User login
High school students using less tobacco, vape products, CDC report shows
TOPLINE:
which have been shown to both entice teens and keep them vaping.
METHODOLOGY:
- The MMRW report from the U.S. Centers for Disease Control and Prevention presents data from an annual survey of U.S. middle and high school students of their use of tobacco products, including vapes.
- The survey is a cross-sectional, school-based, self-administered web-based questionnaire that uses a stratified, three-stage cluster sampling procedure to generate a nationally representative sample based off the responses of 22,069 students in 2023.
- The overall response rate was 30.5%.
- “Ever use” was defined as using a product once or twice previously, and “current use” was defined as use in the past 30 days.
- The survey queried students on their use of e-cigarettes, traditional cigarettes, cigars, smokeless tobacco, nicotine pouches, hookahs, pipe tobacco, and other oral nicotine products.
TAKEAWAY:
- The use of tobacco products by high school students decreased by 540,000 people from 2022 to 2023 (2.51 million vs. 1.97 million students).
- From 2022 to 2023, current e-cigarette use among high school students declined from 14.1% to 10.0%.
- Among middle and high school students, e-cigarettes were the most used nicotine product in 2023 (7.7%; 2.13 million), followed by cigarettes (1.6%), cigars (1.6%), nicotine pouches (1.5%), smokeless tobacco (1.2%), other oral nicotine products (1.2%), hookahs (1.1%), heated tobacco products (1.0%), and pipe tobacco (0.5%).
- Among students reporting current e-cigarette use, 89.4% said that they used flavored products, and 25.2% said they used an e-cigarette daily. The most commonly reported brands were Elf Bar, Esco Bar, Vuse, JUUL, and Mr. Fog. Fruit (63.4%) and candy (35%) were the most commonly reported flavors.
IN PRACTICE:
“Sustained efforts to prevent initiation of tobacco product use among young persons and strategies to help young tobacco users quit are critical to reducing U.S. youth tobacco product use,” the report states.
SOURCE:
The report was produced by the CDC and published in the Morbidity and Mortality Weekly Report for Nov. 3, 2023.
LIMITATIONS:
Data were obtained by students self-reporting their tobacco use, which can result in social desirability and recall biases, the report states. In addition, the responses were from students enrolled in school settings and may not be representative of teens who are in detention centers, alternative schools, have dropped out of school or are homeschooled. The response rate for the 2023 survey was also lower than in the previous year (30.5% in 2023 vs. 45.2% in 2022), increasing the potential for higher standard errors and reducing the power to detect significant differences.
DISCLOSURES:
No potential conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
TOPLINE:
which have been shown to both entice teens and keep them vaping.
METHODOLOGY:
- The MMRW report from the U.S. Centers for Disease Control and Prevention presents data from an annual survey of U.S. middle and high school students of their use of tobacco products, including vapes.
- The survey is a cross-sectional, school-based, self-administered web-based questionnaire that uses a stratified, three-stage cluster sampling procedure to generate a nationally representative sample based off the responses of 22,069 students in 2023.
- The overall response rate was 30.5%.
- “Ever use” was defined as using a product once or twice previously, and “current use” was defined as use in the past 30 days.
- The survey queried students on their use of e-cigarettes, traditional cigarettes, cigars, smokeless tobacco, nicotine pouches, hookahs, pipe tobacco, and other oral nicotine products.
TAKEAWAY:
- The use of tobacco products by high school students decreased by 540,000 people from 2022 to 2023 (2.51 million vs. 1.97 million students).
- From 2022 to 2023, current e-cigarette use among high school students declined from 14.1% to 10.0%.
- Among middle and high school students, e-cigarettes were the most used nicotine product in 2023 (7.7%; 2.13 million), followed by cigarettes (1.6%), cigars (1.6%), nicotine pouches (1.5%), smokeless tobacco (1.2%), other oral nicotine products (1.2%), hookahs (1.1%), heated tobacco products (1.0%), and pipe tobacco (0.5%).
- Among students reporting current e-cigarette use, 89.4% said that they used flavored products, and 25.2% said they used an e-cigarette daily. The most commonly reported brands were Elf Bar, Esco Bar, Vuse, JUUL, and Mr. Fog. Fruit (63.4%) and candy (35%) were the most commonly reported flavors.
IN PRACTICE:
“Sustained efforts to prevent initiation of tobacco product use among young persons and strategies to help young tobacco users quit are critical to reducing U.S. youth tobacco product use,” the report states.
SOURCE:
The report was produced by the CDC and published in the Morbidity and Mortality Weekly Report for Nov. 3, 2023.
LIMITATIONS:
Data were obtained by students self-reporting their tobacco use, which can result in social desirability and recall biases, the report states. In addition, the responses were from students enrolled in school settings and may not be representative of teens who are in detention centers, alternative schools, have dropped out of school or are homeschooled. The response rate for the 2023 survey was also lower than in the previous year (30.5% in 2023 vs. 45.2% in 2022), increasing the potential for higher standard errors and reducing the power to detect significant differences.
DISCLOSURES:
No potential conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
TOPLINE:
which have been shown to both entice teens and keep them vaping.
METHODOLOGY:
- The MMRW report from the U.S. Centers for Disease Control and Prevention presents data from an annual survey of U.S. middle and high school students of their use of tobacco products, including vapes.
- The survey is a cross-sectional, school-based, self-administered web-based questionnaire that uses a stratified, three-stage cluster sampling procedure to generate a nationally representative sample based off the responses of 22,069 students in 2023.
- The overall response rate was 30.5%.
- “Ever use” was defined as using a product once or twice previously, and “current use” was defined as use in the past 30 days.
- The survey queried students on their use of e-cigarettes, traditional cigarettes, cigars, smokeless tobacco, nicotine pouches, hookahs, pipe tobacco, and other oral nicotine products.
TAKEAWAY:
- The use of tobacco products by high school students decreased by 540,000 people from 2022 to 2023 (2.51 million vs. 1.97 million students).
- From 2022 to 2023, current e-cigarette use among high school students declined from 14.1% to 10.0%.
- Among middle and high school students, e-cigarettes were the most used nicotine product in 2023 (7.7%; 2.13 million), followed by cigarettes (1.6%), cigars (1.6%), nicotine pouches (1.5%), smokeless tobacco (1.2%), other oral nicotine products (1.2%), hookahs (1.1%), heated tobacco products (1.0%), and pipe tobacco (0.5%).
- Among students reporting current e-cigarette use, 89.4% said that they used flavored products, and 25.2% said they used an e-cigarette daily. The most commonly reported brands were Elf Bar, Esco Bar, Vuse, JUUL, and Mr. Fog. Fruit (63.4%) and candy (35%) were the most commonly reported flavors.
IN PRACTICE:
“Sustained efforts to prevent initiation of tobacco product use among young persons and strategies to help young tobacco users quit are critical to reducing U.S. youth tobacco product use,” the report states.
SOURCE:
The report was produced by the CDC and published in the Morbidity and Mortality Weekly Report for Nov. 3, 2023.
LIMITATIONS:
Data were obtained by students self-reporting their tobacco use, which can result in social desirability and recall biases, the report states. In addition, the responses were from students enrolled in school settings and may not be representative of teens who are in detention centers, alternative schools, have dropped out of school or are homeschooled. The response rate for the 2023 survey was also lower than in the previous year (30.5% in 2023 vs. 45.2% in 2022), increasing the potential for higher standard errors and reducing the power to detect significant differences.
DISCLOSURES:
No potential conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
FDA to health care providers: Double-check COVID vaccine dose for children
the Food and Drug Administration said in a MedWatch issued Nov. 1, 2023.
That dose is 0.25 mL for children 6 months through 11 years. In the MedWatch, the FDA said that it “has become aware” that the single-dose vial for use in this age group “contains notably more than 0.25 mL of the vaccine.” It added: “Some healthcare providers may be withdrawing the entire contents of the vial to administer to an individual.”
The FDA revised the Fact Sheet for Healthcare Providers Administering Vaccine to clarify that the 0.25 mL should be withdrawn from the vial and that the vial and any excess then should be discarded. It is in a single-dose vial with a blue cap and a green label.
“It is common [for vaccine makers] to put in a little bit of extra vaccine just to make sure everyone gets enough,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University Medical Center, Nashville, Tenn. “The provider is supposed to be looking at the syringe when they withdraw it to make sure they get the right amount,” Dr. Schaffner said.
Recently, parents on social media had expressed concerns that their children may have gotten more than the recommended dose, with some parents noticing more reactions such as soreness and fever with the 2023-2024 vaccine dose than they did with their children’s previous COVID vaccinations.
“Since the beginning of the rollout, parents were telling us of cases where pharmacies accidentally gave their children a double dose, while doctors in our group were pointing out that their vials for children contained twice the amount than what was needed,” said Fatima Khan, a parent and cofounder of the group Protect Their Future, an organization that advocates for pediatric vaccine access. Members contacted the FDA and other officials. “We appreciate that the FDA took our concerns seriously and issued this safety update,” Ms. Khan said.
A spokesperson for Moderna is researching how much more vaccine the single-dose vials might contain.
No safety risks identified
“The FDA has not identified any safety risks associated with administration of the higher dose in individuals 6 months through 11 years of age and no serious adverse events were identified related to a dosing error for the vaccine,” Cherie Duvall-Jones, an FDA spokesperson, said in an email response.
“The FDA received questions from stakeholders about the dosing issue on Oct. 29, and contacted Moderna to discuss and better understand the issue,” Ms. Duvall-Jones said. The agency then alerted health care providers via the safety communication and other means to be sure the correct dosage is given to the children aged 12 years or younger.
One parent’s experience
Jane Jih, MD, an internist in San Francisco, took her 7-year-old daughter to a pharmacy to get the vaccine, and it was the first time the pharmacist had given a pediatric dose. “We both had to double check the dose,” Dr. Jih said. She observed that the vial had about 0.40 mL, which is 0.15 mL above the recommended dose.
A few weeks later, Dr. Jih could access the vaccine for her nearly-3-year-old son. The nurse practitioner who administered it had been giving many pediatric Moderna shots, she said, “so I felt more confident in the second scenario.”
Perhaps more reactions, no danger
“If you get a little bit more [than the recommended 0.25 mL], that certainly is not going to harm the child,” Dr. Schaffner said. “There may be a little bit more local reaction. In terms of the child’s immune system, there really isn’t any harm.”
If an entire adult dose is mistakenly given, he said, “I think the reaction locally in some children may be more evident, they may get more sore arms, redness, maybe a little bit more swelling and tenderness. Fever is also a possibility, but “these vaccines have not been associated with too much fever.”
Could a double dose do more harm than that? “It is unknown,” said Aaron Glatt, MD, chief of infectious diseases and hospital epidemiologist for Mount Sinai South Nassau, Oceanside, N.Y. “But there is the theoretical potential for some more complications. I do not know whether this [excess vaccine] would cause an increased likelihood of cardiac inflammatory problems like myocarditis or other rare complications to occur more frequently.”
The message for health care providers giving the vaccine, Dr. Schaffner said, is: “Look at your syringe to make sure the dose is appropriate.”
A version of this article appeared on Medscape.com.
the Food and Drug Administration said in a MedWatch issued Nov. 1, 2023.
That dose is 0.25 mL for children 6 months through 11 years. In the MedWatch, the FDA said that it “has become aware” that the single-dose vial for use in this age group “contains notably more than 0.25 mL of the vaccine.” It added: “Some healthcare providers may be withdrawing the entire contents of the vial to administer to an individual.”
The FDA revised the Fact Sheet for Healthcare Providers Administering Vaccine to clarify that the 0.25 mL should be withdrawn from the vial and that the vial and any excess then should be discarded. It is in a single-dose vial with a blue cap and a green label.
“It is common [for vaccine makers] to put in a little bit of extra vaccine just to make sure everyone gets enough,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University Medical Center, Nashville, Tenn. “The provider is supposed to be looking at the syringe when they withdraw it to make sure they get the right amount,” Dr. Schaffner said.
Recently, parents on social media had expressed concerns that their children may have gotten more than the recommended dose, with some parents noticing more reactions such as soreness and fever with the 2023-2024 vaccine dose than they did with their children’s previous COVID vaccinations.
“Since the beginning of the rollout, parents were telling us of cases where pharmacies accidentally gave their children a double dose, while doctors in our group were pointing out that their vials for children contained twice the amount than what was needed,” said Fatima Khan, a parent and cofounder of the group Protect Their Future, an organization that advocates for pediatric vaccine access. Members contacted the FDA and other officials. “We appreciate that the FDA took our concerns seriously and issued this safety update,” Ms. Khan said.
A spokesperson for Moderna is researching how much more vaccine the single-dose vials might contain.
No safety risks identified
“The FDA has not identified any safety risks associated with administration of the higher dose in individuals 6 months through 11 years of age and no serious adverse events were identified related to a dosing error for the vaccine,” Cherie Duvall-Jones, an FDA spokesperson, said in an email response.
“The FDA received questions from stakeholders about the dosing issue on Oct. 29, and contacted Moderna to discuss and better understand the issue,” Ms. Duvall-Jones said. The agency then alerted health care providers via the safety communication and other means to be sure the correct dosage is given to the children aged 12 years or younger.
One parent’s experience
Jane Jih, MD, an internist in San Francisco, took her 7-year-old daughter to a pharmacy to get the vaccine, and it was the first time the pharmacist had given a pediatric dose. “We both had to double check the dose,” Dr. Jih said. She observed that the vial had about 0.40 mL, which is 0.15 mL above the recommended dose.
A few weeks later, Dr. Jih could access the vaccine for her nearly-3-year-old son. The nurse practitioner who administered it had been giving many pediatric Moderna shots, she said, “so I felt more confident in the second scenario.”
Perhaps more reactions, no danger
“If you get a little bit more [than the recommended 0.25 mL], that certainly is not going to harm the child,” Dr. Schaffner said. “There may be a little bit more local reaction. In terms of the child’s immune system, there really isn’t any harm.”
If an entire adult dose is mistakenly given, he said, “I think the reaction locally in some children may be more evident, they may get more sore arms, redness, maybe a little bit more swelling and tenderness. Fever is also a possibility, but “these vaccines have not been associated with too much fever.”
Could a double dose do more harm than that? “It is unknown,” said Aaron Glatt, MD, chief of infectious diseases and hospital epidemiologist for Mount Sinai South Nassau, Oceanside, N.Y. “But there is the theoretical potential for some more complications. I do not know whether this [excess vaccine] would cause an increased likelihood of cardiac inflammatory problems like myocarditis or other rare complications to occur more frequently.”
The message for health care providers giving the vaccine, Dr. Schaffner said, is: “Look at your syringe to make sure the dose is appropriate.”
A version of this article appeared on Medscape.com.
the Food and Drug Administration said in a MedWatch issued Nov. 1, 2023.
That dose is 0.25 mL for children 6 months through 11 years. In the MedWatch, the FDA said that it “has become aware” that the single-dose vial for use in this age group “contains notably more than 0.25 mL of the vaccine.” It added: “Some healthcare providers may be withdrawing the entire contents of the vial to administer to an individual.”
The FDA revised the Fact Sheet for Healthcare Providers Administering Vaccine to clarify that the 0.25 mL should be withdrawn from the vial and that the vial and any excess then should be discarded. It is in a single-dose vial with a blue cap and a green label.
“It is common [for vaccine makers] to put in a little bit of extra vaccine just to make sure everyone gets enough,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University Medical Center, Nashville, Tenn. “The provider is supposed to be looking at the syringe when they withdraw it to make sure they get the right amount,” Dr. Schaffner said.
Recently, parents on social media had expressed concerns that their children may have gotten more than the recommended dose, with some parents noticing more reactions such as soreness and fever with the 2023-2024 vaccine dose than they did with their children’s previous COVID vaccinations.
“Since the beginning of the rollout, parents were telling us of cases where pharmacies accidentally gave their children a double dose, while doctors in our group were pointing out that their vials for children contained twice the amount than what was needed,” said Fatima Khan, a parent and cofounder of the group Protect Their Future, an organization that advocates for pediatric vaccine access. Members contacted the FDA and other officials. “We appreciate that the FDA took our concerns seriously and issued this safety update,” Ms. Khan said.
A spokesperson for Moderna is researching how much more vaccine the single-dose vials might contain.
No safety risks identified
“The FDA has not identified any safety risks associated with administration of the higher dose in individuals 6 months through 11 years of age and no serious adverse events were identified related to a dosing error for the vaccine,” Cherie Duvall-Jones, an FDA spokesperson, said in an email response.
“The FDA received questions from stakeholders about the dosing issue on Oct. 29, and contacted Moderna to discuss and better understand the issue,” Ms. Duvall-Jones said. The agency then alerted health care providers via the safety communication and other means to be sure the correct dosage is given to the children aged 12 years or younger.
One parent’s experience
Jane Jih, MD, an internist in San Francisco, took her 7-year-old daughter to a pharmacy to get the vaccine, and it was the first time the pharmacist had given a pediatric dose. “We both had to double check the dose,” Dr. Jih said. She observed that the vial had about 0.40 mL, which is 0.15 mL above the recommended dose.
A few weeks later, Dr. Jih could access the vaccine for her nearly-3-year-old son. The nurse practitioner who administered it had been giving many pediatric Moderna shots, she said, “so I felt more confident in the second scenario.”
Perhaps more reactions, no danger
“If you get a little bit more [than the recommended 0.25 mL], that certainly is not going to harm the child,” Dr. Schaffner said. “There may be a little bit more local reaction. In terms of the child’s immune system, there really isn’t any harm.”
If an entire adult dose is mistakenly given, he said, “I think the reaction locally in some children may be more evident, they may get more sore arms, redness, maybe a little bit more swelling and tenderness. Fever is also a possibility, but “these vaccines have not been associated with too much fever.”
Could a double dose do more harm than that? “It is unknown,” said Aaron Glatt, MD, chief of infectious diseases and hospital epidemiologist for Mount Sinai South Nassau, Oceanside, N.Y. “But there is the theoretical potential for some more complications. I do not know whether this [excess vaccine] would cause an increased likelihood of cardiac inflammatory problems like myocarditis or other rare complications to occur more frequently.”
The message for health care providers giving the vaccine, Dr. Schaffner said, is: “Look at your syringe to make sure the dose is appropriate.”
A version of this article appeared on Medscape.com.
In myasthenia gravis, antibodies pass open-label tests
PHOENIX – . The two drugs, rozanolixizumab (Rystiggo, UCB) and efgartigimod PH20 (Vyvgart, Argenx SE), received Food and Drug Administration approval in June 2023 and December 2021, respectively, for the treatment of MG.
The neonatal Fc receptor binds to IgG within cells and recycles it back into the blood, leading to increased serum levels. The antibodies bind to the neonatal Fc receptor and promote its degradation, therefore preventing IgG recycling without interfering with its production. They do not affect the levels of other immunoglobulin isotypes.
At the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM), researchers presented data from an open-label extension study following the phase 3 MycarinG trial of rozanolixizumab and the ADAPT-SC+ study of efgartigimod.
In the MycarinG study, rozanolixizumab “showed both statistically significant and clinically meaningful improvements in multiple endpoints,” said Vera Bril, MD, during a presentation of the results.
Rozanolixizumab is approved for MG patients who are anti–acetylcholine receptor (AchR) or anti–muscle-specific tyrosine kinase (MuSK) antibody positive. Efgartigimod is approved for MG patients who are AChR positive.
After completing MycarinG, patients were eligible to enroll in one of two open-label studies, one of each dose.
The new efficacy analysis focused on 110 patients who underwent two or more consecutive symptom-driven treatment cycles. A safety analysis focused on 188 patients who received at least one cycle of treatment.
“The post hoc analysis showed that the clinically meaningful improvements in the generalized myasthenia gravis symptoms were maintained over time for the cohort across rozanolixizumab cycles, while individual patients move through the consecutive treatment cycles, rozanolixizumab had an acceptable safety profile that was maintained across repeated treatments cycles. This is consistent with previous results of rozanolixizumab,” said Dr. Bril, who is a clinical investigator at University of Toronto
A reduction in steroid use?
During the Q&A session, George Small, MD, asked if the study had shown a reduction in steroid use among patients with MG.
As clinical associate director of neurology at Allegheny Medical Center, Pittsburgh, he has overseen the care of several hundred patients with generalized MG, as well as participated in clinical trials. “Many physicians use steroids for very quick responses in their patients. I love steroids and I hate steroids. I’ve helped save people’s lives with them, but I’ve also probably hastened their demise, unfortunately, because of the long-term side effects of the medications. Many neurologists in the community will over-utilize steroids because they don’t have access to these more expensive therapies. I look forward to both using these medications more as they become FDA approved and being an advocate for them, because it is my belief that they help decrease the use of steroids,” said Dr. Small in an interview.
Even if new medications do reduce steroid use, there remains a hurdle with insurance companies. “I’ve felt I’ve been forced to use treatments that I know may not be efficacious in the short term in order to get authorization for more expensive therapies that I use now. I’ve had patients admitted to the hospital as I’ve tried to jump through hoops that the insurance companies demanded,” said Dr. Small.
Clinical improvements seen
In the MycarinG study, patients received weekly injections of 7 mg/kg, 10 mg/kg rozanolixizumab, or placebo over a 6-week period. Both treatment groups had reductions in Myasthenia Gravis Activities of Daily Living (MG-ADL) scores compared with placebo.
MG-ADL and Quantitative Myasthenia Gravis (QMG) scores were consistent across treatment cycles. The mean MG-ADL score dropped by about 4 points at week 6. At around week 10, the mean improvement declined to 2 points, and then by week 14 it increased to 3 points, where it remained consistent out to 50 weeks. A similar pattern was seen in QMG scores, with an approximate 5-point drop at 6 weeks, then about a 1.5-point improvement at 10 weeks, then a mean decrease of around 4 points that stayed stable out to 50 weeks.
Over all cycles of treatment, 89.9% of patients experienced a treatment-emergent adverse event, including 22.3% who had a serious TEAE; the study dropout rate because of TEAEs was 15.4%.
In the ADAPT-SC study, 110 patients were randomized to 10 mg/kg intravenous efgartigimod or 1,000 mg efgartigimod PH20, which is formulated with recombinant human hyaluronidase to allow for rapid administration of larger volumes. After completion of ADAPT-SC, 105 patients from both groups and an additional 79 patients entered the open-label extension study with 1,000 mg efgartigimod PH20.
The study included 141 patients who were AChR-Ab positive and 38 who were AChR-Ab negative. In the first cycle, 34.6% experienced an injection-site reaction, which steadily dropped to 11.5% by the sixth cycle. After each cycle of treatment, the mean change in MG-ADL from study baseline at week 4 was between 4.1 and 4.7 points. The mean change in Myasthenia Gravis Quality of Life 15-Item Questionnaire revisited from study baseline at week 4 was between 5.1 and 6.5 points. The mean change in EuroQoL 5-dimension, 5-level visual analog scale was between 12.3 and 16.0 points at week 4.
MyCarinG was funded by UCB Pharma. ADAPT-SC was funded by Argenx SE. Dr. Bril has financial relationships with Behring, Argenyx, Alexion, Immunovant, Alnylam, Akcea, Takeda, Sanofi, Ionis, Roche, Novo Nordisk, Octapharma, Momenta, Pfizer, CSL Behring, Grifols, Powell Mansfield, UCB, and Viela Bio. Dr. Small is on the speaker’s bureau for Alexion.
PHOENIX – . The two drugs, rozanolixizumab (Rystiggo, UCB) and efgartigimod PH20 (Vyvgart, Argenx SE), received Food and Drug Administration approval in June 2023 and December 2021, respectively, for the treatment of MG.
The neonatal Fc receptor binds to IgG within cells and recycles it back into the blood, leading to increased serum levels. The antibodies bind to the neonatal Fc receptor and promote its degradation, therefore preventing IgG recycling without interfering with its production. They do not affect the levels of other immunoglobulin isotypes.
At the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM), researchers presented data from an open-label extension study following the phase 3 MycarinG trial of rozanolixizumab and the ADAPT-SC+ study of efgartigimod.
In the MycarinG study, rozanolixizumab “showed both statistically significant and clinically meaningful improvements in multiple endpoints,” said Vera Bril, MD, during a presentation of the results.
Rozanolixizumab is approved for MG patients who are anti–acetylcholine receptor (AchR) or anti–muscle-specific tyrosine kinase (MuSK) antibody positive. Efgartigimod is approved for MG patients who are AChR positive.
After completing MycarinG, patients were eligible to enroll in one of two open-label studies, one of each dose.
The new efficacy analysis focused on 110 patients who underwent two or more consecutive symptom-driven treatment cycles. A safety analysis focused on 188 patients who received at least one cycle of treatment.
“The post hoc analysis showed that the clinically meaningful improvements in the generalized myasthenia gravis symptoms were maintained over time for the cohort across rozanolixizumab cycles, while individual patients move through the consecutive treatment cycles, rozanolixizumab had an acceptable safety profile that was maintained across repeated treatments cycles. This is consistent with previous results of rozanolixizumab,” said Dr. Bril, who is a clinical investigator at University of Toronto
A reduction in steroid use?
During the Q&A session, George Small, MD, asked if the study had shown a reduction in steroid use among patients with MG.
As clinical associate director of neurology at Allegheny Medical Center, Pittsburgh, he has overseen the care of several hundred patients with generalized MG, as well as participated in clinical trials. “Many physicians use steroids for very quick responses in their patients. I love steroids and I hate steroids. I’ve helped save people’s lives with them, but I’ve also probably hastened their demise, unfortunately, because of the long-term side effects of the medications. Many neurologists in the community will over-utilize steroids because they don’t have access to these more expensive therapies. I look forward to both using these medications more as they become FDA approved and being an advocate for them, because it is my belief that they help decrease the use of steroids,” said Dr. Small in an interview.
Even if new medications do reduce steroid use, there remains a hurdle with insurance companies. “I’ve felt I’ve been forced to use treatments that I know may not be efficacious in the short term in order to get authorization for more expensive therapies that I use now. I’ve had patients admitted to the hospital as I’ve tried to jump through hoops that the insurance companies demanded,” said Dr. Small.
Clinical improvements seen
In the MycarinG study, patients received weekly injections of 7 mg/kg, 10 mg/kg rozanolixizumab, or placebo over a 6-week period. Both treatment groups had reductions in Myasthenia Gravis Activities of Daily Living (MG-ADL) scores compared with placebo.
MG-ADL and Quantitative Myasthenia Gravis (QMG) scores were consistent across treatment cycles. The mean MG-ADL score dropped by about 4 points at week 6. At around week 10, the mean improvement declined to 2 points, and then by week 14 it increased to 3 points, where it remained consistent out to 50 weeks. A similar pattern was seen in QMG scores, with an approximate 5-point drop at 6 weeks, then about a 1.5-point improvement at 10 weeks, then a mean decrease of around 4 points that stayed stable out to 50 weeks.
Over all cycles of treatment, 89.9% of patients experienced a treatment-emergent adverse event, including 22.3% who had a serious TEAE; the study dropout rate because of TEAEs was 15.4%.
In the ADAPT-SC study, 110 patients were randomized to 10 mg/kg intravenous efgartigimod or 1,000 mg efgartigimod PH20, which is formulated with recombinant human hyaluronidase to allow for rapid administration of larger volumes. After completion of ADAPT-SC, 105 patients from both groups and an additional 79 patients entered the open-label extension study with 1,000 mg efgartigimod PH20.
The study included 141 patients who were AChR-Ab positive and 38 who were AChR-Ab negative. In the first cycle, 34.6% experienced an injection-site reaction, which steadily dropped to 11.5% by the sixth cycle. After each cycle of treatment, the mean change in MG-ADL from study baseline at week 4 was between 4.1 and 4.7 points. The mean change in Myasthenia Gravis Quality of Life 15-Item Questionnaire revisited from study baseline at week 4 was between 5.1 and 6.5 points. The mean change in EuroQoL 5-dimension, 5-level visual analog scale was between 12.3 and 16.0 points at week 4.
MyCarinG was funded by UCB Pharma. ADAPT-SC was funded by Argenx SE. Dr. Bril has financial relationships with Behring, Argenyx, Alexion, Immunovant, Alnylam, Akcea, Takeda, Sanofi, Ionis, Roche, Novo Nordisk, Octapharma, Momenta, Pfizer, CSL Behring, Grifols, Powell Mansfield, UCB, and Viela Bio. Dr. Small is on the speaker’s bureau for Alexion.
PHOENIX – . The two drugs, rozanolixizumab (Rystiggo, UCB) and efgartigimod PH20 (Vyvgart, Argenx SE), received Food and Drug Administration approval in June 2023 and December 2021, respectively, for the treatment of MG.
The neonatal Fc receptor binds to IgG within cells and recycles it back into the blood, leading to increased serum levels. The antibodies bind to the neonatal Fc receptor and promote its degradation, therefore preventing IgG recycling without interfering with its production. They do not affect the levels of other immunoglobulin isotypes.
At the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM), researchers presented data from an open-label extension study following the phase 3 MycarinG trial of rozanolixizumab and the ADAPT-SC+ study of efgartigimod.
In the MycarinG study, rozanolixizumab “showed both statistically significant and clinically meaningful improvements in multiple endpoints,” said Vera Bril, MD, during a presentation of the results.
Rozanolixizumab is approved for MG patients who are anti–acetylcholine receptor (AchR) or anti–muscle-specific tyrosine kinase (MuSK) antibody positive. Efgartigimod is approved for MG patients who are AChR positive.
After completing MycarinG, patients were eligible to enroll in one of two open-label studies, one of each dose.
The new efficacy analysis focused on 110 patients who underwent two or more consecutive symptom-driven treatment cycles. A safety analysis focused on 188 patients who received at least one cycle of treatment.
“The post hoc analysis showed that the clinically meaningful improvements in the generalized myasthenia gravis symptoms were maintained over time for the cohort across rozanolixizumab cycles, while individual patients move through the consecutive treatment cycles, rozanolixizumab had an acceptable safety profile that was maintained across repeated treatments cycles. This is consistent with previous results of rozanolixizumab,” said Dr. Bril, who is a clinical investigator at University of Toronto
A reduction in steroid use?
During the Q&A session, George Small, MD, asked if the study had shown a reduction in steroid use among patients with MG.
As clinical associate director of neurology at Allegheny Medical Center, Pittsburgh, he has overseen the care of several hundred patients with generalized MG, as well as participated in clinical trials. “Many physicians use steroids for very quick responses in their patients. I love steroids and I hate steroids. I’ve helped save people’s lives with them, but I’ve also probably hastened their demise, unfortunately, because of the long-term side effects of the medications. Many neurologists in the community will over-utilize steroids because they don’t have access to these more expensive therapies. I look forward to both using these medications more as they become FDA approved and being an advocate for them, because it is my belief that they help decrease the use of steroids,” said Dr. Small in an interview.
Even if new medications do reduce steroid use, there remains a hurdle with insurance companies. “I’ve felt I’ve been forced to use treatments that I know may not be efficacious in the short term in order to get authorization for more expensive therapies that I use now. I’ve had patients admitted to the hospital as I’ve tried to jump through hoops that the insurance companies demanded,” said Dr. Small.
Clinical improvements seen
In the MycarinG study, patients received weekly injections of 7 mg/kg, 10 mg/kg rozanolixizumab, or placebo over a 6-week period. Both treatment groups had reductions in Myasthenia Gravis Activities of Daily Living (MG-ADL) scores compared with placebo.
MG-ADL and Quantitative Myasthenia Gravis (QMG) scores were consistent across treatment cycles. The mean MG-ADL score dropped by about 4 points at week 6. At around week 10, the mean improvement declined to 2 points, and then by week 14 it increased to 3 points, where it remained consistent out to 50 weeks. A similar pattern was seen in QMG scores, with an approximate 5-point drop at 6 weeks, then about a 1.5-point improvement at 10 weeks, then a mean decrease of around 4 points that stayed stable out to 50 weeks.
Over all cycles of treatment, 89.9% of patients experienced a treatment-emergent adverse event, including 22.3% who had a serious TEAE; the study dropout rate because of TEAEs was 15.4%.
In the ADAPT-SC study, 110 patients were randomized to 10 mg/kg intravenous efgartigimod or 1,000 mg efgartigimod PH20, which is formulated with recombinant human hyaluronidase to allow for rapid administration of larger volumes. After completion of ADAPT-SC, 105 patients from both groups and an additional 79 patients entered the open-label extension study with 1,000 mg efgartigimod PH20.
The study included 141 patients who were AChR-Ab positive and 38 who were AChR-Ab negative. In the first cycle, 34.6% experienced an injection-site reaction, which steadily dropped to 11.5% by the sixth cycle. After each cycle of treatment, the mean change in MG-ADL from study baseline at week 4 was between 4.1 and 4.7 points. The mean change in Myasthenia Gravis Quality of Life 15-Item Questionnaire revisited from study baseline at week 4 was between 5.1 and 6.5 points. The mean change in EuroQoL 5-dimension, 5-level visual analog scale was between 12.3 and 16.0 points at week 4.
MyCarinG was funded by UCB Pharma. ADAPT-SC was funded by Argenx SE. Dr. Bril has financial relationships with Behring, Argenyx, Alexion, Immunovant, Alnylam, Akcea, Takeda, Sanofi, Ionis, Roche, Novo Nordisk, Octapharma, Momenta, Pfizer, CSL Behring, Grifols, Powell Mansfield, UCB, and Viela Bio. Dr. Small is on the speaker’s bureau for Alexion.
AT AANEM 2023
Birch bark–derived treatment reduces daily dressings in patients with epidermolysis bullosa
Additional when compared with a control gel.
In a final, post hoc analysis to come from the trial, 15 of 45 (33%) patients treated with Oleogel-S10 versus 5 of 48 (10.4%) treated with the control gel were reported as no longer needing daily dressing changes at 45 days of follow-up.
Moreover, the effect was sustained, with similar percentages of patients no longer requiring daily dressing changes at 60 days (34% vs. 13%, respectively) and 90 days (36% vs. 11%) of follow-up.
The mean reduction in daily dressing changes was 1.36 for Oleogel-S10 and 0.41 for the control gel (P = .005).
“Patients who, in the beginning, had daily dressing changes had almost three fewer dressing changes every 2 weeks if they were treated with Oleogel-S10,” Dimitra Kiritsi, MD, PhD, of the department of dermatology at the University of Freiburg (Germany), reported at the annual congress of the European Academy of Dermatology and Venereology. By comparison, patients in the control group had just one fewer daily dressing change in 2 weeks.
“You might say okay, but what does this mean in terms of time?” added Dr. Kiritsi. Using historical data on the time required for whole body care (Orphanet J Rare Dis. 2020 Jan 3. doi: 10.1186/s13023-019-1279-y), it was estimated that treatment with Oleogel-S10 was associated with an overall time-saving per week of 11 hours (6.6 hours for the patient and 4.4 hours for the caregiver) and use of the control gel was associated with an overall time-saving of 4 hours (2.4 hours for the patient and 1.6 hours for the caregiver).
“This is, for our patients, important,” said Dr. Kiritsi, as “it is time that they can spend doing something nice with the family” instead, avoiding the pain and distress associated with frequent dressing changes.
Approved in Europe, not in the United States
Oleogel-S10, classified as an herbal product, contains triterpenes derived from birch bark extract, which have been formulated with sunflower oil to form a gel.
Despite being approved for use in Europe, Oleogel-S10 has not yet been approved to treat EB in the United States. The FDA did not approve Amryt Pharma’s new drug application in February 2022. The application had included data from the EASE trial.
EASE included 223 patients with dystrophic or junctional EB, including 156 children, at 58 sites in 28 countries. As such, this makes it the largest treatment study in this rare genetic disease to date.
The trial had consisted of an initial 90-day, double-blind treatment period, during which time 109 patients had used Oleogel-S10 and 114 had used a control gel. This was followed by a 24-month open-label phase, during which time all remaining patients (n = 205) had used Oleogel-S10 on top of their standard of care.
Dr. Kiritsi summarized the main results of the EASE trial as follows.
- Complete healing of target wounds (primary endpoint) in 41.3% of patients treated with Oleogel-S10 and 28.9% of patients treated with the control gel (P = .013).
- Improved total body wound burden measured by both Epidermolysis Bullosa Disease Activity and Scarring Index and Body Surface Area Percentage scores.
- Reduced frequency of dressing changes (1 less per 2 weeks for Oleogel-S10 versus 0 less per 2 weeks for control gel).
- Improved pain among participants aged 4 years and older while their dressings were being changed.
- Reduced rates of wound infection (0.9% Oleogel-S10 vs. 4.4% control gel).
- Similar rates of treatment-emergent adverse events (24.8% vs. 22.8%, respectively), which were mostly deemed to be mild or moderate.
The EASE study – an important trial for EB
EASE is an important trial for EB, the study’s principal investigator Dédée Murrell, MD, DSc, University of New South Wales, Sydney, has pointed out previously.
“This was the first EB study to meet its primary endpoint and demonstrated a statistically significant acceleration of target wound healing by day 45,” Dr. Murrell said in a press release issued by Amryt Pharma to coincide with the online publication of the trial results.
“In addition, the favorable trends we see with key secondary endpoints such as reduced wound burden, pain, and frequency of dressing changes are considered as being very meaningful for patients,” Dr. Murrell said.
The EASE study was funded by Amryt Research Limited. Dr. Kiritsi reported receiving honoraria or consultation fees from Amryt, RHEACELL GmbH, and Fibrx Derm. She also acknowledged grant or research support from DEBRA International, EB Research Partnership, Fritz-Thyssen Foundation, German Research Foundation, and RHEACELL. Dr. Murrell has ties to Amryt and Amicus and is a co-owner of the patent for topical sirolimus for EB simplex.
A version of this article appeared on Medscape.com.
Additional when compared with a control gel.
In a final, post hoc analysis to come from the trial, 15 of 45 (33%) patients treated with Oleogel-S10 versus 5 of 48 (10.4%) treated with the control gel were reported as no longer needing daily dressing changes at 45 days of follow-up.
Moreover, the effect was sustained, with similar percentages of patients no longer requiring daily dressing changes at 60 days (34% vs. 13%, respectively) and 90 days (36% vs. 11%) of follow-up.
The mean reduction in daily dressing changes was 1.36 for Oleogel-S10 and 0.41 for the control gel (P = .005).
“Patients who, in the beginning, had daily dressing changes had almost three fewer dressing changes every 2 weeks if they were treated with Oleogel-S10,” Dimitra Kiritsi, MD, PhD, of the department of dermatology at the University of Freiburg (Germany), reported at the annual congress of the European Academy of Dermatology and Venereology. By comparison, patients in the control group had just one fewer daily dressing change in 2 weeks.
“You might say okay, but what does this mean in terms of time?” added Dr. Kiritsi. Using historical data on the time required for whole body care (Orphanet J Rare Dis. 2020 Jan 3. doi: 10.1186/s13023-019-1279-y), it was estimated that treatment with Oleogel-S10 was associated with an overall time-saving per week of 11 hours (6.6 hours for the patient and 4.4 hours for the caregiver) and use of the control gel was associated with an overall time-saving of 4 hours (2.4 hours for the patient and 1.6 hours for the caregiver).
“This is, for our patients, important,” said Dr. Kiritsi, as “it is time that they can spend doing something nice with the family” instead, avoiding the pain and distress associated with frequent dressing changes.
Approved in Europe, not in the United States
Oleogel-S10, classified as an herbal product, contains triterpenes derived from birch bark extract, which have been formulated with sunflower oil to form a gel.
Despite being approved for use in Europe, Oleogel-S10 has not yet been approved to treat EB in the United States. The FDA did not approve Amryt Pharma’s new drug application in February 2022. The application had included data from the EASE trial.
EASE included 223 patients with dystrophic or junctional EB, including 156 children, at 58 sites in 28 countries. As such, this makes it the largest treatment study in this rare genetic disease to date.
The trial had consisted of an initial 90-day, double-blind treatment period, during which time 109 patients had used Oleogel-S10 and 114 had used a control gel. This was followed by a 24-month open-label phase, during which time all remaining patients (n = 205) had used Oleogel-S10 on top of their standard of care.
Dr. Kiritsi summarized the main results of the EASE trial as follows.
- Complete healing of target wounds (primary endpoint) in 41.3% of patients treated with Oleogel-S10 and 28.9% of patients treated with the control gel (P = .013).
- Improved total body wound burden measured by both Epidermolysis Bullosa Disease Activity and Scarring Index and Body Surface Area Percentage scores.
- Reduced frequency of dressing changes (1 less per 2 weeks for Oleogel-S10 versus 0 less per 2 weeks for control gel).
- Improved pain among participants aged 4 years and older while their dressings were being changed.
- Reduced rates of wound infection (0.9% Oleogel-S10 vs. 4.4% control gel).
- Similar rates of treatment-emergent adverse events (24.8% vs. 22.8%, respectively), which were mostly deemed to be mild or moderate.
The EASE study – an important trial for EB
EASE is an important trial for EB, the study’s principal investigator Dédée Murrell, MD, DSc, University of New South Wales, Sydney, has pointed out previously.
“This was the first EB study to meet its primary endpoint and demonstrated a statistically significant acceleration of target wound healing by day 45,” Dr. Murrell said in a press release issued by Amryt Pharma to coincide with the online publication of the trial results.
“In addition, the favorable trends we see with key secondary endpoints such as reduced wound burden, pain, and frequency of dressing changes are considered as being very meaningful for patients,” Dr. Murrell said.
The EASE study was funded by Amryt Research Limited. Dr. Kiritsi reported receiving honoraria or consultation fees from Amryt, RHEACELL GmbH, and Fibrx Derm. She also acknowledged grant or research support from DEBRA International, EB Research Partnership, Fritz-Thyssen Foundation, German Research Foundation, and RHEACELL. Dr. Murrell has ties to Amryt and Amicus and is a co-owner of the patent for topical sirolimus for EB simplex.
A version of this article appeared on Medscape.com.
Additional when compared with a control gel.
In a final, post hoc analysis to come from the trial, 15 of 45 (33%) patients treated with Oleogel-S10 versus 5 of 48 (10.4%) treated with the control gel were reported as no longer needing daily dressing changes at 45 days of follow-up.
Moreover, the effect was sustained, with similar percentages of patients no longer requiring daily dressing changes at 60 days (34% vs. 13%, respectively) and 90 days (36% vs. 11%) of follow-up.
The mean reduction in daily dressing changes was 1.36 for Oleogel-S10 and 0.41 for the control gel (P = .005).
“Patients who, in the beginning, had daily dressing changes had almost three fewer dressing changes every 2 weeks if they were treated with Oleogel-S10,” Dimitra Kiritsi, MD, PhD, of the department of dermatology at the University of Freiburg (Germany), reported at the annual congress of the European Academy of Dermatology and Venereology. By comparison, patients in the control group had just one fewer daily dressing change in 2 weeks.
“You might say okay, but what does this mean in terms of time?” added Dr. Kiritsi. Using historical data on the time required for whole body care (Orphanet J Rare Dis. 2020 Jan 3. doi: 10.1186/s13023-019-1279-y), it was estimated that treatment with Oleogel-S10 was associated with an overall time-saving per week of 11 hours (6.6 hours for the patient and 4.4 hours for the caregiver) and use of the control gel was associated with an overall time-saving of 4 hours (2.4 hours for the patient and 1.6 hours for the caregiver).
“This is, for our patients, important,” said Dr. Kiritsi, as “it is time that they can spend doing something nice with the family” instead, avoiding the pain and distress associated with frequent dressing changes.
Approved in Europe, not in the United States
Oleogel-S10, classified as an herbal product, contains triterpenes derived from birch bark extract, which have been formulated with sunflower oil to form a gel.
Despite being approved for use in Europe, Oleogel-S10 has not yet been approved to treat EB in the United States. The FDA did not approve Amryt Pharma’s new drug application in February 2022. The application had included data from the EASE trial.
EASE included 223 patients with dystrophic or junctional EB, including 156 children, at 58 sites in 28 countries. As such, this makes it the largest treatment study in this rare genetic disease to date.
The trial had consisted of an initial 90-day, double-blind treatment period, during which time 109 patients had used Oleogel-S10 and 114 had used a control gel. This was followed by a 24-month open-label phase, during which time all remaining patients (n = 205) had used Oleogel-S10 on top of their standard of care.
Dr. Kiritsi summarized the main results of the EASE trial as follows.
- Complete healing of target wounds (primary endpoint) in 41.3% of patients treated with Oleogel-S10 and 28.9% of patients treated with the control gel (P = .013).
- Improved total body wound burden measured by both Epidermolysis Bullosa Disease Activity and Scarring Index and Body Surface Area Percentage scores.
- Reduced frequency of dressing changes (1 less per 2 weeks for Oleogel-S10 versus 0 less per 2 weeks for control gel).
- Improved pain among participants aged 4 years and older while their dressings were being changed.
- Reduced rates of wound infection (0.9% Oleogel-S10 vs. 4.4% control gel).
- Similar rates of treatment-emergent adverse events (24.8% vs. 22.8%, respectively), which were mostly deemed to be mild or moderate.
The EASE study – an important trial for EB
EASE is an important trial for EB, the study’s principal investigator Dédée Murrell, MD, DSc, University of New South Wales, Sydney, has pointed out previously.
“This was the first EB study to meet its primary endpoint and demonstrated a statistically significant acceleration of target wound healing by day 45,” Dr. Murrell said in a press release issued by Amryt Pharma to coincide with the online publication of the trial results.
“In addition, the favorable trends we see with key secondary endpoints such as reduced wound burden, pain, and frequency of dressing changes are considered as being very meaningful for patients,” Dr. Murrell said.
The EASE study was funded by Amryt Research Limited. Dr. Kiritsi reported receiving honoraria or consultation fees from Amryt, RHEACELL GmbH, and Fibrx Derm. She also acknowledged grant or research support from DEBRA International, EB Research Partnership, Fritz-Thyssen Foundation, German Research Foundation, and RHEACELL. Dr. Murrell has ties to Amryt and Amicus and is a co-owner of the patent for topical sirolimus for EB simplex.
A version of this article appeared on Medscape.com.
FROM THE EADV CONGRESS
FDA OKs first ustekinumab biosimilar
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
FDA approves abatacept for pediatric patients with psoriatic arthritis
The Food and Drug Administration has approved an expanded indication for abatacept (Orencia) for treatment of psoriatic arthritis (PsA) in pediatric patients aged 2 years and older.
Juvenile psoriatic arthritis (JPsA) is a form of juvenile idiopathic arthritis (JIA). It is a rare condition, and it is estimated that as many as 5% of children with JIA have JPsA.
“The FDA’s approval of expanding Orencia’s indication adds a much-needed treatment option for children with JPsA, a rare, potentially serious condition characterized by chronic inflammation and joint damage,” said Carlos Dortrait, senior vice president of U.S. immunology at Bristol-Myers Squibb in a statement. BMS is the manufacturer of abatacept.
Abatacept was first approved in 2005 for the treatment of moderate to severe rheumatoid arthritis and was approved for treating active PsA in adults in 2017. In 2008, the drug was the first intravenous biologic approved for patients 6 years old and older to treat moderately to severely active polyarticular juvenile idiopathic arthritis (pJIA). In 2017, a subcutaneous administration option was approved for children 2 years old and older with pJIA, according to a BMS press release.
This expanded approval was based on controlled studies of abatacept in adults with PsA; pharmacokinetic data from adults with RA, adults with PsA, and children with pJIA; and safety data from clinical studies in patients aged 2-17 years with pJIA.
“Children living with psoriatic arthritis can experience a number of challenging symptoms including swollen and painful joints,” Steven Taylor, president and CEO of the Arthritis Foundation, said in a BMS statement. “The FDA’s approval of Orencia for JPsA in patients 2 years of age and older means another treatment option is available to manage this rare chronic disease, which is exciting news for the arthritis community of young patients, their caregivers, and health care professionals.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved an expanded indication for abatacept (Orencia) for treatment of psoriatic arthritis (PsA) in pediatric patients aged 2 years and older.
Juvenile psoriatic arthritis (JPsA) is a form of juvenile idiopathic arthritis (JIA). It is a rare condition, and it is estimated that as many as 5% of children with JIA have JPsA.
“The FDA’s approval of expanding Orencia’s indication adds a much-needed treatment option for children with JPsA, a rare, potentially serious condition characterized by chronic inflammation and joint damage,” said Carlos Dortrait, senior vice president of U.S. immunology at Bristol-Myers Squibb in a statement. BMS is the manufacturer of abatacept.
Abatacept was first approved in 2005 for the treatment of moderate to severe rheumatoid arthritis and was approved for treating active PsA in adults in 2017. In 2008, the drug was the first intravenous biologic approved for patients 6 years old and older to treat moderately to severely active polyarticular juvenile idiopathic arthritis (pJIA). In 2017, a subcutaneous administration option was approved for children 2 years old and older with pJIA, according to a BMS press release.
This expanded approval was based on controlled studies of abatacept in adults with PsA; pharmacokinetic data from adults with RA, adults with PsA, and children with pJIA; and safety data from clinical studies in patients aged 2-17 years with pJIA.
“Children living with psoriatic arthritis can experience a number of challenging symptoms including swollen and painful joints,” Steven Taylor, president and CEO of the Arthritis Foundation, said in a BMS statement. “The FDA’s approval of Orencia for JPsA in patients 2 years of age and older means another treatment option is available to manage this rare chronic disease, which is exciting news for the arthritis community of young patients, their caregivers, and health care professionals.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved an expanded indication for abatacept (Orencia) for treatment of psoriatic arthritis (PsA) in pediatric patients aged 2 years and older.
Juvenile psoriatic arthritis (JPsA) is a form of juvenile idiopathic arthritis (JIA). It is a rare condition, and it is estimated that as many as 5% of children with JIA have JPsA.
“The FDA’s approval of expanding Orencia’s indication adds a much-needed treatment option for children with JPsA, a rare, potentially serious condition characterized by chronic inflammation and joint damage,” said Carlos Dortrait, senior vice president of U.S. immunology at Bristol-Myers Squibb in a statement. BMS is the manufacturer of abatacept.
Abatacept was first approved in 2005 for the treatment of moderate to severe rheumatoid arthritis and was approved for treating active PsA in adults in 2017. In 2008, the drug was the first intravenous biologic approved for patients 6 years old and older to treat moderately to severely active polyarticular juvenile idiopathic arthritis (pJIA). In 2017, a subcutaneous administration option was approved for children 2 years old and older with pJIA, according to a BMS press release.
This expanded approval was based on controlled studies of abatacept in adults with PsA; pharmacokinetic data from adults with RA, adults with PsA, and children with pJIA; and safety data from clinical studies in patients aged 2-17 years with pJIA.
“Children living with psoriatic arthritis can experience a number of challenging symptoms including swollen and painful joints,” Steven Taylor, president and CEO of the Arthritis Foundation, said in a BMS statement. “The FDA’s approval of Orencia for JPsA in patients 2 years of age and older means another treatment option is available to manage this rare chronic disease, which is exciting news for the arthritis community of young patients, their caregivers, and health care professionals.”
A version of this article first appeared on Medscape.com.
Phase 3 trial supports topical JAK inhibitor for AD in young children
BERLIN – as previously shown in adolescents and adults for whom it already has an approved indication.
In this study – TRUE-AD3 – systemic exposure to ruxolitinib, which is selective for JAK1 and 2, was followed closely, and the low mean plasma concentrations “suggest systemic JAK inhibition is highly unlikely,” Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the annual congress of the European Academy of Dermatology and Venereology.
For example, at a plasma concentration no greater than 27 nM in both younger and older patients at 4 weeks and again at 8 weeks, the systemic exposure was about a tenth of that (281 nM) previously associated with myelosuppression, he reported.
Given the boxed warning for oral JAK inhibitors, which was based largely on a 2022 study in adults with rheumatoid arthritis that associated tofacitinib, a nonspecific JAK inhibitor, with an increased risk of thrombotic events in adults already at risk for these events, safety was a focus of this phase 3 trial. The boxed warning is also in the labeling for topical ruxolitinib, 1.5% (Opzelura), approved for treating to mild to moderate atopic dermatitis in patients 12 years of age and older.
Dr. Eichenfield said there were no significant safety signals in the younger pediatric population. “There were no treatment-emergent adverse events suggestive of systemic JAK inhibition,” he said. This not only included the absence of serious infections, cardiac events, thromboses, or malignancies, but there was no signal of hematologic abnormalities, such as change in hemoglobin or neutrophil count.
Application site reactions
Rather, in the study of children ages 2-11, the only adverse events associated with topical ruxolitinib not observed in the control arm, which received the vehicle alone, were application site reactions, such as pain, erythema, and irritation. None of these occurred in more than 3% of those randomized to ruxolitinib regardless of dose.
Overall, in the trial, which randomized 329 patients ages from 2 to under 12 years with mild to moderate AD to ruxolitinib 1.5% cream, ruxolitinib 0.75% cream, or vehicle in a 2:2:1 fashion, there were just two (0.8%) discontinuations in the ruxolitinib groups (one in each dosing arm). There were none in the vehicle arm.
The safety supports an expansion of the AD indication for topical ruxolitinib in young children, because the rates of response were very similar to that seen in adolescents and adults in the previously published TRUE AD-1 and TRUE AD-2 trials, he said.
For the primary endpoint of Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) with at least a 2 grade improvement in IGA score from baseline, the response rates were 56.5%, 36.6%, and 10.8% for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively, at 8 weeks (P < .0001 for both doses relative to vehicle).
For the secondary efficacy endpoint of 75% or greater clearance on the Eczema Area and Severity Index, the rates were 67.2%, 51.5%, and 15.4%, for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively. Again, the advantage of both doses of ruxolitinib relative to vehicle was highly statistically significant (P < .0001).
Control of itch, evaluated with the Numerical Rating Scale was only evaluated in children 6-2 because of concern of the reliability of reporting in younger children. Control was defined as at least a 4-point improvement from baseline. It was achieved by 43.4%, 37.5%, and 29.7% by week 8 in the arms receiving the higher dose of ruxolitinib, the lower dose, and vehicle, respectively. The median time to achieving itch control was 11 days, 13 days, and 23 days, respectively. For all of these endpoints, the separation of the curves was readily apparent within the first 2 weeks.
The efficacy and tolerability of ruxolitinib appeared to be similar in younger children (ages 2-6) relative to older children.
Extension study in children near completion
Most of the patients who participated in TRUE AD-3 have been rolled over to the open-label extension trial, which is nearing completion. Those originally randomized to vehicle have been rerandomized to the lower or higher dose of ruxolitinib.
While this trial was focused on ruxolitinib as monotherapy, Thrasyvoulos Tzellos, MD, head of the department of dermatology, Nordland Hospital Trust, Bødo, Norway, questioned whether this is will be how it will be used in clinical practice. With the increasing array of therapies for AD, the “concept of combination therapy becomes more and more relevant,” he said after Dr. Eichenfield’s presentation.
Questioning whether an effective nonsteroidal anti-inflammatory agent like ruxolitinib should be considered a first-line treatment in mild disease or an adjunctive treatment for AD of any severity, he suggested that it might be best considered within a combination.
Dr. Eichenfield agreed. “Once we get the drug approved in a controlled trial, I think we then figure out how to use it in clinical practice.” Based on his own use of ruxolitinib in adults, he noted that he has not seen this drug replace other therapies so much as provide another option for control.
“We have an increasing armamentarium of drugs to use for involvement in different areas of the body in order to get more long-term control of disease,” he said. As an effective topical nonsteroidal drug, he believes its addition to clinical care in younger children, if approved, will be meaningful.
Dr. Eichenfield disclosed financial relationships with more multiple pharmaceutical companies, including Incyte, the manufacturer of ruxolitinib cream that provided funding for the True-AD trials. Dr. Tzellos reported financial relationships with AbbVie and UCB.
BERLIN – as previously shown in adolescents and adults for whom it already has an approved indication.
In this study – TRUE-AD3 – systemic exposure to ruxolitinib, which is selective for JAK1 and 2, was followed closely, and the low mean plasma concentrations “suggest systemic JAK inhibition is highly unlikely,” Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the annual congress of the European Academy of Dermatology and Venereology.
For example, at a plasma concentration no greater than 27 nM in both younger and older patients at 4 weeks and again at 8 weeks, the systemic exposure was about a tenth of that (281 nM) previously associated with myelosuppression, he reported.
Given the boxed warning for oral JAK inhibitors, which was based largely on a 2022 study in adults with rheumatoid arthritis that associated tofacitinib, a nonspecific JAK inhibitor, with an increased risk of thrombotic events in adults already at risk for these events, safety was a focus of this phase 3 trial. The boxed warning is also in the labeling for topical ruxolitinib, 1.5% (Opzelura), approved for treating to mild to moderate atopic dermatitis in patients 12 years of age and older.
Dr. Eichenfield said there were no significant safety signals in the younger pediatric population. “There were no treatment-emergent adverse events suggestive of systemic JAK inhibition,” he said. This not only included the absence of serious infections, cardiac events, thromboses, or malignancies, but there was no signal of hematologic abnormalities, such as change in hemoglobin or neutrophil count.
Application site reactions
Rather, in the study of children ages 2-11, the only adverse events associated with topical ruxolitinib not observed in the control arm, which received the vehicle alone, were application site reactions, such as pain, erythema, and irritation. None of these occurred in more than 3% of those randomized to ruxolitinib regardless of dose.
Overall, in the trial, which randomized 329 patients ages from 2 to under 12 years with mild to moderate AD to ruxolitinib 1.5% cream, ruxolitinib 0.75% cream, or vehicle in a 2:2:1 fashion, there were just two (0.8%) discontinuations in the ruxolitinib groups (one in each dosing arm). There were none in the vehicle arm.
The safety supports an expansion of the AD indication for topical ruxolitinib in young children, because the rates of response were very similar to that seen in adolescents and adults in the previously published TRUE AD-1 and TRUE AD-2 trials, he said.
For the primary endpoint of Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) with at least a 2 grade improvement in IGA score from baseline, the response rates were 56.5%, 36.6%, and 10.8% for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively, at 8 weeks (P < .0001 for both doses relative to vehicle).
For the secondary efficacy endpoint of 75% or greater clearance on the Eczema Area and Severity Index, the rates were 67.2%, 51.5%, and 15.4%, for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively. Again, the advantage of both doses of ruxolitinib relative to vehicle was highly statistically significant (P < .0001).
Control of itch, evaluated with the Numerical Rating Scale was only evaluated in children 6-2 because of concern of the reliability of reporting in younger children. Control was defined as at least a 4-point improvement from baseline. It was achieved by 43.4%, 37.5%, and 29.7% by week 8 in the arms receiving the higher dose of ruxolitinib, the lower dose, and vehicle, respectively. The median time to achieving itch control was 11 days, 13 days, and 23 days, respectively. For all of these endpoints, the separation of the curves was readily apparent within the first 2 weeks.
The efficacy and tolerability of ruxolitinib appeared to be similar in younger children (ages 2-6) relative to older children.
Extension study in children near completion
Most of the patients who participated in TRUE AD-3 have been rolled over to the open-label extension trial, which is nearing completion. Those originally randomized to vehicle have been rerandomized to the lower or higher dose of ruxolitinib.
While this trial was focused on ruxolitinib as monotherapy, Thrasyvoulos Tzellos, MD, head of the department of dermatology, Nordland Hospital Trust, Bødo, Norway, questioned whether this is will be how it will be used in clinical practice. With the increasing array of therapies for AD, the “concept of combination therapy becomes more and more relevant,” he said after Dr. Eichenfield’s presentation.
Questioning whether an effective nonsteroidal anti-inflammatory agent like ruxolitinib should be considered a first-line treatment in mild disease or an adjunctive treatment for AD of any severity, he suggested that it might be best considered within a combination.
Dr. Eichenfield agreed. “Once we get the drug approved in a controlled trial, I think we then figure out how to use it in clinical practice.” Based on his own use of ruxolitinib in adults, he noted that he has not seen this drug replace other therapies so much as provide another option for control.
“We have an increasing armamentarium of drugs to use for involvement in different areas of the body in order to get more long-term control of disease,” he said. As an effective topical nonsteroidal drug, he believes its addition to clinical care in younger children, if approved, will be meaningful.
Dr. Eichenfield disclosed financial relationships with more multiple pharmaceutical companies, including Incyte, the manufacturer of ruxolitinib cream that provided funding for the True-AD trials. Dr. Tzellos reported financial relationships with AbbVie and UCB.
BERLIN – as previously shown in adolescents and adults for whom it already has an approved indication.
In this study – TRUE-AD3 – systemic exposure to ruxolitinib, which is selective for JAK1 and 2, was followed closely, and the low mean plasma concentrations “suggest systemic JAK inhibition is highly unlikely,” Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the annual congress of the European Academy of Dermatology and Venereology.
For example, at a plasma concentration no greater than 27 nM in both younger and older patients at 4 weeks and again at 8 weeks, the systemic exposure was about a tenth of that (281 nM) previously associated with myelosuppression, he reported.
Given the boxed warning for oral JAK inhibitors, which was based largely on a 2022 study in adults with rheumatoid arthritis that associated tofacitinib, a nonspecific JAK inhibitor, with an increased risk of thrombotic events in adults already at risk for these events, safety was a focus of this phase 3 trial. The boxed warning is also in the labeling for topical ruxolitinib, 1.5% (Opzelura), approved for treating to mild to moderate atopic dermatitis in patients 12 years of age and older.
Dr. Eichenfield said there were no significant safety signals in the younger pediatric population. “There were no treatment-emergent adverse events suggestive of systemic JAK inhibition,” he said. This not only included the absence of serious infections, cardiac events, thromboses, or malignancies, but there was no signal of hematologic abnormalities, such as change in hemoglobin or neutrophil count.
Application site reactions
Rather, in the study of children ages 2-11, the only adverse events associated with topical ruxolitinib not observed in the control arm, which received the vehicle alone, were application site reactions, such as pain, erythema, and irritation. None of these occurred in more than 3% of those randomized to ruxolitinib regardless of dose.
Overall, in the trial, which randomized 329 patients ages from 2 to under 12 years with mild to moderate AD to ruxolitinib 1.5% cream, ruxolitinib 0.75% cream, or vehicle in a 2:2:1 fashion, there were just two (0.8%) discontinuations in the ruxolitinib groups (one in each dosing arm). There were none in the vehicle arm.
The safety supports an expansion of the AD indication for topical ruxolitinib in young children, because the rates of response were very similar to that seen in adolescents and adults in the previously published TRUE AD-1 and TRUE AD-2 trials, he said.
For the primary endpoint of Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) with at least a 2 grade improvement in IGA score from baseline, the response rates were 56.5%, 36.6%, and 10.8% for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively, at 8 weeks (P < .0001 for both doses relative to vehicle).
For the secondary efficacy endpoint of 75% or greater clearance on the Eczema Area and Severity Index, the rates were 67.2%, 51.5%, and 15.4%, for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively. Again, the advantage of both doses of ruxolitinib relative to vehicle was highly statistically significant (P < .0001).
Control of itch, evaluated with the Numerical Rating Scale was only evaluated in children 6-2 because of concern of the reliability of reporting in younger children. Control was defined as at least a 4-point improvement from baseline. It was achieved by 43.4%, 37.5%, and 29.7% by week 8 in the arms receiving the higher dose of ruxolitinib, the lower dose, and vehicle, respectively. The median time to achieving itch control was 11 days, 13 days, and 23 days, respectively. For all of these endpoints, the separation of the curves was readily apparent within the first 2 weeks.
The efficacy and tolerability of ruxolitinib appeared to be similar in younger children (ages 2-6) relative to older children.
Extension study in children near completion
Most of the patients who participated in TRUE AD-3 have been rolled over to the open-label extension trial, which is nearing completion. Those originally randomized to vehicle have been rerandomized to the lower or higher dose of ruxolitinib.
While this trial was focused on ruxolitinib as monotherapy, Thrasyvoulos Tzellos, MD, head of the department of dermatology, Nordland Hospital Trust, Bødo, Norway, questioned whether this is will be how it will be used in clinical practice. With the increasing array of therapies for AD, the “concept of combination therapy becomes more and more relevant,” he said after Dr. Eichenfield’s presentation.
Questioning whether an effective nonsteroidal anti-inflammatory agent like ruxolitinib should be considered a first-line treatment in mild disease or an adjunctive treatment for AD of any severity, he suggested that it might be best considered within a combination.
Dr. Eichenfield agreed. “Once we get the drug approved in a controlled trial, I think we then figure out how to use it in clinical practice.” Based on his own use of ruxolitinib in adults, he noted that he has not seen this drug replace other therapies so much as provide another option for control.
“We have an increasing armamentarium of drugs to use for involvement in different areas of the body in order to get more long-term control of disease,” he said. As an effective topical nonsteroidal drug, he believes its addition to clinical care in younger children, if approved, will be meaningful.
Dr. Eichenfield disclosed financial relationships with more multiple pharmaceutical companies, including Incyte, the manufacturer of ruxolitinib cream that provided funding for the True-AD trials. Dr. Tzellos reported financial relationships with AbbVie and UCB.
AT THE EADV CONGRESS
Teens streaming on Twitch vulnerable to predators
WASHINGTON – Half of youth broadcasting live streams on the online platform Twitch revealed their real-world location, and nearly half provided their name to viewers, according to research presented at the annual meeting of the American Academy of Pediatrics. It took researchers less than 5 minutes – and sometimes as little as 12 seconds – to find minors in different video game categories, suggesting the environment offers opportunities to predators to gain sensitive information about minors, reported Fiona Dubrosa, BS, BA, a visiting scholar at Cohen Children’s Medical Center, New York, and colleagues.
A ‘clandestine, threatening digital environment’
the authors concluded. “The nature of live streaming makes it particularly dangerous, as there is no way to take back information that has been revealed or regulate content or viewers. Parents and pediatricians should be aware of the dangers presented by Twitch and other live-streaming platforms and counsel children on best practices for Internet safety.”
Twitch is an online streaming platform where people can watch creator’s live content, such as music performances or narrating real-time video game playing. The platform requires live streamers to be 13 years old with a valid email address or phone number to create an account, but no age restrictions or identification requirements exist for viewers, “potentially putting minors in danger of being watched, followed, and groomed by predators,” the researchers noted. They added that people following different streamers receive notifications when those streamers are live. Further, “viewers can donate money to streamers, which can make it easier for predators to manipulate, track, and encourage risky behaviors from minors.”
To better understand the risks the platform might pose to minors, the researchers searched for and analyzed popular video game live streams that appeared to be streamed by minors who had their cameras on and their faces visible. Then the researchers noted the name of the video game, the topics discussed by the streamers, the time it took to find minors under each game, and each streamer’s age, name, follower count, location, streaming schedule, and social media links for money donations.
The researchers analyzed 100 Twitch streamers who were minors, who had a combined 1,755,452 million followers. Nearly half the streamers (47%) provided their presumably real names, and half (50%) gave out their location. Nearly two-thirds (64%) linked other social media accounts they had and encouraged viewers to follow them. Detailed schedules of when they would be live were available for 38% of the streamers, and 37% of the minor streamers were accepting money donations.
Only 11% of the discussion on the streams revealed personal details, most often related to trying on different outfits for viewers and talking about real-world locations they liked to visit. The researchers needed anywhere from 12 seconds to 5 minutes to find a minor in each game category.
”Young users clearly feel a false sense of safety on the platform; a significant proportion were willing to reveal personal information despite having no knowledge of who might be listening,” the researchers said. “The donation system provides a menacing avenue for manipulation and continued exploitation of minors. Our findings reveal the need for stricter age limitations for streamers and more stringent identity verification of audience members on Twitch.”
Open-minded parental guidance is warranted
Jenny Radesky, MD, a developmental behavioral pediatrician and media researcher at University of Michigan Medicine, Ann Arbor, was not surprised that many teens live stream on Twitch since it’s a popular platform for video gaming, but she was surprised at how many revealed their locations and other personal details.
“I suspect that they do this to build closeness with their viewers, by seeming more authentic,” said Dr. Radesky, who was not involved in the study. “It is this type of parasocial relationship with influencers and gamers that keeps an audience engaged, and encourages future viewing and purchases.”
Their willingness to share personal details suggests it’s important to conduct qualitative research to find out how teen live streamers think about privacy risks, what privacy settings they can use and choose to use, and how they handle inappropriate contact from adults, Dr. Radesky said.
Meanwhile, parents should talk with their kids in an open-minded way about what platforms they use and what they like and dislike about them. She recommended parents read the Common Sense Media guide about different social platforms ”to understand what attracts kids to content on specific sites, what their pitfalls are, and what types of privacy and safety settings are available.”
“A child or teen is much more likely to be honest about negative experiences online if they think their parent will hear them out – not judge them or take away their tech. No teen wants to talk with a panicky parent,” Dr. Radesky said.
David Hill, MD, a hospitalist pediatrician for Goldsboro Pediatrics in Wayne County, North Carolina, who also specializes in media communication, said that Twitch is just one example of a social media platform where children can encounter a variety of dangers, including sometimes adult predators.
“This just highlights the importance of parents having an ongoing conversation with their children about how they use their social media platforms and ensuring, just as we do with learning to ride a bicycle or learning to drive a car, that they apply some basic rules of safety,” Dr. Hill said. Then it’s important to keep coming back to that conversation “again and again as they grow and change and as those platforms change to ensure that those kids are continuing to apply those rules consistently.
“The best way for parents to keep up is ask your kids,” he said. “They love to share. They love to teach. They love to be in a position to show you something, especially if it’s something that interests them.”
An example of a rule would be setting personal accounts to private, not public, by default, Dr. Radesky said. “When interviewed, teens often say that they feel intruded upon by older people ‘stalking’ them or trying to connect with them on social platforms,” so making an account private can reduce those opportunities.
For teens who specifically want to create content on social platforms, parent oversight is needed, she said, but she acknowledged it can be a lot of work. “This might take the form of talking about what a teen plans to post before they do, expectations for positive behaviors or language, plans for privacy settings (such as public vs. private accounts), and what to do with trolls or hateful comment,” she said. “Parents may want to follow their child’s account to check in on it.”
Useful advice
Dr. Radesky also provided a handful of talking points that pediatricians can use in talking with patients who use these platforms:
- Keep your account private to just your friends and people you want to interact with. There are a lot of people on the Internet that you don’t want intruding upon your social life.
- Maintain your feed and the accounts you follow to keep it positive, entertaining, and not a source of stress or self-doubt. Content creators are always trying to grab your attention in new ways, some of which are rude or dehumanizing, so don’t waste your time on things that bring you down.
- Talk about why you want to post or live stream. Is it to get reactions or feel validated? If so, can you find other ways to feel validated that don’t require performing for other people? Is it to share a special skill? If so, how do you keep your posts creative and community building rather than attention grabbing? And how can you keep your parents involved so that they can help you navigate challenges?”
Ms. Dubrosa and Dr. Hill had no disclosures. Dr. Radesky is a consultant for Melissa & Doug. No information on external funding was provided.
WASHINGTON – Half of youth broadcasting live streams on the online platform Twitch revealed their real-world location, and nearly half provided their name to viewers, according to research presented at the annual meeting of the American Academy of Pediatrics. It took researchers less than 5 minutes – and sometimes as little as 12 seconds – to find minors in different video game categories, suggesting the environment offers opportunities to predators to gain sensitive information about minors, reported Fiona Dubrosa, BS, BA, a visiting scholar at Cohen Children’s Medical Center, New York, and colleagues.
A ‘clandestine, threatening digital environment’
the authors concluded. “The nature of live streaming makes it particularly dangerous, as there is no way to take back information that has been revealed or regulate content or viewers. Parents and pediatricians should be aware of the dangers presented by Twitch and other live-streaming platforms and counsel children on best practices for Internet safety.”
Twitch is an online streaming platform where people can watch creator’s live content, such as music performances or narrating real-time video game playing. The platform requires live streamers to be 13 years old with a valid email address or phone number to create an account, but no age restrictions or identification requirements exist for viewers, “potentially putting minors in danger of being watched, followed, and groomed by predators,” the researchers noted. They added that people following different streamers receive notifications when those streamers are live. Further, “viewers can donate money to streamers, which can make it easier for predators to manipulate, track, and encourage risky behaviors from minors.”
To better understand the risks the platform might pose to minors, the researchers searched for and analyzed popular video game live streams that appeared to be streamed by minors who had their cameras on and their faces visible. Then the researchers noted the name of the video game, the topics discussed by the streamers, the time it took to find minors under each game, and each streamer’s age, name, follower count, location, streaming schedule, and social media links for money donations.
The researchers analyzed 100 Twitch streamers who were minors, who had a combined 1,755,452 million followers. Nearly half the streamers (47%) provided their presumably real names, and half (50%) gave out their location. Nearly two-thirds (64%) linked other social media accounts they had and encouraged viewers to follow them. Detailed schedules of when they would be live were available for 38% of the streamers, and 37% of the minor streamers were accepting money donations.
Only 11% of the discussion on the streams revealed personal details, most often related to trying on different outfits for viewers and talking about real-world locations they liked to visit. The researchers needed anywhere from 12 seconds to 5 minutes to find a minor in each game category.
”Young users clearly feel a false sense of safety on the platform; a significant proportion were willing to reveal personal information despite having no knowledge of who might be listening,” the researchers said. “The donation system provides a menacing avenue for manipulation and continued exploitation of minors. Our findings reveal the need for stricter age limitations for streamers and more stringent identity verification of audience members on Twitch.”
Open-minded parental guidance is warranted
Jenny Radesky, MD, a developmental behavioral pediatrician and media researcher at University of Michigan Medicine, Ann Arbor, was not surprised that many teens live stream on Twitch since it’s a popular platform for video gaming, but she was surprised at how many revealed their locations and other personal details.
“I suspect that they do this to build closeness with their viewers, by seeming more authentic,” said Dr. Radesky, who was not involved in the study. “It is this type of parasocial relationship with influencers and gamers that keeps an audience engaged, and encourages future viewing and purchases.”
Their willingness to share personal details suggests it’s important to conduct qualitative research to find out how teen live streamers think about privacy risks, what privacy settings they can use and choose to use, and how they handle inappropriate contact from adults, Dr. Radesky said.
Meanwhile, parents should talk with their kids in an open-minded way about what platforms they use and what they like and dislike about them. She recommended parents read the Common Sense Media guide about different social platforms ”to understand what attracts kids to content on specific sites, what their pitfalls are, and what types of privacy and safety settings are available.”
“A child or teen is much more likely to be honest about negative experiences online if they think their parent will hear them out – not judge them or take away their tech. No teen wants to talk with a panicky parent,” Dr. Radesky said.
David Hill, MD, a hospitalist pediatrician for Goldsboro Pediatrics in Wayne County, North Carolina, who also specializes in media communication, said that Twitch is just one example of a social media platform where children can encounter a variety of dangers, including sometimes adult predators.
“This just highlights the importance of parents having an ongoing conversation with their children about how they use their social media platforms and ensuring, just as we do with learning to ride a bicycle or learning to drive a car, that they apply some basic rules of safety,” Dr. Hill said. Then it’s important to keep coming back to that conversation “again and again as they grow and change and as those platforms change to ensure that those kids are continuing to apply those rules consistently.
“The best way for parents to keep up is ask your kids,” he said. “They love to share. They love to teach. They love to be in a position to show you something, especially if it’s something that interests them.”
An example of a rule would be setting personal accounts to private, not public, by default, Dr. Radesky said. “When interviewed, teens often say that they feel intruded upon by older people ‘stalking’ them or trying to connect with them on social platforms,” so making an account private can reduce those opportunities.
For teens who specifically want to create content on social platforms, parent oversight is needed, she said, but she acknowledged it can be a lot of work. “This might take the form of talking about what a teen plans to post before they do, expectations for positive behaviors or language, plans for privacy settings (such as public vs. private accounts), and what to do with trolls or hateful comment,” she said. “Parents may want to follow their child’s account to check in on it.”
Useful advice
Dr. Radesky also provided a handful of talking points that pediatricians can use in talking with patients who use these platforms:
- Keep your account private to just your friends and people you want to interact with. There are a lot of people on the Internet that you don’t want intruding upon your social life.
- Maintain your feed and the accounts you follow to keep it positive, entertaining, and not a source of stress or self-doubt. Content creators are always trying to grab your attention in new ways, some of which are rude or dehumanizing, so don’t waste your time on things that bring you down.
- Talk about why you want to post or live stream. Is it to get reactions or feel validated? If so, can you find other ways to feel validated that don’t require performing for other people? Is it to share a special skill? If so, how do you keep your posts creative and community building rather than attention grabbing? And how can you keep your parents involved so that they can help you navigate challenges?”
Ms. Dubrosa and Dr. Hill had no disclosures. Dr. Radesky is a consultant for Melissa & Doug. No information on external funding was provided.
WASHINGTON – Half of youth broadcasting live streams on the online platform Twitch revealed their real-world location, and nearly half provided their name to viewers, according to research presented at the annual meeting of the American Academy of Pediatrics. It took researchers less than 5 minutes – and sometimes as little as 12 seconds – to find minors in different video game categories, suggesting the environment offers opportunities to predators to gain sensitive information about minors, reported Fiona Dubrosa, BS, BA, a visiting scholar at Cohen Children’s Medical Center, New York, and colleagues.
A ‘clandestine, threatening digital environment’
the authors concluded. “The nature of live streaming makes it particularly dangerous, as there is no way to take back information that has been revealed or regulate content or viewers. Parents and pediatricians should be aware of the dangers presented by Twitch and other live-streaming platforms and counsel children on best practices for Internet safety.”
Twitch is an online streaming platform where people can watch creator’s live content, such as music performances or narrating real-time video game playing. The platform requires live streamers to be 13 years old with a valid email address or phone number to create an account, but no age restrictions or identification requirements exist for viewers, “potentially putting minors in danger of being watched, followed, and groomed by predators,” the researchers noted. They added that people following different streamers receive notifications when those streamers are live. Further, “viewers can donate money to streamers, which can make it easier for predators to manipulate, track, and encourage risky behaviors from minors.”
To better understand the risks the platform might pose to minors, the researchers searched for and analyzed popular video game live streams that appeared to be streamed by minors who had their cameras on and their faces visible. Then the researchers noted the name of the video game, the topics discussed by the streamers, the time it took to find minors under each game, and each streamer’s age, name, follower count, location, streaming schedule, and social media links for money donations.
The researchers analyzed 100 Twitch streamers who were minors, who had a combined 1,755,452 million followers. Nearly half the streamers (47%) provided their presumably real names, and half (50%) gave out their location. Nearly two-thirds (64%) linked other social media accounts they had and encouraged viewers to follow them. Detailed schedules of when they would be live were available for 38% of the streamers, and 37% of the minor streamers were accepting money donations.
Only 11% of the discussion on the streams revealed personal details, most often related to trying on different outfits for viewers and talking about real-world locations they liked to visit. The researchers needed anywhere from 12 seconds to 5 minutes to find a minor in each game category.
”Young users clearly feel a false sense of safety on the platform; a significant proportion were willing to reveal personal information despite having no knowledge of who might be listening,” the researchers said. “The donation system provides a menacing avenue for manipulation and continued exploitation of minors. Our findings reveal the need for stricter age limitations for streamers and more stringent identity verification of audience members on Twitch.”
Open-minded parental guidance is warranted
Jenny Radesky, MD, a developmental behavioral pediatrician and media researcher at University of Michigan Medicine, Ann Arbor, was not surprised that many teens live stream on Twitch since it’s a popular platform for video gaming, but she was surprised at how many revealed their locations and other personal details.
“I suspect that they do this to build closeness with their viewers, by seeming more authentic,” said Dr. Radesky, who was not involved in the study. “It is this type of parasocial relationship with influencers and gamers that keeps an audience engaged, and encourages future viewing and purchases.”
Their willingness to share personal details suggests it’s important to conduct qualitative research to find out how teen live streamers think about privacy risks, what privacy settings they can use and choose to use, and how they handle inappropriate contact from adults, Dr. Radesky said.
Meanwhile, parents should talk with their kids in an open-minded way about what platforms they use and what they like and dislike about them. She recommended parents read the Common Sense Media guide about different social platforms ”to understand what attracts kids to content on specific sites, what their pitfalls are, and what types of privacy and safety settings are available.”
“A child or teen is much more likely to be honest about negative experiences online if they think their parent will hear them out – not judge them or take away their tech. No teen wants to talk with a panicky parent,” Dr. Radesky said.
David Hill, MD, a hospitalist pediatrician for Goldsboro Pediatrics in Wayne County, North Carolina, who also specializes in media communication, said that Twitch is just one example of a social media platform where children can encounter a variety of dangers, including sometimes adult predators.
“This just highlights the importance of parents having an ongoing conversation with their children about how they use their social media platforms and ensuring, just as we do with learning to ride a bicycle or learning to drive a car, that they apply some basic rules of safety,” Dr. Hill said. Then it’s important to keep coming back to that conversation “again and again as they grow and change and as those platforms change to ensure that those kids are continuing to apply those rules consistently.
“The best way for parents to keep up is ask your kids,” he said. “They love to share. They love to teach. They love to be in a position to show you something, especially if it’s something that interests them.”
An example of a rule would be setting personal accounts to private, not public, by default, Dr. Radesky said. “When interviewed, teens often say that they feel intruded upon by older people ‘stalking’ them or trying to connect with them on social platforms,” so making an account private can reduce those opportunities.
For teens who specifically want to create content on social platforms, parent oversight is needed, she said, but she acknowledged it can be a lot of work. “This might take the form of talking about what a teen plans to post before they do, expectations for positive behaviors or language, plans for privacy settings (such as public vs. private accounts), and what to do with trolls or hateful comment,” she said. “Parents may want to follow their child’s account to check in on it.”
Useful advice
Dr. Radesky also provided a handful of talking points that pediatricians can use in talking with patients who use these platforms:
- Keep your account private to just your friends and people you want to interact with. There are a lot of people on the Internet that you don’t want intruding upon your social life.
- Maintain your feed and the accounts you follow to keep it positive, entertaining, and not a source of stress or self-doubt. Content creators are always trying to grab your attention in new ways, some of which are rude or dehumanizing, so don’t waste your time on things that bring you down.
- Talk about why you want to post or live stream. Is it to get reactions or feel validated? If so, can you find other ways to feel validated that don’t require performing for other people? Is it to share a special skill? If so, how do you keep your posts creative and community building rather than attention grabbing? And how can you keep your parents involved so that they can help you navigate challenges?”
Ms. Dubrosa and Dr. Hill had no disclosures. Dr. Radesky is a consultant for Melissa & Doug. No information on external funding was provided.
AT AAP 2023
Breastfeeding and colorectal cancer
I, like every pediatrician I know, believe that breast milk is the best nutrition for human newborns. Its balance of nutritive elements and its role in preventing of a wide range of illnesses are so great that we are still learning the extent of their magnitude. It just makes sense that a mother’s milk is most well suited for her baby.
I am a bit less unambiguous about breastfeeding. By that I mean the process of providing breast milk to an infant directly from its mother’s breast. Before you yank my AAP membership card, let me make it clear that I think every woman should consider breastfeeding her infant. But we must accept that in a few situations, even with help from caring and enlightened health care providers and family members, breastfeeding doesn’t work as well as we would have hoped. Fortunately, there are alternatives.
My reservations about the process are few, and until recently I have had an unwaveringly positive attitude toward the safety of breast milk. The cause of my little bit of uncertainty arrived in a recent study by two researchers at the Dana Farber Institute in Boston, in which the A younger cohort within that larger group had a dramatic 40% increased risk of developing high-risk cancer before reaching age 55.
The population the investigators studied came from the large Nurses’ Health Study II, a well-known repository of longitudinal health data. The researchers reported that they included biometric data and a large collection of lifestyle factors including smoking, alcohol intake, and diet in their calculations. However, breastfeeding continued to register the highest association. Interestingly, the investigators found that women who were breastfed for 9 months or longer had twice the risk of colorectal cancer as those who breastfed for from 4 to 8 months.
The study population was all women and predominantly white. However, in the general population it is the non-Hispanic white population that is experiencing the greatest increase in incidence. Of course, the study could not answer whether this association with breastfeeding also existed in minority populations.
The researchers suspect that what they are seeing is a reflection of the Westernization of the American lifestyle. One of the researchers is interested in the gut biome of infants and plans to further the investigation in that direction. Could some substance from the environment be concentrating in breast milk? Or is something missing in breast milk? She points out that, while formulas are generally fortified with vitamin D, breast milk is not.
As concerning as the results of this study may sound, the authors are very careful to urge mothers to continue to breastfeed and choose it as their first choice for feeding their babies. I have been pleasantly surprised that this study has not gotten widespread media attention because bad news travels fast. I have chosen to share it with you because at some point you may begin getting questions from concerned parents.
While apparently well done, this study is just the beginning. Like any good research, it poses more questions than it answers. For us as pediatricians it means we should continue to recommend breast milk as the first food. But, we must stay alert as further research looks deeper into this association.
We should also take advantage of our special access to young parents, a demographic that less frequently sees a physician for preventive care. For whatever reason colorectal cancer is occurring at younger ages. When we have the opportunity we should be reminding 40-year-olds not to wait until age 50 to screen for colorectal cancer, particularly if they have a family history of the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I, like every pediatrician I know, believe that breast milk is the best nutrition for human newborns. Its balance of nutritive elements and its role in preventing of a wide range of illnesses are so great that we are still learning the extent of their magnitude. It just makes sense that a mother’s milk is most well suited for her baby.
I am a bit less unambiguous about breastfeeding. By that I mean the process of providing breast milk to an infant directly from its mother’s breast. Before you yank my AAP membership card, let me make it clear that I think every woman should consider breastfeeding her infant. But we must accept that in a few situations, even with help from caring and enlightened health care providers and family members, breastfeeding doesn’t work as well as we would have hoped. Fortunately, there are alternatives.
My reservations about the process are few, and until recently I have had an unwaveringly positive attitude toward the safety of breast milk. The cause of my little bit of uncertainty arrived in a recent study by two researchers at the Dana Farber Institute in Boston, in which the A younger cohort within that larger group had a dramatic 40% increased risk of developing high-risk cancer before reaching age 55.
The population the investigators studied came from the large Nurses’ Health Study II, a well-known repository of longitudinal health data. The researchers reported that they included biometric data and a large collection of lifestyle factors including smoking, alcohol intake, and diet in their calculations. However, breastfeeding continued to register the highest association. Interestingly, the investigators found that women who were breastfed for 9 months or longer had twice the risk of colorectal cancer as those who breastfed for from 4 to 8 months.
The study population was all women and predominantly white. However, in the general population it is the non-Hispanic white population that is experiencing the greatest increase in incidence. Of course, the study could not answer whether this association with breastfeeding also existed in minority populations.
The researchers suspect that what they are seeing is a reflection of the Westernization of the American lifestyle. One of the researchers is interested in the gut biome of infants and plans to further the investigation in that direction. Could some substance from the environment be concentrating in breast milk? Or is something missing in breast milk? She points out that, while formulas are generally fortified with vitamin D, breast milk is not.
As concerning as the results of this study may sound, the authors are very careful to urge mothers to continue to breastfeed and choose it as their first choice for feeding their babies. I have been pleasantly surprised that this study has not gotten widespread media attention because bad news travels fast. I have chosen to share it with you because at some point you may begin getting questions from concerned parents.
While apparently well done, this study is just the beginning. Like any good research, it poses more questions than it answers. For us as pediatricians it means we should continue to recommend breast milk as the first food. But, we must stay alert as further research looks deeper into this association.
We should also take advantage of our special access to young parents, a demographic that less frequently sees a physician for preventive care. For whatever reason colorectal cancer is occurring at younger ages. When we have the opportunity we should be reminding 40-year-olds not to wait until age 50 to screen for colorectal cancer, particularly if they have a family history of the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I, like every pediatrician I know, believe that breast milk is the best nutrition for human newborns. Its balance of nutritive elements and its role in preventing of a wide range of illnesses are so great that we are still learning the extent of their magnitude. It just makes sense that a mother’s milk is most well suited for her baby.
I am a bit less unambiguous about breastfeeding. By that I mean the process of providing breast milk to an infant directly from its mother’s breast. Before you yank my AAP membership card, let me make it clear that I think every woman should consider breastfeeding her infant. But we must accept that in a few situations, even with help from caring and enlightened health care providers and family members, breastfeeding doesn’t work as well as we would have hoped. Fortunately, there are alternatives.
My reservations about the process are few, and until recently I have had an unwaveringly positive attitude toward the safety of breast milk. The cause of my little bit of uncertainty arrived in a recent study by two researchers at the Dana Farber Institute in Boston, in which the A younger cohort within that larger group had a dramatic 40% increased risk of developing high-risk cancer before reaching age 55.
The population the investigators studied came from the large Nurses’ Health Study II, a well-known repository of longitudinal health data. The researchers reported that they included biometric data and a large collection of lifestyle factors including smoking, alcohol intake, and diet in their calculations. However, breastfeeding continued to register the highest association. Interestingly, the investigators found that women who were breastfed for 9 months or longer had twice the risk of colorectal cancer as those who breastfed for from 4 to 8 months.
The study population was all women and predominantly white. However, in the general population it is the non-Hispanic white population that is experiencing the greatest increase in incidence. Of course, the study could not answer whether this association with breastfeeding also existed in minority populations.
The researchers suspect that what they are seeing is a reflection of the Westernization of the American lifestyle. One of the researchers is interested in the gut biome of infants and plans to further the investigation in that direction. Could some substance from the environment be concentrating in breast milk? Or is something missing in breast milk? She points out that, while formulas are generally fortified with vitamin D, breast milk is not.
As concerning as the results of this study may sound, the authors are very careful to urge mothers to continue to breastfeed and choose it as their first choice for feeding their babies. I have been pleasantly surprised that this study has not gotten widespread media attention because bad news travels fast. I have chosen to share it with you because at some point you may begin getting questions from concerned parents.
While apparently well done, this study is just the beginning. Like any good research, it poses more questions than it answers. For us as pediatricians it means we should continue to recommend breast milk as the first food. But, we must stay alert as further research looks deeper into this association.
We should also take advantage of our special access to young parents, a demographic that less frequently sees a physician for preventive care. For whatever reason colorectal cancer is occurring at younger ages. When we have the opportunity we should be reminding 40-year-olds not to wait until age 50 to screen for colorectal cancer, particularly if they have a family history of the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Teens have easy online access to Delta-8 cannabinoid products
WASHINGTON – , researchers reported at the 2023 annual meeting of the American Academy of Pediatrics. Most of the products identified came in bright, colorful, kid-friendly packaging and cost less than $10, the researchers found, and only 2 out of 45 sites had a third-party age verification requirement for purchases.
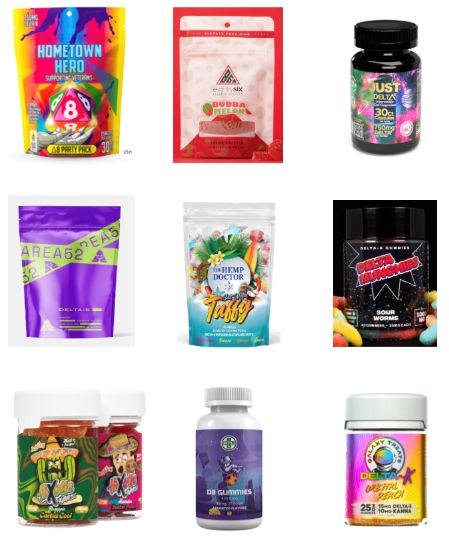
Delta-8 THC, also called D8, is a synthetically produced cannabinoid whose chemical structure and effects are nearly identical to traditional THC, the authors explained, and past research has found that D8 products, such as e-cigarettes, can contain toxic byproducts and contaminants.
”Since D8 is not traditional THC, minors may underestimate its strength and potential danger,” wrote lead author Abhijeet Grewal, BS, a research assistant at Cohen Children’s Medical Center, New York, and senior author Ruth Milanaik, DO, director of the Neonatal Neurodevelopmental Program at Cohen Children’s and a developmental/behavioral pediatrician at Northwell Health, also in New York. “Although traditional THC is a federally banned substance, D8 is legal on a federal level and less restricted on a state by state basis, making it easier for individuals to acquire D8.”
Easily accessible
During the first seven moments of 2021, 77% of reports of accidental exposure occurred in people under age 18, including some children who required ICU admission. The U.S. Food and Drug Administration also received 104 reports of adverse events from products containing D8 between December 2020-February 2022, and more than half of those required medical intervention.
To better understand how easy it is to access D8, the authors collected data on 45 websites they identified that sold D8. The researchers looked for age verification questions for accessing the site, third-party age certification, what kinds of products (edibles, smoke products, or tinctures) were sold, the price and dosage of the cheapest product, and examples of packaging, flavors, marketing claims, and warning statements at each site.
More than a third of the sites (36%) did not ask for customers’ age and almost none of the sites asked for proof: 96% of the sites lacked formal third-party age verification procedures. All but one of the sites sold D8 edibles, and most (82%) sold D8 vaping or smoking products. Only 42% sold tinctures, a mix of concentrated D8 with oil that’s orally consumed.
The cheapest product was priced under $5 on one-third of the sites and under $10 on another third of the sites. The cheapest product was between $10-20 on 16% of the sites while the remaining nine sites’ cheapest product was more than $20. In assessing only the cheapest D8 products on each site, nearly half (47%) contained 51 mg or more of D8, and 20% of the products didn’t report the dosage. Another 22% contained 41-50 mg of D8, and the remaining five products contained 20-40 mg.
Kid-friendly D8
More than half of the D8 products were sold in kid-friendly packaging – packages with bright, colorful designs and fonts that resemble candy or snack food, sometimes cartoon characters or fun items like dice on the packaging. Further, 24% of the websites did not include any warnings or other health information about D8.
“The low prices, high dosages available, and eye-popping packaging make these products extremely attractive to teens who are looking for a high,” the researchers concluded. They advised clinicians to talk with teen patients about the dangers of D8 and advocated for policymakers to more strictly regulate online distributors of D8 products, particularly in requiring age verification procedures and prohibiting kid-friendly packaging.
Megan Moreno, MD, MSEd, MPH, an adolescent medicine physician and researcher at the University of Wisconsin, Madison, School of Medicine and Public Health and UWHealthKids, was particularly struck by how eye-catching the packaging was. “The bright colors and font choices are really designed to attract adolescents,” commented Dr. Moreno, who was not involved in the study. But she was not surprised overall by the findings.
“Other studies have found that the cannabis industry leverages online tools and social media, alongside youth-friendly packaging, to attract youth to their products,” she said. “What is disappointing is that these companies do not use industry standard approaches, such as the alcohol industry, to age-gate their websites.”
It’s important for providers who care for adolescents to ask about substance use but to especially include questions about substances that teens might not think of as “drugs,” such as Delta 8, Dr. Moreno said.
“Prior research on other types of substance such as these has found that teens can think these are less dangerous versions of cannabis, so providing accurate information and asking about these products can prevent harm to kids,” Dr. Moreno said. Although this study focused on websites that sell D8 products, she said that “another important area of influence to consider is social media messaging around these products, which may drive traffic to the purchasing site.” It’s clear this industry is not going to self-regulate without policy changes, Dr. Moreno added, so she noted the importance of advocating for policy that regulates these sites.
Mr. Grewal, Dr. Milanaik and Dr. Moreno had no disclosures. No external funding sources were noted.
WASHINGTON – , researchers reported at the 2023 annual meeting of the American Academy of Pediatrics. Most of the products identified came in bright, colorful, kid-friendly packaging and cost less than $10, the researchers found, and only 2 out of 45 sites had a third-party age verification requirement for purchases.

Delta-8 THC, also called D8, is a synthetically produced cannabinoid whose chemical structure and effects are nearly identical to traditional THC, the authors explained, and past research has found that D8 products, such as e-cigarettes, can contain toxic byproducts and contaminants.
”Since D8 is not traditional THC, minors may underestimate its strength and potential danger,” wrote lead author Abhijeet Grewal, BS, a research assistant at Cohen Children’s Medical Center, New York, and senior author Ruth Milanaik, DO, director of the Neonatal Neurodevelopmental Program at Cohen Children’s and a developmental/behavioral pediatrician at Northwell Health, also in New York. “Although traditional THC is a federally banned substance, D8 is legal on a federal level and less restricted on a state by state basis, making it easier for individuals to acquire D8.”
Easily accessible
During the first seven moments of 2021, 77% of reports of accidental exposure occurred in people under age 18, including some children who required ICU admission. The U.S. Food and Drug Administration also received 104 reports of adverse events from products containing D8 between December 2020-February 2022, and more than half of those required medical intervention.
To better understand how easy it is to access D8, the authors collected data on 45 websites they identified that sold D8. The researchers looked for age verification questions for accessing the site, third-party age certification, what kinds of products (edibles, smoke products, or tinctures) were sold, the price and dosage of the cheapest product, and examples of packaging, flavors, marketing claims, and warning statements at each site.
More than a third of the sites (36%) did not ask for customers’ age and almost none of the sites asked for proof: 96% of the sites lacked formal third-party age verification procedures. All but one of the sites sold D8 edibles, and most (82%) sold D8 vaping or smoking products. Only 42% sold tinctures, a mix of concentrated D8 with oil that’s orally consumed.
The cheapest product was priced under $5 on one-third of the sites and under $10 on another third of the sites. The cheapest product was between $10-20 on 16% of the sites while the remaining nine sites’ cheapest product was more than $20. In assessing only the cheapest D8 products on each site, nearly half (47%) contained 51 mg or more of D8, and 20% of the products didn’t report the dosage. Another 22% contained 41-50 mg of D8, and the remaining five products contained 20-40 mg.
Kid-friendly D8
More than half of the D8 products were sold in kid-friendly packaging – packages with bright, colorful designs and fonts that resemble candy or snack food, sometimes cartoon characters or fun items like dice on the packaging. Further, 24% of the websites did not include any warnings or other health information about D8.
“The low prices, high dosages available, and eye-popping packaging make these products extremely attractive to teens who are looking for a high,” the researchers concluded. They advised clinicians to talk with teen patients about the dangers of D8 and advocated for policymakers to more strictly regulate online distributors of D8 products, particularly in requiring age verification procedures and prohibiting kid-friendly packaging.
Megan Moreno, MD, MSEd, MPH, an adolescent medicine physician and researcher at the University of Wisconsin, Madison, School of Medicine and Public Health and UWHealthKids, was particularly struck by how eye-catching the packaging was. “The bright colors and font choices are really designed to attract adolescents,” commented Dr. Moreno, who was not involved in the study. But she was not surprised overall by the findings.
“Other studies have found that the cannabis industry leverages online tools and social media, alongside youth-friendly packaging, to attract youth to their products,” she said. “What is disappointing is that these companies do not use industry standard approaches, such as the alcohol industry, to age-gate their websites.”
It’s important for providers who care for adolescents to ask about substance use but to especially include questions about substances that teens might not think of as “drugs,” such as Delta 8, Dr. Moreno said.
“Prior research on other types of substance such as these has found that teens can think these are less dangerous versions of cannabis, so providing accurate information and asking about these products can prevent harm to kids,” Dr. Moreno said. Although this study focused on websites that sell D8 products, she said that “another important area of influence to consider is social media messaging around these products, which may drive traffic to the purchasing site.” It’s clear this industry is not going to self-regulate without policy changes, Dr. Moreno added, so she noted the importance of advocating for policy that regulates these sites.
Mr. Grewal, Dr. Milanaik and Dr. Moreno had no disclosures. No external funding sources were noted.
WASHINGTON – , researchers reported at the 2023 annual meeting of the American Academy of Pediatrics. Most of the products identified came in bright, colorful, kid-friendly packaging and cost less than $10, the researchers found, and only 2 out of 45 sites had a third-party age verification requirement for purchases.

Delta-8 THC, also called D8, is a synthetically produced cannabinoid whose chemical structure and effects are nearly identical to traditional THC, the authors explained, and past research has found that D8 products, such as e-cigarettes, can contain toxic byproducts and contaminants.
”Since D8 is not traditional THC, minors may underestimate its strength and potential danger,” wrote lead author Abhijeet Grewal, BS, a research assistant at Cohen Children’s Medical Center, New York, and senior author Ruth Milanaik, DO, director of the Neonatal Neurodevelopmental Program at Cohen Children’s and a developmental/behavioral pediatrician at Northwell Health, also in New York. “Although traditional THC is a federally banned substance, D8 is legal on a federal level and less restricted on a state by state basis, making it easier for individuals to acquire D8.”
Easily accessible
During the first seven moments of 2021, 77% of reports of accidental exposure occurred in people under age 18, including some children who required ICU admission. The U.S. Food and Drug Administration also received 104 reports of adverse events from products containing D8 between December 2020-February 2022, and more than half of those required medical intervention.
To better understand how easy it is to access D8, the authors collected data on 45 websites they identified that sold D8. The researchers looked for age verification questions for accessing the site, third-party age certification, what kinds of products (edibles, smoke products, or tinctures) were sold, the price and dosage of the cheapest product, and examples of packaging, flavors, marketing claims, and warning statements at each site.
More than a third of the sites (36%) did not ask for customers’ age and almost none of the sites asked for proof: 96% of the sites lacked formal third-party age verification procedures. All but one of the sites sold D8 edibles, and most (82%) sold D8 vaping or smoking products. Only 42% sold tinctures, a mix of concentrated D8 with oil that’s orally consumed.
The cheapest product was priced under $5 on one-third of the sites and under $10 on another third of the sites. The cheapest product was between $10-20 on 16% of the sites while the remaining nine sites’ cheapest product was more than $20. In assessing only the cheapest D8 products on each site, nearly half (47%) contained 51 mg or more of D8, and 20% of the products didn’t report the dosage. Another 22% contained 41-50 mg of D8, and the remaining five products contained 20-40 mg.
Kid-friendly D8
More than half of the D8 products were sold in kid-friendly packaging – packages with bright, colorful designs and fonts that resemble candy or snack food, sometimes cartoon characters or fun items like dice on the packaging. Further, 24% of the websites did not include any warnings or other health information about D8.
“The low prices, high dosages available, and eye-popping packaging make these products extremely attractive to teens who are looking for a high,” the researchers concluded. They advised clinicians to talk with teen patients about the dangers of D8 and advocated for policymakers to more strictly regulate online distributors of D8 products, particularly in requiring age verification procedures and prohibiting kid-friendly packaging.
Megan Moreno, MD, MSEd, MPH, an adolescent medicine physician and researcher at the University of Wisconsin, Madison, School of Medicine and Public Health and UWHealthKids, was particularly struck by how eye-catching the packaging was. “The bright colors and font choices are really designed to attract adolescents,” commented Dr. Moreno, who was not involved in the study. But she was not surprised overall by the findings.
“Other studies have found that the cannabis industry leverages online tools and social media, alongside youth-friendly packaging, to attract youth to their products,” she said. “What is disappointing is that these companies do not use industry standard approaches, such as the alcohol industry, to age-gate their websites.”
It’s important for providers who care for adolescents to ask about substance use but to especially include questions about substances that teens might not think of as “drugs,” such as Delta 8, Dr. Moreno said.
“Prior research on other types of substance such as these has found that teens can think these are less dangerous versions of cannabis, so providing accurate information and asking about these products can prevent harm to kids,” Dr. Moreno said. Although this study focused on websites that sell D8 products, she said that “another important area of influence to consider is social media messaging around these products, which may drive traffic to the purchasing site.” It’s clear this industry is not going to self-regulate without policy changes, Dr. Moreno added, so she noted the importance of advocating for policy that regulates these sites.
Mr. Grewal, Dr. Milanaik and Dr. Moreno had no disclosures. No external funding sources were noted.
At AAP 2023








