User login
Oncologists’ wealth and debt: COVID had little impact
concludes the latest Medscape Oncologist Wealth & Debt Report 2022.
Comparing the findings with those in the larger Medscape Physician Wealth & Debt Report 2022, which surveyed more than 13,000 physicians in 29 specialties, the findings for oncologists show how they compare with those who chose other paths in medicine.
Oncologists’ income rose, on average, by 2% in the past year and now stands at an average of $411,000 annually, up from $403,000 in the 2021 report.
This puts oncologists in the top third of specialties, with plastic surgeons again in the top slot (with average income of $576,000 in 2022).
One-fifth (20%) of oncologists surveyed reported a family worth of more than $5 million, which represents substantial family wealth, the report comments.
However, 22% of oncologists reported that their family net worth was less than $500,000, and another 10% estimated that it to fall between $500,000 and $1 million.
For comparison, the average U.S. family net worth is about $749,000, according to data from the Federal Reserve.
Most live ‘within their means’
Most oncologists (94%) and also most (94%) of all of the physicians surveyed said that they live within or below their means.
How does one do this? Just paying off credit cards each month and contributing enough to a 401(k) account to receive an employer match does not meet this standard, said Joel Greenwald MD, CFP, a wealth management advisor for physicians. To live within or below your means, you also need to be saving at least 20% toward retirement, pay down student loans, contribute to your kids’ college savings, and set aside rainy day cash, he explained.
When physicians were asked about their favorite cost-cutting tactics, replies included bringing lunch to work, keeping a car for 15 years, and carrying out their own household maintenance and repairs. One doctor described a “24-hour rule” when it comes to shopping: “Revisit the desired purchase after 24 hours to see if it’s still desired.”
But how well do these tactics go down with ‘the other half’ and the rest of the household? Two-thirds (66%) of oncologists, and a similar proportion of all physicians, said that they argue with their significant other about spending. This appears to be high in comparison with the finding from a recent survey that across the United States, about one in four couples (25%) argue about money at least once a month.
Regarding spending, the top expense among oncologists was for childcare (16%), private tuition for offspring (14%), mortgage on a second home (14%), college tuition for offspring (14%), and a car lease (12%).
Around 17% of oncologists reported that they are still paying off their own college or medical school loans. For this statistic, they are about in the middle of all specialties.
The report notes that freeing oneself from medical school debt is very costly. Physicians in the United States pay an average of $356,000-$440,000, about half of which is interest.
Little change over 2021
The COVID pandemic had much less of an impact on physicians than it had on the general population when it comes to keeping up with payments, and most physicians were not affected. Only 3% of oncologists said they fell behind with payments for mortgage; 6% fell behind with payments for other bills.
In comparison, nearly half (46%) of Americans missed one or more payments of rent or mortgage because of COVID, according to a 2021 industry survey.
Over the past year, most oncologists (70%) did not change their spending habits, and only 11% cut expenses by deferring or refinancing loans. Also, most oncologists (75%) avoided major financial loses. Only 8% reported financial losses because of problems at their medical practice.
However, a slightly higher percentage of oncologists reported a stock or company investment that had turned sour in 2022 (37%) in comparison with 2021 (28%).
A version of this article first appeared on Medscape.com.
concludes the latest Medscape Oncologist Wealth & Debt Report 2022.
Comparing the findings with those in the larger Medscape Physician Wealth & Debt Report 2022, which surveyed more than 13,000 physicians in 29 specialties, the findings for oncologists show how they compare with those who chose other paths in medicine.
Oncologists’ income rose, on average, by 2% in the past year and now stands at an average of $411,000 annually, up from $403,000 in the 2021 report.
This puts oncologists in the top third of specialties, with plastic surgeons again in the top slot (with average income of $576,000 in 2022).
One-fifth (20%) of oncologists surveyed reported a family worth of more than $5 million, which represents substantial family wealth, the report comments.
However, 22% of oncologists reported that their family net worth was less than $500,000, and another 10% estimated that it to fall between $500,000 and $1 million.
For comparison, the average U.S. family net worth is about $749,000, according to data from the Federal Reserve.
Most live ‘within their means’
Most oncologists (94%) and also most (94%) of all of the physicians surveyed said that they live within or below their means.
How does one do this? Just paying off credit cards each month and contributing enough to a 401(k) account to receive an employer match does not meet this standard, said Joel Greenwald MD, CFP, a wealth management advisor for physicians. To live within or below your means, you also need to be saving at least 20% toward retirement, pay down student loans, contribute to your kids’ college savings, and set aside rainy day cash, he explained.
When physicians were asked about their favorite cost-cutting tactics, replies included bringing lunch to work, keeping a car for 15 years, and carrying out their own household maintenance and repairs. One doctor described a “24-hour rule” when it comes to shopping: “Revisit the desired purchase after 24 hours to see if it’s still desired.”
But how well do these tactics go down with ‘the other half’ and the rest of the household? Two-thirds (66%) of oncologists, and a similar proportion of all physicians, said that they argue with their significant other about spending. This appears to be high in comparison with the finding from a recent survey that across the United States, about one in four couples (25%) argue about money at least once a month.
Regarding spending, the top expense among oncologists was for childcare (16%), private tuition for offspring (14%), mortgage on a second home (14%), college tuition for offspring (14%), and a car lease (12%).
Around 17% of oncologists reported that they are still paying off their own college or medical school loans. For this statistic, they are about in the middle of all specialties.
The report notes that freeing oneself from medical school debt is very costly. Physicians in the United States pay an average of $356,000-$440,000, about half of which is interest.
Little change over 2021
The COVID pandemic had much less of an impact on physicians than it had on the general population when it comes to keeping up with payments, and most physicians were not affected. Only 3% of oncologists said they fell behind with payments for mortgage; 6% fell behind with payments for other bills.
In comparison, nearly half (46%) of Americans missed one or more payments of rent or mortgage because of COVID, according to a 2021 industry survey.
Over the past year, most oncologists (70%) did not change their spending habits, and only 11% cut expenses by deferring or refinancing loans. Also, most oncologists (75%) avoided major financial loses. Only 8% reported financial losses because of problems at their medical practice.
However, a slightly higher percentage of oncologists reported a stock or company investment that had turned sour in 2022 (37%) in comparison with 2021 (28%).
A version of this article first appeared on Medscape.com.
concludes the latest Medscape Oncologist Wealth & Debt Report 2022.
Comparing the findings with those in the larger Medscape Physician Wealth & Debt Report 2022, which surveyed more than 13,000 physicians in 29 specialties, the findings for oncologists show how they compare with those who chose other paths in medicine.
Oncologists’ income rose, on average, by 2% in the past year and now stands at an average of $411,000 annually, up from $403,000 in the 2021 report.
This puts oncologists in the top third of specialties, with plastic surgeons again in the top slot (with average income of $576,000 in 2022).
One-fifth (20%) of oncologists surveyed reported a family worth of more than $5 million, which represents substantial family wealth, the report comments.
However, 22% of oncologists reported that their family net worth was less than $500,000, and another 10% estimated that it to fall between $500,000 and $1 million.
For comparison, the average U.S. family net worth is about $749,000, according to data from the Federal Reserve.
Most live ‘within their means’
Most oncologists (94%) and also most (94%) of all of the physicians surveyed said that they live within or below their means.
How does one do this? Just paying off credit cards each month and contributing enough to a 401(k) account to receive an employer match does not meet this standard, said Joel Greenwald MD, CFP, a wealth management advisor for physicians. To live within or below your means, you also need to be saving at least 20% toward retirement, pay down student loans, contribute to your kids’ college savings, and set aside rainy day cash, he explained.
When physicians were asked about their favorite cost-cutting tactics, replies included bringing lunch to work, keeping a car for 15 years, and carrying out their own household maintenance and repairs. One doctor described a “24-hour rule” when it comes to shopping: “Revisit the desired purchase after 24 hours to see if it’s still desired.”
But how well do these tactics go down with ‘the other half’ and the rest of the household? Two-thirds (66%) of oncologists, and a similar proportion of all physicians, said that they argue with their significant other about spending. This appears to be high in comparison with the finding from a recent survey that across the United States, about one in four couples (25%) argue about money at least once a month.
Regarding spending, the top expense among oncologists was for childcare (16%), private tuition for offspring (14%), mortgage on a second home (14%), college tuition for offspring (14%), and a car lease (12%).
Around 17% of oncologists reported that they are still paying off their own college or medical school loans. For this statistic, they are about in the middle of all specialties.
The report notes that freeing oneself from medical school debt is very costly. Physicians in the United States pay an average of $356,000-$440,000, about half of which is interest.
Little change over 2021
The COVID pandemic had much less of an impact on physicians than it had on the general population when it comes to keeping up with payments, and most physicians were not affected. Only 3% of oncologists said they fell behind with payments for mortgage; 6% fell behind with payments for other bills.
In comparison, nearly half (46%) of Americans missed one or more payments of rent or mortgage because of COVID, according to a 2021 industry survey.
Over the past year, most oncologists (70%) did not change their spending habits, and only 11% cut expenses by deferring or refinancing loans. Also, most oncologists (75%) avoided major financial loses. Only 8% reported financial losses because of problems at their medical practice.
However, a slightly higher percentage of oncologists reported a stock or company investment that had turned sour in 2022 (37%) in comparison with 2021 (28%).
A version of this article first appeared on Medscape.com.
Doctors using fake positive reviews to boost business
Five years ago, Kay Dean relied upon Yelp! and Google reviews in her search for a doctor in her area. After finding a physician with fairly high reviews, Ms. Dean was shocked when her personal experience was significantly worse than patients on the review platforms.
Following her experience, Ms. Dean, a former federal government investigator, became skeptical and used her skills to investigate the practice on all review platforms. She uncovered that the practice had a review from an individual who was involved in a review trading group on Facebook, where organizations openly barter their services in exchange for positive reviews fraud.
“I discovered that the online review world was just saturated with fake reviews, much more so than I think most people are aware ... and law enforcement regulators aren’t doing anything to address the problem,” said Ms. Dean. “In this online space, it’s the Wild West; cheating is rewarded.”
Ms. Dean decided to take matters into her own hands. She created a YouTube channel called Fake Review Watch, where she exposes real businesses and their attempts to dupe potential consumers with fake positive reviews.
For example, one video analyzes an orthopedic surgeon in Manhattan with an abundance of five-star reviews. Through her detailed analysis, Ms. Dean created a spreadsheet of the 26 alleged patients of the orthopedic surgeon that had submitted glowing reviews. She looked into other businesses that the individuals had left reviews for and found a significant amount of overlap.
According to the video, 19 of the doctor’s reviewers had left high reviews for the same moving company in Las Vegas, and 18 of them reviewed the same locksmith in Texas. Overall, eight of the patients reviewed the same mover, locksmith, and hotel in New Zealand.
A matter of trust
Ms. Dean expressed the gravity of this phenomenon, especially in health care, as patients often head online first when searching for care options. Based on a survey by Software Advice, about 84% of patients use online reviews to assess a physician, and 77% use review sites as the first step in finding a doctor.
Patient trust has continued to diminish in recent years, particularly following the pandemic. In a 2021 global ranking of trust levels towards health care by country, the U.S. health care system ranked 19th, far below those of several developing countries.
Owing to the rise of fake patient reviews and their inscrutable nature, Ms. Dean advises staying away from online review platforms. Instead, she suggests sticking to the old-fashioned method of getting recommendations from friends and relatives, not virtual people.
Ms. Dean explained a few indicators that she looks for when trying to identify a fake review.
“The business has all five-star reviews, negative reviews are followed by five-star reviews, or the business has an abnormal number of positive reviews in a short period of time,” she noted. “Some businesses try to bury legitimate negative reviews by obtaining more recent, fake, positive ones. The recent reviews will contradict the specific criticisms in the negative review.”
She warned that consumers should not give credibility to reviews simply because the reviewer is dubbed “Elite” or a Google Local Guide, because she has seen plenty of these individuals posting fake reviews.
Unfortunately, review platforms haven’t been doing much self-policing. Google and Healthgrades have a series of policies against fake engagement, impersonation, misinformation, and misrepresentation, according to their websites. However, the only consequence of these violations is review removal.
Both Yelp! and Google say they have automated software that distinguishes real versus fake reviews. When Yelp! uncovers users engaging in compensation review activity, it removes their reviews, closes their account, and blocks those users from creating future Yelp! accounts.
Physicians’ basis
Moreover,
“I think there’s an erosion of business ethics because cheating is rewarded. You can’t compete in an environment where your competition is allowed to accumulate numerous fake reviews while you’re still trying to fill chairs in your business,” said Ms. Dean. “Your competition is then getting the business because the tech companies are allowing this fraud.”
Family physician and practice owner Mike Woo-Ming, MD, MPH, provides career coaching for physicians, including maintaining a good reputation – in-person and online. He has seen physicians bumping up their own five-star reviews personally as well as posting negative reviews for their competition.
“I’ve seen where they’re going to lose business, as many practices were affected through COVID,” he said. “Business owners can become desperate and may decide to start posting or buying reviews because they know people will choose certain services these days based upon reviews.”
Dr. Woo-Ming expressed his frustration with fellow physicians who give in to purchasing fake reviews, because the patients have no idea whether reviews are genuine or not.
To encourage genuine positive reviews, Dr. Woo-Ming’s practice uses a third-party app system that sends patients a follow-up email or text asking about their experience with a link to review sites.
“Honest reviews are a reflection of what I can do to improve my business. At the end of the day, if you’re truly providing great service and you’re helping people by providing great medical care, those are going to win out,” he said. “I would rather, as a responsible practice owner, improve the experience and outcome for the patient.”
A version of this article first appeared on Medscape.com.
Five years ago, Kay Dean relied upon Yelp! and Google reviews in her search for a doctor in her area. After finding a physician with fairly high reviews, Ms. Dean was shocked when her personal experience was significantly worse than patients on the review platforms.
Following her experience, Ms. Dean, a former federal government investigator, became skeptical and used her skills to investigate the practice on all review platforms. She uncovered that the practice had a review from an individual who was involved in a review trading group on Facebook, where organizations openly barter their services in exchange for positive reviews fraud.
“I discovered that the online review world was just saturated with fake reviews, much more so than I think most people are aware ... and law enforcement regulators aren’t doing anything to address the problem,” said Ms. Dean. “In this online space, it’s the Wild West; cheating is rewarded.”
Ms. Dean decided to take matters into her own hands. She created a YouTube channel called Fake Review Watch, where she exposes real businesses and their attempts to dupe potential consumers with fake positive reviews.
For example, one video analyzes an orthopedic surgeon in Manhattan with an abundance of five-star reviews. Through her detailed analysis, Ms. Dean created a spreadsheet of the 26 alleged patients of the orthopedic surgeon that had submitted glowing reviews. She looked into other businesses that the individuals had left reviews for and found a significant amount of overlap.
According to the video, 19 of the doctor’s reviewers had left high reviews for the same moving company in Las Vegas, and 18 of them reviewed the same locksmith in Texas. Overall, eight of the patients reviewed the same mover, locksmith, and hotel in New Zealand.
A matter of trust
Ms. Dean expressed the gravity of this phenomenon, especially in health care, as patients often head online first when searching for care options. Based on a survey by Software Advice, about 84% of patients use online reviews to assess a physician, and 77% use review sites as the first step in finding a doctor.
Patient trust has continued to diminish in recent years, particularly following the pandemic. In a 2021 global ranking of trust levels towards health care by country, the U.S. health care system ranked 19th, far below those of several developing countries.
Owing to the rise of fake patient reviews and their inscrutable nature, Ms. Dean advises staying away from online review platforms. Instead, she suggests sticking to the old-fashioned method of getting recommendations from friends and relatives, not virtual people.
Ms. Dean explained a few indicators that she looks for when trying to identify a fake review.
“The business has all five-star reviews, negative reviews are followed by five-star reviews, or the business has an abnormal number of positive reviews in a short period of time,” she noted. “Some businesses try to bury legitimate negative reviews by obtaining more recent, fake, positive ones. The recent reviews will contradict the specific criticisms in the negative review.”
She warned that consumers should not give credibility to reviews simply because the reviewer is dubbed “Elite” or a Google Local Guide, because she has seen plenty of these individuals posting fake reviews.
Unfortunately, review platforms haven’t been doing much self-policing. Google and Healthgrades have a series of policies against fake engagement, impersonation, misinformation, and misrepresentation, according to their websites. However, the only consequence of these violations is review removal.
Both Yelp! and Google say they have automated software that distinguishes real versus fake reviews. When Yelp! uncovers users engaging in compensation review activity, it removes their reviews, closes their account, and blocks those users from creating future Yelp! accounts.
Physicians’ basis
Moreover,
“I think there’s an erosion of business ethics because cheating is rewarded. You can’t compete in an environment where your competition is allowed to accumulate numerous fake reviews while you’re still trying to fill chairs in your business,” said Ms. Dean. “Your competition is then getting the business because the tech companies are allowing this fraud.”
Family physician and practice owner Mike Woo-Ming, MD, MPH, provides career coaching for physicians, including maintaining a good reputation – in-person and online. He has seen physicians bumping up their own five-star reviews personally as well as posting negative reviews for their competition.
“I’ve seen where they’re going to lose business, as many practices were affected through COVID,” he said. “Business owners can become desperate and may decide to start posting or buying reviews because they know people will choose certain services these days based upon reviews.”
Dr. Woo-Ming expressed his frustration with fellow physicians who give in to purchasing fake reviews, because the patients have no idea whether reviews are genuine or not.
To encourage genuine positive reviews, Dr. Woo-Ming’s practice uses a third-party app system that sends patients a follow-up email or text asking about their experience with a link to review sites.
“Honest reviews are a reflection of what I can do to improve my business. At the end of the day, if you’re truly providing great service and you’re helping people by providing great medical care, those are going to win out,” he said. “I would rather, as a responsible practice owner, improve the experience and outcome for the patient.”
A version of this article first appeared on Medscape.com.
Five years ago, Kay Dean relied upon Yelp! and Google reviews in her search for a doctor in her area. After finding a physician with fairly high reviews, Ms. Dean was shocked when her personal experience was significantly worse than patients on the review platforms.
Following her experience, Ms. Dean, a former federal government investigator, became skeptical and used her skills to investigate the practice on all review platforms. She uncovered that the practice had a review from an individual who was involved in a review trading group on Facebook, where organizations openly barter their services in exchange for positive reviews fraud.
“I discovered that the online review world was just saturated with fake reviews, much more so than I think most people are aware ... and law enforcement regulators aren’t doing anything to address the problem,” said Ms. Dean. “In this online space, it’s the Wild West; cheating is rewarded.”
Ms. Dean decided to take matters into her own hands. She created a YouTube channel called Fake Review Watch, where she exposes real businesses and their attempts to dupe potential consumers with fake positive reviews.
For example, one video analyzes an orthopedic surgeon in Manhattan with an abundance of five-star reviews. Through her detailed analysis, Ms. Dean created a spreadsheet of the 26 alleged patients of the orthopedic surgeon that had submitted glowing reviews. She looked into other businesses that the individuals had left reviews for and found a significant amount of overlap.
According to the video, 19 of the doctor’s reviewers had left high reviews for the same moving company in Las Vegas, and 18 of them reviewed the same locksmith in Texas. Overall, eight of the patients reviewed the same mover, locksmith, and hotel in New Zealand.
A matter of trust
Ms. Dean expressed the gravity of this phenomenon, especially in health care, as patients often head online first when searching for care options. Based on a survey by Software Advice, about 84% of patients use online reviews to assess a physician, and 77% use review sites as the first step in finding a doctor.
Patient trust has continued to diminish in recent years, particularly following the pandemic. In a 2021 global ranking of trust levels towards health care by country, the U.S. health care system ranked 19th, far below those of several developing countries.
Owing to the rise of fake patient reviews and their inscrutable nature, Ms. Dean advises staying away from online review platforms. Instead, she suggests sticking to the old-fashioned method of getting recommendations from friends and relatives, not virtual people.
Ms. Dean explained a few indicators that she looks for when trying to identify a fake review.
“The business has all five-star reviews, negative reviews are followed by five-star reviews, or the business has an abnormal number of positive reviews in a short period of time,” she noted. “Some businesses try to bury legitimate negative reviews by obtaining more recent, fake, positive ones. The recent reviews will contradict the specific criticisms in the negative review.”
She warned that consumers should not give credibility to reviews simply because the reviewer is dubbed “Elite” or a Google Local Guide, because she has seen plenty of these individuals posting fake reviews.
Unfortunately, review platforms haven’t been doing much self-policing. Google and Healthgrades have a series of policies against fake engagement, impersonation, misinformation, and misrepresentation, according to their websites. However, the only consequence of these violations is review removal.
Both Yelp! and Google say they have automated software that distinguishes real versus fake reviews. When Yelp! uncovers users engaging in compensation review activity, it removes their reviews, closes their account, and blocks those users from creating future Yelp! accounts.
Physicians’ basis
Moreover,
“I think there’s an erosion of business ethics because cheating is rewarded. You can’t compete in an environment where your competition is allowed to accumulate numerous fake reviews while you’re still trying to fill chairs in your business,” said Ms. Dean. “Your competition is then getting the business because the tech companies are allowing this fraud.”
Family physician and practice owner Mike Woo-Ming, MD, MPH, provides career coaching for physicians, including maintaining a good reputation – in-person and online. He has seen physicians bumping up their own five-star reviews personally as well as posting negative reviews for their competition.
“I’ve seen where they’re going to lose business, as many practices were affected through COVID,” he said. “Business owners can become desperate and may decide to start posting or buying reviews because they know people will choose certain services these days based upon reviews.”
Dr. Woo-Ming expressed his frustration with fellow physicians who give in to purchasing fake reviews, because the patients have no idea whether reviews are genuine or not.
To encourage genuine positive reviews, Dr. Woo-Ming’s practice uses a third-party app system that sends patients a follow-up email or text asking about their experience with a link to review sites.
“Honest reviews are a reflection of what I can do to improve my business. At the end of the day, if you’re truly providing great service and you’re helping people by providing great medical care, those are going to win out,” he said. “I would rather, as a responsible practice owner, improve the experience and outcome for the patient.”
A version of this article first appeared on Medscape.com.
Patients who won’t pay: What’s your recourse?
Owing to the pandemic, job loss, and the possible loss of health insurance, patients have had more difficulty managing copays, coinsurance, and deductibles, not to mention other out-of-pocket health care charges.
“Many of our patients have lost their jobs or have had their hours cut back, and as a result, they are struggling to make ends meet,” said Ahmad Chaudhry, MD, a cardiothoracic surgeon in Lexington, Ky. “However, we cannot continue to provide care if our patients do not pay their bills.”
This news organization asked physicians what they do when their patients don’t pay. About 43% said that they continue to treat them and develop a payment plan; 13% send their bill to collections; 12% continue their care and write off their balance, and 25% choose other actions. Only 8% of physicians drop patients if they don’t pay.
Because you need to pay your own bills, what can you do about nonpaying patients?
Start with price transparency
In the past, patients never knew what their lab work or a chest EKG would cost because it wasn’t listed anywhere, and it was usually more than expected. Because of new legislation concerning health care price transparency, hospitals, health plans, and insurers must pony up with the actual fees, making them transparent to patients. Physician practices should follow suit and keep prices transparent too. Patients are more likely to pay their bills when prepared for the expense.
Patients with insurance often don’t know what they’ll be paying for their visit or their tests because they don’t know how much insurance will cover and what will be left for them to pay. Also, they may not know if they’ve met their deductible yet so they’re unsure whether insurance will even kick in. And patients without insurance still need to know what their costs will be upfront.
According to 10 insights from the Primary Care Consumer Choice Survey, 74% of health care consumers were willing to pay a $50 out-of-pocket charge to know the cost of their primary care visit.
Provide payment plans
Many patients have always needed payment plans. It’s one thing to post a sign at check-in telling patients that all monies are due at the time of service, but it’s another reality for a patient who can’t fork over the $250 charge they just unexpectedly spent in your office.
Discover Financial Services recently ran a survey, with results presented in the press release Americans are Delaying Non-Emergency Medical Care in Higher Numbers than Last Year, and found that many Americans with medical debt are delaying nonemergency medical care. For example, they put off seeing a specialist (52%), seeing a doctor for sickness (41%), and undergoing treatment plans recommended by their doctor (31%).
Turning an account over to collections should be a last resort. In addition, agencies typically charge 30%-40% of the total collected off the top.
Though collecting that amount is better than nothing, using a collection agency may have unexpected consequences. For instance, you’re trusting the agency you hire to collect to represent you and act on your practice’s behalf. If they’re rude or their tactics are harsh in the eyes of the patient or their relatives, it’s your reputation that is on the line.
Rather than use a collection agency, you could collect the payments yourself. When a patient fails to pay within about 3 months, begin mailing statements from the office, followed by firm but generous phone calls trying to collect. Industry estimates put the average cost of sending an invoice, including staff labor, printing, and postage, at about $35 per mailer. Some practices combat the added costs by offering a 20% prompt-pay discount. Offering payment plans is another option that helps garner eventual payment. Plus, practices should direct patients to third-party lenders such as CareCredit for larger bills.
On occasion, some small practices may allow a swap, such as allowing a patient to provide a service such as plumbing, electrical, or painting in exchange for working off the bill. Though it’s not ideal when it comes to finances, you may find it can work in a pinch for a cash-strapped patient. Make sure to keep records of what bills the patient’s work goes toward.
It often helps to incentivize your billing staff to follow up regularly, with various suggestions and tactics, to get patients to pay their bills. The incentive amount you offer will probably be less than if you had to use a collection agency.
Have a payment policy
Because your practice’s primary job is caring for patients’ physical and emotional needs, payment collection without coming off as insensitive can be tricky. “We understand these are difficult times for everyone, and we are doing our best to work with our patients,” said Dr. Chaudhry. Having a written payment policy can help build the bridge. A policy lets patients know what they can expect and can help prevent surprises over what occurs in the event of nonpayment. Your written policy should include:
- When payment is due.
- How the practice handles copays and deductibles.
- What forms of payment are accepted.
- Your policy regarding nonpayment.
Why patients don’t pay
A 2021 Healthcare Consumer Experience Study from Cedar found that medical bills are a source of anxiety and frustration for most patients, affecting their financial experience. More than half of the respondents said that paying a medical bill is stressful. Complicating matters, many health care practices rely on outdated payment systems, which may not provide patients with a clear view of what they owe and how to pay it.
The study found that 53% of respondents find understanding their plan’s coverage and benefits stressful, and 37% of patients won’t pay their bill if they can’t understand it.
People may think the patient is trying to get out of paying, which, of course, is sometimes true, but most of the time they want to pay, concluded the study. Most patients need a better explanation, communication, and accurate accounting of their out-of-pocket costs.
What can doctors do?
If you’re a physician who regularly sees patients who have problems paying their bills, you can take a few steps to minimize the financial impact on your practice:
- Bill the patient’s insurance directly to ensure you receive at least partial payment.
- Keep adequate records of services in case you need to pursue legal action.
- “Be understanding and flexible when it comes to payment arrangements, as this can often be the difference between getting paid and not getting paid at all,” said Dr. Chaudhry.
Distance yourself
When discussing payment policies, physicians should try to distance themselves from the actual collection process as much as possible. Well-meaning physicians often tell patients things like they can “figure something out “ financially or “work them in” during a scheduling conflict, but that often undermines the authority and credibility of the practice’s office staff. Plus, it teaches patients they can get their way if they work on the doctor’s soft spot – something you don’t want to encourage.
By following some of these measures, you can help ensure that your practice continues to thrive despite the challenges posed by nonpaying patients.
A version of this article first appeared on Medscape.com.
Owing to the pandemic, job loss, and the possible loss of health insurance, patients have had more difficulty managing copays, coinsurance, and deductibles, not to mention other out-of-pocket health care charges.
“Many of our patients have lost their jobs or have had their hours cut back, and as a result, they are struggling to make ends meet,” said Ahmad Chaudhry, MD, a cardiothoracic surgeon in Lexington, Ky. “However, we cannot continue to provide care if our patients do not pay their bills.”
This news organization asked physicians what they do when their patients don’t pay. About 43% said that they continue to treat them and develop a payment plan; 13% send their bill to collections; 12% continue their care and write off their balance, and 25% choose other actions. Only 8% of physicians drop patients if they don’t pay.
Because you need to pay your own bills, what can you do about nonpaying patients?
Start with price transparency
In the past, patients never knew what their lab work or a chest EKG would cost because it wasn’t listed anywhere, and it was usually more than expected. Because of new legislation concerning health care price transparency, hospitals, health plans, and insurers must pony up with the actual fees, making them transparent to patients. Physician practices should follow suit and keep prices transparent too. Patients are more likely to pay their bills when prepared for the expense.
Patients with insurance often don’t know what they’ll be paying for their visit or their tests because they don’t know how much insurance will cover and what will be left for them to pay. Also, they may not know if they’ve met their deductible yet so they’re unsure whether insurance will even kick in. And patients without insurance still need to know what their costs will be upfront.
According to 10 insights from the Primary Care Consumer Choice Survey, 74% of health care consumers were willing to pay a $50 out-of-pocket charge to know the cost of their primary care visit.
Provide payment plans
Many patients have always needed payment plans. It’s one thing to post a sign at check-in telling patients that all monies are due at the time of service, but it’s another reality for a patient who can’t fork over the $250 charge they just unexpectedly spent in your office.
Discover Financial Services recently ran a survey, with results presented in the press release Americans are Delaying Non-Emergency Medical Care in Higher Numbers than Last Year, and found that many Americans with medical debt are delaying nonemergency medical care. For example, they put off seeing a specialist (52%), seeing a doctor for sickness (41%), and undergoing treatment plans recommended by their doctor (31%).
Turning an account over to collections should be a last resort. In addition, agencies typically charge 30%-40% of the total collected off the top.
Though collecting that amount is better than nothing, using a collection agency may have unexpected consequences. For instance, you’re trusting the agency you hire to collect to represent you and act on your practice’s behalf. If they’re rude or their tactics are harsh in the eyes of the patient or their relatives, it’s your reputation that is on the line.
Rather than use a collection agency, you could collect the payments yourself. When a patient fails to pay within about 3 months, begin mailing statements from the office, followed by firm but generous phone calls trying to collect. Industry estimates put the average cost of sending an invoice, including staff labor, printing, and postage, at about $35 per mailer. Some practices combat the added costs by offering a 20% prompt-pay discount. Offering payment plans is another option that helps garner eventual payment. Plus, practices should direct patients to third-party lenders such as CareCredit for larger bills.
On occasion, some small practices may allow a swap, such as allowing a patient to provide a service such as plumbing, electrical, or painting in exchange for working off the bill. Though it’s not ideal when it comes to finances, you may find it can work in a pinch for a cash-strapped patient. Make sure to keep records of what bills the patient’s work goes toward.
It often helps to incentivize your billing staff to follow up regularly, with various suggestions and tactics, to get patients to pay their bills. The incentive amount you offer will probably be less than if you had to use a collection agency.
Have a payment policy
Because your practice’s primary job is caring for patients’ physical and emotional needs, payment collection without coming off as insensitive can be tricky. “We understand these are difficult times for everyone, and we are doing our best to work with our patients,” said Dr. Chaudhry. Having a written payment policy can help build the bridge. A policy lets patients know what they can expect and can help prevent surprises over what occurs in the event of nonpayment. Your written policy should include:
- When payment is due.
- How the practice handles copays and deductibles.
- What forms of payment are accepted.
- Your policy regarding nonpayment.
Why patients don’t pay
A 2021 Healthcare Consumer Experience Study from Cedar found that medical bills are a source of anxiety and frustration for most patients, affecting their financial experience. More than half of the respondents said that paying a medical bill is stressful. Complicating matters, many health care practices rely on outdated payment systems, which may not provide patients with a clear view of what they owe and how to pay it.
The study found that 53% of respondents find understanding their plan’s coverage and benefits stressful, and 37% of patients won’t pay their bill if they can’t understand it.
People may think the patient is trying to get out of paying, which, of course, is sometimes true, but most of the time they want to pay, concluded the study. Most patients need a better explanation, communication, and accurate accounting of their out-of-pocket costs.
What can doctors do?
If you’re a physician who regularly sees patients who have problems paying their bills, you can take a few steps to minimize the financial impact on your practice:
- Bill the patient’s insurance directly to ensure you receive at least partial payment.
- Keep adequate records of services in case you need to pursue legal action.
- “Be understanding and flexible when it comes to payment arrangements, as this can often be the difference between getting paid and not getting paid at all,” said Dr. Chaudhry.
Distance yourself
When discussing payment policies, physicians should try to distance themselves from the actual collection process as much as possible. Well-meaning physicians often tell patients things like they can “figure something out “ financially or “work them in” during a scheduling conflict, but that often undermines the authority and credibility of the practice’s office staff. Plus, it teaches patients they can get their way if they work on the doctor’s soft spot – something you don’t want to encourage.
By following some of these measures, you can help ensure that your practice continues to thrive despite the challenges posed by nonpaying patients.
A version of this article first appeared on Medscape.com.
Owing to the pandemic, job loss, and the possible loss of health insurance, patients have had more difficulty managing copays, coinsurance, and deductibles, not to mention other out-of-pocket health care charges.
“Many of our patients have lost their jobs or have had their hours cut back, and as a result, they are struggling to make ends meet,” said Ahmad Chaudhry, MD, a cardiothoracic surgeon in Lexington, Ky. “However, we cannot continue to provide care if our patients do not pay their bills.”
This news organization asked physicians what they do when their patients don’t pay. About 43% said that they continue to treat them and develop a payment plan; 13% send their bill to collections; 12% continue their care and write off their balance, and 25% choose other actions. Only 8% of physicians drop patients if they don’t pay.
Because you need to pay your own bills, what can you do about nonpaying patients?
Start with price transparency
In the past, patients never knew what their lab work or a chest EKG would cost because it wasn’t listed anywhere, and it was usually more than expected. Because of new legislation concerning health care price transparency, hospitals, health plans, and insurers must pony up with the actual fees, making them transparent to patients. Physician practices should follow suit and keep prices transparent too. Patients are more likely to pay their bills when prepared for the expense.
Patients with insurance often don’t know what they’ll be paying for their visit or their tests because they don’t know how much insurance will cover and what will be left for them to pay. Also, they may not know if they’ve met their deductible yet so they’re unsure whether insurance will even kick in. And patients without insurance still need to know what their costs will be upfront.
According to 10 insights from the Primary Care Consumer Choice Survey, 74% of health care consumers were willing to pay a $50 out-of-pocket charge to know the cost of their primary care visit.
Provide payment plans
Many patients have always needed payment plans. It’s one thing to post a sign at check-in telling patients that all monies are due at the time of service, but it’s another reality for a patient who can’t fork over the $250 charge they just unexpectedly spent in your office.
Discover Financial Services recently ran a survey, with results presented in the press release Americans are Delaying Non-Emergency Medical Care in Higher Numbers than Last Year, and found that many Americans with medical debt are delaying nonemergency medical care. For example, they put off seeing a specialist (52%), seeing a doctor for sickness (41%), and undergoing treatment plans recommended by their doctor (31%).
Turning an account over to collections should be a last resort. In addition, agencies typically charge 30%-40% of the total collected off the top.
Though collecting that amount is better than nothing, using a collection agency may have unexpected consequences. For instance, you’re trusting the agency you hire to collect to represent you and act on your practice’s behalf. If they’re rude or their tactics are harsh in the eyes of the patient or their relatives, it’s your reputation that is on the line.
Rather than use a collection agency, you could collect the payments yourself. When a patient fails to pay within about 3 months, begin mailing statements from the office, followed by firm but generous phone calls trying to collect. Industry estimates put the average cost of sending an invoice, including staff labor, printing, and postage, at about $35 per mailer. Some practices combat the added costs by offering a 20% prompt-pay discount. Offering payment plans is another option that helps garner eventual payment. Plus, practices should direct patients to third-party lenders such as CareCredit for larger bills.
On occasion, some small practices may allow a swap, such as allowing a patient to provide a service such as plumbing, electrical, or painting in exchange for working off the bill. Though it’s not ideal when it comes to finances, you may find it can work in a pinch for a cash-strapped patient. Make sure to keep records of what bills the patient’s work goes toward.
It often helps to incentivize your billing staff to follow up regularly, with various suggestions and tactics, to get patients to pay their bills. The incentive amount you offer will probably be less than if you had to use a collection agency.
Have a payment policy
Because your practice’s primary job is caring for patients’ physical and emotional needs, payment collection without coming off as insensitive can be tricky. “We understand these are difficult times for everyone, and we are doing our best to work with our patients,” said Dr. Chaudhry. Having a written payment policy can help build the bridge. A policy lets patients know what they can expect and can help prevent surprises over what occurs in the event of nonpayment. Your written policy should include:
- When payment is due.
- How the practice handles copays and deductibles.
- What forms of payment are accepted.
- Your policy regarding nonpayment.
Why patients don’t pay
A 2021 Healthcare Consumer Experience Study from Cedar found that medical bills are a source of anxiety and frustration for most patients, affecting their financial experience. More than half of the respondents said that paying a medical bill is stressful. Complicating matters, many health care practices rely on outdated payment systems, which may not provide patients with a clear view of what they owe and how to pay it.
The study found that 53% of respondents find understanding their plan’s coverage and benefits stressful, and 37% of patients won’t pay their bill if they can’t understand it.
People may think the patient is trying to get out of paying, which, of course, is sometimes true, but most of the time they want to pay, concluded the study. Most patients need a better explanation, communication, and accurate accounting of their out-of-pocket costs.
What can doctors do?
If you’re a physician who regularly sees patients who have problems paying their bills, you can take a few steps to minimize the financial impact on your practice:
- Bill the patient’s insurance directly to ensure you receive at least partial payment.
- Keep adequate records of services in case you need to pursue legal action.
- “Be understanding and flexible when it comes to payment arrangements, as this can often be the difference between getting paid and not getting paid at all,” said Dr. Chaudhry.
Distance yourself
When discussing payment policies, physicians should try to distance themselves from the actual collection process as much as possible. Well-meaning physicians often tell patients things like they can “figure something out “ financially or “work them in” during a scheduling conflict, but that often undermines the authority and credibility of the practice’s office staff. Plus, it teaches patients they can get their way if they work on the doctor’s soft spot – something you don’t want to encourage.
By following some of these measures, you can help ensure that your practice continues to thrive despite the challenges posed by nonpaying patients.
A version of this article first appeared on Medscape.com.
Getting cancer research on track again may require a ‘behemoth’ effort
In 2016, as vice president, Joe Biden launched the Cancer Moonshot program just 1 year after his son Beau died from glioblastoma multiforme. His objective, he said, was to “cure” cancer, but to get close to that goal, to get cancer research just back up to pre-COVID-19 pandemic levels.
There has been a significant decrease in the launch of new clinical trials for cancer and biologic therapies since 2020. “That can affect every aspect of our research operation. It really affected our capacity to continue to move forward at a fast pace. It will require a behemoth effort to get back to pre-COVID times,” said Tanios S. Bekaii-Saab, MD, leader of the gastrointestinal cancer program at Mayo Clinic in Phoenix.
Congress passed the 21st Century Cures Act in 2016 authorizing $1.8 billion for Cancer Moonshot over 7 years. More recently, the program received $194 million from the $6.9 billion National Cancer Institute budget in FY 2022.
Joseph Alvarnas, MD, a hematologist oncologist and vice president of government affairs at City of Hope, Duarte, Calif., sees the Moonshot budget as a potential shortcoming.
“The priorities are well founded and based on what we would think are the most important things to cover, but, if we’re going to achieve these extraordinarily ambitious goals of halving cancer mortality and serving communities more equitably, it’s going to need more funding positioned at making these things real,” he said.
Moonshot is being positioned as an opportunity to double down on efforts started in 2016, but treating cancer is complex and goes well beyond funding new research.
“We know that we have amazing research and progress around innovations that will drive us toward the goal of reducing the death rate from cancer. But we also know that we have tools that aren’t reaching all parts of the country, so we have a great opportunity to make sure that we’re doing all we can to prevent, detect and treat cancer,” Dr. Carnival said.
Can cancer be cured?
The Biden administration relaunched Moonshot in 2022 with newly defined goals: Cut the rate of cancer-related deaths in half within 25 years; improve the experience of people with cancer, cancer survivors, and their families; and “end cancer as we know it,” President Biden said in a press conference in February.
Cancer is the second leading cause of death in the United States after heart disease, but it may indeed be possible to cut the total number of cancer-related deaths in half over the next 25 years.
“As a hematologist who’s been involved in both research and clinical care, I think it’s important to realize this is actually doable. Between 1990 and 2020 cancer mortality rates decreased by 31%, and in the last American Cancer Society’s annual report, mortality rates dropped by the largest percentages for 2 consecutive years in a row. The question shifts now from ‘Is this possible? to ‘How do we ensure that it’s possible?’ The spirit of Cancer Moonshot 2.0 is identifying the multiple paths to move this effort forward,” Dr. Alvarnas said.
But without a significant infusion of cash for research, it’s doubtful cancer-related deaths will drop by 50% over the next 25 years.
“There are a lot of big and lofty goals in Cancer Moonshot, and the words ‘ending cancer,’ well those are big words,” Dr. Bekaii-Saab said. “The reality is how do we measure in 25 years the impact of this today? I think it will require significantly more funding over the next few years to achieve the goals set by the Moonshot. Otherwise it will be a 7-year done deal that will accrue a lot of great numbers but won’t make a dent in those goals for the next 25 years. To stop it at some point and not invest more into it, we will probably lose most of the benefit.”
Closing the loop on data sharing
Moonshot has been instrumental in fostering research collaborations by encouraging data sharing among scientists.
“It also brought together a new way for the National Cancer Institute and Department of Energy to drive progress on some of the big data initiatives. The initial Cancer Moonshot infused a sense of urgency and hope into this effort,” said Danielle Carnival, PhD, coordinator of Cancer Moonshot.
Between 2017 and 2022, Cancer Moonshot created more than 70 consortiums or programs, and funded about 240 research projects. Its fundamental goals of improving data sharing and encouraging collaboration are very important, Dr. Bekaii-Saab said.
“Because, historically, what happens with cancer is that researchers compete for resources...and they become very protective of their data. Sharing gets more difficult, collaborations become more onerous, and it becomes counterproductive,” he said.
Dr. Bekaii-Saab highlighted two networks created specifically for data sharing. They include the Human Tumor Atlas for cellular, morphological, and molecular tumor data, and PDXNet, a patient derived xenograft research network.
A shift in funding priorities?
Cancer funding has been stagnant for years. When adjusted for growth, it hasn’t had a significant infusion of funding since at least 2003—at least in relative terms, Dr. Bekaii-Saab said. “This affects a lot of the things we do, including NCI-funded clinical trials. It pushes us to work with the private sector, which is not necessarily a detriment, but it doesn’t advance the academic mission at the same level. So, overall, I wouldn’t call it tragic, but I do think we’re falling behind,” he said.
“I think when we do the process for the budget for FY24 and after we’ve had time to really explore the best ideas and build the foundation for some of these new aspects of the Cancer Moonshot, we hope to have something more concrete going toward these efforts,” Dr. Carnival said.
But in addition to funding, Dr. Alvarnas says, it is equally important to address gaps in care. Not all patients have access to existing cancer treatments.
“The great challenge to us in the 2020s is not only about developing new and more effective technologies, but also in doing a better job of getting existing life-saving treatments into the hands of underserved populations. One of the really positive challenges set forth by the Biden administration is the idea that financing care equity is as important, if not more so, than advancing technologies. If there’s been stagnation, it’s because from a government and resourcing point of view, that priority has been ineffectively supported financially.”
The pandemic stymies cancer research
The pandemic has had a significant impact on cancer research. As in other fields, it disrupted ongoing research, but it may have also contributed to the loss of employees who resigned in what’s been called the “Great Resignation.” “A lot of employees just decided to change jobs in the middle of the pandemic, which led to a cancer research staffing crisis,” Dr. Bekaii-Saab said.
“We all recognized that turning so much of the attention of the entire biomedical research engine and health system to the COVID-19 pandemic would have an impact across cancer research, screenings and care,” Dr. Carnival said. “There is work to do to get us back to whole, but from a research perspective, we’ve seen a reorientation of the trial networks we were using for COVID-19 research, back to their initial purpose. Some of those are cancer and oncology networks, so we’re excited about that and fully believe that we can catch up.”
But then there’s also the impact the pandemic has had on cancer patients who delayed their care at the primary level. This, Dr. Bekaii-Saab fears, will lead to more patients presenting with more advanced disease in years to come. “One of the biggest problems was that a lot of patients delayed their care at the primary level. My biggest concern is that in the years to come we will see a lot more patients presenting with more advanced cancer.”
In 2016, as vice president, Joe Biden launched the Cancer Moonshot program just 1 year after his son Beau died from glioblastoma multiforme. His objective, he said, was to “cure” cancer, but to get close to that goal, to get cancer research just back up to pre-COVID-19 pandemic levels.
There has been a significant decrease in the launch of new clinical trials for cancer and biologic therapies since 2020. “That can affect every aspect of our research operation. It really affected our capacity to continue to move forward at a fast pace. It will require a behemoth effort to get back to pre-COVID times,” said Tanios S. Bekaii-Saab, MD, leader of the gastrointestinal cancer program at Mayo Clinic in Phoenix.
Congress passed the 21st Century Cures Act in 2016 authorizing $1.8 billion for Cancer Moonshot over 7 years. More recently, the program received $194 million from the $6.9 billion National Cancer Institute budget in FY 2022.
Joseph Alvarnas, MD, a hematologist oncologist and vice president of government affairs at City of Hope, Duarte, Calif., sees the Moonshot budget as a potential shortcoming.
“The priorities are well founded and based on what we would think are the most important things to cover, but, if we’re going to achieve these extraordinarily ambitious goals of halving cancer mortality and serving communities more equitably, it’s going to need more funding positioned at making these things real,” he said.
Moonshot is being positioned as an opportunity to double down on efforts started in 2016, but treating cancer is complex and goes well beyond funding new research.
“We know that we have amazing research and progress around innovations that will drive us toward the goal of reducing the death rate from cancer. But we also know that we have tools that aren’t reaching all parts of the country, so we have a great opportunity to make sure that we’re doing all we can to prevent, detect and treat cancer,” Dr. Carnival said.
Can cancer be cured?
The Biden administration relaunched Moonshot in 2022 with newly defined goals: Cut the rate of cancer-related deaths in half within 25 years; improve the experience of people with cancer, cancer survivors, and their families; and “end cancer as we know it,” President Biden said in a press conference in February.
Cancer is the second leading cause of death in the United States after heart disease, but it may indeed be possible to cut the total number of cancer-related deaths in half over the next 25 years.
“As a hematologist who’s been involved in both research and clinical care, I think it’s important to realize this is actually doable. Between 1990 and 2020 cancer mortality rates decreased by 31%, and in the last American Cancer Society’s annual report, mortality rates dropped by the largest percentages for 2 consecutive years in a row. The question shifts now from ‘Is this possible? to ‘How do we ensure that it’s possible?’ The spirit of Cancer Moonshot 2.0 is identifying the multiple paths to move this effort forward,” Dr. Alvarnas said.
But without a significant infusion of cash for research, it’s doubtful cancer-related deaths will drop by 50% over the next 25 years.
“There are a lot of big and lofty goals in Cancer Moonshot, and the words ‘ending cancer,’ well those are big words,” Dr. Bekaii-Saab said. “The reality is how do we measure in 25 years the impact of this today? I think it will require significantly more funding over the next few years to achieve the goals set by the Moonshot. Otherwise it will be a 7-year done deal that will accrue a lot of great numbers but won’t make a dent in those goals for the next 25 years. To stop it at some point and not invest more into it, we will probably lose most of the benefit.”
Closing the loop on data sharing
Moonshot has been instrumental in fostering research collaborations by encouraging data sharing among scientists.
“It also brought together a new way for the National Cancer Institute and Department of Energy to drive progress on some of the big data initiatives. The initial Cancer Moonshot infused a sense of urgency and hope into this effort,” said Danielle Carnival, PhD, coordinator of Cancer Moonshot.
Between 2017 and 2022, Cancer Moonshot created more than 70 consortiums or programs, and funded about 240 research projects. Its fundamental goals of improving data sharing and encouraging collaboration are very important, Dr. Bekaii-Saab said.
“Because, historically, what happens with cancer is that researchers compete for resources...and they become very protective of their data. Sharing gets more difficult, collaborations become more onerous, and it becomes counterproductive,” he said.
Dr. Bekaii-Saab highlighted two networks created specifically for data sharing. They include the Human Tumor Atlas for cellular, morphological, and molecular tumor data, and PDXNet, a patient derived xenograft research network.
A shift in funding priorities?
Cancer funding has been stagnant for years. When adjusted for growth, it hasn’t had a significant infusion of funding since at least 2003—at least in relative terms, Dr. Bekaii-Saab said. “This affects a lot of the things we do, including NCI-funded clinical trials. It pushes us to work with the private sector, which is not necessarily a detriment, but it doesn’t advance the academic mission at the same level. So, overall, I wouldn’t call it tragic, but I do think we’re falling behind,” he said.
“I think when we do the process for the budget for FY24 and after we’ve had time to really explore the best ideas and build the foundation for some of these new aspects of the Cancer Moonshot, we hope to have something more concrete going toward these efforts,” Dr. Carnival said.
But in addition to funding, Dr. Alvarnas says, it is equally important to address gaps in care. Not all patients have access to existing cancer treatments.
“The great challenge to us in the 2020s is not only about developing new and more effective technologies, but also in doing a better job of getting existing life-saving treatments into the hands of underserved populations. One of the really positive challenges set forth by the Biden administration is the idea that financing care equity is as important, if not more so, than advancing technologies. If there’s been stagnation, it’s because from a government and resourcing point of view, that priority has been ineffectively supported financially.”
The pandemic stymies cancer research
The pandemic has had a significant impact on cancer research. As in other fields, it disrupted ongoing research, but it may have also contributed to the loss of employees who resigned in what’s been called the “Great Resignation.” “A lot of employees just decided to change jobs in the middle of the pandemic, which led to a cancer research staffing crisis,” Dr. Bekaii-Saab said.
“We all recognized that turning so much of the attention of the entire biomedical research engine and health system to the COVID-19 pandemic would have an impact across cancer research, screenings and care,” Dr. Carnival said. “There is work to do to get us back to whole, but from a research perspective, we’ve seen a reorientation of the trial networks we were using for COVID-19 research, back to their initial purpose. Some of those are cancer and oncology networks, so we’re excited about that and fully believe that we can catch up.”
But then there’s also the impact the pandemic has had on cancer patients who delayed their care at the primary level. This, Dr. Bekaii-Saab fears, will lead to more patients presenting with more advanced disease in years to come. “One of the biggest problems was that a lot of patients delayed their care at the primary level. My biggest concern is that in the years to come we will see a lot more patients presenting with more advanced cancer.”
In 2016, as vice president, Joe Biden launched the Cancer Moonshot program just 1 year after his son Beau died from glioblastoma multiforme. His objective, he said, was to “cure” cancer, but to get close to that goal, to get cancer research just back up to pre-COVID-19 pandemic levels.
There has been a significant decrease in the launch of new clinical trials for cancer and biologic therapies since 2020. “That can affect every aspect of our research operation. It really affected our capacity to continue to move forward at a fast pace. It will require a behemoth effort to get back to pre-COVID times,” said Tanios S. Bekaii-Saab, MD, leader of the gastrointestinal cancer program at Mayo Clinic in Phoenix.
Congress passed the 21st Century Cures Act in 2016 authorizing $1.8 billion for Cancer Moonshot over 7 years. More recently, the program received $194 million from the $6.9 billion National Cancer Institute budget in FY 2022.
Joseph Alvarnas, MD, a hematologist oncologist and vice president of government affairs at City of Hope, Duarte, Calif., sees the Moonshot budget as a potential shortcoming.
“The priorities are well founded and based on what we would think are the most important things to cover, but, if we’re going to achieve these extraordinarily ambitious goals of halving cancer mortality and serving communities more equitably, it’s going to need more funding positioned at making these things real,” he said.
Moonshot is being positioned as an opportunity to double down on efforts started in 2016, but treating cancer is complex and goes well beyond funding new research.
“We know that we have amazing research and progress around innovations that will drive us toward the goal of reducing the death rate from cancer. But we also know that we have tools that aren’t reaching all parts of the country, so we have a great opportunity to make sure that we’re doing all we can to prevent, detect and treat cancer,” Dr. Carnival said.
Can cancer be cured?
The Biden administration relaunched Moonshot in 2022 with newly defined goals: Cut the rate of cancer-related deaths in half within 25 years; improve the experience of people with cancer, cancer survivors, and their families; and “end cancer as we know it,” President Biden said in a press conference in February.
Cancer is the second leading cause of death in the United States after heart disease, but it may indeed be possible to cut the total number of cancer-related deaths in half over the next 25 years.
“As a hematologist who’s been involved in both research and clinical care, I think it’s important to realize this is actually doable. Between 1990 and 2020 cancer mortality rates decreased by 31%, and in the last American Cancer Society’s annual report, mortality rates dropped by the largest percentages for 2 consecutive years in a row. The question shifts now from ‘Is this possible? to ‘How do we ensure that it’s possible?’ The spirit of Cancer Moonshot 2.0 is identifying the multiple paths to move this effort forward,” Dr. Alvarnas said.
But without a significant infusion of cash for research, it’s doubtful cancer-related deaths will drop by 50% over the next 25 years.
“There are a lot of big and lofty goals in Cancer Moonshot, and the words ‘ending cancer,’ well those are big words,” Dr. Bekaii-Saab said. “The reality is how do we measure in 25 years the impact of this today? I think it will require significantly more funding over the next few years to achieve the goals set by the Moonshot. Otherwise it will be a 7-year done deal that will accrue a lot of great numbers but won’t make a dent in those goals for the next 25 years. To stop it at some point and not invest more into it, we will probably lose most of the benefit.”
Closing the loop on data sharing
Moonshot has been instrumental in fostering research collaborations by encouraging data sharing among scientists.
“It also brought together a new way for the National Cancer Institute and Department of Energy to drive progress on some of the big data initiatives. The initial Cancer Moonshot infused a sense of urgency and hope into this effort,” said Danielle Carnival, PhD, coordinator of Cancer Moonshot.
Between 2017 and 2022, Cancer Moonshot created more than 70 consortiums or programs, and funded about 240 research projects. Its fundamental goals of improving data sharing and encouraging collaboration are very important, Dr. Bekaii-Saab said.
“Because, historically, what happens with cancer is that researchers compete for resources...and they become very protective of their data. Sharing gets more difficult, collaborations become more onerous, and it becomes counterproductive,” he said.
Dr. Bekaii-Saab highlighted two networks created specifically for data sharing. They include the Human Tumor Atlas for cellular, morphological, and molecular tumor data, and PDXNet, a patient derived xenograft research network.
A shift in funding priorities?
Cancer funding has been stagnant for years. When adjusted for growth, it hasn’t had a significant infusion of funding since at least 2003—at least in relative terms, Dr. Bekaii-Saab said. “This affects a lot of the things we do, including NCI-funded clinical trials. It pushes us to work with the private sector, which is not necessarily a detriment, but it doesn’t advance the academic mission at the same level. So, overall, I wouldn’t call it tragic, but I do think we’re falling behind,” he said.
“I think when we do the process for the budget for FY24 and after we’ve had time to really explore the best ideas and build the foundation for some of these new aspects of the Cancer Moonshot, we hope to have something more concrete going toward these efforts,” Dr. Carnival said.
But in addition to funding, Dr. Alvarnas says, it is equally important to address gaps in care. Not all patients have access to existing cancer treatments.
“The great challenge to us in the 2020s is not only about developing new and more effective technologies, but also in doing a better job of getting existing life-saving treatments into the hands of underserved populations. One of the really positive challenges set forth by the Biden administration is the idea that financing care equity is as important, if not more so, than advancing technologies. If there’s been stagnation, it’s because from a government and resourcing point of view, that priority has been ineffectively supported financially.”
The pandemic stymies cancer research
The pandemic has had a significant impact on cancer research. As in other fields, it disrupted ongoing research, but it may have also contributed to the loss of employees who resigned in what’s been called the “Great Resignation.” “A lot of employees just decided to change jobs in the middle of the pandemic, which led to a cancer research staffing crisis,” Dr. Bekaii-Saab said.
“We all recognized that turning so much of the attention of the entire biomedical research engine and health system to the COVID-19 pandemic would have an impact across cancer research, screenings and care,” Dr. Carnival said. “There is work to do to get us back to whole, but from a research perspective, we’ve seen a reorientation of the trial networks we were using for COVID-19 research, back to their initial purpose. Some of those are cancer and oncology networks, so we’re excited about that and fully believe that we can catch up.”
But then there’s also the impact the pandemic has had on cancer patients who delayed their care at the primary level. This, Dr. Bekaii-Saab fears, will lead to more patients presenting with more advanced disease in years to come. “One of the biggest problems was that a lot of patients delayed their care at the primary level. My biggest concern is that in the years to come we will see a lot more patients presenting with more advanced cancer.”
Monkeypox: Another emerging threat?
CASE Pregnant woman’s husband is ill after traveling
A 29-year-old primigravid woman at 18 weeks’ gestation just returned from a 10-day trip to Nigeria with her husband. While in Nigeria, the couple went on safari. On several occasions during the safari, they consumed bushmeat prepared by their guides. Her husband now has severe malaise, fever, chills, myalgias, cough, and prominent submandibular, cervical, and inguinal adenopathy. In addition, he has developed a diffuse papular-vesicular rash on his trunk and extremities.
- What is the most likely diagnosis?
- Does this condition pose a danger to his wife?
- What treatment is indicated for his wife?
What we know
In recent weeks, the specter of another poorly understood biological threat has emerged in the medical literature and lay press: monkeypox. This article will first review the epidemiology, clinical manifestations, and diagnosis of this infection, followed by a discussion of how to prevent and treat the condition, with special emphasis on the risks that this infection poses in pregnant women.
Virology
The monkeypox virus is a member of the orthopoxvirus genus. The variola (smallpox) virus and vaccinia virus are included in this genus. It is one of the largest of all viruses, measuring 200-250 nm. It is enveloped and contains double-stranded DNA. Its natural reservoir is probably African rodents. Two distinct strains of monkeypox exist in different geographical regions of Africa: the Central African clade and the West African clade. The Central African clade is significantly more virulent than the latter, with a mortality rate approaching 10%, versus 1% in the West African clade. The incubation period of the virus ranges from 4-20 days and averages 12 days.1,2
Epidemiology
Monkeypox was first discovered in 1958 by Preben von Magnus in a colony of research monkeys in Copenhagen, Denmark. The first case of monkeypox in humans occurred in the Democratic Republic of Congo in 1970 in a 9-year-old boy. Subsequently, cases were reported in the Ivory Coast, Liberia, Nigeria, and Sierra Leone. The infection was limited to the rain forests of central and western Africa until 2003. At that time, the first cases in the United States were reported. The US cases occurred in the Midwest and were traced to exposure to pet prairie dogs. These animals all came from a single distributor, and they apparently were infected when they were housed in the same space with Gambian rats, which are well recognized reservoirs of monkeypox in their native habitat in Africa.1-3
A limited outbreak of monkeypox occurred in the United Kingdom in 2018. Seventy-one cases, with no fatalities, were reported. In 2021 another US case of monkeypox was reported in Dallas, Texas, in an individual who had recently traveled to the United States from Nigeria. A second US case was reported in November 2021 from a patient in Maryland who had returned from a visit to Nigeria. Those were the only 2 reported cases of monkeypox in the United States in 2021.1-3
Then in early May 2022, the United Kingdom reported 9 cases of monkeypox. The first infected patient had recently traveled to Nigeria and, subsequently, infected 2 members of his family.4 On May 18, the Massachusetts Department of Public Health confirmed a case of monkeypox in an adult man who had recently traveled to Canada. As of July 7, 6,027 cases have been reported from at least 39 countries.
The current outbreak is unusual in that, previously, almost all cases occurred in western and central Africa in remote tropical rain forests. Infection usually resulted from close exposure to rats, rabbits, squirrels, monkeys, porcupines, and gazelles. Exposure occurred when persons captured, slaughtered, prepared, and then ate these animals for food without properly cooking the flesh.
The leading theory is that the present outbreak originated among men who had sex with men at 2 raves held in Spain and Belgium. The virus appears to have been spread by skin-to-skin contact, by respiratory droplets, by contact with contaminated bedding, and probably by sperm.2,4,6
Continue to: Clinical manifestations...
Clinical manifestations
Monkeypox evolves through 2 stages: a pre-eruptive stage and an eruptive stage. Prodromal symptoms include malaise, severe headache, myalgias, fever, drenching sweats, backache, fatigue, sore throat, dyspnea, and cough. Within 2-3 days, the characteristic skin eruption develops. The lesions usually begin on the face and then spread in a centrifugal manner to the trunk and extremities, including the palms of the hands and soles of the feet. The lesions typically progress from macules to papules to vesicles to pustules. They then crust and scab over. An interesting additional finding is the presence of prominent lymphadenopathy behind the ear, beneath the mandible, in the neck, and in the groin.1
Several different illnesses must be considered in the differential diagnosis of monkeypox infection. They include measles, scabies, secondary syphilis, and medication-associated allergic reactions. However, the 2 conditions most likely to be confused with monkeypox are chickenpox (varicella) and smallpox. Lymphadenopathy is much more prominent in monkeypox compared with chickenpox. Moreover, with monkeypox, all lesions tend to be at the same stage of evolution as opposed to appearing in crops as they do in chickenpox. Smallpox would be extremely unlikely in the absence of a recognized laboratory accident or a bioterrorism incident.7
Diagnosis
The presumptive diagnosis of monkeypox infection is made primarily based on clinical examination. However, laboratory testing is indicated to definitively differentiate monkeypox from other orthopoxvirus infections such as varicella and smallpox.
In specialized laboratories that employ highly trained personnel and maintain strict safety precautions, the virus can be isolated in mammalian cell cultures. Electron microscopy is a valuable tool for identifying the characteristic brick-shaped poxvirus virions. Routine histologic examination of a lesion will show ballooning degeneration of keratinocytes, prominent spongiosis, dermal edema, and acute inflammation, although these findings are not unique to monkeypox.1
The Centers for Disease Control and Prevention (CDC) has developed serologic tests that detect immunoglobulin (Ig) M- and IgG-specific antibody. However, the most useful and practical diagnostic test is assessment of a skin scraping by polymerase chain reaction (PCR). This test is more sensitive than assessment of serum PCR.1
When the diagnosis of monkeypox is being considered, the clinician should coordinate testing through the local and state public health departments and through the CDC. Effective communication with all agencies will ensure that laboratory specimens are processed in a timely and efficient manner. The CDC website presents information on specimen collection.8
How do we manage monkeypox?
Prevention
The first step in prevention of infection is to isolate infected individuals until all lesions have dried and crusted over. Susceptible people should avoid close contact with skin lesions, respiratory and genital secretions, and bedding of patients who are infected.
The ultimate preventive measure, however, is vaccination of susceptible people either immediately before exposure (eg, military personnel, first responders, infection control investigators, health care workers) or immediately after exposure (general population). Older individuals who received the original smallpox vaccine likely have immunity to monkeypox infection. Unfortunately, very few women who currently are of reproductive age received this vaccine because its use was discontinued in the United States in the early 1970s. Therefore, the vast majority of our patients are uniquely susceptible to this infection and should be vaccinated if there is an outbreak of monkeypox in their locality.7,9
The current preferred vaccine for prevention of both smallpox and monkeypox is the Jynneos (Bavarian Nordic A/S) vaccine.10 This agent incorporates a replication-deficient live virus and does not pose the same risk for adverse events as the original versions of the smallpox vaccine. Jynneos is administered subcutaneously rather than by scarification. Two 0.5-mL doses, delivered 28 days apart, are required for optimal effect. The vaccine must be obtained from local and state health departments, in consultation with the CDC.7,9
There is very little published information on the safety of the Jynneos vaccine in pregnant or lactating women, although animal data are reassuring. Moreover, the dangers of monkeypox infection are significant, and in the event of an outbreak, vaccination of susceptible individuals, including pregnant women, is indicated.
- Monkeypox is a member of the orthopoxvirus genus and is closely related to the smallpox virus. It is a large, double-stranded, enveloped DNA virus.
- The virus is transmitted primarily by close contact with infected animals or other humans or by consumption of contaminated bushmeat.
- The infection evolves in 2 phases. The pre-eruptive phase is characterized by severe flu-like symptoms and signs. The eruptive phase is distinguished by a diffuse papular-vesicular rash.
- The most valuable test for confirming the diagnosis is a polymerase chain reaction test of a fresh skin lesion.
- In women who are pregnant, monkeypox has been associated with spontaneous abortion and fetal death.
- Three antiviral agents may be of value in treating infected patients: cidofovir, brincidofovir, and tecovirimat. Only the latter has an acceptable safety profile for women who are pregnant or lactating.
- The new nonreplicating smallpox vaccine Jynneos (Bavarian Nordic A/S) is of great value for pre- and post-exposure prophylaxis.
Continue to: Treatment...
Treatment
Infected pregnant women should receive acetaminophen 1,000 mg orally every 8 hours, to control fever and provide analgesia. An antihistamine such as diphenhydramine 25 mg orally every 6-8 hours, may be used to control pruritus and provide mild sedation. Adequate fluid intake and optimal nutrition should be encouraged. Skin lesions should be inspected regularly to detect signs of superimposed bacterial infections. Small, localized bacterial skin infections can be treated with topical application of mupirocin ointment 2%, 3 times daily for 7-14 days. For diffuse and more severe bacterial skin infections, a systemic antibiotic may be necessary. Reasonable choices include amoxicillin-clavulanate 875 mg/125 mg orally every 12 hours, or trimethoprim-sulfamethoxazole double strength 800 mg/160 mg orally every 12 hours.11 The latter agent should be avoided in the first trimester of pregnancy because of potential teratogenic effects.
Several specific agents are available through the CDC for treatment of orthopoxvirus infections such as smallpox and monkeypox. Information about these agents is summarized in the TABLE.12-16
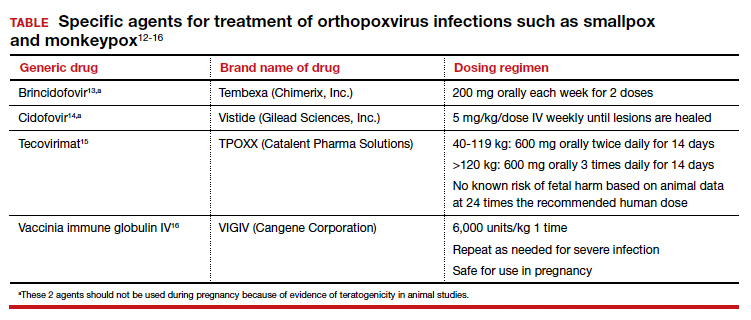
Unique considerations in pregnancy
Because monkeypox is so rare, there is very little information about the effects of this infection in pregnant women. The report most commonly cited in the literature is that by Mbala et al, which was published in 2017.17 These authors described 4 pregnant patients in the Democratic Republic of Congo who contracted monkeypox infection over a 4-year period. All 4 women were hospitalized and treated with systemic antibiotics, antiparasitic medications, and analgesics. One patient delivered a healthy infant. Two women had spontaneous abortions in the first trimester. The fourth patient experienced a stillbirth at 22 weeks’ gestation. At postmortem examination, the fetus had diffuse cutaneous lesions, prominent hepatomegaly, and hydrops. No structural malformations were noted. The placenta demonstrated numerous punctate hemorrhages, and high concentrations of virus were recovered from the placenta and from fetal tissue.
Although the information on pregnancy outcome is quite limited, it seems clear that the virus can cross the placenta and cause adverse effects such as spontaneous abortion and fetal death. Accordingly, I think the following guidelines are a reasonable approach to a pregnant patient who has been exposed to monkeypox or who has developed manifestations of infection.3,7,9
- In the event of a community outbreak, bioterrorism event, or exposure to a person with suspected or confirmed monkeypox infection, the pregnant patient should receive the Jynneos vaccine.
- The pregnant patient should be isolated from any individual with suspected or confirmed monkeypox.
- If infection develops despite these measures, the patient should be treated with either tecovirimat or vaccinia immune globulin IV. Hospitalization may be necessary for seriously ill individuals.
- Within 2 weeks of infection, a comprehensive ultrasound examination should be performed to assess for structural abnormalities in the fetus.
- Subsequently, serial ultrasound examinations should be performed at intervals of 4-6 weeks to assess fetal growth and re-evaluate fetal anatomy.
- Following delivery, a detailed neonatal examination should be performed to assess for evidence of viral injury. Neonatal skin lesions and neonatal serum can be assessed by PCR for monkeypox virus. The newborn should be isolated from the mother until all the mother’s lesions have dried and crusted over.
CASE Resolved
Given the husband’s recent travel to Nigeria and consumption of bushmeat, he most likely has monkeypox. The infection can be spread from person to person by close contact; thus, his wife is at risk. The couple should isolate until all of his lesions have dried and crusted over. The woman also should receive the Jynneos vaccine. If she becomes symptomatic, she should be treated with tecovirimat or vaccinia immune globulin IV. ●
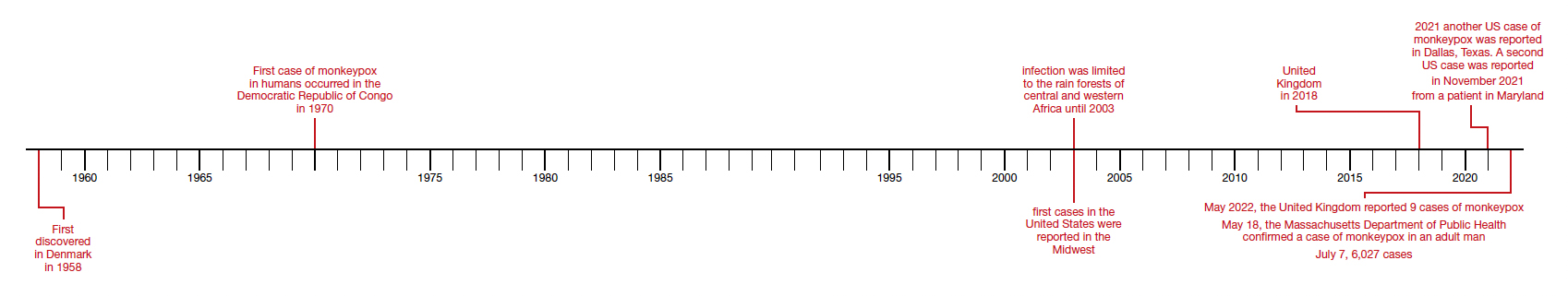
- Isaacs SN, Shenoy ES. Monkeypox. UpToDate. Updated June 28,2022. Accessed July 1, 2022. https://www.uptodate.com /contents/monkeypox?topicRef=8349&source=see_link
- Graham MB. Monkeypox. Medscape. Updated June 29, 2022. Accessed July 1, 2022. https://emedicine.medscape.com /article/1134714-overview.
- Khalil A, Samara A, O’Brien P, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022;60:22-27. doi:10.1002/uog.24968.
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. May 18, 2022. Accessed July 1, 2022. https://www.who.int/emergencies/diseaseoutbreak-news/item/2022-DON383.
- WHO reports two new monkeypox deaths, cases in new areas. Reuters. July 7, 2022. https://www.reuters.com/world /who-reports-two-new-monkeypox-deaths-2022-07-07/. Accessed July 19, 2022.
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. Accessed July 1, 2022. https://www.who.int /emergencies/disease-outbreak-news/item/2022 -DON388#:~:text=Multi%2Dcountry%20monkeypox%20 outbreak%20in%20non%2Dendemic%20countries%3A%20 Update,-29%20May%202022&text=Since%2013%20 May%202022%2C%20monkeypox,Epidemiological%20 investigations%20are%20ongoing.
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections andbioterrorism emergencies. Emerg Infect Dis. 2006;12:16311637. doi:10.3201/eid1211.060618.
- Centers for Disease Control and Prevention. Preparation and collection of specimens. Reviewed June 29, 2022. Accessed July 6, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/prep-collection-specimens.html.
- Rao AK, Petersen BW, Whitehill F, et al. Monkeypox vaccination. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585/mmwr.mm7122e1.
- Smallpox and monkeypox vaccine, live, nonreplicating. Package insert. Bavarian Nordic A/S; 2021. Accessed July 1, 2022. https://www.fda.gov/media/131078/download.
- Duff P. Commonly used antibiotics in ObGyn practice. OBG Manag. 2022;34:29, 36-40. doi:10.12788/obgm.0191.
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals: interim clinical guidance for the treatment of monkeypox. Reviewed June 17, 2022. Accessed July 1, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/treatment.html.
- Brincidofovir. Prescribing information. Chimerix, Inc.; 2021. Accessed July 1, 2022. https://www.accessdata.fda.gov /drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- Cidofovir. Package insert. Gilead Sciences, Inc.; 2010. Accessed July 1, 2022. https://www.gilead.com/~/media /Files/pdfs/medicines/other/vistide/vistide.pdf.
- Tecovirimat. Prescribing information. Catalent Pharma Solutions; 2022. Accessed July 1, 2022. https://www.accessdata.fda.gov/drugsatfda_docs /label/2022/214518s000lbl.pdf.
- Vaccinia immune globulin IV. Prescribing information. Cangene Corporation; 2010. Accessed July 1, 2022. https: //www.fda.gov/media/77004/download.
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260.
CASE Pregnant woman’s husband is ill after traveling
A 29-year-old primigravid woman at 18 weeks’ gestation just returned from a 10-day trip to Nigeria with her husband. While in Nigeria, the couple went on safari. On several occasions during the safari, they consumed bushmeat prepared by their guides. Her husband now has severe malaise, fever, chills, myalgias, cough, and prominent submandibular, cervical, and inguinal adenopathy. In addition, he has developed a diffuse papular-vesicular rash on his trunk and extremities.
- What is the most likely diagnosis?
- Does this condition pose a danger to his wife?
- What treatment is indicated for his wife?
What we know
In recent weeks, the specter of another poorly understood biological threat has emerged in the medical literature and lay press: monkeypox. This article will first review the epidemiology, clinical manifestations, and diagnosis of this infection, followed by a discussion of how to prevent and treat the condition, with special emphasis on the risks that this infection poses in pregnant women.
Virology
The monkeypox virus is a member of the orthopoxvirus genus. The variola (smallpox) virus and vaccinia virus are included in this genus. It is one of the largest of all viruses, measuring 200-250 nm. It is enveloped and contains double-stranded DNA. Its natural reservoir is probably African rodents. Two distinct strains of monkeypox exist in different geographical regions of Africa: the Central African clade and the West African clade. The Central African clade is significantly more virulent than the latter, with a mortality rate approaching 10%, versus 1% in the West African clade. The incubation period of the virus ranges from 4-20 days and averages 12 days.1,2
Epidemiology
Monkeypox was first discovered in 1958 by Preben von Magnus in a colony of research monkeys in Copenhagen, Denmark. The first case of monkeypox in humans occurred in the Democratic Republic of Congo in 1970 in a 9-year-old boy. Subsequently, cases were reported in the Ivory Coast, Liberia, Nigeria, and Sierra Leone. The infection was limited to the rain forests of central and western Africa until 2003. At that time, the first cases in the United States were reported. The US cases occurred in the Midwest and were traced to exposure to pet prairie dogs. These animals all came from a single distributor, and they apparently were infected when they were housed in the same space with Gambian rats, which are well recognized reservoirs of monkeypox in their native habitat in Africa.1-3
A limited outbreak of monkeypox occurred in the United Kingdom in 2018. Seventy-one cases, with no fatalities, were reported. In 2021 another US case of monkeypox was reported in Dallas, Texas, in an individual who had recently traveled to the United States from Nigeria. A second US case was reported in November 2021 from a patient in Maryland who had returned from a visit to Nigeria. Those were the only 2 reported cases of monkeypox in the United States in 2021.1-3
Then in early May 2022, the United Kingdom reported 9 cases of monkeypox. The first infected patient had recently traveled to Nigeria and, subsequently, infected 2 members of his family.4 On May 18, the Massachusetts Department of Public Health confirmed a case of monkeypox in an adult man who had recently traveled to Canada. As of July 7, 6,027 cases have been reported from at least 39 countries.
The current outbreak is unusual in that, previously, almost all cases occurred in western and central Africa in remote tropical rain forests. Infection usually resulted from close exposure to rats, rabbits, squirrels, monkeys, porcupines, and gazelles. Exposure occurred when persons captured, slaughtered, prepared, and then ate these animals for food without properly cooking the flesh.
The leading theory is that the present outbreak originated among men who had sex with men at 2 raves held in Spain and Belgium. The virus appears to have been spread by skin-to-skin contact, by respiratory droplets, by contact with contaminated bedding, and probably by sperm.2,4,6
Continue to: Clinical manifestations...
Clinical manifestations
Monkeypox evolves through 2 stages: a pre-eruptive stage and an eruptive stage. Prodromal symptoms include malaise, severe headache, myalgias, fever, drenching sweats, backache, fatigue, sore throat, dyspnea, and cough. Within 2-3 days, the characteristic skin eruption develops. The lesions usually begin on the face and then spread in a centrifugal manner to the trunk and extremities, including the palms of the hands and soles of the feet. The lesions typically progress from macules to papules to vesicles to pustules. They then crust and scab over. An interesting additional finding is the presence of prominent lymphadenopathy behind the ear, beneath the mandible, in the neck, and in the groin.1
Several different illnesses must be considered in the differential diagnosis of monkeypox infection. They include measles, scabies, secondary syphilis, and medication-associated allergic reactions. However, the 2 conditions most likely to be confused with monkeypox are chickenpox (varicella) and smallpox. Lymphadenopathy is much more prominent in monkeypox compared with chickenpox. Moreover, with monkeypox, all lesions tend to be at the same stage of evolution as opposed to appearing in crops as they do in chickenpox. Smallpox would be extremely unlikely in the absence of a recognized laboratory accident or a bioterrorism incident.7
Diagnosis
The presumptive diagnosis of monkeypox infection is made primarily based on clinical examination. However, laboratory testing is indicated to definitively differentiate monkeypox from other orthopoxvirus infections such as varicella and smallpox.
In specialized laboratories that employ highly trained personnel and maintain strict safety precautions, the virus can be isolated in mammalian cell cultures. Electron microscopy is a valuable tool for identifying the characteristic brick-shaped poxvirus virions. Routine histologic examination of a lesion will show ballooning degeneration of keratinocytes, prominent spongiosis, dermal edema, and acute inflammation, although these findings are not unique to monkeypox.1
The Centers for Disease Control and Prevention (CDC) has developed serologic tests that detect immunoglobulin (Ig) M- and IgG-specific antibody. However, the most useful and practical diagnostic test is assessment of a skin scraping by polymerase chain reaction (PCR). This test is more sensitive than assessment of serum PCR.1
When the diagnosis of monkeypox is being considered, the clinician should coordinate testing through the local and state public health departments and through the CDC. Effective communication with all agencies will ensure that laboratory specimens are processed in a timely and efficient manner. The CDC website presents information on specimen collection.8
How do we manage monkeypox?
Prevention
The first step in prevention of infection is to isolate infected individuals until all lesions have dried and crusted over. Susceptible people should avoid close contact with skin lesions, respiratory and genital secretions, and bedding of patients who are infected.
The ultimate preventive measure, however, is vaccination of susceptible people either immediately before exposure (eg, military personnel, first responders, infection control investigators, health care workers) or immediately after exposure (general population). Older individuals who received the original smallpox vaccine likely have immunity to monkeypox infection. Unfortunately, very few women who currently are of reproductive age received this vaccine because its use was discontinued in the United States in the early 1970s. Therefore, the vast majority of our patients are uniquely susceptible to this infection and should be vaccinated if there is an outbreak of monkeypox in their locality.7,9
The current preferred vaccine for prevention of both smallpox and monkeypox is the Jynneos (Bavarian Nordic A/S) vaccine.10 This agent incorporates a replication-deficient live virus and does not pose the same risk for adverse events as the original versions of the smallpox vaccine. Jynneos is administered subcutaneously rather than by scarification. Two 0.5-mL doses, delivered 28 days apart, are required for optimal effect. The vaccine must be obtained from local and state health departments, in consultation with the CDC.7,9
There is very little published information on the safety of the Jynneos vaccine in pregnant or lactating women, although animal data are reassuring. Moreover, the dangers of monkeypox infection are significant, and in the event of an outbreak, vaccination of susceptible individuals, including pregnant women, is indicated.
- Monkeypox is a member of the orthopoxvirus genus and is closely related to the smallpox virus. It is a large, double-stranded, enveloped DNA virus.
- The virus is transmitted primarily by close contact with infected animals or other humans or by consumption of contaminated bushmeat.
- The infection evolves in 2 phases. The pre-eruptive phase is characterized by severe flu-like symptoms and signs. The eruptive phase is distinguished by a diffuse papular-vesicular rash.
- The most valuable test for confirming the diagnosis is a polymerase chain reaction test of a fresh skin lesion.
- In women who are pregnant, monkeypox has been associated with spontaneous abortion and fetal death.
- Three antiviral agents may be of value in treating infected patients: cidofovir, brincidofovir, and tecovirimat. Only the latter has an acceptable safety profile for women who are pregnant or lactating.
- The new nonreplicating smallpox vaccine Jynneos (Bavarian Nordic A/S) is of great value for pre- and post-exposure prophylaxis.
Continue to: Treatment...
Treatment
Infected pregnant women should receive acetaminophen 1,000 mg orally every 8 hours, to control fever and provide analgesia. An antihistamine such as diphenhydramine 25 mg orally every 6-8 hours, may be used to control pruritus and provide mild sedation. Adequate fluid intake and optimal nutrition should be encouraged. Skin lesions should be inspected regularly to detect signs of superimposed bacterial infections. Small, localized bacterial skin infections can be treated with topical application of mupirocin ointment 2%, 3 times daily for 7-14 days. For diffuse and more severe bacterial skin infections, a systemic antibiotic may be necessary. Reasonable choices include amoxicillin-clavulanate 875 mg/125 mg orally every 12 hours, or trimethoprim-sulfamethoxazole double strength 800 mg/160 mg orally every 12 hours.11 The latter agent should be avoided in the first trimester of pregnancy because of potential teratogenic effects.
Several specific agents are available through the CDC for treatment of orthopoxvirus infections such as smallpox and monkeypox. Information about these agents is summarized in the TABLE.12-16

Unique considerations in pregnancy
Because monkeypox is so rare, there is very little information about the effects of this infection in pregnant women. The report most commonly cited in the literature is that by Mbala et al, which was published in 2017.17 These authors described 4 pregnant patients in the Democratic Republic of Congo who contracted monkeypox infection over a 4-year period. All 4 women were hospitalized and treated with systemic antibiotics, antiparasitic medications, and analgesics. One patient delivered a healthy infant. Two women had spontaneous abortions in the first trimester. The fourth patient experienced a stillbirth at 22 weeks’ gestation. At postmortem examination, the fetus had diffuse cutaneous lesions, prominent hepatomegaly, and hydrops. No structural malformations were noted. The placenta demonstrated numerous punctate hemorrhages, and high concentrations of virus were recovered from the placenta and from fetal tissue.
Although the information on pregnancy outcome is quite limited, it seems clear that the virus can cross the placenta and cause adverse effects such as spontaneous abortion and fetal death. Accordingly, I think the following guidelines are a reasonable approach to a pregnant patient who has been exposed to monkeypox or who has developed manifestations of infection.3,7,9
- In the event of a community outbreak, bioterrorism event, or exposure to a person with suspected or confirmed monkeypox infection, the pregnant patient should receive the Jynneos vaccine.
- The pregnant patient should be isolated from any individual with suspected or confirmed monkeypox.
- If infection develops despite these measures, the patient should be treated with either tecovirimat or vaccinia immune globulin IV. Hospitalization may be necessary for seriously ill individuals.
- Within 2 weeks of infection, a comprehensive ultrasound examination should be performed to assess for structural abnormalities in the fetus.
- Subsequently, serial ultrasound examinations should be performed at intervals of 4-6 weeks to assess fetal growth and re-evaluate fetal anatomy.
- Following delivery, a detailed neonatal examination should be performed to assess for evidence of viral injury. Neonatal skin lesions and neonatal serum can be assessed by PCR for monkeypox virus. The newborn should be isolated from the mother until all the mother’s lesions have dried and crusted over.
CASE Resolved
Given the husband’s recent travel to Nigeria and consumption of bushmeat, he most likely has monkeypox. The infection can be spread from person to person by close contact; thus, his wife is at risk. The couple should isolate until all of his lesions have dried and crusted over. The woman also should receive the Jynneos vaccine. If she becomes symptomatic, she should be treated with tecovirimat or vaccinia immune globulin IV. ●

CASE Pregnant woman’s husband is ill after traveling
A 29-year-old primigravid woman at 18 weeks’ gestation just returned from a 10-day trip to Nigeria with her husband. While in Nigeria, the couple went on safari. On several occasions during the safari, they consumed bushmeat prepared by their guides. Her husband now has severe malaise, fever, chills, myalgias, cough, and prominent submandibular, cervical, and inguinal adenopathy. In addition, he has developed a diffuse papular-vesicular rash on his trunk and extremities.
- What is the most likely diagnosis?
- Does this condition pose a danger to his wife?
- What treatment is indicated for his wife?
What we know
In recent weeks, the specter of another poorly understood biological threat has emerged in the medical literature and lay press: monkeypox. This article will first review the epidemiology, clinical manifestations, and diagnosis of this infection, followed by a discussion of how to prevent and treat the condition, with special emphasis on the risks that this infection poses in pregnant women.
Virology
The monkeypox virus is a member of the orthopoxvirus genus. The variola (smallpox) virus and vaccinia virus are included in this genus. It is one of the largest of all viruses, measuring 200-250 nm. It is enveloped and contains double-stranded DNA. Its natural reservoir is probably African rodents. Two distinct strains of monkeypox exist in different geographical regions of Africa: the Central African clade and the West African clade. The Central African clade is significantly more virulent than the latter, with a mortality rate approaching 10%, versus 1% in the West African clade. The incubation period of the virus ranges from 4-20 days and averages 12 days.1,2
Epidemiology
Monkeypox was first discovered in 1958 by Preben von Magnus in a colony of research monkeys in Copenhagen, Denmark. The first case of monkeypox in humans occurred in the Democratic Republic of Congo in 1970 in a 9-year-old boy. Subsequently, cases were reported in the Ivory Coast, Liberia, Nigeria, and Sierra Leone. The infection was limited to the rain forests of central and western Africa until 2003. At that time, the first cases in the United States were reported. The US cases occurred in the Midwest and were traced to exposure to pet prairie dogs. These animals all came from a single distributor, and they apparently were infected when they were housed in the same space with Gambian rats, which are well recognized reservoirs of monkeypox in their native habitat in Africa.1-3
A limited outbreak of monkeypox occurred in the United Kingdom in 2018. Seventy-one cases, with no fatalities, were reported. In 2021 another US case of monkeypox was reported in Dallas, Texas, in an individual who had recently traveled to the United States from Nigeria. A second US case was reported in November 2021 from a patient in Maryland who had returned from a visit to Nigeria. Those were the only 2 reported cases of monkeypox in the United States in 2021.1-3
Then in early May 2022, the United Kingdom reported 9 cases of monkeypox. The first infected patient had recently traveled to Nigeria and, subsequently, infected 2 members of his family.4 On May 18, the Massachusetts Department of Public Health confirmed a case of monkeypox in an adult man who had recently traveled to Canada. As of July 7, 6,027 cases have been reported from at least 39 countries.
The current outbreak is unusual in that, previously, almost all cases occurred in western and central Africa in remote tropical rain forests. Infection usually resulted from close exposure to rats, rabbits, squirrels, monkeys, porcupines, and gazelles. Exposure occurred when persons captured, slaughtered, prepared, and then ate these animals for food without properly cooking the flesh.
The leading theory is that the present outbreak originated among men who had sex with men at 2 raves held in Spain and Belgium. The virus appears to have been spread by skin-to-skin contact, by respiratory droplets, by contact with contaminated bedding, and probably by sperm.2,4,6
Continue to: Clinical manifestations...
Clinical manifestations
Monkeypox evolves through 2 stages: a pre-eruptive stage and an eruptive stage. Prodromal symptoms include malaise, severe headache, myalgias, fever, drenching sweats, backache, fatigue, sore throat, dyspnea, and cough. Within 2-3 days, the characteristic skin eruption develops. The lesions usually begin on the face and then spread in a centrifugal manner to the trunk and extremities, including the palms of the hands and soles of the feet. The lesions typically progress from macules to papules to vesicles to pustules. They then crust and scab over. An interesting additional finding is the presence of prominent lymphadenopathy behind the ear, beneath the mandible, in the neck, and in the groin.1
Several different illnesses must be considered in the differential diagnosis of monkeypox infection. They include measles, scabies, secondary syphilis, and medication-associated allergic reactions. However, the 2 conditions most likely to be confused with monkeypox are chickenpox (varicella) and smallpox. Lymphadenopathy is much more prominent in monkeypox compared with chickenpox. Moreover, with monkeypox, all lesions tend to be at the same stage of evolution as opposed to appearing in crops as they do in chickenpox. Smallpox would be extremely unlikely in the absence of a recognized laboratory accident or a bioterrorism incident.7
Diagnosis
The presumptive diagnosis of monkeypox infection is made primarily based on clinical examination. However, laboratory testing is indicated to definitively differentiate monkeypox from other orthopoxvirus infections such as varicella and smallpox.
In specialized laboratories that employ highly trained personnel and maintain strict safety precautions, the virus can be isolated in mammalian cell cultures. Electron microscopy is a valuable tool for identifying the characteristic brick-shaped poxvirus virions. Routine histologic examination of a lesion will show ballooning degeneration of keratinocytes, prominent spongiosis, dermal edema, and acute inflammation, although these findings are not unique to monkeypox.1
The Centers for Disease Control and Prevention (CDC) has developed serologic tests that detect immunoglobulin (Ig) M- and IgG-specific antibody. However, the most useful and practical diagnostic test is assessment of a skin scraping by polymerase chain reaction (PCR). This test is more sensitive than assessment of serum PCR.1
When the diagnosis of monkeypox is being considered, the clinician should coordinate testing through the local and state public health departments and through the CDC. Effective communication with all agencies will ensure that laboratory specimens are processed in a timely and efficient manner. The CDC website presents information on specimen collection.8
How do we manage monkeypox?
Prevention
The first step in prevention of infection is to isolate infected individuals until all lesions have dried and crusted over. Susceptible people should avoid close contact with skin lesions, respiratory and genital secretions, and bedding of patients who are infected.
The ultimate preventive measure, however, is vaccination of susceptible people either immediately before exposure (eg, military personnel, first responders, infection control investigators, health care workers) or immediately after exposure (general population). Older individuals who received the original smallpox vaccine likely have immunity to monkeypox infection. Unfortunately, very few women who currently are of reproductive age received this vaccine because its use was discontinued in the United States in the early 1970s. Therefore, the vast majority of our patients are uniquely susceptible to this infection and should be vaccinated if there is an outbreak of monkeypox in their locality.7,9
The current preferred vaccine for prevention of both smallpox and monkeypox is the Jynneos (Bavarian Nordic A/S) vaccine.10 This agent incorporates a replication-deficient live virus and does not pose the same risk for adverse events as the original versions of the smallpox vaccine. Jynneos is administered subcutaneously rather than by scarification. Two 0.5-mL doses, delivered 28 days apart, are required for optimal effect. The vaccine must be obtained from local and state health departments, in consultation with the CDC.7,9
There is very little published information on the safety of the Jynneos vaccine in pregnant or lactating women, although animal data are reassuring. Moreover, the dangers of monkeypox infection are significant, and in the event of an outbreak, vaccination of susceptible individuals, including pregnant women, is indicated.
- Monkeypox is a member of the orthopoxvirus genus and is closely related to the smallpox virus. It is a large, double-stranded, enveloped DNA virus.
- The virus is transmitted primarily by close contact with infected animals or other humans or by consumption of contaminated bushmeat.
- The infection evolves in 2 phases. The pre-eruptive phase is characterized by severe flu-like symptoms and signs. The eruptive phase is distinguished by a diffuse papular-vesicular rash.
- The most valuable test for confirming the diagnosis is a polymerase chain reaction test of a fresh skin lesion.
- In women who are pregnant, monkeypox has been associated with spontaneous abortion and fetal death.
- Three antiviral agents may be of value in treating infected patients: cidofovir, brincidofovir, and tecovirimat. Only the latter has an acceptable safety profile for women who are pregnant or lactating.
- The new nonreplicating smallpox vaccine Jynneos (Bavarian Nordic A/S) is of great value for pre- and post-exposure prophylaxis.
Continue to: Treatment...
Treatment
Infected pregnant women should receive acetaminophen 1,000 mg orally every 8 hours, to control fever and provide analgesia. An antihistamine such as diphenhydramine 25 mg orally every 6-8 hours, may be used to control pruritus and provide mild sedation. Adequate fluid intake and optimal nutrition should be encouraged. Skin lesions should be inspected regularly to detect signs of superimposed bacterial infections. Small, localized bacterial skin infections can be treated with topical application of mupirocin ointment 2%, 3 times daily for 7-14 days. For diffuse and more severe bacterial skin infections, a systemic antibiotic may be necessary. Reasonable choices include amoxicillin-clavulanate 875 mg/125 mg orally every 12 hours, or trimethoprim-sulfamethoxazole double strength 800 mg/160 mg orally every 12 hours.11 The latter agent should be avoided in the first trimester of pregnancy because of potential teratogenic effects.
Several specific agents are available through the CDC for treatment of orthopoxvirus infections such as smallpox and monkeypox. Information about these agents is summarized in the TABLE.12-16

Unique considerations in pregnancy
Because monkeypox is so rare, there is very little information about the effects of this infection in pregnant women. The report most commonly cited in the literature is that by Mbala et al, which was published in 2017.17 These authors described 4 pregnant patients in the Democratic Republic of Congo who contracted monkeypox infection over a 4-year period. All 4 women were hospitalized and treated with systemic antibiotics, antiparasitic medications, and analgesics. One patient delivered a healthy infant. Two women had spontaneous abortions in the first trimester. The fourth patient experienced a stillbirth at 22 weeks’ gestation. At postmortem examination, the fetus had diffuse cutaneous lesions, prominent hepatomegaly, and hydrops. No structural malformations were noted. The placenta demonstrated numerous punctate hemorrhages, and high concentrations of virus were recovered from the placenta and from fetal tissue.
Although the information on pregnancy outcome is quite limited, it seems clear that the virus can cross the placenta and cause adverse effects such as spontaneous abortion and fetal death. Accordingly, I think the following guidelines are a reasonable approach to a pregnant patient who has been exposed to monkeypox or who has developed manifestations of infection.3,7,9
- In the event of a community outbreak, bioterrorism event, or exposure to a person with suspected or confirmed monkeypox infection, the pregnant patient should receive the Jynneos vaccine.
- The pregnant patient should be isolated from any individual with suspected or confirmed monkeypox.
- If infection develops despite these measures, the patient should be treated with either tecovirimat or vaccinia immune globulin IV. Hospitalization may be necessary for seriously ill individuals.
- Within 2 weeks of infection, a comprehensive ultrasound examination should be performed to assess for structural abnormalities in the fetus.
- Subsequently, serial ultrasound examinations should be performed at intervals of 4-6 weeks to assess fetal growth and re-evaluate fetal anatomy.
- Following delivery, a detailed neonatal examination should be performed to assess for evidence of viral injury. Neonatal skin lesions and neonatal serum can be assessed by PCR for monkeypox virus. The newborn should be isolated from the mother until all the mother’s lesions have dried and crusted over.
CASE Resolved
Given the husband’s recent travel to Nigeria and consumption of bushmeat, he most likely has monkeypox. The infection can be spread from person to person by close contact; thus, his wife is at risk. The couple should isolate until all of his lesions have dried and crusted over. The woman also should receive the Jynneos vaccine. If she becomes symptomatic, she should be treated with tecovirimat or vaccinia immune globulin IV. ●

- Isaacs SN, Shenoy ES. Monkeypox. UpToDate. Updated June 28,2022. Accessed July 1, 2022. https://www.uptodate.com /contents/monkeypox?topicRef=8349&source=see_link
- Graham MB. Monkeypox. Medscape. Updated June 29, 2022. Accessed July 1, 2022. https://emedicine.medscape.com /article/1134714-overview.
- Khalil A, Samara A, O’Brien P, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022;60:22-27. doi:10.1002/uog.24968.
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. May 18, 2022. Accessed July 1, 2022. https://www.who.int/emergencies/diseaseoutbreak-news/item/2022-DON383.
- WHO reports two new monkeypox deaths, cases in new areas. Reuters. July 7, 2022. https://www.reuters.com/world /who-reports-two-new-monkeypox-deaths-2022-07-07/. Accessed July 19, 2022.
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. Accessed July 1, 2022. https://www.who.int /emergencies/disease-outbreak-news/item/2022 -DON388#:~:text=Multi%2Dcountry%20monkeypox%20 outbreak%20in%20non%2Dendemic%20countries%3A%20 Update,-29%20May%202022&text=Since%2013%20 May%202022%2C%20monkeypox,Epidemiological%20 investigations%20are%20ongoing.
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections andbioterrorism emergencies. Emerg Infect Dis. 2006;12:16311637. doi:10.3201/eid1211.060618.
- Centers for Disease Control and Prevention. Preparation and collection of specimens. Reviewed June 29, 2022. Accessed July 6, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/prep-collection-specimens.html.
- Rao AK, Petersen BW, Whitehill F, et al. Monkeypox vaccination. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585/mmwr.mm7122e1.
- Smallpox and monkeypox vaccine, live, nonreplicating. Package insert. Bavarian Nordic A/S; 2021. Accessed July 1, 2022. https://www.fda.gov/media/131078/download.
- Duff P. Commonly used antibiotics in ObGyn practice. OBG Manag. 2022;34:29, 36-40. doi:10.12788/obgm.0191.
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals: interim clinical guidance for the treatment of monkeypox. Reviewed June 17, 2022. Accessed July 1, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/treatment.html.
- Brincidofovir. Prescribing information. Chimerix, Inc.; 2021. Accessed July 1, 2022. https://www.accessdata.fda.gov /drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- Cidofovir. Package insert. Gilead Sciences, Inc.; 2010. Accessed July 1, 2022. https://www.gilead.com/~/media /Files/pdfs/medicines/other/vistide/vistide.pdf.
- Tecovirimat. Prescribing information. Catalent Pharma Solutions; 2022. Accessed July 1, 2022. https://www.accessdata.fda.gov/drugsatfda_docs /label/2022/214518s000lbl.pdf.
- Vaccinia immune globulin IV. Prescribing information. Cangene Corporation; 2010. Accessed July 1, 2022. https: //www.fda.gov/media/77004/download.
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260.
- Isaacs SN, Shenoy ES. Monkeypox. UpToDate. Updated June 28,2022. Accessed July 1, 2022. https://www.uptodate.com /contents/monkeypox?topicRef=8349&source=see_link
- Graham MB. Monkeypox. Medscape. Updated June 29, 2022. Accessed July 1, 2022. https://emedicine.medscape.com /article/1134714-overview.
- Khalil A, Samara A, O’Brien P, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022;60:22-27. doi:10.1002/uog.24968.
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. May 18, 2022. Accessed July 1, 2022. https://www.who.int/emergencies/diseaseoutbreak-news/item/2022-DON383.
- WHO reports two new monkeypox deaths, cases in new areas. Reuters. July 7, 2022. https://www.reuters.com/world /who-reports-two-new-monkeypox-deaths-2022-07-07/. Accessed July 19, 2022.
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. Accessed July 1, 2022. https://www.who.int /emergencies/disease-outbreak-news/item/2022 -DON388#:~:text=Multi%2Dcountry%20monkeypox%20 outbreak%20in%20non%2Dendemic%20countries%3A%20 Update,-29%20May%202022&text=Since%2013%20 May%202022%2C%20monkeypox,Epidemiological%20 investigations%20are%20ongoing.
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections andbioterrorism emergencies. Emerg Infect Dis. 2006;12:16311637. doi:10.3201/eid1211.060618.
- Centers for Disease Control and Prevention. Preparation and collection of specimens. Reviewed June 29, 2022. Accessed July 6, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/prep-collection-specimens.html.
- Rao AK, Petersen BW, Whitehill F, et al. Monkeypox vaccination. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585/mmwr.mm7122e1.
- Smallpox and monkeypox vaccine, live, nonreplicating. Package insert. Bavarian Nordic A/S; 2021. Accessed July 1, 2022. https://www.fda.gov/media/131078/download.
- Duff P. Commonly used antibiotics in ObGyn practice. OBG Manag. 2022;34:29, 36-40. doi:10.12788/obgm.0191.
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals: interim clinical guidance for the treatment of monkeypox. Reviewed June 17, 2022. Accessed July 1, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/treatment.html.
- Brincidofovir. Prescribing information. Chimerix, Inc.; 2021. Accessed July 1, 2022. https://www.accessdata.fda.gov /drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- Cidofovir. Package insert. Gilead Sciences, Inc.; 2010. Accessed July 1, 2022. https://www.gilead.com/~/media /Files/pdfs/medicines/other/vistide/vistide.pdf.
- Tecovirimat. Prescribing information. Catalent Pharma Solutions; 2022. Accessed July 1, 2022. https://www.accessdata.fda.gov/drugsatfda_docs /label/2022/214518s000lbl.pdf.
- Vaccinia immune globulin IV. Prescribing information. Cangene Corporation; 2010. Accessed July 1, 2022. https: //www.fda.gov/media/77004/download.
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260.
Some GIs receive more industry money than others
Industry payments to U.S. gastroenterologists and hepatologists increased from 2014 to 2016 before beginning to steadily decrease after 2016, but they're largely concentrated among a small few, according to new research published in Gastroenterology.
The study aimed to identify trends in these specialties in the years after the Sunshine Act, enacted in 2010, and the federal program Open Payments, established in 2013.
“Although Open Payments launched in September of 2014, all the joinpoints in our study occurred more than a year later in 2016, suggesting a delay in observable changes in behavior on industry physician relationships,” wrote Xiaohan Ying, MD, of Weill Cornell Medicine in New York, and colleagues. “Since 2016, we have seen a sustained reduction in general industry payments to physicians while research payments remained stable, which is likely the desired outcome of this program.”
That’s also the conclusion of Lawrence Kosinski, MD, MBA, a spokesperson for the American Gastroenterological Association, who was not involved in the study.
“Most all of us are aware of the Sunshine Act and have reacted accordingly, so I am not surprised that reimbursement per physician has declined over the time period,” Dr. Kosinski told this news organization. “Many physicians are very sensitive to their reporting and have decreased their exposures,” said Dr. Kosinski, founder of SonarMD and a member of the Health & Human Services Advisory Committee on Value-Based Payment. “What does surprise me is the marked disparity in payments with a very small number of physicians receiving tremendous reimbursement from speaking engagements and promotions.”
The researchers retrospectively analyzed industry payments to 26,981 practicing pediatric and adults gastroenterologists and hepatologists using the National Plan and Provider Enumeration System and data from Open Payments between January 2014 and December 2020. The researchers excluded education payments and focused on general payments, which “include charitable contribution, speaker fees, consulting fees, ownership and investments, education, entertainment, food and beverages, gift, honoraria, royalty and license, and travel and lodging,” they reported.
Who gets paid, and how much?
While $27.5 million was going to research and grants, most of the payments ($403.3 million) were general payments; out of the total payments to specialists, $30 million went to hepatology, and $400.8 million went to gastroenterology. Nearly all of the general payments ($398.1 million) were for noneducation purposes; 90.5% of general payments went to men and 9.5% went to women, at an average of $17,167 per person. Nearly half the payments (43.8%) were for speaker fees, totaling $174.3 million, followed by 18.4% going to consulting ($73.1 million) and 12.9% going to food and beverages ($51.5 million).
Most of the physicians accepting payments (86.6%) received less than $10,000, but this made up only 8.3% of all payments. Meanwhile, 74% of all the payments, $294.6 million, went to just 3.1% of the physicians, all of whom received more than $100,000.
That breakdown is what most caught Dr. Kosinki’s attention.
“It’s one thing for a speaker to declare that they are receiving funds from pharma, but they never let us know how much,” Dr. Kosinski said. “Some of these speakers are realizing a very significant payment, which could change the opinions of those listening to their presentations.”
The authors reported that a group of 50 top earners (0.2%) received more than $1 million between 2014 and 2020. Their payments totaled $94.8 million and accounted for nearly a quarter (23.8%) of all the payments. All but one of these physicians were men, and one physician has received more than $1 million every year since 2014.
Payments for guideline authors explored
The authors examined payments to practicing U.S. gastroenterologists and hepatologists who helped write clinical guidelines for the following organizations:
- American Gastroenterological Association (AGA).
- American College of Gastroenterology (ACG).
- American Association for the Study of Liver Disease (AASLD).
- North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN).
- American Society for Gastrointestinal Endoscopy (ASGE).
The 186 guidelines published between 2014 and 2020 had 632 physician authors, 415 of whom were practicing gastroenterologists and hepatologists in the United States. Most of these physicians (85.8%) received at least one industry payment, with payments to guideline authors totaling $43.6 million.
Similar to the lopsided breakdown for total payments across all physicians, the majority of the payments (87.4%, or $38.1 million) went to one-quarter of the authors, who each received more than $100,000 per person. Meanwhile, 38.2% of the guideline authors received less than $10,000.
“However, these numbers are likely to decrease in the future as professional societies, such as AASLD, require a majority of the guideline authors to be free of conflict of interest relevant to the subject matter,” the authors wrote. They added that members selected as part of the AGA’s guideline development group (GDG) must report all conflicts of interest, including indirect and intellectual ones, and are recused or excluded when appropriate. These guideline development group participants must also forgo speaking and consulting arrangements until one year after the guideline’s publication.
Trends have been shifting
Total industry payments initially grew at a rate of 11.4% a year between 2014 and 2016 before decreasing at a rate of 5.8% per year after 2016 (P = .03). Though a similar trend occurred at the individual level, it did not reach significance.
However, the trend differed slightly between men and women: Payments to men increased 10.4% annually until 2016 then decreased 6.8% per year thereafter, but women’s payments increased 11.3% per year until 2019. Between 2014 and 2019, the amount per person payment dropped 3.5% annually to physicians overall, but payments to women initially increased 35.4% a year between 2014 and 2016 before decreasing.
Although not statistically significant, trends for types of payments showed that speaker and food/beverage fees have been declining since 2016 while consulting fees have been declining since 2014.
“The reduction in industry payments could be due to the Hawthorne effect, where physicians alter their behavior after becoming aware that their payments were being monitored,” the authors wrote. “Although many physicians see themselves as less vulnerable to be biased by industry compensation, studies have shown that even small payments can affect behavior such as prescription pattern. Additionally, studies have found that patients are less likely to trust physicians who have received industry payments.”
The authors acknowledged the role of industry payments in funding clinical trials but noted that pharmaceutical companies themselves have been taking on more design and execution of trials in recent decades. Further, only 6% of all payments went to research and grant funding, a little more than half the payments for food and beverages.
“While industry research funding is undeniably crucial, it simply plays a very small role in total industry compensation for physicians,” the authors wrote. “While speaker events could be beneficial and educational for physicians and other audiences, these events could also be utilized as means to promote specific products. While it is beneficial to seek input from experienced gastroenterologists for novel therapies and devices, actions should be taken to place limitations on industry payments to physicians, especially for the top earners.”
One author reported speaker fees from W.L. Gore & Associates and Cook Medical. The other two others had no disclosures. No external funding was noted. Dr. Kosinski reported having no relevant disclosures.
This article was updated Aug. 9, 2022.
Industry payments to U.S. gastroenterologists and hepatologists increased from 2014 to 2016 before beginning to steadily decrease after 2016, but they're largely concentrated among a small few, according to new research published in Gastroenterology.
The study aimed to identify trends in these specialties in the years after the Sunshine Act, enacted in 2010, and the federal program Open Payments, established in 2013.
“Although Open Payments launched in September of 2014, all the joinpoints in our study occurred more than a year later in 2016, suggesting a delay in observable changes in behavior on industry physician relationships,” wrote Xiaohan Ying, MD, of Weill Cornell Medicine in New York, and colleagues. “Since 2016, we have seen a sustained reduction in general industry payments to physicians while research payments remained stable, which is likely the desired outcome of this program.”
That’s also the conclusion of Lawrence Kosinski, MD, MBA, a spokesperson for the American Gastroenterological Association, who was not involved in the study.
“Most all of us are aware of the Sunshine Act and have reacted accordingly, so I am not surprised that reimbursement per physician has declined over the time period,” Dr. Kosinski told this news organization. “Many physicians are very sensitive to their reporting and have decreased their exposures,” said Dr. Kosinski, founder of SonarMD and a member of the Health & Human Services Advisory Committee on Value-Based Payment. “What does surprise me is the marked disparity in payments with a very small number of physicians receiving tremendous reimbursement from speaking engagements and promotions.”
The researchers retrospectively analyzed industry payments to 26,981 practicing pediatric and adults gastroenterologists and hepatologists using the National Plan and Provider Enumeration System and data from Open Payments between January 2014 and December 2020. The researchers excluded education payments and focused on general payments, which “include charitable contribution, speaker fees, consulting fees, ownership and investments, education, entertainment, food and beverages, gift, honoraria, royalty and license, and travel and lodging,” they reported.
Who gets paid, and how much?
While $27.5 million was going to research and grants, most of the payments ($403.3 million) were general payments; out of the total payments to specialists, $30 million went to hepatology, and $400.8 million went to gastroenterology. Nearly all of the general payments ($398.1 million) were for noneducation purposes; 90.5% of general payments went to men and 9.5% went to women, at an average of $17,167 per person. Nearly half the payments (43.8%) were for speaker fees, totaling $174.3 million, followed by 18.4% going to consulting ($73.1 million) and 12.9% going to food and beverages ($51.5 million).
Most of the physicians accepting payments (86.6%) received less than $10,000, but this made up only 8.3% of all payments. Meanwhile, 74% of all the payments, $294.6 million, went to just 3.1% of the physicians, all of whom received more than $100,000.
That breakdown is what most caught Dr. Kosinki’s attention.
“It’s one thing for a speaker to declare that they are receiving funds from pharma, but they never let us know how much,” Dr. Kosinski said. “Some of these speakers are realizing a very significant payment, which could change the opinions of those listening to their presentations.”
The authors reported that a group of 50 top earners (0.2%) received more than $1 million between 2014 and 2020. Their payments totaled $94.8 million and accounted for nearly a quarter (23.8%) of all the payments. All but one of these physicians were men, and one physician has received more than $1 million every year since 2014.
Payments for guideline authors explored
The authors examined payments to practicing U.S. gastroenterologists and hepatologists who helped write clinical guidelines for the following organizations:
- American Gastroenterological Association (AGA).
- American College of Gastroenterology (ACG).
- American Association for the Study of Liver Disease (AASLD).
- North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN).
- American Society for Gastrointestinal Endoscopy (ASGE).
The 186 guidelines published between 2014 and 2020 had 632 physician authors, 415 of whom were practicing gastroenterologists and hepatologists in the United States. Most of these physicians (85.8%) received at least one industry payment, with payments to guideline authors totaling $43.6 million.
Similar to the lopsided breakdown for total payments across all physicians, the majority of the payments (87.4%, or $38.1 million) went to one-quarter of the authors, who each received more than $100,000 per person. Meanwhile, 38.2% of the guideline authors received less than $10,000.
“However, these numbers are likely to decrease in the future as professional societies, such as AASLD, require a majority of the guideline authors to be free of conflict of interest relevant to the subject matter,” the authors wrote. They added that members selected as part of the AGA’s guideline development group (GDG) must report all conflicts of interest, including indirect and intellectual ones, and are recused or excluded when appropriate. These guideline development group participants must also forgo speaking and consulting arrangements until one year after the guideline’s publication.
Trends have been shifting
Total industry payments initially grew at a rate of 11.4% a year between 2014 and 2016 before decreasing at a rate of 5.8% per year after 2016 (P = .03). Though a similar trend occurred at the individual level, it did not reach significance.
However, the trend differed slightly between men and women: Payments to men increased 10.4% annually until 2016 then decreased 6.8% per year thereafter, but women’s payments increased 11.3% per year until 2019. Between 2014 and 2019, the amount per person payment dropped 3.5% annually to physicians overall, but payments to women initially increased 35.4% a year between 2014 and 2016 before decreasing.
Although not statistically significant, trends for types of payments showed that speaker and food/beverage fees have been declining since 2016 while consulting fees have been declining since 2014.
“The reduction in industry payments could be due to the Hawthorne effect, where physicians alter their behavior after becoming aware that their payments were being monitored,” the authors wrote. “Although many physicians see themselves as less vulnerable to be biased by industry compensation, studies have shown that even small payments can affect behavior such as prescription pattern. Additionally, studies have found that patients are less likely to trust physicians who have received industry payments.”
The authors acknowledged the role of industry payments in funding clinical trials but noted that pharmaceutical companies themselves have been taking on more design and execution of trials in recent decades. Further, only 6% of all payments went to research and grant funding, a little more than half the payments for food and beverages.
“While industry research funding is undeniably crucial, it simply plays a very small role in total industry compensation for physicians,” the authors wrote. “While speaker events could be beneficial and educational for physicians and other audiences, these events could also be utilized as means to promote specific products. While it is beneficial to seek input from experienced gastroenterologists for novel therapies and devices, actions should be taken to place limitations on industry payments to physicians, especially for the top earners.”
One author reported speaker fees from W.L. Gore & Associates and Cook Medical. The other two others had no disclosures. No external funding was noted. Dr. Kosinski reported having no relevant disclosures.
This article was updated Aug. 9, 2022.
Industry payments to U.S. gastroenterologists and hepatologists increased from 2014 to 2016 before beginning to steadily decrease after 2016, but they're largely concentrated among a small few, according to new research published in Gastroenterology.
The study aimed to identify trends in these specialties in the years after the Sunshine Act, enacted in 2010, and the federal program Open Payments, established in 2013.
“Although Open Payments launched in September of 2014, all the joinpoints in our study occurred more than a year later in 2016, suggesting a delay in observable changes in behavior on industry physician relationships,” wrote Xiaohan Ying, MD, of Weill Cornell Medicine in New York, and colleagues. “Since 2016, we have seen a sustained reduction in general industry payments to physicians while research payments remained stable, which is likely the desired outcome of this program.”
That’s also the conclusion of Lawrence Kosinski, MD, MBA, a spokesperson for the American Gastroenterological Association, who was not involved in the study.
“Most all of us are aware of the Sunshine Act and have reacted accordingly, so I am not surprised that reimbursement per physician has declined over the time period,” Dr. Kosinski told this news organization. “Many physicians are very sensitive to their reporting and have decreased their exposures,” said Dr. Kosinski, founder of SonarMD and a member of the Health & Human Services Advisory Committee on Value-Based Payment. “What does surprise me is the marked disparity in payments with a very small number of physicians receiving tremendous reimbursement from speaking engagements and promotions.”
The researchers retrospectively analyzed industry payments to 26,981 practicing pediatric and adults gastroenterologists and hepatologists using the National Plan and Provider Enumeration System and data from Open Payments between January 2014 and December 2020. The researchers excluded education payments and focused on general payments, which “include charitable contribution, speaker fees, consulting fees, ownership and investments, education, entertainment, food and beverages, gift, honoraria, royalty and license, and travel and lodging,” they reported.
Who gets paid, and how much?
While $27.5 million was going to research and grants, most of the payments ($403.3 million) were general payments; out of the total payments to specialists, $30 million went to hepatology, and $400.8 million went to gastroenterology. Nearly all of the general payments ($398.1 million) were for noneducation purposes; 90.5% of general payments went to men and 9.5% went to women, at an average of $17,167 per person. Nearly half the payments (43.8%) were for speaker fees, totaling $174.3 million, followed by 18.4% going to consulting ($73.1 million) and 12.9% going to food and beverages ($51.5 million).
Most of the physicians accepting payments (86.6%) received less than $10,000, but this made up only 8.3% of all payments. Meanwhile, 74% of all the payments, $294.6 million, went to just 3.1% of the physicians, all of whom received more than $100,000.
That breakdown is what most caught Dr. Kosinki’s attention.
“It’s one thing for a speaker to declare that they are receiving funds from pharma, but they never let us know how much,” Dr. Kosinski said. “Some of these speakers are realizing a very significant payment, which could change the opinions of those listening to their presentations.”
The authors reported that a group of 50 top earners (0.2%) received more than $1 million between 2014 and 2020. Their payments totaled $94.8 million and accounted for nearly a quarter (23.8%) of all the payments. All but one of these physicians were men, and one physician has received more than $1 million every year since 2014.
Payments for guideline authors explored
The authors examined payments to practicing U.S. gastroenterologists and hepatologists who helped write clinical guidelines for the following organizations:
- American Gastroenterological Association (AGA).
- American College of Gastroenterology (ACG).
- American Association for the Study of Liver Disease (AASLD).
- North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN).
- American Society for Gastrointestinal Endoscopy (ASGE).
The 186 guidelines published between 2014 and 2020 had 632 physician authors, 415 of whom were practicing gastroenterologists and hepatologists in the United States. Most of these physicians (85.8%) received at least one industry payment, with payments to guideline authors totaling $43.6 million.
Similar to the lopsided breakdown for total payments across all physicians, the majority of the payments (87.4%, or $38.1 million) went to one-quarter of the authors, who each received more than $100,000 per person. Meanwhile, 38.2% of the guideline authors received less than $10,000.
“However, these numbers are likely to decrease in the future as professional societies, such as AASLD, require a majority of the guideline authors to be free of conflict of interest relevant to the subject matter,” the authors wrote. They added that members selected as part of the AGA’s guideline development group (GDG) must report all conflicts of interest, including indirect and intellectual ones, and are recused or excluded when appropriate. These guideline development group participants must also forgo speaking and consulting arrangements until one year after the guideline’s publication.
Trends have been shifting
Total industry payments initially grew at a rate of 11.4% a year between 2014 and 2016 before decreasing at a rate of 5.8% per year after 2016 (P = .03). Though a similar trend occurred at the individual level, it did not reach significance.
However, the trend differed slightly between men and women: Payments to men increased 10.4% annually until 2016 then decreased 6.8% per year thereafter, but women’s payments increased 11.3% per year until 2019. Between 2014 and 2019, the amount per person payment dropped 3.5% annually to physicians overall, but payments to women initially increased 35.4% a year between 2014 and 2016 before decreasing.
Although not statistically significant, trends for types of payments showed that speaker and food/beverage fees have been declining since 2016 while consulting fees have been declining since 2014.
“The reduction in industry payments could be due to the Hawthorne effect, where physicians alter their behavior after becoming aware that their payments were being monitored,” the authors wrote. “Although many physicians see themselves as less vulnerable to be biased by industry compensation, studies have shown that even small payments can affect behavior such as prescription pattern. Additionally, studies have found that patients are less likely to trust physicians who have received industry payments.”
The authors acknowledged the role of industry payments in funding clinical trials but noted that pharmaceutical companies themselves have been taking on more design and execution of trials in recent decades. Further, only 6% of all payments went to research and grant funding, a little more than half the payments for food and beverages.
“While industry research funding is undeniably crucial, it simply plays a very small role in total industry compensation for physicians,” the authors wrote. “While speaker events could be beneficial and educational for physicians and other audiences, these events could also be utilized as means to promote specific products. While it is beneficial to seek input from experienced gastroenterologists for novel therapies and devices, actions should be taken to place limitations on industry payments to physicians, especially for the top earners.”
One author reported speaker fees from W.L. Gore & Associates and Cook Medical. The other two others had no disclosures. No external funding was noted. Dr. Kosinski reported having no relevant disclosures.
This article was updated Aug. 9, 2022.
FROM GASTROENTEROLOGY
My patient planned to murder me
San Diego internist David B. Bittleman, MD, was finishing an appointment with a patient when the man’s caregiver slipped Dr. Bittleman a note as the patient walked out of the room.
“Call me tomorrow,” the mysterious message read.
Dr. Bittleman phoned the caregiver, who was the patient’s ex-wife, the next day. He assumed she wanted to discuss a routine issue, such as the patient’s treatment. But the reason she wanted to talk privately was far more ominous.
“He wants to kill you,” she said.
Dr. Bittleman was shocked. The caregiver told Dr. Bittleman she believed her ex-husband was serious.
“The ex-wife and two adult sons were very alarmed by his erratic behavior,” Dr. Bittleman recalled. “She made it very clear that he said he planned to kill me. I feared for my life because I took his threat at face value.”
Patient sends alarming message, makes threats
When he went into medicine, Dr. Bittleman never imagined that he’d have to worry about being attacked or killed by a patient.
After spending 20 years in private practice, Dr. Bittleman was excited to accept a position at the Veterans Affairs San Diego health system. His extended family lived in the area, and he looked forward to helping veterans and to working with students, he said.
Dr. Bittleman had practiced primary care at the VA for about 5 years when he encountered the threatening patient, a veteran in his 60’s. The man was suffering from musculoskeletal pain and mental illness.
The patient had taken opioids on and off for many years. Dr. Bittleman felt that to continue the medication would not be safe, considering the man’s lifestyle.
“He had been maintained on oxycodone for chronic pain by previous providers, but I thought that was dangerous, given that he was mixing it with alcohol and marijuana,” he said. “I met with him and a substance use disorder physician for a conference call, and we explained we would need to taper the medication and eventually stop the opioids.”
Dr. Bittleman pleaded with the patient to enter drug rehab, and he offered him inpatient care for treatment of withdrawal. The man refused.
A few weeks later, Dr. Bittleman was checking the health center’s electronic messaging system. He found a disturbing message from the patient.
“You better learn jiu jitsu and hand-to-hand combat if you ever take my opioids away,” the message read. “You better learn how to defend yourself!”
Dr. Bittleman contacted the VA police and reported the message. The patient was interviewed by mental health professionals, but they did not believe he was dangerous, according to Dr. Bittleman.
“They are pretty limited to what they can do,” he said. “At a private practice, the patient might be fired or no longer allowed to come into the building, but the VA is a safety net institution. I’m not sure if he was even reprimanded.”
Two months later, the patient’s ex-wife shared the alarming news that the patient wanted to kill the doctor.
Dr. Bittleman went back to the police. They suggested he file a restraining order, which he sought that afternoon. By the end of the day, the judge had issued the restraining order, according to Dr. Bittleman and court records. The patient could not come within 100 yards of the physician, his clinic, car, or home.
But there was one frightening caveat. The order was temporary. It would last for only 2 weeks. To make the order permanent, Dr. Bittleman would have to go before the judge and argue why it was needed.
He wouldn’t be alone at the hearing. Someone else would be just paces away – the patient who wanted to murder him.
Doctor and patient face off before judge
As the hearing neared, Dr. Bittleman felt anxious, outraged, and fearful. He wondered whether the patient might make good on his threat.
Some colleagues suggested that Bittleman buy a gun, while others recommended he carry pepper spray. Dr. Bittleman had no interest in learning how to use a gun, he said. He took comfort in the fact that there were armed guards and metal detectors in his building, and there was a panic button under his desk.
“I was not sure I wanted to take care of patients anymore, especially chronic pain patients,” he said. “However, I went for some counseling with the Employee Assistance Program, and the therapist was helpful in normalizing my anxiety and acknowledging my fear.”
On the day of the hearing, Dr. Bittleman sat in the back of the courtroom. The patient, who sat near the front, glanced at Dr. Bittleman with a slight smile.
When his case was called, the judge explained that as the plaintiff, the burden was on Dr. Bittleman to prove the patient was a threat to his safety. He provided the judge a copy of the threatening message and a copy of the ex-wife’s note.
After reading the documents, the judge asked the patient to explain his side. The patient complained that the VA had denied him certain benefits and that he was forced to receive mental health treatment rehab that he “didn’t need.” The judge eventually interrupted the man to ask if he had threatened to kill Dr. Bittleman.
“Oh yes, your honor, I did say that, but I was only joking,” he told the judge.
The admission was enough. The judge issued a restraining order against the patient that would last 1 year. He could not have firearms, and if he violated the order, he would be arrested.
The terrifying saga was finally over.
“I never heard from the patient again,” Dr. Bittleman said. “His [care] location was changed, and police were required to come to all his visits with his new provider. I was relieved that if he ever came near me, he was going to jail.”
To raise awareness about such ordeals and the hassles that can follow, Dr. Bittleman wrote an article about his experience, which was published in the Annals of Family Medicine. He continues to treat patients at the VA, including those with chronic pain, but the memory of the menacing patient resurfaces from time to time.
“I do still think about it,” he said. “I know how to use my panic button, and I test it every 90 days. If there is a patient who concerns me, I will have the VA police wait nearby. I am very aware and upset by violence. When I hear about a doctor getting killed, I feel a clutch in my chest. How could I not relate? Here is a doctor who worked hard, who dedicated their life to help patients, and it comes to this? It’s so revolting. It makes me sick.”
Can you identify a violent patient?
Concern over threatening patients has grown across the country after recent violent attacks against physicians in Oklahoma and California. Two physicians were shot to death in June 2022 when a patient opened fire inside a Tulsa medical building. The primary target of the shooting was a surgeon who had performed surgery on the patient. Also in June, two nurses and an emergency physician were stabbed by a patient inside the Encino Hospital Medical Center. They survived.
The attacks raise questions about how to identify potentially violent patients and how to mitigate possible violence.
Threats and violence against health care professionals are nothing new, but they’re finally getting the attention they deserve, says Derek Schaller, MD, an emergency physician and assistant professor of emergency medicine at Central Michigan University in Mount Pleasant.
“Violence against personnel in medicine has been an issue for a long time; it’s just finally making headlines,” he said. “Way back when, it almost seemed like it was part of the job, part of the gig. But it shouldn’t be part of the gig. It’s not something we should be dealing with.”
It’s common for health care professionals and health centers to take a reactive approach to violent patients, but Dr. Schaller encourages a more proactive strategy. Central Michigan University Health, for example, recently studied its past violent encounters and analyzed the characteristics of violent patients. The analysis came after an increase in violent patient episodes at the health center in the past year, Dr. Schaller said.
The study yielded some interesting results, including that a large percentage of patients who became violent in the emergency department did so within the first hour they were in the hospital, he said.
“You would have thought it’s the patients who have been there and have been stuck in the emergency department for a while and who became disgruntled, but that was not the case,” Dr. Schaller said.
He recommends that physicians, medical practices, and hospitals carry out similar assessments of their patient populations and of past violent encounters to determine trends. His institution will be implementing a screening tool in triage to identify patients more likely to become violent so that health care professionals can intervene earlier, he said.
Such a screening tool is already demonstrating success in a variety of medical settings.
About 10 years ago, a research team led by Son Chae Kim, PhD, RN, found that the 10-item Aggressive Behavior Risk Assessment Tool (ABRAT) was able to identify potentially violent patients with reasonable sensitivity and specificity in hospital medical-surgical units.
Subsequently, the tool was modified for long-term care facilities, and again, researchers found that ABRAT was able to identify potentially violent residents with reasonable sensitivity and specificity, said Dr. Kim, ABRAT developer and a professor at Point Loma Nazarene University, San Diego.
In 2021, researchers embedded the checklist into an electronic health record (EHR) system and tested ABRAT in emergency departments.
“Currently, we are working with computer programmers to build an app that would make the ABRAT very easy to use in conjunction with EHR,” Dr. Kim said. “Instead of a nurse searching the EHR to find out if the patient has history of mental illness or aggressive behavior in the past, the app would automatically search the EHR and combine the nurse’s quick observation whether the patient is confused, agitated, staring, or threatening, to automatically calculate the violence risk.”
Dr. Kim and her team also developed a tool called VEST (Violent Event Severity Tool), a standardized objective workplace violence severity assessment. They are working with programmers to incorporate VEST into the app as well.
Dr. Kim’s hope is that the ABRAT tool can be modified for use in a range of health care settings.
A version of this article first appeared on Medscape.com.
San Diego internist David B. Bittleman, MD, was finishing an appointment with a patient when the man’s caregiver slipped Dr. Bittleman a note as the patient walked out of the room.
“Call me tomorrow,” the mysterious message read.
Dr. Bittleman phoned the caregiver, who was the patient’s ex-wife, the next day. He assumed she wanted to discuss a routine issue, such as the patient’s treatment. But the reason she wanted to talk privately was far more ominous.
“He wants to kill you,” she said.
Dr. Bittleman was shocked. The caregiver told Dr. Bittleman she believed her ex-husband was serious.
“The ex-wife and two adult sons were very alarmed by his erratic behavior,” Dr. Bittleman recalled. “She made it very clear that he said he planned to kill me. I feared for my life because I took his threat at face value.”
Patient sends alarming message, makes threats
When he went into medicine, Dr. Bittleman never imagined that he’d have to worry about being attacked or killed by a patient.
After spending 20 years in private practice, Dr. Bittleman was excited to accept a position at the Veterans Affairs San Diego health system. His extended family lived in the area, and he looked forward to helping veterans and to working with students, he said.
Dr. Bittleman had practiced primary care at the VA for about 5 years when he encountered the threatening patient, a veteran in his 60’s. The man was suffering from musculoskeletal pain and mental illness.
The patient had taken opioids on and off for many years. Dr. Bittleman felt that to continue the medication would not be safe, considering the man’s lifestyle.
“He had been maintained on oxycodone for chronic pain by previous providers, but I thought that was dangerous, given that he was mixing it with alcohol and marijuana,” he said. “I met with him and a substance use disorder physician for a conference call, and we explained we would need to taper the medication and eventually stop the opioids.”
Dr. Bittleman pleaded with the patient to enter drug rehab, and he offered him inpatient care for treatment of withdrawal. The man refused.
A few weeks later, Dr. Bittleman was checking the health center’s electronic messaging system. He found a disturbing message from the patient.
“You better learn jiu jitsu and hand-to-hand combat if you ever take my opioids away,” the message read. “You better learn how to defend yourself!”
Dr. Bittleman contacted the VA police and reported the message. The patient was interviewed by mental health professionals, but they did not believe he was dangerous, according to Dr. Bittleman.
“They are pretty limited to what they can do,” he said. “At a private practice, the patient might be fired or no longer allowed to come into the building, but the VA is a safety net institution. I’m not sure if he was even reprimanded.”
Two months later, the patient’s ex-wife shared the alarming news that the patient wanted to kill the doctor.
Dr. Bittleman went back to the police. They suggested he file a restraining order, which he sought that afternoon. By the end of the day, the judge had issued the restraining order, according to Dr. Bittleman and court records. The patient could not come within 100 yards of the physician, his clinic, car, or home.
But there was one frightening caveat. The order was temporary. It would last for only 2 weeks. To make the order permanent, Dr. Bittleman would have to go before the judge and argue why it was needed.
He wouldn’t be alone at the hearing. Someone else would be just paces away – the patient who wanted to murder him.
Doctor and patient face off before judge
As the hearing neared, Dr. Bittleman felt anxious, outraged, and fearful. He wondered whether the patient might make good on his threat.
Some colleagues suggested that Bittleman buy a gun, while others recommended he carry pepper spray. Dr. Bittleman had no interest in learning how to use a gun, he said. He took comfort in the fact that there were armed guards and metal detectors in his building, and there was a panic button under his desk.
“I was not sure I wanted to take care of patients anymore, especially chronic pain patients,” he said. “However, I went for some counseling with the Employee Assistance Program, and the therapist was helpful in normalizing my anxiety and acknowledging my fear.”
On the day of the hearing, Dr. Bittleman sat in the back of the courtroom. The patient, who sat near the front, glanced at Dr. Bittleman with a slight smile.
When his case was called, the judge explained that as the plaintiff, the burden was on Dr. Bittleman to prove the patient was a threat to his safety. He provided the judge a copy of the threatening message and a copy of the ex-wife’s note.
After reading the documents, the judge asked the patient to explain his side. The patient complained that the VA had denied him certain benefits and that he was forced to receive mental health treatment rehab that he “didn’t need.” The judge eventually interrupted the man to ask if he had threatened to kill Dr. Bittleman.
“Oh yes, your honor, I did say that, but I was only joking,” he told the judge.
The admission was enough. The judge issued a restraining order against the patient that would last 1 year. He could not have firearms, and if he violated the order, he would be arrested.
The terrifying saga was finally over.
“I never heard from the patient again,” Dr. Bittleman said. “His [care] location was changed, and police were required to come to all his visits with his new provider. I was relieved that if he ever came near me, he was going to jail.”
To raise awareness about such ordeals and the hassles that can follow, Dr. Bittleman wrote an article about his experience, which was published in the Annals of Family Medicine. He continues to treat patients at the VA, including those with chronic pain, but the memory of the menacing patient resurfaces from time to time.
“I do still think about it,” he said. “I know how to use my panic button, and I test it every 90 days. If there is a patient who concerns me, I will have the VA police wait nearby. I am very aware and upset by violence. When I hear about a doctor getting killed, I feel a clutch in my chest. How could I not relate? Here is a doctor who worked hard, who dedicated their life to help patients, and it comes to this? It’s so revolting. It makes me sick.”
Can you identify a violent patient?
Concern over threatening patients has grown across the country after recent violent attacks against physicians in Oklahoma and California. Two physicians were shot to death in June 2022 when a patient opened fire inside a Tulsa medical building. The primary target of the shooting was a surgeon who had performed surgery on the patient. Also in June, two nurses and an emergency physician were stabbed by a patient inside the Encino Hospital Medical Center. They survived.
The attacks raise questions about how to identify potentially violent patients and how to mitigate possible violence.
Threats and violence against health care professionals are nothing new, but they’re finally getting the attention they deserve, says Derek Schaller, MD, an emergency physician and assistant professor of emergency medicine at Central Michigan University in Mount Pleasant.
“Violence against personnel in medicine has been an issue for a long time; it’s just finally making headlines,” he said. “Way back when, it almost seemed like it was part of the job, part of the gig. But it shouldn’t be part of the gig. It’s not something we should be dealing with.”
It’s common for health care professionals and health centers to take a reactive approach to violent patients, but Dr. Schaller encourages a more proactive strategy. Central Michigan University Health, for example, recently studied its past violent encounters and analyzed the characteristics of violent patients. The analysis came after an increase in violent patient episodes at the health center in the past year, Dr. Schaller said.
The study yielded some interesting results, including that a large percentage of patients who became violent in the emergency department did so within the first hour they were in the hospital, he said.
“You would have thought it’s the patients who have been there and have been stuck in the emergency department for a while and who became disgruntled, but that was not the case,” Dr. Schaller said.
He recommends that physicians, medical practices, and hospitals carry out similar assessments of their patient populations and of past violent encounters to determine trends. His institution will be implementing a screening tool in triage to identify patients more likely to become violent so that health care professionals can intervene earlier, he said.
Such a screening tool is already demonstrating success in a variety of medical settings.
About 10 years ago, a research team led by Son Chae Kim, PhD, RN, found that the 10-item Aggressive Behavior Risk Assessment Tool (ABRAT) was able to identify potentially violent patients with reasonable sensitivity and specificity in hospital medical-surgical units.
Subsequently, the tool was modified for long-term care facilities, and again, researchers found that ABRAT was able to identify potentially violent residents with reasonable sensitivity and specificity, said Dr. Kim, ABRAT developer and a professor at Point Loma Nazarene University, San Diego.
In 2021, researchers embedded the checklist into an electronic health record (EHR) system and tested ABRAT in emergency departments.
“Currently, we are working with computer programmers to build an app that would make the ABRAT very easy to use in conjunction with EHR,” Dr. Kim said. “Instead of a nurse searching the EHR to find out if the patient has history of mental illness or aggressive behavior in the past, the app would automatically search the EHR and combine the nurse’s quick observation whether the patient is confused, agitated, staring, or threatening, to automatically calculate the violence risk.”
Dr. Kim and her team also developed a tool called VEST (Violent Event Severity Tool), a standardized objective workplace violence severity assessment. They are working with programmers to incorporate VEST into the app as well.
Dr. Kim’s hope is that the ABRAT tool can be modified for use in a range of health care settings.
A version of this article first appeared on Medscape.com.
San Diego internist David B. Bittleman, MD, was finishing an appointment with a patient when the man’s caregiver slipped Dr. Bittleman a note as the patient walked out of the room.
“Call me tomorrow,” the mysterious message read.
Dr. Bittleman phoned the caregiver, who was the patient’s ex-wife, the next day. He assumed she wanted to discuss a routine issue, such as the patient’s treatment. But the reason she wanted to talk privately was far more ominous.
“He wants to kill you,” she said.
Dr. Bittleman was shocked. The caregiver told Dr. Bittleman she believed her ex-husband was serious.
“The ex-wife and two adult sons were very alarmed by his erratic behavior,” Dr. Bittleman recalled. “She made it very clear that he said he planned to kill me. I feared for my life because I took his threat at face value.”
Patient sends alarming message, makes threats
When he went into medicine, Dr. Bittleman never imagined that he’d have to worry about being attacked or killed by a patient.
After spending 20 years in private practice, Dr. Bittleman was excited to accept a position at the Veterans Affairs San Diego health system. His extended family lived in the area, and he looked forward to helping veterans and to working with students, he said.
Dr. Bittleman had practiced primary care at the VA for about 5 years when he encountered the threatening patient, a veteran in his 60’s. The man was suffering from musculoskeletal pain and mental illness.
The patient had taken opioids on and off for many years. Dr. Bittleman felt that to continue the medication would not be safe, considering the man’s lifestyle.
“He had been maintained on oxycodone for chronic pain by previous providers, but I thought that was dangerous, given that he was mixing it with alcohol and marijuana,” he said. “I met with him and a substance use disorder physician for a conference call, and we explained we would need to taper the medication and eventually stop the opioids.”
Dr. Bittleman pleaded with the patient to enter drug rehab, and he offered him inpatient care for treatment of withdrawal. The man refused.
A few weeks later, Dr. Bittleman was checking the health center’s electronic messaging system. He found a disturbing message from the patient.
“You better learn jiu jitsu and hand-to-hand combat if you ever take my opioids away,” the message read. “You better learn how to defend yourself!”
Dr. Bittleman contacted the VA police and reported the message. The patient was interviewed by mental health professionals, but they did not believe he was dangerous, according to Dr. Bittleman.
“They are pretty limited to what they can do,” he said. “At a private practice, the patient might be fired or no longer allowed to come into the building, but the VA is a safety net institution. I’m not sure if he was even reprimanded.”
Two months later, the patient’s ex-wife shared the alarming news that the patient wanted to kill the doctor.
Dr. Bittleman went back to the police. They suggested he file a restraining order, which he sought that afternoon. By the end of the day, the judge had issued the restraining order, according to Dr. Bittleman and court records. The patient could not come within 100 yards of the physician, his clinic, car, or home.
But there was one frightening caveat. The order was temporary. It would last for only 2 weeks. To make the order permanent, Dr. Bittleman would have to go before the judge and argue why it was needed.
He wouldn’t be alone at the hearing. Someone else would be just paces away – the patient who wanted to murder him.
Doctor and patient face off before judge
As the hearing neared, Dr. Bittleman felt anxious, outraged, and fearful. He wondered whether the patient might make good on his threat.
Some colleagues suggested that Bittleman buy a gun, while others recommended he carry pepper spray. Dr. Bittleman had no interest in learning how to use a gun, he said. He took comfort in the fact that there were armed guards and metal detectors in his building, and there was a panic button under his desk.
“I was not sure I wanted to take care of patients anymore, especially chronic pain patients,” he said. “However, I went for some counseling with the Employee Assistance Program, and the therapist was helpful in normalizing my anxiety and acknowledging my fear.”
On the day of the hearing, Dr. Bittleman sat in the back of the courtroom. The patient, who sat near the front, glanced at Dr. Bittleman with a slight smile.
When his case was called, the judge explained that as the plaintiff, the burden was on Dr. Bittleman to prove the patient was a threat to his safety. He provided the judge a copy of the threatening message and a copy of the ex-wife’s note.
After reading the documents, the judge asked the patient to explain his side. The patient complained that the VA had denied him certain benefits and that he was forced to receive mental health treatment rehab that he “didn’t need.” The judge eventually interrupted the man to ask if he had threatened to kill Dr. Bittleman.
“Oh yes, your honor, I did say that, but I was only joking,” he told the judge.
The admission was enough. The judge issued a restraining order against the patient that would last 1 year. He could not have firearms, and if he violated the order, he would be arrested.
The terrifying saga was finally over.
“I never heard from the patient again,” Dr. Bittleman said. “His [care] location was changed, and police were required to come to all his visits with his new provider. I was relieved that if he ever came near me, he was going to jail.”
To raise awareness about such ordeals and the hassles that can follow, Dr. Bittleman wrote an article about his experience, which was published in the Annals of Family Medicine. He continues to treat patients at the VA, including those with chronic pain, but the memory of the menacing patient resurfaces from time to time.
“I do still think about it,” he said. “I know how to use my panic button, and I test it every 90 days. If there is a patient who concerns me, I will have the VA police wait nearby. I am very aware and upset by violence. When I hear about a doctor getting killed, I feel a clutch in my chest. How could I not relate? Here is a doctor who worked hard, who dedicated their life to help patients, and it comes to this? It’s so revolting. It makes me sick.”
Can you identify a violent patient?
Concern over threatening patients has grown across the country after recent violent attacks against physicians in Oklahoma and California. Two physicians were shot to death in June 2022 when a patient opened fire inside a Tulsa medical building. The primary target of the shooting was a surgeon who had performed surgery on the patient. Also in June, two nurses and an emergency physician were stabbed by a patient inside the Encino Hospital Medical Center. They survived.
The attacks raise questions about how to identify potentially violent patients and how to mitigate possible violence.
Threats and violence against health care professionals are nothing new, but they’re finally getting the attention they deserve, says Derek Schaller, MD, an emergency physician and assistant professor of emergency medicine at Central Michigan University in Mount Pleasant.
“Violence against personnel in medicine has been an issue for a long time; it’s just finally making headlines,” he said. “Way back when, it almost seemed like it was part of the job, part of the gig. But it shouldn’t be part of the gig. It’s not something we should be dealing with.”
It’s common for health care professionals and health centers to take a reactive approach to violent patients, but Dr. Schaller encourages a more proactive strategy. Central Michigan University Health, for example, recently studied its past violent encounters and analyzed the characteristics of violent patients. The analysis came after an increase in violent patient episodes at the health center in the past year, Dr. Schaller said.
The study yielded some interesting results, including that a large percentage of patients who became violent in the emergency department did so within the first hour they were in the hospital, he said.
“You would have thought it’s the patients who have been there and have been stuck in the emergency department for a while and who became disgruntled, but that was not the case,” Dr. Schaller said.
He recommends that physicians, medical practices, and hospitals carry out similar assessments of their patient populations and of past violent encounters to determine trends. His institution will be implementing a screening tool in triage to identify patients more likely to become violent so that health care professionals can intervene earlier, he said.
Such a screening tool is already demonstrating success in a variety of medical settings.
About 10 years ago, a research team led by Son Chae Kim, PhD, RN, found that the 10-item Aggressive Behavior Risk Assessment Tool (ABRAT) was able to identify potentially violent patients with reasonable sensitivity and specificity in hospital medical-surgical units.
Subsequently, the tool was modified for long-term care facilities, and again, researchers found that ABRAT was able to identify potentially violent residents with reasonable sensitivity and specificity, said Dr. Kim, ABRAT developer and a professor at Point Loma Nazarene University, San Diego.
In 2021, researchers embedded the checklist into an electronic health record (EHR) system and tested ABRAT in emergency departments.
“Currently, we are working with computer programmers to build an app that would make the ABRAT very easy to use in conjunction with EHR,” Dr. Kim said. “Instead of a nurse searching the EHR to find out if the patient has history of mental illness or aggressive behavior in the past, the app would automatically search the EHR and combine the nurse’s quick observation whether the patient is confused, agitated, staring, or threatening, to automatically calculate the violence risk.”
Dr. Kim and her team also developed a tool called VEST (Violent Event Severity Tool), a standardized objective workplace violence severity assessment. They are working with programmers to incorporate VEST into the app as well.
Dr. Kim’s hope is that the ABRAT tool can be modified for use in a range of health care settings.
A version of this article first appeared on Medscape.com.
Medical assistants identify strategies and barriers to clinic efficiency
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
Continue to: Methods...
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
Continue to: RESULTS...
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.
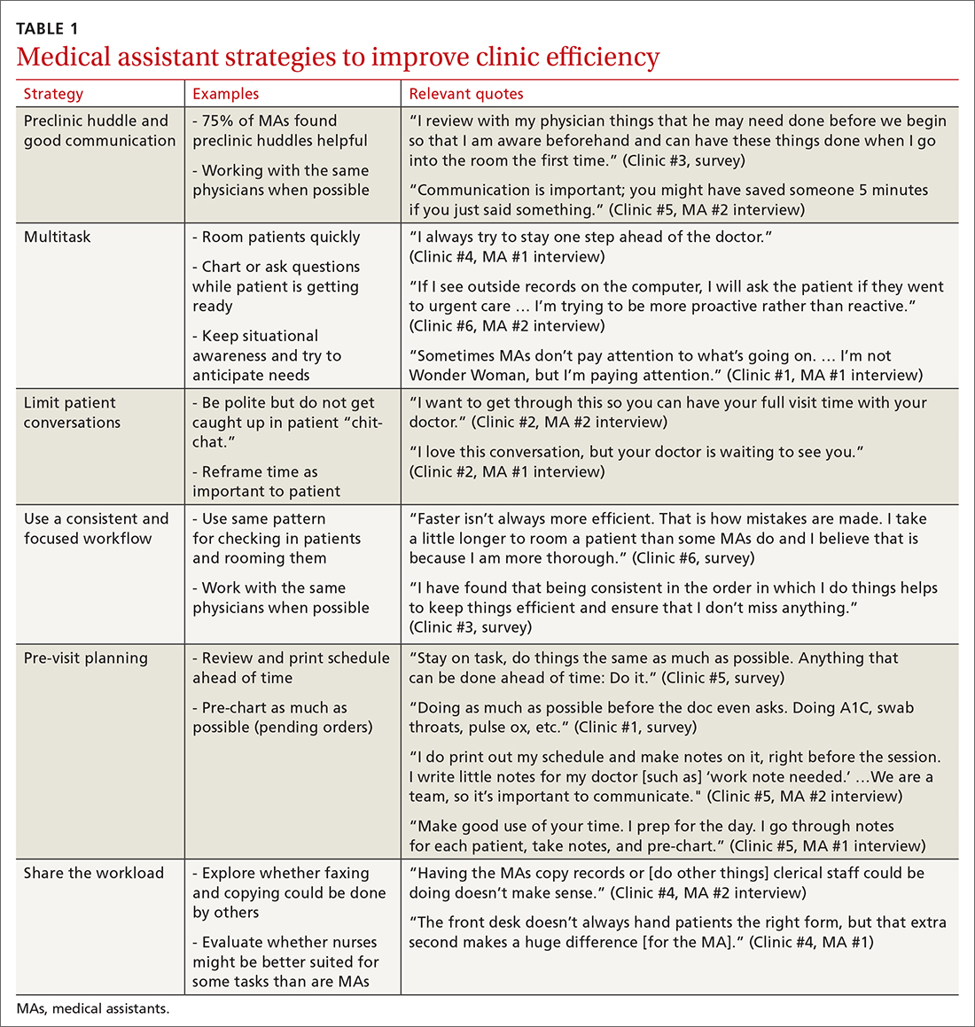
Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)
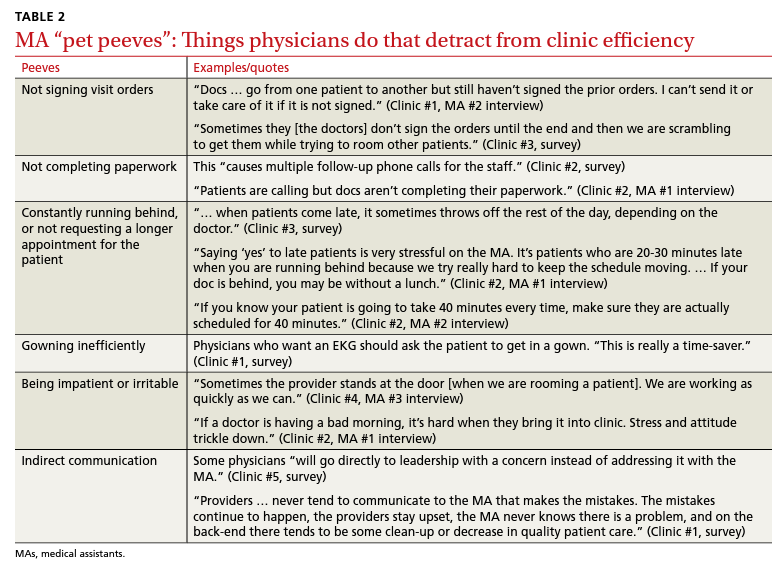
Continue to: Clinic environment...
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“[Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
Continue to: DISCUSSION...
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male- dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries. 19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs. 8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; [email protected]
- Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
- Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
- Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/ articles/medical-assisting-trends/
- Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
- Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medicalassisting-history/
- Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
- Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
- Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
- Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
- STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/ fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_ combined.pdf
- Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
- Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
- Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
- Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
- Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
- US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/ oes/current/oes319092.htm
- Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
- Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
- US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/ healthcare/medical-assistants.htm
- Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
- Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
- Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11: 187-201.
- Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
- O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
Continue to: Methods...
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
Continue to: RESULTS...
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.

Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)

Continue to: Clinic environment...
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“[Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
Continue to: DISCUSSION...
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male- dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries. 19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs. 8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; [email protected]
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
Continue to: Methods...
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
Continue to: RESULTS...
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.

Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)

Continue to: Clinic environment...
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“[Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
Continue to: DISCUSSION...
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male- dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries. 19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs. 8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; [email protected]
- Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
- Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
- Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/ articles/medical-assisting-trends/
- Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
- Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medicalassisting-history/
- Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
- Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
- Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
- Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
- STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/ fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_ combined.pdf
- Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
- Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
- Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
- Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
- Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
- US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/ oes/current/oes319092.htm
- Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
- Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
- US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/ healthcare/medical-assistants.htm
- Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
- Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
- Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11: 187-201.
- Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
- O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
- Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
- Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
- Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/ articles/medical-assisting-trends/
- Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
- Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medicalassisting-history/
- Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
- Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
- Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
- Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
- STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/ fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_ combined.pdf
- Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
- Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
- Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
- Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
- Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
- US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/ oes/current/oes319092.htm
- Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
- Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
- US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/ healthcare/medical-assistants.htm
- Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
- Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
- Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11: 187-201.
- Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
- O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
Burnout and stress of today: How do we cope?
Interestingly, the group that seems to be least impacted by this was health care administrators (with 12% of them planning on leaving their jobs).
I couldn’t stop thinking about these percentages.
I am reminded every day of the commitment and excellence of my colleagues in the health care field, and I do not want to lose them. I am hoping the following information and my thoughts on this topic will be helpful for those thinking about leaving health care.
Surgeon general’s burnout report
The surgeon general recently released a report on addressing health care worker burnout.2 It includes several very interesting and appropriate observations. I will summarize the most important ones here:
1. Our health depends on the well-being of our health workforce.
2. Direct harm to health care workers can lead to anxiety, depression, insomnia, and interpersonal and relationship struggles.
3. Health care workers experience exhaustion from providing overwhelming care and empathy.
4. Health care workers spend less time with patients and too much time with EHRs.
5. There are health workforce shortages.
The report is comprehensive, and everything in it is correct. The real issue is how does it go from being a report to true actionable items that we as health care professionals benefit from? I think in regards to exhaustion from overwhelming care responsibilities, and empathy fatigue, we need better boundaries.
Those who go into medicine, and especially those who go into primary care, always put the patients’ needs first. When operating in a broken system, it stays broken when individuals cover for the deficiencies in the system. Adding four extra patients every day because there is no one to refer them to with availability is injurious to the health care provider, and those providers who accept these additional patients will eventually be part of the 23% who want to leave their jobs. It feels awful to say no, but until the system stops accommodating there will not be substantial change.
The empathy drain
One of the unreported stresses of open access for patients through EHR communications is the empathy drain on physicians. When I see a patient in clinic with chronic symptoms or issues, I spend important time making sure we have a plan and an agreed upon time frame.
With the EHR, patients frequently send multiple messages for the same symptoms between visits. It is okay to redirect the patient and share that these issues will be discussed at length at appointments. My reasoning on this is that I think it is better for me to better care for myself and stay as the doctor for my patients, than always say yes to limitless needs and soon be looking for the off ramp.
The following statistic in the surgeon general’s report really hit home. For every hour of direct patient care, physicians currently spend 2 hours on the EHR system. Most practices allow 10%-20% of time for catch up, where with statistics like this it should be 50%. This concept is fully lost on administrators, or ignored.
It is only when we refuse to continue to accept and follow a broken system that it will change. A minority of internal medicine and family doctors (4.5% in 2018) practice in direct primary care models, where these issues are addressed. Unfortunately, this model as it is currently available is not an option for lower income patients.
A major theme in the surgeon general’s report was that administrative burdens need to be reduced by 75% by 2025. When I look at the report, I see the suggestions, I just don’t see how it will be achieved. Despite almost all clinics moving to the EHR, paperwork in the form of faxes and forms has increased.
A sweeping reform would be needed to eliminate daily faxes from PT offices, visiting nurse services, prior authorization, patients reminders from insurance companies, and disability forms from patients. I am glad that there is acknowledgment of the problem, but this change will take more than 3 years.
Takeaways
So what do we do?
Be good to yourself, and your colleagues. The pandemic has isolated us, which accelerates burnout.
Reach out to people you care about.
We are all feeling this. Set boundaries that allow you to care for yourself, and accept that you are doing your best, even if you can’t meet the needs of all your patients all the time.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Sinsky CA et al. Covid-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021 Dec;5(6):1165-73.
2. Addressing health worker burnout. The U.S. Surgeon General’s advisory on building a thriving health workforce.
Interestingly, the group that seems to be least impacted by this was health care administrators (with 12% of them planning on leaving their jobs).
I couldn’t stop thinking about these percentages.
I am reminded every day of the commitment and excellence of my colleagues in the health care field, and I do not want to lose them. I am hoping the following information and my thoughts on this topic will be helpful for those thinking about leaving health care.
Surgeon general’s burnout report
The surgeon general recently released a report on addressing health care worker burnout.2 It includes several very interesting and appropriate observations. I will summarize the most important ones here:
1. Our health depends on the well-being of our health workforce.
2. Direct harm to health care workers can lead to anxiety, depression, insomnia, and interpersonal and relationship struggles.
3. Health care workers experience exhaustion from providing overwhelming care and empathy.
4. Health care workers spend less time with patients and too much time with EHRs.
5. There are health workforce shortages.
The report is comprehensive, and everything in it is correct. The real issue is how does it go from being a report to true actionable items that we as health care professionals benefit from? I think in regards to exhaustion from overwhelming care responsibilities, and empathy fatigue, we need better boundaries.
Those who go into medicine, and especially those who go into primary care, always put the patients’ needs first. When operating in a broken system, it stays broken when individuals cover for the deficiencies in the system. Adding four extra patients every day because there is no one to refer them to with availability is injurious to the health care provider, and those providers who accept these additional patients will eventually be part of the 23% who want to leave their jobs. It feels awful to say no, but until the system stops accommodating there will not be substantial change.
The empathy drain
One of the unreported stresses of open access for patients through EHR communications is the empathy drain on physicians. When I see a patient in clinic with chronic symptoms or issues, I spend important time making sure we have a plan and an agreed upon time frame.
With the EHR, patients frequently send multiple messages for the same symptoms between visits. It is okay to redirect the patient and share that these issues will be discussed at length at appointments. My reasoning on this is that I think it is better for me to better care for myself and stay as the doctor for my patients, than always say yes to limitless needs and soon be looking for the off ramp.
The following statistic in the surgeon general’s report really hit home. For every hour of direct patient care, physicians currently spend 2 hours on the EHR system. Most practices allow 10%-20% of time for catch up, where with statistics like this it should be 50%. This concept is fully lost on administrators, or ignored.
It is only when we refuse to continue to accept and follow a broken system that it will change. A minority of internal medicine and family doctors (4.5% in 2018) practice in direct primary care models, where these issues are addressed. Unfortunately, this model as it is currently available is not an option for lower income patients.
A major theme in the surgeon general’s report was that administrative burdens need to be reduced by 75% by 2025. When I look at the report, I see the suggestions, I just don’t see how it will be achieved. Despite almost all clinics moving to the EHR, paperwork in the form of faxes and forms has increased.
A sweeping reform would be needed to eliminate daily faxes from PT offices, visiting nurse services, prior authorization, patients reminders from insurance companies, and disability forms from patients. I am glad that there is acknowledgment of the problem, but this change will take more than 3 years.
Takeaways
So what do we do?
Be good to yourself, and your colleagues. The pandemic has isolated us, which accelerates burnout.
Reach out to people you care about.
We are all feeling this. Set boundaries that allow you to care for yourself, and accept that you are doing your best, even if you can’t meet the needs of all your patients all the time.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Sinsky CA et al. Covid-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021 Dec;5(6):1165-73.
2. Addressing health worker burnout. The U.S. Surgeon General’s advisory on building a thriving health workforce.
Interestingly, the group that seems to be least impacted by this was health care administrators (with 12% of them planning on leaving their jobs).
I couldn’t stop thinking about these percentages.
I am reminded every day of the commitment and excellence of my colleagues in the health care field, and I do not want to lose them. I am hoping the following information and my thoughts on this topic will be helpful for those thinking about leaving health care.
Surgeon general’s burnout report
The surgeon general recently released a report on addressing health care worker burnout.2 It includes several very interesting and appropriate observations. I will summarize the most important ones here:
1. Our health depends on the well-being of our health workforce.
2. Direct harm to health care workers can lead to anxiety, depression, insomnia, and interpersonal and relationship struggles.
3. Health care workers experience exhaustion from providing overwhelming care and empathy.
4. Health care workers spend less time with patients and too much time with EHRs.
5. There are health workforce shortages.
The report is comprehensive, and everything in it is correct. The real issue is how does it go from being a report to true actionable items that we as health care professionals benefit from? I think in regards to exhaustion from overwhelming care responsibilities, and empathy fatigue, we need better boundaries.
Those who go into medicine, and especially those who go into primary care, always put the patients’ needs first. When operating in a broken system, it stays broken when individuals cover for the deficiencies in the system. Adding four extra patients every day because there is no one to refer them to with availability is injurious to the health care provider, and those providers who accept these additional patients will eventually be part of the 23% who want to leave their jobs. It feels awful to say no, but until the system stops accommodating there will not be substantial change.
The empathy drain
One of the unreported stresses of open access for patients through EHR communications is the empathy drain on physicians. When I see a patient in clinic with chronic symptoms or issues, I spend important time making sure we have a plan and an agreed upon time frame.
With the EHR, patients frequently send multiple messages for the same symptoms between visits. It is okay to redirect the patient and share that these issues will be discussed at length at appointments. My reasoning on this is that I think it is better for me to better care for myself and stay as the doctor for my patients, than always say yes to limitless needs and soon be looking for the off ramp.
The following statistic in the surgeon general’s report really hit home. For every hour of direct patient care, physicians currently spend 2 hours on the EHR system. Most practices allow 10%-20% of time for catch up, where with statistics like this it should be 50%. This concept is fully lost on administrators, or ignored.
It is only when we refuse to continue to accept and follow a broken system that it will change. A minority of internal medicine and family doctors (4.5% in 2018) practice in direct primary care models, where these issues are addressed. Unfortunately, this model as it is currently available is not an option for lower income patients.
A major theme in the surgeon general’s report was that administrative burdens need to be reduced by 75% by 2025. When I look at the report, I see the suggestions, I just don’t see how it will be achieved. Despite almost all clinics moving to the EHR, paperwork in the form of faxes and forms has increased.
A sweeping reform would be needed to eliminate daily faxes from PT offices, visiting nurse services, prior authorization, patients reminders from insurance companies, and disability forms from patients. I am glad that there is acknowledgment of the problem, but this change will take more than 3 years.
Takeaways
So what do we do?
Be good to yourself, and your colleagues. The pandemic has isolated us, which accelerates burnout.
Reach out to people you care about.
We are all feeling this. Set boundaries that allow you to care for yourself, and accept that you are doing your best, even if you can’t meet the needs of all your patients all the time.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Sinsky CA et al. Covid-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021 Dec;5(6):1165-73.
2. Addressing health worker burnout. The U.S. Surgeon General’s advisory on building a thriving health workforce.
Author Q&A: Intravenous Immunoglobulin for Treatment of COVID-19 in Select Patients
Dr. George Sakoulas is an infectious diseases clinician at Sharp Memorial Hospital in San Diego and professor of pediatrics at the University of California, San Diego School of Medicine. He was the lead investigator in a study published in the May/June 2022 issue of JCOM that found that, when allocated to the appropriate patient type, intravenous immunoglobulin can reduce hospital costs for COVID-19 care. 1 He joined JCOM’s Editor-in-Chief, Dr. Ebrahim Barkoudah, to discuss the study’s background and highlight its main findings.
The following has been edited for length and clarity.
Dr. Barkoudah Dr. Sakoulas is an investigator and a clinician, bridging both worlds to bring the best evidence to our patients. We’re discussing his new article regarding intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia. Dr. Sakoulas, could you please share with our readers the clinical question your study addressed and what your work around COVID-19 management means for clinical practice?
Dr. Sakoulas Thank you. I’m an infectious disease physician. I’ve been treating patients with viral acute respiratory distress syndrome for almost 20 years as an ID doctor. Most of these cases are due to influenza or other viruses. And from time to time, anecdotally and supported by some literature, we’ve been using IVIG, or intravenous immunoglobulin, in some of these cases. And again, I can report anecdotal success with that over the years.
So when COVID emerged in March of 2020, we deployed IVIG in a couple of patients early who were heading downhill. Remember, in March of 2020, we didn’t have the knowledge of steroids helping, patients being ventilated very promptly, and we saw some patients who made a turnaround after treatment with IVIG. We were able to get some support from an industry sponsor and perform and publish a pilot study, enrolling patients early in the pandemic. That study actually showed benefits, which then led the sponsor to fund a phase 3 multicenter clinical trial. Unfortunately, a couple of things happened. First, the trial was designed with the knowledge we had in April of 2020, and again, this is before steroids, before we incorporated proning patients in the ICU, or started ventilating people early. So there were some management changes and evolutions and improvements that happened. And second, the trial was enrolling a very broad repertoire of patients. There were no age limitations, and the trial, ultimately a phase 3 multicenter trial, failed to meet its endpoint.
There were some trends for benefit in younger patients, and as the trial was ongoing, we continued to evolve our knowledge, and we really honed it down to seeing a benefit of using IVIG in patients with COVID with specific criteria in mind. They had to be relatively younger patients, under 65, and not have any major comorbidities. In other words, they weren’t dialysis patients or end-stage disease patients, heart failure patients, cancer or malignancy patients. So, you know, we’re looking at the patients under 65 with obesity, diabetes, and hypertension, who are rapidly declining, going from room air to BiPAP or high-flow oxygen in a short amount of time. And we learned that when using IVIG early, we actually saw patients improve and turn around.
What this article in JCOM highlighted was, number one, incorporating that outcome or that patient type and then looking at the cost of hospitalization of patients who received IVIG versus those that did not. There were 2 groups that were studied. One was the group of patients in that original pilot trial that I discussed who were randomized to receive 1 or the other prospectively; it was an unblinded randomized study. And the second group was a matched case-control study where we had patients treated with IVIG matched by age and comorbidity status and level of hypoxia to patients that did not receive IVIG. We saw a financial benefit in shortening or reducing hospitalizations, really coming down to getting rid of that 20% tail of patients that wound up going to the ICU, getting intubated, and using a high amount of hospital resources that would ramp up the cost of hospitalization. We saw great mitigation of that with IVIG, and even with a small subset of patients, we were able to show a benefit.
Dr. Barkoudah Any thoughts on where we can implement the new findings from your article in our practice at the moment, knowing we now have practice guidelines and protocols to treat COVID-19? There was a tangible benefit in treating the patients the way you approached it in your important work. Could you share with us what would be implementable at the moment?
Dr. Sakoulas I think, fortunately, with the increasing host immunity in the population and decreased virulence of the virus, perhaps we won’t see as many patients of the type that were in these trials going forward, but I suspect we will perhaps in the unvaccinated patients that remain. I believe one-third of the United States is not vaccinated. So there is certainly a vulnerable group of people out there. Potentially, an unvaccinated patient who winds up getting very sick, the patient who is relatively young—what I’m looking at is the 30- to 65-year-old obese, hypertensive, or diabetic patient who comes in and, despite the steroids and the antivirals, rapidly deteriorates into requiring high-flow oxygen. I think implementing IVIG in that patient type would be helpful. I don’t think it’s going to be as helpful in patients who are very elderly, because I think the mechanism of the disease is different in an 80-year-old versus a 50-year-old patient. So again, hopefully, it will not amount to a lot of patients, but I still suspect hospitals are going to see, perhaps in the fall, when they’re expecting a greater number of cases, a trickling of patients that do meet the criteria that I described.
Dr. Barkoudah JCOM’s audience are the QI implementers and hospital leadership. And what caught my eye in your article is your perspective on the pharmacoeconomics of treating COVID-19, and I really appreciate your looking at the cost aspect. Would you talk about the economics of inpatient care, the total care that we provide now that we’re in the age of tocilizumab, and the current state of multiple layers of therapy?
Dr. Sakoulas The reason to look at the economics of it is because IVIG—which is actually not a drug, it’s a blood product—is very expensive. So, we received a considerable amount of administrative pushback implementing this treatment at the beginning outside of the clinical trial setting because it hadn’t been studied on a large scale and because the cost was so high, even though, as a clinician at the bedside, I was seeing a benefit in patients. This study came out of my trying to demonstrate to the folks that are keeping the economics of medicine in mind that, in fact, investing several thousand dollars of treatment in IVIG will save you cost of care, the cost of an ICU bed, the cost of a ventilator, and the cost even of ECMO, which is hugely expensive.
If you look at the numbers in the study, for two-thirds or three-quarters of the patients, your cost of care is actually greater than the controls because you’re giving them IVIG, and it’s increasing the cost of their care, even though three-quarters of the patients are going to do just as well without it. It’s that 20% to 25% of patients that really are going to benefit from it, where you’re reducing your cost of care so much, and you’re getting rid of that very, very expensive 20%, that there’s a cost savings across the board per patient. So, it’s hard to understand when you say you’re losing money on three-quarters of the patients, you’re only saving money on a quarter of the patients, but that cost of saving on that small subset is so substantial it’s really impacting all numbers.
Also, abandoning the outlier principle is sort of an underlying theme in how we think of things. We tend to ignore outliers, not consider them, but I think we really have to pay attention to the more extreme cases because those patients are the ones that drive not just the financial cost of care. Remember, if you’re down to 1 ventilator and you can cut down the use of scarce ICU resources, the cost is sort of even beyond the cost of money. It’s the cost of resources that may become scarce in some settings. So, I think it speaks to that as well.
A lot of the drugs that we use, for example, tocilizumab, were able to be studied in thousands of patients. If you look at the absolute numbers, the benefit of tocilizumab from a magnitude standpoint—low to mid twenties to high twenties—you know, reducing mortality from 29% to 24%. I mean, just take a step back and think about that. Even though it’s statistically significant, try telling a patient, “Well, I’m going to give you this treatment that’s going to reduce mortality from 29% to 24%.” You know, that doesn’t really change anything from a clinical significance standpoint. But they have a P value less than .05, which is our standard, and they were able to do a study with thousands of patients. We didn’t have that luxury with IVIG. No one studied thousands of patients, only retrospectively, and those retrospective studies don’t get the attention because they’re considered biased with all their limitations. But I think one of the difficulties we have here is the balance between statistical and clinical significance. For example, in our pilot study, our ventilation rate was 58% with the non-IVIG patients versus 14% for IVIG patients. So you might say, magnitude-wise, that’s a big number, but the statistical significance of it is borderline because of small numbers.
Anyway, that’s a challenge that we have as clinicians trying to incorporate what’s published—the balancing of statistics, absolute numbers, and practicalities of delivering care. And I think this study highlights some of the nuances that go into that incorporation and those clinical decisions.
Dr. Barkoudah Would you mind sharing with our audience how we can make the connection between the medical outcomes and pharmacoeconomics findings from your article and link it to the bedside and treatment of our patients?
Dr. Sakoulas One of the points this article brings out is the importance of bringing together not just level 1A data, but also small studies with data such as this, where the magnitude of the effect is pretty big but you lose the statistics because of the small numbers. And then also the patients’ aspects of things. I think, as a bedside clinician, you appreciate things, the nuances, much sooner than what percolates out from a level 1A study. Case in point, in the sponsored phase 3 study that we did, and in some other studies that were prospectively done as well, these studies of IVIG simply had an enrollment of patients that was very broad, and not every patient benefits from the same therapy. A great example of this is the sepsis trials with Xigris and those types of agents that failed. You know, there are clinicians to this day who believe that there is a subset of patients that benefit from agents like this. The IVIG story falls a little bit into that category. It comes down to trying to identify the subset of patients that might benefit. And I think we’ve outlined this subset pretty well in our study: the younger, obese diabetic or hypertensive patient who’s rapidly declining.
It really brings together the need to not necessarily toss out these smaller studies, but kind of summarize everything together, and clinicians who are bedside, who are more in tune with the nuances of individual decisions at the individual patient level, might better appreciate these kinds of data. But I think we all have to put it together. IVIG does not make treatment guidelines at national levels and so forth. It’s not even listed in many of them. But there are patients out there who, if you ask them specifically how they felt, including a friend of mine who received the medication, there’s no question from their end, how they felt about this treatment option. Now, some people will get it and will not benefit. We just have to be really tuned into the fact that the same drug does not have the same result for every patient. And just to consider this in the high-risk patients that we talked about in our study.
Dr. Barkoudah While we were prepping for this interview, you made an analogy regarding clinical evidence along the lines of, “Do we need randomized clinical trials to do a parachute-type of experiment,” and we chatted about clinical wisdom. Would you mind sharing with our readers your thoughts on that?
Dr. Sakoulas Sometimes, we try a treatment and it’s very obvious for that particular patient that it helped them. Then you study the treatment in a large trial setting and it doesn’t work. For us bedside clinicians, there are some interventions sometimes that do appear as beneficial as a parachute would be, but yet, there has never been a randomized clinical trial proving that parachutes work. Again, a part of the challenge we have is patients are so different, their immunology is different, the pathogen infecting them is different, the time they present is different. Some present early, some present late. There are just so many moving parts to treating an infection that only a subset of people are going to benefit. And sometimes as clinicians, we’re so nuanced, that we identify a specific subset of patients where we know we can help them. And it’s so obvious for us, like a parachute would be, but to people who are looking at the world from 30,000 feet, they don’t necessarily grasp that because, when you look at all comers, it doesn’t show a benefit.
So the problem is that now those treatments that might help a subset of patients are being denied, and the subset of patients that are going to benefit never get the treatment. Now we have to balance that with a lot of stuff that went on during the pandemic with, you know, ivermectin, hydroxychloroquine, and people pushing those things. Someone asked me once what I thought about hydroxychloroquine, and I said, “Well, somebody in the lab probably showed that it was beneficial, analogous to lighting tissue paper on fire on a plate and taking a cup of water and putting the fire out. Well, now, if you take that cup of water to the Caldor fire that’s burning in California on thousands of acres, you’re not going to be able to put the fire out with that cup of water.” So while it might work in the lab, it’s truly not going to work in a clinical setting. We have to balance individualizing care for patients with some information people are pushing out there that may not be necessarily translatable to the clinical setting.
I think there’s nothing better than being at the bedside, though, and being able to implement something and seeing what works. And really, experience goes a long way in being able to individually treat a patient optimally.
Dr. Barkoudah Thank you for everything you do at the bedside and your work on improving the treatment we have and how we can leverage knowledge to treat our patients. Thank you very much for your time and your scholarly contribution. We appreciate it and I hope the work will continue. We will keep working on treating COVID-19 patients with the best knowledge we have.
Q&A participants: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, La Jolla, CA, and University of California San Diego School of Medicine, San Diego, CA; and Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Disclosures: None reported.
1. Poremba M, Dehner M, Perreiter A, et al. Intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia: a pharmacoeconomic analysis. J Clin Outcomes Manage. 2022;29(3):123-129. doi:10.12788/jcom.0094
Dr. George Sakoulas is an infectious diseases clinician at Sharp Memorial Hospital in San Diego and professor of pediatrics at the University of California, San Diego School of Medicine. He was the lead investigator in a study published in the May/June 2022 issue of JCOM that found that, when allocated to the appropriate patient type, intravenous immunoglobulin can reduce hospital costs for COVID-19 care. 1 He joined JCOM’s Editor-in-Chief, Dr. Ebrahim Barkoudah, to discuss the study’s background and highlight its main findings.
The following has been edited for length and clarity.
Dr. Barkoudah Dr. Sakoulas is an investigator and a clinician, bridging both worlds to bring the best evidence to our patients. We’re discussing his new article regarding intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia. Dr. Sakoulas, could you please share with our readers the clinical question your study addressed and what your work around COVID-19 management means for clinical practice?
Dr. Sakoulas Thank you. I’m an infectious disease physician. I’ve been treating patients with viral acute respiratory distress syndrome for almost 20 years as an ID doctor. Most of these cases are due to influenza or other viruses. And from time to time, anecdotally and supported by some literature, we’ve been using IVIG, or intravenous immunoglobulin, in some of these cases. And again, I can report anecdotal success with that over the years.
So when COVID emerged in March of 2020, we deployed IVIG in a couple of patients early who were heading downhill. Remember, in March of 2020, we didn’t have the knowledge of steroids helping, patients being ventilated very promptly, and we saw some patients who made a turnaround after treatment with IVIG. We were able to get some support from an industry sponsor and perform and publish a pilot study, enrolling patients early in the pandemic. That study actually showed benefits, which then led the sponsor to fund a phase 3 multicenter clinical trial. Unfortunately, a couple of things happened. First, the trial was designed with the knowledge we had in April of 2020, and again, this is before steroids, before we incorporated proning patients in the ICU, or started ventilating people early. So there were some management changes and evolutions and improvements that happened. And second, the trial was enrolling a very broad repertoire of patients. There were no age limitations, and the trial, ultimately a phase 3 multicenter trial, failed to meet its endpoint.
There were some trends for benefit in younger patients, and as the trial was ongoing, we continued to evolve our knowledge, and we really honed it down to seeing a benefit of using IVIG in patients with COVID with specific criteria in mind. They had to be relatively younger patients, under 65, and not have any major comorbidities. In other words, they weren’t dialysis patients or end-stage disease patients, heart failure patients, cancer or malignancy patients. So, you know, we’re looking at the patients under 65 with obesity, diabetes, and hypertension, who are rapidly declining, going from room air to BiPAP or high-flow oxygen in a short amount of time. And we learned that when using IVIG early, we actually saw patients improve and turn around.
What this article in JCOM highlighted was, number one, incorporating that outcome or that patient type and then looking at the cost of hospitalization of patients who received IVIG versus those that did not. There were 2 groups that were studied. One was the group of patients in that original pilot trial that I discussed who were randomized to receive 1 or the other prospectively; it was an unblinded randomized study. And the second group was a matched case-control study where we had patients treated with IVIG matched by age and comorbidity status and level of hypoxia to patients that did not receive IVIG. We saw a financial benefit in shortening or reducing hospitalizations, really coming down to getting rid of that 20% tail of patients that wound up going to the ICU, getting intubated, and using a high amount of hospital resources that would ramp up the cost of hospitalization. We saw great mitigation of that with IVIG, and even with a small subset of patients, we were able to show a benefit.
Dr. Barkoudah Any thoughts on where we can implement the new findings from your article in our practice at the moment, knowing we now have practice guidelines and protocols to treat COVID-19? There was a tangible benefit in treating the patients the way you approached it in your important work. Could you share with us what would be implementable at the moment?
Dr. Sakoulas I think, fortunately, with the increasing host immunity in the population and decreased virulence of the virus, perhaps we won’t see as many patients of the type that were in these trials going forward, but I suspect we will perhaps in the unvaccinated patients that remain. I believe one-third of the United States is not vaccinated. So there is certainly a vulnerable group of people out there. Potentially, an unvaccinated patient who winds up getting very sick, the patient who is relatively young—what I’m looking at is the 30- to 65-year-old obese, hypertensive, or diabetic patient who comes in and, despite the steroids and the antivirals, rapidly deteriorates into requiring high-flow oxygen. I think implementing IVIG in that patient type would be helpful. I don’t think it’s going to be as helpful in patients who are very elderly, because I think the mechanism of the disease is different in an 80-year-old versus a 50-year-old patient. So again, hopefully, it will not amount to a lot of patients, but I still suspect hospitals are going to see, perhaps in the fall, when they’re expecting a greater number of cases, a trickling of patients that do meet the criteria that I described.
Dr. Barkoudah JCOM’s audience are the QI implementers and hospital leadership. And what caught my eye in your article is your perspective on the pharmacoeconomics of treating COVID-19, and I really appreciate your looking at the cost aspect. Would you talk about the economics of inpatient care, the total care that we provide now that we’re in the age of tocilizumab, and the current state of multiple layers of therapy?
Dr. Sakoulas The reason to look at the economics of it is because IVIG—which is actually not a drug, it’s a blood product—is very expensive. So, we received a considerable amount of administrative pushback implementing this treatment at the beginning outside of the clinical trial setting because it hadn’t been studied on a large scale and because the cost was so high, even though, as a clinician at the bedside, I was seeing a benefit in patients. This study came out of my trying to demonstrate to the folks that are keeping the economics of medicine in mind that, in fact, investing several thousand dollars of treatment in IVIG will save you cost of care, the cost of an ICU bed, the cost of a ventilator, and the cost even of ECMO, which is hugely expensive.
If you look at the numbers in the study, for two-thirds or three-quarters of the patients, your cost of care is actually greater than the controls because you’re giving them IVIG, and it’s increasing the cost of their care, even though three-quarters of the patients are going to do just as well without it. It’s that 20% to 25% of patients that really are going to benefit from it, where you’re reducing your cost of care so much, and you’re getting rid of that very, very expensive 20%, that there’s a cost savings across the board per patient. So, it’s hard to understand when you say you’re losing money on three-quarters of the patients, you’re only saving money on a quarter of the patients, but that cost of saving on that small subset is so substantial it’s really impacting all numbers.
Also, abandoning the outlier principle is sort of an underlying theme in how we think of things. We tend to ignore outliers, not consider them, but I think we really have to pay attention to the more extreme cases because those patients are the ones that drive not just the financial cost of care. Remember, if you’re down to 1 ventilator and you can cut down the use of scarce ICU resources, the cost is sort of even beyond the cost of money. It’s the cost of resources that may become scarce in some settings. So, I think it speaks to that as well.
A lot of the drugs that we use, for example, tocilizumab, were able to be studied in thousands of patients. If you look at the absolute numbers, the benefit of tocilizumab from a magnitude standpoint—low to mid twenties to high twenties—you know, reducing mortality from 29% to 24%. I mean, just take a step back and think about that. Even though it’s statistically significant, try telling a patient, “Well, I’m going to give you this treatment that’s going to reduce mortality from 29% to 24%.” You know, that doesn’t really change anything from a clinical significance standpoint. But they have a P value less than .05, which is our standard, and they were able to do a study with thousands of patients. We didn’t have that luxury with IVIG. No one studied thousands of patients, only retrospectively, and those retrospective studies don’t get the attention because they’re considered biased with all their limitations. But I think one of the difficulties we have here is the balance between statistical and clinical significance. For example, in our pilot study, our ventilation rate was 58% with the non-IVIG patients versus 14% for IVIG patients. So you might say, magnitude-wise, that’s a big number, but the statistical significance of it is borderline because of small numbers.
Anyway, that’s a challenge that we have as clinicians trying to incorporate what’s published—the balancing of statistics, absolute numbers, and practicalities of delivering care. And I think this study highlights some of the nuances that go into that incorporation and those clinical decisions.
Dr. Barkoudah Would you mind sharing with our audience how we can make the connection between the medical outcomes and pharmacoeconomics findings from your article and link it to the bedside and treatment of our patients?
Dr. Sakoulas One of the points this article brings out is the importance of bringing together not just level 1A data, but also small studies with data such as this, where the magnitude of the effect is pretty big but you lose the statistics because of the small numbers. And then also the patients’ aspects of things. I think, as a bedside clinician, you appreciate things, the nuances, much sooner than what percolates out from a level 1A study. Case in point, in the sponsored phase 3 study that we did, and in some other studies that were prospectively done as well, these studies of IVIG simply had an enrollment of patients that was very broad, and not every patient benefits from the same therapy. A great example of this is the sepsis trials with Xigris and those types of agents that failed. You know, there are clinicians to this day who believe that there is a subset of patients that benefit from agents like this. The IVIG story falls a little bit into that category. It comes down to trying to identify the subset of patients that might benefit. And I think we’ve outlined this subset pretty well in our study: the younger, obese diabetic or hypertensive patient who’s rapidly declining.
It really brings together the need to not necessarily toss out these smaller studies, but kind of summarize everything together, and clinicians who are bedside, who are more in tune with the nuances of individual decisions at the individual patient level, might better appreciate these kinds of data. But I think we all have to put it together. IVIG does not make treatment guidelines at national levels and so forth. It’s not even listed in many of them. But there are patients out there who, if you ask them specifically how they felt, including a friend of mine who received the medication, there’s no question from their end, how they felt about this treatment option. Now, some people will get it and will not benefit. We just have to be really tuned into the fact that the same drug does not have the same result for every patient. And just to consider this in the high-risk patients that we talked about in our study.
Dr. Barkoudah While we were prepping for this interview, you made an analogy regarding clinical evidence along the lines of, “Do we need randomized clinical trials to do a parachute-type of experiment,” and we chatted about clinical wisdom. Would you mind sharing with our readers your thoughts on that?
Dr. Sakoulas Sometimes, we try a treatment and it’s very obvious for that particular patient that it helped them. Then you study the treatment in a large trial setting and it doesn’t work. For us bedside clinicians, there are some interventions sometimes that do appear as beneficial as a parachute would be, but yet, there has never been a randomized clinical trial proving that parachutes work. Again, a part of the challenge we have is patients are so different, their immunology is different, the pathogen infecting them is different, the time they present is different. Some present early, some present late. There are just so many moving parts to treating an infection that only a subset of people are going to benefit. And sometimes as clinicians, we’re so nuanced, that we identify a specific subset of patients where we know we can help them. And it’s so obvious for us, like a parachute would be, but to people who are looking at the world from 30,000 feet, they don’t necessarily grasp that because, when you look at all comers, it doesn’t show a benefit.
So the problem is that now those treatments that might help a subset of patients are being denied, and the subset of patients that are going to benefit never get the treatment. Now we have to balance that with a lot of stuff that went on during the pandemic with, you know, ivermectin, hydroxychloroquine, and people pushing those things. Someone asked me once what I thought about hydroxychloroquine, and I said, “Well, somebody in the lab probably showed that it was beneficial, analogous to lighting tissue paper on fire on a plate and taking a cup of water and putting the fire out. Well, now, if you take that cup of water to the Caldor fire that’s burning in California on thousands of acres, you’re not going to be able to put the fire out with that cup of water.” So while it might work in the lab, it’s truly not going to work in a clinical setting. We have to balance individualizing care for patients with some information people are pushing out there that may not be necessarily translatable to the clinical setting.
I think there’s nothing better than being at the bedside, though, and being able to implement something and seeing what works. And really, experience goes a long way in being able to individually treat a patient optimally.
Dr. Barkoudah Thank you for everything you do at the bedside and your work on improving the treatment we have and how we can leverage knowledge to treat our patients. Thank you very much for your time and your scholarly contribution. We appreciate it and I hope the work will continue. We will keep working on treating COVID-19 patients with the best knowledge we have.
Q&A participants: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, La Jolla, CA, and University of California San Diego School of Medicine, San Diego, CA; and Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Disclosures: None reported.
Dr. George Sakoulas is an infectious diseases clinician at Sharp Memorial Hospital in San Diego and professor of pediatrics at the University of California, San Diego School of Medicine. He was the lead investigator in a study published in the May/June 2022 issue of JCOM that found that, when allocated to the appropriate patient type, intravenous immunoglobulin can reduce hospital costs for COVID-19 care. 1 He joined JCOM’s Editor-in-Chief, Dr. Ebrahim Barkoudah, to discuss the study’s background and highlight its main findings.
The following has been edited for length and clarity.
Dr. Barkoudah Dr. Sakoulas is an investigator and a clinician, bridging both worlds to bring the best evidence to our patients. We’re discussing his new article regarding intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia. Dr. Sakoulas, could you please share with our readers the clinical question your study addressed and what your work around COVID-19 management means for clinical practice?
Dr. Sakoulas Thank you. I’m an infectious disease physician. I’ve been treating patients with viral acute respiratory distress syndrome for almost 20 years as an ID doctor. Most of these cases are due to influenza or other viruses. And from time to time, anecdotally and supported by some literature, we’ve been using IVIG, or intravenous immunoglobulin, in some of these cases. And again, I can report anecdotal success with that over the years.
So when COVID emerged in March of 2020, we deployed IVIG in a couple of patients early who were heading downhill. Remember, in March of 2020, we didn’t have the knowledge of steroids helping, patients being ventilated very promptly, and we saw some patients who made a turnaround after treatment with IVIG. We were able to get some support from an industry sponsor and perform and publish a pilot study, enrolling patients early in the pandemic. That study actually showed benefits, which then led the sponsor to fund a phase 3 multicenter clinical trial. Unfortunately, a couple of things happened. First, the trial was designed with the knowledge we had in April of 2020, and again, this is before steroids, before we incorporated proning patients in the ICU, or started ventilating people early. So there were some management changes and evolutions and improvements that happened. And second, the trial was enrolling a very broad repertoire of patients. There were no age limitations, and the trial, ultimately a phase 3 multicenter trial, failed to meet its endpoint.
There were some trends for benefit in younger patients, and as the trial was ongoing, we continued to evolve our knowledge, and we really honed it down to seeing a benefit of using IVIG in patients with COVID with specific criteria in mind. They had to be relatively younger patients, under 65, and not have any major comorbidities. In other words, they weren’t dialysis patients or end-stage disease patients, heart failure patients, cancer or malignancy patients. So, you know, we’re looking at the patients under 65 with obesity, diabetes, and hypertension, who are rapidly declining, going from room air to BiPAP or high-flow oxygen in a short amount of time. And we learned that when using IVIG early, we actually saw patients improve and turn around.
What this article in JCOM highlighted was, number one, incorporating that outcome or that patient type and then looking at the cost of hospitalization of patients who received IVIG versus those that did not. There were 2 groups that were studied. One was the group of patients in that original pilot trial that I discussed who were randomized to receive 1 or the other prospectively; it was an unblinded randomized study. And the second group was a matched case-control study where we had patients treated with IVIG matched by age and comorbidity status and level of hypoxia to patients that did not receive IVIG. We saw a financial benefit in shortening or reducing hospitalizations, really coming down to getting rid of that 20% tail of patients that wound up going to the ICU, getting intubated, and using a high amount of hospital resources that would ramp up the cost of hospitalization. We saw great mitigation of that with IVIG, and even with a small subset of patients, we were able to show a benefit.
Dr. Barkoudah Any thoughts on where we can implement the new findings from your article in our practice at the moment, knowing we now have practice guidelines and protocols to treat COVID-19? There was a tangible benefit in treating the patients the way you approached it in your important work. Could you share with us what would be implementable at the moment?
Dr. Sakoulas I think, fortunately, with the increasing host immunity in the population and decreased virulence of the virus, perhaps we won’t see as many patients of the type that were in these trials going forward, but I suspect we will perhaps in the unvaccinated patients that remain. I believe one-third of the United States is not vaccinated. So there is certainly a vulnerable group of people out there. Potentially, an unvaccinated patient who winds up getting very sick, the patient who is relatively young—what I’m looking at is the 30- to 65-year-old obese, hypertensive, or diabetic patient who comes in and, despite the steroids and the antivirals, rapidly deteriorates into requiring high-flow oxygen. I think implementing IVIG in that patient type would be helpful. I don’t think it’s going to be as helpful in patients who are very elderly, because I think the mechanism of the disease is different in an 80-year-old versus a 50-year-old patient. So again, hopefully, it will not amount to a lot of patients, but I still suspect hospitals are going to see, perhaps in the fall, when they’re expecting a greater number of cases, a trickling of patients that do meet the criteria that I described.
Dr. Barkoudah JCOM’s audience are the QI implementers and hospital leadership. And what caught my eye in your article is your perspective on the pharmacoeconomics of treating COVID-19, and I really appreciate your looking at the cost aspect. Would you talk about the economics of inpatient care, the total care that we provide now that we’re in the age of tocilizumab, and the current state of multiple layers of therapy?
Dr. Sakoulas The reason to look at the economics of it is because IVIG—which is actually not a drug, it’s a blood product—is very expensive. So, we received a considerable amount of administrative pushback implementing this treatment at the beginning outside of the clinical trial setting because it hadn’t been studied on a large scale and because the cost was so high, even though, as a clinician at the bedside, I was seeing a benefit in patients. This study came out of my trying to demonstrate to the folks that are keeping the economics of medicine in mind that, in fact, investing several thousand dollars of treatment in IVIG will save you cost of care, the cost of an ICU bed, the cost of a ventilator, and the cost even of ECMO, which is hugely expensive.
If you look at the numbers in the study, for two-thirds or three-quarters of the patients, your cost of care is actually greater than the controls because you’re giving them IVIG, and it’s increasing the cost of their care, even though three-quarters of the patients are going to do just as well without it. It’s that 20% to 25% of patients that really are going to benefit from it, where you’re reducing your cost of care so much, and you’re getting rid of that very, very expensive 20%, that there’s a cost savings across the board per patient. So, it’s hard to understand when you say you’re losing money on three-quarters of the patients, you’re only saving money on a quarter of the patients, but that cost of saving on that small subset is so substantial it’s really impacting all numbers.
Also, abandoning the outlier principle is sort of an underlying theme in how we think of things. We tend to ignore outliers, not consider them, but I think we really have to pay attention to the more extreme cases because those patients are the ones that drive not just the financial cost of care. Remember, if you’re down to 1 ventilator and you can cut down the use of scarce ICU resources, the cost is sort of even beyond the cost of money. It’s the cost of resources that may become scarce in some settings. So, I think it speaks to that as well.
A lot of the drugs that we use, for example, tocilizumab, were able to be studied in thousands of patients. If you look at the absolute numbers, the benefit of tocilizumab from a magnitude standpoint—low to mid twenties to high twenties—you know, reducing mortality from 29% to 24%. I mean, just take a step back and think about that. Even though it’s statistically significant, try telling a patient, “Well, I’m going to give you this treatment that’s going to reduce mortality from 29% to 24%.” You know, that doesn’t really change anything from a clinical significance standpoint. But they have a P value less than .05, which is our standard, and they were able to do a study with thousands of patients. We didn’t have that luxury with IVIG. No one studied thousands of patients, only retrospectively, and those retrospective studies don’t get the attention because they’re considered biased with all their limitations. But I think one of the difficulties we have here is the balance between statistical and clinical significance. For example, in our pilot study, our ventilation rate was 58% with the non-IVIG patients versus 14% for IVIG patients. So you might say, magnitude-wise, that’s a big number, but the statistical significance of it is borderline because of small numbers.
Anyway, that’s a challenge that we have as clinicians trying to incorporate what’s published—the balancing of statistics, absolute numbers, and practicalities of delivering care. And I think this study highlights some of the nuances that go into that incorporation and those clinical decisions.
Dr. Barkoudah Would you mind sharing with our audience how we can make the connection between the medical outcomes and pharmacoeconomics findings from your article and link it to the bedside and treatment of our patients?
Dr. Sakoulas One of the points this article brings out is the importance of bringing together not just level 1A data, but also small studies with data such as this, where the magnitude of the effect is pretty big but you lose the statistics because of the small numbers. And then also the patients’ aspects of things. I think, as a bedside clinician, you appreciate things, the nuances, much sooner than what percolates out from a level 1A study. Case in point, in the sponsored phase 3 study that we did, and in some other studies that were prospectively done as well, these studies of IVIG simply had an enrollment of patients that was very broad, and not every patient benefits from the same therapy. A great example of this is the sepsis trials with Xigris and those types of agents that failed. You know, there are clinicians to this day who believe that there is a subset of patients that benefit from agents like this. The IVIG story falls a little bit into that category. It comes down to trying to identify the subset of patients that might benefit. And I think we’ve outlined this subset pretty well in our study: the younger, obese diabetic or hypertensive patient who’s rapidly declining.
It really brings together the need to not necessarily toss out these smaller studies, but kind of summarize everything together, and clinicians who are bedside, who are more in tune with the nuances of individual decisions at the individual patient level, might better appreciate these kinds of data. But I think we all have to put it together. IVIG does not make treatment guidelines at national levels and so forth. It’s not even listed in many of them. But there are patients out there who, if you ask them specifically how they felt, including a friend of mine who received the medication, there’s no question from their end, how they felt about this treatment option. Now, some people will get it and will not benefit. We just have to be really tuned into the fact that the same drug does not have the same result for every patient. And just to consider this in the high-risk patients that we talked about in our study.
Dr. Barkoudah While we were prepping for this interview, you made an analogy regarding clinical evidence along the lines of, “Do we need randomized clinical trials to do a parachute-type of experiment,” and we chatted about clinical wisdom. Would you mind sharing with our readers your thoughts on that?
Dr. Sakoulas Sometimes, we try a treatment and it’s very obvious for that particular patient that it helped them. Then you study the treatment in a large trial setting and it doesn’t work. For us bedside clinicians, there are some interventions sometimes that do appear as beneficial as a parachute would be, but yet, there has never been a randomized clinical trial proving that parachutes work. Again, a part of the challenge we have is patients are so different, their immunology is different, the pathogen infecting them is different, the time they present is different. Some present early, some present late. There are just so many moving parts to treating an infection that only a subset of people are going to benefit. And sometimes as clinicians, we’re so nuanced, that we identify a specific subset of patients where we know we can help them. And it’s so obvious for us, like a parachute would be, but to people who are looking at the world from 30,000 feet, they don’t necessarily grasp that because, when you look at all comers, it doesn’t show a benefit.
So the problem is that now those treatments that might help a subset of patients are being denied, and the subset of patients that are going to benefit never get the treatment. Now we have to balance that with a lot of stuff that went on during the pandemic with, you know, ivermectin, hydroxychloroquine, and people pushing those things. Someone asked me once what I thought about hydroxychloroquine, and I said, “Well, somebody in the lab probably showed that it was beneficial, analogous to lighting tissue paper on fire on a plate and taking a cup of water and putting the fire out. Well, now, if you take that cup of water to the Caldor fire that’s burning in California on thousands of acres, you’re not going to be able to put the fire out with that cup of water.” So while it might work in the lab, it’s truly not going to work in a clinical setting. We have to balance individualizing care for patients with some information people are pushing out there that may not be necessarily translatable to the clinical setting.
I think there’s nothing better than being at the bedside, though, and being able to implement something and seeing what works. And really, experience goes a long way in being able to individually treat a patient optimally.
Dr. Barkoudah Thank you for everything you do at the bedside and your work on improving the treatment we have and how we can leverage knowledge to treat our patients. Thank you very much for your time and your scholarly contribution. We appreciate it and I hope the work will continue. We will keep working on treating COVID-19 patients with the best knowledge we have.
Q&A participants: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, La Jolla, CA, and University of California San Diego School of Medicine, San Diego, CA; and Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Disclosures: None reported.
1. Poremba M, Dehner M, Perreiter A, et al. Intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia: a pharmacoeconomic analysis. J Clin Outcomes Manage. 2022;29(3):123-129. doi:10.12788/jcom.0094
1. Poremba M, Dehner M, Perreiter A, et al. Intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia: a pharmacoeconomic analysis. J Clin Outcomes Manage. 2022;29(3):123-129. doi:10.12788/jcom.0094