User login
For MD-IQ only
FDA OKs combo therapy of niraparib, abiraterone acetate for prostate cancer
The Food and Drug Administration has approved niraparib and abiraterone acetate (Akeega, Janssen Pharmaceuticals) to treat BRCA-positive, metastatic castration-resistant prostate cancer in adult patients with deleterious or suspected deleterious disease, as determined by an FDA-approved test.
The FDA’s approval was based on findings from the phase 3 MAGNITUDE precision medicine study, a randomized, placebo-controlled trial with 423 patients, 225 (53%) of whom had BRCA gene mutations as determined using a tissue assay such as FoundationOne CDx.
Among the subgroup with a BRCA mutation, radiographic progression-free survival was a median of 16.6 months vs. 10.9 months (hazard ratio [HR], 0.53; 95% confidence interval [CI], 0.36-0.79; P = .0014). In this subgroup, an exploratory overall survival analysis demonstrated a median of 30.4 months vs. 28.6 months (HR, 0.79; 95% CI, 0.55-1.12), favoring the treatment arm.
Although the overall cohort (those with and without BRCA mutations) demonstrated a significant improvement in radiographic progression-free survival, the subgroup with non-BRCA homologous recombination repair mutations did not demonstrate a significant improvement in radiographic progression-free survival, which indicates that the benefit observed was “primarily attributed” to the results in the subgroup of patients with BRCA mutations, according to the FDA.
The safety profile of niraparib and abiraterone acetate plus prednisone was consistent with the known safety profile of each FDA-approved monotherapy. Serious adverse events occurred in 41% of patients in the treatment arm. These most often included musculoskeletal pain (44% vs. 42%), fatigue (43% vs. 30%), constipation (34% vs. 20%), hypertension (33% vs. 27%), and nausea (33% vs. 21%).
An adverse reaction led to permanent discontinuation of treatment in 15% of patients.
“As a physician, identifying patients with a worse prognosis is a priority, especially those whose cancers have a BRCA mutation,” principal investigator Kim Chi, MD, stated in the Janssen press release. “We prospectively designed the MAGNITUDE study to identify the subset of patients most likely to benefit from targeted treatment with AKEEGA and to help us understand how we can potentially achieve better health outcomes for patients.”
About 10%-15% of patients who develop metastatic castration-resistant prostate cancer have BRCA gene alterations, and those patients are more likely to have aggressive disease, poor outcomes, and shorter survival. Therefore, this new agent “brings an important treatment option to patients with prostate cancer as they consider their road ahead,” said Shelby Moneer, vice president of patient programs and education at ZERO Prostate Cancer.
The prescribing information lists the recommended dose at 200 mg niraparib and 1,000 mg abiraterone once daily in combination with 10 mg of prednisone daily until disease progression or unacceptable toxicity. Patients should also receive a gonadotropin-releasing hormone analog concurrently or should have had bilateral orchiectomy.
Health care professionals should report all serious adverse events suspected to be associated with the use of any medicine and device by using the FDA’s MedWatch Reporting System or by calling 1-800-FDA-1088.
A version of this article appeared on Medscape.com.
The Food and Drug Administration has approved niraparib and abiraterone acetate (Akeega, Janssen Pharmaceuticals) to treat BRCA-positive, metastatic castration-resistant prostate cancer in adult patients with deleterious or suspected deleterious disease, as determined by an FDA-approved test.
The FDA’s approval was based on findings from the phase 3 MAGNITUDE precision medicine study, a randomized, placebo-controlled trial with 423 patients, 225 (53%) of whom had BRCA gene mutations as determined using a tissue assay such as FoundationOne CDx.
Among the subgroup with a BRCA mutation, radiographic progression-free survival was a median of 16.6 months vs. 10.9 months (hazard ratio [HR], 0.53; 95% confidence interval [CI], 0.36-0.79; P = .0014). In this subgroup, an exploratory overall survival analysis demonstrated a median of 30.4 months vs. 28.6 months (HR, 0.79; 95% CI, 0.55-1.12), favoring the treatment arm.
Although the overall cohort (those with and without BRCA mutations) demonstrated a significant improvement in radiographic progression-free survival, the subgroup with non-BRCA homologous recombination repair mutations did not demonstrate a significant improvement in radiographic progression-free survival, which indicates that the benefit observed was “primarily attributed” to the results in the subgroup of patients with BRCA mutations, according to the FDA.
The safety profile of niraparib and abiraterone acetate plus prednisone was consistent with the known safety profile of each FDA-approved monotherapy. Serious adverse events occurred in 41% of patients in the treatment arm. These most often included musculoskeletal pain (44% vs. 42%), fatigue (43% vs. 30%), constipation (34% vs. 20%), hypertension (33% vs. 27%), and nausea (33% vs. 21%).
An adverse reaction led to permanent discontinuation of treatment in 15% of patients.
“As a physician, identifying patients with a worse prognosis is a priority, especially those whose cancers have a BRCA mutation,” principal investigator Kim Chi, MD, stated in the Janssen press release. “We prospectively designed the MAGNITUDE study to identify the subset of patients most likely to benefit from targeted treatment with AKEEGA and to help us understand how we can potentially achieve better health outcomes for patients.”
About 10%-15% of patients who develop metastatic castration-resistant prostate cancer have BRCA gene alterations, and those patients are more likely to have aggressive disease, poor outcomes, and shorter survival. Therefore, this new agent “brings an important treatment option to patients with prostate cancer as they consider their road ahead,” said Shelby Moneer, vice president of patient programs and education at ZERO Prostate Cancer.
The prescribing information lists the recommended dose at 200 mg niraparib and 1,000 mg abiraterone once daily in combination with 10 mg of prednisone daily until disease progression or unacceptable toxicity. Patients should also receive a gonadotropin-releasing hormone analog concurrently or should have had bilateral orchiectomy.
Health care professionals should report all serious adverse events suspected to be associated with the use of any medicine and device by using the FDA’s MedWatch Reporting System or by calling 1-800-FDA-1088.
A version of this article appeared on Medscape.com.
The Food and Drug Administration has approved niraparib and abiraterone acetate (Akeega, Janssen Pharmaceuticals) to treat BRCA-positive, metastatic castration-resistant prostate cancer in adult patients with deleterious or suspected deleterious disease, as determined by an FDA-approved test.
The FDA’s approval was based on findings from the phase 3 MAGNITUDE precision medicine study, a randomized, placebo-controlled trial with 423 patients, 225 (53%) of whom had BRCA gene mutations as determined using a tissue assay such as FoundationOne CDx.
Among the subgroup with a BRCA mutation, radiographic progression-free survival was a median of 16.6 months vs. 10.9 months (hazard ratio [HR], 0.53; 95% confidence interval [CI], 0.36-0.79; P = .0014). In this subgroup, an exploratory overall survival analysis demonstrated a median of 30.4 months vs. 28.6 months (HR, 0.79; 95% CI, 0.55-1.12), favoring the treatment arm.
Although the overall cohort (those with and without BRCA mutations) demonstrated a significant improvement in radiographic progression-free survival, the subgroup with non-BRCA homologous recombination repair mutations did not demonstrate a significant improvement in radiographic progression-free survival, which indicates that the benefit observed was “primarily attributed” to the results in the subgroup of patients with BRCA mutations, according to the FDA.
The safety profile of niraparib and abiraterone acetate plus prednisone was consistent with the known safety profile of each FDA-approved monotherapy. Serious adverse events occurred in 41% of patients in the treatment arm. These most often included musculoskeletal pain (44% vs. 42%), fatigue (43% vs. 30%), constipation (34% vs. 20%), hypertension (33% vs. 27%), and nausea (33% vs. 21%).
An adverse reaction led to permanent discontinuation of treatment in 15% of patients.
“As a physician, identifying patients with a worse prognosis is a priority, especially those whose cancers have a BRCA mutation,” principal investigator Kim Chi, MD, stated in the Janssen press release. “We prospectively designed the MAGNITUDE study to identify the subset of patients most likely to benefit from targeted treatment with AKEEGA and to help us understand how we can potentially achieve better health outcomes for patients.”
About 10%-15% of patients who develop metastatic castration-resistant prostate cancer have BRCA gene alterations, and those patients are more likely to have aggressive disease, poor outcomes, and shorter survival. Therefore, this new agent “brings an important treatment option to patients with prostate cancer as they consider their road ahead,” said Shelby Moneer, vice president of patient programs and education at ZERO Prostate Cancer.
The prescribing information lists the recommended dose at 200 mg niraparib and 1,000 mg abiraterone once daily in combination with 10 mg of prednisone daily until disease progression or unacceptable toxicity. Patients should also receive a gonadotropin-releasing hormone analog concurrently or should have had bilateral orchiectomy.
Health care professionals should report all serious adverse events suspected to be associated with the use of any medicine and device by using the FDA’s MedWatch Reporting System or by calling 1-800-FDA-1088.
A version of this article appeared on Medscape.com.
MRI-guided SBRT cuts radiation toxicity in prostate cancer
TOPLINE
The use of magnetic resonance–guided daily adaptive stereotactic body radiotherapy for patients with prostate cancer reduces the risk of acute urinary side effects of grade 2 or higher by 44% and the risk of acute bowel side effects of grade 2 or higher by 60%, compared with standard CT-guided SBRT (CT‐SBRT).
METHODOLOGY
- With the use of magnetic resonance–guided daily adaptive SBRT, clinicians can customize radiation dosing to accommodate changes in prostate anatomy during treatment, which may also make SBRT safer and less toxic for patients.
- To determine whether this approach does reduce patient side effects, investigators ran a meta-analysis that included 29 studies with 2547 patients comparing the incidence of short-term, physician-assessed bowel and genitourinary side effects between the MRI-guided approach and standard CT-SBRT.
- The investigators reported no statistically significant differences in age, prescribed radiation doses, planning target volumes, or International Prostatism Symptom Scores between the two groups; the use of rectal spacers and the number of patients who received pelvic lymph node radiation were low in both.
- The average window for collecting acute toxicity data was 70 days in the MRI-guided investigations and 94 days in CT-SBRT investigations.
TAKEAWAY
- (odds ratio, 0.56; P = .04).
- The pooled estimate for grade 2 or higher gastrointestinal toxicity was 4% with the MRI approach versus 9% with CT-SBRT (OR, 0.40; P = .04).
- There were no differences in grade 3 or higher events, which were rare, between the groups.
- There was also no difference in toxicity among CT‐SBRT studies that used fiducial markers and those that did not.
IN PRACTICE
“These findings suggest that the technical advantages in precision of radiotherapy delivery afforded by [MRI-guided] SBRT translate to measurable clinical benefit,” the authors concluded. Potential reasons for the reduced risk of acute toxicity with the MRI-guided approach include “daily online adaptive planning, MRI‐based contouring that results in smaller treatment volumes, and MRI tracking, all of which may facilitate the precision and accuracy of treatment delivery.”
SOURCE
The study was led by Jonathan Leeman, MD, of the Dana-Farber Cancer Institute, Boston, and was published July 24 in Cancer.
LIMITATIONS
- The analysis did not account for differences in dosimetry, radiation planning, and toxicity management and assessment between the studies.
- Late toxicity and cancer control rates were not tracked and may have differed between the two approaches.
DISCLOSURES
- No external funding was reported.
- The investigators reported grants and consulting, personal, and other payments from Novartis, AstraZeneca, Janssen, and other companies.
A version of this article appeared on Medscape.com.
TOPLINE
The use of magnetic resonance–guided daily adaptive stereotactic body radiotherapy for patients with prostate cancer reduces the risk of acute urinary side effects of grade 2 or higher by 44% and the risk of acute bowel side effects of grade 2 or higher by 60%, compared with standard CT-guided SBRT (CT‐SBRT).
METHODOLOGY
- With the use of magnetic resonance–guided daily adaptive SBRT, clinicians can customize radiation dosing to accommodate changes in prostate anatomy during treatment, which may also make SBRT safer and less toxic for patients.
- To determine whether this approach does reduce patient side effects, investigators ran a meta-analysis that included 29 studies with 2547 patients comparing the incidence of short-term, physician-assessed bowel and genitourinary side effects between the MRI-guided approach and standard CT-SBRT.
- The investigators reported no statistically significant differences in age, prescribed radiation doses, planning target volumes, or International Prostatism Symptom Scores between the two groups; the use of rectal spacers and the number of patients who received pelvic lymph node radiation were low in both.
- The average window for collecting acute toxicity data was 70 days in the MRI-guided investigations and 94 days in CT-SBRT investigations.
TAKEAWAY
- (odds ratio, 0.56; P = .04).
- The pooled estimate for grade 2 or higher gastrointestinal toxicity was 4% with the MRI approach versus 9% with CT-SBRT (OR, 0.40; P = .04).
- There were no differences in grade 3 or higher events, which were rare, between the groups.
- There was also no difference in toxicity among CT‐SBRT studies that used fiducial markers and those that did not.
IN PRACTICE
“These findings suggest that the technical advantages in precision of radiotherapy delivery afforded by [MRI-guided] SBRT translate to measurable clinical benefit,” the authors concluded. Potential reasons for the reduced risk of acute toxicity with the MRI-guided approach include “daily online adaptive planning, MRI‐based contouring that results in smaller treatment volumes, and MRI tracking, all of which may facilitate the precision and accuracy of treatment delivery.”
SOURCE
The study was led by Jonathan Leeman, MD, of the Dana-Farber Cancer Institute, Boston, and was published July 24 in Cancer.
LIMITATIONS
- The analysis did not account for differences in dosimetry, radiation planning, and toxicity management and assessment between the studies.
- Late toxicity and cancer control rates were not tracked and may have differed between the two approaches.
DISCLOSURES
- No external funding was reported.
- The investigators reported grants and consulting, personal, and other payments from Novartis, AstraZeneca, Janssen, and other companies.
A version of this article appeared on Medscape.com.
TOPLINE
The use of magnetic resonance–guided daily adaptive stereotactic body radiotherapy for patients with prostate cancer reduces the risk of acute urinary side effects of grade 2 or higher by 44% and the risk of acute bowel side effects of grade 2 or higher by 60%, compared with standard CT-guided SBRT (CT‐SBRT).
METHODOLOGY
- With the use of magnetic resonance–guided daily adaptive SBRT, clinicians can customize radiation dosing to accommodate changes in prostate anatomy during treatment, which may also make SBRT safer and less toxic for patients.
- To determine whether this approach does reduce patient side effects, investigators ran a meta-analysis that included 29 studies with 2547 patients comparing the incidence of short-term, physician-assessed bowel and genitourinary side effects between the MRI-guided approach and standard CT-SBRT.
- The investigators reported no statistically significant differences in age, prescribed radiation doses, planning target volumes, or International Prostatism Symptom Scores between the two groups; the use of rectal spacers and the number of patients who received pelvic lymph node radiation were low in both.
- The average window for collecting acute toxicity data was 70 days in the MRI-guided investigations and 94 days in CT-SBRT investigations.
TAKEAWAY
- (odds ratio, 0.56; P = .04).
- The pooled estimate for grade 2 or higher gastrointestinal toxicity was 4% with the MRI approach versus 9% with CT-SBRT (OR, 0.40; P = .04).
- There were no differences in grade 3 or higher events, which were rare, between the groups.
- There was also no difference in toxicity among CT‐SBRT studies that used fiducial markers and those that did not.
IN PRACTICE
“These findings suggest that the technical advantages in precision of radiotherapy delivery afforded by [MRI-guided] SBRT translate to measurable clinical benefit,” the authors concluded. Potential reasons for the reduced risk of acute toxicity with the MRI-guided approach include “daily online adaptive planning, MRI‐based contouring that results in smaller treatment volumes, and MRI tracking, all of which may facilitate the precision and accuracy of treatment delivery.”
SOURCE
The study was led by Jonathan Leeman, MD, of the Dana-Farber Cancer Institute, Boston, and was published July 24 in Cancer.
LIMITATIONS
- The analysis did not account for differences in dosimetry, radiation planning, and toxicity management and assessment between the studies.
- Late toxicity and cancer control rates were not tracked and may have differed between the two approaches.
DISCLOSURES
- No external funding was reported.
- The investigators reported grants and consulting, personal, and other payments from Novartis, AstraZeneca, Janssen, and other companies.
A version of this article appeared on Medscape.com.
FROM CANCER
‘Treatment holiday’ in prostate cancer with tailored dosing
and improve patient outcomes, new research suggests.
The findings indicate that implementing a personalized dosing strategy with the radioligand therapy “allowed for treatment holidays in excellent responders, continuous 6-weekly treatments in moderate responders, and [allowed us] to consider changing or adding treatment in limited responders,” said study author Andrew Nguyen, MBBS, FRACP, AANMS, senior staff specialist in the department of theranostics and nuclear medicine at St. Vincent’s Hospital in Sydney.
The research was presented at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging.
Although clinical trials have demonstrated that 177Lu-PSMA is an effective treatment for metastatic castration-resistant prostate cancer, the question remains: Can patient outcomes be improved through the use of biomarkers and by escalating or deescalating treatment as appropriate? asked Dr. Nguyen, who presented the findings at the meeting.
Clinical trials use standardized dosing intervals. Adjusting treatment intervals through the use of early-biomarker responses could give some patients a break from treatment and improve overall survival outcomes, Dr. Nguyen explained. For example, the 2021 REALITY study showed that overall survival was significantly better for patients who received 177Lu-PSMA plus standard care, compared with patients who received standard care alone (median, 15.3 vs. 11.3 months), and that overall survival was better among patients with early prostate-specific antigen (PSA) responses.
In the current study, Dr. Nguyen and colleagues used composite early biomarkers of PSA, imaging with 177Lu-PSMA SPECT, and diagnostic CT to guide a personalized dosing interval strategy for patients with metastatic castration-resistant prostate cancer receiving 177Lu-PSMA. The team evaluated progression-free survival and overall survival among these patients to determine whether personalizing dosing on the basis of early biomarker levels was associated with survival outcomes.
The cohort included 125 men who received six weekly doses of 177Lu-PSMA and who underwent imaging with 177Lu-SPECT/CT after each dose. After the second dose, investigators used the composite of PSA and 177Lu SPECT/CT response to determine which patients had a partial response, which had stable disease, and which had progressive disease.
The men were divided into three groups on the basis of their level of response. Group 1, which included 35% of participants, achieved a significant reduction in PSA levels and a partial response on 177Lu-SPECT. These patients were advised to discontinue treatment until PSA levels increased. This treatment holiday lasted a median of about 6 months.
Group 2, which represented 34% of the cohort, had stable or reduced PSA levels as well as stable disease on SPECT imaging. For these patients, the treatment regimen continued.
Group 3 demonstrated rising PSA levels and progressive disease on SPECT imaging. These men were offered an alternative therapy.
Overall, median PSA progression-free survival was 12.1 months in group 1, 6.1 months in group 2, and 2.6 months in group 3. Median overall survival was also significantly better among patients who showed early responses to therapy: 19.2 months in group 1, 13.2 months in group 2, and 11. 2 months in group 3.
Dr. Nguyen noted several limitations to the findings, including the study’s retrospective nature and the fact that some patients in group 1 chose not to resume further treatment after their PSA levels rose.
“Personalizing dosing intervals using early-response biomarkers with 177Lu-PSMA has the potential to achieve similar overall treatment responses to that published for continuous dosing, while allowing treatment holidays in responders and early crossover to potentially more effective therapies in nonresponders,” the authors conclude.
Given the effectiveness of this strategy, Dr. Nguyen says his team “now routinely uses these composite biomarkers when treating clinical patients.”
A version of this article appeared on Medscape.com.
and improve patient outcomes, new research suggests.
The findings indicate that implementing a personalized dosing strategy with the radioligand therapy “allowed for treatment holidays in excellent responders, continuous 6-weekly treatments in moderate responders, and [allowed us] to consider changing or adding treatment in limited responders,” said study author Andrew Nguyen, MBBS, FRACP, AANMS, senior staff specialist in the department of theranostics and nuclear medicine at St. Vincent’s Hospital in Sydney.
The research was presented at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging.
Although clinical trials have demonstrated that 177Lu-PSMA is an effective treatment for metastatic castration-resistant prostate cancer, the question remains: Can patient outcomes be improved through the use of biomarkers and by escalating or deescalating treatment as appropriate? asked Dr. Nguyen, who presented the findings at the meeting.
Clinical trials use standardized dosing intervals. Adjusting treatment intervals through the use of early-biomarker responses could give some patients a break from treatment and improve overall survival outcomes, Dr. Nguyen explained. For example, the 2021 REALITY study showed that overall survival was significantly better for patients who received 177Lu-PSMA plus standard care, compared with patients who received standard care alone (median, 15.3 vs. 11.3 months), and that overall survival was better among patients with early prostate-specific antigen (PSA) responses.
In the current study, Dr. Nguyen and colleagues used composite early biomarkers of PSA, imaging with 177Lu-PSMA SPECT, and diagnostic CT to guide a personalized dosing interval strategy for patients with metastatic castration-resistant prostate cancer receiving 177Lu-PSMA. The team evaluated progression-free survival and overall survival among these patients to determine whether personalizing dosing on the basis of early biomarker levels was associated with survival outcomes.
The cohort included 125 men who received six weekly doses of 177Lu-PSMA and who underwent imaging with 177Lu-SPECT/CT after each dose. After the second dose, investigators used the composite of PSA and 177Lu SPECT/CT response to determine which patients had a partial response, which had stable disease, and which had progressive disease.
The men were divided into three groups on the basis of their level of response. Group 1, which included 35% of participants, achieved a significant reduction in PSA levels and a partial response on 177Lu-SPECT. These patients were advised to discontinue treatment until PSA levels increased. This treatment holiday lasted a median of about 6 months.
Group 2, which represented 34% of the cohort, had stable or reduced PSA levels as well as stable disease on SPECT imaging. For these patients, the treatment regimen continued.
Group 3 demonstrated rising PSA levels and progressive disease on SPECT imaging. These men were offered an alternative therapy.
Overall, median PSA progression-free survival was 12.1 months in group 1, 6.1 months in group 2, and 2.6 months in group 3. Median overall survival was also significantly better among patients who showed early responses to therapy: 19.2 months in group 1, 13.2 months in group 2, and 11. 2 months in group 3.
Dr. Nguyen noted several limitations to the findings, including the study’s retrospective nature and the fact that some patients in group 1 chose not to resume further treatment after their PSA levels rose.
“Personalizing dosing intervals using early-response biomarkers with 177Lu-PSMA has the potential to achieve similar overall treatment responses to that published for continuous dosing, while allowing treatment holidays in responders and early crossover to potentially more effective therapies in nonresponders,” the authors conclude.
Given the effectiveness of this strategy, Dr. Nguyen says his team “now routinely uses these composite biomarkers when treating clinical patients.”
A version of this article appeared on Medscape.com.
and improve patient outcomes, new research suggests.
The findings indicate that implementing a personalized dosing strategy with the radioligand therapy “allowed for treatment holidays in excellent responders, continuous 6-weekly treatments in moderate responders, and [allowed us] to consider changing or adding treatment in limited responders,” said study author Andrew Nguyen, MBBS, FRACP, AANMS, senior staff specialist in the department of theranostics and nuclear medicine at St. Vincent’s Hospital in Sydney.
The research was presented at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging.
Although clinical trials have demonstrated that 177Lu-PSMA is an effective treatment for metastatic castration-resistant prostate cancer, the question remains: Can patient outcomes be improved through the use of biomarkers and by escalating or deescalating treatment as appropriate? asked Dr. Nguyen, who presented the findings at the meeting.
Clinical trials use standardized dosing intervals. Adjusting treatment intervals through the use of early-biomarker responses could give some patients a break from treatment and improve overall survival outcomes, Dr. Nguyen explained. For example, the 2021 REALITY study showed that overall survival was significantly better for patients who received 177Lu-PSMA plus standard care, compared with patients who received standard care alone (median, 15.3 vs. 11.3 months), and that overall survival was better among patients with early prostate-specific antigen (PSA) responses.
In the current study, Dr. Nguyen and colleagues used composite early biomarkers of PSA, imaging with 177Lu-PSMA SPECT, and diagnostic CT to guide a personalized dosing interval strategy for patients with metastatic castration-resistant prostate cancer receiving 177Lu-PSMA. The team evaluated progression-free survival and overall survival among these patients to determine whether personalizing dosing on the basis of early biomarker levels was associated with survival outcomes.
The cohort included 125 men who received six weekly doses of 177Lu-PSMA and who underwent imaging with 177Lu-SPECT/CT after each dose. After the second dose, investigators used the composite of PSA and 177Lu SPECT/CT response to determine which patients had a partial response, which had stable disease, and which had progressive disease.
The men were divided into three groups on the basis of their level of response. Group 1, which included 35% of participants, achieved a significant reduction in PSA levels and a partial response on 177Lu-SPECT. These patients were advised to discontinue treatment until PSA levels increased. This treatment holiday lasted a median of about 6 months.
Group 2, which represented 34% of the cohort, had stable or reduced PSA levels as well as stable disease on SPECT imaging. For these patients, the treatment regimen continued.
Group 3 demonstrated rising PSA levels and progressive disease on SPECT imaging. These men were offered an alternative therapy.
Overall, median PSA progression-free survival was 12.1 months in group 1, 6.1 months in group 2, and 2.6 months in group 3. Median overall survival was also significantly better among patients who showed early responses to therapy: 19.2 months in group 1, 13.2 months in group 2, and 11. 2 months in group 3.
Dr. Nguyen noted several limitations to the findings, including the study’s retrospective nature and the fact that some patients in group 1 chose not to resume further treatment after their PSA levels rose.
“Personalizing dosing intervals using early-response biomarkers with 177Lu-PSMA has the potential to achieve similar overall treatment responses to that published for continuous dosing, while allowing treatment holidays in responders and early crossover to potentially more effective therapies in nonresponders,” the authors conclude.
Given the effectiveness of this strategy, Dr. Nguyen says his team “now routinely uses these composite biomarkers when treating clinical patients.”
A version of this article appeared on Medscape.com.
FROM SNMMI 2023
Cancer Data Trends 2023
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
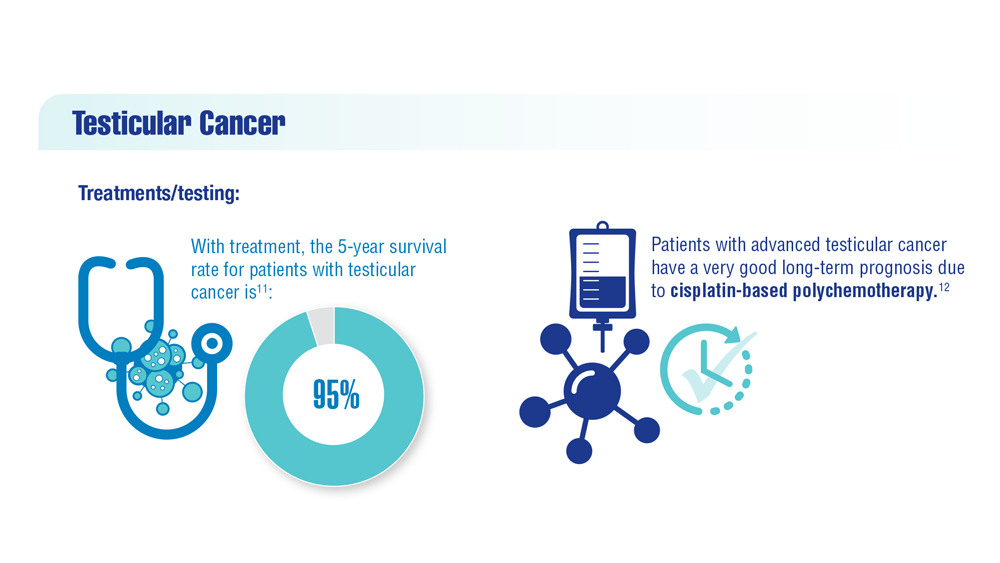
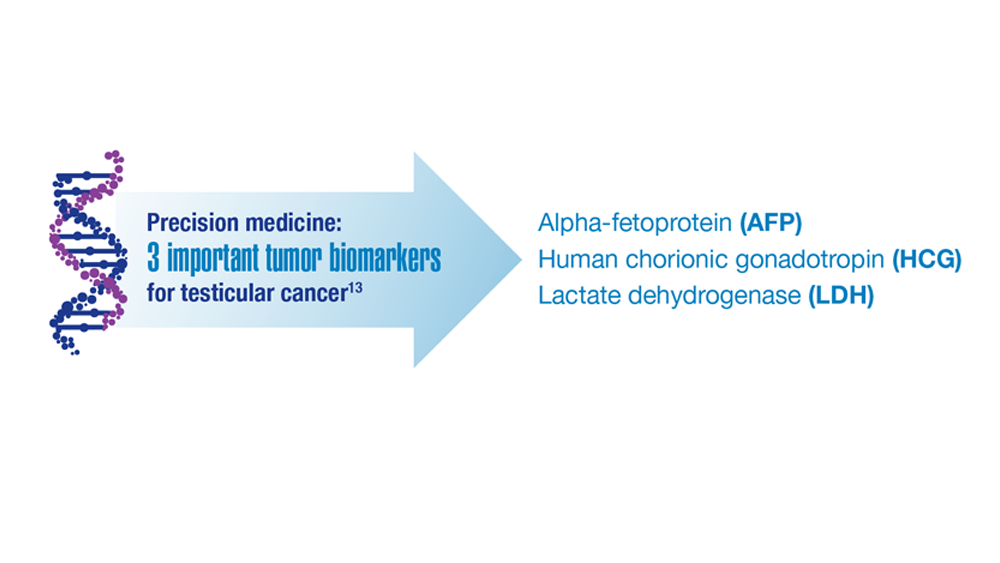
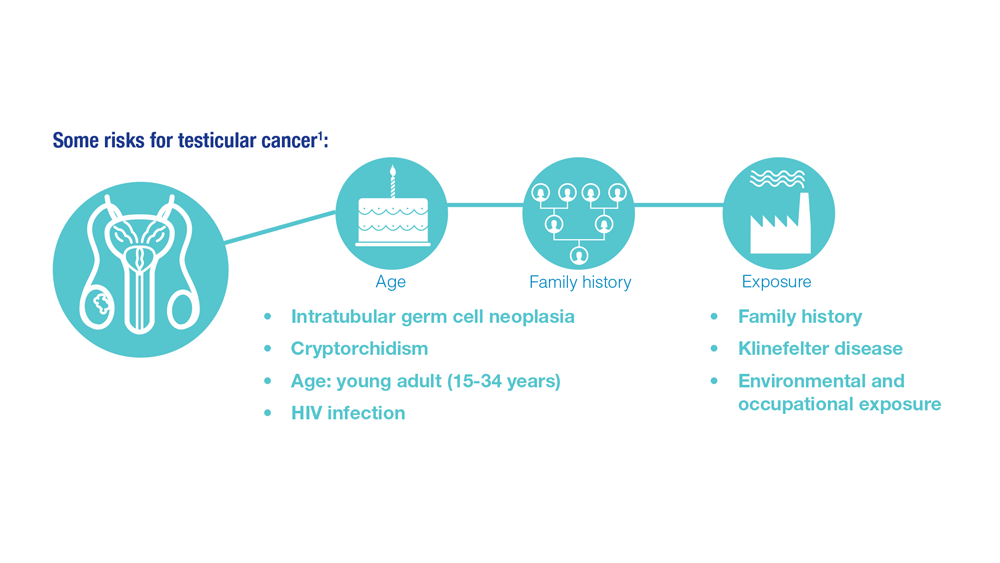
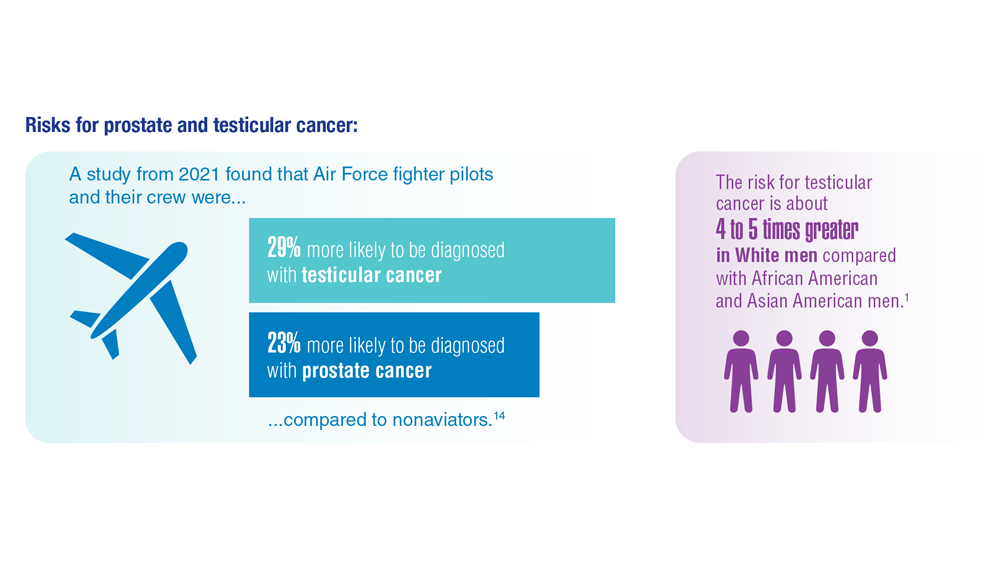
Promising New Approaches for Testicular and Prostate Cancer
- Risk factors for testicular cancer. American Cancer Society. Updated May 17, 2018. Accessed December 15, 2022. https://www.cancer.org/cancer/testicular-cancer/causes-risks-prevention/risk-factors.html
- Chovanec M, Cheng L. BMJ. 2022;379:e070499. doi:10.1136/bmj-2022-070499
- Tavares NT et al. J Pathol. 2022. doi:10.1002/path.6037
- Bryant AK et al. JAMA Oncol. 2022;e224319. doi:10.1001/jamaoncol.2022.4319
- Kabasakal L et al. Nucl Med Commun. 2017;38(2):149-155. doi:10.1097/MNM.0000000000000617
- Sartor O et al; VISION Investigators. N Engl J Med. 2021;385(12):1091-1103. doi:10.1056/NEJMoa2107322
- Rowe SP et al. Annu Rev Med. 2019;70:461-477. doi:10.1146/annurev-med-062117-073027
- Pomykala KL et al. Eur Urol Oncol. 2022;S2588-9311(22)00177-8. doi:10.1016/j.euo.2022.10.007
- Keam SJ. Mol Diagn Ther. 2022;26(4):467-475. doi:10.1007/s40291-022-00594-2
- Lovejoy LA et al. Mil Med. 2022:usac297. doi:10.1093/milmed/usac297
- Smith ZL et al. Med Clin North Am. 2018;102(2):251-264. doi:10.1016/j.mcna.2017.10.003
- Hohnloser JH et al. Eur J Med Res.1996;1(11):509-514.
- Johns Hopkins Medicine website. Testicular Cancer tumor Markers. Accessed December 2022. https://www.hopkinsmedicine.org/health/conditions-and-diseases/testicular-cancer/testicular-cancer-tumor-markers
- Webber BJ et al. J Occup Environ Med. 2022;64(1):71-78. doi:10.1097/JOM.0000000000002353
- Risk factors for testicular cancer. American Cancer Society. Updated May 17, 2018. Accessed December 15, 2022. https://www.cancer.org/cancer/testicular-cancer/causes-risks-prevention/risk-factors.html
- Chovanec M, Cheng L. BMJ. 2022;379:e070499. doi:10.1136/bmj-2022-070499
- Tavares NT et al. J Pathol. 2022. doi:10.1002/path.6037
- Bryant AK et al. JAMA Oncol. 2022;e224319. doi:10.1001/jamaoncol.2022.4319
- Kabasakal L et al. Nucl Med Commun. 2017;38(2):149-155. doi:10.1097/MNM.0000000000000617
- Sartor O et al; VISION Investigators. N Engl J Med. 2021;385(12):1091-1103. doi:10.1056/NEJMoa2107322
- Rowe SP et al. Annu Rev Med. 2019;70:461-477. doi:10.1146/annurev-med-062117-073027
- Pomykala KL et al. Eur Urol Oncol. 2022;S2588-9311(22)00177-8. doi:10.1016/j.euo.2022.10.007
- Keam SJ. Mol Diagn Ther. 2022;26(4):467-475. doi:10.1007/s40291-022-00594-2
- Lovejoy LA et al. Mil Med. 2022:usac297. doi:10.1093/milmed/usac297
- Smith ZL et al. Med Clin North Am. 2018;102(2):251-264. doi:10.1016/j.mcna.2017.10.003
- Hohnloser JH et al. Eur J Med Res.1996;1(11):509-514.
- Johns Hopkins Medicine website. Testicular Cancer tumor Markers. Accessed December 2022. https://www.hopkinsmedicine.org/health/conditions-and-diseases/testicular-cancer/testicular-cancer-tumor-markers
- Webber BJ et al. J Occup Environ Med. 2022;64(1):71-78. doi:10.1097/JOM.0000000000002353
- Risk factors for testicular cancer. American Cancer Society. Updated May 17, 2018. Accessed December 15, 2022. https://www.cancer.org/cancer/testicular-cancer/causes-risks-prevention/risk-factors.html
- Chovanec M, Cheng L. BMJ. 2022;379:e070499. doi:10.1136/bmj-2022-070499
- Tavares NT et al. J Pathol. 2022. doi:10.1002/path.6037
- Bryant AK et al. JAMA Oncol. 2022;e224319. doi:10.1001/jamaoncol.2022.4319
- Kabasakal L et al. Nucl Med Commun. 2017;38(2):149-155. doi:10.1097/MNM.0000000000000617
- Sartor O et al; VISION Investigators. N Engl J Med. 2021;385(12):1091-1103. doi:10.1056/NEJMoa2107322
- Rowe SP et al. Annu Rev Med. 2019;70:461-477. doi:10.1146/annurev-med-062117-073027
- Pomykala KL et al. Eur Urol Oncol. 2022;S2588-9311(22)00177-8. doi:10.1016/j.euo.2022.10.007
- Keam SJ. Mol Diagn Ther. 2022;26(4):467-475. doi:10.1007/s40291-022-00594-2
- Lovejoy LA et al. Mil Med. 2022:usac297. doi:10.1093/milmed/usac297
- Smith ZL et al. Med Clin North Am. 2018;102(2):251-264. doi:10.1016/j.mcna.2017.10.003
- Hohnloser JH et al. Eur J Med Res.1996;1(11):509-514.
- Johns Hopkins Medicine website. Testicular Cancer tumor Markers. Accessed December 2022. https://www.hopkinsmedicine.org/health/conditions-and-diseases/testicular-cancer/testicular-cancer-tumor-markers
- Webber BJ et al. J Occup Environ Med. 2022;64(1):71-78. doi:10.1097/JOM.0000000000002353
Risk of falls seen with newer antiandrogens for prostate cancer
Second-generation antiandrogens (AAs) – abiraterone, apalutamide, darolutamide, and enzalutamide – are a cornerstone of modern prostate cancer treatment, improving outcomes and survival.
However, they carry a significant caveat, according to a new meta-analysis of 12 clinical trials with over 13,000 patients.
the authors reported.
These findings carry “important public health indications” because use of second-generation AAs, currently first-line treatment for advanced and castration-resistant prostate cancer, is expanding with new indications, meaning that the pool of men at risk for such problems is large and growing, the team wrote.
The take-home message is that the findings give men – and the physicians who counsel them – a fuller idea of what to expect when considering using the agents, the researchers comment. This information is key at a time when so much of prostate cancer treatment involves carefully weighing the risks and benefits, they added.
The study was published in JAMA Oncology. It was conducted by a team of researchers from the University of Texas MD Anderson Cancer Center, Houston, and was led by Malgorzata Nowakowska, a medical student at Baylor College of Medicine, Houston.
Two prostate cancer specialists agreed and gave an example to bring the point home in an accompanying editorial.
The risk-benefit ratio of adding a second-generation AA to treatment may be different for a patient who wants to stay alert and sharp to keep a complex job “versus someone whose primary goal is to see their young children graduate high school,” Alexandra Sokolova, MD, of the Oregon Health and Science University, Portland, and Julie Graff, MD, of the VA Portland (Ore.) Health Care System, wrote in their editorial.
The study fills a “critical gap” when it comes to counseling men about the drugs and will help guide discussions, they said.
The investigators said their study also highlights the need for additional research to identify who is most at risk for the side effects and the best way to prevent and treat them. “Interventions currently under investigation include donepezil, methylphenidate, low-fat diet, acupuncture, martial arts, and high-intensity exercise, among many others,” Ms. Nowakowska and colleagues noted.
Study details
The 12 trials in the meta-analysis, which compared second-generation AAs with placebo, were conducted from 2008 to 2021. These trials were multinational investigations that included patients with metastatic disease as well as those with nonmetastatic disease. The median age across the studies ranged from 67 to 74 years, and trial follow-up ranged from 3.9 to 48 months.
The rates of adverse cognitive effects and attention disorders and disturbances ranged from 2% to 8% among patients who received second-generation AAs versus 2%-3% among those who received placebo, a more than doubling of the risk of cognitive toxic effects (P = .002).
Fatigue of any grade was reported in 5%-45% of participants taking second-generation AAs versus 2%-42% of patients taking placebos, which translates to a 34% higher risk (P < .001).
The use of AAs was associated with an 87% increase in the risk of falls in comparison with placebo, regardless of severity. For falls of grade 3 or higher that required hospitalization or invasive treatment, the increase in risk with second-generation AAs was 72% (P = .05).
The findings were consistent for cognitive toxicity and fatigue in studies that included traditional hormone therapy in both the treatment and control arms. Increased age was associated with a greater risk of fatigue.
Study limits include the fact that it was not known how long patients were taking the drugs before they encountered problems. In addition, the findings were not broken down with respect to medication, so it’s unknown whether such problems are worse with some second-generation AAs than with others.
The editorialists noted that real-world patients tend to be older and sicker than patients in trials, so the risk of falls, fatigue, and cognition problems might be higher among everyday patients.
The study was funded by the National Institutes of Health and others. The investigators disclosed no relevant financial relationships. Dr. Sokolova has received personal fees from Lantheus and travel grants from AstraZeneca. Dr. Graff has received nonfinancial support from Janssen, Pfizer/Astellas, and Sanofi.
A version of this article first appeared on Medscape.com.
Second-generation antiandrogens (AAs) – abiraterone, apalutamide, darolutamide, and enzalutamide – are a cornerstone of modern prostate cancer treatment, improving outcomes and survival.
However, they carry a significant caveat, according to a new meta-analysis of 12 clinical trials with over 13,000 patients.
the authors reported.
These findings carry “important public health indications” because use of second-generation AAs, currently first-line treatment for advanced and castration-resistant prostate cancer, is expanding with new indications, meaning that the pool of men at risk for such problems is large and growing, the team wrote.
The take-home message is that the findings give men – and the physicians who counsel them – a fuller idea of what to expect when considering using the agents, the researchers comment. This information is key at a time when so much of prostate cancer treatment involves carefully weighing the risks and benefits, they added.
The study was published in JAMA Oncology. It was conducted by a team of researchers from the University of Texas MD Anderson Cancer Center, Houston, and was led by Malgorzata Nowakowska, a medical student at Baylor College of Medicine, Houston.
Two prostate cancer specialists agreed and gave an example to bring the point home in an accompanying editorial.
The risk-benefit ratio of adding a second-generation AA to treatment may be different for a patient who wants to stay alert and sharp to keep a complex job “versus someone whose primary goal is to see their young children graduate high school,” Alexandra Sokolova, MD, of the Oregon Health and Science University, Portland, and Julie Graff, MD, of the VA Portland (Ore.) Health Care System, wrote in their editorial.
The study fills a “critical gap” when it comes to counseling men about the drugs and will help guide discussions, they said.
The investigators said their study also highlights the need for additional research to identify who is most at risk for the side effects and the best way to prevent and treat them. “Interventions currently under investigation include donepezil, methylphenidate, low-fat diet, acupuncture, martial arts, and high-intensity exercise, among many others,” Ms. Nowakowska and colleagues noted.
Study details
The 12 trials in the meta-analysis, which compared second-generation AAs with placebo, were conducted from 2008 to 2021. These trials were multinational investigations that included patients with metastatic disease as well as those with nonmetastatic disease. The median age across the studies ranged from 67 to 74 years, and trial follow-up ranged from 3.9 to 48 months.
The rates of adverse cognitive effects and attention disorders and disturbances ranged from 2% to 8% among patients who received second-generation AAs versus 2%-3% among those who received placebo, a more than doubling of the risk of cognitive toxic effects (P = .002).
Fatigue of any grade was reported in 5%-45% of participants taking second-generation AAs versus 2%-42% of patients taking placebos, which translates to a 34% higher risk (P < .001).
The use of AAs was associated with an 87% increase in the risk of falls in comparison with placebo, regardless of severity. For falls of grade 3 or higher that required hospitalization or invasive treatment, the increase in risk with second-generation AAs was 72% (P = .05).
The findings were consistent for cognitive toxicity and fatigue in studies that included traditional hormone therapy in both the treatment and control arms. Increased age was associated with a greater risk of fatigue.
Study limits include the fact that it was not known how long patients were taking the drugs before they encountered problems. In addition, the findings were not broken down with respect to medication, so it’s unknown whether such problems are worse with some second-generation AAs than with others.
The editorialists noted that real-world patients tend to be older and sicker than patients in trials, so the risk of falls, fatigue, and cognition problems might be higher among everyday patients.
The study was funded by the National Institutes of Health and others. The investigators disclosed no relevant financial relationships. Dr. Sokolova has received personal fees from Lantheus and travel grants from AstraZeneca. Dr. Graff has received nonfinancial support from Janssen, Pfizer/Astellas, and Sanofi.
A version of this article first appeared on Medscape.com.
Second-generation antiandrogens (AAs) – abiraterone, apalutamide, darolutamide, and enzalutamide – are a cornerstone of modern prostate cancer treatment, improving outcomes and survival.
However, they carry a significant caveat, according to a new meta-analysis of 12 clinical trials with over 13,000 patients.
the authors reported.
These findings carry “important public health indications” because use of second-generation AAs, currently first-line treatment for advanced and castration-resistant prostate cancer, is expanding with new indications, meaning that the pool of men at risk for such problems is large and growing, the team wrote.
The take-home message is that the findings give men – and the physicians who counsel them – a fuller idea of what to expect when considering using the agents, the researchers comment. This information is key at a time when so much of prostate cancer treatment involves carefully weighing the risks and benefits, they added.
The study was published in JAMA Oncology. It was conducted by a team of researchers from the University of Texas MD Anderson Cancer Center, Houston, and was led by Malgorzata Nowakowska, a medical student at Baylor College of Medicine, Houston.
Two prostate cancer specialists agreed and gave an example to bring the point home in an accompanying editorial.
The risk-benefit ratio of adding a second-generation AA to treatment may be different for a patient who wants to stay alert and sharp to keep a complex job “versus someone whose primary goal is to see their young children graduate high school,” Alexandra Sokolova, MD, of the Oregon Health and Science University, Portland, and Julie Graff, MD, of the VA Portland (Ore.) Health Care System, wrote in their editorial.
The study fills a “critical gap” when it comes to counseling men about the drugs and will help guide discussions, they said.
The investigators said their study also highlights the need for additional research to identify who is most at risk for the side effects and the best way to prevent and treat them. “Interventions currently under investigation include donepezil, methylphenidate, low-fat diet, acupuncture, martial arts, and high-intensity exercise, among many others,” Ms. Nowakowska and colleagues noted.
Study details
The 12 trials in the meta-analysis, which compared second-generation AAs with placebo, were conducted from 2008 to 2021. These trials were multinational investigations that included patients with metastatic disease as well as those with nonmetastatic disease. The median age across the studies ranged from 67 to 74 years, and trial follow-up ranged from 3.9 to 48 months.
The rates of adverse cognitive effects and attention disorders and disturbances ranged from 2% to 8% among patients who received second-generation AAs versus 2%-3% among those who received placebo, a more than doubling of the risk of cognitive toxic effects (P = .002).
Fatigue of any grade was reported in 5%-45% of participants taking second-generation AAs versus 2%-42% of patients taking placebos, which translates to a 34% higher risk (P < .001).
The use of AAs was associated with an 87% increase in the risk of falls in comparison with placebo, regardless of severity. For falls of grade 3 or higher that required hospitalization or invasive treatment, the increase in risk with second-generation AAs was 72% (P = .05).
The findings were consistent for cognitive toxicity and fatigue in studies that included traditional hormone therapy in both the treatment and control arms. Increased age was associated with a greater risk of fatigue.
Study limits include the fact that it was not known how long patients were taking the drugs before they encountered problems. In addition, the findings were not broken down with respect to medication, so it’s unknown whether such problems are worse with some second-generation AAs than with others.
The editorialists noted that real-world patients tend to be older and sicker than patients in trials, so the risk of falls, fatigue, and cognition problems might be higher among everyday patients.
The study was funded by the National Institutes of Health and others. The investigators disclosed no relevant financial relationships. Dr. Sokolova has received personal fees from Lantheus and travel grants from AstraZeneca. Dr. Graff has received nonfinancial support from Janssen, Pfizer/Astellas, and Sanofi.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
COVID can mimic prostate cancer symptoms
This patient has a strong likelihood of aggressive prostate cancer, right? If that same patient also presents with severe, burning bone pain with no precipitating trauma to the area and rest and over-the-counter painkillers are not helping, you’d think, “check for metastases,” right?
That patient was me in late January 2023.
As a research scientist member of the American Urological Association, I knew enough to know I had to consult my urologist ASAP.
With the above symptoms, I’ll admit I was scared. Fortunately, if that’s the right word, I was no stranger to a rapid, dramatic spike in PSA. In 2021 I was temporarily living in a new city, and I wanted to form a relationship with a good local urologist. The urologist that I was referred to gave me a thorough consultation, including a vigorous digital rectal exam (DRE) and sent me across the street for a blood draw.
To my shock, my PSA had spiked over 2 points, to 9.9 from 7.8 a few months earlier. I freaked. Had my 3-cm tumor burst out into an aggressive cancer? Research on PubMed provided an array of studies showing what could cause PSA to suddenly rise, including a DRE performed 72 hours before the blood draw.1 A week later, my PSA was back down to its normal 7.6.
But in January 2023, I had none of those previously reported experiences that could suddenly trigger a spike in PSA, like a DRE or riding on a thin bicycle seat for a few hours before the lab visit.
The COVID effect
I went back to PubMed and found a new circumstance that could cause a surge in PSA: COVID-19. A recent study2 of 91 men with benign prostatic hypertrophy by researchers in Turkey found that PSA spiked from 0 to 5 points during the COVID infection period and up to 2 points higher 3 months after the infection had cleared. I had tested positive for COVID-19 in mid-December 2022, 4 weeks before my 9.9 PSA reading.
Using Google translate, I communicated with the team in Turkey and found out that the PSA spike can last up to 6 months.
That study helps explain why my PSA dropped over 1.5 points to 8.5 just 2 weeks after the 9.9 reading, with the expectation that it would return to its previous normal of 7.8 within 6 months of infection with SARS-CoV-2. To be safe, my urologist scheduled another PSA test in May, along with an updated multiparametric MRI, which may be followed by an in-bore MRI-guided biopsy of the 3-cm tumor if the mass has enlarged.
COVID-19 pain
What about my burning bone pain in my upper right humerus and right rotator cuff that was not precipitated by trauma or strain? A radiograph found no evidence of metastasis, thank goodness. And my research showed that several studies3 have found that COVID-19 can cause burning musculoskeletal pain, including enthesopathy, which is what I had per the radiology report. So my PSA spike and searing pain were likely consequences of the infection.
To avoid the risk for a gross misdiagnosis after a radical spike in PSA, the informed urologist should ask the patient if he has had COVID-19 in the previous 6 months. Overlooking that question could lead to the wrong diagnostic decisions about a rapid jump in PSA or unexplained bone pain.
References
1. Bossens MM et al. Eur J Cancer. 1995;31A:682-5.
2. Cinislioglu AE et al. Urology. 2022;159:16-21.
3. Ciaffi J et al. Joint Bone Spine. 2021;88:105158.
Dr. Keller is founder of the Keller Research Institute, Jacksonville, Fla. He reported serving as a research scientist for the American Urological Association, serving on the advisory board of Active Surveillance Patient’s International, and serving on the boards of numerous nonprofit organizations.
A version of this article first appeared on Medscape.com.
This patient has a strong likelihood of aggressive prostate cancer, right? If that same patient also presents with severe, burning bone pain with no precipitating trauma to the area and rest and over-the-counter painkillers are not helping, you’d think, “check for metastases,” right?
That patient was me in late January 2023.
As a research scientist member of the American Urological Association, I knew enough to know I had to consult my urologist ASAP.
With the above symptoms, I’ll admit I was scared. Fortunately, if that’s the right word, I was no stranger to a rapid, dramatic spike in PSA. In 2021 I was temporarily living in a new city, and I wanted to form a relationship with a good local urologist. The urologist that I was referred to gave me a thorough consultation, including a vigorous digital rectal exam (DRE) and sent me across the street for a blood draw.
To my shock, my PSA had spiked over 2 points, to 9.9 from 7.8 a few months earlier. I freaked. Had my 3-cm tumor burst out into an aggressive cancer? Research on PubMed provided an array of studies showing what could cause PSA to suddenly rise, including a DRE performed 72 hours before the blood draw.1 A week later, my PSA was back down to its normal 7.6.
But in January 2023, I had none of those previously reported experiences that could suddenly trigger a spike in PSA, like a DRE or riding on a thin bicycle seat for a few hours before the lab visit.
The COVID effect
I went back to PubMed and found a new circumstance that could cause a surge in PSA: COVID-19. A recent study2 of 91 men with benign prostatic hypertrophy by researchers in Turkey found that PSA spiked from 0 to 5 points during the COVID infection period and up to 2 points higher 3 months after the infection had cleared. I had tested positive for COVID-19 in mid-December 2022, 4 weeks before my 9.9 PSA reading.
Using Google translate, I communicated with the team in Turkey and found out that the PSA spike can last up to 6 months.
That study helps explain why my PSA dropped over 1.5 points to 8.5 just 2 weeks after the 9.9 reading, with the expectation that it would return to its previous normal of 7.8 within 6 months of infection with SARS-CoV-2. To be safe, my urologist scheduled another PSA test in May, along with an updated multiparametric MRI, which may be followed by an in-bore MRI-guided biopsy of the 3-cm tumor if the mass has enlarged.
COVID-19 pain
What about my burning bone pain in my upper right humerus and right rotator cuff that was not precipitated by trauma or strain? A radiograph found no evidence of metastasis, thank goodness. And my research showed that several studies3 have found that COVID-19 can cause burning musculoskeletal pain, including enthesopathy, which is what I had per the radiology report. So my PSA spike and searing pain were likely consequences of the infection.
To avoid the risk for a gross misdiagnosis after a radical spike in PSA, the informed urologist should ask the patient if he has had COVID-19 in the previous 6 months. Overlooking that question could lead to the wrong diagnostic decisions about a rapid jump in PSA or unexplained bone pain.
References
1. Bossens MM et al. Eur J Cancer. 1995;31A:682-5.
2. Cinislioglu AE et al. Urology. 2022;159:16-21.
3. Ciaffi J et al. Joint Bone Spine. 2021;88:105158.
Dr. Keller is founder of the Keller Research Institute, Jacksonville, Fla. He reported serving as a research scientist for the American Urological Association, serving on the advisory board of Active Surveillance Patient’s International, and serving on the boards of numerous nonprofit organizations.
A version of this article first appeared on Medscape.com.
This patient has a strong likelihood of aggressive prostate cancer, right? If that same patient also presents with severe, burning bone pain with no precipitating trauma to the area and rest and over-the-counter painkillers are not helping, you’d think, “check for metastases,” right?
That patient was me in late January 2023.
As a research scientist member of the American Urological Association, I knew enough to know I had to consult my urologist ASAP.
With the above symptoms, I’ll admit I was scared. Fortunately, if that’s the right word, I was no stranger to a rapid, dramatic spike in PSA. In 2021 I was temporarily living in a new city, and I wanted to form a relationship with a good local urologist. The urologist that I was referred to gave me a thorough consultation, including a vigorous digital rectal exam (DRE) and sent me across the street for a blood draw.
To my shock, my PSA had spiked over 2 points, to 9.9 from 7.8 a few months earlier. I freaked. Had my 3-cm tumor burst out into an aggressive cancer? Research on PubMed provided an array of studies showing what could cause PSA to suddenly rise, including a DRE performed 72 hours before the blood draw.1 A week later, my PSA was back down to its normal 7.6.
But in January 2023, I had none of those previously reported experiences that could suddenly trigger a spike in PSA, like a DRE or riding on a thin bicycle seat for a few hours before the lab visit.
The COVID effect
I went back to PubMed and found a new circumstance that could cause a surge in PSA: COVID-19. A recent study2 of 91 men with benign prostatic hypertrophy by researchers in Turkey found that PSA spiked from 0 to 5 points during the COVID infection period and up to 2 points higher 3 months after the infection had cleared. I had tested positive for COVID-19 in mid-December 2022, 4 weeks before my 9.9 PSA reading.
Using Google translate, I communicated with the team in Turkey and found out that the PSA spike can last up to 6 months.
That study helps explain why my PSA dropped over 1.5 points to 8.5 just 2 weeks after the 9.9 reading, with the expectation that it would return to its previous normal of 7.8 within 6 months of infection with SARS-CoV-2. To be safe, my urologist scheduled another PSA test in May, along with an updated multiparametric MRI, which may be followed by an in-bore MRI-guided biopsy of the 3-cm tumor if the mass has enlarged.
COVID-19 pain
What about my burning bone pain in my upper right humerus and right rotator cuff that was not precipitated by trauma or strain? A radiograph found no evidence of metastasis, thank goodness. And my research showed that several studies3 have found that COVID-19 can cause burning musculoskeletal pain, including enthesopathy, which is what I had per the radiology report. So my PSA spike and searing pain were likely consequences of the infection.
To avoid the risk for a gross misdiagnosis after a radical spike in PSA, the informed urologist should ask the patient if he has had COVID-19 in the previous 6 months. Overlooking that question could lead to the wrong diagnostic decisions about a rapid jump in PSA or unexplained bone pain.
References
1. Bossens MM et al. Eur J Cancer. 1995;31A:682-5.
2. Cinislioglu AE et al. Urology. 2022;159:16-21.
3. Ciaffi J et al. Joint Bone Spine. 2021;88:105158.
Dr. Keller is founder of the Keller Research Institute, Jacksonville, Fla. He reported serving as a research scientist for the American Urological Association, serving on the advisory board of Active Surveillance Patient’s International, and serving on the boards of numerous nonprofit organizations.
A version of this article first appeared on Medscape.com.
Reports of dysuria and nocturia
The history and findings in this case are suggestive of small cell carcinoma of the prostate (SCCP).
SCCP is a rare and aggressive cancer that comprises 1%–5% of all prostate cancers (if mixed cases with adenocarcinoma are included). Similar to small cell carcinoma of the lung or other small cell primaries, SCCP is characterized by a primary tumor of the prostate gland that expresses small cell morphology and high-grade features, including minimal cytoplasm, nuclear molding, fine chromatin pattern, extensive tumor necrosis and apoptosis, variable tumor giant cells, and a high mitotic rate. Patients often have disproportionally low PSA levels despite having large metastatic burden and visceral disease. Pathologic diagnosis is made on the basis of prostate biopsy using characteristics of small cell tumors and immunohistochemical staining for neuroendocrine markers, such as CD56, chromogranin A, synaptophysin, and neuron-specific enolase.
SCCP arises de novo in approximately 50% of cases; it also occurs in patients with previous or concomitant prostate adenocarcinoma. Patients are often symptomatic at diagnosis because of the extent of the tumor. The aggressive nature and high proliferation rate associated with SCCP result in an increased risk for lytic or blastic bone, visceral, and brain metastases. In addition, paraneoplastic syndromes (eg, the syndrome of inappropriate antidiuretic hormone secretion, Cushing syndrome, and hypercalcemia) frequently occur as a result of the release of peptides.
SCCP metastasizes early in its course and is associated with a poor prognosis. It has a median survival of < 1 year. Fluorodeoxyglucose PET-CT are useful for staging and monitoring treatment response; in addition, given the disease's predilection for brain metastases, MRI of the brain should be considered.
The optimal treatment for patients with metastatic SCCP has not yet been determined. Localized SCCP is treated aggressively, typically with a multimodality approach involving chemotherapy with concurrent or consolidative radiotherapy.
According to 2023 guidelines from the National Comprehensive Cancer Network (NCCN), platinum-based combination chemotherapy (cisplatin-etoposide, carboplatin-etoposide, docetaxel-carboplatin, cabazitaxel-carboplatin) is the first-line approach for patients with metastatic disease.
Physicians are also advised to consult the NCCN guidelines for small cell lung cancer because the behavior of SCCP is similar to that of small cell carcinoma of the lung. Immunotherapy with pembrolizumab may be used for platinum-resistant extrapulmonary small cell carcinoma. However, sipuleucel-T is not recommended for patients with SCCP.
Chad R. Tracy, MD, Professor; Director, Minimally Invasive Surgery, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, Iowa
Chad R. Tracy, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: CVICO Medical Solutions.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of small cell carcinoma of the prostate (SCCP).
SCCP is a rare and aggressive cancer that comprises 1%–5% of all prostate cancers (if mixed cases with adenocarcinoma are included). Similar to small cell carcinoma of the lung or other small cell primaries, SCCP is characterized by a primary tumor of the prostate gland that expresses small cell morphology and high-grade features, including minimal cytoplasm, nuclear molding, fine chromatin pattern, extensive tumor necrosis and apoptosis, variable tumor giant cells, and a high mitotic rate. Patients often have disproportionally low PSA levels despite having large metastatic burden and visceral disease. Pathologic diagnosis is made on the basis of prostate biopsy using characteristics of small cell tumors and immunohistochemical staining for neuroendocrine markers, such as CD56, chromogranin A, synaptophysin, and neuron-specific enolase.
SCCP arises de novo in approximately 50% of cases; it also occurs in patients with previous or concomitant prostate adenocarcinoma. Patients are often symptomatic at diagnosis because of the extent of the tumor. The aggressive nature and high proliferation rate associated with SCCP result in an increased risk for lytic or blastic bone, visceral, and brain metastases. In addition, paraneoplastic syndromes (eg, the syndrome of inappropriate antidiuretic hormone secretion, Cushing syndrome, and hypercalcemia) frequently occur as a result of the release of peptides.
SCCP metastasizes early in its course and is associated with a poor prognosis. It has a median survival of < 1 year. Fluorodeoxyglucose PET-CT are useful for staging and monitoring treatment response; in addition, given the disease's predilection for brain metastases, MRI of the brain should be considered.
The optimal treatment for patients with metastatic SCCP has not yet been determined. Localized SCCP is treated aggressively, typically with a multimodality approach involving chemotherapy with concurrent or consolidative radiotherapy.
According to 2023 guidelines from the National Comprehensive Cancer Network (NCCN), platinum-based combination chemotherapy (cisplatin-etoposide, carboplatin-etoposide, docetaxel-carboplatin, cabazitaxel-carboplatin) is the first-line approach for patients with metastatic disease.
Physicians are also advised to consult the NCCN guidelines for small cell lung cancer because the behavior of SCCP is similar to that of small cell carcinoma of the lung. Immunotherapy with pembrolizumab may be used for platinum-resistant extrapulmonary small cell carcinoma. However, sipuleucel-T is not recommended for patients with SCCP.
Chad R. Tracy, MD, Professor; Director, Minimally Invasive Surgery, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, Iowa
Chad R. Tracy, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: CVICO Medical Solutions.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of small cell carcinoma of the prostate (SCCP).
SCCP is a rare and aggressive cancer that comprises 1%–5% of all prostate cancers (if mixed cases with adenocarcinoma are included). Similar to small cell carcinoma of the lung or other small cell primaries, SCCP is characterized by a primary tumor of the prostate gland that expresses small cell morphology and high-grade features, including minimal cytoplasm, nuclear molding, fine chromatin pattern, extensive tumor necrosis and apoptosis, variable tumor giant cells, and a high mitotic rate. Patients often have disproportionally low PSA levels despite having large metastatic burden and visceral disease. Pathologic diagnosis is made on the basis of prostate biopsy using characteristics of small cell tumors and immunohistochemical staining for neuroendocrine markers, such as CD56, chromogranin A, synaptophysin, and neuron-specific enolase.
SCCP arises de novo in approximately 50% of cases; it also occurs in patients with previous or concomitant prostate adenocarcinoma. Patients are often symptomatic at diagnosis because of the extent of the tumor. The aggressive nature and high proliferation rate associated with SCCP result in an increased risk for lytic or blastic bone, visceral, and brain metastases. In addition, paraneoplastic syndromes (eg, the syndrome of inappropriate antidiuretic hormone secretion, Cushing syndrome, and hypercalcemia) frequently occur as a result of the release of peptides.
SCCP metastasizes early in its course and is associated with a poor prognosis. It has a median survival of < 1 year. Fluorodeoxyglucose PET-CT are useful for staging and monitoring treatment response; in addition, given the disease's predilection for brain metastases, MRI of the brain should be considered.
The optimal treatment for patients with metastatic SCCP has not yet been determined. Localized SCCP is treated aggressively, typically with a multimodality approach involving chemotherapy with concurrent or consolidative radiotherapy.
According to 2023 guidelines from the National Comprehensive Cancer Network (NCCN), platinum-based combination chemotherapy (cisplatin-etoposide, carboplatin-etoposide, docetaxel-carboplatin, cabazitaxel-carboplatin) is the first-line approach for patients with metastatic disease.
Physicians are also advised to consult the NCCN guidelines for small cell lung cancer because the behavior of SCCP is similar to that of small cell carcinoma of the lung. Immunotherapy with pembrolizumab may be used for platinum-resistant extrapulmonary small cell carcinoma. However, sipuleucel-T is not recommended for patients with SCCP.
Chad R. Tracy, MD, Professor; Director, Minimally Invasive Surgery, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, Iowa
Chad R. Tracy, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: CVICO Medical Solutions.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
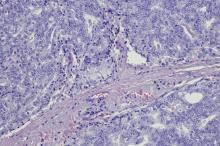
A 69-year-old nonsmoking African American man presents with reports of dysuria, nocturia, and unintentional weight loss. He reveals no other lower urinary tract symptoms, pelvic pain, night sweats, back pain, or excessive fatigue. Digital rectal exam reveals an enlarged prostate with a firm, irregular nodule at the right side of the gland. Laboratory tests reveal a prostate-specific antigen (PSA) level of 2.22 ng/mL; a comprehensive metabolic panel and CBC are within normal limits. The patient is 6 ft 1 in and weighs 187 lb.
A transrectal ultrasound-guided prostate biopsy is performed. Histologic examination reveals immunoreactivity for the neuroendocrine markers synaptophysin, chromogranin A, and expression of transcription factor 1. A proliferation of small cells (> 4 lymphocytes in diameter) is noted, with scant cytoplasm, poorly defined borders, finely granular salt-and-pepper chromatin, inconspicuous nucleoli, and a high mitotic count. Evidence of perineural invasion is noted.
Plant-based diet linked to better outcomes in prostate cancer
The findings, which were reported at the 2023 ASCO Genitourinary Cancers Symposium in February, are based on a study of 2,038 men (median age, 64 years) with prostate cancer at stage T1, T2, or T3a.
“Consuming a whole foods plant-based diet may be an option to decrease risk for recurrence and improve overall survivorship,” said Vivian N. Liu, a clinical research coordinator at the University of California, San Francisco, who presented the findings.
The patients were interviewed about their diets at about 31.5 months after diagnosis. The study group was broken down into four groups based on how much of their diet consisted of a plant-based diet. Men in the highest quintile group who consumed at least 2.4 servings daily of fruit, 4.2 servings of vegetables, 2.6 servings of dairy, and 1.2 servings of meat (not seafood), had a 52% lower risk of progression (hazard ratio, 0.48; 95% confidence interval, 0.36-0.65; P-trend < 0.001) and a 53% lower risk of recurrence (HR, 0.47; 95% CI, 0.32-0.68; P-trend < 0.001), which was statistically significant. This compares with men in the lowest quintile who consumed 0.8 servings a day of fruit, 2.1 servings of vegetables, 3.1 servings of dairy, and 1.4 servings of meat. The findings were adjusted for total caloric intake, race, and smoking status.
For men over 65 years old, researchers found that a plant-based diet was associated with lower risk of recurrence (HR, 0.41; 95% CI, 0.24-0.7; P-trend = 0.03). And for those who exercised daily – in this case walking at a fast pace more than 3 times a week – a plant-based diet had a 56% (HR, 0.33; 95% CI, 0.26-0.73) lower risk of progression in the highest quintile group and a 59% decrease in recurrence (HR, 0.41; 95% CI, 0.25-0.68).
A new analysis like this, Ms. Liu said, “could guide people to make better, more healthful choices across their whole diet rather than adding or removing select foods.”
The primary endpoint was progression including recurrence, secondary treatment, bone metastases, and death due to prostate cancer, and the secondary endpoint was recurrence (PSA > 0.2ng/mL at 2 consecutive follow-up visits or during secondary treatment). At 7.4 years follow-up, there were 204 cases of progression.
“Fruits and vegetables contain antioxidants and anti-inflammatory components as well as dietary fiber that improve glucose control and reduce inflammation,” Ms. Liu said. In contrast, she said, animal-based foods may increase insulin resistance and insulin levels and boost levels of insulin-like growth factor 1, which is associated with prostate cancer risk. More studies, especially randomized controlled trials, are needed to provide evidence whether healthful plant-based foods and prostate cancer progression are connected.
NYU Langone Health urologist Natasha Gupta, MD, published a systematic review in 2022 on the impact of a plant-based diet on prostate cancer.* The review, which included 5 interventional studies and 11 observational studies, found that consuming a plant-based diet was associated with improvements in general health for men with prostate cancer. The observational studies found either a lower risk of prostate cancer or no significant difference.
“Patients often ask if there is anything that they can do to reduce the risk of recurrence, and it is great to be able to tell patients that a healthy lifestyle including plant-based foods and physical activity is helpful,” Dr. Gupta said.
The review’s coauthor, Stacy Loeb, MD, also of NYU Langone Health, said the new study was “a well-done observational study by experts in nutritional epidemiology from UCSF. It adds to a large body of evidence showing that plant-based diets improve health outcomes.”
“In the short-term, purchasing plant-based protein sources, such as beans and lentils, is less expensive than buying meat. Plant-based diets also reduce the risk of obesity, diabetes, and cardiovascular disease, which are associated with hundreds of thousands of dollars over a lifetime,” she said.
Limitations of the new study included the small number of non-White participants and self-reporting of diet. The study doesn’t examine the cost of various diets or the availability of plant-based foods like fresh produce, which can be limited in some neighborhoods.
Ms. Liu and colleagues plan to conduct a study that examines postdiagnostic plant-based diets in relation to prostate cancer–specific mortality. She and her team will also examine the plant-based dietary indices in relation to prostate cancer–specific quality of life at 2, 5, and 10 years from baseline.
The study authors, Dr. Loeb, and Dr. Gupta report no disclosures.
Correction, 3/17/23: An earlier version of this article misstated the name of NYU Langone Health.
The findings, which were reported at the 2023 ASCO Genitourinary Cancers Symposium in February, are based on a study of 2,038 men (median age, 64 years) with prostate cancer at stage T1, T2, or T3a.
“Consuming a whole foods plant-based diet may be an option to decrease risk for recurrence and improve overall survivorship,” said Vivian N. Liu, a clinical research coordinator at the University of California, San Francisco, who presented the findings.
The patients were interviewed about their diets at about 31.5 months after diagnosis. The study group was broken down into four groups based on how much of their diet consisted of a plant-based diet. Men in the highest quintile group who consumed at least 2.4 servings daily of fruit, 4.2 servings of vegetables, 2.6 servings of dairy, and 1.2 servings of meat (not seafood), had a 52% lower risk of progression (hazard ratio, 0.48; 95% confidence interval, 0.36-0.65; P-trend < 0.001) and a 53% lower risk of recurrence (HR, 0.47; 95% CI, 0.32-0.68; P-trend < 0.001), which was statistically significant. This compares with men in the lowest quintile who consumed 0.8 servings a day of fruit, 2.1 servings of vegetables, 3.1 servings of dairy, and 1.4 servings of meat. The findings were adjusted for total caloric intake, race, and smoking status.
For men over 65 years old, researchers found that a plant-based diet was associated with lower risk of recurrence (HR, 0.41; 95% CI, 0.24-0.7; P-trend = 0.03). And for those who exercised daily – in this case walking at a fast pace more than 3 times a week – a plant-based diet had a 56% (HR, 0.33; 95% CI, 0.26-0.73) lower risk of progression in the highest quintile group and a 59% decrease in recurrence (HR, 0.41; 95% CI, 0.25-0.68).
A new analysis like this, Ms. Liu said, “could guide people to make better, more healthful choices across their whole diet rather than adding or removing select foods.”
The primary endpoint was progression including recurrence, secondary treatment, bone metastases, and death due to prostate cancer, and the secondary endpoint was recurrence (PSA > 0.2ng/mL at 2 consecutive follow-up visits or during secondary treatment). At 7.4 years follow-up, there were 204 cases of progression.
“Fruits and vegetables contain antioxidants and anti-inflammatory components as well as dietary fiber that improve glucose control and reduce inflammation,” Ms. Liu said. In contrast, she said, animal-based foods may increase insulin resistance and insulin levels and boost levels of insulin-like growth factor 1, which is associated with prostate cancer risk. More studies, especially randomized controlled trials, are needed to provide evidence whether healthful plant-based foods and prostate cancer progression are connected.
NYU Langone Health urologist Natasha Gupta, MD, published a systematic review in 2022 on the impact of a plant-based diet on prostate cancer.* The review, which included 5 interventional studies and 11 observational studies, found that consuming a plant-based diet was associated with improvements in general health for men with prostate cancer. The observational studies found either a lower risk of prostate cancer or no significant difference.
“Patients often ask if there is anything that they can do to reduce the risk of recurrence, and it is great to be able to tell patients that a healthy lifestyle including plant-based foods and physical activity is helpful,” Dr. Gupta said.
The review’s coauthor, Stacy Loeb, MD, also of NYU Langone Health, said the new study was “a well-done observational study by experts in nutritional epidemiology from UCSF. It adds to a large body of evidence showing that plant-based diets improve health outcomes.”
“In the short-term, purchasing plant-based protein sources, such as beans and lentils, is less expensive than buying meat. Plant-based diets also reduce the risk of obesity, diabetes, and cardiovascular disease, which are associated with hundreds of thousands of dollars over a lifetime,” she said.
Limitations of the new study included the small number of non-White participants and self-reporting of diet. The study doesn’t examine the cost of various diets or the availability of plant-based foods like fresh produce, which can be limited in some neighborhoods.
Ms. Liu and colleagues plan to conduct a study that examines postdiagnostic plant-based diets in relation to prostate cancer–specific mortality. She and her team will also examine the plant-based dietary indices in relation to prostate cancer–specific quality of life at 2, 5, and 10 years from baseline.
The study authors, Dr. Loeb, and Dr. Gupta report no disclosures.
Correction, 3/17/23: An earlier version of this article misstated the name of NYU Langone Health.
The findings, which were reported at the 2023 ASCO Genitourinary Cancers Symposium in February, are based on a study of 2,038 men (median age, 64 years) with prostate cancer at stage T1, T2, or T3a.
“Consuming a whole foods plant-based diet may be an option to decrease risk for recurrence and improve overall survivorship,” said Vivian N. Liu, a clinical research coordinator at the University of California, San Francisco, who presented the findings.
The patients were interviewed about their diets at about 31.5 months after diagnosis. The study group was broken down into four groups based on how much of their diet consisted of a plant-based diet. Men in the highest quintile group who consumed at least 2.4 servings daily of fruit, 4.2 servings of vegetables, 2.6 servings of dairy, and 1.2 servings of meat (not seafood), had a 52% lower risk of progression (hazard ratio, 0.48; 95% confidence interval, 0.36-0.65; P-trend < 0.001) and a 53% lower risk of recurrence (HR, 0.47; 95% CI, 0.32-0.68; P-trend < 0.001), which was statistically significant. This compares with men in the lowest quintile who consumed 0.8 servings a day of fruit, 2.1 servings of vegetables, 3.1 servings of dairy, and 1.4 servings of meat. The findings were adjusted for total caloric intake, race, and smoking status.
For men over 65 years old, researchers found that a plant-based diet was associated with lower risk of recurrence (HR, 0.41; 95% CI, 0.24-0.7; P-trend = 0.03). And for those who exercised daily – in this case walking at a fast pace more than 3 times a week – a plant-based diet had a 56% (HR, 0.33; 95% CI, 0.26-0.73) lower risk of progression in the highest quintile group and a 59% decrease in recurrence (HR, 0.41; 95% CI, 0.25-0.68).
A new analysis like this, Ms. Liu said, “could guide people to make better, more healthful choices across their whole diet rather than adding or removing select foods.”
The primary endpoint was progression including recurrence, secondary treatment, bone metastases, and death due to prostate cancer, and the secondary endpoint was recurrence (PSA > 0.2ng/mL at 2 consecutive follow-up visits or during secondary treatment). At 7.4 years follow-up, there were 204 cases of progression.
“Fruits and vegetables contain antioxidants and anti-inflammatory components as well as dietary fiber that improve glucose control and reduce inflammation,” Ms. Liu said. In contrast, she said, animal-based foods may increase insulin resistance and insulin levels and boost levels of insulin-like growth factor 1, which is associated with prostate cancer risk. More studies, especially randomized controlled trials, are needed to provide evidence whether healthful plant-based foods and prostate cancer progression are connected.
NYU Langone Health urologist Natasha Gupta, MD, published a systematic review in 2022 on the impact of a plant-based diet on prostate cancer.* The review, which included 5 interventional studies and 11 observational studies, found that consuming a plant-based diet was associated with improvements in general health for men with prostate cancer. The observational studies found either a lower risk of prostate cancer or no significant difference.
“Patients often ask if there is anything that they can do to reduce the risk of recurrence, and it is great to be able to tell patients that a healthy lifestyle including plant-based foods and physical activity is helpful,” Dr. Gupta said.
The review’s coauthor, Stacy Loeb, MD, also of NYU Langone Health, said the new study was “a well-done observational study by experts in nutritional epidemiology from UCSF. It adds to a large body of evidence showing that plant-based diets improve health outcomes.”
“In the short-term, purchasing plant-based protein sources, such as beans and lentils, is less expensive than buying meat. Plant-based diets also reduce the risk of obesity, diabetes, and cardiovascular disease, which are associated with hundreds of thousands of dollars over a lifetime,” she said.
Limitations of the new study included the small number of non-White participants and self-reporting of diet. The study doesn’t examine the cost of various diets or the availability of plant-based foods like fresh produce, which can be limited in some neighborhoods.
Ms. Liu and colleagues plan to conduct a study that examines postdiagnostic plant-based diets in relation to prostate cancer–specific mortality. She and her team will also examine the plant-based dietary indices in relation to prostate cancer–specific quality of life at 2, 5, and 10 years from baseline.
The study authors, Dr. Loeb, and Dr. Gupta report no disclosures.
Correction, 3/17/23: An earlier version of this article misstated the name of NYU Langone Health.
FROM ASCO GU 2023
Prostate cancer subgroup may benefit from intensified therapy
SAN FRANCISCO – For patients with prostate cancer who have unfavorable features and a detectable PSA following a radical prostatectomy, the standard of care is treatment with 6 months of a gonadotropin-releasing hormone (GnRH) agonist with salvage radiation therapy (SRT), as established by the GETUG-AFU 16 trial.
A new trial, dubbed FORMULA-509, explored whether outcomes could be improved by intensifying the drug treatment by adding 6 months of abiraterone acetate plus prednisone as well as apalutamide on top of the GnRH agonist alongside the salvage radiotherapy.
However, the combination did significantly improve PFS and MFS in a subset of men with PSA levels greater than 0.5 ng/mL.
“Although this primary analysis did not meet the prespecified threshold for statistical significance, it does strongly suggest that the addition of abiraterone acetate/prednisone/apalutamide to salvage radiotherapy plus 6 months of ADT [androgen deprivation therapy] may improve progression-free survival and metastasis-free survival,” said lead author Paul L. Nguyen, MD, of the Dana-Farber Cancer Institute in Boston, and professor of radiation oncology at Harvard Medical School.
“This may be particularly evident in the subgroup of patients with PSA greater that 0.5 ng/mL where a preplanned subgroup analysis by stratification factors observed a statistically significant benefit for both progression-free survival and metastasis-free survival,” he said. “Six months of intensified ADT with next generation anti-androgens may provide an attractive alternative to lengthening ADT for patients with rising PSA and unfavorable features after radical prostatectomy.”
The study results were presented at the ASCO Genitourinary Cancers Symposium.
Benefit in subset
The FORMULA-509 trial included 305 patients with PSA ≥ 0.1 ng/mL who had undergone a radical prostatectomy, and who had one or more unfavorable risk features (Gleason 8-10 disease, PSA > 0.5 ng/mL, pT3/T4, pN1 or radiographic N1, PSA doubling time < 10 months, negative margins, persistent PSA, gross local/regional disease).
“This was a pretty high-risk population,” Dr. Nguyen emphasized, as 35% had Gleason score of 9, about a third (31%) a PSA >0.5, and 29% were pathologic node positive.
All patients received salvage radiotherapy plus 6 months of GnRH agonist (bicalutamide 50 mg), and half were randomly assigned to also receive abiraterone acetate/prednisone 1,000 mg/5 mg + apalutamide 240 mg daily.
At a median follow-up of 34 months, the 3-year PFS rate was 74.9% in the AAP-apalutamide arm vs. 68.5% for the control group (hazard ratio [HR], 0.71; P = .06), and the 3-year MFS rate was 90.6% vs. 87.2%, respectively (HR, 0.57; P = .05).
In the subset of patients with a PSA greater than 0.5 ng/mL, the 3-year PFS and MFS rates were significantly higher with in the AAP-apalutamide group: the 3-year PFS rate was 67.2% vs. 46.8% (HR, 0.50; P = .03), and the 3-year MFS rate was 84.3% vs. 66.1% (HR, 0.32; P = .02).
Adverse events were consistent with the known safety profiles of the agents being studied, Dr. Nguyen noted. The most common toxicities for AAP-apalutamide vs. controls were hypertension (21.8% vs. 13.3%), maculopapular rash (11.5% vs. 0.6%), diarrhea (8.5% vs. 4.8%), and fatigue (7.9% vs. 6.1%).
Dr. Nguyen noted that even though “we’re not supposed to compare clinical trials,” the results of this study appeared to compare favorably with those of another trial, RADICALS-HD, which was presented at the 2022 European Society of Medical Oncology Congress. That study showed that in patients undergoing postoperative radiation therapy, 24 months of ADT was superior to 6 months of ADT in improving both time to salvage ADT and MFS.
However, Dr. Nguyen emphasized that it would have to be formally tested, to see if “FORMULA-509 is performing in the ballpark of what 24 months of ADT would do.
“And I think that compared to 6 months of ADT, we can say it is certainly performing in the ballpark,” he said. “So, for patients with higher risk features, intensifying 6 months of ADT, I think, may be an appealing alternative to lengthening the ADT duration to 24 months.”
He added that this concept would be formally tested in the upcoming PROSTATE IQ study.
Strong evidence, standardization needed
In a discussion of the paper, Tyler Seibert, MD, PhD, of the University of California San Diego, said that “escalation by 24 months has the strongest evidence today, specifically from the RADICALS-HD trial, with more than 1,500 men with 10 years of follow-up and a clear statistically significant result.
“Intensification for 6 months is a very compelling concept, as most patients are not getting 2 years of androgen deprivation therapy at this point post prostatectomy,” he continued. “While we await the long term follow-up of this study and the pending PROSTATE IQ trial, and if only 6 months of therapy is acceptable or feasible, the FORMULA-509 [trial] provides convincing evidence that select patients would benefit from intensification with AAP and apalutamide.”
Another expert weighed in on the data. Approached by this news organization for an independent comment, Jeff M. Michalski, MD, MBA, professor of radiation oncology at Washington University, St Louis, and president of the American Society of Radiation Oncology, noted a few issues in the study.
He said that standards had changed since this study was first approved and had begun accrual several years ago. “In context of today’s era, the current standard is to do a PET scan if patients have a chemical failure after surgery,” he said. “The PSA levels of patients who were treated [in this trial] were very high, and many patients do not want to wait until they reach that level.”
Dr. Michalski also pointed out the number of patients getting radiation was less than the number who had node-positive disease. “This shows that patients had received suboptimal therapy late in the disease,” he said.
Overall, most patients in the study did not receive lymph node radiation, even though they had high-risk features. “A recent study of almost 1,800 patients that was published in The Lancet found that there is a benefit to pelvic lymph node radiation,” he said. “Because it wasn’t mandated, most of the patients did not receive pelvic lymph node radiation, which we now understand offers some benefit.”
The reasons for not giving pelvic radiation to these men is unclear. “Treatment was left at the discretion of the physician and this could create bias,” Dr. Michalski said. “It could drive one arm more than another.”
The study also wasn’t controlled for pelvic radiation. “Most of the nodal positive patients received it, but the other patients were undertreated,” he noted.
Dr. Michalski added that he hopes that in the forthcoming PROSTATE IQ study, lymph node radiation and imaging are standardized.
The trial was supported by Janssen Oncology. Dr. Nguyen disclosed relationships with, and/or support from, Volatilyx, Bayer, Blue Earth Diagnostics, Boston Scientific, Janssen Oncology, Myovant Sciences, Astellas Pharma, and Janssen. Dr. Seibert disclosed relationships with, and/or support from, CorTechs Labs, Varian Medical Systems, and GE Healthcare.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – For patients with prostate cancer who have unfavorable features and a detectable PSA following a radical prostatectomy, the standard of care is treatment with 6 months of a gonadotropin-releasing hormone (GnRH) agonist with salvage radiation therapy (SRT), as established by the GETUG-AFU 16 trial.
A new trial, dubbed FORMULA-509, explored whether outcomes could be improved by intensifying the drug treatment by adding 6 months of abiraterone acetate plus prednisone as well as apalutamide on top of the GnRH agonist alongside the salvage radiotherapy.
However, the combination did significantly improve PFS and MFS in a subset of men with PSA levels greater than 0.5 ng/mL.
“Although this primary analysis did not meet the prespecified threshold for statistical significance, it does strongly suggest that the addition of abiraterone acetate/prednisone/apalutamide to salvage radiotherapy plus 6 months of ADT [androgen deprivation therapy] may improve progression-free survival and metastasis-free survival,” said lead author Paul L. Nguyen, MD, of the Dana-Farber Cancer Institute in Boston, and professor of radiation oncology at Harvard Medical School.
“This may be particularly evident in the subgroup of patients with PSA greater that 0.5 ng/mL where a preplanned subgroup analysis by stratification factors observed a statistically significant benefit for both progression-free survival and metastasis-free survival,” he said. “Six months of intensified ADT with next generation anti-androgens may provide an attractive alternative to lengthening ADT for patients with rising PSA and unfavorable features after radical prostatectomy.”
The study results were presented at the ASCO Genitourinary Cancers Symposium.
Benefit in subset
The FORMULA-509 trial included 305 patients with PSA ≥ 0.1 ng/mL who had undergone a radical prostatectomy, and who had one or more unfavorable risk features (Gleason 8-10 disease, PSA > 0.5 ng/mL, pT3/T4, pN1 or radiographic N1, PSA doubling time < 10 months, negative margins, persistent PSA, gross local/regional disease).
“This was a pretty high-risk population,” Dr. Nguyen emphasized, as 35% had Gleason score of 9, about a third (31%) a PSA >0.5, and 29% were pathologic node positive.
All patients received salvage radiotherapy plus 6 months of GnRH agonist (bicalutamide 50 mg), and half were randomly assigned to also receive abiraterone acetate/prednisone 1,000 mg/5 mg + apalutamide 240 mg daily.
At a median follow-up of 34 months, the 3-year PFS rate was 74.9% in the AAP-apalutamide arm vs. 68.5% for the control group (hazard ratio [HR], 0.71; P = .06), and the 3-year MFS rate was 90.6% vs. 87.2%, respectively (HR, 0.57; P = .05).
In the subset of patients with a PSA greater than 0.5 ng/mL, the 3-year PFS and MFS rates were significantly higher with in the AAP-apalutamide group: the 3-year PFS rate was 67.2% vs. 46.8% (HR, 0.50; P = .03), and the 3-year MFS rate was 84.3% vs. 66.1% (HR, 0.32; P = .02).
Adverse events were consistent with the known safety profiles of the agents being studied, Dr. Nguyen noted. The most common toxicities for AAP-apalutamide vs. controls were hypertension (21.8% vs. 13.3%), maculopapular rash (11.5% vs. 0.6%), diarrhea (8.5% vs. 4.8%), and fatigue (7.9% vs. 6.1%).
Dr. Nguyen noted that even though “we’re not supposed to compare clinical trials,” the results of this study appeared to compare favorably with those of another trial, RADICALS-HD, which was presented at the 2022 European Society of Medical Oncology Congress. That study showed that in patients undergoing postoperative radiation therapy, 24 months of ADT was superior to 6 months of ADT in improving both time to salvage ADT and MFS.
However, Dr. Nguyen emphasized that it would have to be formally tested, to see if “FORMULA-509 is performing in the ballpark of what 24 months of ADT would do.
“And I think that compared to 6 months of ADT, we can say it is certainly performing in the ballpark,” he said. “So, for patients with higher risk features, intensifying 6 months of ADT, I think, may be an appealing alternative to lengthening the ADT duration to 24 months.”
He added that this concept would be formally tested in the upcoming PROSTATE IQ study.
Strong evidence, standardization needed
In a discussion of the paper, Tyler Seibert, MD, PhD, of the University of California San Diego, said that “escalation by 24 months has the strongest evidence today, specifically from the RADICALS-HD trial, with more than 1,500 men with 10 years of follow-up and a clear statistically significant result.
“Intensification for 6 months is a very compelling concept, as most patients are not getting 2 years of androgen deprivation therapy at this point post prostatectomy,” he continued. “While we await the long term follow-up of this study and the pending PROSTATE IQ trial, and if only 6 months of therapy is acceptable or feasible, the FORMULA-509 [trial] provides convincing evidence that select patients would benefit from intensification with AAP and apalutamide.”
Another expert weighed in on the data. Approached by this news organization for an independent comment, Jeff M. Michalski, MD, MBA, professor of radiation oncology at Washington University, St Louis, and president of the American Society of Radiation Oncology, noted a few issues in the study.
He said that standards had changed since this study was first approved and had begun accrual several years ago. “In context of today’s era, the current standard is to do a PET scan if patients have a chemical failure after surgery,” he said. “The PSA levels of patients who were treated [in this trial] were very high, and many patients do not want to wait until they reach that level.”
Dr. Michalski also pointed out the number of patients getting radiation was less than the number who had node-positive disease. “This shows that patients had received suboptimal therapy late in the disease,” he said.
Overall, most patients in the study did not receive lymph node radiation, even though they had high-risk features. “A recent study of almost 1,800 patients that was published in The Lancet found that there is a benefit to pelvic lymph node radiation,” he said. “Because it wasn’t mandated, most of the patients did not receive pelvic lymph node radiation, which we now understand offers some benefit.”
The reasons for not giving pelvic radiation to these men is unclear. “Treatment was left at the discretion of the physician and this could create bias,” Dr. Michalski said. “It could drive one arm more than another.”
The study also wasn’t controlled for pelvic radiation. “Most of the nodal positive patients received it, but the other patients were undertreated,” he noted.
Dr. Michalski added that he hopes that in the forthcoming PROSTATE IQ study, lymph node radiation and imaging are standardized.
The trial was supported by Janssen Oncology. Dr. Nguyen disclosed relationships with, and/or support from, Volatilyx, Bayer, Blue Earth Diagnostics, Boston Scientific, Janssen Oncology, Myovant Sciences, Astellas Pharma, and Janssen. Dr. Seibert disclosed relationships with, and/or support from, CorTechs Labs, Varian Medical Systems, and GE Healthcare.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – For patients with prostate cancer who have unfavorable features and a detectable PSA following a radical prostatectomy, the standard of care is treatment with 6 months of a gonadotropin-releasing hormone (GnRH) agonist with salvage radiation therapy (SRT), as established by the GETUG-AFU 16 trial.
A new trial, dubbed FORMULA-509, explored whether outcomes could be improved by intensifying the drug treatment by adding 6 months of abiraterone acetate plus prednisone as well as apalutamide on top of the GnRH agonist alongside the salvage radiotherapy.
However, the combination did significantly improve PFS and MFS in a subset of men with PSA levels greater than 0.5 ng/mL.
“Although this primary analysis did not meet the prespecified threshold for statistical significance, it does strongly suggest that the addition of abiraterone acetate/prednisone/apalutamide to salvage radiotherapy plus 6 months of ADT [androgen deprivation therapy] may improve progression-free survival and metastasis-free survival,” said lead author Paul L. Nguyen, MD, of the Dana-Farber Cancer Institute in Boston, and professor of radiation oncology at Harvard Medical School.
“This may be particularly evident in the subgroup of patients with PSA greater that 0.5 ng/mL where a preplanned subgroup analysis by stratification factors observed a statistically significant benefit for both progression-free survival and metastasis-free survival,” he said. “Six months of intensified ADT with next generation anti-androgens may provide an attractive alternative to lengthening ADT for patients with rising PSA and unfavorable features after radical prostatectomy.”
The study results were presented at the ASCO Genitourinary Cancers Symposium.
Benefit in subset
The FORMULA-509 trial included 305 patients with PSA ≥ 0.1 ng/mL who had undergone a radical prostatectomy, and who had one or more unfavorable risk features (Gleason 8-10 disease, PSA > 0.5 ng/mL, pT3/T4, pN1 or radiographic N1, PSA doubling time < 10 months, negative margins, persistent PSA, gross local/regional disease).
“This was a pretty high-risk population,” Dr. Nguyen emphasized, as 35% had Gleason score of 9, about a third (31%) a PSA >0.5, and 29% were pathologic node positive.
All patients received salvage radiotherapy plus 6 months of GnRH agonist (bicalutamide 50 mg), and half were randomly assigned to also receive abiraterone acetate/prednisone 1,000 mg/5 mg + apalutamide 240 mg daily.
At a median follow-up of 34 months, the 3-year PFS rate was 74.9% in the AAP-apalutamide arm vs. 68.5% for the control group (hazard ratio [HR], 0.71; P = .06), and the 3-year MFS rate was 90.6% vs. 87.2%, respectively (HR, 0.57; P = .05).
In the subset of patients with a PSA greater than 0.5 ng/mL, the 3-year PFS and MFS rates were significantly higher with in the AAP-apalutamide group: the 3-year PFS rate was 67.2% vs. 46.8% (HR, 0.50; P = .03), and the 3-year MFS rate was 84.3% vs. 66.1% (HR, 0.32; P = .02).
Adverse events were consistent with the known safety profiles of the agents being studied, Dr. Nguyen noted. The most common toxicities for AAP-apalutamide vs. controls were hypertension (21.8% vs. 13.3%), maculopapular rash (11.5% vs. 0.6%), diarrhea (8.5% vs. 4.8%), and fatigue (7.9% vs. 6.1%).
Dr. Nguyen noted that even though “we’re not supposed to compare clinical trials,” the results of this study appeared to compare favorably with those of another trial, RADICALS-HD, which was presented at the 2022 European Society of Medical Oncology Congress. That study showed that in patients undergoing postoperative radiation therapy, 24 months of ADT was superior to 6 months of ADT in improving both time to salvage ADT and MFS.
However, Dr. Nguyen emphasized that it would have to be formally tested, to see if “FORMULA-509 is performing in the ballpark of what 24 months of ADT would do.
“And I think that compared to 6 months of ADT, we can say it is certainly performing in the ballpark,” he said. “So, for patients with higher risk features, intensifying 6 months of ADT, I think, may be an appealing alternative to lengthening the ADT duration to 24 months.”
He added that this concept would be formally tested in the upcoming PROSTATE IQ study.
Strong evidence, standardization needed
In a discussion of the paper, Tyler Seibert, MD, PhD, of the University of California San Diego, said that “escalation by 24 months has the strongest evidence today, specifically from the RADICALS-HD trial, with more than 1,500 men with 10 years of follow-up and a clear statistically significant result.
“Intensification for 6 months is a very compelling concept, as most patients are not getting 2 years of androgen deprivation therapy at this point post prostatectomy,” he continued. “While we await the long term follow-up of this study and the pending PROSTATE IQ trial, and if only 6 months of therapy is acceptable or feasible, the FORMULA-509 [trial] provides convincing evidence that select patients would benefit from intensification with AAP and apalutamide.”
Another expert weighed in on the data. Approached by this news organization for an independent comment, Jeff M. Michalski, MD, MBA, professor of radiation oncology at Washington University, St Louis, and president of the American Society of Radiation Oncology, noted a few issues in the study.
He said that standards had changed since this study was first approved and had begun accrual several years ago. “In context of today’s era, the current standard is to do a PET scan if patients have a chemical failure after surgery,” he said. “The PSA levels of patients who were treated [in this trial] were very high, and many patients do not want to wait until they reach that level.”
Dr. Michalski also pointed out the number of patients getting radiation was less than the number who had node-positive disease. “This shows that patients had received suboptimal therapy late in the disease,” he said.
Overall, most patients in the study did not receive lymph node radiation, even though they had high-risk features. “A recent study of almost 1,800 patients that was published in The Lancet found that there is a benefit to pelvic lymph node radiation,” he said. “Because it wasn’t mandated, most of the patients did not receive pelvic lymph node radiation, which we now understand offers some benefit.”
The reasons for not giving pelvic radiation to these men is unclear. “Treatment was left at the discretion of the physician and this could create bias,” Dr. Michalski said. “It could drive one arm more than another.”
The study also wasn’t controlled for pelvic radiation. “Most of the nodal positive patients received it, but the other patients were undertreated,” he noted.
Dr. Michalski added that he hopes that in the forthcoming PROSTATE IQ study, lymph node radiation and imaging are standardized.
The trial was supported by Janssen Oncology. Dr. Nguyen disclosed relationships with, and/or support from, Volatilyx, Bayer, Blue Earth Diagnostics, Boston Scientific, Janssen Oncology, Myovant Sciences, Astellas Pharma, and Janssen. Dr. Seibert disclosed relationships with, and/or support from, CorTechs Labs, Varian Medical Systems, and GE Healthcare.
A version of this article first appeared on Medscape.com.
AT ASCO GU 2023