User login
Caution is key when pregnancy and psoriasis mix
NEWPORT BEACH, CALIF. – Psoriasis often clears in pregnant women, giving them a rare break from the skin disease. But
Data from 2011 found 45% of pregnancies in U.S. women aged 15-44 years were unintended (N Engl J Med. 2016 Mar 3;374[9]:843-52), cautioned Jashin J. Wu, MD, of Dermatology Research and Education Foundation, Irvine, Calif.
In a presentation at the Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar, Dr. Wu offered these tips about pregnancy and psoriasis:
Counsel patients before pregnancy
There’s conflicting data about the risks of psoriasis in pregnancy, Dr. Wu said. One 23-year-old study suggests a link to adverse outcomes such as preterm and low-birth-weight babies. But another more recent study found no sign of increased risk (Int J Dermatol. 1996;35:169-72; J Am Acad Dermatol. 2011;64:71-7).
Counseling can include information about risks such as hospitalization during pregnancy because of undertreatment of psoriasis, he said. Discuss lowering medication doses to the lowest effective dose, he recommended, and talk about alternatives to systemic medications.
Make adjustments to timing as needed
In patients with severe cases, it may be appropriate to recommend that they postpone pregnancy until their psoriasis is under better control. As for treatment of psoriasis, “you may want to consider timing medication to end around the first trimester to get the medication out of them during the greatest risk period for the baby,” Dr. Wu said.
Adjust steroids as necessary
There are no “good” studies about the use of steroids in pregnant women with psoriasis, Dr. Wu said. “We can probably assume they are safe overall. Weaker steroids may have less risk,” and some of the stronger steroids may raise concerns.
Dr. Wu made these recommendations: Limit mild-potency topical corticosteroids to less than 100 g/week, potent topical corticosteroids to less than 50 g/week, and superpotent topical corticosteroids to less than 30 g/week.
Some topical drugs appear to be OK
Vitamin D analogues have not been well-studied in pregnancy, he said, but “we consider topical use to be fairly safe.”
There’s no data on calcineurin inhibitors in pregnancy, he said, but topical use is considered to be safe because there’s limited systemic absorption.
Beware of certain drugs in pregnancyTazarotene is considered to be dangerous in pregnancy, Dr. Wu said, and females of childbearing age who take it should use effective contraception, and have a recent negative pregnancy test (within 2 weeks before treatment begins). “In general, I’d probably not use this,” he said. “We have so many other options.”
Data about pregnancy safety for three topical drugs – coal tar, anthralin, and salicylic acid – is limited or nonexistent, Dr. Wu said, and he recommends against their use in pregnancy.
Phototherapy is OK in pregnancy
Phototherapy is considered safe because UVB doesn’t penetrate the superficial layer of the skin, he said. But phototherapy brings a potential risk of lowered folic acid levels, and he urges folic acid supplementation in women undergoing the treatment who are considering pregnancy or who are in the first trimester.
Avoid certain systemic drugs
Dr. Wu offered these recommendations:
- Methotrexate: Do not take during pregnancy, or 3 months prior to conception.
- Acitretin (Soriatane): Avoid all use in women who may become pregnant.
- Cyclosporine: Be aware of reports of prematurity and low birth weight linked to the drug.
- Apremilast (Otezla): Animal studies have shown a risk in pregnancy. Stop the drug at least 2 days before conception.
Avoid monoclonal antibodies
These drugs “result in therapeutic levels in the fetus, which is not a good thing,” Dr. Wu said. “You obviously don’t want to have monoclonal antibodies in the baby.”
Nix the PUVA
While one study found no link between psoralen plus UVA (PUVA) and birth defects (Arch Dermatol. 1993 Mar;129[3]:320-3), there’s still a theoretical risk, Dr. Wu said. He recommended that the treatment be avoided during pregnancy.
Watch for waxing and waning
Dr. Wu pointed to a small 2005 study that suggested that psoriasis activity declines during pregnancy. The study used different measures, finding that psoriasis improved by 30% (based on at least a 3% change in body surface area) or 55% (based on patient self-reporting). But it flares after pregnancy as reported by 65% of women surveyed; a body surface area analysis found that psoriasis worsened in 41% (Arch Dermatol. 2005 May;141[5]:601-6).
Dr. Wu reports various relationships (research, consultation and speaking) with 15 pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
NEWPORT BEACH, CALIF. – Psoriasis often clears in pregnant women, giving them a rare break from the skin disease. But
Data from 2011 found 45% of pregnancies in U.S. women aged 15-44 years were unintended (N Engl J Med. 2016 Mar 3;374[9]:843-52), cautioned Jashin J. Wu, MD, of Dermatology Research and Education Foundation, Irvine, Calif.
In a presentation at the Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar, Dr. Wu offered these tips about pregnancy and psoriasis:
Counsel patients before pregnancy
There’s conflicting data about the risks of psoriasis in pregnancy, Dr. Wu said. One 23-year-old study suggests a link to adverse outcomes such as preterm and low-birth-weight babies. But another more recent study found no sign of increased risk (Int J Dermatol. 1996;35:169-72; J Am Acad Dermatol. 2011;64:71-7).
Counseling can include information about risks such as hospitalization during pregnancy because of undertreatment of psoriasis, he said. Discuss lowering medication doses to the lowest effective dose, he recommended, and talk about alternatives to systemic medications.
Make adjustments to timing as needed
In patients with severe cases, it may be appropriate to recommend that they postpone pregnancy until their psoriasis is under better control. As for treatment of psoriasis, “you may want to consider timing medication to end around the first trimester to get the medication out of them during the greatest risk period for the baby,” Dr. Wu said.
Adjust steroids as necessary
There are no “good” studies about the use of steroids in pregnant women with psoriasis, Dr. Wu said. “We can probably assume they are safe overall. Weaker steroids may have less risk,” and some of the stronger steroids may raise concerns.
Dr. Wu made these recommendations: Limit mild-potency topical corticosteroids to less than 100 g/week, potent topical corticosteroids to less than 50 g/week, and superpotent topical corticosteroids to less than 30 g/week.
Some topical drugs appear to be OK
Vitamin D analogues have not been well-studied in pregnancy, he said, but “we consider topical use to be fairly safe.”
There’s no data on calcineurin inhibitors in pregnancy, he said, but topical use is considered to be safe because there’s limited systemic absorption.
Beware of certain drugs in pregnancyTazarotene is considered to be dangerous in pregnancy, Dr. Wu said, and females of childbearing age who take it should use effective contraception, and have a recent negative pregnancy test (within 2 weeks before treatment begins). “In general, I’d probably not use this,” he said. “We have so many other options.”
Data about pregnancy safety for three topical drugs – coal tar, anthralin, and salicylic acid – is limited or nonexistent, Dr. Wu said, and he recommends against their use in pregnancy.
Phototherapy is OK in pregnancy
Phototherapy is considered safe because UVB doesn’t penetrate the superficial layer of the skin, he said. But phototherapy brings a potential risk of lowered folic acid levels, and he urges folic acid supplementation in women undergoing the treatment who are considering pregnancy or who are in the first trimester.
Avoid certain systemic drugs
Dr. Wu offered these recommendations:
- Methotrexate: Do not take during pregnancy, or 3 months prior to conception.
- Acitretin (Soriatane): Avoid all use in women who may become pregnant.
- Cyclosporine: Be aware of reports of prematurity and low birth weight linked to the drug.
- Apremilast (Otezla): Animal studies have shown a risk in pregnancy. Stop the drug at least 2 days before conception.
Avoid monoclonal antibodies
These drugs “result in therapeutic levels in the fetus, which is not a good thing,” Dr. Wu said. “You obviously don’t want to have monoclonal antibodies in the baby.”
Nix the PUVA
While one study found no link between psoralen plus UVA (PUVA) and birth defects (Arch Dermatol. 1993 Mar;129[3]:320-3), there’s still a theoretical risk, Dr. Wu said. He recommended that the treatment be avoided during pregnancy.
Watch for waxing and waning
Dr. Wu pointed to a small 2005 study that suggested that psoriasis activity declines during pregnancy. The study used different measures, finding that psoriasis improved by 30% (based on at least a 3% change in body surface area) or 55% (based on patient self-reporting). But it flares after pregnancy as reported by 65% of women surveyed; a body surface area analysis found that psoriasis worsened in 41% (Arch Dermatol. 2005 May;141[5]:601-6).
Dr. Wu reports various relationships (research, consultation and speaking) with 15 pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
NEWPORT BEACH, CALIF. – Psoriasis often clears in pregnant women, giving them a rare break from the skin disease. But
Data from 2011 found 45% of pregnancies in U.S. women aged 15-44 years were unintended (N Engl J Med. 2016 Mar 3;374[9]:843-52), cautioned Jashin J. Wu, MD, of Dermatology Research and Education Foundation, Irvine, Calif.
In a presentation at the Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar, Dr. Wu offered these tips about pregnancy and psoriasis:
Counsel patients before pregnancy
There’s conflicting data about the risks of psoriasis in pregnancy, Dr. Wu said. One 23-year-old study suggests a link to adverse outcomes such as preterm and low-birth-weight babies. But another more recent study found no sign of increased risk (Int J Dermatol. 1996;35:169-72; J Am Acad Dermatol. 2011;64:71-7).
Counseling can include information about risks such as hospitalization during pregnancy because of undertreatment of psoriasis, he said. Discuss lowering medication doses to the lowest effective dose, he recommended, and talk about alternatives to systemic medications.
Make adjustments to timing as needed
In patients with severe cases, it may be appropriate to recommend that they postpone pregnancy until their psoriasis is under better control. As for treatment of psoriasis, “you may want to consider timing medication to end around the first trimester to get the medication out of them during the greatest risk period for the baby,” Dr. Wu said.
Adjust steroids as necessary
There are no “good” studies about the use of steroids in pregnant women with psoriasis, Dr. Wu said. “We can probably assume they are safe overall. Weaker steroids may have less risk,” and some of the stronger steroids may raise concerns.
Dr. Wu made these recommendations: Limit mild-potency topical corticosteroids to less than 100 g/week, potent topical corticosteroids to less than 50 g/week, and superpotent topical corticosteroids to less than 30 g/week.
Some topical drugs appear to be OK
Vitamin D analogues have not been well-studied in pregnancy, he said, but “we consider topical use to be fairly safe.”
There’s no data on calcineurin inhibitors in pregnancy, he said, but topical use is considered to be safe because there’s limited systemic absorption.
Beware of certain drugs in pregnancyTazarotene is considered to be dangerous in pregnancy, Dr. Wu said, and females of childbearing age who take it should use effective contraception, and have a recent negative pregnancy test (within 2 weeks before treatment begins). “In general, I’d probably not use this,” he said. “We have so many other options.”
Data about pregnancy safety for three topical drugs – coal tar, anthralin, and salicylic acid – is limited or nonexistent, Dr. Wu said, and he recommends against their use in pregnancy.
Phototherapy is OK in pregnancy
Phototherapy is considered safe because UVB doesn’t penetrate the superficial layer of the skin, he said. But phototherapy brings a potential risk of lowered folic acid levels, and he urges folic acid supplementation in women undergoing the treatment who are considering pregnancy or who are in the first trimester.
Avoid certain systemic drugs
Dr. Wu offered these recommendations:
- Methotrexate: Do not take during pregnancy, or 3 months prior to conception.
- Acitretin (Soriatane): Avoid all use in women who may become pregnant.
- Cyclosporine: Be aware of reports of prematurity and low birth weight linked to the drug.
- Apremilast (Otezla): Animal studies have shown a risk in pregnancy. Stop the drug at least 2 days before conception.
Avoid monoclonal antibodies
These drugs “result in therapeutic levels in the fetus, which is not a good thing,” Dr. Wu said. “You obviously don’t want to have monoclonal antibodies in the baby.”
Nix the PUVA
While one study found no link between psoralen plus UVA (PUVA) and birth defects (Arch Dermatol. 1993 Mar;129[3]:320-3), there’s still a theoretical risk, Dr. Wu said. He recommended that the treatment be avoided during pregnancy.
Watch for waxing and waning
Dr. Wu pointed to a small 2005 study that suggested that psoriasis activity declines during pregnancy. The study used different measures, finding that psoriasis improved by 30% (based on at least a 3% change in body surface area) or 55% (based on patient self-reporting). But it flares after pregnancy as reported by 65% of women surveyed; a body surface area analysis found that psoriasis worsened in 41% (Arch Dermatol. 2005 May;141[5]:601-6).
Dr. Wu reports various relationships (research, consultation and speaking) with 15 pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
In psoriasis, risankizumab outperforms adalimumab
Results of the IMMvent trial were published online ahead of print July 4 in the Lancet.
Risankizumab targets the p19 subunit of the cytokine IL-23. Selectivity for p19 has the potential to be safer than some other approaches that target the p40 subunit, because p19 is specific to IL-23, and many immune defense processes can function without IL-23. The p40 subunit is shared with IL-12, and blocking it can therefore lead to off-target effects.
Risankizumab was previously shown to have superior safety and efficacy over ustekinumab, which inhibits a subunit shared by IL-23 and IL-12 (Gordon KB et al. Lancet. 2018;392[10148]:650-61). Adalimumab is a TNF-alpha inhibitor that is frequently used to treat psoriasis, and which became available in biosimilar form in Europe in 2018.
The researchers randomized 605 adult patients from 66 sites in 11 countries to receive either risankizumab or adalimumab. The first phase (Part A) of the trial lasted up to 16 weeks, and tested the general superiority of risankizumab over adalimumab. The second phase (Part B), from week 16 to 44, evaluated the efficacy of risankizumab in participants who experienced an intermediate response, defined as Psoriasis Area and Severity Index (PASI) score of 50-90.
At the start of Part B, subjects initially receiving adalimumab who had at least a 90% improvement in PASI stayed on adalimumab (PASI 90), while those who had less than 50% improvement in PASI were switched to risankizumab. The remaining intermediate responders (PASI 50-90) were re-randomized to continue adalimumab or switch to risankizumab. All subjects initially randomized to risankizumab continued risankizumab during part B.
At the end of Part A, 72% of the risankizumab group achieved PASI 90, compared with 47% in the adalimumab group (p < 0.0001). A total of 84% in the risankizumab group had a static Physician’s Global Assessment (sPGA) score of 0 or 1 at the end of Part A, compared with 60% in the adalimumab group (p < 0.0001).
During Part B, among intermediate adalimumab responders, 66% of those switched to risankizumab achieved PASI 90, compared with 21% of continued on adalimumab (p < 0.0001).
In Part A, 56% of patients taking risankizumab experienced an adverse event, as did 57% of those taking adalimumab. Among adalimumab intermediate responders, 75% of those who switched to risankizumab during Part B had an adverse event, compared with 66% of those who continued adalimumab.
SOURCE: Reich K, et al. Lancet 2019, July 4 .
Until recently, TNF-alpha inhibitors have been the most commonly prescribed biologic agents for psoriasis. They are more targeted than small molecules like cyclosporine or methotrexate, but still are associated with immune side effects like infection and malignancy. Drugs that specifically target IL-23 home in on the pathogenicity of psoriasis, and they are not associated with infection and malignancy. The results of this study offer evidence that IL-23 inhibitors represent another effective and convenient option for the treatment of psoriasis. Physicians can select from IL-23 inhibitors, IL-17 inhibitors, and TNF-alpha inhibitors to determine the optimal treatment for patients based on patient weight, childbearing status, age, and comorbid conditions.
Mark Lebwohl, MD, is in the department of dermatology at Icahn School of Medicine at Mount Sinai, New York.
Until recently, TNF-alpha inhibitors have been the most commonly prescribed biologic agents for psoriasis. They are more targeted than small molecules like cyclosporine or methotrexate, but still are associated with immune side effects like infection and malignancy. Drugs that specifically target IL-23 home in on the pathogenicity of psoriasis, and they are not associated with infection and malignancy. The results of this study offer evidence that IL-23 inhibitors represent another effective and convenient option for the treatment of psoriasis. Physicians can select from IL-23 inhibitors, IL-17 inhibitors, and TNF-alpha inhibitors to determine the optimal treatment for patients based on patient weight, childbearing status, age, and comorbid conditions.
Mark Lebwohl, MD, is in the department of dermatology at Icahn School of Medicine at Mount Sinai, New York.
Until recently, TNF-alpha inhibitors have been the most commonly prescribed biologic agents for psoriasis. They are more targeted than small molecules like cyclosporine or methotrexate, but still are associated with immune side effects like infection and malignancy. Drugs that specifically target IL-23 home in on the pathogenicity of psoriasis, and they are not associated with infection and malignancy. The results of this study offer evidence that IL-23 inhibitors represent another effective and convenient option for the treatment of psoriasis. Physicians can select from IL-23 inhibitors, IL-17 inhibitors, and TNF-alpha inhibitors to determine the optimal treatment for patients based on patient weight, childbearing status, age, and comorbid conditions.
Mark Lebwohl, MD, is in the department of dermatology at Icahn School of Medicine at Mount Sinai, New York.
Results of the IMMvent trial were published online ahead of print July 4 in the Lancet.
Risankizumab targets the p19 subunit of the cytokine IL-23. Selectivity for p19 has the potential to be safer than some other approaches that target the p40 subunit, because p19 is specific to IL-23, and many immune defense processes can function without IL-23. The p40 subunit is shared with IL-12, and blocking it can therefore lead to off-target effects.
Risankizumab was previously shown to have superior safety and efficacy over ustekinumab, which inhibits a subunit shared by IL-23 and IL-12 (Gordon KB et al. Lancet. 2018;392[10148]:650-61). Adalimumab is a TNF-alpha inhibitor that is frequently used to treat psoriasis, and which became available in biosimilar form in Europe in 2018.
The researchers randomized 605 adult patients from 66 sites in 11 countries to receive either risankizumab or adalimumab. The first phase (Part A) of the trial lasted up to 16 weeks, and tested the general superiority of risankizumab over adalimumab. The second phase (Part B), from week 16 to 44, evaluated the efficacy of risankizumab in participants who experienced an intermediate response, defined as Psoriasis Area and Severity Index (PASI) score of 50-90.
At the start of Part B, subjects initially receiving adalimumab who had at least a 90% improvement in PASI stayed on adalimumab (PASI 90), while those who had less than 50% improvement in PASI were switched to risankizumab. The remaining intermediate responders (PASI 50-90) were re-randomized to continue adalimumab or switch to risankizumab. All subjects initially randomized to risankizumab continued risankizumab during part B.
At the end of Part A, 72% of the risankizumab group achieved PASI 90, compared with 47% in the adalimumab group (p < 0.0001). A total of 84% in the risankizumab group had a static Physician’s Global Assessment (sPGA) score of 0 or 1 at the end of Part A, compared with 60% in the adalimumab group (p < 0.0001).
During Part B, among intermediate adalimumab responders, 66% of those switched to risankizumab achieved PASI 90, compared with 21% of continued on adalimumab (p < 0.0001).
In Part A, 56% of patients taking risankizumab experienced an adverse event, as did 57% of those taking adalimumab. Among adalimumab intermediate responders, 75% of those who switched to risankizumab during Part B had an adverse event, compared with 66% of those who continued adalimumab.
SOURCE: Reich K, et al. Lancet 2019, July 4 .
Results of the IMMvent trial were published online ahead of print July 4 in the Lancet.
Risankizumab targets the p19 subunit of the cytokine IL-23. Selectivity for p19 has the potential to be safer than some other approaches that target the p40 subunit, because p19 is specific to IL-23, and many immune defense processes can function without IL-23. The p40 subunit is shared with IL-12, and blocking it can therefore lead to off-target effects.
Risankizumab was previously shown to have superior safety and efficacy over ustekinumab, which inhibits a subunit shared by IL-23 and IL-12 (Gordon KB et al. Lancet. 2018;392[10148]:650-61). Adalimumab is a TNF-alpha inhibitor that is frequently used to treat psoriasis, and which became available in biosimilar form in Europe in 2018.
The researchers randomized 605 adult patients from 66 sites in 11 countries to receive either risankizumab or adalimumab. The first phase (Part A) of the trial lasted up to 16 weeks, and tested the general superiority of risankizumab over adalimumab. The second phase (Part B), from week 16 to 44, evaluated the efficacy of risankizumab in participants who experienced an intermediate response, defined as Psoriasis Area and Severity Index (PASI) score of 50-90.
At the start of Part B, subjects initially receiving adalimumab who had at least a 90% improvement in PASI stayed on adalimumab (PASI 90), while those who had less than 50% improvement in PASI were switched to risankizumab. The remaining intermediate responders (PASI 50-90) were re-randomized to continue adalimumab or switch to risankizumab. All subjects initially randomized to risankizumab continued risankizumab during part B.
At the end of Part A, 72% of the risankizumab group achieved PASI 90, compared with 47% in the adalimumab group (p < 0.0001). A total of 84% in the risankizumab group had a static Physician’s Global Assessment (sPGA) score of 0 or 1 at the end of Part A, compared with 60% in the adalimumab group (p < 0.0001).
During Part B, among intermediate adalimumab responders, 66% of those switched to risankizumab achieved PASI 90, compared with 21% of continued on adalimumab (p < 0.0001).
In Part A, 56% of patients taking risankizumab experienced an adverse event, as did 57% of those taking adalimumab. Among adalimumab intermediate responders, 75% of those who switched to risankizumab during Part B had an adverse event, compared with 66% of those who continued adalimumab.
SOURCE: Reich K, et al. Lancet 2019, July 4 .
FROM THE LANCET
Psoriasis Treatment in Patients With HIV
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
Novel topical psoriasis treatment targets nerve pathways
MILAN – A novel topical nonsteroidal treatment for psoriasis showed sufficient efficacy in phase 2b clinical trials to proceed to phase 3 studies, with improvements in severity, pain, and burning in adults with mild to moderate psoriasis.
At the end of 12 weeks of treatment, 29% of patients receiving the medication – which targets nerve pathways – experienced a decrease of at least 2 grades on the 5-point Investigator’s Global Assessment (IGA) scale, compared with 13% of those receiving the topical vehicle only (P = .036). A similar proportion of patients achieved 75% improvement on the Psoriasis Area and Severity Index (PASI-75)compared with those on vehicle alone (27% versus 13%; P = .045).
, said Paul F. Lizzul, MD, PhD, presenting the findings during a late-breaking abstract session at the World Congress of Dermatology.
Pruritus severity also dropped by about 60%, but the decrease did not differ significantly from the change seen with vehicle alone, said Dr. Lizzul, chief medical officer for Sienna Biopharmaceuticals, Westlake Village, Calif., which funded the study. He and his coinvestigators found this “interesting, surprising, and different from what we had seen previously,” he said. “We think a few things happened here,” including intensive querying on itch by means of daily diaries, a different approach than had been taken in the investigator’s earlier SNA-120 trials. “We think in this way we probably biased patients’ expectations, altering reporting on this subjective measure,” he added.
“There’s been really a lack of innovation in the topical world in developing nonsteroidal therapies for the majority of patients who are treated with topicals, said Dr. Lizzul. Keratinocytes within psoriatic plaques are known to have elevated levels of nerve growth factor (NGF), he explained. Together with tropomyosin receptor kinase A (TrkA), NGF is implicated in the pathogenesis of psoriasis; it stimulates keratinocyte hyperproliferation, is a factor in neurogenic inflammation, and contributes to pruritus. Upregulation of TrkA expression is seen in nerve fibers within pruritic psoriasis plaques as well, said Dr. Lizzul, senior author of the study. (The first author was Kristina Callis Duffin, MD, cochair of the dermatology department at the University of Utah, Salt Lake City.)
In fact, the pruritus that plagues many psoriasis patients, said Dr. Lizzul, may “serve as a clinical biomarker for elevated NGF/TrkA expression.” And certain clinical phenomena observed in psoriasis, such as the Koebner phenomenon and plaque resolution along the path of damaged nerves, provide other clues. “Clearly, astute clinicians going back many, many years have recognized the very important role that nerves and neuropeptides play in psoriasis,” he added.
SNA-120 targets NGF TrKA activity, and “achieves high local drug concentration in the skin, with low systemic availability,” he said.
The randomized, double-blind, vehicle-controlled study enrolled 208 adults with mild to moderate psoriasis (scores of 2 or 3 on the IGA), with pruritus of at least moderate intensity (5 or higher on a 10-point itch numeric rating scale, or I-NRS). The mean age of the patients was 50 years, and about half were male. Most (84%-90% across study arms) were white. At baseline, the mean I-NRS was 7.3-7.4, and the mean PASI score at baseline ranged from 5.9 to 6.5.
Patients were randomized to receive SNA-120 twice daily at either 0.05% (70 patients) or 0.5% (69 patients) in an ointment formulation, or vehicle alone twice daily (69 patients). Efficacy was tracked by measuring decrease in IGA by one or two grades, the number of patients achieving PASI-50 and PASI-75, reduction in itch, and a composite of a decrease of at least 2 grades on the IGA and having clear or almost clear skin.
The investigators also tracked reduction in burning and pain as measured on a 10-point numeric rating scale. Though itch scores didn’t differ significantly from reductions seen with the topical vehicle alone, pain and burning were both reduced significantly compared with vehicle by week 12 of the study (P = .033 for pain; P = .043 for burning).
All improvements were seen only with the lower dose, not the 0.5% dose of SNA-120, noted Dr. Lizzul, adding: “This is not necessarily surprising in the world of kinase inhibitors, where you can see these J-shaped or inverse dose-response curves.”
In addition to recording adverse events, the researchers assessed safety by obtaining laboratory values and electrocardiograms. Plasma SNA-120 levels at study weeks 2, 4, and 8 were obtained for pharmacokinetic analysis. Systemic uptake was virtually nil, and the safety profile overall was good, said Dr. Lizzul.
Next steps are phase 3 clinical trials that will evaluate global improvement as well as pain, burning, and itch in psoriasis, he noted.
Dr. Lizzul is an employee of Sienna Biopharmaceuticals, which is developing SNA-120.
MILAN – A novel topical nonsteroidal treatment for psoriasis showed sufficient efficacy in phase 2b clinical trials to proceed to phase 3 studies, with improvements in severity, pain, and burning in adults with mild to moderate psoriasis.
At the end of 12 weeks of treatment, 29% of patients receiving the medication – which targets nerve pathways – experienced a decrease of at least 2 grades on the 5-point Investigator’s Global Assessment (IGA) scale, compared with 13% of those receiving the topical vehicle only (P = .036). A similar proportion of patients achieved 75% improvement on the Psoriasis Area and Severity Index (PASI-75)compared with those on vehicle alone (27% versus 13%; P = .045).
, said Paul F. Lizzul, MD, PhD, presenting the findings during a late-breaking abstract session at the World Congress of Dermatology.
Pruritus severity also dropped by about 60%, but the decrease did not differ significantly from the change seen with vehicle alone, said Dr. Lizzul, chief medical officer for Sienna Biopharmaceuticals, Westlake Village, Calif., which funded the study. He and his coinvestigators found this “interesting, surprising, and different from what we had seen previously,” he said. “We think a few things happened here,” including intensive querying on itch by means of daily diaries, a different approach than had been taken in the investigator’s earlier SNA-120 trials. “We think in this way we probably biased patients’ expectations, altering reporting on this subjective measure,” he added.
“There’s been really a lack of innovation in the topical world in developing nonsteroidal therapies for the majority of patients who are treated with topicals, said Dr. Lizzul. Keratinocytes within psoriatic plaques are known to have elevated levels of nerve growth factor (NGF), he explained. Together with tropomyosin receptor kinase A (TrkA), NGF is implicated in the pathogenesis of psoriasis; it stimulates keratinocyte hyperproliferation, is a factor in neurogenic inflammation, and contributes to pruritus. Upregulation of TrkA expression is seen in nerve fibers within pruritic psoriasis plaques as well, said Dr. Lizzul, senior author of the study. (The first author was Kristina Callis Duffin, MD, cochair of the dermatology department at the University of Utah, Salt Lake City.)
In fact, the pruritus that plagues many psoriasis patients, said Dr. Lizzul, may “serve as a clinical biomarker for elevated NGF/TrkA expression.” And certain clinical phenomena observed in psoriasis, such as the Koebner phenomenon and plaque resolution along the path of damaged nerves, provide other clues. “Clearly, astute clinicians going back many, many years have recognized the very important role that nerves and neuropeptides play in psoriasis,” he added.
SNA-120 targets NGF TrKA activity, and “achieves high local drug concentration in the skin, with low systemic availability,” he said.
The randomized, double-blind, vehicle-controlled study enrolled 208 adults with mild to moderate psoriasis (scores of 2 or 3 on the IGA), with pruritus of at least moderate intensity (5 or higher on a 10-point itch numeric rating scale, or I-NRS). The mean age of the patients was 50 years, and about half were male. Most (84%-90% across study arms) were white. At baseline, the mean I-NRS was 7.3-7.4, and the mean PASI score at baseline ranged from 5.9 to 6.5.
Patients were randomized to receive SNA-120 twice daily at either 0.05% (70 patients) or 0.5% (69 patients) in an ointment formulation, or vehicle alone twice daily (69 patients). Efficacy was tracked by measuring decrease in IGA by one or two grades, the number of patients achieving PASI-50 and PASI-75, reduction in itch, and a composite of a decrease of at least 2 grades on the IGA and having clear or almost clear skin.
The investigators also tracked reduction in burning and pain as measured on a 10-point numeric rating scale. Though itch scores didn’t differ significantly from reductions seen with the topical vehicle alone, pain and burning were both reduced significantly compared with vehicle by week 12 of the study (P = .033 for pain; P = .043 for burning).
All improvements were seen only with the lower dose, not the 0.5% dose of SNA-120, noted Dr. Lizzul, adding: “This is not necessarily surprising in the world of kinase inhibitors, where you can see these J-shaped or inverse dose-response curves.”
In addition to recording adverse events, the researchers assessed safety by obtaining laboratory values and electrocardiograms. Plasma SNA-120 levels at study weeks 2, 4, and 8 were obtained for pharmacokinetic analysis. Systemic uptake was virtually nil, and the safety profile overall was good, said Dr. Lizzul.
Next steps are phase 3 clinical trials that will evaluate global improvement as well as pain, burning, and itch in psoriasis, he noted.
Dr. Lizzul is an employee of Sienna Biopharmaceuticals, which is developing SNA-120.
MILAN – A novel topical nonsteroidal treatment for psoriasis showed sufficient efficacy in phase 2b clinical trials to proceed to phase 3 studies, with improvements in severity, pain, and burning in adults with mild to moderate psoriasis.
At the end of 12 weeks of treatment, 29% of patients receiving the medication – which targets nerve pathways – experienced a decrease of at least 2 grades on the 5-point Investigator’s Global Assessment (IGA) scale, compared with 13% of those receiving the topical vehicle only (P = .036). A similar proportion of patients achieved 75% improvement on the Psoriasis Area and Severity Index (PASI-75)compared with those on vehicle alone (27% versus 13%; P = .045).
, said Paul F. Lizzul, MD, PhD, presenting the findings during a late-breaking abstract session at the World Congress of Dermatology.
Pruritus severity also dropped by about 60%, but the decrease did not differ significantly from the change seen with vehicle alone, said Dr. Lizzul, chief medical officer for Sienna Biopharmaceuticals, Westlake Village, Calif., which funded the study. He and his coinvestigators found this “interesting, surprising, and different from what we had seen previously,” he said. “We think a few things happened here,” including intensive querying on itch by means of daily diaries, a different approach than had been taken in the investigator’s earlier SNA-120 trials. “We think in this way we probably biased patients’ expectations, altering reporting on this subjective measure,” he added.
“There’s been really a lack of innovation in the topical world in developing nonsteroidal therapies for the majority of patients who are treated with topicals, said Dr. Lizzul. Keratinocytes within psoriatic plaques are known to have elevated levels of nerve growth factor (NGF), he explained. Together with tropomyosin receptor kinase A (TrkA), NGF is implicated in the pathogenesis of psoriasis; it stimulates keratinocyte hyperproliferation, is a factor in neurogenic inflammation, and contributes to pruritus. Upregulation of TrkA expression is seen in nerve fibers within pruritic psoriasis plaques as well, said Dr. Lizzul, senior author of the study. (The first author was Kristina Callis Duffin, MD, cochair of the dermatology department at the University of Utah, Salt Lake City.)
In fact, the pruritus that plagues many psoriasis patients, said Dr. Lizzul, may “serve as a clinical biomarker for elevated NGF/TrkA expression.” And certain clinical phenomena observed in psoriasis, such as the Koebner phenomenon and plaque resolution along the path of damaged nerves, provide other clues. “Clearly, astute clinicians going back many, many years have recognized the very important role that nerves and neuropeptides play in psoriasis,” he added.
SNA-120 targets NGF TrKA activity, and “achieves high local drug concentration in the skin, with low systemic availability,” he said.
The randomized, double-blind, vehicle-controlled study enrolled 208 adults with mild to moderate psoriasis (scores of 2 or 3 on the IGA), with pruritus of at least moderate intensity (5 or higher on a 10-point itch numeric rating scale, or I-NRS). The mean age of the patients was 50 years, and about half were male. Most (84%-90% across study arms) were white. At baseline, the mean I-NRS was 7.3-7.4, and the mean PASI score at baseline ranged from 5.9 to 6.5.
Patients were randomized to receive SNA-120 twice daily at either 0.05% (70 patients) or 0.5% (69 patients) in an ointment formulation, or vehicle alone twice daily (69 patients). Efficacy was tracked by measuring decrease in IGA by one or two grades, the number of patients achieving PASI-50 and PASI-75, reduction in itch, and a composite of a decrease of at least 2 grades on the IGA and having clear or almost clear skin.
The investigators also tracked reduction in burning and pain as measured on a 10-point numeric rating scale. Though itch scores didn’t differ significantly from reductions seen with the topical vehicle alone, pain and burning were both reduced significantly compared with vehicle by week 12 of the study (P = .033 for pain; P = .043 for burning).
All improvements were seen only with the lower dose, not the 0.5% dose of SNA-120, noted Dr. Lizzul, adding: “This is not necessarily surprising in the world of kinase inhibitors, where you can see these J-shaped or inverse dose-response curves.”
In addition to recording adverse events, the researchers assessed safety by obtaining laboratory values and electrocardiograms. Plasma SNA-120 levels at study weeks 2, 4, and 8 were obtained for pharmacokinetic analysis. Systemic uptake was virtually nil, and the safety profile overall was good, said Dr. Lizzul.
Next steps are phase 3 clinical trials that will evaluate global improvement as well as pain, burning, and itch in psoriasis, he noted.
Dr. Lizzul is an employee of Sienna Biopharmaceuticals, which is developing SNA-120.
REPORTING FROM WCD2019
Ixekizumab boosts quality of life in genital psoriasis
MILAN – , according to data presented at the World Congress of Dermatology.
After 12 weeks of ixekizumab administration, nearly half of patients who received ixekizumab in the randomized, double-blind, placebo-controlled trial achieved a Dermatology Life Quality Index (DLQI) score of 0 or 1, compared with fewer than 5% of those receiving placebo. At the end of one year, 46.7% of the original ixekizumab group and 50.8% of those who transitioned to ixekizumab from placebo via an open-label extension arm achieved a DLQI of 0 or 1. A DLQI score of 0 or 1 on a 30-point scale indicated that psoriasis had no effect on health-related quality of life.
Among patients with plaque psoriasis, genital involvement is common – present in over 60% at some point during the disease course, Lyn Guenther, MD, of Guenther Dermatology Research Centre, London, Ont., and her colleagues wrote in the poster accompanying the presentation. Sexual health and overall quality of life can be negatively affected by genital psoriasis, they added.
In the study, adult patients were included if they had chronic plaque psoriasis present in genital and nongenital areas. For overall psoriasis and genital involvement, the Static Physician’s Global Assessment (sPGA) was 3 or greater, and total body surface area involvement was at least 1%. Also, patients had to have failed or been intolerant to at least one topical therapy for genital psoriasis.
Patients were excluded if they had genital pustules or vesicles, significant uncontrolled medical or psychiatric comorbidities, recent infection, or prior interleukin-17 antagonist treatment.
In all, patients received ixekizumab 160 mg (75 patients) or placebo (74) subcutaneously every 2 weeks during the initial 12-week study period. For the open-label extension arm, 74 patients in the active arm and 65 patients in the placebo arm went on to receive 80 mg of ixekizumab every 4 weeks, with a step-up option to every other week dosing depending on clinical response.
Patients were given the Short Form Health Survey (SF-36) at baseline and at week 12 and week 52; investigators recorded the mean change for baseline for both the physical and mental component.
Participants also completed the DLQI, which was administered nine times over the 52-week study period.
From baseline, SF-36 scores climbed in both the physical and mental domains for those on ixekizumab, wrote Dr. Guenther and her coinvestigators, with “improvements in all SF-36 domains” for those on ixekizumab at 12 weeks, which continued through week 52. For patients who transitioned to ixekizumab from placebo, “improvements in all SF-36 domains were achieved at week 52,” they wrote.
The mean change from baseline on the SF-36 for the ixekizumab population was 3.5 on the physical domain and 4.8 on the mental component. For the placebo-ixekizumab group, scores improved by a mean 4.5 on the physical domain and 4.9 on the mental domain. Those who stayed on ixekizumab saw some decline in SF-36 scores, with a physical component improvement of 2.5 and mental component improvement of 3.6 at 52 weeks.
Ixekizumab (Taltz), a monoclonal antibody targeting interleukin-17A, is approved for moderate to severe plaque psoriasis, and active psoriatic arthritis..
“Ixekizumab provided clinically meaningful and persistent improvements in HRQoL in patients with moderate to severe genital psoriasis,” wrote Dr. Guenther and her colleagues.
Dr. Guenther and two coauthors reported receiving remuneration from several pharmaceutical companies, including Eli Lilly, which funded the study. Four coauthors are employees and shareholders of Eli Lilly.
MILAN – , according to data presented at the World Congress of Dermatology.
After 12 weeks of ixekizumab administration, nearly half of patients who received ixekizumab in the randomized, double-blind, placebo-controlled trial achieved a Dermatology Life Quality Index (DLQI) score of 0 or 1, compared with fewer than 5% of those receiving placebo. At the end of one year, 46.7% of the original ixekizumab group and 50.8% of those who transitioned to ixekizumab from placebo via an open-label extension arm achieved a DLQI of 0 or 1. A DLQI score of 0 or 1 on a 30-point scale indicated that psoriasis had no effect on health-related quality of life.
Among patients with plaque psoriasis, genital involvement is common – present in over 60% at some point during the disease course, Lyn Guenther, MD, of Guenther Dermatology Research Centre, London, Ont., and her colleagues wrote in the poster accompanying the presentation. Sexual health and overall quality of life can be negatively affected by genital psoriasis, they added.
In the study, adult patients were included if they had chronic plaque psoriasis present in genital and nongenital areas. For overall psoriasis and genital involvement, the Static Physician’s Global Assessment (sPGA) was 3 or greater, and total body surface area involvement was at least 1%. Also, patients had to have failed or been intolerant to at least one topical therapy for genital psoriasis.
Patients were excluded if they had genital pustules or vesicles, significant uncontrolled medical or psychiatric comorbidities, recent infection, or prior interleukin-17 antagonist treatment.
In all, patients received ixekizumab 160 mg (75 patients) or placebo (74) subcutaneously every 2 weeks during the initial 12-week study period. For the open-label extension arm, 74 patients in the active arm and 65 patients in the placebo arm went on to receive 80 mg of ixekizumab every 4 weeks, with a step-up option to every other week dosing depending on clinical response.
Patients were given the Short Form Health Survey (SF-36) at baseline and at week 12 and week 52; investigators recorded the mean change for baseline for both the physical and mental component.
Participants also completed the DLQI, which was administered nine times over the 52-week study period.
From baseline, SF-36 scores climbed in both the physical and mental domains for those on ixekizumab, wrote Dr. Guenther and her coinvestigators, with “improvements in all SF-36 domains” for those on ixekizumab at 12 weeks, which continued through week 52. For patients who transitioned to ixekizumab from placebo, “improvements in all SF-36 domains were achieved at week 52,” they wrote.
The mean change from baseline on the SF-36 for the ixekizumab population was 3.5 on the physical domain and 4.8 on the mental component. For the placebo-ixekizumab group, scores improved by a mean 4.5 on the physical domain and 4.9 on the mental domain. Those who stayed on ixekizumab saw some decline in SF-36 scores, with a physical component improvement of 2.5 and mental component improvement of 3.6 at 52 weeks.
Ixekizumab (Taltz), a monoclonal antibody targeting interleukin-17A, is approved for moderate to severe plaque psoriasis, and active psoriatic arthritis..
“Ixekizumab provided clinically meaningful and persistent improvements in HRQoL in patients with moderate to severe genital psoriasis,” wrote Dr. Guenther and her colleagues.
Dr. Guenther and two coauthors reported receiving remuneration from several pharmaceutical companies, including Eli Lilly, which funded the study. Four coauthors are employees and shareholders of Eli Lilly.
MILAN – , according to data presented at the World Congress of Dermatology.
After 12 weeks of ixekizumab administration, nearly half of patients who received ixekizumab in the randomized, double-blind, placebo-controlled trial achieved a Dermatology Life Quality Index (DLQI) score of 0 or 1, compared with fewer than 5% of those receiving placebo. At the end of one year, 46.7% of the original ixekizumab group and 50.8% of those who transitioned to ixekizumab from placebo via an open-label extension arm achieved a DLQI of 0 or 1. A DLQI score of 0 or 1 on a 30-point scale indicated that psoriasis had no effect on health-related quality of life.
Among patients with plaque psoriasis, genital involvement is common – present in over 60% at some point during the disease course, Lyn Guenther, MD, of Guenther Dermatology Research Centre, London, Ont., and her colleagues wrote in the poster accompanying the presentation. Sexual health and overall quality of life can be negatively affected by genital psoriasis, they added.
In the study, adult patients were included if they had chronic plaque psoriasis present in genital and nongenital areas. For overall psoriasis and genital involvement, the Static Physician’s Global Assessment (sPGA) was 3 or greater, and total body surface area involvement was at least 1%. Also, patients had to have failed or been intolerant to at least one topical therapy for genital psoriasis.
Patients were excluded if they had genital pustules or vesicles, significant uncontrolled medical or psychiatric comorbidities, recent infection, or prior interleukin-17 antagonist treatment.
In all, patients received ixekizumab 160 mg (75 patients) or placebo (74) subcutaneously every 2 weeks during the initial 12-week study period. For the open-label extension arm, 74 patients in the active arm and 65 patients in the placebo arm went on to receive 80 mg of ixekizumab every 4 weeks, with a step-up option to every other week dosing depending on clinical response.
Patients were given the Short Form Health Survey (SF-36) at baseline and at week 12 and week 52; investigators recorded the mean change for baseline for both the physical and mental component.
Participants also completed the DLQI, which was administered nine times over the 52-week study period.
From baseline, SF-36 scores climbed in both the physical and mental domains for those on ixekizumab, wrote Dr. Guenther and her coinvestigators, with “improvements in all SF-36 domains” for those on ixekizumab at 12 weeks, which continued through week 52. For patients who transitioned to ixekizumab from placebo, “improvements in all SF-36 domains were achieved at week 52,” they wrote.
The mean change from baseline on the SF-36 for the ixekizumab population was 3.5 on the physical domain and 4.8 on the mental component. For the placebo-ixekizumab group, scores improved by a mean 4.5 on the physical domain and 4.9 on the mental domain. Those who stayed on ixekizumab saw some decline in SF-36 scores, with a physical component improvement of 2.5 and mental component improvement of 3.6 at 52 weeks.
Ixekizumab (Taltz), a monoclonal antibody targeting interleukin-17A, is approved for moderate to severe plaque psoriasis, and active psoriatic arthritis..
“Ixekizumab provided clinically meaningful and persistent improvements in HRQoL in patients with moderate to severe genital psoriasis,” wrote Dr. Guenther and her colleagues.
Dr. Guenther and two coauthors reported receiving remuneration from several pharmaceutical companies, including Eli Lilly, which funded the study. Four coauthors are employees and shareholders of Eli Lilly.
REPORTING FROM WCD2019
VIDEO: Did You Know? Psoriasis and quality of life



Psoriasis Journal Scan: June 2019
Management of psoriasis as a systemic disease: What is the evidence?
Korman NJ. Br J Dermatol. 2019 Jun 21.
This narrative review explores the pathophysiological relationship between psoriasis and its common comorbidities and discusses the need for new treatment paradigms that include strategies to reduce systemic inflammation in patients with moderate-to-severe psoriasis.
Managing Psoriasis in Patients with HBV or HCV Infection: Practical Considerations.
Piaserico S, Messina F, Russo FP. Am J Clin Dermatol. 2019 Jun 20.
It has been estimated that two billion individuals are infected with HBV worldwide and approximately 240 million have chronic HBV infection. Moreover, there are approximately 71 million individuals with chronic HCV infection worldwide, with a high percentage of them unaware of being infected. As patients with HBV and HCV infections are excluded from controlled clinical trials investigating new drugs, data regarding their safety in patients with psoriasis are based almost exclusively on case reports and small retrospective cohort studies and need to be constantly updated.
Effects of Online Care on Functional and Psychological Outcomes in Patients with Psoriasis: A Randomized Controlled Trial.
Young PM, Chen AY, Ford AR, Cheng MY, Lane CJ, Armstrong AW. J Am Acad Dermatol. 2019 Jun 5.
The impact of online care on patients' functional and psychological outcomes is critical to determine yet still unknown. This 12-month randomized controlled equivalency trial evaluated how a novel online health model that facilitates physician-patient collaboration compares with in-person care for improving psoriasis patients' functional status and mental health.
Feasibility and Utility of the Psoriasis Symptom Inventory (PSI) in Clinical Care Settings: A Study from the International Psoriasis Council.
Strober B, van de Kerkhof PCM, Callis Duffin K, et al. Am J Clin Dermatol. 2019 Jun 21.
The Psoriasis Symptom Inventory (PSI) is a patient-reported outcome measure designed to assess psoriasis signs and symptoms. The aim of the study was to assess the usefulness of the PSI in enhancing patient care in the clinical setting. Eight dermatology clinics in six countries enrolled adults representing the full spectrum of psoriasis severity who regularly received care at the clinic. Key benefits of PSI discussions included the following: new information regarding symptom location and severity for physicians; prompting of quality-of-life discussions; better understanding of patient treatment priorities; change in treatment regimens to target specific symptoms or areas; and improvement of patient-physician relationship.
Socioeconomic Costs and Health Inequalities from Psoriasis: A Cohort Study.
Thomsen SF, Skov L, Dodge R, Hedegaard MS, Kjellberg J. Dermatology. 2019 Jun 25:1-8.
Incentives for health care management based on patient-related outcomes and value (IMPROVE) in psoriasis and psoriatic arthritis is a project aimed at assisting movement from activity-based to outcome-based health care management. One of the key objectives in IMPROVE is to describe the disease-associated socioeconomic burden of psoriasis. The IMPROVE study was a retrospective analysis of patients with a hospital diagnosis of psoriasis identified from the Danish National Patient Registry.
Management of psoriasis as a systemic disease: What is the evidence?
Korman NJ. Br J Dermatol. 2019 Jun 21.
This narrative review explores the pathophysiological relationship between psoriasis and its common comorbidities and discusses the need for new treatment paradigms that include strategies to reduce systemic inflammation in patients with moderate-to-severe psoriasis.
Managing Psoriasis in Patients with HBV or HCV Infection: Practical Considerations.
Piaserico S, Messina F, Russo FP. Am J Clin Dermatol. 2019 Jun 20.
It has been estimated that two billion individuals are infected with HBV worldwide and approximately 240 million have chronic HBV infection. Moreover, there are approximately 71 million individuals with chronic HCV infection worldwide, with a high percentage of them unaware of being infected. As patients with HBV and HCV infections are excluded from controlled clinical trials investigating new drugs, data regarding their safety in patients with psoriasis are based almost exclusively on case reports and small retrospective cohort studies and need to be constantly updated.
Effects of Online Care on Functional and Psychological Outcomes in Patients with Psoriasis: A Randomized Controlled Trial.
Young PM, Chen AY, Ford AR, Cheng MY, Lane CJ, Armstrong AW. J Am Acad Dermatol. 2019 Jun 5.
The impact of online care on patients' functional and psychological outcomes is critical to determine yet still unknown. This 12-month randomized controlled equivalency trial evaluated how a novel online health model that facilitates physician-patient collaboration compares with in-person care for improving psoriasis patients' functional status and mental health.
Feasibility and Utility of the Psoriasis Symptom Inventory (PSI) in Clinical Care Settings: A Study from the International Psoriasis Council.
Strober B, van de Kerkhof PCM, Callis Duffin K, et al. Am J Clin Dermatol. 2019 Jun 21.
The Psoriasis Symptom Inventory (PSI) is a patient-reported outcome measure designed to assess psoriasis signs and symptoms. The aim of the study was to assess the usefulness of the PSI in enhancing patient care in the clinical setting. Eight dermatology clinics in six countries enrolled adults representing the full spectrum of psoriasis severity who regularly received care at the clinic. Key benefits of PSI discussions included the following: new information regarding symptom location and severity for physicians; prompting of quality-of-life discussions; better understanding of patient treatment priorities; change in treatment regimens to target specific symptoms or areas; and improvement of patient-physician relationship.
Socioeconomic Costs and Health Inequalities from Psoriasis: A Cohort Study.
Thomsen SF, Skov L, Dodge R, Hedegaard MS, Kjellberg J. Dermatology. 2019 Jun 25:1-8.
Incentives for health care management based on patient-related outcomes and value (IMPROVE) in psoriasis and psoriatic arthritis is a project aimed at assisting movement from activity-based to outcome-based health care management. One of the key objectives in IMPROVE is to describe the disease-associated socioeconomic burden of psoriasis. The IMPROVE study was a retrospective analysis of patients with a hospital diagnosis of psoriasis identified from the Danish National Patient Registry.
Management of psoriasis as a systemic disease: What is the evidence?
Korman NJ. Br J Dermatol. 2019 Jun 21.
This narrative review explores the pathophysiological relationship between psoriasis and its common comorbidities and discusses the need for new treatment paradigms that include strategies to reduce systemic inflammation in patients with moderate-to-severe psoriasis.
Managing Psoriasis in Patients with HBV or HCV Infection: Practical Considerations.
Piaserico S, Messina F, Russo FP. Am J Clin Dermatol. 2019 Jun 20.
It has been estimated that two billion individuals are infected with HBV worldwide and approximately 240 million have chronic HBV infection. Moreover, there are approximately 71 million individuals with chronic HCV infection worldwide, with a high percentage of them unaware of being infected. As patients with HBV and HCV infections are excluded from controlled clinical trials investigating new drugs, data regarding their safety in patients with psoriasis are based almost exclusively on case reports and small retrospective cohort studies and need to be constantly updated.
Effects of Online Care on Functional and Psychological Outcomes in Patients with Psoriasis: A Randomized Controlled Trial.
Young PM, Chen AY, Ford AR, Cheng MY, Lane CJ, Armstrong AW. J Am Acad Dermatol. 2019 Jun 5.
The impact of online care on patients' functional and psychological outcomes is critical to determine yet still unknown. This 12-month randomized controlled equivalency trial evaluated how a novel online health model that facilitates physician-patient collaboration compares with in-person care for improving psoriasis patients' functional status and mental health.
Feasibility and Utility of the Psoriasis Symptom Inventory (PSI) in Clinical Care Settings: A Study from the International Psoriasis Council.
Strober B, van de Kerkhof PCM, Callis Duffin K, et al. Am J Clin Dermatol. 2019 Jun 21.
The Psoriasis Symptom Inventory (PSI) is a patient-reported outcome measure designed to assess psoriasis signs and symptoms. The aim of the study was to assess the usefulness of the PSI in enhancing patient care in the clinical setting. Eight dermatology clinics in six countries enrolled adults representing the full spectrum of psoriasis severity who regularly received care at the clinic. Key benefits of PSI discussions included the following: new information regarding symptom location and severity for physicians; prompting of quality-of-life discussions; better understanding of patient treatment priorities; change in treatment regimens to target specific symptoms or areas; and improvement of patient-physician relationship.
Socioeconomic Costs and Health Inequalities from Psoriasis: A Cohort Study.
Thomsen SF, Skov L, Dodge R, Hedegaard MS, Kjellberg J. Dermatology. 2019 Jun 25:1-8.
Incentives for health care management based on patient-related outcomes and value (IMPROVE) in psoriasis and psoriatic arthritis is a project aimed at assisting movement from activity-based to outcome-based health care management. One of the key objectives in IMPROVE is to describe the disease-associated socioeconomic burden of psoriasis. The IMPROVE study was a retrospective analysis of patients with a hospital diagnosis of psoriasis identified from the Danish National Patient Registry.
Allergic Contact Dermatitis With Sparing of Exposed Psoriasis Plaques
To the Editor:
Allergic contact dermatitis (ACD) is a delayed-type hypersensitivity reaction against antigens to which the skin’s immune system was previously sensitized. The initial sensitization requires penetration of the antigen through the stratum corneum. Thus, the ability of a particle to cause ACD is related to its molecular structure and size, lipophilicity, and protein-binding affinity, as well as the dose and duration of exposure.1 Psoriasis typically presents as well-demarcated areas of skin that may be erythematous, indurated, and scaly to variable degrees. Histologically, psoriasis plaques are characterized by epidermal hyperplasia in the presence of a T-cell infiltrate and neutrophilic microabscesses. We report a case of a patient with plaque-type psoriasis who experienced ACD with sparing of exposed psoriatic plaques.
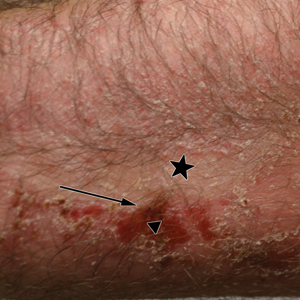
A 45-year-old man with a 5-year history of generalized moderate to severe psoriasis undergoing therapy with ustekinumab 45 mg subcutaneously once every 12 weeks presented to the emergency department with intensely erythematous, pruritic, vesicular lesions on the trunk, arms, and legs within 24 hours of exposure to poison oak while hiking. The patient reported pruritus, pain, and swelling of the affected areas. On physical examination, he was afebrile. Widespread erythematous vesicular lesions were noted on the face, trunk, arms, and legs, sparing the well-demarcated scaly psoriatic plaques on the arms and legs (Figure). The patient was given intravenous fluids and intravenous diphenhydramine. After responding to initial treatment, the patient was discharged with ibuprofen and a tapering dose of oral prednisone from 60 mg 5 times daily, to 40 mg 5 times daily, to 20 mg 5 times daily over 15 days.
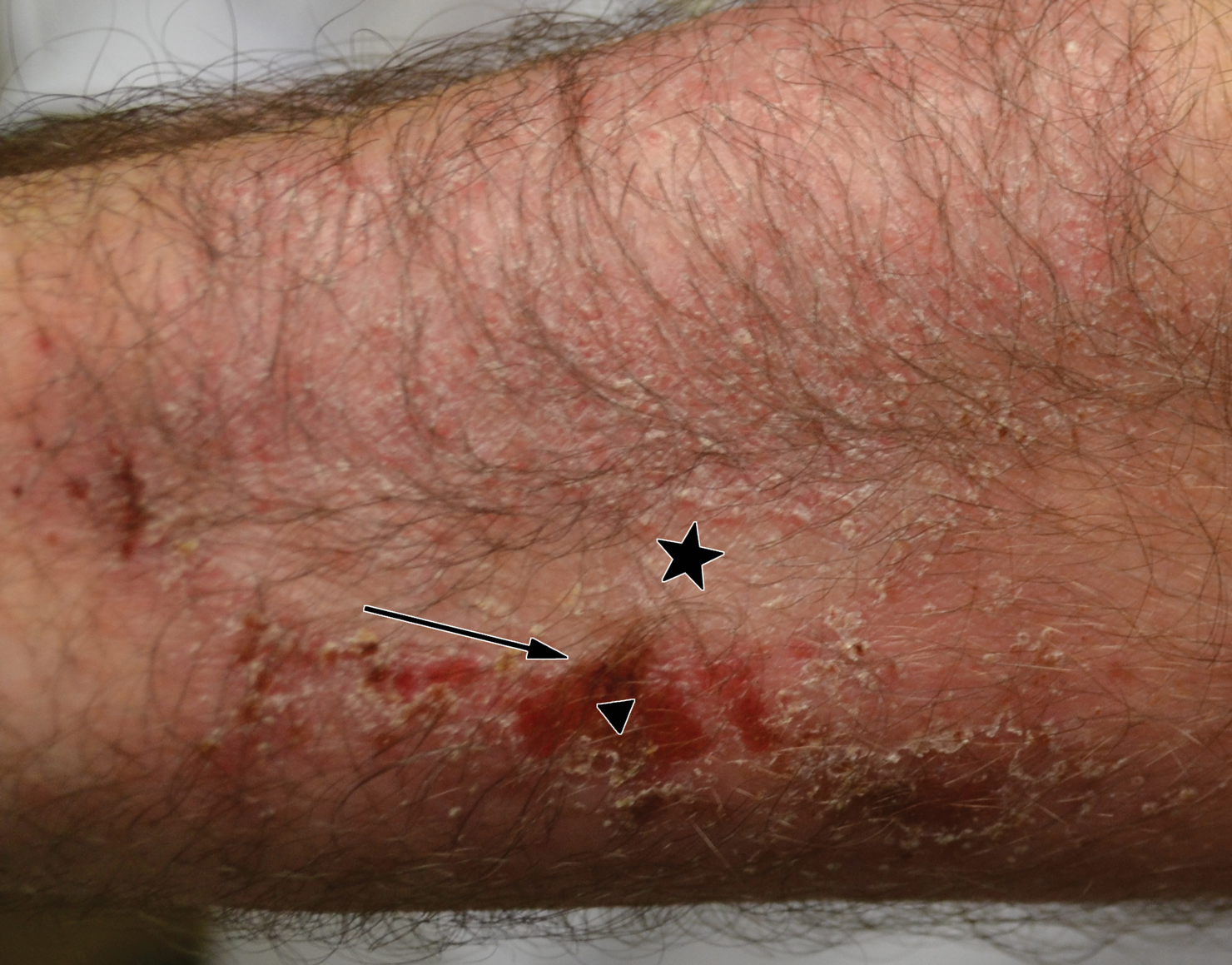
star), with a linear border demarcating the ACD lesion and the unaffected psoriatic plaque (black arrow).
Allergic contact dermatitis occurs after sensitization to environmental allergens or haptens. Clinically, ACD is characterized by pruritic, erythematous, vesicular papules and plaques. The predominant effector cells in ACD are CD8+ T cells, along with contributions from helper T cells (TH2). Together, these cell types produce an environment enriched in IFN-γ, IL-2, IL-4, IL-10, IL-17, and tumor necrosis factor α.2 Ultimately, the ACD response induces keratinocyte apoptosis via cytotoxic effects.3,4
Plaque psoriasis is a chronic, immune-mediated, inflammatory disease that presents clinically as erythematous well-demarcated plaques with a micaceous scale. The immunologic environment of psoriasis plaques is characterized by infiltration of CD4+ TH17 cells and elevated levels of IL-17, IL-23, tumor necrosis factor α, and IL-1β, which induce keratinocyte hyperproliferation through a complex mechanism resulting in hyperkeratosis composed of orthokeratosis and parakeratosis, a neutrophilic infiltrate, and Munro microabscesses.5
The predominant effector cells and the final effects on keratinocyte survival are divergent in psoriasis and ACD. The possibly antagonistic relationship between these immunologic processes is further supported by epidemiologic studies demonstrating a decreased incidence of ACD in patients with psoriasis.6,7
Our patient demonstrated a typical ACD reaction in response to exposure to urushiol, the allergen present in poison oak, in areas unaffected by psoriasis plaques. Interestingly, the patient displayed this response even while undergoing therapy with ustekinumab, a fully humanized antibody that binds IL-12 and IL-23 and ultimately downregulates TH17 cell-mediated release of IL-17 in the treatment of psoriasis. Although IL-17 also has been implicated in ACD, the lack of inhibition of ACD with ustekinumab treatment was previously demonstrated in a small retrospective study, indicating a potentially different source of IL-17 in ACD.8
Our patient did not demonstrate a typical ACD response in areas of active psoriasis plaques. This phenomenon was of great interest to us. It is possible that the presence of hyperkeratosis, manifested clinically as scaling, served as a mechanical barrier preventing the diffusion and exposure of cutaneous immune cells to urushiol. On the other hand, it is possible that the immunologic environment of the active psoriasis plaque was altered in such a way that it did not demonstrate the typical response to allergen exposure.
We hypothesize that the lack of a typical ACD response at sites of psoriatic plaques in our patient may be attributed to the intensity and duration of exposure to the allergen. Quaranta et al9 reported a typical ACD clinical response and a mixed immunohistologic response to nickel patch testing at sites of active plaques in nickel-sensitized psoriasis patients. Patch testing involves 48 hours of direct contact with an allergen, while our patient experienced an estimated 8 to 10 hours of exposure to the allergen prior to removal via washing. Supporting this line of reasoning, a proportion of patients who are responsive to nickel patch testing do not exhibit clinical symptoms in response to casual nickel exposure.10 Although a physical barrier effect due to hyperkeratosis may have contributed to the lack of ACD response in sites of psoriasis plaques in our patient, it remains possible that a more limited duration of exposure to the allergen is not sufficient to overcome the native immunologic milieu of the psoriasis plaque and induce the immunologic cascade resulting in ACD. Further research into the potentially antagonistic relationship of psoriasis and ACD should be performed to elucidate the interaction between these two common conditions.
- Kimber I, Basketter DA, Gerberick GF, et al. Allergic contact dermatitis. Int Immunopharmacol. 2002;2:201-211.
- Vocanson M, Hennino A, Cluzel-Tailhardat M, et al. CD8+ T cells are effector cells of contact dermatitis to common skin allergens in mice. J Invest Dermatol. 2006;126:815-820.
- Akiba H, Kehren J, Ducluzeau MT, et al. Skin inflammation during contact hypersensitivity is mediated by early recruitment of CD8+ T cytotoxic 1 cells inducing keratinocyte apoptosis. J Immunol. 2002;168:3079-3087.
- Trautmann A, Akdis M, Kleemann D, et al. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest. 2000;106:25-35.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Bangsgaard N, Engkilde K, Thyssen JP, et al. Inverse relationship between contact allergy and psoriasis: results from a patient- and a population-based study. Br J Dermatol. 2009;161:1119-1123.
- Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol. 1995;32:982-986.
- Bangsgaard N, Zachariae C, Menne T, et al. Lack of effect of ustekinumab in treatment of allergic contact dermatitis. Contact Dermatitis. 2011;65:227-230.
- Quaranta M, Eyerich S, Knapp B, et al. Allergic contact dermatitis in psoriasis patients: typical, delayed, and non-interacting. PLoS One. 2014;9:e101814.
- Kimber I, Basketter DA, Gerberick GF, et al. Allergic contact dermatitis. Int Immunopharmacol. 2002;2:201-211.
To the Editor:
Allergic contact dermatitis (ACD) is a delayed-type hypersensitivity reaction against antigens to which the skin’s immune system was previously sensitized. The initial sensitization requires penetration of the antigen through the stratum corneum. Thus, the ability of a particle to cause ACD is related to its molecular structure and size, lipophilicity, and protein-binding affinity, as well as the dose and duration of exposure.1 Psoriasis typically presents as well-demarcated areas of skin that may be erythematous, indurated, and scaly to variable degrees. Histologically, psoriasis plaques are characterized by epidermal hyperplasia in the presence of a T-cell infiltrate and neutrophilic microabscesses. We report a case of a patient with plaque-type psoriasis who experienced ACD with sparing of exposed psoriatic plaques.
A 45-year-old man with a 5-year history of generalized moderate to severe psoriasis undergoing therapy with ustekinumab 45 mg subcutaneously once every 12 weeks presented to the emergency department with intensely erythematous, pruritic, vesicular lesions on the trunk, arms, and legs within 24 hours of exposure to poison oak while hiking. The patient reported pruritus, pain, and swelling of the affected areas. On physical examination, he was afebrile. Widespread erythematous vesicular lesions were noted on the face, trunk, arms, and legs, sparing the well-demarcated scaly psoriatic plaques on the arms and legs (Figure). The patient was given intravenous fluids and intravenous diphenhydramine. After responding to initial treatment, the patient was discharged with ibuprofen and a tapering dose of oral prednisone from 60 mg 5 times daily, to 40 mg 5 times daily, to 20 mg 5 times daily over 15 days.

star), with a linear border demarcating the ACD lesion and the unaffected psoriatic plaque (black arrow).
Allergic contact dermatitis occurs after sensitization to environmental allergens or haptens. Clinically, ACD is characterized by pruritic, erythematous, vesicular papules and plaques. The predominant effector cells in ACD are CD8+ T cells, along with contributions from helper T cells (TH2). Together, these cell types produce an environment enriched in IFN-γ, IL-2, IL-4, IL-10, IL-17, and tumor necrosis factor α.2 Ultimately, the ACD response induces keratinocyte apoptosis via cytotoxic effects.3,4
Plaque psoriasis is a chronic, immune-mediated, inflammatory disease that presents clinically as erythematous well-demarcated plaques with a micaceous scale. The immunologic environment of psoriasis plaques is characterized by infiltration of CD4+ TH17 cells and elevated levels of IL-17, IL-23, tumor necrosis factor α, and IL-1β, which induce keratinocyte hyperproliferation through a complex mechanism resulting in hyperkeratosis composed of orthokeratosis and parakeratosis, a neutrophilic infiltrate, and Munro microabscesses.5
The predominant effector cells and the final effects on keratinocyte survival are divergent in psoriasis and ACD. The possibly antagonistic relationship between these immunologic processes is further supported by epidemiologic studies demonstrating a decreased incidence of ACD in patients with psoriasis.6,7
Our patient demonstrated a typical ACD reaction in response to exposure to urushiol, the allergen present in poison oak, in areas unaffected by psoriasis plaques. Interestingly, the patient displayed this response even while undergoing therapy with ustekinumab, a fully humanized antibody that binds IL-12 and IL-23 and ultimately downregulates TH17 cell-mediated release of IL-17 in the treatment of psoriasis. Although IL-17 also has been implicated in ACD, the lack of inhibition of ACD with ustekinumab treatment was previously demonstrated in a small retrospective study, indicating a potentially different source of IL-17 in ACD.8
Our patient did not demonstrate a typical ACD response in areas of active psoriasis plaques. This phenomenon was of great interest to us. It is possible that the presence of hyperkeratosis, manifested clinically as scaling, served as a mechanical barrier preventing the diffusion and exposure of cutaneous immune cells to urushiol. On the other hand, it is possible that the immunologic environment of the active psoriasis plaque was altered in such a way that it did not demonstrate the typical response to allergen exposure.
We hypothesize that the lack of a typical ACD response at sites of psoriatic plaques in our patient may be attributed to the intensity and duration of exposure to the allergen. Quaranta et al9 reported a typical ACD clinical response and a mixed immunohistologic response to nickel patch testing at sites of active plaques in nickel-sensitized psoriasis patients. Patch testing involves 48 hours of direct contact with an allergen, while our patient experienced an estimated 8 to 10 hours of exposure to the allergen prior to removal via washing. Supporting this line of reasoning, a proportion of patients who are responsive to nickel patch testing do not exhibit clinical symptoms in response to casual nickel exposure.10 Although a physical barrier effect due to hyperkeratosis may have contributed to the lack of ACD response in sites of psoriasis plaques in our patient, it remains possible that a more limited duration of exposure to the allergen is not sufficient to overcome the native immunologic milieu of the psoriasis plaque and induce the immunologic cascade resulting in ACD. Further research into the potentially antagonistic relationship of psoriasis and ACD should be performed to elucidate the interaction between these two common conditions.
To the Editor:
Allergic contact dermatitis (ACD) is a delayed-type hypersensitivity reaction against antigens to which the skin’s immune system was previously sensitized. The initial sensitization requires penetration of the antigen through the stratum corneum. Thus, the ability of a particle to cause ACD is related to its molecular structure and size, lipophilicity, and protein-binding affinity, as well as the dose and duration of exposure.1 Psoriasis typically presents as well-demarcated areas of skin that may be erythematous, indurated, and scaly to variable degrees. Histologically, psoriasis plaques are characterized by epidermal hyperplasia in the presence of a T-cell infiltrate and neutrophilic microabscesses. We report a case of a patient with plaque-type psoriasis who experienced ACD with sparing of exposed psoriatic plaques.
A 45-year-old man with a 5-year history of generalized moderate to severe psoriasis undergoing therapy with ustekinumab 45 mg subcutaneously once every 12 weeks presented to the emergency department with intensely erythematous, pruritic, vesicular lesions on the trunk, arms, and legs within 24 hours of exposure to poison oak while hiking. The patient reported pruritus, pain, and swelling of the affected areas. On physical examination, he was afebrile. Widespread erythematous vesicular lesions were noted on the face, trunk, arms, and legs, sparing the well-demarcated scaly psoriatic plaques on the arms and legs (Figure). The patient was given intravenous fluids and intravenous diphenhydramine. After responding to initial treatment, the patient was discharged with ibuprofen and a tapering dose of oral prednisone from 60 mg 5 times daily, to 40 mg 5 times daily, to 20 mg 5 times daily over 15 days.

star), with a linear border demarcating the ACD lesion and the unaffected psoriatic plaque (black arrow).
Allergic contact dermatitis occurs after sensitization to environmental allergens or haptens. Clinically, ACD is characterized by pruritic, erythematous, vesicular papules and plaques. The predominant effector cells in ACD are CD8+ T cells, along with contributions from helper T cells (TH2). Together, these cell types produce an environment enriched in IFN-γ, IL-2, IL-4, IL-10, IL-17, and tumor necrosis factor α.2 Ultimately, the ACD response induces keratinocyte apoptosis via cytotoxic effects.3,4
Plaque psoriasis is a chronic, immune-mediated, inflammatory disease that presents clinically as erythematous well-demarcated plaques with a micaceous scale. The immunologic environment of psoriasis plaques is characterized by infiltration of CD4+ TH17 cells and elevated levels of IL-17, IL-23, tumor necrosis factor α, and IL-1β, which induce keratinocyte hyperproliferation through a complex mechanism resulting in hyperkeratosis composed of orthokeratosis and parakeratosis, a neutrophilic infiltrate, and Munro microabscesses.5
The predominant effector cells and the final effects on keratinocyte survival are divergent in psoriasis and ACD. The possibly antagonistic relationship between these immunologic processes is further supported by epidemiologic studies demonstrating a decreased incidence of ACD in patients with psoriasis.6,7
Our patient demonstrated a typical ACD reaction in response to exposure to urushiol, the allergen present in poison oak, in areas unaffected by psoriasis plaques. Interestingly, the patient displayed this response even while undergoing therapy with ustekinumab, a fully humanized antibody that binds IL-12 and IL-23 and ultimately downregulates TH17 cell-mediated release of IL-17 in the treatment of psoriasis. Although IL-17 also has been implicated in ACD, the lack of inhibition of ACD with ustekinumab treatment was previously demonstrated in a small retrospective study, indicating a potentially different source of IL-17 in ACD.8
Our patient did not demonstrate a typical ACD response in areas of active psoriasis plaques. This phenomenon was of great interest to us. It is possible that the presence of hyperkeratosis, manifested clinically as scaling, served as a mechanical barrier preventing the diffusion and exposure of cutaneous immune cells to urushiol. On the other hand, it is possible that the immunologic environment of the active psoriasis plaque was altered in such a way that it did not demonstrate the typical response to allergen exposure.
We hypothesize that the lack of a typical ACD response at sites of psoriatic plaques in our patient may be attributed to the intensity and duration of exposure to the allergen. Quaranta et al9 reported a typical ACD clinical response and a mixed immunohistologic response to nickel patch testing at sites of active plaques in nickel-sensitized psoriasis patients. Patch testing involves 48 hours of direct contact with an allergen, while our patient experienced an estimated 8 to 10 hours of exposure to the allergen prior to removal via washing. Supporting this line of reasoning, a proportion of patients who are responsive to nickel patch testing do not exhibit clinical symptoms in response to casual nickel exposure.10 Although a physical barrier effect due to hyperkeratosis may have contributed to the lack of ACD response in sites of psoriasis plaques in our patient, it remains possible that a more limited duration of exposure to the allergen is not sufficient to overcome the native immunologic milieu of the psoriasis plaque and induce the immunologic cascade resulting in ACD. Further research into the potentially antagonistic relationship of psoriasis and ACD should be performed to elucidate the interaction between these two common conditions.
- Kimber I, Basketter DA, Gerberick GF, et al. Allergic contact dermatitis. Int Immunopharmacol. 2002;2:201-211.
- Vocanson M, Hennino A, Cluzel-Tailhardat M, et al. CD8+ T cells are effector cells of contact dermatitis to common skin allergens in mice. J Invest Dermatol. 2006;126:815-820.
- Akiba H, Kehren J, Ducluzeau MT, et al. Skin inflammation during contact hypersensitivity is mediated by early recruitment of CD8+ T cytotoxic 1 cells inducing keratinocyte apoptosis. J Immunol. 2002;168:3079-3087.
- Trautmann A, Akdis M, Kleemann D, et al. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest. 2000;106:25-35.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Bangsgaard N, Engkilde K, Thyssen JP, et al. Inverse relationship between contact allergy and psoriasis: results from a patient- and a population-based study. Br J Dermatol. 2009;161:1119-1123.
- Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol. 1995;32:982-986.
- Bangsgaard N, Zachariae C, Menne T, et al. Lack of effect of ustekinumab in treatment of allergic contact dermatitis. Contact Dermatitis. 2011;65:227-230.
- Quaranta M, Eyerich S, Knapp B, et al. Allergic contact dermatitis in psoriasis patients: typical, delayed, and non-interacting. PLoS One. 2014;9:e101814.
- Kimber I, Basketter DA, Gerberick GF, et al. Allergic contact dermatitis. Int Immunopharmacol. 2002;2:201-211.
- Kimber I, Basketter DA, Gerberick GF, et al. Allergic contact dermatitis. Int Immunopharmacol. 2002;2:201-211.
- Vocanson M, Hennino A, Cluzel-Tailhardat M, et al. CD8+ T cells are effector cells of contact dermatitis to common skin allergens in mice. J Invest Dermatol. 2006;126:815-820.
- Akiba H, Kehren J, Ducluzeau MT, et al. Skin inflammation during contact hypersensitivity is mediated by early recruitment of CD8+ T cytotoxic 1 cells inducing keratinocyte apoptosis. J Immunol. 2002;168:3079-3087.
- Trautmann A, Akdis M, Kleemann D, et al. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest. 2000;106:25-35.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Bangsgaard N, Engkilde K, Thyssen JP, et al. Inverse relationship between contact allergy and psoriasis: results from a patient- and a population-based study. Br J Dermatol. 2009;161:1119-1123.
- Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol. 1995;32:982-986.
- Bangsgaard N, Zachariae C, Menne T, et al. Lack of effect of ustekinumab in treatment of allergic contact dermatitis. Contact Dermatitis. 2011;65:227-230.
- Quaranta M, Eyerich S, Knapp B, et al. Allergic contact dermatitis in psoriasis patients: typical, delayed, and non-interacting. PLoS One. 2014;9:e101814.
- Kimber I, Basketter DA, Gerberick GF, et al. Allergic contact dermatitis. Int Immunopharmacol. 2002;2:201-211.
Practice Points
- Patients with plaque-type psoriasis who experience allergic contact dermatitis (ACD) may present with sparing of exposed psoriatic plaques.
- The divergent immunologic milieus present in ACD and psoriasis likely underly the decreased incidence of ACD in patients with psoriasis.
Systemic psoriasis treatments less often prescribed in elderly with psoriasis, despite comparable response rates
MILAN – an analysis of German and Swiss registry data shows.
There was an “imbalance” in the types of medications prescribed for older and younger patients in the registry, with biologics used more frequently in younger patients, according to investigator Matthias Augustin, MD, director of the Institute For Health Services Research in Dermatology and Nursing in Hamburg, Germany.
However, the efficacy of systemic treatments, including nonbiologic therapies, was comparable between older and younger patients, other than a few differences in response rates early in treatment that disappeared with longer follow-up, Dr. Augustin said at the World Congress of Dermatology. Coupled with evidence from the medical literature, results of this registry data analysis suggest there are “very few reasons” to avoid use of systemic drugs in elderly patients.
“I think we should create awareness and discuss possible reasons that deter dermatologists from prescribing systemic antipsoriatics in elderly patients,” he said.
Concerns about safety and drug interactions in the elderly may be one barrier to prescribing systemic therapy in this patient population: More data on this issue are needed, since the elderly are taking more medications than younger patients and have more contraindications, Dr. Augustin said. “I think this is a job for all registries for the future.”
Older individuals have typically been excluded from psoriasis clinical trials, making it difficult to extrapolate existing safety and efficacy data to those patients, he pointed out.
Accordingly, Dr. Augustin and coinvestigators evaluated prospectively collected data for patients with moderate to severe psoriasis who were included in either the German Psoriasis registry (PsoBest) or the Swiss Dermatology Network for Targeted Therapies (SDNTT). They split the cohort into a control group of those younger than 65 years (about 4,600 individuals) and those 65 years or older (about 740 individuals).
A few systemic drugs were used more frequently in the elderly, including apremilast and methotrexate, while most other drugs, including biologics, were used more frequently in younger patients, Dr. Augustin and colleagues found in their analysis. There were a few differences between the elderly and controls related to weight, smoking, and other factors, but not so pronounced that they would explain differences in the use of the systemic therapy.
Response rates to systemic therapies were generally comparable between the elderly and controls, as measured by Psoriasis Area Severity Index (PASI) 75 responses, PASI scores of 3 or less, and Dermatology Life Quality Index scores of one or less, he added.
One exception was methotrexate, which was more effective in the elderly after 3 and 6 months of treatment, but that difference was no longer apparent after 12 months of treatment, he said. Likewise, cyclosporine showed a higher response rate in younger patients at 3 months, but not at 6 or 12 months.
Based on the findings, “overall, we observed comparable responses between the controls and the elderly,” Dr. Augustin concluded.
The PsoBest registry is sponsored by CVderm, DDG, and BVDD, and “has been established and is operated in close cooperation with the involved pharmaceutical companies whose statutory pharmacovigilance requirements are taken into account,” according to a statement on the PsoBest website. The Swiss registry is supported by Janssen, AbbVie, Pfizer, Celgene, Lilly, and Novartis. The investigators did not report any disclosures.
MILAN – an analysis of German and Swiss registry data shows.
There was an “imbalance” in the types of medications prescribed for older and younger patients in the registry, with biologics used more frequently in younger patients, according to investigator Matthias Augustin, MD, director of the Institute For Health Services Research in Dermatology and Nursing in Hamburg, Germany.
However, the efficacy of systemic treatments, including nonbiologic therapies, was comparable between older and younger patients, other than a few differences in response rates early in treatment that disappeared with longer follow-up, Dr. Augustin said at the World Congress of Dermatology. Coupled with evidence from the medical literature, results of this registry data analysis suggest there are “very few reasons” to avoid use of systemic drugs in elderly patients.
“I think we should create awareness and discuss possible reasons that deter dermatologists from prescribing systemic antipsoriatics in elderly patients,” he said.
Concerns about safety and drug interactions in the elderly may be one barrier to prescribing systemic therapy in this patient population: More data on this issue are needed, since the elderly are taking more medications than younger patients and have more contraindications, Dr. Augustin said. “I think this is a job for all registries for the future.”
Older individuals have typically been excluded from psoriasis clinical trials, making it difficult to extrapolate existing safety and efficacy data to those patients, he pointed out.
Accordingly, Dr. Augustin and coinvestigators evaluated prospectively collected data for patients with moderate to severe psoriasis who were included in either the German Psoriasis registry (PsoBest) or the Swiss Dermatology Network for Targeted Therapies (SDNTT). They split the cohort into a control group of those younger than 65 years (about 4,600 individuals) and those 65 years or older (about 740 individuals).
A few systemic drugs were used more frequently in the elderly, including apremilast and methotrexate, while most other drugs, including biologics, were used more frequently in younger patients, Dr. Augustin and colleagues found in their analysis. There were a few differences between the elderly and controls related to weight, smoking, and other factors, but not so pronounced that they would explain differences in the use of the systemic therapy.
Response rates to systemic therapies were generally comparable between the elderly and controls, as measured by Psoriasis Area Severity Index (PASI) 75 responses, PASI scores of 3 or less, and Dermatology Life Quality Index scores of one or less, he added.
One exception was methotrexate, which was more effective in the elderly after 3 and 6 months of treatment, but that difference was no longer apparent after 12 months of treatment, he said. Likewise, cyclosporine showed a higher response rate in younger patients at 3 months, but not at 6 or 12 months.
Based on the findings, “overall, we observed comparable responses between the controls and the elderly,” Dr. Augustin concluded.
The PsoBest registry is sponsored by CVderm, DDG, and BVDD, and “has been established and is operated in close cooperation with the involved pharmaceutical companies whose statutory pharmacovigilance requirements are taken into account,” according to a statement on the PsoBest website. The Swiss registry is supported by Janssen, AbbVie, Pfizer, Celgene, Lilly, and Novartis. The investigators did not report any disclosures.
MILAN – an analysis of German and Swiss registry data shows.
There was an “imbalance” in the types of medications prescribed for older and younger patients in the registry, with biologics used more frequently in younger patients, according to investigator Matthias Augustin, MD, director of the Institute For Health Services Research in Dermatology and Nursing in Hamburg, Germany.
However, the efficacy of systemic treatments, including nonbiologic therapies, was comparable between older and younger patients, other than a few differences in response rates early in treatment that disappeared with longer follow-up, Dr. Augustin said at the World Congress of Dermatology. Coupled with evidence from the medical literature, results of this registry data analysis suggest there are “very few reasons” to avoid use of systemic drugs in elderly patients.
“I think we should create awareness and discuss possible reasons that deter dermatologists from prescribing systemic antipsoriatics in elderly patients,” he said.
Concerns about safety and drug interactions in the elderly may be one barrier to prescribing systemic therapy in this patient population: More data on this issue are needed, since the elderly are taking more medications than younger patients and have more contraindications, Dr. Augustin said. “I think this is a job for all registries for the future.”
Older individuals have typically been excluded from psoriasis clinical trials, making it difficult to extrapolate existing safety and efficacy data to those patients, he pointed out.
Accordingly, Dr. Augustin and coinvestigators evaluated prospectively collected data for patients with moderate to severe psoriasis who were included in either the German Psoriasis registry (PsoBest) or the Swiss Dermatology Network for Targeted Therapies (SDNTT). They split the cohort into a control group of those younger than 65 years (about 4,600 individuals) and those 65 years or older (about 740 individuals).
A few systemic drugs were used more frequently in the elderly, including apremilast and methotrexate, while most other drugs, including biologics, were used more frequently in younger patients, Dr. Augustin and colleagues found in their analysis. There were a few differences between the elderly and controls related to weight, smoking, and other factors, but not so pronounced that they would explain differences in the use of the systemic therapy.
Response rates to systemic therapies were generally comparable between the elderly and controls, as measured by Psoriasis Area Severity Index (PASI) 75 responses, PASI scores of 3 or less, and Dermatology Life Quality Index scores of one or less, he added.
One exception was methotrexate, which was more effective in the elderly after 3 and 6 months of treatment, but that difference was no longer apparent after 12 months of treatment, he said. Likewise, cyclosporine showed a higher response rate in younger patients at 3 months, but not at 6 or 12 months.
Based on the findings, “overall, we observed comparable responses between the controls and the elderly,” Dr. Augustin concluded.
The PsoBest registry is sponsored by CVderm, DDG, and BVDD, and “has been established and is operated in close cooperation with the involved pharmaceutical companies whose statutory pharmacovigilance requirements are taken into account,” according to a statement on the PsoBest website. The Swiss registry is supported by Janssen, AbbVie, Pfizer, Celgene, Lilly, and Novartis. The investigators did not report any disclosures.
REPORTING FROM WCD2019
Phototherapy: Is It Still Important?
Phototherapy has been used to treat skin diseases for millennia. From the Incas to the ancient Greeks and Egyptians, nearly every major civilization has attempted to harness the sun, with some even worshipping it for its healing powers.1 Today, phototherapy remains as important as ever. Despite the technological advances that have brought about biologic medications, small molecule inhibitors, and elegant vehicle delivery systems, phototherapy continues to be a valuable tool in the dermatologist’s armamentarium.
Patient Access to Phototherapy
An important step in successfully managing any disease is access to treatment. In today’s health care landscape, therapeutic decisions frequently are dictated by a patient’s financial situation as well as by the discretion of payers. Costly medications such as biologics often are not accessible to patients on government insurance who fall into the Medicare “donut hole” and may be denied by insurance companies for a myriad of reasons. Luckily, phototherapy typically is well covered and is even a first-line treatment option for some conditions, such as mycosis fungoides.
Nevertheless, phototherapy also has its own unique accessibility hurdles. The time-consuming nature of office-based phototherapy treatment is the main barrier, and many patients find it difficult to incorporate treatments into their daily lives. Additionally, office-based phototherapy units often are clustered in major cities, making access more difficult for rural patients. Because light-responsive conditions often are chronic and may require a lifetime of treatment, home phototherapy units are now being recognized as cost-effective treatment options and are increasingly covered by insurance. In fact, one study comparing psoriasis patients treated with home narrowband UVB (NB-UVB) vs outpatient NB-UVB found that in-home treatment was equally as effective as office-based treatment at a similar cost.2 Because studies comparing the effectiveness of office-based vs home-based phototherapy treatment are underway for various other diseases, hopefully more patients will be able to receive home units, thus increasing access to safe and effective treatment.
Wide Range of Treatment Indications
Another merit of phototherapy is its ability to be used in almost all patient populations. It is one of the few modalities whose indications span the entire length of the human lifetime—from pediatric atopic dermatitis to chronic pruritus in elderly patients. Phototherapy also is one of the few treatment options that is safe to use in patients with an active malignancy or in patients who have multiple other medical conditions. Comorbidities including congestive heart failure, chronic infections, and demyelinating disorders often prevent the use of oral and injectable medications for immune-mediated disorders such as psoriasis or atopic dermatitis. In patients with multiple comorbidities whose disease remains uncontrolled despite an adequate topical regimen, phototherapy is one of the few effective treatment options that remain. Additionally, there is a considerable number of patients who prefer external treatments for cutaneous diseases. For these patients, phototherapy offers the opportunity to control skin conditions without the use of an internal medication.
Favorable Safety Profile
Phototherapy is a largely benign intervention with an excellent safety profile. Its main potential adverse events include erythema, pruritus, xerosis, recurrence of herpes simplex virus infection, and premature skin aging. The effects of phototherapy on skin carcinogenesis have long been controversial; however, data suggest a clear distinction in risk between treatment with NB-UVB and psoralen plus UVA (PUVA). A systematic review of psoriasis patients treated with phototherapy found no evidence to suggest an increased risk of melanoma or nonmelanoma skin cancer with NB-UVB treatment.3 The same cannot be said for psoriasis patients treated with PUVA, who were noted to have a higher incidence of nonmelanoma skin cancer than the general population. This increased risk was more substantial in American cohorts than in European cohorts, likely due to multiple factors including variable skin types and treatment regimens. Increased rates of melanoma also were noted in American PUVA cohorts, with no similar increase seen in their European counterparts.3
Broad vs Targeted Therapies
Targeted therapies have dominated the health care landscape over the last few years, with the majority of new medications being highly focused and only efficacious in a few conditions. One of phototherapy’s greatest strengths is its lack of specificity. Because the field of dermatology is filled with rare, overlapping, and often poorly understood diseases, nonspecific treatment options are needed to fill the gaps. Many generalized skin conditions may lack treatment options indicated by the US Food and Drug Administration. Phototherapy is the ultimate untargeted intervention and may be broadly used for a wide range of cutaneous conditions. Although classically utilized for atopic dermatitis and psoriasis, NB-UVB also can effectively treat generalized pruritus, vitiligo, urticaria, and seborrheic dermatitis.4 Not to be outdone, PUVA has shown success in treating more than 50 different dermatologic conditions including lichen planus, alopecia areata, and mycosis fungoides.
Final Thoughts
Phototherapy is a safe, accessible, and widely applicable treatment for a range of cutaneous disorders. Although more precisely engineered internal therapies have begun to replace UV light in psoriasis and atopic dermatitis, phototherapy likely will always remain an ideal treatment for a wide cohort of patients. Between increased access to home units and the continued validation of its excellent safety record, the future of phototherapy is looking bright.
- Grzybowski A, Sak J, Pawlikowski J. A brief report on the history of phototherapy. Clin Dermatol. 2016;34:532-537.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomised controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):22-31.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ledo E, Ledo A. Phototherapy, photochemotherapy, and photodynamic therapy: unapproved uses or indications. Clin Dermatol. 2000;18:77-86.
Phototherapy has been used to treat skin diseases for millennia. From the Incas to the ancient Greeks and Egyptians, nearly every major civilization has attempted to harness the sun, with some even worshipping it for its healing powers.1 Today, phototherapy remains as important as ever. Despite the technological advances that have brought about biologic medications, small molecule inhibitors, and elegant vehicle delivery systems, phototherapy continues to be a valuable tool in the dermatologist’s armamentarium.
Patient Access to Phototherapy
An important step in successfully managing any disease is access to treatment. In today’s health care landscape, therapeutic decisions frequently are dictated by a patient’s financial situation as well as by the discretion of payers. Costly medications such as biologics often are not accessible to patients on government insurance who fall into the Medicare “donut hole” and may be denied by insurance companies for a myriad of reasons. Luckily, phototherapy typically is well covered and is even a first-line treatment option for some conditions, such as mycosis fungoides.
Nevertheless, phototherapy also has its own unique accessibility hurdles. The time-consuming nature of office-based phototherapy treatment is the main barrier, and many patients find it difficult to incorporate treatments into their daily lives. Additionally, office-based phototherapy units often are clustered in major cities, making access more difficult for rural patients. Because light-responsive conditions often are chronic and may require a lifetime of treatment, home phototherapy units are now being recognized as cost-effective treatment options and are increasingly covered by insurance. In fact, one study comparing psoriasis patients treated with home narrowband UVB (NB-UVB) vs outpatient NB-UVB found that in-home treatment was equally as effective as office-based treatment at a similar cost.2 Because studies comparing the effectiveness of office-based vs home-based phototherapy treatment are underway for various other diseases, hopefully more patients will be able to receive home units, thus increasing access to safe and effective treatment.
Wide Range of Treatment Indications
Another merit of phototherapy is its ability to be used in almost all patient populations. It is one of the few modalities whose indications span the entire length of the human lifetime—from pediatric atopic dermatitis to chronic pruritus in elderly patients. Phototherapy also is one of the few treatment options that is safe to use in patients with an active malignancy or in patients who have multiple other medical conditions. Comorbidities including congestive heart failure, chronic infections, and demyelinating disorders often prevent the use of oral and injectable medications for immune-mediated disorders such as psoriasis or atopic dermatitis. In patients with multiple comorbidities whose disease remains uncontrolled despite an adequate topical regimen, phototherapy is one of the few effective treatment options that remain. Additionally, there is a considerable number of patients who prefer external treatments for cutaneous diseases. For these patients, phototherapy offers the opportunity to control skin conditions without the use of an internal medication.
Favorable Safety Profile
Phototherapy is a largely benign intervention with an excellent safety profile. Its main potential adverse events include erythema, pruritus, xerosis, recurrence of herpes simplex virus infection, and premature skin aging. The effects of phototherapy on skin carcinogenesis have long been controversial; however, data suggest a clear distinction in risk between treatment with NB-UVB and psoralen plus UVA (PUVA). A systematic review of psoriasis patients treated with phototherapy found no evidence to suggest an increased risk of melanoma or nonmelanoma skin cancer with NB-UVB treatment.3 The same cannot be said for psoriasis patients treated with PUVA, who were noted to have a higher incidence of nonmelanoma skin cancer than the general population. This increased risk was more substantial in American cohorts than in European cohorts, likely due to multiple factors including variable skin types and treatment regimens. Increased rates of melanoma also were noted in American PUVA cohorts, with no similar increase seen in their European counterparts.3
Broad vs Targeted Therapies
Targeted therapies have dominated the health care landscape over the last few years, with the majority of new medications being highly focused and only efficacious in a few conditions. One of phototherapy’s greatest strengths is its lack of specificity. Because the field of dermatology is filled with rare, overlapping, and often poorly understood diseases, nonspecific treatment options are needed to fill the gaps. Many generalized skin conditions may lack treatment options indicated by the US Food and Drug Administration. Phototherapy is the ultimate untargeted intervention and may be broadly used for a wide range of cutaneous conditions. Although classically utilized for atopic dermatitis and psoriasis, NB-UVB also can effectively treat generalized pruritus, vitiligo, urticaria, and seborrheic dermatitis.4 Not to be outdone, PUVA has shown success in treating more than 50 different dermatologic conditions including lichen planus, alopecia areata, and mycosis fungoides.
Final Thoughts
Phototherapy is a safe, accessible, and widely applicable treatment for a range of cutaneous disorders. Although more precisely engineered internal therapies have begun to replace UV light in psoriasis and atopic dermatitis, phototherapy likely will always remain an ideal treatment for a wide cohort of patients. Between increased access to home units and the continued validation of its excellent safety record, the future of phototherapy is looking bright.
Phototherapy has been used to treat skin diseases for millennia. From the Incas to the ancient Greeks and Egyptians, nearly every major civilization has attempted to harness the sun, with some even worshipping it for its healing powers.1 Today, phototherapy remains as important as ever. Despite the technological advances that have brought about biologic medications, small molecule inhibitors, and elegant vehicle delivery systems, phototherapy continues to be a valuable tool in the dermatologist’s armamentarium.
Patient Access to Phototherapy
An important step in successfully managing any disease is access to treatment. In today’s health care landscape, therapeutic decisions frequently are dictated by a patient’s financial situation as well as by the discretion of payers. Costly medications such as biologics often are not accessible to patients on government insurance who fall into the Medicare “donut hole” and may be denied by insurance companies for a myriad of reasons. Luckily, phototherapy typically is well covered and is even a first-line treatment option for some conditions, such as mycosis fungoides.
Nevertheless, phototherapy also has its own unique accessibility hurdles. The time-consuming nature of office-based phototherapy treatment is the main barrier, and many patients find it difficult to incorporate treatments into their daily lives. Additionally, office-based phototherapy units often are clustered in major cities, making access more difficult for rural patients. Because light-responsive conditions often are chronic and may require a lifetime of treatment, home phototherapy units are now being recognized as cost-effective treatment options and are increasingly covered by insurance. In fact, one study comparing psoriasis patients treated with home narrowband UVB (NB-UVB) vs outpatient NB-UVB found that in-home treatment was equally as effective as office-based treatment at a similar cost.2 Because studies comparing the effectiveness of office-based vs home-based phototherapy treatment are underway for various other diseases, hopefully more patients will be able to receive home units, thus increasing access to safe and effective treatment.
Wide Range of Treatment Indications
Another merit of phototherapy is its ability to be used in almost all patient populations. It is one of the few modalities whose indications span the entire length of the human lifetime—from pediatric atopic dermatitis to chronic pruritus in elderly patients. Phototherapy also is one of the few treatment options that is safe to use in patients with an active malignancy or in patients who have multiple other medical conditions. Comorbidities including congestive heart failure, chronic infections, and demyelinating disorders often prevent the use of oral and injectable medications for immune-mediated disorders such as psoriasis or atopic dermatitis. In patients with multiple comorbidities whose disease remains uncontrolled despite an adequate topical regimen, phototherapy is one of the few effective treatment options that remain. Additionally, there is a considerable number of patients who prefer external treatments for cutaneous diseases. For these patients, phototherapy offers the opportunity to control skin conditions without the use of an internal medication.
Favorable Safety Profile
Phototherapy is a largely benign intervention with an excellent safety profile. Its main potential adverse events include erythema, pruritus, xerosis, recurrence of herpes simplex virus infection, and premature skin aging. The effects of phototherapy on skin carcinogenesis have long been controversial; however, data suggest a clear distinction in risk between treatment with NB-UVB and psoralen plus UVA (PUVA). A systematic review of psoriasis patients treated with phototherapy found no evidence to suggest an increased risk of melanoma or nonmelanoma skin cancer with NB-UVB treatment.3 The same cannot be said for psoriasis patients treated with PUVA, who were noted to have a higher incidence of nonmelanoma skin cancer than the general population. This increased risk was more substantial in American cohorts than in European cohorts, likely due to multiple factors including variable skin types and treatment regimens. Increased rates of melanoma also were noted in American PUVA cohorts, with no similar increase seen in their European counterparts.3
Broad vs Targeted Therapies
Targeted therapies have dominated the health care landscape over the last few years, with the majority of new medications being highly focused and only efficacious in a few conditions. One of phototherapy’s greatest strengths is its lack of specificity. Because the field of dermatology is filled with rare, overlapping, and often poorly understood diseases, nonspecific treatment options are needed to fill the gaps. Many generalized skin conditions may lack treatment options indicated by the US Food and Drug Administration. Phototherapy is the ultimate untargeted intervention and may be broadly used for a wide range of cutaneous conditions. Although classically utilized for atopic dermatitis and psoriasis, NB-UVB also can effectively treat generalized pruritus, vitiligo, urticaria, and seborrheic dermatitis.4 Not to be outdone, PUVA has shown success in treating more than 50 different dermatologic conditions including lichen planus, alopecia areata, and mycosis fungoides.
Final Thoughts
Phototherapy is a safe, accessible, and widely applicable treatment for a range of cutaneous disorders. Although more precisely engineered internal therapies have begun to replace UV light in psoriasis and atopic dermatitis, phototherapy likely will always remain an ideal treatment for a wide cohort of patients. Between increased access to home units and the continued validation of its excellent safety record, the future of phototherapy is looking bright.
- Grzybowski A, Sak J, Pawlikowski J. A brief report on the history of phototherapy. Clin Dermatol. 2016;34:532-537.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomised controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):22-31.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ledo E, Ledo A. Phototherapy, photochemotherapy, and photodynamic therapy: unapproved uses or indications. Clin Dermatol. 2000;18:77-86.
- Grzybowski A, Sak J, Pawlikowski J. A brief report on the history of phototherapy. Clin Dermatol. 2016;34:532-537.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomised controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):22-31.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ledo E, Ledo A. Phototherapy, photochemotherapy, and photodynamic therapy: unapproved uses or indications. Clin Dermatol. 2000;18:77-86.