User login
Med school to pay $1.2 million to students in refunds and debt cancellation in FTC settlement
Although it disputed the allegations, The complaint referenced the school’s medical license exam test pass rate and residency matches along with violations of rules that protect consumers, including those dealing with credit contracts.
The school, based in the Caribbean with operations in Illinois, agreed to pay $1.2 million toward refunds and debt cancellation for students harmed by the marketing in the past 5 years.
“While we strongly disagree with the FTC’s approach to this matter, we did not want a lengthy legal process to distract from our mission of providing a quality medical education at an affordable cost,” Kaushik Guha, executive vice president of the parent of the school, Human Resources Development Services, said in a YouTube statement posted on the school’s website.
“Saint James lured students by lying about their chances of success,” Samuel Levine, director of the FTC’s Bureau of Consumer Protection, said in a press release. The settlement agreement was with HRDS, which bills itself as providing students from “non-traditional backgrounds the opportunity to pursue a medical degree and practice in the U.S. or Canada,” according to the school’s statement.
The complaint alleges that, since at least April 2018, the school, HRDS, and its operator Mr. Guha has lured students using “phony claims about the standardized test pass rate and students’ residency or job prospects. They lured consumers with false guarantees of student success at passing a critical medical school standardized test, the United States Medical Licensing Examination Step 1 Exam.”
For example, a brochure distributed at open houses claimed a first-time Step 1 pass rate of about 96.8%. The brochure further claimed: “Saint James is the first and only medical school to offer a USMLE Step 1 Pass Guarantee,” according to the FTC complaint.
The FTC said the USMLE rate is lower than touted and lower than reported by other U.S. and Canadian medical schools. “Since 2017, only 35% of Saint James students who have completed the necessary coursework to take the USMLE Step 1 exam passed the test.”
The school also misrepresented the residency match rate as “the same” as American medical schools, according to the complaint. For example, the school instructed telemarketers to tell consumers that the match rate for the school’s students was 85%-90%. The school stated on its website that the residency match rate for Saint James students was 83%. “In fact, the match rate for SJSM students is lower than touted and lower than that reported by U.S. medical schools. Since 2018, defendants’ average match rate has been 63%.”
The FTC also claims the school used illegal credit contracts when marketing financing for tuition and living expenses for students. “The financing contracts contained language attempting to waive consumers’ rights under federal law and omit legally mandated disclosures.”
Saint James’ tuition ranges from about $6,650 to $9,859 per trimester, depending on campus and course study, the complaint states. Between 2016 and 2020, about 1,300 students were enrolled each year in Saint James’ schools. Students who attended the schools between 2016 and 2022 are eligible for a refund under the settlement.
Saint James is required to notify consumers whose debts are being canceled through Delta Financial Solutions, Saint James’ financing partner. The debt will also be deleted from consumers’ credit reports.
“We have chosen to settle with the FTC over its allegations that disclosures on our website and in Delta’s loan agreements were insufficient,” Mr. Guha stated on the school website. “However, we have added additional language and clarifications any time the USMLE pass rate and placement rates are mentioned.”
He said he hopes the school will be “an industry leader for transparency and accountability” and that the school’s “efforts will lead to lasting change throughout the for-profit educational industry.”
Mr. Guha added that more than 600 of the school’s alumni are serving as doctors, including many “working to bridge the health equity gap in underserved areas in North America.”
The FTC has been cracking down on deceptive practices by for-profit institutions. In October, the FTC put 70 for-profit colleges on notice that it would investigate false promises the schools make about their graduates’ job prospects, expected earnings, and other educational outcomes and would levy significant financial penalties against violators. Saint James was not on that list, which included several of the largest for-profit universities in the nation, including Capella University, DeVry University, Strayer University, and Walden University.
A version of this article first appeared on Medscape.com.
Although it disputed the allegations, The complaint referenced the school’s medical license exam test pass rate and residency matches along with violations of rules that protect consumers, including those dealing with credit contracts.
The school, based in the Caribbean with operations in Illinois, agreed to pay $1.2 million toward refunds and debt cancellation for students harmed by the marketing in the past 5 years.
“While we strongly disagree with the FTC’s approach to this matter, we did not want a lengthy legal process to distract from our mission of providing a quality medical education at an affordable cost,” Kaushik Guha, executive vice president of the parent of the school, Human Resources Development Services, said in a YouTube statement posted on the school’s website.
“Saint James lured students by lying about their chances of success,” Samuel Levine, director of the FTC’s Bureau of Consumer Protection, said in a press release. The settlement agreement was with HRDS, which bills itself as providing students from “non-traditional backgrounds the opportunity to pursue a medical degree and practice in the U.S. or Canada,” according to the school’s statement.
The complaint alleges that, since at least April 2018, the school, HRDS, and its operator Mr. Guha has lured students using “phony claims about the standardized test pass rate and students’ residency or job prospects. They lured consumers with false guarantees of student success at passing a critical medical school standardized test, the United States Medical Licensing Examination Step 1 Exam.”
For example, a brochure distributed at open houses claimed a first-time Step 1 pass rate of about 96.8%. The brochure further claimed: “Saint James is the first and only medical school to offer a USMLE Step 1 Pass Guarantee,” according to the FTC complaint.
The FTC said the USMLE rate is lower than touted and lower than reported by other U.S. and Canadian medical schools. “Since 2017, only 35% of Saint James students who have completed the necessary coursework to take the USMLE Step 1 exam passed the test.”
The school also misrepresented the residency match rate as “the same” as American medical schools, according to the complaint. For example, the school instructed telemarketers to tell consumers that the match rate for the school’s students was 85%-90%. The school stated on its website that the residency match rate for Saint James students was 83%. “In fact, the match rate for SJSM students is lower than touted and lower than that reported by U.S. medical schools. Since 2018, defendants’ average match rate has been 63%.”
The FTC also claims the school used illegal credit contracts when marketing financing for tuition and living expenses for students. “The financing contracts contained language attempting to waive consumers’ rights under federal law and omit legally mandated disclosures.”
Saint James’ tuition ranges from about $6,650 to $9,859 per trimester, depending on campus and course study, the complaint states. Between 2016 and 2020, about 1,300 students were enrolled each year in Saint James’ schools. Students who attended the schools between 2016 and 2022 are eligible for a refund under the settlement.
Saint James is required to notify consumers whose debts are being canceled through Delta Financial Solutions, Saint James’ financing partner. The debt will also be deleted from consumers’ credit reports.
“We have chosen to settle with the FTC over its allegations that disclosures on our website and in Delta’s loan agreements were insufficient,” Mr. Guha stated on the school website. “However, we have added additional language and clarifications any time the USMLE pass rate and placement rates are mentioned.”
He said he hopes the school will be “an industry leader for transparency and accountability” and that the school’s “efforts will lead to lasting change throughout the for-profit educational industry.”
Mr. Guha added that more than 600 of the school’s alumni are serving as doctors, including many “working to bridge the health equity gap in underserved areas in North America.”
The FTC has been cracking down on deceptive practices by for-profit institutions. In October, the FTC put 70 for-profit colleges on notice that it would investigate false promises the schools make about their graduates’ job prospects, expected earnings, and other educational outcomes and would levy significant financial penalties against violators. Saint James was not on that list, which included several of the largest for-profit universities in the nation, including Capella University, DeVry University, Strayer University, and Walden University.
A version of this article first appeared on Medscape.com.
Although it disputed the allegations, The complaint referenced the school’s medical license exam test pass rate and residency matches along with violations of rules that protect consumers, including those dealing with credit contracts.
The school, based in the Caribbean with operations in Illinois, agreed to pay $1.2 million toward refunds and debt cancellation for students harmed by the marketing in the past 5 years.
“While we strongly disagree with the FTC’s approach to this matter, we did not want a lengthy legal process to distract from our mission of providing a quality medical education at an affordable cost,” Kaushik Guha, executive vice president of the parent of the school, Human Resources Development Services, said in a YouTube statement posted on the school’s website.
“Saint James lured students by lying about their chances of success,” Samuel Levine, director of the FTC’s Bureau of Consumer Protection, said in a press release. The settlement agreement was with HRDS, which bills itself as providing students from “non-traditional backgrounds the opportunity to pursue a medical degree and practice in the U.S. or Canada,” according to the school’s statement.
The complaint alleges that, since at least April 2018, the school, HRDS, and its operator Mr. Guha has lured students using “phony claims about the standardized test pass rate and students’ residency or job prospects. They lured consumers with false guarantees of student success at passing a critical medical school standardized test, the United States Medical Licensing Examination Step 1 Exam.”
For example, a brochure distributed at open houses claimed a first-time Step 1 pass rate of about 96.8%. The brochure further claimed: “Saint James is the first and only medical school to offer a USMLE Step 1 Pass Guarantee,” according to the FTC complaint.
The FTC said the USMLE rate is lower than touted and lower than reported by other U.S. and Canadian medical schools. “Since 2017, only 35% of Saint James students who have completed the necessary coursework to take the USMLE Step 1 exam passed the test.”
The school also misrepresented the residency match rate as “the same” as American medical schools, according to the complaint. For example, the school instructed telemarketers to tell consumers that the match rate for the school’s students was 85%-90%. The school stated on its website that the residency match rate for Saint James students was 83%. “In fact, the match rate for SJSM students is lower than touted and lower than that reported by U.S. medical schools. Since 2018, defendants’ average match rate has been 63%.”
The FTC also claims the school used illegal credit contracts when marketing financing for tuition and living expenses for students. “The financing contracts contained language attempting to waive consumers’ rights under federal law and omit legally mandated disclosures.”
Saint James’ tuition ranges from about $6,650 to $9,859 per trimester, depending on campus and course study, the complaint states. Between 2016 and 2020, about 1,300 students were enrolled each year in Saint James’ schools. Students who attended the schools between 2016 and 2022 are eligible for a refund under the settlement.
Saint James is required to notify consumers whose debts are being canceled through Delta Financial Solutions, Saint James’ financing partner. The debt will also be deleted from consumers’ credit reports.
“We have chosen to settle with the FTC over its allegations that disclosures on our website and in Delta’s loan agreements were insufficient,” Mr. Guha stated on the school website. “However, we have added additional language and clarifications any time the USMLE pass rate and placement rates are mentioned.”
He said he hopes the school will be “an industry leader for transparency and accountability” and that the school’s “efforts will lead to lasting change throughout the for-profit educational industry.”
Mr. Guha added that more than 600 of the school’s alumni are serving as doctors, including many “working to bridge the health equity gap in underserved areas in North America.”
The FTC has been cracking down on deceptive practices by for-profit institutions. In October, the FTC put 70 for-profit colleges on notice that it would investigate false promises the schools make about their graduates’ job prospects, expected earnings, and other educational outcomes and would levy significant financial penalties against violators. Saint James was not on that list, which included several of the largest for-profit universities in the nation, including Capella University, DeVry University, Strayer University, and Walden University.
A version of this article first appeared on Medscape.com.
New York NPs join half of states with full practice authority
according to leading national nurse organizations.
New York joins 24 other states, the District of Columbia, and two U.S. territories that have adopted FPA legislation, as reported by the American Association of Nurse Practitioners (AANP). Like other states, New York has been under an emergency order during the pandemic that allowed NPs to practice to their full authority because of staffing shortages. That order was extended multiple times and was expected to expire this month, AANP reports.
“This has been in the making for nurse practitioners in New York since 2014, trying to get full practice authority,” Michelle Jones, RN, MSN, ANP-C, director at large for the New York State Nurses Association, said in an interview.
NPs who were allowed to practice independently during the pandemic campaigned for that provision to become permanent once the emergency order expired, she said. Ms. Jones explained that the FPA law expands the scope of practice and “removes unnecessary barriers,” namely an agreement with doctors to oversee NPs’ actions.
FPA gives NPs the authority to evaluate patients; diagnose, order, and interpret diagnostic tests; and initiate and manage treatments – including prescribing medications – without oversight by a doctor or state medical board, according to AANP.
Before the pandemic, New York NPs had “reduced” practice authority with those who had more than 3,600 hours of experience required to maintain a collaborative practice agreement with doctors and those with less experience maintaining a written agreement. The change gives full practice authority to those with more than 3,600 hours of experience, Stephen A. Ferrara, DNP, FNP-BC, AANP regional director, said in an interview.
Ferrara, who practices in New York, said the state is the largest to change to FPA. He said the state and others that have moved to FPA have determined that there “has been no lapse in quality care” during the emergency order period and that the regulatory barriers kept NPs from providing access to care.
Jones said that the law also will allow NPs to open private practices and serve underserved patients in areas that lack access to health care. “This is a step to improve access to health care and health equity of the New York population.”
It’s been a while since another state passed FPA legislation, Massachusetts in January 2021 and Delaware in August 2021, according to AANP.
Earlier this month, AANP released new data showing a 9% increase in NPs licensed to practice in the United States, rising from 325,000 in May 2021 to 355,000.
The New York legislation “will help New York attract and retain nurse practitioners and provide New Yorkers better access to quality care,” AANP President April Kapu, DNP, APRN, said in a statement.
A version of this article first appeared on Medscape.com.
according to leading national nurse organizations.
New York joins 24 other states, the District of Columbia, and two U.S. territories that have adopted FPA legislation, as reported by the American Association of Nurse Practitioners (AANP). Like other states, New York has been under an emergency order during the pandemic that allowed NPs to practice to their full authority because of staffing shortages. That order was extended multiple times and was expected to expire this month, AANP reports.
“This has been in the making for nurse practitioners in New York since 2014, trying to get full practice authority,” Michelle Jones, RN, MSN, ANP-C, director at large for the New York State Nurses Association, said in an interview.
NPs who were allowed to practice independently during the pandemic campaigned for that provision to become permanent once the emergency order expired, she said. Ms. Jones explained that the FPA law expands the scope of practice and “removes unnecessary barriers,” namely an agreement with doctors to oversee NPs’ actions.
FPA gives NPs the authority to evaluate patients; diagnose, order, and interpret diagnostic tests; and initiate and manage treatments – including prescribing medications – without oversight by a doctor or state medical board, according to AANP.
Before the pandemic, New York NPs had “reduced” practice authority with those who had more than 3,600 hours of experience required to maintain a collaborative practice agreement with doctors and those with less experience maintaining a written agreement. The change gives full practice authority to those with more than 3,600 hours of experience, Stephen A. Ferrara, DNP, FNP-BC, AANP regional director, said in an interview.
Ferrara, who practices in New York, said the state is the largest to change to FPA. He said the state and others that have moved to FPA have determined that there “has been no lapse in quality care” during the emergency order period and that the regulatory barriers kept NPs from providing access to care.
Jones said that the law also will allow NPs to open private practices and serve underserved patients in areas that lack access to health care. “This is a step to improve access to health care and health equity of the New York population.”
It’s been a while since another state passed FPA legislation, Massachusetts in January 2021 and Delaware in August 2021, according to AANP.
Earlier this month, AANP released new data showing a 9% increase in NPs licensed to practice in the United States, rising from 325,000 in May 2021 to 355,000.
The New York legislation “will help New York attract and retain nurse practitioners and provide New Yorkers better access to quality care,” AANP President April Kapu, DNP, APRN, said in a statement.
A version of this article first appeared on Medscape.com.
according to leading national nurse organizations.
New York joins 24 other states, the District of Columbia, and two U.S. territories that have adopted FPA legislation, as reported by the American Association of Nurse Practitioners (AANP). Like other states, New York has been under an emergency order during the pandemic that allowed NPs to practice to their full authority because of staffing shortages. That order was extended multiple times and was expected to expire this month, AANP reports.
“This has been in the making for nurse practitioners in New York since 2014, trying to get full practice authority,” Michelle Jones, RN, MSN, ANP-C, director at large for the New York State Nurses Association, said in an interview.
NPs who were allowed to practice independently during the pandemic campaigned for that provision to become permanent once the emergency order expired, she said. Ms. Jones explained that the FPA law expands the scope of practice and “removes unnecessary barriers,” namely an agreement with doctors to oversee NPs’ actions.
FPA gives NPs the authority to evaluate patients; diagnose, order, and interpret diagnostic tests; and initiate and manage treatments – including prescribing medications – without oversight by a doctor or state medical board, according to AANP.
Before the pandemic, New York NPs had “reduced” practice authority with those who had more than 3,600 hours of experience required to maintain a collaborative practice agreement with doctors and those with less experience maintaining a written agreement. The change gives full practice authority to those with more than 3,600 hours of experience, Stephen A. Ferrara, DNP, FNP-BC, AANP regional director, said in an interview.
Ferrara, who practices in New York, said the state is the largest to change to FPA. He said the state and others that have moved to FPA have determined that there “has been no lapse in quality care” during the emergency order period and that the regulatory barriers kept NPs from providing access to care.
Jones said that the law also will allow NPs to open private practices and serve underserved patients in areas that lack access to health care. “This is a step to improve access to health care and health equity of the New York population.”
It’s been a while since another state passed FPA legislation, Massachusetts in January 2021 and Delaware in August 2021, according to AANP.
Earlier this month, AANP released new data showing a 9% increase in NPs licensed to practice in the United States, rising from 325,000 in May 2021 to 355,000.
The New York legislation “will help New York attract and retain nurse practitioners and provide New Yorkers better access to quality care,” AANP President April Kapu, DNP, APRN, said in a statement.
A version of this article first appeared on Medscape.com.
University of Washington, Harvard ranked top medical schools for second year
It may seem like déjà vu, as not much has changed regarding the rankings of top U.S. medical schools over the past 2 years.
The University of Washington, Seattle retained its ranking from the U.S. News & World Report as the top medical school for primary care for 2023. Also repeating its 2022 standing as the top medical school for research is Harvard University.
In the primary care ranking, the top 10 schools after the University of Washington were the University of California, San Francisco; the University of Minnesota; Oregon Health and Science University; the University of North Carolina at Chapel Hill; the University of Colorado; the University of Nebraska Medical Center; the University of California, Davis; and Harvard. Three schools tied for the no. 10 slot: the University of Kansas Medical Center, the University of Massachusetts Chan Medical Center, and the University of Pittsburgh.
The top five schools with the most graduates practicing in primary care specialties are Des Moines University, Iowa (50.6%); the University of Pikeville (Ky.) (46.8%); Western University of Health Sciences, Pomona, California (46%); William Carey University College of Osteopathic Medicine, Hattiesburg, Mississippi (44.7%); and A.T. Still University of Health Sciences, Kirksville, Missouri (44.3%).
Best for research
When it comes to schools ranking the highest for research, the Grossman School of Medicine at New York University takes the no. 2 spot after Harvard. Three schools were tied for the no. 3 spot: Columbia University, Johns Hopkins University, and the University of California, San Francisco; and two schools for no. 6: Duke University and the Perelman School of Medicine at the University of Pennsylvania, Philadelphia. No. 8 goes to Stanford University, followed by the University of Washington. Rounding out the top 10 is Yale University.
Specialty ranks
The top-ranked schools in eight specialties are as follows:
- Anesthesiology: Harvard
- Family medicine: the University of Washington
- Internal medicine: Johns Hopkins
- Obstetrics/gynecology: Harvard
- Pediatrics: the University of Pennsylvania (Perelman)
- Psychiatry: Harvard
- Radiology: Johns Hopkins
- Surgery: Harvard
Most diverse student body
If you’re looking for a school with significant minority representation, Howard University, Washington, D.C., ranked highest (76.8%), followed by the Wertheim College of Medicine at Florida International University, Miami (43.2%). The University of California, Davis (40%), Sacramento, California, and the University of Vermont (Larner), Burlington (14.1%), tied for third.
Three southern schools take top honors for the most graduates practicing in underserved areas, starting with the University of South Carolina (70.9%), followed by the University of Mississippi (66.2%), and East Tennessee State University (Quillen), Johnson City, Tennessee (65.8%).
The colleges with the most graduates practicing in rural areas are William Carey University College of Osteopathic Medicine (28%), the University of Pikesville (25.6%), and the University of Mississippi (22.1%).
College debt
The medical school where graduates have the most debt is Nova Southeastern University Patel College of Osteopathic Medicine, Fort Lauderdale, Florida. Graduates incurred an average debt of $309,206. Western University of Health Sciences graduates racked up $276,840 in debt, followed by graduates of West Virginia School of Osteopathic Medicine, owing $268,416.
Ranking criteria
Each year, U.S. News ranks hundreds of U.S. colleges and universities. Medical schools fall under the rankings for best graduate schools.
U.S. News surveyed 192 medical and osteopathic schools accredited in 2021 by the Liaison Committee on Medical Education or the American Osteopathic Association. Among the schools surveyed in fall 2021 and early 2022, 130 schools responded. Of those, 124 were included in both the research and primary care rankings.
The criteria for ranking include faculty resources, academic achievements of entering students, and qualitative assessments by schools and residency directors.
A version of this article first appeared on Medscape.com.
It may seem like déjà vu, as not much has changed regarding the rankings of top U.S. medical schools over the past 2 years.
The University of Washington, Seattle retained its ranking from the U.S. News & World Report as the top medical school for primary care for 2023. Also repeating its 2022 standing as the top medical school for research is Harvard University.
In the primary care ranking, the top 10 schools after the University of Washington were the University of California, San Francisco; the University of Minnesota; Oregon Health and Science University; the University of North Carolina at Chapel Hill; the University of Colorado; the University of Nebraska Medical Center; the University of California, Davis; and Harvard. Three schools tied for the no. 10 slot: the University of Kansas Medical Center, the University of Massachusetts Chan Medical Center, and the University of Pittsburgh.
The top five schools with the most graduates practicing in primary care specialties are Des Moines University, Iowa (50.6%); the University of Pikeville (Ky.) (46.8%); Western University of Health Sciences, Pomona, California (46%); William Carey University College of Osteopathic Medicine, Hattiesburg, Mississippi (44.7%); and A.T. Still University of Health Sciences, Kirksville, Missouri (44.3%).
Best for research
When it comes to schools ranking the highest for research, the Grossman School of Medicine at New York University takes the no. 2 spot after Harvard. Three schools were tied for the no. 3 spot: Columbia University, Johns Hopkins University, and the University of California, San Francisco; and two schools for no. 6: Duke University and the Perelman School of Medicine at the University of Pennsylvania, Philadelphia. No. 8 goes to Stanford University, followed by the University of Washington. Rounding out the top 10 is Yale University.
Specialty ranks
The top-ranked schools in eight specialties are as follows:
- Anesthesiology: Harvard
- Family medicine: the University of Washington
- Internal medicine: Johns Hopkins
- Obstetrics/gynecology: Harvard
- Pediatrics: the University of Pennsylvania (Perelman)
- Psychiatry: Harvard
- Radiology: Johns Hopkins
- Surgery: Harvard
Most diverse student body
If you’re looking for a school with significant minority representation, Howard University, Washington, D.C., ranked highest (76.8%), followed by the Wertheim College of Medicine at Florida International University, Miami (43.2%). The University of California, Davis (40%), Sacramento, California, and the University of Vermont (Larner), Burlington (14.1%), tied for third.
Three southern schools take top honors for the most graduates practicing in underserved areas, starting with the University of South Carolina (70.9%), followed by the University of Mississippi (66.2%), and East Tennessee State University (Quillen), Johnson City, Tennessee (65.8%).
The colleges with the most graduates practicing in rural areas are William Carey University College of Osteopathic Medicine (28%), the University of Pikesville (25.6%), and the University of Mississippi (22.1%).
College debt
The medical school where graduates have the most debt is Nova Southeastern University Patel College of Osteopathic Medicine, Fort Lauderdale, Florida. Graduates incurred an average debt of $309,206. Western University of Health Sciences graduates racked up $276,840 in debt, followed by graduates of West Virginia School of Osteopathic Medicine, owing $268,416.
Ranking criteria
Each year, U.S. News ranks hundreds of U.S. colleges and universities. Medical schools fall under the rankings for best graduate schools.
U.S. News surveyed 192 medical and osteopathic schools accredited in 2021 by the Liaison Committee on Medical Education or the American Osteopathic Association. Among the schools surveyed in fall 2021 and early 2022, 130 schools responded. Of those, 124 were included in both the research and primary care rankings.
The criteria for ranking include faculty resources, academic achievements of entering students, and qualitative assessments by schools and residency directors.
A version of this article first appeared on Medscape.com.
It may seem like déjà vu, as not much has changed regarding the rankings of top U.S. medical schools over the past 2 years.
The University of Washington, Seattle retained its ranking from the U.S. News & World Report as the top medical school for primary care for 2023. Also repeating its 2022 standing as the top medical school for research is Harvard University.
In the primary care ranking, the top 10 schools after the University of Washington were the University of California, San Francisco; the University of Minnesota; Oregon Health and Science University; the University of North Carolina at Chapel Hill; the University of Colorado; the University of Nebraska Medical Center; the University of California, Davis; and Harvard. Three schools tied for the no. 10 slot: the University of Kansas Medical Center, the University of Massachusetts Chan Medical Center, and the University of Pittsburgh.
The top five schools with the most graduates practicing in primary care specialties are Des Moines University, Iowa (50.6%); the University of Pikeville (Ky.) (46.8%); Western University of Health Sciences, Pomona, California (46%); William Carey University College of Osteopathic Medicine, Hattiesburg, Mississippi (44.7%); and A.T. Still University of Health Sciences, Kirksville, Missouri (44.3%).
Best for research
When it comes to schools ranking the highest for research, the Grossman School of Medicine at New York University takes the no. 2 spot after Harvard. Three schools were tied for the no. 3 spot: Columbia University, Johns Hopkins University, and the University of California, San Francisco; and two schools for no. 6: Duke University and the Perelman School of Medicine at the University of Pennsylvania, Philadelphia. No. 8 goes to Stanford University, followed by the University of Washington. Rounding out the top 10 is Yale University.
Specialty ranks
The top-ranked schools in eight specialties are as follows:
- Anesthesiology: Harvard
- Family medicine: the University of Washington
- Internal medicine: Johns Hopkins
- Obstetrics/gynecology: Harvard
- Pediatrics: the University of Pennsylvania (Perelman)
- Psychiatry: Harvard
- Radiology: Johns Hopkins
- Surgery: Harvard
Most diverse student body
If you’re looking for a school with significant minority representation, Howard University, Washington, D.C., ranked highest (76.8%), followed by the Wertheim College of Medicine at Florida International University, Miami (43.2%). The University of California, Davis (40%), Sacramento, California, and the University of Vermont (Larner), Burlington (14.1%), tied for third.
Three southern schools take top honors for the most graduates practicing in underserved areas, starting with the University of South Carolina (70.9%), followed by the University of Mississippi (66.2%), and East Tennessee State University (Quillen), Johnson City, Tennessee (65.8%).
The colleges with the most graduates practicing in rural areas are William Carey University College of Osteopathic Medicine (28%), the University of Pikesville (25.6%), and the University of Mississippi (22.1%).
College debt
The medical school where graduates have the most debt is Nova Southeastern University Patel College of Osteopathic Medicine, Fort Lauderdale, Florida. Graduates incurred an average debt of $309,206. Western University of Health Sciences graduates racked up $276,840 in debt, followed by graduates of West Virginia School of Osteopathic Medicine, owing $268,416.
Ranking criteria
Each year, U.S. News ranks hundreds of U.S. colleges and universities. Medical schools fall under the rankings for best graduate schools.
U.S. News surveyed 192 medical and osteopathic schools accredited in 2021 by the Liaison Committee on Medical Education or the American Osteopathic Association. Among the schools surveyed in fall 2021 and early 2022, 130 schools responded. Of those, 124 were included in both the research and primary care rankings.
The criteria for ranking include faculty resources, academic achievements of entering students, and qualitative assessments by schools and residency directors.
A version of this article first appeared on Medscape.com.
The power of the pause to prevent diagnostic error
None of us like being wrong, especially about a patient’s diagnosis. To help you avoid diagnostic errors for 4 difficult diagnoses, read and study the article in this issue of JFP by Rosen and colleagues.1 They discuss misdiagnosis of polymyalgia rheumatica, fibromyalgia, ovarian cancer, and Lewy body dementia to illustrate how we can go astray if we do not take care to pause and think through things carefully. They point out that, for quick and mostly accurate diagnoses, pattern recognition or type 1 thinking serves us well in a busy office practice. However, we must frequently pause and reflect, using type 2 thinking—especially when the puzzle pieces don’t quite fit together.
I still recall vividly a diagnostic error I made many years ago. One of my patients, whom I had diagnosed and was treating for hyperlipidemia, returned for follow-up while I was on vacation. My partner conducted the follow-up visit. To my chagrin, he noticed her puffy face and weight gain and ordered thyroid studies. Sure enough, my patient was severely hypothyroid, and her lipid levels normalized with thyroid replacement therapy.
A happier tale for me was making the correct diagnosis for a woman with chronic cough. She had been evaluated by multiple specialists during the prior year and treated with a nasal steroid for allergies, a proton pump inhibitor for reflux, and a steroid inhaler for possible asthma. None of these relieved her cough. After reviewing her medication list and noting that it included amitriptyline, which has anticholinergic adverse effects, I recommended she stop taking that medication and the cough resolved.
John Ely, MD, MPH, a family physician who has spent his academic career investigating causes of and solutions to diagnostic errors, has outlined important steps we can take. These include: (1) obtaining your own complete medical history, (2) performing a “focused and purposeful” physical exam, (3) generating initial hypotheses and differentiating them through additional history taking, exams, and diagnostic tests, (4) pausing to reflect [my emphasis], and (5) embarking on a plan (while acknowledging uncertainty) and ensuring there is a pathway for follow-up.2
To help avoid diagnostic errors, Dr. Ely developed and uses a set of checklists that cover the differential diagnosis for 72 presenting complaints/conditions, including syncope, back pain, insomnia, and headache.2 When you are faced with diagnostic uncertainty, it takes just a few minutes to run through the checklist for the patient’s presenting complaint.
1. Rosen PD, Klenzak S, Baptista S. Diagnostic challenges in primary care: identifying and avoiding cognitive bias. J Fam Pract. 2022;71:124-132.
2. Ely JW, Graber ML, Croskerry P. Checklists to reduce diagnostic errors. Acad Med. 2011;86:307-313. doi: 10.1097/ACM.0b013e31820824cd
None of us like being wrong, especially about a patient’s diagnosis. To help you avoid diagnostic errors for 4 difficult diagnoses, read and study the article in this issue of JFP by Rosen and colleagues.1 They discuss misdiagnosis of polymyalgia rheumatica, fibromyalgia, ovarian cancer, and Lewy body dementia to illustrate how we can go astray if we do not take care to pause and think through things carefully. They point out that, for quick and mostly accurate diagnoses, pattern recognition or type 1 thinking serves us well in a busy office practice. However, we must frequently pause and reflect, using type 2 thinking—especially when the puzzle pieces don’t quite fit together.
I still recall vividly a diagnostic error I made many years ago. One of my patients, whom I had diagnosed and was treating for hyperlipidemia, returned for follow-up while I was on vacation. My partner conducted the follow-up visit. To my chagrin, he noticed her puffy face and weight gain and ordered thyroid studies. Sure enough, my patient was severely hypothyroid, and her lipid levels normalized with thyroid replacement therapy.
A happier tale for me was making the correct diagnosis for a woman with chronic cough. She had been evaluated by multiple specialists during the prior year and treated with a nasal steroid for allergies, a proton pump inhibitor for reflux, and a steroid inhaler for possible asthma. None of these relieved her cough. After reviewing her medication list and noting that it included amitriptyline, which has anticholinergic adverse effects, I recommended she stop taking that medication and the cough resolved.
John Ely, MD, MPH, a family physician who has spent his academic career investigating causes of and solutions to diagnostic errors, has outlined important steps we can take. These include: (1) obtaining your own complete medical history, (2) performing a “focused and purposeful” physical exam, (3) generating initial hypotheses and differentiating them through additional history taking, exams, and diagnostic tests, (4) pausing to reflect [my emphasis], and (5) embarking on a plan (while acknowledging uncertainty) and ensuring there is a pathway for follow-up.2
To help avoid diagnostic errors, Dr. Ely developed and uses a set of checklists that cover the differential diagnosis for 72 presenting complaints/conditions, including syncope, back pain, insomnia, and headache.2 When you are faced with diagnostic uncertainty, it takes just a few minutes to run through the checklist for the patient’s presenting complaint.
None of us like being wrong, especially about a patient’s diagnosis. To help you avoid diagnostic errors for 4 difficult diagnoses, read and study the article in this issue of JFP by Rosen and colleagues.1 They discuss misdiagnosis of polymyalgia rheumatica, fibromyalgia, ovarian cancer, and Lewy body dementia to illustrate how we can go astray if we do not take care to pause and think through things carefully. They point out that, for quick and mostly accurate diagnoses, pattern recognition or type 1 thinking serves us well in a busy office practice. However, we must frequently pause and reflect, using type 2 thinking—especially when the puzzle pieces don’t quite fit together.
I still recall vividly a diagnostic error I made many years ago. One of my patients, whom I had diagnosed and was treating for hyperlipidemia, returned for follow-up while I was on vacation. My partner conducted the follow-up visit. To my chagrin, he noticed her puffy face and weight gain and ordered thyroid studies. Sure enough, my patient was severely hypothyroid, and her lipid levels normalized with thyroid replacement therapy.
A happier tale for me was making the correct diagnosis for a woman with chronic cough. She had been evaluated by multiple specialists during the prior year and treated with a nasal steroid for allergies, a proton pump inhibitor for reflux, and a steroid inhaler for possible asthma. None of these relieved her cough. After reviewing her medication list and noting that it included amitriptyline, which has anticholinergic adverse effects, I recommended she stop taking that medication and the cough resolved.
John Ely, MD, MPH, a family physician who has spent his academic career investigating causes of and solutions to diagnostic errors, has outlined important steps we can take. These include: (1) obtaining your own complete medical history, (2) performing a “focused and purposeful” physical exam, (3) generating initial hypotheses and differentiating them through additional history taking, exams, and diagnostic tests, (4) pausing to reflect [my emphasis], and (5) embarking on a plan (while acknowledging uncertainty) and ensuring there is a pathway for follow-up.2
To help avoid diagnostic errors, Dr. Ely developed and uses a set of checklists that cover the differential diagnosis for 72 presenting complaints/conditions, including syncope, back pain, insomnia, and headache.2 When you are faced with diagnostic uncertainty, it takes just a few minutes to run through the checklist for the patient’s presenting complaint.
1. Rosen PD, Klenzak S, Baptista S. Diagnostic challenges in primary care: identifying and avoiding cognitive bias. J Fam Pract. 2022;71:124-132.
2. Ely JW, Graber ML, Croskerry P. Checklists to reduce diagnostic errors. Acad Med. 2011;86:307-313. doi: 10.1097/ACM.0b013e31820824cd
1. Rosen PD, Klenzak S, Baptista S. Diagnostic challenges in primary care: identifying and avoiding cognitive bias. J Fam Pract. 2022;71:124-132.
2. Ely JW, Graber ML, Croskerry P. Checklists to reduce diagnostic errors. Acad Med. 2011;86:307-313. doi: 10.1097/ACM.0b013e31820824cd
Medical assistants identify strategies and barriers to clinic efficiency
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
Continue to: We developed and piloted...
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Continue to: Efficiency and competing priorities
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.
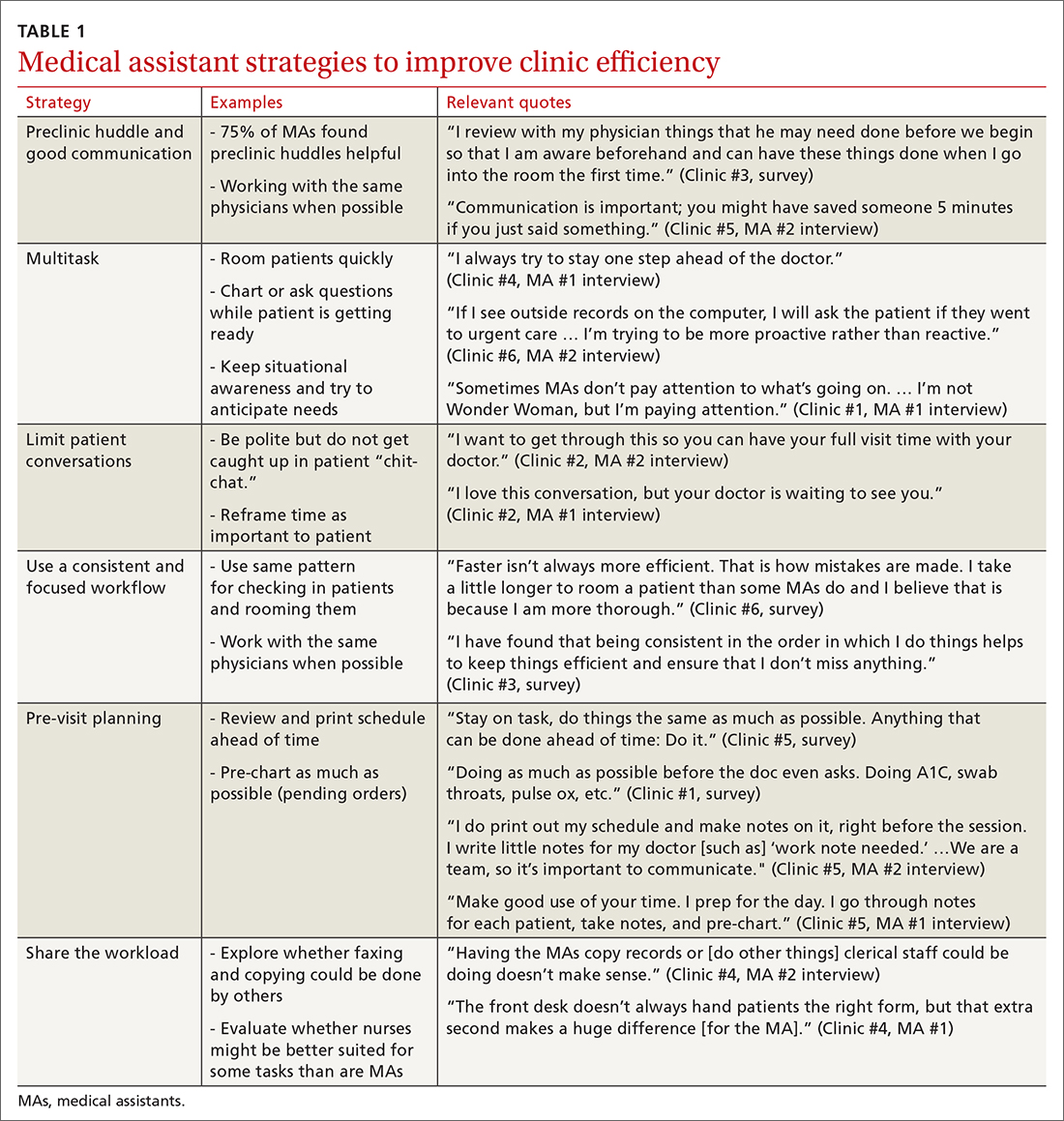
Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
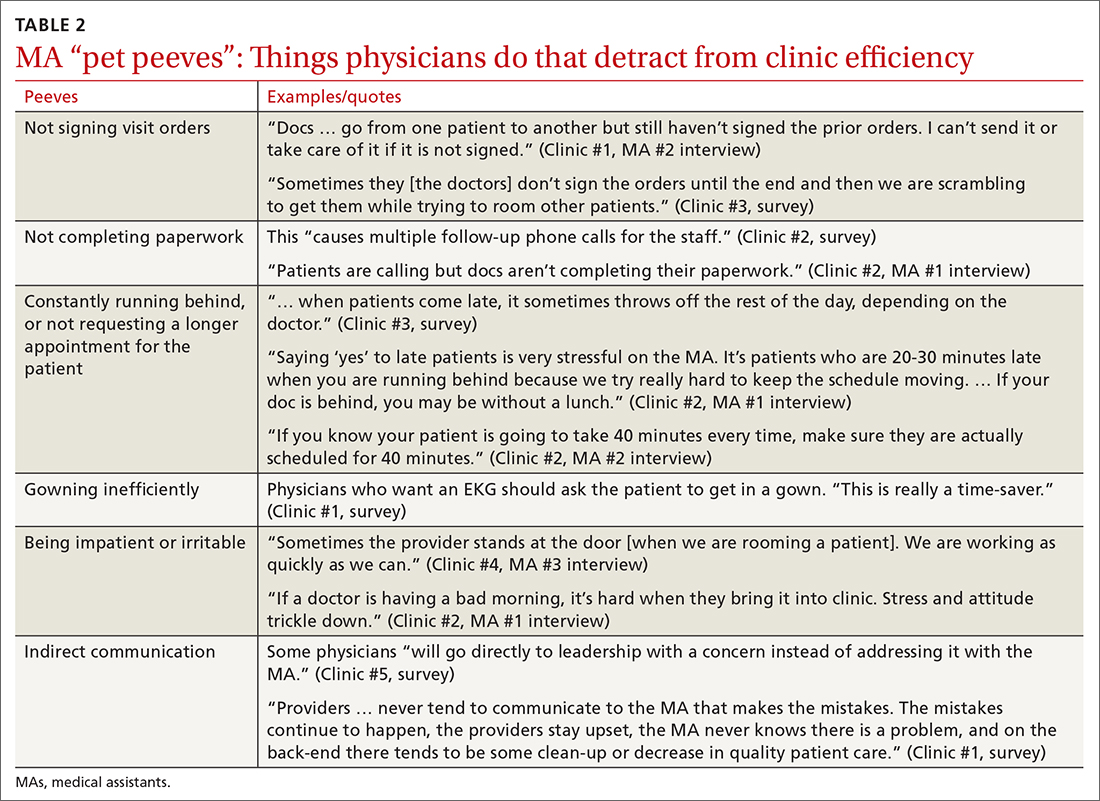
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“ Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“ [Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
Continue to: A minority of staff...
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Continue to: Almost all respondents...
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male-dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries.19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs.8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE
Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; [email protected]
1. Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
2. Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
3. Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/articles/medical-assisting-trends/
4. Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
5. Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medical-assisting-history/
6. Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
7. Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
8. Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
9. Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
10. STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_combined.pdf
11. Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
12. Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
13. Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
14. Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
15. Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
16. US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/oes/current/oes319092.htm
17. Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
18. Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
19. US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/healthcare/medical-assistants.htm
20. Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
21. Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
22. Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11:187-201.
23. Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
24. O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
Continue to: We developed and piloted...
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Continue to: Efficiency and competing priorities
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.

Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)

Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“ Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“ [Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
Continue to: A minority of staff...
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Continue to: Almost all respondents...
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male-dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries.19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs.8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE
Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; [email protected]
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
Continue to: We developed and piloted...
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Continue to: Efficiency and competing priorities
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.

Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)

Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“ Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“ [Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
Continue to: A minority of staff...
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Continue to: Almost all respondents...
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male-dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries.19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs.8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE
Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; [email protected]
1. Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
2. Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
3. Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/articles/medical-assisting-trends/
4. Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
5. Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medical-assisting-history/
6. Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
7. Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
8. Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
9. Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
10. STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_combined.pdf
11. Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
12. Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
13. Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
14. Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
15. Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
16. US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/oes/current/oes319092.htm
17. Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
18. Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
19. US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/healthcare/medical-assistants.htm
20. Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
21. Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
22. Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11:187-201.
23. Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
24. O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
1. Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
2. Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
3. Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/articles/medical-assisting-trends/
4. Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
5. Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medical-assisting-history/
6. Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
7. Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
8. Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
9. Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
10. STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_combined.pdf
11. Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
12. Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
13. Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
14. Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
15. Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
16. US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/oes/current/oes319092.htm
17. Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
18. Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
19. US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/healthcare/medical-assistants.htm
20. Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
21. Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
22. Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11:187-201.
23. Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
24. O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
Diagnostic challenges in primary care: Identifying and avoiding cognitive bias
Medical errors in all settings contributed to as many as 250,000 deaths per year in the United States between 2000 and 2008, according to a 2016 study.1 Diagnostic error, in particular, remains a leading cause of morbidity and mortality in the United States and worldwide. In 2017, 12 million patients (roughly 5% of all US adults) who sought outpatient care experienced missed, delayed, or incorrect diagnosis at least once.2
In his classic work, How Doctors Think, Jerome Groopman, MD, explored the diagnostic process with a focus on the role of cognitive bias in clinical decision-making. Groopman examined how physicians can become sidetracked in their thinking and “blinded” to potential alternative diagnoses.3 Medical error is not necessarily because of a deficiency in medical knowledge; rather, physicians become susceptible to medical error when defective and faulty reasoning distort their diagnostic ability.4
Cognitive bias in the diagnostic process has been extensively studied, and a full review is beyond the scope of this article.5 However, here we will examine pathways leading to diagnostic errors in the primary care setting, specifically the role of cognitive bias in the work-up of polymyalgia rheumatica (PMR), ovarian cancer (OC), Lewy body dementia (LBD), and fibromyalgia (FM). As these 4 disease states are seen with low-to-moderate frequency in primary care, cognitive bias can complicate accurate diagnosis. But first, a word about how to understand clinical reasoning.
There are 2 types of reasoning (and 1 is more prone to error)
Physician clinical reasoning can be divided into 2 different cognitive approaches.
Type 1 reasoning employs intuition and heuristics; this type is automatic, reflexive, and quick.5 While the use of mental shortcuts in type 1 increases the speed with which decisions are made, it also makes this form of reasoning more prone to error.
Type 2 reasoning requires conscious effort. It is goal directed and rigorous and therefore slower than type 1 reasoning. Extrapolated to the clinical context, clinicians transition from type 2 to type 1 reasoning as they gain experience and training throughout their careers and develop their own conscious and subconscious heuristics. Deviations from accurate decision-making occur in a systematic manner due to cognitive biases and result in medical error.6table 17 lists common types of cognitive bias.
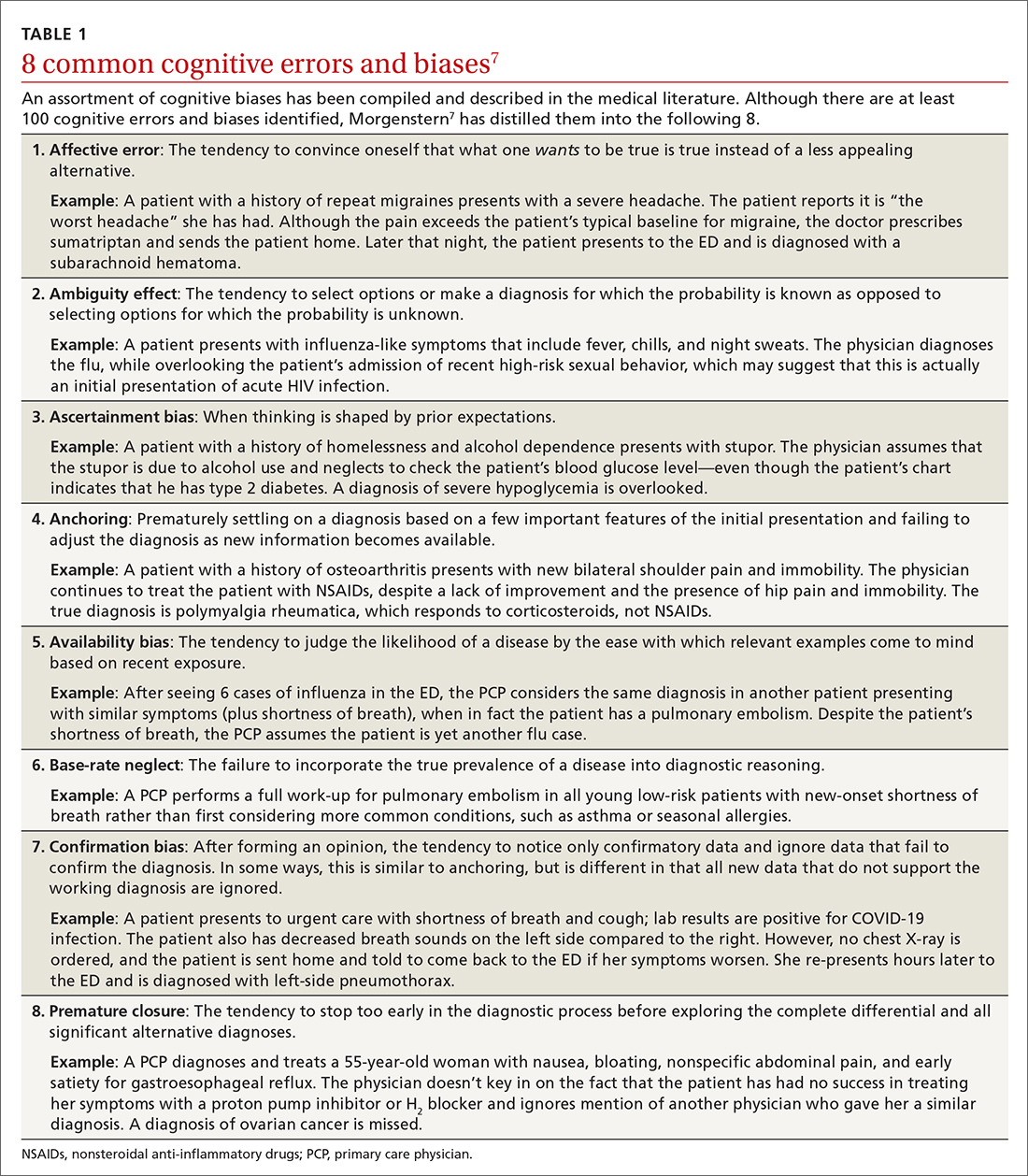
An important question to ask. Physicians tend to fall into a pattern of quick, type 1 reasoning. However, it’s important to strive to maintain a broad differential diagnosis and avoid premature closure of the diagnostic process. It’s critical that we consider alternative diagnoses (ie, consciously move from type 1 to type 2 thinking) and continue to ask ourselves, “What else?” while working through differential diagnoses. This can be a powerful debiasing technique.
Continue to: The discussion...
The discussion of the following 4 disease states demonstrates how cognitive bias can lead to diagnostic error.
The patient is barely able to ambulate and appears to be in considerable pain. She is relying heavily on her walker and is assisted by her granddaughter. The primary care physician (PCP) obtains a detailed history that includes chronic shoulder and hip pain. Given that the patient has not responded to NSAID treatment over the previous 6 months, the PCP takes a moment to reconsider the diagnosis of OA and considers other options.
In light of the high prevalence of PMR in older women, the physician pursues a more specific physical examination tailored to ferret out PMR. He had learned this diagnostic shortcut as a resident, remembered it, and adeptly applied it whenever circumstances warranted. He asks the patient to raise her arms above her head (goalpost sign). She is unable to perform this task and experiences severe bilateral shoulder pain on trial. The PCP then places the patient on the examining table and attempts to assist her in rolling toward him. The patient is also unable to perform this maneuver and experiences significant bilateral hip pain on trial.
Based primarily on the patient’s history and physical exam findings, the PCP makes a presumptive diagnosis of PMR vs OA vs combined PMR with OA, orders an erythrocyte sedimentation rate (ESR) and basic rheumatologic
PMR can be mistaken for OA
PMR is the most common inflammatory rheumatic disease in older patients.8 It is a debilitating illness with simple, effective treatment but has devastating consequences if missed or left untreated.9 PMR typically manifests in patients older than age 50, with a peak incidence at 80 years of age. It is also far more common in women.10
Approximately 80% of patients with PMR initially present to their PCP, often posing a diagnostic challenge to many clinicians.11 Due to overlap in symptoms, the condition is often misdiagnosed as OA, a more common condition seen by PCPs. Also, there are no specific diagnostic tests for PMR. An elevated ESR can help confirm the diagnosis, but one-third of patients with PMR have a normal ESR.12 Therefore, the diagnostic conundrum the physician faces is OA vs rheumatoid arthritis (RA), PMR, or another condition.
Continue to: The consequences...
The consequences of a missed and delayed PMR diagnosis range from seriously impaired quality of life to significantly increased risk of vascular events (eg, blindness, stroke) due to temporal arteritis.13 Early diagnosis is even more critical as the risk of a vascular event and death is highest during initial phases of the disease course.14
FPs often miss this Dx. A timely diagnosis relies almost exclusively on an accurate, thorough history and physical exam. However, PCPs often struggle to correctly diagnose PMR. According to a study by Bahlas and colleagues,15 the accuracy rate for correctly diagnosing PMR was 24% among a cohort of family physicians.
The differential diagnosis for PMR is broad and includes seronegative spondyloarthropathies, malignancy, Lyme disease, hypothyroidism, and both RA and OA.16
PCPs are extremely adept at correctly diagnosing RA, but not PMR. A study by Blaauw and colleagues17 comparing PCPs and rheumatologists found PCPs correctly identified 92% of RA cases but only 55% of PMR cases. When rheumatologists reviewed these same cases, they correctly identified PMR and RA almost 100% of the time.17 The difference in diagnostic accuracy between rheumatologists and PCPs suggests limited experience and gaps in fund of knowledge.
Making the diagnosis. The diagnosis of PMR is often made on empiric response to corticosteroid treatment, but doing so based solely on a patient’s response is controversial.18 There are rare instances in which patients with PMR fail to respond to treatment. On the other hand, some inflammatory conditions that mimic or share symptoms with PMR also respond to corticosteroids, potentially resulting in erroneous confirmation bias.
Some classification criteria use rapid response to low-dose prednisone/prednisolone (≤ 20 mg) to confirm the diagnosis,19 while other more recent guidelines no longer include this approach.20 If PMR continues to be suspected after a trial of steroids is unsuccessful, the PCP can try another course of higher dose steroids or consult with Rheumatology.
Continue to: A full history...
A full history and physical exam revealed a myriad of gastrointestinal (GI) complaints, such as diarrhea. But the PCP recalled a recent roundtable discussion on debiasing techniques specifically related to gynecologic disorders, including OC. Therefore, he decided to include OC in the differential diagnosis—something he would not routinely have done given the preponderance of GI symptoms. Despite the patient’s reluctance and time constraints, the PCP ordered a transvaginal ultrasound. Findings from the ultrasound study revealed stage II OC, which carries a good prognosis. The patient is currently undergoing treatment and was last reported as doing well.
Early signs of ovarian cancer can be chalked up to a “GI issue”
OC is the second most common gynecologic cancer21 and the fifth leading cause of cancer-related death22 in US women. Compared to other cancers, the prognosis for localized early-stage OC is surprisingly good, with a 5-year survival rate approaching 93%.23 However, most disease is detected in later stages, and the 5-year survival rate drops to a low of 29%.24
There remains no established screening protocol for OC. Fewer than a quarter of all cases are diagnosed in stage I, and detection of OC relies heavily on the physician’s ability to decipher vague symptomatology that overlaps with other, more common maladies. This poses an obvious diagnostic challenge and, not surprisingly, a high level of susceptibility to cognitive bias.
More than 90% of patients with OC present with some combination of the following symptoms prior to diagnosis: abdominal (77%), GI (70%), pain (58%), constitutional (50%), urinary (34%), and pelvic (26%).25 The 3 most common isolated symptoms in patients with OC are abdominal bloating, decrease in appetite, and frank abdominal pain.26 Patients with biopsy-confirmed OC experience these symptoms an average of 6 months prior to actual diagnosis.27
Knowledge gaps play a role. Studies assessing the ability of health care providers to identify presenting symptoms of OC reveal specific knowledge gaps. For instance, in a survey by Gajjar and colleagues,28 most PCPs correctly identified bloating as a key symptom of OC; however, they weren’t as good at identifying less common symptoms, such as inability to finish a meal and early satiety. Moreover, survey participants misinterpreted or missed GI symptoms as an important manifestation of early OC disease.28 These specific knowledge gaps combine with physician errors in thinking, further obscuring and extending the diagnostic process. The point prevalence for OC is relatively low, and many PCPs only encounter a few cases during their entire career.29 This low pre-test probability may also fuel the delay in diagnosis.
Watch for these forms of bias. Since nonspecific symptoms of early-stage OC resemble those of other more benign conditions, a form of anchoring error known as multiple alternatives bias can arise. In this scenario, clinicians investigate only 1 potential plausible diagnosis and remain focused on that single, often faulty, conclusion. This persists despite other equally plausible alternatives that arise as the investigation proceeds.28
Affective error may also play a role in missed or delayed diagnosis. For example, a physician would prefer to diagnose and treat a common GI illness than consider OC. Another distortion involves outcome bias wherein the physician gives more significance to benign conditions such as irritable bowel syndrome because they have a more favorable outcome and clear treatment path. Physicians also favor these benign conditions because they encounter them more frequently than OC in the clinic setting. (This is known as availability bias.) Outcome bias and multiple alternatives bias can result in noninvestigation of symptoms and inefficient or improper management, leading to a delay in arriving at the correct diagnosis or anchoring on a plausible but incorrect diagnosis.
Continue to: An incorrect initial diagnostic...
An incorrect initial diagnostic path often triggers a cascade of subsequent errors. The physician orders additional unhelpful and expensive tests in an effort to characterize the suspected GI pathology. This then leads the physician to prematurely terminate the work-up and accept the most favored diagnosis. Lastly, sunk-cost fallacy comes into play: The physician has “invested” time and energy investigating a particular diagnosis and rather than abandon the presumed diagnosis, continues to put more time and effort in going down an incorrect diagnostic path.
A series of failures. These biases and miscues have been observed in several studies. For example, a survey of 1725 women by Goff and colleagues30 sought to identify factors related to delayed OC diagnosis. The authors found that the following factors were significantly associated with a delayed diagnosis: omission of a pelvic exam at initial presentation, a separate exploration of a multitude of collateral symptoms, a failure to order ultrasound/computed tomography/CA-125 test, and a failure to consider age as a factor (especially if the patient was outside the norm).
Responses from the survey also revealed that physicians initially ordered work-ups related to GI etiology and only later considered a pelvic work-up. This suggests that well-known presenting signs and symptoms or a constellation of typical and atypical symptoms of OC often failed to trigger physician recognition. Understandably, patients presenting with menorrhagia or gynecologic complaints are more likely to have OC detected at an earlier stage than patients who present with GI or abdominal signs alone.31 table 27 summarizes some of the cognitive biases seen in the diagnostic path of OC.
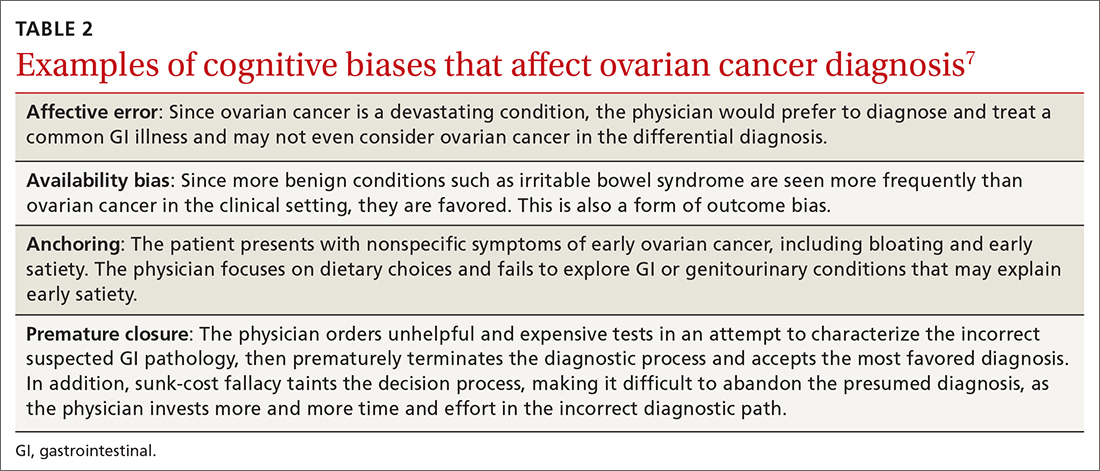
While in the hospital, he becomes acutely upset by the hallucinations and is given haloperidol and lorazepam by house staff. In the morning, the patient exhibits severe signs of Parkinson disease that include rigidity and masked facies.
Given the patient’s poor response to haloperidol and continued confusion, the team consulted Neurology and Psychiatry. Gathering a more detailed history from the patient and family, the patient is given a diagnosis of classic LBD. The antipsychotic medications are stopped. The patient and his family receive education about LBD treatment and management, and the patient is discharged to outpatient care.
Psychiatric symptoms can be an early “misdirect” in cases of Lewy body disease
LBD, the second leading neurodegenerative dementia after Alzheimer disease (AD), affects 1.5 million Americans,32 representing about 10% of all dementia cases. LBD and AD overlap in 25% of dementia cases.33 In patients older than 85 years, the prevalence jumps to 5% of the general population and 22% of all cases of dementia.33 Despite its prevalence, a recent study showed that only 6% of PCPs correctly identified LBD as the primary diagnosis when presented with typical case examples.32
Continue to: 3 stages of presentation
3 stages of presentation. Unlike other forms of dementia, LBD typically presents first with psychiatric symptoms, then with cognitive impairment, and last with parkinsonian symptoms. Additionally, rapid eye movement sleep behavior disorder and often subtle elements of nonmemory cognitive impairment distinguish LBD from both AD and vascular dementia.32 The primary cognitive deficit in LBD is not in memory but in attention, executive functioning, and visuospatial ability.34 Only in the later stages of the disease do patients exhibit gradual and progressive memory loss.
Mistaken for many things. When evaluating patients exhibiting signs of dementia, it’s important to include LBD in the differential, with increased suspicion for patients experiencing episodes of psychosis or delirium. The uniqueness of LBD lies in its psychotic symptomatology, particularly during earlier stages of the disease. This feature helps distinguish LBD from both AD and vascular dementia. As seen in the case, LBD can also be confused with acute delirium.
Older adult patients presenting to the ED or clinic with visual hallucinations, delirium, and mental confusion may receive a false diagnosis of schizophrenia, medication- or substance-induced psychosis, Parkinson disease, or delirium of unknown etiology.35 Unfortunately, LBD is often overlooked and not considered in the differential diagnosis. Due to underrecognition, patients may receive treatment with typical antipsychotics. The addition of a neuroleptic to help control the psychotic symptoms causes patients with LBD to develop severe extrapyramidal symptoms and worsening mental status,36 leading to severe parkinsonian signs, which further muddies the diagnostic process. In addition, treatment for suspected Parkinson disease, including carbidopa-levodopa, has no benefit for patients with LBD and may increase psychotic symptoms.37
First-line treatment for LBD includes psychoeducation for the patient and family, cholinesterase inhibitors (eg, rivastigmine), and avoidance of high-potency antipsychotics, such as haloperidol. Although persistent hallucinations and psychosis remain difficult to treat in LBD, low-dose quetiapine is 1 option. Incorrectly diagnosing and prescribing treatment for another condition exacerbates symptoms in this patient population.
The patient has been experiencing chronic pain for the past few years after a motor vehicle accident. She has seen a physiatrist and another provider, both of whom found no “objective” causes of her chronic pain. They started the patient on sertraline for depression and an analgesic, both of which were ineffective.
The patient likes to exercise at a gym twice a week by doing light cardio (treadmill) exercise and light weightlifting. Lately, however, she has been unable to exercise due to the pain. At this visit, she mentions having low energy, poor sleep, frequent fatigue, and generalized soreness and pain in multiple areas of her body. The PCP recognizes the patient’s presenting symptoms as significant for FM and starts her on pregabalin and hydrotherapy, with positive results.
Continue to: Fibromyalgia skepticism may lead to a Dx of depression
Fibromyalgia skepticism may lead to a Dx of depression
FM, the second most common disorder seen in rheumatologic practice after OA, is estimated to affect approximately 1 in 20 patients (approximately 5 million Americans) in the primary care setting.38,39 The condition has a high female-to-male preponderance (3.4% vs 0.5%).40 While the primary symptom of FM is chronic pain, patients commonly present with fatigue and sleep disturbance.41 Comorbid conditions include headaches, irritable bowel syndrome, and mood disturbances (most commonly anxiety and depression).
Several studies have explored reasons for the misdiagnosis and underdiagnosis of FM. One important factor is ongoing skepticism among some physicians and the public, in general, as to whether FM is a real disease. This issue was addressed by a study by White and colleagues,42 who estimated and compared the point prevalence of FM and related disorders in Amish vs non-Amish adults. The authors hypothesized that if litigation and/or compensation availability have a major impact on FM prevalence, then there would be a near zero prevalence of FM in the Amish community. And yet, researchers found an overall age- and sex-adjusted FM prevalence of 7.3% (95% CI; 5.3%-9.7%); this was both statistically greater than zero (P < .0001) and greater than 2 control populations of non-Amish adults (both P < .05).
Many physicians consider FM fundamentally an emotional disturbance, and the high preponderance of FM in female patients may contribute to this misconception as reports of pain and emotional distress by women are often dismissed as hysteria.43 Physicians often explore the emotional aspects of FM, incorrectly diagnosing patients with depression and subsequently treating them with a psychotropic drug.39 Alternatively, they may focus on the musculoskeletal presentations of FM and prescribe analgesics or physical therapy, both of which do little to alleviate FM.
To make the correct diagnosis of FM, the American College of Rheumatology created a specific set of criteria in 1990, which was updated in 2010.44 For a diagnosis of FM, a patient must have at least a 3-month history of bilateral pain above and below the waist and along the axial skeletal spine. Although not included in the updated 2010 criteria, many clinicians continue to check for tender points, following the 1990 criteria requiring the presence of 11 of 18 points to make the diagnosis.
At least 3 cognitive biases relating to FM apply: anchoring, availability, and fundamental attribution error (see table 3).7 Anchoring occurs when the PCP settles on a psychiatric diagnosis of exaggerated pain syndrome, muscle overuse, or OA and fails to explore alternative etiology. Availability bias may obscure the true diagnosis of FM. Since PCPs see many patients with RA or OA, they may overlook or dismiss the possibility of FM. Attribution error happens when physicians dismiss the complaints of patients with FM as merely due to psychological distress, hysteria, or acting out.43
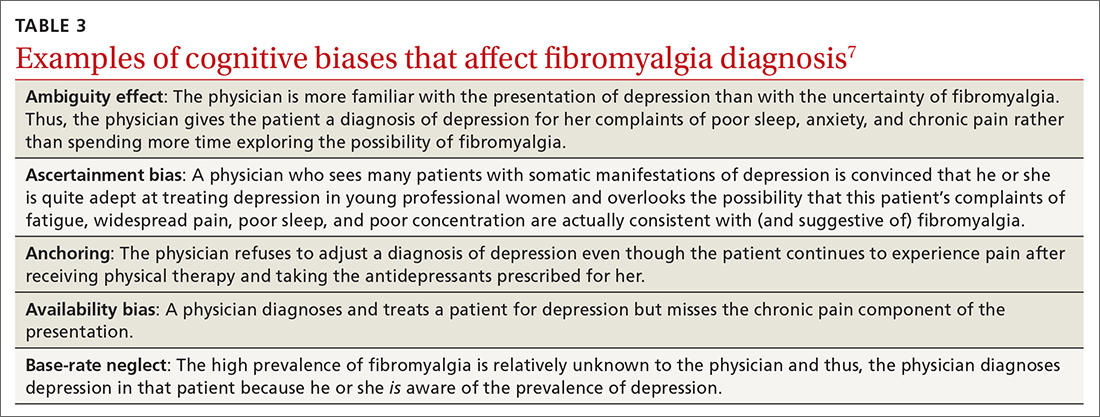
Patients with FM, who are often otherwise healthy, often present multiple times to the same PCP with a chief complaint of chronic pain. These repeat presentations can result in compassion fatigue and impact care. As Aloush and colleagues40 noted in their study, “FM patients were perceived as more difficult than RA patients, with a high level of concern and emotional response. A high proportion of physicians were reluctant to accept them because they feel emotional/psychological difficulties meeting and coping with these patients.”In response, patients with undiagnosed FM or inadequately treated FM may visit other PCPs, which may or may not result in a correct diagnosis and treatment.
We can do better
Primary care physicians face the daunting task of diagnosing and treating a wide range of common conditions while also trying to recognize less-common conditions with atypical presentations—all during a busy clinic workday. Nonetheless, we should strive to overcome internal (eg, cognitive bias and fund-of-knowledge deficits) and external (eg, time constraints, limited resources) pressures to improve diagnostic accuracy and care.
Each of the 4 disease states we’ve discussed have high rates of missed and/or delayed diagnosis. Each presents a unique set of confounders: PMR with its overlapping symptoms of many other rheumatologic diseases; OC with its often vague and misleading GI symptomatology; LBD with overlapping features of AD and Parkinson disease; and FM with skepticism. As gatekeepers to health care, it falls on PCPs to sort out these diagnostic dilemmas to avoid medical errors. Fundamental knowledge of each disease, its unique pathophysiology and symptoms, and varying presentations can be learned, internalized, and subsequently put into clinical practice to improve patient outcomes.
CORRESPONDENCE
Paul D. Rosen MD, Brooklyn Hospital Center, Department of Family Medicine, 121 Dekalb Avenue, Brooklyn, New York 11201; [email protected]
1. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi: 10.1136/bmj.i2139
2. Van Such M, Lohr R, Beckman T, et al. Extent of diagnostic agreement among medical referrals. J Eval Clin Pract. 2017;23:870-874. doi: 10.1111/jep.12747
3. Groopman JE. How Doctors Think. Houghton Mifflin; 2007.
4. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124-1131. doi: 10.1126/science.185.4157.1124
5. Norman GR, Monteiro SD, Sherbino J, et al. The causes of errors in clinical reasoning: Cognitive biases, knowledge deficits, and dual process thinking. Acad Med. 2017;92:23-30. doi: 10.1097/ACM.0000000000001421
6. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780. doi: 10.1097/00001888-200308000-00003
7. Morgenstern J. Cognitive errors in medicine: The common errors. First10EM blog. September 15, 2015. Updated September 22, 2019. Accessed February 8, 2022. https://first10em.com/cognitive-errors/
8. Gazitt T, Zisman D, Gardner G. Polymyalgia rheumatica: a common disease in seniors. Curr Rheumatol Rep. 2020;22:40. doi: 10.1007/s11926-020-00919-2
9. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35. doi: 10.1002/art.23176
10. Doran MF, Crowson CS, O’Fallon WM, et al. Trends in the incidence of polymyalgia rheumatica over a 30 year period in Olmsted County, Minnesota, USA. J Rheumatol. 2002;29:1694-1697.
11. Barraclough K, Liddell WG, du Toit J, et al. Polymyalgia rheumatica in primary care: a cohort study of the diagnostic criteria and outcome. Fam Pract. 2008;25:328-33. doi: 10.1093/fampra/cmn044
12. Manzo C. Polymyalgia rheumatica (PMR) with normal values of both erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) concentration at the time of diagnosis in a centenarian man: a case report. Diseases. 2018;6:84. doi: 10.3390/diseases6040084
13. Crowson CS, Matteson EL. Contemporary prevalence estimates for giant cell arteritis and polymyalgia rheumatica, 2015. Semin Arthritis Rheum. 2017;47:253-256. doi: 10.1016/j.semarthrit.2017.04.001
14. Nordborg E, Bengtsson BA. Death rates and causes of death in 284 consecutive patients with giant cell arteritis confirmed by biopsy. BMJ. 1989;299:549-550. doi: 10.1136/bmj.299.6698.549
15. Bahlas S, Ramos-Remus C, Davis P. Utilisation and costs of investigations, and accuracy of diagnosis of polymyalgia rheumatica by family physicians. Clin Rheumatol. 2000;19:278-280. doi: 10.1007/s100670070045
16. Brooks RC, McGee SR. Diagnostic dilemmas in polymyalgia rheumatica. Arch Intern Med. 1997;157:162-168.
17. Blaauw AA, Schuwirth LW, van der Vleuten CP, et al. Assessing clinical competence: recognition of case descriptions of rheumatic diseases by general practitioners. Br J Rheumatol. 1995;34:375-379. doi:10.1093/rheumatology/34.4.375
18. Mager DR. Polymylagia rheumatica: common disease, elusive diagnosis. Home Healthc Now. 2015;33:132-138. doi:10.1097/NHH.0000000000000199
19. Kermani TA, Warrington KJ. Polymyalgia rheumatica. Lancet. 381;63-72. doi: 10.1016/S0140-6736(12)60680-1. Published correction appears in Lancet. 20135;381:28.
20. Liew DF, Owen CE, Buchanan RR. Prescribing for polymyalgia rheumatica. Aust Prescr. 2018;41:14-19. doi: 10.18773/austprescr.2018.001
21. Ovarian cancer statistics. Centers for Disease Control and Prevention. Reviewed June 8, 2021. Accessed February 22, 2022. www.cdc.gov/cancer/ovarian/statistics/index.htm
22. Key statistics for ovarian cancer. American Cancer Society. Revised January 12, 2022. Accessed February 22, 2022. www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html
23. Survival rates for ovarian cancer. American Cancer Society. Revised January 25, 2021. Accessed February 22, 2022. www.cancer.org/cancer/ovarian-cancer/detection-diagnosis-staging/survival-rates.html
24. Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14:9-32. doi: 10.20892/j.issn.2095-3941.2016.0084
25. Goff BA, Mandel LS, Melancon CH, et al. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705-2712. doi: 10.1001/jama.291.22.2705
26. Olson SH, Mignone L, Nakraseive C, et al. Symptoms of ovarian cancer. Obstet Gynecol. 2001;98:212-217. doi: 10.1016/s0029-7844(01)01457-0
27. Allgar VL, Neal RD. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS patients: Cancer. Br J Cancer. 2005;92:1959-1970. doi: 10.1038/sj.bjc.6602587
28. Gajjar K, Ogden G, Mujahid MI, et al. Symptoms and risk factors of ovarian cancer: a survey in primary care. Obstet Gynecol. 2012:754197. doi: 10.5402/2012/754197
29. Austoker J. Diagnosis of ovarian cancer in primary care. BMJ. 2009;339:b3286. doi: 10.1136/bmj.b3286
30. Goff BA, Mandel L, Muntz HG, et al. Ovarian carcinoma diagnosis: results of a national ovarian cancer survey. Cancer. 2000;89:2068-2075. doi: 10.1002/1097-0142(20001115)89:10<2068::aid-cncr6>3.0.co;2-z
31. Smith EM, Anderson B. The effects of symptoms and delay in seeking diagnosis on stage of disease at diagnosis among women with cancers of the ovary. Cancer. 1985;56:2727-2732. doi: 10.1002/1097-0142(19851201)56:11<2727::aid-cncr2820561138>3.0.co;2-8
32. Galvin JE, Duda JE, Kaufer DI, et al. Lewy body dementia: the caregiver experience of clinical care. Parkinsonism Relat Disord. 2010;16:388-392. doi: 10.1016/j.parkreldis.2010.03.007
33. McKeith I. Dementia with Lewy bodies. Dialogues Clin Neurosci. 2004;6:333-341. doi: 10.31887/DCNS.2004.6.3/imckeith
34. Mrak RE, Griffin WS. Dementia with Lewy bodies: definitions, diagnosis, and pathogenic relationship to Alzheimer’s disease. Neuropsychiatr Dis Treat. 2007;3:619-625.
35. Khotianov N, Singh R, Singh S. Lewy body dementia: case report and discussion. J Am Board Fam Pract. 2002;15:50-54.
36. Stinton C, McKeith I, Taylor JP, et al. Pharmacological management of Lewy body dementia: a systematic review and meta-analysis. Am J Psychiatry. 2015;172:731-742. doi: 10.1176/appi.ajp.2015.14121582
37. Velayudhan L, Ffytche D, Ballard C, et al. New therapeutic strategies of Lewy body dementias. Curr Neurol Neurosci Rep. 2017;17:68. doi: 10.1007/s11910-017-0778-2
38. Arnold LM, Clauw DJ, McCarberg BH; FibroCollaborative. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin Proc. 2011;86:457-464. doi: 10.4065/mcp.2010.0738
39. Arnold LM, Gebke KB, Choy EHS. Fibromyalgia: management strategies for primary care providers. Int J Clin Pract. 2016;70:99-112. doi: 10.1111/ijcp.12757
40. Aloush V, Niv D, Ablin JN, et al. Good pain, bad pain: illness perception and physician attitudes towards rheumatoid arthritis and fibromyalgia patients. Clin Exp Rheumatol. 2021;39(suppl 130):54-60.
41. Vincent A, Lahr BD, Wolfe F, et al. Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res (Hoboken). 2013;65:786-792. doi: 10.1002/acr.21896
42. White KP, Thompson J. Fibromyalgia syndrome in an Amish community: a controlled study to determine disease and symptom prevalence. J Rheumatol. 2003;30:1835-1840.
43. Lobo CP, Pfalzgraf AR, Giannetti V, et al. Impact of invalidation and trust in physicians on health outcomes in fibromyalgia patients. Prim Care Companion CNS Disord. 2014;16:10.4088/PCC.14m01664. doi: 10.4088/PCC.14m01664
44. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62:600-610. doi:10.1002/acr.20140
Medical errors in all settings contributed to as many as 250,000 deaths per year in the United States between 2000 and 2008, according to a 2016 study.1 Diagnostic error, in particular, remains a leading cause of morbidity and mortality in the United States and worldwide. In 2017, 12 million patients (roughly 5% of all US adults) who sought outpatient care experienced missed, delayed, or incorrect diagnosis at least once.2
In his classic work, How Doctors Think, Jerome Groopman, MD, explored the diagnostic process with a focus on the role of cognitive bias in clinical decision-making. Groopman examined how physicians can become sidetracked in their thinking and “blinded” to potential alternative diagnoses.3 Medical error is not necessarily because of a deficiency in medical knowledge; rather, physicians become susceptible to medical error when defective and faulty reasoning distort their diagnostic ability.4
Cognitive bias in the diagnostic process has been extensively studied, and a full review is beyond the scope of this article.5 However, here we will examine pathways leading to diagnostic errors in the primary care setting, specifically the role of cognitive bias in the work-up of polymyalgia rheumatica (PMR), ovarian cancer (OC), Lewy body dementia (LBD), and fibromyalgia (FM). As these 4 disease states are seen with low-to-moderate frequency in primary care, cognitive bias can complicate accurate diagnosis. But first, a word about how to understand clinical reasoning.
There are 2 types of reasoning (and 1 is more prone to error)
Physician clinical reasoning can be divided into 2 different cognitive approaches.
Type 1 reasoning employs intuition and heuristics; this type is automatic, reflexive, and quick.5 While the use of mental shortcuts in type 1 increases the speed with which decisions are made, it also makes this form of reasoning more prone to error.
Type 2 reasoning requires conscious effort. It is goal directed and rigorous and therefore slower than type 1 reasoning. Extrapolated to the clinical context, clinicians transition from type 2 to type 1 reasoning as they gain experience and training throughout their careers and develop their own conscious and subconscious heuristics. Deviations from accurate decision-making occur in a systematic manner due to cognitive biases and result in medical error.6table 17 lists common types of cognitive bias.

An important question to ask. Physicians tend to fall into a pattern of quick, type 1 reasoning. However, it’s important to strive to maintain a broad differential diagnosis and avoid premature closure of the diagnostic process. It’s critical that we consider alternative diagnoses (ie, consciously move from type 1 to type 2 thinking) and continue to ask ourselves, “What else?” while working through differential diagnoses. This can be a powerful debiasing technique.
Continue to: The discussion...
The discussion of the following 4 disease states demonstrates how cognitive bias can lead to diagnostic error.
The patient is barely able to ambulate and appears to be in considerable pain. She is relying heavily on her walker and is assisted by her granddaughter. The primary care physician (PCP) obtains a detailed history that includes chronic shoulder and hip pain. Given that the patient has not responded to NSAID treatment over the previous 6 months, the PCP takes a moment to reconsider the diagnosis of OA and considers other options.
In light of the high prevalence of PMR in older women, the physician pursues a more specific physical examination tailored to ferret out PMR. He had learned this diagnostic shortcut as a resident, remembered it, and adeptly applied it whenever circumstances warranted. He asks the patient to raise her arms above her head (goalpost sign). She is unable to perform this task and experiences severe bilateral shoulder pain on trial. The PCP then places the patient on the examining table and attempts to assist her in rolling toward him. The patient is also unable to perform this maneuver and experiences significant bilateral hip pain on trial.
Based primarily on the patient’s history and physical exam findings, the PCP makes a presumptive diagnosis of PMR vs OA vs combined PMR with OA, orders an erythrocyte sedimentation rate (ESR) and basic rheumatologic
PMR can be mistaken for OA
PMR is the most common inflammatory rheumatic disease in older patients.8 It is a debilitating illness with simple, effective treatment but has devastating consequences if missed or left untreated.9 PMR typically manifests in patients older than age 50, with a peak incidence at 80 years of age. It is also far more common in women.10
Approximately 80% of patients with PMR initially present to their PCP, often posing a diagnostic challenge to many clinicians.11 Due to overlap in symptoms, the condition is often misdiagnosed as OA, a more common condition seen by PCPs. Also, there are no specific diagnostic tests for PMR. An elevated ESR can help confirm the diagnosis, but one-third of patients with PMR have a normal ESR.12 Therefore, the diagnostic conundrum the physician faces is OA vs rheumatoid arthritis (RA), PMR, or another condition.
Continue to: The consequences...
The consequences of a missed and delayed PMR diagnosis range from seriously impaired quality of life to significantly increased risk of vascular events (eg, blindness, stroke) due to temporal arteritis.13 Early diagnosis is even more critical as the risk of a vascular event and death is highest during initial phases of the disease course.14
FPs often miss this Dx. A timely diagnosis relies almost exclusively on an accurate, thorough history and physical exam. However, PCPs often struggle to correctly diagnose PMR. According to a study by Bahlas and colleagues,15 the accuracy rate for correctly diagnosing PMR was 24% among a cohort of family physicians.
The differential diagnosis for PMR is broad and includes seronegative spondyloarthropathies, malignancy, Lyme disease, hypothyroidism, and both RA and OA.16
PCPs are extremely adept at correctly diagnosing RA, but not PMR. A study by Blaauw and colleagues17 comparing PCPs and rheumatologists found PCPs correctly identified 92% of RA cases but only 55% of PMR cases. When rheumatologists reviewed these same cases, they correctly identified PMR and RA almost 100% of the time.17 The difference in diagnostic accuracy between rheumatologists and PCPs suggests limited experience and gaps in fund of knowledge.
Making the diagnosis. The diagnosis of PMR is often made on empiric response to corticosteroid treatment, but doing so based solely on a patient’s response is controversial.18 There are rare instances in which patients with PMR fail to respond to treatment. On the other hand, some inflammatory conditions that mimic or share symptoms with PMR also respond to corticosteroids, potentially resulting in erroneous confirmation bias.
Some classification criteria use rapid response to low-dose prednisone/prednisolone (≤ 20 mg) to confirm the diagnosis,19 while other more recent guidelines no longer include this approach.20 If PMR continues to be suspected after a trial of steroids is unsuccessful, the PCP can try another course of higher dose steroids or consult with Rheumatology.
Continue to: A full history...
A full history and physical exam revealed a myriad of gastrointestinal (GI) complaints, such as diarrhea. But the PCP recalled a recent roundtable discussion on debiasing techniques specifically related to gynecologic disorders, including OC. Therefore, he decided to include OC in the differential diagnosis—something he would not routinely have done given the preponderance of GI symptoms. Despite the patient’s reluctance and time constraints, the PCP ordered a transvaginal ultrasound. Findings from the ultrasound study revealed stage II OC, which carries a good prognosis. The patient is currently undergoing treatment and was last reported as doing well.
Early signs of ovarian cancer can be chalked up to a “GI issue”
OC is the second most common gynecologic cancer21 and the fifth leading cause of cancer-related death22 in US women. Compared to other cancers, the prognosis for localized early-stage OC is surprisingly good, with a 5-year survival rate approaching 93%.23 However, most disease is detected in later stages, and the 5-year survival rate drops to a low of 29%.24
There remains no established screening protocol for OC. Fewer than a quarter of all cases are diagnosed in stage I, and detection of OC relies heavily on the physician’s ability to decipher vague symptomatology that overlaps with other, more common maladies. This poses an obvious diagnostic challenge and, not surprisingly, a high level of susceptibility to cognitive bias.
More than 90% of patients with OC present with some combination of the following symptoms prior to diagnosis: abdominal (77%), GI (70%), pain (58%), constitutional (50%), urinary (34%), and pelvic (26%).25 The 3 most common isolated symptoms in patients with OC are abdominal bloating, decrease in appetite, and frank abdominal pain.26 Patients with biopsy-confirmed OC experience these symptoms an average of 6 months prior to actual diagnosis.27
Knowledge gaps play a role. Studies assessing the ability of health care providers to identify presenting symptoms of OC reveal specific knowledge gaps. For instance, in a survey by Gajjar and colleagues,28 most PCPs correctly identified bloating as a key symptom of OC; however, they weren’t as good at identifying less common symptoms, such as inability to finish a meal and early satiety. Moreover, survey participants misinterpreted or missed GI symptoms as an important manifestation of early OC disease.28 These specific knowledge gaps combine with physician errors in thinking, further obscuring and extending the diagnostic process. The point prevalence for OC is relatively low, and many PCPs only encounter a few cases during their entire career.29 This low pre-test probability may also fuel the delay in diagnosis.
Watch for these forms of bias. Since nonspecific symptoms of early-stage OC resemble those of other more benign conditions, a form of anchoring error known as multiple alternatives bias can arise. In this scenario, clinicians investigate only 1 potential plausible diagnosis and remain focused on that single, often faulty, conclusion. This persists despite other equally plausible alternatives that arise as the investigation proceeds.28
Affective error may also play a role in missed or delayed diagnosis. For example, a physician would prefer to diagnose and treat a common GI illness than consider OC. Another distortion involves outcome bias wherein the physician gives more significance to benign conditions such as irritable bowel syndrome because they have a more favorable outcome and clear treatment path. Physicians also favor these benign conditions because they encounter them more frequently than OC in the clinic setting. (This is known as availability bias.) Outcome bias and multiple alternatives bias can result in noninvestigation of symptoms and inefficient or improper management, leading to a delay in arriving at the correct diagnosis or anchoring on a plausible but incorrect diagnosis.
Continue to: An incorrect initial diagnostic...
An incorrect initial diagnostic path often triggers a cascade of subsequent errors. The physician orders additional unhelpful and expensive tests in an effort to characterize the suspected GI pathology. This then leads the physician to prematurely terminate the work-up and accept the most favored diagnosis. Lastly, sunk-cost fallacy comes into play: The physician has “invested” time and energy investigating a particular diagnosis and rather than abandon the presumed diagnosis, continues to put more time and effort in going down an incorrect diagnostic path.
A series of failures. These biases and miscues have been observed in several studies. For example, a survey of 1725 women by Goff and colleagues30 sought to identify factors related to delayed OC diagnosis. The authors found that the following factors were significantly associated with a delayed diagnosis: omission of a pelvic exam at initial presentation, a separate exploration of a multitude of collateral symptoms, a failure to order ultrasound/computed tomography/CA-125 test, and a failure to consider age as a factor (especially if the patient was outside the norm).
Responses from the survey also revealed that physicians initially ordered work-ups related to GI etiology and only later considered a pelvic work-up. This suggests that well-known presenting signs and symptoms or a constellation of typical and atypical symptoms of OC often failed to trigger physician recognition. Understandably, patients presenting with menorrhagia or gynecologic complaints are more likely to have OC detected at an earlier stage than patients who present with GI or abdominal signs alone.31 table 27 summarizes some of the cognitive biases seen in the diagnostic path of OC.

While in the hospital, he becomes acutely upset by the hallucinations and is given haloperidol and lorazepam by house staff. In the morning, the patient exhibits severe signs of Parkinson disease that include rigidity and masked facies.
Given the patient’s poor response to haloperidol and continued confusion, the team consulted Neurology and Psychiatry. Gathering a more detailed history from the patient and family, the patient is given a diagnosis of classic LBD. The antipsychotic medications are stopped. The patient and his family receive education about LBD treatment and management, and the patient is discharged to outpatient care.
Psychiatric symptoms can be an early “misdirect” in cases of Lewy body disease
LBD, the second leading neurodegenerative dementia after Alzheimer disease (AD), affects 1.5 million Americans,32 representing about 10% of all dementia cases. LBD and AD overlap in 25% of dementia cases.33 In patients older than 85 years, the prevalence jumps to 5% of the general population and 22% of all cases of dementia.33 Despite its prevalence, a recent study showed that only 6% of PCPs correctly identified LBD as the primary diagnosis when presented with typical case examples.32
Continue to: 3 stages of presentation
3 stages of presentation. Unlike other forms of dementia, LBD typically presents first with psychiatric symptoms, then with cognitive impairment, and last with parkinsonian symptoms. Additionally, rapid eye movement sleep behavior disorder and often subtle elements of nonmemory cognitive impairment distinguish LBD from both AD and vascular dementia.32 The primary cognitive deficit in LBD is not in memory but in attention, executive functioning, and visuospatial ability.34 Only in the later stages of the disease do patients exhibit gradual and progressive memory loss.
Mistaken for many things. When evaluating patients exhibiting signs of dementia, it’s important to include LBD in the differential, with increased suspicion for patients experiencing episodes of psychosis or delirium. The uniqueness of LBD lies in its psychotic symptomatology, particularly during earlier stages of the disease. This feature helps distinguish LBD from both AD and vascular dementia. As seen in the case, LBD can also be confused with acute delirium.
Older adult patients presenting to the ED or clinic with visual hallucinations, delirium, and mental confusion may receive a false diagnosis of schizophrenia, medication- or substance-induced psychosis, Parkinson disease, or delirium of unknown etiology.35 Unfortunately, LBD is often overlooked and not considered in the differential diagnosis. Due to underrecognition, patients may receive treatment with typical antipsychotics. The addition of a neuroleptic to help control the psychotic symptoms causes patients with LBD to develop severe extrapyramidal symptoms and worsening mental status,36 leading to severe parkinsonian signs, which further muddies the diagnostic process. In addition, treatment for suspected Parkinson disease, including carbidopa-levodopa, has no benefit for patients with LBD and may increase psychotic symptoms.37
First-line treatment for LBD includes psychoeducation for the patient and family, cholinesterase inhibitors (eg, rivastigmine), and avoidance of high-potency antipsychotics, such as haloperidol. Although persistent hallucinations and psychosis remain difficult to treat in LBD, low-dose quetiapine is 1 option. Incorrectly diagnosing and prescribing treatment for another condition exacerbates symptoms in this patient population.
The patient has been experiencing chronic pain for the past few years after a motor vehicle accident. She has seen a physiatrist and another provider, both of whom found no “objective” causes of her chronic pain. They started the patient on sertraline for depression and an analgesic, both of which were ineffective.
The patient likes to exercise at a gym twice a week by doing light cardio (treadmill) exercise and light weightlifting. Lately, however, she has been unable to exercise due to the pain. At this visit, she mentions having low energy, poor sleep, frequent fatigue, and generalized soreness and pain in multiple areas of her body. The PCP recognizes the patient’s presenting symptoms as significant for FM and starts her on pregabalin and hydrotherapy, with positive results.
Continue to: Fibromyalgia skepticism may lead to a Dx of depression
Fibromyalgia skepticism may lead to a Dx of depression
FM, the second most common disorder seen in rheumatologic practice after OA, is estimated to affect approximately 1 in 20 patients (approximately 5 million Americans) in the primary care setting.38,39 The condition has a high female-to-male preponderance (3.4% vs 0.5%).40 While the primary symptom of FM is chronic pain, patients commonly present with fatigue and sleep disturbance.41 Comorbid conditions include headaches, irritable bowel syndrome, and mood disturbances (most commonly anxiety and depression).
Several studies have explored reasons for the misdiagnosis and underdiagnosis of FM. One important factor is ongoing skepticism among some physicians and the public, in general, as to whether FM is a real disease. This issue was addressed by a study by White and colleagues,42 who estimated and compared the point prevalence of FM and related disorders in Amish vs non-Amish adults. The authors hypothesized that if litigation and/or compensation availability have a major impact on FM prevalence, then there would be a near zero prevalence of FM in the Amish community. And yet, researchers found an overall age- and sex-adjusted FM prevalence of 7.3% (95% CI; 5.3%-9.7%); this was both statistically greater than zero (P < .0001) and greater than 2 control populations of non-Amish adults (both P < .05).
Many physicians consider FM fundamentally an emotional disturbance, and the high preponderance of FM in female patients may contribute to this misconception as reports of pain and emotional distress by women are often dismissed as hysteria.43 Physicians often explore the emotional aspects of FM, incorrectly diagnosing patients with depression and subsequently treating them with a psychotropic drug.39 Alternatively, they may focus on the musculoskeletal presentations of FM and prescribe analgesics or physical therapy, both of which do little to alleviate FM.
To make the correct diagnosis of FM, the American College of Rheumatology created a specific set of criteria in 1990, which was updated in 2010.44 For a diagnosis of FM, a patient must have at least a 3-month history of bilateral pain above and below the waist and along the axial skeletal spine. Although not included in the updated 2010 criteria, many clinicians continue to check for tender points, following the 1990 criteria requiring the presence of 11 of 18 points to make the diagnosis.
At least 3 cognitive biases relating to FM apply: anchoring, availability, and fundamental attribution error (see table 3).7 Anchoring occurs when the PCP settles on a psychiatric diagnosis of exaggerated pain syndrome, muscle overuse, or OA and fails to explore alternative etiology. Availability bias may obscure the true diagnosis of FM. Since PCPs see many patients with RA or OA, they may overlook or dismiss the possibility of FM. Attribution error happens when physicians dismiss the complaints of patients with FM as merely due to psychological distress, hysteria, or acting out.43

Patients with FM, who are often otherwise healthy, often present multiple times to the same PCP with a chief complaint of chronic pain. These repeat presentations can result in compassion fatigue and impact care. As Aloush and colleagues40 noted in their study, “FM patients were perceived as more difficult than RA patients, with a high level of concern and emotional response. A high proportion of physicians were reluctant to accept them because they feel emotional/psychological difficulties meeting and coping with these patients.”In response, patients with undiagnosed FM or inadequately treated FM may visit other PCPs, which may or may not result in a correct diagnosis and treatment.
We can do better
Primary care physicians face the daunting task of diagnosing and treating a wide range of common conditions while also trying to recognize less-common conditions with atypical presentations—all during a busy clinic workday. Nonetheless, we should strive to overcome internal (eg, cognitive bias and fund-of-knowledge deficits) and external (eg, time constraints, limited resources) pressures to improve diagnostic accuracy and care.
Each of the 4 disease states we’ve discussed have high rates of missed and/or delayed diagnosis. Each presents a unique set of confounders: PMR with its overlapping symptoms of many other rheumatologic diseases; OC with its often vague and misleading GI symptomatology; LBD with overlapping features of AD and Parkinson disease; and FM with skepticism. As gatekeepers to health care, it falls on PCPs to sort out these diagnostic dilemmas to avoid medical errors. Fundamental knowledge of each disease, its unique pathophysiology and symptoms, and varying presentations can be learned, internalized, and subsequently put into clinical practice to improve patient outcomes.
CORRESPONDENCE
Paul D. Rosen MD, Brooklyn Hospital Center, Department of Family Medicine, 121 Dekalb Avenue, Brooklyn, New York 11201; [email protected]
Medical errors in all settings contributed to as many as 250,000 deaths per year in the United States between 2000 and 2008, according to a 2016 study.1 Diagnostic error, in particular, remains a leading cause of morbidity and mortality in the United States and worldwide. In 2017, 12 million patients (roughly 5% of all US adults) who sought outpatient care experienced missed, delayed, or incorrect diagnosis at least once.2
In his classic work, How Doctors Think, Jerome Groopman, MD, explored the diagnostic process with a focus on the role of cognitive bias in clinical decision-making. Groopman examined how physicians can become sidetracked in their thinking and “blinded” to potential alternative diagnoses.3 Medical error is not necessarily because of a deficiency in medical knowledge; rather, physicians become susceptible to medical error when defective and faulty reasoning distort their diagnostic ability.4
Cognitive bias in the diagnostic process has been extensively studied, and a full review is beyond the scope of this article.5 However, here we will examine pathways leading to diagnostic errors in the primary care setting, specifically the role of cognitive bias in the work-up of polymyalgia rheumatica (PMR), ovarian cancer (OC), Lewy body dementia (LBD), and fibromyalgia (FM). As these 4 disease states are seen with low-to-moderate frequency in primary care, cognitive bias can complicate accurate diagnosis. But first, a word about how to understand clinical reasoning.
There are 2 types of reasoning (and 1 is more prone to error)
Physician clinical reasoning can be divided into 2 different cognitive approaches.
Type 1 reasoning employs intuition and heuristics; this type is automatic, reflexive, and quick.5 While the use of mental shortcuts in type 1 increases the speed with which decisions are made, it also makes this form of reasoning more prone to error.
Type 2 reasoning requires conscious effort. It is goal directed and rigorous and therefore slower than type 1 reasoning. Extrapolated to the clinical context, clinicians transition from type 2 to type 1 reasoning as they gain experience and training throughout their careers and develop their own conscious and subconscious heuristics. Deviations from accurate decision-making occur in a systematic manner due to cognitive biases and result in medical error.6table 17 lists common types of cognitive bias.

An important question to ask. Physicians tend to fall into a pattern of quick, type 1 reasoning. However, it’s important to strive to maintain a broad differential diagnosis and avoid premature closure of the diagnostic process. It’s critical that we consider alternative diagnoses (ie, consciously move from type 1 to type 2 thinking) and continue to ask ourselves, “What else?” while working through differential diagnoses. This can be a powerful debiasing technique.
Continue to: The discussion...
The discussion of the following 4 disease states demonstrates how cognitive bias can lead to diagnostic error.
The patient is barely able to ambulate and appears to be in considerable pain. She is relying heavily on her walker and is assisted by her granddaughter. The primary care physician (PCP) obtains a detailed history that includes chronic shoulder and hip pain. Given that the patient has not responded to NSAID treatment over the previous 6 months, the PCP takes a moment to reconsider the diagnosis of OA and considers other options.
In light of the high prevalence of PMR in older women, the physician pursues a more specific physical examination tailored to ferret out PMR. He had learned this diagnostic shortcut as a resident, remembered it, and adeptly applied it whenever circumstances warranted. He asks the patient to raise her arms above her head (goalpost sign). She is unable to perform this task and experiences severe bilateral shoulder pain on trial. The PCP then places the patient on the examining table and attempts to assist her in rolling toward him. The patient is also unable to perform this maneuver and experiences significant bilateral hip pain on trial.
Based primarily on the patient’s history and physical exam findings, the PCP makes a presumptive diagnosis of PMR vs OA vs combined PMR with OA, orders an erythrocyte sedimentation rate (ESR) and basic rheumatologic
PMR can be mistaken for OA
PMR is the most common inflammatory rheumatic disease in older patients.8 It is a debilitating illness with simple, effective treatment but has devastating consequences if missed or left untreated.9 PMR typically manifests in patients older than age 50, with a peak incidence at 80 years of age. It is also far more common in women.10
Approximately 80% of patients with PMR initially present to their PCP, often posing a diagnostic challenge to many clinicians.11 Due to overlap in symptoms, the condition is often misdiagnosed as OA, a more common condition seen by PCPs. Also, there are no specific diagnostic tests for PMR. An elevated ESR can help confirm the diagnosis, but one-third of patients with PMR have a normal ESR.12 Therefore, the diagnostic conundrum the physician faces is OA vs rheumatoid arthritis (RA), PMR, or another condition.
Continue to: The consequences...
The consequences of a missed and delayed PMR diagnosis range from seriously impaired quality of life to significantly increased risk of vascular events (eg, blindness, stroke) due to temporal arteritis.13 Early diagnosis is even more critical as the risk of a vascular event and death is highest during initial phases of the disease course.14
FPs often miss this Dx. A timely diagnosis relies almost exclusively on an accurate, thorough history and physical exam. However, PCPs often struggle to correctly diagnose PMR. According to a study by Bahlas and colleagues,15 the accuracy rate for correctly diagnosing PMR was 24% among a cohort of family physicians.
The differential diagnosis for PMR is broad and includes seronegative spondyloarthropathies, malignancy, Lyme disease, hypothyroidism, and both RA and OA.16
PCPs are extremely adept at correctly diagnosing RA, but not PMR. A study by Blaauw and colleagues17 comparing PCPs and rheumatologists found PCPs correctly identified 92% of RA cases but only 55% of PMR cases. When rheumatologists reviewed these same cases, they correctly identified PMR and RA almost 100% of the time.17 The difference in diagnostic accuracy between rheumatologists and PCPs suggests limited experience and gaps in fund of knowledge.
Making the diagnosis. The diagnosis of PMR is often made on empiric response to corticosteroid treatment, but doing so based solely on a patient’s response is controversial.18 There are rare instances in which patients with PMR fail to respond to treatment. On the other hand, some inflammatory conditions that mimic or share symptoms with PMR also respond to corticosteroids, potentially resulting in erroneous confirmation bias.
Some classification criteria use rapid response to low-dose prednisone/prednisolone (≤ 20 mg) to confirm the diagnosis,19 while other more recent guidelines no longer include this approach.20 If PMR continues to be suspected after a trial of steroids is unsuccessful, the PCP can try another course of higher dose steroids or consult with Rheumatology.
Continue to: A full history...
A full history and physical exam revealed a myriad of gastrointestinal (GI) complaints, such as diarrhea. But the PCP recalled a recent roundtable discussion on debiasing techniques specifically related to gynecologic disorders, including OC. Therefore, he decided to include OC in the differential diagnosis—something he would not routinely have done given the preponderance of GI symptoms. Despite the patient’s reluctance and time constraints, the PCP ordered a transvaginal ultrasound. Findings from the ultrasound study revealed stage II OC, which carries a good prognosis. The patient is currently undergoing treatment and was last reported as doing well.
Early signs of ovarian cancer can be chalked up to a “GI issue”
OC is the second most common gynecologic cancer21 and the fifth leading cause of cancer-related death22 in US women. Compared to other cancers, the prognosis for localized early-stage OC is surprisingly good, with a 5-year survival rate approaching 93%.23 However, most disease is detected in later stages, and the 5-year survival rate drops to a low of 29%.24
There remains no established screening protocol for OC. Fewer than a quarter of all cases are diagnosed in stage I, and detection of OC relies heavily on the physician’s ability to decipher vague symptomatology that overlaps with other, more common maladies. This poses an obvious diagnostic challenge and, not surprisingly, a high level of susceptibility to cognitive bias.
More than 90% of patients with OC present with some combination of the following symptoms prior to diagnosis: abdominal (77%), GI (70%), pain (58%), constitutional (50%), urinary (34%), and pelvic (26%).25 The 3 most common isolated symptoms in patients with OC are abdominal bloating, decrease in appetite, and frank abdominal pain.26 Patients with biopsy-confirmed OC experience these symptoms an average of 6 months prior to actual diagnosis.27
Knowledge gaps play a role. Studies assessing the ability of health care providers to identify presenting symptoms of OC reveal specific knowledge gaps. For instance, in a survey by Gajjar and colleagues,28 most PCPs correctly identified bloating as a key symptom of OC; however, they weren’t as good at identifying less common symptoms, such as inability to finish a meal and early satiety. Moreover, survey participants misinterpreted or missed GI symptoms as an important manifestation of early OC disease.28 These specific knowledge gaps combine with physician errors in thinking, further obscuring and extending the diagnostic process. The point prevalence for OC is relatively low, and many PCPs only encounter a few cases during their entire career.29 This low pre-test probability may also fuel the delay in diagnosis.
Watch for these forms of bias. Since nonspecific symptoms of early-stage OC resemble those of other more benign conditions, a form of anchoring error known as multiple alternatives bias can arise. In this scenario, clinicians investigate only 1 potential plausible diagnosis and remain focused on that single, often faulty, conclusion. This persists despite other equally plausible alternatives that arise as the investigation proceeds.28
Affective error may also play a role in missed or delayed diagnosis. For example, a physician would prefer to diagnose and treat a common GI illness than consider OC. Another distortion involves outcome bias wherein the physician gives more significance to benign conditions such as irritable bowel syndrome because they have a more favorable outcome and clear treatment path. Physicians also favor these benign conditions because they encounter them more frequently than OC in the clinic setting. (This is known as availability bias.) Outcome bias and multiple alternatives bias can result in noninvestigation of symptoms and inefficient or improper management, leading to a delay in arriving at the correct diagnosis or anchoring on a plausible but incorrect diagnosis.
Continue to: An incorrect initial diagnostic...
An incorrect initial diagnostic path often triggers a cascade of subsequent errors. The physician orders additional unhelpful and expensive tests in an effort to characterize the suspected GI pathology. This then leads the physician to prematurely terminate the work-up and accept the most favored diagnosis. Lastly, sunk-cost fallacy comes into play: The physician has “invested” time and energy investigating a particular diagnosis and rather than abandon the presumed diagnosis, continues to put more time and effort in going down an incorrect diagnostic path.
A series of failures. These biases and miscues have been observed in several studies. For example, a survey of 1725 women by Goff and colleagues30 sought to identify factors related to delayed OC diagnosis. The authors found that the following factors were significantly associated with a delayed diagnosis: omission of a pelvic exam at initial presentation, a separate exploration of a multitude of collateral symptoms, a failure to order ultrasound/computed tomography/CA-125 test, and a failure to consider age as a factor (especially if the patient was outside the norm).
Responses from the survey also revealed that physicians initially ordered work-ups related to GI etiology and only later considered a pelvic work-up. This suggests that well-known presenting signs and symptoms or a constellation of typical and atypical symptoms of OC often failed to trigger physician recognition. Understandably, patients presenting with menorrhagia or gynecologic complaints are more likely to have OC detected at an earlier stage than patients who present with GI or abdominal signs alone.31 table 27 summarizes some of the cognitive biases seen in the diagnostic path of OC.

While in the hospital, he becomes acutely upset by the hallucinations and is given haloperidol and lorazepam by house staff. In the morning, the patient exhibits severe signs of Parkinson disease that include rigidity and masked facies.
Given the patient’s poor response to haloperidol and continued confusion, the team consulted Neurology and Psychiatry. Gathering a more detailed history from the patient and family, the patient is given a diagnosis of classic LBD. The antipsychotic medications are stopped. The patient and his family receive education about LBD treatment and management, and the patient is discharged to outpatient care.
Psychiatric symptoms can be an early “misdirect” in cases of Lewy body disease
LBD, the second leading neurodegenerative dementia after Alzheimer disease (AD), affects 1.5 million Americans,32 representing about 10% of all dementia cases. LBD and AD overlap in 25% of dementia cases.33 In patients older than 85 years, the prevalence jumps to 5% of the general population and 22% of all cases of dementia.33 Despite its prevalence, a recent study showed that only 6% of PCPs correctly identified LBD as the primary diagnosis when presented with typical case examples.32
Continue to: 3 stages of presentation
3 stages of presentation. Unlike other forms of dementia, LBD typically presents first with psychiatric symptoms, then with cognitive impairment, and last with parkinsonian symptoms. Additionally, rapid eye movement sleep behavior disorder and often subtle elements of nonmemory cognitive impairment distinguish LBD from both AD and vascular dementia.32 The primary cognitive deficit in LBD is not in memory but in attention, executive functioning, and visuospatial ability.34 Only in the later stages of the disease do patients exhibit gradual and progressive memory loss.
Mistaken for many things. When evaluating patients exhibiting signs of dementia, it’s important to include LBD in the differential, with increased suspicion for patients experiencing episodes of psychosis or delirium. The uniqueness of LBD lies in its psychotic symptomatology, particularly during earlier stages of the disease. This feature helps distinguish LBD from both AD and vascular dementia. As seen in the case, LBD can also be confused with acute delirium.
Older adult patients presenting to the ED or clinic with visual hallucinations, delirium, and mental confusion may receive a false diagnosis of schizophrenia, medication- or substance-induced psychosis, Parkinson disease, or delirium of unknown etiology.35 Unfortunately, LBD is often overlooked and not considered in the differential diagnosis. Due to underrecognition, patients may receive treatment with typical antipsychotics. The addition of a neuroleptic to help control the psychotic symptoms causes patients with LBD to develop severe extrapyramidal symptoms and worsening mental status,36 leading to severe parkinsonian signs, which further muddies the diagnostic process. In addition, treatment for suspected Parkinson disease, including carbidopa-levodopa, has no benefit for patients with LBD and may increase psychotic symptoms.37
First-line treatment for LBD includes psychoeducation for the patient and family, cholinesterase inhibitors (eg, rivastigmine), and avoidance of high-potency antipsychotics, such as haloperidol. Although persistent hallucinations and psychosis remain difficult to treat in LBD, low-dose quetiapine is 1 option. Incorrectly diagnosing and prescribing treatment for another condition exacerbates symptoms in this patient population.
The patient has been experiencing chronic pain for the past few years after a motor vehicle accident. She has seen a physiatrist and another provider, both of whom found no “objective” causes of her chronic pain. They started the patient on sertraline for depression and an analgesic, both of which were ineffective.
The patient likes to exercise at a gym twice a week by doing light cardio (treadmill) exercise and light weightlifting. Lately, however, she has been unable to exercise due to the pain. At this visit, she mentions having low energy, poor sleep, frequent fatigue, and generalized soreness and pain in multiple areas of her body. The PCP recognizes the patient’s presenting symptoms as significant for FM and starts her on pregabalin and hydrotherapy, with positive results.
Continue to: Fibromyalgia skepticism may lead to a Dx of depression
Fibromyalgia skepticism may lead to a Dx of depression
FM, the second most common disorder seen in rheumatologic practice after OA, is estimated to affect approximately 1 in 20 patients (approximately 5 million Americans) in the primary care setting.38,39 The condition has a high female-to-male preponderance (3.4% vs 0.5%).40 While the primary symptom of FM is chronic pain, patients commonly present with fatigue and sleep disturbance.41 Comorbid conditions include headaches, irritable bowel syndrome, and mood disturbances (most commonly anxiety and depression).
Several studies have explored reasons for the misdiagnosis and underdiagnosis of FM. One important factor is ongoing skepticism among some physicians and the public, in general, as to whether FM is a real disease. This issue was addressed by a study by White and colleagues,42 who estimated and compared the point prevalence of FM and related disorders in Amish vs non-Amish adults. The authors hypothesized that if litigation and/or compensation availability have a major impact on FM prevalence, then there would be a near zero prevalence of FM in the Amish community. And yet, researchers found an overall age- and sex-adjusted FM prevalence of 7.3% (95% CI; 5.3%-9.7%); this was both statistically greater than zero (P < .0001) and greater than 2 control populations of non-Amish adults (both P < .05).
Many physicians consider FM fundamentally an emotional disturbance, and the high preponderance of FM in female patients may contribute to this misconception as reports of pain and emotional distress by women are often dismissed as hysteria.43 Physicians often explore the emotional aspects of FM, incorrectly diagnosing patients with depression and subsequently treating them with a psychotropic drug.39 Alternatively, they may focus on the musculoskeletal presentations of FM and prescribe analgesics or physical therapy, both of which do little to alleviate FM.
To make the correct diagnosis of FM, the American College of Rheumatology created a specific set of criteria in 1990, which was updated in 2010.44 For a diagnosis of FM, a patient must have at least a 3-month history of bilateral pain above and below the waist and along the axial skeletal spine. Although not included in the updated 2010 criteria, many clinicians continue to check for tender points, following the 1990 criteria requiring the presence of 11 of 18 points to make the diagnosis.
At least 3 cognitive biases relating to FM apply: anchoring, availability, and fundamental attribution error (see table 3).7 Anchoring occurs when the PCP settles on a psychiatric diagnosis of exaggerated pain syndrome, muscle overuse, or OA and fails to explore alternative etiology. Availability bias may obscure the true diagnosis of FM. Since PCPs see many patients with RA or OA, they may overlook or dismiss the possibility of FM. Attribution error happens when physicians dismiss the complaints of patients with FM as merely due to psychological distress, hysteria, or acting out.43

Patients with FM, who are often otherwise healthy, often present multiple times to the same PCP with a chief complaint of chronic pain. These repeat presentations can result in compassion fatigue and impact care. As Aloush and colleagues40 noted in their study, “FM patients were perceived as more difficult than RA patients, with a high level of concern and emotional response. A high proportion of physicians were reluctant to accept them because they feel emotional/psychological difficulties meeting and coping with these patients.”In response, patients with undiagnosed FM or inadequately treated FM may visit other PCPs, which may or may not result in a correct diagnosis and treatment.
We can do better
Primary care physicians face the daunting task of diagnosing and treating a wide range of common conditions while also trying to recognize less-common conditions with atypical presentations—all during a busy clinic workday. Nonetheless, we should strive to overcome internal (eg, cognitive bias and fund-of-knowledge deficits) and external (eg, time constraints, limited resources) pressures to improve diagnostic accuracy and care.
Each of the 4 disease states we’ve discussed have high rates of missed and/or delayed diagnosis. Each presents a unique set of confounders: PMR with its overlapping symptoms of many other rheumatologic diseases; OC with its often vague and misleading GI symptomatology; LBD with overlapping features of AD and Parkinson disease; and FM with skepticism. As gatekeepers to health care, it falls on PCPs to sort out these diagnostic dilemmas to avoid medical errors. Fundamental knowledge of each disease, its unique pathophysiology and symptoms, and varying presentations can be learned, internalized, and subsequently put into clinical practice to improve patient outcomes.
CORRESPONDENCE
Paul D. Rosen MD, Brooklyn Hospital Center, Department of Family Medicine, 121 Dekalb Avenue, Brooklyn, New York 11201; [email protected]
1. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi: 10.1136/bmj.i2139
2. Van Such M, Lohr R, Beckman T, et al. Extent of diagnostic agreement among medical referrals. J Eval Clin Pract. 2017;23:870-874. doi: 10.1111/jep.12747
3. Groopman JE. How Doctors Think. Houghton Mifflin; 2007.
4. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124-1131. doi: 10.1126/science.185.4157.1124
5. Norman GR, Monteiro SD, Sherbino J, et al. The causes of errors in clinical reasoning: Cognitive biases, knowledge deficits, and dual process thinking. Acad Med. 2017;92:23-30. doi: 10.1097/ACM.0000000000001421
6. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780. doi: 10.1097/00001888-200308000-00003
7. Morgenstern J. Cognitive errors in medicine: The common errors. First10EM blog. September 15, 2015. Updated September 22, 2019. Accessed February 8, 2022. https://first10em.com/cognitive-errors/
8. Gazitt T, Zisman D, Gardner G. Polymyalgia rheumatica: a common disease in seniors. Curr Rheumatol Rep. 2020;22:40. doi: 10.1007/s11926-020-00919-2
9. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35. doi: 10.1002/art.23176
10. Doran MF, Crowson CS, O’Fallon WM, et al. Trends in the incidence of polymyalgia rheumatica over a 30 year period in Olmsted County, Minnesota, USA. J Rheumatol. 2002;29:1694-1697.
11. Barraclough K, Liddell WG, du Toit J, et al. Polymyalgia rheumatica in primary care: a cohort study of the diagnostic criteria and outcome. Fam Pract. 2008;25:328-33. doi: 10.1093/fampra/cmn044
12. Manzo C. Polymyalgia rheumatica (PMR) with normal values of both erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) concentration at the time of diagnosis in a centenarian man: a case report. Diseases. 2018;6:84. doi: 10.3390/diseases6040084
13. Crowson CS, Matteson EL. Contemporary prevalence estimates for giant cell arteritis and polymyalgia rheumatica, 2015. Semin Arthritis Rheum. 2017;47:253-256. doi: 10.1016/j.semarthrit.2017.04.001
14. Nordborg E, Bengtsson BA. Death rates and causes of death in 284 consecutive patients with giant cell arteritis confirmed by biopsy. BMJ. 1989;299:549-550. doi: 10.1136/bmj.299.6698.549
15. Bahlas S, Ramos-Remus C, Davis P. Utilisation and costs of investigations, and accuracy of diagnosis of polymyalgia rheumatica by family physicians. Clin Rheumatol. 2000;19:278-280. doi: 10.1007/s100670070045
16. Brooks RC, McGee SR. Diagnostic dilemmas in polymyalgia rheumatica. Arch Intern Med. 1997;157:162-168.
17. Blaauw AA, Schuwirth LW, van der Vleuten CP, et al. Assessing clinical competence: recognition of case descriptions of rheumatic diseases by general practitioners. Br J Rheumatol. 1995;34:375-379. doi:10.1093/rheumatology/34.4.375
18. Mager DR. Polymylagia rheumatica: common disease, elusive diagnosis. Home Healthc Now. 2015;33:132-138. doi:10.1097/NHH.0000000000000199
19. Kermani TA, Warrington KJ. Polymyalgia rheumatica. Lancet. 381;63-72. doi: 10.1016/S0140-6736(12)60680-1. Published correction appears in Lancet. 20135;381:28.
20. Liew DF, Owen CE, Buchanan RR. Prescribing for polymyalgia rheumatica. Aust Prescr. 2018;41:14-19. doi: 10.18773/austprescr.2018.001
21. Ovarian cancer statistics. Centers for Disease Control and Prevention. Reviewed June 8, 2021. Accessed February 22, 2022. www.cdc.gov/cancer/ovarian/statistics/index.htm
22. Key statistics for ovarian cancer. American Cancer Society. Revised January 12, 2022. Accessed February 22, 2022. www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html
23. Survival rates for ovarian cancer. American Cancer Society. Revised January 25, 2021. Accessed February 22, 2022. www.cancer.org/cancer/ovarian-cancer/detection-diagnosis-staging/survival-rates.html
24. Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14:9-32. doi: 10.20892/j.issn.2095-3941.2016.0084
25. Goff BA, Mandel LS, Melancon CH, et al. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705-2712. doi: 10.1001/jama.291.22.2705
26. Olson SH, Mignone L, Nakraseive C, et al. Symptoms of ovarian cancer. Obstet Gynecol. 2001;98:212-217. doi: 10.1016/s0029-7844(01)01457-0
27. Allgar VL, Neal RD. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS patients: Cancer. Br J Cancer. 2005;92:1959-1970. doi: 10.1038/sj.bjc.6602587
28. Gajjar K, Ogden G, Mujahid MI, et al. Symptoms and risk factors of ovarian cancer: a survey in primary care. Obstet Gynecol. 2012:754197. doi: 10.5402/2012/754197
29. Austoker J. Diagnosis of ovarian cancer in primary care. BMJ. 2009;339:b3286. doi: 10.1136/bmj.b3286
30. Goff BA, Mandel L, Muntz HG, et al. Ovarian carcinoma diagnosis: results of a national ovarian cancer survey. Cancer. 2000;89:2068-2075. doi: 10.1002/1097-0142(20001115)89:10<2068::aid-cncr6>3.0.co;2-z
31. Smith EM, Anderson B. The effects of symptoms and delay in seeking diagnosis on stage of disease at diagnosis among women with cancers of the ovary. Cancer. 1985;56:2727-2732. doi: 10.1002/1097-0142(19851201)56:11<2727::aid-cncr2820561138>3.0.co;2-8
32. Galvin JE, Duda JE, Kaufer DI, et al. Lewy body dementia: the caregiver experience of clinical care. Parkinsonism Relat Disord. 2010;16:388-392. doi: 10.1016/j.parkreldis.2010.03.007
33. McKeith I. Dementia with Lewy bodies. Dialogues Clin Neurosci. 2004;6:333-341. doi: 10.31887/DCNS.2004.6.3/imckeith
34. Mrak RE, Griffin WS. Dementia with Lewy bodies: definitions, diagnosis, and pathogenic relationship to Alzheimer’s disease. Neuropsychiatr Dis Treat. 2007;3:619-625.
35. Khotianov N, Singh R, Singh S. Lewy body dementia: case report and discussion. J Am Board Fam Pract. 2002;15:50-54.
36. Stinton C, McKeith I, Taylor JP, et al. Pharmacological management of Lewy body dementia: a systematic review and meta-analysis. Am J Psychiatry. 2015;172:731-742. doi: 10.1176/appi.ajp.2015.14121582
37. Velayudhan L, Ffytche D, Ballard C, et al. New therapeutic strategies of Lewy body dementias. Curr Neurol Neurosci Rep. 2017;17:68. doi: 10.1007/s11910-017-0778-2
38. Arnold LM, Clauw DJ, McCarberg BH; FibroCollaborative. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin Proc. 2011;86:457-464. doi: 10.4065/mcp.2010.0738
39. Arnold LM, Gebke KB, Choy EHS. Fibromyalgia: management strategies for primary care providers. Int J Clin Pract. 2016;70:99-112. doi: 10.1111/ijcp.12757
40. Aloush V, Niv D, Ablin JN, et al. Good pain, bad pain: illness perception and physician attitudes towards rheumatoid arthritis and fibromyalgia patients. Clin Exp Rheumatol. 2021;39(suppl 130):54-60.
41. Vincent A, Lahr BD, Wolfe F, et al. Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res (Hoboken). 2013;65:786-792. doi: 10.1002/acr.21896
42. White KP, Thompson J. Fibromyalgia syndrome in an Amish community: a controlled study to determine disease and symptom prevalence. J Rheumatol. 2003;30:1835-1840.
43. Lobo CP, Pfalzgraf AR, Giannetti V, et al. Impact of invalidation and trust in physicians on health outcomes in fibromyalgia patients. Prim Care Companion CNS Disord. 2014;16:10.4088/PCC.14m01664. doi: 10.4088/PCC.14m01664
44. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62:600-610. doi:10.1002/acr.20140
1. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi: 10.1136/bmj.i2139
2. Van Such M, Lohr R, Beckman T, et al. Extent of diagnostic agreement among medical referrals. J Eval Clin Pract. 2017;23:870-874. doi: 10.1111/jep.12747
3. Groopman JE. How Doctors Think. Houghton Mifflin; 2007.
4. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124-1131. doi: 10.1126/science.185.4157.1124
5. Norman GR, Monteiro SD, Sherbino J, et al. The causes of errors in clinical reasoning: Cognitive biases, knowledge deficits, and dual process thinking. Acad Med. 2017;92:23-30. doi: 10.1097/ACM.0000000000001421
6. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780. doi: 10.1097/00001888-200308000-00003
7. Morgenstern J. Cognitive errors in medicine: The common errors. First10EM blog. September 15, 2015. Updated September 22, 2019. Accessed February 8, 2022. https://first10em.com/cognitive-errors/
8. Gazitt T, Zisman D, Gardner G. Polymyalgia rheumatica: a common disease in seniors. Curr Rheumatol Rep. 2020;22:40. doi: 10.1007/s11926-020-00919-2
9. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35. doi: 10.1002/art.23176
10. Doran MF, Crowson CS, O’Fallon WM, et al. Trends in the incidence of polymyalgia rheumatica over a 30 year period in Olmsted County, Minnesota, USA. J Rheumatol. 2002;29:1694-1697.
11. Barraclough K, Liddell WG, du Toit J, et al. Polymyalgia rheumatica in primary care: a cohort study of the diagnostic criteria and outcome. Fam Pract. 2008;25:328-33. doi: 10.1093/fampra/cmn044
12. Manzo C. Polymyalgia rheumatica (PMR) with normal values of both erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) concentration at the time of diagnosis in a centenarian man: a case report. Diseases. 2018;6:84. doi: 10.3390/diseases6040084
13. Crowson CS, Matteson EL. Contemporary prevalence estimates for giant cell arteritis and polymyalgia rheumatica, 2015. Semin Arthritis Rheum. 2017;47:253-256. doi: 10.1016/j.semarthrit.2017.04.001
14. Nordborg E, Bengtsson BA. Death rates and causes of death in 284 consecutive patients with giant cell arteritis confirmed by biopsy. BMJ. 1989;299:549-550. doi: 10.1136/bmj.299.6698.549
15. Bahlas S, Ramos-Remus C, Davis P. Utilisation and costs of investigations, and accuracy of diagnosis of polymyalgia rheumatica by family physicians. Clin Rheumatol. 2000;19:278-280. doi: 10.1007/s100670070045
16. Brooks RC, McGee SR. Diagnostic dilemmas in polymyalgia rheumatica. Arch Intern Med. 1997;157:162-168.
17. Blaauw AA, Schuwirth LW, van der Vleuten CP, et al. Assessing clinical competence: recognition of case descriptions of rheumatic diseases by general practitioners. Br J Rheumatol. 1995;34:375-379. doi:10.1093/rheumatology/34.4.375
18. Mager DR. Polymylagia rheumatica: common disease, elusive diagnosis. Home Healthc Now. 2015;33:132-138. doi:10.1097/NHH.0000000000000199
19. Kermani TA, Warrington KJ. Polymyalgia rheumatica. Lancet. 381;63-72. doi: 10.1016/S0140-6736(12)60680-1. Published correction appears in Lancet. 20135;381:28.
20. Liew DF, Owen CE, Buchanan RR. Prescribing for polymyalgia rheumatica. Aust Prescr. 2018;41:14-19. doi: 10.18773/austprescr.2018.001
21. Ovarian cancer statistics. Centers for Disease Control and Prevention. Reviewed June 8, 2021. Accessed February 22, 2022. www.cdc.gov/cancer/ovarian/statistics/index.htm
22. Key statistics for ovarian cancer. American Cancer Society. Revised January 12, 2022. Accessed February 22, 2022. www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html
23. Survival rates for ovarian cancer. American Cancer Society. Revised January 25, 2021. Accessed February 22, 2022. www.cancer.org/cancer/ovarian-cancer/detection-diagnosis-staging/survival-rates.html
24. Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14:9-32. doi: 10.20892/j.issn.2095-3941.2016.0084
25. Goff BA, Mandel LS, Melancon CH, et al. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705-2712. doi: 10.1001/jama.291.22.2705
26. Olson SH, Mignone L, Nakraseive C, et al. Symptoms of ovarian cancer. Obstet Gynecol. 2001;98:212-217. doi: 10.1016/s0029-7844(01)01457-0
27. Allgar VL, Neal RD. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS patients: Cancer. Br J Cancer. 2005;92:1959-1970. doi: 10.1038/sj.bjc.6602587
28. Gajjar K, Ogden G, Mujahid MI, et al. Symptoms and risk factors of ovarian cancer: a survey in primary care. Obstet Gynecol. 2012:754197. doi: 10.5402/2012/754197
29. Austoker J. Diagnosis of ovarian cancer in primary care. BMJ. 2009;339:b3286. doi: 10.1136/bmj.b3286
30. Goff BA, Mandel L, Muntz HG, et al. Ovarian carcinoma diagnosis: results of a national ovarian cancer survey. Cancer. 2000;89:2068-2075. doi: 10.1002/1097-0142(20001115)89:10<2068::aid-cncr6>3.0.co;2-z
31. Smith EM, Anderson B. The effects of symptoms and delay in seeking diagnosis on stage of disease at diagnosis among women with cancers of the ovary. Cancer. 1985;56:2727-2732. doi: 10.1002/1097-0142(19851201)56:11<2727::aid-cncr2820561138>3.0.co;2-8
32. Galvin JE, Duda JE, Kaufer DI, et al. Lewy body dementia: the caregiver experience of clinical care. Parkinsonism Relat Disord. 2010;16:388-392. doi: 10.1016/j.parkreldis.2010.03.007
33. McKeith I. Dementia with Lewy bodies. Dialogues Clin Neurosci. 2004;6:333-341. doi: 10.31887/DCNS.2004.6.3/imckeith
34. Mrak RE, Griffin WS. Dementia with Lewy bodies: definitions, diagnosis, and pathogenic relationship to Alzheimer’s disease. Neuropsychiatr Dis Treat. 2007;3:619-625.
35. Khotianov N, Singh R, Singh S. Lewy body dementia: case report and discussion. J Am Board Fam Pract. 2002;15:50-54.
36. Stinton C, McKeith I, Taylor JP, et al. Pharmacological management of Lewy body dementia: a systematic review and meta-analysis. Am J Psychiatry. 2015;172:731-742. doi: 10.1176/appi.ajp.2015.14121582
37. Velayudhan L, Ffytche D, Ballard C, et al. New therapeutic strategies of Lewy body dementias. Curr Neurol Neurosci Rep. 2017;17:68. doi: 10.1007/s11910-017-0778-2
38. Arnold LM, Clauw DJ, McCarberg BH; FibroCollaborative. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin Proc. 2011;86:457-464. doi: 10.4065/mcp.2010.0738
39. Arnold LM, Gebke KB, Choy EHS. Fibromyalgia: management strategies for primary care providers. Int J Clin Pract. 2016;70:99-112. doi: 10.1111/ijcp.12757
40. Aloush V, Niv D, Ablin JN, et al. Good pain, bad pain: illness perception and physician attitudes towards rheumatoid arthritis and fibromyalgia patients. Clin Exp Rheumatol. 2021;39(suppl 130):54-60.
41. Vincent A, Lahr BD, Wolfe F, et al. Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res (Hoboken). 2013;65:786-792. doi: 10.1002/acr.21896
42. White KP, Thompson J. Fibromyalgia syndrome in an Amish community: a controlled study to determine disease and symptom prevalence. J Rheumatol. 2003;30:1835-1840.
43. Lobo CP, Pfalzgraf AR, Giannetti V, et al. Impact of invalidation and trust in physicians on health outcomes in fibromyalgia patients. Prim Care Companion CNS Disord. 2014;16:10.4088/PCC.14m01664. doi: 10.4088/PCC.14m01664
44. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62:600-610. doi:10.1002/acr.20140
Developing and Measuring Effectiveness of a Distance Learning Dermatology Course: A Prospective Observational Study
Medical education has seen major changes over the last decade. The allotted time for preclinical education has decreased from 24 months to 18 months or less at most institutions, with an increased focus on content associated with health care delivery and health system science.1,2 Many schools now include at least some blended learning with online delivery of preclinical education.3 On the other hand, the clinical portion of medical education has remained largely unchanged prior to the COVID-19 pandemic, with the apprenticeship framework allowing the experienced physician to observe, mentor, and pass on practical knowledge so that the apprentice can one day gain independence after demonstrating adequate proficiency.4
With respect to dermatology education, skin disorders are in the top 5 reported reasons for visits to primary care5; however, a 2009 survey found that only 0.24% to 0.30% of medical schools’ curricula are spent on dermatology.6 Moreover, one institution found that fourth-year medical students received an average of 46.6% on a 15-item quiz designed to assess the ability to diagnose and treat common dermatologic conditions, and within that same cohort, 87.6% of students felt that they received inadequate training in dermatology during medical school.7
COVID-19 caused an unprecedented paradigm shift when medical schools throughout the country, including our own, canceled clinical rotations at the end of March 2020 to protect students and control the spread of infection. To enable clinical and preclinical learning to continue, institutions around the globe turned to either online learning or participation in telehealth as a substitute for clinical rotations.8-10 At the Uniformed Services University of the Health Sciences (Bethesda, Maryland), one of the many online clinical courses offered included a distance learning (DL) dermatology course. Herein, we describe the results of a prospective study evaluating short-term information recall and comprehension as well as students’ confidence in their ability to apply course objectives over 3 months of an online DL dermatology course.
Methods
Between April and July 2020, 14 students at the Uniformed Services University of the Health Sciences (Table 1) enrolled in 1 of 3 four-week DL dermatology classes. The students independently completed the Basic Dermatology Curriculum, a set of online modules with demonstrated efficacy from the American Academy of Dermatology, over 4 weeks.11 Additionally, students were instructed to review an hour of clinical dermatology images daily from online dermatology atlases and e-books accessed through our medical school’s virtual library. Optional Free Open Access Meducation resources also were provided. The course syllabus provided the students with clear expectations, links to the resources, and a recommended daily schedule.
An online video conferencing platform was utilized for an orientation session and 4 subsequent weekly 1.5-hour virtual meetings. The weekly DL meetings focused on a discussion of clinical images pertinent to the American Academy of Dermatology modules covered for the week. These interactive analytic sessions were referred to as Clinpic sessions. With instructor guidance, the students learned to describe images, and they provided differential diagnoses, workup, and treatments for various skin diseases. The virtual meetings included supplemental lectures detailing the use of teledermatology and laser therapy in the Military Health System and a journal review on the cutaneous manifestations of COVID-19.
A 40-question, image-based pretest and posttest utilized during clinical rotations evaluated knowledge recall and comprehension. A precourse and postcourse survey using a 5-point Likert scale (1=not confident; 5=extremely confident) assessed students’ confidence levels across course objectives: general knowledge of dermatology, working knowledge of teledermatology, ability to accurately describe skin lesions, generate sound differential diagnoses, and formulate a reasonable treatment plan. Statistical analysis was performed using free online statistical software at statskingdom.com.12
Results
All 14 student enrollees completed the precourse and postcourse tests and surveys. Pretest and posttest scores followed a normal distribution and therefore met criteria for utilization of a parametric test. The precourse test average of 67% (range, 40%–90%) improved to 84% postcourse (range, 70%–98%; P<.001; 95% CI, 11-23 by paired t test). Not surprisingly, the 2 students who had completed a dermatology rotation had higher average pretest and posttest scores (pretest, 87%; posttest, 94%). Students’ confidence with the course objectives were mostly at the somewhat confident level on the 5-point Likert scale precourse survey. By the end of the course, student survey responses increased to confident and very confident levels, corresponding to an overall improvement of 1.3 points (P<.001 by paired t test)(Table 2) when the mean of the survey results was aggregated across every question. Instructor evaluation of student performance mirrored student assessments.
Comment
The DL dermatology course succeeded in helping the enrolled students attain course objectives and offered a reasonable solution when in-person interaction was restricted. The students in the DL course made notable improvements in their dermatology knowledge and improved their communication, diagnosis, and management skills. Although a blended dermatology curriculum with e-learning combined with clinical experience has been shown to increase knowledge acquisition,13,14 our results suggest that an online-only program also can increase comprehension as well as students’ confidence in their abilities.
A major challenge for the DL course was the lack of opportunity to perform common dermatology procedures. The addition of a hands-on skin procedure module would have been a great supplement to the course but was not possible due to social distancing guidelines during the COVID-19 pandemic. The small sample size and voluntary enrollment were limitations to this study.
Conclusion
Although the traditional dermatology rotation remains the gold standard for clinical instruction, a well-organized DL teaching environment allowed for a more controlled learning experience with a broader coverage of topics to include potentially greater exposure to rare skin disorders not typically encountered in everyday practice. A DL dermatology course may serve as an enduring curriculum for those who wish to learn dermatology more broadly and are not interested in performing skin procedures or direct patient exposure (eg, those pursuing non–primary care specialties, pathology, or radiology). It also may be attractive to students who have had a prior clinical dermatology rotation and desire a different learning experience with a wide coverage of topics.
Acknowledgments—The authors thank Thomas Darling, MD, PhD (Bethesda, Maryland), for coining the term Clinpic and providing critical feedback throughout the course. The authors also thank Sorana Raiciulescu, MS (Bethesda, Maryland), for assistance with the statistical analysis.
- Emanuel EJ. The inevitable reimagining of medical education. JAMA. 2020;323:1127-1128.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Vallée A, Blacher J, Cariou A, et al. Blended learning compared to traditional learning in medical education: systematic review and meta-analysis. J Med Internet Res. 2020;22:E16504.
- Rangachari D, Brown LE, Kern DE, et al. Clinical coaching: evolving the apprenticeship model for modern housestaff. Med Teach. 2017;39:780-782.
- Finley CR, Chan DS, Garrison S, et al. What are the most common conditions in primary care? Can Fam Physician. 2018;64:832-840.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
- Ulman CA, Binder SB, Borges NJ. Assessment of medical students’ proficiency in dermatology: are medical students adequately prepared to diagnose and treat common dermatologic conditions in the United States? J Educ Eval Health Prof. 2015;12:18.
- Loh TY, Hsiao JL, Shi VY. COVID-19 and its effect on medical student education in dermatology. J Am Acad Dermatol. 2020;83:E163-E164.
- Hilburg R, Patel N, Ambruso S, et al. Medical education during the coronavirus disease-2019 pandemic: learning from a distance. Adv Chronic Kidney Dis. 2020;27:412-417.
- Rose S. Medical student education in the time of COVID-19. JAMA. 2020;323:2131-2132.
- McCleskey PE. Clinic teaching made easy: a prospective study of the American Academy of Dermatology core curriculum in primary care learners. J Am Acad Dermatol. 2013;69:273-279.e1.
- Paired T Test calculator. Statistics Kingdom website. Accessed February 7, 2022. http://www.statskingdom.com/160MeanT2pair.html
- Fransen F, Martens H, Nagtzaam I, et al. Use of e-learning in clinical clerkships: effects on acquisition of dermatological knowledge and learning processes. Int J Med Educ. 2018;9:11-17.
- Silva CS, Souza MB, Silva Filho RS, et al. E-learning program for medical students in dermatology. Clinics. 2011;66:619-622.
Medical education has seen major changes over the last decade. The allotted time for preclinical education has decreased from 24 months to 18 months or less at most institutions, with an increased focus on content associated with health care delivery and health system science.1,2 Many schools now include at least some blended learning with online delivery of preclinical education.3 On the other hand, the clinical portion of medical education has remained largely unchanged prior to the COVID-19 pandemic, with the apprenticeship framework allowing the experienced physician to observe, mentor, and pass on practical knowledge so that the apprentice can one day gain independence after demonstrating adequate proficiency.4
With respect to dermatology education, skin disorders are in the top 5 reported reasons for visits to primary care5; however, a 2009 survey found that only 0.24% to 0.30% of medical schools’ curricula are spent on dermatology.6 Moreover, one institution found that fourth-year medical students received an average of 46.6% on a 15-item quiz designed to assess the ability to diagnose and treat common dermatologic conditions, and within that same cohort, 87.6% of students felt that they received inadequate training in dermatology during medical school.7
COVID-19 caused an unprecedented paradigm shift when medical schools throughout the country, including our own, canceled clinical rotations at the end of March 2020 to protect students and control the spread of infection. To enable clinical and preclinical learning to continue, institutions around the globe turned to either online learning or participation in telehealth as a substitute for clinical rotations.8-10 At the Uniformed Services University of the Health Sciences (Bethesda, Maryland), one of the many online clinical courses offered included a distance learning (DL) dermatology course. Herein, we describe the results of a prospective study evaluating short-term information recall and comprehension as well as students’ confidence in their ability to apply course objectives over 3 months of an online DL dermatology course.
Methods
Between April and July 2020, 14 students at the Uniformed Services University of the Health Sciences (Table 1) enrolled in 1 of 3 four-week DL dermatology classes. The students independently completed the Basic Dermatology Curriculum, a set of online modules with demonstrated efficacy from the American Academy of Dermatology, over 4 weeks.11 Additionally, students were instructed to review an hour of clinical dermatology images daily from online dermatology atlases and e-books accessed through our medical school’s virtual library. Optional Free Open Access Meducation resources also were provided. The course syllabus provided the students with clear expectations, links to the resources, and a recommended daily schedule.

An online video conferencing platform was utilized for an orientation session and 4 subsequent weekly 1.5-hour virtual meetings. The weekly DL meetings focused on a discussion of clinical images pertinent to the American Academy of Dermatology modules covered for the week. These interactive analytic sessions were referred to as Clinpic sessions. With instructor guidance, the students learned to describe images, and they provided differential diagnoses, workup, and treatments for various skin diseases. The virtual meetings included supplemental lectures detailing the use of teledermatology and laser therapy in the Military Health System and a journal review on the cutaneous manifestations of COVID-19.
A 40-question, image-based pretest and posttest utilized during clinical rotations evaluated knowledge recall and comprehension. A precourse and postcourse survey using a 5-point Likert scale (1=not confident; 5=extremely confident) assessed students’ confidence levels across course objectives: general knowledge of dermatology, working knowledge of teledermatology, ability to accurately describe skin lesions, generate sound differential diagnoses, and formulate a reasonable treatment plan. Statistical analysis was performed using free online statistical software at statskingdom.com.12
Results
All 14 student enrollees completed the precourse and postcourse tests and surveys. Pretest and posttest scores followed a normal distribution and therefore met criteria for utilization of a parametric test. The precourse test average of 67% (range, 40%–90%) improved to 84% postcourse (range, 70%–98%; P<.001; 95% CI, 11-23 by paired t test). Not surprisingly, the 2 students who had completed a dermatology rotation had higher average pretest and posttest scores (pretest, 87%; posttest, 94%). Students’ confidence with the course objectives were mostly at the somewhat confident level on the 5-point Likert scale precourse survey. By the end of the course, student survey responses increased to confident and very confident levels, corresponding to an overall improvement of 1.3 points (P<.001 by paired t test)(Table 2) when the mean of the survey results was aggregated across every question. Instructor evaluation of student performance mirrored student assessments.

Comment
The DL dermatology course succeeded in helping the enrolled students attain course objectives and offered a reasonable solution when in-person interaction was restricted. The students in the DL course made notable improvements in their dermatology knowledge and improved their communication, diagnosis, and management skills. Although a blended dermatology curriculum with e-learning combined with clinical experience has been shown to increase knowledge acquisition,13,14 our results suggest that an online-only program also can increase comprehension as well as students’ confidence in their abilities.
A major challenge for the DL course was the lack of opportunity to perform common dermatology procedures. The addition of a hands-on skin procedure module would have been a great supplement to the course but was not possible due to social distancing guidelines during the COVID-19 pandemic. The small sample size and voluntary enrollment were limitations to this study.
Conclusion
Although the traditional dermatology rotation remains the gold standard for clinical instruction, a well-organized DL teaching environment allowed for a more controlled learning experience with a broader coverage of topics to include potentially greater exposure to rare skin disorders not typically encountered in everyday practice. A DL dermatology course may serve as an enduring curriculum for those who wish to learn dermatology more broadly and are not interested in performing skin procedures or direct patient exposure (eg, those pursuing non–primary care specialties, pathology, or radiology). It also may be attractive to students who have had a prior clinical dermatology rotation and desire a different learning experience with a wide coverage of topics.
Acknowledgments—The authors thank Thomas Darling, MD, PhD (Bethesda, Maryland), for coining the term Clinpic and providing critical feedback throughout the course. The authors also thank Sorana Raiciulescu, MS (Bethesda, Maryland), for assistance with the statistical analysis.
Medical education has seen major changes over the last decade. The allotted time for preclinical education has decreased from 24 months to 18 months or less at most institutions, with an increased focus on content associated with health care delivery and health system science.1,2 Many schools now include at least some blended learning with online delivery of preclinical education.3 On the other hand, the clinical portion of medical education has remained largely unchanged prior to the COVID-19 pandemic, with the apprenticeship framework allowing the experienced physician to observe, mentor, and pass on practical knowledge so that the apprentice can one day gain independence after demonstrating adequate proficiency.4
With respect to dermatology education, skin disorders are in the top 5 reported reasons for visits to primary care5; however, a 2009 survey found that only 0.24% to 0.30% of medical schools’ curricula are spent on dermatology.6 Moreover, one institution found that fourth-year medical students received an average of 46.6% on a 15-item quiz designed to assess the ability to diagnose and treat common dermatologic conditions, and within that same cohort, 87.6% of students felt that they received inadequate training in dermatology during medical school.7
COVID-19 caused an unprecedented paradigm shift when medical schools throughout the country, including our own, canceled clinical rotations at the end of March 2020 to protect students and control the spread of infection. To enable clinical and preclinical learning to continue, institutions around the globe turned to either online learning or participation in telehealth as a substitute for clinical rotations.8-10 At the Uniformed Services University of the Health Sciences (Bethesda, Maryland), one of the many online clinical courses offered included a distance learning (DL) dermatology course. Herein, we describe the results of a prospective study evaluating short-term information recall and comprehension as well as students’ confidence in their ability to apply course objectives over 3 months of an online DL dermatology course.
Methods
Between April and July 2020, 14 students at the Uniformed Services University of the Health Sciences (Table 1) enrolled in 1 of 3 four-week DL dermatology classes. The students independently completed the Basic Dermatology Curriculum, a set of online modules with demonstrated efficacy from the American Academy of Dermatology, over 4 weeks.11 Additionally, students were instructed to review an hour of clinical dermatology images daily from online dermatology atlases and e-books accessed through our medical school’s virtual library. Optional Free Open Access Meducation resources also were provided. The course syllabus provided the students with clear expectations, links to the resources, and a recommended daily schedule.

An online video conferencing platform was utilized for an orientation session and 4 subsequent weekly 1.5-hour virtual meetings. The weekly DL meetings focused on a discussion of clinical images pertinent to the American Academy of Dermatology modules covered for the week. These interactive analytic sessions were referred to as Clinpic sessions. With instructor guidance, the students learned to describe images, and they provided differential diagnoses, workup, and treatments for various skin diseases. The virtual meetings included supplemental lectures detailing the use of teledermatology and laser therapy in the Military Health System and a journal review on the cutaneous manifestations of COVID-19.
A 40-question, image-based pretest and posttest utilized during clinical rotations evaluated knowledge recall and comprehension. A precourse and postcourse survey using a 5-point Likert scale (1=not confident; 5=extremely confident) assessed students’ confidence levels across course objectives: general knowledge of dermatology, working knowledge of teledermatology, ability to accurately describe skin lesions, generate sound differential diagnoses, and formulate a reasonable treatment plan. Statistical analysis was performed using free online statistical software at statskingdom.com.12
Results
All 14 student enrollees completed the precourse and postcourse tests and surveys. Pretest and posttest scores followed a normal distribution and therefore met criteria for utilization of a parametric test. The precourse test average of 67% (range, 40%–90%) improved to 84% postcourse (range, 70%–98%; P<.001; 95% CI, 11-23 by paired t test). Not surprisingly, the 2 students who had completed a dermatology rotation had higher average pretest and posttest scores (pretest, 87%; posttest, 94%). Students’ confidence with the course objectives were mostly at the somewhat confident level on the 5-point Likert scale precourse survey. By the end of the course, student survey responses increased to confident and very confident levels, corresponding to an overall improvement of 1.3 points (P<.001 by paired t test)(Table 2) when the mean of the survey results was aggregated across every question. Instructor evaluation of student performance mirrored student assessments.

Comment
The DL dermatology course succeeded in helping the enrolled students attain course objectives and offered a reasonable solution when in-person interaction was restricted. The students in the DL course made notable improvements in their dermatology knowledge and improved their communication, diagnosis, and management skills. Although a blended dermatology curriculum with e-learning combined with clinical experience has been shown to increase knowledge acquisition,13,14 our results suggest that an online-only program also can increase comprehension as well as students’ confidence in their abilities.
A major challenge for the DL course was the lack of opportunity to perform common dermatology procedures. The addition of a hands-on skin procedure module would have been a great supplement to the course but was not possible due to social distancing guidelines during the COVID-19 pandemic. The small sample size and voluntary enrollment were limitations to this study.
Conclusion
Although the traditional dermatology rotation remains the gold standard for clinical instruction, a well-organized DL teaching environment allowed for a more controlled learning experience with a broader coverage of topics to include potentially greater exposure to rare skin disorders not typically encountered in everyday practice. A DL dermatology course may serve as an enduring curriculum for those who wish to learn dermatology more broadly and are not interested in performing skin procedures or direct patient exposure (eg, those pursuing non–primary care specialties, pathology, or radiology). It also may be attractive to students who have had a prior clinical dermatology rotation and desire a different learning experience with a wide coverage of topics.
Acknowledgments—The authors thank Thomas Darling, MD, PhD (Bethesda, Maryland), for coining the term Clinpic and providing critical feedback throughout the course. The authors also thank Sorana Raiciulescu, MS (Bethesda, Maryland), for assistance with the statistical analysis.
- Emanuel EJ. The inevitable reimagining of medical education. JAMA. 2020;323:1127-1128.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Vallée A, Blacher J, Cariou A, et al. Blended learning compared to traditional learning in medical education: systematic review and meta-analysis. J Med Internet Res. 2020;22:E16504.
- Rangachari D, Brown LE, Kern DE, et al. Clinical coaching: evolving the apprenticeship model for modern housestaff. Med Teach. 2017;39:780-782.
- Finley CR, Chan DS, Garrison S, et al. What are the most common conditions in primary care? Can Fam Physician. 2018;64:832-840.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
- Ulman CA, Binder SB, Borges NJ. Assessment of medical students’ proficiency in dermatology: are medical students adequately prepared to diagnose and treat common dermatologic conditions in the United States? J Educ Eval Health Prof. 2015;12:18.
- Loh TY, Hsiao JL, Shi VY. COVID-19 and its effect on medical student education in dermatology. J Am Acad Dermatol. 2020;83:E163-E164.
- Hilburg R, Patel N, Ambruso S, et al. Medical education during the coronavirus disease-2019 pandemic: learning from a distance. Adv Chronic Kidney Dis. 2020;27:412-417.
- Rose S. Medical student education in the time of COVID-19. JAMA. 2020;323:2131-2132.
- McCleskey PE. Clinic teaching made easy: a prospective study of the American Academy of Dermatology core curriculum in primary care learners. J Am Acad Dermatol. 2013;69:273-279.e1.
- Paired T Test calculator. Statistics Kingdom website. Accessed February 7, 2022. http://www.statskingdom.com/160MeanT2pair.html
- Fransen F, Martens H, Nagtzaam I, et al. Use of e-learning in clinical clerkships: effects on acquisition of dermatological knowledge and learning processes. Int J Med Educ. 2018;9:11-17.
- Silva CS, Souza MB, Silva Filho RS, et al. E-learning program for medical students in dermatology. Clinics. 2011;66:619-622.
- Emanuel EJ. The inevitable reimagining of medical education. JAMA. 2020;323:1127-1128.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Vallée A, Blacher J, Cariou A, et al. Blended learning compared to traditional learning in medical education: systematic review and meta-analysis. J Med Internet Res. 2020;22:E16504.
- Rangachari D, Brown LE, Kern DE, et al. Clinical coaching: evolving the apprenticeship model for modern housestaff. Med Teach. 2017;39:780-782.
- Finley CR, Chan DS, Garrison S, et al. What are the most common conditions in primary care? Can Fam Physician. 2018;64:832-840.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
- Ulman CA, Binder SB, Borges NJ. Assessment of medical students’ proficiency in dermatology: are medical students adequately prepared to diagnose and treat common dermatologic conditions in the United States? J Educ Eval Health Prof. 2015;12:18.
- Loh TY, Hsiao JL, Shi VY. COVID-19 and its effect on medical student education in dermatology. J Am Acad Dermatol. 2020;83:E163-E164.
- Hilburg R, Patel N, Ambruso S, et al. Medical education during the coronavirus disease-2019 pandemic: learning from a distance. Adv Chronic Kidney Dis. 2020;27:412-417.
- Rose S. Medical student education in the time of COVID-19. JAMA. 2020;323:2131-2132.
- McCleskey PE. Clinic teaching made easy: a prospective study of the American Academy of Dermatology core curriculum in primary care learners. J Am Acad Dermatol. 2013;69:273-279.e1.
- Paired T Test calculator. Statistics Kingdom website. Accessed February 7, 2022. http://www.statskingdom.com/160MeanT2pair.html
- Fransen F, Martens H, Nagtzaam I, et al. Use of e-learning in clinical clerkships: effects on acquisition of dermatological knowledge and learning processes. Int J Med Educ. 2018;9:11-17.
- Silva CS, Souza MB, Silva Filho RS, et al. E-learning program for medical students in dermatology. Clinics. 2011;66:619-622.
Practice Points
- An e-learning distance learning (DL) dermatology course can substantially improve clinically relevant skills and knowledge in dermatology.
- A DL dermatology course may serve as an alternative to clinical rotations for those who wish to learn dermatology more broadly and are not interested in performing skin procedures or direct patient exposure.
Direct-to-Consumer Teledermatology Growth: A Review and Outlook for the Future
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.
The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.
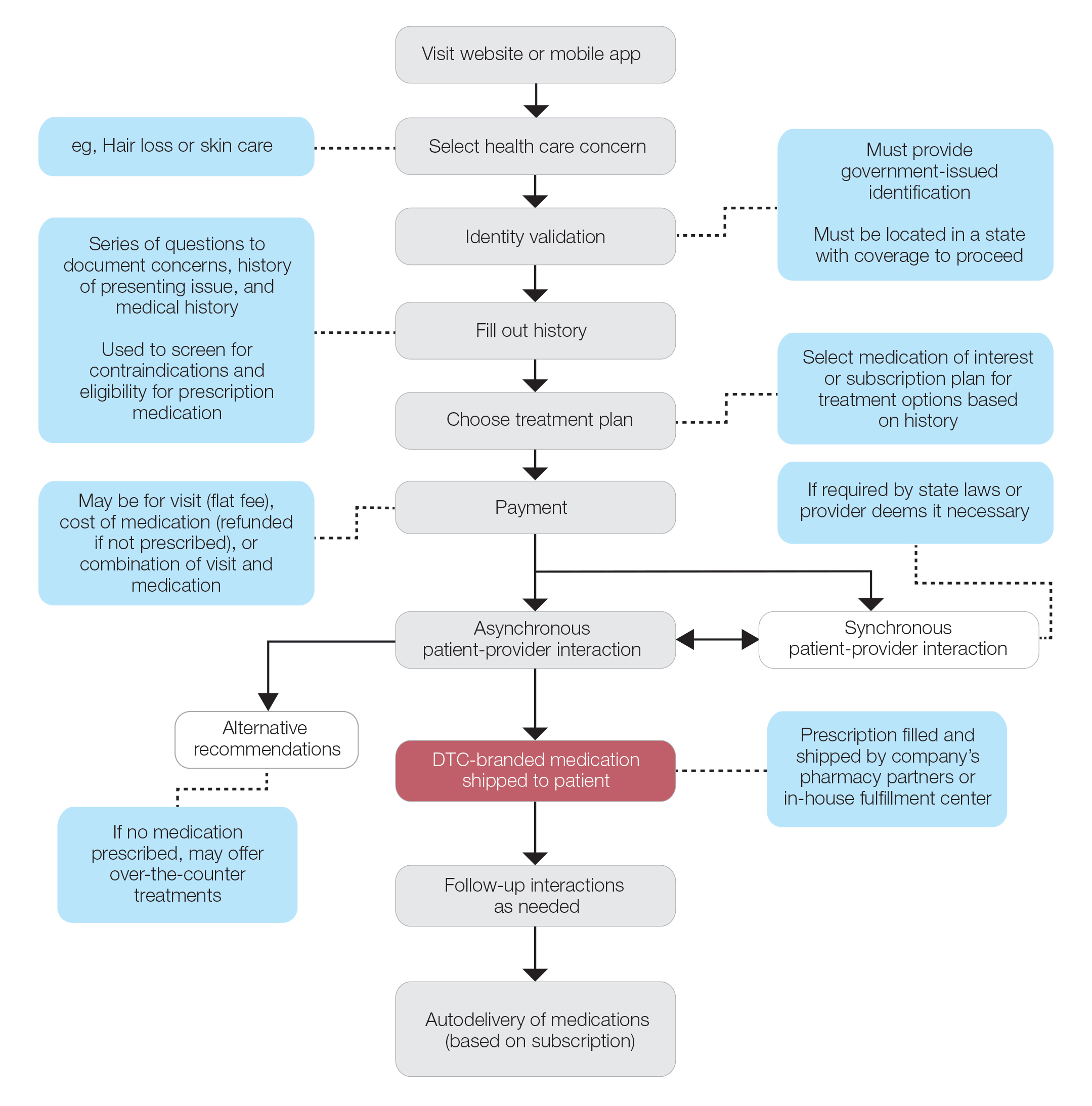
Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.
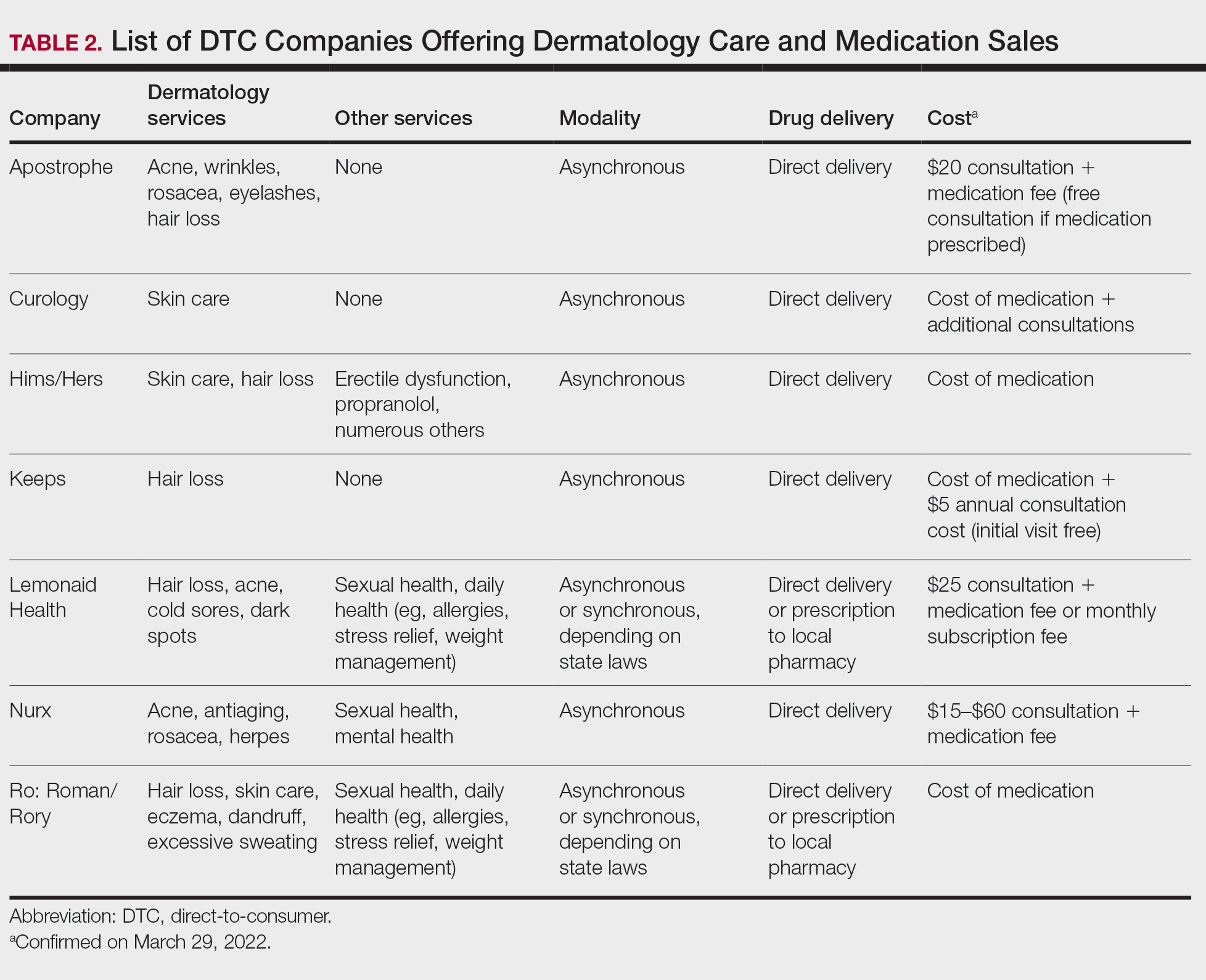
Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.

The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.

Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.

Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.

The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.

Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.

Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
Practice Points
- Direct-to-consumer (DTC) teledermatology platforms are for-profit companies that provide telemedicine visits and sell prescription drugs directly to patients.
- Although they are growing in popularity, DTC teledermatology platforms may lead to overdiagnosis, overtreatment, and fragmentation of health care. Knowledge of teledermatology will be vital to counsel patients on the risks and benefits of these platforms.
‘Outbid on three houses!’ Doc frustrated by crazy market
After more than a decade of moving because of medical school, residencies, and international fellowships, Abhi Kole, MD, PhD, is ready to put down roots. But he’s learning that buying a house in today’s housing market is easier said than done.
In the past 6 months, Dr. Kole, an internist at Grady Hospital in Atlanta, put in offers on three houses. None resulted in a purchase. Dr. Kole says he’s learned how to be more competitive with each subsequent offer, starting out with a bid significantly above the asking price and waiving his right to an appraisal or financing contingencies.
The experience has been surprising and disappointing.
“I knew the market was bad when I started looking and that home prices had gone up,” Dr. Kole says. “What I didn’t realize was that it would still be so hard for me. I have a good job, no debt, and great credit.”
Another frustration for Dr. Kole: He’s been approved for a physician’s loan (a type of mortgage that requires a lower down payment and does not count student loans in debt-to-income calculations) from a national bank, but sellers seem to prefer buyers who work with local lenders. Dr. Kole has been willing to waive the appraisal and mortgage contingency on the right home, but he draws the line at waiving the inspection, a trend that some other buyers in his area are going along with.
“With each house, I learn more about how this works and what amount of risk I can safely assume,” Dr. Kobe says. “There are certain things I definitely wouldn’t give up.”
“Potential homebuyers are really facing a triple threat right now,” says Clare Losey, an assistant research economist with the Texas Real Estate Research Center. “There’s high home appreciation, high mortgage rates, and low inventory of homes for sale.”
It’s still possible to find — and buy — your dream home, even in today’s market with all its challenges. Here are some important steps that can help you.
1. Do not low ball.
There may be some cases in which you can save money by making an offer significantly below the asking price on a property. However, with most housing areas across the country experiencing a seller’s market, you run the risk of offending the buyer or being dismissed as not having a serious offer.
In today’s market, a better strategy is to go in with close to your best and final offer from the start, realtors say. It can help to waive the appraisal or financing contingency as well, although it’s important to understand the risk associated with doing so. Last month, the average home sold for 103% of the list price, according to data compiled from Statista.
2. Get credit ready.
The better your credit, the easier time you’ll have getting a mortgage — and the lower the rate you’ll pay for the loan. The average first-time homebuyer has a credit score of 746, according to a recent paper by Fannie Mae. If you know you’re going to buy a home in the next few months, you can improve your credit by making sure to pay all your bills on time and by avoiding taking on any new debt.
This is also a good opportunity to check your credit report (get all three reports for free from AnnualCreditReport.com) to see whether there are any mistakes or other problems that you’ll need to clear up before applying for a loan. Also, take a look at your credit-utilization ratio (the amount of credit you use compared to the amount available to you). Experts recommend keeping this number below 30%.
3. Prepare to move quickly.
Among homes that closed in March, the average number of days on the market (the amount of time between listing and closing) was just 38 days, according to Realtor.com. In busy markets, homes are moving even faster, realtors say, with sellers commonly accepting offers within days of listing their house for sale.
“It’s crazy,” says Sarah Scattini, president of the Reno/Sparks Association of Realtors. “The market is moving extremely fast here. If you list your home, your sale is pending within 5 days.”
In addition to moving quickly to make your initial offer, do the same if a buyer counters with a negotiation. A speedy response will show the buyer that you’re very interested — and to beat out any other bidders who may have also received a counteroffer.
4. Shop around for mortgages.
Especially for first-time homebuyers, the process will go much more smoothly if you’ve got a team of professionals to help you. Look for a realtor and a mortgage lender who have experience working with first-time homebuyers and with physicians, if possible.
Since mortgage rates can vary wildly, you’ll want to shop around a bit before settling on a lender. Get quotes from a local lender, an online lender, and, potentially, a credit union or a mortgage broker to get a sense of the types of mortgages and rates available to you.
“With multiple offers on every single listing, you really want to align yourself with a great realtor who can negotiate for you on your behalf and navigate you through this very tricky market,” says Ms. Scattini.
For both your realtor and your lender, you’ll want to know up front how they get paid and how they calculate their fees. Typically, the real estate agents for buyers and sellers split a 6% commission on home sales, meaning that your realtor will likely take home 3% of the purchase price.
5. Get preapproved.
Once you’ve settled on a lender, getting preapproved for a mortgage can make your offer more appealing to potential buyers. Preapproval is an in-depth process in which lenders pull your credit and look at other financial factors, such as your income and assets, to tell you ahead of time how much you could borrow under their standards and how much that might cost you.
These days, a large number of buyers are coming in with a cash offer, which in former times was considered very appealing to sellers. However, preapproval helps equalize buyers, and as one seller noted, “I don’t care if it’s cash or mortgage, as long as I get the money.”
If, like most homebuyers, you need a mortgage to finance the purchase, having preapproval can provide some assurance to sellers that your offer won’t fall through because you can’t qualify for the mortgage you expected. Once you’ve received preapproval, don’t open any new credit accounts. If your credit score goes down, the amount you can borrow could decline as well.
6. Firm up your budget.
While the preapproval process will tell you how much a lender thinks you can afford, it typically makes sense to come up with your own budget as well. That’s because banks and other mortgage lenders may approve you for much more than you want or are able to pay for a home.
You’ll want to factor in future costs of homeowners as well as any other (current or future) expenses for which the lender may not have accounted. For example, if you’re planning to have children soon, you may want to lower your budget to factor in the cost of childcare.
Knowing your budget ahead of time, and looking only at houses that fall within it, will prevent you from falling in love with a house that you really can’t afford.
7. Stick with it.
Buying a house in today’s market is no easy task. The first part of the process requires simply looking at multiple houses to get a sense of how far your budget will go and whether there are homes that meet your requirements.
If you’re sure that purchasing a home is the best financial move for you, don’t give up. Instead, consider whether you can make adjustments that could widen your pool of potential homes. That may mean changing your budget, moving a little further out geographically, or opting for a house that needs a little more work than you expected.
That said, while the pace of price increases will likely moderate, it’s unlikely prices will go down significantly in the future.
“We might see home price appreciation subside to levels close to 10% to 15% [from 20% last year] or even just 5% to 10%,” Ms. Losey says. “When you do the math, home prices just can’t continue to go up 20% year over year.”
Dr. Kobe is planning to keep looking for his home for at least the next several months.
“Prices are still going up, but we are hearing that the inventory will increase over the summer,” he says. “I’m still out looking for the right house, and I’m ready to make an offer.”
A version of this article first appeared on Medscape.com.
After more than a decade of moving because of medical school, residencies, and international fellowships, Abhi Kole, MD, PhD, is ready to put down roots. But he’s learning that buying a house in today’s housing market is easier said than done.
In the past 6 months, Dr. Kole, an internist at Grady Hospital in Atlanta, put in offers on three houses. None resulted in a purchase. Dr. Kole says he’s learned how to be more competitive with each subsequent offer, starting out with a bid significantly above the asking price and waiving his right to an appraisal or financing contingencies.
The experience has been surprising and disappointing.
“I knew the market was bad when I started looking and that home prices had gone up,” Dr. Kole says. “What I didn’t realize was that it would still be so hard for me. I have a good job, no debt, and great credit.”
Another frustration for Dr. Kole: He’s been approved for a physician’s loan (a type of mortgage that requires a lower down payment and does not count student loans in debt-to-income calculations) from a national bank, but sellers seem to prefer buyers who work with local lenders. Dr. Kole has been willing to waive the appraisal and mortgage contingency on the right home, but he draws the line at waiving the inspection, a trend that some other buyers in his area are going along with.
“With each house, I learn more about how this works and what amount of risk I can safely assume,” Dr. Kobe says. “There are certain things I definitely wouldn’t give up.”
“Potential homebuyers are really facing a triple threat right now,” says Clare Losey, an assistant research economist with the Texas Real Estate Research Center. “There’s high home appreciation, high mortgage rates, and low inventory of homes for sale.”
It’s still possible to find — and buy — your dream home, even in today’s market with all its challenges. Here are some important steps that can help you.
1. Do not low ball.
There may be some cases in which you can save money by making an offer significantly below the asking price on a property. However, with most housing areas across the country experiencing a seller’s market, you run the risk of offending the buyer or being dismissed as not having a serious offer.
In today’s market, a better strategy is to go in with close to your best and final offer from the start, realtors say. It can help to waive the appraisal or financing contingency as well, although it’s important to understand the risk associated with doing so. Last month, the average home sold for 103% of the list price, according to data compiled from Statista.
2. Get credit ready.
The better your credit, the easier time you’ll have getting a mortgage — and the lower the rate you’ll pay for the loan. The average first-time homebuyer has a credit score of 746, according to a recent paper by Fannie Mae. If you know you’re going to buy a home in the next few months, you can improve your credit by making sure to pay all your bills on time and by avoiding taking on any new debt.
This is also a good opportunity to check your credit report (get all three reports for free from AnnualCreditReport.com) to see whether there are any mistakes or other problems that you’ll need to clear up before applying for a loan. Also, take a look at your credit-utilization ratio (the amount of credit you use compared to the amount available to you). Experts recommend keeping this number below 30%.
3. Prepare to move quickly.
Among homes that closed in March, the average number of days on the market (the amount of time between listing and closing) was just 38 days, according to Realtor.com. In busy markets, homes are moving even faster, realtors say, with sellers commonly accepting offers within days of listing their house for sale.
“It’s crazy,” says Sarah Scattini, president of the Reno/Sparks Association of Realtors. “The market is moving extremely fast here. If you list your home, your sale is pending within 5 days.”
In addition to moving quickly to make your initial offer, do the same if a buyer counters with a negotiation. A speedy response will show the buyer that you’re very interested — and to beat out any other bidders who may have also received a counteroffer.
4. Shop around for mortgages.
Especially for first-time homebuyers, the process will go much more smoothly if you’ve got a team of professionals to help you. Look for a realtor and a mortgage lender who have experience working with first-time homebuyers and with physicians, if possible.
Since mortgage rates can vary wildly, you’ll want to shop around a bit before settling on a lender. Get quotes from a local lender, an online lender, and, potentially, a credit union or a mortgage broker to get a sense of the types of mortgages and rates available to you.
“With multiple offers on every single listing, you really want to align yourself with a great realtor who can negotiate for you on your behalf and navigate you through this very tricky market,” says Ms. Scattini.
For both your realtor and your lender, you’ll want to know up front how they get paid and how they calculate their fees. Typically, the real estate agents for buyers and sellers split a 6% commission on home sales, meaning that your realtor will likely take home 3% of the purchase price.
5. Get preapproved.
Once you’ve settled on a lender, getting preapproved for a mortgage can make your offer more appealing to potential buyers. Preapproval is an in-depth process in which lenders pull your credit and look at other financial factors, such as your income and assets, to tell you ahead of time how much you could borrow under their standards and how much that might cost you.
These days, a large number of buyers are coming in with a cash offer, which in former times was considered very appealing to sellers. However, preapproval helps equalize buyers, and as one seller noted, “I don’t care if it’s cash or mortgage, as long as I get the money.”
If, like most homebuyers, you need a mortgage to finance the purchase, having preapproval can provide some assurance to sellers that your offer won’t fall through because you can’t qualify for the mortgage you expected. Once you’ve received preapproval, don’t open any new credit accounts. If your credit score goes down, the amount you can borrow could decline as well.
6. Firm up your budget.
While the preapproval process will tell you how much a lender thinks you can afford, it typically makes sense to come up with your own budget as well. That’s because banks and other mortgage lenders may approve you for much more than you want or are able to pay for a home.
You’ll want to factor in future costs of homeowners as well as any other (current or future) expenses for which the lender may not have accounted. For example, if you’re planning to have children soon, you may want to lower your budget to factor in the cost of childcare.
Knowing your budget ahead of time, and looking only at houses that fall within it, will prevent you from falling in love with a house that you really can’t afford.
7. Stick with it.
Buying a house in today’s market is no easy task. The first part of the process requires simply looking at multiple houses to get a sense of how far your budget will go and whether there are homes that meet your requirements.
If you’re sure that purchasing a home is the best financial move for you, don’t give up. Instead, consider whether you can make adjustments that could widen your pool of potential homes. That may mean changing your budget, moving a little further out geographically, or opting for a house that needs a little more work than you expected.
That said, while the pace of price increases will likely moderate, it’s unlikely prices will go down significantly in the future.
“We might see home price appreciation subside to levels close to 10% to 15% [from 20% last year] or even just 5% to 10%,” Ms. Losey says. “When you do the math, home prices just can’t continue to go up 20% year over year.”
Dr. Kobe is planning to keep looking for his home for at least the next several months.
“Prices are still going up, but we are hearing that the inventory will increase over the summer,” he says. “I’m still out looking for the right house, and I’m ready to make an offer.”
A version of this article first appeared on Medscape.com.
After more than a decade of moving because of medical school, residencies, and international fellowships, Abhi Kole, MD, PhD, is ready to put down roots. But he’s learning that buying a house in today’s housing market is easier said than done.
In the past 6 months, Dr. Kole, an internist at Grady Hospital in Atlanta, put in offers on three houses. None resulted in a purchase. Dr. Kole says he’s learned how to be more competitive with each subsequent offer, starting out with a bid significantly above the asking price and waiving his right to an appraisal or financing contingencies.
The experience has been surprising and disappointing.
“I knew the market was bad when I started looking and that home prices had gone up,” Dr. Kole says. “What I didn’t realize was that it would still be so hard for me. I have a good job, no debt, and great credit.”
Another frustration for Dr. Kole: He’s been approved for a physician’s loan (a type of mortgage that requires a lower down payment and does not count student loans in debt-to-income calculations) from a national bank, but sellers seem to prefer buyers who work with local lenders. Dr. Kole has been willing to waive the appraisal and mortgage contingency on the right home, but he draws the line at waiving the inspection, a trend that some other buyers in his area are going along with.
“With each house, I learn more about how this works and what amount of risk I can safely assume,” Dr. Kobe says. “There are certain things I definitely wouldn’t give up.”
“Potential homebuyers are really facing a triple threat right now,” says Clare Losey, an assistant research economist with the Texas Real Estate Research Center. “There’s high home appreciation, high mortgage rates, and low inventory of homes for sale.”
It’s still possible to find — and buy — your dream home, even in today’s market with all its challenges. Here are some important steps that can help you.
1. Do not low ball.
There may be some cases in which you can save money by making an offer significantly below the asking price on a property. However, with most housing areas across the country experiencing a seller’s market, you run the risk of offending the buyer or being dismissed as not having a serious offer.
In today’s market, a better strategy is to go in with close to your best and final offer from the start, realtors say. It can help to waive the appraisal or financing contingency as well, although it’s important to understand the risk associated with doing so. Last month, the average home sold for 103% of the list price, according to data compiled from Statista.
2. Get credit ready.
The better your credit, the easier time you’ll have getting a mortgage — and the lower the rate you’ll pay for the loan. The average first-time homebuyer has a credit score of 746, according to a recent paper by Fannie Mae. If you know you’re going to buy a home in the next few months, you can improve your credit by making sure to pay all your bills on time and by avoiding taking on any new debt.
This is also a good opportunity to check your credit report (get all three reports for free from AnnualCreditReport.com) to see whether there are any mistakes or other problems that you’ll need to clear up before applying for a loan. Also, take a look at your credit-utilization ratio (the amount of credit you use compared to the amount available to you). Experts recommend keeping this number below 30%.
3. Prepare to move quickly.
Among homes that closed in March, the average number of days on the market (the amount of time between listing and closing) was just 38 days, according to Realtor.com. In busy markets, homes are moving even faster, realtors say, with sellers commonly accepting offers within days of listing their house for sale.
“It’s crazy,” says Sarah Scattini, president of the Reno/Sparks Association of Realtors. “The market is moving extremely fast here. If you list your home, your sale is pending within 5 days.”
In addition to moving quickly to make your initial offer, do the same if a buyer counters with a negotiation. A speedy response will show the buyer that you’re very interested — and to beat out any other bidders who may have also received a counteroffer.
4. Shop around for mortgages.
Especially for first-time homebuyers, the process will go much more smoothly if you’ve got a team of professionals to help you. Look for a realtor and a mortgage lender who have experience working with first-time homebuyers and with physicians, if possible.
Since mortgage rates can vary wildly, you’ll want to shop around a bit before settling on a lender. Get quotes from a local lender, an online lender, and, potentially, a credit union or a mortgage broker to get a sense of the types of mortgages and rates available to you.
“With multiple offers on every single listing, you really want to align yourself with a great realtor who can negotiate for you on your behalf and navigate you through this very tricky market,” says Ms. Scattini.
For both your realtor and your lender, you’ll want to know up front how they get paid and how they calculate their fees. Typically, the real estate agents for buyers and sellers split a 6% commission on home sales, meaning that your realtor will likely take home 3% of the purchase price.
5. Get preapproved.
Once you’ve settled on a lender, getting preapproved for a mortgage can make your offer more appealing to potential buyers. Preapproval is an in-depth process in which lenders pull your credit and look at other financial factors, such as your income and assets, to tell you ahead of time how much you could borrow under their standards and how much that might cost you.
These days, a large number of buyers are coming in with a cash offer, which in former times was considered very appealing to sellers. However, preapproval helps equalize buyers, and as one seller noted, “I don’t care if it’s cash or mortgage, as long as I get the money.”
If, like most homebuyers, you need a mortgage to finance the purchase, having preapproval can provide some assurance to sellers that your offer won’t fall through because you can’t qualify for the mortgage you expected. Once you’ve received preapproval, don’t open any new credit accounts. If your credit score goes down, the amount you can borrow could decline as well.
6. Firm up your budget.
While the preapproval process will tell you how much a lender thinks you can afford, it typically makes sense to come up with your own budget as well. That’s because banks and other mortgage lenders may approve you for much more than you want or are able to pay for a home.
You’ll want to factor in future costs of homeowners as well as any other (current or future) expenses for which the lender may not have accounted. For example, if you’re planning to have children soon, you may want to lower your budget to factor in the cost of childcare.
Knowing your budget ahead of time, and looking only at houses that fall within it, will prevent you from falling in love with a house that you really can’t afford.
7. Stick with it.
Buying a house in today’s market is no easy task. The first part of the process requires simply looking at multiple houses to get a sense of how far your budget will go and whether there are homes that meet your requirements.
If you’re sure that purchasing a home is the best financial move for you, don’t give up. Instead, consider whether you can make adjustments that could widen your pool of potential homes. That may mean changing your budget, moving a little further out geographically, or opting for a house that needs a little more work than you expected.
That said, while the pace of price increases will likely moderate, it’s unlikely prices will go down significantly in the future.
“We might see home price appreciation subside to levels close to 10% to 15% [from 20% last year] or even just 5% to 10%,” Ms. Losey says. “When you do the math, home prices just can’t continue to go up 20% year over year.”
Dr. Kobe is planning to keep looking for his home for at least the next several months.
“Prices are still going up, but we are hearing that the inventory will increase over the summer,” he says. “I’m still out looking for the right house, and I’m ready to make an offer.”
A version of this article first appeared on Medscape.com.
Clinician experience has been cited for declining operative vaginal delivery rates. Are you comfortable performing OVD as an alternative to cesarean?
[polldaddy:11086729]
[polldaddy:11086729]
[polldaddy:11086729]


