User login
Elevated PCSK9 levels associated with psoriasis suggest new treatment target
A Mendelian randomization study employing data from nearly 300,000 individuals has linked elevated levels of the PCSK9 enzyme with an increased risk of psoriasis, suggesting it might be targetable as an intervention.
. Conversely, psoriasis risk did not appear to be affected when LDL-C was reduced by other pathways of lipid control.
This study “suggests that PCSK9 inhibition is causally associated with reduced risk of psoriasis,” reported a team of investigators led by Sizheng Steven Zhao, MD, PhD, of the division of musculoskeletal and dermatological sciences, University of Manchester (England). “Existing PCSK9 inhibitors hold potential as therapeutic targets for prevention, and possibly treatment, of psoriasis, although further clinical studies are needed,” they concluded.
In an interview, Dr. Zhao also noted that it will be interesting to look at psoriasis susceptibility in post hoc analyses of large randomized controlled trials of PCSK9 inhibitors for cardiovascular disease.
“Genetically proxied” inhibition of HMG-CoA reductase, which is targeted by statins, and NPC1L1 which is targeted by ezetimibe, “were not associated with psoriasis risk,” the investigators reported in the study, published in JAMA Dermatology.
Abnormal lipid metabolism is sufficiently common among people with psoriasis that screening in patients with moderate to severe disease is recommended in 2019 psoriasis guidelines from the American Academy of Dermatology and the National Psoriasis Foundation. However, the link between these diseases is unclear. This study was launched to explore genetically proxied relationships between psoriasis and LDL-C reductions as well as specific treatments for elevated LDL-C.
Mendelian randomizations were applied to deidentified data from two sources, a UK biobank and FinnGen, a Finnish-based project for identifying genotype-to-phenotype correlations. Genetic proxies for these variables were established on the basis of genomewide association studies on large population samples.
Ultimately, 34 genetic variants were selected to proxy for lipid lowering by PCSK9, 19 were selected to proxy for HMG-CoA reductase, and 9 for NPC1L1. In the Mendelian analyses performed on the two sources, genetically proxied PCSK9 inhibition was associated with about a 30% reduction in the odds ratio of psoriasis (OR, 0.69; P = .003). There were no robust associations with proxies for reductions in either HMG-CoA reductase or NPC1L1.
In sensitivity analyses, there was no evidence of bias from pleiotropy or genetic confounding, according to Dr. Zhao and his coauthors, who noted that the relationship between reductions in PCSK9 and reduced risk of psoriasis appeared to be independent of change in circulating LDL-C.
Given the prior evidence implicating the PCSK9 enzyme in psoriasis risk, “this is an exciting study that really highlights the importance of studying and targeting lipid metabolism in psoriasis for a few reasons,” according to Michael S. Garshick, MD, a researcher, cardiologist, and director of the cardio-rheumatology program, New York University Langone Health.
An investigator who has participated in several studies evaluating the relationship between cardiovascular risk and psoriasis, Dr. Garshick said there is increasing interest in PCSK9 as a biomarker or even a mediator of inflammation independent of blood lipid levels.
“In psoriasis regarding PCSK9, we and others have shown PCSK9 is elevated in psoriatic lesion skin, and studies are starting to investigate the unique lipidomic profile in psoriasis,” Dr. Garshick said in an interview. The study he led that showed elevated PCSK9 levels in psoriatic skin was published in 2021 in the Journal of Investigative Dermatology.
While the Mendelian randomization provides only “an inference” that PCSK9 plays a role in mediating risk of psoriasis, Dr. Zhao and coauthors cited numerous studies linking elevated PCSK9 to psoriasis pathophysiology. This not only includes the elevated PCSK9 expression in psoriatic plaques as shown by Dr. Garshick and others but several sets of experimental evidence linking PCSK9 to inflammatory pathways, including upregulation of interleukin-17 and stimulation of macrophage activation.
While Dr. Zhao and coauthors suggested that clinical trials are now needed to test the potential of PCSK9 inhibitors to modify the risk of psoriasis, Dr. Garshick indicated that there are numerous variables to unravel in the relationship between elevated lipids, PCSK9, and psoriasis.
“In our own studies, we did see a statistical correlation between circulating PCSK9 and psoriasis severity,” Dr. Garshick said. But he added, “I think we are just beginning to understand the functions of circulating (extrahepatic) PCSK9 independent of lipid metabolism.”
While he is intrigued by the evidence that PCSK9 is linked to systemic inflammation, he pointed out that several medications used to treat dyslipidemias, such as statins, are associated with an anti-inflammatory effect.
This study “further emphasizes the need to conduct clinical trials treating dyslipidemia in psoriasis, including the targeting of PCSK9, whether it is with statins with lipid lowering and potential pleiotropic anti-inflammatory properties or PCSK9 inhibition,” he said. If positive, “both would be exciting.“
From a cardiologist’s point of view, there is an upside for including patients with psoriasis in lipid-lowering trials even if the effect on psoriasis is modest. Either way, “you still get the lipid-lowering benefit, which is important for reducing atherosclerotic cardiovascular disease,” Dr. Garshick said.
Dr. Zhao reported financial relationships with UCB, although UCB did not provide funding for this study. One author reported grants from Versus Arthritis and the National Institute for Health Research Manchester Biomedical Research Centre during the study, grants from Bristol Myers Squibb, Galapagos, and Pfizer, and personal fees from Chugai Roche outside the submitted work. No other disclosures were reported. The study was supported by grants from Versus Arthritis and the NIHR Manchester Biomedical Research Centre. Dr. Garshick reported financial relationships with AbbVie and Horizon Therapeutics.
A Mendelian randomization study employing data from nearly 300,000 individuals has linked elevated levels of the PCSK9 enzyme with an increased risk of psoriasis, suggesting it might be targetable as an intervention.
. Conversely, psoriasis risk did not appear to be affected when LDL-C was reduced by other pathways of lipid control.
This study “suggests that PCSK9 inhibition is causally associated with reduced risk of psoriasis,” reported a team of investigators led by Sizheng Steven Zhao, MD, PhD, of the division of musculoskeletal and dermatological sciences, University of Manchester (England). “Existing PCSK9 inhibitors hold potential as therapeutic targets for prevention, and possibly treatment, of psoriasis, although further clinical studies are needed,” they concluded.
In an interview, Dr. Zhao also noted that it will be interesting to look at psoriasis susceptibility in post hoc analyses of large randomized controlled trials of PCSK9 inhibitors for cardiovascular disease.
“Genetically proxied” inhibition of HMG-CoA reductase, which is targeted by statins, and NPC1L1 which is targeted by ezetimibe, “were not associated with psoriasis risk,” the investigators reported in the study, published in JAMA Dermatology.
Abnormal lipid metabolism is sufficiently common among people with psoriasis that screening in patients with moderate to severe disease is recommended in 2019 psoriasis guidelines from the American Academy of Dermatology and the National Psoriasis Foundation. However, the link between these diseases is unclear. This study was launched to explore genetically proxied relationships between psoriasis and LDL-C reductions as well as specific treatments for elevated LDL-C.
Mendelian randomizations were applied to deidentified data from two sources, a UK biobank and FinnGen, a Finnish-based project for identifying genotype-to-phenotype correlations. Genetic proxies for these variables were established on the basis of genomewide association studies on large population samples.
Ultimately, 34 genetic variants were selected to proxy for lipid lowering by PCSK9, 19 were selected to proxy for HMG-CoA reductase, and 9 for NPC1L1. In the Mendelian analyses performed on the two sources, genetically proxied PCSK9 inhibition was associated with about a 30% reduction in the odds ratio of psoriasis (OR, 0.69; P = .003). There were no robust associations with proxies for reductions in either HMG-CoA reductase or NPC1L1.
In sensitivity analyses, there was no evidence of bias from pleiotropy or genetic confounding, according to Dr. Zhao and his coauthors, who noted that the relationship between reductions in PCSK9 and reduced risk of psoriasis appeared to be independent of change in circulating LDL-C.
Given the prior evidence implicating the PCSK9 enzyme in psoriasis risk, “this is an exciting study that really highlights the importance of studying and targeting lipid metabolism in psoriasis for a few reasons,” according to Michael S. Garshick, MD, a researcher, cardiologist, and director of the cardio-rheumatology program, New York University Langone Health.
An investigator who has participated in several studies evaluating the relationship between cardiovascular risk and psoriasis, Dr. Garshick said there is increasing interest in PCSK9 as a biomarker or even a mediator of inflammation independent of blood lipid levels.
“In psoriasis regarding PCSK9, we and others have shown PCSK9 is elevated in psoriatic lesion skin, and studies are starting to investigate the unique lipidomic profile in psoriasis,” Dr. Garshick said in an interview. The study he led that showed elevated PCSK9 levels in psoriatic skin was published in 2021 in the Journal of Investigative Dermatology.
While the Mendelian randomization provides only “an inference” that PCSK9 plays a role in mediating risk of psoriasis, Dr. Zhao and coauthors cited numerous studies linking elevated PCSK9 to psoriasis pathophysiology. This not only includes the elevated PCSK9 expression in psoriatic plaques as shown by Dr. Garshick and others but several sets of experimental evidence linking PCSK9 to inflammatory pathways, including upregulation of interleukin-17 and stimulation of macrophage activation.
While Dr. Zhao and coauthors suggested that clinical trials are now needed to test the potential of PCSK9 inhibitors to modify the risk of psoriasis, Dr. Garshick indicated that there are numerous variables to unravel in the relationship between elevated lipids, PCSK9, and psoriasis.
“In our own studies, we did see a statistical correlation between circulating PCSK9 and psoriasis severity,” Dr. Garshick said. But he added, “I think we are just beginning to understand the functions of circulating (extrahepatic) PCSK9 independent of lipid metabolism.”
While he is intrigued by the evidence that PCSK9 is linked to systemic inflammation, he pointed out that several medications used to treat dyslipidemias, such as statins, are associated with an anti-inflammatory effect.
This study “further emphasizes the need to conduct clinical trials treating dyslipidemia in psoriasis, including the targeting of PCSK9, whether it is with statins with lipid lowering and potential pleiotropic anti-inflammatory properties or PCSK9 inhibition,” he said. If positive, “both would be exciting.“
From a cardiologist’s point of view, there is an upside for including patients with psoriasis in lipid-lowering trials even if the effect on psoriasis is modest. Either way, “you still get the lipid-lowering benefit, which is important for reducing atherosclerotic cardiovascular disease,” Dr. Garshick said.
Dr. Zhao reported financial relationships with UCB, although UCB did not provide funding for this study. One author reported grants from Versus Arthritis and the National Institute for Health Research Manchester Biomedical Research Centre during the study, grants from Bristol Myers Squibb, Galapagos, and Pfizer, and personal fees from Chugai Roche outside the submitted work. No other disclosures were reported. The study was supported by grants from Versus Arthritis and the NIHR Manchester Biomedical Research Centre. Dr. Garshick reported financial relationships with AbbVie and Horizon Therapeutics.
A Mendelian randomization study employing data from nearly 300,000 individuals has linked elevated levels of the PCSK9 enzyme with an increased risk of psoriasis, suggesting it might be targetable as an intervention.
. Conversely, psoriasis risk did not appear to be affected when LDL-C was reduced by other pathways of lipid control.
This study “suggests that PCSK9 inhibition is causally associated with reduced risk of psoriasis,” reported a team of investigators led by Sizheng Steven Zhao, MD, PhD, of the division of musculoskeletal and dermatological sciences, University of Manchester (England). “Existing PCSK9 inhibitors hold potential as therapeutic targets for prevention, and possibly treatment, of psoriasis, although further clinical studies are needed,” they concluded.
In an interview, Dr. Zhao also noted that it will be interesting to look at psoriasis susceptibility in post hoc analyses of large randomized controlled trials of PCSK9 inhibitors for cardiovascular disease.
“Genetically proxied” inhibition of HMG-CoA reductase, which is targeted by statins, and NPC1L1 which is targeted by ezetimibe, “were not associated with psoriasis risk,” the investigators reported in the study, published in JAMA Dermatology.
Abnormal lipid metabolism is sufficiently common among people with psoriasis that screening in patients with moderate to severe disease is recommended in 2019 psoriasis guidelines from the American Academy of Dermatology and the National Psoriasis Foundation. However, the link between these diseases is unclear. This study was launched to explore genetically proxied relationships between psoriasis and LDL-C reductions as well as specific treatments for elevated LDL-C.
Mendelian randomizations were applied to deidentified data from two sources, a UK biobank and FinnGen, a Finnish-based project for identifying genotype-to-phenotype correlations. Genetic proxies for these variables were established on the basis of genomewide association studies on large population samples.
Ultimately, 34 genetic variants were selected to proxy for lipid lowering by PCSK9, 19 were selected to proxy for HMG-CoA reductase, and 9 for NPC1L1. In the Mendelian analyses performed on the two sources, genetically proxied PCSK9 inhibition was associated with about a 30% reduction in the odds ratio of psoriasis (OR, 0.69; P = .003). There were no robust associations with proxies for reductions in either HMG-CoA reductase or NPC1L1.
In sensitivity analyses, there was no evidence of bias from pleiotropy or genetic confounding, according to Dr. Zhao and his coauthors, who noted that the relationship between reductions in PCSK9 and reduced risk of psoriasis appeared to be independent of change in circulating LDL-C.
Given the prior evidence implicating the PCSK9 enzyme in psoriasis risk, “this is an exciting study that really highlights the importance of studying and targeting lipid metabolism in psoriasis for a few reasons,” according to Michael S. Garshick, MD, a researcher, cardiologist, and director of the cardio-rheumatology program, New York University Langone Health.
An investigator who has participated in several studies evaluating the relationship between cardiovascular risk and psoriasis, Dr. Garshick said there is increasing interest in PCSK9 as a biomarker or even a mediator of inflammation independent of blood lipid levels.
“In psoriasis regarding PCSK9, we and others have shown PCSK9 is elevated in psoriatic lesion skin, and studies are starting to investigate the unique lipidomic profile in psoriasis,” Dr. Garshick said in an interview. The study he led that showed elevated PCSK9 levels in psoriatic skin was published in 2021 in the Journal of Investigative Dermatology.
While the Mendelian randomization provides only “an inference” that PCSK9 plays a role in mediating risk of psoriasis, Dr. Zhao and coauthors cited numerous studies linking elevated PCSK9 to psoriasis pathophysiology. This not only includes the elevated PCSK9 expression in psoriatic plaques as shown by Dr. Garshick and others but several sets of experimental evidence linking PCSK9 to inflammatory pathways, including upregulation of interleukin-17 and stimulation of macrophage activation.
While Dr. Zhao and coauthors suggested that clinical trials are now needed to test the potential of PCSK9 inhibitors to modify the risk of psoriasis, Dr. Garshick indicated that there are numerous variables to unravel in the relationship between elevated lipids, PCSK9, and psoriasis.
“In our own studies, we did see a statistical correlation between circulating PCSK9 and psoriasis severity,” Dr. Garshick said. But he added, “I think we are just beginning to understand the functions of circulating (extrahepatic) PCSK9 independent of lipid metabolism.”
While he is intrigued by the evidence that PCSK9 is linked to systemic inflammation, he pointed out that several medications used to treat dyslipidemias, such as statins, are associated with an anti-inflammatory effect.
This study “further emphasizes the need to conduct clinical trials treating dyslipidemia in psoriasis, including the targeting of PCSK9, whether it is with statins with lipid lowering and potential pleiotropic anti-inflammatory properties or PCSK9 inhibition,” he said. If positive, “both would be exciting.“
From a cardiologist’s point of view, there is an upside for including patients with psoriasis in lipid-lowering trials even if the effect on psoriasis is modest. Either way, “you still get the lipid-lowering benefit, which is important for reducing atherosclerotic cardiovascular disease,” Dr. Garshick said.
Dr. Zhao reported financial relationships with UCB, although UCB did not provide funding for this study. One author reported grants from Versus Arthritis and the National Institute for Health Research Manchester Biomedical Research Centre during the study, grants from Bristol Myers Squibb, Galapagos, and Pfizer, and personal fees from Chugai Roche outside the submitted work. No other disclosures were reported. The study was supported by grants from Versus Arthritis and the NIHR Manchester Biomedical Research Centre. Dr. Garshick reported financial relationships with AbbVie and Horizon Therapeutics.
FROM JAMA DERMATOLOGY
Fluorescence-optical imaging may detect preclinical PsA
Fluorescence-optical imaging (FOI) identified early signs of psoriatic arthritis, based on data from 2 years of follow-up of a cohort of 389 adults at 14 rheumatology centers.
Approximately 25% of individuals with psoriasis go on to develop psoriatic arthritis (PsA), but there are no validated biomarkers to identify patients at risk for progression to PsA, Michaela Koehm, MD, of Goethe University, Frankfurt am Main, Germany, and colleagues wrote in RMD Open.
FOI is a technique that allows assessment of changes in microvascularization and subdermal skin inflammation, and because individuals with psoriasis who develop PsA have shown changes in blood vessel formation in the early stages of disease, the researchers sought to determine if FOI could be used to predict early PsA.
The researchers conducted a multicenter, two-part observational cohort study. The two parts, known as XCITING and XTEND, included 389 adults aged 18-75 years with plaque psoriasis deemed at increased risk for PsA. The patients were seen at rheumatology sites in Germany between Jan. 28, 2014, and March 16, 2017. The XTEND study included clinic visits 18-24 months after the XCITING study.
Participants underwent a complete clinical examination, with musculoskeletal ultrasound (MSUS) and FOI on both hands at a single visit. Those with positive FOI findings not seen with clinical exam or MSUS underwent MRI within 7 days. Patients with positive FOI but negative findings on clinical exam, MSUS, and MRI were followed for 2 years in the XTEND study.
The primary outcome was the ability of FOI to detect musculoskeletal inflammation, compared with clinical examination and MSUS.
Overall, 50% of the patients were diagnosed with PsA. A total of 116 (30%) had positive FOI findings; complete MRI data were available for 108 of these patients, including 68 negative MRIs and 40 positive MRIs.
In the XTEND study, another 12% of patients who were positive on FOI but not on MRI also developed PsA by the end of the 2-year follow-up. In comparison, the researchers noted that “literature data on yearly incidence rates [of PsA] in different national cohorts indicate an incidence rate of approximately 4.3% per year.”
A total of 149 of the 196 patients with PsA confirmed by either clinical exam or MSUS were also positive on FOI, yielding a sensitivity of 76.0%. The specificity of FOI was 39.5%.
The sensitive visualization of musculoskeletal inflammation possible with FOI “may exceed its ability to detect clinically manifest PsA at high sensitivity or specificity, but early visualization is arguably of greater value as other imaging methods are currently available for detection of later stages of PsA,” the researchers wrote in their discussion. “A technique allowing early identification of PsA may be especially valuable for nonrheumatologists, including dermatologists and general practitioners, and help expedite more efficient referral to specialists.”
The findings were limited by several factors, including the nonrandomized design and small subgroup numbers, the researchers noted. Other limitations include the presence of alternative conditions such as osteoarthritis that might have complicated the imaging; the focus only on the hands; and potential variation in FOI assessment related to technical standards such as temperature and positioning.
However, the results support FOI as a safe and effective method of detecting early signs of joint inflammation that could predict increased risk for PsA in psoriasis patients, the researchers said.
The researchers added that more work is needed to evaluate FOI in clinical practice, but FOI has the potential to identify vascularization changes earlier than other imaging modalities and in advance of clinical symptoms.
“Accordingly, FOI may have the potential to improve patient outcomes in PsA by reducing the time to initiation of early treatment,” they concluded.
The study was supported by Fraunhofer ITMP, a nonprofit organization, and a research grant from Pfizer Germany. Some of the researchers disclosed financial relationships with many pharmaceutical companies, including Pfizer.
Fluorescence-optical imaging (FOI) identified early signs of psoriatic arthritis, based on data from 2 years of follow-up of a cohort of 389 adults at 14 rheumatology centers.
Approximately 25% of individuals with psoriasis go on to develop psoriatic arthritis (PsA), but there are no validated biomarkers to identify patients at risk for progression to PsA, Michaela Koehm, MD, of Goethe University, Frankfurt am Main, Germany, and colleagues wrote in RMD Open.
FOI is a technique that allows assessment of changes in microvascularization and subdermal skin inflammation, and because individuals with psoriasis who develop PsA have shown changes in blood vessel formation in the early stages of disease, the researchers sought to determine if FOI could be used to predict early PsA.
The researchers conducted a multicenter, two-part observational cohort study. The two parts, known as XCITING and XTEND, included 389 adults aged 18-75 years with plaque psoriasis deemed at increased risk for PsA. The patients were seen at rheumatology sites in Germany between Jan. 28, 2014, and March 16, 2017. The XTEND study included clinic visits 18-24 months after the XCITING study.
Participants underwent a complete clinical examination, with musculoskeletal ultrasound (MSUS) and FOI on both hands at a single visit. Those with positive FOI findings not seen with clinical exam or MSUS underwent MRI within 7 days. Patients with positive FOI but negative findings on clinical exam, MSUS, and MRI were followed for 2 years in the XTEND study.
The primary outcome was the ability of FOI to detect musculoskeletal inflammation, compared with clinical examination and MSUS.
Overall, 50% of the patients were diagnosed with PsA. A total of 116 (30%) had positive FOI findings; complete MRI data were available for 108 of these patients, including 68 negative MRIs and 40 positive MRIs.
In the XTEND study, another 12% of patients who were positive on FOI but not on MRI also developed PsA by the end of the 2-year follow-up. In comparison, the researchers noted that “literature data on yearly incidence rates [of PsA] in different national cohorts indicate an incidence rate of approximately 4.3% per year.”
A total of 149 of the 196 patients with PsA confirmed by either clinical exam or MSUS were also positive on FOI, yielding a sensitivity of 76.0%. The specificity of FOI was 39.5%.
The sensitive visualization of musculoskeletal inflammation possible with FOI “may exceed its ability to detect clinically manifest PsA at high sensitivity or specificity, but early visualization is arguably of greater value as other imaging methods are currently available for detection of later stages of PsA,” the researchers wrote in their discussion. “A technique allowing early identification of PsA may be especially valuable for nonrheumatologists, including dermatologists and general practitioners, and help expedite more efficient referral to specialists.”
The findings were limited by several factors, including the nonrandomized design and small subgroup numbers, the researchers noted. Other limitations include the presence of alternative conditions such as osteoarthritis that might have complicated the imaging; the focus only on the hands; and potential variation in FOI assessment related to technical standards such as temperature and positioning.
However, the results support FOI as a safe and effective method of detecting early signs of joint inflammation that could predict increased risk for PsA in psoriasis patients, the researchers said.
The researchers added that more work is needed to evaluate FOI in clinical practice, but FOI has the potential to identify vascularization changes earlier than other imaging modalities and in advance of clinical symptoms.
“Accordingly, FOI may have the potential to improve patient outcomes in PsA by reducing the time to initiation of early treatment,” they concluded.
The study was supported by Fraunhofer ITMP, a nonprofit organization, and a research grant from Pfizer Germany. Some of the researchers disclosed financial relationships with many pharmaceutical companies, including Pfizer.
Fluorescence-optical imaging (FOI) identified early signs of psoriatic arthritis, based on data from 2 years of follow-up of a cohort of 389 adults at 14 rheumatology centers.
Approximately 25% of individuals with psoriasis go on to develop psoriatic arthritis (PsA), but there are no validated biomarkers to identify patients at risk for progression to PsA, Michaela Koehm, MD, of Goethe University, Frankfurt am Main, Germany, and colleagues wrote in RMD Open.
FOI is a technique that allows assessment of changes in microvascularization and subdermal skin inflammation, and because individuals with psoriasis who develop PsA have shown changes in blood vessel formation in the early stages of disease, the researchers sought to determine if FOI could be used to predict early PsA.
The researchers conducted a multicenter, two-part observational cohort study. The two parts, known as XCITING and XTEND, included 389 adults aged 18-75 years with plaque psoriasis deemed at increased risk for PsA. The patients were seen at rheumatology sites in Germany between Jan. 28, 2014, and March 16, 2017. The XTEND study included clinic visits 18-24 months after the XCITING study.
Participants underwent a complete clinical examination, with musculoskeletal ultrasound (MSUS) and FOI on both hands at a single visit. Those with positive FOI findings not seen with clinical exam or MSUS underwent MRI within 7 days. Patients with positive FOI but negative findings on clinical exam, MSUS, and MRI were followed for 2 years in the XTEND study.
The primary outcome was the ability of FOI to detect musculoskeletal inflammation, compared with clinical examination and MSUS.
Overall, 50% of the patients were diagnosed with PsA. A total of 116 (30%) had positive FOI findings; complete MRI data were available for 108 of these patients, including 68 negative MRIs and 40 positive MRIs.
In the XTEND study, another 12% of patients who were positive on FOI but not on MRI also developed PsA by the end of the 2-year follow-up. In comparison, the researchers noted that “literature data on yearly incidence rates [of PsA] in different national cohorts indicate an incidence rate of approximately 4.3% per year.”
A total of 149 of the 196 patients with PsA confirmed by either clinical exam or MSUS were also positive on FOI, yielding a sensitivity of 76.0%. The specificity of FOI was 39.5%.
The sensitive visualization of musculoskeletal inflammation possible with FOI “may exceed its ability to detect clinically manifest PsA at high sensitivity or specificity, but early visualization is arguably of greater value as other imaging methods are currently available for detection of later stages of PsA,” the researchers wrote in their discussion. “A technique allowing early identification of PsA may be especially valuable for nonrheumatologists, including dermatologists and general practitioners, and help expedite more efficient referral to specialists.”
The findings were limited by several factors, including the nonrandomized design and small subgroup numbers, the researchers noted. Other limitations include the presence of alternative conditions such as osteoarthritis that might have complicated the imaging; the focus only on the hands; and potential variation in FOI assessment related to technical standards such as temperature and positioning.
However, the results support FOI as a safe and effective method of detecting early signs of joint inflammation that could predict increased risk for PsA in psoriasis patients, the researchers said.
The researchers added that more work is needed to evaluate FOI in clinical practice, but FOI has the potential to identify vascularization changes earlier than other imaging modalities and in advance of clinical symptoms.
“Accordingly, FOI may have the potential to improve patient outcomes in PsA by reducing the time to initiation of early treatment,” they concluded.
The study was supported by Fraunhofer ITMP, a nonprofit organization, and a research grant from Pfizer Germany. Some of the researchers disclosed financial relationships with many pharmaceutical companies, including Pfizer.
FROM RMD OPEN
75 years: A look back on the fascinating history of methotrexate and folate antagonists
If you could go back in time 75 years and tell Dr. Sidney Farber, the developer of methotrexate for cancer therapy, that 21st-century medicine would utilize his specially designed drug more in rheumatology than oncology, he might be surprised. He might scratch his head even more, hearing of his drug sparking interest in still other medical fields, like cardiology.
But drug repurposing is not so uncommon. One classic example is aspirin. Once the most common pain medication and used also in rheumatology, aspirin now finds a range of applications, from colorectal cancer to the prevention of cardiovascular and cerebrovascular thrombosis. Minoxidil is another example, developed for hypertension but used today mostly to stop hair loss. Perhaps most ironic is thalidomide, utilized today for leprosy and multiple myeloma, yet actually contraindicated for its original application, nausea of pregnancy.
Methotrexate, thus, has much in common with other medical treatments, and yet its origin story is as unique and as fascinating as the story of Dr. Farber himself. While this is a rheumatology article, it’s also a story about the origin of a particular rheumatologic treatment, and so the story of that origin will take us mostly through a discussion of hematologic malignancy and of the clinical researcher who dared search for a cure.
Born in 1903, in Buffalo, New York, third of fourteen children of Jewish immigrants from Poland, Dr. Farber grew up in a household that was crowded but academically rigorous. His father, Simon, routinely brought home textbooks, assigning each child a book to read and on which to write a report. His mother, Matilda, was as devoted as her husband to raising the children to succeed in their adopted new country. Upstairs, the children were permitted to speak Yiddish, but downstairs they were required to use only English and German.
As a teen, Dr. Farber lived through the 1918 influenza pandemic that killed at least 50 million people worldwide, including more than 2,000 Buffalonians. This probably helped motivate him to study medicine, but with antisemitism overt in the America of the early 1920s, securing admission to a U.S. medical school was close to impossible. So, in what now seems like the greatest of ironies, Dr. Farber began medical studies in Germany, then transferred for the second year to a U.S. program that seemed adequate – Harvard Medical School, from which he graduated in 1927. From there, he trained as a pathologist, focusing ultimately on pediatric pathology. But, frustrated by case after case of malignancy, whose young victims he’d often have to autopsy, Dr. Farber decided that he wanted to advance the pitiful state of cancer therapeutics, especially for hematologic malignancy.
This was a tall order in the 1930s and early 1940s, when cancer therapeutics consisted only of surgical resection and very primitive forms of radiation therapy. Applicable only to neoplasia that was localized, these options were useless against malignancies in the blood, like acute lymphoblastic leukemia (ALL), but by January 1948 there was at least one glimmer of hope. At that time, one patient with ALL, 2-year-old Robert Sandler, was too ill to join his twin brother Elliott for snow play outside their home in the Dorchester section of Boston. Diagnosed back in August, Robert had suffered multiple episodes of fever, anemia, and thrombocytopenia. His illness had enlarged his spleen dramatically and caused pathologic bone fractures with excruciating bone pain, and for a while he couldn’t walk because of pressure on his lower spinal cord. All of this was the result of uncontrolled mitosis and cell division of lymphoblasts, immature lymphocytes. By December, these out-of-control cells had elevated the boy’s white blood cell count to a peak of 70,000/mcL, more than six times the high end of the normal range (4,500-11,000/mcL). This had happened despite treatment with an experimental drug, developed at Boston Children’s Hospital by Dr. Farber and his team, working on the assumption that inhibition of folate metabolism should slow the growth of tumor cells. On Dec. 28, however, Dr. Farber had switched the child to a new drug with a chemical structure just slightly different from the other agent’s.
Merely another chemical modification in a series of attempts by the research team, the new drug, aminopterin, was not expected to do anything dramatic, but Dr. Farber and the team had come such a long way since the middle of 1947, when he’d actually done the opposite of what he was doing now. On the basis of British research from India showing folic acid deficiency as the basis of a common type of anemia in malnourished people, Dr. Farber had reasoned that children with leukemia, who also suffered from anemia, might also benefit from folic acid supplementation. Even without prior rodent testing, Dr. Farber had tried giving the nutrient to patients with ALL, a strategy made possible by the presence of a spectacular chemist working on folic acid synthesis at Farber’s own hospital to help combat folate deficiency. Born into a poor Brahmin family in India, the chemist, Dr. Yellapragada SubbaRow, had begun life with so much stacked against him as to appear even less likely during childhood than the young Dr. Farber to grow up to make major contributions to medicine. Going through childhood with death all around him, Dr. SubbaRow was motivated to study medicine, but getting into medical school had been an uphill fight, given his family’s economic difficulty. Knowing that he’d also face discrimination on account of his low status after receiving admission to a medical program, SubbaRow could have made things a bit easier for himself by living within the norms of the British Imperial system, but as a supporter of Mohandas Gandhi’s nationalist movement, he boycotted British goods. As a medical student, this meant doing things like wearing Indian-made surgical gloves, instead of the English products that were expected of the students. Such actions led Dr. SubbaRow to receive a kind of second-rate medical degree, rather than the prestigious MBBS.
The political situation also led Dr. SubbaRow to emigrate to the United States, where, ironically, his medical degree initially was taken less seriously than it had been taken in his British-occupied homeland. He thus worked in the capacity of a hospital night porter at Peter Bent Brigham Hospital (the future Brigham and Women’s Hospital), doing menial tasks like changing sheets to make ends meet. He studied, however, and made enough of an impression to gain admission to the same institution that also admitted Farber through the backdoor, Harvard Medical School. This launched him into a research career in which he not only would be instrumental in developing folate antagonists and other classes of drugs, but also would make him the codiscoverer of the role of creatine phosphate and ATP in cellular energy metabolism. Sadly, even after obtaining his top-notch American credentials and contributing through his research to what you might say is a good chunk of the biochemistry pathways that first year medical students memorize without ever learning who discovered them, Dr. SubbaRow still faced prejudice for the rest of his life, which turned out to last only until the age of 53. To add insult to injury, he is rarely remembered for his role.
Dr. Farber proceeded with the folic acid supplementation idea in patients with ALL, even though ALL caused a hypoproliferative anemia, whereas anemia from folate deficiency was megaloblastic, meaning that erythrocytes were produced but they were oversized and dysfunctional. Tragically, folic acid had accelerated the disease process in children with ALL, but the process of chemical experimentation aimed at synthesizing folate also produced some compounds that mimicked chemical precursors of folate in a way that made them antifolates, inhibitors of folate metabolism. If folic acid made lymphoblasts grow faster, Dr. Farber had reasoned that antifolates should inhibit their growth. He thus asked the chemistry lab to focus on folate inhibitors. Testing aminopterin, beginning with young Robert Sandler at the end of December, is what proved his hypothesis correct. By late January, aminopterin had brought the child’s WBC count down to the realm of 12,000, just slightly above normal, with symptoms and signs abating as well, and by February, the child could play with his twin brother. It was not a cure; malignant lymphoblasts still showed on microscopy of Robert’s blood. While he and some 15 other children whom Dr. Farber treated in this early trial would all succumb to ALL, they experienced remission lasting several months.
This was a big deal because the concept of chemotherapy was based only on serendipitous observations of WBC counts dropping in soldiers exposed to nitrogen mustard gas during World War I and during an incident in World War II, yet aminopterin had been designed from the ground up. Though difficult to synthesize in quantities, there was no reason for Dr. Farber’s team not to keep tweaking the drug, and so they did. Replacing one hydrogen atom with a methyl group, they turned it into methotrexate.
Proving easier to synthesize and less toxic, methotrexate would become a workhorse for chemotherapy over the next couple of decades, but the capability of both methotrexate and aminopterin to blunt the growth of white blood cells and other cells did not go unnoticed outside the realm of oncology. As early as the 1950s, dermatologists were using aminopterin to treat psoriasis. This led to the approval of methotrexate for psoriasis in 1972.
Meanwhile, like oncology, infectious diseases, aviation medicine, and so many other areas of practice, rheumatology had gotten a major boost from research stemming from World War II. During the war, Dr. Philip Hench of the Mayo Clinic developed cortisone, which pilots used to stay alert and energetic during trans-Atlantic flights. But it turned out that cortisone had a powerful immunosuppressive effect that dramatically improved rheumatoid arthritis, leading Dr. Hench to receive the Nobel Prize in Physiology or Medicine in 1950. By the end of the 1950s, however, the significant side effects of long-term corticosteroid therapy were very clear, so over the next few decades there was a major effort to develop different treatments for RA and other rheumatologic diseases.
Top on the list of such agents was methotrexate, developed for RA in part by Dr. Michael Weinblatt of Brigham and Women’s Hospital in Boston. In the 1980s, Dr. Weinblatt published the first clinical trial showing the benefits of methotrexate for RA patients. This has since developed into a standard treatment, noticeably different from the original malignancy application in that it is a low-dose regimen. Patients taking methotrexate for RA typically receive no more than 25 mg per week orally, and often much less. Rheumatology today includes expertise in keeping long-term methotrexate therapy safe by monitoring liver function and through other routine tests. The routine nature of the therapy has brought methotrexate to the point of beckoning in a realm that Dr. Farber might not have predicted in his wildest imagination: cardiology. This is on account of the growing appreciation of the inflammatory process in the pathophysiology of atherosclerotic heart disease.
Meanwhile, being an antimetabolite, harmful to rapidly dividing cells, the danger of methotrexate to the embryo and fetus was recognized early. This made methotrexate off-limits to pregnant women, yet it also has made the drug useful as an abortifacient. Though not as good for medication abortion in unwanted but thriving pregnancies, where mifepristone/misoprostol has become the regimen of choice, methotrexate has become a workhorse in other obstetrical settings, such as for ending ectopic pregnancy.
Looking at the present and into the future, the potential for this very old medication looks wide open, as if it could go in any direction, so let’s wind up the discussion with the thought that we may be in for some surprises. Rather than jumping deeply into any rheumatologic issue, we spent most of this article weaving through other medical issues, but does this not make today’s story fairly analogous to rheumatology itself?
Dr. Warmflash is a physician from Portland, Ore. He reported no conflicts of interest.
This story was updated 2/10/2023.
A version of this article first appeared on Medscape.com.
If you could go back in time 75 years and tell Dr. Sidney Farber, the developer of methotrexate for cancer therapy, that 21st-century medicine would utilize his specially designed drug more in rheumatology than oncology, he might be surprised. He might scratch his head even more, hearing of his drug sparking interest in still other medical fields, like cardiology.
But drug repurposing is not so uncommon. One classic example is aspirin. Once the most common pain medication and used also in rheumatology, aspirin now finds a range of applications, from colorectal cancer to the prevention of cardiovascular and cerebrovascular thrombosis. Minoxidil is another example, developed for hypertension but used today mostly to stop hair loss. Perhaps most ironic is thalidomide, utilized today for leprosy and multiple myeloma, yet actually contraindicated for its original application, nausea of pregnancy.
Methotrexate, thus, has much in common with other medical treatments, and yet its origin story is as unique and as fascinating as the story of Dr. Farber himself. While this is a rheumatology article, it’s also a story about the origin of a particular rheumatologic treatment, and so the story of that origin will take us mostly through a discussion of hematologic malignancy and of the clinical researcher who dared search for a cure.
Born in 1903, in Buffalo, New York, third of fourteen children of Jewish immigrants from Poland, Dr. Farber grew up in a household that was crowded but academically rigorous. His father, Simon, routinely brought home textbooks, assigning each child a book to read and on which to write a report. His mother, Matilda, was as devoted as her husband to raising the children to succeed in their adopted new country. Upstairs, the children were permitted to speak Yiddish, but downstairs they were required to use only English and German.
As a teen, Dr. Farber lived through the 1918 influenza pandemic that killed at least 50 million people worldwide, including more than 2,000 Buffalonians. This probably helped motivate him to study medicine, but with antisemitism overt in the America of the early 1920s, securing admission to a U.S. medical school was close to impossible. So, in what now seems like the greatest of ironies, Dr. Farber began medical studies in Germany, then transferred for the second year to a U.S. program that seemed adequate – Harvard Medical School, from which he graduated in 1927. From there, he trained as a pathologist, focusing ultimately on pediatric pathology. But, frustrated by case after case of malignancy, whose young victims he’d often have to autopsy, Dr. Farber decided that he wanted to advance the pitiful state of cancer therapeutics, especially for hematologic malignancy.
This was a tall order in the 1930s and early 1940s, when cancer therapeutics consisted only of surgical resection and very primitive forms of radiation therapy. Applicable only to neoplasia that was localized, these options were useless against malignancies in the blood, like acute lymphoblastic leukemia (ALL), but by January 1948 there was at least one glimmer of hope. At that time, one patient with ALL, 2-year-old Robert Sandler, was too ill to join his twin brother Elliott for snow play outside their home in the Dorchester section of Boston. Diagnosed back in August, Robert had suffered multiple episodes of fever, anemia, and thrombocytopenia. His illness had enlarged his spleen dramatically and caused pathologic bone fractures with excruciating bone pain, and for a while he couldn’t walk because of pressure on his lower spinal cord. All of this was the result of uncontrolled mitosis and cell division of lymphoblasts, immature lymphocytes. By December, these out-of-control cells had elevated the boy’s white blood cell count to a peak of 70,000/mcL, more than six times the high end of the normal range (4,500-11,000/mcL). This had happened despite treatment with an experimental drug, developed at Boston Children’s Hospital by Dr. Farber and his team, working on the assumption that inhibition of folate metabolism should slow the growth of tumor cells. On Dec. 28, however, Dr. Farber had switched the child to a new drug with a chemical structure just slightly different from the other agent’s.
Merely another chemical modification in a series of attempts by the research team, the new drug, aminopterin, was not expected to do anything dramatic, but Dr. Farber and the team had come such a long way since the middle of 1947, when he’d actually done the opposite of what he was doing now. On the basis of British research from India showing folic acid deficiency as the basis of a common type of anemia in malnourished people, Dr. Farber had reasoned that children with leukemia, who also suffered from anemia, might also benefit from folic acid supplementation. Even without prior rodent testing, Dr. Farber had tried giving the nutrient to patients with ALL, a strategy made possible by the presence of a spectacular chemist working on folic acid synthesis at Farber’s own hospital to help combat folate deficiency. Born into a poor Brahmin family in India, the chemist, Dr. Yellapragada SubbaRow, had begun life with so much stacked against him as to appear even less likely during childhood than the young Dr. Farber to grow up to make major contributions to medicine. Going through childhood with death all around him, Dr. SubbaRow was motivated to study medicine, but getting into medical school had been an uphill fight, given his family’s economic difficulty. Knowing that he’d also face discrimination on account of his low status after receiving admission to a medical program, SubbaRow could have made things a bit easier for himself by living within the norms of the British Imperial system, but as a supporter of Mohandas Gandhi’s nationalist movement, he boycotted British goods. As a medical student, this meant doing things like wearing Indian-made surgical gloves, instead of the English products that were expected of the students. Such actions led Dr. SubbaRow to receive a kind of second-rate medical degree, rather than the prestigious MBBS.
The political situation also led Dr. SubbaRow to emigrate to the United States, where, ironically, his medical degree initially was taken less seriously than it had been taken in his British-occupied homeland. He thus worked in the capacity of a hospital night porter at Peter Bent Brigham Hospital (the future Brigham and Women’s Hospital), doing menial tasks like changing sheets to make ends meet. He studied, however, and made enough of an impression to gain admission to the same institution that also admitted Farber through the backdoor, Harvard Medical School. This launched him into a research career in which he not only would be instrumental in developing folate antagonists and other classes of drugs, but also would make him the codiscoverer of the role of creatine phosphate and ATP in cellular energy metabolism. Sadly, even after obtaining his top-notch American credentials and contributing through his research to what you might say is a good chunk of the biochemistry pathways that first year medical students memorize without ever learning who discovered them, Dr. SubbaRow still faced prejudice for the rest of his life, which turned out to last only until the age of 53. To add insult to injury, he is rarely remembered for his role.
Dr. Farber proceeded with the folic acid supplementation idea in patients with ALL, even though ALL caused a hypoproliferative anemia, whereas anemia from folate deficiency was megaloblastic, meaning that erythrocytes were produced but they were oversized and dysfunctional. Tragically, folic acid had accelerated the disease process in children with ALL, but the process of chemical experimentation aimed at synthesizing folate also produced some compounds that mimicked chemical precursors of folate in a way that made them antifolates, inhibitors of folate metabolism. If folic acid made lymphoblasts grow faster, Dr. Farber had reasoned that antifolates should inhibit their growth. He thus asked the chemistry lab to focus on folate inhibitors. Testing aminopterin, beginning with young Robert Sandler at the end of December, is what proved his hypothesis correct. By late January, aminopterin had brought the child’s WBC count down to the realm of 12,000, just slightly above normal, with symptoms and signs abating as well, and by February, the child could play with his twin brother. It was not a cure; malignant lymphoblasts still showed on microscopy of Robert’s blood. While he and some 15 other children whom Dr. Farber treated in this early trial would all succumb to ALL, they experienced remission lasting several months.
This was a big deal because the concept of chemotherapy was based only on serendipitous observations of WBC counts dropping in soldiers exposed to nitrogen mustard gas during World War I and during an incident in World War II, yet aminopterin had been designed from the ground up. Though difficult to synthesize in quantities, there was no reason for Dr. Farber’s team not to keep tweaking the drug, and so they did. Replacing one hydrogen atom with a methyl group, they turned it into methotrexate.
Proving easier to synthesize and less toxic, methotrexate would become a workhorse for chemotherapy over the next couple of decades, but the capability of both methotrexate and aminopterin to blunt the growth of white blood cells and other cells did not go unnoticed outside the realm of oncology. As early as the 1950s, dermatologists were using aminopterin to treat psoriasis. This led to the approval of methotrexate for psoriasis in 1972.
Meanwhile, like oncology, infectious diseases, aviation medicine, and so many other areas of practice, rheumatology had gotten a major boost from research stemming from World War II. During the war, Dr. Philip Hench of the Mayo Clinic developed cortisone, which pilots used to stay alert and energetic during trans-Atlantic flights. But it turned out that cortisone had a powerful immunosuppressive effect that dramatically improved rheumatoid arthritis, leading Dr. Hench to receive the Nobel Prize in Physiology or Medicine in 1950. By the end of the 1950s, however, the significant side effects of long-term corticosteroid therapy were very clear, so over the next few decades there was a major effort to develop different treatments for RA and other rheumatologic diseases.
Top on the list of such agents was methotrexate, developed for RA in part by Dr. Michael Weinblatt of Brigham and Women’s Hospital in Boston. In the 1980s, Dr. Weinblatt published the first clinical trial showing the benefits of methotrexate for RA patients. This has since developed into a standard treatment, noticeably different from the original malignancy application in that it is a low-dose regimen. Patients taking methotrexate for RA typically receive no more than 25 mg per week orally, and often much less. Rheumatology today includes expertise in keeping long-term methotrexate therapy safe by monitoring liver function and through other routine tests. The routine nature of the therapy has brought methotrexate to the point of beckoning in a realm that Dr. Farber might not have predicted in his wildest imagination: cardiology. This is on account of the growing appreciation of the inflammatory process in the pathophysiology of atherosclerotic heart disease.
Meanwhile, being an antimetabolite, harmful to rapidly dividing cells, the danger of methotrexate to the embryo and fetus was recognized early. This made methotrexate off-limits to pregnant women, yet it also has made the drug useful as an abortifacient. Though not as good for medication abortion in unwanted but thriving pregnancies, where mifepristone/misoprostol has become the regimen of choice, methotrexate has become a workhorse in other obstetrical settings, such as for ending ectopic pregnancy.
Looking at the present and into the future, the potential for this very old medication looks wide open, as if it could go in any direction, so let’s wind up the discussion with the thought that we may be in for some surprises. Rather than jumping deeply into any rheumatologic issue, we spent most of this article weaving through other medical issues, but does this not make today’s story fairly analogous to rheumatology itself?
Dr. Warmflash is a physician from Portland, Ore. He reported no conflicts of interest.
This story was updated 2/10/2023.
A version of this article first appeared on Medscape.com.
If you could go back in time 75 years and tell Dr. Sidney Farber, the developer of methotrexate for cancer therapy, that 21st-century medicine would utilize his specially designed drug more in rheumatology than oncology, he might be surprised. He might scratch his head even more, hearing of his drug sparking interest in still other medical fields, like cardiology.
But drug repurposing is not so uncommon. One classic example is aspirin. Once the most common pain medication and used also in rheumatology, aspirin now finds a range of applications, from colorectal cancer to the prevention of cardiovascular and cerebrovascular thrombosis. Minoxidil is another example, developed for hypertension but used today mostly to stop hair loss. Perhaps most ironic is thalidomide, utilized today for leprosy and multiple myeloma, yet actually contraindicated for its original application, nausea of pregnancy.
Methotrexate, thus, has much in common with other medical treatments, and yet its origin story is as unique and as fascinating as the story of Dr. Farber himself. While this is a rheumatology article, it’s also a story about the origin of a particular rheumatologic treatment, and so the story of that origin will take us mostly through a discussion of hematologic malignancy and of the clinical researcher who dared search for a cure.
Born in 1903, in Buffalo, New York, third of fourteen children of Jewish immigrants from Poland, Dr. Farber grew up in a household that was crowded but academically rigorous. His father, Simon, routinely brought home textbooks, assigning each child a book to read and on which to write a report. His mother, Matilda, was as devoted as her husband to raising the children to succeed in their adopted new country. Upstairs, the children were permitted to speak Yiddish, but downstairs they were required to use only English and German.
As a teen, Dr. Farber lived through the 1918 influenza pandemic that killed at least 50 million people worldwide, including more than 2,000 Buffalonians. This probably helped motivate him to study medicine, but with antisemitism overt in the America of the early 1920s, securing admission to a U.S. medical school was close to impossible. So, in what now seems like the greatest of ironies, Dr. Farber began medical studies in Germany, then transferred for the second year to a U.S. program that seemed adequate – Harvard Medical School, from which he graduated in 1927. From there, he trained as a pathologist, focusing ultimately on pediatric pathology. But, frustrated by case after case of malignancy, whose young victims he’d often have to autopsy, Dr. Farber decided that he wanted to advance the pitiful state of cancer therapeutics, especially for hematologic malignancy.
This was a tall order in the 1930s and early 1940s, when cancer therapeutics consisted only of surgical resection and very primitive forms of radiation therapy. Applicable only to neoplasia that was localized, these options were useless against malignancies in the blood, like acute lymphoblastic leukemia (ALL), but by January 1948 there was at least one glimmer of hope. At that time, one patient with ALL, 2-year-old Robert Sandler, was too ill to join his twin brother Elliott for snow play outside their home in the Dorchester section of Boston. Diagnosed back in August, Robert had suffered multiple episodes of fever, anemia, and thrombocytopenia. His illness had enlarged his spleen dramatically and caused pathologic bone fractures with excruciating bone pain, and for a while he couldn’t walk because of pressure on his lower spinal cord. All of this was the result of uncontrolled mitosis and cell division of lymphoblasts, immature lymphocytes. By December, these out-of-control cells had elevated the boy’s white blood cell count to a peak of 70,000/mcL, more than six times the high end of the normal range (4,500-11,000/mcL). This had happened despite treatment with an experimental drug, developed at Boston Children’s Hospital by Dr. Farber and his team, working on the assumption that inhibition of folate metabolism should slow the growth of tumor cells. On Dec. 28, however, Dr. Farber had switched the child to a new drug with a chemical structure just slightly different from the other agent’s.
Merely another chemical modification in a series of attempts by the research team, the new drug, aminopterin, was not expected to do anything dramatic, but Dr. Farber and the team had come such a long way since the middle of 1947, when he’d actually done the opposite of what he was doing now. On the basis of British research from India showing folic acid deficiency as the basis of a common type of anemia in malnourished people, Dr. Farber had reasoned that children with leukemia, who also suffered from anemia, might also benefit from folic acid supplementation. Even without prior rodent testing, Dr. Farber had tried giving the nutrient to patients with ALL, a strategy made possible by the presence of a spectacular chemist working on folic acid synthesis at Farber’s own hospital to help combat folate deficiency. Born into a poor Brahmin family in India, the chemist, Dr. Yellapragada SubbaRow, had begun life with so much stacked against him as to appear even less likely during childhood than the young Dr. Farber to grow up to make major contributions to medicine. Going through childhood with death all around him, Dr. SubbaRow was motivated to study medicine, but getting into medical school had been an uphill fight, given his family’s economic difficulty. Knowing that he’d also face discrimination on account of his low status after receiving admission to a medical program, SubbaRow could have made things a bit easier for himself by living within the norms of the British Imperial system, but as a supporter of Mohandas Gandhi’s nationalist movement, he boycotted British goods. As a medical student, this meant doing things like wearing Indian-made surgical gloves, instead of the English products that were expected of the students. Such actions led Dr. SubbaRow to receive a kind of second-rate medical degree, rather than the prestigious MBBS.
The political situation also led Dr. SubbaRow to emigrate to the United States, where, ironically, his medical degree initially was taken less seriously than it had been taken in his British-occupied homeland. He thus worked in the capacity of a hospital night porter at Peter Bent Brigham Hospital (the future Brigham and Women’s Hospital), doing menial tasks like changing sheets to make ends meet. He studied, however, and made enough of an impression to gain admission to the same institution that also admitted Farber through the backdoor, Harvard Medical School. This launched him into a research career in which he not only would be instrumental in developing folate antagonists and other classes of drugs, but also would make him the codiscoverer of the role of creatine phosphate and ATP in cellular energy metabolism. Sadly, even after obtaining his top-notch American credentials and contributing through his research to what you might say is a good chunk of the biochemistry pathways that first year medical students memorize without ever learning who discovered them, Dr. SubbaRow still faced prejudice for the rest of his life, which turned out to last only until the age of 53. To add insult to injury, he is rarely remembered for his role.
Dr. Farber proceeded with the folic acid supplementation idea in patients with ALL, even though ALL caused a hypoproliferative anemia, whereas anemia from folate deficiency was megaloblastic, meaning that erythrocytes were produced but they were oversized and dysfunctional. Tragically, folic acid had accelerated the disease process in children with ALL, but the process of chemical experimentation aimed at synthesizing folate also produced some compounds that mimicked chemical precursors of folate in a way that made them antifolates, inhibitors of folate metabolism. If folic acid made lymphoblasts grow faster, Dr. Farber had reasoned that antifolates should inhibit their growth. He thus asked the chemistry lab to focus on folate inhibitors. Testing aminopterin, beginning with young Robert Sandler at the end of December, is what proved his hypothesis correct. By late January, aminopterin had brought the child’s WBC count down to the realm of 12,000, just slightly above normal, with symptoms and signs abating as well, and by February, the child could play with his twin brother. It was not a cure; malignant lymphoblasts still showed on microscopy of Robert’s blood. While he and some 15 other children whom Dr. Farber treated in this early trial would all succumb to ALL, they experienced remission lasting several months.
This was a big deal because the concept of chemotherapy was based only on serendipitous observations of WBC counts dropping in soldiers exposed to nitrogen mustard gas during World War I and during an incident in World War II, yet aminopterin had been designed from the ground up. Though difficult to synthesize in quantities, there was no reason for Dr. Farber’s team not to keep tweaking the drug, and so they did. Replacing one hydrogen atom with a methyl group, they turned it into methotrexate.
Proving easier to synthesize and less toxic, methotrexate would become a workhorse for chemotherapy over the next couple of decades, but the capability of both methotrexate and aminopterin to blunt the growth of white blood cells and other cells did not go unnoticed outside the realm of oncology. As early as the 1950s, dermatologists were using aminopterin to treat psoriasis. This led to the approval of methotrexate for psoriasis in 1972.
Meanwhile, like oncology, infectious diseases, aviation medicine, and so many other areas of practice, rheumatology had gotten a major boost from research stemming from World War II. During the war, Dr. Philip Hench of the Mayo Clinic developed cortisone, which pilots used to stay alert and energetic during trans-Atlantic flights. But it turned out that cortisone had a powerful immunosuppressive effect that dramatically improved rheumatoid arthritis, leading Dr. Hench to receive the Nobel Prize in Physiology or Medicine in 1950. By the end of the 1950s, however, the significant side effects of long-term corticosteroid therapy were very clear, so over the next few decades there was a major effort to develop different treatments for RA and other rheumatologic diseases.
Top on the list of such agents was methotrexate, developed for RA in part by Dr. Michael Weinblatt of Brigham and Women’s Hospital in Boston. In the 1980s, Dr. Weinblatt published the first clinical trial showing the benefits of methotrexate for RA patients. This has since developed into a standard treatment, noticeably different from the original malignancy application in that it is a low-dose regimen. Patients taking methotrexate for RA typically receive no more than 25 mg per week orally, and often much less. Rheumatology today includes expertise in keeping long-term methotrexate therapy safe by monitoring liver function and through other routine tests. The routine nature of the therapy has brought methotrexate to the point of beckoning in a realm that Dr. Farber might not have predicted in his wildest imagination: cardiology. This is on account of the growing appreciation of the inflammatory process in the pathophysiology of atherosclerotic heart disease.
Meanwhile, being an antimetabolite, harmful to rapidly dividing cells, the danger of methotrexate to the embryo and fetus was recognized early. This made methotrexate off-limits to pregnant women, yet it also has made the drug useful as an abortifacient. Though not as good for medication abortion in unwanted but thriving pregnancies, where mifepristone/misoprostol has become the regimen of choice, methotrexate has become a workhorse in other obstetrical settings, such as for ending ectopic pregnancy.
Looking at the present and into the future, the potential for this very old medication looks wide open, as if it could go in any direction, so let’s wind up the discussion with the thought that we may be in for some surprises. Rather than jumping deeply into any rheumatologic issue, we spent most of this article weaving through other medical issues, but does this not make today’s story fairly analogous to rheumatology itself?
Dr. Warmflash is a physician from Portland, Ore. He reported no conflicts of interest.
This story was updated 2/10/2023.
A version of this article first appeared on Medscape.com.
Psoriasis, psoriatic arthritis show distinctive skin microbiomes
The bacterial diversity in lesional and nonlesional skin of patients with psoriasis (PsO) with or without psoriatic arthritis (PsA) was significantly lower than that of healthy control skin, based on data from 74 individuals.
Previous studies in humans and animals have suggested that microbes play a role in PsO pathogenesis, but microbial analyses of PsA are lacking, wrote Alba Boix-Amorós, PhD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues.
“The passage from PsO to PsA may, in part, be driven by microbial triggers, which deserves further investigation,” they wrote.
In a study published in Annals of the Rheumatic Diseases, the researchers recruited 23 patients with PsO and 31 with PsA from the dermatology and rheumatology clinics at the NYU Grossman School of Medicine/NYU Langone Health in New York. An additional 20 healthy individuals with no history of PsA or PsO were recruited from within NYU to serve as controls. All participants were aged 18 years and older, and more than 75% were White. Males made up 65.4%, 47.8%, and 55.0% of the PsA, PsO, and control groups.
The researchers collected skin swabs from lesional and nonlesional skin of individuals with PsO and PsA and from the upper and lower extremities of the healthy controls. The microbiota analysis included 148 samples that were analyzed using 16S rRNA sequencing.
The microbiome diversity was significantly greater in healthy skin, compared with lesional and nonlesional psoriatic skin (P < .05 for both). Specifically, levels of Cutibacterium and Kocuria were significantly higher in healthy skin than in psoriatic skin (P = .016 and P = .011, respectively), while psoriatic skin showed higher levels of Staphylococcus.
No significant microbiome differences were noted between lesional and nonlesional PsO and PsA samples. The finding that the microbiome of nonlesional psoriatic skin was more similar to lesional psoriatic skin than to healthy skin was unexpected, and suggests the development of microbial dysbiosis in psoriatic skin independent of the presence of lesions, the researchers wrote.
The researchers also found that levels of Corynebacterium in nonlesional PsA samples were significantly elevated, compared with nonlesional PsO samples (P < .05), which suggests a possible role for the microbe as a biomarker for disease progression, the researchers said.
“One important application of these data is the potential development of therapeutic options for the treatment of psoriatic disease and/or the prevention of PsA,” the researchers wrote in their discussion.
The findings were limited by several factors, including the combination of samples from upper and lower extremities and the exclusion of data from the scalp, the researchers noted. Other limitations included the use of only 16S rRNA gene sequencing, which presents a less comprehensive view of the microbiome, they said.
However, the results support the role of the skin microbiome in psoriasis pathogenesis, with details on microbiota across the psoriatic disease spectrum, they said.
The study received no outside funding. Dr. Boix-Amorós had no financial conflicts to disclose. Several coauthors disclosed financial relationships with pharmaceutical companies including Janssen, AbbVie, Bristol-Myers Squibb, Johnson & Johnson, Eli Lilly, Pfizer, Novartis, Sanofi, and UCB.
The bacterial diversity in lesional and nonlesional skin of patients with psoriasis (PsO) with or without psoriatic arthritis (PsA) was significantly lower than that of healthy control skin, based on data from 74 individuals.
Previous studies in humans and animals have suggested that microbes play a role in PsO pathogenesis, but microbial analyses of PsA are lacking, wrote Alba Boix-Amorós, PhD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues.
“The passage from PsO to PsA may, in part, be driven by microbial triggers, which deserves further investigation,” they wrote.
In a study published in Annals of the Rheumatic Diseases, the researchers recruited 23 patients with PsO and 31 with PsA from the dermatology and rheumatology clinics at the NYU Grossman School of Medicine/NYU Langone Health in New York. An additional 20 healthy individuals with no history of PsA or PsO were recruited from within NYU to serve as controls. All participants were aged 18 years and older, and more than 75% were White. Males made up 65.4%, 47.8%, and 55.0% of the PsA, PsO, and control groups.
The researchers collected skin swabs from lesional and nonlesional skin of individuals with PsO and PsA and from the upper and lower extremities of the healthy controls. The microbiota analysis included 148 samples that were analyzed using 16S rRNA sequencing.
The microbiome diversity was significantly greater in healthy skin, compared with lesional and nonlesional psoriatic skin (P < .05 for both). Specifically, levels of Cutibacterium and Kocuria were significantly higher in healthy skin than in psoriatic skin (P = .016 and P = .011, respectively), while psoriatic skin showed higher levels of Staphylococcus.
No significant microbiome differences were noted between lesional and nonlesional PsO and PsA samples. The finding that the microbiome of nonlesional psoriatic skin was more similar to lesional psoriatic skin than to healthy skin was unexpected, and suggests the development of microbial dysbiosis in psoriatic skin independent of the presence of lesions, the researchers wrote.
The researchers also found that levels of Corynebacterium in nonlesional PsA samples were significantly elevated, compared with nonlesional PsO samples (P < .05), which suggests a possible role for the microbe as a biomarker for disease progression, the researchers said.
“One important application of these data is the potential development of therapeutic options for the treatment of psoriatic disease and/or the prevention of PsA,” the researchers wrote in their discussion.
The findings were limited by several factors, including the combination of samples from upper and lower extremities and the exclusion of data from the scalp, the researchers noted. Other limitations included the use of only 16S rRNA gene sequencing, which presents a less comprehensive view of the microbiome, they said.
However, the results support the role of the skin microbiome in psoriasis pathogenesis, with details on microbiota across the psoriatic disease spectrum, they said.
The study received no outside funding. Dr. Boix-Amorós had no financial conflicts to disclose. Several coauthors disclosed financial relationships with pharmaceutical companies including Janssen, AbbVie, Bristol-Myers Squibb, Johnson & Johnson, Eli Lilly, Pfizer, Novartis, Sanofi, and UCB.
The bacterial diversity in lesional and nonlesional skin of patients with psoriasis (PsO) with or without psoriatic arthritis (PsA) was significantly lower than that of healthy control skin, based on data from 74 individuals.
Previous studies in humans and animals have suggested that microbes play a role in PsO pathogenesis, but microbial analyses of PsA are lacking, wrote Alba Boix-Amorós, PhD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues.
“The passage from PsO to PsA may, in part, be driven by microbial triggers, which deserves further investigation,” they wrote.
In a study published in Annals of the Rheumatic Diseases, the researchers recruited 23 patients with PsO and 31 with PsA from the dermatology and rheumatology clinics at the NYU Grossman School of Medicine/NYU Langone Health in New York. An additional 20 healthy individuals with no history of PsA or PsO were recruited from within NYU to serve as controls. All participants were aged 18 years and older, and more than 75% were White. Males made up 65.4%, 47.8%, and 55.0% of the PsA, PsO, and control groups.
The researchers collected skin swabs from lesional and nonlesional skin of individuals with PsO and PsA and from the upper and lower extremities of the healthy controls. The microbiota analysis included 148 samples that were analyzed using 16S rRNA sequencing.
The microbiome diversity was significantly greater in healthy skin, compared with lesional and nonlesional psoriatic skin (P < .05 for both). Specifically, levels of Cutibacterium and Kocuria were significantly higher in healthy skin than in psoriatic skin (P = .016 and P = .011, respectively), while psoriatic skin showed higher levels of Staphylococcus.
No significant microbiome differences were noted between lesional and nonlesional PsO and PsA samples. The finding that the microbiome of nonlesional psoriatic skin was more similar to lesional psoriatic skin than to healthy skin was unexpected, and suggests the development of microbial dysbiosis in psoriatic skin independent of the presence of lesions, the researchers wrote.
The researchers also found that levels of Corynebacterium in nonlesional PsA samples were significantly elevated, compared with nonlesional PsO samples (P < .05), which suggests a possible role for the microbe as a biomarker for disease progression, the researchers said.
“One important application of these data is the potential development of therapeutic options for the treatment of psoriatic disease and/or the prevention of PsA,” the researchers wrote in their discussion.
The findings were limited by several factors, including the combination of samples from upper and lower extremities and the exclusion of data from the scalp, the researchers noted. Other limitations included the use of only 16S rRNA gene sequencing, which presents a less comprehensive view of the microbiome, they said.
However, the results support the role of the skin microbiome in psoriasis pathogenesis, with details on microbiota across the psoriatic disease spectrum, they said.
The study received no outside funding. Dr. Boix-Amorós had no financial conflicts to disclose. Several coauthors disclosed financial relationships with pharmaceutical companies including Janssen, AbbVie, Bristol-Myers Squibb, Johnson & Johnson, Eli Lilly, Pfizer, Novartis, Sanofi, and UCB.
FROM ANNALS OF THE RHEUMATIC DISEASES
Topical psoriasis treatments
LAS VEGAS – ,” said Linda Stein Gold, MD, in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
However, when using topical treatments, combination therapy is generally more effective than monotherapy for psoriasis, especially for plaque psoriasis, said Dr. Stein Gold, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit.
Two combination products, calcipotriene/betamethasone (CAL/BDP) and tazarotene/halobetasol lotion, each offer a complimentary mechanism of action that minimizes side effects, with decreased irritation and less atrophy, she said. Calcipotriene/betamethasone (CAL/BDP) is available as a cream or foam, Dr. Stein Gold noted. The cream is engineered for rapid onset, as well as enhanced penetration, she said. CAL/BDP foam also is designed for enhanced penetration, and has been shown to have long-term maintenance efficacy, she said.
The currently available CAL/BDP cream is made using a patented technology known as “PAD,” in which the internal oil of the cream vehicle is stabilized by encapsulation in “a robust aqueous film,” Dr. Stein Gold said, noting that the greater solubility enhances skin penetration. The creation of “a robust oil droplet” addresses the problems associated with the surfactants present in many cream vehicles, namely irritation and impedance of skin penetration of the cream, she said.
In an 8-week study published in 2021, researchers compared CAL/BDP cream with PAD technology to CAL/BDP topical suspension in adults with mild to moderate psoriasis.
Patients randomized to treatment with CAL/BDP cream were significantly more likely to achieve the primary endpoint of Physician Global Assessment (PGA) treatment success than those randomized to the topical solution or vehicle (37.4%, 22.8%, and 3.7%, respectively).
Get proactive to maintain results
With topical psoriasis treatment, a proactive strategy helps maintain results over time, Dr. Stein Gold said. As an example, she cited a study published in 2021. In that study, known as PSO-LONG, which evaluated topical CAL/BDP foam, proactive management with the CAL/BDP foam formulation, “reduced the risk of experiencing relapse by 43%,” compared with reactive management (treatment with the vehicle foam), she said. Patients in the proactive-management group experienced an average of 41 more days in remission, compared with those in the reactive management group over a 1-year period.
Dr. Stein Gold also highlighted the value of tazarotene/halobetasol lotion for psoriasis, which she described as having synergistic efficacy,
She shared data presented at the 2021 Maui Dermatology meeting showing treatment success by 8 weeks with halobetasol/tazarotene with significantly reduced mean scores on measures of itching, dryness, and burning/stinging, compared with those on vehicle.
What’s new and approved
Joining the current topical treatment options for psoriasis is tapinarof, a small molecule that works by down-regulating Th17 cytokines, said Dr. Stein Gold. Tapinarof is Food and Drug Administration approved for treating psoriasis and is being studied in clinical trials for atopic dermatitis, she noted.
Dr. Stein Gold reviewed data from the PSOARING program published in the New England Journal of Medicine that served as a foundation for the FDA approval of tapinarof 1% cream. In the PSOARING 1 and 2 studies, patients with PSORIASIS showed significant improvement compared with vehicle over 12 weeks for the primary endpoint of Physicians’ Global Assessment scores of 0 or 1 (clear or almost clear). In the two studies, 60.7% and 56.9% of patients randomized to tapinarof met the patient-reported outcome of a minimum 4-point improvement in peak pruritus on the numerical rating scale (NRS) from baseline vs. 43.2% and 29.7% of placebo patients in the two studies, respectively.
In PSOARING 1 and 2, folliculitis (mostly mild or moderate), contact dermatitis, headache, pruritus, and dermatitis were the most common treatment-emergent adverse events, occurring in 1% or more of patients. Adverse event profiles for tapinarof are similar to those seen in previous studies, and a long-term extension showed a consistent safety profile, Dr. Stein Gold said.
Another recently approved topical treatment for psoriasis, a cream formulation of roflumilast, a phosphodiesterase (PDE)-4 inhibitor, has shown efficacy for treating plaque psoriasis, she said.
Patients with psoriasis in the DERMIS 1 and DERMIS 2 phase 3 studies randomized to 0.3% roflumilast cream showed significant improvement compared with those randomized to vehicle in terms of Investigator Global Assessment scores of clear or almost clear with an improvement of at least 2 grades from baseline.
Roflumilast foam also has shown success in improving scalp and body psoriasis, but this vehicle and indication has not yet been approved, Dr. Stein Gold said.
Dr. Stein Gold disclosed serving as a consultant or adviser for companies including AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, EPI Health, Galderma, Janssen, Incyte, Ortho Dermatologics, Pfizer, Regeneron, Sanofi; UCB, and serving as a speaker or member of speakers’ bureau for Amgen, AbbVie, Incyte, Pfizer, Regeneron, Sanofi, and Sun Research. She also disclosed receiving funding from AbbVie Amgen, Arcutis, Dermata, Dermavant, Galderma, Incyte, Ortho Dermatologics, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – ,” said Linda Stein Gold, MD, in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
However, when using topical treatments, combination therapy is generally more effective than monotherapy for psoriasis, especially for plaque psoriasis, said Dr. Stein Gold, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit.
Two combination products, calcipotriene/betamethasone (CAL/BDP) and tazarotene/halobetasol lotion, each offer a complimentary mechanism of action that minimizes side effects, with decreased irritation and less atrophy, she said. Calcipotriene/betamethasone (CAL/BDP) is available as a cream or foam, Dr. Stein Gold noted. The cream is engineered for rapid onset, as well as enhanced penetration, she said. CAL/BDP foam also is designed for enhanced penetration, and has been shown to have long-term maintenance efficacy, she said.
The currently available CAL/BDP cream is made using a patented technology known as “PAD,” in which the internal oil of the cream vehicle is stabilized by encapsulation in “a robust aqueous film,” Dr. Stein Gold said, noting that the greater solubility enhances skin penetration. The creation of “a robust oil droplet” addresses the problems associated with the surfactants present in many cream vehicles, namely irritation and impedance of skin penetration of the cream, she said.
In an 8-week study published in 2021, researchers compared CAL/BDP cream with PAD technology to CAL/BDP topical suspension in adults with mild to moderate psoriasis.
Patients randomized to treatment with CAL/BDP cream were significantly more likely to achieve the primary endpoint of Physician Global Assessment (PGA) treatment success than those randomized to the topical solution or vehicle (37.4%, 22.8%, and 3.7%, respectively).
Get proactive to maintain results
With topical psoriasis treatment, a proactive strategy helps maintain results over time, Dr. Stein Gold said. As an example, she cited a study published in 2021. In that study, known as PSO-LONG, which evaluated topical CAL/BDP foam, proactive management with the CAL/BDP foam formulation, “reduced the risk of experiencing relapse by 43%,” compared with reactive management (treatment with the vehicle foam), she said. Patients in the proactive-management group experienced an average of 41 more days in remission, compared with those in the reactive management group over a 1-year period.
Dr. Stein Gold also highlighted the value of tazarotene/halobetasol lotion for psoriasis, which she described as having synergistic efficacy,
She shared data presented at the 2021 Maui Dermatology meeting showing treatment success by 8 weeks with halobetasol/tazarotene with significantly reduced mean scores on measures of itching, dryness, and burning/stinging, compared with those on vehicle.
What’s new and approved
Joining the current topical treatment options for psoriasis is tapinarof, a small molecule that works by down-regulating Th17 cytokines, said Dr. Stein Gold. Tapinarof is Food and Drug Administration approved for treating psoriasis and is being studied in clinical trials for atopic dermatitis, she noted.
Dr. Stein Gold reviewed data from the PSOARING program published in the New England Journal of Medicine that served as a foundation for the FDA approval of tapinarof 1% cream. In the PSOARING 1 and 2 studies, patients with PSORIASIS showed significant improvement compared with vehicle over 12 weeks for the primary endpoint of Physicians’ Global Assessment scores of 0 or 1 (clear or almost clear). In the two studies, 60.7% and 56.9% of patients randomized to tapinarof met the patient-reported outcome of a minimum 4-point improvement in peak pruritus on the numerical rating scale (NRS) from baseline vs. 43.2% and 29.7% of placebo patients in the two studies, respectively.
In PSOARING 1 and 2, folliculitis (mostly mild or moderate), contact dermatitis, headache, pruritus, and dermatitis were the most common treatment-emergent adverse events, occurring in 1% or more of patients. Adverse event profiles for tapinarof are similar to those seen in previous studies, and a long-term extension showed a consistent safety profile, Dr. Stein Gold said.
Another recently approved topical treatment for psoriasis, a cream formulation of roflumilast, a phosphodiesterase (PDE)-4 inhibitor, has shown efficacy for treating plaque psoriasis, she said.
Patients with psoriasis in the DERMIS 1 and DERMIS 2 phase 3 studies randomized to 0.3% roflumilast cream showed significant improvement compared with those randomized to vehicle in terms of Investigator Global Assessment scores of clear or almost clear with an improvement of at least 2 grades from baseline.
Roflumilast foam also has shown success in improving scalp and body psoriasis, but this vehicle and indication has not yet been approved, Dr. Stein Gold said.
Dr. Stein Gold disclosed serving as a consultant or adviser for companies including AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, EPI Health, Galderma, Janssen, Incyte, Ortho Dermatologics, Pfizer, Regeneron, Sanofi; UCB, and serving as a speaker or member of speakers’ bureau for Amgen, AbbVie, Incyte, Pfizer, Regeneron, Sanofi, and Sun Research. She also disclosed receiving funding from AbbVie Amgen, Arcutis, Dermata, Dermavant, Galderma, Incyte, Ortho Dermatologics, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – ,” said Linda Stein Gold, MD, in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
However, when using topical treatments, combination therapy is generally more effective than monotherapy for psoriasis, especially for plaque psoriasis, said Dr. Stein Gold, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit.
Two combination products, calcipotriene/betamethasone (CAL/BDP) and tazarotene/halobetasol lotion, each offer a complimentary mechanism of action that minimizes side effects, with decreased irritation and less atrophy, she said. Calcipotriene/betamethasone (CAL/BDP) is available as a cream or foam, Dr. Stein Gold noted. The cream is engineered for rapid onset, as well as enhanced penetration, she said. CAL/BDP foam also is designed for enhanced penetration, and has been shown to have long-term maintenance efficacy, she said.
The currently available CAL/BDP cream is made using a patented technology known as “PAD,” in which the internal oil of the cream vehicle is stabilized by encapsulation in “a robust aqueous film,” Dr. Stein Gold said, noting that the greater solubility enhances skin penetration. The creation of “a robust oil droplet” addresses the problems associated with the surfactants present in many cream vehicles, namely irritation and impedance of skin penetration of the cream, she said.
In an 8-week study published in 2021, researchers compared CAL/BDP cream with PAD technology to CAL/BDP topical suspension in adults with mild to moderate psoriasis.
Patients randomized to treatment with CAL/BDP cream were significantly more likely to achieve the primary endpoint of Physician Global Assessment (PGA) treatment success than those randomized to the topical solution or vehicle (37.4%, 22.8%, and 3.7%, respectively).
Get proactive to maintain results
With topical psoriasis treatment, a proactive strategy helps maintain results over time, Dr. Stein Gold said. As an example, she cited a study published in 2021. In that study, known as PSO-LONG, which evaluated topical CAL/BDP foam, proactive management with the CAL/BDP foam formulation, “reduced the risk of experiencing relapse by 43%,” compared with reactive management (treatment with the vehicle foam), she said. Patients in the proactive-management group experienced an average of 41 more days in remission, compared with those in the reactive management group over a 1-year period.
Dr. Stein Gold also highlighted the value of tazarotene/halobetasol lotion for psoriasis, which she described as having synergistic efficacy,
She shared data presented at the 2021 Maui Dermatology meeting showing treatment success by 8 weeks with halobetasol/tazarotene with significantly reduced mean scores on measures of itching, dryness, and burning/stinging, compared with those on vehicle.
What’s new and approved
Joining the current topical treatment options for psoriasis is tapinarof, a small molecule that works by down-regulating Th17 cytokines, said Dr. Stein Gold. Tapinarof is Food and Drug Administration approved for treating psoriasis and is being studied in clinical trials for atopic dermatitis, she noted.
Dr. Stein Gold reviewed data from the PSOARING program published in the New England Journal of Medicine that served as a foundation for the FDA approval of tapinarof 1% cream. In the PSOARING 1 and 2 studies, patients with PSORIASIS showed significant improvement compared with vehicle over 12 weeks for the primary endpoint of Physicians’ Global Assessment scores of 0 or 1 (clear or almost clear). In the two studies, 60.7% and 56.9% of patients randomized to tapinarof met the patient-reported outcome of a minimum 4-point improvement in peak pruritus on the numerical rating scale (NRS) from baseline vs. 43.2% and 29.7% of placebo patients in the two studies, respectively.
In PSOARING 1 and 2, folliculitis (mostly mild or moderate), contact dermatitis, headache, pruritus, and dermatitis were the most common treatment-emergent adverse events, occurring in 1% or more of patients. Adverse event profiles for tapinarof are similar to those seen in previous studies, and a long-term extension showed a consistent safety profile, Dr. Stein Gold said.
Another recently approved topical treatment for psoriasis, a cream formulation of roflumilast, a phosphodiesterase (PDE)-4 inhibitor, has shown efficacy for treating plaque psoriasis, she said.
Patients with psoriasis in the DERMIS 1 and DERMIS 2 phase 3 studies randomized to 0.3% roflumilast cream showed significant improvement compared with those randomized to vehicle in terms of Investigator Global Assessment scores of clear or almost clear with an improvement of at least 2 grades from baseline.
Roflumilast foam also has shown success in improving scalp and body psoriasis, but this vehicle and indication has not yet been approved, Dr. Stein Gold said.
Dr. Stein Gold disclosed serving as a consultant or adviser for companies including AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, EPI Health, Galderma, Janssen, Incyte, Ortho Dermatologics, Pfizer, Regeneron, Sanofi; UCB, and serving as a speaker or member of speakers’ bureau for Amgen, AbbVie, Incyte, Pfizer, Regeneron, Sanofi, and Sun Research. She also disclosed receiving funding from AbbVie Amgen, Arcutis, Dermata, Dermavant, Galderma, Incyte, Ortho Dermatologics, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Factors Influencing Patient Preferences for Phototherapy: A Survey Study
Phototherapy—particularly UVB phototherapy, which utilizes UVB rays of specific wavelengths within the UV spectrum—is indicated for a wide variety of dermatoses. In-office and at-home UVB treatments commonly are used, as are salon tanning and sunbathing. When selecting a form of phototherapy, patients are likely to consider safety, cost, effectiveness, insurance issues, and convenience. Research on patient preferences; the reasons for these preferences; and which options patients perceive to be the safest, most cost-effective, efficacious, and convenient is lacking. We aimed to assess the forms of phototherapy that patients would most consider using; the factors influencing patient preferences; and the forms patients perceived as the safest and most cost-effective, efficacious, and convenient.
Methods
Study Participants—We recruited 500 Amazon Mechanical Turk users who were 18 years or older to complete our REDCap-generated survey. The study was approved by the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Evaluation—Participants were asked, “If you were diagnosed with a skin disease that benefited from UV therapy, which of the following forms of UV therapy would you consider choosing?” Participants were instructed to choose all of the forms they would consider using. Available options included in-office UV, at-home UV, home tanning, salon tanning, sunbathing, and other. Participants were asked to select which factors—from safety, cost, effectiveness, issues with insurance, convenience, and other—influenced their decision-making; which form of phototherapy they would most consider along with the factors that influenced their preference for this specific form of phototherapy; and which options they considered to be safest and most cost-effective, efficacious, and convenient. Participants were asked to provide basic sociodemographic information, level of education, income, insurance status (private, Medicare, Medicaid, Veterans Affairs, and uninsured), and distance from the nearest dermatologist.
Statistical Analysis—Descriptive and inferential statistics (χ2 test) were used to analyze the data with a significance set at P<.05.
Results
Five hundred participants completed the survey (Table 1).
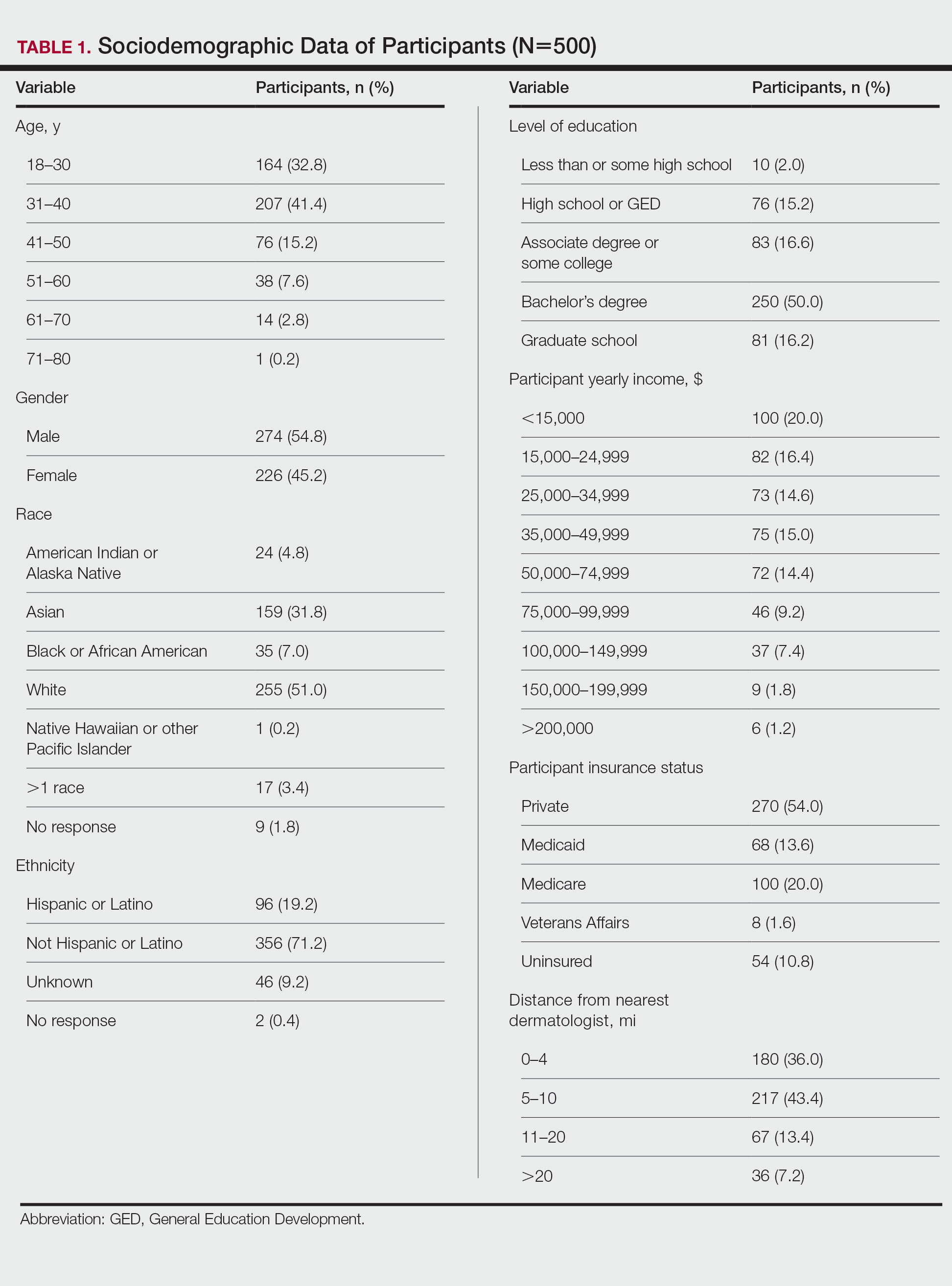
Factors Influencing Patient Preferences—When asked to select all forms of phototherapy they would consider, 186 (37.2%) participants selected in-office UVB, 263 (52.6%) selected at-home UV, 141 (28.2%) selected home tanning, 117 (23.4%) selected salon tanning, 191 (38.2%) selected sunbathing, and 3 (0.6%) selected other. Participants who selected in-office UVB as an option were more likely to also select salon tanning (P<.012). No other relationship was found between the UVB options and the tanning options. When asked which factors influenced their phototherapy preferences, 295 (59%) selected convenience, 266 (53.2%) selected effectiveness, 220 (44%) selected safety, 218 (43.6%) selected cost, 72 (14.4%) selected issues with insurance, and 4 (0.8%) selected other. Forms of Phototherapy Patients Consider Using—When asked which form of phototherapy they would most consider using, 179 (35.8%) participants selected at-home UVB, 108 (21.6%) selected sunbathing, 92 (18.4%) selected in-office UVB, 62 (12.4%) selected home-tanning, 57 (11.4%) selected salon tanning, 1 (0.2%) selected other, and 1 participant provided no response (P<.001).
Reasons for Using Phototherapy—Of the 179 who selected at-home UVB, 125 (70%) cited convenience as a reason. Of the 108 who selected salon tanning as their top choice, 62 (57%) cited cost as a reason. Convenience (P<.001), cost (P<.001), and safety (P=.023) were related to top preference. Issues with insurance did not have a statistically significant relationship with the top preference. However, participant insurance type was related to top phototherapy preference (P=.021), with privately insured patients more likely to select in-office UVB, whereas those with Medicaid and Medicare were more likely to select home or salon tanning. Efficacy was not related to top preference. Furthermore, age, gender, education, income, and distance from nearest dermatologist were not related to top preference.
In-office UVB was perceived to be safest (P<.001) and most efficacious (P<.001). Meanwhile, at-home UVB was selected as most convenient (P<.001). Lastly, sunbathing was determined to be most cost-effective (P<.001)(Table 2). Cost-effectiveness had a relationship (P<.001) with the participant’s insurance, as those with private insurance were more likely to select at-home UVB, whereas those with Medicare or Medicaid were more likely to select the tanning options. Additionally, of the54 uninsured participants in the survey, 29 selected sunbathing as the most cost-effective option.
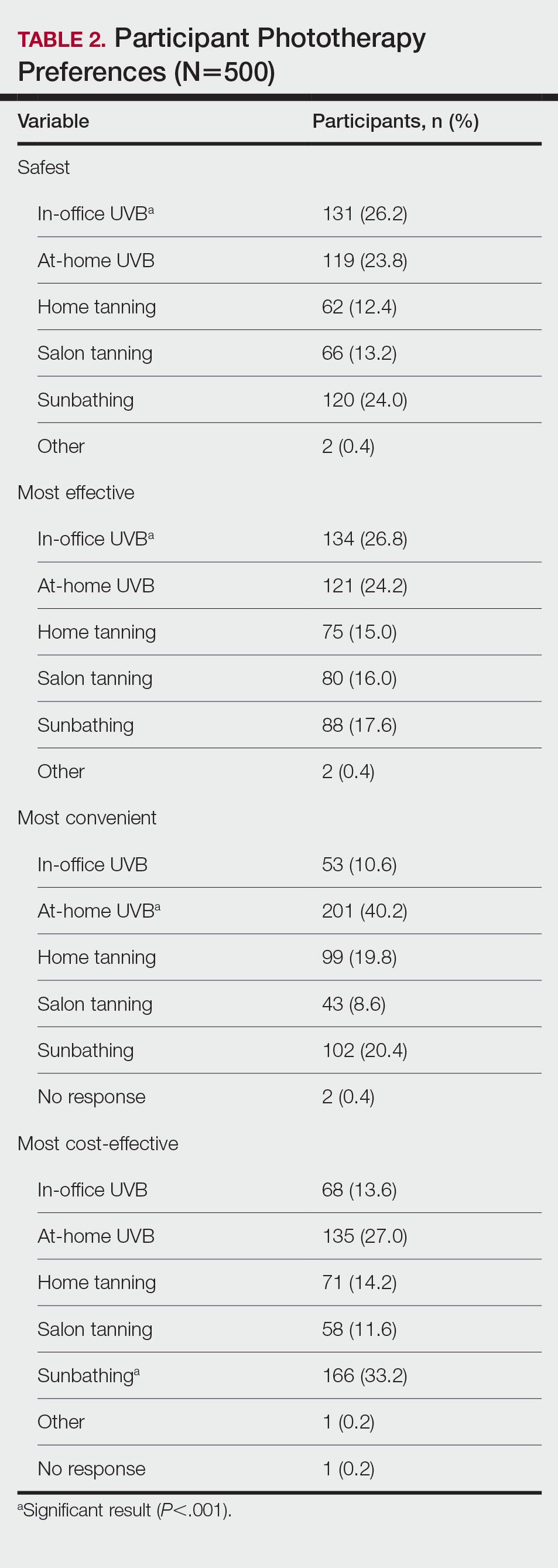
Comment
Phototherapy Treatment—UVB phototherapy at a wavelength of 290 to 320 nm (311–313 nm for narrowband UVB) is used to treat various dermatoses, including psoriasis and atopic dermatitis. UVB alters skin cytokines, induces apoptosis, promotes immunosuppression, causes DNA damage, and decreases the proliferation of dendritic cells and other cells of the innate immune system.1 In-office and at-home UV therapies make use of UVB wavelengths for treatment, while tanning and sunbathing contain not only UVB but also potentially harmful UVA rays. The wavelengths for indoor tanning devices include UVB at 280 to 315 nm and UVA at 315 to 400 nm, which are similar to those of the sun but with a different ratio of UVB to UVA and more intense total UV.2 When in-office and at-home UVB options are not available, various forms of tanning such as salon tanning and sunbathing may be alternatives that are widely used.3 One of the main reasons patients consider alternative phototherapy options is cost, as 1 in-office UVB treatment may cost $140, but a month of unlimited tanning may cost $30 or perhaps nothing if a patient has a gym membership with access to a tanning bed. Lack of insurance benefits covering phototherapy can exacerbate cost burden.4 However, tanning beds are associated with an increased risk for melanoma and nonmelanoma cancers.5,6 Additionally, all forms of phototherapy are associated with photoaging, but it is more intense with tanning and heliotherapy because of the presence of UVA, which penetrates deeper into the dermis.7 Meanwhile, for those who choose UVB therapy, deciding between an in-office and at-home UVB treatment could be a matter of convenience, as patients must consider long trips to the physician’s office; insurance status, as some insurances may not cover at-home UVB; or efficacy, which might be influenced by the presence of a physician or other medical staff. In many cases, patients may not be informed that at-home UVB is an option.
Patient Preferences—At-home UVB therapy was the most popular option in our study population, with most participants (52.6%) considering using it, and 35.9% choosing it as their top choice over all other phototherapy options. Safety, cost, and convenience were all found to be related to the option participants would most consider using. Prior analysis between at-home UVB and in-office UVB for the treatment of psoriasis determined that at-home UVB is as safe and cost-effective as in-office UVB without the inconvenience of the patient having to take time out of the week to visit the physician’s office,8,9 making at-home UVB an option dermatologists may strongly consider for patients who value safety, cost, and convenience. Oddly, efficacy was not related to the top preference, despite being the second highest–cited factor (53.2%) for which forms of phototherapy participants would consider using. For insurance coverage, those with Medicaid and Medicare selected the cheaper tanning options with higher-than-expected frequencies. Although problems with insurance were not related to the top preference, insurance status was related, suggesting that preferences are tied to cost. Of note, while the number of dermatologists that accept Medicare has increased in the last few years, there still remains an uneven distribution of phototherapy clinics. As of 2015, there were 19 million individuals who qualified for Medicare without a clinic within driving distance.10 This problem likely also exists for many Medicaid patients who may not qualify for at-home UVB. In this scenario, tanning or heliotherapy may be effective alternatives.
In-Office vs At-Home Options—Although in-office UVB was the option considered safest (26.2%) and most efficacious (26.8%), it was followed closely by at-home UVB in both categories (safest, 23.8%; most efficacious, 24.2%). Meanwhile, at-home UVB (40.2%) was chosen as the most convenient. Some patients consider tanning options over in-office UVB because of the inconvenience of traveling to an appointment.11 Therefore, at-home tanning may be a convenient alternative for these patients.
Considerations—Although our study was limited to an adult population, issues with convenience exist for the pediatric population as well, as children may need to miss multiple days of school each week to be treated in the office. For these pediatric patients, an at-home unit is preferable; however; issues with insurance coverage remain a challenge.12 Increasing insurance coverage of at-home units for the pediatric population therefore would be most prudent. However, when other options have been exhausted, including in-office UVB, tanning and sunbathing may be viable alternatives because of cost and convenience. In our study, sunbathing (33.2%) was considered the most cost-effective, likely because it does not require expensive equipment or a visit to a salon or physician’s office. Sunbathing has been effective in treating some dermatologic conditions, such as atopic dermatitis.13 However, it may only be effective during certain months and at different latitudes—conditions that make UVB sun rays more accessible—particularly when treating psoriasis.14 Furthermore, sunbathing may not be as cost-effective in patients with average-severity psoriasis compared with conventional psoriasis therapy because of the costs of travel to areas with sufficient UVB rays for treatment.15 Additionally, insurance status was related to which option was selected as the most cost-effective, as 29 (53.7%) of 54 uninsured participants chose sunbathing as the most cost-effective option, while only 92 (34.2%) of 269 privately insured patients selected sunbathing. Therefore, insurance status may be a factor for dermatologists to consider if a patient prefers a treatment that is cost-effective. Overall, dermatologists could perhaps consider guiding patients and optimizing their treatment plans based on the factors most important to the patients while understanding that costs and insurance status may ultimately determine the treatment option.
Limitations—Survey participants were recruited on Amazon Mechanical Turk, which could create sampling bias. Furthermore, these participants were representative of the general public and not exclusively patients on phototherapy, therefore representing the opinions of the general public and not those who may require phototherapy. Furthermore, given the nature of the survey, the study was limited to the adult population.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix-x.
- Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730-740.
- Su J, Pearce DJ, Feldman SR. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J Dermatolog Treat. 2005;16:324-326.
- Anderson KL, Huang KE, Huang WW, et al. Dermatology residents are prescribing tanning bed treatment. Dermatol Online J. 2016;22:13030/qt19h4k7sx.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanomaattributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:E4757.
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomized controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679.
- Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
- Juarez MC, Grossberg AL. Phototherapy in the pediatric population. Dermatol Clin. 2020;38:91-108.
- Autio P, Komulainen P, Larni HM. Heliotherapy in atopic dermatitis: a prospective study on climatotherapy using the SCORAD index. Acta Derm Venereol. 2002;82:436-440.
- Krzys´cin JW, Jarosławski J, Rajewska-Wie˛ch B, et al. Effectiveness of heliotherapy for psoriasis clearance in low and mid-latitudinal regions: a theoretical approach. J Photochem Photobiol B. 2012;115:35-41.
- Snellman E, Maljanen T, Aromaa A, et al. Effect of heliotherapy on the cost of psoriasis. Br J Dermatol. 1998;138:288-292.
Phototherapy—particularly UVB phototherapy, which utilizes UVB rays of specific wavelengths within the UV spectrum—is indicated for a wide variety of dermatoses. In-office and at-home UVB treatments commonly are used, as are salon tanning and sunbathing. When selecting a form of phototherapy, patients are likely to consider safety, cost, effectiveness, insurance issues, and convenience. Research on patient preferences; the reasons for these preferences; and which options patients perceive to be the safest, most cost-effective, efficacious, and convenient is lacking. We aimed to assess the forms of phototherapy that patients would most consider using; the factors influencing patient preferences; and the forms patients perceived as the safest and most cost-effective, efficacious, and convenient.
Methods
Study Participants—We recruited 500 Amazon Mechanical Turk users who were 18 years or older to complete our REDCap-generated survey. The study was approved by the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Evaluation—Participants were asked, “If you were diagnosed with a skin disease that benefited from UV therapy, which of the following forms of UV therapy would you consider choosing?” Participants were instructed to choose all of the forms they would consider using. Available options included in-office UV, at-home UV, home tanning, salon tanning, sunbathing, and other. Participants were asked to select which factors—from safety, cost, effectiveness, issues with insurance, convenience, and other—influenced their decision-making; which form of phototherapy they would most consider along with the factors that influenced their preference for this specific form of phototherapy; and which options they considered to be safest and most cost-effective, efficacious, and convenient. Participants were asked to provide basic sociodemographic information, level of education, income, insurance status (private, Medicare, Medicaid, Veterans Affairs, and uninsured), and distance from the nearest dermatologist.
Statistical Analysis—Descriptive and inferential statistics (χ2 test) were used to analyze the data with a significance set at P<.05.
Results
Five hundred participants completed the survey (Table 1).

Factors Influencing Patient Preferences—When asked to select all forms of phototherapy they would consider, 186 (37.2%) participants selected in-office UVB, 263 (52.6%) selected at-home UV, 141 (28.2%) selected home tanning, 117 (23.4%) selected salon tanning, 191 (38.2%) selected sunbathing, and 3 (0.6%) selected other. Participants who selected in-office UVB as an option were more likely to also select salon tanning (P<.012). No other relationship was found between the UVB options and the tanning options. When asked which factors influenced their phototherapy preferences, 295 (59%) selected convenience, 266 (53.2%) selected effectiveness, 220 (44%) selected safety, 218 (43.6%) selected cost, 72 (14.4%) selected issues with insurance, and 4 (0.8%) selected other. Forms of Phototherapy Patients Consider Using—When asked which form of phototherapy they would most consider using, 179 (35.8%) participants selected at-home UVB, 108 (21.6%) selected sunbathing, 92 (18.4%) selected in-office UVB, 62 (12.4%) selected home-tanning, 57 (11.4%) selected salon tanning, 1 (0.2%) selected other, and 1 participant provided no response (P<.001).
Reasons for Using Phototherapy—Of the 179 who selected at-home UVB, 125 (70%) cited convenience as a reason. Of the 108 who selected salon tanning as their top choice, 62 (57%) cited cost as a reason. Convenience (P<.001), cost (P<.001), and safety (P=.023) were related to top preference. Issues with insurance did not have a statistically significant relationship with the top preference. However, participant insurance type was related to top phototherapy preference (P=.021), with privately insured patients more likely to select in-office UVB, whereas those with Medicaid and Medicare were more likely to select home or salon tanning. Efficacy was not related to top preference. Furthermore, age, gender, education, income, and distance from nearest dermatologist were not related to top preference.
In-office UVB was perceived to be safest (P<.001) and most efficacious (P<.001). Meanwhile, at-home UVB was selected as most convenient (P<.001). Lastly, sunbathing was determined to be most cost-effective (P<.001)(Table 2). Cost-effectiveness had a relationship (P<.001) with the participant’s insurance, as those with private insurance were more likely to select at-home UVB, whereas those with Medicare or Medicaid were more likely to select the tanning options. Additionally, of the54 uninsured participants in the survey, 29 selected sunbathing as the most cost-effective option.

Comment
Phototherapy Treatment—UVB phototherapy at a wavelength of 290 to 320 nm (311–313 nm for narrowband UVB) is used to treat various dermatoses, including psoriasis and atopic dermatitis. UVB alters skin cytokines, induces apoptosis, promotes immunosuppression, causes DNA damage, and decreases the proliferation of dendritic cells and other cells of the innate immune system.1 In-office and at-home UV therapies make use of UVB wavelengths for treatment, while tanning and sunbathing contain not only UVB but also potentially harmful UVA rays. The wavelengths for indoor tanning devices include UVB at 280 to 315 nm and UVA at 315 to 400 nm, which are similar to those of the sun but with a different ratio of UVB to UVA and more intense total UV.2 When in-office and at-home UVB options are not available, various forms of tanning such as salon tanning and sunbathing may be alternatives that are widely used.3 One of the main reasons patients consider alternative phototherapy options is cost, as 1 in-office UVB treatment may cost $140, but a month of unlimited tanning may cost $30 or perhaps nothing if a patient has a gym membership with access to a tanning bed. Lack of insurance benefits covering phototherapy can exacerbate cost burden.4 However, tanning beds are associated with an increased risk for melanoma and nonmelanoma cancers.5,6 Additionally, all forms of phototherapy are associated with photoaging, but it is more intense with tanning and heliotherapy because of the presence of UVA, which penetrates deeper into the dermis.7 Meanwhile, for those who choose UVB therapy, deciding between an in-office and at-home UVB treatment could be a matter of convenience, as patients must consider long trips to the physician’s office; insurance status, as some insurances may not cover at-home UVB; or efficacy, which might be influenced by the presence of a physician or other medical staff. In many cases, patients may not be informed that at-home UVB is an option.
Patient Preferences—At-home UVB therapy was the most popular option in our study population, with most participants (52.6%) considering using it, and 35.9% choosing it as their top choice over all other phototherapy options. Safety, cost, and convenience were all found to be related to the option participants would most consider using. Prior analysis between at-home UVB and in-office UVB for the treatment of psoriasis determined that at-home UVB is as safe and cost-effective as in-office UVB without the inconvenience of the patient having to take time out of the week to visit the physician’s office,8,9 making at-home UVB an option dermatologists may strongly consider for patients who value safety, cost, and convenience. Oddly, efficacy was not related to the top preference, despite being the second highest–cited factor (53.2%) for which forms of phototherapy participants would consider using. For insurance coverage, those with Medicaid and Medicare selected the cheaper tanning options with higher-than-expected frequencies. Although problems with insurance were not related to the top preference, insurance status was related, suggesting that preferences are tied to cost. Of note, while the number of dermatologists that accept Medicare has increased in the last few years, there still remains an uneven distribution of phototherapy clinics. As of 2015, there were 19 million individuals who qualified for Medicare without a clinic within driving distance.10 This problem likely also exists for many Medicaid patients who may not qualify for at-home UVB. In this scenario, tanning or heliotherapy may be effective alternatives.
In-Office vs At-Home Options—Although in-office UVB was the option considered safest (26.2%) and most efficacious (26.8%), it was followed closely by at-home UVB in both categories (safest, 23.8%; most efficacious, 24.2%). Meanwhile, at-home UVB (40.2%) was chosen as the most convenient. Some patients consider tanning options over in-office UVB because of the inconvenience of traveling to an appointment.11 Therefore, at-home tanning may be a convenient alternative for these patients.
Considerations—Although our study was limited to an adult population, issues with convenience exist for the pediatric population as well, as children may need to miss multiple days of school each week to be treated in the office. For these pediatric patients, an at-home unit is preferable; however; issues with insurance coverage remain a challenge.12 Increasing insurance coverage of at-home units for the pediatric population therefore would be most prudent. However, when other options have been exhausted, including in-office UVB, tanning and sunbathing may be viable alternatives because of cost and convenience. In our study, sunbathing (33.2%) was considered the most cost-effective, likely because it does not require expensive equipment or a visit to a salon or physician’s office. Sunbathing has been effective in treating some dermatologic conditions, such as atopic dermatitis.13 However, it may only be effective during certain months and at different latitudes—conditions that make UVB sun rays more accessible—particularly when treating psoriasis.14 Furthermore, sunbathing may not be as cost-effective in patients with average-severity psoriasis compared with conventional psoriasis therapy because of the costs of travel to areas with sufficient UVB rays for treatment.15 Additionally, insurance status was related to which option was selected as the most cost-effective, as 29 (53.7%) of 54 uninsured participants chose sunbathing as the most cost-effective option, while only 92 (34.2%) of 269 privately insured patients selected sunbathing. Therefore, insurance status may be a factor for dermatologists to consider if a patient prefers a treatment that is cost-effective. Overall, dermatologists could perhaps consider guiding patients and optimizing their treatment plans based on the factors most important to the patients while understanding that costs and insurance status may ultimately determine the treatment option.
Limitations—Survey participants were recruited on Amazon Mechanical Turk, which could create sampling bias. Furthermore, these participants were representative of the general public and not exclusively patients on phototherapy, therefore representing the opinions of the general public and not those who may require phototherapy. Furthermore, given the nature of the survey, the study was limited to the adult population.
Phototherapy—particularly UVB phototherapy, which utilizes UVB rays of specific wavelengths within the UV spectrum—is indicated for a wide variety of dermatoses. In-office and at-home UVB treatments commonly are used, as are salon tanning and sunbathing. When selecting a form of phototherapy, patients are likely to consider safety, cost, effectiveness, insurance issues, and convenience. Research on patient preferences; the reasons for these preferences; and which options patients perceive to be the safest, most cost-effective, efficacious, and convenient is lacking. We aimed to assess the forms of phototherapy that patients would most consider using; the factors influencing patient preferences; and the forms patients perceived as the safest and most cost-effective, efficacious, and convenient.
Methods
Study Participants—We recruited 500 Amazon Mechanical Turk users who were 18 years or older to complete our REDCap-generated survey. The study was approved by the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Evaluation—Participants were asked, “If you were diagnosed with a skin disease that benefited from UV therapy, which of the following forms of UV therapy would you consider choosing?” Participants were instructed to choose all of the forms they would consider using. Available options included in-office UV, at-home UV, home tanning, salon tanning, sunbathing, and other. Participants were asked to select which factors—from safety, cost, effectiveness, issues with insurance, convenience, and other—influenced their decision-making; which form of phototherapy they would most consider along with the factors that influenced their preference for this specific form of phototherapy; and which options they considered to be safest and most cost-effective, efficacious, and convenient. Participants were asked to provide basic sociodemographic information, level of education, income, insurance status (private, Medicare, Medicaid, Veterans Affairs, and uninsured), and distance from the nearest dermatologist.
Statistical Analysis—Descriptive and inferential statistics (χ2 test) were used to analyze the data with a significance set at P<.05.
Results
Five hundred participants completed the survey (Table 1).

Factors Influencing Patient Preferences—When asked to select all forms of phototherapy they would consider, 186 (37.2%) participants selected in-office UVB, 263 (52.6%) selected at-home UV, 141 (28.2%) selected home tanning, 117 (23.4%) selected salon tanning, 191 (38.2%) selected sunbathing, and 3 (0.6%) selected other. Participants who selected in-office UVB as an option were more likely to also select salon tanning (P<.012). No other relationship was found between the UVB options and the tanning options. When asked which factors influenced their phototherapy preferences, 295 (59%) selected convenience, 266 (53.2%) selected effectiveness, 220 (44%) selected safety, 218 (43.6%) selected cost, 72 (14.4%) selected issues with insurance, and 4 (0.8%) selected other. Forms of Phototherapy Patients Consider Using—When asked which form of phototherapy they would most consider using, 179 (35.8%) participants selected at-home UVB, 108 (21.6%) selected sunbathing, 92 (18.4%) selected in-office UVB, 62 (12.4%) selected home-tanning, 57 (11.4%) selected salon tanning, 1 (0.2%) selected other, and 1 participant provided no response (P<.001).
Reasons for Using Phototherapy—Of the 179 who selected at-home UVB, 125 (70%) cited convenience as a reason. Of the 108 who selected salon tanning as their top choice, 62 (57%) cited cost as a reason. Convenience (P<.001), cost (P<.001), and safety (P=.023) were related to top preference. Issues with insurance did not have a statistically significant relationship with the top preference. However, participant insurance type was related to top phototherapy preference (P=.021), with privately insured patients more likely to select in-office UVB, whereas those with Medicaid and Medicare were more likely to select home or salon tanning. Efficacy was not related to top preference. Furthermore, age, gender, education, income, and distance from nearest dermatologist were not related to top preference.
In-office UVB was perceived to be safest (P<.001) and most efficacious (P<.001). Meanwhile, at-home UVB was selected as most convenient (P<.001). Lastly, sunbathing was determined to be most cost-effective (P<.001)(Table 2). Cost-effectiveness had a relationship (P<.001) with the participant’s insurance, as those with private insurance were more likely to select at-home UVB, whereas those with Medicare or Medicaid were more likely to select the tanning options. Additionally, of the54 uninsured participants in the survey, 29 selected sunbathing as the most cost-effective option.

Comment
Phototherapy Treatment—UVB phototherapy at a wavelength of 290 to 320 nm (311–313 nm for narrowband UVB) is used to treat various dermatoses, including psoriasis and atopic dermatitis. UVB alters skin cytokines, induces apoptosis, promotes immunosuppression, causes DNA damage, and decreases the proliferation of dendritic cells and other cells of the innate immune system.1 In-office and at-home UV therapies make use of UVB wavelengths for treatment, while tanning and sunbathing contain not only UVB but also potentially harmful UVA rays. The wavelengths for indoor tanning devices include UVB at 280 to 315 nm and UVA at 315 to 400 nm, which are similar to those of the sun but with a different ratio of UVB to UVA and more intense total UV.2 When in-office and at-home UVB options are not available, various forms of tanning such as salon tanning and sunbathing may be alternatives that are widely used.3 One of the main reasons patients consider alternative phototherapy options is cost, as 1 in-office UVB treatment may cost $140, but a month of unlimited tanning may cost $30 or perhaps nothing if a patient has a gym membership with access to a tanning bed. Lack of insurance benefits covering phototherapy can exacerbate cost burden.4 However, tanning beds are associated with an increased risk for melanoma and nonmelanoma cancers.5,6 Additionally, all forms of phototherapy are associated with photoaging, but it is more intense with tanning and heliotherapy because of the presence of UVA, which penetrates deeper into the dermis.7 Meanwhile, for those who choose UVB therapy, deciding between an in-office and at-home UVB treatment could be a matter of convenience, as patients must consider long trips to the physician’s office; insurance status, as some insurances may not cover at-home UVB; or efficacy, which might be influenced by the presence of a physician or other medical staff. In many cases, patients may not be informed that at-home UVB is an option.
Patient Preferences—At-home UVB therapy was the most popular option in our study population, with most participants (52.6%) considering using it, and 35.9% choosing it as their top choice over all other phototherapy options. Safety, cost, and convenience were all found to be related to the option participants would most consider using. Prior analysis between at-home UVB and in-office UVB for the treatment of psoriasis determined that at-home UVB is as safe and cost-effective as in-office UVB without the inconvenience of the patient having to take time out of the week to visit the physician’s office,8,9 making at-home UVB an option dermatologists may strongly consider for patients who value safety, cost, and convenience. Oddly, efficacy was not related to the top preference, despite being the second highest–cited factor (53.2%) for which forms of phototherapy participants would consider using. For insurance coverage, those with Medicaid and Medicare selected the cheaper tanning options with higher-than-expected frequencies. Although problems with insurance were not related to the top preference, insurance status was related, suggesting that preferences are tied to cost. Of note, while the number of dermatologists that accept Medicare has increased in the last few years, there still remains an uneven distribution of phototherapy clinics. As of 2015, there were 19 million individuals who qualified for Medicare without a clinic within driving distance.10 This problem likely also exists for many Medicaid patients who may not qualify for at-home UVB. In this scenario, tanning or heliotherapy may be effective alternatives.
In-Office vs At-Home Options—Although in-office UVB was the option considered safest (26.2%) and most efficacious (26.8%), it was followed closely by at-home UVB in both categories (safest, 23.8%; most efficacious, 24.2%). Meanwhile, at-home UVB (40.2%) was chosen as the most convenient. Some patients consider tanning options over in-office UVB because of the inconvenience of traveling to an appointment.11 Therefore, at-home tanning may be a convenient alternative for these patients.
Considerations—Although our study was limited to an adult population, issues with convenience exist for the pediatric population as well, as children may need to miss multiple days of school each week to be treated in the office. For these pediatric patients, an at-home unit is preferable; however; issues with insurance coverage remain a challenge.12 Increasing insurance coverage of at-home units for the pediatric population therefore would be most prudent. However, when other options have been exhausted, including in-office UVB, tanning and sunbathing may be viable alternatives because of cost and convenience. In our study, sunbathing (33.2%) was considered the most cost-effective, likely because it does not require expensive equipment or a visit to a salon or physician’s office. Sunbathing has been effective in treating some dermatologic conditions, such as atopic dermatitis.13 However, it may only be effective during certain months and at different latitudes—conditions that make UVB sun rays more accessible—particularly when treating psoriasis.14 Furthermore, sunbathing may not be as cost-effective in patients with average-severity psoriasis compared with conventional psoriasis therapy because of the costs of travel to areas with sufficient UVB rays for treatment.15 Additionally, insurance status was related to which option was selected as the most cost-effective, as 29 (53.7%) of 54 uninsured participants chose sunbathing as the most cost-effective option, while only 92 (34.2%) of 269 privately insured patients selected sunbathing. Therefore, insurance status may be a factor for dermatologists to consider if a patient prefers a treatment that is cost-effective. Overall, dermatologists could perhaps consider guiding patients and optimizing their treatment plans based on the factors most important to the patients while understanding that costs and insurance status may ultimately determine the treatment option.
Limitations—Survey participants were recruited on Amazon Mechanical Turk, which could create sampling bias. Furthermore, these participants were representative of the general public and not exclusively patients on phototherapy, therefore representing the opinions of the general public and not those who may require phototherapy. Furthermore, given the nature of the survey, the study was limited to the adult population.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix-x.
- Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730-740.
- Su J, Pearce DJ, Feldman SR. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J Dermatolog Treat. 2005;16:324-326.
- Anderson KL, Huang KE, Huang WW, et al. Dermatology residents are prescribing tanning bed treatment. Dermatol Online J. 2016;22:13030/qt19h4k7sx.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanomaattributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:E4757.
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomized controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679.
- Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
- Juarez MC, Grossberg AL. Phototherapy in the pediatric population. Dermatol Clin. 2020;38:91-108.
- Autio P, Komulainen P, Larni HM. Heliotherapy in atopic dermatitis: a prospective study on climatotherapy using the SCORAD index. Acta Derm Venereol. 2002;82:436-440.
- Krzys´cin JW, Jarosławski J, Rajewska-Wie˛ch B, et al. Effectiveness of heliotherapy for psoriasis clearance in low and mid-latitudinal regions: a theoretical approach. J Photochem Photobiol B. 2012;115:35-41.
- Snellman E, Maljanen T, Aromaa A, et al. Effect of heliotherapy on the cost of psoriasis. Br J Dermatol. 1998;138:288-292.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix-x.
- Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730-740.
- Su J, Pearce DJ, Feldman SR. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J Dermatolog Treat. 2005;16:324-326.
- Anderson KL, Huang KE, Huang WW, et al. Dermatology residents are prescribing tanning bed treatment. Dermatol Online J. 2016;22:13030/qt19h4k7sx.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanomaattributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:E4757.
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomized controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679.
- Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
- Juarez MC, Grossberg AL. Phototherapy in the pediatric population. Dermatol Clin. 2020;38:91-108.
- Autio P, Komulainen P, Larni HM. Heliotherapy in atopic dermatitis: a prospective study on climatotherapy using the SCORAD index. Acta Derm Venereol. 2002;82:436-440.
- Krzys´cin JW, Jarosławski J, Rajewska-Wie˛ch B, et al. Effectiveness of heliotherapy for psoriasis clearance in low and mid-latitudinal regions: a theoretical approach. J Photochem Photobiol B. 2012;115:35-41.
- Snellman E, Maljanen T, Aromaa A, et al. Effect of heliotherapy on the cost of psoriasis. Br J Dermatol. 1998;138:288-292.
Practice Points
- Patients have different priorities when selecting phototherapy, including safety, costs, effectiveness, insurance issues, and convenience.
- By offering and educating patients on all forms of phototherapy, dermatologists may help guide patients to their optimal treatment plan according to patient priorities.
FDA will review pediatric indication for roflumilast cream
, according to a press release from the manufacturer.
The company, Arcutis Biotherapeutics, announced the submission of a supplemental new drug application for approval of roflumilast cream (Zoryve), a topical phosphodiesterase-4 (PDE-4) inhibitor, to treat psoriasis in children aged 2-11 years. If approved, this would be the first such product for young children with plaque psoriasis, according to the press release. In July 2022, the FDA approved roflumilast cream 0.3% for the treatment of plaque psoriasis in people 12 years of age and older, including in intertriginous areas, based on data from the phase 3 DERMIS-1 and DERMIS-2 trials.
The new submission is supported by data from two 4-week Maximal Usage Systemic Exposure (MUSE) studies in children ages 2-11 years with plaque psoriasis. In these phase 2, open-label studies, one study of children aged 2-5 years and another study of children aged 6-11 years, participants were treated with roflumilast cream 0.3% once daily for 4 weeks. The MUSE studies are also intended to fulfill postmarketing requirements for roflumilast, according to the company. The MUSE results were consistent with those from DERMIS-1 and DERMIS-2, according to the company press release. In DERMIS-1 and DERMIS-2, significantly more patients randomized to roflumilast met criteria for Investigators Global Success (IGA) scores after 8 weeks of daily treatment compared with placebo patients, and significantly more achieved a 75% reduction in Psoriasis Area and Severity Index (PASI) scores compared with those on placebo.
Common adverse events associated with roflumilast include diarrhea, headache, insomnia, nausea, application site pain, upper respiratory tract infection, and urinary tract infection. None of these have been reported in more than 3% of patients, the press release noted.
, according to a press release from the manufacturer.
The company, Arcutis Biotherapeutics, announced the submission of a supplemental new drug application for approval of roflumilast cream (Zoryve), a topical phosphodiesterase-4 (PDE-4) inhibitor, to treat psoriasis in children aged 2-11 years. If approved, this would be the first such product for young children with plaque psoriasis, according to the press release. In July 2022, the FDA approved roflumilast cream 0.3% for the treatment of plaque psoriasis in people 12 years of age and older, including in intertriginous areas, based on data from the phase 3 DERMIS-1 and DERMIS-2 trials.
The new submission is supported by data from two 4-week Maximal Usage Systemic Exposure (MUSE) studies in children ages 2-11 years with plaque psoriasis. In these phase 2, open-label studies, one study of children aged 2-5 years and another study of children aged 6-11 years, participants were treated with roflumilast cream 0.3% once daily for 4 weeks. The MUSE studies are also intended to fulfill postmarketing requirements for roflumilast, according to the company. The MUSE results were consistent with those from DERMIS-1 and DERMIS-2, according to the company press release. In DERMIS-1 and DERMIS-2, significantly more patients randomized to roflumilast met criteria for Investigators Global Success (IGA) scores after 8 weeks of daily treatment compared with placebo patients, and significantly more achieved a 75% reduction in Psoriasis Area and Severity Index (PASI) scores compared with those on placebo.
Common adverse events associated with roflumilast include diarrhea, headache, insomnia, nausea, application site pain, upper respiratory tract infection, and urinary tract infection. None of these have been reported in more than 3% of patients, the press release noted.
, according to a press release from the manufacturer.
The company, Arcutis Biotherapeutics, announced the submission of a supplemental new drug application for approval of roflumilast cream (Zoryve), a topical phosphodiesterase-4 (PDE-4) inhibitor, to treat psoriasis in children aged 2-11 years. If approved, this would be the first such product for young children with plaque psoriasis, according to the press release. In July 2022, the FDA approved roflumilast cream 0.3% for the treatment of plaque psoriasis in people 12 years of age and older, including in intertriginous areas, based on data from the phase 3 DERMIS-1 and DERMIS-2 trials.
The new submission is supported by data from two 4-week Maximal Usage Systemic Exposure (MUSE) studies in children ages 2-11 years with plaque psoriasis. In these phase 2, open-label studies, one study of children aged 2-5 years and another study of children aged 6-11 years, participants were treated with roflumilast cream 0.3% once daily for 4 weeks. The MUSE studies are also intended to fulfill postmarketing requirements for roflumilast, according to the company. The MUSE results were consistent with those from DERMIS-1 and DERMIS-2, according to the company press release. In DERMIS-1 and DERMIS-2, significantly more patients randomized to roflumilast met criteria for Investigators Global Success (IGA) scores after 8 weeks of daily treatment compared with placebo patients, and significantly more achieved a 75% reduction in Psoriasis Area and Severity Index (PASI) scores compared with those on placebo.
Common adverse events associated with roflumilast include diarrhea, headache, insomnia, nausea, application site pain, upper respiratory tract infection, and urinary tract infection. None of these have been reported in more than 3% of patients, the press release noted.
FDA approves Idacio as eighth adalimumab biosimilar in U.S.
A biosimilar drug to the tumor necrosis factor inhibitor adalimumab, marketed as Idacio (adalimumab-aacf), has been approved by the Food and Drug Administration for use in the United States, according to a press release from manufacturer Fresenius Kabi.
Idacio is a citrate-free, low-concentration formulation of adalimumab and is now approved for use for all but three of the indications that currently apply to the reference adalimumab product (Humira): rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis in adults, ankylosing spondylitis, Crohn’s disease in adults and children aged 6 years or older, ulcerative colitis in adults, and plaque psoriasis in adults. It does not apply to Humira’s indications for hidradenitis suppurativa, uveitis, or ulcerative colitis in pediatric patients aged 5 years and older.
Idacio is the eighth adalimumab biosimilar to be approved in the United States. Its approval was based on evidence of a similar profile of pharmacokinetics, safety, efficacy, and immunogenicity to Humira.
Idacio was first launched in 2019 and has been marketed in more than 37 countries worldwide, according to Fresenius Kabi. The U.S. launch is scheduled for July, and Idacio will be available as a self-administered prefilled syringe or prefilled pen.
A version of this article first appeared on Medscape.com.
A biosimilar drug to the tumor necrosis factor inhibitor adalimumab, marketed as Idacio (adalimumab-aacf), has been approved by the Food and Drug Administration for use in the United States, according to a press release from manufacturer Fresenius Kabi.
Idacio is a citrate-free, low-concentration formulation of adalimumab and is now approved for use for all but three of the indications that currently apply to the reference adalimumab product (Humira): rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis in adults, ankylosing spondylitis, Crohn’s disease in adults and children aged 6 years or older, ulcerative colitis in adults, and plaque psoriasis in adults. It does not apply to Humira’s indications for hidradenitis suppurativa, uveitis, or ulcerative colitis in pediatric patients aged 5 years and older.
Idacio is the eighth adalimumab biosimilar to be approved in the United States. Its approval was based on evidence of a similar profile of pharmacokinetics, safety, efficacy, and immunogenicity to Humira.
Idacio was first launched in 2019 and has been marketed in more than 37 countries worldwide, according to Fresenius Kabi. The U.S. launch is scheduled for July, and Idacio will be available as a self-administered prefilled syringe or prefilled pen.
A version of this article first appeared on Medscape.com.
A biosimilar drug to the tumor necrosis factor inhibitor adalimumab, marketed as Idacio (adalimumab-aacf), has been approved by the Food and Drug Administration for use in the United States, according to a press release from manufacturer Fresenius Kabi.
Idacio is a citrate-free, low-concentration formulation of adalimumab and is now approved for use for all but three of the indications that currently apply to the reference adalimumab product (Humira): rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis in adults, ankylosing spondylitis, Crohn’s disease in adults and children aged 6 years or older, ulcerative colitis in adults, and plaque psoriasis in adults. It does not apply to Humira’s indications for hidradenitis suppurativa, uveitis, or ulcerative colitis in pediatric patients aged 5 years and older.
Idacio is the eighth adalimumab biosimilar to be approved in the United States. Its approval was based on evidence of a similar profile of pharmacokinetics, safety, efficacy, and immunogenicity to Humira.
Idacio was first launched in 2019 and has been marketed in more than 37 countries worldwide, according to Fresenius Kabi. The U.S. launch is scheduled for July, and Idacio will be available as a self-administered prefilled syringe or prefilled pen.
A version of this article first appeared on Medscape.com.
Consider gaps in access and knowledge in diagnosis and treatment in skin of color
LAS VEGAS – and patients, Susan C. Taylor, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Additionally, some disparities occur because of gaps in access to health care, said Dr. Taylor, vice chair, diversity, equity and inclusion, in the department of dermatology at the University of Pennsylvania, Philadelphia, who moderated an expert panel discussion of treatment tips for several common dermatologic conditions in skin of color patients.
Atopic dermatitis angles
Atopic dermatitis (AD) is the fourth most common dermatologic complaint in Black patients, based on data from the United States National Ambulatory Medical Care Survey. Also, data from the National Health and Nutrition Examination Survey show that Black children are nearly twice as likely as White children to develop AD after controlling for socioeconomic factors, Dr. Taylor said.
When Black patients present with AD, “you may not see the erythema,” said Valerie D. Callender, MD, of Howard University, Washington, who presented on AD. Instead, “you may see more follicular and papular presentations.” Erythema and erythroderma can present as shades of violet, gray, or dark brown in patients with rich skin tones, added Dr. Callender, who practices in Glenn Dale, Md.
Consequently, disease severity can be misinterpreted, she said, noting that data suggest that scoring systems such as the Eczema Area and Severity Index and Scoring Atopic Dermatitis underestimate AD severity in dark skin.
As for treatment, skin of color patients with AD are often as bothered by postinflammatory hyperpigmentation (PIH) as by active lesions, so treatment should take these concerns into account, Dr. Callender said. Studies evaluating the effectiveness of AD treatments in diverse populations are limited by lack of representation of racial groups in clinical trials and lack of subset analyses by race.
Acne awareness
An important consideration of acne in skin of color patients is that the acne “might not be red, it might just be darker,” said Andrew F. Alexis, MD, vice-chair for diversity and inclusion in the department of dermatology, and professor of clinical dermatology at Weill Cornell Medicine, New York. A study published in JAMA Dermatology of nearly 30,000 patients with acne from 2007 to 2017 found that non-Hispanic Black patients were more likely than non-Hispanic White patients to see a dermatologist for acne, but Black patients received fewer prescriptions for acne medications than White patients.
The study also showed that Black patients who received prescriptions for acne were more likely to receive topical retinoids and topical antibiotics, and less likely to receive oral antibiotics, spironolactone, or isotretinoin, compared with White patients. Similarly, Asian patients were more likely to receive topical antibiotics and less likely to receive oral antibiotics, compared with White patients.
Other panelists shared some of their best practices for acne in patients with skin of color, including treatment with topical retinoids (for inflammation) and spironolactone, and therapies that address both inflammation and pigmentation, such as salicylic acid and azelaic acid. Dr. Callender also advised asking patients about makeup, as they may not know that many types of makeup used to cover acne are in fact comedogenic.
Melanoma misconceptions
One of the most common misperceptions about melanoma among skin of color patients is that they don’t think they can get it, Dr. Taylor said. Many health care providers don’t think about melanoma in skin of color patients because of the dramatically lower incidence in this population, but as a result, cases may go undiagnosed, and as studies have shown, the mortality rate from melanoma is higher in Black patients.
Consider the palms, soles, nails, and web spaces as possible melanoma sites, Dr. Taylor added.
Educating skin of color patients about melanoma is important, although the incidence is 20 to 30 times lower than in non-Hispanic Whites, said Nada Elbuluk, MD, the founder and director of the University of Southern California Skin of Color Center and Pigmentary Disorders Clinic, Los Angeles. A 2020 editorial published in Cancer Cytopathology pointed out that 1 in 3 Black men or women with a melanoma diagnosis in the United States dies of the disease, compared with 1 in 7 non-Hispanic White men and 1 in 11 non-Hispanic White women with melanoma.
Don’t skip the total body skin exam in these patients, Dr. Elbuluk emphasized. Many patients will only partially undress, and areas such as toes can be missed.
Rosacea review
For patients with skin of color, clinicians need to look for different signs of rosacea than those typically seen in White patients, Dr. Elbuluk said. “The most common presentation of rosacea in skin of color is papulopustular,” and the granulomatous variant.
“These patients will often give you a history of sensitivity to products,” Dr. Elbuluk noted. They may not always have the flushing, but they may report warmth or itching, in addition to product sensitivity.
When considering rosacea in skin of color patients, be sure to have good lighting for close examination, as skin thickening is another subtle sign of rosacea in these patients, she said. Skin thickening “is a very early sign that will present in skin of color with no erythema, so keep that in mind.”
Stinging and burning sensations may be reported by skin of color patients with rosacea. Use patient history to confirm the diagnosis of rosacea, which is often delayed in skin of color patients because of a low index of suspicion, she said.
Psoriasis pointers
Psoriasis in skin of color patients used to be considered rare, “but that is far from true,” Dr. Alexis said. In fact, many cases of psoriasis are undiagnosed or the diagnosis is delayed in these patients.
The panelists noted that current guidelines for psoriasis treatment are based on clinical trials composed mainly of White patients, and do not contain specific recommendations for skin of color patients.
Notably, the morphology, location, and color of psoriasis lesions may be different for patients with darker skin, such as thicker plaques and more scaling over larger areas, they said. Also, skin of color patients may experience long-lasting dyspigmentation from psoriasis lesions that have resolved.
When developing a strategy for psoriasis in skin of color patients, consider not only disease severity, but also comorbidities and medications, response (if any) to prior therapies, patient preferences, and quality of life, the panelists said.
Dr. Callender, Dr. Elbuluk, Dr. Taylor, and Dr. Alexis reported conflicts of interest from numerous sources in industry. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – and patients, Susan C. Taylor, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Additionally, some disparities occur because of gaps in access to health care, said Dr. Taylor, vice chair, diversity, equity and inclusion, in the department of dermatology at the University of Pennsylvania, Philadelphia, who moderated an expert panel discussion of treatment tips for several common dermatologic conditions in skin of color patients.
Atopic dermatitis angles
Atopic dermatitis (AD) is the fourth most common dermatologic complaint in Black patients, based on data from the United States National Ambulatory Medical Care Survey. Also, data from the National Health and Nutrition Examination Survey show that Black children are nearly twice as likely as White children to develop AD after controlling for socioeconomic factors, Dr. Taylor said.
When Black patients present with AD, “you may not see the erythema,” said Valerie D. Callender, MD, of Howard University, Washington, who presented on AD. Instead, “you may see more follicular and papular presentations.” Erythema and erythroderma can present as shades of violet, gray, or dark brown in patients with rich skin tones, added Dr. Callender, who practices in Glenn Dale, Md.
Consequently, disease severity can be misinterpreted, she said, noting that data suggest that scoring systems such as the Eczema Area and Severity Index and Scoring Atopic Dermatitis underestimate AD severity in dark skin.
As for treatment, skin of color patients with AD are often as bothered by postinflammatory hyperpigmentation (PIH) as by active lesions, so treatment should take these concerns into account, Dr. Callender said. Studies evaluating the effectiveness of AD treatments in diverse populations are limited by lack of representation of racial groups in clinical trials and lack of subset analyses by race.
Acne awareness
An important consideration of acne in skin of color patients is that the acne “might not be red, it might just be darker,” said Andrew F. Alexis, MD, vice-chair for diversity and inclusion in the department of dermatology, and professor of clinical dermatology at Weill Cornell Medicine, New York. A study published in JAMA Dermatology of nearly 30,000 patients with acne from 2007 to 2017 found that non-Hispanic Black patients were more likely than non-Hispanic White patients to see a dermatologist for acne, but Black patients received fewer prescriptions for acne medications than White patients.
The study also showed that Black patients who received prescriptions for acne were more likely to receive topical retinoids and topical antibiotics, and less likely to receive oral antibiotics, spironolactone, or isotretinoin, compared with White patients. Similarly, Asian patients were more likely to receive topical antibiotics and less likely to receive oral antibiotics, compared with White patients.
Other panelists shared some of their best practices for acne in patients with skin of color, including treatment with topical retinoids (for inflammation) and spironolactone, and therapies that address both inflammation and pigmentation, such as salicylic acid and azelaic acid. Dr. Callender also advised asking patients about makeup, as they may not know that many types of makeup used to cover acne are in fact comedogenic.
Melanoma misconceptions
One of the most common misperceptions about melanoma among skin of color patients is that they don’t think they can get it, Dr. Taylor said. Many health care providers don’t think about melanoma in skin of color patients because of the dramatically lower incidence in this population, but as a result, cases may go undiagnosed, and as studies have shown, the mortality rate from melanoma is higher in Black patients.
Consider the palms, soles, nails, and web spaces as possible melanoma sites, Dr. Taylor added.
Educating skin of color patients about melanoma is important, although the incidence is 20 to 30 times lower than in non-Hispanic Whites, said Nada Elbuluk, MD, the founder and director of the University of Southern California Skin of Color Center and Pigmentary Disorders Clinic, Los Angeles. A 2020 editorial published in Cancer Cytopathology pointed out that 1 in 3 Black men or women with a melanoma diagnosis in the United States dies of the disease, compared with 1 in 7 non-Hispanic White men and 1 in 11 non-Hispanic White women with melanoma.
Don’t skip the total body skin exam in these patients, Dr. Elbuluk emphasized. Many patients will only partially undress, and areas such as toes can be missed.
Rosacea review
For patients with skin of color, clinicians need to look for different signs of rosacea than those typically seen in White patients, Dr. Elbuluk said. “The most common presentation of rosacea in skin of color is papulopustular,” and the granulomatous variant.
“These patients will often give you a history of sensitivity to products,” Dr. Elbuluk noted. They may not always have the flushing, but they may report warmth or itching, in addition to product sensitivity.
When considering rosacea in skin of color patients, be sure to have good lighting for close examination, as skin thickening is another subtle sign of rosacea in these patients, she said. Skin thickening “is a very early sign that will present in skin of color with no erythema, so keep that in mind.”
Stinging and burning sensations may be reported by skin of color patients with rosacea. Use patient history to confirm the diagnosis of rosacea, which is often delayed in skin of color patients because of a low index of suspicion, she said.
Psoriasis pointers
Psoriasis in skin of color patients used to be considered rare, “but that is far from true,” Dr. Alexis said. In fact, many cases of psoriasis are undiagnosed or the diagnosis is delayed in these patients.
The panelists noted that current guidelines for psoriasis treatment are based on clinical trials composed mainly of White patients, and do not contain specific recommendations for skin of color patients.
Notably, the morphology, location, and color of psoriasis lesions may be different for patients with darker skin, such as thicker plaques and more scaling over larger areas, they said. Also, skin of color patients may experience long-lasting dyspigmentation from psoriasis lesions that have resolved.
When developing a strategy for psoriasis in skin of color patients, consider not only disease severity, but also comorbidities and medications, response (if any) to prior therapies, patient preferences, and quality of life, the panelists said.
Dr. Callender, Dr. Elbuluk, Dr. Taylor, and Dr. Alexis reported conflicts of interest from numerous sources in industry. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – and patients, Susan C. Taylor, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Additionally, some disparities occur because of gaps in access to health care, said Dr. Taylor, vice chair, diversity, equity and inclusion, in the department of dermatology at the University of Pennsylvania, Philadelphia, who moderated an expert panel discussion of treatment tips for several common dermatologic conditions in skin of color patients.
Atopic dermatitis angles
Atopic dermatitis (AD) is the fourth most common dermatologic complaint in Black patients, based on data from the United States National Ambulatory Medical Care Survey. Also, data from the National Health and Nutrition Examination Survey show that Black children are nearly twice as likely as White children to develop AD after controlling for socioeconomic factors, Dr. Taylor said.
When Black patients present with AD, “you may not see the erythema,” said Valerie D. Callender, MD, of Howard University, Washington, who presented on AD. Instead, “you may see more follicular and papular presentations.” Erythema and erythroderma can present as shades of violet, gray, or dark brown in patients with rich skin tones, added Dr. Callender, who practices in Glenn Dale, Md.
Consequently, disease severity can be misinterpreted, she said, noting that data suggest that scoring systems such as the Eczema Area and Severity Index and Scoring Atopic Dermatitis underestimate AD severity in dark skin.
As for treatment, skin of color patients with AD are often as bothered by postinflammatory hyperpigmentation (PIH) as by active lesions, so treatment should take these concerns into account, Dr. Callender said. Studies evaluating the effectiveness of AD treatments in diverse populations are limited by lack of representation of racial groups in clinical trials and lack of subset analyses by race.
Acne awareness
An important consideration of acne in skin of color patients is that the acne “might not be red, it might just be darker,” said Andrew F. Alexis, MD, vice-chair for diversity and inclusion in the department of dermatology, and professor of clinical dermatology at Weill Cornell Medicine, New York. A study published in JAMA Dermatology of nearly 30,000 patients with acne from 2007 to 2017 found that non-Hispanic Black patients were more likely than non-Hispanic White patients to see a dermatologist for acne, but Black patients received fewer prescriptions for acne medications than White patients.
The study also showed that Black patients who received prescriptions for acne were more likely to receive topical retinoids and topical antibiotics, and less likely to receive oral antibiotics, spironolactone, or isotretinoin, compared with White patients. Similarly, Asian patients were more likely to receive topical antibiotics and less likely to receive oral antibiotics, compared with White patients.
Other panelists shared some of their best practices for acne in patients with skin of color, including treatment with topical retinoids (for inflammation) and spironolactone, and therapies that address both inflammation and pigmentation, such as salicylic acid and azelaic acid. Dr. Callender also advised asking patients about makeup, as they may not know that many types of makeup used to cover acne are in fact comedogenic.
Melanoma misconceptions
One of the most common misperceptions about melanoma among skin of color patients is that they don’t think they can get it, Dr. Taylor said. Many health care providers don’t think about melanoma in skin of color patients because of the dramatically lower incidence in this population, but as a result, cases may go undiagnosed, and as studies have shown, the mortality rate from melanoma is higher in Black patients.
Consider the palms, soles, nails, and web spaces as possible melanoma sites, Dr. Taylor added.
Educating skin of color patients about melanoma is important, although the incidence is 20 to 30 times lower than in non-Hispanic Whites, said Nada Elbuluk, MD, the founder and director of the University of Southern California Skin of Color Center and Pigmentary Disorders Clinic, Los Angeles. A 2020 editorial published in Cancer Cytopathology pointed out that 1 in 3 Black men or women with a melanoma diagnosis in the United States dies of the disease, compared with 1 in 7 non-Hispanic White men and 1 in 11 non-Hispanic White women with melanoma.
Don’t skip the total body skin exam in these patients, Dr. Elbuluk emphasized. Many patients will only partially undress, and areas such as toes can be missed.
Rosacea review
For patients with skin of color, clinicians need to look for different signs of rosacea than those typically seen in White patients, Dr. Elbuluk said. “The most common presentation of rosacea in skin of color is papulopustular,” and the granulomatous variant.
“These patients will often give you a history of sensitivity to products,” Dr. Elbuluk noted. They may not always have the flushing, but they may report warmth or itching, in addition to product sensitivity.
When considering rosacea in skin of color patients, be sure to have good lighting for close examination, as skin thickening is another subtle sign of rosacea in these patients, she said. Skin thickening “is a very early sign that will present in skin of color with no erythema, so keep that in mind.”
Stinging and burning sensations may be reported by skin of color patients with rosacea. Use patient history to confirm the diagnosis of rosacea, which is often delayed in skin of color patients because of a low index of suspicion, she said.
Psoriasis pointers
Psoriasis in skin of color patients used to be considered rare, “but that is far from true,” Dr. Alexis said. In fact, many cases of psoriasis are undiagnosed or the diagnosis is delayed in these patients.
The panelists noted that current guidelines for psoriasis treatment are based on clinical trials composed mainly of White patients, and do not contain specific recommendations for skin of color patients.
Notably, the morphology, location, and color of psoriasis lesions may be different for patients with darker skin, such as thicker plaques and more scaling over larger areas, they said. Also, skin of color patients may experience long-lasting dyspigmentation from psoriasis lesions that have resolved.
When developing a strategy for psoriasis in skin of color patients, consider not only disease severity, but also comorbidities and medications, response (if any) to prior therapies, patient preferences, and quality of life, the panelists said.
Dr. Callender, Dr. Elbuluk, Dr. Taylor, and Dr. Alexis reported conflicts of interest from numerous sources in industry. MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Skinny-label biosimilars provide substantial savings to Medicare
Recent court rulings could put such saving under threat
Competition between five biologic drugs and their skinny-label biosimilars saved Medicare an estimated $1.5 billion during 2015-2020. But these savings accruing to Medicare and the availability of those and other biosimilars through skinny labeling is under threat from recent court rulings, according to a research letter published online in JAMA Internal Medicine.
The authors highlighted the need for such savings by noting that, while biologics comprise less than 5% of prescription drug use, their price tag amounts to about 40% of U.S. drug spending, Biologic manufacturers often delay the availability of biosimilars for additional years beyond the original patent expiration through further patents for supplemental indications. To provide a counterbalance, federal law allows the Food and Drug Administration to approve “skinny-label” generics and biosimilars that carve out patent-protected indications or regulatory exclusivities. But once a generic drug reaches the market through this process with a skinny label, it may often be substituted for indications that go beyond the ones listed on the skinny label. In fact, some state laws mandate that pharmacists substitute interchangeable generics for brand-name drugs, helping to decrease drug prices. In response to legal threats to the skinny-label pathway, Alexander C. Egilman and colleagues at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, assessed the frequency of approval and marketing of skinny-label biosimilars from 2015 to 2021 and the resultant savings to Medicare.
The authors estimated annual Part B (clinician-administered) savings from skinny-label biosimilars through 2020 by comparing actual biologic and skinny-label biosimilar spending with estimated biologic spending without competition using the Medicare Dashboard. They assumed that the unit price of the biologic would increase at its 5-year compound annual growth rate prior to competition.
In that period, the FDA approved 33 biosimilars linked to 11 biologics. Among them, 22 (66.7%) had a skinny label. Of 21 biosimilars marketed before 2022, 13 (61.9%) were launched with a skinny label. Of the 8 biologics linked to these 21 biosimilars, 5 of the first-to-market biosimilars had skinny labels (bevacizumab, filgrastim, infliximab, pegfilgrastim, and rituximab), leading to earlier competition through 2021.
The estimated $1.5 billion in savings to Medicare from these skinny-label biosimilars over the 2015-2020 span represents 4.9% of the $30.2 billion that Medicare spent on the five biologics during this period. The researchers pointed out that once adalimumab (Humira) faces skinny-label biosimilar competition in 2023, savings will likely grow substantially.
In response to the research letter, an editor’s note by JAMA Internal Medicine Editorial Fellow Eric Ward, MD, and JAMA Internal Medicine Editor at Large and Online Editor Robert Steinbrook, MD, stated that, between 2015 and 2019, 24 (43%) of 56 brand-name drugs had competition from skinny-labeled generic formulations after first becoming available as generics.
The editors also referenced a JAMA Viewpoints article from 2021 that reviewed the most recent case challenging the skinny-label pathway in which GlaxoSmithKline sued Teva for its marketing of a skinny-label generic of the brand-name beta-blocker carvedilol (Coreg) that the plaintive claimed “induced physicians to prescribe carvedilol for indications that had been carved out by Teva’s skinny label, thus infringing GlaxoSmithKline’s patents.” A $235 million judgment against Teva was overturned by a district court and then reversed again by a Federal Circuit court that, after receiving criticism, reconsidered the case, and a panel affirmed the judgment against Teva.
“The Federal Circuit panel’s decision has the potential to put generic drugs that fail to adequately carve out indications from the brand name labeling at risk for damages related to infringement,” the authors wrote. Similar claims of infringement are being heard in other courts, they wrote, and they urged careful targeting of skinny-label carveouts, and suggest also that challenges to the arguments used against Teva focus on preservation of First Amendment rights as protection for lawful and accurate speech in drug labels.
“The legal uncertainties are likely to continue, as manufacturers pursue novel and complex strategies to protect the patents and regulatory exclusivities of brand-name drugs and biologics,” Dr. Ward and Dr. Steinbrook wrote, adding that “the path forward is for Congress to enact additional legislation that reaffirms and strengthens the permissibility of skinny labeling.”
The research letter’s corresponding author, Ameet Sarpatwari, PhD, JD, assistant professor at Harvard Medical School, and assistant director for the Harvard Program On Regulation, Therapeutics, And Law, echoed concerns over the Teva case in an interview. “There has certainly been concern that should the appellate decision stand, there will be a chilling effect. As the lone dissenter in that case noted, ‘no skinny-label generic is safe.’ I think many generic and biosimilar manufacturers are awaiting to see whether the Supreme Court will take the case.”
He added: “I do not believe the likelihood of skinny-label-supportive legislation making it through Congress will be greatly diminished in a divided Congress. Democrats and Republicans alike should seek to promote competition in the marketplace, which is what the skinny-labeling pathway accomplishes.”
The authors reported no relevant conflicts of interest. The research was funded by a grant from Arnold Ventures.
Recent court rulings could put such saving under threat
Recent court rulings could put such saving under threat
Competition between five biologic drugs and their skinny-label biosimilars saved Medicare an estimated $1.5 billion during 2015-2020. But these savings accruing to Medicare and the availability of those and other biosimilars through skinny labeling is under threat from recent court rulings, according to a research letter published online in JAMA Internal Medicine.
The authors highlighted the need for such savings by noting that, while biologics comprise less than 5% of prescription drug use, their price tag amounts to about 40% of U.S. drug spending, Biologic manufacturers often delay the availability of biosimilars for additional years beyond the original patent expiration through further patents for supplemental indications. To provide a counterbalance, federal law allows the Food and Drug Administration to approve “skinny-label” generics and biosimilars that carve out patent-protected indications or regulatory exclusivities. But once a generic drug reaches the market through this process with a skinny label, it may often be substituted for indications that go beyond the ones listed on the skinny label. In fact, some state laws mandate that pharmacists substitute interchangeable generics for brand-name drugs, helping to decrease drug prices. In response to legal threats to the skinny-label pathway, Alexander C. Egilman and colleagues at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, assessed the frequency of approval and marketing of skinny-label biosimilars from 2015 to 2021 and the resultant savings to Medicare.
The authors estimated annual Part B (clinician-administered) savings from skinny-label biosimilars through 2020 by comparing actual biologic and skinny-label biosimilar spending with estimated biologic spending without competition using the Medicare Dashboard. They assumed that the unit price of the biologic would increase at its 5-year compound annual growth rate prior to competition.
In that period, the FDA approved 33 biosimilars linked to 11 biologics. Among them, 22 (66.7%) had a skinny label. Of 21 biosimilars marketed before 2022, 13 (61.9%) were launched with a skinny label. Of the 8 biologics linked to these 21 biosimilars, 5 of the first-to-market biosimilars had skinny labels (bevacizumab, filgrastim, infliximab, pegfilgrastim, and rituximab), leading to earlier competition through 2021.
The estimated $1.5 billion in savings to Medicare from these skinny-label biosimilars over the 2015-2020 span represents 4.9% of the $30.2 billion that Medicare spent on the five biologics during this period. The researchers pointed out that once adalimumab (Humira) faces skinny-label biosimilar competition in 2023, savings will likely grow substantially.
In response to the research letter, an editor’s note by JAMA Internal Medicine Editorial Fellow Eric Ward, MD, and JAMA Internal Medicine Editor at Large and Online Editor Robert Steinbrook, MD, stated that, between 2015 and 2019, 24 (43%) of 56 brand-name drugs had competition from skinny-labeled generic formulations after first becoming available as generics.
The editors also referenced a JAMA Viewpoints article from 2021 that reviewed the most recent case challenging the skinny-label pathway in which GlaxoSmithKline sued Teva for its marketing of a skinny-label generic of the brand-name beta-blocker carvedilol (Coreg) that the plaintive claimed “induced physicians to prescribe carvedilol for indications that had been carved out by Teva’s skinny label, thus infringing GlaxoSmithKline’s patents.” A $235 million judgment against Teva was overturned by a district court and then reversed again by a Federal Circuit court that, after receiving criticism, reconsidered the case, and a panel affirmed the judgment against Teva.
“The Federal Circuit panel’s decision has the potential to put generic drugs that fail to adequately carve out indications from the brand name labeling at risk for damages related to infringement,” the authors wrote. Similar claims of infringement are being heard in other courts, they wrote, and they urged careful targeting of skinny-label carveouts, and suggest also that challenges to the arguments used against Teva focus on preservation of First Amendment rights as protection for lawful and accurate speech in drug labels.
“The legal uncertainties are likely to continue, as manufacturers pursue novel and complex strategies to protect the patents and regulatory exclusivities of brand-name drugs and biologics,” Dr. Ward and Dr. Steinbrook wrote, adding that “the path forward is for Congress to enact additional legislation that reaffirms and strengthens the permissibility of skinny labeling.”
The research letter’s corresponding author, Ameet Sarpatwari, PhD, JD, assistant professor at Harvard Medical School, and assistant director for the Harvard Program On Regulation, Therapeutics, And Law, echoed concerns over the Teva case in an interview. “There has certainly been concern that should the appellate decision stand, there will be a chilling effect. As the lone dissenter in that case noted, ‘no skinny-label generic is safe.’ I think many generic and biosimilar manufacturers are awaiting to see whether the Supreme Court will take the case.”
He added: “I do not believe the likelihood of skinny-label-supportive legislation making it through Congress will be greatly diminished in a divided Congress. Democrats and Republicans alike should seek to promote competition in the marketplace, which is what the skinny-labeling pathway accomplishes.”
The authors reported no relevant conflicts of interest. The research was funded by a grant from Arnold Ventures.
Competition between five biologic drugs and their skinny-label biosimilars saved Medicare an estimated $1.5 billion during 2015-2020. But these savings accruing to Medicare and the availability of those and other biosimilars through skinny labeling is under threat from recent court rulings, according to a research letter published online in JAMA Internal Medicine.
The authors highlighted the need for such savings by noting that, while biologics comprise less than 5% of prescription drug use, their price tag amounts to about 40% of U.S. drug spending, Biologic manufacturers often delay the availability of biosimilars for additional years beyond the original patent expiration through further patents for supplemental indications. To provide a counterbalance, federal law allows the Food and Drug Administration to approve “skinny-label” generics and biosimilars that carve out patent-protected indications or regulatory exclusivities. But once a generic drug reaches the market through this process with a skinny label, it may often be substituted for indications that go beyond the ones listed on the skinny label. In fact, some state laws mandate that pharmacists substitute interchangeable generics for brand-name drugs, helping to decrease drug prices. In response to legal threats to the skinny-label pathway, Alexander C. Egilman and colleagues at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, assessed the frequency of approval and marketing of skinny-label biosimilars from 2015 to 2021 and the resultant savings to Medicare.
The authors estimated annual Part B (clinician-administered) savings from skinny-label biosimilars through 2020 by comparing actual biologic and skinny-label biosimilar spending with estimated biologic spending without competition using the Medicare Dashboard. They assumed that the unit price of the biologic would increase at its 5-year compound annual growth rate prior to competition.
In that period, the FDA approved 33 biosimilars linked to 11 biologics. Among them, 22 (66.7%) had a skinny label. Of 21 biosimilars marketed before 2022, 13 (61.9%) were launched with a skinny label. Of the 8 biologics linked to these 21 biosimilars, 5 of the first-to-market biosimilars had skinny labels (bevacizumab, filgrastim, infliximab, pegfilgrastim, and rituximab), leading to earlier competition through 2021.
The estimated $1.5 billion in savings to Medicare from these skinny-label biosimilars over the 2015-2020 span represents 4.9% of the $30.2 billion that Medicare spent on the five biologics during this period. The researchers pointed out that once adalimumab (Humira) faces skinny-label biosimilar competition in 2023, savings will likely grow substantially.
In response to the research letter, an editor’s note by JAMA Internal Medicine Editorial Fellow Eric Ward, MD, and JAMA Internal Medicine Editor at Large and Online Editor Robert Steinbrook, MD, stated that, between 2015 and 2019, 24 (43%) of 56 brand-name drugs had competition from skinny-labeled generic formulations after first becoming available as generics.
The editors also referenced a JAMA Viewpoints article from 2021 that reviewed the most recent case challenging the skinny-label pathway in which GlaxoSmithKline sued Teva for its marketing of a skinny-label generic of the brand-name beta-blocker carvedilol (Coreg) that the plaintive claimed “induced physicians to prescribe carvedilol for indications that had been carved out by Teva’s skinny label, thus infringing GlaxoSmithKline’s patents.” A $235 million judgment against Teva was overturned by a district court and then reversed again by a Federal Circuit court that, after receiving criticism, reconsidered the case, and a panel affirmed the judgment against Teva.
“The Federal Circuit panel’s decision has the potential to put generic drugs that fail to adequately carve out indications from the brand name labeling at risk for damages related to infringement,” the authors wrote. Similar claims of infringement are being heard in other courts, they wrote, and they urged careful targeting of skinny-label carveouts, and suggest also that challenges to the arguments used against Teva focus on preservation of First Amendment rights as protection for lawful and accurate speech in drug labels.
“The legal uncertainties are likely to continue, as manufacturers pursue novel and complex strategies to protect the patents and regulatory exclusivities of brand-name drugs and biologics,” Dr. Ward and Dr. Steinbrook wrote, adding that “the path forward is for Congress to enact additional legislation that reaffirms and strengthens the permissibility of skinny labeling.”
The research letter’s corresponding author, Ameet Sarpatwari, PhD, JD, assistant professor at Harvard Medical School, and assistant director for the Harvard Program On Regulation, Therapeutics, And Law, echoed concerns over the Teva case in an interview. “There has certainly been concern that should the appellate decision stand, there will be a chilling effect. As the lone dissenter in that case noted, ‘no skinny-label generic is safe.’ I think many generic and biosimilar manufacturers are awaiting to see whether the Supreme Court will take the case.”
He added: “I do not believe the likelihood of skinny-label-supportive legislation making it through Congress will be greatly diminished in a divided Congress. Democrats and Republicans alike should seek to promote competition in the marketplace, which is what the skinny-labeling pathway accomplishes.”
The authors reported no relevant conflicts of interest. The research was funded by a grant from Arnold Ventures.
FROM JAMA INTERNAL MEDICINE