User login
PASI-75 with ixekizumab approaches 90% in pediatric psoriasis study
MADRID – The interleukin-17A inhibitor , Kim A. Papp, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The results bode well for an underserved population.
“I think all of us know that there is still a vulnerable population that remains a high-risk population because of the limited number of therapies available for them, and that is children,” said Dr. Papp, a dermatologist and president of Probity Medical Research, Inc., of Waterloo, Ont.
At present, etanercept, one of the earliest biologics to become available, and a relatively less effective one, is the only biologic approved for treatment of pediatric psoriasis. However, Lilly, which sponsored the phase 3 ixekizumab study, has announced that based upon the highly positive findings the company plans to seek Food and Drug Administration approval for an expanded indication for the medication in pediatric psoriasis. The company now markets ixekizumab for the approved indications of treatment of adults with moderate to severe plaque psoriasis, active psoriatic arthritis, or active ankylosing spondylitis.
The 12-week, double-blind, multicenter phase 3 trial known as IXORA-PEDS included 115 pediatric psoriasis patients randomized to weight-based ixekizumab, 30 on weight-based etanercept, and 58 on placebo. At the 12-week mark, everyone was switched to open-label ixekizumab in a long-term extension study. Children weighing less than 25 kg received a 40-mg loading dose of ixekizumab, followed by a maintenance dose of 20 mg by subcutaneous injection every 4 weeks. Patients weighing 25-50 kg got a starting dose of 80 mg, then 40 mg for maintenance therapy. Those who weighed more than 50 kg got the usual adult dosing: a 160-mg loading dose followed by 80 mg every 4 weeks. Etanercept was dosed at 0.8 mg/kg once weekly.
The coprimary endpoints were the proportion of subjects achieving a static Physician’s Global Assessment (sPGA) of 0 or 1 – that is, clear or almost clear skin – at week 12, and the PASI 75 response rate.
An sPGA of 0 or 1 at week 12 was documented in 81% of the ixekizumab group, 11% on placebo, and 40% of etanercept-treated patients, who on average had more severe baseline disease than did the other two groups.
The PASI 75 rate was 89% with ixekizumab, 25% for placebo, and 63% on etanercept. But Dr. Papp indicated that’s too low a bar. “I don’t think PASI 75s are the standard any longer,” he said.
More revealing was the PASI 90 rate: 78% with the IL-17A inhibitor, 5% in placebo-treated controls, and 40% with etanercept.
And then there’s the PASI 100 response rate: 50% with ixekizumab, 2% for placebo, and 17% for etanercept.
“I think this is very telling. I’ll leave it as a tantalizing comment that if one looks at the slope of the curve, it doesn’t yet seem to have reached its plateau at week 12 – and this is very similar to the pattern that we see in the adult population. I don’t have the long-term extension efficacy data, but I am, like you, very interested in seeing where this PASI 100 response rate finally plateaus,” Dr. Papp said.
He did, however, have the combined safety data for the 12-week double-blind phase plus the open-label extension, which he described as essentially the same as the adult experience. Injection-site reactions occurred in 19% of pediatric patients on ixekizumab, but they were generally mild and there were few if any treatment discontinuations for that reason. There was a 2% incidence of Crohn’s disease. Candidiasis and other infections were rare.
Seventy-one percent of the ixekizumab group had at least a 4-point improvement in itch on a 10-point self-rated scale by week 12, as did 20% of placebo-treated controls. A Dermatologic Life Quality Index score of 0 or 1 at week 12, indicative of no or minimal impact of psoriasis on quality of life, was documented in 64% of the ixekizumab group and 23% of controls.
Dr. Papp reported serving as a consultant, investigator, and/or speaker for Lilly and more than three dozen other pharmaceutical companies.
SOURCE: Papp KA. EADV Late breaker.
MADRID – The interleukin-17A inhibitor , Kim A. Papp, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The results bode well for an underserved population.
“I think all of us know that there is still a vulnerable population that remains a high-risk population because of the limited number of therapies available for them, and that is children,” said Dr. Papp, a dermatologist and president of Probity Medical Research, Inc., of Waterloo, Ont.
At present, etanercept, one of the earliest biologics to become available, and a relatively less effective one, is the only biologic approved for treatment of pediatric psoriasis. However, Lilly, which sponsored the phase 3 ixekizumab study, has announced that based upon the highly positive findings the company plans to seek Food and Drug Administration approval for an expanded indication for the medication in pediatric psoriasis. The company now markets ixekizumab for the approved indications of treatment of adults with moderate to severe plaque psoriasis, active psoriatic arthritis, or active ankylosing spondylitis.
The 12-week, double-blind, multicenter phase 3 trial known as IXORA-PEDS included 115 pediatric psoriasis patients randomized to weight-based ixekizumab, 30 on weight-based etanercept, and 58 on placebo. At the 12-week mark, everyone was switched to open-label ixekizumab in a long-term extension study. Children weighing less than 25 kg received a 40-mg loading dose of ixekizumab, followed by a maintenance dose of 20 mg by subcutaneous injection every 4 weeks. Patients weighing 25-50 kg got a starting dose of 80 mg, then 40 mg for maintenance therapy. Those who weighed more than 50 kg got the usual adult dosing: a 160-mg loading dose followed by 80 mg every 4 weeks. Etanercept was dosed at 0.8 mg/kg once weekly.
The coprimary endpoints were the proportion of subjects achieving a static Physician’s Global Assessment (sPGA) of 0 or 1 – that is, clear or almost clear skin – at week 12, and the PASI 75 response rate.
An sPGA of 0 or 1 at week 12 was documented in 81% of the ixekizumab group, 11% on placebo, and 40% of etanercept-treated patients, who on average had more severe baseline disease than did the other two groups.
The PASI 75 rate was 89% with ixekizumab, 25% for placebo, and 63% on etanercept. But Dr. Papp indicated that’s too low a bar. “I don’t think PASI 75s are the standard any longer,” he said.
More revealing was the PASI 90 rate: 78% with the IL-17A inhibitor, 5% in placebo-treated controls, and 40% with etanercept.
And then there’s the PASI 100 response rate: 50% with ixekizumab, 2% for placebo, and 17% for etanercept.
“I think this is very telling. I’ll leave it as a tantalizing comment that if one looks at the slope of the curve, it doesn’t yet seem to have reached its plateau at week 12 – and this is very similar to the pattern that we see in the adult population. I don’t have the long-term extension efficacy data, but I am, like you, very interested in seeing where this PASI 100 response rate finally plateaus,” Dr. Papp said.
He did, however, have the combined safety data for the 12-week double-blind phase plus the open-label extension, which he described as essentially the same as the adult experience. Injection-site reactions occurred in 19% of pediatric patients on ixekizumab, but they were generally mild and there were few if any treatment discontinuations for that reason. There was a 2% incidence of Crohn’s disease. Candidiasis and other infections were rare.
Seventy-one percent of the ixekizumab group had at least a 4-point improvement in itch on a 10-point self-rated scale by week 12, as did 20% of placebo-treated controls. A Dermatologic Life Quality Index score of 0 or 1 at week 12, indicative of no or minimal impact of psoriasis on quality of life, was documented in 64% of the ixekizumab group and 23% of controls.
Dr. Papp reported serving as a consultant, investigator, and/or speaker for Lilly and more than three dozen other pharmaceutical companies.
SOURCE: Papp KA. EADV Late breaker.
MADRID – The interleukin-17A inhibitor , Kim A. Papp, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The results bode well for an underserved population.
“I think all of us know that there is still a vulnerable population that remains a high-risk population because of the limited number of therapies available for them, and that is children,” said Dr. Papp, a dermatologist and president of Probity Medical Research, Inc., of Waterloo, Ont.
At present, etanercept, one of the earliest biologics to become available, and a relatively less effective one, is the only biologic approved for treatment of pediatric psoriasis. However, Lilly, which sponsored the phase 3 ixekizumab study, has announced that based upon the highly positive findings the company plans to seek Food and Drug Administration approval for an expanded indication for the medication in pediatric psoriasis. The company now markets ixekizumab for the approved indications of treatment of adults with moderate to severe plaque psoriasis, active psoriatic arthritis, or active ankylosing spondylitis.
The 12-week, double-blind, multicenter phase 3 trial known as IXORA-PEDS included 115 pediatric psoriasis patients randomized to weight-based ixekizumab, 30 on weight-based etanercept, and 58 on placebo. At the 12-week mark, everyone was switched to open-label ixekizumab in a long-term extension study. Children weighing less than 25 kg received a 40-mg loading dose of ixekizumab, followed by a maintenance dose of 20 mg by subcutaneous injection every 4 weeks. Patients weighing 25-50 kg got a starting dose of 80 mg, then 40 mg for maintenance therapy. Those who weighed more than 50 kg got the usual adult dosing: a 160-mg loading dose followed by 80 mg every 4 weeks. Etanercept was dosed at 0.8 mg/kg once weekly.
The coprimary endpoints were the proportion of subjects achieving a static Physician’s Global Assessment (sPGA) of 0 or 1 – that is, clear or almost clear skin – at week 12, and the PASI 75 response rate.
An sPGA of 0 or 1 at week 12 was documented in 81% of the ixekizumab group, 11% on placebo, and 40% of etanercept-treated patients, who on average had more severe baseline disease than did the other two groups.
The PASI 75 rate was 89% with ixekizumab, 25% for placebo, and 63% on etanercept. But Dr. Papp indicated that’s too low a bar. “I don’t think PASI 75s are the standard any longer,” he said.
More revealing was the PASI 90 rate: 78% with the IL-17A inhibitor, 5% in placebo-treated controls, and 40% with etanercept.
And then there’s the PASI 100 response rate: 50% with ixekizumab, 2% for placebo, and 17% for etanercept.
“I think this is very telling. I’ll leave it as a tantalizing comment that if one looks at the slope of the curve, it doesn’t yet seem to have reached its plateau at week 12 – and this is very similar to the pattern that we see in the adult population. I don’t have the long-term extension efficacy data, but I am, like you, very interested in seeing where this PASI 100 response rate finally plateaus,” Dr. Papp said.
He did, however, have the combined safety data for the 12-week double-blind phase plus the open-label extension, which he described as essentially the same as the adult experience. Injection-site reactions occurred in 19% of pediatric patients on ixekizumab, but they were generally mild and there were few if any treatment discontinuations for that reason. There was a 2% incidence of Crohn’s disease. Candidiasis and other infections were rare.
Seventy-one percent of the ixekizumab group had at least a 4-point improvement in itch on a 10-point self-rated scale by week 12, as did 20% of placebo-treated controls. A Dermatologic Life Quality Index score of 0 or 1 at week 12, indicative of no or minimal impact of psoriasis on quality of life, was documented in 64% of the ixekizumab group and 23% of controls.
Dr. Papp reported serving as a consultant, investigator, and/or speaker for Lilly and more than three dozen other pharmaceutical companies.
SOURCE: Papp KA. EADV Late breaker.
REPORTING FROM THE EADV CONGRESS
Understanding your LGBTQ patients’ needs
NEW ORLEANS – One of the most important things pediatricians can do to support their lesbian, gay, bisexual, transgender (LGBT) and other gender-nonconforming patients is to ask all their patients about their feelings, preferences and experiences when it comes to gender and sexuality, according to Julie Finger, MD, MPH.
It’s equally important not to make assumptions, she told attendees at the annual meeting of the American Academy of Pediatrics. Biology and sexual and gender identity and expression can be very diverse, she said. Specifically, doctors should not assume patients are heterosexual, that bisexuality is a phase, that orientation or attraction translates directly to behavior or vice versa, or that LGBTQ patients have unsupportive families or are engaging in risky behavior. Research suggests LGB youth have slightly higher rates of early sexual debut, sexual activity or multiple partners than straight or uncertain youth, but only marginally so.
Pediatricians also cannot assume a patient’s sexual orientation based on their partner’s gender or determine a patient’s sexual orientation or gender identity based on appearance – or even that either is the same as it was on the previous visit.
What doctors can be sure of is that they do have LGBTQ patients, said Dr. Finger, and assistant professor of clinical pediatrics at Tulane University in New Orleans. According to a 2016 Morbidity and Mortality Weekly Report (2016 Aug 12; 65[9]), about 1 in 10 students in grades 9-12 are a sexual minority. About 2% of respondents identify as gay or lesbian, 6% identify as bisexual and 3% say they aren’t sure.
Knowing the terminology
Dr. Finger defined key terminology regarding gender and sexuality. She first clarified that LGBT is not the full spectrum for sexual orientation. Pansexual (fluid attraction to any sex or gender) and asexual (lack of feeling sexual attraction) can also describe sexuality, and the Q on the end of LGBTQ is often an umbrella term for “queer” or “questioning” that encompasses anyone who fits outside conventional social norms of sexual identity and gender expression.
Sexual behaviors – which include “young men who have sex with men” and “young women who have sex with women” – do not necessarily correspond as one might expect with sexual orientation or identity, which is one’s concept of their romantic or sexual feelings, attractions and desires, again reinforcing the importance of asking patients their identity and preferences.
In terms of gender, a person’s natal or biologic gender is the one assigned people at birth based on their body parts and hormones. Gender identity is a person’s understanding of their own gender, and gender expression refers to how someone acts or presents themselves and communicates their gender within their culture.
Those who identify as “gender nonconforming, genderqueer, gender fluid, or nonbinary” see their gender on a spectrum, not within the binary “male” or “female.” A cisgender person’s gender identity matches both their biological sex assigned at birth and conventional cultural norms, while a transgender person’s gender differs from the sex they were assigned at birth. Transgender women (male to female, MTF) and men (female to men, FTM) go through the process of transition, a time that can occur in weeks or years when they shift from living as one gender to another.
While it’s unclear what leads to a person’s sexual orientation – likely a combination of genetic, hormonal and environmental factors—there is no question that sexual orientation is not a “choice,” Dr Finger said. Research has also clarified that one’s sexual orientation does not result from parenting behaviors or a history of sexual abuse.
“But I would urge all of you, instead of focusing on why someone is LGBTQ, to focus on what that means for them in their life,” Dr Finger said. “How is this bearing out in terms of their relationships and their behaviors, and how do they feel about it? How are they being supported by their family or their community, and how is it impacting their lives?”
She cited findings from a Human Rights Campaign survey in 2012 of 10,000 youth aged 13-17, which found that most LGBTQ respondents became aware of their same-sex attraction at 9 years of age, though the average age of disclosures is 16, an improvement from age 21 in the 1980s.
How and what to ask
Although children start becoming conscious of gender at ages 1-2, their sense of gender usually stabilizes by age 4.
“Who should we be screening for gender nonconformity? Quite frankly, all children, because all of them have some gender identity, so we should be asking them about that,” Dr Finger said.
When children are younger, doctors can ask parents about their child’s social interactions, forms of play, dress preferences, and mood. Questions for patients themselves, adapted for their age, might include, “Do you feel more like a girl, boy, neither or both?”, “How would you like to play, cut your hair and dress?” And “What name or pronoun (he or she) fits you?”
While such conversations do not necessarily need to happen annually, doctors should especially ask youth who dress or behave in non–gender-conforming ways or who appear to have mood, behavior or social difficulties.
To understand a patient’s sexuality, ask whether they are attracted to people of their own gender or sex, a different gender or sex, both or all genders or no one, or if they’re not sure yet. Doctors can then ask how comfortable they are with their attraction and whether they have told family members or friends about them.
Sexual behavior questions should be developmentally appropriate and lead to counsel but not judgment, Dr Finger said. Her method, with adjustments for age and development, starts, “There are many way of being sexual or intimate with someone: kissing, hugging and touching, and oral sex, anal sex and vaginal sex. Have you ever had any of these experiences? Which ones? With males or females or both, or other genders?”
Then she gets more specific while remaining sensitive. Doctors can ask younger children if they have held hands or cuddled with someone, if they have kissed someone, or if they have touched another person’s private parts. They can ask teens about oral sex, vaginal sex and anal sex and then gather more details about what parts went where, which helps determine what screenings or treatment options a patient may need or desire.
Doctors can use their judgment about whether to ask questions with parents in the room or not, but as kids grow older, it’s good practice to speak to patients without their caregivers present. Doctors should also explain the rules of confidentiality to their patients and be aware of the risks of “coming out,” including family discord or rejection, problems at school or work, social stigma, bullying and harassment, physical violence and risk-taking behaviors, such as substance use, self-injury and risky sexual behaviors. A HEADSSS screen can help doctors learn if any of these are present.
Making your practice inclusive and welcoming
Fewer than one in five teens who are “out” as LGBTQ have come out to their doctor, Dr Finger cited. Most are out to their friends and classmates, and more than half are out to their family, but teens are less likely to tell their doctors.
Research suggests one reason for this is the fact that pediatricians often don’t ask. One study found that only 20% of pediatricians discussed sexual orientation with their patients (Pediatrics 2010 Apr;125:e741-7). Similarly, only 30% of family physicians brought up sexual orientation, found another study (Fam. Med. 2001 May;33[5]:376-81). The studies found physicians more often discussed condoms, HIV, sexually transmitted infections, abstinence, violence, contraception or, in the case of family physicians, sexual behaviors, and relationships.
But another reason for not being out to doctors is a history of poor experiences. A Lambda Legal Survey in 2009 of 4,916 LGBT respondents found that 8% of LGB and 27% of transgender and gender nonconforming patients had been denied care because of their identity of orientation. Eleven percent said “providers refused to touch them or used excessive precautions,” Dr Finger reported. LGBTQ patients may fear the doctor’s reaction or not keeping their identity confidential. Patients may also have internalized shame or guilt due to societal norms or homophobia, and all these barriers can reduce LGBTQ people’s willingness to seek and access to competent care.
The first step to making LGBTQ patients comfortable in your practice is to confront your own personal biases, Dr Finger said. Understand what they are and that a provider’s discomfort, even unconscious, can be damaging to the patient-provider relationship.
“If you find that this is just not something that you’re going to be comfortable doing, at the very least, I would suggest that you find providers in your area who are comfortable working with this patient population and you refer your patients to them so that they can have a good, trusting patient-provider relationship with somebody who can provide the care that they need,” Dr Finger said.
The next step is creating a safe place with zero tolerance for insensitivity by training staff to be welcoming and inclusive, assuring patients confidentiality, providing support and resources and displaying LGBTQ-affirming materials. These youth need active, visible evidence that the office will be a safe place for them.
Ways pediatricians can communicate an inclusive environment include having gender-neutral restrooms, using “parent” instead of “mother/father” and using forms and EMR prompts with gender-neutral language or multiple options for gender selection.
Screening and LGBTQ patients’ health needs
LGB youth and those who aren’t sure of their sexual orientation tend to have higher rates of substance use, including tobacco, alcohol and illicit drugs, and are more often victims of rape and other sexual violence. Their rates of depressive symptoms, bullying victimization, and suicidality are also significantly higher than in their heterosexual cisgender peers. Homelessness rates are also considerably higher in LGBTQ youth than in heterosexual cisgender youth.
One thing pediatricians can do is work with parents to ensure a patient’s school is meeting their needs. The greater risks LGBTQ youth typically face are mediated by social support, resiliency, supportive friends and family and a supportive school environment, including inclusive curricula and supportive staff.
Lesbian and bisexual women are considerably more at risk for poor sexual or reproductive outcomes, Dr Finger said. Their rates of unplanned pregnancy are double that of straight women, contributing to their higher rates of emergency contraception and abortion. They are also more likely to have more partners (male and females), to have a younger sexual debut and to be forced into sex by a male partner—yet they are far less likely to perceive themselves as at risk for a sexually transmitted infection than their peers.
This patient population therefore may need contraception counseling, including discussing their current methods and reviewing their options, including emergency contraception and possibly an advance prescription. Dr Finger also suggests having male and female condoms available in the office.
Doctors should screen all their female patients, regardless of sexuality, for chlamydia and gonorrhea, and offer routine cervical cancer screening and the HPV vaccine, as recommended by the CDC. They might consider screening for trichomoniasis, bacterial vaginosis, herpes simplex, human papillomavirus and HIV.
For men who have sex with men, the CDC recommends HIV and syphilis serology, urine/pharyngeal/rectal gonorrhea nucleic acid amplification test (NAAT), urine/rectal chlamydia NAAT, and hepatitis C screening for those who are HIV-positive—all at least once a year.
For transgender patients, doctors need to assess their STI- and HIV-related risks based on their current anatomy and sexual behaviors.
Doctors should also consider discussing pre-exposure prophylaxis (PrEP) for any youth at high risk for HIV infection if they are at least 77 pounds (35 kg). Emtricitabine/tenofovir (Truvada, Descovy) reduces the chance of sexually acquired infection by 99%, and infection acquired via drug injection by 74% when taken as prescribed.
Resources
Dr Finger noted a range of resources for LGBTQ youth and their families and providers, including the Family Acceptance Project, Gay and Lesbian Medical Association, Gay, Lesbian and Straight Education Network, GLBTQ Legal Advocates and Defenders (GLAD), Human Rights Campaign, It Gets Better Project, LGBTQ Student Resources and Support, National Center for Lesbian Rights, Parents and Friends of Lesbians and Gays (PFLAG), Safe Schools Coalition and The Trevor Project (concerning suicide risk).
NEW ORLEANS – One of the most important things pediatricians can do to support their lesbian, gay, bisexual, transgender (LGBT) and other gender-nonconforming patients is to ask all their patients about their feelings, preferences and experiences when it comes to gender and sexuality, according to Julie Finger, MD, MPH.
It’s equally important not to make assumptions, she told attendees at the annual meeting of the American Academy of Pediatrics. Biology and sexual and gender identity and expression can be very diverse, she said. Specifically, doctors should not assume patients are heterosexual, that bisexuality is a phase, that orientation or attraction translates directly to behavior or vice versa, or that LGBTQ patients have unsupportive families or are engaging in risky behavior. Research suggests LGB youth have slightly higher rates of early sexual debut, sexual activity or multiple partners than straight or uncertain youth, but only marginally so.
Pediatricians also cannot assume a patient’s sexual orientation based on their partner’s gender or determine a patient’s sexual orientation or gender identity based on appearance – or even that either is the same as it was on the previous visit.
What doctors can be sure of is that they do have LGBTQ patients, said Dr. Finger, and assistant professor of clinical pediatrics at Tulane University in New Orleans. According to a 2016 Morbidity and Mortality Weekly Report (2016 Aug 12; 65[9]), about 1 in 10 students in grades 9-12 are a sexual minority. About 2% of respondents identify as gay or lesbian, 6% identify as bisexual and 3% say they aren’t sure.
Knowing the terminology
Dr. Finger defined key terminology regarding gender and sexuality. She first clarified that LGBT is not the full spectrum for sexual orientation. Pansexual (fluid attraction to any sex or gender) and asexual (lack of feeling sexual attraction) can also describe sexuality, and the Q on the end of LGBTQ is often an umbrella term for “queer” or “questioning” that encompasses anyone who fits outside conventional social norms of sexual identity and gender expression.
Sexual behaviors – which include “young men who have sex with men” and “young women who have sex with women” – do not necessarily correspond as one might expect with sexual orientation or identity, which is one’s concept of their romantic or sexual feelings, attractions and desires, again reinforcing the importance of asking patients their identity and preferences.
In terms of gender, a person’s natal or biologic gender is the one assigned people at birth based on their body parts and hormones. Gender identity is a person’s understanding of their own gender, and gender expression refers to how someone acts or presents themselves and communicates their gender within their culture.
Those who identify as “gender nonconforming, genderqueer, gender fluid, or nonbinary” see their gender on a spectrum, not within the binary “male” or “female.” A cisgender person’s gender identity matches both their biological sex assigned at birth and conventional cultural norms, while a transgender person’s gender differs from the sex they were assigned at birth. Transgender women (male to female, MTF) and men (female to men, FTM) go through the process of transition, a time that can occur in weeks or years when they shift from living as one gender to another.
While it’s unclear what leads to a person’s sexual orientation – likely a combination of genetic, hormonal and environmental factors—there is no question that sexual orientation is not a “choice,” Dr Finger said. Research has also clarified that one’s sexual orientation does not result from parenting behaviors or a history of sexual abuse.
“But I would urge all of you, instead of focusing on why someone is LGBTQ, to focus on what that means for them in their life,” Dr Finger said. “How is this bearing out in terms of their relationships and their behaviors, and how do they feel about it? How are they being supported by their family or their community, and how is it impacting their lives?”
She cited findings from a Human Rights Campaign survey in 2012 of 10,000 youth aged 13-17, which found that most LGBTQ respondents became aware of their same-sex attraction at 9 years of age, though the average age of disclosures is 16, an improvement from age 21 in the 1980s.
How and what to ask
Although children start becoming conscious of gender at ages 1-2, their sense of gender usually stabilizes by age 4.
“Who should we be screening for gender nonconformity? Quite frankly, all children, because all of them have some gender identity, so we should be asking them about that,” Dr Finger said.
When children are younger, doctors can ask parents about their child’s social interactions, forms of play, dress preferences, and mood. Questions for patients themselves, adapted for their age, might include, “Do you feel more like a girl, boy, neither or both?”, “How would you like to play, cut your hair and dress?” And “What name or pronoun (he or she) fits you?”
While such conversations do not necessarily need to happen annually, doctors should especially ask youth who dress or behave in non–gender-conforming ways or who appear to have mood, behavior or social difficulties.
To understand a patient’s sexuality, ask whether they are attracted to people of their own gender or sex, a different gender or sex, both or all genders or no one, or if they’re not sure yet. Doctors can then ask how comfortable they are with their attraction and whether they have told family members or friends about them.
Sexual behavior questions should be developmentally appropriate and lead to counsel but not judgment, Dr Finger said. Her method, with adjustments for age and development, starts, “There are many way of being sexual or intimate with someone: kissing, hugging and touching, and oral sex, anal sex and vaginal sex. Have you ever had any of these experiences? Which ones? With males or females or both, or other genders?”
Then she gets more specific while remaining sensitive. Doctors can ask younger children if they have held hands or cuddled with someone, if they have kissed someone, or if they have touched another person’s private parts. They can ask teens about oral sex, vaginal sex and anal sex and then gather more details about what parts went where, which helps determine what screenings or treatment options a patient may need or desire.
Doctors can use their judgment about whether to ask questions with parents in the room or not, but as kids grow older, it’s good practice to speak to patients without their caregivers present. Doctors should also explain the rules of confidentiality to their patients and be aware of the risks of “coming out,” including family discord or rejection, problems at school or work, social stigma, bullying and harassment, physical violence and risk-taking behaviors, such as substance use, self-injury and risky sexual behaviors. A HEADSSS screen can help doctors learn if any of these are present.
Making your practice inclusive and welcoming
Fewer than one in five teens who are “out” as LGBTQ have come out to their doctor, Dr Finger cited. Most are out to their friends and classmates, and more than half are out to their family, but teens are less likely to tell their doctors.
Research suggests one reason for this is the fact that pediatricians often don’t ask. One study found that only 20% of pediatricians discussed sexual orientation with their patients (Pediatrics 2010 Apr;125:e741-7). Similarly, only 30% of family physicians brought up sexual orientation, found another study (Fam. Med. 2001 May;33[5]:376-81). The studies found physicians more often discussed condoms, HIV, sexually transmitted infections, abstinence, violence, contraception or, in the case of family physicians, sexual behaviors, and relationships.
But another reason for not being out to doctors is a history of poor experiences. A Lambda Legal Survey in 2009 of 4,916 LGBT respondents found that 8% of LGB and 27% of transgender and gender nonconforming patients had been denied care because of their identity of orientation. Eleven percent said “providers refused to touch them or used excessive precautions,” Dr Finger reported. LGBTQ patients may fear the doctor’s reaction or not keeping their identity confidential. Patients may also have internalized shame or guilt due to societal norms or homophobia, and all these barriers can reduce LGBTQ people’s willingness to seek and access to competent care.
The first step to making LGBTQ patients comfortable in your practice is to confront your own personal biases, Dr Finger said. Understand what they are and that a provider’s discomfort, even unconscious, can be damaging to the patient-provider relationship.
“If you find that this is just not something that you’re going to be comfortable doing, at the very least, I would suggest that you find providers in your area who are comfortable working with this patient population and you refer your patients to them so that they can have a good, trusting patient-provider relationship with somebody who can provide the care that they need,” Dr Finger said.
The next step is creating a safe place with zero tolerance for insensitivity by training staff to be welcoming and inclusive, assuring patients confidentiality, providing support and resources and displaying LGBTQ-affirming materials. These youth need active, visible evidence that the office will be a safe place for them.
Ways pediatricians can communicate an inclusive environment include having gender-neutral restrooms, using “parent” instead of “mother/father” and using forms and EMR prompts with gender-neutral language or multiple options for gender selection.
Screening and LGBTQ patients’ health needs
LGB youth and those who aren’t sure of their sexual orientation tend to have higher rates of substance use, including tobacco, alcohol and illicit drugs, and are more often victims of rape and other sexual violence. Their rates of depressive symptoms, bullying victimization, and suicidality are also significantly higher than in their heterosexual cisgender peers. Homelessness rates are also considerably higher in LGBTQ youth than in heterosexual cisgender youth.
One thing pediatricians can do is work with parents to ensure a patient’s school is meeting their needs. The greater risks LGBTQ youth typically face are mediated by social support, resiliency, supportive friends and family and a supportive school environment, including inclusive curricula and supportive staff.
Lesbian and bisexual women are considerably more at risk for poor sexual or reproductive outcomes, Dr Finger said. Their rates of unplanned pregnancy are double that of straight women, contributing to their higher rates of emergency contraception and abortion. They are also more likely to have more partners (male and females), to have a younger sexual debut and to be forced into sex by a male partner—yet they are far less likely to perceive themselves as at risk for a sexually transmitted infection than their peers.
This patient population therefore may need contraception counseling, including discussing their current methods and reviewing their options, including emergency contraception and possibly an advance prescription. Dr Finger also suggests having male and female condoms available in the office.
Doctors should screen all their female patients, regardless of sexuality, for chlamydia and gonorrhea, and offer routine cervical cancer screening and the HPV vaccine, as recommended by the CDC. They might consider screening for trichomoniasis, bacterial vaginosis, herpes simplex, human papillomavirus and HIV.
For men who have sex with men, the CDC recommends HIV and syphilis serology, urine/pharyngeal/rectal gonorrhea nucleic acid amplification test (NAAT), urine/rectal chlamydia NAAT, and hepatitis C screening for those who are HIV-positive—all at least once a year.
For transgender patients, doctors need to assess their STI- and HIV-related risks based on their current anatomy and sexual behaviors.
Doctors should also consider discussing pre-exposure prophylaxis (PrEP) for any youth at high risk for HIV infection if they are at least 77 pounds (35 kg). Emtricitabine/tenofovir (Truvada, Descovy) reduces the chance of sexually acquired infection by 99%, and infection acquired via drug injection by 74% when taken as prescribed.
Resources
Dr Finger noted a range of resources for LGBTQ youth and their families and providers, including the Family Acceptance Project, Gay and Lesbian Medical Association, Gay, Lesbian and Straight Education Network, GLBTQ Legal Advocates and Defenders (GLAD), Human Rights Campaign, It Gets Better Project, LGBTQ Student Resources and Support, National Center for Lesbian Rights, Parents and Friends of Lesbians and Gays (PFLAG), Safe Schools Coalition and The Trevor Project (concerning suicide risk).
NEW ORLEANS – One of the most important things pediatricians can do to support their lesbian, gay, bisexual, transgender (LGBT) and other gender-nonconforming patients is to ask all their patients about their feelings, preferences and experiences when it comes to gender and sexuality, according to Julie Finger, MD, MPH.
It’s equally important not to make assumptions, she told attendees at the annual meeting of the American Academy of Pediatrics. Biology and sexual and gender identity and expression can be very diverse, she said. Specifically, doctors should not assume patients are heterosexual, that bisexuality is a phase, that orientation or attraction translates directly to behavior or vice versa, or that LGBTQ patients have unsupportive families or are engaging in risky behavior. Research suggests LGB youth have slightly higher rates of early sexual debut, sexual activity or multiple partners than straight or uncertain youth, but only marginally so.
Pediatricians also cannot assume a patient’s sexual orientation based on their partner’s gender or determine a patient’s sexual orientation or gender identity based on appearance – or even that either is the same as it was on the previous visit.
What doctors can be sure of is that they do have LGBTQ patients, said Dr. Finger, and assistant professor of clinical pediatrics at Tulane University in New Orleans. According to a 2016 Morbidity and Mortality Weekly Report (2016 Aug 12; 65[9]), about 1 in 10 students in grades 9-12 are a sexual minority. About 2% of respondents identify as gay or lesbian, 6% identify as bisexual and 3% say they aren’t sure.
Knowing the terminology
Dr. Finger defined key terminology regarding gender and sexuality. She first clarified that LGBT is not the full spectrum for sexual orientation. Pansexual (fluid attraction to any sex or gender) and asexual (lack of feeling sexual attraction) can also describe sexuality, and the Q on the end of LGBTQ is often an umbrella term for “queer” or “questioning” that encompasses anyone who fits outside conventional social norms of sexual identity and gender expression.
Sexual behaviors – which include “young men who have sex with men” and “young women who have sex with women” – do not necessarily correspond as one might expect with sexual orientation or identity, which is one’s concept of their romantic or sexual feelings, attractions and desires, again reinforcing the importance of asking patients their identity and preferences.
In terms of gender, a person’s natal or biologic gender is the one assigned people at birth based on their body parts and hormones. Gender identity is a person’s understanding of their own gender, and gender expression refers to how someone acts or presents themselves and communicates their gender within their culture.
Those who identify as “gender nonconforming, genderqueer, gender fluid, or nonbinary” see their gender on a spectrum, not within the binary “male” or “female.” A cisgender person’s gender identity matches both their biological sex assigned at birth and conventional cultural norms, while a transgender person’s gender differs from the sex they were assigned at birth. Transgender women (male to female, MTF) and men (female to men, FTM) go through the process of transition, a time that can occur in weeks or years when they shift from living as one gender to another.
While it’s unclear what leads to a person’s sexual orientation – likely a combination of genetic, hormonal and environmental factors—there is no question that sexual orientation is not a “choice,” Dr Finger said. Research has also clarified that one’s sexual orientation does not result from parenting behaviors or a history of sexual abuse.
“But I would urge all of you, instead of focusing on why someone is LGBTQ, to focus on what that means for them in their life,” Dr Finger said. “How is this bearing out in terms of their relationships and their behaviors, and how do they feel about it? How are they being supported by their family or their community, and how is it impacting their lives?”
She cited findings from a Human Rights Campaign survey in 2012 of 10,000 youth aged 13-17, which found that most LGBTQ respondents became aware of their same-sex attraction at 9 years of age, though the average age of disclosures is 16, an improvement from age 21 in the 1980s.
How and what to ask
Although children start becoming conscious of gender at ages 1-2, their sense of gender usually stabilizes by age 4.
“Who should we be screening for gender nonconformity? Quite frankly, all children, because all of them have some gender identity, so we should be asking them about that,” Dr Finger said.
When children are younger, doctors can ask parents about their child’s social interactions, forms of play, dress preferences, and mood. Questions for patients themselves, adapted for their age, might include, “Do you feel more like a girl, boy, neither or both?”, “How would you like to play, cut your hair and dress?” And “What name or pronoun (he or she) fits you?”
While such conversations do not necessarily need to happen annually, doctors should especially ask youth who dress or behave in non–gender-conforming ways or who appear to have mood, behavior or social difficulties.
To understand a patient’s sexuality, ask whether they are attracted to people of their own gender or sex, a different gender or sex, both or all genders or no one, or if they’re not sure yet. Doctors can then ask how comfortable they are with their attraction and whether they have told family members or friends about them.
Sexual behavior questions should be developmentally appropriate and lead to counsel but not judgment, Dr Finger said. Her method, with adjustments for age and development, starts, “There are many way of being sexual or intimate with someone: kissing, hugging and touching, and oral sex, anal sex and vaginal sex. Have you ever had any of these experiences? Which ones? With males or females or both, or other genders?”
Then she gets more specific while remaining sensitive. Doctors can ask younger children if they have held hands or cuddled with someone, if they have kissed someone, or if they have touched another person’s private parts. They can ask teens about oral sex, vaginal sex and anal sex and then gather more details about what parts went where, which helps determine what screenings or treatment options a patient may need or desire.
Doctors can use their judgment about whether to ask questions with parents in the room or not, but as kids grow older, it’s good practice to speak to patients without their caregivers present. Doctors should also explain the rules of confidentiality to their patients and be aware of the risks of “coming out,” including family discord or rejection, problems at school or work, social stigma, bullying and harassment, physical violence and risk-taking behaviors, such as substance use, self-injury and risky sexual behaviors. A HEADSSS screen can help doctors learn if any of these are present.
Making your practice inclusive and welcoming
Fewer than one in five teens who are “out” as LGBTQ have come out to their doctor, Dr Finger cited. Most are out to their friends and classmates, and more than half are out to their family, but teens are less likely to tell their doctors.
Research suggests one reason for this is the fact that pediatricians often don’t ask. One study found that only 20% of pediatricians discussed sexual orientation with their patients (Pediatrics 2010 Apr;125:e741-7). Similarly, only 30% of family physicians brought up sexual orientation, found another study (Fam. Med. 2001 May;33[5]:376-81). The studies found physicians more often discussed condoms, HIV, sexually transmitted infections, abstinence, violence, contraception or, in the case of family physicians, sexual behaviors, and relationships.
But another reason for not being out to doctors is a history of poor experiences. A Lambda Legal Survey in 2009 of 4,916 LGBT respondents found that 8% of LGB and 27% of transgender and gender nonconforming patients had been denied care because of their identity of orientation. Eleven percent said “providers refused to touch them or used excessive precautions,” Dr Finger reported. LGBTQ patients may fear the doctor’s reaction or not keeping their identity confidential. Patients may also have internalized shame or guilt due to societal norms or homophobia, and all these barriers can reduce LGBTQ people’s willingness to seek and access to competent care.
The first step to making LGBTQ patients comfortable in your practice is to confront your own personal biases, Dr Finger said. Understand what they are and that a provider’s discomfort, even unconscious, can be damaging to the patient-provider relationship.
“If you find that this is just not something that you’re going to be comfortable doing, at the very least, I would suggest that you find providers in your area who are comfortable working with this patient population and you refer your patients to them so that they can have a good, trusting patient-provider relationship with somebody who can provide the care that they need,” Dr Finger said.
The next step is creating a safe place with zero tolerance for insensitivity by training staff to be welcoming and inclusive, assuring patients confidentiality, providing support and resources and displaying LGBTQ-affirming materials. These youth need active, visible evidence that the office will be a safe place for them.
Ways pediatricians can communicate an inclusive environment include having gender-neutral restrooms, using “parent” instead of “mother/father” and using forms and EMR prompts with gender-neutral language or multiple options for gender selection.
Screening and LGBTQ patients’ health needs
LGB youth and those who aren’t sure of their sexual orientation tend to have higher rates of substance use, including tobacco, alcohol and illicit drugs, and are more often victims of rape and other sexual violence. Their rates of depressive symptoms, bullying victimization, and suicidality are also significantly higher than in their heterosexual cisgender peers. Homelessness rates are also considerably higher in LGBTQ youth than in heterosexual cisgender youth.
One thing pediatricians can do is work with parents to ensure a patient’s school is meeting their needs. The greater risks LGBTQ youth typically face are mediated by social support, resiliency, supportive friends and family and a supportive school environment, including inclusive curricula and supportive staff.
Lesbian and bisexual women are considerably more at risk for poor sexual or reproductive outcomes, Dr Finger said. Their rates of unplanned pregnancy are double that of straight women, contributing to their higher rates of emergency contraception and abortion. They are also more likely to have more partners (male and females), to have a younger sexual debut and to be forced into sex by a male partner—yet they are far less likely to perceive themselves as at risk for a sexually transmitted infection than their peers.
This patient population therefore may need contraception counseling, including discussing their current methods and reviewing their options, including emergency contraception and possibly an advance prescription. Dr Finger also suggests having male and female condoms available in the office.
Doctors should screen all their female patients, regardless of sexuality, for chlamydia and gonorrhea, and offer routine cervical cancer screening and the HPV vaccine, as recommended by the CDC. They might consider screening for trichomoniasis, bacterial vaginosis, herpes simplex, human papillomavirus and HIV.
For men who have sex with men, the CDC recommends HIV and syphilis serology, urine/pharyngeal/rectal gonorrhea nucleic acid amplification test (NAAT), urine/rectal chlamydia NAAT, and hepatitis C screening for those who are HIV-positive—all at least once a year.
For transgender patients, doctors need to assess their STI- and HIV-related risks based on their current anatomy and sexual behaviors.
Doctors should also consider discussing pre-exposure prophylaxis (PrEP) for any youth at high risk for HIV infection if they are at least 77 pounds (35 kg). Emtricitabine/tenofovir (Truvada, Descovy) reduces the chance of sexually acquired infection by 99%, and infection acquired via drug injection by 74% when taken as prescribed.
Resources
Dr Finger noted a range of resources for LGBTQ youth and their families and providers, including the Family Acceptance Project, Gay and Lesbian Medical Association, Gay, Lesbian and Straight Education Network, GLBTQ Legal Advocates and Defenders (GLAD), Human Rights Campaign, It Gets Better Project, LGBTQ Student Resources and Support, National Center for Lesbian Rights, Parents and Friends of Lesbians and Gays (PFLAG), Safe Schools Coalition and The Trevor Project (concerning suicide risk).
EXPERT ANALYSIS FROM AAP 2019
Recurrent intussusception rare in young children
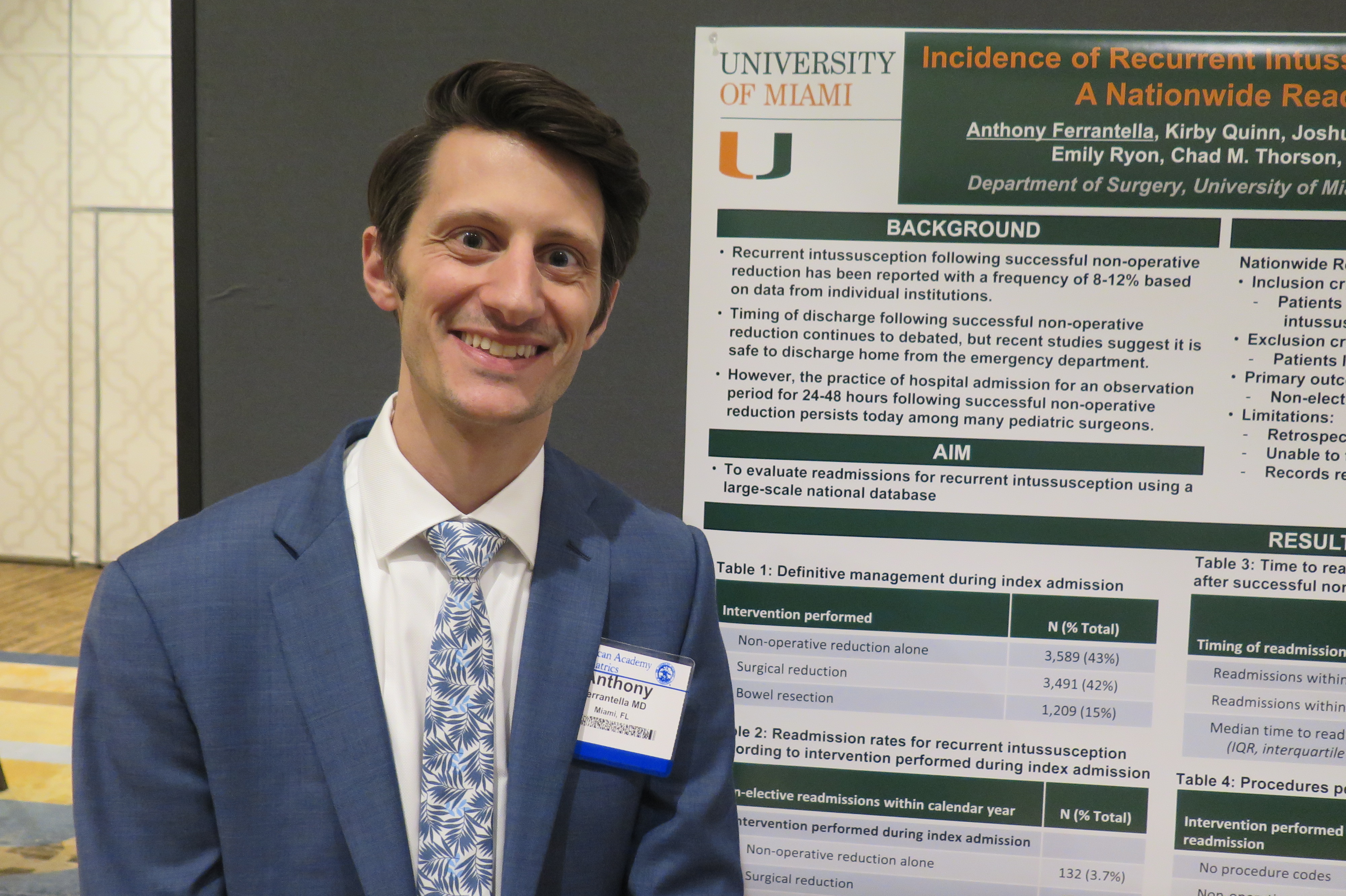
NEW ORLEANS – Recurrent intussusception after discharge may be far less common in young children than previously reported, and significant morbidity associated with the condition is rare, results from an analysis of national data show.
“Recurrent intussusception following successful nonoperative reduction has previously been reported with a frequency of 8-12% based on data from individual institutions,” researchers led by Anthony R. Ferrantella, MD, wrote in an abstract presented at the annual meeting of the American Academy of Pediatrics. “Timing of discharge following successful non-operative reduction continues to be debated, but recent studies suggest it is safe to discharge home from the emergency department. However, the practice of hospital admission for an observation period of 24-48 hours following successful nonoperative reduction persists today among many pediatric surgeons.”
In an effort to evaluate readmissions for recurrent intussusception in young children on a large scale, Dr. Ferrantella, a surgery resident at the University of Miami, and his colleagues queried the Nationwide Readmissions Database during 2010-2014 to identify children younger than 5 years of age diagnosed with ileocolic intussusception. They compared the management during index admission and frequency of readmissions for recurrent intussusception up to one year after discharge. They excluded patients lacking procedure data, weighted the results for national estimates, and used chi-square analysis to compare cohorts.
The search yielded 8,289 young children who were diagnosed with ileocolic intussusception during an index admission. Of these, 43% received definitive treatment with nonoperative reduction alone, 42% underwent surgical reduction without bowel resection, and 15% underwent surgery with bowel resection. Among the hospitals where patients were treated, 75% were large, 80% were not-for-profit, and 94% were metropolitan teaching hospitals.
The researchers found that readmission for recurrent intussusception was required for only 4% of patients managed with nonoperative reduction alone, 2% of patients who underwent surgical reduction, and 0% of those who underwent bowel resection. The median time to readmission was 4 days for those managed with nonoperative reduction only and 64 days for those managed with surgery.
Among patients managed with nonoperative reduction alone during index admission, 71% were again managed successfully with nonoperative reduction alone, 24 underwent surgical reduction, and only 5% required bowel resection. No deaths occurred during any readmissions.
The findings “suggest and support the idea that if you can successfully perform a nonoperative reduction on a child that comes in with an intussusception, you can safely discharge them,” Dr. Ferrantella said in an interview. “If you were to keep them in the hospital, the [rate] of recurrences are very low. Even when they do recur, only 30%-40% will happen within the first 24-48 hours, so the majority will not benefit from a hospital admission.”
He acknowledged certain limitations of the analysis, including that the data came from a retrospectively collected database and that he and his colleagues were unable to track readmissions across state lines.
Dr. Ferrantella reported having no financial disclosures.
SOURCE: Ferrantella A. AAP 2019, Section on Surgery session.
NEW ORLEANS – Recurrent intussusception after discharge may be far less common in young children than previously reported, and significant morbidity associated with the condition is rare, results from an analysis of national data show.
“Recurrent intussusception following successful nonoperative reduction has previously been reported with a frequency of 8-12% based on data from individual institutions,” researchers led by Anthony R. Ferrantella, MD, wrote in an abstract presented at the annual meeting of the American Academy of Pediatrics. “Timing of discharge following successful non-operative reduction continues to be debated, but recent studies suggest it is safe to discharge home from the emergency department. However, the practice of hospital admission for an observation period of 24-48 hours following successful nonoperative reduction persists today among many pediatric surgeons.”
In an effort to evaluate readmissions for recurrent intussusception in young children on a large scale, Dr. Ferrantella, a surgery resident at the University of Miami, and his colleagues queried the Nationwide Readmissions Database during 2010-2014 to identify children younger than 5 years of age diagnosed with ileocolic intussusception. They compared the management during index admission and frequency of readmissions for recurrent intussusception up to one year after discharge. They excluded patients lacking procedure data, weighted the results for national estimates, and used chi-square analysis to compare cohorts.
The search yielded 8,289 young children who were diagnosed with ileocolic intussusception during an index admission. Of these, 43% received definitive treatment with nonoperative reduction alone, 42% underwent surgical reduction without bowel resection, and 15% underwent surgery with bowel resection. Among the hospitals where patients were treated, 75% were large, 80% were not-for-profit, and 94% were metropolitan teaching hospitals.
The researchers found that readmission for recurrent intussusception was required for only 4% of patients managed with nonoperative reduction alone, 2% of patients who underwent surgical reduction, and 0% of those who underwent bowel resection. The median time to readmission was 4 days for those managed with nonoperative reduction only and 64 days for those managed with surgery.
Among patients managed with nonoperative reduction alone during index admission, 71% were again managed successfully with nonoperative reduction alone, 24 underwent surgical reduction, and only 5% required bowel resection. No deaths occurred during any readmissions.
The findings “suggest and support the idea that if you can successfully perform a nonoperative reduction on a child that comes in with an intussusception, you can safely discharge them,” Dr. Ferrantella said in an interview. “If you were to keep them in the hospital, the [rate] of recurrences are very low. Even when they do recur, only 30%-40% will happen within the first 24-48 hours, so the majority will not benefit from a hospital admission.”
He acknowledged certain limitations of the analysis, including that the data came from a retrospectively collected database and that he and his colleagues were unable to track readmissions across state lines.
Dr. Ferrantella reported having no financial disclosures.
SOURCE: Ferrantella A. AAP 2019, Section on Surgery session.
NEW ORLEANS – Recurrent intussusception after discharge may be far less common in young children than previously reported, and significant morbidity associated with the condition is rare, results from an analysis of national data show.
“Recurrent intussusception following successful nonoperative reduction has previously been reported with a frequency of 8-12% based on data from individual institutions,” researchers led by Anthony R. Ferrantella, MD, wrote in an abstract presented at the annual meeting of the American Academy of Pediatrics. “Timing of discharge following successful non-operative reduction continues to be debated, but recent studies suggest it is safe to discharge home from the emergency department. However, the practice of hospital admission for an observation period of 24-48 hours following successful nonoperative reduction persists today among many pediatric surgeons.”
In an effort to evaluate readmissions for recurrent intussusception in young children on a large scale, Dr. Ferrantella, a surgery resident at the University of Miami, and his colleagues queried the Nationwide Readmissions Database during 2010-2014 to identify children younger than 5 years of age diagnosed with ileocolic intussusception. They compared the management during index admission and frequency of readmissions for recurrent intussusception up to one year after discharge. They excluded patients lacking procedure data, weighted the results for national estimates, and used chi-square analysis to compare cohorts.
The search yielded 8,289 young children who were diagnosed with ileocolic intussusception during an index admission. Of these, 43% received definitive treatment with nonoperative reduction alone, 42% underwent surgical reduction without bowel resection, and 15% underwent surgery with bowel resection. Among the hospitals where patients were treated, 75% were large, 80% were not-for-profit, and 94% were metropolitan teaching hospitals.
The researchers found that readmission for recurrent intussusception was required for only 4% of patients managed with nonoperative reduction alone, 2% of patients who underwent surgical reduction, and 0% of those who underwent bowel resection. The median time to readmission was 4 days for those managed with nonoperative reduction only and 64 days for those managed with surgery.
Among patients managed with nonoperative reduction alone during index admission, 71% were again managed successfully with nonoperative reduction alone, 24 underwent surgical reduction, and only 5% required bowel resection. No deaths occurred during any readmissions.
The findings “suggest and support the idea that if you can successfully perform a nonoperative reduction on a child that comes in with an intussusception, you can safely discharge them,” Dr. Ferrantella said in an interview. “If you were to keep them in the hospital, the [rate] of recurrences are very low. Even when they do recur, only 30%-40% will happen within the first 24-48 hours, so the majority will not benefit from a hospital admission.”
He acknowledged certain limitations of the analysis, including that the data came from a retrospectively collected database and that he and his colleagues were unable to track readmissions across state lines.
Dr. Ferrantella reported having no financial disclosures.
SOURCE: Ferrantella A. AAP 2019, Section on Surgery session.
AT AAP 2016
Wandering is underrecognized, serious problem for autistic children
NEW ORLEANS – Nearly half of all children with autism spectrum disorder wander off from safe supervision at some point in their childhood or adolescence, reported Paul Lipkin, MD, at the annual meeting of the American Academy of Pediatrics.
Though such behavior is developmentally normal in toddlers, it’s rarer for older children to leave a supervised, safe space for a longer period than just running away for a bit, he said.
Far more than an inconvenience, wandering, also called elopement, puts these children at high risk for injury or victimization. In fact, statistics from a survey by the National Autism Foundation suggest that nearly a third of autism-related wandering cases resulted in death or serious enough injury to require medical attention, said Dr. Lipkin, an associate professor of pediatrics at the Kennedy Krieger Institute and Johns Hopkins Medicine in Baltimore.
“Drowning is overwhelmingly the main cause of death in children with autism,” he said, sharing the data from National Autism Association, which relied on parent report and media reports. In that data, 71% of deaths from autistic children who wandered from 2011-2016 were drowning, and of those deaths, 76% of the drownings occurred in a natural body of water or drainage water. At a distant second, 18% of deaths were traffic accidents. The remaining causes were being hit by a train (4%), hypothermia or hyperthermia (3%), falling (1%) or other trauma (3%) (J Autism Dev Disord. 2019 Mar 5. doi: 10.1007/s10803-019-03961-x).
Academic research has found similar statistics to those from the National Autism Association. In one study, 53% of autistic youth who attempted to run off succeeded and were missing long enough to cause safety concerns (Pediatrics. 2012 Nov;130[5]:870-7). Among these youth – representing about a quarter of all families surveyed in the study – the police were called in 31% of cases. In addition, 65% had a “close call” with a traffic injury and 24% had a close call with drowning.
The children wandered off in various settings, including home; another’s home; a store or other public place; or school, daycare or camp. A 2019 study found that 70% of parents reported their children wandering off from home at least once in the past 2 years (J Autism Dev Disorders. 2019 Mar 5; doi: 10.1107/s10803-019-03961-x).
Although most cases occur in children and teens, with the highest rate of death among children aged 5-9, the National Autism Association has received reports of wandering occur throughout autistic people’s lifetime.
Yet this issue doesn’t appear to be on the radar of many pediatricians, and those who are aware of it may not know the best strategies to share with parents to prevent wandering and subsequent injury, Dr Lipkin explained. In one study, only one-third of parents reported receiving guidance from a health provider related to wandering (J Dev Behav Pediatr. 2018 Sep;39[7]:538-46).
That research found that only 10% received advice from a pediatrician or other primary care provider, 12% received advice from a developmental pediatrician or neurologist and 10% received advance from a psychologist or psychiatrist. The largest source of guidance in that study was autism advocacy organizations, whom 22% of parents cited. Others included a teacher or other school staff member (15%), a personal contact (13%), law enforcement (8%) or another source (1%).
Role of the pediatrician
Pediatricians have an important role to play in prevention of elopement, Dr Lipkin said. They can screen autistic patients for wandering and elopement during visits, work with community stakeholders such as schools and law enforcement, advocate for awareness, and provider education and resources for families.
Perhaps the most valuable resource, he said, is the Big Red Safety Box, available from the National Autism Association. This resource, sponsored by more than a half dozen autism advocacy organizations, includes three digital safety toolkits: one for caregivers, one for first responders, and one for teachers. Parents can therefore share the toolkits for first responders and teachers with those respective community members.
Pediatricians can also help families develop a Family Wandering Emergency Plan (FWEP), a template for which is in the Big Red Safety Box. Parents and community members should know the steps to take if someone wanders: Stay calm, call 911, search nearby water first and then implement the FWEP.
It’s first helpful to understand why these youth wander off. In the National Autism Association survey, the most common reasons were to escape an anxious situation, particularly for those with Asperger’s, or simply to run, explore, or go to a favorite place, particularly among those with autism or pervasive developmental disorder-not otherwise specified (PDD-NOS).
Researchers have found similar reasons: 43% of elopement situations occurred when children were trying to escape an anxious situation, 39% left while in a stressful environment, and 24% were in an environment with conflict, found one study (J Autism Dev Disord. 2019 Mar 5. doi: 10.1007/s10803-019-03961-x).
Sensory overload was also a trigger, with 38% of elopements occurring when it was too noisy, and 34% when it was a generally uncomfortable sensory experience. Just over a quarter (27%) of children left when they were understimulated or in a “boring” environment, Dr Lipkin reported. The remaining reason was goal-directed: 27% left to pursue a special interest, 18% sought a place where they enjoyed playing, and 11% were after their favorite food.
Prevention Strategies
Most data about effective strategies to prevent wandering comes from research that relies on parents, Dr Lipkin said. In general, environmental interventions tend to be the most effective, and medication tends to be the least effective.
One study on elopement prevention found that 96% of caregivers use at least some type of intervention, and the vast majority (83%) were using environmental interventions such as dead bolts (51%), latches (49%) and gates (36%). An equal proportion used behavioral services (83%), such as a behavioral psychologist (41%), social stories (40%) or an aide (39%). Just under a third used an ID bracelet or shoe tag (31%), and 19% used GPS trackers, according to Dr. Lipkin.
Although parents reported environmental interventions to be very effective, 68% said they were highly burdensome, though the median cost over 2 years was less than $1,000. The least expensive intervention was home behavioral specialists (when covered by insurance) and school aides, and the most expensive and burdensome – albeit highly effective – was a service animal.
Interventions with the least cost effectiveness included security cameras and GPS trackers, which only 15% of parents reported as being effective.
Although nearly half of parents reported their child had taken any psychiatric medication (48%), only 16% had taken medication explicitly to prevent wandering. Few reported the medication was very effective, however. Among the small number who did (less than 10), lorazepam, diazepam and atomoxetine appeared best.
Teaching children survival skills, as developmentally appropriate and possible, can also help. These include swimming lessons as well as learning how to interact in traffic, knowing their home address, and learning how to navigate around their neighborhood.
Dr. Lipkin no disclosures and used no external funding for this presentation.
NEW ORLEANS – Nearly half of all children with autism spectrum disorder wander off from safe supervision at some point in their childhood or adolescence, reported Paul Lipkin, MD, at the annual meeting of the American Academy of Pediatrics.
Though such behavior is developmentally normal in toddlers, it’s rarer for older children to leave a supervised, safe space for a longer period than just running away for a bit, he said.
Far more than an inconvenience, wandering, also called elopement, puts these children at high risk for injury or victimization. In fact, statistics from a survey by the National Autism Foundation suggest that nearly a third of autism-related wandering cases resulted in death or serious enough injury to require medical attention, said Dr. Lipkin, an associate professor of pediatrics at the Kennedy Krieger Institute and Johns Hopkins Medicine in Baltimore.
“Drowning is overwhelmingly the main cause of death in children with autism,” he said, sharing the data from National Autism Association, which relied on parent report and media reports. In that data, 71% of deaths from autistic children who wandered from 2011-2016 were drowning, and of those deaths, 76% of the drownings occurred in a natural body of water or drainage water. At a distant second, 18% of deaths were traffic accidents. The remaining causes were being hit by a train (4%), hypothermia or hyperthermia (3%), falling (1%) or other trauma (3%) (J Autism Dev Disord. 2019 Mar 5. doi: 10.1007/s10803-019-03961-x).
Academic research has found similar statistics to those from the National Autism Association. In one study, 53% of autistic youth who attempted to run off succeeded and were missing long enough to cause safety concerns (Pediatrics. 2012 Nov;130[5]:870-7). Among these youth – representing about a quarter of all families surveyed in the study – the police were called in 31% of cases. In addition, 65% had a “close call” with a traffic injury and 24% had a close call with drowning.
The children wandered off in various settings, including home; another’s home; a store or other public place; or school, daycare or camp. A 2019 study found that 70% of parents reported their children wandering off from home at least once in the past 2 years (J Autism Dev Disorders. 2019 Mar 5; doi: 10.1107/s10803-019-03961-x).
Although most cases occur in children and teens, with the highest rate of death among children aged 5-9, the National Autism Association has received reports of wandering occur throughout autistic people’s lifetime.
Yet this issue doesn’t appear to be on the radar of many pediatricians, and those who are aware of it may not know the best strategies to share with parents to prevent wandering and subsequent injury, Dr Lipkin explained. In one study, only one-third of parents reported receiving guidance from a health provider related to wandering (J Dev Behav Pediatr. 2018 Sep;39[7]:538-46).
That research found that only 10% received advice from a pediatrician or other primary care provider, 12% received advice from a developmental pediatrician or neurologist and 10% received advance from a psychologist or psychiatrist. The largest source of guidance in that study was autism advocacy organizations, whom 22% of parents cited. Others included a teacher or other school staff member (15%), a personal contact (13%), law enforcement (8%) or another source (1%).
Role of the pediatrician
Pediatricians have an important role to play in prevention of elopement, Dr Lipkin said. They can screen autistic patients for wandering and elopement during visits, work with community stakeholders such as schools and law enforcement, advocate for awareness, and provider education and resources for families.
Perhaps the most valuable resource, he said, is the Big Red Safety Box, available from the National Autism Association. This resource, sponsored by more than a half dozen autism advocacy organizations, includes three digital safety toolkits: one for caregivers, one for first responders, and one for teachers. Parents can therefore share the toolkits for first responders and teachers with those respective community members.
Pediatricians can also help families develop a Family Wandering Emergency Plan (FWEP), a template for which is in the Big Red Safety Box. Parents and community members should know the steps to take if someone wanders: Stay calm, call 911, search nearby water first and then implement the FWEP.
It’s first helpful to understand why these youth wander off. In the National Autism Association survey, the most common reasons were to escape an anxious situation, particularly for those with Asperger’s, or simply to run, explore, or go to a favorite place, particularly among those with autism or pervasive developmental disorder-not otherwise specified (PDD-NOS).
Researchers have found similar reasons: 43% of elopement situations occurred when children were trying to escape an anxious situation, 39% left while in a stressful environment, and 24% were in an environment with conflict, found one study (J Autism Dev Disord. 2019 Mar 5. doi: 10.1007/s10803-019-03961-x).
Sensory overload was also a trigger, with 38% of elopements occurring when it was too noisy, and 34% when it was a generally uncomfortable sensory experience. Just over a quarter (27%) of children left when they were understimulated or in a “boring” environment, Dr Lipkin reported. The remaining reason was goal-directed: 27% left to pursue a special interest, 18% sought a place where they enjoyed playing, and 11% were after their favorite food.
Prevention Strategies
Most data about effective strategies to prevent wandering comes from research that relies on parents, Dr Lipkin said. In general, environmental interventions tend to be the most effective, and medication tends to be the least effective.
One study on elopement prevention found that 96% of caregivers use at least some type of intervention, and the vast majority (83%) were using environmental interventions such as dead bolts (51%), latches (49%) and gates (36%). An equal proportion used behavioral services (83%), such as a behavioral psychologist (41%), social stories (40%) or an aide (39%). Just under a third used an ID bracelet or shoe tag (31%), and 19% used GPS trackers, according to Dr. Lipkin.
Although parents reported environmental interventions to be very effective, 68% said they were highly burdensome, though the median cost over 2 years was less than $1,000. The least expensive intervention was home behavioral specialists (when covered by insurance) and school aides, and the most expensive and burdensome – albeit highly effective – was a service animal.
Interventions with the least cost effectiveness included security cameras and GPS trackers, which only 15% of parents reported as being effective.
Although nearly half of parents reported their child had taken any psychiatric medication (48%), only 16% had taken medication explicitly to prevent wandering. Few reported the medication was very effective, however. Among the small number who did (less than 10), lorazepam, diazepam and atomoxetine appeared best.
Teaching children survival skills, as developmentally appropriate and possible, can also help. These include swimming lessons as well as learning how to interact in traffic, knowing their home address, and learning how to navigate around their neighborhood.
Dr. Lipkin no disclosures and used no external funding for this presentation.
NEW ORLEANS – Nearly half of all children with autism spectrum disorder wander off from safe supervision at some point in their childhood or adolescence, reported Paul Lipkin, MD, at the annual meeting of the American Academy of Pediatrics.
Though such behavior is developmentally normal in toddlers, it’s rarer for older children to leave a supervised, safe space for a longer period than just running away for a bit, he said.
Far more than an inconvenience, wandering, also called elopement, puts these children at high risk for injury or victimization. In fact, statistics from a survey by the National Autism Foundation suggest that nearly a third of autism-related wandering cases resulted in death or serious enough injury to require medical attention, said Dr. Lipkin, an associate professor of pediatrics at the Kennedy Krieger Institute and Johns Hopkins Medicine in Baltimore.
“Drowning is overwhelmingly the main cause of death in children with autism,” he said, sharing the data from National Autism Association, which relied on parent report and media reports. In that data, 71% of deaths from autistic children who wandered from 2011-2016 were drowning, and of those deaths, 76% of the drownings occurred in a natural body of water or drainage water. At a distant second, 18% of deaths were traffic accidents. The remaining causes were being hit by a train (4%), hypothermia or hyperthermia (3%), falling (1%) or other trauma (3%) (J Autism Dev Disord. 2019 Mar 5. doi: 10.1007/s10803-019-03961-x).
Academic research has found similar statistics to those from the National Autism Association. In one study, 53% of autistic youth who attempted to run off succeeded and were missing long enough to cause safety concerns (Pediatrics. 2012 Nov;130[5]:870-7). Among these youth – representing about a quarter of all families surveyed in the study – the police were called in 31% of cases. In addition, 65% had a “close call” with a traffic injury and 24% had a close call with drowning.
The children wandered off in various settings, including home; another’s home; a store or other public place; or school, daycare or camp. A 2019 study found that 70% of parents reported their children wandering off from home at least once in the past 2 years (J Autism Dev Disorders. 2019 Mar 5; doi: 10.1107/s10803-019-03961-x).
Although most cases occur in children and teens, with the highest rate of death among children aged 5-9, the National Autism Association has received reports of wandering occur throughout autistic people’s lifetime.
Yet this issue doesn’t appear to be on the radar of many pediatricians, and those who are aware of it may not know the best strategies to share with parents to prevent wandering and subsequent injury, Dr Lipkin explained. In one study, only one-third of parents reported receiving guidance from a health provider related to wandering (J Dev Behav Pediatr. 2018 Sep;39[7]:538-46).
That research found that only 10% received advice from a pediatrician or other primary care provider, 12% received advice from a developmental pediatrician or neurologist and 10% received advance from a psychologist or psychiatrist. The largest source of guidance in that study was autism advocacy organizations, whom 22% of parents cited. Others included a teacher or other school staff member (15%), a personal contact (13%), law enforcement (8%) or another source (1%).
Role of the pediatrician
Pediatricians have an important role to play in prevention of elopement, Dr Lipkin said. They can screen autistic patients for wandering and elopement during visits, work with community stakeholders such as schools and law enforcement, advocate for awareness, and provider education and resources for families.
Perhaps the most valuable resource, he said, is the Big Red Safety Box, available from the National Autism Association. This resource, sponsored by more than a half dozen autism advocacy organizations, includes three digital safety toolkits: one for caregivers, one for first responders, and one for teachers. Parents can therefore share the toolkits for first responders and teachers with those respective community members.
Pediatricians can also help families develop a Family Wandering Emergency Plan (FWEP), a template for which is in the Big Red Safety Box. Parents and community members should know the steps to take if someone wanders: Stay calm, call 911, search nearby water first and then implement the FWEP.
It’s first helpful to understand why these youth wander off. In the National Autism Association survey, the most common reasons were to escape an anxious situation, particularly for those with Asperger’s, or simply to run, explore, or go to a favorite place, particularly among those with autism or pervasive developmental disorder-not otherwise specified (PDD-NOS).
Researchers have found similar reasons: 43% of elopement situations occurred when children were trying to escape an anxious situation, 39% left while in a stressful environment, and 24% were in an environment with conflict, found one study (J Autism Dev Disord. 2019 Mar 5. doi: 10.1007/s10803-019-03961-x).
Sensory overload was also a trigger, with 38% of elopements occurring when it was too noisy, and 34% when it was a generally uncomfortable sensory experience. Just over a quarter (27%) of children left when they were understimulated or in a “boring” environment, Dr Lipkin reported. The remaining reason was goal-directed: 27% left to pursue a special interest, 18% sought a place where they enjoyed playing, and 11% were after their favorite food.
Prevention Strategies
Most data about effective strategies to prevent wandering comes from research that relies on parents, Dr Lipkin said. In general, environmental interventions tend to be the most effective, and medication tends to be the least effective.
One study on elopement prevention found that 96% of caregivers use at least some type of intervention, and the vast majority (83%) were using environmental interventions such as dead bolts (51%), latches (49%) and gates (36%). An equal proportion used behavioral services (83%), such as a behavioral psychologist (41%), social stories (40%) or an aide (39%). Just under a third used an ID bracelet or shoe tag (31%), and 19% used GPS trackers, according to Dr. Lipkin.
Although parents reported environmental interventions to be very effective, 68% said they were highly burdensome, though the median cost over 2 years was less than $1,000. The least expensive intervention was home behavioral specialists (when covered by insurance) and school aides, and the most expensive and burdensome – albeit highly effective – was a service animal.
Interventions with the least cost effectiveness included security cameras and GPS trackers, which only 15% of parents reported as being effective.
Although nearly half of parents reported their child had taken any psychiatric medication (48%), only 16% had taken medication explicitly to prevent wandering. Few reported the medication was very effective, however. Among the small number who did (less than 10), lorazepam, diazepam and atomoxetine appeared best.
Teaching children survival skills, as developmentally appropriate and possible, can also help. These include swimming lessons as well as learning how to interact in traffic, knowing their home address, and learning how to navigate around their neighborhood.
Dr. Lipkin no disclosures and used no external funding for this presentation.
FROM AAP
Urban or rural, children’s gun-related injuries equally deadly
NEW ORLEANS – Accidental firearm-related injuries among children occur more frequently in rural than in urban locations, and nearly 60% of such cases are potentially preventable, results from a single-center study suggest.
Furthermore, these gun injuries carry the same mortality and disability risk.
“Firearm-related injury is an understudied topic,” lead study author Amelia Lucisano, MD, said in an interview in advance of the annual meeting of the American Academy of Pediatrics. “In particular there is a lack of granular level research on firearm-related injury in the population.”
At the meeting, she presented findings from an analysis which set out to investigate the location, preventability, and temporal trends of pediatric firearm-related injury in 184 patients age 18 and younger who were treated in the pediatric trauma program at University of Pittsburgh Medical Center during 2008-2017. Dr. Lucisano, a surgical resident at the university, and her colleagues focused their work on efforts to illustrate the differences and similarities in the demographics, injury-related characteristics, and outcomes between the rural and urban populations of children who are injured by firearms in Southwestern Pennsylvania. They classified the location as rural if the injury occurred outside the region’s central metropolitan county, and classified the injury as potentially preventable if the firearm was not stored securely and was used without permission. Statistical analyses included Wilcoxon rank-sum and chi-square analyses.
Of the 184 children who sustained a firearm-related injury during the study period, 43% occurred in a rural location. Compared with children who were injured in an urban setting, those who were injured in a rural setting were younger (a mean of 13 vs. 14 years; P = 0.0003), were more frequently white (81% vs. 14%; P less than 0.0001), and were more frequently injured by accident (70% vs. 15%; P less than 0.0001). They were also more likely to be injured by rifle or shotgun (24.1% vs. 6.67%; P = 0.001).
The rates of death or disability and lengths of stay did not differ significantly based on location of injury, occurring in 16.5% of rural and 13.3% of urban patients.
Nearly three-quarters of accidental injuries (72%) occurred on the gun-owner’s property and 58% were considered by the researchers to be potentially preventable.
“As expected, rural injuries are more frequently unintentional while urban injuries are more frequently assaults,” Dr. Lucisano said. “However, markers of injury severity and outcomes are equivalent between the groups, meaning that morbidity and mortality of injuries in the rural setting are similar to those in the urban setting.”
She emphasized that while clinician bias may be to consider rural firearm-based injuries as less severe, “our study shows that they carry the same burden of morbidity and mortality as urban injuries and thus should be cared for with the same intensity and anticipation of a possible poor outcome. Furthermore, the large number of potentially preventable injuries among those that were unintentional represents a significant burden of morbidity and mortality that could have been avoided through safer firearm storage. Programs to promote safe firearm storage should be targeted to populations that have high rates of potentially preventable injuries.”
Dr. Lucisano and her colleagues observed that the rates of all forms of firearm-related injury appear to be on the rise in both rural and urban areas: accidental, self-inflicted, and assault, in particular. She acknowledged certain limitations of the study, including its retrospective, single-center design. “We did not capture children who died in the field or who were treated at other hospitals, though as our center is the only pediatric Level 1 trauma center, we capture a large majority of pediatric trauma patients in the region,” she said.
The researchers reported having no disclosures.
SOURCE: Lucisano A. AAP 2019, Section on Surgery program.
NEW ORLEANS – Accidental firearm-related injuries among children occur more frequently in rural than in urban locations, and nearly 60% of such cases are potentially preventable, results from a single-center study suggest.
Furthermore, these gun injuries carry the same mortality and disability risk.
“Firearm-related injury is an understudied topic,” lead study author Amelia Lucisano, MD, said in an interview in advance of the annual meeting of the American Academy of Pediatrics. “In particular there is a lack of granular level research on firearm-related injury in the population.”
At the meeting, she presented findings from an analysis which set out to investigate the location, preventability, and temporal trends of pediatric firearm-related injury in 184 patients age 18 and younger who were treated in the pediatric trauma program at University of Pittsburgh Medical Center during 2008-2017. Dr. Lucisano, a surgical resident at the university, and her colleagues focused their work on efforts to illustrate the differences and similarities in the demographics, injury-related characteristics, and outcomes between the rural and urban populations of children who are injured by firearms in Southwestern Pennsylvania. They classified the location as rural if the injury occurred outside the region’s central metropolitan county, and classified the injury as potentially preventable if the firearm was not stored securely and was used without permission. Statistical analyses included Wilcoxon rank-sum and chi-square analyses.
Of the 184 children who sustained a firearm-related injury during the study period, 43% occurred in a rural location. Compared with children who were injured in an urban setting, those who were injured in a rural setting were younger (a mean of 13 vs. 14 years; P = 0.0003), were more frequently white (81% vs. 14%; P less than 0.0001), and were more frequently injured by accident (70% vs. 15%; P less than 0.0001). They were also more likely to be injured by rifle or shotgun (24.1% vs. 6.67%; P = 0.001).
The rates of death or disability and lengths of stay did not differ significantly based on location of injury, occurring in 16.5% of rural and 13.3% of urban patients.
Nearly three-quarters of accidental injuries (72%) occurred on the gun-owner’s property and 58% were considered by the researchers to be potentially preventable.
“As expected, rural injuries are more frequently unintentional while urban injuries are more frequently assaults,” Dr. Lucisano said. “However, markers of injury severity and outcomes are equivalent between the groups, meaning that morbidity and mortality of injuries in the rural setting are similar to those in the urban setting.”
She emphasized that while clinician bias may be to consider rural firearm-based injuries as less severe, “our study shows that they carry the same burden of morbidity and mortality as urban injuries and thus should be cared for with the same intensity and anticipation of a possible poor outcome. Furthermore, the large number of potentially preventable injuries among those that were unintentional represents a significant burden of morbidity and mortality that could have been avoided through safer firearm storage. Programs to promote safe firearm storage should be targeted to populations that have high rates of potentially preventable injuries.”
Dr. Lucisano and her colleagues observed that the rates of all forms of firearm-related injury appear to be on the rise in both rural and urban areas: accidental, self-inflicted, and assault, in particular. She acknowledged certain limitations of the study, including its retrospective, single-center design. “We did not capture children who died in the field or who were treated at other hospitals, though as our center is the only pediatric Level 1 trauma center, we capture a large majority of pediatric trauma patients in the region,” she said.
The researchers reported having no disclosures.
SOURCE: Lucisano A. AAP 2019, Section on Surgery program.
NEW ORLEANS – Accidental firearm-related injuries among children occur more frequently in rural than in urban locations, and nearly 60% of such cases are potentially preventable, results from a single-center study suggest.
Furthermore, these gun injuries carry the same mortality and disability risk.
“Firearm-related injury is an understudied topic,” lead study author Amelia Lucisano, MD, said in an interview in advance of the annual meeting of the American Academy of Pediatrics. “In particular there is a lack of granular level research on firearm-related injury in the population.”
At the meeting, she presented findings from an analysis which set out to investigate the location, preventability, and temporal trends of pediatric firearm-related injury in 184 patients age 18 and younger who were treated in the pediatric trauma program at University of Pittsburgh Medical Center during 2008-2017. Dr. Lucisano, a surgical resident at the university, and her colleagues focused their work on efforts to illustrate the differences and similarities in the demographics, injury-related characteristics, and outcomes between the rural and urban populations of children who are injured by firearms in Southwestern Pennsylvania. They classified the location as rural if the injury occurred outside the region’s central metropolitan county, and classified the injury as potentially preventable if the firearm was not stored securely and was used without permission. Statistical analyses included Wilcoxon rank-sum and chi-square analyses.
Of the 184 children who sustained a firearm-related injury during the study period, 43% occurred in a rural location. Compared with children who were injured in an urban setting, those who were injured in a rural setting were younger (a mean of 13 vs. 14 years; P = 0.0003), were more frequently white (81% vs. 14%; P less than 0.0001), and were more frequently injured by accident (70% vs. 15%; P less than 0.0001). They were also more likely to be injured by rifle or shotgun (24.1% vs. 6.67%; P = 0.001).
The rates of death or disability and lengths of stay did not differ significantly based on location of injury, occurring in 16.5% of rural and 13.3% of urban patients.
Nearly three-quarters of accidental injuries (72%) occurred on the gun-owner’s property and 58% were considered by the researchers to be potentially preventable.
“As expected, rural injuries are more frequently unintentional while urban injuries are more frequently assaults,” Dr. Lucisano said. “However, markers of injury severity and outcomes are equivalent between the groups, meaning that morbidity and mortality of injuries in the rural setting are similar to those in the urban setting.”
She emphasized that while clinician bias may be to consider rural firearm-based injuries as less severe, “our study shows that they carry the same burden of morbidity and mortality as urban injuries and thus should be cared for with the same intensity and anticipation of a possible poor outcome. Furthermore, the large number of potentially preventable injuries among those that were unintentional represents a significant burden of morbidity and mortality that could have been avoided through safer firearm storage. Programs to promote safe firearm storage should be targeted to populations that have high rates of potentially preventable injuries.”
Dr. Lucisano and her colleagues observed that the rates of all forms of firearm-related injury appear to be on the rise in both rural and urban areas: accidental, self-inflicted, and assault, in particular. She acknowledged certain limitations of the study, including its retrospective, single-center design. “We did not capture children who died in the field or who were treated at other hospitals, though as our center is the only pediatric Level 1 trauma center, we capture a large majority of pediatric trauma patients in the region,” she said.
The researchers reported having no disclosures.
SOURCE: Lucisano A. AAP 2019, Section on Surgery program.
AT AAP 2019
Adolescent lung inflammation may trigger later MS
STOCKHOLM – Scott Montgomery, PhD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
This is speculative, he readily acknowledged, but it is a hypothesis supported by multiple lines of evidence provided by separate Swedish national health care registry studies he has led that showed associations between pneumonia or infectious mononucleosis occurring in early adolescence and increased risk of later MS.
These findings are consistent with the well-established observations that two other causes of lung irritation – cigarette smoking and exposure to organic solvents – are also linked to increased risk of MS (Neurology. 2018 Jul 31;91[5]:e455-62), noted Dr. Montgomery, head of the clinical epidemiology research group at Örebro (Sweden) University.
Moreover, he and his coinvestigators also found in yet another Swedish national registry cohort study that one concussion during adolescence was independently associated with a statistically significant 1.22-fold increased risk of later MS, while two or more were linked to a 2.33-fold increased risk. In contrast, concussions occurring before age 11 years were not associated with any increased risk of MS, which suggests an age-defined period of susceptibility (Ann Neurol. 2017 Oct;82[4]:554-61).
“There seems to be greater brain resilience in childhood as compared to adolescence,” Dr. Montgomery commented.
The new Swedish registry pneumonia study included 6,109 Swedish MS patients and 49,479 controls matched for age, gender, and locale. In an analysis adjusted for education level and history of infectious mononucleosis, history of having pneumonia at age 11-15 years was independently associated with a 2.8-fold increased risk of subsequent MS. Pneumonia occurring at age 16-20 years was associated with a more modest 1.38-fold increased risk, which did not achieve statistical significance, while pneumonia up to age 5 years or at age 6-10 years conferred no increased risk. The investigators restricted their analysis to cases of pneumonia occurring up to age 20 years because that is younger than the typical age of MS onset. The age restriction sidestepped the potential for confounding by reverse causation since it is known that pneumonia occurs with increased frequency in patients with MS.
Because MS patients also have an increased risk of urinary tract infections, Dr. Montgomery and coinvestigators also analyzed the same pediatric data set for UTI rates broken down by 5-year age groups. Rates were similar in individuals who later developed MS and in controls, which suggests that the observed increase in MS risk associated with pneumonia in early adolescence was not an expression of an MS prodromal illness, he explained.
The investigators focused on pneumonia in childhood and adolescence as a potential trigger for MS because pneumonia results in more profound and prolonged inflammation than do other common respiratory illnesses. For example, pneumonia has been shown to be linked to increased risks of cardiovascular disease and chronic kidney disease for up to 5 years after the infection.
Developmentally, age 11-15 years is a period defined by peripubertal reorganization and synaptogenesis, while synaptic pruning and axonal myelination are on the agenda at age 16-20 years, Dr. Montgomery observed.
The study of infectious mononucleosis as a potential risk factor for MS included 4,527 Swedish MS patients and 3.2 million controls, all born during 1970-2000 and followed until 2014. In this analysis, infectious mononucleosis occurring at age 11-15 years was associated with the greatest risk of subsequent MS, with an associated 3.47-fold greater risk of the neurologic disease versus that seen in patients who did not have infectious mononucleosis at age 11-15 years
“It does look like a causal association between Epstein-Barr virus infection and subsequent MS,” according to Dr. Montgomery.
He noted that a plausible mechanism by which lung inflammation could predispose future MS has been put forth by German investigators. Using an animal model, they demonstrated that autoreactive T cells are prepared in bronchus-associated lymphoid tissue and attain a migratory profile allowing them to cross the blood-brain barrier and induce CNS autoimmune disease (Nature. 2012 Aug 30;488[7413]:675-9).
All of this, as Dr. Montgomery emphasized, is speculative at this point in regard to MS pathogenesis. What is not speculative, he continued, is the solid evidence that infection-related mortality after diagnosis of MS has gone down substantially in the current era of newer disease-modifying treatments, as he and his coinvestigators have demonstrated (Neurology. 2017 Aug 8;89[6]:555-62).
“People with MS, compared to the general population, are still at increased risk, but not nearly as much as the infection-related mortality risk present back in the 1960s-80s. So things have improved somewhat,” Dr. Montgomery said.
Which MS patients are at increased risk for mortality caused by infection? His Swedish national registry research demonstrates that the risk is essentially confined to patients with secondary or primary progressive MS or an Expanded Disability Status Scale score of 6 or more.
Another new study he presented at the meeting focused on the types of infections that are more common in a contemporary MS population than in MS-free individuals. This Swedish national cohort study included 6,602 patients diagnosed with MS during 2008-2016 and 61,828 age-, sex-, and location-matched controls. Infections serious enough to have resulted in hospitalization occurred 2.59 times more frequently in the MS population. The risk of meningitis and encephalitis was increased 6.16-fold, opportunistic infections were 2.72-fold more frequent, the risk of urinary tract and kidney infections was increased 2.44-fold, herpes virus infections were increased 2.32-fold, and the combined rate of pneumonia and influenza was roughly double that seen in the matched general population.
Dr. Montgomery reported receiving research funding from F. Hoffmann–La Roche, Novartis, and AstraZeneca and serving on an advisory board for IQVIA.
SOURCE: Montgomery S. ECTRIMS 2019, Abstract 270.
STOCKHOLM – Scott Montgomery, PhD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
This is speculative, he readily acknowledged, but it is a hypothesis supported by multiple lines of evidence provided by separate Swedish national health care registry studies he has led that showed associations between pneumonia or infectious mononucleosis occurring in early adolescence and increased risk of later MS.
These findings are consistent with the well-established observations that two other causes of lung irritation – cigarette smoking and exposure to organic solvents – are also linked to increased risk of MS (Neurology. 2018 Jul 31;91[5]:e455-62), noted Dr. Montgomery, head of the clinical epidemiology research group at Örebro (Sweden) University.
Moreover, he and his coinvestigators also found in yet another Swedish national registry cohort study that one concussion during adolescence was independently associated with a statistically significant 1.22-fold increased risk of later MS, while two or more were linked to a 2.33-fold increased risk. In contrast, concussions occurring before age 11 years were not associated with any increased risk of MS, which suggests an age-defined period of susceptibility (Ann Neurol. 2017 Oct;82[4]:554-61).
“There seems to be greater brain resilience in childhood as compared to adolescence,” Dr. Montgomery commented.
The new Swedish registry pneumonia study included 6,109 Swedish MS patients and 49,479 controls matched for age, gender, and locale. In an analysis adjusted for education level and history of infectious mononucleosis, history of having pneumonia at age 11-15 years was independently associated with a 2.8-fold increased risk of subsequent MS. Pneumonia occurring at age 16-20 years was associated with a more modest 1.38-fold increased risk, which did not achieve statistical significance, while pneumonia up to age 5 years or at age 6-10 years conferred no increased risk. The investigators restricted their analysis to cases of pneumonia occurring up to age 20 years because that is younger than the typical age of MS onset. The age restriction sidestepped the potential for confounding by reverse causation since it is known that pneumonia occurs with increased frequency in patients with MS.
Because MS patients also have an increased risk of urinary tract infections, Dr. Montgomery and coinvestigators also analyzed the same pediatric data set for UTI rates broken down by 5-year age groups. Rates were similar in individuals who later developed MS and in controls, which suggests that the observed increase in MS risk associated with pneumonia in early adolescence was not an expression of an MS prodromal illness, he explained.
The investigators focused on pneumonia in childhood and adolescence as a potential trigger for MS because pneumonia results in more profound and prolonged inflammation than do other common respiratory illnesses. For example, pneumonia has been shown to be linked to increased risks of cardiovascular disease and chronic kidney disease for up to 5 years after the infection.
Developmentally, age 11-15 years is a period defined by peripubertal reorganization and synaptogenesis, while synaptic pruning and axonal myelination are on the agenda at age 16-20 years, Dr. Montgomery observed.
The study of infectious mononucleosis as a potential risk factor for MS included 4,527 Swedish MS patients and 3.2 million controls, all born during 1970-2000 and followed until 2014. In this analysis, infectious mononucleosis occurring at age 11-15 years was associated with the greatest risk of subsequent MS, with an associated 3.47-fold greater risk of the neurologic disease versus that seen in patients who did not have infectious mononucleosis at age 11-15 years
“It does look like a causal association between Epstein-Barr virus infection and subsequent MS,” according to Dr. Montgomery.
He noted that a plausible mechanism by which lung inflammation could predispose future MS has been put forth by German investigators. Using an animal model, they demonstrated that autoreactive T cells are prepared in bronchus-associated lymphoid tissue and attain a migratory profile allowing them to cross the blood-brain barrier and induce CNS autoimmune disease (Nature. 2012 Aug 30;488[7413]:675-9).
All of this, as Dr. Montgomery emphasized, is speculative at this point in regard to MS pathogenesis. What is not speculative, he continued, is the solid evidence that infection-related mortality after diagnosis of MS has gone down substantially in the current era of newer disease-modifying treatments, as he and his coinvestigators have demonstrated (Neurology. 2017 Aug 8;89[6]:555-62).
“People with MS, compared to the general population, are still at increased risk, but not nearly as much as the infection-related mortality risk present back in the 1960s-80s. So things have improved somewhat,” Dr. Montgomery said.
Which MS patients are at increased risk for mortality caused by infection? His Swedish national registry research demonstrates that the risk is essentially confined to patients with secondary or primary progressive MS or an Expanded Disability Status Scale score of 6 or more.
Another new study he presented at the meeting focused on the types of infections that are more common in a contemporary MS population than in MS-free individuals. This Swedish national cohort study included 6,602 patients diagnosed with MS during 2008-2016 and 61,828 age-, sex-, and location-matched controls. Infections serious enough to have resulted in hospitalization occurred 2.59 times more frequently in the MS population. The risk of meningitis and encephalitis was increased 6.16-fold, opportunistic infections were 2.72-fold more frequent, the risk of urinary tract and kidney infections was increased 2.44-fold, herpes virus infections were increased 2.32-fold, and the combined rate of pneumonia and influenza was roughly double that seen in the matched general population.
Dr. Montgomery reported receiving research funding from F. Hoffmann–La Roche, Novartis, and AstraZeneca and serving on an advisory board for IQVIA.
SOURCE: Montgomery S. ECTRIMS 2019, Abstract 270.
STOCKHOLM – Scott Montgomery, PhD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
This is speculative, he readily acknowledged, but it is a hypothesis supported by multiple lines of evidence provided by separate Swedish national health care registry studies he has led that showed associations between pneumonia or infectious mononucleosis occurring in early adolescence and increased risk of later MS.
These findings are consistent with the well-established observations that two other causes of lung irritation – cigarette smoking and exposure to organic solvents – are also linked to increased risk of MS (Neurology. 2018 Jul 31;91[5]:e455-62), noted Dr. Montgomery, head of the clinical epidemiology research group at Örebro (Sweden) University.
Moreover, he and his coinvestigators also found in yet another Swedish national registry cohort study that one concussion during adolescence was independently associated with a statistically significant 1.22-fold increased risk of later MS, while two or more were linked to a 2.33-fold increased risk. In contrast, concussions occurring before age 11 years were not associated with any increased risk of MS, which suggests an age-defined period of susceptibility (Ann Neurol. 2017 Oct;82[4]:554-61).
“There seems to be greater brain resilience in childhood as compared to adolescence,” Dr. Montgomery commented.
The new Swedish registry pneumonia study included 6,109 Swedish MS patients and 49,479 controls matched for age, gender, and locale. In an analysis adjusted for education level and history of infectious mononucleosis, history of having pneumonia at age 11-15 years was independently associated with a 2.8-fold increased risk of subsequent MS. Pneumonia occurring at age 16-20 years was associated with a more modest 1.38-fold increased risk, which did not achieve statistical significance, while pneumonia up to age 5 years or at age 6-10 years conferred no increased risk. The investigators restricted their analysis to cases of pneumonia occurring up to age 20 years because that is younger than the typical age of MS onset. The age restriction sidestepped the potential for confounding by reverse causation since it is known that pneumonia occurs with increased frequency in patients with MS.
Because MS patients also have an increased risk of urinary tract infections, Dr. Montgomery and coinvestigators also analyzed the same pediatric data set for UTI rates broken down by 5-year age groups. Rates were similar in individuals who later developed MS and in controls, which suggests that the observed increase in MS risk associated with pneumonia in early adolescence was not an expression of an MS prodromal illness, he explained.
The investigators focused on pneumonia in childhood and adolescence as a potential trigger for MS because pneumonia results in more profound and prolonged inflammation than do other common respiratory illnesses. For example, pneumonia has been shown to be linked to increased risks of cardiovascular disease and chronic kidney disease for up to 5 years after the infection.
Developmentally, age 11-15 years is a period defined by peripubertal reorganization and synaptogenesis, while synaptic pruning and axonal myelination are on the agenda at age 16-20 years, Dr. Montgomery observed.
The study of infectious mononucleosis as a potential risk factor for MS included 4,527 Swedish MS patients and 3.2 million controls, all born during 1970-2000 and followed until 2014. In this analysis, infectious mononucleosis occurring at age 11-15 years was associated with the greatest risk of subsequent MS, with an associated 3.47-fold greater risk of the neurologic disease versus that seen in patients who did not have infectious mononucleosis at age 11-15 years
“It does look like a causal association between Epstein-Barr virus infection and subsequent MS,” according to Dr. Montgomery.
He noted that a plausible mechanism by which lung inflammation could predispose future MS has been put forth by German investigators. Using an animal model, they demonstrated that autoreactive T cells are prepared in bronchus-associated lymphoid tissue and attain a migratory profile allowing them to cross the blood-brain barrier and induce CNS autoimmune disease (Nature. 2012 Aug 30;488[7413]:675-9).
All of this, as Dr. Montgomery emphasized, is speculative at this point in regard to MS pathogenesis. What is not speculative, he continued, is the solid evidence that infection-related mortality after diagnosis of MS has gone down substantially in the current era of newer disease-modifying treatments, as he and his coinvestigators have demonstrated (Neurology. 2017 Aug 8;89[6]:555-62).
“People with MS, compared to the general population, are still at increased risk, but not nearly as much as the infection-related mortality risk present back in the 1960s-80s. So things have improved somewhat,” Dr. Montgomery said.
Which MS patients are at increased risk for mortality caused by infection? His Swedish national registry research demonstrates that the risk is essentially confined to patients with secondary or primary progressive MS or an Expanded Disability Status Scale score of 6 or more.
Another new study he presented at the meeting focused on the types of infections that are more common in a contemporary MS population than in MS-free individuals. This Swedish national cohort study included 6,602 patients diagnosed with MS during 2008-2016 and 61,828 age-, sex-, and location-matched controls. Infections serious enough to have resulted in hospitalization occurred 2.59 times more frequently in the MS population. The risk of meningitis and encephalitis was increased 6.16-fold, opportunistic infections were 2.72-fold more frequent, the risk of urinary tract and kidney infections was increased 2.44-fold, herpes virus infections were increased 2.32-fold, and the combined rate of pneumonia and influenza was roughly double that seen in the matched general population.
Dr. Montgomery reported receiving research funding from F. Hoffmann–La Roche, Novartis, and AstraZeneca and serving on an advisory board for IQVIA.
SOURCE: Montgomery S. ECTRIMS 2019, Abstract 270.
REPORTING FROM ECTRIMS 2019
Researchers seek to characterize pediatric new daily persistent headache
CHARLOTTE, N.C. – according to research presented at the 48th national meeting of the Child Neurology Society. Most children with NDPH fulfill criteria for its migraine subtype, and one-third of pediatric patients with NDPH have comorbid medication overuse headache (MOH).
NDPH is defined as a daily, unremitting headache that lasts for at least 3 months. “Not many studies on NDPH focus on pediatrics,” said Emily Pierce, from Children’s National Medical Center in Washington. NDPH “is considered to be one of the most intractable headaches in children. Children are able to tell that they’ve had this different type of headache because there’s some kind of onset that is very memorable.”
Ms. Pierce and colleagues conducted an observational study to describe the characteristics of NDPH in pediatric patients who presented to a headache program at a tertiary referral center. The researchers included pediatric patients who visited the headache clinic at Children’s National Medical Center between 2016 and 2018 in their analysis. All patients were enrolled in patient registry that had been approved by an independent review board. Ms. Pierce and colleagues queried the registry for NDPH and reviewed these records to examine participants’ clinical presentations.
The investigators identified 3,260 patient encounters during the study period. Of these encounters, 454 patients (13.9%) were identified as having NDPH. Patients with NDPH were predominantly female (78%) and white (72%). The median age of the sample was 14.8 years.
The frontal head region was the most common location of headache pain, which often had a throbbing quality and was associated with photophobia, phonophobia, nausea, and decreased activity. The median pain intensity was 6 of 10. Approximately 72% of patients had tried abortive medication, and 56% of patients had failed at least one abortive medication. Excedrin, ibuprofen, and acetaminophen were among the common failed abortive medications.
Furthermore, 36% of patients were diagnosed with MOH. The most commonly overused medication was ibuprofen. MOH “is also considered to be intractable for patients with NDPH,” said Ms. Pierce. “Typically, if the patient stops overusing that medication, they’ll find relief from their headaches. However, with our NDPH patients, when they stop overusing that medication, they still are having headaches associated with NDPH.”
The data indicated “a strong difference between our male and female patients,” said Ms. Pierce. Female patients reported significantly more instances of photophobia, phonophobia, nausea, and dizziness than did male patients. Overall, 78% of participants had a diagnosis of an additional comorbidity, such as head trauma (18%), anxiety (14%), depression (8%), or other (37%).
Observational studies of pediatric NDPH offer “a better way for our providers to diagnose these patients, and also to better understand them and help them figure out a treatment that may work,” said Ms. Pierce. In future research, she and her colleagues intend to examine blood work and potential genetic associations in pediatric patients with NDPH.
The study was not supported by funding, and the investigators had no disclosures.
SOURCE: Pierce E et al. CNS 2019, Abstract 100.
CHARLOTTE, N.C. – according to research presented at the 48th national meeting of the Child Neurology Society. Most children with NDPH fulfill criteria for its migraine subtype, and one-third of pediatric patients with NDPH have comorbid medication overuse headache (MOH).
NDPH is defined as a daily, unremitting headache that lasts for at least 3 months. “Not many studies on NDPH focus on pediatrics,” said Emily Pierce, from Children’s National Medical Center in Washington. NDPH “is considered to be one of the most intractable headaches in children. Children are able to tell that they’ve had this different type of headache because there’s some kind of onset that is very memorable.”
Ms. Pierce and colleagues conducted an observational study to describe the characteristics of NDPH in pediatric patients who presented to a headache program at a tertiary referral center. The researchers included pediatric patients who visited the headache clinic at Children’s National Medical Center between 2016 and 2018 in their analysis. All patients were enrolled in patient registry that had been approved by an independent review board. Ms. Pierce and colleagues queried the registry for NDPH and reviewed these records to examine participants’ clinical presentations.
The investigators identified 3,260 patient encounters during the study period. Of these encounters, 454 patients (13.9%) were identified as having NDPH. Patients with NDPH were predominantly female (78%) and white (72%). The median age of the sample was 14.8 years.
The frontal head region was the most common location of headache pain, which often had a throbbing quality and was associated with photophobia, phonophobia, nausea, and decreased activity. The median pain intensity was 6 of 10. Approximately 72% of patients had tried abortive medication, and 56% of patients had failed at least one abortive medication. Excedrin, ibuprofen, and acetaminophen were among the common failed abortive medications.
Furthermore, 36% of patients were diagnosed with MOH. The most commonly overused medication was ibuprofen. MOH “is also considered to be intractable for patients with NDPH,” said Ms. Pierce. “Typically, if the patient stops overusing that medication, they’ll find relief from their headaches. However, with our NDPH patients, when they stop overusing that medication, they still are having headaches associated with NDPH.”
The data indicated “a strong difference between our male and female patients,” said Ms. Pierce. Female patients reported significantly more instances of photophobia, phonophobia, nausea, and dizziness than did male patients. Overall, 78% of participants had a diagnosis of an additional comorbidity, such as head trauma (18%), anxiety (14%), depression (8%), or other (37%).
Observational studies of pediatric NDPH offer “a better way for our providers to diagnose these patients, and also to better understand them and help them figure out a treatment that may work,” said Ms. Pierce. In future research, she and her colleagues intend to examine blood work and potential genetic associations in pediatric patients with NDPH.
The study was not supported by funding, and the investigators had no disclosures.
SOURCE: Pierce E et al. CNS 2019, Abstract 100.
CHARLOTTE, N.C. – according to research presented at the 48th national meeting of the Child Neurology Society. Most children with NDPH fulfill criteria for its migraine subtype, and one-third of pediatric patients with NDPH have comorbid medication overuse headache (MOH).
NDPH is defined as a daily, unremitting headache that lasts for at least 3 months. “Not many studies on NDPH focus on pediatrics,” said Emily Pierce, from Children’s National Medical Center in Washington. NDPH “is considered to be one of the most intractable headaches in children. Children are able to tell that they’ve had this different type of headache because there’s some kind of onset that is very memorable.”
Ms. Pierce and colleagues conducted an observational study to describe the characteristics of NDPH in pediatric patients who presented to a headache program at a tertiary referral center. The researchers included pediatric patients who visited the headache clinic at Children’s National Medical Center between 2016 and 2018 in their analysis. All patients were enrolled in patient registry that had been approved by an independent review board. Ms. Pierce and colleagues queried the registry for NDPH and reviewed these records to examine participants’ clinical presentations.
The investigators identified 3,260 patient encounters during the study period. Of these encounters, 454 patients (13.9%) were identified as having NDPH. Patients with NDPH were predominantly female (78%) and white (72%). The median age of the sample was 14.8 years.
The frontal head region was the most common location of headache pain, which often had a throbbing quality and was associated with photophobia, phonophobia, nausea, and decreased activity. The median pain intensity was 6 of 10. Approximately 72% of patients had tried abortive medication, and 56% of patients had failed at least one abortive medication. Excedrin, ibuprofen, and acetaminophen were among the common failed abortive medications.
Furthermore, 36% of patients were diagnosed with MOH. The most commonly overused medication was ibuprofen. MOH “is also considered to be intractable for patients with NDPH,” said Ms. Pierce. “Typically, if the patient stops overusing that medication, they’ll find relief from their headaches. However, with our NDPH patients, when they stop overusing that medication, they still are having headaches associated with NDPH.”
The data indicated “a strong difference between our male and female patients,” said Ms. Pierce. Female patients reported significantly more instances of photophobia, phonophobia, nausea, and dizziness than did male patients. Overall, 78% of participants had a diagnosis of an additional comorbidity, such as head trauma (18%), anxiety (14%), depression (8%), or other (37%).
Observational studies of pediatric NDPH offer “a better way for our providers to diagnose these patients, and also to better understand them and help them figure out a treatment that may work,” said Ms. Pierce. In future research, she and her colleagues intend to examine blood work and potential genetic associations in pediatric patients with NDPH.
The study was not supported by funding, and the investigators had no disclosures.
SOURCE: Pierce E et al. CNS 2019, Abstract 100.
REPORTING FROM CNS 2019
Many children who present to headache clinics have joint hypermobility
CHARLOTTE, N.C. – according to data presented at the 48th national meeting of the Child Neurology Society. Furthermore, patients with joint hypermobility have a high rate of headache disability, while patients without joint hypermobility have less headache disability, according to Dhwani Sahjwani, MD, a resident at Inova Fairfax Hospital in Falls Church, Va., and colleagues.
While conducting research in the headache clinic at Children’s National Hospital in Washington, D.C., Dr. Sahjwani saw several children with joint hypermobility and a diagnosis of a disorder such as Ehlers-Danlos syndrome. She and her colleagues began analyzing patients to evaluate the potential association between joint hypermobility and headache disability in children. The investigators included pediatric patients examined in the headache clinic at Children’s National Medical Center between October 2018 and January 2019 in their study. All headache clinic patients were enrolled in a patient registry that had been approved by an independent review board.
Dr. Sahjwani and colleagues measured patients’ headache disability with the Headache Impact Test–6 (HIT-6) questionnaire. Scores of 60 or greater on this questionnaire indicate severe headache disability. The researchers assessed joint hypermobility using the Beighton scoring system. In this system, scores greater than 4 indicate joint hypermobility.
Dr. Sahjwani’s group scored 76 patients using the Beighton system and HIT-6 questionnaire. Participants’ median age was 13.7 years. Approximately 26% of patients had Beighton scores that indicated joint hypermobility. About 65% of the patients with joint hypermobility had a diagnosis of migraine without aura. In addition, 80% of patients with joint hypermobility had severe headache disability, according to the HIT-6 disability criteria. The average pain intensity in patients with hypermobile joints was 6.1 out of 10. Among participants without significant joint hypermobility, 90% had mild headache disability.
Patients with joint hypermobility and increased tissue elasticity “tend to have a lower threshold for pain, in general, in all parts of their bodies,” said Dr. Sahjwani. Greater headache severity might be expected in this population, “because they have more pain if they have hypermobile joints or tissue.”
Headache treatments for this population are based solely on the type of headache that each patient has. Patients with joint hypermobility and migraine, for example, are candidates for rescue medication and long-term prophylactic medications. “I don’t think the joint hypermobility is going to change how you manage their headaches,” said Dr. Sahjwani.
The study results suggest that, when children present with severely debilitating headaches, a neurologist should consider examining them for joint hypermobility “to see if they have another diagnosis, such as Ehlers-Danlos syndrome ... that has to be managed in addition to their headaches,” Dr. Sahjwani concluded.
The study was not supported by funding. The authors did not report any disclosures.
SOURCE: Sahjwani D et al. CNS 2019, Abstract 101.
CHARLOTTE, N.C. – according to data presented at the 48th national meeting of the Child Neurology Society. Furthermore, patients with joint hypermobility have a high rate of headache disability, while patients without joint hypermobility have less headache disability, according to Dhwani Sahjwani, MD, a resident at Inova Fairfax Hospital in Falls Church, Va., and colleagues.
While conducting research in the headache clinic at Children’s National Hospital in Washington, D.C., Dr. Sahjwani saw several children with joint hypermobility and a diagnosis of a disorder such as Ehlers-Danlos syndrome. She and her colleagues began analyzing patients to evaluate the potential association between joint hypermobility and headache disability in children. The investigators included pediatric patients examined in the headache clinic at Children’s National Medical Center between October 2018 and January 2019 in their study. All headache clinic patients were enrolled in a patient registry that had been approved by an independent review board.
Dr. Sahjwani and colleagues measured patients’ headache disability with the Headache Impact Test–6 (HIT-6) questionnaire. Scores of 60 or greater on this questionnaire indicate severe headache disability. The researchers assessed joint hypermobility using the Beighton scoring system. In this system, scores greater than 4 indicate joint hypermobility.
Dr. Sahjwani’s group scored 76 patients using the Beighton system and HIT-6 questionnaire. Participants’ median age was 13.7 years. Approximately 26% of patients had Beighton scores that indicated joint hypermobility. About 65% of the patients with joint hypermobility had a diagnosis of migraine without aura. In addition, 80% of patients with joint hypermobility had severe headache disability, according to the HIT-6 disability criteria. The average pain intensity in patients with hypermobile joints was 6.1 out of 10. Among participants without significant joint hypermobility, 90% had mild headache disability.
Patients with joint hypermobility and increased tissue elasticity “tend to have a lower threshold for pain, in general, in all parts of their bodies,” said Dr. Sahjwani. Greater headache severity might be expected in this population, “because they have more pain if they have hypermobile joints or tissue.”
Headache treatments for this population are based solely on the type of headache that each patient has. Patients with joint hypermobility and migraine, for example, are candidates for rescue medication and long-term prophylactic medications. “I don’t think the joint hypermobility is going to change how you manage their headaches,” said Dr. Sahjwani.
The study results suggest that, when children present with severely debilitating headaches, a neurologist should consider examining them for joint hypermobility “to see if they have another diagnosis, such as Ehlers-Danlos syndrome ... that has to be managed in addition to their headaches,” Dr. Sahjwani concluded.
The study was not supported by funding. The authors did not report any disclosures.
SOURCE: Sahjwani D et al. CNS 2019, Abstract 101.
CHARLOTTE, N.C. – according to data presented at the 48th national meeting of the Child Neurology Society. Furthermore, patients with joint hypermobility have a high rate of headache disability, while patients without joint hypermobility have less headache disability, according to Dhwani Sahjwani, MD, a resident at Inova Fairfax Hospital in Falls Church, Va., and colleagues.
While conducting research in the headache clinic at Children’s National Hospital in Washington, D.C., Dr. Sahjwani saw several children with joint hypermobility and a diagnosis of a disorder such as Ehlers-Danlos syndrome. She and her colleagues began analyzing patients to evaluate the potential association between joint hypermobility and headache disability in children. The investigators included pediatric patients examined in the headache clinic at Children’s National Medical Center between October 2018 and January 2019 in their study. All headache clinic patients were enrolled in a patient registry that had been approved by an independent review board.
Dr. Sahjwani and colleagues measured patients’ headache disability with the Headache Impact Test–6 (HIT-6) questionnaire. Scores of 60 or greater on this questionnaire indicate severe headache disability. The researchers assessed joint hypermobility using the Beighton scoring system. In this system, scores greater than 4 indicate joint hypermobility.
Dr. Sahjwani’s group scored 76 patients using the Beighton system and HIT-6 questionnaire. Participants’ median age was 13.7 years. Approximately 26% of patients had Beighton scores that indicated joint hypermobility. About 65% of the patients with joint hypermobility had a diagnosis of migraine without aura. In addition, 80% of patients with joint hypermobility had severe headache disability, according to the HIT-6 disability criteria. The average pain intensity in patients with hypermobile joints was 6.1 out of 10. Among participants without significant joint hypermobility, 90% had mild headache disability.
Patients with joint hypermobility and increased tissue elasticity “tend to have a lower threshold for pain, in general, in all parts of their bodies,” said Dr. Sahjwani. Greater headache severity might be expected in this population, “because they have more pain if they have hypermobile joints or tissue.”
Headache treatments for this population are based solely on the type of headache that each patient has. Patients with joint hypermobility and migraine, for example, are candidates for rescue medication and long-term prophylactic medications. “I don’t think the joint hypermobility is going to change how you manage their headaches,” said Dr. Sahjwani.
The study results suggest that, when children present with severely debilitating headaches, a neurologist should consider examining them for joint hypermobility “to see if they have another diagnosis, such as Ehlers-Danlos syndrome ... that has to be managed in addition to their headaches,” Dr. Sahjwani concluded.
The study was not supported by funding. The authors did not report any disclosures.
SOURCE: Sahjwani D et al. CNS 2019, Abstract 101.
REPORTING FROM CNS 2019
Suicide deaths rising in children aged 10-19 years
according to the National Center for Health Statistics.
Death rates from suicide for children aged 10-14 years jumped by 178% from 2007 to 2017, while teenagers aged 15-19 years experienced a 76% increase over that period, with both changes reaching significance, the NCHS said in a recent data brief based on data from the National Vital Statistics System.
The actual rate for teens was higher to begin with, however, so in absolute terms the increase is larger for the older group. In 2007, deaths from suicide occurred at a rate of 6.7 per 100,000 persons for persons aged 15-19 years, and by 2017 that rate was up significantly to 11.8 per 100,000. Among children aged 10-14 years, the suicide-related death rate climbed from 0.9 per 100,000 in 2007 to 2.5 in 2014, the NCHS investigators reported.
The news was somewhat better on the other side of the violent death coin. Homicides are down by a significant 18% since 2000 among children aged 10-14 years, as the rate dropped from 1.1 per 100,000 in 2000 to 0.9 in 2017. The homicide rate since 2000 is down slightly for teens aged 15-19 years, but it has risen 32% in recent years, going from 6.6 deaths per 100,000 in 2013 to 8.7 in 2017, they said.
Suicide was the second-leading cause of death in both age groups in 2017, and homicide was third for those aged 15-19 and fifth among 10- to 14-year-olds, the investigators noted.
according to the National Center for Health Statistics.

Death rates from suicide for children aged 10-14 years jumped by 178% from 2007 to 2017, while teenagers aged 15-19 years experienced a 76% increase over that period, with both changes reaching significance, the NCHS said in a recent data brief based on data from the National Vital Statistics System.
The actual rate for teens was higher to begin with, however, so in absolute terms the increase is larger for the older group. In 2007, deaths from suicide occurred at a rate of 6.7 per 100,000 persons for persons aged 15-19 years, and by 2017 that rate was up significantly to 11.8 per 100,000. Among children aged 10-14 years, the suicide-related death rate climbed from 0.9 per 100,000 in 2007 to 2.5 in 2014, the NCHS investigators reported.
The news was somewhat better on the other side of the violent death coin. Homicides are down by a significant 18% since 2000 among children aged 10-14 years, as the rate dropped from 1.1 per 100,000 in 2000 to 0.9 in 2017. The homicide rate since 2000 is down slightly for teens aged 15-19 years, but it has risen 32% in recent years, going from 6.6 deaths per 100,000 in 2013 to 8.7 in 2017, they said.
Suicide was the second-leading cause of death in both age groups in 2017, and homicide was third for those aged 15-19 and fifth among 10- to 14-year-olds, the investigators noted.
according to the National Center for Health Statistics.

Death rates from suicide for children aged 10-14 years jumped by 178% from 2007 to 2017, while teenagers aged 15-19 years experienced a 76% increase over that period, with both changes reaching significance, the NCHS said in a recent data brief based on data from the National Vital Statistics System.
The actual rate for teens was higher to begin with, however, so in absolute terms the increase is larger for the older group. In 2007, deaths from suicide occurred at a rate of 6.7 per 100,000 persons for persons aged 15-19 years, and by 2017 that rate was up significantly to 11.8 per 100,000. Among children aged 10-14 years, the suicide-related death rate climbed from 0.9 per 100,000 in 2007 to 2.5 in 2014, the NCHS investigators reported.
The news was somewhat better on the other side of the violent death coin. Homicides are down by a significant 18% since 2000 among children aged 10-14 years, as the rate dropped from 1.1 per 100,000 in 2000 to 0.9 in 2017. The homicide rate since 2000 is down slightly for teens aged 15-19 years, but it has risen 32% in recent years, going from 6.6 deaths per 100,000 in 2013 to 8.7 in 2017, they said.
Suicide was the second-leading cause of death in both age groups in 2017, and homicide was third for those aged 15-19 and fifth among 10- to 14-year-olds, the investigators noted.
Supporting elimination of nonmedical vaccine exemptions
Let’s suppose your first patient of the morning is a 2-month-old you have never seen before. The family arrives 10 minutes late because they are still getting the dressing-undressing-diaper change-car seat–adjusting thing worked out. Father is a computer programmer. Mother lists her occupation as nutrition counselor. The child is gaining. Breastfeeding seems to come naturally to the dyad.
As the visit draws to a close, you take the matter-of-fact approach and say, “The nurse will be in shortly with the vaccines do you have any questions.” Well ... it turns out the parents don’t feel comfortable with vaccines. They claim to understand the science and feel that vaccines make sense for some families. But they feel that for themselves, with a healthy lifestyle and God’s benevolence their son will be protected without having to introduce a host of foreign substances into his body.
What word best describes your reaction? Anger? Frustration? Disappointment (in our education system)? Maybe you’re angry at yourself for failing to make it clear in your office pamphlet and social media feeds that to protect your other patients, you no longer accept families who refuse immunizations for the common childhood diseases.
The American Academy of Pediatrics says it feels your pain, and its Annual Leadership Forum made eliminating nonmedical vaccine exemption laws its top priority in 2019. As part of its effort to help, the AAP Board of Directors was asked to advocate for the creation of a toolkit of strategies for Academy chapters facing the challenge of nonmedical exemptions. As an initial step to this process, three physicians in the department of pediatrics at the Denver Health Medical Center have begun interviewing religious leaders in hopes of developing “clergy-specific vaccine educational materials and deriv[ing] best practices for engaging them as vaccination advocates.” The investigators describe their plan and initial findings in Pediatrics (2019 Oct. doi: 10.1542/peds.2019-0933). Although they acknowledged that their efforts may not provide a quick solution to the nonmedical exemption problem, they hope that including more stakeholders and engendering trust will help future discussions.
Fourteen pages deeper into that issue of Pediatrics is the runner-up submission of this year’s Section on Pediatric Trainees essay competition titled “What I Learned From the Antivaccine Movement” (2019 Oct. doi: 10.1542/peds.2019-2384). Alana C. Ju, MD, describes the 2-hour ordeal she endured to testify at the California State Capitol in support of a state Senate bill aimed at tightening the regulations for vaccine medical exemptions. Totally unprepared for the “level of vitriol” aimed at her and other supporters of the bill, she was “accused of violating her duty as” a pediatrician because she was failing to protect children. The supporters were called “greedy, ignorant, and negligent.”
To her credit, Dr. Ju was able to step back from this assault and began looking at the faces of her accusers and learned that, “they too, felt strongly about children’s health.” She realized that “focusing on perceived ignorance is counterproductive.” She now hopes that by focusing on the shared goal of what is best for children, “we can all be better advocates.”
Both of these articles have a warm sort of kumbaya feel about them. It never is a bad idea to learn more about those with whom we disagree. But before huddling up too close to the campfire, we must realize that there is good evidence that sharing the scientific data with vaccine-hesitant parents doesn’t convert them into vaccine acceptors. In fact, it may strengthen their resolve to resist (Nyhan et al. “Effective Messages in Vaccine Promotion: A Randomized Trial,” Pediatrics. 2014 Apr;133[4] e835-42).
We are unlikely to convert many anti-vaxxers by sitting down together. Our target audience needs to be legislators and the majority of people who do vaccinate their children. These are the voters who will support legislation to eliminate nonmedical vaccine exemptions. To characterize anti-vaxxers as despicable ignorants is untrue and serves no purpose. We all do care about the health of children. However,
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
*This article has been updated 1/22/2020.
Let’s suppose your first patient of the morning is a 2-month-old you have never seen before. The family arrives 10 minutes late because they are still getting the dressing-undressing-diaper change-car seat–adjusting thing worked out. Father is a computer programmer. Mother lists her occupation as nutrition counselor. The child is gaining. Breastfeeding seems to come naturally to the dyad.
As the visit draws to a close, you take the matter-of-fact approach and say, “The nurse will be in shortly with the vaccines do you have any questions.” Well ... it turns out the parents don’t feel comfortable with vaccines. They claim to understand the science and feel that vaccines make sense for some families. But they feel that for themselves, with a healthy lifestyle and God’s benevolence their son will be protected without having to introduce a host of foreign substances into his body.
What word best describes your reaction? Anger? Frustration? Disappointment (in our education system)? Maybe you’re angry at yourself for failing to make it clear in your office pamphlet and social media feeds that to protect your other patients, you no longer accept families who refuse immunizations for the common childhood diseases.
The American Academy of Pediatrics says it feels your pain, and its Annual Leadership Forum made eliminating nonmedical vaccine exemption laws its top priority in 2019. As part of its effort to help, the AAP Board of Directors was asked to advocate for the creation of a toolkit of strategies for Academy chapters facing the challenge of nonmedical exemptions. As an initial step to this process, three physicians in the department of pediatrics at the Denver Health Medical Center have begun interviewing religious leaders in hopes of developing “clergy-specific vaccine educational materials and deriv[ing] best practices for engaging them as vaccination advocates.” The investigators describe their plan and initial findings in Pediatrics (2019 Oct. doi: 10.1542/peds.2019-0933). Although they acknowledged that their efforts may not provide a quick solution to the nonmedical exemption problem, they hope that including more stakeholders and engendering trust will help future discussions.
Fourteen pages deeper into that issue of Pediatrics is the runner-up submission of this year’s Section on Pediatric Trainees essay competition titled “What I Learned From the Antivaccine Movement” (2019 Oct. doi: 10.1542/peds.2019-2384). Alana C. Ju, MD, describes the 2-hour ordeal she endured to testify at the California State Capitol in support of a state Senate bill aimed at tightening the regulations for vaccine medical exemptions. Totally unprepared for the “level of vitriol” aimed at her and other supporters of the bill, she was “accused of violating her duty as” a pediatrician because she was failing to protect children. The supporters were called “greedy, ignorant, and negligent.”
To her credit, Dr. Ju was able to step back from this assault and began looking at the faces of her accusers and learned that, “they too, felt strongly about children’s health.” She realized that “focusing on perceived ignorance is counterproductive.” She now hopes that by focusing on the shared goal of what is best for children, “we can all be better advocates.”
Both of these articles have a warm sort of kumbaya feel about them. It never is a bad idea to learn more about those with whom we disagree. But before huddling up too close to the campfire, we must realize that there is good evidence that sharing the scientific data with vaccine-hesitant parents doesn’t convert them into vaccine acceptors. In fact, it may strengthen their resolve to resist (Nyhan et al. “Effective Messages in Vaccine Promotion: A Randomized Trial,” Pediatrics. 2014 Apr;133[4] e835-42).
We are unlikely to convert many anti-vaxxers by sitting down together. Our target audience needs to be legislators and the majority of people who do vaccinate their children. These are the voters who will support legislation to eliminate nonmedical vaccine exemptions. To characterize anti-vaxxers as despicable ignorants is untrue and serves no purpose. We all do care about the health of children. However,
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
*This article has been updated 1/22/2020.
Let’s suppose your first patient of the morning is a 2-month-old you have never seen before. The family arrives 10 minutes late because they are still getting the dressing-undressing-diaper change-car seat–adjusting thing worked out. Father is a computer programmer. Mother lists her occupation as nutrition counselor. The child is gaining. Breastfeeding seems to come naturally to the dyad.
As the visit draws to a close, you take the matter-of-fact approach and say, “The nurse will be in shortly with the vaccines do you have any questions.” Well ... it turns out the parents don’t feel comfortable with vaccines. They claim to understand the science and feel that vaccines make sense for some families. But they feel that for themselves, with a healthy lifestyle and God’s benevolence their son will be protected without having to introduce a host of foreign substances into his body.
What word best describes your reaction? Anger? Frustration? Disappointment (in our education system)? Maybe you’re angry at yourself for failing to make it clear in your office pamphlet and social media feeds that to protect your other patients, you no longer accept families who refuse immunizations for the common childhood diseases.
The American Academy of Pediatrics says it feels your pain, and its Annual Leadership Forum made eliminating nonmedical vaccine exemption laws its top priority in 2019. As part of its effort to help, the AAP Board of Directors was asked to advocate for the creation of a toolkit of strategies for Academy chapters facing the challenge of nonmedical exemptions. As an initial step to this process, three physicians in the department of pediatrics at the Denver Health Medical Center have begun interviewing religious leaders in hopes of developing “clergy-specific vaccine educational materials and deriv[ing] best practices for engaging them as vaccination advocates.” The investigators describe their plan and initial findings in Pediatrics (2019 Oct. doi: 10.1542/peds.2019-0933). Although they acknowledged that their efforts may not provide a quick solution to the nonmedical exemption problem, they hope that including more stakeholders and engendering trust will help future discussions.
Fourteen pages deeper into that issue of Pediatrics is the runner-up submission of this year’s Section on Pediatric Trainees essay competition titled “What I Learned From the Antivaccine Movement” (2019 Oct. doi: 10.1542/peds.2019-2384). Alana C. Ju, MD, describes the 2-hour ordeal she endured to testify at the California State Capitol in support of a state Senate bill aimed at tightening the regulations for vaccine medical exemptions. Totally unprepared for the “level of vitriol” aimed at her and other supporters of the bill, she was “accused of violating her duty as” a pediatrician because she was failing to protect children. The supporters were called “greedy, ignorant, and negligent.”
To her credit, Dr. Ju was able to step back from this assault and began looking at the faces of her accusers and learned that, “they too, felt strongly about children’s health.” She realized that “focusing on perceived ignorance is counterproductive.” She now hopes that by focusing on the shared goal of what is best for children, “we can all be better advocates.”
Both of these articles have a warm sort of kumbaya feel about them. It never is a bad idea to learn more about those with whom we disagree. But before huddling up too close to the campfire, we must realize that there is good evidence that sharing the scientific data with vaccine-hesitant parents doesn’t convert them into vaccine acceptors. In fact, it may strengthen their resolve to resist (Nyhan et al. “Effective Messages in Vaccine Promotion: A Randomized Trial,” Pediatrics. 2014 Apr;133[4] e835-42).
We are unlikely to convert many anti-vaxxers by sitting down together. Our target audience needs to be legislators and the majority of people who do vaccinate their children. These are the voters who will support legislation to eliminate nonmedical vaccine exemptions. To characterize anti-vaxxers as despicable ignorants is untrue and serves no purpose. We all do care about the health of children. However,
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
*This article has been updated 1/22/2020.