manifestations were not appreciated.
There are no current guidelines for effective treatment of BASCULE syndrome. Given the possible role of mast cells in the condition, H1 antihistamines are proposed as first-line treatment. Desloratadine (10 mg/d for 7 days) has been found to be associated with improvement of pruritus. However, a recent literature review found little evidence to support the use of H1 antihistamines for resolution of other symptoms.2
The differential diagnosis includes livedo reticularis, Bier spots, Sneddon syndrome, and urticarial vasculitis. Livedo reticularis presents as distinct, netlike, blue-erythematousviolaceous discoloration, which differs from the distinct orange-red macules in BASCULE syndrome.5 In addition to distinct variances in dermatologic presentation, livedo reticularis typically is associated with cold exposure as a causative agent, with cold avoidance as the treatment for this benign and often transient condition.6 This phenomenon was not appreciated in our patient. Livedo reticularis commonly occurs with antiphospholipid syndrome.5 This association in combination with our patient's positive ANA findings and her mother's history of miscarriages resulted in the misdiagnosis as livedo reticularis.
Bier spots manifest as white macules with surrounding erythema and typically present in young adults. When first described in the literature, it was debated if BASCULE syndrome was simply another manifestation of Bier spots or postural orthostatic intolerance,4 as there was a large consensus that postural orthostatic intolerance was associated with BASCULE syndrome, with the majority of patients not meeting criteria for the condition. Heymann4 addressed the differences in BASCULE manifestations vs typical Bier spots. The author extended the syndrome to include cyanosis, an urticarialike eruption of red-orange macules with central papules located centrally, pruritus, tenderness, and partial or diffuse edema, in addition to Bier spots.4
Sneddon syndrome is a rare progressive disorder that affects small- to medium-sized blood vessels resulting in multiple episodes of ischemia in the brain. Skin manifestations of these repeated strokes are similar to livedo reticularis, typically manifesting as livedo racemosa—irregular reticular patterns of skin mottling with reddish-blue hues.6 However, Sneddon syndrome is more generalized and widespread and differs from BASCULE syndrome in shape and histologic findings. Our patient presented with findings on the legs, which is more characteristic of livedo reticularis vs livedo racemosa. Our patient experienced resolution upon laying down and sitting, and Sneddon syndrome persists beyond postural changes. Furthermore, patients with Sneddon syndrome present with neurologic symptoms such as prodromal headaches.6
Urticarial vasculitis was ruled out in our patient because of the duration of symptoms as well as the spatial changes. Urticarial vasculitis is a rare skin condition characterized by chronic recurring urticarial lesions that may persist for more than a day. This condition typically presents in middle-aged women and rarely in children. Urticarial vasculitis is thought to be immune-complex mediated, but its cause is largely unknown. It is a common manifestation of underlying conditions such as systemic lupus erythematosus.6 Our patient had a positive ANA and possible autoimmune history from her mother; however, urticarial vasculitis does not present transiently on the legs or in the rash pattern appreciated in our patient.
- Bessis D, Jeziorski E, Rigau V, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome: a new entity? Br J Dermatol. 2016;175:218-220. doi:10.1111/bjd.14589
- Baurens N, Briand C, Giovannini-Chami L, et al. Case report, practices survey and literature review of an under-recognized pediatric vascular disorder: the BASCULE syndrome. Front Pediatr. 2022;10:849914. doi:10.3389/fped.2022.849914
- Jiménez-Gallo D, Collantes-Rodríguez C, Ossorio-García L, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome on trunk and upper limbs. Pediatr Dermatol. 2018;35:E313-E315. doi:10.1111/pde.13558
- Heymann WR. BASCULE syndrome: is something brewing with Bier spots? Dermatology World Insights and Inquiries. September 7, 2022. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2022/bascule-syndrome
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Gu SL, Jorizzo JL. Urticarial vasculitis. Int J Womens Dermatol. 2021;7:290-297. doi:10.1016/j.ijwd.2021.01.021
The Diagnosis: BASCULE Syndrome
The patient had previously been thought to have livedo reticularis by primary care. Repeat antinuclear antibody (ANA) testing was positive (1:1280 homogeneous [reflexive titers all negative]). However, upon dermatologic evaluation, the manifestation of the rash in addition to onset occurring with postural changes challenged the livedo reticularis diagnosis. Extensive research and consultation with dermatologic colleagues led to the diagnosis of the rare entity BASCULE syndrome. BASCULE (Bier anemic spots, cyanosis, and urticarialike eruption) syndrome was described by Bessis et al1 in 2016. It is a rare condition but may be underreported.2 It is a benign pediatric disorder in the vascular acrosyndrome family that is characterized by underlying vasomotor dysfunction in distal regions of the body. Raynaud phenomenon is a widely known member of this family. As seen in our patient, it typically presents on the distal legs and feet with numerous irregular hypopigmented macules on a cyanotic background. Red-orange papules may appear on the hypopigmented macules and often are pruritic. Lesions on the distal upper extremities are less common, and a case involving the trunk has been reported.3 Onset generally begins within a couple of minutes of standing or mechanical compression of the lower legs, with full reversal of symptoms occurring within minutes of laying down or walking. Commonly reported associated symptoms include tenderness, pruritus, edema, and pain; however, the cutaneous lesions may be asymptomatic. The condition tends to affect adolescents, as seen in our patient; however, there have been reports in infants as young as 3 months to adults aged 19 years.2
The pathophysiology behind BASCULE syndrome remains unclear but is believed to be centered around the role of physiologic venous stasis that occurs when standing. The hypoxia secondary to stasis is thought to induce amplified vasoconstriction of arterioles. These responses are further exaggerated due to absence of venoarteriolar reflexes in dermal ascending arterioles, leading to Bier spots.2 The role of mast cells and eosinophils remains unclear. It is a clinical diagnosis without clear histologic findings; therefore, biopsy was not pursued in our patient.
Although BASCULE syndrome is a benign entity, it is imperative that it be recognized to avoid a time consuming, expensive, and anxiety-producing diagnostic workup, as occurred in our patient. Although not a manifestation of systemic disease, BASCULE syndrome may be associated with orthostatic hypotension in up to 20% of cases.2,4 Therefore, these patients should undergo orthostatic testing, including the tilt table test. In our patient, these manifestations were not appreciated.
There are no current guidelines for effective treatment of BASCULE syndrome. Given the possible role of mast cells in the condition, H1 antihistamines are proposed as first-line treatment. Desloratadine (10 mg/d for 7 days) has been found to be associated with improvement of pruritus. However, a recent literature review found little evidence to support the use of H1 antihistamines for resolution of other symptoms.2
The differential diagnosis includes livedo reticularis, Bier spots, Sneddon syndrome, and urticarial vasculitis. Livedo reticularis presents as distinct, netlike, blue-erythematousviolaceous discoloration, which differs from the distinct orange-red macules in BASCULE syndrome.5 In addition to distinct variances in dermatologic presentation, livedo reticularis typically is associated with cold exposure as a causative agent, with cold avoidance as the treatment for this benign and often transient condition.6 This phenomenon was not appreciated in our patient. Livedo reticularis commonly occurs with antiphospholipid syndrome.5 This association in combination with our patient's positive ANA findings and her mother's history of miscarriages resulted in the misdiagnosis as livedo reticularis.
Bier spots manifest as white macules with surrounding erythema and typically present in young adults. When first described in the literature, it was debated if BASCULE syndrome was simply another manifestation of Bier spots or postural orthostatic intolerance,4 as there was a large consensus that postural orthostatic intolerance was associated with BASCULE syndrome, with the majority of patients not meeting criteria for the condition. Heymann4 addressed the differences in BASCULE manifestations vs typical Bier spots. The author extended the syndrome to include cyanosis, an urticarialike eruption of red-orange macules with central papules located centrally, pruritus, tenderness, and partial or diffuse edema, in addition to Bier spots.4
Sneddon syndrome is a rare progressive disorder that affects small- to medium-sized blood vessels resulting in multiple episodes of ischemia in the brain. Skin manifestations of these repeated strokes are similar to livedo reticularis, typically manifesting as livedo racemosa—irregular reticular patterns of skin mottling with reddish-blue hues.6 However, Sneddon syndrome is more generalized and widespread and differs from BASCULE syndrome in shape and histologic findings. Our patient presented with findings on the legs, which is more characteristic of livedo reticularis vs livedo racemosa. Our patient experienced resolution upon laying down and sitting, and Sneddon syndrome persists beyond postural changes. Furthermore, patients with Sneddon syndrome present with neurologic symptoms such as prodromal headaches.6
Urticarial vasculitis was ruled out in our patient because of the duration of symptoms as well as the spatial changes. Urticarial vasculitis is a rare skin condition characterized by chronic recurring urticarial lesions that may persist for more than a day. This condition typically presents in middle-aged women and rarely in children. Urticarial vasculitis is thought to be immune-complex mediated, but its cause is largely unknown. It is a common manifestation of underlying conditions such as systemic lupus erythematosus.6 Our patient had a positive ANA and possible autoimmune history from her mother; however, urticarial vasculitis does not present transiently on the legs or in the rash pattern appreciated in our patient.
The Diagnosis: BASCULE Syndrome
The patient had previously been thought to have livedo reticularis by primary care. Repeat antinuclear antibody (ANA) testing was positive (1:1280 homogeneous [reflexive titers all negative]). However, upon dermatologic evaluation, the manifestation of the rash in addition to onset occurring with postural changes challenged the livedo reticularis diagnosis. Extensive research and consultation with dermatologic colleagues led to the diagnosis of the rare entity BASCULE syndrome. BASCULE (Bier anemic spots, cyanosis, and urticarialike eruption) syndrome was described by Bessis et al1 in 2016. It is a rare condition but may be underreported.2 It is a benign pediatric disorder in the vascular acrosyndrome family that is characterized by underlying vasomotor dysfunction in distal regions of the body. Raynaud phenomenon is a widely known member of this family. As seen in our patient, it typically presents on the distal legs and feet with numerous irregular hypopigmented macules on a cyanotic background. Red-orange papules may appear on the hypopigmented macules and often are pruritic. Lesions on the distal upper extremities are less common, and a case involving the trunk has been reported.3 Onset generally begins within a couple of minutes of standing or mechanical compression of the lower legs, with full reversal of symptoms occurring within minutes of laying down or walking. Commonly reported associated symptoms include tenderness, pruritus, edema, and pain; however, the cutaneous lesions may be asymptomatic. The condition tends to affect adolescents, as seen in our patient; however, there have been reports in infants as young as 3 months to adults aged 19 years.2
The pathophysiology behind BASCULE syndrome remains unclear but is believed to be centered around the role of physiologic venous stasis that occurs when standing. The hypoxia secondary to stasis is thought to induce amplified vasoconstriction of arterioles. These responses are further exaggerated due to absence of venoarteriolar reflexes in dermal ascending arterioles, leading to Bier spots.2 The role of mast cells and eosinophils remains unclear. It is a clinical diagnosis without clear histologic findings; therefore, biopsy was not pursued in our patient.
Although BASCULE syndrome is a benign entity, it is imperative that it be recognized to avoid a time consuming, expensive, and anxiety-producing diagnostic workup, as occurred in our patient. Although not a manifestation of systemic disease, BASCULE syndrome may be associated with orthostatic hypotension in up to 20% of cases.2,4 Therefore, these patients should undergo orthostatic testing, including the tilt table test. In our patient, these manifestations were not appreciated.
There are no current guidelines for effective treatment of BASCULE syndrome. Given the possible role of mast cells in the condition, H1 antihistamines are proposed as first-line treatment. Desloratadine (10 mg/d for 7 days) has been found to be associated with improvement of pruritus. However, a recent literature review found little evidence to support the use of H1 antihistamines for resolution of other symptoms.2
The differential diagnosis includes livedo reticularis, Bier spots, Sneddon syndrome, and urticarial vasculitis. Livedo reticularis presents as distinct, netlike, blue-erythematousviolaceous discoloration, which differs from the distinct orange-red macules in BASCULE syndrome.5 In addition to distinct variances in dermatologic presentation, livedo reticularis typically is associated with cold exposure as a causative agent, with cold avoidance as the treatment for this benign and often transient condition.6 This phenomenon was not appreciated in our patient. Livedo reticularis commonly occurs with antiphospholipid syndrome.5 This association in combination with our patient's positive ANA findings and her mother's history of miscarriages resulted in the misdiagnosis as livedo reticularis.
Bier spots manifest as white macules with surrounding erythema and typically present in young adults. When first described in the literature, it was debated if BASCULE syndrome was simply another manifestation of Bier spots or postural orthostatic intolerance,4 as there was a large consensus that postural orthostatic intolerance was associated with BASCULE syndrome, with the majority of patients not meeting criteria for the condition. Heymann4 addressed the differences in BASCULE manifestations vs typical Bier spots. The author extended the syndrome to include cyanosis, an urticarialike eruption of red-orange macules with central papules located centrally, pruritus, tenderness, and partial or diffuse edema, in addition to Bier spots.4
Sneddon syndrome is a rare progressive disorder that affects small- to medium-sized blood vessels resulting in multiple episodes of ischemia in the brain. Skin manifestations of these repeated strokes are similar to livedo reticularis, typically manifesting as livedo racemosa—irregular reticular patterns of skin mottling with reddish-blue hues.6 However, Sneddon syndrome is more generalized and widespread and differs from BASCULE syndrome in shape and histologic findings. Our patient presented with findings on the legs, which is more characteristic of livedo reticularis vs livedo racemosa. Our patient experienced resolution upon laying down and sitting, and Sneddon syndrome persists beyond postural changes. Furthermore, patients with Sneddon syndrome present with neurologic symptoms such as prodromal headaches.6
Urticarial vasculitis was ruled out in our patient because of the duration of symptoms as well as the spatial changes. Urticarial vasculitis is a rare skin condition characterized by chronic recurring urticarial lesions that may persist for more than a day. This condition typically presents in middle-aged women and rarely in children. Urticarial vasculitis is thought to be immune-complex mediated, but its cause is largely unknown. It is a common manifestation of underlying conditions such as systemic lupus erythematosus.6 Our patient had a positive ANA and possible autoimmune history from her mother; however, urticarial vasculitis does not present transiently on the legs or in the rash pattern appreciated in our patient.
- Bessis D, Jeziorski E, Rigau V, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome: a new entity? Br J Dermatol. 2016;175:218-220. doi:10.1111/bjd.14589
- Baurens N, Briand C, Giovannini-Chami L, et al. Case report, practices survey and literature review of an under-recognized pediatric vascular disorder: the BASCULE syndrome. Front Pediatr. 2022;10:849914. doi:10.3389/fped.2022.849914
- Jiménez-Gallo D, Collantes-Rodríguez C, Ossorio-García L, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome on trunk and upper limbs. Pediatr Dermatol. 2018;35:E313-E315. doi:10.1111/pde.13558
- Heymann WR. BASCULE syndrome: is something brewing with Bier spots? Dermatology World Insights and Inquiries. September 7, 2022. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2022/bascule-syndrome
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Gu SL, Jorizzo JL. Urticarial vasculitis. Int J Womens Dermatol. 2021;7:290-297. doi:10.1016/j.ijwd.2021.01.021
- Bessis D, Jeziorski E, Rigau V, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome: a new entity? Br J Dermatol. 2016;175:218-220. doi:10.1111/bjd.14589
- Baurens N, Briand C, Giovannini-Chami L, et al. Case report, practices survey and literature review of an under-recognized pediatric vascular disorder: the BASCULE syndrome. Front Pediatr. 2022;10:849914. doi:10.3389/fped.2022.849914
- Jiménez-Gallo D, Collantes-Rodríguez C, Ossorio-García L, et al. Bier anaemic spots, cyanosis with urticaria-like eruption (BASCULE) syndrome on trunk and upper limbs. Pediatr Dermatol. 2018;35:E313-E315. doi:10.1111/pde.13558
- Heymann WR. BASCULE syndrome: is something brewing with Bier spots? Dermatology World Insights and Inquiries. September 7, 2022. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2022/bascule-syndrome
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Gu SL, Jorizzo JL. Urticarial vasculitis. Int J Womens Dermatol. 2021;7:290-297. doi:10.1016/j.ijwd.2021.01.021
An 11-year-old girl was referred to the dermatology clinic for evaluation of a rash on the legs and feet of 1 year’s duration. The rash appeared every time she was standing for longer than 10 to 15 minutes and resolved when sitting or laying down. After the initial onset, the rash did not spread to other body areas but became more prominent in appearance. The patient endorsed intense pruritus associated with the rash. A review of systems was negative for fever, headaches, history of blood clots, and joint pain. She did not have any known medical conditions or take any medications. The patient’s mother reported that the patient experienced episodes of leg numbness while sitting in vehicles from 6 to 10 years of age. There was no family history of rheumatologic, hematologic, or cardiac conditions. The patient’s mother had experienced 2 miscarriages but denied any other obstetric complications. The patient had 1 sibling who was unaffected. Physical examination revealed reticulate erythema on the calves with scattered regions of blanching and evanescent pink macules as well as dermatographism.
One month prior to presenting to dermatology, the patient was evaluated by rheumatology, endocrinology, and hematology. Laboratory workup completed at age 3 years included antinuclear antibody, anticardiolipin antibody, and antithrombin III activity; factor V Leiden; cryoglobulins; quantitation (human chorionic gonadotropin); proteins S and C activity; antineutrophil cytoplasmic antibody screen; thyroid studies; prothrombin time; and partial thromboplastin time. All laboratory results were within reference range.
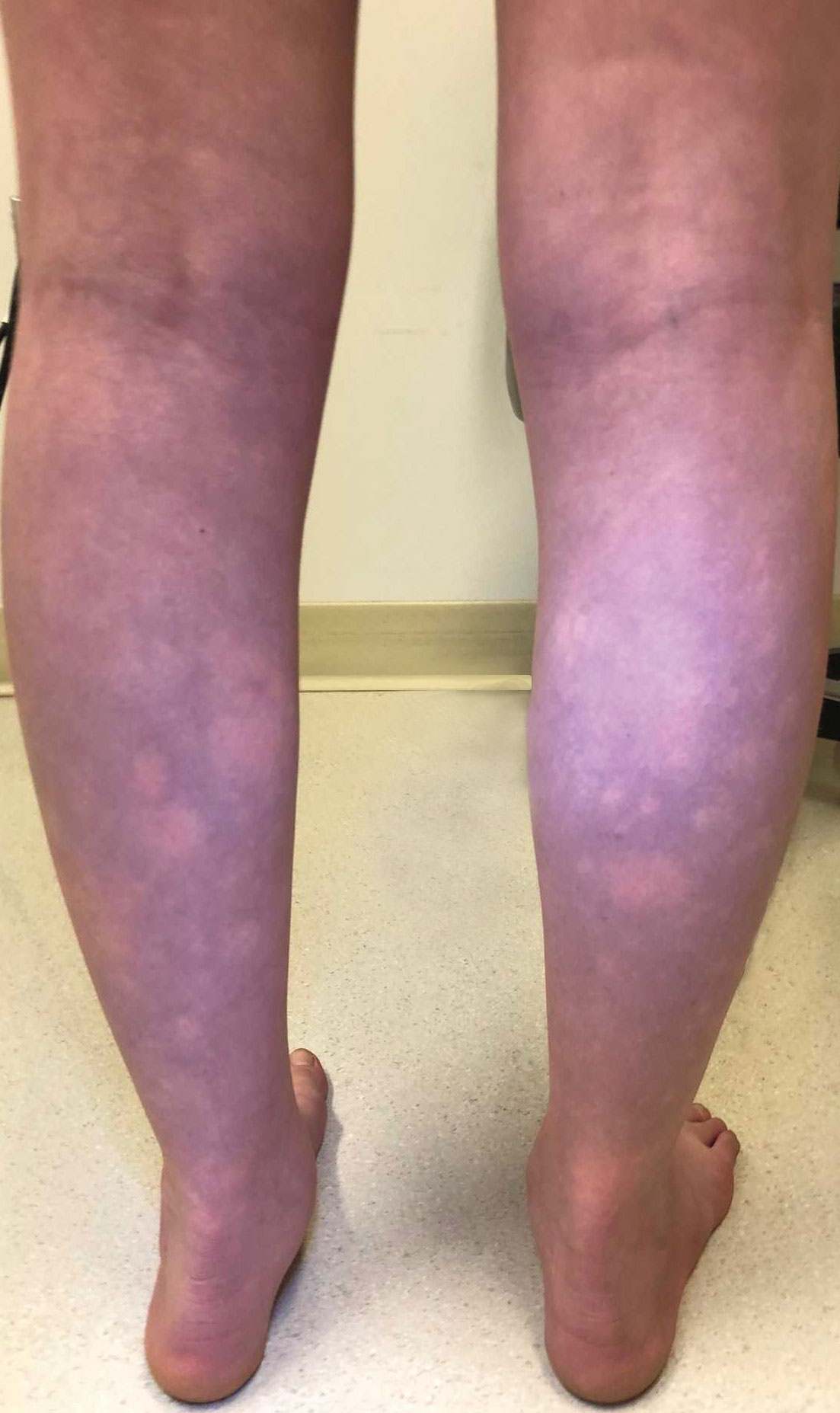
AAP Shifts Stance, Updates Guidance on Breastfeeding With HIV
aside from avoiding breastfeeding altogether, according to a new clinical report from the American Academy of Pediatrics (AAP).
“The risk of HIV transmission via breastfeeding from a parent with HIV who is receiving antiretroviral treatment (ART) and is virally suppressed is estimated to be less than 1%,” Lisa Abuogi, MD, an associate professor of pediatric infectious disease at the University of Colorado Anschutz Medical Campus, and her colleagues wrote in Pediatrics. For people living with HIV in the United States, however, the AAP recommends that “avoidance of breastfeeding is the only infant feeding option with 0% risk of HIV transmission.”
The authors go on to suggest that pediatricians “be prepared to offer a family-centered, nonjudgmental, harm reduction approach” to support people with HIV who do want to breastfeed and have sustained viral suppression. Parents with HIV who are not on ART or who do not have adequate viral suppression should be advised against breastfeeding, the report states. Members of the AAP Committee on Pediatric and Adolescent HIV and of the AAP Section on Breastfeeding coauthored the clinical report.
“The new guidelines emphasize the importance of patient-centered counseling as the foundation for shared decision-making, allowing patients and pediatric providers to make feeding decisions together and for the first time really giving support to people with HIV in the U.S. who want to breastfeed,” Danna Biala, MD, MS, an attending pediatrician at Children’s Hospital at Montefiore and an assistant professor at Albert Einstein College of Medicine, told MDedge News.
Dr. Biala was not involved in the development of the report, but she said the AAP’s guidance reflects the recent shift in the stance of the Centers for Disease Control and Prevention (CDC) regarding breastfeeding among people who are HIV+. Recommendations from the CDC and the U.S. Department of Health and Human Services (HHS) were updated in 2023.
“I’m glad that the AAP is putting out guidelines on infant feeding for people with HIV,” Dr. Biala said. “For so long in the U.S., pediatricians have been advising all mothers with HIV to avoid breastfeeding, believing that the risk of transmission outweighed the benefits of breastfeeding.”
The updated guidance from HHS in 2023 “was revolutionary in supporting people with HIV in low-risk situations who want to breastfeed,” Dr. Biala said, but “clear protocols for monitoring and follow-up were not in place,” which these AAP guidelines help address.
Prior Discordance Between Global, U.S. Guidance
An estimated 5,000 people with HIV give birth each year in the United States, and up to one third of pregnant people with HIV may be unaware of their HIV status, the AAP report notes. Pediatric healthcare professionals in the United States therefore need to be aware of recommendations related to HIV testing of pregnant people and of counseling the feeding of infants exposed to HIV. The report recommends opt-out HIV testing at the first prenatal visit and then possibly retesting in the third trimester in areas with high HIV incidence or for people at high risk for HIV or with signs or symptoms of acute HIV infection.
The report also highlights the health benefits of breastfeeding to both the infant and the breastfeeding parent, but notes the CDC’s historical recommendation against breastfeeding for people who are HIV+. The WHO, meanwhile, began recommending in 2016 that infants be breastfed through 12 to 24 months old if the parent was on ART and/or the infant was receiving antiretroviral (ARV) prophylaxis, since research showed those treatments were effective in reducing transmission risk.
Still, an estimated 30% of perinatal HIV transmission occurs via breastfeeding worldwide, primarily from people with HIV who are not on ART or are not adequately virally suppressed. Without parental ART or infant ARV prophylaxis, HIV transmission risk to infants via breastfeeding is highest, about 5%-6%, in the first 4-6 weeks of life. Risk then declines to about 0.9% a month thereafter. The AAP report goes on to describe factors that increase or decrease the likelihood of transmission during breastfeeding, but it notes that neither ART in the breastfeeding person nor ARV prophylaxis in the infant completely eliminates the risk of HIV transmission during breastfeeding. There have been rare cases where transmission occurred despite viral suppression in the person with HIV.
Among the reasons people with HIV have expressed a desire to breastfeed are wanting to bond with their infant, wanting to provide optimal nutrition and health benefits to their baby, and meeting cultural expectations, including the desire not to disclose their HIV infection status to family or friends by virtue of not breastfeeding.
“Among immigrant and refugee populations, the discordance between infant feeding guidelines in the United States and their country of birth may result in confusion, especially among parents who breastfed previous infants,” the AAP report also notes. Further, not breastfeeding could compound health disparities already more likely to be present among those living with HIV.
Discussions about infant feeding with parents with HIV should therefore “begin as early as possible and involve a multidisciplinary team that might include the pediatric primary care provider (once identified), a pediatric HIV expert, the breastfeeding parent’s HIV care and obstetric providers, and lactation consultants,” the report states. ”The parent’s motivations for breastfeeding should be explored and counseling provided on the risks and benefits of each feeding option, including breastfeeding, formula feeding, or certified, banked donor human milk.” The statement emphasizes the need for providing counseling in a “non-judgmental, respectful way, recognizing potential drivers for their decisions such as avoidance of stigma, prior experience with breastfeeding, and cultural contributors.”
Clear Recommendations Can Help Providers
The AAP’s statement that “replacement feeding (with formula or certified, banked donor human milk) is the only option that is 100% certain to prevent postnatal transmission of HIV” feels like it takes a “more conservative or discouraging approach” to breastfeeding than the CDC or WHO guidelines, Alissa Parker-Smith, APRN, DNP, CPNP-PC, IBCLC, a nurse practitioner and lactation consultant at PrimaryPlus, a Federally Qualified Health Clinic in Ashland, Kentucky, told MDedge. But she said they do clearly align with the CDC guidelines, and their differences from the WHO guidelines make sense in light of the different populations served by the WHO versus the U.S. agencies.
“Unclean water for formula preparation and a reduced or lack of access to formula in general can lead to many other risks of death for the infant other than the very small risk of HIV infection from breastfeeding from an HIV+ parent,” Ms. Parker-Smith said. “In the U.S. we generally have consistent access to clean water and safe formula as well as social structures to help families have access to formula, so the very small risk of HIV being passed to the infant is far greater than an infant in the U.S. dying as a result of unclean water or formula contamination.”
Ms. Parker-Smith also said the AAP recommendations seem thorough in helping pediatric practitioners counsel and support parents with HIV. “If I had a parent who is HIV+ walk in the door today wanting to breastfeed their infant, the AAP guidelines give me specific steps to make me feel confident in helping that parent breastfeed as safely as possible as well as providing education to assist that parent through the decision process,” she said.
Dr. Biala agreed, noting that the clinical report “very clearly delineates recommendations for different groups of people: those in labor or postpartum with undocumented HIV infection status, pregnant and postpartum people with HIV, those without HIV but at high risk of acquiring it, and those with suspected acute HIV infection while breastfeeding.” Dr. Biala said the report “provides concrete, detailed, and easy-to-follow guidance on comprehensive counseling, strategies to minimize risk of transmission, and infant screening timelines.”
How easily the guidelines can be implemented will depend on the existing resources at different institutions in the United States, Dr. Biala added.
“In hospitals and clinics that have, or could easily have, systems in place to ensure follow-up and regular assessment during breastfeeding, the guidelines could be implemented fairly quickly,” she said. “It might be more challenging in areas with inadequate or limited access to multidisciplinary team members, including HIV care providers and lactation consultants.”
The report did not use external funding, and the authors reported no disclosures. Dr. Abuogi and Ms. Parker-Smith have no disclosures.
aside from avoiding breastfeeding altogether, according to a new clinical report from the American Academy of Pediatrics (AAP).
“The risk of HIV transmission via breastfeeding from a parent with HIV who is receiving antiretroviral treatment (ART) and is virally suppressed is estimated to be less than 1%,” Lisa Abuogi, MD, an associate professor of pediatric infectious disease at the University of Colorado Anschutz Medical Campus, and her colleagues wrote in Pediatrics. For people living with HIV in the United States, however, the AAP recommends that “avoidance of breastfeeding is the only infant feeding option with 0% risk of HIV transmission.”
The authors go on to suggest that pediatricians “be prepared to offer a family-centered, nonjudgmental, harm reduction approach” to support people with HIV who do want to breastfeed and have sustained viral suppression. Parents with HIV who are not on ART or who do not have adequate viral suppression should be advised against breastfeeding, the report states. Members of the AAP Committee on Pediatric and Adolescent HIV and of the AAP Section on Breastfeeding coauthored the clinical report.
“The new guidelines emphasize the importance of patient-centered counseling as the foundation for shared decision-making, allowing patients and pediatric providers to make feeding decisions together and for the first time really giving support to people with HIV in the U.S. who want to breastfeed,” Danna Biala, MD, MS, an attending pediatrician at Children’s Hospital at Montefiore and an assistant professor at Albert Einstein College of Medicine, told MDedge News.
Dr. Biala was not involved in the development of the report, but she said the AAP’s guidance reflects the recent shift in the stance of the Centers for Disease Control and Prevention (CDC) regarding breastfeeding among people who are HIV+. Recommendations from the CDC and the U.S. Department of Health and Human Services (HHS) were updated in 2023.
“I’m glad that the AAP is putting out guidelines on infant feeding for people with HIV,” Dr. Biala said. “For so long in the U.S., pediatricians have been advising all mothers with HIV to avoid breastfeeding, believing that the risk of transmission outweighed the benefits of breastfeeding.”
The updated guidance from HHS in 2023 “was revolutionary in supporting people with HIV in low-risk situations who want to breastfeed,” Dr. Biala said, but “clear protocols for monitoring and follow-up were not in place,” which these AAP guidelines help address.
Prior Discordance Between Global, U.S. Guidance
An estimated 5,000 people with HIV give birth each year in the United States, and up to one third of pregnant people with HIV may be unaware of their HIV status, the AAP report notes. Pediatric healthcare professionals in the United States therefore need to be aware of recommendations related to HIV testing of pregnant people and of counseling the feeding of infants exposed to HIV. The report recommends opt-out HIV testing at the first prenatal visit and then possibly retesting in the third trimester in areas with high HIV incidence or for people at high risk for HIV or with signs or symptoms of acute HIV infection.
The report also highlights the health benefits of breastfeeding to both the infant and the breastfeeding parent, but notes the CDC’s historical recommendation against breastfeeding for people who are HIV+. The WHO, meanwhile, began recommending in 2016 that infants be breastfed through 12 to 24 months old if the parent was on ART and/or the infant was receiving antiretroviral (ARV) prophylaxis, since research showed those treatments were effective in reducing transmission risk.
Still, an estimated 30% of perinatal HIV transmission occurs via breastfeeding worldwide, primarily from people with HIV who are not on ART or are not adequately virally suppressed. Without parental ART or infant ARV prophylaxis, HIV transmission risk to infants via breastfeeding is highest, about 5%-6%, in the first 4-6 weeks of life. Risk then declines to about 0.9% a month thereafter. The AAP report goes on to describe factors that increase or decrease the likelihood of transmission during breastfeeding, but it notes that neither ART in the breastfeeding person nor ARV prophylaxis in the infant completely eliminates the risk of HIV transmission during breastfeeding. There have been rare cases where transmission occurred despite viral suppression in the person with HIV.
Among the reasons people with HIV have expressed a desire to breastfeed are wanting to bond with their infant, wanting to provide optimal nutrition and health benefits to their baby, and meeting cultural expectations, including the desire not to disclose their HIV infection status to family or friends by virtue of not breastfeeding.
“Among immigrant and refugee populations, the discordance between infant feeding guidelines in the United States and their country of birth may result in confusion, especially among parents who breastfed previous infants,” the AAP report also notes. Further, not breastfeeding could compound health disparities already more likely to be present among those living with HIV.
Discussions about infant feeding with parents with HIV should therefore “begin as early as possible and involve a multidisciplinary team that might include the pediatric primary care provider (once identified), a pediatric HIV expert, the breastfeeding parent’s HIV care and obstetric providers, and lactation consultants,” the report states. ”The parent’s motivations for breastfeeding should be explored and counseling provided on the risks and benefits of each feeding option, including breastfeeding, formula feeding, or certified, banked donor human milk.” The statement emphasizes the need for providing counseling in a “non-judgmental, respectful way, recognizing potential drivers for their decisions such as avoidance of stigma, prior experience with breastfeeding, and cultural contributors.”
Clear Recommendations Can Help Providers
The AAP’s statement that “replacement feeding (with formula or certified, banked donor human milk) is the only option that is 100% certain to prevent postnatal transmission of HIV” feels like it takes a “more conservative or discouraging approach” to breastfeeding than the CDC or WHO guidelines, Alissa Parker-Smith, APRN, DNP, CPNP-PC, IBCLC, a nurse practitioner and lactation consultant at PrimaryPlus, a Federally Qualified Health Clinic in Ashland, Kentucky, told MDedge. But she said they do clearly align with the CDC guidelines, and their differences from the WHO guidelines make sense in light of the different populations served by the WHO versus the U.S. agencies.
“Unclean water for formula preparation and a reduced or lack of access to formula in general can lead to many other risks of death for the infant other than the very small risk of HIV infection from breastfeeding from an HIV+ parent,” Ms. Parker-Smith said. “In the U.S. we generally have consistent access to clean water and safe formula as well as social structures to help families have access to formula, so the very small risk of HIV being passed to the infant is far greater than an infant in the U.S. dying as a result of unclean water or formula contamination.”
Ms. Parker-Smith also said the AAP recommendations seem thorough in helping pediatric practitioners counsel and support parents with HIV. “If I had a parent who is HIV+ walk in the door today wanting to breastfeed their infant, the AAP guidelines give me specific steps to make me feel confident in helping that parent breastfeed as safely as possible as well as providing education to assist that parent through the decision process,” she said.
Dr. Biala agreed, noting that the clinical report “very clearly delineates recommendations for different groups of people: those in labor or postpartum with undocumented HIV infection status, pregnant and postpartum people with HIV, those without HIV but at high risk of acquiring it, and those with suspected acute HIV infection while breastfeeding.” Dr. Biala said the report “provides concrete, detailed, and easy-to-follow guidance on comprehensive counseling, strategies to minimize risk of transmission, and infant screening timelines.”
How easily the guidelines can be implemented will depend on the existing resources at different institutions in the United States, Dr. Biala added.
“In hospitals and clinics that have, or could easily have, systems in place to ensure follow-up and regular assessment during breastfeeding, the guidelines could be implemented fairly quickly,” she said. “It might be more challenging in areas with inadequate or limited access to multidisciplinary team members, including HIV care providers and lactation consultants.”
The report did not use external funding, and the authors reported no disclosures. Dr. Abuogi and Ms. Parker-Smith have no disclosures.
aside from avoiding breastfeeding altogether, according to a new clinical report from the American Academy of Pediatrics (AAP).
“The risk of HIV transmission via breastfeeding from a parent with HIV who is receiving antiretroviral treatment (ART) and is virally suppressed is estimated to be less than 1%,” Lisa Abuogi, MD, an associate professor of pediatric infectious disease at the University of Colorado Anschutz Medical Campus, and her colleagues wrote in Pediatrics. For people living with HIV in the United States, however, the AAP recommends that “avoidance of breastfeeding is the only infant feeding option with 0% risk of HIV transmission.”
The authors go on to suggest that pediatricians “be prepared to offer a family-centered, nonjudgmental, harm reduction approach” to support people with HIV who do want to breastfeed and have sustained viral suppression. Parents with HIV who are not on ART or who do not have adequate viral suppression should be advised against breastfeeding, the report states. Members of the AAP Committee on Pediatric and Adolescent HIV and of the AAP Section on Breastfeeding coauthored the clinical report.
“The new guidelines emphasize the importance of patient-centered counseling as the foundation for shared decision-making, allowing patients and pediatric providers to make feeding decisions together and for the first time really giving support to people with HIV in the U.S. who want to breastfeed,” Danna Biala, MD, MS, an attending pediatrician at Children’s Hospital at Montefiore and an assistant professor at Albert Einstein College of Medicine, told MDedge News.
Dr. Biala was not involved in the development of the report, but she said the AAP’s guidance reflects the recent shift in the stance of the Centers for Disease Control and Prevention (CDC) regarding breastfeeding among people who are HIV+. Recommendations from the CDC and the U.S. Department of Health and Human Services (HHS) were updated in 2023.
“I’m glad that the AAP is putting out guidelines on infant feeding for people with HIV,” Dr. Biala said. “For so long in the U.S., pediatricians have been advising all mothers with HIV to avoid breastfeeding, believing that the risk of transmission outweighed the benefits of breastfeeding.”
The updated guidance from HHS in 2023 “was revolutionary in supporting people with HIV in low-risk situations who want to breastfeed,” Dr. Biala said, but “clear protocols for monitoring and follow-up were not in place,” which these AAP guidelines help address.
Prior Discordance Between Global, U.S. Guidance
An estimated 5,000 people with HIV give birth each year in the United States, and up to one third of pregnant people with HIV may be unaware of their HIV status, the AAP report notes. Pediatric healthcare professionals in the United States therefore need to be aware of recommendations related to HIV testing of pregnant people and of counseling the feeding of infants exposed to HIV. The report recommends opt-out HIV testing at the first prenatal visit and then possibly retesting in the third trimester in areas with high HIV incidence or for people at high risk for HIV or with signs or symptoms of acute HIV infection.
The report also highlights the health benefits of breastfeeding to both the infant and the breastfeeding parent, but notes the CDC’s historical recommendation against breastfeeding for people who are HIV+. The WHO, meanwhile, began recommending in 2016 that infants be breastfed through 12 to 24 months old if the parent was on ART and/or the infant was receiving antiretroviral (ARV) prophylaxis, since research showed those treatments were effective in reducing transmission risk.
Still, an estimated 30% of perinatal HIV transmission occurs via breastfeeding worldwide, primarily from people with HIV who are not on ART or are not adequately virally suppressed. Without parental ART or infant ARV prophylaxis, HIV transmission risk to infants via breastfeeding is highest, about 5%-6%, in the first 4-6 weeks of life. Risk then declines to about 0.9% a month thereafter. The AAP report goes on to describe factors that increase or decrease the likelihood of transmission during breastfeeding, but it notes that neither ART in the breastfeeding person nor ARV prophylaxis in the infant completely eliminates the risk of HIV transmission during breastfeeding. There have been rare cases where transmission occurred despite viral suppression in the person with HIV.
Among the reasons people with HIV have expressed a desire to breastfeed are wanting to bond with their infant, wanting to provide optimal nutrition and health benefits to their baby, and meeting cultural expectations, including the desire not to disclose their HIV infection status to family or friends by virtue of not breastfeeding.
“Among immigrant and refugee populations, the discordance between infant feeding guidelines in the United States and their country of birth may result in confusion, especially among parents who breastfed previous infants,” the AAP report also notes. Further, not breastfeeding could compound health disparities already more likely to be present among those living with HIV.
Discussions about infant feeding with parents with HIV should therefore “begin as early as possible and involve a multidisciplinary team that might include the pediatric primary care provider (once identified), a pediatric HIV expert, the breastfeeding parent’s HIV care and obstetric providers, and lactation consultants,” the report states. ”The parent’s motivations for breastfeeding should be explored and counseling provided on the risks and benefits of each feeding option, including breastfeeding, formula feeding, or certified, banked donor human milk.” The statement emphasizes the need for providing counseling in a “non-judgmental, respectful way, recognizing potential drivers for their decisions such as avoidance of stigma, prior experience with breastfeeding, and cultural contributors.”
Clear Recommendations Can Help Providers
The AAP’s statement that “replacement feeding (with formula or certified, banked donor human milk) is the only option that is 100% certain to prevent postnatal transmission of HIV” feels like it takes a “more conservative or discouraging approach” to breastfeeding than the CDC or WHO guidelines, Alissa Parker-Smith, APRN, DNP, CPNP-PC, IBCLC, a nurse practitioner and lactation consultant at PrimaryPlus, a Federally Qualified Health Clinic in Ashland, Kentucky, told MDedge. But she said they do clearly align with the CDC guidelines, and their differences from the WHO guidelines make sense in light of the different populations served by the WHO versus the U.S. agencies.
“Unclean water for formula preparation and a reduced or lack of access to formula in general can lead to many other risks of death for the infant other than the very small risk of HIV infection from breastfeeding from an HIV+ parent,” Ms. Parker-Smith said. “In the U.S. we generally have consistent access to clean water and safe formula as well as social structures to help families have access to formula, so the very small risk of HIV being passed to the infant is far greater than an infant in the U.S. dying as a result of unclean water or formula contamination.”
Ms. Parker-Smith also said the AAP recommendations seem thorough in helping pediatric practitioners counsel and support parents with HIV. “If I had a parent who is HIV+ walk in the door today wanting to breastfeed their infant, the AAP guidelines give me specific steps to make me feel confident in helping that parent breastfeed as safely as possible as well as providing education to assist that parent through the decision process,” she said.
Dr. Biala agreed, noting that the clinical report “very clearly delineates recommendations for different groups of people: those in labor or postpartum with undocumented HIV infection status, pregnant and postpartum people with HIV, those without HIV but at high risk of acquiring it, and those with suspected acute HIV infection while breastfeeding.” Dr. Biala said the report “provides concrete, detailed, and easy-to-follow guidance on comprehensive counseling, strategies to minimize risk of transmission, and infant screening timelines.”
How easily the guidelines can be implemented will depend on the existing resources at different institutions in the United States, Dr. Biala added.
“In hospitals and clinics that have, or could easily have, systems in place to ensure follow-up and regular assessment during breastfeeding, the guidelines could be implemented fairly quickly,” she said. “It might be more challenging in areas with inadequate or limited access to multidisciplinary team members, including HIV care providers and lactation consultants.”
The report did not use external funding, and the authors reported no disclosures. Dr. Abuogi and Ms. Parker-Smith have no disclosures.
FROM PEDIATRICS
An 8-year-old girl presented with papules on her bilateral eyelid margins
, with an equal distribution across genders and ethnicities.1 It is caused by mutations in the ECM1 gene2 on chromosome 1q21. This leads to the abnormal deposition of hyaline material in various tissues across different organ systems, with the classic manifestations known as the “string of pearls” sign and a hoarse cry or voice.
The rarity of lipoid proteinosis often leads to challenges in diagnosis. Particularly when deviating from the common association with consanguinity, the potential for de novo mutations or a broader genetic variability in disease expression is highlighted. Our patient presents with symptoms that are pathognomonic to LP with moniliform blepharosis and hoarseness of the voice, in addition to scarring of the extremities.
Other common clinical manifestations in patients with LP include cobblestoning of the mucosa; hyperkeratosis of the elbows, knees, and hands; and calcification of the amygdala with neuroimaging.3
Genetic testing that identifies a loss-of-function mutation in ECM1 offers diagnostic confirmation. Patients often need multidisciplinary care involving dermatology; ear, nose, throat; neurology; and genetics. Treatment of LP is mostly symptomatic with unsatisfactory resolution of cutaneous changes, with retinoids such as acitretin used as the first-line option and surgery as a consideration for laryngeal hyaline deposits.2 Although LP can affect different organ systems, patients tend to have a normal lifespan.
LP is a rare disorder that dermatologists often learn about during textbook sessions or didactics in residency but do not see in practice for decades, or if ever. This case highlights the need to review the classic presentations of rare conditions.
This case and the photos were submitted by Ms. Chang, BS, Western University of Health Sciences, College of Osteopathic Medicine, Pomona, California; Dr. Connie Chang, Verdugo Dermatology, Glendale, California; and Dr. Yuchieh Kathryn Chang, MD Anderson Cancer Center, Houston, Texas. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Mcgrath JA. Handb Clin Neurol. 2015:132:317-22. doi: 10.1016/B978-0-444-62702-5.00023-8.
2. Hamada Tet al. Hum Mol Genet. 2002 Apr 1;11(7):833-40. doi: 10.1093/hmg/11.7.833.
3. Frenkel B et al. Clin Oral Investig. 2017 Sep;21(7):2245-51 doi: 10.1007/s00784-016-2017-7.
, with an equal distribution across genders and ethnicities.1 It is caused by mutations in the ECM1 gene2 on chromosome 1q21. This leads to the abnormal deposition of hyaline material in various tissues across different organ systems, with the classic manifestations known as the “string of pearls” sign and a hoarse cry or voice.
The rarity of lipoid proteinosis often leads to challenges in diagnosis. Particularly when deviating from the common association with consanguinity, the potential for de novo mutations or a broader genetic variability in disease expression is highlighted. Our patient presents with symptoms that are pathognomonic to LP with moniliform blepharosis and hoarseness of the voice, in addition to scarring of the extremities.
Other common clinical manifestations in patients with LP include cobblestoning of the mucosa; hyperkeratosis of the elbows, knees, and hands; and calcification of the amygdala with neuroimaging.3
Genetic testing that identifies a loss-of-function mutation in ECM1 offers diagnostic confirmation. Patients often need multidisciplinary care involving dermatology; ear, nose, throat; neurology; and genetics. Treatment of LP is mostly symptomatic with unsatisfactory resolution of cutaneous changes, with retinoids such as acitretin used as the first-line option and surgery as a consideration for laryngeal hyaline deposits.2 Although LP can affect different organ systems, patients tend to have a normal lifespan.
LP is a rare disorder that dermatologists often learn about during textbook sessions or didactics in residency but do not see in practice for decades, or if ever. This case highlights the need to review the classic presentations of rare conditions.
This case and the photos were submitted by Ms. Chang, BS, Western University of Health Sciences, College of Osteopathic Medicine, Pomona, California; Dr. Connie Chang, Verdugo Dermatology, Glendale, California; and Dr. Yuchieh Kathryn Chang, MD Anderson Cancer Center, Houston, Texas. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Mcgrath JA. Handb Clin Neurol. 2015:132:317-22. doi: 10.1016/B978-0-444-62702-5.00023-8.
2. Hamada Tet al. Hum Mol Genet. 2002 Apr 1;11(7):833-40. doi: 10.1093/hmg/11.7.833.
3. Frenkel B et al. Clin Oral Investig. 2017 Sep;21(7):2245-51 doi: 10.1007/s00784-016-2017-7.
, with an equal distribution across genders and ethnicities.1 It is caused by mutations in the ECM1 gene2 on chromosome 1q21. This leads to the abnormal deposition of hyaline material in various tissues across different organ systems, with the classic manifestations known as the “string of pearls” sign and a hoarse cry or voice.
The rarity of lipoid proteinosis often leads to challenges in diagnosis. Particularly when deviating from the common association with consanguinity, the potential for de novo mutations or a broader genetic variability in disease expression is highlighted. Our patient presents with symptoms that are pathognomonic to LP with moniliform blepharosis and hoarseness of the voice, in addition to scarring of the extremities.
Other common clinical manifestations in patients with LP include cobblestoning of the mucosa; hyperkeratosis of the elbows, knees, and hands; and calcification of the amygdala with neuroimaging.3
Genetic testing that identifies a loss-of-function mutation in ECM1 offers diagnostic confirmation. Patients often need multidisciplinary care involving dermatology; ear, nose, throat; neurology; and genetics. Treatment of LP is mostly symptomatic with unsatisfactory resolution of cutaneous changes, with retinoids such as acitretin used as the first-line option and surgery as a consideration for laryngeal hyaline deposits.2 Although LP can affect different organ systems, patients tend to have a normal lifespan.
LP is a rare disorder that dermatologists often learn about during textbook sessions or didactics in residency but do not see in practice for decades, or if ever. This case highlights the need to review the classic presentations of rare conditions.
This case and the photos were submitted by Ms. Chang, BS, Western University of Health Sciences, College of Osteopathic Medicine, Pomona, California; Dr. Connie Chang, Verdugo Dermatology, Glendale, California; and Dr. Yuchieh Kathryn Chang, MD Anderson Cancer Center, Houston, Texas. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Mcgrath JA. Handb Clin Neurol. 2015:132:317-22. doi: 10.1016/B978-0-444-62702-5.00023-8.
2. Hamada Tet al. Hum Mol Genet. 2002 Apr 1;11(7):833-40. doi: 10.1093/hmg/11.7.833.
3. Frenkel B et al. Clin Oral Investig. 2017 Sep;21(7):2245-51 doi: 10.1007/s00784-016-2017-7.
Resource Menu Gives Choice to Caregivers Struggling to Meet Basic Needs
Screenings may not be the way to get needed resources to children and their caregivers, according to new research presented at the annual meeting of the Pediatric Academic Societies (PAS).
Caregivers and parents who were asked if they wanted assistance in several areas of need, including transportation and childcare, were nearly twice as likely to say they wanted such help than those who received a screening on current hardships. Generally, each questionnaire is administered in front of their children in primary care or pediatric hospital settings.
“Families have a lot of concern about being seen a different way by their healthcare team, being seen as unfit, and having child protective services involved in their childcare for issues related to poverty,” said Danielle Cullen, MD, a pediatric emergency medicine specialist at Children’s Hospital of Philadelphia (CHOP) and assistant professor of pediatrics at the University of Pennsylvania in Philadelphia.
Dr. Cullen and her colleagues analyzed data from nearly 4000 caregivers of children up to age 21 at emergency departments or primary care clinics at CHOP between 2021 and 2023.
Caregivers were randomly assigned to one of three arms — screening with a version of WE CARE (Well Child Care, Evaluation, Community Resources, Advocacy, Referral, Education), use of an online menu of options for help in areas like housing, or neither approach.
Caregivers in all three arms received a map of resources and a follow-up text from a resource navigator to assist them as needed.
Nearly 40% of caregivers who presented with the digital menu said they wanted resources compared with 29% of those who were screened (P < .001). Non-native English speakers given the menu were 2.5 times more likely to say yes to resources compared with those who were screened.
“We need to be thoughtful about these mandates to screen for social determinants of health: It’s not that straightforward,” said Esther K. Chung, MD, a pediatrician and professor of pediatrics at the University of Washington Medicine in Seattle, who was not involved in the study. “What we’re getting from this study is that patients want choice, and the menu provides them choice.”
Dr. Cullen said the menu option allows caregivers to make choices based on their priorities and not on whether they meet the screening thresholds for need.
While some health clinics utilize tablet forms for screenings to offer more privacy with questions, asking direct questions about income, food insecurity, and housing stability can be stigmatizing, Dr. Cullen said.
“Screening positive for social risk doesn’t mean that you actually want resources, and on the flip side, the literature shows that about half of the people who screen negative want resources,” she said.
Dr. Cullen and her team also conducted follow-up interviews with caregivers and found many feared that their clinician would assume a medical condition was connected to living conditions. They also had concerns about insurance companies gaining access to the data and using it to deny coverage or raise costs.
Spanish-speaking caregivers cited fears about their immigration status, experiences of discrimination, and language barriers when trying to access resources.
Participants said a few key strategies could make screening less intimidating, such as abstaining from screening during a serious medical visit, asking for consent to record answers in medical records, and communicating in an empathetic manner.
“Some families are a bit surprised when we ask about things like housing and food insecurity, but I think as long as we contextualize it, we can minimize the stigma associated with it,” Dr. Chung said. “That takes quite a bit of nuance and skill.”
The study was funded by the William T. Grant Foundation and the Emergency Medicine Foundation. The authors reported no disclosures.
A version of this article appeared on Medscape.com.
Screenings may not be the way to get needed resources to children and their caregivers, according to new research presented at the annual meeting of the Pediatric Academic Societies (PAS).
Caregivers and parents who were asked if they wanted assistance in several areas of need, including transportation and childcare, were nearly twice as likely to say they wanted such help than those who received a screening on current hardships. Generally, each questionnaire is administered in front of their children in primary care or pediatric hospital settings.
“Families have a lot of concern about being seen a different way by their healthcare team, being seen as unfit, and having child protective services involved in their childcare for issues related to poverty,” said Danielle Cullen, MD, a pediatric emergency medicine specialist at Children’s Hospital of Philadelphia (CHOP) and assistant professor of pediatrics at the University of Pennsylvania in Philadelphia.
Dr. Cullen and her colleagues analyzed data from nearly 4000 caregivers of children up to age 21 at emergency departments or primary care clinics at CHOP between 2021 and 2023.
Caregivers were randomly assigned to one of three arms — screening with a version of WE CARE (Well Child Care, Evaluation, Community Resources, Advocacy, Referral, Education), use of an online menu of options for help in areas like housing, or neither approach.
Caregivers in all three arms received a map of resources and a follow-up text from a resource navigator to assist them as needed.
Nearly 40% of caregivers who presented with the digital menu said they wanted resources compared with 29% of those who were screened (P < .001). Non-native English speakers given the menu were 2.5 times more likely to say yes to resources compared with those who were screened.
“We need to be thoughtful about these mandates to screen for social determinants of health: It’s not that straightforward,” said Esther K. Chung, MD, a pediatrician and professor of pediatrics at the University of Washington Medicine in Seattle, who was not involved in the study. “What we’re getting from this study is that patients want choice, and the menu provides them choice.”
Dr. Cullen said the menu option allows caregivers to make choices based on their priorities and not on whether they meet the screening thresholds for need.
While some health clinics utilize tablet forms for screenings to offer more privacy with questions, asking direct questions about income, food insecurity, and housing stability can be stigmatizing, Dr. Cullen said.
“Screening positive for social risk doesn’t mean that you actually want resources, and on the flip side, the literature shows that about half of the people who screen negative want resources,” she said.
Dr. Cullen and her team also conducted follow-up interviews with caregivers and found many feared that their clinician would assume a medical condition was connected to living conditions. They also had concerns about insurance companies gaining access to the data and using it to deny coverage or raise costs.
Spanish-speaking caregivers cited fears about their immigration status, experiences of discrimination, and language barriers when trying to access resources.
Participants said a few key strategies could make screening less intimidating, such as abstaining from screening during a serious medical visit, asking for consent to record answers in medical records, and communicating in an empathetic manner.
“Some families are a bit surprised when we ask about things like housing and food insecurity, but I think as long as we contextualize it, we can minimize the stigma associated with it,” Dr. Chung said. “That takes quite a bit of nuance and skill.”
The study was funded by the William T. Grant Foundation and the Emergency Medicine Foundation. The authors reported no disclosures.
A version of this article appeared on Medscape.com.
Screenings may not be the way to get needed resources to children and their caregivers, according to new research presented at the annual meeting of the Pediatric Academic Societies (PAS).
Caregivers and parents who were asked if they wanted assistance in several areas of need, including transportation and childcare, were nearly twice as likely to say they wanted such help than those who received a screening on current hardships. Generally, each questionnaire is administered in front of their children in primary care or pediatric hospital settings.
“Families have a lot of concern about being seen a different way by their healthcare team, being seen as unfit, and having child protective services involved in their childcare for issues related to poverty,” said Danielle Cullen, MD, a pediatric emergency medicine specialist at Children’s Hospital of Philadelphia (CHOP) and assistant professor of pediatrics at the University of Pennsylvania in Philadelphia.
Dr. Cullen and her colleagues analyzed data from nearly 4000 caregivers of children up to age 21 at emergency departments or primary care clinics at CHOP between 2021 and 2023.
Caregivers were randomly assigned to one of three arms — screening with a version of WE CARE (Well Child Care, Evaluation, Community Resources, Advocacy, Referral, Education), use of an online menu of options for help in areas like housing, or neither approach.
Caregivers in all three arms received a map of resources and a follow-up text from a resource navigator to assist them as needed.
Nearly 40% of caregivers who presented with the digital menu said they wanted resources compared with 29% of those who were screened (P < .001). Non-native English speakers given the menu were 2.5 times more likely to say yes to resources compared with those who were screened.
“We need to be thoughtful about these mandates to screen for social determinants of health: It’s not that straightforward,” said Esther K. Chung, MD, a pediatrician and professor of pediatrics at the University of Washington Medicine in Seattle, who was not involved in the study. “What we’re getting from this study is that patients want choice, and the menu provides them choice.”
Dr. Cullen said the menu option allows caregivers to make choices based on their priorities and not on whether they meet the screening thresholds for need.
While some health clinics utilize tablet forms for screenings to offer more privacy with questions, asking direct questions about income, food insecurity, and housing stability can be stigmatizing, Dr. Cullen said.
“Screening positive for social risk doesn’t mean that you actually want resources, and on the flip side, the literature shows that about half of the people who screen negative want resources,” she said.
Dr. Cullen and her team also conducted follow-up interviews with caregivers and found many feared that their clinician would assume a medical condition was connected to living conditions. They also had concerns about insurance companies gaining access to the data and using it to deny coverage or raise costs.
Spanish-speaking caregivers cited fears about their immigration status, experiences of discrimination, and language barriers when trying to access resources.
Participants said a few key strategies could make screening less intimidating, such as abstaining from screening during a serious medical visit, asking for consent to record answers in medical records, and communicating in an empathetic manner.
“Some families are a bit surprised when we ask about things like housing and food insecurity, but I think as long as we contextualize it, we can minimize the stigma associated with it,” Dr. Chung said. “That takes quite a bit of nuance and skill.”
The study was funded by the William T. Grant Foundation and the Emergency Medicine Foundation. The authors reported no disclosures.
A version of this article appeared on Medscape.com.
FROM PAS 2024
Little Less Talk and a Lot More Action
No matter where one looks for the statistics, no matter what words one chooses to describe it, this country has a child and adolescent mental health crisis. Almost 20% of young people in the 3-17 age bracket have a mental, emotional, developmental, or behavioral disorder. COVID-19 has certainly exacerbated the problem, but the downward trend in the mental health of this nation has been going on for decades.
The voices calling for more services to address the problem are getting more numerous and louder. But, what exactly should those services look like and who should be delivering them?
When considered together, two recent research papers suggest that we should be venturing well beyond the usual mental health strategies if we are going to be successful in addressing the current crisis.
The first paper is an analysis by two psychologists who contend that our efforts to raise the awareness of mental issues may be contributing to the increase in reported mental health problems. The authors agree that more attention paid to mental health conditions can result in “more accurate reporting of previous under-recognized symptoms” and would seem to be a positive. However, the investigators also observe that when exposed to this flood of information, some individuals who are only experiencing minor distress may report their symptoms as mental problems. The authors of the paper have coined the term for this phenomenon as “prevalence inflation.” Their preliminary investigation suggests it may be much more common than once believed and they present numerous situations in which prevalence inflation seems to have occurred.
A New York Times article about this hypothesis reports on a British study in which nearly 30,000 teenagers were instructed by their teachers to “direct their attentions to the present moment” and utilize other mindfulness strategies. The educators had hoped that after 8 years of this indoctrination, the students’ mental health would have improved. The bottom line was that this mindfulness-based program was of no help and may have actually made things worse for a subgroup of students who were at greatest risk for mental health challenges.
Dr. Jack Andrews, one of the authors, feels that mindfulness training may encourage what he calls “co-rumination,” which he describes as “the kind of long, unresolved group discussion that churns up problems without finding solutions.” One has to wonder if “prevalence inflation” and “co-rumination,” if they do exist, may be playing a role in the hotly debated phenomenon some have termed “late-onset gender dysphoria.”
Never having been a fan of mindfulness training as an effective strategy, I am relieved to learn that serious investigators are finding evidence that supports my gut reaction.
If raising awareness, “education,” and group discussion aren’t working, and in some cases are actually contributing to the crisis, or at least making the data difficult to interpret, what should we be doing to turn this foundering ship around?
A second paper, coming from Taiwan, may provide an answer. Huey-Ling Chiang and fellow investigators have reported on a study of nearly two million children and adolescents in which they found improved performance in a variety of physical fitness challenges “was linked with a lower risk of mental health disorder.” The dose-dependent effect resulted in less anxiety and depressive disorders as well as less attention-deficit/hyperactivity disorder when cardio-respiratory, muscle endurance, and power indices improved.
There have been other observers who have suggested a link between physical fitness and improved mental health, but this Taiwanese study is by far one of the largest. And, the discovery of a dose-dependent effect makes it particularly convincing.
As I reviewed these two papers, I became increasingly frustrated because this is another example in which one of the answers is staring us in the face and we continue to do nothing more than talk about it.
We already know that physically active people are healthier both physically and mentally, but we do little more than talk. It may be helpful for some people to become a bit more self-aware. However, it is becoming increasingly clear that you can’t talk yourself into being mentally healthy without a concurrent effort to actually do the things that can improve your overall health, such as being physically active and adopting healthy sleep habits. A political advisor once said, “It’s the economy, stupid.” As a community interested in the health of our children and the adults they will become, we need to remind ourselves again, “It’s the old Mind-Body Thing, Stupid.” Our children need a little less talk and a lot more action.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
No matter where one looks for the statistics, no matter what words one chooses to describe it, this country has a child and adolescent mental health crisis. Almost 20% of young people in the 3-17 age bracket have a mental, emotional, developmental, or behavioral disorder. COVID-19 has certainly exacerbated the problem, but the downward trend in the mental health of this nation has been going on for decades.
The voices calling for more services to address the problem are getting more numerous and louder. But, what exactly should those services look like and who should be delivering them?
When considered together, two recent research papers suggest that we should be venturing well beyond the usual mental health strategies if we are going to be successful in addressing the current crisis.
The first paper is an analysis by two psychologists who contend that our efforts to raise the awareness of mental issues may be contributing to the increase in reported mental health problems. The authors agree that more attention paid to mental health conditions can result in “more accurate reporting of previous under-recognized symptoms” and would seem to be a positive. However, the investigators also observe that when exposed to this flood of information, some individuals who are only experiencing minor distress may report their symptoms as mental problems. The authors of the paper have coined the term for this phenomenon as “prevalence inflation.” Their preliminary investigation suggests it may be much more common than once believed and they present numerous situations in which prevalence inflation seems to have occurred.
A New York Times article about this hypothesis reports on a British study in which nearly 30,000 teenagers were instructed by their teachers to “direct their attentions to the present moment” and utilize other mindfulness strategies. The educators had hoped that after 8 years of this indoctrination, the students’ mental health would have improved. The bottom line was that this mindfulness-based program was of no help and may have actually made things worse for a subgroup of students who were at greatest risk for mental health challenges.
Dr. Jack Andrews, one of the authors, feels that mindfulness training may encourage what he calls “co-rumination,” which he describes as “the kind of long, unresolved group discussion that churns up problems without finding solutions.” One has to wonder if “prevalence inflation” and “co-rumination,” if they do exist, may be playing a role in the hotly debated phenomenon some have termed “late-onset gender dysphoria.”
Never having been a fan of mindfulness training as an effective strategy, I am relieved to learn that serious investigators are finding evidence that supports my gut reaction.
If raising awareness, “education,” and group discussion aren’t working, and in some cases are actually contributing to the crisis, or at least making the data difficult to interpret, what should we be doing to turn this foundering ship around?
A second paper, coming from Taiwan, may provide an answer. Huey-Ling Chiang and fellow investigators have reported on a study of nearly two million children and adolescents in which they found improved performance in a variety of physical fitness challenges “was linked with a lower risk of mental health disorder.” The dose-dependent effect resulted in less anxiety and depressive disorders as well as less attention-deficit/hyperactivity disorder when cardio-respiratory, muscle endurance, and power indices improved.
There have been other observers who have suggested a link between physical fitness and improved mental health, but this Taiwanese study is by far one of the largest. And, the discovery of a dose-dependent effect makes it particularly convincing.
As I reviewed these two papers, I became increasingly frustrated because this is another example in which one of the answers is staring us in the face and we continue to do nothing more than talk about it.
We already know that physically active people are healthier both physically and mentally, but we do little more than talk. It may be helpful for some people to become a bit more self-aware. However, it is becoming increasingly clear that you can’t talk yourself into being mentally healthy without a concurrent effort to actually do the things that can improve your overall health, such as being physically active and adopting healthy sleep habits. A political advisor once said, “It’s the economy, stupid.” As a community interested in the health of our children and the adults they will become, we need to remind ourselves again, “It’s the old Mind-Body Thing, Stupid.” Our children need a little less talk and a lot more action.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
No matter where one looks for the statistics, no matter what words one chooses to describe it, this country has a child and adolescent mental health crisis. Almost 20% of young people in the 3-17 age bracket have a mental, emotional, developmental, or behavioral disorder. COVID-19 has certainly exacerbated the problem, but the downward trend in the mental health of this nation has been going on for decades.
The voices calling for more services to address the problem are getting more numerous and louder. But, what exactly should those services look like and who should be delivering them?
When considered together, two recent research papers suggest that we should be venturing well beyond the usual mental health strategies if we are going to be successful in addressing the current crisis.
The first paper is an analysis by two psychologists who contend that our efforts to raise the awareness of mental issues may be contributing to the increase in reported mental health problems. The authors agree that more attention paid to mental health conditions can result in “more accurate reporting of previous under-recognized symptoms” and would seem to be a positive. However, the investigators also observe that when exposed to this flood of information, some individuals who are only experiencing minor distress may report their symptoms as mental problems. The authors of the paper have coined the term for this phenomenon as “prevalence inflation.” Their preliminary investigation suggests it may be much more common than once believed and they present numerous situations in which prevalence inflation seems to have occurred.
A New York Times article about this hypothesis reports on a British study in which nearly 30,000 teenagers were instructed by their teachers to “direct their attentions to the present moment” and utilize other mindfulness strategies. The educators had hoped that after 8 years of this indoctrination, the students’ mental health would have improved. The bottom line was that this mindfulness-based program was of no help and may have actually made things worse for a subgroup of students who were at greatest risk for mental health challenges.
Dr. Jack Andrews, one of the authors, feels that mindfulness training may encourage what he calls “co-rumination,” which he describes as “the kind of long, unresolved group discussion that churns up problems without finding solutions.” One has to wonder if “prevalence inflation” and “co-rumination,” if they do exist, may be playing a role in the hotly debated phenomenon some have termed “late-onset gender dysphoria.”
Never having been a fan of mindfulness training as an effective strategy, I am relieved to learn that serious investigators are finding evidence that supports my gut reaction.
If raising awareness, “education,” and group discussion aren’t working, and in some cases are actually contributing to the crisis, or at least making the data difficult to interpret, what should we be doing to turn this foundering ship around?
A second paper, coming from Taiwan, may provide an answer. Huey-Ling Chiang and fellow investigators have reported on a study of nearly two million children and adolescents in which they found improved performance in a variety of physical fitness challenges “was linked with a lower risk of mental health disorder.” The dose-dependent effect resulted in less anxiety and depressive disorders as well as less attention-deficit/hyperactivity disorder when cardio-respiratory, muscle endurance, and power indices improved.
There have been other observers who have suggested a link between physical fitness and improved mental health, but this Taiwanese study is by far one of the largest. And, the discovery of a dose-dependent effect makes it particularly convincing.
As I reviewed these two papers, I became increasingly frustrated because this is another example in which one of the answers is staring us in the face and we continue to do nothing more than talk about it.
We already know that physically active people are healthier both physically and mentally, but we do little more than talk. It may be helpful for some people to become a bit more self-aware. However, it is becoming increasingly clear that you can’t talk yourself into being mentally healthy without a concurrent effort to actually do the things that can improve your overall health, such as being physically active and adopting healthy sleep habits. A political advisor once said, “It’s the economy, stupid.” As a community interested in the health of our children and the adults they will become, we need to remind ourselves again, “It’s the old Mind-Body Thing, Stupid.” Our children need a little less talk and a lot more action.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Hypopigmented Cutaneous Langerhans Cell Histiocytosis in a Hispanic Infant
To the Editor:
Langerhans cell histiocytosis (LCH) is a rare inflammatory neoplasia caused by accumulation of clonal Langerhans cells in 1 or more organs. The clinical spectrum is diverse, ranging from mild, single-organ involvement that may resolve spontaneously to severe progressive multisystem disease that can be fatal. It is most prevalent in children, affecting an estimated 4 to 5 children for every 1 million annually, with male predominance.1 The pathogenesis is driven by activating mutations in the mitogen-activated protein kinase pathway, with the BRAF V600E mutation detected in most LCH patients, resulting in proliferation of pathologic Langerhans cells and dysregulated expression of inflammatory cytokines in LCH lesions.2 A biopsy of lesional tissue is required for definitive diagnosis. Histopathology reveals a mixed inflammatory infiltrate and characteristic mononuclear cells with reniform nuclei that are positive for CD1a and CD207 proteins on immunohistochemical staining.3
Langerhans cell histiocytosis is categorized by the extent of organ involvement. It commonly affects the bones, skin, pituitary gland, liver, lungs, bone marrow, and lymph nodes.4 Single-system LCH involves a single organ with unifocal or multifocal lesions; multisystem LCH involves 2 or more organs and has a worse prognosis if risk organs (eg, liver, spleen, bone marrow) are involved.4
Skin lesions are reported in more than half of LCH cases and are the most common initial manifestation in patients younger than 2 years.4 Cutaneous findings are highly variable, which poses a diagnostic challenge. Common morphologies include erythematous papules, pustules, papulovesicles, scaly plaques, erosions, and petechiae. Lesions can be solitary or widespread and favor the trunk, head, and face.4 We describe an atypical case of hypopigmented cutaneous LCH and review the literature on this morphology in patients with skin of color.
A 7-month-old Hispanic male infant who was otherwise healthy presented with numerous hypopigmented macules and pink papules on the trunk and groin that had progressed since birth. A review of systems was unremarkable. Physical examination revealed 1- to 3-mm, discrete, hypopigmented macules intermixed with 1- to 2-mm pearly pink papules scattered on the back, chest, abdomen, and inguinal folds (Figure 1). Some lesions appeared koebnerized; however, the parents denied a history of scratching or trauma.
Histopathology of a lesion in the inguinal fold showed aggregates of mononuclear cells with reniform nuclei and abundant amphophilic cytoplasm in the papillary dermis, with focal extension into the epidermis. Scattered eosinophils and multinucleated giant cells were present in the dermal inflammatory infiltrate (Figure 2). Immunohistochemical staining was positive for CD1a (Figure 3) and S-100 protein (Figure 4). Although epidermal Langerhans cell collections also can be seen in allergic contact dermatitis,5 predominant involvement of the papillary dermis and the presence of multinucleated giant cells are characteristic of LCH.4 Given these findings, which were consistent with LCH, the dermatopathology deemed BRAF V600E immunostaining unnecessary for diagnostic purposes.
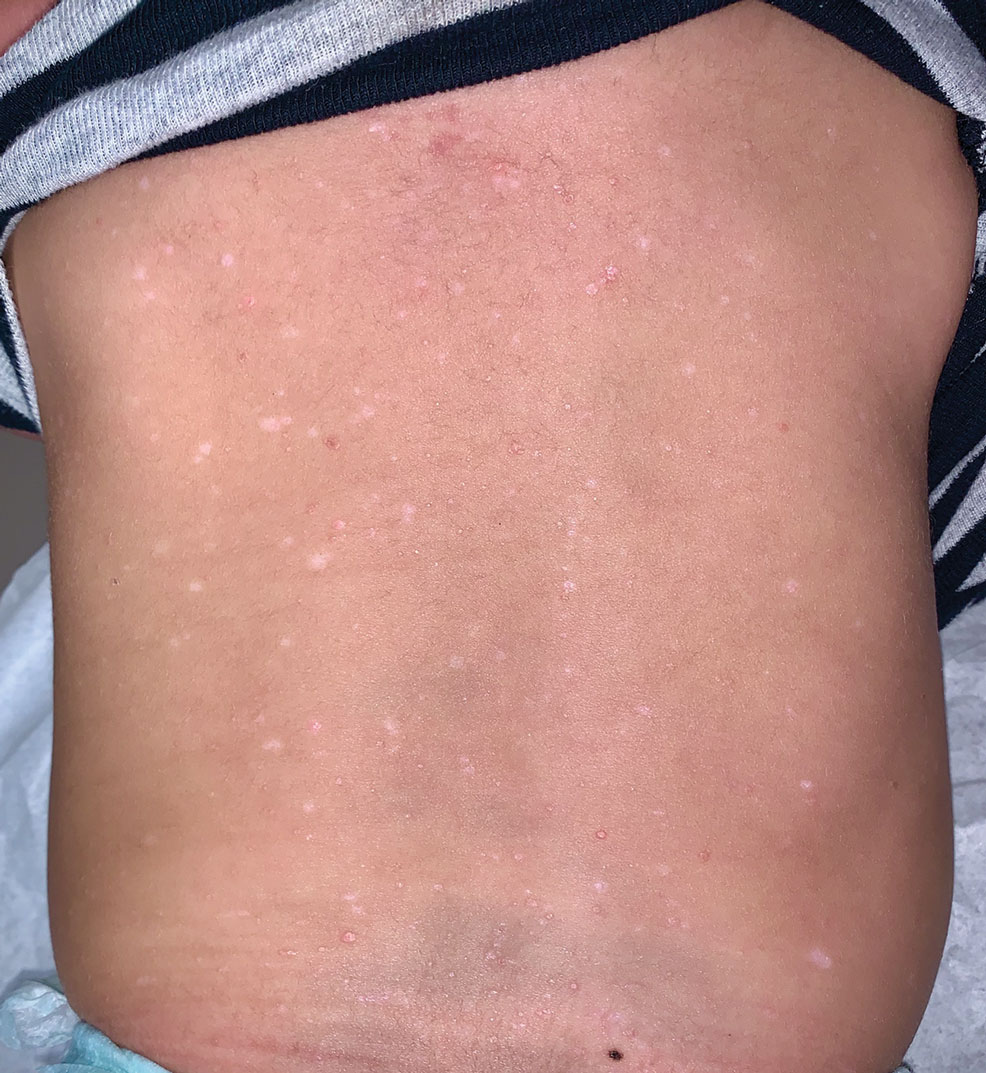
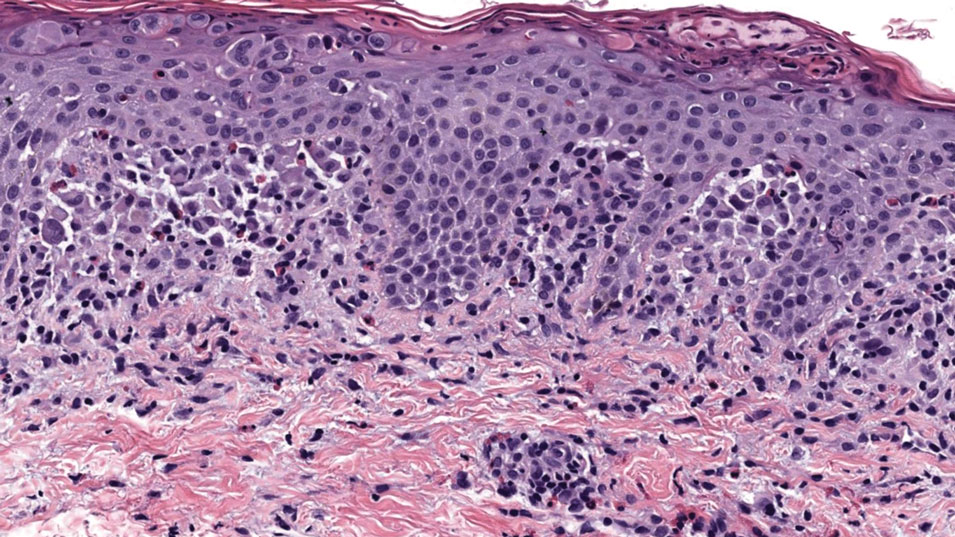
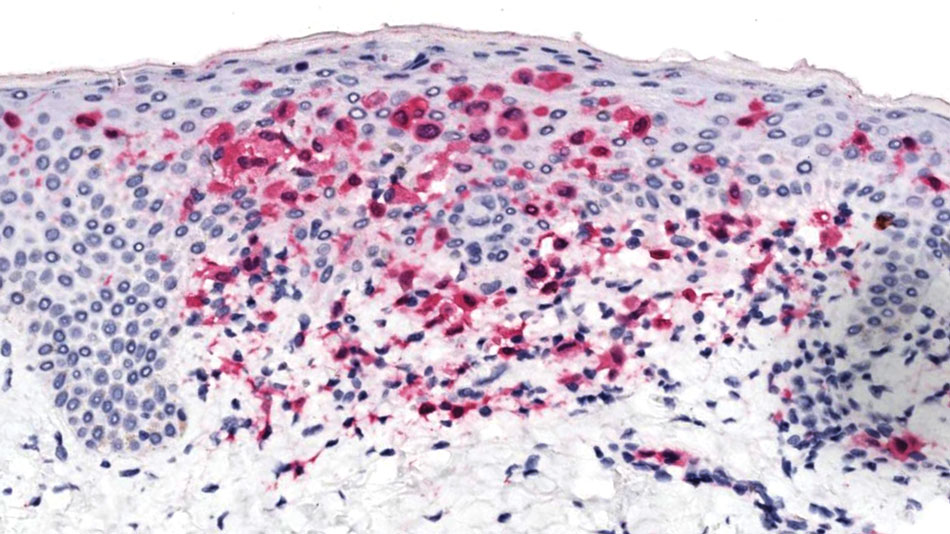
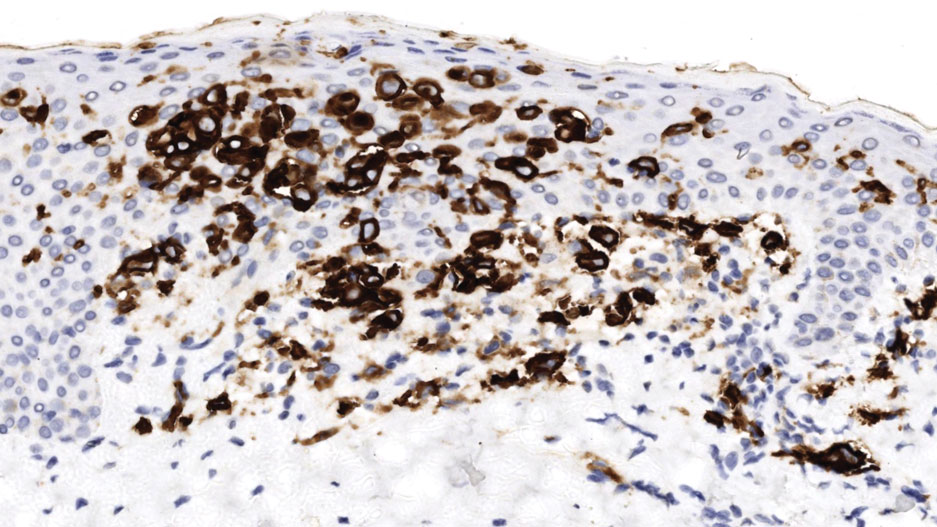
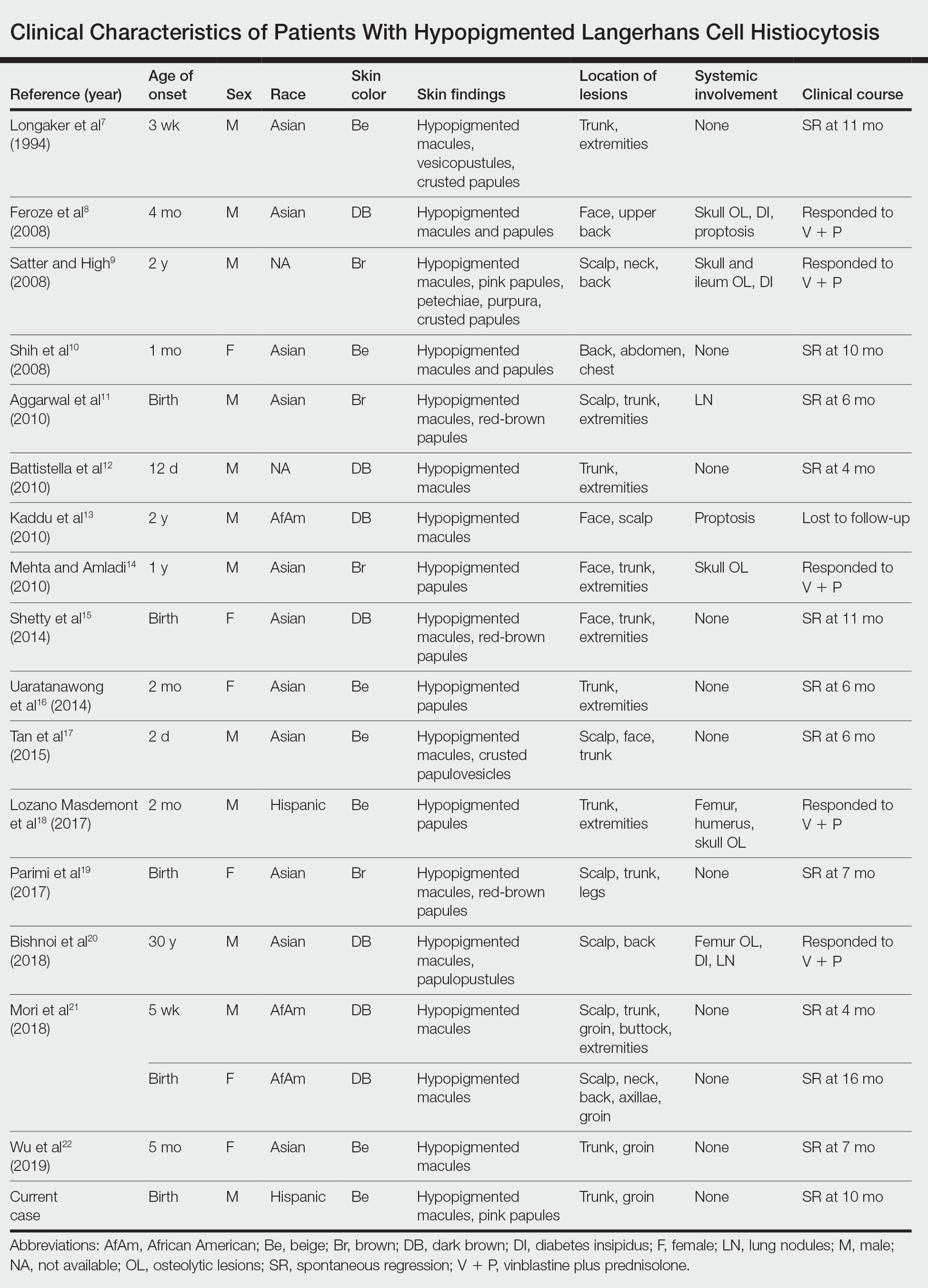
The patient was referred to the hematology and oncology department to undergo thorough evaluation for extracutaneous involvement. The workup included a complete blood cell count, liver function testing, electrolyte assessment, skeletal survey, chest radiography, and ultrasonography of the liver and spleen. All results were negative, suggesting a diagnosis of single-system cutaneous LCH.
Three months later, the patient presented to dermatology with spontaneous regression of all skin lesions. Continued follow-up—every 6 months for 5 years—was recommended to monitor for disease recurrence or progression to multisystem disease.
Cutaneous LCH is a clinically heterogeneous disease with the potential for multisystem involvement and long-term sequelae; therefore, timely diagnosis is paramount to optimize outcomes. However, delayed diagnosis is common because of the spectrum of skin findings that can mimic common pediatric dermatoses, such as seborrheic dermatitis, atopic dermatitis, and diaper dermatitis.4 In one study, the median time from onset of skin lesions to diagnostic biopsy was longer than 3 months (maximum, 5 years).6 Our patient was referred to dermatology 7 months after onset of hypopigmented macules, a rarely reported cutaneous manifestation of LCH.
A PubMed search of articles indexed for MEDLINE from 1994 to 2019 using the terms Langerhans cell histiocytotis and hypopigmented yielded 17 cases of LCH presenting as hypopigmented skin lesions (Table).7-22 All cases occurred in patients with skin of color (ie, patients of Asian, Hispanic, or African descent). Hypopigmented macules were the only cutaneous manifestation in 10 (59%) cases. Lesions most commonly were distributed on the trunk (16/17 [94%]) and extremities (8/17 [47%]). The median age of onset was 1 month; 76% (13/17) of patients developed skin lesions before 1 year of age, indicating that this morphology may be more common in newborns. In most patients, the diagnosis was single-system cutaneous LCH; they exhibited spontaneous regression by 8 months of age on average, suggesting that this variant may be associated with a better prognosis. Mori and colleagues21 hypothesized that hypopigmented lesions may represent the resolving stage of active LCH based on histopathologic findings of dermal pallor and fibrosis in a hypopigmented LCH lesion. However, systemic involvement was reported in 7 cases of hypopigmented LCH, highlighting the importance of assessing for multisystem disease regardless of cutaneous morphology.21Langerhans cell histiocytosis should be considered in the differential diagnosis when evaluating hypopigmented skin eruptions in infants with darker skin types. Prompt diagnosis of this atypical variant requires a higher index of suspicion because of its rarity and the polymorphic nature of cutaneous LCH. This morphology may go undiagnosed in the setting of mild or spontaneously resolving disease; notwithstanding, accurate diagnosis and longitudinal surveillance are necessary given the potential for progressive systemic involvement.
1. Guyot-Goubin A, Donadieu J, Barkaoui M, et al. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000–2004. Pediatr Blood Cancer. 2008;51:71-75. doi:10.1002/pbc.21498
2. Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
3. Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
4. Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: history, classification, pathobiology, clinical manifestations, and prognosis. J Am Acad Dermatol. 2018;78:1035-1044. doi:10.1016/j.jaad.2017.05.059
5. Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504. doi:10.1111/cup.12707
6. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996. doi:10.1016/j.jpeds.2014.07.063
7. Longaker MA, Frieden IJ, LeBoit PE, et al. Congenital “self-healing” Langerhans cell histiocytosis: the need for long-term follow-up. J Am Acad Dermatol. 1994;31(5, pt 2):910-916. doi:10.1016/s0190-9622(94)70258-6
8. Feroze K, Unni M, Jayasree MG, et al. Langerhans cell histiocytosis presenting with hypopigmented macules. Indian J Dermatol Venereol Leprol. 2008;74:670-672. doi:10.4103/0378-6323.45128
9. Satter EK, High WA. Langerhans cell histiocytosis: a case report and summary of the current recommendations of the Histiocyte Society. Dermatol Online J. 2008;14:3.
10. Chang SL, Shih IH, Kuo TT, et al. Congenital self-healing reticulohistiocytosis presenting as hypopigmented macules and papules in a neonate. Dermatologica Sinica 2008;26:80-84.
11. Aggarwal V, Seth A, Jain M, et al. Congenital Langerhans cell histiocytosis with skin and lung involvement: spontaneous regression. Indian J Pediatr. 2010;77:811-812.
12. Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156. doi:10.1001/archdermatol.2009.360
13. Kaddu S, Mulyowa G, Kovarik C. Hypopigmented scaly, scalp and facial lesions and disfiguring exopthalmus. Clin Exp Dermatol. 2010;3:E52-E53. doi:10.1111/j.1365-2230.2009.03336.x
14. Mehta B, Amladi S. Langerhans cell histiocytosis presenting as hypopigmented papules. Pediatr Dermatol. 2010;27:215-217. doi:10.1111/j.1525-1470.2010.01104.x
15. Shetty S, Monappa V, Pai K, et al. Congenital self-healing reticulohistiocytosis: a case report. Our Dermatol Online. 2014;5:264-266.
16. Uaratanawong R, Kootiratrakarn T, Sudtikoonaseth P, et al. Congenital self-healing reticulohistiocytosis presented with multiple hypopigmented flat-topped papules: a case report and review of literatures. J Med Assoc Thai. 2014;97:993-997.
17. Tan Q, Gan LQ, Wang H. Congenital self-healing Langerhans cell histiocytosis in a male neonate. Indian J Dermatol Venereol Leprol. 2015;81:75-77. doi:10.4103/0378-6323.148587
18. Lozano Masdemont B, Gómez‐Recuero Muñoz L, Villanueva Álvarez‐Santullano A, et al. Langerhans cell histiocytosis mimicking lichen nitidus with bone involvement. Australas J Dermatol. 2017;58:231-233. doi:10.1111/ajd.12467
19. Parimi LR, You J, Hong L, et al. Congenital self-healing reticulohistiocytosis with spontaneous regression. An Bras Dermatol. 2017;92:553-555. doi:10.1590/abd1806-4841.20175432
20. Bishnoi A, De D, Khullar G, et al. Hypopigmented and acneiform lesions: an unusual initial presentation of adult-onset multisystem Langerhans cell histiocytosis. Indian J Dermatol Venereol Leprol. 2018;84:621-626. doi:10.4103/ijdvl.IJDVL_639_17
21. Mori S, Adar T, Kazlouskaya V, et al. Cutaneous Langerhans cell histiocytosis presenting with hypopigmented lesions: report of two cases and review of literature. Pediatr Dermatol. 2018;35:502-506. doi:10.1111/pde.13509
22. Wu X, Huang J, Jiang L, et al. Congenital self‐healing reticulohistiocytosis with BRAF V600E mutation in an infant. Clin Exp Dermatol. 2019;44:647-650. doi:10.1111/ced.13880
To the Editor:
Langerhans cell histiocytosis (LCH) is a rare inflammatory neoplasia caused by accumulation of clonal Langerhans cells in 1 or more organs. The clinical spectrum is diverse, ranging from mild, single-organ involvement that may resolve spontaneously to severe progressive multisystem disease that can be fatal. It is most prevalent in children, affecting an estimated 4 to 5 children for every 1 million annually, with male predominance.1 The pathogenesis is driven by activating mutations in the mitogen-activated protein kinase pathway, with the BRAF V600E mutation detected in most LCH patients, resulting in proliferation of pathologic Langerhans cells and dysregulated expression of inflammatory cytokines in LCH lesions.2 A biopsy of lesional tissue is required for definitive diagnosis. Histopathology reveals a mixed inflammatory infiltrate and characteristic mononuclear cells with reniform nuclei that are positive for CD1a and CD207 proteins on immunohistochemical staining.3
Langerhans cell histiocytosis is categorized by the extent of organ involvement. It commonly affects the bones, skin, pituitary gland, liver, lungs, bone marrow, and lymph nodes.4 Single-system LCH involves a single organ with unifocal or multifocal lesions; multisystem LCH involves 2 or more organs and has a worse prognosis if risk organs (eg, liver, spleen, bone marrow) are involved.4
Skin lesions are reported in more than half of LCH cases and are the most common initial manifestation in patients younger than 2 years.4 Cutaneous findings are highly variable, which poses a diagnostic challenge. Common morphologies include erythematous papules, pustules, papulovesicles, scaly plaques, erosions, and petechiae. Lesions can be solitary or widespread and favor the trunk, head, and face.4 We describe an atypical case of hypopigmented cutaneous LCH and review the literature on this morphology in patients with skin of color.
A 7-month-old Hispanic male infant who was otherwise healthy presented with numerous hypopigmented macules and pink papules on the trunk and groin that had progressed since birth. A review of systems was unremarkable. Physical examination revealed 1- to 3-mm, discrete, hypopigmented macules intermixed with 1- to 2-mm pearly pink papules scattered on the back, chest, abdomen, and inguinal folds (Figure 1). Some lesions appeared koebnerized; however, the parents denied a history of scratching or trauma.
Histopathology of a lesion in the inguinal fold showed aggregates of mononuclear cells with reniform nuclei and abundant amphophilic cytoplasm in the papillary dermis, with focal extension into the epidermis. Scattered eosinophils and multinucleated giant cells were present in the dermal inflammatory infiltrate (Figure 2). Immunohistochemical staining was positive for CD1a (Figure 3) and S-100 protein (Figure 4). Although epidermal Langerhans cell collections also can be seen in allergic contact dermatitis,5 predominant involvement of the papillary dermis and the presence of multinucleated giant cells are characteristic of LCH.4 Given these findings, which were consistent with LCH, the dermatopathology deemed BRAF V600E immunostaining unnecessary for diagnostic purposes.





The patient was referred to the hematology and oncology department to undergo thorough evaluation for extracutaneous involvement. The workup included a complete blood cell count, liver function testing, electrolyte assessment, skeletal survey, chest radiography, and ultrasonography of the liver and spleen. All results were negative, suggesting a diagnosis of single-system cutaneous LCH.
Three months later, the patient presented to dermatology with spontaneous regression of all skin lesions. Continued follow-up—every 6 months for 5 years—was recommended to monitor for disease recurrence or progression to multisystem disease.
Cutaneous LCH is a clinically heterogeneous disease with the potential for multisystem involvement and long-term sequelae; therefore, timely diagnosis is paramount to optimize outcomes. However, delayed diagnosis is common because of the spectrum of skin findings that can mimic common pediatric dermatoses, such as seborrheic dermatitis, atopic dermatitis, and diaper dermatitis.4 In one study, the median time from onset of skin lesions to diagnostic biopsy was longer than 3 months (maximum, 5 years).6 Our patient was referred to dermatology 7 months after onset of hypopigmented macules, a rarely reported cutaneous manifestation of LCH.
A PubMed search of articles indexed for MEDLINE from 1994 to 2019 using the terms Langerhans cell histiocytotis and hypopigmented yielded 17 cases of LCH presenting as hypopigmented skin lesions (Table).7-22 All cases occurred in patients with skin of color (ie, patients of Asian, Hispanic, or African descent). Hypopigmented macules were the only cutaneous manifestation in 10 (59%) cases. Lesions most commonly were distributed on the trunk (16/17 [94%]) and extremities (8/17 [47%]). The median age of onset was 1 month; 76% (13/17) of patients developed skin lesions before 1 year of age, indicating that this morphology may be more common in newborns. In most patients, the diagnosis was single-system cutaneous LCH; they exhibited spontaneous regression by 8 months of age on average, suggesting that this variant may be associated with a better prognosis. Mori and colleagues21 hypothesized that hypopigmented lesions may represent the resolving stage of active LCH based on histopathologic findings of dermal pallor and fibrosis in a hypopigmented LCH lesion. However, systemic involvement was reported in 7 cases of hypopigmented LCH, highlighting the importance of assessing for multisystem disease regardless of cutaneous morphology.21Langerhans cell histiocytosis should be considered in the differential diagnosis when evaluating hypopigmented skin eruptions in infants with darker skin types. Prompt diagnosis of this atypical variant requires a higher index of suspicion because of its rarity and the polymorphic nature of cutaneous LCH. This morphology may go undiagnosed in the setting of mild or spontaneously resolving disease; notwithstanding, accurate diagnosis and longitudinal surveillance are necessary given the potential for progressive systemic involvement.
To the Editor:
Langerhans cell histiocytosis (LCH) is a rare inflammatory neoplasia caused by accumulation of clonal Langerhans cells in 1 or more organs. The clinical spectrum is diverse, ranging from mild, single-organ involvement that may resolve spontaneously to severe progressive multisystem disease that can be fatal. It is most prevalent in children, affecting an estimated 4 to 5 children for every 1 million annually, with male predominance.1 The pathogenesis is driven by activating mutations in the mitogen-activated protein kinase pathway, with the BRAF V600E mutation detected in most LCH patients, resulting in proliferation of pathologic Langerhans cells and dysregulated expression of inflammatory cytokines in LCH lesions.2 A biopsy of lesional tissue is required for definitive diagnosis. Histopathology reveals a mixed inflammatory infiltrate and characteristic mononuclear cells with reniform nuclei that are positive for CD1a and CD207 proteins on immunohistochemical staining.3
Langerhans cell histiocytosis is categorized by the extent of organ involvement. It commonly affects the bones, skin, pituitary gland, liver, lungs, bone marrow, and lymph nodes.4 Single-system LCH involves a single organ with unifocal or multifocal lesions; multisystem LCH involves 2 or more organs and has a worse prognosis if risk organs (eg, liver, spleen, bone marrow) are involved.4
Skin lesions are reported in more than half of LCH cases and are the most common initial manifestation in patients younger than 2 years.4 Cutaneous findings are highly variable, which poses a diagnostic challenge. Common morphologies include erythematous papules, pustules, papulovesicles, scaly plaques, erosions, and petechiae. Lesions can be solitary or widespread and favor the trunk, head, and face.4 We describe an atypical case of hypopigmented cutaneous LCH and review the literature on this morphology in patients with skin of color.
A 7-month-old Hispanic male infant who was otherwise healthy presented with numerous hypopigmented macules and pink papules on the trunk and groin that had progressed since birth. A review of systems was unremarkable. Physical examination revealed 1- to 3-mm, discrete, hypopigmented macules intermixed with 1- to 2-mm pearly pink papules scattered on the back, chest, abdomen, and inguinal folds (Figure 1). Some lesions appeared koebnerized; however, the parents denied a history of scratching or trauma.
Histopathology of a lesion in the inguinal fold showed aggregates of mononuclear cells with reniform nuclei and abundant amphophilic cytoplasm in the papillary dermis, with focal extension into the epidermis. Scattered eosinophils and multinucleated giant cells were present in the dermal inflammatory infiltrate (Figure 2). Immunohistochemical staining was positive for CD1a (Figure 3) and S-100 protein (Figure 4). Although epidermal Langerhans cell collections also can be seen in allergic contact dermatitis,5 predominant involvement of the papillary dermis and the presence of multinucleated giant cells are characteristic of LCH.4 Given these findings, which were consistent with LCH, the dermatopathology deemed BRAF V600E immunostaining unnecessary for diagnostic purposes.





The patient was referred to the hematology and oncology department to undergo thorough evaluation for extracutaneous involvement. The workup included a complete blood cell count, liver function testing, electrolyte assessment, skeletal survey, chest radiography, and ultrasonography of the liver and spleen. All results were negative, suggesting a diagnosis of single-system cutaneous LCH.
Three months later, the patient presented to dermatology with spontaneous regression of all skin lesions. Continued follow-up—every 6 months for 5 years—was recommended to monitor for disease recurrence or progression to multisystem disease.
Cutaneous LCH is a clinically heterogeneous disease with the potential for multisystem involvement and long-term sequelae; therefore, timely diagnosis is paramount to optimize outcomes. However, delayed diagnosis is common because of the spectrum of skin findings that can mimic common pediatric dermatoses, such as seborrheic dermatitis, atopic dermatitis, and diaper dermatitis.4 In one study, the median time from onset of skin lesions to diagnostic biopsy was longer than 3 months (maximum, 5 years).6 Our patient was referred to dermatology 7 months after onset of hypopigmented macules, a rarely reported cutaneous manifestation of LCH.
A PubMed search of articles indexed for MEDLINE from 1994 to 2019 using the terms Langerhans cell histiocytotis and hypopigmented yielded 17 cases of LCH presenting as hypopigmented skin lesions (Table).7-22 All cases occurred in patients with skin of color (ie, patients of Asian, Hispanic, or African descent). Hypopigmented macules were the only cutaneous manifestation in 10 (59%) cases. Lesions most commonly were distributed on the trunk (16/17 [94%]) and extremities (8/17 [47%]). The median age of onset was 1 month; 76% (13/17) of patients developed skin lesions before 1 year of age, indicating that this morphology may be more common in newborns. In most patients, the diagnosis was single-system cutaneous LCH; they exhibited spontaneous regression by 8 months of age on average, suggesting that this variant may be associated with a better prognosis. Mori and colleagues21 hypothesized that hypopigmented lesions may represent the resolving stage of active LCH based on histopathologic findings of dermal pallor and fibrosis in a hypopigmented LCH lesion. However, systemic involvement was reported in 7 cases of hypopigmented LCH, highlighting the importance of assessing for multisystem disease regardless of cutaneous morphology.21Langerhans cell histiocytosis should be considered in the differential diagnosis when evaluating hypopigmented skin eruptions in infants with darker skin types. Prompt diagnosis of this atypical variant requires a higher index of suspicion because of its rarity and the polymorphic nature of cutaneous LCH. This morphology may go undiagnosed in the setting of mild or spontaneously resolving disease; notwithstanding, accurate diagnosis and longitudinal surveillance are necessary given the potential for progressive systemic involvement.
1. Guyot-Goubin A, Donadieu J, Barkaoui M, et al. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000–2004. Pediatr Blood Cancer. 2008;51:71-75. doi:10.1002/pbc.21498
2. Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
3. Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
4. Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: history, classification, pathobiology, clinical manifestations, and prognosis. J Am Acad Dermatol. 2018;78:1035-1044. doi:10.1016/j.jaad.2017.05.059
5. Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504. doi:10.1111/cup.12707
6. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996. doi:10.1016/j.jpeds.2014.07.063
7. Longaker MA, Frieden IJ, LeBoit PE, et al. Congenital “self-healing” Langerhans cell histiocytosis: the need for long-term follow-up. J Am Acad Dermatol. 1994;31(5, pt 2):910-916. doi:10.1016/s0190-9622(94)70258-6
8. Feroze K, Unni M, Jayasree MG, et al. Langerhans cell histiocytosis presenting with hypopigmented macules. Indian J Dermatol Venereol Leprol. 2008;74:670-672. doi:10.4103/0378-6323.45128
9. Satter EK, High WA. Langerhans cell histiocytosis: a case report and summary of the current recommendations of the Histiocyte Society. Dermatol Online J. 2008;14:3.
10. Chang SL, Shih IH, Kuo TT, et al. Congenital self-healing reticulohistiocytosis presenting as hypopigmented macules and papules in a neonate. Dermatologica Sinica 2008;26:80-84.
11. Aggarwal V, Seth A, Jain M, et al. Congenital Langerhans cell histiocytosis with skin and lung involvement: spontaneous regression. Indian J Pediatr. 2010;77:811-812.
12. Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156. doi:10.1001/archdermatol.2009.360
13. Kaddu S, Mulyowa G, Kovarik C. Hypopigmented scaly, scalp and facial lesions and disfiguring exopthalmus. Clin Exp Dermatol. 2010;3:E52-E53. doi:10.1111/j.1365-2230.2009.03336.x
14. Mehta B, Amladi S. Langerhans cell histiocytosis presenting as hypopigmented papules. Pediatr Dermatol. 2010;27:215-217. doi:10.1111/j.1525-1470.2010.01104.x
15. Shetty S, Monappa V, Pai K, et al. Congenital self-healing reticulohistiocytosis: a case report. Our Dermatol Online. 2014;5:264-266.
16. Uaratanawong R, Kootiratrakarn T, Sudtikoonaseth P, et al. Congenital self-healing reticulohistiocytosis presented with multiple hypopigmented flat-topped papules: a case report and review of literatures. J Med Assoc Thai. 2014;97:993-997.
17. Tan Q, Gan LQ, Wang H. Congenital self-healing Langerhans cell histiocytosis in a male neonate. Indian J Dermatol Venereol Leprol. 2015;81:75-77. doi:10.4103/0378-6323.148587
18. Lozano Masdemont B, Gómez‐Recuero Muñoz L, Villanueva Álvarez‐Santullano A, et al. Langerhans cell histiocytosis mimicking lichen nitidus with bone involvement. Australas J Dermatol. 2017;58:231-233. doi:10.1111/ajd.12467
19. Parimi LR, You J, Hong L, et al. Congenital self-healing reticulohistiocytosis with spontaneous regression. An Bras Dermatol. 2017;92:553-555. doi:10.1590/abd1806-4841.20175432
20. Bishnoi A, De D, Khullar G, et al. Hypopigmented and acneiform lesions: an unusual initial presentation of adult-onset multisystem Langerhans cell histiocytosis. Indian J Dermatol Venereol Leprol. 2018;84:621-626. doi:10.4103/ijdvl.IJDVL_639_17
21. Mori S, Adar T, Kazlouskaya V, et al. Cutaneous Langerhans cell histiocytosis presenting with hypopigmented lesions: report of two cases and review of literature. Pediatr Dermatol. 2018;35:502-506. doi:10.1111/pde.13509
22. Wu X, Huang J, Jiang L, et al. Congenital self‐healing reticulohistiocytosis with BRAF V600E mutation in an infant. Clin Exp Dermatol. 2019;44:647-650. doi:10.1111/ced.13880
1. Guyot-Goubin A, Donadieu J, Barkaoui M, et al. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000–2004. Pediatr Blood Cancer. 2008;51:71-75. doi:10.1002/pbc.21498
2. Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
3. Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
4. Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: history, classification, pathobiology, clinical manifestations, and prognosis. J Am Acad Dermatol. 2018;78:1035-1044. doi:10.1016/j.jaad.2017.05.059
5. Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504. doi:10.1111/cup.12707
6. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996. doi:10.1016/j.jpeds.2014.07.063
7. Longaker MA, Frieden IJ, LeBoit PE, et al. Congenital “self-healing” Langerhans cell histiocytosis: the need for long-term follow-up. J Am Acad Dermatol. 1994;31(5, pt 2):910-916. doi:10.1016/s0190-9622(94)70258-6
8. Feroze K, Unni M, Jayasree MG, et al. Langerhans cell histiocytosis presenting with hypopigmented macules. Indian J Dermatol Venereol Leprol. 2008;74:670-672. doi:10.4103/0378-6323.45128
9. Satter EK, High WA. Langerhans cell histiocytosis: a case report and summary of the current recommendations of the Histiocyte Society. Dermatol Online J. 2008;14:3.
10. Chang SL, Shih IH, Kuo TT, et al. Congenital self-healing reticulohistiocytosis presenting as hypopigmented macules and papules in a neonate. Dermatologica Sinica 2008;26:80-84.
11. Aggarwal V, Seth A, Jain M, et al. Congenital Langerhans cell histiocytosis with skin and lung involvement: spontaneous regression. Indian J Pediatr. 2010;77:811-812.
12. Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156. doi:10.1001/archdermatol.2009.360
13. Kaddu S, Mulyowa G, Kovarik C. Hypopigmented scaly, scalp and facial lesions and disfiguring exopthalmus. Clin Exp Dermatol. 2010;3:E52-E53. doi:10.1111/j.1365-2230.2009.03336.x
14. Mehta B, Amladi S. Langerhans cell histiocytosis presenting as hypopigmented papules. Pediatr Dermatol. 2010;27:215-217. doi:10.1111/j.1525-1470.2010.01104.x
15. Shetty S, Monappa V, Pai K, et al. Congenital self-healing reticulohistiocytosis: a case report. Our Dermatol Online. 2014;5:264-266.
16. Uaratanawong R, Kootiratrakarn T, Sudtikoonaseth P, et al. Congenital self-healing reticulohistiocytosis presented with multiple hypopigmented flat-topped papules: a case report and review of literatures. J Med Assoc Thai. 2014;97:993-997.
17. Tan Q, Gan LQ, Wang H. Congenital self-healing Langerhans cell histiocytosis in a male neonate. Indian J Dermatol Venereol Leprol. 2015;81:75-77. doi:10.4103/0378-6323.148587
18. Lozano Masdemont B, Gómez‐Recuero Muñoz L, Villanueva Álvarez‐Santullano A, et al. Langerhans cell histiocytosis mimicking lichen nitidus with bone involvement. Australas J Dermatol. 2017;58:231-233. doi:10.1111/ajd.12467
19. Parimi LR, You J, Hong L, et al. Congenital self-healing reticulohistiocytosis with spontaneous regression. An Bras Dermatol. 2017;92:553-555. doi:10.1590/abd1806-4841.20175432
20. Bishnoi A, De D, Khullar G, et al. Hypopigmented and acneiform lesions: an unusual initial presentation of adult-onset multisystem Langerhans cell histiocytosis. Indian J Dermatol Venereol Leprol. 2018;84:621-626. doi:10.4103/ijdvl.IJDVL_639_17
21. Mori S, Adar T, Kazlouskaya V, et al. Cutaneous Langerhans cell histiocytosis presenting with hypopigmented lesions: report of two cases and review of literature. Pediatr Dermatol. 2018;35:502-506. doi:10.1111/pde.13509
22. Wu X, Huang J, Jiang L, et al. Congenital self‐healing reticulohistiocytosis with BRAF V600E mutation in an infant. Clin Exp Dermatol. 2019;44:647-650. doi:10.1111/ced.13880
Practice Points
- Dermatologists should be aware of the hypopigmented variant of cutaneous Langerhans cell histiocytosis (LCH), which has been reported exclusively in patients with skin of color.
- Langerhans cell histiocytosis should be included in the differential diagnosis of hypopigmented macules, which may be the only cutaneous manifestation or may coincide with typical lesions of LCH.
- Hypopigmented cutaneous LCH may be more common in newborns and associated with a better prognosis.
Chatbots Seem More Empathetic Than Docs in Cancer Discussions
Large language models (LLM) such as ChatGPT have shown mixed results in the quality of their responses to consumer questions about cancer.
One recent study found AI chatbots to churn out incomplete, inaccurate, or even nonsensical cancer treatment recommendations, while another found them to generate largely accurate — if technical — responses to the most common cancer questions.
While researchers have seen success with purpose-built chatbots created to address patient concerns about specific cancers, the consensus to date has been that the generalized models like ChatGPT remain works in progress and that physicians should avoid pointing patients to them, for now.
Yet new findings suggest that these chatbots may do better than individual physicians, at least on some measures, when it comes to answering queries about cancer. For research published May 16 in JAMA Oncology (doi: 10.1001/jamaoncol.2024.0836), David Chen, a medical student at the University of Toronto, and his colleagues, isolated a random sample of 200 questions related to cancer care addressed to doctors on the public online forum Reddit. They then compared responses from oncologists with responses generated by three different AI chatbots. The blinded responses were rated for quality, readability, and empathy by six physicians, including oncologists and palliative and supportive care specialists.
Mr. Chen and colleagues’ research was modeled after a 2023 study that measured the quality of physician responses compared with chatbots for general medicine questions addressed to doctors on Reddit. That study found that the chatbots produced more empathetic-sounding answers, something Mr. Chen’s study also found. : quality, empathy, and readability.
Q&A With Author of New Research
Mr. Chen discussed his new study’s implications during an interview with this news organization.
Question: What is novel about this study?
Mr. Chen: We’ve seen many evaluations of chatbots that test for medical accuracy, but this study occurs in the domain of oncology care, where there are unique psychosocial and emotional considerations that are not precisely reflected in a general medicine setting. In effect, this study is putting these chatbots through a harder challenge.
Question: Why would chatbot responses seem more empathetic than those of physicians?
Mr. Chen: With the physician responses that we observed in our sample data set, we saw that there was very high variation of amount of apparent effort [in the physician responses]. Some physicians would put in a lot of time and effort, thinking through their response, and others wouldn’t do so as much. These chatbots don’t face fatigue the way humans do, or burnout. So they’re able to consistently provide responses with less variation in empathy.
Question: Do chatbots just seem empathetic because they are chattier?
Mr. Chen: We did think of verbosity as a potential confounder in this study. So we set a word count limit for the chatbot responses to keep it in the range of the physician responses. That way, verbosity was no longer a significant factor.
Question: How were quality and empathy measured by the reviewers?
Mr. Chen: For our study we used two teams of readers, each team composed of three physicians. In terms of the actual metrics we used, they were pilot metrics. There are no well-defined measurement scales or checklists that we could use to measure empathy. This is an emerging field of research. So we came up by consensus with our own set of ratings, and we feel that this is an area for the research to define a standardized set of guidelines.
Another novel aspect of this study is that we separated out different dimensions of quality and empathy. A quality response didn’t just mean it was medically accurate — quality also had to do with the focus and completeness of the response.
With empathy there are cognitive and emotional dimensions. Cognitive empathy uses critical thinking to understand the person’s emotions and thoughts and then adjusting a response to fit that. A patient may not want the best medically indicated treatment for their condition, because they want to preserve their quality of life. The chatbot may be able to adjust its recommendation with consideration of some of those humanistic elements that the patient is presenting with.
Emotional empathy is more about being supportive of the patient’s emotions by using expressions like ‘I understand where you’re coming from.’ or, ‘I can see how that makes you feel.’
Question: Why would physicians, not patients, be the best evaluators of empathy?
Mr. Chen: We’re actually very interested in evaluating patient ratings of empathy. We are conducting a follow-up study that evaluates patient ratings of empathy to the same set of chatbot and physician responses,to see if there are differences.
Question: Should cancer patients go ahead and consult chatbots?
Mr. Chen: Although we did observe increases in all of the metrics compared with physicians, this is a very specialized evaluation scenario where we’re using these Reddit questions and responses.
Naturally, we would need to do a trial, a head to head randomized comparison of physicians versus chatbots.
This pilot study does highlight the promising potential of these chatbots to suggest responses. But we can’t fully recommend that they should be used as standalone clinical tools without physicians.
This Q&A was edited for clarity.
Large language models (LLM) such as ChatGPT have shown mixed results in the quality of their responses to consumer questions about cancer.
One recent study found AI chatbots to churn out incomplete, inaccurate, or even nonsensical cancer treatment recommendations, while another found them to generate largely accurate — if technical — responses to the most common cancer questions.
While researchers have seen success with purpose-built chatbots created to address patient concerns about specific cancers, the consensus to date has been that the generalized models like ChatGPT remain works in progress and that physicians should avoid pointing patients to them, for now.
Yet new findings suggest that these chatbots may do better than individual physicians, at least on some measures, when it comes to answering queries about cancer. For research published May 16 in JAMA Oncology (doi: 10.1001/jamaoncol.2024.0836), David Chen, a medical student at the University of Toronto, and his colleagues, isolated a random sample of 200 questions related to cancer care addressed to doctors on the public online forum Reddit. They then compared responses from oncologists with responses generated by three different AI chatbots. The blinded responses were rated for quality, readability, and empathy by six physicians, including oncologists and palliative and supportive care specialists.
Mr. Chen and colleagues’ research was modeled after a 2023 study that measured the quality of physician responses compared with chatbots for general medicine questions addressed to doctors on Reddit. That study found that the chatbots produced more empathetic-sounding answers, something Mr. Chen’s study also found. : quality, empathy, and readability.
Q&A With Author of New Research
Mr. Chen discussed his new study’s implications during an interview with this news organization.
Question: What is novel about this study?
Mr. Chen: We’ve seen many evaluations of chatbots that test for medical accuracy, but this study occurs in the domain of oncology care, where there are unique psychosocial and emotional considerations that are not precisely reflected in a general medicine setting. In effect, this study is putting these chatbots through a harder challenge.
Question: Why would chatbot responses seem more empathetic than those of physicians?
Mr. Chen: With the physician responses that we observed in our sample data set, we saw that there was very high variation of amount of apparent effort [in the physician responses]. Some physicians would put in a lot of time and effort, thinking through their response, and others wouldn’t do so as much. These chatbots don’t face fatigue the way humans do, or burnout. So they’re able to consistently provide responses with less variation in empathy.
Question: Do chatbots just seem empathetic because they are chattier?
Mr. Chen: We did think of verbosity as a potential confounder in this study. So we set a word count limit for the chatbot responses to keep it in the range of the physician responses. That way, verbosity was no longer a significant factor.
Question: How were quality and empathy measured by the reviewers?
Mr. Chen: For our study we used two teams of readers, each team composed of three physicians. In terms of the actual metrics we used, they were pilot metrics. There are no well-defined measurement scales or checklists that we could use to measure empathy. This is an emerging field of research. So we came up by consensus with our own set of ratings, and we feel that this is an area for the research to define a standardized set of guidelines.
Another novel aspect of this study is that we separated out different dimensions of quality and empathy. A quality response didn’t just mean it was medically accurate — quality also had to do with the focus and completeness of the response.
With empathy there are cognitive and emotional dimensions. Cognitive empathy uses critical thinking to understand the person’s emotions and thoughts and then adjusting a response to fit that. A patient may not want the best medically indicated treatment for their condition, because they want to preserve their quality of life. The chatbot may be able to adjust its recommendation with consideration of some of those humanistic elements that the patient is presenting with.
Emotional empathy is more about being supportive of the patient’s emotions by using expressions like ‘I understand where you’re coming from.’ or, ‘I can see how that makes you feel.’
Question: Why would physicians, not patients, be the best evaluators of empathy?
Mr. Chen: We’re actually very interested in evaluating patient ratings of empathy. We are conducting a follow-up study that evaluates patient ratings of empathy to the same set of chatbot and physician responses,to see if there are differences.
Question: Should cancer patients go ahead and consult chatbots?
Mr. Chen: Although we did observe increases in all of the metrics compared with physicians, this is a very specialized evaluation scenario where we’re using these Reddit questions and responses.
Naturally, we would need to do a trial, a head to head randomized comparison of physicians versus chatbots.
This pilot study does highlight the promising potential of these chatbots to suggest responses. But we can’t fully recommend that they should be used as standalone clinical tools without physicians.
This Q&A was edited for clarity.
Large language models (LLM) such as ChatGPT have shown mixed results in the quality of their responses to consumer questions about cancer.
One recent study found AI chatbots to churn out incomplete, inaccurate, or even nonsensical cancer treatment recommendations, while another found them to generate largely accurate — if technical — responses to the most common cancer questions.
While researchers have seen success with purpose-built chatbots created to address patient concerns about specific cancers, the consensus to date has been that the generalized models like ChatGPT remain works in progress and that physicians should avoid pointing patients to them, for now.
Yet new findings suggest that these chatbots may do better than individual physicians, at least on some measures, when it comes to answering queries about cancer. For research published May 16 in JAMA Oncology (doi: 10.1001/jamaoncol.2024.0836), David Chen, a medical student at the University of Toronto, and his colleagues, isolated a random sample of 200 questions related to cancer care addressed to doctors on the public online forum Reddit. They then compared responses from oncologists with responses generated by three different AI chatbots. The blinded responses were rated for quality, readability, and empathy by six physicians, including oncologists and palliative and supportive care specialists.
Mr. Chen and colleagues’ research was modeled after a 2023 study that measured the quality of physician responses compared with chatbots for general medicine questions addressed to doctors on Reddit. That study found that the chatbots produced more empathetic-sounding answers, something Mr. Chen’s study also found. : quality, empathy, and readability.
Q&A With Author of New Research
Mr. Chen discussed his new study’s implications during an interview with this news organization.
Question: What is novel about this study?
Mr. Chen: We’ve seen many evaluations of chatbots that test for medical accuracy, but this study occurs in the domain of oncology care, where there are unique psychosocial and emotional considerations that are not precisely reflected in a general medicine setting. In effect, this study is putting these chatbots through a harder challenge.
Question: Why would chatbot responses seem more empathetic than those of physicians?
Mr. Chen: With the physician responses that we observed in our sample data set, we saw that there was very high variation of amount of apparent effort [in the physician responses]. Some physicians would put in a lot of time and effort, thinking through their response, and others wouldn’t do so as much. These chatbots don’t face fatigue the way humans do, or burnout. So they’re able to consistently provide responses with less variation in empathy.
Question: Do chatbots just seem empathetic because they are chattier?
Mr. Chen: We did think of verbosity as a potential confounder in this study. So we set a word count limit for the chatbot responses to keep it in the range of the physician responses. That way, verbosity was no longer a significant factor.
Question: How were quality and empathy measured by the reviewers?
Mr. Chen: For our study we used two teams of readers, each team composed of three physicians. In terms of the actual metrics we used, they were pilot metrics. There are no well-defined measurement scales or checklists that we could use to measure empathy. This is an emerging field of research. So we came up by consensus with our own set of ratings, and we feel that this is an area for the research to define a standardized set of guidelines.
Another novel aspect of this study is that we separated out different dimensions of quality and empathy. A quality response didn’t just mean it was medically accurate — quality also had to do with the focus and completeness of the response.
With empathy there are cognitive and emotional dimensions. Cognitive empathy uses critical thinking to understand the person’s emotions and thoughts and then adjusting a response to fit that. A patient may not want the best medically indicated treatment for their condition, because they want to preserve their quality of life. The chatbot may be able to adjust its recommendation with consideration of some of those humanistic elements that the patient is presenting with.
Emotional empathy is more about being supportive of the patient’s emotions by using expressions like ‘I understand where you’re coming from.’ or, ‘I can see how that makes you feel.’
Question: Why would physicians, not patients, be the best evaluators of empathy?
Mr. Chen: We’re actually very interested in evaluating patient ratings of empathy. We are conducting a follow-up study that evaluates patient ratings of empathy to the same set of chatbot and physician responses,to see if there are differences.
Question: Should cancer patients go ahead and consult chatbots?
Mr. Chen: Although we did observe increases in all of the metrics compared with physicians, this is a very specialized evaluation scenario where we’re using these Reddit questions and responses.
Naturally, we would need to do a trial, a head to head randomized comparison of physicians versus chatbots.
This pilot study does highlight the promising potential of these chatbots to suggest responses. But we can’t fully recommend that they should be used as standalone clinical tools without physicians.
This Q&A was edited for clarity.
FROM JAMA ONCOLOGY
Pediatric Dermatologists Beat ChatGPT on Board Questions
In an experiment that pitted the wits of results from a small single-center study showed.
“We were relieved to find that the pediatric dermatologists in our study performed better than ChatGPT on both multiple choice and case-based questions; however, the latest iteration of ChatGPT (4.0) was very close,” one of the study’s first authors Charles Huang, a fourth-year medical student at Thomas Jefferson University, Philadelphia, said in an interview. “Something else that was interesting in our data was that the pediatric dermatologists performed much better than ChatGPT on questions related to procedural dermatology/surgical techniques, perhaps indicating that knowledge/reasoning gained through practical experience isn’t easily replicated in AI tools such as ChatGPT.”
For the study, which was published on May 9 in Pediatric Dermatology, Mr. Huang, and co-first author Esther Zhang, BS, a medical student at the University of Pennsylvania, Philadelphia, and coauthors from the Department of Dermatology, Children’s Hospital of Philadelphia, asked five pediatric dermatologists to answer 24 text-based questions including 16 single-answer, multiple-choice questions and two multiple answer questions drawn from the American Board of Dermatology 2021 Certification Sample Test and six free-response case-based questions drawn from the “Photoquiz” section of Pediatric Dermatology between July 2022 and July 2023. The researchers then processed the same set of questions through ChatGPT versions 3.5 and 4.0 and used statistical analysis to compare responses between the pediatric dermatologists and ChatGPT. A 5-point scale adapted from current AI tools was used to score replies to case-based questions.
On average, study participants had 5.6 years of clinical experience. Pediatric dermatologists performed significantly better than ChatGPT version 3.5 on multiple-choice and multiple answer questions (91.4% vs 76.2%, respectively; P = .021) but not significantly better than ChatGPT version 4.0 (90.5%; P = .44). As for replies to case-based questions, the average performance based on the 5-point scale was 3.81 for pediatric dermatologists and 3.53 for ChatGPT overall. The mean scores were significantly greater for pediatric dermatologists than for ChatGPT version 3.5 (P = .039) but not ChatGPT version 4.0 (P = .43).
The researchers acknowledged certain limitations of the analysis, including the evolving nature of AI tools, which may affect the reproducibility of results with subsequent model updates. And, while participating pediatric dermatologists said they were unfamiliar with the questions and cases used in the study, “there is potential for prior exposure through other dermatology board examination review processes,” they wrote.
“AI tools such as ChatGPT and similar large language models can be a valuable tool in your clinical practice, but be aware of potential pitfalls such as patient privacy, medical inaccuracies, [and] intrinsic biases in the tools,” Mr. Huang told this news organization. “As these technologies continue to advance, it is essential for all of us as medical clinicians to gain familiarity and stay abreast of new developments, just as we adapted to electronic health records and the use of the Internet.”
Maria Buethe, MD, PhD, a pediatric dermatology fellow at Rady Children’s Hospital–San Diego, who was asked to comment on the study, said she found it “interesting” that ChatGPT’s version 4.0 started to produce comparable results to clinician responses in some of the tested scenarios.
“The authors propose a set of best practices for pediatric dermatology clinicians using ChatGPT and other AI tools,” said Dr. Buethe, who was senior author of a recent literature review on AI and its application to pediatric dermatology. It was published in SKIN The Journal of Cutaneous Medicine. “One interesting recommended use for AI tools is to utilize it to generate differential diagnosis, which can broaden the list of pathologies previously considered.”
Asked to comment on the study, Erum Ilyas, MD, who practices dermatology in King of Prussia, Pennsylvania, and is a member of the Society for Pediatric Dermatology, said she was not surprised that ChatGPT “can perform fairly well on multiple-choice questions as we find available in testing circumstances,” as presented in the study. “Just as board questions only support testing a base of medical knowledge and facts for clinicians to master, they do not necessarily provide real-life circumstances that apply to caring for patients, which is inherently nuanced.”
In addition, the study “highlights that ChatGPT can be an aid to support thinking through differentials based on data entered by a clinician who understands how to phrase queries, especially if provided with enough data while respecting patient privacy, in the context of fact checking responses,” Dr. Ilyas said. “This underscores the fact that AI tools can be helpful to clinicians in assimilating various data points entered. However, ultimately, the tool is only able to support an output based on the information it has access to.” She added, “ChatGPT cannot be relied on to provide a single diagnosis with the clinician still responsible for making a final diagnosis. The tool is not definitive and cannot assimilate data that is not entered correctly.”
The study was not funded, and the study authors reported having no disclosures. Dr. Buethe and Dr. Ilyas, who were not involved with the study, had no disclosures.
A version of this article appeared on Medscape.com .
In an experiment that pitted the wits of results from a small single-center study showed.
“We were relieved to find that the pediatric dermatologists in our study performed better than ChatGPT on both multiple choice and case-based questions; however, the latest iteration of ChatGPT (4.0) was very close,” one of the study’s first authors Charles Huang, a fourth-year medical student at Thomas Jefferson University, Philadelphia, said in an interview. “Something else that was interesting in our data was that the pediatric dermatologists performed much better than ChatGPT on questions related to procedural dermatology/surgical techniques, perhaps indicating that knowledge/reasoning gained through practical experience isn’t easily replicated in AI tools such as ChatGPT.”
For the study, which was published on May 9 in Pediatric Dermatology, Mr. Huang, and co-first author Esther Zhang, BS, a medical student at the University of Pennsylvania, Philadelphia, and coauthors from the Department of Dermatology, Children’s Hospital of Philadelphia, asked five pediatric dermatologists to answer 24 text-based questions including 16 single-answer, multiple-choice questions and two multiple answer questions drawn from the American Board of Dermatology 2021 Certification Sample Test and six free-response case-based questions drawn from the “Photoquiz” section of Pediatric Dermatology between July 2022 and July 2023. The researchers then processed the same set of questions through ChatGPT versions 3.5 and 4.0 and used statistical analysis to compare responses between the pediatric dermatologists and ChatGPT. A 5-point scale adapted from current AI tools was used to score replies to case-based questions.
On average, study participants had 5.6 years of clinical experience. Pediatric dermatologists performed significantly better than ChatGPT version 3.5 on multiple-choice and multiple answer questions (91.4% vs 76.2%, respectively; P = .021) but not significantly better than ChatGPT version 4.0 (90.5%; P = .44). As for replies to case-based questions, the average performance based on the 5-point scale was 3.81 for pediatric dermatologists and 3.53 for ChatGPT overall. The mean scores were significantly greater for pediatric dermatologists than for ChatGPT version 3.5 (P = .039) but not ChatGPT version 4.0 (P = .43).
The researchers acknowledged certain limitations of the analysis, including the evolving nature of AI tools, which may affect the reproducibility of results with subsequent model updates. And, while participating pediatric dermatologists said they were unfamiliar with the questions and cases used in the study, “there is potential for prior exposure through other dermatology board examination review processes,” they wrote.
“AI tools such as ChatGPT and similar large language models can be a valuable tool in your clinical practice, but be aware of potential pitfalls such as patient privacy, medical inaccuracies, [and] intrinsic biases in the tools,” Mr. Huang told this news organization. “As these technologies continue to advance, it is essential for all of us as medical clinicians to gain familiarity and stay abreast of new developments, just as we adapted to electronic health records and the use of the Internet.”
Maria Buethe, MD, PhD, a pediatric dermatology fellow at Rady Children’s Hospital–San Diego, who was asked to comment on the study, said she found it “interesting” that ChatGPT’s version 4.0 started to produce comparable results to clinician responses in some of the tested scenarios.
“The authors propose a set of best practices for pediatric dermatology clinicians using ChatGPT and other AI tools,” said Dr. Buethe, who was senior author of a recent literature review on AI and its application to pediatric dermatology. It was published in SKIN The Journal of Cutaneous Medicine. “One interesting recommended use for AI tools is to utilize it to generate differential diagnosis, which can broaden the list of pathologies previously considered.”
Asked to comment on the study, Erum Ilyas, MD, who practices dermatology in King of Prussia, Pennsylvania, and is a member of the Society for Pediatric Dermatology, said she was not surprised that ChatGPT “can perform fairly well on multiple-choice questions as we find available in testing circumstances,” as presented in the study. “Just as board questions only support testing a base of medical knowledge and facts for clinicians to master, they do not necessarily provide real-life circumstances that apply to caring for patients, which is inherently nuanced.”
In addition, the study “highlights that ChatGPT can be an aid to support thinking through differentials based on data entered by a clinician who understands how to phrase queries, especially if provided with enough data while respecting patient privacy, in the context of fact checking responses,” Dr. Ilyas said. “This underscores the fact that AI tools can be helpful to clinicians in assimilating various data points entered. However, ultimately, the tool is only able to support an output based on the information it has access to.” She added, “ChatGPT cannot be relied on to provide a single diagnosis with the clinician still responsible for making a final diagnosis. The tool is not definitive and cannot assimilate data that is not entered correctly.”
The study was not funded, and the study authors reported having no disclosures. Dr. Buethe and Dr. Ilyas, who were not involved with the study, had no disclosures.
A version of this article appeared on Medscape.com .
In an experiment that pitted the wits of results from a small single-center study showed.
“We were relieved to find that the pediatric dermatologists in our study performed better than ChatGPT on both multiple choice and case-based questions; however, the latest iteration of ChatGPT (4.0) was very close,” one of the study’s first authors Charles Huang, a fourth-year medical student at Thomas Jefferson University, Philadelphia, said in an interview. “Something else that was interesting in our data was that the pediatric dermatologists performed much better than ChatGPT on questions related to procedural dermatology/surgical techniques, perhaps indicating that knowledge/reasoning gained through practical experience isn’t easily replicated in AI tools such as ChatGPT.”
For the study, which was published on May 9 in Pediatric Dermatology, Mr. Huang, and co-first author Esther Zhang, BS, a medical student at the University of Pennsylvania, Philadelphia, and coauthors from the Department of Dermatology, Children’s Hospital of Philadelphia, asked five pediatric dermatologists to answer 24 text-based questions including 16 single-answer, multiple-choice questions and two multiple answer questions drawn from the American Board of Dermatology 2021 Certification Sample Test and six free-response case-based questions drawn from the “Photoquiz” section of Pediatric Dermatology between July 2022 and July 2023. The researchers then processed the same set of questions through ChatGPT versions 3.5 and 4.0 and used statistical analysis to compare responses between the pediatric dermatologists and ChatGPT. A 5-point scale adapted from current AI tools was used to score replies to case-based questions.
On average, study participants had 5.6 years of clinical experience. Pediatric dermatologists performed significantly better than ChatGPT version 3.5 on multiple-choice and multiple answer questions (91.4% vs 76.2%, respectively; P = .021) but not significantly better than ChatGPT version 4.0 (90.5%; P = .44). As for replies to case-based questions, the average performance based on the 5-point scale was 3.81 for pediatric dermatologists and 3.53 for ChatGPT overall. The mean scores were significantly greater for pediatric dermatologists than for ChatGPT version 3.5 (P = .039) but not ChatGPT version 4.0 (P = .43).
The researchers acknowledged certain limitations of the analysis, including the evolving nature of AI tools, which may affect the reproducibility of results with subsequent model updates. And, while participating pediatric dermatologists said they were unfamiliar with the questions and cases used in the study, “there is potential for prior exposure through other dermatology board examination review processes,” they wrote.
“AI tools such as ChatGPT and similar large language models can be a valuable tool in your clinical practice, but be aware of potential pitfalls such as patient privacy, medical inaccuracies, [and] intrinsic biases in the tools,” Mr. Huang told this news organization. “As these technologies continue to advance, it is essential for all of us as medical clinicians to gain familiarity and stay abreast of new developments, just as we adapted to electronic health records and the use of the Internet.”
Maria Buethe, MD, PhD, a pediatric dermatology fellow at Rady Children’s Hospital–San Diego, who was asked to comment on the study, said she found it “interesting” that ChatGPT’s version 4.0 started to produce comparable results to clinician responses in some of the tested scenarios.
“The authors propose a set of best practices for pediatric dermatology clinicians using ChatGPT and other AI tools,” said Dr. Buethe, who was senior author of a recent literature review on AI and its application to pediatric dermatology. It was published in SKIN The Journal of Cutaneous Medicine. “One interesting recommended use for AI tools is to utilize it to generate differential diagnosis, which can broaden the list of pathologies previously considered.”
Asked to comment on the study, Erum Ilyas, MD, who practices dermatology in King of Prussia, Pennsylvania, and is a member of the Society for Pediatric Dermatology, said she was not surprised that ChatGPT “can perform fairly well on multiple-choice questions as we find available in testing circumstances,” as presented in the study. “Just as board questions only support testing a base of medical knowledge and facts for clinicians to master, they do not necessarily provide real-life circumstances that apply to caring for patients, which is inherently nuanced.”
In addition, the study “highlights that ChatGPT can be an aid to support thinking through differentials based on data entered by a clinician who understands how to phrase queries, especially if provided with enough data while respecting patient privacy, in the context of fact checking responses,” Dr. Ilyas said. “This underscores the fact that AI tools can be helpful to clinicians in assimilating various data points entered. However, ultimately, the tool is only able to support an output based on the information it has access to.” She added, “ChatGPT cannot be relied on to provide a single diagnosis with the clinician still responsible for making a final diagnosis. The tool is not definitive and cannot assimilate data that is not entered correctly.”
The study was not funded, and the study authors reported having no disclosures. Dr. Buethe and Dr. Ilyas, who were not involved with the study, had no disclosures.
A version of this article appeared on Medscape.com .