User login
New psoriasis guidelines focus on topical and alternative treatments, and severity measures
and the National Psoriasis Foundation.
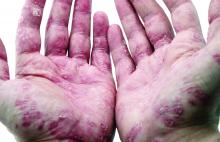
The guidelines, published in the Journal of the American Academy of Dermatology, focus on treatment for adults, and follow the release of other AAD-NPF guidelines on biologics for psoriasis, psoriasis-related comorbidities, pediatric psoriasis, and phototherapy in 2019, and earlier this year, guidelines for systemic nonbiologic treatments. The latest guidelines’ section on topical treatment outlines evidence for the efficacy, effectiveness, and adverse events related to topical steroids, topical tacrolimus and pimecrolimus, vitamin D analogues, tazarotene, moisturizers, salicylic acid, anthralin, coal tar, combinations with biologic agents, and combinations with nonbiologic treatments (methotrexate, cyclosporine, acitretin, and apremilast).
The guidelines noted the “key role” of topical corticosteroids in treating psoriasis “especially for localized disease,” and include a review of the data on low-, moderate-, high-, and ultrahigh-potency topical steroids for psoriasis.
In general, all topical steroids can be used in combination with biologics, according to the guidelines, but the strongest recommendations based on the latest evidence include the addition of an ultra-high potency topical corticosteroid to standard dose etanercept for 12 weeks. Currently, 11 biologics are approved by the Food and Drug Administration for the treatment of psoriasis.
In addition, “while not FDA approved for psoriasis, the topical calcineurin inhibitors tacrolimus and pimecrolimus are often employed in the treatment of psoriasis,” can be helpful for “thinner skin such as facial and intertriginous areas,” and can be steroid sparing when used for more than 4 weeks, according to the guidelines.
Don’t discount the role of patient preferences when choosing topical treatments, the authors noted. “The optimal vehicle choice is the one the patient is mostly likely to use.”
The guidelines also address the evidence for effectiveness, and adverse events in the use of several alternative medicines for psoriasis including traditional Chinese medicine, and the herbal therapies aloe vera and St. John’s wort, as well as the potential role of dietary supplements including fish oil, vitamin D, turmeric, and zinc in managing psoriasis, and the potential role of a gluten-free diet.
In general, research on the efficacy, effectiveness, and potential adverse effects of these strategies are limited, according to the guidelines, although many patients express interest in supplements and herbal products. For example, “Many patients ask about the overall role of vitamin D in skin health. Rather than adding oral vitamin D supplementation, topical therapy with vitamin D agents is effective for the treatment of psoriasis,” the authors noted.
In addition, they noted that mind/body strategies, namely hypnosis and stress reduction or meditation techniques, have been shown to improve symptoms and can be helpful for some patients, but clinical evidence is limited.
The guidelines also addressed methods for assessing disease severity in psoriasis. They recommended using body surface area (BSA) to assess psoriasis severity and patient response to treatment in the clinical setting. However, BSA is a provider assessment tool that “does not take into account location on the body, clinical characteristics of the plaques, symptoms, or quality of life issues,” the authors noted. The Psoriasis Area and Severity Index (PASI) measures erythema, induration, and scaling and is more suited to assessing psoriasis severity and response to treatment in clinical trials rather than in practice, they said.
Prior AAD guidelines on psoriasis were published more than 10 years ago, and major developments including the availability of new biologic drugs and new data on comorbidities have been recognized in the past decade, working group cochair and author of the guidelines Alan Menter, MD, said in an interview.
The key game-changers from previous guidelines include the full section published on comorbidities plus the development of two new important cytokine classes: three IL-17 drugs and three new IL-23 drugs now available for moderate to severe psoriasis, said Dr. Menter, chairman of the division of dermatology at Baylor University Medical Center, Dallas.
Barriers to implementing the guidelines in practice may occur when “third party payers make the decision on which of the 11 biologic drugs now approved for moderate to severe psoriasis should be used,” he noted.
As for next steps in psoriasis studies, “new biomarker research is currently underway,” Dr. Menter said. With 11 biologic agents new formally approved by the FDA for moderate to severe psoriasis, the next steps are to determine which drug is likely to be the most appropriate for each individual patient.
Dr. Menter disclosed relationships with multiple companies that develop and manufacture psoriasis therapies, including Abbott Labs, AbbVie, Amgen, Eli Lilly and Company, Galderma USA, Janssen Pharmaceuticals, LEO Pharma US, Menlo Therapeutics, and Novartis. The updated guidelines were designed by a multidisciplinary work group of psoriasis experts including dermatologists, a rheumatologist, a cardiologist, and representatives from a patient advocacy organization.
SOURCE: Elmets CA et al. J Am Acad Dermatol. 2020 Jul 29. doi: 10.1016/j.jaad.2020.07.087.
and the National Psoriasis Foundation.
The guidelines, published in the Journal of the American Academy of Dermatology, focus on treatment for adults, and follow the release of other AAD-NPF guidelines on biologics for psoriasis, psoriasis-related comorbidities, pediatric psoriasis, and phototherapy in 2019, and earlier this year, guidelines for systemic nonbiologic treatments. The latest guidelines’ section on topical treatment outlines evidence for the efficacy, effectiveness, and adverse events related to topical steroids, topical tacrolimus and pimecrolimus, vitamin D analogues, tazarotene, moisturizers, salicylic acid, anthralin, coal tar, combinations with biologic agents, and combinations with nonbiologic treatments (methotrexate, cyclosporine, acitretin, and apremilast).
The guidelines noted the “key role” of topical corticosteroids in treating psoriasis “especially for localized disease,” and include a review of the data on low-, moderate-, high-, and ultrahigh-potency topical steroids for psoriasis.
In general, all topical steroids can be used in combination with biologics, according to the guidelines, but the strongest recommendations based on the latest evidence include the addition of an ultra-high potency topical corticosteroid to standard dose etanercept for 12 weeks. Currently, 11 biologics are approved by the Food and Drug Administration for the treatment of psoriasis.
In addition, “while not FDA approved for psoriasis, the topical calcineurin inhibitors tacrolimus and pimecrolimus are often employed in the treatment of psoriasis,” can be helpful for “thinner skin such as facial and intertriginous areas,” and can be steroid sparing when used for more than 4 weeks, according to the guidelines.
Don’t discount the role of patient preferences when choosing topical treatments, the authors noted. “The optimal vehicle choice is the one the patient is mostly likely to use.”
The guidelines also address the evidence for effectiveness, and adverse events in the use of several alternative medicines for psoriasis including traditional Chinese medicine, and the herbal therapies aloe vera and St. John’s wort, as well as the potential role of dietary supplements including fish oil, vitamin D, turmeric, and zinc in managing psoriasis, and the potential role of a gluten-free diet.
In general, research on the efficacy, effectiveness, and potential adverse effects of these strategies are limited, according to the guidelines, although many patients express interest in supplements and herbal products. For example, “Many patients ask about the overall role of vitamin D in skin health. Rather than adding oral vitamin D supplementation, topical therapy with vitamin D agents is effective for the treatment of psoriasis,” the authors noted.
In addition, they noted that mind/body strategies, namely hypnosis and stress reduction or meditation techniques, have been shown to improve symptoms and can be helpful for some patients, but clinical evidence is limited.
The guidelines also addressed methods for assessing disease severity in psoriasis. They recommended using body surface area (BSA) to assess psoriasis severity and patient response to treatment in the clinical setting. However, BSA is a provider assessment tool that “does not take into account location on the body, clinical characteristics of the plaques, symptoms, or quality of life issues,” the authors noted. The Psoriasis Area and Severity Index (PASI) measures erythema, induration, and scaling and is more suited to assessing psoriasis severity and response to treatment in clinical trials rather than in practice, they said.
Prior AAD guidelines on psoriasis were published more than 10 years ago, and major developments including the availability of new biologic drugs and new data on comorbidities have been recognized in the past decade, working group cochair and author of the guidelines Alan Menter, MD, said in an interview.
The key game-changers from previous guidelines include the full section published on comorbidities plus the development of two new important cytokine classes: three IL-17 drugs and three new IL-23 drugs now available for moderate to severe psoriasis, said Dr. Menter, chairman of the division of dermatology at Baylor University Medical Center, Dallas.
Barriers to implementing the guidelines in practice may occur when “third party payers make the decision on which of the 11 biologic drugs now approved for moderate to severe psoriasis should be used,” he noted.
As for next steps in psoriasis studies, “new biomarker research is currently underway,” Dr. Menter said. With 11 biologic agents new formally approved by the FDA for moderate to severe psoriasis, the next steps are to determine which drug is likely to be the most appropriate for each individual patient.
Dr. Menter disclosed relationships with multiple companies that develop and manufacture psoriasis therapies, including Abbott Labs, AbbVie, Amgen, Eli Lilly and Company, Galderma USA, Janssen Pharmaceuticals, LEO Pharma US, Menlo Therapeutics, and Novartis. The updated guidelines were designed by a multidisciplinary work group of psoriasis experts including dermatologists, a rheumatologist, a cardiologist, and representatives from a patient advocacy organization.
SOURCE: Elmets CA et al. J Am Acad Dermatol. 2020 Jul 29. doi: 10.1016/j.jaad.2020.07.087.
and the National Psoriasis Foundation.
The guidelines, published in the Journal of the American Academy of Dermatology, focus on treatment for adults, and follow the release of other AAD-NPF guidelines on biologics for psoriasis, psoriasis-related comorbidities, pediatric psoriasis, and phototherapy in 2019, and earlier this year, guidelines for systemic nonbiologic treatments. The latest guidelines’ section on topical treatment outlines evidence for the efficacy, effectiveness, and adverse events related to topical steroids, topical tacrolimus and pimecrolimus, vitamin D analogues, tazarotene, moisturizers, salicylic acid, anthralin, coal tar, combinations with biologic agents, and combinations with nonbiologic treatments (methotrexate, cyclosporine, acitretin, and apremilast).
The guidelines noted the “key role” of topical corticosteroids in treating psoriasis “especially for localized disease,” and include a review of the data on low-, moderate-, high-, and ultrahigh-potency topical steroids for psoriasis.
In general, all topical steroids can be used in combination with biologics, according to the guidelines, but the strongest recommendations based on the latest evidence include the addition of an ultra-high potency topical corticosteroid to standard dose etanercept for 12 weeks. Currently, 11 biologics are approved by the Food and Drug Administration for the treatment of psoriasis.
In addition, “while not FDA approved for psoriasis, the topical calcineurin inhibitors tacrolimus and pimecrolimus are often employed in the treatment of psoriasis,” can be helpful for “thinner skin such as facial and intertriginous areas,” and can be steroid sparing when used for more than 4 weeks, according to the guidelines.
Don’t discount the role of patient preferences when choosing topical treatments, the authors noted. “The optimal vehicle choice is the one the patient is mostly likely to use.”
The guidelines also address the evidence for effectiveness, and adverse events in the use of several alternative medicines for psoriasis including traditional Chinese medicine, and the herbal therapies aloe vera and St. John’s wort, as well as the potential role of dietary supplements including fish oil, vitamin D, turmeric, and zinc in managing psoriasis, and the potential role of a gluten-free diet.
In general, research on the efficacy, effectiveness, and potential adverse effects of these strategies are limited, according to the guidelines, although many patients express interest in supplements and herbal products. For example, “Many patients ask about the overall role of vitamin D in skin health. Rather than adding oral vitamin D supplementation, topical therapy with vitamin D agents is effective for the treatment of psoriasis,” the authors noted.
In addition, they noted that mind/body strategies, namely hypnosis and stress reduction or meditation techniques, have been shown to improve symptoms and can be helpful for some patients, but clinical evidence is limited.
The guidelines also addressed methods for assessing disease severity in psoriasis. They recommended using body surface area (BSA) to assess psoriasis severity and patient response to treatment in the clinical setting. However, BSA is a provider assessment tool that “does not take into account location on the body, clinical characteristics of the plaques, symptoms, or quality of life issues,” the authors noted. The Psoriasis Area and Severity Index (PASI) measures erythema, induration, and scaling and is more suited to assessing psoriasis severity and response to treatment in clinical trials rather than in practice, they said.
Prior AAD guidelines on psoriasis were published more than 10 years ago, and major developments including the availability of new biologic drugs and new data on comorbidities have been recognized in the past decade, working group cochair and author of the guidelines Alan Menter, MD, said in an interview.
The key game-changers from previous guidelines include the full section published on comorbidities plus the development of two new important cytokine classes: three IL-17 drugs and three new IL-23 drugs now available for moderate to severe psoriasis, said Dr. Menter, chairman of the division of dermatology at Baylor University Medical Center, Dallas.
Barriers to implementing the guidelines in practice may occur when “third party payers make the decision on which of the 11 biologic drugs now approved for moderate to severe psoriasis should be used,” he noted.
As for next steps in psoriasis studies, “new biomarker research is currently underway,” Dr. Menter said. With 11 biologic agents new formally approved by the FDA for moderate to severe psoriasis, the next steps are to determine which drug is likely to be the most appropriate for each individual patient.
Dr. Menter disclosed relationships with multiple companies that develop and manufacture psoriasis therapies, including Abbott Labs, AbbVie, Amgen, Eli Lilly and Company, Galderma USA, Janssen Pharmaceuticals, LEO Pharma US, Menlo Therapeutics, and Novartis. The updated guidelines were designed by a multidisciplinary work group of psoriasis experts including dermatologists, a rheumatologist, a cardiologist, and representatives from a patient advocacy organization.
SOURCE: Elmets CA et al. J Am Acad Dermatol. 2020 Jul 29. doi: 10.1016/j.jaad.2020.07.087.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Biologics may delay psoriatic arthritis, study finds
(DMARDs), in a single center retrospective analysis in Argentina that followed patients for almost 2 decades.
About 30%-40% of patients with psoriasis go on to develop psoriatic arthritis (PsA), usually on average about 10 years after the onset of psoriasis. One potential mechanism of PsA onset is through enthesitis, which has been described at subclinical levels in psoriasis.
“It could be speculated that treatment with biologics in patients with psoriasis could prevent the development of psoriatic arthritis, perhaps by inhibiting the subclinical development of enthesitis,” Luciano Lo Giudice, MD, a rheumatology fellow at Hospital Italiano de Buenos Aires, said during his presentation at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Although these results do not prove that treatment of the underlying disease delays progression to PsA, it is suggestive, and highlights an emerging field of research, according to Diamant Thaçi, MD, PhD, professor of medicine at University Hospital Schleswig-Holstein, Germany, who led a live discussion following a prerecorded presentation of the results. “We’re going in this direction – how can we prevent psoriatic arthritis, how can we delay it. We are just starting to think about this,” Dr. Thaçi said in an interview.
The researchers examined medical records of 1,626 patients with psoriasis treated at their center between 2000 and 2019, with a total of 15,152 years of follow-up. Of these patients, 1,293 were treated with topical medication, 229 with conventional DMARDs (methotrexate in 77%, cyclosporine in 13%, and both in 10%), and 104 with biologics, including etanercept (34%), secukinumab (20%), adalimumab (20%), ustekinumab (12%), ixekizumab (9%), and infliximab (5%).
They found that 11% in the topical treatment group developed PsA, as did 3.5% in the conventional DMARD group, 1.9% in the biologics group, and 9.1% overall. Treatment with biologics was associated with a significantly lower odds of developing PsA compared with treatment with conventional DMARDs (3 versus 17.2 per 1,000 patient-years; incidence rate ratio [IRR], 0.17; P = .0177). There was a trend toward reduced odds of developing PsA among those on biologic therapy compared with those on topicals (3 versus 9.8 per 1,000 patient-years; IRR, 0.3; P = .0588).
The researchers confirmed all medical encounters using electronic medical records and the study had a long follow-up time, but was limited by the single center and its retrospective nature. It also could not associate reduced risk with specific biologics.
The findings probably reflect the presence of subclinical PsA that many clinicians don’t see, according to Dr. Thaçi. While a dermatology practice might find PsA in 2% or 3%, or at most, 10% of patients with psoriasis, “in our department it’s about 50 to 60 percent of patients who have psoriatic arthritis, because we diagnose it early,” he said.
He found the results of the study encouraging. “It looks like some of the biologics, for example IL [interleukin]-17 or even IL-23 [blockers] may have an influence on occurrence or delay the occurrence of psoriatic arthritis.”
Dr. Thaçi noted that early treatment of skin lesions can increase the probability of longer remissions, especially with IL-23 blockers. Still, that’s no guarantee the same would hold true for PsA risk. “Skin is skin and joints are joints,” Dr. Thaçi said.
Dr. Thaçi and Dr. Lo Giudice had no relevant financial disclosures.
(DMARDs), in a single center retrospective analysis in Argentina that followed patients for almost 2 decades.
About 30%-40% of patients with psoriasis go on to develop psoriatic arthritis (PsA), usually on average about 10 years after the onset of psoriasis. One potential mechanism of PsA onset is through enthesitis, which has been described at subclinical levels in psoriasis.
“It could be speculated that treatment with biologics in patients with psoriasis could prevent the development of psoriatic arthritis, perhaps by inhibiting the subclinical development of enthesitis,” Luciano Lo Giudice, MD, a rheumatology fellow at Hospital Italiano de Buenos Aires, said during his presentation at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Although these results do not prove that treatment of the underlying disease delays progression to PsA, it is suggestive, and highlights an emerging field of research, according to Diamant Thaçi, MD, PhD, professor of medicine at University Hospital Schleswig-Holstein, Germany, who led a live discussion following a prerecorded presentation of the results. “We’re going in this direction – how can we prevent psoriatic arthritis, how can we delay it. We are just starting to think about this,” Dr. Thaçi said in an interview.
The researchers examined medical records of 1,626 patients with psoriasis treated at their center between 2000 and 2019, with a total of 15,152 years of follow-up. Of these patients, 1,293 were treated with topical medication, 229 with conventional DMARDs (methotrexate in 77%, cyclosporine in 13%, and both in 10%), and 104 with biologics, including etanercept (34%), secukinumab (20%), adalimumab (20%), ustekinumab (12%), ixekizumab (9%), and infliximab (5%).
They found that 11% in the topical treatment group developed PsA, as did 3.5% in the conventional DMARD group, 1.9% in the biologics group, and 9.1% overall. Treatment with biologics was associated with a significantly lower odds of developing PsA compared with treatment with conventional DMARDs (3 versus 17.2 per 1,000 patient-years; incidence rate ratio [IRR], 0.17; P = .0177). There was a trend toward reduced odds of developing PsA among those on biologic therapy compared with those on topicals (3 versus 9.8 per 1,000 patient-years; IRR, 0.3; P = .0588).
The researchers confirmed all medical encounters using electronic medical records and the study had a long follow-up time, but was limited by the single center and its retrospective nature. It also could not associate reduced risk with specific biologics.
The findings probably reflect the presence of subclinical PsA that many clinicians don’t see, according to Dr. Thaçi. While a dermatology practice might find PsA in 2% or 3%, or at most, 10% of patients with psoriasis, “in our department it’s about 50 to 60 percent of patients who have psoriatic arthritis, because we diagnose it early,” he said.
He found the results of the study encouraging. “It looks like some of the biologics, for example IL [interleukin]-17 or even IL-23 [blockers] may have an influence on occurrence or delay the occurrence of psoriatic arthritis.”
Dr. Thaçi noted that early treatment of skin lesions can increase the probability of longer remissions, especially with IL-23 blockers. Still, that’s no guarantee the same would hold true for PsA risk. “Skin is skin and joints are joints,” Dr. Thaçi said.
Dr. Thaçi and Dr. Lo Giudice had no relevant financial disclosures.
(DMARDs), in a single center retrospective analysis in Argentina that followed patients for almost 2 decades.
About 30%-40% of patients with psoriasis go on to develop psoriatic arthritis (PsA), usually on average about 10 years after the onset of psoriasis. One potential mechanism of PsA onset is through enthesitis, which has been described at subclinical levels in psoriasis.
“It could be speculated that treatment with biologics in patients with psoriasis could prevent the development of psoriatic arthritis, perhaps by inhibiting the subclinical development of enthesitis,” Luciano Lo Giudice, MD, a rheumatology fellow at Hospital Italiano de Buenos Aires, said during his presentation at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Although these results do not prove that treatment of the underlying disease delays progression to PsA, it is suggestive, and highlights an emerging field of research, according to Diamant Thaçi, MD, PhD, professor of medicine at University Hospital Schleswig-Holstein, Germany, who led a live discussion following a prerecorded presentation of the results. “We’re going in this direction – how can we prevent psoriatic arthritis, how can we delay it. We are just starting to think about this,” Dr. Thaçi said in an interview.
The researchers examined medical records of 1,626 patients with psoriasis treated at their center between 2000 and 2019, with a total of 15,152 years of follow-up. Of these patients, 1,293 were treated with topical medication, 229 with conventional DMARDs (methotrexate in 77%, cyclosporine in 13%, and both in 10%), and 104 with biologics, including etanercept (34%), secukinumab (20%), adalimumab (20%), ustekinumab (12%), ixekizumab (9%), and infliximab (5%).
They found that 11% in the topical treatment group developed PsA, as did 3.5% in the conventional DMARD group, 1.9% in the biologics group, and 9.1% overall. Treatment with biologics was associated with a significantly lower odds of developing PsA compared with treatment with conventional DMARDs (3 versus 17.2 per 1,000 patient-years; incidence rate ratio [IRR], 0.17; P = .0177). There was a trend toward reduced odds of developing PsA among those on biologic therapy compared with those on topicals (3 versus 9.8 per 1,000 patient-years; IRR, 0.3; P = .0588).
The researchers confirmed all medical encounters using electronic medical records and the study had a long follow-up time, but was limited by the single center and its retrospective nature. It also could not associate reduced risk with specific biologics.
The findings probably reflect the presence of subclinical PsA that many clinicians don’t see, according to Dr. Thaçi. While a dermatology practice might find PsA in 2% or 3%, or at most, 10% of patients with psoriasis, “in our department it’s about 50 to 60 percent of patients who have psoriatic arthritis, because we diagnose it early,” he said.
He found the results of the study encouraging. “It looks like some of the biologics, for example IL [interleukin]-17 or even IL-23 [blockers] may have an influence on occurrence or delay the occurrence of psoriatic arthritis.”
Dr. Thaçi noted that early treatment of skin lesions can increase the probability of longer remissions, especially with IL-23 blockers. Still, that’s no guarantee the same would hold true for PsA risk. “Skin is skin and joints are joints,” Dr. Thaçi said.
Dr. Thaçi and Dr. Lo Giudice had no relevant financial disclosures.
FROM GRAPPA 2020 VIRTUAL ANNUAL MEETING
New developments in pustular psoriasis
It has various dermatologic and rheumatologic manifestations and sometimes overlaps with plaque psoriasis. Pustular palmoplantar psoriasis (PPP) affects the palmar and plantar areas of the skin, while generalized pustular psoriasis (GPP) can affect large areas of skin and tends to be more severe, even life threatening. PPP can accompany psoriatic arthritis or can be a side effect of tumor necrosis factor (TNF) inhibitor therapy, or a non–drug-induced component of rheumatologic syndromes, according to Kristina Callis Duffin, MD, an associate professor and chair of dermatology at the University of Utah, Salt Lake City.
“Each phenotype could be considered an orphan disease, and the response to therapy is often unpredictable,” Dr. Duffin said during a session on pustular psoriasis at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
But there is some positive news. A study in 2011 of several people with GPP opened the door to better understanding the pathophysiology of pustular psoriasis. Researchers identified a causal autosomal mutation in the IL36RN gene, which encodes an antagonist to the interleukin-36 receptor (Am J Hum Genet. 2011 Sep 9;89[3]:432-7). “As a result of this paper and others, drug development in this space has recently accelerated,” Dr. Duffin said.
In fact, she added,“it’s my opinion that pustular psoriasis is now where plaque psoriasis was 20 years ago, when accelerated drug development was driving a better understanding of the pathogenesis of psoriatic disease and its comorbidities, and also driving outcome measure development.”
In another presentation at the meeting, Hervé Bachelez, MD, PhD, professor of dermatology and immunologist at the University of Paris and Saint-Louis Hospital, Paris, discussed recent advances in drug development for pustular psoriasis. He noted other recent findings of genetic variants related to the disease, including AP1S3, CARD14, and SERPINA3.
For GPP, he said, the current algorithm for management is based on weak evidence for treatments like acitretin, cyclosporine, methotrexate, and infliximab. The story is similar for other biologics, with evidence in the form of case series; open-label studies; controlled, prospective studies; or retrospective analyses. Most of the evidence has been amassed for TNF inhibitors. A retrospective study of all TNF inhibitors suggested they may be effective as induction and maintenance therapy, he noted.
Among IL-17A inhibitors, a prospective study of 12 patients in Japan found secukinumab showed efficacy against GPP, as did studies of ixekizumab and brodalumab. A small phase 3 study in Japan demonstrated efficacy for the IL-23 inhibitor guselkumab in patients with erythrodermic psoriasis and GPP (J Dermatol. 2018 May;45[5]:529-39).
The limited data are a reflection in part of the difficulty in studying GPP, since its flares tend to be more self-remitting than with psoriasis vulgaris or PPP.
There are two monoclonal antibodies against the IL-36 receptor currently being developed. A proof-of-concept study of one of them, spesolimab, showed promise against GPP, with five of seven patients reaching “clear” or “almost clear” scores on the Generalized Pustular Psoriasis Physician Global Assessment within a week after infusion and in all seven by the fourth week (N Engl J Med. 2019 Mar 7;380[10]:981-3).
With respect to PPP, the strongest evidence for conventional therapies comes from two randomized, controlled trials of cyclosporine, with response rates of 48% and 89%, compared with 19% and 21%, respectively, in the placebo groups, although the primary endpoint was poorly designed, according to Dr. Bachelez. Retinoids like etretinate and acitretin, combined with psoralen and UVA, also have some supporting evidence regarding efficacy.
Among biologics, secukinumab did not fare well in a phase 3 study of patients with PPP. A subset of patients may benefit from it, but there are no biomarkers available to identify them, Dr. Bachelez said. A phase 2 study of guselkumab in Japan told a similar story, with only weak signs of efficacy. While there are many more ongoing clinical trials evaluating treatments for PPP, which is encouraging, PPP seems to be more challenging at this stage to tackle than GPP, Dr. Bachelez added. “The genetically inherited IL-36 antagonist abnormalities are clearly driving the advances regarding the pathogenesis of the disease, mainly for GPP rather than PPP.”
Part of the efforts to develop therapies for pustular psoriasis relies on the development of new outcome measures, or adaptation of existing ones. “We have a need to adapt or develop new investigator-reported measures, we need to adapt or develop new patient-reported outcomes,” Dr. Duffin said.
Many existing measures use inconsistent language and anchoring definitions, and some may be proprietary, she added. “The language varies by sponsor and is sometimes tweaked or modified by the agencies. Often synonyms are being used … it raises questions, does it change the validity of the instrument?”
Dr. Duffin called for the research community to use the pause in clinical research during the COVID-19 pandemic to reassess the research agenda, develop consensus on performing and training for GPP and PPP assessments, develop patient-reported outcomes, and strengthen connections to industry.
Dr. Duffin and Dr. Bachelez have consulted, served on the advisory board, been a speaker for, and/or received research support from a wide range of pharmaceutical companies, including those that manufacture and develop psoriasis treatments.
It has various dermatologic and rheumatologic manifestations and sometimes overlaps with plaque psoriasis. Pustular palmoplantar psoriasis (PPP) affects the palmar and plantar areas of the skin, while generalized pustular psoriasis (GPP) can affect large areas of skin and tends to be more severe, even life threatening. PPP can accompany psoriatic arthritis or can be a side effect of tumor necrosis factor (TNF) inhibitor therapy, or a non–drug-induced component of rheumatologic syndromes, according to Kristina Callis Duffin, MD, an associate professor and chair of dermatology at the University of Utah, Salt Lake City.
“Each phenotype could be considered an orphan disease, and the response to therapy is often unpredictable,” Dr. Duffin said during a session on pustular psoriasis at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
But there is some positive news. A study in 2011 of several people with GPP opened the door to better understanding the pathophysiology of pustular psoriasis. Researchers identified a causal autosomal mutation in the IL36RN gene, which encodes an antagonist to the interleukin-36 receptor (Am J Hum Genet. 2011 Sep 9;89[3]:432-7). “As a result of this paper and others, drug development in this space has recently accelerated,” Dr. Duffin said.
In fact, she added,“it’s my opinion that pustular psoriasis is now where plaque psoriasis was 20 years ago, when accelerated drug development was driving a better understanding of the pathogenesis of psoriatic disease and its comorbidities, and also driving outcome measure development.”
In another presentation at the meeting, Hervé Bachelez, MD, PhD, professor of dermatology and immunologist at the University of Paris and Saint-Louis Hospital, Paris, discussed recent advances in drug development for pustular psoriasis. He noted other recent findings of genetic variants related to the disease, including AP1S3, CARD14, and SERPINA3.
For GPP, he said, the current algorithm for management is based on weak evidence for treatments like acitretin, cyclosporine, methotrexate, and infliximab. The story is similar for other biologics, with evidence in the form of case series; open-label studies; controlled, prospective studies; or retrospective analyses. Most of the evidence has been amassed for TNF inhibitors. A retrospective study of all TNF inhibitors suggested they may be effective as induction and maintenance therapy, he noted.
Among IL-17A inhibitors, a prospective study of 12 patients in Japan found secukinumab showed efficacy against GPP, as did studies of ixekizumab and brodalumab. A small phase 3 study in Japan demonstrated efficacy for the IL-23 inhibitor guselkumab in patients with erythrodermic psoriasis and GPP (J Dermatol. 2018 May;45[5]:529-39).
The limited data are a reflection in part of the difficulty in studying GPP, since its flares tend to be more self-remitting than with psoriasis vulgaris or PPP.
There are two monoclonal antibodies against the IL-36 receptor currently being developed. A proof-of-concept study of one of them, spesolimab, showed promise against GPP, with five of seven patients reaching “clear” or “almost clear” scores on the Generalized Pustular Psoriasis Physician Global Assessment within a week after infusion and in all seven by the fourth week (N Engl J Med. 2019 Mar 7;380[10]:981-3).
With respect to PPP, the strongest evidence for conventional therapies comes from two randomized, controlled trials of cyclosporine, with response rates of 48% and 89%, compared with 19% and 21%, respectively, in the placebo groups, although the primary endpoint was poorly designed, according to Dr. Bachelez. Retinoids like etretinate and acitretin, combined with psoralen and UVA, also have some supporting evidence regarding efficacy.
Among biologics, secukinumab did not fare well in a phase 3 study of patients with PPP. A subset of patients may benefit from it, but there are no biomarkers available to identify them, Dr. Bachelez said. A phase 2 study of guselkumab in Japan told a similar story, with only weak signs of efficacy. While there are many more ongoing clinical trials evaluating treatments for PPP, which is encouraging, PPP seems to be more challenging at this stage to tackle than GPP, Dr. Bachelez added. “The genetically inherited IL-36 antagonist abnormalities are clearly driving the advances regarding the pathogenesis of the disease, mainly for GPP rather than PPP.”
Part of the efforts to develop therapies for pustular psoriasis relies on the development of new outcome measures, or adaptation of existing ones. “We have a need to adapt or develop new investigator-reported measures, we need to adapt or develop new patient-reported outcomes,” Dr. Duffin said.
Many existing measures use inconsistent language and anchoring definitions, and some may be proprietary, she added. “The language varies by sponsor and is sometimes tweaked or modified by the agencies. Often synonyms are being used … it raises questions, does it change the validity of the instrument?”
Dr. Duffin called for the research community to use the pause in clinical research during the COVID-19 pandemic to reassess the research agenda, develop consensus on performing and training for GPP and PPP assessments, develop patient-reported outcomes, and strengthen connections to industry.
Dr. Duffin and Dr. Bachelez have consulted, served on the advisory board, been a speaker for, and/or received research support from a wide range of pharmaceutical companies, including those that manufacture and develop psoriasis treatments.
It has various dermatologic and rheumatologic manifestations and sometimes overlaps with plaque psoriasis. Pustular palmoplantar psoriasis (PPP) affects the palmar and plantar areas of the skin, while generalized pustular psoriasis (GPP) can affect large areas of skin and tends to be more severe, even life threatening. PPP can accompany psoriatic arthritis or can be a side effect of tumor necrosis factor (TNF) inhibitor therapy, or a non–drug-induced component of rheumatologic syndromes, according to Kristina Callis Duffin, MD, an associate professor and chair of dermatology at the University of Utah, Salt Lake City.
“Each phenotype could be considered an orphan disease, and the response to therapy is often unpredictable,” Dr. Duffin said during a session on pustular psoriasis at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
But there is some positive news. A study in 2011 of several people with GPP opened the door to better understanding the pathophysiology of pustular psoriasis. Researchers identified a causal autosomal mutation in the IL36RN gene, which encodes an antagonist to the interleukin-36 receptor (Am J Hum Genet. 2011 Sep 9;89[3]:432-7). “As a result of this paper and others, drug development in this space has recently accelerated,” Dr. Duffin said.
In fact, she added,“it’s my opinion that pustular psoriasis is now where plaque psoriasis was 20 years ago, when accelerated drug development was driving a better understanding of the pathogenesis of psoriatic disease and its comorbidities, and also driving outcome measure development.”
In another presentation at the meeting, Hervé Bachelez, MD, PhD, professor of dermatology and immunologist at the University of Paris and Saint-Louis Hospital, Paris, discussed recent advances in drug development for pustular psoriasis. He noted other recent findings of genetic variants related to the disease, including AP1S3, CARD14, and SERPINA3.
For GPP, he said, the current algorithm for management is based on weak evidence for treatments like acitretin, cyclosporine, methotrexate, and infliximab. The story is similar for other biologics, with evidence in the form of case series; open-label studies; controlled, prospective studies; or retrospective analyses. Most of the evidence has been amassed for TNF inhibitors. A retrospective study of all TNF inhibitors suggested they may be effective as induction and maintenance therapy, he noted.
Among IL-17A inhibitors, a prospective study of 12 patients in Japan found secukinumab showed efficacy against GPP, as did studies of ixekizumab and brodalumab. A small phase 3 study in Japan demonstrated efficacy for the IL-23 inhibitor guselkumab in patients with erythrodermic psoriasis and GPP (J Dermatol. 2018 May;45[5]:529-39).
The limited data are a reflection in part of the difficulty in studying GPP, since its flares tend to be more self-remitting than with psoriasis vulgaris or PPP.
There are two monoclonal antibodies against the IL-36 receptor currently being developed. A proof-of-concept study of one of them, spesolimab, showed promise against GPP, with five of seven patients reaching “clear” or “almost clear” scores on the Generalized Pustular Psoriasis Physician Global Assessment within a week after infusion and in all seven by the fourth week (N Engl J Med. 2019 Mar 7;380[10]:981-3).
With respect to PPP, the strongest evidence for conventional therapies comes from two randomized, controlled trials of cyclosporine, with response rates of 48% and 89%, compared with 19% and 21%, respectively, in the placebo groups, although the primary endpoint was poorly designed, according to Dr. Bachelez. Retinoids like etretinate and acitretin, combined with psoralen and UVA, also have some supporting evidence regarding efficacy.
Among biologics, secukinumab did not fare well in a phase 3 study of patients with PPP. A subset of patients may benefit from it, but there are no biomarkers available to identify them, Dr. Bachelez said. A phase 2 study of guselkumab in Japan told a similar story, with only weak signs of efficacy. While there are many more ongoing clinical trials evaluating treatments for PPP, which is encouraging, PPP seems to be more challenging at this stage to tackle than GPP, Dr. Bachelez added. “The genetically inherited IL-36 antagonist abnormalities are clearly driving the advances regarding the pathogenesis of the disease, mainly for GPP rather than PPP.”
Part of the efforts to develop therapies for pustular psoriasis relies on the development of new outcome measures, or adaptation of existing ones. “We have a need to adapt or develop new investigator-reported measures, we need to adapt or develop new patient-reported outcomes,” Dr. Duffin said.
Many existing measures use inconsistent language and anchoring definitions, and some may be proprietary, she added. “The language varies by sponsor and is sometimes tweaked or modified by the agencies. Often synonyms are being used … it raises questions, does it change the validity of the instrument?”
Dr. Duffin called for the research community to use the pause in clinical research during the COVID-19 pandemic to reassess the research agenda, develop consensus on performing and training for GPP and PPP assessments, develop patient-reported outcomes, and strengthen connections to industry.
Dr. Duffin and Dr. Bachelez have consulted, served on the advisory board, been a speaker for, and/or received research support from a wide range of pharmaceutical companies, including those that manufacture and develop psoriasis treatments.
FROM THE GRAPPA 2020 VIRTUAL ANNUAL MEETING
Psoriatic disease inflammation linked to heart failure
Patients with psoriatic disease are known to be at increased risk of heart failure. A new cohort study suggests that part of the risk may be attributable to the disease itself, not just traditional cardiovascular risk factors like obesity and metabolic abnormalities that are common comorbidities in psoriatic disease. There may also be differences in the risk profiles of patients with ischemic and nonischemic heart failure.
Previous studies have shown that heart failure risk in patients with psoriatic arthritis is 32% higher than in the general population, and with psoriasis, it is 22%-53% higher. However, those studies were based on administrative databases with no clinical information to back up the accuracy of diagnoses, Sahil Koppikar, MD, from the University of Toronto, said during a presentation of the research at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
The finding that psoriatic disease inflammation may be a direct risk factor for heart failure might be good news for patients. “By controlling inflammation, we may be able to reduce the risk of heart failure in these patients,” Dr. Koppikar said.
During a question and answer session, discussant Deepak Jadon, MBChB, PhD, director of the rheumatology research unit and lead for psoriatic arthritis at Addenbrooke’s Hospital, Cambridge (England), noted that patients with conditions like lupus and systemic sclerosis may undergo regular echocardiograms, chest CTs, or other surveillance, and asked if Dr. Koppikar could recommend a framework for similar surveillance in psoriatic arthritis.
“With the current data we have, I don’t know if we can make recommendations. What we learned from our study is that patients that have elevated inflammatory disease, with elevated inflammatory markers for a prolonged period of time, were at higher risk than [if they had elevated markers only] just before the event. So poorly controlled patients might be something you should be more aware of, and maybe get cardiology involved. But I don’t think it’s something we should be doing right now for all patients,” Dr. Koppikar said.
The researchers analyzed data from a psoriasis cohort at the University of Toronto that began in 2006. Every 6-12 months, they were assessed by a rheumatologist and underwent imaging assessment and laboratory tests. The primary outcome of the study was the first heart failure event, which the researchers identified by linking the cohort database with provincial hospitalization and mortality databases. They verified all events by examining medical records. They also assessed the association between heart failure and disease activity over time rather than just before the event.
The analysis included 1,994 patients. A total of 64 new heart failure events occurred during a mean follow-up of 11.3 years (2.85 per 1,000 person-years), including 38 ischemic and 26 nonischemic events. A multivariate analysis found that heart failure was associated with adjusted mean (AM) tender joint count (hazard ratio, 1.51; P = .02), AM swollen joint count (HR, 1.82; P = .04), AM erythrocyte sedimentation rate (HR, 1.26; P = .009), AM C-reactive protein (HR, 1.27; P = .001), Health Assessment Questionnaire (HR, 1.95; P = .001), and minimum disease activity state (HR, 0.40; P = .04). The multivariate analysis was adjusted for sex, hypertension, diabetes mellitus, body mass index, ischemic heart disease, lipids, and smoking status.
When the researchers separated the analysis into ischemic and nonischemic heart failure, some interesting associations popped out. Nonischemic heart failure was associated with AM tender joint count (HR, 1.83; P = .004), but ischemic heart failure was not. Other factors associated with nonischemic but not ischemic heart failure included AM swollen joint count (HR, 3.56; P = .0003), damaged joint count (HR, 1.29; P = .04), and pain score (HR, 1.22; P = .047). Minimum disease activity had the opposite result: It was associated with only ischemic heart failure (HR, 0.40; P = .04).
The study cohort more closely resembles a rheumatology cohort than a dermatology cohort, and it suggests that patients with psoriatic arthritis have different cardiovascular comorbidities than those with pure psoriasis, according to Diamant Thaçi, MD, PhD, professor and chairman of the department of dermatology at the University of Lübeck (Germany). “It shows how it important it is to look for comorbidity in the rheumatologic setting,” Dr. Thaçi said in an interview.
The study was supported by the Arthritis Society. Dr. Koppikar and Dr. Thaçi have no relevant financial disclosures.
SOURCE: Koppikar S et al. GRAPPA 2020 Virtual Annual Meeting.
Patients with psoriatic disease are known to be at increased risk of heart failure. A new cohort study suggests that part of the risk may be attributable to the disease itself, not just traditional cardiovascular risk factors like obesity and metabolic abnormalities that are common comorbidities in psoriatic disease. There may also be differences in the risk profiles of patients with ischemic and nonischemic heart failure.
Previous studies have shown that heart failure risk in patients with psoriatic arthritis is 32% higher than in the general population, and with psoriasis, it is 22%-53% higher. However, those studies were based on administrative databases with no clinical information to back up the accuracy of diagnoses, Sahil Koppikar, MD, from the University of Toronto, said during a presentation of the research at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
The finding that psoriatic disease inflammation may be a direct risk factor for heart failure might be good news for patients. “By controlling inflammation, we may be able to reduce the risk of heart failure in these patients,” Dr. Koppikar said.
During a question and answer session, discussant Deepak Jadon, MBChB, PhD, director of the rheumatology research unit and lead for psoriatic arthritis at Addenbrooke’s Hospital, Cambridge (England), noted that patients with conditions like lupus and systemic sclerosis may undergo regular echocardiograms, chest CTs, or other surveillance, and asked if Dr. Koppikar could recommend a framework for similar surveillance in psoriatic arthritis.
“With the current data we have, I don’t know if we can make recommendations. What we learned from our study is that patients that have elevated inflammatory disease, with elevated inflammatory markers for a prolonged period of time, were at higher risk than [if they had elevated markers only] just before the event. So poorly controlled patients might be something you should be more aware of, and maybe get cardiology involved. But I don’t think it’s something we should be doing right now for all patients,” Dr. Koppikar said.
The researchers analyzed data from a psoriasis cohort at the University of Toronto that began in 2006. Every 6-12 months, they were assessed by a rheumatologist and underwent imaging assessment and laboratory tests. The primary outcome of the study was the first heart failure event, which the researchers identified by linking the cohort database with provincial hospitalization and mortality databases. They verified all events by examining medical records. They also assessed the association between heart failure and disease activity over time rather than just before the event.
The analysis included 1,994 patients. A total of 64 new heart failure events occurred during a mean follow-up of 11.3 years (2.85 per 1,000 person-years), including 38 ischemic and 26 nonischemic events. A multivariate analysis found that heart failure was associated with adjusted mean (AM) tender joint count (hazard ratio, 1.51; P = .02), AM swollen joint count (HR, 1.82; P = .04), AM erythrocyte sedimentation rate (HR, 1.26; P = .009), AM C-reactive protein (HR, 1.27; P = .001), Health Assessment Questionnaire (HR, 1.95; P = .001), and minimum disease activity state (HR, 0.40; P = .04). The multivariate analysis was adjusted for sex, hypertension, diabetes mellitus, body mass index, ischemic heart disease, lipids, and smoking status.
When the researchers separated the analysis into ischemic and nonischemic heart failure, some interesting associations popped out. Nonischemic heart failure was associated with AM tender joint count (HR, 1.83; P = .004), but ischemic heart failure was not. Other factors associated with nonischemic but not ischemic heart failure included AM swollen joint count (HR, 3.56; P = .0003), damaged joint count (HR, 1.29; P = .04), and pain score (HR, 1.22; P = .047). Minimum disease activity had the opposite result: It was associated with only ischemic heart failure (HR, 0.40; P = .04).
The study cohort more closely resembles a rheumatology cohort than a dermatology cohort, and it suggests that patients with psoriatic arthritis have different cardiovascular comorbidities than those with pure psoriasis, according to Diamant Thaçi, MD, PhD, professor and chairman of the department of dermatology at the University of Lübeck (Germany). “It shows how it important it is to look for comorbidity in the rheumatologic setting,” Dr. Thaçi said in an interview.
The study was supported by the Arthritis Society. Dr. Koppikar and Dr. Thaçi have no relevant financial disclosures.
SOURCE: Koppikar S et al. GRAPPA 2020 Virtual Annual Meeting.
Patients with psoriatic disease are known to be at increased risk of heart failure. A new cohort study suggests that part of the risk may be attributable to the disease itself, not just traditional cardiovascular risk factors like obesity and metabolic abnormalities that are common comorbidities in psoriatic disease. There may also be differences in the risk profiles of patients with ischemic and nonischemic heart failure.
Previous studies have shown that heart failure risk in patients with psoriatic arthritis is 32% higher than in the general population, and with psoriasis, it is 22%-53% higher. However, those studies were based on administrative databases with no clinical information to back up the accuracy of diagnoses, Sahil Koppikar, MD, from the University of Toronto, said during a presentation of the research at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
The finding that psoriatic disease inflammation may be a direct risk factor for heart failure might be good news for patients. “By controlling inflammation, we may be able to reduce the risk of heart failure in these patients,” Dr. Koppikar said.
During a question and answer session, discussant Deepak Jadon, MBChB, PhD, director of the rheumatology research unit and lead for psoriatic arthritis at Addenbrooke’s Hospital, Cambridge (England), noted that patients with conditions like lupus and systemic sclerosis may undergo regular echocardiograms, chest CTs, or other surveillance, and asked if Dr. Koppikar could recommend a framework for similar surveillance in psoriatic arthritis.
“With the current data we have, I don’t know if we can make recommendations. What we learned from our study is that patients that have elevated inflammatory disease, with elevated inflammatory markers for a prolonged period of time, were at higher risk than [if they had elevated markers only] just before the event. So poorly controlled patients might be something you should be more aware of, and maybe get cardiology involved. But I don’t think it’s something we should be doing right now for all patients,” Dr. Koppikar said.
The researchers analyzed data from a psoriasis cohort at the University of Toronto that began in 2006. Every 6-12 months, they were assessed by a rheumatologist and underwent imaging assessment and laboratory tests. The primary outcome of the study was the first heart failure event, which the researchers identified by linking the cohort database with provincial hospitalization and mortality databases. They verified all events by examining medical records. They also assessed the association between heart failure and disease activity over time rather than just before the event.
The analysis included 1,994 patients. A total of 64 new heart failure events occurred during a mean follow-up of 11.3 years (2.85 per 1,000 person-years), including 38 ischemic and 26 nonischemic events. A multivariate analysis found that heart failure was associated with adjusted mean (AM) tender joint count (hazard ratio, 1.51; P = .02), AM swollen joint count (HR, 1.82; P = .04), AM erythrocyte sedimentation rate (HR, 1.26; P = .009), AM C-reactive protein (HR, 1.27; P = .001), Health Assessment Questionnaire (HR, 1.95; P = .001), and minimum disease activity state (HR, 0.40; P = .04). The multivariate analysis was adjusted for sex, hypertension, diabetes mellitus, body mass index, ischemic heart disease, lipids, and smoking status.
When the researchers separated the analysis into ischemic and nonischemic heart failure, some interesting associations popped out. Nonischemic heart failure was associated with AM tender joint count (HR, 1.83; P = .004), but ischemic heart failure was not. Other factors associated with nonischemic but not ischemic heart failure included AM swollen joint count (HR, 3.56; P = .0003), damaged joint count (HR, 1.29; P = .04), and pain score (HR, 1.22; P = .047). Minimum disease activity had the opposite result: It was associated with only ischemic heart failure (HR, 0.40; P = .04).
The study cohort more closely resembles a rheumatology cohort than a dermatology cohort, and it suggests that patients with psoriatic arthritis have different cardiovascular comorbidities than those with pure psoriasis, according to Diamant Thaçi, MD, PhD, professor and chairman of the department of dermatology at the University of Lübeck (Germany). “It shows how it important it is to look for comorbidity in the rheumatologic setting,” Dr. Thaçi said in an interview.
The study was supported by the Arthritis Society. Dr. Koppikar and Dr. Thaçi have no relevant financial disclosures.
SOURCE: Koppikar S et al. GRAPPA 2020 Virtual Annual Meeting.
FROM GRAPPA 2020 VIRTUAL ANNUAL MEETING
Doctors hesitated to embrace biosimilar infliximab in first 2 years
Physicians have been slow to embrace biosimilar versions of infliximab, but are more likely to prescribe it to new patients, based on data from a review of nearly 50,000 infliximab claims through Medicare in the first 2 years that biosimilars were available in the United States.
“Although biosimilar versions are as safe and effective as the biologic, patients and physicians may be more reluctant to switch from a working biologic regimen in a chronic setting than an acute one,” wrote Alice J. Chen, PhD, of the University of Southern California, Los Angeles, and colleagues.
In a research letter published in JAMA Internal Medicine, the investigators examined prescribing patterns of physicians switching between the originator infliximab (Remicade) and two of its biosimilars (Inflectra and Renflexis).
They reviewed infliximab use and reimbursement in the 100% Medicare Part B quarterly claims database from Jan. 1, 2017, to Dec. 31, 2018. The study population included Medicare patients classified as new if they had no infliximab claims in the prior 6 months; those with claims were considered returning patients.
In a comparison of claims reflecting 49,771 patients and 4,289 physicians in 2018, a total of 1,418 new patients (17.4%) and 4,495 (10.8%) returning patients used a biosimilar. “Of returning patients, half used the biosimilar version exclusively, whereas the other half switched between biologic and biosimilar versions,” the researchers noted.
Of the 4,289 physicians who prescribed infliximab, 3,124 prescribed no biosimilars, 1,015 prescribed both biologics and biosimilars, and 150 prescribed biosimilars only. Of the physicians who prescribed both, approximately 61% switched some patients from the biologic to the biosimilar; “the remainder kept individual patients on only 1 version of the drug but treated patients with both versions,” the researchers wrote.
The adoption of biosimilars may be slower for chronic vs. acute conditions, the researchers noted. “Prescribers may hesitate to switch clinically stable chronic patients from biologic regimens if they are unfamiliar with the biosimilar or face financial disincentives from prescribing it.”
The study findings were limited by several factors including the use of only 2 years of data and a focus only on Medicare Part B. Switching medications may have been influenced by factors such as lower copays for patients and rebates or discounts for physicians; however, “further research is needed to better understand biosimilar pricing dynamics and the barriers to adopting biosimilars for chronic conditions,” they concluded.
The study was supported by the Leonard D. Schaeffer Center for Health Policy & Economics at the University of Southern California, Los Angeles, and the National Institute on Aging. Lead author Dr. Chen also disclosed receiving personal fees from Amgen outside of the current study.
SOURCE: Chen AJ et al. JAMA Intern Med. 2020 July 20. doi: 10.1001/jamainternmed.2020.3188.
Physicians have been slow to embrace biosimilar versions of infliximab, but are more likely to prescribe it to new patients, based on data from a review of nearly 50,000 infliximab claims through Medicare in the first 2 years that biosimilars were available in the United States.
“Although biosimilar versions are as safe and effective as the biologic, patients and physicians may be more reluctant to switch from a working biologic regimen in a chronic setting than an acute one,” wrote Alice J. Chen, PhD, of the University of Southern California, Los Angeles, and colleagues.
In a research letter published in JAMA Internal Medicine, the investigators examined prescribing patterns of physicians switching between the originator infliximab (Remicade) and two of its biosimilars (Inflectra and Renflexis).
They reviewed infliximab use and reimbursement in the 100% Medicare Part B quarterly claims database from Jan. 1, 2017, to Dec. 31, 2018. The study population included Medicare patients classified as new if they had no infliximab claims in the prior 6 months; those with claims were considered returning patients.
In a comparison of claims reflecting 49,771 patients and 4,289 physicians in 2018, a total of 1,418 new patients (17.4%) and 4,495 (10.8%) returning patients used a biosimilar. “Of returning patients, half used the biosimilar version exclusively, whereas the other half switched between biologic and biosimilar versions,” the researchers noted.
Of the 4,289 physicians who prescribed infliximab, 3,124 prescribed no biosimilars, 1,015 prescribed both biologics and biosimilars, and 150 prescribed biosimilars only. Of the physicians who prescribed both, approximately 61% switched some patients from the biologic to the biosimilar; “the remainder kept individual patients on only 1 version of the drug but treated patients with both versions,” the researchers wrote.
The adoption of biosimilars may be slower for chronic vs. acute conditions, the researchers noted. “Prescribers may hesitate to switch clinically stable chronic patients from biologic regimens if they are unfamiliar with the biosimilar or face financial disincentives from prescribing it.”
The study findings were limited by several factors including the use of only 2 years of data and a focus only on Medicare Part B. Switching medications may have been influenced by factors such as lower copays for patients and rebates or discounts for physicians; however, “further research is needed to better understand biosimilar pricing dynamics and the barriers to adopting biosimilars for chronic conditions,” they concluded.
The study was supported by the Leonard D. Schaeffer Center for Health Policy & Economics at the University of Southern California, Los Angeles, and the National Institute on Aging. Lead author Dr. Chen also disclosed receiving personal fees from Amgen outside of the current study.
SOURCE: Chen AJ et al. JAMA Intern Med. 2020 July 20. doi: 10.1001/jamainternmed.2020.3188.
Physicians have been slow to embrace biosimilar versions of infliximab, but are more likely to prescribe it to new patients, based on data from a review of nearly 50,000 infliximab claims through Medicare in the first 2 years that biosimilars were available in the United States.
“Although biosimilar versions are as safe and effective as the biologic, patients and physicians may be more reluctant to switch from a working biologic regimen in a chronic setting than an acute one,” wrote Alice J. Chen, PhD, of the University of Southern California, Los Angeles, and colleagues.
In a research letter published in JAMA Internal Medicine, the investigators examined prescribing patterns of physicians switching between the originator infliximab (Remicade) and two of its biosimilars (Inflectra and Renflexis).
They reviewed infliximab use and reimbursement in the 100% Medicare Part B quarterly claims database from Jan. 1, 2017, to Dec. 31, 2018. The study population included Medicare patients classified as new if they had no infliximab claims in the prior 6 months; those with claims were considered returning patients.
In a comparison of claims reflecting 49,771 patients and 4,289 physicians in 2018, a total of 1,418 new patients (17.4%) and 4,495 (10.8%) returning patients used a biosimilar. “Of returning patients, half used the biosimilar version exclusively, whereas the other half switched between biologic and biosimilar versions,” the researchers noted.
Of the 4,289 physicians who prescribed infliximab, 3,124 prescribed no biosimilars, 1,015 prescribed both biologics and biosimilars, and 150 prescribed biosimilars only. Of the physicians who prescribed both, approximately 61% switched some patients from the biologic to the biosimilar; “the remainder kept individual patients on only 1 version of the drug but treated patients with both versions,” the researchers wrote.
The adoption of biosimilars may be slower for chronic vs. acute conditions, the researchers noted. “Prescribers may hesitate to switch clinically stable chronic patients from biologic regimens if they are unfamiliar with the biosimilar or face financial disincentives from prescribing it.”
The study findings were limited by several factors including the use of only 2 years of data and a focus only on Medicare Part B. Switching medications may have been influenced by factors such as lower copays for patients and rebates or discounts for physicians; however, “further research is needed to better understand biosimilar pricing dynamics and the barriers to adopting biosimilars for chronic conditions,” they concluded.
The study was supported by the Leonard D. Schaeffer Center for Health Policy & Economics at the University of Southern California, Los Angeles, and the National Institute on Aging. Lead author Dr. Chen also disclosed receiving personal fees from Amgen outside of the current study.
SOURCE: Chen AJ et al. JAMA Intern Med. 2020 July 20. doi: 10.1001/jamainternmed.2020.3188.
FROM JAMA INTERNAL MEDICINE
Key clinical point: A total of 17% of patients new to infliximab received a biosimilar in 2018, compared with 11% of returning patients.
Major finding: Biosimilar infliximab accounted for 10% of the market share 2 years after the product was introduced.
Study details: The data come from a review of infliximab claims across 49,771 patients and 4,289 physicians who prescribed infliximab in 2018.
Disclosures: The study was supported by the Leonard D. Schaeffer Center for Health Policy & Economics at the University of Southern California, Los Angeles, and the National Institute on Aging. Lead author Dr. Chen also disclosed receiving personal fees from Amgen outside of the current study.
Source: Chen AJ et al. JAMA Intern Med. 2020 July 20. doi: 10.1001/jamainternmed.2020.3188.
Clinicians address psoriatic disease risk in the era of COVID-19
COVID-19 has posed serious questions for patients with psoriatic disease and the clinicians who treat them. Both have serious concerns over whether psoriasis or the medications used to treat it pose additional risk for contracting COVID-19 or experiencing worse outcomes with illness.
At the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, experts gathered to discuss these concerns and what is known about the special risk factors for psoriatic disease patients.
Studies from a few registries have been done already among patients with autoimmune disease, and the results so far suggest that patients may be able to breathe a little easier. “I don’t see any data that suggests that use of immunosuppressives or having autoimmune disease increases your risk of acquiring it. I think most of the risk is driven by risk of exposure,” said Kevin Winthrop, MD, MPH, a professor of public health, infectious diseases, ophthalmology at Oregon Health & Science University, Portland, during a presentation.
That assertion was reinforced by data presented by Rebecca Haberman, MD, a rheumatologist at New York University Langone Health. Her group created the Web-Based Assessment of Autoimmune, Immune-Mediated, and Rheumatic Patients during the COVID-19 Pandemic (WARCOV) cohort study to address the question of whether patients with immune-mediated inflammatory disease (IMID), including inflammatory arthritis, psoriasis, or inflammatory bowel disease, should discontinue or modify their immunotherapy regimens in the face of potential exposure to COVID-19.
To date, the study has data on 1,122 patients; 604 with inflammatory arthritis, 128 of whom have tested positive for COVID-19. The team established a cohort using the first 86 IMID patients confirmed to have contracted COVID-19. The hospitalization rate was 16% overall, and use of corticosteroids was associated with increased hospitalization risk. A follow-up analysis looking at the first 103 inflammatory arthritis patients who contracted COVID-19 showed a hospitalization rate of 26% and a mortality of 4%. That hospitalization rate is similar to the general hospitalization rate estimated by the New York Department of Health, Dr. Haberman said in her presentation.
Risk factors associated with hospitalization included being older and having asthma or COPD, which is similar to the general population. Use of oral glucocorticoids was linked to a big increase in risk for hospitalization, even with doses less than 10 mg prednisone daily (odds ratio, 14.31; 95% confidence interval, 3.55-57.70). There were no links between use of any cytokine therapy and risk, but use of TNF inhibitors was associated with a reduced risk (OR, 0.35; 95% CI, 0.13-0.97), while use of JAK inhibitors was associated with greater risk (OR, 6.30; 95% CI, 1.68-23.69). The latter result is tentative because of a small sample size, and it was driven largely by the experiences of patients with psoriatic arthritis.
Another study, run by the COVID-19 Global Rheumatology Alliance, looked at 600 patients with rheumatic disease from 40 countries, and “found no smoking gun,” said Leonard Calabrese, DO, who leads the Cleveland Clinic’s section of clinical immunology, during his presentation. “People can develop this when they’re on hydroxychloroquine. They seem to do not remarkably bad or remarkably good. There is no adverse signal for biologics, but being on prednisone [at a dose of] more than 10 mg is not great,” said Dr. Calabrese, who also noted that other publications have supported these conclusions.
So given these findings, how should clinicians address patient concerns? In the absence of probable exposure, “we say it’s better to have a well-controlled IMID on therapy than a poorly-controlled IMID on submaximal therapy. We say stick to therapy and try to wean the prednisone down as low as possible,” Dr. Calabrese said.
More controversially, what should patients do if they have had a significant exposure, such as a close proximity, prolonged exposure encounter with an individual with documented COVID-19, or at high-risk of disease? Dr. Calabrese noted that the American College of Rheumatology (ACR) guidelines recommend that low-level immunomodulation can be continued, “with an asterisk if it’s hydroxychloroquine, and it is in most of our minds now that we know that it is not effective, and the toxicity in the COVID setting is still being worked out,” he said.
With respect to other immunosuppressants, the ACR recommends stopping them temporarily, although IL-6 inhibitors may be continued in select circumstances. Resumption of the therapeutics can resume after a negative COVID test or completion of a 2-week observation period.
When patients contract COVID-19, antimalarial medications can be continued because they have been studied. “But medium-level immunomodulators, in particular methotrexate, I have grave concerns about because it can inhibit the adaptive immune response and antibody formation,” he said. COVID-19 is a serious infection, and all serious biologics have a package insert saying to stop them in a serious infection. Again, IL-6 inhibitors may be considered an exception in the right circumstances. When to resume these medications remains unknown. “I think that’s a work in progress. Test-based versus clinic-based strategies are a matter of controversy,” Dr. Calabrese said.
Ultimately, the question of what to do with immunosuppressive therapies in this population will continue to be a challenge. “The only good answer is to follow the rules of social distancing and to wear a mask,” said Kristina Callis Duffin, MD, a cochair of the department of dermatology and associate professor of dermatology at the University of Utah, Salt Lake City.
COVID-19 has posed serious questions for patients with psoriatic disease and the clinicians who treat them. Both have serious concerns over whether psoriasis or the medications used to treat it pose additional risk for contracting COVID-19 or experiencing worse outcomes with illness.
At the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, experts gathered to discuss these concerns and what is known about the special risk factors for psoriatic disease patients.
Studies from a few registries have been done already among patients with autoimmune disease, and the results so far suggest that patients may be able to breathe a little easier. “I don’t see any data that suggests that use of immunosuppressives or having autoimmune disease increases your risk of acquiring it. I think most of the risk is driven by risk of exposure,” said Kevin Winthrop, MD, MPH, a professor of public health, infectious diseases, ophthalmology at Oregon Health & Science University, Portland, during a presentation.
That assertion was reinforced by data presented by Rebecca Haberman, MD, a rheumatologist at New York University Langone Health. Her group created the Web-Based Assessment of Autoimmune, Immune-Mediated, and Rheumatic Patients during the COVID-19 Pandemic (WARCOV) cohort study to address the question of whether patients with immune-mediated inflammatory disease (IMID), including inflammatory arthritis, psoriasis, or inflammatory bowel disease, should discontinue or modify their immunotherapy regimens in the face of potential exposure to COVID-19.
To date, the study has data on 1,122 patients; 604 with inflammatory arthritis, 128 of whom have tested positive for COVID-19. The team established a cohort using the first 86 IMID patients confirmed to have contracted COVID-19. The hospitalization rate was 16% overall, and use of corticosteroids was associated with increased hospitalization risk. A follow-up analysis looking at the first 103 inflammatory arthritis patients who contracted COVID-19 showed a hospitalization rate of 26% and a mortality of 4%. That hospitalization rate is similar to the general hospitalization rate estimated by the New York Department of Health, Dr. Haberman said in her presentation.
Risk factors associated with hospitalization included being older and having asthma or COPD, which is similar to the general population. Use of oral glucocorticoids was linked to a big increase in risk for hospitalization, even with doses less than 10 mg prednisone daily (odds ratio, 14.31; 95% confidence interval, 3.55-57.70). There were no links between use of any cytokine therapy and risk, but use of TNF inhibitors was associated with a reduced risk (OR, 0.35; 95% CI, 0.13-0.97), while use of JAK inhibitors was associated with greater risk (OR, 6.30; 95% CI, 1.68-23.69). The latter result is tentative because of a small sample size, and it was driven largely by the experiences of patients with psoriatic arthritis.
Another study, run by the COVID-19 Global Rheumatology Alliance, looked at 600 patients with rheumatic disease from 40 countries, and “found no smoking gun,” said Leonard Calabrese, DO, who leads the Cleveland Clinic’s section of clinical immunology, during his presentation. “People can develop this when they’re on hydroxychloroquine. They seem to do not remarkably bad or remarkably good. There is no adverse signal for biologics, but being on prednisone [at a dose of] more than 10 mg is not great,” said Dr. Calabrese, who also noted that other publications have supported these conclusions.
So given these findings, how should clinicians address patient concerns? In the absence of probable exposure, “we say it’s better to have a well-controlled IMID on therapy than a poorly-controlled IMID on submaximal therapy. We say stick to therapy and try to wean the prednisone down as low as possible,” Dr. Calabrese said.
More controversially, what should patients do if they have had a significant exposure, such as a close proximity, prolonged exposure encounter with an individual with documented COVID-19, or at high-risk of disease? Dr. Calabrese noted that the American College of Rheumatology (ACR) guidelines recommend that low-level immunomodulation can be continued, “with an asterisk if it’s hydroxychloroquine, and it is in most of our minds now that we know that it is not effective, and the toxicity in the COVID setting is still being worked out,” he said.
With respect to other immunosuppressants, the ACR recommends stopping them temporarily, although IL-6 inhibitors may be continued in select circumstances. Resumption of the therapeutics can resume after a negative COVID test or completion of a 2-week observation period.
When patients contract COVID-19, antimalarial medications can be continued because they have been studied. “But medium-level immunomodulators, in particular methotrexate, I have grave concerns about because it can inhibit the adaptive immune response and antibody formation,” he said. COVID-19 is a serious infection, and all serious biologics have a package insert saying to stop them in a serious infection. Again, IL-6 inhibitors may be considered an exception in the right circumstances. When to resume these medications remains unknown. “I think that’s a work in progress. Test-based versus clinic-based strategies are a matter of controversy,” Dr. Calabrese said.
Ultimately, the question of what to do with immunosuppressive therapies in this population will continue to be a challenge. “The only good answer is to follow the rules of social distancing and to wear a mask,” said Kristina Callis Duffin, MD, a cochair of the department of dermatology and associate professor of dermatology at the University of Utah, Salt Lake City.
COVID-19 has posed serious questions for patients with psoriatic disease and the clinicians who treat them. Both have serious concerns over whether psoriasis or the medications used to treat it pose additional risk for contracting COVID-19 or experiencing worse outcomes with illness.
At the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, experts gathered to discuss these concerns and what is known about the special risk factors for psoriatic disease patients.
Studies from a few registries have been done already among patients with autoimmune disease, and the results so far suggest that patients may be able to breathe a little easier. “I don’t see any data that suggests that use of immunosuppressives or having autoimmune disease increases your risk of acquiring it. I think most of the risk is driven by risk of exposure,” said Kevin Winthrop, MD, MPH, a professor of public health, infectious diseases, ophthalmology at Oregon Health & Science University, Portland, during a presentation.
That assertion was reinforced by data presented by Rebecca Haberman, MD, a rheumatologist at New York University Langone Health. Her group created the Web-Based Assessment of Autoimmune, Immune-Mediated, and Rheumatic Patients during the COVID-19 Pandemic (WARCOV) cohort study to address the question of whether patients with immune-mediated inflammatory disease (IMID), including inflammatory arthritis, psoriasis, or inflammatory bowel disease, should discontinue or modify their immunotherapy regimens in the face of potential exposure to COVID-19.
To date, the study has data on 1,122 patients; 604 with inflammatory arthritis, 128 of whom have tested positive for COVID-19. The team established a cohort using the first 86 IMID patients confirmed to have contracted COVID-19. The hospitalization rate was 16% overall, and use of corticosteroids was associated with increased hospitalization risk. A follow-up analysis looking at the first 103 inflammatory arthritis patients who contracted COVID-19 showed a hospitalization rate of 26% and a mortality of 4%. That hospitalization rate is similar to the general hospitalization rate estimated by the New York Department of Health, Dr. Haberman said in her presentation.
Risk factors associated with hospitalization included being older and having asthma or COPD, which is similar to the general population. Use of oral glucocorticoids was linked to a big increase in risk for hospitalization, even with doses less than 10 mg prednisone daily (odds ratio, 14.31; 95% confidence interval, 3.55-57.70). There were no links between use of any cytokine therapy and risk, but use of TNF inhibitors was associated with a reduced risk (OR, 0.35; 95% CI, 0.13-0.97), while use of JAK inhibitors was associated with greater risk (OR, 6.30; 95% CI, 1.68-23.69). The latter result is tentative because of a small sample size, and it was driven largely by the experiences of patients with psoriatic arthritis.
Another study, run by the COVID-19 Global Rheumatology Alliance, looked at 600 patients with rheumatic disease from 40 countries, and “found no smoking gun,” said Leonard Calabrese, DO, who leads the Cleveland Clinic’s section of clinical immunology, during his presentation. “People can develop this when they’re on hydroxychloroquine. They seem to do not remarkably bad or remarkably good. There is no adverse signal for biologics, but being on prednisone [at a dose of] more than 10 mg is not great,” said Dr. Calabrese, who also noted that other publications have supported these conclusions.
So given these findings, how should clinicians address patient concerns? In the absence of probable exposure, “we say it’s better to have a well-controlled IMID on therapy than a poorly-controlled IMID on submaximal therapy. We say stick to therapy and try to wean the prednisone down as low as possible,” Dr. Calabrese said.
More controversially, what should patients do if they have had a significant exposure, such as a close proximity, prolonged exposure encounter with an individual with documented COVID-19, or at high-risk of disease? Dr. Calabrese noted that the American College of Rheumatology (ACR) guidelines recommend that low-level immunomodulation can be continued, “with an asterisk if it’s hydroxychloroquine, and it is in most of our minds now that we know that it is not effective, and the toxicity in the COVID setting is still being worked out,” he said.
With respect to other immunosuppressants, the ACR recommends stopping them temporarily, although IL-6 inhibitors may be continued in select circumstances. Resumption of the therapeutics can resume after a negative COVID test or completion of a 2-week observation period.
When patients contract COVID-19, antimalarial medications can be continued because they have been studied. “But medium-level immunomodulators, in particular methotrexate, I have grave concerns about because it can inhibit the adaptive immune response and antibody formation,” he said. COVID-19 is a serious infection, and all serious biologics have a package insert saying to stop them in a serious infection. Again, IL-6 inhibitors may be considered an exception in the right circumstances. When to resume these medications remains unknown. “I think that’s a work in progress. Test-based versus clinic-based strategies are a matter of controversy,” Dr. Calabrese said.
Ultimately, the question of what to do with immunosuppressive therapies in this population will continue to be a challenge. “The only good answer is to follow the rules of social distancing and to wear a mask,” said Kristina Callis Duffin, MD, a cochair of the department of dermatology and associate professor of dermatology at the University of Utah, Salt Lake City.
FROM THE GRAPPA 2020 VIRTUAL ANNUAL MEETING
Rupioid Psoriasis and Psoriatic Arthritis in a Patient With Skin of Color
To the Editor:
A 49-year-old black woman presented with multiple hyperkeratotic papules that progressed over the last 2 months to circular plaques with central thick black crust resembling eschar. She first noticed these lesions as firm, small, black papules on the legs and continued to develop new lesions that eventually evolved into large, coin-shaped, hyperkeratotic plaques. Her medical history was notable for stage III non-Hodgkin follicular lymphoma in remission after treatment with rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone 7 months earlier, and chronic hepatitis B infection being treated with entecavir. Her family history was not remarkable for psoriasis or inflammatory arthritis.
She initially was seen by internal medicine and was started on topical triamcinolone with no improvement of the lesions. At presentation to dermatology, physical examination revealed firm, small, black, hyperkeratotic papules (Figure 1A) and circular plaques with a rim of erythema and central thick, smooth, black crust resembling eschar (Figure 1B). No other skin changes were noted at the time. The bilateral metacarpophalangeal, bilateral proximal interphalangeal, left wrist, and bilateral ankle joints were remarkable for tenderness, swelling, and reduced range of motion. She noted concomitant arthralgia and stiffness but denied fever. She had no other systemic symptoms including night sweats, weight loss, fatigue, malaise, sun sensitivity, oral ulcers, or hair loss. A radiograph of the hand was negative for erosive changes but showed mild periarticular osteopenia and fusiform soft tissue swelling of the third digit. Given the central appearance of eschar in the larger lesions, the initial differential diagnosis included Sweet syndrome, invasive fungal infection, vasculitis, and recurrent lymphoma.
A 4-mm punch biopsy specimen of a representative lesion on the right leg revealed psoriasiform epidermal hyperplasia, parakeratosis, neutrophils in the stratum corneum and spinosum, elongation of the rete ridges, and superficial vascular ectasia, which favored a diagnosis of psoriasis (Figure 2). A periodic acid-Schiff stain was negative for fungal hyphae. Fungal culture, bacterial tissue culture, and acid-fast bacilli smear were negative. Absence of deep dermal inflammation precluded a diagnosis of Sweet syndrome. Further notable laboratory studies included negative human immunodeficiency virus (HIV) antibody, rapid plasma reagin, hepatitis C antibody, and rheumatoid factor.
At follow-up 2 weeks later, the initial lesions were still present, and she had developed new widespread, well-demarcated, erythematous plaques with silver scale along the scalp, back, chest, and abdomen that were more typical of psoriasis. Oil spots were noted on several fingernails and toenails. Based on the clinicopathologic findings, nail changes, and asymmetric inflammatory arthritis, a diagnosis of rupioid psoriasis with psoriatic arthritis (PsA) was established. Treatment with clobetasol ointment 0.05% twice daily to active lesions was started. Initiation of systemic therapy with a steroid-sparing agent was deferred in anticipation of care coordination with rheumatology, hepatology, and hematology/oncology due to the patient's history of follicular lymphoma and chronic hepatitis B. Although attempts were made to avoid systemic corticosteroids due to the risk for a psoriasis flare upon discontinuation, because of the severity of arthralgia she was started on oral prednisone 20 mg daily by rheumatology with plans for a slow taper once an alternative systemic agent was started.1
At 10-week follow-up, the patient had marked improvement of psoriatic plaques with no active lesions while only on prednisone 20 mg daily. In consultation with her care team, she subsequently was started on methotrexate 10 mg weekly for 2 weeks followed by titration to 15 mg weekly. Plans were to start a prednisone taper after a month of methotrexate to allow her new treatment time for therapeutic effect. Notably, the patient chose to discontinue prednisone 2 weeks into methotrexate therapy after only two 10-mg doses of methotrexate weekly and well before therapeutic levels were achieved. Despite stopping prednisone early and without a taper, she did not experience a relapse in psoriatic skin lesions. Three months following initiation of methotrexate, she sustained resolution of the cutaneous lesions with only residual postinflammatory hyperpigmentation.
Psoriasis is a common chronic inflammatory skin disorder with multiple clinical presentations. There are several variants of psoriasis that are classified by their morphologic appearance including chronic plaque, guttate, erythrodermic, and pustular, with more than 90% of cases representing the plaque variant. Less common clinical presentations of psoriasis include rupioid, ostraceous, inverse, elephantine, and HIV associated.2 Rupioid psoriasis is a rare variant that presents with cone-shaped, limpetlike lesions.3,4 Similar to the limited epidemiological and clinical data pertaining to psoriasis in nonwhite racial groups, there also is a paucity of documented reports of rupioid psoriasis in skin of color.
Rupioid comes from the Greek word rhupos, meaning dirt or filth, and is used to describe well-demarcated lesions with thick, yellow, dirty-appearing, adherent crusts resembling oyster shells with a surrounding rim of erythema.5 Rupioid psoriasis initially was reported in 1948 and remains an uncommon and infrequently reported variant.6 The majority of reported cases have been associated with arthropathy, similar to our patient.3,4 Rupioid lesions also have been observed in an array of other diseases, such as secondary syphilis, crusted scabies, disseminated histoplasmosis, HIV, reactive arthritis, and aminoaciduria.7-11
Diagnosis of rupioid psoriasis can be confirmed with a skin biopsy, which demonstrates characteristic histopathologic findings of psoriasis.3 Laboratory analysis should be performed to rule out other causes of rupioid lesions, and PsA should be differentiated from rheumatoid arthritis if arthropathy is present. In our case, serum rapid plasma reagin, anti-HIV antibody, rheumatoid factor, and fungal cultures were negative. Usin0)g clinical findings, histopathology, laboratory analyses, and radiograph findings, the diagnosis of rupioid psoriasis with PsA was confirmed in our patient.
Psoriasis was not originally suspected in our patient due to the noncharacteristic lesions with smooth black crust--similar appearing to eschar--and the patient's complicated medical history. Variations in the presentation of psoriasis among white individuals and those with skin of color have been reported in the literature.12,13 Psoriatic lesions in darker skin tones may appear more violaceous or hyperpigmented with more conspicuous erythema and thicker plaques. Our patient lacked the classic rupioid appearance of concentric circular layers of dirty, yellow, oysterlike scale, and instead had thick, lamellate, black crust. A PubMed search of articles indexed for MEDLINE using the terms rupioid, coral reef psoriasis, rupioides, and rhupus revealed no other cases of rupioid psoriasis reported in black patients and no cases detailing the variations of rupioid lesions in skin of color. A case of rupioid psoriasis has been reported in a Hispanic patient, but the described psoriatic lesions were more characteristic of the dirty-appearing, conic plaques previously reported.14 Our case highlights a unique example of the variable presentations of cutaneous disorders in skin of color and black patients.
Our patient's case of rupioid psoriasis with PsA presented unique challenges for systemic treatment due to her multiple comorbidities. Rupioid psoriasis most often is treated with combination topical and systemic therapy, with agents such as methotrexate and cyclosporine having prior success.3,4 This variant of psoriasis is highly responsive to treatment, and marked improvement of lesions has been achieved with topical steroids alone with proper adherence.15 Our patient was started on clobetasol ointment 0.05% while a systemic agent was debated for her PsA. Although she did not have improvement with topical therapy alone, she experienced rapid resolution of the skin lesions after initiation of low-dose prednisone 20 mg daily. Interestingly, our patient did not experience a flare of the skin lesions upon discontinuation of systemic steroids despite the lack of an appropriate taper and methotrexate not having reached therapeutic levels.
The clinical nuances of rupioid psoriasis in skin of color have not yet been described and remain an important diagnostic consideration. Our patient achieved remission of skin lesions with sequential treatment of topical clobetasol, a low-dose systemic steroid, and methotrexate. Based on available reports, rupioid psoriasis may represent a variant of psoriasis that is highly responsive to treatment.
- Mrowietz U, Domm S. Systemic steroids in the treatment of psoriasis: what is fact, what is fiction? J Eur Acad Dermatol Venereol. 2013;27:1022-1025.
- Goldsmith LA, Katz SI, Gilchrest BA, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Wang JL, Yang JH. Rupioid psoriasis associated with arthropathy. J Dermatol. 1997;24:46-49.
- Murakami T, Ohtsuki M, Nakagawa H. Rupioid psoriasis with arthropathy. Clin Exp Dermatol. 2000;25:409-412.
- Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis. 2014;94:119-121.
- Salamon M, Omulecki A, Sysa-Jedrzejowska A, et al. Psoriasisrupioides: a rare variant of a common disease. Cutis. 2011;88:135-137.
- Krase IZ, Cavanaugh K, Curiel-Lewandrowski C. A case of rupioid syphilis. JAAD Case Rep. 2016;2:141-143.
- Garofalo V, Saraceno R, Milana M, et al. Crusted scabies in a liver transplant patient mimicking rupioid psoriasis. Eur J Dermatol. 2016;26:495-496.
- Corti M, Villafane MF, Palmieri O, et al. Rupioid histoplasmosis: first case reported in an AIDS patient in Argentina. Rev Inst Med Trop Sao Paulo. 2010;52:279-280.
- Sehgal VN, Koranne RV, Shyam Prasad AL. Unusual manifestations of Reiter's disease in a child. Dermatologica. 1985;170:77-79.
- Haim S, Gilhar A, Cohen A. Cutaneous manifestations associated with aminoaciduria. report of two cases. Dermatologica. 1978;156:244-250.
- McMichael AJ, Vachiramon V, Guzman-Sanchez DA, et al. Psoriasis in African-Americans: a caregivers' survey. J Drugs Dermatol. 2012;11:478-482.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Posligua A, Maldonado C, Gonzalez MG. Rupioid psoriasis preceded by varicella presenting as Koebner phenomenon. J Am Acad Dermatol. 2016;74(5 suppl 1):AB268.
- Feldman SR, Feldman S, Brown K, et al. "Coral reef" psoriasis: a marker of resistance to topical treatment. J Dermatolog Treat. 2008;19:257-258.
To the Editor:
A 49-year-old black woman presented with multiple hyperkeratotic papules that progressed over the last 2 months to circular plaques with central thick black crust resembling eschar. She first noticed these lesions as firm, small, black papules on the legs and continued to develop new lesions that eventually evolved into large, coin-shaped, hyperkeratotic plaques. Her medical history was notable for stage III non-Hodgkin follicular lymphoma in remission after treatment with rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone 7 months earlier, and chronic hepatitis B infection being treated with entecavir. Her family history was not remarkable for psoriasis or inflammatory arthritis.
She initially was seen by internal medicine and was started on topical triamcinolone with no improvement of the lesions. At presentation to dermatology, physical examination revealed firm, small, black, hyperkeratotic papules (Figure 1A) and circular plaques with a rim of erythema and central thick, smooth, black crust resembling eschar (Figure 1B). No other skin changes were noted at the time. The bilateral metacarpophalangeal, bilateral proximal interphalangeal, left wrist, and bilateral ankle joints were remarkable for tenderness, swelling, and reduced range of motion. She noted concomitant arthralgia and stiffness but denied fever. She had no other systemic symptoms including night sweats, weight loss, fatigue, malaise, sun sensitivity, oral ulcers, or hair loss. A radiograph of the hand was negative for erosive changes but showed mild periarticular osteopenia and fusiform soft tissue swelling of the third digit. Given the central appearance of eschar in the larger lesions, the initial differential diagnosis included Sweet syndrome, invasive fungal infection, vasculitis, and recurrent lymphoma.

A 4-mm punch biopsy specimen of a representative lesion on the right leg revealed psoriasiform epidermal hyperplasia, parakeratosis, neutrophils in the stratum corneum and spinosum, elongation of the rete ridges, and superficial vascular ectasia, which favored a diagnosis of psoriasis (Figure 2). A periodic acid-Schiff stain was negative for fungal hyphae. Fungal culture, bacterial tissue culture, and acid-fast bacilli smear were negative. Absence of deep dermal inflammation precluded a diagnosis of Sweet syndrome. Further notable laboratory studies included negative human immunodeficiency virus (HIV) antibody, rapid plasma reagin, hepatitis C antibody, and rheumatoid factor.

At follow-up 2 weeks later, the initial lesions were still present, and she had developed new widespread, well-demarcated, erythematous plaques with silver scale along the scalp, back, chest, and abdomen that were more typical of psoriasis. Oil spots were noted on several fingernails and toenails. Based on the clinicopathologic findings, nail changes, and asymmetric inflammatory arthritis, a diagnosis of rupioid psoriasis with psoriatic arthritis (PsA) was established. Treatment with clobetasol ointment 0.05% twice daily to active lesions was started. Initiation of systemic therapy with a steroid-sparing agent was deferred in anticipation of care coordination with rheumatology, hepatology, and hematology/oncology due to the patient's history of follicular lymphoma and chronic hepatitis B. Although attempts were made to avoid systemic corticosteroids due to the risk for a psoriasis flare upon discontinuation, because of the severity of arthralgia she was started on oral prednisone 20 mg daily by rheumatology with plans for a slow taper once an alternative systemic agent was started.1
At 10-week follow-up, the patient had marked improvement of psoriatic plaques with no active lesions while only on prednisone 20 mg daily. In consultation with her care team, she subsequently was started on methotrexate 10 mg weekly for 2 weeks followed by titration to 15 mg weekly. Plans were to start a prednisone taper after a month of methotrexate to allow her new treatment time for therapeutic effect. Notably, the patient chose to discontinue prednisone 2 weeks into methotrexate therapy after only two 10-mg doses of methotrexate weekly and well before therapeutic levels were achieved. Despite stopping prednisone early and without a taper, she did not experience a relapse in psoriatic skin lesions. Three months following initiation of methotrexate, she sustained resolution of the cutaneous lesions with only residual postinflammatory hyperpigmentation.
Psoriasis is a common chronic inflammatory skin disorder with multiple clinical presentations. There are several variants of psoriasis that are classified by their morphologic appearance including chronic plaque, guttate, erythrodermic, and pustular, with more than 90% of cases representing the plaque variant. Less common clinical presentations of psoriasis include rupioid, ostraceous, inverse, elephantine, and HIV associated.2 Rupioid psoriasis is a rare variant that presents with cone-shaped, limpetlike lesions.3,4 Similar to the limited epidemiological and clinical data pertaining to psoriasis in nonwhite racial groups, there also is a paucity of documented reports of rupioid psoriasis in skin of color.
Rupioid comes from the Greek word rhupos, meaning dirt or filth, and is used to describe well-demarcated lesions with thick, yellow, dirty-appearing, adherent crusts resembling oyster shells with a surrounding rim of erythema.5 Rupioid psoriasis initially was reported in 1948 and remains an uncommon and infrequently reported variant.6 The majority of reported cases have been associated with arthropathy, similar to our patient.3,4 Rupioid lesions also have been observed in an array of other diseases, such as secondary syphilis, crusted scabies, disseminated histoplasmosis, HIV, reactive arthritis, and aminoaciduria.7-11
Diagnosis of rupioid psoriasis can be confirmed with a skin biopsy, which demonstrates characteristic histopathologic findings of psoriasis.3 Laboratory analysis should be performed to rule out other causes of rupioid lesions, and PsA should be differentiated from rheumatoid arthritis if arthropathy is present. In our case, serum rapid plasma reagin, anti-HIV antibody, rheumatoid factor, and fungal cultures were negative. Usin0)g clinical findings, histopathology, laboratory analyses, and radiograph findings, the diagnosis of rupioid psoriasis with PsA was confirmed in our patient.
Psoriasis was not originally suspected in our patient due to the noncharacteristic lesions with smooth black crust--similar appearing to eschar--and the patient's complicated medical history. Variations in the presentation of psoriasis among white individuals and those with skin of color have been reported in the literature.12,13 Psoriatic lesions in darker skin tones may appear more violaceous or hyperpigmented with more conspicuous erythema and thicker plaques. Our patient lacked the classic rupioid appearance of concentric circular layers of dirty, yellow, oysterlike scale, and instead had thick, lamellate, black crust. A PubMed search of articles indexed for MEDLINE using the terms rupioid, coral reef psoriasis, rupioides, and rhupus revealed no other cases of rupioid psoriasis reported in black patients and no cases detailing the variations of rupioid lesions in skin of color. A case of rupioid psoriasis has been reported in a Hispanic patient, but the described psoriatic lesions were more characteristic of the dirty-appearing, conic plaques previously reported.14 Our case highlights a unique example of the variable presentations of cutaneous disorders in skin of color and black patients.
Our patient's case of rupioid psoriasis with PsA presented unique challenges for systemic treatment due to her multiple comorbidities. Rupioid psoriasis most often is treated with combination topical and systemic therapy, with agents such as methotrexate and cyclosporine having prior success.3,4 This variant of psoriasis is highly responsive to treatment, and marked improvement of lesions has been achieved with topical steroids alone with proper adherence.15 Our patient was started on clobetasol ointment 0.05% while a systemic agent was debated for her PsA. Although she did not have improvement with topical therapy alone, she experienced rapid resolution of the skin lesions after initiation of low-dose prednisone 20 mg daily. Interestingly, our patient did not experience a flare of the skin lesions upon discontinuation of systemic steroids despite the lack of an appropriate taper and methotrexate not having reached therapeutic levels.
The clinical nuances of rupioid psoriasis in skin of color have not yet been described and remain an important diagnostic consideration. Our patient achieved remission of skin lesions with sequential treatment of topical clobetasol, a low-dose systemic steroid, and methotrexate. Based on available reports, rupioid psoriasis may represent a variant of psoriasis that is highly responsive to treatment.
To the Editor:
A 49-year-old black woman presented with multiple hyperkeratotic papules that progressed over the last 2 months to circular plaques with central thick black crust resembling eschar. She first noticed these lesions as firm, small, black papules on the legs and continued to develop new lesions that eventually evolved into large, coin-shaped, hyperkeratotic plaques. Her medical history was notable for stage III non-Hodgkin follicular lymphoma in remission after treatment with rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone 7 months earlier, and chronic hepatitis B infection being treated with entecavir. Her family history was not remarkable for psoriasis or inflammatory arthritis.
She initially was seen by internal medicine and was started on topical triamcinolone with no improvement of the lesions. At presentation to dermatology, physical examination revealed firm, small, black, hyperkeratotic papules (Figure 1A) and circular plaques with a rim of erythema and central thick, smooth, black crust resembling eschar (Figure 1B). No other skin changes were noted at the time. The bilateral metacarpophalangeal, bilateral proximal interphalangeal, left wrist, and bilateral ankle joints were remarkable for tenderness, swelling, and reduced range of motion. She noted concomitant arthralgia and stiffness but denied fever. She had no other systemic symptoms including night sweats, weight loss, fatigue, malaise, sun sensitivity, oral ulcers, or hair loss. A radiograph of the hand was negative for erosive changes but showed mild periarticular osteopenia and fusiform soft tissue swelling of the third digit. Given the central appearance of eschar in the larger lesions, the initial differential diagnosis included Sweet syndrome, invasive fungal infection, vasculitis, and recurrent lymphoma.

A 4-mm punch biopsy specimen of a representative lesion on the right leg revealed psoriasiform epidermal hyperplasia, parakeratosis, neutrophils in the stratum corneum and spinosum, elongation of the rete ridges, and superficial vascular ectasia, which favored a diagnosis of psoriasis (Figure 2). A periodic acid-Schiff stain was negative for fungal hyphae. Fungal culture, bacterial tissue culture, and acid-fast bacilli smear were negative. Absence of deep dermal inflammation precluded a diagnosis of Sweet syndrome. Further notable laboratory studies included negative human immunodeficiency virus (HIV) antibody, rapid plasma reagin, hepatitis C antibody, and rheumatoid factor.

At follow-up 2 weeks later, the initial lesions were still present, and she had developed new widespread, well-demarcated, erythematous plaques with silver scale along the scalp, back, chest, and abdomen that were more typical of psoriasis. Oil spots were noted on several fingernails and toenails. Based on the clinicopathologic findings, nail changes, and asymmetric inflammatory arthritis, a diagnosis of rupioid psoriasis with psoriatic arthritis (PsA) was established. Treatment with clobetasol ointment 0.05% twice daily to active lesions was started. Initiation of systemic therapy with a steroid-sparing agent was deferred in anticipation of care coordination with rheumatology, hepatology, and hematology/oncology due to the patient's history of follicular lymphoma and chronic hepatitis B. Although attempts were made to avoid systemic corticosteroids due to the risk for a psoriasis flare upon discontinuation, because of the severity of arthralgia she was started on oral prednisone 20 mg daily by rheumatology with plans for a slow taper once an alternative systemic agent was started.1
At 10-week follow-up, the patient had marked improvement of psoriatic plaques with no active lesions while only on prednisone 20 mg daily. In consultation with her care team, she subsequently was started on methotrexate 10 mg weekly for 2 weeks followed by titration to 15 mg weekly. Plans were to start a prednisone taper after a month of methotrexate to allow her new treatment time for therapeutic effect. Notably, the patient chose to discontinue prednisone 2 weeks into methotrexate therapy after only two 10-mg doses of methotrexate weekly and well before therapeutic levels were achieved. Despite stopping prednisone early and without a taper, she did not experience a relapse in psoriatic skin lesions. Three months following initiation of methotrexate, she sustained resolution of the cutaneous lesions with only residual postinflammatory hyperpigmentation.
Psoriasis is a common chronic inflammatory skin disorder with multiple clinical presentations. There are several variants of psoriasis that are classified by their morphologic appearance including chronic plaque, guttate, erythrodermic, and pustular, with more than 90% of cases representing the plaque variant. Less common clinical presentations of psoriasis include rupioid, ostraceous, inverse, elephantine, and HIV associated.2 Rupioid psoriasis is a rare variant that presents with cone-shaped, limpetlike lesions.3,4 Similar to the limited epidemiological and clinical data pertaining to psoriasis in nonwhite racial groups, there also is a paucity of documented reports of rupioid psoriasis in skin of color.
Rupioid comes from the Greek word rhupos, meaning dirt or filth, and is used to describe well-demarcated lesions with thick, yellow, dirty-appearing, adherent crusts resembling oyster shells with a surrounding rim of erythema.5 Rupioid psoriasis initially was reported in 1948 and remains an uncommon and infrequently reported variant.6 The majority of reported cases have been associated with arthropathy, similar to our patient.3,4 Rupioid lesions also have been observed in an array of other diseases, such as secondary syphilis, crusted scabies, disseminated histoplasmosis, HIV, reactive arthritis, and aminoaciduria.7-11
Diagnosis of rupioid psoriasis can be confirmed with a skin biopsy, which demonstrates characteristic histopathologic findings of psoriasis.3 Laboratory analysis should be performed to rule out other causes of rupioid lesions, and PsA should be differentiated from rheumatoid arthritis if arthropathy is present. In our case, serum rapid plasma reagin, anti-HIV antibody, rheumatoid factor, and fungal cultures were negative. Usin0)g clinical findings, histopathology, laboratory analyses, and radiograph findings, the diagnosis of rupioid psoriasis with PsA was confirmed in our patient.
Psoriasis was not originally suspected in our patient due to the noncharacteristic lesions with smooth black crust--similar appearing to eschar--and the patient's complicated medical history. Variations in the presentation of psoriasis among white individuals and those with skin of color have been reported in the literature.12,13 Psoriatic lesions in darker skin tones may appear more violaceous or hyperpigmented with more conspicuous erythema and thicker plaques. Our patient lacked the classic rupioid appearance of concentric circular layers of dirty, yellow, oysterlike scale, and instead had thick, lamellate, black crust. A PubMed search of articles indexed for MEDLINE using the terms rupioid, coral reef psoriasis, rupioides, and rhupus revealed no other cases of rupioid psoriasis reported in black patients and no cases detailing the variations of rupioid lesions in skin of color. A case of rupioid psoriasis has been reported in a Hispanic patient, but the described psoriatic lesions were more characteristic of the dirty-appearing, conic plaques previously reported.14 Our case highlights a unique example of the variable presentations of cutaneous disorders in skin of color and black patients.
Our patient's case of rupioid psoriasis with PsA presented unique challenges for systemic treatment due to her multiple comorbidities. Rupioid psoriasis most often is treated with combination topical and systemic therapy, with agents such as methotrexate and cyclosporine having prior success.3,4 This variant of psoriasis is highly responsive to treatment, and marked improvement of lesions has been achieved with topical steroids alone with proper adherence.15 Our patient was started on clobetasol ointment 0.05% while a systemic agent was debated for her PsA. Although she did not have improvement with topical therapy alone, she experienced rapid resolution of the skin lesions after initiation of low-dose prednisone 20 mg daily. Interestingly, our patient did not experience a flare of the skin lesions upon discontinuation of systemic steroids despite the lack of an appropriate taper and methotrexate not having reached therapeutic levels.
The clinical nuances of rupioid psoriasis in skin of color have not yet been described and remain an important diagnostic consideration. Our patient achieved remission of skin lesions with sequential treatment of topical clobetasol, a low-dose systemic steroid, and methotrexate. Based on available reports, rupioid psoriasis may represent a variant of psoriasis that is highly responsive to treatment.
- Mrowietz U, Domm S. Systemic steroids in the treatment of psoriasis: what is fact, what is fiction? J Eur Acad Dermatol Venereol. 2013;27:1022-1025.
- Goldsmith LA, Katz SI, Gilchrest BA, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Wang JL, Yang JH. Rupioid psoriasis associated with arthropathy. J Dermatol. 1997;24:46-49.
- Murakami T, Ohtsuki M, Nakagawa H. Rupioid psoriasis with arthropathy. Clin Exp Dermatol. 2000;25:409-412.
- Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis. 2014;94:119-121.
- Salamon M, Omulecki A, Sysa-Jedrzejowska A, et al. Psoriasisrupioides: a rare variant of a common disease. Cutis. 2011;88:135-137.
- Krase IZ, Cavanaugh K, Curiel-Lewandrowski C. A case of rupioid syphilis. JAAD Case Rep. 2016;2:141-143.
- Garofalo V, Saraceno R, Milana M, et al. Crusted scabies in a liver transplant patient mimicking rupioid psoriasis. Eur J Dermatol. 2016;26:495-496.
- Corti M, Villafane MF, Palmieri O, et al. Rupioid histoplasmosis: first case reported in an AIDS patient in Argentina. Rev Inst Med Trop Sao Paulo. 2010;52:279-280.
- Sehgal VN, Koranne RV, Shyam Prasad AL. Unusual manifestations of Reiter's disease in a child. Dermatologica. 1985;170:77-79.
- Haim S, Gilhar A, Cohen A. Cutaneous manifestations associated with aminoaciduria. report of two cases. Dermatologica. 1978;156:244-250.
- McMichael AJ, Vachiramon V, Guzman-Sanchez DA, et al. Psoriasis in African-Americans: a caregivers' survey. J Drugs Dermatol. 2012;11:478-482.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Posligua A, Maldonado C, Gonzalez MG. Rupioid psoriasis preceded by varicella presenting as Koebner phenomenon. J Am Acad Dermatol. 2016;74(5 suppl 1):AB268.
- Feldman SR, Feldman S, Brown K, et al. "Coral reef" psoriasis: a marker of resistance to topical treatment. J Dermatolog Treat. 2008;19:257-258.
- Mrowietz U, Domm S. Systemic steroids in the treatment of psoriasis: what is fact, what is fiction? J Eur Acad Dermatol Venereol. 2013;27:1022-1025.
- Goldsmith LA, Katz SI, Gilchrest BA, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Wang JL, Yang JH. Rupioid psoriasis associated with arthropathy. J Dermatol. 1997;24:46-49.
- Murakami T, Ohtsuki M, Nakagawa H. Rupioid psoriasis with arthropathy. Clin Exp Dermatol. 2000;25:409-412.
- Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis. 2014;94:119-121.
- Salamon M, Omulecki A, Sysa-Jedrzejowska A, et al. Psoriasisrupioides: a rare variant of a common disease. Cutis. 2011;88:135-137.
- Krase IZ, Cavanaugh K, Curiel-Lewandrowski C. A case of rupioid syphilis. JAAD Case Rep. 2016;2:141-143.
- Garofalo V, Saraceno R, Milana M, et al. Crusted scabies in a liver transplant patient mimicking rupioid psoriasis. Eur J Dermatol. 2016;26:495-496.
- Corti M, Villafane MF, Palmieri O, et al. Rupioid histoplasmosis: first case reported in an AIDS patient in Argentina. Rev Inst Med Trop Sao Paulo. 2010;52:279-280.
- Sehgal VN, Koranne RV, Shyam Prasad AL. Unusual manifestations of Reiter's disease in a child. Dermatologica. 1985;170:77-79.
- Haim S, Gilhar A, Cohen A. Cutaneous manifestations associated with aminoaciduria. report of two cases. Dermatologica. 1978;156:244-250.
- McMichael AJ, Vachiramon V, Guzman-Sanchez DA, et al. Psoriasis in African-Americans: a caregivers' survey. J Drugs Dermatol. 2012;11:478-482.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Posligua A, Maldonado C, Gonzalez MG. Rupioid psoriasis preceded by varicella presenting as Koebner phenomenon. J Am Acad Dermatol. 2016;74(5 suppl 1):AB268.
- Feldman SR, Feldman S, Brown K, et al. "Coral reef" psoriasis: a marker of resistance to topical treatment. J Dermatolog Treat. 2008;19:257-258.
Practice Points
- Rupioid psoriasis in skin of color may present a diagnostic challenge for health care providers.
- Rupioid psoriasis may represent a psoriasis variant that is highly responsive to treatment.
Topical PDE-4 inhibitor for psoriasis effective in phase 2b trial
Once-daily – including challenging lesions in tough-to-treat intertriginous areas – in a phase 2b, randomized, double-blind, vehicle-controlled clinical trial, Mark G. Lebwohl, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
The clinical improvement occurred rapidly. And topical roflumilast’s side effect profile was essentially the same as in vehicle-treated controls, which suggests a potential major advantage for the novel drug in future clinical practice. After all, topical treatment is the mainstay of psoriasis therapy, but the current topical agents – high-potency corticosteroids, vitamin D derivatives, and retinoids – have long-term tolerability, efficacy, or side effect issues, especially in treating sensitive skin areas, including the face and intertriginous areas.
“Roflumilast cream could really be a game changer,” predicted Dr. Lebwohl, professor and chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Phosphodiesterase-4 (PDE-4) activity is elevated in psoriatic skin. Indeed, inhibition of PDE-4 via oral apremilast (Otezla) is an established strategy for improving psoriasis through down-regulation of inflammatory cytokines including tumor necrosis factor–alpha, interleukins-17 and -23, and interferon-gamma. Notably, however, roflumilast is orders of magnitude more potent than any other PDE-4 inhibitor. An oral version marketed as Daliresp has been available for treatment of chronic obstructive pulmonary disease for nearly a decade.
The 12-week, multicenter, phase 2b study included 331 patients with chronic plaque psoriasis who were randomized to once-daily 0.3% roflumilast cream, 0.15% roflumilast cream, or vehicle. Three-quarters of participants had a baseline Investigator Global Assessment (IGA) score of 3, indicative of moderate disease.
The primary endpoint was achievement of an IGA score of 0 or 1 (clear or almost clear) at week 6. The observed improvement was dose related, although both doses of roflumilast were significantly more effective than vehicle. However, peak improvement occurred at week 8, not week 6, with subsequent plateauing of response through week 12. A week 8 IGA of 0 or 1 plus at least a 2-grade improvement from baseline occurred in 32% of the high-dose roflumilast group, 25% of those on the 0.15% formulation, and 10% of controls.
“The effect in improvement was very rapid, with a statistically significant improvement compared to vehicle for both concentrations as early as week 2,” Dr. Lebwohl said.
A key secondary endpoint focused on treatment response in intertriginous areas, since “those are the areas where we really don’t want to use steroids because of major irritation problems,” the dermatologist explained. At week 12, treatment success as defined by an intertriginous IGA score of 0 or 1 plus at least a 2-point improvement from baseline was seen in 86% of the 0.3% roflumilast cream group, 50% on low-dose therapy, and 29% of controls.
About 65% of subjects on high-dose roflumilast cream reported at least a 4-point reduction in the Worst Itch–Numerical Rating Scale by week 8, as did 58% of those on the low-dose version and 42% of controls. Another secondary endpoint – patient-reported burden of disease as captured in a Psoriasis Symptoms Diary – showed a significant divergence between both doses of roflumilast and vehicle as early as week 2.
“Adverse events were negligible,” Dr. Lebwohl said. “In fact, there was only one discontinuation in the 0.3% arm, compared to none with 0.15% and two with vehicle.”
The phase 3 program is now recruiting participants.
The phase 2b study was funded by Arcutis Biotherapeutics. Dr. Lebwohl reported receiving research funding from and serving as a consultant to that company and numerous others.
Once-daily – including challenging lesions in tough-to-treat intertriginous areas – in a phase 2b, randomized, double-blind, vehicle-controlled clinical trial, Mark G. Lebwohl, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
The clinical improvement occurred rapidly. And topical roflumilast’s side effect profile was essentially the same as in vehicle-treated controls, which suggests a potential major advantage for the novel drug in future clinical practice. After all, topical treatment is the mainstay of psoriasis therapy, but the current topical agents – high-potency corticosteroids, vitamin D derivatives, and retinoids – have long-term tolerability, efficacy, or side effect issues, especially in treating sensitive skin areas, including the face and intertriginous areas.
“Roflumilast cream could really be a game changer,” predicted Dr. Lebwohl, professor and chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Phosphodiesterase-4 (PDE-4) activity is elevated in psoriatic skin. Indeed, inhibition of PDE-4 via oral apremilast (Otezla) is an established strategy for improving psoriasis through down-regulation of inflammatory cytokines including tumor necrosis factor–alpha, interleukins-17 and -23, and interferon-gamma. Notably, however, roflumilast is orders of magnitude more potent than any other PDE-4 inhibitor. An oral version marketed as Daliresp has been available for treatment of chronic obstructive pulmonary disease for nearly a decade.
The 12-week, multicenter, phase 2b study included 331 patients with chronic plaque psoriasis who were randomized to once-daily 0.3% roflumilast cream, 0.15% roflumilast cream, or vehicle. Three-quarters of participants had a baseline Investigator Global Assessment (IGA) score of 3, indicative of moderate disease.
The primary endpoint was achievement of an IGA score of 0 or 1 (clear or almost clear) at week 6. The observed improvement was dose related, although both doses of roflumilast were significantly more effective than vehicle. However, peak improvement occurred at week 8, not week 6, with subsequent plateauing of response through week 12. A week 8 IGA of 0 or 1 plus at least a 2-grade improvement from baseline occurred in 32% of the high-dose roflumilast group, 25% of those on the 0.15% formulation, and 10% of controls.
“The effect in improvement was very rapid, with a statistically significant improvement compared to vehicle for both concentrations as early as week 2,” Dr. Lebwohl said.
A key secondary endpoint focused on treatment response in intertriginous areas, since “those are the areas where we really don’t want to use steroids because of major irritation problems,” the dermatologist explained. At week 12, treatment success as defined by an intertriginous IGA score of 0 or 1 plus at least a 2-point improvement from baseline was seen in 86% of the 0.3% roflumilast cream group, 50% on low-dose therapy, and 29% of controls.
About 65% of subjects on high-dose roflumilast cream reported at least a 4-point reduction in the Worst Itch–Numerical Rating Scale by week 8, as did 58% of those on the low-dose version and 42% of controls. Another secondary endpoint – patient-reported burden of disease as captured in a Psoriasis Symptoms Diary – showed a significant divergence between both doses of roflumilast and vehicle as early as week 2.
“Adverse events were negligible,” Dr. Lebwohl said. “In fact, there was only one discontinuation in the 0.3% arm, compared to none with 0.15% and two with vehicle.”
The phase 3 program is now recruiting participants.
The phase 2b study was funded by Arcutis Biotherapeutics. Dr. Lebwohl reported receiving research funding from and serving as a consultant to that company and numerous others.
Once-daily – including challenging lesions in tough-to-treat intertriginous areas – in a phase 2b, randomized, double-blind, vehicle-controlled clinical trial, Mark G. Lebwohl, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
The clinical improvement occurred rapidly. And topical roflumilast’s side effect profile was essentially the same as in vehicle-treated controls, which suggests a potential major advantage for the novel drug in future clinical practice. After all, topical treatment is the mainstay of psoriasis therapy, but the current topical agents – high-potency corticosteroids, vitamin D derivatives, and retinoids – have long-term tolerability, efficacy, or side effect issues, especially in treating sensitive skin areas, including the face and intertriginous areas.
“Roflumilast cream could really be a game changer,” predicted Dr. Lebwohl, professor and chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Phosphodiesterase-4 (PDE-4) activity is elevated in psoriatic skin. Indeed, inhibition of PDE-4 via oral apremilast (Otezla) is an established strategy for improving psoriasis through down-regulation of inflammatory cytokines including tumor necrosis factor–alpha, interleukins-17 and -23, and interferon-gamma. Notably, however, roflumilast is orders of magnitude more potent than any other PDE-4 inhibitor. An oral version marketed as Daliresp has been available for treatment of chronic obstructive pulmonary disease for nearly a decade.
The 12-week, multicenter, phase 2b study included 331 patients with chronic plaque psoriasis who were randomized to once-daily 0.3% roflumilast cream, 0.15% roflumilast cream, or vehicle. Three-quarters of participants had a baseline Investigator Global Assessment (IGA) score of 3, indicative of moderate disease.
The primary endpoint was achievement of an IGA score of 0 or 1 (clear or almost clear) at week 6. The observed improvement was dose related, although both doses of roflumilast were significantly more effective than vehicle. However, peak improvement occurred at week 8, not week 6, with subsequent plateauing of response through week 12. A week 8 IGA of 0 or 1 plus at least a 2-grade improvement from baseline occurred in 32% of the high-dose roflumilast group, 25% of those on the 0.15% formulation, and 10% of controls.
“The effect in improvement was very rapid, with a statistically significant improvement compared to vehicle for both concentrations as early as week 2,” Dr. Lebwohl said.
A key secondary endpoint focused on treatment response in intertriginous areas, since “those are the areas where we really don’t want to use steroids because of major irritation problems,” the dermatologist explained. At week 12, treatment success as defined by an intertriginous IGA score of 0 or 1 plus at least a 2-point improvement from baseline was seen in 86% of the 0.3% roflumilast cream group, 50% on low-dose therapy, and 29% of controls.
About 65% of subjects on high-dose roflumilast cream reported at least a 4-point reduction in the Worst Itch–Numerical Rating Scale by week 8, as did 58% of those on the low-dose version and 42% of controls. Another secondary endpoint – patient-reported burden of disease as captured in a Psoriasis Symptoms Diary – showed a significant divergence between both doses of roflumilast and vehicle as early as week 2.
“Adverse events were negligible,” Dr. Lebwohl said. “In fact, there was only one discontinuation in the 0.3% arm, compared to none with 0.15% and two with vehicle.”
The phase 3 program is now recruiting participants.
The phase 2b study was funded by Arcutis Biotherapeutics. Dr. Lebwohl reported receiving research funding from and serving as a consultant to that company and numerous others.
FROM AAD 20
Eczema Herpeticum in a Patient With Hailey-Hailey Disease Confounded by Coexistent Psoriasis
To the Editor:
Hailey-Hailey disease (HHD), also known as benign familial pemphigus, is an uncommon autosomal-dominant skin disease.1 Defects in the ATPase type 2C member 1 gene, ATP2C1, result in abnormal intracellular epidermal adherence, and patients experience recurring blisters in skin folds. Longitudinal white streaks of the fingernails also may be present.1 The illness does not appear until puberty and is heightened by the second or third decade of life. Family history often suggests the presence of disease.2 Misdiagnosis of HHD occurs because of a wide spectrum of presentations. The presence of superimposed infections and carcinomas may both obscure and exacerbate this disease.2
Herpes simplex viruse types 1 and 2 (HSV-1 and HSV-2) are DNA viruses that cause common recurrent diseases. Usually, HSV-1 is associated with infection of the mouth and HSV-2 is associated with infection of the genitalia.3 Longitudinal cutaneous lesions manifest as grouped vesicles on an erythematous base. Tzanck smear of herpetic vesicles will reveal the presence of multinucleated giant cells. A direct fluorescent antibody technique also may be used to confirm the diagnosis.3
Erythrodermic HHD disease is a rare condition; moreover, there are only a few reported cases with coexistence of HHD and HSV in the literature.3-6 We report a rare presentation of erythrodermic HHD and coexistent psoriasis with HSV superinfection.
A 69-year-old man presented to an outpatient dermatology clinic for evaluation and treatment of a rash on the scalp, face, back, and lower legs. The patient confirmed a dandruff diagnosis on the scalp and face as well as psoriasis on the trunk and extremities for the last 45 years. He described a history of successful treatment with topical agents and UV light therapy. A family history revealed that the patient’s father and 1 of 2 siblings had a similar rash and “skin problems.” The patient had a medical history of thyroid cancer treated with radiation treatment and a partial thyroidectomy 35 years prior to the current presentation as well as incompletely treated chronic hepatitis C.
A search of medical records revealed a punch biopsy from the posterior neck that demonstrated an acantholytic dyskeratosis with suprabasal acantholysis. Clinicians were unable to differentiate if it was Darier disease (DAR) or HHD. Treatment of the patient’s seborrheic dermatitis and acantholytic disorder was successful at that time with ketoconazole shampoo, ketoconazole cream, desonide cream, and triamcinolone cream. The patient remained stable for 5 years before presenting again to the dermatology clinic for worsening rash despite topical therapies.
At the current presentation, physical examination at the outpatient dermatology clinic revealed few scaly, erythematous, eroded papules distributed on the mid-back; erythematous greasy scaling on the scalp, face, and chest; and pink scaly plaques with white-silvery scale on the anterior lower legs. Histopathology of a specimen from the right mid-back demonstrated acantholysis with suprabasal clefting, hyperkeratosis, and parakeratosis with no dyskeratotic cells identified. The pathologic differential diagnosis included primary acantholytic processes including Grover disease, DAR, HHD, and pemphigus. Pathology from the right shin demonstrated acanthosis, confluent parakeratosis with associated decreased granular cell layer and collections of neutrophils within the stratum corneum, spongiosis, and superficial dermal perivascular chronic inflammation with focal exocytosis and dilated blood vessels in the papillary dermis. The clinical and pathological diagnosis on the lower legs was consistent with psoriasis. Diagnoses of seborrheic dermatitis, psoriasis on the lower legs, and HHD vs DAR on the back and chest were made. The patient was instructed to continue ketoconazole shampoo, ketoconazole cream, and desonide for seborrheic dermatitis; fluocinonide ointment 0.05% to the lower legs for psoriasis; and triamcinolone cream and a bland moisturizer to the back and chest for HHD.
Over the ensuing months, the rash worsened with erythema and scaling affecting more than half of the body surface area. Topical corticosteroids and bland emollients resulted in minimal success. Biologics and acitretin were considered for the psoriasiform dermatitis but avoided due to the patient’s medical history of thyroid cancer and chronic hepatitis C infection. Because the patient described prior success with UV light therapy for psoriasis, he requested light therapy. A subsequent trial of narrowband UVB light therapy initially improved some of the psoriasiform dermatitis on the trunk and extremities; however, after 4 weeks of treatment, the patient described pain in some of the skin and felt he was burned by minimal exposure to light therapy on one particular visit, which caused him to stop light therapy.
Approximately 2 weeks later, the patient presented to the emergency department stating his psoriasis was infected; he was diagnosed with psoriasis with secondary cellulitis and received intravenous vancomycin and piperacillin-tazobactam, with bacterial cultures demonstrating Corynebacterium and methicillin-resistant Staphylococcus aureus. Some improvement was noted in the patient’s skin after antibiotics were initiated, but he continued to describe worsening “burning and pain” throughout the psoriasis lesions. The patient’s care was transferred to the Veterans Affairs hospital where a dermatology inpatient consultation was placed.
Our initial dermatologic examination revealed generalized scaly erythroderma on the neck, trunk, and extremities, sparing the face, palms, and soles (Figure 1). Multiple crusted and intact vesicles also were present overlying the erythematous plaques on the chest, back, and proximal extremities, most grouped in clusters. The patient endorsed new symptoms of pain and burning. Tzanck smear from the abdomen along with shave biopsies from the left flank and right abdomen were performed, and intravenous acyclovir was initiated immediately after these procedures.
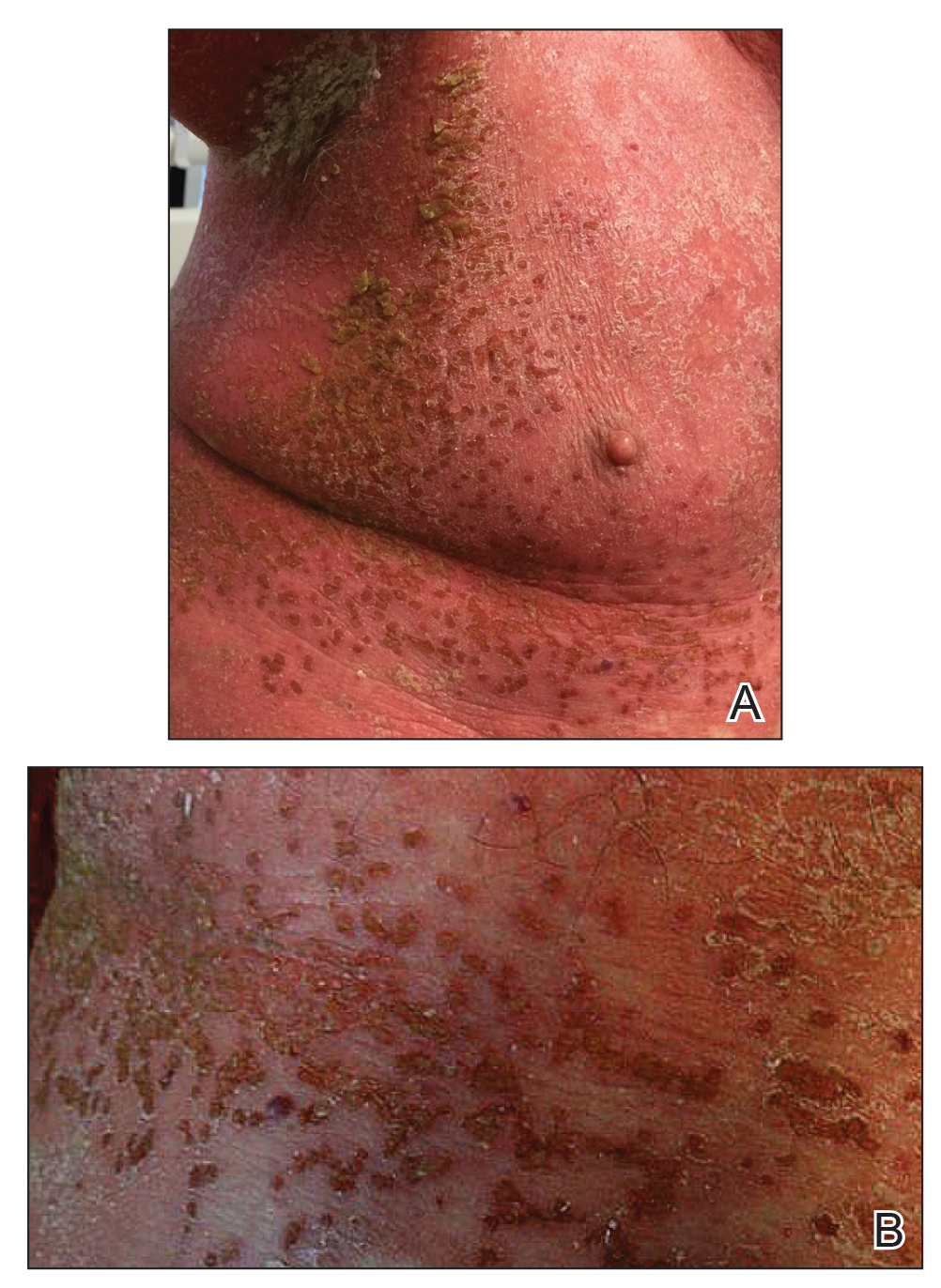
Viral cultures were taken but were incorrectly processed by the laboratory. Tzanck smear showed severe acute inflammation with numerous neutrophils, multinucleated giant cells with viral nuclear changes, and positive immunostaining for HSV and negative immunostaining for herpes zoster. Both pathology specimens revealed an intense acute mixed, mainly neutrophilic, inflammatory infiltrate extending into the deeper dermis as well as distorted and necrotic hair follicles, some of which displayed multinucleated epithelial cells with margination of chromatin that were positive for both HSV-1 and HSV-2 and negative for herpes zoster (Figure 2). The positivity of both HSV strains might represent co-infection or could be a cross-reaction of antibodies used in immunohistochemistry to the HSV antigens. There was acantholysis surrounding the ulceration and extending through the full thickness of the epidermis with a dilapidated brick wall pattern (Figure 3) as well as negative immunohistochemical staining for HSV-1 and HSV-2 antigens. The clinical and histological picture together, along with prior clinical and pathological reports, confirmed the diagnoses of acute erythrodermic HHD with HSV superinfection.
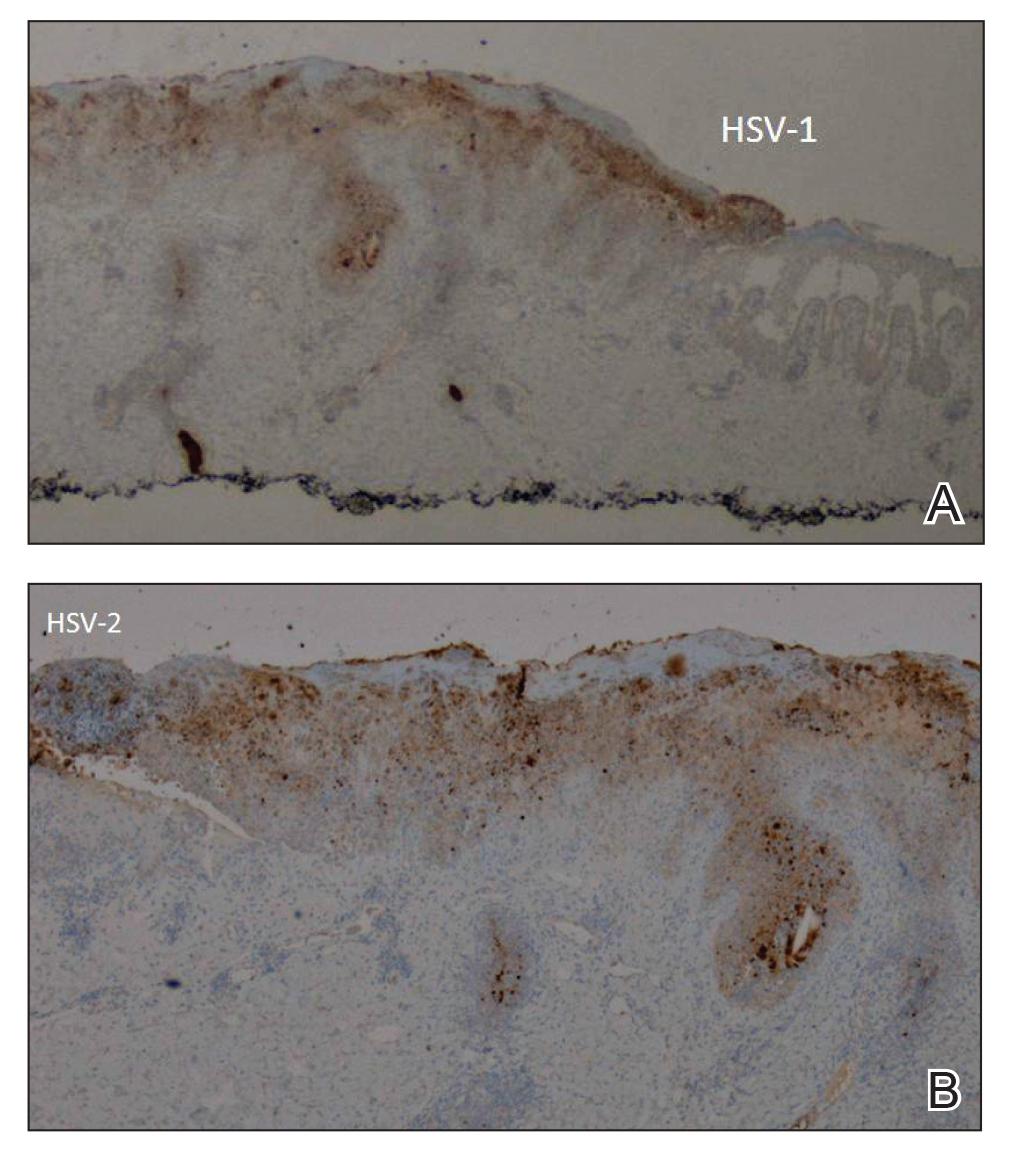
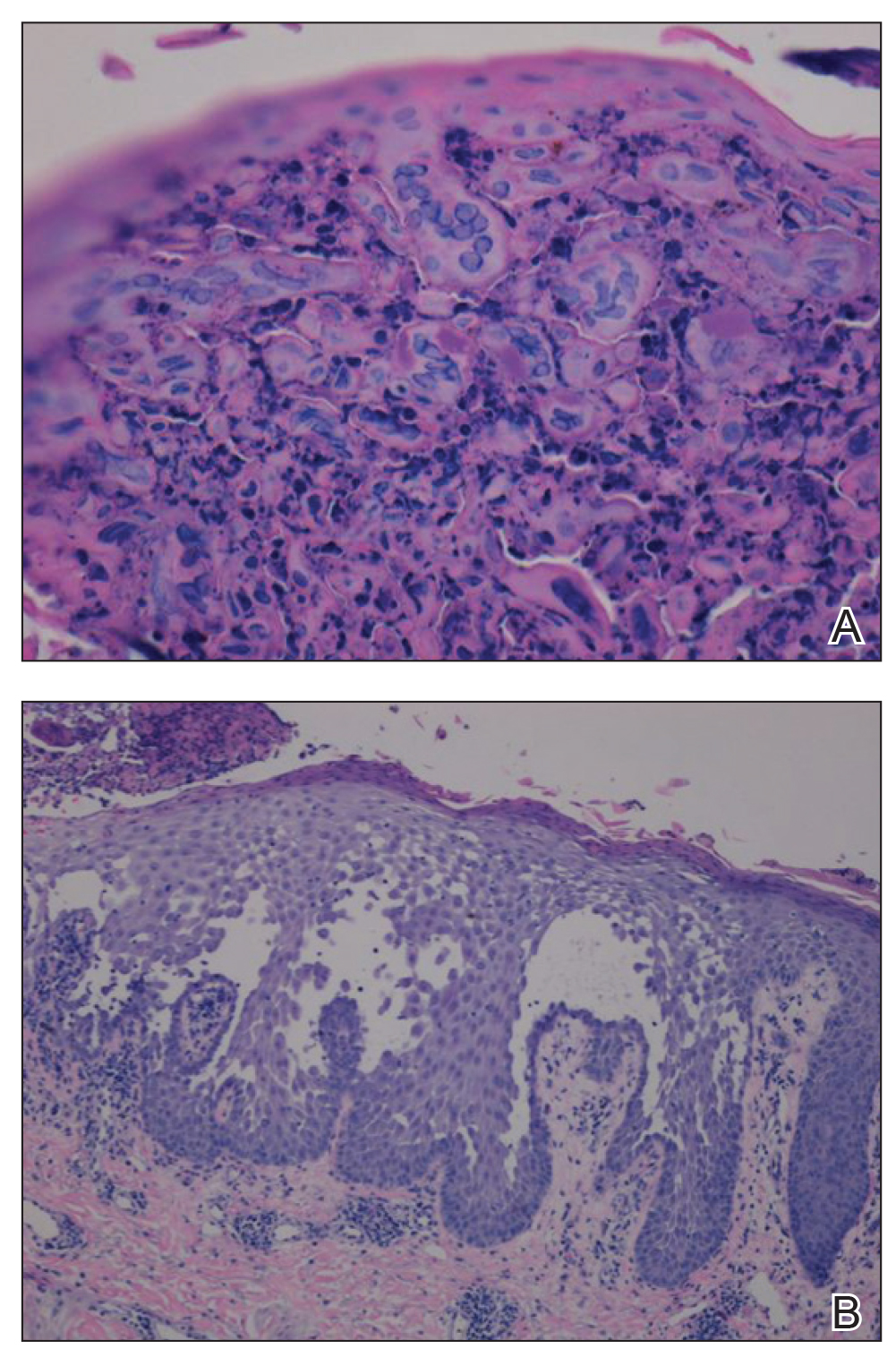
The patient’s condition and pain improved within 24 hours on intravenous acyclovir. On the third day, his lesions were resolving and symptoms improved, so he was transitioned to oral acyclovir and discharged from the hospital. Follow-up in the dermatology outpatient clinic 1 week later revealed that all vesicles and papules had cleared, but the patient was still erythrodermic. Because HHD cannot always be distinguished histologically from other forms of pemphigus but yields a negative immunofluorescence, direct immunofluorescence and indirect immunofluorescence were obtained upon patient follow-up in the clinic and were both negative. Hepatitis C viral loads were undetectable. Consultations to gastroenterology and oncology teams were placed for consideration of systemic agents, and the patient was initiated on oral acitretin 25 mg daily, along with clobetasol as adjuvant therapy for any residual skin plaques. The laboratory results were closely monitored. Within 4 weeks after starting acitretin, the patient’s erythroderma had completely resolved. The patient has remained stable since then, except for one episode of secondary Staphylococcus infection that cleared on oral antibiotics. The patient remains stable and clear on oral acitretin 25 mg daily, with concomitant desonide cream and fluocinonide ointment as needed.
Hailey-Hailey disease is characterized by recurrent episodes of erythema, blisters, and plaques localized to intertriginous and perianal areas.1,2 Patients display a spectrum of lesions that vary in severity.8 Typical histologic examination reveals a dilapidated brick wall appearance. Pathology of well-developed lesions will show suprabasal acantholysis with minimal dyskeratosis.2
The generalized form of HHD is an extremely rare variant of the disease.10 Generalized HHD may resemble acute hypersensitivity reaction, erythema multiforme, and toxic epidermal necrolysis.1 Chronic diseases, such as psoriasis (as in this patient), also may contribute to a clinically confusing picture.8 Hailey-Hailey disease and psoriasis are thought to occasionally koebnerize (isomorphic response) to areas of trauma.16 Our patient experienced widespread erythematous papules and plaques not restricted to skin folds. His skin lesions continued to worsen over several months progressing to erythroderma. The presence of suprabasal acantholysis in a dilapidated brick wall pattern, along with the patient’s history, prior pathology reports, clinical picture, and negative direct immunofluorescence and indirect immunofluorescence studies helped to confirm the diagnosis of erythrodermic HHD.
Hailey-Hailey disease is caused by heterozygous mutations in the ATP2C1 gene on chromosome 3q21-24 coding for a Golgi ATPase called SPCA1 (secretory pathway calcium/manganese-ATPase).9 Subsequent disturbances in cytosolic-Golgi calcium concentrations interfere with epidermal keratinocyte adherence resulting in acantholytic disease. Studies of interfamilial and intrafamilial mutations fail to pinpoint a common mutation pattern among patients with generalized phenotypes,9 which further supports theories that intrinsic or extrinsic factors such as friction, heat, radiation, contact allergens, and infection affect the severity of HHD disease and not the type of mutation.3,9
Generalization of HHD is likely caused by nonspecific triggers in an already genetically disturbed epidermis.10 Interrupted epithelial function exposes skin to infections that exacerbate the underlying disease. Superimposing bacterial infections are commonly reported in HHD. Staphylococcus, Streptococcus, and Candida species colonize the skin and aggravate the disease.11 Much less commonly, HSV superinfection can complicate HHD.3-7 No data are currently available about the frequency or incidence of Herpesviridae in HHD.7 Some studies suggest that UVB light therapy can be an exacerbating factor in DAR and some but not all HHD patients,12,13 while other case reports14,15 document clinically improved responses using phototherapy for patients with HHD. Clinicians should remain suspicious and evaluate for HSV infection in refractory or sudden exacerbation of HHD.7 Furthermore, coexistent psoriasis and HHD also is a rare entity but has been described,8 which illustrates the importance of not attributing all skin manifestations to a previously diagnosed disorder but instead keeping an open mind in case new dermatologic conditions present themselves at a later time.
We present a rare case of erythrodermic HHD and coexistent psoriasis with HSV superinfection. We hope to draw awareness to this association of generalized HHD with both HSV and psoriasis to help clinicians make the correct diagnosis promptly in similar cases in the future.
- Chave TA, Milligan A. Acute generalized Hailey-Hailey disease. Clin Exp Dermatol. 2002;27:290-292.
- Mohr MR, Erdag G, Shada Al, et al. Two patients with Hailey-Hailey disease, multiple primary melanomas, and other cancers. Arch Dermatol. 2011;147:211-215.
- Lee GM, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey Disease. Ann Dermatol. 2009;21:311-314.
- Zaim MT, Bickers DR. Herpes simplex associated with Hailey-Hailey disease. J Am Acad Dermatol. 1987;17:701-702.
- Peppiatt T, Keefe M, White JE. Hailey-Hailey disease-exacerbation by herpes simplex virus and patch tests. Clin Exp Dermatol. 2006;17:201-202.
- Almeida L, Grossman ME. Benign familial pemphigus complicated by herpes simplex virus. Cutis. 1989;44:261-262.
- Nikkels AF, Delvenne P, Herfs M, et al. Occult herpes simplex virus colonization of bullous dermatitides. Am J Clin Dermatol. 2008;9:163-168.
- Chao SC, Lee JY, Wu MC, et al. A novel splice mutation in the ATP2C1 gene in a woman with concomitant psoriasis vulgaris and disseminated Hailey-Hailey disease. Int J Dermatol. 2012;51:947-951.
- Ikeda S, Shigihara T, Mayuzumi N, et al. Mutations of ATP2C1 in Japanese patients with Hailey-Hailey disease: intrafamilial and interfamilial phenotype variations and lack of correlation with mutation patterns. J Invest Dermatol. 2001;117:1654-1656.
- Marsch W, Stuttgen G. Generalized Hailey-Hailey disease. Br J Dermatol. 1978;99:553-559.
- Friedman-Birnbaum R, Haim S, Marcus S. Generalized familial benign chronic pemphigus. Dermatologica. 1980;161:112-115.
- Richard G, Linse R, Harth W. Hailey-Hailey disease. early detection of heterozygotes by an ultraviolet provocation tests—clinical relevance of the method. Hautarzt. 1993;44:376-379.
- Mayuzumi N, Ikeda S, Kawada H, et al. Effects of ultraviolet B irradiation, proinflammatory cytokines and raised extracellular calcium concentration on the expression of ATP2A2 and ATP2C1. Br J Dermatol. 2005;152:697-701.
- Vanderbeck KA, Giroux L, Murugan NJ, et al. Combined therapeutic use of oral alitretinoin and narrowband ultraviolet-B therapy in the treatment of Hailey-Hailey disease. Dermatol Rep. 2014;6:5604.
- Mizuno K, Hamada T, Hasimoto T, et al. Successful treatment with narrow-band UVB therapy for a case of generalized Hailey-Hailey disease with a novel splice-site mutation in ATP2C1 gene. Dermatol Ther. 2014;27:233-235.
- Thappa DM. The isomorphic phenomenon of Koebner. Indian J Dermatol Venereol Leprol. 2004;70:187-189.
To the Editor:
Hailey-Hailey disease (HHD), also known as benign familial pemphigus, is an uncommon autosomal-dominant skin disease.1 Defects in the ATPase type 2C member 1 gene, ATP2C1, result in abnormal intracellular epidermal adherence, and patients experience recurring blisters in skin folds. Longitudinal white streaks of the fingernails also may be present.1 The illness does not appear until puberty and is heightened by the second or third decade of life. Family history often suggests the presence of disease.2 Misdiagnosis of HHD occurs because of a wide spectrum of presentations. The presence of superimposed infections and carcinomas may both obscure and exacerbate this disease.2
Herpes simplex viruse types 1 and 2 (HSV-1 and HSV-2) are DNA viruses that cause common recurrent diseases. Usually, HSV-1 is associated with infection of the mouth and HSV-2 is associated with infection of the genitalia.3 Longitudinal cutaneous lesions manifest as grouped vesicles on an erythematous base. Tzanck smear of herpetic vesicles will reveal the presence of multinucleated giant cells. A direct fluorescent antibody technique also may be used to confirm the diagnosis.3
Erythrodermic HHD disease is a rare condition; moreover, there are only a few reported cases with coexistence of HHD and HSV in the literature.3-6 We report a rare presentation of erythrodermic HHD and coexistent psoriasis with HSV superinfection.
A 69-year-old man presented to an outpatient dermatology clinic for evaluation and treatment of a rash on the scalp, face, back, and lower legs. The patient confirmed a dandruff diagnosis on the scalp and face as well as psoriasis on the trunk and extremities for the last 45 years. He described a history of successful treatment with topical agents and UV light therapy. A family history revealed that the patient’s father and 1 of 2 siblings had a similar rash and “skin problems.” The patient had a medical history of thyroid cancer treated with radiation treatment and a partial thyroidectomy 35 years prior to the current presentation as well as incompletely treated chronic hepatitis C.
A search of medical records revealed a punch biopsy from the posterior neck that demonstrated an acantholytic dyskeratosis with suprabasal acantholysis. Clinicians were unable to differentiate if it was Darier disease (DAR) or HHD. Treatment of the patient’s seborrheic dermatitis and acantholytic disorder was successful at that time with ketoconazole shampoo, ketoconazole cream, desonide cream, and triamcinolone cream. The patient remained stable for 5 years before presenting again to the dermatology clinic for worsening rash despite topical therapies.
At the current presentation, physical examination at the outpatient dermatology clinic revealed few scaly, erythematous, eroded papules distributed on the mid-back; erythematous greasy scaling on the scalp, face, and chest; and pink scaly plaques with white-silvery scale on the anterior lower legs. Histopathology of a specimen from the right mid-back demonstrated acantholysis with suprabasal clefting, hyperkeratosis, and parakeratosis with no dyskeratotic cells identified. The pathologic differential diagnosis included primary acantholytic processes including Grover disease, DAR, HHD, and pemphigus. Pathology from the right shin demonstrated acanthosis, confluent parakeratosis with associated decreased granular cell layer and collections of neutrophils within the stratum corneum, spongiosis, and superficial dermal perivascular chronic inflammation with focal exocytosis and dilated blood vessels in the papillary dermis. The clinical and pathological diagnosis on the lower legs was consistent with psoriasis. Diagnoses of seborrheic dermatitis, psoriasis on the lower legs, and HHD vs DAR on the back and chest were made. The patient was instructed to continue ketoconazole shampoo, ketoconazole cream, and desonide for seborrheic dermatitis; fluocinonide ointment 0.05% to the lower legs for psoriasis; and triamcinolone cream and a bland moisturizer to the back and chest for HHD.
Over the ensuing months, the rash worsened with erythema and scaling affecting more than half of the body surface area. Topical corticosteroids and bland emollients resulted in minimal success. Biologics and acitretin were considered for the psoriasiform dermatitis but avoided due to the patient’s medical history of thyroid cancer and chronic hepatitis C infection. Because the patient described prior success with UV light therapy for psoriasis, he requested light therapy. A subsequent trial of narrowband UVB light therapy initially improved some of the psoriasiform dermatitis on the trunk and extremities; however, after 4 weeks of treatment, the patient described pain in some of the skin and felt he was burned by minimal exposure to light therapy on one particular visit, which caused him to stop light therapy.
Approximately 2 weeks later, the patient presented to the emergency department stating his psoriasis was infected; he was diagnosed with psoriasis with secondary cellulitis and received intravenous vancomycin and piperacillin-tazobactam, with bacterial cultures demonstrating Corynebacterium and methicillin-resistant Staphylococcus aureus. Some improvement was noted in the patient’s skin after antibiotics were initiated, but he continued to describe worsening “burning and pain” throughout the psoriasis lesions. The patient’s care was transferred to the Veterans Affairs hospital where a dermatology inpatient consultation was placed.
Our initial dermatologic examination revealed generalized scaly erythroderma on the neck, trunk, and extremities, sparing the face, palms, and soles (Figure 1). Multiple crusted and intact vesicles also were present overlying the erythematous plaques on the chest, back, and proximal extremities, most grouped in clusters. The patient endorsed new symptoms of pain and burning. Tzanck smear from the abdomen along with shave biopsies from the left flank and right abdomen were performed, and intravenous acyclovir was initiated immediately after these procedures.

Viral cultures were taken but were incorrectly processed by the laboratory. Tzanck smear showed severe acute inflammation with numerous neutrophils, multinucleated giant cells with viral nuclear changes, and positive immunostaining for HSV and negative immunostaining for herpes zoster. Both pathology specimens revealed an intense acute mixed, mainly neutrophilic, inflammatory infiltrate extending into the deeper dermis as well as distorted and necrotic hair follicles, some of which displayed multinucleated epithelial cells with margination of chromatin that were positive for both HSV-1 and HSV-2 and negative for herpes zoster (Figure 2). The positivity of both HSV strains might represent co-infection or could be a cross-reaction of antibodies used in immunohistochemistry to the HSV antigens. There was acantholysis surrounding the ulceration and extending through the full thickness of the epidermis with a dilapidated brick wall pattern (Figure 3) as well as negative immunohistochemical staining for HSV-1 and HSV-2 antigens. The clinical and histological picture together, along with prior clinical and pathological reports, confirmed the diagnoses of acute erythrodermic HHD with HSV superinfection.


The patient’s condition and pain improved within 24 hours on intravenous acyclovir. On the third day, his lesions were resolving and symptoms improved, so he was transitioned to oral acyclovir and discharged from the hospital. Follow-up in the dermatology outpatient clinic 1 week later revealed that all vesicles and papules had cleared, but the patient was still erythrodermic. Because HHD cannot always be distinguished histologically from other forms of pemphigus but yields a negative immunofluorescence, direct immunofluorescence and indirect immunofluorescence were obtained upon patient follow-up in the clinic and were both negative. Hepatitis C viral loads were undetectable. Consultations to gastroenterology and oncology teams were placed for consideration of systemic agents, and the patient was initiated on oral acitretin 25 mg daily, along with clobetasol as adjuvant therapy for any residual skin plaques. The laboratory results were closely monitored. Within 4 weeks after starting acitretin, the patient’s erythroderma had completely resolved. The patient has remained stable since then, except for one episode of secondary Staphylococcus infection that cleared on oral antibiotics. The patient remains stable and clear on oral acitretin 25 mg daily, with concomitant desonide cream and fluocinonide ointment as needed.
Hailey-Hailey disease is characterized by recurrent episodes of erythema, blisters, and plaques localized to intertriginous and perianal areas.1,2 Patients display a spectrum of lesions that vary in severity.8 Typical histologic examination reveals a dilapidated brick wall appearance. Pathology of well-developed lesions will show suprabasal acantholysis with minimal dyskeratosis.2
The generalized form of HHD is an extremely rare variant of the disease.10 Generalized HHD may resemble acute hypersensitivity reaction, erythema multiforme, and toxic epidermal necrolysis.1 Chronic diseases, such as psoriasis (as in this patient), also may contribute to a clinically confusing picture.8 Hailey-Hailey disease and psoriasis are thought to occasionally koebnerize (isomorphic response) to areas of trauma.16 Our patient experienced widespread erythematous papules and plaques not restricted to skin folds. His skin lesions continued to worsen over several months progressing to erythroderma. The presence of suprabasal acantholysis in a dilapidated brick wall pattern, along with the patient’s history, prior pathology reports, clinical picture, and negative direct immunofluorescence and indirect immunofluorescence studies helped to confirm the diagnosis of erythrodermic HHD.
Hailey-Hailey disease is caused by heterozygous mutations in the ATP2C1 gene on chromosome 3q21-24 coding for a Golgi ATPase called SPCA1 (secretory pathway calcium/manganese-ATPase).9 Subsequent disturbances in cytosolic-Golgi calcium concentrations interfere with epidermal keratinocyte adherence resulting in acantholytic disease. Studies of interfamilial and intrafamilial mutations fail to pinpoint a common mutation pattern among patients with generalized phenotypes,9 which further supports theories that intrinsic or extrinsic factors such as friction, heat, radiation, contact allergens, and infection affect the severity of HHD disease and not the type of mutation.3,9
Generalization of HHD is likely caused by nonspecific triggers in an already genetically disturbed epidermis.10 Interrupted epithelial function exposes skin to infections that exacerbate the underlying disease. Superimposing bacterial infections are commonly reported in HHD. Staphylococcus, Streptococcus, and Candida species colonize the skin and aggravate the disease.11 Much less commonly, HSV superinfection can complicate HHD.3-7 No data are currently available about the frequency or incidence of Herpesviridae in HHD.7 Some studies suggest that UVB light therapy can be an exacerbating factor in DAR and some but not all HHD patients,12,13 while other case reports14,15 document clinically improved responses using phototherapy for patients with HHD. Clinicians should remain suspicious and evaluate for HSV infection in refractory or sudden exacerbation of HHD.7 Furthermore, coexistent psoriasis and HHD also is a rare entity but has been described,8 which illustrates the importance of not attributing all skin manifestations to a previously diagnosed disorder but instead keeping an open mind in case new dermatologic conditions present themselves at a later time.
We present a rare case of erythrodermic HHD and coexistent psoriasis with HSV superinfection. We hope to draw awareness to this association of generalized HHD with both HSV and psoriasis to help clinicians make the correct diagnosis promptly in similar cases in the future.
To the Editor:
Hailey-Hailey disease (HHD), also known as benign familial pemphigus, is an uncommon autosomal-dominant skin disease.1 Defects in the ATPase type 2C member 1 gene, ATP2C1, result in abnormal intracellular epidermal adherence, and patients experience recurring blisters in skin folds. Longitudinal white streaks of the fingernails also may be present.1 The illness does not appear until puberty and is heightened by the second or third decade of life. Family history often suggests the presence of disease.2 Misdiagnosis of HHD occurs because of a wide spectrum of presentations. The presence of superimposed infections and carcinomas may both obscure and exacerbate this disease.2
Herpes simplex viruse types 1 and 2 (HSV-1 and HSV-2) are DNA viruses that cause common recurrent diseases. Usually, HSV-1 is associated with infection of the mouth and HSV-2 is associated with infection of the genitalia.3 Longitudinal cutaneous lesions manifest as grouped vesicles on an erythematous base. Tzanck smear of herpetic vesicles will reveal the presence of multinucleated giant cells. A direct fluorescent antibody technique also may be used to confirm the diagnosis.3
Erythrodermic HHD disease is a rare condition; moreover, there are only a few reported cases with coexistence of HHD and HSV in the literature.3-6 We report a rare presentation of erythrodermic HHD and coexistent psoriasis with HSV superinfection.
A 69-year-old man presented to an outpatient dermatology clinic for evaluation and treatment of a rash on the scalp, face, back, and lower legs. The patient confirmed a dandruff diagnosis on the scalp and face as well as psoriasis on the trunk and extremities for the last 45 years. He described a history of successful treatment with topical agents and UV light therapy. A family history revealed that the patient’s father and 1 of 2 siblings had a similar rash and “skin problems.” The patient had a medical history of thyroid cancer treated with radiation treatment and a partial thyroidectomy 35 years prior to the current presentation as well as incompletely treated chronic hepatitis C.
A search of medical records revealed a punch biopsy from the posterior neck that demonstrated an acantholytic dyskeratosis with suprabasal acantholysis. Clinicians were unable to differentiate if it was Darier disease (DAR) or HHD. Treatment of the patient’s seborrheic dermatitis and acantholytic disorder was successful at that time with ketoconazole shampoo, ketoconazole cream, desonide cream, and triamcinolone cream. The patient remained stable for 5 years before presenting again to the dermatology clinic for worsening rash despite topical therapies.
At the current presentation, physical examination at the outpatient dermatology clinic revealed few scaly, erythematous, eroded papules distributed on the mid-back; erythematous greasy scaling on the scalp, face, and chest; and pink scaly plaques with white-silvery scale on the anterior lower legs. Histopathology of a specimen from the right mid-back demonstrated acantholysis with suprabasal clefting, hyperkeratosis, and parakeratosis with no dyskeratotic cells identified. The pathologic differential diagnosis included primary acantholytic processes including Grover disease, DAR, HHD, and pemphigus. Pathology from the right shin demonstrated acanthosis, confluent parakeratosis with associated decreased granular cell layer and collections of neutrophils within the stratum corneum, spongiosis, and superficial dermal perivascular chronic inflammation with focal exocytosis and dilated blood vessels in the papillary dermis. The clinical and pathological diagnosis on the lower legs was consistent with psoriasis. Diagnoses of seborrheic dermatitis, psoriasis on the lower legs, and HHD vs DAR on the back and chest were made. The patient was instructed to continue ketoconazole shampoo, ketoconazole cream, and desonide for seborrheic dermatitis; fluocinonide ointment 0.05% to the lower legs for psoriasis; and triamcinolone cream and a bland moisturizer to the back and chest for HHD.
Over the ensuing months, the rash worsened with erythema and scaling affecting more than half of the body surface area. Topical corticosteroids and bland emollients resulted in minimal success. Biologics and acitretin were considered for the psoriasiform dermatitis but avoided due to the patient’s medical history of thyroid cancer and chronic hepatitis C infection. Because the patient described prior success with UV light therapy for psoriasis, he requested light therapy. A subsequent trial of narrowband UVB light therapy initially improved some of the psoriasiform dermatitis on the trunk and extremities; however, after 4 weeks of treatment, the patient described pain in some of the skin and felt he was burned by minimal exposure to light therapy on one particular visit, which caused him to stop light therapy.
Approximately 2 weeks later, the patient presented to the emergency department stating his psoriasis was infected; he was diagnosed with psoriasis with secondary cellulitis and received intravenous vancomycin and piperacillin-tazobactam, with bacterial cultures demonstrating Corynebacterium and methicillin-resistant Staphylococcus aureus. Some improvement was noted in the patient’s skin after antibiotics were initiated, but he continued to describe worsening “burning and pain” throughout the psoriasis lesions. The patient’s care was transferred to the Veterans Affairs hospital where a dermatology inpatient consultation was placed.
Our initial dermatologic examination revealed generalized scaly erythroderma on the neck, trunk, and extremities, sparing the face, palms, and soles (Figure 1). Multiple crusted and intact vesicles also were present overlying the erythematous plaques on the chest, back, and proximal extremities, most grouped in clusters. The patient endorsed new symptoms of pain and burning. Tzanck smear from the abdomen along with shave biopsies from the left flank and right abdomen were performed, and intravenous acyclovir was initiated immediately after these procedures.

Viral cultures were taken but were incorrectly processed by the laboratory. Tzanck smear showed severe acute inflammation with numerous neutrophils, multinucleated giant cells with viral nuclear changes, and positive immunostaining for HSV and negative immunostaining for herpes zoster. Both pathology specimens revealed an intense acute mixed, mainly neutrophilic, inflammatory infiltrate extending into the deeper dermis as well as distorted and necrotic hair follicles, some of which displayed multinucleated epithelial cells with margination of chromatin that were positive for both HSV-1 and HSV-2 and negative for herpes zoster (Figure 2). The positivity of both HSV strains might represent co-infection or could be a cross-reaction of antibodies used in immunohistochemistry to the HSV antigens. There was acantholysis surrounding the ulceration and extending through the full thickness of the epidermis with a dilapidated brick wall pattern (Figure 3) as well as negative immunohistochemical staining for HSV-1 and HSV-2 antigens. The clinical and histological picture together, along with prior clinical and pathological reports, confirmed the diagnoses of acute erythrodermic HHD with HSV superinfection.


The patient’s condition and pain improved within 24 hours on intravenous acyclovir. On the third day, his lesions were resolving and symptoms improved, so he was transitioned to oral acyclovir and discharged from the hospital. Follow-up in the dermatology outpatient clinic 1 week later revealed that all vesicles and papules had cleared, but the patient was still erythrodermic. Because HHD cannot always be distinguished histologically from other forms of pemphigus but yields a negative immunofluorescence, direct immunofluorescence and indirect immunofluorescence were obtained upon patient follow-up in the clinic and were both negative. Hepatitis C viral loads were undetectable. Consultations to gastroenterology and oncology teams were placed for consideration of systemic agents, and the patient was initiated on oral acitretin 25 mg daily, along with clobetasol as adjuvant therapy for any residual skin plaques. The laboratory results were closely monitored. Within 4 weeks after starting acitretin, the patient’s erythroderma had completely resolved. The patient has remained stable since then, except for one episode of secondary Staphylococcus infection that cleared on oral antibiotics. The patient remains stable and clear on oral acitretin 25 mg daily, with concomitant desonide cream and fluocinonide ointment as needed.
Hailey-Hailey disease is characterized by recurrent episodes of erythema, blisters, and plaques localized to intertriginous and perianal areas.1,2 Patients display a spectrum of lesions that vary in severity.8 Typical histologic examination reveals a dilapidated brick wall appearance. Pathology of well-developed lesions will show suprabasal acantholysis with minimal dyskeratosis.2
The generalized form of HHD is an extremely rare variant of the disease.10 Generalized HHD may resemble acute hypersensitivity reaction, erythema multiforme, and toxic epidermal necrolysis.1 Chronic diseases, such as psoriasis (as in this patient), also may contribute to a clinically confusing picture.8 Hailey-Hailey disease and psoriasis are thought to occasionally koebnerize (isomorphic response) to areas of trauma.16 Our patient experienced widespread erythematous papules and plaques not restricted to skin folds. His skin lesions continued to worsen over several months progressing to erythroderma. The presence of suprabasal acantholysis in a dilapidated brick wall pattern, along with the patient’s history, prior pathology reports, clinical picture, and negative direct immunofluorescence and indirect immunofluorescence studies helped to confirm the diagnosis of erythrodermic HHD.
Hailey-Hailey disease is caused by heterozygous mutations in the ATP2C1 gene on chromosome 3q21-24 coding for a Golgi ATPase called SPCA1 (secretory pathway calcium/manganese-ATPase).9 Subsequent disturbances in cytosolic-Golgi calcium concentrations interfere with epidermal keratinocyte adherence resulting in acantholytic disease. Studies of interfamilial and intrafamilial mutations fail to pinpoint a common mutation pattern among patients with generalized phenotypes,9 which further supports theories that intrinsic or extrinsic factors such as friction, heat, radiation, contact allergens, and infection affect the severity of HHD disease and not the type of mutation.3,9
Generalization of HHD is likely caused by nonspecific triggers in an already genetically disturbed epidermis.10 Interrupted epithelial function exposes skin to infections that exacerbate the underlying disease. Superimposing bacterial infections are commonly reported in HHD. Staphylococcus, Streptococcus, and Candida species colonize the skin and aggravate the disease.11 Much less commonly, HSV superinfection can complicate HHD.3-7 No data are currently available about the frequency or incidence of Herpesviridae in HHD.7 Some studies suggest that UVB light therapy can be an exacerbating factor in DAR and some but not all HHD patients,12,13 while other case reports14,15 document clinically improved responses using phototherapy for patients with HHD. Clinicians should remain suspicious and evaluate for HSV infection in refractory or sudden exacerbation of HHD.7 Furthermore, coexistent psoriasis and HHD also is a rare entity but has been described,8 which illustrates the importance of not attributing all skin manifestations to a previously diagnosed disorder but instead keeping an open mind in case new dermatologic conditions present themselves at a later time.
We present a rare case of erythrodermic HHD and coexistent psoriasis with HSV superinfection. We hope to draw awareness to this association of generalized HHD with both HSV and psoriasis to help clinicians make the correct diagnosis promptly in similar cases in the future.
- Chave TA, Milligan A. Acute generalized Hailey-Hailey disease. Clin Exp Dermatol. 2002;27:290-292.
- Mohr MR, Erdag G, Shada Al, et al. Two patients with Hailey-Hailey disease, multiple primary melanomas, and other cancers. Arch Dermatol. 2011;147:211-215.
- Lee GM, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey Disease. Ann Dermatol. 2009;21:311-314.
- Zaim MT, Bickers DR. Herpes simplex associated with Hailey-Hailey disease. J Am Acad Dermatol. 1987;17:701-702.
- Peppiatt T, Keefe M, White JE. Hailey-Hailey disease-exacerbation by herpes simplex virus and patch tests. Clin Exp Dermatol. 2006;17:201-202.
- Almeida L, Grossman ME. Benign familial pemphigus complicated by herpes simplex virus. Cutis. 1989;44:261-262.
- Nikkels AF, Delvenne P, Herfs M, et al. Occult herpes simplex virus colonization of bullous dermatitides. Am J Clin Dermatol. 2008;9:163-168.
- Chao SC, Lee JY, Wu MC, et al. A novel splice mutation in the ATP2C1 gene in a woman with concomitant psoriasis vulgaris and disseminated Hailey-Hailey disease. Int J Dermatol. 2012;51:947-951.
- Ikeda S, Shigihara T, Mayuzumi N, et al. Mutations of ATP2C1 in Japanese patients with Hailey-Hailey disease: intrafamilial and interfamilial phenotype variations and lack of correlation with mutation patterns. J Invest Dermatol. 2001;117:1654-1656.
- Marsch W, Stuttgen G. Generalized Hailey-Hailey disease. Br J Dermatol. 1978;99:553-559.
- Friedman-Birnbaum R, Haim S, Marcus S. Generalized familial benign chronic pemphigus. Dermatologica. 1980;161:112-115.
- Richard G, Linse R, Harth W. Hailey-Hailey disease. early detection of heterozygotes by an ultraviolet provocation tests—clinical relevance of the method. Hautarzt. 1993;44:376-379.
- Mayuzumi N, Ikeda S, Kawada H, et al. Effects of ultraviolet B irradiation, proinflammatory cytokines and raised extracellular calcium concentration on the expression of ATP2A2 and ATP2C1. Br J Dermatol. 2005;152:697-701.
- Vanderbeck KA, Giroux L, Murugan NJ, et al. Combined therapeutic use of oral alitretinoin and narrowband ultraviolet-B therapy in the treatment of Hailey-Hailey disease. Dermatol Rep. 2014;6:5604.
- Mizuno K, Hamada T, Hasimoto T, et al. Successful treatment with narrow-band UVB therapy for a case of generalized Hailey-Hailey disease with a novel splice-site mutation in ATP2C1 gene. Dermatol Ther. 2014;27:233-235.
- Thappa DM. The isomorphic phenomenon of Koebner. Indian J Dermatol Venereol Leprol. 2004;70:187-189.
- Chave TA, Milligan A. Acute generalized Hailey-Hailey disease. Clin Exp Dermatol. 2002;27:290-292.
- Mohr MR, Erdag G, Shada Al, et al. Two patients with Hailey-Hailey disease, multiple primary melanomas, and other cancers. Arch Dermatol. 2011;147:211-215.
- Lee GM, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey Disease. Ann Dermatol. 2009;21:311-314.
- Zaim MT, Bickers DR. Herpes simplex associated with Hailey-Hailey disease. J Am Acad Dermatol. 1987;17:701-702.
- Peppiatt T, Keefe M, White JE. Hailey-Hailey disease-exacerbation by herpes simplex virus and patch tests. Clin Exp Dermatol. 2006;17:201-202.
- Almeida L, Grossman ME. Benign familial pemphigus complicated by herpes simplex virus. Cutis. 1989;44:261-262.
- Nikkels AF, Delvenne P, Herfs M, et al. Occult herpes simplex virus colonization of bullous dermatitides. Am J Clin Dermatol. 2008;9:163-168.
- Chao SC, Lee JY, Wu MC, et al. A novel splice mutation in the ATP2C1 gene in a woman with concomitant psoriasis vulgaris and disseminated Hailey-Hailey disease. Int J Dermatol. 2012;51:947-951.
- Ikeda S, Shigihara T, Mayuzumi N, et al. Mutations of ATP2C1 in Japanese patients with Hailey-Hailey disease: intrafamilial and interfamilial phenotype variations and lack of correlation with mutation patterns. J Invest Dermatol. 2001;117:1654-1656.
- Marsch W, Stuttgen G. Generalized Hailey-Hailey disease. Br J Dermatol. 1978;99:553-559.
- Friedman-Birnbaum R, Haim S, Marcus S. Generalized familial benign chronic pemphigus. Dermatologica. 1980;161:112-115.
- Richard G, Linse R, Harth W. Hailey-Hailey disease. early detection of heterozygotes by an ultraviolet provocation tests—clinical relevance of the method. Hautarzt. 1993;44:376-379.
- Mayuzumi N, Ikeda S, Kawada H, et al. Effects of ultraviolet B irradiation, proinflammatory cytokines and raised extracellular calcium concentration on the expression of ATP2A2 and ATP2C1. Br J Dermatol. 2005;152:697-701.
- Vanderbeck KA, Giroux L, Murugan NJ, et al. Combined therapeutic use of oral alitretinoin and narrowband ultraviolet-B therapy in the treatment of Hailey-Hailey disease. Dermatol Rep. 2014;6:5604.
- Mizuno K, Hamada T, Hasimoto T, et al. Successful treatment with narrow-band UVB therapy for a case of generalized Hailey-Hailey disease with a novel splice-site mutation in ATP2C1 gene. Dermatol Ther. 2014;27:233-235.
- Thappa DM. The isomorphic phenomenon of Koebner. Indian J Dermatol Venereol Leprol. 2004;70:187-189.
Practice Points
- Misdiagnosis of Hailey-Hailey disease (HHD) occurs because of a wide spectrum of presentations.
- Hailey-Hailey disease and psoriasis are thought to occasionally koebnerize (isomorphic response) to areas of trauma.
- Clinicians should remain suspicious and evaluate for herpes simplex virus infection in refractory or sudden exacerbation of HHD.
Variations in Preference for Topical Vehicles Among Demographic Groups
Topical medication is a mainstay in the treatment of dermatologic conditions. Adherence to medication regimens can be challenging in patients requiring long-term topical treatment, and nonadherence is multifactorial. A major modifiable contributing factor is patient dissatisfaction with the vehicle used. Medications often have options for different topical preparations. Therefore, it is important to consider patient preference when prescribing topical treatments to maximize adherence, ensure patient satisfaction, and optimize outcomes.
We hypothesized that notable differences exist among demographic groups regarding preference for topical vehicles. Little research has been conducted to delineate trends. This study aimed to identify variations in preference for creams, lotions, and ointments by age, gender, and ethnicity.
Methods
Data were collected through surveys distributed to all patients seen at the Truman Medical Center University Health Dermatology Clinic in Kansas City, Missouri, between September 2018 and June 2019. The study was approved by the University of Missouri Kansas City institutional review board. An estimated response rate of 95% was achieved. Each patient was informed that the survey was voluntary and anonymous, and declining to complete the survey had no effect on the care provided. Each patient completed only 1 survey and returned it to a collection box before departing from clinic.
In the survey, patients provided demographic information, including age, gender, and ethnicity. Age groups included patients younger than 40 years, 40 to 60 years, and older than 60 years. Gender groups included male and female. Ethnicity included white, black, Hispanic/Latino, and Asian/Pacific Islander or other. Patients then chose 1 of 3 options for topical vehicle preference: cream, lotion, or ointment. Each of these options was accompanied by a brief description of the vehicle, a photograph, and examples of common commercial products to aid in decision-making. The expected values were calculated based on a probability distribution under the assumption that variables have no association. Therefore, the discrepancy between the expected value and the observed value was used to describe the significance of the association between variables.
Data were analyzed using χ2 tests with the aid of a statistician. P<.05 was considered statistically significant.
Results
A total of 404 surveys were collected and recorded. Data showed statistically significant trends in each demographic parameter.
Age
First, we analyzed differences in preference based on age (Table 1). Of 404 patients, 163 were younger than 40 years, 171 were aged 40 to 60 years, and 70 were older than 60 years. Patients younger than 40 years preferred lotion (68 vs 46.0 expected). Patients aged 40 to 60 years showed preference for cream (83 vs 76.6 expected) and ointment (56 vs 46.1 expected). Patients older than 60 years preferred cream (41 vs 31.4 expected). These findings were statistically significant (P<.0001).
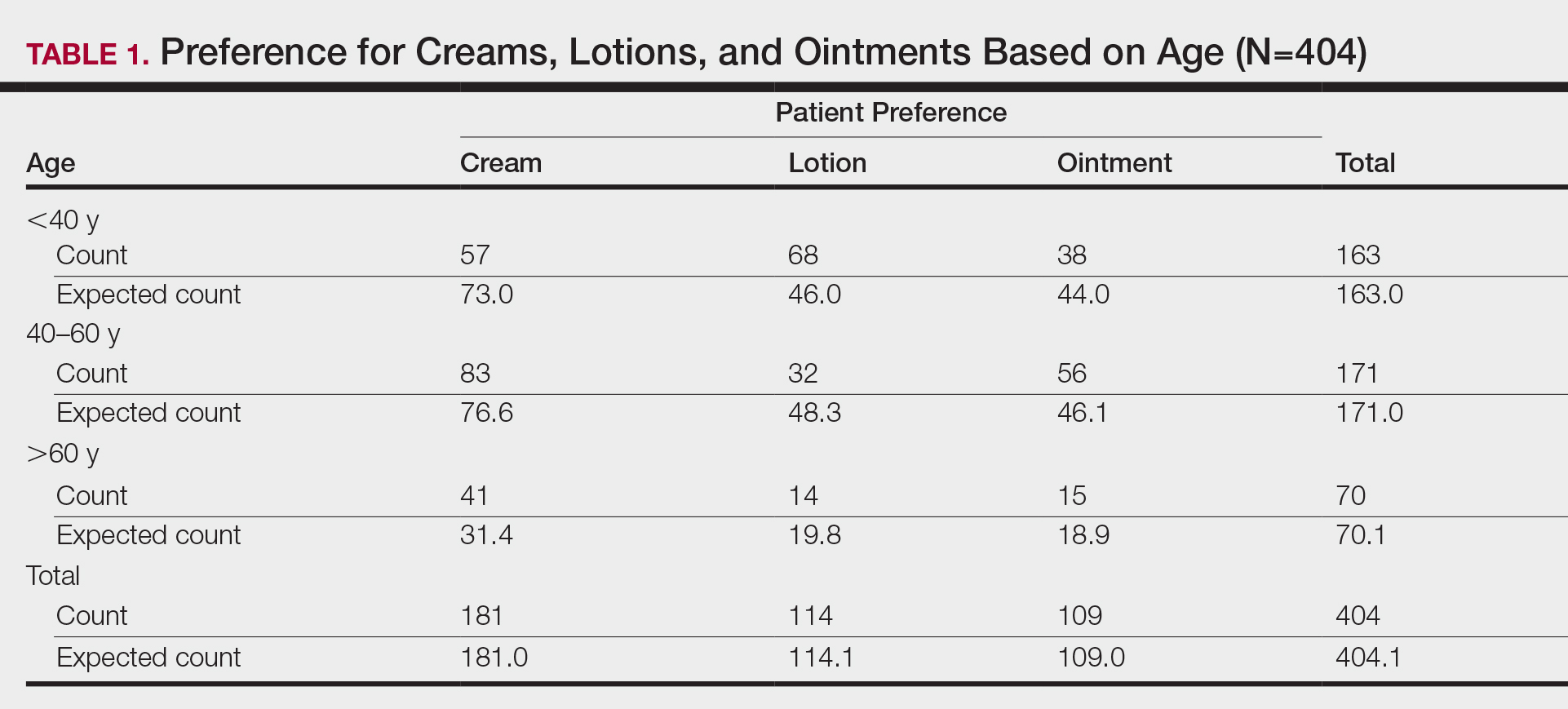
Gender
Next, we evaluated variations based on gender (Table 2). Of 404 patients, 254 were female and 150 were male. Females preferred cream (127 vs 113.8 expected). Males exhibited preference for lotion (50 vs 42.3 expected) and ointment (46 vs 40.5 expected). Differences between genders were statistically significant (P=.023).

Ethnicity
We then analyzed preferences based on ethnicity (Table 3). Of 404 patients, 30 were Hispanic/Latino, 26 were Asian/Pacific Islander or other, 227 were white, and 121 were black. Hispanic/Latino patients showed equivocal findings, aligning with expected counts. Asian/Pacific Islander or other patients exhibited slight preferences for cream (14 vs 11.6 expected) and lotion (10 vs 7.3 expected). White patients preferred cream (119 vs 101.7 expected) and lotion (82 vs 64.1 expected). Black patients showed strong preference for ointment (72 vs 32.6 expected). Differences in preferences based on ethnicity were statistically significant (P<.0001).

Comment
Topical medication is a mainstay of dermatologic therapy. Many topical preparations (or vehicles) exist, including ointments, creams, lotions, gels, solutions, and foams. Vehicle type not only influences bioavailability of the prepared medication but also has a notable impact on adherence and subsequent efficacy of the topical therapy.
Medication adherence is especially challenging in dermatology, as topical medications play a central role in treatment. Compliance with the medication regimen is paramount in treatment efficacy.1 In dermatology, adherence with oral medications is higher than it is for topical medications2; various factors contribute to this difference. Compliance may decline with topical treatment due to time-consuming application, misunderstanding about the disease or the treatment regimen, frequency of administration, dissatisfaction with efficacy or appearance, and other variables.3
Other factors have been found to be important to topical medication adherence; younger age, female gender, marriage, employment, nonsmoking, nondrinking, and higher cognitive ability were associated with higher topical medication adherence.4 Our study focused on one factor: identification of demographic-specific preferences that might have implications on adherence within the studied demographic groups.
It is known that individual preferences exist when patients are choosing a topical preparation. However, a PubMed search of articles indexed for MEDLINE using the terms topical, vehicle, preparation, adherence, and preference revealed few studies that examined the preference for topical vehicle by age, gender, or ethnicity.
Existing studies have examined preferences for topical preparations based on specific disease states; this literature, albeit limited, demonstrates that preferences for topical product formulations vary among acne, atopic dermatitis, and plaque psoriasis patients.5 Other studies focus on specific patient populations or medications. For example, one study found that preference for corticosteroid vehicles among psoriasis patients was highly variable and choice of vehicle was critical to adherence.6 Another study highlighted differences in vehicle choice between younger and older age groups with psoriasis.7
Given the limited data overall, it was our goal to determine if any patterns of preference existed by age, gender, or ethnicity, regardless of disease state or indication for topical product. Importantly, over-the-counter products—cosmetic or otherwise—were not differentiated from prescribed topical medications. Our survey elucidated significant differences in preference by age, gender, and ethnicity.
Notable Findings
Regarding age, patients younger than 40 years preferred lotion, patients aged 40 to 60 years preferred cream, and patients older than 60 years preferred cream. Analysis based on gender showed that females preferred cream, and males preferred lotion and ointment. Analysis based on ethnicity most notably demonstrated a strong preference for ointment in black patients while showing preference for cream in white patients.
Potential Biases and Pitfalls
Limitations of this study included the small Hispanic/Latino and Asian/Pacific Islander populations surveyed, possible misunderstanding of the survey by respondents, and the potential for surveys being filled out twice by the same patient. Future surveys could be conducted over a longer period to increase the total sample size and to better characterize less-represented populations, such as Hispanic and Asian patients. To avoid repeat participation, the first question of the survey asked patients to indicate if they had previously completed the survey and instructed patients who had to return the repeat survey to the front desk.
To limit other errors, our survey included concise accessible descriptions of each preparation along with clear representative photographs and examples of common brands. Still, it is possible that some mistakes could have been made while patients filled out the survey based on comprehension deficits, oversight, or other reasons. It also is possible that preference might vary individually depending on the indication of the topical product—cosmetic or therapeutic—or even by anatomic site of application. Neither of these considerations was assessed specifically in our survey.
Conclusion
Our hope is that this study helps practitioners better anticipate topical preferences among patients with the ultimate goal of increasing medication adherence and patient outcomes. Nevertheless, although these general trends can provide helpful guidance, we acknowledge that individual preferences vary, and care should always be patient centered.
Acknowledgment
We thank An-Lin Cheng, PhD (Kansas City, Missouri), for assistance with the statistical analysis.
- Kircik LH. Vehicles always matter. J Drugs Dermatol. 2019;18:s99.
- Furue M, Onozuka D, Takeuchi S, et al. Poor adherence to oral andtopical medication in 3096 dermatological patients as assessed by the Morisky Medication Adherence Scale-8. Br J Dermatol. 2015;172:272-275.
- Tan X, Feldman SR, Chang, J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Ahn CS, Culp L, Huang WW, et al. Adherence in dermatology. J Dermatolog Treat. 2017;28:94-103.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Felix K, Unrue E, Inyang M, et al. Patients preferences for different corticosteroid vehicles are highly variable. J Dermatolog Treat. 2019;31:147-151.
- Hong C-H, Papp KA, Lophaven KW, et al. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study. J Eur Acad Dermatol Venereol. 2017;31:1876-1883.
Topical medication is a mainstay in the treatment of dermatologic conditions. Adherence to medication regimens can be challenging in patients requiring long-term topical treatment, and nonadherence is multifactorial. A major modifiable contributing factor is patient dissatisfaction with the vehicle used. Medications often have options for different topical preparations. Therefore, it is important to consider patient preference when prescribing topical treatments to maximize adherence, ensure patient satisfaction, and optimize outcomes.
We hypothesized that notable differences exist among demographic groups regarding preference for topical vehicles. Little research has been conducted to delineate trends. This study aimed to identify variations in preference for creams, lotions, and ointments by age, gender, and ethnicity.
Methods
Data were collected through surveys distributed to all patients seen at the Truman Medical Center University Health Dermatology Clinic in Kansas City, Missouri, between September 2018 and June 2019. The study was approved by the University of Missouri Kansas City institutional review board. An estimated response rate of 95% was achieved. Each patient was informed that the survey was voluntary and anonymous, and declining to complete the survey had no effect on the care provided. Each patient completed only 1 survey and returned it to a collection box before departing from clinic.
In the survey, patients provided demographic information, including age, gender, and ethnicity. Age groups included patients younger than 40 years, 40 to 60 years, and older than 60 years. Gender groups included male and female. Ethnicity included white, black, Hispanic/Latino, and Asian/Pacific Islander or other. Patients then chose 1 of 3 options for topical vehicle preference: cream, lotion, or ointment. Each of these options was accompanied by a brief description of the vehicle, a photograph, and examples of common commercial products to aid in decision-making. The expected values were calculated based on a probability distribution under the assumption that variables have no association. Therefore, the discrepancy between the expected value and the observed value was used to describe the significance of the association between variables.
Data were analyzed using χ2 tests with the aid of a statistician. P<.05 was considered statistically significant.
Results
A total of 404 surveys were collected and recorded. Data showed statistically significant trends in each demographic parameter.
Age
First, we analyzed differences in preference based on age (Table 1). Of 404 patients, 163 were younger than 40 years, 171 were aged 40 to 60 years, and 70 were older than 60 years. Patients younger than 40 years preferred lotion (68 vs 46.0 expected). Patients aged 40 to 60 years showed preference for cream (83 vs 76.6 expected) and ointment (56 vs 46.1 expected). Patients older than 60 years preferred cream (41 vs 31.4 expected). These findings were statistically significant (P<.0001).

Gender
Next, we evaluated variations based on gender (Table 2). Of 404 patients, 254 were female and 150 were male. Females preferred cream (127 vs 113.8 expected). Males exhibited preference for lotion (50 vs 42.3 expected) and ointment (46 vs 40.5 expected). Differences between genders were statistically significant (P=.023).

Ethnicity
We then analyzed preferences based on ethnicity (Table 3). Of 404 patients, 30 were Hispanic/Latino, 26 were Asian/Pacific Islander or other, 227 were white, and 121 were black. Hispanic/Latino patients showed equivocal findings, aligning with expected counts. Asian/Pacific Islander or other patients exhibited slight preferences for cream (14 vs 11.6 expected) and lotion (10 vs 7.3 expected). White patients preferred cream (119 vs 101.7 expected) and lotion (82 vs 64.1 expected). Black patients showed strong preference for ointment (72 vs 32.6 expected). Differences in preferences based on ethnicity were statistically significant (P<.0001).

Comment
Topical medication is a mainstay of dermatologic therapy. Many topical preparations (or vehicles) exist, including ointments, creams, lotions, gels, solutions, and foams. Vehicle type not only influences bioavailability of the prepared medication but also has a notable impact on adherence and subsequent efficacy of the topical therapy.
Medication adherence is especially challenging in dermatology, as topical medications play a central role in treatment. Compliance with the medication regimen is paramount in treatment efficacy.1 In dermatology, adherence with oral medications is higher than it is for topical medications2; various factors contribute to this difference. Compliance may decline with topical treatment due to time-consuming application, misunderstanding about the disease or the treatment regimen, frequency of administration, dissatisfaction with efficacy or appearance, and other variables.3
Other factors have been found to be important to topical medication adherence; younger age, female gender, marriage, employment, nonsmoking, nondrinking, and higher cognitive ability were associated with higher topical medication adherence.4 Our study focused on one factor: identification of demographic-specific preferences that might have implications on adherence within the studied demographic groups.
It is known that individual preferences exist when patients are choosing a topical preparation. However, a PubMed search of articles indexed for MEDLINE using the terms topical, vehicle, preparation, adherence, and preference revealed few studies that examined the preference for topical vehicle by age, gender, or ethnicity.
Existing studies have examined preferences for topical preparations based on specific disease states; this literature, albeit limited, demonstrates that preferences for topical product formulations vary among acne, atopic dermatitis, and plaque psoriasis patients.5 Other studies focus on specific patient populations or medications. For example, one study found that preference for corticosteroid vehicles among psoriasis patients was highly variable and choice of vehicle was critical to adherence.6 Another study highlighted differences in vehicle choice between younger and older age groups with psoriasis.7
Given the limited data overall, it was our goal to determine if any patterns of preference existed by age, gender, or ethnicity, regardless of disease state or indication for topical product. Importantly, over-the-counter products—cosmetic or otherwise—were not differentiated from prescribed topical medications. Our survey elucidated significant differences in preference by age, gender, and ethnicity.
Notable Findings
Regarding age, patients younger than 40 years preferred lotion, patients aged 40 to 60 years preferred cream, and patients older than 60 years preferred cream. Analysis based on gender showed that females preferred cream, and males preferred lotion and ointment. Analysis based on ethnicity most notably demonstrated a strong preference for ointment in black patients while showing preference for cream in white patients.
Potential Biases and Pitfalls
Limitations of this study included the small Hispanic/Latino and Asian/Pacific Islander populations surveyed, possible misunderstanding of the survey by respondents, and the potential for surveys being filled out twice by the same patient. Future surveys could be conducted over a longer period to increase the total sample size and to better characterize less-represented populations, such as Hispanic and Asian patients. To avoid repeat participation, the first question of the survey asked patients to indicate if they had previously completed the survey and instructed patients who had to return the repeat survey to the front desk.
To limit other errors, our survey included concise accessible descriptions of each preparation along with clear representative photographs and examples of common brands. Still, it is possible that some mistakes could have been made while patients filled out the survey based on comprehension deficits, oversight, or other reasons. It also is possible that preference might vary individually depending on the indication of the topical product—cosmetic or therapeutic—or even by anatomic site of application. Neither of these considerations was assessed specifically in our survey.
Conclusion
Our hope is that this study helps practitioners better anticipate topical preferences among patients with the ultimate goal of increasing medication adherence and patient outcomes. Nevertheless, although these general trends can provide helpful guidance, we acknowledge that individual preferences vary, and care should always be patient centered.
Acknowledgment
We thank An-Lin Cheng, PhD (Kansas City, Missouri), for assistance with the statistical analysis.
Topical medication is a mainstay in the treatment of dermatologic conditions. Adherence to medication regimens can be challenging in patients requiring long-term topical treatment, and nonadherence is multifactorial. A major modifiable contributing factor is patient dissatisfaction with the vehicle used. Medications often have options for different topical preparations. Therefore, it is important to consider patient preference when prescribing topical treatments to maximize adherence, ensure patient satisfaction, and optimize outcomes.
We hypothesized that notable differences exist among demographic groups regarding preference for topical vehicles. Little research has been conducted to delineate trends. This study aimed to identify variations in preference for creams, lotions, and ointments by age, gender, and ethnicity.
Methods
Data were collected through surveys distributed to all patients seen at the Truman Medical Center University Health Dermatology Clinic in Kansas City, Missouri, between September 2018 and June 2019. The study was approved by the University of Missouri Kansas City institutional review board. An estimated response rate of 95% was achieved. Each patient was informed that the survey was voluntary and anonymous, and declining to complete the survey had no effect on the care provided. Each patient completed only 1 survey and returned it to a collection box before departing from clinic.
In the survey, patients provided demographic information, including age, gender, and ethnicity. Age groups included patients younger than 40 years, 40 to 60 years, and older than 60 years. Gender groups included male and female. Ethnicity included white, black, Hispanic/Latino, and Asian/Pacific Islander or other. Patients then chose 1 of 3 options for topical vehicle preference: cream, lotion, or ointment. Each of these options was accompanied by a brief description of the vehicle, a photograph, and examples of common commercial products to aid in decision-making. The expected values were calculated based on a probability distribution under the assumption that variables have no association. Therefore, the discrepancy between the expected value and the observed value was used to describe the significance of the association between variables.
Data were analyzed using χ2 tests with the aid of a statistician. P<.05 was considered statistically significant.
Results
A total of 404 surveys were collected and recorded. Data showed statistically significant trends in each demographic parameter.
Age
First, we analyzed differences in preference based on age (Table 1). Of 404 patients, 163 were younger than 40 years, 171 were aged 40 to 60 years, and 70 were older than 60 years. Patients younger than 40 years preferred lotion (68 vs 46.0 expected). Patients aged 40 to 60 years showed preference for cream (83 vs 76.6 expected) and ointment (56 vs 46.1 expected). Patients older than 60 years preferred cream (41 vs 31.4 expected). These findings were statistically significant (P<.0001).

Gender
Next, we evaluated variations based on gender (Table 2). Of 404 patients, 254 were female and 150 were male. Females preferred cream (127 vs 113.8 expected). Males exhibited preference for lotion (50 vs 42.3 expected) and ointment (46 vs 40.5 expected). Differences between genders were statistically significant (P=.023).

Ethnicity
We then analyzed preferences based on ethnicity (Table 3). Of 404 patients, 30 were Hispanic/Latino, 26 were Asian/Pacific Islander or other, 227 were white, and 121 were black. Hispanic/Latino patients showed equivocal findings, aligning with expected counts. Asian/Pacific Islander or other patients exhibited slight preferences for cream (14 vs 11.6 expected) and lotion (10 vs 7.3 expected). White patients preferred cream (119 vs 101.7 expected) and lotion (82 vs 64.1 expected). Black patients showed strong preference for ointment (72 vs 32.6 expected). Differences in preferences based on ethnicity were statistically significant (P<.0001).

Comment
Topical medication is a mainstay of dermatologic therapy. Many topical preparations (or vehicles) exist, including ointments, creams, lotions, gels, solutions, and foams. Vehicle type not only influences bioavailability of the prepared medication but also has a notable impact on adherence and subsequent efficacy of the topical therapy.
Medication adherence is especially challenging in dermatology, as topical medications play a central role in treatment. Compliance with the medication regimen is paramount in treatment efficacy.1 In dermatology, adherence with oral medications is higher than it is for topical medications2; various factors contribute to this difference. Compliance may decline with topical treatment due to time-consuming application, misunderstanding about the disease or the treatment regimen, frequency of administration, dissatisfaction with efficacy or appearance, and other variables.3
Other factors have been found to be important to topical medication adherence; younger age, female gender, marriage, employment, nonsmoking, nondrinking, and higher cognitive ability were associated with higher topical medication adherence.4 Our study focused on one factor: identification of demographic-specific preferences that might have implications on adherence within the studied demographic groups.
It is known that individual preferences exist when patients are choosing a topical preparation. However, a PubMed search of articles indexed for MEDLINE using the terms topical, vehicle, preparation, adherence, and preference revealed few studies that examined the preference for topical vehicle by age, gender, or ethnicity.
Existing studies have examined preferences for topical preparations based on specific disease states; this literature, albeit limited, demonstrates that preferences for topical product formulations vary among acne, atopic dermatitis, and plaque psoriasis patients.5 Other studies focus on specific patient populations or medications. For example, one study found that preference for corticosteroid vehicles among psoriasis patients was highly variable and choice of vehicle was critical to adherence.6 Another study highlighted differences in vehicle choice between younger and older age groups with psoriasis.7
Given the limited data overall, it was our goal to determine if any patterns of preference existed by age, gender, or ethnicity, regardless of disease state or indication for topical product. Importantly, over-the-counter products—cosmetic or otherwise—were not differentiated from prescribed topical medications. Our survey elucidated significant differences in preference by age, gender, and ethnicity.
Notable Findings
Regarding age, patients younger than 40 years preferred lotion, patients aged 40 to 60 years preferred cream, and patients older than 60 years preferred cream. Analysis based on gender showed that females preferred cream, and males preferred lotion and ointment. Analysis based on ethnicity most notably demonstrated a strong preference for ointment in black patients while showing preference for cream in white patients.
Potential Biases and Pitfalls
Limitations of this study included the small Hispanic/Latino and Asian/Pacific Islander populations surveyed, possible misunderstanding of the survey by respondents, and the potential for surveys being filled out twice by the same patient. Future surveys could be conducted over a longer period to increase the total sample size and to better characterize less-represented populations, such as Hispanic and Asian patients. To avoid repeat participation, the first question of the survey asked patients to indicate if they had previously completed the survey and instructed patients who had to return the repeat survey to the front desk.
To limit other errors, our survey included concise accessible descriptions of each preparation along with clear representative photographs and examples of common brands. Still, it is possible that some mistakes could have been made while patients filled out the survey based on comprehension deficits, oversight, or other reasons. It also is possible that preference might vary individually depending on the indication of the topical product—cosmetic or therapeutic—or even by anatomic site of application. Neither of these considerations was assessed specifically in our survey.
Conclusion
Our hope is that this study helps practitioners better anticipate topical preferences among patients with the ultimate goal of increasing medication adherence and patient outcomes. Nevertheless, although these general trends can provide helpful guidance, we acknowledge that individual preferences vary, and care should always be patient centered.
Acknowledgment
We thank An-Lin Cheng, PhD (Kansas City, Missouri), for assistance with the statistical analysis.
- Kircik LH. Vehicles always matter. J Drugs Dermatol. 2019;18:s99.
- Furue M, Onozuka D, Takeuchi S, et al. Poor adherence to oral andtopical medication in 3096 dermatological patients as assessed by the Morisky Medication Adherence Scale-8. Br J Dermatol. 2015;172:272-275.
- Tan X, Feldman SR, Chang, J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Ahn CS, Culp L, Huang WW, et al. Adherence in dermatology. J Dermatolog Treat. 2017;28:94-103.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Felix K, Unrue E, Inyang M, et al. Patients preferences for different corticosteroid vehicles are highly variable. J Dermatolog Treat. 2019;31:147-151.
- Hong C-H, Papp KA, Lophaven KW, et al. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study. J Eur Acad Dermatol Venereol. 2017;31:1876-1883.
- Kircik LH. Vehicles always matter. J Drugs Dermatol. 2019;18:s99.
- Furue M, Onozuka D, Takeuchi S, et al. Poor adherence to oral andtopical medication in 3096 dermatological patients as assessed by the Morisky Medication Adherence Scale-8. Br J Dermatol. 2015;172:272-275.
- Tan X, Feldman SR, Chang, J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Ahn CS, Culp L, Huang WW, et al. Adherence in dermatology. J Dermatolog Treat. 2017;28:94-103.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Felix K, Unrue E, Inyang M, et al. Patients preferences for different corticosteroid vehicles are highly variable. J Dermatolog Treat. 2019;31:147-151.
- Hong C-H, Papp KA, Lophaven KW, et al. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study. J Eur Acad Dermatol Venereol. 2017;31:1876-1883.
Practice Points
- Variations exist in preference for topical vehicles by age group, gender, and ethnicity.
- Identifying and utilizing preferred treatment options can help maximize patient outcomes.