User login
FDA strengthens mammography regulations: Final rule
A final rule, updating the regulations issued under the Mammography Quality Standards Act of 1992, requires that mammography facilities notify patients about the density of their breasts, strengthens the FDA’s oversight of facilities, and provides guidance to help physicians better categorize and assess mammograms, according to a March 9 press release.
The rule requires implementation of the changes within 18 months.
According to the final rule document, the updates are “intended to improve the delivery of mammography services” in ways that reflect changes in mammography technology, quality standards, and the way results are categorized, reported, and communicated to patients and providers.
For instance, mammography reports must include an assessment of breast density to provide greater detail on the potential limitations of the mammogram results and allow patients and physicians to make more informed decisions, such as the possibility of additional imaging for women with dense breast tissue.
“Today’s action represents the agency’s broader commitment to support innovation to prevent, detect and treat cancer,” said Hilary Marston, MD, MPH, FDA’s chief medical officer, in the agency’s press release. The FDA remains “committed to advancing efforts to improve the health of women and strengthen the fight against breast cancer.”
A version of this article first appeared on Medscape.com.
A final rule, updating the regulations issued under the Mammography Quality Standards Act of 1992, requires that mammography facilities notify patients about the density of their breasts, strengthens the FDA’s oversight of facilities, and provides guidance to help physicians better categorize and assess mammograms, according to a March 9 press release.
The rule requires implementation of the changes within 18 months.
According to the final rule document, the updates are “intended to improve the delivery of mammography services” in ways that reflect changes in mammography technology, quality standards, and the way results are categorized, reported, and communicated to patients and providers.
For instance, mammography reports must include an assessment of breast density to provide greater detail on the potential limitations of the mammogram results and allow patients and physicians to make more informed decisions, such as the possibility of additional imaging for women with dense breast tissue.
“Today’s action represents the agency’s broader commitment to support innovation to prevent, detect and treat cancer,” said Hilary Marston, MD, MPH, FDA’s chief medical officer, in the agency’s press release. The FDA remains “committed to advancing efforts to improve the health of women and strengthen the fight against breast cancer.”
A version of this article first appeared on Medscape.com.
A final rule, updating the regulations issued under the Mammography Quality Standards Act of 1992, requires that mammography facilities notify patients about the density of their breasts, strengthens the FDA’s oversight of facilities, and provides guidance to help physicians better categorize and assess mammograms, according to a March 9 press release.
The rule requires implementation of the changes within 18 months.
According to the final rule document, the updates are “intended to improve the delivery of mammography services” in ways that reflect changes in mammography technology, quality standards, and the way results are categorized, reported, and communicated to patients and providers.
For instance, mammography reports must include an assessment of breast density to provide greater detail on the potential limitations of the mammogram results and allow patients and physicians to make more informed decisions, such as the possibility of additional imaging for women with dense breast tissue.
“Today’s action represents the agency’s broader commitment to support innovation to prevent, detect and treat cancer,” said Hilary Marston, MD, MPH, FDA’s chief medical officer, in the agency’s press release. The FDA remains “committed to advancing efforts to improve the health of women and strengthen the fight against breast cancer.”
A version of this article first appeared on Medscape.com.
Telehealth doctor indicted on health care fraud, opioid distribution charges
Sangita Patel, MD, 50, practiced at Advance Medical Home Physicians in Troy.
According to court documents, between July 2020 and June 2022 Patel was responsible for submitting Medicare claims for improper telehealth visits she didn’t conduct herself.
Dr. Patel, who accepted patients who paid in cash as well as those with Medicare and Medicaid coverage, billed approximately $3.4 million to Medicare between 2018 and 2022, according to court documents. An unusual number of these visits were billed using complex codes, an indication of health care fraud. The investigation also found that on many days, Dr. Patel billed for more than 24 hours of services. During this period, according to the document, 76% of Dr. Patel’s Medicare reimbursements were for telehealth.
Prosecutors say that Dr. Patel prescribed Schedule II controlled substances to more than 90% of the patients in these telehealth visits. She delegated her prescription authority to an unlicensed medical assistant. Through undercover visits and cell site search warrant data, the investigation found that Dr. Patel directed patients to contact, via cell phone, this assistant, who then entered electronic prescriptions into the electronic medical records system. Dr. Patel then signed the prescriptions and sent them to the pharmacies without ever interacting with the patients. Prosecutors also used text messages, obtained by search warrant, between Dr. Patel and her assistant and between the assistant and undercover informers to build their case.
Dr. Patel is also accused of referring patients to other providers, who in turn billed Medicare for claims associated with those patients. Advance Medical received $143,000 from these providers, potentially in violation of anti-kickback laws, according to bank records obtained by subpoena.
If convicted, Dr. Patel could be sentenced to up to 10 years in federal prison.
A version of this article first appeared on Medscape.com.
Sangita Patel, MD, 50, practiced at Advance Medical Home Physicians in Troy.
According to court documents, between July 2020 and June 2022 Patel was responsible for submitting Medicare claims for improper telehealth visits she didn’t conduct herself.
Dr. Patel, who accepted patients who paid in cash as well as those with Medicare and Medicaid coverage, billed approximately $3.4 million to Medicare between 2018 and 2022, according to court documents. An unusual number of these visits were billed using complex codes, an indication of health care fraud. The investigation also found that on many days, Dr. Patel billed for more than 24 hours of services. During this period, according to the document, 76% of Dr. Patel’s Medicare reimbursements were for telehealth.
Prosecutors say that Dr. Patel prescribed Schedule II controlled substances to more than 90% of the patients in these telehealth visits. She delegated her prescription authority to an unlicensed medical assistant. Through undercover visits and cell site search warrant data, the investigation found that Dr. Patel directed patients to contact, via cell phone, this assistant, who then entered electronic prescriptions into the electronic medical records system. Dr. Patel then signed the prescriptions and sent them to the pharmacies without ever interacting with the patients. Prosecutors also used text messages, obtained by search warrant, between Dr. Patel and her assistant and between the assistant and undercover informers to build their case.
Dr. Patel is also accused of referring patients to other providers, who in turn billed Medicare for claims associated with those patients. Advance Medical received $143,000 from these providers, potentially in violation of anti-kickback laws, according to bank records obtained by subpoena.
If convicted, Dr. Patel could be sentenced to up to 10 years in federal prison.
A version of this article first appeared on Medscape.com.
Sangita Patel, MD, 50, practiced at Advance Medical Home Physicians in Troy.
According to court documents, between July 2020 and June 2022 Patel was responsible for submitting Medicare claims for improper telehealth visits she didn’t conduct herself.
Dr. Patel, who accepted patients who paid in cash as well as those with Medicare and Medicaid coverage, billed approximately $3.4 million to Medicare between 2018 and 2022, according to court documents. An unusual number of these visits were billed using complex codes, an indication of health care fraud. The investigation also found that on many days, Dr. Patel billed for more than 24 hours of services. During this period, according to the document, 76% of Dr. Patel’s Medicare reimbursements were for telehealth.
Prosecutors say that Dr. Patel prescribed Schedule II controlled substances to more than 90% of the patients in these telehealth visits. She delegated her prescription authority to an unlicensed medical assistant. Through undercover visits and cell site search warrant data, the investigation found that Dr. Patel directed patients to contact, via cell phone, this assistant, who then entered electronic prescriptions into the electronic medical records system. Dr. Patel then signed the prescriptions and sent them to the pharmacies without ever interacting with the patients. Prosecutors also used text messages, obtained by search warrant, between Dr. Patel and her assistant and between the assistant and undercover informers to build their case.
Dr. Patel is also accused of referring patients to other providers, who in turn billed Medicare for claims associated with those patients. Advance Medical received $143,000 from these providers, potentially in violation of anti-kickback laws, according to bank records obtained by subpoena.
If convicted, Dr. Patel could be sentenced to up to 10 years in federal prison.
A version of this article first appeared on Medscape.com.
Specialty and age may contribute to suicidal thoughts among physicians
A physician’s specialty can make a difference when it comes to having suicidal thoughts. Doctors who specialize in family medicine, obstetrics-gynecology, and psychiatry reported double the rates of suicidal thoughts than doctors in oncology, rheumatology, and pulmonary medicine, according to Doctors’ Burden: Medscape Physician Suicide Report 2023.
“The specialties with the highest reporting of physician suicidal thoughts are also those with the greatest physician shortages, based on the number of job openings posted by recruiting sites,” said Peter Yellowlees, MD, professor of psychiatry and chief wellness officer at UC Davis Health.
Doctors in those specialties are overworked, which can lead to burnout, he said.
There’s also a generational divide among physicians who reported suicidal thoughts. Millennials (age 27-41) and Gen-X physicians (age 42-56) were more likely to report these thoughts than were Baby Boomers (age 57-75) and the Silent Generation (age 76-95).
“Younger physicians are more burned out – they may have less control over their lives and less meaning than some older doctors who can do what they want,” said Dr. Yellowlees.
One millennial respondent commented that being on call and being required to chart detailed notes in the EHR has contributed to her burnout. “I’m more impatient and make less time and effort to see my friends and family.”
One Silent Generation respondent commented, “I am semi-retired, I take no call, I work no weekends, I provide anesthesia care in my area of special expertise, I work clinically about 46 days a year. Life is good, particularly compared to my younger colleagues who are working 60-plus hours a week with evening work, weekend work, and call. I feel really sorry for them.”
When young people enter medical school, they’re quite healthy, with low rates of depression and burnout, said Dr. Yellowlees. Yet, studies have shown that rates of burnout and suicidal thoughts increased within 2 years. “That reflects what happens when a group of idealistic young people hit a horrible system,” he said.
Who’s responsible?
Millennials were three times as likely as baby boomers to say that a medical school or health care organization should be responsible when a student or physician commits suicide.
“Young physicians may expect more of their employers than my generation did, which we see in residency programs that have unionized,” said Dr. Yellowlees, a Baby Boomer.
“As more young doctors are employed by health care organizations, they also may expect more resources to be available to them, such as wellness programs,” he added.
Younger doctors also focus more on work-life balance than older doctors, including time off and having hobbies, he said. “They are much more rational in terms of their overall beliefs and expectations than the older generation.”
Whom doctors confide in
Nearly 60% of physician-respondents with suicidal thoughts said they confided in a professional or someone they knew. Men were just as likely as women to reach out to a therapist (38%), whereas men were slightly more likely to confide in a family member and women were slightly more likely to confide in a colleague.
“It’s interesting that women are more active in seeking support at work – they often have developed a network of colleagues to support each other’s careers and whom they can confide in,” said Dr. Yellowlees.
He emphasized that 40% of physicians said they didn’t confide in anyone when they had suicidal thoughts. Of those, just over half said they could cope without professional help.
One respondent commented, “It’s just a thought; nothing I would actually do.” Another commented, “Mental health professionals can’t fix the underlying reason for the problem.”
Many doctors were concerned about risking disclosure to their medical boards (42%); that it would show up on their insurance records (33%); and that their colleagues would find out (25%), according to the report.
One respondent commented, “I don’t trust doctors to keep it to themselves.”
Another barrier doctors mentioned was a lack of time to seek help. One commented, “Time. I have none, when am I supposed to find an hour for counseling?”
A version of this article originally appeared on Medscape.com.
A physician’s specialty can make a difference when it comes to having suicidal thoughts. Doctors who specialize in family medicine, obstetrics-gynecology, and psychiatry reported double the rates of suicidal thoughts than doctors in oncology, rheumatology, and pulmonary medicine, according to Doctors’ Burden: Medscape Physician Suicide Report 2023.
“The specialties with the highest reporting of physician suicidal thoughts are also those with the greatest physician shortages, based on the number of job openings posted by recruiting sites,” said Peter Yellowlees, MD, professor of psychiatry and chief wellness officer at UC Davis Health.
Doctors in those specialties are overworked, which can lead to burnout, he said.
There’s also a generational divide among physicians who reported suicidal thoughts. Millennials (age 27-41) and Gen-X physicians (age 42-56) were more likely to report these thoughts than were Baby Boomers (age 57-75) and the Silent Generation (age 76-95).
“Younger physicians are more burned out – they may have less control over their lives and less meaning than some older doctors who can do what they want,” said Dr. Yellowlees.
One millennial respondent commented that being on call and being required to chart detailed notes in the EHR has contributed to her burnout. “I’m more impatient and make less time and effort to see my friends and family.”
One Silent Generation respondent commented, “I am semi-retired, I take no call, I work no weekends, I provide anesthesia care in my area of special expertise, I work clinically about 46 days a year. Life is good, particularly compared to my younger colleagues who are working 60-plus hours a week with evening work, weekend work, and call. I feel really sorry for them.”
When young people enter medical school, they’re quite healthy, with low rates of depression and burnout, said Dr. Yellowlees. Yet, studies have shown that rates of burnout and suicidal thoughts increased within 2 years. “That reflects what happens when a group of idealistic young people hit a horrible system,” he said.
Who’s responsible?
Millennials were three times as likely as baby boomers to say that a medical school or health care organization should be responsible when a student or physician commits suicide.
“Young physicians may expect more of their employers than my generation did, which we see in residency programs that have unionized,” said Dr. Yellowlees, a Baby Boomer.
“As more young doctors are employed by health care organizations, they also may expect more resources to be available to them, such as wellness programs,” he added.
Younger doctors also focus more on work-life balance than older doctors, including time off and having hobbies, he said. “They are much more rational in terms of their overall beliefs and expectations than the older generation.”
Whom doctors confide in
Nearly 60% of physician-respondents with suicidal thoughts said they confided in a professional or someone they knew. Men were just as likely as women to reach out to a therapist (38%), whereas men were slightly more likely to confide in a family member and women were slightly more likely to confide in a colleague.
“It’s interesting that women are more active in seeking support at work – they often have developed a network of colleagues to support each other’s careers and whom they can confide in,” said Dr. Yellowlees.
He emphasized that 40% of physicians said they didn’t confide in anyone when they had suicidal thoughts. Of those, just over half said they could cope without professional help.
One respondent commented, “It’s just a thought; nothing I would actually do.” Another commented, “Mental health professionals can’t fix the underlying reason for the problem.”
Many doctors were concerned about risking disclosure to their medical boards (42%); that it would show up on their insurance records (33%); and that their colleagues would find out (25%), according to the report.
One respondent commented, “I don’t trust doctors to keep it to themselves.”
Another barrier doctors mentioned was a lack of time to seek help. One commented, “Time. I have none, when am I supposed to find an hour for counseling?”
A version of this article originally appeared on Medscape.com.
A physician’s specialty can make a difference when it comes to having suicidal thoughts. Doctors who specialize in family medicine, obstetrics-gynecology, and psychiatry reported double the rates of suicidal thoughts than doctors in oncology, rheumatology, and pulmonary medicine, according to Doctors’ Burden: Medscape Physician Suicide Report 2023.
“The specialties with the highest reporting of physician suicidal thoughts are also those with the greatest physician shortages, based on the number of job openings posted by recruiting sites,” said Peter Yellowlees, MD, professor of psychiatry and chief wellness officer at UC Davis Health.
Doctors in those specialties are overworked, which can lead to burnout, he said.
There’s also a generational divide among physicians who reported suicidal thoughts. Millennials (age 27-41) and Gen-X physicians (age 42-56) were more likely to report these thoughts than were Baby Boomers (age 57-75) and the Silent Generation (age 76-95).
“Younger physicians are more burned out – they may have less control over their lives and less meaning than some older doctors who can do what they want,” said Dr. Yellowlees.
One millennial respondent commented that being on call and being required to chart detailed notes in the EHR has contributed to her burnout. “I’m more impatient and make less time and effort to see my friends and family.”
One Silent Generation respondent commented, “I am semi-retired, I take no call, I work no weekends, I provide anesthesia care in my area of special expertise, I work clinically about 46 days a year. Life is good, particularly compared to my younger colleagues who are working 60-plus hours a week with evening work, weekend work, and call. I feel really sorry for them.”
When young people enter medical school, they’re quite healthy, with low rates of depression and burnout, said Dr. Yellowlees. Yet, studies have shown that rates of burnout and suicidal thoughts increased within 2 years. “That reflects what happens when a group of idealistic young people hit a horrible system,” he said.
Who’s responsible?
Millennials were three times as likely as baby boomers to say that a medical school or health care organization should be responsible when a student or physician commits suicide.
“Young physicians may expect more of their employers than my generation did, which we see in residency programs that have unionized,” said Dr. Yellowlees, a Baby Boomer.
“As more young doctors are employed by health care organizations, they also may expect more resources to be available to them, such as wellness programs,” he added.
Younger doctors also focus more on work-life balance than older doctors, including time off and having hobbies, he said. “They are much more rational in terms of their overall beliefs and expectations than the older generation.”
Whom doctors confide in
Nearly 60% of physician-respondents with suicidal thoughts said they confided in a professional or someone they knew. Men were just as likely as women to reach out to a therapist (38%), whereas men were slightly more likely to confide in a family member and women were slightly more likely to confide in a colleague.
“It’s interesting that women are more active in seeking support at work – they often have developed a network of colleagues to support each other’s careers and whom they can confide in,” said Dr. Yellowlees.
He emphasized that 40% of physicians said they didn’t confide in anyone when they had suicidal thoughts. Of those, just over half said they could cope without professional help.
One respondent commented, “It’s just a thought; nothing I would actually do.” Another commented, “Mental health professionals can’t fix the underlying reason for the problem.”
Many doctors were concerned about risking disclosure to their medical boards (42%); that it would show up on their insurance records (33%); and that their colleagues would find out (25%), according to the report.
One respondent commented, “I don’t trust doctors to keep it to themselves.”
Another barrier doctors mentioned was a lack of time to seek help. One commented, “Time. I have none, when am I supposed to find an hour for counseling?”
A version of this article originally appeared on Medscape.com.
Popular book by USC oncologist pulled because of plagiarism
The Los Angeles Times reported earlier this week that it identified at least 95 instances of plagiarism by author David B. Agus, MD, in “The Book of Animal Secrets: Nature’s Lessons for a Long and Happy Life.”
According to the LA Times, Dr. Agus copied passages from numerous sources, including The New York Times, National Geographic, Wikipedia, and smaller niche sites. Some instances involved a sentence or two; others involved multiparagraph, word-for-word copying without attribution.
The book by Dr. Agus – who interviews celebrities for a health-related miniseries on Paramount Plus – had reached the top spot on Amazon’s list of best-selling books about animals a week before its planned March 7 release.
Publisher Simon & Schuster released a statement announcing a recall of the book at Dr. Agus’ expense “until a fully revised and corrected edition can be released.”
Dr. Agus included his own statement apologizing “to the scientists and writers whose work or words were used or not fully attributed,” and said he will “rewrite the passages in question with new language, will provide proper and full attribution, and when ready will announce a new publication date.”
“Writers should always be credited for their work, and I deeply regret these mistakes and the lack of rigor in finalizing the book,” he stated, adding that “[t]his book contains important lessons, messages, and guidance about health that I wanted to convey to the readers. I do not want these mistakes to interfere with that effort.”
A version of this article first appeared on Medscape.com.
The Los Angeles Times reported earlier this week that it identified at least 95 instances of plagiarism by author David B. Agus, MD, in “The Book of Animal Secrets: Nature’s Lessons for a Long and Happy Life.”
According to the LA Times, Dr. Agus copied passages from numerous sources, including The New York Times, National Geographic, Wikipedia, and smaller niche sites. Some instances involved a sentence or two; others involved multiparagraph, word-for-word copying without attribution.
The book by Dr. Agus – who interviews celebrities for a health-related miniseries on Paramount Plus – had reached the top spot on Amazon’s list of best-selling books about animals a week before its planned March 7 release.
Publisher Simon & Schuster released a statement announcing a recall of the book at Dr. Agus’ expense “until a fully revised and corrected edition can be released.”
Dr. Agus included his own statement apologizing “to the scientists and writers whose work or words were used or not fully attributed,” and said he will “rewrite the passages in question with new language, will provide proper and full attribution, and when ready will announce a new publication date.”
“Writers should always be credited for their work, and I deeply regret these mistakes and the lack of rigor in finalizing the book,” he stated, adding that “[t]his book contains important lessons, messages, and guidance about health that I wanted to convey to the readers. I do not want these mistakes to interfere with that effort.”
A version of this article first appeared on Medscape.com.
The Los Angeles Times reported earlier this week that it identified at least 95 instances of plagiarism by author David B. Agus, MD, in “The Book of Animal Secrets: Nature’s Lessons for a Long and Happy Life.”
According to the LA Times, Dr. Agus copied passages from numerous sources, including The New York Times, National Geographic, Wikipedia, and smaller niche sites. Some instances involved a sentence or two; others involved multiparagraph, word-for-word copying without attribution.
The book by Dr. Agus – who interviews celebrities for a health-related miniseries on Paramount Plus – had reached the top spot on Amazon’s list of best-selling books about animals a week before its planned March 7 release.
Publisher Simon & Schuster released a statement announcing a recall of the book at Dr. Agus’ expense “until a fully revised and corrected edition can be released.”
Dr. Agus included his own statement apologizing “to the scientists and writers whose work or words were used or not fully attributed,” and said he will “rewrite the passages in question with new language, will provide proper and full attribution, and when ready will announce a new publication date.”
“Writers should always be credited for their work, and I deeply regret these mistakes and the lack of rigor in finalizing the book,” he stated, adding that “[t]his book contains important lessons, messages, and guidance about health that I wanted to convey to the readers. I do not want these mistakes to interfere with that effort.”
A version of this article first appeared on Medscape.com.
HER2-low breast cancer is not a separate clinical entity: Study
Much attention has been focused recently on the idea that breast cancer with a low expression of HER2 can be treated with HER2-targeted agents. Not surprisingly, manufacturers of these drugs have pounced on this idea, as it opens up a whole new patient population: previously these drugs were only for tumors with a high HER2 expression.
There is a large potential market at stake: HER2-low (also referred to as ERBB2-low), as defined by a score of 0 to 3+ on immunohistochemistry (IHC), is seen in approximately 50%-60% of all breast cancers
However, The authors argued that it actually it represents a series of biological differences from HER2-negative disease that do not have a strong bearing on outcomes.
The analysis was published online in JAMA Oncology.
For the study, researchers from the University of Chicago examined data on more than 1.1 million breast cancer patients recorded as HER2-negative in the U.S. National Cancer Database, and re-classified almost two thirds as HER2-low on further analysis.
They found that HER2-low status was associated with higher estrogen receptor (ER) expression, as well as a lower rate of pathologic complete response, compared with HER2-negative disease. It was also linked to an improvement in overall survival on multivariate analysis of up to 9% in advance stage triple negative tumors.
“However, the clinical significance of these differences is questionable,” the researchers commented.
HER2-low status alone “should not influence neoadjuvant treatment decisions with currently approved regimens,” they added.
These results “do not support classification of HER2-low breast cancer as a distinct clinical subtype,” the team concluded.
Not necessarily, according to Giuseppe Curigliano, MD, PhD, director of the new drugs and early drug development for innovative therapies division at the European Institute of Oncology, Milan. He argued the opposite case, that HER2-low is a separate clinical entity, in a recent debate on the topic held at the 2022 San Antonio Breast Cancer Symposium.
In a comment, Dr. Curigliano said that a “major strength” of the current study is its large patient cohort, which reflects the majority of cancer diagnoses in the United States, but that it nevertheless has “important limitations that should be considered when interpreting the results.”
The inclusion of only overall survival in the dataset limits the ability to make associations between HER2-low status and cancer-specific prognosis, as “survival may lag years behind recurrence.”
The lack of centralized assessment of IHC results is also an issue, as “some of the results may be associated with regional variation in practice of classifying cases as HER2 0 versus HER2 1+.”
“In my opinion, this is a great limitation,” he said, in being able to conclude that there is “no prognostic difference between ERBB2-low and -negative patients.”
He also noted that, from a molecular point of view, “the key determinant of the gene expression profile is the expression of hormone receptors, [and] if we perform a correction for hormone receptor expression, only marginal differences in gene expression are found” between HER2-low and HER2-negative tumors.
“Similarly, large genomic studies have identified no specific and consistent difference in genomic profiles,” Dr. Curigliano said, and so, HER2-low disease, “as currently defined, should not be considered a distinct molecular entity, but rather a heterogeneous group of tumors, with biology primarily driven by hormone receptor expression.”
Analysis quoted by both sides
Senior author of the new analysis, Frederick M. Howard, MD, from the section of hematology-oncology in the department of medicine at the University of Chicago, said that his team’s work was quoted by both sides of the debate at SABCS 2022.
This reflects the fact that, while differences between ERBB2-low and -negative are present, it is “questionable how clinically significant those differences are,” he said in an interview. It’s a matter of “the eye of the beholder.”
Dr. Howard does not think that clinicians are going to modify standard treatment regimens based solely on HER2-low status, and that low expression of the protein “is probably just a reflection of some underlying biologic processes.”
Dr. Howard agreed with Dr. Curigliano that a “caveat” of their study is that the IHC analyses were performed locally, especially as it has shown that there can be discordance between pathologists in around 40% of cases, and that the associations they found might therefore be “strengthened” by more precise quantification of HER2 expression.
“But, even then,” Dr. Howard continued, “I doubt that [ERBB2-low status] is going to be that strong a prognostic factor.”
He believes that advances in analytic techniques will, in the future, allow tumors to be characterized more precisely, and it may be that HER2-low tumors end up being called something else in 5 years.
Renewed interest in this subgroup
In their paper, Dr. Howard and colleagues pointed out that, as a group, HER2-low tumors are heterogeneous, with HER2-low found in both hormone receptor–positive and triple-negative breast cancers. Also, various studies have come to different conclusions about what HER2 low means prognostically, with conclusions ranging from negative to neutral and to positive prognoses.
However, new research has shown that patients with HER2-low tumors can benefit from HER2-targeted drugs. In particular, the recent report that the antibody-drug conjugate trastuzumab deruxtecan doubled progression-free survival versus chemotherapy in ERBB2-low tumors led to “renewed interest in the subgroup,” the researchers noted.
To examine this subgroup further, they embarked on their analysis. Examining the National Cancer Database, they gathered information on more than 1.1 million U.S. patients diagnosed with HER2-negative invasive breast cancer in the 10-year period 2010-2019, and for whom IHC results were available.
The patients were reclassified as having HER2-low disease if they had an IHC score of 1+, or 2+ with a negative in situ hybridization test, while those with an IHC score of 0 were deemed to be HER2-negative. They were followed up until Nov. 30, 2022.
These patients had a mean age of 62.4 years, and 99.1% were female. The majority (78.6%) were non-Hispanic white. HER2-low was identified in 65.5% of the cohort, while 34.5% were HER2-negative.
The proportion of HER2-low disease was lower in non-Hispanic black (62.8%) and Hispanic (61%) patients than in non-Hispanic White patients, at 66.1%.
HER2-low disease was also more common in hormone receptor–positive than triple-negative tumors, at just 51.5%, rising to 58.6% for progesterone receptor–positive, ER-negative, and 69.1% for PR-positive, ER-positive tumors.
Multivariate logistic regression analysis taking into account age, sex, race and ethnicity, comorbidity score, and treatment facility type, among other factors, revealed that the likelihood of HER2-low disease was significantly with increased ER expression, at an adjusted odds ratio of 1.15 for each 10% increase (P < .001).
Non-Hispanic Black, Asian, and Pacific Islander patients had similar rates of HER2-low disease as non-Hispanic White patients after adjustment, whereas Native American patients had an increased rate, at an aOR of 1.22 (P < .001), and Hispanic patients had a lower rate, at an aOR of 0.85 (P < .001).
HER2-low status was associated with a slightly reduced likelihood of having a pathologic complete response (aOR, 0.89; P < .001), with similar results when restricting the analysis to patients with triple negative or hormone receptor-positive tumors.
After a median follow-up of 54 months, HER2-low disease was associated with a minor improvement in survival on multivariate analysis, at an adjusted hazard ratio for death of 0.98 (P < .001).
The greatest improvement in survival was seen in patients with stage III and IV triple-negative breast cancer, at HRs of 0.92 and 0.91, respectively, although the researchers noted that this represented only a 2% and 0.4% improvement in 5-year overall survival, respectively.
The study was supported by the Breast Cancer Research Foundation, the American Society of Clinical Oncology/Conquer Cancer Foundation, the Department of Defense, the National Institutes of Health/National Cancer Institute, Susan G. Komen, the Breast Cancer Research Foundation, and the University of Chicago Elwood V. Jensen Scholars Program. Dr. Howard reported no relevant financial relationships. Dr. Curigliano has relationships with Pfizer, Novartis, Lilly, Roche, Seattle Genetics, Celltrion, Veracyte, Daiichi Sankyo, AstraZeneca, Merck, Seagen, Exact Sciences, Gilead, Bristol-Myers Squibb, Scientific Affairs Group, and Ellipsis.
A version of this article first appeared on Medscape.com.
Much attention has been focused recently on the idea that breast cancer with a low expression of HER2 can be treated with HER2-targeted agents. Not surprisingly, manufacturers of these drugs have pounced on this idea, as it opens up a whole new patient population: previously these drugs were only for tumors with a high HER2 expression.
There is a large potential market at stake: HER2-low (also referred to as ERBB2-low), as defined by a score of 0 to 3+ on immunohistochemistry (IHC), is seen in approximately 50%-60% of all breast cancers
However, The authors argued that it actually it represents a series of biological differences from HER2-negative disease that do not have a strong bearing on outcomes.
The analysis was published online in JAMA Oncology.
For the study, researchers from the University of Chicago examined data on more than 1.1 million breast cancer patients recorded as HER2-negative in the U.S. National Cancer Database, and re-classified almost two thirds as HER2-low on further analysis.
They found that HER2-low status was associated with higher estrogen receptor (ER) expression, as well as a lower rate of pathologic complete response, compared with HER2-negative disease. It was also linked to an improvement in overall survival on multivariate analysis of up to 9% in advance stage triple negative tumors.
“However, the clinical significance of these differences is questionable,” the researchers commented.
HER2-low status alone “should not influence neoadjuvant treatment decisions with currently approved regimens,” they added.
These results “do not support classification of HER2-low breast cancer as a distinct clinical subtype,” the team concluded.
Not necessarily, according to Giuseppe Curigliano, MD, PhD, director of the new drugs and early drug development for innovative therapies division at the European Institute of Oncology, Milan. He argued the opposite case, that HER2-low is a separate clinical entity, in a recent debate on the topic held at the 2022 San Antonio Breast Cancer Symposium.
In a comment, Dr. Curigliano said that a “major strength” of the current study is its large patient cohort, which reflects the majority of cancer diagnoses in the United States, but that it nevertheless has “important limitations that should be considered when interpreting the results.”
The inclusion of only overall survival in the dataset limits the ability to make associations between HER2-low status and cancer-specific prognosis, as “survival may lag years behind recurrence.”
The lack of centralized assessment of IHC results is also an issue, as “some of the results may be associated with regional variation in practice of classifying cases as HER2 0 versus HER2 1+.”
“In my opinion, this is a great limitation,” he said, in being able to conclude that there is “no prognostic difference between ERBB2-low and -negative patients.”
He also noted that, from a molecular point of view, “the key determinant of the gene expression profile is the expression of hormone receptors, [and] if we perform a correction for hormone receptor expression, only marginal differences in gene expression are found” between HER2-low and HER2-negative tumors.
“Similarly, large genomic studies have identified no specific and consistent difference in genomic profiles,” Dr. Curigliano said, and so, HER2-low disease, “as currently defined, should not be considered a distinct molecular entity, but rather a heterogeneous group of tumors, with biology primarily driven by hormone receptor expression.”
Analysis quoted by both sides
Senior author of the new analysis, Frederick M. Howard, MD, from the section of hematology-oncology in the department of medicine at the University of Chicago, said that his team’s work was quoted by both sides of the debate at SABCS 2022.
This reflects the fact that, while differences between ERBB2-low and -negative are present, it is “questionable how clinically significant those differences are,” he said in an interview. It’s a matter of “the eye of the beholder.”
Dr. Howard does not think that clinicians are going to modify standard treatment regimens based solely on HER2-low status, and that low expression of the protein “is probably just a reflection of some underlying biologic processes.”
Dr. Howard agreed with Dr. Curigliano that a “caveat” of their study is that the IHC analyses were performed locally, especially as it has shown that there can be discordance between pathologists in around 40% of cases, and that the associations they found might therefore be “strengthened” by more precise quantification of HER2 expression.
“But, even then,” Dr. Howard continued, “I doubt that [ERBB2-low status] is going to be that strong a prognostic factor.”
He believes that advances in analytic techniques will, in the future, allow tumors to be characterized more precisely, and it may be that HER2-low tumors end up being called something else in 5 years.
Renewed interest in this subgroup
In their paper, Dr. Howard and colleagues pointed out that, as a group, HER2-low tumors are heterogeneous, with HER2-low found in both hormone receptor–positive and triple-negative breast cancers. Also, various studies have come to different conclusions about what HER2 low means prognostically, with conclusions ranging from negative to neutral and to positive prognoses.
However, new research has shown that patients with HER2-low tumors can benefit from HER2-targeted drugs. In particular, the recent report that the antibody-drug conjugate trastuzumab deruxtecan doubled progression-free survival versus chemotherapy in ERBB2-low tumors led to “renewed interest in the subgroup,” the researchers noted.
To examine this subgroup further, they embarked on their analysis. Examining the National Cancer Database, they gathered information on more than 1.1 million U.S. patients diagnosed with HER2-negative invasive breast cancer in the 10-year period 2010-2019, and for whom IHC results were available.
The patients were reclassified as having HER2-low disease if they had an IHC score of 1+, or 2+ with a negative in situ hybridization test, while those with an IHC score of 0 were deemed to be HER2-negative. They were followed up until Nov. 30, 2022.
These patients had a mean age of 62.4 years, and 99.1% were female. The majority (78.6%) were non-Hispanic white. HER2-low was identified in 65.5% of the cohort, while 34.5% were HER2-negative.
The proportion of HER2-low disease was lower in non-Hispanic black (62.8%) and Hispanic (61%) patients than in non-Hispanic White patients, at 66.1%.
HER2-low disease was also more common in hormone receptor–positive than triple-negative tumors, at just 51.5%, rising to 58.6% for progesterone receptor–positive, ER-negative, and 69.1% for PR-positive, ER-positive tumors.
Multivariate logistic regression analysis taking into account age, sex, race and ethnicity, comorbidity score, and treatment facility type, among other factors, revealed that the likelihood of HER2-low disease was significantly with increased ER expression, at an adjusted odds ratio of 1.15 for each 10% increase (P < .001).
Non-Hispanic Black, Asian, and Pacific Islander patients had similar rates of HER2-low disease as non-Hispanic White patients after adjustment, whereas Native American patients had an increased rate, at an aOR of 1.22 (P < .001), and Hispanic patients had a lower rate, at an aOR of 0.85 (P < .001).
HER2-low status was associated with a slightly reduced likelihood of having a pathologic complete response (aOR, 0.89; P < .001), with similar results when restricting the analysis to patients with triple negative or hormone receptor-positive tumors.
After a median follow-up of 54 months, HER2-low disease was associated with a minor improvement in survival on multivariate analysis, at an adjusted hazard ratio for death of 0.98 (P < .001).
The greatest improvement in survival was seen in patients with stage III and IV triple-negative breast cancer, at HRs of 0.92 and 0.91, respectively, although the researchers noted that this represented only a 2% and 0.4% improvement in 5-year overall survival, respectively.
The study was supported by the Breast Cancer Research Foundation, the American Society of Clinical Oncology/Conquer Cancer Foundation, the Department of Defense, the National Institutes of Health/National Cancer Institute, Susan G. Komen, the Breast Cancer Research Foundation, and the University of Chicago Elwood V. Jensen Scholars Program. Dr. Howard reported no relevant financial relationships. Dr. Curigliano has relationships with Pfizer, Novartis, Lilly, Roche, Seattle Genetics, Celltrion, Veracyte, Daiichi Sankyo, AstraZeneca, Merck, Seagen, Exact Sciences, Gilead, Bristol-Myers Squibb, Scientific Affairs Group, and Ellipsis.
A version of this article first appeared on Medscape.com.
Much attention has been focused recently on the idea that breast cancer with a low expression of HER2 can be treated with HER2-targeted agents. Not surprisingly, manufacturers of these drugs have pounced on this idea, as it opens up a whole new patient population: previously these drugs were only for tumors with a high HER2 expression.
There is a large potential market at stake: HER2-low (also referred to as ERBB2-low), as defined by a score of 0 to 3+ on immunohistochemistry (IHC), is seen in approximately 50%-60% of all breast cancers
However, The authors argued that it actually it represents a series of biological differences from HER2-negative disease that do not have a strong bearing on outcomes.
The analysis was published online in JAMA Oncology.
For the study, researchers from the University of Chicago examined data on more than 1.1 million breast cancer patients recorded as HER2-negative in the U.S. National Cancer Database, and re-classified almost two thirds as HER2-low on further analysis.
They found that HER2-low status was associated with higher estrogen receptor (ER) expression, as well as a lower rate of pathologic complete response, compared with HER2-negative disease. It was also linked to an improvement in overall survival on multivariate analysis of up to 9% in advance stage triple negative tumors.
“However, the clinical significance of these differences is questionable,” the researchers commented.
HER2-low status alone “should not influence neoadjuvant treatment decisions with currently approved regimens,” they added.
These results “do not support classification of HER2-low breast cancer as a distinct clinical subtype,” the team concluded.
Not necessarily, according to Giuseppe Curigliano, MD, PhD, director of the new drugs and early drug development for innovative therapies division at the European Institute of Oncology, Milan. He argued the opposite case, that HER2-low is a separate clinical entity, in a recent debate on the topic held at the 2022 San Antonio Breast Cancer Symposium.
In a comment, Dr. Curigliano said that a “major strength” of the current study is its large patient cohort, which reflects the majority of cancer diagnoses in the United States, but that it nevertheless has “important limitations that should be considered when interpreting the results.”
The inclusion of only overall survival in the dataset limits the ability to make associations between HER2-low status and cancer-specific prognosis, as “survival may lag years behind recurrence.”
The lack of centralized assessment of IHC results is also an issue, as “some of the results may be associated with regional variation in practice of classifying cases as HER2 0 versus HER2 1+.”
“In my opinion, this is a great limitation,” he said, in being able to conclude that there is “no prognostic difference between ERBB2-low and -negative patients.”
He also noted that, from a molecular point of view, “the key determinant of the gene expression profile is the expression of hormone receptors, [and] if we perform a correction for hormone receptor expression, only marginal differences in gene expression are found” between HER2-low and HER2-negative tumors.
“Similarly, large genomic studies have identified no specific and consistent difference in genomic profiles,” Dr. Curigliano said, and so, HER2-low disease, “as currently defined, should not be considered a distinct molecular entity, but rather a heterogeneous group of tumors, with biology primarily driven by hormone receptor expression.”
Analysis quoted by both sides
Senior author of the new analysis, Frederick M. Howard, MD, from the section of hematology-oncology in the department of medicine at the University of Chicago, said that his team’s work was quoted by both sides of the debate at SABCS 2022.
This reflects the fact that, while differences between ERBB2-low and -negative are present, it is “questionable how clinically significant those differences are,” he said in an interview. It’s a matter of “the eye of the beholder.”
Dr. Howard does not think that clinicians are going to modify standard treatment regimens based solely on HER2-low status, and that low expression of the protein “is probably just a reflection of some underlying biologic processes.”
Dr. Howard agreed with Dr. Curigliano that a “caveat” of their study is that the IHC analyses were performed locally, especially as it has shown that there can be discordance between pathologists in around 40% of cases, and that the associations they found might therefore be “strengthened” by more precise quantification of HER2 expression.
“But, even then,” Dr. Howard continued, “I doubt that [ERBB2-low status] is going to be that strong a prognostic factor.”
He believes that advances in analytic techniques will, in the future, allow tumors to be characterized more precisely, and it may be that HER2-low tumors end up being called something else in 5 years.
Renewed interest in this subgroup
In their paper, Dr. Howard and colleagues pointed out that, as a group, HER2-low tumors are heterogeneous, with HER2-low found in both hormone receptor–positive and triple-negative breast cancers. Also, various studies have come to different conclusions about what HER2 low means prognostically, with conclusions ranging from negative to neutral and to positive prognoses.
However, new research has shown that patients with HER2-low tumors can benefit from HER2-targeted drugs. In particular, the recent report that the antibody-drug conjugate trastuzumab deruxtecan doubled progression-free survival versus chemotherapy in ERBB2-low tumors led to “renewed interest in the subgroup,” the researchers noted.
To examine this subgroup further, they embarked on their analysis. Examining the National Cancer Database, they gathered information on more than 1.1 million U.S. patients diagnosed with HER2-negative invasive breast cancer in the 10-year period 2010-2019, and for whom IHC results were available.
The patients were reclassified as having HER2-low disease if they had an IHC score of 1+, or 2+ with a negative in situ hybridization test, while those with an IHC score of 0 were deemed to be HER2-negative. They were followed up until Nov. 30, 2022.
These patients had a mean age of 62.4 years, and 99.1% were female. The majority (78.6%) were non-Hispanic white. HER2-low was identified in 65.5% of the cohort, while 34.5% were HER2-negative.
The proportion of HER2-low disease was lower in non-Hispanic black (62.8%) and Hispanic (61%) patients than in non-Hispanic White patients, at 66.1%.
HER2-low disease was also more common in hormone receptor–positive than triple-negative tumors, at just 51.5%, rising to 58.6% for progesterone receptor–positive, ER-negative, and 69.1% for PR-positive, ER-positive tumors.
Multivariate logistic regression analysis taking into account age, sex, race and ethnicity, comorbidity score, and treatment facility type, among other factors, revealed that the likelihood of HER2-low disease was significantly with increased ER expression, at an adjusted odds ratio of 1.15 for each 10% increase (P < .001).
Non-Hispanic Black, Asian, and Pacific Islander patients had similar rates of HER2-low disease as non-Hispanic White patients after adjustment, whereas Native American patients had an increased rate, at an aOR of 1.22 (P < .001), and Hispanic patients had a lower rate, at an aOR of 0.85 (P < .001).
HER2-low status was associated with a slightly reduced likelihood of having a pathologic complete response (aOR, 0.89; P < .001), with similar results when restricting the analysis to patients with triple negative or hormone receptor-positive tumors.
After a median follow-up of 54 months, HER2-low disease was associated with a minor improvement in survival on multivariate analysis, at an adjusted hazard ratio for death of 0.98 (P < .001).
The greatest improvement in survival was seen in patients with stage III and IV triple-negative breast cancer, at HRs of 0.92 and 0.91, respectively, although the researchers noted that this represented only a 2% and 0.4% improvement in 5-year overall survival, respectively.
The study was supported by the Breast Cancer Research Foundation, the American Society of Clinical Oncology/Conquer Cancer Foundation, the Department of Defense, the National Institutes of Health/National Cancer Institute, Susan G. Komen, the Breast Cancer Research Foundation, and the University of Chicago Elwood V. Jensen Scholars Program. Dr. Howard reported no relevant financial relationships. Dr. Curigliano has relationships with Pfizer, Novartis, Lilly, Roche, Seattle Genetics, Celltrion, Veracyte, Daiichi Sankyo, AstraZeneca, Merck, Seagen, Exact Sciences, Gilead, Bristol-Myers Squibb, Scientific Affairs Group, and Ellipsis.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
FDA expands abemaciclib use in high-risk early breast cancer
Abemaciclib was previously approved for this group of high-risk patients with the requirement that they have a Ki-67 score of at least 20%. The new expansion removes the Ki-67 testing requirement, meaning more patients are now eligible to receive this drug. High-risk patients eligible for the CDK4/6 inhibitor can now be identified solely on the basis of nodal status, tumor size, and tumor grade.
The FDA’s decision to expand the approval was based on 4-year data from the phase 3 monarchE trial of adjuvant abemaciclib, which showed benefit in invasive disease-free survival beyond the 2-year treatment course.
At 4 years, 85.5% of patients remained recurrence free with abemaciclib plus endocrine therapy, compared with 78.6% who received endocrine therapy alone, an absolute difference in invasive disease-free survival of 6.9%.
“The initial Verzenio FDA approval in early breast cancer was practice changing and now, through this indication expansion, we have the potential to reduce the risk of breast cancer recurrence for many more patients, relying solely on commonly utilized clinicopathologic features to identify them,” Erika P. Hamilton, MD, an investigator on the monarchE clinical trial, said in a press release.
A version of this article first appeared on Medscape.com.
Abemaciclib was previously approved for this group of high-risk patients with the requirement that they have a Ki-67 score of at least 20%. The new expansion removes the Ki-67 testing requirement, meaning more patients are now eligible to receive this drug. High-risk patients eligible for the CDK4/6 inhibitor can now be identified solely on the basis of nodal status, tumor size, and tumor grade.
The FDA’s decision to expand the approval was based on 4-year data from the phase 3 monarchE trial of adjuvant abemaciclib, which showed benefit in invasive disease-free survival beyond the 2-year treatment course.
At 4 years, 85.5% of patients remained recurrence free with abemaciclib plus endocrine therapy, compared with 78.6% who received endocrine therapy alone, an absolute difference in invasive disease-free survival of 6.9%.
“The initial Verzenio FDA approval in early breast cancer was practice changing and now, through this indication expansion, we have the potential to reduce the risk of breast cancer recurrence for many more patients, relying solely on commonly utilized clinicopathologic features to identify them,” Erika P. Hamilton, MD, an investigator on the monarchE clinical trial, said in a press release.
A version of this article first appeared on Medscape.com.
Abemaciclib was previously approved for this group of high-risk patients with the requirement that they have a Ki-67 score of at least 20%. The new expansion removes the Ki-67 testing requirement, meaning more patients are now eligible to receive this drug. High-risk patients eligible for the CDK4/6 inhibitor can now be identified solely on the basis of nodal status, tumor size, and tumor grade.
The FDA’s decision to expand the approval was based on 4-year data from the phase 3 monarchE trial of adjuvant abemaciclib, which showed benefit in invasive disease-free survival beyond the 2-year treatment course.
At 4 years, 85.5% of patients remained recurrence free with abemaciclib plus endocrine therapy, compared with 78.6% who received endocrine therapy alone, an absolute difference in invasive disease-free survival of 6.9%.
“The initial Verzenio FDA approval in early breast cancer was practice changing and now, through this indication expansion, we have the potential to reduce the risk of breast cancer recurrence for many more patients, relying solely on commonly utilized clinicopathologic features to identify them,” Erika P. Hamilton, MD, an investigator on the monarchE clinical trial, said in a press release.
A version of this article first appeared on Medscape.com.
Med center and top cardio surgeon must pay $8.5 million for fraud, concurrent surgeries
the Department of Justice (DOJ) announced.
The lawsuit alleges that James L. Luketich, MD, the longtime chair of the school’s cardiothoracic surgery department, regularly performed up to three complex surgical procedures simultaneously, moving among multiple operating rooms and attending to matters other than patient care. The investigation began after Jonathan D’Cunha, MD, a former UPMC surgeon, raised concerns about his colleague’s surgical scheduling and billing practices.
Dr. Luketich’s overbooking of procedures led to patients enduring hours of medically unnecessary anesthesia time and risking surgical complications, according to court documents.
In addition, the complaint states that these practices violated the False Claims Act, which prohibits “teaching physicians” like Dr. Luketich from billing Medicare and other government health plans for “concurrent surgeries” – regulations federal authorities say UPMC leadership were aware of and the University of Pittsburgh Physicians (UPP), also named in the suit, permitted Dr. Luketich to skirt.
The whistleblower provision of the False Claims Act allows private parties to file an action on behalf of the United States and receive a portion of the recovery to help deter health care fraud, says the DOJ.
The defendants previously asked the court to dismiss the case, but a judge denied the request in June 2022.
Paul Wood, vice president and chief communications officer for UPMC, told this news organization that the lawsuit pertained to Dr. Luketich’s “most complicated, team-based surgical procedures.”
“At issue was compliance with the Centers for Medicare & Medicaid Services’ (CMS’s) Teaching Physician Regulation and related billing guidance as well as with UPMC’s internal surgical policies,” he said.
“While UPMC continues to believe Dr. Luketich’s surgical practice complies with CMS requirements, it has agreed to [the settlement] to avoid the distraction and expense of further litigation,” said Mr. Wood, adding that all parties agree that UPMC can seek clarity from CMS regarding future billing of these surgeries.
Efrem Grail, JD, Dr. Luketich’s attorney, said in an interview that he and Dr. Luketich are pleased that the settlement puts an end to the case and that he hopes the United States will issue “authoritative guidance” on billing regulations for teaching physicians, something medical schools and hospitals have sought for years.
Dr. Luketich, UPMC, and UPP face more legal challenges from a separate medical malpractice lawsuit. In March 2018, Bernadette Fedorka underwent a lung transplant at UPMC. Although Dr. Luketich did not perform the surgery, Ms. Fedorka alleges that his poor leadership caused understaffing of the lung transplant program and contributed to surgical complications, including a 4-inch piece of wire left in her neck.
Ms. Fedorka claims that suboxone impaired Dr. Luketich’s decision-making. He began taking the drug in 2008 to manage the pain from a slipped disc injury after a history of prescription drug abuse. Both UPMC and Dr. Luketich have denied the validity of Ms. Fedorka’s claims.
The malpractice suit centers on a recording of a conversation between Dr. Luketich and David Wilson, MD, who prescribed the suboxone and treated the surgeon’s opioid use disorder for several years. Dr. Luketich has accused former colleagues, Dr. D’Cunha and Lara Schaheen, MD, of illegally recording the private conversation that discussed Dr. Luketich’s suboxone prescription – something both physicians deny.
For the billing fraud case, Dr. Luketich has agreed to complete a corrective action plan and submit to a third-party audit of his Medicare billings for 1 year.
“This is an important settlement and a just conclusion to the United States’ investigation into Dr. Luketich’s surgical and billing practices and UPMC and UPP’s acceptance of those practices,” Acting U.S. Attorney Troy Rivetti said in a statement that, “no medical provider – however renowned – is excepted from scrutiny or above the law.”
A version of this article first appeared on Medscape.com.
the Department of Justice (DOJ) announced.
The lawsuit alleges that James L. Luketich, MD, the longtime chair of the school’s cardiothoracic surgery department, regularly performed up to three complex surgical procedures simultaneously, moving among multiple operating rooms and attending to matters other than patient care. The investigation began after Jonathan D’Cunha, MD, a former UPMC surgeon, raised concerns about his colleague’s surgical scheduling and billing practices.
Dr. Luketich’s overbooking of procedures led to patients enduring hours of medically unnecessary anesthesia time and risking surgical complications, according to court documents.
In addition, the complaint states that these practices violated the False Claims Act, which prohibits “teaching physicians” like Dr. Luketich from billing Medicare and other government health plans for “concurrent surgeries” – regulations federal authorities say UPMC leadership were aware of and the University of Pittsburgh Physicians (UPP), also named in the suit, permitted Dr. Luketich to skirt.
The whistleblower provision of the False Claims Act allows private parties to file an action on behalf of the United States and receive a portion of the recovery to help deter health care fraud, says the DOJ.
The defendants previously asked the court to dismiss the case, but a judge denied the request in June 2022.
Paul Wood, vice president and chief communications officer for UPMC, told this news organization that the lawsuit pertained to Dr. Luketich’s “most complicated, team-based surgical procedures.”
“At issue was compliance with the Centers for Medicare & Medicaid Services’ (CMS’s) Teaching Physician Regulation and related billing guidance as well as with UPMC’s internal surgical policies,” he said.
“While UPMC continues to believe Dr. Luketich’s surgical practice complies with CMS requirements, it has agreed to [the settlement] to avoid the distraction and expense of further litigation,” said Mr. Wood, adding that all parties agree that UPMC can seek clarity from CMS regarding future billing of these surgeries.
Efrem Grail, JD, Dr. Luketich’s attorney, said in an interview that he and Dr. Luketich are pleased that the settlement puts an end to the case and that he hopes the United States will issue “authoritative guidance” on billing regulations for teaching physicians, something medical schools and hospitals have sought for years.
Dr. Luketich, UPMC, and UPP face more legal challenges from a separate medical malpractice lawsuit. In March 2018, Bernadette Fedorka underwent a lung transplant at UPMC. Although Dr. Luketich did not perform the surgery, Ms. Fedorka alleges that his poor leadership caused understaffing of the lung transplant program and contributed to surgical complications, including a 4-inch piece of wire left in her neck.
Ms. Fedorka claims that suboxone impaired Dr. Luketich’s decision-making. He began taking the drug in 2008 to manage the pain from a slipped disc injury after a history of prescription drug abuse. Both UPMC and Dr. Luketich have denied the validity of Ms. Fedorka’s claims.
The malpractice suit centers on a recording of a conversation between Dr. Luketich and David Wilson, MD, who prescribed the suboxone and treated the surgeon’s opioid use disorder for several years. Dr. Luketich has accused former colleagues, Dr. D’Cunha and Lara Schaheen, MD, of illegally recording the private conversation that discussed Dr. Luketich’s suboxone prescription – something both physicians deny.
For the billing fraud case, Dr. Luketich has agreed to complete a corrective action plan and submit to a third-party audit of his Medicare billings for 1 year.
“This is an important settlement and a just conclusion to the United States’ investigation into Dr. Luketich’s surgical and billing practices and UPMC and UPP’s acceptance of those practices,” Acting U.S. Attorney Troy Rivetti said in a statement that, “no medical provider – however renowned – is excepted from scrutiny or above the law.”
A version of this article first appeared on Medscape.com.
the Department of Justice (DOJ) announced.
The lawsuit alleges that James L. Luketich, MD, the longtime chair of the school’s cardiothoracic surgery department, regularly performed up to three complex surgical procedures simultaneously, moving among multiple operating rooms and attending to matters other than patient care. The investigation began after Jonathan D’Cunha, MD, a former UPMC surgeon, raised concerns about his colleague’s surgical scheduling and billing practices.
Dr. Luketich’s overbooking of procedures led to patients enduring hours of medically unnecessary anesthesia time and risking surgical complications, according to court documents.
In addition, the complaint states that these practices violated the False Claims Act, which prohibits “teaching physicians” like Dr. Luketich from billing Medicare and other government health plans for “concurrent surgeries” – regulations federal authorities say UPMC leadership were aware of and the University of Pittsburgh Physicians (UPP), also named in the suit, permitted Dr. Luketich to skirt.
The whistleblower provision of the False Claims Act allows private parties to file an action on behalf of the United States and receive a portion of the recovery to help deter health care fraud, says the DOJ.
The defendants previously asked the court to dismiss the case, but a judge denied the request in June 2022.
Paul Wood, vice president and chief communications officer for UPMC, told this news organization that the lawsuit pertained to Dr. Luketich’s “most complicated, team-based surgical procedures.”
“At issue was compliance with the Centers for Medicare & Medicaid Services’ (CMS’s) Teaching Physician Regulation and related billing guidance as well as with UPMC’s internal surgical policies,” he said.
“While UPMC continues to believe Dr. Luketich’s surgical practice complies with CMS requirements, it has agreed to [the settlement] to avoid the distraction and expense of further litigation,” said Mr. Wood, adding that all parties agree that UPMC can seek clarity from CMS regarding future billing of these surgeries.
Efrem Grail, JD, Dr. Luketich’s attorney, said in an interview that he and Dr. Luketich are pleased that the settlement puts an end to the case and that he hopes the United States will issue “authoritative guidance” on billing regulations for teaching physicians, something medical schools and hospitals have sought for years.
Dr. Luketich, UPMC, and UPP face more legal challenges from a separate medical malpractice lawsuit. In March 2018, Bernadette Fedorka underwent a lung transplant at UPMC. Although Dr. Luketich did not perform the surgery, Ms. Fedorka alleges that his poor leadership caused understaffing of the lung transplant program and contributed to surgical complications, including a 4-inch piece of wire left in her neck.
Ms. Fedorka claims that suboxone impaired Dr. Luketich’s decision-making. He began taking the drug in 2008 to manage the pain from a slipped disc injury after a history of prescription drug abuse. Both UPMC and Dr. Luketich have denied the validity of Ms. Fedorka’s claims.
The malpractice suit centers on a recording of a conversation between Dr. Luketich and David Wilson, MD, who prescribed the suboxone and treated the surgeon’s opioid use disorder for several years. Dr. Luketich has accused former colleagues, Dr. D’Cunha and Lara Schaheen, MD, of illegally recording the private conversation that discussed Dr. Luketich’s suboxone prescription – something both physicians deny.
For the billing fraud case, Dr. Luketich has agreed to complete a corrective action plan and submit to a third-party audit of his Medicare billings for 1 year.
“This is an important settlement and a just conclusion to the United States’ investigation into Dr. Luketich’s surgical and billing practices and UPMC and UPP’s acceptance of those practices,” Acting U.S. Attorney Troy Rivetti said in a statement that, “no medical provider – however renowned – is excepted from scrutiny or above the law.”
A version of this article first appeared on Medscape.com.
Bridging the Digital Divide in Teledermatology Usage: A Retrospective Review of Patient Visits
Teledermatology is an effective patient care model for the delivery of high-quality dermatologic care.1 Teledermatology can occur using synchronous, asynchronous, and hybrid models of care. In asynchronous visits (AVs), patients or health professionals submit photographs and information for dermatologists to review and provide treatment recommendations. With synchronous visits (SVs), patients have a visit with a dermatology health professional in real time via live video conferencing software. Hybrid models incorporate asynchronous strategies for patient intake forms and skin photograph submissions as well as synchronous methods for live video consultation in a single visit.1 However, remarkable inequities in internet access limit telemedicine usage among medically marginalized patient populations, including racialized, elderly, and low socioeconomic status groups.2
Synchronous visits, a relatively newer teledermatology format, allow for communication with dermatology professionals from the convenience of a patient’s selected location. The live interaction of SVs allows dermatology professionals to answer questions, provide treatment recommendations, and build therapeutic relationships with patients. Concerns for dermatologist reimbursement, malpractice/liability, and technological challenges stalled large-scale uptake of teledermatology platforms.3 The COVID-19 pandemic led to a drastic increase in teledermatology usage of approximately 587.2%, largely due to public safety measures and Medicaid reimbursement parity between SV and in-office visits (IVs).3,4
With the implementation of SVs as a patient care model, we investigated the demographics of patients who utilized SVs, AVs, or IVs, and we propose strategies to promote equity in dermatologic care access.
Methods
This study was approved by the University of Pittsburgh institutional review board (STUDY20110043). We performed a retrospective electronic medical record review of deidentified data from the University of Pittsburgh Medical Center, a tertiary care center in Allegheny County, Pennsylvania, with an established asynchronous teledermatology program. Hybrid SVs were integrated into the University of Pittsburgh Medical Center patient care visit options in March 2020. Patients were instructed to upload photographs of their skin conditions prior to SV appointments. The study included visits occurring between July and December 2020. Visit types included SVs, AVs, and IVs.
We analyzed the initial dermatology visits of 17,130 patients aged 17.5 years and older. Recorded data included diagnosis, age, sex, race, ethnicity, and insurance type for each visit type. Patients without a reported race (990 patients) or ethnicity (1712 patients) were excluded from analysis of race/ethnicity data. Patient zip codes were compared with the zip codes of Allegheny County municipalities as reported by the Allegheny County Elections Division.
Statistical Analysis—Descriptive statistics were calculated; frequency with percentage was used to report categorical variables, and the mean (SD) was used for normally distributed continuous variables. Univariate analysis was performed using the χ2 test for categorical variables. One-way analysis of variance was used to compare age among visit types. Statistical significance was defined as P<.05. IBM SPSS Statistics for Windows, Version 24 (IBM Corp) was used for all statistical analyses.
Results
In our study population, 81.2% (13,916) of patients were residents of Allegheny County, where 51.6% of residents are female and 81.4% are older than 18 years according to data from 2020.5 The racial and ethnic demographics of Allegheny County were 13.4% African American/Black, 0.2% American Indian/Alaska Native, 4.2% Asian, 2.3% Hispanic/Latino, and 79.6% White. The percentage of residents who identified as Native Hawaiian/Pacific Islander was reported to be greater than 0% but less than 0.5%.5
In our analysis, IVs were the most utilized visit type, accounting for 71.5% (12,240) of visits, followed by 15.0% (2577) for SVs and 13.5% (2313) for AVs. The mean age (SD) of IV patients was 51.0 (18.8) years compared with 39.9 (16.9) years for SV patients and 37.5 (14.3) years for AV patients (eTable). The majority of patients for all visits were female: 62.1% (7599) for IVs, 71.4% (1652) for AVs, and 72.8% (1877) for SVs. The largest racial or ethnic group for all visit types included White patients (83.8% [13,524] of all patients), followed by Black (12.4% [2007]), Hispanic/Latino (1.4% [209]), Asian (3.4% [555]), American Indian/Alaska Native (0.2% [35]), and Native Hawaiian/Other Pacific Islander patients (0.1% [19]).
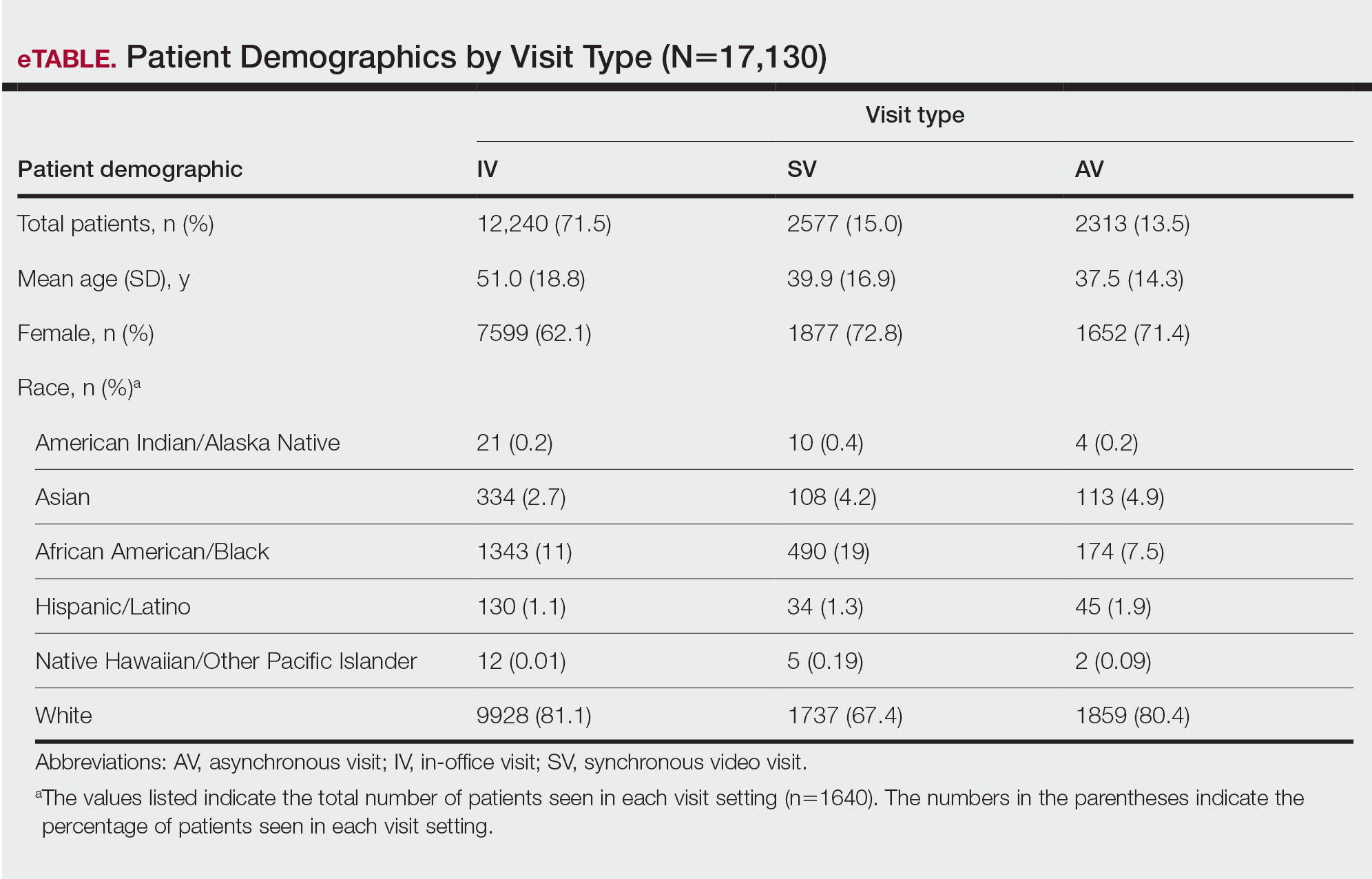
Asian patients, who comprised 4.2% of Allegheny County residents,5 accounted for 2.7% (334) of IVs, 4.9% (113) of AVs, and 4.2% (108) of SVs. Black patients, who were reported as 13.4% of the Allegheny County population,5 were more likely to utilize SVs (19% [490])compared with AVs (7.5% [174]) and IVs (11% [1343]). Hispanic/Latino patients had a disproportionally lower utilization of dermatologic care in all settings, comprising 1.4% (209) of all patients in our study compared with 2.3% of Allegheny County residents.5 White patients, who comprised 79.6% of Allegheny County residents, accounted for 81.1% (9928) of IVs, 67.4% (1737) of SVs, and 80.4% (1859) of AVs. There was no significant difference in the percentage of American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients among visit types.
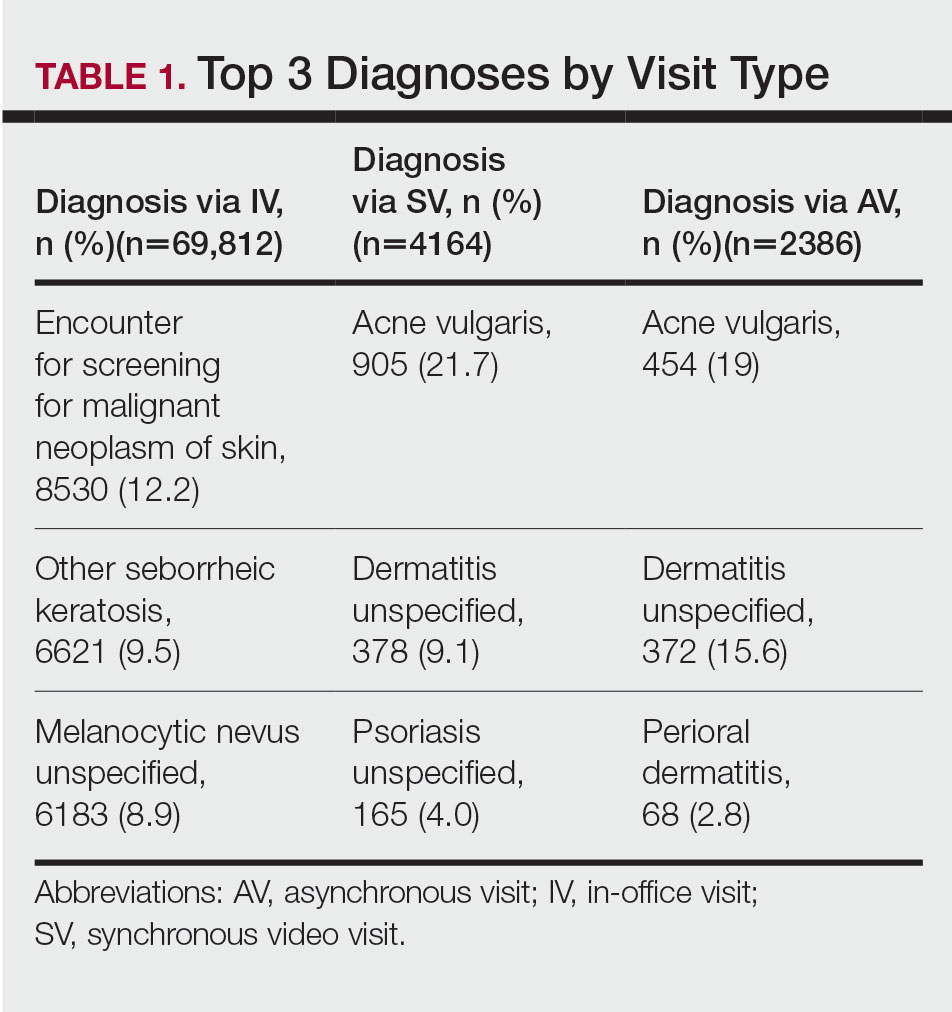
The 3 most common diagnoses for IVs were skin cancer screening, seborrheic keratosis, and melanocytic nevus (Table 1). Skin cancer screening was the most common diagnosis, accounting for 12.2% (8530) of 69,812 IVs. The 3 most common diagnoses for SVs were acne vulgaris, dermatitis, and psoriasis. The 3 most common diagnoses for AVs were acne vulgaris, dermatitis, and perioral dermatitis.
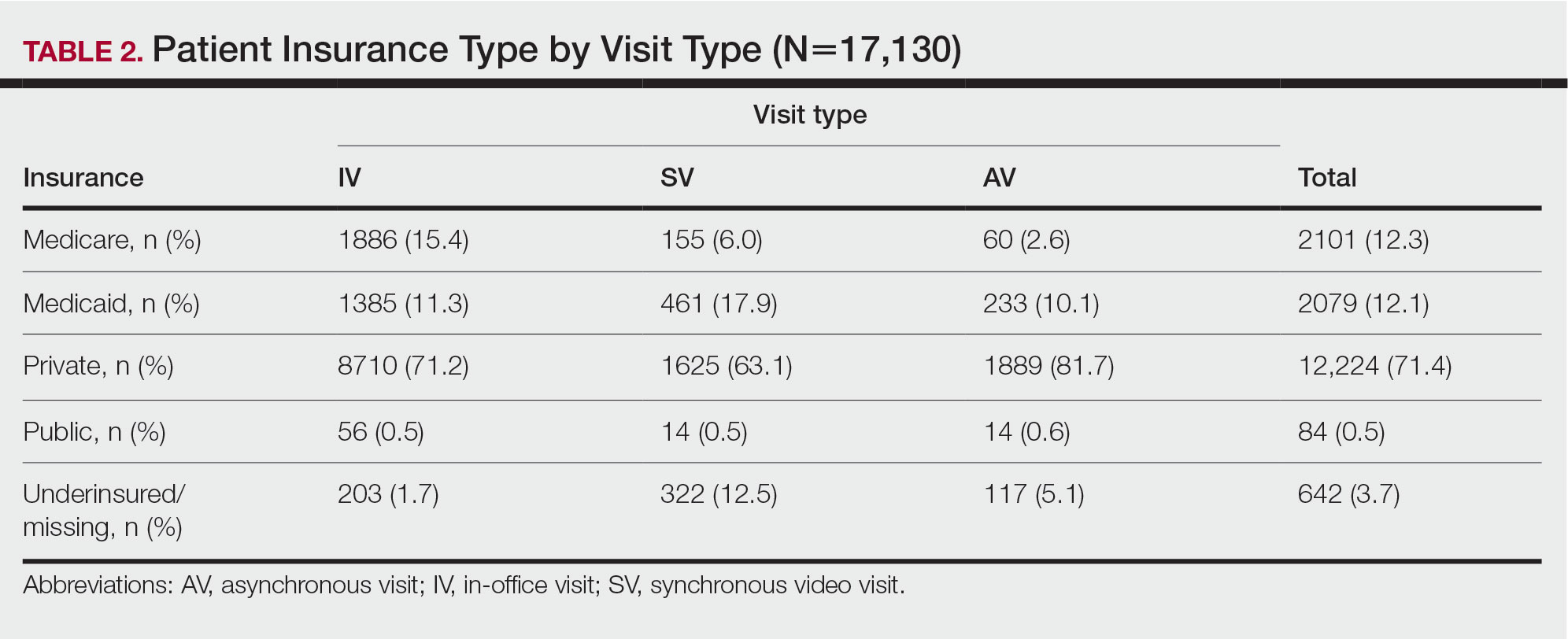
Private insurance was the most common insurance type among all patients (71.4% [12,224])(Table 2). A higher percentage of patients with Medicaid insurance (17.9% [461]) utilized SVs compared with AVs (10.1% [233]) and IVs (11.3% 1385]). Similarly, a higher percentage of patients with no insurance or no insurance listed were seen via SVs (12.5% [322]) compared with AVs (5.1% [117]) and IVs (1.7% [203]). Patients with Medicare insurance used IVs (15.4% [1886]) more than SVs (6.0% [155]) or AVs (2.6% [60]). There was no significant difference among visit type usage for patients with public insurance.
Comment
Teledermatology Benefits—In this retrospective review of medical records of patients who obtained dermatologic care after the implementation of SVs at our institution, we found a proportionally higher use of SVs among Black patients, patients with Medicaid, and patients who are underinsured. Benefits of teledermatology include decreases in patient transportation and associated costs, time away from work or home, and need for childcare.6 The SV format provides the additional advantage of direct live interaction and the development of a patient-physician or patient–physician assistant relationship. Although the prerequisite technology, internet, and broadband connectivity preclude use of teledermatology for many vulnerable patients,2 its convenience ultimately may reduce inequities in access.
Disparities in Dermatologic Care—Hispanic ethnicity and male sex are among described patient demographics associated with decreased rates of outpatient dermatologic care.7 We reported disparities in dermatologic care utilization across all visit types among Hispanic patients and males. Patients identifying as Hispanic/Latino composed only 1.4% (n=209) of our study population compared with 2.3% of Allegheny County residents.5 During our study period, most patients seen were female, accounting for 62.1% to 72.8% of visits, compared with 51.6% of Allegheny County residents.5 These disparities in dermatologic care use may have implications for increased skin-associated morbidity and provide impetus for dermatologists to increase engagement with these patient groups.
Characteristics of Patients Using Teledermatology—Patients using SVs and AVs were significantly younger (mean age [SD], 39.9 [16.9] years and 37.5 [14.3] years, respectively) compared with those using IVs (51.0 [18.8] years). This finding reflects known digital knowledge barriers among older patients.8,9 The synchronous communication format of SVs simulates the traditional visit style of IVs, which may be preferable for some patients. Continued patient education and advocacy for broadband access may increase teledermatology use among older patients and patients with limited technology resources.8
Teledermatology visits were used most frequently for acne and dermatitis, while IVs were used for skin cancer screenings and examination of concerning lesions. This usage pattern is consistent with a previously described consensus among dermatologists on the conditions most amenable to teledermatology evaluation.3
Medicaid reimbursement parity for SVs is in effect nationally until the end of the COVID-19 public health emergency declaration in the United States.10 As of February 2023, the public health emergency declaration has been renewed 12 times since January 2020, with the most recent renewal on January 11, 2023.11 As of January 2023, 21 states have enacted legislation providing permanent reimbursement parity for SV services. Six additional states have some payment parity in place, each with its own qualifying criteria, and 23 states have no payment parity.12 Only 25 Medicaid programs currently provide reimbursement for AV services.13
Study Limitations—Our study was limited by lack of data on patients who are multiracial and those who identify as nonbinary and transgender. Because of the low numbers of Hispanic patients associated with each race category and a high number of patients who did not report an ethnicity or race, race and ethnicity data were analyzed separately. For SVs, patients were instructed to upload photographs prior to their visit; however, the percentage of patients who uploaded photographs was not analyzed.
Conclusion
Expansion of teledermatology services, including SVs and AVs, patient outreach and education, advocacy for broadband access, and Medicaid payment parity, may improve dermatologic care access for medically marginalized groups. Teledermatology has the potential to serve as an effective health care option for patients who are racially minoritized, older, and underinsured. To further assess the effectiveness of teledermatology, we plan to analyze the number of SVs and AVs that were referred to IVs. Future studies also will investigate the impact of implementing patient education and patient-reported outcomes of teledermatology visits.
- Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Kennedy J, Arey S, Hopkins Z, et al. dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed February 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
- United States Census Bureau. QuickFacts: Allegheny County, Pennsylvania. Accessed August 12, 2021. https://www.census.gov/quickfacts/alleghenycountypennsylvania
- Moore HW. Teledermatology—access to specialized care via a different model. Dermatology Advisor. November 12, 2019. Accessed February 10, 2023. https://www.dermatologyadvisor.com/home/topics/practice-management/teledermatology-access-to-specialized-care-via-a-different-model/
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Nouri S, Khoong EC, Lyles CR, et al. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic [published online May 4, 2020]. NEJM Catal Innov Care Deliv. doi:10.1056/CAT.20.0123
- Swenson K, Ghertner R. People in low-income households have less access to internet services—2019 update. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health and Human Services. March 2021. Accessed February 10, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/263601/internet-access-among-low-income-2019.pdf
- Centers for Medicare and Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. Updated August 16, 2022. Accessed February 10, 2023. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf
- US Department of Health and Human Services. Renewal of determination that a public health emergency exists. Updated February 9, 2023. Accessed February 20, 2023. https://aspr.hhs.gov/legal/PHE/Pages/COVID19-9Feb2023.aspx?
- Augenstein J, Smith JM. Executive summary: tracking telehealth changes state-by-state in response to COVID-19. Updated January 27, 2023. Accessed February 10, 2023. https://www.manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
- Center for Connected Health Policy. Policy trend maps: store and forward Medicaid reimbursement. Accessed June 23, 2022. https://www.cchpca.org/policy-trends/
Teledermatology is an effective patient care model for the delivery of high-quality dermatologic care.1 Teledermatology can occur using synchronous, asynchronous, and hybrid models of care. In asynchronous visits (AVs), patients or health professionals submit photographs and information for dermatologists to review and provide treatment recommendations. With synchronous visits (SVs), patients have a visit with a dermatology health professional in real time via live video conferencing software. Hybrid models incorporate asynchronous strategies for patient intake forms and skin photograph submissions as well as synchronous methods for live video consultation in a single visit.1 However, remarkable inequities in internet access limit telemedicine usage among medically marginalized patient populations, including racialized, elderly, and low socioeconomic status groups.2
Synchronous visits, a relatively newer teledermatology format, allow for communication with dermatology professionals from the convenience of a patient’s selected location. The live interaction of SVs allows dermatology professionals to answer questions, provide treatment recommendations, and build therapeutic relationships with patients. Concerns for dermatologist reimbursement, malpractice/liability, and technological challenges stalled large-scale uptake of teledermatology platforms.3 The COVID-19 pandemic led to a drastic increase in teledermatology usage of approximately 587.2%, largely due to public safety measures and Medicaid reimbursement parity between SV and in-office visits (IVs).3,4
With the implementation of SVs as a patient care model, we investigated the demographics of patients who utilized SVs, AVs, or IVs, and we propose strategies to promote equity in dermatologic care access.
Methods
This study was approved by the University of Pittsburgh institutional review board (STUDY20110043). We performed a retrospective electronic medical record review of deidentified data from the University of Pittsburgh Medical Center, a tertiary care center in Allegheny County, Pennsylvania, with an established asynchronous teledermatology program. Hybrid SVs were integrated into the University of Pittsburgh Medical Center patient care visit options in March 2020. Patients were instructed to upload photographs of their skin conditions prior to SV appointments. The study included visits occurring between July and December 2020. Visit types included SVs, AVs, and IVs.
We analyzed the initial dermatology visits of 17,130 patients aged 17.5 years and older. Recorded data included diagnosis, age, sex, race, ethnicity, and insurance type for each visit type. Patients without a reported race (990 patients) or ethnicity (1712 patients) were excluded from analysis of race/ethnicity data. Patient zip codes were compared with the zip codes of Allegheny County municipalities as reported by the Allegheny County Elections Division.
Statistical Analysis—Descriptive statistics were calculated; frequency with percentage was used to report categorical variables, and the mean (SD) was used for normally distributed continuous variables. Univariate analysis was performed using the χ2 test for categorical variables. One-way analysis of variance was used to compare age among visit types. Statistical significance was defined as P<.05. IBM SPSS Statistics for Windows, Version 24 (IBM Corp) was used for all statistical analyses.
Results
In our study population, 81.2% (13,916) of patients were residents of Allegheny County, where 51.6% of residents are female and 81.4% are older than 18 years according to data from 2020.5 The racial and ethnic demographics of Allegheny County were 13.4% African American/Black, 0.2% American Indian/Alaska Native, 4.2% Asian, 2.3% Hispanic/Latino, and 79.6% White. The percentage of residents who identified as Native Hawaiian/Pacific Islander was reported to be greater than 0% but less than 0.5%.5
In our analysis, IVs were the most utilized visit type, accounting for 71.5% (12,240) of visits, followed by 15.0% (2577) for SVs and 13.5% (2313) for AVs. The mean age (SD) of IV patients was 51.0 (18.8) years compared with 39.9 (16.9) years for SV patients and 37.5 (14.3) years for AV patients (eTable). The majority of patients for all visits were female: 62.1% (7599) for IVs, 71.4% (1652) for AVs, and 72.8% (1877) for SVs. The largest racial or ethnic group for all visit types included White patients (83.8% [13,524] of all patients), followed by Black (12.4% [2007]), Hispanic/Latino (1.4% [209]), Asian (3.4% [555]), American Indian/Alaska Native (0.2% [35]), and Native Hawaiian/Other Pacific Islander patients (0.1% [19]).

Asian patients, who comprised 4.2% of Allegheny County residents,5 accounted for 2.7% (334) of IVs, 4.9% (113) of AVs, and 4.2% (108) of SVs. Black patients, who were reported as 13.4% of the Allegheny County population,5 were more likely to utilize SVs (19% [490])compared with AVs (7.5% [174]) and IVs (11% [1343]). Hispanic/Latino patients had a disproportionally lower utilization of dermatologic care in all settings, comprising 1.4% (209) of all patients in our study compared with 2.3% of Allegheny County residents.5 White patients, who comprised 79.6% of Allegheny County residents, accounted for 81.1% (9928) of IVs, 67.4% (1737) of SVs, and 80.4% (1859) of AVs. There was no significant difference in the percentage of American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients among visit types.
The 3 most common diagnoses for IVs were skin cancer screening, seborrheic keratosis, and melanocytic nevus (Table 1). Skin cancer screening was the most common diagnosis, accounting for 12.2% (8530) of 69,812 IVs. The 3 most common diagnoses for SVs were acne vulgaris, dermatitis, and psoriasis. The 3 most common diagnoses for AVs were acne vulgaris, dermatitis, and perioral dermatitis.

Private insurance was the most common insurance type among all patients (71.4% [12,224])(Table 2). A higher percentage of patients with Medicaid insurance (17.9% [461]) utilized SVs compared with AVs (10.1% [233]) and IVs (11.3% 1385]). Similarly, a higher percentage of patients with no insurance or no insurance listed were seen via SVs (12.5% [322]) compared with AVs (5.1% [117]) and IVs (1.7% [203]). Patients with Medicare insurance used IVs (15.4% [1886]) more than SVs (6.0% [155]) or AVs (2.6% [60]). There was no significant difference among visit type usage for patients with public insurance.

Comment
Teledermatology Benefits—In this retrospective review of medical records of patients who obtained dermatologic care after the implementation of SVs at our institution, we found a proportionally higher use of SVs among Black patients, patients with Medicaid, and patients who are underinsured. Benefits of teledermatology include decreases in patient transportation and associated costs, time away from work or home, and need for childcare.6 The SV format provides the additional advantage of direct live interaction and the development of a patient-physician or patient–physician assistant relationship. Although the prerequisite technology, internet, and broadband connectivity preclude use of teledermatology for many vulnerable patients,2 its convenience ultimately may reduce inequities in access.
Disparities in Dermatologic Care—Hispanic ethnicity and male sex are among described patient demographics associated with decreased rates of outpatient dermatologic care.7 We reported disparities in dermatologic care utilization across all visit types among Hispanic patients and males. Patients identifying as Hispanic/Latino composed only 1.4% (n=209) of our study population compared with 2.3% of Allegheny County residents.5 During our study period, most patients seen were female, accounting for 62.1% to 72.8% of visits, compared with 51.6% of Allegheny County residents.5 These disparities in dermatologic care use may have implications for increased skin-associated morbidity and provide impetus for dermatologists to increase engagement with these patient groups.
Characteristics of Patients Using Teledermatology—Patients using SVs and AVs were significantly younger (mean age [SD], 39.9 [16.9] years and 37.5 [14.3] years, respectively) compared with those using IVs (51.0 [18.8] years). This finding reflects known digital knowledge barriers among older patients.8,9 The synchronous communication format of SVs simulates the traditional visit style of IVs, which may be preferable for some patients. Continued patient education and advocacy for broadband access may increase teledermatology use among older patients and patients with limited technology resources.8
Teledermatology visits were used most frequently for acne and dermatitis, while IVs were used for skin cancer screenings and examination of concerning lesions. This usage pattern is consistent with a previously described consensus among dermatologists on the conditions most amenable to teledermatology evaluation.3
Medicaid reimbursement parity for SVs is in effect nationally until the end of the COVID-19 public health emergency declaration in the United States.10 As of February 2023, the public health emergency declaration has been renewed 12 times since January 2020, with the most recent renewal on January 11, 2023.11 As of January 2023, 21 states have enacted legislation providing permanent reimbursement parity for SV services. Six additional states have some payment parity in place, each with its own qualifying criteria, and 23 states have no payment parity.12 Only 25 Medicaid programs currently provide reimbursement for AV services.13
Study Limitations—Our study was limited by lack of data on patients who are multiracial and those who identify as nonbinary and transgender. Because of the low numbers of Hispanic patients associated with each race category and a high number of patients who did not report an ethnicity or race, race and ethnicity data were analyzed separately. For SVs, patients were instructed to upload photographs prior to their visit; however, the percentage of patients who uploaded photographs was not analyzed.
Conclusion
Expansion of teledermatology services, including SVs and AVs, patient outreach and education, advocacy for broadband access, and Medicaid payment parity, may improve dermatologic care access for medically marginalized groups. Teledermatology has the potential to serve as an effective health care option for patients who are racially minoritized, older, and underinsured. To further assess the effectiveness of teledermatology, we plan to analyze the number of SVs and AVs that were referred to IVs. Future studies also will investigate the impact of implementing patient education and patient-reported outcomes of teledermatology visits.
Teledermatology is an effective patient care model for the delivery of high-quality dermatologic care.1 Teledermatology can occur using synchronous, asynchronous, and hybrid models of care. In asynchronous visits (AVs), patients or health professionals submit photographs and information for dermatologists to review and provide treatment recommendations. With synchronous visits (SVs), patients have a visit with a dermatology health professional in real time via live video conferencing software. Hybrid models incorporate asynchronous strategies for patient intake forms and skin photograph submissions as well as synchronous methods for live video consultation in a single visit.1 However, remarkable inequities in internet access limit telemedicine usage among medically marginalized patient populations, including racialized, elderly, and low socioeconomic status groups.2
Synchronous visits, a relatively newer teledermatology format, allow for communication with dermatology professionals from the convenience of a patient’s selected location. The live interaction of SVs allows dermatology professionals to answer questions, provide treatment recommendations, and build therapeutic relationships with patients. Concerns for dermatologist reimbursement, malpractice/liability, and technological challenges stalled large-scale uptake of teledermatology platforms.3 The COVID-19 pandemic led to a drastic increase in teledermatology usage of approximately 587.2%, largely due to public safety measures and Medicaid reimbursement parity between SV and in-office visits (IVs).3,4
With the implementation of SVs as a patient care model, we investigated the demographics of patients who utilized SVs, AVs, or IVs, and we propose strategies to promote equity in dermatologic care access.
Methods
This study was approved by the University of Pittsburgh institutional review board (STUDY20110043). We performed a retrospective electronic medical record review of deidentified data from the University of Pittsburgh Medical Center, a tertiary care center in Allegheny County, Pennsylvania, with an established asynchronous teledermatology program. Hybrid SVs were integrated into the University of Pittsburgh Medical Center patient care visit options in March 2020. Patients were instructed to upload photographs of their skin conditions prior to SV appointments. The study included visits occurring between July and December 2020. Visit types included SVs, AVs, and IVs.
We analyzed the initial dermatology visits of 17,130 patients aged 17.5 years and older. Recorded data included diagnosis, age, sex, race, ethnicity, and insurance type for each visit type. Patients without a reported race (990 patients) or ethnicity (1712 patients) were excluded from analysis of race/ethnicity data. Patient zip codes were compared with the zip codes of Allegheny County municipalities as reported by the Allegheny County Elections Division.
Statistical Analysis—Descriptive statistics were calculated; frequency with percentage was used to report categorical variables, and the mean (SD) was used for normally distributed continuous variables. Univariate analysis was performed using the χ2 test for categorical variables. One-way analysis of variance was used to compare age among visit types. Statistical significance was defined as P<.05. IBM SPSS Statistics for Windows, Version 24 (IBM Corp) was used for all statistical analyses.
Results
In our study population, 81.2% (13,916) of patients were residents of Allegheny County, where 51.6% of residents are female and 81.4% are older than 18 years according to data from 2020.5 The racial and ethnic demographics of Allegheny County were 13.4% African American/Black, 0.2% American Indian/Alaska Native, 4.2% Asian, 2.3% Hispanic/Latino, and 79.6% White. The percentage of residents who identified as Native Hawaiian/Pacific Islander was reported to be greater than 0% but less than 0.5%.5
In our analysis, IVs were the most utilized visit type, accounting for 71.5% (12,240) of visits, followed by 15.0% (2577) for SVs and 13.5% (2313) for AVs. The mean age (SD) of IV patients was 51.0 (18.8) years compared with 39.9 (16.9) years for SV patients and 37.5 (14.3) years for AV patients (eTable). The majority of patients for all visits were female: 62.1% (7599) for IVs, 71.4% (1652) for AVs, and 72.8% (1877) for SVs. The largest racial or ethnic group for all visit types included White patients (83.8% [13,524] of all patients), followed by Black (12.4% [2007]), Hispanic/Latino (1.4% [209]), Asian (3.4% [555]), American Indian/Alaska Native (0.2% [35]), and Native Hawaiian/Other Pacific Islander patients (0.1% [19]).

Asian patients, who comprised 4.2% of Allegheny County residents,5 accounted for 2.7% (334) of IVs, 4.9% (113) of AVs, and 4.2% (108) of SVs. Black patients, who were reported as 13.4% of the Allegheny County population,5 were more likely to utilize SVs (19% [490])compared with AVs (7.5% [174]) and IVs (11% [1343]). Hispanic/Latino patients had a disproportionally lower utilization of dermatologic care in all settings, comprising 1.4% (209) of all patients in our study compared with 2.3% of Allegheny County residents.5 White patients, who comprised 79.6% of Allegheny County residents, accounted for 81.1% (9928) of IVs, 67.4% (1737) of SVs, and 80.4% (1859) of AVs. There was no significant difference in the percentage of American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients among visit types.
The 3 most common diagnoses for IVs were skin cancer screening, seborrheic keratosis, and melanocytic nevus (Table 1). Skin cancer screening was the most common diagnosis, accounting for 12.2% (8530) of 69,812 IVs. The 3 most common diagnoses for SVs were acne vulgaris, dermatitis, and psoriasis. The 3 most common diagnoses for AVs were acne vulgaris, dermatitis, and perioral dermatitis.

Private insurance was the most common insurance type among all patients (71.4% [12,224])(Table 2). A higher percentage of patients with Medicaid insurance (17.9% [461]) utilized SVs compared with AVs (10.1% [233]) and IVs (11.3% 1385]). Similarly, a higher percentage of patients with no insurance or no insurance listed were seen via SVs (12.5% [322]) compared with AVs (5.1% [117]) and IVs (1.7% [203]). Patients with Medicare insurance used IVs (15.4% [1886]) more than SVs (6.0% [155]) or AVs (2.6% [60]). There was no significant difference among visit type usage for patients with public insurance.

Comment
Teledermatology Benefits—In this retrospective review of medical records of patients who obtained dermatologic care after the implementation of SVs at our institution, we found a proportionally higher use of SVs among Black patients, patients with Medicaid, and patients who are underinsured. Benefits of teledermatology include decreases in patient transportation and associated costs, time away from work or home, and need for childcare.6 The SV format provides the additional advantage of direct live interaction and the development of a patient-physician or patient–physician assistant relationship. Although the prerequisite technology, internet, and broadband connectivity preclude use of teledermatology for many vulnerable patients,2 its convenience ultimately may reduce inequities in access.
Disparities in Dermatologic Care—Hispanic ethnicity and male sex are among described patient demographics associated with decreased rates of outpatient dermatologic care.7 We reported disparities in dermatologic care utilization across all visit types among Hispanic patients and males. Patients identifying as Hispanic/Latino composed only 1.4% (n=209) of our study population compared with 2.3% of Allegheny County residents.5 During our study period, most patients seen were female, accounting for 62.1% to 72.8% of visits, compared with 51.6% of Allegheny County residents.5 These disparities in dermatologic care use may have implications for increased skin-associated morbidity and provide impetus for dermatologists to increase engagement with these patient groups.
Characteristics of Patients Using Teledermatology—Patients using SVs and AVs were significantly younger (mean age [SD], 39.9 [16.9] years and 37.5 [14.3] years, respectively) compared with those using IVs (51.0 [18.8] years). This finding reflects known digital knowledge barriers among older patients.8,9 The synchronous communication format of SVs simulates the traditional visit style of IVs, which may be preferable for some patients. Continued patient education and advocacy for broadband access may increase teledermatology use among older patients and patients with limited technology resources.8
Teledermatology visits were used most frequently for acne and dermatitis, while IVs were used for skin cancer screenings and examination of concerning lesions. This usage pattern is consistent with a previously described consensus among dermatologists on the conditions most amenable to teledermatology evaluation.3
Medicaid reimbursement parity for SVs is in effect nationally until the end of the COVID-19 public health emergency declaration in the United States.10 As of February 2023, the public health emergency declaration has been renewed 12 times since January 2020, with the most recent renewal on January 11, 2023.11 As of January 2023, 21 states have enacted legislation providing permanent reimbursement parity for SV services. Six additional states have some payment parity in place, each with its own qualifying criteria, and 23 states have no payment parity.12 Only 25 Medicaid programs currently provide reimbursement for AV services.13
Study Limitations—Our study was limited by lack of data on patients who are multiracial and those who identify as nonbinary and transgender. Because of the low numbers of Hispanic patients associated with each race category and a high number of patients who did not report an ethnicity or race, race and ethnicity data were analyzed separately. For SVs, patients were instructed to upload photographs prior to their visit; however, the percentage of patients who uploaded photographs was not analyzed.
Conclusion
Expansion of teledermatology services, including SVs and AVs, patient outreach and education, advocacy for broadband access, and Medicaid payment parity, may improve dermatologic care access for medically marginalized groups. Teledermatology has the potential to serve as an effective health care option for patients who are racially minoritized, older, and underinsured. To further assess the effectiveness of teledermatology, we plan to analyze the number of SVs and AVs that were referred to IVs. Future studies also will investigate the impact of implementing patient education and patient-reported outcomes of teledermatology visits.
- Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Kennedy J, Arey S, Hopkins Z, et al. dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed February 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
- United States Census Bureau. QuickFacts: Allegheny County, Pennsylvania. Accessed August 12, 2021. https://www.census.gov/quickfacts/alleghenycountypennsylvania
- Moore HW. Teledermatology—access to specialized care via a different model. Dermatology Advisor. November 12, 2019. Accessed February 10, 2023. https://www.dermatologyadvisor.com/home/topics/practice-management/teledermatology-access-to-specialized-care-via-a-different-model/
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Nouri S, Khoong EC, Lyles CR, et al. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic [published online May 4, 2020]. NEJM Catal Innov Care Deliv. doi:10.1056/CAT.20.0123
- Swenson K, Ghertner R. People in low-income households have less access to internet services—2019 update. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health and Human Services. March 2021. Accessed February 10, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/263601/internet-access-among-low-income-2019.pdf
- Centers for Medicare and Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. Updated August 16, 2022. Accessed February 10, 2023. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf
- US Department of Health and Human Services. Renewal of determination that a public health emergency exists. Updated February 9, 2023. Accessed February 20, 2023. https://aspr.hhs.gov/legal/PHE/Pages/COVID19-9Feb2023.aspx?
- Augenstein J, Smith JM. Executive summary: tracking telehealth changes state-by-state in response to COVID-19. Updated January 27, 2023. Accessed February 10, 2023. https://www.manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
- Center for Connected Health Policy. Policy trend maps: store and forward Medicaid reimbursement. Accessed June 23, 2022. https://www.cchpca.org/policy-trends/
- Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Kennedy J, Arey S, Hopkins Z, et al. dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed February 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
- United States Census Bureau. QuickFacts: Allegheny County, Pennsylvania. Accessed August 12, 2021. https://www.census.gov/quickfacts/alleghenycountypennsylvania
- Moore HW. Teledermatology—access to specialized care via a different model. Dermatology Advisor. November 12, 2019. Accessed February 10, 2023. https://www.dermatologyadvisor.com/home/topics/practice-management/teledermatology-access-to-specialized-care-via-a-different-model/
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Nouri S, Khoong EC, Lyles CR, et al. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic [published online May 4, 2020]. NEJM Catal Innov Care Deliv. doi:10.1056/CAT.20.0123
- Swenson K, Ghertner R. People in low-income households have less access to internet services—2019 update. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health and Human Services. March 2021. Accessed February 10, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/263601/internet-access-among-low-income-2019.pdf
- Centers for Medicare and Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. Updated August 16, 2022. Accessed February 10, 2023. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf
- US Department of Health and Human Services. Renewal of determination that a public health emergency exists. Updated February 9, 2023. Accessed February 20, 2023. https://aspr.hhs.gov/legal/PHE/Pages/COVID19-9Feb2023.aspx?
- Augenstein J, Smith JM. Executive summary: tracking telehealth changes state-by-state in response to COVID-19. Updated January 27, 2023. Accessed February 10, 2023. https://www.manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
- Center for Connected Health Policy. Policy trend maps: store and forward Medicaid reimbursement. Accessed June 23, 2022. https://www.cchpca.org/policy-trends/
Practice Points
- There is increased use of synchronous video visits (SVs) among Black patients, patients with Medicaid, and patients who are underinsured.
- Synchronous video visits may increase dermatologic care utilization for medically marginalized groups.
- Efforts are needed to increase engagement with dermatologic care for Hispanic and male patients.
NP-PA turf fights: Where the relationship can improve
– The U.S. Bureau of Labor Statistics forecasts a 40% increase in the NP workforce by 2031, coupled with a 28% rise in PAs.
In recent reports on the quality of the relationships involving these health care professions, survey respondents mostly gave positive accounts of collaboration, using words such as like “comradery,” “teamwork,” “congenial,” and “cohesion.” But all was not perfect. Where and how could these important health care provider relationships improve?
PAs: “Competition and collaboration’ with RNs
In a Medscape survey of more than 770 PAs about their working relationships with other health care professionals; 83% of them supported the idea of PAs and NPs practicing more independently from physicians, but sometimes it’s not easy to stay in their individual lanes.
One PA respondent complained that NPs get “more opportunities and preference,” another pointed to PA-NP “turf issues,” and a third griped about NPs’ “strong unions,” which have stoked more fighting about practice abilities and available settings.
Robert Blumm, MA, PA-C, a retired surgical and emergency medicine PA who regards himself as an advocate for both PAs and NPs, describes their interaction as a “mixture of competition and collaboration.”
On one hand, the two groups typically “cooperate and do an excellent job, incurring patient errors similar to or less than physician colleagues or senior residents.” On the other hand, Mr. Blumm conceded, there is some jealousy among PAs over NPs’ advantage in staffing and hiring decisions, “since they don’t need [direct physician] supervision ... and there are limits on how many PAs can be supervised by one physician.”
Most PA-NP interactions are collaborative, although many people emphasize the relatively few conflicts, said Jennifer Orozco, DMSc, PA-C, president and chair of the American Academy of PAs.
“We see that a lot in this country,” she said. “People try to drive a wedge, but it’s often a misnomer that there’s a lot of arguing and infighting.”
NPs: Different backgrounds, same goal
The Medscape survey also included information from 750 NPs on working relationships; 93% of them favored nurses and PAs working more independently from doctors.
April Kapu, DNP, ARPN, has worked closely with PAs for more than 20 years. “In my experience ... they complement one another as health team members, although the education and training are somewhat different,” said Ms. Kapu, , president of the American Association of Nurse Practitioners.
Some respondents noted the different educational trajectories for NPs and PAs. “Doctors and PAs are taught using the same model, but NPs are taught under the nursing model,” wrote a family medicine PA.
In emergency departments where Mr. Blumm has worked, ICU NPs have an edge over PAs in terms of preparation, organization, and the tabulation of formulas. On the other hand, some of Mr. Blumm’s fellow PAs were also emergency medicine technicians or respiratory therapists, who had “2 years of classroom training, on par with that of medical students.”
Must these differences in training and education foment conflict between NPs and PAs? “We all bring something different to the table,” said Ms. Kapu, who also is associate dean for clinical and community partnerships at Vanderbilt University, Nashville, Tenn. “It is important to respect each person’s entry point, education, and training.”
Differing personalities and environments
Numerous PA respondents said that individual personalities and work environments are more likely to trigger issues with NPs than are differences in training.
“It depends on the team and situation and who the people are, not the letters behind their names,” an emergency medicine PA wrote. A surgical PA noted that “group dynamics and work culture differ from place to place,” while a third PA agreed that “it’s personality dependent, not title dependent.”
No single formula will resolve areas of NP-PA conflict, Ms. Orozco said. “What works in Chicago might not work in rural Colorado or Texas or California, but we do have to come together. The overall focus should be on greater flexibility for PAs and NPs. Patients will fare better.”
Joint research, publishing could help
About a decade ago, Mr. Blumm joined with another PA and an NP to form the American College of Clinicians, the first joint PA-NP national professional organization. Although it disbanded after 6 years, owing to low membership, he hopes a similar collaboration will take off in the future.
“I also recommend that PAs and NPs publish articles together, with research as an excellent place to start,” he added. “PAs and NPs should stand together and be a source of healing for all our patients. Regardless of our titles, our responsibility is to bring healing together.”
A version of this article first appeared on Medscape.com.
– The U.S. Bureau of Labor Statistics forecasts a 40% increase in the NP workforce by 2031, coupled with a 28% rise in PAs.
In recent reports on the quality of the relationships involving these health care professions, survey respondents mostly gave positive accounts of collaboration, using words such as like “comradery,” “teamwork,” “congenial,” and “cohesion.” But all was not perfect. Where and how could these important health care provider relationships improve?
PAs: “Competition and collaboration’ with RNs
In a Medscape survey of more than 770 PAs about their working relationships with other health care professionals; 83% of them supported the idea of PAs and NPs practicing more independently from physicians, but sometimes it’s not easy to stay in their individual lanes.
One PA respondent complained that NPs get “more opportunities and preference,” another pointed to PA-NP “turf issues,” and a third griped about NPs’ “strong unions,” which have stoked more fighting about practice abilities and available settings.
Robert Blumm, MA, PA-C, a retired surgical and emergency medicine PA who regards himself as an advocate for both PAs and NPs, describes their interaction as a “mixture of competition and collaboration.”
On one hand, the two groups typically “cooperate and do an excellent job, incurring patient errors similar to or less than physician colleagues or senior residents.” On the other hand, Mr. Blumm conceded, there is some jealousy among PAs over NPs’ advantage in staffing and hiring decisions, “since they don’t need [direct physician] supervision ... and there are limits on how many PAs can be supervised by one physician.”
Most PA-NP interactions are collaborative, although many people emphasize the relatively few conflicts, said Jennifer Orozco, DMSc, PA-C, president and chair of the American Academy of PAs.
“We see that a lot in this country,” she said. “People try to drive a wedge, but it’s often a misnomer that there’s a lot of arguing and infighting.”
NPs: Different backgrounds, same goal
The Medscape survey also included information from 750 NPs on working relationships; 93% of them favored nurses and PAs working more independently from doctors.
April Kapu, DNP, ARPN, has worked closely with PAs for more than 20 years. “In my experience ... they complement one another as health team members, although the education and training are somewhat different,” said Ms. Kapu, , president of the American Association of Nurse Practitioners.
Some respondents noted the different educational trajectories for NPs and PAs. “Doctors and PAs are taught using the same model, but NPs are taught under the nursing model,” wrote a family medicine PA.
In emergency departments where Mr. Blumm has worked, ICU NPs have an edge over PAs in terms of preparation, organization, and the tabulation of formulas. On the other hand, some of Mr. Blumm’s fellow PAs were also emergency medicine technicians or respiratory therapists, who had “2 years of classroom training, on par with that of medical students.”
Must these differences in training and education foment conflict between NPs and PAs? “We all bring something different to the table,” said Ms. Kapu, who also is associate dean for clinical and community partnerships at Vanderbilt University, Nashville, Tenn. “It is important to respect each person’s entry point, education, and training.”
Differing personalities and environments
Numerous PA respondents said that individual personalities and work environments are more likely to trigger issues with NPs than are differences in training.
“It depends on the team and situation and who the people are, not the letters behind their names,” an emergency medicine PA wrote. A surgical PA noted that “group dynamics and work culture differ from place to place,” while a third PA agreed that “it’s personality dependent, not title dependent.”
No single formula will resolve areas of NP-PA conflict, Ms. Orozco said. “What works in Chicago might not work in rural Colorado or Texas or California, but we do have to come together. The overall focus should be on greater flexibility for PAs and NPs. Patients will fare better.”
Joint research, publishing could help
About a decade ago, Mr. Blumm joined with another PA and an NP to form the American College of Clinicians, the first joint PA-NP national professional organization. Although it disbanded after 6 years, owing to low membership, he hopes a similar collaboration will take off in the future.
“I also recommend that PAs and NPs publish articles together, with research as an excellent place to start,” he added. “PAs and NPs should stand together and be a source of healing for all our patients. Regardless of our titles, our responsibility is to bring healing together.”
A version of this article first appeared on Medscape.com.
– The U.S. Bureau of Labor Statistics forecasts a 40% increase in the NP workforce by 2031, coupled with a 28% rise in PAs.
In recent reports on the quality of the relationships involving these health care professions, survey respondents mostly gave positive accounts of collaboration, using words such as like “comradery,” “teamwork,” “congenial,” and “cohesion.” But all was not perfect. Where and how could these important health care provider relationships improve?
PAs: “Competition and collaboration’ with RNs
In a Medscape survey of more than 770 PAs about their working relationships with other health care professionals; 83% of them supported the idea of PAs and NPs practicing more independently from physicians, but sometimes it’s not easy to stay in their individual lanes.
One PA respondent complained that NPs get “more opportunities and preference,” another pointed to PA-NP “turf issues,” and a third griped about NPs’ “strong unions,” which have stoked more fighting about practice abilities and available settings.
Robert Blumm, MA, PA-C, a retired surgical and emergency medicine PA who regards himself as an advocate for both PAs and NPs, describes their interaction as a “mixture of competition and collaboration.”
On one hand, the two groups typically “cooperate and do an excellent job, incurring patient errors similar to or less than physician colleagues or senior residents.” On the other hand, Mr. Blumm conceded, there is some jealousy among PAs over NPs’ advantage in staffing and hiring decisions, “since they don’t need [direct physician] supervision ... and there are limits on how many PAs can be supervised by one physician.”
Most PA-NP interactions are collaborative, although many people emphasize the relatively few conflicts, said Jennifer Orozco, DMSc, PA-C, president and chair of the American Academy of PAs.
“We see that a lot in this country,” she said. “People try to drive a wedge, but it’s often a misnomer that there’s a lot of arguing and infighting.”
NPs: Different backgrounds, same goal
The Medscape survey also included information from 750 NPs on working relationships; 93% of them favored nurses and PAs working more independently from doctors.
April Kapu, DNP, ARPN, has worked closely with PAs for more than 20 years. “In my experience ... they complement one another as health team members, although the education and training are somewhat different,” said Ms. Kapu, , president of the American Association of Nurse Practitioners.
Some respondents noted the different educational trajectories for NPs and PAs. “Doctors and PAs are taught using the same model, but NPs are taught under the nursing model,” wrote a family medicine PA.
In emergency departments where Mr. Blumm has worked, ICU NPs have an edge over PAs in terms of preparation, organization, and the tabulation of formulas. On the other hand, some of Mr. Blumm’s fellow PAs were also emergency medicine technicians or respiratory therapists, who had “2 years of classroom training, on par with that of medical students.”
Must these differences in training and education foment conflict between NPs and PAs? “We all bring something different to the table,” said Ms. Kapu, who also is associate dean for clinical and community partnerships at Vanderbilt University, Nashville, Tenn. “It is important to respect each person’s entry point, education, and training.”
Differing personalities and environments
Numerous PA respondents said that individual personalities and work environments are more likely to trigger issues with NPs than are differences in training.
“It depends on the team and situation and who the people are, not the letters behind their names,” an emergency medicine PA wrote. A surgical PA noted that “group dynamics and work culture differ from place to place,” while a third PA agreed that “it’s personality dependent, not title dependent.”
No single formula will resolve areas of NP-PA conflict, Ms. Orozco said. “What works in Chicago might not work in rural Colorado or Texas or California, but we do have to come together. The overall focus should be on greater flexibility for PAs and NPs. Patients will fare better.”
Joint research, publishing could help
About a decade ago, Mr. Blumm joined with another PA and an NP to form the American College of Clinicians, the first joint PA-NP national professional organization. Although it disbanded after 6 years, owing to low membership, he hopes a similar collaboration will take off in the future.
“I also recommend that PAs and NPs publish articles together, with research as an excellent place to start,” he added. “PAs and NPs should stand together and be a source of healing for all our patients. Regardless of our titles, our responsibility is to bring healing together.”
A version of this article first appeared on Medscape.com.
Docs struggle to keep up with the flood of new medical knowledge. Here’s advice
making it much tougher for physicians to identify innovative findings and newer guidelines for helping patients. Yet not keeping up with the latest information can put doctors at risk.
“Most doctors are feeling lost about keeping up to date,” said John P.A. Ioannidis, MD, professor of medicine at Stanford (Calif.) University School of Medicine. “The vast majority of new studies are either wrong or not useful, but physicians cannot sort out which are those studies.”
The sheer number of new studies may even force some doctors to retreat from areas where they have not kept up, said Stephen A. Martin, MD, professor of family medicine and community health at the University of Massachusetts, Worcester. “When doctors don’t feel they can stay current, they may refer more cases to specialists or narrow their focus,” he said.
Some specialties have a greater challenge than others
Dr. Martin said the deluge of studies heavily impacts generalists because they have a wider field of information to keep up with. However, certain specialties like oncology are particularly flooded with new findings.
Specialties with the greatest number of published studies are reportedly oncology, cardiology, and neurology. A 2021 study found that the number of articles with the word “stroke” in them increased five times from 2000 to 2020. And investigative treatments targeting cancer nearly quadrupled just between 2010 and 2020.
What’s more, physicians spend a great deal of time sifting through studies that are ultimately useless. In a survey of internists by Univadis, which is part of WebMD/Medscape, 82% said that fewer than half of the studies they read actually had an impact on how they practice medicine.
“You often have to dig into an article and learn more about a finding before you now whether it’s useful,” Dr. Martin said. “And in the end, relatively few new findings are truly novel ones that are useful for patient care.”
So what can a physician do? First, find out what you don’t know
Looking for new findings needs to be carried out systematically, according to William B. Cutrer, MD, MEd, a pediatric intensivist who is associate dean for undergraduate medical education at Vanderbilt University School of Medicine, Nashville, Tenn.
“Before you start, you have to know what you don’t know, and that’s often not so easy,” he said. “You may get a spark about what you don’t know in an encounter with a patient or colleague or through patient outcomes data,” he said.
Dr. Martin, on the other hand, advocates a broad approach that involves finding out at least a little about everything in one’s field. “If you have a good base, you’re not starting from zero when you encounter a new clinical situation,” he said.
“The idea is that you don’t need to memorize most things, but you do need to know how to access them,” Dr. Martin said. “I memorize the things I do all the time, such as dosing or indicated testing, but I look up things that I don’t see that often and ones that have some complexity.”
Updating the old ways
For generations, doctors have stayed current by going to meetings, conversing with colleagues, and reading journals, but many physicians have updated these methods through various resources on the internet.
For example, meetings went virtual during the pandemic, and now that face-to-face meetings are back, many of them retain a virtual option, said Kevin Campbell, MD, a cardiologist at Health First Medical Group, Melbourne, Fla. “I typically go to one or two conferences a year, but I also learn a lot digitally,” he said.
As to journal reading, “assessing an article is an essential skill,” Dr. Cutrer said. “It’s important to quickly decide whether a journal article is worth reading or not. One answer to this problem is to consult summaries of important articles. But summaries are sometimes unhelpful, and it is hard to know which articles are significant. Therefore, doctors have been reaching out to others who can research the articles for them.”
For many years, some physicians have pooled their resources in journal clubs. “You get a chance to cross-cultivate your skills with others,” Dr. Ioannidis said. “But you need someone who is well informed and dedicated to run the journal club, using evidence-based principles.”
Dr. Cutrer said physicians like to cast their net wide because they are understandably wary of changing their practice based on one study. “Unless there is one large study that is really well designed, doctors will need two or more findings to be convinced,” he said. This requires having the ability to match studies across many journals.
Using research summaries
In the past two decades, physicians have gained access to countless summaries of journal articles prepared by armies of clinical experts working for review services such as the New England Journal of Medicine’s “Journal Watch,” Annals of Internal Medicine’s “In the Clinic,” and BMJ’s “State of the Arts.”
In addition to summarizing findings from a wide variety of journals in plain language, reviewers may compare them to similar studies and assess the validity of the finding by assigning a level of evidence.
Some commercial ventures provide similar services. Betsy Jones, executive vice president of clinical decisions at EBSCO, said the DynaMed service is now available through an app on the physician’s smartphone or through the electronic health record.
Physicians like this approach. Many specialists have noted that reading full-length articles was not an efficient use of their time, while even more said that reviews are efficient.
Exchanging information online
Physicians are increasingly keeping current by using the internet, especially on social media, Dr. Cutrer said. “Young doctors in particular are more likely to keep up digitally,” he said.
Internet-based information has become so widespread that disparities in health care from region to region have somewhat abated, according to Stuart J. Fischer, MD, an orthopedic surgeon at Summit Orthopaedics and Sports Medicine, New Jersey. “One positive outcome of this plethora of information today is that geographic disparities in clinical practice are not as great as they used to be,” he said.
Rather than chatting up colleagues in the hallway, many physicians have come to rely on internet-based discussion boards.
Blogs, podcasts, and Twitter
Blogs and podcasts, often focused on a specialty, can be a great way for physicians to keep up, said UMass Chan professor Dr. Martin. “Podcasts in particular have enhanced the ability to stay current,” he said. “You want to find someone you trust.”
Internal medicine podcasts include Annals on Call, where doctors discuss articles in the Annals of Internal Medicine, and the Curbsiders, where two internists interview a guest expert.
Orthopedic surgeons can visit podcasts like Nailed it, Orthobullets, the Ortho Show, and Inside Orthopedics. Neurologists can consult Brainwaves, Neurology Podcast, Practical Neurology Podcast, and Clinical Neurology with KD. And pediatricians can drop in on Talking Pediatrics, The Cribsiders, and PedsCases.
Meanwhile, Twitter has become a particularly effective way to broadcast new findings, speeding up the transition from the bench to the bedside, said Dr. Campbell, the Florida cardiologist.
“I visit cardio-specific resources on Twitter,” he said. “They can be real-time video chats or posted messages. They spur discussion like a journal club. Colleagues present cases and drop in and out of the discussion.”
Others are not as enthusiastic. Although Stanford’s Dr. Ioannidis is in the heart of the Silicon Valley, he is leery of some of the new digital methods. “I don’t use Twitter,” he says. “You just add more people to the process, which could only make things more confusing. I want to be able to think a lot about it.”
Cutting-edge knowledge at the point of care
Consulting the literature often takes place at the point of care, when a particular patient requires treatment. This can be done by using clinical decision support (CDS) and by using clinical practice guidelines (CPGs), which are typically developed by panels of doctors at specialty societies.
“It used to be that the doctor was expected to know everything,” said Ms. Jones at DynaMed. “Today there is no way to keep up with it all. Doctors often need a quick memory jog.”
Ms. Jones said the CDS result always requires the doctor’s interpretation. “It is up to the doctor to decide whether a new finding is the best choice for his or her patient,” she said.
Dr. Martin recommends going easy on point-of-care resources. “They can be used for showing a patient a differential diagnosis list or checking the cost of a procedure, but they are harder to use for novel developments that require time and context to evaluate their impact,” he said.
CPGs, meanwhile, have a high profile in the research world. In a 2018 study, Dr. Ioannidis found that 8 of the 15 most-cited articles were CPGs, disease definitions, or disease statistics.
Dr. Fischer said CPGs are typically based on thorough reviews of the literature, but they do involve experts’ interpretation of the science. “It can be difficult to obtain specific answers to some medical questions, especially for problems with complex treatments or variations,” he said.
As a result, Dr. Fischer said doctors have to use their judgment in applying CPGs to a specific patient. “For example, the orthopedic surgeon would normally recommend a total hip replacement for patients with a bad hip, but it might not be appropriate for an overweight patient.”
Stay skeptical
There are many novel ways for physicians to keep current, including summaries of articles, discussion boards, blogs, podcasts, Twitter, clinical decision support, and clinical practice guidelines.
Even with all these new services, though, doctors need to retain a healthy amount of skepticism about new research findings, Dr. Ioannidis said. “Ask yourself questions such as: Does it deal with a real problem? Am I getting the real information? Is it relevant to real patients? Is it offering good value for money?”
A version of this article first appeared on Medscape.com.
making it much tougher for physicians to identify innovative findings and newer guidelines for helping patients. Yet not keeping up with the latest information can put doctors at risk.
“Most doctors are feeling lost about keeping up to date,” said John P.A. Ioannidis, MD, professor of medicine at Stanford (Calif.) University School of Medicine. “The vast majority of new studies are either wrong or not useful, but physicians cannot sort out which are those studies.”
The sheer number of new studies may even force some doctors to retreat from areas where they have not kept up, said Stephen A. Martin, MD, professor of family medicine and community health at the University of Massachusetts, Worcester. “When doctors don’t feel they can stay current, they may refer more cases to specialists or narrow their focus,” he said.
Some specialties have a greater challenge than others
Dr. Martin said the deluge of studies heavily impacts generalists because they have a wider field of information to keep up with. However, certain specialties like oncology are particularly flooded with new findings.
Specialties with the greatest number of published studies are reportedly oncology, cardiology, and neurology. A 2021 study found that the number of articles with the word “stroke” in them increased five times from 2000 to 2020. And investigative treatments targeting cancer nearly quadrupled just between 2010 and 2020.
What’s more, physicians spend a great deal of time sifting through studies that are ultimately useless. In a survey of internists by Univadis, which is part of WebMD/Medscape, 82% said that fewer than half of the studies they read actually had an impact on how they practice medicine.
“You often have to dig into an article and learn more about a finding before you now whether it’s useful,” Dr. Martin said. “And in the end, relatively few new findings are truly novel ones that are useful for patient care.”
So what can a physician do? First, find out what you don’t know
Looking for new findings needs to be carried out systematically, according to William B. Cutrer, MD, MEd, a pediatric intensivist who is associate dean for undergraduate medical education at Vanderbilt University School of Medicine, Nashville, Tenn.
“Before you start, you have to know what you don’t know, and that’s often not so easy,” he said. “You may get a spark about what you don’t know in an encounter with a patient or colleague or through patient outcomes data,” he said.
Dr. Martin, on the other hand, advocates a broad approach that involves finding out at least a little about everything in one’s field. “If you have a good base, you’re not starting from zero when you encounter a new clinical situation,” he said.
“The idea is that you don’t need to memorize most things, but you do need to know how to access them,” Dr. Martin said. “I memorize the things I do all the time, such as dosing or indicated testing, but I look up things that I don’t see that often and ones that have some complexity.”
Updating the old ways
For generations, doctors have stayed current by going to meetings, conversing with colleagues, and reading journals, but many physicians have updated these methods through various resources on the internet.
For example, meetings went virtual during the pandemic, and now that face-to-face meetings are back, many of them retain a virtual option, said Kevin Campbell, MD, a cardiologist at Health First Medical Group, Melbourne, Fla. “I typically go to one or two conferences a year, but I also learn a lot digitally,” he said.
As to journal reading, “assessing an article is an essential skill,” Dr. Cutrer said. “It’s important to quickly decide whether a journal article is worth reading or not. One answer to this problem is to consult summaries of important articles. But summaries are sometimes unhelpful, and it is hard to know which articles are significant. Therefore, doctors have been reaching out to others who can research the articles for them.”
For many years, some physicians have pooled their resources in journal clubs. “You get a chance to cross-cultivate your skills with others,” Dr. Ioannidis said. “But you need someone who is well informed and dedicated to run the journal club, using evidence-based principles.”
Dr. Cutrer said physicians like to cast their net wide because they are understandably wary of changing their practice based on one study. “Unless there is one large study that is really well designed, doctors will need two or more findings to be convinced,” he said. This requires having the ability to match studies across many journals.
Using research summaries
In the past two decades, physicians have gained access to countless summaries of journal articles prepared by armies of clinical experts working for review services such as the New England Journal of Medicine’s “Journal Watch,” Annals of Internal Medicine’s “In the Clinic,” and BMJ’s “State of the Arts.”
In addition to summarizing findings from a wide variety of journals in plain language, reviewers may compare them to similar studies and assess the validity of the finding by assigning a level of evidence.
Some commercial ventures provide similar services. Betsy Jones, executive vice president of clinical decisions at EBSCO, said the DynaMed service is now available through an app on the physician’s smartphone or through the electronic health record.
Physicians like this approach. Many specialists have noted that reading full-length articles was not an efficient use of their time, while even more said that reviews are efficient.
Exchanging information online
Physicians are increasingly keeping current by using the internet, especially on social media, Dr. Cutrer said. “Young doctors in particular are more likely to keep up digitally,” he said.
Internet-based information has become so widespread that disparities in health care from region to region have somewhat abated, according to Stuart J. Fischer, MD, an orthopedic surgeon at Summit Orthopaedics and Sports Medicine, New Jersey. “One positive outcome of this plethora of information today is that geographic disparities in clinical practice are not as great as they used to be,” he said.
Rather than chatting up colleagues in the hallway, many physicians have come to rely on internet-based discussion boards.
Blogs, podcasts, and Twitter
Blogs and podcasts, often focused on a specialty, can be a great way for physicians to keep up, said UMass Chan professor Dr. Martin. “Podcasts in particular have enhanced the ability to stay current,” he said. “You want to find someone you trust.”
Internal medicine podcasts include Annals on Call, where doctors discuss articles in the Annals of Internal Medicine, and the Curbsiders, where two internists interview a guest expert.
Orthopedic surgeons can visit podcasts like Nailed it, Orthobullets, the Ortho Show, and Inside Orthopedics. Neurologists can consult Brainwaves, Neurology Podcast, Practical Neurology Podcast, and Clinical Neurology with KD. And pediatricians can drop in on Talking Pediatrics, The Cribsiders, and PedsCases.
Meanwhile, Twitter has become a particularly effective way to broadcast new findings, speeding up the transition from the bench to the bedside, said Dr. Campbell, the Florida cardiologist.
“I visit cardio-specific resources on Twitter,” he said. “They can be real-time video chats or posted messages. They spur discussion like a journal club. Colleagues present cases and drop in and out of the discussion.”
Others are not as enthusiastic. Although Stanford’s Dr. Ioannidis is in the heart of the Silicon Valley, he is leery of some of the new digital methods. “I don’t use Twitter,” he says. “You just add more people to the process, which could only make things more confusing. I want to be able to think a lot about it.”
Cutting-edge knowledge at the point of care
Consulting the literature often takes place at the point of care, when a particular patient requires treatment. This can be done by using clinical decision support (CDS) and by using clinical practice guidelines (CPGs), which are typically developed by panels of doctors at specialty societies.
“It used to be that the doctor was expected to know everything,” said Ms. Jones at DynaMed. “Today there is no way to keep up with it all. Doctors often need a quick memory jog.”
Ms. Jones said the CDS result always requires the doctor’s interpretation. “It is up to the doctor to decide whether a new finding is the best choice for his or her patient,” she said.
Dr. Martin recommends going easy on point-of-care resources. “They can be used for showing a patient a differential diagnosis list or checking the cost of a procedure, but they are harder to use for novel developments that require time and context to evaluate their impact,” he said.
CPGs, meanwhile, have a high profile in the research world. In a 2018 study, Dr. Ioannidis found that 8 of the 15 most-cited articles were CPGs, disease definitions, or disease statistics.
Dr. Fischer said CPGs are typically based on thorough reviews of the literature, but they do involve experts’ interpretation of the science. “It can be difficult to obtain specific answers to some medical questions, especially for problems with complex treatments or variations,” he said.
As a result, Dr. Fischer said doctors have to use their judgment in applying CPGs to a specific patient. “For example, the orthopedic surgeon would normally recommend a total hip replacement for patients with a bad hip, but it might not be appropriate for an overweight patient.”
Stay skeptical
There are many novel ways for physicians to keep current, including summaries of articles, discussion boards, blogs, podcasts, Twitter, clinical decision support, and clinical practice guidelines.
Even with all these new services, though, doctors need to retain a healthy amount of skepticism about new research findings, Dr. Ioannidis said. “Ask yourself questions such as: Does it deal with a real problem? Am I getting the real information? Is it relevant to real patients? Is it offering good value for money?”
A version of this article first appeared on Medscape.com.
making it much tougher for physicians to identify innovative findings and newer guidelines for helping patients. Yet not keeping up with the latest information can put doctors at risk.
“Most doctors are feeling lost about keeping up to date,” said John P.A. Ioannidis, MD, professor of medicine at Stanford (Calif.) University School of Medicine. “The vast majority of new studies are either wrong or not useful, but physicians cannot sort out which are those studies.”
The sheer number of new studies may even force some doctors to retreat from areas where they have not kept up, said Stephen A. Martin, MD, professor of family medicine and community health at the University of Massachusetts, Worcester. “When doctors don’t feel they can stay current, they may refer more cases to specialists or narrow their focus,” he said.
Some specialties have a greater challenge than others
Dr. Martin said the deluge of studies heavily impacts generalists because they have a wider field of information to keep up with. However, certain specialties like oncology are particularly flooded with new findings.
Specialties with the greatest number of published studies are reportedly oncology, cardiology, and neurology. A 2021 study found that the number of articles with the word “stroke” in them increased five times from 2000 to 2020. And investigative treatments targeting cancer nearly quadrupled just between 2010 and 2020.
What’s more, physicians spend a great deal of time sifting through studies that are ultimately useless. In a survey of internists by Univadis, which is part of WebMD/Medscape, 82% said that fewer than half of the studies they read actually had an impact on how they practice medicine.
“You often have to dig into an article and learn more about a finding before you now whether it’s useful,” Dr. Martin said. “And in the end, relatively few new findings are truly novel ones that are useful for patient care.”
So what can a physician do? First, find out what you don’t know
Looking for new findings needs to be carried out systematically, according to William B. Cutrer, MD, MEd, a pediatric intensivist who is associate dean for undergraduate medical education at Vanderbilt University School of Medicine, Nashville, Tenn.
“Before you start, you have to know what you don’t know, and that’s often not so easy,” he said. “You may get a spark about what you don’t know in an encounter with a patient or colleague or through patient outcomes data,” he said.
Dr. Martin, on the other hand, advocates a broad approach that involves finding out at least a little about everything in one’s field. “If you have a good base, you’re not starting from zero when you encounter a new clinical situation,” he said.
“The idea is that you don’t need to memorize most things, but you do need to know how to access them,” Dr. Martin said. “I memorize the things I do all the time, such as dosing or indicated testing, but I look up things that I don’t see that often and ones that have some complexity.”
Updating the old ways
For generations, doctors have stayed current by going to meetings, conversing with colleagues, and reading journals, but many physicians have updated these methods through various resources on the internet.
For example, meetings went virtual during the pandemic, and now that face-to-face meetings are back, many of them retain a virtual option, said Kevin Campbell, MD, a cardiologist at Health First Medical Group, Melbourne, Fla. “I typically go to one or two conferences a year, but I also learn a lot digitally,” he said.
As to journal reading, “assessing an article is an essential skill,” Dr. Cutrer said. “It’s important to quickly decide whether a journal article is worth reading or not. One answer to this problem is to consult summaries of important articles. But summaries are sometimes unhelpful, and it is hard to know which articles are significant. Therefore, doctors have been reaching out to others who can research the articles for them.”
For many years, some physicians have pooled their resources in journal clubs. “You get a chance to cross-cultivate your skills with others,” Dr. Ioannidis said. “But you need someone who is well informed and dedicated to run the journal club, using evidence-based principles.”
Dr. Cutrer said physicians like to cast their net wide because they are understandably wary of changing their practice based on one study. “Unless there is one large study that is really well designed, doctors will need two or more findings to be convinced,” he said. This requires having the ability to match studies across many journals.
Using research summaries
In the past two decades, physicians have gained access to countless summaries of journal articles prepared by armies of clinical experts working for review services such as the New England Journal of Medicine’s “Journal Watch,” Annals of Internal Medicine’s “In the Clinic,” and BMJ’s “State of the Arts.”
In addition to summarizing findings from a wide variety of journals in plain language, reviewers may compare them to similar studies and assess the validity of the finding by assigning a level of evidence.
Some commercial ventures provide similar services. Betsy Jones, executive vice president of clinical decisions at EBSCO, said the DynaMed service is now available through an app on the physician’s smartphone or through the electronic health record.
Physicians like this approach. Many specialists have noted that reading full-length articles was not an efficient use of their time, while even more said that reviews are efficient.
Exchanging information online
Physicians are increasingly keeping current by using the internet, especially on social media, Dr. Cutrer said. “Young doctors in particular are more likely to keep up digitally,” he said.
Internet-based information has become so widespread that disparities in health care from region to region have somewhat abated, according to Stuart J. Fischer, MD, an orthopedic surgeon at Summit Orthopaedics and Sports Medicine, New Jersey. “One positive outcome of this plethora of information today is that geographic disparities in clinical practice are not as great as they used to be,” he said.
Rather than chatting up colleagues in the hallway, many physicians have come to rely on internet-based discussion boards.
Blogs, podcasts, and Twitter
Blogs and podcasts, often focused on a specialty, can be a great way for physicians to keep up, said UMass Chan professor Dr. Martin. “Podcasts in particular have enhanced the ability to stay current,” he said. “You want to find someone you trust.”
Internal medicine podcasts include Annals on Call, where doctors discuss articles in the Annals of Internal Medicine, and the Curbsiders, where two internists interview a guest expert.
Orthopedic surgeons can visit podcasts like Nailed it, Orthobullets, the Ortho Show, and Inside Orthopedics. Neurologists can consult Brainwaves, Neurology Podcast, Practical Neurology Podcast, and Clinical Neurology with KD. And pediatricians can drop in on Talking Pediatrics, The Cribsiders, and PedsCases.
Meanwhile, Twitter has become a particularly effective way to broadcast new findings, speeding up the transition from the bench to the bedside, said Dr. Campbell, the Florida cardiologist.
“I visit cardio-specific resources on Twitter,” he said. “They can be real-time video chats or posted messages. They spur discussion like a journal club. Colleagues present cases and drop in and out of the discussion.”
Others are not as enthusiastic. Although Stanford’s Dr. Ioannidis is in the heart of the Silicon Valley, he is leery of some of the new digital methods. “I don’t use Twitter,” he says. “You just add more people to the process, which could only make things more confusing. I want to be able to think a lot about it.”
Cutting-edge knowledge at the point of care
Consulting the literature often takes place at the point of care, when a particular patient requires treatment. This can be done by using clinical decision support (CDS) and by using clinical practice guidelines (CPGs), which are typically developed by panels of doctors at specialty societies.
“It used to be that the doctor was expected to know everything,” said Ms. Jones at DynaMed. “Today there is no way to keep up with it all. Doctors often need a quick memory jog.”
Ms. Jones said the CDS result always requires the doctor’s interpretation. “It is up to the doctor to decide whether a new finding is the best choice for his or her patient,” she said.
Dr. Martin recommends going easy on point-of-care resources. “They can be used for showing a patient a differential diagnosis list or checking the cost of a procedure, but they are harder to use for novel developments that require time and context to evaluate their impact,” he said.
CPGs, meanwhile, have a high profile in the research world. In a 2018 study, Dr. Ioannidis found that 8 of the 15 most-cited articles were CPGs, disease definitions, or disease statistics.
Dr. Fischer said CPGs are typically based on thorough reviews of the literature, but they do involve experts’ interpretation of the science. “It can be difficult to obtain specific answers to some medical questions, especially for problems with complex treatments or variations,” he said.
As a result, Dr. Fischer said doctors have to use their judgment in applying CPGs to a specific patient. “For example, the orthopedic surgeon would normally recommend a total hip replacement for patients with a bad hip, but it might not be appropriate for an overweight patient.”
Stay skeptical
There are many novel ways for physicians to keep current, including summaries of articles, discussion boards, blogs, podcasts, Twitter, clinical decision support, and clinical practice guidelines.
Even with all these new services, though, doctors need to retain a healthy amount of skepticism about new research findings, Dr. Ioannidis said. “Ask yourself questions such as: Does it deal with a real problem? Am I getting the real information? Is it relevant to real patients? Is it offering good value for money?”
A version of this article first appeared on Medscape.com.
