User login
FDA okays spesolimab, first treatment for generalized pustular psoriasis
The U.S. Food and Drug Administration has approved the biologic agent spesolimab (Spevigo) for the treatment of flares in adults with generalized pustular psoriasis (GPP), the company that manufactures the drug has announced.
Until this approval, “there were no FDA-approved options to treat patients experiencing a GPP flare,” Mark Lebwohl, MD, principal investigator in the pivotal spesolimab trial, told this news organization. The approval “is a turning point for dermatologists and clinicians who treat patients living with this devastating and debilitating disease,” he said. Treatment with spesolimab “rapidly improves the clinical symptoms of GPP flares and will greatly improve our ability to help our patients manage painful flares,” noted Dr. Lebwohl, dean of clinical therapeutics and professor of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Spesolimab, manufactured by Boehringer Ingelheim, is a novel, selective monoclonal antibody that blocks interleukin-36 signaling known to be involved in GPP. It received priority review and had orphan drug and breakthrough therapy designation.
GPP affects an estimated 1 of every 10,000 people in the United States.
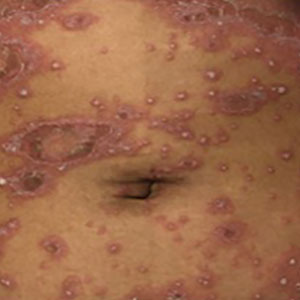
Though rare, GPP is a potentially life-threatening disease that is distinct from plaque psoriasis. GPP is caused by the accumulation of neutrophils in the skin. Throughout the course of the disease, patients may suffer recurring episodes of widespread eruptions of painful, sterile pustules across all parts of the body.
Spesolimab was evaluated in a global, 12-week, placebo-controlled clinical trial that involved 53 adults experiencing a GPP flare. After 1 week, significantly more patients treated with spesolimab than placebo showed no visible pustules (54% vs 6%), according to the company.
The most common adverse reactions, seen in at least 5% of patients treated with spesolimab, were asthenia and fatigue; nausea and vomiting; headache; pruritus and prurigo; hematoma and bruising at the infusion site; and urinary tract infection.
Dr. Lebwohl is a paid consultant to Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
This article was updated 9/6/22.
The U.S. Food and Drug Administration has approved the biologic agent spesolimab (Spevigo) for the treatment of flares in adults with generalized pustular psoriasis (GPP), the company that manufactures the drug has announced.
Until this approval, “there were no FDA-approved options to treat patients experiencing a GPP flare,” Mark Lebwohl, MD, principal investigator in the pivotal spesolimab trial, told this news organization. The approval “is a turning point for dermatologists and clinicians who treat patients living with this devastating and debilitating disease,” he said. Treatment with spesolimab “rapidly improves the clinical symptoms of GPP flares and will greatly improve our ability to help our patients manage painful flares,” noted Dr. Lebwohl, dean of clinical therapeutics and professor of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Spesolimab, manufactured by Boehringer Ingelheim, is a novel, selective monoclonal antibody that blocks interleukin-36 signaling known to be involved in GPP. It received priority review and had orphan drug and breakthrough therapy designation.
GPP affects an estimated 1 of every 10,000 people in the United States.
Though rare, GPP is a potentially life-threatening disease that is distinct from plaque psoriasis. GPP is caused by the accumulation of neutrophils in the skin. Throughout the course of the disease, patients may suffer recurring episodes of widespread eruptions of painful, sterile pustules across all parts of the body.
Spesolimab was evaluated in a global, 12-week, placebo-controlled clinical trial that involved 53 adults experiencing a GPP flare. After 1 week, significantly more patients treated with spesolimab than placebo showed no visible pustules (54% vs 6%), according to the company.
The most common adverse reactions, seen in at least 5% of patients treated with spesolimab, were asthenia and fatigue; nausea and vomiting; headache; pruritus and prurigo; hematoma and bruising at the infusion site; and urinary tract infection.
Dr. Lebwohl is a paid consultant to Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
This article was updated 9/6/22.
The U.S. Food and Drug Administration has approved the biologic agent spesolimab (Spevigo) for the treatment of flares in adults with generalized pustular psoriasis (GPP), the company that manufactures the drug has announced.
Until this approval, “there were no FDA-approved options to treat patients experiencing a GPP flare,” Mark Lebwohl, MD, principal investigator in the pivotal spesolimab trial, told this news organization. The approval “is a turning point for dermatologists and clinicians who treat patients living with this devastating and debilitating disease,” he said. Treatment with spesolimab “rapidly improves the clinical symptoms of GPP flares and will greatly improve our ability to help our patients manage painful flares,” noted Dr. Lebwohl, dean of clinical therapeutics and professor of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Spesolimab, manufactured by Boehringer Ingelheim, is a novel, selective monoclonal antibody that blocks interleukin-36 signaling known to be involved in GPP. It received priority review and had orphan drug and breakthrough therapy designation.
GPP affects an estimated 1 of every 10,000 people in the United States.
Though rare, GPP is a potentially life-threatening disease that is distinct from plaque psoriasis. GPP is caused by the accumulation of neutrophils in the skin. Throughout the course of the disease, patients may suffer recurring episodes of widespread eruptions of painful, sterile pustules across all parts of the body.
Spesolimab was evaluated in a global, 12-week, placebo-controlled clinical trial that involved 53 adults experiencing a GPP flare. After 1 week, significantly more patients treated with spesolimab than placebo showed no visible pustules (54% vs 6%), according to the company.
The most common adverse reactions, seen in at least 5% of patients treated with spesolimab, were asthenia and fatigue; nausea and vomiting; headache; pruritus and prurigo; hematoma and bruising at the infusion site; and urinary tract infection.
Dr. Lebwohl is a paid consultant to Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
This article was updated 9/6/22.
Autoimmune disease patients’ waxing, waning response to COVID vaccination studied in-depth
A new study in The Lancet Rheumatology examines the strength and duration of SARS-CoV-2 vaccine–induced immunoglobulin-G antibody responses over time for patients with a variety of autoimmune diseases, compared with healthy controls.
The presence of humoral antibodies to SARS-CoV-2 has been shown to correlate with protection against COVID infection. But for patients with immune-mediated inflammatory diseases (IMIDs), host response to COVID infection or to vaccination is affected by the immune dysfunction imposed by the IMID and by the use of immune-modulating drugs to treat it.
This new study finds a weaker – as shown previously – and less sustained immune response to SARS-CoV-2 vaccines in patients with a variety of IMIDs, including rheumatoid arthritis, spondyloarthritis, psoriasis, inflammatory bowel diseases, and other systemic autoimmune diseases such as lupus. It also points toward the possibility of adjusting treatment and vaccination schedules and strategies for these patients based on their antibody levels, among other factors, to preserve best protection against severe COVID.
“It is important to assess immune response in these patients to see if they still have protection against severe COVID infection,” said lead author David Simon, MD, senior clinical scientist in clinical immunology and rheumatology at University Hospital Erlangen (Germany). “We know that antibody response is an immune correlate. Therefore, it is important to see how large and durable the immune response is to the coronavirus vaccine in these IMID patients, and whether specific drugs or therapies have negative effects on their immune response.”
What was studied?
For this large prospective cohort study, researchers registered 5076 coronavirus-vaccinated individuals. They analyzed serum samples obtained between December 15, 2020, and December 1, 2021, from 2,535 patients diagnosed with IMIDs and participating in a prospective coronavirus study program at the Deutsches Zentrum Immuntherapie in Erlangen. The IMID patients had a mean age of 55.0 years, and 58.9% were women.
A healthy control group of 1,198 individuals without IMID who had a mean age of 40.7 years, including 53.8% men, was also recruited for the analysis. All approved coronavirus vaccines were included, following standard vaccination schedules. Antibody response was measured over time by an enzyme-linked immunosorbent assay from 8 weeks after first vaccination to week 40.
Among the findings, the healthy controls had higher postvaccine antibody levels than did those with IMIDs. But the majority of vaccinated patients with IMID were able to build up a humoral immune response to SARS-CoV-2. Patients who were taking B-cell inhibitors like rituximab (Rituxan, Genentech; and biosimilars) and T-cell inhibitors like abatacept (Orencia, Bristol Myers Squibb) for IMIDs had significantly poorer antibody response.
Greater age and the use of combination therapies for IMIDs, compared with monotherapy, further reduced immune response to the vaccine. In terms of vaccination modality, messenger RNA–based vaccines induced higher antibody levels than did vector-based vaccines. The researchers noted that patients with IMID who were given a third vaccine dose could actually catch up well with the antibody responses observed in healthy controls.
“We looked at whether different IMIDs had a different humoral response, and we also assessed if there are effects from different therapeutic strategies,” Dr. Simon explained. “It doesn’t matter so much what kind of IMID patients have; much more important is the specific drug treatment and its impact on their antibody response.” Some participants were advised to briefly stop taking some immunosuppressive treatments before or after vaccination.
One of Dr. Simon’s coauthors, statistician and rheumatologist Koray Tascilar, MD, added, “This research is important because we looked not only at who responded less, which has been previously established, but who are at greater risk of losing their immune response, and how quickly.”
Need to take care
“Most treatments we as rheumatologists give to our patients don’t affect their SARS-CoV-2 humoral response,” Dr. Simon said. “However, there are specific drugs that are associated with lower antibody response. With respect to those drugs, we have to be more careful.”
It is important to be able to tell patients which drugs are safe and won’t have a negative impact on their immune response to vaccinations, Dr. Tascilar said. “But it would be too strong to say we’re ready to choose therapies based on their potential impact on protection against COVID. Yes, there is a risk from catching COVID, but we need to balance that risk with the risk of not giving patients the medications that are necessary to treat their rheumatologic condition.”
These diseases are serious, sometimes life-threatening. “We might think of strategies for how to mitigate the risk of underprotection from COVID that is brought about by these treatments,” he said. For example, offering boosters sooner or more frequently, or prophylactically treating with monoclonal antibodies.
“This study, along other recent studies, has found that antibody levels in patients with immune-mediated diseases wane more rapidly than in healthy controls, and this is especially true of those on medications that interfere with the B and T cells and anticytokine therapies,” Rebecca Haberman, MD, assistant professor, division of rheumatology, New York University Langone Health, noted in an email to this news organization.
“While there is no known antibody level that specifically correlates with clinical protection, and each patient needs to be thought of individually, these findings support the use of supplemental booster dosing in patients with immune-mediated inflammatory diseases,” Dr. Haberman said, adding that her own research in this area has shown similar results.
“As a rheumatologist, I would be more likely to encourage my patients – especially those on immunomodulatory medications – to get boosted.”
Dr. Tascilar said his study does not directly answer the question of whether an earlier booster shot would be an effective strategy for patients with IMID. “In our department, we have an early boosting strategy, based on level of immune response.” But the decision of revaccination or not, and when, is based on a number of factors, not only on the level of antibodies. “It’s just part of the instruments we are using.”
The study was supported by the Deutsche Forschungsgemeinschaft. Dr. Simon and Dr. Tascilar declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study in The Lancet Rheumatology examines the strength and duration of SARS-CoV-2 vaccine–induced immunoglobulin-G antibody responses over time for patients with a variety of autoimmune diseases, compared with healthy controls.
The presence of humoral antibodies to SARS-CoV-2 has been shown to correlate with protection against COVID infection. But for patients with immune-mediated inflammatory diseases (IMIDs), host response to COVID infection or to vaccination is affected by the immune dysfunction imposed by the IMID and by the use of immune-modulating drugs to treat it.
This new study finds a weaker – as shown previously – and less sustained immune response to SARS-CoV-2 vaccines in patients with a variety of IMIDs, including rheumatoid arthritis, spondyloarthritis, psoriasis, inflammatory bowel diseases, and other systemic autoimmune diseases such as lupus. It also points toward the possibility of adjusting treatment and vaccination schedules and strategies for these patients based on their antibody levels, among other factors, to preserve best protection against severe COVID.
“It is important to assess immune response in these patients to see if they still have protection against severe COVID infection,” said lead author David Simon, MD, senior clinical scientist in clinical immunology and rheumatology at University Hospital Erlangen (Germany). “We know that antibody response is an immune correlate. Therefore, it is important to see how large and durable the immune response is to the coronavirus vaccine in these IMID patients, and whether specific drugs or therapies have negative effects on their immune response.”
What was studied?
For this large prospective cohort study, researchers registered 5076 coronavirus-vaccinated individuals. They analyzed serum samples obtained between December 15, 2020, and December 1, 2021, from 2,535 patients diagnosed with IMIDs and participating in a prospective coronavirus study program at the Deutsches Zentrum Immuntherapie in Erlangen. The IMID patients had a mean age of 55.0 years, and 58.9% were women.
A healthy control group of 1,198 individuals without IMID who had a mean age of 40.7 years, including 53.8% men, was also recruited for the analysis. All approved coronavirus vaccines were included, following standard vaccination schedules. Antibody response was measured over time by an enzyme-linked immunosorbent assay from 8 weeks after first vaccination to week 40.
Among the findings, the healthy controls had higher postvaccine antibody levels than did those with IMIDs. But the majority of vaccinated patients with IMID were able to build up a humoral immune response to SARS-CoV-2. Patients who were taking B-cell inhibitors like rituximab (Rituxan, Genentech; and biosimilars) and T-cell inhibitors like abatacept (Orencia, Bristol Myers Squibb) for IMIDs had significantly poorer antibody response.
Greater age and the use of combination therapies for IMIDs, compared with monotherapy, further reduced immune response to the vaccine. In terms of vaccination modality, messenger RNA–based vaccines induced higher antibody levels than did vector-based vaccines. The researchers noted that patients with IMID who were given a third vaccine dose could actually catch up well with the antibody responses observed in healthy controls.
“We looked at whether different IMIDs had a different humoral response, and we also assessed if there are effects from different therapeutic strategies,” Dr. Simon explained. “It doesn’t matter so much what kind of IMID patients have; much more important is the specific drug treatment and its impact on their antibody response.” Some participants were advised to briefly stop taking some immunosuppressive treatments before or after vaccination.
One of Dr. Simon’s coauthors, statistician and rheumatologist Koray Tascilar, MD, added, “This research is important because we looked not only at who responded less, which has been previously established, but who are at greater risk of losing their immune response, and how quickly.”
Need to take care
“Most treatments we as rheumatologists give to our patients don’t affect their SARS-CoV-2 humoral response,” Dr. Simon said. “However, there are specific drugs that are associated with lower antibody response. With respect to those drugs, we have to be more careful.”
It is important to be able to tell patients which drugs are safe and won’t have a negative impact on their immune response to vaccinations, Dr. Tascilar said. “But it would be too strong to say we’re ready to choose therapies based on their potential impact on protection against COVID. Yes, there is a risk from catching COVID, but we need to balance that risk with the risk of not giving patients the medications that are necessary to treat their rheumatologic condition.”
These diseases are serious, sometimes life-threatening. “We might think of strategies for how to mitigate the risk of underprotection from COVID that is brought about by these treatments,” he said. For example, offering boosters sooner or more frequently, or prophylactically treating with monoclonal antibodies.
“This study, along other recent studies, has found that antibody levels in patients with immune-mediated diseases wane more rapidly than in healthy controls, and this is especially true of those on medications that interfere with the B and T cells and anticytokine therapies,” Rebecca Haberman, MD, assistant professor, division of rheumatology, New York University Langone Health, noted in an email to this news organization.
“While there is no known antibody level that specifically correlates with clinical protection, and each patient needs to be thought of individually, these findings support the use of supplemental booster dosing in patients with immune-mediated inflammatory diseases,” Dr. Haberman said, adding that her own research in this area has shown similar results.
“As a rheumatologist, I would be more likely to encourage my patients – especially those on immunomodulatory medications – to get boosted.”
Dr. Tascilar said his study does not directly answer the question of whether an earlier booster shot would be an effective strategy for patients with IMID. “In our department, we have an early boosting strategy, based on level of immune response.” But the decision of revaccination or not, and when, is based on a number of factors, not only on the level of antibodies. “It’s just part of the instruments we are using.”
The study was supported by the Deutsche Forschungsgemeinschaft. Dr. Simon and Dr. Tascilar declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study in The Lancet Rheumatology examines the strength and duration of SARS-CoV-2 vaccine–induced immunoglobulin-G antibody responses over time for patients with a variety of autoimmune diseases, compared with healthy controls.
The presence of humoral antibodies to SARS-CoV-2 has been shown to correlate with protection against COVID infection. But for patients with immune-mediated inflammatory diseases (IMIDs), host response to COVID infection or to vaccination is affected by the immune dysfunction imposed by the IMID and by the use of immune-modulating drugs to treat it.
This new study finds a weaker – as shown previously – and less sustained immune response to SARS-CoV-2 vaccines in patients with a variety of IMIDs, including rheumatoid arthritis, spondyloarthritis, psoriasis, inflammatory bowel diseases, and other systemic autoimmune diseases such as lupus. It also points toward the possibility of adjusting treatment and vaccination schedules and strategies for these patients based on their antibody levels, among other factors, to preserve best protection against severe COVID.
“It is important to assess immune response in these patients to see if they still have protection against severe COVID infection,” said lead author David Simon, MD, senior clinical scientist in clinical immunology and rheumatology at University Hospital Erlangen (Germany). “We know that antibody response is an immune correlate. Therefore, it is important to see how large and durable the immune response is to the coronavirus vaccine in these IMID patients, and whether specific drugs or therapies have negative effects on their immune response.”
What was studied?
For this large prospective cohort study, researchers registered 5076 coronavirus-vaccinated individuals. They analyzed serum samples obtained between December 15, 2020, and December 1, 2021, from 2,535 patients diagnosed with IMIDs and participating in a prospective coronavirus study program at the Deutsches Zentrum Immuntherapie in Erlangen. The IMID patients had a mean age of 55.0 years, and 58.9% were women.
A healthy control group of 1,198 individuals without IMID who had a mean age of 40.7 years, including 53.8% men, was also recruited for the analysis. All approved coronavirus vaccines were included, following standard vaccination schedules. Antibody response was measured over time by an enzyme-linked immunosorbent assay from 8 weeks after first vaccination to week 40.
Among the findings, the healthy controls had higher postvaccine antibody levels than did those with IMIDs. But the majority of vaccinated patients with IMID were able to build up a humoral immune response to SARS-CoV-2. Patients who were taking B-cell inhibitors like rituximab (Rituxan, Genentech; and biosimilars) and T-cell inhibitors like abatacept (Orencia, Bristol Myers Squibb) for IMIDs had significantly poorer antibody response.
Greater age and the use of combination therapies for IMIDs, compared with monotherapy, further reduced immune response to the vaccine. In terms of vaccination modality, messenger RNA–based vaccines induced higher antibody levels than did vector-based vaccines. The researchers noted that patients with IMID who were given a third vaccine dose could actually catch up well with the antibody responses observed in healthy controls.
“We looked at whether different IMIDs had a different humoral response, and we also assessed if there are effects from different therapeutic strategies,” Dr. Simon explained. “It doesn’t matter so much what kind of IMID patients have; much more important is the specific drug treatment and its impact on their antibody response.” Some participants were advised to briefly stop taking some immunosuppressive treatments before or after vaccination.
One of Dr. Simon’s coauthors, statistician and rheumatologist Koray Tascilar, MD, added, “This research is important because we looked not only at who responded less, which has been previously established, but who are at greater risk of losing their immune response, and how quickly.”
Need to take care
“Most treatments we as rheumatologists give to our patients don’t affect their SARS-CoV-2 humoral response,” Dr. Simon said. “However, there are specific drugs that are associated with lower antibody response. With respect to those drugs, we have to be more careful.”
It is important to be able to tell patients which drugs are safe and won’t have a negative impact on their immune response to vaccinations, Dr. Tascilar said. “But it would be too strong to say we’re ready to choose therapies based on their potential impact on protection against COVID. Yes, there is a risk from catching COVID, but we need to balance that risk with the risk of not giving patients the medications that are necessary to treat their rheumatologic condition.”
These diseases are serious, sometimes life-threatening. “We might think of strategies for how to mitigate the risk of underprotection from COVID that is brought about by these treatments,” he said. For example, offering boosters sooner or more frequently, or prophylactically treating with monoclonal antibodies.
“This study, along other recent studies, has found that antibody levels in patients with immune-mediated diseases wane more rapidly than in healthy controls, and this is especially true of those on medications that interfere with the B and T cells and anticytokine therapies,” Rebecca Haberman, MD, assistant professor, division of rheumatology, New York University Langone Health, noted in an email to this news organization.
“While there is no known antibody level that specifically correlates with clinical protection, and each patient needs to be thought of individually, these findings support the use of supplemental booster dosing in patients with immune-mediated inflammatory diseases,” Dr. Haberman said, adding that her own research in this area has shown similar results.
“As a rheumatologist, I would be more likely to encourage my patients – especially those on immunomodulatory medications – to get boosted.”
Dr. Tascilar said his study does not directly answer the question of whether an earlier booster shot would be an effective strategy for patients with IMID. “In our department, we have an early boosting strategy, based on level of immune response.” But the decision of revaccination or not, and when, is based on a number of factors, not only on the level of antibodies. “It’s just part of the instruments we are using.”
The study was supported by the Deutsche Forschungsgemeinschaft. Dr. Simon and Dr. Tascilar declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET RHEUMATOLOGY
Asian patients with psoriasis have shortest visits, study shows
(NAMCS) from 2010 to 2016.
Yet the reasons for the difference are unclear and in need of further research, said the investigators and dermatologists who were asked to comment on the research.
The study covered over 4 million visits for psoriasis and found that the mean duration of visits for Asian patients was 9.2 minutes, compared with 15.7 minutes for Hispanic or Latino patients, 20.7 minutes for non-Hispanic Black patients, and 15.4 minutes for non-Hispanic White patients.
The mean duration of visits with Asian patients was 39.9% shorter, compared with visits with White patients (beta coefficient, –5,747; 95% confidence interval, –11.026 to –0.469; P = .03), and 40.6% shorter, compared with visits with non-Asian patients combined (beta coefficient, –5.908; 95% CI, –11.147 to –0.669, P = .03), April W. Armstrong, MD, MPH, professor of dermatology and director of the psoriasis program at the University of Southern California, Los Angeles, and Kevin K. Wu, MD, a dermatology resident at USC, said in a research letter published in JAMA Dermatology.
“The etiology of these differences is unclear,” they wrote. “It is possible that factors such as unconscious bias, cultural differences in communication, or residual confounding may be responsible for the observed findings.”
Their findings came from multivariable linear regression analyses that adjusted for age, sex, type of visit (new or follow-up), visit complexity based on the number of reasons for the visit, insurance status (such as private insurance or Medicaid), psoriasis severity on the basis of systemic psoriasis treatment or phototherapy, and complex topical regimens (three or more topical agents).
Commenting on the results, Deborah A. Scott, MD, codirector of the skin of color dermatology program at Brigham and Women’s Hospital and assistant professor at Harvard Medical School, both in Boston, said in an interview that visit length “is a reasonable parameter to look at among many others” when investigating potential disparities in care.
“They’re equating [shorter visit times] with lack of time spent counseling patients,” said Dr. Scott, who was not involved in the research. But there are “many variables” that can affect visit time, such as language differences, time spent with interpreters, and differences in patient educational levels.
Clarissa Yang, MD, dermatologist-in-chief at Tufts Medical Center, Boston, agreed. “We’re worried about there being a quality of care issue. However, there could also be differences culturally in how [the patients] interact with their physicians – their styles and the questions they ask,” she said in an interview. “The study is a good first step to noting that there may be a disparity,” and there is a need to break down the differences “into more granularity.”
Previous research, the authors wrote, has found that Asian patients were less likely to receive counseling from physicians, compared with White patients. And “paradoxically,” they noted, Asian individuals tend to present with more severe psoriasis than patients of other races and ethnicities.
Dr. Scott said the tendency to present with more severe psoriasis has been documented in patients with skin of color broadly – likely because of delays in recognition and treatment.
Race and ethnicity in the study were self-reported by patients, and missing data were imputed by NAMCS researchers using a sequential regression method. Patients who did not report race and ethnicity may have different characteristics affecting visit duration than those who did report the information, Dr. Armstrong and Dr. Wu said in describing their study’s limitations.
Other differences found
In addition to visit length, they found significant differences in mean age and in the use of complex topical regimens. The mean ages of Asian, Hispanic or Latino, and non-Hispanic Black patients were 37.2, 44.7, and 33.3 years, respectively. Complex topical regimens were prescribed to 11.8% of Asian patients, compared with 1.5% of Black and 1.1% of White patients.
For practicing dermatologists, knowing for now that Asian patients have shorter visits “may bring to light some consciousness to how we practice,” Dr. Yang noted. “We may counsel differently, we may spend differing amounts of time – for reasons still unknown. But being generally aware can help us to shift any unconscious bias that may be there.”
Dermatologists, Dr. Armstrong and Dr. Wu wrote, “need to allow sufficient time to develop strong physician-patient communication regardless of patient background.”
The NAMCS – administered by the Center for Disease Control and Prevention’s National Center for Health Statistics – collects data on a sample of visits provided by non–federally employed office-based physicians.
Dr. Armstrong disclosed receiving personal fees from AbbVie and Regeneron for research funding and serving as a scientific adviser and speaker for additional pharmaceutical and therapeutic companies. Dr. Wu, Dr. Scott, and Dr. Yang did not report any disclosures.
(NAMCS) from 2010 to 2016.
Yet the reasons for the difference are unclear and in need of further research, said the investigators and dermatologists who were asked to comment on the research.
The study covered over 4 million visits for psoriasis and found that the mean duration of visits for Asian patients was 9.2 minutes, compared with 15.7 minutes for Hispanic or Latino patients, 20.7 minutes for non-Hispanic Black patients, and 15.4 minutes for non-Hispanic White patients.
The mean duration of visits with Asian patients was 39.9% shorter, compared with visits with White patients (beta coefficient, –5,747; 95% confidence interval, –11.026 to –0.469; P = .03), and 40.6% shorter, compared with visits with non-Asian patients combined (beta coefficient, –5.908; 95% CI, –11.147 to –0.669, P = .03), April W. Armstrong, MD, MPH, professor of dermatology and director of the psoriasis program at the University of Southern California, Los Angeles, and Kevin K. Wu, MD, a dermatology resident at USC, said in a research letter published in JAMA Dermatology.
“The etiology of these differences is unclear,” they wrote. “It is possible that factors such as unconscious bias, cultural differences in communication, or residual confounding may be responsible for the observed findings.”
Their findings came from multivariable linear regression analyses that adjusted for age, sex, type of visit (new or follow-up), visit complexity based on the number of reasons for the visit, insurance status (such as private insurance or Medicaid), psoriasis severity on the basis of systemic psoriasis treatment or phototherapy, and complex topical regimens (three or more topical agents).
Commenting on the results, Deborah A. Scott, MD, codirector of the skin of color dermatology program at Brigham and Women’s Hospital and assistant professor at Harvard Medical School, both in Boston, said in an interview that visit length “is a reasonable parameter to look at among many others” when investigating potential disparities in care.
“They’re equating [shorter visit times] with lack of time spent counseling patients,” said Dr. Scott, who was not involved in the research. But there are “many variables” that can affect visit time, such as language differences, time spent with interpreters, and differences in patient educational levels.
Clarissa Yang, MD, dermatologist-in-chief at Tufts Medical Center, Boston, agreed. “We’re worried about there being a quality of care issue. However, there could also be differences culturally in how [the patients] interact with their physicians – their styles and the questions they ask,” she said in an interview. “The study is a good first step to noting that there may be a disparity,” and there is a need to break down the differences “into more granularity.”
Previous research, the authors wrote, has found that Asian patients were less likely to receive counseling from physicians, compared with White patients. And “paradoxically,” they noted, Asian individuals tend to present with more severe psoriasis than patients of other races and ethnicities.
Dr. Scott said the tendency to present with more severe psoriasis has been documented in patients with skin of color broadly – likely because of delays in recognition and treatment.
Race and ethnicity in the study were self-reported by patients, and missing data were imputed by NAMCS researchers using a sequential regression method. Patients who did not report race and ethnicity may have different characteristics affecting visit duration than those who did report the information, Dr. Armstrong and Dr. Wu said in describing their study’s limitations.
Other differences found
In addition to visit length, they found significant differences in mean age and in the use of complex topical regimens. The mean ages of Asian, Hispanic or Latino, and non-Hispanic Black patients were 37.2, 44.7, and 33.3 years, respectively. Complex topical regimens were prescribed to 11.8% of Asian patients, compared with 1.5% of Black and 1.1% of White patients.
For practicing dermatologists, knowing for now that Asian patients have shorter visits “may bring to light some consciousness to how we practice,” Dr. Yang noted. “We may counsel differently, we may spend differing amounts of time – for reasons still unknown. But being generally aware can help us to shift any unconscious bias that may be there.”
Dermatologists, Dr. Armstrong and Dr. Wu wrote, “need to allow sufficient time to develop strong physician-patient communication regardless of patient background.”
The NAMCS – administered by the Center for Disease Control and Prevention’s National Center for Health Statistics – collects data on a sample of visits provided by non–federally employed office-based physicians.
Dr. Armstrong disclosed receiving personal fees from AbbVie and Regeneron for research funding and serving as a scientific adviser and speaker for additional pharmaceutical and therapeutic companies. Dr. Wu, Dr. Scott, and Dr. Yang did not report any disclosures.
(NAMCS) from 2010 to 2016.
Yet the reasons for the difference are unclear and in need of further research, said the investigators and dermatologists who were asked to comment on the research.
The study covered over 4 million visits for psoriasis and found that the mean duration of visits for Asian patients was 9.2 minutes, compared with 15.7 minutes for Hispanic or Latino patients, 20.7 minutes for non-Hispanic Black patients, and 15.4 minutes for non-Hispanic White patients.
The mean duration of visits with Asian patients was 39.9% shorter, compared with visits with White patients (beta coefficient, –5,747; 95% confidence interval, –11.026 to –0.469; P = .03), and 40.6% shorter, compared with visits with non-Asian patients combined (beta coefficient, –5.908; 95% CI, –11.147 to –0.669, P = .03), April W. Armstrong, MD, MPH, professor of dermatology and director of the psoriasis program at the University of Southern California, Los Angeles, and Kevin K. Wu, MD, a dermatology resident at USC, said in a research letter published in JAMA Dermatology.
“The etiology of these differences is unclear,” they wrote. “It is possible that factors such as unconscious bias, cultural differences in communication, or residual confounding may be responsible for the observed findings.”
Their findings came from multivariable linear regression analyses that adjusted for age, sex, type of visit (new or follow-up), visit complexity based on the number of reasons for the visit, insurance status (such as private insurance or Medicaid), psoriasis severity on the basis of systemic psoriasis treatment or phototherapy, and complex topical regimens (three or more topical agents).
Commenting on the results, Deborah A. Scott, MD, codirector of the skin of color dermatology program at Brigham and Women’s Hospital and assistant professor at Harvard Medical School, both in Boston, said in an interview that visit length “is a reasonable parameter to look at among many others” when investigating potential disparities in care.
“They’re equating [shorter visit times] with lack of time spent counseling patients,” said Dr. Scott, who was not involved in the research. But there are “many variables” that can affect visit time, such as language differences, time spent with interpreters, and differences in patient educational levels.
Clarissa Yang, MD, dermatologist-in-chief at Tufts Medical Center, Boston, agreed. “We’re worried about there being a quality of care issue. However, there could also be differences culturally in how [the patients] interact with their physicians – their styles and the questions they ask,” she said in an interview. “The study is a good first step to noting that there may be a disparity,” and there is a need to break down the differences “into more granularity.”
Previous research, the authors wrote, has found that Asian patients were less likely to receive counseling from physicians, compared with White patients. And “paradoxically,” they noted, Asian individuals tend to present with more severe psoriasis than patients of other races and ethnicities.
Dr. Scott said the tendency to present with more severe psoriasis has been documented in patients with skin of color broadly – likely because of delays in recognition and treatment.
Race and ethnicity in the study were self-reported by patients, and missing data were imputed by NAMCS researchers using a sequential regression method. Patients who did not report race and ethnicity may have different characteristics affecting visit duration than those who did report the information, Dr. Armstrong and Dr. Wu said in describing their study’s limitations.
Other differences found
In addition to visit length, they found significant differences in mean age and in the use of complex topical regimens. The mean ages of Asian, Hispanic or Latino, and non-Hispanic Black patients were 37.2, 44.7, and 33.3 years, respectively. Complex topical regimens were prescribed to 11.8% of Asian patients, compared with 1.5% of Black and 1.1% of White patients.
For practicing dermatologists, knowing for now that Asian patients have shorter visits “may bring to light some consciousness to how we practice,” Dr. Yang noted. “We may counsel differently, we may spend differing amounts of time – for reasons still unknown. But being generally aware can help us to shift any unconscious bias that may be there.”
Dermatologists, Dr. Armstrong and Dr. Wu wrote, “need to allow sufficient time to develop strong physician-patient communication regardless of patient background.”
The NAMCS – administered by the Center for Disease Control and Prevention’s National Center for Health Statistics – collects data on a sample of visits provided by non–federally employed office-based physicians.
Dr. Armstrong disclosed receiving personal fees from AbbVie and Regeneron for research funding and serving as a scientific adviser and speaker for additional pharmaceutical and therapeutic companies. Dr. Wu, Dr. Scott, and Dr. Yang did not report any disclosures.
FROM JAMA DERMATOLOGY
FDA approves adalimumab-bwwd biosimilar (Hadlima) in high-concentration form
The U.S. Food and Drug Administration today approved a citrate-free, high-concentration formulation of adalimumab-bwwd (Hadlima), the manufacturer, Samsung Bioepis, and its commercialization partner Organon said in an announcement.
Hadlima is a biosimilar of the tumor necrosis factor inhibitor reference product adalimumab (Humira).
Hadlima was first approved in July 2019 in a citrated, 50-mg/mL formulation. The new citrate-free, 100-mg/mL version will be available in prefilled syringe and autoinjector options.
The 100-mg/mL formulation is indicated for the same seven conditions as its 50-mg/mL counterpart: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, adult and pediatric Crohn’s disease, and ulcerative colitis.
The approval was based on clinical data from a randomized, single-blind, two-arm, parallel group, single-dose study that compared the pharmacokinetics, safety, tolerability, and immunogenicity of the 100-mg/mL and 50-mg/mL formulations of Hadlima in healthy volunteers.
Both low- and high-concentration formulations of Humira are currently marketed in the United States. Organon said that it expects to market Hadlima in the United States on or after July 1, 2023, in accordance with a licensing agreement with AbbVie.
The prescribing information for Hadlima includes specific warnings and areas of concern. The drug should not be administered to individuals who are known to be hypersensitive to adalimumab. The drug may lower the ability of the immune system to fight infections and may increase risk of infections, including serious infections leading to hospitalization or death, such as tuberculosis, bacterial sepsis, invasive fungal infections (such as histoplasmosis), and infections attributable to other opportunistic pathogens.
A test for latent TB infection should be given before administration, and treatment of TB should begin before administration of Hadlima.
Patients taking Hadlima should not take a live vaccine.
The most common adverse effects (incidence > 10%) include infections (for example, upper respiratory infections, sinusitis), injection site reactions, headache, and rash.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration today approved a citrate-free, high-concentration formulation of adalimumab-bwwd (Hadlima), the manufacturer, Samsung Bioepis, and its commercialization partner Organon said in an announcement.
Hadlima is a biosimilar of the tumor necrosis factor inhibitor reference product adalimumab (Humira).
Hadlima was first approved in July 2019 in a citrated, 50-mg/mL formulation. The new citrate-free, 100-mg/mL version will be available in prefilled syringe and autoinjector options.
The 100-mg/mL formulation is indicated for the same seven conditions as its 50-mg/mL counterpart: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, adult and pediatric Crohn’s disease, and ulcerative colitis.
The approval was based on clinical data from a randomized, single-blind, two-arm, parallel group, single-dose study that compared the pharmacokinetics, safety, tolerability, and immunogenicity of the 100-mg/mL and 50-mg/mL formulations of Hadlima in healthy volunteers.
Both low- and high-concentration formulations of Humira are currently marketed in the United States. Organon said that it expects to market Hadlima in the United States on or after July 1, 2023, in accordance with a licensing agreement with AbbVie.
The prescribing information for Hadlima includes specific warnings and areas of concern. The drug should not be administered to individuals who are known to be hypersensitive to adalimumab. The drug may lower the ability of the immune system to fight infections and may increase risk of infections, including serious infections leading to hospitalization or death, such as tuberculosis, bacterial sepsis, invasive fungal infections (such as histoplasmosis), and infections attributable to other opportunistic pathogens.
A test for latent TB infection should be given before administration, and treatment of TB should begin before administration of Hadlima.
Patients taking Hadlima should not take a live vaccine.
The most common adverse effects (incidence > 10%) include infections (for example, upper respiratory infections, sinusitis), injection site reactions, headache, and rash.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration today approved a citrate-free, high-concentration formulation of adalimumab-bwwd (Hadlima), the manufacturer, Samsung Bioepis, and its commercialization partner Organon said in an announcement.
Hadlima is a biosimilar of the tumor necrosis factor inhibitor reference product adalimumab (Humira).
Hadlima was first approved in July 2019 in a citrated, 50-mg/mL formulation. The new citrate-free, 100-mg/mL version will be available in prefilled syringe and autoinjector options.
The 100-mg/mL formulation is indicated for the same seven conditions as its 50-mg/mL counterpart: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, adult and pediatric Crohn’s disease, and ulcerative colitis.
The approval was based on clinical data from a randomized, single-blind, two-arm, parallel group, single-dose study that compared the pharmacokinetics, safety, tolerability, and immunogenicity of the 100-mg/mL and 50-mg/mL formulations of Hadlima in healthy volunteers.
Both low- and high-concentration formulations of Humira are currently marketed in the United States. Organon said that it expects to market Hadlima in the United States on or after July 1, 2023, in accordance with a licensing agreement with AbbVie.
The prescribing information for Hadlima includes specific warnings and areas of concern. The drug should not be administered to individuals who are known to be hypersensitive to adalimumab. The drug may lower the ability of the immune system to fight infections and may increase risk of infections, including serious infections leading to hospitalization or death, such as tuberculosis, bacterial sepsis, invasive fungal infections (such as histoplasmosis), and infections attributable to other opportunistic pathogens.
A test for latent TB infection should be given before administration, and treatment of TB should begin before administration of Hadlima.
Patients taking Hadlima should not take a live vaccine.
The most common adverse effects (incidence > 10%) include infections (for example, upper respiratory infections, sinusitis), injection site reactions, headache, and rash.
A version of this article first appeared on Medscape.com.
Biosimilar-to-biosimilar switches deemed safe and effective, systematic review reveals
Switching from one biosimilar medication to another is safe and effective, a new systematic review indicates, even though this clinical practice is not governed by current health authority regulations or guidance.
“No reduction in effectiveness or increase in adverse events was detected in biosimilar-to-biosimilar switching studies conducted to date,” the review’s authors noted in their study, published online in BioDrugs.
“The possibility of multiple switches between biosimilars of the same reference biologic is already a reality, and these types of switches are expected to become more common in the future. ... Although it is not covered by current health authority regulations or guidance,” added the authors, led by Hillel P. Cohen, PhD, executive director of scientific affairs at Sandoz, a division of Novartis.
The researchers searched electronic databases through December 2021 and found 23 observational studies that met their search criteria, of which 13 were published in peer-reviewed journals; the remainder appeared in abstract form. The studies totaled 3,657 patients. The researchers did not identify any randomized clinical trials.
“The studies were heterogeneous in size, design, and endpoints, providing data on safety, effectiveness, immunogenicity, pharmacokinetics, patient retention, patient and physician perceptions, and drug-use patterns,” the authors wrote.
The authors found that the majority of studies evaluated switches between biosimilars of infliximab, but they also identified switches between biosimilars of adalimumab, etanercept, and rituximab.
“Some health care providers are hesitant to switch patients from one biosimilar to another biosimilar because of a perceived lack of clinical data on such switches,” Dr. Cohen said in an interview.
The review’s findings – that there were no clinically relevant differences when switching patients from one biosimilar to another – are consistent with the science, Dr. Cohen said. “Physicians should have confidence that the data demonstrate that safety and effectiveness are not impacted if patients switch from one biosimilar to another biosimilar of the same reference biologic,” he said.
Currently, the published data include biosimilars to only four reference biologics. “However, I anticipate additional biosimilar-to-biosimilar switching data will become available in the future,” Dr. Cohen said. “In fact, several new studies have been published in recent months, after the cut-off date for inclusion in our systematic review.”
Switching common in rheumatology, dermatology, and gastroenterology
Biosimilar-to-biosimilar switching was observed most commonly in rheumatology practice, but also was seen in the specialties of dermatology and gastroenterology.
Jeffrey Weinberg, MD, clinical professor of dermatology, Icahn School of Medicine at Mount Sinai, New York City, said in an interview that the study is among the best to date showing that switching biosimilars does not compromise efficacy or safety.
“I would hypothesize that the interchangeability would apply to psoriasis patients,” Dr. Weinberg said. However, “over the next few years, we will have an increasing number of biosimilars for an increasing number of different molecules. We will need to be vigilant to observe if similar behavior is observed with the biosimilars yet to come.”
Keith Choate, MD, PhD, professor of dermatology, pathology, and genetics, and associate dean for physician-scientist development at Yale University, New Haven, Conn., said that biosimilars have comparable efficacy to the branded medication they replace. “If response is lost to an individual agent, we would not typically then switch to a biosimilar, but would favor another class of therapy or a distinct therapeutic which targets the same pathway.”
When physicians prescribe a biosimilar for rheumatoid arthritis or psoriatic arthritis, in 9 out 10 people, “it’s going to work as well, and it’s not going to cause any more side effects,” said Stanford Shoor, MD, clinical professor of medicine and rheumatology, Stanford (Calif.) University.
The systematic review, even within its limitations, reinforces confidence in the antitumor necrosis factor biosimilars, said Jean-Frederic Colombel, MD, codirector of the Feinstein Inflammatory Bowel Disease Clinical Center at Mount Sinai, New York, and professor of medicine, division of gastroenterology, Icahn School of Medicine at Mount Sinai.
“Still, studies with longer follow-up are needed,” Dr. Colombel said, adding that the remaining questions relate to the efficacy and safety of switching multiple times, which will likely occur in the near future. There will be a “need to provide information to the patient regarding what originator or biosimilar(s) he has been exposed to during the course of his disease.”
Switching will increasingly become the norm, said Miguel Regueiro, MD, chair of the Digestive Disease & Surgery Institute, Cleveland Clinic. In his clinical practice, he has the most experience with Crohn’s disease and ulcerative colitis, and biosimilar-to-biosimilar infliximab switches. “Unless there are data that emerge, I have no concerns with this.”
He added that it’s an “interesting study that affirms my findings in clinical practice – that one can switch from a biosimilar to biosimilar (of the same reference product).”
The review’s results also make sense from an economic standpoint, said Rajat Bhatt, MD, owner of Prime Rheumatology in Richmond, Tex., and an adjunct faculty member at Caribbean Medical University, Willemstad, Curaçao. “Switching to biosimilars will result in cost savings for the health care system.” Patients on certain insurances also will save by switching to a biosimilar with a lower copay.
However, the review is limited by a relatively small number of studies that have provided primary data on this topic, and most of these were switching from infliximab to a biosimilar for inflammatory bowel disease, said Alfred Kim, MD, PhD, an adult rheumatologist at Barnes-Jewish Hospital, St. Louis, and assistant professor of medicine at Washington University in St. Louis.
As with any meta-analysis evaluating a small number of studies, “broad applicability to all conditions and reference/biosimilar pair can only be assumed. Also, many of the studies used for this meta-analysis are observational, which can introduce a variety of biases that can be difficult to adjust for,” Dr. Kim said. “Nevertheless, these analyses are an important first step in validating the [Food and Drug Administration’s] approach to evaluating biosimilars, as the clinical outcomes are consistent between different biosimilars.”
This systematic review is not enough to prove that all patients will do fine when switching from one biosimilar to another, said Florence Aslinia, MD, a gastroenterologist at the University of Kansas Health System in Kansas City. It’s possible that some patients may not do as well, she said, noting that, in one study of patients with inflammatory bowel disease, 10% of patients on a biosimilar infliximab needed to switch back to the originator infliximab (Remicade, Janssen) because of side effects attributed to the biosimilar. The same thing may or may not happen with biosimilar-to-biosimilar switching, and it requires further study.
The authors did not receive any funding for writing this review. Dr. Cohen is an employee of Sandoz, a division of Novartis. He may own stock in Novartis. Two coauthors are also employees of Sandoz. The other three coauthors reported having financial relationships with numerous pharmaceutical companies, including Sandoz and/or Novartis. Dr. Colombel reported financial relationships with many pharmaceutical companies, including Novartis and other manufacturers of biosimilars. Dr. Regueiro reports financial relationships with numerous pharmaceutical companies, including some manufacturers of biosimilars. Dr. Weinberg reported financial relationships with Celgene, AbbVie, Eli Lilly, and Novartis. Kim reports financial relationships with GlaxoSmithKline, Pfizer, and AstraZeneca. Dr. Aslinia, Dr. Shoor, Dr. Choate, and Dr. Bhatt reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Switching from one biosimilar medication to another is safe and effective, a new systematic review indicates, even though this clinical practice is not governed by current health authority regulations or guidance.
“No reduction in effectiveness or increase in adverse events was detected in biosimilar-to-biosimilar switching studies conducted to date,” the review’s authors noted in their study, published online in BioDrugs.
“The possibility of multiple switches between biosimilars of the same reference biologic is already a reality, and these types of switches are expected to become more common in the future. ... Although it is not covered by current health authority regulations or guidance,” added the authors, led by Hillel P. Cohen, PhD, executive director of scientific affairs at Sandoz, a division of Novartis.
The researchers searched electronic databases through December 2021 and found 23 observational studies that met their search criteria, of which 13 were published in peer-reviewed journals; the remainder appeared in abstract form. The studies totaled 3,657 patients. The researchers did not identify any randomized clinical trials.
“The studies were heterogeneous in size, design, and endpoints, providing data on safety, effectiveness, immunogenicity, pharmacokinetics, patient retention, patient and physician perceptions, and drug-use patterns,” the authors wrote.
The authors found that the majority of studies evaluated switches between biosimilars of infliximab, but they also identified switches between biosimilars of adalimumab, etanercept, and rituximab.
“Some health care providers are hesitant to switch patients from one biosimilar to another biosimilar because of a perceived lack of clinical data on such switches,” Dr. Cohen said in an interview.
The review’s findings – that there were no clinically relevant differences when switching patients from one biosimilar to another – are consistent with the science, Dr. Cohen said. “Physicians should have confidence that the data demonstrate that safety and effectiveness are not impacted if patients switch from one biosimilar to another biosimilar of the same reference biologic,” he said.
Currently, the published data include biosimilars to only four reference biologics. “However, I anticipate additional biosimilar-to-biosimilar switching data will become available in the future,” Dr. Cohen said. “In fact, several new studies have been published in recent months, after the cut-off date for inclusion in our systematic review.”
Switching common in rheumatology, dermatology, and gastroenterology
Biosimilar-to-biosimilar switching was observed most commonly in rheumatology practice, but also was seen in the specialties of dermatology and gastroenterology.
Jeffrey Weinberg, MD, clinical professor of dermatology, Icahn School of Medicine at Mount Sinai, New York City, said in an interview that the study is among the best to date showing that switching biosimilars does not compromise efficacy or safety.
“I would hypothesize that the interchangeability would apply to psoriasis patients,” Dr. Weinberg said. However, “over the next few years, we will have an increasing number of biosimilars for an increasing number of different molecules. We will need to be vigilant to observe if similar behavior is observed with the biosimilars yet to come.”
Keith Choate, MD, PhD, professor of dermatology, pathology, and genetics, and associate dean for physician-scientist development at Yale University, New Haven, Conn., said that biosimilars have comparable efficacy to the branded medication they replace. “If response is lost to an individual agent, we would not typically then switch to a biosimilar, but would favor another class of therapy or a distinct therapeutic which targets the same pathway.”
When physicians prescribe a biosimilar for rheumatoid arthritis or psoriatic arthritis, in 9 out 10 people, “it’s going to work as well, and it’s not going to cause any more side effects,” said Stanford Shoor, MD, clinical professor of medicine and rheumatology, Stanford (Calif.) University.
The systematic review, even within its limitations, reinforces confidence in the antitumor necrosis factor biosimilars, said Jean-Frederic Colombel, MD, codirector of the Feinstein Inflammatory Bowel Disease Clinical Center at Mount Sinai, New York, and professor of medicine, division of gastroenterology, Icahn School of Medicine at Mount Sinai.
“Still, studies with longer follow-up are needed,” Dr. Colombel said, adding that the remaining questions relate to the efficacy and safety of switching multiple times, which will likely occur in the near future. There will be a “need to provide information to the patient regarding what originator or biosimilar(s) he has been exposed to during the course of his disease.”
Switching will increasingly become the norm, said Miguel Regueiro, MD, chair of the Digestive Disease & Surgery Institute, Cleveland Clinic. In his clinical practice, he has the most experience with Crohn’s disease and ulcerative colitis, and biosimilar-to-biosimilar infliximab switches. “Unless there are data that emerge, I have no concerns with this.”
He added that it’s an “interesting study that affirms my findings in clinical practice – that one can switch from a biosimilar to biosimilar (of the same reference product).”
The review’s results also make sense from an economic standpoint, said Rajat Bhatt, MD, owner of Prime Rheumatology in Richmond, Tex., and an adjunct faculty member at Caribbean Medical University, Willemstad, Curaçao. “Switching to biosimilars will result in cost savings for the health care system.” Patients on certain insurances also will save by switching to a biosimilar with a lower copay.
However, the review is limited by a relatively small number of studies that have provided primary data on this topic, and most of these were switching from infliximab to a biosimilar for inflammatory bowel disease, said Alfred Kim, MD, PhD, an adult rheumatologist at Barnes-Jewish Hospital, St. Louis, and assistant professor of medicine at Washington University in St. Louis.
As with any meta-analysis evaluating a small number of studies, “broad applicability to all conditions and reference/biosimilar pair can only be assumed. Also, many of the studies used for this meta-analysis are observational, which can introduce a variety of biases that can be difficult to adjust for,” Dr. Kim said. “Nevertheless, these analyses are an important first step in validating the [Food and Drug Administration’s] approach to evaluating biosimilars, as the clinical outcomes are consistent between different biosimilars.”
This systematic review is not enough to prove that all patients will do fine when switching from one biosimilar to another, said Florence Aslinia, MD, a gastroenterologist at the University of Kansas Health System in Kansas City. It’s possible that some patients may not do as well, she said, noting that, in one study of patients with inflammatory bowel disease, 10% of patients on a biosimilar infliximab needed to switch back to the originator infliximab (Remicade, Janssen) because of side effects attributed to the biosimilar. The same thing may or may not happen with biosimilar-to-biosimilar switching, and it requires further study.
The authors did not receive any funding for writing this review. Dr. Cohen is an employee of Sandoz, a division of Novartis. He may own stock in Novartis. Two coauthors are also employees of Sandoz. The other three coauthors reported having financial relationships with numerous pharmaceutical companies, including Sandoz and/or Novartis. Dr. Colombel reported financial relationships with many pharmaceutical companies, including Novartis and other manufacturers of biosimilars. Dr. Regueiro reports financial relationships with numerous pharmaceutical companies, including some manufacturers of biosimilars. Dr. Weinberg reported financial relationships with Celgene, AbbVie, Eli Lilly, and Novartis. Kim reports financial relationships with GlaxoSmithKline, Pfizer, and AstraZeneca. Dr. Aslinia, Dr. Shoor, Dr. Choate, and Dr. Bhatt reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Switching from one biosimilar medication to another is safe and effective, a new systematic review indicates, even though this clinical practice is not governed by current health authority regulations or guidance.
“No reduction in effectiveness or increase in adverse events was detected in biosimilar-to-biosimilar switching studies conducted to date,” the review’s authors noted in their study, published online in BioDrugs.
“The possibility of multiple switches between biosimilars of the same reference biologic is already a reality, and these types of switches are expected to become more common in the future. ... Although it is not covered by current health authority regulations or guidance,” added the authors, led by Hillel P. Cohen, PhD, executive director of scientific affairs at Sandoz, a division of Novartis.
The researchers searched electronic databases through December 2021 and found 23 observational studies that met their search criteria, of which 13 were published in peer-reviewed journals; the remainder appeared in abstract form. The studies totaled 3,657 patients. The researchers did not identify any randomized clinical trials.
“The studies were heterogeneous in size, design, and endpoints, providing data on safety, effectiveness, immunogenicity, pharmacokinetics, patient retention, patient and physician perceptions, and drug-use patterns,” the authors wrote.
The authors found that the majority of studies evaluated switches between biosimilars of infliximab, but they also identified switches between biosimilars of adalimumab, etanercept, and rituximab.
“Some health care providers are hesitant to switch patients from one biosimilar to another biosimilar because of a perceived lack of clinical data on such switches,” Dr. Cohen said in an interview.
The review’s findings – that there were no clinically relevant differences when switching patients from one biosimilar to another – are consistent with the science, Dr. Cohen said. “Physicians should have confidence that the data demonstrate that safety and effectiveness are not impacted if patients switch from one biosimilar to another biosimilar of the same reference biologic,” he said.
Currently, the published data include biosimilars to only four reference biologics. “However, I anticipate additional biosimilar-to-biosimilar switching data will become available in the future,” Dr. Cohen said. “In fact, several new studies have been published in recent months, after the cut-off date for inclusion in our systematic review.”
Switching common in rheumatology, dermatology, and gastroenterology
Biosimilar-to-biosimilar switching was observed most commonly in rheumatology practice, but also was seen in the specialties of dermatology and gastroenterology.
Jeffrey Weinberg, MD, clinical professor of dermatology, Icahn School of Medicine at Mount Sinai, New York City, said in an interview that the study is among the best to date showing that switching biosimilars does not compromise efficacy or safety.
“I would hypothesize that the interchangeability would apply to psoriasis patients,” Dr. Weinberg said. However, “over the next few years, we will have an increasing number of biosimilars for an increasing number of different molecules. We will need to be vigilant to observe if similar behavior is observed with the biosimilars yet to come.”
Keith Choate, MD, PhD, professor of dermatology, pathology, and genetics, and associate dean for physician-scientist development at Yale University, New Haven, Conn., said that biosimilars have comparable efficacy to the branded medication they replace. “If response is lost to an individual agent, we would not typically then switch to a biosimilar, but would favor another class of therapy or a distinct therapeutic which targets the same pathway.”
When physicians prescribe a biosimilar for rheumatoid arthritis or psoriatic arthritis, in 9 out 10 people, “it’s going to work as well, and it’s not going to cause any more side effects,” said Stanford Shoor, MD, clinical professor of medicine and rheumatology, Stanford (Calif.) University.
The systematic review, even within its limitations, reinforces confidence in the antitumor necrosis factor biosimilars, said Jean-Frederic Colombel, MD, codirector of the Feinstein Inflammatory Bowel Disease Clinical Center at Mount Sinai, New York, and professor of medicine, division of gastroenterology, Icahn School of Medicine at Mount Sinai.
“Still, studies with longer follow-up are needed,” Dr. Colombel said, adding that the remaining questions relate to the efficacy and safety of switching multiple times, which will likely occur in the near future. There will be a “need to provide information to the patient regarding what originator or biosimilar(s) he has been exposed to during the course of his disease.”
Switching will increasingly become the norm, said Miguel Regueiro, MD, chair of the Digestive Disease & Surgery Institute, Cleveland Clinic. In his clinical practice, he has the most experience with Crohn’s disease and ulcerative colitis, and biosimilar-to-biosimilar infliximab switches. “Unless there are data that emerge, I have no concerns with this.”
He added that it’s an “interesting study that affirms my findings in clinical practice – that one can switch from a biosimilar to biosimilar (of the same reference product).”
The review’s results also make sense from an economic standpoint, said Rajat Bhatt, MD, owner of Prime Rheumatology in Richmond, Tex., and an adjunct faculty member at Caribbean Medical University, Willemstad, Curaçao. “Switching to biosimilars will result in cost savings for the health care system.” Patients on certain insurances also will save by switching to a biosimilar with a lower copay.
However, the review is limited by a relatively small number of studies that have provided primary data on this topic, and most of these were switching from infliximab to a biosimilar for inflammatory bowel disease, said Alfred Kim, MD, PhD, an adult rheumatologist at Barnes-Jewish Hospital, St. Louis, and assistant professor of medicine at Washington University in St. Louis.
As with any meta-analysis evaluating a small number of studies, “broad applicability to all conditions and reference/biosimilar pair can only be assumed. Also, many of the studies used for this meta-analysis are observational, which can introduce a variety of biases that can be difficult to adjust for,” Dr. Kim said. “Nevertheless, these analyses are an important first step in validating the [Food and Drug Administration’s] approach to evaluating biosimilars, as the clinical outcomes are consistent between different biosimilars.”
This systematic review is not enough to prove that all patients will do fine when switching from one biosimilar to another, said Florence Aslinia, MD, a gastroenterologist at the University of Kansas Health System in Kansas City. It’s possible that some patients may not do as well, she said, noting that, in one study of patients with inflammatory bowel disease, 10% of patients on a biosimilar infliximab needed to switch back to the originator infliximab (Remicade, Janssen) because of side effects attributed to the biosimilar. The same thing may or may not happen with biosimilar-to-biosimilar switching, and it requires further study.
The authors did not receive any funding for writing this review. Dr. Cohen is an employee of Sandoz, a division of Novartis. He may own stock in Novartis. Two coauthors are also employees of Sandoz. The other three coauthors reported having financial relationships with numerous pharmaceutical companies, including Sandoz and/or Novartis. Dr. Colombel reported financial relationships with many pharmaceutical companies, including Novartis and other manufacturers of biosimilars. Dr. Regueiro reports financial relationships with numerous pharmaceutical companies, including some manufacturers of biosimilars. Dr. Weinberg reported financial relationships with Celgene, AbbVie, Eli Lilly, and Novartis. Kim reports financial relationships with GlaxoSmithKline, Pfizer, and AstraZeneca. Dr. Aslinia, Dr. Shoor, Dr. Choate, and Dr. Bhatt reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BIODRUGS
Study suggests psoriasis and PsA are underdiagnosed in underserved groups
, a study based on national registry data suggests.
“Using the All of Us dataset, we identified lower rates of psoriasis and psoriatic arthritis in participants with skin of color, lower education levels, and no health insurance,” lead author Megan M. Tran said in her oral presentation at the annual meeting of the Society for Investigative Dermatology.
“This suggests psoriasis and psoriatic arthritis underdiagnosis in these underserved populations, possibly due to limited dermatologic care access,” added Ms. Tran, a second-year medical student at Brown University in Providence, R.I.
Ms. Tran and colleagues used the ongoing National Institutes of Health All of Us Research Program registry that contains a large proportion of participants from groups in the United States who have historically been underrepresented in biomedical research, she said in her talk.
Of the 329,038 participants with data in version 5 (released this past March) of the All of Us database, 150,158 (45.6%) had skin of color, and 251,597 (76.5%) had available electronic health records (EHRs).
Underserved groups need better access to health care
Linking data from EHRs, surveys, and physical measurements at enrollment, the researchers used several variables to estimate psoriasis and psoriatic arthritis (PsA) prevalence, and they used multivariate logistic regression to adjust for the variables. They found:
- Twenty-two percent of patients with psoriasis had PsA. Odds of psoriasis and PsA were lower among Black (psoriasis odds ratio [OR], 0.32, 95% confidence interval [CI], 0.28-0.36; PsA OR, 0.20, 95% CI, 0.15-0.26) and Hispanic participants (psoriasis OR, 0.77, 95% CI, 0.71-0.84; PsA OR, 0.74, 95% CI, 0.61-0.89) compared with White participants.
- Psoriasis prevalence increased linearly with age (topping off at age 70 and older [OR, 3.35, 95% CI, 2.91-3.88], with 18-29 years as the reference). The same trend was found with PsA (70 years and above [OR, 4.41, 95% CI, 3.07-6.55] compared with those aged 18-29 years).
- Psoriasis prevalence increased linearly with body mass index (BMI 40 and above [OR, 1.71, 95% CI, 1.54-1.90], with 20-24.9 as the reference). The same trend was found with PsA (BMI 40 and above [OR, 2.09, 95% CI, 1.68-2.59], with 20-24.9 as the reference).
- Former smokers were at increased risk for disease, compared with people who had never smoked (psoriasis OR, 1.30, 95% CI, 1.22-1.39; PsA OR, 2.15, 95% CI, 1.33-3.78).
- Lower odds were found in uninsured adults (psoriasis OR, 0.43, 95% CI, 0.35-0.52; PsA OR, 0.37, 95% CI, 0.22-0.58) compared with those who were insured, and in those with less than a high school degree (psoriasis OR, 0.72, 95% CI, 0.63-0.82; PsA OR, 0.65, 95% CI, 0.47-0.87) compared with those with a college degree.
“The All of Us research program has demonstrated to be a valuable resource to gain unique dermatologic insights on diverse participant populations,” Ms. Tran said.
“There needs to be improvement in access to quality dermatologic care, as this may help to reduce underdiagnosis of psoriasis and psoriatic arthritis,” she added. Access can be increased in various ways, including “outreach to underserved communities, equitable distribution of resources, and increased awareness of clinical variations in skin of color.”
Laura Korb Ferris, MD, PhD, professor of dermatology and director of clinical trials for the department of dermatology at University of Pittsburgh Medical Center, said the study is interesting.
“Because All of Us uses electronic health records to identify cases, while these findings could suggest that these patients are less likely to develop psoriasis and psoriatic arthritis, it more likely shows that they are less likely to receive care for these conditions,” she told this news organization.
“This is concerning, as psoriasis is associated with other comorbidities such as cardiovascular disease and depression, and psoriatic arthritis if left untreated can cause irreversible joint damage that limits function,” she explained in an email. “Both conditions profoundly impact a patient’s quality of life.
“It is important to know whether the diagnoses are simply being missed in these patients or are being neglected,” noted Dr. Ferris, who was not involved in the study and was asked to comment on the results. “It is also important to find strategies to improve diagnosis and treatment, improve quality of life, and allow for interventions to improve long-term sequelae of these diseases and their comorbid conditions.”
The NIH All of Us Research Program, which aims to build a diverse database from at least 1 million adult participants in the United States as a part of the agency’s precision medicine initiative, is open to researchers and to the public. Researchers can access All of Us data and tools to conduct studies at the All of Us Research Hub, and adults who live in the United States can contribute their health data at the All of Us Research Program website and at participating health care provider organizations.
Ms. Tran, study coauthors, and Dr. Ferris reported no relevant relationships. The All of Us Research Program is supported by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
, a study based on national registry data suggests.
“Using the All of Us dataset, we identified lower rates of psoriasis and psoriatic arthritis in participants with skin of color, lower education levels, and no health insurance,” lead author Megan M. Tran said in her oral presentation at the annual meeting of the Society for Investigative Dermatology.
“This suggests psoriasis and psoriatic arthritis underdiagnosis in these underserved populations, possibly due to limited dermatologic care access,” added Ms. Tran, a second-year medical student at Brown University in Providence, R.I.
Ms. Tran and colleagues used the ongoing National Institutes of Health All of Us Research Program registry that contains a large proportion of participants from groups in the United States who have historically been underrepresented in biomedical research, she said in her talk.
Of the 329,038 participants with data in version 5 (released this past March) of the All of Us database, 150,158 (45.6%) had skin of color, and 251,597 (76.5%) had available electronic health records (EHRs).
Underserved groups need better access to health care
Linking data from EHRs, surveys, and physical measurements at enrollment, the researchers used several variables to estimate psoriasis and psoriatic arthritis (PsA) prevalence, and they used multivariate logistic regression to adjust for the variables. They found:
- Twenty-two percent of patients with psoriasis had PsA. Odds of psoriasis and PsA were lower among Black (psoriasis odds ratio [OR], 0.32, 95% confidence interval [CI], 0.28-0.36; PsA OR, 0.20, 95% CI, 0.15-0.26) and Hispanic participants (psoriasis OR, 0.77, 95% CI, 0.71-0.84; PsA OR, 0.74, 95% CI, 0.61-0.89) compared with White participants.
- Psoriasis prevalence increased linearly with age (topping off at age 70 and older [OR, 3.35, 95% CI, 2.91-3.88], with 18-29 years as the reference). The same trend was found with PsA (70 years and above [OR, 4.41, 95% CI, 3.07-6.55] compared with those aged 18-29 years).
- Psoriasis prevalence increased linearly with body mass index (BMI 40 and above [OR, 1.71, 95% CI, 1.54-1.90], with 20-24.9 as the reference). The same trend was found with PsA (BMI 40 and above [OR, 2.09, 95% CI, 1.68-2.59], with 20-24.9 as the reference).
- Former smokers were at increased risk for disease, compared with people who had never smoked (psoriasis OR, 1.30, 95% CI, 1.22-1.39; PsA OR, 2.15, 95% CI, 1.33-3.78).
- Lower odds were found in uninsured adults (psoriasis OR, 0.43, 95% CI, 0.35-0.52; PsA OR, 0.37, 95% CI, 0.22-0.58) compared with those who were insured, and in those with less than a high school degree (psoriasis OR, 0.72, 95% CI, 0.63-0.82; PsA OR, 0.65, 95% CI, 0.47-0.87) compared with those with a college degree.
“The All of Us research program has demonstrated to be a valuable resource to gain unique dermatologic insights on diverse participant populations,” Ms. Tran said.
“There needs to be improvement in access to quality dermatologic care, as this may help to reduce underdiagnosis of psoriasis and psoriatic arthritis,” she added. Access can be increased in various ways, including “outreach to underserved communities, equitable distribution of resources, and increased awareness of clinical variations in skin of color.”
Laura Korb Ferris, MD, PhD, professor of dermatology and director of clinical trials for the department of dermatology at University of Pittsburgh Medical Center, said the study is interesting.
“Because All of Us uses electronic health records to identify cases, while these findings could suggest that these patients are less likely to develop psoriasis and psoriatic arthritis, it more likely shows that they are less likely to receive care for these conditions,” she told this news organization.
“This is concerning, as psoriasis is associated with other comorbidities such as cardiovascular disease and depression, and psoriatic arthritis if left untreated can cause irreversible joint damage that limits function,” she explained in an email. “Both conditions profoundly impact a patient’s quality of life.
“It is important to know whether the diagnoses are simply being missed in these patients or are being neglected,” noted Dr. Ferris, who was not involved in the study and was asked to comment on the results. “It is also important to find strategies to improve diagnosis and treatment, improve quality of life, and allow for interventions to improve long-term sequelae of these diseases and their comorbid conditions.”
The NIH All of Us Research Program, which aims to build a diverse database from at least 1 million adult participants in the United States as a part of the agency’s precision medicine initiative, is open to researchers and to the public. Researchers can access All of Us data and tools to conduct studies at the All of Us Research Hub, and adults who live in the United States can contribute their health data at the All of Us Research Program website and at participating health care provider organizations.
Ms. Tran, study coauthors, and Dr. Ferris reported no relevant relationships. The All of Us Research Program is supported by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
, a study based on national registry data suggests.
“Using the All of Us dataset, we identified lower rates of psoriasis and psoriatic arthritis in participants with skin of color, lower education levels, and no health insurance,” lead author Megan M. Tran said in her oral presentation at the annual meeting of the Society for Investigative Dermatology.
“This suggests psoriasis and psoriatic arthritis underdiagnosis in these underserved populations, possibly due to limited dermatologic care access,” added Ms. Tran, a second-year medical student at Brown University in Providence, R.I.
Ms. Tran and colleagues used the ongoing National Institutes of Health All of Us Research Program registry that contains a large proportion of participants from groups in the United States who have historically been underrepresented in biomedical research, she said in her talk.
Of the 329,038 participants with data in version 5 (released this past March) of the All of Us database, 150,158 (45.6%) had skin of color, and 251,597 (76.5%) had available electronic health records (EHRs).
Underserved groups need better access to health care
Linking data from EHRs, surveys, and physical measurements at enrollment, the researchers used several variables to estimate psoriasis and psoriatic arthritis (PsA) prevalence, and they used multivariate logistic regression to adjust for the variables. They found:
- Twenty-two percent of patients with psoriasis had PsA. Odds of psoriasis and PsA were lower among Black (psoriasis odds ratio [OR], 0.32, 95% confidence interval [CI], 0.28-0.36; PsA OR, 0.20, 95% CI, 0.15-0.26) and Hispanic participants (psoriasis OR, 0.77, 95% CI, 0.71-0.84; PsA OR, 0.74, 95% CI, 0.61-0.89) compared with White participants.
- Psoriasis prevalence increased linearly with age (topping off at age 70 and older [OR, 3.35, 95% CI, 2.91-3.88], with 18-29 years as the reference). The same trend was found with PsA (70 years and above [OR, 4.41, 95% CI, 3.07-6.55] compared with those aged 18-29 years).
- Psoriasis prevalence increased linearly with body mass index (BMI 40 and above [OR, 1.71, 95% CI, 1.54-1.90], with 20-24.9 as the reference). The same trend was found with PsA (BMI 40 and above [OR, 2.09, 95% CI, 1.68-2.59], with 20-24.9 as the reference).
- Former smokers were at increased risk for disease, compared with people who had never smoked (psoriasis OR, 1.30, 95% CI, 1.22-1.39; PsA OR, 2.15, 95% CI, 1.33-3.78).
- Lower odds were found in uninsured adults (psoriasis OR, 0.43, 95% CI, 0.35-0.52; PsA OR, 0.37, 95% CI, 0.22-0.58) compared with those who were insured, and in those with less than a high school degree (psoriasis OR, 0.72, 95% CI, 0.63-0.82; PsA OR, 0.65, 95% CI, 0.47-0.87) compared with those with a college degree.
“The All of Us research program has demonstrated to be a valuable resource to gain unique dermatologic insights on diverse participant populations,” Ms. Tran said.
“There needs to be improvement in access to quality dermatologic care, as this may help to reduce underdiagnosis of psoriasis and psoriatic arthritis,” she added. Access can be increased in various ways, including “outreach to underserved communities, equitable distribution of resources, and increased awareness of clinical variations in skin of color.”
Laura Korb Ferris, MD, PhD, professor of dermatology and director of clinical trials for the department of dermatology at University of Pittsburgh Medical Center, said the study is interesting.
“Because All of Us uses electronic health records to identify cases, while these findings could suggest that these patients are less likely to develop psoriasis and psoriatic arthritis, it more likely shows that they are less likely to receive care for these conditions,” she told this news organization.
“This is concerning, as psoriasis is associated with other comorbidities such as cardiovascular disease and depression, and psoriatic arthritis if left untreated can cause irreversible joint damage that limits function,” she explained in an email. “Both conditions profoundly impact a patient’s quality of life.
“It is important to know whether the diagnoses are simply being missed in these patients or are being neglected,” noted Dr. Ferris, who was not involved in the study and was asked to comment on the results. “It is also important to find strategies to improve diagnosis and treatment, improve quality of life, and allow for interventions to improve long-term sequelae of these diseases and their comorbid conditions.”
The NIH All of Us Research Program, which aims to build a diverse database from at least 1 million adult participants in the United States as a part of the agency’s precision medicine initiative, is open to researchers and to the public. Researchers can access All of Us data and tools to conduct studies at the All of Us Research Hub, and adults who live in the United States can contribute their health data at the All of Us Research Program website and at participating health care provider organizations.
Ms. Tran, study coauthors, and Dr. Ferris reported no relevant relationships. The All of Us Research Program is supported by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM SID 2022
Generalized Pustular Psoriasis: A Review of the Pathophysiology, Clinical Manifestations, Diagnosis, and Treatment
Acute generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis characterized by the sudden widespread eruption of sterile pustules.1,2 The cutaneous manifestations of GPP also may be accompanied by signs of systemic inflammation, including fever, malaise, and leukocytosis.2 Complications are common and may be life-threatening, especially in older patients with comorbid diseases.3 Generalized pustular psoriasis most commonly occurs in patients with a preceding history of psoriasis, but it also may occur de novo.4 Generalized pustular psoriasis is associated with notable morbidity and mortality, and relapses are common.3,4 Many triggers of GPP have been identified, including initiation and withdrawal of various medications, infections, pregnancy, and other conditions.5,6 Although GPP most often occurs in adults, it also may arise in children and infants.3 In pregnancy, GPP is referred to as impetigo herpetiformis, despite having no etiologic ties with either herpes simplex virus or staphylococcal or streptococcal infection. Impetigo herpetiformis is considered one of the most dangerous dermatoses of pregnancy because of high rates of associated maternal and fetal morbidity.6,7
Acute GPP has proven to be a challenging disease to treat due to the rarity and relapsing-remitting nature of the disease; additionally, there are relatively few randomized controlled trials investigating the efficacy and safety of treatments for GPP. This review summarizes the features of GPP, including the pathophysiology of the disease, clinical and histological manifestations, and recommendations for management based on a PubMed search of articles indexed for MEDLINE using MeSH terms pertaining to the disease, including generalized pustular psoriasis, impetigo herpetiformis, and von Zumbusch psoriasis.
Pathophysiology
The pathophysiology of GPP is only partially understood, but it is thought to have a distinct pattern of immune activation compared with plaque psoriasis.8 Although there is a considerable amount of overlap and cross-talk among cytokine pathways, GPP generally is driven by innate immunity and unrestrained IL-36 cytokine activity. In contrast, adaptive immune responses—namely the tumor necrosis factor (TNF) α, IL-23, IL-17, and IL-22 axes—underlie plaque psoriasis.8-10
Proinflammatory IL-36 cytokines α, β, and γ, which are all part of the IL-1 superfamily, bind to the IL-36 receptor (IL-36R) to recruit and activate immune cells via various mediators, including IL-1β; IL-8; and chemokines CXCL1, CXCL2, and CXCL8.3 The IL-36 receptor antagonist (IL-36ra) acts to inhibit this inflammatory cascade.3,8 Microarray analyses of skin biopsy samples have shown that overexpression of IL-17A, TNF-α, IL-1, and IL-36 are seen in both GPP and plaque psoriasis lesions, but GPP lesions had higher expression of IL-1β, IL-36α, and IL-36γ and elevated neutrophil chemokines—CXCL1, CXCL2, and CXCL8—compared with plaque psoriasis lesions.8
Gene Mutations Associated With GPP
There are 3 gene mutations that have been associated with pustular variants of psoriasis, though these mutations account for a minority of cases of GPP.4 Genetic screenings are not routinely indicated in patients with GPP, but they may be warranted in severe cases when a familial pattern of inheritance is suspected.4
IL36RN—The gene IL36RN codes the anti-inflammatory IL-36ra. Loss-of-function mutations in IL36RN lead to impairment of IL-36ra and consequently hyperactivity of the proinflammatory responses triggered by IL-36.3 Homozygous and heterozygous mutations in IL36RN have been observed in both familial and sporadic cases of GPP.11-13 Subsequent retrospective analyses have identified the presence of IL36RN mutations in patients with GPP with frequencies ranging from 23% to 37%.14-17IL36RN mutations are thought to be more common in patients without concomitant plaque psoriasis and have been associated with severe disease and early disease onset.15
CARD14—A gain-of-function mutation in CARD14 results in overactivation of the proinflammatory nuclear factor κB pathway and has been implicated in cases of GPP with concurrent psoriasis vulgaris. Interestingly, this may suggest distinct etiologies underlying GPP de novo and GPP in patients with a history of psoriasis.18,19
AP1S3—A loss-of-function mutation in AP1S3 results in abnormal endosomal trafficking and autophagy as well as increased expression of IL-36α.20,21
Clinical Presentation and Diagnosis Cutaneous Manifestations of GPP
Generalized pustular psoriasis is characterized by the onset of widespread 2- to 3-mm sterile pustules on erythematous skin or within psoriasiform plaques4 (Figure). In patients with skin of color, the erythema may appear less obvious or perhaps slightly violaceous compared to White skin. Pustules may coalesce to form “lakes” of pus.5 Cutaneous symptoms include pain, burning, and pruritus. Associated mucosal findings may include cheilitis, geographic tongue, conjunctivitis, and uveitis.4
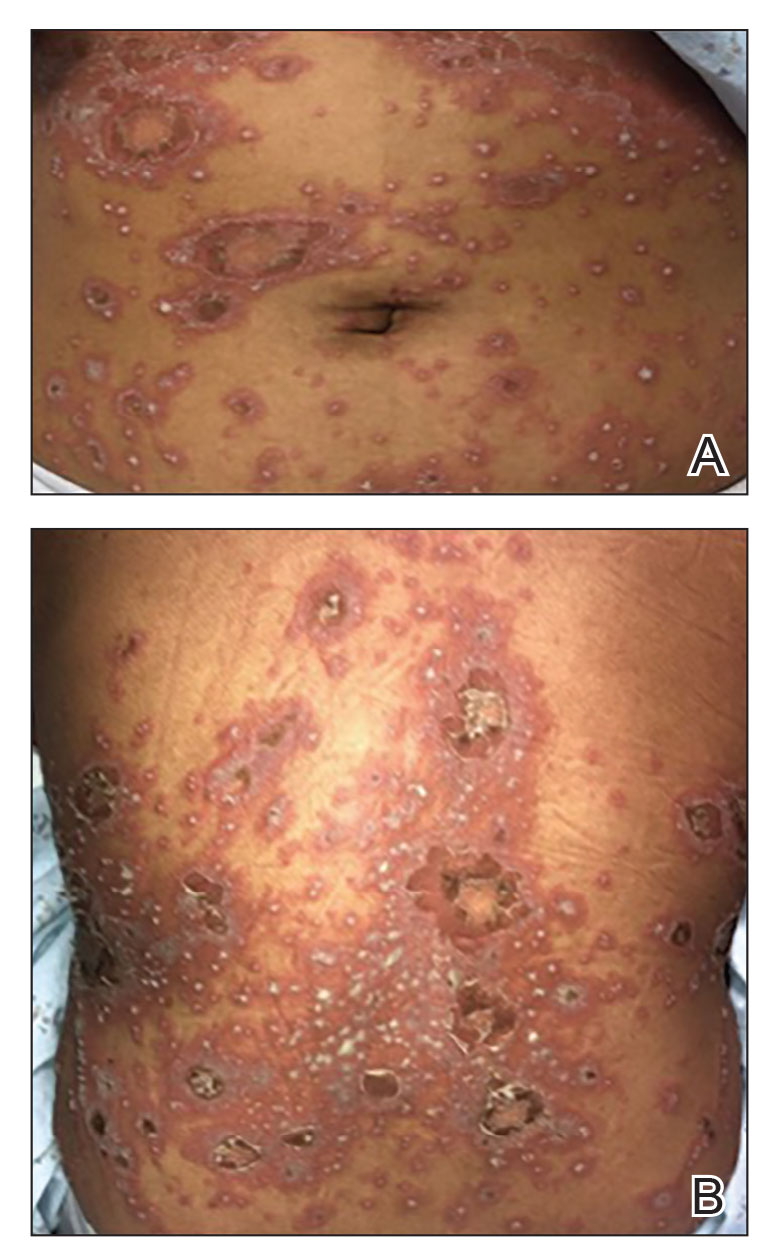
The severity of symptoms can vary greatly among patients as well as between flares within the same patient.2,3 Four distinct patterns of GPP have been described. The von Zumbusch pattern is characterized by a rapid, generalized, painful, erythematous and pustular eruption accompanied by fever and asthenia. The pustules usually resolve after several days with extensive scaling. The annular pattern is characterized by annular, erythematous, scaly lesions with pustules present centrifugally. The lesions enlarge by centrifugal expansion over a period of hours to days, while healing occurs centrally. The exanthematic type is an acute eruption of small pustules that abruptly appear and disappear within a few days, usually from infection or medication initiation. Sometimes pustules appear within or at the edge of existing psoriatic plaques in a localized pattern—the fourth pattern—often following the exposure to irritants (eg, tars, anthralin).5
Impetigo Herpetiformis—Impetigo herpetiformis is a form of GPP associated with pregnancy. It generally presents early in the third trimester with symmetric erythematous plaques in flexural and intertriginous areas with pustules present at lesion margins. Lesions expand centrifugally, with pustulation present at the advancing edge.6,7 Patients often are acutely ill with fever, delirium, vomiting, and tetany. Mucous membranes, including the tongue, mouth, and esophagus, also may be involved. The eruption typically resolves after delivery, though it often recurs with subsequent pregnancies, with the morbidity risk rising with each successive pregnancy.7
Systemic and Extracutaneous Manifestations of GPP
Although the severity of GPP is highly variable, skin manifestations often are accompanied by systemic manifestations of inflammation, including fever and malaise. Common laboratory abnormalities include leukocytosis with peripheral neutrophilia, a high serum C-reactive protein level, hypocalcemia, and hypoalbuminemia.22 Abnormal liver enzymes often are present and result from neutrophilic cholangitis, with alternating strictures and dilations of biliary ducts observed on magnetic resonance imaging.23 Additional laboratory abnormalities are provided in Table 2. Other extracutaneous findings associated with GPP include arthralgia, edema, and characteristic psoriatic nail changes.4 Fatal complications include acute respiratory distress syndrome, renal dysfunction, cardiovascular shock, and sepsis.24,25
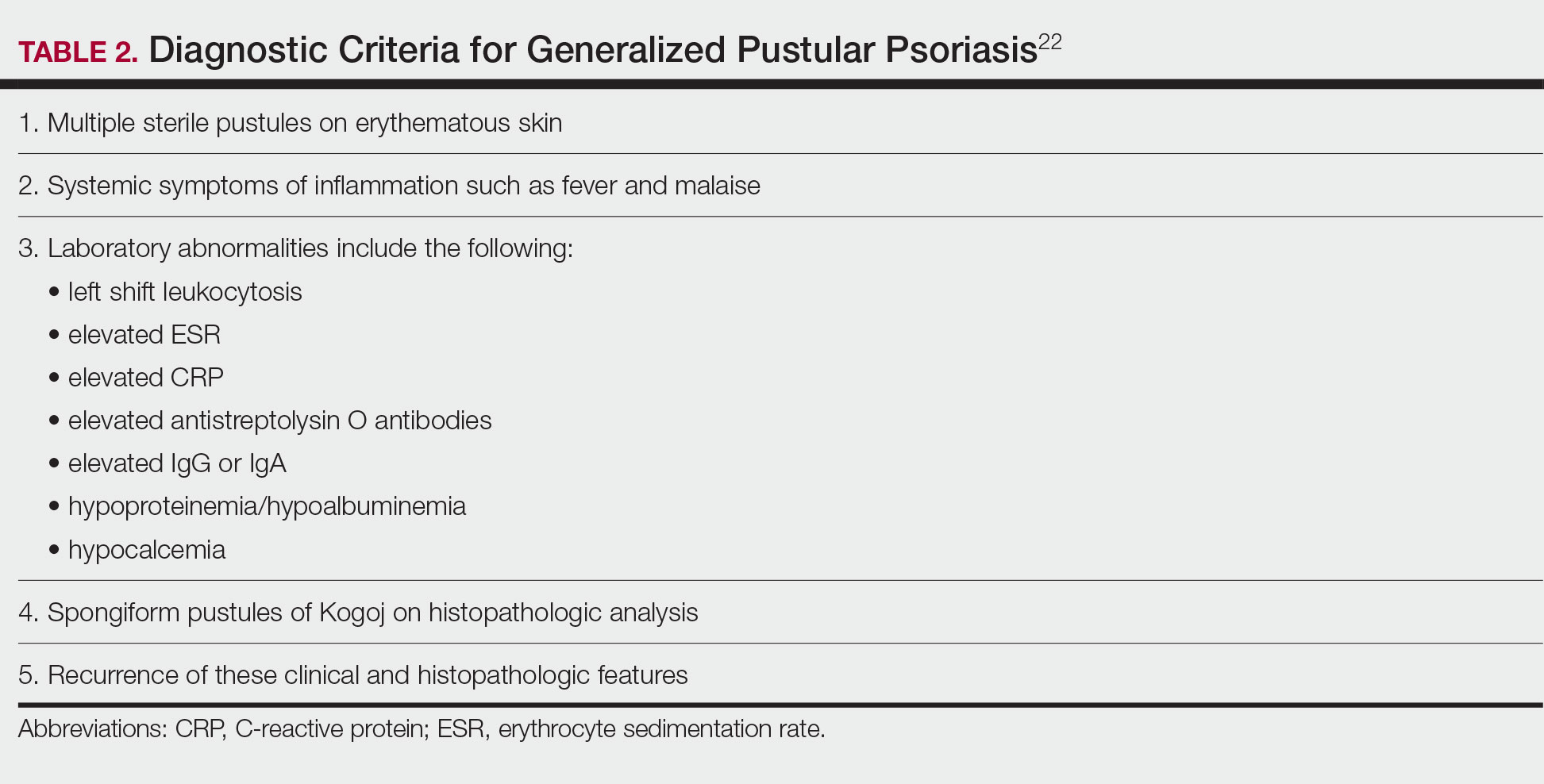
Histologic Features
Given the potential for the skin manifestations of GPP to mimic other disorders, a skin biopsy is warranted to confirm the diagnosis. Generalized pustular psoriasis is histologically characterized by the presence of subcorneal macropustules (ie, spongiform pustules of Kogoj) formed by neutrophil infiltration into the spongelike network of the epidermis.6 Otherwise, the architecture of the epithelium in GPP is similar to that seen with plaque psoriasis, with parakeratosis, acanthosis, rete-ridge elongation, diminished stratum granulosum, and thinning of the suprapapillary epidermis, though the inflammatory cell infiltrate and edema are markedly more severe in GPP than plaque psoriasis.3,4
Differential Diagnosis
There are many other cutaneous pustular diagnoses that must be ruled out when evaluating a patient with GPP (Table 1).26 Acute generalized exanthematous pustulosis (AGEP) is a common mimicker of GPP that is differentiated histologically by the presence of eosinophils and necrotic keratinocytes.4 In addition to its distinct histopathologic findings, AGEP is classically associated with recent initiation of certain medications, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, and diltiazem.27 In contrast, GPP more commonly is related to withdrawal of corticosteroids as well as initiation of some biologic medications, including anti-TNF agents.3 Generalized pustular psoriasis should be suspected over AGEP in patients with a personal or family history of psoriasis, though GPP may arise in patients with or without a history of psoriasis. Acute generalized exanthematous pustulosis usually is more abrupt in both onset and resolution compared with GPP, with clearance of pustules within a few days to weeks following cessation of the triggering factor.4
Other pustular variants of psoriasis (eg, palmoplantar pustular psoriasis, acrodermatitis continua of Hallopeau) are differentiated from GPP by their chronicity and localization to palmoplantar and/or ungual surfaces.5 Other differential diagnoses are listed in Table 1.
Diagnostic Criteria for GPP
Diagnostic criteria have been proposed for GPP (Table 2), including (1) the presence of sterile pustules, (2) systemic signs of inflammation, (3) laboratory abnormalities, (4) histopathologic confirmation of spongiform pustules of Kogoj, and (5) recurrence of symptoms.22 To definitively diagnose GPP, all 5 criteria must be met. To rule out mimickers, it may be worthwhile to perform Gram staining, potassium hydroxide preparation, in vitro cultures, and/or immunofluorescence testing.6
Treatment
Given the high potential for mortality associated with GPP, the most essential component of management is to ensure adequate supportive care. Any temperature, fluid, or electrolyte imbalances should be corrected as they arise. Secondary infections also must be identified and treated, if present, to reduce the risk for fatal complications, including systemic infection and sepsis. Precautions must be taken to ensure that serious end-organ damage, including hepatic, renal, and respiratory dysfunction, is avoided.
Adjunctive topical intervention often is initiated with bland emollients, corticosteroids, calcineurin inhibitors, and/or vitamin D derivatives to help soothe skin symptoms, but treatment with systemic therapies usually is warranted to achieve symptom control.2,25 Importantly, there are no systemic or topical agents that have specifically been approved for the treatment of GPP in Europe or the United States.3 Given the absence of universally accepted treatment guidelines, therapeutic agents for GPP usually are selected based on clinical experience while also taking the extent of involvement and disease severity into consideration.3
Treatment Recommendations for Adults
Oral Systemic Agents—Treatment guidelines set forth by the National Psoriasis Foundation (NPF) in 2012 proposed that first-line therapies for GPP should be acitretin, cyclosporine, methotrexate, and infliximab.28 However, since those guidelines were established, many new biologic therapies have been approved for the treatment of psoriasis and often are considered in the treatment of psoriasis subtypes, including GPP.29 Although retinoids previously were considered to be a preferred first-line therapy, they are associated with a high incidence of adverse effects and must be used with caution in women of childbearing age.6 Oral acitretin at a dosage of 0.75 to 1.0 mg/kg/d has been shown to result in clinical improvement within 1 to 2 weeks, and a maintenance dosage of 0.125 to 0.25 mg/kg/d is required for several months to prevent recurrence.30 Methotrexate—5.0 to 15.0 mg/wk, or perhaps higher in patients with refractory disease, increased by 2.5-mg intervals until symptoms improve—is recommended by the NPF in patients who are unresponsive or cannot tolerate retinoids, though close monitoring for hematologic abnormalities is required. Cyclosporine 2.5 to 5.0 mg/kg/d is considered an alternative to methotrexate and retinoids; it has a faster onset of action, with improvement reported as early as 2 weeks after initiation of therapy.1,28 Although cyclosporine may be effective in the acute phase, especially in severe cases of GPP, long-term use of cyclosporine is not recommended because of the potential for renal dysfunction and hypertension.31
Biologic Agents—More recent evidence has accumulated supporting the efficacy of anti-TNF agents in the treatment of GPP, suggesting the positioning of these agents as first line. A number of case series have shown dramatic and rapid improvement of GPP with intravenous infliximab 3 to 5 mg/kg, with results observed hours to days after the first infusion.32-37 Thus, infliximab is recommended as first-line treatment in severe acute cases, though its efficacy as a maintenance therapy has not been sufficiently investigated.6 Case reports and case series document the safety and efficacy of adalimumab 40 to 80 mg every 1 to 2 weeks38,39 and etanercept 25 to 50 mg twice weekly40-42 in patients with recalcitrantGPP. Therefore, these anti-TNF agents may be considered in patients who are nonresponsive to treatment with infliximab.
Rarely, there have been reports of paradoxical induction of GPP with the use of some anti-TNF agents,43-45 which may be due to a cytokine imbalance characterized by unopposed IFN-α activation.6 In patients with a history of GPP after initiation of a biologic, treatment with agents from within the offending class should be avoided.
The IL-17A monoclonal antibodies secukinumab, ixekizumab, and brodalumab have been shown in open-label phase 3 studies to result in disease remission at 12 weeks.46-48 Treatment with guselkumab, an IL-23 monoclonal antibody, also has demonstrated efficacy in patients with GPP.49 Ustekinumab, an IL-12/23 inhibitor, in combination with acitretin also has been shown to be successful in achieving disease remission after a few weeks of treatment.50
More recent case reports have shown the efficacy of IL-1 inhibitors including gevokizumab, canakinumab, and anakinra in achieving GPP clearance, though more prospective studies are needed to evaluate their efficacy.51-53 Given the etiologic association between IL-1 disinhibition and GPP, future investigations of these therapies as well as those that target the IL-36 pathway may prove to be particularly interesting.
Phototherapy and Combination Therapies—Phototherapy may be considered as maintenance therapy after disease control is achieved, though it is not considered appropriate for acute cases.28 Combination therapies with a biologic plus a nonbiologic systemic agent or alternating among various biologics may allow physicians to maximize benefits and minimize adverse effects in the long term, though there is insufficient evidence to suggest any specific combination treatment algorithm for GPP.28
Treatment Recommendations for Pediatric Patients
Based on a small number of case series and case reports, the first-line treatment strategy for children with GPP is similar to adults. Given the notable adverse events of most oral systemic agents, biologic therapies may emerge as first-line therapy in the pediatric population as more evidence accumulates.28
Treatment Recommendations for Pregnant Patients
Systemic corticosteroids are widely considered to be the first-line treatments for the management of impetigo herpetiformis.7 Low-dose prednisone (15–30 mg/d) usually is effective, but severe cases may require increasing the dosage to 60 mg/d.6 Given the potential for rebound flares upon withdrawal of systemic corticosteroids, these agents must be gradually tapered after the resolution of symptoms.
Certolizumab pegol also is an attractive option in pregnant patients with impetigo herpetiformis because of its favorable safety profile and negligible mother-to-infant transfer through the placenta or breast milk. It has been shown to be effective in treating GPP and impetigo herpetiformis during pregnancy in recently published case reports.54,55 In refractory cases, other TNF-α inhibitors (eg, adalimumab, infliximab, etanercept) or cyclosporine may be considered. However, cautious medical monitoring is warranted, as little is known about the potential adverse effects of these agents to the mother and fetus.28,56 Data from transplant recipients along with several case reports indicate that cyclosporine is not associated with an increased risk for adverse effects during pregnancy at a dose of 2 to 3 mg/kg.57-59 Both methotrexate and retinoids are known teratogens and are therefore contraindicated in pregnant patients.56
If pustules do not resolve in the postpartum period, patients should be treated with standard GPP therapies. However, long-term and population studies are lacking regarding the potential for infant exposure to systemic agents in breast milk. Therefore, the NPF recommends avoiding breastfeeding while taking systemic medications, if possible.56
Limitations of Treatment Recommendations
The ability to generate an evidence-based treatment strategy for GPP is limited by a lack of high-quality studies investigating the efficacy and safety of treatments in patients with GPP due to the rarity and relapsing-remitting nature of the disease, which makes randomized controlled trials difficult to conduct. The quality of the available research is further limited by the lack of validated outcome measures to specifically assess improvements in patients with GPP, such that results are difficult to synthesize and compare among studies.31
Conclusion
Although limited, the available research suggests that treatment with various biologics, especially infliximab, is effective in achieving rapid clearance in patients with GPP. In general, biologics may be the most appropriate treatment option in patients with GPP given their relatively favorable safety profiles. Other oral systemic agents, including acitretin, cyclosporine, and methotrexate, have limited evidence to support their use in the acute phase, but their safety profiles often limit their utility in the long-term. Emerging evidence regarding the association of GPP with IL36RN mutations suggests a unique role for agents targeting the IL-36 or IL-1 pathways, though this has yet to be thoroughly investigated.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131‐144.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614‐618.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37‐42.
- van de Kerkhof PCM, Nestle FO. Psoriasis. In: Bolognia JL, Jorizzo JJ, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012:138-160.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645‐1651.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101‐104.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5-13.
- Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264-272.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432-437.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514-2521.
- Hussain S, Berki DM, Choon SE, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1070.e9.
- Körber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133:2634-2637.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021-1026.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-192
- Wang Y, Cheng R, Lu Z, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235-240.
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and upregulating IL-36 production. J Invest Dermatol. 2016;136:2251-2259.
- Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43-S54.
- Viguier M, Allez M, Zagdanski AM, et al. High frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tract. Hepatology. 2004;40:452-458.
- Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971;85:407-411.
- Kalb RE. Pustular psoriasis: management. In: Ofori AO, Duffin KC, eds. UpToDate. UpToDate; 2014. Accessed July 20, 2022. https://www.uptodate.com/contents/pustular-psoriasis-management/print
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-425.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol 2002;3:389-400.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591‐601.
- Elewski BE. Infliximab for the treatment of severe pustular psoriasis. J Am Acad Dermatol. 2002;47:796-797.
- Kim HS, You HS, Cho HH, et al. Two cases of generalized pustular psoriasis: successful treatment with infliximab. Ann Dermatol. 2014;26:787-788.
- Trent JT, Kerdel FA. Successful treatment of Von Zumbusch pustular psoriasis with infliximab. J Cutan Med Surg. 2004;8:224-228.
- Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329-336.
- Routhouska S, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: case series. J Cutan Med Surg. 2008;12:184-188.
- Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84:247-248.
- Zangrilli A, Papoutsaki M, Talamonti M, et al. Long-term efficacy of adalimumab in generalized pustular psoriasis. J Dermatol Treat. 2008;19:185-187.
- Matsumoto A, Komine M, Karakawa M, et al. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44:202-204.
- Kamarashev J, Lor P, Forster A, et al. Generalized pustular psoriasis induced by cyclosporin in a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213-216.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different dosages in the treatment of generalized pustular psoriasis: a case series. Dermatology. 2008;216:355-360.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233-234.
- Goiriz R, Daudén E, Pérez-Gala S, et al. Flare and change of psoriasis morphology during the course of treatment with tumor necrosis factor blockers. Clin Exp Dermatol. 2006;32:176-179.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Almutairi D, Sheasgreen C, Weizman A, et al. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;1:507-510.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis, and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45:529‐539.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825-828.
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br J Dermatol. 2015;173:239-241.
- Skendros P, Papagoras C, Lefaki I, et al. Successful response in a case of severe pustular psoriasis after interleukin-1 beta inhibition. Br J Dermatol. 2017;176:212-215.
- Viguier M, Guigue P, Pagès C, et al. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66-67.
- Fukushima H, Iwata Y, Arima M, et al. Efficacy and safety of treatment with anti-tumor necrosis factor‐α drugs for severe impetigo herpetiformis. J Dermatol. 2021;48:207-210.
- Mizutani Y, Mizutani YH, Matsuyama K, et al. Generalized pustular psoriasis in pregnancy, successfully treated with certolizumab pegol. J Dermatol. 2021;47:e262-e263.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459‐477.
- Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporin A. Br J Dermatol. 2000;142:582-584.
- Gaughan WJ, Moritz MJ, Radomski JS, et al. National Transplantation Pregnancy Registry: report on outcomes of cyclosporine-treated female kidney transplant recipients with an interval from transplantation to pregnancy of greater than five years. Am J Kidney Dis. 1996;28:266-269.
- Kura MM, Surjushe AU. Generalized pustular psoriasis of pregnancy treated with oral cyclosporin. Indian J Dermatol Venereol Leprol. 2006;72:458-459.
Acute generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis characterized by the sudden widespread eruption of sterile pustules.1,2 The cutaneous manifestations of GPP also may be accompanied by signs of systemic inflammation, including fever, malaise, and leukocytosis.2 Complications are common and may be life-threatening, especially in older patients with comorbid diseases.3 Generalized pustular psoriasis most commonly occurs in patients with a preceding history of psoriasis, but it also may occur de novo.4 Generalized pustular psoriasis is associated with notable morbidity and mortality, and relapses are common.3,4 Many triggers of GPP have been identified, including initiation and withdrawal of various medications, infections, pregnancy, and other conditions.5,6 Although GPP most often occurs in adults, it also may arise in children and infants.3 In pregnancy, GPP is referred to as impetigo herpetiformis, despite having no etiologic ties with either herpes simplex virus or staphylococcal or streptococcal infection. Impetigo herpetiformis is considered one of the most dangerous dermatoses of pregnancy because of high rates of associated maternal and fetal morbidity.6,7
Acute GPP has proven to be a challenging disease to treat due to the rarity and relapsing-remitting nature of the disease; additionally, there are relatively few randomized controlled trials investigating the efficacy and safety of treatments for GPP. This review summarizes the features of GPP, including the pathophysiology of the disease, clinical and histological manifestations, and recommendations for management based on a PubMed search of articles indexed for MEDLINE using MeSH terms pertaining to the disease, including generalized pustular psoriasis, impetigo herpetiformis, and von Zumbusch psoriasis.
Pathophysiology
The pathophysiology of GPP is only partially understood, but it is thought to have a distinct pattern of immune activation compared with plaque psoriasis.8 Although there is a considerable amount of overlap and cross-talk among cytokine pathways, GPP generally is driven by innate immunity and unrestrained IL-36 cytokine activity. In contrast, adaptive immune responses—namely the tumor necrosis factor (TNF) α, IL-23, IL-17, and IL-22 axes—underlie plaque psoriasis.8-10
Proinflammatory IL-36 cytokines α, β, and γ, which are all part of the IL-1 superfamily, bind to the IL-36 receptor (IL-36R) to recruit and activate immune cells via various mediators, including IL-1β; IL-8; and chemokines CXCL1, CXCL2, and CXCL8.3 The IL-36 receptor antagonist (IL-36ra) acts to inhibit this inflammatory cascade.3,8 Microarray analyses of skin biopsy samples have shown that overexpression of IL-17A, TNF-α, IL-1, and IL-36 are seen in both GPP and plaque psoriasis lesions, but GPP lesions had higher expression of IL-1β, IL-36α, and IL-36γ and elevated neutrophil chemokines—CXCL1, CXCL2, and CXCL8—compared with plaque psoriasis lesions.8
Gene Mutations Associated With GPP
There are 3 gene mutations that have been associated with pustular variants of psoriasis, though these mutations account for a minority of cases of GPP.4 Genetic screenings are not routinely indicated in patients with GPP, but they may be warranted in severe cases when a familial pattern of inheritance is suspected.4
IL36RN—The gene IL36RN codes the anti-inflammatory IL-36ra. Loss-of-function mutations in IL36RN lead to impairment of IL-36ra and consequently hyperactivity of the proinflammatory responses triggered by IL-36.3 Homozygous and heterozygous mutations in IL36RN have been observed in both familial and sporadic cases of GPP.11-13 Subsequent retrospective analyses have identified the presence of IL36RN mutations in patients with GPP with frequencies ranging from 23% to 37%.14-17IL36RN mutations are thought to be more common in patients without concomitant plaque psoriasis and have been associated with severe disease and early disease onset.15
CARD14—A gain-of-function mutation in CARD14 results in overactivation of the proinflammatory nuclear factor κB pathway and has been implicated in cases of GPP with concurrent psoriasis vulgaris. Interestingly, this may suggest distinct etiologies underlying GPP de novo and GPP in patients with a history of psoriasis.18,19
AP1S3—A loss-of-function mutation in AP1S3 results in abnormal endosomal trafficking and autophagy as well as increased expression of IL-36α.20,21
Clinical Presentation and Diagnosis Cutaneous Manifestations of GPP
Generalized pustular psoriasis is characterized by the onset of widespread 2- to 3-mm sterile pustules on erythematous skin or within psoriasiform plaques4 (Figure). In patients with skin of color, the erythema may appear less obvious or perhaps slightly violaceous compared to White skin. Pustules may coalesce to form “lakes” of pus.5 Cutaneous symptoms include pain, burning, and pruritus. Associated mucosal findings may include cheilitis, geographic tongue, conjunctivitis, and uveitis.4

The severity of symptoms can vary greatly among patients as well as between flares within the same patient.2,3 Four distinct patterns of GPP have been described. The von Zumbusch pattern is characterized by a rapid, generalized, painful, erythematous and pustular eruption accompanied by fever and asthenia. The pustules usually resolve after several days with extensive scaling. The annular pattern is characterized by annular, erythematous, scaly lesions with pustules present centrifugally. The lesions enlarge by centrifugal expansion over a period of hours to days, while healing occurs centrally. The exanthematic type is an acute eruption of small pustules that abruptly appear and disappear within a few days, usually from infection or medication initiation. Sometimes pustules appear within or at the edge of existing psoriatic plaques in a localized pattern—the fourth pattern—often following the exposure to irritants (eg, tars, anthralin).5
Impetigo Herpetiformis—Impetigo herpetiformis is a form of GPP associated with pregnancy. It generally presents early in the third trimester with symmetric erythematous plaques in flexural and intertriginous areas with pustules present at lesion margins. Lesions expand centrifugally, with pustulation present at the advancing edge.6,7 Patients often are acutely ill with fever, delirium, vomiting, and tetany. Mucous membranes, including the tongue, mouth, and esophagus, also may be involved. The eruption typically resolves after delivery, though it often recurs with subsequent pregnancies, with the morbidity risk rising with each successive pregnancy.7
Systemic and Extracutaneous Manifestations of GPP
Although the severity of GPP is highly variable, skin manifestations often are accompanied by systemic manifestations of inflammation, including fever and malaise. Common laboratory abnormalities include leukocytosis with peripheral neutrophilia, a high serum C-reactive protein level, hypocalcemia, and hypoalbuminemia.22 Abnormal liver enzymes often are present and result from neutrophilic cholangitis, with alternating strictures and dilations of biliary ducts observed on magnetic resonance imaging.23 Additional laboratory abnormalities are provided in Table 2. Other extracutaneous findings associated with GPP include arthralgia, edema, and characteristic psoriatic nail changes.4 Fatal complications include acute respiratory distress syndrome, renal dysfunction, cardiovascular shock, and sepsis.24,25

Histologic Features
Given the potential for the skin manifestations of GPP to mimic other disorders, a skin biopsy is warranted to confirm the diagnosis. Generalized pustular psoriasis is histologically characterized by the presence of subcorneal macropustules (ie, spongiform pustules of Kogoj) formed by neutrophil infiltration into the spongelike network of the epidermis.6 Otherwise, the architecture of the epithelium in GPP is similar to that seen with plaque psoriasis, with parakeratosis, acanthosis, rete-ridge elongation, diminished stratum granulosum, and thinning of the suprapapillary epidermis, though the inflammatory cell infiltrate and edema are markedly more severe in GPP than plaque psoriasis.3,4
Differential Diagnosis
There are many other cutaneous pustular diagnoses that must be ruled out when evaluating a patient with GPP (Table 1).26 Acute generalized exanthematous pustulosis (AGEP) is a common mimicker of GPP that is differentiated histologically by the presence of eosinophils and necrotic keratinocytes.4 In addition to its distinct histopathologic findings, AGEP is classically associated with recent initiation of certain medications, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, and diltiazem.27 In contrast, GPP more commonly is related to withdrawal of corticosteroids as well as initiation of some biologic medications, including anti-TNF agents.3 Generalized pustular psoriasis should be suspected over AGEP in patients with a personal or family history of psoriasis, though GPP may arise in patients with or without a history of psoriasis. Acute generalized exanthematous pustulosis usually is more abrupt in both onset and resolution compared with GPP, with clearance of pustules within a few days to weeks following cessation of the triggering factor.4

Other pustular variants of psoriasis (eg, palmoplantar pustular psoriasis, acrodermatitis continua of Hallopeau) are differentiated from GPP by their chronicity and localization to palmoplantar and/or ungual surfaces.5 Other differential diagnoses are listed in Table 1.
Diagnostic Criteria for GPP
Diagnostic criteria have been proposed for GPP (Table 2), including (1) the presence of sterile pustules, (2) systemic signs of inflammation, (3) laboratory abnormalities, (4) histopathologic confirmation of spongiform pustules of Kogoj, and (5) recurrence of symptoms.22 To definitively diagnose GPP, all 5 criteria must be met. To rule out mimickers, it may be worthwhile to perform Gram staining, potassium hydroxide preparation, in vitro cultures, and/or immunofluorescence testing.6
Treatment
Given the high potential for mortality associated with GPP, the most essential component of management is to ensure adequate supportive care. Any temperature, fluid, or electrolyte imbalances should be corrected as they arise. Secondary infections also must be identified and treated, if present, to reduce the risk for fatal complications, including systemic infection and sepsis. Precautions must be taken to ensure that serious end-organ damage, including hepatic, renal, and respiratory dysfunction, is avoided.
Adjunctive topical intervention often is initiated with bland emollients, corticosteroids, calcineurin inhibitors, and/or vitamin D derivatives to help soothe skin symptoms, but treatment with systemic therapies usually is warranted to achieve symptom control.2,25 Importantly, there are no systemic or topical agents that have specifically been approved for the treatment of GPP in Europe or the United States.3 Given the absence of universally accepted treatment guidelines, therapeutic agents for GPP usually are selected based on clinical experience while also taking the extent of involvement and disease severity into consideration.3
Treatment Recommendations for Adults
Oral Systemic Agents—Treatment guidelines set forth by the National Psoriasis Foundation (NPF) in 2012 proposed that first-line therapies for GPP should be acitretin, cyclosporine, methotrexate, and infliximab.28 However, since those guidelines were established, many new biologic therapies have been approved for the treatment of psoriasis and often are considered in the treatment of psoriasis subtypes, including GPP.29 Although retinoids previously were considered to be a preferred first-line therapy, they are associated with a high incidence of adverse effects and must be used with caution in women of childbearing age.6 Oral acitretin at a dosage of 0.75 to 1.0 mg/kg/d has been shown to result in clinical improvement within 1 to 2 weeks, and a maintenance dosage of 0.125 to 0.25 mg/kg/d is required for several months to prevent recurrence.30 Methotrexate—5.0 to 15.0 mg/wk, or perhaps higher in patients with refractory disease, increased by 2.5-mg intervals until symptoms improve—is recommended by the NPF in patients who are unresponsive or cannot tolerate retinoids, though close monitoring for hematologic abnormalities is required. Cyclosporine 2.5 to 5.0 mg/kg/d is considered an alternative to methotrexate and retinoids; it has a faster onset of action, with improvement reported as early as 2 weeks after initiation of therapy.1,28 Although cyclosporine may be effective in the acute phase, especially in severe cases of GPP, long-term use of cyclosporine is not recommended because of the potential for renal dysfunction and hypertension.31
Biologic Agents—More recent evidence has accumulated supporting the efficacy of anti-TNF agents in the treatment of GPP, suggesting the positioning of these agents as first line. A number of case series have shown dramatic and rapid improvement of GPP with intravenous infliximab 3 to 5 mg/kg, with results observed hours to days after the first infusion.32-37 Thus, infliximab is recommended as first-line treatment in severe acute cases, though its efficacy as a maintenance therapy has not been sufficiently investigated.6 Case reports and case series document the safety and efficacy of adalimumab 40 to 80 mg every 1 to 2 weeks38,39 and etanercept 25 to 50 mg twice weekly40-42 in patients with recalcitrantGPP. Therefore, these anti-TNF agents may be considered in patients who are nonresponsive to treatment with infliximab.
Rarely, there have been reports of paradoxical induction of GPP with the use of some anti-TNF agents,43-45 which may be due to a cytokine imbalance characterized by unopposed IFN-α activation.6 In patients with a history of GPP after initiation of a biologic, treatment with agents from within the offending class should be avoided.
The IL-17A monoclonal antibodies secukinumab, ixekizumab, and brodalumab have been shown in open-label phase 3 studies to result in disease remission at 12 weeks.46-48 Treatment with guselkumab, an IL-23 monoclonal antibody, also has demonstrated efficacy in patients with GPP.49 Ustekinumab, an IL-12/23 inhibitor, in combination with acitretin also has been shown to be successful in achieving disease remission after a few weeks of treatment.50
More recent case reports have shown the efficacy of IL-1 inhibitors including gevokizumab, canakinumab, and anakinra in achieving GPP clearance, though more prospective studies are needed to evaluate their efficacy.51-53 Given the etiologic association between IL-1 disinhibition and GPP, future investigations of these therapies as well as those that target the IL-36 pathway may prove to be particularly interesting.
Phototherapy and Combination Therapies—Phototherapy may be considered as maintenance therapy after disease control is achieved, though it is not considered appropriate for acute cases.28 Combination therapies with a biologic plus a nonbiologic systemic agent or alternating among various biologics may allow physicians to maximize benefits and minimize adverse effects in the long term, though there is insufficient evidence to suggest any specific combination treatment algorithm for GPP.28
Treatment Recommendations for Pediatric Patients
Based on a small number of case series and case reports, the first-line treatment strategy for children with GPP is similar to adults. Given the notable adverse events of most oral systemic agents, biologic therapies may emerge as first-line therapy in the pediatric population as more evidence accumulates.28
Treatment Recommendations for Pregnant Patients
Systemic corticosteroids are widely considered to be the first-line treatments for the management of impetigo herpetiformis.7 Low-dose prednisone (15–30 mg/d) usually is effective, but severe cases may require increasing the dosage to 60 mg/d.6 Given the potential for rebound flares upon withdrawal of systemic corticosteroids, these agents must be gradually tapered after the resolution of symptoms.
Certolizumab pegol also is an attractive option in pregnant patients with impetigo herpetiformis because of its favorable safety profile and negligible mother-to-infant transfer through the placenta or breast milk. It has been shown to be effective in treating GPP and impetigo herpetiformis during pregnancy in recently published case reports.54,55 In refractory cases, other TNF-α inhibitors (eg, adalimumab, infliximab, etanercept) or cyclosporine may be considered. However, cautious medical monitoring is warranted, as little is known about the potential adverse effects of these agents to the mother and fetus.28,56 Data from transplant recipients along with several case reports indicate that cyclosporine is not associated with an increased risk for adverse effects during pregnancy at a dose of 2 to 3 mg/kg.57-59 Both methotrexate and retinoids are known teratogens and are therefore contraindicated in pregnant patients.56
If pustules do not resolve in the postpartum period, patients should be treated with standard GPP therapies. However, long-term and population studies are lacking regarding the potential for infant exposure to systemic agents in breast milk. Therefore, the NPF recommends avoiding breastfeeding while taking systemic medications, if possible.56
Limitations of Treatment Recommendations
The ability to generate an evidence-based treatment strategy for GPP is limited by a lack of high-quality studies investigating the efficacy and safety of treatments in patients with GPP due to the rarity and relapsing-remitting nature of the disease, which makes randomized controlled trials difficult to conduct. The quality of the available research is further limited by the lack of validated outcome measures to specifically assess improvements in patients with GPP, such that results are difficult to synthesize and compare among studies.31
Conclusion
Although limited, the available research suggests that treatment with various biologics, especially infliximab, is effective in achieving rapid clearance in patients with GPP. In general, biologics may be the most appropriate treatment option in patients with GPP given their relatively favorable safety profiles. Other oral systemic agents, including acitretin, cyclosporine, and methotrexate, have limited evidence to support their use in the acute phase, but their safety profiles often limit their utility in the long-term. Emerging evidence regarding the association of GPP with IL36RN mutations suggests a unique role for agents targeting the IL-36 or IL-1 pathways, though this has yet to be thoroughly investigated.
Acute generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis characterized by the sudden widespread eruption of sterile pustules.1,2 The cutaneous manifestations of GPP also may be accompanied by signs of systemic inflammation, including fever, malaise, and leukocytosis.2 Complications are common and may be life-threatening, especially in older patients with comorbid diseases.3 Generalized pustular psoriasis most commonly occurs in patients with a preceding history of psoriasis, but it also may occur de novo.4 Generalized pustular psoriasis is associated with notable morbidity and mortality, and relapses are common.3,4 Many triggers of GPP have been identified, including initiation and withdrawal of various medications, infections, pregnancy, and other conditions.5,6 Although GPP most often occurs in adults, it also may arise in children and infants.3 In pregnancy, GPP is referred to as impetigo herpetiformis, despite having no etiologic ties with either herpes simplex virus or staphylococcal or streptococcal infection. Impetigo herpetiformis is considered one of the most dangerous dermatoses of pregnancy because of high rates of associated maternal and fetal morbidity.6,7
Acute GPP has proven to be a challenging disease to treat due to the rarity and relapsing-remitting nature of the disease; additionally, there are relatively few randomized controlled trials investigating the efficacy and safety of treatments for GPP. This review summarizes the features of GPP, including the pathophysiology of the disease, clinical and histological manifestations, and recommendations for management based on a PubMed search of articles indexed for MEDLINE using MeSH terms pertaining to the disease, including generalized pustular psoriasis, impetigo herpetiformis, and von Zumbusch psoriasis.
Pathophysiology
The pathophysiology of GPP is only partially understood, but it is thought to have a distinct pattern of immune activation compared with plaque psoriasis.8 Although there is a considerable amount of overlap and cross-talk among cytokine pathways, GPP generally is driven by innate immunity and unrestrained IL-36 cytokine activity. In contrast, adaptive immune responses—namely the tumor necrosis factor (TNF) α, IL-23, IL-17, and IL-22 axes—underlie plaque psoriasis.8-10
Proinflammatory IL-36 cytokines α, β, and γ, which are all part of the IL-1 superfamily, bind to the IL-36 receptor (IL-36R) to recruit and activate immune cells via various mediators, including IL-1β; IL-8; and chemokines CXCL1, CXCL2, and CXCL8.3 The IL-36 receptor antagonist (IL-36ra) acts to inhibit this inflammatory cascade.3,8 Microarray analyses of skin biopsy samples have shown that overexpression of IL-17A, TNF-α, IL-1, and IL-36 are seen in both GPP and plaque psoriasis lesions, but GPP lesions had higher expression of IL-1β, IL-36α, and IL-36γ and elevated neutrophil chemokines—CXCL1, CXCL2, and CXCL8—compared with plaque psoriasis lesions.8
Gene Mutations Associated With GPP
There are 3 gene mutations that have been associated with pustular variants of psoriasis, though these mutations account for a minority of cases of GPP.4 Genetic screenings are not routinely indicated in patients with GPP, but they may be warranted in severe cases when a familial pattern of inheritance is suspected.4
IL36RN—The gene IL36RN codes the anti-inflammatory IL-36ra. Loss-of-function mutations in IL36RN lead to impairment of IL-36ra and consequently hyperactivity of the proinflammatory responses triggered by IL-36.3 Homozygous and heterozygous mutations in IL36RN have been observed in both familial and sporadic cases of GPP.11-13 Subsequent retrospective analyses have identified the presence of IL36RN mutations in patients with GPP with frequencies ranging from 23% to 37%.14-17IL36RN mutations are thought to be more common in patients without concomitant plaque psoriasis and have been associated with severe disease and early disease onset.15
CARD14—A gain-of-function mutation in CARD14 results in overactivation of the proinflammatory nuclear factor κB pathway and has been implicated in cases of GPP with concurrent psoriasis vulgaris. Interestingly, this may suggest distinct etiologies underlying GPP de novo and GPP in patients with a history of psoriasis.18,19
AP1S3—A loss-of-function mutation in AP1S3 results in abnormal endosomal trafficking and autophagy as well as increased expression of IL-36α.20,21
Clinical Presentation and Diagnosis Cutaneous Manifestations of GPP
Generalized pustular psoriasis is characterized by the onset of widespread 2- to 3-mm sterile pustules on erythematous skin or within psoriasiform plaques4 (Figure). In patients with skin of color, the erythema may appear less obvious or perhaps slightly violaceous compared to White skin. Pustules may coalesce to form “lakes” of pus.5 Cutaneous symptoms include pain, burning, and pruritus. Associated mucosal findings may include cheilitis, geographic tongue, conjunctivitis, and uveitis.4

The severity of symptoms can vary greatly among patients as well as between flares within the same patient.2,3 Four distinct patterns of GPP have been described. The von Zumbusch pattern is characterized by a rapid, generalized, painful, erythematous and pustular eruption accompanied by fever and asthenia. The pustules usually resolve after several days with extensive scaling. The annular pattern is characterized by annular, erythematous, scaly lesions with pustules present centrifugally. The lesions enlarge by centrifugal expansion over a period of hours to days, while healing occurs centrally. The exanthematic type is an acute eruption of small pustules that abruptly appear and disappear within a few days, usually from infection or medication initiation. Sometimes pustules appear within or at the edge of existing psoriatic plaques in a localized pattern—the fourth pattern—often following the exposure to irritants (eg, tars, anthralin).5
Impetigo Herpetiformis—Impetigo herpetiformis is a form of GPP associated with pregnancy. It generally presents early in the third trimester with symmetric erythematous plaques in flexural and intertriginous areas with pustules present at lesion margins. Lesions expand centrifugally, with pustulation present at the advancing edge.6,7 Patients often are acutely ill with fever, delirium, vomiting, and tetany. Mucous membranes, including the tongue, mouth, and esophagus, also may be involved. The eruption typically resolves after delivery, though it often recurs with subsequent pregnancies, with the morbidity risk rising with each successive pregnancy.7
Systemic and Extracutaneous Manifestations of GPP
Although the severity of GPP is highly variable, skin manifestations often are accompanied by systemic manifestations of inflammation, including fever and malaise. Common laboratory abnormalities include leukocytosis with peripheral neutrophilia, a high serum C-reactive protein level, hypocalcemia, and hypoalbuminemia.22 Abnormal liver enzymes often are present and result from neutrophilic cholangitis, with alternating strictures and dilations of biliary ducts observed on magnetic resonance imaging.23 Additional laboratory abnormalities are provided in Table 2. Other extracutaneous findings associated with GPP include arthralgia, edema, and characteristic psoriatic nail changes.4 Fatal complications include acute respiratory distress syndrome, renal dysfunction, cardiovascular shock, and sepsis.24,25

Histologic Features
Given the potential for the skin manifestations of GPP to mimic other disorders, a skin biopsy is warranted to confirm the diagnosis. Generalized pustular psoriasis is histologically characterized by the presence of subcorneal macropustules (ie, spongiform pustules of Kogoj) formed by neutrophil infiltration into the spongelike network of the epidermis.6 Otherwise, the architecture of the epithelium in GPP is similar to that seen with plaque psoriasis, with parakeratosis, acanthosis, rete-ridge elongation, diminished stratum granulosum, and thinning of the suprapapillary epidermis, though the inflammatory cell infiltrate and edema are markedly more severe in GPP than plaque psoriasis.3,4
Differential Diagnosis
There are many other cutaneous pustular diagnoses that must be ruled out when evaluating a patient with GPP (Table 1).26 Acute generalized exanthematous pustulosis (AGEP) is a common mimicker of GPP that is differentiated histologically by the presence of eosinophils and necrotic keratinocytes.4 In addition to its distinct histopathologic findings, AGEP is classically associated with recent initiation of certain medications, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, and diltiazem.27 In contrast, GPP more commonly is related to withdrawal of corticosteroids as well as initiation of some biologic medications, including anti-TNF agents.3 Generalized pustular psoriasis should be suspected over AGEP in patients with a personal or family history of psoriasis, though GPP may arise in patients with or without a history of psoriasis. Acute generalized exanthematous pustulosis usually is more abrupt in both onset and resolution compared with GPP, with clearance of pustules within a few days to weeks following cessation of the triggering factor.4

Other pustular variants of psoriasis (eg, palmoplantar pustular psoriasis, acrodermatitis continua of Hallopeau) are differentiated from GPP by their chronicity and localization to palmoplantar and/or ungual surfaces.5 Other differential diagnoses are listed in Table 1.
Diagnostic Criteria for GPP
Diagnostic criteria have been proposed for GPP (Table 2), including (1) the presence of sterile pustules, (2) systemic signs of inflammation, (3) laboratory abnormalities, (4) histopathologic confirmation of spongiform pustules of Kogoj, and (5) recurrence of symptoms.22 To definitively diagnose GPP, all 5 criteria must be met. To rule out mimickers, it may be worthwhile to perform Gram staining, potassium hydroxide preparation, in vitro cultures, and/or immunofluorescence testing.6
Treatment
Given the high potential for mortality associated with GPP, the most essential component of management is to ensure adequate supportive care. Any temperature, fluid, or electrolyte imbalances should be corrected as they arise. Secondary infections also must be identified and treated, if present, to reduce the risk for fatal complications, including systemic infection and sepsis. Precautions must be taken to ensure that serious end-organ damage, including hepatic, renal, and respiratory dysfunction, is avoided.
Adjunctive topical intervention often is initiated with bland emollients, corticosteroids, calcineurin inhibitors, and/or vitamin D derivatives to help soothe skin symptoms, but treatment with systemic therapies usually is warranted to achieve symptom control.2,25 Importantly, there are no systemic or topical agents that have specifically been approved for the treatment of GPP in Europe or the United States.3 Given the absence of universally accepted treatment guidelines, therapeutic agents for GPP usually are selected based on clinical experience while also taking the extent of involvement and disease severity into consideration.3
Treatment Recommendations for Adults
Oral Systemic Agents—Treatment guidelines set forth by the National Psoriasis Foundation (NPF) in 2012 proposed that first-line therapies for GPP should be acitretin, cyclosporine, methotrexate, and infliximab.28 However, since those guidelines were established, many new biologic therapies have been approved for the treatment of psoriasis and often are considered in the treatment of psoriasis subtypes, including GPP.29 Although retinoids previously were considered to be a preferred first-line therapy, they are associated with a high incidence of adverse effects and must be used with caution in women of childbearing age.6 Oral acitretin at a dosage of 0.75 to 1.0 mg/kg/d has been shown to result in clinical improvement within 1 to 2 weeks, and a maintenance dosage of 0.125 to 0.25 mg/kg/d is required for several months to prevent recurrence.30 Methotrexate—5.0 to 15.0 mg/wk, or perhaps higher in patients with refractory disease, increased by 2.5-mg intervals until symptoms improve—is recommended by the NPF in patients who are unresponsive or cannot tolerate retinoids, though close monitoring for hematologic abnormalities is required. Cyclosporine 2.5 to 5.0 mg/kg/d is considered an alternative to methotrexate and retinoids; it has a faster onset of action, with improvement reported as early as 2 weeks after initiation of therapy.1,28 Although cyclosporine may be effective in the acute phase, especially in severe cases of GPP, long-term use of cyclosporine is not recommended because of the potential for renal dysfunction and hypertension.31
Biologic Agents—More recent evidence has accumulated supporting the efficacy of anti-TNF agents in the treatment of GPP, suggesting the positioning of these agents as first line. A number of case series have shown dramatic and rapid improvement of GPP with intravenous infliximab 3 to 5 mg/kg, with results observed hours to days after the first infusion.32-37 Thus, infliximab is recommended as first-line treatment in severe acute cases, though its efficacy as a maintenance therapy has not been sufficiently investigated.6 Case reports and case series document the safety and efficacy of adalimumab 40 to 80 mg every 1 to 2 weeks38,39 and etanercept 25 to 50 mg twice weekly40-42 in patients with recalcitrantGPP. Therefore, these anti-TNF agents may be considered in patients who are nonresponsive to treatment with infliximab.
Rarely, there have been reports of paradoxical induction of GPP with the use of some anti-TNF agents,43-45 which may be due to a cytokine imbalance characterized by unopposed IFN-α activation.6 In patients with a history of GPP after initiation of a biologic, treatment with agents from within the offending class should be avoided.
The IL-17A monoclonal antibodies secukinumab, ixekizumab, and brodalumab have been shown in open-label phase 3 studies to result in disease remission at 12 weeks.46-48 Treatment with guselkumab, an IL-23 monoclonal antibody, also has demonstrated efficacy in patients with GPP.49 Ustekinumab, an IL-12/23 inhibitor, in combination with acitretin also has been shown to be successful in achieving disease remission after a few weeks of treatment.50
More recent case reports have shown the efficacy of IL-1 inhibitors including gevokizumab, canakinumab, and anakinra in achieving GPP clearance, though more prospective studies are needed to evaluate their efficacy.51-53 Given the etiologic association between IL-1 disinhibition and GPP, future investigations of these therapies as well as those that target the IL-36 pathway may prove to be particularly interesting.
Phototherapy and Combination Therapies—Phototherapy may be considered as maintenance therapy after disease control is achieved, though it is not considered appropriate for acute cases.28 Combination therapies with a biologic plus a nonbiologic systemic agent or alternating among various biologics may allow physicians to maximize benefits and minimize adverse effects in the long term, though there is insufficient evidence to suggest any specific combination treatment algorithm for GPP.28
Treatment Recommendations for Pediatric Patients
Based on a small number of case series and case reports, the first-line treatment strategy for children with GPP is similar to adults. Given the notable adverse events of most oral systemic agents, biologic therapies may emerge as first-line therapy in the pediatric population as more evidence accumulates.28
Treatment Recommendations for Pregnant Patients
Systemic corticosteroids are widely considered to be the first-line treatments for the management of impetigo herpetiformis.7 Low-dose prednisone (15–30 mg/d) usually is effective, but severe cases may require increasing the dosage to 60 mg/d.6 Given the potential for rebound flares upon withdrawal of systemic corticosteroids, these agents must be gradually tapered after the resolution of symptoms.
Certolizumab pegol also is an attractive option in pregnant patients with impetigo herpetiformis because of its favorable safety profile and negligible mother-to-infant transfer through the placenta or breast milk. It has been shown to be effective in treating GPP and impetigo herpetiformis during pregnancy in recently published case reports.54,55 In refractory cases, other TNF-α inhibitors (eg, adalimumab, infliximab, etanercept) or cyclosporine may be considered. However, cautious medical monitoring is warranted, as little is known about the potential adverse effects of these agents to the mother and fetus.28,56 Data from transplant recipients along with several case reports indicate that cyclosporine is not associated with an increased risk for adverse effects during pregnancy at a dose of 2 to 3 mg/kg.57-59 Both methotrexate and retinoids are known teratogens and are therefore contraindicated in pregnant patients.56
If pustules do not resolve in the postpartum period, patients should be treated with standard GPP therapies. However, long-term and population studies are lacking regarding the potential for infant exposure to systemic agents in breast milk. Therefore, the NPF recommends avoiding breastfeeding while taking systemic medications, if possible.56
Limitations of Treatment Recommendations
The ability to generate an evidence-based treatment strategy for GPP is limited by a lack of high-quality studies investigating the efficacy and safety of treatments in patients with GPP due to the rarity and relapsing-remitting nature of the disease, which makes randomized controlled trials difficult to conduct. The quality of the available research is further limited by the lack of validated outcome measures to specifically assess improvements in patients with GPP, such that results are difficult to synthesize and compare among studies.31
Conclusion
Although limited, the available research suggests that treatment with various biologics, especially infliximab, is effective in achieving rapid clearance in patients with GPP. In general, biologics may be the most appropriate treatment option in patients with GPP given their relatively favorable safety profiles. Other oral systemic agents, including acitretin, cyclosporine, and methotrexate, have limited evidence to support their use in the acute phase, but their safety profiles often limit their utility in the long-term. Emerging evidence regarding the association of GPP with IL36RN mutations suggests a unique role for agents targeting the IL-36 or IL-1 pathways, though this has yet to be thoroughly investigated.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131‐144.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614‐618.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37‐42.
- van de Kerkhof PCM, Nestle FO. Psoriasis. In: Bolognia JL, Jorizzo JJ, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012:138-160.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645‐1651.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101‐104.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5-13.
- Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264-272.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432-437.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514-2521.
- Hussain S, Berki DM, Choon SE, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1070.e9.
- Körber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133:2634-2637.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021-1026.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-192
- Wang Y, Cheng R, Lu Z, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235-240.
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and upregulating IL-36 production. J Invest Dermatol. 2016;136:2251-2259.
- Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43-S54.
- Viguier M, Allez M, Zagdanski AM, et al. High frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tract. Hepatology. 2004;40:452-458.
- Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971;85:407-411.
- Kalb RE. Pustular psoriasis: management. In: Ofori AO, Duffin KC, eds. UpToDate. UpToDate; 2014. Accessed July 20, 2022. https://www.uptodate.com/contents/pustular-psoriasis-management/print
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-425.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol 2002;3:389-400.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591‐601.
- Elewski BE. Infliximab for the treatment of severe pustular psoriasis. J Am Acad Dermatol. 2002;47:796-797.
- Kim HS, You HS, Cho HH, et al. Two cases of generalized pustular psoriasis: successful treatment with infliximab. Ann Dermatol. 2014;26:787-788.
- Trent JT, Kerdel FA. Successful treatment of Von Zumbusch pustular psoriasis with infliximab. J Cutan Med Surg. 2004;8:224-228.
- Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329-336.
- Routhouska S, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: case series. J Cutan Med Surg. 2008;12:184-188.
- Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84:247-248.
- Zangrilli A, Papoutsaki M, Talamonti M, et al. Long-term efficacy of adalimumab in generalized pustular psoriasis. J Dermatol Treat. 2008;19:185-187.
- Matsumoto A, Komine M, Karakawa M, et al. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44:202-204.
- Kamarashev J, Lor P, Forster A, et al. Generalized pustular psoriasis induced by cyclosporin in a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213-216.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different dosages in the treatment of generalized pustular psoriasis: a case series. Dermatology. 2008;216:355-360.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233-234.
- Goiriz R, Daudén E, Pérez-Gala S, et al. Flare and change of psoriasis morphology during the course of treatment with tumor necrosis factor blockers. Clin Exp Dermatol. 2006;32:176-179.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Almutairi D, Sheasgreen C, Weizman A, et al. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;1:507-510.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis, and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45:529‐539.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825-828.
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br J Dermatol. 2015;173:239-241.
- Skendros P, Papagoras C, Lefaki I, et al. Successful response in a case of severe pustular psoriasis after interleukin-1 beta inhibition. Br J Dermatol. 2017;176:212-215.
- Viguier M, Guigue P, Pagès C, et al. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66-67.
- Fukushima H, Iwata Y, Arima M, et al. Efficacy and safety of treatment with anti-tumor necrosis factor‐α drugs for severe impetigo herpetiformis. J Dermatol. 2021;48:207-210.
- Mizutani Y, Mizutani YH, Matsuyama K, et al. Generalized pustular psoriasis in pregnancy, successfully treated with certolizumab pegol. J Dermatol. 2021;47:e262-e263.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459‐477.
- Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporin A. Br J Dermatol. 2000;142:582-584.
- Gaughan WJ, Moritz MJ, Radomski JS, et al. National Transplantation Pregnancy Registry: report on outcomes of cyclosporine-treated female kidney transplant recipients with an interval from transplantation to pregnancy of greater than five years. Am J Kidney Dis. 1996;28:266-269.
- Kura MM, Surjushe AU. Generalized pustular psoriasis of pregnancy treated with oral cyclosporin. Indian J Dermatol Venereol Leprol. 2006;72:458-459.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131‐144.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614‐618.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37‐42.
- van de Kerkhof PCM, Nestle FO. Psoriasis. In: Bolognia JL, Jorizzo JJ, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012:138-160.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645‐1651.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101‐104.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5-13.
- Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264-272.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432-437.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514-2521.
- Hussain S, Berki DM, Choon SE, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1070.e9.
- Körber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133:2634-2637.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021-1026.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-192
- Wang Y, Cheng R, Lu Z, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235-240.
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and upregulating IL-36 production. J Invest Dermatol. 2016;136:2251-2259.
- Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43-S54.
- Viguier M, Allez M, Zagdanski AM, et al. High frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tract. Hepatology. 2004;40:452-458.
- Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971;85:407-411.
- Kalb RE. Pustular psoriasis: management. In: Ofori AO, Duffin KC, eds. UpToDate. UpToDate; 2014. Accessed July 20, 2022. https://www.uptodate.com/contents/pustular-psoriasis-management/print
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-425.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol 2002;3:389-400.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591‐601.
- Elewski BE. Infliximab for the treatment of severe pustular psoriasis. J Am Acad Dermatol. 2002;47:796-797.
- Kim HS, You HS, Cho HH, et al. Two cases of generalized pustular psoriasis: successful treatment with infliximab. Ann Dermatol. 2014;26:787-788.
- Trent JT, Kerdel FA. Successful treatment of Von Zumbusch pustular psoriasis with infliximab. J Cutan Med Surg. 2004;8:224-228.
- Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329-336.
- Routhouska S, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: case series. J Cutan Med Surg. 2008;12:184-188.
- Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84:247-248.
- Zangrilli A, Papoutsaki M, Talamonti M, et al. Long-term efficacy of adalimumab in generalized pustular psoriasis. J Dermatol Treat. 2008;19:185-187.
- Matsumoto A, Komine M, Karakawa M, et al. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44:202-204.
- Kamarashev J, Lor P, Forster A, et al. Generalized pustular psoriasis induced by cyclosporin in a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213-216.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different dosages in the treatment of generalized pustular psoriasis: a case series. Dermatology. 2008;216:355-360.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233-234.
- Goiriz R, Daudén E, Pérez-Gala S, et al. Flare and change of psoriasis morphology during the course of treatment with tumor necrosis factor blockers. Clin Exp Dermatol. 2006;32:176-179.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Almutairi D, Sheasgreen C, Weizman A, et al. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;1:507-510.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis, and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45:529‐539.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825-828.
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br J Dermatol. 2015;173:239-241.
- Skendros P, Papagoras C, Lefaki I, et al. Successful response in a case of severe pustular psoriasis after interleukin-1 beta inhibition. Br J Dermatol. 2017;176:212-215.
- Viguier M, Guigue P, Pagès C, et al. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66-67.
- Fukushima H, Iwata Y, Arima M, et al. Efficacy and safety of treatment with anti-tumor necrosis factor‐α drugs for severe impetigo herpetiformis. J Dermatol. 2021;48:207-210.
- Mizutani Y, Mizutani YH, Matsuyama K, et al. Generalized pustular psoriasis in pregnancy, successfully treated with certolizumab pegol. J Dermatol. 2021;47:e262-e263.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459‐477.
- Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporin A. Br J Dermatol. 2000;142:582-584.
- Gaughan WJ, Moritz MJ, Radomski JS, et al. National Transplantation Pregnancy Registry: report on outcomes of cyclosporine-treated female kidney transplant recipients with an interval from transplantation to pregnancy of greater than five years. Am J Kidney Dis. 1996;28:266-269.
- Kura MM, Surjushe AU. Generalized pustular psoriasis of pregnancy treated with oral cyclosporin. Indian J Dermatol Venereol Leprol. 2006;72:458-459.
Practice Points
- Generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis that is characterized by the abrupt widespread onset of small pustules.
- Although no treatments have specifically been approved for GPP, various biologics, especially infliximab, may be effective in achieving rapid clearance in patients with GPP. Other oral systemic agents including acitretin, cyclosporine, and methotrexate also have been shown to be effective.
Racial Disparities in the Diagnosis of Psoriasis
To the Editor:
Psoriasis affects 2% to 3% of the US population and is one of the more commonly diagnosed dermatologic conditions.1-3 Experts agree that common cutaneous diseases such as psoriasis present differently in patients with skin of color (SOC) compared to non-SOC patients.3,4 Despite the prevalence of psoriasis, data on these morphologic differences are limited.3-5 We performed a retrospective chart review comparing characteristics of psoriasis in SOC and non-SOC patients.
Through a search of electronic health records, we identified patients with an International Classification of Diseases, 10th Revision, diagnosis of psoriasis who were 18 years or older and were evaluated in the dermatology department between August 2015 and June 2020 at University Medical Center, an academic institution in New Orleans, Louisiana. Photographs and descriptions of lesions from these patients were reviewed. Patient data collected included age, sex, psoriasis classification, insurance status, self-identified race and ethnicity, location of lesion(s), biopsy, final diagnosis, and average number of visits or days required for accurate diagnosis. Self-identified SOC race and ethnicity categories included Black or African American, Hispanic, Asian, American Indian and Alaskan Native, Native Hawaiian and Other Pacific Islander, and “other.”
All analyses were conducted using R-4.0.1 statistics software. Categorical variables were compared in SOC and non-SOC groups using Fisher exact tests. Continuous covariates were conducted using a Wilcoxon rank sum test.
In total, we reviewed 557 charts. Four patients who declined to identify their race or ethnicity were excluded, yielding 286 SOC and 267 non-SOC patients (N=553). A total of 276 patients (131 SOC; 145 non-SOC) with a prior diagnosis of psoriasis were excluded in the days to diagnosis analysis. Twenty patients (15, SOC; 5, non-SOC) were given a diagnosis of a disease other than psoriasis when evaluated in the dermatology department.
Distributions between racial groups differed for insurance status, sex, psoriasis classification, biopsy status, and days between first dermatology visit and diagnosis. Skin of color patients had significantly longer days between initial presentation to dermatology and final diagnosis vs non-SOC patients (180.11 and 60.27 days, respectively; P=.001). Skin of color patients had a higher rate of palmoplantar psoriasis and severe plaque psoriasis (ie, >10% body surface area involvement) at presentation.
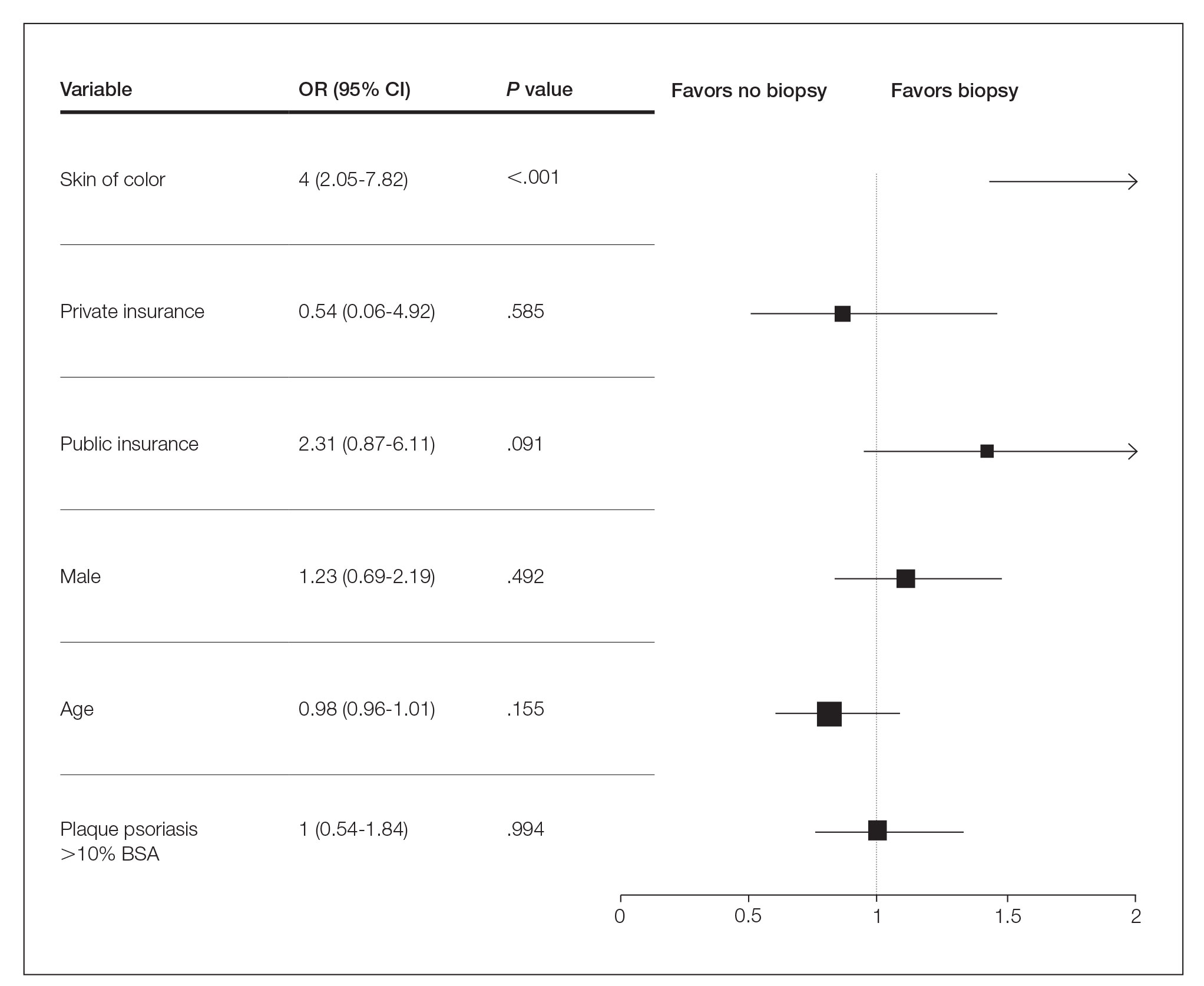
Several multivariable regression analyses were performed. Skin of color patients had significantly higher odds of biopsy compared to non-SOC patients (adjusted odds ratio [95% CI]=4 [2.05-7.82]; P<.001)(Figure 1). There were no significant predictors for severe plaque psoriasis involving more than 10% body surface area. Skin of color patients had a significantly longer time to diagnosis than non-SOC patients (P=.006)(Figure 2). On average, patients with SOC waited 3.23 times longer for a diagnosis than their non-SOC counterparts (95% CI, 1.42-7.36).
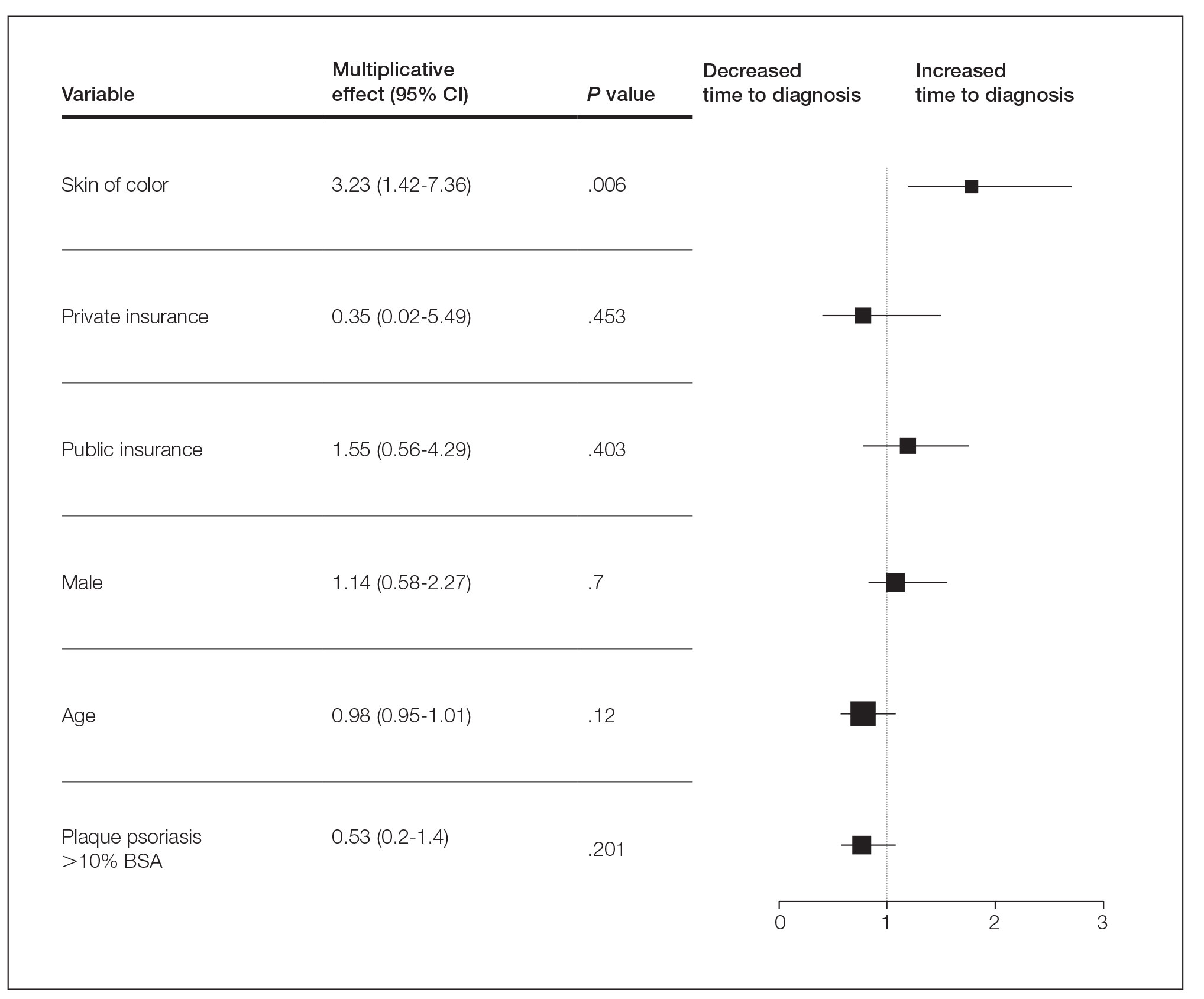
Our data reveal striking racial disparities in psoriasis care. Worse outcomes for patients with SOC compared to non-SOC patients may result from physicians’ inadequate familiarity with diverse presentations of psoriasis, including more frequent involvement of special body sites in SOC. Other likely contributing factors that we did not evaluate include socioeconomic barriers to health care, lack of physician diversity, missed appointments, and a paucity of literature on the topic of differentiating morphologies of psoriasis in SOC and non-SOC patients. Our study did not examine the effects of sex, tobacco use, or prior or current therapy, and it excluded pediatric patients.
To improve dermatologic outcomes for our increasingly diverse patient population, more studies must be undertaken to elucidate and document disparities in care for SOC populations.
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26. doi:10.1016/j.jaad.2004.07.045
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136-139. doi:10.1046/j.1087-0024.2003.09102.x
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423. doi:10.1007/s40257-017-0332-7
To the Editor:
Psoriasis affects 2% to 3% of the US population and is one of the more commonly diagnosed dermatologic conditions.1-3 Experts agree that common cutaneous diseases such as psoriasis present differently in patients with skin of color (SOC) compared to non-SOC patients.3,4 Despite the prevalence of psoriasis, data on these morphologic differences are limited.3-5 We performed a retrospective chart review comparing characteristics of psoriasis in SOC and non-SOC patients.
Through a search of electronic health records, we identified patients with an International Classification of Diseases, 10th Revision, diagnosis of psoriasis who were 18 years or older and were evaluated in the dermatology department between August 2015 and June 2020 at University Medical Center, an academic institution in New Orleans, Louisiana. Photographs and descriptions of lesions from these patients were reviewed. Patient data collected included age, sex, psoriasis classification, insurance status, self-identified race and ethnicity, location of lesion(s), biopsy, final diagnosis, and average number of visits or days required for accurate diagnosis. Self-identified SOC race and ethnicity categories included Black or African American, Hispanic, Asian, American Indian and Alaskan Native, Native Hawaiian and Other Pacific Islander, and “other.”
All analyses were conducted using R-4.0.1 statistics software. Categorical variables were compared in SOC and non-SOC groups using Fisher exact tests. Continuous covariates were conducted using a Wilcoxon rank sum test.
In total, we reviewed 557 charts. Four patients who declined to identify their race or ethnicity were excluded, yielding 286 SOC and 267 non-SOC patients (N=553). A total of 276 patients (131 SOC; 145 non-SOC) with a prior diagnosis of psoriasis were excluded in the days to diagnosis analysis. Twenty patients (15, SOC; 5, non-SOC) were given a diagnosis of a disease other than psoriasis when evaluated in the dermatology department.
Distributions between racial groups differed for insurance status, sex, psoriasis classification, biopsy status, and days between first dermatology visit and diagnosis. Skin of color patients had significantly longer days between initial presentation to dermatology and final diagnosis vs non-SOC patients (180.11 and 60.27 days, respectively; P=.001). Skin of color patients had a higher rate of palmoplantar psoriasis and severe plaque psoriasis (ie, >10% body surface area involvement) at presentation.

Several multivariable regression analyses were performed. Skin of color patients had significantly higher odds of biopsy compared to non-SOC patients (adjusted odds ratio [95% CI]=4 [2.05-7.82]; P<.001)(Figure 1). There were no significant predictors for severe plaque psoriasis involving more than 10% body surface area. Skin of color patients had a significantly longer time to diagnosis than non-SOC patients (P=.006)(Figure 2). On average, patients with SOC waited 3.23 times longer for a diagnosis than their non-SOC counterparts (95% CI, 1.42-7.36).

Our data reveal striking racial disparities in psoriasis care. Worse outcomes for patients with SOC compared to non-SOC patients may result from physicians’ inadequate familiarity with diverse presentations of psoriasis, including more frequent involvement of special body sites in SOC. Other likely contributing factors that we did not evaluate include socioeconomic barriers to health care, lack of physician diversity, missed appointments, and a paucity of literature on the topic of differentiating morphologies of psoriasis in SOC and non-SOC patients. Our study did not examine the effects of sex, tobacco use, or prior or current therapy, and it excluded pediatric patients.
To improve dermatologic outcomes for our increasingly diverse patient population, more studies must be undertaken to elucidate and document disparities in care for SOC populations.
To the Editor:
Psoriasis affects 2% to 3% of the US population and is one of the more commonly diagnosed dermatologic conditions.1-3 Experts agree that common cutaneous diseases such as psoriasis present differently in patients with skin of color (SOC) compared to non-SOC patients.3,4 Despite the prevalence of psoriasis, data on these morphologic differences are limited.3-5 We performed a retrospective chart review comparing characteristics of psoriasis in SOC and non-SOC patients.
Through a search of electronic health records, we identified patients with an International Classification of Diseases, 10th Revision, diagnosis of psoriasis who were 18 years or older and were evaluated in the dermatology department between August 2015 and June 2020 at University Medical Center, an academic institution in New Orleans, Louisiana. Photographs and descriptions of lesions from these patients were reviewed. Patient data collected included age, sex, psoriasis classification, insurance status, self-identified race and ethnicity, location of lesion(s), biopsy, final diagnosis, and average number of visits or days required for accurate diagnosis. Self-identified SOC race and ethnicity categories included Black or African American, Hispanic, Asian, American Indian and Alaskan Native, Native Hawaiian and Other Pacific Islander, and “other.”
All analyses were conducted using R-4.0.1 statistics software. Categorical variables were compared in SOC and non-SOC groups using Fisher exact tests. Continuous covariates were conducted using a Wilcoxon rank sum test.
In total, we reviewed 557 charts. Four patients who declined to identify their race or ethnicity were excluded, yielding 286 SOC and 267 non-SOC patients (N=553). A total of 276 patients (131 SOC; 145 non-SOC) with a prior diagnosis of psoriasis were excluded in the days to diagnosis analysis. Twenty patients (15, SOC; 5, non-SOC) were given a diagnosis of a disease other than psoriasis when evaluated in the dermatology department.
Distributions between racial groups differed for insurance status, sex, psoriasis classification, biopsy status, and days between first dermatology visit and diagnosis. Skin of color patients had significantly longer days between initial presentation to dermatology and final diagnosis vs non-SOC patients (180.11 and 60.27 days, respectively; P=.001). Skin of color patients had a higher rate of palmoplantar psoriasis and severe plaque psoriasis (ie, >10% body surface area involvement) at presentation.

Several multivariable regression analyses were performed. Skin of color patients had significantly higher odds of biopsy compared to non-SOC patients (adjusted odds ratio [95% CI]=4 [2.05-7.82]; P<.001)(Figure 1). There were no significant predictors for severe plaque psoriasis involving more than 10% body surface area. Skin of color patients had a significantly longer time to diagnosis than non-SOC patients (P=.006)(Figure 2). On average, patients with SOC waited 3.23 times longer for a diagnosis than their non-SOC counterparts (95% CI, 1.42-7.36).

Our data reveal striking racial disparities in psoriasis care. Worse outcomes for patients with SOC compared to non-SOC patients may result from physicians’ inadequate familiarity with diverse presentations of psoriasis, including more frequent involvement of special body sites in SOC. Other likely contributing factors that we did not evaluate include socioeconomic barriers to health care, lack of physician diversity, missed appointments, and a paucity of literature on the topic of differentiating morphologies of psoriasis in SOC and non-SOC patients. Our study did not examine the effects of sex, tobacco use, or prior or current therapy, and it excluded pediatric patients.
To improve dermatologic outcomes for our increasingly diverse patient population, more studies must be undertaken to elucidate and document disparities in care for SOC populations.
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26. doi:10.1016/j.jaad.2004.07.045
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136-139. doi:10.1046/j.1087-0024.2003.09102.x
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423. doi:10.1007/s40257-017-0332-7
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26. doi:10.1016/j.jaad.2004.07.045
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136-139. doi:10.1046/j.1087-0024.2003.09102.x
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423. doi:10.1007/s40257-017-0332-7
Practice Points
- Skin of color (SOC) patients can wait 3 times longer to receive a diagnosis of psoriasis than non-SOC patients.
- Patients with SOC more often present with severe forms of psoriasis and are more likely to have palmoplantar psoriasis.
- Skin of color patients can be 4 times as likely to require a biopsy to confirm psoriasis diagnosis compared to non-SOC patients.
Management of Psoriasis With Topicals: Applying the 2020 AAD-NPF Guidelines of Care to Clinical Practice
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
Practice Points
- Topical medications collectively represent the most common form of psoriasis treatment. Depending on disease severity and distribution, topical agents can be used as monotherapy or adjunct therapy, offering the benefit of localized treatment without systemic side effects.
- Dermatologists should base the selection of an appropriate topical medication on factors including adverse effects, potency, vehicle, and anatomic localization of disease.
Guidance From the National Psoriasis Foundation COVID-19 Task Force
When COVID-19 emerged in March 2020, physicians were forced to evaluate the potential impacts of the pandemic on our patients and the conditions that we treat. For dermatologists, psoriasis came into particular focus, as many patients were being treated with biologic therapies. The initial concern was that these biologics might render our patients more susceptible to both COVID-19 infection and/or a more severe disease course.
In early 2020, the National Psoriasis Foundation (NPF) presented its own recommendations for treating patients with psoriatic disease during the pandemic.1 Some highlights included the following1:
• At the time, it was stipulated that patients with COVID-19 infection should stop taking a biologic.
• Psoriasis patients in high-risk groups (eg, concomitant systemic disease) should discuss with their dermatologist if their therapeutic regimen should be continued or altered.
• Patients taking oral immunosuppressive therapy may be at greater risk for COVID-19 infection, though there is no strong COVID-19–related evidence to provide specific guidelines or risk level.
In May 2020, the NPF COVID-19 Task Force was formed. This group—chaired by dermatologist Joel M. Gelfand, MD, MSCE (Philadelphia, Pennsylvania), and rheumatologist Christopher T. Ritchlin, MD, MPH (Rochester, New York)—was comprised of members from both the NPF Medical Board and Scientific Advisory Committee in dermatology, rheumatology, infectious disease, and critical care. The NPF COVID-19 Task Force has been critical in keeping the dermatology community apprised of the latest scientific thinking related to COVID-19 and publishing guidance statements that are updated and amended on a regular basis as new data becomes available.2 Key recommendations most relevant to the daily care of patients with psoriatic disease included the following2:
• Patients with psoriasis and/or psoriatic arthritis have similar rates of SARS-CoV-2 infection and COVID-19 outcomes as the general population based on existing data, with some exceptions.
• Therapies for psoriasis and/or psoriatic arthritis do not meaningfully alter the risk for acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.
• Patients should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases, unless they become infected with SARS-CoV-2.
• Chronic systemic steroid use for psoriatic disease in the setting of acute infection with COVID-19 may be associated with worse outcomes; however, steroids may improve outcomes for COVID-19 when initiated in hospitalized patients who require oxygen therapy.
• When local restrictions or pandemic conditions limit the ability for in-person visits, offer telemedicine to manage patients.
• Patients with psoriatic disease who do not have contraindications to vaccination should receive a messenger RNA (mRNA)–based COVID-19 vaccine and boosters, based on federal, state, and local guidance. Systemic medications for psoriasis or psoriatic arthritis are not a contraindication to the mRNA-based COVID-19 vaccine.
• Patients who are to receive an mRNA-based COVID-19 vaccine should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases.
• The use of hydroxychloroquine, chloroquine, and ivermectin is not suggested for the prevention or treatment of COVID-19 disease.
These guidelines have been critical in addressing some of the most pressing issues in psoriasis patient care, particularly the susceptibility to COVID-19, the role of psoriasis therapies in initial infection and health outcomes, and issues related to the administration of vaccines in those on systemic therapies. Based on these recommendations, we have been given a solid foundation that our current standard of care can (for the most part) continue with the continued presence of COVID-19 in our society. I encourage all providers to familiarize themselves with the NPF COVID-19 Task Force guidelines and keep abreast of updates as they become available (https://www.psoriasis.org/covid-19-task-force-guidance-statements/).
- Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: version 1. J Am Acad Dermatol. 2020;83:1704-1716.
- COVID-19 Task Force guidance statements. National Psoriasis Foundation website. Updated April 28, 2022. Accessed July 12, 2022. https://www.psoriasis.org/covid-19-task-force-guidance-statements/
When COVID-19 emerged in March 2020, physicians were forced to evaluate the potential impacts of the pandemic on our patients and the conditions that we treat. For dermatologists, psoriasis came into particular focus, as many patients were being treated with biologic therapies. The initial concern was that these biologics might render our patients more susceptible to both COVID-19 infection and/or a more severe disease course.
In early 2020, the National Psoriasis Foundation (NPF) presented its own recommendations for treating patients with psoriatic disease during the pandemic.1 Some highlights included the following1:
• At the time, it was stipulated that patients with COVID-19 infection should stop taking a biologic.
• Psoriasis patients in high-risk groups (eg, concomitant systemic disease) should discuss with their dermatologist if their therapeutic regimen should be continued or altered.
• Patients taking oral immunosuppressive therapy may be at greater risk for COVID-19 infection, though there is no strong COVID-19–related evidence to provide specific guidelines or risk level.
In May 2020, the NPF COVID-19 Task Force was formed. This group—chaired by dermatologist Joel M. Gelfand, MD, MSCE (Philadelphia, Pennsylvania), and rheumatologist Christopher T. Ritchlin, MD, MPH (Rochester, New York)—was comprised of members from both the NPF Medical Board and Scientific Advisory Committee in dermatology, rheumatology, infectious disease, and critical care. The NPF COVID-19 Task Force has been critical in keeping the dermatology community apprised of the latest scientific thinking related to COVID-19 and publishing guidance statements that are updated and amended on a regular basis as new data becomes available.2 Key recommendations most relevant to the daily care of patients with psoriatic disease included the following2:
• Patients with psoriasis and/or psoriatic arthritis have similar rates of SARS-CoV-2 infection and COVID-19 outcomes as the general population based on existing data, with some exceptions.
• Therapies for psoriasis and/or psoriatic arthritis do not meaningfully alter the risk for acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.
• Patients should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases, unless they become infected with SARS-CoV-2.
• Chronic systemic steroid use for psoriatic disease in the setting of acute infection with COVID-19 may be associated with worse outcomes; however, steroids may improve outcomes for COVID-19 when initiated in hospitalized patients who require oxygen therapy.
• When local restrictions or pandemic conditions limit the ability for in-person visits, offer telemedicine to manage patients.
• Patients with psoriatic disease who do not have contraindications to vaccination should receive a messenger RNA (mRNA)–based COVID-19 vaccine and boosters, based on federal, state, and local guidance. Systemic medications for psoriasis or psoriatic arthritis are not a contraindication to the mRNA-based COVID-19 vaccine.
• Patients who are to receive an mRNA-based COVID-19 vaccine should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases.
• The use of hydroxychloroquine, chloroquine, and ivermectin is not suggested for the prevention or treatment of COVID-19 disease.
These guidelines have been critical in addressing some of the most pressing issues in psoriasis patient care, particularly the susceptibility to COVID-19, the role of psoriasis therapies in initial infection and health outcomes, and issues related to the administration of vaccines in those on systemic therapies. Based on these recommendations, we have been given a solid foundation that our current standard of care can (for the most part) continue with the continued presence of COVID-19 in our society. I encourage all providers to familiarize themselves with the NPF COVID-19 Task Force guidelines and keep abreast of updates as they become available (https://www.psoriasis.org/covid-19-task-force-guidance-statements/).
When COVID-19 emerged in March 2020, physicians were forced to evaluate the potential impacts of the pandemic on our patients and the conditions that we treat. For dermatologists, psoriasis came into particular focus, as many patients were being treated with biologic therapies. The initial concern was that these biologics might render our patients more susceptible to both COVID-19 infection and/or a more severe disease course.
In early 2020, the National Psoriasis Foundation (NPF) presented its own recommendations for treating patients with psoriatic disease during the pandemic.1 Some highlights included the following1:
• At the time, it was stipulated that patients with COVID-19 infection should stop taking a biologic.
• Psoriasis patients in high-risk groups (eg, concomitant systemic disease) should discuss with their dermatologist if their therapeutic regimen should be continued or altered.
• Patients taking oral immunosuppressive therapy may be at greater risk for COVID-19 infection, though there is no strong COVID-19–related evidence to provide specific guidelines or risk level.
In May 2020, the NPF COVID-19 Task Force was formed. This group—chaired by dermatologist Joel M. Gelfand, MD, MSCE (Philadelphia, Pennsylvania), and rheumatologist Christopher T. Ritchlin, MD, MPH (Rochester, New York)—was comprised of members from both the NPF Medical Board and Scientific Advisory Committee in dermatology, rheumatology, infectious disease, and critical care. The NPF COVID-19 Task Force has been critical in keeping the dermatology community apprised of the latest scientific thinking related to COVID-19 and publishing guidance statements that are updated and amended on a regular basis as new data becomes available.2 Key recommendations most relevant to the daily care of patients with psoriatic disease included the following2:
• Patients with psoriasis and/or psoriatic arthritis have similar rates of SARS-CoV-2 infection and COVID-19 outcomes as the general population based on existing data, with some exceptions.
• Therapies for psoriasis and/or psoriatic arthritis do not meaningfully alter the risk for acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.
• Patients should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases, unless they become infected with SARS-CoV-2.
• Chronic systemic steroid use for psoriatic disease in the setting of acute infection with COVID-19 may be associated with worse outcomes; however, steroids may improve outcomes for COVID-19 when initiated in hospitalized patients who require oxygen therapy.
• When local restrictions or pandemic conditions limit the ability for in-person visits, offer telemedicine to manage patients.
• Patients with psoriatic disease who do not have contraindications to vaccination should receive a messenger RNA (mRNA)–based COVID-19 vaccine and boosters, based on federal, state, and local guidance. Systemic medications for psoriasis or psoriatic arthritis are not a contraindication to the mRNA-based COVID-19 vaccine.
• Patients who are to receive an mRNA-based COVID-19 vaccine should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases.
• The use of hydroxychloroquine, chloroquine, and ivermectin is not suggested for the prevention or treatment of COVID-19 disease.
These guidelines have been critical in addressing some of the most pressing issues in psoriasis patient care, particularly the susceptibility to COVID-19, the role of psoriasis therapies in initial infection and health outcomes, and issues related to the administration of vaccines in those on systemic therapies. Based on these recommendations, we have been given a solid foundation that our current standard of care can (for the most part) continue with the continued presence of COVID-19 in our society. I encourage all providers to familiarize themselves with the NPF COVID-19 Task Force guidelines and keep abreast of updates as they become available (https://www.psoriasis.org/covid-19-task-force-guidance-statements/).
- Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: version 1. J Am Acad Dermatol. 2020;83:1704-1716.
- COVID-19 Task Force guidance statements. National Psoriasis Foundation website. Updated April 28, 2022. Accessed July 12, 2022. https://www.psoriasis.org/covid-19-task-force-guidance-statements/
- Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: version 1. J Am Acad Dermatol. 2020;83:1704-1716.
- COVID-19 Task Force guidance statements. National Psoriasis Foundation website. Updated April 28, 2022. Accessed July 12, 2022. https://www.psoriasis.org/covid-19-task-force-guidance-statements/