User login
Study Links Maternal Hidradenitis Suppurativa to Risk for Childhood Morbidity
SAN DIEGO — , and other conditions.
Those are key findings from a longitudinal cohort study that was presented during a poster session at the annual meeting of the American Academy of Dermatology.
“HS is associated with morbidity in women of reproductive age and adverse pregnancy outcomes, [but] its effect on offspring outcomes remains unclear,” corresponding author Kaiyang Li, a third-year medical student at McGill University, Quebec, Canada, and coauthors wrote in their abstract.
To investigate the association between maternal HS and offspring outcomes at birth and with up to 16 years of follow-up, the researchers drew from a longitudinal cohort of 1,275,593 children born in Quebec between April 1, 2006 and March 31, 2022. They matched children with their mothers and used identification numbers to follow the children to note morbidities that led to hospital admissions before age 16 years. The exposure of interest was HS, and the main outcome measure was childhood hospitalizations for respiratory, cardiovascular, metabolic, and other morbidities prior to age 16 years.
Next, they estimated hazard ratios (HR) and 95% CIs for the association of maternal HS with childhood morbidity in adjusted Cox proportional hazards regression models. “As prenatal exposure to hyperandrogenism may influence boys and girls differently, we carried out subgroup analyses stratified by child sex,” they wrote.
The study population included 1283 children whose mothers had HS and 1,274,310 unexposed children. As for infant outcomes, compared with no exposure, maternal HS was associated with an increased risk for preterm birth (relative risk [RR], 1.29; 95% CI, 1,08-1.55), neonatal death (RR, 2.07; 95% CI, 1.03-14.13), birth defects (RR, 1.29; 95% CI, 1.07-1.56), congenital heart defects (RR, 1.57; 95% CI, 1.02-2.44), and orofacial defects (RR 4.29; 95% CI, 1.85-9.97).
As for long-term outcomes in the children, compared with those whose mothers did not have HS, maternal HS was associated with an increased risk for any childhood hospitalization (HR, 1.31; 95% CI, 1.19-1.44), respiratory hospitalization (HR, 1.21; 95% CI, 1.05-1.40), metabolic hospitalization (HR, 2.64; 95% CI, 1.67-4.20), gastrointestinal hospitalization (HR, 1.34; 95% CI, 1.03-1.74), and developmental hospitalization (HR, 1.92; 95% CI, 1.43-2.58).
Commenting on the results after the meeting, Ms. Li said that the findings support the need for timely management of HS in expectant mothers and people planning to conceive, and for “interdisciplinary care and follow up for both the mother and the baby, involving the dermatologist, the obstetrician, and the neonatologist or pediatrician if needed.”
“HS is a multidisciplinary disease, plain and simple,” Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, who was asked to comment on the study, said in an interview. “This study highlights the importance of collaboration between dermatology and obstetrician-gynecologist given the potential negative pregnancy outcomes, but to me raising alarm bells given the known gaps and delays in diagnosis matched to disease onset,” said Dr. Friedman, who was not involved with the study. “We need to do better to ensure the safety of both patient and patient-to-be.”
The researchers reported having no financial disclosures. The abstract was selected as the second-place winner in the AAD’s poster competition. Dr. Friedman has no relevant disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — , and other conditions.
Those are key findings from a longitudinal cohort study that was presented during a poster session at the annual meeting of the American Academy of Dermatology.
“HS is associated with morbidity in women of reproductive age and adverse pregnancy outcomes, [but] its effect on offspring outcomes remains unclear,” corresponding author Kaiyang Li, a third-year medical student at McGill University, Quebec, Canada, and coauthors wrote in their abstract.
To investigate the association between maternal HS and offspring outcomes at birth and with up to 16 years of follow-up, the researchers drew from a longitudinal cohort of 1,275,593 children born in Quebec between April 1, 2006 and March 31, 2022. They matched children with their mothers and used identification numbers to follow the children to note morbidities that led to hospital admissions before age 16 years. The exposure of interest was HS, and the main outcome measure was childhood hospitalizations for respiratory, cardiovascular, metabolic, and other morbidities prior to age 16 years.
Next, they estimated hazard ratios (HR) and 95% CIs for the association of maternal HS with childhood morbidity in adjusted Cox proportional hazards regression models. “As prenatal exposure to hyperandrogenism may influence boys and girls differently, we carried out subgroup analyses stratified by child sex,” they wrote.
The study population included 1283 children whose mothers had HS and 1,274,310 unexposed children. As for infant outcomes, compared with no exposure, maternal HS was associated with an increased risk for preterm birth (relative risk [RR], 1.29; 95% CI, 1,08-1.55), neonatal death (RR, 2.07; 95% CI, 1.03-14.13), birth defects (RR, 1.29; 95% CI, 1.07-1.56), congenital heart defects (RR, 1.57; 95% CI, 1.02-2.44), and orofacial defects (RR 4.29; 95% CI, 1.85-9.97).
As for long-term outcomes in the children, compared with those whose mothers did not have HS, maternal HS was associated with an increased risk for any childhood hospitalization (HR, 1.31; 95% CI, 1.19-1.44), respiratory hospitalization (HR, 1.21; 95% CI, 1.05-1.40), metabolic hospitalization (HR, 2.64; 95% CI, 1.67-4.20), gastrointestinal hospitalization (HR, 1.34; 95% CI, 1.03-1.74), and developmental hospitalization (HR, 1.92; 95% CI, 1.43-2.58).
Commenting on the results after the meeting, Ms. Li said that the findings support the need for timely management of HS in expectant mothers and people planning to conceive, and for “interdisciplinary care and follow up for both the mother and the baby, involving the dermatologist, the obstetrician, and the neonatologist or pediatrician if needed.”
“HS is a multidisciplinary disease, plain and simple,” Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, who was asked to comment on the study, said in an interview. “This study highlights the importance of collaboration between dermatology and obstetrician-gynecologist given the potential negative pregnancy outcomes, but to me raising alarm bells given the known gaps and delays in diagnosis matched to disease onset,” said Dr. Friedman, who was not involved with the study. “We need to do better to ensure the safety of both patient and patient-to-be.”
The researchers reported having no financial disclosures. The abstract was selected as the second-place winner in the AAD’s poster competition. Dr. Friedman has no relevant disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — , and other conditions.
Those are key findings from a longitudinal cohort study that was presented during a poster session at the annual meeting of the American Academy of Dermatology.
“HS is associated with morbidity in women of reproductive age and adverse pregnancy outcomes, [but] its effect on offspring outcomes remains unclear,” corresponding author Kaiyang Li, a third-year medical student at McGill University, Quebec, Canada, and coauthors wrote in their abstract.
To investigate the association between maternal HS and offspring outcomes at birth and with up to 16 years of follow-up, the researchers drew from a longitudinal cohort of 1,275,593 children born in Quebec between April 1, 2006 and March 31, 2022. They matched children with their mothers and used identification numbers to follow the children to note morbidities that led to hospital admissions before age 16 years. The exposure of interest was HS, and the main outcome measure was childhood hospitalizations for respiratory, cardiovascular, metabolic, and other morbidities prior to age 16 years.
Next, they estimated hazard ratios (HR) and 95% CIs for the association of maternal HS with childhood morbidity in adjusted Cox proportional hazards regression models. “As prenatal exposure to hyperandrogenism may influence boys and girls differently, we carried out subgroup analyses stratified by child sex,” they wrote.
The study population included 1283 children whose mothers had HS and 1,274,310 unexposed children. As for infant outcomes, compared with no exposure, maternal HS was associated with an increased risk for preterm birth (relative risk [RR], 1.29; 95% CI, 1,08-1.55), neonatal death (RR, 2.07; 95% CI, 1.03-14.13), birth defects (RR, 1.29; 95% CI, 1.07-1.56), congenital heart defects (RR, 1.57; 95% CI, 1.02-2.44), and orofacial defects (RR 4.29; 95% CI, 1.85-9.97).
As for long-term outcomes in the children, compared with those whose mothers did not have HS, maternal HS was associated with an increased risk for any childhood hospitalization (HR, 1.31; 95% CI, 1.19-1.44), respiratory hospitalization (HR, 1.21; 95% CI, 1.05-1.40), metabolic hospitalization (HR, 2.64; 95% CI, 1.67-4.20), gastrointestinal hospitalization (HR, 1.34; 95% CI, 1.03-1.74), and developmental hospitalization (HR, 1.92; 95% CI, 1.43-2.58).
Commenting on the results after the meeting, Ms. Li said that the findings support the need for timely management of HS in expectant mothers and people planning to conceive, and for “interdisciplinary care and follow up for both the mother and the baby, involving the dermatologist, the obstetrician, and the neonatologist or pediatrician if needed.”
“HS is a multidisciplinary disease, plain and simple,” Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, who was asked to comment on the study, said in an interview. “This study highlights the importance of collaboration between dermatology and obstetrician-gynecologist given the potential negative pregnancy outcomes, but to me raising alarm bells given the known gaps and delays in diagnosis matched to disease onset,” said Dr. Friedman, who was not involved with the study. “We need to do better to ensure the safety of both patient and patient-to-be.”
The researchers reported having no financial disclosures. The abstract was selected as the second-place winner in the AAD’s poster competition. Dr. Friedman has no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM AAD 2024
Most Cancer Trial Centers Located Closer to White, Affluent Populations
This inequity may be potentiating the underrepresentation of racially minoritized and socioeconomically disadvantaged populations in clinical trials, suggesting that employment of satellite hospitals is needed to expand access to investigational therapies, reported lead author Hassal Lee, MD, PhD, of Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, and colleagues.
“Minoritized and socioeconomically disadvantaged populations are underrepresented in clinical trials,” the investigators wrote in JAMA Oncology. “This may reduce the generalizability of trial results and propagate health disparities. Contributors to inequitable trial participation include individual-level factors and structural factors.”
Specifically, travel time to trial centers, as well as socioeconomic deprivation, can reduce likelihood of trial participation.
“Data on these parameters and population data on self-identified race exist, but their interrelation with clinical research facilities has not been systematically analyzed,” they wrote.
To try to draw comparisons between the distribution of patients of different races and socioeconomic statuses and the locations of clinical research facilities, Dr. Lee and colleagues aggregated data from the US Census, National Trial registry, Nature Index of Cancer Research Health Institutions, OpenStreetMap, National Cancer Institute–designated Cancer Centers list, and National Homeland Infrastructure Foundation. They then characterized catchment population demographics within 30-, 60-, and 120-minute driving commute times of all US hospitals, along with a more focused look at centers capable of conducting phase 1, phase 2, and phase 3 trials.
These efforts revealed broad geographic inequity.The 78 major centers that conduct 94% of all US cancer trials are located within 30 minutes of populations that have a 10.1% higher proportion of self-identified White individuals than the average US county, and a median income $18,900 higher than average (unpaired mean differences).
The publication also includes several maps characterizing racial and socioeconomic demographics within various catchment areas. For example, centers in New York City, Houston, and Chicago have the most diverse catchment populations within a 30-minute commute. Maps of all cities in the United States with populations greater than 500,000 are available in a supplementary index.
“This study indicates that geographical population distributions may present barriers to equitable clinical trial access and that data are available to proactively strategize about reduction of such barriers,” Dr. Lee and colleagues wrote.
The findings call attention to modifiable socioeconomic factors associated with trial participation, they added, like financial toxicity and affordable transportation, noting that ethnic and racial groups consent to trials at similar rates after controlling for income.
In addition, Dr. Lee and colleagues advised clinical trial designers to enlist satellite hospitals to increase participant diversity, since long commutes exacerbate “socioeconomic burdens associated with clinical trial participation,” with trial participation decreasing as commute time increases.
“Existing clinical trial centers may build collaborative efforts with nearby hospitals closer to underrepresented populations or set up community centers to support new collaborative networks to improve geographical access equity,” they wrote. “Methodologically, our approach is transferable to any country, region, or global effort with sufficient source data and can inform decision-making along the continuum of cancer care, from screening to implementing specialist care.”
A coauthor disclosed relationships with Flagship Therapeutics, Leidos Holding Ltd, Pershing Square Foundation, and others.
This inequity may be potentiating the underrepresentation of racially minoritized and socioeconomically disadvantaged populations in clinical trials, suggesting that employment of satellite hospitals is needed to expand access to investigational therapies, reported lead author Hassal Lee, MD, PhD, of Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, and colleagues.
“Minoritized and socioeconomically disadvantaged populations are underrepresented in clinical trials,” the investigators wrote in JAMA Oncology. “This may reduce the generalizability of trial results and propagate health disparities. Contributors to inequitable trial participation include individual-level factors and structural factors.”
Specifically, travel time to trial centers, as well as socioeconomic deprivation, can reduce likelihood of trial participation.
“Data on these parameters and population data on self-identified race exist, but their interrelation with clinical research facilities has not been systematically analyzed,” they wrote.
To try to draw comparisons between the distribution of patients of different races and socioeconomic statuses and the locations of clinical research facilities, Dr. Lee and colleagues aggregated data from the US Census, National Trial registry, Nature Index of Cancer Research Health Institutions, OpenStreetMap, National Cancer Institute–designated Cancer Centers list, and National Homeland Infrastructure Foundation. They then characterized catchment population demographics within 30-, 60-, and 120-minute driving commute times of all US hospitals, along with a more focused look at centers capable of conducting phase 1, phase 2, and phase 3 trials.
These efforts revealed broad geographic inequity.The 78 major centers that conduct 94% of all US cancer trials are located within 30 minutes of populations that have a 10.1% higher proportion of self-identified White individuals than the average US county, and a median income $18,900 higher than average (unpaired mean differences).
The publication also includes several maps characterizing racial and socioeconomic demographics within various catchment areas. For example, centers in New York City, Houston, and Chicago have the most diverse catchment populations within a 30-minute commute. Maps of all cities in the United States with populations greater than 500,000 are available in a supplementary index.
“This study indicates that geographical population distributions may present barriers to equitable clinical trial access and that data are available to proactively strategize about reduction of such barriers,” Dr. Lee and colleagues wrote.
The findings call attention to modifiable socioeconomic factors associated with trial participation, they added, like financial toxicity and affordable transportation, noting that ethnic and racial groups consent to trials at similar rates after controlling for income.
In addition, Dr. Lee and colleagues advised clinical trial designers to enlist satellite hospitals to increase participant diversity, since long commutes exacerbate “socioeconomic burdens associated with clinical trial participation,” with trial participation decreasing as commute time increases.
“Existing clinical trial centers may build collaborative efforts with nearby hospitals closer to underrepresented populations or set up community centers to support new collaborative networks to improve geographical access equity,” they wrote. “Methodologically, our approach is transferable to any country, region, or global effort with sufficient source data and can inform decision-making along the continuum of cancer care, from screening to implementing specialist care.”
A coauthor disclosed relationships with Flagship Therapeutics, Leidos Holding Ltd, Pershing Square Foundation, and others.
This inequity may be potentiating the underrepresentation of racially minoritized and socioeconomically disadvantaged populations in clinical trials, suggesting that employment of satellite hospitals is needed to expand access to investigational therapies, reported lead author Hassal Lee, MD, PhD, of Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, and colleagues.
“Minoritized and socioeconomically disadvantaged populations are underrepresented in clinical trials,” the investigators wrote in JAMA Oncology. “This may reduce the generalizability of trial results and propagate health disparities. Contributors to inequitable trial participation include individual-level factors and structural factors.”
Specifically, travel time to trial centers, as well as socioeconomic deprivation, can reduce likelihood of trial participation.
“Data on these parameters and population data on self-identified race exist, but their interrelation with clinical research facilities has not been systematically analyzed,” they wrote.
To try to draw comparisons between the distribution of patients of different races and socioeconomic statuses and the locations of clinical research facilities, Dr. Lee and colleagues aggregated data from the US Census, National Trial registry, Nature Index of Cancer Research Health Institutions, OpenStreetMap, National Cancer Institute–designated Cancer Centers list, and National Homeland Infrastructure Foundation. They then characterized catchment population demographics within 30-, 60-, and 120-minute driving commute times of all US hospitals, along with a more focused look at centers capable of conducting phase 1, phase 2, and phase 3 trials.
These efforts revealed broad geographic inequity.The 78 major centers that conduct 94% of all US cancer trials are located within 30 minutes of populations that have a 10.1% higher proportion of self-identified White individuals than the average US county, and a median income $18,900 higher than average (unpaired mean differences).
The publication also includes several maps characterizing racial and socioeconomic demographics within various catchment areas. For example, centers in New York City, Houston, and Chicago have the most diverse catchment populations within a 30-minute commute. Maps of all cities in the United States with populations greater than 500,000 are available in a supplementary index.
“This study indicates that geographical population distributions may present barriers to equitable clinical trial access and that data are available to proactively strategize about reduction of such barriers,” Dr. Lee and colleagues wrote.
The findings call attention to modifiable socioeconomic factors associated with trial participation, they added, like financial toxicity and affordable transportation, noting that ethnic and racial groups consent to trials at similar rates after controlling for income.
In addition, Dr. Lee and colleagues advised clinical trial designers to enlist satellite hospitals to increase participant diversity, since long commutes exacerbate “socioeconomic burdens associated with clinical trial participation,” with trial participation decreasing as commute time increases.
“Existing clinical trial centers may build collaborative efforts with nearby hospitals closer to underrepresented populations or set up community centers to support new collaborative networks to improve geographical access equity,” they wrote. “Methodologically, our approach is transferable to any country, region, or global effort with sufficient source data and can inform decision-making along the continuum of cancer care, from screening to implementing specialist care.”
A coauthor disclosed relationships with Flagship Therapeutics, Leidos Holding Ltd, Pershing Square Foundation, and others.
FROM JAMA ONCOLOGY
Acne Risk With Progestin-Only Long-Acting Reversible Contraceptives Evaluated
TOPLINE:
Despite the .
METHODOLOGY:
- Progestin-only LARC may increase the risk for acne, but this has not been well studied in adolescents and young adults.
- In the study, researchers evaluated the incidence of acne, acne as a reason for removal, and strategies used to manage acne after insertion of a progestin-only intrauterine device (IUD) or contraceptive implant in 1319 adolescents and young adults across four Adolescent Medicine LARC Collaborative study sites from January 2017 to June 2021.The mean age at insertion was 18.6 years.
- Overall, 24% of participants had acne at the time of LARC insertion.
- Worsening acne was defined as new patient reports of concern about acne, observations of acne, or addition of an acne medication after insertion; increased severity noted on an exam during follow-up or at the time of LARC removal; or acne reported as a side effect and/or reason for LARC removal.
TAKEAWAY:
- During the study period, 376 participants (28.5%) experienced worsening acne after LARC insertion, and 17% reported acne as a new concern, with no differences between those who received an IUD or an implant.
- Only 44 of the 376 participants (11.7%) who reported worsening acne were being treated with an oral agent at follow-up.
- Of the 542 individuals (41% of the total) who had the LARC device removed, 40 (7.4%) cited concerns about acne for removing the device, although just 5 (0.92%) said that acne was the only reason for removal. Of the 40 with concerns about acne when the device was removed, 18 (45%) had documented acne at the time of insertion.
IN PRACTICE:
The authors recommend that clinicians prescribing progestin-only LARC should counsel patients that acne may be a side effect, reassuring them that if they develop acne, “it typically is not problematic enough to warrant discontinuation,” and concluded that “concerns about the development or worsening of acne should not be cause to avoid these forms of contraception.”
SOURCE:
The study, led by Markus D. Boos, MD, PhD, of the division of dermatology in the Department of Pediatrics, University of Washington in Seattle and Seattle Children’s Hospital, was published in Pediatric Dermatology.
LIMITATIONS:
Individuals without documented acne were assumed to be acne-free, creating potential bias. Acne evaluation and treatment were not standardized and were not performed by dermatologists; acne severity was not recorded for many participants, possibly underestimating severity, and excluding LARC insertions without follow-up or with removal within 8 weeks may have underestimated the percentage of participants who developed new or worsening acne.
DISCLOSURES:
The study was supported by Investigator-Initiated Studies Program of Organon and by the Health Resources and Services Administration of the US Department of Health and Human Services. Many authors received grants for this work. The authors did not disclose any other competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
Despite the .
METHODOLOGY:
- Progestin-only LARC may increase the risk for acne, but this has not been well studied in adolescents and young adults.
- In the study, researchers evaluated the incidence of acne, acne as a reason for removal, and strategies used to manage acne after insertion of a progestin-only intrauterine device (IUD) or contraceptive implant in 1319 adolescents and young adults across four Adolescent Medicine LARC Collaborative study sites from January 2017 to June 2021.The mean age at insertion was 18.6 years.
- Overall, 24% of participants had acne at the time of LARC insertion.
- Worsening acne was defined as new patient reports of concern about acne, observations of acne, or addition of an acne medication after insertion; increased severity noted on an exam during follow-up or at the time of LARC removal; or acne reported as a side effect and/or reason for LARC removal.
TAKEAWAY:
- During the study period, 376 participants (28.5%) experienced worsening acne after LARC insertion, and 17% reported acne as a new concern, with no differences between those who received an IUD or an implant.
- Only 44 of the 376 participants (11.7%) who reported worsening acne were being treated with an oral agent at follow-up.
- Of the 542 individuals (41% of the total) who had the LARC device removed, 40 (7.4%) cited concerns about acne for removing the device, although just 5 (0.92%) said that acne was the only reason for removal. Of the 40 with concerns about acne when the device was removed, 18 (45%) had documented acne at the time of insertion.
IN PRACTICE:
The authors recommend that clinicians prescribing progestin-only LARC should counsel patients that acne may be a side effect, reassuring them that if they develop acne, “it typically is not problematic enough to warrant discontinuation,” and concluded that “concerns about the development or worsening of acne should not be cause to avoid these forms of contraception.”
SOURCE:
The study, led by Markus D. Boos, MD, PhD, of the division of dermatology in the Department of Pediatrics, University of Washington in Seattle and Seattle Children’s Hospital, was published in Pediatric Dermatology.
LIMITATIONS:
Individuals without documented acne were assumed to be acne-free, creating potential bias. Acne evaluation and treatment were not standardized and were not performed by dermatologists; acne severity was not recorded for many participants, possibly underestimating severity, and excluding LARC insertions without follow-up or with removal within 8 weeks may have underestimated the percentage of participants who developed new or worsening acne.
DISCLOSURES:
The study was supported by Investigator-Initiated Studies Program of Organon and by the Health Resources and Services Administration of the US Department of Health and Human Services. Many authors received grants for this work. The authors did not disclose any other competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
Despite the .
METHODOLOGY:
- Progestin-only LARC may increase the risk for acne, but this has not been well studied in adolescents and young adults.
- In the study, researchers evaluated the incidence of acne, acne as a reason for removal, and strategies used to manage acne after insertion of a progestin-only intrauterine device (IUD) or contraceptive implant in 1319 adolescents and young adults across four Adolescent Medicine LARC Collaborative study sites from January 2017 to June 2021.The mean age at insertion was 18.6 years.
- Overall, 24% of participants had acne at the time of LARC insertion.
- Worsening acne was defined as new patient reports of concern about acne, observations of acne, or addition of an acne medication after insertion; increased severity noted on an exam during follow-up or at the time of LARC removal; or acne reported as a side effect and/or reason for LARC removal.
TAKEAWAY:
- During the study period, 376 participants (28.5%) experienced worsening acne after LARC insertion, and 17% reported acne as a new concern, with no differences between those who received an IUD or an implant.
- Only 44 of the 376 participants (11.7%) who reported worsening acne were being treated with an oral agent at follow-up.
- Of the 542 individuals (41% of the total) who had the LARC device removed, 40 (7.4%) cited concerns about acne for removing the device, although just 5 (0.92%) said that acne was the only reason for removal. Of the 40 with concerns about acne when the device was removed, 18 (45%) had documented acne at the time of insertion.
IN PRACTICE:
The authors recommend that clinicians prescribing progestin-only LARC should counsel patients that acne may be a side effect, reassuring them that if they develop acne, “it typically is not problematic enough to warrant discontinuation,” and concluded that “concerns about the development or worsening of acne should not be cause to avoid these forms of contraception.”
SOURCE:
The study, led by Markus D. Boos, MD, PhD, of the division of dermatology in the Department of Pediatrics, University of Washington in Seattle and Seattle Children’s Hospital, was published in Pediatric Dermatology.
LIMITATIONS:
Individuals without documented acne were assumed to be acne-free, creating potential bias. Acne evaluation and treatment were not standardized and were not performed by dermatologists; acne severity was not recorded for many participants, possibly underestimating severity, and excluding LARC insertions without follow-up or with removal within 8 weeks may have underestimated the percentage of participants who developed new or worsening acne.
DISCLOSURES:
The study was supported by Investigator-Initiated Studies Program of Organon and by the Health Resources and Services Administration of the US Department of Health and Human Services. Many authors received grants for this work. The authors did not disclose any other competing interests.
A version of this article appeared on Medscape.com.
New Drug Approvals Are the Wrong Metric for Cancer Policy
How should we define success in cancer policy — what should the endpoint be?
It’s debatable. Is it fewer cancer deaths? Perhaps improved access to therapies or a reduction in disparities?
One thing I know with certainty: The number of new cancer drugs approved by the US Food and Drug Administration (FDA) is not and should not be our primary endpoint in and of itself.
I’ll go a step further: It is not even a surrogate marker for success.
Unfortunately, a new drug approval does not necessarily mean improved patient outcomes. In fact, the majority of cancer drugs approved these days improve neither survival nor quality of life. Our previous work has shown better mortality outcomes in other high-income countries that have not approved or do not fund several cancer drugs that the FDA has approved.
Even if a drug has a meaningful benefit, at an average cost of more than $250,000 per year, if a new drug cannot reach patients because of access or cost issues, it’s meaningless.
However, regulators and media celebrate the number (and speed) of drug approvals every year as if it were a marker of success in and of itself. But approving more drugs should not be the goal; improving outcomes should. The FDA’s current approach is akin to a university celebrating its graduation rate by lowering the requirements to pass.
When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine ‘ending cancer as we know it’ is premature and even embarrassing.
This is exactly what the FDA has been doing with our regulatory standards for drug approval. They have gradually lowered the requirements for approval from two randomized trials to one randomized trial, then further to one randomized trial with a surrogate endpoint. In many instances, they have gone even further, demanding merely single-arm trials. They’ve also gone from requiring overall survival benefits to celebrating nondetrimental effects on overall survival. It’s no wonder that we approve more drugs today than we did in the past — the bar for approval is pretty low nowadays.
In 2019, our lab found an interesting phenomenon: The number of approvals based on surrogate endpoints has been increasing while the number of accelerated approvals has been decreasing. This made no sense at first, because you’d think surrogate-based approvals and accelerated approvals would be collinear. However, we realized that the recent approvals based on surrogate endpoints were regular approvals instead of accelerated approvals, which explained the phenomenon. Not only is the FDA approving more drugs on the basis of lower levels of evidence, but the agency is also offering regular instead of accelerated approval, thereby removing the safety net of a confirmatory trial.
Nearly everybody sees this as a cause for celebration. Pharma celebrates record profits, regulators celebrate record numbers of drug approvals, insurance companies celebrate because they can pass these costs on as insurance premiums and make even more money, and physicians and patients celebrate access to the shiniest, sexiest new cancer drug.
Everybody is happy in this system. The only problem is that patient outcomes don’t improve, resources are taken away from other priorities, and society suffers a net harm.
When you contrast this celebration with the reality on the ground, the difference is stark and sobering. In our clinics, patients lack access to even old chemotherapeutic drugs that are already generic and cheap but make a meaningful difference in patient outcomes. Citing a current lack of incentives, several generic cancer drug manufacturers have stopped making these drugs; the US supply now relies heavily on importing them from emerging economies such as India. When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine “ending cancer as we know it” is premature and even embarrassing.
5-Fluorouracil, methotrexate, and the platinums are backbones of cancer treatment. Cisplatin and carboplatin are not drugs we use with the hope of improving survival by a couple of months; these drugs are the difference between life and death for patients with testicular and ovarian cancers. In a survey of 948 global oncologists, these were considered among the most essential cancer drugs by oncologists in high-income and low- and middle-income countries alike. Although oncologists in low- and middle-income countries sometimes argue that even these cheap generic drugs may be unaffordable to their patients, they usually remain available; access is a function of both availability and affordability. However, the shortage situation in the US is unique in that availability — rather than affordability — is impacting access.
Our profit-over-patients policy has landed us in a terrible paradox.
Generic drugs are cheap, and any industrialized country can manufacture them. This is why so few companies actually do so; the profit margins are low and companies have little incentive to produce them, despite their benefit. Meanwhile, the FDA is approving and offering access to new shiny molecules that cost more than $15,000 per month yet offer less than a month of progression-free survival benefit and no overall survival benefit (see margetuximab in breast cancer). We have a literal fatal attraction to everything new and shiny.
This is a clear misalignment of priorities in US cancer drug policy. Our profit-over-patients policy has landed us in a terrible paradox: If a drug is cheap and meaningful, it won’t be available, but if it is marginal and expensive, we will do everything to ensure patients can get it. It’s no wonder that patients on Medicaid are disproportionately affected by these drug shortages. Unless all patients have easy access to cisplatin, carboplatin, and 5-fluorouracil, it is frankly embarrassing to celebrate the number of new cancer drugs approved each year.
We all have a responsibility in this — policymakers and lawmakers, regulators and payers, manufacturers and distributors, the American Society of Clinical Oncology and other oncology societies, and physicians and patients. This is where our advocacy work should focus. The primary endpoint of our cancer policy should not be how many new treatments we can approve or how many expensive drugs a rich person with the best insurance can get at a leading cancer center. The true measure of our civilization is how it treats its most vulnerable members.
Dr. Gyawali has disclosed the following relevant financial relationship: Received consulting fees from Vivio Health.
Dr. Gyawali is an associate professor in the Departments of Oncology and Public Health Sciences and a scientist in the Division of Cancer Care and Epidemiology at Queen’s University in Kingston, Ontario, Canada, and is also affiliated faculty at the Program on Regulation, Therapeutics, and Law in the Department of Medicine at Brigham and Women’s Hospital in Boston. His clinical and research interests revolve around cancer policy, global oncology, evidence-based oncology, financial toxicities of cancer treatment, clinical trial methods, and supportive care. He tweets at @oncology_bg.
A version of this article appeared on Medscape.com.
How should we define success in cancer policy — what should the endpoint be?
It’s debatable. Is it fewer cancer deaths? Perhaps improved access to therapies or a reduction in disparities?
One thing I know with certainty: The number of new cancer drugs approved by the US Food and Drug Administration (FDA) is not and should not be our primary endpoint in and of itself.
I’ll go a step further: It is not even a surrogate marker for success.
Unfortunately, a new drug approval does not necessarily mean improved patient outcomes. In fact, the majority of cancer drugs approved these days improve neither survival nor quality of life. Our previous work has shown better mortality outcomes in other high-income countries that have not approved or do not fund several cancer drugs that the FDA has approved.
Even if a drug has a meaningful benefit, at an average cost of more than $250,000 per year, if a new drug cannot reach patients because of access or cost issues, it’s meaningless.
However, regulators and media celebrate the number (and speed) of drug approvals every year as if it were a marker of success in and of itself. But approving more drugs should not be the goal; improving outcomes should. The FDA’s current approach is akin to a university celebrating its graduation rate by lowering the requirements to pass.
When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine ‘ending cancer as we know it’ is premature and even embarrassing.
This is exactly what the FDA has been doing with our regulatory standards for drug approval. They have gradually lowered the requirements for approval from two randomized trials to one randomized trial, then further to one randomized trial with a surrogate endpoint. In many instances, they have gone even further, demanding merely single-arm trials. They’ve also gone from requiring overall survival benefits to celebrating nondetrimental effects on overall survival. It’s no wonder that we approve more drugs today than we did in the past — the bar for approval is pretty low nowadays.
In 2019, our lab found an interesting phenomenon: The number of approvals based on surrogate endpoints has been increasing while the number of accelerated approvals has been decreasing. This made no sense at first, because you’d think surrogate-based approvals and accelerated approvals would be collinear. However, we realized that the recent approvals based on surrogate endpoints were regular approvals instead of accelerated approvals, which explained the phenomenon. Not only is the FDA approving more drugs on the basis of lower levels of evidence, but the agency is also offering regular instead of accelerated approval, thereby removing the safety net of a confirmatory trial.
Nearly everybody sees this as a cause for celebration. Pharma celebrates record profits, regulators celebrate record numbers of drug approvals, insurance companies celebrate because they can pass these costs on as insurance premiums and make even more money, and physicians and patients celebrate access to the shiniest, sexiest new cancer drug.
Everybody is happy in this system. The only problem is that patient outcomes don’t improve, resources are taken away from other priorities, and society suffers a net harm.
When you contrast this celebration with the reality on the ground, the difference is stark and sobering. In our clinics, patients lack access to even old chemotherapeutic drugs that are already generic and cheap but make a meaningful difference in patient outcomes. Citing a current lack of incentives, several generic cancer drug manufacturers have stopped making these drugs; the US supply now relies heavily on importing them from emerging economies such as India. When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine “ending cancer as we know it” is premature and even embarrassing.
5-Fluorouracil, methotrexate, and the platinums are backbones of cancer treatment. Cisplatin and carboplatin are not drugs we use with the hope of improving survival by a couple of months; these drugs are the difference between life and death for patients with testicular and ovarian cancers. In a survey of 948 global oncologists, these were considered among the most essential cancer drugs by oncologists in high-income and low- and middle-income countries alike. Although oncologists in low- and middle-income countries sometimes argue that even these cheap generic drugs may be unaffordable to their patients, they usually remain available; access is a function of both availability and affordability. However, the shortage situation in the US is unique in that availability — rather than affordability — is impacting access.
Our profit-over-patients policy has landed us in a terrible paradox.
Generic drugs are cheap, and any industrialized country can manufacture them. This is why so few companies actually do so; the profit margins are low and companies have little incentive to produce them, despite their benefit. Meanwhile, the FDA is approving and offering access to new shiny molecules that cost more than $15,000 per month yet offer less than a month of progression-free survival benefit and no overall survival benefit (see margetuximab in breast cancer). We have a literal fatal attraction to everything new and shiny.
This is a clear misalignment of priorities in US cancer drug policy. Our profit-over-patients policy has landed us in a terrible paradox: If a drug is cheap and meaningful, it won’t be available, but if it is marginal and expensive, we will do everything to ensure patients can get it. It’s no wonder that patients on Medicaid are disproportionately affected by these drug shortages. Unless all patients have easy access to cisplatin, carboplatin, and 5-fluorouracil, it is frankly embarrassing to celebrate the number of new cancer drugs approved each year.
We all have a responsibility in this — policymakers and lawmakers, regulators and payers, manufacturers and distributors, the American Society of Clinical Oncology and other oncology societies, and physicians and patients. This is where our advocacy work should focus. The primary endpoint of our cancer policy should not be how many new treatments we can approve or how many expensive drugs a rich person with the best insurance can get at a leading cancer center. The true measure of our civilization is how it treats its most vulnerable members.
Dr. Gyawali has disclosed the following relevant financial relationship: Received consulting fees from Vivio Health.
Dr. Gyawali is an associate professor in the Departments of Oncology and Public Health Sciences and a scientist in the Division of Cancer Care and Epidemiology at Queen’s University in Kingston, Ontario, Canada, and is also affiliated faculty at the Program on Regulation, Therapeutics, and Law in the Department of Medicine at Brigham and Women’s Hospital in Boston. His clinical and research interests revolve around cancer policy, global oncology, evidence-based oncology, financial toxicities of cancer treatment, clinical trial methods, and supportive care. He tweets at @oncology_bg.
A version of this article appeared on Medscape.com.
How should we define success in cancer policy — what should the endpoint be?
It’s debatable. Is it fewer cancer deaths? Perhaps improved access to therapies or a reduction in disparities?
One thing I know with certainty: The number of new cancer drugs approved by the US Food and Drug Administration (FDA) is not and should not be our primary endpoint in and of itself.
I’ll go a step further: It is not even a surrogate marker for success.
Unfortunately, a new drug approval does not necessarily mean improved patient outcomes. In fact, the majority of cancer drugs approved these days improve neither survival nor quality of life. Our previous work has shown better mortality outcomes in other high-income countries that have not approved or do not fund several cancer drugs that the FDA has approved.
Even if a drug has a meaningful benefit, at an average cost of more than $250,000 per year, if a new drug cannot reach patients because of access or cost issues, it’s meaningless.
However, regulators and media celebrate the number (and speed) of drug approvals every year as if it were a marker of success in and of itself. But approving more drugs should not be the goal; improving outcomes should. The FDA’s current approach is akin to a university celebrating its graduation rate by lowering the requirements to pass.
When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine ‘ending cancer as we know it’ is premature and even embarrassing.
This is exactly what the FDA has been doing with our regulatory standards for drug approval. They have gradually lowered the requirements for approval from two randomized trials to one randomized trial, then further to one randomized trial with a surrogate endpoint. In many instances, they have gone even further, demanding merely single-arm trials. They’ve also gone from requiring overall survival benefits to celebrating nondetrimental effects on overall survival. It’s no wonder that we approve more drugs today than we did in the past — the bar for approval is pretty low nowadays.
In 2019, our lab found an interesting phenomenon: The number of approvals based on surrogate endpoints has been increasing while the number of accelerated approvals has been decreasing. This made no sense at first, because you’d think surrogate-based approvals and accelerated approvals would be collinear. However, we realized that the recent approvals based on surrogate endpoints were regular approvals instead of accelerated approvals, which explained the phenomenon. Not only is the FDA approving more drugs on the basis of lower levels of evidence, but the agency is also offering regular instead of accelerated approval, thereby removing the safety net of a confirmatory trial.
Nearly everybody sees this as a cause for celebration. Pharma celebrates record profits, regulators celebrate record numbers of drug approvals, insurance companies celebrate because they can pass these costs on as insurance premiums and make even more money, and physicians and patients celebrate access to the shiniest, sexiest new cancer drug.
Everybody is happy in this system. The only problem is that patient outcomes don’t improve, resources are taken away from other priorities, and society suffers a net harm.
When you contrast this celebration with the reality on the ground, the difference is stark and sobering. In our clinics, patients lack access to even old chemotherapeutic drugs that are already generic and cheap but make a meaningful difference in patient outcomes. Citing a current lack of incentives, several generic cancer drug manufacturers have stopped making these drugs; the US supply now relies heavily on importing them from emerging economies such as India. When US patients lack access to cisplatin and carboplatin, any talk of a Moonshot or precision medicine “ending cancer as we know it” is premature and even embarrassing.
5-Fluorouracil, methotrexate, and the platinums are backbones of cancer treatment. Cisplatin and carboplatin are not drugs we use with the hope of improving survival by a couple of months; these drugs are the difference between life and death for patients with testicular and ovarian cancers. In a survey of 948 global oncologists, these were considered among the most essential cancer drugs by oncologists in high-income and low- and middle-income countries alike. Although oncologists in low- and middle-income countries sometimes argue that even these cheap generic drugs may be unaffordable to their patients, they usually remain available; access is a function of both availability and affordability. However, the shortage situation in the US is unique in that availability — rather than affordability — is impacting access.
Our profit-over-patients policy has landed us in a terrible paradox.
Generic drugs are cheap, and any industrialized country can manufacture them. This is why so few companies actually do so; the profit margins are low and companies have little incentive to produce them, despite their benefit. Meanwhile, the FDA is approving and offering access to new shiny molecules that cost more than $15,000 per month yet offer less than a month of progression-free survival benefit and no overall survival benefit (see margetuximab in breast cancer). We have a literal fatal attraction to everything new and shiny.
This is a clear misalignment of priorities in US cancer drug policy. Our profit-over-patients policy has landed us in a terrible paradox: If a drug is cheap and meaningful, it won’t be available, but if it is marginal and expensive, we will do everything to ensure patients can get it. It’s no wonder that patients on Medicaid are disproportionately affected by these drug shortages. Unless all patients have easy access to cisplatin, carboplatin, and 5-fluorouracil, it is frankly embarrassing to celebrate the number of new cancer drugs approved each year.
We all have a responsibility in this — policymakers and lawmakers, regulators and payers, manufacturers and distributors, the American Society of Clinical Oncology and other oncology societies, and physicians and patients. This is where our advocacy work should focus. The primary endpoint of our cancer policy should not be how many new treatments we can approve or how many expensive drugs a rich person with the best insurance can get at a leading cancer center. The true measure of our civilization is how it treats its most vulnerable members.
Dr. Gyawali has disclosed the following relevant financial relationship: Received consulting fees from Vivio Health.
Dr. Gyawali is an associate professor in the Departments of Oncology and Public Health Sciences and a scientist in the Division of Cancer Care and Epidemiology at Queen’s University in Kingston, Ontario, Canada, and is also affiliated faculty at the Program on Regulation, Therapeutics, and Law in the Department of Medicine at Brigham and Women’s Hospital in Boston. His clinical and research interests revolve around cancer policy, global oncology, evidence-based oncology, financial toxicities of cancer treatment, clinical trial methods, and supportive care. He tweets at @oncology_bg.
A version of this article appeared on Medscape.com.
Very Low-Energy Diet Safe, Acceptable for Adolescents
More research is needed to understand which patients are best suited for the diet; “however, given the associated rapid weight loss, the use of [very low-energy diets] should be emphasized in clinical practice guidelines for the treatment of severe obesity and obesity-related complications in adolescents, especially before pharmacological or surgical intervention,” first author Megan Gow, PhD, of Children’s Hospital Westmead Clinical School, The University of Sydney, Westmead, Australia, said in a press statement.
The study will be presented in May at the upcoming European Congress on Obesity, in Venice, Italy.
While very low-calorie diets have been shown to promote rapid weight loss in adolescents, research is lacking on general side effects and acceptability of the regimens. Data is also lacking on important issues including the diet’s effect on growth, heart health, and psychological wellbeing.
To investigate, Dr. Gow and colleagues conducted a subanalysis of the 52-week Fast Track to Health study evaluating the acceptability of different dietary plans for adolescents with obesity.
The analysis included 141 adolescents between the ages of 13 and 17 years with moderate to severe obesity (average body mass index, 35 kg/m2) and at least one obesity-related complication, such as high blood pressure or insulin resistance.
The participants were placed on a nutritionally balanced very low-energy diet consisting of 800 calories per day.
The diet involved one of two regimens — either four Optifast-formulated meal replacement products per day, including shakes, soups, bars, and/or dessert, along with low carbohydrate vegetables, such as broccoli, celery, capsicum, mushrooms, and tomatoes, with one teaspoon of vegetable oil, or a regimen of three Optifast-formulated meal replacements and one meal consisting of 100-150 g lean cooked meat, low-carbohydrate vegetables, and one teaspoon of vegetable oil.
Participants, about half of whom were women, also received dietitian support at least weekly.
After 4 weeks, most of the adolescents, ie, 134 of the 141, with an average age of 14.9 years, completed the diet, with an average weight loss of 5.5 kg or 12 pounds (P < .001).
Most patients (95%) experienced at least one side effect, and 70% reported at least 3 of the side effects, with the most common side effects including hunger, fatigue, headache, irritability, loose stools, constipation, nausea, and a lack of concentration.
Viral infections occurred in seven participants.
While most side effects occurred at the end of week 1, the development of side effects earlier, at day 3-4, was associated with higher levels of weight loss at the 4-week cut-off, which the authors noted could suggest a greater adherence to the diet at that stage.
One adverse event occurred, consisting of a single fainting episode determined to be potentially related to the dietary intervention.
In surveys, the adolescents gave the intervention an acceptability rating of 61 on a scale of 100, the score was 53 of 100 in terms of being “enjoyable to follow.”
The most-liked aspects of the intervention were losing weight (described by 34% of participants) and the prescriptive structure (listed by 28% of participants).
The least-liked aspects included the diet’s restrictive nature, described by 45% of participants, and the taste of meal replacement products, listed by 20% of participants.
Alternative to Weight Loss Drugs?
While weight loss drugs are transforming the obesity treatment and semaglutide is now approved for adolescents as young as age 12 years, “access to these medications is limited, and not all families want to commence on medication for their child›s obesity,” Dr. Gow said.
As an alternative, a very low-energy diet, with the interaction of a dietitian, can enable adolescents “to develop a healthier relationship with food, including encouraging the consumption of more fruits and vegetables in their diet, not only to assist in weight loss but for good health,” she said.
Very Low-Calorie Diet Concerns for Adolescents Addressed
Early studies suggested concerns of health effects from very low-calorie diets in adolescents, including potential cardiac effects; however, subsequent studies, including a systematic review published by Dr. Gow and her team, have shown that such results were likely the result of nutritional deficiencies in the diets, which can be overcome with careful food selection and dietary counseling.
Another key concern has been a potential effect on growth, but Dr. Gow noted that “in our short-term study we saw small increase in height (0.1 cm), and other more recent studies suggest that a short-term very low-energy diet does not impact growth.”
And in an earlier pilot study, the authors also found an association between the very low-calorie diet and an improvement in the quality of life for youth with type 2 diabetes.
A key caveat with the findings is that participants in the study all received supervision and monitoring from a trained dietitian, and Gow noted that that element is essential.
“We therefore do not recommend adolescents in the community undertake this type of diet without appropriate support,” she said.
“Close monitoring of adolescents by a health professional following a very low-energy diet is essential to ensure that the very low-energy diet is leading to holistic health benefits for the individual.”
Following the 4-week regimen, participants were randomized to transition to interventions of either continuous energy restriction or intermittent energy restriction over the 52 weeks, and further findings from the study will be presented at the obesity meeting in May.
The authors had no disclosures to report.
A version of this article appeared on Medscape.com.
More research is needed to understand which patients are best suited for the diet; “however, given the associated rapid weight loss, the use of [very low-energy diets] should be emphasized in clinical practice guidelines for the treatment of severe obesity and obesity-related complications in adolescents, especially before pharmacological or surgical intervention,” first author Megan Gow, PhD, of Children’s Hospital Westmead Clinical School, The University of Sydney, Westmead, Australia, said in a press statement.
The study will be presented in May at the upcoming European Congress on Obesity, in Venice, Italy.
While very low-calorie diets have been shown to promote rapid weight loss in adolescents, research is lacking on general side effects and acceptability of the regimens. Data is also lacking on important issues including the diet’s effect on growth, heart health, and psychological wellbeing.
To investigate, Dr. Gow and colleagues conducted a subanalysis of the 52-week Fast Track to Health study evaluating the acceptability of different dietary plans for adolescents with obesity.
The analysis included 141 adolescents between the ages of 13 and 17 years with moderate to severe obesity (average body mass index, 35 kg/m2) and at least one obesity-related complication, such as high blood pressure or insulin resistance.
The participants were placed on a nutritionally balanced very low-energy diet consisting of 800 calories per day.
The diet involved one of two regimens — either four Optifast-formulated meal replacement products per day, including shakes, soups, bars, and/or dessert, along with low carbohydrate vegetables, such as broccoli, celery, capsicum, mushrooms, and tomatoes, with one teaspoon of vegetable oil, or a regimen of three Optifast-formulated meal replacements and one meal consisting of 100-150 g lean cooked meat, low-carbohydrate vegetables, and one teaspoon of vegetable oil.
Participants, about half of whom were women, also received dietitian support at least weekly.
After 4 weeks, most of the adolescents, ie, 134 of the 141, with an average age of 14.9 years, completed the diet, with an average weight loss of 5.5 kg or 12 pounds (P < .001).
Most patients (95%) experienced at least one side effect, and 70% reported at least 3 of the side effects, with the most common side effects including hunger, fatigue, headache, irritability, loose stools, constipation, nausea, and a lack of concentration.
Viral infections occurred in seven participants.
While most side effects occurred at the end of week 1, the development of side effects earlier, at day 3-4, was associated with higher levels of weight loss at the 4-week cut-off, which the authors noted could suggest a greater adherence to the diet at that stage.
One adverse event occurred, consisting of a single fainting episode determined to be potentially related to the dietary intervention.
In surveys, the adolescents gave the intervention an acceptability rating of 61 on a scale of 100, the score was 53 of 100 in terms of being “enjoyable to follow.”
The most-liked aspects of the intervention were losing weight (described by 34% of participants) and the prescriptive structure (listed by 28% of participants).
The least-liked aspects included the diet’s restrictive nature, described by 45% of participants, and the taste of meal replacement products, listed by 20% of participants.
Alternative to Weight Loss Drugs?
While weight loss drugs are transforming the obesity treatment and semaglutide is now approved for adolescents as young as age 12 years, “access to these medications is limited, and not all families want to commence on medication for their child›s obesity,” Dr. Gow said.
As an alternative, a very low-energy diet, with the interaction of a dietitian, can enable adolescents “to develop a healthier relationship with food, including encouraging the consumption of more fruits and vegetables in their diet, not only to assist in weight loss but for good health,” she said.
Very Low-Calorie Diet Concerns for Adolescents Addressed
Early studies suggested concerns of health effects from very low-calorie diets in adolescents, including potential cardiac effects; however, subsequent studies, including a systematic review published by Dr. Gow and her team, have shown that such results were likely the result of nutritional deficiencies in the diets, which can be overcome with careful food selection and dietary counseling.
Another key concern has been a potential effect on growth, but Dr. Gow noted that “in our short-term study we saw small increase in height (0.1 cm), and other more recent studies suggest that a short-term very low-energy diet does not impact growth.”
And in an earlier pilot study, the authors also found an association between the very low-calorie diet and an improvement in the quality of life for youth with type 2 diabetes.
A key caveat with the findings is that participants in the study all received supervision and monitoring from a trained dietitian, and Gow noted that that element is essential.
“We therefore do not recommend adolescents in the community undertake this type of diet without appropriate support,” she said.
“Close monitoring of adolescents by a health professional following a very low-energy diet is essential to ensure that the very low-energy diet is leading to holistic health benefits for the individual.”
Following the 4-week regimen, participants were randomized to transition to interventions of either continuous energy restriction or intermittent energy restriction over the 52 weeks, and further findings from the study will be presented at the obesity meeting in May.
The authors had no disclosures to report.
A version of this article appeared on Medscape.com.
More research is needed to understand which patients are best suited for the diet; “however, given the associated rapid weight loss, the use of [very low-energy diets] should be emphasized in clinical practice guidelines for the treatment of severe obesity and obesity-related complications in adolescents, especially before pharmacological or surgical intervention,” first author Megan Gow, PhD, of Children’s Hospital Westmead Clinical School, The University of Sydney, Westmead, Australia, said in a press statement.
The study will be presented in May at the upcoming European Congress on Obesity, in Venice, Italy.
While very low-calorie diets have been shown to promote rapid weight loss in adolescents, research is lacking on general side effects and acceptability of the regimens. Data is also lacking on important issues including the diet’s effect on growth, heart health, and psychological wellbeing.
To investigate, Dr. Gow and colleagues conducted a subanalysis of the 52-week Fast Track to Health study evaluating the acceptability of different dietary plans for adolescents with obesity.
The analysis included 141 adolescents between the ages of 13 and 17 years with moderate to severe obesity (average body mass index, 35 kg/m2) and at least one obesity-related complication, such as high blood pressure or insulin resistance.
The participants were placed on a nutritionally balanced very low-energy diet consisting of 800 calories per day.
The diet involved one of two regimens — either four Optifast-formulated meal replacement products per day, including shakes, soups, bars, and/or dessert, along with low carbohydrate vegetables, such as broccoli, celery, capsicum, mushrooms, and tomatoes, with one teaspoon of vegetable oil, or a regimen of three Optifast-formulated meal replacements and one meal consisting of 100-150 g lean cooked meat, low-carbohydrate vegetables, and one teaspoon of vegetable oil.
Participants, about half of whom were women, also received dietitian support at least weekly.
After 4 weeks, most of the adolescents, ie, 134 of the 141, with an average age of 14.9 years, completed the diet, with an average weight loss of 5.5 kg or 12 pounds (P < .001).
Most patients (95%) experienced at least one side effect, and 70% reported at least 3 of the side effects, with the most common side effects including hunger, fatigue, headache, irritability, loose stools, constipation, nausea, and a lack of concentration.
Viral infections occurred in seven participants.
While most side effects occurred at the end of week 1, the development of side effects earlier, at day 3-4, was associated with higher levels of weight loss at the 4-week cut-off, which the authors noted could suggest a greater adherence to the diet at that stage.
One adverse event occurred, consisting of a single fainting episode determined to be potentially related to the dietary intervention.
In surveys, the adolescents gave the intervention an acceptability rating of 61 on a scale of 100, the score was 53 of 100 in terms of being “enjoyable to follow.”
The most-liked aspects of the intervention were losing weight (described by 34% of participants) and the prescriptive structure (listed by 28% of participants).
The least-liked aspects included the diet’s restrictive nature, described by 45% of participants, and the taste of meal replacement products, listed by 20% of participants.
Alternative to Weight Loss Drugs?
While weight loss drugs are transforming the obesity treatment and semaglutide is now approved for adolescents as young as age 12 years, “access to these medications is limited, and not all families want to commence on medication for their child›s obesity,” Dr. Gow said.
As an alternative, a very low-energy diet, with the interaction of a dietitian, can enable adolescents “to develop a healthier relationship with food, including encouraging the consumption of more fruits and vegetables in their diet, not only to assist in weight loss but for good health,” she said.
Very Low-Calorie Diet Concerns for Adolescents Addressed
Early studies suggested concerns of health effects from very low-calorie diets in adolescents, including potential cardiac effects; however, subsequent studies, including a systematic review published by Dr. Gow and her team, have shown that such results were likely the result of nutritional deficiencies in the diets, which can be overcome with careful food selection and dietary counseling.
Another key concern has been a potential effect on growth, but Dr. Gow noted that “in our short-term study we saw small increase in height (0.1 cm), and other more recent studies suggest that a short-term very low-energy diet does not impact growth.”
And in an earlier pilot study, the authors also found an association between the very low-calorie diet and an improvement in the quality of life for youth with type 2 diabetes.
A key caveat with the findings is that participants in the study all received supervision and monitoring from a trained dietitian, and Gow noted that that element is essential.
“We therefore do not recommend adolescents in the community undertake this type of diet without appropriate support,” she said.
“Close monitoring of adolescents by a health professional following a very low-energy diet is essential to ensure that the very low-energy diet is leading to holistic health benefits for the individual.”
Following the 4-week regimen, participants were randomized to transition to interventions of either continuous energy restriction or intermittent energy restriction over the 52 weeks, and further findings from the study will be presented at the obesity meeting in May.
The authors had no disclosures to report.
A version of this article appeared on Medscape.com.
FROM THE EUROPEAN CONGRESS ON OBESITY
Mental Health and Slow Concussion Recovery
Those of you who are regular readers of Letters from Maine have probably noticed that concussion is one of my favorite topics. The explanation for this perseveration is personal and may lie in the fact that I played two contact sports in college. In high school we still wore leather helmets and in college the lacrosse helmets were constructed of plastic-coated cardboard. I can recall just a few of what might be now labeled as sports-related concussions. Ironically, my only loss of consciousness came on the first dinner date with the woman who would eventually become my wife. A hypotensive episode resulting from the combination of sweat loss (2 hours of basketball) and blood loss from selling some platelets earlier in the day (to pay for the dinner) led to the unfortunate meeting of my head and the beautifully tiled floor at the restaurant.
Postconcussion Recovery
The phenomenon of delayed symptomatic recovery has been a particular interest of mine. Within the last 12 months I have written about an excellent companion commentary in Pediatricsby Talin Babikian PhD, a psychologist at University of California, Los Angeles, in which he urges us to “Consider the comorbidities or premorbidities,” including, among others, anxiety and/or depression, post-traumatic stress, and poor sleep when we are faced with a patient who is slow in shedding his postconcussion symptoms. A short 6 months after reading Dr. Babikian’s prescient commentary, I have encountered some evidence supporting his advice.
Investigators at the Sports Medicine and Performance Center at the Children’s Hospital of Philadelphia have recently published a study in which they have found “Preexisting mental health diagnoses are associated with greater postinjury emotional symptom burden and longer concussion recovery in a dose-response fashion.” In their prospective study of over 3000 children and adolescents, they found that, although patients with more mental health diagnoses were at greater risk of increased emotional symptoms after concussion, “Children and adolescents with any preexisting mental health diagnosis took longer to recover.”
Female patients and those with abnormal visio-vestibular test results at the initial postinjury evaluation took longer to recover, although boys with prolonged recovery had more emotional symptoms. In general, patients with preexisting mental health diagnoses returned to exercise later, a known factor in delayed concussion recovery.
Making Sense of It All
There are a couple of ways to look at this paper’s findings. The first is through the lens that focuses on the population of children and adolescents who have known mental health conditions. If our patient has a mental health diagnosis, we shouldn’t be surprised that he/she is taking longer to recover from his/her concussion and is experiencing an increase in symptoms. Most of us probably suspected this already. However, we should be particularly aware of this phenomenon if the patient is male.
The other perspective is probably more valuable to us as primary care physicians. Even one diagnosis so subtle that we may have overlooked it is likely to slow his/her progress toward recovery. And, of course, it may be that injury has triggered an underlying condition that none — not even the most astute diagnostician — would have found without a focused investigation.
I can’t leave this subject without wondering whether the findings in this paper should be extrapolated to other conditions of delayed recovery, including Lyme disease and COVID 19. Patients with these conditions are understandably resistant to the suggestion that their mental health may be contributing to the situation. Too many have been told too often it is “all in their head.” However, I think we as clinicians should keep open minds when symptoms are resolving more slowly than we would expect.
Finally, in their conclusion the authors of this paper reinforce a principle that has unfortunately taken some of us a while to accept. Early introduction of symptom-limited exercise should be a standard of postconcussion management, especially for patients with a mental health diagnosis.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Those of you who are regular readers of Letters from Maine have probably noticed that concussion is one of my favorite topics. The explanation for this perseveration is personal and may lie in the fact that I played two contact sports in college. In high school we still wore leather helmets and in college the lacrosse helmets were constructed of plastic-coated cardboard. I can recall just a few of what might be now labeled as sports-related concussions. Ironically, my only loss of consciousness came on the first dinner date with the woman who would eventually become my wife. A hypotensive episode resulting from the combination of sweat loss (2 hours of basketball) and blood loss from selling some platelets earlier in the day (to pay for the dinner) led to the unfortunate meeting of my head and the beautifully tiled floor at the restaurant.
Postconcussion Recovery
The phenomenon of delayed symptomatic recovery has been a particular interest of mine. Within the last 12 months I have written about an excellent companion commentary in Pediatricsby Talin Babikian PhD, a psychologist at University of California, Los Angeles, in which he urges us to “Consider the comorbidities or premorbidities,” including, among others, anxiety and/or depression, post-traumatic stress, and poor sleep when we are faced with a patient who is slow in shedding his postconcussion symptoms. A short 6 months after reading Dr. Babikian’s prescient commentary, I have encountered some evidence supporting his advice.
Investigators at the Sports Medicine and Performance Center at the Children’s Hospital of Philadelphia have recently published a study in which they have found “Preexisting mental health diagnoses are associated with greater postinjury emotional symptom burden and longer concussion recovery in a dose-response fashion.” In their prospective study of over 3000 children and adolescents, they found that, although patients with more mental health diagnoses were at greater risk of increased emotional symptoms after concussion, “Children and adolescents with any preexisting mental health diagnosis took longer to recover.”
Female patients and those with abnormal visio-vestibular test results at the initial postinjury evaluation took longer to recover, although boys with prolonged recovery had more emotional symptoms. In general, patients with preexisting mental health diagnoses returned to exercise later, a known factor in delayed concussion recovery.
Making Sense of It All
There are a couple of ways to look at this paper’s findings. The first is through the lens that focuses on the population of children and adolescents who have known mental health conditions. If our patient has a mental health diagnosis, we shouldn’t be surprised that he/she is taking longer to recover from his/her concussion and is experiencing an increase in symptoms. Most of us probably suspected this already. However, we should be particularly aware of this phenomenon if the patient is male.
The other perspective is probably more valuable to us as primary care physicians. Even one diagnosis so subtle that we may have overlooked it is likely to slow his/her progress toward recovery. And, of course, it may be that injury has triggered an underlying condition that none — not even the most astute diagnostician — would have found without a focused investigation.
I can’t leave this subject without wondering whether the findings in this paper should be extrapolated to other conditions of delayed recovery, including Lyme disease and COVID 19. Patients with these conditions are understandably resistant to the suggestion that their mental health may be contributing to the situation. Too many have been told too often it is “all in their head.” However, I think we as clinicians should keep open minds when symptoms are resolving more slowly than we would expect.
Finally, in their conclusion the authors of this paper reinforce a principle that has unfortunately taken some of us a while to accept. Early introduction of symptom-limited exercise should be a standard of postconcussion management, especially for patients with a mental health diagnosis.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Those of you who are regular readers of Letters from Maine have probably noticed that concussion is one of my favorite topics. The explanation for this perseveration is personal and may lie in the fact that I played two contact sports in college. In high school we still wore leather helmets and in college the lacrosse helmets were constructed of plastic-coated cardboard. I can recall just a few of what might be now labeled as sports-related concussions. Ironically, my only loss of consciousness came on the first dinner date with the woman who would eventually become my wife. A hypotensive episode resulting from the combination of sweat loss (2 hours of basketball) and blood loss from selling some platelets earlier in the day (to pay for the dinner) led to the unfortunate meeting of my head and the beautifully tiled floor at the restaurant.
Postconcussion Recovery
The phenomenon of delayed symptomatic recovery has been a particular interest of mine. Within the last 12 months I have written about an excellent companion commentary in Pediatricsby Talin Babikian PhD, a psychologist at University of California, Los Angeles, in which he urges us to “Consider the comorbidities or premorbidities,” including, among others, anxiety and/or depression, post-traumatic stress, and poor sleep when we are faced with a patient who is slow in shedding his postconcussion symptoms. A short 6 months after reading Dr. Babikian’s prescient commentary, I have encountered some evidence supporting his advice.
Investigators at the Sports Medicine and Performance Center at the Children’s Hospital of Philadelphia have recently published a study in which they have found “Preexisting mental health diagnoses are associated with greater postinjury emotional symptom burden and longer concussion recovery in a dose-response fashion.” In their prospective study of over 3000 children and adolescents, they found that, although patients with more mental health diagnoses were at greater risk of increased emotional symptoms after concussion, “Children and adolescents with any preexisting mental health diagnosis took longer to recover.”
Female patients and those with abnormal visio-vestibular test results at the initial postinjury evaluation took longer to recover, although boys with prolonged recovery had more emotional symptoms. In general, patients with preexisting mental health diagnoses returned to exercise later, a known factor in delayed concussion recovery.
Making Sense of It All
There are a couple of ways to look at this paper’s findings. The first is through the lens that focuses on the population of children and adolescents who have known mental health conditions. If our patient has a mental health diagnosis, we shouldn’t be surprised that he/she is taking longer to recover from his/her concussion and is experiencing an increase in symptoms. Most of us probably suspected this already. However, we should be particularly aware of this phenomenon if the patient is male.
The other perspective is probably more valuable to us as primary care physicians. Even one diagnosis so subtle that we may have overlooked it is likely to slow his/her progress toward recovery. And, of course, it may be that injury has triggered an underlying condition that none — not even the most astute diagnostician — would have found without a focused investigation.
I can’t leave this subject without wondering whether the findings in this paper should be extrapolated to other conditions of delayed recovery, including Lyme disease and COVID 19. Patients with these conditions are understandably resistant to the suggestion that their mental health may be contributing to the situation. Too many have been told too often it is “all in their head.” However, I think we as clinicians should keep open minds when symptoms are resolving more slowly than we would expect.
Finally, in their conclusion the authors of this paper reinforce a principle that has unfortunately taken some of us a while to accept. Early introduction of symptom-limited exercise should be a standard of postconcussion management, especially for patients with a mental health diagnosis.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
USPSTF: Insufficient Evidence for Primary Care Interventions to Prevent Child Maltreatment
While primary care physicians are uniquely positioned to identify mistreated minors, there is insufficient evidence of benefits and harms to support primary care interventions to prevent maltreatment in children who have no indicative signs or symptoms. That is the conclusion of the US Preventive Services Task Force (USPSTF) in an update of its 2018 statement published in JAMA Network Open.
This gap, however, might be partially filled by addressing in young patients the known social determinants of health such as economic stability, food, shelter, and healthcare access. The USPSTF statement is based on a simultaneously published evidence review and synthesis compiled by Meera Viswanathan, PhD, of the RTI International-University of North Carolina at Chapel Hill Evidence-Based Practice Center in Triangle Park, NC, and colleagues.
The review included 14,355 participants in 25 trials, of which 23 included home visits. It measured such things as direct reports to Child Protective Services or removal of children from the home and proxy measures of abuse or neglect such as injury, emergency department visits, and hospitalizations. In addition, it looked at behavioral, developmental, emotional, mental or physical health and well-being, mortality, and harms.
More than 50% of the studies analyzed consisted of children with no prior reports of maltreatment. In addition to limited and inconsistent findings, the researchers noted wide variance in screening, identifying, and reporting child maltreatment to authorities, including variations by race or ethnicity, as well as wide variance in the accuracy of screening instruments.
“Contextual evidence pointed to the potential for bias or inaccuracy in screening, identification, and reporting of child maltreatment but also highlighted the importance of addressing social determinants when intervening to prevent child maltreatment,” Dr. Viswanathan’s group wrote.
The USPSTF panel, chaired by Michael J. Barry, MD, of Harvard Medical School, Boston, Massachusetts (now immediate past chair of the Task Force), stressed that the current statement applies only to children with no signs of maltreatment: Those with direct signs should be assessed and appropriately reported.
A Common and Costly Problem
Child abuse or neglect is widespread and has long-lasting adverse effects. In 2021, the statement noted, Child Protective Services identified 600,000 children as abused or neglected, with 1821 related deaths. Most (76%) experienced neglect, but many were subjected to physical abuse (16%), sexual abuse (10%), and sex trafficking (0.2%). Of the 1820 who died, 78% experienced neglect and 43% experienced physical abuse alone or combined with maltreatment such as neglect and psychological abuse.
Benefits aside, among the potential harms of intervention, the USPSTF noted, is family stigma and bias toward non-White and low-income groups. There may be a greater probability of clinicians’ disproportionately reporting abuse for the children of Black, Hispanic, indigenous, and one-parent households. Some studies indicate that more cases of maltreatment are missed in White children, the review authors noted.
“Additional evidence is needed to clarify potential linkages between improvements in social determinants of health and child maltreatment prevention,” the USPSTF panelists concluded. They acknowledged that their recommendation does not address the effectiveness of interventions such as home visits to improve family well-being.
In an accompanying editorial Samantha Schilling, MD, MSHP, of the Department of Pediatrics at the University of North Carolina at Chapel Hill, and colleagues from the Children’s Hospital of Philadelphia in Pennsylvania admitted they were “disheartened, but not surprised” at the USPSTF’s conclusions and urged that prevention measures be continued. “It is not yet time to wave the white flag of surrender and abandon primary care–based efforts to mitigate risks for child abuse and neglect.
They sent a heartfelt message to primary care doctors: “Know this: while additional evidence is amassed, do not stop your ongoing efforts to protect vulnerable children. You are an important component of child maltreatment prevention, although your actions and support cannot be delivered (or measured) in isolation.”
Dr. Schilling and associates argued that insufficient evidence does not mean that primary care prevention efforts are ineffective, only that evidence is lacking. They pointed out that proximal outcomes along a causal pathway have been used to assess the effectiveness of preventive measures and should be considered in this context. “For example, based on evidence that counseling about minimizing exposure to UV radiation is associated with a moderate increase in use of sunscreen protection, the USPSTF recommends that counseling be provided to certain populations,” they wrote. “The USPSTF did not require direct evidence that counseling decreases skin cancer.”
More high-quality research is needed, as the USPSTF recognized. “Given the inadequacy of the current gold standard measures of child maltreatment, proximal outcomes on the complex, multifactorial, causal pathway to child abuse and neglect should be considered,” the commentators wrote.
The commentators also acknowledged that patients’ caregivers often struggle to do their best with sparse resources and that resources such as food and housing, treatment for substance use and mental health disorders, appropriate strategies to manage typical child behavior, and affordable child care too often fall short.
They argued, therefore, that consequential prevention is not possible without sustained investment in policies and programs that provide tangible support to families, reduce childhood poverty, and target relevant risk factors.
The Agency for Healthcare Research and Quality of the US Department of Health and Human Services supports the operations of the USPSTF. Dr. Barry reported grants from Healthwise, a nonprofit organization, outside of the submitted work. Dr. Silverstein reported receiving a research grant on approaches to child maltreatment prevention. Dr. Lee reported grants from the National Institute on Aging. The evidence review was supported by a grant from the Agency for Healthcare Research. Dr. Viswanathan and colleagues disclosed no conflicts of interest. Dr. Wood reported grants from the Annie E. Casey Foundation outside of the submitted work. Dr. Christian reported personal fees from multiple government agencies and legal firms and provides medical-legal expert work in child abuse cases outside of the submitted work.
While primary care physicians are uniquely positioned to identify mistreated minors, there is insufficient evidence of benefits and harms to support primary care interventions to prevent maltreatment in children who have no indicative signs or symptoms. That is the conclusion of the US Preventive Services Task Force (USPSTF) in an update of its 2018 statement published in JAMA Network Open.
This gap, however, might be partially filled by addressing in young patients the known social determinants of health such as economic stability, food, shelter, and healthcare access. The USPSTF statement is based on a simultaneously published evidence review and synthesis compiled by Meera Viswanathan, PhD, of the RTI International-University of North Carolina at Chapel Hill Evidence-Based Practice Center in Triangle Park, NC, and colleagues.
The review included 14,355 participants in 25 trials, of which 23 included home visits. It measured such things as direct reports to Child Protective Services or removal of children from the home and proxy measures of abuse or neglect such as injury, emergency department visits, and hospitalizations. In addition, it looked at behavioral, developmental, emotional, mental or physical health and well-being, mortality, and harms.
More than 50% of the studies analyzed consisted of children with no prior reports of maltreatment. In addition to limited and inconsistent findings, the researchers noted wide variance in screening, identifying, and reporting child maltreatment to authorities, including variations by race or ethnicity, as well as wide variance in the accuracy of screening instruments.
“Contextual evidence pointed to the potential for bias or inaccuracy in screening, identification, and reporting of child maltreatment but also highlighted the importance of addressing social determinants when intervening to prevent child maltreatment,” Dr. Viswanathan’s group wrote.
The USPSTF panel, chaired by Michael J. Barry, MD, of Harvard Medical School, Boston, Massachusetts (now immediate past chair of the Task Force), stressed that the current statement applies only to children with no signs of maltreatment: Those with direct signs should be assessed and appropriately reported.
A Common and Costly Problem
Child abuse or neglect is widespread and has long-lasting adverse effects. In 2021, the statement noted, Child Protective Services identified 600,000 children as abused or neglected, with 1821 related deaths. Most (76%) experienced neglect, but many were subjected to physical abuse (16%), sexual abuse (10%), and sex trafficking (0.2%). Of the 1820 who died, 78% experienced neglect and 43% experienced physical abuse alone or combined with maltreatment such as neglect and psychological abuse.
Benefits aside, among the potential harms of intervention, the USPSTF noted, is family stigma and bias toward non-White and low-income groups. There may be a greater probability of clinicians’ disproportionately reporting abuse for the children of Black, Hispanic, indigenous, and one-parent households. Some studies indicate that more cases of maltreatment are missed in White children, the review authors noted.
“Additional evidence is needed to clarify potential linkages between improvements in social determinants of health and child maltreatment prevention,” the USPSTF panelists concluded. They acknowledged that their recommendation does not address the effectiveness of interventions such as home visits to improve family well-being.
In an accompanying editorial Samantha Schilling, MD, MSHP, of the Department of Pediatrics at the University of North Carolina at Chapel Hill, and colleagues from the Children’s Hospital of Philadelphia in Pennsylvania admitted they were “disheartened, but not surprised” at the USPSTF’s conclusions and urged that prevention measures be continued. “It is not yet time to wave the white flag of surrender and abandon primary care–based efforts to mitigate risks for child abuse and neglect.
They sent a heartfelt message to primary care doctors: “Know this: while additional evidence is amassed, do not stop your ongoing efforts to protect vulnerable children. You are an important component of child maltreatment prevention, although your actions and support cannot be delivered (or measured) in isolation.”
Dr. Schilling and associates argued that insufficient evidence does not mean that primary care prevention efforts are ineffective, only that evidence is lacking. They pointed out that proximal outcomes along a causal pathway have been used to assess the effectiveness of preventive measures and should be considered in this context. “For example, based on evidence that counseling about minimizing exposure to UV radiation is associated with a moderate increase in use of sunscreen protection, the USPSTF recommends that counseling be provided to certain populations,” they wrote. “The USPSTF did not require direct evidence that counseling decreases skin cancer.”
More high-quality research is needed, as the USPSTF recognized. “Given the inadequacy of the current gold standard measures of child maltreatment, proximal outcomes on the complex, multifactorial, causal pathway to child abuse and neglect should be considered,” the commentators wrote.
The commentators also acknowledged that patients’ caregivers often struggle to do their best with sparse resources and that resources such as food and housing, treatment for substance use and mental health disorders, appropriate strategies to manage typical child behavior, and affordable child care too often fall short.
They argued, therefore, that consequential prevention is not possible without sustained investment in policies and programs that provide tangible support to families, reduce childhood poverty, and target relevant risk factors.
The Agency for Healthcare Research and Quality of the US Department of Health and Human Services supports the operations of the USPSTF. Dr. Barry reported grants from Healthwise, a nonprofit organization, outside of the submitted work. Dr. Silverstein reported receiving a research grant on approaches to child maltreatment prevention. Dr. Lee reported grants from the National Institute on Aging. The evidence review was supported by a grant from the Agency for Healthcare Research. Dr. Viswanathan and colleagues disclosed no conflicts of interest. Dr. Wood reported grants from the Annie E. Casey Foundation outside of the submitted work. Dr. Christian reported personal fees from multiple government agencies and legal firms and provides medical-legal expert work in child abuse cases outside of the submitted work.
While primary care physicians are uniquely positioned to identify mistreated minors, there is insufficient evidence of benefits and harms to support primary care interventions to prevent maltreatment in children who have no indicative signs or symptoms. That is the conclusion of the US Preventive Services Task Force (USPSTF) in an update of its 2018 statement published in JAMA Network Open.
This gap, however, might be partially filled by addressing in young patients the known social determinants of health such as economic stability, food, shelter, and healthcare access. The USPSTF statement is based on a simultaneously published evidence review and synthesis compiled by Meera Viswanathan, PhD, of the RTI International-University of North Carolina at Chapel Hill Evidence-Based Practice Center in Triangle Park, NC, and colleagues.
The review included 14,355 participants in 25 trials, of which 23 included home visits. It measured such things as direct reports to Child Protective Services or removal of children from the home and proxy measures of abuse or neglect such as injury, emergency department visits, and hospitalizations. In addition, it looked at behavioral, developmental, emotional, mental or physical health and well-being, mortality, and harms.
More than 50% of the studies analyzed consisted of children with no prior reports of maltreatment. In addition to limited and inconsistent findings, the researchers noted wide variance in screening, identifying, and reporting child maltreatment to authorities, including variations by race or ethnicity, as well as wide variance in the accuracy of screening instruments.
“Contextual evidence pointed to the potential for bias or inaccuracy in screening, identification, and reporting of child maltreatment but also highlighted the importance of addressing social determinants when intervening to prevent child maltreatment,” Dr. Viswanathan’s group wrote.
The USPSTF panel, chaired by Michael J. Barry, MD, of Harvard Medical School, Boston, Massachusetts (now immediate past chair of the Task Force), stressed that the current statement applies only to children with no signs of maltreatment: Those with direct signs should be assessed and appropriately reported.
A Common and Costly Problem
Child abuse or neglect is widespread and has long-lasting adverse effects. In 2021, the statement noted, Child Protective Services identified 600,000 children as abused or neglected, with 1821 related deaths. Most (76%) experienced neglect, but many were subjected to physical abuse (16%), sexual abuse (10%), and sex trafficking (0.2%). Of the 1820 who died, 78% experienced neglect and 43% experienced physical abuse alone or combined with maltreatment such as neglect and psychological abuse.
Benefits aside, among the potential harms of intervention, the USPSTF noted, is family stigma and bias toward non-White and low-income groups. There may be a greater probability of clinicians’ disproportionately reporting abuse for the children of Black, Hispanic, indigenous, and one-parent households. Some studies indicate that more cases of maltreatment are missed in White children, the review authors noted.
“Additional evidence is needed to clarify potential linkages between improvements in social determinants of health and child maltreatment prevention,” the USPSTF panelists concluded. They acknowledged that their recommendation does not address the effectiveness of interventions such as home visits to improve family well-being.
In an accompanying editorial Samantha Schilling, MD, MSHP, of the Department of Pediatrics at the University of North Carolina at Chapel Hill, and colleagues from the Children’s Hospital of Philadelphia in Pennsylvania admitted they were “disheartened, but not surprised” at the USPSTF’s conclusions and urged that prevention measures be continued. “It is not yet time to wave the white flag of surrender and abandon primary care–based efforts to mitigate risks for child abuse and neglect.
They sent a heartfelt message to primary care doctors: “Know this: while additional evidence is amassed, do not stop your ongoing efforts to protect vulnerable children. You are an important component of child maltreatment prevention, although your actions and support cannot be delivered (or measured) in isolation.”
Dr. Schilling and associates argued that insufficient evidence does not mean that primary care prevention efforts are ineffective, only that evidence is lacking. They pointed out that proximal outcomes along a causal pathway have been used to assess the effectiveness of preventive measures and should be considered in this context. “For example, based on evidence that counseling about minimizing exposure to UV radiation is associated with a moderate increase in use of sunscreen protection, the USPSTF recommends that counseling be provided to certain populations,” they wrote. “The USPSTF did not require direct evidence that counseling decreases skin cancer.”
More high-quality research is needed, as the USPSTF recognized. “Given the inadequacy of the current gold standard measures of child maltreatment, proximal outcomes on the complex, multifactorial, causal pathway to child abuse and neglect should be considered,” the commentators wrote.
The commentators also acknowledged that patients’ caregivers often struggle to do their best with sparse resources and that resources such as food and housing, treatment for substance use and mental health disorders, appropriate strategies to manage typical child behavior, and affordable child care too often fall short.
They argued, therefore, that consequential prevention is not possible without sustained investment in policies and programs that provide tangible support to families, reduce childhood poverty, and target relevant risk factors.
The Agency for Healthcare Research and Quality of the US Department of Health and Human Services supports the operations of the USPSTF. Dr. Barry reported grants from Healthwise, a nonprofit organization, outside of the submitted work. Dr. Silverstein reported receiving a research grant on approaches to child maltreatment prevention. Dr. Lee reported grants from the National Institute on Aging. The evidence review was supported by a grant from the Agency for Healthcare Research. Dr. Viswanathan and colleagues disclosed no conflicts of interest. Dr. Wood reported grants from the Annie E. Casey Foundation outside of the submitted work. Dr. Christian reported personal fees from multiple government agencies and legal firms and provides medical-legal expert work in child abuse cases outside of the submitted work.
FROM JAMA NETWORK OPEN
Paid Parental Leave: Impact on Maternal Mental Health and Child Wellbeing
Maternal mental health has a profound impact on the health and wellbeing of the child. Since the onset of the pandemic, rates of postpartum depression have increased, affecting an estimated 1 in 5 women.1 Numerous studies show the impact of postpartum depression on the newborn child across multiple domains, from bonding to healthy weight gain to meeting developmental milestones.
While new medications are being studied and approved to specifically target postpartum depression, these treatments are inaccessible to many because of high costs and long wait lists. Beyond medication, structural changes such as paid parental leave have been shown to have a substantial impact on maternal mental health, thus impacting the health of children as well.
Implications for Mothers and Children
Psychiatric diagnoses such as postpartum depression are on the rise.1,2 This is likely attributable to a combination of factors, including increased isolation since the start of the pandemic, worsening health inequities across race and socioeconomic status, and difficulty accessing mental health care.3-5 The effect that postpartum depression has on the family is significant for the newborn as well as other children in the home.
Data suggest that postpartum depression impacts both the physical and mental health of the child. Infants of mothers with postpartum depression may experience challenges with weight gain, decreased breastfeeding, sleep disruptions, and delays in achieving developmental milestones.6-9 They may also show decreased maternal infant bonding, challenges with cognitive development including language and IQ, and increased risk of behavioral disturbances.10,11 These effects are likely attributable to a combination of factors, including decreased maternal responsiveness to infant cues.7,12 Many of these effects are mediated by the chronicity and severity of depressive symptoms, suggesting the importance of screening and treatment of postpartum depression.10,11 However, treatment for postpartum depression can be difficult to access, particularly given the increased level of need.
It is therefore critical to consider what structural interventions and policy changes can decrease the risk of developing postpartum depression. Data consistently show that access to paid parental leave improves maternal mental health outcomes. Among patients with access to parental leave, research shows that paid leave of longer duration, at least 2-3 months, is the most protective.13 Studies have identified decreased depressive symptoms, decreased stress, decreased use of mental health services, and decreased hospital admissions among women with longer parental leave.13 The positive effects of paid parental leave on maternal mental health can extend beyond the postpartum period, solidifying its impact on the long-term health outcomes of both mother and child.13
Advocacy Is Imperative
In 2024, the United States is the only high-income country, and one of only seven countries in the world, that does not guarantee access to paid parental leave. The Family Medical Leave Act is a 31-year-old federal law that requires some employers to provide unpaid leave to eligible employees. It is narrow in scope, and it excludes many low-wage workers and LGBTQ+ families. Thirteen states — California, Colorado, Connecticut, Delaware, Maine, Massachusetts, Maryland, Minnesota, New Jersey, New York, Oregon, Rhode Island, and Washington — as well as the District of Columbia, have enacted their own paid leave policies. However, there are no federal laws requiring access to paid parental leave. As of 2023, fewer than 30% of workers in the United States have access to paid parental leave, and only 16% of employees in the service industry have access to paid parental leave.14 This disproportionately affects families from lower income backgrounds, and further exacerbates socioeconomic, racial, and gender inequities. From a health systems lens, this increases risk of adverse maternal mental health outcomes among those who already have decreased access to mental health services, worsening health disparities.
Paid parental leave has strong public support across party lines, with polls showing the majority of Americans support comprehensive paid family and medical leave.15 Despite this, the United States has failed to enact legislation on this issue since 1993. Multiple attempts at expanding leave have not come to fruition. In the past year, both the house and the senate have announced bipartisan efforts to expand access to paid parental leave. However, legislative frameworks are still in early stages.
As physicians, it is crucial that we advocate for expanded access to paid parental leave. We must use our expertise to speak to the impact that paid parental leave can have on the mental and physical health of parents, children, and families. By advocating for paid parental leave, we can help create a more just and equitable healthcare system.
Dr. Shannon is a second-year psychiatry resident at University of California, Los Angeles. She attended Stanford University for her undergraduate degree and Dartmouth Geisel School of Medicine for medical school. Her interests include perinatal psychiatry, health systems research, and mental health policy advocacy. Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences; program director of the child and adolescent psychiatry fellowship; and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
References
1. Wang Z et al. Mapping Global Prevalence of Depression Among Postpartum Women. Transl Psychiatry. 2021 Oct 20. doi: 10.1038/s41398-021-01663-6.
2. Iyengar U et al. One Year Into the Pandemic: A Systematic Review of Perinatal Mental Health Outcomes During COVID-19. Front Psychiatry. 2021 Jun 24. doi: 10.3389/fpsyt.2021.674194.
3. World Health Organization. Mental Health and COVID-19: Early Evidence of the Pandemic’s Impact: Scientific Brief. 2022 Mar 2. www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Mental_health-2022.1.
4. Masters GA et al. Impact of the COVID-19 Pandemic on Mental Health, Access to Care, and Health Disparities in the Perinatal Period. J Psychiatr Res. 2021 May. doi: 10.1016/j.jpsychires.2021.02.056.
5. Shuffrey LC et al. Improving Perinatal Maternal Mental Health Starts With Addressing Structural Inequities. JAMA Psychiatry. 2022 May 1. doi: 10.1001/jamapsychiatry.2022.0097.
6. Lubotzky-Gete S et al. Postpartum Depression and Infant Development Up to 24 months: A Nationwide Population-Based Study. J Affect Disord. 2021 Apr 15. doi: 10.1016/j.jad.2021.02.042.
7. Saharoy R et al. Postpartum Depression and Maternal Care: Exploring the Complex Effects on Mothers and Infants. Cureus. 2023 Jul 4. doi: 10.7759/cureus.41381..
8. Gress-Smith JL et al. Postpartum Depression Prevalence and Impact on Infant Health, Weight, and Sleep in Low-Income and Ethnic Minority Women and Infants. Matern Child Health J. 2012 May. doi: 10.1007/s10995-011-0812-y.
9. Kim S et al. The Impact of Antepartum Depression and Postpartum Depression on Exclusive Breastfeeding: A Systematic Review and Meta-Analysis. Clin Nurs Res. 2022 Jun. doi: 10.1177/10547738211053507.
10. Mirhosseini H et al. Cognitive Behavioral Development in Children Following Maternal Postpartum Depression: A Review Article. Electron Physician. 2015 Dec 20. doi: 10.19082/1673.
11. Grace SL et al. The Effect of Postpartum Depression on Child Cognitive Development and Behavior: A Review and Critical Analysis of the Literature. Arch Womens Ment Health. 2003 Nov. doi: 10.1007/s00737-003-0024-6.
12. Milgrom J et al. The Mediating Role of Maternal Responsiveness in Some Longer Term Effects of Postnatal Depression on Infant Development. Infant Behavior and Development. 2004 Sep 11. doi.org/10.1016/j.infbeh.2004.03.003.
13. Heshmati A et al. The Effect of Parental Leave on Parents’ Mental Health: A Systematic Review. Lancet Public Health. 2023 Jan. doi: 10.1016/S2468-2667(22)00311-5.
14. U.S. Bureau of Labor Statistics, What Data Does the BLS Publish on Family Leave? 2023 Sept 21. www.bls.gov/ebs/factsheets/family-leave-benefits-fact-sheet.htm.
15. Horowitz JM et al. Americans Widely Support Paid Family and Medical Leave, But Differ Over Specific Policies. Pew Research Center’s Social & Demographic Trends Project, Pew Research Center. 2017 Mar 23. www.pewresearch.org/social-trends/2017/03/23/americans-widely-support-paid-family-and-medical-leave-but-differ-over-specific-policies/.
Maternal mental health has a profound impact on the health and wellbeing of the child. Since the onset of the pandemic, rates of postpartum depression have increased, affecting an estimated 1 in 5 women.1 Numerous studies show the impact of postpartum depression on the newborn child across multiple domains, from bonding to healthy weight gain to meeting developmental milestones.
While new medications are being studied and approved to specifically target postpartum depression, these treatments are inaccessible to many because of high costs and long wait lists. Beyond medication, structural changes such as paid parental leave have been shown to have a substantial impact on maternal mental health, thus impacting the health of children as well.
Implications for Mothers and Children
Psychiatric diagnoses such as postpartum depression are on the rise.1,2 This is likely attributable to a combination of factors, including increased isolation since the start of the pandemic, worsening health inequities across race and socioeconomic status, and difficulty accessing mental health care.3-5 The effect that postpartum depression has on the family is significant for the newborn as well as other children in the home.
Data suggest that postpartum depression impacts both the physical and mental health of the child. Infants of mothers with postpartum depression may experience challenges with weight gain, decreased breastfeeding, sleep disruptions, and delays in achieving developmental milestones.6-9 They may also show decreased maternal infant bonding, challenges with cognitive development including language and IQ, and increased risk of behavioral disturbances.10,11 These effects are likely attributable to a combination of factors, including decreased maternal responsiveness to infant cues.7,12 Many of these effects are mediated by the chronicity and severity of depressive symptoms, suggesting the importance of screening and treatment of postpartum depression.10,11 However, treatment for postpartum depression can be difficult to access, particularly given the increased level of need.
It is therefore critical to consider what structural interventions and policy changes can decrease the risk of developing postpartum depression. Data consistently show that access to paid parental leave improves maternal mental health outcomes. Among patients with access to parental leave, research shows that paid leave of longer duration, at least 2-3 months, is the most protective.13 Studies have identified decreased depressive symptoms, decreased stress, decreased use of mental health services, and decreased hospital admissions among women with longer parental leave.13 The positive effects of paid parental leave on maternal mental health can extend beyond the postpartum period, solidifying its impact on the long-term health outcomes of both mother and child.13
Advocacy Is Imperative
In 2024, the United States is the only high-income country, and one of only seven countries in the world, that does not guarantee access to paid parental leave. The Family Medical Leave Act is a 31-year-old federal law that requires some employers to provide unpaid leave to eligible employees. It is narrow in scope, and it excludes many low-wage workers and LGBTQ+ families. Thirteen states — California, Colorado, Connecticut, Delaware, Maine, Massachusetts, Maryland, Minnesota, New Jersey, New York, Oregon, Rhode Island, and Washington — as well as the District of Columbia, have enacted their own paid leave policies. However, there are no federal laws requiring access to paid parental leave. As of 2023, fewer than 30% of workers in the United States have access to paid parental leave, and only 16% of employees in the service industry have access to paid parental leave.14 This disproportionately affects families from lower income backgrounds, and further exacerbates socioeconomic, racial, and gender inequities. From a health systems lens, this increases risk of adverse maternal mental health outcomes among those who already have decreased access to mental health services, worsening health disparities.
Paid parental leave has strong public support across party lines, with polls showing the majority of Americans support comprehensive paid family and medical leave.15 Despite this, the United States has failed to enact legislation on this issue since 1993. Multiple attempts at expanding leave have not come to fruition. In the past year, both the house and the senate have announced bipartisan efforts to expand access to paid parental leave. However, legislative frameworks are still in early stages.
As physicians, it is crucial that we advocate for expanded access to paid parental leave. We must use our expertise to speak to the impact that paid parental leave can have on the mental and physical health of parents, children, and families. By advocating for paid parental leave, we can help create a more just and equitable healthcare system.
Dr. Shannon is a second-year psychiatry resident at University of California, Los Angeles. She attended Stanford University for her undergraduate degree and Dartmouth Geisel School of Medicine for medical school. Her interests include perinatal psychiatry, health systems research, and mental health policy advocacy. Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences; program director of the child and adolescent psychiatry fellowship; and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
References
1. Wang Z et al. Mapping Global Prevalence of Depression Among Postpartum Women. Transl Psychiatry. 2021 Oct 20. doi: 10.1038/s41398-021-01663-6.
2. Iyengar U et al. One Year Into the Pandemic: A Systematic Review of Perinatal Mental Health Outcomes During COVID-19. Front Psychiatry. 2021 Jun 24. doi: 10.3389/fpsyt.2021.674194.
3. World Health Organization. Mental Health and COVID-19: Early Evidence of the Pandemic’s Impact: Scientific Brief. 2022 Mar 2. www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Mental_health-2022.1.
4. Masters GA et al. Impact of the COVID-19 Pandemic on Mental Health, Access to Care, and Health Disparities in the Perinatal Period. J Psychiatr Res. 2021 May. doi: 10.1016/j.jpsychires.2021.02.056.
5. Shuffrey LC et al. Improving Perinatal Maternal Mental Health Starts With Addressing Structural Inequities. JAMA Psychiatry. 2022 May 1. doi: 10.1001/jamapsychiatry.2022.0097.
6. Lubotzky-Gete S et al. Postpartum Depression and Infant Development Up to 24 months: A Nationwide Population-Based Study. J Affect Disord. 2021 Apr 15. doi: 10.1016/j.jad.2021.02.042.
7. Saharoy R et al. Postpartum Depression and Maternal Care: Exploring the Complex Effects on Mothers and Infants. Cureus. 2023 Jul 4. doi: 10.7759/cureus.41381..
8. Gress-Smith JL et al. Postpartum Depression Prevalence and Impact on Infant Health, Weight, and Sleep in Low-Income and Ethnic Minority Women and Infants. Matern Child Health J. 2012 May. doi: 10.1007/s10995-011-0812-y.
9. Kim S et al. The Impact of Antepartum Depression and Postpartum Depression on Exclusive Breastfeeding: A Systematic Review and Meta-Analysis. Clin Nurs Res. 2022 Jun. doi: 10.1177/10547738211053507.
10. Mirhosseini H et al. Cognitive Behavioral Development in Children Following Maternal Postpartum Depression: A Review Article. Electron Physician. 2015 Dec 20. doi: 10.19082/1673.
11. Grace SL et al. The Effect of Postpartum Depression on Child Cognitive Development and Behavior: A Review and Critical Analysis of the Literature. Arch Womens Ment Health. 2003 Nov. doi: 10.1007/s00737-003-0024-6.
12. Milgrom J et al. The Mediating Role of Maternal Responsiveness in Some Longer Term Effects of Postnatal Depression on Infant Development. Infant Behavior and Development. 2004 Sep 11. doi.org/10.1016/j.infbeh.2004.03.003.
13. Heshmati A et al. The Effect of Parental Leave on Parents’ Mental Health: A Systematic Review. Lancet Public Health. 2023 Jan. doi: 10.1016/S2468-2667(22)00311-5.
14. U.S. Bureau of Labor Statistics, What Data Does the BLS Publish on Family Leave? 2023 Sept 21. www.bls.gov/ebs/factsheets/family-leave-benefits-fact-sheet.htm.
15. Horowitz JM et al. Americans Widely Support Paid Family and Medical Leave, But Differ Over Specific Policies. Pew Research Center’s Social & Demographic Trends Project, Pew Research Center. 2017 Mar 23. www.pewresearch.org/social-trends/2017/03/23/americans-widely-support-paid-family-and-medical-leave-but-differ-over-specific-policies/.
Maternal mental health has a profound impact on the health and wellbeing of the child. Since the onset of the pandemic, rates of postpartum depression have increased, affecting an estimated 1 in 5 women.1 Numerous studies show the impact of postpartum depression on the newborn child across multiple domains, from bonding to healthy weight gain to meeting developmental milestones.
While new medications are being studied and approved to specifically target postpartum depression, these treatments are inaccessible to many because of high costs and long wait lists. Beyond medication, structural changes such as paid parental leave have been shown to have a substantial impact on maternal mental health, thus impacting the health of children as well.
Implications for Mothers and Children
Psychiatric diagnoses such as postpartum depression are on the rise.1,2 This is likely attributable to a combination of factors, including increased isolation since the start of the pandemic, worsening health inequities across race and socioeconomic status, and difficulty accessing mental health care.3-5 The effect that postpartum depression has on the family is significant for the newborn as well as other children in the home.
Data suggest that postpartum depression impacts both the physical and mental health of the child. Infants of mothers with postpartum depression may experience challenges with weight gain, decreased breastfeeding, sleep disruptions, and delays in achieving developmental milestones.6-9 They may also show decreased maternal infant bonding, challenges with cognitive development including language and IQ, and increased risk of behavioral disturbances.10,11 These effects are likely attributable to a combination of factors, including decreased maternal responsiveness to infant cues.7,12 Many of these effects are mediated by the chronicity and severity of depressive symptoms, suggesting the importance of screening and treatment of postpartum depression.10,11 However, treatment for postpartum depression can be difficult to access, particularly given the increased level of need.
It is therefore critical to consider what structural interventions and policy changes can decrease the risk of developing postpartum depression. Data consistently show that access to paid parental leave improves maternal mental health outcomes. Among patients with access to parental leave, research shows that paid leave of longer duration, at least 2-3 months, is the most protective.13 Studies have identified decreased depressive symptoms, decreased stress, decreased use of mental health services, and decreased hospital admissions among women with longer parental leave.13 The positive effects of paid parental leave on maternal mental health can extend beyond the postpartum period, solidifying its impact on the long-term health outcomes of both mother and child.13
Advocacy Is Imperative
In 2024, the United States is the only high-income country, and one of only seven countries in the world, that does not guarantee access to paid parental leave. The Family Medical Leave Act is a 31-year-old federal law that requires some employers to provide unpaid leave to eligible employees. It is narrow in scope, and it excludes many low-wage workers and LGBTQ+ families. Thirteen states — California, Colorado, Connecticut, Delaware, Maine, Massachusetts, Maryland, Minnesota, New Jersey, New York, Oregon, Rhode Island, and Washington — as well as the District of Columbia, have enacted their own paid leave policies. However, there are no federal laws requiring access to paid parental leave. As of 2023, fewer than 30% of workers in the United States have access to paid parental leave, and only 16% of employees in the service industry have access to paid parental leave.14 This disproportionately affects families from lower income backgrounds, and further exacerbates socioeconomic, racial, and gender inequities. From a health systems lens, this increases risk of adverse maternal mental health outcomes among those who already have decreased access to mental health services, worsening health disparities.
Paid parental leave has strong public support across party lines, with polls showing the majority of Americans support comprehensive paid family and medical leave.15 Despite this, the United States has failed to enact legislation on this issue since 1993. Multiple attempts at expanding leave have not come to fruition. In the past year, both the house and the senate have announced bipartisan efforts to expand access to paid parental leave. However, legislative frameworks are still in early stages.
As physicians, it is crucial that we advocate for expanded access to paid parental leave. We must use our expertise to speak to the impact that paid parental leave can have on the mental and physical health of parents, children, and families. By advocating for paid parental leave, we can help create a more just and equitable healthcare system.
Dr. Shannon is a second-year psychiatry resident at University of California, Los Angeles. She attended Stanford University for her undergraduate degree and Dartmouth Geisel School of Medicine for medical school. Her interests include perinatal psychiatry, health systems research, and mental health policy advocacy. Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences; program director of the child and adolescent psychiatry fellowship; and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
References
1. Wang Z et al. Mapping Global Prevalence of Depression Among Postpartum Women. Transl Psychiatry. 2021 Oct 20. doi: 10.1038/s41398-021-01663-6.
2. Iyengar U et al. One Year Into the Pandemic: A Systematic Review of Perinatal Mental Health Outcomes During COVID-19. Front Psychiatry. 2021 Jun 24. doi: 10.3389/fpsyt.2021.674194.
3. World Health Organization. Mental Health and COVID-19: Early Evidence of the Pandemic’s Impact: Scientific Brief. 2022 Mar 2. www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Mental_health-2022.1.
4. Masters GA et al. Impact of the COVID-19 Pandemic on Mental Health, Access to Care, and Health Disparities in the Perinatal Period. J Psychiatr Res. 2021 May. doi: 10.1016/j.jpsychires.2021.02.056.
5. Shuffrey LC et al. Improving Perinatal Maternal Mental Health Starts With Addressing Structural Inequities. JAMA Psychiatry. 2022 May 1. doi: 10.1001/jamapsychiatry.2022.0097.
6. Lubotzky-Gete S et al. Postpartum Depression and Infant Development Up to 24 months: A Nationwide Population-Based Study. J Affect Disord. 2021 Apr 15. doi: 10.1016/j.jad.2021.02.042.
7. Saharoy R et al. Postpartum Depression and Maternal Care: Exploring the Complex Effects on Mothers and Infants. Cureus. 2023 Jul 4. doi: 10.7759/cureus.41381..
8. Gress-Smith JL et al. Postpartum Depression Prevalence and Impact on Infant Health, Weight, and Sleep in Low-Income and Ethnic Minority Women and Infants. Matern Child Health J. 2012 May. doi: 10.1007/s10995-011-0812-y.
9. Kim S et al. The Impact of Antepartum Depression and Postpartum Depression on Exclusive Breastfeeding: A Systematic Review and Meta-Analysis. Clin Nurs Res. 2022 Jun. doi: 10.1177/10547738211053507.
10. Mirhosseini H et al. Cognitive Behavioral Development in Children Following Maternal Postpartum Depression: A Review Article. Electron Physician. 2015 Dec 20. doi: 10.19082/1673.
11. Grace SL et al. The Effect of Postpartum Depression on Child Cognitive Development and Behavior: A Review and Critical Analysis of the Literature. Arch Womens Ment Health. 2003 Nov. doi: 10.1007/s00737-003-0024-6.
12. Milgrom J et al. The Mediating Role of Maternal Responsiveness in Some Longer Term Effects of Postnatal Depression on Infant Development. Infant Behavior and Development. 2004 Sep 11. doi.org/10.1016/j.infbeh.2004.03.003.
13. Heshmati A et al. The Effect of Parental Leave on Parents’ Mental Health: A Systematic Review. Lancet Public Health. 2023 Jan. doi: 10.1016/S2468-2667(22)00311-5.
14. U.S. Bureau of Labor Statistics, What Data Does the BLS Publish on Family Leave? 2023 Sept 21. www.bls.gov/ebs/factsheets/family-leave-benefits-fact-sheet.htm.
15. Horowitz JM et al. Americans Widely Support Paid Family and Medical Leave, But Differ Over Specific Policies. Pew Research Center’s Social & Demographic Trends Project, Pew Research Center. 2017 Mar 23. www.pewresearch.org/social-trends/2017/03/23/americans-widely-support-paid-family-and-medical-leave-but-differ-over-specific-policies/.
Topical Roflumilast Effective in 4 Weeks for Atopic Dermatitis in Young Children
SAN DIEGO — Treatment with (AD), according to the results of a phase 3 study reported at the annual meeting of the American Academy of Dermatology.
Among patients treated with roflumilast cream, 0.05%, 25.4% reached the primary endpoint of “clear” or “almost clear” plus a two-grade improvement from baseline at week 4 vs 10.7% among those in the vehicle group (P < .0001) in a phase 3 randomized controlled trial of children. The findings were released in a late-breaker session at the meeting.
Roflumilast cream, 0.3% (Zoryve), is approved by the Food and Drug Administration (FDA) for treating psoriasis in patients 6 years and older, and lower doses are being evaluated for AD: 0.15% for adults and children ages 6 and older, and 0.05% for ages 2-5. Roflumilast is a phosphodiesterase-4 inhibitor. In 2023, the FDA accepted a supplemental drug application from the manufacturer, Arcutis, for roflumilast, 0.15%, for treating AD in patients ages 6 and older, based on the results from two recently published phase 3 trials, INTEGUMENT-1 and INTEGUMENT-2.
The study of younger children, INTEGUMENT-PED, recruited 652 patients aged 2-5 with mild to moderate AD, with a Validated Investigator Global Assessment scale for AD (vlGA-AD) score of 2 or 3, a mean body surface area of 22% overall (range, 3%-82%), and an Eczema Area and Severity Index (EASI) score of at least 5. Of the patients enrolled, 437 were assigned to 0.05% roflumilast cream, applied once a day for 4 weeks (mean age, 3.3 years; 51.6% male; 67.4% White; 15.6% Black; 8.5% Asian; 8.5% other or more than one race; 80.5% not Latino/Hispanic). The remaining 215 children were assigned to vehicle cream and had similar characteristics.
About 52% of the patients in both groups had an inadequate response, intolerance, or contraindications to topical corticosteroids (and about 17% for topical calcineurin inhibitors and about 9% for crisaborole).
The proportions of patients who reached “clear” (0) or “almost clear” (1) on the vlGA-AD scale were 35.4% and 14.6%, respectively, at week 4 (P < .0001) for roflumilast and vehicle, respectively, according to the lead author of the study, Lawrence M. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, who presented the results at the meeting. In addition, 39.4% and 20.6% achieved an EASI-75 (a secondary endpoint), respectively (P < .0001), and itch also improved within 24 hours of starting treatment.
With regard to safety, 29.7% of patients taking roflumilast had treatment-emergent adverse effects (including upper respiratory tract infections in 4.1%) vs 21.9% of those in the vehicle arm (including upper respiratory tract infections in 1.4%). Reports of pain at the administration site were low (1.6% for roflumilast vs 1.9% for vehicle). Only one patient, a 2-year-old girl, had a treatment-emergent serious adverse event. The child, who was in the roflumilast group, had cellulitis involving noneczematous skin and was treated with antibiotics in the hospital for 3 days. The event was not attributed to roflumilast, which was stopped for 5 days, according to Dr. Eichenfield.
In an interview, Fairfield, Connecticut–based dermatologist Brittany Craiglow, MD, who was not involved in the study, said topical roflumilast would be an “important” new treatment because there are still few nonsteroidal options for the treatment of AD in children under 12. “The excellent local tolerability combined with early improvements in itch and skin clearance will make this a particularly attractive option, if approved,” she said.
Dr. Eichenfield disclosed multiple relationships with various drugmakers. He and several other study authors are investigators and/or consultants for Arcutis and received grants/research funding and/or honoraria. Two authors are Arcutis employees. Other disclosure information for the authors was not immediately available. Dr. Craiglow had no disclosures.
A version of this article appeared on Medscape.com .
SAN DIEGO — Treatment with (AD), according to the results of a phase 3 study reported at the annual meeting of the American Academy of Dermatology.
Among patients treated with roflumilast cream, 0.05%, 25.4% reached the primary endpoint of “clear” or “almost clear” plus a two-grade improvement from baseline at week 4 vs 10.7% among those in the vehicle group (P < .0001) in a phase 3 randomized controlled trial of children. The findings were released in a late-breaker session at the meeting.
Roflumilast cream, 0.3% (Zoryve), is approved by the Food and Drug Administration (FDA) for treating psoriasis in patients 6 years and older, and lower doses are being evaluated for AD: 0.15% for adults and children ages 6 and older, and 0.05% for ages 2-5. Roflumilast is a phosphodiesterase-4 inhibitor. In 2023, the FDA accepted a supplemental drug application from the manufacturer, Arcutis, for roflumilast, 0.15%, for treating AD in patients ages 6 and older, based on the results from two recently published phase 3 trials, INTEGUMENT-1 and INTEGUMENT-2.
The study of younger children, INTEGUMENT-PED, recruited 652 patients aged 2-5 with mild to moderate AD, with a Validated Investigator Global Assessment scale for AD (vlGA-AD) score of 2 or 3, a mean body surface area of 22% overall (range, 3%-82%), and an Eczema Area and Severity Index (EASI) score of at least 5. Of the patients enrolled, 437 were assigned to 0.05% roflumilast cream, applied once a day for 4 weeks (mean age, 3.3 years; 51.6% male; 67.4% White; 15.6% Black; 8.5% Asian; 8.5% other or more than one race; 80.5% not Latino/Hispanic). The remaining 215 children were assigned to vehicle cream and had similar characteristics.
About 52% of the patients in both groups had an inadequate response, intolerance, or contraindications to topical corticosteroids (and about 17% for topical calcineurin inhibitors and about 9% for crisaborole).
The proportions of patients who reached “clear” (0) or “almost clear” (1) on the vlGA-AD scale were 35.4% and 14.6%, respectively, at week 4 (P < .0001) for roflumilast and vehicle, respectively, according to the lead author of the study, Lawrence M. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, who presented the results at the meeting. In addition, 39.4% and 20.6% achieved an EASI-75 (a secondary endpoint), respectively (P < .0001), and itch also improved within 24 hours of starting treatment.
With regard to safety, 29.7% of patients taking roflumilast had treatment-emergent adverse effects (including upper respiratory tract infections in 4.1%) vs 21.9% of those in the vehicle arm (including upper respiratory tract infections in 1.4%). Reports of pain at the administration site were low (1.6% for roflumilast vs 1.9% for vehicle). Only one patient, a 2-year-old girl, had a treatment-emergent serious adverse event. The child, who was in the roflumilast group, had cellulitis involving noneczematous skin and was treated with antibiotics in the hospital for 3 days. The event was not attributed to roflumilast, which was stopped for 5 days, according to Dr. Eichenfield.
In an interview, Fairfield, Connecticut–based dermatologist Brittany Craiglow, MD, who was not involved in the study, said topical roflumilast would be an “important” new treatment because there are still few nonsteroidal options for the treatment of AD in children under 12. “The excellent local tolerability combined with early improvements in itch and skin clearance will make this a particularly attractive option, if approved,” she said.
Dr. Eichenfield disclosed multiple relationships with various drugmakers. He and several other study authors are investigators and/or consultants for Arcutis and received grants/research funding and/or honoraria. Two authors are Arcutis employees. Other disclosure information for the authors was not immediately available. Dr. Craiglow had no disclosures.
A version of this article appeared on Medscape.com .
SAN DIEGO — Treatment with (AD), according to the results of a phase 3 study reported at the annual meeting of the American Academy of Dermatology.
Among patients treated with roflumilast cream, 0.05%, 25.4% reached the primary endpoint of “clear” or “almost clear” plus a two-grade improvement from baseline at week 4 vs 10.7% among those in the vehicle group (P < .0001) in a phase 3 randomized controlled trial of children. The findings were released in a late-breaker session at the meeting.
Roflumilast cream, 0.3% (Zoryve), is approved by the Food and Drug Administration (FDA) for treating psoriasis in patients 6 years and older, and lower doses are being evaluated for AD: 0.15% for adults and children ages 6 and older, and 0.05% for ages 2-5. Roflumilast is a phosphodiesterase-4 inhibitor. In 2023, the FDA accepted a supplemental drug application from the manufacturer, Arcutis, for roflumilast, 0.15%, for treating AD in patients ages 6 and older, based on the results from two recently published phase 3 trials, INTEGUMENT-1 and INTEGUMENT-2.
The study of younger children, INTEGUMENT-PED, recruited 652 patients aged 2-5 with mild to moderate AD, with a Validated Investigator Global Assessment scale for AD (vlGA-AD) score of 2 or 3, a mean body surface area of 22% overall (range, 3%-82%), and an Eczema Area and Severity Index (EASI) score of at least 5. Of the patients enrolled, 437 were assigned to 0.05% roflumilast cream, applied once a day for 4 weeks (mean age, 3.3 years; 51.6% male; 67.4% White; 15.6% Black; 8.5% Asian; 8.5% other or more than one race; 80.5% not Latino/Hispanic). The remaining 215 children were assigned to vehicle cream and had similar characteristics.
About 52% of the patients in both groups had an inadequate response, intolerance, or contraindications to topical corticosteroids (and about 17% for topical calcineurin inhibitors and about 9% for crisaborole).
The proportions of patients who reached “clear” (0) or “almost clear” (1) on the vlGA-AD scale were 35.4% and 14.6%, respectively, at week 4 (P < .0001) for roflumilast and vehicle, respectively, according to the lead author of the study, Lawrence M. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, who presented the results at the meeting. In addition, 39.4% and 20.6% achieved an EASI-75 (a secondary endpoint), respectively (P < .0001), and itch also improved within 24 hours of starting treatment.
With regard to safety, 29.7% of patients taking roflumilast had treatment-emergent adverse effects (including upper respiratory tract infections in 4.1%) vs 21.9% of those in the vehicle arm (including upper respiratory tract infections in 1.4%). Reports of pain at the administration site were low (1.6% for roflumilast vs 1.9% for vehicle). Only one patient, a 2-year-old girl, had a treatment-emergent serious adverse event. The child, who was in the roflumilast group, had cellulitis involving noneczematous skin and was treated with antibiotics in the hospital for 3 days. The event was not attributed to roflumilast, which was stopped for 5 days, according to Dr. Eichenfield.
In an interview, Fairfield, Connecticut–based dermatologist Brittany Craiglow, MD, who was not involved in the study, said topical roflumilast would be an “important” new treatment because there are still few nonsteroidal options for the treatment of AD in children under 12. “The excellent local tolerability combined with early improvements in itch and skin clearance will make this a particularly attractive option, if approved,” she said.
Dr. Eichenfield disclosed multiple relationships with various drugmakers. He and several other study authors are investigators and/or consultants for Arcutis and received grants/research funding and/or honoraria. Two authors are Arcutis employees. Other disclosure information for the authors was not immediately available. Dr. Craiglow had no disclosures.
A version of this article appeared on Medscape.com .
FROM AAD 2024
New Research Dissects Transgenerational Obesity and Diabetes
FAIRFAX, VIRGINIA — Nearly 30 years ago, in a 1995 paper, the British physician-epidemiologist David Barker, MD, PhD, wrote about his fetal origins hypothesis — the idea that programs to address fetal undernutrition and low birth weight produced later coronary heart disease (BMJ 1995;311:171-4).
His hypothesis and subsequent research led to the concept of adult diseases of fetal origins, which today extends beyond low birth weight and implicates the in utero environment as a significant determinant of risk for adverse childhood and adult metabolic outcomes and for major chronic diseases, including diabetes and obesity. Studies have shown that the offspring of pregnant mothers with diabetes have a higher risk of developing obesity and diabetes themselves.
“It’s a whole discipline [of research],” E. Albert Reece, MD, PhD, MBA, of the University of Maryland School of Medicine (UMSOM), said in an interview. “But what we’ve never quite understood is the ‘how’ and ‘why’? What are the mechanisms driving the fetal origins of such adverse outcomes in offspring?
At the biennial meeting of the Diabetes in Pregnancy Study Group of North America (DPSG), investigators described studies underway that are digging deeper into the associations between the intrauterine milieu and longer-term offspring health — and that are searching for biological and molecular processes that may be involved.
The studies are like “branches of the Barker hypothesis,” said Dr. Reece, former dean of UMSOM and current director of the UMSOM Center for Advanced Research Training and Innovation, who co-organized the DPSG meeting. “They’re taking the hypothesis and dissecting it by asking, for instance, it is possible that transgenerational obesity may align with the Barker hypothesis? Is it possible that it involves epigenetics regulation? Could we find biomarkers?”
The need for a better understanding of the fetal origins framework — and its subsequent transgenerational impact — is urgent. From 2000 to 2018, the prevalence of childhood obesity increased from 14.7% to 19.2% (a 31% increase) and the prevalence of severe childhood obesity rose from 3.9% to 6.1% (a 56% increase), according to data from the U.S. National Health and Nutrition Examination Survey (Obes Facts. 2022;15[4]:560-9).
Children aged 2-5 years have had an especially sharp increase in obesity (Pediatrics 2018;141[3]:e20173459), Christine Wey Hockett, PhD, of the University of South Dakota School of Medicine, said at the DPSG meeting (Figure 1).
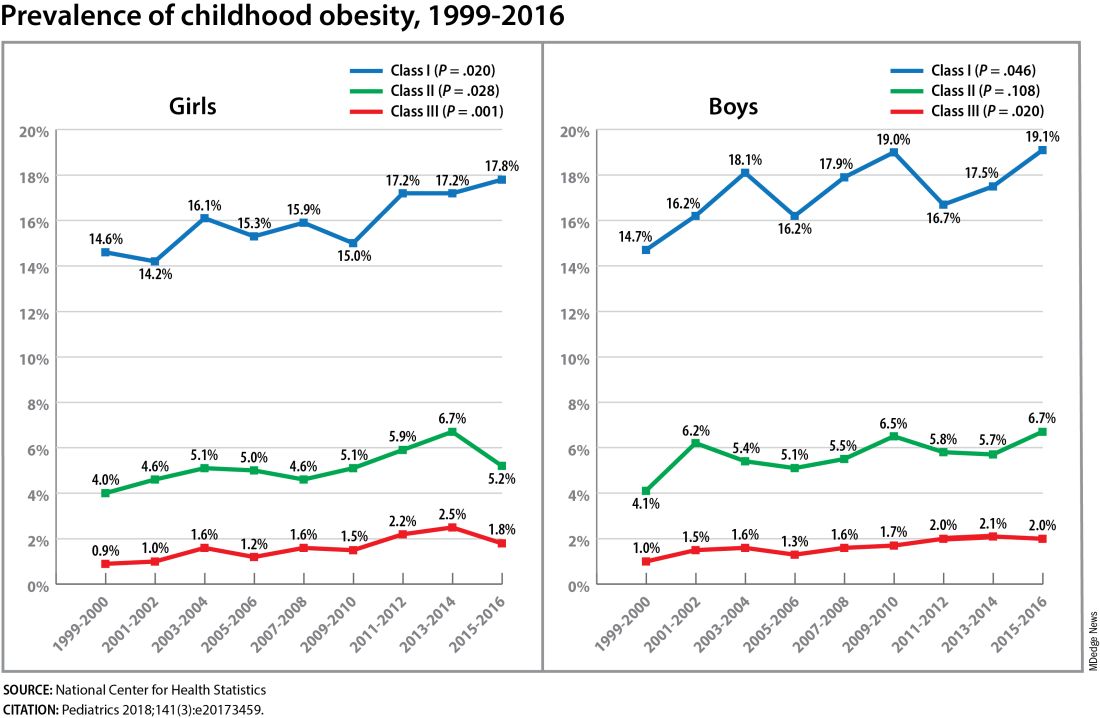
Also notable, she said, is that one-quarter of today’s pediatric diabetes cases are type 2 diabetes, which “is significant as there is a higher prevalence of early complications and comorbidities in youth with type 2 diabetes compared to type 1 diabetes.”
Moreover, recent projections estimate that 57% of today’s children will be obese at 35 years of age (N Engl J Med. 2017;377[22]:2145-53) and that 45% will have diabetes or prediabetes by 2030 (Popul Health Manag. 2017;20[1]:6-12), said Dr. Hockett, assistant professor in the university’s department of pediatrics. An investigator of the Exploring Perinatal Outcomes Among Children (EPOCH) study, which looked at gestational diabetes (GDM) and offspring cardiometabolic risks, she said more chronic disease “at increasingly younger ages [points toward] prebirth influences.”
She noted that there are critical periods postnatally — such as infancy and puberty — that can “impact or further shift the trajectory of chronic disease.” The developmental origins theory posits that life events and biological and environmental processes during the lifespan can modify the effects of intrauterine exposures.
The transgenerational implications “are clear,” she said. “As the number of reproductive-aged individuals with chronic diseases rises, the number of exposed offspring also rises ... It leads to a vicious cycle.”
Deeper Dives Into Associations, Potential Mechanisms
The EPOCH prospective cohort study with which Dr. Hockett was involved gave her a front-seat view of the transgenerational adverse effects of in utero exposure to hyperglycemia. The study recruited ethnically diverse maternal/child dyads from the Kaiser Permanente of Colorado perinatal database from 1992 to 2002 and assessed 418 offspring at two points — a mean age of 10.5 years and 16.5 years — for fasting blood glucose, adiposity, and diet and physical activity. The second visit also involved an oral glucose tolerance test.
The 77 offspring who had been exposed in utero to GDM had a homeostatic model assessment of insulin resistance (HOMA-IR) that was 18% higher, a 19% lower Matsuda index, and a 9% greater HOMA of β-cell function (HOMA-β) than the 341 offspring whose mothers did not have diabetes. Each 5-kg/m2 increase in prepregnancy body mass index predicted increased insulin resistance, but there was no combined effect of both maternal obesity and diabetes in utero.
Exposed offspring had a higher BMI and increased adiposity, but when BMI was controlled for in the analysis of metabolic outcomes, maternal diabetes was still associated with 12% higher HOMA-IR and a 17% lower Matsuda index. “So [the metabolic outcomes] are a direct effect of maternal diabetes,” Dr. Hockett said at the DPSG meeting, noting the fetal overnutrition hypothesis in which maternal glucose, but not maternal insulin, freely passes through the placenta, promoting growth and adiposity in the fetus.
[The EPOCH results on metabolic outcomes and offspring adiposity were published in 2017 and 2019, respectively (Diabet Med. 2017;34:1392-9; Diabetologia. 2019;62:2017-24). In 2020, EPOCH researchers reported sex-specific effects on cardiovascular outcomes, with GDM exposure associated with higher total and LDL cholesterol in girls and higher systolic blood pressure in boys (Pediatr Obes. 2020;15[5]:e12611).]
Now, a new longitudinal cohort study underway in Phoenix, is taking a deeper dive, trying to pinpoint what exactly influences childhood obesity and metabolic risk by following Hispanic and American Indian maternal/child dyads from pregnancy until 18 years postpartum. Researchers are looking not only at associations between maternal risk factors (pregnancy BMI, gestational weight gain, and diabetes in pregnancy) and offspring BMI, adiposity, and growth patterns, but also how various factors during pregnancy — clinical, genetic, lifestyle, biochemical — ”may mediate the associations,” said lead investigator Madhumita Sinha, MD.
“We need a better understanding at the molecular level of the biological processes that lead to obesity in children and that cause metabolic dysfunction,” said Dr. Sinha, who heads the Diabetes Epidemiology and Clinical Research Section of the of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) branch in Phoenix.
The populations being enrolled in the ETCHED study (for Early Tracking of Childhood Health Determinants) are at especially high risk of childhood obesity and metabolic dysfunction. Research conducted decades ago by the NIDDK in Phoenix showed that approximately 50% of Pima Indian children from diabetic pregnancies develop type 2 diabetes by age 25 (N Engl J Med. 1983;308:242-5). Years later, to tease out possible genetic factors, researchers compared siblings born before and after their mother was found to have type 2 diabetes, and found significantly higher rates of diabetes in those born after the mother’s diagnosis, affirming the role of in utero toxicity (Diabetes 2000;49:2208-11).
In the new study, the researchers will look at adipokines and inflammatory biomarkers in the mothers and offspring in addition to traditional anthropometric and glycemic measures. They’ll analyze placental tissue, breast milk, and the gut microbiome longitudinally, and they’ll lean heavily on genomics/epigenomics, proteomics, and metabolomics. “There’s potential,” Dr. Sinha said, “to develop a more accurate predictive and prognostic model of childhood obesity.”
The researchers also will study the role of family, socioeconomics, and environmental factors in influencing child growth patterns and they’ll look at neurodevelopment in infancy and childhood. As of October 2023, almost 80 pregnant women, most with obesity and almost one-third with type 2 diabetes, had enrolled in the study. Over the next several years, the study aims to enroll 750 dyads.
The Timing of In Utero Exposure
Shelley Ehrlich, MD, ScD, MPH, of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center, is aiming, meanwhile, to learn how the timing of in utero exposure to hyperglycemia predicts specific metabolic and cardiovascular morbidities in the adult offspring of diabetic mothers.
“While we know that exposure to maternal diabetes, regardless of type, increases the risk of obesity, insulin resistance, diabetes, renal compromise, and cardiovascular disease in the offspring, there is little known about the level and timing of hyperglycemic exposure during fetal development that triggers these adverse outcomes,” said Dr. Ehrlich. A goal, she said, is to identify gestational profiles that predict phenotypes of offspring at risk for morbidity in later life.
She and other investigators with the TEAM (Transgenerational Effect on Adult Morbidity) study have recruited over 170 offspring of mothers who participated in the Diabetes in Pregnancy Program Project Grant (PPG) at the University of Cincinnati Medical Center from 1978 to 1995 — a landmark study that demonstrated the effect of strict glucose control in reducing major congenital malformations.
The women in the PPG study had frequent glucose monitoring (up to 6-8 times a day) throughout their pregnancies, and now, their recruited offspring, who are up to 43 years of age, are being assessed for obesity, diabetes/metabolic health, cardiovascular disease/cardiac and peripheral vascular structure and function, and other outcomes including those that may be amenable to secondary prevention (J Diabetes Res. Nov 1;2021:6590431).
Preliminary findings from over 170 offspring recruited between 2017 and 2022 suggest that in utero exposure to dysglycemia (as measured by standard deviations of glycohemoglobin) in the third trimester appears to increase the risk of morbid obesity in adulthood, while exposure to dysglycemia in the first trimester increases the risk of impaired glucose tolerance. The risk of B-cell dysfunction, meanwhile, appears to be linked to dysglycemia in the first and third trimesters — particularly the first — Dr. Ehrlich reported.
Cognitive outcomes in offspring have also been assessed and here it appears that dysglycemia in the third trimester is linked to worse scores on the Wechsler Abbreviated Scale of Intelligence (WASI-II), said Katherine Bowers, PhD, MPH, a TEAM study coinvestigator, also of Cincinnati Children’s Hospital Medical Center.
“We’ve already observed [an association between] diabetes in pregnancy and cognition in early childhood and through adolescence, but [the question has been] does this association persist into adulthood?” she said.
Preliminary analyses of 104 offspring show no statistically significant associations between maternal dysglycemia in the first or second trimesters and offspring cognition, but “consistent inverse associations between maternal glycohemoglobin in the third trimester across two [WASI-II] subscales and composite measures of cognition,” Dr. Bowers said.
Their analysis adjusted for a variety of factors, including maternal age, prepregnancy and first trimester BMI, race, family history of diabetes, and diabetes severity/macrovascular complications.
Back In The Laboratory
At the other end of the research spectrum, basic research scientists are also investigating the mechanisms and sequelae of in utero hyperglycemia and other injuries, including congenital malformations, placental adaptive responses and fetal programming. Researchers are asking, for instance, what does placental metabolic reprogramming entail? What role do placental extracellular vesicles play in GDM? Can we alter the in utero environment and thus improve the short and long-term fetal/infant outcomes?
Animal research done at the UMSOM Center for Birth Defects Research, led by Dr. Reece and Peixin Yang, PhD, suggests that “a good portion of in utero injury is due to epigenetics,” Dr. Reece said in the interview. “We’ve shown that under conditions of hyperglycemia, for example, genetic regulation and genetic function can be altered.”
Through in vivo research, they have also shown that antioxidants or membrane stabilizers such as arachidonic acid or myo-inositol, or experimental inhibitors to certain pro-apoptotic intermediates, can individually or collectively result in reduced malformations. “It is highly likely that understanding the biological impact of various altered in utero environments, and then modifying or reversing those environments, will result in short and long-term outcome improvements similar to those shown with congenital malformations,” Dr. Reece said.
FAIRFAX, VIRGINIA — Nearly 30 years ago, in a 1995 paper, the British physician-epidemiologist David Barker, MD, PhD, wrote about his fetal origins hypothesis — the idea that programs to address fetal undernutrition and low birth weight produced later coronary heart disease (BMJ 1995;311:171-4).
His hypothesis and subsequent research led to the concept of adult diseases of fetal origins, which today extends beyond low birth weight and implicates the in utero environment as a significant determinant of risk for adverse childhood and adult metabolic outcomes and for major chronic diseases, including diabetes and obesity. Studies have shown that the offspring of pregnant mothers with diabetes have a higher risk of developing obesity and diabetes themselves.
“It’s a whole discipline [of research],” E. Albert Reece, MD, PhD, MBA, of the University of Maryland School of Medicine (UMSOM), said in an interview. “But what we’ve never quite understood is the ‘how’ and ‘why’? What are the mechanisms driving the fetal origins of such adverse outcomes in offspring?
At the biennial meeting of the Diabetes in Pregnancy Study Group of North America (DPSG), investigators described studies underway that are digging deeper into the associations between the intrauterine milieu and longer-term offspring health — and that are searching for biological and molecular processes that may be involved.
The studies are like “branches of the Barker hypothesis,” said Dr. Reece, former dean of UMSOM and current director of the UMSOM Center for Advanced Research Training and Innovation, who co-organized the DPSG meeting. “They’re taking the hypothesis and dissecting it by asking, for instance, it is possible that transgenerational obesity may align with the Barker hypothesis? Is it possible that it involves epigenetics regulation? Could we find biomarkers?”
The need for a better understanding of the fetal origins framework — and its subsequent transgenerational impact — is urgent. From 2000 to 2018, the prevalence of childhood obesity increased from 14.7% to 19.2% (a 31% increase) and the prevalence of severe childhood obesity rose from 3.9% to 6.1% (a 56% increase), according to data from the U.S. National Health and Nutrition Examination Survey (Obes Facts. 2022;15[4]:560-9).
Children aged 2-5 years have had an especially sharp increase in obesity (Pediatrics 2018;141[3]:e20173459), Christine Wey Hockett, PhD, of the University of South Dakota School of Medicine, said at the DPSG meeting (Figure 1).

Also notable, she said, is that one-quarter of today’s pediatric diabetes cases are type 2 diabetes, which “is significant as there is a higher prevalence of early complications and comorbidities in youth with type 2 diabetes compared to type 1 diabetes.”
Moreover, recent projections estimate that 57% of today’s children will be obese at 35 years of age (N Engl J Med. 2017;377[22]:2145-53) and that 45% will have diabetes or prediabetes by 2030 (Popul Health Manag. 2017;20[1]:6-12), said Dr. Hockett, assistant professor in the university’s department of pediatrics. An investigator of the Exploring Perinatal Outcomes Among Children (EPOCH) study, which looked at gestational diabetes (GDM) and offspring cardiometabolic risks, she said more chronic disease “at increasingly younger ages [points toward] prebirth influences.”
She noted that there are critical periods postnatally — such as infancy and puberty — that can “impact or further shift the trajectory of chronic disease.” The developmental origins theory posits that life events and biological and environmental processes during the lifespan can modify the effects of intrauterine exposures.
The transgenerational implications “are clear,” she said. “As the number of reproductive-aged individuals with chronic diseases rises, the number of exposed offspring also rises ... It leads to a vicious cycle.”
Deeper Dives Into Associations, Potential Mechanisms
The EPOCH prospective cohort study with which Dr. Hockett was involved gave her a front-seat view of the transgenerational adverse effects of in utero exposure to hyperglycemia. The study recruited ethnically diverse maternal/child dyads from the Kaiser Permanente of Colorado perinatal database from 1992 to 2002 and assessed 418 offspring at two points — a mean age of 10.5 years and 16.5 years — for fasting blood glucose, adiposity, and diet and physical activity. The second visit also involved an oral glucose tolerance test.
The 77 offspring who had been exposed in utero to GDM had a homeostatic model assessment of insulin resistance (HOMA-IR) that was 18% higher, a 19% lower Matsuda index, and a 9% greater HOMA of β-cell function (HOMA-β) than the 341 offspring whose mothers did not have diabetes. Each 5-kg/m2 increase in prepregnancy body mass index predicted increased insulin resistance, but there was no combined effect of both maternal obesity and diabetes in utero.
Exposed offspring had a higher BMI and increased adiposity, but when BMI was controlled for in the analysis of metabolic outcomes, maternal diabetes was still associated with 12% higher HOMA-IR and a 17% lower Matsuda index. “So [the metabolic outcomes] are a direct effect of maternal diabetes,” Dr. Hockett said at the DPSG meeting, noting the fetal overnutrition hypothesis in which maternal glucose, but not maternal insulin, freely passes through the placenta, promoting growth and adiposity in the fetus.
[The EPOCH results on metabolic outcomes and offspring adiposity were published in 2017 and 2019, respectively (Diabet Med. 2017;34:1392-9; Diabetologia. 2019;62:2017-24). In 2020, EPOCH researchers reported sex-specific effects on cardiovascular outcomes, with GDM exposure associated with higher total and LDL cholesterol in girls and higher systolic blood pressure in boys (Pediatr Obes. 2020;15[5]:e12611).]
Now, a new longitudinal cohort study underway in Phoenix, is taking a deeper dive, trying to pinpoint what exactly influences childhood obesity and metabolic risk by following Hispanic and American Indian maternal/child dyads from pregnancy until 18 years postpartum. Researchers are looking not only at associations between maternal risk factors (pregnancy BMI, gestational weight gain, and diabetes in pregnancy) and offspring BMI, adiposity, and growth patterns, but also how various factors during pregnancy — clinical, genetic, lifestyle, biochemical — ”may mediate the associations,” said lead investigator Madhumita Sinha, MD.
“We need a better understanding at the molecular level of the biological processes that lead to obesity in children and that cause metabolic dysfunction,” said Dr. Sinha, who heads the Diabetes Epidemiology and Clinical Research Section of the of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) branch in Phoenix.
The populations being enrolled in the ETCHED study (for Early Tracking of Childhood Health Determinants) are at especially high risk of childhood obesity and metabolic dysfunction. Research conducted decades ago by the NIDDK in Phoenix showed that approximately 50% of Pima Indian children from diabetic pregnancies develop type 2 diabetes by age 25 (N Engl J Med. 1983;308:242-5). Years later, to tease out possible genetic factors, researchers compared siblings born before and after their mother was found to have type 2 diabetes, and found significantly higher rates of diabetes in those born after the mother’s diagnosis, affirming the role of in utero toxicity (Diabetes 2000;49:2208-11).
In the new study, the researchers will look at adipokines and inflammatory biomarkers in the mothers and offspring in addition to traditional anthropometric and glycemic measures. They’ll analyze placental tissue, breast milk, and the gut microbiome longitudinally, and they’ll lean heavily on genomics/epigenomics, proteomics, and metabolomics. “There’s potential,” Dr. Sinha said, “to develop a more accurate predictive and prognostic model of childhood obesity.”
The researchers also will study the role of family, socioeconomics, and environmental factors in influencing child growth patterns and they’ll look at neurodevelopment in infancy and childhood. As of October 2023, almost 80 pregnant women, most with obesity and almost one-third with type 2 diabetes, had enrolled in the study. Over the next several years, the study aims to enroll 750 dyads.
The Timing of In Utero Exposure
Shelley Ehrlich, MD, ScD, MPH, of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center, is aiming, meanwhile, to learn how the timing of in utero exposure to hyperglycemia predicts specific metabolic and cardiovascular morbidities in the adult offspring of diabetic mothers.
“While we know that exposure to maternal diabetes, regardless of type, increases the risk of obesity, insulin resistance, diabetes, renal compromise, and cardiovascular disease in the offspring, there is little known about the level and timing of hyperglycemic exposure during fetal development that triggers these adverse outcomes,” said Dr. Ehrlich. A goal, she said, is to identify gestational profiles that predict phenotypes of offspring at risk for morbidity in later life.
She and other investigators with the TEAM (Transgenerational Effect on Adult Morbidity) study have recruited over 170 offspring of mothers who participated in the Diabetes in Pregnancy Program Project Grant (PPG) at the University of Cincinnati Medical Center from 1978 to 1995 — a landmark study that demonstrated the effect of strict glucose control in reducing major congenital malformations.
The women in the PPG study had frequent glucose monitoring (up to 6-8 times a day) throughout their pregnancies, and now, their recruited offspring, who are up to 43 years of age, are being assessed for obesity, diabetes/metabolic health, cardiovascular disease/cardiac and peripheral vascular structure and function, and other outcomes including those that may be amenable to secondary prevention (J Diabetes Res. Nov 1;2021:6590431).
Preliminary findings from over 170 offspring recruited between 2017 and 2022 suggest that in utero exposure to dysglycemia (as measured by standard deviations of glycohemoglobin) in the third trimester appears to increase the risk of morbid obesity in adulthood, while exposure to dysglycemia in the first trimester increases the risk of impaired glucose tolerance. The risk of B-cell dysfunction, meanwhile, appears to be linked to dysglycemia in the first and third trimesters — particularly the first — Dr. Ehrlich reported.
Cognitive outcomes in offspring have also been assessed and here it appears that dysglycemia in the third trimester is linked to worse scores on the Wechsler Abbreviated Scale of Intelligence (WASI-II), said Katherine Bowers, PhD, MPH, a TEAM study coinvestigator, also of Cincinnati Children’s Hospital Medical Center.
“We’ve already observed [an association between] diabetes in pregnancy and cognition in early childhood and through adolescence, but [the question has been] does this association persist into adulthood?” she said.
Preliminary analyses of 104 offspring show no statistically significant associations between maternal dysglycemia in the first or second trimesters and offspring cognition, but “consistent inverse associations between maternal glycohemoglobin in the third trimester across two [WASI-II] subscales and composite measures of cognition,” Dr. Bowers said.
Their analysis adjusted for a variety of factors, including maternal age, prepregnancy and first trimester BMI, race, family history of diabetes, and diabetes severity/macrovascular complications.
Back In The Laboratory
At the other end of the research spectrum, basic research scientists are also investigating the mechanisms and sequelae of in utero hyperglycemia and other injuries, including congenital malformations, placental adaptive responses and fetal programming. Researchers are asking, for instance, what does placental metabolic reprogramming entail? What role do placental extracellular vesicles play in GDM? Can we alter the in utero environment and thus improve the short and long-term fetal/infant outcomes?
Animal research done at the UMSOM Center for Birth Defects Research, led by Dr. Reece and Peixin Yang, PhD, suggests that “a good portion of in utero injury is due to epigenetics,” Dr. Reece said in the interview. “We’ve shown that under conditions of hyperglycemia, for example, genetic regulation and genetic function can be altered.”
Through in vivo research, they have also shown that antioxidants or membrane stabilizers such as arachidonic acid or myo-inositol, or experimental inhibitors to certain pro-apoptotic intermediates, can individually or collectively result in reduced malformations. “It is highly likely that understanding the biological impact of various altered in utero environments, and then modifying or reversing those environments, will result in short and long-term outcome improvements similar to those shown with congenital malformations,” Dr. Reece said.
FAIRFAX, VIRGINIA — Nearly 30 years ago, in a 1995 paper, the British physician-epidemiologist David Barker, MD, PhD, wrote about his fetal origins hypothesis — the idea that programs to address fetal undernutrition and low birth weight produced later coronary heart disease (BMJ 1995;311:171-4).
His hypothesis and subsequent research led to the concept of adult diseases of fetal origins, which today extends beyond low birth weight and implicates the in utero environment as a significant determinant of risk for adverse childhood and adult metabolic outcomes and for major chronic diseases, including diabetes and obesity. Studies have shown that the offspring of pregnant mothers with diabetes have a higher risk of developing obesity and diabetes themselves.
“It’s a whole discipline [of research],” E. Albert Reece, MD, PhD, MBA, of the University of Maryland School of Medicine (UMSOM), said in an interview. “But what we’ve never quite understood is the ‘how’ and ‘why’? What are the mechanisms driving the fetal origins of such adverse outcomes in offspring?
At the biennial meeting of the Diabetes in Pregnancy Study Group of North America (DPSG), investigators described studies underway that are digging deeper into the associations between the intrauterine milieu and longer-term offspring health — and that are searching for biological and molecular processes that may be involved.
The studies are like “branches of the Barker hypothesis,” said Dr. Reece, former dean of UMSOM and current director of the UMSOM Center for Advanced Research Training and Innovation, who co-organized the DPSG meeting. “They’re taking the hypothesis and dissecting it by asking, for instance, it is possible that transgenerational obesity may align with the Barker hypothesis? Is it possible that it involves epigenetics regulation? Could we find biomarkers?”
The need for a better understanding of the fetal origins framework — and its subsequent transgenerational impact — is urgent. From 2000 to 2018, the prevalence of childhood obesity increased from 14.7% to 19.2% (a 31% increase) and the prevalence of severe childhood obesity rose from 3.9% to 6.1% (a 56% increase), according to data from the U.S. National Health and Nutrition Examination Survey (Obes Facts. 2022;15[4]:560-9).
Children aged 2-5 years have had an especially sharp increase in obesity (Pediatrics 2018;141[3]:e20173459), Christine Wey Hockett, PhD, of the University of South Dakota School of Medicine, said at the DPSG meeting (Figure 1).

Also notable, she said, is that one-quarter of today’s pediatric diabetes cases are type 2 diabetes, which “is significant as there is a higher prevalence of early complications and comorbidities in youth with type 2 diabetes compared to type 1 diabetes.”
Moreover, recent projections estimate that 57% of today’s children will be obese at 35 years of age (N Engl J Med. 2017;377[22]:2145-53) and that 45% will have diabetes or prediabetes by 2030 (Popul Health Manag. 2017;20[1]:6-12), said Dr. Hockett, assistant professor in the university’s department of pediatrics. An investigator of the Exploring Perinatal Outcomes Among Children (EPOCH) study, which looked at gestational diabetes (GDM) and offspring cardiometabolic risks, she said more chronic disease “at increasingly younger ages [points toward] prebirth influences.”
She noted that there are critical periods postnatally — such as infancy and puberty — that can “impact or further shift the trajectory of chronic disease.” The developmental origins theory posits that life events and biological and environmental processes during the lifespan can modify the effects of intrauterine exposures.
The transgenerational implications “are clear,” she said. “As the number of reproductive-aged individuals with chronic diseases rises, the number of exposed offspring also rises ... It leads to a vicious cycle.”
Deeper Dives Into Associations, Potential Mechanisms
The EPOCH prospective cohort study with which Dr. Hockett was involved gave her a front-seat view of the transgenerational adverse effects of in utero exposure to hyperglycemia. The study recruited ethnically diverse maternal/child dyads from the Kaiser Permanente of Colorado perinatal database from 1992 to 2002 and assessed 418 offspring at two points — a mean age of 10.5 years and 16.5 years — for fasting blood glucose, adiposity, and diet and physical activity. The second visit also involved an oral glucose tolerance test.
The 77 offspring who had been exposed in utero to GDM had a homeostatic model assessment of insulin resistance (HOMA-IR) that was 18% higher, a 19% lower Matsuda index, and a 9% greater HOMA of β-cell function (HOMA-β) than the 341 offspring whose mothers did not have diabetes. Each 5-kg/m2 increase in prepregnancy body mass index predicted increased insulin resistance, but there was no combined effect of both maternal obesity and diabetes in utero.
Exposed offspring had a higher BMI and increased adiposity, but when BMI was controlled for in the analysis of metabolic outcomes, maternal diabetes was still associated with 12% higher HOMA-IR and a 17% lower Matsuda index. “So [the metabolic outcomes] are a direct effect of maternal diabetes,” Dr. Hockett said at the DPSG meeting, noting the fetal overnutrition hypothesis in which maternal glucose, but not maternal insulin, freely passes through the placenta, promoting growth and adiposity in the fetus.
[The EPOCH results on metabolic outcomes and offspring adiposity were published in 2017 and 2019, respectively (Diabet Med. 2017;34:1392-9; Diabetologia. 2019;62:2017-24). In 2020, EPOCH researchers reported sex-specific effects on cardiovascular outcomes, with GDM exposure associated with higher total and LDL cholesterol in girls and higher systolic blood pressure in boys (Pediatr Obes. 2020;15[5]:e12611).]
Now, a new longitudinal cohort study underway in Phoenix, is taking a deeper dive, trying to pinpoint what exactly influences childhood obesity and metabolic risk by following Hispanic and American Indian maternal/child dyads from pregnancy until 18 years postpartum. Researchers are looking not only at associations between maternal risk factors (pregnancy BMI, gestational weight gain, and diabetes in pregnancy) and offspring BMI, adiposity, and growth patterns, but also how various factors during pregnancy — clinical, genetic, lifestyle, biochemical — ”may mediate the associations,” said lead investigator Madhumita Sinha, MD.
“We need a better understanding at the molecular level of the biological processes that lead to obesity in children and that cause metabolic dysfunction,” said Dr. Sinha, who heads the Diabetes Epidemiology and Clinical Research Section of the of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) branch in Phoenix.
The populations being enrolled in the ETCHED study (for Early Tracking of Childhood Health Determinants) are at especially high risk of childhood obesity and metabolic dysfunction. Research conducted decades ago by the NIDDK in Phoenix showed that approximately 50% of Pima Indian children from diabetic pregnancies develop type 2 diabetes by age 25 (N Engl J Med. 1983;308:242-5). Years later, to tease out possible genetic factors, researchers compared siblings born before and after their mother was found to have type 2 diabetes, and found significantly higher rates of diabetes in those born after the mother’s diagnosis, affirming the role of in utero toxicity (Diabetes 2000;49:2208-11).
In the new study, the researchers will look at adipokines and inflammatory biomarkers in the mothers and offspring in addition to traditional anthropometric and glycemic measures. They’ll analyze placental tissue, breast milk, and the gut microbiome longitudinally, and they’ll lean heavily on genomics/epigenomics, proteomics, and metabolomics. “There’s potential,” Dr. Sinha said, “to develop a more accurate predictive and prognostic model of childhood obesity.”
The researchers also will study the role of family, socioeconomics, and environmental factors in influencing child growth patterns and they’ll look at neurodevelopment in infancy and childhood. As of October 2023, almost 80 pregnant women, most with obesity and almost one-third with type 2 diabetes, had enrolled in the study. Over the next several years, the study aims to enroll 750 dyads.
The Timing of In Utero Exposure
Shelley Ehrlich, MD, ScD, MPH, of the University of Cincinnati and Cincinnati Children’s Hospital Medical Center, is aiming, meanwhile, to learn how the timing of in utero exposure to hyperglycemia predicts specific metabolic and cardiovascular morbidities in the adult offspring of diabetic mothers.
“While we know that exposure to maternal diabetes, regardless of type, increases the risk of obesity, insulin resistance, diabetes, renal compromise, and cardiovascular disease in the offspring, there is little known about the level and timing of hyperglycemic exposure during fetal development that triggers these adverse outcomes,” said Dr. Ehrlich. A goal, she said, is to identify gestational profiles that predict phenotypes of offspring at risk for morbidity in later life.
She and other investigators with the TEAM (Transgenerational Effect on Adult Morbidity) study have recruited over 170 offspring of mothers who participated in the Diabetes in Pregnancy Program Project Grant (PPG) at the University of Cincinnati Medical Center from 1978 to 1995 — a landmark study that demonstrated the effect of strict glucose control in reducing major congenital malformations.
The women in the PPG study had frequent glucose monitoring (up to 6-8 times a day) throughout their pregnancies, and now, their recruited offspring, who are up to 43 years of age, are being assessed for obesity, diabetes/metabolic health, cardiovascular disease/cardiac and peripheral vascular structure and function, and other outcomes including those that may be amenable to secondary prevention (J Diabetes Res. Nov 1;2021:6590431).
Preliminary findings from over 170 offspring recruited between 2017 and 2022 suggest that in utero exposure to dysglycemia (as measured by standard deviations of glycohemoglobin) in the third trimester appears to increase the risk of morbid obesity in adulthood, while exposure to dysglycemia in the first trimester increases the risk of impaired glucose tolerance. The risk of B-cell dysfunction, meanwhile, appears to be linked to dysglycemia in the first and third trimesters — particularly the first — Dr. Ehrlich reported.
Cognitive outcomes in offspring have also been assessed and here it appears that dysglycemia in the third trimester is linked to worse scores on the Wechsler Abbreviated Scale of Intelligence (WASI-II), said Katherine Bowers, PhD, MPH, a TEAM study coinvestigator, also of Cincinnati Children’s Hospital Medical Center.
“We’ve already observed [an association between] diabetes in pregnancy and cognition in early childhood and through adolescence, but [the question has been] does this association persist into adulthood?” she said.
Preliminary analyses of 104 offspring show no statistically significant associations between maternal dysglycemia in the first or second trimesters and offspring cognition, but “consistent inverse associations between maternal glycohemoglobin in the third trimester across two [WASI-II] subscales and composite measures of cognition,” Dr. Bowers said.
Their analysis adjusted for a variety of factors, including maternal age, prepregnancy and first trimester BMI, race, family history of diabetes, and diabetes severity/macrovascular complications.
Back In The Laboratory
At the other end of the research spectrum, basic research scientists are also investigating the mechanisms and sequelae of in utero hyperglycemia and other injuries, including congenital malformations, placental adaptive responses and fetal programming. Researchers are asking, for instance, what does placental metabolic reprogramming entail? What role do placental extracellular vesicles play in GDM? Can we alter the in utero environment and thus improve the short and long-term fetal/infant outcomes?
Animal research done at the UMSOM Center for Birth Defects Research, led by Dr. Reece and Peixin Yang, PhD, suggests that “a good portion of in utero injury is due to epigenetics,” Dr. Reece said in the interview. “We’ve shown that under conditions of hyperglycemia, for example, genetic regulation and genetic function can be altered.”
Through in vivo research, they have also shown that antioxidants or membrane stabilizers such as arachidonic acid or myo-inositol, or experimental inhibitors to certain pro-apoptotic intermediates, can individually or collectively result in reduced malformations. “It is highly likely that understanding the biological impact of various altered in utero environments, and then modifying or reversing those environments, will result in short and long-term outcome improvements similar to those shown with congenital malformations,” Dr. Reece said.
FROM DPSG-NA 2023








