User login
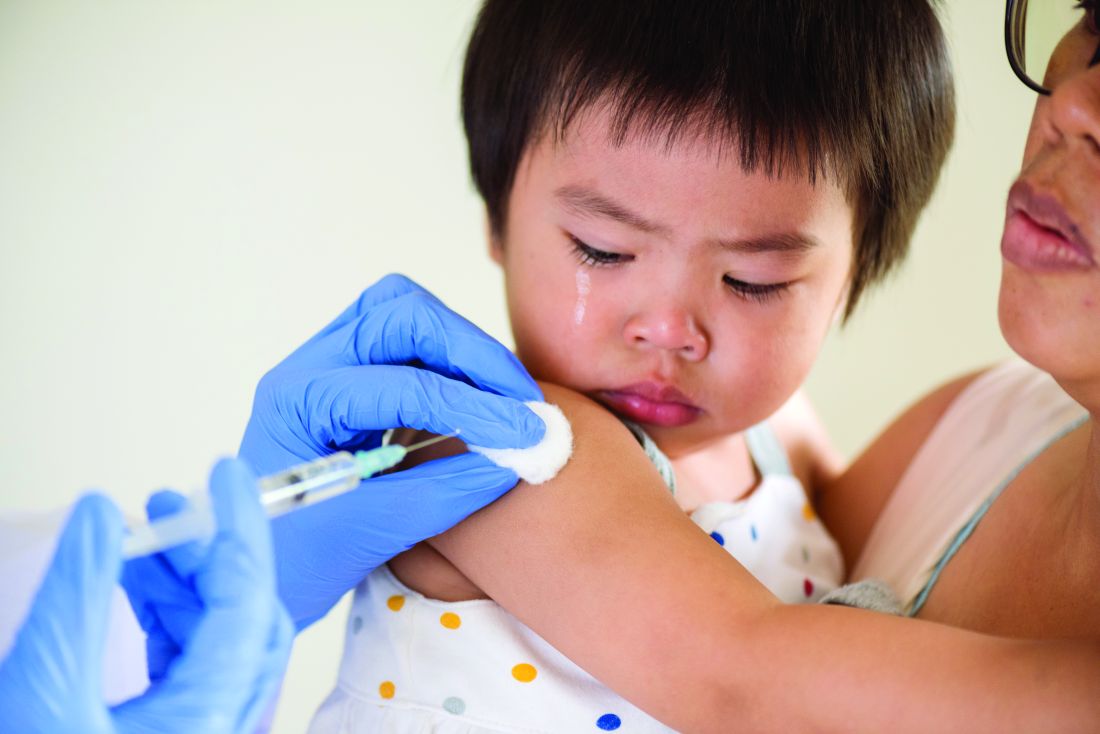
ACIP approves child and adolescent vaccination schedule for 2020
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the child and adolescent immunization schedule for 2020.
by busy providers,” Candice Robinson, MD, MPH, of the CDC’s National Center for Immunization and Respiratory Diseases, said at the CDC’s October meeting of ACIP. Updates reflect changes in language in the adult vaccination schedule, notably the change in the definition of “contraindication.” The updated wording in the Notes substitutes “not recommended or contraindicated” instead of the word “contraindicated” only.
Another notable change was the addition of information on adolescent vaccination of children who received the meningococcal ACWY vaccine before 10 years of age. For “children in whom boosters are not recommended due to an ongoing or increased risk of meningococcal disease” (such as a healthy child traveling to an endemic area), they should receive MenACWY according to the recommended adolescent schedule. But those children for whom boosters are recommended because of increased disease risk from conditions including complement deficiency, HIV, or asplenia should “follow the booster schedule for persons at increased risk.”
Other changes include restructuring of the notes for the live attenuated influenza vaccine (LAIV) in special situations. The schedule now uses a bulleted list to show that LAIV should not be used in the following circumstances:
- Having history of severe allergic reaction to a previous vaccine or vaccine component.
- Using aspirin or a salicylate-containing medication.
- Being aged 2-4 years with a history of asthma or wheezing.
- Having immunocompromised conditions.
- Having anatomic or functional asplenia.
- Having cochlear implants.
- Experiencing cerebrospinal fluid–oropharyngeal communication.
- Having immunocompromised close contacts or caregivers.
- Being pregnant.
- Having received flu antivirals within the previous 48 hours.
In addition, language on shared clinical decision-making was added to the notes on the meningococcal B vaccine for adolescents and young adults aged 18-23 years not at increased risk. Based on shared clinical decision making, the recommendation is a “two-dose series of Bexsero at least 1 month apart” or “two-dose series of Trumenba at least 6 months apart; if dose two is administered earlier than 6 months, administer a third dose at least 4 months after dose two.”
Several vaccines’ Notes sections, including hepatitis B and meningococcal disease, added links to detailed recommendations in the corresponding issues of the CDC’s Morbidity and Mortality Weekly Report, to allow clinicians easy access to additional information.
View the current Child & Adolescent Vaccination Schedule here.
The ACIP members had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the child and adolescent immunization schedule for 2020.
by busy providers,” Candice Robinson, MD, MPH, of the CDC’s National Center for Immunization and Respiratory Diseases, said at the CDC’s October meeting of ACIP. Updates reflect changes in language in the adult vaccination schedule, notably the change in the definition of “contraindication.” The updated wording in the Notes substitutes “not recommended or contraindicated” instead of the word “contraindicated” only.
Another notable change was the addition of information on adolescent vaccination of children who received the meningococcal ACWY vaccine before 10 years of age. For “children in whom boosters are not recommended due to an ongoing or increased risk of meningococcal disease” (such as a healthy child traveling to an endemic area), they should receive MenACWY according to the recommended adolescent schedule. But those children for whom boosters are recommended because of increased disease risk from conditions including complement deficiency, HIV, or asplenia should “follow the booster schedule for persons at increased risk.”
Other changes include restructuring of the notes for the live attenuated influenza vaccine (LAIV) in special situations. The schedule now uses a bulleted list to show that LAIV should not be used in the following circumstances:
- Having history of severe allergic reaction to a previous vaccine or vaccine component.
- Using aspirin or a salicylate-containing medication.
- Being aged 2-4 years with a history of asthma or wheezing.
- Having immunocompromised conditions.
- Having anatomic or functional asplenia.
- Having cochlear implants.
- Experiencing cerebrospinal fluid–oropharyngeal communication.
- Having immunocompromised close contacts or caregivers.
- Being pregnant.
- Having received flu antivirals within the previous 48 hours.
In addition, language on shared clinical decision-making was added to the notes on the meningococcal B vaccine for adolescents and young adults aged 18-23 years not at increased risk. Based on shared clinical decision making, the recommendation is a “two-dose series of Bexsero at least 1 month apart” or “two-dose series of Trumenba at least 6 months apart; if dose two is administered earlier than 6 months, administer a third dose at least 4 months after dose two.”
Several vaccines’ Notes sections, including hepatitis B and meningococcal disease, added links to detailed recommendations in the corresponding issues of the CDC’s Morbidity and Mortality Weekly Report, to allow clinicians easy access to additional information.
View the current Child & Adolescent Vaccination Schedule here.
The ACIP members had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the child and adolescent immunization schedule for 2020.
by busy providers,” Candice Robinson, MD, MPH, of the CDC’s National Center for Immunization and Respiratory Diseases, said at the CDC’s October meeting of ACIP. Updates reflect changes in language in the adult vaccination schedule, notably the change in the definition of “contraindication.” The updated wording in the Notes substitutes “not recommended or contraindicated” instead of the word “contraindicated” only.
Another notable change was the addition of information on adolescent vaccination of children who received the meningococcal ACWY vaccine before 10 years of age. For “children in whom boosters are not recommended due to an ongoing or increased risk of meningococcal disease” (such as a healthy child traveling to an endemic area), they should receive MenACWY according to the recommended adolescent schedule. But those children for whom boosters are recommended because of increased disease risk from conditions including complement deficiency, HIV, or asplenia should “follow the booster schedule for persons at increased risk.”
Other changes include restructuring of the notes for the live attenuated influenza vaccine (LAIV) in special situations. The schedule now uses a bulleted list to show that LAIV should not be used in the following circumstances:
- Having history of severe allergic reaction to a previous vaccine or vaccine component.
- Using aspirin or a salicylate-containing medication.
- Being aged 2-4 years with a history of asthma or wheezing.
- Having immunocompromised conditions.
- Having anatomic or functional asplenia.
- Having cochlear implants.
- Experiencing cerebrospinal fluid–oropharyngeal communication.
- Having immunocompromised close contacts or caregivers.
- Being pregnant.
- Having received flu antivirals within the previous 48 hours.
In addition, language on shared clinical decision-making was added to the notes on the meningococcal B vaccine for adolescents and young adults aged 18-23 years not at increased risk. Based on shared clinical decision making, the recommendation is a “two-dose series of Bexsero at least 1 month apart” or “two-dose series of Trumenba at least 6 months apart; if dose two is administered earlier than 6 months, administer a third dose at least 4 months after dose two.”
Several vaccines’ Notes sections, including hepatitis B and meningococcal disease, added links to detailed recommendations in the corresponding issues of the CDC’s Morbidity and Mortality Weekly Report, to allow clinicians easy access to additional information.
View the current Child & Adolescent Vaccination Schedule here.
The ACIP members had no financial conflicts to disclose.
FROM AN ACIP MEETING
PHM 19: PREP yourself for the PHM boards
Get ready for the first-ever ABP PHM exam
Presenters
Jared Austin, MD, FAAP
Ryan Bode, MD, FAAP
Jeremy Kern, MD, FAAP
Mary Ottolini, MD, MPH, MEd, FAAP
Stacy Pierson, MD, FAAP
Mary Rocha, MD, MPH, FAAP
Susan Walley, MD, CTTS, FAAP
Session summary
Professional development sessions at the Pediatric Hospital Medicine 2019 conference intended to further educate pediatric hospitalists and advance their careers. In November 2019, many pediatric hospitalists will be taking subspecialty PHM boards for the very first time. This PHM19 session had clear objectives: to describe the American Board of Pediatrics (ABP) PHM board content areas, to analyze common knowledge gaps in PREP PHM, and to examine different approaches to clinical management of PHM patients.
The session opened with a brief history of a vision of PHM and the story of its realization. In 2016, a group of eight stalwart writers, four new writers, and three editors created PREP 2018 and 2019 questions that were released in full prior to November 2019. The ABP will offer the board exam in 2019, 2021, and 2023
The exam content domains include the following:
- Medical conditions.
- Behavioral and mental health conditions.
- Newborn care.
- Children with medical complexity.
- Medical procedures.
- Patient and family centered care.
- Transitions of care.
- Quality improvement, patient safety and system based improvement.
- Evidence-based, high-value care.
- Advocacy and leadership.
- Ethics, legal issues, and human rights.
- Teaching and education.
- Core knowledge in scholarly activities.
Each question consists of a case vignette, question, response choices, critiques, PREP PEARLs, and references. There are also additional PREP Ponder Points that intend to prompt reflection on practice change.
For the remainder of the session presenters reviewed the PHM PREP questions that were most frequently answered incorrectly. Some of the topics included: asthma vs. anaphylaxis, venous thromboembolism prophylaxis in surgical patients, postoperative feeding regimens, transmission-based precautions, febrile neonates, Ebola, medical child abuse, absolute indications for intubation, toxic megacolon, palivizumab prophylaxis guidelines, key driver diagrams, and infantile hemangiomas.
Key takeaway
Pediatric hospitalists all over the United States will for the first time ever take PHM boards in November 2019. The exam content domains were demonstrated in detail, and several often incorrectly answered PREP questions were presented and discussed.
Dr. Giordano is assistant professor in pediatrics at Columbia University Medical Center, New York.
Get ready for the first-ever ABP PHM exam
Get ready for the first-ever ABP PHM exam
Presenters
Jared Austin, MD, FAAP
Ryan Bode, MD, FAAP
Jeremy Kern, MD, FAAP
Mary Ottolini, MD, MPH, MEd, FAAP
Stacy Pierson, MD, FAAP
Mary Rocha, MD, MPH, FAAP
Susan Walley, MD, CTTS, FAAP
Session summary
Professional development sessions at the Pediatric Hospital Medicine 2019 conference intended to further educate pediatric hospitalists and advance their careers. In November 2019, many pediatric hospitalists will be taking subspecialty PHM boards for the very first time. This PHM19 session had clear objectives: to describe the American Board of Pediatrics (ABP) PHM board content areas, to analyze common knowledge gaps in PREP PHM, and to examine different approaches to clinical management of PHM patients.
The session opened with a brief history of a vision of PHM and the story of its realization. In 2016, a group of eight stalwart writers, four new writers, and three editors created PREP 2018 and 2019 questions that were released in full prior to November 2019. The ABP will offer the board exam in 2019, 2021, and 2023
The exam content domains include the following:
- Medical conditions.
- Behavioral and mental health conditions.
- Newborn care.
- Children with medical complexity.
- Medical procedures.
- Patient and family centered care.
- Transitions of care.
- Quality improvement, patient safety and system based improvement.
- Evidence-based, high-value care.
- Advocacy and leadership.
- Ethics, legal issues, and human rights.
- Teaching and education.
- Core knowledge in scholarly activities.
Each question consists of a case vignette, question, response choices, critiques, PREP PEARLs, and references. There are also additional PREP Ponder Points that intend to prompt reflection on practice change.
For the remainder of the session presenters reviewed the PHM PREP questions that were most frequently answered incorrectly. Some of the topics included: asthma vs. anaphylaxis, venous thromboembolism prophylaxis in surgical patients, postoperative feeding regimens, transmission-based precautions, febrile neonates, Ebola, medical child abuse, absolute indications for intubation, toxic megacolon, palivizumab prophylaxis guidelines, key driver diagrams, and infantile hemangiomas.
Key takeaway
Pediatric hospitalists all over the United States will for the first time ever take PHM boards in November 2019. The exam content domains were demonstrated in detail, and several often incorrectly answered PREP questions were presented and discussed.
Dr. Giordano is assistant professor in pediatrics at Columbia University Medical Center, New York.
Presenters
Jared Austin, MD, FAAP
Ryan Bode, MD, FAAP
Jeremy Kern, MD, FAAP
Mary Ottolini, MD, MPH, MEd, FAAP
Stacy Pierson, MD, FAAP
Mary Rocha, MD, MPH, FAAP
Susan Walley, MD, CTTS, FAAP
Session summary
Professional development sessions at the Pediatric Hospital Medicine 2019 conference intended to further educate pediatric hospitalists and advance their careers. In November 2019, many pediatric hospitalists will be taking subspecialty PHM boards for the very first time. This PHM19 session had clear objectives: to describe the American Board of Pediatrics (ABP) PHM board content areas, to analyze common knowledge gaps in PREP PHM, and to examine different approaches to clinical management of PHM patients.
The session opened with a brief history of a vision of PHM and the story of its realization. In 2016, a group of eight stalwart writers, four new writers, and three editors created PREP 2018 and 2019 questions that were released in full prior to November 2019. The ABP will offer the board exam in 2019, 2021, and 2023
The exam content domains include the following:
- Medical conditions.
- Behavioral and mental health conditions.
- Newborn care.
- Children with medical complexity.
- Medical procedures.
- Patient and family centered care.
- Transitions of care.
- Quality improvement, patient safety and system based improvement.
- Evidence-based, high-value care.
- Advocacy and leadership.
- Ethics, legal issues, and human rights.
- Teaching and education.
- Core knowledge in scholarly activities.
Each question consists of a case vignette, question, response choices, critiques, PREP PEARLs, and references. There are also additional PREP Ponder Points that intend to prompt reflection on practice change.
For the remainder of the session presenters reviewed the PHM PREP questions that were most frequently answered incorrectly. Some of the topics included: asthma vs. anaphylaxis, venous thromboembolism prophylaxis in surgical patients, postoperative feeding regimens, transmission-based precautions, febrile neonates, Ebola, medical child abuse, absolute indications for intubation, toxic megacolon, palivizumab prophylaxis guidelines, key driver diagrams, and infantile hemangiomas.
Key takeaway
Pediatric hospitalists all over the United States will for the first time ever take PHM boards in November 2019. The exam content domains were demonstrated in detail, and several often incorrectly answered PREP questions were presented and discussed.
Dr. Giordano is assistant professor in pediatrics at Columbia University Medical Center, New York.
FDA approves onabotulinumtoxinA for pediatric lower limb spasticity
The Food and Drug Administration has approved onabotulinumtoxinA (Botox) for treatment of pediatric lower limb spasticity in patients aged 2-17 years, excluding those in whom it is associated with cerebral palsy, according to an announcement from Allergan.
The approval is based on a phase 3 study evaluating safety and efficacy in more than 300 patients with lower limb spasticity. Although patients with cerebral palsy were included in the study, they’re excluded from this indication. Orphan Drug Exclusivity prevents it from being indicated for lower limb spasticity in cerebral palsy because abobotulinumtoxinA (Dysport) already has marketing exclusivity for the indication. Botox also is indicated for children aged 2-17 years of age with upper limb spasticity, as well as nine other indications.
OnabotulinumtoxinA comes with warnings, including problems of swallowing, speaking, or breathing and even risk of spread of the toxin. It also may cause loss of strength or general muscle weakness, vision problems, or dizziness within hours or weeks of administration. Serious and sometimes immediate allergic reactions have been reported. Patients and health care professionals should discuss various concerns before treatment, including whether the patient has recently received antibiotics by injection, or has taken muscle relaxants, allergy or cold medicine, sleep medicine, and aspirinlike products or blood thinners. It’s important to note that the dose of onabotulinumtoxinA is not the same as that for other botulinum toxin products. The full prescribing information is available on the Allergan website.
The Food and Drug Administration has approved onabotulinumtoxinA (Botox) for treatment of pediatric lower limb spasticity in patients aged 2-17 years, excluding those in whom it is associated with cerebral palsy, according to an announcement from Allergan.
The approval is based on a phase 3 study evaluating safety and efficacy in more than 300 patients with lower limb spasticity. Although patients with cerebral palsy were included in the study, they’re excluded from this indication. Orphan Drug Exclusivity prevents it from being indicated for lower limb spasticity in cerebral palsy because abobotulinumtoxinA (Dysport) already has marketing exclusivity for the indication. Botox also is indicated for children aged 2-17 years of age with upper limb spasticity, as well as nine other indications.
OnabotulinumtoxinA comes with warnings, including problems of swallowing, speaking, or breathing and even risk of spread of the toxin. It also may cause loss of strength or general muscle weakness, vision problems, or dizziness within hours or weeks of administration. Serious and sometimes immediate allergic reactions have been reported. Patients and health care professionals should discuss various concerns before treatment, including whether the patient has recently received antibiotics by injection, or has taken muscle relaxants, allergy or cold medicine, sleep medicine, and aspirinlike products or blood thinners. It’s important to note that the dose of onabotulinumtoxinA is not the same as that for other botulinum toxin products. The full prescribing information is available on the Allergan website.
The Food and Drug Administration has approved onabotulinumtoxinA (Botox) for treatment of pediatric lower limb spasticity in patients aged 2-17 years, excluding those in whom it is associated with cerebral palsy, according to an announcement from Allergan.
The approval is based on a phase 3 study evaluating safety and efficacy in more than 300 patients with lower limb spasticity. Although patients with cerebral palsy were included in the study, they’re excluded from this indication. Orphan Drug Exclusivity prevents it from being indicated for lower limb spasticity in cerebral palsy because abobotulinumtoxinA (Dysport) already has marketing exclusivity for the indication. Botox also is indicated for children aged 2-17 years of age with upper limb spasticity, as well as nine other indications.
OnabotulinumtoxinA comes with warnings, including problems of swallowing, speaking, or breathing and even risk of spread of the toxin. It also may cause loss of strength or general muscle weakness, vision problems, or dizziness within hours or weeks of administration. Serious and sometimes immediate allergic reactions have been reported. Patients and health care professionals should discuss various concerns before treatment, including whether the patient has recently received antibiotics by injection, or has taken muscle relaxants, allergy or cold medicine, sleep medicine, and aspirinlike products or blood thinners. It’s important to note that the dose of onabotulinumtoxinA is not the same as that for other botulinum toxin products. The full prescribing information is available on the Allergan website.
ACIP approves 2020 adult vaccination schedule
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the adult immunization schedule for 2020, although some fine-tuning may occur before publication.
“Some of the wordsmithing may be done later,” ACIP executive secretary Amanda Cohn, MD, said at the ACIP October meeting.
Key updates to the schedule included a change in wording for the definition of the red bars on the table to include “not recommended or contraindicated” instead of only the word “contraindicated.” Committee members were especially interested in changing this wording to guide clinicians in use of the live attenuated influenza vaccine because of its potential value in vaccinating health care personnel.
Other updates include language that vaccination of adolescents and young adults aged 16-23 years who are not at increased risk for meningococcal disease should be vaccinated as follows: “Based on shared clinical decision making, 2-dose series MenB-4C at least 1 month apart or 2-dose series MenB-FHbp at 0, 6 months.”
Similarly, clinical decision-making language was added to the notes for the pneumococcal polysaccharide vaccine (PPSV23) and the 13-valent pneumococcal conjugate vaccine (PCV13).
The routine vaccination calls for only one dose of PPSV23 given on or after the individual’s 65th birthday. Then, based on shared clinical decision making, a dose of PCV13 is recommended for immunocompetent individuals aged 65 years and older. The notes also state that, based on shared clinical decision making, PCV13 and PPSV23 should not be given in the same visit and, if both will be given, PCV13 should be first and should be given 1 year before PPSV23. In addition, “PPSV23 should be given at least 5 years after any previous PPSV23 dose.”
The schedule also adds shared clinical decision making to the notes on human papillomavirus vaccination for adults aged 27-45 years.
The committee members acknowledged the increasing complexity of the adult vaccination schedule, but several members agreed that it is accessible to many clinicians.
“We can’t let the perfect be the enemy of the good” said Jason Goldman, MD, liaison representing the American College of Physicians. “Those who want to learn the schedule will learn it; the health system will learn it,” even if not every specialist does.
The table “is something to draw you in,” said Sandra Fryhofer, MD, an internist who is liaison for the American Medical Association. The notes provide more details.
More specific information about contraindications for patients with cochlear implants, which also came up in the discussion, may be added to the schedule at a later date.
View the current adult vaccination schedule here.
The ACIP members had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the adult immunization schedule for 2020, although some fine-tuning may occur before publication.
“Some of the wordsmithing may be done later,” ACIP executive secretary Amanda Cohn, MD, said at the ACIP October meeting.
Key updates to the schedule included a change in wording for the definition of the red bars on the table to include “not recommended or contraindicated” instead of only the word “contraindicated.” Committee members were especially interested in changing this wording to guide clinicians in use of the live attenuated influenza vaccine because of its potential value in vaccinating health care personnel.
Other updates include language that vaccination of adolescents and young adults aged 16-23 years who are not at increased risk for meningococcal disease should be vaccinated as follows: “Based on shared clinical decision making, 2-dose series MenB-4C at least 1 month apart or 2-dose series MenB-FHbp at 0, 6 months.”
Similarly, clinical decision-making language was added to the notes for the pneumococcal polysaccharide vaccine (PPSV23) and the 13-valent pneumococcal conjugate vaccine (PCV13).
The routine vaccination calls for only one dose of PPSV23 given on or after the individual’s 65th birthday. Then, based on shared clinical decision making, a dose of PCV13 is recommended for immunocompetent individuals aged 65 years and older. The notes also state that, based on shared clinical decision making, PCV13 and PPSV23 should not be given in the same visit and, if both will be given, PCV13 should be first and should be given 1 year before PPSV23. In addition, “PPSV23 should be given at least 5 years after any previous PPSV23 dose.”
The schedule also adds shared clinical decision making to the notes on human papillomavirus vaccination for adults aged 27-45 years.
The committee members acknowledged the increasing complexity of the adult vaccination schedule, but several members agreed that it is accessible to many clinicians.
“We can’t let the perfect be the enemy of the good” said Jason Goldman, MD, liaison representing the American College of Physicians. “Those who want to learn the schedule will learn it; the health system will learn it,” even if not every specialist does.
The table “is something to draw you in,” said Sandra Fryhofer, MD, an internist who is liaison for the American Medical Association. The notes provide more details.
More specific information about contraindications for patients with cochlear implants, which also came up in the discussion, may be added to the schedule at a later date.
View the current adult vaccination schedule here.
The ACIP members had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the adult immunization schedule for 2020, although some fine-tuning may occur before publication.
“Some of the wordsmithing may be done later,” ACIP executive secretary Amanda Cohn, MD, said at the ACIP October meeting.
Key updates to the schedule included a change in wording for the definition of the red bars on the table to include “not recommended or contraindicated” instead of only the word “contraindicated.” Committee members were especially interested in changing this wording to guide clinicians in use of the live attenuated influenza vaccine because of its potential value in vaccinating health care personnel.
Other updates include language that vaccination of adolescents and young adults aged 16-23 years who are not at increased risk for meningococcal disease should be vaccinated as follows: “Based on shared clinical decision making, 2-dose series MenB-4C at least 1 month apart or 2-dose series MenB-FHbp at 0, 6 months.”
Similarly, clinical decision-making language was added to the notes for the pneumococcal polysaccharide vaccine (PPSV23) and the 13-valent pneumococcal conjugate vaccine (PCV13).
The routine vaccination calls for only one dose of PPSV23 given on or after the individual’s 65th birthday. Then, based on shared clinical decision making, a dose of PCV13 is recommended for immunocompetent individuals aged 65 years and older. The notes also state that, based on shared clinical decision making, PCV13 and PPSV23 should not be given in the same visit and, if both will be given, PCV13 should be first and should be given 1 year before PPSV23. In addition, “PPSV23 should be given at least 5 years after any previous PPSV23 dose.”
The schedule also adds shared clinical decision making to the notes on human papillomavirus vaccination for adults aged 27-45 years.
The committee members acknowledged the increasing complexity of the adult vaccination schedule, but several members agreed that it is accessible to many clinicians.
“We can’t let the perfect be the enemy of the good” said Jason Goldman, MD, liaison representing the American College of Physicians. “Those who want to learn the schedule will learn it; the health system will learn it,” even if not every specialist does.
The table “is something to draw you in,” said Sandra Fryhofer, MD, an internist who is liaison for the American Medical Association. The notes provide more details.
More specific information about contraindications for patients with cochlear implants, which also came up in the discussion, may be added to the schedule at a later date.
View the current adult vaccination schedule here.
The ACIP members had no financial conflicts to disclose.
FDA approves Trikafta for treatment of cystic fibrosis
in patients aged 12 years or older, the first triple-combination therapy approved for that indication.
Approval for Trikafta was based on results from two clinical trials in patients with cystic fibrosis with an F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. In the first trial, a 24-week, randomized, double-blind, placebo-controlled study of 403 patients, the mean percent predicted forced expiratory volume in 1 second increased by 14% from baseline, compared with placebo. In the second trial, a 4-week, randomized, double-blind, active-controlled study of 107 patients, mean percent predicted forced expiratory volume in 1 second was increased 10% from baseline, compared with tezacaftor/ivacaftor, according to the FDA press release.
In the first trial, patients who received Trikafta also saw improvement in sweat chloride, reduction in the number of pulmonary exacerbations, and reduction of body mass index, compared with placebo.
The most common adverse events associated with Trikafta during the trials were headaches, upper respiratory tract infections, abdominal pains, diarrhea, rashes, and rhinorrhea, among others. The label includes a warning related to elevated liver function tests, use at the same time with products that induce or inhibit a liver enzyme called cytochrome P450 3A4, and cataract risk.
“At the FDA, we’re consistently looking for ways to help speed the development of new therapies for complex diseases, while maintaining our high standards of review. Today’s landmark approval is a testament to these efforts, making a novel treatment available to most cystic fibrosis patients, including adolescents, who previously had no options and giving others in the cystic fibrosis community access to an additional effective therapy,” said acting FDA Commissioner Ned Sharpless, MD.
Find the full press release on the FDA website.
in patients aged 12 years or older, the first triple-combination therapy approved for that indication.
Approval for Trikafta was based on results from two clinical trials in patients with cystic fibrosis with an F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. In the first trial, a 24-week, randomized, double-blind, placebo-controlled study of 403 patients, the mean percent predicted forced expiratory volume in 1 second increased by 14% from baseline, compared with placebo. In the second trial, a 4-week, randomized, double-blind, active-controlled study of 107 patients, mean percent predicted forced expiratory volume in 1 second was increased 10% from baseline, compared with tezacaftor/ivacaftor, according to the FDA press release.
In the first trial, patients who received Trikafta also saw improvement in sweat chloride, reduction in the number of pulmonary exacerbations, and reduction of body mass index, compared with placebo.
The most common adverse events associated with Trikafta during the trials were headaches, upper respiratory tract infections, abdominal pains, diarrhea, rashes, and rhinorrhea, among others. The label includes a warning related to elevated liver function tests, use at the same time with products that induce or inhibit a liver enzyme called cytochrome P450 3A4, and cataract risk.
“At the FDA, we’re consistently looking for ways to help speed the development of new therapies for complex diseases, while maintaining our high standards of review. Today’s landmark approval is a testament to these efforts, making a novel treatment available to most cystic fibrosis patients, including adolescents, who previously had no options and giving others in the cystic fibrosis community access to an additional effective therapy,” said acting FDA Commissioner Ned Sharpless, MD.
Find the full press release on the FDA website.
in patients aged 12 years or older, the first triple-combination therapy approved for that indication.
Approval for Trikafta was based on results from two clinical trials in patients with cystic fibrosis with an F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. In the first trial, a 24-week, randomized, double-blind, placebo-controlled study of 403 patients, the mean percent predicted forced expiratory volume in 1 second increased by 14% from baseline, compared with placebo. In the second trial, a 4-week, randomized, double-blind, active-controlled study of 107 patients, mean percent predicted forced expiratory volume in 1 second was increased 10% from baseline, compared with tezacaftor/ivacaftor, according to the FDA press release.
In the first trial, patients who received Trikafta also saw improvement in sweat chloride, reduction in the number of pulmonary exacerbations, and reduction of body mass index, compared with placebo.
The most common adverse events associated with Trikafta during the trials were headaches, upper respiratory tract infections, abdominal pains, diarrhea, rashes, and rhinorrhea, among others. The label includes a warning related to elevated liver function tests, use at the same time with products that induce or inhibit a liver enzyme called cytochrome P450 3A4, and cataract risk.
“At the FDA, we’re consistently looking for ways to help speed the development of new therapies for complex diseases, while maintaining our high standards of review. Today’s landmark approval is a testament to these efforts, making a novel treatment available to most cystic fibrosis patients, including adolescents, who previously had no options and giving others in the cystic fibrosis community access to an additional effective therapy,” said acting FDA Commissioner Ned Sharpless, MD.
Find the full press release on the FDA website.
Flu vaccine: Larger impact on influenza burden than you thought?
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at [email protected].
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at [email protected].
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at [email protected].
EEG asymmetry predicts poor pediatric ECMO outcomes
ST. LOUIS – Children who have background EEG asymmetry while on extracorporeal membrane oxygenation (ECMO) have worse outcomes even after adjustment for recent cardiac arrest and EEG suppression, according to a review of 41 children treated at Washington University, St. Louis.
ECMO is a last-ditch heart/lung bypass for patients near death, be it from infection, trauma, cardiac abnormalities, or any other issue. Children can be on it for days or weeks while problems are addressed and the body attempts to recover. Sometimes ECMO works, and children make a remarkable recovery, but other times they die or are left with severe disabilities, and no one really knows why.
Because of this, the investigators in this review sought to identify predictors of poor outcomes with an eye toward identifying modifiable risk factors, said senior investigator Kristin Guilliams, MD, an assistant professor of pediatric critical care medicine.
“We are trying to figure out why some kids do fantastically, and others don’t. We were looking at whether EEG can give us any clues and new ways to think about modifiable risk factors so that every kid rescued by ECMO can go back to their normal life,” she said at the American Neurological Association annual meeting.
The 41 children had an EEG within a day or 2 of starting ECMO; 22 did well, but 19 had bad outcomes, defined in the study as either dying in the hospital or being discharged with a Functional Status Score above 12, meaning mild dysfunction across six domains or more severe disability in particular ones.
The finding that all four children with EEG suppression – overall low brain activity – did poorly was not surprising, but the fact that EEG background asymmetry – one side of the brain being much less active than the other or giving different signals – in five children predicted poor outcomes, even after adjustment for cardiac arrest and overall suppression, was “a big surprise,” Dr. Guilliams said (odds ratio, 29.3; 95% confidence interval, 2.2-398.3; P = .003).
“The asymmetry tells me that we need to look more closely into brain blood flow patterns on ECMO,” she said. There might be a way to change delivery that could help, but “it’s not obvious right now.” The issue warrants further investigation, Dr. Guilliams said.
Twelve children had ECMO during chest compressions for cardiac arrest, which as expected, also predicted poor outcomes (OR, 9.5; 95% CI 1.6-58.2; P = .008).
Neuroimaging was available for 34 children. Abnormalities (n = 13; P = .2), including ischemia (n = 8; P = .1), hemorrhage (n = 8; P = .06), and seizures (n = 4; P = .2) did not predict poor outcomes, nor did sex, age, and mode of ECMO delivery (veno-arterial versus veno-venous).
As of about a year ago, EEGs at the university are now standard for children on ECMO, with special software to pick out asymmetries. “We are paying more attention to” EEGs, Dr. Guilliams said.
Children were a median of about 10 years old, and subjects were at least 1 year old. There were about equal numbers of boys and girls; 25 children were alive at discharge.
There was no external funding, and Dr. Guilliams didn’t have any disclosures.
ST. LOUIS – Children who have background EEG asymmetry while on extracorporeal membrane oxygenation (ECMO) have worse outcomes even after adjustment for recent cardiac arrest and EEG suppression, according to a review of 41 children treated at Washington University, St. Louis.
ECMO is a last-ditch heart/lung bypass for patients near death, be it from infection, trauma, cardiac abnormalities, or any other issue. Children can be on it for days or weeks while problems are addressed and the body attempts to recover. Sometimes ECMO works, and children make a remarkable recovery, but other times they die or are left with severe disabilities, and no one really knows why.
Because of this, the investigators in this review sought to identify predictors of poor outcomes with an eye toward identifying modifiable risk factors, said senior investigator Kristin Guilliams, MD, an assistant professor of pediatric critical care medicine.
“We are trying to figure out why some kids do fantastically, and others don’t. We were looking at whether EEG can give us any clues and new ways to think about modifiable risk factors so that every kid rescued by ECMO can go back to their normal life,” she said at the American Neurological Association annual meeting.
The 41 children had an EEG within a day or 2 of starting ECMO; 22 did well, but 19 had bad outcomes, defined in the study as either dying in the hospital or being discharged with a Functional Status Score above 12, meaning mild dysfunction across six domains or more severe disability in particular ones.
The finding that all four children with EEG suppression – overall low brain activity – did poorly was not surprising, but the fact that EEG background asymmetry – one side of the brain being much less active than the other or giving different signals – in five children predicted poor outcomes, even after adjustment for cardiac arrest and overall suppression, was “a big surprise,” Dr. Guilliams said (odds ratio, 29.3; 95% confidence interval, 2.2-398.3; P = .003).
“The asymmetry tells me that we need to look more closely into brain blood flow patterns on ECMO,” she said. There might be a way to change delivery that could help, but “it’s not obvious right now.” The issue warrants further investigation, Dr. Guilliams said.
Twelve children had ECMO during chest compressions for cardiac arrest, which as expected, also predicted poor outcomes (OR, 9.5; 95% CI 1.6-58.2; P = .008).
Neuroimaging was available for 34 children. Abnormalities (n = 13; P = .2), including ischemia (n = 8; P = .1), hemorrhage (n = 8; P = .06), and seizures (n = 4; P = .2) did not predict poor outcomes, nor did sex, age, and mode of ECMO delivery (veno-arterial versus veno-venous).
As of about a year ago, EEGs at the university are now standard for children on ECMO, with special software to pick out asymmetries. “We are paying more attention to” EEGs, Dr. Guilliams said.
Children were a median of about 10 years old, and subjects were at least 1 year old. There were about equal numbers of boys and girls; 25 children were alive at discharge.
There was no external funding, and Dr. Guilliams didn’t have any disclosures.
ST. LOUIS – Children who have background EEG asymmetry while on extracorporeal membrane oxygenation (ECMO) have worse outcomes even after adjustment for recent cardiac arrest and EEG suppression, according to a review of 41 children treated at Washington University, St. Louis.
ECMO is a last-ditch heart/lung bypass for patients near death, be it from infection, trauma, cardiac abnormalities, or any other issue. Children can be on it for days or weeks while problems are addressed and the body attempts to recover. Sometimes ECMO works, and children make a remarkable recovery, but other times they die or are left with severe disabilities, and no one really knows why.
Because of this, the investigators in this review sought to identify predictors of poor outcomes with an eye toward identifying modifiable risk factors, said senior investigator Kristin Guilliams, MD, an assistant professor of pediatric critical care medicine.
“We are trying to figure out why some kids do fantastically, and others don’t. We were looking at whether EEG can give us any clues and new ways to think about modifiable risk factors so that every kid rescued by ECMO can go back to their normal life,” she said at the American Neurological Association annual meeting.
The 41 children had an EEG within a day or 2 of starting ECMO; 22 did well, but 19 had bad outcomes, defined in the study as either dying in the hospital or being discharged with a Functional Status Score above 12, meaning mild dysfunction across six domains or more severe disability in particular ones.
The finding that all four children with EEG suppression – overall low brain activity – did poorly was not surprising, but the fact that EEG background asymmetry – one side of the brain being much less active than the other or giving different signals – in five children predicted poor outcomes, even after adjustment for cardiac arrest and overall suppression, was “a big surprise,” Dr. Guilliams said (odds ratio, 29.3; 95% confidence interval, 2.2-398.3; P = .003).
“The asymmetry tells me that we need to look more closely into brain blood flow patterns on ECMO,” she said. There might be a way to change delivery that could help, but “it’s not obvious right now.” The issue warrants further investigation, Dr. Guilliams said.
Twelve children had ECMO during chest compressions for cardiac arrest, which as expected, also predicted poor outcomes (OR, 9.5; 95% CI 1.6-58.2; P = .008).
Neuroimaging was available for 34 children. Abnormalities (n = 13; P = .2), including ischemia (n = 8; P = .1), hemorrhage (n = 8; P = .06), and seizures (n = 4; P = .2) did not predict poor outcomes, nor did sex, age, and mode of ECMO delivery (veno-arterial versus veno-venous).
As of about a year ago, EEGs at the university are now standard for children on ECMO, with special software to pick out asymmetries. “We are paying more attention to” EEGs, Dr. Guilliams said.
Children were a median of about 10 years old, and subjects were at least 1 year old. There were about equal numbers of boys and girls; 25 children were alive at discharge.
There was no external funding, and Dr. Guilliams didn’t have any disclosures.
REPORTING FROM ANA 2019
One monoclonal dose gives preterm neonates season-long RSV protection
WASHINGTON – A single dose of a novel monoclonal antibody against a respiratory syncytial virus surface protein safely protected preterm infants against severe infections for 150 days during their first winter season in a randomized trial with more than 1,400 children.
One intramuscular injection of nirsevimab (also known as MEDI8897) administered to infants born at 29-35 weeks’ gestation at the start of the local respiratory syncytial virus (RSV) season (November in the Northern hemisphere) led to a 70% relative reduction in the rate of medically attended lower respiratory tract infections with RSV during the subsequent 150 days, compared with placebo, the study’s primary efficacy outcome, M. Pamela Griffin, MD, said at an annual scientific meeting on infectious diseases.
In a secondary efficacy measure, the rate of hospitalizations for RSV-caused lower respiratory tract infections, a single injection of nirsevimab dropped the incidence by 78%, relative to placebo. Both effects were statistically significant. The rate of total adverse events and serious adverse events was similar in the two treatment arms, reported Dr. Griffin, a clinical development lead with AstraZeneca.
These positive results for a single intramuscular injection of nirsevimab are the first findings from a series of studies aimed at getting the monoclonal antibody onto the U.S. market as a superior alternative to palivizumab (Synagis), which acts in a similar way to block RSV infection (albeit by targeting a different viral surface protein) but which requires administration every 30 days. This need for serial dosing of palivizumab in children younger than 1 year old for complete seasonal protection against RSV is probably a reason why the American Academy of Pediatrics, as well as other medical societies, have targeted using palivizumab only on certain types of high-risk infants: those born before 29 weeks’ gestational age, with chronic lung disease of prematurity, or with hemodynamically significant congenital heart disease (Pediatrics. 2014 Aug;134[2]:415-20). “It’s not feasible for most infants to come for five treatments during RSV season,” Dr. Griffin noted. A tweak in the structure of nirsevimab gives it a much longer blood half-life than palivizumab and allows a single dose to maintain efficacy for 5 months, the duration of RSV season.
“The big advantage of nirsevimab is one dose instead of five,” she said in an interview.
The study randomized 969 preterm infants to nirsevimab and 484 to placebo when the children averaged 3 months old and 4.5 kg. The incidence of the primary endpoint was 2.6% in the nirsevimab-treated infants and 9.5% in those who received placebo. The incidence of hospitalizations associated with an RSV lower respiratory tract infection was 0.8% in the nirsevimab group and 4.1% on placebo. Nirsevimab was equally effective regardless of RSV subtype, infant age, or sex. The rate of hypersensitivity reactions was low, less than 1%, and similar in the two treatment arms, as was the rate of detection of antidrug antibody, 3.8% with placebo and 5.6% with nirsevimab.
Two other large trials are underway to document the performance of nirsevimab in other types of infants. One study is examining the drug’s performance compared with placebo in term infants with a gestational age of at least 36 weeks, while another is comparing nirsevimab against a five-dose regimen of palivizumab in high-risk infants who are recommended to receive palivizumab by local medical societies. In the United States, this would be infants born at less than 29 weeks’ gestation, and those with either hemodynamically significant congenital heart disease or chronic lung disease of prematurity. In these studies, the researchers also will assess the cost effectiveness of nirsevimab relative to the costs for medical care needed by infants who receive comparator treatments, Dr. Griffin said.
The study was funded by AstraZeneca, the company developing nirsevimab. Dr. Griffin is an employee of and shareholder in AstraZeneca.
SOURCE: ClinicalTrials.gov identifier: NCT02878330.
WASHINGTON – A single dose of a novel monoclonal antibody against a respiratory syncytial virus surface protein safely protected preterm infants against severe infections for 150 days during their first winter season in a randomized trial with more than 1,400 children.
One intramuscular injection of nirsevimab (also known as MEDI8897) administered to infants born at 29-35 weeks’ gestation at the start of the local respiratory syncytial virus (RSV) season (November in the Northern hemisphere) led to a 70% relative reduction in the rate of medically attended lower respiratory tract infections with RSV during the subsequent 150 days, compared with placebo, the study’s primary efficacy outcome, M. Pamela Griffin, MD, said at an annual scientific meeting on infectious diseases.
In a secondary efficacy measure, the rate of hospitalizations for RSV-caused lower respiratory tract infections, a single injection of nirsevimab dropped the incidence by 78%, relative to placebo. Both effects were statistically significant. The rate of total adverse events and serious adverse events was similar in the two treatment arms, reported Dr. Griffin, a clinical development lead with AstraZeneca.
These positive results for a single intramuscular injection of nirsevimab are the first findings from a series of studies aimed at getting the monoclonal antibody onto the U.S. market as a superior alternative to palivizumab (Synagis), which acts in a similar way to block RSV infection (albeit by targeting a different viral surface protein) but which requires administration every 30 days. This need for serial dosing of palivizumab in children younger than 1 year old for complete seasonal protection against RSV is probably a reason why the American Academy of Pediatrics, as well as other medical societies, have targeted using palivizumab only on certain types of high-risk infants: those born before 29 weeks’ gestational age, with chronic lung disease of prematurity, or with hemodynamically significant congenital heart disease (Pediatrics. 2014 Aug;134[2]:415-20). “It’s not feasible for most infants to come for five treatments during RSV season,” Dr. Griffin noted. A tweak in the structure of nirsevimab gives it a much longer blood half-life than palivizumab and allows a single dose to maintain efficacy for 5 months, the duration of RSV season.
“The big advantage of nirsevimab is one dose instead of five,” she said in an interview.
The study randomized 969 preterm infants to nirsevimab and 484 to placebo when the children averaged 3 months old and 4.5 kg. The incidence of the primary endpoint was 2.6% in the nirsevimab-treated infants and 9.5% in those who received placebo. The incidence of hospitalizations associated with an RSV lower respiratory tract infection was 0.8% in the nirsevimab group and 4.1% on placebo. Nirsevimab was equally effective regardless of RSV subtype, infant age, or sex. The rate of hypersensitivity reactions was low, less than 1%, and similar in the two treatment arms, as was the rate of detection of antidrug antibody, 3.8% with placebo and 5.6% with nirsevimab.
Two other large trials are underway to document the performance of nirsevimab in other types of infants. One study is examining the drug’s performance compared with placebo in term infants with a gestational age of at least 36 weeks, while another is comparing nirsevimab against a five-dose regimen of palivizumab in high-risk infants who are recommended to receive palivizumab by local medical societies. In the United States, this would be infants born at less than 29 weeks’ gestation, and those with either hemodynamically significant congenital heart disease or chronic lung disease of prematurity. In these studies, the researchers also will assess the cost effectiveness of nirsevimab relative to the costs for medical care needed by infants who receive comparator treatments, Dr. Griffin said.
The study was funded by AstraZeneca, the company developing nirsevimab. Dr. Griffin is an employee of and shareholder in AstraZeneca.
SOURCE: ClinicalTrials.gov identifier: NCT02878330.
WASHINGTON – A single dose of a novel monoclonal antibody against a respiratory syncytial virus surface protein safely protected preterm infants against severe infections for 150 days during their first winter season in a randomized trial with more than 1,400 children.
One intramuscular injection of nirsevimab (also known as MEDI8897) administered to infants born at 29-35 weeks’ gestation at the start of the local respiratory syncytial virus (RSV) season (November in the Northern hemisphere) led to a 70% relative reduction in the rate of medically attended lower respiratory tract infections with RSV during the subsequent 150 days, compared with placebo, the study’s primary efficacy outcome, M. Pamela Griffin, MD, said at an annual scientific meeting on infectious diseases.
In a secondary efficacy measure, the rate of hospitalizations for RSV-caused lower respiratory tract infections, a single injection of nirsevimab dropped the incidence by 78%, relative to placebo. Both effects were statistically significant. The rate of total adverse events and serious adverse events was similar in the two treatment arms, reported Dr. Griffin, a clinical development lead with AstraZeneca.
These positive results for a single intramuscular injection of nirsevimab are the first findings from a series of studies aimed at getting the monoclonal antibody onto the U.S. market as a superior alternative to palivizumab (Synagis), which acts in a similar way to block RSV infection (albeit by targeting a different viral surface protein) but which requires administration every 30 days. This need for serial dosing of palivizumab in children younger than 1 year old for complete seasonal protection against RSV is probably a reason why the American Academy of Pediatrics, as well as other medical societies, have targeted using palivizumab only on certain types of high-risk infants: those born before 29 weeks’ gestational age, with chronic lung disease of prematurity, or with hemodynamically significant congenital heart disease (Pediatrics. 2014 Aug;134[2]:415-20). “It’s not feasible for most infants to come for five treatments during RSV season,” Dr. Griffin noted. A tweak in the structure of nirsevimab gives it a much longer blood half-life than palivizumab and allows a single dose to maintain efficacy for 5 months, the duration of RSV season.
“The big advantage of nirsevimab is one dose instead of five,” she said in an interview.
The study randomized 969 preterm infants to nirsevimab and 484 to placebo when the children averaged 3 months old and 4.5 kg. The incidence of the primary endpoint was 2.6% in the nirsevimab-treated infants and 9.5% in those who received placebo. The incidence of hospitalizations associated with an RSV lower respiratory tract infection was 0.8% in the nirsevimab group and 4.1% on placebo. Nirsevimab was equally effective regardless of RSV subtype, infant age, or sex. The rate of hypersensitivity reactions was low, less than 1%, and similar in the two treatment arms, as was the rate of detection of antidrug antibody, 3.8% with placebo and 5.6% with nirsevimab.
Two other large trials are underway to document the performance of nirsevimab in other types of infants. One study is examining the drug’s performance compared with placebo in term infants with a gestational age of at least 36 weeks, while another is comparing nirsevimab against a five-dose regimen of palivizumab in high-risk infants who are recommended to receive palivizumab by local medical societies. In the United States, this would be infants born at less than 29 weeks’ gestation, and those with either hemodynamically significant congenital heart disease or chronic lung disease of prematurity. In these studies, the researchers also will assess the cost effectiveness of nirsevimab relative to the costs for medical care needed by infants who receive comparator treatments, Dr. Griffin said.
The study was funded by AstraZeneca, the company developing nirsevimab. Dr. Griffin is an employee of and shareholder in AstraZeneca.
SOURCE: ClinicalTrials.gov identifier: NCT02878330.
REPORTING FROM ID WEEK 2019
Robot-assisted, gamelike tool effective for classifying ADHD
A novel robot-assisted, gamelike test accurately classified ADHD type in elementary school–aged children, according to Mun-Taek Choi, PhD, and associates.
A total of 326 children in the third and fourth grades were included in the study, 35 of whom had been diagnosed with ADHD and 26 of whom were at risk. For the 10- to 12-minute test, participants followed a robot on a path across a numbered mat while stimuli were shown on a TV with both images and sound, and completed a task at each numbered square, reported Dr. Choi, of Sungkyunkwan University, Suwan, South Korea, and associates. The study was published in the Journal of Intelligent & Robotic Systems.
Inattentive and hyperactive-impulsive behavior was measured by the number of omission and commission errors. Response time and task completion time contributed to the measure of inattentive and hyperactive-impulsive behavior. Working memory deficits were measured as deviations in the prescribed route.
This figure improved over the course of the study as the tool learned more, indicating that generalization errors were not a serious issue for the tool, the investigators noted.
“Unlike conventional questionnaire-based tests, the robot-assisted test increases the accuracy of ADHD diagnosis by directly reflecting the quality of children’s behavior during the activity game with the robot involved in the action. Since the test obtains behavioral patterns and levels using robotic sensing technologies, it can reliably determine the three key elements of ADHD diagnosis: hyperactivity, inattentive behavior, and working memory,” the investigators wrote. Ultimately, Dr. Choi and associates wrote, the tool could help clinicians diagnose childhood ADHD.
The study was funded by the South Korean Ministry of Trade, Industry, & Energy. No disclosures were reported.
SOURCE: Choi M-T et al. J Intell Robot Syst. 2018 Jun 19. doi: 10.1007/s10846-018-0890-9.
A novel robot-assisted, gamelike test accurately classified ADHD type in elementary school–aged children, according to Mun-Taek Choi, PhD, and associates.
A total of 326 children in the third and fourth grades were included in the study, 35 of whom had been diagnosed with ADHD and 26 of whom were at risk. For the 10- to 12-minute test, participants followed a robot on a path across a numbered mat while stimuli were shown on a TV with both images and sound, and completed a task at each numbered square, reported Dr. Choi, of Sungkyunkwan University, Suwan, South Korea, and associates. The study was published in the Journal of Intelligent & Robotic Systems.
Inattentive and hyperactive-impulsive behavior was measured by the number of omission and commission errors. Response time and task completion time contributed to the measure of inattentive and hyperactive-impulsive behavior. Working memory deficits were measured as deviations in the prescribed route.
This figure improved over the course of the study as the tool learned more, indicating that generalization errors were not a serious issue for the tool, the investigators noted.
“Unlike conventional questionnaire-based tests, the robot-assisted test increases the accuracy of ADHD diagnosis by directly reflecting the quality of children’s behavior during the activity game with the robot involved in the action. Since the test obtains behavioral patterns and levels using robotic sensing technologies, it can reliably determine the three key elements of ADHD diagnosis: hyperactivity, inattentive behavior, and working memory,” the investigators wrote. Ultimately, Dr. Choi and associates wrote, the tool could help clinicians diagnose childhood ADHD.
The study was funded by the South Korean Ministry of Trade, Industry, & Energy. No disclosures were reported.
SOURCE: Choi M-T et al. J Intell Robot Syst. 2018 Jun 19. doi: 10.1007/s10846-018-0890-9.
A novel robot-assisted, gamelike test accurately classified ADHD type in elementary school–aged children, according to Mun-Taek Choi, PhD, and associates.
A total of 326 children in the third and fourth grades were included in the study, 35 of whom had been diagnosed with ADHD and 26 of whom were at risk. For the 10- to 12-minute test, participants followed a robot on a path across a numbered mat while stimuli were shown on a TV with both images and sound, and completed a task at each numbered square, reported Dr. Choi, of Sungkyunkwan University, Suwan, South Korea, and associates. The study was published in the Journal of Intelligent & Robotic Systems.
Inattentive and hyperactive-impulsive behavior was measured by the number of omission and commission errors. Response time and task completion time contributed to the measure of inattentive and hyperactive-impulsive behavior. Working memory deficits were measured as deviations in the prescribed route.
This figure improved over the course of the study as the tool learned more, indicating that generalization errors were not a serious issue for the tool, the investigators noted.
“Unlike conventional questionnaire-based tests, the robot-assisted test increases the accuracy of ADHD diagnosis by directly reflecting the quality of children’s behavior during the activity game with the robot involved in the action. Since the test obtains behavioral patterns and levels using robotic sensing technologies, it can reliably determine the three key elements of ADHD diagnosis: hyperactivity, inattentive behavior, and working memory,” the investigators wrote. Ultimately, Dr. Choi and associates wrote, the tool could help clinicians diagnose childhood ADHD.
The study was funded by the South Korean Ministry of Trade, Industry, & Energy. No disclosures were reported.
SOURCE: Choi M-T et al. J Intell Robot Syst. 2018 Jun 19. doi: 10.1007/s10846-018-0890-9.
FROM THE JOURNAL OF INTELLIGENT & ROBOTIC SYSTEMS
Research on pediatric firearms deaths is underfunded
new research has found.
For the period 2008-2017, an average of $88 million per year was granted to study motor vehicle crashes, the leading cause of death in this age group. Cancer, the third leading cause of mortality, received on average $335 million per year. However, research into mortality from firearms, the second leading cause of death in this age group, received $12 million total during the entire research period across a total of 32 research grants.
This translates to $26,136 in research funding per death for the 33,577 deaths of children and adolescents in motor vehicle crashes from 2008-2017, $195,508 per death from cancer (17,111 deaths recorded), and just $597 per death from firearm injury (20,719 deaths recorded).
Pediatric firearm injury prevention “is substantially underfunded in relation to the magnitude of the public health problem,” Rebecca Cunningham, MD, from the University of Michigan, Ann Arbor, and colleagues wrote in the October 2019 issue of Health Affairs.
“According to our analysis, federal funding for this leading cause of pediatric mortality is 3.3 percent of what would be needed for it to be commensurate with the funding for other common causes of pediatric death,” the authors continued.
Dr. Cunningham and colleagues said that the “lack of an evidence base for firearm safety prevention has likely contributed to the lack of progress on, and recent increase in, firearm deaths among children and adolescents since 2013.”
They did note that there was an increase in federal research funding following the shooting in Newtown, Conn., with an increase from $136,224 in 2012 to $4.5 million in 2017, but it clearly is not enough.
“Our analysis, using other major diseases and the country’s history of federal funding as a guide, demonstrates that approximately $37 million per year over the next decade is needed to realize a reduction in pediatric firearm mortality that is comparable to that observed for other pediatric causes of death,” the authors state.
The group also suggests the development of a group similar to the National Highway Traffic Safety Administration that is focused specifically on firearm safety that could “begin to address the large gaps in foundational epidemiological and multidisciplinary behavioral research that the nation needs. It could have a transformational impact on the reduction of firearm injuries among children and adolescents parallel to what has been seen for other major causes of pediatric death in the U.S.”
SOURCE: Cunningham R et al. Health Affairs. 2019. doi: 10.1377/hlthaff.2019.00476.
new research has found.
For the period 2008-2017, an average of $88 million per year was granted to study motor vehicle crashes, the leading cause of death in this age group. Cancer, the third leading cause of mortality, received on average $335 million per year. However, research into mortality from firearms, the second leading cause of death in this age group, received $12 million total during the entire research period across a total of 32 research grants.
This translates to $26,136 in research funding per death for the 33,577 deaths of children and adolescents in motor vehicle crashes from 2008-2017, $195,508 per death from cancer (17,111 deaths recorded), and just $597 per death from firearm injury (20,719 deaths recorded).
Pediatric firearm injury prevention “is substantially underfunded in relation to the magnitude of the public health problem,” Rebecca Cunningham, MD, from the University of Michigan, Ann Arbor, and colleagues wrote in the October 2019 issue of Health Affairs.
“According to our analysis, federal funding for this leading cause of pediatric mortality is 3.3 percent of what would be needed for it to be commensurate with the funding for other common causes of pediatric death,” the authors continued.
Dr. Cunningham and colleagues said that the “lack of an evidence base for firearm safety prevention has likely contributed to the lack of progress on, and recent increase in, firearm deaths among children and adolescents since 2013.”
They did note that there was an increase in federal research funding following the shooting in Newtown, Conn., with an increase from $136,224 in 2012 to $4.5 million in 2017, but it clearly is not enough.
“Our analysis, using other major diseases and the country’s history of federal funding as a guide, demonstrates that approximately $37 million per year over the next decade is needed to realize a reduction in pediatric firearm mortality that is comparable to that observed for other pediatric causes of death,” the authors state.
The group also suggests the development of a group similar to the National Highway Traffic Safety Administration that is focused specifically on firearm safety that could “begin to address the large gaps in foundational epidemiological and multidisciplinary behavioral research that the nation needs. It could have a transformational impact on the reduction of firearm injuries among children and adolescents parallel to what has been seen for other major causes of pediatric death in the U.S.”
SOURCE: Cunningham R et al. Health Affairs. 2019. doi: 10.1377/hlthaff.2019.00476.
new research has found.
For the period 2008-2017, an average of $88 million per year was granted to study motor vehicle crashes, the leading cause of death in this age group. Cancer, the third leading cause of mortality, received on average $335 million per year. However, research into mortality from firearms, the second leading cause of death in this age group, received $12 million total during the entire research period across a total of 32 research grants.
This translates to $26,136 in research funding per death for the 33,577 deaths of children and adolescents in motor vehicle crashes from 2008-2017, $195,508 per death from cancer (17,111 deaths recorded), and just $597 per death from firearm injury (20,719 deaths recorded).
Pediatric firearm injury prevention “is substantially underfunded in relation to the magnitude of the public health problem,” Rebecca Cunningham, MD, from the University of Michigan, Ann Arbor, and colleagues wrote in the October 2019 issue of Health Affairs.
“According to our analysis, federal funding for this leading cause of pediatric mortality is 3.3 percent of what would be needed for it to be commensurate with the funding for other common causes of pediatric death,” the authors continued.
Dr. Cunningham and colleagues said that the “lack of an evidence base for firearm safety prevention has likely contributed to the lack of progress on, and recent increase in, firearm deaths among children and adolescents since 2013.”
They did note that there was an increase in federal research funding following the shooting in Newtown, Conn., with an increase from $136,224 in 2012 to $4.5 million in 2017, but it clearly is not enough.
“Our analysis, using other major diseases and the country’s history of federal funding as a guide, demonstrates that approximately $37 million per year over the next decade is needed to realize a reduction in pediatric firearm mortality that is comparable to that observed for other pediatric causes of death,” the authors state.
The group also suggests the development of a group similar to the National Highway Traffic Safety Administration that is focused specifically on firearm safety that could “begin to address the large gaps in foundational epidemiological and multidisciplinary behavioral research that the nation needs. It could have a transformational impact on the reduction of firearm injuries among children and adolescents parallel to what has been seen for other major causes of pediatric death in the U.S.”
SOURCE: Cunningham R et al. Health Affairs. 2019. doi: 10.1377/hlthaff.2019.00476.
FROM HEALTH AFFAIRS