User login
‘Malicious peer review’ destroyed doc’s career, he says
Cardiothoracic surgeon J. Marvin Smith III, MD, had always thrived on a busy practice schedule, often performing 20-30 surgeries a week. A practicing surgeon for more than 40 years, Dr. Smith said he had no plans to slow down anytime soon.
But Dr. Smith said his career was derailed when leaders at Methodist Healthcare System of San Antonio initiated a sudden peer review proceeding against him. The hospital system alleged certain surgeries performed by Dr. Smith had excessive mortality rates. When he proved the data inaccurate, Dr. Smith said administrators next claimed he was cognitively impaired and wasn’t safe to practice.
Dr. Smith has now been embroiled in a peer review dispute with the hospital system for more than 2 years and says the conflict has essentially forced him out of surgical practice. He believes the peer review was “malicious” and was really launched because of complaints he made about nurse staffing and other issues at the hospital.
“I think it is absolutely in bad faith and is disingenuous what they’ve told me along the way,” said Dr. Smith, 73. “It’s because I pointed out deficiencies in nursing care, and they want to get rid of me. It would be a lot easier for them if I had a contract and they could control me better. But the fact that I was independent, meant they had to resort to a malicious peer review to try and push me out.”
Dr. Smith had a peer review hearing with Methodist in March 2021, and in April, a panel found in Dr. Smith’s favor, according to Dr. Smith. The findings were sent to the hospital’s medical board for review, which issued a decision in early May.
Eric A. Pullen, an attorney for Dr. Smith, said he could not go into detail about the board’s decision for legal reasons, but that “the medical board’s decision did not completely resolve the matter, and Dr. Smith intends to exercise his procedural rights, which could include an appeal.”
Methodist Hospital Texsan and its parent company, Methodist Health System of San Antonio, did not respond to messages seeking comment about the case. Without hearing from the hospital system, its side is unknown and it is unclear if there is more to the story from Methodist’s view.
The problem is not new, but some experts, such as Lawrence Huntoon, MD, PhD, say the practice has become more common in recent years, particularly against independent doctors.
Dr. Huntoon believes there is a nationwide trend at many hospitals to get rid of independent physicians and replace them with employed doctors, he said.
However, because most sham peer reviews go on behind closed doors, there are no data to pinpoint its prevalence or measure its growth.
“Independent physicians are basically being purged from medical staffs across the United States,” said Dr. Huntoon, who is chair of the Association of American Physicians and Surgeons’ Committee to Combat Sham Peer Review. “The hospitals want more control over how physicians practice and who they refer to, and they do that by having employees.”
Anthony P. Weiss, MD, MBA, chief medical officer for Beth Israel Deaconess Medical Center said it has not been his experience that independent physicians are being targeted in such a way. Dr. Weiss responded to an inquiry sent to the American Hospital Association for this story.
“As the authority for peer review rests with the organized medical staff (i.e., physicians), and not formally with the hospital per se, the peer review lever is not typically available as a management tool for hospital administration,” said Dr. Weiss, who is a former member of the AHA’s Committee on Clinical Leadership, but who was speaking on behalf of himself.
A spokesman for the AHA said the organization stands behinds Dr. Weiss’ comments.
Peer review remains a foundational aspect of overseeing the safety and appropriateness of healthcare provided by physicians, Dr. Weiss said. Peer review likely varies from hospital to hospital, he added, although the Healthcare Quality Improvement Act provides some level of guidance as does the American Medical Association Code of Medical Ethics (section 9.4.1).
“In essence, both require that the evaluation be conducted in good faith with the intention to improve care, by physicians with adequate training and knowledge, using a process that is fair and inclusive of the physician under review,” he said. “I believe that most medical staffs abide by these ethical principles, but we have little data to confirm this supposition.”
Did hospital target doc for being vocal?
When members of Methodist’s medical staff first approached Dr. Smith with concerns about his surgery outcomes in November 2018, the physician says he was surprised, but that he was open to an assessment.
“They came to me and said they thought my numbers were bad, and I said: ‘Well my gosh, I certainly don’t want that to be the case. I need to see what numbers you are talking about,’ ” Dr. Smith recalled. “I’ve been president of the Bexar County Medical Society; I’ve been involved with standards and ethics for the Society of Thoracic Surgeons. Quality health care means a whole lot to me.”
The statistical information provided by hospital administrators indicated that Dr. Smith’s mortality rates for coronary artery surgery in 2018 were “excessive” and that his rates for aortic surgery were “unacceptable,” according to a lawsuit Dr. Smith filed against the hospital system. Dr. Smith, who is double boarded with the American Board of Surgery and the American Board of Thoracic Surgery, said his outcomes had never come into question in the past. Dr. Smith said the timing was suspicious to him, however, considering he had recently raised concerns with the hospital through letters about nursing performance, staffing, and compensation.
A peer review investigation was initiated. In the meantime, Dr. Smith agreed to intensivist consults on his postoperative patients and consults with the hospital’s “Heart Team” on all preoperative cardiac, valve, and aortic cases. A vocal critic of the Heart Team, Dr. Smith had long contended the entity provided no meaningful benefit to his patients in most cases and, rather, increased hospital stays and raised medical expenses. Despite his agreement, Dr. Smith was later asked to voluntarily stop performing surgeries at the hospital.
“I agreed, convinced that we’d get this all settled,” he said.
Another report issued by the hospital in 2019 also indicated elevated mortality rates associated with some of Smith’s surgeries, although the document differed from the first report, according to the lawsuit. Dr. Smith says he was ignored when he pointed out problems with the data, including a lack of appropriate risk stratification in the report, departure from Society of Thoracic Surgeons data rules, and improper inclusion of his cases in the denominator of the ratio when a comparison was made of his outcomes with those hospitalwide. A subsequent report from Methodist in March 2019 indicated Dr. Smith’s surgery outcomes were “within the expected parameters of performance,” according to court documents.
The surgery accusations were dropped, but the peer review proceeding against Dr. Smith wasn’t over. The hospital next requested that Dr. Smith undergo a competency evaluation.
“When they realized the data was bad, they then changed their argument in the peer review proceeding and essentially started to argue that Dr. Smith had some sort of cognitive disability that prevented him from continuing to practice,” said Mr. Pullen. “The way I look at it, when the initial basis for the peer review was proven false, the hospital found something else and some other reason to try to keep Dr. Smith from practicing.”
Thus began a lengthy disagreement about which entity would conduct the evaluation, who would pay, and the type of acceptable assessment. An evaluation by the hospital’s preferred organization resulted in a finding of mild cognitive impairment, Dr. Smith said. He hired his own experts who conducted separate evaluations, finding no impairment and no basis for the former evaluation’s conclusion.
“Literally, the determinant as to whether I was normal or below normal on their test was one point, which was associated with a finding that I didn’t draw a clock correctly,” Dr. Smith claimed. “The reviewer said my minute hand was a little too short and docked me a point. It was purely subjective. To me, the gold standard of whether you are learned in thoracic surgery is the American Board of Thoracic Surgery’s test. The board’s test shows my cognitive ability is entirely in keeping with my practice. That contrasts with the one point off I got for drawing a clock wrong in somebody’s estimation.”
Conflict leads to legal case
In September 2020, Dr. Smith filed a lawsuit against Methodist Healthcare System of San Antonio, alleging business disparagement by Methodist for allegedly publishing false and disparaging information about Dr. Smith and tortious interference with business relations. The latter claim stems from Methodist refusing to provide documents to other hospitals about the status of Dr. Smith’s privileges at Methodist, Mr. Pullen said.
Because Methodist refused to confirm his status, the renewal process for Baptist Health System could not be completed and Dr. Smith lost his privileges at Baptist Health System facilities, according to the lawsuit.
Notably, Dr. Smith’s legal challenge also asks the court to take a stance against alleged amendments by Methodist to its Unified Medical Staff Bylaws. The hospital allegedly proposed changes that would prevent physicians from seeking legal action against the hospital for malicious peer review, according to Dr. Smith’s lawsuit.
The amendments would make the peer review process itself the “sole and exclusive remedy with respect to any action or recommendation taken at the hospital affecting medical staff appointment and/or clinical privileges,” according to an excerpt of the proposed amendments included in Dr. Smith’s lawsuit. In addition, the changes would hold practitioners liable for lost revenues if the doctor initiates “any type of legal action challenging credentialing, privileging, or other medical peer review or professional review activity,” according to the lawsuit.
Dr. Smith’s lawsuit seeks a declaration that the proposed amendments to the bylaws are “void as against public policy,” and a declaration that the proposed amendments to the bylaws cannot take away physicians’ statutory right to bring litigation against Methodist for malicious peer review.
“The proposed amendments have a tendency to and will injure the public good,” Dr. Smith argued in the lawsuit. “The proposed amendments allow Methodist to act with malice and in bad faith in conducting peer review proceedings and face no legal repercussions.”
Regardless of the final outcome of the peer review proceeding, Mr. Pullen said the harm Dr. Smith has already endured cannot be reversed.
“Even if comes out in his favor, the damage is already done,” he said. “It will not remedy the damage Dr. Smith has incurred.”
Fighting sham peer review is difficult
Battling a malicious peer review has long been an uphill battle for physicians, according to Dr. Huntoon. That’s because the Health Care Quality Improvement Act (HCQIA), a federal law passed in 1986, provides near absolute immunity to hospitals and peer reviewers in legal disputes.
The HCQIA was created by Congress to extend immunity to good-faith peer review of doctors and to increase overall participation in peer review by removing fear of litigation. However, the act has also enabled abuse of peer review by shielding bad-faith reviewers from accountability, said Dr. Huntoon.
“The Health Care Quality Improvement Act presumes that what the hospital did was warranted and reasonable and shifts the burden to the physician to prove his innocence by a preponderance of evidence,” he said. “That’s an entirely foreign concept to most people who think a person should be considered innocent until proven guilty. Here, it’s the exact opposite.”
The HCQIA has been challenged numerous times over the years and tested at the appellate level, but continues to survive and remain settled law, added Richard B. Willner, DPM, founder and director of the Center for Peer Review Justice, which assists and counsels physicians about sham peer review.
In 2011, former Rep. Joe Heck, DO, (R-Nev.) introduced a bill that would have amended the HCQIA to prohibit a professional review entity from submitting a report to the National Practitioner Data Bank (NPDB) while the doctor was still under investigation and before the doctor was afforded adequate notice and a hearing. Although the measure had 16 cosponsors and plenty of support from the physician community, it failed.
In addition to a heavy legal burden, physicians who experience malicious peer reviews also face ramifications from being reported to the NPDB. Peer review organizations are required to report certain negative actions or findings to the NPDB.
“A databank entry is a scarlet letter on your forehead,” Dr. Willner said. “The rules at a lot of institutions are not to take anyone who has been databanked, rightfully or wrongfully. And what is the evidence necessary to databank you? None. There’s no evidence needed to databank somebody.”
Despite the bleak landscape, experts say progress has been made on a case-by-case basis by physicians who have succeeded in fighting back against questionable peer reviews in recent years.
In January 2020, Indiana ob.gyn. Rebecca Denman, MD, prevailed in her defamation lawsuit against St Vincent Carmel Hospital and St Vincent Carmel Medical Group, winning $4.75 million in damages. Dr. Denman alleged administrators failed to conduct a proper peer review investigation after a false allegation by a nurse that she was under the influence while on the job.
Indianapolis attorney Kathleen A. DeLaney, who represented Dr. Denman, said hospital leaders misled Dr. Denman into believing a peer review had occurred when no formal peer review hearing or proceeding took place.
“The CMO of the medical group claimed that he performed a peer review ‘screening,’ but he never informed the other members of the peer review executive committee of the matter until after he had placed Dr. Denman on administrative leave,” Ms. DeLaney said. “He also neglected to tell the peer review executive committee that the substance abuse policy had not been followed, or that Dr. Denman had not been tested for alcohol use – due to the 12-hour delay in report.”
Dr. Denman was ultimately required to undergo an alcohol abuse evaluation, enter a treatment program, and sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit. She claimed repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
She sued the hospital in July 2018, alleging fraud, defamation, tortious interference with an employment relationship, and negligent misrepresentation. After a 4-day trial, jurors found in her favor, awarding Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
A hospital spokesperson said Ascension St Vincent is pursuing an appeal, and that it looks “forward to the opportunity to bring this matter before the Indiana Court of Appeals in June.”
In another case, South Dakota surgeon Linda Miller, MD, was awarded $1.1 million in 2017 after a federal jury found Huron Regional Medical Center breached her contract and violated her due process rights. Dr. Miller became the subject of a peer review at Huron Regional Medical Center when the hospital began analyzing some of her surgery outcomes.
Ken Barker, an attorney for Dr. Miller, said he feels it became evident at trial that the campaign to force Dr. Miller to either resign or lose her privileges was led by the lay board of directors of the hospital and upper-level administration at the hospital.
“They began the process by ordering an unprecedented 90-day review of her medical charts, looking for errors in the medical care she provided patients,” he said. “They could find nothing, so they did a second 90-day review, waiting for a patient’s ‘bad outcome.’ As any general surgeon will say, a ‘bad outcome’ is inevitable. And so it was. Upon that occurrence, they had a medical review committee review the patient’s chart and use it as an excuse to force her to reduce her privileges. Unbeknown to Dr. Miller, an external review had been conducted on another patient’s chart, in which the external review found her care above the standards and, in some measure, ‘exemplary.’ ”
Dr. Miller was eventually pressured to resign, according to her claim. Because of reports made to the NPDB by the medical center, including a patient complication that was allegedly falsified by the hospital, Dr. Miller said she was unable to find work as a general surgeon and went to work as a wound care doctor. At trial, jurors awarded Dr. Miller $586,617 in lost wages, $343,640 for lost future earning capacity, and $250,000 for mental anguish. (The mental anguish award was subsequently struck by a district court.)
Attorneys for Huron Regional Medical Center argued the jury improperly awarded damages and requested a new trial, which was denied by an appeals court.
In the end, the evidence came to light and the jury’s verdict spoke loudly that the hospital had taken unfair advantage of Dr. Miller, Mr. Barker said. But he emphasized that such cases often end differently.
“There are a handful of cases in which physicians like Dr. Miller have challenged the system and won,” he said. “In most cases, however, it is a ‘David vs. Goliath’ scenario where the giant prevails.”
What to do if faced with malicious peer review
An important step when doctors encounter a peer review that they believe is malicious is to consult with an experienced attorney as early as possible, Dr. Huntoon said. “Not all attorneys who set themselves out to be health law attorneys necessarily have knowledge and expertise in sham peer review. And before such a thing happens, I always encourage physicians to read their medical staff bylaws. That’s where everything is set forth, [such as] the corrective action section that tells how peer review is to take place.”
Mr. Barker added that documentation is also key in the event of a potential malicious peer review.
“When a physician senses [the] administration has targeted them, they should start documenting their conversations and actions very carefully, and if possible, recruit another ‘observer’ who can provide a third-party perspective, if necessary,” Mr. Barker said.
Dr. Huntoon recently wrote an article with advice about preparedness and defense of sham peer reviews. The guidance includes that physicians educate themselves about the tactics used by some hospitals to conduct sham peer reviews and the factors that place doctors more at risk. Factors that may raise a doctor’s danger of being targeted include being in solo practice or a small group, being new on staff, or being an older physician approaching retirement as some bad-actor hospitals may view older physicians as being less likely to fight back, said Dr. Huntoon.
Doctors should also keep detailed records and a timeline in the event of a malicious peer review and insist that an independent court reporter record all peer review hearings, even if that means the physician has to pay for the reporter him or herself, according to the guidance. An independent record is invaluable should the physician ultimately issue a future legal challenge against the hospital.
Mr. Willner encourages physicians to call the Center for Peer Review Justice hotline at (504) 621-1670 or visit the website for help with peer review and NPDB issues.
As for Dr. Smith, his days are much quieter and slower today, compared with the active practice he was accustomed to for more than half his life. He misses the fast pace, the patients, and the work that always brought him great joy.
“I hope to get back to doing surgeries eventually,” he said. “I graduated medical school in 1972. Practicing surgery has been my whole life and my career. They have taken my identity and my livelihood away from me based on false numbers and false premises. I want it back.”
A version of this article first appeared on Medscape.com.
Cardiothoracic surgeon J. Marvin Smith III, MD, had always thrived on a busy practice schedule, often performing 20-30 surgeries a week. A practicing surgeon for more than 40 years, Dr. Smith said he had no plans to slow down anytime soon.
But Dr. Smith said his career was derailed when leaders at Methodist Healthcare System of San Antonio initiated a sudden peer review proceeding against him. The hospital system alleged certain surgeries performed by Dr. Smith had excessive mortality rates. When he proved the data inaccurate, Dr. Smith said administrators next claimed he was cognitively impaired and wasn’t safe to practice.
Dr. Smith has now been embroiled in a peer review dispute with the hospital system for more than 2 years and says the conflict has essentially forced him out of surgical practice. He believes the peer review was “malicious” and was really launched because of complaints he made about nurse staffing and other issues at the hospital.
“I think it is absolutely in bad faith and is disingenuous what they’ve told me along the way,” said Dr. Smith, 73. “It’s because I pointed out deficiencies in nursing care, and they want to get rid of me. It would be a lot easier for them if I had a contract and they could control me better. But the fact that I was independent, meant they had to resort to a malicious peer review to try and push me out.”
Dr. Smith had a peer review hearing with Methodist in March 2021, and in April, a panel found in Dr. Smith’s favor, according to Dr. Smith. The findings were sent to the hospital’s medical board for review, which issued a decision in early May.
Eric A. Pullen, an attorney for Dr. Smith, said he could not go into detail about the board’s decision for legal reasons, but that “the medical board’s decision did not completely resolve the matter, and Dr. Smith intends to exercise his procedural rights, which could include an appeal.”
Methodist Hospital Texsan and its parent company, Methodist Health System of San Antonio, did not respond to messages seeking comment about the case. Without hearing from the hospital system, its side is unknown and it is unclear if there is more to the story from Methodist’s view.
The problem is not new, but some experts, such as Lawrence Huntoon, MD, PhD, say the practice has become more common in recent years, particularly against independent doctors.
Dr. Huntoon believes there is a nationwide trend at many hospitals to get rid of independent physicians and replace them with employed doctors, he said.
However, because most sham peer reviews go on behind closed doors, there are no data to pinpoint its prevalence or measure its growth.
“Independent physicians are basically being purged from medical staffs across the United States,” said Dr. Huntoon, who is chair of the Association of American Physicians and Surgeons’ Committee to Combat Sham Peer Review. “The hospitals want more control over how physicians practice and who they refer to, and they do that by having employees.”
Anthony P. Weiss, MD, MBA, chief medical officer for Beth Israel Deaconess Medical Center said it has not been his experience that independent physicians are being targeted in such a way. Dr. Weiss responded to an inquiry sent to the American Hospital Association for this story.
“As the authority for peer review rests with the organized medical staff (i.e., physicians), and not formally with the hospital per se, the peer review lever is not typically available as a management tool for hospital administration,” said Dr. Weiss, who is a former member of the AHA’s Committee on Clinical Leadership, but who was speaking on behalf of himself.
A spokesman for the AHA said the organization stands behinds Dr. Weiss’ comments.
Peer review remains a foundational aspect of overseeing the safety and appropriateness of healthcare provided by physicians, Dr. Weiss said. Peer review likely varies from hospital to hospital, he added, although the Healthcare Quality Improvement Act provides some level of guidance as does the American Medical Association Code of Medical Ethics (section 9.4.1).
“In essence, both require that the evaluation be conducted in good faith with the intention to improve care, by physicians with adequate training and knowledge, using a process that is fair and inclusive of the physician under review,” he said. “I believe that most medical staffs abide by these ethical principles, but we have little data to confirm this supposition.”
Did hospital target doc for being vocal?
When members of Methodist’s medical staff first approached Dr. Smith with concerns about his surgery outcomes in November 2018, the physician says he was surprised, but that he was open to an assessment.
“They came to me and said they thought my numbers were bad, and I said: ‘Well my gosh, I certainly don’t want that to be the case. I need to see what numbers you are talking about,’ ” Dr. Smith recalled. “I’ve been president of the Bexar County Medical Society; I’ve been involved with standards and ethics for the Society of Thoracic Surgeons. Quality health care means a whole lot to me.”
The statistical information provided by hospital administrators indicated that Dr. Smith’s mortality rates for coronary artery surgery in 2018 were “excessive” and that his rates for aortic surgery were “unacceptable,” according to a lawsuit Dr. Smith filed against the hospital system. Dr. Smith, who is double boarded with the American Board of Surgery and the American Board of Thoracic Surgery, said his outcomes had never come into question in the past. Dr. Smith said the timing was suspicious to him, however, considering he had recently raised concerns with the hospital through letters about nursing performance, staffing, and compensation.
A peer review investigation was initiated. In the meantime, Dr. Smith agreed to intensivist consults on his postoperative patients and consults with the hospital’s “Heart Team” on all preoperative cardiac, valve, and aortic cases. A vocal critic of the Heart Team, Dr. Smith had long contended the entity provided no meaningful benefit to his patients in most cases and, rather, increased hospital stays and raised medical expenses. Despite his agreement, Dr. Smith was later asked to voluntarily stop performing surgeries at the hospital.
“I agreed, convinced that we’d get this all settled,” he said.
Another report issued by the hospital in 2019 also indicated elevated mortality rates associated with some of Smith’s surgeries, although the document differed from the first report, according to the lawsuit. Dr. Smith says he was ignored when he pointed out problems with the data, including a lack of appropriate risk stratification in the report, departure from Society of Thoracic Surgeons data rules, and improper inclusion of his cases in the denominator of the ratio when a comparison was made of his outcomes with those hospitalwide. A subsequent report from Methodist in March 2019 indicated Dr. Smith’s surgery outcomes were “within the expected parameters of performance,” according to court documents.
The surgery accusations were dropped, but the peer review proceeding against Dr. Smith wasn’t over. The hospital next requested that Dr. Smith undergo a competency evaluation.
“When they realized the data was bad, they then changed their argument in the peer review proceeding and essentially started to argue that Dr. Smith had some sort of cognitive disability that prevented him from continuing to practice,” said Mr. Pullen. “The way I look at it, when the initial basis for the peer review was proven false, the hospital found something else and some other reason to try to keep Dr. Smith from practicing.”
Thus began a lengthy disagreement about which entity would conduct the evaluation, who would pay, and the type of acceptable assessment. An evaluation by the hospital’s preferred organization resulted in a finding of mild cognitive impairment, Dr. Smith said. He hired his own experts who conducted separate evaluations, finding no impairment and no basis for the former evaluation’s conclusion.
“Literally, the determinant as to whether I was normal or below normal on their test was one point, which was associated with a finding that I didn’t draw a clock correctly,” Dr. Smith claimed. “The reviewer said my minute hand was a little too short and docked me a point. It was purely subjective. To me, the gold standard of whether you are learned in thoracic surgery is the American Board of Thoracic Surgery’s test. The board’s test shows my cognitive ability is entirely in keeping with my practice. That contrasts with the one point off I got for drawing a clock wrong in somebody’s estimation.”
Conflict leads to legal case
In September 2020, Dr. Smith filed a lawsuit against Methodist Healthcare System of San Antonio, alleging business disparagement by Methodist for allegedly publishing false and disparaging information about Dr. Smith and tortious interference with business relations. The latter claim stems from Methodist refusing to provide documents to other hospitals about the status of Dr. Smith’s privileges at Methodist, Mr. Pullen said.
Because Methodist refused to confirm his status, the renewal process for Baptist Health System could not be completed and Dr. Smith lost his privileges at Baptist Health System facilities, according to the lawsuit.
Notably, Dr. Smith’s legal challenge also asks the court to take a stance against alleged amendments by Methodist to its Unified Medical Staff Bylaws. The hospital allegedly proposed changes that would prevent physicians from seeking legal action against the hospital for malicious peer review, according to Dr. Smith’s lawsuit.
The amendments would make the peer review process itself the “sole and exclusive remedy with respect to any action or recommendation taken at the hospital affecting medical staff appointment and/or clinical privileges,” according to an excerpt of the proposed amendments included in Dr. Smith’s lawsuit. In addition, the changes would hold practitioners liable for lost revenues if the doctor initiates “any type of legal action challenging credentialing, privileging, or other medical peer review or professional review activity,” according to the lawsuit.
Dr. Smith’s lawsuit seeks a declaration that the proposed amendments to the bylaws are “void as against public policy,” and a declaration that the proposed amendments to the bylaws cannot take away physicians’ statutory right to bring litigation against Methodist for malicious peer review.
“The proposed amendments have a tendency to and will injure the public good,” Dr. Smith argued in the lawsuit. “The proposed amendments allow Methodist to act with malice and in bad faith in conducting peer review proceedings and face no legal repercussions.”
Regardless of the final outcome of the peer review proceeding, Mr. Pullen said the harm Dr. Smith has already endured cannot be reversed.
“Even if comes out in his favor, the damage is already done,” he said. “It will not remedy the damage Dr. Smith has incurred.”
Fighting sham peer review is difficult
Battling a malicious peer review has long been an uphill battle for physicians, according to Dr. Huntoon. That’s because the Health Care Quality Improvement Act (HCQIA), a federal law passed in 1986, provides near absolute immunity to hospitals and peer reviewers in legal disputes.
The HCQIA was created by Congress to extend immunity to good-faith peer review of doctors and to increase overall participation in peer review by removing fear of litigation. However, the act has also enabled abuse of peer review by shielding bad-faith reviewers from accountability, said Dr. Huntoon.
“The Health Care Quality Improvement Act presumes that what the hospital did was warranted and reasonable and shifts the burden to the physician to prove his innocence by a preponderance of evidence,” he said. “That’s an entirely foreign concept to most people who think a person should be considered innocent until proven guilty. Here, it’s the exact opposite.”
The HCQIA has been challenged numerous times over the years and tested at the appellate level, but continues to survive and remain settled law, added Richard B. Willner, DPM, founder and director of the Center for Peer Review Justice, which assists and counsels physicians about sham peer review.
In 2011, former Rep. Joe Heck, DO, (R-Nev.) introduced a bill that would have amended the HCQIA to prohibit a professional review entity from submitting a report to the National Practitioner Data Bank (NPDB) while the doctor was still under investigation and before the doctor was afforded adequate notice and a hearing. Although the measure had 16 cosponsors and plenty of support from the physician community, it failed.
In addition to a heavy legal burden, physicians who experience malicious peer reviews also face ramifications from being reported to the NPDB. Peer review organizations are required to report certain negative actions or findings to the NPDB.
“A databank entry is a scarlet letter on your forehead,” Dr. Willner said. “The rules at a lot of institutions are not to take anyone who has been databanked, rightfully or wrongfully. And what is the evidence necessary to databank you? None. There’s no evidence needed to databank somebody.”
Despite the bleak landscape, experts say progress has been made on a case-by-case basis by physicians who have succeeded in fighting back against questionable peer reviews in recent years.
In January 2020, Indiana ob.gyn. Rebecca Denman, MD, prevailed in her defamation lawsuit against St Vincent Carmel Hospital and St Vincent Carmel Medical Group, winning $4.75 million in damages. Dr. Denman alleged administrators failed to conduct a proper peer review investigation after a false allegation by a nurse that she was under the influence while on the job.
Indianapolis attorney Kathleen A. DeLaney, who represented Dr. Denman, said hospital leaders misled Dr. Denman into believing a peer review had occurred when no formal peer review hearing or proceeding took place.
“The CMO of the medical group claimed that he performed a peer review ‘screening,’ but he never informed the other members of the peer review executive committee of the matter until after he had placed Dr. Denman on administrative leave,” Ms. DeLaney said. “He also neglected to tell the peer review executive committee that the substance abuse policy had not been followed, or that Dr. Denman had not been tested for alcohol use – due to the 12-hour delay in report.”
Dr. Denman was ultimately required to undergo an alcohol abuse evaluation, enter a treatment program, and sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit. She claimed repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
She sued the hospital in July 2018, alleging fraud, defamation, tortious interference with an employment relationship, and negligent misrepresentation. After a 4-day trial, jurors found in her favor, awarding Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
A hospital spokesperson said Ascension St Vincent is pursuing an appeal, and that it looks “forward to the opportunity to bring this matter before the Indiana Court of Appeals in June.”
In another case, South Dakota surgeon Linda Miller, MD, was awarded $1.1 million in 2017 after a federal jury found Huron Regional Medical Center breached her contract and violated her due process rights. Dr. Miller became the subject of a peer review at Huron Regional Medical Center when the hospital began analyzing some of her surgery outcomes.
Ken Barker, an attorney for Dr. Miller, said he feels it became evident at trial that the campaign to force Dr. Miller to either resign or lose her privileges was led by the lay board of directors of the hospital and upper-level administration at the hospital.
“They began the process by ordering an unprecedented 90-day review of her medical charts, looking for errors in the medical care she provided patients,” he said. “They could find nothing, so they did a second 90-day review, waiting for a patient’s ‘bad outcome.’ As any general surgeon will say, a ‘bad outcome’ is inevitable. And so it was. Upon that occurrence, they had a medical review committee review the patient’s chart and use it as an excuse to force her to reduce her privileges. Unbeknown to Dr. Miller, an external review had been conducted on another patient’s chart, in which the external review found her care above the standards and, in some measure, ‘exemplary.’ ”
Dr. Miller was eventually pressured to resign, according to her claim. Because of reports made to the NPDB by the medical center, including a patient complication that was allegedly falsified by the hospital, Dr. Miller said she was unable to find work as a general surgeon and went to work as a wound care doctor. At trial, jurors awarded Dr. Miller $586,617 in lost wages, $343,640 for lost future earning capacity, and $250,000 for mental anguish. (The mental anguish award was subsequently struck by a district court.)
Attorneys for Huron Regional Medical Center argued the jury improperly awarded damages and requested a new trial, which was denied by an appeals court.
In the end, the evidence came to light and the jury’s verdict spoke loudly that the hospital had taken unfair advantage of Dr. Miller, Mr. Barker said. But he emphasized that such cases often end differently.
“There are a handful of cases in which physicians like Dr. Miller have challenged the system and won,” he said. “In most cases, however, it is a ‘David vs. Goliath’ scenario where the giant prevails.”
What to do if faced with malicious peer review
An important step when doctors encounter a peer review that they believe is malicious is to consult with an experienced attorney as early as possible, Dr. Huntoon said. “Not all attorneys who set themselves out to be health law attorneys necessarily have knowledge and expertise in sham peer review. And before such a thing happens, I always encourage physicians to read their medical staff bylaws. That’s where everything is set forth, [such as] the corrective action section that tells how peer review is to take place.”
Mr. Barker added that documentation is also key in the event of a potential malicious peer review.
“When a physician senses [the] administration has targeted them, they should start documenting their conversations and actions very carefully, and if possible, recruit another ‘observer’ who can provide a third-party perspective, if necessary,” Mr. Barker said.
Dr. Huntoon recently wrote an article with advice about preparedness and defense of sham peer reviews. The guidance includes that physicians educate themselves about the tactics used by some hospitals to conduct sham peer reviews and the factors that place doctors more at risk. Factors that may raise a doctor’s danger of being targeted include being in solo practice or a small group, being new on staff, or being an older physician approaching retirement as some bad-actor hospitals may view older physicians as being less likely to fight back, said Dr. Huntoon.
Doctors should also keep detailed records and a timeline in the event of a malicious peer review and insist that an independent court reporter record all peer review hearings, even if that means the physician has to pay for the reporter him or herself, according to the guidance. An independent record is invaluable should the physician ultimately issue a future legal challenge against the hospital.
Mr. Willner encourages physicians to call the Center for Peer Review Justice hotline at (504) 621-1670 or visit the website for help with peer review and NPDB issues.
As for Dr. Smith, his days are much quieter and slower today, compared with the active practice he was accustomed to for more than half his life. He misses the fast pace, the patients, and the work that always brought him great joy.
“I hope to get back to doing surgeries eventually,” he said. “I graduated medical school in 1972. Practicing surgery has been my whole life and my career. They have taken my identity and my livelihood away from me based on false numbers and false premises. I want it back.”
A version of this article first appeared on Medscape.com.
Cardiothoracic surgeon J. Marvin Smith III, MD, had always thrived on a busy practice schedule, often performing 20-30 surgeries a week. A practicing surgeon for more than 40 years, Dr. Smith said he had no plans to slow down anytime soon.
But Dr. Smith said his career was derailed when leaders at Methodist Healthcare System of San Antonio initiated a sudden peer review proceeding against him. The hospital system alleged certain surgeries performed by Dr. Smith had excessive mortality rates. When he proved the data inaccurate, Dr. Smith said administrators next claimed he was cognitively impaired and wasn’t safe to practice.
Dr. Smith has now been embroiled in a peer review dispute with the hospital system for more than 2 years and says the conflict has essentially forced him out of surgical practice. He believes the peer review was “malicious” and was really launched because of complaints he made about nurse staffing and other issues at the hospital.
“I think it is absolutely in bad faith and is disingenuous what they’ve told me along the way,” said Dr. Smith, 73. “It’s because I pointed out deficiencies in nursing care, and they want to get rid of me. It would be a lot easier for them if I had a contract and they could control me better. But the fact that I was independent, meant they had to resort to a malicious peer review to try and push me out.”
Dr. Smith had a peer review hearing with Methodist in March 2021, and in April, a panel found in Dr. Smith’s favor, according to Dr. Smith. The findings were sent to the hospital’s medical board for review, which issued a decision in early May.
Eric A. Pullen, an attorney for Dr. Smith, said he could not go into detail about the board’s decision for legal reasons, but that “the medical board’s decision did not completely resolve the matter, and Dr. Smith intends to exercise his procedural rights, which could include an appeal.”
Methodist Hospital Texsan and its parent company, Methodist Health System of San Antonio, did not respond to messages seeking comment about the case. Without hearing from the hospital system, its side is unknown and it is unclear if there is more to the story from Methodist’s view.
The problem is not new, but some experts, such as Lawrence Huntoon, MD, PhD, say the practice has become more common in recent years, particularly against independent doctors.
Dr. Huntoon believes there is a nationwide trend at many hospitals to get rid of independent physicians and replace them with employed doctors, he said.
However, because most sham peer reviews go on behind closed doors, there are no data to pinpoint its prevalence or measure its growth.
“Independent physicians are basically being purged from medical staffs across the United States,” said Dr. Huntoon, who is chair of the Association of American Physicians and Surgeons’ Committee to Combat Sham Peer Review. “The hospitals want more control over how physicians practice and who they refer to, and they do that by having employees.”
Anthony P. Weiss, MD, MBA, chief medical officer for Beth Israel Deaconess Medical Center said it has not been his experience that independent physicians are being targeted in such a way. Dr. Weiss responded to an inquiry sent to the American Hospital Association for this story.
“As the authority for peer review rests with the organized medical staff (i.e., physicians), and not formally with the hospital per se, the peer review lever is not typically available as a management tool for hospital administration,” said Dr. Weiss, who is a former member of the AHA’s Committee on Clinical Leadership, but who was speaking on behalf of himself.
A spokesman for the AHA said the organization stands behinds Dr. Weiss’ comments.
Peer review remains a foundational aspect of overseeing the safety and appropriateness of healthcare provided by physicians, Dr. Weiss said. Peer review likely varies from hospital to hospital, he added, although the Healthcare Quality Improvement Act provides some level of guidance as does the American Medical Association Code of Medical Ethics (section 9.4.1).
“In essence, both require that the evaluation be conducted in good faith with the intention to improve care, by physicians with adequate training and knowledge, using a process that is fair and inclusive of the physician under review,” he said. “I believe that most medical staffs abide by these ethical principles, but we have little data to confirm this supposition.”
Did hospital target doc for being vocal?
When members of Methodist’s medical staff first approached Dr. Smith with concerns about his surgery outcomes in November 2018, the physician says he was surprised, but that he was open to an assessment.
“They came to me and said they thought my numbers were bad, and I said: ‘Well my gosh, I certainly don’t want that to be the case. I need to see what numbers you are talking about,’ ” Dr. Smith recalled. “I’ve been president of the Bexar County Medical Society; I’ve been involved with standards and ethics for the Society of Thoracic Surgeons. Quality health care means a whole lot to me.”
The statistical information provided by hospital administrators indicated that Dr. Smith’s mortality rates for coronary artery surgery in 2018 were “excessive” and that his rates for aortic surgery were “unacceptable,” according to a lawsuit Dr. Smith filed against the hospital system. Dr. Smith, who is double boarded with the American Board of Surgery and the American Board of Thoracic Surgery, said his outcomes had never come into question in the past. Dr. Smith said the timing was suspicious to him, however, considering he had recently raised concerns with the hospital through letters about nursing performance, staffing, and compensation.
A peer review investigation was initiated. In the meantime, Dr. Smith agreed to intensivist consults on his postoperative patients and consults with the hospital’s “Heart Team” on all preoperative cardiac, valve, and aortic cases. A vocal critic of the Heart Team, Dr. Smith had long contended the entity provided no meaningful benefit to his patients in most cases and, rather, increased hospital stays and raised medical expenses. Despite his agreement, Dr. Smith was later asked to voluntarily stop performing surgeries at the hospital.
“I agreed, convinced that we’d get this all settled,” he said.
Another report issued by the hospital in 2019 also indicated elevated mortality rates associated with some of Smith’s surgeries, although the document differed from the first report, according to the lawsuit. Dr. Smith says he was ignored when he pointed out problems with the data, including a lack of appropriate risk stratification in the report, departure from Society of Thoracic Surgeons data rules, and improper inclusion of his cases in the denominator of the ratio when a comparison was made of his outcomes with those hospitalwide. A subsequent report from Methodist in March 2019 indicated Dr. Smith’s surgery outcomes were “within the expected parameters of performance,” according to court documents.
The surgery accusations were dropped, but the peer review proceeding against Dr. Smith wasn’t over. The hospital next requested that Dr. Smith undergo a competency evaluation.
“When they realized the data was bad, they then changed their argument in the peer review proceeding and essentially started to argue that Dr. Smith had some sort of cognitive disability that prevented him from continuing to practice,” said Mr. Pullen. “The way I look at it, when the initial basis for the peer review was proven false, the hospital found something else and some other reason to try to keep Dr. Smith from practicing.”
Thus began a lengthy disagreement about which entity would conduct the evaluation, who would pay, and the type of acceptable assessment. An evaluation by the hospital’s preferred organization resulted in a finding of mild cognitive impairment, Dr. Smith said. He hired his own experts who conducted separate evaluations, finding no impairment and no basis for the former evaluation’s conclusion.
“Literally, the determinant as to whether I was normal or below normal on their test was one point, which was associated with a finding that I didn’t draw a clock correctly,” Dr. Smith claimed. “The reviewer said my minute hand was a little too short and docked me a point. It was purely subjective. To me, the gold standard of whether you are learned in thoracic surgery is the American Board of Thoracic Surgery’s test. The board’s test shows my cognitive ability is entirely in keeping with my practice. That contrasts with the one point off I got for drawing a clock wrong in somebody’s estimation.”
Conflict leads to legal case
In September 2020, Dr. Smith filed a lawsuit against Methodist Healthcare System of San Antonio, alleging business disparagement by Methodist for allegedly publishing false and disparaging information about Dr. Smith and tortious interference with business relations. The latter claim stems from Methodist refusing to provide documents to other hospitals about the status of Dr. Smith’s privileges at Methodist, Mr. Pullen said.
Because Methodist refused to confirm his status, the renewal process for Baptist Health System could not be completed and Dr. Smith lost his privileges at Baptist Health System facilities, according to the lawsuit.
Notably, Dr. Smith’s legal challenge also asks the court to take a stance against alleged amendments by Methodist to its Unified Medical Staff Bylaws. The hospital allegedly proposed changes that would prevent physicians from seeking legal action against the hospital for malicious peer review, according to Dr. Smith’s lawsuit.
The amendments would make the peer review process itself the “sole and exclusive remedy with respect to any action or recommendation taken at the hospital affecting medical staff appointment and/or clinical privileges,” according to an excerpt of the proposed amendments included in Dr. Smith’s lawsuit. In addition, the changes would hold practitioners liable for lost revenues if the doctor initiates “any type of legal action challenging credentialing, privileging, or other medical peer review or professional review activity,” according to the lawsuit.
Dr. Smith’s lawsuit seeks a declaration that the proposed amendments to the bylaws are “void as against public policy,” and a declaration that the proposed amendments to the bylaws cannot take away physicians’ statutory right to bring litigation against Methodist for malicious peer review.
“The proposed amendments have a tendency to and will injure the public good,” Dr. Smith argued in the lawsuit. “The proposed amendments allow Methodist to act with malice and in bad faith in conducting peer review proceedings and face no legal repercussions.”
Regardless of the final outcome of the peer review proceeding, Mr. Pullen said the harm Dr. Smith has already endured cannot be reversed.
“Even if comes out in his favor, the damage is already done,” he said. “It will not remedy the damage Dr. Smith has incurred.”
Fighting sham peer review is difficult
Battling a malicious peer review has long been an uphill battle for physicians, according to Dr. Huntoon. That’s because the Health Care Quality Improvement Act (HCQIA), a federal law passed in 1986, provides near absolute immunity to hospitals and peer reviewers in legal disputes.
The HCQIA was created by Congress to extend immunity to good-faith peer review of doctors and to increase overall participation in peer review by removing fear of litigation. However, the act has also enabled abuse of peer review by shielding bad-faith reviewers from accountability, said Dr. Huntoon.
“The Health Care Quality Improvement Act presumes that what the hospital did was warranted and reasonable and shifts the burden to the physician to prove his innocence by a preponderance of evidence,” he said. “That’s an entirely foreign concept to most people who think a person should be considered innocent until proven guilty. Here, it’s the exact opposite.”
The HCQIA has been challenged numerous times over the years and tested at the appellate level, but continues to survive and remain settled law, added Richard B. Willner, DPM, founder and director of the Center for Peer Review Justice, which assists and counsels physicians about sham peer review.
In 2011, former Rep. Joe Heck, DO, (R-Nev.) introduced a bill that would have amended the HCQIA to prohibit a professional review entity from submitting a report to the National Practitioner Data Bank (NPDB) while the doctor was still under investigation and before the doctor was afforded adequate notice and a hearing. Although the measure had 16 cosponsors and plenty of support from the physician community, it failed.
In addition to a heavy legal burden, physicians who experience malicious peer reviews also face ramifications from being reported to the NPDB. Peer review organizations are required to report certain negative actions or findings to the NPDB.
“A databank entry is a scarlet letter on your forehead,” Dr. Willner said. “The rules at a lot of institutions are not to take anyone who has been databanked, rightfully or wrongfully. And what is the evidence necessary to databank you? None. There’s no evidence needed to databank somebody.”
Despite the bleak landscape, experts say progress has been made on a case-by-case basis by physicians who have succeeded in fighting back against questionable peer reviews in recent years.
In January 2020, Indiana ob.gyn. Rebecca Denman, MD, prevailed in her defamation lawsuit against St Vincent Carmel Hospital and St Vincent Carmel Medical Group, winning $4.75 million in damages. Dr. Denman alleged administrators failed to conduct a proper peer review investigation after a false allegation by a nurse that she was under the influence while on the job.
Indianapolis attorney Kathleen A. DeLaney, who represented Dr. Denman, said hospital leaders misled Dr. Denman into believing a peer review had occurred when no formal peer review hearing or proceeding took place.
“The CMO of the medical group claimed that he performed a peer review ‘screening,’ but he never informed the other members of the peer review executive committee of the matter until after he had placed Dr. Denman on administrative leave,” Ms. DeLaney said. “He also neglected to tell the peer review executive committee that the substance abuse policy had not been followed, or that Dr. Denman had not been tested for alcohol use – due to the 12-hour delay in report.”
Dr. Denman was ultimately required to undergo an alcohol abuse evaluation, enter a treatment program, and sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit. She claimed repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
She sued the hospital in July 2018, alleging fraud, defamation, tortious interference with an employment relationship, and negligent misrepresentation. After a 4-day trial, jurors found in her favor, awarding Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
A hospital spokesperson said Ascension St Vincent is pursuing an appeal, and that it looks “forward to the opportunity to bring this matter before the Indiana Court of Appeals in June.”
In another case, South Dakota surgeon Linda Miller, MD, was awarded $1.1 million in 2017 after a federal jury found Huron Regional Medical Center breached her contract and violated her due process rights. Dr. Miller became the subject of a peer review at Huron Regional Medical Center when the hospital began analyzing some of her surgery outcomes.
Ken Barker, an attorney for Dr. Miller, said he feels it became evident at trial that the campaign to force Dr. Miller to either resign or lose her privileges was led by the lay board of directors of the hospital and upper-level administration at the hospital.
“They began the process by ordering an unprecedented 90-day review of her medical charts, looking for errors in the medical care she provided patients,” he said. “They could find nothing, so they did a second 90-day review, waiting for a patient’s ‘bad outcome.’ As any general surgeon will say, a ‘bad outcome’ is inevitable. And so it was. Upon that occurrence, they had a medical review committee review the patient’s chart and use it as an excuse to force her to reduce her privileges. Unbeknown to Dr. Miller, an external review had been conducted on another patient’s chart, in which the external review found her care above the standards and, in some measure, ‘exemplary.’ ”
Dr. Miller was eventually pressured to resign, according to her claim. Because of reports made to the NPDB by the medical center, including a patient complication that was allegedly falsified by the hospital, Dr. Miller said she was unable to find work as a general surgeon and went to work as a wound care doctor. At trial, jurors awarded Dr. Miller $586,617 in lost wages, $343,640 for lost future earning capacity, and $250,000 for mental anguish. (The mental anguish award was subsequently struck by a district court.)
Attorneys for Huron Regional Medical Center argued the jury improperly awarded damages and requested a new trial, which was denied by an appeals court.
In the end, the evidence came to light and the jury’s verdict spoke loudly that the hospital had taken unfair advantage of Dr. Miller, Mr. Barker said. But he emphasized that such cases often end differently.
“There are a handful of cases in which physicians like Dr. Miller have challenged the system and won,” he said. “In most cases, however, it is a ‘David vs. Goliath’ scenario where the giant prevails.”
What to do if faced with malicious peer review
An important step when doctors encounter a peer review that they believe is malicious is to consult with an experienced attorney as early as possible, Dr. Huntoon said. “Not all attorneys who set themselves out to be health law attorneys necessarily have knowledge and expertise in sham peer review. And before such a thing happens, I always encourage physicians to read their medical staff bylaws. That’s where everything is set forth, [such as] the corrective action section that tells how peer review is to take place.”
Mr. Barker added that documentation is also key in the event of a potential malicious peer review.
“When a physician senses [the] administration has targeted them, they should start documenting their conversations and actions very carefully, and if possible, recruit another ‘observer’ who can provide a third-party perspective, if necessary,” Mr. Barker said.
Dr. Huntoon recently wrote an article with advice about preparedness and defense of sham peer reviews. The guidance includes that physicians educate themselves about the tactics used by some hospitals to conduct sham peer reviews and the factors that place doctors more at risk. Factors that may raise a doctor’s danger of being targeted include being in solo practice or a small group, being new on staff, or being an older physician approaching retirement as some bad-actor hospitals may view older physicians as being less likely to fight back, said Dr. Huntoon.
Doctors should also keep detailed records and a timeline in the event of a malicious peer review and insist that an independent court reporter record all peer review hearings, even if that means the physician has to pay for the reporter him or herself, according to the guidance. An independent record is invaluable should the physician ultimately issue a future legal challenge against the hospital.
Mr. Willner encourages physicians to call the Center for Peer Review Justice hotline at (504) 621-1670 or visit the website for help with peer review and NPDB issues.
As for Dr. Smith, his days are much quieter and slower today, compared with the active practice he was accustomed to for more than half his life. He misses the fast pace, the patients, and the work that always brought him great joy.
“I hope to get back to doing surgeries eventually,” he said. “I graduated medical school in 1972. Practicing surgery has been my whole life and my career. They have taken my identity and my livelihood away from me based on false numbers and false premises. I want it back.”
A version of this article first appeared on Medscape.com.
FDA and power morcellation, gel for vaginal odor, and an intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
Communication Strategies in Mohs Micrographic Surgery: A Survey of Methods, Time Savings, and Perceived Patient Satisfaction
Mohs micrographic surgery (MMS) entails multiple time-consuming surgical and histological examinations for each patient. As surgical stages are performed and histological sections are processed, an efficient communication method among providers, medical assistants, histotechnologists, and patients is necessary to avoid delays. To address these and other communication issues, providers have focused on ways to increase clinic efficiency and improve patient-reported outcomes by utilizing new or repurposed communication technologies in their Mohs practice.
Prior reports have highlighted the utility of hands-free headsets that allow real-time communication among staff members as a means of increasing clinic efficiency and decreasing patient wait times.1-4 These systems may mediate a more rapid turnover between stages by mitigating the need for surgeons and support staff to assemble within a designated workspace.1,3,4 However, there is no single or standardized communication method that best suits all surgical suites and MMS practices. Our study aimed to identify the current communication strategies employed by Mohs surgeons and thereby ascertain which method(s) portend(s) the highest benefit in average daily time savings and provider-perceived patient satisfaction.
Materials and Methods
Survey Instrument
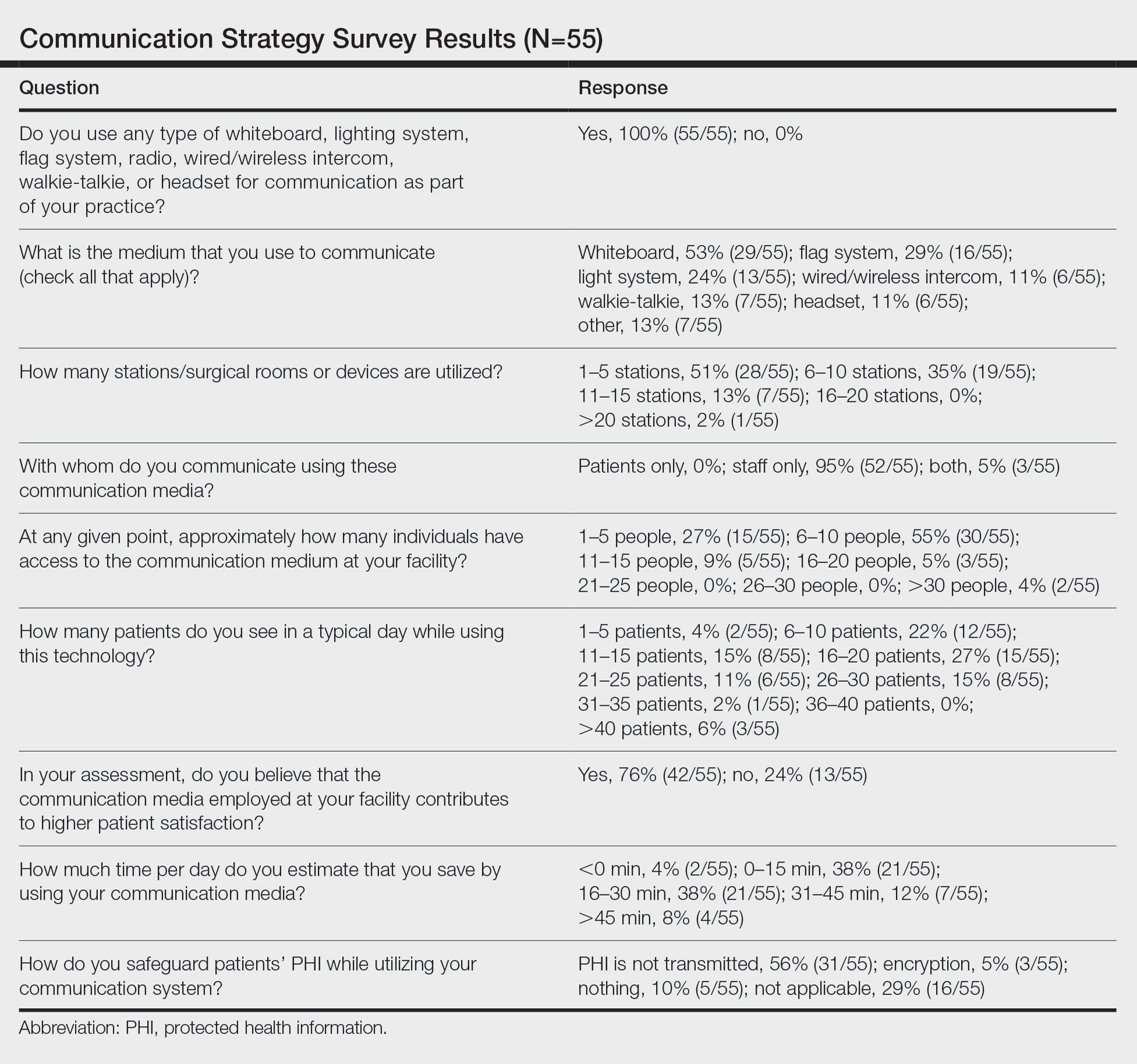
A new 10-question electronic survey was published on the SurveyMonkey website, and a link to the survey was provided in a quarterly email that originated from the American College of Mohs Surgery and was distributed to all 1735 active members. Responses were obtained from January 2019 to February 2019.
Statistical Analysis
A statistical analysis was done to determine any significant associations among the providers’ responses. P<.05 was used to determine statistical significance. A Cochran-Armitage test for trend was used to identify significant associations between the number of rooms and the communication systems that were used. Thus, 7 total tests—1 for each device (whiteboard, light system, flag system, wired intercom, wireless intercom, walkie-talkie, or headset)—were conducted. The Cochran-Armitage test also was used to determine whether the probability of using the device was affected by the number of stations/surgical rooms that were attended by the Mohs surgeons. To determine whether the communication devices used were associated with higher patient satisfaction, a χ2 test was conducted for each device (7 total tests), testing the categories of using that device (yes/no) and patient satisfaction (yes/no). A Fisher exact test of independence was used in any case where the proportion for the device and patient satisfaction was 25% or higher. To determine whether the communication method was associated with increased time savings, 7 total Cochran-Armitage tests were conducted, 1 for each device. A logistic regression model was used to determine whether there was a significant association between the number of stations and the likelihood of reporting patient satisfaction.
Results
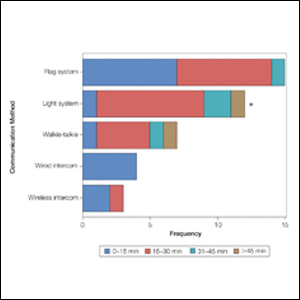
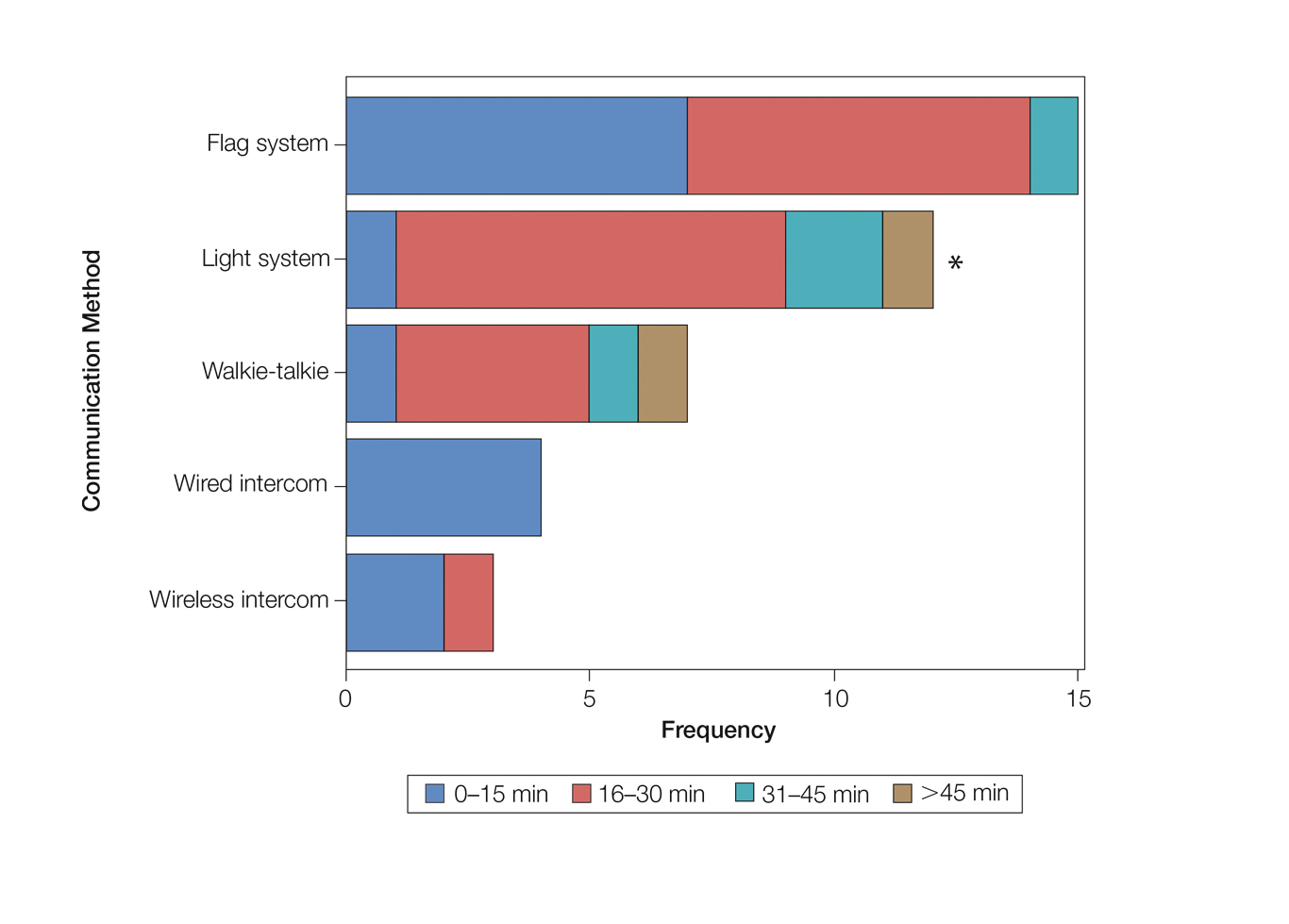
Eighty-eight surgeons responded to the survey, with a response rate of 5% (88/1735). A total of 55 surgeons completed the survey in its entirety and were included in the data analysis. The most commonly used communication mediums were whiteboards (29/55 [53%]), followed by a flag system (16/55 [29%]) and a light system (13/55 [24%]). Most Mohs surgeons (52/55 [95%]) used the communication media to communicate with their staff only, and 76% (42/55) of Mohs surgeons believed that their communication media contributed to higher patient satisfaction. Overall, 58% (32/55) of Mohs surgeons stated that their communication media saved more than 15 minutes (on average) per day. The use of a whiteboard and/or flag system was reported as the least efficient method, with average daily time savings of 13 minutes. With the introduction of newer technology (wired or wireless intercoms, headsets, walkie-talkies, or internal messaging systems such as Skype) to the whiteboard and/or flag system, the time savings increased by 10 minutes per day. Nearly 25% (14/55) of surgeons utilized more than 1 communication system.
As the number of stations in an MMS suite increased, the probability of using a whiteboard to track the progress of the cases decreased. There were no statistically significant associations identified between the number of stations and the use of other communication devices (ie, flag system, light system, wireless intercom, wired intercom, walkie-talkie, headset). The stratified percentages of the amount of time savings for each communication modality are presented in the Figure (whiteboards and headsets were excluded because they did not increase time savings). The use of a light system was the only communication modality found to be statistically associated with an increase in provider-reported time savings (P=.0482; Figure). In addition, our analysis did not show an improvement in provider-reported patient satisfaction with any of the current systems used in MMS clinics.
Comment
The process of transmitting information among the medical team during MMS is a complex interplay involving the relay of crucial information, with many opportunities for the introduction of distraction and error. Despite numerous improvements in the efficiency of the preparation of histological specimens and implementation of various time-saving and tissue-saving surgical interventions, relatively little attention has been given to address the sometimes chaotic and challenging process of organizing results from each stage of multiple patients in an MMS surgical suite.5
As demonstrated by our survey, incorporation of a light-based system into an MMS clinic may improve workplace efficiency by decreasing the redundant use of support staff and allowing Mohs surgeons to transition from one station to the next seamlessly. Light-based communication systems provide an immediate notification for support staff via color-coded and/or numerically coded indicators on input switches located outside and inside the examination/surgery rooms. The switch indicators can be depressed with minimal disruption from station to station, thereby foregoing the need to interrupt an ongoing excision or closure to convey the status of the case. These systems may then permit enhanced clinic and workflow efficiency, which may help to shorten patient wait times.
Study Limitation
Although all members of the American College of Mohs Surgery were invited to participate in this online survey, only a small number (N=55) completed it in its entirety. Moreover, sample sizes for some of the communication devices were small. As a result, many of the tests might be lacking sufficient power to detect possible relationships, which might be identified in future larger-scale studies.
Conclusion
Our study supports the use of light-based communication systems in MMS suites to improve efficiency in the clinic. Based on our analysis, light-based communication methods were significantly associated with improved time savings (P=.0482). Our study did not show an improvement in provider-reported satisfaction with any of the current systems used in MMS clinics. We hope that this information will help guide providers in implementing new communication techniques to improve clinic efficiency.
Acknowledgments
The authors would like to thank Ms. Kathy Kyler (Oklahoma City, Oklahoma) for her assistance in preparing this manuscript. Support for Dr. Chen and Mr. Stubblefield was provided through National Institutes of Health, National Institute of General Medical Sciences [Grant 2U54GM104938-06, PI Judith James].
- Chen T, Vines L, Wanitphakdeedecha R, et al. Electronically linked: wireless, discrete, hands-free communication to improve surgical workflow in Mohs and dermasurgery clinic. Dermatol Surg. 2009;35:248-252.
- Lanto AB, Yano EM, Fink A, et al. Anatomy of an outpatient visit. An evaluation of clinic efficiency in general and subspecialty clinics. Med Group Manage J. 1995;42:18-25.
- Kantor J. Application of Google Glass to Mohs micrographic surgery: a pilot study in 120 patients. Dermatol Surg. 2015;41:288-289.
- Spurk PA, Mohr ML, Seroka AM, et al. The impact of a wireless telecommunication system on efficiency. J Nurs Admin. 1995;25:21-26.
- Dietert JB, MacFarlane DF. A survey of Mohs tissue tracking practices. Dermatol Surg. 2019;45:514-518.
Mohs micrographic surgery (MMS) entails multiple time-consuming surgical and histological examinations for each patient. As surgical stages are performed and histological sections are processed, an efficient communication method among providers, medical assistants, histotechnologists, and patients is necessary to avoid delays. To address these and other communication issues, providers have focused on ways to increase clinic efficiency and improve patient-reported outcomes by utilizing new or repurposed communication technologies in their Mohs practice.
Prior reports have highlighted the utility of hands-free headsets that allow real-time communication among staff members as a means of increasing clinic efficiency and decreasing patient wait times.1-4 These systems may mediate a more rapid turnover between stages by mitigating the need for surgeons and support staff to assemble within a designated workspace.1,3,4 However, there is no single or standardized communication method that best suits all surgical suites and MMS practices. Our study aimed to identify the current communication strategies employed by Mohs surgeons and thereby ascertain which method(s) portend(s) the highest benefit in average daily time savings and provider-perceived patient satisfaction.
Materials and Methods
Survey Instrument
A new 10-question electronic survey was published on the SurveyMonkey website, and a link to the survey was provided in a quarterly email that originated from the American College of Mohs Surgery and was distributed to all 1735 active members. Responses were obtained from January 2019 to February 2019.
Statistical Analysis
A statistical analysis was done to determine any significant associations among the providers’ responses. P<.05 was used to determine statistical significance. A Cochran-Armitage test for trend was used to identify significant associations between the number of rooms and the communication systems that were used. Thus, 7 total tests—1 for each device (whiteboard, light system, flag system, wired intercom, wireless intercom, walkie-talkie, or headset)—were conducted. The Cochran-Armitage test also was used to determine whether the probability of using the device was affected by the number of stations/surgical rooms that were attended by the Mohs surgeons. To determine whether the communication devices used were associated with higher patient satisfaction, a χ2 test was conducted for each device (7 total tests), testing the categories of using that device (yes/no) and patient satisfaction (yes/no). A Fisher exact test of independence was used in any case where the proportion for the device and patient satisfaction was 25% or higher. To determine whether the communication method was associated with increased time savings, 7 total Cochran-Armitage tests were conducted, 1 for each device. A logistic regression model was used to determine whether there was a significant association between the number of stations and the likelihood of reporting patient satisfaction.
Results
Eighty-eight surgeons responded to the survey, with a response rate of 5% (88/1735). A total of 55 surgeons completed the survey in its entirety and were included in the data analysis. The most commonly used communication mediums were whiteboards (29/55 [53%]), followed by a flag system (16/55 [29%]) and a light system (13/55 [24%]). Most Mohs surgeons (52/55 [95%]) used the communication media to communicate with their staff only, and 76% (42/55) of Mohs surgeons believed that their communication media contributed to higher patient satisfaction. Overall, 58% (32/55) of Mohs surgeons stated that their communication media saved more than 15 minutes (on average) per day. The use of a whiteboard and/or flag system was reported as the least efficient method, with average daily time savings of 13 minutes. With the introduction of newer technology (wired or wireless intercoms, headsets, walkie-talkies, or internal messaging systems such as Skype) to the whiteboard and/or flag system, the time savings increased by 10 minutes per day. Nearly 25% (14/55) of surgeons utilized more than 1 communication system.
As the number of stations in an MMS suite increased, the probability of using a whiteboard to track the progress of the cases decreased. There were no statistically significant associations identified between the number of stations and the use of other communication devices (ie, flag system, light system, wireless intercom, wired intercom, walkie-talkie, headset). The stratified percentages of the amount of time savings for each communication modality are presented in the Figure (whiteboards and headsets were excluded because they did not increase time savings). The use of a light system was the only communication modality found to be statistically associated with an increase in provider-reported time savings (P=.0482; Figure). In addition, our analysis did not show an improvement in provider-reported patient satisfaction with any of the current systems used in MMS clinics.

Comment
The process of transmitting information among the medical team during MMS is a complex interplay involving the relay of crucial information, with many opportunities for the introduction of distraction and error. Despite numerous improvements in the efficiency of the preparation of histological specimens and implementation of various time-saving and tissue-saving surgical interventions, relatively little attention has been given to address the sometimes chaotic and challenging process of organizing results from each stage of multiple patients in an MMS surgical suite.5
As demonstrated by our survey, incorporation of a light-based system into an MMS clinic may improve workplace efficiency by decreasing the redundant use of support staff and allowing Mohs surgeons to transition from one station to the next seamlessly. Light-based communication systems provide an immediate notification for support staff via color-coded and/or numerically coded indicators on input switches located outside and inside the examination/surgery rooms. The switch indicators can be depressed with minimal disruption from station to station, thereby foregoing the need to interrupt an ongoing excision or closure to convey the status of the case. These systems may then permit enhanced clinic and workflow efficiency, which may help to shorten patient wait times.

Study Limitation
Although all members of the American College of Mohs Surgery were invited to participate in this online survey, only a small number (N=55) completed it in its entirety. Moreover, sample sizes for some of the communication devices were small. As a result, many of the tests might be lacking sufficient power to detect possible relationships, which might be identified in future larger-scale studies.
Conclusion
Our study supports the use of light-based communication systems in MMS suites to improve efficiency in the clinic. Based on our analysis, light-based communication methods were significantly associated with improved time savings (P=.0482). Our study did not show an improvement in provider-reported satisfaction with any of the current systems used in MMS clinics. We hope that this information will help guide providers in implementing new communication techniques to improve clinic efficiency.
Acknowledgments
The authors would like to thank Ms. Kathy Kyler (Oklahoma City, Oklahoma) for her assistance in preparing this manuscript. Support for Dr. Chen and Mr. Stubblefield was provided through National Institutes of Health, National Institute of General Medical Sciences [Grant 2U54GM104938-06, PI Judith James].
Mohs micrographic surgery (MMS) entails multiple time-consuming surgical and histological examinations for each patient. As surgical stages are performed and histological sections are processed, an efficient communication method among providers, medical assistants, histotechnologists, and patients is necessary to avoid delays. To address these and other communication issues, providers have focused on ways to increase clinic efficiency and improve patient-reported outcomes by utilizing new or repurposed communication technologies in their Mohs practice.
Prior reports have highlighted the utility of hands-free headsets that allow real-time communication among staff members as a means of increasing clinic efficiency and decreasing patient wait times.1-4 These systems may mediate a more rapid turnover between stages by mitigating the need for surgeons and support staff to assemble within a designated workspace.1,3,4 However, there is no single or standardized communication method that best suits all surgical suites and MMS practices. Our study aimed to identify the current communication strategies employed by Mohs surgeons and thereby ascertain which method(s) portend(s) the highest benefit in average daily time savings and provider-perceived patient satisfaction.
Materials and Methods
Survey Instrument
A new 10-question electronic survey was published on the SurveyMonkey website, and a link to the survey was provided in a quarterly email that originated from the American College of Mohs Surgery and was distributed to all 1735 active members. Responses were obtained from January 2019 to February 2019.
Statistical Analysis
A statistical analysis was done to determine any significant associations among the providers’ responses. P<.05 was used to determine statistical significance. A Cochran-Armitage test for trend was used to identify significant associations between the number of rooms and the communication systems that were used. Thus, 7 total tests—1 for each device (whiteboard, light system, flag system, wired intercom, wireless intercom, walkie-talkie, or headset)—were conducted. The Cochran-Armitage test also was used to determine whether the probability of using the device was affected by the number of stations/surgical rooms that were attended by the Mohs surgeons. To determine whether the communication devices used were associated with higher patient satisfaction, a χ2 test was conducted for each device (7 total tests), testing the categories of using that device (yes/no) and patient satisfaction (yes/no). A Fisher exact test of independence was used in any case where the proportion for the device and patient satisfaction was 25% or higher. To determine whether the communication method was associated with increased time savings, 7 total Cochran-Armitage tests were conducted, 1 for each device. A logistic regression model was used to determine whether there was a significant association between the number of stations and the likelihood of reporting patient satisfaction.
Results
Eighty-eight surgeons responded to the survey, with a response rate of 5% (88/1735). A total of 55 surgeons completed the survey in its entirety and were included in the data analysis. The most commonly used communication mediums were whiteboards (29/55 [53%]), followed by a flag system (16/55 [29%]) and a light system (13/55 [24%]). Most Mohs surgeons (52/55 [95%]) used the communication media to communicate with their staff only, and 76% (42/55) of Mohs surgeons believed that their communication media contributed to higher patient satisfaction. Overall, 58% (32/55) of Mohs surgeons stated that their communication media saved more than 15 minutes (on average) per day. The use of a whiteboard and/or flag system was reported as the least efficient method, with average daily time savings of 13 minutes. With the introduction of newer technology (wired or wireless intercoms, headsets, walkie-talkies, or internal messaging systems such as Skype) to the whiteboard and/or flag system, the time savings increased by 10 minutes per day. Nearly 25% (14/55) of surgeons utilized more than 1 communication system.
As the number of stations in an MMS suite increased, the probability of using a whiteboard to track the progress of the cases decreased. There were no statistically significant associations identified between the number of stations and the use of other communication devices (ie, flag system, light system, wireless intercom, wired intercom, walkie-talkie, headset). The stratified percentages of the amount of time savings for each communication modality are presented in the Figure (whiteboards and headsets were excluded because they did not increase time savings). The use of a light system was the only communication modality found to be statistically associated with an increase in provider-reported time savings (P=.0482; Figure). In addition, our analysis did not show an improvement in provider-reported patient satisfaction with any of the current systems used in MMS clinics.

Comment
The process of transmitting information among the medical team during MMS is a complex interplay involving the relay of crucial information, with many opportunities for the introduction of distraction and error. Despite numerous improvements in the efficiency of the preparation of histological specimens and implementation of various time-saving and tissue-saving surgical interventions, relatively little attention has been given to address the sometimes chaotic and challenging process of organizing results from each stage of multiple patients in an MMS surgical suite.5
As demonstrated by our survey, incorporation of a light-based system into an MMS clinic may improve workplace efficiency by decreasing the redundant use of support staff and allowing Mohs surgeons to transition from one station to the next seamlessly. Light-based communication systems provide an immediate notification for support staff via color-coded and/or numerically coded indicators on input switches located outside and inside the examination/surgery rooms. The switch indicators can be depressed with minimal disruption from station to station, thereby foregoing the need to interrupt an ongoing excision or closure to convey the status of the case. These systems may then permit enhanced clinic and workflow efficiency, which may help to shorten patient wait times.

Study Limitation
Although all members of the American College of Mohs Surgery were invited to participate in this online survey, only a small number (N=55) completed it in its entirety. Moreover, sample sizes for some of the communication devices were small. As a result, many of the tests might be lacking sufficient power to detect possible relationships, which might be identified in future larger-scale studies.
Conclusion
Our study supports the use of light-based communication systems in MMS suites to improve efficiency in the clinic. Based on our analysis, light-based communication methods were significantly associated with improved time savings (P=.0482). Our study did not show an improvement in provider-reported satisfaction with any of the current systems used in MMS clinics. We hope that this information will help guide providers in implementing new communication techniques to improve clinic efficiency.
Acknowledgments
The authors would like to thank Ms. Kathy Kyler (Oklahoma City, Oklahoma) for her assistance in preparing this manuscript. Support for Dr. Chen and Mr. Stubblefield was provided through National Institutes of Health, National Institute of General Medical Sciences [Grant 2U54GM104938-06, PI Judith James].
- Chen T, Vines L, Wanitphakdeedecha R, et al. Electronically linked: wireless, discrete, hands-free communication to improve surgical workflow in Mohs and dermasurgery clinic. Dermatol Surg. 2009;35:248-252.
- Lanto AB, Yano EM, Fink A, et al. Anatomy of an outpatient visit. An evaluation of clinic efficiency in general and subspecialty clinics. Med Group Manage J. 1995;42:18-25.
- Kantor J. Application of Google Glass to Mohs micrographic surgery: a pilot study in 120 patients. Dermatol Surg. 2015;41:288-289.
- Spurk PA, Mohr ML, Seroka AM, et al. The impact of a wireless telecommunication system on efficiency. J Nurs Admin. 1995;25:21-26.
- Dietert JB, MacFarlane DF. A survey of Mohs tissue tracking practices. Dermatol Surg. 2019;45:514-518.
- Chen T, Vines L, Wanitphakdeedecha R, et al. Electronically linked: wireless, discrete, hands-free communication to improve surgical workflow in Mohs and dermasurgery clinic. Dermatol Surg. 2009;35:248-252.
- Lanto AB, Yano EM, Fink A, et al. Anatomy of an outpatient visit. An evaluation of clinic efficiency in general and subspecialty clinics. Med Group Manage J. 1995;42:18-25.
- Kantor J. Application of Google Glass to Mohs micrographic surgery: a pilot study in 120 patients. Dermatol Surg. 2015;41:288-289.
- Spurk PA, Mohr ML, Seroka AM, et al. The impact of a wireless telecommunication system on efficiency. J Nurs Admin. 1995;25:21-26.
- Dietert JB, MacFarlane DF. A survey of Mohs tissue tracking practices. Dermatol Surg. 2019;45:514-518.
Practice Points
- There are limited studies evaluating the efficacy of different communication methods in Mohs micrographic surgery (MMS) clinics.
- This study suggests that incorporation of a light-based system into an MMS clinic improves workplace efficiency.
Who can call themselves ‘doctor’? The debate heats up
Who Should Get to Be Called ‘Doctor’? shows. The topic has clearly struck a nerve, since a record number of respondents – over 12,000 – voted in the poll.
Most physicians think it’s appropriate for people with other doctorate degrees such as a PhD or EdD to call themselves ‘doctor,’ although slightly more than half said it depends on the context.
The controversy over who gets to be called a doctor was reignited when a Wall Street Journal opinion piece criticized First Lady Jill Biden, EdD, for wanting to be called “Dr Biden.” The piece also challenged the idea that having a PhD is worth the honorific of ‘doctor.’
Medical ethicist Arthur Caplan, PhD, disagreed with that viewpoint, saying the context matters. For example, he prefers to be called “professor” when he’s introduced to the public rather than “doctor” to avoid any confusion about his professional status.
More than 12,000 clinicians including physicians, medical students, nurses, pharmacists, and other health care professionals responded to the poll. The non-MD clinicians were the most likely to say it was always appropriate to be called “doctor” while physicians were the least likely.
Context matters
Large percentages of clinicians – 54% of doctors, 62% of medical students, and 41% of nurses – said that the context matters for being called “doctor.’’
“I earned my PhD in 1995 and my MD in 2000. I think it is contextual. In a research or University setting, “Dr.” seems appropriate for a PhD. That same person in public should probably not hold themselves out as “Dr.” So, maybe MDs and DOs can choose, while others maintain the title in their specific setting.”
Some readers proposed that people with MDs call themselves physicians rather than doctors. Said one: “Anyone with a terminal doctorate degree has the right to use the word doctor. As a physician when someone asks what I do, I say: ‘I am a physician.’ Problem solved. There can only be one physician but there are many types of doctors.”
Physicians and nurses differed most in their views. Just 24% of physicians said it was always appropriate for people with other doctorate degrees to call themselves doctor whereas about an equal number (22%) thought it was never appropriate.
In contrast, 43% of nurses (including advance practice nurses) said it was always appropriate for people with non-MD doctorates to be called doctor. Only 16% said it’s never appropriate.
This difference may reflect the growing number of nurses with doctorate degrees, either a DNP or PhD, who want to be called doctor in clinical settings.
Age made a difference too. Only 16% of physicians younger than age 45 said it was always appropriate for people with non-MD doctorate degrees to be called doctor, compared with 27% of physicians aged 45 and up.
Medical students (31%) were also more likely than physicians to say it was always appropriate for non-MD doctorates to use the title “doctor” and 64% said it depends on the context. This was noteworthy because twice as many medical students as physicians (16% vs. 8%) said they work in academia, research, or military government settings.
Too many ‘doctors’ confuse the public
Physicians (70%) were also more likely to say it was always or often confusing for the public to hear someone without a medical degree addressed as “doctor.” Only 6% of physicians thought it was never or rarely confusing.
Nurses disagreed. Just 45% said that it was always or often confusing while 16% said it was never or rarely confusing.
Medical students were more aligned with physicians on this issue – 60% said it was always or often confusing to the public and just 10% said it was never or rarely confusing.
One reader commented, “The problem is the confusion the ‘doctor’ title causes for patients, especially in a hospital setting. Is the ‘doctor’ a physician, a pharmacist, a psychologist, a nurse, etc., etc.? We need to think not of our own egos but if and how the confusion about this plethora of titles may be hindering good patient care.”
These concerns are not unfounded. The American Medical Association reported in its Truth in Advertising campaign that “patients mistake physicians with nonphysician providers” based on an online survey of 802 adults in 2018. The participants thought these specialists were MDs: dentists (61%), podiatrists (67%), optometrists (47%), psychologists (43%), doctors of nursing (39%), and chiropractors (27%).
The AMA has advocated that states pass the “Health Care Professional Transparency Act,” which New Jersey has enacted. The law requires all health care professionals dealing with patients to wear a name tag that clearly identifies their licensure. Health care professionals must also display their education, training, and licensure in their office.
A version of this article first appeared on Medscape.com.
Who Should Get to Be Called ‘Doctor’? shows. The topic has clearly struck a nerve, since a record number of respondents – over 12,000 – voted in the poll.
Most physicians think it’s appropriate for people with other doctorate degrees such as a PhD or EdD to call themselves ‘doctor,’ although slightly more than half said it depends on the context.
The controversy over who gets to be called a doctor was reignited when a Wall Street Journal opinion piece criticized First Lady Jill Biden, EdD, for wanting to be called “Dr Biden.” The piece also challenged the idea that having a PhD is worth the honorific of ‘doctor.’
Medical ethicist Arthur Caplan, PhD, disagreed with that viewpoint, saying the context matters. For example, he prefers to be called “professor” when he’s introduced to the public rather than “doctor” to avoid any confusion about his professional status.
More than 12,000 clinicians including physicians, medical students, nurses, pharmacists, and other health care professionals responded to the poll. The non-MD clinicians were the most likely to say it was always appropriate to be called “doctor” while physicians were the least likely.
Context matters
Large percentages of clinicians – 54% of doctors, 62% of medical students, and 41% of nurses – said that the context matters for being called “doctor.’’
“I earned my PhD in 1995 and my MD in 2000. I think it is contextual. In a research or University setting, “Dr.” seems appropriate for a PhD. That same person in public should probably not hold themselves out as “Dr.” So, maybe MDs and DOs can choose, while others maintain the title in their specific setting.”
Some readers proposed that people with MDs call themselves physicians rather than doctors. Said one: “Anyone with a terminal doctorate degree has the right to use the word doctor. As a physician when someone asks what I do, I say: ‘I am a physician.’ Problem solved. There can only be one physician but there are many types of doctors.”
Physicians and nurses differed most in their views. Just 24% of physicians said it was always appropriate for people with other doctorate degrees to call themselves doctor whereas about an equal number (22%) thought it was never appropriate.
In contrast, 43% of nurses (including advance practice nurses) said it was always appropriate for people with non-MD doctorates to be called doctor. Only 16% said it’s never appropriate.
This difference may reflect the growing number of nurses with doctorate degrees, either a DNP or PhD, who want to be called doctor in clinical settings.
Age made a difference too. Only 16% of physicians younger than age 45 said it was always appropriate for people with non-MD doctorate degrees to be called doctor, compared with 27% of physicians aged 45 and up.
Medical students (31%) were also more likely than physicians to say it was always appropriate for non-MD doctorates to use the title “doctor” and 64% said it depends on the context. This was noteworthy because twice as many medical students as physicians (16% vs. 8%) said they work in academia, research, or military government settings.
Too many ‘doctors’ confuse the public
Physicians (70%) were also more likely to say it was always or often confusing for the public to hear someone without a medical degree addressed as “doctor.” Only 6% of physicians thought it was never or rarely confusing.
Nurses disagreed. Just 45% said that it was always or often confusing while 16% said it was never or rarely confusing.
Medical students were more aligned with physicians on this issue – 60% said it was always or often confusing to the public and just 10% said it was never or rarely confusing.
One reader commented, “The problem is the confusion the ‘doctor’ title causes for patients, especially in a hospital setting. Is the ‘doctor’ a physician, a pharmacist, a psychologist, a nurse, etc., etc.? We need to think not of our own egos but if and how the confusion about this plethora of titles may be hindering good patient care.”
These concerns are not unfounded. The American Medical Association reported in its Truth in Advertising campaign that “patients mistake physicians with nonphysician providers” based on an online survey of 802 adults in 2018. The participants thought these specialists were MDs: dentists (61%), podiatrists (67%), optometrists (47%), psychologists (43%), doctors of nursing (39%), and chiropractors (27%).
The AMA has advocated that states pass the “Health Care Professional Transparency Act,” which New Jersey has enacted. The law requires all health care professionals dealing with patients to wear a name tag that clearly identifies their licensure. Health care professionals must also display their education, training, and licensure in their office.
A version of this article first appeared on Medscape.com.
Who Should Get to Be Called ‘Doctor’? shows. The topic has clearly struck a nerve, since a record number of respondents – over 12,000 – voted in the poll.
Most physicians think it’s appropriate for people with other doctorate degrees such as a PhD or EdD to call themselves ‘doctor,’ although slightly more than half said it depends on the context.
The controversy over who gets to be called a doctor was reignited when a Wall Street Journal opinion piece criticized First Lady Jill Biden, EdD, for wanting to be called “Dr Biden.” The piece also challenged the idea that having a PhD is worth the honorific of ‘doctor.’
Medical ethicist Arthur Caplan, PhD, disagreed with that viewpoint, saying the context matters. For example, he prefers to be called “professor” when he’s introduced to the public rather than “doctor” to avoid any confusion about his professional status.
More than 12,000 clinicians including physicians, medical students, nurses, pharmacists, and other health care professionals responded to the poll. The non-MD clinicians were the most likely to say it was always appropriate to be called “doctor” while physicians were the least likely.
Context matters
Large percentages of clinicians – 54% of doctors, 62% of medical students, and 41% of nurses – said that the context matters for being called “doctor.’’
“I earned my PhD in 1995 and my MD in 2000. I think it is contextual. In a research or University setting, “Dr.” seems appropriate for a PhD. That same person in public should probably not hold themselves out as “Dr.” So, maybe MDs and DOs can choose, while others maintain the title in their specific setting.”
Some readers proposed that people with MDs call themselves physicians rather than doctors. Said one: “Anyone with a terminal doctorate degree has the right to use the word doctor. As a physician when someone asks what I do, I say: ‘I am a physician.’ Problem solved. There can only be one physician but there are many types of doctors.”
Physicians and nurses differed most in their views. Just 24% of physicians said it was always appropriate for people with other doctorate degrees to call themselves doctor whereas about an equal number (22%) thought it was never appropriate.
In contrast, 43% of nurses (including advance practice nurses) said it was always appropriate for people with non-MD doctorates to be called doctor. Only 16% said it’s never appropriate.
This difference may reflect the growing number of nurses with doctorate degrees, either a DNP or PhD, who want to be called doctor in clinical settings.
Age made a difference too. Only 16% of physicians younger than age 45 said it was always appropriate for people with non-MD doctorate degrees to be called doctor, compared with 27% of physicians aged 45 and up.
Medical students (31%) were also more likely than physicians to say it was always appropriate for non-MD doctorates to use the title “doctor” and 64% said it depends on the context. This was noteworthy because twice as many medical students as physicians (16% vs. 8%) said they work in academia, research, or military government settings.
Too many ‘doctors’ confuse the public
Physicians (70%) were also more likely to say it was always or often confusing for the public to hear someone without a medical degree addressed as “doctor.” Only 6% of physicians thought it was never or rarely confusing.
Nurses disagreed. Just 45% said that it was always or often confusing while 16% said it was never or rarely confusing.
Medical students were more aligned with physicians on this issue – 60% said it was always or often confusing to the public and just 10% said it was never or rarely confusing.
One reader commented, “The problem is the confusion the ‘doctor’ title causes for patients, especially in a hospital setting. Is the ‘doctor’ a physician, a pharmacist, a psychologist, a nurse, etc., etc.? We need to think not of our own egos but if and how the confusion about this plethora of titles may be hindering good patient care.”
These concerns are not unfounded. The American Medical Association reported in its Truth in Advertising campaign that “patients mistake physicians with nonphysician providers” based on an online survey of 802 adults in 2018. The participants thought these specialists were MDs: dentists (61%), podiatrists (67%), optometrists (47%), psychologists (43%), doctors of nursing (39%), and chiropractors (27%).
The AMA has advocated that states pass the “Health Care Professional Transparency Act,” which New Jersey has enacted. The law requires all health care professionals dealing with patients to wear a name tag that clearly identifies their licensure. Health care professionals must also display their education, training, and licensure in their office.
A version of this article first appeared on Medscape.com.
The power and promise of social media in oncology
Mark A. Lewis, MD, explained to the COSMO meeting audience how storytelling on social media can educate and engage patients, advocates, and professional colleagues – advancing knowledge, dispelling misinformation, and promoting clinical research.
Dr. Lewis, an oncologist at Intermountain Healthcare in Salt Lake City, reflected on the bifid roles of oncologists as scientists engaged in life-long learning and humanists who can internalize and appreciate the unique character and circumstances of their patients.
Patients who have serious illnesses are necessarily aggregated by statistics. However, in an essay published in 2011, Dr. Lewis noted that “each individual patient partakes in a unique, irreproducible experiment where n = 1” (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
Dr. Lewis highlighted the duality of individual data points on a survival curve as descriptors of common disease trajectories and treatment effects. However, those data points also conceal important narratives regarding the most highly valued aspects of the doctor-patient relationship and the impact of cancer treatment on patients’ lives.
In referring to the futuristic essay “Ars Brevis,” Dr. Lewis contrasted the humanism of oncology specialists in the present day with the fictional image of data-regurgitating robots programmed to maximize the efficiency of each patient encounter (J Clin Oncol. 2013 May 10;31[14]:1792-4).
Dr. Lewis reminded attendees that to practice medicine without using both “head and heart” undermines the inherent nature of medical care.
Unfortunately, that perspective may not match the public perception of oncologists. Dr. Lewis described his experience of typing “oncologists are” into an Internet search engine and seeing the auto-complete function prompt words such as “criminals,” “evil,” “murderers,” and “confused.”
Obviously, it is hard to establish a trusting patient-doctor relationship if that is the prima facie perception of the oncology specialty.
Dispelling myths and creating community via social media
A primary goal of consultation with a newly-diagnosed cancer patient is for the patient to feel that the oncologist will be there to take care of them, regardless of what the future holds.
Dr. Lewis has found that social media can potentially extend that feeling to a global community of patients, caregivers, and others seeking information relevant to a cancer diagnosis. He believes that oncologists have an opportunity to dispel myths and fears by being attentive to the real-life concerns of patients.
Dr. Lewis took advantage of this opportunity when he underwent a Whipple procedure (pancreaticoduodenectomy) for a pancreatic neuroendocrine tumor. He and the hospital’s media services staff “live-tweeted” his surgery and recovery.
With those tweets, Dr. Lewis demystified each step of a major surgical procedure. From messages he received on social media, Dr. Lewis knows he made the decision to have a Whipple procedure more acceptable to other patients.
His personal medical experience notwithstanding, Dr. Lewis acknowledged that every patient’s circumstances are unique.
Oncologists cannot possibly empathize with every circumstance. However, when they show sensitivity to personal elements of the cancer experience, they shed light on the complicated role they play in patient care and can facilitate good decision-making among patients across the globe.
Social media for professional development and patient care
The publication of his 2011 essay was gratifying for Dr. Lewis, but the finite number of comments he received thereafter illustrated the rather limited audience that traditional academic publications have and the laborious process for subsequent interaction (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
First as an observer and later as a participant on social media, Dr. Lewis appreciated that teaching points and publications can be amplified by global distribution and the potential for informal bidirectional communication.
Social media platforms enable physicians to connect with a larger audience through participative communication, in which users develop, share, and react to content (N Engl J Med. 2009 Aug 13;361[7]:649-51).
Dr. Lewis reflected on how oncologists are challenged to sort through the thousands of oncology-focused publications annually. Through social media, one can see the studies on which the experts are commenting and appreciate the nuances that contextualize the results. Focused interactions with renowned doctors, at regular intervals, require little formality.
Online journal clubs enable the sharing of ideas, opinions, multimedia resources, and references across institutional and international borders (J Gen Intern Med. 2014 Oct;29[10]:1317-8).
Social media in oncology: Accomplishments and promise
The development of broadband Internet, wireless connectivity, and social media for peer-to-peer and general communication are among the major technological advances that have transformed medical communication.
As an organization, COSMO aims to describe, understand, and improve the use of social media to increase the penetration of evidence-based guidelines and research insights into clinical practice (Future Oncol. 2017 Jun;13[15]:1281-5).
At the inaugural COSMO meeting, areas of progress since COSMO’s inception in 2015 were highlighted, including:
- The involvement of cancer professionals and advocates in multiple distinctive platforms.
- The development of hashtag libraries to aggregate interest groups and topics.
- The refinement of strategies for engaging advocates with attention to inclusiveness.
- A steady trajectory of growth in tweeting at scientific conferences.
An overarching theme of the COSMO meeting was “authenticity,” a virtue that is easy to admire but requires conscious, consistent effort to achieve.
Disclosure of conflicts of interest and avoiding using social media simply as a recruitment tool for clinical trials are basic components of accurate self-representation.
In addition, Dr. Lewis advocated for sharing personal experiences in a component of social media posts so oncologists can show humanity as a feature of their professional online identity and inherent nature.
Dr. Lewis disclosed consultancy with Medscape/WebMD, which are owned by the same parent company as MDedge. He also disclosed relationships with Foundation Medicine, Natera, Exelixis, QED, HalioDX, and Ipsen.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Mark A. Lewis, MD, explained to the COSMO meeting audience how storytelling on social media can educate and engage patients, advocates, and professional colleagues – advancing knowledge, dispelling misinformation, and promoting clinical research.
Dr. Lewis, an oncologist at Intermountain Healthcare in Salt Lake City, reflected on the bifid roles of oncologists as scientists engaged in life-long learning and humanists who can internalize and appreciate the unique character and circumstances of their patients.
Patients who have serious illnesses are necessarily aggregated by statistics. However, in an essay published in 2011, Dr. Lewis noted that “each individual patient partakes in a unique, irreproducible experiment where n = 1” (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
Dr. Lewis highlighted the duality of individual data points on a survival curve as descriptors of common disease trajectories and treatment effects. However, those data points also conceal important narratives regarding the most highly valued aspects of the doctor-patient relationship and the impact of cancer treatment on patients’ lives.
In referring to the futuristic essay “Ars Brevis,” Dr. Lewis contrasted the humanism of oncology specialists in the present day with the fictional image of data-regurgitating robots programmed to maximize the efficiency of each patient encounter (J Clin Oncol. 2013 May 10;31[14]:1792-4).
Dr. Lewis reminded attendees that to practice medicine without using both “head and heart” undermines the inherent nature of medical care.
Unfortunately, that perspective may not match the public perception of oncologists. Dr. Lewis described his experience of typing “oncologists are” into an Internet search engine and seeing the auto-complete function prompt words such as “criminals,” “evil,” “murderers,” and “confused.”
Obviously, it is hard to establish a trusting patient-doctor relationship if that is the prima facie perception of the oncology specialty.
Dispelling myths and creating community via social media
A primary goal of consultation with a newly-diagnosed cancer patient is for the patient to feel that the oncologist will be there to take care of them, regardless of what the future holds.
Dr. Lewis has found that social media can potentially extend that feeling to a global community of patients, caregivers, and others seeking information relevant to a cancer diagnosis. He believes that oncologists have an opportunity to dispel myths and fears by being attentive to the real-life concerns of patients.
Dr. Lewis took advantage of this opportunity when he underwent a Whipple procedure (pancreaticoduodenectomy) for a pancreatic neuroendocrine tumor. He and the hospital’s media services staff “live-tweeted” his surgery and recovery.
With those tweets, Dr. Lewis demystified each step of a major surgical procedure. From messages he received on social media, Dr. Lewis knows he made the decision to have a Whipple procedure more acceptable to other patients.
His personal medical experience notwithstanding, Dr. Lewis acknowledged that every patient’s circumstances are unique.
Oncologists cannot possibly empathize with every circumstance. However, when they show sensitivity to personal elements of the cancer experience, they shed light on the complicated role they play in patient care and can facilitate good decision-making among patients across the globe.
Social media for professional development and patient care
The publication of his 2011 essay was gratifying for Dr. Lewis, but the finite number of comments he received thereafter illustrated the rather limited audience that traditional academic publications have and the laborious process for subsequent interaction (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
First as an observer and later as a participant on social media, Dr. Lewis appreciated that teaching points and publications can be amplified by global distribution and the potential for informal bidirectional communication.
Social media platforms enable physicians to connect with a larger audience through participative communication, in which users develop, share, and react to content (N Engl J Med. 2009 Aug 13;361[7]:649-51).
Dr. Lewis reflected on how oncologists are challenged to sort through the thousands of oncology-focused publications annually. Through social media, one can see the studies on which the experts are commenting and appreciate the nuances that contextualize the results. Focused interactions with renowned doctors, at regular intervals, require little formality.
Online journal clubs enable the sharing of ideas, opinions, multimedia resources, and references across institutional and international borders (J Gen Intern Med. 2014 Oct;29[10]:1317-8).
Social media in oncology: Accomplishments and promise
The development of broadband Internet, wireless connectivity, and social media for peer-to-peer and general communication are among the major technological advances that have transformed medical communication.
As an organization, COSMO aims to describe, understand, and improve the use of social media to increase the penetration of evidence-based guidelines and research insights into clinical practice (Future Oncol. 2017 Jun;13[15]:1281-5).
At the inaugural COSMO meeting, areas of progress since COSMO’s inception in 2015 were highlighted, including:
- The involvement of cancer professionals and advocates in multiple distinctive platforms.
- The development of hashtag libraries to aggregate interest groups and topics.
- The refinement of strategies for engaging advocates with attention to inclusiveness.
- A steady trajectory of growth in tweeting at scientific conferences.
An overarching theme of the COSMO meeting was “authenticity,” a virtue that is easy to admire but requires conscious, consistent effort to achieve.
Disclosure of conflicts of interest and avoiding using social media simply as a recruitment tool for clinical trials are basic components of accurate self-representation.
In addition, Dr. Lewis advocated for sharing personal experiences in a component of social media posts so oncologists can show humanity as a feature of their professional online identity and inherent nature.
Dr. Lewis disclosed consultancy with Medscape/WebMD, which are owned by the same parent company as MDedge. He also disclosed relationships with Foundation Medicine, Natera, Exelixis, QED, HalioDX, and Ipsen.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Mark A. Lewis, MD, explained to the COSMO meeting audience how storytelling on social media can educate and engage patients, advocates, and professional colleagues – advancing knowledge, dispelling misinformation, and promoting clinical research.
Dr. Lewis, an oncologist at Intermountain Healthcare in Salt Lake City, reflected on the bifid roles of oncologists as scientists engaged in life-long learning and humanists who can internalize and appreciate the unique character and circumstances of their patients.
Patients who have serious illnesses are necessarily aggregated by statistics. However, in an essay published in 2011, Dr. Lewis noted that “each individual patient partakes in a unique, irreproducible experiment where n = 1” (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
Dr. Lewis highlighted the duality of individual data points on a survival curve as descriptors of common disease trajectories and treatment effects. However, those data points also conceal important narratives regarding the most highly valued aspects of the doctor-patient relationship and the impact of cancer treatment on patients’ lives.
In referring to the futuristic essay “Ars Brevis,” Dr. Lewis contrasted the humanism of oncology specialists in the present day with the fictional image of data-regurgitating robots programmed to maximize the efficiency of each patient encounter (J Clin Oncol. 2013 May 10;31[14]:1792-4).
Dr. Lewis reminded attendees that to practice medicine without using both “head and heart” undermines the inherent nature of medical care.
Unfortunately, that perspective may not match the public perception of oncologists. Dr. Lewis described his experience of typing “oncologists are” into an Internet search engine and seeing the auto-complete function prompt words such as “criminals,” “evil,” “murderers,” and “confused.”
Obviously, it is hard to establish a trusting patient-doctor relationship if that is the prima facie perception of the oncology specialty.
Dispelling myths and creating community via social media
A primary goal of consultation with a newly-diagnosed cancer patient is for the patient to feel that the oncologist will be there to take care of them, regardless of what the future holds.
Dr. Lewis has found that social media can potentially extend that feeling to a global community of patients, caregivers, and others seeking information relevant to a cancer diagnosis. He believes that oncologists have an opportunity to dispel myths and fears by being attentive to the real-life concerns of patients.
Dr. Lewis took advantage of this opportunity when he underwent a Whipple procedure (pancreaticoduodenectomy) for a pancreatic neuroendocrine tumor. He and the hospital’s media services staff “live-tweeted” his surgery and recovery.
With those tweets, Dr. Lewis demystified each step of a major surgical procedure. From messages he received on social media, Dr. Lewis knows he made the decision to have a Whipple procedure more acceptable to other patients.
His personal medical experience notwithstanding, Dr. Lewis acknowledged that every patient’s circumstances are unique.
Oncologists cannot possibly empathize with every circumstance. However, when they show sensitivity to personal elements of the cancer experience, they shed light on the complicated role they play in patient care and can facilitate good decision-making among patients across the globe.
Social media for professional development and patient care
The publication of his 2011 essay was gratifying for Dr. Lewis, but the finite number of comments he received thereafter illustrated the rather limited audience that traditional academic publications have and the laborious process for subsequent interaction (J Clin Oncol. 2011 Aug 1;29[22]:3103-4).
First as an observer and later as a participant on social media, Dr. Lewis appreciated that teaching points and publications can be amplified by global distribution and the potential for informal bidirectional communication.
Social media platforms enable physicians to connect with a larger audience through participative communication, in which users develop, share, and react to content (N Engl J Med. 2009 Aug 13;361[7]:649-51).
Dr. Lewis reflected on how oncologists are challenged to sort through the thousands of oncology-focused publications annually. Through social media, one can see the studies on which the experts are commenting and appreciate the nuances that contextualize the results. Focused interactions with renowned doctors, at regular intervals, require little formality.
Online journal clubs enable the sharing of ideas, opinions, multimedia resources, and references across institutional and international borders (J Gen Intern Med. 2014 Oct;29[10]:1317-8).
Social media in oncology: Accomplishments and promise
The development of broadband Internet, wireless connectivity, and social media for peer-to-peer and general communication are among the major technological advances that have transformed medical communication.
As an organization, COSMO aims to describe, understand, and improve the use of social media to increase the penetration of evidence-based guidelines and research insights into clinical practice (Future Oncol. 2017 Jun;13[15]:1281-5).
At the inaugural COSMO meeting, areas of progress since COSMO’s inception in 2015 were highlighted, including:
- The involvement of cancer professionals and advocates in multiple distinctive platforms.
- The development of hashtag libraries to aggregate interest groups and topics.
- The refinement of strategies for engaging advocates with attention to inclusiveness.
- A steady trajectory of growth in tweeting at scientific conferences.
An overarching theme of the COSMO meeting was “authenticity,” a virtue that is easy to admire but requires conscious, consistent effort to achieve.
Disclosure of conflicts of interest and avoiding using social media simply as a recruitment tool for clinical trials are basic components of accurate self-representation.
In addition, Dr. Lewis advocated for sharing personal experiences in a component of social media posts so oncologists can show humanity as a feature of their professional online identity and inherent nature.
Dr. Lewis disclosed consultancy with Medscape/WebMD, which are owned by the same parent company as MDedge. He also disclosed relationships with Foundation Medicine, Natera, Exelixis, QED, HalioDX, and Ipsen.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM COSMO 2021
Doctors lose jobs after speaking out about unsafe conditions
In April 2020, hospitalist Samantha Houston, MD, lost her job at Baptist Memorial Hospital–North, in Oxford, Miss., after she publicly campaigned to get donations of N95 masks for nurses. Dr. Houston filed a lawsuit against the hospital, saying she was improperly fired for speaking out. The lawsuit has not yet gone to trial.
In January 2017, emergency physician Raymond Brovont, MD, was fired by EmCare, an emergency physician staffing company, after reporting understaffing at hospitals with which it contracted in the Kansas City, Mo., area. Dr. Brovont sued EmCare, and the company lost the case. In February 2019, it was ordered to pay him $13.1 million in damages.
These are just two of several cases in recent years in which physicians have spoken out about problems involving patient care and have been sanctioned. Other physicians who see problems choose to stay silent.
Doctors often hesitate to speak out because of the prospect of losing their jobs. A 2013 study of emergency physicians found that nearly 20% reported a possible or real threat to their employment if they expressed concerns about quality of care.
When physicians do not speak openly about important medical issues, the quality of care in their institutions suffers, said a coauthor of the study, Larry D. Weiss, MD, JD, a retired professor of emergency medicine at the University of Maryland, Baltimore.
“Physicians can’t effectively represent patients if they are always thinking they can get fired for what they say,” Dr. Weiss said. “If you don’t have protections like due process, which is often the case, you are less likely to speak out.”
In the first few weeks, health care facilities were struggling to obtain personal protective equipment (PPE) and to create policies that would keep patients and caregivers safe.
Physicians such as Dr. Houston took the initiative to make sure their institutions were taking the right steps against COVID-19 and found themselves at loggerheads with administrators who were concerned that their organizations were being portrayed as unsafe.
The case of one physician who spoke out
One of the highest-profile cases of a physician speaking out and being removed from work during the pandemic is that of Ming Lin, MD, an emergency physician who lost a job he had held for 17 years at St. Joseph Medical Center, in Bellingham, Wash. Dr. Lin lost his job after he made a series of Facebook posts that criticized the hospital’s COVID-19 preparedness efforts.
In an interview, Dr. Lin discussed the details of his situation to a degree that rarely occurs in such cases. This is one of the most extensive interviews he has granted.
Postings on Facebook
Dr. Lin said that on the basis of an intense study of the virus at the onset of the pandemic, he developed many ideas as to what could be done to mitigate its spread. While working as a locum tenens physician on his time off, he could see how others dealt with COVID-19.
Dr. Lin said from past experiences he did not feel that he could present his ideas directly to administration and be heard, so he decided to air his ideas about how his hospital could handle COVID-19 on his Facebook page, which drew a large audience.
He said he was certain that hospital administrators were reading his posts. He said receptionists at this hospital were advised not to wear masks, evidently because it would alarm patients. Dr. Lin said he posted concerns about their safety and called for them to wear masks. Soon after, the hospital directed receptionists to wear masks.
Dr. Lin’s Facebook posts also criticized the hospital for taking what he felt was too long to get results on COVID-19 tests. “It was taking them up to 10 days to get test results, because samples were being sent to a lab in California,” he said. He suggested it would be faster to send samples to the University of Washington. Soon after, the hospital started sending samples there.
In just a couple of weeks, Dr. Lin said, he voiced almost a dozen concerns. Each time the hospital made changes in line with his recommendations. Although he didn’t get any direct acknowledgment from the hospital for his help, he said he felt he was making a positive impact.
How employers react to physicians who speak out
Physicians who speak out about conditions tend to deeply disturb administrators, said William P. Sullivan, DO, JD, an emergency physician and lawyer in Frankfort, Ill., who has written about physicians being terminated by hospitals.
“These physicians go to the news media or they use social media,” Dr. Sullivan said, “but hospital administrators don’t want the public to hear bad things about their hospital.”
Then the public might not come to the hospital, which is an administrator’s worst nightmare. Even if physicians think their criticisms are reasonable, administrators may still fear a resulting drop in patients.
Dr. Houston, for example, was helping her Mississippi hospital by collecting donations of N95 masks for nurses, but to administrators, it showed that the hospital did not have enough masks.
“It is not helpful to stoke fear and anxiety, even if the intent is sincere,” a spokesperson for the hospital said.
Administrator fires back
Dr. Lin’s posts were deeply concerning to Richard DeCarlo, chief operating officer of PeaceHealth, which runs St. Joseph Hospital. Mr. DeCarlo discussed his concerns in a video interview in April with the blogger Zubin Damania, MD, known as ZDoggMD.
Comments on Dr. Lin’s Facebook posts showed that people “were fearful to go to the hospital,” he told Dr. Damania. “They were concluding that they would need to drive to another hospital.”
Mr. DeCarlo said he was also unhappy that Dr. Lin did not directly contact administrators about his concerns. “He didn’t communicate with his medical director,” Mr. DeCarlo said in the interview. “The ED staff had been meeting three times a week with the chief medical officer to make sure they had everything they needed, but he only attended one of these meetings and didn’t ask any questions.”
Dr. Lin maintains he did ask questions at the first meeting but stopped attending because he felt he wasn’t being heeded. “I found their tone not very receptive,” he said.
Doctor allegedly offered “misinformation”
At the start of the pandemic, some hospitals made it clear what would happen to doctors who brought up lack of PPE or other problems to the media. For example, NYU Langone Medical Center in New York sent an email to staff warning that speaking to the media without permission “will be subject to disciplinary action, including termination.”
PeaceHealth took a different tack. “It’s not that we have a policy that says don’t ever talk to the media,” Mr. DeCarlo said in the ZDoggMD interview, but in Dr. Lin’s case, “what was at issue was the misinformation. His leader went to him and said, ‘Look, you’re posting things that aren’t accurate.’ ”
Dr. Lin disputes that he provided any misinformation. In the interview, Mr. DeCarlo cited just one example of alleged misinformation. He said Dr. Lin called for a tent outside the emergency department (ED) to protect patients entering the department from aerosol exposure to COVID-19. Mr. DeCarlo said the tent was not needed because fewer people were using the ED.
“To put it in an extreme way,” Mr. DeCarlo said of Dr. Lin’s posts, “it was like yelling fire in a theater where there is not a fire.”
Dr. Lin said the hospital did briefly erect a tent and then removed it, and he still insisted that a tent was a good idea. He added that Mr. DeCarlo never mentioned any of the other suggestions Dr. Lin made, nor did he state that the hospital adopted them.
Doctor gets a warning
Dr. Lin said that after he started posting his concerns, he got a call from the emergency department director who worked for TeamHealth, an emergency medicine staffing firm that contracted with PeaceHealth and employed Dr. Lin, too.
Dr. Lin said his immediate supervisor at TeamHealth told him the hospital was unhappy with his posts and that he should take them down and suggested he might be fired. Dr. Lin said the supervisor also asked him to apologize to the hospital administration for these posts, but he refused to do so.
“Retracting and apologizing was not only wrong but would have left me vulnerable to being terminated with no repercussions,” he said.
“At that point, I realized I had crossed the Rubicon,” Dr. Lin said. He thought he might well be fired, no matter what he did, so he took his story to The Seattle Times, which had a much wider platform than his Facebook page had.
Dr. Lin lost his job at St. Joseph a week after The Seattle Times story about him appeared. “About 10 minutes before my shift was supposed to start, I received a text message from TeamHealth saying that someone else would be taking the shift,” he said.
In a release, TeamHealth insisted Dr. Lin was not fired and that he was scheduled to be reassigned to work at other hospitals. Dr. Lin, however, said he was not told this at the time and that he found out later that the new assignment would involve a pay cut and a significant commute. He said he has not taken any new assignments from TeamHealth since he lost his job at St. Joseph.
Dr. Lin has filed a lawsuit against PeaceHealth, TeamHealth, and Mr. DeCarlo, asking for his job back and for an apology. He said he has not asked for any financial damages at this point.
Since leaving St. Joseph, Dr. Lin has been working as an administrator for the Indian Health Service in the upper plains states. He said he can do some of the work at home in Washington State, which allows him to be with his wife and three young children.
Dr. Lin no longer sees patients. “I feel I have lost my confidence as a clinician,” he said. “I’m not sure why, but I find it hard to make quick judgments when taking care of patients.”
He said many doctors have told him about their own troubles with speaking out, but they did not want to come forward and talk about it because they feared more repercussions.
Do doctors who speak out have any rights?
Because TeamHealth, Dr. Lin’s actual employer, asserts he was never actually terminated, Dr. Lin has not been able to appeal his case internally in accordance with due process, an option that allows doctors to get a fair hearing and to appeal decisions against them.
The American Academy of Emergency Medicine pointed out this problem. “Dr. Lin, as a member of the medical staff, is entitled to full due process and a fair hearing from his peers on the medical staff,” the academy said in a statement supporting him.
The Joint Commission, the hospital accreditor, requires that hospitals provide due process to doctors before they can be terminated. However, Dr. Sullivan said employers often make physicians waive their due process rights in the employment contract. “The result is that the employer can terminate doctors for no reason,” he said.
In the 2013 survey of emergency physicians, 62% reported that their employers could terminate them without full due process.
Dr. Weiss, the Maryland MD-JD, said that when he advises doctors on their contracts, he generally tells them to cross out the waiver language. The applicant, he says, may also tell the employer that the waivers are considered unethical by many physician professional societies. In some cases, he said, the hospital will back down.
Conclusion
To maintain quality of care, it is essential that physicians feel free to speak out about issues that concern them. They can improve their chances of being heard by working directly with management and attending meetings, but in some cases, management may be unwilling to listen.
A version of this article first appeared on Medscape.com.
In April 2020, hospitalist Samantha Houston, MD, lost her job at Baptist Memorial Hospital–North, in Oxford, Miss., after she publicly campaigned to get donations of N95 masks for nurses. Dr. Houston filed a lawsuit against the hospital, saying she was improperly fired for speaking out. The lawsuit has not yet gone to trial.
In January 2017, emergency physician Raymond Brovont, MD, was fired by EmCare, an emergency physician staffing company, after reporting understaffing at hospitals with which it contracted in the Kansas City, Mo., area. Dr. Brovont sued EmCare, and the company lost the case. In February 2019, it was ordered to pay him $13.1 million in damages.
These are just two of several cases in recent years in which physicians have spoken out about problems involving patient care and have been sanctioned. Other physicians who see problems choose to stay silent.
Doctors often hesitate to speak out because of the prospect of losing their jobs. A 2013 study of emergency physicians found that nearly 20% reported a possible or real threat to their employment if they expressed concerns about quality of care.
When physicians do not speak openly about important medical issues, the quality of care in their institutions suffers, said a coauthor of the study, Larry D. Weiss, MD, JD, a retired professor of emergency medicine at the University of Maryland, Baltimore.
“Physicians can’t effectively represent patients if they are always thinking they can get fired for what they say,” Dr. Weiss said. “If you don’t have protections like due process, which is often the case, you are less likely to speak out.”
In the first few weeks, health care facilities were struggling to obtain personal protective equipment (PPE) and to create policies that would keep patients and caregivers safe.
Physicians such as Dr. Houston took the initiative to make sure their institutions were taking the right steps against COVID-19 and found themselves at loggerheads with administrators who were concerned that their organizations were being portrayed as unsafe.
The case of one physician who spoke out
One of the highest-profile cases of a physician speaking out and being removed from work during the pandemic is that of Ming Lin, MD, an emergency physician who lost a job he had held for 17 years at St. Joseph Medical Center, in Bellingham, Wash. Dr. Lin lost his job after he made a series of Facebook posts that criticized the hospital’s COVID-19 preparedness efforts.
In an interview, Dr. Lin discussed the details of his situation to a degree that rarely occurs in such cases. This is one of the most extensive interviews he has granted.
Postings on Facebook
Dr. Lin said that on the basis of an intense study of the virus at the onset of the pandemic, he developed many ideas as to what could be done to mitigate its spread. While working as a locum tenens physician on his time off, he could see how others dealt with COVID-19.
Dr. Lin said from past experiences he did not feel that he could present his ideas directly to administration and be heard, so he decided to air his ideas about how his hospital could handle COVID-19 on his Facebook page, which drew a large audience.
He said he was certain that hospital administrators were reading his posts. He said receptionists at this hospital were advised not to wear masks, evidently because it would alarm patients. Dr. Lin said he posted concerns about their safety and called for them to wear masks. Soon after, the hospital directed receptionists to wear masks.
Dr. Lin’s Facebook posts also criticized the hospital for taking what he felt was too long to get results on COVID-19 tests. “It was taking them up to 10 days to get test results, because samples were being sent to a lab in California,” he said. He suggested it would be faster to send samples to the University of Washington. Soon after, the hospital started sending samples there.
In just a couple of weeks, Dr. Lin said, he voiced almost a dozen concerns. Each time the hospital made changes in line with his recommendations. Although he didn’t get any direct acknowledgment from the hospital for his help, he said he felt he was making a positive impact.
How employers react to physicians who speak out
Physicians who speak out about conditions tend to deeply disturb administrators, said William P. Sullivan, DO, JD, an emergency physician and lawyer in Frankfort, Ill., who has written about physicians being terminated by hospitals.
“These physicians go to the news media or they use social media,” Dr. Sullivan said, “but hospital administrators don’t want the public to hear bad things about their hospital.”
Then the public might not come to the hospital, which is an administrator’s worst nightmare. Even if physicians think their criticisms are reasonable, administrators may still fear a resulting drop in patients.
Dr. Houston, for example, was helping her Mississippi hospital by collecting donations of N95 masks for nurses, but to administrators, it showed that the hospital did not have enough masks.
“It is not helpful to stoke fear and anxiety, even if the intent is sincere,” a spokesperson for the hospital said.
Administrator fires back
Dr. Lin’s posts were deeply concerning to Richard DeCarlo, chief operating officer of PeaceHealth, which runs St. Joseph Hospital. Mr. DeCarlo discussed his concerns in a video interview in April with the blogger Zubin Damania, MD, known as ZDoggMD.
Comments on Dr. Lin’s Facebook posts showed that people “were fearful to go to the hospital,” he told Dr. Damania. “They were concluding that they would need to drive to another hospital.”
Mr. DeCarlo said he was also unhappy that Dr. Lin did not directly contact administrators about his concerns. “He didn’t communicate with his medical director,” Mr. DeCarlo said in the interview. “The ED staff had been meeting three times a week with the chief medical officer to make sure they had everything they needed, but he only attended one of these meetings and didn’t ask any questions.”
Dr. Lin maintains he did ask questions at the first meeting but stopped attending because he felt he wasn’t being heeded. “I found their tone not very receptive,” he said.
Doctor allegedly offered “misinformation”
At the start of the pandemic, some hospitals made it clear what would happen to doctors who brought up lack of PPE or other problems to the media. For example, NYU Langone Medical Center in New York sent an email to staff warning that speaking to the media without permission “will be subject to disciplinary action, including termination.”
PeaceHealth took a different tack. “It’s not that we have a policy that says don’t ever talk to the media,” Mr. DeCarlo said in the ZDoggMD interview, but in Dr. Lin’s case, “what was at issue was the misinformation. His leader went to him and said, ‘Look, you’re posting things that aren’t accurate.’ ”
Dr. Lin disputes that he provided any misinformation. In the interview, Mr. DeCarlo cited just one example of alleged misinformation. He said Dr. Lin called for a tent outside the emergency department (ED) to protect patients entering the department from aerosol exposure to COVID-19. Mr. DeCarlo said the tent was not needed because fewer people were using the ED.
“To put it in an extreme way,” Mr. DeCarlo said of Dr. Lin’s posts, “it was like yelling fire in a theater where there is not a fire.”
Dr. Lin said the hospital did briefly erect a tent and then removed it, and he still insisted that a tent was a good idea. He added that Mr. DeCarlo never mentioned any of the other suggestions Dr. Lin made, nor did he state that the hospital adopted them.
Doctor gets a warning
Dr. Lin said that after he started posting his concerns, he got a call from the emergency department director who worked for TeamHealth, an emergency medicine staffing firm that contracted with PeaceHealth and employed Dr. Lin, too.
Dr. Lin said his immediate supervisor at TeamHealth told him the hospital was unhappy with his posts and that he should take them down and suggested he might be fired. Dr. Lin said the supervisor also asked him to apologize to the hospital administration for these posts, but he refused to do so.
“Retracting and apologizing was not only wrong but would have left me vulnerable to being terminated with no repercussions,” he said.
“At that point, I realized I had crossed the Rubicon,” Dr. Lin said. He thought he might well be fired, no matter what he did, so he took his story to The Seattle Times, which had a much wider platform than his Facebook page had.
Dr. Lin lost his job at St. Joseph a week after The Seattle Times story about him appeared. “About 10 minutes before my shift was supposed to start, I received a text message from TeamHealth saying that someone else would be taking the shift,” he said.
In a release, TeamHealth insisted Dr. Lin was not fired and that he was scheduled to be reassigned to work at other hospitals. Dr. Lin, however, said he was not told this at the time and that he found out later that the new assignment would involve a pay cut and a significant commute. He said he has not taken any new assignments from TeamHealth since he lost his job at St. Joseph.
Dr. Lin has filed a lawsuit against PeaceHealth, TeamHealth, and Mr. DeCarlo, asking for his job back and for an apology. He said he has not asked for any financial damages at this point.
Since leaving St. Joseph, Dr. Lin has been working as an administrator for the Indian Health Service in the upper plains states. He said he can do some of the work at home in Washington State, which allows him to be with his wife and three young children.
Dr. Lin no longer sees patients. “I feel I have lost my confidence as a clinician,” he said. “I’m not sure why, but I find it hard to make quick judgments when taking care of patients.”
He said many doctors have told him about their own troubles with speaking out, but they did not want to come forward and talk about it because they feared more repercussions.
Do doctors who speak out have any rights?
Because TeamHealth, Dr. Lin’s actual employer, asserts he was never actually terminated, Dr. Lin has not been able to appeal his case internally in accordance with due process, an option that allows doctors to get a fair hearing and to appeal decisions against them.
The American Academy of Emergency Medicine pointed out this problem. “Dr. Lin, as a member of the medical staff, is entitled to full due process and a fair hearing from his peers on the medical staff,” the academy said in a statement supporting him.
The Joint Commission, the hospital accreditor, requires that hospitals provide due process to doctors before they can be terminated. However, Dr. Sullivan said employers often make physicians waive their due process rights in the employment contract. “The result is that the employer can terminate doctors for no reason,” he said.
In the 2013 survey of emergency physicians, 62% reported that their employers could terminate them without full due process.
Dr. Weiss, the Maryland MD-JD, said that when he advises doctors on their contracts, he generally tells them to cross out the waiver language. The applicant, he says, may also tell the employer that the waivers are considered unethical by many physician professional societies. In some cases, he said, the hospital will back down.
Conclusion
To maintain quality of care, it is essential that physicians feel free to speak out about issues that concern them. They can improve their chances of being heard by working directly with management and attending meetings, but in some cases, management may be unwilling to listen.
A version of this article first appeared on Medscape.com.
In April 2020, hospitalist Samantha Houston, MD, lost her job at Baptist Memorial Hospital–North, in Oxford, Miss., after she publicly campaigned to get donations of N95 masks for nurses. Dr. Houston filed a lawsuit against the hospital, saying she was improperly fired for speaking out. The lawsuit has not yet gone to trial.
In January 2017, emergency physician Raymond Brovont, MD, was fired by EmCare, an emergency physician staffing company, after reporting understaffing at hospitals with which it contracted in the Kansas City, Mo., area. Dr. Brovont sued EmCare, and the company lost the case. In February 2019, it was ordered to pay him $13.1 million in damages.
These are just two of several cases in recent years in which physicians have spoken out about problems involving patient care and have been sanctioned. Other physicians who see problems choose to stay silent.
Doctors often hesitate to speak out because of the prospect of losing their jobs. A 2013 study of emergency physicians found that nearly 20% reported a possible or real threat to their employment if they expressed concerns about quality of care.
When physicians do not speak openly about important medical issues, the quality of care in their institutions suffers, said a coauthor of the study, Larry D. Weiss, MD, JD, a retired professor of emergency medicine at the University of Maryland, Baltimore.
“Physicians can’t effectively represent patients if they are always thinking they can get fired for what they say,” Dr. Weiss said. “If you don’t have protections like due process, which is often the case, you are less likely to speak out.”
In the first few weeks, health care facilities were struggling to obtain personal protective equipment (PPE) and to create policies that would keep patients and caregivers safe.
Physicians such as Dr. Houston took the initiative to make sure their institutions were taking the right steps against COVID-19 and found themselves at loggerheads with administrators who were concerned that their organizations were being portrayed as unsafe.
The case of one physician who spoke out
One of the highest-profile cases of a physician speaking out and being removed from work during the pandemic is that of Ming Lin, MD, an emergency physician who lost a job he had held for 17 years at St. Joseph Medical Center, in Bellingham, Wash. Dr. Lin lost his job after he made a series of Facebook posts that criticized the hospital’s COVID-19 preparedness efforts.
In an interview, Dr. Lin discussed the details of his situation to a degree that rarely occurs in such cases. This is one of the most extensive interviews he has granted.
Postings on Facebook
Dr. Lin said that on the basis of an intense study of the virus at the onset of the pandemic, he developed many ideas as to what could be done to mitigate its spread. While working as a locum tenens physician on his time off, he could see how others dealt with COVID-19.
Dr. Lin said from past experiences he did not feel that he could present his ideas directly to administration and be heard, so he decided to air his ideas about how his hospital could handle COVID-19 on his Facebook page, which drew a large audience.
He said he was certain that hospital administrators were reading his posts. He said receptionists at this hospital were advised not to wear masks, evidently because it would alarm patients. Dr. Lin said he posted concerns about their safety and called for them to wear masks. Soon after, the hospital directed receptionists to wear masks.
Dr. Lin’s Facebook posts also criticized the hospital for taking what he felt was too long to get results on COVID-19 tests. “It was taking them up to 10 days to get test results, because samples were being sent to a lab in California,” he said. He suggested it would be faster to send samples to the University of Washington. Soon after, the hospital started sending samples there.
In just a couple of weeks, Dr. Lin said, he voiced almost a dozen concerns. Each time the hospital made changes in line with his recommendations. Although he didn’t get any direct acknowledgment from the hospital for his help, he said he felt he was making a positive impact.
How employers react to physicians who speak out
Physicians who speak out about conditions tend to deeply disturb administrators, said William P. Sullivan, DO, JD, an emergency physician and lawyer in Frankfort, Ill., who has written about physicians being terminated by hospitals.
“These physicians go to the news media or they use social media,” Dr. Sullivan said, “but hospital administrators don’t want the public to hear bad things about their hospital.”
Then the public might not come to the hospital, which is an administrator’s worst nightmare. Even if physicians think their criticisms are reasonable, administrators may still fear a resulting drop in patients.
Dr. Houston, for example, was helping her Mississippi hospital by collecting donations of N95 masks for nurses, but to administrators, it showed that the hospital did not have enough masks.
“It is not helpful to stoke fear and anxiety, even if the intent is sincere,” a spokesperson for the hospital said.
Administrator fires back
Dr. Lin’s posts were deeply concerning to Richard DeCarlo, chief operating officer of PeaceHealth, which runs St. Joseph Hospital. Mr. DeCarlo discussed his concerns in a video interview in April with the blogger Zubin Damania, MD, known as ZDoggMD.
Comments on Dr. Lin’s Facebook posts showed that people “were fearful to go to the hospital,” he told Dr. Damania. “They were concluding that they would need to drive to another hospital.”
Mr. DeCarlo said he was also unhappy that Dr. Lin did not directly contact administrators about his concerns. “He didn’t communicate with his medical director,” Mr. DeCarlo said in the interview. “The ED staff had been meeting three times a week with the chief medical officer to make sure they had everything they needed, but he only attended one of these meetings and didn’t ask any questions.”
Dr. Lin maintains he did ask questions at the first meeting but stopped attending because he felt he wasn’t being heeded. “I found their tone not very receptive,” he said.
Doctor allegedly offered “misinformation”
At the start of the pandemic, some hospitals made it clear what would happen to doctors who brought up lack of PPE or other problems to the media. For example, NYU Langone Medical Center in New York sent an email to staff warning that speaking to the media without permission “will be subject to disciplinary action, including termination.”
PeaceHealth took a different tack. “It’s not that we have a policy that says don’t ever talk to the media,” Mr. DeCarlo said in the ZDoggMD interview, but in Dr. Lin’s case, “what was at issue was the misinformation. His leader went to him and said, ‘Look, you’re posting things that aren’t accurate.’ ”
Dr. Lin disputes that he provided any misinformation. In the interview, Mr. DeCarlo cited just one example of alleged misinformation. He said Dr. Lin called for a tent outside the emergency department (ED) to protect patients entering the department from aerosol exposure to COVID-19. Mr. DeCarlo said the tent was not needed because fewer people were using the ED.
“To put it in an extreme way,” Mr. DeCarlo said of Dr. Lin’s posts, “it was like yelling fire in a theater where there is not a fire.”
Dr. Lin said the hospital did briefly erect a tent and then removed it, and he still insisted that a tent was a good idea. He added that Mr. DeCarlo never mentioned any of the other suggestions Dr. Lin made, nor did he state that the hospital adopted them.
Doctor gets a warning
Dr. Lin said that after he started posting his concerns, he got a call from the emergency department director who worked for TeamHealth, an emergency medicine staffing firm that contracted with PeaceHealth and employed Dr. Lin, too.
Dr. Lin said his immediate supervisor at TeamHealth told him the hospital was unhappy with his posts and that he should take them down and suggested he might be fired. Dr. Lin said the supervisor also asked him to apologize to the hospital administration for these posts, but he refused to do so.
“Retracting and apologizing was not only wrong but would have left me vulnerable to being terminated with no repercussions,” he said.
“At that point, I realized I had crossed the Rubicon,” Dr. Lin said. He thought he might well be fired, no matter what he did, so he took his story to The Seattle Times, which had a much wider platform than his Facebook page had.
Dr. Lin lost his job at St. Joseph a week after The Seattle Times story about him appeared. “About 10 minutes before my shift was supposed to start, I received a text message from TeamHealth saying that someone else would be taking the shift,” he said.
In a release, TeamHealth insisted Dr. Lin was not fired and that he was scheduled to be reassigned to work at other hospitals. Dr. Lin, however, said he was not told this at the time and that he found out later that the new assignment would involve a pay cut and a significant commute. He said he has not taken any new assignments from TeamHealth since he lost his job at St. Joseph.
Dr. Lin has filed a lawsuit against PeaceHealth, TeamHealth, and Mr. DeCarlo, asking for his job back and for an apology. He said he has not asked for any financial damages at this point.
Since leaving St. Joseph, Dr. Lin has been working as an administrator for the Indian Health Service in the upper plains states. He said he can do some of the work at home in Washington State, which allows him to be with his wife and three young children.
Dr. Lin no longer sees patients. “I feel I have lost my confidence as a clinician,” he said. “I’m not sure why, but I find it hard to make quick judgments when taking care of patients.”
He said many doctors have told him about their own troubles with speaking out, but they did not want to come forward and talk about it because they feared more repercussions.
Do doctors who speak out have any rights?
Because TeamHealth, Dr. Lin’s actual employer, asserts he was never actually terminated, Dr. Lin has not been able to appeal his case internally in accordance with due process, an option that allows doctors to get a fair hearing and to appeal decisions against them.
The American Academy of Emergency Medicine pointed out this problem. “Dr. Lin, as a member of the medical staff, is entitled to full due process and a fair hearing from his peers on the medical staff,” the academy said in a statement supporting him.
The Joint Commission, the hospital accreditor, requires that hospitals provide due process to doctors before they can be terminated. However, Dr. Sullivan said employers often make physicians waive their due process rights in the employment contract. “The result is that the employer can terminate doctors for no reason,” he said.
In the 2013 survey of emergency physicians, 62% reported that their employers could terminate them without full due process.
Dr. Weiss, the Maryland MD-JD, said that when he advises doctors on their contracts, he generally tells them to cross out the waiver language. The applicant, he says, may also tell the employer that the waivers are considered unethical by many physician professional societies. In some cases, he said, the hospital will back down.
Conclusion
To maintain quality of care, it is essential that physicians feel free to speak out about issues that concern them. They can improve their chances of being heard by working directly with management and attending meetings, but in some cases, management may be unwilling to listen.
A version of this article first appeared on Medscape.com.
Long-Distance Dermatology: Lessons From an Interview on Remote Practice During a Pandemic and Beyond
For the US health care system, the year 2020 was one of great change as well as extreme pain and hardship: some physical, but much emotional and financial. Dermatologists nationwide have not been sheltered from the winds of change. Yet as with most great challenges, one also can discern great change for the better if you look for it. One area of major growth in the wake of the COVID-19 pandemic is the expansion of telehealth, specifically teledermatology.
Prior to the pandemic, teledermatology was in a phase of modest expansion.1 Since the start of the pandemic, however, the adoption of telemedicine services in the United States has been beyond exponential. Before the pandemic, an estimated 15,000 Medicare recipients received telehealth services on a weekly basis. Yet by the end of April 2020, only 3 months after the first reported case of COVID-19 in the United States, nearly 1.3 million Medicare beneficiaries were utilizing telehealth services on a weekly basis.2 The Centers for Medicare & Medicaid Services has recognized the vast increase in need and responded with the addition of 144 new telehealth services covered by Medicare in the last year. In December 2020, the Centers for Medicare & Medicaid Services moved to make many of the previously provisional policies permanent, expanding long-term coverage for telehealth services,2 and use of teledermatology has expanded in parallel. Although the impetus for this change was simple necessity, the benefits of expanded teledermatology are likely to drive its continued incorporation into our daily practices.
Kevin Wright, MD, is a staff dermatologist at the Naval Medical Center San Diego (San Diego, California) and an Associate Professor of Dermatology at the Uniformed Services University of the Health Sciences (Bethesda, Maryland). In this interview, we discussed his experience incorporating a teledermatology component into his postresidency practice, the pros and cons of teledermatology practice, and ways that residents can prepare for a future in teledermatology.
Would you start by briefly describing your work model now?
My primary job is a Monday-through-Friday classic dermatology clinic job. On the weekends or days off, I see asynchronous and synchronous teledermatology through a specialized platform. On weekends, I tend to see anywhere between 20 and 40 patients in about a 6-hour period with breaks in between.
What does a typical “weekend” day of work look like?
In general, I’ll wake up early before my family and spend maybe an hour working. Oftentimes, that will be in my truck parked down by the beach, where I will go for a run or surf before logging on. If I have 40 visits scheduled that day, I can spend a few hours, message most of them, clarify some aspects of the visit, then go and have breakfast with my family before logging back on and completing the encounters.
Is most of your interaction with patients asynchronous, messaging back and forth to take history?
A few states require a phone call, so those are synchronous, and every Medicaid patient requires a video call. I do synchronous visits with all of my isotretinoin patients at first. It’s a mixed bag, but a lot of my visits are done entirely asynchronously.
What attracted you to this model?
During residency, I always felt that many of the ways we saw patients seemed extraordinarily inefficient. My best example of this is isotretinoin follow-ups. Before this year, most of my colleagues were uncomfortable with virtual isotretinoin follow-ups or thought it was a ridiculous idea. Frankly, I never shared this sentiment. Once I had my own board certification, I knew I was going to pursue teledermatology, because seeing kids take a half day off of school to come in for a 10-minute isotretinoin appointment (that’s mainly just a conversation about sports) just didn’t make sense to me. So I knew I wanted to pursue this idea, I just didn’t know exactly how. One day I was approached by a close friend and mentor of mine who had just purchased a teledermatology platform. She asked me if I would like to moonlight once I graduated and I jumped at the opportunity.
What steps did you take prior to graduating to help prepare you to practice teledermatology?
The most important thing I did—and the most important thing I think for third-year residents to do—is to set myself up for success by starting the US Drug Enforcement Administration (DEA) licensure and certification process. Once you have a DEA number, you can apply for Medicare and Medicaid. The nice thing about Medicare is you can start billing immediately after you apply, which is important. The reimbursement isn’t as high, but they pay faster, which allows you to start seeing patients through teledermatology right away. In a pinch, you could see all Medicare patients and make a living until you’ve completed the rest of the process. Once you have a Medicare and Medicaid number, you can apply for credentialing through private payers. However, the Medicare process takes 3 months, and private-payers credentialing takes about 90 days as well. That’s a lot of time! Before finishing residency, I recommend you make sure you have an unrestricted DEA license and you apply for Medicare/Medicaid credentials. Then, when you’re looking at future employment, you can start getting state licenses almost immediately in whatever states you anticipate needing them.
What are the top 3 benefits of incorporating teledermatology into your practice?
Accessibility is one huge benefit. If you’re practicing in a rural area, you’re basically giving [patients] back their time. Teledermatology takes patients much less time, and they get the same level of care. That’s a big selling point. Your patients will be very happy and loyal because of that.
The other thing I never would have foreseen before starting teledermatology is the amazing follow-up you can get. I think many dermatology residents will agree that there are those patients where you think, “Wow, I wish I could see them back. I wonder how they did,” but you never see them again. That’s not the case with teledermatology. I have a running list of all my interesting cases, and I’ll just shoot them a message 2 or 4 weeks later and at their convenience, they can submit a quick photo. I get that excellent feedback, and that’s huge to me for my own personal education and growth.
The third would be experience. I have 24 state medical licenses, and I see patients of all varieties: all socioeconomic backgrounds and skin types and many with severe skin conditions never managed before by a specialist. That, frankly, has increased my comfort level for seeing patients of all types. It forces me to expand my utilization of certain therapies because some people can’t afford 95% of medications we prescribe commonly. I find that challenge very rewarding. It’s something I’m not sure you can achieve by just practicing within your bubble. Inevitably you are going to see a certain type of patient that your hospital or practice attracts by merit of its geography or catchment area. Teledermatology allows you to see the full spectrum of dermatology.
What are the biggest cons to incorporating teledermatology into your practice?
To start off, some patients have boundary issues. Every 200 patients or so, I’ll have someone who submits a visit at 11:30
The second is reimbursement. In other practice models I can bill more in half the time by seeing a patient in person, doing a skin screening and a few biopsies. I believe there’s always a role for teledermatology in any practice, but ultimately dermatologists are pragmatic people who need to be smart about time management. At some point, it becomes difficult to pay the bills if reimbursement is lacking. That’s one of the bigger downsides to teledermatology. We still need to figure out how to reimburse to incentivize what’s best for the patient.
Could you talk more about the effect on work-life balance?
I think the things that make teledermatology appealing are the same things that could end up disrupting your work-life balance. On the positive side, you can vacation in Hawaii, work for 2 hours each morning, and pay for the whole thing. That’s very appealing to me! The downside is that there are always patients in the queue. In some sense, your waiting room is always half-full, 24/7. Mentally, you have to become comfortable with that, and you have to develop boundaries. I have very specific times I do teledermatology and then I log off. This helps me establish boundaries and creates balance.
You touched on it earlier regarding isotretinoin visits, but what other facets of practice do you think are particularly well-suited to teledermatology?
There are a few that I’ve incorporated into my practice quite aggressively. Almost all acne is going to go to a teledermatology visit. That’s in large part due to payer parity. For the most part, you make the same doing an acne visit online as you will doing it in person. Your patients will be getting the same level of care, better follow-up, and you’ll make the same amount of money. Another thing I do as a patient courtesy is wound checks postsurgery or post-Mohs [micrographic surgery]. There is a huge benefit there to seeing your patients because you can identify infections early, answer simple questions, and reduce in-person clinic visits. That’s a win.
What are visit types you feel are not well-suited to teledermatology or that you approach with more caution?
This will be different for everyone to some degree. I think practitioners need to be alert and use their best judgement when approaching any new patient or new concern. Pigmented lesions certainly give me pause. Although the technology is getting better every day, I believe there is still a gap between seeing a photo of a lesion and seeing a pigmented lesion in person, being able to get up close and examine it dermoscopically. Teledermoscopy, however, is an emerging business model as well, and it will be interesting to see what role this can play as it gets incorporated.
You mentioned having medical licenses in several states. Can you describe the process you went through to obtain these licenses?
It’s a painful process. I started realizing this was something I wanted to incorporate after residency, so I started looking into applying for medical licenses early. Teledermatology companies often will reimburse you and help you to get licenses. I was lucky enough to get assistance, which was essential because it is an onerous process. If you can work that into your contract during negotiations that would be ideal. Not everyone will be as lucky as I was, though. If that doesn’t pertain to you, pick a few states that have larger populations around you, where you know that they have a lot of need and start applying there. Be aware that medical licensure takes about 6 months. Having this started around mid–third year is important.
Employers want someone they can use right away, so I found it very beneficial to approach an employer and be able to explain to them tangibly where you are in the process. For example, “I’ve got my DEA license, Medicare, Medicaid number, and I have licensure in your state and all the surrounding states.” You then have a leg to stand on with your negotiating. If you do the legwork and can then negotiate a higher percentage, you’ll make up the licensure fees in a half day of work. It’s an investment toward your professional career.
Any final thoughts?
I think that insurers are very interested in teledermatology because there’s a potential for huge cost savings. As the dust settles with COVID-19 and we see how telemedicine has changed medicine in general, I really think that payers are going to be more aggressive about requiring teledermatology from their dermatologists. I think residents need to anticipate that teledermatology will be some part of their practice in the future and should start planning now to be prepared for this brave new world going forward.
- Yim KM, Florek AG, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era. Telemed J E Health. 2018;24:691-697.
- Shatzkes MM, Borha EL. Permanent expansion of Medicare telehealth services. The National Law Review website. Published December 7, 2020. Accessed April 13, 2021. https://www.natlawreview.com/article/permanent-expansion-medicare-telehealth-services
For the US health care system, the year 2020 was one of great change as well as extreme pain and hardship: some physical, but much emotional and financial. Dermatologists nationwide have not been sheltered from the winds of change. Yet as with most great challenges, one also can discern great change for the better if you look for it. One area of major growth in the wake of the COVID-19 pandemic is the expansion of telehealth, specifically teledermatology.
Prior to the pandemic, teledermatology was in a phase of modest expansion.1 Since the start of the pandemic, however, the adoption of telemedicine services in the United States has been beyond exponential. Before the pandemic, an estimated 15,000 Medicare recipients received telehealth services on a weekly basis. Yet by the end of April 2020, only 3 months after the first reported case of COVID-19 in the United States, nearly 1.3 million Medicare beneficiaries were utilizing telehealth services on a weekly basis.2 The Centers for Medicare & Medicaid Services has recognized the vast increase in need and responded with the addition of 144 new telehealth services covered by Medicare in the last year. In December 2020, the Centers for Medicare & Medicaid Services moved to make many of the previously provisional policies permanent, expanding long-term coverage for telehealth services,2 and use of teledermatology has expanded in parallel. Although the impetus for this change was simple necessity, the benefits of expanded teledermatology are likely to drive its continued incorporation into our daily practices.
Kevin Wright, MD, is a staff dermatologist at the Naval Medical Center San Diego (San Diego, California) and an Associate Professor of Dermatology at the Uniformed Services University of the Health Sciences (Bethesda, Maryland). In this interview, we discussed his experience incorporating a teledermatology component into his postresidency practice, the pros and cons of teledermatology practice, and ways that residents can prepare for a future in teledermatology.
Would you start by briefly describing your work model now?
My primary job is a Monday-through-Friday classic dermatology clinic job. On the weekends or days off, I see asynchronous and synchronous teledermatology through a specialized platform. On weekends, I tend to see anywhere between 20 and 40 patients in about a 6-hour period with breaks in between.
What does a typical “weekend” day of work look like?
In general, I’ll wake up early before my family and spend maybe an hour working. Oftentimes, that will be in my truck parked down by the beach, where I will go for a run or surf before logging on. If I have 40 visits scheduled that day, I can spend a few hours, message most of them, clarify some aspects of the visit, then go and have breakfast with my family before logging back on and completing the encounters.
Is most of your interaction with patients asynchronous, messaging back and forth to take history?
A few states require a phone call, so those are synchronous, and every Medicaid patient requires a video call. I do synchronous visits with all of my isotretinoin patients at first. It’s a mixed bag, but a lot of my visits are done entirely asynchronously.
What attracted you to this model?
During residency, I always felt that many of the ways we saw patients seemed extraordinarily inefficient. My best example of this is isotretinoin follow-ups. Before this year, most of my colleagues were uncomfortable with virtual isotretinoin follow-ups or thought it was a ridiculous idea. Frankly, I never shared this sentiment. Once I had my own board certification, I knew I was going to pursue teledermatology, because seeing kids take a half day off of school to come in for a 10-minute isotretinoin appointment (that’s mainly just a conversation about sports) just didn’t make sense to me. So I knew I wanted to pursue this idea, I just didn’t know exactly how. One day I was approached by a close friend and mentor of mine who had just purchased a teledermatology platform. She asked me if I would like to moonlight once I graduated and I jumped at the opportunity.
What steps did you take prior to graduating to help prepare you to practice teledermatology?
The most important thing I did—and the most important thing I think for third-year residents to do—is to set myself up for success by starting the US Drug Enforcement Administration (DEA) licensure and certification process. Once you have a DEA number, you can apply for Medicare and Medicaid. The nice thing about Medicare is you can start billing immediately after you apply, which is important. The reimbursement isn’t as high, but they pay faster, which allows you to start seeing patients through teledermatology right away. In a pinch, you could see all Medicare patients and make a living until you’ve completed the rest of the process. Once you have a Medicare and Medicaid number, you can apply for credentialing through private payers. However, the Medicare process takes 3 months, and private-payers credentialing takes about 90 days as well. That’s a lot of time! Before finishing residency, I recommend you make sure you have an unrestricted DEA license and you apply for Medicare/Medicaid credentials. Then, when you’re looking at future employment, you can start getting state licenses almost immediately in whatever states you anticipate needing them.
What are the top 3 benefits of incorporating teledermatology into your practice?
Accessibility is one huge benefit. If you’re practicing in a rural area, you’re basically giving [patients] back their time. Teledermatology takes patients much less time, and they get the same level of care. That’s a big selling point. Your patients will be very happy and loyal because of that.
The other thing I never would have foreseen before starting teledermatology is the amazing follow-up you can get. I think many dermatology residents will agree that there are those patients where you think, “Wow, I wish I could see them back. I wonder how they did,” but you never see them again. That’s not the case with teledermatology. I have a running list of all my interesting cases, and I’ll just shoot them a message 2 or 4 weeks later and at their convenience, they can submit a quick photo. I get that excellent feedback, and that’s huge to me for my own personal education and growth.
The third would be experience. I have 24 state medical licenses, and I see patients of all varieties: all socioeconomic backgrounds and skin types and many with severe skin conditions never managed before by a specialist. That, frankly, has increased my comfort level for seeing patients of all types. It forces me to expand my utilization of certain therapies because some people can’t afford 95% of medications we prescribe commonly. I find that challenge very rewarding. It’s something I’m not sure you can achieve by just practicing within your bubble. Inevitably you are going to see a certain type of patient that your hospital or practice attracts by merit of its geography or catchment area. Teledermatology allows you to see the full spectrum of dermatology.
What are the biggest cons to incorporating teledermatology into your practice?
To start off, some patients have boundary issues. Every 200 patients or so, I’ll have someone who submits a visit at 11:30
The second is reimbursement. In other practice models I can bill more in half the time by seeing a patient in person, doing a skin screening and a few biopsies. I believe there’s always a role for teledermatology in any practice, but ultimately dermatologists are pragmatic people who need to be smart about time management. At some point, it becomes difficult to pay the bills if reimbursement is lacking. That’s one of the bigger downsides to teledermatology. We still need to figure out how to reimburse to incentivize what’s best for the patient.
Could you talk more about the effect on work-life balance?
I think the things that make teledermatology appealing are the same things that could end up disrupting your work-life balance. On the positive side, you can vacation in Hawaii, work for 2 hours each morning, and pay for the whole thing. That’s very appealing to me! The downside is that there are always patients in the queue. In some sense, your waiting room is always half-full, 24/7. Mentally, you have to become comfortable with that, and you have to develop boundaries. I have very specific times I do teledermatology and then I log off. This helps me establish boundaries and creates balance.
You touched on it earlier regarding isotretinoin visits, but what other facets of practice do you think are particularly well-suited to teledermatology?
There are a few that I’ve incorporated into my practice quite aggressively. Almost all acne is going to go to a teledermatology visit. That’s in large part due to payer parity. For the most part, you make the same doing an acne visit online as you will doing it in person. Your patients will be getting the same level of care, better follow-up, and you’ll make the same amount of money. Another thing I do as a patient courtesy is wound checks postsurgery or post-Mohs [micrographic surgery]. There is a huge benefit there to seeing your patients because you can identify infections early, answer simple questions, and reduce in-person clinic visits. That’s a win.
What are visit types you feel are not well-suited to teledermatology or that you approach with more caution?
This will be different for everyone to some degree. I think practitioners need to be alert and use their best judgement when approaching any new patient or new concern. Pigmented lesions certainly give me pause. Although the technology is getting better every day, I believe there is still a gap between seeing a photo of a lesion and seeing a pigmented lesion in person, being able to get up close and examine it dermoscopically. Teledermoscopy, however, is an emerging business model as well, and it will be interesting to see what role this can play as it gets incorporated.
You mentioned having medical licenses in several states. Can you describe the process you went through to obtain these licenses?
It’s a painful process. I started realizing this was something I wanted to incorporate after residency, so I started looking into applying for medical licenses early. Teledermatology companies often will reimburse you and help you to get licenses. I was lucky enough to get assistance, which was essential because it is an onerous process. If you can work that into your contract during negotiations that would be ideal. Not everyone will be as lucky as I was, though. If that doesn’t pertain to you, pick a few states that have larger populations around you, where you know that they have a lot of need and start applying there. Be aware that medical licensure takes about 6 months. Having this started around mid–third year is important.
Employers want someone they can use right away, so I found it very beneficial to approach an employer and be able to explain to them tangibly where you are in the process. For example, “I’ve got my DEA license, Medicare, Medicaid number, and I have licensure in your state and all the surrounding states.” You then have a leg to stand on with your negotiating. If you do the legwork and can then negotiate a higher percentage, you’ll make up the licensure fees in a half day of work. It’s an investment toward your professional career.
Any final thoughts?
I think that insurers are very interested in teledermatology because there’s a potential for huge cost savings. As the dust settles with COVID-19 and we see how telemedicine has changed medicine in general, I really think that payers are going to be more aggressive about requiring teledermatology from their dermatologists. I think residents need to anticipate that teledermatology will be some part of their practice in the future and should start planning now to be prepared for this brave new world going forward.
For the US health care system, the year 2020 was one of great change as well as extreme pain and hardship: some physical, but much emotional and financial. Dermatologists nationwide have not been sheltered from the winds of change. Yet as with most great challenges, one also can discern great change for the better if you look for it. One area of major growth in the wake of the COVID-19 pandemic is the expansion of telehealth, specifically teledermatology.
Prior to the pandemic, teledermatology was in a phase of modest expansion.1 Since the start of the pandemic, however, the adoption of telemedicine services in the United States has been beyond exponential. Before the pandemic, an estimated 15,000 Medicare recipients received telehealth services on a weekly basis. Yet by the end of April 2020, only 3 months after the first reported case of COVID-19 in the United States, nearly 1.3 million Medicare beneficiaries were utilizing telehealth services on a weekly basis.2 The Centers for Medicare & Medicaid Services has recognized the vast increase in need and responded with the addition of 144 new telehealth services covered by Medicare in the last year. In December 2020, the Centers for Medicare & Medicaid Services moved to make many of the previously provisional policies permanent, expanding long-term coverage for telehealth services,2 and use of teledermatology has expanded in parallel. Although the impetus for this change was simple necessity, the benefits of expanded teledermatology are likely to drive its continued incorporation into our daily practices.
Kevin Wright, MD, is a staff dermatologist at the Naval Medical Center San Diego (San Diego, California) and an Associate Professor of Dermatology at the Uniformed Services University of the Health Sciences (Bethesda, Maryland). In this interview, we discussed his experience incorporating a teledermatology component into his postresidency practice, the pros and cons of teledermatology practice, and ways that residents can prepare for a future in teledermatology.
Would you start by briefly describing your work model now?
My primary job is a Monday-through-Friday classic dermatology clinic job. On the weekends or days off, I see asynchronous and synchronous teledermatology through a specialized platform. On weekends, I tend to see anywhere between 20 and 40 patients in about a 6-hour period with breaks in between.
What does a typical “weekend” day of work look like?
In general, I’ll wake up early before my family and spend maybe an hour working. Oftentimes, that will be in my truck parked down by the beach, where I will go for a run or surf before logging on. If I have 40 visits scheduled that day, I can spend a few hours, message most of them, clarify some aspects of the visit, then go and have breakfast with my family before logging back on and completing the encounters.
Is most of your interaction with patients asynchronous, messaging back and forth to take history?
A few states require a phone call, so those are synchronous, and every Medicaid patient requires a video call. I do synchronous visits with all of my isotretinoin patients at first. It’s a mixed bag, but a lot of my visits are done entirely asynchronously.
What attracted you to this model?
During residency, I always felt that many of the ways we saw patients seemed extraordinarily inefficient. My best example of this is isotretinoin follow-ups. Before this year, most of my colleagues were uncomfortable with virtual isotretinoin follow-ups or thought it was a ridiculous idea. Frankly, I never shared this sentiment. Once I had my own board certification, I knew I was going to pursue teledermatology, because seeing kids take a half day off of school to come in for a 10-minute isotretinoin appointment (that’s mainly just a conversation about sports) just didn’t make sense to me. So I knew I wanted to pursue this idea, I just didn’t know exactly how. One day I was approached by a close friend and mentor of mine who had just purchased a teledermatology platform. She asked me if I would like to moonlight once I graduated and I jumped at the opportunity.
What steps did you take prior to graduating to help prepare you to practice teledermatology?
The most important thing I did—and the most important thing I think for third-year residents to do—is to set myself up for success by starting the US Drug Enforcement Administration (DEA) licensure and certification process. Once you have a DEA number, you can apply for Medicare and Medicaid. The nice thing about Medicare is you can start billing immediately after you apply, which is important. The reimbursement isn’t as high, but they pay faster, which allows you to start seeing patients through teledermatology right away. In a pinch, you could see all Medicare patients and make a living until you’ve completed the rest of the process. Once you have a Medicare and Medicaid number, you can apply for credentialing through private payers. However, the Medicare process takes 3 months, and private-payers credentialing takes about 90 days as well. That’s a lot of time! Before finishing residency, I recommend you make sure you have an unrestricted DEA license and you apply for Medicare/Medicaid credentials. Then, when you’re looking at future employment, you can start getting state licenses almost immediately in whatever states you anticipate needing them.
What are the top 3 benefits of incorporating teledermatology into your practice?
Accessibility is one huge benefit. If you’re practicing in a rural area, you’re basically giving [patients] back their time. Teledermatology takes patients much less time, and they get the same level of care. That’s a big selling point. Your patients will be very happy and loyal because of that.
The other thing I never would have foreseen before starting teledermatology is the amazing follow-up you can get. I think many dermatology residents will agree that there are those patients where you think, “Wow, I wish I could see them back. I wonder how they did,” but you never see them again. That’s not the case with teledermatology. I have a running list of all my interesting cases, and I’ll just shoot them a message 2 or 4 weeks later and at their convenience, they can submit a quick photo. I get that excellent feedback, and that’s huge to me for my own personal education and growth.
The third would be experience. I have 24 state medical licenses, and I see patients of all varieties: all socioeconomic backgrounds and skin types and many with severe skin conditions never managed before by a specialist. That, frankly, has increased my comfort level for seeing patients of all types. It forces me to expand my utilization of certain therapies because some people can’t afford 95% of medications we prescribe commonly. I find that challenge very rewarding. It’s something I’m not sure you can achieve by just practicing within your bubble. Inevitably you are going to see a certain type of patient that your hospital or practice attracts by merit of its geography or catchment area. Teledermatology allows you to see the full spectrum of dermatology.
What are the biggest cons to incorporating teledermatology into your practice?
To start off, some patients have boundary issues. Every 200 patients or so, I’ll have someone who submits a visit at 11:30
The second is reimbursement. In other practice models I can bill more in half the time by seeing a patient in person, doing a skin screening and a few biopsies. I believe there’s always a role for teledermatology in any practice, but ultimately dermatologists are pragmatic people who need to be smart about time management. At some point, it becomes difficult to pay the bills if reimbursement is lacking. That’s one of the bigger downsides to teledermatology. We still need to figure out how to reimburse to incentivize what’s best for the patient.
Could you talk more about the effect on work-life balance?
I think the things that make teledermatology appealing are the same things that could end up disrupting your work-life balance. On the positive side, you can vacation in Hawaii, work for 2 hours each morning, and pay for the whole thing. That’s very appealing to me! The downside is that there are always patients in the queue. In some sense, your waiting room is always half-full, 24/7. Mentally, you have to become comfortable with that, and you have to develop boundaries. I have very specific times I do teledermatology and then I log off. This helps me establish boundaries and creates balance.
You touched on it earlier regarding isotretinoin visits, but what other facets of practice do you think are particularly well-suited to teledermatology?
There are a few that I’ve incorporated into my practice quite aggressively. Almost all acne is going to go to a teledermatology visit. That’s in large part due to payer parity. For the most part, you make the same doing an acne visit online as you will doing it in person. Your patients will be getting the same level of care, better follow-up, and you’ll make the same amount of money. Another thing I do as a patient courtesy is wound checks postsurgery or post-Mohs [micrographic surgery]. There is a huge benefit there to seeing your patients because you can identify infections early, answer simple questions, and reduce in-person clinic visits. That’s a win.
What are visit types you feel are not well-suited to teledermatology or that you approach with more caution?
This will be different for everyone to some degree. I think practitioners need to be alert and use their best judgement when approaching any new patient or new concern. Pigmented lesions certainly give me pause. Although the technology is getting better every day, I believe there is still a gap between seeing a photo of a lesion and seeing a pigmented lesion in person, being able to get up close and examine it dermoscopically. Teledermoscopy, however, is an emerging business model as well, and it will be interesting to see what role this can play as it gets incorporated.
You mentioned having medical licenses in several states. Can you describe the process you went through to obtain these licenses?
It’s a painful process. I started realizing this was something I wanted to incorporate after residency, so I started looking into applying for medical licenses early. Teledermatology companies often will reimburse you and help you to get licenses. I was lucky enough to get assistance, which was essential because it is an onerous process. If you can work that into your contract during negotiations that would be ideal. Not everyone will be as lucky as I was, though. If that doesn’t pertain to you, pick a few states that have larger populations around you, where you know that they have a lot of need and start applying there. Be aware that medical licensure takes about 6 months. Having this started around mid–third year is important.
Employers want someone they can use right away, so I found it very beneficial to approach an employer and be able to explain to them tangibly where you are in the process. For example, “I’ve got my DEA license, Medicare, Medicaid number, and I have licensure in your state and all the surrounding states.” You then have a leg to stand on with your negotiating. If you do the legwork and can then negotiate a higher percentage, you’ll make up the licensure fees in a half day of work. It’s an investment toward your professional career.
Any final thoughts?
I think that insurers are very interested in teledermatology because there’s a potential for huge cost savings. As the dust settles with COVID-19 and we see how telemedicine has changed medicine in general, I really think that payers are going to be more aggressive about requiring teledermatology from their dermatologists. I think residents need to anticipate that teledermatology will be some part of their practice in the future and should start planning now to be prepared for this brave new world going forward.
- Yim KM, Florek AG, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era. Telemed J E Health. 2018;24:691-697.
- Shatzkes MM, Borha EL. Permanent expansion of Medicare telehealth services. The National Law Review website. Published December 7, 2020. Accessed April 13, 2021. https://www.natlawreview.com/article/permanent-expansion-medicare-telehealth-services
- Yim KM, Florek AG, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era. Telemed J E Health. 2018;24:691-697.
- Shatzkes MM, Borha EL. Permanent expansion of Medicare telehealth services. The National Law Review website. Published December 7, 2020. Accessed April 13, 2021. https://www.natlawreview.com/article/permanent-expansion-medicare-telehealth-services
Resident Pearl
- One result of the COVID-19 pandemic is the aggressive adoption of teledermatology across the United States. Graduating residents should be preparing for a scope of practice that incorporates teledermatology.
Percentage of doctors who are Black barely changed in 120 years
according to a new study.
In 1900, 1.3% of physicians were Black. In 1940, 2.8% of physicians were Black, and by 2018 – when almost 13% of the population was Black – 5.4% of doctors were Black, reports Dan Ly, MD, PhD, MPP, an assistant professor of medicine at the University of California, Los Angeles, in a study published online April 19, 2021, in the Journal of General Internal Medicine.
The proportion of male Black physicians was 2.7% in 1940 and 2.6% in 2018.
Dr. Ly also found a significant wage gap. The median income earned by White doctors was $50,000 more than the median income of Black physicians in 2018. Dr. Ly based his findings on the U.S. Census Decennial Census long form, accessed via IPUMS, a free database funded by the National Institutes of Health and other organizations.
“If we care about the health of the population, particularly the health of Black patients, we should care about how small the proportion of our physicians who are Black is and the extremely slow progress we have made as a medical system in increasing that proportion,” Dr. Ly said in an interview.
Dr. Ly said he took on this research in part because previous studies have shown that Black patients are more likely to seek preventive care from Black doctors. Thus, increasing the numbers of Black physicians could narrow gaps in life expectancy between Whites and Blacks.
He also wanted to see whether progress had been made as a result of various medical organizations and the Association of American Medical Colleges undertaking initiatives to increase workforce diversity. There has been “very, very little” progress, he said.
Norma Poll-Hunter, PhD, the AAMC’s senior director of workforce diversity, said Dr. Ly’s report “was not surprising at all.”
The AAMC reported in 2014 that the number of Black men who apply to and matriculate into medical schools has been declining since 1978. That year, there were 1,410 Black male applicants and 542 Black enrollees. In 2014, there were 1,337 applicants and 515 enrollees.
Since 2014, Black male enrollment has increased slightly, rising from 2.4% in the 2014-2015 school year to 2.9% in the 2019-2020 year, the AAMC reported last year.
In addition, among other historically underrepresented minorities, “we really have seen very small progress” despite the increase in the number of medical schools, Dr. Poll-Hunter said in an interview.
The AAMC and the National Medical Association consider the lack of Black male applicants and matriculants to be a national crisis. The two groups started an alliance in 2020 aimed at finding ways to amplify and support Black men’s interest in medicine and the biomedical sciences and to “develop systems-based solutions to address exclusionary practices that create barriers for Black men and prevent them from having equitable opportunities to successfully enroll in medical school.”
Solutions include requiring medical school admissions committees and application screeners to undergo implicit bias awareness and mitigation training, adopting holistic admissions reviews, and incentivizing institutions of higher learning to partner with Black communities in urban and rural school systems to establish K-12 health sciences academies, said NMA President Leon McDougle, MD, MPH.
“There are the systems factors, and racism is a big one that we have to tackle,” said Dr. Poll-Hunter.
Diversity isn’t just about numbers, said Dr. McDougle, a professor of family medicine and associate dean for diversity and inclusion at Ohio State University, Columbus. “We know that medical school graduates who are African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native are more likely to serve those communities as practicing physicians.
“The COVID-19 pandemic highlighted the urgent need for more African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native physicians,” he said. “Inadequate access to culturally competent care has exacerbated existing health disparities, resulting in death and hospitalization rates up to three to four times the rates of European American or White people.”
Dr. Poll-Hunter also said that studies have shown that diversity in the classroom creates a more enriched learning environment and increases civic mindedness and cognitive complexity, “as well as helps us understand people who are different than ourselves.”
The diversity goal “is not about quotas, it’s about excellence,” she said. “We know that there’s talent that exists, and we want to make sure that everyone has an opportunity to be successful.”
Dr. Ly has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study.
In 1900, 1.3% of physicians were Black. In 1940, 2.8% of physicians were Black, and by 2018 – when almost 13% of the population was Black – 5.4% of doctors were Black, reports Dan Ly, MD, PhD, MPP, an assistant professor of medicine at the University of California, Los Angeles, in a study published online April 19, 2021, in the Journal of General Internal Medicine.
The proportion of male Black physicians was 2.7% in 1940 and 2.6% in 2018.
Dr. Ly also found a significant wage gap. The median income earned by White doctors was $50,000 more than the median income of Black physicians in 2018. Dr. Ly based his findings on the U.S. Census Decennial Census long form, accessed via IPUMS, a free database funded by the National Institutes of Health and other organizations.
“If we care about the health of the population, particularly the health of Black patients, we should care about how small the proportion of our physicians who are Black is and the extremely slow progress we have made as a medical system in increasing that proportion,” Dr. Ly said in an interview.
Dr. Ly said he took on this research in part because previous studies have shown that Black patients are more likely to seek preventive care from Black doctors. Thus, increasing the numbers of Black physicians could narrow gaps in life expectancy between Whites and Blacks.
He also wanted to see whether progress had been made as a result of various medical organizations and the Association of American Medical Colleges undertaking initiatives to increase workforce diversity. There has been “very, very little” progress, he said.
Norma Poll-Hunter, PhD, the AAMC’s senior director of workforce diversity, said Dr. Ly’s report “was not surprising at all.”
The AAMC reported in 2014 that the number of Black men who apply to and matriculate into medical schools has been declining since 1978. That year, there were 1,410 Black male applicants and 542 Black enrollees. In 2014, there were 1,337 applicants and 515 enrollees.
Since 2014, Black male enrollment has increased slightly, rising from 2.4% in the 2014-2015 school year to 2.9% in the 2019-2020 year, the AAMC reported last year.
In addition, among other historically underrepresented minorities, “we really have seen very small progress” despite the increase in the number of medical schools, Dr. Poll-Hunter said in an interview.
The AAMC and the National Medical Association consider the lack of Black male applicants and matriculants to be a national crisis. The two groups started an alliance in 2020 aimed at finding ways to amplify and support Black men’s interest in medicine and the biomedical sciences and to “develop systems-based solutions to address exclusionary practices that create barriers for Black men and prevent them from having equitable opportunities to successfully enroll in medical school.”
Solutions include requiring medical school admissions committees and application screeners to undergo implicit bias awareness and mitigation training, adopting holistic admissions reviews, and incentivizing institutions of higher learning to partner with Black communities in urban and rural school systems to establish K-12 health sciences academies, said NMA President Leon McDougle, MD, MPH.
“There are the systems factors, and racism is a big one that we have to tackle,” said Dr. Poll-Hunter.
Diversity isn’t just about numbers, said Dr. McDougle, a professor of family medicine and associate dean for diversity and inclusion at Ohio State University, Columbus. “We know that medical school graduates who are African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native are more likely to serve those communities as practicing physicians.
“The COVID-19 pandemic highlighted the urgent need for more African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native physicians,” he said. “Inadequate access to culturally competent care has exacerbated existing health disparities, resulting in death and hospitalization rates up to three to four times the rates of European American or White people.”
Dr. Poll-Hunter also said that studies have shown that diversity in the classroom creates a more enriched learning environment and increases civic mindedness and cognitive complexity, “as well as helps us understand people who are different than ourselves.”
The diversity goal “is not about quotas, it’s about excellence,” she said. “We know that there’s talent that exists, and we want to make sure that everyone has an opportunity to be successful.”
Dr. Ly has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study.
In 1900, 1.3% of physicians were Black. In 1940, 2.8% of physicians were Black, and by 2018 – when almost 13% of the population was Black – 5.4% of doctors were Black, reports Dan Ly, MD, PhD, MPP, an assistant professor of medicine at the University of California, Los Angeles, in a study published online April 19, 2021, in the Journal of General Internal Medicine.
The proportion of male Black physicians was 2.7% in 1940 and 2.6% in 2018.
Dr. Ly also found a significant wage gap. The median income earned by White doctors was $50,000 more than the median income of Black physicians in 2018. Dr. Ly based his findings on the U.S. Census Decennial Census long form, accessed via IPUMS, a free database funded by the National Institutes of Health and other organizations.
“If we care about the health of the population, particularly the health of Black patients, we should care about how small the proportion of our physicians who are Black is and the extremely slow progress we have made as a medical system in increasing that proportion,” Dr. Ly said in an interview.
Dr. Ly said he took on this research in part because previous studies have shown that Black patients are more likely to seek preventive care from Black doctors. Thus, increasing the numbers of Black physicians could narrow gaps in life expectancy between Whites and Blacks.
He also wanted to see whether progress had been made as a result of various medical organizations and the Association of American Medical Colleges undertaking initiatives to increase workforce diversity. There has been “very, very little” progress, he said.
Norma Poll-Hunter, PhD, the AAMC’s senior director of workforce diversity, said Dr. Ly’s report “was not surprising at all.”
The AAMC reported in 2014 that the number of Black men who apply to and matriculate into medical schools has been declining since 1978. That year, there were 1,410 Black male applicants and 542 Black enrollees. In 2014, there were 1,337 applicants and 515 enrollees.
Since 2014, Black male enrollment has increased slightly, rising from 2.4% in the 2014-2015 school year to 2.9% in the 2019-2020 year, the AAMC reported last year.
In addition, among other historically underrepresented minorities, “we really have seen very small progress” despite the increase in the number of medical schools, Dr. Poll-Hunter said in an interview.
The AAMC and the National Medical Association consider the lack of Black male applicants and matriculants to be a national crisis. The two groups started an alliance in 2020 aimed at finding ways to amplify and support Black men’s interest in medicine and the biomedical sciences and to “develop systems-based solutions to address exclusionary practices that create barriers for Black men and prevent them from having equitable opportunities to successfully enroll in medical school.”
Solutions include requiring medical school admissions committees and application screeners to undergo implicit bias awareness and mitigation training, adopting holistic admissions reviews, and incentivizing institutions of higher learning to partner with Black communities in urban and rural school systems to establish K-12 health sciences academies, said NMA President Leon McDougle, MD, MPH.
“There are the systems factors, and racism is a big one that we have to tackle,” said Dr. Poll-Hunter.
Diversity isn’t just about numbers, said Dr. McDougle, a professor of family medicine and associate dean for diversity and inclusion at Ohio State University, Columbus. “We know that medical school graduates who are African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native are more likely to serve those communities as practicing physicians.
“The COVID-19 pandemic highlighted the urgent need for more African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native physicians,” he said. “Inadequate access to culturally competent care has exacerbated existing health disparities, resulting in death and hospitalization rates up to three to four times the rates of European American or White people.”
Dr. Poll-Hunter also said that studies have shown that diversity in the classroom creates a more enriched learning environment and increases civic mindedness and cognitive complexity, “as well as helps us understand people who are different than ourselves.”
The diversity goal “is not about quotas, it’s about excellence,” she said. “We know that there’s talent that exists, and we want to make sure that everyone has an opportunity to be successful.”
Dr. Ly has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Vaccinating homebound patients is an uphill battle

There are about 2 million to 4 million homebound patients in the United States, according to a webinar from The Trust for America’s Health, which was broadcast in March. But many of these individuals have not been vaccinated yet because of logistical challenges.
Some homebound COVID-19 immunization programs are administering Moderna and Pfizer vaccines to their patients, but many state, city, and local programs administered the Johnson & Johnson vaccine after it was cleared for use by the Food and Drug Administration in February 2021. The efficacy of the one-shot vaccine, as well as it being easier to store and ship than the Moderna and Pfizer vaccines, makes getting it to homebound patients less challenging.
“With Pfizer and Moderna, transportation is a challenge because the temperature demands and the fragility of [messenger] RNA–based vaccines,” Brent Feorene, executive director of the American Academy of Home Care Medicine, said in an interview. That’s why [the Johnson & Johnson] vaccine held such promise – it’s less fragile, [can be stored in] higher temperatures, and was a one shot.”
Other hurdles to getting homebound patients vaccinated had already been in place prior to the 10-day-pause on using the J&J vaccine that occurred for federal agencies to consider possible serious side effects linked to it.
Many roadblocks to vaccination
Although many homebound patients can’t readily go out into the community and be exposed to the COVID-19 virus themselves, they are dependent on caregivers and family members who do go out into the community.
“Their friends, family, neighbors, home health aides, and other kinds of health care workers come into the home,” said Shawn Amer, clinical program director at Central Ohio Primary Care in Columbus.
Nurses from Ms. Amer’s practice vaccinated approximately ten homebound patients with the J&J vaccine through a pilot program in March. Then on April 24, nurses from Central Ohio Primary Care vaccinated just under 40 homebound patients and about a handful of their caregivers who were not able to get their vaccines elsewhere, according to Ms. Amer. This time they used the Pfizer vaccine and will be returning to these patients’ homes on May 15 to administer the second dose.
“Any time you are getting in the car and adding miles, it adds complexity,” Ms. Amer said.
“We called patients 24 to 36 hours before coming to their homes to make sure they were ready, but we learned that just because the healthcare power of attorney agrees to a patient getting vaccinated does not mean that patient will be willing to get the vaccine when the nurse shows up," she noted.
Ms. Amer elaborated that three patients with dementia refused the vaccine when nurses arrived at their home on April 24.
“We had to pivot and find other people,” Ms. Amer. Her practice ended up having to waste one shot.
Expenses are greater
The higher costs of getting homebound patients vaccinated is an additional hurdle to getting these vulnerable individuals protected by COVID-19 shots.
Vaccinating patients in their homes “doesn’t require a lot of technology, but it does require a lot of time” and the staffing expense becomes part of the challenge, Ms. Amer noted.
For each of the two days that Central Ohio Primary Care provides the Pfizer vaccine to homebound patients, the practice needs to pay seven nurses to administer the vaccine, Ms. Amer explained.
There have also been reports of organizations that administer the vaccines – which are free for patients because the federal government is paying for them – not being paid enough by Medicare to cover staff time and efforts to vaccinate patients in their homes, Kaiser Health News reported. According to the Centers for Medicare & Medicaid Services, they pay $40 for the administration of a single-dose COVID-19 vaccine and, for COVID-19 vaccines requiring multiple doses, Medicare pays approximately $40 for each dose in the series. These rates were implemented after March 15. Before that date, the rates were even lower, with the Medicare reimbursement rates for initial doses of COVID-19 vaccines being $16.94 and final doses being $28.39.
William Dombi, president of the National Association for Home Care & Hospice, told Kaiser Health News that the actual cost of these homebound visits are closer to $150 or $160.
“The reimbursement for the injection is pretty minimal,” Mr. Feorene said. “So unless you’re a larger organization and able to have staff to deploy some of your smaller practices, just couldn’t afford to do it.”
Many homebound patients have also been unable to get the lifesaving shots because of logistical roadblocks and many practices not being able to do home visits.
“I think that initially when the [Centers for Disease Control and Prevention] came out with vaccine guidance for medical providers, they offered no guidance for in-home medical providers and we had to go back and ask for that, which they did produce,” Mr. Feorene said. “And we’re grateful for that. But I think just this general understanding that there is a population of folks that are [limited to their home], that they do receive medical care and other care in the home, and that we have to remember that the medical providers who provide care in the home are also primary care providers.”
Furthermore, trying to navigate or find programs delivering vaccines to the homebound can be difficult depending on where a patient lives.
While some programs have been launched on the country or city level – the New York Fire Department launched a pilot program to bring the Johnson & Johnson vaccine to homebound seniors – other programs have been spearheaded by hospital networks like Northwell and Mount Sinai. However, many of these hospital networks only reach out to people who already have a relationship with the hospital.
Ms Amer said identifying homebound patients and reaching out to them can be tough and can contribute to the logistics and time involved in setting patients up for the vaccine.
“Reaching some of these patients is difficult,” Ms. Amer noted. “Sometimes the best way to reach them or get a hold of them is through their caregiver. And so do you have the right phone number? Do you have the right name?”
Overcoming the challenges
With the absence of a national plan targeting homebound patients, many local initiatives were launched to help these individuals get vaccinated. Local fire department paramedics have gone door to door to administer the COVID-19 vaccine in cities like Chicago, New York, and Miami. The suspension of the Johnson & Johnson vaccine resulted in the suspension of in-home vaccinations for some people in New York City. However, the program resumed after the FDA and CDC lifted the pause on April 24.

Health systems like Mount Sinai vaccinated approximately 530 people through the Mount Sinai Visiting Doctors Program, including patients and their caregivers, according to Peter Gliatto, MD, associate director of the Mount Sinai Visiting Doctors Program.
“In different cities, townships, and jurisdictions, different health departments and different provider groups are approaching [the distribution of the COVID-19 vaccine] slightly differently,” Ms. Amer said. So a lot of the decisions surrounding the distribution of shots are local or dependent on local resourcing.
People who live in rural areas present a unique challenge, but Mr. Feorene said reaching out to local emergency medical services or the local health departments can provide some insight on what their town is doing to vaccinate homebound patients.
“I think understanding what a [public health department] is doing would be the very first place to start,” Mr. Feorene said in an interview.
If a patient is bedridden and is mobile enough to sit in a car, Mr. Feorene also recommends finding out if there are vaccine fairs “within a reasonable driving distance.”
Ms. Amer said continuing this mission of getting homebound patients vaccinated is necessary for public health.
“Even if it’s going to take longer to vaccinate these homebound patients, we still have to make an effort. So much of the country’s vaccine efforts have been focused on getting as many shots in as many arms as quickly as possible. And that is definitely super important,” she said.
Ms. Amer is working with her practice’s primary care physicians to try to identify all of those patients who are functionally debilitated or unable to leave their home to get vaccinated and that Central Ohio Primary Care will vaccinate more homebound patients, she added.
The experts interviewed in this article have no conflicts.
Katie Lennon contributed to this report.
This article was updated 4/29/21.

There are about 2 million to 4 million homebound patients in the United States, according to a webinar from The Trust for America’s Health, which was broadcast in March. But many of these individuals have not been vaccinated yet because of logistical challenges.
Some homebound COVID-19 immunization programs are administering Moderna and Pfizer vaccines to their patients, but many state, city, and local programs administered the Johnson & Johnson vaccine after it was cleared for use by the Food and Drug Administration in February 2021. The efficacy of the one-shot vaccine, as well as it being easier to store and ship than the Moderna and Pfizer vaccines, makes getting it to homebound patients less challenging.
“With Pfizer and Moderna, transportation is a challenge because the temperature demands and the fragility of [messenger] RNA–based vaccines,” Brent Feorene, executive director of the American Academy of Home Care Medicine, said in an interview. That’s why [the Johnson & Johnson] vaccine held such promise – it’s less fragile, [can be stored in] higher temperatures, and was a one shot.”
Other hurdles to getting homebound patients vaccinated had already been in place prior to the 10-day-pause on using the J&J vaccine that occurred for federal agencies to consider possible serious side effects linked to it.
Many roadblocks to vaccination
Although many homebound patients can’t readily go out into the community and be exposed to the COVID-19 virus themselves, they are dependent on caregivers and family members who do go out into the community.
“Their friends, family, neighbors, home health aides, and other kinds of health care workers come into the home,” said Shawn Amer, clinical program director at Central Ohio Primary Care in Columbus.
Nurses from Ms. Amer’s practice vaccinated approximately ten homebound patients with the J&J vaccine through a pilot program in March. Then on April 24, nurses from Central Ohio Primary Care vaccinated just under 40 homebound patients and about a handful of their caregivers who were not able to get their vaccines elsewhere, according to Ms. Amer. This time they used the Pfizer vaccine and will be returning to these patients’ homes on May 15 to administer the second dose.

“Any time you are getting in the car and adding miles, it adds complexity,” Ms. Amer said.
“We called patients 24 to 36 hours before coming to their homes to make sure they were ready, but we learned that just because the healthcare power of attorney agrees to a patient getting vaccinated does not mean that patient will be willing to get the vaccine when the nurse shows up," she noted.
Ms. Amer elaborated that three patients with dementia refused the vaccine when nurses arrived at their home on April 24.
“We had to pivot and find other people,” Ms. Amer. Her practice ended up having to waste one shot.
Expenses are greater
The higher costs of getting homebound patients vaccinated is an additional hurdle to getting these vulnerable individuals protected by COVID-19 shots.
Vaccinating patients in their homes “doesn’t require a lot of technology, but it does require a lot of time” and the staffing expense becomes part of the challenge, Ms. Amer noted.
For each of the two days that Central Ohio Primary Care provides the Pfizer vaccine to homebound patients, the practice needs to pay seven nurses to administer the vaccine, Ms. Amer explained.
There have also been reports of organizations that administer the vaccines – which are free for patients because the federal government is paying for them – not being paid enough by Medicare to cover staff time and efforts to vaccinate patients in their homes, Kaiser Health News reported. According to the Centers for Medicare & Medicaid Services, they pay $40 for the administration of a single-dose COVID-19 vaccine and, for COVID-19 vaccines requiring multiple doses, Medicare pays approximately $40 for each dose in the series. These rates were implemented after March 15. Before that date, the rates were even lower, with the Medicare reimbursement rates for initial doses of COVID-19 vaccines being $16.94 and final doses being $28.39.
William Dombi, president of the National Association for Home Care & Hospice, told Kaiser Health News that the actual cost of these homebound visits are closer to $150 or $160.
“The reimbursement for the injection is pretty minimal,” Mr. Feorene said. “So unless you’re a larger organization and able to have staff to deploy some of your smaller practices, just couldn’t afford to do it.”
Many homebound patients have also been unable to get the lifesaving shots because of logistical roadblocks and many practices not being able to do home visits.
“I think that initially when the [Centers for Disease Control and Prevention] came out with vaccine guidance for medical providers, they offered no guidance for in-home medical providers and we had to go back and ask for that, which they did produce,” Mr. Feorene said. “And we’re grateful for that. But I think just this general understanding that there is a population of folks that are [limited to their home], that they do receive medical care and other care in the home, and that we have to remember that the medical providers who provide care in the home are also primary care providers.”
Furthermore, trying to navigate or find programs delivering vaccines to the homebound can be difficult depending on where a patient lives.
While some programs have been launched on the country or city level – the New York Fire Department launched a pilot program to bring the Johnson & Johnson vaccine to homebound seniors – other programs have been spearheaded by hospital networks like Northwell and Mount Sinai. However, many of these hospital networks only reach out to people who already have a relationship with the hospital.
Ms Amer said identifying homebound patients and reaching out to them can be tough and can contribute to the logistics and time involved in setting patients up for the vaccine.
“Reaching some of these patients is difficult,” Ms. Amer noted. “Sometimes the best way to reach them or get a hold of them is through their caregiver. And so do you have the right phone number? Do you have the right name?”
Overcoming the challenges
With the absence of a national plan targeting homebound patients, many local initiatives were launched to help these individuals get vaccinated. Local fire department paramedics have gone door to door to administer the COVID-19 vaccine in cities like Chicago, New York, and Miami. The suspension of the Johnson & Johnson vaccine resulted in the suspension of in-home vaccinations for some people in New York City. However, the program resumed after the FDA and CDC lifted the pause on April 24.

Health systems like Mount Sinai vaccinated approximately 530 people through the Mount Sinai Visiting Doctors Program, including patients and their caregivers, according to Peter Gliatto, MD, associate director of the Mount Sinai Visiting Doctors Program.
“In different cities, townships, and jurisdictions, different health departments and different provider groups are approaching [the distribution of the COVID-19 vaccine] slightly differently,” Ms. Amer said. So a lot of the decisions surrounding the distribution of shots are local or dependent on local resourcing.
People who live in rural areas present a unique challenge, but Mr. Feorene said reaching out to local emergency medical services or the local health departments can provide some insight on what their town is doing to vaccinate homebound patients.
“I think understanding what a [public health department] is doing would be the very first place to start,” Mr. Feorene said in an interview.
If a patient is bedridden and is mobile enough to sit in a car, Mr. Feorene also recommends finding out if there are vaccine fairs “within a reasonable driving distance.”
Ms. Amer said continuing this mission of getting homebound patients vaccinated is necessary for public health.
“Even if it’s going to take longer to vaccinate these homebound patients, we still have to make an effort. So much of the country’s vaccine efforts have been focused on getting as many shots in as many arms as quickly as possible. And that is definitely super important,” she said.
Ms. Amer is working with her practice’s primary care physicians to try to identify all of those patients who are functionally debilitated or unable to leave their home to get vaccinated and that Central Ohio Primary Care will vaccinate more homebound patients, she added.
The experts interviewed in this article have no conflicts.
Katie Lennon contributed to this report.
This article was updated 4/29/21.

There are about 2 million to 4 million homebound patients in the United States, according to a webinar from The Trust for America’s Health, which was broadcast in March. But many of these individuals have not been vaccinated yet because of logistical challenges.
Some homebound COVID-19 immunization programs are administering Moderna and Pfizer vaccines to their patients, but many state, city, and local programs administered the Johnson & Johnson vaccine after it was cleared for use by the Food and Drug Administration in February 2021. The efficacy of the one-shot vaccine, as well as it being easier to store and ship than the Moderna and Pfizer vaccines, makes getting it to homebound patients less challenging.
“With Pfizer and Moderna, transportation is a challenge because the temperature demands and the fragility of [messenger] RNA–based vaccines,” Brent Feorene, executive director of the American Academy of Home Care Medicine, said in an interview. That’s why [the Johnson & Johnson] vaccine held such promise – it’s less fragile, [can be stored in] higher temperatures, and was a one shot.”
Other hurdles to getting homebound patients vaccinated had already been in place prior to the 10-day-pause on using the J&J vaccine that occurred for federal agencies to consider possible serious side effects linked to it.
Many roadblocks to vaccination
Although many homebound patients can’t readily go out into the community and be exposed to the COVID-19 virus themselves, they are dependent on caregivers and family members who do go out into the community.
“Their friends, family, neighbors, home health aides, and other kinds of health care workers come into the home,” said Shawn Amer, clinical program director at Central Ohio Primary Care in Columbus.
Nurses from Ms. Amer’s practice vaccinated approximately ten homebound patients with the J&J vaccine through a pilot program in March. Then on April 24, nurses from Central Ohio Primary Care vaccinated just under 40 homebound patients and about a handful of their caregivers who were not able to get their vaccines elsewhere, according to Ms. Amer. This time they used the Pfizer vaccine and will be returning to these patients’ homes on May 15 to administer the second dose.

“Any time you are getting in the car and adding miles, it adds complexity,” Ms. Amer said.
“We called patients 24 to 36 hours before coming to their homes to make sure they were ready, but we learned that just because the healthcare power of attorney agrees to a patient getting vaccinated does not mean that patient will be willing to get the vaccine when the nurse shows up," she noted.
Ms. Amer elaborated that three patients with dementia refused the vaccine when nurses arrived at their home on April 24.
“We had to pivot and find other people,” Ms. Amer. Her practice ended up having to waste one shot.
Expenses are greater
The higher costs of getting homebound patients vaccinated is an additional hurdle to getting these vulnerable individuals protected by COVID-19 shots.
Vaccinating patients in their homes “doesn’t require a lot of technology, but it does require a lot of time” and the staffing expense becomes part of the challenge, Ms. Amer noted.
For each of the two days that Central Ohio Primary Care provides the Pfizer vaccine to homebound patients, the practice needs to pay seven nurses to administer the vaccine, Ms. Amer explained.
There have also been reports of organizations that administer the vaccines – which are free for patients because the federal government is paying for them – not being paid enough by Medicare to cover staff time and efforts to vaccinate patients in their homes, Kaiser Health News reported. According to the Centers for Medicare & Medicaid Services, they pay $40 for the administration of a single-dose COVID-19 vaccine and, for COVID-19 vaccines requiring multiple doses, Medicare pays approximately $40 for each dose in the series. These rates were implemented after March 15. Before that date, the rates were even lower, with the Medicare reimbursement rates for initial doses of COVID-19 vaccines being $16.94 and final doses being $28.39.
William Dombi, president of the National Association for Home Care & Hospice, told Kaiser Health News that the actual cost of these homebound visits are closer to $150 or $160.
“The reimbursement for the injection is pretty minimal,” Mr. Feorene said. “So unless you’re a larger organization and able to have staff to deploy some of your smaller practices, just couldn’t afford to do it.”
Many homebound patients have also been unable to get the lifesaving shots because of logistical roadblocks and many practices not being able to do home visits.
“I think that initially when the [Centers for Disease Control and Prevention] came out with vaccine guidance for medical providers, they offered no guidance for in-home medical providers and we had to go back and ask for that, which they did produce,” Mr. Feorene said. “And we’re grateful for that. But I think just this general understanding that there is a population of folks that are [limited to their home], that they do receive medical care and other care in the home, and that we have to remember that the medical providers who provide care in the home are also primary care providers.”
Furthermore, trying to navigate or find programs delivering vaccines to the homebound can be difficult depending on where a patient lives.
While some programs have been launched on the country or city level – the New York Fire Department launched a pilot program to bring the Johnson & Johnson vaccine to homebound seniors – other programs have been spearheaded by hospital networks like Northwell and Mount Sinai. However, many of these hospital networks only reach out to people who already have a relationship with the hospital.
Ms Amer said identifying homebound patients and reaching out to them can be tough and can contribute to the logistics and time involved in setting patients up for the vaccine.
“Reaching some of these patients is difficult,” Ms. Amer noted. “Sometimes the best way to reach them or get a hold of them is through their caregiver. And so do you have the right phone number? Do you have the right name?”
Overcoming the challenges
With the absence of a national plan targeting homebound patients, many local initiatives were launched to help these individuals get vaccinated. Local fire department paramedics have gone door to door to administer the COVID-19 vaccine in cities like Chicago, New York, and Miami. The suspension of the Johnson & Johnson vaccine resulted in the suspension of in-home vaccinations for some people in New York City. However, the program resumed after the FDA and CDC lifted the pause on April 24.

Health systems like Mount Sinai vaccinated approximately 530 people through the Mount Sinai Visiting Doctors Program, including patients and their caregivers, according to Peter Gliatto, MD, associate director of the Mount Sinai Visiting Doctors Program.
“In different cities, townships, and jurisdictions, different health departments and different provider groups are approaching [the distribution of the COVID-19 vaccine] slightly differently,” Ms. Amer said. So a lot of the decisions surrounding the distribution of shots are local or dependent on local resourcing.
People who live in rural areas present a unique challenge, but Mr. Feorene said reaching out to local emergency medical services or the local health departments can provide some insight on what their town is doing to vaccinate homebound patients.
“I think understanding what a [public health department] is doing would be the very first place to start,” Mr. Feorene said in an interview.
If a patient is bedridden and is mobile enough to sit in a car, Mr. Feorene also recommends finding out if there are vaccine fairs “within a reasonable driving distance.”
Ms. Amer said continuing this mission of getting homebound patients vaccinated is necessary for public health.
“Even if it’s going to take longer to vaccinate these homebound patients, we still have to make an effort. So much of the country’s vaccine efforts have been focused on getting as many shots in as many arms as quickly as possible. And that is definitely super important,” she said.
Ms. Amer is working with her practice’s primary care physicians to try to identify all of those patients who are functionally debilitated or unable to leave their home to get vaccinated and that Central Ohio Primary Care will vaccinate more homebound patients, she added.
The experts interviewed in this article have no conflicts.
Katie Lennon contributed to this report.
This article was updated 4/29/21.
US methods of delivery, a snapshot