User login
Erythematous Plaque on the Back of a Newborn
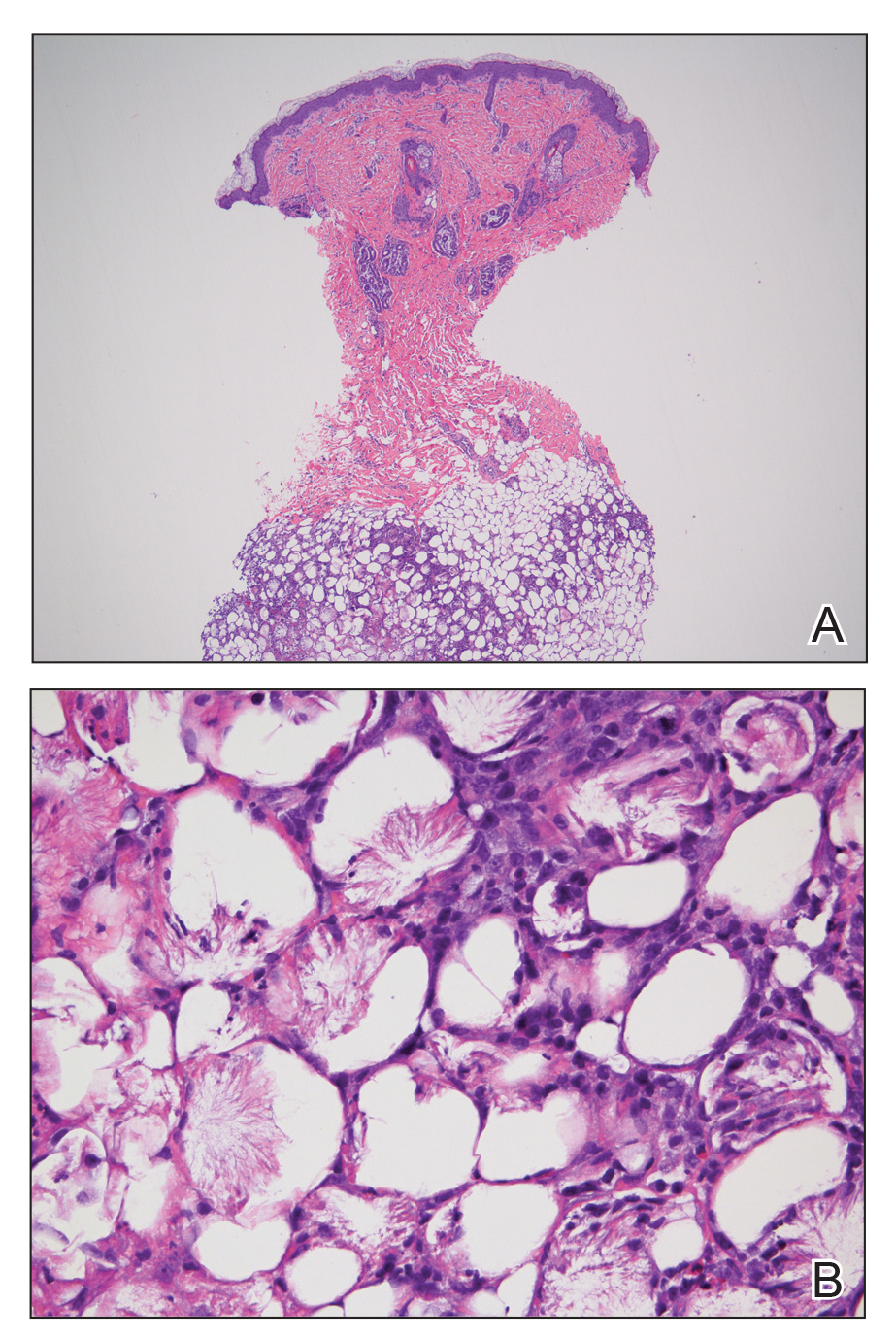
Subcutaneous fat necrosis of the newborn is a benign and self-limited condition that commonly occurs in term to postterm infants.1 However, it is an important diagnosis to recognize, as the potential exists for co-occurring metabolic derangements, most commonly hypercalcemia.1-4 Subcutaneous fat necrosis of the newborn is characterized by a panniculitis, most often on the back, shoulders, face, and buttocks. Lesions commonly present as erythematous nodules and plaques with overlying induration and can appear from birth to up to the first 6 weeks of life; calcification can be present in long-standing cases.2 Biopsy is diagnostic, showing a normal epidermis and dermis with a diffuse lobular panniculitis (Figure, A). Fat degeneration, radial crystal formation, and interstitial histiocytes also can be seen (Figure, B).
Patients with suspected subcutaneous fat necrosis should have their calcium levels checked, as up to 25% of patients may have coexisting hypercalcemia, which can contribute to morbidity and mortality.2 The hypercalcemia can occur with the onset of the lesions; however, it may be seen after they resolve completely.3 Thus, it is recommended that calcium levels be monitored for at least 1 month after lesions resolve. The exact etiology of subcutaneous fat necrosis is unknown, but it has been associated with perinatal stress and neonatal and maternal risk factors such as umbilical cord prolapse, meconium aspiration, neonatal sepsis, preeclampsia, and Rh incompatibility.1 The prognosis generally is excellent, with no treatment necessary for the skin lesions, as they resolve within a few months without subsequent sequelae or scarring.1,2 Patients with hypercalcemia should be treated appropriately with measures such as hydration and restriction of vitamin D; severe cases can be treated with bisphosphonates or loop diuretics.4
Cutis marmorata presents symmetrically on the trunk and may affect the upper and lower extremities as a reticulated erythema, often in response to cold temperature. Lesions are transient and resolve with warming. The isolated location of the skin lesions on the back, consistent course, and induration is unlikely to be seen in cutis marmorata. Infantile hemangiomas present several weeks to months after birth, and they undergo a rapid growth phase and subsequent slower involution phase. Furthermore, infantile hemangiomas have a rubbery feel and typically are not hard plaques, as seen in our patient.5 Patients with bacterial cellulitis often have systemic symptoms such as fever or chills, and the lesion generally is an ill-defined area of erythema and edema that can enlarge and become fluctuant.6 Sclerema neonatorum is a rare condition characterized by diffuse thickening of the skin that occurs in premature infants.7 These patients often are severely ill, as opposed to our asymptomatic full-term patient.
- Rubin G, Spagnut G, Morandi F, et al. Subcutaneous fat necrosis of the newborn. Clin Case Rep. 2015;3:1017-1020.
- de Campos Luciano Gomes MP, Porro AM, Simões da Silva Enokihara MM, et al. Subcutaneous fat necrosis of the newborn: clinical manifestations in two cases. An Bras Dermatol. 2013;88(6 suppl 1):154-157.
- Karochristou K, Siahanidou T, Kakourou-Tsivitanidou T, et al. Subcutaneous fat necrosis associated with severe hypocalcemia in a neonate. J Perinatol. 2005;26:64-66.
- Salas IV, Miralbell AR, Peinado CM, et al. Subcutaneous fat necrosis of the newborn and hypercalcemia: a case report. J Am Acad Dermatol. 2014;70:AB149.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:E1060-E1104.
- Linder KA, Malani PN. Cellulitis. JAMA. 2017;317:2142.
- Jardine D, Atherton DJ, Trompeter RS. Sclerema neonaturm and subcutaneous fat necrosis of the newborn in the same infant. Eur J Pediatr. 1990;150:125-126.
Subcutaneous fat necrosis of the newborn is a benign and self-limited condition that commonly occurs in term to postterm infants.1 However, it is an important diagnosis to recognize, as the potential exists for co-occurring metabolic derangements, most commonly hypercalcemia.1-4 Subcutaneous fat necrosis of the newborn is characterized by a panniculitis, most often on the back, shoulders, face, and buttocks. Lesions commonly present as erythematous nodules and plaques with overlying induration and can appear from birth to up to the first 6 weeks of life; calcification can be present in long-standing cases.2 Biopsy is diagnostic, showing a normal epidermis and dermis with a diffuse lobular panniculitis (Figure, A). Fat degeneration, radial crystal formation, and interstitial histiocytes also can be seen (Figure, B).

Patients with suspected subcutaneous fat necrosis should have their calcium levels checked, as up to 25% of patients may have coexisting hypercalcemia, which can contribute to morbidity and mortality.2 The hypercalcemia can occur with the onset of the lesions; however, it may be seen after they resolve completely.3 Thus, it is recommended that calcium levels be monitored for at least 1 month after lesions resolve. The exact etiology of subcutaneous fat necrosis is unknown, but it has been associated with perinatal stress and neonatal and maternal risk factors such as umbilical cord prolapse, meconium aspiration, neonatal sepsis, preeclampsia, and Rh incompatibility.1 The prognosis generally is excellent, with no treatment necessary for the skin lesions, as they resolve within a few months without subsequent sequelae or scarring.1,2 Patients with hypercalcemia should be treated appropriately with measures such as hydration and restriction of vitamin D; severe cases can be treated with bisphosphonates or loop diuretics.4
Cutis marmorata presents symmetrically on the trunk and may affect the upper and lower extremities as a reticulated erythema, often in response to cold temperature. Lesions are transient and resolve with warming. The isolated location of the skin lesions on the back, consistent course, and induration is unlikely to be seen in cutis marmorata. Infantile hemangiomas present several weeks to months after birth, and they undergo a rapid growth phase and subsequent slower involution phase. Furthermore, infantile hemangiomas have a rubbery feel and typically are not hard plaques, as seen in our patient.5 Patients with bacterial cellulitis often have systemic symptoms such as fever or chills, and the lesion generally is an ill-defined area of erythema and edema that can enlarge and become fluctuant.6 Sclerema neonatorum is a rare condition characterized by diffuse thickening of the skin that occurs in premature infants.7 These patients often are severely ill, as opposed to our asymptomatic full-term patient.
Subcutaneous fat necrosis of the newborn is a benign and self-limited condition that commonly occurs in term to postterm infants.1 However, it is an important diagnosis to recognize, as the potential exists for co-occurring metabolic derangements, most commonly hypercalcemia.1-4 Subcutaneous fat necrosis of the newborn is characterized by a panniculitis, most often on the back, shoulders, face, and buttocks. Lesions commonly present as erythematous nodules and plaques with overlying induration and can appear from birth to up to the first 6 weeks of life; calcification can be present in long-standing cases.2 Biopsy is diagnostic, showing a normal epidermis and dermis with a diffuse lobular panniculitis (Figure, A). Fat degeneration, radial crystal formation, and interstitial histiocytes also can be seen (Figure, B).

Patients with suspected subcutaneous fat necrosis should have their calcium levels checked, as up to 25% of patients may have coexisting hypercalcemia, which can contribute to morbidity and mortality.2 The hypercalcemia can occur with the onset of the lesions; however, it may be seen after they resolve completely.3 Thus, it is recommended that calcium levels be monitored for at least 1 month after lesions resolve. The exact etiology of subcutaneous fat necrosis is unknown, but it has been associated with perinatal stress and neonatal and maternal risk factors such as umbilical cord prolapse, meconium aspiration, neonatal sepsis, preeclampsia, and Rh incompatibility.1 The prognosis generally is excellent, with no treatment necessary for the skin lesions, as they resolve within a few months without subsequent sequelae or scarring.1,2 Patients with hypercalcemia should be treated appropriately with measures such as hydration and restriction of vitamin D; severe cases can be treated with bisphosphonates or loop diuretics.4
Cutis marmorata presents symmetrically on the trunk and may affect the upper and lower extremities as a reticulated erythema, often in response to cold temperature. Lesions are transient and resolve with warming. The isolated location of the skin lesions on the back, consistent course, and induration is unlikely to be seen in cutis marmorata. Infantile hemangiomas present several weeks to months after birth, and they undergo a rapid growth phase and subsequent slower involution phase. Furthermore, infantile hemangiomas have a rubbery feel and typically are not hard plaques, as seen in our patient.5 Patients with bacterial cellulitis often have systemic symptoms such as fever or chills, and the lesion generally is an ill-defined area of erythema and edema that can enlarge and become fluctuant.6 Sclerema neonatorum is a rare condition characterized by diffuse thickening of the skin that occurs in premature infants.7 These patients often are severely ill, as opposed to our asymptomatic full-term patient.
- Rubin G, Spagnut G, Morandi F, et al. Subcutaneous fat necrosis of the newborn. Clin Case Rep. 2015;3:1017-1020.
- de Campos Luciano Gomes MP, Porro AM, Simões da Silva Enokihara MM, et al. Subcutaneous fat necrosis of the newborn: clinical manifestations in two cases. An Bras Dermatol. 2013;88(6 suppl 1):154-157.
- Karochristou K, Siahanidou T, Kakourou-Tsivitanidou T, et al. Subcutaneous fat necrosis associated with severe hypocalcemia in a neonate. J Perinatol. 2005;26:64-66.
- Salas IV, Miralbell AR, Peinado CM, et al. Subcutaneous fat necrosis of the newborn and hypercalcemia: a case report. J Am Acad Dermatol. 2014;70:AB149.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:E1060-E1104.
- Linder KA, Malani PN. Cellulitis. JAMA. 2017;317:2142.
- Jardine D, Atherton DJ, Trompeter RS. Sclerema neonaturm and subcutaneous fat necrosis of the newborn in the same infant. Eur J Pediatr. 1990;150:125-126.
- Rubin G, Spagnut G, Morandi F, et al. Subcutaneous fat necrosis of the newborn. Clin Case Rep. 2015;3:1017-1020.
- de Campos Luciano Gomes MP, Porro AM, Simões da Silva Enokihara MM, et al. Subcutaneous fat necrosis of the newborn: clinical manifestations in two cases. An Bras Dermatol. 2013;88(6 suppl 1):154-157.
- Karochristou K, Siahanidou T, Kakourou-Tsivitanidou T, et al. Subcutaneous fat necrosis associated with severe hypocalcemia in a neonate. J Perinatol. 2005;26:64-66.
- Salas IV, Miralbell AR, Peinado CM, et al. Subcutaneous fat necrosis of the newborn and hypercalcemia: a case report. J Am Acad Dermatol. 2014;70:AB149.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:E1060-E1104.
- Linder KA, Malani PN. Cellulitis. JAMA. 2017;317:2142.
- Jardine D, Atherton DJ, Trompeter RS. Sclerema neonaturm and subcutaneous fat necrosis of the newborn in the same infant. Eur J Pediatr. 1990;150:125-126.
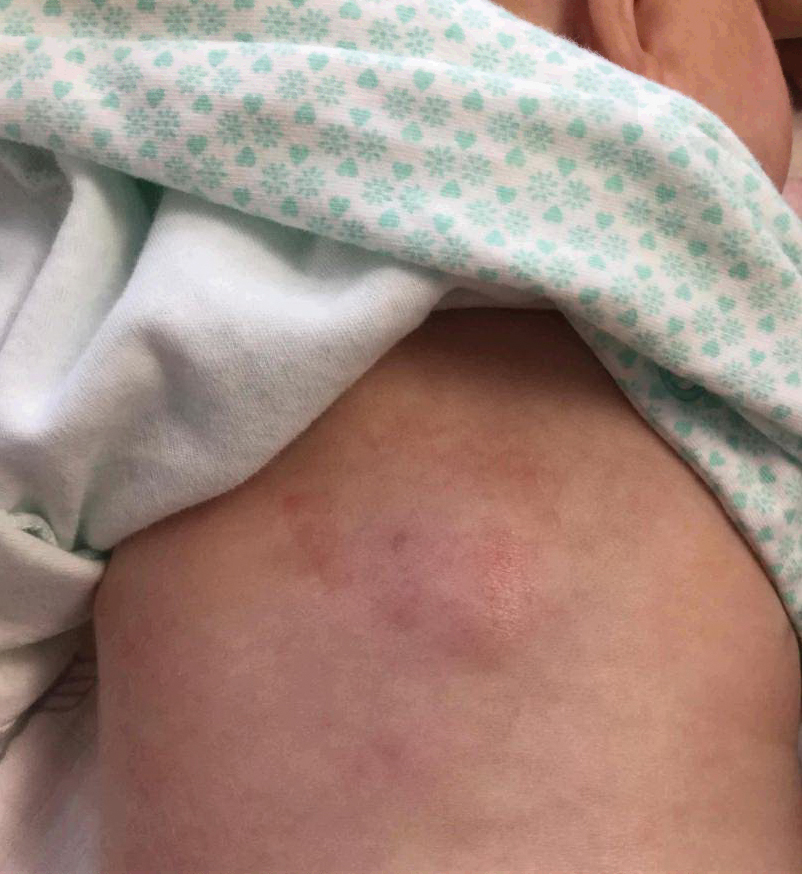
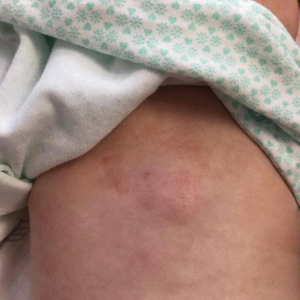
An 8-day-old female infant presented with a mass on the lower back that had been present since birth. The patient was well appearing, alert, and active. Physical examination revealed a 6×5-cm, erythematous, ill-defined, indurated plaque on the lower thoracic back. There was no associated family history of similar findings. According to the mother, the patient was feeding well with no recent fever, irritability, or lethargy. The patient was born via elective induction of labor at term due to maternal intrauterine infection from chorioamnionitis. The birth was complicated by shoulder dystocia with an Erb palsy, and she was hospitalized for 5 days after delivery for management of hypotension and ABO isoimmunization and to rule out sepsis; blood cultures were negative for neonatal infection.
Helping families understand internalized racism
Ms. Jones brings her 15-year-old daughter, Angela, to the resident clinic. Angela is becoming increasingly anxious, withdrawn, and difficult to manage. As part of the initial interview, the resident, Dr. Sota, asks about the sociocultural background of the family. Ms. Jones is African American and recently began a relationship with a white man. Her daughter, Angela, is biracial; her biological father is white and has moved out of state with little ongoing contact with Angela and her mother.
At interview, Angela expresses a lot of anger at her mother, her biological father, and her new “stepfather.” Ms. Jones says: “I do not want Angela growing up as an ‘angry black woman.’ ” When asked for an explanation, she stated that she doesn’t want her daughter to be stereotyped, to be perceived as an angry black person. “She needs to fit in with our new life. She has lots of opportunities if only she would take them.”
Dr. Sota recognizes that Angela’s struggle, and perhaps also the struggle of Ms. Jones, has a component of internalized racism. How should Dr. Sota proceed? Dr. Sota puts herself in Angela’s shoes: How does Angela see herself? Angela has light brown skin, and
The term internalized racism (IR) first appeared in the 1980s. IR was compared to the oppression of black people in the 1800s: “The slavery that captures the mind and incarcerates the motivation, perception, aspiration, and identity in a web of anti-self images, generating a personal and collective self destruction, is more cruel than the shackles on the wrists and ankles.”1 According to Susanne Lipsky,2 IR “in African Americans manifests as internalizing stereotypes, mistrusting the self and other Blacks, and narrows one’s view of authentic Black culture.”
IR refers to the internalization and acceptance of the dominant white culture’s actions and beliefs, while rejecting one’s own cultural background. There is a long history of negative cultural representations of African Americans in popular American culture, and IR has a detrimental impact on the emotional well-being of African Americans.3
IR is associated with poorer metabolic health4 and psychological distress, depression and anxiety,5-8 and decreased self-esteem.9 However, protective processes can reduce one’s response to risk and can be developed through the psychotherapeutic relationship.
Interventions at an individual, family, or community levels
Angela: Tell me about yourself: What type of person are you? How do you identify? How do you feel about yourself/your appearance/your language?
Tell me about your friends/family? What interests do you have?
“Tell me more” questions can reveal conflicted feelings, etc., even if Angela does not answer. A good therapist can talk about IR; even if Angela does not bring it up, it is important for the therapist to find language suitable for the age of the patient.
Dr. Sota has some luck with Angela, who nods her head but says little. Dr. Sota then turns to Ms. Jones and asks whether she can answer these questions, too, and rephrases the questions for an adult. Interviewing parents in the presence of their children gives Dr. Sota and Angela an idea of what is permitted to talk about in the family.
A therapist can also note other permissions in the family: How do Angela and her mother use language? Do they claim or reject words and phrases such as “angry black woman” and choose, instead, to use language to “fit in” with the dominant white culture?
Dr. Sota notices that Ms. Jones presents herself as keen to fit in with her new future husband’s life. She wants Angela to do likewise. Dr. Sota notices that Angela vacillates between wanting to claim her black identity and having to navigate what that means in this family (not a good thing) – and wanting to assimilate into white culture. Her peers fall into two separate groups: a set of black friends and a set of white friends. Her mother prefers that she see her white friends, mistrusting her black friends.
Dr. Sota’s supervisor suggests that she introduce IR more forcefully because this seems to be a major course of conflict for Angela and encourage a frank discussion between mother and daughter. Dr. Sota starts the next session in the following way: “I noticed last week that the way you each identify yourselves is quite different. Ms. Jones, you want Angela to ‘fit in’ and perhaps just embrace white culture, whereas Angela, perhaps you vacillate between a white identity and a black identity?”
The following questions can help Dr. Sota elicit IR:
- What information about yourself would you like others to know – about your heritage, country of origin, family, class background, and so on?
- What makes you proud about being a member of this group, and what do you love about other members of this group?
- What has been hard about being a member of this group, and what don’t you like about others in this group?
- What were your early life experiences with people in this group? How were you treated? How did you feel about others in your group when you were young?
At a community level, family workshops support positive cultural identities that strengthen family functioning and reducing behavioral health risks. In a study of 575 urban American Indian (AI) families from diverse tribal backgrounds, the AI families who participated in such a workshop had significant increases in their ethnic identity, improved sense of spirituality, and a more positive cultural identification. The workshops provided culturally adaptive parenting interventions.10
IR is a serious determinant of both physical and mental health. Assessment of IR can be done using rating scales, such as the Nadanolitization Scale11 or the Internalized Racial Oppression Scale.12 IR also can also be assessed using a more formalized interview guide, such as the DSM-5 Cultural Formulation Interview (CFI).13 This 16-question interview guide helps behavioral health providers better understand the way service users and their social networks (e.g., families, friends) understand what is happening to them and why, as well as the barriers they experience, such as racism, discrimination, stigma, and financial stressors.
Individuals’ cultures and experiences have a profound impact on their understanding of their symptoms and their engagement in care. The American Psychiatric Association considers it to be part of mental health providers’ duty of care to engage all individuals in culturally relevant conversations about their past experiences and care expectations. More relevant, I submit that you cannot treat someone without having made this inquiry. A cultural assessment improves understanding but also shifts power relationships between providers and patients. The DSM-5 CFI and training guides are widely available and provide additional information for those who want to improve their cultural literacy.
Conclusion
Internalized racism is the component of racism that is the most difficult to discern. Psychiatrists and mental health professionals are uniquely poised to address IR, and any subsequent internal conflict and identity difficulties. Each program, office, and clinic can easily find the resources to do this through the APA. If you would like help providing education, contact me at [email protected].
References
1. Akbar N. J Black Studies. 1984. doi: 10.11771002193478401400401.
2. Lipsky S. Internalized Racism. Seattle: Rational Island Publishers, 1987.
3. Williams DR and Mohammed SA. Am Behav Sci. 2013 May 8. doi: 10.1177/00027642134873340.
4. DeLilly CR and Flaskerud JH. Issues Ment Health Nurs. 2012 Nov;33(11):804-11.
5. Molina KM and James D. Group Process Intergroup Relat. 2016 Jul;19(4):439-61.
6. Szymanski D and Obiri O. Couns Psychologist. 2011;39(3):438-62.
7. Carter RT et al. J Multicul Couns Dev. 2017 Oct 5;45(4):232-59.
8. Mouzon DM and McLean JS. Ethn Health. 2017 Feb;22(1):36-48.
9. Szymanski DM and Gupta A. J Couns Psychol. 2009;56(1):110-18.
10. Kulis SS et al. Cultural Diversity and Ethnic Minority Psychol. 2019. doi: 10.1037/cpd000315.
11. Taylor J and Grundy C. “Measuring black internalization of white stereotypes about African Americans: The Nadanolization Scale.” In: Jones RL, ed. Handbook of Tests and Measurements of Black Populations. Hampton, Va.: Cobb & Henry, 1996.
12. Bailey T-K M et al. J Couns Psychol. 2011 Oct;58(4):481-93.
13. American Psychiatric Association. Cultural Formulation Interview. DSM-5. American Psychiatric Association Publishing: Arlington, Va. 2013.
Various aspects about the case described above have been changed to protect the clinician’s and patients’ identities. Thanks to the following individuals for their contributions to this article: Suzanne Huberty, MD, and Shiona Heru, JD.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (Routledge, 2013). She has no conflicts of interest to disclose.
Ms. Jones brings her 15-year-old daughter, Angela, to the resident clinic. Angela is becoming increasingly anxious, withdrawn, and difficult to manage. As part of the initial interview, the resident, Dr. Sota, asks about the sociocultural background of the family. Ms. Jones is African American and recently began a relationship with a white man. Her daughter, Angela, is biracial; her biological father is white and has moved out of state with little ongoing contact with Angela and her mother.
At interview, Angela expresses a lot of anger at her mother, her biological father, and her new “stepfather.” Ms. Jones says: “I do not want Angela growing up as an ‘angry black woman.’ ” When asked for an explanation, she stated that she doesn’t want her daughter to be stereotyped, to be perceived as an angry black person. “She needs to fit in with our new life. She has lots of opportunities if only she would take them.”
Dr. Sota recognizes that Angela’s struggle, and perhaps also the struggle of Ms. Jones, has a component of internalized racism. How should Dr. Sota proceed? Dr. Sota puts herself in Angela’s shoes: How does Angela see herself? Angela has light brown skin, and
The term internalized racism (IR) first appeared in the 1980s. IR was compared to the oppression of black people in the 1800s: “The slavery that captures the mind and incarcerates the motivation, perception, aspiration, and identity in a web of anti-self images, generating a personal and collective self destruction, is more cruel than the shackles on the wrists and ankles.”1 According to Susanne Lipsky,2 IR “in African Americans manifests as internalizing stereotypes, mistrusting the self and other Blacks, and narrows one’s view of authentic Black culture.”
IR refers to the internalization and acceptance of the dominant white culture’s actions and beliefs, while rejecting one’s own cultural background. There is a long history of negative cultural representations of African Americans in popular American culture, and IR has a detrimental impact on the emotional well-being of African Americans.3
IR is associated with poorer metabolic health4 and psychological distress, depression and anxiety,5-8 and decreased self-esteem.9 However, protective processes can reduce one’s response to risk and can be developed through the psychotherapeutic relationship.
Interventions at an individual, family, or community levels
Angela: Tell me about yourself: What type of person are you? How do you identify? How do you feel about yourself/your appearance/your language?
Tell me about your friends/family? What interests do you have?
“Tell me more” questions can reveal conflicted feelings, etc., even if Angela does not answer. A good therapist can talk about IR; even if Angela does not bring it up, it is important for the therapist to find language suitable for the age of the patient.
Dr. Sota has some luck with Angela, who nods her head but says little. Dr. Sota then turns to Ms. Jones and asks whether she can answer these questions, too, and rephrases the questions for an adult. Interviewing parents in the presence of their children gives Dr. Sota and Angela an idea of what is permitted to talk about in the family.
A therapist can also note other permissions in the family: How do Angela and her mother use language? Do they claim or reject words and phrases such as “angry black woman” and choose, instead, to use language to “fit in” with the dominant white culture?
Dr. Sota notices that Ms. Jones presents herself as keen to fit in with her new future husband’s life. She wants Angela to do likewise. Dr. Sota notices that Angela vacillates between wanting to claim her black identity and having to navigate what that means in this family (not a good thing) – and wanting to assimilate into white culture. Her peers fall into two separate groups: a set of black friends and a set of white friends. Her mother prefers that she see her white friends, mistrusting her black friends.
Dr. Sota’s supervisor suggests that she introduce IR more forcefully because this seems to be a major course of conflict for Angela and encourage a frank discussion between mother and daughter. Dr. Sota starts the next session in the following way: “I noticed last week that the way you each identify yourselves is quite different. Ms. Jones, you want Angela to ‘fit in’ and perhaps just embrace white culture, whereas Angela, perhaps you vacillate between a white identity and a black identity?”
The following questions can help Dr. Sota elicit IR:
- What information about yourself would you like others to know – about your heritage, country of origin, family, class background, and so on?
- What makes you proud about being a member of this group, and what do you love about other members of this group?
- What has been hard about being a member of this group, and what don’t you like about others in this group?
- What were your early life experiences with people in this group? How were you treated? How did you feel about others in your group when you were young?
At a community level, family workshops support positive cultural identities that strengthen family functioning and reducing behavioral health risks. In a study of 575 urban American Indian (AI) families from diverse tribal backgrounds, the AI families who participated in such a workshop had significant increases in their ethnic identity, improved sense of spirituality, and a more positive cultural identification. The workshops provided culturally adaptive parenting interventions.10
IR is a serious determinant of both physical and mental health. Assessment of IR can be done using rating scales, such as the Nadanolitization Scale11 or the Internalized Racial Oppression Scale.12 IR also can also be assessed using a more formalized interview guide, such as the DSM-5 Cultural Formulation Interview (CFI).13 This 16-question interview guide helps behavioral health providers better understand the way service users and their social networks (e.g., families, friends) understand what is happening to them and why, as well as the barriers they experience, such as racism, discrimination, stigma, and financial stressors.
Individuals’ cultures and experiences have a profound impact on their understanding of their symptoms and their engagement in care. The American Psychiatric Association considers it to be part of mental health providers’ duty of care to engage all individuals in culturally relevant conversations about their past experiences and care expectations. More relevant, I submit that you cannot treat someone without having made this inquiry. A cultural assessment improves understanding but also shifts power relationships between providers and patients. The DSM-5 CFI and training guides are widely available and provide additional information for those who want to improve their cultural literacy.
Conclusion
Internalized racism is the component of racism that is the most difficult to discern. Psychiatrists and mental health professionals are uniquely poised to address IR, and any subsequent internal conflict and identity difficulties. Each program, office, and clinic can easily find the resources to do this through the APA. If you would like help providing education, contact me at [email protected].
References
1. Akbar N. J Black Studies. 1984. doi: 10.11771002193478401400401.
2. Lipsky S. Internalized Racism. Seattle: Rational Island Publishers, 1987.
3. Williams DR and Mohammed SA. Am Behav Sci. 2013 May 8. doi: 10.1177/00027642134873340.
4. DeLilly CR and Flaskerud JH. Issues Ment Health Nurs. 2012 Nov;33(11):804-11.
5. Molina KM and James D. Group Process Intergroup Relat. 2016 Jul;19(4):439-61.
6. Szymanski D and Obiri O. Couns Psychologist. 2011;39(3):438-62.
7. Carter RT et al. J Multicul Couns Dev. 2017 Oct 5;45(4):232-59.
8. Mouzon DM and McLean JS. Ethn Health. 2017 Feb;22(1):36-48.
9. Szymanski DM and Gupta A. J Couns Psychol. 2009;56(1):110-18.
10. Kulis SS et al. Cultural Diversity and Ethnic Minority Psychol. 2019. doi: 10.1037/cpd000315.
11. Taylor J and Grundy C. “Measuring black internalization of white stereotypes about African Americans: The Nadanolization Scale.” In: Jones RL, ed. Handbook of Tests and Measurements of Black Populations. Hampton, Va.: Cobb & Henry, 1996.
12. Bailey T-K M et al. J Couns Psychol. 2011 Oct;58(4):481-93.
13. American Psychiatric Association. Cultural Formulation Interview. DSM-5. American Psychiatric Association Publishing: Arlington, Va. 2013.
Various aspects about the case described above have been changed to protect the clinician’s and patients’ identities. Thanks to the following individuals for their contributions to this article: Suzanne Huberty, MD, and Shiona Heru, JD.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (Routledge, 2013). She has no conflicts of interest to disclose.
Ms. Jones brings her 15-year-old daughter, Angela, to the resident clinic. Angela is becoming increasingly anxious, withdrawn, and difficult to manage. As part of the initial interview, the resident, Dr. Sota, asks about the sociocultural background of the family. Ms. Jones is African American and recently began a relationship with a white man. Her daughter, Angela, is biracial; her biological father is white and has moved out of state with little ongoing contact with Angela and her mother.
At interview, Angela expresses a lot of anger at her mother, her biological father, and her new “stepfather.” Ms. Jones says: “I do not want Angela growing up as an ‘angry black woman.’ ” When asked for an explanation, she stated that she doesn’t want her daughter to be stereotyped, to be perceived as an angry black person. “She needs to fit in with our new life. She has lots of opportunities if only she would take them.”
Dr. Sota recognizes that Angela’s struggle, and perhaps also the struggle of Ms. Jones, has a component of internalized racism. How should Dr. Sota proceed? Dr. Sota puts herself in Angela’s shoes: How does Angela see herself? Angela has light brown skin, and
The term internalized racism (IR) first appeared in the 1980s. IR was compared to the oppression of black people in the 1800s: “The slavery that captures the mind and incarcerates the motivation, perception, aspiration, and identity in a web of anti-self images, generating a personal and collective self destruction, is more cruel than the shackles on the wrists and ankles.”1 According to Susanne Lipsky,2 IR “in African Americans manifests as internalizing stereotypes, mistrusting the self and other Blacks, and narrows one’s view of authentic Black culture.”
IR refers to the internalization and acceptance of the dominant white culture’s actions and beliefs, while rejecting one’s own cultural background. There is a long history of negative cultural representations of African Americans in popular American culture, and IR has a detrimental impact on the emotional well-being of African Americans.3
IR is associated with poorer metabolic health4 and psychological distress, depression and anxiety,5-8 and decreased self-esteem.9 However, protective processes can reduce one’s response to risk and can be developed through the psychotherapeutic relationship.
Interventions at an individual, family, or community levels
Angela: Tell me about yourself: What type of person are you? How do you identify? How do you feel about yourself/your appearance/your language?
Tell me about your friends/family? What interests do you have?
“Tell me more” questions can reveal conflicted feelings, etc., even if Angela does not answer. A good therapist can talk about IR; even if Angela does not bring it up, it is important for the therapist to find language suitable for the age of the patient.
Dr. Sota has some luck with Angela, who nods her head but says little. Dr. Sota then turns to Ms. Jones and asks whether she can answer these questions, too, and rephrases the questions for an adult. Interviewing parents in the presence of their children gives Dr. Sota and Angela an idea of what is permitted to talk about in the family.
A therapist can also note other permissions in the family: How do Angela and her mother use language? Do they claim or reject words and phrases such as “angry black woman” and choose, instead, to use language to “fit in” with the dominant white culture?
Dr. Sota notices that Ms. Jones presents herself as keen to fit in with her new future husband’s life. She wants Angela to do likewise. Dr. Sota notices that Angela vacillates between wanting to claim her black identity and having to navigate what that means in this family (not a good thing) – and wanting to assimilate into white culture. Her peers fall into two separate groups: a set of black friends and a set of white friends. Her mother prefers that she see her white friends, mistrusting her black friends.
Dr. Sota’s supervisor suggests that she introduce IR more forcefully because this seems to be a major course of conflict for Angela and encourage a frank discussion between mother and daughter. Dr. Sota starts the next session in the following way: “I noticed last week that the way you each identify yourselves is quite different. Ms. Jones, you want Angela to ‘fit in’ and perhaps just embrace white culture, whereas Angela, perhaps you vacillate between a white identity and a black identity?”
The following questions can help Dr. Sota elicit IR:
- What information about yourself would you like others to know – about your heritage, country of origin, family, class background, and so on?
- What makes you proud about being a member of this group, and what do you love about other members of this group?
- What has been hard about being a member of this group, and what don’t you like about others in this group?
- What were your early life experiences with people in this group? How were you treated? How did you feel about others in your group when you were young?
At a community level, family workshops support positive cultural identities that strengthen family functioning and reducing behavioral health risks. In a study of 575 urban American Indian (AI) families from diverse tribal backgrounds, the AI families who participated in such a workshop had significant increases in their ethnic identity, improved sense of spirituality, and a more positive cultural identification. The workshops provided culturally adaptive parenting interventions.10
IR is a serious determinant of both physical and mental health. Assessment of IR can be done using rating scales, such as the Nadanolitization Scale11 or the Internalized Racial Oppression Scale.12 IR also can also be assessed using a more formalized interview guide, such as the DSM-5 Cultural Formulation Interview (CFI).13 This 16-question interview guide helps behavioral health providers better understand the way service users and their social networks (e.g., families, friends) understand what is happening to them and why, as well as the barriers they experience, such as racism, discrimination, stigma, and financial stressors.
Individuals’ cultures and experiences have a profound impact on their understanding of their symptoms and their engagement in care. The American Psychiatric Association considers it to be part of mental health providers’ duty of care to engage all individuals in culturally relevant conversations about their past experiences and care expectations. More relevant, I submit that you cannot treat someone without having made this inquiry. A cultural assessment improves understanding but also shifts power relationships between providers and patients. The DSM-5 CFI and training guides are widely available and provide additional information for those who want to improve their cultural literacy.
Conclusion
Internalized racism is the component of racism that is the most difficult to discern. Psychiatrists and mental health professionals are uniquely poised to address IR, and any subsequent internal conflict and identity difficulties. Each program, office, and clinic can easily find the resources to do this through the APA. If you would like help providing education, contact me at [email protected].
References
1. Akbar N. J Black Studies. 1984. doi: 10.11771002193478401400401.
2. Lipsky S. Internalized Racism. Seattle: Rational Island Publishers, 1987.
3. Williams DR and Mohammed SA. Am Behav Sci. 2013 May 8. doi: 10.1177/00027642134873340.
4. DeLilly CR and Flaskerud JH. Issues Ment Health Nurs. 2012 Nov;33(11):804-11.
5. Molina KM and James D. Group Process Intergroup Relat. 2016 Jul;19(4):439-61.
6. Szymanski D and Obiri O. Couns Psychologist. 2011;39(3):438-62.
7. Carter RT et al. J Multicul Couns Dev. 2017 Oct 5;45(4):232-59.
8. Mouzon DM and McLean JS. Ethn Health. 2017 Feb;22(1):36-48.
9. Szymanski DM and Gupta A. J Couns Psychol. 2009;56(1):110-18.
10. Kulis SS et al. Cultural Diversity and Ethnic Minority Psychol. 2019. doi: 10.1037/cpd000315.
11. Taylor J and Grundy C. “Measuring black internalization of white stereotypes about African Americans: The Nadanolization Scale.” In: Jones RL, ed. Handbook of Tests and Measurements of Black Populations. Hampton, Va.: Cobb & Henry, 1996.
12. Bailey T-K M et al. J Couns Psychol. 2011 Oct;58(4):481-93.
13. American Psychiatric Association. Cultural Formulation Interview. DSM-5. American Psychiatric Association Publishing: Arlington, Va. 2013.
Various aspects about the case described above have been changed to protect the clinician’s and patients’ identities. Thanks to the following individuals for their contributions to this article: Suzanne Huberty, MD, and Shiona Heru, JD.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (Routledge, 2013). She has no conflicts of interest to disclose.
FDA expands Dysport use for cerebral palsy–related spasticity
– for patients as young as 2 years and older, according to manufacturer Ipsen Biopharmaceuticals.
When Dysport (abobotulinumtoxinA) initially was approved for treating pediatric lower limb spasticity by the FDA in 2016, Ipsen was granted Orphan Drug exclusivity for children whose lower-limb spasticity was caused by cerebral palsy. In 2019, Dysport was approved by the FDA for treating of upper-limb spasticity in children 2 years older. But if that spasticity was caused by cerebral palsy, Dysport could be used to treat it only through Orphan Drug exclusivity granted to another manufacturer, according to an Ipsen press release.
“The proactive step to resolve the uncertainty created by the previous CP [cerebral palsy] carve out enables us as physicians to prescribe consistent therapy for pediatric patients experiencing both upper- and lower-limb spasticity,” Sarah Helen Evans, MD, division chief of rehabilitation medicine in the department of pediatrics at the Children’s Hospital of Philadelphia, said in the press release.
The most common adverse effects among children with lower-limb spasticity treated with Dysport were nasopharyngitis, cough, and pyrexia. Among children with upper-limb spasticity, the most common effects associated with Dysport treatment were upper respiratory tract infection and pharyngitis.
The press release also included a warning of the distant spread of the botulinum toxin from the area of injection hours to weeks afterward, causing symptoms including blurred vision, generalized muscle weakness, and swallowing and breathing difficulties that can be life threatening; there have been reports of death.
Suspected adverse effects can be reported to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
– for patients as young as 2 years and older, according to manufacturer Ipsen Biopharmaceuticals.
When Dysport (abobotulinumtoxinA) initially was approved for treating pediatric lower limb spasticity by the FDA in 2016, Ipsen was granted Orphan Drug exclusivity for children whose lower-limb spasticity was caused by cerebral palsy. In 2019, Dysport was approved by the FDA for treating of upper-limb spasticity in children 2 years older. But if that spasticity was caused by cerebral palsy, Dysport could be used to treat it only through Orphan Drug exclusivity granted to another manufacturer, according to an Ipsen press release.
“The proactive step to resolve the uncertainty created by the previous CP [cerebral palsy] carve out enables us as physicians to prescribe consistent therapy for pediatric patients experiencing both upper- and lower-limb spasticity,” Sarah Helen Evans, MD, division chief of rehabilitation medicine in the department of pediatrics at the Children’s Hospital of Philadelphia, said in the press release.
The most common adverse effects among children with lower-limb spasticity treated with Dysport were nasopharyngitis, cough, and pyrexia. Among children with upper-limb spasticity, the most common effects associated with Dysport treatment were upper respiratory tract infection and pharyngitis.
The press release also included a warning of the distant spread of the botulinum toxin from the area of injection hours to weeks afterward, causing symptoms including blurred vision, generalized muscle weakness, and swallowing and breathing difficulties that can be life threatening; there have been reports of death.
Suspected adverse effects can be reported to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
– for patients as young as 2 years and older, according to manufacturer Ipsen Biopharmaceuticals.
When Dysport (abobotulinumtoxinA) initially was approved for treating pediatric lower limb spasticity by the FDA in 2016, Ipsen was granted Orphan Drug exclusivity for children whose lower-limb spasticity was caused by cerebral palsy. In 2019, Dysport was approved by the FDA for treating of upper-limb spasticity in children 2 years older. But if that spasticity was caused by cerebral palsy, Dysport could be used to treat it only through Orphan Drug exclusivity granted to another manufacturer, according to an Ipsen press release.
“The proactive step to resolve the uncertainty created by the previous CP [cerebral palsy] carve out enables us as physicians to prescribe consistent therapy for pediatric patients experiencing both upper- and lower-limb spasticity,” Sarah Helen Evans, MD, division chief of rehabilitation medicine in the department of pediatrics at the Children’s Hospital of Philadelphia, said in the press release.
The most common adverse effects among children with lower-limb spasticity treated with Dysport were nasopharyngitis, cough, and pyrexia. Among children with upper-limb spasticity, the most common effects associated with Dysport treatment were upper respiratory tract infection and pharyngitis.
The press release also included a warning of the distant spread of the botulinum toxin from the area of injection hours to weeks afterward, causing symptoms including blurred vision, generalized muscle weakness, and swallowing and breathing difficulties that can be life threatening; there have been reports of death.
Suspected adverse effects can be reported to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Subcutaneous nemolizumab eases itching for atopic dermatitis
of 215 patients in Japan.
Controlling the pruritus associated with atopic dermatitis (AD) can have a significant impact on patients’ quality of life, wrote Kenji Kabashima, MD, PhD, of the department of dermatology at Kyoto University, and coauthors. Frequent scratching can cause not only mechanical skin damage, but also may enhance inflammatory reactions and contribute to sleep problems.
In earlier phase studies, nemolizumab, a humanized monoclonal antibody against interleukin-31 receptor A, showed efficacy in reducing pruritus in patients with AD, but has not been well studied in patients who are also using topical agents, they wrote.
In the study published in the New England Journal of Medicine, the researchers randomized 143 patients with AD and moderate to severe pruritus to 60 mg of subcutaneous nemolizumab and 72 patients to a placebo every 4 weeks for 16 weeks. All patients were aged 13 years and older with a confirmed AD diagnosis and a history of inadequate response to or inability to use treatments, including topical glucocorticoids and oral antihistamines. Their average age was 40 years, approximately two-thirds were male, and the average disease duration was approximately 30 years. Topical treatments included a medium potency glucocorticoid in 97% of patients in both groups, and a topical calcineurin inhibitor in 41% of those on nemolizumab, and 40% of those on placebo; almost 90% of the patients in both groups were on oral antihistamines.
At 16 weeks, scores on the visual analog scale for pruritus (the primary outcome) significantly improved from baseline in the nemolizumab group, compared with the placebo group (a mean change of –42.8% and –21.4%, respectively, P < .001).
In addition, more patients in the nemolizumab group, compared with the placebo group (40% vs. 22%) achieved a score of 4 or less on the Dermatology Life Quality Index, with lower scores reflecting less impact of disease on daily life. In addition, more patients in the nemolizumab group, compared with the placebo group (55% vs. 21%) achieved a score of 7 or less on the Insomnia Severity Index.
During the study, 71% of the patients in each group reported adverse events, most were mild or moderate. The most common adverse event was worsening AD, reported by 24% of the nemolizumab patients and 21% of the placebo patients. Reactions related to the injection occurred in 8% of nemolizumab patients and 3% of placebo patients. Cytokine abnormalities, which included an increased level of thymus and activation regulated chemokine, were reported in 10 (7%) of the patients on nemolizumab, none of which occurred in those on placebo. “Most were not accompanied by a worsening of signs of or the extent of atopic dermatitis,” the authors wrote.
Severe adverse events were reported in three patients (2%) in the nemolizumab group, which were Meniere’s disease, acute pancreatitis, and AD in one patient each. No severe adverse events were reported in the placebo group. In addition, three patients in the nemolizumab group experienced four treatment-related adverse events that led them to discontinue treatment: AD, Meniere’s disease, alopecia, and peripheral edema.
The study findings were limited by several factors including the relatively short treatment period, inclusion only of Japanese patients, inclusion of patients aged as young as 13 years, and the inability to draw conclusions from the secondary endpoints such as quality of life and sleep issues, the researchers noted.
However, the results suggest that “nemolizumab plus topical agents may ameliorate both pruritus and signs of eczema and may lessen the severity of atopic dermatitis by disrupting the itch-scratch cycle,” they added.
“Novel therapies [for AD] are needed, as there are still patients who need better disease control despite current therapies, and AD is a heterogeneous disease that may need different treatment approaches,” Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, said in an interview.
Dr. Simpson, who was not an investigator in this study, said that he was somewhat surprised that the itch reduction was lower in the current study, compared with previous studies by the same group. Also surprising was the increase in cytokine abnormalities in the nemolizumab group, which “needs further study.”
Overall, the data “provide support that blockade of the IL-31 receptor improves itch in AD and appears to have some effect on inflammation,” Dr. Simpson said.
One challenge to the clinical use of nemolizumab will be identifying “where this type of drug fits into the treatment paradigm,” and determining whether specific patients whose disease is driven more by this neuroimmune pathway could benefit more than with the traditional IL-4 or IL-13 blockade, he said.
The study was supported by Maruho. Dr. Kabashima disclosed consulting fees from Maruho and two coauthors were Maruho employees. Dr. Simpson had no financial conflicts relevant to this study, but he reported receiving research grants and other financial relationships with manufacturers of AD therapies.
SOURCE: Kabashima K et al. N Engl J Med. 2020 Jul 9. doi: 10.1056/NEJMoa1917006.
of 215 patients in Japan.
Controlling the pruritus associated with atopic dermatitis (AD) can have a significant impact on patients’ quality of life, wrote Kenji Kabashima, MD, PhD, of the department of dermatology at Kyoto University, and coauthors. Frequent scratching can cause not only mechanical skin damage, but also may enhance inflammatory reactions and contribute to sleep problems.
In earlier phase studies, nemolizumab, a humanized monoclonal antibody against interleukin-31 receptor A, showed efficacy in reducing pruritus in patients with AD, but has not been well studied in patients who are also using topical agents, they wrote.
In the study published in the New England Journal of Medicine, the researchers randomized 143 patients with AD and moderate to severe pruritus to 60 mg of subcutaneous nemolizumab and 72 patients to a placebo every 4 weeks for 16 weeks. All patients were aged 13 years and older with a confirmed AD diagnosis and a history of inadequate response to or inability to use treatments, including topical glucocorticoids and oral antihistamines. Their average age was 40 years, approximately two-thirds were male, and the average disease duration was approximately 30 years. Topical treatments included a medium potency glucocorticoid in 97% of patients in both groups, and a topical calcineurin inhibitor in 41% of those on nemolizumab, and 40% of those on placebo; almost 90% of the patients in both groups were on oral antihistamines.
At 16 weeks, scores on the visual analog scale for pruritus (the primary outcome) significantly improved from baseline in the nemolizumab group, compared with the placebo group (a mean change of –42.8% and –21.4%, respectively, P < .001).
In addition, more patients in the nemolizumab group, compared with the placebo group (40% vs. 22%) achieved a score of 4 or less on the Dermatology Life Quality Index, with lower scores reflecting less impact of disease on daily life. In addition, more patients in the nemolizumab group, compared with the placebo group (55% vs. 21%) achieved a score of 7 or less on the Insomnia Severity Index.
During the study, 71% of the patients in each group reported adverse events, most were mild or moderate. The most common adverse event was worsening AD, reported by 24% of the nemolizumab patients and 21% of the placebo patients. Reactions related to the injection occurred in 8% of nemolizumab patients and 3% of placebo patients. Cytokine abnormalities, which included an increased level of thymus and activation regulated chemokine, were reported in 10 (7%) of the patients on nemolizumab, none of which occurred in those on placebo. “Most were not accompanied by a worsening of signs of or the extent of atopic dermatitis,” the authors wrote.
Severe adverse events were reported in three patients (2%) in the nemolizumab group, which were Meniere’s disease, acute pancreatitis, and AD in one patient each. No severe adverse events were reported in the placebo group. In addition, three patients in the nemolizumab group experienced four treatment-related adverse events that led them to discontinue treatment: AD, Meniere’s disease, alopecia, and peripheral edema.
The study findings were limited by several factors including the relatively short treatment period, inclusion only of Japanese patients, inclusion of patients aged as young as 13 years, and the inability to draw conclusions from the secondary endpoints such as quality of life and sleep issues, the researchers noted.
However, the results suggest that “nemolizumab plus topical agents may ameliorate both pruritus and signs of eczema and may lessen the severity of atopic dermatitis by disrupting the itch-scratch cycle,” they added.
“Novel therapies [for AD] are needed, as there are still patients who need better disease control despite current therapies, and AD is a heterogeneous disease that may need different treatment approaches,” Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, said in an interview.
Dr. Simpson, who was not an investigator in this study, said that he was somewhat surprised that the itch reduction was lower in the current study, compared with previous studies by the same group. Also surprising was the increase in cytokine abnormalities in the nemolizumab group, which “needs further study.”
Overall, the data “provide support that blockade of the IL-31 receptor improves itch in AD and appears to have some effect on inflammation,” Dr. Simpson said.
One challenge to the clinical use of nemolizumab will be identifying “where this type of drug fits into the treatment paradigm,” and determining whether specific patients whose disease is driven more by this neuroimmune pathway could benefit more than with the traditional IL-4 or IL-13 blockade, he said.
The study was supported by Maruho. Dr. Kabashima disclosed consulting fees from Maruho and two coauthors were Maruho employees. Dr. Simpson had no financial conflicts relevant to this study, but he reported receiving research grants and other financial relationships with manufacturers of AD therapies.
SOURCE: Kabashima K et al. N Engl J Med. 2020 Jul 9. doi: 10.1056/NEJMoa1917006.
of 215 patients in Japan.
Controlling the pruritus associated with atopic dermatitis (AD) can have a significant impact on patients’ quality of life, wrote Kenji Kabashima, MD, PhD, of the department of dermatology at Kyoto University, and coauthors. Frequent scratching can cause not only mechanical skin damage, but also may enhance inflammatory reactions and contribute to sleep problems.
In earlier phase studies, nemolizumab, a humanized monoclonal antibody against interleukin-31 receptor A, showed efficacy in reducing pruritus in patients with AD, but has not been well studied in patients who are also using topical agents, they wrote.
In the study published in the New England Journal of Medicine, the researchers randomized 143 patients with AD and moderate to severe pruritus to 60 mg of subcutaneous nemolizumab and 72 patients to a placebo every 4 weeks for 16 weeks. All patients were aged 13 years and older with a confirmed AD diagnosis and a history of inadequate response to or inability to use treatments, including topical glucocorticoids and oral antihistamines. Their average age was 40 years, approximately two-thirds were male, and the average disease duration was approximately 30 years. Topical treatments included a medium potency glucocorticoid in 97% of patients in both groups, and a topical calcineurin inhibitor in 41% of those on nemolizumab, and 40% of those on placebo; almost 90% of the patients in both groups were on oral antihistamines.
At 16 weeks, scores on the visual analog scale for pruritus (the primary outcome) significantly improved from baseline in the nemolizumab group, compared with the placebo group (a mean change of –42.8% and –21.4%, respectively, P < .001).
In addition, more patients in the nemolizumab group, compared with the placebo group (40% vs. 22%) achieved a score of 4 or less on the Dermatology Life Quality Index, with lower scores reflecting less impact of disease on daily life. In addition, more patients in the nemolizumab group, compared with the placebo group (55% vs. 21%) achieved a score of 7 or less on the Insomnia Severity Index.
During the study, 71% of the patients in each group reported adverse events, most were mild or moderate. The most common adverse event was worsening AD, reported by 24% of the nemolizumab patients and 21% of the placebo patients. Reactions related to the injection occurred in 8% of nemolizumab patients and 3% of placebo patients. Cytokine abnormalities, which included an increased level of thymus and activation regulated chemokine, were reported in 10 (7%) of the patients on nemolizumab, none of which occurred in those on placebo. “Most were not accompanied by a worsening of signs of or the extent of atopic dermatitis,” the authors wrote.
Severe adverse events were reported in three patients (2%) in the nemolizumab group, which were Meniere’s disease, acute pancreatitis, and AD in one patient each. No severe adverse events were reported in the placebo group. In addition, three patients in the nemolizumab group experienced four treatment-related adverse events that led them to discontinue treatment: AD, Meniere’s disease, alopecia, and peripheral edema.
The study findings were limited by several factors including the relatively short treatment period, inclusion only of Japanese patients, inclusion of patients aged as young as 13 years, and the inability to draw conclusions from the secondary endpoints such as quality of life and sleep issues, the researchers noted.
However, the results suggest that “nemolizumab plus topical agents may ameliorate both pruritus and signs of eczema and may lessen the severity of atopic dermatitis by disrupting the itch-scratch cycle,” they added.
“Novel therapies [for AD] are needed, as there are still patients who need better disease control despite current therapies, and AD is a heterogeneous disease that may need different treatment approaches,” Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, said in an interview.
Dr. Simpson, who was not an investigator in this study, said that he was somewhat surprised that the itch reduction was lower in the current study, compared with previous studies by the same group. Also surprising was the increase in cytokine abnormalities in the nemolizumab group, which “needs further study.”
Overall, the data “provide support that blockade of the IL-31 receptor improves itch in AD and appears to have some effect on inflammation,” Dr. Simpson said.
One challenge to the clinical use of nemolizumab will be identifying “where this type of drug fits into the treatment paradigm,” and determining whether specific patients whose disease is driven more by this neuroimmune pathway could benefit more than with the traditional IL-4 or IL-13 blockade, he said.
The study was supported by Maruho. Dr. Kabashima disclosed consulting fees from Maruho and two coauthors were Maruho employees. Dr. Simpson had no financial conflicts relevant to this study, but he reported receiving research grants and other financial relationships with manufacturers of AD therapies.
SOURCE: Kabashima K et al. N Engl J Med. 2020 Jul 9. doi: 10.1056/NEJMoa1917006.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Infants around the world with bronchiolitis received excess tests despite guidelines
While guidelines for bronchiolitis aim to reduce gratuitous tests and treatments, one-third of infants presenting at EDs with bronchiolitis receive an unnecessary intervention, according to a new global study.

For infants with symptoms of bronchiolitis, viral testing, blood tests, and chest x-rays are discouraged in most cases. Antibiotics are not recommended as treatment.
In a study published in Pediatrics, Amy Zipursky, MD, of the Hospital for Sick Children and the University of Toronto, and colleagues, reviewed records for 2,359 infants aged 2-11 months diagnosed with bronchiolitis during the year 2013. The data came from a network of 38 EDs in the Australia, Canada, Ireland, New Zealand, Portugal, Spain, the United Kingdom, and the United States.
Dr. Zipursky and colleagues found that, while 8% of infants in the cohort had been treated with antibiotics, 33% had received at least one nonrecommended test, with rates ranging widely across regions. In the United Kingdom and Ireland, for example, only 15% received such a test, compared with 50% in Spain and Portugal.
Of the children given antibiotics, two-thirds had suspected bacterial infections, the researchers found. Antibiotic use was highest in the United States, at 11% of infants seen for bronchiolitis, and lowest in the United Kingdom and Ireland at 4%. Administration of chest x-rays – which occurred in nearly a quarter of the cohort – increased the likelihood of antibiotics being administered (odds ratio, 2.29; 95% confidence interval, 1.62-3.24) independent of fever or severe symptoms.
The most common nonrecommended tests performed in the study were:
- Nasopharyngeal viral testing without admission to hospital (n = 591).
- Chest x-ray without ICU admission (n = 507).
- Complete blood counts (n = 222).
- Blood cultures (n = 129).
- Urinalysis in the absence of fever (n = 86).
- Febrile infants 3 months of age or less had blood cultures (n = 49).
In some treatment centers the rate of nonrecommended tests performed was 6%, while others saw rates of 74%.
“Despite the evidence that laboratory testing rarely impacts bronchiolitis management and that bacterial infections in bronchiolitis are uncommon, our study reveals that these tests continue to be performed frequently in many parts of the world,” Dr. Zipursky and colleagues wrote in their analysis.
“Plausible reasons may include ‘automatic’ blood draws with intravenous placement, uncertainty about institutional policies, perceived need for reassurance about the diagnosis, perception of ‘doing something,’ and parental desire for a viral label,” the authors surmised. “Because parental pressure to provide interventions may be a driver of care in infants with bronchiolitis in some countries, ED clinicians need to have higher confidence in the evidence-based bronchiolitis care and convey this trust to families.”
The researchers listed among the weaknesses of their study its retrospective design, and that results from x-rays and lab tests performed were not available.
In an editorial comment accompanying the study, Joseph J. Zorc, MD, of Children’s Hospital of Philadelphia and the University of Pennsylvania in Philadelphia, noted that some of the regional differences seen in the study may be attributable to different clinical criteria used to diagnose bronchiolitis. In the United Kingdom, for example, national guidelines include the presence of crackles, while in North America guidelines focus on wheeze. “Perhaps clinicians in the United Kingdom accept the presence of crackles as an expected finding in infant with bronchiolitis and are less likely to order imaging,” Dr. Zorc said (Pediatrics. 2020 Jul 13;146[2]:e20193684).
He also pointed out that the coronavirus pandemic caused by SARS-CoV-2 (COVID- 19) could have an impact on global testing and treatment practices for bronchiolitis, as coronaviruses are a known cause of bronchiolitis. The Pediatric Emergency Research Network, comprising the 38 EDs from which Dr. Zipursky and colleagues drew their data, is conducting a prospective study looking at pediatric disease caused by SARS-CoV-2.
The “collaboration of international networks of pediatric emergency providers is an encouraging sign of potential opportunities to come ... [providing] an opportunity to evaluate variation that can lead to innovation,” Dr. Zorc concluded.
Dr. Zipursky and colleagues reported no external funding or relevant financial disclosures. Dr. Zorc reported no relevant conflicts of interest.
SOURCE: Zipursky A et al. Pediatrics. 2020 Jul 13;146(2):e2020002311.
While guidelines for bronchiolitis aim to reduce gratuitous tests and treatments, one-third of infants presenting at EDs with bronchiolitis receive an unnecessary intervention, according to a new global study.

For infants with symptoms of bronchiolitis, viral testing, blood tests, and chest x-rays are discouraged in most cases. Antibiotics are not recommended as treatment.
In a study published in Pediatrics, Amy Zipursky, MD, of the Hospital for Sick Children and the University of Toronto, and colleagues, reviewed records for 2,359 infants aged 2-11 months diagnosed with bronchiolitis during the year 2013. The data came from a network of 38 EDs in the Australia, Canada, Ireland, New Zealand, Portugal, Spain, the United Kingdom, and the United States.
Dr. Zipursky and colleagues found that, while 8% of infants in the cohort had been treated with antibiotics, 33% had received at least one nonrecommended test, with rates ranging widely across regions. In the United Kingdom and Ireland, for example, only 15% received such a test, compared with 50% in Spain and Portugal.
Of the children given antibiotics, two-thirds had suspected bacterial infections, the researchers found. Antibiotic use was highest in the United States, at 11% of infants seen for bronchiolitis, and lowest in the United Kingdom and Ireland at 4%. Administration of chest x-rays – which occurred in nearly a quarter of the cohort – increased the likelihood of antibiotics being administered (odds ratio, 2.29; 95% confidence interval, 1.62-3.24) independent of fever or severe symptoms.
The most common nonrecommended tests performed in the study were:
- Nasopharyngeal viral testing without admission to hospital (n = 591).
- Chest x-ray without ICU admission (n = 507).
- Complete blood counts (n = 222).
- Blood cultures (n = 129).
- Urinalysis in the absence of fever (n = 86).
- Febrile infants 3 months of age or less had blood cultures (n = 49).
In some treatment centers the rate of nonrecommended tests performed was 6%, while others saw rates of 74%.
“Despite the evidence that laboratory testing rarely impacts bronchiolitis management and that bacterial infections in bronchiolitis are uncommon, our study reveals that these tests continue to be performed frequently in many parts of the world,” Dr. Zipursky and colleagues wrote in their analysis.
“Plausible reasons may include ‘automatic’ blood draws with intravenous placement, uncertainty about institutional policies, perceived need for reassurance about the diagnosis, perception of ‘doing something,’ and parental desire for a viral label,” the authors surmised. “Because parental pressure to provide interventions may be a driver of care in infants with bronchiolitis in some countries, ED clinicians need to have higher confidence in the evidence-based bronchiolitis care and convey this trust to families.”
The researchers listed among the weaknesses of their study its retrospective design, and that results from x-rays and lab tests performed were not available.
In an editorial comment accompanying the study, Joseph J. Zorc, MD, of Children’s Hospital of Philadelphia and the University of Pennsylvania in Philadelphia, noted that some of the regional differences seen in the study may be attributable to different clinical criteria used to diagnose bronchiolitis. In the United Kingdom, for example, national guidelines include the presence of crackles, while in North America guidelines focus on wheeze. “Perhaps clinicians in the United Kingdom accept the presence of crackles as an expected finding in infant with bronchiolitis and are less likely to order imaging,” Dr. Zorc said (Pediatrics. 2020 Jul 13;146[2]:e20193684).
He also pointed out that the coronavirus pandemic caused by SARS-CoV-2 (COVID- 19) could have an impact on global testing and treatment practices for bronchiolitis, as coronaviruses are a known cause of bronchiolitis. The Pediatric Emergency Research Network, comprising the 38 EDs from which Dr. Zipursky and colleagues drew their data, is conducting a prospective study looking at pediatric disease caused by SARS-CoV-2.
The “collaboration of international networks of pediatric emergency providers is an encouraging sign of potential opportunities to come ... [providing] an opportunity to evaluate variation that can lead to innovation,” Dr. Zorc concluded.
Dr. Zipursky and colleagues reported no external funding or relevant financial disclosures. Dr. Zorc reported no relevant conflicts of interest.
SOURCE: Zipursky A et al. Pediatrics. 2020 Jul 13;146(2):e2020002311.
While guidelines for bronchiolitis aim to reduce gratuitous tests and treatments, one-third of infants presenting at EDs with bronchiolitis receive an unnecessary intervention, according to a new global study.

For infants with symptoms of bronchiolitis, viral testing, blood tests, and chest x-rays are discouraged in most cases. Antibiotics are not recommended as treatment.
In a study published in Pediatrics, Amy Zipursky, MD, of the Hospital for Sick Children and the University of Toronto, and colleagues, reviewed records for 2,359 infants aged 2-11 months diagnosed with bronchiolitis during the year 2013. The data came from a network of 38 EDs in the Australia, Canada, Ireland, New Zealand, Portugal, Spain, the United Kingdom, and the United States.
Dr. Zipursky and colleagues found that, while 8% of infants in the cohort had been treated with antibiotics, 33% had received at least one nonrecommended test, with rates ranging widely across regions. In the United Kingdom and Ireland, for example, only 15% received such a test, compared with 50% in Spain and Portugal.
Of the children given antibiotics, two-thirds had suspected bacterial infections, the researchers found. Antibiotic use was highest in the United States, at 11% of infants seen for bronchiolitis, and lowest in the United Kingdom and Ireland at 4%. Administration of chest x-rays – which occurred in nearly a quarter of the cohort – increased the likelihood of antibiotics being administered (odds ratio, 2.29; 95% confidence interval, 1.62-3.24) independent of fever or severe symptoms.
The most common nonrecommended tests performed in the study were:
- Nasopharyngeal viral testing without admission to hospital (n = 591).
- Chest x-ray without ICU admission (n = 507).
- Complete blood counts (n = 222).
- Blood cultures (n = 129).
- Urinalysis in the absence of fever (n = 86).
- Febrile infants 3 months of age or less had blood cultures (n = 49).
In some treatment centers the rate of nonrecommended tests performed was 6%, while others saw rates of 74%.
“Despite the evidence that laboratory testing rarely impacts bronchiolitis management and that bacterial infections in bronchiolitis are uncommon, our study reveals that these tests continue to be performed frequently in many parts of the world,” Dr. Zipursky and colleagues wrote in their analysis.
“Plausible reasons may include ‘automatic’ blood draws with intravenous placement, uncertainty about institutional policies, perceived need for reassurance about the diagnosis, perception of ‘doing something,’ and parental desire for a viral label,” the authors surmised. “Because parental pressure to provide interventions may be a driver of care in infants with bronchiolitis in some countries, ED clinicians need to have higher confidence in the evidence-based bronchiolitis care and convey this trust to families.”
The researchers listed among the weaknesses of their study its retrospective design, and that results from x-rays and lab tests performed were not available.
In an editorial comment accompanying the study, Joseph J. Zorc, MD, of Children’s Hospital of Philadelphia and the University of Pennsylvania in Philadelphia, noted that some of the regional differences seen in the study may be attributable to different clinical criteria used to diagnose bronchiolitis. In the United Kingdom, for example, national guidelines include the presence of crackles, while in North America guidelines focus on wheeze. “Perhaps clinicians in the United Kingdom accept the presence of crackles as an expected finding in infant with bronchiolitis and are less likely to order imaging,” Dr. Zorc said (Pediatrics. 2020 Jul 13;146[2]:e20193684).
He also pointed out that the coronavirus pandemic caused by SARS-CoV-2 (COVID- 19) could have an impact on global testing and treatment practices for bronchiolitis, as coronaviruses are a known cause of bronchiolitis. The Pediatric Emergency Research Network, comprising the 38 EDs from which Dr. Zipursky and colleagues drew their data, is conducting a prospective study looking at pediatric disease caused by SARS-CoV-2.
The “collaboration of international networks of pediatric emergency providers is an encouraging sign of potential opportunities to come ... [providing] an opportunity to evaluate variation that can lead to innovation,” Dr. Zorc concluded.
Dr. Zipursky and colleagues reported no external funding or relevant financial disclosures. Dr. Zorc reported no relevant conflicts of interest.
SOURCE: Zipursky A et al. Pediatrics. 2020 Jul 13;146(2):e2020002311.
FROM PEDIATRICS
Key clinical point:
Major finding: In a global cohort, 33% of infants received at least one nonrecommended test, most commonly viral tests, chest x-rays, and blood cultures.
Study details: A retrospective cohort of 2,359 infants aged 2-11 months seen in 38 EDs in developed countries.
Disclosures: Dr. Zipursky and colleagues reported no external funding or relevant financial disclosures.
Source: Zipursky A et al. Pediatrics. 2020 Jul 13;146(2):e2020002311.
Diagnosing molluscum contagiosum can be tricky
The way James R. Treat, MD, sees it, if there ever were a truism in the field of dermatology, it’s that everyone hates molluscum contagiosum.
“It tortures all of us,” Dr. Treat, a pediatric dermatologist at Children’s Hospital of Philadelphia, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “It’s very distressing to parents, but often more distressing to parents than to kids.”
A viral disorder of the skin and mucous membranes characterized by discrete single or multiple, flesh-colored papules, “When you look at inflamed molluscum it can be very difficult to recognize because it looks like a more complicated infection,” said Dr. Treat, who is also associate professor of clinical pediatrics and dermatology, at the University of Pennsylvania, Philadelphia.
An epidemiologic review of 302 MC cases found that 80% of patients were aged younger than 8 years, 63% had more than 15 lesions, and 24% had concomitant atopic dermatitis (J Am Acad Dermatol. 2006; 2006;54[1]:47-54). “Children with atopic dermatitis often have their molluscum last longer,” he said. “The average time course for molluscum is 18 months, but it can certainly be longer than that. So if you say, ‘it’s probably going to go away in a few months,’ that’s probably not going to happen.”
The telltale MC lesion is glossy and contains a white core in the center that can be revealed by shining an otoscope sideways on the lesion. “Umbilication doesn’t always occur, but if the center part looks white, that will help with diagnosis,” Dr. Treat said. “If they’re inflamed and they’re red and you’re worried that there’s a bacterial infection, do a culture, pop the lesion open, and get some of the pus out. If you’re concerned, start them on antibiotics. It’s always worse to miss an infection than to overtreat molluscum. But once you’ve done it a few times and you realize that the cultures are coming back negative, then you’ll probably have your threshold a little higher.”
The most useful clinical sign of MC is the so-called “BOTE” (beginning of the end) sign, which is characterized by erythema and swelling of MC skin lesions. “When the parents come to us in pediatric dermatology, often it’s because their kids have had molluscum for a while,” he said. “It spreads and becomes inflamed and the parents ask, ‘Is it infected?’ The answer is, yes, it’s an infection, but it’s not infected with what you think it is [which is Staphylococcus or Streptococcus], it’s the virus being recognized by the body. When the virus is recognized by the body, it creates a huge inflammatory reaction. That’s usually the time at which the body has had enough of the virus, and it eradicates the rest of it. It means the inflammatory response is finding the molluscum and it’s going to take care of it.”
MC brings its own eczematous response, which can complicate efforts to confirm the diagnosis. Dr. Treat spoke of a young patient he recently saw who had an eczematous reaction on the inner parts of the arms and the upper flank – with no such clinical history. “It kind of came out of the blue,” he said. “You think about contact allergies and other types of dermatitis, but molluscum brings its own eczema. Often what the parents recognize is the eczematous eruption and not the little dots of molluscum. So if you see someone with a new eruption in typical molluscum areas – the flank and your thighs and the back of the legs – and they’ve never had eczema in the past, or they’ve only had mild eczema, think about eczema as a response to molluscum.”
MC can also result in a Gianotti-Crosti syndrome-like reactions (Arch Dermatol. 2012;148[11]:1257-64). “These are angry, inflamed red papules on the knees and on the elbows and on the buttocks and on the cheeks,” Dr. Treat said. “It typically spares the trunk, and they look like molluscum.”
He went on to note that MC can present as cysts, and that MC in the gluteal cleft is a mimicker of condyloma. MC can also cause conjunctivitis, which is increased in HIV patients and in those with atopic dermatitis. “These are patients who should probably see an ophthalmologist” to make no damage has occurred, Dr. Treat said.
He closed his remarks by noting that rarely, MC can be the presenting sign of an immunodeficiency. “The immune system dysregulation that shows up this way is called a DOCK8 mutation, which have eczema and widespread viral disease including warts and molluscum,” Dr. Treat said.
He reported having no financial disclosures.
The way James R. Treat, MD, sees it, if there ever were a truism in the field of dermatology, it’s that everyone hates molluscum contagiosum.
“It tortures all of us,” Dr. Treat, a pediatric dermatologist at Children’s Hospital of Philadelphia, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “It’s very distressing to parents, but often more distressing to parents than to kids.”
A viral disorder of the skin and mucous membranes characterized by discrete single or multiple, flesh-colored papules, “When you look at inflamed molluscum it can be very difficult to recognize because it looks like a more complicated infection,” said Dr. Treat, who is also associate professor of clinical pediatrics and dermatology, at the University of Pennsylvania, Philadelphia.
An epidemiologic review of 302 MC cases found that 80% of patients were aged younger than 8 years, 63% had more than 15 lesions, and 24% had concomitant atopic dermatitis (J Am Acad Dermatol. 2006; 2006;54[1]:47-54). “Children with atopic dermatitis often have their molluscum last longer,” he said. “The average time course for molluscum is 18 months, but it can certainly be longer than that. So if you say, ‘it’s probably going to go away in a few months,’ that’s probably not going to happen.”
The telltale MC lesion is glossy and contains a white core in the center that can be revealed by shining an otoscope sideways on the lesion. “Umbilication doesn’t always occur, but if the center part looks white, that will help with diagnosis,” Dr. Treat said. “If they’re inflamed and they’re red and you’re worried that there’s a bacterial infection, do a culture, pop the lesion open, and get some of the pus out. If you’re concerned, start them on antibiotics. It’s always worse to miss an infection than to overtreat molluscum. But once you’ve done it a few times and you realize that the cultures are coming back negative, then you’ll probably have your threshold a little higher.”
The most useful clinical sign of MC is the so-called “BOTE” (beginning of the end) sign, which is characterized by erythema and swelling of MC skin lesions. “When the parents come to us in pediatric dermatology, often it’s because their kids have had molluscum for a while,” he said. “It spreads and becomes inflamed and the parents ask, ‘Is it infected?’ The answer is, yes, it’s an infection, but it’s not infected with what you think it is [which is Staphylococcus or Streptococcus], it’s the virus being recognized by the body. When the virus is recognized by the body, it creates a huge inflammatory reaction. That’s usually the time at which the body has had enough of the virus, and it eradicates the rest of it. It means the inflammatory response is finding the molluscum and it’s going to take care of it.”
MC brings its own eczematous response, which can complicate efforts to confirm the diagnosis. Dr. Treat spoke of a young patient he recently saw who had an eczematous reaction on the inner parts of the arms and the upper flank – with no such clinical history. “It kind of came out of the blue,” he said. “You think about contact allergies and other types of dermatitis, but molluscum brings its own eczema. Often what the parents recognize is the eczematous eruption and not the little dots of molluscum. So if you see someone with a new eruption in typical molluscum areas – the flank and your thighs and the back of the legs – and they’ve never had eczema in the past, or they’ve only had mild eczema, think about eczema as a response to molluscum.”
MC can also result in a Gianotti-Crosti syndrome-like reactions (Arch Dermatol. 2012;148[11]:1257-64). “These are angry, inflamed red papules on the knees and on the elbows and on the buttocks and on the cheeks,” Dr. Treat said. “It typically spares the trunk, and they look like molluscum.”
He went on to note that MC can present as cysts, and that MC in the gluteal cleft is a mimicker of condyloma. MC can also cause conjunctivitis, which is increased in HIV patients and in those with atopic dermatitis. “These are patients who should probably see an ophthalmologist” to make no damage has occurred, Dr. Treat said.
He closed his remarks by noting that rarely, MC can be the presenting sign of an immunodeficiency. “The immune system dysregulation that shows up this way is called a DOCK8 mutation, which have eczema and widespread viral disease including warts and molluscum,” Dr. Treat said.
He reported having no financial disclosures.
The way James R. Treat, MD, sees it, if there ever were a truism in the field of dermatology, it’s that everyone hates molluscum contagiosum.
“It tortures all of us,” Dr. Treat, a pediatric dermatologist at Children’s Hospital of Philadelphia, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “It’s very distressing to parents, but often more distressing to parents than to kids.”
A viral disorder of the skin and mucous membranes characterized by discrete single or multiple, flesh-colored papules, “When you look at inflamed molluscum it can be very difficult to recognize because it looks like a more complicated infection,” said Dr. Treat, who is also associate professor of clinical pediatrics and dermatology, at the University of Pennsylvania, Philadelphia.
An epidemiologic review of 302 MC cases found that 80% of patients were aged younger than 8 years, 63% had more than 15 lesions, and 24% had concomitant atopic dermatitis (J Am Acad Dermatol. 2006; 2006;54[1]:47-54). “Children with atopic dermatitis often have their molluscum last longer,” he said. “The average time course for molluscum is 18 months, but it can certainly be longer than that. So if you say, ‘it’s probably going to go away in a few months,’ that’s probably not going to happen.”
The telltale MC lesion is glossy and contains a white core in the center that can be revealed by shining an otoscope sideways on the lesion. “Umbilication doesn’t always occur, but if the center part looks white, that will help with diagnosis,” Dr. Treat said. “If they’re inflamed and they’re red and you’re worried that there’s a bacterial infection, do a culture, pop the lesion open, and get some of the pus out. If you’re concerned, start them on antibiotics. It’s always worse to miss an infection than to overtreat molluscum. But once you’ve done it a few times and you realize that the cultures are coming back negative, then you’ll probably have your threshold a little higher.”
The most useful clinical sign of MC is the so-called “BOTE” (beginning of the end) sign, which is characterized by erythema and swelling of MC skin lesions. “When the parents come to us in pediatric dermatology, often it’s because their kids have had molluscum for a while,” he said. “It spreads and becomes inflamed and the parents ask, ‘Is it infected?’ The answer is, yes, it’s an infection, but it’s not infected with what you think it is [which is Staphylococcus or Streptococcus], it’s the virus being recognized by the body. When the virus is recognized by the body, it creates a huge inflammatory reaction. That’s usually the time at which the body has had enough of the virus, and it eradicates the rest of it. It means the inflammatory response is finding the molluscum and it’s going to take care of it.”
MC brings its own eczematous response, which can complicate efforts to confirm the diagnosis. Dr. Treat spoke of a young patient he recently saw who had an eczematous reaction on the inner parts of the arms and the upper flank – with no such clinical history. “It kind of came out of the blue,” he said. “You think about contact allergies and other types of dermatitis, but molluscum brings its own eczema. Often what the parents recognize is the eczematous eruption and not the little dots of molluscum. So if you see someone with a new eruption in typical molluscum areas – the flank and your thighs and the back of the legs – and they’ve never had eczema in the past, or they’ve only had mild eczema, think about eczema as a response to molluscum.”
MC can also result in a Gianotti-Crosti syndrome-like reactions (Arch Dermatol. 2012;148[11]:1257-64). “These are angry, inflamed red papules on the knees and on the elbows and on the buttocks and on the cheeks,” Dr. Treat said. “It typically spares the trunk, and they look like molluscum.”
He went on to note that MC can present as cysts, and that MC in the gluteal cleft is a mimicker of condyloma. MC can also cause conjunctivitis, which is increased in HIV patients and in those with atopic dermatitis. “These are patients who should probably see an ophthalmologist” to make no damage has occurred, Dr. Treat said.
He closed his remarks by noting that rarely, MC can be the presenting sign of an immunodeficiency. “The immune system dysregulation that shows up this way is called a DOCK8 mutation, which have eczema and widespread viral disease including warts and molluscum,” Dr. Treat said.
He reported having no financial disclosures.
FROM PEDIATRIC DERMATOLOGY 2020
PHM fellowship changes in response to the COVID-19 pandemic
The COVID-19 surge and pandemic have changed many things in medicine, from how we round on the wards to our increased use of telemedicine in outpatient and inpatient care. This not only changed our interactions with patients, but it also changed our learners’ education. From March to May 2020 during the COVID-19 pandemic, many Pediatric Hospital Medicine (PHM) fellowship directors were required to adjust clinical responsibilities and scholarly activities. These changes led to unique challenges and learning opportunities for fellows and faculty.
Many fellowships were asked to make changes to patient care for patient/provider safety. Because of low censuses, the University of Pittsburgh Medical Center’s Pittsburgh Children’s Hospital closed their observation unit; as a result, Elise Lu, MD, PhD, PHM fellow, spent time rounding on inpatient units instead of the observation unit. At the Children’s Hospital of Los Angeles, PHM fellow Brandon Palmer, MD, said his in-person child abuse elective was switched to a virtual elective. Dr. Adam Cohen, chief PHM fellow at Baylor College of Medicine/Texas Children’s Hospital, both in Houston, offered to care for adult patients and provide telehealth services to pediatric patients, but was never called to participate.
At other programs, fellows experienced greater changes to patient care and systems-based practice. Carlos Plancarte, MD, PHM fellow at Children’s Hospital of Montefiore, New York, provided care for patients up to the age of 30 years as his training hospital began admitting adult patients. Jeremiah Cleveland, MD, PHM fellowship director at Maimonides Children’s Hospital, New York, shared that his fellow’s “away elective” at a pediatric long-term acute care facility was canceled. Dr. Cleveland changed the fellow’s rotation to an infectious disease rotation, which gave her a unique opportunity to evaluate the clinical and nonclinical aspects of pandemic and disaster response.
PHM fellows also experienced changes to their scholarly activities. Matthew Shapiro, MD, a fellow at Anne and Robert H. Lurie Children’s Hospital in Chicago, had to place his quality improvement research on hold and is writing a commentary with his mentors. Marie Pfarr, MD, a fellow at Cincinnati Children’s Hospital and Medical Center, changed her plans from a simulation-based research project to studying compassion fatigue. Many fellows had workshops, platform and/or poster presentations at the Pediatric Academic Societies Meeting and/or the Pediatric Hospital Medicine Conference, both of which were canceled.
Some fellows felt the pandemic provided them an opportunity to learn communication and interpersonal skills. Dr. Shapiro observed his mentors effectively communicating while managing a crisis. Dr. Plancarte shared that he learned that saying “I don’t know” can be helpful when effectively leading a team.
Despite the changes, the COVID-19 pandemic’s most important lesson was the creation of a supportive community. Across the country, PHM fellows were supported by faculty, and faculty by their fellows. Dr. Plancarte observed how his colleagues united during a challenging situation to support each other. Ritu Patel, MD, PHM fellowship director at Kaiser Oakland shared that her fellowship’s weekly informal meetings helped bring her fellowship’s fellows and faculty closer. Joel Forman, MD, PHM fellowship director and vice chair of education at Mount Sinai Kravis Children’s Hospital in New York stressed the importance of camaraderie amongst faculty and learners.
While the pandemic continues, and long after it has passed, fellowship programs have learned that fostering community across training lines is important for both fellows and faculty.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and serves as the pediatrics editor for the Hospitalist.
The COVID-19 surge and pandemic have changed many things in medicine, from how we round on the wards to our increased use of telemedicine in outpatient and inpatient care. This not only changed our interactions with patients, but it also changed our learners’ education. From March to May 2020 during the COVID-19 pandemic, many Pediatric Hospital Medicine (PHM) fellowship directors were required to adjust clinical responsibilities and scholarly activities. These changes led to unique challenges and learning opportunities for fellows and faculty.
Many fellowships were asked to make changes to patient care for patient/provider safety. Because of low censuses, the University of Pittsburgh Medical Center’s Pittsburgh Children’s Hospital closed their observation unit; as a result, Elise Lu, MD, PhD, PHM fellow, spent time rounding on inpatient units instead of the observation unit. At the Children’s Hospital of Los Angeles, PHM fellow Brandon Palmer, MD, said his in-person child abuse elective was switched to a virtual elective. Dr. Adam Cohen, chief PHM fellow at Baylor College of Medicine/Texas Children’s Hospital, both in Houston, offered to care for adult patients and provide telehealth services to pediatric patients, but was never called to participate.
At other programs, fellows experienced greater changes to patient care and systems-based practice. Carlos Plancarte, MD, PHM fellow at Children’s Hospital of Montefiore, New York, provided care for patients up to the age of 30 years as his training hospital began admitting adult patients. Jeremiah Cleveland, MD, PHM fellowship director at Maimonides Children’s Hospital, New York, shared that his fellow’s “away elective” at a pediatric long-term acute care facility was canceled. Dr. Cleveland changed the fellow’s rotation to an infectious disease rotation, which gave her a unique opportunity to evaluate the clinical and nonclinical aspects of pandemic and disaster response.
PHM fellows also experienced changes to their scholarly activities. Matthew Shapiro, MD, a fellow at Anne and Robert H. Lurie Children’s Hospital in Chicago, had to place his quality improvement research on hold and is writing a commentary with his mentors. Marie Pfarr, MD, a fellow at Cincinnati Children’s Hospital and Medical Center, changed her plans from a simulation-based research project to studying compassion fatigue. Many fellows had workshops, platform and/or poster presentations at the Pediatric Academic Societies Meeting and/or the Pediatric Hospital Medicine Conference, both of which were canceled.
Some fellows felt the pandemic provided them an opportunity to learn communication and interpersonal skills. Dr. Shapiro observed his mentors effectively communicating while managing a crisis. Dr. Plancarte shared that he learned that saying “I don’t know” can be helpful when effectively leading a team.
Despite the changes, the COVID-19 pandemic’s most important lesson was the creation of a supportive community. Across the country, PHM fellows were supported by faculty, and faculty by their fellows. Dr. Plancarte observed how his colleagues united during a challenging situation to support each other. Ritu Patel, MD, PHM fellowship director at Kaiser Oakland shared that her fellowship’s weekly informal meetings helped bring her fellowship’s fellows and faculty closer. Joel Forman, MD, PHM fellowship director and vice chair of education at Mount Sinai Kravis Children’s Hospital in New York stressed the importance of camaraderie amongst faculty and learners.
While the pandemic continues, and long after it has passed, fellowship programs have learned that fostering community across training lines is important for both fellows and faculty.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and serves as the pediatrics editor for the Hospitalist.
The COVID-19 surge and pandemic have changed many things in medicine, from how we round on the wards to our increased use of telemedicine in outpatient and inpatient care. This not only changed our interactions with patients, but it also changed our learners’ education. From March to May 2020 during the COVID-19 pandemic, many Pediatric Hospital Medicine (PHM) fellowship directors were required to adjust clinical responsibilities and scholarly activities. These changes led to unique challenges and learning opportunities for fellows and faculty.
Many fellowships were asked to make changes to patient care for patient/provider safety. Because of low censuses, the University of Pittsburgh Medical Center’s Pittsburgh Children’s Hospital closed their observation unit; as a result, Elise Lu, MD, PhD, PHM fellow, spent time rounding on inpatient units instead of the observation unit. At the Children’s Hospital of Los Angeles, PHM fellow Brandon Palmer, MD, said his in-person child abuse elective was switched to a virtual elective. Dr. Adam Cohen, chief PHM fellow at Baylor College of Medicine/Texas Children’s Hospital, both in Houston, offered to care for adult patients and provide telehealth services to pediatric patients, but was never called to participate.
At other programs, fellows experienced greater changes to patient care and systems-based practice. Carlos Plancarte, MD, PHM fellow at Children’s Hospital of Montefiore, New York, provided care for patients up to the age of 30 years as his training hospital began admitting adult patients. Jeremiah Cleveland, MD, PHM fellowship director at Maimonides Children’s Hospital, New York, shared that his fellow’s “away elective” at a pediatric long-term acute care facility was canceled. Dr. Cleveland changed the fellow’s rotation to an infectious disease rotation, which gave her a unique opportunity to evaluate the clinical and nonclinical aspects of pandemic and disaster response.
PHM fellows also experienced changes to their scholarly activities. Matthew Shapiro, MD, a fellow at Anne and Robert H. Lurie Children’s Hospital in Chicago, had to place his quality improvement research on hold and is writing a commentary with his mentors. Marie Pfarr, MD, a fellow at Cincinnati Children’s Hospital and Medical Center, changed her plans from a simulation-based research project to studying compassion fatigue. Many fellows had workshops, platform and/or poster presentations at the Pediatric Academic Societies Meeting and/or the Pediatric Hospital Medicine Conference, both of which were canceled.
Some fellows felt the pandemic provided them an opportunity to learn communication and interpersonal skills. Dr. Shapiro observed his mentors effectively communicating while managing a crisis. Dr. Plancarte shared that he learned that saying “I don’t know” can be helpful when effectively leading a team.
Despite the changes, the COVID-19 pandemic’s most important lesson was the creation of a supportive community. Across the country, PHM fellows were supported by faculty, and faculty by their fellows. Dr. Plancarte observed how his colleagues united during a challenging situation to support each other. Ritu Patel, MD, PHM fellowship director at Kaiser Oakland shared that her fellowship’s weekly informal meetings helped bring her fellowship’s fellows and faculty closer. Joel Forman, MD, PHM fellowship director and vice chair of education at Mount Sinai Kravis Children’s Hospital in New York stressed the importance of camaraderie amongst faculty and learners.
While the pandemic continues, and long after it has passed, fellowship programs have learned that fostering community across training lines is important for both fellows and faculty.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and serves as the pediatrics editor for the Hospitalist.
Early childhood overweight, obesity tied to high cardiometabolic syndrome risk
Children who were overweight or obese at ages 2-3 years and at 6-7 years were significantly more likely than were healthy-weight children to show cardiometabolic risk factors at 11-12 years in a population-based study of more than 5,000 children.
Previous studies of the impact of childhood body mass index on cardiovascular disease have used a single BMI measurement, wrote Kate Lycett, PhD, of Deakin University, Victoria, Australia, and colleagues. “This overlooks the considerable physiologic changes in BMI throughout childhood as part of typical growth.”
In a study published in Pediatrics, the researchers examined overweight and obesity at five time points in a cohort of 5,107 infants by measuring BMI every 2 years between the ages of 2-3 years and 10-11 years.
Overall, children with consistently high BMI trajectories from age 3 years had the highest risk of metabolic syndrome. At age 6-7 years, overweight and obese children had, respectively, higher metabolic syndrome risk scores by 0.23 and 0.76 mean standard deviation (SD) units, compared with healthy-weight children; these associations approximately doubled by age 11-12 years.
In addition, obese children had higher pulse wave velocity (PWV) from age 6-7 years (0.64-0.73 standard deviation units) and slightly higher carotid artery intima-media thickness (cIMT) at all measured ages, compared with healthy-weight children (0.20-0.30 SD units).
The findings were limited by several factors, including the inability to evaluate the effects of BMI on actual cardiovascular disease because of the young age of the study population, the researchers noted.
However, the “results are in keeping with previous studies but provide additional important insights that suggest BMI from as early as 2 to 3 years of age is predictive of preclinical cardiometabolic phenotypes by ages 11 to 12 years,” Dr. Lycett and associates said. The results have implications for public health by highlighting the subclinical effects of obesity in childhood and the importance of early intervention, they concluded.
“This important and comprehensive study has two important implications: first, high BMI by age 2 to 3 tends to stay high, and second, normal BMI occasionally increases to high BMI, but the reverse is rarely true,” Sarah Armstrong, MD, Jennifer S. Li, MD, and Asheley C. Skinner, PhD, wrote in an accompanying editorial (Pediatrics. 2020 Jul 6. doi: 10.1542/peds.2020-1353).
noted the editorialists, who are affiliated with Duke University, Durham, N.C.
“An important caveat is that although the relationships were significant, the amount of variance attributable directly to child BMI was small,” which highlights the complex relationship between obesity and health, they noted.
“Early-onset obesity is unlikely to change and, if it persists, will lead to detectable precursors of atherosclerosis by the time a child enters middle school,” and parents and primary care providers have an opportunity to “flatten the curve” by addressing BMI increases early in life to delay or prevent obesity, the editorialists concluded.
The study was supported by Australia’s National Health and Medical Research Council, The Royal Children’s Hospital Foundation, Murdoch Children’s Research Institute, The University of Melbourne, National Heart Foundation of Australia, Financial Markets Foundation for Children, and Victorian Deaf Education Institute. A number of the researchers were supported by grants from these and other universities and organizations. The researchers had no relevant financial disclosures. The editorialists had no financial conflicts to disclose.
SOURCE: Lycett K et al. Pediatrics. 2020 Jul 6. doi: 10.1542/peds.2019-3666.
Children who were overweight or obese at ages 2-3 years and at 6-7 years were significantly more likely than were healthy-weight children to show cardiometabolic risk factors at 11-12 years in a population-based study of more than 5,000 children.
Previous studies of the impact of childhood body mass index on cardiovascular disease have used a single BMI measurement, wrote Kate Lycett, PhD, of Deakin University, Victoria, Australia, and colleagues. “This overlooks the considerable physiologic changes in BMI throughout childhood as part of typical growth.”
In a study published in Pediatrics, the researchers examined overweight and obesity at five time points in a cohort of 5,107 infants by measuring BMI every 2 years between the ages of 2-3 years and 10-11 years.
Overall, children with consistently high BMI trajectories from age 3 years had the highest risk of metabolic syndrome. At age 6-7 years, overweight and obese children had, respectively, higher metabolic syndrome risk scores by 0.23 and 0.76 mean standard deviation (SD) units, compared with healthy-weight children; these associations approximately doubled by age 11-12 years.
In addition, obese children had higher pulse wave velocity (PWV) from age 6-7 years (0.64-0.73 standard deviation units) and slightly higher carotid artery intima-media thickness (cIMT) at all measured ages, compared with healthy-weight children (0.20-0.30 SD units).
The findings were limited by several factors, including the inability to evaluate the effects of BMI on actual cardiovascular disease because of the young age of the study population, the researchers noted.
However, the “results are in keeping with previous studies but provide additional important insights that suggest BMI from as early as 2 to 3 years of age is predictive of preclinical cardiometabolic phenotypes by ages 11 to 12 years,” Dr. Lycett and associates said. The results have implications for public health by highlighting the subclinical effects of obesity in childhood and the importance of early intervention, they concluded.
“This important and comprehensive study has two important implications: first, high BMI by age 2 to 3 tends to stay high, and second, normal BMI occasionally increases to high BMI, but the reverse is rarely true,” Sarah Armstrong, MD, Jennifer S. Li, MD, and Asheley C. Skinner, PhD, wrote in an accompanying editorial (Pediatrics. 2020 Jul 6. doi: 10.1542/peds.2020-1353).
noted the editorialists, who are affiliated with Duke University, Durham, N.C.
“An important caveat is that although the relationships were significant, the amount of variance attributable directly to child BMI was small,” which highlights the complex relationship between obesity and health, they noted.
“Early-onset obesity is unlikely to change and, if it persists, will lead to detectable precursors of atherosclerosis by the time a child enters middle school,” and parents and primary care providers have an opportunity to “flatten the curve” by addressing BMI increases early in life to delay or prevent obesity, the editorialists concluded.
The study was supported by Australia’s National Health and Medical Research Council, The Royal Children’s Hospital Foundation, Murdoch Children’s Research Institute, The University of Melbourne, National Heart Foundation of Australia, Financial Markets Foundation for Children, and Victorian Deaf Education Institute. A number of the researchers were supported by grants from these and other universities and organizations. The researchers had no relevant financial disclosures. The editorialists had no financial conflicts to disclose.
SOURCE: Lycett K et al. Pediatrics. 2020 Jul 6. doi: 10.1542/peds.2019-3666.
Children who were overweight or obese at ages 2-3 years and at 6-7 years were significantly more likely than were healthy-weight children to show cardiometabolic risk factors at 11-12 years in a population-based study of more than 5,000 children.
Previous studies of the impact of childhood body mass index on cardiovascular disease have used a single BMI measurement, wrote Kate Lycett, PhD, of Deakin University, Victoria, Australia, and colleagues. “This overlooks the considerable physiologic changes in BMI throughout childhood as part of typical growth.”
In a study published in Pediatrics, the researchers examined overweight and obesity at five time points in a cohort of 5,107 infants by measuring BMI every 2 years between the ages of 2-3 years and 10-11 years.
Overall, children with consistently high BMI trajectories from age 3 years had the highest risk of metabolic syndrome. At age 6-7 years, overweight and obese children had, respectively, higher metabolic syndrome risk scores by 0.23 and 0.76 mean standard deviation (SD) units, compared with healthy-weight children; these associations approximately doubled by age 11-12 years.
In addition, obese children had higher pulse wave velocity (PWV) from age 6-7 years (0.64-0.73 standard deviation units) and slightly higher carotid artery intima-media thickness (cIMT) at all measured ages, compared with healthy-weight children (0.20-0.30 SD units).
The findings were limited by several factors, including the inability to evaluate the effects of BMI on actual cardiovascular disease because of the young age of the study population, the researchers noted.
However, the “results are in keeping with previous studies but provide additional important insights that suggest BMI from as early as 2 to 3 years of age is predictive of preclinical cardiometabolic phenotypes by ages 11 to 12 years,” Dr. Lycett and associates said. The results have implications for public health by highlighting the subclinical effects of obesity in childhood and the importance of early intervention, they concluded.
“This important and comprehensive study has two important implications: first, high BMI by age 2 to 3 tends to stay high, and second, normal BMI occasionally increases to high BMI, but the reverse is rarely true,” Sarah Armstrong, MD, Jennifer S. Li, MD, and Asheley C. Skinner, PhD, wrote in an accompanying editorial (Pediatrics. 2020 Jul 6. doi: 10.1542/peds.2020-1353).
noted the editorialists, who are affiliated with Duke University, Durham, N.C.
“An important caveat is that although the relationships were significant, the amount of variance attributable directly to child BMI was small,” which highlights the complex relationship between obesity and health, they noted.
“Early-onset obesity is unlikely to change and, if it persists, will lead to detectable precursors of atherosclerosis by the time a child enters middle school,” and parents and primary care providers have an opportunity to “flatten the curve” by addressing BMI increases early in life to delay or prevent obesity, the editorialists concluded.
The study was supported by Australia’s National Health and Medical Research Council, The Royal Children’s Hospital Foundation, Murdoch Children’s Research Institute, The University of Melbourne, National Heart Foundation of Australia, Financial Markets Foundation for Children, and Victorian Deaf Education Institute. A number of the researchers were supported by grants from these and other universities and organizations. The researchers had no relevant financial disclosures. The editorialists had no financial conflicts to disclose.
SOURCE: Lycett K et al. Pediatrics. 2020 Jul 6. doi: 10.1542/peds.2019-3666.
FROM PEDIATRICS
Children rarely transmit SARS-CoV-2 within households
“Unlike with other viral respiratory infections, children do not seem to be a major vector of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission, with most pediatric cases described inside familial clusters and no documentation of child-to-child or child-to-adult transmission,” said Klara M. Posfay-Barbe, MD, of the University of Geneva, Switzerland, and colleagues.
In a study published in Pediatrics, the researchers analyzed data from all COVID-19 patients younger than 16 years who were identified between March 10, 2020, and April 10, 2020, through a hospital surveillance network. Parents and household contacts were called for contact tracing.
In 31 of 39 (79%) households, at least one adult family member had a suspected or confirmed SARS-CoV-2 infection before onset of symptoms in the child. These findings support data from previous studies suggesting that children mainly become infected from adult family members rather than transmitting the virus to them, the researchers said
In only 3 of 39 (8%) households was the study child the first to develop symptoms. “Surprisingly, in 33% of households, symptomatic HHCs [household contacts] tested negative despite belonging to a familial cluster with confirmed SARS-CoV-2 cases, suggesting an underreporting of cases,” Dr. Posfay-Barbe and associates noted.
The findings were limited by several factors including potential underreporting of cases because those with mild or atypical presentations may not have sought medical care, and the inability to confirm child-to-adult transmission. The results were strengthened by the extensive contact tracing and very few individuals lost to follow-up, they said; however, more diagnostic screening and contact tracing are needed to improve understanding of household transmission of SARS-CoV-2, they concluded.
Resolving the issue of how much children contribute to transmission of SARS-CoV-2 is essential to making informed decisions about public health, including how to structure schools and child-care facility reopening, Benjamin Lee, MD, and William V. Raszka Jr., MD, both of the University of Vermont, Burlington, said in an accompanying editorial (Pediatrics. 2020 Jul 10. doi: 10.1542/peds/2020-004879).
The data in the current study support other studies of transmission among household contacts in China suggesting that, in most cases of childhood infections, “the child was not the source of infection and that children most frequently acquire COVID-19 from adults, rather than transmitting it to them,” they wrote.
In addition, the limited data on transmission of SARS-CoV-2 by children outside of the household show few cases of secondary infection from children identified with SARS-CoV-2 in school settings in studies from France and Australia, Dr. Lee and Dr. Raszka noted.
the editorialists wrote. “This would be another manner by which SARS-CoV2 differs drastically from influenza, for which school-based transmission is well recognized as a significant driver of epidemic disease and forms the basis for most evidence regarding school closures as public health strategy.”
“Therefore, serious consideration should be paid toward strategies that allow schools to remain open, even during periods of COVID-19 spread,” the editorialists concluded. “In doing so, we could minimize the potentially profound adverse social, developmental, and health costs that our children will continue to suffer until an effective treatment or vaccine can be developed and distributed or, failing that, until we reach herd immunity,” Dr. Lee and Dr. Raszka emphasized.
The study received no outside funding. The researchers and editorialists had no financial conflicts to disclose.
SOURCE: Posfay-Barbe KM et al. Pediatrics. 2020 Jul 10. doi: 10.1542/peds.2020-1576.
“Unlike with other viral respiratory infections, children do not seem to be a major vector of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission, with most pediatric cases described inside familial clusters and no documentation of child-to-child or child-to-adult transmission,” said Klara M. Posfay-Barbe, MD, of the University of Geneva, Switzerland, and colleagues.
In a study published in Pediatrics, the researchers analyzed data from all COVID-19 patients younger than 16 years who were identified between March 10, 2020, and April 10, 2020, through a hospital surveillance network. Parents and household contacts were called for contact tracing.
In 31 of 39 (79%) households, at least one adult family member had a suspected or confirmed SARS-CoV-2 infection before onset of symptoms in the child. These findings support data from previous studies suggesting that children mainly become infected from adult family members rather than transmitting the virus to them, the researchers said
In only 3 of 39 (8%) households was the study child the first to develop symptoms. “Surprisingly, in 33% of households, symptomatic HHCs [household contacts] tested negative despite belonging to a familial cluster with confirmed SARS-CoV-2 cases, suggesting an underreporting of cases,” Dr. Posfay-Barbe and associates noted.
The findings were limited by several factors including potential underreporting of cases because those with mild or atypical presentations may not have sought medical care, and the inability to confirm child-to-adult transmission. The results were strengthened by the extensive contact tracing and very few individuals lost to follow-up, they said; however, more diagnostic screening and contact tracing are needed to improve understanding of household transmission of SARS-CoV-2, they concluded.
Resolving the issue of how much children contribute to transmission of SARS-CoV-2 is essential to making informed decisions about public health, including how to structure schools and child-care facility reopening, Benjamin Lee, MD, and William V. Raszka Jr., MD, both of the University of Vermont, Burlington, said in an accompanying editorial (Pediatrics. 2020 Jul 10. doi: 10.1542/peds/2020-004879).
The data in the current study support other studies of transmission among household contacts in China suggesting that, in most cases of childhood infections, “the child was not the source of infection and that children most frequently acquire COVID-19 from adults, rather than transmitting it to them,” they wrote.
In addition, the limited data on transmission of SARS-CoV-2 by children outside of the household show few cases of secondary infection from children identified with SARS-CoV-2 in school settings in studies from France and Australia, Dr. Lee and Dr. Raszka noted.
the editorialists wrote. “This would be another manner by which SARS-CoV2 differs drastically from influenza, for which school-based transmission is well recognized as a significant driver of epidemic disease and forms the basis for most evidence regarding school closures as public health strategy.”
“Therefore, serious consideration should be paid toward strategies that allow schools to remain open, even during periods of COVID-19 spread,” the editorialists concluded. “In doing so, we could minimize the potentially profound adverse social, developmental, and health costs that our children will continue to suffer until an effective treatment or vaccine can be developed and distributed or, failing that, until we reach herd immunity,” Dr. Lee and Dr. Raszka emphasized.
The study received no outside funding. The researchers and editorialists had no financial conflicts to disclose.
SOURCE: Posfay-Barbe KM et al. Pediatrics. 2020 Jul 10. doi: 10.1542/peds.2020-1576.
“Unlike with other viral respiratory infections, children do not seem to be a major vector of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission, with most pediatric cases described inside familial clusters and no documentation of child-to-child or child-to-adult transmission,” said Klara M. Posfay-Barbe, MD, of the University of Geneva, Switzerland, and colleagues.
In a study published in Pediatrics, the researchers analyzed data from all COVID-19 patients younger than 16 years who were identified between March 10, 2020, and April 10, 2020, through a hospital surveillance network. Parents and household contacts were called for contact tracing.
In 31 of 39 (79%) households, at least one adult family member had a suspected or confirmed SARS-CoV-2 infection before onset of symptoms in the child. These findings support data from previous studies suggesting that children mainly become infected from adult family members rather than transmitting the virus to them, the researchers said
In only 3 of 39 (8%) households was the study child the first to develop symptoms. “Surprisingly, in 33% of households, symptomatic HHCs [household contacts] tested negative despite belonging to a familial cluster with confirmed SARS-CoV-2 cases, suggesting an underreporting of cases,” Dr. Posfay-Barbe and associates noted.
The findings were limited by several factors including potential underreporting of cases because those with mild or atypical presentations may not have sought medical care, and the inability to confirm child-to-adult transmission. The results were strengthened by the extensive contact tracing and very few individuals lost to follow-up, they said; however, more diagnostic screening and contact tracing are needed to improve understanding of household transmission of SARS-CoV-2, they concluded.
Resolving the issue of how much children contribute to transmission of SARS-CoV-2 is essential to making informed decisions about public health, including how to structure schools and child-care facility reopening, Benjamin Lee, MD, and William V. Raszka Jr., MD, both of the University of Vermont, Burlington, said in an accompanying editorial (Pediatrics. 2020 Jul 10. doi: 10.1542/peds/2020-004879).
The data in the current study support other studies of transmission among household contacts in China suggesting that, in most cases of childhood infections, “the child was not the source of infection and that children most frequently acquire COVID-19 from adults, rather than transmitting it to them,” they wrote.
In addition, the limited data on transmission of SARS-CoV-2 by children outside of the household show few cases of secondary infection from children identified with SARS-CoV-2 in school settings in studies from France and Australia, Dr. Lee and Dr. Raszka noted.
the editorialists wrote. “This would be another manner by which SARS-CoV2 differs drastically from influenza, for which school-based transmission is well recognized as a significant driver of epidemic disease and forms the basis for most evidence regarding school closures as public health strategy.”
“Therefore, serious consideration should be paid toward strategies that allow schools to remain open, even during periods of COVID-19 spread,” the editorialists concluded. “In doing so, we could minimize the potentially profound adverse social, developmental, and health costs that our children will continue to suffer until an effective treatment or vaccine can be developed and distributed or, failing that, until we reach herd immunity,” Dr. Lee and Dr. Raszka emphasized.
The study received no outside funding. The researchers and editorialists had no financial conflicts to disclose.
SOURCE: Posfay-Barbe KM et al. Pediatrics. 2020 Jul 10. doi: 10.1542/peds.2020-1576.
FROM PEDIATRICS
Delayed diagnoses seen in children during COVID-19
There were also nine deaths where delayed presentation was considered a contributing factor, resulting mainly from sepsis and malignancy.
By comparison, over the same 2-week period of the survey there were three child deaths from COVID-19 directly, according to senior study author Shamez Ladhani, MRCPCH, PhD, chair of the British Paediatric Surveillance Unit (BPSU), Royal College of Paediatrics and Child Health, London.
“The unintended consequences of COVID are far greater, in children, than the disease itself. The way we are trying to prevent this is causing more harm than the disease,” he lamented.
One-third of senior U.K. pediatric specialists who responded to the survey reported dealing with so-called emergency delayed presentations in children who they would normally have expected to present much earlier.
After diabetes, the most commonly reported delayed diagnoses were sepsis and child protection issues. Cancer also featured prominently.
“We’ve found that there is great concern that children are not accessing healthcare as they should during lockdown and after,” Dr. Ladhani stressed. “Our emergency departments saw a 50% reduction during the peak, and now it is still 40% less than expected. The problem is improving but it remains.”
The survey findings were recently published online in Archives of Disease in Childhood, by first author Richard M. Lynn, MSc, of the Institute of Child Health, department of epidemiology and public health, University College London Research, and colleagues.
New diabetes cases presented very late during lockdown
Over the 2-week reporting period in mid-April 2020, type 1 diabetes was the most frequently reported delayed diagnosis, with 44 cases overall, 23 of which involved diabetic ketoacidosis.
“If you talk to the diabetes specialists, they tell us that generally, most cases of new diabetes arrive late because it has very nonspecific symptoms,” Dr. Ladhani explained.
However, he added, “pediatricians on the frontline know what to expect with diabetes. Those children who would have come in late prior to the pandemic are now arriving very late. Those consultants surveyed were not junior doctors but consultant pediatricians with many years of experience.”
In a recent article looking at pediatric delayed presentations, one patient with diabetes entered intensive care, and the BPSU report recorded one death possibly associated with diabetes, Dr. Ladhani pointed out.
“Pediatricians are worried that children are coming in late. We need to raise awareness that parents need to access healthcare and this message needs to go out now,” he said. “We can’t wait until a second wave. It has to be now because A&E [accident and emergency] attendance is still 40% [lower than] ... expected.”
BPSU survey covers over 90% of pediatricians in U.K. and Ireland
After numerous anecdotal reports of delayed presentations in the United Kingdom and abroad, the snapshot survey was conducted as part of routine monthly reports where pediatricians are asked to document any cases of rare conditions seen.
“We had heard stories of delayed presentations, but we wanted to know was this a real problem or just anecdotal?” Dr. Ladhani said.
The regular BPSU survey covers over 90% of U.K.- and Ireland-based pediatric consultants (numbering 4,075). On the back of this established communication, the BPSU decided to gauge the extent of delayed presentations during the peak weeks of the COVID-19 pandemic.
Over the next 7 days, 2,433 pediatricians, representing 60% of BPSU participants, responded.
“This response rate in 7 days highlights the importance given to the survey by pediatricians ... and the widespread professional concern about delayed presentations,” the authors wrote.
Participants were asked whether they had seen any children during the previous 14 days who, in their opinion, presented later than they would have expected prior to the COVID-19 pandemic.
“There’s no one definition for this but these senior clinicians know when something is unusual,” said Dr. Ladhani.
ED attendances were compared with figures for the same period last year. Overall, a total of 32% of 752 pediatricians working in EDs and pediatric assessment units reported witnessing delayed presentations, with 57 (8%) reporting at least three patients with delayed presentation.
“It was clear that those doctors on the frontline were seeing a lot of delayed presentations. Also, neonatologists reported women arriving late for labor, and community physicians said they just weren’t witnessing child protection cases anymore,” added Dr. Ladhani.
Other issues included early discharges following births because of COVID-19 concerns, before feeding had been established, prompting return visits because of feeding problems and dehydration.
The top five delayed diagnoses were diabetes (n = 44), sepsis (n = 21), child protection (n = 14), malignancy (n = 8), and appendicitis (n = 6). There were 10 delayed perinatal presentations.
Of the nine deaths, for which delayed presentation was considered to play a role, three were caused by sepsis, three were caused by new malignancy diagnoses, one was caused by new diagnosis of metabolic disease, and two did not have the cause reported.
The delays in presentation are likely to have been influenced by the U.K. government’s message to “stay at home” during the strict lockdown period, which perhaps was sometimes interpreted too literally, Dr. Ladhani suggested. “It was the right message socially, but not medically.”
Russell Viner, MB, PhD, president of the Royal College of Paediatrics and Child Health, said in a statement: “The impact for children is what we call ‘collateral damage’, including long absences from school and delays or interruptions to vital services. We know that parents adhered very strongly to the ‘stay at home’ [message] and we need to say clearly that this doesn’t apply if your child is very sick. Should we experience a second wave or regional outbreaks, it is vital that we get the message out to parents that we want to see unwell children at the earliest possible stage.”
Dr. Ladhani reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
There were also nine deaths where delayed presentation was considered a contributing factor, resulting mainly from sepsis and malignancy.
By comparison, over the same 2-week period of the survey there were three child deaths from COVID-19 directly, according to senior study author Shamez Ladhani, MRCPCH, PhD, chair of the British Paediatric Surveillance Unit (BPSU), Royal College of Paediatrics and Child Health, London.
“The unintended consequences of COVID are far greater, in children, than the disease itself. The way we are trying to prevent this is causing more harm than the disease,” he lamented.
One-third of senior U.K. pediatric specialists who responded to the survey reported dealing with so-called emergency delayed presentations in children who they would normally have expected to present much earlier.
After diabetes, the most commonly reported delayed diagnoses were sepsis and child protection issues. Cancer also featured prominently.
“We’ve found that there is great concern that children are not accessing healthcare as they should during lockdown and after,” Dr. Ladhani stressed. “Our emergency departments saw a 50% reduction during the peak, and now it is still 40% less than expected. The problem is improving but it remains.”
The survey findings were recently published online in Archives of Disease in Childhood, by first author Richard M. Lynn, MSc, of the Institute of Child Health, department of epidemiology and public health, University College London Research, and colleagues.
New diabetes cases presented very late during lockdown
Over the 2-week reporting period in mid-April 2020, type 1 diabetes was the most frequently reported delayed diagnosis, with 44 cases overall, 23 of which involved diabetic ketoacidosis.
“If you talk to the diabetes specialists, they tell us that generally, most cases of new diabetes arrive late because it has very nonspecific symptoms,” Dr. Ladhani explained.
However, he added, “pediatricians on the frontline know what to expect with diabetes. Those children who would have come in late prior to the pandemic are now arriving very late. Those consultants surveyed were not junior doctors but consultant pediatricians with many years of experience.”
In a recent article looking at pediatric delayed presentations, one patient with diabetes entered intensive care, and the BPSU report recorded one death possibly associated with diabetes, Dr. Ladhani pointed out.
“Pediatricians are worried that children are coming in late. We need to raise awareness that parents need to access healthcare and this message needs to go out now,” he said. “We can’t wait until a second wave. It has to be now because A&E [accident and emergency] attendance is still 40% [lower than] ... expected.”
BPSU survey covers over 90% of pediatricians in U.K. and Ireland
After numerous anecdotal reports of delayed presentations in the United Kingdom and abroad, the snapshot survey was conducted as part of routine monthly reports where pediatricians are asked to document any cases of rare conditions seen.
“We had heard stories of delayed presentations, but we wanted to know was this a real problem or just anecdotal?” Dr. Ladhani said.
The regular BPSU survey covers over 90% of U.K.- and Ireland-based pediatric consultants (numbering 4,075). On the back of this established communication, the BPSU decided to gauge the extent of delayed presentations during the peak weeks of the COVID-19 pandemic.
Over the next 7 days, 2,433 pediatricians, representing 60% of BPSU participants, responded.
“This response rate in 7 days highlights the importance given to the survey by pediatricians ... and the widespread professional concern about delayed presentations,” the authors wrote.
Participants were asked whether they had seen any children during the previous 14 days who, in their opinion, presented later than they would have expected prior to the COVID-19 pandemic.
“There’s no one definition for this but these senior clinicians know when something is unusual,” said Dr. Ladhani.
ED attendances were compared with figures for the same period last year. Overall, a total of 32% of 752 pediatricians working in EDs and pediatric assessment units reported witnessing delayed presentations, with 57 (8%) reporting at least three patients with delayed presentation.
“It was clear that those doctors on the frontline were seeing a lot of delayed presentations. Also, neonatologists reported women arriving late for labor, and community physicians said they just weren’t witnessing child protection cases anymore,” added Dr. Ladhani.
Other issues included early discharges following births because of COVID-19 concerns, before feeding had been established, prompting return visits because of feeding problems and dehydration.
The top five delayed diagnoses were diabetes (n = 44), sepsis (n = 21), child protection (n = 14), malignancy (n = 8), and appendicitis (n = 6). There were 10 delayed perinatal presentations.
Of the nine deaths, for which delayed presentation was considered to play a role, three were caused by sepsis, three were caused by new malignancy diagnoses, one was caused by new diagnosis of metabolic disease, and two did not have the cause reported.
The delays in presentation are likely to have been influenced by the U.K. government’s message to “stay at home” during the strict lockdown period, which perhaps was sometimes interpreted too literally, Dr. Ladhani suggested. “It was the right message socially, but not medically.”
Russell Viner, MB, PhD, president of the Royal College of Paediatrics and Child Health, said in a statement: “The impact for children is what we call ‘collateral damage’, including long absences from school and delays or interruptions to vital services. We know that parents adhered very strongly to the ‘stay at home’ [message] and we need to say clearly that this doesn’t apply if your child is very sick. Should we experience a second wave or regional outbreaks, it is vital that we get the message out to parents that we want to see unwell children at the earliest possible stage.”
Dr. Ladhani reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
There were also nine deaths where delayed presentation was considered a contributing factor, resulting mainly from sepsis and malignancy.
By comparison, over the same 2-week period of the survey there were three child deaths from COVID-19 directly, according to senior study author Shamez Ladhani, MRCPCH, PhD, chair of the British Paediatric Surveillance Unit (BPSU), Royal College of Paediatrics and Child Health, London.
“The unintended consequences of COVID are far greater, in children, than the disease itself. The way we are trying to prevent this is causing more harm than the disease,” he lamented.
One-third of senior U.K. pediatric specialists who responded to the survey reported dealing with so-called emergency delayed presentations in children who they would normally have expected to present much earlier.
After diabetes, the most commonly reported delayed diagnoses were sepsis and child protection issues. Cancer also featured prominently.
“We’ve found that there is great concern that children are not accessing healthcare as they should during lockdown and after,” Dr. Ladhani stressed. “Our emergency departments saw a 50% reduction during the peak, and now it is still 40% less than expected. The problem is improving but it remains.”
The survey findings were recently published online in Archives of Disease in Childhood, by first author Richard M. Lynn, MSc, of the Institute of Child Health, department of epidemiology and public health, University College London Research, and colleagues.
New diabetes cases presented very late during lockdown
Over the 2-week reporting period in mid-April 2020, type 1 diabetes was the most frequently reported delayed diagnosis, with 44 cases overall, 23 of which involved diabetic ketoacidosis.
“If you talk to the diabetes specialists, they tell us that generally, most cases of new diabetes arrive late because it has very nonspecific symptoms,” Dr. Ladhani explained.
However, he added, “pediatricians on the frontline know what to expect with diabetes. Those children who would have come in late prior to the pandemic are now arriving very late. Those consultants surveyed were not junior doctors but consultant pediatricians with many years of experience.”
In a recent article looking at pediatric delayed presentations, one patient with diabetes entered intensive care, and the BPSU report recorded one death possibly associated with diabetes, Dr. Ladhani pointed out.
“Pediatricians are worried that children are coming in late. We need to raise awareness that parents need to access healthcare and this message needs to go out now,” he said. “We can’t wait until a second wave. It has to be now because A&E [accident and emergency] attendance is still 40% [lower than] ... expected.”
BPSU survey covers over 90% of pediatricians in U.K. and Ireland
After numerous anecdotal reports of delayed presentations in the United Kingdom and abroad, the snapshot survey was conducted as part of routine monthly reports where pediatricians are asked to document any cases of rare conditions seen.
“We had heard stories of delayed presentations, but we wanted to know was this a real problem or just anecdotal?” Dr. Ladhani said.
The regular BPSU survey covers over 90% of U.K.- and Ireland-based pediatric consultants (numbering 4,075). On the back of this established communication, the BPSU decided to gauge the extent of delayed presentations during the peak weeks of the COVID-19 pandemic.
Over the next 7 days, 2,433 pediatricians, representing 60% of BPSU participants, responded.
“This response rate in 7 days highlights the importance given to the survey by pediatricians ... and the widespread professional concern about delayed presentations,” the authors wrote.
Participants were asked whether they had seen any children during the previous 14 days who, in their opinion, presented later than they would have expected prior to the COVID-19 pandemic.
“There’s no one definition for this but these senior clinicians know when something is unusual,” said Dr. Ladhani.
ED attendances were compared with figures for the same period last year. Overall, a total of 32% of 752 pediatricians working in EDs and pediatric assessment units reported witnessing delayed presentations, with 57 (8%) reporting at least three patients with delayed presentation.
“It was clear that those doctors on the frontline were seeing a lot of delayed presentations. Also, neonatologists reported women arriving late for labor, and community physicians said they just weren’t witnessing child protection cases anymore,” added Dr. Ladhani.
Other issues included early discharges following births because of COVID-19 concerns, before feeding had been established, prompting return visits because of feeding problems and dehydration.
The top five delayed diagnoses were diabetes (n = 44), sepsis (n = 21), child protection (n = 14), malignancy (n = 8), and appendicitis (n = 6). There were 10 delayed perinatal presentations.
Of the nine deaths, for which delayed presentation was considered to play a role, three were caused by sepsis, three were caused by new malignancy diagnoses, one was caused by new diagnosis of metabolic disease, and two did not have the cause reported.
The delays in presentation are likely to have been influenced by the U.K. government’s message to “stay at home” during the strict lockdown period, which perhaps was sometimes interpreted too literally, Dr. Ladhani suggested. “It was the right message socially, but not medically.”
Russell Viner, MB, PhD, president of the Royal College of Paediatrics and Child Health, said in a statement: “The impact for children is what we call ‘collateral damage’, including long absences from school and delays or interruptions to vital services. We know that parents adhered very strongly to the ‘stay at home’ [message] and we need to say clearly that this doesn’t apply if your child is very sick. Should we experience a second wave or regional outbreaks, it is vital that we get the message out to parents that we want to see unwell children at the earliest possible stage.”
Dr. Ladhani reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.