User login
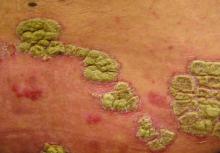
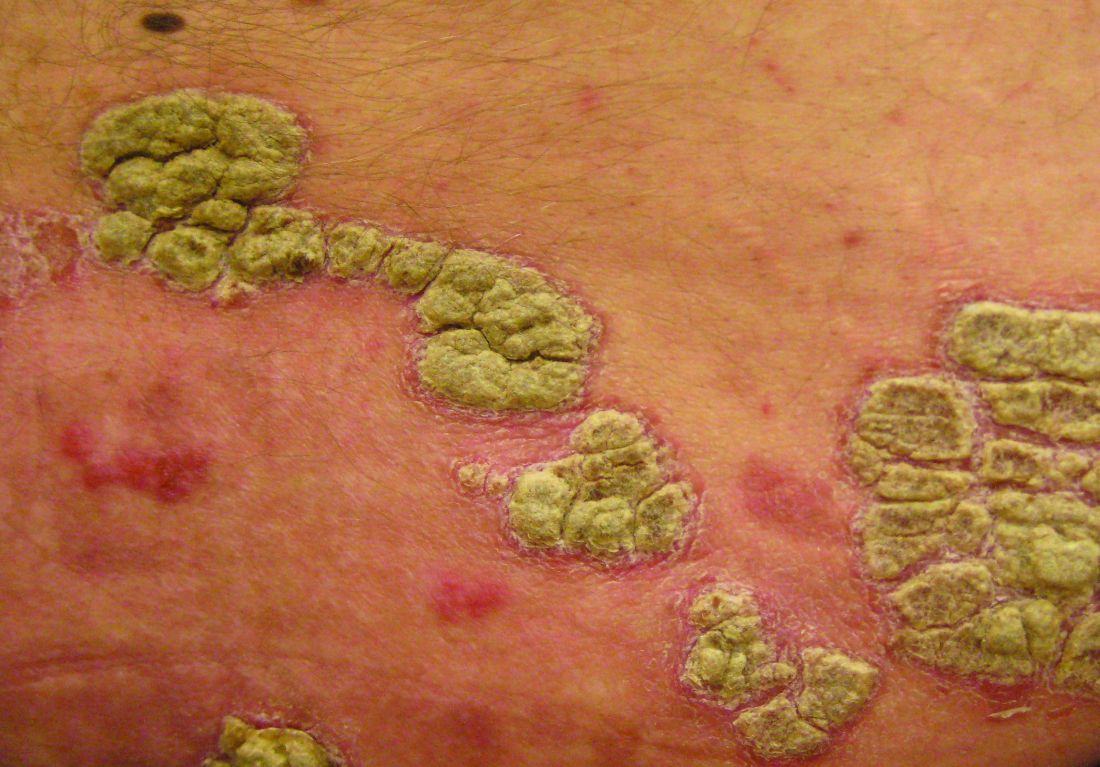
Extended data support ixekizumab for plaque psoriasis
followed over a 4-year extension of a randomized trial.
Patients from a previous study of ixekizumab were treated with 120 mg at the start of the extension, and then 80 mg subcutaneously every 4 weeks, Claus Zachariae, MD, of the University Hospital of Copenhagen Gentofte, and his coauthors reported in the Journal of the American Academy of Dermatology.
At week 208, 82% of the patients achieved Psoriasis Area and Severity Index (PASI) 75, 65% achieved PASI 90, and 45% achieved PASI 100; 65% scored a 0 or 1 on the Physician’s Global Assessment Scale. In addition, 45% of patients reported a score of 0 on the Physician Global Assessment. Patients also reported a decrease in itching from baseline.
A total of 17% of patients experienced a serious adverse event and 87% of the patients experienced at least one treatment-related adverse event by the end of the 4-year extension period. Most of the reported events were mild to moderate; the most common were nasopharyngitis (23%), sinusitis (13%), upper respiratory tract infection (13%), and headache (10%).
The study findings were limited by several factors including the lack of blinding and lack of a placebo, the researchers noted.
However, the results demonstrate “that efficacy can be maintained at high levels for up to 4 years of ixekizumab therapy without apparent increases in health risks or safety issues,” for psoriasis patients, Dr. Zachariae and his associates said. “Longer treatment periods in larger numbers of patients will be reported for patients enrolled in the 5-year phase 3 ixekizumab studies.”
The study was supported by Eli Lilly. Dr. Zachariae disclosed relationships with Eli Lilly and other companies including Janssen, Novartis, AbbVie, and Amgen. His coauthors had financial relationships with multiple companies.
SOURCE: Zachariae C et al. J Am Acad Dermatol. 2018 Aug;79(2):294-301.e6.
followed over a 4-year extension of a randomized trial.
Patients from a previous study of ixekizumab were treated with 120 mg at the start of the extension, and then 80 mg subcutaneously every 4 weeks, Claus Zachariae, MD, of the University Hospital of Copenhagen Gentofte, and his coauthors reported in the Journal of the American Academy of Dermatology.
At week 208, 82% of the patients achieved Psoriasis Area and Severity Index (PASI) 75, 65% achieved PASI 90, and 45% achieved PASI 100; 65% scored a 0 or 1 on the Physician’s Global Assessment Scale. In addition, 45% of patients reported a score of 0 on the Physician Global Assessment. Patients also reported a decrease in itching from baseline.
A total of 17% of patients experienced a serious adverse event and 87% of the patients experienced at least one treatment-related adverse event by the end of the 4-year extension period. Most of the reported events were mild to moderate; the most common were nasopharyngitis (23%), sinusitis (13%), upper respiratory tract infection (13%), and headache (10%).
The study findings were limited by several factors including the lack of blinding and lack of a placebo, the researchers noted.
However, the results demonstrate “that efficacy can be maintained at high levels for up to 4 years of ixekizumab therapy without apparent increases in health risks or safety issues,” for psoriasis patients, Dr. Zachariae and his associates said. “Longer treatment periods in larger numbers of patients will be reported for patients enrolled in the 5-year phase 3 ixekizumab studies.”
The study was supported by Eli Lilly. Dr. Zachariae disclosed relationships with Eli Lilly and other companies including Janssen, Novartis, AbbVie, and Amgen. His coauthors had financial relationships with multiple companies.
SOURCE: Zachariae C et al. J Am Acad Dermatol. 2018 Aug;79(2):294-301.e6.
followed over a 4-year extension of a randomized trial.
Patients from a previous study of ixekizumab were treated with 120 mg at the start of the extension, and then 80 mg subcutaneously every 4 weeks, Claus Zachariae, MD, of the University Hospital of Copenhagen Gentofte, and his coauthors reported in the Journal of the American Academy of Dermatology.
At week 208, 82% of the patients achieved Psoriasis Area and Severity Index (PASI) 75, 65% achieved PASI 90, and 45% achieved PASI 100; 65% scored a 0 or 1 on the Physician’s Global Assessment Scale. In addition, 45% of patients reported a score of 0 on the Physician Global Assessment. Patients also reported a decrease in itching from baseline.
A total of 17% of patients experienced a serious adverse event and 87% of the patients experienced at least one treatment-related adverse event by the end of the 4-year extension period. Most of the reported events were mild to moderate; the most common were nasopharyngitis (23%), sinusitis (13%), upper respiratory tract infection (13%), and headache (10%).
The study findings were limited by several factors including the lack of blinding and lack of a placebo, the researchers noted.
However, the results demonstrate “that efficacy can be maintained at high levels for up to 4 years of ixekizumab therapy without apparent increases in health risks or safety issues,” for psoriasis patients, Dr. Zachariae and his associates said. “Longer treatment periods in larger numbers of patients will be reported for patients enrolled in the 5-year phase 3 ixekizumab studies.”
The study was supported by Eli Lilly. Dr. Zachariae disclosed relationships with Eli Lilly and other companies including Janssen, Novartis, AbbVie, and Amgen. His coauthors had financial relationships with multiple companies.
SOURCE: Zachariae C et al. J Am Acad Dermatol. 2018 Aug;79(2):294-301.e6.
FROM JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Ixekizumab appears to be an effective option for long-term treatment of patients with moderate to severe psoriasis.
Major finding: A total of 82% of patients achieved PASI 75 at week 208 of the open-label extension study.
Study details: The data come from a 4-year open-label extension of a phase 2 randomized, placebo-controlled trial including 120 adults with psoriasis.
Disclosures: The study was supported by Eli Lilly. Dr. Zachariae disclosed relationships with Eli Lilly and other companies including Janssen, Novartis, AbbVie, and Amgen. His coauthors had financial relationships with multiple companies.
Source: Zachariae C et al. J Am Acad Dermatol. 2018 Aug;79(2):294-301.e6.
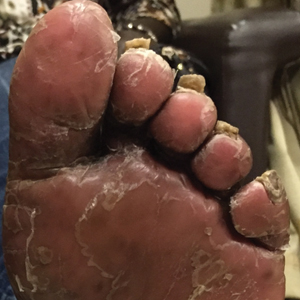
Scalp Psoriasis With Increased Hair Density
Case Report
A 19-year-old man first presented to our outpatient dermatology clinic for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. His medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made, and he was treated with topical corticosteroids for 2 weeks and as needed thereafter.
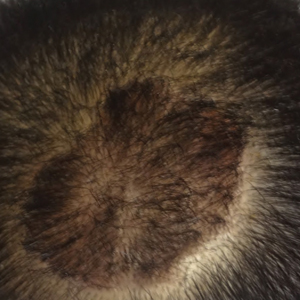
The patient remained stable for 5 years before presenting again to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye. We prescribed clobetasol solution 0.05% twice daily to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.

Comment
The scalp is a site of predilection in psoriasis, as approximately 80% of psoriasis patients report involvement of the scalp.1 Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.1 Alopecia in the setting of scalp psoriasis is common but is not well understood.2 First described by Shuster3 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.4 It clinically presents as pink scaly plaques consistent with psoriasis with overlying alopecia. There are few instances of psoriatic alopecia reported as cicatricial hair loss and generalized telogen effluvium.2 It is known that a higher proportion of telogen and catagen hairs exist in patients with psoriatic alopecia.5 Additionally, psoriasis patients have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients. Many patients achieve hair regrowth following treatment of psoriasis.2
We described a patient with scalp psoriasis who had increased and preserved hair density. Our case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.2 Additionally, the presence of increased hair density in scalp psoriasis can further complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
Case Report
A 19-year-old man first presented to our outpatient dermatology clinic for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. His medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made, and he was treated with topical corticosteroids for 2 weeks and as needed thereafter.
The patient remained stable for 5 years before presenting again to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye. We prescribed clobetasol solution 0.05% twice daily to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.

Comment
The scalp is a site of predilection in psoriasis, as approximately 80% of psoriasis patients report involvement of the scalp.1 Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.1 Alopecia in the setting of scalp psoriasis is common but is not well understood.2 First described by Shuster3 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.4 It clinically presents as pink scaly plaques consistent with psoriasis with overlying alopecia. There are few instances of psoriatic alopecia reported as cicatricial hair loss and generalized telogen effluvium.2 It is known that a higher proportion of telogen and catagen hairs exist in patients with psoriatic alopecia.5 Additionally, psoriasis patients have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients. Many patients achieve hair regrowth following treatment of psoriasis.2
We described a patient with scalp psoriasis who had increased and preserved hair density. Our case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.2 Additionally, the presence of increased hair density in scalp psoriasis can further complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.
Case Report
A 19-year-old man first presented to our outpatient dermatology clinic for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. His medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made, and he was treated with topical corticosteroids for 2 weeks and as needed thereafter.
The patient remained stable for 5 years before presenting again to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye. We prescribed clobetasol solution 0.05% twice daily to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.

Comment
The scalp is a site of predilection in psoriasis, as approximately 80% of psoriasis patients report involvement of the scalp.1 Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.1 Alopecia in the setting of scalp psoriasis is common but is not well understood.2 First described by Shuster3 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.4 It clinically presents as pink scaly plaques consistent with psoriasis with overlying alopecia. There are few instances of psoriatic alopecia reported as cicatricial hair loss and generalized telogen effluvium.2 It is known that a higher proportion of telogen and catagen hairs exist in patients with psoriatic alopecia.5 Additionally, psoriasis patients have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients. Many patients achieve hair regrowth following treatment of psoriasis.2
We described a patient with scalp psoriasis who had increased and preserved hair density. Our case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.2 Additionally, the presence of increased hair density in scalp psoriasis can further complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
Practice Points
- Scalp psoriasis may present with hair loss or increased hair density.
- Psoriasis with increased hair density may make topical medications more difficult to apply.
Diet and Dermatology: Google Search Results for Acne, Psoriasis, and Eczema
Researching medical information currently is the third most common use of the Internet in the United States,1 with the majority of adults using the Web as their first source for health information before seeing a physician.2 When assessing health-related information online, resources can be grouped into 4 categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational.3 Access to such a wide range of sources may give readers the opportunity to share personal anecdotes and opinions, thereby serving as a forum for information that essentially cannot be validated. Although such websites may include useful information and cite current literature, in other instances health-related information may be misleading or fabricated.3
In a study evaluating 291 skin conditions and related Google trends, acne, psoriasis, and eczema were among the most burdensome diseases, with acne yielding the highest number of search results.4 Results of the study indicated a positive correlation between disease burden and online search interest.4 The impact of these online searches and the validity of Google search results are topics worth considering, as more dermatology patients are relying on holistic and nonpharmaceutical approaches to treatment and disease management.5 The purpose of this study was to evaluate content on diet and dermatology available on the Internet for acne, psoriasis, and eczema.
Methods
Google searches were performed in December 2017 using the terms diet and acne, diet and psoriasis, and diet and eczema. The first 10 results for each respective search were reviewed for recommendations about which foods to incorporate in the diet and which to avoid. They also were classified according to the following 4 website categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational. The recommendations gathered from the 30 websites were then compared to the current literature assessing the impact of diet on these respective conditions by conducting PubMed searches of articles indexed for MEDLINE using the same terms.
Results
The results of this study are outlined in the eTable.

Acne
Our Google search using the term diet and acne produced 17,500,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 40% (4/10) were educational resources, and 20% (2/10) were promotional websites. Most of the websites advised acne patients to avoid high glycemic index foods (90% [9/10]) and dairy products (90% [9/10]). When discussing which foods to include in the diet, 70% (7/10) of websites recommended that patients incorporate omega-3 fatty acids and antioxidants in the diet.
Research has shown that a low glycemic index diet can lead to a decrease in patients’ acne lesion counts in some instances.6,7 In a case-controlled study of 2258 patients on a popular weight loss diet that emphasized low glycemic index foods, 87% of participants reported a reduction in acne and 91% reported a decrease in their dosage or number of acne medications.7 Still, the exact correlation between acne development and consumption of glycemic index foods has not been confirmed. However, high glycemic index diets have been linked to hyperinsulinemia, indicating that insulin levels may play a role in acne formation.8 The majority of other currently available studies evaluated the potential link between dairy consumption and acne. A retrospective analysis of 47,355 women spanning 12 weeks showed a positive link between increased dairy consumption, specifically skim milk, and acne formation. Despite the positive trend, limitations such as recall bias made it difficult to draw a conclusion based on these findings.9 However, results of a longitudinal questionnaire-based population study evaluating the impact of dairy consumption on acne in 2489 adolescent patients confirmed a positive correlation.10 Studies conducted in 2009 and 2011 concluded that milk consumption results in elevated insulinlike growth factor 1 levels, which were linked to comedogenesis.8,11
Currently, there are well-described mechanisms to explain the association of dairy consumption and glycemic index with acne. Confirming a correlation between acne development and dairy consumption suggests that a dairy-free diet may benefit acne patients.5 Other trials indicate that low glycemic index diets are beneficial in treating acne.6,7 Therefore, some of the recommendations made in our search results may be of merit; however, there is minimal evidence proving the benefits of the other dietary recommendations made in the websites we evaluated.
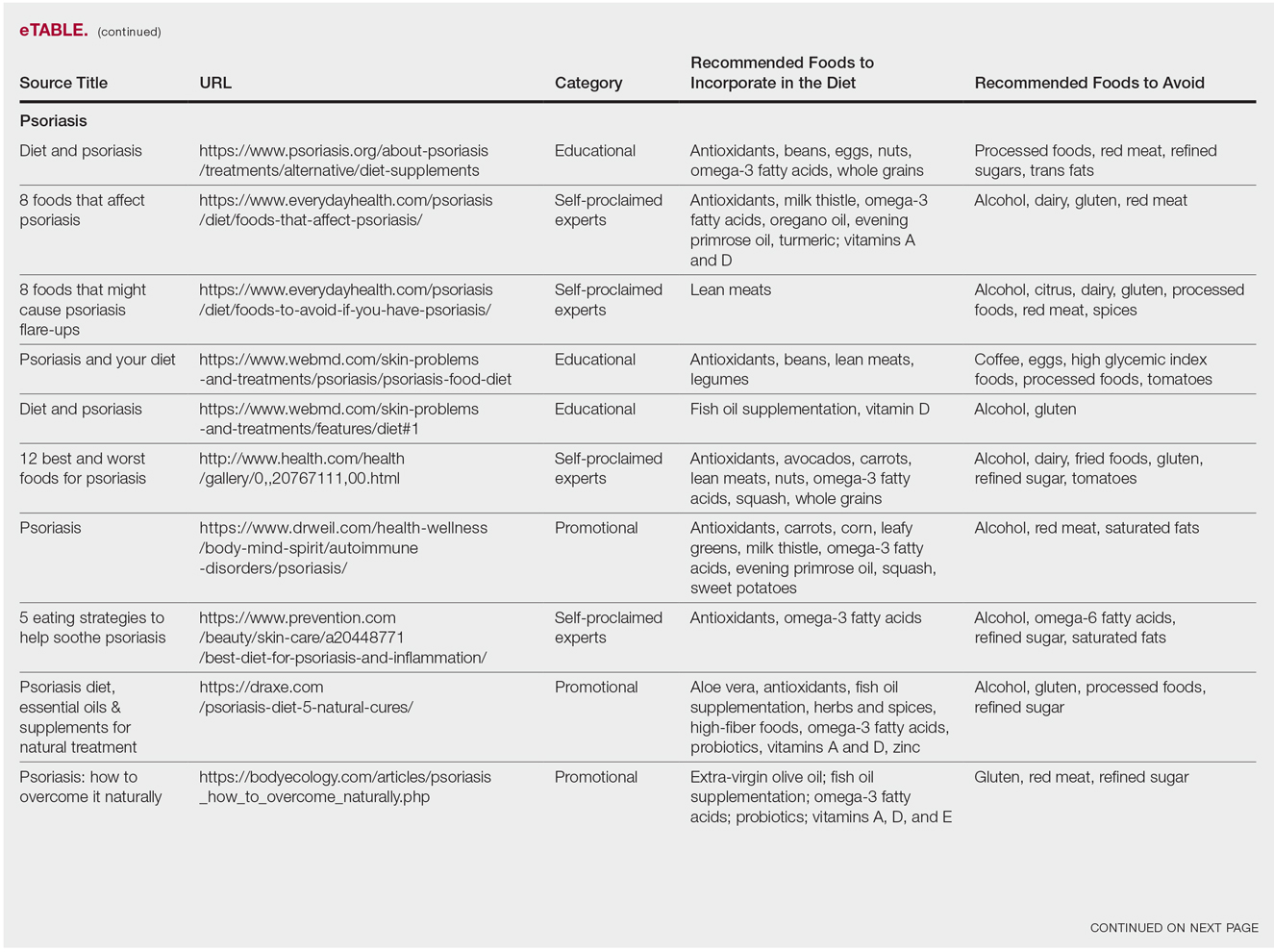
Psoriasis
Our Google search using the term diet and psoriasis yielded a total of 9,420,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 30% (3/10) were promotional, and 30% (3/10) were educational. Seventy percent (7/10) of websites recommended avoiding alcohol and 60% (6/10) recommended avoiding gluten, with others discouraging consumption of red meat. Most of the websites encouraged patients to consume omega-3 fatty acids and antioxidants, while a few also recommended vitamins A, D, and E, as well as evening primrose oil supplements.
Although current research indicates a positive correlation between excessive alcohol use and psoriasis severity, it is still unclear whether alcohol consumption can be directly linked to the disease.12-14 Likewise, despite belief that increased oxidative stress likely contributes to inflammation in psoriasis, there is little evidence linking antioxidants to improvement in psoriasis symptoms.12 However, the current literature is inconsistent regarding the effects of fish oil supplementation on psoriasis.12 In a randomized double-blind study of 145 patients, there was no significant difference in psoriasis area and severity index scores between a control group and a treatment group receiving fish oil supplementation.15 In another RCT of 45 participants, those given daily very long-chain omega-3 fatty acid supplements saw no difference in psoriasis symptoms.15 Despite debate, literature assessing the impact of gluten-free diets has described improvement in psoriasis lesions in patients with celiac-specific antibodies.16 Although some observational studies described vitamin D supplementation to be beneficial in the treatment of psoriatic lesions, a more recent RCT found no significant difference between control and treatment groups.17-19
Studies also have revealed that certain eating patterns, such as those associated with the Mediterranean diet that is rich in fruits, vegetables, whole grains, and omega-3 fatty acids may be linked to improved endothelial function scores and reduced C-reactive protein and IL-18levels.20,21 In a double-blind RCT of 75 patients with plaque psoriasis, mean (SD) psoriasis area and severity index scores decreased by 11.2 (9.8) in a group treated with omega-3 fatty acids compared to 7.5 (8.8) with omega-6 fatty acids (P=.048).22
Although excessive alcohol use may be linked to psoriasis, there is no conclusive evidence indicating causation, thereby discrediting online claims.12-14 Research has revealed that gluten-free diets in psoriasis patients with celiac disease may improve psoriasis treatment16; however, sufficient evidence is lacking for diets low in gluten and high in polyunsaturated fatty acids or antioxidant supplementation. Of the dietary supplements recommended in the search results we reviewed, fish oil appears to be the most promising, but no recommendations can be made based on the current research.
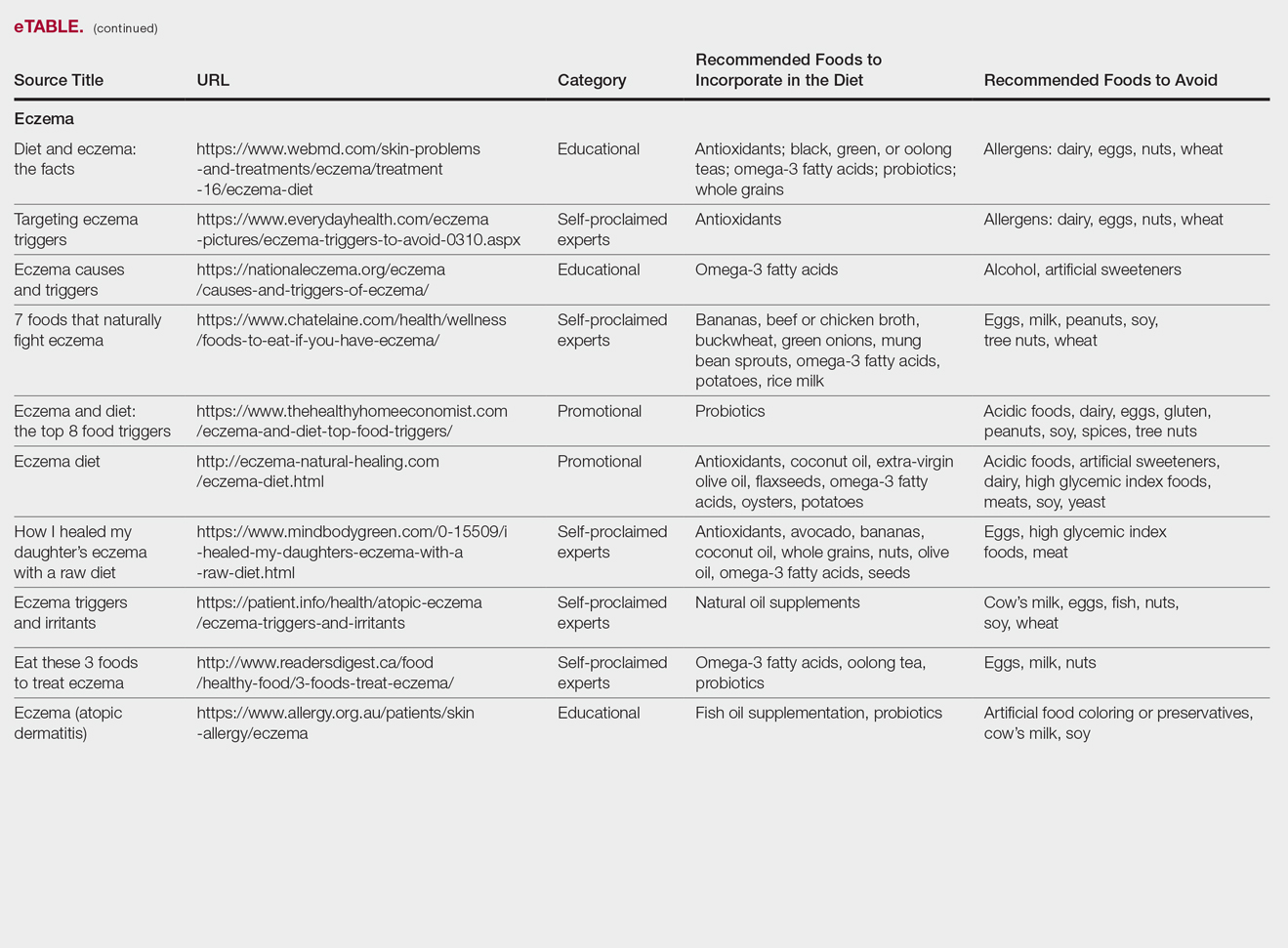
Eczema
Our Google search using the term diet and eczema yielded 1,160,000 results, with 50% (5/10) of websites attributed to self-proclaimed experts, 30% (3/10) to educational websites, and 20% (2/10) to promotional sites. Of the first 10 results, 80% (8/10) recommended that patients with eczema avoid milk/dairy and 50% (5/10) advised to avoid soy and wheat/gluten. Other websites indicated to avoid eggs, nuts, and artificial sweeteners. Patients were encouraged to incorporate omega-3 fatty acids in their diets, and a few sites recommended bananas, coconut oil, olive oil, and various teas.
In a review of 11 studies with a total of 596 participants, supplementation with vitamins D and E, fish oil, olive oil, and linoleic acid was evaluated for the treatment of eczema.23 Although results indicated modest improvement of eczema severity with supplementation of fish oil, evidence favoring this treatment is limited and unconvincing. Furthermore, some evidence indicates that elimination diets are only appropriate for patients with food allergies.24 In a study evaluating an egg-free and dairy-free diet for eczema patients, only participants with positive egg-specific serum IgE levels saw improvement in disease severity.23 Even though IgE-mediated food allergies have been reported in 40% of children with moderate eczema, the contribution of these allergies to eczema is questionable.25
There is little evidence in the literature to indicate a definitive correlation between the foods mentioned in the search results we evaluated and the development of eczema; however, for patients with food allergies and eczema, elimination diets may decrease disease severity.25,26 There is insufficient evidence to suggest a benefit from evening primrose oil or fish oil supplementation, thereby debunking claims found online.
Comment
Although our Google search results included a wide range of sources and information regarding diet and dermatologic conditions such as acne, psoriasis, and eczema, most of the information we found was either unfounded or misleading. Study limitations in the current literature include small sample size, potential recall bias, lack of appropriate controls, incomplete reported results, and the failure to clearly define skin changes.
When considering the accuracy and type of information regarding skin conditions that is available on the Internet, it is important to note that most of the results we reviewed were webpages attributed to self-proclaimed experts. Although educational websites also were included in the search results, whether or not patients prefer or understand the content of such websites is still unknown; therefore, health organizations should consider revising online patient education materials to allow universal comprehension.27
Furthermore, it is important to consider the impact that widespread Internet access may have on the physician-patient relationship. Having access to health-related information online and being able to potentially self-diagnose could delay or deter patients from seeking professional advice or care.3 A study evaluating the impact of online searches on the physician-patient relationship among 175 patients determined that 36.5% of patients gathered information online prior to their consultation with a physician, while 67.3% chose to complement the information given to them by their physician with online resources.28 Based on these statistics, it is important that physicians be up-to-date with Internet discourse to discredit unfounded recommendations. Ultimately, when it comes to diet and dermatology, patients ought to be skeptical of the information currently available on the Internet, given that most of it is unsubstantiated by medical research.
- Fox S. Online health search 2006. Pew Research Center website. http://www.pewinternet.org/2006/10/29/online-health-search-2006/. Published October 29, 2006. Accessed May 3, 2018.
- Prestin A, Vieux SN, Chou WY. Is online health activity alive and well or flatlining? findings from 10 years of the health information national trends survey. J Health Commun. 2015;20:790-798.
- Zeichner JA, Del Rosso JQ. Acne and the internet. Dermatol Clin. 2016;34:129-132.
- Whitsitt J, Karimkhani C, Boyers LN, et al. Comparing burden of dermatologic disease to search interest on Google trends. Dermatol Online J. 2015;21. pii:13030/qt5xg811qp.
- Shokeen D. Influence of diet in acne vulgaris and atopic dermatitis. Cutis. 2016;98:E28-E29.
- Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84-91.
- Rouhani P. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(3, suppl 1):P706.
- Melnick BC. Evidence for acne-promoting effect of milk and other insulinotropic dairy products. Nestle Nutr Worksop Ser Pediatr Program. 2011;67:131-145.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. High school dietary diary intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Ulvestad M, Bjertness E, Dalgard F, et al. Acne and dairy products in adolescence: results from a Norwegian longitudinal study [published online July 16, 2016]. J Eur Acad Dermatol Venereol. 2017;31:530-535.
- Melnick BC, Schmitz G. Role of insulin, insulin like growth factor 1, hyperglycemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Murzaku EC, Bronsnick T, Rao BK. Diet in dermatology: part II. melanoma, chronic urticaria, and psoriasis. J Am Acad Dermatol. 2014;71:1053.E1-1053.E16.
- Tobin AM, Higgins EM, Norris S, et al. Prevalence of psoriasis in patients with alcoholic liver disease. Clin Exp Dermatol. 2009;34:698-701.
- Kirby B, Richards HL, Mason DL, et al. Alcohol consumption and psychological distress in patients with psoriasis. Br J Dermatol. 2008;158:138-140.
- Søyland E, Funk J, Rajika G, et al. Effect of dietary supplementation with very long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Morimoto S, Yoshikawa K. Psoriasis and vitamin D3. a review of our experience. Arch Dermatol. 1989;125:231-234.
- Smith EL, Pincus SH, Donovan L, et al. A novel approach for the evaluation and treatment of psoriasis. oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis. J Am Acad Dermatol. 1988;19:516-528.
- Siddiqui MA, Al-Khawajah MM. Vitamin D3 and psoriasis: a randomized double-blind placebo-controlled study. J Dermatol Treat. 1990;1:243-245.
- Wang Y, Gao H, Loyd CM, et al. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol. 2012;132:2067-2075.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13. pii:E743. doi:10.3390/ijerph13070743.
- Mayser P, Mrowietz U, Arenberger P, et al. Omega-3 fatty acid-based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38:539-547.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012;2:CD005205.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer [published online November 15, 2014]. J Am Acad Dermatol. 2014;71:1039.E1-1039.E12.
- Campbell DE. The role of food allergy in childhood atopic dermatitis. J Paediatr Child Health. 2012;48:1058-1064.
- Werfel T, Erdmann S, Fuchs T, et al. Approach to suspected food allergy in atopic dermatitis. guideline of the Task Force on Food Allergy of the German Society of Allergology and Clinical Immunology (DGAKI) and the Medical Association of German Allergologists (ADA) and the German Society of Pediatric Allergology (GPA). J Dtsch Dermatol Ges. 2009;3:265-271.
- John AM, John ES, Hansberry DR, et al. Assessment of online patient education materials from major dermatologic associations. J Clin Aesthet Dermatol. 2016;9:23-28.
- Orgaz-Molina J, Cotugno M, Girón-Prieto MS, et al. A study of internet searches for medical information in dermatology patients: the patient-physician relationship. Actas Dermosifiliogr. 2015;106:493-499.
Researching medical information currently is the third most common use of the Internet in the United States,1 with the majority of adults using the Web as their first source for health information before seeing a physician.2 When assessing health-related information online, resources can be grouped into 4 categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational.3 Access to such a wide range of sources may give readers the opportunity to share personal anecdotes and opinions, thereby serving as a forum for information that essentially cannot be validated. Although such websites may include useful information and cite current literature, in other instances health-related information may be misleading or fabricated.3
In a study evaluating 291 skin conditions and related Google trends, acne, psoriasis, and eczema were among the most burdensome diseases, with acne yielding the highest number of search results.4 Results of the study indicated a positive correlation between disease burden and online search interest.4 The impact of these online searches and the validity of Google search results are topics worth considering, as more dermatology patients are relying on holistic and nonpharmaceutical approaches to treatment and disease management.5 The purpose of this study was to evaluate content on diet and dermatology available on the Internet for acne, psoriasis, and eczema.
Methods
Google searches were performed in December 2017 using the terms diet and acne, diet and psoriasis, and diet and eczema. The first 10 results for each respective search were reviewed for recommendations about which foods to incorporate in the diet and which to avoid. They also were classified according to the following 4 website categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational. The recommendations gathered from the 30 websites were then compared to the current literature assessing the impact of diet on these respective conditions by conducting PubMed searches of articles indexed for MEDLINE using the same terms.
Results
The results of this study are outlined in the eTable.



Acne
Our Google search using the term diet and acne produced 17,500,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 40% (4/10) were educational resources, and 20% (2/10) were promotional websites. Most of the websites advised acne patients to avoid high glycemic index foods (90% [9/10]) and dairy products (90% [9/10]). When discussing which foods to include in the diet, 70% (7/10) of websites recommended that patients incorporate omega-3 fatty acids and antioxidants in the diet.
Research has shown that a low glycemic index diet can lead to a decrease in patients’ acne lesion counts in some instances.6,7 In a case-controlled study of 2258 patients on a popular weight loss diet that emphasized low glycemic index foods, 87% of participants reported a reduction in acne and 91% reported a decrease in their dosage or number of acne medications.7 Still, the exact correlation between acne development and consumption of glycemic index foods has not been confirmed. However, high glycemic index diets have been linked to hyperinsulinemia, indicating that insulin levels may play a role in acne formation.8 The majority of other currently available studies evaluated the potential link between dairy consumption and acne. A retrospective analysis of 47,355 women spanning 12 weeks showed a positive link between increased dairy consumption, specifically skim milk, and acne formation. Despite the positive trend, limitations such as recall bias made it difficult to draw a conclusion based on these findings.9 However, results of a longitudinal questionnaire-based population study evaluating the impact of dairy consumption on acne in 2489 adolescent patients confirmed a positive correlation.10 Studies conducted in 2009 and 2011 concluded that milk consumption results in elevated insulinlike growth factor 1 levels, which were linked to comedogenesis.8,11
Currently, there are well-described mechanisms to explain the association of dairy consumption and glycemic index with acne. Confirming a correlation between acne development and dairy consumption suggests that a dairy-free diet may benefit acne patients.5 Other trials indicate that low glycemic index diets are beneficial in treating acne.6,7 Therefore, some of the recommendations made in our search results may be of merit; however, there is minimal evidence proving the benefits of the other dietary recommendations made in the websites we evaluated.
Psoriasis
Our Google search using the term diet and psoriasis yielded a total of 9,420,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 30% (3/10) were promotional, and 30% (3/10) were educational. Seventy percent (7/10) of websites recommended avoiding alcohol and 60% (6/10) recommended avoiding gluten, with others discouraging consumption of red meat. Most of the websites encouraged patients to consume omega-3 fatty acids and antioxidants, while a few also recommended vitamins A, D, and E, as well as evening primrose oil supplements.
Although current research indicates a positive correlation between excessive alcohol use and psoriasis severity, it is still unclear whether alcohol consumption can be directly linked to the disease.12-14 Likewise, despite belief that increased oxidative stress likely contributes to inflammation in psoriasis, there is little evidence linking antioxidants to improvement in psoriasis symptoms.12 However, the current literature is inconsistent regarding the effects of fish oil supplementation on psoriasis.12 In a randomized double-blind study of 145 patients, there was no significant difference in psoriasis area and severity index scores between a control group and a treatment group receiving fish oil supplementation.15 In another RCT of 45 participants, those given daily very long-chain omega-3 fatty acid supplements saw no difference in psoriasis symptoms.15 Despite debate, literature assessing the impact of gluten-free diets has described improvement in psoriasis lesions in patients with celiac-specific antibodies.16 Although some observational studies described vitamin D supplementation to be beneficial in the treatment of psoriatic lesions, a more recent RCT found no significant difference between control and treatment groups.17-19
Studies also have revealed that certain eating patterns, such as those associated with the Mediterranean diet that is rich in fruits, vegetables, whole grains, and omega-3 fatty acids may be linked to improved endothelial function scores and reduced C-reactive protein and IL-18levels.20,21 In a double-blind RCT of 75 patients with plaque psoriasis, mean (SD) psoriasis area and severity index scores decreased by 11.2 (9.8) in a group treated with omega-3 fatty acids compared to 7.5 (8.8) with omega-6 fatty acids (P=.048).22
Although excessive alcohol use may be linked to psoriasis, there is no conclusive evidence indicating causation, thereby discrediting online claims.12-14 Research has revealed that gluten-free diets in psoriasis patients with celiac disease may improve psoriasis treatment16; however, sufficient evidence is lacking for diets low in gluten and high in polyunsaturated fatty acids or antioxidant supplementation. Of the dietary supplements recommended in the search results we reviewed, fish oil appears to be the most promising, but no recommendations can be made based on the current research.
Eczema
Our Google search using the term diet and eczema yielded 1,160,000 results, with 50% (5/10) of websites attributed to self-proclaimed experts, 30% (3/10) to educational websites, and 20% (2/10) to promotional sites. Of the first 10 results, 80% (8/10) recommended that patients with eczema avoid milk/dairy and 50% (5/10) advised to avoid soy and wheat/gluten. Other websites indicated to avoid eggs, nuts, and artificial sweeteners. Patients were encouraged to incorporate omega-3 fatty acids in their diets, and a few sites recommended bananas, coconut oil, olive oil, and various teas.
In a review of 11 studies with a total of 596 participants, supplementation with vitamins D and E, fish oil, olive oil, and linoleic acid was evaluated for the treatment of eczema.23 Although results indicated modest improvement of eczema severity with supplementation of fish oil, evidence favoring this treatment is limited and unconvincing. Furthermore, some evidence indicates that elimination diets are only appropriate for patients with food allergies.24 In a study evaluating an egg-free and dairy-free diet for eczema patients, only participants with positive egg-specific serum IgE levels saw improvement in disease severity.23 Even though IgE-mediated food allergies have been reported in 40% of children with moderate eczema, the contribution of these allergies to eczema is questionable.25
There is little evidence in the literature to indicate a definitive correlation between the foods mentioned in the search results we evaluated and the development of eczema; however, for patients with food allergies and eczema, elimination diets may decrease disease severity.25,26 There is insufficient evidence to suggest a benefit from evening primrose oil or fish oil supplementation, thereby debunking claims found online.
Comment
Although our Google search results included a wide range of sources and information regarding diet and dermatologic conditions such as acne, psoriasis, and eczema, most of the information we found was either unfounded or misleading. Study limitations in the current literature include small sample size, potential recall bias, lack of appropriate controls, incomplete reported results, and the failure to clearly define skin changes.
When considering the accuracy and type of information regarding skin conditions that is available on the Internet, it is important to note that most of the results we reviewed were webpages attributed to self-proclaimed experts. Although educational websites also were included in the search results, whether or not patients prefer or understand the content of such websites is still unknown; therefore, health organizations should consider revising online patient education materials to allow universal comprehension.27
Furthermore, it is important to consider the impact that widespread Internet access may have on the physician-patient relationship. Having access to health-related information online and being able to potentially self-diagnose could delay or deter patients from seeking professional advice or care.3 A study evaluating the impact of online searches on the physician-patient relationship among 175 patients determined that 36.5% of patients gathered information online prior to their consultation with a physician, while 67.3% chose to complement the information given to them by their physician with online resources.28 Based on these statistics, it is important that physicians be up-to-date with Internet discourse to discredit unfounded recommendations. Ultimately, when it comes to diet and dermatology, patients ought to be skeptical of the information currently available on the Internet, given that most of it is unsubstantiated by medical research.
Researching medical information currently is the third most common use of the Internet in the United States,1 with the majority of adults using the Web as their first source for health information before seeing a physician.2 When assessing health-related information online, resources can be grouped into 4 categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational.3 Access to such a wide range of sources may give readers the opportunity to share personal anecdotes and opinions, thereby serving as a forum for information that essentially cannot be validated. Although such websites may include useful information and cite current literature, in other instances health-related information may be misleading or fabricated.3
In a study evaluating 291 skin conditions and related Google trends, acne, psoriasis, and eczema were among the most burdensome diseases, with acne yielding the highest number of search results.4 Results of the study indicated a positive correlation between disease burden and online search interest.4 The impact of these online searches and the validity of Google search results are topics worth considering, as more dermatology patients are relying on holistic and nonpharmaceutical approaches to treatment and disease management.5 The purpose of this study was to evaluate content on diet and dermatology available on the Internet for acne, psoriasis, and eczema.
Methods
Google searches were performed in December 2017 using the terms diet and acne, diet and psoriasis, and diet and eczema. The first 10 results for each respective search were reviewed for recommendations about which foods to incorporate in the diet and which to avoid. They also were classified according to the following 4 website categories: (1) those attributed to self-proclaimed experts, (2) promotional, (3) social media, and (4) educational. The recommendations gathered from the 30 websites were then compared to the current literature assessing the impact of diet on these respective conditions by conducting PubMed searches of articles indexed for MEDLINE using the same terms.
Results
The results of this study are outlined in the eTable.



Acne
Our Google search using the term diet and acne produced 17,500,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 40% (4/10) were educational resources, and 20% (2/10) were promotional websites. Most of the websites advised acne patients to avoid high glycemic index foods (90% [9/10]) and dairy products (90% [9/10]). When discussing which foods to include in the diet, 70% (7/10) of websites recommended that patients incorporate omega-3 fatty acids and antioxidants in the diet.
Research has shown that a low glycemic index diet can lead to a decrease in patients’ acne lesion counts in some instances.6,7 In a case-controlled study of 2258 patients on a popular weight loss diet that emphasized low glycemic index foods, 87% of participants reported a reduction in acne and 91% reported a decrease in their dosage or number of acne medications.7 Still, the exact correlation between acne development and consumption of glycemic index foods has not been confirmed. However, high glycemic index diets have been linked to hyperinsulinemia, indicating that insulin levels may play a role in acne formation.8 The majority of other currently available studies evaluated the potential link between dairy consumption and acne. A retrospective analysis of 47,355 women spanning 12 weeks showed a positive link between increased dairy consumption, specifically skim milk, and acne formation. Despite the positive trend, limitations such as recall bias made it difficult to draw a conclusion based on these findings.9 However, results of a longitudinal questionnaire-based population study evaluating the impact of dairy consumption on acne in 2489 adolescent patients confirmed a positive correlation.10 Studies conducted in 2009 and 2011 concluded that milk consumption results in elevated insulinlike growth factor 1 levels, which were linked to comedogenesis.8,11
Currently, there are well-described mechanisms to explain the association of dairy consumption and glycemic index with acne. Confirming a correlation between acne development and dairy consumption suggests that a dairy-free diet may benefit acne patients.5 Other trials indicate that low glycemic index diets are beneficial in treating acne.6,7 Therefore, some of the recommendations made in our search results may be of merit; however, there is minimal evidence proving the benefits of the other dietary recommendations made in the websites we evaluated.
Psoriasis
Our Google search using the term diet and psoriasis yielded a total of 9,420,000 results. Of the first 10 search results, 40% (4/10) were websites attributed to self-proclaimed experts, 30% (3/10) were promotional, and 30% (3/10) were educational. Seventy percent (7/10) of websites recommended avoiding alcohol and 60% (6/10) recommended avoiding gluten, with others discouraging consumption of red meat. Most of the websites encouraged patients to consume omega-3 fatty acids and antioxidants, while a few also recommended vitamins A, D, and E, as well as evening primrose oil supplements.
Although current research indicates a positive correlation between excessive alcohol use and psoriasis severity, it is still unclear whether alcohol consumption can be directly linked to the disease.12-14 Likewise, despite belief that increased oxidative stress likely contributes to inflammation in psoriasis, there is little evidence linking antioxidants to improvement in psoriasis symptoms.12 However, the current literature is inconsistent regarding the effects of fish oil supplementation on psoriasis.12 In a randomized double-blind study of 145 patients, there was no significant difference in psoriasis area and severity index scores between a control group and a treatment group receiving fish oil supplementation.15 In another RCT of 45 participants, those given daily very long-chain omega-3 fatty acid supplements saw no difference in psoriasis symptoms.15 Despite debate, literature assessing the impact of gluten-free diets has described improvement in psoriasis lesions in patients with celiac-specific antibodies.16 Although some observational studies described vitamin D supplementation to be beneficial in the treatment of psoriatic lesions, a more recent RCT found no significant difference between control and treatment groups.17-19
Studies also have revealed that certain eating patterns, such as those associated with the Mediterranean diet that is rich in fruits, vegetables, whole grains, and omega-3 fatty acids may be linked to improved endothelial function scores and reduced C-reactive protein and IL-18levels.20,21 In a double-blind RCT of 75 patients with plaque psoriasis, mean (SD) psoriasis area and severity index scores decreased by 11.2 (9.8) in a group treated with omega-3 fatty acids compared to 7.5 (8.8) with omega-6 fatty acids (P=.048).22
Although excessive alcohol use may be linked to psoriasis, there is no conclusive evidence indicating causation, thereby discrediting online claims.12-14 Research has revealed that gluten-free diets in psoriasis patients with celiac disease may improve psoriasis treatment16; however, sufficient evidence is lacking for diets low in gluten and high in polyunsaturated fatty acids or antioxidant supplementation. Of the dietary supplements recommended in the search results we reviewed, fish oil appears to be the most promising, but no recommendations can be made based on the current research.
Eczema
Our Google search using the term diet and eczema yielded 1,160,000 results, with 50% (5/10) of websites attributed to self-proclaimed experts, 30% (3/10) to educational websites, and 20% (2/10) to promotional sites. Of the first 10 results, 80% (8/10) recommended that patients with eczema avoid milk/dairy and 50% (5/10) advised to avoid soy and wheat/gluten. Other websites indicated to avoid eggs, nuts, and artificial sweeteners. Patients were encouraged to incorporate omega-3 fatty acids in their diets, and a few sites recommended bananas, coconut oil, olive oil, and various teas.
In a review of 11 studies with a total of 596 participants, supplementation with vitamins D and E, fish oil, olive oil, and linoleic acid was evaluated for the treatment of eczema.23 Although results indicated modest improvement of eczema severity with supplementation of fish oil, evidence favoring this treatment is limited and unconvincing. Furthermore, some evidence indicates that elimination diets are only appropriate for patients with food allergies.24 In a study evaluating an egg-free and dairy-free diet for eczema patients, only participants with positive egg-specific serum IgE levels saw improvement in disease severity.23 Even though IgE-mediated food allergies have been reported in 40% of children with moderate eczema, the contribution of these allergies to eczema is questionable.25
There is little evidence in the literature to indicate a definitive correlation between the foods mentioned in the search results we evaluated and the development of eczema; however, for patients with food allergies and eczema, elimination diets may decrease disease severity.25,26 There is insufficient evidence to suggest a benefit from evening primrose oil or fish oil supplementation, thereby debunking claims found online.
Comment
Although our Google search results included a wide range of sources and information regarding diet and dermatologic conditions such as acne, psoriasis, and eczema, most of the information we found was either unfounded or misleading. Study limitations in the current literature include small sample size, potential recall bias, lack of appropriate controls, incomplete reported results, and the failure to clearly define skin changes.
When considering the accuracy and type of information regarding skin conditions that is available on the Internet, it is important to note that most of the results we reviewed were webpages attributed to self-proclaimed experts. Although educational websites also were included in the search results, whether or not patients prefer or understand the content of such websites is still unknown; therefore, health organizations should consider revising online patient education materials to allow universal comprehension.27
Furthermore, it is important to consider the impact that widespread Internet access may have on the physician-patient relationship. Having access to health-related information online and being able to potentially self-diagnose could delay or deter patients from seeking professional advice or care.3 A study evaluating the impact of online searches on the physician-patient relationship among 175 patients determined that 36.5% of patients gathered information online prior to their consultation with a physician, while 67.3% chose to complement the information given to them by their physician with online resources.28 Based on these statistics, it is important that physicians be up-to-date with Internet discourse to discredit unfounded recommendations. Ultimately, when it comes to diet and dermatology, patients ought to be skeptical of the information currently available on the Internet, given that most of it is unsubstantiated by medical research.
- Fox S. Online health search 2006. Pew Research Center website. http://www.pewinternet.org/2006/10/29/online-health-search-2006/. Published October 29, 2006. Accessed May 3, 2018.
- Prestin A, Vieux SN, Chou WY. Is online health activity alive and well or flatlining? findings from 10 years of the health information national trends survey. J Health Commun. 2015;20:790-798.
- Zeichner JA, Del Rosso JQ. Acne and the internet. Dermatol Clin. 2016;34:129-132.
- Whitsitt J, Karimkhani C, Boyers LN, et al. Comparing burden of dermatologic disease to search interest on Google trends. Dermatol Online J. 2015;21. pii:13030/qt5xg811qp.
- Shokeen D. Influence of diet in acne vulgaris and atopic dermatitis. Cutis. 2016;98:E28-E29.
- Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84-91.
- Rouhani P. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(3, suppl 1):P706.
- Melnick BC. Evidence for acne-promoting effect of milk and other insulinotropic dairy products. Nestle Nutr Worksop Ser Pediatr Program. 2011;67:131-145.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. High school dietary diary intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Ulvestad M, Bjertness E, Dalgard F, et al. Acne and dairy products in adolescence: results from a Norwegian longitudinal study [published online July 16, 2016]. J Eur Acad Dermatol Venereol. 2017;31:530-535.
- Melnick BC, Schmitz G. Role of insulin, insulin like growth factor 1, hyperglycemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Murzaku EC, Bronsnick T, Rao BK. Diet in dermatology: part II. melanoma, chronic urticaria, and psoriasis. J Am Acad Dermatol. 2014;71:1053.E1-1053.E16.
- Tobin AM, Higgins EM, Norris S, et al. Prevalence of psoriasis in patients with alcoholic liver disease. Clin Exp Dermatol. 2009;34:698-701.
- Kirby B, Richards HL, Mason DL, et al. Alcohol consumption and psychological distress in patients with psoriasis. Br J Dermatol. 2008;158:138-140.
- Søyland E, Funk J, Rajika G, et al. Effect of dietary supplementation with very long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Morimoto S, Yoshikawa K. Psoriasis and vitamin D3. a review of our experience. Arch Dermatol. 1989;125:231-234.
- Smith EL, Pincus SH, Donovan L, et al. A novel approach for the evaluation and treatment of psoriasis. oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis. J Am Acad Dermatol. 1988;19:516-528.
- Siddiqui MA, Al-Khawajah MM. Vitamin D3 and psoriasis: a randomized double-blind placebo-controlled study. J Dermatol Treat. 1990;1:243-245.
- Wang Y, Gao H, Loyd CM, et al. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol. 2012;132:2067-2075.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13. pii:E743. doi:10.3390/ijerph13070743.
- Mayser P, Mrowietz U, Arenberger P, et al. Omega-3 fatty acid-based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38:539-547.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012;2:CD005205.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer [published online November 15, 2014]. J Am Acad Dermatol. 2014;71:1039.E1-1039.E12.
- Campbell DE. The role of food allergy in childhood atopic dermatitis. J Paediatr Child Health. 2012;48:1058-1064.
- Werfel T, Erdmann S, Fuchs T, et al. Approach to suspected food allergy in atopic dermatitis. guideline of the Task Force on Food Allergy of the German Society of Allergology and Clinical Immunology (DGAKI) and the Medical Association of German Allergologists (ADA) and the German Society of Pediatric Allergology (GPA). J Dtsch Dermatol Ges. 2009;3:265-271.
- John AM, John ES, Hansberry DR, et al. Assessment of online patient education materials from major dermatologic associations. J Clin Aesthet Dermatol. 2016;9:23-28.
- Orgaz-Molina J, Cotugno M, Girón-Prieto MS, et al. A study of internet searches for medical information in dermatology patients: the patient-physician relationship. Actas Dermosifiliogr. 2015;106:493-499.
- Fox S. Online health search 2006. Pew Research Center website. http://www.pewinternet.org/2006/10/29/online-health-search-2006/. Published October 29, 2006. Accessed May 3, 2018.
- Prestin A, Vieux SN, Chou WY. Is online health activity alive and well or flatlining? findings from 10 years of the health information national trends survey. J Health Commun. 2015;20:790-798.
- Zeichner JA, Del Rosso JQ. Acne and the internet. Dermatol Clin. 2016;34:129-132.
- Whitsitt J, Karimkhani C, Boyers LN, et al. Comparing burden of dermatologic disease to search interest on Google trends. Dermatol Online J. 2015;21. pii:13030/qt5xg811qp.
- Shokeen D. Influence of diet in acne vulgaris and atopic dermatitis. Cutis. 2016;98:E28-E29.
- Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84-91.
- Rouhani P. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(3, suppl 1):P706.
- Melnick BC. Evidence for acne-promoting effect of milk and other insulinotropic dairy products. Nestle Nutr Worksop Ser Pediatr Program. 2011;67:131-145.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. High school dietary diary intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Ulvestad M, Bjertness E, Dalgard F, et al. Acne and dairy products in adolescence: results from a Norwegian longitudinal study [published online July 16, 2016]. J Eur Acad Dermatol Venereol. 2017;31:530-535.
- Melnick BC, Schmitz G. Role of insulin, insulin like growth factor 1, hyperglycemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Murzaku EC, Bronsnick T, Rao BK. Diet in dermatology: part II. melanoma, chronic urticaria, and psoriasis. J Am Acad Dermatol. 2014;71:1053.E1-1053.E16.
- Tobin AM, Higgins EM, Norris S, et al. Prevalence of psoriasis in patients with alcoholic liver disease. Clin Exp Dermatol. 2009;34:698-701.
- Kirby B, Richards HL, Mason DL, et al. Alcohol consumption and psychological distress in patients with psoriasis. Br J Dermatol. 2008;158:138-140.
- Søyland E, Funk J, Rajika G, et al. Effect of dietary supplementation with very long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Morimoto S, Yoshikawa K. Psoriasis and vitamin D3. a review of our experience. Arch Dermatol. 1989;125:231-234.
- Smith EL, Pincus SH, Donovan L, et al. A novel approach for the evaluation and treatment of psoriasis. oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis. J Am Acad Dermatol. 1988;19:516-528.
- Siddiqui MA, Al-Khawajah MM. Vitamin D3 and psoriasis: a randomized double-blind placebo-controlled study. J Dermatol Treat. 1990;1:243-245.
- Wang Y, Gao H, Loyd CM, et al. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol. 2012;132:2067-2075.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13. pii:E743. doi:10.3390/ijerph13070743.
- Mayser P, Mrowietz U, Arenberger P, et al. Omega-3 fatty acid-based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38:539-547.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012;2:CD005205.
- Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer [published online November 15, 2014]. J Am Acad Dermatol. 2014;71:1039.E1-1039.E12.
- Campbell DE. The role of food allergy in childhood atopic dermatitis. J Paediatr Child Health. 2012;48:1058-1064.
- Werfel T, Erdmann S, Fuchs T, et al. Approach to suspected food allergy in atopic dermatitis. guideline of the Task Force on Food Allergy of the German Society of Allergology and Clinical Immunology (DGAKI) and the Medical Association of German Allergologists (ADA) and the German Society of Pediatric Allergology (GPA). J Dtsch Dermatol Ges. 2009;3:265-271.
- John AM, John ES, Hansberry DR, et al. Assessment of online patient education materials from major dermatologic associations. J Clin Aesthet Dermatol. 2016;9:23-28.
- Orgaz-Molina J, Cotugno M, Girón-Prieto MS, et al. A study of internet searches for medical information in dermatology patients: the patient-physician relationship. Actas Dermosifiliogr. 2015;106:493-499.
Practice Points
- It is important physicians be well-informed regarding Internet discourse to discredit unfounded recommendations.
- It is likely that patients seeking medical advice regarding their dermatologic condition and treatment will have done prior research on the Internet.
- Oftentimes, the information on educational health websites can be confusing to patients.
- Because of widespread Internet access to health-related information, patients may opt to self-diagnose and therefore delay seeking professional care.
Elderly patients with psoriasis can benefit from biologics with low rates of adverse events
according to a new retrospective study.
Among 266 older patients, 65% achieved a 75% improvement in Psoriasis Area Severity Index score (PASI 75) after 1 year of therapy; 50% reached a PASI 90, and 40% a PASI 100, Francesca Prignano MD, PhD, and her colleagues reported in the Journal of the European Academy of Dermatology and Venereology. The rate of serious adverse events was less than 10%.
Elderly patients – those aged 65 years and older – are commonly excluded from studies on biologic treatments because they have more medical comorbidities and are thought to be more at risk for serious adverse events, like infections and malignancy, wrote Dr. Prignano of the dermatology unit, University of Florence, Italy, and her colleagues.
As a result, they noted, there is a “lack of information concerning safety and effectiveness of available treatments for psoriasis in the elderly, particularly about new biologic agents. Disease remission should be an objective for both younger patients and older patients, and biologic therapy should be considered a treatment option for all patients.”
To examine both the benefit and risk of biologics in this population, the team reviewed the records of 266 elderly psoriasis patients; everyone had been on a biologic treatment for at least 1 year.
The primary outcome was PASI score at weeks 8, 16, 28, and 52. The secondary outcomes were the rate and types of biologic-associated adverse events.
The study comprised 266 patients (mean age 72 years). Their mean psoriasis duration was 25.7 years. Comorbidities included psoriatic arthritis; hypertension and dyslipidemia; diabetes mellitus; cardiovascular, gastrointestinal and respiratory diseases; osteoporosis; thyroid dysfunction; depression; and cancer.
Adalimumab was the most commonly prescribed biologic (31%), followed by ustekinumab (28.9%), etanercept (20%), and secukinumab (15%). A smaller proportion of patients were taking infliximab, golimumab, or certolizumab pegol.
The mean baseline PASI was 16.5, although the range was wide (4-54). At the time of review, the average biologic treatment duration was 44 months. Almost half of the cohort (128) were on their second biologic, and 20 more had been on three biologics. A few patients were taking concomitant medications, including steroids, cyclosporine, and acitretin.
The mean PASI scores decreased to 3.7 at week 16, 1.6 at week 28, and 1.2 at week 52. The group exhibited a rapid response to biologic treatment. By 16 weeks, about 55% had achieved a PASI 75, about 28% a PASI 90, and about 20% a PASI 100. By 28 weeks, these numbers were about 64%, 45%, and 35%, respectively. At 1 year, they were about 65%, 50%, and 40%, respectively.
The rate of adverse events was 9.4%. There were 25 events in the cohort, the majority of which (48%) were infections; these included four respiratory infections, three urinary tract infections, two cases of mucocutaneous candidiasis, two cases of herpes zoster infection, and one case of erysipelas.
There were four malignancies: three nonmelanoma skin cancers and one vocal cord cancer.
Noting that, to date, their study represented “the broadest experience on the use of biological drugs” for elderly patients with psoriasis, they wrote that while “comorbidities should be taken into consideration when a long-term treatment is proposed, for the higher risk of side effects and drug interactions,” they wrote, noting that none of the 266 patients had a serious infection and the malignancy rate was low (1.5%).
None of the authors had financial disclosures, and the study had no funding source.
SOURCE: Ricceri F et al. J Eur Acad Dermatol Venereol. 2018 Jun 15. doi: 10.1111/jdv.15139.
according to a new retrospective study.
Among 266 older patients, 65% achieved a 75% improvement in Psoriasis Area Severity Index score (PASI 75) after 1 year of therapy; 50% reached a PASI 90, and 40% a PASI 100, Francesca Prignano MD, PhD, and her colleagues reported in the Journal of the European Academy of Dermatology and Venereology. The rate of serious adverse events was less than 10%.
Elderly patients – those aged 65 years and older – are commonly excluded from studies on biologic treatments because they have more medical comorbidities and are thought to be more at risk for serious adverse events, like infections and malignancy, wrote Dr. Prignano of the dermatology unit, University of Florence, Italy, and her colleagues.
As a result, they noted, there is a “lack of information concerning safety and effectiveness of available treatments for psoriasis in the elderly, particularly about new biologic agents. Disease remission should be an objective for both younger patients and older patients, and biologic therapy should be considered a treatment option for all patients.”
To examine both the benefit and risk of biologics in this population, the team reviewed the records of 266 elderly psoriasis patients; everyone had been on a biologic treatment for at least 1 year.
The primary outcome was PASI score at weeks 8, 16, 28, and 52. The secondary outcomes were the rate and types of biologic-associated adverse events.
The study comprised 266 patients (mean age 72 years). Their mean psoriasis duration was 25.7 years. Comorbidities included psoriatic arthritis; hypertension and dyslipidemia; diabetes mellitus; cardiovascular, gastrointestinal and respiratory diseases; osteoporosis; thyroid dysfunction; depression; and cancer.
Adalimumab was the most commonly prescribed biologic (31%), followed by ustekinumab (28.9%), etanercept (20%), and secukinumab (15%). A smaller proportion of patients were taking infliximab, golimumab, or certolizumab pegol.
The mean baseline PASI was 16.5, although the range was wide (4-54). At the time of review, the average biologic treatment duration was 44 months. Almost half of the cohort (128) were on their second biologic, and 20 more had been on three biologics. A few patients were taking concomitant medications, including steroids, cyclosporine, and acitretin.
The mean PASI scores decreased to 3.7 at week 16, 1.6 at week 28, and 1.2 at week 52. The group exhibited a rapid response to biologic treatment. By 16 weeks, about 55% had achieved a PASI 75, about 28% a PASI 90, and about 20% a PASI 100. By 28 weeks, these numbers were about 64%, 45%, and 35%, respectively. At 1 year, they were about 65%, 50%, and 40%, respectively.
The rate of adverse events was 9.4%. There were 25 events in the cohort, the majority of which (48%) were infections; these included four respiratory infections, three urinary tract infections, two cases of mucocutaneous candidiasis, two cases of herpes zoster infection, and one case of erysipelas.
There were four malignancies: three nonmelanoma skin cancers and one vocal cord cancer.
Noting that, to date, their study represented “the broadest experience on the use of biological drugs” for elderly patients with psoriasis, they wrote that while “comorbidities should be taken into consideration when a long-term treatment is proposed, for the higher risk of side effects and drug interactions,” they wrote, noting that none of the 266 patients had a serious infection and the malignancy rate was low (1.5%).
None of the authors had financial disclosures, and the study had no funding source.
SOURCE: Ricceri F et al. J Eur Acad Dermatol Venereol. 2018 Jun 15. doi: 10.1111/jdv.15139.
according to a new retrospective study.
Among 266 older patients, 65% achieved a 75% improvement in Psoriasis Area Severity Index score (PASI 75) after 1 year of therapy; 50% reached a PASI 90, and 40% a PASI 100, Francesca Prignano MD, PhD, and her colleagues reported in the Journal of the European Academy of Dermatology and Venereology. The rate of serious adverse events was less than 10%.
Elderly patients – those aged 65 years and older – are commonly excluded from studies on biologic treatments because they have more medical comorbidities and are thought to be more at risk for serious adverse events, like infections and malignancy, wrote Dr. Prignano of the dermatology unit, University of Florence, Italy, and her colleagues.
As a result, they noted, there is a “lack of information concerning safety and effectiveness of available treatments for psoriasis in the elderly, particularly about new biologic agents. Disease remission should be an objective for both younger patients and older patients, and biologic therapy should be considered a treatment option for all patients.”
To examine both the benefit and risk of biologics in this population, the team reviewed the records of 266 elderly psoriasis patients; everyone had been on a biologic treatment for at least 1 year.
The primary outcome was PASI score at weeks 8, 16, 28, and 52. The secondary outcomes were the rate and types of biologic-associated adverse events.
The study comprised 266 patients (mean age 72 years). Their mean psoriasis duration was 25.7 years. Comorbidities included psoriatic arthritis; hypertension and dyslipidemia; diabetes mellitus; cardiovascular, gastrointestinal and respiratory diseases; osteoporosis; thyroid dysfunction; depression; and cancer.
Adalimumab was the most commonly prescribed biologic (31%), followed by ustekinumab (28.9%), etanercept (20%), and secukinumab (15%). A smaller proportion of patients were taking infliximab, golimumab, or certolizumab pegol.
The mean baseline PASI was 16.5, although the range was wide (4-54). At the time of review, the average biologic treatment duration was 44 months. Almost half of the cohort (128) were on their second biologic, and 20 more had been on three biologics. A few patients were taking concomitant medications, including steroids, cyclosporine, and acitretin.
The mean PASI scores decreased to 3.7 at week 16, 1.6 at week 28, and 1.2 at week 52. The group exhibited a rapid response to biologic treatment. By 16 weeks, about 55% had achieved a PASI 75, about 28% a PASI 90, and about 20% a PASI 100. By 28 weeks, these numbers were about 64%, 45%, and 35%, respectively. At 1 year, they were about 65%, 50%, and 40%, respectively.
The rate of adverse events was 9.4%. There were 25 events in the cohort, the majority of which (48%) were infections; these included four respiratory infections, three urinary tract infections, two cases of mucocutaneous candidiasis, two cases of herpes zoster infection, and one case of erysipelas.
There were four malignancies: three nonmelanoma skin cancers and one vocal cord cancer.
Noting that, to date, their study represented “the broadest experience on the use of biological drugs” for elderly patients with psoriasis, they wrote that while “comorbidities should be taken into consideration when a long-term treatment is proposed, for the higher risk of side effects and drug interactions,” they wrote, noting that none of the 266 patients had a serious infection and the malignancy rate was low (1.5%).
None of the authors had financial disclosures, and the study had no funding source.
SOURCE: Ricceri F et al. J Eur Acad Dermatol Venereol. 2018 Jun 15. doi: 10.1111/jdv.15139.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Key clinical point: Patients aged 65 years and older responded well to biologics and had a low rate of serious adverse events.
Major finding: At 1 year, 65% achieved a PASI 75, 50% achieved a reached PASI 90, and 40% achieved a PASI 100, with a 9.4% rate of serious adverse events.
Study details: The retrospective study comprised 266 patients aged 65 years and older treated with biologics for psoriasis.
Disclosures: None of the authors had financial disclosures, and the study had no funding source.
Source: Ricceri F et al. J Eur Acad Dermatol Venereol. 2018. doi: 10.1111/jdv.15139.
Adalimumab strikes out for aortic inflammation in psoriasis
ORLANDO – Adalimumab (Humira) did not reduce aortic inflammation in a year-long, randomized trial that pitted the tumor necrosis factor (TNF) blocker against phototherapy and placebo in 97 psoriasis patients.
a risk factor for cardiovascular events, said senior investigator Joel M. Gelfand, MD, a dermatology professor at the University of Pennsylvania, Philadelphia.
The other trial, from Canada, also found no effect in the ascending aorta after 52 weeks of treatment, but it did find a modest increase in carotid inflammation (J Invest Dermatol. 2017 Aug;137[8]:1638-45).
Both studies used positron emission tomography/computed tomography to assess vascular inflammation.
“We know patients with psoriasis are at increased risk for cardiovascular disease. We think the same kind of inflammation that occurs in atherosclerosis occurs in psoriasis, but we are still teasing out the impact of therapy and which one is most likely to lower the risk of heart attacks, strokes, and things of that nature,” Dr. Gelfand said at the International Investigative Dermatology meeting. The study was published when he gave his presentation (Circ Cardiovasc Imaging. 2018 Jun. doi: 10.1161/CIRCIMAGING.117.007394).
Although it didn’t reduce aortic inflammation, adalimumab had a positive effect on glycoprotein acetylation, a marker of inflammation and subclinical cardiovascular disease in psoriasis. Observational studies have also reported a drop in cardiovascular events with adalimumab. Taken together, the mixed findings “give us pause for thought,” Dr. Gelfand said.
In a previous trial, he and his colleagues had found that the interleukin blocker ustekinumab (Stelara) reduced aortic inflammation in psoriasis by about 19%, but Dr. Gelfand said at the meeting that it’s too early to opt for ustekinumab over adalimumab for cardiovascular protection: “I don’t think we are quite there yet; we are still not certain.”
Following washout of psoriasis treatments, the 97 subjects were randomized to either adalimumab for 12 months or ultraviolet B phototherapy or placebo for 12 weeks, followed by adalimumab for 12 months.
Aortic inflammation was used as a proxy for cardiovascular events because trials to assess actual event rates would require thousands of patients treated for several years. “They’re not likely to be done in psoriasis any time soon,” Dr. Gelfand said.
At both 12 and 52 weeks, adalimumab patients had no change in aortic inflammation, compared with placebo and baseline. Phototherapy patients had a 4% drop from baseline at 12 weeks, but it was not statistically significant when compared with placebo.
Both adalimumab and phototherapy decreased systemic inflammation as gauged by serum C-reactive protein and interleukin-6 levels, but only adalimumab reduced TNF levels and glycoprotein acetylation at 12 and 52 weeks. Neither treatment affected insulin, adiponectin, or leptin levels. Adalimumab dropped HDL cholesterol a bit, a known side effect, while phototherapy increased it.
About half of the patients in both treatment arms had a 75% reduction in the Psoriasis Area and Severity Index at 12 weeks, compared with 7% of those on placebo. Subjects were aged 43 years, on average, and more than two-thirds were men. They had a mean psoriasis duration of 17 years and a baseline PASI score of 19.
The work was funded by the National Institutes of Health and AbbVie, adalimumab’s maker. Among many industry ties, Dr. Gelfand is a consultant for Janssen, maker of ustekinumab, and receives research grants from Janssen and AbbVie.
SOURCE: Gelfand JM et al. IID 2018, Abstract 393.
ORLANDO – Adalimumab (Humira) did not reduce aortic inflammation in a year-long, randomized trial that pitted the tumor necrosis factor (TNF) blocker against phototherapy and placebo in 97 psoriasis patients.
a risk factor for cardiovascular events, said senior investigator Joel M. Gelfand, MD, a dermatology professor at the University of Pennsylvania, Philadelphia.
The other trial, from Canada, also found no effect in the ascending aorta after 52 weeks of treatment, but it did find a modest increase in carotid inflammation (J Invest Dermatol. 2017 Aug;137[8]:1638-45).
Both studies used positron emission tomography/computed tomography to assess vascular inflammation.
“We know patients with psoriasis are at increased risk for cardiovascular disease. We think the same kind of inflammation that occurs in atherosclerosis occurs in psoriasis, but we are still teasing out the impact of therapy and which one is most likely to lower the risk of heart attacks, strokes, and things of that nature,” Dr. Gelfand said at the International Investigative Dermatology meeting. The study was published when he gave his presentation (Circ Cardiovasc Imaging. 2018 Jun. doi: 10.1161/CIRCIMAGING.117.007394).
Although it didn’t reduce aortic inflammation, adalimumab had a positive effect on glycoprotein acetylation, a marker of inflammation and subclinical cardiovascular disease in psoriasis. Observational studies have also reported a drop in cardiovascular events with adalimumab. Taken together, the mixed findings “give us pause for thought,” Dr. Gelfand said.
In a previous trial, he and his colleagues had found that the interleukin blocker ustekinumab (Stelara) reduced aortic inflammation in psoriasis by about 19%, but Dr. Gelfand said at the meeting that it’s too early to opt for ustekinumab over adalimumab for cardiovascular protection: “I don’t think we are quite there yet; we are still not certain.”
Following washout of psoriasis treatments, the 97 subjects were randomized to either adalimumab for 12 months or ultraviolet B phototherapy or placebo for 12 weeks, followed by adalimumab for 12 months.
Aortic inflammation was used as a proxy for cardiovascular events because trials to assess actual event rates would require thousands of patients treated for several years. “They’re not likely to be done in psoriasis any time soon,” Dr. Gelfand said.
At both 12 and 52 weeks, adalimumab patients had no change in aortic inflammation, compared with placebo and baseline. Phototherapy patients had a 4% drop from baseline at 12 weeks, but it was not statistically significant when compared with placebo.
Both adalimumab and phototherapy decreased systemic inflammation as gauged by serum C-reactive protein and interleukin-6 levels, but only adalimumab reduced TNF levels and glycoprotein acetylation at 12 and 52 weeks. Neither treatment affected insulin, adiponectin, or leptin levels. Adalimumab dropped HDL cholesterol a bit, a known side effect, while phototherapy increased it.
About half of the patients in both treatment arms had a 75% reduction in the Psoriasis Area and Severity Index at 12 weeks, compared with 7% of those on placebo. Subjects were aged 43 years, on average, and more than two-thirds were men. They had a mean psoriasis duration of 17 years and a baseline PASI score of 19.
The work was funded by the National Institutes of Health and AbbVie, adalimumab’s maker. Among many industry ties, Dr. Gelfand is a consultant for Janssen, maker of ustekinumab, and receives research grants from Janssen and AbbVie.
SOURCE: Gelfand JM et al. IID 2018, Abstract 393.
ORLANDO – Adalimumab (Humira) did not reduce aortic inflammation in a year-long, randomized trial that pitted the tumor necrosis factor (TNF) blocker against phototherapy and placebo in 97 psoriasis patients.
a risk factor for cardiovascular events, said senior investigator Joel M. Gelfand, MD, a dermatology professor at the University of Pennsylvania, Philadelphia.
The other trial, from Canada, also found no effect in the ascending aorta after 52 weeks of treatment, but it did find a modest increase in carotid inflammation (J Invest Dermatol. 2017 Aug;137[8]:1638-45).
Both studies used positron emission tomography/computed tomography to assess vascular inflammation.
“We know patients with psoriasis are at increased risk for cardiovascular disease. We think the same kind of inflammation that occurs in atherosclerosis occurs in psoriasis, but we are still teasing out the impact of therapy and which one is most likely to lower the risk of heart attacks, strokes, and things of that nature,” Dr. Gelfand said at the International Investigative Dermatology meeting. The study was published when he gave his presentation (Circ Cardiovasc Imaging. 2018 Jun. doi: 10.1161/CIRCIMAGING.117.007394).
Although it didn’t reduce aortic inflammation, adalimumab had a positive effect on glycoprotein acetylation, a marker of inflammation and subclinical cardiovascular disease in psoriasis. Observational studies have also reported a drop in cardiovascular events with adalimumab. Taken together, the mixed findings “give us pause for thought,” Dr. Gelfand said.
In a previous trial, he and his colleagues had found that the interleukin blocker ustekinumab (Stelara) reduced aortic inflammation in psoriasis by about 19%, but Dr. Gelfand said at the meeting that it’s too early to opt for ustekinumab over adalimumab for cardiovascular protection: “I don’t think we are quite there yet; we are still not certain.”
Following washout of psoriasis treatments, the 97 subjects were randomized to either adalimumab for 12 months or ultraviolet B phototherapy or placebo for 12 weeks, followed by adalimumab for 12 months.
Aortic inflammation was used as a proxy for cardiovascular events because trials to assess actual event rates would require thousands of patients treated for several years. “They’re not likely to be done in psoriasis any time soon,” Dr. Gelfand said.
At both 12 and 52 weeks, adalimumab patients had no change in aortic inflammation, compared with placebo and baseline. Phototherapy patients had a 4% drop from baseline at 12 weeks, but it was not statistically significant when compared with placebo.
Both adalimumab and phototherapy decreased systemic inflammation as gauged by serum C-reactive protein and interleukin-6 levels, but only adalimumab reduced TNF levels and glycoprotein acetylation at 12 and 52 weeks. Neither treatment affected insulin, adiponectin, or leptin levels. Adalimumab dropped HDL cholesterol a bit, a known side effect, while phototherapy increased it.
About half of the patients in both treatment arms had a 75% reduction in the Psoriasis Area and Severity Index at 12 weeks, compared with 7% of those on placebo. Subjects were aged 43 years, on average, and more than two-thirds were men. They had a mean psoriasis duration of 17 years and a baseline PASI score of 19.
The work was funded by the National Institutes of Health and AbbVie, adalimumab’s maker. Among many industry ties, Dr. Gelfand is a consultant for Janssen, maker of ustekinumab, and receives research grants from Janssen and AbbVie.
SOURCE: Gelfand JM et al. IID 2018, Abstract 393.
REPORTING FROM IID 2018
Key clinical point: Adalimumab does not appear to reduce aortic inflammation in psoriasis, a risk factor for cardiovascular events.
Major finding: After a year of treatment, patients had no change in aortic inflammation, compared with placebo and baseline.
Study details: Randomized, controlled trial of 97 patients
Disclosures: The National Institutes of Health and AbbVie, adalimumab’s maker, funded the work. Among many industry ties, Dr. Gelfand is a consultant for Janssen, maker of ustekinumab, and receives research grants from Janssen and AbbVie.
Source: Gelfand JM et al. IID 2018, abstract 393
Psoriasis therapy with biologics not linked to increased cancer risk
Biologic treatments were not associated with an increased risk of cancer among patients with psoriasis in the medium term, in a study that analyzed data from patient registries.
“Cumulative length of exposure to biologics was not associated with the risk of developing cancers, even after controlling for the effect of age, gender, location,” as well as for previous exposure to methotrexate, cyclosporine, and phototherapy; duration of psoriasis; and comorbidities, reported Ignacio García-Doval, MD, of the Fundación Academia Española de Dermatología y Venereología, Madrid, and his associates.
The pooled adjusted odds ratio of cancer per year of biologic exposure was 1.02 (95% confidence interval, 0.92-1.13), demonstrating no significantly increased risk of cancer per cumulative year of biologic exposure for psoriasis therapy, Dr. García-Doval and his associates reported in the study, published in the British Journal of Dermatology. This was true even when broken down within the registries for comparison, and when analyzed by type of cancers, such as squamous cell carcinoma and basal cell carcinoma.
A limitation of the study was inadequate power to detect and compare risk between individual biologics, they said. Also, “as our data describe limited follow-up and latencies, it is still possible that a risk after longer periods of exposure and latencies exists.”
Most of the authors had numerous financial disclosures related to pharmaceutical companies. Psonet was supported with funds from the European Association of Venereology and Dermatology and the Italian Drug Agency. Funding for the individual registries includes support from pharmaceutical companies.
SOURCE: García-Doval I et al. Br J Dermatol. 2018 May 3. doi: 10.1111/bjd.16715.
Biologic treatments were not associated with an increased risk of cancer among patients with psoriasis in the medium term, in a study that analyzed data from patient registries.
“Cumulative length of exposure to biologics was not associated with the risk of developing cancers, even after controlling for the effect of age, gender, location,” as well as for previous exposure to methotrexate, cyclosporine, and phototherapy; duration of psoriasis; and comorbidities, reported Ignacio García-Doval, MD, of the Fundación Academia Española de Dermatología y Venereología, Madrid, and his associates.
The pooled adjusted odds ratio of cancer per year of biologic exposure was 1.02 (95% confidence interval, 0.92-1.13), demonstrating no significantly increased risk of cancer per cumulative year of biologic exposure for psoriasis therapy, Dr. García-Doval and his associates reported in the study, published in the British Journal of Dermatology. This was true even when broken down within the registries for comparison, and when analyzed by type of cancers, such as squamous cell carcinoma and basal cell carcinoma.
A limitation of the study was inadequate power to detect and compare risk between individual biologics, they said. Also, “as our data describe limited follow-up and latencies, it is still possible that a risk after longer periods of exposure and latencies exists.”
Most of the authors had numerous financial disclosures related to pharmaceutical companies. Psonet was supported with funds from the European Association of Venereology and Dermatology and the Italian Drug Agency. Funding for the individual registries includes support from pharmaceutical companies.
SOURCE: García-Doval I et al. Br J Dermatol. 2018 May 3. doi: 10.1111/bjd.16715.
Biologic treatments were not associated with an increased risk of cancer among patients with psoriasis in the medium term, in a study that analyzed data from patient registries.
“Cumulative length of exposure to biologics was not associated with the risk of developing cancers, even after controlling for the effect of age, gender, location,” as well as for previous exposure to methotrexate, cyclosporine, and phototherapy; duration of psoriasis; and comorbidities, reported Ignacio García-Doval, MD, of the Fundación Academia Española de Dermatología y Venereología, Madrid, and his associates.
The pooled adjusted odds ratio of cancer per year of biologic exposure was 1.02 (95% confidence interval, 0.92-1.13), demonstrating no significantly increased risk of cancer per cumulative year of biologic exposure for psoriasis therapy, Dr. García-Doval and his associates reported in the study, published in the British Journal of Dermatology. This was true even when broken down within the registries for comparison, and when analyzed by type of cancers, such as squamous cell carcinoma and basal cell carcinoma.
A limitation of the study was inadequate power to detect and compare risk between individual biologics, they said. Also, “as our data describe limited follow-up and latencies, it is still possible that a risk after longer periods of exposure and latencies exists.”
Most of the authors had numerous financial disclosures related to pharmaceutical companies. Psonet was supported with funds from the European Association of Venereology and Dermatology and the Italian Drug Agency. Funding for the individual registries includes support from pharmaceutical companies.
SOURCE: García-Doval I et al. Br J Dermatol. 2018 May 3. doi: 10.1111/bjd.16715.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point:
Major finding: The pooled adjusted odds ratio of cancer per year of biologic exposure was 1.02.
Study details: Patient data were drawn from four national databases within Psonet, which included 579 cancer cases and 2,671 matched controls.
Disclosures: Most of the authors had numerous financial disclosures related to pharmaceutical companies. Psonet was supported with funds from the European Association of Venereology and Dermatology and the Italian Drug Agency. Funding for the individual registries includes support from pharmaceutical companies.
Source: García-Doval I et al. Br J Dermatol. 2018 May 3. doi: 10.1111/bjd.16715.
CBC values linked to CVD risk in psoriasis
ORLANDO – conducted by researchers at Case Western Reserve University, Cleveland.*
It’s generally accepted that psoriasis increases the risk of cardiovascular disease (CVD), but it’s not clear who’s most at risk. “We really wanted to find something that is cheap and easy to risk stratify these patients” said lead investigator Rosalynn Conic, MD, of Case Western’s department of dermatology.
What they found was “very impressive, for sure,” Dr. Conic said at the International Investigative Dermatology meeting.
The incidence of MI was highest among the 1,920 patients (5%) with elevated RDW and MPV (odds ratio, 3.4; 95% confidence interval, 2.7-4.2; P less than .001), followed by the 7,060 (18%) patients with high RDW and normal MPV (OR, 2.4; 95% CI, 2.1-2.8; P less than .001), as compared with normal/low MPV and RDW patients.
Elevated RDW or elevated RDW plus MPV increased the odds of atrial fibrillation, coronary artery disease, heart failure, and peripheral vascular disease anywhere from 2 to 8.3 times (P less than .001). Among psoriatic arthritis patients, elevated RDW almost doubled the risk of MI (OR, 1.8; P less than .001). Results were adjusted for age, gender, and hypertension.
In a subanalysis of treatment effects, 4 of 23 psoriasis patients at Case Western had elevated RDWs at baseline. Values normalized in the three patients who achieved a 75% reduction in the Psoriasis Area and Severity Index score after about a year of systemic treatment.
“We aim to validate [the study results] with a Veterans Administration data set,” Dr. Conic said. If it pans out, “one use would be to send [patients with elevated values] to a cardiologist earlier” so other CVD risk factors can be monitored and treated. The findings also add to the case for good control, she noted.
Systemic inflammation is the common denominator between the blood value elevations and CVD. The same inflammatory cytokines that cause skin problems in psoriasis also stimulate bone marrow to release immature red blood cells, which are larger than mature cells, leading to an increased RDW. Similarly, elevated MPV indicates a higher number of larger, younger platelets in the blood.
“It’s probably something along those lines, but I think we need to go back to basic science and really figure it out,” Dr. Conic said.
Patients were 18-65 years old. The study excluded patients with diabetes, Crohn’s disease, RA, and generalized atherosclerosis.
The National Institutes of Health funded the work. Dr. Conic reported no relevant financial disclosures.
*This article was updated on May 30, 2018.
SOURCE: Conic R et al. IID 2018, Abstract 550.
ORLANDO – conducted by researchers at Case Western Reserve University, Cleveland.*
It’s generally accepted that psoriasis increases the risk of cardiovascular disease (CVD), but it’s not clear who’s most at risk. “We really wanted to find something that is cheap and easy to risk stratify these patients” said lead investigator Rosalynn Conic, MD, of Case Western’s department of dermatology.
What they found was “very impressive, for sure,” Dr. Conic said at the International Investigative Dermatology meeting.
The incidence of MI was highest among the 1,920 patients (5%) with elevated RDW and MPV (odds ratio, 3.4; 95% confidence interval, 2.7-4.2; P less than .001), followed by the 7,060 (18%) patients with high RDW and normal MPV (OR, 2.4; 95% CI, 2.1-2.8; P less than .001), as compared with normal/low MPV and RDW patients.
Elevated RDW or elevated RDW plus MPV increased the odds of atrial fibrillation, coronary artery disease, heart failure, and peripheral vascular disease anywhere from 2 to 8.3 times (P less than .001). Among psoriatic arthritis patients, elevated RDW almost doubled the risk of MI (OR, 1.8; P less than .001). Results were adjusted for age, gender, and hypertension.
In a subanalysis of treatment effects, 4 of 23 psoriasis patients at Case Western had elevated RDWs at baseline. Values normalized in the three patients who achieved a 75% reduction in the Psoriasis Area and Severity Index score after about a year of systemic treatment.
“We aim to validate [the study results] with a Veterans Administration data set,” Dr. Conic said. If it pans out, “one use would be to send [patients with elevated values] to a cardiologist earlier” so other CVD risk factors can be monitored and treated. The findings also add to the case for good control, she noted.
Systemic inflammation is the common denominator between the blood value elevations and CVD. The same inflammatory cytokines that cause skin problems in psoriasis also stimulate bone marrow to release immature red blood cells, which are larger than mature cells, leading to an increased RDW. Similarly, elevated MPV indicates a higher number of larger, younger platelets in the blood.
“It’s probably something along those lines, but I think we need to go back to basic science and really figure it out,” Dr. Conic said.
Patients were 18-65 years old. The study excluded patients with diabetes, Crohn’s disease, RA, and generalized atherosclerosis.
The National Institutes of Health funded the work. Dr. Conic reported no relevant financial disclosures.
*This article was updated on May 30, 2018.
SOURCE: Conic R et al. IID 2018, Abstract 550.
ORLANDO – conducted by researchers at Case Western Reserve University, Cleveland.*
It’s generally accepted that psoriasis increases the risk of cardiovascular disease (CVD), but it’s not clear who’s most at risk. “We really wanted to find something that is cheap and easy to risk stratify these patients” said lead investigator Rosalynn Conic, MD, of Case Western’s department of dermatology.
What they found was “very impressive, for sure,” Dr. Conic said at the International Investigative Dermatology meeting.
The incidence of MI was highest among the 1,920 patients (5%) with elevated RDW and MPV (odds ratio, 3.4; 95% confidence interval, 2.7-4.2; P less than .001), followed by the 7,060 (18%) patients with high RDW and normal MPV (OR, 2.4; 95% CI, 2.1-2.8; P less than .001), as compared with normal/low MPV and RDW patients.
Elevated RDW or elevated RDW plus MPV increased the odds of atrial fibrillation, coronary artery disease, heart failure, and peripheral vascular disease anywhere from 2 to 8.3 times (P less than .001). Among psoriatic arthritis patients, elevated RDW almost doubled the risk of MI (OR, 1.8; P less than .001). Results were adjusted for age, gender, and hypertension.
In a subanalysis of treatment effects, 4 of 23 psoriasis patients at Case Western had elevated RDWs at baseline. Values normalized in the three patients who achieved a 75% reduction in the Psoriasis Area and Severity Index score after about a year of systemic treatment.
“We aim to validate [the study results] with a Veterans Administration data set,” Dr. Conic said. If it pans out, “one use would be to send [patients with elevated values] to a cardiologist earlier” so other CVD risk factors can be monitored and treated. The findings also add to the case for good control, she noted.
Systemic inflammation is the common denominator between the blood value elevations and CVD. The same inflammatory cytokines that cause skin problems in psoriasis also stimulate bone marrow to release immature red blood cells, which are larger than mature cells, leading to an increased RDW. Similarly, elevated MPV indicates a higher number of larger, younger platelets in the blood.
“It’s probably something along those lines, but I think we need to go back to basic science and really figure it out,” Dr. Conic said.
Patients were 18-65 years old. The study excluded patients with diabetes, Crohn’s disease, RA, and generalized atherosclerosis.
The National Institutes of Health funded the work. Dr. Conic reported no relevant financial disclosures.
*This article was updated on May 30, 2018.
SOURCE: Conic R et al. IID 2018, Abstract 550.
REPORTING FROM IID 2018
Key clinical point: Elevated red blood cell distribution width and mean platelet volume might identify psoriasis patients at risk for cardiovascular disease.
Major finding: The incidence of MI was highest among the 1,920 patients with elevated red cell distribution width and mean platelet volume (odds ratio, 3.4; 95% confidence interval, 2.7-4.2; P less than .001).
Study details: A database review of 39,510 patients with psoriasis.
Disclosures: The National Institutes of Health funded the work. The lead investigator had no disclosures to report.
Source: Conic R et al. IID 2018, Abstract 550.
Topical corticosteroid-retinoid combination effective in moderate to severe psoriasis
The combination of a topical corticosteroid and a topical retinoid for the treatment of plaque psoriasis resulted in significant improvements in clinical signs, in two multicenter, double-blind, vehicle-controlled phase 3 studies.
In the two studies, investigators randomized a total of 418 or vehicle lotion, applied once a day to affected areas. After 8 weeks of treatment, 35.8% of adults in the first study and 45.3% of those in the second study had achieved the primary outcome of at least a two-grade improvement in the Investigator’s Global Assessment score and reaching “clear” or “almost clear,” compared with 7.0% and 12.5%, respectively, of patients treated with the vehicle (P less than .001). The report was published online in the Journal of the American Academy of Dermatology.
At 8 weeks, reduction in erythema was achieved by 44.2% and 49.6% of patients in the treatment arms, compared with 10% and 18.7% of patients in the control arms. Plaque elevation was reduced in 59.3% and 59.7% of patients in the treatment arms, compared with 17.9% and 21.3% of patients in the control arms; and scaling was reduced in 59.4% and 62.9% of those on treatment, compared with 20.6% and 21.0%, respectively. All differences between the treatment and control groups were statistically significant (P less than .001).
Participants who received the treatment also reported significantly lower scores for itching, dryness, and burning or stinging compared with those who received the vehicle lotion.
Dr. Linda Stein Gold of Henry Ford Hospital in Detroit, and her coauthors, wrote that while clinical studies have established the benefit of using a topical corticosteroid as an adjunct to tazarotene for plaque psoriasis, data on their combined use was limited. This combination “was consistently more effective than vehicle in achieving treatment success; effectively reducing affected area and psoriasis signs at the target lesion, and improving QoL [quality of life],” they wrote.
Most patients maintained these improvements over the 4-week posttreatment period.
Patients who received the halobetasol propionate/tazarotene lotion reported more adverse events than did those who received the control lotion, but most were mild to moderate and included contact dermatitis (6.3%), pruritus (2.2%) and application site pain (2.6%). Three serious adverse events were not related to treatment.
The studies were funded by Dow Pharmaceutical Sciences, a division of Valeant Pharmaceuticals North America. Four authors disclosed advisory, consultancy and speaking positions and other funding from the pharmaceutical industry, including with Valeant Pharmaceuticals. Five authors are employees of the company.
SOURCE: Gold L et al. J Am Acad Dermatol. 2018 Mar 31. pii: S0190-9622(18)30494-8. doi: 10.1016/j.jaad.2018.03.040.
The combination of a topical corticosteroid and a topical retinoid for the treatment of plaque psoriasis resulted in significant improvements in clinical signs, in two multicenter, double-blind, vehicle-controlled phase 3 studies.
In the two studies, investigators randomized a total of 418 or vehicle lotion, applied once a day to affected areas. After 8 weeks of treatment, 35.8% of adults in the first study and 45.3% of those in the second study had achieved the primary outcome of at least a two-grade improvement in the Investigator’s Global Assessment score and reaching “clear” or “almost clear,” compared with 7.0% and 12.5%, respectively, of patients treated with the vehicle (P less than .001). The report was published online in the Journal of the American Academy of Dermatology.
At 8 weeks, reduction in erythema was achieved by 44.2% and 49.6% of patients in the treatment arms, compared with 10% and 18.7% of patients in the control arms. Plaque elevation was reduced in 59.3% and 59.7% of patients in the treatment arms, compared with 17.9% and 21.3% of patients in the control arms; and scaling was reduced in 59.4% and 62.9% of those on treatment, compared with 20.6% and 21.0%, respectively. All differences between the treatment and control groups were statistically significant (P less than .001).
Participants who received the treatment also reported significantly lower scores for itching, dryness, and burning or stinging compared with those who received the vehicle lotion.
Dr. Linda Stein Gold of Henry Ford Hospital in Detroit, and her coauthors, wrote that while clinical studies have established the benefit of using a topical corticosteroid as an adjunct to tazarotene for plaque psoriasis, data on their combined use was limited. This combination “was consistently more effective than vehicle in achieving treatment success; effectively reducing affected area and psoriasis signs at the target lesion, and improving QoL [quality of life],” they wrote.
Most patients maintained these improvements over the 4-week posttreatment period.
Patients who received the halobetasol propionate/tazarotene lotion reported more adverse events than did those who received the control lotion, but most were mild to moderate and included contact dermatitis (6.3%), pruritus (2.2%) and application site pain (2.6%). Three serious adverse events were not related to treatment.
The studies were funded by Dow Pharmaceutical Sciences, a division of Valeant Pharmaceuticals North America. Four authors disclosed advisory, consultancy and speaking positions and other funding from the pharmaceutical industry, including with Valeant Pharmaceuticals. Five authors are employees of the company.
SOURCE: Gold L et al. J Am Acad Dermatol. 2018 Mar 31. pii: S0190-9622(18)30494-8. doi: 10.1016/j.jaad.2018.03.040.
The combination of a topical corticosteroid and a topical retinoid for the treatment of plaque psoriasis resulted in significant improvements in clinical signs, in two multicenter, double-blind, vehicle-controlled phase 3 studies.
In the two studies, investigators randomized a total of 418 or vehicle lotion, applied once a day to affected areas. After 8 weeks of treatment, 35.8% of adults in the first study and 45.3% of those in the second study had achieved the primary outcome of at least a two-grade improvement in the Investigator’s Global Assessment score and reaching “clear” or “almost clear,” compared with 7.0% and 12.5%, respectively, of patients treated with the vehicle (P less than .001). The report was published online in the Journal of the American Academy of Dermatology.
At 8 weeks, reduction in erythema was achieved by 44.2% and 49.6% of patients in the treatment arms, compared with 10% and 18.7% of patients in the control arms. Plaque elevation was reduced in 59.3% and 59.7% of patients in the treatment arms, compared with 17.9% and 21.3% of patients in the control arms; and scaling was reduced in 59.4% and 62.9% of those on treatment, compared with 20.6% and 21.0%, respectively. All differences between the treatment and control groups were statistically significant (P less than .001).
Participants who received the treatment also reported significantly lower scores for itching, dryness, and burning or stinging compared with those who received the vehicle lotion.
Dr. Linda Stein Gold of Henry Ford Hospital in Detroit, and her coauthors, wrote that while clinical studies have established the benefit of using a topical corticosteroid as an adjunct to tazarotene for plaque psoriasis, data on their combined use was limited. This combination “was consistently more effective than vehicle in achieving treatment success; effectively reducing affected area and psoriasis signs at the target lesion, and improving QoL [quality of life],” they wrote.
Most patients maintained these improvements over the 4-week posttreatment period.
Patients who received the halobetasol propionate/tazarotene lotion reported more adverse events than did those who received the control lotion, but most were mild to moderate and included contact dermatitis (6.3%), pruritus (2.2%) and application site pain (2.6%). Three serious adverse events were not related to treatment.
The studies were funded by Dow Pharmaceutical Sciences, a division of Valeant Pharmaceuticals North America. Four authors disclosed advisory, consultancy and speaking positions and other funding from the pharmaceutical industry, including with Valeant Pharmaceuticals. Five authors are employees of the company.
SOURCE: Gold L et al. J Am Acad Dermatol. 2018 Mar 31. pii: S0190-9622(18)30494-8. doi: 10.1016/j.jaad.2018.03.040.
FROM JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Topical halobetasol propionate and tazarotene can significantly improve plaque psoriasis symptoms.
Major finding: Nearly half of adults treated with topical halobetasol propionate and tazarotene were “clear” or “almost clear” after 8 weeks.
Study details: Two multicenter, double-blind, vehicle-controlled phase 3 studies of 418 adults with psoriasis.
Disclosures: The studies were funded by Dow Pharmaceutical Sciences, a division of Valeant Pharmaceuticals North America. Four authors disclosed advisory, consultancy and speaking positions and other funding from the pharmaceutical industry, including Valeant Pharmaceuticals. Five authors are employees of the company.
Source: Gold L et al. J Am Acad Dermatol. 2018 Mar 31. pii: S0190-9622(18)30494-8. doi: 10.1016/j.jaad.2018.03.040.
Secukinumab Emerges as a Rapidly Effective Therapy for Pityriasis Rubra Pilaris
Although there currently are no formal guidelines for the treatment of refractory pityriasis rubra pilaris (PRP), successful off-label treatment of the condition with multiple biologics approved for psoriasis has been reported.1,2 Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP in 2 recent case reports.3,4 We report 2 additional cases of severe refractory PRP that responded rapidly to treatment with secukinumab. In both cases, the patients’ erythematous plaques resolved or had nearly resolved by week 4 of treatment. Our findings suggest that IL-17 plays an important role in PRP pathogenesis and support future clinical trials of anti–IL-17 agents for treatment of this entity.
Case Reports
Patient 1
A 60-year-old man with a history of biopsy-proven PRP presented with persistent generalized erythema, scattered patches of normal skin, and hyperkeratotic plaques on the bilateral palms of 1 year’s duration. Previous therapies included topical steroids, topical calcipotriene, adalimumab 40 mg once every other week, infliximab 5 mg/kg once every 8 weeks, ustekinumab 90 mg once every 12 weeks, acitretin 25 mg once daily, and most recently cyclosporine 200 mg twice daily. Of these treatments, infliximab was the
At 4 weeks’ follow-up, there was a marked decrease in erythema and scaling. The body surface area affected had decreased to 5%, and improvement of palmar keratoderma was noted. The patient continued with maintenance dosing of secukinumab 300 mg once every 4 weeks. By week 8, the erythema had fully resolved (Figure 1B), and he remained clear at week 24. No adverse events were noted since initiation of therapy.
Patient 2
A 74-year-old woman with a history of PRP that had previously been misdiagnosed as psoriasis by an outside physician presented for evaluation of palmoplantar keratoderma (Figure 2A), follicular hyperkeratosis, and erythematous plaques on the trunk and arms of 5 years’ duration. Previous therapies included topical steroids, topical urea, methotrexate 20 mg once weekly, adalimumab 40 mg once every other week, infliximab 10 mg/kg once every 4 weeks, ustekinumab 90 mg once every 12 weeks, and most recently acitretin 50 mg once daily.
The patient had been maintained on ustekinumab and acitretin for 2 years with only mild improvement. Ustekinumab was then discontinued, and after 3 months treatment with secukinumab was added to the once-daily acitretin. Similar to Patient 1, loading doses of secukinumab 300 mg were administered once weekly for 5 weeks. The plaques on the trunk and arms had resolved by week 4, but the palmoplantar keratoderma persisted. The patient continued with the maintenance dose of secukinumab 300 mg once every 4 weeks and reported an increase in peeling of the palms and soles at week 8.
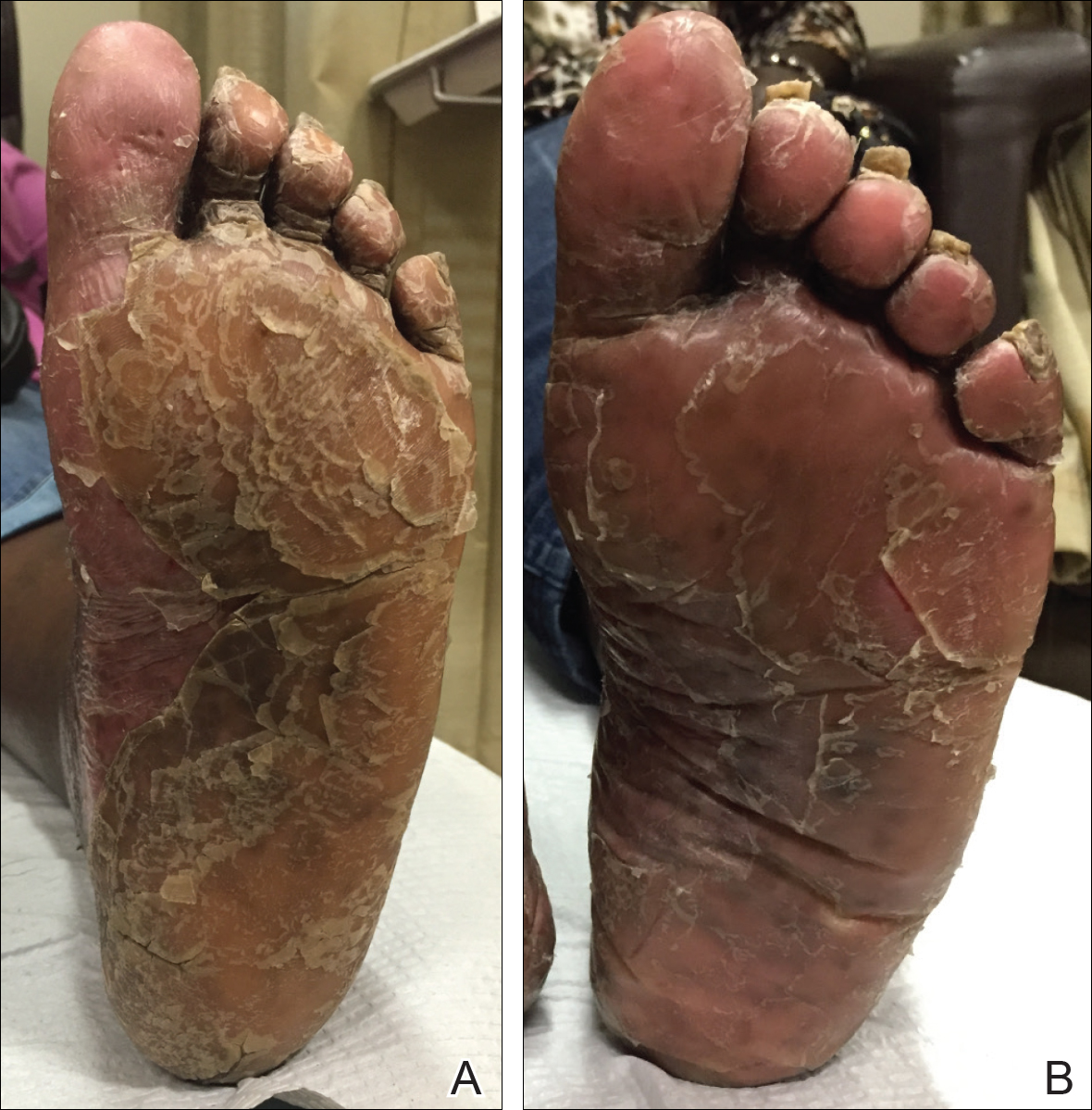
By week 12 of treatment, the palmar keratoderma had resolved, and debridement of the soles revealed patches of normal skin (Figure 2B). By week 52, no adverse events had been noted. The patient continued to experience mild keratoderma of the soles, making us reluctant to discontinue acitretin; however, she has maintained her maximal response, and her quality of life has significantly improved. The patient was continued on acitretin and secukinumab, and her condition remained stable.
Comment
Because there are no formal treatment guidelines for refractory PRP, case reports play an important role in clinical decision-making. When a patient is unresponsive to topical medications and first-line traditional systemic therapies (eg, methotrexate, cyclosporine, acitretin), biologic drugs effective in the treatment of psoriasis are widely accepted as the next therapeutic step.1 The biologic medications that are most often reported in the treatment of PRP are the TNF-α antagonists, as they have been available the longest.1-2 In a systematic review of 15 patients with PRP who were treated with TNF-α antagonists,2 80% of patients achieved complete response (mean time to maximal response, 5 months). There also are a number of reports of successful treatment of PRP with the IL-12/23 antagonist ustekinumab, which has been commercially available since 2009.5-9 Although improvement was noted in most of these patients at the time of the second injection (week 4 of therapy), maximal response with ustekinumab typically occurs between weeks 12 and 28.10
In our cases of PRP treated with secukinumab as well as 2 others that were recently reported in the literature, resolution of erythema and plaques was rapid. This superiority of the response rate parallels the performance of secukinumab relative to ustekinumab in patients with psoriasis11 In one case of a 67-year-old man with PRP treated with secukinumab, scaling and pruritus were reduced by week 3 of treatment and erythema had cleared by week 8.3 In another case of a 33-year-old woman with PRP, pruritus resolved after 1 week of treatment and erythematous plaques and palmoplantar keratoderma improved by week 2.4 In both of our cases, plaques had resolved or nearly resolved by week 4 of follow-up. Patient 1 achieved complete response at week 8 of therapy. Patient 2 never attained complete response, but by week 12 she achieved maximal response, which still resulted in markedly increased quality of life. We do not intend to make additions to her treatment plan because she is currently the clearest she has been since onset of symptoms and is happy with her present condition.
Although it is difficult to predict the long-term prognosis in our 2 patients, we will continue their current regimens indefinitely—as long as the response persists and no adverse events are experienced. This approach is consistent with guidelines for management of plaque psoriasis with secukinumab.12
This accumulation of evidence suggests the importance of the role of IL-17 in the pathogenesis of PRP. The serum level of IL-17 was not evaluated in our patients, but elevation of IL-17 has been reported in a case of PRP.13 Further studies are needed to clarify the role of IL-17 in this disease entity.
Conclusion
Given the refractory nature of PRP and the relative safety of targeted immunotherapy, trials of new biologics and potent small molecules approved for psoriasis treatment are worth exploring for PRP. In light of our reports and those in the literature and given the relative safety of anti–IL-17 agents, it may be reasonable to consider such agents as a first-line therapy for this predictably refractory disease.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris. Am J Clin Dermatol. 2010;11:157-170.
- Petrof G, Almaani N, Archer CB, et al. A systematic review of the literature on the treatment of pityriasis rubra pilaris type 1 with TNF-antagonists. J Eur Acad Dermatol Venereol. 2013;27:E131-E135.
- Schuster D, Pfister-Wartha A, Bruckner-Tuderman L, et al. Successful treatment of refractory pityriasis rubra pilaris with secukinumab. JAMA Dermatol. 2016;152:1278-1280.
- Gauci ML, Jachiet M, Gottlieb J, et al. Successful treatment of type II pityriasis rubra pilaris with secukinumab. JAAD Case Rep. 2016;2:462-264.
- Chowdhary M, Davila U, Cohen DJ. Ustekinumab as an alternative treatment option for chronic pityriasis rubra pilaris. Case Rep Dermatol. 2015;7:46-50.
- Wohlrab J, Kreft B. Treatment of pityriasis rubra pilaris with ustekinumab. Br J Dermatol. 2010;163:655-656.
- Villaverde RR, Cano DS. Successful treatment of type 1 pityriasis rubra pilaris with ustekinumab therapy. Eur J Dermatol. 2010;20:630-631.
- Di Stefani A, Galluzzo M, Talamonti M, et al. Long-term ustekinumab treatment for refractory type I pityriasis rubra pilaris. J Dermatol Case Rep. 2013;7:5-9.
- Eytan O, Sarig O, Sprecher E, et al. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br J Dermatol. 2014;171:420-422.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Adnot-Desanlis L, Antonicelli F, Tabary T, et al. Effectiveness of infliximab in pityriasis rubra pilaris is associated with pro-inflammatory cytokine inhibition. Dermatology. 2013;226:41-46.
Although there currently are no formal guidelines for the treatment of refractory pityriasis rubra pilaris (PRP), successful off-label treatment of the condition with multiple biologics approved for psoriasis has been reported.1,2 Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP in 2 recent case reports.3,4 We report 2 additional cases of severe refractory PRP that responded rapidly to treatment with secukinumab. In both cases, the patients’ erythematous plaques resolved or had nearly resolved by week 4 of treatment. Our findings suggest that IL-17 plays an important role in PRP pathogenesis and support future clinical trials of anti–IL-17 agents for treatment of this entity.
Case Reports
Patient 1
A 60-year-old man with a history of biopsy-proven PRP presented with persistent generalized erythema, scattered patches of normal skin, and hyperkeratotic plaques on the bilateral palms of 1 year’s duration. Previous therapies included topical steroids, topical calcipotriene, adalimumab 40 mg once every other week, infliximab 5 mg/kg once every 8 weeks, ustekinumab 90 mg once every 12 weeks, acitretin 25 mg once daily, and most recently cyclosporine 200 mg twice daily. Of these treatments, infliximab was the
At 4 weeks’ follow-up, there was a marked decrease in erythema and scaling. The body surface area affected had decreased to 5%, and improvement of palmar keratoderma was noted. The patient continued with maintenance dosing of secukinumab 300 mg once every 4 weeks. By week 8, the erythema had fully resolved (Figure 1B), and he remained clear at week 24. No adverse events were noted since initiation of therapy.

Patient 2
A 74-year-old woman with a history of PRP that had previously been misdiagnosed as psoriasis by an outside physician presented for evaluation of palmoplantar keratoderma (Figure 2A), follicular hyperkeratosis, and erythematous plaques on the trunk and arms of 5 years’ duration. Previous therapies included topical steroids, topical urea, methotrexate 20 mg once weekly, adalimumab 40 mg once every other week, infliximab 10 mg/kg once every 4 weeks, ustekinumab 90 mg once every 12 weeks, and most recently acitretin 50 mg once daily.
The patient had been maintained on ustekinumab and acitretin for 2 years with only mild improvement. Ustekinumab was then discontinued, and after 3 months treatment with secukinumab was added to the once-daily acitretin. Similar to Patient 1, loading doses of secukinumab 300 mg were administered once weekly for 5 weeks. The plaques on the trunk and arms had resolved by week 4, but the palmoplantar keratoderma persisted. The patient continued with the maintenance dose of secukinumab 300 mg once every 4 weeks and reported an increase in peeling of the palms and soles at week 8.

By week 12 of treatment, the palmar keratoderma had resolved, and debridement of the soles revealed patches of normal skin (Figure 2B). By week 52, no adverse events had been noted. The patient continued to experience mild keratoderma of the soles, making us reluctant to discontinue acitretin; however, she has maintained her maximal response, and her quality of life has significantly improved. The patient was continued on acitretin and secukinumab, and her condition remained stable.
Comment
Because there are no formal treatment guidelines for refractory PRP, case reports play an important role in clinical decision-making. When a patient is unresponsive to topical medications and first-line traditional systemic therapies (eg, methotrexate, cyclosporine, acitretin), biologic drugs effective in the treatment of psoriasis are widely accepted as the next therapeutic step.1 The biologic medications that are most often reported in the treatment of PRP are the TNF-α antagonists, as they have been available the longest.1-2 In a systematic review of 15 patients with PRP who were treated with TNF-α antagonists,2 80% of patients achieved complete response (mean time to maximal response, 5 months). There also are a number of reports of successful treatment of PRP with the IL-12/23 antagonist ustekinumab, which has been commercially available since 2009.5-9 Although improvement was noted in most of these patients at the time of the second injection (week 4 of therapy), maximal response with ustekinumab typically occurs between weeks 12 and 28.10
In our cases of PRP treated with secukinumab as well as 2 others that were recently reported in the literature, resolution of erythema and plaques was rapid. This superiority of the response rate parallels the performance of secukinumab relative to ustekinumab in patients with psoriasis11 In one case of a 67-year-old man with PRP treated with secukinumab, scaling and pruritus were reduced by week 3 of treatment and erythema had cleared by week 8.3 In another case of a 33-year-old woman with PRP, pruritus resolved after 1 week of treatment and erythematous plaques and palmoplantar keratoderma improved by week 2.4 In both of our cases, plaques had resolved or nearly resolved by week 4 of follow-up. Patient 1 achieved complete response at week 8 of therapy. Patient 2 never attained complete response, but by week 12 she achieved maximal response, which still resulted in markedly increased quality of life. We do not intend to make additions to her treatment plan because she is currently the clearest she has been since onset of symptoms and is happy with her present condition.
Although it is difficult to predict the long-term prognosis in our 2 patients, we will continue their current regimens indefinitely—as long as the response persists and no adverse events are experienced. This approach is consistent with guidelines for management of plaque psoriasis with secukinumab.12
This accumulation of evidence suggests the importance of the role of IL-17 in the pathogenesis of PRP. The serum level of IL-17 was not evaluated in our patients, but elevation of IL-17 has been reported in a case of PRP.13 Further studies are needed to clarify the role of IL-17 in this disease entity.
Conclusion
Given the refractory nature of PRP and the relative safety of targeted immunotherapy, trials of new biologics and potent small molecules approved for psoriasis treatment are worth exploring for PRP. In light of our reports and those in the literature and given the relative safety of anti–IL-17 agents, it may be reasonable to consider such agents as a first-line therapy for this predictably refractory disease.
Although there currently are no formal guidelines for the treatment of refractory pityriasis rubra pilaris (PRP), successful off-label treatment of the condition with multiple biologics approved for psoriasis has been reported.1,2 Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP in 2 recent case reports.3,4 We report 2 additional cases of severe refractory PRP that responded rapidly to treatment with secukinumab. In both cases, the patients’ erythematous plaques resolved or had nearly resolved by week 4 of treatment. Our findings suggest that IL-17 plays an important role in PRP pathogenesis and support future clinical trials of anti–IL-17 agents for treatment of this entity.
Case Reports
Patient 1
A 60-year-old man with a history of biopsy-proven PRP presented with persistent generalized erythema, scattered patches of normal skin, and hyperkeratotic plaques on the bilateral palms of 1 year’s duration. Previous therapies included topical steroids, topical calcipotriene, adalimumab 40 mg once every other week, infliximab 5 mg/kg once every 8 weeks, ustekinumab 90 mg once every 12 weeks, acitretin 25 mg once daily, and most recently cyclosporine 200 mg twice daily. Of these treatments, infliximab was the
At 4 weeks’ follow-up, there was a marked decrease in erythema and scaling. The body surface area affected had decreased to 5%, and improvement of palmar keratoderma was noted. The patient continued with maintenance dosing of secukinumab 300 mg once every 4 weeks. By week 8, the erythema had fully resolved (Figure 1B), and he remained clear at week 24. No adverse events were noted since initiation of therapy.

Patient 2
A 74-year-old woman with a history of PRP that had previously been misdiagnosed as psoriasis by an outside physician presented for evaluation of palmoplantar keratoderma (Figure 2A), follicular hyperkeratosis, and erythematous plaques on the trunk and arms of 5 years’ duration. Previous therapies included topical steroids, topical urea, methotrexate 20 mg once weekly, adalimumab 40 mg once every other week, infliximab 10 mg/kg once every 4 weeks, ustekinumab 90 mg once every 12 weeks, and most recently acitretin 50 mg once daily.
The patient had been maintained on ustekinumab and acitretin for 2 years with only mild improvement. Ustekinumab was then discontinued, and after 3 months treatment with secukinumab was added to the once-daily acitretin. Similar to Patient 1, loading doses of secukinumab 300 mg were administered once weekly for 5 weeks. The plaques on the trunk and arms had resolved by week 4, but the palmoplantar keratoderma persisted. The patient continued with the maintenance dose of secukinumab 300 mg once every 4 weeks and reported an increase in peeling of the palms and soles at week 8.

By week 12 of treatment, the palmar keratoderma had resolved, and debridement of the soles revealed patches of normal skin (Figure 2B). By week 52, no adverse events had been noted. The patient continued to experience mild keratoderma of the soles, making us reluctant to discontinue acitretin; however, she has maintained her maximal response, and her quality of life has significantly improved. The patient was continued on acitretin and secukinumab, and her condition remained stable.
Comment
Because there are no formal treatment guidelines for refractory PRP, case reports play an important role in clinical decision-making. When a patient is unresponsive to topical medications and first-line traditional systemic therapies (eg, methotrexate, cyclosporine, acitretin), biologic drugs effective in the treatment of psoriasis are widely accepted as the next therapeutic step.1 The biologic medications that are most often reported in the treatment of PRP are the TNF-α antagonists, as they have been available the longest.1-2 In a systematic review of 15 patients with PRP who were treated with TNF-α antagonists,2 80% of patients achieved complete response (mean time to maximal response, 5 months). There also are a number of reports of successful treatment of PRP with the IL-12/23 antagonist ustekinumab, which has been commercially available since 2009.5-9 Although improvement was noted in most of these patients at the time of the second injection (week 4 of therapy), maximal response with ustekinumab typically occurs between weeks 12 and 28.10
In our cases of PRP treated with secukinumab as well as 2 others that were recently reported in the literature, resolution of erythema and plaques was rapid. This superiority of the response rate parallels the performance of secukinumab relative to ustekinumab in patients with psoriasis11 In one case of a 67-year-old man with PRP treated with secukinumab, scaling and pruritus were reduced by week 3 of treatment and erythema had cleared by week 8.3 In another case of a 33-year-old woman with PRP, pruritus resolved after 1 week of treatment and erythematous plaques and palmoplantar keratoderma improved by week 2.4 In both of our cases, plaques had resolved or nearly resolved by week 4 of follow-up. Patient 1 achieved complete response at week 8 of therapy. Patient 2 never attained complete response, but by week 12 she achieved maximal response, which still resulted in markedly increased quality of life. We do not intend to make additions to her treatment plan because she is currently the clearest she has been since onset of symptoms and is happy with her present condition.
Although it is difficult to predict the long-term prognosis in our 2 patients, we will continue their current regimens indefinitely—as long as the response persists and no adverse events are experienced. This approach is consistent with guidelines for management of plaque psoriasis with secukinumab.12
This accumulation of evidence suggests the importance of the role of IL-17 in the pathogenesis of PRP. The serum level of IL-17 was not evaluated in our patients, but elevation of IL-17 has been reported in a case of PRP.13 Further studies are needed to clarify the role of IL-17 in this disease entity.
Conclusion
Given the refractory nature of PRP and the relative safety of targeted immunotherapy, trials of new biologics and potent small molecules approved for psoriasis treatment are worth exploring for PRP. In light of our reports and those in the literature and given the relative safety of anti–IL-17 agents, it may be reasonable to consider such agents as a first-line therapy for this predictably refractory disease.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris. Am J Clin Dermatol. 2010;11:157-170.
- Petrof G, Almaani N, Archer CB, et al. A systematic review of the literature on the treatment of pityriasis rubra pilaris type 1 with TNF-antagonists. J Eur Acad Dermatol Venereol. 2013;27:E131-E135.
- Schuster D, Pfister-Wartha A, Bruckner-Tuderman L, et al. Successful treatment of refractory pityriasis rubra pilaris with secukinumab. JAMA Dermatol. 2016;152:1278-1280.
- Gauci ML, Jachiet M, Gottlieb J, et al. Successful treatment of type II pityriasis rubra pilaris with secukinumab. JAAD Case Rep. 2016;2:462-264.
- Chowdhary M, Davila U, Cohen DJ. Ustekinumab as an alternative treatment option for chronic pityriasis rubra pilaris. Case Rep Dermatol. 2015;7:46-50.
- Wohlrab J, Kreft B. Treatment of pityriasis rubra pilaris with ustekinumab. Br J Dermatol. 2010;163:655-656.
- Villaverde RR, Cano DS. Successful treatment of type 1 pityriasis rubra pilaris with ustekinumab therapy. Eur J Dermatol. 2010;20:630-631.
- Di Stefani A, Galluzzo M, Talamonti M, et al. Long-term ustekinumab treatment for refractory type I pityriasis rubra pilaris. J Dermatol Case Rep. 2013;7:5-9.
- Eytan O, Sarig O, Sprecher E, et al. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br J Dermatol. 2014;171:420-422.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Adnot-Desanlis L, Antonicelli F, Tabary T, et al. Effectiveness of infliximab in pityriasis rubra pilaris is associated with pro-inflammatory cytokine inhibition. Dermatology. 2013;226:41-46.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris. Am J Clin Dermatol. 2010;11:157-170.
- Petrof G, Almaani N, Archer CB, et al. A systematic review of the literature on the treatment of pityriasis rubra pilaris type 1 with TNF-antagonists. J Eur Acad Dermatol Venereol. 2013;27:E131-E135.
- Schuster D, Pfister-Wartha A, Bruckner-Tuderman L, et al. Successful treatment of refractory pityriasis rubra pilaris with secukinumab. JAMA Dermatol. 2016;152:1278-1280.
- Gauci ML, Jachiet M, Gottlieb J, et al. Successful treatment of type II pityriasis rubra pilaris with secukinumab. JAAD Case Rep. 2016;2:462-264.
- Chowdhary M, Davila U, Cohen DJ. Ustekinumab as an alternative treatment option for chronic pityriasis rubra pilaris. Case Rep Dermatol. 2015;7:46-50.
- Wohlrab J, Kreft B. Treatment of pityriasis rubra pilaris with ustekinumab. Br J Dermatol. 2010;163:655-656.
- Villaverde RR, Cano DS. Successful treatment of type 1 pityriasis rubra pilaris with ustekinumab therapy. Eur J Dermatol. 2010;20:630-631.
- Di Stefani A, Galluzzo M, Talamonti M, et al. Long-term ustekinumab treatment for refractory type I pityriasis rubra pilaris. J Dermatol Case Rep. 2013;7:5-9.
- Eytan O, Sarig O, Sprecher E, et al. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br J Dermatol. 2014;171:420-422.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Adnot-Desanlis L, Antonicelli F, Tabary T, et al. Effectiveness of infliximab in pityriasis rubra pilaris is associated with pro-inflammatory cytokine inhibition. Dermatology. 2013;226:41-46.
Practice Points
- In patients with pityriasis rubra pilaris (PRP) who have not responded to topical treatments, off-label treatment with systemic therapies approved for plaque psoriasis can be considered.
- Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP.
Hidradenitis suppurativa packs mighty QOL impact
KAUAI, HAWAII – Anyone who has treated patients with hidradenitis suppurativa (HS) recognizes that this can be a debilitating disease. Helping put that into fuller perspective, recent evidence has shown that the quality of life effects of moderate to severe HS are objectively worse than those of moderate to severe psoriasis, according to Iltefat H. Hamzavi, MD, president of the Hidradenitis Suppurativa Foundation and a dermatologist at Henry Ford Hospital in Detroit.
He was lead author of a study in which he and his coinvestigators compared weighted averages of a variety of quality of life measures in patients with moderate to severe HS or psoriasis who participated in five AbbVie-sponsored randomized clinical trials of biologic agents (J Am Acad Dermatol. 2017 Dec;77[6]:1038-46).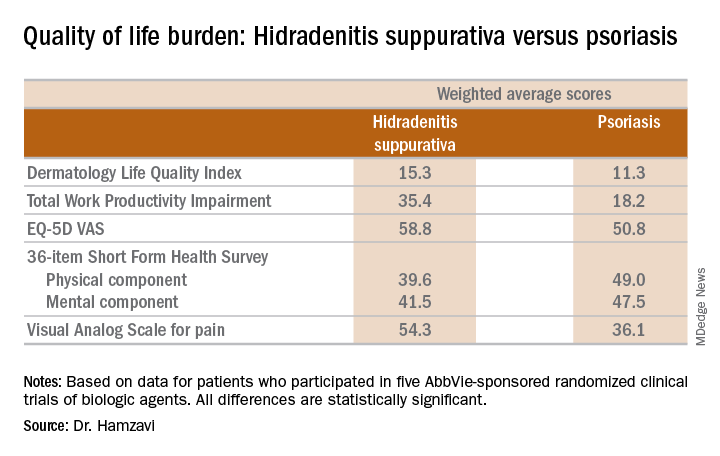
“The number of HS patients who experience downward drift – losing their job and their health insurance and ultimately being unable to move out of a lower socioeconomic group – is staggering,” the dermatologist said.
which underscores the importance of a psychiatric evaluation as part of routine care for patients with this dermatologic disease. “Suicide is much more common in the HS population than in almost any other dermatologic disease,” Dr. Hamzavi added.
He reported serving as a consultant for AbbVie, Incyte, and UCB.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Anyone who has treated patients with hidradenitis suppurativa (HS) recognizes that this can be a debilitating disease. Helping put that into fuller perspective, recent evidence has shown that the quality of life effects of moderate to severe HS are objectively worse than those of moderate to severe psoriasis, according to Iltefat H. Hamzavi, MD, president of the Hidradenitis Suppurativa Foundation and a dermatologist at Henry Ford Hospital in Detroit.
He was lead author of a study in which he and his coinvestigators compared weighted averages of a variety of quality of life measures in patients with moderate to severe HS or psoriasis who participated in five AbbVie-sponsored randomized clinical trials of biologic agents (J Am Acad Dermatol. 2017 Dec;77[6]:1038-46).
“The number of HS patients who experience downward drift – losing their job and their health insurance and ultimately being unable to move out of a lower socioeconomic group – is staggering,” the dermatologist said.
which underscores the importance of a psychiatric evaluation as part of routine care for patients with this dermatologic disease. “Suicide is much more common in the HS population than in almost any other dermatologic disease,” Dr. Hamzavi added.
He reported serving as a consultant for AbbVie, Incyte, and UCB.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Anyone who has treated patients with hidradenitis suppurativa (HS) recognizes that this can be a debilitating disease. Helping put that into fuller perspective, recent evidence has shown that the quality of life effects of moderate to severe HS are objectively worse than those of moderate to severe psoriasis, according to Iltefat H. Hamzavi, MD, president of the Hidradenitis Suppurativa Foundation and a dermatologist at Henry Ford Hospital in Detroit.
He was lead author of a study in which he and his coinvestigators compared weighted averages of a variety of quality of life measures in patients with moderate to severe HS or psoriasis who participated in five AbbVie-sponsored randomized clinical trials of biologic agents (J Am Acad Dermatol. 2017 Dec;77[6]:1038-46).
“The number of HS patients who experience downward drift – losing their job and their health insurance and ultimately being unable to move out of a lower socioeconomic group – is staggering,” the dermatologist said.
which underscores the importance of a psychiatric evaluation as part of routine care for patients with this dermatologic disease. “Suicide is much more common in the HS population than in almost any other dermatologic disease,” Dr. Hamzavi added.
He reported serving as a consultant for AbbVie, Incyte, and UCB.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR