User login
Content Analysis of Psoriasis and Eczema Direct-to-Consumer Advertisements
Direct-to-consumer (DTC) advertisements are an important and influential source of health-related information for Americans. In 1997, the US Food and Drug Administration (FDA) relaxed regulations and permitted DTC drug advertisements to be televised. Now, via television alone, the average American is exposed to more than 30 hours annually of DTC advertisements for drugs,1 which exceeds, by far, the amount of time the average American spends with his/her physician.2 The United States spends $9.6 billion on DTC advertisements per year, of which $605 million is spent exclusively on DTC advertisements for dermatologic conditions—one of the highest amounts of spending for DTC advertisements, second only to diabetes.3
The increase in advertising for dermatologic conditions is reflective of the rapid growth in the number of treatment options available for chronic skin diseases, especially psoriasis. Since 2004, 11 biologics and 1 oral medication were FDA approved for the treatment of moderate to severe psoriasis. Despite the expansion of treatment options for psoriasis, knowledge and understanding of psoriasis and its treatments generally are poor,4,5 and undertreatment of psoriasis continues to be common.6 Data also suggest existing age and racial disparities in psoriasis treatment in the United States, whereby patients who are older or Black are less likely to receive biologic therapies.7-9 Although the exact causes of these disparities remain unclear, one study found that Black patients with psoriasis were less familiar with biologics compared to White patients,10 which suggests that the racial disparity in biologic treatment of psoriasis could be due to less exposure to and thus recognition of biologics as treatments of psoriasis among Black patients.
Some data suggest that DTC advertisements may affect drug uptake by encouraging patients to request advertised medications from their medical providers.11,12 As such, DTC advertisements are a potentially important source of exposure and information for patients. However, is it possible that DTC advertisements also may contribute to widening knowledge gaps among certain populations, and thus treatment disparities, by neglecting certain groups and targeting others with their content? In an effort to answer this question, we performed an analysis of DTC advertisements for psoriasis and eczema with special attention to advertisement placement, character representation, and disease-related content. We specifically targeted advertisements for psoriasis and eczema, as advertisements for the former are rampant and advertisements for the latter are on the rise because of emerging therapies. We hypothesized that age and racial/ethnic diversity among advertisement characters is poor, and disease-related content is lacking.
Materials and Methods
Study Design and Sample
We performed a cross-sectional analysis of televised DTC advertisements for psoriasis and eczema over 14 consecutive days (July 1, 2018, to July 14, 2018). We accessed Nielsen’s top 10 lists, specifically Prime Broadcast Network TV-United States and Prime Broadcast Programs Among African-American, from June 2018 and identified the networks with the greatest potential exposure to American consumers: ABC, CBS, FOX, and NBC.13,14 Each day, programming aired from 5
The FDA identifies DTC advertisement types as product-claim, reminder, and help-seeking advertisements. Product-claim advertisements are required to include the following information for the drug of interest: name; at least 1 FDA-approved indication; the most notable risks; and reference to a toll-free telephone number, website, or print advertisement by which a detailed summary of risks and benefits can be accessed. Reminder advertisements include the name of the drug but no information about the drug’s use.15 Help-seeking advertisements describe a disease or condition without referencing a specific drug treatment. Product-claim, reminder, and help-seeking advertisements for psoriasis or eczema that aired during the recorded time frame were included for analysis; advertisements that aired during sporting events and special programming were excluded.
DTC Advertisement Coding
Advertisement placement (ie, network, day of the week, time, associated television program), type, and target disease were documented for all advertisements included in the study. The content of each unique advertisement for psoriasis and eczema also was documented electronically in REDCap (Research Electronic Data Capture) as follows: characteristics of affected individuals and disease-related content. Advertisement coding was performed independently by 2 graduate students (A.H. and C.W.). First, one-third of the advertisements were randomly selected to be coded by both students. Intercoder agreement between the 2 students was 95.3%. Coding disagreements were primarily due to misunderstanding of definitions and were resolved through consensus. Subsequently, the remaining advertisements were randomly distributed between the 2 students, and each advertisement was coded by 1 student.
Statistical Analysis
All data were summarized descriptively with counts and frequencies using Stata 15 (StataCorp).
Results
We identified 297 DTC advertisements addressing 25 different conditions during our study period. CBS, ABC, NBC, and FOX aired 44.4%, 26.3%, 24.4%, and 5.1% of advertisements, respectively. Overall, DTC advertisements were least likely to air on Saturdays and between the hours of 5
Psoriasis DTC Advertisements
There were 5 unique psoriasis DTC advertisements, all of which were product-claim advertisements, with 1 each for secukinumab (Cosentyx [Novartis]), ixekizumab (Taltz [Eli Lilly and Company]), and guselkumab (Tremfya [Janssen Biotech, Inc]), and 2 for adalimumab (Humira [AbbVie Inc]). The advertisements aired on ABC (n=5 [38.5%]), CBS (n=5 [38.5%]), and NBC (n=3 [23.1%]). Most advertisements aired on weekdays (61.5%) between 6
Psoriasis Character Portrayal and Disease-Related Content
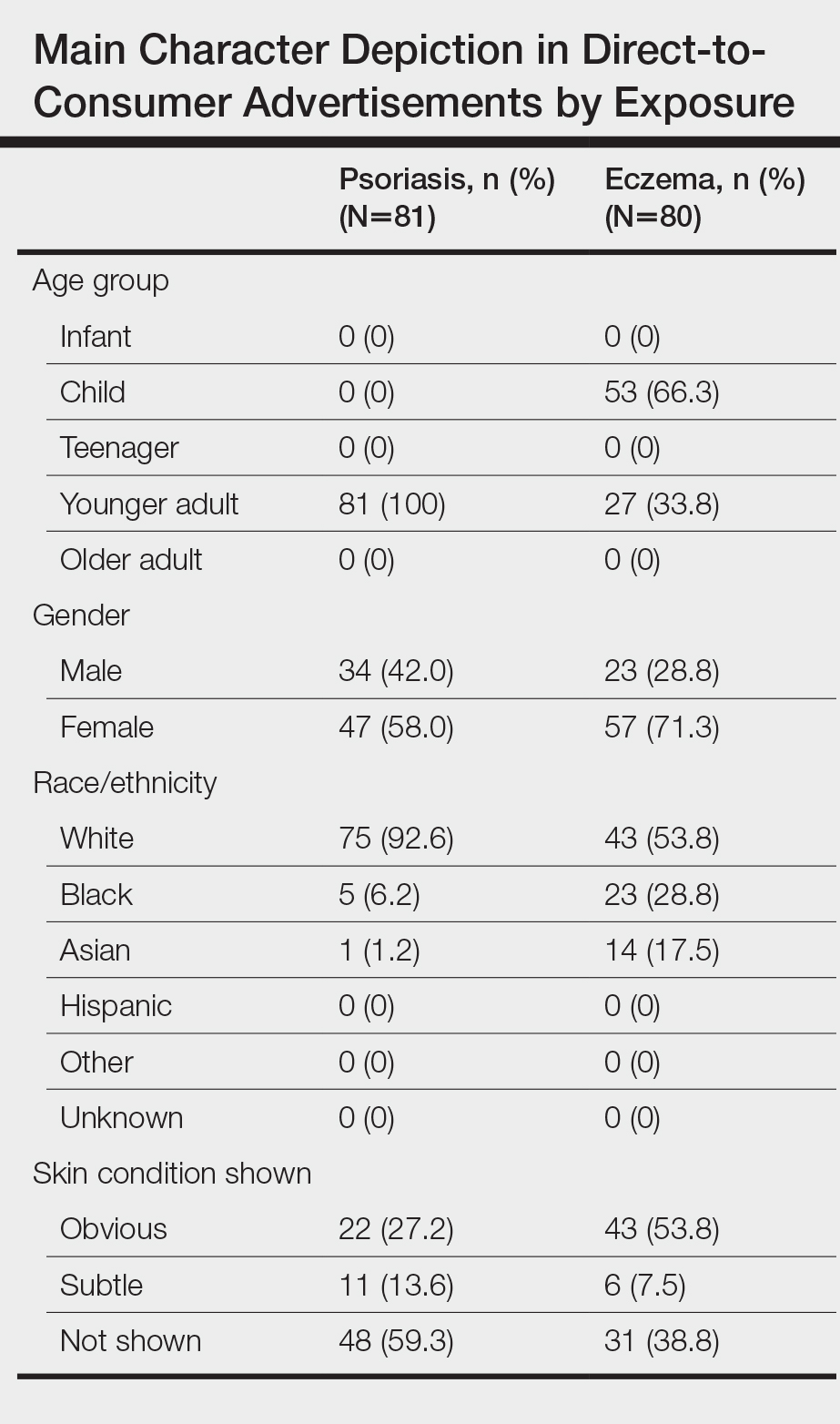
We identified 81 main characters who were depicted as having psoriasis among all advertisements. Characteristics of the affected characters are summarized in the Table. All affected characters were perceived to be younger adults, and there was a slight female predominance (58.0% [47/81]). Most characters were perceived to be White (92.6% [75/81]). Black and Asian characters only represented 6.2% (5/81) and 1.2% (1/81) of all affected individuals, respectively. Notably, the advertisements that featured only White main characters were aired 2.75 times more frequently than the advertisements that included non-White characters.
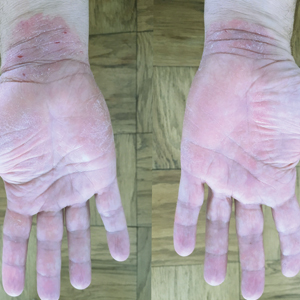
Psoriasis was shown on the skin of at least 1 character in an obvious depiction (ie, did not require more than 1 viewing) in 84.6% (11/13) of the advertisements. Symptoms of psoriasis (communicated either verbally or visually) were included in only 15.4% (2/13) of advertisements. No advertisements included information on the epidemiology of (ie, prevalence, subpopulations at risk), risk factors for, pathophysiology of, or comorbid diseases associated with psoriasis.
Eczema DTC Advertisements
Among the 27 eczema advertisements aired, there were 4 unique advertisements, of which 3 were product-claim advertisements (all for crisaborole [Eucrisa (Pfizer Inc)]), and 1 was a help-seeking advertisement that was sponsored by Sanofi Genzyme and Regeneron Pharmaceuticals. The advertisements aired on ABC (n=2 [7.4%]), CBS (n=17 [63.0%]), and NBC (n=8 [29.6%]). All advertisements aired on weekdays between 7
Eczema Character Portrayal and Disease-Related Content
We identified 80 main characters who were depicted to be affected by eczema among all advertisements. Characteristics of the affected characters are summarized in the Table. Most of the affected characters were perceived to be White (53.8% [43/80]) and female (71.3% [57/80]). Other races depicted included Black (28.8% [23/80]) and Asian (17.5% [14/80]). Each unique eczema advertisement included at least 1 non-White main character. Most eczema main characters were perceived to be children (66.3% [53/80]), followed by younger adults (33.8% [27/80]). No infants, teenagers, or older adults were shown as being affected by eczema.
Skin manifestations of eczema were portrayed on at least 1 character in all of the advertisements; 77.8% (21/27) of the advertisements had at least 1 obvious depiction. Symptoms of eczema and the mechanism of disease (pathophysiology) were each included in 44.4% (12/27) of advertisements. This information was included exclusively in the single help-seeking advertisement, which also referenced a website for additional disease-related information. No advertisements included information on the epidemiology of, risk factors for, or comorbid diseases associated with eczema.
Comment
In our study of televised DTC advertisements for psoriasis and eczema in the United States, we identified underrepresentation of racial/ethnic minorities and specific age groups (older adults for psoriasis and all adults for eczema) across all advertisements. Although psoriasis is suggested to be less prevalent among minority patients (1.3%–1.9% among Black patients and 1.6% among Hispanic patients) compared to White patients (2%–4%),16,17 minority vs White representation in psoriasis DTC advertisements was disproportionately lower than population-based prevalence estimates. Direct-to-consumer advertisements for eczema included more minority characters than psoriasis advertisements; however, minority representation remained inadequate considering that childhood eczema is more prevalent among Black vs White children,18 and adult eczema is at least as prevalent among minority patients compared to White patients.19 Not only was minority representation in all advertisements poor, but advertisement placement also was suboptimal, particularly for reaching Black viewers. FOX network was home to 2 of the top 3 primetime broadcast programs among Black viewers around the study period,13 yet no DTC advertisements were aired on FOX.
The current literature regarding minority representation in DTC advertisements is mixed. Some studies report underrepresentation of Black and other minority patients across a variety of diseases.20 Other studies suggest that representation of Black patients, in particular, generally is adequate, except among select serious health conditions, and that advertisements depict tokenism or stereotypical roles for minorities.21 Our study provides new and specific insight about the state of racial/ethnic and age diversity, or lack thereof, in DTC advertisements for the skin conditions that currently are most commonly targeted—psoriasis and eczema. Although it remains unclear whether DTC advertisements are good or bad, existing data suggest that potential benefits of DTC advertisements include strengthening of patient-provider relationships, reduction of underdiagnosis and undertreatment of disease, and reduction of disease stigma.22 However, in our analyses, we found disease-specific factual content among all DTC advertisements to be sparse and obvious depictions of skin disease and symptoms to be uncommon, especially for psoriasis. As such, it seems unlikely that existing DTC advertisements for psoriasis and eczema can be expected to contribute to meaningful disease education, reduce underdiagnosis, and reduce the stigmatizing attitudes that have been documented for both skin diseases.23-25
Furthermore, it is important to consider our findings in light of the role that social identity theory plays in marketing. Social identity theory supports the idea that a person’s social identity (eg, age, gender, race/ethnicity) influences his/her behavior, perceptions, and performance.26 The principle of homophily—the tendency for individuals to have positive ties to those who are similar to themselves—is a critical concept in social identity theory and suggests that consumers are more likely to pay attention to and be influenced by sources perceived as similar to themselves.20 Thus, even if the potential benefits of DTC advertisements were to be realized for psoriasis and eczema, the lack of adequate minority and older adult representation raises concerns about whether these benefits would reach a diverse population and if the advertisements might further potentiate existing knowledge and treatment disparities.
Limitations
Our study is not without limitations. The sampling period was short and might not reflect advertisement content over a longer time course. We did not evaluate other potential sources of information, such as the Internet and social media. Nevertheless, televised DTC advertisements remain a major source of medical and drug information for the general public. We did not directly evaluate viewers’ reactions to the DTC advertisements of interest; however, other literature lends support to the significance of social identity theory and its impact on consumer behavior.26
Conclusion
Our study highlights a lost opportunity among psoriasis and eczema DTC advertisements for patient reach and disease education that may encourage existing and emerging knowledge and treatment disparities for both conditions. Our findings should serve as a call to action to pharmaceutical companies and other organizations involved in creating and supporting DTC advertisements for psoriasis and eczema to increase the educational content, diversify the depicted characters, and optimize advertisement placement.
- Brownfield ED, Bernhardt JM, Phan JL, et al. Direct-to-consumer drug advertisements on network television: an exploration of quantity, frequency, and placement. J Health Commun. 2004;9:491-497.
- Tai-Seale M, McGuire TG, Zhang W. Time allocation in primary care office visits. Health Serv Res. 2007;42:1871-1894.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Lanigan SW, Farber EM. Patients’ knowledge of psoriasis: pilot study. Cutis. 1990;46:359-362.
- Renzi C, Di Pietro C, Tabolli S. Participation, satisfaction and knowledge level of patients with cutaneous psoriasis or psoriatic arthritis. Clin Exp Dermatol. 2011;36:885-888.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881.e871-830.
- Wu JJ, Lu M, Veverka KA, et al. The journey for US psoriasis patients prescribed a topical: a retrospective database evaluation of patient progression to oral and/or biologic treatment. J Dermatolog Treat. 2019;30:446-453.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
- Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34:1753-1759.
- Takeshita J, Eriksen WT, Raziano VT, et al. Racial differences in perceptions of psoriasis therapies: implications for racial disparities in psoriasis treatment. J Invest Dermatol. 2019;139:1672-1679.e1.
- Wu MH, Bartz D, Avorn J, et al. Trends in direct-to-consumer advertising of prescription contraceptives. Contraception. 2016;93:398-405.
- Mintzes B, Barer ML, Kravitz RL, et al. How does direct-to-consumer advertising (DTCA) affect prescribing? a survey in primary care environments with and without legal DTCA. CMAJ. 2003;169:405-412.
- Topten. Nielson website. https://www.nielsen.com/us/en/top-ten/. Accessed July 22, 2020.
- Leading ad supported broadcast and cable networks in the United States in 2019, by average number of viewers. Statistia website. https://www.statista.com/statistics/530119/tv-networks-viewers-usa/. Accessed July 22, 2020.
- Prescription drug advertisements. Electronic Code of Federal Regulations website. https://www.ecfr.gov/cgi-bin/text-idx?SID=d4f308e364578bda8e55a831638a26c6&mc=true&node=pt21.4.202&rgn=div5. Updated August 12, 2020. Accessed August 12, 2020.
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Centers for Disease Control and Prevention. National Center for Health Statistics, National Health Interview Survey, 2014. https://www.cdc.gov/nchs/data/health_policy/eczema_skin_problems_tables.pdf. Accessed July 22, 2020.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Welch Cline RJ, Young HN. Marketing drugs, marketing health care relationships: a content analysis of visual cues in direct-to-consumer prescription drug advertising. Health Commun. 2004;16:131-157.
- Ball JG, Liang A, Lee WN. Representation of African Americans in direct-to-consumer pharmaceutical commercials: a content analysis with implications for health disparities. Health Mark Q. 2009;26:372-390.
- Ventola CL. Direct-to-consumer pharmaceutical advertising: therapeutic or toxic? P T. 2011;36:669-674, 681-684.
- Pearl RL, Wan MT, Takeshita J, et al. Stigmatizing attitudes toward persons with psoriasis among laypersons and medical students. J Am Acad Dermatol. 2019;80:1556-1563.
- Chernyshov PV. Stigmatization and self-perception in children with atopic dermatitis. Clin Cosmet Investig Dermatol. 2016;9:159-166.
- Wittkowski A, Richards HL, Griffiths CEM, et al. The impact of psychological and clinical factors on quality of life in individuals with atopic dermatitis. J Psychosom Res. 2004;57:195-200.
- Forehand MR, Deshpande R, Reed 2nd A. Identity salience and the influence of differential activation of the social self-schema on advertising response. J Appl Psychol. 2002;87:1086-1099.
Direct-to-consumer (DTC) advertisements are an important and influential source of health-related information for Americans. In 1997, the US Food and Drug Administration (FDA) relaxed regulations and permitted DTC drug advertisements to be televised. Now, via television alone, the average American is exposed to more than 30 hours annually of DTC advertisements for drugs,1 which exceeds, by far, the amount of time the average American spends with his/her physician.2 The United States spends $9.6 billion on DTC advertisements per year, of which $605 million is spent exclusively on DTC advertisements for dermatologic conditions—one of the highest amounts of spending for DTC advertisements, second only to diabetes.3
The increase in advertising for dermatologic conditions is reflective of the rapid growth in the number of treatment options available for chronic skin diseases, especially psoriasis. Since 2004, 11 biologics and 1 oral medication were FDA approved for the treatment of moderate to severe psoriasis. Despite the expansion of treatment options for psoriasis, knowledge and understanding of psoriasis and its treatments generally are poor,4,5 and undertreatment of psoriasis continues to be common.6 Data also suggest existing age and racial disparities in psoriasis treatment in the United States, whereby patients who are older or Black are less likely to receive biologic therapies.7-9 Although the exact causes of these disparities remain unclear, one study found that Black patients with psoriasis were less familiar with biologics compared to White patients,10 which suggests that the racial disparity in biologic treatment of psoriasis could be due to less exposure to and thus recognition of biologics as treatments of psoriasis among Black patients.
Some data suggest that DTC advertisements may affect drug uptake by encouraging patients to request advertised medications from their medical providers.11,12 As such, DTC advertisements are a potentially important source of exposure and information for patients. However, is it possible that DTC advertisements also may contribute to widening knowledge gaps among certain populations, and thus treatment disparities, by neglecting certain groups and targeting others with their content? In an effort to answer this question, we performed an analysis of DTC advertisements for psoriasis and eczema with special attention to advertisement placement, character representation, and disease-related content. We specifically targeted advertisements for psoriasis and eczema, as advertisements for the former are rampant and advertisements for the latter are on the rise because of emerging therapies. We hypothesized that age and racial/ethnic diversity among advertisement characters is poor, and disease-related content is lacking.
Materials and Methods
Study Design and Sample
We performed a cross-sectional analysis of televised DTC advertisements for psoriasis and eczema over 14 consecutive days (July 1, 2018, to July 14, 2018). We accessed Nielsen’s top 10 lists, specifically Prime Broadcast Network TV-United States and Prime Broadcast Programs Among African-American, from June 2018 and identified the networks with the greatest potential exposure to American consumers: ABC, CBS, FOX, and NBC.13,14 Each day, programming aired from 5
The FDA identifies DTC advertisement types as product-claim, reminder, and help-seeking advertisements. Product-claim advertisements are required to include the following information for the drug of interest: name; at least 1 FDA-approved indication; the most notable risks; and reference to a toll-free telephone number, website, or print advertisement by which a detailed summary of risks and benefits can be accessed. Reminder advertisements include the name of the drug but no information about the drug’s use.15 Help-seeking advertisements describe a disease or condition without referencing a specific drug treatment. Product-claim, reminder, and help-seeking advertisements for psoriasis or eczema that aired during the recorded time frame were included for analysis; advertisements that aired during sporting events and special programming were excluded.
DTC Advertisement Coding
Advertisement placement (ie, network, day of the week, time, associated television program), type, and target disease were documented for all advertisements included in the study. The content of each unique advertisement for psoriasis and eczema also was documented electronically in REDCap (Research Electronic Data Capture) as follows: characteristics of affected individuals and disease-related content. Advertisement coding was performed independently by 2 graduate students (A.H. and C.W.). First, one-third of the advertisements were randomly selected to be coded by both students. Intercoder agreement between the 2 students was 95.3%. Coding disagreements were primarily due to misunderstanding of definitions and were resolved through consensus. Subsequently, the remaining advertisements were randomly distributed between the 2 students, and each advertisement was coded by 1 student.
Statistical Analysis
All data were summarized descriptively with counts and frequencies using Stata 15 (StataCorp).
Results
We identified 297 DTC advertisements addressing 25 different conditions during our study period. CBS, ABC, NBC, and FOX aired 44.4%, 26.3%, 24.4%, and 5.1% of advertisements, respectively. Overall, DTC advertisements were least likely to air on Saturdays and between the hours of 5
Psoriasis DTC Advertisements
There were 5 unique psoriasis DTC advertisements, all of which were product-claim advertisements, with 1 each for secukinumab (Cosentyx [Novartis]), ixekizumab (Taltz [Eli Lilly and Company]), and guselkumab (Tremfya [Janssen Biotech, Inc]), and 2 for adalimumab (Humira [AbbVie Inc]). The advertisements aired on ABC (n=5 [38.5%]), CBS (n=5 [38.5%]), and NBC (n=3 [23.1%]). Most advertisements aired on weekdays (61.5%) between 6
Psoriasis Character Portrayal and Disease-Related Content
We identified 81 main characters who were depicted as having psoriasis among all advertisements. Characteristics of the affected characters are summarized in the Table. All affected characters were perceived to be younger adults, and there was a slight female predominance (58.0% [47/81]). Most characters were perceived to be White (92.6% [75/81]). Black and Asian characters only represented 6.2% (5/81) and 1.2% (1/81) of all affected individuals, respectively. Notably, the advertisements that featured only White main characters were aired 2.75 times more frequently than the advertisements that included non-White characters.

Psoriasis was shown on the skin of at least 1 character in an obvious depiction (ie, did not require more than 1 viewing) in 84.6% (11/13) of the advertisements. Symptoms of psoriasis (communicated either verbally or visually) were included in only 15.4% (2/13) of advertisements. No advertisements included information on the epidemiology of (ie, prevalence, subpopulations at risk), risk factors for, pathophysiology of, or comorbid diseases associated with psoriasis.
Eczema DTC Advertisements
Among the 27 eczema advertisements aired, there were 4 unique advertisements, of which 3 were product-claim advertisements (all for crisaborole [Eucrisa (Pfizer Inc)]), and 1 was a help-seeking advertisement that was sponsored by Sanofi Genzyme and Regeneron Pharmaceuticals. The advertisements aired on ABC (n=2 [7.4%]), CBS (n=17 [63.0%]), and NBC (n=8 [29.6%]). All advertisements aired on weekdays between 7
Eczema Character Portrayal and Disease-Related Content
We identified 80 main characters who were depicted to be affected by eczema among all advertisements. Characteristics of the affected characters are summarized in the Table. Most of the affected characters were perceived to be White (53.8% [43/80]) and female (71.3% [57/80]). Other races depicted included Black (28.8% [23/80]) and Asian (17.5% [14/80]). Each unique eczema advertisement included at least 1 non-White main character. Most eczema main characters were perceived to be children (66.3% [53/80]), followed by younger adults (33.8% [27/80]). No infants, teenagers, or older adults were shown as being affected by eczema.
Skin manifestations of eczema were portrayed on at least 1 character in all of the advertisements; 77.8% (21/27) of the advertisements had at least 1 obvious depiction. Symptoms of eczema and the mechanism of disease (pathophysiology) were each included in 44.4% (12/27) of advertisements. This information was included exclusively in the single help-seeking advertisement, which also referenced a website for additional disease-related information. No advertisements included information on the epidemiology of, risk factors for, or comorbid diseases associated with eczema.
Comment
In our study of televised DTC advertisements for psoriasis and eczema in the United States, we identified underrepresentation of racial/ethnic minorities and specific age groups (older adults for psoriasis and all adults for eczema) across all advertisements. Although psoriasis is suggested to be less prevalent among minority patients (1.3%–1.9% among Black patients and 1.6% among Hispanic patients) compared to White patients (2%–4%),16,17 minority vs White representation in psoriasis DTC advertisements was disproportionately lower than population-based prevalence estimates. Direct-to-consumer advertisements for eczema included more minority characters than psoriasis advertisements; however, minority representation remained inadequate considering that childhood eczema is more prevalent among Black vs White children,18 and adult eczema is at least as prevalent among minority patients compared to White patients.19 Not only was minority representation in all advertisements poor, but advertisement placement also was suboptimal, particularly for reaching Black viewers. FOX network was home to 2 of the top 3 primetime broadcast programs among Black viewers around the study period,13 yet no DTC advertisements were aired on FOX.
The current literature regarding minority representation in DTC advertisements is mixed. Some studies report underrepresentation of Black and other minority patients across a variety of diseases.20 Other studies suggest that representation of Black patients, in particular, generally is adequate, except among select serious health conditions, and that advertisements depict tokenism or stereotypical roles for minorities.21 Our study provides new and specific insight about the state of racial/ethnic and age diversity, or lack thereof, in DTC advertisements for the skin conditions that currently are most commonly targeted—psoriasis and eczema. Although it remains unclear whether DTC advertisements are good or bad, existing data suggest that potential benefits of DTC advertisements include strengthening of patient-provider relationships, reduction of underdiagnosis and undertreatment of disease, and reduction of disease stigma.22 However, in our analyses, we found disease-specific factual content among all DTC advertisements to be sparse and obvious depictions of skin disease and symptoms to be uncommon, especially for psoriasis. As such, it seems unlikely that existing DTC advertisements for psoriasis and eczema can be expected to contribute to meaningful disease education, reduce underdiagnosis, and reduce the stigmatizing attitudes that have been documented for both skin diseases.23-25
Furthermore, it is important to consider our findings in light of the role that social identity theory plays in marketing. Social identity theory supports the idea that a person’s social identity (eg, age, gender, race/ethnicity) influences his/her behavior, perceptions, and performance.26 The principle of homophily—the tendency for individuals to have positive ties to those who are similar to themselves—is a critical concept in social identity theory and suggests that consumers are more likely to pay attention to and be influenced by sources perceived as similar to themselves.20 Thus, even if the potential benefits of DTC advertisements were to be realized for psoriasis and eczema, the lack of adequate minority and older adult representation raises concerns about whether these benefits would reach a diverse population and if the advertisements might further potentiate existing knowledge and treatment disparities.
Limitations
Our study is not without limitations. The sampling period was short and might not reflect advertisement content over a longer time course. We did not evaluate other potential sources of information, such as the Internet and social media. Nevertheless, televised DTC advertisements remain a major source of medical and drug information for the general public. We did not directly evaluate viewers’ reactions to the DTC advertisements of interest; however, other literature lends support to the significance of social identity theory and its impact on consumer behavior.26
Conclusion
Our study highlights a lost opportunity among psoriasis and eczema DTC advertisements for patient reach and disease education that may encourage existing and emerging knowledge and treatment disparities for both conditions. Our findings should serve as a call to action to pharmaceutical companies and other organizations involved in creating and supporting DTC advertisements for psoriasis and eczema to increase the educational content, diversify the depicted characters, and optimize advertisement placement.
Direct-to-consumer (DTC) advertisements are an important and influential source of health-related information for Americans. In 1997, the US Food and Drug Administration (FDA) relaxed regulations and permitted DTC drug advertisements to be televised. Now, via television alone, the average American is exposed to more than 30 hours annually of DTC advertisements for drugs,1 which exceeds, by far, the amount of time the average American spends with his/her physician.2 The United States spends $9.6 billion on DTC advertisements per year, of which $605 million is spent exclusively on DTC advertisements for dermatologic conditions—one of the highest amounts of spending for DTC advertisements, second only to diabetes.3
The increase in advertising for dermatologic conditions is reflective of the rapid growth in the number of treatment options available for chronic skin diseases, especially psoriasis. Since 2004, 11 biologics and 1 oral medication were FDA approved for the treatment of moderate to severe psoriasis. Despite the expansion of treatment options for psoriasis, knowledge and understanding of psoriasis and its treatments generally are poor,4,5 and undertreatment of psoriasis continues to be common.6 Data also suggest existing age and racial disparities in psoriasis treatment in the United States, whereby patients who are older or Black are less likely to receive biologic therapies.7-9 Although the exact causes of these disparities remain unclear, one study found that Black patients with psoriasis were less familiar with biologics compared to White patients,10 which suggests that the racial disparity in biologic treatment of psoriasis could be due to less exposure to and thus recognition of biologics as treatments of psoriasis among Black patients.
Some data suggest that DTC advertisements may affect drug uptake by encouraging patients to request advertised medications from their medical providers.11,12 As such, DTC advertisements are a potentially important source of exposure and information for patients. However, is it possible that DTC advertisements also may contribute to widening knowledge gaps among certain populations, and thus treatment disparities, by neglecting certain groups and targeting others with their content? In an effort to answer this question, we performed an analysis of DTC advertisements for psoriasis and eczema with special attention to advertisement placement, character representation, and disease-related content. We specifically targeted advertisements for psoriasis and eczema, as advertisements for the former are rampant and advertisements for the latter are on the rise because of emerging therapies. We hypothesized that age and racial/ethnic diversity among advertisement characters is poor, and disease-related content is lacking.
Materials and Methods
Study Design and Sample
We performed a cross-sectional analysis of televised DTC advertisements for psoriasis and eczema over 14 consecutive days (July 1, 2018, to July 14, 2018). We accessed Nielsen’s top 10 lists, specifically Prime Broadcast Network TV-United States and Prime Broadcast Programs Among African-American, from June 2018 and identified the networks with the greatest potential exposure to American consumers: ABC, CBS, FOX, and NBC.13,14 Each day, programming aired from 5
The FDA identifies DTC advertisement types as product-claim, reminder, and help-seeking advertisements. Product-claim advertisements are required to include the following information for the drug of interest: name; at least 1 FDA-approved indication; the most notable risks; and reference to a toll-free telephone number, website, or print advertisement by which a detailed summary of risks and benefits can be accessed. Reminder advertisements include the name of the drug but no information about the drug’s use.15 Help-seeking advertisements describe a disease or condition without referencing a specific drug treatment. Product-claim, reminder, and help-seeking advertisements for psoriasis or eczema that aired during the recorded time frame were included for analysis; advertisements that aired during sporting events and special programming were excluded.
DTC Advertisement Coding
Advertisement placement (ie, network, day of the week, time, associated television program), type, and target disease were documented for all advertisements included in the study. The content of each unique advertisement for psoriasis and eczema also was documented electronically in REDCap (Research Electronic Data Capture) as follows: characteristics of affected individuals and disease-related content. Advertisement coding was performed independently by 2 graduate students (A.H. and C.W.). First, one-third of the advertisements were randomly selected to be coded by both students. Intercoder agreement between the 2 students was 95.3%. Coding disagreements were primarily due to misunderstanding of definitions and were resolved through consensus. Subsequently, the remaining advertisements were randomly distributed between the 2 students, and each advertisement was coded by 1 student.
Statistical Analysis
All data were summarized descriptively with counts and frequencies using Stata 15 (StataCorp).
Results
We identified 297 DTC advertisements addressing 25 different conditions during our study period. CBS, ABC, NBC, and FOX aired 44.4%, 26.3%, 24.4%, and 5.1% of advertisements, respectively. Overall, DTC advertisements were least likely to air on Saturdays and between the hours of 5
Psoriasis DTC Advertisements
There were 5 unique psoriasis DTC advertisements, all of which were product-claim advertisements, with 1 each for secukinumab (Cosentyx [Novartis]), ixekizumab (Taltz [Eli Lilly and Company]), and guselkumab (Tremfya [Janssen Biotech, Inc]), and 2 for adalimumab (Humira [AbbVie Inc]). The advertisements aired on ABC (n=5 [38.5%]), CBS (n=5 [38.5%]), and NBC (n=3 [23.1%]). Most advertisements aired on weekdays (61.5%) between 6
Psoriasis Character Portrayal and Disease-Related Content
We identified 81 main characters who were depicted as having psoriasis among all advertisements. Characteristics of the affected characters are summarized in the Table. All affected characters were perceived to be younger adults, and there was a slight female predominance (58.0% [47/81]). Most characters were perceived to be White (92.6% [75/81]). Black and Asian characters only represented 6.2% (5/81) and 1.2% (1/81) of all affected individuals, respectively. Notably, the advertisements that featured only White main characters were aired 2.75 times more frequently than the advertisements that included non-White characters.

Psoriasis was shown on the skin of at least 1 character in an obvious depiction (ie, did not require more than 1 viewing) in 84.6% (11/13) of the advertisements. Symptoms of psoriasis (communicated either verbally or visually) were included in only 15.4% (2/13) of advertisements. No advertisements included information on the epidemiology of (ie, prevalence, subpopulations at risk), risk factors for, pathophysiology of, or comorbid diseases associated with psoriasis.
Eczema DTC Advertisements
Among the 27 eczema advertisements aired, there were 4 unique advertisements, of which 3 were product-claim advertisements (all for crisaborole [Eucrisa (Pfizer Inc)]), and 1 was a help-seeking advertisement that was sponsored by Sanofi Genzyme and Regeneron Pharmaceuticals. The advertisements aired on ABC (n=2 [7.4%]), CBS (n=17 [63.0%]), and NBC (n=8 [29.6%]). All advertisements aired on weekdays between 7
Eczema Character Portrayal and Disease-Related Content
We identified 80 main characters who were depicted to be affected by eczema among all advertisements. Characteristics of the affected characters are summarized in the Table. Most of the affected characters were perceived to be White (53.8% [43/80]) and female (71.3% [57/80]). Other races depicted included Black (28.8% [23/80]) and Asian (17.5% [14/80]). Each unique eczema advertisement included at least 1 non-White main character. Most eczema main characters were perceived to be children (66.3% [53/80]), followed by younger adults (33.8% [27/80]). No infants, teenagers, or older adults were shown as being affected by eczema.
Skin manifestations of eczema were portrayed on at least 1 character in all of the advertisements; 77.8% (21/27) of the advertisements had at least 1 obvious depiction. Symptoms of eczema and the mechanism of disease (pathophysiology) were each included in 44.4% (12/27) of advertisements. This information was included exclusively in the single help-seeking advertisement, which also referenced a website for additional disease-related information. No advertisements included information on the epidemiology of, risk factors for, or comorbid diseases associated with eczema.
Comment
In our study of televised DTC advertisements for psoriasis and eczema in the United States, we identified underrepresentation of racial/ethnic minorities and specific age groups (older adults for psoriasis and all adults for eczema) across all advertisements. Although psoriasis is suggested to be less prevalent among minority patients (1.3%–1.9% among Black patients and 1.6% among Hispanic patients) compared to White patients (2%–4%),16,17 minority vs White representation in psoriasis DTC advertisements was disproportionately lower than population-based prevalence estimates. Direct-to-consumer advertisements for eczema included more minority characters than psoriasis advertisements; however, minority representation remained inadequate considering that childhood eczema is more prevalent among Black vs White children,18 and adult eczema is at least as prevalent among minority patients compared to White patients.19 Not only was minority representation in all advertisements poor, but advertisement placement also was suboptimal, particularly for reaching Black viewers. FOX network was home to 2 of the top 3 primetime broadcast programs among Black viewers around the study period,13 yet no DTC advertisements were aired on FOX.
The current literature regarding minority representation in DTC advertisements is mixed. Some studies report underrepresentation of Black and other minority patients across a variety of diseases.20 Other studies suggest that representation of Black patients, in particular, generally is adequate, except among select serious health conditions, and that advertisements depict tokenism or stereotypical roles for minorities.21 Our study provides new and specific insight about the state of racial/ethnic and age diversity, or lack thereof, in DTC advertisements for the skin conditions that currently are most commonly targeted—psoriasis and eczema. Although it remains unclear whether DTC advertisements are good or bad, existing data suggest that potential benefits of DTC advertisements include strengthening of patient-provider relationships, reduction of underdiagnosis and undertreatment of disease, and reduction of disease stigma.22 However, in our analyses, we found disease-specific factual content among all DTC advertisements to be sparse and obvious depictions of skin disease and symptoms to be uncommon, especially for psoriasis. As such, it seems unlikely that existing DTC advertisements for psoriasis and eczema can be expected to contribute to meaningful disease education, reduce underdiagnosis, and reduce the stigmatizing attitudes that have been documented for both skin diseases.23-25
Furthermore, it is important to consider our findings in light of the role that social identity theory plays in marketing. Social identity theory supports the idea that a person’s social identity (eg, age, gender, race/ethnicity) influences his/her behavior, perceptions, and performance.26 The principle of homophily—the tendency for individuals to have positive ties to those who are similar to themselves—is a critical concept in social identity theory and suggests that consumers are more likely to pay attention to and be influenced by sources perceived as similar to themselves.20 Thus, even if the potential benefits of DTC advertisements were to be realized for psoriasis and eczema, the lack of adequate minority and older adult representation raises concerns about whether these benefits would reach a diverse population and if the advertisements might further potentiate existing knowledge and treatment disparities.
Limitations
Our study is not without limitations. The sampling period was short and might not reflect advertisement content over a longer time course. We did not evaluate other potential sources of information, such as the Internet and social media. Nevertheless, televised DTC advertisements remain a major source of medical and drug information for the general public. We did not directly evaluate viewers’ reactions to the DTC advertisements of interest; however, other literature lends support to the significance of social identity theory and its impact on consumer behavior.26
Conclusion
Our study highlights a lost opportunity among psoriasis and eczema DTC advertisements for patient reach and disease education that may encourage existing and emerging knowledge and treatment disparities for both conditions. Our findings should serve as a call to action to pharmaceutical companies and other organizations involved in creating and supporting DTC advertisements for psoriasis and eczema to increase the educational content, diversify the depicted characters, and optimize advertisement placement.
- Brownfield ED, Bernhardt JM, Phan JL, et al. Direct-to-consumer drug advertisements on network television: an exploration of quantity, frequency, and placement. J Health Commun. 2004;9:491-497.
- Tai-Seale M, McGuire TG, Zhang W. Time allocation in primary care office visits. Health Serv Res. 2007;42:1871-1894.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Lanigan SW, Farber EM. Patients’ knowledge of psoriasis: pilot study. Cutis. 1990;46:359-362.
- Renzi C, Di Pietro C, Tabolli S. Participation, satisfaction and knowledge level of patients with cutaneous psoriasis or psoriatic arthritis. Clin Exp Dermatol. 2011;36:885-888.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881.e871-830.
- Wu JJ, Lu M, Veverka KA, et al. The journey for US psoriasis patients prescribed a topical: a retrospective database evaluation of patient progression to oral and/or biologic treatment. J Dermatolog Treat. 2019;30:446-453.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
- Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34:1753-1759.
- Takeshita J, Eriksen WT, Raziano VT, et al. Racial differences in perceptions of psoriasis therapies: implications for racial disparities in psoriasis treatment. J Invest Dermatol. 2019;139:1672-1679.e1.
- Wu MH, Bartz D, Avorn J, et al. Trends in direct-to-consumer advertising of prescription contraceptives. Contraception. 2016;93:398-405.
- Mintzes B, Barer ML, Kravitz RL, et al. How does direct-to-consumer advertising (DTCA) affect prescribing? a survey in primary care environments with and without legal DTCA. CMAJ. 2003;169:405-412.
- Topten. Nielson website. https://www.nielsen.com/us/en/top-ten/. Accessed July 22, 2020.
- Leading ad supported broadcast and cable networks in the United States in 2019, by average number of viewers. Statistia website. https://www.statista.com/statistics/530119/tv-networks-viewers-usa/. Accessed July 22, 2020.
- Prescription drug advertisements. Electronic Code of Federal Regulations website. https://www.ecfr.gov/cgi-bin/text-idx?SID=d4f308e364578bda8e55a831638a26c6&mc=true&node=pt21.4.202&rgn=div5. Updated August 12, 2020. Accessed August 12, 2020.
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Centers for Disease Control and Prevention. National Center for Health Statistics, National Health Interview Survey, 2014. https://www.cdc.gov/nchs/data/health_policy/eczema_skin_problems_tables.pdf. Accessed July 22, 2020.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Welch Cline RJ, Young HN. Marketing drugs, marketing health care relationships: a content analysis of visual cues in direct-to-consumer prescription drug advertising. Health Commun. 2004;16:131-157.
- Ball JG, Liang A, Lee WN. Representation of African Americans in direct-to-consumer pharmaceutical commercials: a content analysis with implications for health disparities. Health Mark Q. 2009;26:372-390.
- Ventola CL. Direct-to-consumer pharmaceutical advertising: therapeutic or toxic? P T. 2011;36:669-674, 681-684.
- Pearl RL, Wan MT, Takeshita J, et al. Stigmatizing attitudes toward persons with psoriasis among laypersons and medical students. J Am Acad Dermatol. 2019;80:1556-1563.
- Chernyshov PV. Stigmatization and self-perception in children with atopic dermatitis. Clin Cosmet Investig Dermatol. 2016;9:159-166.
- Wittkowski A, Richards HL, Griffiths CEM, et al. The impact of psychological and clinical factors on quality of life in individuals with atopic dermatitis. J Psychosom Res. 2004;57:195-200.
- Forehand MR, Deshpande R, Reed 2nd A. Identity salience and the influence of differential activation of the social self-schema on advertising response. J Appl Psychol. 2002;87:1086-1099.
- Brownfield ED, Bernhardt JM, Phan JL, et al. Direct-to-consumer drug advertisements on network television: an exploration of quantity, frequency, and placement. J Health Commun. 2004;9:491-497.
- Tai-Seale M, McGuire TG, Zhang W. Time allocation in primary care office visits. Health Serv Res. 2007;42:1871-1894.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Lanigan SW, Farber EM. Patients’ knowledge of psoriasis: pilot study. Cutis. 1990;46:359-362.
- Renzi C, Di Pietro C, Tabolli S. Participation, satisfaction and knowledge level of patients with cutaneous psoriasis or psoriatic arthritis. Clin Exp Dermatol. 2011;36:885-888.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881.e871-830.
- Wu JJ, Lu M, Veverka KA, et al. The journey for US psoriasis patients prescribed a topical: a retrospective database evaluation of patient progression to oral and/or biologic treatment. J Dermatolog Treat. 2019;30:446-453.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
- Kerr GS, Qaiyumi S, Richards J, et al. Psoriasis and psoriatic arthritis in African-American patients—the need to measure disease burden. Clin Rheumatol. 2015;34:1753-1759.
- Takeshita J, Eriksen WT, Raziano VT, et al. Racial differences in perceptions of psoriasis therapies: implications for racial disparities in psoriasis treatment. J Invest Dermatol. 2019;139:1672-1679.e1.
- Wu MH, Bartz D, Avorn J, et al. Trends in direct-to-consumer advertising of prescription contraceptives. Contraception. 2016;93:398-405.
- Mintzes B, Barer ML, Kravitz RL, et al. How does direct-to-consumer advertising (DTCA) affect prescribing? a survey in primary care environments with and without legal DTCA. CMAJ. 2003;169:405-412.
- Topten. Nielson website. https://www.nielsen.com/us/en/top-ten/. Accessed July 22, 2020.
- Leading ad supported broadcast and cable networks in the United States in 2019, by average number of viewers. Statistia website. https://www.statista.com/statistics/530119/tv-networks-viewers-usa/. Accessed July 22, 2020.
- Prescription drug advertisements. Electronic Code of Federal Regulations website. https://www.ecfr.gov/cgi-bin/text-idx?SID=d4f308e364578bda8e55a831638a26c6&mc=true&node=pt21.4.202&rgn=div5. Updated August 12, 2020. Accessed August 12, 2020.
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Centers for Disease Control and Prevention. National Center for Health Statistics, National Health Interview Survey, 2014. https://www.cdc.gov/nchs/data/health_policy/eczema_skin_problems_tables.pdf. Accessed July 22, 2020.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Welch Cline RJ, Young HN. Marketing drugs, marketing health care relationships: a content analysis of visual cues in direct-to-consumer prescription drug advertising. Health Commun. 2004;16:131-157.
- Ball JG, Liang A, Lee WN. Representation of African Americans in direct-to-consumer pharmaceutical commercials: a content analysis with implications for health disparities. Health Mark Q. 2009;26:372-390.
- Ventola CL. Direct-to-consumer pharmaceutical advertising: therapeutic or toxic? P T. 2011;36:669-674, 681-684.
- Pearl RL, Wan MT, Takeshita J, et al. Stigmatizing attitudes toward persons with psoriasis among laypersons and medical students. J Am Acad Dermatol. 2019;80:1556-1563.
- Chernyshov PV. Stigmatization and self-perception in children with atopic dermatitis. Clin Cosmet Investig Dermatol. 2016;9:159-166.
- Wittkowski A, Richards HL, Griffiths CEM, et al. The impact of psychological and clinical factors on quality of life in individuals with atopic dermatitis. J Psychosom Res. 2004;57:195-200.
- Forehand MR, Deshpande R, Reed 2nd A. Identity salience and the influence of differential activation of the social self-schema on advertising response. J Appl Psychol. 2002;87:1086-1099.
Practice Points
- Racial/ethnic minorities and older adults are underrepresented in direct-to-consumer (DTC) advertisements for psoriasis and eczema.
- Character representation in psoriasis DTC advertisements, in particular, mirrors existing age and racial disparities in treatment with biologics.
- Disease-specific factual content was sparse, and obvious depictions of skin disease and symptoms were uncommon, especially among psoriasis DTC advertisements.
- Dermatologists should be aware of these deficiencies in psoriasis and eczema DTC advertisements and take care not to further reinforce existing knowledge gaps and inequitable treatment patterns among patients.
Psoriasis, PsA, and pregnancy: Tailoring treatment with increasing data
With an average age of diagnosis of 28 years, and one of two incidence peaks occurring at 15-30 years, psoriasis affects many women in the midst of their reproductive years. The prospect of pregnancy – or the reality of a surprise pregnancy – drives questions about heritability of the disease in offspring, the impact of the disease on pregnancy outcomes and breastfeeding, and how to best balance risks of treatments with risks of uncontrolled psoriasis and/or psoriatic arthritis (PsA).
While answers to these questions are not always clear, discussions about pregnancy and psoriasis management “shouldn’t be scary,” said Jenny E. Murase, MD, a dermatologist who speaks and writes widely about her research and experience with psoriasis and pregnancy. “We have access to information and data and educational resources to [work with] and reassure our patients – we just need to use it. Right now, there’s unnecessary suffering [with some patients unnecessarily stopping all treatment].”
Much has been learned in the past 2 decades about the course of psoriasis in pregnancy, and pregnancy outcomes data on the safety of biologics during pregnancy are increasingly emerging – particularly for tumor necrosis factor (TNF)–alpha inhibitors.
Ideally, since half of all pregnancies are unplanned, the implications of therapeutic options should be discussed with all women with psoriasis who are of reproductive age, whether they are sexually active or not. “The onus is on us to make sure that we’re considering the possibility [that our patient] could become pregnant without consulting us first,” said Dr. Murase, associate professor of dermatology at the University of California, San Francisco, and director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif.
Lisa R. Sammaritano, MD, associate professor of clinical medicine at Weill Cornell Medicine and a rheumatologist at the Hospital for Special Surgery, both in New York, urges similar attention for PsA. “Pregnancy is best planned while patients have quiescent disease on pregnancy-compatible medications,” she said. “We encourage [more] rheumatologists to be actively involved in pregnancy planning [in order] to guide therapy.”
The impact of estrogen
Dr. Murase was inspired to study psoriasis and pregnancy in part by a patient she met as a medical student. “She had severe psoriasis covering her body, and she said that the only times her psoriasis cleared was during her three pregnancies,” Dr. Murase recalled. “I wondered: What about the pregnancies resulted in such a substantial reduction of her psoriasis?”
She subsequently led a study, published in 2005, of 47 pregnant and 27 nonpregnant patients with psoriasis. More than half of the patients – 55% – reported improvements in their psoriasis during pregnancy, 21% reported no change, and 23% reported worsening. Among the 16 patients who had 10% or greater psoriatic body surface area (BSA) involvement and reported improvements, lesions decreased by 84%.
In the postpartum period, only 9% reported improvement, 26% reported no change, and 65% reported worsening. The increased BSA values observed 6 weeks postpartum did not exceed those of the first trimester, suggesting a return to the patients’ baseline status.
Earlier and smaller retrospective studies had also shown that approximately half of patients improve during pregnancy, and it was believed that progesterone was most likely responsible for this improvement. Dr. Murase’s study moved the needle in that it examined BSA in pregnancy and the postpartum period. It also turned the spotlight on estrogen: Patients who had higher levels of improvement also had higher levels of estradiol, estrone, and the ratio of estrogen to progesterone. However, there was no correlation between psoriatic change and levels of progesterone.
To promote fetal survival, pregnancy triggers a shift from Th1 cell–mediated immunity – and Th17 immunity – to Th2 immunity. While there’s no proof of a causative effect, increased estrogen appears to play a role in this shift and in the reduced production of Th1 and Th17 cytokines. Psoriasis is believed to be primarily a Th17-mediated disease, with some Th1 involvement, so this down-regulation can result in improved disease status, Dr. Murase said. (A host of other autoimmune diseases categorized as Th1 mediated similarly tend to improve during pregnancy, she added.)
Information on the effect of pregnancy on PsA is “conflicting,” Dr. Sammaritano said. “Some [of a limited number of studies] suggest a beneficial effect as is generally seen for rheumatoid arthritis. Others, however, have found an increased risk of disease activity during pregnancy ... It may be that psoriatic arthritis can be quite variable from patient to patient in its clinical presentation.”
At least one study, Dr. Sammaritano added, “has shown that the arthritis in pregnancy patients with PsA did not improve, compared to control nonpregnant patients, while the psoriasis rash did improve.”
The mixed findings don’t surprise Dr. Murase. “It harder to quantify joint disease in general,” she said. “And during pregnancy, physiologic changes relating to the pregnancy itself can cause discomfort – your joints ache. The numbers [of improved] cases aren’t as high with PsA, but it’s a more complex question.”
In the postpartum period, however, research findings “all suggest an increased risk of flare” of PsA, Dr. Sammaritano said, just as with psoriasis.
Assessing risk of treatment
Understanding the immunologic effects of pregnancy on psoriasis and PsA – and appreciating the concept of a hormonal component – is an important part of treatment decision making. So is understanding pregnancy outcomes data.
Researchers have looked at a host of pregnancy outcomes – including congenital malformations, preterm birth, spontaneous abortion, low birth weight, macrosomia, and gestational diabetes and hypertension – in women with psoriasis or psoriasis/PsA, compared with control groups. Some studies have suggested a link between disease activity and pregnancy complications or adverse pregnancy outcomes, “just as a result of having moderate to severe disease,” while others have found no evidence of increased risk, Dr. Murase said.
“It’s a bit unclear and a difficult question to answer; it depends on what study you look at and what data you believe. It would be nice to have some clarity, but basically the jury is still out,” said Dr. Murase, who, with coauthors Alice B. Gottlieb, MD, PhD, of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and Caitriona Ryan, MD, of the Blackrock Clinic and Charles Institute of Dermatology, University College Dublin, discussed the pregnancy outcomes data in a recently published review of psoriasis in women.
“In my opinion, because we have therapies that are so low risk and well tolerated, it’s better to make sure that the inflammatory cascade and inflammation created by psoriasis is under control,” she said. “So whether or not the pregnancy itself causes the patient to go into remission, or whether you have to use therapy to help the patient stay in remission, it’s important to control the inflammation.”
Contraindicated in pregnancy are oral psoralen, methotrexate, and acitretin, the latter of which should be avoided for several years before pregnancy and “therefore shouldn’t be used in a woman of childbearing age,” said Dr. Murase. Methotrexate, said Dr. Sammaritano, should generally be stopped 1-3 months prior to conception.
For psoriasis, the therapy that’s “classically considered the safest in pregnancy is UVB light therapy, specifically the 300-nm wavelength of light, which works really well as an anti-inflammatory,” Dr. Murase said. Because of the potential for maternal folate degradation with phototherapy and the long-known association of folate deficiency with neural tube defects, women of childbearing age who are receiving light therapy should take daily folic acid supplementation. (She prescribes a daily prenatal vitamin containing at least 1 mg of folic acid for women who are utilizing light therapy.)
Many topical agents can be used during pregnancy, Dr. Murase said. Topical corticosteroids, she noted, have the most safety-affirming data of any topical medication.
Regarding oral therapies, Dr. Murase recommends against the use of apremilast (Otezla) for her patients. “It’s not contraindicated, but the animal studies don’t look promising, so I don’t use that one in women of childbearing age just in case. There’s just very little data to support the safety of this medication [in pregnancy].”
There are no therapeutic guidelines in the United States for guiding the management of psoriasis in women who are considering pregnancy. In 2012, the medical board of the National Psoriasis Foundation published a review of treatment options for psoriasis in pregnant or lactating women, the “closest thing to guidelines that we’ve had,” said Dr. Murase. (Now almost a decade old, the review addresses TNF inhibitors but does not cover the anti-interleukin agents more recently approved for moderate to severe psoriasis and PsA.)
For treating PsA, rheumatologists now have the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases to reference. The 2020 guideline does not address PsA specifically, but its section on pregnancy and lactation includes recommendations on biologic and other therapies used to treat the disease.
Guidelines aside, physician-patient discussions over drug safety have the potential to be much more meaningful now that drug labels offer clinical summaries, data, and risk summaries regarding potential use in pregnancy. The labels have “more of a narrative, which is a more useful way to counsel patients and make risk-benefit decisions” than the former system of five-letter categories, said Dr. Murase. (The changes were made per the Pregnancy and Lactation Labeling Rule of 2015.)
MothertoBaby, a service of the nonprofit Organization of Teratology Information Specialists, also provides good evidence-based information to physicians and mothers, Dr. Sammaritano noted.
The use of biologic therapies
In a 2017 review of biologic safety for patients with psoriasis during pregnancy, Alexa B. Kimball, MD, MPH, professor of dermatology at Harvard Medical School, Boston; Martina L. Porter, MD, currently with the department of dermatology at Beth Israel Deaconess Medical Center, Boston; and Stephen J. Lockwood, MD, MPH, of the department of dermatology at Harvard Medical School, concluded that an increasing body of literature suggests that biologic agents can be used during pregnancy and breastfeeding. Anti-TNF agents “should be considered over IL-12/23 and IL-17 inhibitors due to the increased availability of long-term data,” they wrote.
“In general,” said Dr. Murase, “there’s more and more data coming out from gastroenterology and rheumatology to reassure patients and prescribing physicians that the TNF-blocker class is likely safe to use in pregnancy,” particularly during the first trimester and early second trimester, when the transport of maternal antibodies across the placenta is “essentially nonexistent.” In the third trimester, the active transport of IgG antibodies increases rapidly.
If possible, said Dr. Sammaritano, who served as lead author of the ACR’s reproductive health guideline, TNF inhibitors “will be stopped prior to the third trimester to avoid [the possibility of] high drug levels in the infant at birth, which raises concern for immunosuppression in the newborn. If disease is very active, however, they can be continued throughout the pregnancy.”
The TNF inhibitor certolizumab pegol (Cimzia) has the advantage of being transported only minimally across the placenta, if at all, she and Dr. Murase both explained. “To be actively carried across, antibodies need what’s called an Fc region for the placenta to grab onto,” Dr. Murase said. Certolizumab – a pegylated anti–binding fragment antibody – lacks this Fc region.
Two recent studies – CRIB and a UCB Pharma safety database analysis – showed “essentially no medication crossing – there were barely detectable levels,” Dr. Murase said. Certolizumab’s label contains this information and other clinical trial data as well as findings from safety database analyses/surveillance registries.
“Before we had much data for the biologics, I’d advise transitioning patients to light therapy from their biologics and a lot of times their psoriasis would improve, but it was more of a dance,” she said. “Now we tend to look at [certolizumab] when they’re of childbearing age and keep them on the treatment. I know that the baby is not being immunosuppressed.”
Consideration of the use of certolizumab when treatment with biologic agents is required throughout the pregnancy is a recommendation included in Dr. Kimball’s 2017 review.
As newer anti-interleukin agents – the IL-12/23 and IL-17 inhibitors – play a growing role in the treatment of psoriasis and PsA, questions loom about their safety profile. Dr. Murase and Dr. Sammaritano are waiting for more data. “In general,” Dr. Sammaritano said, “we recommend stopping them at the time pregnancy is detected, based on a lack of data at this time.”
Small-molecule drugs are also less well studied, she noted. “Because of their low molecular weight, we anticipate they will easily cross the placenta, so we recommend avoiding use during pregnancy until more information is available.”
Postpartum care
The good news, both experts say, is that the vast majority of medications, including biologics, are safe to use during breastfeeding. Methotrexate should be avoided, Dr. Sammaritano pointed out, and the impact of novel small-molecule therapies on breast milk has not been studied.
In her 2019 review of psoriasis in women, Dr. Murase and coauthors wrote that too many dermatologists believe that breastfeeding women should either not be on biologics or are uncertain about biologic use during breastfeeding. However, “biologics are considered compatible for use while breastfeeding due to their large molecular size and the proteolytic environment in the neonatal gastrointestinal tract,” they added.
Counseling and support for breastfeeding is especially important for women with psoriasis, Dr. Murase emphasized. “Breastfeeding is very traumatizing to the skin, and psoriasis can form in skin that’s injured. I have my patients set up an office visit very soon after the pregnancy to make sure they’re doing alright with their breastfeeding and that they’re coating their nipple area with some type of moisturizer and keeping the health of their nipples in good shape.”
Timely reviews of therapy and adjustments are also a priority, she said. “We need to prepare for 6 weeks post partum” when psoriasis will often flare without treatment.
Dr. Murase disclosed that she is a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron. She is also coeditor in chief of the International Journal of Women’s Dermatology. Dr. Sammaritano reported that she has no disclosures relating to the treatment of PsA.
With an average age of diagnosis of 28 years, and one of two incidence peaks occurring at 15-30 years, psoriasis affects many women in the midst of their reproductive years. The prospect of pregnancy – or the reality of a surprise pregnancy – drives questions about heritability of the disease in offspring, the impact of the disease on pregnancy outcomes and breastfeeding, and how to best balance risks of treatments with risks of uncontrolled psoriasis and/or psoriatic arthritis (PsA).
While answers to these questions are not always clear, discussions about pregnancy and psoriasis management “shouldn’t be scary,” said Jenny E. Murase, MD, a dermatologist who speaks and writes widely about her research and experience with psoriasis and pregnancy. “We have access to information and data and educational resources to [work with] and reassure our patients – we just need to use it. Right now, there’s unnecessary suffering [with some patients unnecessarily stopping all treatment].”
Much has been learned in the past 2 decades about the course of psoriasis in pregnancy, and pregnancy outcomes data on the safety of biologics during pregnancy are increasingly emerging – particularly for tumor necrosis factor (TNF)–alpha inhibitors.
Ideally, since half of all pregnancies are unplanned, the implications of therapeutic options should be discussed with all women with psoriasis who are of reproductive age, whether they are sexually active or not. “The onus is on us to make sure that we’re considering the possibility [that our patient] could become pregnant without consulting us first,” said Dr. Murase, associate professor of dermatology at the University of California, San Francisco, and director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif.
Lisa R. Sammaritano, MD, associate professor of clinical medicine at Weill Cornell Medicine and a rheumatologist at the Hospital for Special Surgery, both in New York, urges similar attention for PsA. “Pregnancy is best planned while patients have quiescent disease on pregnancy-compatible medications,” she said. “We encourage [more] rheumatologists to be actively involved in pregnancy planning [in order] to guide therapy.”
The impact of estrogen
Dr. Murase was inspired to study psoriasis and pregnancy in part by a patient she met as a medical student. “She had severe psoriasis covering her body, and she said that the only times her psoriasis cleared was during her three pregnancies,” Dr. Murase recalled. “I wondered: What about the pregnancies resulted in such a substantial reduction of her psoriasis?”
She subsequently led a study, published in 2005, of 47 pregnant and 27 nonpregnant patients with psoriasis. More than half of the patients – 55% – reported improvements in their psoriasis during pregnancy, 21% reported no change, and 23% reported worsening. Among the 16 patients who had 10% or greater psoriatic body surface area (BSA) involvement and reported improvements, lesions decreased by 84%.
In the postpartum period, only 9% reported improvement, 26% reported no change, and 65% reported worsening. The increased BSA values observed 6 weeks postpartum did not exceed those of the first trimester, suggesting a return to the patients’ baseline status.
Earlier and smaller retrospective studies had also shown that approximately half of patients improve during pregnancy, and it was believed that progesterone was most likely responsible for this improvement. Dr. Murase’s study moved the needle in that it examined BSA in pregnancy and the postpartum period. It also turned the spotlight on estrogen: Patients who had higher levels of improvement also had higher levels of estradiol, estrone, and the ratio of estrogen to progesterone. However, there was no correlation between psoriatic change and levels of progesterone.
To promote fetal survival, pregnancy triggers a shift from Th1 cell–mediated immunity – and Th17 immunity – to Th2 immunity. While there’s no proof of a causative effect, increased estrogen appears to play a role in this shift and in the reduced production of Th1 and Th17 cytokines. Psoriasis is believed to be primarily a Th17-mediated disease, with some Th1 involvement, so this down-regulation can result in improved disease status, Dr. Murase said. (A host of other autoimmune diseases categorized as Th1 mediated similarly tend to improve during pregnancy, she added.)
Information on the effect of pregnancy on PsA is “conflicting,” Dr. Sammaritano said. “Some [of a limited number of studies] suggest a beneficial effect as is generally seen for rheumatoid arthritis. Others, however, have found an increased risk of disease activity during pregnancy ... It may be that psoriatic arthritis can be quite variable from patient to patient in its clinical presentation.”
At least one study, Dr. Sammaritano added, “has shown that the arthritis in pregnancy patients with PsA did not improve, compared to control nonpregnant patients, while the psoriasis rash did improve.”
The mixed findings don’t surprise Dr. Murase. “It harder to quantify joint disease in general,” she said. “And during pregnancy, physiologic changes relating to the pregnancy itself can cause discomfort – your joints ache. The numbers [of improved] cases aren’t as high with PsA, but it’s a more complex question.”
In the postpartum period, however, research findings “all suggest an increased risk of flare” of PsA, Dr. Sammaritano said, just as with psoriasis.
Assessing risk of treatment
Understanding the immunologic effects of pregnancy on psoriasis and PsA – and appreciating the concept of a hormonal component – is an important part of treatment decision making. So is understanding pregnancy outcomes data.
Researchers have looked at a host of pregnancy outcomes – including congenital malformations, preterm birth, spontaneous abortion, low birth weight, macrosomia, and gestational diabetes and hypertension – in women with psoriasis or psoriasis/PsA, compared with control groups. Some studies have suggested a link between disease activity and pregnancy complications or adverse pregnancy outcomes, “just as a result of having moderate to severe disease,” while others have found no evidence of increased risk, Dr. Murase said.
“It’s a bit unclear and a difficult question to answer; it depends on what study you look at and what data you believe. It would be nice to have some clarity, but basically the jury is still out,” said Dr. Murase, who, with coauthors Alice B. Gottlieb, MD, PhD, of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and Caitriona Ryan, MD, of the Blackrock Clinic and Charles Institute of Dermatology, University College Dublin, discussed the pregnancy outcomes data in a recently published review of psoriasis in women.
“In my opinion, because we have therapies that are so low risk and well tolerated, it’s better to make sure that the inflammatory cascade and inflammation created by psoriasis is under control,” she said. “So whether or not the pregnancy itself causes the patient to go into remission, or whether you have to use therapy to help the patient stay in remission, it’s important to control the inflammation.”
Contraindicated in pregnancy are oral psoralen, methotrexate, and acitretin, the latter of which should be avoided for several years before pregnancy and “therefore shouldn’t be used in a woman of childbearing age,” said Dr. Murase. Methotrexate, said Dr. Sammaritano, should generally be stopped 1-3 months prior to conception.
For psoriasis, the therapy that’s “classically considered the safest in pregnancy is UVB light therapy, specifically the 300-nm wavelength of light, which works really well as an anti-inflammatory,” Dr. Murase said. Because of the potential for maternal folate degradation with phototherapy and the long-known association of folate deficiency with neural tube defects, women of childbearing age who are receiving light therapy should take daily folic acid supplementation. (She prescribes a daily prenatal vitamin containing at least 1 mg of folic acid for women who are utilizing light therapy.)
Many topical agents can be used during pregnancy, Dr. Murase said. Topical corticosteroids, she noted, have the most safety-affirming data of any topical medication.
Regarding oral therapies, Dr. Murase recommends against the use of apremilast (Otezla) for her patients. “It’s not contraindicated, but the animal studies don’t look promising, so I don’t use that one in women of childbearing age just in case. There’s just very little data to support the safety of this medication [in pregnancy].”
There are no therapeutic guidelines in the United States for guiding the management of psoriasis in women who are considering pregnancy. In 2012, the medical board of the National Psoriasis Foundation published a review of treatment options for psoriasis in pregnant or lactating women, the “closest thing to guidelines that we’ve had,” said Dr. Murase. (Now almost a decade old, the review addresses TNF inhibitors but does not cover the anti-interleukin agents more recently approved for moderate to severe psoriasis and PsA.)
For treating PsA, rheumatologists now have the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases to reference. The 2020 guideline does not address PsA specifically, but its section on pregnancy and lactation includes recommendations on biologic and other therapies used to treat the disease.
Guidelines aside, physician-patient discussions over drug safety have the potential to be much more meaningful now that drug labels offer clinical summaries, data, and risk summaries regarding potential use in pregnancy. The labels have “more of a narrative, which is a more useful way to counsel patients and make risk-benefit decisions” than the former system of five-letter categories, said Dr. Murase. (The changes were made per the Pregnancy and Lactation Labeling Rule of 2015.)
MothertoBaby, a service of the nonprofit Organization of Teratology Information Specialists, also provides good evidence-based information to physicians and mothers, Dr. Sammaritano noted.
The use of biologic therapies
In a 2017 review of biologic safety for patients with psoriasis during pregnancy, Alexa B. Kimball, MD, MPH, professor of dermatology at Harvard Medical School, Boston; Martina L. Porter, MD, currently with the department of dermatology at Beth Israel Deaconess Medical Center, Boston; and Stephen J. Lockwood, MD, MPH, of the department of dermatology at Harvard Medical School, concluded that an increasing body of literature suggests that biologic agents can be used during pregnancy and breastfeeding. Anti-TNF agents “should be considered over IL-12/23 and IL-17 inhibitors due to the increased availability of long-term data,” they wrote.
“In general,” said Dr. Murase, “there’s more and more data coming out from gastroenterology and rheumatology to reassure patients and prescribing physicians that the TNF-blocker class is likely safe to use in pregnancy,” particularly during the first trimester and early second trimester, when the transport of maternal antibodies across the placenta is “essentially nonexistent.” In the third trimester, the active transport of IgG antibodies increases rapidly.
If possible, said Dr. Sammaritano, who served as lead author of the ACR’s reproductive health guideline, TNF inhibitors “will be stopped prior to the third trimester to avoid [the possibility of] high drug levels in the infant at birth, which raises concern for immunosuppression in the newborn. If disease is very active, however, they can be continued throughout the pregnancy.”
The TNF inhibitor certolizumab pegol (Cimzia) has the advantage of being transported only minimally across the placenta, if at all, she and Dr. Murase both explained. “To be actively carried across, antibodies need what’s called an Fc region for the placenta to grab onto,” Dr. Murase said. Certolizumab – a pegylated anti–binding fragment antibody – lacks this Fc region.
Two recent studies – CRIB and a UCB Pharma safety database analysis – showed “essentially no medication crossing – there were barely detectable levels,” Dr. Murase said. Certolizumab’s label contains this information and other clinical trial data as well as findings from safety database analyses/surveillance registries.
“Before we had much data for the biologics, I’d advise transitioning patients to light therapy from their biologics and a lot of times their psoriasis would improve, but it was more of a dance,” she said. “Now we tend to look at [certolizumab] when they’re of childbearing age and keep them on the treatment. I know that the baby is not being immunosuppressed.”
Consideration of the use of certolizumab when treatment with biologic agents is required throughout the pregnancy is a recommendation included in Dr. Kimball’s 2017 review.
As newer anti-interleukin agents – the IL-12/23 and IL-17 inhibitors – play a growing role in the treatment of psoriasis and PsA, questions loom about their safety profile. Dr. Murase and Dr. Sammaritano are waiting for more data. “In general,” Dr. Sammaritano said, “we recommend stopping them at the time pregnancy is detected, based on a lack of data at this time.”
Small-molecule drugs are also less well studied, she noted. “Because of their low molecular weight, we anticipate they will easily cross the placenta, so we recommend avoiding use during pregnancy until more information is available.”
Postpartum care
The good news, both experts say, is that the vast majority of medications, including biologics, are safe to use during breastfeeding. Methotrexate should be avoided, Dr. Sammaritano pointed out, and the impact of novel small-molecule therapies on breast milk has not been studied.
In her 2019 review of psoriasis in women, Dr. Murase and coauthors wrote that too many dermatologists believe that breastfeeding women should either not be on biologics or are uncertain about biologic use during breastfeeding. However, “biologics are considered compatible for use while breastfeeding due to their large molecular size and the proteolytic environment in the neonatal gastrointestinal tract,” they added.
Counseling and support for breastfeeding is especially important for women with psoriasis, Dr. Murase emphasized. “Breastfeeding is very traumatizing to the skin, and psoriasis can form in skin that’s injured. I have my patients set up an office visit very soon after the pregnancy to make sure they’re doing alright with their breastfeeding and that they’re coating their nipple area with some type of moisturizer and keeping the health of their nipples in good shape.”
Timely reviews of therapy and adjustments are also a priority, she said. “We need to prepare for 6 weeks post partum” when psoriasis will often flare without treatment.
Dr. Murase disclosed that she is a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron. She is also coeditor in chief of the International Journal of Women’s Dermatology. Dr. Sammaritano reported that she has no disclosures relating to the treatment of PsA.
With an average age of diagnosis of 28 years, and one of two incidence peaks occurring at 15-30 years, psoriasis affects many women in the midst of their reproductive years. The prospect of pregnancy – or the reality of a surprise pregnancy – drives questions about heritability of the disease in offspring, the impact of the disease on pregnancy outcomes and breastfeeding, and how to best balance risks of treatments with risks of uncontrolled psoriasis and/or psoriatic arthritis (PsA).
While answers to these questions are not always clear, discussions about pregnancy and psoriasis management “shouldn’t be scary,” said Jenny E. Murase, MD, a dermatologist who speaks and writes widely about her research and experience with psoriasis and pregnancy. “We have access to information and data and educational resources to [work with] and reassure our patients – we just need to use it. Right now, there’s unnecessary suffering [with some patients unnecessarily stopping all treatment].”
Much has been learned in the past 2 decades about the course of psoriasis in pregnancy, and pregnancy outcomes data on the safety of biologics during pregnancy are increasingly emerging – particularly for tumor necrosis factor (TNF)–alpha inhibitors.
Ideally, since half of all pregnancies are unplanned, the implications of therapeutic options should be discussed with all women with psoriasis who are of reproductive age, whether they are sexually active or not. “The onus is on us to make sure that we’re considering the possibility [that our patient] could become pregnant without consulting us first,” said Dr. Murase, associate professor of dermatology at the University of California, San Francisco, and director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif.
Lisa R. Sammaritano, MD, associate professor of clinical medicine at Weill Cornell Medicine and a rheumatologist at the Hospital for Special Surgery, both in New York, urges similar attention for PsA. “Pregnancy is best planned while patients have quiescent disease on pregnancy-compatible medications,” she said. “We encourage [more] rheumatologists to be actively involved in pregnancy planning [in order] to guide therapy.”
The impact of estrogen
Dr. Murase was inspired to study psoriasis and pregnancy in part by a patient she met as a medical student. “She had severe psoriasis covering her body, and she said that the only times her psoriasis cleared was during her three pregnancies,” Dr. Murase recalled. “I wondered: What about the pregnancies resulted in such a substantial reduction of her psoriasis?”
She subsequently led a study, published in 2005, of 47 pregnant and 27 nonpregnant patients with psoriasis. More than half of the patients – 55% – reported improvements in their psoriasis during pregnancy, 21% reported no change, and 23% reported worsening. Among the 16 patients who had 10% or greater psoriatic body surface area (BSA) involvement and reported improvements, lesions decreased by 84%.
In the postpartum period, only 9% reported improvement, 26% reported no change, and 65% reported worsening. The increased BSA values observed 6 weeks postpartum did not exceed those of the first trimester, suggesting a return to the patients’ baseline status.
Earlier and smaller retrospective studies had also shown that approximately half of patients improve during pregnancy, and it was believed that progesterone was most likely responsible for this improvement. Dr. Murase’s study moved the needle in that it examined BSA in pregnancy and the postpartum period. It also turned the spotlight on estrogen: Patients who had higher levels of improvement also had higher levels of estradiol, estrone, and the ratio of estrogen to progesterone. However, there was no correlation between psoriatic change and levels of progesterone.
To promote fetal survival, pregnancy triggers a shift from Th1 cell–mediated immunity – and Th17 immunity – to Th2 immunity. While there’s no proof of a causative effect, increased estrogen appears to play a role in this shift and in the reduced production of Th1 and Th17 cytokines. Psoriasis is believed to be primarily a Th17-mediated disease, with some Th1 involvement, so this down-regulation can result in improved disease status, Dr. Murase said. (A host of other autoimmune diseases categorized as Th1 mediated similarly tend to improve during pregnancy, she added.)
Information on the effect of pregnancy on PsA is “conflicting,” Dr. Sammaritano said. “Some [of a limited number of studies] suggest a beneficial effect as is generally seen for rheumatoid arthritis. Others, however, have found an increased risk of disease activity during pregnancy ... It may be that psoriatic arthritis can be quite variable from patient to patient in its clinical presentation.”
At least one study, Dr. Sammaritano added, “has shown that the arthritis in pregnancy patients with PsA did not improve, compared to control nonpregnant patients, while the psoriasis rash did improve.”
The mixed findings don’t surprise Dr. Murase. “It harder to quantify joint disease in general,” she said. “And during pregnancy, physiologic changes relating to the pregnancy itself can cause discomfort – your joints ache. The numbers [of improved] cases aren’t as high with PsA, but it’s a more complex question.”
In the postpartum period, however, research findings “all suggest an increased risk of flare” of PsA, Dr. Sammaritano said, just as with psoriasis.
Assessing risk of treatment
Understanding the immunologic effects of pregnancy on psoriasis and PsA – and appreciating the concept of a hormonal component – is an important part of treatment decision making. So is understanding pregnancy outcomes data.
Researchers have looked at a host of pregnancy outcomes – including congenital malformations, preterm birth, spontaneous abortion, low birth weight, macrosomia, and gestational diabetes and hypertension – in women with psoriasis or psoriasis/PsA, compared with control groups. Some studies have suggested a link between disease activity and pregnancy complications or adverse pregnancy outcomes, “just as a result of having moderate to severe disease,” while others have found no evidence of increased risk, Dr. Murase said.
“It’s a bit unclear and a difficult question to answer; it depends on what study you look at and what data you believe. It would be nice to have some clarity, but basically the jury is still out,” said Dr. Murase, who, with coauthors Alice B. Gottlieb, MD, PhD, of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and Caitriona Ryan, MD, of the Blackrock Clinic and Charles Institute of Dermatology, University College Dublin, discussed the pregnancy outcomes data in a recently published review of psoriasis in women.
“In my opinion, because we have therapies that are so low risk and well tolerated, it’s better to make sure that the inflammatory cascade and inflammation created by psoriasis is under control,” she said. “So whether or not the pregnancy itself causes the patient to go into remission, or whether you have to use therapy to help the patient stay in remission, it’s important to control the inflammation.”
Contraindicated in pregnancy are oral psoralen, methotrexate, and acitretin, the latter of which should be avoided for several years before pregnancy and “therefore shouldn’t be used in a woman of childbearing age,” said Dr. Murase. Methotrexate, said Dr. Sammaritano, should generally be stopped 1-3 months prior to conception.
For psoriasis, the therapy that’s “classically considered the safest in pregnancy is UVB light therapy, specifically the 300-nm wavelength of light, which works really well as an anti-inflammatory,” Dr. Murase said. Because of the potential for maternal folate degradation with phototherapy and the long-known association of folate deficiency with neural tube defects, women of childbearing age who are receiving light therapy should take daily folic acid supplementation. (She prescribes a daily prenatal vitamin containing at least 1 mg of folic acid for women who are utilizing light therapy.)
Many topical agents can be used during pregnancy, Dr. Murase said. Topical corticosteroids, she noted, have the most safety-affirming data of any topical medication.
Regarding oral therapies, Dr. Murase recommends against the use of apremilast (Otezla) for her patients. “It’s not contraindicated, but the animal studies don’t look promising, so I don’t use that one in women of childbearing age just in case. There’s just very little data to support the safety of this medication [in pregnancy].”
There are no therapeutic guidelines in the United States for guiding the management of psoriasis in women who are considering pregnancy. In 2012, the medical board of the National Psoriasis Foundation published a review of treatment options for psoriasis in pregnant or lactating women, the “closest thing to guidelines that we’ve had,” said Dr. Murase. (Now almost a decade old, the review addresses TNF inhibitors but does not cover the anti-interleukin agents more recently approved for moderate to severe psoriasis and PsA.)
For treating PsA, rheumatologists now have the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases to reference. The 2020 guideline does not address PsA specifically, but its section on pregnancy and lactation includes recommendations on biologic and other therapies used to treat the disease.
Guidelines aside, physician-patient discussions over drug safety have the potential to be much more meaningful now that drug labels offer clinical summaries, data, and risk summaries regarding potential use in pregnancy. The labels have “more of a narrative, which is a more useful way to counsel patients and make risk-benefit decisions” than the former system of five-letter categories, said Dr. Murase. (The changes were made per the Pregnancy and Lactation Labeling Rule of 2015.)
MothertoBaby, a service of the nonprofit Organization of Teratology Information Specialists, also provides good evidence-based information to physicians and mothers, Dr. Sammaritano noted.
The use of biologic therapies
In a 2017 review of biologic safety for patients with psoriasis during pregnancy, Alexa B. Kimball, MD, MPH, professor of dermatology at Harvard Medical School, Boston; Martina L. Porter, MD, currently with the department of dermatology at Beth Israel Deaconess Medical Center, Boston; and Stephen J. Lockwood, MD, MPH, of the department of dermatology at Harvard Medical School, concluded that an increasing body of literature suggests that biologic agents can be used during pregnancy and breastfeeding. Anti-TNF agents “should be considered over IL-12/23 and IL-17 inhibitors due to the increased availability of long-term data,” they wrote.
“In general,” said Dr. Murase, “there’s more and more data coming out from gastroenterology and rheumatology to reassure patients and prescribing physicians that the TNF-blocker class is likely safe to use in pregnancy,” particularly during the first trimester and early second trimester, when the transport of maternal antibodies across the placenta is “essentially nonexistent.” In the third trimester, the active transport of IgG antibodies increases rapidly.
If possible, said Dr. Sammaritano, who served as lead author of the ACR’s reproductive health guideline, TNF inhibitors “will be stopped prior to the third trimester to avoid [the possibility of] high drug levels in the infant at birth, which raises concern for immunosuppression in the newborn. If disease is very active, however, they can be continued throughout the pregnancy.”
The TNF inhibitor certolizumab pegol (Cimzia) has the advantage of being transported only minimally across the placenta, if at all, she and Dr. Murase both explained. “To be actively carried across, antibodies need what’s called an Fc region for the placenta to grab onto,” Dr. Murase said. Certolizumab – a pegylated anti–binding fragment antibody – lacks this Fc region.
Two recent studies – CRIB and a UCB Pharma safety database analysis – showed “essentially no medication crossing – there were barely detectable levels,” Dr. Murase said. Certolizumab’s label contains this information and other clinical trial data as well as findings from safety database analyses/surveillance registries.
“Before we had much data for the biologics, I’d advise transitioning patients to light therapy from their biologics and a lot of times their psoriasis would improve, but it was more of a dance,” she said. “Now we tend to look at [certolizumab] when they’re of childbearing age and keep them on the treatment. I know that the baby is not being immunosuppressed.”
Consideration of the use of certolizumab when treatment with biologic agents is required throughout the pregnancy is a recommendation included in Dr. Kimball’s 2017 review.
As newer anti-interleukin agents – the IL-12/23 and IL-17 inhibitors – play a growing role in the treatment of psoriasis and PsA, questions loom about their safety profile. Dr. Murase and Dr. Sammaritano are waiting for more data. “In general,” Dr. Sammaritano said, “we recommend stopping them at the time pregnancy is detected, based on a lack of data at this time.”
Small-molecule drugs are also less well studied, she noted. “Because of their low molecular weight, we anticipate they will easily cross the placenta, so we recommend avoiding use during pregnancy until more information is available.”
Postpartum care
The good news, both experts say, is that the vast majority of medications, including biologics, are safe to use during breastfeeding. Methotrexate should be avoided, Dr. Sammaritano pointed out, and the impact of novel small-molecule therapies on breast milk has not been studied.
In her 2019 review of psoriasis in women, Dr. Murase and coauthors wrote that too many dermatologists believe that breastfeeding women should either not be on biologics or are uncertain about biologic use during breastfeeding. However, “biologics are considered compatible for use while breastfeeding due to their large molecular size and the proteolytic environment in the neonatal gastrointestinal tract,” they added.
Counseling and support for breastfeeding is especially important for women with psoriasis, Dr. Murase emphasized. “Breastfeeding is very traumatizing to the skin, and psoriasis can form in skin that’s injured. I have my patients set up an office visit very soon after the pregnancy to make sure they’re doing alright with their breastfeeding and that they’re coating their nipple area with some type of moisturizer and keeping the health of their nipples in good shape.”
Timely reviews of therapy and adjustments are also a priority, she said. “We need to prepare for 6 weeks post partum” when psoriasis will often flare without treatment.
Dr. Murase disclosed that she is a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron. She is also coeditor in chief of the International Journal of Women’s Dermatology. Dr. Sammaritano reported that she has no disclosures relating to the treatment of PsA.
The long road to a PsA prevention trial
About one-third of all patients with psoriasis will develop psoriatic arthritis (PsA), a condition that comes with a host of vague symptoms and no definitive blood test for diagnosis. Prevention trials could help to identify higher-risk groups for PsA, with a goal to catch disease early and improve outcomes. The challenge is finding enough participants in a disease that lacks a clear clinical profile, then tracking them for long periods of time to generate any significant data.
Researchers have been taking several approaches to improve outcomes in PsA, Christopher Ritchlin, MD, MPH, chief of the allergy/immunology and rheumatology division at the University of Rochester (N.Y.), said in an interview. “We are in the process of identifying biomarkers and imaging findings that characterize psoriasis patients at high risk to develop PsA.”
The next step would be to design an interventional trial to treat high-risk patients before they develop musculoskeletal inflammation, with a goal to delay onset, attenuate severity, or completely prevent arthritis. The issue now is “we don’t know which agents would be most effective in this prevention effort,” Dr. Ritchlin said. Biologics that target specific pathways significant in PsA pathogenesis are an appealing prospect. However, “it may be that alternative therapies such as methotrexate or ultraviolet A radiation therapy, for example, may help arrest the development of joint inflammation.”
Underdiagnosis impedes research
Several factors may undermine this important research.
For one, psoriasis patients are often unaware that they have PsA. “Many times they are diagnosed incorrectly by nonspecialists. As a consequence, they do not get access to appropriate medications,” said Lihi Eder, MD, PhD, staff rheumatologist and director of the psoriatic arthritis research program at the University of Toronto’s Women’s College Research Institute.
The condition also lacks a good diagnostic tool, Dr. Eder said. There’s no blood test that identifies this condition in the same manner as RA and lupus, for example. For these conditions, a general practitioner such as a family physician may conduct a blood test, and if positive, refer them to a rheumatologist. Such a system doesn’t exist for PsA. “Instead, nonspecialists are ordering tests and when they’re negative, they assume wrongly that these patients don’t have a rheumatic condition,” she explained.
Many clinicians aren’t that well versed in PsA, although dermatology has taken steps to become better educated. As a result, more dermatologists are referring patients to rheumatologists for PsA. Despite this small step forward, the heterogeneous clinical presentation of this condition makes diagnosis especially difficult. Unlike RA, which presents with inflammation in the joints, PsA can present as back or joint pain, “which makes our life as rheumatologists much more complex,” Dr. Eder said.
Defining a risk group
Most experts agree that the presence of psoriasis isn’t sufficient to conduct a prevention trial. Ideally, the goal of a prevention study would be to identify a critical subgroup of psoriasis patients at high risk of developing PsA.
However, this presents a challenging task, Dr. Eder said. Psoriasis is a risk factor for PsA, but most patients with psoriasis won’t actually develop it. Given that there’s an incidence rate of 2.7% a year, “you would need to recruit many hundreds of psoriasis patients and follow them for a long period of time until you have enough events.”
Moving forward with prevention studies calls for a better definition of the PsA risk group, according to Georg Schett, MD, chair of internal medicine in the department of internal medicine, rheumatology, and immunology at Friedrich‐Alexander University, Erlangen, Germany. “That’s very important, because you need to define such a group to make a prevention trial feasible. The whole benefit of such an approach is to catch the disease early, to say that psoriasis is a biomarker that’s linked to psoriatic arthritis.”
Indicators of risk other than psoriasis, such as pain, inflammation seen in ultrasound or MRI, and other specificities of psoriasis, could be used to define a population where interception can take place, Dr. Schett added. Although it’s not always clinically recognized, the combination of pain and structural lesions can be an indicator for developing PsA.
One prospective study he and his colleagues conducted in 114 psoriasis patients cited structural entheseal lesions and low cortical volumetric bone mineral density as risk factors in developing PsA. Keeping these factors in mind, Dr. Schett expects to see more studies in biointervention in these populations, “with the idea to prevent the onset of PsA and also decrease pain and subcutaneous inflammation.”
Researchers are currently working to identify those high-risk patients to include in an interventional trial, Dr. Ritchlin said.
That said, there’s been a great deal of “clinical trial angst” among investigators, Dr. Ritchlin noted. Outcomes in clinical trials for a wide range of biologic agents have not demonstrated significant advances in outcomes, compared with initial studies with anti–tumor necrosis factor–alpha (TNF-alpha) agents 20 years ago.
Combination biologics
One approach that’s generated some interest is the use of combination biologics medications. Sequential inhibition of cytokines such as interleukin-17A and TNF-alpha is of interest given their central contribution in joint inflammation and damage. “The challenge here of course is toxicity,” Dr. Ritchlin said. Trials that combined blockade of IL-1 and TNF-alpha in a RA trial years ago resulted in significant adverse events without improving outcomes.
Comparatively, a recent study in The Lancet Rheumatology reported success in using the IL-17A inhibitor secukinumab (Cosentyx) to reduce PsA symptoms. Tested on patients in the FUTURE 2 trial, investigators demonstrated that secukinumab in 300- and 150-mg doses safely reduced PsA signs and symptoms over a period of 5 years. Secukinumab also outperformed the TNF-alpha inhibitor adalimumab in 853 PsA patients in the 52-week, randomized, head-to-head, phase 3b EXCEED study, which was recently reported in The Lancet. Articular outcomes were similar between the two therapies, yet the secukinumab group did markedly better in Psoriasis Area and Severity Index scores, compared with the adalimumab group.
Based on these findings, “I suspect that studies examining the efficacy of combination biologics for treatment of PsA will surface in the near future,” Dr. Ritchlin said.
Yet another approach encompasses the spirit of personalized medicine. Clinicians often treat PsA patients empirically because they lack biomarkers that indicate which drug may be most effective for an individual patient, Dr. Ritchlin said. However, the technologies for investigating specific cell subsets in both the blood and tissues have advanced greatly over the last decade. “I am confident that a more precision medicine–based approach to the diagnosis and treatment of PsA is on the near horizon.”
Diet as an intervention
Other research has looked at the strong link between metabolic abnormalities and psoriasis and PsA. Some diets, such as the Mediterranean diet, show promise in improving the metabolic profile of these patients, making it a candidate as a potential intervention to reduce PsA risk. Another strategy would be to focus on limiting calories and promoting weight reduction.
One study in the British Journal of Dermatology looked at the associations between PsA and smoking, alcohol, and body mass index, identifying obesity as an important risk factor. Analyzing more than 90,000 psoriasis cases from the U.K. Clinical Practice Research Datalink between 1998 and 2014, researchers identified 1,409 PsA diagnoses. Among this cohort, researchers found an association between PsA and increased body mass index and moderate drinking. This finding underscores the need to support weight-reduction programs to reduce risk, Dr. Eder and Alexis Ogdie, MD, of the University of Pennsylvania, Philadelphia, wrote in a related editorial.
While observational studies such as this one provide further guidance for interventional trials, confounders can affect results. “Patients who lost weight could have made a positive lifestyle change (e.g., a dietary change) that was associated with the decreased risk for PsA rather than weight loss specifically, or they could have lost weight for unhealthy reasons,” Dr. Eder and Dr. Ogdie explained. Future research could address whether weight loss or other interventional factors may reduce PsA progression.
To make this work, “we would need to select patients that would benefit from diet. Secondly, we’d need to identify what kind of diet would be good for preventing PsA. And we don’t know that yet,” Dr. Eder further elaborated.
As with any prevention trial, the challenge is to follow patients over a long period of time, making sure they comply with the restrictions of the prescribed diet, Dr. Eder noted. “I do think it’s a really exciting type of intervention because it’s something that people are very interested in. There’s little risk of side effects, and it’s not very expensive.”
In other research on weight-loss methods, an observational study from Denmark found that bariatric surgery, especially gastric bypass, reduced the risk of developing PsA. This suggests that weight reduction by itself is important, “although we don’t know that yet,” Dr. Eder said.
A risk model for PsA
Dr. Eder and colleagues have been working on an algorithm that will incorporate clinical information (for example, the presence of nail lesions and the severity of psoriasis) to provide an estimated risk of developing PsA over the next 5 years. Subsequently, this information could be used to identify high-risk psoriasis patients as candidates for a prevention trial.
Other groups are looking at laboratory or imaging biomarkers to help develop PsA prediction models, she said. “Once we have these tools, we can move to next steps of prevention trials. What kinds of interventions should we apply? Are we talking biologic medications or other lifestyle interventions like diet? We are still at the early stages. However, with an improved understanding of the underlying mechanisms and risk factors we are expected to see prevention trials for PsA in the future.”
About one-third of all patients with psoriasis will develop psoriatic arthritis (PsA), a condition that comes with a host of vague symptoms and no definitive blood test for diagnosis. Prevention trials could help to identify higher-risk groups for PsA, with a goal to catch disease early and improve outcomes. The challenge is finding enough participants in a disease that lacks a clear clinical profile, then tracking them for long periods of time to generate any significant data.
Researchers have been taking several approaches to improve outcomes in PsA, Christopher Ritchlin, MD, MPH, chief of the allergy/immunology and rheumatology division at the University of Rochester (N.Y.), said in an interview. “We are in the process of identifying biomarkers and imaging findings that characterize psoriasis patients at high risk to develop PsA.”
The next step would be to design an interventional trial to treat high-risk patients before they develop musculoskeletal inflammation, with a goal to delay onset, attenuate severity, or completely prevent arthritis. The issue now is “we don’t know which agents would be most effective in this prevention effort,” Dr. Ritchlin said. Biologics that target specific pathways significant in PsA pathogenesis are an appealing prospect. However, “it may be that alternative therapies such as methotrexate or ultraviolet A radiation therapy, for example, may help arrest the development of joint inflammation.”
Underdiagnosis impedes research
Several factors may undermine this important research.
For one, psoriasis patients are often unaware that they have PsA. “Many times they are diagnosed incorrectly by nonspecialists. As a consequence, they do not get access to appropriate medications,” said Lihi Eder, MD, PhD, staff rheumatologist and director of the psoriatic arthritis research program at the University of Toronto’s Women’s College Research Institute.
The condition also lacks a good diagnostic tool, Dr. Eder said. There’s no blood test that identifies this condition in the same manner as RA and lupus, for example. For these conditions, a general practitioner such as a family physician may conduct a blood test, and if positive, refer them to a rheumatologist. Such a system doesn’t exist for PsA. “Instead, nonspecialists are ordering tests and when they’re negative, they assume wrongly that these patients don’t have a rheumatic condition,” she explained.
Many clinicians aren’t that well versed in PsA, although dermatology has taken steps to become better educated. As a result, more dermatologists are referring patients to rheumatologists for PsA. Despite this small step forward, the heterogeneous clinical presentation of this condition makes diagnosis especially difficult. Unlike RA, which presents with inflammation in the joints, PsA can present as back or joint pain, “which makes our life as rheumatologists much more complex,” Dr. Eder said.
Defining a risk group
Most experts agree that the presence of psoriasis isn’t sufficient to conduct a prevention trial. Ideally, the goal of a prevention study would be to identify a critical subgroup of psoriasis patients at high risk of developing PsA.
However, this presents a challenging task, Dr. Eder said. Psoriasis is a risk factor for PsA, but most patients with psoriasis won’t actually develop it. Given that there’s an incidence rate of 2.7% a year, “you would need to recruit many hundreds of psoriasis patients and follow them for a long period of time until you have enough events.”
Moving forward with prevention studies calls for a better definition of the PsA risk group, according to Georg Schett, MD, chair of internal medicine in the department of internal medicine, rheumatology, and immunology at Friedrich‐Alexander University, Erlangen, Germany. “That’s very important, because you need to define such a group to make a prevention trial feasible. The whole benefit of such an approach is to catch the disease early, to say that psoriasis is a biomarker that’s linked to psoriatic arthritis.”
Indicators of risk other than psoriasis, such as pain, inflammation seen in ultrasound or MRI, and other specificities of psoriasis, could be used to define a population where interception can take place, Dr. Schett added. Although it’s not always clinically recognized, the combination of pain and structural lesions can be an indicator for developing PsA.
One prospective study he and his colleagues conducted in 114 psoriasis patients cited structural entheseal lesions and low cortical volumetric bone mineral density as risk factors in developing PsA. Keeping these factors in mind, Dr. Schett expects to see more studies in biointervention in these populations, “with the idea to prevent the onset of PsA and also decrease pain and subcutaneous inflammation.”
Researchers are currently working to identify those high-risk patients to include in an interventional trial, Dr. Ritchlin said.
That said, there’s been a great deal of “clinical trial angst” among investigators, Dr. Ritchlin noted. Outcomes in clinical trials for a wide range of biologic agents have not demonstrated significant advances in outcomes, compared with initial studies with anti–tumor necrosis factor–alpha (TNF-alpha) agents 20 years ago.
Combination biologics
One approach that’s generated some interest is the use of combination biologics medications. Sequential inhibition of cytokines such as interleukin-17A and TNF-alpha is of interest given their central contribution in joint inflammation and damage. “The challenge here of course is toxicity,” Dr. Ritchlin said. Trials that combined blockade of IL-1 and TNF-alpha in a RA trial years ago resulted in significant adverse events without improving outcomes.
Comparatively, a recent study in The Lancet Rheumatology reported success in using the IL-17A inhibitor secukinumab (Cosentyx) to reduce PsA symptoms. Tested on patients in the FUTURE 2 trial, investigators demonstrated that secukinumab in 300- and 150-mg doses safely reduced PsA signs and symptoms over a period of 5 years. Secukinumab also outperformed the TNF-alpha inhibitor adalimumab in 853 PsA patients in the 52-week, randomized, head-to-head, phase 3b EXCEED study, which was recently reported in The Lancet. Articular outcomes were similar between the two therapies, yet the secukinumab group did markedly better in Psoriasis Area and Severity Index scores, compared with the adalimumab group.
Based on these findings, “I suspect that studies examining the efficacy of combination biologics for treatment of PsA will surface in the near future,” Dr. Ritchlin said.
Yet another approach encompasses the spirit of personalized medicine. Clinicians often treat PsA patients empirically because they lack biomarkers that indicate which drug may be most effective for an individual patient, Dr. Ritchlin said. However, the technologies for investigating specific cell subsets in both the blood and tissues have advanced greatly over the last decade. “I am confident that a more precision medicine–based approach to the diagnosis and treatment of PsA is on the near horizon.”
Diet as an intervention
Other research has looked at the strong link between metabolic abnormalities and psoriasis and PsA. Some diets, such as the Mediterranean diet, show promise in improving the metabolic profile of these patients, making it a candidate as a potential intervention to reduce PsA risk. Another strategy would be to focus on limiting calories and promoting weight reduction.
One study in the British Journal of Dermatology looked at the associations between PsA and smoking, alcohol, and body mass index, identifying obesity as an important risk factor. Analyzing more than 90,000 psoriasis cases from the U.K. Clinical Practice Research Datalink between 1998 and 2014, researchers identified 1,409 PsA diagnoses. Among this cohort, researchers found an association between PsA and increased body mass index and moderate drinking. This finding underscores the need to support weight-reduction programs to reduce risk, Dr. Eder and Alexis Ogdie, MD, of the University of Pennsylvania, Philadelphia, wrote in a related editorial.
While observational studies such as this one provide further guidance for interventional trials, confounders can affect results. “Patients who lost weight could have made a positive lifestyle change (e.g., a dietary change) that was associated with the decreased risk for PsA rather than weight loss specifically, or they could have lost weight for unhealthy reasons,” Dr. Eder and Dr. Ogdie explained. Future research could address whether weight loss or other interventional factors may reduce PsA progression.
To make this work, “we would need to select patients that would benefit from diet. Secondly, we’d need to identify what kind of diet would be good for preventing PsA. And we don’t know that yet,” Dr. Eder further elaborated.
As with any prevention trial, the challenge is to follow patients over a long period of time, making sure they comply with the restrictions of the prescribed diet, Dr. Eder noted. “I do think it’s a really exciting type of intervention because it’s something that people are very interested in. There’s little risk of side effects, and it’s not very expensive.”
In other research on weight-loss methods, an observational study from Denmark found that bariatric surgery, especially gastric bypass, reduced the risk of developing PsA. This suggests that weight reduction by itself is important, “although we don’t know that yet,” Dr. Eder said.
A risk model for PsA
Dr. Eder and colleagues have been working on an algorithm that will incorporate clinical information (for example, the presence of nail lesions and the severity of psoriasis) to provide an estimated risk of developing PsA over the next 5 years. Subsequently, this information could be used to identify high-risk psoriasis patients as candidates for a prevention trial.
Other groups are looking at laboratory or imaging biomarkers to help develop PsA prediction models, she said. “Once we have these tools, we can move to next steps of prevention trials. What kinds of interventions should we apply? Are we talking biologic medications or other lifestyle interventions like diet? We are still at the early stages. However, with an improved understanding of the underlying mechanisms and risk factors we are expected to see prevention trials for PsA in the future.”
About one-third of all patients with psoriasis will develop psoriatic arthritis (PsA), a condition that comes with a host of vague symptoms and no definitive blood test for diagnosis. Prevention trials could help to identify higher-risk groups for PsA, with a goal to catch disease early and improve outcomes. The challenge is finding enough participants in a disease that lacks a clear clinical profile, then tracking them for long periods of time to generate any significant data.
Researchers have been taking several approaches to improve outcomes in PsA, Christopher Ritchlin, MD, MPH, chief of the allergy/immunology and rheumatology division at the University of Rochester (N.Y.), said in an interview. “We are in the process of identifying biomarkers and imaging findings that characterize psoriasis patients at high risk to develop PsA.”
The next step would be to design an interventional trial to treat high-risk patients before they develop musculoskeletal inflammation, with a goal to delay onset, attenuate severity, or completely prevent arthritis. The issue now is “we don’t know which agents would be most effective in this prevention effort,” Dr. Ritchlin said. Biologics that target specific pathways significant in PsA pathogenesis are an appealing prospect. However, “it may be that alternative therapies such as methotrexate or ultraviolet A radiation therapy, for example, may help arrest the development of joint inflammation.”
Underdiagnosis impedes research
Several factors may undermine this important research.
For one, psoriasis patients are often unaware that they have PsA. “Many times they are diagnosed incorrectly by nonspecialists. As a consequence, they do not get access to appropriate medications,” said Lihi Eder, MD, PhD, staff rheumatologist and director of the psoriatic arthritis research program at the University of Toronto’s Women’s College Research Institute.
The condition also lacks a good diagnostic tool, Dr. Eder said. There’s no blood test that identifies this condition in the same manner as RA and lupus, for example. For these conditions, a general practitioner such as a family physician may conduct a blood test, and if positive, refer them to a rheumatologist. Such a system doesn’t exist for PsA. “Instead, nonspecialists are ordering tests and when they’re negative, they assume wrongly that these patients don’t have a rheumatic condition,” she explained.
Many clinicians aren’t that well versed in PsA, although dermatology has taken steps to become better educated. As a result, more dermatologists are referring patients to rheumatologists for PsA. Despite this small step forward, the heterogeneous clinical presentation of this condition makes diagnosis especially difficult. Unlike RA, which presents with inflammation in the joints, PsA can present as back or joint pain, “which makes our life as rheumatologists much more complex,” Dr. Eder said.
Defining a risk group
Most experts agree that the presence of psoriasis isn’t sufficient to conduct a prevention trial. Ideally, the goal of a prevention study would be to identify a critical subgroup of psoriasis patients at high risk of developing PsA.
However, this presents a challenging task, Dr. Eder said. Psoriasis is a risk factor for PsA, but most patients with psoriasis won’t actually develop it. Given that there’s an incidence rate of 2.7% a year, “you would need to recruit many hundreds of psoriasis patients and follow them for a long period of time until you have enough events.”
Moving forward with prevention studies calls for a better definition of the PsA risk group, according to Georg Schett, MD, chair of internal medicine in the department of internal medicine, rheumatology, and immunology at Friedrich‐Alexander University, Erlangen, Germany. “That’s very important, because you need to define such a group to make a prevention trial feasible. The whole benefit of such an approach is to catch the disease early, to say that psoriasis is a biomarker that’s linked to psoriatic arthritis.”
Indicators of risk other than psoriasis, such as pain, inflammation seen in ultrasound or MRI, and other specificities of psoriasis, could be used to define a population where interception can take place, Dr. Schett added. Although it’s not always clinically recognized, the combination of pain and structural lesions can be an indicator for developing PsA.
One prospective study he and his colleagues conducted in 114 psoriasis patients cited structural entheseal lesions and low cortical volumetric bone mineral density as risk factors in developing PsA. Keeping these factors in mind, Dr. Schett expects to see more studies in biointervention in these populations, “with the idea to prevent the onset of PsA and also decrease pain and subcutaneous inflammation.”
Researchers are currently working to identify those high-risk patients to include in an interventional trial, Dr. Ritchlin said.
That said, there’s been a great deal of “clinical trial angst” among investigators, Dr. Ritchlin noted. Outcomes in clinical trials for a wide range of biologic agents have not demonstrated significant advances in outcomes, compared with initial studies with anti–tumor necrosis factor–alpha (TNF-alpha) agents 20 years ago.
Combination biologics
One approach that’s generated some interest is the use of combination biologics medications. Sequential inhibition of cytokines such as interleukin-17A and TNF-alpha is of interest given their central contribution in joint inflammation and damage. “The challenge here of course is toxicity,” Dr. Ritchlin said. Trials that combined blockade of IL-1 and TNF-alpha in a RA trial years ago resulted in significant adverse events without improving outcomes.
Comparatively, a recent study in The Lancet Rheumatology reported success in using the IL-17A inhibitor secukinumab (Cosentyx) to reduce PsA symptoms. Tested on patients in the FUTURE 2 trial, investigators demonstrated that secukinumab in 300- and 150-mg doses safely reduced PsA signs and symptoms over a period of 5 years. Secukinumab also outperformed the TNF-alpha inhibitor adalimumab in 853 PsA patients in the 52-week, randomized, head-to-head, phase 3b EXCEED study, which was recently reported in The Lancet. Articular outcomes were similar between the two therapies, yet the secukinumab group did markedly better in Psoriasis Area and Severity Index scores, compared with the adalimumab group.
Based on these findings, “I suspect that studies examining the efficacy of combination biologics for treatment of PsA will surface in the near future,” Dr. Ritchlin said.
Yet another approach encompasses the spirit of personalized medicine. Clinicians often treat PsA patients empirically because they lack biomarkers that indicate which drug may be most effective for an individual patient, Dr. Ritchlin said. However, the technologies for investigating specific cell subsets in both the blood and tissues have advanced greatly over the last decade. “I am confident that a more precision medicine–based approach to the diagnosis and treatment of PsA is on the near horizon.”
Diet as an intervention
Other research has looked at the strong link between metabolic abnormalities and psoriasis and PsA. Some diets, such as the Mediterranean diet, show promise in improving the metabolic profile of these patients, making it a candidate as a potential intervention to reduce PsA risk. Another strategy would be to focus on limiting calories and promoting weight reduction.
One study in the British Journal of Dermatology looked at the associations between PsA and smoking, alcohol, and body mass index, identifying obesity as an important risk factor. Analyzing more than 90,000 psoriasis cases from the U.K. Clinical Practice Research Datalink between 1998 and 2014, researchers identified 1,409 PsA diagnoses. Among this cohort, researchers found an association between PsA and increased body mass index and moderate drinking. This finding underscores the need to support weight-reduction programs to reduce risk, Dr. Eder and Alexis Ogdie, MD, of the University of Pennsylvania, Philadelphia, wrote in a related editorial.
While observational studies such as this one provide further guidance for interventional trials, confounders can affect results. “Patients who lost weight could have made a positive lifestyle change (e.g., a dietary change) that was associated with the decreased risk for PsA rather than weight loss specifically, or they could have lost weight for unhealthy reasons,” Dr. Eder and Dr. Ogdie explained. Future research could address whether weight loss or other interventional factors may reduce PsA progression.
To make this work, “we would need to select patients that would benefit from diet. Secondly, we’d need to identify what kind of diet would be good for preventing PsA. And we don’t know that yet,” Dr. Eder further elaborated.
As with any prevention trial, the challenge is to follow patients over a long period of time, making sure they comply with the restrictions of the prescribed diet, Dr. Eder noted. “I do think it’s a really exciting type of intervention because it’s something that people are very interested in. There’s little risk of side effects, and it’s not very expensive.”
In other research on weight-loss methods, an observational study from Denmark found that bariatric surgery, especially gastric bypass, reduced the risk of developing PsA. This suggests that weight reduction by itself is important, “although we don’t know that yet,” Dr. Eder said.
A risk model for PsA
Dr. Eder and colleagues have been working on an algorithm that will incorporate clinical information (for example, the presence of nail lesions and the severity of psoriasis) to provide an estimated risk of developing PsA over the next 5 years. Subsequently, this information could be used to identify high-risk psoriasis patients as candidates for a prevention trial.
Other groups are looking at laboratory or imaging biomarkers to help develop PsA prediction models, she said. “Once we have these tools, we can move to next steps of prevention trials. What kinds of interventions should we apply? Are we talking biologic medications or other lifestyle interventions like diet? We are still at the early stages. However, with an improved understanding of the underlying mechanisms and risk factors we are expected to see prevention trials for PsA in the future.”
TNF inhibitors linked to inflammatory CNS events
, new research suggests
The nested case-control study included more than 200 participants with diseases such as rheumatoid arthritis, psoriasis, and Crohn’s disease. Results showed that exposure to TNF inhibitors was significantly associated with increased risk for demyelinating CNS events, such as multiple sclerosis, and nondemyelinating events, such as meningitis and encephalitis.
Interestingly, disease-specific secondary analyses showed that the strongest association for inflammatory events was in patients with rheumatoid arthritis.
Lead author Amy Kunchok, MD, of Mayo Clinic, Rochester, Minn., noted that “these are highly effective therapies for patients” and that these CNS events are likely uncommon.
“Our study has observed an association, but this does not imply causality. Therefore, we are not cautioning against using these therapies in appropriate patients,” Dr. Kunchok said in an interview.
“Rather, we recommend that clinicians assessing patients with both inflammatory demyelinating and nondemyelinating CNS events consider a detailed evaluation of the medication history, particularly in patients with coexistent autoimmune diseases who may have a current or past history of biological therapies,” she said.
The findings were published in JAMA Neurology.
Poorly understood
TNF inhibitors “are common therapies for certain autoimmune diseases,” the investigators noted.
Previously, a link between exposure to these inhibitors and inflammatory CNS events “has been postulated but is poorly understood,” they wrote.
In the current study, they examined records for 106 patients who were treated at Mayo clinics in Minnesota, Arizona, or Florida from January 2003 through February 2019. All participants had been diagnosed with an autoimmune disease that the Food and Drug Administration has listed as an indication for TNF inhibitor use. This included rheumatoid arthritis (n = 48), ankylosing spondylitis (n = 4), psoriasis and psoriatic arthritis (n = 21), Crohn’s disease (n = 27), and ulcerative colitis (n = 6). Their records also showed diagnostic codes for the inflammatory demyelinating CNS events of relapsing-remitting or primary progressive MS, clinically isolated syndrome, radiologically isolated syndrome, neuromyelitis optica spectrum disorder, and transverse myelitis or for the inflammatory nondemyelinating CNS events of meningitis, meningoencephalitis, encephalitis, neurosarcoidosis, and CNS vasculitis. The investigators also included 106 age-, sex-, and autoimmune disease–matched participants 1:1 to act as the control group.
In the total study population, 64% were women and the median age at disease onset was 52 years. In addition, 60% of the patient group and 40% of the control group were exposed to TNF inhibitors.
Novel finding?
Results showed that TNF inhibitor exposure was significantly linked to increased risk for developing any inflammatory CNS event (adjusted odds ratio, 3.01; 95% CI, 1.55-5.82; P = .001). When the outcomes were stratified by class of inflammatory event, these results were similar. The aOR was 3.09 (95% CI, 1.19-8.04; P = .02) for inflammatory demyelinating CNS events and was 2.97 (95% CI, 1.15-7.65; P = .02) for inflammatory nondemyelinating events.
Dr. Kunchok noted that the association between the inhibitors and nondemyelinating events was “a novel finding from this study.”
In secondary analyses, patients with rheumatoid arthritis and exposure to TNF inhibitors had the strongest association with any inflammatory CNS event (aOR, 4.82; 95% CI, 1.62-14.36; P = .005).
A pooled cohort comprising only the participants with the other autoimmune diseases did not show a significant association between exposure to TNF inhibitors and development of CNS events (P = .09).
“Because of the lack of power, further stratification by individual autoimmune diseases was not analyzed,” the investigators reported.
Although the overall findings showed that exposure to TNF inhibitors was linked to increased risk for inflammatory events, whether this association “represents de novo or exacerbated inflammatory pathways requires further research,” the authors wrote.
Dr. Kunchok added that more research, especially population-based studies, is also needed to examine the incidence of these inflammatory CNS events in patients exposed to TNF-alpha inhibitors.
Adds to the literature
In an accompanying editorial, Jeffrey M. Gelfand, MD, department of neurology at the University of California, San Francisco, and Jinoos Yazdany, MD, Zuckerberg San Francisco General Hospital at UCSF, noted that although the study adds to the literature, the magnitude of the risk found “remains unclear.”
“Randomized clinical trials are not suited to the study of rare adverse events,” Dr. Gelfand and Dr. Yazdany wrote. They agree with Dr. Kunchok that “next steps should include population-based observational studies that control for disease severity.”
Still, the current study provides additional evidence of rare adverse events in patients receiving TNF inhibitors, they noted. So how should prescribers proceed?
“As with all treatments, the risk-benefit ratio for the individual patient’s situation must be weighed and appropriate counseling must be given to facilitate shared decision-making discussions,” wrote the editorialists.
“Given what is known about the risk of harm, avoiding TNF inhibitors is advisable in patients with known MS,” they wrote.
In addition, neurologic consultation can be helpful for clarifying diagnoses and providing advice on monitoring strategies for TNF inhibitor treatment in those with possible MS or other demyelinating conditions, noted the editorialists.
“In patients who develop new concerning neurological symptoms while receiving TNF inhibitor treatment, timely evaluation is indicated, including consideration of neuroinflammatory, infectious, and neurological diagnoses that may be unrelated to treatment,” they added.
“Broader awareness of risks that studies such as this one by Kunchok et al provide can ... encourage timelier recognition of potential TNF inhibitor–associated neuroinflammatory events and may improve outcomes for patients,” Dr. Gelfand and Dr. Yazdany concluded.
The study was funded by a grant from the National Center for Advancing Translational Sciences. Dr. Kunchok reports having received research funding from Biogen outside this study. A full list of disclosures for the other study authors is in the original article. Dr. Gelfand reports having received g rants for a clinical trial from Genentech and consulting fees from Biogen, Alexion, Theranica, Impel Neuropharma, Advanced Clinical, Biohaven, and Satsuma. Dr. Yazdany reports having received grants from Pfizer and consulting fees from AstraZeneca and Eli Lilly outside the submitted work.
A version of this article originally appeared on Medscape.com.
, new research suggests
The nested case-control study included more than 200 participants with diseases such as rheumatoid arthritis, psoriasis, and Crohn’s disease. Results showed that exposure to TNF inhibitors was significantly associated with increased risk for demyelinating CNS events, such as multiple sclerosis, and nondemyelinating events, such as meningitis and encephalitis.
Interestingly, disease-specific secondary analyses showed that the strongest association for inflammatory events was in patients with rheumatoid arthritis.
Lead author Amy Kunchok, MD, of Mayo Clinic, Rochester, Minn., noted that “these are highly effective therapies for patients” and that these CNS events are likely uncommon.
“Our study has observed an association, but this does not imply causality. Therefore, we are not cautioning against using these therapies in appropriate patients,” Dr. Kunchok said in an interview.
“Rather, we recommend that clinicians assessing patients with both inflammatory demyelinating and nondemyelinating CNS events consider a detailed evaluation of the medication history, particularly in patients with coexistent autoimmune diseases who may have a current or past history of biological therapies,” she said.
The findings were published in JAMA Neurology.
Poorly understood
TNF inhibitors “are common therapies for certain autoimmune diseases,” the investigators noted.
Previously, a link between exposure to these inhibitors and inflammatory CNS events “has been postulated but is poorly understood,” they wrote.
In the current study, they examined records for 106 patients who were treated at Mayo clinics in Minnesota, Arizona, or Florida from January 2003 through February 2019. All participants had been diagnosed with an autoimmune disease that the Food and Drug Administration has listed as an indication for TNF inhibitor use. This included rheumatoid arthritis (n = 48), ankylosing spondylitis (n = 4), psoriasis and psoriatic arthritis (n = 21), Crohn’s disease (n = 27), and ulcerative colitis (n = 6). Their records also showed diagnostic codes for the inflammatory demyelinating CNS events of relapsing-remitting or primary progressive MS, clinically isolated syndrome, radiologically isolated syndrome, neuromyelitis optica spectrum disorder, and transverse myelitis or for the inflammatory nondemyelinating CNS events of meningitis, meningoencephalitis, encephalitis, neurosarcoidosis, and CNS vasculitis. The investigators also included 106 age-, sex-, and autoimmune disease–matched participants 1:1 to act as the control group.
In the total study population, 64% were women and the median age at disease onset was 52 years. In addition, 60% of the patient group and 40% of the control group were exposed to TNF inhibitors.
Novel finding?
Results showed that TNF inhibitor exposure was significantly linked to increased risk for developing any inflammatory CNS event (adjusted odds ratio, 3.01; 95% CI, 1.55-5.82; P = .001). When the outcomes were stratified by class of inflammatory event, these results were similar. The aOR was 3.09 (95% CI, 1.19-8.04; P = .02) for inflammatory demyelinating CNS events and was 2.97 (95% CI, 1.15-7.65; P = .02) for inflammatory nondemyelinating events.
Dr. Kunchok noted that the association between the inhibitors and nondemyelinating events was “a novel finding from this study.”
In secondary analyses, patients with rheumatoid arthritis and exposure to TNF inhibitors had the strongest association with any inflammatory CNS event (aOR, 4.82; 95% CI, 1.62-14.36; P = .005).
A pooled cohort comprising only the participants with the other autoimmune diseases did not show a significant association between exposure to TNF inhibitors and development of CNS events (P = .09).
“Because of the lack of power, further stratification by individual autoimmune diseases was not analyzed,” the investigators reported.
Although the overall findings showed that exposure to TNF inhibitors was linked to increased risk for inflammatory events, whether this association “represents de novo or exacerbated inflammatory pathways requires further research,” the authors wrote.
Dr. Kunchok added that more research, especially population-based studies, is also needed to examine the incidence of these inflammatory CNS events in patients exposed to TNF-alpha inhibitors.
Adds to the literature
In an accompanying editorial, Jeffrey M. Gelfand, MD, department of neurology at the University of California, San Francisco, and Jinoos Yazdany, MD, Zuckerberg San Francisco General Hospital at UCSF, noted that although the study adds to the literature, the magnitude of the risk found “remains unclear.”
“Randomized clinical trials are not suited to the study of rare adverse events,” Dr. Gelfand and Dr. Yazdany wrote. They agree with Dr. Kunchok that “next steps should include population-based observational studies that control for disease severity.”
Still, the current study provides additional evidence of rare adverse events in patients receiving TNF inhibitors, they noted. So how should prescribers proceed?
“As with all treatments, the risk-benefit ratio for the individual patient’s situation must be weighed and appropriate counseling must be given to facilitate shared decision-making discussions,” wrote the editorialists.
“Given what is known about the risk of harm, avoiding TNF inhibitors is advisable in patients with known MS,” they wrote.
In addition, neurologic consultation can be helpful for clarifying diagnoses and providing advice on monitoring strategies for TNF inhibitor treatment in those with possible MS or other demyelinating conditions, noted the editorialists.
“In patients who develop new concerning neurological symptoms while receiving TNF inhibitor treatment, timely evaluation is indicated, including consideration of neuroinflammatory, infectious, and neurological diagnoses that may be unrelated to treatment,” they added.
“Broader awareness of risks that studies such as this one by Kunchok et al provide can ... encourage timelier recognition of potential TNF inhibitor–associated neuroinflammatory events and may improve outcomes for patients,” Dr. Gelfand and Dr. Yazdany concluded.
The study was funded by a grant from the National Center for Advancing Translational Sciences. Dr. Kunchok reports having received research funding from Biogen outside this study. A full list of disclosures for the other study authors is in the original article. Dr. Gelfand reports having received g rants for a clinical trial from Genentech and consulting fees from Biogen, Alexion, Theranica, Impel Neuropharma, Advanced Clinical, Biohaven, and Satsuma. Dr. Yazdany reports having received grants from Pfizer and consulting fees from AstraZeneca and Eli Lilly outside the submitted work.
A version of this article originally appeared on Medscape.com.
, new research suggests
The nested case-control study included more than 200 participants with diseases such as rheumatoid arthritis, psoriasis, and Crohn’s disease. Results showed that exposure to TNF inhibitors was significantly associated with increased risk for demyelinating CNS events, such as multiple sclerosis, and nondemyelinating events, such as meningitis and encephalitis.
Interestingly, disease-specific secondary analyses showed that the strongest association for inflammatory events was in patients with rheumatoid arthritis.
Lead author Amy Kunchok, MD, of Mayo Clinic, Rochester, Minn., noted that “these are highly effective therapies for patients” and that these CNS events are likely uncommon.
“Our study has observed an association, but this does not imply causality. Therefore, we are not cautioning against using these therapies in appropriate patients,” Dr. Kunchok said in an interview.
“Rather, we recommend that clinicians assessing patients with both inflammatory demyelinating and nondemyelinating CNS events consider a detailed evaluation of the medication history, particularly in patients with coexistent autoimmune diseases who may have a current or past history of biological therapies,” she said.
The findings were published in JAMA Neurology.
Poorly understood
TNF inhibitors “are common therapies for certain autoimmune diseases,” the investigators noted.
Previously, a link between exposure to these inhibitors and inflammatory CNS events “has been postulated but is poorly understood,” they wrote.
In the current study, they examined records for 106 patients who were treated at Mayo clinics in Minnesota, Arizona, or Florida from January 2003 through February 2019. All participants had been diagnosed with an autoimmune disease that the Food and Drug Administration has listed as an indication for TNF inhibitor use. This included rheumatoid arthritis (n = 48), ankylosing spondylitis (n = 4), psoriasis and psoriatic arthritis (n = 21), Crohn’s disease (n = 27), and ulcerative colitis (n = 6). Their records also showed diagnostic codes for the inflammatory demyelinating CNS events of relapsing-remitting or primary progressive MS, clinically isolated syndrome, radiologically isolated syndrome, neuromyelitis optica spectrum disorder, and transverse myelitis or for the inflammatory nondemyelinating CNS events of meningitis, meningoencephalitis, encephalitis, neurosarcoidosis, and CNS vasculitis. The investigators also included 106 age-, sex-, and autoimmune disease–matched participants 1:1 to act as the control group.
In the total study population, 64% were women and the median age at disease onset was 52 years. In addition, 60% of the patient group and 40% of the control group were exposed to TNF inhibitors.
Novel finding?
Results showed that TNF inhibitor exposure was significantly linked to increased risk for developing any inflammatory CNS event (adjusted odds ratio, 3.01; 95% CI, 1.55-5.82; P = .001). When the outcomes were stratified by class of inflammatory event, these results were similar. The aOR was 3.09 (95% CI, 1.19-8.04; P = .02) for inflammatory demyelinating CNS events and was 2.97 (95% CI, 1.15-7.65; P = .02) for inflammatory nondemyelinating events.
Dr. Kunchok noted that the association between the inhibitors and nondemyelinating events was “a novel finding from this study.”
In secondary analyses, patients with rheumatoid arthritis and exposure to TNF inhibitors had the strongest association with any inflammatory CNS event (aOR, 4.82; 95% CI, 1.62-14.36; P = .005).
A pooled cohort comprising only the participants with the other autoimmune diseases did not show a significant association between exposure to TNF inhibitors and development of CNS events (P = .09).
“Because of the lack of power, further stratification by individual autoimmune diseases was not analyzed,” the investigators reported.
Although the overall findings showed that exposure to TNF inhibitors was linked to increased risk for inflammatory events, whether this association “represents de novo or exacerbated inflammatory pathways requires further research,” the authors wrote.
Dr. Kunchok added that more research, especially population-based studies, is also needed to examine the incidence of these inflammatory CNS events in patients exposed to TNF-alpha inhibitors.
Adds to the literature
In an accompanying editorial, Jeffrey M. Gelfand, MD, department of neurology at the University of California, San Francisco, and Jinoos Yazdany, MD, Zuckerberg San Francisco General Hospital at UCSF, noted that although the study adds to the literature, the magnitude of the risk found “remains unclear.”
“Randomized clinical trials are not suited to the study of rare adverse events,” Dr. Gelfand and Dr. Yazdany wrote. They agree with Dr. Kunchok that “next steps should include population-based observational studies that control for disease severity.”
Still, the current study provides additional evidence of rare adverse events in patients receiving TNF inhibitors, they noted. So how should prescribers proceed?
“As with all treatments, the risk-benefit ratio for the individual patient’s situation must be weighed and appropriate counseling must be given to facilitate shared decision-making discussions,” wrote the editorialists.
“Given what is known about the risk of harm, avoiding TNF inhibitors is advisable in patients with known MS,” they wrote.
In addition, neurologic consultation can be helpful for clarifying diagnoses and providing advice on monitoring strategies for TNF inhibitor treatment in those with possible MS or other demyelinating conditions, noted the editorialists.
“In patients who develop new concerning neurological symptoms while receiving TNF inhibitor treatment, timely evaluation is indicated, including consideration of neuroinflammatory, infectious, and neurological diagnoses that may be unrelated to treatment,” they added.
“Broader awareness of risks that studies such as this one by Kunchok et al provide can ... encourage timelier recognition of potential TNF inhibitor–associated neuroinflammatory events and may improve outcomes for patients,” Dr. Gelfand and Dr. Yazdany concluded.
The study was funded by a grant from the National Center for Advancing Translational Sciences. Dr. Kunchok reports having received research funding from Biogen outside this study. A full list of disclosures for the other study authors is in the original article. Dr. Gelfand reports having received g rants for a clinical trial from Genentech and consulting fees from Biogen, Alexion, Theranica, Impel Neuropharma, Advanced Clinical, Biohaven, and Satsuma. Dr. Yazdany reports having received grants from Pfizer and consulting fees from AstraZeneca and Eli Lilly outside the submitted work.
A version of this article originally appeared on Medscape.com.
Humira topped drug-revenue list for 2019
Humira outsold all other drugs in 2019 in terms of revenue as cytokine inhibitor medications earned their way to three of the first four spots on the pharmaceutical best-seller list, according to a new analysis from the IQVIA Institute for Human Data Science.

Sales of Humira (adalimumab) amounted to $21.4 billion before discounting, Murray Aitken, the institute’s executive director, and associates wrote in their analysis. That’s more than double the total of the anticoagulant Eliquis (apixaban), which brought in $9.9 billion in its last year before generic forms became available.
The next two spots were filled by the tumor necrosis factor inhibitor Enbrel (etanercept) with $8.1 billion in sales and the interleukin 12/23 inhibitor Stelara (ustekinumab) with sales totaling $6.6 billion, followed by the chemotherapy drug Keytruda (pembrolizumab) close behind after racking up $6.5 billion in sales, the researchers reported.
Total nondiscounted spending on all drugs in the U.S. market came to $511 billion in 2019, an increase of 5.7% over the $484 billion spent in 2018, based on data from the July 2020 IQVIA National Sales Perspectives.
These figures are “not adjusted for estimates of off-invoice discounts and rebates,” the authors noted, but they include “prescription and insulin products sold into chain and independent pharmacies, food store pharmacies, mail service pharmacies, long-term care facilities, hospitals, clinics, and other institutional settings.”
Those “discounts and rebates” do exist, however, and they can add up. Drug sales for 2019, “after deducting negotiated rebates, discounts, and other forms of price concessions, such as patient coupons or vouchers that offset out-of-pocket costs,” were $235 billion less than overall nondiscounted spending, the report noted.
Now that we’ve shown you the money, let’s take a quick look at volume. The leading drugs by number of dispensed prescriptions in 2019 were, not surprisingly, quite different. First, with 118 million prescriptions, was atorvastatin, followed by levothyroxine (113 million), lisinopril (96), amlodipine (89), and metoprolol (85), Mr. Aitken and associates reported.
Altogether, over 4.2 billion prescriptions were dispensed last year, with a couple of caveats: 90-day and 30-day fills were both counted as one prescription, and OTC drugs were not included, they pointed out.
Humira outsold all other drugs in 2019 in terms of revenue as cytokine inhibitor medications earned their way to three of the first four spots on the pharmaceutical best-seller list, according to a new analysis from the IQVIA Institute for Human Data Science.

Sales of Humira (adalimumab) amounted to $21.4 billion before discounting, Murray Aitken, the institute’s executive director, and associates wrote in their analysis. That’s more than double the total of the anticoagulant Eliquis (apixaban), which brought in $9.9 billion in its last year before generic forms became available.
The next two spots were filled by the tumor necrosis factor inhibitor Enbrel (etanercept) with $8.1 billion in sales and the interleukin 12/23 inhibitor Stelara (ustekinumab) with sales totaling $6.6 billion, followed by the chemotherapy drug Keytruda (pembrolizumab) close behind after racking up $6.5 billion in sales, the researchers reported.
Total nondiscounted spending on all drugs in the U.S. market came to $511 billion in 2019, an increase of 5.7% over the $484 billion spent in 2018, based on data from the July 2020 IQVIA National Sales Perspectives.
These figures are “not adjusted for estimates of off-invoice discounts and rebates,” the authors noted, but they include “prescription and insulin products sold into chain and independent pharmacies, food store pharmacies, mail service pharmacies, long-term care facilities, hospitals, clinics, and other institutional settings.”
Those “discounts and rebates” do exist, however, and they can add up. Drug sales for 2019, “after deducting negotiated rebates, discounts, and other forms of price concessions, such as patient coupons or vouchers that offset out-of-pocket costs,” were $235 billion less than overall nondiscounted spending, the report noted.
Now that we’ve shown you the money, let’s take a quick look at volume. The leading drugs by number of dispensed prescriptions in 2019 were, not surprisingly, quite different. First, with 118 million prescriptions, was atorvastatin, followed by levothyroxine (113 million), lisinopril (96), amlodipine (89), and metoprolol (85), Mr. Aitken and associates reported.
Altogether, over 4.2 billion prescriptions were dispensed last year, with a couple of caveats: 90-day and 30-day fills were both counted as one prescription, and OTC drugs were not included, they pointed out.
Humira outsold all other drugs in 2019 in terms of revenue as cytokine inhibitor medications earned their way to three of the first four spots on the pharmaceutical best-seller list, according to a new analysis from the IQVIA Institute for Human Data Science.

Sales of Humira (adalimumab) amounted to $21.4 billion before discounting, Murray Aitken, the institute’s executive director, and associates wrote in their analysis. That’s more than double the total of the anticoagulant Eliquis (apixaban), which brought in $9.9 billion in its last year before generic forms became available.
The next two spots were filled by the tumor necrosis factor inhibitor Enbrel (etanercept) with $8.1 billion in sales and the interleukin 12/23 inhibitor Stelara (ustekinumab) with sales totaling $6.6 billion, followed by the chemotherapy drug Keytruda (pembrolizumab) close behind after racking up $6.5 billion in sales, the researchers reported.
Total nondiscounted spending on all drugs in the U.S. market came to $511 billion in 2019, an increase of 5.7% over the $484 billion spent in 2018, based on data from the July 2020 IQVIA National Sales Perspectives.
These figures are “not adjusted for estimates of off-invoice discounts and rebates,” the authors noted, but they include “prescription and insulin products sold into chain and independent pharmacies, food store pharmacies, mail service pharmacies, long-term care facilities, hospitals, clinics, and other institutional settings.”
Those “discounts and rebates” do exist, however, and they can add up. Drug sales for 2019, “after deducting negotiated rebates, discounts, and other forms of price concessions, such as patient coupons or vouchers that offset out-of-pocket costs,” were $235 billion less than overall nondiscounted spending, the report noted.
Now that we’ve shown you the money, let’s take a quick look at volume. The leading drugs by number of dispensed prescriptions in 2019 were, not surprisingly, quite different. First, with 118 million prescriptions, was atorvastatin, followed by levothyroxine (113 million), lisinopril (96), amlodipine (89), and metoprolol (85), Mr. Aitken and associates reported.
Altogether, over 4.2 billion prescriptions were dispensed last year, with a couple of caveats: 90-day and 30-day fills were both counted as one prescription, and OTC drugs were not included, they pointed out.
Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis With Phototherapy
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 Although topical therapies often are the first-line treatment of mild to moderate psoriasis, approximately 1 in 6 individuals has moderate to severe disease that requires systemic treatment such as biologics or phototherapy.2 In patients with localized disease that is refractory to treatment or who have moderate to severe psoriasis requiring systemic treatment, phototherapy should be considered as a potential low-risk treatment option.
In July 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of phototherapy in treating adult patients with psoriasis.3 Since the prior guidelines were released in 2010, there have been numerous studies affirming the efficacy of phototherapy, with several large meta-analyses helping to refine clinical recommendations.4,5 Each treatment was ranked using Strength of Recommendation Taxonomy, with a score of A, B, or C based on the strength of the evidence supporting the given modality. With the ever-increasing number of treatment options for patients with psoriasis, these guidelines inform dermatologists of the recommendations for the initiation, maintenance, and optimization of phototherapy in the treatment of psoriasis.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and frequency of adverse events of 10 commonly used phototherapy/photochemotherapy modalities. They also address dosing regimens, the potential to combine phototherapy with other therapies, and the efficacy of treatment modalities for different types of psoriasis.3 The purpose of this discussion is to present these guidelines in a condensed form for prescribers of phototherapy and to review the most clinically significant considerations during each step of treatment. Of note, we only highlight the treatment of adult patients and do not discuss information relevant to pediatric patients with psoriasis.
Choosing a Phototherapy Modality
Phototherapy may be considered for patients with psoriasis that affects more than 3% body surface area or for localized disease refractory to conventional treatments. UV light is believed to provide relief from psoriasis via multiple mechanisms, such as through favorable alterations in cytokine profiles, initiation of apoptosis, and local immunosupression.6 There is no single first-line phototherapeutic modality recommended for all patients with psoriasis. Rather, the decision to implement a particular modality should be individualized to the patient, considering factors such as percentage of body surface area affected by disease, quality-of-life assessment, comorbidities, lifestyle, and cost of treatment.
Of the 10 phototherapy modalities reviewed in these guidelines, 4 were ranked by the AAD and NPF as having grade A evidence for efficacy in the treatment of moderate to severe plaque psoriasis. Treatments with a grade A level of recommendation included narrowband UVB (NB-UVB), broadband UVB (BB-UVB), targeted phototherapy (excimer laser and excimer lamp), and
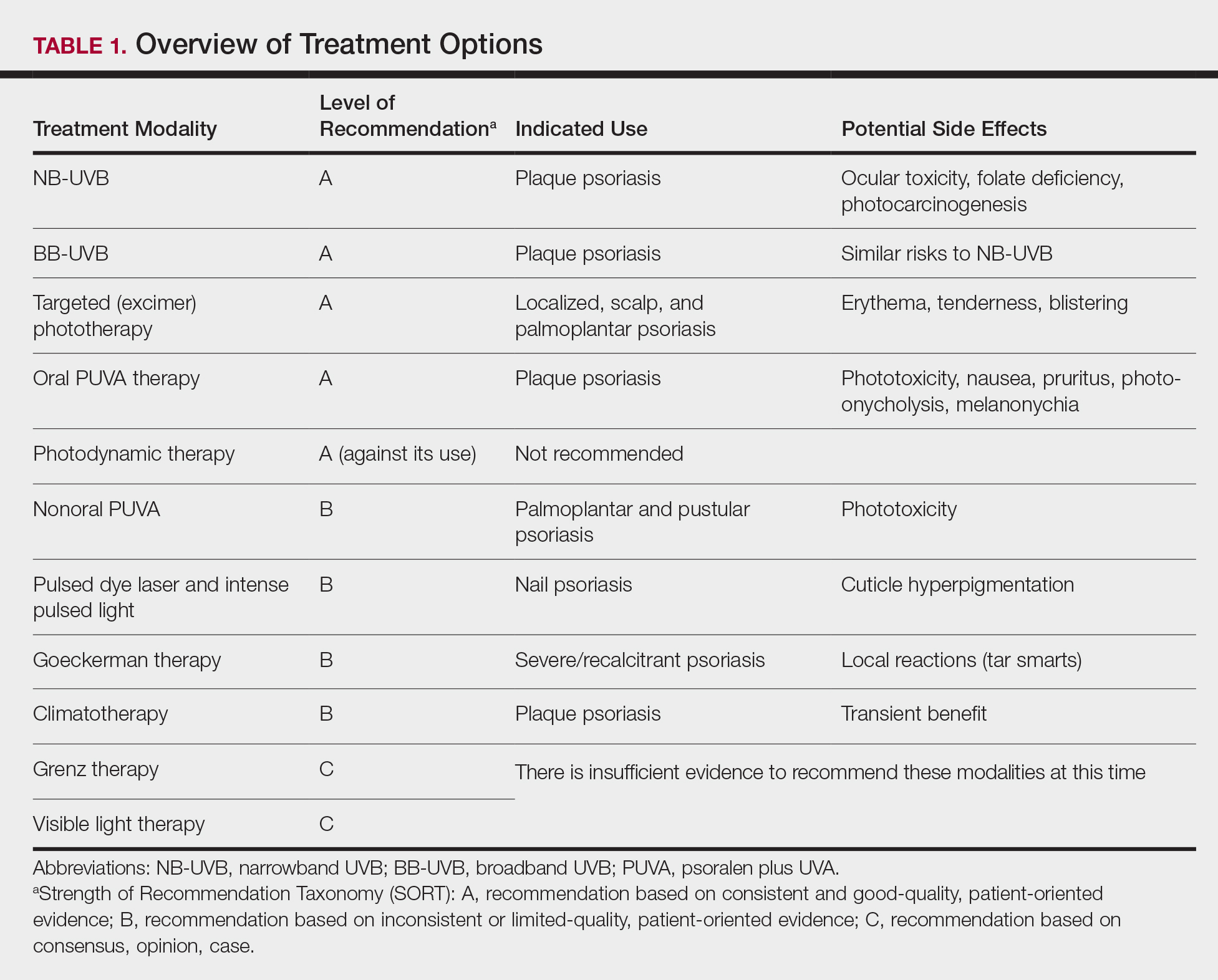
Studies have shown that the ideal wavelength needed to produce a therapeutic effect (ie, clearance of psoriatic plaques) is 304 to 313 nm. Wavelengths of 290 to 300 nm were found to be less therapeutic and more harmful, as they contributed to the development of sunburns.7 Broadband UVB phototherapy, with wavelengths ranging from 270 to 390 nm, exposes patients to a greater spectrum of radiation, thus making it more likely to cause sunburn and any theoretical form of sun-related damage, such as dysplasia and cancer. Compared with NB-UVB phototherapy, BB-UVB phototherapy is associated with a greater degree of sun damage–related side effects. Narrowband UVB, with a wavelength range of 311 to 313 nm, carries a grade A level of recommendation and should be considered as first-line monotherapy in patients with generalized plaque psoriasis, given its efficacy and promising safety profile. Multiple studies have shown that NB-UVB phototherapy is superior to BB-UVB phototherapy in the treatment of moderate to severe psoriasis in adults.8,9 In facilities where access to NB-UVB is limited, BB-UVB monotherapy is recommended as the treatment of generalized plaque psoriasis.
Psoralen plus UVA, which may be used topically (ie, bathwater PUVA) or taken orally, refers to treatment with photosensitizing psoralens. Psoralens are agents that intercalate with DNA and enhance the efficacy of phototherapy.10 Topical PUVA, with a grade B level of recommendation, is an effective treatment option for patients with localized disease and has been shown to be particularly efficacious in the treatment of palmoplantar pustular psoriasis. Oral PUVA is an effective option for psoriasis with a grade A recommendation, while bathwater PUVA has a grade B level of recommendation for moderate to severe plaque psoriasis. Oral PUVA is associated with greater systemic side effects (both acute and subacute) compared with NB-UVB and also is associated with photocarcinogenesis, particularly squamous cell carcinoma in white patients.11 Other side effects from PUVA include pigmented macules in sun-protected areas (known as PUVA lentigines), which may make evaluation of skin lesions challenging. Because of the increased risk for cancer with oral PUVA, NB-UVB is preferable as a first-line treatment vs PUVA, especially in patients with a history of skin cancer.12,13
Goeckerman therapy, which involves the synergistic combination of UVB and crude coal tar, is an older treatment that has shown efficacy in the treatment of severe or recalcitrant psoriasis (grade B level of recommendation). One prior case-control study comparing the efficacy of Goeckerman therapy with newer treatments, such as biologic therapies, steroids, and oral immunosuppressants, found a similar reduction in symptoms among both treatment groups, with longer disease-free periods in patients who received Goeckerman therapy than those who received newer therapies (22.3 years vs 4.6 months).14 However, Goeckerman therapy is utilized less frequently than more modern therapies because of the time required for treatment and declining insurance reimbursements for it. Climatotherapy, another older established therapy, involves the temporary or permanent relocation of patients to an environment that is favorable for disease control (grade B level of recommendation). Locations such as the Dead Sea and Canary Islands have been studied and shown to provide both subjective and objective improvement in patients’ psoriasis disease course. Patients had notable improvement in both their psoriasis area and severity index score and quality of life after a 3- to 4-week relocation to these areas.15,16 Access to climatotherapy and the transient nature of disease relief are apparent limitations of this treatment modality.
Grenz ray is a type of phototherapy that uses longer-wavelength ionizing radiation, which has low penetrance into surrounding tissues and a 95% absorption rate within the first 3 mm of the skin depth. This treatment has been used less frequently since the development of newer alternatives but should still be considered as a second line to UV therapy, especially in cases of recalcitrant disease and palmoplantar psoriasis, and when access to other treatment options is limited. Grenz ray has a grade C level of recommendation due to the paucity of evidence that supports its efficacy. Thus, it is not recommended as first-line therapy for the treatment of moderate to severe psoriasis. Visible light therapy is another treatment option that uses light in the visible wavelength spectrum but predominantly utilizes blue and red light. Psoriatic lesions are sensitive to light therapy because of the elevated levels of naturally occurring photosensitizing agents, called protoporphyrins, in these lesions.17 Several small studies have shown improvement in psoriatic lesions treated with visible light therapy, with blue light showing greater efficacy in lesional clearance than red light.18,19
Pulsed dye laser is a phototherapy modality that has shown efficacy in the treatment of nail psoriasis (grade B level of recommendation). One study comparing the effects of tazarotene cream 0.1% with pulsed dye laser and tazarotene cream 0.1% alone showed that patients receiving combination therapy had a greater decrease in
Initiation of Phototherapy
Prior to initiating phototherapy, it is important to assess the patient for any personal or family history of skin cancer, as phototherapy carries an increased risk for cutaneous malignancy, especially in patients with a history of skin cancer.22,23 All patients also should be evaluated for their Fitzpatrick skin type, and the minimal erythema dose should be defined for those initiating UVB treatment. These classifications can be useful for the initial determination of treatment dose and the prevention of treatment-related adverse events (TRAEs). A careful drug history also should be taken before the initiation of phototherapy to avoid photosensitizing reactions. Thiazide diuretics and tetracyclines are 2 such examples of medications commonly associated with photosensitizing reactions.24
Fitzpatrick skin type and/or minimal erythema dose testing also are essential in determining the appropriate initial NB-UVB dose required for treatment initiation (Table 2). Patient response to the initial NB-UVB trial will determine the optimal dosage for subsequent maintenance treatments.
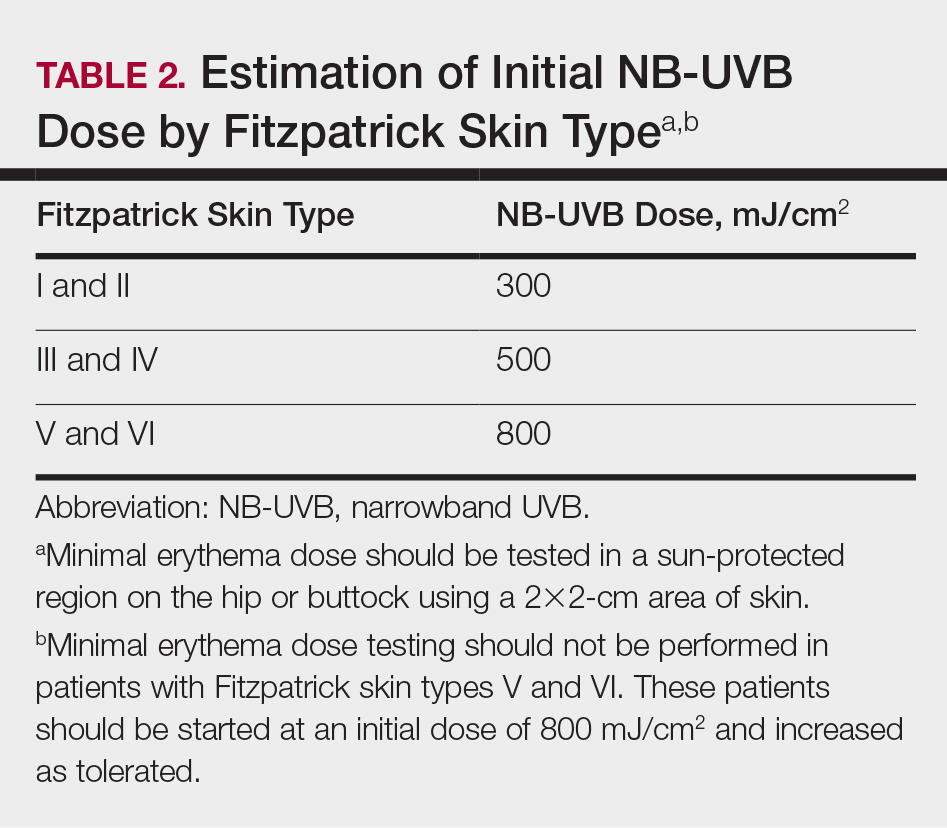
For patients unable or unwilling to commit to office-based or institution-based treatments, home NB-UVB is another therapeutic option. One study comparing patients with moderate to severe psoriasis who received home NB-UVB vs in-office treatment showed comparable psoriasis area and severity index scores and quality-of-life indices and no difference in the frequency of TRAE indices. It is important to note that patients who received home treatment had a significantly lower treatment burden (P≤.001) and greater treatment satisfaction than those receiving treatment in an office-based setting (P=.001).25
Assessment and Optimization of Phototherapy
After an appropriate starting dosage has been established, patients should be evaluated at each subsequent visit for the degree of treatment response. Excessive erythema (lasting more than 48 hours) or adverse effects, such as itching, stinging, or burning, are indications that the patient should have their dose adjusted back to the last dose without such adverse responses. Because tolerance to treatment develops over time, patients who miss a scheduled dose of NB-UVB phototherapy require their dose to be temporarily lowered. Targeted dosage of UVB phototherapy at a frequency of 2 to 3 times weekly is preferred over treatment 1 to 2 times weekly; however, consideration should be given toward patient preference.26 Dosing may be increased at a rate of 5% to 10% after each treatment, as tolerated, if there is no clearance of skin lesions with the original treatment dose. Patient skin type also is helpful in dictating the maximum phototherapy dose for each patient (Table 3).
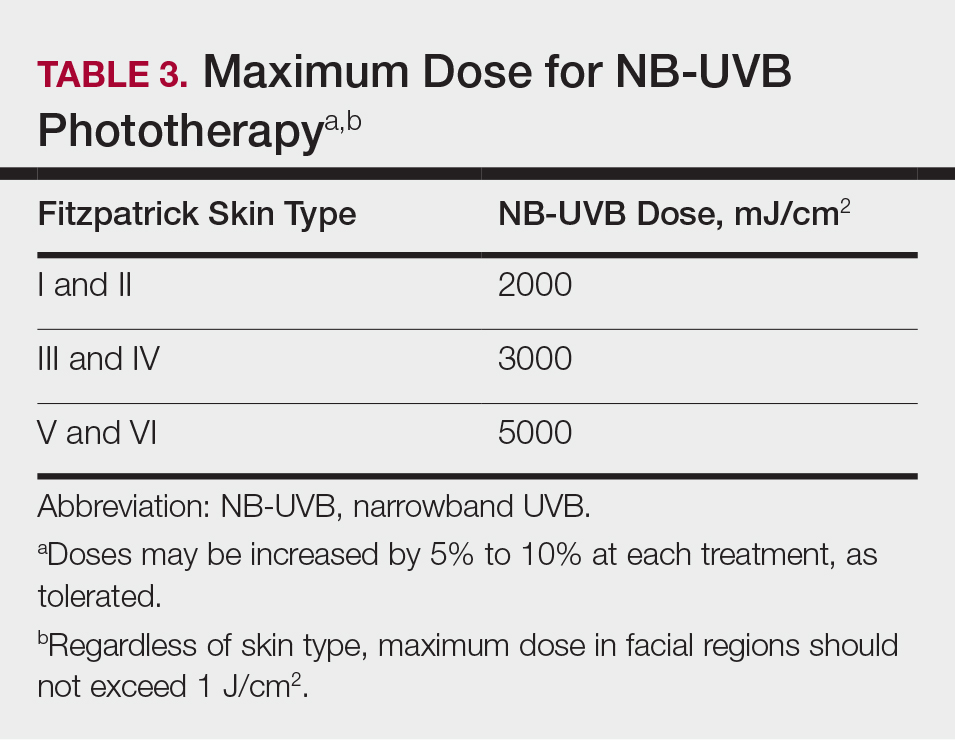
Once a patient’s psoriatic lesions have cleared, the patient has the option to taper or indefinitely continue maintenance therapy. The established protocol for patients who choose to taper therapy is treatment twice weekly for 4 weeks, followed by once-weekly treatment for the second month. The maintenance dosage is held constant during the taper. For patients who prefer indefinite maintenance therapy, treatment is administered every 1 to 2 weeks, with a maintenance dosage that is approximately 25% lower than the original maintenance dosage.
Treatment Considerations
Efforts should be made to ensure that the long-term sequalae of phototherapy are minimized (Table 1). Development of cataracts is a recognized consequence of prolonged UVB exposure; therefore, eye protection is recommended during all UVB treatment sessions to reduce the risk for ocular toxicity. Although pregnancy is not a contraindication to phototherapy, except for PUVA, there is a dose-dependent degradation of folate with NB-UVB treatment, so folate supplementation (0.8 mg) is recommended during NB-UVB treatment to prevent development of neural tube defects in fetuses of patients who are pregnant or who may become pregnant.27
Although phototherapy carries the theoretical risk for photocarcinogenesis, multiple studies have shown no increased risk for malignancy with either NB-UVB or BB-UVB phototherapy.22,23 Regardless, patients who develop new-onset skin cancer while receiving any phototherapeutic treatment should discuss the potential risks and benefits of continued treatment with their physician. Providers should take extra caution prior to initiating treatment, especially in patients with a history of cutaneous malignancy. Because oral PUVA is a systemic therapy, it is associated with a greater risk for photocarcinogenesis than any other modality, particularly in fair-skinned individuals. Patients younger than 10 years; pregnant or nursing patients; and those with a history of lupus, xeroderma pigmentosum, or melanoma should not receive PUVA therapy because of their increased risk for photocarcinogenesis and TRAEs.
The decision to switch patients between different phototherapy modalities during treatment should be individualized to each patient based on factors such as disease severity, quality of life, and treatment burden. Other factors to consider include dosing frequency, treatment cost, and logistical factors, such as proximity to a treatment facility. Physicians should have a detailed discussion about the risks and benefits of continuing therapy for patients who develop new-onset skin cancer during phototherapy.
Final Thoughts
Phototherapy is an effective and safe treatment for patients with psoriasis who have localized and systemic disease. There are several treatment modalities that can be tailored to patient needs and preferences, such as home NB-UVB for patients who are unable or unwilling to undergo office-based treatments. Phototherapy has few absolute contraindications; however, relative contraindications to phototherapy exist. Patients with a history of skin cancer, photosensitivity disorders, and autoimmune diseases (eg, lupus) carry greater risks for adverse events, such as sun-related damage, cancer, and dysplasia. Because these conditions may preclude patients from pursuing phototherapy as a safe and effective approach to treating moderate to severe psoriasis, these patients should be considered for other therapies, such as biologic medications, which may carry a more favorable risk-benefit ratio given that individual’s background.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173-1179.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Archier E, Devaux S, Castela E, et al. Efficacy of psoralen UV-A therapy vs. narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):11-21.
- Chen X, Yang M, Cheng Y, et al. Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis. Cochrane Database Syst Rev. 2013;10:CD009481.
- Wong T, Hsu L, Liao W. Phototherapy in psoriasis: a review of mechanisms of action. J Cutan Med Surg. 2013;17:6-12.
- Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359-362.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- El-Mofty M, Mostafa WZ, Bosseila M, et al. A large scale analytical study on efficacy of different photo(chemo)therapeutic modalities in the treatment of psoriasis, vitiligo and mycosis fungoides. Dermatol Ther. 2010;23:428-434.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
- Bruynzeel I, Bergman W, Hartevelt HM, et al. 'High single-dose' European PUVA regimen also causes an excess of non-melanoma skin cancer. Br J Dermatol. 1991;124:49-55.
- Lindelöf B, Sigurgeirsson B, Tegner E, et al. PUVA and cancer risk: the Swedish follow-up study. Br J Dermatol. 1999;141:108-112.
- Chern E, Yau D, Ho JC, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Harari M, Czarnowicki T, Fluss R, et al. Patients with early-onset psoriasis achieve better results following Dead Sea climatotherapy. J Eur Acad Dermatol Venereol. 2012;26:554-559.
- Wahl AK, Langeland E, Larsen MH, et al. Positive changes in self-management and disease severity following climate therapy in people with psoriasis. Acta Dermatol Venereol. 2015;95:317-321.
- Bissonnette R, Zeng H, McLean DI, et al. Psoriatic plaques exhibit red autofluorescence that is due to protoporphyrin IX. J Invest Dermatol. 1998;111:586-591.
- Kleinpenning MM, Otero ME, van Erp PE, et al. Efficacy of blue light vs. red light in the treatment of psoriasis: a double-blind, randomized comparative study. J Eur Acad Dermatol Venereol. 2012;26:219-225.
- Weinstabl A, Hoff-Lesch S, Merk HF, et al. Prospective randomized study on the efficacy of blue light in the treatment of psoriasis vulgaris. Dermatology. 2011;223:251-259.
- Huang YC, Chou CL, Chiang YY. Efficacy of pulsed dye laser plus topical tazarotene versus topical tazarotene alone in psoriatic nail disease: a single-blind, intrapatient left-to-right controlled study. Lasers Surg Med. 2013;45:102-107.
- Tawfik AA. Novel treatment of nail psoriasis using the intense pulsed light: a one-year follow-up study. Dermatol Surg. 2014;40:763-768.
- Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):22-31.
- Osmancevic A, Gillstedt M, Wennberg AM, et al. The risk of skin cancer in psoriasis patients treated with UVB therapy. Acta Dermatol Venereol. 2014;94:425-430.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:B1542.
- Almutawa F, Thalib L, Hekman D, et al. Efficacy of localized phototherapy and photodynamic therapy for psoriasis: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2015;31:5-14.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 Although topical therapies often are the first-line treatment of mild to moderate psoriasis, approximately 1 in 6 individuals has moderate to severe disease that requires systemic treatment such as biologics or phototherapy.2 In patients with localized disease that is refractory to treatment or who have moderate to severe psoriasis requiring systemic treatment, phototherapy should be considered as a potential low-risk treatment option.
In July 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of phototherapy in treating adult patients with psoriasis.3 Since the prior guidelines were released in 2010, there have been numerous studies affirming the efficacy of phototherapy, with several large meta-analyses helping to refine clinical recommendations.4,5 Each treatment was ranked using Strength of Recommendation Taxonomy, with a score of A, B, or C based on the strength of the evidence supporting the given modality. With the ever-increasing number of treatment options for patients with psoriasis, these guidelines inform dermatologists of the recommendations for the initiation, maintenance, and optimization of phototherapy in the treatment of psoriasis.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and frequency of adverse events of 10 commonly used phototherapy/photochemotherapy modalities. They also address dosing regimens, the potential to combine phototherapy with other therapies, and the efficacy of treatment modalities for different types of psoriasis.3 The purpose of this discussion is to present these guidelines in a condensed form for prescribers of phototherapy and to review the most clinically significant considerations during each step of treatment. Of note, we only highlight the treatment of adult patients and do not discuss information relevant to pediatric patients with psoriasis.
Choosing a Phototherapy Modality
Phototherapy may be considered for patients with psoriasis that affects more than 3% body surface area or for localized disease refractory to conventional treatments. UV light is believed to provide relief from psoriasis via multiple mechanisms, such as through favorable alterations in cytokine profiles, initiation of apoptosis, and local immunosupression.6 There is no single first-line phototherapeutic modality recommended for all patients with psoriasis. Rather, the decision to implement a particular modality should be individualized to the patient, considering factors such as percentage of body surface area affected by disease, quality-of-life assessment, comorbidities, lifestyle, and cost of treatment.
Of the 10 phototherapy modalities reviewed in these guidelines, 4 were ranked by the AAD and NPF as having grade A evidence for efficacy in the treatment of moderate to severe plaque psoriasis. Treatments with a grade A level of recommendation included narrowband UVB (NB-UVB), broadband UVB (BB-UVB), targeted phototherapy (excimer laser and excimer lamp), and

Studies have shown that the ideal wavelength needed to produce a therapeutic effect (ie, clearance of psoriatic plaques) is 304 to 313 nm. Wavelengths of 290 to 300 nm were found to be less therapeutic and more harmful, as they contributed to the development of sunburns.7 Broadband UVB phototherapy, with wavelengths ranging from 270 to 390 nm, exposes patients to a greater spectrum of radiation, thus making it more likely to cause sunburn and any theoretical form of sun-related damage, such as dysplasia and cancer. Compared with NB-UVB phototherapy, BB-UVB phototherapy is associated with a greater degree of sun damage–related side effects. Narrowband UVB, with a wavelength range of 311 to 313 nm, carries a grade A level of recommendation and should be considered as first-line monotherapy in patients with generalized plaque psoriasis, given its efficacy and promising safety profile. Multiple studies have shown that NB-UVB phototherapy is superior to BB-UVB phototherapy in the treatment of moderate to severe psoriasis in adults.8,9 In facilities where access to NB-UVB is limited, BB-UVB monotherapy is recommended as the treatment of generalized plaque psoriasis.
Psoralen plus UVA, which may be used topically (ie, bathwater PUVA) or taken orally, refers to treatment with photosensitizing psoralens. Psoralens are agents that intercalate with DNA and enhance the efficacy of phototherapy.10 Topical PUVA, with a grade B level of recommendation, is an effective treatment option for patients with localized disease and has been shown to be particularly efficacious in the treatment of palmoplantar pustular psoriasis. Oral PUVA is an effective option for psoriasis with a grade A recommendation, while bathwater PUVA has a grade B level of recommendation for moderate to severe plaque psoriasis. Oral PUVA is associated with greater systemic side effects (both acute and subacute) compared with NB-UVB and also is associated with photocarcinogenesis, particularly squamous cell carcinoma in white patients.11 Other side effects from PUVA include pigmented macules in sun-protected areas (known as PUVA lentigines), which may make evaluation of skin lesions challenging. Because of the increased risk for cancer with oral PUVA, NB-UVB is preferable as a first-line treatment vs PUVA, especially in patients with a history of skin cancer.12,13
Goeckerman therapy, which involves the synergistic combination of UVB and crude coal tar, is an older treatment that has shown efficacy in the treatment of severe or recalcitrant psoriasis (grade B level of recommendation). One prior case-control study comparing the efficacy of Goeckerman therapy with newer treatments, such as biologic therapies, steroids, and oral immunosuppressants, found a similar reduction in symptoms among both treatment groups, with longer disease-free periods in patients who received Goeckerman therapy than those who received newer therapies (22.3 years vs 4.6 months).14 However, Goeckerman therapy is utilized less frequently than more modern therapies because of the time required for treatment and declining insurance reimbursements for it. Climatotherapy, another older established therapy, involves the temporary or permanent relocation of patients to an environment that is favorable for disease control (grade B level of recommendation). Locations such as the Dead Sea and Canary Islands have been studied and shown to provide both subjective and objective improvement in patients’ psoriasis disease course. Patients had notable improvement in both their psoriasis area and severity index score and quality of life after a 3- to 4-week relocation to these areas.15,16 Access to climatotherapy and the transient nature of disease relief are apparent limitations of this treatment modality.
Grenz ray is a type of phototherapy that uses longer-wavelength ionizing radiation, which has low penetrance into surrounding tissues and a 95% absorption rate within the first 3 mm of the skin depth. This treatment has been used less frequently since the development of newer alternatives but should still be considered as a second line to UV therapy, especially in cases of recalcitrant disease and palmoplantar psoriasis, and when access to other treatment options is limited. Grenz ray has a grade C level of recommendation due to the paucity of evidence that supports its efficacy. Thus, it is not recommended as first-line therapy for the treatment of moderate to severe psoriasis. Visible light therapy is another treatment option that uses light in the visible wavelength spectrum but predominantly utilizes blue and red light. Psoriatic lesions are sensitive to light therapy because of the elevated levels of naturally occurring photosensitizing agents, called protoporphyrins, in these lesions.17 Several small studies have shown improvement in psoriatic lesions treated with visible light therapy, with blue light showing greater efficacy in lesional clearance than red light.18,19
Pulsed dye laser is a phototherapy modality that has shown efficacy in the treatment of nail psoriasis (grade B level of recommendation). One study comparing the effects of tazarotene cream 0.1% with pulsed dye laser and tazarotene cream 0.1% alone showed that patients receiving combination therapy had a greater decrease in
Initiation of Phototherapy
Prior to initiating phototherapy, it is important to assess the patient for any personal or family history of skin cancer, as phototherapy carries an increased risk for cutaneous malignancy, especially in patients with a history of skin cancer.22,23 All patients also should be evaluated for their Fitzpatrick skin type, and the minimal erythema dose should be defined for those initiating UVB treatment. These classifications can be useful for the initial determination of treatment dose and the prevention of treatment-related adverse events (TRAEs). A careful drug history also should be taken before the initiation of phototherapy to avoid photosensitizing reactions. Thiazide diuretics and tetracyclines are 2 such examples of medications commonly associated with photosensitizing reactions.24
Fitzpatrick skin type and/or minimal erythema dose testing also are essential in determining the appropriate initial NB-UVB dose required for treatment initiation (Table 2). Patient response to the initial NB-UVB trial will determine the optimal dosage for subsequent maintenance treatments.

For patients unable or unwilling to commit to office-based or institution-based treatments, home NB-UVB is another therapeutic option. One study comparing patients with moderate to severe psoriasis who received home NB-UVB vs in-office treatment showed comparable psoriasis area and severity index scores and quality-of-life indices and no difference in the frequency of TRAE indices. It is important to note that patients who received home treatment had a significantly lower treatment burden (P≤.001) and greater treatment satisfaction than those receiving treatment in an office-based setting (P=.001).25
Assessment and Optimization of Phototherapy
After an appropriate starting dosage has been established, patients should be evaluated at each subsequent visit for the degree of treatment response. Excessive erythema (lasting more than 48 hours) or adverse effects, such as itching, stinging, or burning, are indications that the patient should have their dose adjusted back to the last dose without such adverse responses. Because tolerance to treatment develops over time, patients who miss a scheduled dose of NB-UVB phototherapy require their dose to be temporarily lowered. Targeted dosage of UVB phototherapy at a frequency of 2 to 3 times weekly is preferred over treatment 1 to 2 times weekly; however, consideration should be given toward patient preference.26 Dosing may be increased at a rate of 5% to 10% after each treatment, as tolerated, if there is no clearance of skin lesions with the original treatment dose. Patient skin type also is helpful in dictating the maximum phototherapy dose for each patient (Table 3).

Once a patient’s psoriatic lesions have cleared, the patient has the option to taper or indefinitely continue maintenance therapy. The established protocol for patients who choose to taper therapy is treatment twice weekly for 4 weeks, followed by once-weekly treatment for the second month. The maintenance dosage is held constant during the taper. For patients who prefer indefinite maintenance therapy, treatment is administered every 1 to 2 weeks, with a maintenance dosage that is approximately 25% lower than the original maintenance dosage.
Treatment Considerations
Efforts should be made to ensure that the long-term sequalae of phototherapy are minimized (Table 1). Development of cataracts is a recognized consequence of prolonged UVB exposure; therefore, eye protection is recommended during all UVB treatment sessions to reduce the risk for ocular toxicity. Although pregnancy is not a contraindication to phototherapy, except for PUVA, there is a dose-dependent degradation of folate with NB-UVB treatment, so folate supplementation (0.8 mg) is recommended during NB-UVB treatment to prevent development of neural tube defects in fetuses of patients who are pregnant or who may become pregnant.27
Although phototherapy carries the theoretical risk for photocarcinogenesis, multiple studies have shown no increased risk for malignancy with either NB-UVB or BB-UVB phototherapy.22,23 Regardless, patients who develop new-onset skin cancer while receiving any phototherapeutic treatment should discuss the potential risks and benefits of continued treatment with their physician. Providers should take extra caution prior to initiating treatment, especially in patients with a history of cutaneous malignancy. Because oral PUVA is a systemic therapy, it is associated with a greater risk for photocarcinogenesis than any other modality, particularly in fair-skinned individuals. Patients younger than 10 years; pregnant or nursing patients; and those with a history of lupus, xeroderma pigmentosum, or melanoma should not receive PUVA therapy because of their increased risk for photocarcinogenesis and TRAEs.
The decision to switch patients between different phototherapy modalities during treatment should be individualized to each patient based on factors such as disease severity, quality of life, and treatment burden. Other factors to consider include dosing frequency, treatment cost, and logistical factors, such as proximity to a treatment facility. Physicians should have a detailed discussion about the risks and benefits of continuing therapy for patients who develop new-onset skin cancer during phototherapy.
Final Thoughts
Phototherapy is an effective and safe treatment for patients with psoriasis who have localized and systemic disease. There are several treatment modalities that can be tailored to patient needs and preferences, such as home NB-UVB for patients who are unable or unwilling to undergo office-based treatments. Phototherapy has few absolute contraindications; however, relative contraindications to phototherapy exist. Patients with a history of skin cancer, photosensitivity disorders, and autoimmune diseases (eg, lupus) carry greater risks for adverse events, such as sun-related damage, cancer, and dysplasia. Because these conditions may preclude patients from pursuing phototherapy as a safe and effective approach to treating moderate to severe psoriasis, these patients should be considered for other therapies, such as biologic medications, which may carry a more favorable risk-benefit ratio given that individual’s background.
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 Although topical therapies often are the first-line treatment of mild to moderate psoriasis, approximately 1 in 6 individuals has moderate to severe disease that requires systemic treatment such as biologics or phototherapy.2 In patients with localized disease that is refractory to treatment or who have moderate to severe psoriasis requiring systemic treatment, phototherapy should be considered as a potential low-risk treatment option.
In July 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of phototherapy in treating adult patients with psoriasis.3 Since the prior guidelines were released in 2010, there have been numerous studies affirming the efficacy of phototherapy, with several large meta-analyses helping to refine clinical recommendations.4,5 Each treatment was ranked using Strength of Recommendation Taxonomy, with a score of A, B, or C based on the strength of the evidence supporting the given modality. With the ever-increasing number of treatment options for patients with psoriasis, these guidelines inform dermatologists of the recommendations for the initiation, maintenance, and optimization of phototherapy in the treatment of psoriasis.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and frequency of adverse events of 10 commonly used phototherapy/photochemotherapy modalities. They also address dosing regimens, the potential to combine phototherapy with other therapies, and the efficacy of treatment modalities for different types of psoriasis.3 The purpose of this discussion is to present these guidelines in a condensed form for prescribers of phototherapy and to review the most clinically significant considerations during each step of treatment. Of note, we only highlight the treatment of adult patients and do not discuss information relevant to pediatric patients with psoriasis.
Choosing a Phototherapy Modality
Phototherapy may be considered for patients with psoriasis that affects more than 3% body surface area or for localized disease refractory to conventional treatments. UV light is believed to provide relief from psoriasis via multiple mechanisms, such as through favorable alterations in cytokine profiles, initiation of apoptosis, and local immunosupression.6 There is no single first-line phototherapeutic modality recommended for all patients with psoriasis. Rather, the decision to implement a particular modality should be individualized to the patient, considering factors such as percentage of body surface area affected by disease, quality-of-life assessment, comorbidities, lifestyle, and cost of treatment.
Of the 10 phototherapy modalities reviewed in these guidelines, 4 were ranked by the AAD and NPF as having grade A evidence for efficacy in the treatment of moderate to severe plaque psoriasis. Treatments with a grade A level of recommendation included narrowband UVB (NB-UVB), broadband UVB (BB-UVB), targeted phototherapy (excimer laser and excimer lamp), and

Studies have shown that the ideal wavelength needed to produce a therapeutic effect (ie, clearance of psoriatic plaques) is 304 to 313 nm. Wavelengths of 290 to 300 nm were found to be less therapeutic and more harmful, as they contributed to the development of sunburns.7 Broadband UVB phototherapy, with wavelengths ranging from 270 to 390 nm, exposes patients to a greater spectrum of radiation, thus making it more likely to cause sunburn and any theoretical form of sun-related damage, such as dysplasia and cancer. Compared with NB-UVB phototherapy, BB-UVB phototherapy is associated with a greater degree of sun damage–related side effects. Narrowband UVB, with a wavelength range of 311 to 313 nm, carries a grade A level of recommendation and should be considered as first-line monotherapy in patients with generalized plaque psoriasis, given its efficacy and promising safety profile. Multiple studies have shown that NB-UVB phototherapy is superior to BB-UVB phototherapy in the treatment of moderate to severe psoriasis in adults.8,9 In facilities where access to NB-UVB is limited, BB-UVB monotherapy is recommended as the treatment of generalized plaque psoriasis.
Psoralen plus UVA, which may be used topically (ie, bathwater PUVA) or taken orally, refers to treatment with photosensitizing psoralens. Psoralens are agents that intercalate with DNA and enhance the efficacy of phototherapy.10 Topical PUVA, with a grade B level of recommendation, is an effective treatment option for patients with localized disease and has been shown to be particularly efficacious in the treatment of palmoplantar pustular psoriasis. Oral PUVA is an effective option for psoriasis with a grade A recommendation, while bathwater PUVA has a grade B level of recommendation for moderate to severe plaque psoriasis. Oral PUVA is associated with greater systemic side effects (both acute and subacute) compared with NB-UVB and also is associated with photocarcinogenesis, particularly squamous cell carcinoma in white patients.11 Other side effects from PUVA include pigmented macules in sun-protected areas (known as PUVA lentigines), which may make evaluation of skin lesions challenging. Because of the increased risk for cancer with oral PUVA, NB-UVB is preferable as a first-line treatment vs PUVA, especially in patients with a history of skin cancer.12,13
Goeckerman therapy, which involves the synergistic combination of UVB and crude coal tar, is an older treatment that has shown efficacy in the treatment of severe or recalcitrant psoriasis (grade B level of recommendation). One prior case-control study comparing the efficacy of Goeckerman therapy with newer treatments, such as biologic therapies, steroids, and oral immunosuppressants, found a similar reduction in symptoms among both treatment groups, with longer disease-free periods in patients who received Goeckerman therapy than those who received newer therapies (22.3 years vs 4.6 months).14 However, Goeckerman therapy is utilized less frequently than more modern therapies because of the time required for treatment and declining insurance reimbursements for it. Climatotherapy, another older established therapy, involves the temporary or permanent relocation of patients to an environment that is favorable for disease control (grade B level of recommendation). Locations such as the Dead Sea and Canary Islands have been studied and shown to provide both subjective and objective improvement in patients’ psoriasis disease course. Patients had notable improvement in both their psoriasis area and severity index score and quality of life after a 3- to 4-week relocation to these areas.15,16 Access to climatotherapy and the transient nature of disease relief are apparent limitations of this treatment modality.
Grenz ray is a type of phototherapy that uses longer-wavelength ionizing radiation, which has low penetrance into surrounding tissues and a 95% absorption rate within the first 3 mm of the skin depth. This treatment has been used less frequently since the development of newer alternatives but should still be considered as a second line to UV therapy, especially in cases of recalcitrant disease and palmoplantar psoriasis, and when access to other treatment options is limited. Grenz ray has a grade C level of recommendation due to the paucity of evidence that supports its efficacy. Thus, it is not recommended as first-line therapy for the treatment of moderate to severe psoriasis. Visible light therapy is another treatment option that uses light in the visible wavelength spectrum but predominantly utilizes blue and red light. Psoriatic lesions are sensitive to light therapy because of the elevated levels of naturally occurring photosensitizing agents, called protoporphyrins, in these lesions.17 Several small studies have shown improvement in psoriatic lesions treated with visible light therapy, with blue light showing greater efficacy in lesional clearance than red light.18,19
Pulsed dye laser is a phototherapy modality that has shown efficacy in the treatment of nail psoriasis (grade B level of recommendation). One study comparing the effects of tazarotene cream 0.1% with pulsed dye laser and tazarotene cream 0.1% alone showed that patients receiving combination therapy had a greater decrease in
Initiation of Phototherapy
Prior to initiating phototherapy, it is important to assess the patient for any personal or family history of skin cancer, as phototherapy carries an increased risk for cutaneous malignancy, especially in patients with a history of skin cancer.22,23 All patients also should be evaluated for their Fitzpatrick skin type, and the minimal erythema dose should be defined for those initiating UVB treatment. These classifications can be useful for the initial determination of treatment dose and the prevention of treatment-related adverse events (TRAEs). A careful drug history also should be taken before the initiation of phototherapy to avoid photosensitizing reactions. Thiazide diuretics and tetracyclines are 2 such examples of medications commonly associated with photosensitizing reactions.24
Fitzpatrick skin type and/or minimal erythema dose testing also are essential in determining the appropriate initial NB-UVB dose required for treatment initiation (Table 2). Patient response to the initial NB-UVB trial will determine the optimal dosage for subsequent maintenance treatments.

For patients unable or unwilling to commit to office-based or institution-based treatments, home NB-UVB is another therapeutic option. One study comparing patients with moderate to severe psoriasis who received home NB-UVB vs in-office treatment showed comparable psoriasis area and severity index scores and quality-of-life indices and no difference in the frequency of TRAE indices. It is important to note that patients who received home treatment had a significantly lower treatment burden (P≤.001) and greater treatment satisfaction than those receiving treatment in an office-based setting (P=.001).25
Assessment and Optimization of Phototherapy
After an appropriate starting dosage has been established, patients should be evaluated at each subsequent visit for the degree of treatment response. Excessive erythema (lasting more than 48 hours) or adverse effects, such as itching, stinging, or burning, are indications that the patient should have their dose adjusted back to the last dose without such adverse responses. Because tolerance to treatment develops over time, patients who miss a scheduled dose of NB-UVB phototherapy require their dose to be temporarily lowered. Targeted dosage of UVB phototherapy at a frequency of 2 to 3 times weekly is preferred over treatment 1 to 2 times weekly; however, consideration should be given toward patient preference.26 Dosing may be increased at a rate of 5% to 10% after each treatment, as tolerated, if there is no clearance of skin lesions with the original treatment dose. Patient skin type also is helpful in dictating the maximum phototherapy dose for each patient (Table 3).

Once a patient’s psoriatic lesions have cleared, the patient has the option to taper or indefinitely continue maintenance therapy. The established protocol for patients who choose to taper therapy is treatment twice weekly for 4 weeks, followed by once-weekly treatment for the second month. The maintenance dosage is held constant during the taper. For patients who prefer indefinite maintenance therapy, treatment is administered every 1 to 2 weeks, with a maintenance dosage that is approximately 25% lower than the original maintenance dosage.
Treatment Considerations
Efforts should be made to ensure that the long-term sequalae of phototherapy are minimized (Table 1). Development of cataracts is a recognized consequence of prolonged UVB exposure; therefore, eye protection is recommended during all UVB treatment sessions to reduce the risk for ocular toxicity. Although pregnancy is not a contraindication to phototherapy, except for PUVA, there is a dose-dependent degradation of folate with NB-UVB treatment, so folate supplementation (0.8 mg) is recommended during NB-UVB treatment to prevent development of neural tube defects in fetuses of patients who are pregnant or who may become pregnant.27
Although phototherapy carries the theoretical risk for photocarcinogenesis, multiple studies have shown no increased risk for malignancy with either NB-UVB or BB-UVB phototherapy.22,23 Regardless, patients who develop new-onset skin cancer while receiving any phototherapeutic treatment should discuss the potential risks and benefits of continued treatment with their physician. Providers should take extra caution prior to initiating treatment, especially in patients with a history of cutaneous malignancy. Because oral PUVA is a systemic therapy, it is associated with a greater risk for photocarcinogenesis than any other modality, particularly in fair-skinned individuals. Patients younger than 10 years; pregnant or nursing patients; and those with a history of lupus, xeroderma pigmentosum, or melanoma should not receive PUVA therapy because of their increased risk for photocarcinogenesis and TRAEs.
The decision to switch patients between different phototherapy modalities during treatment should be individualized to each patient based on factors such as disease severity, quality of life, and treatment burden. Other factors to consider include dosing frequency, treatment cost, and logistical factors, such as proximity to a treatment facility. Physicians should have a detailed discussion about the risks and benefits of continuing therapy for patients who develop new-onset skin cancer during phototherapy.
Final Thoughts
Phototherapy is an effective and safe treatment for patients with psoriasis who have localized and systemic disease. There are several treatment modalities that can be tailored to patient needs and preferences, such as home NB-UVB for patients who are unable or unwilling to undergo office-based treatments. Phototherapy has few absolute contraindications; however, relative contraindications to phototherapy exist. Patients with a history of skin cancer, photosensitivity disorders, and autoimmune diseases (eg, lupus) carry greater risks for adverse events, such as sun-related damage, cancer, and dysplasia. Because these conditions may preclude patients from pursuing phototherapy as a safe and effective approach to treating moderate to severe psoriasis, these patients should be considered for other therapies, such as biologic medications, which may carry a more favorable risk-benefit ratio given that individual’s background.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173-1179.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Archier E, Devaux S, Castela E, et al. Efficacy of psoralen UV-A therapy vs. narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):11-21.
- Chen X, Yang M, Cheng Y, et al. Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis. Cochrane Database Syst Rev. 2013;10:CD009481.
- Wong T, Hsu L, Liao W. Phototherapy in psoriasis: a review of mechanisms of action. J Cutan Med Surg. 2013;17:6-12.
- Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359-362.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- El-Mofty M, Mostafa WZ, Bosseila M, et al. A large scale analytical study on efficacy of different photo(chemo)therapeutic modalities in the treatment of psoriasis, vitiligo and mycosis fungoides. Dermatol Ther. 2010;23:428-434.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
- Bruynzeel I, Bergman W, Hartevelt HM, et al. 'High single-dose' European PUVA regimen also causes an excess of non-melanoma skin cancer. Br J Dermatol. 1991;124:49-55.
- Lindelöf B, Sigurgeirsson B, Tegner E, et al. PUVA and cancer risk: the Swedish follow-up study. Br J Dermatol. 1999;141:108-112.
- Chern E, Yau D, Ho JC, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Harari M, Czarnowicki T, Fluss R, et al. Patients with early-onset psoriasis achieve better results following Dead Sea climatotherapy. J Eur Acad Dermatol Venereol. 2012;26:554-559.
- Wahl AK, Langeland E, Larsen MH, et al. Positive changes in self-management and disease severity following climate therapy in people with psoriasis. Acta Dermatol Venereol. 2015;95:317-321.
- Bissonnette R, Zeng H, McLean DI, et al. Psoriatic plaques exhibit red autofluorescence that is due to protoporphyrin IX. J Invest Dermatol. 1998;111:586-591.
- Kleinpenning MM, Otero ME, van Erp PE, et al. Efficacy of blue light vs. red light in the treatment of psoriasis: a double-blind, randomized comparative study. J Eur Acad Dermatol Venereol. 2012;26:219-225.
- Weinstabl A, Hoff-Lesch S, Merk HF, et al. Prospective randomized study on the efficacy of blue light in the treatment of psoriasis vulgaris. Dermatology. 2011;223:251-259.
- Huang YC, Chou CL, Chiang YY. Efficacy of pulsed dye laser plus topical tazarotene versus topical tazarotene alone in psoriatic nail disease: a single-blind, intrapatient left-to-right controlled study. Lasers Surg Med. 2013;45:102-107.
- Tawfik AA. Novel treatment of nail psoriasis using the intense pulsed light: a one-year follow-up study. Dermatol Surg. 2014;40:763-768.
- Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):22-31.
- Osmancevic A, Gillstedt M, Wennberg AM, et al. The risk of skin cancer in psoriasis patients treated with UVB therapy. Acta Dermatol Venereol. 2014;94:425-430.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:B1542.
- Almutawa F, Thalib L, Hekman D, et al. Efficacy of localized phototherapy and photodynamic therapy for psoriasis: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2015;31:5-14.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173-1179.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Archier E, Devaux S, Castela E, et al. Efficacy of psoralen UV-A therapy vs. narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):11-21.
- Chen X, Yang M, Cheng Y, et al. Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis. Cochrane Database Syst Rev. 2013;10:CD009481.
- Wong T, Hsu L, Liao W. Phototherapy in psoriasis: a review of mechanisms of action. J Cutan Med Surg. 2013;17:6-12.
- Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359-362.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- El-Mofty M, Mostafa WZ, Bosseila M, et al. A large scale analytical study on efficacy of different photo(chemo)therapeutic modalities in the treatment of psoriasis, vitiligo and mycosis fungoides. Dermatol Ther. 2010;23:428-434.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
- Bruynzeel I, Bergman W, Hartevelt HM, et al. 'High single-dose' European PUVA regimen also causes an excess of non-melanoma skin cancer. Br J Dermatol. 1991;124:49-55.
- Lindelöf B, Sigurgeirsson B, Tegner E, et al. PUVA and cancer risk: the Swedish follow-up study. Br J Dermatol. 1999;141:108-112.
- Chern E, Yau D, Ho JC, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Harari M, Czarnowicki T, Fluss R, et al. Patients with early-onset psoriasis achieve better results following Dead Sea climatotherapy. J Eur Acad Dermatol Venereol. 2012;26:554-559.
- Wahl AK, Langeland E, Larsen MH, et al. Positive changes in self-management and disease severity following climate therapy in people with psoriasis. Acta Dermatol Venereol. 2015;95:317-321.
- Bissonnette R, Zeng H, McLean DI, et al. Psoriatic plaques exhibit red autofluorescence that is due to protoporphyrin IX. J Invest Dermatol. 1998;111:586-591.
- Kleinpenning MM, Otero ME, van Erp PE, et al. Efficacy of blue light vs. red light in the treatment of psoriasis: a double-blind, randomized comparative study. J Eur Acad Dermatol Venereol. 2012;26:219-225.
- Weinstabl A, Hoff-Lesch S, Merk HF, et al. Prospective randomized study on the efficacy of blue light in the treatment of psoriasis vulgaris. Dermatology. 2011;223:251-259.
- Huang YC, Chou CL, Chiang YY. Efficacy of pulsed dye laser plus topical tazarotene versus topical tazarotene alone in psoriatic nail disease: a single-blind, intrapatient left-to-right controlled study. Lasers Surg Med. 2013;45:102-107.
- Tawfik AA. Novel treatment of nail psoriasis using the intense pulsed light: a one-year follow-up study. Dermatol Surg. 2014;40:763-768.
- Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):22-31.
- Osmancevic A, Gillstedt M, Wennberg AM, et al. The risk of skin cancer in psoriasis patients treated with UVB therapy. Acta Dermatol Venereol. 2014;94:425-430.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:B1542.
- Almutawa F, Thalib L, Hekman D, et al. Efficacy of localized phototherapy and photodynamic therapy for psoriasis: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2015;31:5-14.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
Practice Points
- Phototherapy should be considered as an effective and low-risk treatment of psoriasis.
- Narrowband UVB, broadband UVB, targeted phototherapy (excimer laser and excimer lamp), and oral psoralen plus UVA have all received a grade A level of recommendation for the treatment of psoriasis in adults.
Apremilast and Systemic Retinoid Combination Treatment for Moderate to Severe Palmoplantar Psoriasis
To the Editor:
Psoriasis is a chronic inflammatory papulosquamous skin disease affecting 2% to 3% of the population.1 Its pathogenesis is multifactorial, consisting of a disrupted skin barrier and dysregulated immune activation.2
A wide armamentarium of topical and systemic treatments targeting different aspects of the disease pathogenesis have been developed over the years.3,4 Psoriasis was once considered a skin disease exclusively, but accumulating evidence suggests that it is accompanied by a multitude of systemic inflammatory comorbidities.5 This insight supports the concept of systemic treatment for patients with moderate to severe psoriasis. As a chronic disease, psoriasis requires continuous therapy. The treatment approach should focus on achieving efficacy and minimizing side effects. These goals can be achieved by combination, rotational, and sequential treatment approaches.6 Many therapeutic combinations have proven effective, using beneficially different mechanisms of action (MOAs) and toxicity profiles.7 We present a patient with moderate to severe recalcitrant palmoplantar psoriasis who demonstrated improvement with combination therapy.
A 50-year-old man presented with palmoplantar psoriasis of 7 years’ duration. His medical history included mild hyperlipidemia treated with atorvastatin. Prior topical treatments including calcipotriene, betamethasone dipropionate, and tacrolimus ointment did not result in improvement. Persistent acral involvement required further intervention, and the excimer laser was added to the therapeutic regimen with a minor additive therapeutic value. Acitretin (25 mg/d) was initiated; however, the disease flared up soon after. Acitretin was discontinued, and the patient was treated with apremilast (30 mg twice daily) for 9 months with a slight improvement. Physical examination revealed erythematous, fissured, scaly plaques involving both the palms and soles. Acitretin (25 mg/d) was reintroduced to the therapeutic regimen, and the acitretin-apremilast combination was used for 2 months. With this regimen, the patient experienced 90% improvement (Figures 1 and 2).

Palmoplantar psoriasis is a debilitating dermatosis that is extremely challenging to treat and is unresponsive to many modalities.8 Increased understanding of psoriasis mechanisms paved the path for the development of highly targeted biologic therapies9 with fewer side effects than drugs such as cyclosporine that indiscriminately neutralize multiple components of the immune system. Although highly specific, these targeted approaches are not without side effects10 and lead to diverse therapeutic outcomes, particularly when prescribed for palmoplantar psoriasis.11,12
The small-molecule inhibitor of phosphodiesterase 4—apremilast—was approved for plaque psoriasis treatment in late 2014. Although not fully elucidated, its MOA involves interfering with intracellular signaling, leading to increased intracellular cyclic adenosine monophosphate levels in inflammatory cells and keratinocytes.13 Proximal interruption of the pathologic cascade leads to the reduction of multiple proinflammatory cytokines with a simultaneous increase in anti-inflammatory mediators.13 Its efficacy and safety in the treatment of psoriasis have been shown in phase 2 and 3 clinical trials.14,15 In contrast to traditional oral therapies for psoriasis (ie, methotrexate, cyclosporine, acitretin), no laboratory test monitoring is needed and the safety profile is notably better.16
Acitretin, the active metabolite of etretinate, modulates epidermal differentiation and has immunomodulating activities.17 It commonly is used for treating palmoplantar psoriasis.8 Until recently, it was the only nonimmunosuppressive systemic treatment for psoriasis, and its combination with other systemic treatments, particularly biologics, has been advocated.18 Prior reports showed remarkable disease improvement when combining acitretin with alefacept, etanercept, infliximab, adalimumab, and ustekinumab.19 The optimal combination should include modalities with different MOAs without overlapping toxicities.19 Apremilast and acitretin have different MOAs and side-effect profiles, but another theoretical advantage is that they both interfere with intracellular signaling on the transcription level rather than affecting extracellular targets.13
Our patient with moderate to severe recalcitrant palmoplantar psoriasis demonstrated approximately 90% improvement following apremilast and acitretin combination therapy. This treatment regimen should be considered in cases of persistent acral disease resistant to other therapeutic efforts.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg. 2010;29:3-9.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin. 2015;33:41-44.
- Lebwohl M, Menter A, Koo J, et al. Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol. 2004;50:416-430.
- Cather JC, Menter A. Combining traditional agents and biologics for the treatment of psoriasis. Semin Cutan Med Surg. 2005;24:37-45.
- Janagond AB, Kanwar AJ, Handa S. Efficacy and safety of systemic methotrexate vs. acitretin in psoriasis patients with significant palmoplantar involvement: a prospective, randomized study. J Eur Acad Dermatol Venereol. 2013;27:E384-E389.
- Campa M, Mansouri B, Warren R, et al. A review of biologic therapies targeting IL-23 and IL-17 for use in moderate-to-severe plaque psoriasis [published online December 29, 2015]. Dermatol Ther (Heidelb). 2015;6:1-12.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Jacobi A, Schuler G, Hertl M. Differential clinical response to alefacept in combination with methotrexate in two patients with refractory palmar psoriasis. Br J Dermatol. 2007;156:178-180.
- Meyer V, Goerge T, Luger TA, et al. Successful treatment of palmoplantar hyperkeratotic psoriasis with a combination of etanercept and alitretinoin. J Clin Aesthet Dermatol. 2011;4:45-46.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): a new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Pilkington T, Brogden RN. Acitretin—a review of its pharmacology and therapeutic use. Drugs. 1992;43:597-627.
- Lebwohl M. Combining the new biologic agents with our current psoriasis armamentarium. J Am Acad Dermatol. 2003;49:S118-S124.
- Heinecke GM, Luber AJ, Levitt JO, et al. Combination use of ustekinumab with other systemic therapies: a retrospective study in a tertiary referral center. J Drugs Dermatol. 2013;12:1098-1102.
To the Editor:
Psoriasis is a chronic inflammatory papulosquamous skin disease affecting 2% to 3% of the population.1 Its pathogenesis is multifactorial, consisting of a disrupted skin barrier and dysregulated immune activation.2
A wide armamentarium of topical and systemic treatments targeting different aspects of the disease pathogenesis have been developed over the years.3,4 Psoriasis was once considered a skin disease exclusively, but accumulating evidence suggests that it is accompanied by a multitude of systemic inflammatory comorbidities.5 This insight supports the concept of systemic treatment for patients with moderate to severe psoriasis. As a chronic disease, psoriasis requires continuous therapy. The treatment approach should focus on achieving efficacy and minimizing side effects. These goals can be achieved by combination, rotational, and sequential treatment approaches.6 Many therapeutic combinations have proven effective, using beneficially different mechanisms of action (MOAs) and toxicity profiles.7 We present a patient with moderate to severe recalcitrant palmoplantar psoriasis who demonstrated improvement with combination therapy.
A 50-year-old man presented with palmoplantar psoriasis of 7 years’ duration. His medical history included mild hyperlipidemia treated with atorvastatin. Prior topical treatments including calcipotriene, betamethasone dipropionate, and tacrolimus ointment did not result in improvement. Persistent acral involvement required further intervention, and the excimer laser was added to the therapeutic regimen with a minor additive therapeutic value. Acitretin (25 mg/d) was initiated; however, the disease flared up soon after. Acitretin was discontinued, and the patient was treated with apremilast (30 mg twice daily) for 9 months with a slight improvement. Physical examination revealed erythematous, fissured, scaly plaques involving both the palms and soles. Acitretin (25 mg/d) was reintroduced to the therapeutic regimen, and the acitretin-apremilast combination was used for 2 months. With this regimen, the patient experienced 90% improvement (Figures 1 and 2).


Palmoplantar psoriasis is a debilitating dermatosis that is extremely challenging to treat and is unresponsive to many modalities.8 Increased understanding of psoriasis mechanisms paved the path for the development of highly targeted biologic therapies9 with fewer side effects than drugs such as cyclosporine that indiscriminately neutralize multiple components of the immune system. Although highly specific, these targeted approaches are not without side effects10 and lead to diverse therapeutic outcomes, particularly when prescribed for palmoplantar psoriasis.11,12
The small-molecule inhibitor of phosphodiesterase 4—apremilast—was approved for plaque psoriasis treatment in late 2014. Although not fully elucidated, its MOA involves interfering with intracellular signaling, leading to increased intracellular cyclic adenosine monophosphate levels in inflammatory cells and keratinocytes.13 Proximal interruption of the pathologic cascade leads to the reduction of multiple proinflammatory cytokines with a simultaneous increase in anti-inflammatory mediators.13 Its efficacy and safety in the treatment of psoriasis have been shown in phase 2 and 3 clinical trials.14,15 In contrast to traditional oral therapies for psoriasis (ie, methotrexate, cyclosporine, acitretin), no laboratory test monitoring is needed and the safety profile is notably better.16
Acitretin, the active metabolite of etretinate, modulates epidermal differentiation and has immunomodulating activities.17 It commonly is used for treating palmoplantar psoriasis.8 Until recently, it was the only nonimmunosuppressive systemic treatment for psoriasis, and its combination with other systemic treatments, particularly biologics, has been advocated.18 Prior reports showed remarkable disease improvement when combining acitretin with alefacept, etanercept, infliximab, adalimumab, and ustekinumab.19 The optimal combination should include modalities with different MOAs without overlapping toxicities.19 Apremilast and acitretin have different MOAs and side-effect profiles, but another theoretical advantage is that they both interfere with intracellular signaling on the transcription level rather than affecting extracellular targets.13
Our patient with moderate to severe recalcitrant palmoplantar psoriasis demonstrated approximately 90% improvement following apremilast and acitretin combination therapy. This treatment regimen should be considered in cases of persistent acral disease resistant to other therapeutic efforts.
To the Editor:
Psoriasis is a chronic inflammatory papulosquamous skin disease affecting 2% to 3% of the population.1 Its pathogenesis is multifactorial, consisting of a disrupted skin barrier and dysregulated immune activation.2
A wide armamentarium of topical and systemic treatments targeting different aspects of the disease pathogenesis have been developed over the years.3,4 Psoriasis was once considered a skin disease exclusively, but accumulating evidence suggests that it is accompanied by a multitude of systemic inflammatory comorbidities.5 This insight supports the concept of systemic treatment for patients with moderate to severe psoriasis. As a chronic disease, psoriasis requires continuous therapy. The treatment approach should focus on achieving efficacy and minimizing side effects. These goals can be achieved by combination, rotational, and sequential treatment approaches.6 Many therapeutic combinations have proven effective, using beneficially different mechanisms of action (MOAs) and toxicity profiles.7 We present a patient with moderate to severe recalcitrant palmoplantar psoriasis who demonstrated improvement with combination therapy.
A 50-year-old man presented with palmoplantar psoriasis of 7 years’ duration. His medical history included mild hyperlipidemia treated with atorvastatin. Prior topical treatments including calcipotriene, betamethasone dipropionate, and tacrolimus ointment did not result in improvement. Persistent acral involvement required further intervention, and the excimer laser was added to the therapeutic regimen with a minor additive therapeutic value. Acitretin (25 mg/d) was initiated; however, the disease flared up soon after. Acitretin was discontinued, and the patient was treated with apremilast (30 mg twice daily) for 9 months with a slight improvement. Physical examination revealed erythematous, fissured, scaly plaques involving both the palms and soles. Acitretin (25 mg/d) was reintroduced to the therapeutic regimen, and the acitretin-apremilast combination was used for 2 months. With this regimen, the patient experienced 90% improvement (Figures 1 and 2).


Palmoplantar psoriasis is a debilitating dermatosis that is extremely challenging to treat and is unresponsive to many modalities.8 Increased understanding of psoriasis mechanisms paved the path for the development of highly targeted biologic therapies9 with fewer side effects than drugs such as cyclosporine that indiscriminately neutralize multiple components of the immune system. Although highly specific, these targeted approaches are not without side effects10 and lead to diverse therapeutic outcomes, particularly when prescribed for palmoplantar psoriasis.11,12
The small-molecule inhibitor of phosphodiesterase 4—apremilast—was approved for plaque psoriasis treatment in late 2014. Although not fully elucidated, its MOA involves interfering with intracellular signaling, leading to increased intracellular cyclic adenosine monophosphate levels in inflammatory cells and keratinocytes.13 Proximal interruption of the pathologic cascade leads to the reduction of multiple proinflammatory cytokines with a simultaneous increase in anti-inflammatory mediators.13 Its efficacy and safety in the treatment of psoriasis have been shown in phase 2 and 3 clinical trials.14,15 In contrast to traditional oral therapies for psoriasis (ie, methotrexate, cyclosporine, acitretin), no laboratory test monitoring is needed and the safety profile is notably better.16
Acitretin, the active metabolite of etretinate, modulates epidermal differentiation and has immunomodulating activities.17 It commonly is used for treating palmoplantar psoriasis.8 Until recently, it was the only nonimmunosuppressive systemic treatment for psoriasis, and its combination with other systemic treatments, particularly biologics, has been advocated.18 Prior reports showed remarkable disease improvement when combining acitretin with alefacept, etanercept, infliximab, adalimumab, and ustekinumab.19 The optimal combination should include modalities with different MOAs without overlapping toxicities.19 Apremilast and acitretin have different MOAs and side-effect profiles, but another theoretical advantage is that they both interfere with intracellular signaling on the transcription level rather than affecting extracellular targets.13
Our patient with moderate to severe recalcitrant palmoplantar psoriasis demonstrated approximately 90% improvement following apremilast and acitretin combination therapy. This treatment regimen should be considered in cases of persistent acral disease resistant to other therapeutic efforts.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg. 2010;29:3-9.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin. 2015;33:41-44.
- Lebwohl M, Menter A, Koo J, et al. Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol. 2004;50:416-430.
- Cather JC, Menter A. Combining traditional agents and biologics for the treatment of psoriasis. Semin Cutan Med Surg. 2005;24:37-45.
- Janagond AB, Kanwar AJ, Handa S. Efficacy and safety of systemic methotrexate vs. acitretin in psoriasis patients with significant palmoplantar involvement: a prospective, randomized study. J Eur Acad Dermatol Venereol. 2013;27:E384-E389.
- Campa M, Mansouri B, Warren R, et al. A review of biologic therapies targeting IL-23 and IL-17 for use in moderate-to-severe plaque psoriasis [published online December 29, 2015]. Dermatol Ther (Heidelb). 2015;6:1-12.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Jacobi A, Schuler G, Hertl M. Differential clinical response to alefacept in combination with methotrexate in two patients with refractory palmar psoriasis. Br J Dermatol. 2007;156:178-180.
- Meyer V, Goerge T, Luger TA, et al. Successful treatment of palmoplantar hyperkeratotic psoriasis with a combination of etanercept and alitretinoin. J Clin Aesthet Dermatol. 2011;4:45-46.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): a new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Pilkington T, Brogden RN. Acitretin—a review of its pharmacology and therapeutic use. Drugs. 1992;43:597-627.
- Lebwohl M. Combining the new biologic agents with our current psoriasis armamentarium. J Am Acad Dermatol. 2003;49:S118-S124.
- Heinecke GM, Luber AJ, Levitt JO, et al. Combination use of ustekinumab with other systemic therapies: a retrospective study in a tertiary referral center. J Drugs Dermatol. 2013;12:1098-1102.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg. 2010;29:3-9.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin. 2015;33:41-44.
- Lebwohl M, Menter A, Koo J, et al. Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol. 2004;50:416-430.
- Cather JC, Menter A. Combining traditional agents and biologics for the treatment of psoriasis. Semin Cutan Med Surg. 2005;24:37-45.
- Janagond AB, Kanwar AJ, Handa S. Efficacy and safety of systemic methotrexate vs. acitretin in psoriasis patients with significant palmoplantar involvement: a prospective, randomized study. J Eur Acad Dermatol Venereol. 2013;27:E384-E389.
- Campa M, Mansouri B, Warren R, et al. A review of biologic therapies targeting IL-23 and IL-17 for use in moderate-to-severe plaque psoriasis [published online December 29, 2015]. Dermatol Ther (Heidelb). 2015;6:1-12.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Jacobi A, Schuler G, Hertl M. Differential clinical response to alefacept in combination with methotrexate in two patients with refractory palmar psoriasis. Br J Dermatol. 2007;156:178-180.
- Meyer V, Goerge T, Luger TA, et al. Successful treatment of palmoplantar hyperkeratotic psoriasis with a combination of etanercept and alitretinoin. J Clin Aesthet Dermatol. 2011;4:45-46.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): a new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Pilkington T, Brogden RN. Acitretin—a review of its pharmacology and therapeutic use. Drugs. 1992;43:597-627.
- Lebwohl M. Combining the new biologic agents with our current psoriasis armamentarium. J Am Acad Dermatol. 2003;49:S118-S124.
- Heinecke GM, Luber AJ, Levitt JO, et al. Combination use of ustekinumab with other systemic therapies: a retrospective study in a tertiary referral center. J Drugs Dermatol. 2013;12:1098-1102.
Practice Points
- Palmoplantar psoriasis is challenging to treat and is unresponsive to many modalities.
- Combination, rotational, and sequential treatment approaches may minimize side effects and loss of efficacy as well as enhance treatment responses.
- Apremilast and acitretin combination therapy led to 90% skin improvement in a case of severe recalcitrant palmoplantar psoriasis.
Beyond PASI 100: striving for molecular clearance
All PASI 100 responses to psoriasis therapy are not the same, Andrew Blauvelt, MD, declared at the virtual annual meeting of the American Academy of Dermatology.
He presented a first-of-its-kind study that potentially opens the door to a new, more rigorous standard for treatment success in psoriasis: Not simply cleared lesional skin as captured by a Psoriasis Area and Severity Index (PASI) 100 response, but also clearance of residual psoriasis signs and symptoms – as well as what he termed “molecular clearance.”
“We’ve found that clearing skin with drugs utilizing different mechanisms of action may lead to differential consequences for our patients,” observed Dr. Blauvelt, a dermatologist and clinical trialist who is president of the Oregon Medical Research Center, Portland.
A PASI 100 response, traditionally considered an elusive goal for the great majority of patients with severe psoriasis, can now often be achieved using today’s top-tier, high-performance biologics. But Dr. Blauvelt and his coinvestigators are interested in pushing even beyond PASI 100 to a new frontier of therapeutic benefit.
He presented a secondary analysis of the previously reported VOYAGE 1 and 2 head-to-head randomized trials of guselkumab (Tremfya) versus adalimumab (Humira) for treatment of moderate to severe psoriasis. This new analysis, which focused exclusively on PASI 100 responders by week 24, demonstrated that patients with a PASI 100 response to guselkumab, an interleukin (IL)-23 inhibitor, had significantly fewer persistent symptoms and signs of psoriasis than those whose skin clearance was attained using adalimumab, a tumor necrosis factor (TNF) inhibitor.
Moreover,
The analysis included 16 participants in the VOYAGE trials who achieved PASI 100 at week 24 on guselkumab and 5 who did so on adalimumab. At baseline and again at week 24, these individuals completed the Psoriasis Symptoms and Signs Diary (PSSD). Also, biopsies of lesional and nonlesional skin were obtained at baseline and of cleared lesional skin at week 24 for transcriptomic microarray analysis of the expression of many thousands of genes.
Persistent psoriasis symptoms despite cleared skin
The PSSD involves patient ratings of various psoriasis symptoms and signs. Total scores can range from 0 (symptom- and sign-free) up to 100. At week 24, a significantly higher proportion of guselkumab-treated PASI 100 responders had a total PSSD score of zero: 55%, versus 43% in the adalimumab group. This was consistently true across the board for each of the individual signs and symptoms assessed. For example, 61% of the guselkumab group gave themselves a zero for itch, as did 50% of the adalimumab group. Sixty-four percent on guselkumab and 52% on adalimumab reported being free of redness. And 78% of the guselkumab group reported being pain-free, compared with 69% with adalimumab, Dr. Blauvelt reported.
Gene expression analysis
At baseline, more than 2,300 dysregulated genes were identified in lesional skin while functioning normally in nonlesional skin. The great majority of these initially dysregulated genes became normalized in cleared lesional skin in PASI 100 responders at week 24. However, 25 of the genes remained dysregulated in cleared lesional skin, meaning they displayed less than 75% of normal function. Ten of these 25 genes with dysregulated expression at follow-up showed abnormal function in patients with residual symptoms despite cleared skin, but they functioned normally in those without persistent symptoms. This raises the possibility that the residual symptoms of psoriasis were attributable to the abnormal gene functioning, according to Dr. Blauvelt.
Of note, 9 of the 10 dysregulated genes in cleared lesional skin of patients with residual symptoms were present in the adalimumab group; these included two genes localized to the epidermal differentiation complex as well as the psoriasis-specific proline-rich 9 gene known as PRR9, which is induced by IL-17A. In contrast, only four genes, none of which were localized to the epidermal differentiation complex, were insufficiently normalized in the cleared lesional skin of guselkumab-treated PASI-100 responders.
“Nothing like this analysis has ever been done before,” the dermatologist observed. “It’s a pilot study. Perhaps with more data like this, we’ll be using this type of information in clinical practice to go beyond clearing patients’ skin.”
Dr. Blauvelt reported serving as a scientific advisor to and paid clinical investigator for Janssen, which sponsored the study, as well as for roughly two dozen other pharmaceutical companies.
All PASI 100 responses to psoriasis therapy are not the same, Andrew Blauvelt, MD, declared at the virtual annual meeting of the American Academy of Dermatology.
He presented a first-of-its-kind study that potentially opens the door to a new, more rigorous standard for treatment success in psoriasis: Not simply cleared lesional skin as captured by a Psoriasis Area and Severity Index (PASI) 100 response, but also clearance of residual psoriasis signs and symptoms – as well as what he termed “molecular clearance.”
“We’ve found that clearing skin with drugs utilizing different mechanisms of action may lead to differential consequences for our patients,” observed Dr. Blauvelt, a dermatologist and clinical trialist who is president of the Oregon Medical Research Center, Portland.
A PASI 100 response, traditionally considered an elusive goal for the great majority of patients with severe psoriasis, can now often be achieved using today’s top-tier, high-performance biologics. But Dr. Blauvelt and his coinvestigators are interested in pushing even beyond PASI 100 to a new frontier of therapeutic benefit.
He presented a secondary analysis of the previously reported VOYAGE 1 and 2 head-to-head randomized trials of guselkumab (Tremfya) versus adalimumab (Humira) for treatment of moderate to severe psoriasis. This new analysis, which focused exclusively on PASI 100 responders by week 24, demonstrated that patients with a PASI 100 response to guselkumab, an interleukin (IL)-23 inhibitor, had significantly fewer persistent symptoms and signs of psoriasis than those whose skin clearance was attained using adalimumab, a tumor necrosis factor (TNF) inhibitor.
Moreover,
The analysis included 16 participants in the VOYAGE trials who achieved PASI 100 at week 24 on guselkumab and 5 who did so on adalimumab. At baseline and again at week 24, these individuals completed the Psoriasis Symptoms and Signs Diary (PSSD). Also, biopsies of lesional and nonlesional skin were obtained at baseline and of cleared lesional skin at week 24 for transcriptomic microarray analysis of the expression of many thousands of genes.
Persistent psoriasis symptoms despite cleared skin
The PSSD involves patient ratings of various psoriasis symptoms and signs. Total scores can range from 0 (symptom- and sign-free) up to 100. At week 24, a significantly higher proportion of guselkumab-treated PASI 100 responders had a total PSSD score of zero: 55%, versus 43% in the adalimumab group. This was consistently true across the board for each of the individual signs and symptoms assessed. For example, 61% of the guselkumab group gave themselves a zero for itch, as did 50% of the adalimumab group. Sixty-four percent on guselkumab and 52% on adalimumab reported being free of redness. And 78% of the guselkumab group reported being pain-free, compared with 69% with adalimumab, Dr. Blauvelt reported.
Gene expression analysis
At baseline, more than 2,300 dysregulated genes were identified in lesional skin while functioning normally in nonlesional skin. The great majority of these initially dysregulated genes became normalized in cleared lesional skin in PASI 100 responders at week 24. However, 25 of the genes remained dysregulated in cleared lesional skin, meaning they displayed less than 75% of normal function. Ten of these 25 genes with dysregulated expression at follow-up showed abnormal function in patients with residual symptoms despite cleared skin, but they functioned normally in those without persistent symptoms. This raises the possibility that the residual symptoms of psoriasis were attributable to the abnormal gene functioning, according to Dr. Blauvelt.
Of note, 9 of the 10 dysregulated genes in cleared lesional skin of patients with residual symptoms were present in the adalimumab group; these included two genes localized to the epidermal differentiation complex as well as the psoriasis-specific proline-rich 9 gene known as PRR9, which is induced by IL-17A. In contrast, only four genes, none of which were localized to the epidermal differentiation complex, were insufficiently normalized in the cleared lesional skin of guselkumab-treated PASI-100 responders.
“Nothing like this analysis has ever been done before,” the dermatologist observed. “It’s a pilot study. Perhaps with more data like this, we’ll be using this type of information in clinical practice to go beyond clearing patients’ skin.”
Dr. Blauvelt reported serving as a scientific advisor to and paid clinical investigator for Janssen, which sponsored the study, as well as for roughly two dozen other pharmaceutical companies.
All PASI 100 responses to psoriasis therapy are not the same, Andrew Blauvelt, MD, declared at the virtual annual meeting of the American Academy of Dermatology.
He presented a first-of-its-kind study that potentially opens the door to a new, more rigorous standard for treatment success in psoriasis: Not simply cleared lesional skin as captured by a Psoriasis Area and Severity Index (PASI) 100 response, but also clearance of residual psoriasis signs and symptoms – as well as what he termed “molecular clearance.”
“We’ve found that clearing skin with drugs utilizing different mechanisms of action may lead to differential consequences for our patients,” observed Dr. Blauvelt, a dermatologist and clinical trialist who is president of the Oregon Medical Research Center, Portland.
A PASI 100 response, traditionally considered an elusive goal for the great majority of patients with severe psoriasis, can now often be achieved using today’s top-tier, high-performance biologics. But Dr. Blauvelt and his coinvestigators are interested in pushing even beyond PASI 100 to a new frontier of therapeutic benefit.
He presented a secondary analysis of the previously reported VOYAGE 1 and 2 head-to-head randomized trials of guselkumab (Tremfya) versus adalimumab (Humira) for treatment of moderate to severe psoriasis. This new analysis, which focused exclusively on PASI 100 responders by week 24, demonstrated that patients with a PASI 100 response to guselkumab, an interleukin (IL)-23 inhibitor, had significantly fewer persistent symptoms and signs of psoriasis than those whose skin clearance was attained using adalimumab, a tumor necrosis factor (TNF) inhibitor.
Moreover,
The analysis included 16 participants in the VOYAGE trials who achieved PASI 100 at week 24 on guselkumab and 5 who did so on adalimumab. At baseline and again at week 24, these individuals completed the Psoriasis Symptoms and Signs Diary (PSSD). Also, biopsies of lesional and nonlesional skin were obtained at baseline and of cleared lesional skin at week 24 for transcriptomic microarray analysis of the expression of many thousands of genes.
Persistent psoriasis symptoms despite cleared skin
The PSSD involves patient ratings of various psoriasis symptoms and signs. Total scores can range from 0 (symptom- and sign-free) up to 100. At week 24, a significantly higher proportion of guselkumab-treated PASI 100 responders had a total PSSD score of zero: 55%, versus 43% in the adalimumab group. This was consistently true across the board for each of the individual signs and symptoms assessed. For example, 61% of the guselkumab group gave themselves a zero for itch, as did 50% of the adalimumab group. Sixty-four percent on guselkumab and 52% on adalimumab reported being free of redness. And 78% of the guselkumab group reported being pain-free, compared with 69% with adalimumab, Dr. Blauvelt reported.
Gene expression analysis
At baseline, more than 2,300 dysregulated genes were identified in lesional skin while functioning normally in nonlesional skin. The great majority of these initially dysregulated genes became normalized in cleared lesional skin in PASI 100 responders at week 24. However, 25 of the genes remained dysregulated in cleared lesional skin, meaning they displayed less than 75% of normal function. Ten of these 25 genes with dysregulated expression at follow-up showed abnormal function in patients with residual symptoms despite cleared skin, but they functioned normally in those without persistent symptoms. This raises the possibility that the residual symptoms of psoriasis were attributable to the abnormal gene functioning, according to Dr. Blauvelt.
Of note, 9 of the 10 dysregulated genes in cleared lesional skin of patients with residual symptoms were present in the adalimumab group; these included two genes localized to the epidermal differentiation complex as well as the psoriasis-specific proline-rich 9 gene known as PRR9, which is induced by IL-17A. In contrast, only four genes, none of which were localized to the epidermal differentiation complex, were insufficiently normalized in the cleared lesional skin of guselkumab-treated PASI-100 responders.
“Nothing like this analysis has ever been done before,” the dermatologist observed. “It’s a pilot study. Perhaps with more data like this, we’ll be using this type of information in clinical practice to go beyond clearing patients’ skin.”
Dr. Blauvelt reported serving as a scientific advisor to and paid clinical investigator for Janssen, which sponsored the study, as well as for roughly two dozen other pharmaceutical companies.
FROM AAD 20
Study highlights potential advantages of tape strips over biopsy
for monitoring these and potentially other dermatologic diseases, according to the latest advances with this approach.
“Tape strips are not going to fully replace biopsies, but we think they will have an important role in diagnosing and monitoring response to therapy by avoiding the potential scarring and pain of biopsy,” reported Emma Guttman-Yassky, MD, PhD, professor of dermatology and director of the laboratory inflammatory skin diseases at the Icahn School of Medicine at Mount Sinai Medical Center, New York.
The concept of using adhesive strips to remove surface skin cells for clinical study has been around for more than 20 years, but there has been recent progress. A newly published study, which compared skin from patients with atopic dermatitis (AD) or psoriasis with that of controls, was characterized as “the most comprehensive tape strip molecular profiling in any inflammatory skin disease to date and the first to fully characterize and compare AD to psoriasis,” wrote Dr. Guttman-Yassky, the senior author, and coauthors.
It also appears to be a leap forward. RNA sequencing detected thousands of differentially expressed genes reflecting immune and barrier biomarkers characteristic of the molecular phenotypes of atopic dermatitis and psoriasis. These were not only found to be consistent with biopsy studies but identified additional unique genes and pathways relevant to their pathological signature.
“In the past, the success rate for transcriptome sequencing even for a more limited panel of proteins was approaching 50% when considering both lesional, nonlesional skin, and healthy skin, but we are now approaching 100% for sample recovery and for analysis of RNA and genes,” Dr. Guttman-Yassky said in an interview.
Tissue samples were obtained with tape strips from lesional and nonlesional skin from 20 patients with AD and 20 patients with psoriasis. Compared with 20 tape strips from controls, they were evaluated with RNA sequencing followed by quantitative real-time polymerase chain reaction of immune and barrier biomarkers.
The sample recovery rate was 96% overall and 95% or better regardless of whether the skin was lesional or nonlesional.
With RNA sequencing of more than 20,000 transcripts, including multiple cellular, immune, and barrier biomarkers, an enormous amount of data was generated, but the key finding is that these diseases are readily distinguished with profiling based on tape strips.
Although numerous biomarkers were shared, “tape strips completely discriminate between atopic dermatitis and psoriasis with a degree of reliability that is comparable to skin biopsy,” Dr. Guttman-Yassky said.
One of the biomarkers, expression of nitric oxide synthase 2/inducible nitric oxide synthase, distinguished AD from psoriasis with 100% accuracy. As previously reported in biopsy studies, other biomarkers collectively associated AD with a profile related to a Th2-type inflammatory response and psoriasis with a Th17-type inflammatory response.
Tape strips also confirmed significant pathology in the nonlesional as well as the lesional skin of patients with AD or psoriasis. This included an increase in Th2-type products, such as interleukin-4 and IL-13, in nonlesional skin of atopic dermatitis and Th17-type products, such as IL-17, in nonlesional skin of psoriasis.
Some biomarkers of AD and psoriasis had an even greater differentiation in tape strips than previously reported from biopsy studies, according to Dr. Guttman-Yassky. In this study, tape strips also captured more differentially expressed genes than previously reported with biopsies.
One potential limitation of tape strips is that the RNA isolation process is time consuming, but this might be less of an issue in routine clinical use if there is a more refined number of biomarkers that are targeted or if technological improvements simplify processing, Dr. Guttman-Yassky pointed out.
To develop clinical utility for tape strips beyond AD and psoriasis, more work is needed to standardize the depth of sampling, which is variable with tape strips, she noted. Depth is relevant to the analysis of gene expression and mRNA activity of each dermatologic disease.
“Tape strips remain a research tool for now, but we do think that this technique can be refined and employed for clinical purposes, including diagnosis and monitoring response to treatment,” she said.
Relative to biopsy, the advantages are not difficult to envision. Dr. Guttman-Yassky, who recently published a study of tape strips for evaluating AD in children emphasized that tape strips are generally painless.
“Patients really do not mind tape strips,” she said. Although she believes that tape strips are providing unique insight into the pathology of inflammatory diseases not necessarily available with biopsy, she emphasized the practical value. Not least, “these could really help when the goal is to evaluate response to therapy over time.”
Another investigator who has conducted studies with tape strips, Maja-Lisa Clausen, MD, PhD, also thinks tape strips are likely to become routine clinical tools.
“Once the basis research, validation, and data are out, I think numerous companies will be ready to develop machines for more quick and easy processing, compared to the more labor intensive process that is used today for research,” explained Dr. Clausen, who is in the department of dermatology, Bispebjerb Hospital, University of Copenhagen.
She considers tape strips particularly promising for children, but she thinks the biomarker profiling made possible by these strips might be leading to personalized treatment programs for dermatologic diseases.
“What we need is further validation; which tape to use, how deep, and the importance of storage, which is a big issue in the clinic,” Dr. Clausen said in an interview.
Dr. Guttman-Yassky has financial relationships with multiple pharmaceutical companies, including those with therapies for psoriasis.
SOURCE: Guttman-Yassky E et al. J Allergy Clin Immunol. 2020 Jul 9. doi: 10.1016/j.jaci.2020.05.048.
for monitoring these and potentially other dermatologic diseases, according to the latest advances with this approach.
“Tape strips are not going to fully replace biopsies, but we think they will have an important role in diagnosing and monitoring response to therapy by avoiding the potential scarring and pain of biopsy,” reported Emma Guttman-Yassky, MD, PhD, professor of dermatology and director of the laboratory inflammatory skin diseases at the Icahn School of Medicine at Mount Sinai Medical Center, New York.
The concept of using adhesive strips to remove surface skin cells for clinical study has been around for more than 20 years, but there has been recent progress. A newly published study, which compared skin from patients with atopic dermatitis (AD) or psoriasis with that of controls, was characterized as “the most comprehensive tape strip molecular profiling in any inflammatory skin disease to date and the first to fully characterize and compare AD to psoriasis,” wrote Dr. Guttman-Yassky, the senior author, and coauthors.
It also appears to be a leap forward. RNA sequencing detected thousands of differentially expressed genes reflecting immune and barrier biomarkers characteristic of the molecular phenotypes of atopic dermatitis and psoriasis. These were not only found to be consistent with biopsy studies but identified additional unique genes and pathways relevant to their pathological signature.
“In the past, the success rate for transcriptome sequencing even for a more limited panel of proteins was approaching 50% when considering both lesional, nonlesional skin, and healthy skin, but we are now approaching 100% for sample recovery and for analysis of RNA and genes,” Dr. Guttman-Yassky said in an interview.
Tissue samples were obtained with tape strips from lesional and nonlesional skin from 20 patients with AD and 20 patients with psoriasis. Compared with 20 tape strips from controls, they were evaluated with RNA sequencing followed by quantitative real-time polymerase chain reaction of immune and barrier biomarkers.
The sample recovery rate was 96% overall and 95% or better regardless of whether the skin was lesional or nonlesional.
With RNA sequencing of more than 20,000 transcripts, including multiple cellular, immune, and barrier biomarkers, an enormous amount of data was generated, but the key finding is that these diseases are readily distinguished with profiling based on tape strips.
Although numerous biomarkers were shared, “tape strips completely discriminate between atopic dermatitis and psoriasis with a degree of reliability that is comparable to skin biopsy,” Dr. Guttman-Yassky said.
One of the biomarkers, expression of nitric oxide synthase 2/inducible nitric oxide synthase, distinguished AD from psoriasis with 100% accuracy. As previously reported in biopsy studies, other biomarkers collectively associated AD with a profile related to a Th2-type inflammatory response and psoriasis with a Th17-type inflammatory response.
Tape strips also confirmed significant pathology in the nonlesional as well as the lesional skin of patients with AD or psoriasis. This included an increase in Th2-type products, such as interleukin-4 and IL-13, in nonlesional skin of atopic dermatitis and Th17-type products, such as IL-17, in nonlesional skin of psoriasis.
Some biomarkers of AD and psoriasis had an even greater differentiation in tape strips than previously reported from biopsy studies, according to Dr. Guttman-Yassky. In this study, tape strips also captured more differentially expressed genes than previously reported with biopsies.
One potential limitation of tape strips is that the RNA isolation process is time consuming, but this might be less of an issue in routine clinical use if there is a more refined number of biomarkers that are targeted or if technological improvements simplify processing, Dr. Guttman-Yassky pointed out.
To develop clinical utility for tape strips beyond AD and psoriasis, more work is needed to standardize the depth of sampling, which is variable with tape strips, she noted. Depth is relevant to the analysis of gene expression and mRNA activity of each dermatologic disease.
“Tape strips remain a research tool for now, but we do think that this technique can be refined and employed for clinical purposes, including diagnosis and monitoring response to treatment,” she said.
Relative to biopsy, the advantages are not difficult to envision. Dr. Guttman-Yassky, who recently published a study of tape strips for evaluating AD in children emphasized that tape strips are generally painless.
“Patients really do not mind tape strips,” she said. Although she believes that tape strips are providing unique insight into the pathology of inflammatory diseases not necessarily available with biopsy, she emphasized the practical value. Not least, “these could really help when the goal is to evaluate response to therapy over time.”
Another investigator who has conducted studies with tape strips, Maja-Lisa Clausen, MD, PhD, also thinks tape strips are likely to become routine clinical tools.
“Once the basis research, validation, and data are out, I think numerous companies will be ready to develop machines for more quick and easy processing, compared to the more labor intensive process that is used today for research,” explained Dr. Clausen, who is in the department of dermatology, Bispebjerb Hospital, University of Copenhagen.
She considers tape strips particularly promising for children, but she thinks the biomarker profiling made possible by these strips might be leading to personalized treatment programs for dermatologic diseases.
“What we need is further validation; which tape to use, how deep, and the importance of storage, which is a big issue in the clinic,” Dr. Clausen said in an interview.
Dr. Guttman-Yassky has financial relationships with multiple pharmaceutical companies, including those with therapies for psoriasis.
SOURCE: Guttman-Yassky E et al. J Allergy Clin Immunol. 2020 Jul 9. doi: 10.1016/j.jaci.2020.05.048.
for monitoring these and potentially other dermatologic diseases, according to the latest advances with this approach.
“Tape strips are not going to fully replace biopsies, but we think they will have an important role in diagnosing and monitoring response to therapy by avoiding the potential scarring and pain of biopsy,” reported Emma Guttman-Yassky, MD, PhD, professor of dermatology and director of the laboratory inflammatory skin diseases at the Icahn School of Medicine at Mount Sinai Medical Center, New York.
The concept of using adhesive strips to remove surface skin cells for clinical study has been around for more than 20 years, but there has been recent progress. A newly published study, which compared skin from patients with atopic dermatitis (AD) or psoriasis with that of controls, was characterized as “the most comprehensive tape strip molecular profiling in any inflammatory skin disease to date and the first to fully characterize and compare AD to psoriasis,” wrote Dr. Guttman-Yassky, the senior author, and coauthors.
It also appears to be a leap forward. RNA sequencing detected thousands of differentially expressed genes reflecting immune and barrier biomarkers characteristic of the molecular phenotypes of atopic dermatitis and psoriasis. These were not only found to be consistent with biopsy studies but identified additional unique genes and pathways relevant to their pathological signature.
“In the past, the success rate for transcriptome sequencing even for a more limited panel of proteins was approaching 50% when considering both lesional, nonlesional skin, and healthy skin, but we are now approaching 100% for sample recovery and for analysis of RNA and genes,” Dr. Guttman-Yassky said in an interview.
Tissue samples were obtained with tape strips from lesional and nonlesional skin from 20 patients with AD and 20 patients with psoriasis. Compared with 20 tape strips from controls, they were evaluated with RNA sequencing followed by quantitative real-time polymerase chain reaction of immune and barrier biomarkers.
The sample recovery rate was 96% overall and 95% or better regardless of whether the skin was lesional or nonlesional.
With RNA sequencing of more than 20,000 transcripts, including multiple cellular, immune, and barrier biomarkers, an enormous amount of data was generated, but the key finding is that these diseases are readily distinguished with profiling based on tape strips.
Although numerous biomarkers were shared, “tape strips completely discriminate between atopic dermatitis and psoriasis with a degree of reliability that is comparable to skin biopsy,” Dr. Guttman-Yassky said.
One of the biomarkers, expression of nitric oxide synthase 2/inducible nitric oxide synthase, distinguished AD from psoriasis with 100% accuracy. As previously reported in biopsy studies, other biomarkers collectively associated AD with a profile related to a Th2-type inflammatory response and psoriasis with a Th17-type inflammatory response.
Tape strips also confirmed significant pathology in the nonlesional as well as the lesional skin of patients with AD or psoriasis. This included an increase in Th2-type products, such as interleukin-4 and IL-13, in nonlesional skin of atopic dermatitis and Th17-type products, such as IL-17, in nonlesional skin of psoriasis.
Some biomarkers of AD and psoriasis had an even greater differentiation in tape strips than previously reported from biopsy studies, according to Dr. Guttman-Yassky. In this study, tape strips also captured more differentially expressed genes than previously reported with biopsies.
One potential limitation of tape strips is that the RNA isolation process is time consuming, but this might be less of an issue in routine clinical use if there is a more refined number of biomarkers that are targeted or if technological improvements simplify processing, Dr. Guttman-Yassky pointed out.
To develop clinical utility for tape strips beyond AD and psoriasis, more work is needed to standardize the depth of sampling, which is variable with tape strips, she noted. Depth is relevant to the analysis of gene expression and mRNA activity of each dermatologic disease.
“Tape strips remain a research tool for now, but we do think that this technique can be refined and employed for clinical purposes, including diagnosis and monitoring response to treatment,” she said.
Relative to biopsy, the advantages are not difficult to envision. Dr. Guttman-Yassky, who recently published a study of tape strips for evaluating AD in children emphasized that tape strips are generally painless.
“Patients really do not mind tape strips,” she said. Although she believes that tape strips are providing unique insight into the pathology of inflammatory diseases not necessarily available with biopsy, she emphasized the practical value. Not least, “these could really help when the goal is to evaluate response to therapy over time.”
Another investigator who has conducted studies with tape strips, Maja-Lisa Clausen, MD, PhD, also thinks tape strips are likely to become routine clinical tools.
“Once the basis research, validation, and data are out, I think numerous companies will be ready to develop machines for more quick and easy processing, compared to the more labor intensive process that is used today for research,” explained Dr. Clausen, who is in the department of dermatology, Bispebjerb Hospital, University of Copenhagen.
She considers tape strips particularly promising for children, but she thinks the biomarker profiling made possible by these strips might be leading to personalized treatment programs for dermatologic diseases.
“What we need is further validation; which tape to use, how deep, and the importance of storage, which is a big issue in the clinic,” Dr. Clausen said in an interview.
Dr. Guttman-Yassky has financial relationships with multiple pharmaceutical companies, including those with therapies for psoriasis.
SOURCE: Guttman-Yassky E et al. J Allergy Clin Immunol. 2020 Jul 9. doi: 10.1016/j.jaci.2020.05.048.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
Cohort study finds a twofold greater psoriasis risk linked to a PCOS diagnosis
PCOS is characterized by androgen elevation that can lead to insulin resistance and metabolic syndrome, which have also been associated with an increased risk of psoriasis. Previous retrospective analyses have suggested an increased risk of psoriasis associated with PCOS, and psoriasis patients with PCOS have been reported to have more severe skin lesions, compared with those who do not have PCOS.
“The incidence of psoriasis is indeed higher in the PCOS group than in the control group, and the comorbidities related to metabolic syndrome did not modify the adjusted hazard ratio,” said Ming-Li Chen, during her presentation of the study results at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. Dr. Chen is at Chung Shan Medical University in Taiwan.
The researchers analyzed 1 million randomly selected records from Taiwan’s Longitudinal Health Insurance database, a subset of the country’s National Health Insurance Program. Between 2000 and 2012, they identified a case group with at least three outpatient diagnoses or one inpatient diagnosis of PCOS; they then compared each with four patients who did not have PCOS who were matched by age and index year. The mean age in both groups was about 27 years.
The mean follow-up times were 6.99 years for 4,707 cases and 6.94 years for 18,828 controls. Comorbidities were slightly higher in the PCOS group, including asthma (6.7% vs. 4.9%; P less than .001), chronic obstructive pulmonary disease (14% vs. 11%; P less than .001), chronic liver disease (8.0% vs. 5.0%; P less than .001), diabetes mellitus (3.0% vs. 1.4%; P less than .001), hypertension (2.4% vs. 1.5%; P less than .001), hyperlipidemia (5.4% vs. 2.5%; P less than .001), depression (5.4% vs. 3.9%; P less than .001), and sleep apnea (0.23% vs. 0.10%; P = .040).
There was a higher cumulative incidence of psoriasis in the PCOS group (adjusted hazard ratio, 2.07; 95% confidence interval, 1.25-3.44). Other factors associated with increased risk of psoriasis were advanced age (greater than 50 years old; aHR, 14.13; 95% CI, 1.8-110.7) and having a cancer diagnosis (aHR, 11.72; 95% CI, 2.87-47.9).
When PCOS patients were stratified by age, the researchers noted a higher risk of psoriasis among those 20 years or younger (aHR, 4.02; 95% CI, 1.16-13.9) than among those aged 20-50 years (aHR, 1.88; 95% CI, 1.07-3.29). Among those older than 50 years, there was no significantly increased risk, although the number of psoriasis diagnoses and population sizes were small in the latter category. Among patients with PCOS, a cancer diagnosis was not associated with a statistically significant increased risk of psoriasis.
The mechanisms underlying the association between PCOS and psoriasis should be studied further, she noted.
Following Dr. Chen’s prerecorded presentation, there was a live discussion session led by Alice Gottlieb, MD, PhD, medical director of Mount Sinai Beth Israel Dermatology, New York, and Ennio Lubrano, MD, associate professor of rheumatology at the University of Molise (Italy). Dr. Gottlieb noted that the study did not appear to account for weight in the association between PCOS and psoriasis, since heavier people are known to be at greater risk of developing psoriasis. Dr. Chen acknowledged that the study had no records of BMI or weight.
Dr. Gottlieb also wondered if treatment of PCOS led to any improvements in psoriasis in patients with the two diagnoses. “If we treat PCOS, does the psoriasis get better?” Again, the study did not address the question. “We didn’t follow up on therapies,” Dr. Chen said.
Dr. Chen reported no relevant financial disclosures. Dr. Gottlieb is a consultant, advisory board member and/or speaker for AbbVie, Allergan, Avotres Therapeutics, Beiersdorf, Bristol-Myers Squibb, Celgene, Dermira, Eli Lilly, Incyte, Janssen, Leo, Novartis, Reddy Labs, Sun Pharmaceutical Industries, UCB Pharma and Xbiotech. She has received research or educational grants from Boehringer Ingelheim, Incyte, Janssen, Novartis and Xbiotech.
PCOS is characterized by androgen elevation that can lead to insulin resistance and metabolic syndrome, which have also been associated with an increased risk of psoriasis. Previous retrospective analyses have suggested an increased risk of psoriasis associated with PCOS, and psoriasis patients with PCOS have been reported to have more severe skin lesions, compared with those who do not have PCOS.
“The incidence of psoriasis is indeed higher in the PCOS group than in the control group, and the comorbidities related to metabolic syndrome did not modify the adjusted hazard ratio,” said Ming-Li Chen, during her presentation of the study results at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. Dr. Chen is at Chung Shan Medical University in Taiwan.
The researchers analyzed 1 million randomly selected records from Taiwan’s Longitudinal Health Insurance database, a subset of the country’s National Health Insurance Program. Between 2000 and 2012, they identified a case group with at least three outpatient diagnoses or one inpatient diagnosis of PCOS; they then compared each with four patients who did not have PCOS who were matched by age and index year. The mean age in both groups was about 27 years.
The mean follow-up times were 6.99 years for 4,707 cases and 6.94 years for 18,828 controls. Comorbidities were slightly higher in the PCOS group, including asthma (6.7% vs. 4.9%; P less than .001), chronic obstructive pulmonary disease (14% vs. 11%; P less than .001), chronic liver disease (8.0% vs. 5.0%; P less than .001), diabetes mellitus (3.0% vs. 1.4%; P less than .001), hypertension (2.4% vs. 1.5%; P less than .001), hyperlipidemia (5.4% vs. 2.5%; P less than .001), depression (5.4% vs. 3.9%; P less than .001), and sleep apnea (0.23% vs. 0.10%; P = .040).
There was a higher cumulative incidence of psoriasis in the PCOS group (adjusted hazard ratio, 2.07; 95% confidence interval, 1.25-3.44). Other factors associated with increased risk of psoriasis were advanced age (greater than 50 years old; aHR, 14.13; 95% CI, 1.8-110.7) and having a cancer diagnosis (aHR, 11.72; 95% CI, 2.87-47.9).
When PCOS patients were stratified by age, the researchers noted a higher risk of psoriasis among those 20 years or younger (aHR, 4.02; 95% CI, 1.16-13.9) than among those aged 20-50 years (aHR, 1.88; 95% CI, 1.07-3.29). Among those older than 50 years, there was no significantly increased risk, although the number of psoriasis diagnoses and population sizes were small in the latter category. Among patients with PCOS, a cancer diagnosis was not associated with a statistically significant increased risk of psoriasis.
The mechanisms underlying the association between PCOS and psoriasis should be studied further, she noted.
Following Dr. Chen’s prerecorded presentation, there was a live discussion session led by Alice Gottlieb, MD, PhD, medical director of Mount Sinai Beth Israel Dermatology, New York, and Ennio Lubrano, MD, associate professor of rheumatology at the University of Molise (Italy). Dr. Gottlieb noted that the study did not appear to account for weight in the association between PCOS and psoriasis, since heavier people are known to be at greater risk of developing psoriasis. Dr. Chen acknowledged that the study had no records of BMI or weight.
Dr. Gottlieb also wondered if treatment of PCOS led to any improvements in psoriasis in patients with the two diagnoses. “If we treat PCOS, does the psoriasis get better?” Again, the study did not address the question. “We didn’t follow up on therapies,” Dr. Chen said.
Dr. Chen reported no relevant financial disclosures. Dr. Gottlieb is a consultant, advisory board member and/or speaker for AbbVie, Allergan, Avotres Therapeutics, Beiersdorf, Bristol-Myers Squibb, Celgene, Dermira, Eli Lilly, Incyte, Janssen, Leo, Novartis, Reddy Labs, Sun Pharmaceutical Industries, UCB Pharma and Xbiotech. She has received research or educational grants from Boehringer Ingelheim, Incyte, Janssen, Novartis and Xbiotech.
PCOS is characterized by androgen elevation that can lead to insulin resistance and metabolic syndrome, which have also been associated with an increased risk of psoriasis. Previous retrospective analyses have suggested an increased risk of psoriasis associated with PCOS, and psoriasis patients with PCOS have been reported to have more severe skin lesions, compared with those who do not have PCOS.
“The incidence of psoriasis is indeed higher in the PCOS group than in the control group, and the comorbidities related to metabolic syndrome did not modify the adjusted hazard ratio,” said Ming-Li Chen, during her presentation of the study results at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. Dr. Chen is at Chung Shan Medical University in Taiwan.
The researchers analyzed 1 million randomly selected records from Taiwan’s Longitudinal Health Insurance database, a subset of the country’s National Health Insurance Program. Between 2000 and 2012, they identified a case group with at least three outpatient diagnoses or one inpatient diagnosis of PCOS; they then compared each with four patients who did not have PCOS who were matched by age and index year. The mean age in both groups was about 27 years.
The mean follow-up times were 6.99 years for 4,707 cases and 6.94 years for 18,828 controls. Comorbidities were slightly higher in the PCOS group, including asthma (6.7% vs. 4.9%; P less than .001), chronic obstructive pulmonary disease (14% vs. 11%; P less than .001), chronic liver disease (8.0% vs. 5.0%; P less than .001), diabetes mellitus (3.0% vs. 1.4%; P less than .001), hypertension (2.4% vs. 1.5%; P less than .001), hyperlipidemia (5.4% vs. 2.5%; P less than .001), depression (5.4% vs. 3.9%; P less than .001), and sleep apnea (0.23% vs. 0.10%; P = .040).
There was a higher cumulative incidence of psoriasis in the PCOS group (adjusted hazard ratio, 2.07; 95% confidence interval, 1.25-3.44). Other factors associated with increased risk of psoriasis were advanced age (greater than 50 years old; aHR, 14.13; 95% CI, 1.8-110.7) and having a cancer diagnosis (aHR, 11.72; 95% CI, 2.87-47.9).
When PCOS patients were stratified by age, the researchers noted a higher risk of psoriasis among those 20 years or younger (aHR, 4.02; 95% CI, 1.16-13.9) than among those aged 20-50 years (aHR, 1.88; 95% CI, 1.07-3.29). Among those older than 50 years, there was no significantly increased risk, although the number of psoriasis diagnoses and population sizes were small in the latter category. Among patients with PCOS, a cancer diagnosis was not associated with a statistically significant increased risk of psoriasis.
The mechanisms underlying the association between PCOS and psoriasis should be studied further, she noted.
Following Dr. Chen’s prerecorded presentation, there was a live discussion session led by Alice Gottlieb, MD, PhD, medical director of Mount Sinai Beth Israel Dermatology, New York, and Ennio Lubrano, MD, associate professor of rheumatology at the University of Molise (Italy). Dr. Gottlieb noted that the study did not appear to account for weight in the association between PCOS and psoriasis, since heavier people are known to be at greater risk of developing psoriasis. Dr. Chen acknowledged that the study had no records of BMI or weight.
Dr. Gottlieb also wondered if treatment of PCOS led to any improvements in psoriasis in patients with the two diagnoses. “If we treat PCOS, does the psoriasis get better?” Again, the study did not address the question. “We didn’t follow up on therapies,” Dr. Chen said.
Dr. Chen reported no relevant financial disclosures. Dr. Gottlieb is a consultant, advisory board member and/or speaker for AbbVie, Allergan, Avotres Therapeutics, Beiersdorf, Bristol-Myers Squibb, Celgene, Dermira, Eli Lilly, Incyte, Janssen, Leo, Novartis, Reddy Labs, Sun Pharmaceutical Industries, UCB Pharma and Xbiotech. She has received research or educational grants from Boehringer Ingelheim, Incyte, Janssen, Novartis and Xbiotech.
FROM GRAPPA 2020 VIRTUAL ANNUAL MEETING