User login
Even a few days of steroids may be risky, new study suggests
Extended use of corticosteroids for chronic inflammatory conditions puts patients at risk for serious adverse events (AEs), including cardiovascular disease, osteoporosis, cataracts, and diabetes. Now, a growing body of evidence suggests that even short bursts of these drugs are associated with serious risks.
Most recently, a population-based study of more than 2.6 million people found that taking corticosteroids for 14 days or less was associated with a substantially greater risk for gastrointestinal (GI) bleeding, sepsis, and heart failure, particularly within the first 30 days after therapy.
In the study, Tsung-Chieh Yao, MD, PhD, a professor in the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan, Taiwan, and colleagues used a self-controlled case series to analyze data from Taiwan’s National Health Insurance Research Database of medical claims. They compared patients’ conditions in the period from 5 to 90 days before treatment to conditions from the periods from 5 to 30 days and from 31 to 90 days after therapy.
With a median duration of 3 days of treatment, the incidence rate ratios (IRRs) were 1.80 (95% confidence interval, 1.75-1.84) for GI bleeding, 1.99 (95% CI, 1.70-2.32) for sepsis, and 2.37 (95% CI, 2.13-2.63) for heart failure.
Given the findings, physicians should weigh the benefits against the risks of rare but potentially serious consequences of these anti-inflammatory drugs, according to the authors.
“After initiating patients on oral steroid bursts, physicians should be on the lookout for these severe adverse events, particularly within the first month after initiation of steroid therapy,” Dr. Yao said in an interview.
The findings were published online July 6 in Annals of Internal Medicine.
Of the 15,859,129 adult Asians in the Taiwanese database, the study included 2,623,327 adults aged 20-64 years who received single steroid bursts (14 days or less) between Jan. 1, 2013, and Dec. 31, 2015.
Almost 60% of the indications were for skin disorders, such as eczema and urticaria, and for respiratory tract infections, such as sinusitis and acute pharyngitis. Among specialties, dermatology, otolaryngology, family practice, internal medicine, and pediatrics accounted for 88% of prescriptions.
“Our findings are important for physicians and guideline developers because short-term use of oral corticosteroids is common and the real-world safety of this approach remains unclear,” the authors wrote. They acknowledged that the database did not provide information on such potential confounders as disease severity and lifestyle factors, nor did it include children and vulnerable individuals, which may limit the generalizability of the results.
The findings echo those of a 2017 cohort study conducted by researchers at the University of Michigan in Ann Arbor. That study, by Akbar K. Waljee, MD, assistant professor of gastroenterology, University of Michigan, Ann Arbor, and colleagues, included data on more than 1.5 million privately insured U.S. adults. The researchers included somewhat longer steroid bursts of up to 30 days’ duration and found that use of the drugs was associated with a greater than fivefold increased risk for sepsis, a more than threefold increased risk for venous thromboembolism, and a nearly twofold increased risk for fracture within 30 days of starting treatment.
Furthermore, the elevated risk persisted at prednisone-equivalent doses of less than 20 mg/d (IRR, 4.02 for sepsis, 3.61 for venous thromboembolism, and 1.83 for fracture; all P < .001).
The U.S. study also found that during the 3-year period from 2012 to 2014, more than 20% of patients were prescribed short-term oral corticosteroids.
“Both studies indicate that these short-term regimens are more common in the real world than was previously thought and are not risk free,” Dr. Yao said.
Recognition that corticosteroids are associated with adverse events has been building for decades, according to the authors of an editorial that accompanies the new study.
“However, we commonly use short corticosteroid ‘bursts’ for minor ailments despite a lack of evidence for meaningful benefit. We are now learning that bursts as short as 3 days may increase risk for serious AEs, even in young and healthy people,” wrote editorialists Beth I. Wallace, MD, of the Center for Clinical Management Research at the VA Ann Arbor Healthcare System and the Institute for Healthcare Policy and Innovation at Michigan Medicine, Ann Arbor, and Dr. Waljee, who led the 2017 study.
Dr. Wallace and Dr. Waljee drew parallels between corticosteroid bursts and other short-term regimens, such as of antibiotics and opiates, in which prescriber preference and sometimes patient pressure play a role. “All of these treatments have well-defined indications but can cause net harm when used. We can thus conceive of a corticosteroid stewardship model of targeted interventions that aims to reduce inappropriate prescribing,” they wrote.
In an interview, Dr. Wallace, a rheumatologist who prescribes oral steroids fairly frequently, noted that the Taiwan study is the first to investigate steroid bursts. “Up till now, these very short courses have flown under the radar. Clinicians very commonly prescribe short courses to help relieve symptoms of self-limited conditions like bronchitis, and we assume that because the exposure duration is short, the risks are low, especially for patients who are otherwise healthy.”
She warned that the data in the current study indicate that these short bursts – even at the lower end of the 1- to 2-week courses American physicians prescribe most often – carry small but real increases in risk for serious AEs. “And these increases were seen in young, healthy people, not just in people with preexisting conditions,” she said. “So, we might need to start thinking harder about how we are prescribing even these very short courses of steroids and try to use steroids only when their meaningful benefits really outweigh the risk.”
She noted that a patient with a chronic inflammatory condition such as rheumatoid arthritis may benefit substantially from short-term steroids to treat a disease flare. In that specific case, the benefits of short-term steroids may outweigh the risks, Dr. Wallace said.
But not everyone thinks a new strategy is needed. For Whitney A. High, MD, associate professor of dermatology and pathology at the University of Colorado at Denver, Aurora, the overprescribing of short-term corticosteroids is not a problem, and dermatologists are already exercising caution.
“I only prescribe these drugs short term to, at a guess, about 1 in 40 patients and only when a patient is miserable and quality of life is being seriously affected,” he said in an interview. “And that’s something that can’t be measured in a database study like the one from Taiwan but only in a risk-benefit analysis,” he said.
Furthermore, dermatologists have other drugs and technologies in their armamentarium, including topical steroids with occlusion or with wet wraps, phototherapy, phosphodiesterase inhibitors, calcipotriene, methotrexate and other immunosuppressive agents, and biologics. “In fact, many of these agents are specifically referred to as steroid-sparing,” Dr. High said.
Nor does he experience much pressure from patients to prescribe these drugs. “While occasionally I may encounter a patient who places pressure on me for oral steroids, it’s probably not nearly as frequently as providers in other fields are pressured to prescribe antibiotics or narcotics,” he said.
According to the Taiwanese researchers, the next step is to conduct more studies, including clinical trials, to determine optimal use of corticosteroids by monitoring adverse events. In the meantime, for practitioners such as Dr. Wallace and Dr. High, there is ample evidence from several recent studies of the harms of short-term corticosteroids, whereas the benefits for patients with self-limiting conditions remain uncertain. “This and other studies like it quite appropriately remind providers to avoid oral steroids when they’re not necessary and to seek alternatives where possible,” Dr. High said.
The study was supported by the National Health Research Institutes of Taiwan, the Ministry of Science and Technology of Taiwan, the Chang Gung Medical Foundation, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH). Dr. Yao has disclosed no relevant financial relationships. Dr. Wu has received grants from GlaxoSmithKline outside the submitted work. The editorialists and Dr. High have disclosed no relevant financial relationships. Dr. Wallace received an NIH grant during the writing of the editorial.
A version of this article originally appeared on Medscape.com.
Extended use of corticosteroids for chronic inflammatory conditions puts patients at risk for serious adverse events (AEs), including cardiovascular disease, osteoporosis, cataracts, and diabetes. Now, a growing body of evidence suggests that even short bursts of these drugs are associated with serious risks.
Most recently, a population-based study of more than 2.6 million people found that taking corticosteroids for 14 days or less was associated with a substantially greater risk for gastrointestinal (GI) bleeding, sepsis, and heart failure, particularly within the first 30 days after therapy.
In the study, Tsung-Chieh Yao, MD, PhD, a professor in the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan, Taiwan, and colleagues used a self-controlled case series to analyze data from Taiwan’s National Health Insurance Research Database of medical claims. They compared patients’ conditions in the period from 5 to 90 days before treatment to conditions from the periods from 5 to 30 days and from 31 to 90 days after therapy.
With a median duration of 3 days of treatment, the incidence rate ratios (IRRs) were 1.80 (95% confidence interval, 1.75-1.84) for GI bleeding, 1.99 (95% CI, 1.70-2.32) for sepsis, and 2.37 (95% CI, 2.13-2.63) for heart failure.
Given the findings, physicians should weigh the benefits against the risks of rare but potentially serious consequences of these anti-inflammatory drugs, according to the authors.
“After initiating patients on oral steroid bursts, physicians should be on the lookout for these severe adverse events, particularly within the first month after initiation of steroid therapy,” Dr. Yao said in an interview.
The findings were published online July 6 in Annals of Internal Medicine.
Of the 15,859,129 adult Asians in the Taiwanese database, the study included 2,623,327 adults aged 20-64 years who received single steroid bursts (14 days or less) between Jan. 1, 2013, and Dec. 31, 2015.
Almost 60% of the indications were for skin disorders, such as eczema and urticaria, and for respiratory tract infections, such as sinusitis and acute pharyngitis. Among specialties, dermatology, otolaryngology, family practice, internal medicine, and pediatrics accounted for 88% of prescriptions.
“Our findings are important for physicians and guideline developers because short-term use of oral corticosteroids is common and the real-world safety of this approach remains unclear,” the authors wrote. They acknowledged that the database did not provide information on such potential confounders as disease severity and lifestyle factors, nor did it include children and vulnerable individuals, which may limit the generalizability of the results.
The findings echo those of a 2017 cohort study conducted by researchers at the University of Michigan in Ann Arbor. That study, by Akbar K. Waljee, MD, assistant professor of gastroenterology, University of Michigan, Ann Arbor, and colleagues, included data on more than 1.5 million privately insured U.S. adults. The researchers included somewhat longer steroid bursts of up to 30 days’ duration and found that use of the drugs was associated with a greater than fivefold increased risk for sepsis, a more than threefold increased risk for venous thromboembolism, and a nearly twofold increased risk for fracture within 30 days of starting treatment.
Furthermore, the elevated risk persisted at prednisone-equivalent doses of less than 20 mg/d (IRR, 4.02 for sepsis, 3.61 for venous thromboembolism, and 1.83 for fracture; all P < .001).
The U.S. study also found that during the 3-year period from 2012 to 2014, more than 20% of patients were prescribed short-term oral corticosteroids.
“Both studies indicate that these short-term regimens are more common in the real world than was previously thought and are not risk free,” Dr. Yao said.
Recognition that corticosteroids are associated with adverse events has been building for decades, according to the authors of an editorial that accompanies the new study.
“However, we commonly use short corticosteroid ‘bursts’ for minor ailments despite a lack of evidence for meaningful benefit. We are now learning that bursts as short as 3 days may increase risk for serious AEs, even in young and healthy people,” wrote editorialists Beth I. Wallace, MD, of the Center for Clinical Management Research at the VA Ann Arbor Healthcare System and the Institute for Healthcare Policy and Innovation at Michigan Medicine, Ann Arbor, and Dr. Waljee, who led the 2017 study.
Dr. Wallace and Dr. Waljee drew parallels between corticosteroid bursts and other short-term regimens, such as of antibiotics and opiates, in which prescriber preference and sometimes patient pressure play a role. “All of these treatments have well-defined indications but can cause net harm when used. We can thus conceive of a corticosteroid stewardship model of targeted interventions that aims to reduce inappropriate prescribing,” they wrote.
In an interview, Dr. Wallace, a rheumatologist who prescribes oral steroids fairly frequently, noted that the Taiwan study is the first to investigate steroid bursts. “Up till now, these very short courses have flown under the radar. Clinicians very commonly prescribe short courses to help relieve symptoms of self-limited conditions like bronchitis, and we assume that because the exposure duration is short, the risks are low, especially for patients who are otherwise healthy.”
She warned that the data in the current study indicate that these short bursts – even at the lower end of the 1- to 2-week courses American physicians prescribe most often – carry small but real increases in risk for serious AEs. “And these increases were seen in young, healthy people, not just in people with preexisting conditions,” she said. “So, we might need to start thinking harder about how we are prescribing even these very short courses of steroids and try to use steroids only when their meaningful benefits really outweigh the risk.”
She noted that a patient with a chronic inflammatory condition such as rheumatoid arthritis may benefit substantially from short-term steroids to treat a disease flare. In that specific case, the benefits of short-term steroids may outweigh the risks, Dr. Wallace said.
But not everyone thinks a new strategy is needed. For Whitney A. High, MD, associate professor of dermatology and pathology at the University of Colorado at Denver, Aurora, the overprescribing of short-term corticosteroids is not a problem, and dermatologists are already exercising caution.
“I only prescribe these drugs short term to, at a guess, about 1 in 40 patients and only when a patient is miserable and quality of life is being seriously affected,” he said in an interview. “And that’s something that can’t be measured in a database study like the one from Taiwan but only in a risk-benefit analysis,” he said.
Furthermore, dermatologists have other drugs and technologies in their armamentarium, including topical steroids with occlusion or with wet wraps, phototherapy, phosphodiesterase inhibitors, calcipotriene, methotrexate and other immunosuppressive agents, and biologics. “In fact, many of these agents are specifically referred to as steroid-sparing,” Dr. High said.
Nor does he experience much pressure from patients to prescribe these drugs. “While occasionally I may encounter a patient who places pressure on me for oral steroids, it’s probably not nearly as frequently as providers in other fields are pressured to prescribe antibiotics or narcotics,” he said.
According to the Taiwanese researchers, the next step is to conduct more studies, including clinical trials, to determine optimal use of corticosteroids by monitoring adverse events. In the meantime, for practitioners such as Dr. Wallace and Dr. High, there is ample evidence from several recent studies of the harms of short-term corticosteroids, whereas the benefits for patients with self-limiting conditions remain uncertain. “This and other studies like it quite appropriately remind providers to avoid oral steroids when they’re not necessary and to seek alternatives where possible,” Dr. High said.
The study was supported by the National Health Research Institutes of Taiwan, the Ministry of Science and Technology of Taiwan, the Chang Gung Medical Foundation, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH). Dr. Yao has disclosed no relevant financial relationships. Dr. Wu has received grants from GlaxoSmithKline outside the submitted work. The editorialists and Dr. High have disclosed no relevant financial relationships. Dr. Wallace received an NIH grant during the writing of the editorial.
A version of this article originally appeared on Medscape.com.
Extended use of corticosteroids for chronic inflammatory conditions puts patients at risk for serious adverse events (AEs), including cardiovascular disease, osteoporosis, cataracts, and diabetes. Now, a growing body of evidence suggests that even short bursts of these drugs are associated with serious risks.
Most recently, a population-based study of more than 2.6 million people found that taking corticosteroids for 14 days or less was associated with a substantially greater risk for gastrointestinal (GI) bleeding, sepsis, and heart failure, particularly within the first 30 days after therapy.
In the study, Tsung-Chieh Yao, MD, PhD, a professor in the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan, Taiwan, and colleagues used a self-controlled case series to analyze data from Taiwan’s National Health Insurance Research Database of medical claims. They compared patients’ conditions in the period from 5 to 90 days before treatment to conditions from the periods from 5 to 30 days and from 31 to 90 days after therapy.
With a median duration of 3 days of treatment, the incidence rate ratios (IRRs) were 1.80 (95% confidence interval, 1.75-1.84) for GI bleeding, 1.99 (95% CI, 1.70-2.32) for sepsis, and 2.37 (95% CI, 2.13-2.63) for heart failure.
Given the findings, physicians should weigh the benefits against the risks of rare but potentially serious consequences of these anti-inflammatory drugs, according to the authors.
“After initiating patients on oral steroid bursts, physicians should be on the lookout for these severe adverse events, particularly within the first month after initiation of steroid therapy,” Dr. Yao said in an interview.
The findings were published online July 6 in Annals of Internal Medicine.
Of the 15,859,129 adult Asians in the Taiwanese database, the study included 2,623,327 adults aged 20-64 years who received single steroid bursts (14 days or less) between Jan. 1, 2013, and Dec. 31, 2015.
Almost 60% of the indications were for skin disorders, such as eczema and urticaria, and for respiratory tract infections, such as sinusitis and acute pharyngitis. Among specialties, dermatology, otolaryngology, family practice, internal medicine, and pediatrics accounted for 88% of prescriptions.
“Our findings are important for physicians and guideline developers because short-term use of oral corticosteroids is common and the real-world safety of this approach remains unclear,” the authors wrote. They acknowledged that the database did not provide information on such potential confounders as disease severity and lifestyle factors, nor did it include children and vulnerable individuals, which may limit the generalizability of the results.
The findings echo those of a 2017 cohort study conducted by researchers at the University of Michigan in Ann Arbor. That study, by Akbar K. Waljee, MD, assistant professor of gastroenterology, University of Michigan, Ann Arbor, and colleagues, included data on more than 1.5 million privately insured U.S. adults. The researchers included somewhat longer steroid bursts of up to 30 days’ duration and found that use of the drugs was associated with a greater than fivefold increased risk for sepsis, a more than threefold increased risk for venous thromboembolism, and a nearly twofold increased risk for fracture within 30 days of starting treatment.
Furthermore, the elevated risk persisted at prednisone-equivalent doses of less than 20 mg/d (IRR, 4.02 for sepsis, 3.61 for venous thromboembolism, and 1.83 for fracture; all P < .001).
The U.S. study also found that during the 3-year period from 2012 to 2014, more than 20% of patients were prescribed short-term oral corticosteroids.
“Both studies indicate that these short-term regimens are more common in the real world than was previously thought and are not risk free,” Dr. Yao said.
Recognition that corticosteroids are associated with adverse events has been building for decades, according to the authors of an editorial that accompanies the new study.
“However, we commonly use short corticosteroid ‘bursts’ for minor ailments despite a lack of evidence for meaningful benefit. We are now learning that bursts as short as 3 days may increase risk for serious AEs, even in young and healthy people,” wrote editorialists Beth I. Wallace, MD, of the Center for Clinical Management Research at the VA Ann Arbor Healthcare System and the Institute for Healthcare Policy and Innovation at Michigan Medicine, Ann Arbor, and Dr. Waljee, who led the 2017 study.
Dr. Wallace and Dr. Waljee drew parallels between corticosteroid bursts and other short-term regimens, such as of antibiotics and opiates, in which prescriber preference and sometimes patient pressure play a role. “All of these treatments have well-defined indications but can cause net harm when used. We can thus conceive of a corticosteroid stewardship model of targeted interventions that aims to reduce inappropriate prescribing,” they wrote.
In an interview, Dr. Wallace, a rheumatologist who prescribes oral steroids fairly frequently, noted that the Taiwan study is the first to investigate steroid bursts. “Up till now, these very short courses have flown under the radar. Clinicians very commonly prescribe short courses to help relieve symptoms of self-limited conditions like bronchitis, and we assume that because the exposure duration is short, the risks are low, especially for patients who are otherwise healthy.”
She warned that the data in the current study indicate that these short bursts – even at the lower end of the 1- to 2-week courses American physicians prescribe most often – carry small but real increases in risk for serious AEs. “And these increases were seen in young, healthy people, not just in people with preexisting conditions,” she said. “So, we might need to start thinking harder about how we are prescribing even these very short courses of steroids and try to use steroids only when their meaningful benefits really outweigh the risk.”
She noted that a patient with a chronic inflammatory condition such as rheumatoid arthritis may benefit substantially from short-term steroids to treat a disease flare. In that specific case, the benefits of short-term steroids may outweigh the risks, Dr. Wallace said.
But not everyone thinks a new strategy is needed. For Whitney A. High, MD, associate professor of dermatology and pathology at the University of Colorado at Denver, Aurora, the overprescribing of short-term corticosteroids is not a problem, and dermatologists are already exercising caution.
“I only prescribe these drugs short term to, at a guess, about 1 in 40 patients and only when a patient is miserable and quality of life is being seriously affected,” he said in an interview. “And that’s something that can’t be measured in a database study like the one from Taiwan but only in a risk-benefit analysis,” he said.
Furthermore, dermatologists have other drugs and technologies in their armamentarium, including topical steroids with occlusion or with wet wraps, phototherapy, phosphodiesterase inhibitors, calcipotriene, methotrexate and other immunosuppressive agents, and biologics. “In fact, many of these agents are specifically referred to as steroid-sparing,” Dr. High said.
Nor does he experience much pressure from patients to prescribe these drugs. “While occasionally I may encounter a patient who places pressure on me for oral steroids, it’s probably not nearly as frequently as providers in other fields are pressured to prescribe antibiotics or narcotics,” he said.
According to the Taiwanese researchers, the next step is to conduct more studies, including clinical trials, to determine optimal use of corticosteroids by monitoring adverse events. In the meantime, for practitioners such as Dr. Wallace and Dr. High, there is ample evidence from several recent studies of the harms of short-term corticosteroids, whereas the benefits for patients with self-limiting conditions remain uncertain. “This and other studies like it quite appropriately remind providers to avoid oral steroids when they’re not necessary and to seek alternatives where possible,” Dr. High said.
The study was supported by the National Health Research Institutes of Taiwan, the Ministry of Science and Technology of Taiwan, the Chang Gung Medical Foundation, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH). Dr. Yao has disclosed no relevant financial relationships. Dr. Wu has received grants from GlaxoSmithKline outside the submitted work. The editorialists and Dr. High have disclosed no relevant financial relationships. Dr. Wallace received an NIH grant during the writing of the editorial.
A version of this article originally appeared on Medscape.com.
Pediatric hospitalists convene virtually to discuss PHM designation
A recent teleconference brought together an ad hoc panel of pediatric hospitalists, with more than 100 diverse voices discussing whether there ought to be an additional professional recognition or designation for the subspecialty, apart from the new pediatric hospital medicine (PHM) board certification that was launched in 2019.
The heterogeneity of PHM was on display during the discussion, as participants included university-based pediatric hospitalists and those from community hospitals, physicians trained in combined medicine and pediatrics or in family medicine, doctors who completed a general pediatric residency before going straight into PHM, niche practitioners such as newborn hospitalists, trainees, and a small but growing number of graduates of PHM fellowship programs. There are 61 PHM fellowships, and these programs graduate approximately 70 new fellows per year.
Although a route to some kind of professional designation for PHM – separate from board certification – was the centerpiece of the conference call, there is no proposal actively under consideration for developing such a designation, said Weijen W. Chang, MD, FAAP, SFHM, chief of pediatric hospital medicine at Baystate Medical Center in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts–Baystate Campus.
Who might develop such a proposal? “The hope is that the three major professional societies involved in pediatric hospital medicine – the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association – would jointly develop such a designation,” Dr. Chang said. However, it is not clear whether the three societies could agree on this. An online survey of 551 pediatric hospitalists, shared during the conference call, found that the majority would like to see some kind of alternate designation.
The reality of the boards
The pediatric subspecialty of PHM was recognized by the American Board of Medical Specialties in 2015 following a petition by a group of PHM leaders seeking a way to credential their unique skill set. The first PHM board certification exam was offered by the American Board of Pediatrics on Nov. 12, 2019, with 1,491 hospitalists sitting for the exam and 84% passing. An estimated 4,000 pediatric hospitalists currently work in the field.
Certification as a subspecialty typically requires completing a fellowship, but new subspecialties often offer a “practice pathway” allowing those who already have experience working in the field to sit for the exam. A PHM practice pathway, and a combined fellowship and experience option for those whose fellowship training was less than 2 years, was offered for last year’s exam and will be offered again in 2021 and 2023. After that, board certification will only be available to graduates of recognized fellowships.
But concerns began to emerge last summer in advance of ABM’s initial PHM board exam, when some applicants were told that they weren’t eligible to sit for it, said H. Barrett Fromme, MD, associate dean for faculty development in medical education and section chief for pediatric hospital medicine at the University of Chicago. She also chairs the section of hospital medicine for the AAP.
Concerns including unintended gender bias against women, such as those hospitalists whose training is interrupted for maternity leave, were raised in a petition to ABP. The board promptly responded that gender bias was not supported by the facts, although its response did not account for selection bias in the data. But the ABP removed its practice interruption criteria.1,2
There are various reasons why a pediatric hospitalist might not be able or willing to pursue a 2-year fellowship or otherwise qualify for certification, Dr. Fromme said, including time and cost. For some, the practice pathway’s requirements, including a minimum number of hours worked in pediatrics in the previous 4 years, may be impossible to meet. Pediatric hospitalists boarded in family medicine are not eligible.
For hospitalists who can’t achieve board certification, what might that mean in terms of their future salary, employment opportunities, reimbursement, other career goals? Might they find themselves unable to qualify for PHM jobs at some university-based medical centers? The answers are not yet known.
What might self-designation look like?
PHM is distinct from adult hospital medicine by virtue of its designation as a board-certified subspecialty. But it can look to the broader HM field for examples of designations that bestow a kind of professional recognition, Dr. Chang said. These include SHM’s merit-based Fellow in Hospital Medicine program and the American Board of Medical Specialties’ Focused Practice in Hospital Medicine, a pathway for board recertification in internal medicine and family medicine, he said.
But PHM self-designation is not necessarily a pathway to hospital privileges. “If we build it, will they come? If they come, will it mean anything to them? That’s the million-dollar question?” Dr. Chang said.
Hospitalists need to appreciate that this issue is important to all three PHM professional societies, SHM, AAP, and APA, Dr. Fromme said. “We are concerned about how to support all of our members – certified, noncertified, nonphysician. Alternate designation is one idea, but we need time to understand it. We need a lot more conversations and a lot of people thinking about it.”
Dr. Fromme is part of the Council on Pediatric Hospital Medicine, a small circle of leaders of PHM interest groups within the three professional associations. It meets quarterly and will be reviewing the results of the conference call.
“I personally think we don’t understand the scope of the problem or the needs of pediatric hospitalists who are not able to sit for boards or pursue a fellowship,” she said. “We have empathy and concern for our colleagues who can’t take the boards. We don’t want them to feel excluded, and that includes advanced practice nurses and residents. But does an alternative designation actually provide what people think it provides?”
There are other ways to demonstrate that professionals are engaged with and serious about developing their practice. If they are looking to better themselves at quality improvement, leadership, education, and other elements of PHM practice, the associations can endeavor to provide more educational opportunities, Dr. Fromme said. “But if it’s about how they look as a candidate for hire, relative to board-certified candidates, that’s a different beast, and we need to think about what can help them the most.”
References
1. American Board of Pediatrics, Response to the Pediatric Hospital Medicine Petition. 2019 Aug 20. https://www.abp.org/sites/abp/files/phm-petition-response.pdf.
2. Chang WW et al. J Hosp Med. 2019 Oct;14(10):589-90.
A recent teleconference brought together an ad hoc panel of pediatric hospitalists, with more than 100 diverse voices discussing whether there ought to be an additional professional recognition or designation for the subspecialty, apart from the new pediatric hospital medicine (PHM) board certification that was launched in 2019.
The heterogeneity of PHM was on display during the discussion, as participants included university-based pediatric hospitalists and those from community hospitals, physicians trained in combined medicine and pediatrics or in family medicine, doctors who completed a general pediatric residency before going straight into PHM, niche practitioners such as newborn hospitalists, trainees, and a small but growing number of graduates of PHM fellowship programs. There are 61 PHM fellowships, and these programs graduate approximately 70 new fellows per year.
Although a route to some kind of professional designation for PHM – separate from board certification – was the centerpiece of the conference call, there is no proposal actively under consideration for developing such a designation, said Weijen W. Chang, MD, FAAP, SFHM, chief of pediatric hospital medicine at Baystate Medical Center in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts–Baystate Campus.
Who might develop such a proposal? “The hope is that the three major professional societies involved in pediatric hospital medicine – the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association – would jointly develop such a designation,” Dr. Chang said. However, it is not clear whether the three societies could agree on this. An online survey of 551 pediatric hospitalists, shared during the conference call, found that the majority would like to see some kind of alternate designation.
The reality of the boards
The pediatric subspecialty of PHM was recognized by the American Board of Medical Specialties in 2015 following a petition by a group of PHM leaders seeking a way to credential their unique skill set. The first PHM board certification exam was offered by the American Board of Pediatrics on Nov. 12, 2019, with 1,491 hospitalists sitting for the exam and 84% passing. An estimated 4,000 pediatric hospitalists currently work in the field.
Certification as a subspecialty typically requires completing a fellowship, but new subspecialties often offer a “practice pathway” allowing those who already have experience working in the field to sit for the exam. A PHM practice pathway, and a combined fellowship and experience option for those whose fellowship training was less than 2 years, was offered for last year’s exam and will be offered again in 2021 and 2023. After that, board certification will only be available to graduates of recognized fellowships.
But concerns began to emerge last summer in advance of ABM’s initial PHM board exam, when some applicants were told that they weren’t eligible to sit for it, said H. Barrett Fromme, MD, associate dean for faculty development in medical education and section chief for pediatric hospital medicine at the University of Chicago. She also chairs the section of hospital medicine for the AAP.
Concerns including unintended gender bias against women, such as those hospitalists whose training is interrupted for maternity leave, were raised in a petition to ABP. The board promptly responded that gender bias was not supported by the facts, although its response did not account for selection bias in the data. But the ABP removed its practice interruption criteria.1,2
There are various reasons why a pediatric hospitalist might not be able or willing to pursue a 2-year fellowship or otherwise qualify for certification, Dr. Fromme said, including time and cost. For some, the practice pathway’s requirements, including a minimum number of hours worked in pediatrics in the previous 4 years, may be impossible to meet. Pediatric hospitalists boarded in family medicine are not eligible.
For hospitalists who can’t achieve board certification, what might that mean in terms of their future salary, employment opportunities, reimbursement, other career goals? Might they find themselves unable to qualify for PHM jobs at some university-based medical centers? The answers are not yet known.
What might self-designation look like?
PHM is distinct from adult hospital medicine by virtue of its designation as a board-certified subspecialty. But it can look to the broader HM field for examples of designations that bestow a kind of professional recognition, Dr. Chang said. These include SHM’s merit-based Fellow in Hospital Medicine program and the American Board of Medical Specialties’ Focused Practice in Hospital Medicine, a pathway for board recertification in internal medicine and family medicine, he said.
But PHM self-designation is not necessarily a pathway to hospital privileges. “If we build it, will they come? If they come, will it mean anything to them? That’s the million-dollar question?” Dr. Chang said.
Hospitalists need to appreciate that this issue is important to all three PHM professional societies, SHM, AAP, and APA, Dr. Fromme said. “We are concerned about how to support all of our members – certified, noncertified, nonphysician. Alternate designation is one idea, but we need time to understand it. We need a lot more conversations and a lot of people thinking about it.”
Dr. Fromme is part of the Council on Pediatric Hospital Medicine, a small circle of leaders of PHM interest groups within the three professional associations. It meets quarterly and will be reviewing the results of the conference call.
“I personally think we don’t understand the scope of the problem or the needs of pediatric hospitalists who are not able to sit for boards or pursue a fellowship,” she said. “We have empathy and concern for our colleagues who can’t take the boards. We don’t want them to feel excluded, and that includes advanced practice nurses and residents. But does an alternative designation actually provide what people think it provides?”
There are other ways to demonstrate that professionals are engaged with and serious about developing their practice. If they are looking to better themselves at quality improvement, leadership, education, and other elements of PHM practice, the associations can endeavor to provide more educational opportunities, Dr. Fromme said. “But if it’s about how they look as a candidate for hire, relative to board-certified candidates, that’s a different beast, and we need to think about what can help them the most.”
References
1. American Board of Pediatrics, Response to the Pediatric Hospital Medicine Petition. 2019 Aug 20. https://www.abp.org/sites/abp/files/phm-petition-response.pdf.
2. Chang WW et al. J Hosp Med. 2019 Oct;14(10):589-90.
A recent teleconference brought together an ad hoc panel of pediatric hospitalists, with more than 100 diverse voices discussing whether there ought to be an additional professional recognition or designation for the subspecialty, apart from the new pediatric hospital medicine (PHM) board certification that was launched in 2019.
The heterogeneity of PHM was on display during the discussion, as participants included university-based pediatric hospitalists and those from community hospitals, physicians trained in combined medicine and pediatrics or in family medicine, doctors who completed a general pediatric residency before going straight into PHM, niche practitioners such as newborn hospitalists, trainees, and a small but growing number of graduates of PHM fellowship programs. There are 61 PHM fellowships, and these programs graduate approximately 70 new fellows per year.
Although a route to some kind of professional designation for PHM – separate from board certification – was the centerpiece of the conference call, there is no proposal actively under consideration for developing such a designation, said Weijen W. Chang, MD, FAAP, SFHM, chief of pediatric hospital medicine at Baystate Medical Center in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts–Baystate Campus.
Who might develop such a proposal? “The hope is that the three major professional societies involved in pediatric hospital medicine – the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association – would jointly develop such a designation,” Dr. Chang said. However, it is not clear whether the three societies could agree on this. An online survey of 551 pediatric hospitalists, shared during the conference call, found that the majority would like to see some kind of alternate designation.
The reality of the boards
The pediatric subspecialty of PHM was recognized by the American Board of Medical Specialties in 2015 following a petition by a group of PHM leaders seeking a way to credential their unique skill set. The first PHM board certification exam was offered by the American Board of Pediatrics on Nov. 12, 2019, with 1,491 hospitalists sitting for the exam and 84% passing. An estimated 4,000 pediatric hospitalists currently work in the field.
Certification as a subspecialty typically requires completing a fellowship, but new subspecialties often offer a “practice pathway” allowing those who already have experience working in the field to sit for the exam. A PHM practice pathway, and a combined fellowship and experience option for those whose fellowship training was less than 2 years, was offered for last year’s exam and will be offered again in 2021 and 2023. After that, board certification will only be available to graduates of recognized fellowships.
But concerns began to emerge last summer in advance of ABM’s initial PHM board exam, when some applicants were told that they weren’t eligible to sit for it, said H. Barrett Fromme, MD, associate dean for faculty development in medical education and section chief for pediatric hospital medicine at the University of Chicago. She also chairs the section of hospital medicine for the AAP.
Concerns including unintended gender bias against women, such as those hospitalists whose training is interrupted for maternity leave, were raised in a petition to ABP. The board promptly responded that gender bias was not supported by the facts, although its response did not account for selection bias in the data. But the ABP removed its practice interruption criteria.1,2
There are various reasons why a pediatric hospitalist might not be able or willing to pursue a 2-year fellowship or otherwise qualify for certification, Dr. Fromme said, including time and cost. For some, the practice pathway’s requirements, including a minimum number of hours worked in pediatrics in the previous 4 years, may be impossible to meet. Pediatric hospitalists boarded in family medicine are not eligible.
For hospitalists who can’t achieve board certification, what might that mean in terms of their future salary, employment opportunities, reimbursement, other career goals? Might they find themselves unable to qualify for PHM jobs at some university-based medical centers? The answers are not yet known.
What might self-designation look like?
PHM is distinct from adult hospital medicine by virtue of its designation as a board-certified subspecialty. But it can look to the broader HM field for examples of designations that bestow a kind of professional recognition, Dr. Chang said. These include SHM’s merit-based Fellow in Hospital Medicine program and the American Board of Medical Specialties’ Focused Practice in Hospital Medicine, a pathway for board recertification in internal medicine and family medicine, he said.
But PHM self-designation is not necessarily a pathway to hospital privileges. “If we build it, will they come? If they come, will it mean anything to them? That’s the million-dollar question?” Dr. Chang said.
Hospitalists need to appreciate that this issue is important to all three PHM professional societies, SHM, AAP, and APA, Dr. Fromme said. “We are concerned about how to support all of our members – certified, noncertified, nonphysician. Alternate designation is one idea, but we need time to understand it. We need a lot more conversations and a lot of people thinking about it.”
Dr. Fromme is part of the Council on Pediatric Hospital Medicine, a small circle of leaders of PHM interest groups within the three professional associations. It meets quarterly and will be reviewing the results of the conference call.
“I personally think we don’t understand the scope of the problem or the needs of pediatric hospitalists who are not able to sit for boards or pursue a fellowship,” she said. “We have empathy and concern for our colleagues who can’t take the boards. We don’t want them to feel excluded, and that includes advanced practice nurses and residents. But does an alternative designation actually provide what people think it provides?”
There are other ways to demonstrate that professionals are engaged with and serious about developing their practice. If they are looking to better themselves at quality improvement, leadership, education, and other elements of PHM practice, the associations can endeavor to provide more educational opportunities, Dr. Fromme said. “But if it’s about how they look as a candidate for hire, relative to board-certified candidates, that’s a different beast, and we need to think about what can help them the most.”
References
1. American Board of Pediatrics, Response to the Pediatric Hospital Medicine Petition. 2019 Aug 20. https://www.abp.org/sites/abp/files/phm-petition-response.pdf.
2. Chang WW et al. J Hosp Med. 2019 Oct;14(10):589-90.
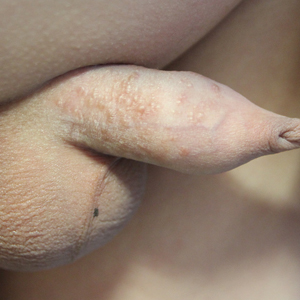
Sorting out the many mimickers of psoriasis
“It has an earlier age of onset, usually in infancy, and can occur with the atopic triad that presents with asthma and seasonal allergies as well,” Israel David “Izzy” Andrews, MD, said at the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “There is typically a very strong family history, as this is an autosomal dominant condition, and it’s far more common than psoriasis. The annual incidence is estimated to be 10%-15% of pediatric patients. It has classic areas of involvement depending on the age of the patient, and lesions are intensely pruritic at all times. There is induration and crust, but it’s important to distinguish crust from scale. Whereas crust is dried exudate, and scale is usually secondary to a hyperproliferation of the skin. Initially, treatments (especially topical) are similar and may also delay the formalized diagnosis of either of the two.”
Another psoriasis mimicker, pityriasis rosea, is thought to be secondary to human herpes virus 6 or 7 infection, said Dr. Andrews, of the department of dermatology at Phoenix Children’s Hospital. It typically appears in the teens and tweens and usually presents as a large herald patch or plaque on the trunk. As the herald patch resolves, smaller lesions will develop on the trunk following skin folds. “It’s rarely symptomatic and it’s very short lived, and clears within 6-12 weeks,” Dr. Andrews noted. “It can present with an inverse pattern involving the face, neck, and groin, but sparing the trunk. This variant, termed inverse pityriasis rosea, can be confused with inverse psoriasis, which has a similar distribution. However, the inverse pattern of pityriasis rosea will still resolve in a similar time frame to its more classic variant.”
Pityriasis lichenoides can also be mistaken for psoriasis. The acute form can present with erythematous, scaly papules and plaques, but lesions are often found in different phases of resolution or healing. “This benign lymphoproliferative skin disorder can be very difficult to distinguish from psoriasis and may require a biopsy to rule in or out,” Dr. Andrews said. “It can last months to years and there are few treatments that are effective. It is typically nonresponsive to topical steroids and other treatments that would be more effective for psoriasis, helping to distinguish the two. It is thought to exist in the spectrum with other lymphoproliferative diseases including cutaneous T-cell lymphoma [CTCL]. However, there are only a few cases in the literature that support a transformation from pityriasis lichenoides to CTCL.”
Seborrheic dermatitis is more common than atopic dermatitis and psoriasis, but it can be mistaken for psoriasis. It is caused by an inflammatory response secondary to overgrowth of Malassezia yeast and has a bimodal age distribution. “Seborrheic dermatitis affects babies, teens, and tweens, and can persist into adulthood,” he said. “Infants with cradle cap usually resolve with moisturization, gentle brushing, and occasional antifungal shampoos.” Petaloid seborrheic dermatitis can predominately involve the face with psoriatic-appearing induration, plaques, and varying degrees of scales. “In skin of color, this can be confused with discoid lupus, sarcoidosis, and psoriasis, occasionally requiring a biopsy to distinguish,” said Dr. Andrews, who is also an assistant professor of pediatrics at the Mayo Clinic College of Medicine and Science in Scottsdale, Ariz.
Another psoriasis mimicker, pityriasis amiantacea, is thought to be a more severe form of seborrheic dermatitis. It presents with concretions of scale around hair follicles that are highly adherent and are sometimes called sebopsoriasis. “It may be associated with cutaneous findings of psoriasis elsewhere, but may also be found with secondarily infected atopic dermatitis and tinea capitis; however, in my clinical experience, it is most often found in isolation,” he said. “There may be a seasonal association with exacerbation in warm temperatures, and treatment often consists of humectants like salicylic acid for loosening scale, topical steroids for inflammation, and gentle combing out of scale.”
Infections can also mimic psoriasis. For example, tinea infections are often misdiagnosed as eczema or psoriasis and treated with topical steroids. “This can lead to tinea incognito, making it harder to diagnose either condition without attention to detail,” Dr. Andrews said. “On the body, look for expanding lesions with more raised peripheral edges, and central flattening, giving a classic annular appearance. It’s also important to inquire about family history and contacts including pets, contact sports/mat sports (think yoga, gymnastics, martial arts), or other contacts with similar rashes.” Work-up typically includes a fungal culture and starting empiric oral antifungal medications. “It is important to be able to distinguish scalp psoriasis from tinea capitis to prevent the more inflammatory form of tinea capitis, kerion (a deeper more symptomatic, painful and purulent dermatitis), which can lead to permanent scarring alopecia,” he said.
Bacterial infections can also mimic psoriasis, specifically nonbullous impetigo and ecthyma, the more ulcerative form of impetigo. The most frequent associations are group A Streptococcus, methicillin-susceptible Staphylococcus aureus and methicillin-resistant S. aureus.
Dr. Andrews closed his presentation by noting that tumor necrosis factor–alpha inhibitor–induced psoriasiform drug eruptions can occur in psoriasis-naive patients or unmask a predilection for psoriasis in patients with Crohn’s disease, juvenile idiopathic arthritis, or other autoinflammatory or autoimmune conditions. “They may improve with continued treatment and resolve with switching treatments,” he said. “Early biopsy in psoriasiform drug eruptions can appear like atopic dermatitis on pathology. When suspecting psoriasis in a pediatric patient, it is important to consider the history and physical exam as well as family history and associated comorbidities. While a biopsy may aide in the work-up, diagnosis can be made clinically.”
Dr. Andrews reported having no financial disclosures.
“It has an earlier age of onset, usually in infancy, and can occur with the atopic triad that presents with asthma and seasonal allergies as well,” Israel David “Izzy” Andrews, MD, said at the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “There is typically a very strong family history, as this is an autosomal dominant condition, and it’s far more common than psoriasis. The annual incidence is estimated to be 10%-15% of pediatric patients. It has classic areas of involvement depending on the age of the patient, and lesions are intensely pruritic at all times. There is induration and crust, but it’s important to distinguish crust from scale. Whereas crust is dried exudate, and scale is usually secondary to a hyperproliferation of the skin. Initially, treatments (especially topical) are similar and may also delay the formalized diagnosis of either of the two.”
Another psoriasis mimicker, pityriasis rosea, is thought to be secondary to human herpes virus 6 or 7 infection, said Dr. Andrews, of the department of dermatology at Phoenix Children’s Hospital. It typically appears in the teens and tweens and usually presents as a large herald patch or plaque on the trunk. As the herald patch resolves, smaller lesions will develop on the trunk following skin folds. “It’s rarely symptomatic and it’s very short lived, and clears within 6-12 weeks,” Dr. Andrews noted. “It can present with an inverse pattern involving the face, neck, and groin, but sparing the trunk. This variant, termed inverse pityriasis rosea, can be confused with inverse psoriasis, which has a similar distribution. However, the inverse pattern of pityriasis rosea will still resolve in a similar time frame to its more classic variant.”
Pityriasis lichenoides can also be mistaken for psoriasis. The acute form can present with erythematous, scaly papules and plaques, but lesions are often found in different phases of resolution or healing. “This benign lymphoproliferative skin disorder can be very difficult to distinguish from psoriasis and may require a biopsy to rule in or out,” Dr. Andrews said. “It can last months to years and there are few treatments that are effective. It is typically nonresponsive to topical steroids and other treatments that would be more effective for psoriasis, helping to distinguish the two. It is thought to exist in the spectrum with other lymphoproliferative diseases including cutaneous T-cell lymphoma [CTCL]. However, there are only a few cases in the literature that support a transformation from pityriasis lichenoides to CTCL.”
Seborrheic dermatitis is more common than atopic dermatitis and psoriasis, but it can be mistaken for psoriasis. It is caused by an inflammatory response secondary to overgrowth of Malassezia yeast and has a bimodal age distribution. “Seborrheic dermatitis affects babies, teens, and tweens, and can persist into adulthood,” he said. “Infants with cradle cap usually resolve with moisturization, gentle brushing, and occasional antifungal shampoos.” Petaloid seborrheic dermatitis can predominately involve the face with psoriatic-appearing induration, plaques, and varying degrees of scales. “In skin of color, this can be confused with discoid lupus, sarcoidosis, and psoriasis, occasionally requiring a biopsy to distinguish,” said Dr. Andrews, who is also an assistant professor of pediatrics at the Mayo Clinic College of Medicine and Science in Scottsdale, Ariz.
Another psoriasis mimicker, pityriasis amiantacea, is thought to be a more severe form of seborrheic dermatitis. It presents with concretions of scale around hair follicles that are highly adherent and are sometimes called sebopsoriasis. “It may be associated with cutaneous findings of psoriasis elsewhere, but may also be found with secondarily infected atopic dermatitis and tinea capitis; however, in my clinical experience, it is most often found in isolation,” he said. “There may be a seasonal association with exacerbation in warm temperatures, and treatment often consists of humectants like salicylic acid for loosening scale, topical steroids for inflammation, and gentle combing out of scale.”
Infections can also mimic psoriasis. For example, tinea infections are often misdiagnosed as eczema or psoriasis and treated with topical steroids. “This can lead to tinea incognito, making it harder to diagnose either condition without attention to detail,” Dr. Andrews said. “On the body, look for expanding lesions with more raised peripheral edges, and central flattening, giving a classic annular appearance. It’s also important to inquire about family history and contacts including pets, contact sports/mat sports (think yoga, gymnastics, martial arts), or other contacts with similar rashes.” Work-up typically includes a fungal culture and starting empiric oral antifungal medications. “It is important to be able to distinguish scalp psoriasis from tinea capitis to prevent the more inflammatory form of tinea capitis, kerion (a deeper more symptomatic, painful and purulent dermatitis), which can lead to permanent scarring alopecia,” he said.
Bacterial infections can also mimic psoriasis, specifically nonbullous impetigo and ecthyma, the more ulcerative form of impetigo. The most frequent associations are group A Streptococcus, methicillin-susceptible Staphylococcus aureus and methicillin-resistant S. aureus.
Dr. Andrews closed his presentation by noting that tumor necrosis factor–alpha inhibitor–induced psoriasiform drug eruptions can occur in psoriasis-naive patients or unmask a predilection for psoriasis in patients with Crohn’s disease, juvenile idiopathic arthritis, or other autoinflammatory or autoimmune conditions. “They may improve with continued treatment and resolve with switching treatments,” he said. “Early biopsy in psoriasiform drug eruptions can appear like atopic dermatitis on pathology. When suspecting psoriasis in a pediatric patient, it is important to consider the history and physical exam as well as family history and associated comorbidities. While a biopsy may aide in the work-up, diagnosis can be made clinically.”
Dr. Andrews reported having no financial disclosures.
“It has an earlier age of onset, usually in infancy, and can occur with the atopic triad that presents with asthma and seasonal allergies as well,” Israel David “Izzy” Andrews, MD, said at the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “There is typically a very strong family history, as this is an autosomal dominant condition, and it’s far more common than psoriasis. The annual incidence is estimated to be 10%-15% of pediatric patients. It has classic areas of involvement depending on the age of the patient, and lesions are intensely pruritic at all times. There is induration and crust, but it’s important to distinguish crust from scale. Whereas crust is dried exudate, and scale is usually secondary to a hyperproliferation of the skin. Initially, treatments (especially topical) are similar and may also delay the formalized diagnosis of either of the two.”
Another psoriasis mimicker, pityriasis rosea, is thought to be secondary to human herpes virus 6 or 7 infection, said Dr. Andrews, of the department of dermatology at Phoenix Children’s Hospital. It typically appears in the teens and tweens and usually presents as a large herald patch or plaque on the trunk. As the herald patch resolves, smaller lesions will develop on the trunk following skin folds. “It’s rarely symptomatic and it’s very short lived, and clears within 6-12 weeks,” Dr. Andrews noted. “It can present with an inverse pattern involving the face, neck, and groin, but sparing the trunk. This variant, termed inverse pityriasis rosea, can be confused with inverse psoriasis, which has a similar distribution. However, the inverse pattern of pityriasis rosea will still resolve in a similar time frame to its more classic variant.”
Pityriasis lichenoides can also be mistaken for psoriasis. The acute form can present with erythematous, scaly papules and plaques, but lesions are often found in different phases of resolution or healing. “This benign lymphoproliferative skin disorder can be very difficult to distinguish from psoriasis and may require a biopsy to rule in or out,” Dr. Andrews said. “It can last months to years and there are few treatments that are effective. It is typically nonresponsive to topical steroids and other treatments that would be more effective for psoriasis, helping to distinguish the two. It is thought to exist in the spectrum with other lymphoproliferative diseases including cutaneous T-cell lymphoma [CTCL]. However, there are only a few cases in the literature that support a transformation from pityriasis lichenoides to CTCL.”
Seborrheic dermatitis is more common than atopic dermatitis and psoriasis, but it can be mistaken for psoriasis. It is caused by an inflammatory response secondary to overgrowth of Malassezia yeast and has a bimodal age distribution. “Seborrheic dermatitis affects babies, teens, and tweens, and can persist into adulthood,” he said. “Infants with cradle cap usually resolve with moisturization, gentle brushing, and occasional antifungal shampoos.” Petaloid seborrheic dermatitis can predominately involve the face with psoriatic-appearing induration, plaques, and varying degrees of scales. “In skin of color, this can be confused with discoid lupus, sarcoidosis, and psoriasis, occasionally requiring a biopsy to distinguish,” said Dr. Andrews, who is also an assistant professor of pediatrics at the Mayo Clinic College of Medicine and Science in Scottsdale, Ariz.
Another psoriasis mimicker, pityriasis amiantacea, is thought to be a more severe form of seborrheic dermatitis. It presents with concretions of scale around hair follicles that are highly adherent and are sometimes called sebopsoriasis. “It may be associated with cutaneous findings of psoriasis elsewhere, but may also be found with secondarily infected atopic dermatitis and tinea capitis; however, in my clinical experience, it is most often found in isolation,” he said. “There may be a seasonal association with exacerbation in warm temperatures, and treatment often consists of humectants like salicylic acid for loosening scale, topical steroids for inflammation, and gentle combing out of scale.”
Infections can also mimic psoriasis. For example, tinea infections are often misdiagnosed as eczema or psoriasis and treated with topical steroids. “This can lead to tinea incognito, making it harder to diagnose either condition without attention to detail,” Dr. Andrews said. “On the body, look for expanding lesions with more raised peripheral edges, and central flattening, giving a classic annular appearance. It’s also important to inquire about family history and contacts including pets, contact sports/mat sports (think yoga, gymnastics, martial arts), or other contacts with similar rashes.” Work-up typically includes a fungal culture and starting empiric oral antifungal medications. “It is important to be able to distinguish scalp psoriasis from tinea capitis to prevent the more inflammatory form of tinea capitis, kerion (a deeper more symptomatic, painful and purulent dermatitis), which can lead to permanent scarring alopecia,” he said.
Bacterial infections can also mimic psoriasis, specifically nonbullous impetigo and ecthyma, the more ulcerative form of impetigo. The most frequent associations are group A Streptococcus, methicillin-susceptible Staphylococcus aureus and methicillin-resistant S. aureus.
Dr. Andrews closed his presentation by noting that tumor necrosis factor–alpha inhibitor–induced psoriasiform drug eruptions can occur in psoriasis-naive patients or unmask a predilection for psoriasis in patients with Crohn’s disease, juvenile idiopathic arthritis, or other autoinflammatory or autoimmune conditions. “They may improve with continued treatment and resolve with switching treatments,” he said. “Early biopsy in psoriasiform drug eruptions can appear like atopic dermatitis on pathology. When suspecting psoriasis in a pediatric patient, it is important to consider the history and physical exam as well as family history and associated comorbidities. While a biopsy may aide in the work-up, diagnosis can be made clinically.”
Dr. Andrews reported having no financial disclosures.
FROM PEDIATRIC DERMATOLOGY 2020
Expert shares his approach to treating warts in children
In the clinical experience of Anthony J. Mancini, MD, one option for children and adolescents who present with common warts is to do nothing, since they may resolve on their own.
“Many effective treatments that we have are painful and poorly tolerated, especially in younger children,” Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “However, while they’re harmless and often self-limited, warts often form a social stigma, and parents often desire therapy.”
Even though warts may spontaneously resolve in up to 65% of patients at 2 years and 80% at 4 years, the goals of treatment are to eradicate them, minimize pain, avoid scarring, and help prevent recurrence.
One effective topical therapy he highlighted is WartPEEL cream, which is a proprietary, compounded formulation of 17% salicylic acid and 2% 5-fluorouracil. “It’s in a sustained release vehicle called Remedium, and is available from a compounding pharmacy, but not FDA approved,” said Dr. Mancini, who is also head of pediatric dermatology at Lurie Children’s Hospital of Chicago. “It’s applied nightly with plastic tape occlusion and rinsed off each morning.”
WartPEEL is available through NuCara Pharmacy at 877-268-2272. It is not covered by most insurance plans and it costs around $80. “It is very effective, tends to be totally painless, and has a much quicker response than over-the-counter salicylic acid-based treatments for warts,” he said.
Another treatment option is oral cimetidine, especially in patients who have multiple or recalcitrant warts. The recommended dosing is 30-40 mg/kg per day, divided into twice-daily dosing. “You have to give it for at least 8-12 weeks to determine whether it’s working or not,” Dr. Mancini said. “In the initial report, [investigators] described an 81% complete response rate, but subsequent randomized, controlled trials were not able to confirm that data against placebo or topical treatments. I will say, though, that cimetidine is well tolerated. It’s always worth a try but, if you do use it, always consider other medications the patient may be taking and potential drug-drug interactions.”
For flat warts, verrucous papules that commonly occur on the face, Dr. Mancini recommends off-label treatment with 5% 5-fluorouracil cream (Efudex), which is normally indicated for actinic keratoses in adults. “I have patients apply this for 3 nights per week and work their way up gradually to nightly application,” he said. “It’s really important that parents and patients understand the importance of sun protection when they’re using Efudex, and they need to know that some irritation is possible. Overall, this treatment seems to be very well tolerated.”
Other treatment options for common warts, in addition to over-the-counter products that contain salicylic acid, are home cryotherapy kits that contain a mixture of diethyl ether and propane. “These can be effective for small warts,” Dr. Mancini said. “But for larger, thicker lesions, they’re not going to quite as effective.”
Treatment options best reserved for dermatologists, he continued, include in-office liquid nitrogen cryotherapy, “if it’s tolerated,” he said. “I have a no-hold policy, so if we have to hold a child down who’s flailing and crying and screaming during treatment, we’re probably not going to use liquid nitrogen.” He also mentioned topical immunotherapy with agents like squaric acid dibutylester. “This is almost like putting poison ivy on your warts to get the immune system revved up,” he said. “It can be very effective.” Other treatment options include intralesional immune therapy, topical cidofovir, and even pulsed-dye laser.
Dr. Mancini disclosed that he is a consultant to and a member of the scientific advisory board for Verrica Pharmaceuticals.
In the clinical experience of Anthony J. Mancini, MD, one option for children and adolescents who present with common warts is to do nothing, since they may resolve on their own.
“Many effective treatments that we have are painful and poorly tolerated, especially in younger children,” Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “However, while they’re harmless and often self-limited, warts often form a social stigma, and parents often desire therapy.”
Even though warts may spontaneously resolve in up to 65% of patients at 2 years and 80% at 4 years, the goals of treatment are to eradicate them, minimize pain, avoid scarring, and help prevent recurrence.
One effective topical therapy he highlighted is WartPEEL cream, which is a proprietary, compounded formulation of 17% salicylic acid and 2% 5-fluorouracil. “It’s in a sustained release vehicle called Remedium, and is available from a compounding pharmacy, but not FDA approved,” said Dr. Mancini, who is also head of pediatric dermatology at Lurie Children’s Hospital of Chicago. “It’s applied nightly with plastic tape occlusion and rinsed off each morning.”
WartPEEL is available through NuCara Pharmacy at 877-268-2272. It is not covered by most insurance plans and it costs around $80. “It is very effective, tends to be totally painless, and has a much quicker response than over-the-counter salicylic acid-based treatments for warts,” he said.
Another treatment option is oral cimetidine, especially in patients who have multiple or recalcitrant warts. The recommended dosing is 30-40 mg/kg per day, divided into twice-daily dosing. “You have to give it for at least 8-12 weeks to determine whether it’s working or not,” Dr. Mancini said. “In the initial report, [investigators] described an 81% complete response rate, but subsequent randomized, controlled trials were not able to confirm that data against placebo or topical treatments. I will say, though, that cimetidine is well tolerated. It’s always worth a try but, if you do use it, always consider other medications the patient may be taking and potential drug-drug interactions.”
For flat warts, verrucous papules that commonly occur on the face, Dr. Mancini recommends off-label treatment with 5% 5-fluorouracil cream (Efudex), which is normally indicated for actinic keratoses in adults. “I have patients apply this for 3 nights per week and work their way up gradually to nightly application,” he said. “It’s really important that parents and patients understand the importance of sun protection when they’re using Efudex, and they need to know that some irritation is possible. Overall, this treatment seems to be very well tolerated.”
Other treatment options for common warts, in addition to over-the-counter products that contain salicylic acid, are home cryotherapy kits that contain a mixture of diethyl ether and propane. “These can be effective for small warts,” Dr. Mancini said. “But for larger, thicker lesions, they’re not going to quite as effective.”
Treatment options best reserved for dermatologists, he continued, include in-office liquid nitrogen cryotherapy, “if it’s tolerated,” he said. “I have a no-hold policy, so if we have to hold a child down who’s flailing and crying and screaming during treatment, we’re probably not going to use liquid nitrogen.” He also mentioned topical immunotherapy with agents like squaric acid dibutylester. “This is almost like putting poison ivy on your warts to get the immune system revved up,” he said. “It can be very effective.” Other treatment options include intralesional immune therapy, topical cidofovir, and even pulsed-dye laser.
Dr. Mancini disclosed that he is a consultant to and a member of the scientific advisory board for Verrica Pharmaceuticals.
In the clinical experience of Anthony J. Mancini, MD, one option for children and adolescents who present with common warts is to do nothing, since they may resolve on their own.
“Many effective treatments that we have are painful and poorly tolerated, especially in younger children,” Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “However, while they’re harmless and often self-limited, warts often form a social stigma, and parents often desire therapy.”
Even though warts may spontaneously resolve in up to 65% of patients at 2 years and 80% at 4 years, the goals of treatment are to eradicate them, minimize pain, avoid scarring, and help prevent recurrence.
One effective topical therapy he highlighted is WartPEEL cream, which is a proprietary, compounded formulation of 17% salicylic acid and 2% 5-fluorouracil. “It’s in a sustained release vehicle called Remedium, and is available from a compounding pharmacy, but not FDA approved,” said Dr. Mancini, who is also head of pediatric dermatology at Lurie Children’s Hospital of Chicago. “It’s applied nightly with plastic tape occlusion and rinsed off each morning.”
WartPEEL is available through NuCara Pharmacy at 877-268-2272. It is not covered by most insurance plans and it costs around $80. “It is very effective, tends to be totally painless, and has a much quicker response than over-the-counter salicylic acid-based treatments for warts,” he said.
Another treatment option is oral cimetidine, especially in patients who have multiple or recalcitrant warts. The recommended dosing is 30-40 mg/kg per day, divided into twice-daily dosing. “You have to give it for at least 8-12 weeks to determine whether it’s working or not,” Dr. Mancini said. “In the initial report, [investigators] described an 81% complete response rate, but subsequent randomized, controlled trials were not able to confirm that data against placebo or topical treatments. I will say, though, that cimetidine is well tolerated. It’s always worth a try but, if you do use it, always consider other medications the patient may be taking and potential drug-drug interactions.”
For flat warts, verrucous papules that commonly occur on the face, Dr. Mancini recommends off-label treatment with 5% 5-fluorouracil cream (Efudex), which is normally indicated for actinic keratoses in adults. “I have patients apply this for 3 nights per week and work their way up gradually to nightly application,” he said. “It’s really important that parents and patients understand the importance of sun protection when they’re using Efudex, and they need to know that some irritation is possible. Overall, this treatment seems to be very well tolerated.”
Other treatment options for common warts, in addition to over-the-counter products that contain salicylic acid, are home cryotherapy kits that contain a mixture of diethyl ether and propane. “These can be effective for small warts,” Dr. Mancini said. “But for larger, thicker lesions, they’re not going to quite as effective.”
Treatment options best reserved for dermatologists, he continued, include in-office liquid nitrogen cryotherapy, “if it’s tolerated,” he said. “I have a no-hold policy, so if we have to hold a child down who’s flailing and crying and screaming during treatment, we’re probably not going to use liquid nitrogen.” He also mentioned topical immunotherapy with agents like squaric acid dibutylester. “This is almost like putting poison ivy on your warts to get the immune system revved up,” he said. “It can be very effective.” Other treatment options include intralesional immune therapy, topical cidofovir, and even pulsed-dye laser.
Dr. Mancini disclosed that he is a consultant to and a member of the scientific advisory board for Verrica Pharmaceuticals.
FROM PEDIATRIC DERMATOLOGY 2020
Daily Recap: Lifestyle vs. genes in breast cancer showdown; Big pharma sues over insulin affordability law
Here are the stories our MDedge editors across specialties think you need to know about today:
Lifestyle choices may reduce breast cancer risk regardless of genetics
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” said William Gradishar, MD, who was not invovled with the study. Read more.
Primary care practices may lose $68K per physician this year
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
Dermatology and rheumatology visits have recovered, but some specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15, and pulmonology visits were down 45% in that time.
This primary care estimate is without a potential second wave of COVID-19, noted Sanjay Basu, MD, director of research and population health at Collective Health in San Francisco, and colleagues.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview. Read more.
Big pharma sues to block Minnesota insulin affordability law
The Pharmaceutical Research and Manufacturers Association (PhRMA) is suing the state of Minnesota in an attempt to overturn a law that requires insulin makers to provide an emergency supply to individuals free of charge.
In the July 1 filing, PhRMA’s attorneys said the law is unconstitutional. It “order[s] pharmaceutical manufacturers to give insulin to state residents, on the state’s prescribed terms, at no charge to the recipients and without compensating the manufacturers in any way.”
The state has estimated that as many as 30,000 Minnesotans would be eligible for free insulin in the first year of the program. The drugmakers strenuously objected, noting that would mean they would “be compelled to provide 173,800 monthly supplies of free insulin” just in the first year.
“There is nothing in the U.S. Constitution that prevents states from saving the lives of its citizens who are in imminent danger,” said Mayo Clinic hematologist S. Vincent Rajkumar, MD. “The only motives for this lawsuit in my opinion are greed and the worry that other states may also choose to put lives of patients ahead of pharma profits.” Read more.
Despite guidelines, kids get opioids & steroids for pneumonia, sinusitis
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.” Read more.
Study supports changing classification of RCC
The definition of stage IV renal cell carcinoma (RCC) should be expanded to include lymph node–positive stage III disease, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Lifestyle choices may reduce breast cancer risk regardless of genetics
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” said William Gradishar, MD, who was not invovled with the study. Read more.
Primary care practices may lose $68K per physician this year
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
Dermatology and rheumatology visits have recovered, but some specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15, and pulmonology visits were down 45% in that time.
This primary care estimate is without a potential second wave of COVID-19, noted Sanjay Basu, MD, director of research and population health at Collective Health in San Francisco, and colleagues.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview. Read more.
Big pharma sues to block Minnesota insulin affordability law
The Pharmaceutical Research and Manufacturers Association (PhRMA) is suing the state of Minnesota in an attempt to overturn a law that requires insulin makers to provide an emergency supply to individuals free of charge.
In the July 1 filing, PhRMA’s attorneys said the law is unconstitutional. It “order[s] pharmaceutical manufacturers to give insulin to state residents, on the state’s prescribed terms, at no charge to the recipients and without compensating the manufacturers in any way.”
The state has estimated that as many as 30,000 Minnesotans would be eligible for free insulin in the first year of the program. The drugmakers strenuously objected, noting that would mean they would “be compelled to provide 173,800 monthly supplies of free insulin” just in the first year.
“There is nothing in the U.S. Constitution that prevents states from saving the lives of its citizens who are in imminent danger,” said Mayo Clinic hematologist S. Vincent Rajkumar, MD. “The only motives for this lawsuit in my opinion are greed and the worry that other states may also choose to put lives of patients ahead of pharma profits.” Read more.
Despite guidelines, kids get opioids & steroids for pneumonia, sinusitis
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.” Read more.
Study supports changing classification of RCC
The definition of stage IV renal cell carcinoma (RCC) should be expanded to include lymph node–positive stage III disease, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Lifestyle choices may reduce breast cancer risk regardless of genetics
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” said William Gradishar, MD, who was not invovled with the study. Read more.
Primary care practices may lose $68K per physician this year
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
Dermatology and rheumatology visits have recovered, but some specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15, and pulmonology visits were down 45% in that time.
This primary care estimate is without a potential second wave of COVID-19, noted Sanjay Basu, MD, director of research and population health at Collective Health in San Francisco, and colleagues.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview. Read more.
Big pharma sues to block Minnesota insulin affordability law
The Pharmaceutical Research and Manufacturers Association (PhRMA) is suing the state of Minnesota in an attempt to overturn a law that requires insulin makers to provide an emergency supply to individuals free of charge.
In the July 1 filing, PhRMA’s attorneys said the law is unconstitutional. It “order[s] pharmaceutical manufacturers to give insulin to state residents, on the state’s prescribed terms, at no charge to the recipients and without compensating the manufacturers in any way.”
The state has estimated that as many as 30,000 Minnesotans would be eligible for free insulin in the first year of the program. The drugmakers strenuously objected, noting that would mean they would “be compelled to provide 173,800 monthly supplies of free insulin” just in the first year.
“There is nothing in the U.S. Constitution that prevents states from saving the lives of its citizens who are in imminent danger,” said Mayo Clinic hematologist S. Vincent Rajkumar, MD. “The only motives for this lawsuit in my opinion are greed and the worry that other states may also choose to put lives of patients ahead of pharma profits.” Read more.
Despite guidelines, kids get opioids & steroids for pneumonia, sinusitis
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.” Read more.
Study supports changing classification of RCC
The definition of stage IV renal cell carcinoma (RCC) should be expanded to include lymph node–positive stage III disease, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Despite guidelines, children receive opioids and steroids for pneumonia and sinusitis
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.”
To compare the frequency of opioid and corticosteroid prescriptions for children with pneumonia or sinusitis in ED and ambulatory care settings, the investigators studied 2016 South Carolina Medicaid claims, examining data for patients aged 5-18 years with pneumonia or sinusitis. They excluded children with chronic conditions and acute secondary diagnoses with potentially appropriate indications for steroids, such as asthma. They also excluded children seen at more than one type of clinical location or hospitalized within a week of the visit. Only the primary diagnosis of pneumonia or sinusitis during the first visit of the year for each patient was included.
The researchers included data from 31,838 children in the study, including 2,140 children with pneumonia and 29,698 with sinusitis.
Pneumonia was linked to an opioid prescription in 6% of ED visits (34 of 542) and 1.5% of ambulatory visits (24 of 1,590) (P ≤ .0001). Pneumonia was linked to a steroid prescription in 20% of ED visits (106 of 542) and 12% of ambulatory visits (196 of 1,590) (P ≤ .0001).
Sinusitis was linked to an opioid prescription in 7.5% of ED visits (202 of 2,705) and 2% of ambulatory visits (568 of 26,866) (P ≤ .0001). Sinusitis was linked to a steroid prescription in 19% of ED visits (510 of 2,705) and 7% of ambulatory visits (1,922 of 26,866) (P ≤ .0001).
In logistic regression analyses, ED visits for pneumonia or sinusitis were more than four times more likely to result in children receiving opioids, relative to ambulatory visits (adjusted odds ratio, 4.69 and 4.02, respectively). ED visits also were more likely to result in steroid prescriptions, with aORs of 1.67 for pneumonia and 3.05 for sinusitis.
“I was disappointed to read of these results, although not necessarily surprised,” Michael E. Pichichero, MD, a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital, said in an interview.
The data suggest that improved prescribing practices may be needed, “especially in the ED,” wrote Dr. Phang and colleagues. “Although more children who are acutely ill may be seen in the ED, national practice guidelines and research remain relevant for these patients.”
Repeated or prolonged courses of systemic corticosteroids put children at risk for adrenal suppression and hypothalamic-pituitary-adrenal axis dysfunction. “Providers for children must also be aware of the trends in opioid abuse and diversion and must mitigate those risks while still providing adequate analgesia and symptom control,” they wrote.
The use of Medicaid data from 1 year in one state limits the generalizability of the findings. Nevertheless, the visits occurred “well after publication of relevant guidelines and after concerns of opioid prescribing had become widespread,” according to Dr. Phang and colleagues.
A post hoc evaluation identified one patient with a secondary diagnosis of fracture and 24 patients with a secondary diagnosis of pain, but none of these patients had received an opioid. “Thus, the small subset of patients who may have had secondary diagnoses that would warrant an opioid prescription would not have changed the overall results,” they wrote.
The study was funded by the National Institutes of Health. The authors had no relevant financial disclosures.
SOURCE: Phang KG et al. Pediatrics. 2020 Jul 2. doi: 10.1542/peds.2019-3690.
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.”
To compare the frequency of opioid and corticosteroid prescriptions for children with pneumonia or sinusitis in ED and ambulatory care settings, the investigators studied 2016 South Carolina Medicaid claims, examining data for patients aged 5-18 years with pneumonia or sinusitis. They excluded children with chronic conditions and acute secondary diagnoses with potentially appropriate indications for steroids, such as asthma. They also excluded children seen at more than one type of clinical location or hospitalized within a week of the visit. Only the primary diagnosis of pneumonia or sinusitis during the first visit of the year for each patient was included.
The researchers included data from 31,838 children in the study, including 2,140 children with pneumonia and 29,698 with sinusitis.
Pneumonia was linked to an opioid prescription in 6% of ED visits (34 of 542) and 1.5% of ambulatory visits (24 of 1,590) (P ≤ .0001). Pneumonia was linked to a steroid prescription in 20% of ED visits (106 of 542) and 12% of ambulatory visits (196 of 1,590) (P ≤ .0001).
Sinusitis was linked to an opioid prescription in 7.5% of ED visits (202 of 2,705) and 2% of ambulatory visits (568 of 26,866) (P ≤ .0001). Sinusitis was linked to a steroid prescription in 19% of ED visits (510 of 2,705) and 7% of ambulatory visits (1,922 of 26,866) (P ≤ .0001).
In logistic regression analyses, ED visits for pneumonia or sinusitis were more than four times more likely to result in children receiving opioids, relative to ambulatory visits (adjusted odds ratio, 4.69 and 4.02, respectively). ED visits also were more likely to result in steroid prescriptions, with aORs of 1.67 for pneumonia and 3.05 for sinusitis.
“I was disappointed to read of these results, although not necessarily surprised,” Michael E. Pichichero, MD, a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital, said in an interview.
The data suggest that improved prescribing practices may be needed, “especially in the ED,” wrote Dr. Phang and colleagues. “Although more children who are acutely ill may be seen in the ED, national practice guidelines and research remain relevant for these patients.”
Repeated or prolonged courses of systemic corticosteroids put children at risk for adrenal suppression and hypothalamic-pituitary-adrenal axis dysfunction. “Providers for children must also be aware of the trends in opioid abuse and diversion and must mitigate those risks while still providing adequate analgesia and symptom control,” they wrote.
The use of Medicaid data from 1 year in one state limits the generalizability of the findings. Nevertheless, the visits occurred “well after publication of relevant guidelines and after concerns of opioid prescribing had become widespread,” according to Dr. Phang and colleagues.
A post hoc evaluation identified one patient with a secondary diagnosis of fracture and 24 patients with a secondary diagnosis of pain, but none of these patients had received an opioid. “Thus, the small subset of patients who may have had secondary diagnoses that would warrant an opioid prescription would not have changed the overall results,” they wrote.
The study was funded by the National Institutes of Health. The authors had no relevant financial disclosures.
SOURCE: Phang KG et al. Pediatrics. 2020 Jul 2. doi: 10.1542/peds.2019-3690.
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.”
To compare the frequency of opioid and corticosteroid prescriptions for children with pneumonia or sinusitis in ED and ambulatory care settings, the investigators studied 2016 South Carolina Medicaid claims, examining data for patients aged 5-18 years with pneumonia or sinusitis. They excluded children with chronic conditions and acute secondary diagnoses with potentially appropriate indications for steroids, such as asthma. They also excluded children seen at more than one type of clinical location or hospitalized within a week of the visit. Only the primary diagnosis of pneumonia or sinusitis during the first visit of the year for each patient was included.
The researchers included data from 31,838 children in the study, including 2,140 children with pneumonia and 29,698 with sinusitis.
Pneumonia was linked to an opioid prescription in 6% of ED visits (34 of 542) and 1.5% of ambulatory visits (24 of 1,590) (P ≤ .0001). Pneumonia was linked to a steroid prescription in 20% of ED visits (106 of 542) and 12% of ambulatory visits (196 of 1,590) (P ≤ .0001).
Sinusitis was linked to an opioid prescription in 7.5% of ED visits (202 of 2,705) and 2% of ambulatory visits (568 of 26,866) (P ≤ .0001). Sinusitis was linked to a steroid prescription in 19% of ED visits (510 of 2,705) and 7% of ambulatory visits (1,922 of 26,866) (P ≤ .0001).
In logistic regression analyses, ED visits for pneumonia or sinusitis were more than four times more likely to result in children receiving opioids, relative to ambulatory visits (adjusted odds ratio, 4.69 and 4.02, respectively). ED visits also were more likely to result in steroid prescriptions, with aORs of 1.67 for pneumonia and 3.05 for sinusitis.
“I was disappointed to read of these results, although not necessarily surprised,” Michael E. Pichichero, MD, a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital, said in an interview.
The data suggest that improved prescribing practices may be needed, “especially in the ED,” wrote Dr. Phang and colleagues. “Although more children who are acutely ill may be seen in the ED, national practice guidelines and research remain relevant for these patients.”
Repeated or prolonged courses of systemic corticosteroids put children at risk for adrenal suppression and hypothalamic-pituitary-adrenal axis dysfunction. “Providers for children must also be aware of the trends in opioid abuse and diversion and must mitigate those risks while still providing adequate analgesia and symptom control,” they wrote.
The use of Medicaid data from 1 year in one state limits the generalizability of the findings. Nevertheless, the visits occurred “well after publication of relevant guidelines and after concerns of opioid prescribing had become widespread,” according to Dr. Phang and colleagues.
A post hoc evaluation identified one patient with a secondary diagnosis of fracture and 24 patients with a secondary diagnosis of pain, but none of these patients had received an opioid. “Thus, the small subset of patients who may have had secondary diagnoses that would warrant an opioid prescription would not have changed the overall results,” they wrote.
The study was funded by the National Institutes of Health. The authors had no relevant financial disclosures.
SOURCE: Phang KG et al. Pediatrics. 2020 Jul 2. doi: 10.1542/peds.2019-3690.
FROM PEDIATRICS
Diagnostic criteria may miss some MIS-C cases, experts say
New data from active surveillance of the severe inflammatory condition associated with COVID-19 in previously healthy children provide further insight into the prevalence and course of the rare syndrome, but experts are concerned that current diagnostic criteria may not capture the true scope of the problem.
In separate reports published online June 29 in the New England Journal of Medicine, researchers from the New York State Department of Health and the Centers for Disease Control and Prevention (CDC) describe the epidemiology and clinical features of multisystem inflammatory syndrome in children (MIS-C) on the basis of information derived from targeted surveillance programs in New York State and across the country.
For the New York study, Elizabeth M. Dufort, MD, from the New York Department of Health in Albany and colleagues analyzed MIS-C surveillance data from 106 hospitals across the state. Of 191 suspected MIS-C cases reported to the Department of Health from March 1 through May 10, 99 met the state’s interim case definition of the condition and were included in the analysis.
The incidence rate for MIS-C was two cases per 100,000 individuals younger than 21 years, whereas the incidence rate of confirmed COVID-19 cases in this age group was 322 per 100,000. Most cases occurred approximately 1 month after the state’s COVID-19 peak.
“Among our patients, predominantly from the New York Metropolitan Region, 40% were black and 36% were Hispanic. This may be a reflection of the well-documented elevated incidence of SARS-CoV-2 infection among black and Hispanic communities,” the authors report.
All children presented with fever or chills, and most had tachycardia (97%) and gastrointestinal symptoms (80%). Rash (60%), conjunctival infection (56%), hypotension (32%), and mucosal changes (27%) were reported. Among all of the children, levels of inflammatory markers were elevated, including levels of C-reactive protein (100%), D-dimer (91%), and troponin (71%). More than one third of the patients (36%) were diagnosed with myocarditis, and an additional 16% had clinical myocarditis.
Of the full cohort, 80% of the children required intensive care, 62% received vasopressor support, and two children died.
The high prevalence of cardiac dysfunction or depression, coagulopathy, gastrointestinal symptoms, mild respiratory symptoms, and indications for supplemental oxygen in patients with MIS-C stands in contrast to the clinical picture observed in most acute cases of COVID-19 in hospitalized children, the authors write.
“Although most children have mild or no illness from SARS-CoV-2 infection, MIS-C may follow Covid-19 or asymptomatic SARS-CoV-2 infection. Recognition of the syndrome and early identification of children with MIS-C, including early monitoring of blood pressure and electrocardiographic and echocardiographic evaluation, could inform appropriate supportive care and other potential therapeutic options,” they continue.
The incidence of MIS-C among children infected with SARS-CoV-2 is unclear because children with COVID-19 often have mild or no symptoms and because children are not tested as frequently, the authors state. For this reason, “[i]t is crucial to establish surveillance for MIS-C cases, particularly in communities with higher levels of SARS-CoV-2 transmission.”
Important Differences From Kawasaki Disease
In a separate study, Leora R. Feldstein, MD, of the CDC, and colleagues report 186 cases of MIS-C collected through targeted surveillance of pediatric health centers in 26 US states from March 15 to May 20, 2020. As with the New York cohort, a disproportionate number of children in this cohort were black (25%) and Hispanic or Latino (31%).
Similar to the New York cohort, 80% of the children in this group required intensive care, 48% received vasoactive support, 20% required invasive mechanical ventilation, and four children died. Skin rashes, gastrointestinal symptoms, cardiovascular and hematologic effects, mucous changes, and elevations of inflammatory biomarkers were also similarly observed.
The researchers note that, although many of the features of MIS-C overlap with Kawasaki disease, there are some important differences, particularly with respect to the nature of cardiovascular involvement. “Approximately 5% of children with Kawasaki’s disease in the United States present with cardiovascular shock leading to vasopressor or inotropic support, as compared with 50% of the patients in our series,” the authors write.
In addition, coronary-artery aneurysms affect approximately one quarter of Kawasaki disease patients within 21 days of disease onset. “In our series, a maximum z score of 2.5 or higher in the left anterior descending or right coronary artery was reported in 8% of the patients overall and in 9% of patients with echocardiograms,” they report.
Additional differentiating features include patient age and race/ethnicity. Kawasaki disease occurs most commonly in children younger than 5 years. The median age in the multistate study was 8.3 years, and nearly half of the children in the New York cohort were in the 6- to 12-year age group. Further, Kawasaki disease is disproportionately prevalent in children of Asian descent.
Despite the differences, “until more is known about long-term cardiac sequelae of MIS-C, providers could consider following Kawasaki’s disease guidelines for follow-up, which recommend repeat echocardiographic imaging at 1 to 2 weeks.”
As was the case in the New York series, treatment in the multistate cohort most commonly included intravenous immunoglobulin and systemic glucocorticoids. Optimal management, however, will require a better understanding of the pathogenesis of MIS-C, Feldstein and colleagues write.
Questions Remain
With the accumulating data on this syndrome, the MIS-C picture seems to be getting incrementally clearer, but there is still much uncertainty, according to Michael Levin, FMedSci, PhD, from the Department of Infectious Disease, Imperial College London, United Kingdom.
“The recognition and description of new diseases often resemble the parable of the blind men and the elephant, with each declaring that the part of the beast they have touched fully defines it,” he writes in an accompanying editorial.
“As the coronavirus disease 2019 (Covid-19) pandemic has evolved, case reports have appeared describing children with unusual febrile illnesses that have features of Kawasaki’s disease, toxic shock syndrome, acute abdominal conditions, and encephalopathy, along with other reports of children with fever, elevated inflammatory markers, and multisystem involvement. It is now apparent that these reports were describing different clinical presentations of a new childhood inflammatory disorder.”
Although a consistent clinical picture is emerging, “[t]he published reports have used a variety of hastily developed case definitions based on the most severe cases, possibly missing less serious cases,” Levin writes. In particular, both the CDC and World Health Organization definitions require evidence of SARS-CoV-2 infection or exposure, which might contribute to underrecognition and underreporting because asymptomatic infections are common and antibody testing is not universally available.
“There is concern that children meeting current diagnostic criteria for MIS-C are the ‘tip of the iceberg,’ and a bigger problem may be lurking below the waterline,” Levin states. With approximately 1000 cases of the syndrome reported worldwide, “do we now have a clear picture of the new disorder, or as in the story of the blind men and the elephant, has only part of the beast been described?”
Adrienne Randolph, MD, of Boston Children’s Hospital, who is a coauthor of the multistate report, agrees that there is still much to learn about MIS-C before the whole beast can be understood. In an interview with Medscape Medical News, she listed the following key questions that have yet to be answered:
- Why do some children get MIS-C and not others?
- What is the long-term outcome of children with MIS-C?
- How can we differentiate MIS-C from acute COVID-19 infection in children with respiratory failure?
- Does MIS-C occur in young adults?
Randolph said her team is taking the best path forward toward answering these questions, including conducting a second study to identify risk factors for MIS-C and longer-term follow-up studies with the National Institutes of Health. “We are also getting consent to collect blood samples and look at other tests to help distinguish MIS-C from acute COVID-19 infection,” she said. She encouraged heightened awareness among physicians who care for young adults to consider MIS-C in patients aged 21 years and older who present with similar signs and symptoms.
On the basis of the answers to these and additional questions, the case definitions for MIS-C may need refinement to capture the wider spectrum of illness, Levin writes in his editorial. “The challenges of this new condition will now be to understand its pathophysiological mechanisms, to develop diagnostics, and to define the best treatment.”
Kleinman has received grants from the Health Services Resources Administration outside the submitted work. Maddux has received grants from the NIH/NICHD and the Francis Family Foundation outside the submitted work. Randolph has received grants from Genentech and personal fees from La Jolla Pharma outside the submitted work and others from the CDC during the conduct of the study.
This article first appeared on Medscape.com.
New data from active surveillance of the severe inflammatory condition associated with COVID-19 in previously healthy children provide further insight into the prevalence and course of the rare syndrome, but experts are concerned that current diagnostic criteria may not capture the true scope of the problem.
In separate reports published online June 29 in the New England Journal of Medicine, researchers from the New York State Department of Health and the Centers for Disease Control and Prevention (CDC) describe the epidemiology and clinical features of multisystem inflammatory syndrome in children (MIS-C) on the basis of information derived from targeted surveillance programs in New York State and across the country.
For the New York study, Elizabeth M. Dufort, MD, from the New York Department of Health in Albany and colleagues analyzed MIS-C surveillance data from 106 hospitals across the state. Of 191 suspected MIS-C cases reported to the Department of Health from March 1 through May 10, 99 met the state’s interim case definition of the condition and were included in the analysis.
The incidence rate for MIS-C was two cases per 100,000 individuals younger than 21 years, whereas the incidence rate of confirmed COVID-19 cases in this age group was 322 per 100,000. Most cases occurred approximately 1 month after the state’s COVID-19 peak.
“Among our patients, predominantly from the New York Metropolitan Region, 40% were black and 36% were Hispanic. This may be a reflection of the well-documented elevated incidence of SARS-CoV-2 infection among black and Hispanic communities,” the authors report.
All children presented with fever or chills, and most had tachycardia (97%) and gastrointestinal symptoms (80%). Rash (60%), conjunctival infection (56%), hypotension (32%), and mucosal changes (27%) were reported. Among all of the children, levels of inflammatory markers were elevated, including levels of C-reactive protein (100%), D-dimer (91%), and troponin (71%). More than one third of the patients (36%) were diagnosed with myocarditis, and an additional 16% had clinical myocarditis.
Of the full cohort, 80% of the children required intensive care, 62% received vasopressor support, and two children died.
The high prevalence of cardiac dysfunction or depression, coagulopathy, gastrointestinal symptoms, mild respiratory symptoms, and indications for supplemental oxygen in patients with MIS-C stands in contrast to the clinical picture observed in most acute cases of COVID-19 in hospitalized children, the authors write.
“Although most children have mild or no illness from SARS-CoV-2 infection, MIS-C may follow Covid-19 or asymptomatic SARS-CoV-2 infection. Recognition of the syndrome and early identification of children with MIS-C, including early monitoring of blood pressure and electrocardiographic and echocardiographic evaluation, could inform appropriate supportive care and other potential therapeutic options,” they continue.
The incidence of MIS-C among children infected with SARS-CoV-2 is unclear because children with COVID-19 often have mild or no symptoms and because children are not tested as frequently, the authors state. For this reason, “[i]t is crucial to establish surveillance for MIS-C cases, particularly in communities with higher levels of SARS-CoV-2 transmission.”
Important Differences From Kawasaki Disease
In a separate study, Leora R. Feldstein, MD, of the CDC, and colleagues report 186 cases of MIS-C collected through targeted surveillance of pediatric health centers in 26 US states from March 15 to May 20, 2020. As with the New York cohort, a disproportionate number of children in this cohort were black (25%) and Hispanic or Latino (31%).
Similar to the New York cohort, 80% of the children in this group required intensive care, 48% received vasoactive support, 20% required invasive mechanical ventilation, and four children died. Skin rashes, gastrointestinal symptoms, cardiovascular and hematologic effects, mucous changes, and elevations of inflammatory biomarkers were also similarly observed.
The researchers note that, although many of the features of MIS-C overlap with Kawasaki disease, there are some important differences, particularly with respect to the nature of cardiovascular involvement. “Approximately 5% of children with Kawasaki’s disease in the United States present with cardiovascular shock leading to vasopressor or inotropic support, as compared with 50% of the patients in our series,” the authors write.
In addition, coronary-artery aneurysms affect approximately one quarter of Kawasaki disease patients within 21 days of disease onset. “In our series, a maximum z score of 2.5 or higher in the left anterior descending or right coronary artery was reported in 8% of the patients overall and in 9% of patients with echocardiograms,” they report.
Additional differentiating features include patient age and race/ethnicity. Kawasaki disease occurs most commonly in children younger than 5 years. The median age in the multistate study was 8.3 years, and nearly half of the children in the New York cohort were in the 6- to 12-year age group. Further, Kawasaki disease is disproportionately prevalent in children of Asian descent.
Despite the differences, “until more is known about long-term cardiac sequelae of MIS-C, providers could consider following Kawasaki’s disease guidelines for follow-up, which recommend repeat echocardiographic imaging at 1 to 2 weeks.”
As was the case in the New York series, treatment in the multistate cohort most commonly included intravenous immunoglobulin and systemic glucocorticoids. Optimal management, however, will require a better understanding of the pathogenesis of MIS-C, Feldstein and colleagues write.
Questions Remain
With the accumulating data on this syndrome, the MIS-C picture seems to be getting incrementally clearer, but there is still much uncertainty, according to Michael Levin, FMedSci, PhD, from the Department of Infectious Disease, Imperial College London, United Kingdom.
“The recognition and description of new diseases often resemble the parable of the blind men and the elephant, with each declaring that the part of the beast they have touched fully defines it,” he writes in an accompanying editorial.
“As the coronavirus disease 2019 (Covid-19) pandemic has evolved, case reports have appeared describing children with unusual febrile illnesses that have features of Kawasaki’s disease, toxic shock syndrome, acute abdominal conditions, and encephalopathy, along with other reports of children with fever, elevated inflammatory markers, and multisystem involvement. It is now apparent that these reports were describing different clinical presentations of a new childhood inflammatory disorder.”
Although a consistent clinical picture is emerging, “[t]he published reports have used a variety of hastily developed case definitions based on the most severe cases, possibly missing less serious cases,” Levin writes. In particular, both the CDC and World Health Organization definitions require evidence of SARS-CoV-2 infection or exposure, which might contribute to underrecognition and underreporting because asymptomatic infections are common and antibody testing is not universally available.
“There is concern that children meeting current diagnostic criteria for MIS-C are the ‘tip of the iceberg,’ and a bigger problem may be lurking below the waterline,” Levin states. With approximately 1000 cases of the syndrome reported worldwide, “do we now have a clear picture of the new disorder, or as in the story of the blind men and the elephant, has only part of the beast been described?”
Adrienne Randolph, MD, of Boston Children’s Hospital, who is a coauthor of the multistate report, agrees that there is still much to learn about MIS-C before the whole beast can be understood. In an interview with Medscape Medical News, she listed the following key questions that have yet to be answered:
- Why do some children get MIS-C and not others?
- What is the long-term outcome of children with MIS-C?
- How can we differentiate MIS-C from acute COVID-19 infection in children with respiratory failure?
- Does MIS-C occur in young adults?
Randolph said her team is taking the best path forward toward answering these questions, including conducting a second study to identify risk factors for MIS-C and longer-term follow-up studies with the National Institutes of Health. “We are also getting consent to collect blood samples and look at other tests to help distinguish MIS-C from acute COVID-19 infection,” she said. She encouraged heightened awareness among physicians who care for young adults to consider MIS-C in patients aged 21 years and older who present with similar signs and symptoms.
On the basis of the answers to these and additional questions, the case definitions for MIS-C may need refinement to capture the wider spectrum of illness, Levin writes in his editorial. “The challenges of this new condition will now be to understand its pathophysiological mechanisms, to develop diagnostics, and to define the best treatment.”
Kleinman has received grants from the Health Services Resources Administration outside the submitted work. Maddux has received grants from the NIH/NICHD and the Francis Family Foundation outside the submitted work. Randolph has received grants from Genentech and personal fees from La Jolla Pharma outside the submitted work and others from the CDC during the conduct of the study.
This article first appeared on Medscape.com.
New data from active surveillance of the severe inflammatory condition associated with COVID-19 in previously healthy children provide further insight into the prevalence and course of the rare syndrome, but experts are concerned that current diagnostic criteria may not capture the true scope of the problem.
In separate reports published online June 29 in the New England Journal of Medicine, researchers from the New York State Department of Health and the Centers for Disease Control and Prevention (CDC) describe the epidemiology and clinical features of multisystem inflammatory syndrome in children (MIS-C) on the basis of information derived from targeted surveillance programs in New York State and across the country.
For the New York study, Elizabeth M. Dufort, MD, from the New York Department of Health in Albany and colleagues analyzed MIS-C surveillance data from 106 hospitals across the state. Of 191 suspected MIS-C cases reported to the Department of Health from March 1 through May 10, 99 met the state’s interim case definition of the condition and were included in the analysis.
The incidence rate for MIS-C was two cases per 100,000 individuals younger than 21 years, whereas the incidence rate of confirmed COVID-19 cases in this age group was 322 per 100,000. Most cases occurred approximately 1 month after the state’s COVID-19 peak.
“Among our patients, predominantly from the New York Metropolitan Region, 40% were black and 36% were Hispanic. This may be a reflection of the well-documented elevated incidence of SARS-CoV-2 infection among black and Hispanic communities,” the authors report.
All children presented with fever or chills, and most had tachycardia (97%) and gastrointestinal symptoms (80%). Rash (60%), conjunctival infection (56%), hypotension (32%), and mucosal changes (27%) were reported. Among all of the children, levels of inflammatory markers were elevated, including levels of C-reactive protein (100%), D-dimer (91%), and troponin (71%). More than one third of the patients (36%) were diagnosed with myocarditis, and an additional 16% had clinical myocarditis.
Of the full cohort, 80% of the children required intensive care, 62% received vasopressor support, and two children died.
The high prevalence of cardiac dysfunction or depression, coagulopathy, gastrointestinal symptoms, mild respiratory symptoms, and indications for supplemental oxygen in patients with MIS-C stands in contrast to the clinical picture observed in most acute cases of COVID-19 in hospitalized children, the authors write.
“Although most children have mild or no illness from SARS-CoV-2 infection, MIS-C may follow Covid-19 or asymptomatic SARS-CoV-2 infection. Recognition of the syndrome and early identification of children with MIS-C, including early monitoring of blood pressure and electrocardiographic and echocardiographic evaluation, could inform appropriate supportive care and other potential therapeutic options,” they continue.
The incidence of MIS-C among children infected with SARS-CoV-2 is unclear because children with COVID-19 often have mild or no symptoms and because children are not tested as frequently, the authors state. For this reason, “[i]t is crucial to establish surveillance for MIS-C cases, particularly in communities with higher levels of SARS-CoV-2 transmission.”
Important Differences From Kawasaki Disease
In a separate study, Leora R. Feldstein, MD, of the CDC, and colleagues report 186 cases of MIS-C collected through targeted surveillance of pediatric health centers in 26 US states from March 15 to May 20, 2020. As with the New York cohort, a disproportionate number of children in this cohort were black (25%) and Hispanic or Latino (31%).
Similar to the New York cohort, 80% of the children in this group required intensive care, 48% received vasoactive support, 20% required invasive mechanical ventilation, and four children died. Skin rashes, gastrointestinal symptoms, cardiovascular and hematologic effects, mucous changes, and elevations of inflammatory biomarkers were also similarly observed.
The researchers note that, although many of the features of MIS-C overlap with Kawasaki disease, there are some important differences, particularly with respect to the nature of cardiovascular involvement. “Approximately 5% of children with Kawasaki’s disease in the United States present with cardiovascular shock leading to vasopressor or inotropic support, as compared with 50% of the patients in our series,” the authors write.
In addition, coronary-artery aneurysms affect approximately one quarter of Kawasaki disease patients within 21 days of disease onset. “In our series, a maximum z score of 2.5 or higher in the left anterior descending or right coronary artery was reported in 8% of the patients overall and in 9% of patients with echocardiograms,” they report.
Additional differentiating features include patient age and race/ethnicity. Kawasaki disease occurs most commonly in children younger than 5 years. The median age in the multistate study was 8.3 years, and nearly half of the children in the New York cohort were in the 6- to 12-year age group. Further, Kawasaki disease is disproportionately prevalent in children of Asian descent.
Despite the differences, “until more is known about long-term cardiac sequelae of MIS-C, providers could consider following Kawasaki’s disease guidelines for follow-up, which recommend repeat echocardiographic imaging at 1 to 2 weeks.”
As was the case in the New York series, treatment in the multistate cohort most commonly included intravenous immunoglobulin and systemic glucocorticoids. Optimal management, however, will require a better understanding of the pathogenesis of MIS-C, Feldstein and colleagues write.
Questions Remain
With the accumulating data on this syndrome, the MIS-C picture seems to be getting incrementally clearer, but there is still much uncertainty, according to Michael Levin, FMedSci, PhD, from the Department of Infectious Disease, Imperial College London, United Kingdom.
“The recognition and description of new diseases often resemble the parable of the blind men and the elephant, with each declaring that the part of the beast they have touched fully defines it,” he writes in an accompanying editorial.
“As the coronavirus disease 2019 (Covid-19) pandemic has evolved, case reports have appeared describing children with unusual febrile illnesses that have features of Kawasaki’s disease, toxic shock syndrome, acute abdominal conditions, and encephalopathy, along with other reports of children with fever, elevated inflammatory markers, and multisystem involvement. It is now apparent that these reports were describing different clinical presentations of a new childhood inflammatory disorder.”
Although a consistent clinical picture is emerging, “[t]he published reports have used a variety of hastily developed case definitions based on the most severe cases, possibly missing less serious cases,” Levin writes. In particular, both the CDC and World Health Organization definitions require evidence of SARS-CoV-2 infection or exposure, which might contribute to underrecognition and underreporting because asymptomatic infections are common and antibody testing is not universally available.
“There is concern that children meeting current diagnostic criteria for MIS-C are the ‘tip of the iceberg,’ and a bigger problem may be lurking below the waterline,” Levin states. With approximately 1000 cases of the syndrome reported worldwide, “do we now have a clear picture of the new disorder, or as in the story of the blind men and the elephant, has only part of the beast been described?”
Adrienne Randolph, MD, of Boston Children’s Hospital, who is a coauthor of the multistate report, agrees that there is still much to learn about MIS-C before the whole beast can be understood. In an interview with Medscape Medical News, she listed the following key questions that have yet to be answered:
- Why do some children get MIS-C and not others?
- What is the long-term outcome of children with MIS-C?
- How can we differentiate MIS-C from acute COVID-19 infection in children with respiratory failure?
- Does MIS-C occur in young adults?
Randolph said her team is taking the best path forward toward answering these questions, including conducting a second study to identify risk factors for MIS-C and longer-term follow-up studies with the National Institutes of Health. “We are also getting consent to collect blood samples and look at other tests to help distinguish MIS-C from acute COVID-19 infection,” she said. She encouraged heightened awareness among physicians who care for young adults to consider MIS-C in patients aged 21 years and older who present with similar signs and symptoms.
On the basis of the answers to these and additional questions, the case definitions for MIS-C may need refinement to capture the wider spectrum of illness, Levin writes in his editorial. “The challenges of this new condition will now be to understand its pathophysiological mechanisms, to develop diagnostics, and to define the best treatment.”
Kleinman has received grants from the Health Services Resources Administration outside the submitted work. Maddux has received grants from the NIH/NICHD and the Francis Family Foundation outside the submitted work. Randolph has received grants from Genentech and personal fees from La Jolla Pharma outside the submitted work and others from the CDC during the conduct of the study.
This article first appeared on Medscape.com.
Declines in infant mortality tempered by disparities
Age-adjusted infant mortality dropped 11% from 2000 to 2017 in the United States, but the even larger decline for infants born to black women still left a death rate more than twice as high as those of white or Hispanic infants, according to a new analysis from the National Center for Health Statistics.

while the crude mortality rate fell 16% from 6.89 to 5.79, reported Anne K. Driscoll, PhD, and Danielle M. Ely, PhD, of the NCHS.
Over that same time period, age-adjusted infant mortality for births to black women went from 13.59 per 1,000 to 11.19, a drop of 18%. By comparison, age-adjusted mortality declined 7% from 5.59 per 1,000 for infants born to Hispanic women to 5.21 in 2017, they said in a National Vital Statistics Report.
Changes in maternal age distribution had an important effect on infant mortality. Women aged under 25 years, who have higher mortality rates, were less likely to give birth in 2017 than in 2000, and women aged 30-39 years, who have the lowest rates, made up a larger share of births in 2017, they pointed out.
It was, however, changes in age-specific mortality rates (ASMRs) that had the largest influence on the overall drop in the crude mortality rate, accounting for about two-thirds of the overall decline, the NCHS researchers said, noting that the effect varied by race and Hispanic origin.
Births to non-Hispanic white women mirrored the national situation: Approximately two-thirds (68.7%) of the decrease in infant mortality came from changes in ASMRs and one-third (31.3%) from changes in maternal age distribution. Among non-Hispanic black women, the distribution was 95.2% ASMRs and 4.8% age distribution, Dr. Driscoll and Dr. Ely reported based on data from the National Vital Statistics System.
The disparity between the two trends went even further for infants born to Hispanic women. Changes in ASMRs were responsible for 133.7% of the overall change in crude mortality versus –33.7% for changes in maternal age distribution. “If no changes occurred in the ASMRs, the changes in the maternal age distribution would have resulted in a higher mortality rate in 2017,” they explained.
The declines in the ASMRs may be related to incremental improved survival of preterm and low-birthweight infants in certain groups. “While little or no progress has been made to lower [these] two key risk factors for poor birth outcomes, progress has been made in lowering the mortality rates of at-risk infants across maternal age and race and Hispanic origin, resulting in lower ASMRs for all age groups,” the investigators suggested.
It also is possible that “changes in other factors, such as maternal education and cigarette smoking during pregnancy, may have indirectly resulted in declining ASMRs for all age groups over time,” they added.
SOURCE: Driscoll AK, Ely DM. National Vital Statistics Reports. 2020;69(5):1-18.
Age-adjusted infant mortality dropped 11% from 2000 to 2017 in the United States, but the even larger decline for infants born to black women still left a death rate more than twice as high as those of white or Hispanic infants, according to a new analysis from the National Center for Health Statistics.

while the crude mortality rate fell 16% from 6.89 to 5.79, reported Anne K. Driscoll, PhD, and Danielle M. Ely, PhD, of the NCHS.
Over that same time period, age-adjusted infant mortality for births to black women went from 13.59 per 1,000 to 11.19, a drop of 18%. By comparison, age-adjusted mortality declined 7% from 5.59 per 1,000 for infants born to Hispanic women to 5.21 in 2017, they said in a National Vital Statistics Report.
Changes in maternal age distribution had an important effect on infant mortality. Women aged under 25 years, who have higher mortality rates, were less likely to give birth in 2017 than in 2000, and women aged 30-39 years, who have the lowest rates, made up a larger share of births in 2017, they pointed out.
It was, however, changes in age-specific mortality rates (ASMRs) that had the largest influence on the overall drop in the crude mortality rate, accounting for about two-thirds of the overall decline, the NCHS researchers said, noting that the effect varied by race and Hispanic origin.
Births to non-Hispanic white women mirrored the national situation: Approximately two-thirds (68.7%) of the decrease in infant mortality came from changes in ASMRs and one-third (31.3%) from changes in maternal age distribution. Among non-Hispanic black women, the distribution was 95.2% ASMRs and 4.8% age distribution, Dr. Driscoll and Dr. Ely reported based on data from the National Vital Statistics System.
The disparity between the two trends went even further for infants born to Hispanic women. Changes in ASMRs were responsible for 133.7% of the overall change in crude mortality versus –33.7% for changes in maternal age distribution. “If no changes occurred in the ASMRs, the changes in the maternal age distribution would have resulted in a higher mortality rate in 2017,” they explained.
The declines in the ASMRs may be related to incremental improved survival of preterm and low-birthweight infants in certain groups. “While little or no progress has been made to lower [these] two key risk factors for poor birth outcomes, progress has been made in lowering the mortality rates of at-risk infants across maternal age and race and Hispanic origin, resulting in lower ASMRs for all age groups,” the investigators suggested.
It also is possible that “changes in other factors, such as maternal education and cigarette smoking during pregnancy, may have indirectly resulted in declining ASMRs for all age groups over time,” they added.
SOURCE: Driscoll AK, Ely DM. National Vital Statistics Reports. 2020;69(5):1-18.
Age-adjusted infant mortality dropped 11% from 2000 to 2017 in the United States, but the even larger decline for infants born to black women still left a death rate more than twice as high as those of white or Hispanic infants, according to a new analysis from the National Center for Health Statistics.

while the crude mortality rate fell 16% from 6.89 to 5.79, reported Anne K. Driscoll, PhD, and Danielle M. Ely, PhD, of the NCHS.
Over that same time period, age-adjusted infant mortality for births to black women went from 13.59 per 1,000 to 11.19, a drop of 18%. By comparison, age-adjusted mortality declined 7% from 5.59 per 1,000 for infants born to Hispanic women to 5.21 in 2017, they said in a National Vital Statistics Report.
Changes in maternal age distribution had an important effect on infant mortality. Women aged under 25 years, who have higher mortality rates, were less likely to give birth in 2017 than in 2000, and women aged 30-39 years, who have the lowest rates, made up a larger share of births in 2017, they pointed out.
It was, however, changes in age-specific mortality rates (ASMRs) that had the largest influence on the overall drop in the crude mortality rate, accounting for about two-thirds of the overall decline, the NCHS researchers said, noting that the effect varied by race and Hispanic origin.
Births to non-Hispanic white women mirrored the national situation: Approximately two-thirds (68.7%) of the decrease in infant mortality came from changes in ASMRs and one-third (31.3%) from changes in maternal age distribution. Among non-Hispanic black women, the distribution was 95.2% ASMRs and 4.8% age distribution, Dr. Driscoll and Dr. Ely reported based on data from the National Vital Statistics System.
The disparity between the two trends went even further for infants born to Hispanic women. Changes in ASMRs were responsible for 133.7% of the overall change in crude mortality versus –33.7% for changes in maternal age distribution. “If no changes occurred in the ASMRs, the changes in the maternal age distribution would have resulted in a higher mortality rate in 2017,” they explained.
The declines in the ASMRs may be related to incremental improved survival of preterm and low-birthweight infants in certain groups. “While little or no progress has been made to lower [these] two key risk factors for poor birth outcomes, progress has been made in lowering the mortality rates of at-risk infants across maternal age and race and Hispanic origin, resulting in lower ASMRs for all age groups,” the investigators suggested.
It also is possible that “changes in other factors, such as maternal education and cigarette smoking during pregnancy, may have indirectly resulted in declining ASMRs for all age groups over time,” they added.
SOURCE: Driscoll AK, Ely DM. National Vital Statistics Reports. 2020;69(5):1-18.
FDA approves new indications for pembrolizumab
The Food and Drug Administration recently announced two new types of cancer that can be treated by the anti–PD-1 antibody pembrolizumab.
The new indications expand the use of pembrolizumab (Keytruda) to include treatment of patients with unresectable or metastatic tumor mutational burden–high (TMB-H) solid tumors as well as patients with cutaneous squamous cell carcinoma (cSCC). The FDA announced the new indications just 8 days apart, on June 16 and June 24.
In addition, on June 29, the FDA approved a third new indication for pembrolizumab, this time as first-line treatment for patients with unresectable or metastatic microsatellite instability–high or mismatch repair–deficient colorectal cancer.
The new approvals add to a wide range of oncology indications for which pembrolizumab can be used.
Accelerated approval to treat solid tumors
The FDA granted accelerated approval for pembrolizumab to treat children and adults with unresectable or metastatic TMB-H solid tumors that progressed after previous treatment or in instances where there are no satisfactory alternative treatment options.
The tumor mutational burden must be confirmed by an FDA-approved test. To that end, the FDA approved the FoundationOneCDx assay, which is designed to help physicians determine which patients meet the threshold for TMB-H malignancies (10 or more mutations per megabase).
The efficacy of pembrolizumab in TMB-H solid tumors was investigated in 10 cohorts from the multicenter, open-label KEYNOTE-158 trial. Participants received 200 mg of pembrolizumab intravenously every 3 weeks until their disease progressed or they experienced unacceptable toxicity.
Within this population, 102 patients had tumors that met the TMB-H definition. In this group, the overall response rate was 29%, including a 25% partial response rate and a 4% complete response rate.
The median duration of response was not reached, but 57% of participants experienced a response lasting 12 months or longer, and 50% had a response lasting 24 months or longer.
The most common adverse events associated with pembrolizumab in this trial were fatigue, musculoskeletal pain, decreased appetite, pruritus, diarrhea, nausea, rash, pyrexia, cough, dyspnea, constipation, pain, and abdominal pain. Pembrolizumab is associated with immune-mediated side effects, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, and skin adverse reactions, the FDA noted.
Safety and efficacy of pembrolizumab in pediatric patients with TMB-H central nervous system cancers have not been established.
New option for recurrent or metastatic cSCC
Physicians treating patients with cSCC that is not curable by surgery or radiation now have pembrolizumab to consider as another treatment option.
The cSCC approval is based on results of the multicenter, open-label KEYNOTE-629 trial. The dosage regimen was 200 mg of pembrolizumab intravenously every 3 weeks until cancer progressed, unacceptable toxicity arose, or 24 months of treatment were completed.
The objective response rate was 34%, and the median duration of response was not reached.
Adverse events were similar to those occurring in patients who received pembrolizumab as a single agent in other clinical trials, the FDA noted.
The Food and Drug Administration recently announced two new types of cancer that can be treated by the anti–PD-1 antibody pembrolizumab.
The new indications expand the use of pembrolizumab (Keytruda) to include treatment of patients with unresectable or metastatic tumor mutational burden–high (TMB-H) solid tumors as well as patients with cutaneous squamous cell carcinoma (cSCC). The FDA announced the new indications just 8 days apart, on June 16 and June 24.
In addition, on June 29, the FDA approved a third new indication for pembrolizumab, this time as first-line treatment for patients with unresectable or metastatic microsatellite instability–high or mismatch repair–deficient colorectal cancer.
The new approvals add to a wide range of oncology indications for which pembrolizumab can be used.
Accelerated approval to treat solid tumors
The FDA granted accelerated approval for pembrolizumab to treat children and adults with unresectable or metastatic TMB-H solid tumors that progressed after previous treatment or in instances where there are no satisfactory alternative treatment options.
The tumor mutational burden must be confirmed by an FDA-approved test. To that end, the FDA approved the FoundationOneCDx assay, which is designed to help physicians determine which patients meet the threshold for TMB-H malignancies (10 or more mutations per megabase).
The efficacy of pembrolizumab in TMB-H solid tumors was investigated in 10 cohorts from the multicenter, open-label KEYNOTE-158 trial. Participants received 200 mg of pembrolizumab intravenously every 3 weeks until their disease progressed or they experienced unacceptable toxicity.
Within this population, 102 patients had tumors that met the TMB-H definition. In this group, the overall response rate was 29%, including a 25% partial response rate and a 4% complete response rate.
The median duration of response was not reached, but 57% of participants experienced a response lasting 12 months or longer, and 50% had a response lasting 24 months or longer.
The most common adverse events associated with pembrolizumab in this trial were fatigue, musculoskeletal pain, decreased appetite, pruritus, diarrhea, nausea, rash, pyrexia, cough, dyspnea, constipation, pain, and abdominal pain. Pembrolizumab is associated with immune-mediated side effects, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, and skin adverse reactions, the FDA noted.
Safety and efficacy of pembrolizumab in pediatric patients with TMB-H central nervous system cancers have not been established.
New option for recurrent or metastatic cSCC
Physicians treating patients with cSCC that is not curable by surgery or radiation now have pembrolizumab to consider as another treatment option.
The cSCC approval is based on results of the multicenter, open-label KEYNOTE-629 trial. The dosage regimen was 200 mg of pembrolizumab intravenously every 3 weeks until cancer progressed, unacceptable toxicity arose, or 24 months of treatment were completed.
The objective response rate was 34%, and the median duration of response was not reached.
Adverse events were similar to those occurring in patients who received pembrolizumab as a single agent in other clinical trials, the FDA noted.
The Food and Drug Administration recently announced two new types of cancer that can be treated by the anti–PD-1 antibody pembrolizumab.
The new indications expand the use of pembrolizumab (Keytruda) to include treatment of patients with unresectable or metastatic tumor mutational burden–high (TMB-H) solid tumors as well as patients with cutaneous squamous cell carcinoma (cSCC). The FDA announced the new indications just 8 days apart, on June 16 and June 24.
In addition, on June 29, the FDA approved a third new indication for pembrolizumab, this time as first-line treatment for patients with unresectable or metastatic microsatellite instability–high or mismatch repair–deficient colorectal cancer.
The new approvals add to a wide range of oncology indications for which pembrolizumab can be used.
Accelerated approval to treat solid tumors
The FDA granted accelerated approval for pembrolizumab to treat children and adults with unresectable or metastatic TMB-H solid tumors that progressed after previous treatment or in instances where there are no satisfactory alternative treatment options.
The tumor mutational burden must be confirmed by an FDA-approved test. To that end, the FDA approved the FoundationOneCDx assay, which is designed to help physicians determine which patients meet the threshold for TMB-H malignancies (10 or more mutations per megabase).
The efficacy of pembrolizumab in TMB-H solid tumors was investigated in 10 cohorts from the multicenter, open-label KEYNOTE-158 trial. Participants received 200 mg of pembrolizumab intravenously every 3 weeks until their disease progressed or they experienced unacceptable toxicity.
Within this population, 102 patients had tumors that met the TMB-H definition. In this group, the overall response rate was 29%, including a 25% partial response rate and a 4% complete response rate.
The median duration of response was not reached, but 57% of participants experienced a response lasting 12 months or longer, and 50% had a response lasting 24 months or longer.
The most common adverse events associated with pembrolizumab in this trial were fatigue, musculoskeletal pain, decreased appetite, pruritus, diarrhea, nausea, rash, pyrexia, cough, dyspnea, constipation, pain, and abdominal pain. Pembrolizumab is associated with immune-mediated side effects, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, and skin adverse reactions, the FDA noted.
Safety and efficacy of pembrolizumab in pediatric patients with TMB-H central nervous system cancers have not been established.
New option for recurrent or metastatic cSCC
Physicians treating patients with cSCC that is not curable by surgery or radiation now have pembrolizumab to consider as another treatment option.
The cSCC approval is based on results of the multicenter, open-label KEYNOTE-629 trial. The dosage regimen was 200 mg of pembrolizumab intravenously every 3 weeks until cancer progressed, unacceptable toxicity arose, or 24 months of treatment were completed.
The objective response rate was 34%, and the median duration of response was not reached.
Adverse events were similar to those occurring in patients who received pembrolizumab as a single agent in other clinical trials, the FDA noted.
Multiple Yellow-Brown Papules on the Penis
The Diagnosis: Eruptive Syringoma
A punch biopsy of a lesion on the penis was performed. Histopathologic examination revealed many tadpole-shaped cords of epithelial cells and small ducts in the dermis (Figure). Based on clinical and histopathological findings, a diagnosis of eruptive syringoma was made. The patient declined treatment.
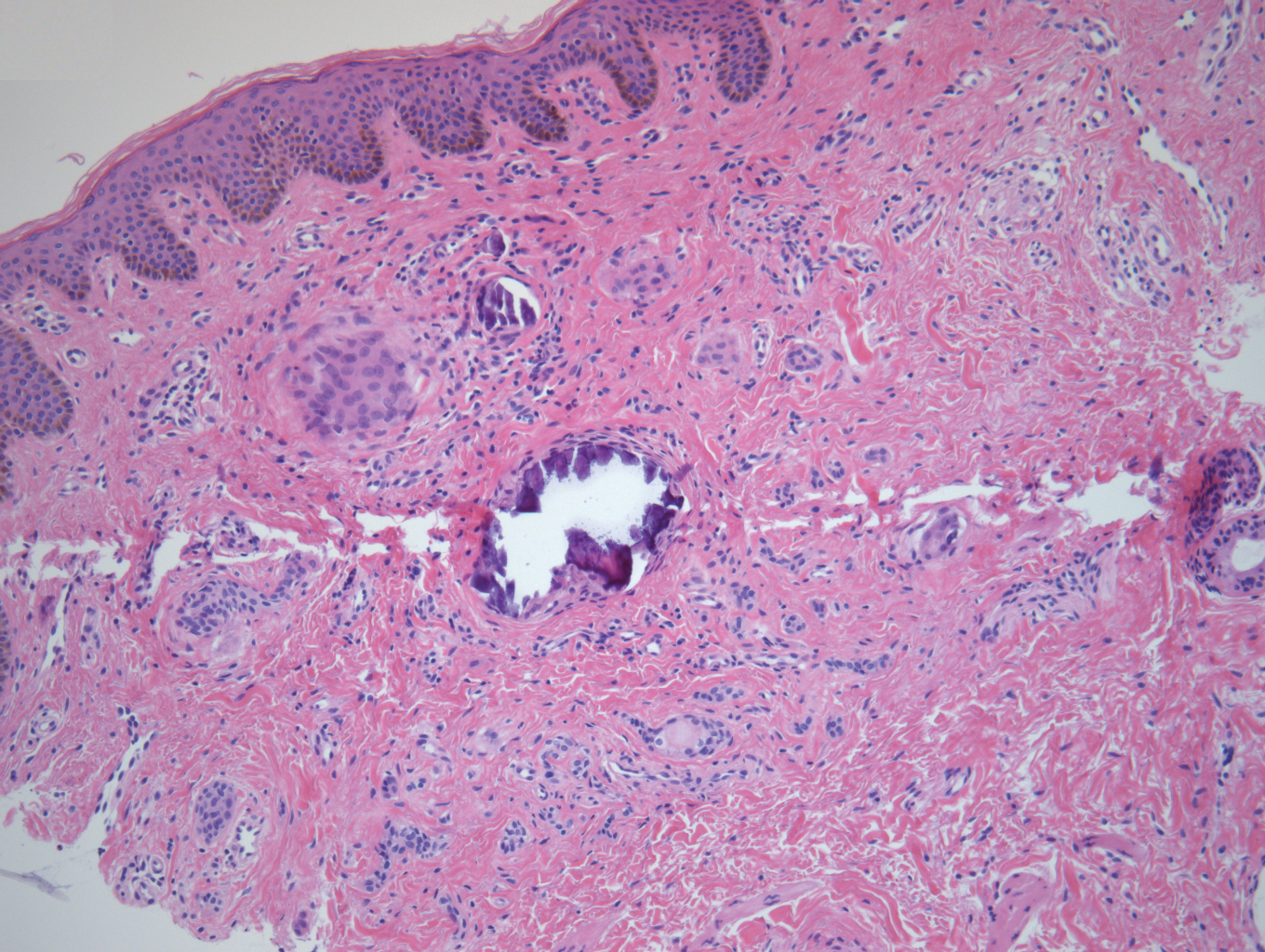
Syringomas are common benign eccrine neoplasms. They present clinically as small flesh-colored to brownish papules symmetrically distributed on the face, neck, trunk, pubic area, arms, and legs.1-3 Classic syringoma occurs more frequently in young adult women.1 Eruptive syringoma is a rare variant, and the age of onset ranges from 3 to 50 years.1-13 Eruptive syringoma is the term for multiple lesions that occur synchronously in any part of the body.1,4,13 The term eruptive is not the opposite of localized and refers to the time of onset of the lesion. There may be both a generalized eruptive syringoma or a localized eruptive syringoma depending on the distribution of the lesions.1 The most common site for syringoma occurrence is the eyelid; penile syringoma is extremely rare. Several cases of penile syringoma have been reported, but eruptive penile syringoma is rare.3,5-10,12,13
Histopathology is essential for the diagnosis of syringoma. Hematoxylin and eosin stain shows multiple small cystic ducts and epithelial cell nests in the dermis. Ductal structures sometimes appear tadpolelike or comma shaped depending on the section.1,2,7,12
The clinical differential diagnosis of syringoma includes sebaceous hyperplasia, verruca plana, molluscum contagiosum, bowenoid papulosis, condyloma acuminatum, lichen planus, lichen nitidus, milia, angiofibroma, epidermal cyst, calcinosis cutis, granuloma annulare, and sarcoidosis.3,8,12
Because syringoma is benign, treatment is not necessary unless there is a cosmetic problem.3,5,7,8,12 There is no satisfactory treatment of eruptive penile syringoma. Treatment options include topical tretinoin and adapalene, oral isotretinoin, cryotherapy, microelectrodesiccation with an epilating needle, dermabrasion, CO2 laser, and surgical excision.2,3,7,8,12
Adult patients with penile syringoma may be concerned about sexually transmitted diseases due to the appearance of the papules. If cosmesis is not an issue, clinicians should reassure the patient after a biopsy that the lesions are benign and self-limiting without recommending treatment.
- Ghanadan A, Khosravi M. Cutaneous syringoma: a clinicopathologic study of 34 new cases and review of the literature. Indian J Dermatol. 2013;58:326.
- Soler-Carrillo J, Estrach T, Mascaro JM. Eruptive syringoma: 27 new cases and review of the literature. J Eur Acad Dermatol Venereol. 2001;15:242-246.
- Baek JO, Jee HJ, Kim TK, et al. Eruptive penile syringomas spreading to the pubic area and lower abdomen. Ann Dermatol. 2013;25:116-118.
- Pruzan DL, Esterly NB, Prose NS. Eruptive syringoma. Arch Dermatol. 1989;125:1119-1120.
- Olson JM, Robles DT, Argenyi ZB, et al. Multiple penile syringomas. J Am Acad Dermatol. 2008;59(2 suppl 1):S46-S47.
- Petersson F, Mjornberg PA, Kazakov DV, et al. Eruptive syringoma of the penis. a report of 2 cases and a review of the literature. Am J Dermatopathol. 2009;31:436-438.
- Huang C, Wang W, Wu B. Multiple brownish papules on the penile shaft. Indian J Dermatol Venereol Leprol. 2011;77:404.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Vaca EE, Mundinger GS, Zelken JA, et al. Surgical excision of multiple penile syringomas with scrotal flap reconstruction. Eplasty. 2014;14:E21.
- Mitkov M, Balagula Y, Taube JM, et al. Plaque-like syringoma with involvement of deep reticular dermis. J Am Acad Dermatol. 2014;71:e206-207.
- Vaca EE, Mundinger GS, Zelken JA, et al. Surgical excision of multiple penile syringomas with scrotal flap reconstruction. Eplasty. 2014;14:e21.
- Dhossche JM, Brodell RT, Al Hmada Y, et al. Skin-colored papules of the penis. Pediatr Dermatol. 2015;32:145-146.
- Todd PS, Gordon SC, Rovner RL, et al. Eruptive penile syringomas in an adolescent: novel approach with serial microexcisions and suture-adhesive repair. Pediatr Dermatol. 2016;33:E57-E60.
The Diagnosis: Eruptive Syringoma
A punch biopsy of a lesion on the penis was performed. Histopathologic examination revealed many tadpole-shaped cords of epithelial cells and small ducts in the dermis (Figure). Based on clinical and histopathological findings, a diagnosis of eruptive syringoma was made. The patient declined treatment.

Syringomas are common benign eccrine neoplasms. They present clinically as small flesh-colored to brownish papules symmetrically distributed on the face, neck, trunk, pubic area, arms, and legs.1-3 Classic syringoma occurs more frequently in young adult women.1 Eruptive syringoma is a rare variant, and the age of onset ranges from 3 to 50 years.1-13 Eruptive syringoma is the term for multiple lesions that occur synchronously in any part of the body.1,4,13 The term eruptive is not the opposite of localized and refers to the time of onset of the lesion. There may be both a generalized eruptive syringoma or a localized eruptive syringoma depending on the distribution of the lesions.1 The most common site for syringoma occurrence is the eyelid; penile syringoma is extremely rare. Several cases of penile syringoma have been reported, but eruptive penile syringoma is rare.3,5-10,12,13
Histopathology is essential for the diagnosis of syringoma. Hematoxylin and eosin stain shows multiple small cystic ducts and epithelial cell nests in the dermis. Ductal structures sometimes appear tadpolelike or comma shaped depending on the section.1,2,7,12
The clinical differential diagnosis of syringoma includes sebaceous hyperplasia, verruca plana, molluscum contagiosum, bowenoid papulosis, condyloma acuminatum, lichen planus, lichen nitidus, milia, angiofibroma, epidermal cyst, calcinosis cutis, granuloma annulare, and sarcoidosis.3,8,12
Because syringoma is benign, treatment is not necessary unless there is a cosmetic problem.3,5,7,8,12 There is no satisfactory treatment of eruptive penile syringoma. Treatment options include topical tretinoin and adapalene, oral isotretinoin, cryotherapy, microelectrodesiccation with an epilating needle, dermabrasion, CO2 laser, and surgical excision.2,3,7,8,12
Adult patients with penile syringoma may be concerned about sexually transmitted diseases due to the appearance of the papules. If cosmesis is not an issue, clinicians should reassure the patient after a biopsy that the lesions are benign and self-limiting without recommending treatment.
The Diagnosis: Eruptive Syringoma
A punch biopsy of a lesion on the penis was performed. Histopathologic examination revealed many tadpole-shaped cords of epithelial cells and small ducts in the dermis (Figure). Based on clinical and histopathological findings, a diagnosis of eruptive syringoma was made. The patient declined treatment.

Syringomas are common benign eccrine neoplasms. They present clinically as small flesh-colored to brownish papules symmetrically distributed on the face, neck, trunk, pubic area, arms, and legs.1-3 Classic syringoma occurs more frequently in young adult women.1 Eruptive syringoma is a rare variant, and the age of onset ranges from 3 to 50 years.1-13 Eruptive syringoma is the term for multiple lesions that occur synchronously in any part of the body.1,4,13 The term eruptive is not the opposite of localized and refers to the time of onset of the lesion. There may be both a generalized eruptive syringoma or a localized eruptive syringoma depending on the distribution of the lesions.1 The most common site for syringoma occurrence is the eyelid; penile syringoma is extremely rare. Several cases of penile syringoma have been reported, but eruptive penile syringoma is rare.3,5-10,12,13
Histopathology is essential for the diagnosis of syringoma. Hematoxylin and eosin stain shows multiple small cystic ducts and epithelial cell nests in the dermis. Ductal structures sometimes appear tadpolelike or comma shaped depending on the section.1,2,7,12
The clinical differential diagnosis of syringoma includes sebaceous hyperplasia, verruca plana, molluscum contagiosum, bowenoid papulosis, condyloma acuminatum, lichen planus, lichen nitidus, milia, angiofibroma, epidermal cyst, calcinosis cutis, granuloma annulare, and sarcoidosis.3,8,12
Because syringoma is benign, treatment is not necessary unless there is a cosmetic problem.3,5,7,8,12 There is no satisfactory treatment of eruptive penile syringoma. Treatment options include topical tretinoin and adapalene, oral isotretinoin, cryotherapy, microelectrodesiccation with an epilating needle, dermabrasion, CO2 laser, and surgical excision.2,3,7,8,12
Adult patients with penile syringoma may be concerned about sexually transmitted diseases due to the appearance of the papules. If cosmesis is not an issue, clinicians should reassure the patient after a biopsy that the lesions are benign and self-limiting without recommending treatment.
- Ghanadan A, Khosravi M. Cutaneous syringoma: a clinicopathologic study of 34 new cases and review of the literature. Indian J Dermatol. 2013;58:326.
- Soler-Carrillo J, Estrach T, Mascaro JM. Eruptive syringoma: 27 new cases and review of the literature. J Eur Acad Dermatol Venereol. 2001;15:242-246.
- Baek JO, Jee HJ, Kim TK, et al. Eruptive penile syringomas spreading to the pubic area and lower abdomen. Ann Dermatol. 2013;25:116-118.
- Pruzan DL, Esterly NB, Prose NS. Eruptive syringoma. Arch Dermatol. 1989;125:1119-1120.
- Olson JM, Robles DT, Argenyi ZB, et al. Multiple penile syringomas. J Am Acad Dermatol. 2008;59(2 suppl 1):S46-S47.
- Petersson F, Mjornberg PA, Kazakov DV, et al. Eruptive syringoma of the penis. a report of 2 cases and a review of the literature. Am J Dermatopathol. 2009;31:436-438.
- Huang C, Wang W, Wu B. Multiple brownish papules on the penile shaft. Indian J Dermatol Venereol Leprol. 2011;77:404.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Vaca EE, Mundinger GS, Zelken JA, et al. Surgical excision of multiple penile syringomas with scrotal flap reconstruction. Eplasty. 2014;14:E21.
- Mitkov M, Balagula Y, Taube JM, et al. Plaque-like syringoma with involvement of deep reticular dermis. J Am Acad Dermatol. 2014;71:e206-207.
- Vaca EE, Mundinger GS, Zelken JA, et al. Surgical excision of multiple penile syringomas with scrotal flap reconstruction. Eplasty. 2014;14:e21.
- Dhossche JM, Brodell RT, Al Hmada Y, et al. Skin-colored papules of the penis. Pediatr Dermatol. 2015;32:145-146.
- Todd PS, Gordon SC, Rovner RL, et al. Eruptive penile syringomas in an adolescent: novel approach with serial microexcisions and suture-adhesive repair. Pediatr Dermatol. 2016;33:E57-E60.
- Ghanadan A, Khosravi M. Cutaneous syringoma: a clinicopathologic study of 34 new cases and review of the literature. Indian J Dermatol. 2013;58:326.
- Soler-Carrillo J, Estrach T, Mascaro JM. Eruptive syringoma: 27 new cases and review of the literature. J Eur Acad Dermatol Venereol. 2001;15:242-246.
- Baek JO, Jee HJ, Kim TK, et al. Eruptive penile syringomas spreading to the pubic area and lower abdomen. Ann Dermatol. 2013;25:116-118.
- Pruzan DL, Esterly NB, Prose NS. Eruptive syringoma. Arch Dermatol. 1989;125:1119-1120.
- Olson JM, Robles DT, Argenyi ZB, et al. Multiple penile syringomas. J Am Acad Dermatol. 2008;59(2 suppl 1):S46-S47.
- Petersson F, Mjornberg PA, Kazakov DV, et al. Eruptive syringoma of the penis. a report of 2 cases and a review of the literature. Am J Dermatopathol. 2009;31:436-438.
- Huang C, Wang W, Wu B. Multiple brownish papules on the penile shaft. Indian J Dermatol Venereol Leprol. 2011;77:404.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Vaca EE, Mundinger GS, Zelken JA, et al. Surgical excision of multiple penile syringomas with scrotal flap reconstruction. Eplasty. 2014;14:E21.
- Mitkov M, Balagula Y, Taube JM, et al. Plaque-like syringoma with involvement of deep reticular dermis. J Am Acad Dermatol. 2014;71:e206-207.
- Vaca EE, Mundinger GS, Zelken JA, et al. Surgical excision of multiple penile syringomas with scrotal flap reconstruction. Eplasty. 2014;14:e21.
- Dhossche JM, Brodell RT, Al Hmada Y, et al. Skin-colored papules of the penis. Pediatr Dermatol. 2015;32:145-146.
- Todd PS, Gordon SC, Rovner RL, et al. Eruptive penile syringomas in an adolescent: novel approach with serial microexcisions and suture-adhesive repair. Pediatr Dermatol. 2016;33:E57-E60.
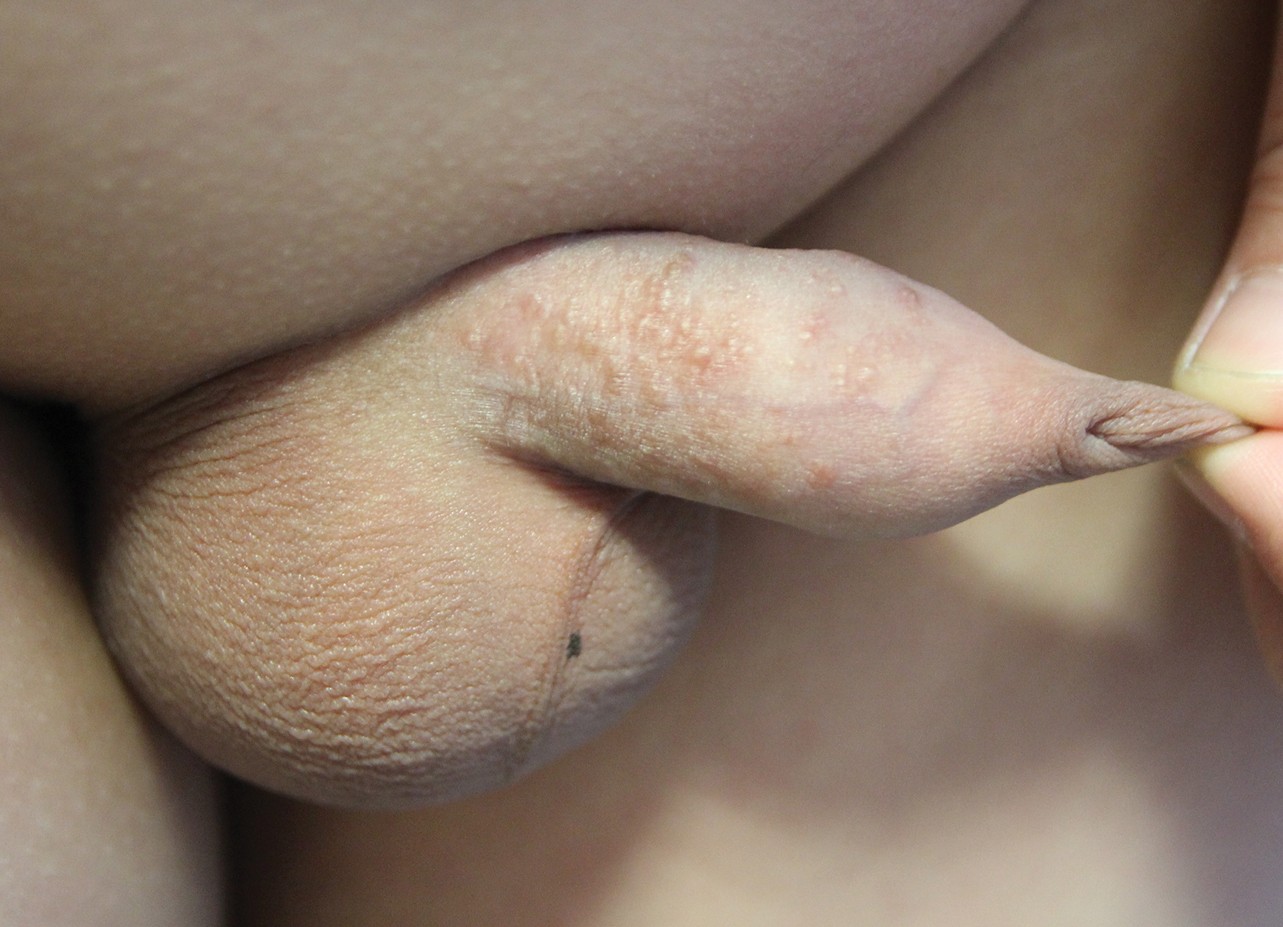
A 12-year-old boy presented with multiple asymptomatic, 0.1-cm, yellow-brown papules on the penile shaft of several years' duration. The lesions appeared suddenly. The patient had no history of trauma, injection, or an underlying disorder.