User login
Psoriasis, psoriatic arthritis insurance coverage remains restrictive
Insurance coverage for specialty drugs to treat psoriasis and psoriatic arthritis varies extensively among insurance companies and often restricts coverage beyond the drug labels, according to a review of data from commercial health plans in the United States.
Although specialty medications have demonstrated effectiveness for psoriasis and psoriatic arthritis, data on insurance coverage for these indications are limited and costs are often a barrier to treatment, Christine Learned, of Tufts Medical Center, Boston, and colleagues wrote.
In a study published in the Journal of Psoriasis and Psoriatic Arthritis, the researchers used the Tufts Medical Center Specialty Drug Evidence and Coverage database, which includes information on 158 specialty drugs covered by 17 U.S. commercial health plans, to review data on a total of 11 medications indicated for psoriasis (etanercept, adalimumab, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, guselkumab, tildrakizumab, risankizumab, and apremilast) and 11 indicated for psoriatic arthritis (etanercept, adalimumab, certolizumab pegol, golimumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tofacitinib, apremilast, and abatacept) at the time of the study.
Overall, an average of 78.6% and 66.8% of insurance plans were more restrictive than the Food and Drug Association label in coverage of specialty medications for psoriasis and psoriatic arthritis, respectively.
Disease severity affected insurance coverage for psoriasis. The percentage of plans with a body surface area requirement for specialty medications ranged from 11% for apremilast to 39% for tildrakizumab, adalimumab, and certolizumab pegol. The percentage of plans with exceptions for special body locations affected by psoriasis ranged from 6% for risankizumab and brodalumab to 39% for certolizumab pegol. In addition, 6% of plans had Psoriasis Area and Severity Index requirements for etanercept and ixekizumab, and 11% had PASI requirements for adalimumab, certolizumab pegol, and tildrakizumab.
The percentage of plans with prescriber restrictions for both psoriasis and psoriatic arthritis ranged from 33% to 50%.
All 11 medications for psoriatic arthritis were approved as first-line treatments by at least one plan, compared with 3 the 11 medications with indications for psoriasis. However, medications for both psoriasis and psoriatic arthritis were approved mainly as second-line therapies.
Study designs may impact insurance coverage, as randomized, controlled trials are often used as the basis for coverage decisions for psoriasis, while coverage for psoriatic arthritis is more often based on clinical guidelines, the researchers explained.
“Our analysis confirms that variability exists for the indications of psoriasis and psoriatic arthritis,” they wrote.
The comorbidities associated with psoriasis are not always considered in insurance coverage, and coverage complications may contribute to the persistent undertreatment of many patients with psoriasis, the researchers added.
“Insurance restrictions may blunt provider and patient autonomy in selection of specialty medications and have the potential to diminish a provider’s ability to tailor regimens so as to optimize outcomes while minimizing risks,” they emphasized.
The study findings were limited by the inclusion only of publicly available policy information; therefore, some plans’ restrictions may have been missed in the analysis, the researchers said.
The results suggest that patients should review their insurance coverage of specialty drugs when choosing a health plan, and clinicians should factor in a patient’s plan a likely drug access when considering treatment options, they concluded.
The study received no outside funding. Ms. Learned had no relevant financial conflicts to disclose, but two coauthors reported financial relationships with pharmaceutical companies that manufacturer drugs for psoriasis and psoriatic arthritis.
Insurance coverage for specialty drugs to treat psoriasis and psoriatic arthritis varies extensively among insurance companies and often restricts coverage beyond the drug labels, according to a review of data from commercial health plans in the United States.
Although specialty medications have demonstrated effectiveness for psoriasis and psoriatic arthritis, data on insurance coverage for these indications are limited and costs are often a barrier to treatment, Christine Learned, of Tufts Medical Center, Boston, and colleagues wrote.
In a study published in the Journal of Psoriasis and Psoriatic Arthritis, the researchers used the Tufts Medical Center Specialty Drug Evidence and Coverage database, which includes information on 158 specialty drugs covered by 17 U.S. commercial health plans, to review data on a total of 11 medications indicated for psoriasis (etanercept, adalimumab, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, guselkumab, tildrakizumab, risankizumab, and apremilast) and 11 indicated for psoriatic arthritis (etanercept, adalimumab, certolizumab pegol, golimumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tofacitinib, apremilast, and abatacept) at the time of the study.
Overall, an average of 78.6% and 66.8% of insurance plans were more restrictive than the Food and Drug Association label in coverage of specialty medications for psoriasis and psoriatic arthritis, respectively.
Disease severity affected insurance coverage for psoriasis. The percentage of plans with a body surface area requirement for specialty medications ranged from 11% for apremilast to 39% for tildrakizumab, adalimumab, and certolizumab pegol. The percentage of plans with exceptions for special body locations affected by psoriasis ranged from 6% for risankizumab and brodalumab to 39% for certolizumab pegol. In addition, 6% of plans had Psoriasis Area and Severity Index requirements for etanercept and ixekizumab, and 11% had PASI requirements for adalimumab, certolizumab pegol, and tildrakizumab.
The percentage of plans with prescriber restrictions for both psoriasis and psoriatic arthritis ranged from 33% to 50%.
All 11 medications for psoriatic arthritis were approved as first-line treatments by at least one plan, compared with 3 the 11 medications with indications for psoriasis. However, medications for both psoriasis and psoriatic arthritis were approved mainly as second-line therapies.
Study designs may impact insurance coverage, as randomized, controlled trials are often used as the basis for coverage decisions for psoriasis, while coverage for psoriatic arthritis is more often based on clinical guidelines, the researchers explained.
“Our analysis confirms that variability exists for the indications of psoriasis and psoriatic arthritis,” they wrote.
The comorbidities associated with psoriasis are not always considered in insurance coverage, and coverage complications may contribute to the persistent undertreatment of many patients with psoriasis, the researchers added.
“Insurance restrictions may blunt provider and patient autonomy in selection of specialty medications and have the potential to diminish a provider’s ability to tailor regimens so as to optimize outcomes while minimizing risks,” they emphasized.
The study findings were limited by the inclusion only of publicly available policy information; therefore, some plans’ restrictions may have been missed in the analysis, the researchers said.
The results suggest that patients should review their insurance coverage of specialty drugs when choosing a health plan, and clinicians should factor in a patient’s plan a likely drug access when considering treatment options, they concluded.
The study received no outside funding. Ms. Learned had no relevant financial conflicts to disclose, but two coauthors reported financial relationships with pharmaceutical companies that manufacturer drugs for psoriasis and psoriatic arthritis.
Insurance coverage for specialty drugs to treat psoriasis and psoriatic arthritis varies extensively among insurance companies and often restricts coverage beyond the drug labels, according to a review of data from commercial health plans in the United States.
Although specialty medications have demonstrated effectiveness for psoriasis and psoriatic arthritis, data on insurance coverage for these indications are limited and costs are often a barrier to treatment, Christine Learned, of Tufts Medical Center, Boston, and colleagues wrote.
In a study published in the Journal of Psoriasis and Psoriatic Arthritis, the researchers used the Tufts Medical Center Specialty Drug Evidence and Coverage database, which includes information on 158 specialty drugs covered by 17 U.S. commercial health plans, to review data on a total of 11 medications indicated for psoriasis (etanercept, adalimumab, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, guselkumab, tildrakizumab, risankizumab, and apremilast) and 11 indicated for psoriatic arthritis (etanercept, adalimumab, certolizumab pegol, golimumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tofacitinib, apremilast, and abatacept) at the time of the study.
Overall, an average of 78.6% and 66.8% of insurance plans were more restrictive than the Food and Drug Association label in coverage of specialty medications for psoriasis and psoriatic arthritis, respectively.
Disease severity affected insurance coverage for psoriasis. The percentage of plans with a body surface area requirement for specialty medications ranged from 11% for apremilast to 39% for tildrakizumab, adalimumab, and certolizumab pegol. The percentage of plans with exceptions for special body locations affected by psoriasis ranged from 6% for risankizumab and brodalumab to 39% for certolizumab pegol. In addition, 6% of plans had Psoriasis Area and Severity Index requirements for etanercept and ixekizumab, and 11% had PASI requirements for adalimumab, certolizumab pegol, and tildrakizumab.
The percentage of plans with prescriber restrictions for both psoriasis and psoriatic arthritis ranged from 33% to 50%.
All 11 medications for psoriatic arthritis were approved as first-line treatments by at least one plan, compared with 3 the 11 medications with indications for psoriasis. However, medications for both psoriasis and psoriatic arthritis were approved mainly as second-line therapies.
Study designs may impact insurance coverage, as randomized, controlled trials are often used as the basis for coverage decisions for psoriasis, while coverage for psoriatic arthritis is more often based on clinical guidelines, the researchers explained.
“Our analysis confirms that variability exists for the indications of psoriasis and psoriatic arthritis,” they wrote.
The comorbidities associated with psoriasis are not always considered in insurance coverage, and coverage complications may contribute to the persistent undertreatment of many patients with psoriasis, the researchers added.
“Insurance restrictions may blunt provider and patient autonomy in selection of specialty medications and have the potential to diminish a provider’s ability to tailor regimens so as to optimize outcomes while minimizing risks,” they emphasized.
The study findings were limited by the inclusion only of publicly available policy information; therefore, some plans’ restrictions may have been missed in the analysis, the researchers said.
The results suggest that patients should review their insurance coverage of specialty drugs when choosing a health plan, and clinicians should factor in a patient’s plan a likely drug access when considering treatment options, they concluded.
The study received no outside funding. Ms. Learned had no relevant financial conflicts to disclose, but two coauthors reported financial relationships with pharmaceutical companies that manufacturer drugs for psoriasis and psoriatic arthritis.
FROM THE JOURNAL OF PSORIASIS AND PSORIATIC ARTHRITIS
Apremilast alleviates severe psoriasis in some children, data show
not controlled by topical therapy, according to the results of a phase 3 trial.
“Unfortunately, there are limited treatment options for pediatric patients with moderate to severe plaque psoriasis” who do not respond to or cannot use topical therapy, said study investigator Anna Belloni Fortina, MD, speaking at the annual meeting of the European Academy of Dermatology and Venereology.
“In this randomized, placebo-controlled trial, oral apremilast demonstrated effectiveness and was well tolerated,” added Dr. Belloni Fortina, of Azienda Ospedale Università Padova (Italy). “I underline oral because for children, oral administration is better than the injection treatment.”
Key findings
Dubbed the SPROUT study, the trial set a primary endpoint of the percentage of children with a Physician’s Global Assessment (sPGA) response after 16 weeks of treatment or placebo. The sPGA is a 5-point scale ranging from 0 (clear) to 4 (severe). The study enrolled children with an sPGA greater than or equal to 3. Response was defined as a sPGA score of 0 or 1, indicating clear or almost clear skin, with at least a 2-point reduction from baseline values.
At week 16, the primary endpoint was met by 33% of 163 children treated with apremilast versus 11% of 82 children who had been given a placebo, a treatment difference of 21.7% (95% confidence interval, 11.2%-32.1%).
A greater proportion of children treated with apremilast also achieved a major secondary endpoint, a 75% or greater reduction in the Psoriasis Area and Severity Index (PASI-75) (45.4% vs. 16.1%), a treatment difference of 29.4% (95% CI, 17.8%-40.9%).
Results unaffected by weight and age
Regarding apremilast, “it’s important to underline that patients were dosed according to their weight,” Dr. Belloni Fortina said.
A dose of 20 mg twice daily was given to children who weighed between 20 kg and less than 50 kg, and a 30-mg twice-daily dose was given to those who weighed greater than or equal to 50 kg.
When the data were analyzed according to weight, proportionately more children on apremilast saw a sPGA response: 47.4% versus 21.8% in the lower weight and dose range and 19.2% versus 1.6% in the higher weight and dose range.
As for PASI-75, a greater proportion of children on apremilast also responded in both the lower and upper weight ranges, a respective 52.4% and 38.7% of patients, compared with 21.4% and 11% of those treated with placebo.
Data were also evaluated according to age, with a younger (aged 6-11 years) and older (age 12-17 years) group. The mean age of children was 12 years overall. Results showed a similar pattern for weight: The psoriasis of more children treated with apremilast was reduced by both measures, sPGA response, and PASI-75.
Safety of apremilast in children
“The overall safety profile during the placebo-controlled phase was comparable with the known safety profile of apremilast,” Dr. Belloni Fontina reported. “No new safety signals were identified.”
The rate of any adverse event was substantially higher in children given the active treatment, however, at 65% versus 41.3% for placebo.
Rates of severe and serious adverse events were low, at around 1.3%, and similar between the groups.
There was also a low rate of withdrawal because of side effects, although this was higher in the apremilast group (3.1% vs. 1.3%).
The primary reason for withdrawal of apremilast treatment were the most commonly reported adverse events: gastrointestinal disorders, including diarrhea, nausea, upper and lower abdominal pain, and vomiting. Headache, pyrexia, and nasopharyngitis were also reported.
Despite being common, most treatment-related adverse effects resolved within 3 days, Dr. Belloni Fontina said.
Expect further data
Further data from the trial are to be expected, because only the 16-week primary endpoint results have been released so far. The trial also included a 36-week extension phase, during which all children who had originally been randomly assigned to placebo were now eligible to be treated with apremilast, and all those who were originally given the active treatment were able to continue. This extension treatment period means that data will be available for a full year of treatment, and there will also be a further 2-week observational follow-up at the end of the trial.
The study was funded by Amgen. Dr. Belloni Fontina reported acting as an investigator and advisory board member for and receiving honoraria from Amgen, Galderma, Leo Pharma, and Pfizer. She also reported speaking on behalf of Pierre-Fabre and Galderma.
A version of this article first appeared on Medscape.com.
not controlled by topical therapy, according to the results of a phase 3 trial.
“Unfortunately, there are limited treatment options for pediatric patients with moderate to severe plaque psoriasis” who do not respond to or cannot use topical therapy, said study investigator Anna Belloni Fortina, MD, speaking at the annual meeting of the European Academy of Dermatology and Venereology.
“In this randomized, placebo-controlled trial, oral apremilast demonstrated effectiveness and was well tolerated,” added Dr. Belloni Fortina, of Azienda Ospedale Università Padova (Italy). “I underline oral because for children, oral administration is better than the injection treatment.”
Key findings
Dubbed the SPROUT study, the trial set a primary endpoint of the percentage of children with a Physician’s Global Assessment (sPGA) response after 16 weeks of treatment or placebo. The sPGA is a 5-point scale ranging from 0 (clear) to 4 (severe). The study enrolled children with an sPGA greater than or equal to 3. Response was defined as a sPGA score of 0 or 1, indicating clear or almost clear skin, with at least a 2-point reduction from baseline values.
At week 16, the primary endpoint was met by 33% of 163 children treated with apremilast versus 11% of 82 children who had been given a placebo, a treatment difference of 21.7% (95% confidence interval, 11.2%-32.1%).
A greater proportion of children treated with apremilast also achieved a major secondary endpoint, a 75% or greater reduction in the Psoriasis Area and Severity Index (PASI-75) (45.4% vs. 16.1%), a treatment difference of 29.4% (95% CI, 17.8%-40.9%).
Results unaffected by weight and age
Regarding apremilast, “it’s important to underline that patients were dosed according to their weight,” Dr. Belloni Fortina said.
A dose of 20 mg twice daily was given to children who weighed between 20 kg and less than 50 kg, and a 30-mg twice-daily dose was given to those who weighed greater than or equal to 50 kg.
When the data were analyzed according to weight, proportionately more children on apremilast saw a sPGA response: 47.4% versus 21.8% in the lower weight and dose range and 19.2% versus 1.6% in the higher weight and dose range.
As for PASI-75, a greater proportion of children on apremilast also responded in both the lower and upper weight ranges, a respective 52.4% and 38.7% of patients, compared with 21.4% and 11% of those treated with placebo.
Data were also evaluated according to age, with a younger (aged 6-11 years) and older (age 12-17 years) group. The mean age of children was 12 years overall. Results showed a similar pattern for weight: The psoriasis of more children treated with apremilast was reduced by both measures, sPGA response, and PASI-75.
Safety of apremilast in children
“The overall safety profile during the placebo-controlled phase was comparable with the known safety profile of apremilast,” Dr. Belloni Fontina reported. “No new safety signals were identified.”
The rate of any adverse event was substantially higher in children given the active treatment, however, at 65% versus 41.3% for placebo.
Rates of severe and serious adverse events were low, at around 1.3%, and similar between the groups.
There was also a low rate of withdrawal because of side effects, although this was higher in the apremilast group (3.1% vs. 1.3%).
The primary reason for withdrawal of apremilast treatment were the most commonly reported adverse events: gastrointestinal disorders, including diarrhea, nausea, upper and lower abdominal pain, and vomiting. Headache, pyrexia, and nasopharyngitis were also reported.
Despite being common, most treatment-related adverse effects resolved within 3 days, Dr. Belloni Fontina said.
Expect further data
Further data from the trial are to be expected, because only the 16-week primary endpoint results have been released so far. The trial also included a 36-week extension phase, during which all children who had originally been randomly assigned to placebo were now eligible to be treated with apremilast, and all those who were originally given the active treatment were able to continue. This extension treatment period means that data will be available for a full year of treatment, and there will also be a further 2-week observational follow-up at the end of the trial.
The study was funded by Amgen. Dr. Belloni Fontina reported acting as an investigator and advisory board member for and receiving honoraria from Amgen, Galderma, Leo Pharma, and Pfizer. She also reported speaking on behalf of Pierre-Fabre and Galderma.
A version of this article first appeared on Medscape.com.
not controlled by topical therapy, according to the results of a phase 3 trial.
“Unfortunately, there are limited treatment options for pediatric patients with moderate to severe plaque psoriasis” who do not respond to or cannot use topical therapy, said study investigator Anna Belloni Fortina, MD, speaking at the annual meeting of the European Academy of Dermatology and Venereology.
“In this randomized, placebo-controlled trial, oral apremilast demonstrated effectiveness and was well tolerated,” added Dr. Belloni Fortina, of Azienda Ospedale Università Padova (Italy). “I underline oral because for children, oral administration is better than the injection treatment.”
Key findings
Dubbed the SPROUT study, the trial set a primary endpoint of the percentage of children with a Physician’s Global Assessment (sPGA) response after 16 weeks of treatment or placebo. The sPGA is a 5-point scale ranging from 0 (clear) to 4 (severe). The study enrolled children with an sPGA greater than or equal to 3. Response was defined as a sPGA score of 0 or 1, indicating clear or almost clear skin, with at least a 2-point reduction from baseline values.
At week 16, the primary endpoint was met by 33% of 163 children treated with apremilast versus 11% of 82 children who had been given a placebo, a treatment difference of 21.7% (95% confidence interval, 11.2%-32.1%).
A greater proportion of children treated with apremilast also achieved a major secondary endpoint, a 75% or greater reduction in the Psoriasis Area and Severity Index (PASI-75) (45.4% vs. 16.1%), a treatment difference of 29.4% (95% CI, 17.8%-40.9%).
Results unaffected by weight and age
Regarding apremilast, “it’s important to underline that patients were dosed according to their weight,” Dr. Belloni Fortina said.
A dose of 20 mg twice daily was given to children who weighed between 20 kg and less than 50 kg, and a 30-mg twice-daily dose was given to those who weighed greater than or equal to 50 kg.
When the data were analyzed according to weight, proportionately more children on apremilast saw a sPGA response: 47.4% versus 21.8% in the lower weight and dose range and 19.2% versus 1.6% in the higher weight and dose range.
As for PASI-75, a greater proportion of children on apremilast also responded in both the lower and upper weight ranges, a respective 52.4% and 38.7% of patients, compared with 21.4% and 11% of those treated with placebo.
Data were also evaluated according to age, with a younger (aged 6-11 years) and older (age 12-17 years) group. The mean age of children was 12 years overall. Results showed a similar pattern for weight: The psoriasis of more children treated with apremilast was reduced by both measures, sPGA response, and PASI-75.
Safety of apremilast in children
“The overall safety profile during the placebo-controlled phase was comparable with the known safety profile of apremilast,” Dr. Belloni Fontina reported. “No new safety signals were identified.”
The rate of any adverse event was substantially higher in children given the active treatment, however, at 65% versus 41.3% for placebo.
Rates of severe and serious adverse events were low, at around 1.3%, and similar between the groups.
There was also a low rate of withdrawal because of side effects, although this was higher in the apremilast group (3.1% vs. 1.3%).
The primary reason for withdrawal of apremilast treatment were the most commonly reported adverse events: gastrointestinal disorders, including diarrhea, nausea, upper and lower abdominal pain, and vomiting. Headache, pyrexia, and nasopharyngitis were also reported.
Despite being common, most treatment-related adverse effects resolved within 3 days, Dr. Belloni Fontina said.
Expect further data
Further data from the trial are to be expected, because only the 16-week primary endpoint results have been released so far. The trial also included a 36-week extension phase, during which all children who had originally been randomly assigned to placebo were now eligible to be treated with apremilast, and all those who were originally given the active treatment were able to continue. This extension treatment period means that data will be available for a full year of treatment, and there will also be a further 2-week observational follow-up at the end of the trial.
The study was funded by Amgen. Dr. Belloni Fontina reported acting as an investigator and advisory board member for and receiving honoraria from Amgen, Galderma, Leo Pharma, and Pfizer. She also reported speaking on behalf of Pierre-Fabre and Galderma.
A version of this article first appeared on Medscape.com.
FROM EADV 2022
Open Clinical Trials for Psoriasis
The psoriasis clinical trials listed below are all phase 3 and recruiting participants as of July 19, 2022. For additional information on the study design, eligibility criteria, and contacts/locations, visit ClinicalTrials.gov.
GENERALIZED PUSTULAR PSORIASIS
Long-Term Safety and Efficacy of Imsidolimab (ANB019) in Subjects With Generalized Pustular Psoriasis (GEMINI2)
ClinicalTrials.gov Identifier: NCT05366855
An Expanded Access Trial in Japan to Provide Spesolimab to People With a Flare-up in Generalized Pustular Psoriasis Who Have no Other Treatment Options
ClinicalTrials.gov Identifier: NCT05200247
An Expanded Access Program in China to Provide Spesolimab to People With a Flare-up in Generalized Pustular Psoriasis Who Have No Other Treatment Options
ClinicalTrials.gov Identifier: NCT05239039
Study to Evaluate the Efficacy and Safety of Imsidolimab (ANB019) in the Treatment of Subjects With GPP (GEMINI1)
ClinicalTrials.gov Identifier: NCT05352893
NAIL PSORIASIS
Efficacy and Safety Study of Tildrakizumab in the Treatment of Nail Psoriasis
ClinicalTrials.gov Identifier: NCT03897075
PALMOPLANTAR PUSTULOSIS
Phase 3, Randomized Study of Apremilast in Japanese Participants With Palmoplantar Pustulosis (PPP)
ClinicalTrials.gov Identifier: NCT05174065
PLAQUE PSORIASIS
A Long-term Extension Study of Apremilast (CC-10004) in Pediatric Subjects From 6 Through 17 Years of Age With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04175613
A Phase III Efficacy and Safety Study of Hemay005 in Subjects With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04839328
A Study of Subcutaneous Risankizumab Injection for Pediatric Participants With Moderate to Severe Plaque Psoriasis to Assess Change in Disease Symptoms
ClinicalTrials.gov Identifier: NCT04435600
A Study to Evaluate the Drug Levels, Efficacy and Safety of Deucravacitinib in Adolescent Participants With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04772079
Investigator Initiated Trial to Study Duobrii® Lotion in the Treatment of Mild Plaque Psoriasis in Adults
ClinicalTrials.gov Identifier: NCT05203315
Comparative Study of BAT2206 With Stelara® in Patients With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04728360
A Study to Evaluate the Efficacy and Safety of Bimekizumab in Adult Korean Study Participants With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT05020249
Comparing Efficacy and Safety of Bmab 1200 and Stelara in Patients With Moderate to Severe Chronic Plaque Psoriasis (STELLAR-2)
ClinicalTrials.gov Identifier: NCT05335356
A Study to Evaluate the Efficacy, Safety, and Drug Concentration of Certolizumab Pegol (CZP) in Children and Adolescent Study Participants With Moderate to Severe Chronic Plaque Psoriasis (PSO)(CIMcare)
ClinicalTrials.gov Identifier: NCT04123795
A Study of Tildrakizumab in Pediatric Subjects With Chronic Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT03997786
Tapinarof for the Treatment of Plaque Psoriasis in Pediatric Subjects
ClinicalTrials.gov Identifier: NCT05172726
A Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of Subcutaneously Administered Guselkumab for the Treatment of Chronic Plaque Psoriasis in Pediatric Participants (PROTOSTAR)
ClinicalTrials.gov Identifier: NCT03451851
PSORIATIC ARTHRITIS
Efficacy of Secukinumab Compared to Ustekinumab in Adults With Active Psoriatic Arthritis and Failure of TNFα-Inhibitor Treatment (AgAIN)
ClinicalTrials.gov Identifier: NCT04632927
A Long-term Extension Study of Ustekinumab in Pediatric Participants (UNITED)
ClinicalTrials.gov Identifier: NCT05092269
A Study of Ustekinumab or Guselkumab in Pediatric Participants With Active Juvenile Psoriatic Arthritis (PSUMMIT-Jr)
ClinicalTrials.gov Identifier: NCT05083182
Comparative Study of BAT2506 With Simponi® in Participants With Active Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT05046431
Long Term Evaluation of Safety and Efficacy of Tildrakizumab in Patients With Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04991116
To Evaluate the Efficacy and Safety of SHR0302 Tablet in Subjects of Active Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04957550
PSORIATIC ARTHRITIS (continued)
Guselkumab in Active Psoriatic Arthritis Participants With Inadequate Response/Intolerance to One Prior Anti-TNF Alpha Agent (SOLSTICE)
ClinicalTrials.gov Identifier: NCT04936308
A Study to Determine the Efficacy and Safety of Deucravacitinib Compared With Placebo in Participants With Active Psoriatic Arthritis (PsA) Who Are Naïve to Biologic Disease-modifying Anti-rheumatic Drugs
ClinicalTrials.gov Identifier: NCT04908202
A Study to Determine the Efficacy and Safety of Deucravacitinib Compared With Placebo in Participants With Active Psoriatic Arthritis (PsA) Who Are Naïve to Biologic Disease Modifying Anti-rheumatic Drugs or Had Previously Received TNFα Inhibitor Treatment
ClinicalTrials.gov Identifier: NCT04908189
A Study of Guselkumab in Participants With Active Psoriatic Arthritis (APEX) ClinicalTrials.gov Identifier: NCT04882098 Apremilast Pediatric Study in Children With Active Juvenile Psoriatic Arthritis (PEAPOD)
ClinicalTrials.gov Identifier: NCT04804553
Impact of Tapering Immunosuppressants on Maintaining Minimal Disease Activity in Adult Subjects With Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04610476
A Study of Ixekizumab (LY2439821) in Children With Juvenile Idiopathic Arthritis Categories of Enthesitis-related Arthritis (Including Juvenile Onset Ankylosing Spondylitis) and Juvenile Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04527380
Efficacy and Safety of Tildrakizumab Compared to Placebo in Subjects With Active Psoriatic Arthritis I (INSPIRE 1)
ClinicalTrials.gov Identifier: NCT04314544
Efficacy and Safety of Tildrakizumab Compared to Placebo in Anti- TNF naïve Subjects With Active Psoriatic Arthritis II (INSPIRE 2)
ClinicalTrials.gov Identifier: NCT04314531
The psoriasis clinical trials listed below are all phase 3 and recruiting participants as of July 19, 2022. For additional information on the study design, eligibility criteria, and contacts/locations, visit ClinicalTrials.gov.
GENERALIZED PUSTULAR PSORIASIS
Long-Term Safety and Efficacy of Imsidolimab (ANB019) in Subjects With Generalized Pustular Psoriasis (GEMINI2)
ClinicalTrials.gov Identifier: NCT05366855
An Expanded Access Trial in Japan to Provide Spesolimab to People With a Flare-up in Generalized Pustular Psoriasis Who Have no Other Treatment Options
ClinicalTrials.gov Identifier: NCT05200247
An Expanded Access Program in China to Provide Spesolimab to People With a Flare-up in Generalized Pustular Psoriasis Who Have No Other Treatment Options
ClinicalTrials.gov Identifier: NCT05239039
Study to Evaluate the Efficacy and Safety of Imsidolimab (ANB019) in the Treatment of Subjects With GPP (GEMINI1)
ClinicalTrials.gov Identifier: NCT05352893
NAIL PSORIASIS
Efficacy and Safety Study of Tildrakizumab in the Treatment of Nail Psoriasis
ClinicalTrials.gov Identifier: NCT03897075
PALMOPLANTAR PUSTULOSIS
Phase 3, Randomized Study of Apremilast in Japanese Participants With Palmoplantar Pustulosis (PPP)
ClinicalTrials.gov Identifier: NCT05174065
PLAQUE PSORIASIS
A Long-term Extension Study of Apremilast (CC-10004) in Pediatric Subjects From 6 Through 17 Years of Age With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04175613
A Phase III Efficacy and Safety Study of Hemay005 in Subjects With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04839328
A Study of Subcutaneous Risankizumab Injection for Pediatric Participants With Moderate to Severe Plaque Psoriasis to Assess Change in Disease Symptoms
ClinicalTrials.gov Identifier: NCT04435600
A Study to Evaluate the Drug Levels, Efficacy and Safety of Deucravacitinib in Adolescent Participants With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04772079
Investigator Initiated Trial to Study Duobrii® Lotion in the Treatment of Mild Plaque Psoriasis in Adults
ClinicalTrials.gov Identifier: NCT05203315
Comparative Study of BAT2206 With Stelara® in Patients With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04728360
A Study to Evaluate the Efficacy and Safety of Bimekizumab in Adult Korean Study Participants With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT05020249
Comparing Efficacy and Safety of Bmab 1200 and Stelara in Patients With Moderate to Severe Chronic Plaque Psoriasis (STELLAR-2)
ClinicalTrials.gov Identifier: NCT05335356
A Study to Evaluate the Efficacy, Safety, and Drug Concentration of Certolizumab Pegol (CZP) in Children and Adolescent Study Participants With Moderate to Severe Chronic Plaque Psoriasis (PSO)(CIMcare)
ClinicalTrials.gov Identifier: NCT04123795
A Study of Tildrakizumab in Pediatric Subjects With Chronic Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT03997786
Tapinarof for the Treatment of Plaque Psoriasis in Pediatric Subjects
ClinicalTrials.gov Identifier: NCT05172726
A Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of Subcutaneously Administered Guselkumab for the Treatment of Chronic Plaque Psoriasis in Pediatric Participants (PROTOSTAR)
ClinicalTrials.gov Identifier: NCT03451851
PSORIATIC ARTHRITIS
Efficacy of Secukinumab Compared to Ustekinumab in Adults With Active Psoriatic Arthritis and Failure of TNFα-Inhibitor Treatment (AgAIN)
ClinicalTrials.gov Identifier: NCT04632927
A Long-term Extension Study of Ustekinumab in Pediatric Participants (UNITED)
ClinicalTrials.gov Identifier: NCT05092269
A Study of Ustekinumab or Guselkumab in Pediatric Participants With Active Juvenile Psoriatic Arthritis (PSUMMIT-Jr)
ClinicalTrials.gov Identifier: NCT05083182
Comparative Study of BAT2506 With Simponi® in Participants With Active Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT05046431
Long Term Evaluation of Safety and Efficacy of Tildrakizumab in Patients With Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04991116
To Evaluate the Efficacy and Safety of SHR0302 Tablet in Subjects of Active Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04957550
PSORIATIC ARTHRITIS (continued)
Guselkumab in Active Psoriatic Arthritis Participants With Inadequate Response/Intolerance to One Prior Anti-TNF Alpha Agent (SOLSTICE)
ClinicalTrials.gov Identifier: NCT04936308
A Study to Determine the Efficacy and Safety of Deucravacitinib Compared With Placebo in Participants With Active Psoriatic Arthritis (PsA) Who Are Naïve to Biologic Disease-modifying Anti-rheumatic Drugs
ClinicalTrials.gov Identifier: NCT04908202
A Study to Determine the Efficacy and Safety of Deucravacitinib Compared With Placebo in Participants With Active Psoriatic Arthritis (PsA) Who Are Naïve to Biologic Disease Modifying Anti-rheumatic Drugs or Had Previously Received TNFα Inhibitor Treatment
ClinicalTrials.gov Identifier: NCT04908189
A Study of Guselkumab in Participants With Active Psoriatic Arthritis (APEX) ClinicalTrials.gov Identifier: NCT04882098 Apremilast Pediatric Study in Children With Active Juvenile Psoriatic Arthritis (PEAPOD)
ClinicalTrials.gov Identifier: NCT04804553
Impact of Tapering Immunosuppressants on Maintaining Minimal Disease Activity in Adult Subjects With Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04610476
A Study of Ixekizumab (LY2439821) in Children With Juvenile Idiopathic Arthritis Categories of Enthesitis-related Arthritis (Including Juvenile Onset Ankylosing Spondylitis) and Juvenile Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04527380
Efficacy and Safety of Tildrakizumab Compared to Placebo in Subjects With Active Psoriatic Arthritis I (INSPIRE 1)
ClinicalTrials.gov Identifier: NCT04314544
Efficacy and Safety of Tildrakizumab Compared to Placebo in Anti- TNF naïve Subjects With Active Psoriatic Arthritis II (INSPIRE 2)
ClinicalTrials.gov Identifier: NCT04314531
The psoriasis clinical trials listed below are all phase 3 and recruiting participants as of July 19, 2022. For additional information on the study design, eligibility criteria, and contacts/locations, visit ClinicalTrials.gov.
GENERALIZED PUSTULAR PSORIASIS
Long-Term Safety and Efficacy of Imsidolimab (ANB019) in Subjects With Generalized Pustular Psoriasis (GEMINI2)
ClinicalTrials.gov Identifier: NCT05366855
An Expanded Access Trial in Japan to Provide Spesolimab to People With a Flare-up in Generalized Pustular Psoriasis Who Have no Other Treatment Options
ClinicalTrials.gov Identifier: NCT05200247
An Expanded Access Program in China to Provide Spesolimab to People With a Flare-up in Generalized Pustular Psoriasis Who Have No Other Treatment Options
ClinicalTrials.gov Identifier: NCT05239039
Study to Evaluate the Efficacy and Safety of Imsidolimab (ANB019) in the Treatment of Subjects With GPP (GEMINI1)
ClinicalTrials.gov Identifier: NCT05352893
NAIL PSORIASIS
Efficacy and Safety Study of Tildrakizumab in the Treatment of Nail Psoriasis
ClinicalTrials.gov Identifier: NCT03897075
PALMOPLANTAR PUSTULOSIS
Phase 3, Randomized Study of Apremilast in Japanese Participants With Palmoplantar Pustulosis (PPP)
ClinicalTrials.gov Identifier: NCT05174065
PLAQUE PSORIASIS
A Long-term Extension Study of Apremilast (CC-10004) in Pediatric Subjects From 6 Through 17 Years of Age With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04175613
A Phase III Efficacy and Safety Study of Hemay005 in Subjects With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04839328
A Study of Subcutaneous Risankizumab Injection for Pediatric Participants With Moderate to Severe Plaque Psoriasis to Assess Change in Disease Symptoms
ClinicalTrials.gov Identifier: NCT04435600
A Study to Evaluate the Drug Levels, Efficacy and Safety of Deucravacitinib in Adolescent Participants With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04772079
Investigator Initiated Trial to Study Duobrii® Lotion in the Treatment of Mild Plaque Psoriasis in Adults
ClinicalTrials.gov Identifier: NCT05203315
Comparative Study of BAT2206 With Stelara® in Patients With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT04728360
A Study to Evaluate the Efficacy and Safety of Bimekizumab in Adult Korean Study Participants With Moderate to Severe Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT05020249
Comparing Efficacy and Safety of Bmab 1200 and Stelara in Patients With Moderate to Severe Chronic Plaque Psoriasis (STELLAR-2)
ClinicalTrials.gov Identifier: NCT05335356
A Study to Evaluate the Efficacy, Safety, and Drug Concentration of Certolizumab Pegol (CZP) in Children and Adolescent Study Participants With Moderate to Severe Chronic Plaque Psoriasis (PSO)(CIMcare)
ClinicalTrials.gov Identifier: NCT04123795
A Study of Tildrakizumab in Pediatric Subjects With Chronic Plaque Psoriasis
ClinicalTrials.gov Identifier: NCT03997786
Tapinarof for the Treatment of Plaque Psoriasis in Pediatric Subjects
ClinicalTrials.gov Identifier: NCT05172726
A Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of Subcutaneously Administered Guselkumab for the Treatment of Chronic Plaque Psoriasis in Pediatric Participants (PROTOSTAR)
ClinicalTrials.gov Identifier: NCT03451851
PSORIATIC ARTHRITIS
Efficacy of Secukinumab Compared to Ustekinumab in Adults With Active Psoriatic Arthritis and Failure of TNFα-Inhibitor Treatment (AgAIN)
ClinicalTrials.gov Identifier: NCT04632927
A Long-term Extension Study of Ustekinumab in Pediatric Participants (UNITED)
ClinicalTrials.gov Identifier: NCT05092269
A Study of Ustekinumab or Guselkumab in Pediatric Participants With Active Juvenile Psoriatic Arthritis (PSUMMIT-Jr)
ClinicalTrials.gov Identifier: NCT05083182
Comparative Study of BAT2506 With Simponi® in Participants With Active Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT05046431
Long Term Evaluation of Safety and Efficacy of Tildrakizumab in Patients With Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04991116
To Evaluate the Efficacy and Safety of SHR0302 Tablet in Subjects of Active Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04957550
PSORIATIC ARTHRITIS (continued)
Guselkumab in Active Psoriatic Arthritis Participants With Inadequate Response/Intolerance to One Prior Anti-TNF Alpha Agent (SOLSTICE)
ClinicalTrials.gov Identifier: NCT04936308
A Study to Determine the Efficacy and Safety of Deucravacitinib Compared With Placebo in Participants With Active Psoriatic Arthritis (PsA) Who Are Naïve to Biologic Disease-modifying Anti-rheumatic Drugs
ClinicalTrials.gov Identifier: NCT04908202
A Study to Determine the Efficacy and Safety of Deucravacitinib Compared With Placebo in Participants With Active Psoriatic Arthritis (PsA) Who Are Naïve to Biologic Disease Modifying Anti-rheumatic Drugs or Had Previously Received TNFα Inhibitor Treatment
ClinicalTrials.gov Identifier: NCT04908189
A Study of Guselkumab in Participants With Active Psoriatic Arthritis (APEX) ClinicalTrials.gov Identifier: NCT04882098 Apremilast Pediatric Study in Children With Active Juvenile Psoriatic Arthritis (PEAPOD)
ClinicalTrials.gov Identifier: NCT04804553
Impact of Tapering Immunosuppressants on Maintaining Minimal Disease Activity in Adult Subjects With Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04610476
A Study of Ixekizumab (LY2439821) in Children With Juvenile Idiopathic Arthritis Categories of Enthesitis-related Arthritis (Including Juvenile Onset Ankylosing Spondylitis) and Juvenile Psoriatic Arthritis
ClinicalTrials.gov Identifier: NCT04527380
Efficacy and Safety of Tildrakizumab Compared to Placebo in Subjects With Active Psoriatic Arthritis I (INSPIRE 1)
ClinicalTrials.gov Identifier: NCT04314544
Efficacy and Safety of Tildrakizumab Compared to Placebo in Anti- TNF naïve Subjects With Active Psoriatic Arthritis II (INSPIRE 2)
ClinicalTrials.gov Identifier: NCT04314531
Brodalumab suicide risk similar to other biologics, postmarket study finds
.
The Food and Drug Administration approved brodalumab (Siliq) in 2017 for treatment of moderate to severe plaque psoriasis with a boxed warning for suicidal ideation and behavior and an associated Risk Evaluation and Mitigation Strategies (REMS) program indicating an increased risk of suicidality.
Half a decade later, “the available worldwide data do not support the notion that brodalumab has a unique risk of increased suicides,” senior investigator John Koo, MD, and coinvestigators at the University of California, San Francisco, wrote in a preproof article in JAAD International, noting that postmarketing data are “often considered a better reflection of real-world outcomes than clinical trials.”
The researchers extracted data through the end of 2021 on the number of completed suicides for brodalumab and ten other biologics approved for psoriasis from the FDA’s Adverse Events Reporting System (FAERS), an international publicly available database. The researchers included suicide data on the biologics for all indications.
The authors contacted pharmaceutical companies to determine the total number of patients prescribed each drug, securing mostly “best estimates” data on 5 of the 11 biologics available for psoriasis. The researchers then calculated the number of completed suicides per total number of prescribed patients.
For brodalumab, across 20,871 total prescriptions, there was only one verifiable suicide. It occurred in a Japanese man with terminal cancer and no nearby relatives 36 days after his first dose. The suicide rate for brodalumab was similar to that of ixekizumab, secukinumab, infliximab, and adalimumab.
“Brodalumab is a very efficacious agent and may have the fastest onset of action, yet its usage is minimal compared to the other agents because of this ‘black box’ warning ... despite the fact that it’s the least expensive of any biologic,” Dr. Koo, professor of dermatology and director of the Psoriasis and Skin Treatment Center, University of California, San Francisco, said in an interview.
Dr. Koo, who is board-certified in both dermatology and psychiatry, said he believes the boxed warning was never warranted. All three of the verified completed suicides that occurred during clinical trials of brodalumab for psoriasis were in people who had underlying psychiatric disorders or significant stressors, such as going to jail in one case, and depression and significant isolation in another, he said.
(An analysis of psychiatric adverse events during the psoriasis clinical trials, involving more than 4,000 patients, was published online Oct. 4, 2017, in the Journal of the American Academy of Dermatology.
George Han, MD, PhD, associate professor and director of research and teledermatology at the Zucker School of Medicine at Hofstra/Northwell, New Hyde Park, N.Y., who was not involved in the research, said the new data is reassuring.
“We sometimes put it into context [in thinking and counseling about risk] that in the trials for brodalumab, the number of suicide attempts [versus completed suicides] was not an outlier,” he said. “But it’s hard to know what to make of that, so this piece of knowledge that the postmarketing data show there’s no safety signal should give people a lot of reassurance.”
Dr. Han said he has used the medication, a fully human anti-interleukin 17 receptor A monoclonal antibody, in many patients who “have not done so well on other biologics and it’s been a lifesaver ... a couple who have switched over have maintained the longest level of clearance they’ve had with anything. It’s quite striking.”
The efficacy stems at least partly from its mechanism of blocking all cytokines in the IL-17 family – including those involved in the “feedback loops that perpetuate psoriasis” – rather than just one as other biologics do, Dr. Han said.
Usage of the drug has been hindered by the black box warning and REMS program, not only because of the extra steps required and hesitation potentially evoked, but because samples are not available, and because the “formulary access is not what it could have been otherwise,” he noted.
The Siliq REMS patient enrollment form requires patients to pledge awareness of the fact that suicidal thoughts and behaviors have occurred in treated patients and that they should seek medical attention if they experience suicidal thoughts or new or worsening depression, anxiety, or other mood changes. Prescribers must be certified with the program and must pledge on each enrollment form that they have counseled their patients.
The box warning states that there is no established causal association between treatment with brodalumab and increased risk for suicidal ideation and behaviors (SIB).
Individuals with psoriasis are an “already vulnerable population” who have been shown in reviews and meta-analyses to have a higher prevalence of depression and a higher risk of SIB than those without the disease, Dr. Koo and colleagues wrote in a narrative review published in Cutis .
Regardless of therapy, they wrote in the review, dermatologists should assess for any history of depression and SIB, and evaluate for signs and symptoms of current depression and SIB, referring patients as necessary to primary care or mental health care.
In the psoriasis trials, brodalumab treatment appeared to improve symptoms of depression and anxiety – a finding consistent with the effects reported for other biologic therapies, they wrote.
The first author on the newly published preproof is Samuel Yeroushalmi, BS, a fourth-year medical student at George Washington University, Washington.
Siliq is marketed by Valeant Pharmaceuticals.
Dr. Koo disclosed that he is an adviser/consultant/speaker for numerous pharmaceutical companies, but not those that were involved in the development of brodalumab. Dr. Han said he has relationships with numerous companies, including those that have developed brodalumab and other biologic agents used for psoriasis. The authors declared funding sources as none.
.
The Food and Drug Administration approved brodalumab (Siliq) in 2017 for treatment of moderate to severe plaque psoriasis with a boxed warning for suicidal ideation and behavior and an associated Risk Evaluation and Mitigation Strategies (REMS) program indicating an increased risk of suicidality.
Half a decade later, “the available worldwide data do not support the notion that brodalumab has a unique risk of increased suicides,” senior investigator John Koo, MD, and coinvestigators at the University of California, San Francisco, wrote in a preproof article in JAAD International, noting that postmarketing data are “often considered a better reflection of real-world outcomes than clinical trials.”
The researchers extracted data through the end of 2021 on the number of completed suicides for brodalumab and ten other biologics approved for psoriasis from the FDA’s Adverse Events Reporting System (FAERS), an international publicly available database. The researchers included suicide data on the biologics for all indications.
The authors contacted pharmaceutical companies to determine the total number of patients prescribed each drug, securing mostly “best estimates” data on 5 of the 11 biologics available for psoriasis. The researchers then calculated the number of completed suicides per total number of prescribed patients.
For brodalumab, across 20,871 total prescriptions, there was only one verifiable suicide. It occurred in a Japanese man with terminal cancer and no nearby relatives 36 days after his first dose. The suicide rate for brodalumab was similar to that of ixekizumab, secukinumab, infliximab, and adalimumab.
“Brodalumab is a very efficacious agent and may have the fastest onset of action, yet its usage is minimal compared to the other agents because of this ‘black box’ warning ... despite the fact that it’s the least expensive of any biologic,” Dr. Koo, professor of dermatology and director of the Psoriasis and Skin Treatment Center, University of California, San Francisco, said in an interview.
Dr. Koo, who is board-certified in both dermatology and psychiatry, said he believes the boxed warning was never warranted. All three of the verified completed suicides that occurred during clinical trials of brodalumab for psoriasis were in people who had underlying psychiatric disorders or significant stressors, such as going to jail in one case, and depression and significant isolation in another, he said.
(An analysis of psychiatric adverse events during the psoriasis clinical trials, involving more than 4,000 patients, was published online Oct. 4, 2017, in the Journal of the American Academy of Dermatology.
George Han, MD, PhD, associate professor and director of research and teledermatology at the Zucker School of Medicine at Hofstra/Northwell, New Hyde Park, N.Y., who was not involved in the research, said the new data is reassuring.
“We sometimes put it into context [in thinking and counseling about risk] that in the trials for brodalumab, the number of suicide attempts [versus completed suicides] was not an outlier,” he said. “But it’s hard to know what to make of that, so this piece of knowledge that the postmarketing data show there’s no safety signal should give people a lot of reassurance.”
Dr. Han said he has used the medication, a fully human anti-interleukin 17 receptor A monoclonal antibody, in many patients who “have not done so well on other biologics and it’s been a lifesaver ... a couple who have switched over have maintained the longest level of clearance they’ve had with anything. It’s quite striking.”
The efficacy stems at least partly from its mechanism of blocking all cytokines in the IL-17 family – including those involved in the “feedback loops that perpetuate psoriasis” – rather than just one as other biologics do, Dr. Han said.
Usage of the drug has been hindered by the black box warning and REMS program, not only because of the extra steps required and hesitation potentially evoked, but because samples are not available, and because the “formulary access is not what it could have been otherwise,” he noted.
The Siliq REMS patient enrollment form requires patients to pledge awareness of the fact that suicidal thoughts and behaviors have occurred in treated patients and that they should seek medical attention if they experience suicidal thoughts or new or worsening depression, anxiety, or other mood changes. Prescribers must be certified with the program and must pledge on each enrollment form that they have counseled their patients.
The box warning states that there is no established causal association between treatment with brodalumab and increased risk for suicidal ideation and behaviors (SIB).
Individuals with psoriasis are an “already vulnerable population” who have been shown in reviews and meta-analyses to have a higher prevalence of depression and a higher risk of SIB than those without the disease, Dr. Koo and colleagues wrote in a narrative review published in Cutis .
Regardless of therapy, they wrote in the review, dermatologists should assess for any history of depression and SIB, and evaluate for signs and symptoms of current depression and SIB, referring patients as necessary to primary care or mental health care.
In the psoriasis trials, brodalumab treatment appeared to improve symptoms of depression and anxiety – a finding consistent with the effects reported for other biologic therapies, they wrote.
The first author on the newly published preproof is Samuel Yeroushalmi, BS, a fourth-year medical student at George Washington University, Washington.
Siliq is marketed by Valeant Pharmaceuticals.
Dr. Koo disclosed that he is an adviser/consultant/speaker for numerous pharmaceutical companies, but not those that were involved in the development of brodalumab. Dr. Han said he has relationships with numerous companies, including those that have developed brodalumab and other biologic agents used for psoriasis. The authors declared funding sources as none.
.
The Food and Drug Administration approved brodalumab (Siliq) in 2017 for treatment of moderate to severe plaque psoriasis with a boxed warning for suicidal ideation and behavior and an associated Risk Evaluation and Mitigation Strategies (REMS) program indicating an increased risk of suicidality.
Half a decade later, “the available worldwide data do not support the notion that brodalumab has a unique risk of increased suicides,” senior investigator John Koo, MD, and coinvestigators at the University of California, San Francisco, wrote in a preproof article in JAAD International, noting that postmarketing data are “often considered a better reflection of real-world outcomes than clinical trials.”
The researchers extracted data through the end of 2021 on the number of completed suicides for brodalumab and ten other biologics approved for psoriasis from the FDA’s Adverse Events Reporting System (FAERS), an international publicly available database. The researchers included suicide data on the biologics for all indications.
The authors contacted pharmaceutical companies to determine the total number of patients prescribed each drug, securing mostly “best estimates” data on 5 of the 11 biologics available for psoriasis. The researchers then calculated the number of completed suicides per total number of prescribed patients.
For brodalumab, across 20,871 total prescriptions, there was only one verifiable suicide. It occurred in a Japanese man with terminal cancer and no nearby relatives 36 days after his first dose. The suicide rate for brodalumab was similar to that of ixekizumab, secukinumab, infliximab, and adalimumab.
“Brodalumab is a very efficacious agent and may have the fastest onset of action, yet its usage is minimal compared to the other agents because of this ‘black box’ warning ... despite the fact that it’s the least expensive of any biologic,” Dr. Koo, professor of dermatology and director of the Psoriasis and Skin Treatment Center, University of California, San Francisco, said in an interview.
Dr. Koo, who is board-certified in both dermatology and psychiatry, said he believes the boxed warning was never warranted. All three of the verified completed suicides that occurred during clinical trials of brodalumab for psoriasis were in people who had underlying psychiatric disorders or significant stressors, such as going to jail in one case, and depression and significant isolation in another, he said.
(An analysis of psychiatric adverse events during the psoriasis clinical trials, involving more than 4,000 patients, was published online Oct. 4, 2017, in the Journal of the American Academy of Dermatology.
George Han, MD, PhD, associate professor and director of research and teledermatology at the Zucker School of Medicine at Hofstra/Northwell, New Hyde Park, N.Y., who was not involved in the research, said the new data is reassuring.
“We sometimes put it into context [in thinking and counseling about risk] that in the trials for brodalumab, the number of suicide attempts [versus completed suicides] was not an outlier,” he said. “But it’s hard to know what to make of that, so this piece of knowledge that the postmarketing data show there’s no safety signal should give people a lot of reassurance.”
Dr. Han said he has used the medication, a fully human anti-interleukin 17 receptor A monoclonal antibody, in many patients who “have not done so well on other biologics and it’s been a lifesaver ... a couple who have switched over have maintained the longest level of clearance they’ve had with anything. It’s quite striking.”
The efficacy stems at least partly from its mechanism of blocking all cytokines in the IL-17 family – including those involved in the “feedback loops that perpetuate psoriasis” – rather than just one as other biologics do, Dr. Han said.
Usage of the drug has been hindered by the black box warning and REMS program, not only because of the extra steps required and hesitation potentially evoked, but because samples are not available, and because the “formulary access is not what it could have been otherwise,” he noted.
The Siliq REMS patient enrollment form requires patients to pledge awareness of the fact that suicidal thoughts and behaviors have occurred in treated patients and that they should seek medical attention if they experience suicidal thoughts or new or worsening depression, anxiety, or other mood changes. Prescribers must be certified with the program and must pledge on each enrollment form that they have counseled their patients.
The box warning states that there is no established causal association between treatment with brodalumab and increased risk for suicidal ideation and behaviors (SIB).
Individuals with psoriasis are an “already vulnerable population” who have been shown in reviews and meta-analyses to have a higher prevalence of depression and a higher risk of SIB than those without the disease, Dr. Koo and colleagues wrote in a narrative review published in Cutis .
Regardless of therapy, they wrote in the review, dermatologists should assess for any history of depression and SIB, and evaluate for signs and symptoms of current depression and SIB, referring patients as necessary to primary care or mental health care.
In the psoriasis trials, brodalumab treatment appeared to improve symptoms of depression and anxiety – a finding consistent with the effects reported for other biologic therapies, they wrote.
The first author on the newly published preproof is Samuel Yeroushalmi, BS, a fourth-year medical student at George Washington University, Washington.
Siliq is marketed by Valeant Pharmaceuticals.
Dr. Koo disclosed that he is an adviser/consultant/speaker for numerous pharmaceutical companies, but not those that were involved in the development of brodalumab. Dr. Han said he has relationships with numerous companies, including those that have developed brodalumab and other biologic agents used for psoriasis. The authors declared funding sources as none.
Artemisia capillaris extract
Melasma is a difficult disorder to treat. With the removal of hydroquinone from the cosmetic market and the prevalence of dyschromia, new skin lightening ingredients are being sought and many new discoveries are coming from Asia.
There are more than 500 species of the genus Artemisia (of the Astraceae or Compositae family) dispersed throughout the temperate areas of Asia, Europe, and North America.1 Various parts of the shrub Artemisia capillaris, found abundantly in China, Japan, and Korea, have been used in traditional medicine in Asia for hundreds of years. A. capillaris (Yin-Chen in Chinese) has been deployed in traditional Chinese medicine as a diuretic, to protect the liver, and to treat skin inflammation.2,3 Antioxidant, anti-inflammatory, antisteatotic, antitumor, and antiviral properties have been associated with this plant,3 and hydrating effects have been recently attributed to it. In Korean medicine, A. capillaris (InJin in Korean) has been used for its hepatoprotective, analgesic, and antipyretic activities.4,5 In this column, the focus will be on recent evidence that suggests possible applications in skin care.
Chemical constituents
In 2008, Kim et al. studied the anticarcinogenic activity of A. capillaris, among other medicinal herbs, using the 7,12-dimethylbenz[a]anthracene (DMBA)-induced mouse skin carcinogenesis model. The researchers found that A. capillaris exhibited the most effective anticarcinogenic activity compared to the other herbs tested, with such properties ascribed to its constituent camphor, 1-borneol, coumarin, and achillin. Notably, the chloroform fraction of A. capillaris significantly lowered the number of tumors/mouse and tumor incidence compared with the other tested herbs.6
The wide range of biological functions associated with A. capillaris, including anti-inflammatory, antioxidant, antidiabetic, antisteatotic, and antitumor activities have, in various studies, been attributed to the bioactive constituents scoparone, scopoletin, capillarisin, capillin, and chlorogenic acids.3
Tyrosinase-related protein 1 (TYRP-1) and its role in skin pigmentation
Tyrosinase related protein 1 (TYRP-1) is structurally similar to tyrosinase, but its role is still being elucidated. Mutations in TYR-1 results in oculocutaneous albinism. TYRP-1 is involved in eumelanin synthesis, but not in pheomelanin synthesis. Mutations in TYRP-1 affect the quality of melanin synthesized rather than the quantity.4 TYRP-1 is being looked at as a target for treatment of hyperpigmentation disorders such as melasma.
Effects on melanin synthesis
A. capillaris reduces the expression of TYRP-1, making it attractive for use in skin lightening products. Although there are not a lot of data, this is a developing area of interest and the following will discuss what is known so far.
Kim et al. investigated the antimelanogenic activity of 10 essential oils, including A. capillaris, utilizing the B16F10 cell line model. A. capillaris was among four extracts found to hinder melanogenesis, and the only one that improved cell proliferation, displayed anti-H2O2 activity, and reduced tyrosinase-related protein (TRP)-1 expression. The researchers determined that A. capillaris extract suppressed melanin production through the downregulation of the TRP 1 translational level. They concluded that while investigations using in vivo models are necessary to buttress and validate these results, A. capillaris extract appears to be suitable as a natural therapeutic antimelanogenic agent as well as a skin-whitening ingredient in cosmeceutical products.7
Tabassum et al. screened A. capillaris for antipigmentary functions using murine cultured cells (B16-F10 malignant melanocytes). They found that the A. capillaris constituent 4,5-O-dicaffeoylquinic acid significantly and dose-dependently diminished melanin production and tyrosinase activity in the melanocytes. The expression of tyrosinase-related protein-1 was also decreased. Further, the researchers observed antipigmentary activity in a zebrafish model, with no toxicity demonstrated by either A. capillaris or its component 4,5-O-dicaffeoylquinic acid. They concluded that this compound could be included as an active ingredient in products intended to address pigmentation disorders.8
Anti-inflammatory activity
Inflammation is well known to trigger the production of melanin. This is why anti-inflammatory ingredients are often included in skin lighting products. A. capillaris displays anti-inflammatory activity and has shown some antioxidant activity.
In 2018, Lee et al. confirmed the therapeutic potential of A. capillaris extract to treat psoriasis in HaCaT cells and imiquimod-induced psoriasis-like mouse models. In the murine models, those treated with the ethanol extract of A. capillaris had a significantly lower Psoriasis Area and Severity Index score than that of the mice not given the topical application of the botanical. Epidermal thickness was noted to be significantly lower compared with the mice not treated with A. capillaris.9 Further studies in mice by the same team later that year supported the use of a cream formulation containing A. capillaris that they developed to treat psoriasis, warranting new investigations in human skin.10
Yeo et al. reported, earlier in 2018, on other anti-inflammatory activity of the herb, finding that the aqueous extract from A. capillaris blocked acute gastric mucosal injury by hindering reactive oxygen species and nuclear factor kappa B. They added that A. capillaris maintains oxidant/antioxidant homeostasis and displays potential as a nutraceutical agent for treating gastric ulcers and gastritis.5
In 2011, Kwon et al. studied the 5-lipoxygenase inhibitory action of a 70% ethanol extract of aerial parts of A. capillaris. They identified esculetin and quercetin as strong inhibitors of 5-lipoxygenase. The botanical agent, and esculetin in particular, robustly suppressed arachidonic acid-induced ear edema in mice as well as delayed-type hypersensitivity reactions. Further, A. capillaris potently blocked 5-lipoxygenase-catalyzed leukotriene synthesis by ionophore-induced rat basophilic leukemia-1 cells. The researchers concluded that their findings may partially account for the use of A. capillaris as a traditional medical treatment for cutaneous inflammatory conditions.2
Atopic dermatitis and A. capillaris
In 2014, Ha et al. used in vitro and in vivo systems to assess the anti-inflammatory effects of A. capillaris as well as its activity against atopic dermatitis. The in vitro studies revealed that A. capillaris hampered NO and cellular histamine synthesis. In Nc/Nga mice sensitized by Dermatophagoides farinae, dermatitis scores as well as hemorrhage, hypertrophy, and hyperkeratosis of the epidermis in the dorsal skin and ear all declined after the topical application of A. capillaris. Plasma levels of histamine and IgE also significantly decreased after treatment with A. capillaris. The investigators concluded that further study of A. capillaris is warranted as a potential therapeutic option for atopic dermatitis.11
Summary
Many botanical ingredients from Asia are making their way into skin care products in the USA. A. capillaris extract is an example and may have utility in treating hyperpigmentation-associated skin issues such as melasma. Its inhibitory effects on both inflammation and melanin production in addition to possible antioxidant activity make it an interesting compound worthy of more scrutiny.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Bora KS and Sharma A. Pharm Biol. 2011 Jan;49(1):101-9.
2. Kwon OS et al. Arch Pharm Res. 2011 Sep;34(9):1561-9.
3. Hsueh TP et al. Biomedicines. 2021 Oct 8;9(10):1412.
4. Dolinska MB et al. Int J Mol Sci. 2020 Jan 3;21(1):331.
5. Yeo D et al. Biomed Pharmacother. 2018 Mar;99:681-7.
6. Kim YS et al. J Food Sci. 2008 Jan;73(1):T16-20.
7. Kim MJ et al. Mol Med Rep. 2022 Apr;25(4):113.
8. Tabassum N et al. Evid Based Complement Alternat Med. 2016;2016:7823541.
9. Lee SY et al. Phytother Res. 2018 May;32(5):923-2.
10. Lee SY et al. Evid Based Complement Alternat Med. 2018 Aug 19;2018:3610494.
11. Ha H et al. BMC Complement Altern Med. 2014 Mar 14;14:100.
Melasma is a difficult disorder to treat. With the removal of hydroquinone from the cosmetic market and the prevalence of dyschromia, new skin lightening ingredients are being sought and many new discoveries are coming from Asia.
There are more than 500 species of the genus Artemisia (of the Astraceae or Compositae family) dispersed throughout the temperate areas of Asia, Europe, and North America.1 Various parts of the shrub Artemisia capillaris, found abundantly in China, Japan, and Korea, have been used in traditional medicine in Asia for hundreds of years. A. capillaris (Yin-Chen in Chinese) has been deployed in traditional Chinese medicine as a diuretic, to protect the liver, and to treat skin inflammation.2,3 Antioxidant, anti-inflammatory, antisteatotic, antitumor, and antiviral properties have been associated with this plant,3 and hydrating effects have been recently attributed to it. In Korean medicine, A. capillaris (InJin in Korean) has been used for its hepatoprotective, analgesic, and antipyretic activities.4,5 In this column, the focus will be on recent evidence that suggests possible applications in skin care.
Chemical constituents
In 2008, Kim et al. studied the anticarcinogenic activity of A. capillaris, among other medicinal herbs, using the 7,12-dimethylbenz[a]anthracene (DMBA)-induced mouse skin carcinogenesis model. The researchers found that A. capillaris exhibited the most effective anticarcinogenic activity compared to the other herbs tested, with such properties ascribed to its constituent camphor, 1-borneol, coumarin, and achillin. Notably, the chloroform fraction of A. capillaris significantly lowered the number of tumors/mouse and tumor incidence compared with the other tested herbs.6
The wide range of biological functions associated with A. capillaris, including anti-inflammatory, antioxidant, antidiabetic, antisteatotic, and antitumor activities have, in various studies, been attributed to the bioactive constituents scoparone, scopoletin, capillarisin, capillin, and chlorogenic acids.3
Tyrosinase-related protein 1 (TYRP-1) and its role in skin pigmentation
Tyrosinase related protein 1 (TYRP-1) is structurally similar to tyrosinase, but its role is still being elucidated. Mutations in TYR-1 results in oculocutaneous albinism. TYRP-1 is involved in eumelanin synthesis, but not in pheomelanin synthesis. Mutations in TYRP-1 affect the quality of melanin synthesized rather than the quantity.4 TYRP-1 is being looked at as a target for treatment of hyperpigmentation disorders such as melasma.
Effects on melanin synthesis
A. capillaris reduces the expression of TYRP-1, making it attractive for use in skin lightening products. Although there are not a lot of data, this is a developing area of interest and the following will discuss what is known so far.
Kim et al. investigated the antimelanogenic activity of 10 essential oils, including A. capillaris, utilizing the B16F10 cell line model. A. capillaris was among four extracts found to hinder melanogenesis, and the only one that improved cell proliferation, displayed anti-H2O2 activity, and reduced tyrosinase-related protein (TRP)-1 expression. The researchers determined that A. capillaris extract suppressed melanin production through the downregulation of the TRP 1 translational level. They concluded that while investigations using in vivo models are necessary to buttress and validate these results, A. capillaris extract appears to be suitable as a natural therapeutic antimelanogenic agent as well as a skin-whitening ingredient in cosmeceutical products.7
Tabassum et al. screened A. capillaris for antipigmentary functions using murine cultured cells (B16-F10 malignant melanocytes). They found that the A. capillaris constituent 4,5-O-dicaffeoylquinic acid significantly and dose-dependently diminished melanin production and tyrosinase activity in the melanocytes. The expression of tyrosinase-related protein-1 was also decreased. Further, the researchers observed antipigmentary activity in a zebrafish model, with no toxicity demonstrated by either A. capillaris or its component 4,5-O-dicaffeoylquinic acid. They concluded that this compound could be included as an active ingredient in products intended to address pigmentation disorders.8
Anti-inflammatory activity
Inflammation is well known to trigger the production of melanin. This is why anti-inflammatory ingredients are often included in skin lighting products. A. capillaris displays anti-inflammatory activity and has shown some antioxidant activity.
In 2018, Lee et al. confirmed the therapeutic potential of A. capillaris extract to treat psoriasis in HaCaT cells and imiquimod-induced psoriasis-like mouse models. In the murine models, those treated with the ethanol extract of A. capillaris had a significantly lower Psoriasis Area and Severity Index score than that of the mice not given the topical application of the botanical. Epidermal thickness was noted to be significantly lower compared with the mice not treated with A. capillaris.9 Further studies in mice by the same team later that year supported the use of a cream formulation containing A. capillaris that they developed to treat psoriasis, warranting new investigations in human skin.10
Yeo et al. reported, earlier in 2018, on other anti-inflammatory activity of the herb, finding that the aqueous extract from A. capillaris blocked acute gastric mucosal injury by hindering reactive oxygen species and nuclear factor kappa B. They added that A. capillaris maintains oxidant/antioxidant homeostasis and displays potential as a nutraceutical agent for treating gastric ulcers and gastritis.5
In 2011, Kwon et al. studied the 5-lipoxygenase inhibitory action of a 70% ethanol extract of aerial parts of A. capillaris. They identified esculetin and quercetin as strong inhibitors of 5-lipoxygenase. The botanical agent, and esculetin in particular, robustly suppressed arachidonic acid-induced ear edema in mice as well as delayed-type hypersensitivity reactions. Further, A. capillaris potently blocked 5-lipoxygenase-catalyzed leukotriene synthesis by ionophore-induced rat basophilic leukemia-1 cells. The researchers concluded that their findings may partially account for the use of A. capillaris as a traditional medical treatment for cutaneous inflammatory conditions.2
Atopic dermatitis and A. capillaris
In 2014, Ha et al. used in vitro and in vivo systems to assess the anti-inflammatory effects of A. capillaris as well as its activity against atopic dermatitis. The in vitro studies revealed that A. capillaris hampered NO and cellular histamine synthesis. In Nc/Nga mice sensitized by Dermatophagoides farinae, dermatitis scores as well as hemorrhage, hypertrophy, and hyperkeratosis of the epidermis in the dorsal skin and ear all declined after the topical application of A. capillaris. Plasma levels of histamine and IgE also significantly decreased after treatment with A. capillaris. The investigators concluded that further study of A. capillaris is warranted as a potential therapeutic option for atopic dermatitis.11
Summary
Many botanical ingredients from Asia are making their way into skin care products in the USA. A. capillaris extract is an example and may have utility in treating hyperpigmentation-associated skin issues such as melasma. Its inhibitory effects on both inflammation and melanin production in addition to possible antioxidant activity make it an interesting compound worthy of more scrutiny.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Bora KS and Sharma A. Pharm Biol. 2011 Jan;49(1):101-9.
2. Kwon OS et al. Arch Pharm Res. 2011 Sep;34(9):1561-9.
3. Hsueh TP et al. Biomedicines. 2021 Oct 8;9(10):1412.
4. Dolinska MB et al. Int J Mol Sci. 2020 Jan 3;21(1):331.
5. Yeo D et al. Biomed Pharmacother. 2018 Mar;99:681-7.
6. Kim YS et al. J Food Sci. 2008 Jan;73(1):T16-20.
7. Kim MJ et al. Mol Med Rep. 2022 Apr;25(4):113.
8. Tabassum N et al. Evid Based Complement Alternat Med. 2016;2016:7823541.
9. Lee SY et al. Phytother Res. 2018 May;32(5):923-2.
10. Lee SY et al. Evid Based Complement Alternat Med. 2018 Aug 19;2018:3610494.
11. Ha H et al. BMC Complement Altern Med. 2014 Mar 14;14:100.
Melasma is a difficult disorder to treat. With the removal of hydroquinone from the cosmetic market and the prevalence of dyschromia, new skin lightening ingredients are being sought and many new discoveries are coming from Asia.
There are more than 500 species of the genus Artemisia (of the Astraceae or Compositae family) dispersed throughout the temperate areas of Asia, Europe, and North America.1 Various parts of the shrub Artemisia capillaris, found abundantly in China, Japan, and Korea, have been used in traditional medicine in Asia for hundreds of years. A. capillaris (Yin-Chen in Chinese) has been deployed in traditional Chinese medicine as a diuretic, to protect the liver, and to treat skin inflammation.2,3 Antioxidant, anti-inflammatory, antisteatotic, antitumor, and antiviral properties have been associated with this plant,3 and hydrating effects have been recently attributed to it. In Korean medicine, A. capillaris (InJin in Korean) has been used for its hepatoprotective, analgesic, and antipyretic activities.4,5 In this column, the focus will be on recent evidence that suggests possible applications in skin care.
Chemical constituents
In 2008, Kim et al. studied the anticarcinogenic activity of A. capillaris, among other medicinal herbs, using the 7,12-dimethylbenz[a]anthracene (DMBA)-induced mouse skin carcinogenesis model. The researchers found that A. capillaris exhibited the most effective anticarcinogenic activity compared to the other herbs tested, with such properties ascribed to its constituent camphor, 1-borneol, coumarin, and achillin. Notably, the chloroform fraction of A. capillaris significantly lowered the number of tumors/mouse and tumor incidence compared with the other tested herbs.6
The wide range of biological functions associated with A. capillaris, including anti-inflammatory, antioxidant, antidiabetic, antisteatotic, and antitumor activities have, in various studies, been attributed to the bioactive constituents scoparone, scopoletin, capillarisin, capillin, and chlorogenic acids.3
Tyrosinase-related protein 1 (TYRP-1) and its role in skin pigmentation
Tyrosinase related protein 1 (TYRP-1) is structurally similar to tyrosinase, but its role is still being elucidated. Mutations in TYR-1 results in oculocutaneous albinism. TYRP-1 is involved in eumelanin synthesis, but not in pheomelanin synthesis. Mutations in TYRP-1 affect the quality of melanin synthesized rather than the quantity.4 TYRP-1 is being looked at as a target for treatment of hyperpigmentation disorders such as melasma.
Effects on melanin synthesis
A. capillaris reduces the expression of TYRP-1, making it attractive for use in skin lightening products. Although there are not a lot of data, this is a developing area of interest and the following will discuss what is known so far.
Kim et al. investigated the antimelanogenic activity of 10 essential oils, including A. capillaris, utilizing the B16F10 cell line model. A. capillaris was among four extracts found to hinder melanogenesis, and the only one that improved cell proliferation, displayed anti-H2O2 activity, and reduced tyrosinase-related protein (TRP)-1 expression. The researchers determined that A. capillaris extract suppressed melanin production through the downregulation of the TRP 1 translational level. They concluded that while investigations using in vivo models are necessary to buttress and validate these results, A. capillaris extract appears to be suitable as a natural therapeutic antimelanogenic agent as well as a skin-whitening ingredient in cosmeceutical products.7
Tabassum et al. screened A. capillaris for antipigmentary functions using murine cultured cells (B16-F10 malignant melanocytes). They found that the A. capillaris constituent 4,5-O-dicaffeoylquinic acid significantly and dose-dependently diminished melanin production and tyrosinase activity in the melanocytes. The expression of tyrosinase-related protein-1 was also decreased. Further, the researchers observed antipigmentary activity in a zebrafish model, with no toxicity demonstrated by either A. capillaris or its component 4,5-O-dicaffeoylquinic acid. They concluded that this compound could be included as an active ingredient in products intended to address pigmentation disorders.8
Anti-inflammatory activity
Inflammation is well known to trigger the production of melanin. This is why anti-inflammatory ingredients are often included in skin lighting products. A. capillaris displays anti-inflammatory activity and has shown some antioxidant activity.
In 2018, Lee et al. confirmed the therapeutic potential of A. capillaris extract to treat psoriasis in HaCaT cells and imiquimod-induced psoriasis-like mouse models. In the murine models, those treated with the ethanol extract of A. capillaris had a significantly lower Psoriasis Area and Severity Index score than that of the mice not given the topical application of the botanical. Epidermal thickness was noted to be significantly lower compared with the mice not treated with A. capillaris.9 Further studies in mice by the same team later that year supported the use of a cream formulation containing A. capillaris that they developed to treat psoriasis, warranting new investigations in human skin.10
Yeo et al. reported, earlier in 2018, on other anti-inflammatory activity of the herb, finding that the aqueous extract from A. capillaris blocked acute gastric mucosal injury by hindering reactive oxygen species and nuclear factor kappa B. They added that A. capillaris maintains oxidant/antioxidant homeostasis and displays potential as a nutraceutical agent for treating gastric ulcers and gastritis.5
In 2011, Kwon et al. studied the 5-lipoxygenase inhibitory action of a 70% ethanol extract of aerial parts of A. capillaris. They identified esculetin and quercetin as strong inhibitors of 5-lipoxygenase. The botanical agent, and esculetin in particular, robustly suppressed arachidonic acid-induced ear edema in mice as well as delayed-type hypersensitivity reactions. Further, A. capillaris potently blocked 5-lipoxygenase-catalyzed leukotriene synthesis by ionophore-induced rat basophilic leukemia-1 cells. The researchers concluded that their findings may partially account for the use of A. capillaris as a traditional medical treatment for cutaneous inflammatory conditions.2
Atopic dermatitis and A. capillaris
In 2014, Ha et al. used in vitro and in vivo systems to assess the anti-inflammatory effects of A. capillaris as well as its activity against atopic dermatitis. The in vitro studies revealed that A. capillaris hampered NO and cellular histamine synthesis. In Nc/Nga mice sensitized by Dermatophagoides farinae, dermatitis scores as well as hemorrhage, hypertrophy, and hyperkeratosis of the epidermis in the dorsal skin and ear all declined after the topical application of A. capillaris. Plasma levels of histamine and IgE also significantly decreased after treatment with A. capillaris. The investigators concluded that further study of A. capillaris is warranted as a potential therapeutic option for atopic dermatitis.11
Summary
Many botanical ingredients from Asia are making their way into skin care products in the USA. A. capillaris extract is an example and may have utility in treating hyperpigmentation-associated skin issues such as melasma. Its inhibitory effects on both inflammation and melanin production in addition to possible antioxidant activity make it an interesting compound worthy of more scrutiny.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Bora KS and Sharma A. Pharm Biol. 2011 Jan;49(1):101-9.
2. Kwon OS et al. Arch Pharm Res. 2011 Sep;34(9):1561-9.
3. Hsueh TP et al. Biomedicines. 2021 Oct 8;9(10):1412.
4. Dolinska MB et al. Int J Mol Sci. 2020 Jan 3;21(1):331.
5. Yeo D et al. Biomed Pharmacother. 2018 Mar;99:681-7.
6. Kim YS et al. J Food Sci. 2008 Jan;73(1):T16-20.
7. Kim MJ et al. Mol Med Rep. 2022 Apr;25(4):113.
8. Tabassum N et al. Evid Based Complement Alternat Med. 2016;2016:7823541.
9. Lee SY et al. Phytother Res. 2018 May;32(5):923-2.
10. Lee SY et al. Evid Based Complement Alternat Med. 2018 Aug 19;2018:3610494.
11. Ha H et al. BMC Complement Altern Med. 2014 Mar 14;14:100.
Sustained response at 2 years reported for newly approved oral psoriasis agent
MILAN – The day after deucravacitinib became the first TYK2 inhibitor approved for the treatment of moderate to severe psoriasis, long-term data were presented at the annual congress of the European Academy of Dermatology and Venereology, suggesting that a high degree of benefit persists for at least 2 years, making this oral drug a potential competitor for biologics.
,” said Mark G. Lebwohl, MD, professor of dermatology and dean of clinical therapeutics, Icahn School of Medicine at Mount Sinai, New York.
Just 2 months after the 52-week data from the phase 3 POETYK PSO-1 trial were published online in the Journal of the American Academy of Dermatology, a long-term extension study found essentially no loss of benefit at 112 weeks, according to Dr. Lebwohl.
One of the two co-primary endpoints was a 75% clearance on the Psoriasis and Severity Index (PASI75) score. At 52 weeks, 80.2% of patients on deucravacitinib had met this criterion of benefit. At 112 weeks, the proportion was 84.4%.
The other primary endpoint was a static Physician’s Global Assessment (sPGA) score of clear or almost clear skin. The proportion of patients meeting this criterion at weeks 52 and 112 weeks were 65.6% and 67.6%, respectively.
When assessed by Treatment Failure Rule (TFR) or modified nonresponder imputation (mNRI), results were similar. For both, the primary endpoints at every time interval were just one or two percentage points lower but not clinically meaningfully different, according to Dr. Lebwohl.
The same type of sustained response out to 112 weeks was observed in multiple analyses. When the researchers isolated the subgroup of patients who had achieved a PASI 75 response at 16 weeks (100%), there was a modest decline in the PASI 75 rate at week 52 (90.2%) but then no additional decline at week 112 (91.3%).
There were essentially no changes in the PASI 90 rates at week 16 (63%), week 52 (65.3%), and week 112 (63.1%), Dr. Lebwohl reported. PASI 100 rates, once achieved, were sustained long term.
The target, TYK2, is one of four Janus kinase (JAK) inhibitors. Until now, almost all JAK inhibitors have had greater relative specificity for JAK 1, JAK 2, and JAK 3, but several inhibitors of TYK2 inhibitors other than deucravacitinib are in development for inflammatory diseases. Deucravacitinib (Sotyktu), approved by the Food and Drug Administration on Sept. 9, is the only TYK2 inhibitor with regulatory approval for plaque psoriasis.
In the POETYK PSO-1 trial, 666 patients were initially randomized in a 2:1:1 ratio to 6 mg deucravacitinib (now the approved dose), placebo, or the oral phosphodiesterase 4 inhibitor apremilast. At week 16, patients on placebo were switched over to deucravacitinib. At week 24, patients who did not achieve a PASI 50 on apremilast (which had been titrated to 10 mg daily to 30 mg twice a day over the first 5 days of dosing) were switched to deucravacitinib.
In the previously reported data, deucravacitinib was superior for all efficacy endpoints at week 16, including an analysis of quality of life when compared with placebo (P < .0001) or apremilast (P = .0088). At week 52, after having been switched to deucravacitinib at week 16, patients on placebo achieved comparable responses on the efficacy measures in this study, including PASI75.
Relative to JAK inhibitors commonly used in rheumatoid arthritis and other inflammatory diseases, the greater specificity of deucravacitinib for TYK2 appears to have meaningful safety advantages, according to Dr. Lebwohl. Targeted mostly on the TYK2 regulatory domain, deucravacitinib largely avoids inhibition of the JAK 1, 2, and 3 subtypes. Dr. Lebwohl said this explains why deucravacitinib labeling does not share the boxed warnings about off-target effects, such as those on the cardiovascular system, that can be found in the labeling of other JAK inhibitors.
In the published 52-week data, the discontinuation rate for adverse events was lower in the group randomized to deucravacitinib arm than in the placebo arm. In the extended follow-up, there were no new signals for adverse events, including those involving the CV system or immune function.
The key message so far from the long-term follow-up, which is ongoing, is that “continuous treatment with deucravacitinib is associated with durable efficacy,” Dr. Lebwohl said. It is this combination of sustained efficacy and safety that led Dr. Lebwohl to suggest it as a reasonable oral competitor to injectable biologics.
“Patients now have a choice,” he said.
Jashin J. Wu, MD, a board member of the National Psoriasis Foundation and an associate professor in the department of dermatology, University of Miami, has been following the development of deucravacitinib. He said that the recent FDA approval validates the clinical evidence of benefit and safety, while the long-term data presented at the EADV congress support its role in expanding treatment options.
“Deucravacitinib is a very effective oral agent for moderate to severe plaque psoriasis with strong maintenance of effect through week 112,” he said. Differentiating it from other JAK inhibitors, the FDA approval “confirms the safety of this agent as there is no boxed warning,” he added.
Dr. Lebwohl reports financial relationships with more than 30 pharmaceutical companies, including Bristol-Myers Squibb, the manufacturer of deucravacitinib. Dr. Wu has financial relationships with 14 pharmaceutical companies, including Bristol-Myers Squibb, but he was not an investigator for the phase 3 trials of deucravacitinib.
MILAN – The day after deucravacitinib became the first TYK2 inhibitor approved for the treatment of moderate to severe psoriasis, long-term data were presented at the annual congress of the European Academy of Dermatology and Venereology, suggesting that a high degree of benefit persists for at least 2 years, making this oral drug a potential competitor for biologics.
,” said Mark G. Lebwohl, MD, professor of dermatology and dean of clinical therapeutics, Icahn School of Medicine at Mount Sinai, New York.
Just 2 months after the 52-week data from the phase 3 POETYK PSO-1 trial were published online in the Journal of the American Academy of Dermatology, a long-term extension study found essentially no loss of benefit at 112 weeks, according to Dr. Lebwohl.
One of the two co-primary endpoints was a 75% clearance on the Psoriasis and Severity Index (PASI75) score. At 52 weeks, 80.2% of patients on deucravacitinib had met this criterion of benefit. At 112 weeks, the proportion was 84.4%.
The other primary endpoint was a static Physician’s Global Assessment (sPGA) score of clear or almost clear skin. The proportion of patients meeting this criterion at weeks 52 and 112 weeks were 65.6% and 67.6%, respectively.
When assessed by Treatment Failure Rule (TFR) or modified nonresponder imputation (mNRI), results were similar. For both, the primary endpoints at every time interval were just one or two percentage points lower but not clinically meaningfully different, according to Dr. Lebwohl.
The same type of sustained response out to 112 weeks was observed in multiple analyses. When the researchers isolated the subgroup of patients who had achieved a PASI 75 response at 16 weeks (100%), there was a modest decline in the PASI 75 rate at week 52 (90.2%) but then no additional decline at week 112 (91.3%).
There were essentially no changes in the PASI 90 rates at week 16 (63%), week 52 (65.3%), and week 112 (63.1%), Dr. Lebwohl reported. PASI 100 rates, once achieved, were sustained long term.
The target, TYK2, is one of four Janus kinase (JAK) inhibitors. Until now, almost all JAK inhibitors have had greater relative specificity for JAK 1, JAK 2, and JAK 3, but several inhibitors of TYK2 inhibitors other than deucravacitinib are in development for inflammatory diseases. Deucravacitinib (Sotyktu), approved by the Food and Drug Administration on Sept. 9, is the only TYK2 inhibitor with regulatory approval for plaque psoriasis.
In the POETYK PSO-1 trial, 666 patients were initially randomized in a 2:1:1 ratio to 6 mg deucravacitinib (now the approved dose), placebo, or the oral phosphodiesterase 4 inhibitor apremilast. At week 16, patients on placebo were switched over to deucravacitinib. At week 24, patients who did not achieve a PASI 50 on apremilast (which had been titrated to 10 mg daily to 30 mg twice a day over the first 5 days of dosing) were switched to deucravacitinib.
In the previously reported data, deucravacitinib was superior for all efficacy endpoints at week 16, including an analysis of quality of life when compared with placebo (P < .0001) or apremilast (P = .0088). At week 52, after having been switched to deucravacitinib at week 16, patients on placebo achieved comparable responses on the efficacy measures in this study, including PASI75.
Relative to JAK inhibitors commonly used in rheumatoid arthritis and other inflammatory diseases, the greater specificity of deucravacitinib for TYK2 appears to have meaningful safety advantages, according to Dr. Lebwohl. Targeted mostly on the TYK2 regulatory domain, deucravacitinib largely avoids inhibition of the JAK 1, 2, and 3 subtypes. Dr. Lebwohl said this explains why deucravacitinib labeling does not share the boxed warnings about off-target effects, such as those on the cardiovascular system, that can be found in the labeling of other JAK inhibitors.
In the published 52-week data, the discontinuation rate for adverse events was lower in the group randomized to deucravacitinib arm than in the placebo arm. In the extended follow-up, there were no new signals for adverse events, including those involving the CV system or immune function.
The key message so far from the long-term follow-up, which is ongoing, is that “continuous treatment with deucravacitinib is associated with durable efficacy,” Dr. Lebwohl said. It is this combination of sustained efficacy and safety that led Dr. Lebwohl to suggest it as a reasonable oral competitor to injectable biologics.
“Patients now have a choice,” he said.
Jashin J. Wu, MD, a board member of the National Psoriasis Foundation and an associate professor in the department of dermatology, University of Miami, has been following the development of deucravacitinib. He said that the recent FDA approval validates the clinical evidence of benefit and safety, while the long-term data presented at the EADV congress support its role in expanding treatment options.
“Deucravacitinib is a very effective oral agent for moderate to severe plaque psoriasis with strong maintenance of effect through week 112,” he said. Differentiating it from other JAK inhibitors, the FDA approval “confirms the safety of this agent as there is no boxed warning,” he added.
Dr. Lebwohl reports financial relationships with more than 30 pharmaceutical companies, including Bristol-Myers Squibb, the manufacturer of deucravacitinib. Dr. Wu has financial relationships with 14 pharmaceutical companies, including Bristol-Myers Squibb, but he was not an investigator for the phase 3 trials of deucravacitinib.
MILAN – The day after deucravacitinib became the first TYK2 inhibitor approved for the treatment of moderate to severe psoriasis, long-term data were presented at the annual congress of the European Academy of Dermatology and Venereology, suggesting that a high degree of benefit persists for at least 2 years, making this oral drug a potential competitor for biologics.
,” said Mark G. Lebwohl, MD, professor of dermatology and dean of clinical therapeutics, Icahn School of Medicine at Mount Sinai, New York.
Just 2 months after the 52-week data from the phase 3 POETYK PSO-1 trial were published online in the Journal of the American Academy of Dermatology, a long-term extension study found essentially no loss of benefit at 112 weeks, according to Dr. Lebwohl.
One of the two co-primary endpoints was a 75% clearance on the Psoriasis and Severity Index (PASI75) score. At 52 weeks, 80.2% of patients on deucravacitinib had met this criterion of benefit. At 112 weeks, the proportion was 84.4%.
The other primary endpoint was a static Physician’s Global Assessment (sPGA) score of clear or almost clear skin. The proportion of patients meeting this criterion at weeks 52 and 112 weeks were 65.6% and 67.6%, respectively.
When assessed by Treatment Failure Rule (TFR) or modified nonresponder imputation (mNRI), results were similar. For both, the primary endpoints at every time interval were just one or two percentage points lower but not clinically meaningfully different, according to Dr. Lebwohl.
The same type of sustained response out to 112 weeks was observed in multiple analyses. When the researchers isolated the subgroup of patients who had achieved a PASI 75 response at 16 weeks (100%), there was a modest decline in the PASI 75 rate at week 52 (90.2%) but then no additional decline at week 112 (91.3%).
There were essentially no changes in the PASI 90 rates at week 16 (63%), week 52 (65.3%), and week 112 (63.1%), Dr. Lebwohl reported. PASI 100 rates, once achieved, were sustained long term.
The target, TYK2, is one of four Janus kinase (JAK) inhibitors. Until now, almost all JAK inhibitors have had greater relative specificity for JAK 1, JAK 2, and JAK 3, but several inhibitors of TYK2 inhibitors other than deucravacitinib are in development for inflammatory diseases. Deucravacitinib (Sotyktu), approved by the Food and Drug Administration on Sept. 9, is the only TYK2 inhibitor with regulatory approval for plaque psoriasis.
In the POETYK PSO-1 trial, 666 patients were initially randomized in a 2:1:1 ratio to 6 mg deucravacitinib (now the approved dose), placebo, or the oral phosphodiesterase 4 inhibitor apremilast. At week 16, patients on placebo were switched over to deucravacitinib. At week 24, patients who did not achieve a PASI 50 on apremilast (which had been titrated to 10 mg daily to 30 mg twice a day over the first 5 days of dosing) were switched to deucravacitinib.
In the previously reported data, deucravacitinib was superior for all efficacy endpoints at week 16, including an analysis of quality of life when compared with placebo (P < .0001) or apremilast (P = .0088). At week 52, after having been switched to deucravacitinib at week 16, patients on placebo achieved comparable responses on the efficacy measures in this study, including PASI75.
Relative to JAK inhibitors commonly used in rheumatoid arthritis and other inflammatory diseases, the greater specificity of deucravacitinib for TYK2 appears to have meaningful safety advantages, according to Dr. Lebwohl. Targeted mostly on the TYK2 regulatory domain, deucravacitinib largely avoids inhibition of the JAK 1, 2, and 3 subtypes. Dr. Lebwohl said this explains why deucravacitinib labeling does not share the boxed warnings about off-target effects, such as those on the cardiovascular system, that can be found in the labeling of other JAK inhibitors.
In the published 52-week data, the discontinuation rate for adverse events was lower in the group randomized to deucravacitinib arm than in the placebo arm. In the extended follow-up, there were no new signals for adverse events, including those involving the CV system or immune function.
The key message so far from the long-term follow-up, which is ongoing, is that “continuous treatment with deucravacitinib is associated with durable efficacy,” Dr. Lebwohl said. It is this combination of sustained efficacy and safety that led Dr. Lebwohl to suggest it as a reasonable oral competitor to injectable biologics.
“Patients now have a choice,” he said.
Jashin J. Wu, MD, a board member of the National Psoriasis Foundation and an associate professor in the department of dermatology, University of Miami, has been following the development of deucravacitinib. He said that the recent FDA approval validates the clinical evidence of benefit and safety, while the long-term data presented at the EADV congress support its role in expanding treatment options.
“Deucravacitinib is a very effective oral agent for moderate to severe plaque psoriasis with strong maintenance of effect through week 112,” he said. Differentiating it from other JAK inhibitors, the FDA approval “confirms the safety of this agent as there is no boxed warning,” he added.
Dr. Lebwohl reports financial relationships with more than 30 pharmaceutical companies, including Bristol-Myers Squibb, the manufacturer of deucravacitinib. Dr. Wu has financial relationships with 14 pharmaceutical companies, including Bristol-Myers Squibb, but he was not an investigator for the phase 3 trials of deucravacitinib.
AT THE EADV CONGRESS
Optimizing Narrowband UVB Phototherapy: Is It More Challenging for Your Older Patients?
Even with recent pharmacologic treatment advances, narrowband UVB (NB-UVB) phototherapy remains a versatile, safe, and efficacious adjunctive or exclusive treatment for multiple dermatologic conditions, including psoriasis and atopic dermatitis.
In a prior study, Matthews et al13 reported that 96% (50/52) of patients older than 65 years achieved medium to high levels of clearance with NB-UVB phototherapy. Nonetheless, 2 other findings in this study related to the number of treatments required to achieve clearance (ie, clearance rates) and erythema rates prompted further investigation. The first finding was higher-than-expected clearance rates. Older adults had a clearance rate with a mean of 33 treatments compared to prior studies featuring mean clearance rates of 20 to 28 treatments.7,8,14-16 This finding resembled a study in the United Kingdom17 with a median clearance rate in older adults of 30 treatments. In contrast, the median clearance rate from a study in Turkey18 was 42 treatments in older adults. We hypothesized that more photosensitizing medications used in older vs younger adults prompted more dose adjustments with NB-UVB phototherapy to avoid burning (ie, erythema) at baseline and throughout the treatment course. These dose adjustments may have increased the overall clearance rates. If true, we predicted that younger adults treated with the same protocol would have cleared more quickly, either because of age-related differences or because they likely had fewer comorbidities and therefore fewer medications.
The second finding from Matthews et al13 that warranted further investigation was a higher erythema rate compared to the older adult study from the United Kingdom.17 We hypothesized that potentially greater use of photosensitizing medications in the United States could explain the higher erythema rates. Although medication-induced photosensitivity is less likely with NB-UVB phototherapy than with UVA, certain medications can cause UVB photosensitivity, including thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.8,19,20 Therefore, photosensitizing medication use either at baseline or during a course of NB-UVB phototherapy could increase the risk for erythema. Age-related skin changes also have been considered as a
This retrospective study aimed to determine if NB-UVB phototherapy is equally effective in both older and younger adults treated with the same protocol; to examine the association between the use of photosensitizing medications and clearance rates in both older and younger adults; and to examine the association between the use of photosensitizing medications and erythema rates in older vs younger adults.
Methods
Study Design and Patients—This retrospective cohort study used billing records to identify patients who received NB-UVB phototherapy at 3 different clinical sites within a large US health care system in Washington (Group Health Cooperative, now Kaiser Permanente Washington), serving more than 600,000 patients between January 1, 2012, and December 31, 2016. The institutional review board of Kaiser Permanente Washington Health Research Institute approved this study (IRB 1498087-4). Younger adults were classified as those 64 years or younger and older adults as those 65 years and older at the start of their phototherapy regimen. A power analysis determined that the optimal sample size for this study was 250 patients.
Individuals were excluded if they had fewer than 6 phototherapy treatments; a diagnosis of vitiligo, photosensitivity dermatitis, morphea, or pityriasis rubra pilaris; and/or treatment of the hands or feet only.
Phototherapy Protocol—Using a 48-lamp NB-UVB unit, trained phototherapy nurses provided all treatments following standardized treatment protocols13 based on previously published phototherapy guidelines.24 Nurses determined each patient’s disease clearance level using a 3-point clearance scale (high, medium, low).13 Each patient’s starting dose was determined based on the estimated MED for their skin phototype.
Statistical Analysis—Data were analyzed using Stata statistical software (StataCorp LLC). Univariate analyses were used to examine the data and identify outliers, bad values, and missing data, as well as to calculate descriptive statistics. Pearson χ2 and Fisher exact statistics were used to calculate differences in categorical variables. Linear multivariate regression models and logistic multivariate models were used to examine statistical relationships between variables. Statistical significance was defined as P≤.05.
Results
Patient Characteristics—Medical records were reviewed for 172 patients who received phototherapy between 2012 and 2016. Patients ranged in age from 23 to 91 years, with 102 patients 64 years and younger and 70 patients 65 years and older. Tables 1 and 2 outline the patient characteristics and conditions treated.

Phototherapy Effectiveness—
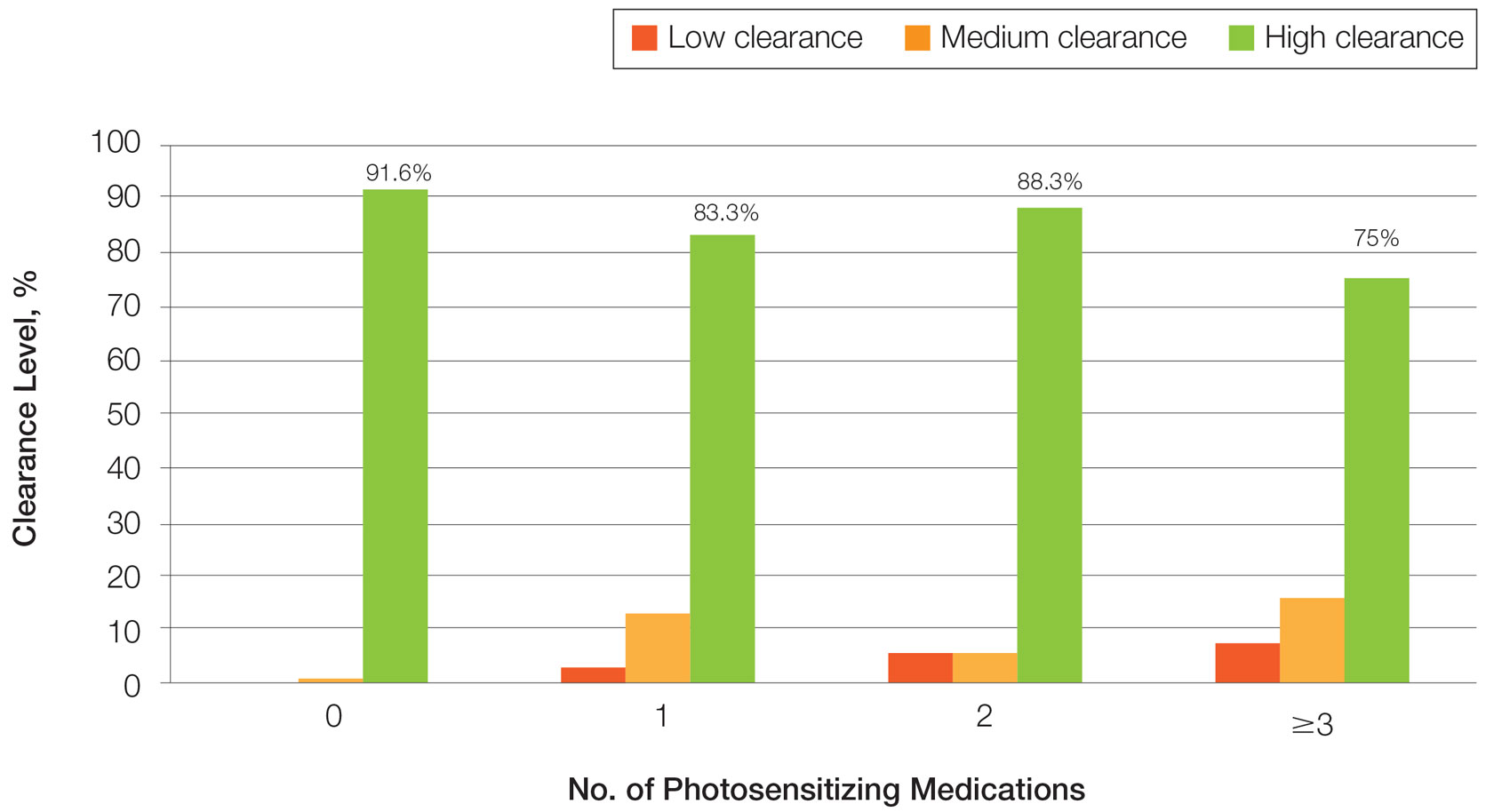
Photosensitizing Medications, Clearance Levels, and Clearance Rates—
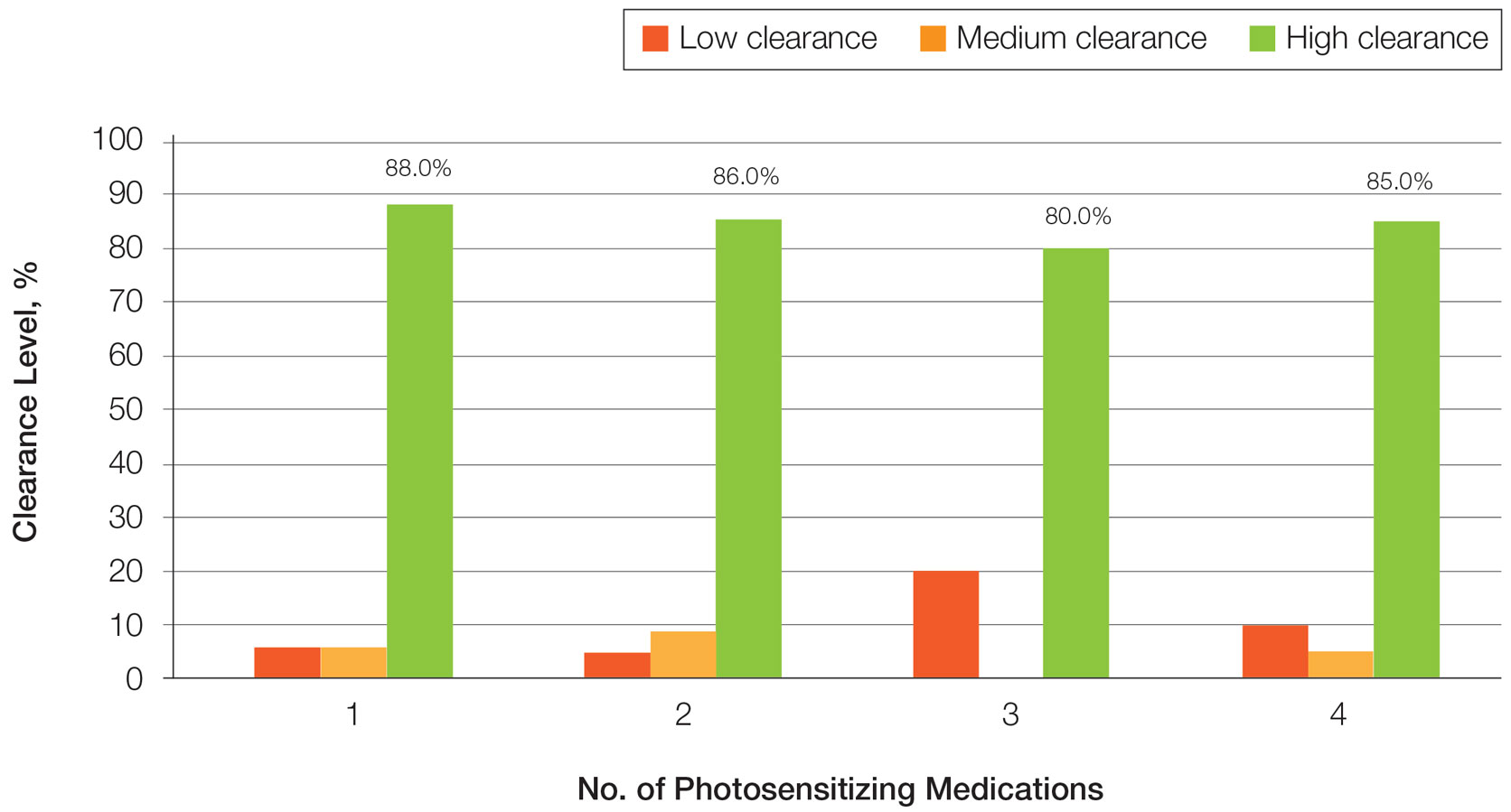
Frequency of Treatments and Clearance Rates—Older adults more consistently completed the recommended frequency of treatments—3 times weekly—compared to younger adults (74.3% vs 58.5%). However, all patients who completed 3 treatments per week required a similar number of treatments to clear (older adults, mean [SD]: 35.7 [21.6]; younger adults, mean [SD]: 34.7 [19.0]; P=.85). Among patients completing 2 or fewer treatments per week, older adults required a mean (SD) of only 31 (9.0) treatments to clear vs 41.5 (21.3) treatments to clear for younger adults, but the difference was not statistically significant (P=.08). However, even those with suboptimal frequency ultimately achieved similar clearance levels.
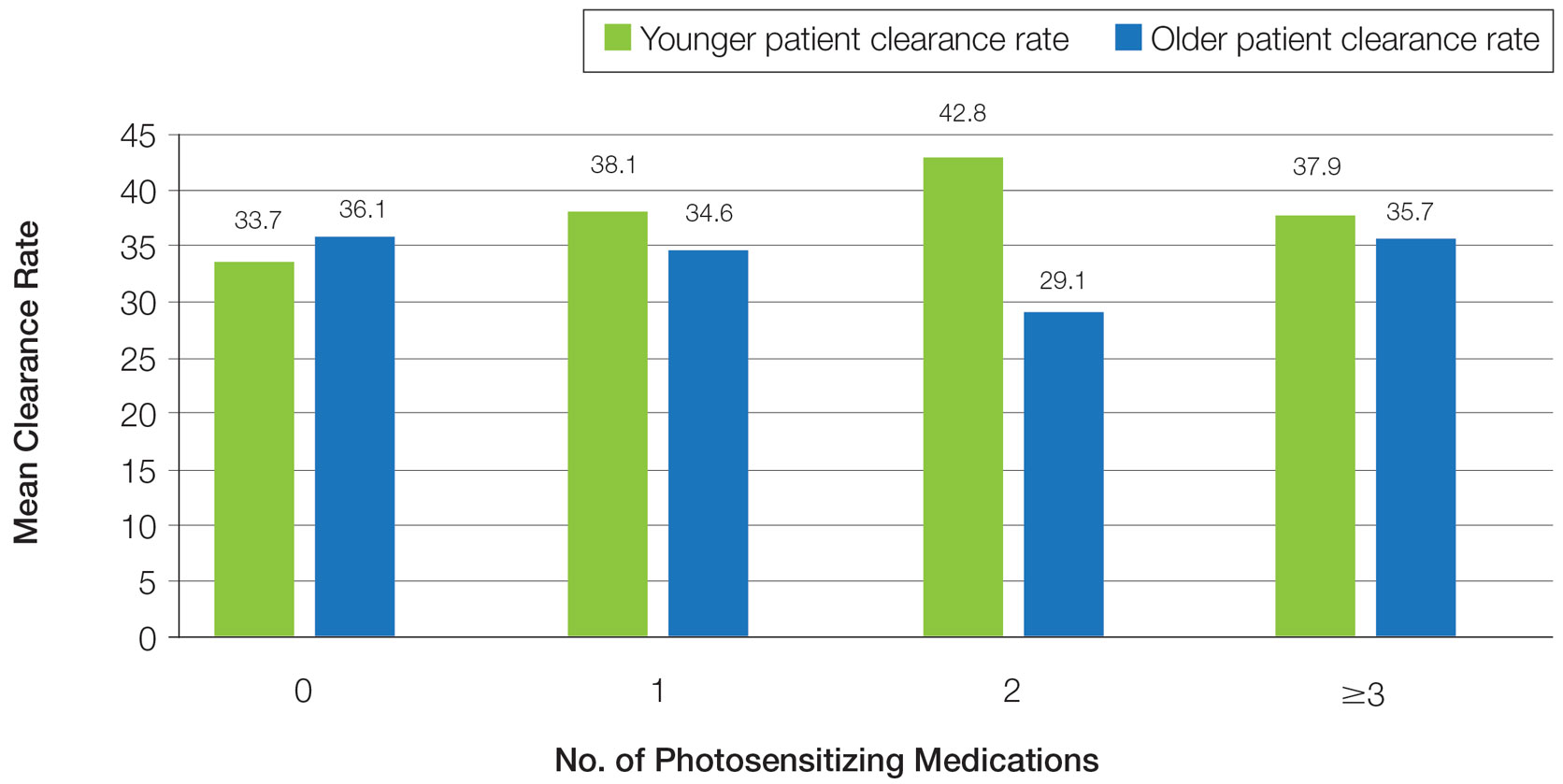
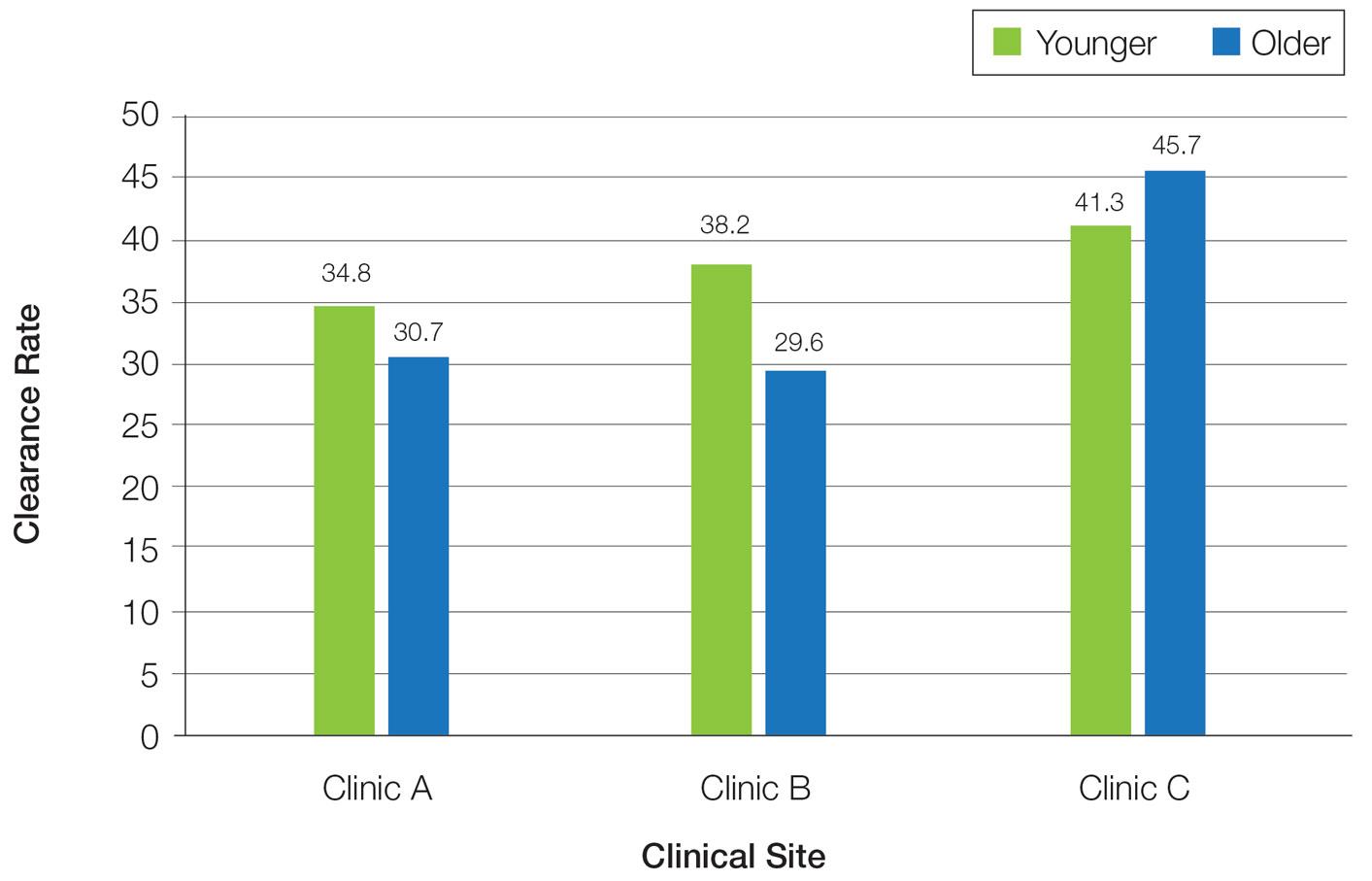
Photosensitizing Medications and Erythema Rates—
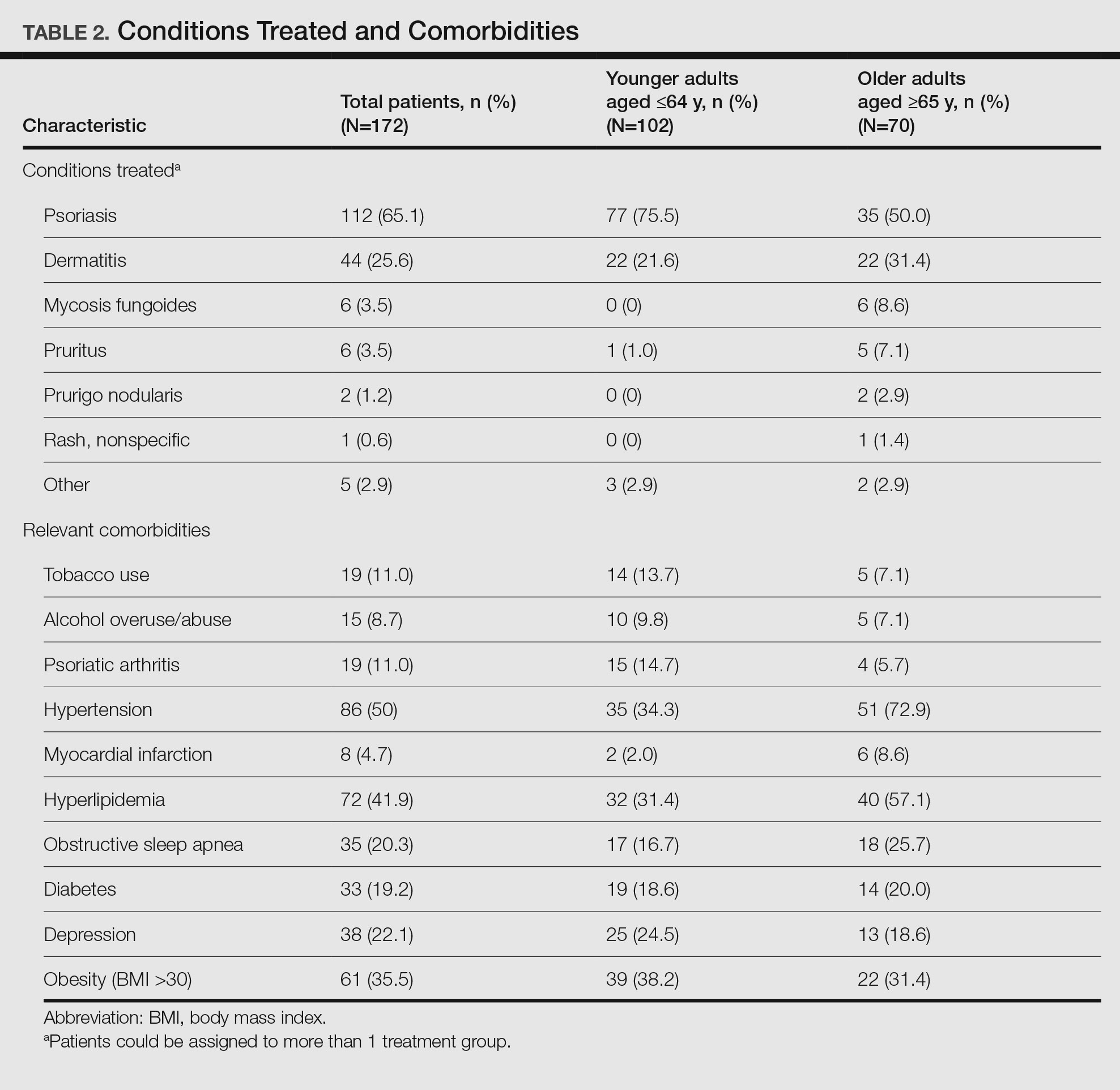
Overall, phototherapy nurses adjusted the starting dose according to the phototype-based protocol an average of 69% of the time for patients on medications with photosensitivity listed as a potential side effect. However, the frequency depended significantly on the clinic (clinic A, 24%; clinic B, 92%; clinic C, 87%)(P≤.001). Nurses across all clinics consistently decreased the treatment dose when patients reported starting new photosensitizing medications. Patients with adjusted starting doses had slightly but not significantly higher clearance rates compared to those without (mean, 37.8 vs 35.5; t(104)=0.58; P=.56).
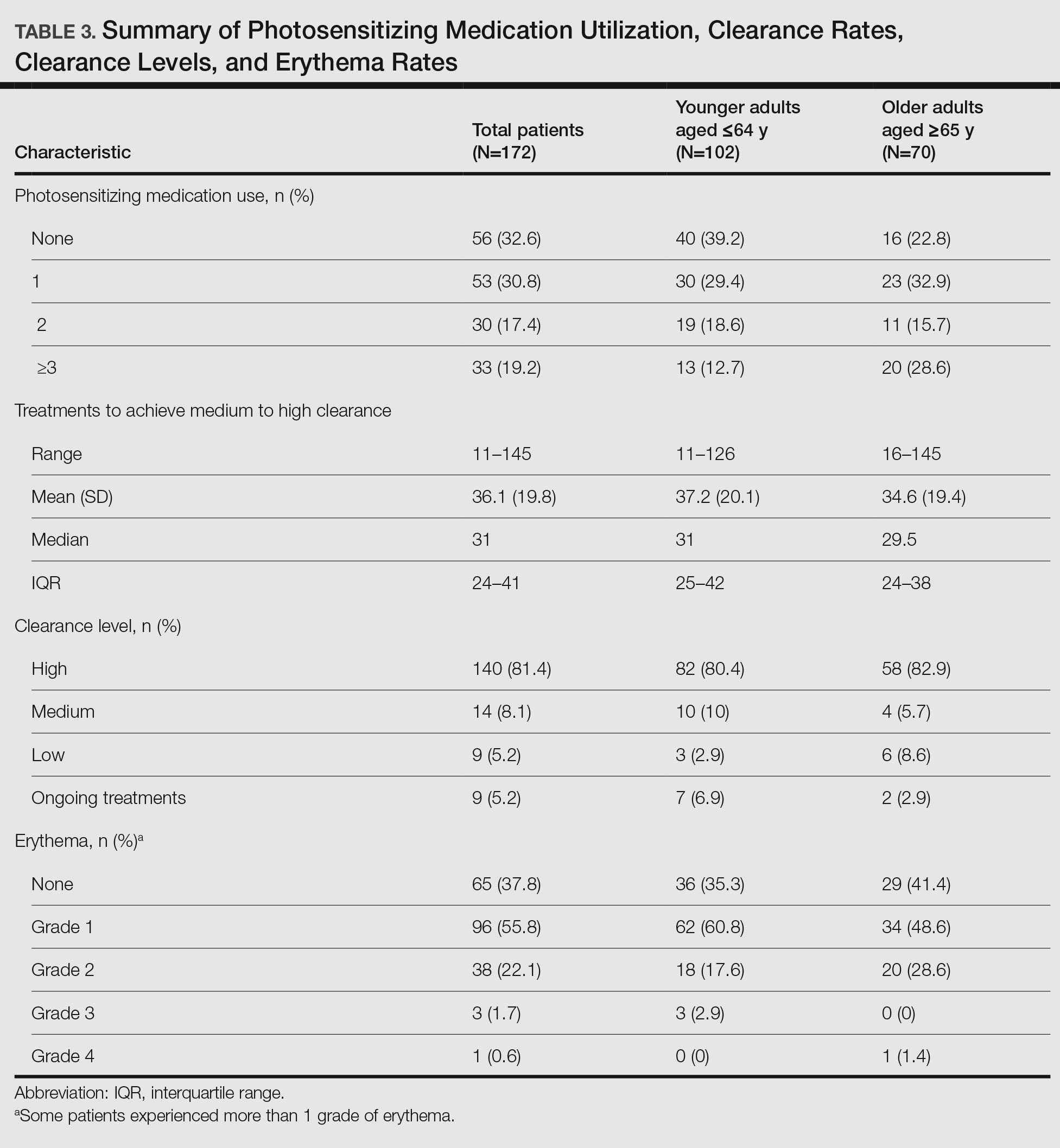
Comment
Impact of Photosensitizing Medications on Clearance—Photosensitizing medications and treatment frequency were 2 factors that might explain the slower clearance rates in younger adults. In this study, both groups of patients used similar numbers of photosensitizing medications, but more older adults were taking 3 or more medications (Table 3). We found no statistically significant relationship between taking photosensitizing medications and either the clearance rates or the level of clearance achieved in either age group.
Impact of Treatment Frequency—Weekly treatment frequency also was examined. One prior study demonstrated that treatments 3 times weekly led to a faster clearance time and higher clearance levels compared with twice-weekly treatment.7 When patients completed treatments twice weekly, it took an average of 1.5 times more days to clear, which impacted cost and clinical resource availability. The patients ranged in age from 17 to 80 years, but outcomes in older patients were not described separately.7 Interestingly, our study seemed to find a difference between age groups when the impact of treatment frequency was examined. Older adults completed nearly 4 fewer mean treatments to clear when treating less often, with more than 80% achieving high levels of clearance, whereas the younger adults required almost 7 more treatments to clear when they came in less frequently, with approximately 80% achieving a high level of clearance. As a result, our study found that in both age groups, slowing the treatment frequency extended the treatment time to clearance—more for the younger adults than the older adults—but did not significantly change the percentage of individuals reaching full clearance in either group.
Erythema Rates—There was no association between photosensitizing medications and erythema rates except when patients were taking at least 3 medications. Most medications that listed photosensitivity as a possible side effect did not specify their relevant range of UV radiation; therefore, all such medications were examined during this analysis. Prior research has shown UVB range photosensitizing medications include thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.19 A sensitivity analysis that focused only on these medications found no association between them and any particular grade of erythema. However, patients taking 3 or more of any medications listing photosensitivity as a side effect had an increased risk for grade 2 erythema.
Erythema rates in this study were consistent with a 2013 systematic review that reported 57% of patients with asymptomatic grade 1 erythema.25 In the 2 other comparative older adult studies, erythema rates varied widely: 35% in a study from Turkey18compared to only1.89% in a study from the United Kingdom.17
The starting dose for NB-UVB may drive erythema rates. The current study’s protocols were based on an estimated MED that is subjectively determined by the dermatology provider’s assessment of the patient’s skin sensitivity via examination and questions to the patient about their response to environmental sun exposure (ie, burning and tanning)26 and is frequently used to determine the starting dose and subsequent dose escalation. Certain medications have been found to increase photosensitivity and erythema,20 which can change an individual’s MED. If photosensitizing medications are started prior to or during a course of NB-UVB without a pretreatment MED, they might increase the risk for erythema. This study did not identify specific erythema-inducing medications but did find that taking 3 or more photosensitizing medications was associated with increased episodes of grade 2 erythema. Similarly, Harrop et al8 found that patients who were taking photosensitizing medications were more likely to have grade 2 or higher erythema, despite baseline MED testing, which is an established safety mechanism to reduce the risk and severity of erythema.14,20,27 The authors of a recent study of older adults in Taiwan specifically recommended MED testing due to the unpredictable influence of polypharmacy on MED calculations in this population.28 Therefore, this study’s use of an estimated MED in older adults may have influenced the starting dose as well as the incidence and severity of erythemic events. Age-related skin changes likely are ruled out as a consideration for mild erythema by the similarity of grade 1 erythema rates in both older and younger adults. Other studies have identified differences between the age groups, where older patients experienced more intense erythema in the late phase of UVB treatments.22,23 This phenomenon could increase the risk for a grade 2 erythema, which may correspond with this study’s findings.
Other potential causes of erythema were ruled out during our study, including erythema related to missed treatments and shielding mishaps. Other factors, however, may impact the level of sensitivity each patient has to phototherapy, including genetics, epigenetics, and cumulative sun damage. With NB-UVB, near-erythemogenic doses are optimal to achieve effective treatments but require a delicate balance to achieve, which may be more problematic for older adults, especially those taking several medications.
Study Limitations—Our study design made it difficult to draw conclusions about rarer dermatologic conditions. Some patients received treatments over years that were not included in the study period. Finally, power calculations suggested that our actual sample size was too small, with approximately one-third of the required sample missing.
Practical Implications—The goals of phototherapy are to achieve a high level of disease clearance with the fewest number of treatments possible and minimal side effects.
The extra staff training and patient monitoring required for MED testing likely is to add value and preserve resources if faster clearance rates could be achieved and may warrant further investigation. Phototherapy centers require standardized treatment protocols, diligent well-trained staff, and program monitoring to ensure consistent care to all patients. This study highlighted the ongoing opportunity for health care organizations to conduct evidence-based practice inquiries to continually optimize care for their patients.
- Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2016;29:19-23.
- Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS One. 2017;12:e0181813.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593.e581-584.
- Cameron H, Dawe RS, Yule S, et al. A randomized, observer-blinded trial of twice vs. three times weekly narrowband ultraviolet B phototherapy for chronic plaque psoriasis. Br J Dermatol. 2002;147:973-978.
- Harrop G, Dawe RS, Ibbotson S. Are photosensitizing medications associated with increased risk of important erythemal reactions during ultraviolet B phototherapy? Br J Dermatol. 2018;179:1184-1185.
- Torres AE, Lyons AB, Hamzavi IH, et al. Role of phototherapy in the era of biologics. J Am Acad Dermatol. 2021;84:479-485.
- Bukvic´ć Mokos Z, Jovic´ A, Cˇeovic´ R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin Biol Ther. 2018;18:897-903.
- Oliveira C, Torres T. More than skin deep: the systemic nature of atopic dermatitis. Eur J Dermatol. 2019;29:250-258.
- Matthews S, Pike K, Chien A. Phototherapy: safe and effective for challenging skin conditions in older adults. Cutis. 2021;108:E15-E21.
- Rodríguez-Granados MT, Estany-Gestal A, Pousa-Martínez M, et al. Is it useful to calculate minimal erythema dose before narrowband UV-B phototherapy? Actas Dermosifiliogr. 2017;108:852-858.
- Parlak N, Kundakci N, Parlak A, et al. Narrowband ultraviolet B phototherapy starting and incremental dose in patients with psoriasis: comparison of percentage dose and fixed dose protocols. Photodermatol Photoimmunol Photomed. 2015;31:90-97.
- Kleinpenning MM, Smits T, Boezeman J, et al. Narrowband ultraviolet B therapy in psoriasis: randomized double-blind comparison of high-dose and low-dose irradiation regimens. Br J Dermatol. 2009;161:1351-1356.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368, ix.
- Cameron H, Dawe RS. Photosensitizing drugs may lower the narrow-band ultraviolet B (TL-01) minimal erythema dose. Br J Dermatol. 2000;142:389-390.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Gloor M, Scherotzke A. Age dependence of ultraviolet light-induced erythema following narrow-band UVB exposure. Photodermatol Photoimmunol Photomed. 2002;18:121-126.
- Cox NH, Diffey BL, Farr PM. The relationship between chronological age and the erythemal response to ultraviolet B radiation. Br J Dermatol. 1992;126:315-319.
- Morrison W. Phototherapy and Photochemotherapy for Skin Disease. 2nd ed. Informa Healthcare; 2005.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Kwon IH, Kwon HH, Na SJ, et al. Could colorimetric method replace the individual minimal erythemal dose (MED) measurements in determining the initial dose of narrow-band UVB treatment for psoriasis patients with skin phototype III-V? J Eur Acad Dermatol Venereol. 2013;27:494-498.
- Chen WA, Chang CM. The minimal erythema dose of narrowband ultraviolet B in elderly Taiwanese [published online September 1, 2021]. Photodermatol Photoimmunol Photomed. doi:10.1111/phpp.12730
Even with recent pharmacologic treatment advances, narrowband UVB (NB-UVB) phototherapy remains a versatile, safe, and efficacious adjunctive or exclusive treatment for multiple dermatologic conditions, including psoriasis and atopic dermatitis.
In a prior study, Matthews et al13 reported that 96% (50/52) of patients older than 65 years achieved medium to high levels of clearance with NB-UVB phototherapy. Nonetheless, 2 other findings in this study related to the number of treatments required to achieve clearance (ie, clearance rates) and erythema rates prompted further investigation. The first finding was higher-than-expected clearance rates. Older adults had a clearance rate with a mean of 33 treatments compared to prior studies featuring mean clearance rates of 20 to 28 treatments.7,8,14-16 This finding resembled a study in the United Kingdom17 with a median clearance rate in older adults of 30 treatments. In contrast, the median clearance rate from a study in Turkey18 was 42 treatments in older adults. We hypothesized that more photosensitizing medications used in older vs younger adults prompted more dose adjustments with NB-UVB phototherapy to avoid burning (ie, erythema) at baseline and throughout the treatment course. These dose adjustments may have increased the overall clearance rates. If true, we predicted that younger adults treated with the same protocol would have cleared more quickly, either because of age-related differences or because they likely had fewer comorbidities and therefore fewer medications.
The second finding from Matthews et al13 that warranted further investigation was a higher erythema rate compared to the older adult study from the United Kingdom.17 We hypothesized that potentially greater use of photosensitizing medications in the United States could explain the higher erythema rates. Although medication-induced photosensitivity is less likely with NB-UVB phototherapy than with UVA, certain medications can cause UVB photosensitivity, including thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.8,19,20 Therefore, photosensitizing medication use either at baseline or during a course of NB-UVB phototherapy could increase the risk for erythema. Age-related skin changes also have been considered as a
This retrospective study aimed to determine if NB-UVB phototherapy is equally effective in both older and younger adults treated with the same protocol; to examine the association between the use of photosensitizing medications and clearance rates in both older and younger adults; and to examine the association between the use of photosensitizing medications and erythema rates in older vs younger adults.
Methods
Study Design and Patients—This retrospective cohort study used billing records to identify patients who received NB-UVB phototherapy at 3 different clinical sites within a large US health care system in Washington (Group Health Cooperative, now Kaiser Permanente Washington), serving more than 600,000 patients between January 1, 2012, and December 31, 2016. The institutional review board of Kaiser Permanente Washington Health Research Institute approved this study (IRB 1498087-4). Younger adults were classified as those 64 years or younger and older adults as those 65 years and older at the start of their phototherapy regimen. A power analysis determined that the optimal sample size for this study was 250 patients.
Individuals were excluded if they had fewer than 6 phototherapy treatments; a diagnosis of vitiligo, photosensitivity dermatitis, morphea, or pityriasis rubra pilaris; and/or treatment of the hands or feet only.
Phototherapy Protocol—Using a 48-lamp NB-UVB unit, trained phototherapy nurses provided all treatments following standardized treatment protocols13 based on previously published phototherapy guidelines.24 Nurses determined each patient’s disease clearance level using a 3-point clearance scale (high, medium, low).13 Each patient’s starting dose was determined based on the estimated MED for their skin phototype.
Statistical Analysis—Data were analyzed using Stata statistical software (StataCorp LLC). Univariate analyses were used to examine the data and identify outliers, bad values, and missing data, as well as to calculate descriptive statistics. Pearson χ2 and Fisher exact statistics were used to calculate differences in categorical variables. Linear multivariate regression models and logistic multivariate models were used to examine statistical relationships between variables. Statistical significance was defined as P≤.05.
Results
Patient Characteristics—Medical records were reviewed for 172 patients who received phototherapy between 2012 and 2016. Patients ranged in age from 23 to 91 years, with 102 patients 64 years and younger and 70 patients 65 years and older. Tables 1 and 2 outline the patient characteristics and conditions treated.

Phototherapy Effectiveness—

Photosensitizing Medications, Clearance Levels, and Clearance Rates—

Frequency of Treatments and Clearance Rates—Older adults more consistently completed the recommended frequency of treatments—3 times weekly—compared to younger adults (74.3% vs 58.5%). However, all patients who completed 3 treatments per week required a similar number of treatments to clear (older adults, mean [SD]: 35.7 [21.6]; younger adults, mean [SD]: 34.7 [19.0]; P=.85). Among patients completing 2 or fewer treatments per week, older adults required a mean (SD) of only 31 (9.0) treatments to clear vs 41.5 (21.3) treatments to clear for younger adults, but the difference was not statistically significant (P=.08). However, even those with suboptimal frequency ultimately achieved similar clearance levels.


Photosensitizing Medications and Erythema Rates—

Overall, phototherapy nurses adjusted the starting dose according to the phototype-based protocol an average of 69% of the time for patients on medications with photosensitivity listed as a potential side effect. However, the frequency depended significantly on the clinic (clinic A, 24%; clinic B, 92%; clinic C, 87%)(P≤.001). Nurses across all clinics consistently decreased the treatment dose when patients reported starting new photosensitizing medications. Patients with adjusted starting doses had slightly but not significantly higher clearance rates compared to those without (mean, 37.8 vs 35.5; t(104)=0.58; P=.56).

Comment
Impact of Photosensitizing Medications on Clearance—Photosensitizing medications and treatment frequency were 2 factors that might explain the slower clearance rates in younger adults. In this study, both groups of patients used similar numbers of photosensitizing medications, but more older adults were taking 3 or more medications (Table 3). We found no statistically significant relationship between taking photosensitizing medications and either the clearance rates or the level of clearance achieved in either age group.
Impact of Treatment Frequency—Weekly treatment frequency also was examined. One prior study demonstrated that treatments 3 times weekly led to a faster clearance time and higher clearance levels compared with twice-weekly treatment.7 When patients completed treatments twice weekly, it took an average of 1.5 times more days to clear, which impacted cost and clinical resource availability. The patients ranged in age from 17 to 80 years, but outcomes in older patients were not described separately.7 Interestingly, our study seemed to find a difference between age groups when the impact of treatment frequency was examined. Older adults completed nearly 4 fewer mean treatments to clear when treating less often, with more than 80% achieving high levels of clearance, whereas the younger adults required almost 7 more treatments to clear when they came in less frequently, with approximately 80% achieving a high level of clearance. As a result, our study found that in both age groups, slowing the treatment frequency extended the treatment time to clearance—more for the younger adults than the older adults—but did not significantly change the percentage of individuals reaching full clearance in either group.
Erythema Rates—There was no association between photosensitizing medications and erythema rates except when patients were taking at least 3 medications. Most medications that listed photosensitivity as a possible side effect did not specify their relevant range of UV radiation; therefore, all such medications were examined during this analysis. Prior research has shown UVB range photosensitizing medications include thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.19 A sensitivity analysis that focused only on these medications found no association between them and any particular grade of erythema. However, patients taking 3 or more of any medications listing photosensitivity as a side effect had an increased risk for grade 2 erythema.
Erythema rates in this study were consistent with a 2013 systematic review that reported 57% of patients with asymptomatic grade 1 erythema.25 In the 2 other comparative older adult studies, erythema rates varied widely: 35% in a study from Turkey18compared to only1.89% in a study from the United Kingdom.17
The starting dose for NB-UVB may drive erythema rates. The current study’s protocols were based on an estimated MED that is subjectively determined by the dermatology provider’s assessment of the patient’s skin sensitivity via examination and questions to the patient about their response to environmental sun exposure (ie, burning and tanning)26 and is frequently used to determine the starting dose and subsequent dose escalation. Certain medications have been found to increase photosensitivity and erythema,20 which can change an individual’s MED. If photosensitizing medications are started prior to or during a course of NB-UVB without a pretreatment MED, they might increase the risk for erythema. This study did not identify specific erythema-inducing medications but did find that taking 3 or more photosensitizing medications was associated with increased episodes of grade 2 erythema. Similarly, Harrop et al8 found that patients who were taking photosensitizing medications were more likely to have grade 2 or higher erythema, despite baseline MED testing, which is an established safety mechanism to reduce the risk and severity of erythema.14,20,27 The authors of a recent study of older adults in Taiwan specifically recommended MED testing due to the unpredictable influence of polypharmacy on MED calculations in this population.28 Therefore, this study’s use of an estimated MED in older adults may have influenced the starting dose as well as the incidence and severity of erythemic events. Age-related skin changes likely are ruled out as a consideration for mild erythema by the similarity of grade 1 erythema rates in both older and younger adults. Other studies have identified differences between the age groups, where older patients experienced more intense erythema in the late phase of UVB treatments.22,23 This phenomenon could increase the risk for a grade 2 erythema, which may correspond with this study’s findings.
Other potential causes of erythema were ruled out during our study, including erythema related to missed treatments and shielding mishaps. Other factors, however, may impact the level of sensitivity each patient has to phototherapy, including genetics, epigenetics, and cumulative sun damage. With NB-UVB, near-erythemogenic doses are optimal to achieve effective treatments but require a delicate balance to achieve, which may be more problematic for older adults, especially those taking several medications.
Study Limitations—Our study design made it difficult to draw conclusions about rarer dermatologic conditions. Some patients received treatments over years that were not included in the study period. Finally, power calculations suggested that our actual sample size was too small, with approximately one-third of the required sample missing.
Practical Implications—The goals of phototherapy are to achieve a high level of disease clearance with the fewest number of treatments possible and minimal side effects.
The extra staff training and patient monitoring required for MED testing likely is to add value and preserve resources if faster clearance rates could be achieved and may warrant further investigation. Phototherapy centers require standardized treatment protocols, diligent well-trained staff, and program monitoring to ensure consistent care to all patients. This study highlighted the ongoing opportunity for health care organizations to conduct evidence-based practice inquiries to continually optimize care for their patients.
Even with recent pharmacologic treatment advances, narrowband UVB (NB-UVB) phototherapy remains a versatile, safe, and efficacious adjunctive or exclusive treatment for multiple dermatologic conditions, including psoriasis and atopic dermatitis.
In a prior study, Matthews et al13 reported that 96% (50/52) of patients older than 65 years achieved medium to high levels of clearance with NB-UVB phototherapy. Nonetheless, 2 other findings in this study related to the number of treatments required to achieve clearance (ie, clearance rates) and erythema rates prompted further investigation. The first finding was higher-than-expected clearance rates. Older adults had a clearance rate with a mean of 33 treatments compared to prior studies featuring mean clearance rates of 20 to 28 treatments.7,8,14-16 This finding resembled a study in the United Kingdom17 with a median clearance rate in older adults of 30 treatments. In contrast, the median clearance rate from a study in Turkey18 was 42 treatments in older adults. We hypothesized that more photosensitizing medications used in older vs younger adults prompted more dose adjustments with NB-UVB phototherapy to avoid burning (ie, erythema) at baseline and throughout the treatment course. These dose adjustments may have increased the overall clearance rates. If true, we predicted that younger adults treated with the same protocol would have cleared more quickly, either because of age-related differences or because they likely had fewer comorbidities and therefore fewer medications.
The second finding from Matthews et al13 that warranted further investigation was a higher erythema rate compared to the older adult study from the United Kingdom.17 We hypothesized that potentially greater use of photosensitizing medications in the United States could explain the higher erythema rates. Although medication-induced photosensitivity is less likely with NB-UVB phototherapy than with UVA, certain medications can cause UVB photosensitivity, including thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.8,19,20 Therefore, photosensitizing medication use either at baseline or during a course of NB-UVB phototherapy could increase the risk for erythema. Age-related skin changes also have been considered as a
This retrospective study aimed to determine if NB-UVB phototherapy is equally effective in both older and younger adults treated with the same protocol; to examine the association between the use of photosensitizing medications and clearance rates in both older and younger adults; and to examine the association between the use of photosensitizing medications and erythema rates in older vs younger adults.
Methods
Study Design and Patients—This retrospective cohort study used billing records to identify patients who received NB-UVB phototherapy at 3 different clinical sites within a large US health care system in Washington (Group Health Cooperative, now Kaiser Permanente Washington), serving more than 600,000 patients between January 1, 2012, and December 31, 2016. The institutional review board of Kaiser Permanente Washington Health Research Institute approved this study (IRB 1498087-4). Younger adults were classified as those 64 years or younger and older adults as those 65 years and older at the start of their phototherapy regimen. A power analysis determined that the optimal sample size for this study was 250 patients.
Individuals were excluded if they had fewer than 6 phototherapy treatments; a diagnosis of vitiligo, photosensitivity dermatitis, morphea, or pityriasis rubra pilaris; and/or treatment of the hands or feet only.
Phototherapy Protocol—Using a 48-lamp NB-UVB unit, trained phototherapy nurses provided all treatments following standardized treatment protocols13 based on previously published phototherapy guidelines.24 Nurses determined each patient’s disease clearance level using a 3-point clearance scale (high, medium, low).13 Each patient’s starting dose was determined based on the estimated MED for their skin phototype.
Statistical Analysis—Data were analyzed using Stata statistical software (StataCorp LLC). Univariate analyses were used to examine the data and identify outliers, bad values, and missing data, as well as to calculate descriptive statistics. Pearson χ2 and Fisher exact statistics were used to calculate differences in categorical variables. Linear multivariate regression models and logistic multivariate models were used to examine statistical relationships between variables. Statistical significance was defined as P≤.05.
Results
Patient Characteristics—Medical records were reviewed for 172 patients who received phototherapy between 2012 and 2016. Patients ranged in age from 23 to 91 years, with 102 patients 64 years and younger and 70 patients 65 years and older. Tables 1 and 2 outline the patient characteristics and conditions treated.

Phototherapy Effectiveness—

Photosensitizing Medications, Clearance Levels, and Clearance Rates—

Frequency of Treatments and Clearance Rates—Older adults more consistently completed the recommended frequency of treatments—3 times weekly—compared to younger adults (74.3% vs 58.5%). However, all patients who completed 3 treatments per week required a similar number of treatments to clear (older adults, mean [SD]: 35.7 [21.6]; younger adults, mean [SD]: 34.7 [19.0]; P=.85). Among patients completing 2 or fewer treatments per week, older adults required a mean (SD) of only 31 (9.0) treatments to clear vs 41.5 (21.3) treatments to clear for younger adults, but the difference was not statistically significant (P=.08). However, even those with suboptimal frequency ultimately achieved similar clearance levels.


Photosensitizing Medications and Erythema Rates—

Overall, phototherapy nurses adjusted the starting dose according to the phototype-based protocol an average of 69% of the time for patients on medications with photosensitivity listed as a potential side effect. However, the frequency depended significantly on the clinic (clinic A, 24%; clinic B, 92%; clinic C, 87%)(P≤.001). Nurses across all clinics consistently decreased the treatment dose when patients reported starting new photosensitizing medications. Patients with adjusted starting doses had slightly but not significantly higher clearance rates compared to those without (mean, 37.8 vs 35.5; t(104)=0.58; P=.56).

Comment
Impact of Photosensitizing Medications on Clearance—Photosensitizing medications and treatment frequency were 2 factors that might explain the slower clearance rates in younger adults. In this study, both groups of patients used similar numbers of photosensitizing medications, but more older adults were taking 3 or more medications (Table 3). We found no statistically significant relationship between taking photosensitizing medications and either the clearance rates or the level of clearance achieved in either age group.
Impact of Treatment Frequency—Weekly treatment frequency also was examined. One prior study demonstrated that treatments 3 times weekly led to a faster clearance time and higher clearance levels compared with twice-weekly treatment.7 When patients completed treatments twice weekly, it took an average of 1.5 times more days to clear, which impacted cost and clinical resource availability. The patients ranged in age from 17 to 80 years, but outcomes in older patients were not described separately.7 Interestingly, our study seemed to find a difference between age groups when the impact of treatment frequency was examined. Older adults completed nearly 4 fewer mean treatments to clear when treating less often, with more than 80% achieving high levels of clearance, whereas the younger adults required almost 7 more treatments to clear when they came in less frequently, with approximately 80% achieving a high level of clearance. As a result, our study found that in both age groups, slowing the treatment frequency extended the treatment time to clearance—more for the younger adults than the older adults—but did not significantly change the percentage of individuals reaching full clearance in either group.
Erythema Rates—There was no association between photosensitizing medications and erythema rates except when patients were taking at least 3 medications. Most medications that listed photosensitivity as a possible side effect did not specify their relevant range of UV radiation; therefore, all such medications were examined during this analysis. Prior research has shown UVB range photosensitizing medications include thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.19 A sensitivity analysis that focused only on these medications found no association between them and any particular grade of erythema. However, patients taking 3 or more of any medications listing photosensitivity as a side effect had an increased risk for grade 2 erythema.
Erythema rates in this study were consistent with a 2013 systematic review that reported 57% of patients with asymptomatic grade 1 erythema.25 In the 2 other comparative older adult studies, erythema rates varied widely: 35% in a study from Turkey18compared to only1.89% in a study from the United Kingdom.17
The starting dose for NB-UVB may drive erythema rates. The current study’s protocols were based on an estimated MED that is subjectively determined by the dermatology provider’s assessment of the patient’s skin sensitivity via examination and questions to the patient about their response to environmental sun exposure (ie, burning and tanning)26 and is frequently used to determine the starting dose and subsequent dose escalation. Certain medications have been found to increase photosensitivity and erythema,20 which can change an individual’s MED. If photosensitizing medications are started prior to or during a course of NB-UVB without a pretreatment MED, they might increase the risk for erythema. This study did not identify specific erythema-inducing medications but did find that taking 3 or more photosensitizing medications was associated with increased episodes of grade 2 erythema. Similarly, Harrop et al8 found that patients who were taking photosensitizing medications were more likely to have grade 2 or higher erythema, despite baseline MED testing, which is an established safety mechanism to reduce the risk and severity of erythema.14,20,27 The authors of a recent study of older adults in Taiwan specifically recommended MED testing due to the unpredictable influence of polypharmacy on MED calculations in this population.28 Therefore, this study’s use of an estimated MED in older adults may have influenced the starting dose as well as the incidence and severity of erythemic events. Age-related skin changes likely are ruled out as a consideration for mild erythema by the similarity of grade 1 erythema rates in both older and younger adults. Other studies have identified differences between the age groups, where older patients experienced more intense erythema in the late phase of UVB treatments.22,23 This phenomenon could increase the risk for a grade 2 erythema, which may correspond with this study’s findings.
Other potential causes of erythema were ruled out during our study, including erythema related to missed treatments and shielding mishaps. Other factors, however, may impact the level of sensitivity each patient has to phototherapy, including genetics, epigenetics, and cumulative sun damage. With NB-UVB, near-erythemogenic doses are optimal to achieve effective treatments but require a delicate balance to achieve, which may be more problematic for older adults, especially those taking several medications.
Study Limitations—Our study design made it difficult to draw conclusions about rarer dermatologic conditions. Some patients received treatments over years that were not included in the study period. Finally, power calculations suggested that our actual sample size was too small, with approximately one-third of the required sample missing.
Practical Implications—The goals of phototherapy are to achieve a high level of disease clearance with the fewest number of treatments possible and minimal side effects.
The extra staff training and patient monitoring required for MED testing likely is to add value and preserve resources if faster clearance rates could be achieved and may warrant further investigation. Phototherapy centers require standardized treatment protocols, diligent well-trained staff, and program monitoring to ensure consistent care to all patients. This study highlighted the ongoing opportunity for health care organizations to conduct evidence-based practice inquiries to continually optimize care for their patients.
- Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2016;29:19-23.
- Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS One. 2017;12:e0181813.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593.e581-584.
- Cameron H, Dawe RS, Yule S, et al. A randomized, observer-blinded trial of twice vs. three times weekly narrowband ultraviolet B phototherapy for chronic plaque psoriasis. Br J Dermatol. 2002;147:973-978.
- Harrop G, Dawe RS, Ibbotson S. Are photosensitizing medications associated with increased risk of important erythemal reactions during ultraviolet B phototherapy? Br J Dermatol. 2018;179:1184-1185.
- Torres AE, Lyons AB, Hamzavi IH, et al. Role of phototherapy in the era of biologics. J Am Acad Dermatol. 2021;84:479-485.
- Bukvic´ć Mokos Z, Jovic´ A, Cˇeovic´ R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin Biol Ther. 2018;18:897-903.
- Oliveira C, Torres T. More than skin deep: the systemic nature of atopic dermatitis. Eur J Dermatol. 2019;29:250-258.
- Matthews S, Pike K, Chien A. Phototherapy: safe and effective for challenging skin conditions in older adults. Cutis. 2021;108:E15-E21.
- Rodríguez-Granados MT, Estany-Gestal A, Pousa-Martínez M, et al. Is it useful to calculate minimal erythema dose before narrowband UV-B phototherapy? Actas Dermosifiliogr. 2017;108:852-858.
- Parlak N, Kundakci N, Parlak A, et al. Narrowband ultraviolet B phototherapy starting and incremental dose in patients with psoriasis: comparison of percentage dose and fixed dose protocols. Photodermatol Photoimmunol Photomed. 2015;31:90-97.
- Kleinpenning MM, Smits T, Boezeman J, et al. Narrowband ultraviolet B therapy in psoriasis: randomized double-blind comparison of high-dose and low-dose irradiation regimens. Br J Dermatol. 2009;161:1351-1356.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368, ix.
- Cameron H, Dawe RS. Photosensitizing drugs may lower the narrow-band ultraviolet B (TL-01) minimal erythema dose. Br J Dermatol. 2000;142:389-390.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Gloor M, Scherotzke A. Age dependence of ultraviolet light-induced erythema following narrow-band UVB exposure. Photodermatol Photoimmunol Photomed. 2002;18:121-126.
- Cox NH, Diffey BL, Farr PM. The relationship between chronological age and the erythemal response to ultraviolet B radiation. Br J Dermatol. 1992;126:315-319.
- Morrison W. Phototherapy and Photochemotherapy for Skin Disease. 2nd ed. Informa Healthcare; 2005.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Kwon IH, Kwon HH, Na SJ, et al. Could colorimetric method replace the individual minimal erythemal dose (MED) measurements in determining the initial dose of narrow-band UVB treatment for psoriasis patients with skin phototype III-V? J Eur Acad Dermatol Venereol. 2013;27:494-498.
- Chen WA, Chang CM. The minimal erythema dose of narrowband ultraviolet B in elderly Taiwanese [published online September 1, 2021]. Photodermatol Photoimmunol Photomed. doi:10.1111/phpp.12730
- Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2016;29:19-23.
- Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS One. 2017;12:e0181813.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593.e581-584.
- Cameron H, Dawe RS, Yule S, et al. A randomized, observer-blinded trial of twice vs. three times weekly narrowband ultraviolet B phototherapy for chronic plaque psoriasis. Br J Dermatol. 2002;147:973-978.
- Harrop G, Dawe RS, Ibbotson S. Are photosensitizing medications associated with increased risk of important erythemal reactions during ultraviolet B phototherapy? Br J Dermatol. 2018;179:1184-1185.
- Torres AE, Lyons AB, Hamzavi IH, et al. Role of phototherapy in the era of biologics. J Am Acad Dermatol. 2021;84:479-485.
- Bukvic´ć Mokos Z, Jovic´ A, Cˇeovic´ R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin Biol Ther. 2018;18:897-903.
- Oliveira C, Torres T. More than skin deep: the systemic nature of atopic dermatitis. Eur J Dermatol. 2019;29:250-258.
- Matthews S, Pike K, Chien A. Phototherapy: safe and effective for challenging skin conditions in older adults. Cutis. 2021;108:E15-E21.
- Rodríguez-Granados MT, Estany-Gestal A, Pousa-Martínez M, et al. Is it useful to calculate minimal erythema dose before narrowband UV-B phototherapy? Actas Dermosifiliogr. 2017;108:852-858.
- Parlak N, Kundakci N, Parlak A, et al. Narrowband ultraviolet B phototherapy starting and incremental dose in patients with psoriasis: comparison of percentage dose and fixed dose protocols. Photodermatol Photoimmunol Photomed. 2015;31:90-97.
- Kleinpenning MM, Smits T, Boezeman J, et al. Narrowband ultraviolet B therapy in psoriasis: randomized double-blind comparison of high-dose and low-dose irradiation regimens. Br J Dermatol. 2009;161:1351-1356.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368, ix.
- Cameron H, Dawe RS. Photosensitizing drugs may lower the narrow-band ultraviolet B (TL-01) minimal erythema dose. Br J Dermatol. 2000;142:389-390.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Gloor M, Scherotzke A. Age dependence of ultraviolet light-induced erythema following narrow-band UVB exposure. Photodermatol Photoimmunol Photomed. 2002;18:121-126.
- Cox NH, Diffey BL, Farr PM. The relationship between chronological age and the erythemal response to ultraviolet B radiation. Br J Dermatol. 1992;126:315-319.
- Morrison W. Phototherapy and Photochemotherapy for Skin Disease. 2nd ed. Informa Healthcare; 2005.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Kwon IH, Kwon HH, Na SJ, et al. Could colorimetric method replace the individual minimal erythemal dose (MED) measurements in determining the initial dose of narrow-band UVB treatment for psoriasis patients with skin phototype III-V? J Eur Acad Dermatol Venereol. 2013;27:494-498.
- Chen WA, Chang CM. The minimal erythema dose of narrowband ultraviolet B in elderly Taiwanese [published online September 1, 2021]. Photodermatol Photoimmunol Photomed. doi:10.1111/phpp.12730
Practice Points
- Narrowband UVB (NB-UVB) phototherapy remains a safe and efficacious nonpharmacologic treatment for dermatologic conditions in older and younger adults.
- Compared to younger adults, older adults using the same protocols need similar or even fewer treatments to achieve high levels of clearance.
- Individuals taking 3 or more photosensitizing medications, regardless of age, may be at higher risk for substantial erythema with NB-UVB phototherapy.
- Phototherapy program monitoring is important to ensure quality care and investigate opportunities for care optimization.
FDA approves oral TYK2 inhibitor deucravacitinib for treating psoriasis
the manufacturer announced on Sept. 9.
Deucravacitinib targets TYK2, which inhibits signaling of interleukin-23, interleukin-12, and type 1 interferons, key cytokines involved in the pathogenesis of multiple immune-mediated diseases, according to Bristol Myers Squibb (BMS). This is the first approval for deucravacitinib, which will be marketed as Sotyktu, and the first drug in this class to be approved.
It is also currently under review for the same indication in Europe and Japan, and elsewhere, and for treating pustular psoriasis and erythrodermic psoriasis in Japan.
FDA approval was based on the results of POETYK PSO-1 and POETYK PSO-2, phase 3 trials of almost 1,700 adults with moderate to severe plaque psoriasis. In these studies, treatment with once-daily deucravacitinib showed significant and clinically meaningful improvements in skin clearance and symptoms, compared with placebo and with apremilast (Otezla), according to the company.
In the two studies, patients were randomly assigned to receive 6 mg daily of deucravacitinib, placebo, or a 30-mg twice-daily dose of apremilast, the oral phosphodiesterase 4 inhibitor approved for psoriasis. The primary endpoints were the percentage of patients who achieved a Psoriasis Area and Severity Index (PASI) 75 response and a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear) at 16 weeks.
At 16 weeks, 58% and 53% of patients receiving deucravacitinib in the POETYK PSO-1 and POETYK PSO-2 studies, respectively, achieved PASI 75 response, compared with 13% and 9% of those receiving placebo (P < .0001 for both) and 35% and 40% receiving apremilast (P < .0001, P = .0004, respectively), according to the company’s announcement of the approval. PASI 75 responses were maintained through 52 weeks among the patients who remained on treatment, in both studies, according to BMS.
In the POETYK PSO-1 and PSO-2 studies, respectively, 54% and 50% of those on deucravacitinib achieved an sPGA of 0/1 at 16 weeks, compared with 7% and 9% of those receiving placebo (P < .0001 for both) and 32% and 34% of those receiving apremilast (P < .0001 for both).
Across the two studies, at 16 weeks, the most common adverse events that affected at least 1% of patients on deucravacitinib and that occurred at higher rates than in the placebo group were upper respiratory infections (19.2%), increases in serum creatine phosphokinase (2.7%), herpes simplex (2%), mouth ulcers (1.9%), folliculitis (1.7%), and acne (1.4%). Adverse events resulting in discontinuation of treatment were reported in 2.4% of persons receiving deucravacitinib and 5.2% of those receiving apremilast, compared with 3.8% of those receiving placebo.
Up to 16 weeks, according to the BMS statement, 28% of persons receiving deucravacitinib had infections, most of which were mild to moderate and not serious and did not result in stopping treatment, compared with 22% of those receiving placebo. In addition, five patients treated with deucravacitinib and five patients receiving placebo had serious infections, and three patients receiving deucravacitinib had cancer (not including nonmelanoma skin cancer).
Deucravacitinib is also being evaluated in clinical trials for psoriatic arthritis, lupus, and inflammatory bowel disease. It is not recommended for use in combination with other potent immunosuppressants, according to BMS.
The prescribing information and patient medication guide are available online.
The POETYK PSO-1 and POETYK PSO-2 studies were funded by Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
the manufacturer announced on Sept. 9.
Deucravacitinib targets TYK2, which inhibits signaling of interleukin-23, interleukin-12, and type 1 interferons, key cytokines involved in the pathogenesis of multiple immune-mediated diseases, according to Bristol Myers Squibb (BMS). This is the first approval for deucravacitinib, which will be marketed as Sotyktu, and the first drug in this class to be approved.
It is also currently under review for the same indication in Europe and Japan, and elsewhere, and for treating pustular psoriasis and erythrodermic psoriasis in Japan.
FDA approval was based on the results of POETYK PSO-1 and POETYK PSO-2, phase 3 trials of almost 1,700 adults with moderate to severe plaque psoriasis. In these studies, treatment with once-daily deucravacitinib showed significant and clinically meaningful improvements in skin clearance and symptoms, compared with placebo and with apremilast (Otezla), according to the company.
In the two studies, patients were randomly assigned to receive 6 mg daily of deucravacitinib, placebo, or a 30-mg twice-daily dose of apremilast, the oral phosphodiesterase 4 inhibitor approved for psoriasis. The primary endpoints were the percentage of patients who achieved a Psoriasis Area and Severity Index (PASI) 75 response and a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear) at 16 weeks.
At 16 weeks, 58% and 53% of patients receiving deucravacitinib in the POETYK PSO-1 and POETYK PSO-2 studies, respectively, achieved PASI 75 response, compared with 13% and 9% of those receiving placebo (P < .0001 for both) and 35% and 40% receiving apremilast (P < .0001, P = .0004, respectively), according to the company’s announcement of the approval. PASI 75 responses were maintained through 52 weeks among the patients who remained on treatment, in both studies, according to BMS.
In the POETYK PSO-1 and PSO-2 studies, respectively, 54% and 50% of those on deucravacitinib achieved an sPGA of 0/1 at 16 weeks, compared with 7% and 9% of those receiving placebo (P < .0001 for both) and 32% and 34% of those receiving apremilast (P < .0001 for both).
Across the two studies, at 16 weeks, the most common adverse events that affected at least 1% of patients on deucravacitinib and that occurred at higher rates than in the placebo group were upper respiratory infections (19.2%), increases in serum creatine phosphokinase (2.7%), herpes simplex (2%), mouth ulcers (1.9%), folliculitis (1.7%), and acne (1.4%). Adverse events resulting in discontinuation of treatment were reported in 2.4% of persons receiving deucravacitinib and 5.2% of those receiving apremilast, compared with 3.8% of those receiving placebo.
Up to 16 weeks, according to the BMS statement, 28% of persons receiving deucravacitinib had infections, most of which were mild to moderate and not serious and did not result in stopping treatment, compared with 22% of those receiving placebo. In addition, five patients treated with deucravacitinib and five patients receiving placebo had serious infections, and three patients receiving deucravacitinib had cancer (not including nonmelanoma skin cancer).
Deucravacitinib is also being evaluated in clinical trials for psoriatic arthritis, lupus, and inflammatory bowel disease. It is not recommended for use in combination with other potent immunosuppressants, according to BMS.
The prescribing information and patient medication guide are available online.
The POETYK PSO-1 and POETYK PSO-2 studies were funded by Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
the manufacturer announced on Sept. 9.
Deucravacitinib targets TYK2, which inhibits signaling of interleukin-23, interleukin-12, and type 1 interferons, key cytokines involved in the pathogenesis of multiple immune-mediated diseases, according to Bristol Myers Squibb (BMS). This is the first approval for deucravacitinib, which will be marketed as Sotyktu, and the first drug in this class to be approved.
It is also currently under review for the same indication in Europe and Japan, and elsewhere, and for treating pustular psoriasis and erythrodermic psoriasis in Japan.
FDA approval was based on the results of POETYK PSO-1 and POETYK PSO-2, phase 3 trials of almost 1,700 adults with moderate to severe plaque psoriasis. In these studies, treatment with once-daily deucravacitinib showed significant and clinically meaningful improvements in skin clearance and symptoms, compared with placebo and with apremilast (Otezla), according to the company.
In the two studies, patients were randomly assigned to receive 6 mg daily of deucravacitinib, placebo, or a 30-mg twice-daily dose of apremilast, the oral phosphodiesterase 4 inhibitor approved for psoriasis. The primary endpoints were the percentage of patients who achieved a Psoriasis Area and Severity Index (PASI) 75 response and a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear) at 16 weeks.
At 16 weeks, 58% and 53% of patients receiving deucravacitinib in the POETYK PSO-1 and POETYK PSO-2 studies, respectively, achieved PASI 75 response, compared with 13% and 9% of those receiving placebo (P < .0001 for both) and 35% and 40% receiving apremilast (P < .0001, P = .0004, respectively), according to the company’s announcement of the approval. PASI 75 responses were maintained through 52 weeks among the patients who remained on treatment, in both studies, according to BMS.
In the POETYK PSO-1 and PSO-2 studies, respectively, 54% and 50% of those on deucravacitinib achieved an sPGA of 0/1 at 16 weeks, compared with 7% and 9% of those receiving placebo (P < .0001 for both) and 32% and 34% of those receiving apremilast (P < .0001 for both).
Across the two studies, at 16 weeks, the most common adverse events that affected at least 1% of patients on deucravacitinib and that occurred at higher rates than in the placebo group were upper respiratory infections (19.2%), increases in serum creatine phosphokinase (2.7%), herpes simplex (2%), mouth ulcers (1.9%), folliculitis (1.7%), and acne (1.4%). Adverse events resulting in discontinuation of treatment were reported in 2.4% of persons receiving deucravacitinib and 5.2% of those receiving apremilast, compared with 3.8% of those receiving placebo.
Up to 16 weeks, according to the BMS statement, 28% of persons receiving deucravacitinib had infections, most of which were mild to moderate and not serious and did not result in stopping treatment, compared with 22% of those receiving placebo. In addition, five patients treated with deucravacitinib and five patients receiving placebo had serious infections, and three patients receiving deucravacitinib had cancer (not including nonmelanoma skin cancer).
Deucravacitinib is also being evaluated in clinical trials for psoriatic arthritis, lupus, and inflammatory bowel disease. It is not recommended for use in combination with other potent immunosuppressants, according to BMS.
The prescribing information and patient medication guide are available online.
The POETYK PSO-1 and POETYK PSO-2 studies were funded by Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
Surgical Deroofing for Hidradenitis Suppurativa
Practice Gap
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by inflammatory nodules, abscesses, sinus tracts, fistulae, and scarring, mainly in intertriginous areas. The extent of disease—classified using the Hurley staging system (stages I–III)—helps guide treatment, which includes medical management and surgical intervention in later stages.
First-line treatment of HS includes topical or systemic medications, or both. Surgical therapy typically is reserved for refractory HS in moderate to severe disease (Hurley stages II and III) and is combined with pharmacotherapy. Specifically, clinical management guidelines issued by an expert committee of the United States and Canadian Hidradenitis Suppurativa Foundations recommend excision or deroofing for recurrent nodules and tunnels.1
Surgical options for HS that are available to the outpatient dermatologist include incision and drainage, electrosurgery, CO2 laser evaporation, excision, and deroofing (also known as unroofing).2 Deroofing is a fairly novel therapy; many dermatologists are unfamiliar with the procedure. A PubMed search of articles indexed for MEDLINE related to HS prior to 2010 revealed only 1 article containing the word deroofing and only 4 articles containing unroofing.
The pathophysiology of HS has important implications for successful treatment. Inflammation of the follicular pilosebaceous unit along with follicular occlusion create challenges with treatment.3 It is postulated that a defect in the glassy membrane of the infra-infundibular wall predisposes the pilosebaceous follicle to lose its structural integrality as pressure builds from plugging of the duct,4 which can result in the clinical hallmarks of HS including tunneling tracts, bridging nodules, abscesses, and fistulae that form with lateral expansion of the plugged follicle.
Leaking of the contents of these plugged follicles into surrounding tissue produces an inflammatory response in characteristic HS lesions. Because debris within the lesions moves laterally instead of being able to burst to the surface, the lesions have difficulty fully healing. Unroofing the lesions and removing built-up debris allows them to heal more expediently and quiets the underlying immune response by removing the stimulus.4
Herein, we describe the benefits, risks, and surgical process of deroofing for HS.
Technique and Tools
Deroofing is performed under local anesthesia, stepwise as follows:
1. Identify sinus tracts and infiltrate the area with lidocaine (Figure, A).
2. Use a blunt probe to define the borders of the area to be unroofed and to evaluate for any communicating sinus tracts (Figure, B).
3. Remove the roof of underlying abscesses and tracts, using a probe as a guide (Figure, C).
4. Enter through the skin or sinus opening using electrocautery or with a scalpel or scissors; perform blunt dissection.
5. Reflect back the entirety of skin overlying the probed areas and remove the skin to expose the base of the lesion (Figure, D).
6. Explore the exposed base and walls of the lesion with the probe again to assess for hidden tracts; take care not to create false tracts.
7. Debride the surgical wound using curettage or rough gauze grattage to remove remaining inflammatory debris or biofilm. To achieve hemostasis, apply aluminum chloride or ferric chloride. Coat the wound with petroleum jelly and gauze and allow it to heal by secondary intention.
8. Educate the patient on wound care—once-daily gentle cleansing with soap and water, followed by application of a moist dressing—which is similar to wound healing by secondary intention from other causes.2,4

Practice Implications
A deroofing procedure has many benefits compared to other surgical modalities for the treatment of HS. Deroofing requires only a probe, curette, and electrocautery device, making the procedure more cost-effective than excision, which requires a full tray of equipment and sutures. Furthermore, margins do not need to be taken with deroofing, and no undermining or closure is needed, which saves time during the operation and minimizes the risk for complications, including dehiscence and formation of new sinus tracts.4 No specialized equipment, such as a CO2 laser, is required, which makes deroofing accessible to every clinical dermatologist in any demographic or geographic setting.
Evidence of Benefit—Saylor and colleagues5 found that deroofing carries a 12.5% complication rate, which includes postoperative bleeding, hypergranulation tissue, and rarely wound infection. This rate is significantly lower than the 26% complication rate associated with local excision, which includes wound dehiscence, infection, and contracture (P<.001). Deroofing also was found to have an HS recurrence rate of 14.5%, which is significantly less than the 30% recurrence rate seen with local excision (P=.015). Saylor et al5 also concluded that incision and drainage was recommended only for immediate relief of HS because of its 100% recurrence rate.
van der Zee2 reported on 88 lesions from 44 patients that were treated by surgical deroofing, resulting in an average defect of 3.0 cm in length and a mean healing time of 14 days. The typical outcome was cosmetically acceptable scarring; this finding was supported by a postoperative survey (>1 year), to which 37 of 44 patients responded and assigned an average satisfaction score of 8 (of a possible 10) and a recommendation rate of 90%.2
Procedural Coding—Specific Current Procedural Terminology codes (11450-11471) from the International Classification of Diseases, Tenth Revision, exist for HS deroofing procedures; the applicable code for a given case depends on the final length of the surgical defect. Documentation to support these codes is similar to the note for an excision procedure, taking care to include location, depth, and length of the excision; healing by secondary intention; and the diagnosis of HS.
Final Thoughts
Deroofing is a surgical option that can be beneficial to patients with HS. It is a relatively simple procedure available to any dermatologist, regardless of setting. We encourage dermatologists to consider deroofing, even in patients with Hurley stage II lesions, because it can yield cosmetically acceptable and definitive results, given the variety of therapies available for HS. Deroofing also can be superior to standard excision, especially because of the potential complications with standard excision and quicker operative time with deroofing. As more providers become familiar with the deroofing procedure for HS, further studies can be undertaken to add to the paucity of data about deroofing and how it compares to other surgical treatments.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j.jaad.2019.02.067
- van der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol. 2010;63:475-480. doi:10.1016/j.jaad.2009.12.018
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi:10.2147/CCID.S111019
- Danby FW. Commentary: unroofing for hidradenitis suppurativa, why and how. J Am Acad Dermatol. 2010;63:481.e1-481.e3. doi:10.1016/j.jaad.2010.01.033
- Saylor DK, Brownstone ND, Naik HB. Office-based surgical intervention for hidradenitis suppurativa (HS): a focused review for dermatologists. Dermatol Ther (Heidelb). 2020;10:529-549. doi:10.1007/s13555-020-00391-x
Practice Gap
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by inflammatory nodules, abscesses, sinus tracts, fistulae, and scarring, mainly in intertriginous areas. The extent of disease—classified using the Hurley staging system (stages I–III)—helps guide treatment, which includes medical management and surgical intervention in later stages.
First-line treatment of HS includes topical or systemic medications, or both. Surgical therapy typically is reserved for refractory HS in moderate to severe disease (Hurley stages II and III) and is combined with pharmacotherapy. Specifically, clinical management guidelines issued by an expert committee of the United States and Canadian Hidradenitis Suppurativa Foundations recommend excision or deroofing for recurrent nodules and tunnels.1
Surgical options for HS that are available to the outpatient dermatologist include incision and drainage, electrosurgery, CO2 laser evaporation, excision, and deroofing (also known as unroofing).2 Deroofing is a fairly novel therapy; many dermatologists are unfamiliar with the procedure. A PubMed search of articles indexed for MEDLINE related to HS prior to 2010 revealed only 1 article containing the word deroofing and only 4 articles containing unroofing.
The pathophysiology of HS has important implications for successful treatment. Inflammation of the follicular pilosebaceous unit along with follicular occlusion create challenges with treatment.3 It is postulated that a defect in the glassy membrane of the infra-infundibular wall predisposes the pilosebaceous follicle to lose its structural integrality as pressure builds from plugging of the duct,4 which can result in the clinical hallmarks of HS including tunneling tracts, bridging nodules, abscesses, and fistulae that form with lateral expansion of the plugged follicle.
Leaking of the contents of these plugged follicles into surrounding tissue produces an inflammatory response in characteristic HS lesions. Because debris within the lesions moves laterally instead of being able to burst to the surface, the lesions have difficulty fully healing. Unroofing the lesions and removing built-up debris allows them to heal more expediently and quiets the underlying immune response by removing the stimulus.4
Herein, we describe the benefits, risks, and surgical process of deroofing for HS.
Technique and Tools
Deroofing is performed under local anesthesia, stepwise as follows:
1. Identify sinus tracts and infiltrate the area with lidocaine (Figure, A).
2. Use a blunt probe to define the borders of the area to be unroofed and to evaluate for any communicating sinus tracts (Figure, B).
3. Remove the roof of underlying abscesses and tracts, using a probe as a guide (Figure, C).
4. Enter through the skin or sinus opening using electrocautery or with a scalpel or scissors; perform blunt dissection.
5. Reflect back the entirety of skin overlying the probed areas and remove the skin to expose the base of the lesion (Figure, D).
6. Explore the exposed base and walls of the lesion with the probe again to assess for hidden tracts; take care not to create false tracts.
7. Debride the surgical wound using curettage or rough gauze grattage to remove remaining inflammatory debris or biofilm. To achieve hemostasis, apply aluminum chloride or ferric chloride. Coat the wound with petroleum jelly and gauze and allow it to heal by secondary intention.
8. Educate the patient on wound care—once-daily gentle cleansing with soap and water, followed by application of a moist dressing—which is similar to wound healing by secondary intention from other causes.2,4

Practice Implications
A deroofing procedure has many benefits compared to other surgical modalities for the treatment of HS. Deroofing requires only a probe, curette, and electrocautery device, making the procedure more cost-effective than excision, which requires a full tray of equipment and sutures. Furthermore, margins do not need to be taken with deroofing, and no undermining or closure is needed, which saves time during the operation and minimizes the risk for complications, including dehiscence and formation of new sinus tracts.4 No specialized equipment, such as a CO2 laser, is required, which makes deroofing accessible to every clinical dermatologist in any demographic or geographic setting.
Evidence of Benefit—Saylor and colleagues5 found that deroofing carries a 12.5% complication rate, which includes postoperative bleeding, hypergranulation tissue, and rarely wound infection. This rate is significantly lower than the 26% complication rate associated with local excision, which includes wound dehiscence, infection, and contracture (P<.001). Deroofing also was found to have an HS recurrence rate of 14.5%, which is significantly less than the 30% recurrence rate seen with local excision (P=.015). Saylor et al5 also concluded that incision and drainage was recommended only for immediate relief of HS because of its 100% recurrence rate.
van der Zee2 reported on 88 lesions from 44 patients that were treated by surgical deroofing, resulting in an average defect of 3.0 cm in length and a mean healing time of 14 days. The typical outcome was cosmetically acceptable scarring; this finding was supported by a postoperative survey (>1 year), to which 37 of 44 patients responded and assigned an average satisfaction score of 8 (of a possible 10) and a recommendation rate of 90%.2
Procedural Coding—Specific Current Procedural Terminology codes (11450-11471) from the International Classification of Diseases, Tenth Revision, exist for HS deroofing procedures; the applicable code for a given case depends on the final length of the surgical defect. Documentation to support these codes is similar to the note for an excision procedure, taking care to include location, depth, and length of the excision; healing by secondary intention; and the diagnosis of HS.
Final Thoughts
Deroofing is a surgical option that can be beneficial to patients with HS. It is a relatively simple procedure available to any dermatologist, regardless of setting. We encourage dermatologists to consider deroofing, even in patients with Hurley stage II lesions, because it can yield cosmetically acceptable and definitive results, given the variety of therapies available for HS. Deroofing also can be superior to standard excision, especially because of the potential complications with standard excision and quicker operative time with deroofing. As more providers become familiar with the deroofing procedure for HS, further studies can be undertaken to add to the paucity of data about deroofing and how it compares to other surgical treatments.
Practice Gap
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by inflammatory nodules, abscesses, sinus tracts, fistulae, and scarring, mainly in intertriginous areas. The extent of disease—classified using the Hurley staging system (stages I–III)—helps guide treatment, which includes medical management and surgical intervention in later stages.
First-line treatment of HS includes topical or systemic medications, or both. Surgical therapy typically is reserved for refractory HS in moderate to severe disease (Hurley stages II and III) and is combined with pharmacotherapy. Specifically, clinical management guidelines issued by an expert committee of the United States and Canadian Hidradenitis Suppurativa Foundations recommend excision or deroofing for recurrent nodules and tunnels.1
Surgical options for HS that are available to the outpatient dermatologist include incision and drainage, electrosurgery, CO2 laser evaporation, excision, and deroofing (also known as unroofing).2 Deroofing is a fairly novel therapy; many dermatologists are unfamiliar with the procedure. A PubMed search of articles indexed for MEDLINE related to HS prior to 2010 revealed only 1 article containing the word deroofing and only 4 articles containing unroofing.
The pathophysiology of HS has important implications for successful treatment. Inflammation of the follicular pilosebaceous unit along with follicular occlusion create challenges with treatment.3 It is postulated that a defect in the glassy membrane of the infra-infundibular wall predisposes the pilosebaceous follicle to lose its structural integrality as pressure builds from plugging of the duct,4 which can result in the clinical hallmarks of HS including tunneling tracts, bridging nodules, abscesses, and fistulae that form with lateral expansion of the plugged follicle.
Leaking of the contents of these plugged follicles into surrounding tissue produces an inflammatory response in characteristic HS lesions. Because debris within the lesions moves laterally instead of being able to burst to the surface, the lesions have difficulty fully healing. Unroofing the lesions and removing built-up debris allows them to heal more expediently and quiets the underlying immune response by removing the stimulus.4
Herein, we describe the benefits, risks, and surgical process of deroofing for HS.
Technique and Tools
Deroofing is performed under local anesthesia, stepwise as follows:
1. Identify sinus tracts and infiltrate the area with lidocaine (Figure, A).
2. Use a blunt probe to define the borders of the area to be unroofed and to evaluate for any communicating sinus tracts (Figure, B).
3. Remove the roof of underlying abscesses and tracts, using a probe as a guide (Figure, C).
4. Enter through the skin or sinus opening using electrocautery or with a scalpel or scissors; perform blunt dissection.
5. Reflect back the entirety of skin overlying the probed areas and remove the skin to expose the base of the lesion (Figure, D).
6. Explore the exposed base and walls of the lesion with the probe again to assess for hidden tracts; take care not to create false tracts.
7. Debride the surgical wound using curettage or rough gauze grattage to remove remaining inflammatory debris or biofilm. To achieve hemostasis, apply aluminum chloride or ferric chloride. Coat the wound with petroleum jelly and gauze and allow it to heal by secondary intention.
8. Educate the patient on wound care—once-daily gentle cleansing with soap and water, followed by application of a moist dressing—which is similar to wound healing by secondary intention from other causes.2,4

Practice Implications
A deroofing procedure has many benefits compared to other surgical modalities for the treatment of HS. Deroofing requires only a probe, curette, and electrocautery device, making the procedure more cost-effective than excision, which requires a full tray of equipment and sutures. Furthermore, margins do not need to be taken with deroofing, and no undermining or closure is needed, which saves time during the operation and minimizes the risk for complications, including dehiscence and formation of new sinus tracts.4 No specialized equipment, such as a CO2 laser, is required, which makes deroofing accessible to every clinical dermatologist in any demographic or geographic setting.
Evidence of Benefit—Saylor and colleagues5 found that deroofing carries a 12.5% complication rate, which includes postoperative bleeding, hypergranulation tissue, and rarely wound infection. This rate is significantly lower than the 26% complication rate associated with local excision, which includes wound dehiscence, infection, and contracture (P<.001). Deroofing also was found to have an HS recurrence rate of 14.5%, which is significantly less than the 30% recurrence rate seen with local excision (P=.015). Saylor et al5 also concluded that incision and drainage was recommended only for immediate relief of HS because of its 100% recurrence rate.
van der Zee2 reported on 88 lesions from 44 patients that were treated by surgical deroofing, resulting in an average defect of 3.0 cm in length and a mean healing time of 14 days. The typical outcome was cosmetically acceptable scarring; this finding was supported by a postoperative survey (>1 year), to which 37 of 44 patients responded and assigned an average satisfaction score of 8 (of a possible 10) and a recommendation rate of 90%.2
Procedural Coding—Specific Current Procedural Terminology codes (11450-11471) from the International Classification of Diseases, Tenth Revision, exist for HS deroofing procedures; the applicable code for a given case depends on the final length of the surgical defect. Documentation to support these codes is similar to the note for an excision procedure, taking care to include location, depth, and length of the excision; healing by secondary intention; and the diagnosis of HS.
Final Thoughts
Deroofing is a surgical option that can be beneficial to patients with HS. It is a relatively simple procedure available to any dermatologist, regardless of setting. We encourage dermatologists to consider deroofing, even in patients with Hurley stage II lesions, because it can yield cosmetically acceptable and definitive results, given the variety of therapies available for HS. Deroofing also can be superior to standard excision, especially because of the potential complications with standard excision and quicker operative time with deroofing. As more providers become familiar with the deroofing procedure for HS, further studies can be undertaken to add to the paucity of data about deroofing and how it compares to other surgical treatments.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j.jaad.2019.02.067
- van der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol. 2010;63:475-480. doi:10.1016/j.jaad.2009.12.018
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi:10.2147/CCID.S111019
- Danby FW. Commentary: unroofing for hidradenitis suppurativa, why and how. J Am Acad Dermatol. 2010;63:481.e1-481.e3. doi:10.1016/j.jaad.2010.01.033
- Saylor DK, Brownstone ND, Naik HB. Office-based surgical intervention for hidradenitis suppurativa (HS): a focused review for dermatologists. Dermatol Ther (Heidelb). 2020;10:529-549. doi:10.1007/s13555-020-00391-x
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j.jaad.2019.02.067
- van der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol. 2010;63:475-480. doi:10.1016/j.jaad.2009.12.018
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi:10.2147/CCID.S111019
- Danby FW. Commentary: unroofing for hidradenitis suppurativa, why and how. J Am Acad Dermatol. 2010;63:481.e1-481.e3. doi:10.1016/j.jaad.2010.01.033
- Saylor DK, Brownstone ND, Naik HB. Office-based surgical intervention for hidradenitis suppurativa (HS): a focused review for dermatologists. Dermatol Ther (Heidelb). 2020;10:529-549. doi:10.1007/s13555-020-00391-x
Should patients with PsA or ankylosing spondylitis with axial disease be ‘lumped’ or ‘split’?
A new study provides evidence that two conditions that fall under the umbrella of spondyloarthritis – isolated axial disease in patients with psoriatic arthritis (PsA) and isolated axial disease in patients with ankylosing spondylitis (AS) accompanied by psoriasis – are different clinical entities and may need different treatments. These relatively rare rheumatologic conditions, defined by their back involvement, have considerable clinical overlap and are often lumped together under the label axial spondyloarthritis.
This is a hot topic and current matter of debate within the scientific community: Are axial PsA and axial AS two separate diseases or just two phenotypes under the spondyloarthritis umbrella? said Fabian Proft, MD, a rheumatologist and researcher at Charité Universitätsmedizin Berlin, commenting on the new study, which was published online in Annals of the Rheumatic Diseases.
Both conditions belong to the spectrum of spondyloarthritis, but with varying viewpoints on nomenclature. They have intersections and overlaps, but not all treatments are equally effective for both. “We need to better understand their differences and similarities,” Dr. Proft said, adding that the new study is noteworthy for the size of the population included, its long-term follow-up data, and the researchers’ depth of experience treating these patients.
The researchers are based at the University of Toronto, which has separate clinics dedicated to PsA and to AS, said Dafna D. Gladman, MD, professor of medicine at the university, codirector of the PsA clinic, and corresponding author for the new study. The two clinics follow the same standardized protocols, including clinical, radiographic, genetic, and laboratory assessments. Even though the patients present quite similarly, she credits referring physicians for recognizing the distinctions by their referrals to the PsA or AS clinic.
According to previous research, pure axial PsA, without peripheral involvement, is rare, affecting about 2%-5% of patients with PsA. For this study, an observational cohort of 1,576 patients from the PsA clinic included 31% (n = 495) with axial disease, 2% (n = 32) with isolated axial PsA, and 29% (n = 463) with both axial and peripheral involvement. A total of 25 of the patients with isolated axial PsA ultimately developed peripheral disease by their most recent clinic follow-up visit. In a second cohort of 1,688 patients with AS, nearly 5% (n = 68) had isolated axial disease with psoriasis.
“In our logistic regression analysis, isolated axial PsA was found to be a different clinical entity than isolated AS with psoriasis. They are not the same patients,” Dr. Gladman said. The patients with isolated axial PsA were older at diagnosis, more likely to have psoriatic nail lesions, and less likely to have inflammatory back pain than were patients with isolated axial AS and accompanying psoriasis.
When interviewed in early September, Dr. Gladman was preparing to fly to Ghent, Belgium, to participate in a debate at the International Congress on Spondyloarthritides, taking the pro position on the thesis: Is axial inflammation in PsA distinct from axial spondyloarthritis? Taking the con position was to be Robert Landewé, MD, PhD, of Amsterdam University Medical Center in the Netherlands.
“This is an old debate, splitters versus lumpers,” Dr. Gladman told this news organization. “My message is that when you place patients in more homogeneous groups, you can learn more and perhaps find better opportunities for treating their disease.” For example, even with the similarities, do these patients need to be treated with different medications? Medications for psoriasis, including those targeting the interleukin-23 cytokine, may not be effective for AS, but patients with axial PsA may not get them because of the association with axial AS.
“Now is the opportunity to really understand what – if any – are the differences between various components of this disease group. If you lump people together, you may miss the forest for the trees,” Dr. Gladman said. “If, at the end of the day, we find out these patients essentially are the same, I will lump. But until we have proved that there are no important differences, I will split.” She added that it is important for practicing rheumatologists to make the correct diagnosis so that they know to access certain drugs.
Dr. Proft credited Dr. Gladman and colleagues’ study for adding another piece of the puzzle to better understand differences and similarities for these two axial diseases. He noted, however, that the study did not include MRI scans for every participating patient, which could have given a deeper picture.
“International efforts are being made to recruit patients for a multinational, multicenter study of axial involvement in PsA,” which will include MRI data, Dr. Gladman said. She and Dr. Proft are both part of AXIS, the Axial Involvement in Psoriatic Arthritis cohort, now recruiting patients for such a study. AXIS is a joint project of the Assessment of SpondyloArthritis international Society and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“We don’t have final answers yet, although we have given evidence to support the differences.” The proof is in the pudding, she said, and that pudding will be the clinical trials.
The University of Toronto Psoriatic Arthritis Program is supported by a grant from the Krembil Foundation. The study authors declared no competing interests. Dr. Proft reported receiving research support from Novartis, Eli Lilly, and UCB, and fees for consulting and serving on speakers bureaus from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Hexal, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and UCB.
A new study provides evidence that two conditions that fall under the umbrella of spondyloarthritis – isolated axial disease in patients with psoriatic arthritis (PsA) and isolated axial disease in patients with ankylosing spondylitis (AS) accompanied by psoriasis – are different clinical entities and may need different treatments. These relatively rare rheumatologic conditions, defined by their back involvement, have considerable clinical overlap and are often lumped together under the label axial spondyloarthritis.
This is a hot topic and current matter of debate within the scientific community: Are axial PsA and axial AS two separate diseases or just two phenotypes under the spondyloarthritis umbrella? said Fabian Proft, MD, a rheumatologist and researcher at Charité Universitätsmedizin Berlin, commenting on the new study, which was published online in Annals of the Rheumatic Diseases.
Both conditions belong to the spectrum of spondyloarthritis, but with varying viewpoints on nomenclature. They have intersections and overlaps, but not all treatments are equally effective for both. “We need to better understand their differences and similarities,” Dr. Proft said, adding that the new study is noteworthy for the size of the population included, its long-term follow-up data, and the researchers’ depth of experience treating these patients.
The researchers are based at the University of Toronto, which has separate clinics dedicated to PsA and to AS, said Dafna D. Gladman, MD, professor of medicine at the university, codirector of the PsA clinic, and corresponding author for the new study. The two clinics follow the same standardized protocols, including clinical, radiographic, genetic, and laboratory assessments. Even though the patients present quite similarly, she credits referring physicians for recognizing the distinctions by their referrals to the PsA or AS clinic.
According to previous research, pure axial PsA, without peripheral involvement, is rare, affecting about 2%-5% of patients with PsA. For this study, an observational cohort of 1,576 patients from the PsA clinic included 31% (n = 495) with axial disease, 2% (n = 32) with isolated axial PsA, and 29% (n = 463) with both axial and peripheral involvement. A total of 25 of the patients with isolated axial PsA ultimately developed peripheral disease by their most recent clinic follow-up visit. In a second cohort of 1,688 patients with AS, nearly 5% (n = 68) had isolated axial disease with psoriasis.
“In our logistic regression analysis, isolated axial PsA was found to be a different clinical entity than isolated AS with psoriasis. They are not the same patients,” Dr. Gladman said. The patients with isolated axial PsA were older at diagnosis, more likely to have psoriatic nail lesions, and less likely to have inflammatory back pain than were patients with isolated axial AS and accompanying psoriasis.
When interviewed in early September, Dr. Gladman was preparing to fly to Ghent, Belgium, to participate in a debate at the International Congress on Spondyloarthritides, taking the pro position on the thesis: Is axial inflammation in PsA distinct from axial spondyloarthritis? Taking the con position was to be Robert Landewé, MD, PhD, of Amsterdam University Medical Center in the Netherlands.
“This is an old debate, splitters versus lumpers,” Dr. Gladman told this news organization. “My message is that when you place patients in more homogeneous groups, you can learn more and perhaps find better opportunities for treating their disease.” For example, even with the similarities, do these patients need to be treated with different medications? Medications for psoriasis, including those targeting the interleukin-23 cytokine, may not be effective for AS, but patients with axial PsA may not get them because of the association with axial AS.
“Now is the opportunity to really understand what – if any – are the differences between various components of this disease group. If you lump people together, you may miss the forest for the trees,” Dr. Gladman said. “If, at the end of the day, we find out these patients essentially are the same, I will lump. But until we have proved that there are no important differences, I will split.” She added that it is important for practicing rheumatologists to make the correct diagnosis so that they know to access certain drugs.
Dr. Proft credited Dr. Gladman and colleagues’ study for adding another piece of the puzzle to better understand differences and similarities for these two axial diseases. He noted, however, that the study did not include MRI scans for every participating patient, which could have given a deeper picture.
“International efforts are being made to recruit patients for a multinational, multicenter study of axial involvement in PsA,” which will include MRI data, Dr. Gladman said. She and Dr. Proft are both part of AXIS, the Axial Involvement in Psoriatic Arthritis cohort, now recruiting patients for such a study. AXIS is a joint project of the Assessment of SpondyloArthritis international Society and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“We don’t have final answers yet, although we have given evidence to support the differences.” The proof is in the pudding, she said, and that pudding will be the clinical trials.
The University of Toronto Psoriatic Arthritis Program is supported by a grant from the Krembil Foundation. The study authors declared no competing interests. Dr. Proft reported receiving research support from Novartis, Eli Lilly, and UCB, and fees for consulting and serving on speakers bureaus from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Hexal, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and UCB.
A new study provides evidence that two conditions that fall under the umbrella of spondyloarthritis – isolated axial disease in patients with psoriatic arthritis (PsA) and isolated axial disease in patients with ankylosing spondylitis (AS) accompanied by psoriasis – are different clinical entities and may need different treatments. These relatively rare rheumatologic conditions, defined by their back involvement, have considerable clinical overlap and are often lumped together under the label axial spondyloarthritis.
This is a hot topic and current matter of debate within the scientific community: Are axial PsA and axial AS two separate diseases or just two phenotypes under the spondyloarthritis umbrella? said Fabian Proft, MD, a rheumatologist and researcher at Charité Universitätsmedizin Berlin, commenting on the new study, which was published online in Annals of the Rheumatic Diseases.
Both conditions belong to the spectrum of spondyloarthritis, but with varying viewpoints on nomenclature. They have intersections and overlaps, but not all treatments are equally effective for both. “We need to better understand their differences and similarities,” Dr. Proft said, adding that the new study is noteworthy for the size of the population included, its long-term follow-up data, and the researchers’ depth of experience treating these patients.
The researchers are based at the University of Toronto, which has separate clinics dedicated to PsA and to AS, said Dafna D. Gladman, MD, professor of medicine at the university, codirector of the PsA clinic, and corresponding author for the new study. The two clinics follow the same standardized protocols, including clinical, radiographic, genetic, and laboratory assessments. Even though the patients present quite similarly, she credits referring physicians for recognizing the distinctions by their referrals to the PsA or AS clinic.
According to previous research, pure axial PsA, without peripheral involvement, is rare, affecting about 2%-5% of patients with PsA. For this study, an observational cohort of 1,576 patients from the PsA clinic included 31% (n = 495) with axial disease, 2% (n = 32) with isolated axial PsA, and 29% (n = 463) with both axial and peripheral involvement. A total of 25 of the patients with isolated axial PsA ultimately developed peripheral disease by their most recent clinic follow-up visit. In a second cohort of 1,688 patients with AS, nearly 5% (n = 68) had isolated axial disease with psoriasis.
“In our logistic regression analysis, isolated axial PsA was found to be a different clinical entity than isolated AS with psoriasis. They are not the same patients,” Dr. Gladman said. The patients with isolated axial PsA were older at diagnosis, more likely to have psoriatic nail lesions, and less likely to have inflammatory back pain than were patients with isolated axial AS and accompanying psoriasis.
When interviewed in early September, Dr. Gladman was preparing to fly to Ghent, Belgium, to participate in a debate at the International Congress on Spondyloarthritides, taking the pro position on the thesis: Is axial inflammation in PsA distinct from axial spondyloarthritis? Taking the con position was to be Robert Landewé, MD, PhD, of Amsterdam University Medical Center in the Netherlands.
“This is an old debate, splitters versus lumpers,” Dr. Gladman told this news organization. “My message is that when you place patients in more homogeneous groups, you can learn more and perhaps find better opportunities for treating their disease.” For example, even with the similarities, do these patients need to be treated with different medications? Medications for psoriasis, including those targeting the interleukin-23 cytokine, may not be effective for AS, but patients with axial PsA may not get them because of the association with axial AS.
“Now is the opportunity to really understand what – if any – are the differences between various components of this disease group. If you lump people together, you may miss the forest for the trees,” Dr. Gladman said. “If, at the end of the day, we find out these patients essentially are the same, I will lump. But until we have proved that there are no important differences, I will split.” She added that it is important for practicing rheumatologists to make the correct diagnosis so that they know to access certain drugs.
Dr. Proft credited Dr. Gladman and colleagues’ study for adding another piece of the puzzle to better understand differences and similarities for these two axial diseases. He noted, however, that the study did not include MRI scans for every participating patient, which could have given a deeper picture.
“International efforts are being made to recruit patients for a multinational, multicenter study of axial involvement in PsA,” which will include MRI data, Dr. Gladman said. She and Dr. Proft are both part of AXIS, the Axial Involvement in Psoriatic Arthritis cohort, now recruiting patients for such a study. AXIS is a joint project of the Assessment of SpondyloArthritis international Society and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“We don’t have final answers yet, although we have given evidence to support the differences.” The proof is in the pudding, she said, and that pudding will be the clinical trials.
The University of Toronto Psoriatic Arthritis Program is supported by a grant from the Krembil Foundation. The study authors declared no competing interests. Dr. Proft reported receiving research support from Novartis, Eli Lilly, and UCB, and fees for consulting and serving on speakers bureaus from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Hexal, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and UCB.
FROM ANNALS OF THE RHEUMATIC DISEASES