User login
Cost of Drugs Can Be Breathtaking for COPD Patients
For patients with chronic obstructive pulmonary disease (COPD), the Global Initiative on Obstructive Lung Disease recommends long-term term pharmacologic and nonpharmacologic therapies based on each patient’s symptoms and disease severity.
Yet even the most effective drugs work only when patients take them as directed, and according to the World Health Organization, fewer than half of all patients worldwide are fully compliant with long-term COPD drug regimens.
And as a recent cross-sectional study showed, nearly one in six patients in the United States reported missing a COPD drug dose, lowering the dose, or delaying filling a prescription for financial reasons.
“I care for patients with COPD as their pulmonologist, and this is a very common problem that we see in clinical practice,” said Meredith McCormack, MD, a pulmonary and critical care medicine physician and associate director of the Pulmonary & Critical Care Medicine Division at Johns Hopkins University in Baltimore.
Dr. McCormack, a national spokesperson for The American Lung Association, said that she shows new patients the photos of all available inhalers and asks which ones they have and how they take them.
“I would say that a majority of the time people are taking their medicines slightly differently than prescribed, and often, this is due to cost,” she said.
Serious Consequences
Cost-related medication nonadherence (CRN), as investigators term it, can have major health effects and can be significantly more costly in the long run due to increased hospitalization rates, higher morbidity, and greater risk for COPD-related death associated with suboptimal care.
“For some patients even a month or two of being off medications increases the risk of having exacerbations, having more symptoms, [and] having a decline in their lung function,” said Douglas M. Beach, MD, a pulmonologist at Beth Israel Deaconess Medical Center in Boston.
In the aforementioned cross-sectional study, published in the open access journal BMC Public Health, Xin Wen, MD, from the Jiamusi (China) University School of Public Health, and colleagues looked at data on a representative sample of US adults who participated in the US National Health Interview Survey from 2013 through 2020.
The sample included 15,928 persons aged 18 years or older with a self-reported history of COPD who completed a CRN survey including the following questions:
During the past 12 months, have you
- Skipped medication doses to save money?
- Taken less medicine than prescribed to save money?
- Delayed filling a prescription to save money?
The investigators found that a weighted 18.56% of participants representing 2.39 million persons with COPD answered “yes” to one of the questions.
Translated into representative population numbers, that works out to an estimated 1.61 million persons with COPD missing doses, 1.72 million taking lower doses than those prescribed, and 2.03 million delaying filling prescriptions to save money.
A multivariable logistic regression analysis showed that those who were most likely to be nonadherent for financial reasons were patients younger than 65 years, women, persons with low family income, those who lacked health insurance, and patients with multiple comorbidities, the authors found.
Financial Barriers
One of the biggest barriers to COPD medication adherence is, somewhat paradoxically, insurance status, particularly Medicare, said Corinne Young, MSN, FNP-C, FCCP, from Colorado Springs Pulmonary Consultants.
“What’s so unfair about Medicare is that patients have to buy a drug plan, so they have to already pay for an extra plan to have access to drugs, and the plans vary because there are so many choices,” she said in an interview.
Elderly patients may be confused about the available options and may choose the Medicare Advantage plan with the lowest monthly premiums, which have the highest annual deductibles, usually in the $5000-and-up range, she said.
In addition, the Medicare Part D prescription coverage gap, commonly known as the “donut hole,” requires patients to pay a percentage of drug costs above a certain limit ($5030 in 2024) until a yearly out-of-pocket limit (currently $8000) is reached, after which the plan will again pick up most of the costs.
Although makers of inhalers have voluntarily agreed to limit monthly co-pays to $35 for uninsured patients, Medicare plans require insured patients to shell out considerably more, with 30 days of Trelegy Ellipta (fluticasone furoate, umeclidinium, and vilanterol) setting patients back nearly $350 per month, according to a recent search of costs for a United Healthcare Medicare Advantage plan.
Chasing Lower-Cost Options
“I have a lot of patients who use Canadian pharmacies to try to get around it, and I have a lot of patients who make a trip to Mexico every year and load up. I have patients who don’t take their inhalers as they are supposed to in order to make them last longer, and I have patients who take the inhalers of other family members,” Ms. Young said.
Humayun Anjum, MD, FCCP, from Dallas Pulmonary and Critical Care in Dallas, Texas, said in an interview that when patients claim that a prescribed drug isn’t working as expected for them, financial pressures may be partly to blame.
“When you investigate a little bit more, that’s where things become a bit more clear, and the patient may say ‘yeah, I stopped using this inhaler because it was costing me 200 bucks a month and I’m already on other medications,’ ” he said.
He noted that, when possible, he will steer patients toward discount prescription services such as GoodRx, which offers discounts at local pharmacies, or Mark Cuban Cost Plus Drugs, an online pharmacy that offers generic versions of Advair Diskus (fluticasone propionate/salmeterol) at a 100-50 mcg dose for $94.70, a savings of $307.30 over retail pharmacies, according to the company’s website.
Dr. Beach noted that Beth Israel Deaconess has a pharmacist embedded in the pulmonary clinic who can help eligible patients get financial assistance to pay for their medications.
“The influencing factors of CRN are multifaceted and necessitating more rigorous research. Health policy interventions focusing on reducing drug costs, delaying disease progression, preventing exacerbations, and reducing the risk of comorbidities may improve the economic burden of COPD and its outcomes,” Dr. Wen and colleagues wrote.
The study by Dr. Wen and colleagues was funded by grants from Chinese national and academic sources. Dr. McCormack has served as a consultant to Aridis, Boehringer Ingelheim, GlaxoSmithKline, MCG Diagnostics, ndd Medical Technologies, and UpToDate. Ms. Young, Dr. Anjum, and Dr. Beach reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
For patients with chronic obstructive pulmonary disease (COPD), the Global Initiative on Obstructive Lung Disease recommends long-term term pharmacologic and nonpharmacologic therapies based on each patient’s symptoms and disease severity.
Yet even the most effective drugs work only when patients take them as directed, and according to the World Health Organization, fewer than half of all patients worldwide are fully compliant with long-term COPD drug regimens.
And as a recent cross-sectional study showed, nearly one in six patients in the United States reported missing a COPD drug dose, lowering the dose, or delaying filling a prescription for financial reasons.
“I care for patients with COPD as their pulmonologist, and this is a very common problem that we see in clinical practice,” said Meredith McCormack, MD, a pulmonary and critical care medicine physician and associate director of the Pulmonary & Critical Care Medicine Division at Johns Hopkins University in Baltimore.
Dr. McCormack, a national spokesperson for The American Lung Association, said that she shows new patients the photos of all available inhalers and asks which ones they have and how they take them.
“I would say that a majority of the time people are taking their medicines slightly differently than prescribed, and often, this is due to cost,” she said.
Serious Consequences
Cost-related medication nonadherence (CRN), as investigators term it, can have major health effects and can be significantly more costly in the long run due to increased hospitalization rates, higher morbidity, and greater risk for COPD-related death associated with suboptimal care.
“For some patients even a month or two of being off medications increases the risk of having exacerbations, having more symptoms, [and] having a decline in their lung function,” said Douglas M. Beach, MD, a pulmonologist at Beth Israel Deaconess Medical Center in Boston.
In the aforementioned cross-sectional study, published in the open access journal BMC Public Health, Xin Wen, MD, from the Jiamusi (China) University School of Public Health, and colleagues looked at data on a representative sample of US adults who participated in the US National Health Interview Survey from 2013 through 2020.
The sample included 15,928 persons aged 18 years or older with a self-reported history of COPD who completed a CRN survey including the following questions:
During the past 12 months, have you
- Skipped medication doses to save money?
- Taken less medicine than prescribed to save money?
- Delayed filling a prescription to save money?
The investigators found that a weighted 18.56% of participants representing 2.39 million persons with COPD answered “yes” to one of the questions.
Translated into representative population numbers, that works out to an estimated 1.61 million persons with COPD missing doses, 1.72 million taking lower doses than those prescribed, and 2.03 million delaying filling prescriptions to save money.
A multivariable logistic regression analysis showed that those who were most likely to be nonadherent for financial reasons were patients younger than 65 years, women, persons with low family income, those who lacked health insurance, and patients with multiple comorbidities, the authors found.
Financial Barriers
One of the biggest barriers to COPD medication adherence is, somewhat paradoxically, insurance status, particularly Medicare, said Corinne Young, MSN, FNP-C, FCCP, from Colorado Springs Pulmonary Consultants.
“What’s so unfair about Medicare is that patients have to buy a drug plan, so they have to already pay for an extra plan to have access to drugs, and the plans vary because there are so many choices,” she said in an interview.
Elderly patients may be confused about the available options and may choose the Medicare Advantage plan with the lowest monthly premiums, which have the highest annual deductibles, usually in the $5000-and-up range, she said.
In addition, the Medicare Part D prescription coverage gap, commonly known as the “donut hole,” requires patients to pay a percentage of drug costs above a certain limit ($5030 in 2024) until a yearly out-of-pocket limit (currently $8000) is reached, after which the plan will again pick up most of the costs.
Although makers of inhalers have voluntarily agreed to limit monthly co-pays to $35 for uninsured patients, Medicare plans require insured patients to shell out considerably more, with 30 days of Trelegy Ellipta (fluticasone furoate, umeclidinium, and vilanterol) setting patients back nearly $350 per month, according to a recent search of costs for a United Healthcare Medicare Advantage plan.
Chasing Lower-Cost Options
“I have a lot of patients who use Canadian pharmacies to try to get around it, and I have a lot of patients who make a trip to Mexico every year and load up. I have patients who don’t take their inhalers as they are supposed to in order to make them last longer, and I have patients who take the inhalers of other family members,” Ms. Young said.
Humayun Anjum, MD, FCCP, from Dallas Pulmonary and Critical Care in Dallas, Texas, said in an interview that when patients claim that a prescribed drug isn’t working as expected for them, financial pressures may be partly to blame.
“When you investigate a little bit more, that’s where things become a bit more clear, and the patient may say ‘yeah, I stopped using this inhaler because it was costing me 200 bucks a month and I’m already on other medications,’ ” he said.
He noted that, when possible, he will steer patients toward discount prescription services such as GoodRx, which offers discounts at local pharmacies, or Mark Cuban Cost Plus Drugs, an online pharmacy that offers generic versions of Advair Diskus (fluticasone propionate/salmeterol) at a 100-50 mcg dose for $94.70, a savings of $307.30 over retail pharmacies, according to the company’s website.
Dr. Beach noted that Beth Israel Deaconess has a pharmacist embedded in the pulmonary clinic who can help eligible patients get financial assistance to pay for their medications.
“The influencing factors of CRN are multifaceted and necessitating more rigorous research. Health policy interventions focusing on reducing drug costs, delaying disease progression, preventing exacerbations, and reducing the risk of comorbidities may improve the economic burden of COPD and its outcomes,” Dr. Wen and colleagues wrote.
The study by Dr. Wen and colleagues was funded by grants from Chinese national and academic sources. Dr. McCormack has served as a consultant to Aridis, Boehringer Ingelheim, GlaxoSmithKline, MCG Diagnostics, ndd Medical Technologies, and UpToDate. Ms. Young, Dr. Anjum, and Dr. Beach reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
For patients with chronic obstructive pulmonary disease (COPD), the Global Initiative on Obstructive Lung Disease recommends long-term term pharmacologic and nonpharmacologic therapies based on each patient’s symptoms and disease severity.
Yet even the most effective drugs work only when patients take them as directed, and according to the World Health Organization, fewer than half of all patients worldwide are fully compliant with long-term COPD drug regimens.
And as a recent cross-sectional study showed, nearly one in six patients in the United States reported missing a COPD drug dose, lowering the dose, or delaying filling a prescription for financial reasons.
“I care for patients with COPD as their pulmonologist, and this is a very common problem that we see in clinical practice,” said Meredith McCormack, MD, a pulmonary and critical care medicine physician and associate director of the Pulmonary & Critical Care Medicine Division at Johns Hopkins University in Baltimore.
Dr. McCormack, a national spokesperson for The American Lung Association, said that she shows new patients the photos of all available inhalers and asks which ones they have and how they take them.
“I would say that a majority of the time people are taking their medicines slightly differently than prescribed, and often, this is due to cost,” she said.
Serious Consequences
Cost-related medication nonadherence (CRN), as investigators term it, can have major health effects and can be significantly more costly in the long run due to increased hospitalization rates, higher morbidity, and greater risk for COPD-related death associated with suboptimal care.
“For some patients even a month or two of being off medications increases the risk of having exacerbations, having more symptoms, [and] having a decline in their lung function,” said Douglas M. Beach, MD, a pulmonologist at Beth Israel Deaconess Medical Center in Boston.
In the aforementioned cross-sectional study, published in the open access journal BMC Public Health, Xin Wen, MD, from the Jiamusi (China) University School of Public Health, and colleagues looked at data on a representative sample of US adults who participated in the US National Health Interview Survey from 2013 through 2020.
The sample included 15,928 persons aged 18 years or older with a self-reported history of COPD who completed a CRN survey including the following questions:
During the past 12 months, have you
- Skipped medication doses to save money?
- Taken less medicine than prescribed to save money?
- Delayed filling a prescription to save money?
The investigators found that a weighted 18.56% of participants representing 2.39 million persons with COPD answered “yes” to one of the questions.
Translated into representative population numbers, that works out to an estimated 1.61 million persons with COPD missing doses, 1.72 million taking lower doses than those prescribed, and 2.03 million delaying filling prescriptions to save money.
A multivariable logistic regression analysis showed that those who were most likely to be nonadherent for financial reasons were patients younger than 65 years, women, persons with low family income, those who lacked health insurance, and patients with multiple comorbidities, the authors found.
Financial Barriers
One of the biggest barriers to COPD medication adherence is, somewhat paradoxically, insurance status, particularly Medicare, said Corinne Young, MSN, FNP-C, FCCP, from Colorado Springs Pulmonary Consultants.
“What’s so unfair about Medicare is that patients have to buy a drug plan, so they have to already pay for an extra plan to have access to drugs, and the plans vary because there are so many choices,” she said in an interview.
Elderly patients may be confused about the available options and may choose the Medicare Advantage plan with the lowest monthly premiums, which have the highest annual deductibles, usually in the $5000-and-up range, she said.
In addition, the Medicare Part D prescription coverage gap, commonly known as the “donut hole,” requires patients to pay a percentage of drug costs above a certain limit ($5030 in 2024) until a yearly out-of-pocket limit (currently $8000) is reached, after which the plan will again pick up most of the costs.
Although makers of inhalers have voluntarily agreed to limit monthly co-pays to $35 for uninsured patients, Medicare plans require insured patients to shell out considerably more, with 30 days of Trelegy Ellipta (fluticasone furoate, umeclidinium, and vilanterol) setting patients back nearly $350 per month, according to a recent search of costs for a United Healthcare Medicare Advantage plan.
Chasing Lower-Cost Options
“I have a lot of patients who use Canadian pharmacies to try to get around it, and I have a lot of patients who make a trip to Mexico every year and load up. I have patients who don’t take their inhalers as they are supposed to in order to make them last longer, and I have patients who take the inhalers of other family members,” Ms. Young said.
Humayun Anjum, MD, FCCP, from Dallas Pulmonary and Critical Care in Dallas, Texas, said in an interview that when patients claim that a prescribed drug isn’t working as expected for them, financial pressures may be partly to blame.
“When you investigate a little bit more, that’s where things become a bit more clear, and the patient may say ‘yeah, I stopped using this inhaler because it was costing me 200 bucks a month and I’m already on other medications,’ ” he said.
He noted that, when possible, he will steer patients toward discount prescription services such as GoodRx, which offers discounts at local pharmacies, or Mark Cuban Cost Plus Drugs, an online pharmacy that offers generic versions of Advair Diskus (fluticasone propionate/salmeterol) at a 100-50 mcg dose for $94.70, a savings of $307.30 over retail pharmacies, according to the company’s website.
Dr. Beach noted that Beth Israel Deaconess has a pharmacist embedded in the pulmonary clinic who can help eligible patients get financial assistance to pay for their medications.
“The influencing factors of CRN are multifaceted and necessitating more rigorous research. Health policy interventions focusing on reducing drug costs, delaying disease progression, preventing exacerbations, and reducing the risk of comorbidities may improve the economic burden of COPD and its outcomes,” Dr. Wen and colleagues wrote.
The study by Dr. Wen and colleagues was funded by grants from Chinese national and academic sources. Dr. McCormack has served as a consultant to Aridis, Boehringer Ingelheim, GlaxoSmithKline, MCG Diagnostics, ndd Medical Technologies, and UpToDate. Ms. Young, Dr. Anjum, and Dr. Beach reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Measuring Restrictive Lung Disease Severity Using FEV1 vs TLC
Respiratory diseases have varied clinical presentations and are classified as restrictive, obstructive, mixed, or normal. Restrictive lung diseases have reduced lung volumes, either due to an alteration in lung parenchyma or a disease of the pleura, chest wall, or neuromuscular apparatus. If caused by parenchymal lung disease, restrictive lung disorders are accompanied by reduced gas transfer, which may be portrayed clinically by desaturation after exercise. Based on anatomical structures, the causes of lung volume reduction may be intrinsic or extrinsic. Intrinsic causes correspond to diseases of the lung parenchyma, such as idiopathic fibrotic diseases, connective-tissue diseases, drug-induced lung diseases, and other primary diseases of the lungs. Extrinsic causes refer to disorders outside the lungs or extra-pulmonary diseases such as neuromuscular and nonmuscular diseases of the chest wall.1 For example, obesity and myasthenia gravis can cause restrictive lung diseases, one through mechanical interference of lung expansion and the other through neuromuscular impedance of thoracic cage expansion. All these diseases eventually result in lung restriction, impaired lung function, and respiratory failure. This heterogenicity of disease makes establishing a single severity criterion difficult.
Laboratory testing, imaging studies, and examinations are important for determining the pulmonary disease and its course and progression. The pulmonary function test (PFT), which consists of multiple procedures that are performed depending on the information needed, has been an essential tool in practice for the pulmonologist. The PFT includes spirometry, lung volume measurement, respiratory muscle strength, diffusion capacity, and a broncho-provocation test. Each test has a particular role in assisting the diagnosis and/or follow-up of the patient. Spirometry is frequently used due to its range of dynamic physiological parameters, ease of use, and accessibility. It is used for the diagnosis of pulmonary symptoms, in the assessment of disability, and preoperatory evaluation, including lung resection surgery, assisting in the diagnosis, monitoring, and therapy response of pulmonary diseases.
A systematic approach to PFT interpretation is recommended by several societies, such as the American Thoracic Society (ATS) and the European Respiratory Society (ERS).2 The pulmonary function test results must be reproducible and meet established standards to ensure reliable and consistent clinical outcomes. A restrictive respiratory disease is defined by a decrease in total lung capacity (TLC) (< 5% of predicted value) and a normal forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio.2 Although other findings—such as a decrease in vital capacity—should prompt an investigation into whether the patient has a possible restrictive respiratory disease, the sole presence of this parameter is not definitive or diagnostic of a restrictive impairment.2-4 The assessment of severity is typically determined by TLC. Unfortunately, the severity of a restrictive respiratory disease and the degree of patient discomfort do not always correlate when utilizing just TLC. Pulmonary sarcoidosis, for example, is a granulomatous lung disease with a restrictive PFT pattern and a disease burden that may vary over time. Having a more consistent method of grading the severity of the restrictive lung disease may help guide treatment. The modified Medical Research Council (mMRC) scale, a 5-point dyspnea scale, is widely used in assessing the severity of dyspnea in various respiratory conditions, including chronic obstructive pulmonary disease (COPD), where its scores have been associated with patient mortality.1,5 The goal of this study was to document the associations between objective parameters obtained through PFT and other variables, with an established measurement of dyspnea to assess the severity grade of restrictive lung diseases.
Methods
This retrospective record review at the Veterans Affairs Caribbean Healthcare System (VACHS) in San Juan, Puerto Rico, wasconducted using the Veterans Health Information Systems and Technology Architecture to identify patients with a PFT, including spirometry, that indicated a restrictive ventilator pattern based on the current ATS/ERS Task Force on Lung Function Testing.2 Patients were included if they were aged ≥ 21 years, PFT with TLC ≤ 80% predicted, mMRC score documented on PFT, and documented diffusing capacity of the lung for carbon monoxide (DLCO). Patients were excluded if their FEV1/vital capacity (VC) was < 70% predicted using the largest VC, or no mMRC score was available. All patients meeting the inclusion criteria were considered regardless of comorbidities.
The PFT results of all adult patients, including those performed between June 1, 2013, and January 6, 2016, were submitted to spirometry, and lung volume measurements were analyzed. Sociodemographic information was collected, including sex, ethnicity, age, height, weight, and basal metabolic index. Other data found in PFTs, such as smoking status, smoking in packs/year, mMRC score, predicted TLC value, imaging present (chest X-ray, computed tomography), and hospitalizations and exacerbations within 1 year were collected. In addition, we examined the predicted values for FEV1, DLCO, and DLCO/VA (calculated using the Ayer equation), FVC (calculated using the Knudson equation), expiratory reserve volume, inspiratory VC, and slow VC. PaO2, PaCO2, and Alveolar-arterial gradients also were collected.6-9 Information about heart failure status was gathered through medical evaluation of notes and cardiac studies. All categorical variables were correlated with Spearman analysis and quantitative variables with average percentages. P values were calculated with analysis of variance.
Results
Of 6461 VACHS patient records reviewed, 415 met the inclusion criteria. Patients were divided according to their mMRC score: 65 had mMRC score of 0, 87 had an mMRC score of 1, 2 had an mMRC score of 2, 146 had an mMRC of 3, and 115 had an mMRC score of 4. The population was primarily male (98.6%) and of Hispanic ethnicity (96.4%), with a mean age of 72 years (Table 1). Most patients (n = 269, 64.0%) were prior smokers, while 135 patients (32.5%) had never smoked, and 11 (2.7%) were current smokers. At baseline, 169 patients (41.4%) had interstitial lung disease, 39 (9.6%) had chest wall disorders, 29 (7.1%) had occupational exposure, 25 (6.1%) had pneumonitis, and 14 (3.4%) had neuromuscular disorders.
There was a statistically significant relationship between mMRC score and hospitalization and FEV1 but not TLC (Table 2). As mMRC increased, so did hospitalizations: a total of 168 patients (40.5%) were hospitalized; 24 patients (36.9%) had an mMRC score of 0, 30 patients (34.0%) had an mMRC score of 1, 2 patients (100%) had an mMRC score of 2, 54 patients (37.0%) had an mMRC score of 3, and 58 patients (50.0%) had an mMRC score of 4 (P = .04). Mean (SD) TLC values increased as mMRC scores increased. Mean (SD) TLC was 70.5% (33.0) for the entire population; 68.8% (7.2) for patients with an mMRC score of 0, 70.8% (5.8) for patients with an mMRC score of 1, 75.0% (1.4) for patients with an mMRC score of 2, 70.1% (7.2) for patients with an mMRC score of 3, and 71.5% (62.1) for patients with an mMRC score of 4 (P = .10) (Figure 1). There was an associated decrease in mean (SD) FEV1 with mMRC. Mean (SD) FEV1 was 76.2% (18.9) for the entire population; 81.7% (19.3) for patients with an mMRC score of 0, 80.9% (18) for patients with an mMRC score of 1, 93.5% (34.6) for patients with an mMRC score of 2, 76.2% (17.1) for patients with an mMRC score of 3, and 69.2% (19.4) for patients with an mMRC score of 4; (P < .001) (Figure 2).
The correlation between mMRC and FEV1 (r = 0.25, P < .001) was stronger than the correlation between mMRC and TLC (r = 0.15, P < .001). The correlations for DLCO (P < .001), DLCO/VA (P < .001), hemoglobin (P < .02), and PaO2 (P < .001) were all statistically significant (P < .005), but with no strong identifiable trend.
Discussion
The patient population of this study was primarily older males of Hispanic ethnicity with a history of smoking. There was no association between body mass index or smoking status with worsening dyspnea as measured with mMRC scores. We observed no significant correlation between mMRC scores and various factors such as comorbidities including heart conditions, and epidemiological factors like the etiology of lung disease, including both intrinsic and extrinsic causes. This lack of association was anticipated, as restrictive lung diseases in our study predominantly arose from intrinsic pulmonary etiologies, such as interstitial lung disease. A difference between more hospitalizations and worsening dyspnea was identified. There was a slightly higher correlation between FEV1 and mMRC scores when compared with TLC and mMRC scores concerning worsening dyspnea, which could indicate that the use of FEV1 should be preferred over previous recommendations to use TLC.10 Other guidelines have utilized exercise capacity via the 6-minute walk test as a marker of severity with spirometry values and found that DLCO was correlated with severity.11
The latest ERS/ATS guidelines recommend z scores for grading the severity of obstructive lung diseases but do not recommend them for the diagnosis of restrictive lung diseases.12 A z score encompasses diverse variables (eg, age, sex, and ethnicity) to provide more uniform and consistent results. Other studies have been done to relate z scores to other spirometry variables with restrictive lung disease. One such study indicates the potential benefit of using FVC alone to grade restrictive lung diseases.13 There continues to be great diversity in the interpretation of pulmonary function tests, and we believe the information gathered can provide valuable insight for managing patients with restrictive lung diseases.
Limitations
Only 2 patients reported an mMRC score of 2 in our study. This may have affected statistical outcomes. It also may reveal possible deficits in the efficacy of patient education on the mMRC scale. This study was also limited by its small sample size, single center location, and the distribution of patients that reported an mMRC favored either low or high values. The patients in this study, who were all veterans, may not be representative of other patient populations.
Conclusions
There continue to be few factors associated with the physiological severity of the defective oxygen delivery and reported dyspnea of a patient with restrictive lung disease that allows for an accurate, repeatable grading of severity. Using FEV1 instead of TLC to determine the severity of a restrictive lung disease should be reconsidered. We could not find any other strong correlation among other factors studied. Further research should be conducted to continue looking for variables that more accurately depict patient dyspnea in restrictive lung disease.
Acknowledgments
This study is based upon work supported by the Veterans Affairs Caribbean Healthcare System in San Juan, Puerto Rico, and is the result of work supported by Pulmonary & Critical Care Medicine service, with resources and the use of its facilities.
1. Hegewald MJ, Crapo RO. Pulmonary function testing. In: Broaddus VC, Ernst JD, King Jr TE, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 5th ed. Saunders; 2010:522-553.
2. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948-968. doi:10.1183/09031936.05.00035205
3. Rabe KF, Beghé B, Luppi F, Fabbri LM. Update in chronic obstructive pulmonary disease 2006. Am J Respir Crit Care Med. 2007;175(12):1222-1232. doi:10.1164/rccm.200704-586UP
4. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Spirometry for health care providers Accessed April 30, 2024. https://goldcopd.org/wp-content/uploads/2016/04/GOLD_Spirometry_2010.pdf
5. Mannino DM, Holguin F, Pavlin BI, Ferdinands JM. Risk factors for prevalence of and mortality related to restriction on spirometry: findings from the First National Health and Nutrition Examination Survey and follow-up. Int J Tuberc Lung Dis. 2005;9(6):613-621.
6. Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127(6):725-734. doi:10.1164/arrd.1983.127.6.725
7. Knudson RJ, Burrows B, Lebowitz MD. The maximal expiratory flow-volume curve: its use in the detection of ventilatory abnormalities in a population study. Am Rev Respir Dis. 1976;114(5):871-879. doi:10.1164/arrd.1976.114.5.871
8. Knudson RJ, Lebowitz MD, Burton AP, Knudson DE. The closing volume test: evaluation of nitrogen and bolus methods in a random population. Am Rev Respir Dis. 1977;115(3):423-434. doi:10.1164/arrd.1977.115.3.423
9. Ayers LN, Ginsberg ML, Fein J, Wasserman K. Diffusing capacity, specific diffusing capacity and interpretation of diffusion defects. West J Med. 1975;123(4):255-264.
10. Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144(5):1202-1218. doi:10.1164/ajrccm/144.5.1202
11. Larson J, Wrzos K, Corazalla E, Wang Q, Kim HJ, Cho RJ. Should FEV1 be used to grade restrictive impairment? A single-center comparison of lung function parameters to 6-minute walk test in patients with restrictive lung disease. HSOA J Pulm Med Respir Res. 2023;9:082. doi:10.24966/PMRR-0177/100082
12. Stanojevic S, Kaminsky DA, Miller MR, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1):2101499. Published 2022 Jul 13. doi:10.1183/13993003.01499-2021
13. Myrberg T, Lindberg A, Eriksson B, et al. Restrictive spirometry versus restrictive lung function using the GLI reference values. Clin Physiol Funct Imaging. 2022;42(3):181-189. doi:10.1111/cpf.12745
Respiratory diseases have varied clinical presentations and are classified as restrictive, obstructive, mixed, or normal. Restrictive lung diseases have reduced lung volumes, either due to an alteration in lung parenchyma or a disease of the pleura, chest wall, or neuromuscular apparatus. If caused by parenchymal lung disease, restrictive lung disorders are accompanied by reduced gas transfer, which may be portrayed clinically by desaturation after exercise. Based on anatomical structures, the causes of lung volume reduction may be intrinsic or extrinsic. Intrinsic causes correspond to diseases of the lung parenchyma, such as idiopathic fibrotic diseases, connective-tissue diseases, drug-induced lung diseases, and other primary diseases of the lungs. Extrinsic causes refer to disorders outside the lungs or extra-pulmonary diseases such as neuromuscular and nonmuscular diseases of the chest wall.1 For example, obesity and myasthenia gravis can cause restrictive lung diseases, one through mechanical interference of lung expansion and the other through neuromuscular impedance of thoracic cage expansion. All these diseases eventually result in lung restriction, impaired lung function, and respiratory failure. This heterogenicity of disease makes establishing a single severity criterion difficult.
Laboratory testing, imaging studies, and examinations are important for determining the pulmonary disease and its course and progression. The pulmonary function test (PFT), which consists of multiple procedures that are performed depending on the information needed, has been an essential tool in practice for the pulmonologist. The PFT includes spirometry, lung volume measurement, respiratory muscle strength, diffusion capacity, and a broncho-provocation test. Each test has a particular role in assisting the diagnosis and/or follow-up of the patient. Spirometry is frequently used due to its range of dynamic physiological parameters, ease of use, and accessibility. It is used for the diagnosis of pulmonary symptoms, in the assessment of disability, and preoperatory evaluation, including lung resection surgery, assisting in the diagnosis, monitoring, and therapy response of pulmonary diseases.
A systematic approach to PFT interpretation is recommended by several societies, such as the American Thoracic Society (ATS) and the European Respiratory Society (ERS).2 The pulmonary function test results must be reproducible and meet established standards to ensure reliable and consistent clinical outcomes. A restrictive respiratory disease is defined by a decrease in total lung capacity (TLC) (< 5% of predicted value) and a normal forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio.2 Although other findings—such as a decrease in vital capacity—should prompt an investigation into whether the patient has a possible restrictive respiratory disease, the sole presence of this parameter is not definitive or diagnostic of a restrictive impairment.2-4 The assessment of severity is typically determined by TLC. Unfortunately, the severity of a restrictive respiratory disease and the degree of patient discomfort do not always correlate when utilizing just TLC. Pulmonary sarcoidosis, for example, is a granulomatous lung disease with a restrictive PFT pattern and a disease burden that may vary over time. Having a more consistent method of grading the severity of the restrictive lung disease may help guide treatment. The modified Medical Research Council (mMRC) scale, a 5-point dyspnea scale, is widely used in assessing the severity of dyspnea in various respiratory conditions, including chronic obstructive pulmonary disease (COPD), where its scores have been associated with patient mortality.1,5 The goal of this study was to document the associations between objective parameters obtained through PFT and other variables, with an established measurement of dyspnea to assess the severity grade of restrictive lung diseases.
Methods
This retrospective record review at the Veterans Affairs Caribbean Healthcare System (VACHS) in San Juan, Puerto Rico, wasconducted using the Veterans Health Information Systems and Technology Architecture to identify patients with a PFT, including spirometry, that indicated a restrictive ventilator pattern based on the current ATS/ERS Task Force on Lung Function Testing.2 Patients were included if they were aged ≥ 21 years, PFT with TLC ≤ 80% predicted, mMRC score documented on PFT, and documented diffusing capacity of the lung for carbon monoxide (DLCO). Patients were excluded if their FEV1/vital capacity (VC) was < 70% predicted using the largest VC, or no mMRC score was available. All patients meeting the inclusion criteria were considered regardless of comorbidities.
The PFT results of all adult patients, including those performed between June 1, 2013, and January 6, 2016, were submitted to spirometry, and lung volume measurements were analyzed. Sociodemographic information was collected, including sex, ethnicity, age, height, weight, and basal metabolic index. Other data found in PFTs, such as smoking status, smoking in packs/year, mMRC score, predicted TLC value, imaging present (chest X-ray, computed tomography), and hospitalizations and exacerbations within 1 year were collected. In addition, we examined the predicted values for FEV1, DLCO, and DLCO/VA (calculated using the Ayer equation), FVC (calculated using the Knudson equation), expiratory reserve volume, inspiratory VC, and slow VC. PaO2, PaCO2, and Alveolar-arterial gradients also were collected.6-9 Information about heart failure status was gathered through medical evaluation of notes and cardiac studies. All categorical variables were correlated with Spearman analysis and quantitative variables with average percentages. P values were calculated with analysis of variance.
Results
Of 6461 VACHS patient records reviewed, 415 met the inclusion criteria. Patients were divided according to their mMRC score: 65 had mMRC score of 0, 87 had an mMRC score of 1, 2 had an mMRC score of 2, 146 had an mMRC of 3, and 115 had an mMRC score of 4. The population was primarily male (98.6%) and of Hispanic ethnicity (96.4%), with a mean age of 72 years (Table 1). Most patients (n = 269, 64.0%) were prior smokers, while 135 patients (32.5%) had never smoked, and 11 (2.7%) were current smokers. At baseline, 169 patients (41.4%) had interstitial lung disease, 39 (9.6%) had chest wall disorders, 29 (7.1%) had occupational exposure, 25 (6.1%) had pneumonitis, and 14 (3.4%) had neuromuscular disorders.
There was a statistically significant relationship between mMRC score and hospitalization and FEV1 but not TLC (Table 2). As mMRC increased, so did hospitalizations: a total of 168 patients (40.5%) were hospitalized; 24 patients (36.9%) had an mMRC score of 0, 30 patients (34.0%) had an mMRC score of 1, 2 patients (100%) had an mMRC score of 2, 54 patients (37.0%) had an mMRC score of 3, and 58 patients (50.0%) had an mMRC score of 4 (P = .04). Mean (SD) TLC values increased as mMRC scores increased. Mean (SD) TLC was 70.5% (33.0) for the entire population; 68.8% (7.2) for patients with an mMRC score of 0, 70.8% (5.8) for patients with an mMRC score of 1, 75.0% (1.4) for patients with an mMRC score of 2, 70.1% (7.2) for patients with an mMRC score of 3, and 71.5% (62.1) for patients with an mMRC score of 4 (P = .10) (Figure 1). There was an associated decrease in mean (SD) FEV1 with mMRC. Mean (SD) FEV1 was 76.2% (18.9) for the entire population; 81.7% (19.3) for patients with an mMRC score of 0, 80.9% (18) for patients with an mMRC score of 1, 93.5% (34.6) for patients with an mMRC score of 2, 76.2% (17.1) for patients with an mMRC score of 3, and 69.2% (19.4) for patients with an mMRC score of 4; (P < .001) (Figure 2).
The correlation between mMRC and FEV1 (r = 0.25, P < .001) was stronger than the correlation between mMRC and TLC (r = 0.15, P < .001). The correlations for DLCO (P < .001), DLCO/VA (P < .001), hemoglobin (P < .02), and PaO2 (P < .001) were all statistically significant (P < .005), but with no strong identifiable trend.
Discussion
The patient population of this study was primarily older males of Hispanic ethnicity with a history of smoking. There was no association between body mass index or smoking status with worsening dyspnea as measured with mMRC scores. We observed no significant correlation between mMRC scores and various factors such as comorbidities including heart conditions, and epidemiological factors like the etiology of lung disease, including both intrinsic and extrinsic causes. This lack of association was anticipated, as restrictive lung diseases in our study predominantly arose from intrinsic pulmonary etiologies, such as interstitial lung disease. A difference between more hospitalizations and worsening dyspnea was identified. There was a slightly higher correlation between FEV1 and mMRC scores when compared with TLC and mMRC scores concerning worsening dyspnea, which could indicate that the use of FEV1 should be preferred over previous recommendations to use TLC.10 Other guidelines have utilized exercise capacity via the 6-minute walk test as a marker of severity with spirometry values and found that DLCO was correlated with severity.11
The latest ERS/ATS guidelines recommend z scores for grading the severity of obstructive lung diseases but do not recommend them for the diagnosis of restrictive lung diseases.12 A z score encompasses diverse variables (eg, age, sex, and ethnicity) to provide more uniform and consistent results. Other studies have been done to relate z scores to other spirometry variables with restrictive lung disease. One such study indicates the potential benefit of using FVC alone to grade restrictive lung diseases.13 There continues to be great diversity in the interpretation of pulmonary function tests, and we believe the information gathered can provide valuable insight for managing patients with restrictive lung diseases.
Limitations
Only 2 patients reported an mMRC score of 2 in our study. This may have affected statistical outcomes. It also may reveal possible deficits in the efficacy of patient education on the mMRC scale. This study was also limited by its small sample size, single center location, and the distribution of patients that reported an mMRC favored either low or high values. The patients in this study, who were all veterans, may not be representative of other patient populations.
Conclusions
There continue to be few factors associated with the physiological severity of the defective oxygen delivery and reported dyspnea of a patient with restrictive lung disease that allows for an accurate, repeatable grading of severity. Using FEV1 instead of TLC to determine the severity of a restrictive lung disease should be reconsidered. We could not find any other strong correlation among other factors studied. Further research should be conducted to continue looking for variables that more accurately depict patient dyspnea in restrictive lung disease.
Acknowledgments
This study is based upon work supported by the Veterans Affairs Caribbean Healthcare System in San Juan, Puerto Rico, and is the result of work supported by Pulmonary & Critical Care Medicine service, with resources and the use of its facilities.
Respiratory diseases have varied clinical presentations and are classified as restrictive, obstructive, mixed, or normal. Restrictive lung diseases have reduced lung volumes, either due to an alteration in lung parenchyma or a disease of the pleura, chest wall, or neuromuscular apparatus. If caused by parenchymal lung disease, restrictive lung disorders are accompanied by reduced gas transfer, which may be portrayed clinically by desaturation after exercise. Based on anatomical structures, the causes of lung volume reduction may be intrinsic or extrinsic. Intrinsic causes correspond to diseases of the lung parenchyma, such as idiopathic fibrotic diseases, connective-tissue diseases, drug-induced lung diseases, and other primary diseases of the lungs. Extrinsic causes refer to disorders outside the lungs or extra-pulmonary diseases such as neuromuscular and nonmuscular diseases of the chest wall.1 For example, obesity and myasthenia gravis can cause restrictive lung diseases, one through mechanical interference of lung expansion and the other through neuromuscular impedance of thoracic cage expansion. All these diseases eventually result in lung restriction, impaired lung function, and respiratory failure. This heterogenicity of disease makes establishing a single severity criterion difficult.
Laboratory testing, imaging studies, and examinations are important for determining the pulmonary disease and its course and progression. The pulmonary function test (PFT), which consists of multiple procedures that are performed depending on the information needed, has been an essential tool in practice for the pulmonologist. The PFT includes spirometry, lung volume measurement, respiratory muscle strength, diffusion capacity, and a broncho-provocation test. Each test has a particular role in assisting the diagnosis and/or follow-up of the patient. Spirometry is frequently used due to its range of dynamic physiological parameters, ease of use, and accessibility. It is used for the diagnosis of pulmonary symptoms, in the assessment of disability, and preoperatory evaluation, including lung resection surgery, assisting in the diagnosis, monitoring, and therapy response of pulmonary diseases.
A systematic approach to PFT interpretation is recommended by several societies, such as the American Thoracic Society (ATS) and the European Respiratory Society (ERS).2 The pulmonary function test results must be reproducible and meet established standards to ensure reliable and consistent clinical outcomes. A restrictive respiratory disease is defined by a decrease in total lung capacity (TLC) (< 5% of predicted value) and a normal forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio.2 Although other findings—such as a decrease in vital capacity—should prompt an investigation into whether the patient has a possible restrictive respiratory disease, the sole presence of this parameter is not definitive or diagnostic of a restrictive impairment.2-4 The assessment of severity is typically determined by TLC. Unfortunately, the severity of a restrictive respiratory disease and the degree of patient discomfort do not always correlate when utilizing just TLC. Pulmonary sarcoidosis, for example, is a granulomatous lung disease with a restrictive PFT pattern and a disease burden that may vary over time. Having a more consistent method of grading the severity of the restrictive lung disease may help guide treatment. The modified Medical Research Council (mMRC) scale, a 5-point dyspnea scale, is widely used in assessing the severity of dyspnea in various respiratory conditions, including chronic obstructive pulmonary disease (COPD), where its scores have been associated with patient mortality.1,5 The goal of this study was to document the associations between objective parameters obtained through PFT and other variables, with an established measurement of dyspnea to assess the severity grade of restrictive lung diseases.
Methods
This retrospective record review at the Veterans Affairs Caribbean Healthcare System (VACHS) in San Juan, Puerto Rico, wasconducted using the Veterans Health Information Systems and Technology Architecture to identify patients with a PFT, including spirometry, that indicated a restrictive ventilator pattern based on the current ATS/ERS Task Force on Lung Function Testing.2 Patients were included if they were aged ≥ 21 years, PFT with TLC ≤ 80% predicted, mMRC score documented on PFT, and documented diffusing capacity of the lung for carbon monoxide (DLCO). Patients were excluded if their FEV1/vital capacity (VC) was < 70% predicted using the largest VC, or no mMRC score was available. All patients meeting the inclusion criteria were considered regardless of comorbidities.
The PFT results of all adult patients, including those performed between June 1, 2013, and January 6, 2016, were submitted to spirometry, and lung volume measurements were analyzed. Sociodemographic information was collected, including sex, ethnicity, age, height, weight, and basal metabolic index. Other data found in PFTs, such as smoking status, smoking in packs/year, mMRC score, predicted TLC value, imaging present (chest X-ray, computed tomography), and hospitalizations and exacerbations within 1 year were collected. In addition, we examined the predicted values for FEV1, DLCO, and DLCO/VA (calculated using the Ayer equation), FVC (calculated using the Knudson equation), expiratory reserve volume, inspiratory VC, and slow VC. PaO2, PaCO2, and Alveolar-arterial gradients also were collected.6-9 Information about heart failure status was gathered through medical evaluation of notes and cardiac studies. All categorical variables were correlated with Spearman analysis and quantitative variables with average percentages. P values were calculated with analysis of variance.
Results
Of 6461 VACHS patient records reviewed, 415 met the inclusion criteria. Patients were divided according to their mMRC score: 65 had mMRC score of 0, 87 had an mMRC score of 1, 2 had an mMRC score of 2, 146 had an mMRC of 3, and 115 had an mMRC score of 4. The population was primarily male (98.6%) and of Hispanic ethnicity (96.4%), with a mean age of 72 years (Table 1). Most patients (n = 269, 64.0%) were prior smokers, while 135 patients (32.5%) had never smoked, and 11 (2.7%) were current smokers. At baseline, 169 patients (41.4%) had interstitial lung disease, 39 (9.6%) had chest wall disorders, 29 (7.1%) had occupational exposure, 25 (6.1%) had pneumonitis, and 14 (3.4%) had neuromuscular disorders.
There was a statistically significant relationship between mMRC score and hospitalization and FEV1 but not TLC (Table 2). As mMRC increased, so did hospitalizations: a total of 168 patients (40.5%) were hospitalized; 24 patients (36.9%) had an mMRC score of 0, 30 patients (34.0%) had an mMRC score of 1, 2 patients (100%) had an mMRC score of 2, 54 patients (37.0%) had an mMRC score of 3, and 58 patients (50.0%) had an mMRC score of 4 (P = .04). Mean (SD) TLC values increased as mMRC scores increased. Mean (SD) TLC was 70.5% (33.0) for the entire population; 68.8% (7.2) for patients with an mMRC score of 0, 70.8% (5.8) for patients with an mMRC score of 1, 75.0% (1.4) for patients with an mMRC score of 2, 70.1% (7.2) for patients with an mMRC score of 3, and 71.5% (62.1) for patients with an mMRC score of 4 (P = .10) (Figure 1). There was an associated decrease in mean (SD) FEV1 with mMRC. Mean (SD) FEV1 was 76.2% (18.9) for the entire population; 81.7% (19.3) for patients with an mMRC score of 0, 80.9% (18) for patients with an mMRC score of 1, 93.5% (34.6) for patients with an mMRC score of 2, 76.2% (17.1) for patients with an mMRC score of 3, and 69.2% (19.4) for patients with an mMRC score of 4; (P < .001) (Figure 2).
The correlation between mMRC and FEV1 (r = 0.25, P < .001) was stronger than the correlation between mMRC and TLC (r = 0.15, P < .001). The correlations for DLCO (P < .001), DLCO/VA (P < .001), hemoglobin (P < .02), and PaO2 (P < .001) were all statistically significant (P < .005), but with no strong identifiable trend.
Discussion
The patient population of this study was primarily older males of Hispanic ethnicity with a history of smoking. There was no association between body mass index or smoking status with worsening dyspnea as measured with mMRC scores. We observed no significant correlation between mMRC scores and various factors such as comorbidities including heart conditions, and epidemiological factors like the etiology of lung disease, including both intrinsic and extrinsic causes. This lack of association was anticipated, as restrictive lung diseases in our study predominantly arose from intrinsic pulmonary etiologies, such as interstitial lung disease. A difference between more hospitalizations and worsening dyspnea was identified. There was a slightly higher correlation between FEV1 and mMRC scores when compared with TLC and mMRC scores concerning worsening dyspnea, which could indicate that the use of FEV1 should be preferred over previous recommendations to use TLC.10 Other guidelines have utilized exercise capacity via the 6-minute walk test as a marker of severity with spirometry values and found that DLCO was correlated with severity.11
The latest ERS/ATS guidelines recommend z scores for grading the severity of obstructive lung diseases but do not recommend them for the diagnosis of restrictive lung diseases.12 A z score encompasses diverse variables (eg, age, sex, and ethnicity) to provide more uniform and consistent results. Other studies have been done to relate z scores to other spirometry variables with restrictive lung disease. One such study indicates the potential benefit of using FVC alone to grade restrictive lung diseases.13 There continues to be great diversity in the interpretation of pulmonary function tests, and we believe the information gathered can provide valuable insight for managing patients with restrictive lung diseases.
Limitations
Only 2 patients reported an mMRC score of 2 in our study. This may have affected statistical outcomes. It also may reveal possible deficits in the efficacy of patient education on the mMRC scale. This study was also limited by its small sample size, single center location, and the distribution of patients that reported an mMRC favored either low or high values. The patients in this study, who were all veterans, may not be representative of other patient populations.
Conclusions
There continue to be few factors associated with the physiological severity of the defective oxygen delivery and reported dyspnea of a patient with restrictive lung disease that allows for an accurate, repeatable grading of severity. Using FEV1 instead of TLC to determine the severity of a restrictive lung disease should be reconsidered. We could not find any other strong correlation among other factors studied. Further research should be conducted to continue looking for variables that more accurately depict patient dyspnea in restrictive lung disease.
Acknowledgments
This study is based upon work supported by the Veterans Affairs Caribbean Healthcare System in San Juan, Puerto Rico, and is the result of work supported by Pulmonary & Critical Care Medicine service, with resources and the use of its facilities.
1. Hegewald MJ, Crapo RO. Pulmonary function testing. In: Broaddus VC, Ernst JD, King Jr TE, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 5th ed. Saunders; 2010:522-553.
2. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948-968. doi:10.1183/09031936.05.00035205
3. Rabe KF, Beghé B, Luppi F, Fabbri LM. Update in chronic obstructive pulmonary disease 2006. Am J Respir Crit Care Med. 2007;175(12):1222-1232. doi:10.1164/rccm.200704-586UP
4. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Spirometry for health care providers Accessed April 30, 2024. https://goldcopd.org/wp-content/uploads/2016/04/GOLD_Spirometry_2010.pdf
5. Mannino DM, Holguin F, Pavlin BI, Ferdinands JM. Risk factors for prevalence of and mortality related to restriction on spirometry: findings from the First National Health and Nutrition Examination Survey and follow-up. Int J Tuberc Lung Dis. 2005;9(6):613-621.
6. Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127(6):725-734. doi:10.1164/arrd.1983.127.6.725
7. Knudson RJ, Burrows B, Lebowitz MD. The maximal expiratory flow-volume curve: its use in the detection of ventilatory abnormalities in a population study. Am Rev Respir Dis. 1976;114(5):871-879. doi:10.1164/arrd.1976.114.5.871
8. Knudson RJ, Lebowitz MD, Burton AP, Knudson DE. The closing volume test: evaluation of nitrogen and bolus methods in a random population. Am Rev Respir Dis. 1977;115(3):423-434. doi:10.1164/arrd.1977.115.3.423
9. Ayers LN, Ginsberg ML, Fein J, Wasserman K. Diffusing capacity, specific diffusing capacity and interpretation of diffusion defects. West J Med. 1975;123(4):255-264.
10. Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144(5):1202-1218. doi:10.1164/ajrccm/144.5.1202
11. Larson J, Wrzos K, Corazalla E, Wang Q, Kim HJ, Cho RJ. Should FEV1 be used to grade restrictive impairment? A single-center comparison of lung function parameters to 6-minute walk test in patients with restrictive lung disease. HSOA J Pulm Med Respir Res. 2023;9:082. doi:10.24966/PMRR-0177/100082
12. Stanojevic S, Kaminsky DA, Miller MR, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1):2101499. Published 2022 Jul 13. doi:10.1183/13993003.01499-2021
13. Myrberg T, Lindberg A, Eriksson B, et al. Restrictive spirometry versus restrictive lung function using the GLI reference values. Clin Physiol Funct Imaging. 2022;42(3):181-189. doi:10.1111/cpf.12745
1. Hegewald MJ, Crapo RO. Pulmonary function testing. In: Broaddus VC, Ernst JD, King Jr TE, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 5th ed. Saunders; 2010:522-553.
2. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948-968. doi:10.1183/09031936.05.00035205
3. Rabe KF, Beghé B, Luppi F, Fabbri LM. Update in chronic obstructive pulmonary disease 2006. Am J Respir Crit Care Med. 2007;175(12):1222-1232. doi:10.1164/rccm.200704-586UP
4. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Spirometry for health care providers Accessed April 30, 2024. https://goldcopd.org/wp-content/uploads/2016/04/GOLD_Spirometry_2010.pdf
5. Mannino DM, Holguin F, Pavlin BI, Ferdinands JM. Risk factors for prevalence of and mortality related to restriction on spirometry: findings from the First National Health and Nutrition Examination Survey and follow-up. Int J Tuberc Lung Dis. 2005;9(6):613-621.
6. Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127(6):725-734. doi:10.1164/arrd.1983.127.6.725
7. Knudson RJ, Burrows B, Lebowitz MD. The maximal expiratory flow-volume curve: its use in the detection of ventilatory abnormalities in a population study. Am Rev Respir Dis. 1976;114(5):871-879. doi:10.1164/arrd.1976.114.5.871
8. Knudson RJ, Lebowitz MD, Burton AP, Knudson DE. The closing volume test: evaluation of nitrogen and bolus methods in a random population. Am Rev Respir Dis. 1977;115(3):423-434. doi:10.1164/arrd.1977.115.3.423
9. Ayers LN, Ginsberg ML, Fein J, Wasserman K. Diffusing capacity, specific diffusing capacity and interpretation of diffusion defects. West J Med. 1975;123(4):255-264.
10. Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144(5):1202-1218. doi:10.1164/ajrccm/144.5.1202
11. Larson J, Wrzos K, Corazalla E, Wang Q, Kim HJ, Cho RJ. Should FEV1 be used to grade restrictive impairment? A single-center comparison of lung function parameters to 6-minute walk test in patients with restrictive lung disease. HSOA J Pulm Med Respir Res. 2023;9:082. doi:10.24966/PMRR-0177/100082
12. Stanojevic S, Kaminsky DA, Miller MR, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1):2101499. Published 2022 Jul 13. doi:10.1183/13993003.01499-2021
13. Myrberg T, Lindberg A, Eriksson B, et al. Restrictive spirometry versus restrictive lung function using the GLI reference values. Clin Physiol Funct Imaging. 2022;42(3):181-189. doi:10.1111/cpf.12745
Quitting Smoking Boosts Life Expectancy at Any Age
TOPLINE:
Quitting smoking at any age increases life expectancy, with the most significant increases observed in younger individuals. But people who quit over age 65 can extend life expectancy.
METHODOLOGY:
- Researchers analyzed the detrimental effects of smoking and the positive impacts of cessation on life expectancy in individuals aged 35-75 years.
- Age-specific death rates by smoking status were calculated using the relative risks for all-cause mortality derived from the Cancer Prevention Study II data, 2018 National Health Interview Survey smoking prevalence data, and 2018 all-cause mortality rates.
- Life tables were constructed to obtain information on the life expectancies of people who never smoked, those who currently smoked, and those who previously smoked but quit at various ages.
- Estimates of years lost due to smoking and years gained by quitting smoking were calculated for people starting at age 35 and over 10-year increments.
TAKEAWAY:
- Compared with people who never smoked, those who smoked at ages 35, 45, 55, 65, and 75 years and continued smoking throughout their lives would lose 9.1, 8.3, 7.3, 5.9, and 4.4 years, respectively.
- People who quit smoking at ages 35, 45, 55, 65, and 75 years would have life expectancies that are shorter by 1.2, 2.7, 3.9, 4.2, and 3.7 years, respectively, than those of same-age individuals who never smoked.
- Individuals who quit smoking at ages 35, 45, 55, 65, and 75 years would experience an additional 8.0, 5.6, 3.4, 1.7, and 0.7 years of life expectancy compared with those who continued smoking.
- People who quit at ages 65 and 75 years would have a 23.4% and 14.2% chance of gaining at least 1 additional year of life.
IN PRACTICE:
“This cessation benefit is not limited to young- and middle-aged adults who smoke; this study demonstrates its applicability to seniors as well. These findings may be valuable for clinicians seeking scientific evidence to motivate their patients who smoke to quit,” the authors wrote.
SOURCE:
The study was led by Thuy T.T. Le, PhD, from the Department of Health Management and Policy at the University of Michigan School of Public Health in Ann Arbor and published online in the American Journal of Preventive Medicine.
LIMITATIONS:
The study’s estimates were according to data from 2018 and may not reflect current trends. The estimates also did not account for variability in smoking intensity among individuals.
DISCLOSURES:
The study was supported by grants from the National Cancer Institute of the US National Institutes of Health and the US Food and Drug Administration Center for Tobacco Products. The authors declared that they had no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Quitting smoking at any age increases life expectancy, with the most significant increases observed in younger individuals. But people who quit over age 65 can extend life expectancy.
METHODOLOGY:
- Researchers analyzed the detrimental effects of smoking and the positive impacts of cessation on life expectancy in individuals aged 35-75 years.
- Age-specific death rates by smoking status were calculated using the relative risks for all-cause mortality derived from the Cancer Prevention Study II data, 2018 National Health Interview Survey smoking prevalence data, and 2018 all-cause mortality rates.
- Life tables were constructed to obtain information on the life expectancies of people who never smoked, those who currently smoked, and those who previously smoked but quit at various ages.
- Estimates of years lost due to smoking and years gained by quitting smoking were calculated for people starting at age 35 and over 10-year increments.
TAKEAWAY:
- Compared with people who never smoked, those who smoked at ages 35, 45, 55, 65, and 75 years and continued smoking throughout their lives would lose 9.1, 8.3, 7.3, 5.9, and 4.4 years, respectively.
- People who quit smoking at ages 35, 45, 55, 65, and 75 years would have life expectancies that are shorter by 1.2, 2.7, 3.9, 4.2, and 3.7 years, respectively, than those of same-age individuals who never smoked.
- Individuals who quit smoking at ages 35, 45, 55, 65, and 75 years would experience an additional 8.0, 5.6, 3.4, 1.7, and 0.7 years of life expectancy compared with those who continued smoking.
- People who quit at ages 65 and 75 years would have a 23.4% and 14.2% chance of gaining at least 1 additional year of life.
IN PRACTICE:
“This cessation benefit is not limited to young- and middle-aged adults who smoke; this study demonstrates its applicability to seniors as well. These findings may be valuable for clinicians seeking scientific evidence to motivate their patients who smoke to quit,” the authors wrote.
SOURCE:
The study was led by Thuy T.T. Le, PhD, from the Department of Health Management and Policy at the University of Michigan School of Public Health in Ann Arbor and published online in the American Journal of Preventive Medicine.
LIMITATIONS:
The study’s estimates were according to data from 2018 and may not reflect current trends. The estimates also did not account for variability in smoking intensity among individuals.
DISCLOSURES:
The study was supported by grants from the National Cancer Institute of the US National Institutes of Health and the US Food and Drug Administration Center for Tobacco Products. The authors declared that they had no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Quitting smoking at any age increases life expectancy, with the most significant increases observed in younger individuals. But people who quit over age 65 can extend life expectancy.
METHODOLOGY:
- Researchers analyzed the detrimental effects of smoking and the positive impacts of cessation on life expectancy in individuals aged 35-75 years.
- Age-specific death rates by smoking status were calculated using the relative risks for all-cause mortality derived from the Cancer Prevention Study II data, 2018 National Health Interview Survey smoking prevalence data, and 2018 all-cause mortality rates.
- Life tables were constructed to obtain information on the life expectancies of people who never smoked, those who currently smoked, and those who previously smoked but quit at various ages.
- Estimates of years lost due to smoking and years gained by quitting smoking were calculated for people starting at age 35 and over 10-year increments.
TAKEAWAY:
- Compared with people who never smoked, those who smoked at ages 35, 45, 55, 65, and 75 years and continued smoking throughout their lives would lose 9.1, 8.3, 7.3, 5.9, and 4.4 years, respectively.
- People who quit smoking at ages 35, 45, 55, 65, and 75 years would have life expectancies that are shorter by 1.2, 2.7, 3.9, 4.2, and 3.7 years, respectively, than those of same-age individuals who never smoked.
- Individuals who quit smoking at ages 35, 45, 55, 65, and 75 years would experience an additional 8.0, 5.6, 3.4, 1.7, and 0.7 years of life expectancy compared with those who continued smoking.
- People who quit at ages 65 and 75 years would have a 23.4% and 14.2% chance of gaining at least 1 additional year of life.
IN PRACTICE:
“This cessation benefit is not limited to young- and middle-aged adults who smoke; this study demonstrates its applicability to seniors as well. These findings may be valuable for clinicians seeking scientific evidence to motivate their patients who smoke to quit,” the authors wrote.
SOURCE:
The study was led by Thuy T.T. Le, PhD, from the Department of Health Management and Policy at the University of Michigan School of Public Health in Ann Arbor and published online in the American Journal of Preventive Medicine.
LIMITATIONS:
The study’s estimates were according to data from 2018 and may not reflect current trends. The estimates also did not account for variability in smoking intensity among individuals.
DISCLOSURES:
The study was supported by grants from the National Cancer Institute of the US National Institutes of Health and the US Food and Drug Administration Center for Tobacco Products. The authors declared that they had no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Specific Antipsychotics Linked to Increased Pneumonia Risk
TOPLINE:
High-dose antipsychotics, particularly quetiapine, clozapine, and olanzapine, are linked to increased pneumonia risk in patients with schizophrenia, new data show. Monotherapy with high anticholinergic burden also raises pneumonia risk.
METHODOLOGY:
- Using several nationwide data registers, investigators pulled data on individuals who received inpatient care for schizophrenia or schizoaffective disorder (n = 61,889) between 1972 and 2014.
- Data on drug use were gathered from a prescription register and included dispensing dates, cost, dose, package size, and drug formulation. Data on dates and causes of death were obtained from the Causes of Death register.
- After entering the cohort, follow-up started in January 1996 or after the first diagnosis of schizophrenia for those diagnosed between 1996 and 2014.
- The primary outcome was hospitalization caused by pneumonia as the main diagnosis for hospital admission.
TAKEAWAY:
- During 22 years of follow-up, 8917 patients (14.4%) had one or more hospitalizations for pneumonia, and 1137 (12.8%) died within 30 days of admission.
- Pneumonia risk was the highest with the use of high-dose (> 440 mg/d) quetiapine (P = .003), followed by high- (≥ 330 mg/d) and medium-dose (180 to < 330 mg/d) clozapine (both P < .001) and high-dose (≥ 11 mg/d) olanzapine (P = .02).
- Compared with no antipsychotic use, antipsychotic monotherapy was associated with an increased pneumonia risk (P = .03), whereas antipsychotic polytherapy was not.
- Only the use of antipsychotics with high anticholinergic potency was associated with pneumonia risk (P < .001).
IN PRACTICE:
“Identification of antipsychotic drugs that are associated with pneumonia risk may better inform prevention programs (eg, vaccinations),” the researchers noted. “Second, the availability of pneumonia risk estimates for individual antipsychotics and for groups of antipsychotics may foster personalized prescribing guidelines.”
SOURCE:
The study was led by Jurjen Luykx, MD, Amsterdam University Medical Center, Amsterdam, the Netherlands. It was published online in JAMA Psychiatry.
LIMITATIONS:
The investigators could not correct for all possible risk factors that may increase pneumonia risk in individuals with schizophrenia, such as smoking and lifestyle habits. Also, cases of pneumonia that didn’t require hospital admission couldn’t be included in the analysis, so the findings may generalize only to cases of severe pneumonia.
DISCLOSURES:
The study was funded by the Finnish Ministry of Social Affairs and Health.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
High-dose antipsychotics, particularly quetiapine, clozapine, and olanzapine, are linked to increased pneumonia risk in patients with schizophrenia, new data show. Monotherapy with high anticholinergic burden also raises pneumonia risk.
METHODOLOGY:
- Using several nationwide data registers, investigators pulled data on individuals who received inpatient care for schizophrenia or schizoaffective disorder (n = 61,889) between 1972 and 2014.
- Data on drug use were gathered from a prescription register and included dispensing dates, cost, dose, package size, and drug formulation. Data on dates and causes of death were obtained from the Causes of Death register.
- After entering the cohort, follow-up started in January 1996 or after the first diagnosis of schizophrenia for those diagnosed between 1996 and 2014.
- The primary outcome was hospitalization caused by pneumonia as the main diagnosis for hospital admission.
TAKEAWAY:
- During 22 years of follow-up, 8917 patients (14.4%) had one or more hospitalizations for pneumonia, and 1137 (12.8%) died within 30 days of admission.
- Pneumonia risk was the highest with the use of high-dose (> 440 mg/d) quetiapine (P = .003), followed by high- (≥ 330 mg/d) and medium-dose (180 to < 330 mg/d) clozapine (both P < .001) and high-dose (≥ 11 mg/d) olanzapine (P = .02).
- Compared with no antipsychotic use, antipsychotic monotherapy was associated with an increased pneumonia risk (P = .03), whereas antipsychotic polytherapy was not.
- Only the use of antipsychotics with high anticholinergic potency was associated with pneumonia risk (P < .001).
IN PRACTICE:
“Identification of antipsychotic drugs that are associated with pneumonia risk may better inform prevention programs (eg, vaccinations),” the researchers noted. “Second, the availability of pneumonia risk estimates for individual antipsychotics and for groups of antipsychotics may foster personalized prescribing guidelines.”
SOURCE:
The study was led by Jurjen Luykx, MD, Amsterdam University Medical Center, Amsterdam, the Netherlands. It was published online in JAMA Psychiatry.
LIMITATIONS:
The investigators could not correct for all possible risk factors that may increase pneumonia risk in individuals with schizophrenia, such as smoking and lifestyle habits. Also, cases of pneumonia that didn’t require hospital admission couldn’t be included in the analysis, so the findings may generalize only to cases of severe pneumonia.
DISCLOSURES:
The study was funded by the Finnish Ministry of Social Affairs and Health.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
High-dose antipsychotics, particularly quetiapine, clozapine, and olanzapine, are linked to increased pneumonia risk in patients with schizophrenia, new data show. Monotherapy with high anticholinergic burden also raises pneumonia risk.
METHODOLOGY:
- Using several nationwide data registers, investigators pulled data on individuals who received inpatient care for schizophrenia or schizoaffective disorder (n = 61,889) between 1972 and 2014.
- Data on drug use were gathered from a prescription register and included dispensing dates, cost, dose, package size, and drug formulation. Data on dates and causes of death were obtained from the Causes of Death register.
- After entering the cohort, follow-up started in January 1996 or after the first diagnosis of schizophrenia for those diagnosed between 1996 and 2014.
- The primary outcome was hospitalization caused by pneumonia as the main diagnosis for hospital admission.
TAKEAWAY:
- During 22 years of follow-up, 8917 patients (14.4%) had one or more hospitalizations for pneumonia, and 1137 (12.8%) died within 30 days of admission.
- Pneumonia risk was the highest with the use of high-dose (> 440 mg/d) quetiapine (P = .003), followed by high- (≥ 330 mg/d) and medium-dose (180 to < 330 mg/d) clozapine (both P < .001) and high-dose (≥ 11 mg/d) olanzapine (P = .02).
- Compared with no antipsychotic use, antipsychotic monotherapy was associated with an increased pneumonia risk (P = .03), whereas antipsychotic polytherapy was not.
- Only the use of antipsychotics with high anticholinergic potency was associated with pneumonia risk (P < .001).
IN PRACTICE:
“Identification of antipsychotic drugs that are associated with pneumonia risk may better inform prevention programs (eg, vaccinations),” the researchers noted. “Second, the availability of pneumonia risk estimates for individual antipsychotics and for groups of antipsychotics may foster personalized prescribing guidelines.”
SOURCE:
The study was led by Jurjen Luykx, MD, Amsterdam University Medical Center, Amsterdam, the Netherlands. It was published online in JAMA Psychiatry.
LIMITATIONS:
The investigators could not correct for all possible risk factors that may increase pneumonia risk in individuals with schizophrenia, such as smoking and lifestyle habits. Also, cases of pneumonia that didn’t require hospital admission couldn’t be included in the analysis, so the findings may generalize only to cases of severe pneumonia.
DISCLOSURES:
The study was funded by the Finnish Ministry of Social Affairs and Health.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Top reads from the CHEST journal portfolio
Understanding RA with COPD, lung cancer prediction models, and chronic cardiac dysfunction
Journal CHEST®
Does Rheumatoid Arthritis Increase the Risk of COPD?
By: Chiwook Chung, MD, and colleagues
Notably, individuals with seropositive RA exhibit a greater risk of COPD onset than those with seronegative RA. Although smoking history didn’t affect the relationship between RA and COPD, monitoring respiratory symptoms and pulmonary function in patients with RA, especially patients who are seropositive, is crucial. These findings underscore the importance of interdisciplinary collaboration between rheumatologists and pulmonologists to enhance early detection and management strategies for pulmonary complications in patients with RA.
– Commentary by Corinne Young, MSN, FNP-C, FCCP, Member of the CHEST Physician® Editorial Board
CHEST Pulmonary®
The Lung Cancer Prediction Model “Stress Test”
By: Brent E. Heideman, MD, and colleagues
Current lung cancer prediction models have limited utility in high-risk patients referred for diagnostic biopsy. In a study of 322 indeterminate pulmonary nodules, the Brock, Mayo Clinic, Herder, and Veterans Affairs models showed modest discrimination between benign and malignant nodules (AUCs 0.67-0.77). The models performed poorly for low-risk patients (negative predictive values 63%-71%) and suboptimally for high-risk patients (positive predictive values 73%-87%), suggesting referring physicians use additional clinical information not captured in these models to identify high-risk patients needing biopsy. New prediction models and biomarkers specifically developed and calibrated for high-risk populations are needed to better inform clinical decision-making. Incorporating interval imaging to assess changes in nodule characteristics could potentially improve model performance. Tailored risk assessment tools are crucial for optimizing management and reducing unnecessary invasive procedures in this challenging patient population.
– Commentary by Russell Miller, MD, Member of the CHEST Physician Editorial Board
CHEST Critical Care ®
Characterizing Cardiac Function in ICU Survivors of Sepsis
By: Kevin Garrity, MBChB, and colleagues
While chronic cardiac dysfunction is one of the proposed mechanisms of long-term impairment post critical illness, its prevalence, mechanisms, and associations with disability following admission for sepsis are not well understood. Garrity and colleagues describe the Characterization of Cardiovascular Function in ICU Survivors of Sepsis (CONDUCT-ICU) protocol, a prospective study including two ICUs in Scotland aimed to better define cardiovascular dysfunction in survivors of sepsis. Designed to enroll 69 patients, demographics, cardiac and inflammatory biomarkers, and echocardiograms will be obtained on ICU discharge with additional laboratory data, cardiac magnetic resonance imaging, and patient-reported outcome measures to be obtained at 6 to 10 weeks. This novel multimodal approach will provide understanding into the role of cardiovascular dysfunction following critical illness as well as offer mechanistic insights. The investigators hope to obtain operational and pilot data for larger future studies.
– Commentary by Eugene Yuriditsky, MD, FCCP, Member of the CHEST Physician Editorial Board
Understanding RA with COPD, lung cancer prediction models, and chronic cardiac dysfunction
Understanding RA with COPD, lung cancer prediction models, and chronic cardiac dysfunction
Journal CHEST®
Does Rheumatoid Arthritis Increase the Risk of COPD?
By: Chiwook Chung, MD, and colleagues
Notably, individuals with seropositive RA exhibit a greater risk of COPD onset than those with seronegative RA. Although smoking history didn’t affect the relationship between RA and COPD, monitoring respiratory symptoms and pulmonary function in patients with RA, especially patients who are seropositive, is crucial. These findings underscore the importance of interdisciplinary collaboration between rheumatologists and pulmonologists to enhance early detection and management strategies for pulmonary complications in patients with RA.
– Commentary by Corinne Young, MSN, FNP-C, FCCP, Member of the CHEST Physician® Editorial Board
CHEST Pulmonary®
The Lung Cancer Prediction Model “Stress Test”
By: Brent E. Heideman, MD, and colleagues
Current lung cancer prediction models have limited utility in high-risk patients referred for diagnostic biopsy. In a study of 322 indeterminate pulmonary nodules, the Brock, Mayo Clinic, Herder, and Veterans Affairs models showed modest discrimination between benign and malignant nodules (AUCs 0.67-0.77). The models performed poorly for low-risk patients (negative predictive values 63%-71%) and suboptimally for high-risk patients (positive predictive values 73%-87%), suggesting referring physicians use additional clinical information not captured in these models to identify high-risk patients needing biopsy. New prediction models and biomarkers specifically developed and calibrated for high-risk populations are needed to better inform clinical decision-making. Incorporating interval imaging to assess changes in nodule characteristics could potentially improve model performance. Tailored risk assessment tools are crucial for optimizing management and reducing unnecessary invasive procedures in this challenging patient population.
– Commentary by Russell Miller, MD, Member of the CHEST Physician Editorial Board
CHEST Critical Care ®
Characterizing Cardiac Function in ICU Survivors of Sepsis
By: Kevin Garrity, MBChB, and colleagues
While chronic cardiac dysfunction is one of the proposed mechanisms of long-term impairment post critical illness, its prevalence, mechanisms, and associations with disability following admission for sepsis are not well understood. Garrity and colleagues describe the Characterization of Cardiovascular Function in ICU Survivors of Sepsis (CONDUCT-ICU) protocol, a prospective study including two ICUs in Scotland aimed to better define cardiovascular dysfunction in survivors of sepsis. Designed to enroll 69 patients, demographics, cardiac and inflammatory biomarkers, and echocardiograms will be obtained on ICU discharge with additional laboratory data, cardiac magnetic resonance imaging, and patient-reported outcome measures to be obtained at 6 to 10 weeks. This novel multimodal approach will provide understanding into the role of cardiovascular dysfunction following critical illness as well as offer mechanistic insights. The investigators hope to obtain operational and pilot data for larger future studies.
– Commentary by Eugene Yuriditsky, MD, FCCP, Member of the CHEST Physician Editorial Board
Journal CHEST®
Does Rheumatoid Arthritis Increase the Risk of COPD?
By: Chiwook Chung, MD, and colleagues
Notably, individuals with seropositive RA exhibit a greater risk of COPD onset than those with seronegative RA. Although smoking history didn’t affect the relationship between RA and COPD, monitoring respiratory symptoms and pulmonary function in patients with RA, especially patients who are seropositive, is crucial. These findings underscore the importance of interdisciplinary collaboration between rheumatologists and pulmonologists to enhance early detection and management strategies for pulmonary complications in patients with RA.
– Commentary by Corinne Young, MSN, FNP-C, FCCP, Member of the CHEST Physician® Editorial Board
CHEST Pulmonary®
The Lung Cancer Prediction Model “Stress Test”
By: Brent E. Heideman, MD, and colleagues
Current lung cancer prediction models have limited utility in high-risk patients referred for diagnostic biopsy. In a study of 322 indeterminate pulmonary nodules, the Brock, Mayo Clinic, Herder, and Veterans Affairs models showed modest discrimination between benign and malignant nodules (AUCs 0.67-0.77). The models performed poorly for low-risk patients (negative predictive values 63%-71%) and suboptimally for high-risk patients (positive predictive values 73%-87%), suggesting referring physicians use additional clinical information not captured in these models to identify high-risk patients needing biopsy. New prediction models and biomarkers specifically developed and calibrated for high-risk populations are needed to better inform clinical decision-making. Incorporating interval imaging to assess changes in nodule characteristics could potentially improve model performance. Tailored risk assessment tools are crucial for optimizing management and reducing unnecessary invasive procedures in this challenging patient population.
– Commentary by Russell Miller, MD, Member of the CHEST Physician Editorial Board
CHEST Critical Care ®
Characterizing Cardiac Function in ICU Survivors of Sepsis
By: Kevin Garrity, MBChB, and colleagues
While chronic cardiac dysfunction is one of the proposed mechanisms of long-term impairment post critical illness, its prevalence, mechanisms, and associations with disability following admission for sepsis are not well understood. Garrity and colleagues describe the Characterization of Cardiovascular Function in ICU Survivors of Sepsis (CONDUCT-ICU) protocol, a prospective study including two ICUs in Scotland aimed to better define cardiovascular dysfunction in survivors of sepsis. Designed to enroll 69 patients, demographics, cardiac and inflammatory biomarkers, and echocardiograms will be obtained on ICU discharge with additional laboratory data, cardiac magnetic resonance imaging, and patient-reported outcome measures to be obtained at 6 to 10 weeks. This novel multimodal approach will provide understanding into the role of cardiovascular dysfunction following critical illness as well as offer mechanistic insights. The investigators hope to obtain operational and pilot data for larger future studies.
– Commentary by Eugene Yuriditsky, MD, FCCP, Member of the CHEST Physician Editorial Board
Sex-Related Differences Found in IgG4-Related Disease Epidemiology
TOPLINE:
Men with immunoglobulin G4 (IgG4)-related disease exhibit significantly lower serum lipase levels and a greater likelihood of organ involvement than women, highlighting significant sex-dependent differences in disease manifestations.
METHODOLOGY:
- Researchers conducted a retrospective study of 328 patients (69% men) diagnosed with IgG4-related disease at the Massachusetts General Hospital – Rheumatology Clinic, Boston, who met the American College of Rheumatology–European Alliance of Associations for Rheumatology (ACR-EULAR) classification criteria between January 2008 and May 2023.
- Among the 328 patients, 69% were men and 31% were women, with a significant male-to-female ratio of 2.2:1.0. Men were typically older at diagnosis (median age, 63.7 vs 58.2 years).
- Data on serum lipase levels, renal involvement, and other clinical and laboratory parameters were collected.
TAKEAWAY:
- Men had higher baseline ACR-EULAR scores, indicating more severe disease (median score of 35.0 vs 29.5; P = .0010).
- Male patients demonstrated a median baseline serum lipase concentration of 24.5 U/L, significantly lower than the 33.5 U/L observed in women.
- Pancreatic (50% vs 26%) or renal (36% vs 18%) involvement was more common in men.
- Men exhibited higher IgG4 levels (P = .0050) and active B-cell responses in the blood (P = .0095).
IN PRACTICE:
According to the authors, this work confirms “the impression of an important sex disparity among patients with IgG4-related disease, with most patients being male, and male patients demonstrating strong tendencies toward more severe disease than female patients.”
SOURCE:
The study was led by Isha Jha, MD, Massachusetts General Hospital, Boston. It was published online on May 30, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The study’s retrospective design may limit the ability to establish causality between sex differences and IgG4-related disease manifestations. A relatively small percentage of patients were assessed before receiving any immunosuppressive treatment, potentially influencing the observed clinical parameters.
DISCLOSURES:
This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases, the Rheumatology Research Foundation, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Some authors declared financial ties outside this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Men with immunoglobulin G4 (IgG4)-related disease exhibit significantly lower serum lipase levels and a greater likelihood of organ involvement than women, highlighting significant sex-dependent differences in disease manifestations.
METHODOLOGY:
- Researchers conducted a retrospective study of 328 patients (69% men) diagnosed with IgG4-related disease at the Massachusetts General Hospital – Rheumatology Clinic, Boston, who met the American College of Rheumatology–European Alliance of Associations for Rheumatology (ACR-EULAR) classification criteria between January 2008 and May 2023.
- Among the 328 patients, 69% were men and 31% were women, with a significant male-to-female ratio of 2.2:1.0. Men were typically older at diagnosis (median age, 63.7 vs 58.2 years).
- Data on serum lipase levels, renal involvement, and other clinical and laboratory parameters were collected.
TAKEAWAY:
- Men had higher baseline ACR-EULAR scores, indicating more severe disease (median score of 35.0 vs 29.5; P = .0010).
- Male patients demonstrated a median baseline serum lipase concentration of 24.5 U/L, significantly lower than the 33.5 U/L observed in women.
- Pancreatic (50% vs 26%) or renal (36% vs 18%) involvement was more common in men.
- Men exhibited higher IgG4 levels (P = .0050) and active B-cell responses in the blood (P = .0095).
IN PRACTICE:
According to the authors, this work confirms “the impression of an important sex disparity among patients with IgG4-related disease, with most patients being male, and male patients demonstrating strong tendencies toward more severe disease than female patients.”
SOURCE:
The study was led by Isha Jha, MD, Massachusetts General Hospital, Boston. It was published online on May 30, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The study’s retrospective design may limit the ability to establish causality between sex differences and IgG4-related disease manifestations. A relatively small percentage of patients were assessed before receiving any immunosuppressive treatment, potentially influencing the observed clinical parameters.
DISCLOSURES:
This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases, the Rheumatology Research Foundation, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Some authors declared financial ties outside this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Men with immunoglobulin G4 (IgG4)-related disease exhibit significantly lower serum lipase levels and a greater likelihood of organ involvement than women, highlighting significant sex-dependent differences in disease manifestations.
METHODOLOGY:
- Researchers conducted a retrospective study of 328 patients (69% men) diagnosed with IgG4-related disease at the Massachusetts General Hospital – Rheumatology Clinic, Boston, who met the American College of Rheumatology–European Alliance of Associations for Rheumatology (ACR-EULAR) classification criteria between January 2008 and May 2023.
- Among the 328 patients, 69% were men and 31% were women, with a significant male-to-female ratio of 2.2:1.0. Men were typically older at diagnosis (median age, 63.7 vs 58.2 years).
- Data on serum lipase levels, renal involvement, and other clinical and laboratory parameters were collected.
TAKEAWAY:
- Men had higher baseline ACR-EULAR scores, indicating more severe disease (median score of 35.0 vs 29.5; P = .0010).
- Male patients demonstrated a median baseline serum lipase concentration of 24.5 U/L, significantly lower than the 33.5 U/L observed in women.
- Pancreatic (50% vs 26%) or renal (36% vs 18%) involvement was more common in men.
- Men exhibited higher IgG4 levels (P = .0050) and active B-cell responses in the blood (P = .0095).
IN PRACTICE:
According to the authors, this work confirms “the impression of an important sex disparity among patients with IgG4-related disease, with most patients being male, and male patients demonstrating strong tendencies toward more severe disease than female patients.”
SOURCE:
The study was led by Isha Jha, MD, Massachusetts General Hospital, Boston. It was published online on May 30, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The study’s retrospective design may limit the ability to establish causality between sex differences and IgG4-related disease manifestations. A relatively small percentage of patients were assessed before receiving any immunosuppressive treatment, potentially influencing the observed clinical parameters.
DISCLOSURES:
This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases, the Rheumatology Research Foundation, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Some authors declared financial ties outside this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Autoantibodies Nonspecific to Systemic Sclerosis May Play Role in ILD Prediction
VIENNA — Anti-Ro/SSA antibodies may help predict which patients with systemic sclerosis (SSc) are at a greater risk for interstitial lung disease (ILD) and may serve as a biomarker to guide screening, according to an analysis of data from a large European cohort.
The researchers were led by Blaž Burja, MD, PhD, a physician-scientist at the Center of Experimental Rheumatology, University Hospital Zürich, Switzerland, who reported that anti-Ro/SSA antibodies are a risk factor for ILD, with an odds ratio of 1.24, in patients with SSc.
At the annual European Congress of Rheumatology, he presented the findings of the study that aimed to find out if SSc-nonspecific antibodies might help better risk-stratify patients with SSc, focusing on lung involvement. “Among them, anti-Ro/SSA antibodies have been shown to be associated with interstitial lung disease in different connective tissue diseases,” Dr. Burja pointed out.
“A total of 15% of all patients in the SSc cohort presented with anti-Ro/SSA antibodies, and this subgroup presented with distinct clinical features: Importantly, higher prevalence of ILD and lower DLCO% [diffusing capacity of the lungs for carbon monoxide] in patients with established ILD,” reported Dr. Burja. “However, these anti-Ro/SSA antibodies do not predict ILD progression, death, or overall disease progression.”
Based on the findings, Dr. Burja suggested that these antibodies be incorporated into routine clinical practice to identify patients with SSc who have a high risk for ILD. He noted that “this has specific importance in clinical settings without availability of high-resolution computed tomography (HRCT), where anti-Ro/SSA antibodies could represent an additional biomarker to guide the screening process, in particular, in patients without SSc-specific antibodies.”
Caroline Ospelt, MD, PhD, co-moderator of the session and scientific program chair of EULAR 2024, told this news organization that the study was unique in its approach to studying ILD risk by “looking outside the box, so not just at specific antibodies but whether cross-disease antibodies may have value in stratifying patients and help predict risk of lung involvement and possibly monitor these patients.”
Dr. Ospelt, professor of experimental rheumatology at University Hospital Zürich, who was not involved in the study, noted: “It might also be the case that we could adapt this concept and use these antibodies in other rheumatic diseases, too, not just systemic sclerosis, to predict lung involvement.”
Risk-Stratifying With SSc-Nonspecific Antibodies
Dr. Burja explained that despite better stratification of patients with SSc with SSc-specific antibodies, “in clinical practice, we see large heterogeneity, and individual prognosis with regards to outcomes is still unpredictable, so we wanted to know whether by using nonspecific autoantibodies we might be better able to risk-stratify these patients.”
A study population of 4421 with at least one follow-up visit, including 3060 patients with available follow-up serologic data, was drawn from the European Scleroderma Trials and Research group database (n = 22,482). Of these 3060 patients, 461 were positive for anti-Ro/SSA antibodies and 2599 were negative. The researchers analyzed the relationships between baseline characteristics and the development or progression of ILD over 2.7 years of follow-up. Incident, de novo ILD was defined based on its presence on HRCT, and progression was defined by whether the percentage of predicted forced vital capacity (FVC%) dropped ≥ 10%, FVC% dropped 5%-9% in association with a DLCO% drop ≥ 15%, or FVC% dropped > 5%. Deaths from all causes and prognostic factors for the progression of lung fibrosis during follow-up were recorded.
High Prevalence of ILD With Anti-Ro/SSA Antibodies in SSc
At baseline, patients with anti-Ro/SSA antibodies were aged 55-56 years, 84%-87% were women, and muscular involvement was present in 18% of patients positive for anti-Ro/SSA antibodies and 12.5% of those who were negative (P < .001). According to HRCT, ILD was present in 56.2% of patients positive for anti-Ro/SSA antibodies and in 47.8% of those who were negative (P = .001). FVC% was 92.5% in patients positive for anti-Ro/SSA antibodies and 95.7% in those who were negative (P = .002). DLCO% was 66.9% in patients positive for anti-Ro/SSA antibodies and 71% in those who were negative (P < .001).
“A total of 15% of all SSc patients presented as positive for anti-Ro/SSA antibodies, and these patients all presented with higher prevalence of SSA-nonspecific antibodies, too: Of note, those with anti-La/SSB and anti-U1/RNP and rheumatoid factor,” Dr. Burja reported.
In patients with anti-U1/RNP autoantibodies, 1% were positive and 4% were negative for anti-Ro/SSA antibodies; in those with anti-La/SSB autoantibodies, 17% were positive and 1% were negative for anti-Ro/SSA antibodies; and in those with rheumatoid factor, 28% were positive and 14% were negative for anti-Ro/SSA antibodies.
Dr. Burja pointed out that the average disease duration in the study cohort at baseline was 7 years, “and at this timepoint, we expect to see some common disease manifestations. Specifically, higher muscular involvement and higher ILD based on HRCT.
“We decided to focus on patients with established ILD at baseline,” said Dr. Burja. “Anti-Ro/SSA-positive patients with established ILD at baseline presented with lower DLCO values at 59% in patients positive for anti-Ro/SSA antibodies and 61% for those who were negative.”
After conducting a multivariable analysis of 14,066 healthcare visits and adjusting for known risk factors for ILD, the researchers concluded that anti-Ro/SSA antibodies are an independent risk factor for ILD, with an odds ratio of 1.24 (95% CI, 1.07-1.44; P = .006). They also determined that anti-Ro/SSA antibodies are a risk factor for lower DLCO values in patients with ILD, with a regression coefficient of −1.93.
The researchers then explored the progression of ILD and overall disease progression and survival during the follow-up period in a longitudinal analysis. “However, anti-Ro/SSA antibodies were not found to predict the progression of ILD,” reported Dr. Burja, adding that this was true regardless of the definition of ILD progression used. “Nor did anti-Ro/SSA antibodies do not predict survival or overall disease progression.”
Dr. Burja pointed out the limitations in his study, including the lack of standardized criteria for all centers to assess anti-Ro/SSA positivity; there was a lack of discrimination between anti-Ro52 and anti-Ro60 subtypes, and there were no standardized applicable criteria to study lung progression in SSc.
Dr. Burja and Dr. Ospelt had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
VIENNA — Anti-Ro/SSA antibodies may help predict which patients with systemic sclerosis (SSc) are at a greater risk for interstitial lung disease (ILD) and may serve as a biomarker to guide screening, according to an analysis of data from a large European cohort.
The researchers were led by Blaž Burja, MD, PhD, a physician-scientist at the Center of Experimental Rheumatology, University Hospital Zürich, Switzerland, who reported that anti-Ro/SSA antibodies are a risk factor for ILD, with an odds ratio of 1.24, in patients with SSc.
At the annual European Congress of Rheumatology, he presented the findings of the study that aimed to find out if SSc-nonspecific antibodies might help better risk-stratify patients with SSc, focusing on lung involvement. “Among them, anti-Ro/SSA antibodies have been shown to be associated with interstitial lung disease in different connective tissue diseases,” Dr. Burja pointed out.
“A total of 15% of all patients in the SSc cohort presented with anti-Ro/SSA antibodies, and this subgroup presented with distinct clinical features: Importantly, higher prevalence of ILD and lower DLCO% [diffusing capacity of the lungs for carbon monoxide] in patients with established ILD,” reported Dr. Burja. “However, these anti-Ro/SSA antibodies do not predict ILD progression, death, or overall disease progression.”
Based on the findings, Dr. Burja suggested that these antibodies be incorporated into routine clinical practice to identify patients with SSc who have a high risk for ILD. He noted that “this has specific importance in clinical settings without availability of high-resolution computed tomography (HRCT), where anti-Ro/SSA antibodies could represent an additional biomarker to guide the screening process, in particular, in patients without SSc-specific antibodies.”
Caroline Ospelt, MD, PhD, co-moderator of the session and scientific program chair of EULAR 2024, told this news organization that the study was unique in its approach to studying ILD risk by “looking outside the box, so not just at specific antibodies but whether cross-disease antibodies may have value in stratifying patients and help predict risk of lung involvement and possibly monitor these patients.”
Dr. Ospelt, professor of experimental rheumatology at University Hospital Zürich, who was not involved in the study, noted: “It might also be the case that we could adapt this concept and use these antibodies in other rheumatic diseases, too, not just systemic sclerosis, to predict lung involvement.”
Risk-Stratifying With SSc-Nonspecific Antibodies
Dr. Burja explained that despite better stratification of patients with SSc with SSc-specific antibodies, “in clinical practice, we see large heterogeneity, and individual prognosis with regards to outcomes is still unpredictable, so we wanted to know whether by using nonspecific autoantibodies we might be better able to risk-stratify these patients.”
A study population of 4421 with at least one follow-up visit, including 3060 patients with available follow-up serologic data, was drawn from the European Scleroderma Trials and Research group database (n = 22,482). Of these 3060 patients, 461 were positive for anti-Ro/SSA antibodies and 2599 were negative. The researchers analyzed the relationships between baseline characteristics and the development or progression of ILD over 2.7 years of follow-up. Incident, de novo ILD was defined based on its presence on HRCT, and progression was defined by whether the percentage of predicted forced vital capacity (FVC%) dropped ≥ 10%, FVC% dropped 5%-9% in association with a DLCO% drop ≥ 15%, or FVC% dropped > 5%. Deaths from all causes and prognostic factors for the progression of lung fibrosis during follow-up were recorded.
High Prevalence of ILD With Anti-Ro/SSA Antibodies in SSc
At baseline, patients with anti-Ro/SSA antibodies were aged 55-56 years, 84%-87% were women, and muscular involvement was present in 18% of patients positive for anti-Ro/SSA antibodies and 12.5% of those who were negative (P < .001). According to HRCT, ILD was present in 56.2% of patients positive for anti-Ro/SSA antibodies and in 47.8% of those who were negative (P = .001). FVC% was 92.5% in patients positive for anti-Ro/SSA antibodies and 95.7% in those who were negative (P = .002). DLCO% was 66.9% in patients positive for anti-Ro/SSA antibodies and 71% in those who were negative (P < .001).
“A total of 15% of all SSc patients presented as positive for anti-Ro/SSA antibodies, and these patients all presented with higher prevalence of SSA-nonspecific antibodies, too: Of note, those with anti-La/SSB and anti-U1/RNP and rheumatoid factor,” Dr. Burja reported.
In patients with anti-U1/RNP autoantibodies, 1% were positive and 4% were negative for anti-Ro/SSA antibodies; in those with anti-La/SSB autoantibodies, 17% were positive and 1% were negative for anti-Ro/SSA antibodies; and in those with rheumatoid factor, 28% were positive and 14% were negative for anti-Ro/SSA antibodies.
Dr. Burja pointed out that the average disease duration in the study cohort at baseline was 7 years, “and at this timepoint, we expect to see some common disease manifestations. Specifically, higher muscular involvement and higher ILD based on HRCT.
“We decided to focus on patients with established ILD at baseline,” said Dr. Burja. “Anti-Ro/SSA-positive patients with established ILD at baseline presented with lower DLCO values at 59% in patients positive for anti-Ro/SSA antibodies and 61% for those who were negative.”
After conducting a multivariable analysis of 14,066 healthcare visits and adjusting for known risk factors for ILD, the researchers concluded that anti-Ro/SSA antibodies are an independent risk factor for ILD, with an odds ratio of 1.24 (95% CI, 1.07-1.44; P = .006). They also determined that anti-Ro/SSA antibodies are a risk factor for lower DLCO values in patients with ILD, with a regression coefficient of −1.93.
The researchers then explored the progression of ILD and overall disease progression and survival during the follow-up period in a longitudinal analysis. “However, anti-Ro/SSA antibodies were not found to predict the progression of ILD,” reported Dr. Burja, adding that this was true regardless of the definition of ILD progression used. “Nor did anti-Ro/SSA antibodies do not predict survival or overall disease progression.”
Dr. Burja pointed out the limitations in his study, including the lack of standardized criteria for all centers to assess anti-Ro/SSA positivity; there was a lack of discrimination between anti-Ro52 and anti-Ro60 subtypes, and there were no standardized applicable criteria to study lung progression in SSc.
Dr. Burja and Dr. Ospelt had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
VIENNA — Anti-Ro/SSA antibodies may help predict which patients with systemic sclerosis (SSc) are at a greater risk for interstitial lung disease (ILD) and may serve as a biomarker to guide screening, according to an analysis of data from a large European cohort.
The researchers were led by Blaž Burja, MD, PhD, a physician-scientist at the Center of Experimental Rheumatology, University Hospital Zürich, Switzerland, who reported that anti-Ro/SSA antibodies are a risk factor for ILD, with an odds ratio of 1.24, in patients with SSc.
At the annual European Congress of Rheumatology, he presented the findings of the study that aimed to find out if SSc-nonspecific antibodies might help better risk-stratify patients with SSc, focusing on lung involvement. “Among them, anti-Ro/SSA antibodies have been shown to be associated with interstitial lung disease in different connective tissue diseases,” Dr. Burja pointed out.
“A total of 15% of all patients in the SSc cohort presented with anti-Ro/SSA antibodies, and this subgroup presented with distinct clinical features: Importantly, higher prevalence of ILD and lower DLCO% [diffusing capacity of the lungs for carbon monoxide] in patients with established ILD,” reported Dr. Burja. “However, these anti-Ro/SSA antibodies do not predict ILD progression, death, or overall disease progression.”
Based on the findings, Dr. Burja suggested that these antibodies be incorporated into routine clinical practice to identify patients with SSc who have a high risk for ILD. He noted that “this has specific importance in clinical settings without availability of high-resolution computed tomography (HRCT), where anti-Ro/SSA antibodies could represent an additional biomarker to guide the screening process, in particular, in patients without SSc-specific antibodies.”
Caroline Ospelt, MD, PhD, co-moderator of the session and scientific program chair of EULAR 2024, told this news organization that the study was unique in its approach to studying ILD risk by “looking outside the box, so not just at specific antibodies but whether cross-disease antibodies may have value in stratifying patients and help predict risk of lung involvement and possibly monitor these patients.”
Dr. Ospelt, professor of experimental rheumatology at University Hospital Zürich, who was not involved in the study, noted: “It might also be the case that we could adapt this concept and use these antibodies in other rheumatic diseases, too, not just systemic sclerosis, to predict lung involvement.”
Risk-Stratifying With SSc-Nonspecific Antibodies
Dr. Burja explained that despite better stratification of patients with SSc with SSc-specific antibodies, “in clinical practice, we see large heterogeneity, and individual prognosis with regards to outcomes is still unpredictable, so we wanted to know whether by using nonspecific autoantibodies we might be better able to risk-stratify these patients.”
A study population of 4421 with at least one follow-up visit, including 3060 patients with available follow-up serologic data, was drawn from the European Scleroderma Trials and Research group database (n = 22,482). Of these 3060 patients, 461 were positive for anti-Ro/SSA antibodies and 2599 were negative. The researchers analyzed the relationships between baseline characteristics and the development or progression of ILD over 2.7 years of follow-up. Incident, de novo ILD was defined based on its presence on HRCT, and progression was defined by whether the percentage of predicted forced vital capacity (FVC%) dropped ≥ 10%, FVC% dropped 5%-9% in association with a DLCO% drop ≥ 15%, or FVC% dropped > 5%. Deaths from all causes and prognostic factors for the progression of lung fibrosis during follow-up were recorded.
High Prevalence of ILD With Anti-Ro/SSA Antibodies in SSc
At baseline, patients with anti-Ro/SSA antibodies were aged 55-56 years, 84%-87% were women, and muscular involvement was present in 18% of patients positive for anti-Ro/SSA antibodies and 12.5% of those who were negative (P < .001). According to HRCT, ILD was present in 56.2% of patients positive for anti-Ro/SSA antibodies and in 47.8% of those who were negative (P = .001). FVC% was 92.5% in patients positive for anti-Ro/SSA antibodies and 95.7% in those who were negative (P = .002). DLCO% was 66.9% in patients positive for anti-Ro/SSA antibodies and 71% in those who were negative (P < .001).
“A total of 15% of all SSc patients presented as positive for anti-Ro/SSA antibodies, and these patients all presented with higher prevalence of SSA-nonspecific antibodies, too: Of note, those with anti-La/SSB and anti-U1/RNP and rheumatoid factor,” Dr. Burja reported.
In patients with anti-U1/RNP autoantibodies, 1% were positive and 4% were negative for anti-Ro/SSA antibodies; in those with anti-La/SSB autoantibodies, 17% were positive and 1% were negative for anti-Ro/SSA antibodies; and in those with rheumatoid factor, 28% were positive and 14% were negative for anti-Ro/SSA antibodies.
Dr. Burja pointed out that the average disease duration in the study cohort at baseline was 7 years, “and at this timepoint, we expect to see some common disease manifestations. Specifically, higher muscular involvement and higher ILD based on HRCT.
“We decided to focus on patients with established ILD at baseline,” said Dr. Burja. “Anti-Ro/SSA-positive patients with established ILD at baseline presented with lower DLCO values at 59% in patients positive for anti-Ro/SSA antibodies and 61% for those who were negative.”
After conducting a multivariable analysis of 14,066 healthcare visits and adjusting for known risk factors for ILD, the researchers concluded that anti-Ro/SSA antibodies are an independent risk factor for ILD, with an odds ratio of 1.24 (95% CI, 1.07-1.44; P = .006). They also determined that anti-Ro/SSA antibodies are a risk factor for lower DLCO values in patients with ILD, with a regression coefficient of −1.93.
The researchers then explored the progression of ILD and overall disease progression and survival during the follow-up period in a longitudinal analysis. “However, anti-Ro/SSA antibodies were not found to predict the progression of ILD,” reported Dr. Burja, adding that this was true regardless of the definition of ILD progression used. “Nor did anti-Ro/SSA antibodies do not predict survival or overall disease progression.”
Dr. Burja pointed out the limitations in his study, including the lack of standardized criteria for all centers to assess anti-Ro/SSA positivity; there was a lack of discrimination between anti-Ro52 and anti-Ro60 subtypes, and there were no standardized applicable criteria to study lung progression in SSc.
Dr. Burja and Dr. Ospelt had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
FROM EULAR 2024
The Tyranny of Beta-Blockers
Beta-blockers are excellent drugs. They’re cheap and effective; feature prominently in hypertension guidelines; and remain a sine qua non for coronary artery disease, myocardial infarction, and heart failure treatment. They’ve been around forever, and we know they work. Good luck finding an adult medicine patient who isn’t on one.
Beta-blockers act by slowing resting heart rate (and blunting the heart rate response to exercise. The latter is a pernicious cause of activity intolerance that often goes unchecked. Even when the adverse effects of beta-blockers are appreciated, providers are loath to alter dosing, much less stop the drug. After all, beta-blockers are an integral part of guideline-directed medical therapy (GDMT), and GDMT saves lives.
Balancing Heart Rate and Stroke Volume Effects
To augment cardiac output and optimize oxygen uptake (VO2) during exercise, we need the heart rate response. In fact, the heart rate response contributes more to cardiac output than augmenting stroke volume (SV) and more to VO2 than the increase in arteriovenous (AV) oxygen difference. An inability to increase the heart rate commensurate with physiologic work is called chronotropic incompetence (CI). That’s what beta-blockers do ─ they cause CI.
Physiology dictates that CI will cause activity intolerance. That said, it’s hard to quantify the impact from beta-blockers at the individual patient level. Data suggest the heart rate effect is profound. A study in patients without heart failure found that 22% of participants on beta-blockers had CI, and the investigators used a conservative CI definition (≤ 62% of heart rate reserve used). A recent report published in JAMA Cardiology found that stopping beta-blockers in patients with heart failure allowed for an extra 30 beats/min at max exercise.
Wasserman and Whipp’s textbook, the last word on all things exercise, presents a sample subject who undergoes two separate cardiopulmonary exercise tests (CPETs). Before the first, he’s given a placebo, and before the second, he gets an intravenous beta-blocker. He’s a 23-year-old otherwise healthy male — the perfect test case for isolating beta-blocker impact without confounding by comorbid diseases, other medications, or deconditioning. His max heart rate dropped by 30 beats/min after the beta-blocker, identical to what we saw in the JAMA Cardiology study (with the heart rate increasing by 30 beats/min following withdrawal). Case closed. Stop the beta-blockers on your patients so they can meet their exercise goals and get healthy!
Such pithy enthusiasm discounts physiology’s complexities. When blunting our patient’s heart rate response with beta-blockers, we also increase diastolic filling time, which increases SV. For the 23-year-old in Wasserman and Whipp’s physiology textbook, the beta-blocker increased O2 pulse (the product of SV and AV difference). Presumably, this is mediated by the increased SV. There was a net reduction in VO2 peak, but it was nominal, suggesting that the drop in heart rate was largely offset by the increase in O2 pulse. For the patients in the JAMA Cardiology study, the entire group had a small increase in VO2 peak with beta-blocker withdrawal, but the effect differed by left ventricular function. Across different studies, the beta-blocker effect on heart rate is consistent but the change in overall exercise capacity is not.
Patient Variability in Beta-Blocker Response
In addition to left ventricular function, there are other factors likely to drive variability at the patient level. We’ve treated the response to beta-blockers as a class effect — an obvious oversimplification. The impact on exercise and the heart will vary by dose and drug (eg, atenolol vs metoprolol vs carvedilol, and so on). Beta-blockers can also affect the lungs, and we’re still debating how cautious to be in the presence of asthma or chronic obstructive pulmonary disease.
In a world of infinite time, resources, and expertise, we’d CPET everyone before and after beta-blocker use. Our current reality requires the unthinkable: We’ll have to talk to each other and our patients. For example, heart failure guidelines recommend titrating drugs to match the dose from trials that proved efficacy. These doses are quite high. Simple discussion with the cardiologist and the patient may allow for an adjustment back down with careful monitoring and close attention to activity tolerance. With any luck, you’ll preserve the benefits from GDMT while optimizing your patient›s ability to meet their exercise goals.
Dr. Holley, professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center, Washington, disclosed ties with Metapharm, CHEST College, and WebMD.
A version of this article appeared on Medscape.com.
Beta-blockers are excellent drugs. They’re cheap and effective; feature prominently in hypertension guidelines; and remain a sine qua non for coronary artery disease, myocardial infarction, and heart failure treatment. They’ve been around forever, and we know they work. Good luck finding an adult medicine patient who isn’t on one.
Beta-blockers act by slowing resting heart rate (and blunting the heart rate response to exercise. The latter is a pernicious cause of activity intolerance that often goes unchecked. Even when the adverse effects of beta-blockers are appreciated, providers are loath to alter dosing, much less stop the drug. After all, beta-blockers are an integral part of guideline-directed medical therapy (GDMT), and GDMT saves lives.
Balancing Heart Rate and Stroke Volume Effects
To augment cardiac output and optimize oxygen uptake (VO2) during exercise, we need the heart rate response. In fact, the heart rate response contributes more to cardiac output than augmenting stroke volume (SV) and more to VO2 than the increase in arteriovenous (AV) oxygen difference. An inability to increase the heart rate commensurate with physiologic work is called chronotropic incompetence (CI). That’s what beta-blockers do ─ they cause CI.
Physiology dictates that CI will cause activity intolerance. That said, it’s hard to quantify the impact from beta-blockers at the individual patient level. Data suggest the heart rate effect is profound. A study in patients without heart failure found that 22% of participants on beta-blockers had CI, and the investigators used a conservative CI definition (≤ 62% of heart rate reserve used). A recent report published in JAMA Cardiology found that stopping beta-blockers in patients with heart failure allowed for an extra 30 beats/min at max exercise.
Wasserman and Whipp’s textbook, the last word on all things exercise, presents a sample subject who undergoes two separate cardiopulmonary exercise tests (CPETs). Before the first, he’s given a placebo, and before the second, he gets an intravenous beta-blocker. He’s a 23-year-old otherwise healthy male — the perfect test case for isolating beta-blocker impact without confounding by comorbid diseases, other medications, or deconditioning. His max heart rate dropped by 30 beats/min after the beta-blocker, identical to what we saw in the JAMA Cardiology study (with the heart rate increasing by 30 beats/min following withdrawal). Case closed. Stop the beta-blockers on your patients so they can meet their exercise goals and get healthy!
Such pithy enthusiasm discounts physiology’s complexities. When blunting our patient’s heart rate response with beta-blockers, we also increase diastolic filling time, which increases SV. For the 23-year-old in Wasserman and Whipp’s physiology textbook, the beta-blocker increased O2 pulse (the product of SV and AV difference). Presumably, this is mediated by the increased SV. There was a net reduction in VO2 peak, but it was nominal, suggesting that the drop in heart rate was largely offset by the increase in O2 pulse. For the patients in the JAMA Cardiology study, the entire group had a small increase in VO2 peak with beta-blocker withdrawal, but the effect differed by left ventricular function. Across different studies, the beta-blocker effect on heart rate is consistent but the change in overall exercise capacity is not.
Patient Variability in Beta-Blocker Response
In addition to left ventricular function, there are other factors likely to drive variability at the patient level. We’ve treated the response to beta-blockers as a class effect — an obvious oversimplification. The impact on exercise and the heart will vary by dose and drug (eg, atenolol vs metoprolol vs carvedilol, and so on). Beta-blockers can also affect the lungs, and we’re still debating how cautious to be in the presence of asthma or chronic obstructive pulmonary disease.
In a world of infinite time, resources, and expertise, we’d CPET everyone before and after beta-blocker use. Our current reality requires the unthinkable: We’ll have to talk to each other and our patients. For example, heart failure guidelines recommend titrating drugs to match the dose from trials that proved efficacy. These doses are quite high. Simple discussion with the cardiologist and the patient may allow for an adjustment back down with careful monitoring and close attention to activity tolerance. With any luck, you’ll preserve the benefits from GDMT while optimizing your patient›s ability to meet their exercise goals.
Dr. Holley, professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center, Washington, disclosed ties with Metapharm, CHEST College, and WebMD.
A version of this article appeared on Medscape.com.
Beta-blockers are excellent drugs. They’re cheap and effective; feature prominently in hypertension guidelines; and remain a sine qua non for coronary artery disease, myocardial infarction, and heart failure treatment. They’ve been around forever, and we know they work. Good luck finding an adult medicine patient who isn’t on one.
Beta-blockers act by slowing resting heart rate (and blunting the heart rate response to exercise. The latter is a pernicious cause of activity intolerance that often goes unchecked. Even when the adverse effects of beta-blockers are appreciated, providers are loath to alter dosing, much less stop the drug. After all, beta-blockers are an integral part of guideline-directed medical therapy (GDMT), and GDMT saves lives.
Balancing Heart Rate and Stroke Volume Effects
To augment cardiac output and optimize oxygen uptake (VO2) during exercise, we need the heart rate response. In fact, the heart rate response contributes more to cardiac output than augmenting stroke volume (SV) and more to VO2 than the increase in arteriovenous (AV) oxygen difference. An inability to increase the heart rate commensurate with physiologic work is called chronotropic incompetence (CI). That’s what beta-blockers do ─ they cause CI.
Physiology dictates that CI will cause activity intolerance. That said, it’s hard to quantify the impact from beta-blockers at the individual patient level. Data suggest the heart rate effect is profound. A study in patients without heart failure found that 22% of participants on beta-blockers had CI, and the investigators used a conservative CI definition (≤ 62% of heart rate reserve used). A recent report published in JAMA Cardiology found that stopping beta-blockers in patients with heart failure allowed for an extra 30 beats/min at max exercise.
Wasserman and Whipp’s textbook, the last word on all things exercise, presents a sample subject who undergoes two separate cardiopulmonary exercise tests (CPETs). Before the first, he’s given a placebo, and before the second, he gets an intravenous beta-blocker. He’s a 23-year-old otherwise healthy male — the perfect test case for isolating beta-blocker impact without confounding by comorbid diseases, other medications, or deconditioning. His max heart rate dropped by 30 beats/min after the beta-blocker, identical to what we saw in the JAMA Cardiology study (with the heart rate increasing by 30 beats/min following withdrawal). Case closed. Stop the beta-blockers on your patients so they can meet their exercise goals and get healthy!
Such pithy enthusiasm discounts physiology’s complexities. When blunting our patient’s heart rate response with beta-blockers, we also increase diastolic filling time, which increases SV. For the 23-year-old in Wasserman and Whipp’s physiology textbook, the beta-blocker increased O2 pulse (the product of SV and AV difference). Presumably, this is mediated by the increased SV. There was a net reduction in VO2 peak, but it was nominal, suggesting that the drop in heart rate was largely offset by the increase in O2 pulse. For the patients in the JAMA Cardiology study, the entire group had a small increase in VO2 peak with beta-blocker withdrawal, but the effect differed by left ventricular function. Across different studies, the beta-blocker effect on heart rate is consistent but the change in overall exercise capacity is not.
Patient Variability in Beta-Blocker Response
In addition to left ventricular function, there are other factors likely to drive variability at the patient level. We’ve treated the response to beta-blockers as a class effect — an obvious oversimplification. The impact on exercise and the heart will vary by dose and drug (eg, atenolol vs metoprolol vs carvedilol, and so on). Beta-blockers can also affect the lungs, and we’re still debating how cautious to be in the presence of asthma or chronic obstructive pulmonary disease.
In a world of infinite time, resources, and expertise, we’d CPET everyone before and after beta-blocker use. Our current reality requires the unthinkable: We’ll have to talk to each other and our patients. For example, heart failure guidelines recommend titrating drugs to match the dose from trials that proved efficacy. These doses are quite high. Simple discussion with the cardiologist and the patient may allow for an adjustment back down with careful monitoring and close attention to activity tolerance. With any luck, you’ll preserve the benefits from GDMT while optimizing your patient›s ability to meet their exercise goals.
Dr. Holley, professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center, Washington, disclosed ties with Metapharm, CHEST College, and WebMD.
A version of this article appeared on Medscape.com.
Chronotherapy: Why Timing Drugs to Our Body Clocks May Work
Do drugs work better if taken by the clock?
A new analysis published in The Lancet journal’s eClinicalMedicine suggests: Yes, they do — if you consider the patient’s individual body clock. The study is the first to find that timing blood pressure drugs to a person’s personal “chronotype” — that is, whether they are a night owl or an early bird — may reduce the risk for a heart attack.
The findings represent a significant advance in the field of circadian medicine or “chronotherapy” — timing drug administration to circadian rhythms. A growing stack of research suggests this approach could reduce side effects and improve the effectiveness of a wide range of therapies, including vaccines, cancer treatments, and drugs for depression, glaucoma, pain, seizures, and other conditions. Still, despite decades of research, time of day is rarely considered in writing prescriptions.
“We are really just at the beginning of an exciting new way of looking at patient care,” said Kenneth A. Dyar, PhD, whose lab at Helmholtz Zentrum München’s Institute for Diabetes and Cancer focuses on metabolic physiology. Dr. Dyar is co-lead author of the new blood pressure analysis.
“Chronotherapy is a rapidly growing field,” he said, “and I suspect we are soon going to see more and more studies focused on ‘personalized chronotherapy,’ not only in hypertension but also potentially in other clinical areas.”
The ‘Missing Piece’ in Chronotherapy Research
Blood pressure drugs have long been chronotherapy’s battleground. After all, blood pressure follows a circadian rhythm, peaking in the morning and dropping at night.
That healthy overnight dip can disappear in people with diabetes, kidney disease, and obstructive sleep apnea. Some physicians have suggested a bed-time dose to restore that dip. But studies have had mixed results, so “take at bedtime” has become a less common recommendation in recent years.
But the debate continued. After a large 2019 Spanish study found that bedtime doses had benefits so big that the results drew questions, an even larger, 2022 randomized, controlled trial from the University of Dundee in Dundee, Scotland — called the TIME study — aimed to settle the question.
Researchers assigned over 21,000 people to take morning or night hypertension drugs for several years and found no difference in cardiovascular outcomes.
“We did this study thinking nocturnal blood pressure tablets might be better,” said Thomas MacDonald, MD, professor emeritus of clinical pharmacology and pharmacoepidemiology at the University of Dundee and principal investigator for the TIME study and the recent chronotype analysis. “But there was no difference for heart attacks, strokes, or vascular death.”
So, the researchers then looked at participants’ chronotypes, sorting outcomes based on whether the participants were late-to-bed, late-to-rise “night owls” or early-to-bed, early-to-rise “morning larks.”
Their analysis of these 5358 TIME participants found the following results: Risk for hospitalization for a heart attack was at least 34% lower for “owls” who took their drugs at bedtime. By contrast, owls’ heart attack risk was at least 62% higher with morning doses. For “larks,” the opposite was true. Morning doses were associated with an 11% lower heart attack risk and night doses with an 11% higher risk, according to supplemental data.
The personalized approach could explain why some previous chronotherapy studies have failed to show a benefit. Those studies did not individualize drug timing as this one did. But personalization could be key to circadian medicine’s success.
“Our ‘internal personal time’ appears to be an important variable to consider when dosing antihypertensives,” said co-lead author Filippo Pigazzani, MD, PhD, clinical senior lecturer and honorary consultant cardiologist at the University of Dundee School of Medicine. “Chronotherapy research has been going on for decades. We knew there was something important with time of day. But researchers haven’t considered the internal time of individual people. I think that is the missing piece.”
The analysis has several important limitations, the researchers said. A total of 95% of participants were White. And it was an observational study, not a true randomized comparison. “We started it late in the original TIME study,” Dr. MacDonald said. “You could argue we were reporting on those who survived long enough to get into the analysis.” More research is needed, they concluded.
Looking Beyond Blood Pressure
What about the rest of the body? “Almost all the cells of our body contain ‘circadian clocks’ that are synchronized by daily environmental cues, including light-dark, activity-rest, and feeding-fasting cycles,” said Dr. Dyar.
An estimated 50% of prescription drugs hit targets in the body that have circadian patterns. So, experts suspect that syncing a drug with a person’s body clock might increase effectiveness of many drugs.
A handful of US Food and Drug Administration–approved drugs already have time-of-day recommendations on the label for effectiveness or to limit side effects, including bedtime or evening for the insomnia drug Ambien, the HIV antiviral Atripla, and cholesterol-lowering Zocor. Others are intended to be taken with or after your last meal of the day, such as the long-acting insulin Levemir and the cardiovascular drug Xarelto. A morning recommendation comes with the proton pump inhibitor Nexium and the attention-deficit/hyperactivity disorder drug Ritalin.
Interest is expanding. About one third of the papers published about chronotherapy in the past 25 years have come out in the past 5 years. The May 2024 meeting of the Society for Research on Biological Rhythms featured a day-long session aimed at bringing clinicians up to speed. An organization called the International Association of Circadian Health Clinics is trying to bring circadian medicine findings to clinicians and their patients and to support research.
Moreover, while recent research suggests minding the clock could have benefits for a wide range of treatments, ignoring it could cause problems.
In a Massachusetts Institute of Technology study published in April in Science Advances, researchers looked at engineered livers made from human donor cells and found more than 300 genes that operate on a circadian schedule, many with roles in drug metabolism. They also found that circadian patterns affected the toxicity of acetaminophen and atorvastatin. Identifying the time of day to take these drugs could maximize effectiveness and minimize adverse effects, the researchers said.
Timing and the Immune System
Circadian rhythms are also seen in immune processes. In a 2023 study in The Journal of Clinical Investigation of vaccine data from 1.5 million people in Israel, researchers found that children and older adults who got their second dose of the Pfizer mRNA COVID vaccine earlier in the day were about 36% less likely to be hospitalized with SARS-CoV-2 infection than those who got an evening shot.
“The sweet spot in our data was somewhere around late morning to late afternoon,” said lead researcher Jeffrey Haspel, MD, PhD, associate professor of medicine in the division of pulmonary and critical care medicine at Washington University School of Medicine in St. Louis.
In a multicenter, 2024 analysis of 13 studies of immunotherapy for advanced cancers in 1663 people, researchers found treatment earlier in the day was associated with longer survival time and longer survival without cancer progression.
“Patients with selected metastatic cancers seemed to largely benefit from early [time of day] infusions, which is consistent with circadian mechanisms in immune-cell functions and trafficking,” the researchers noted. But “retrospective randomized trials are needed to establish recommendations for optimal circadian timing.”
Other research suggests or is investigating possible chronotherapy benefits for depression, glaucoma, respiratory diseases, stroke treatment, epilepsy, and sedatives used in surgery. So why aren’t healthcare providers adding time of day to more prescriptions? “What’s missing is more reliable data,” Dr. Dyar said.
Should You Use Chronotherapy Now?
Experts emphasize that more research is needed before doctors use chronotherapy and before medical organizations include it in treatment recommendations. But for some patients, circadian dosing may be worth a try:
Night owls whose blood pressure isn’t well controlled. Dr. Dyar and Dr. Pigazzani said night-time blood pressure drugs may be helpful for people with a “late chronotype.” Of course, patients shouldn’t change their medication schedule on their own, they said. And doctors may want to consider other concerns, like more overnight bathroom visits with evening diuretics.
In their study, the researchers determined participants’ chronotype with a few questions from the Munich Chronotype Questionnaire about what time they fell asleep and woke up on workdays and days off and whether they considered themselves “morning types” or “evening types.” (The questions can be found in supplementary data for the study.)
If a physician thinks matching the timing of a dose with chronotype would help, they can consider it, Dr. Pigazzani said. “However, I must add that this was an observational study, so I would advise healthcare practitioners to wait for our data to be confirmed in new RCTs of personalized chronotherapy of hypertension.”
Children and older adults getting vaccines. Timing COVID shots and possibly other vaccines from late morning to mid-afternoon could have a small benefit for individuals and a bigger public-health benefit, Dr. Haspel said. But the most important thing is getting vaccinated. “If you can only get one in the evening, it’s still worthwhile. Timing may add oomph at a public-health level for more vulnerable groups.”
A version of this article appeared on Medscape.com.
Do drugs work better if taken by the clock?
A new analysis published in The Lancet journal’s eClinicalMedicine suggests: Yes, they do — if you consider the patient’s individual body clock. The study is the first to find that timing blood pressure drugs to a person’s personal “chronotype” — that is, whether they are a night owl or an early bird — may reduce the risk for a heart attack.
The findings represent a significant advance in the field of circadian medicine or “chronotherapy” — timing drug administration to circadian rhythms. A growing stack of research suggests this approach could reduce side effects and improve the effectiveness of a wide range of therapies, including vaccines, cancer treatments, and drugs for depression, glaucoma, pain, seizures, and other conditions. Still, despite decades of research, time of day is rarely considered in writing prescriptions.
“We are really just at the beginning of an exciting new way of looking at patient care,” said Kenneth A. Dyar, PhD, whose lab at Helmholtz Zentrum München’s Institute for Diabetes and Cancer focuses on metabolic physiology. Dr. Dyar is co-lead author of the new blood pressure analysis.
“Chronotherapy is a rapidly growing field,” he said, “and I suspect we are soon going to see more and more studies focused on ‘personalized chronotherapy,’ not only in hypertension but also potentially in other clinical areas.”
The ‘Missing Piece’ in Chronotherapy Research
Blood pressure drugs have long been chronotherapy’s battleground. After all, blood pressure follows a circadian rhythm, peaking in the morning and dropping at night.
That healthy overnight dip can disappear in people with diabetes, kidney disease, and obstructive sleep apnea. Some physicians have suggested a bed-time dose to restore that dip. But studies have had mixed results, so “take at bedtime” has become a less common recommendation in recent years.
But the debate continued. After a large 2019 Spanish study found that bedtime doses had benefits so big that the results drew questions, an even larger, 2022 randomized, controlled trial from the University of Dundee in Dundee, Scotland — called the TIME study — aimed to settle the question.
Researchers assigned over 21,000 people to take morning or night hypertension drugs for several years and found no difference in cardiovascular outcomes.
“We did this study thinking nocturnal blood pressure tablets might be better,” said Thomas MacDonald, MD, professor emeritus of clinical pharmacology and pharmacoepidemiology at the University of Dundee and principal investigator for the TIME study and the recent chronotype analysis. “But there was no difference for heart attacks, strokes, or vascular death.”
So, the researchers then looked at participants’ chronotypes, sorting outcomes based on whether the participants were late-to-bed, late-to-rise “night owls” or early-to-bed, early-to-rise “morning larks.”
Their analysis of these 5358 TIME participants found the following results: Risk for hospitalization for a heart attack was at least 34% lower for “owls” who took their drugs at bedtime. By contrast, owls’ heart attack risk was at least 62% higher with morning doses. For “larks,” the opposite was true. Morning doses were associated with an 11% lower heart attack risk and night doses with an 11% higher risk, according to supplemental data.
The personalized approach could explain why some previous chronotherapy studies have failed to show a benefit. Those studies did not individualize drug timing as this one did. But personalization could be key to circadian medicine’s success.
“Our ‘internal personal time’ appears to be an important variable to consider when dosing antihypertensives,” said co-lead author Filippo Pigazzani, MD, PhD, clinical senior lecturer and honorary consultant cardiologist at the University of Dundee School of Medicine. “Chronotherapy research has been going on for decades. We knew there was something important with time of day. But researchers haven’t considered the internal time of individual people. I think that is the missing piece.”
The analysis has several important limitations, the researchers said. A total of 95% of participants were White. And it was an observational study, not a true randomized comparison. “We started it late in the original TIME study,” Dr. MacDonald said. “You could argue we were reporting on those who survived long enough to get into the analysis.” More research is needed, they concluded.
Looking Beyond Blood Pressure
What about the rest of the body? “Almost all the cells of our body contain ‘circadian clocks’ that are synchronized by daily environmental cues, including light-dark, activity-rest, and feeding-fasting cycles,” said Dr. Dyar.
An estimated 50% of prescription drugs hit targets in the body that have circadian patterns. So, experts suspect that syncing a drug with a person’s body clock might increase effectiveness of many drugs.
A handful of US Food and Drug Administration–approved drugs already have time-of-day recommendations on the label for effectiveness or to limit side effects, including bedtime or evening for the insomnia drug Ambien, the HIV antiviral Atripla, and cholesterol-lowering Zocor. Others are intended to be taken with or after your last meal of the day, such as the long-acting insulin Levemir and the cardiovascular drug Xarelto. A morning recommendation comes with the proton pump inhibitor Nexium and the attention-deficit/hyperactivity disorder drug Ritalin.
Interest is expanding. About one third of the papers published about chronotherapy in the past 25 years have come out in the past 5 years. The May 2024 meeting of the Society for Research on Biological Rhythms featured a day-long session aimed at bringing clinicians up to speed. An organization called the International Association of Circadian Health Clinics is trying to bring circadian medicine findings to clinicians and their patients and to support research.
Moreover, while recent research suggests minding the clock could have benefits for a wide range of treatments, ignoring it could cause problems.
In a Massachusetts Institute of Technology study published in April in Science Advances, researchers looked at engineered livers made from human donor cells and found more than 300 genes that operate on a circadian schedule, many with roles in drug metabolism. They also found that circadian patterns affected the toxicity of acetaminophen and atorvastatin. Identifying the time of day to take these drugs could maximize effectiveness and minimize adverse effects, the researchers said.
Timing and the Immune System
Circadian rhythms are also seen in immune processes. In a 2023 study in The Journal of Clinical Investigation of vaccine data from 1.5 million people in Israel, researchers found that children and older adults who got their second dose of the Pfizer mRNA COVID vaccine earlier in the day were about 36% less likely to be hospitalized with SARS-CoV-2 infection than those who got an evening shot.
“The sweet spot in our data was somewhere around late morning to late afternoon,” said lead researcher Jeffrey Haspel, MD, PhD, associate professor of medicine in the division of pulmonary and critical care medicine at Washington University School of Medicine in St. Louis.
In a multicenter, 2024 analysis of 13 studies of immunotherapy for advanced cancers in 1663 people, researchers found treatment earlier in the day was associated with longer survival time and longer survival without cancer progression.
“Patients with selected metastatic cancers seemed to largely benefit from early [time of day] infusions, which is consistent with circadian mechanisms in immune-cell functions and trafficking,” the researchers noted. But “retrospective randomized trials are needed to establish recommendations for optimal circadian timing.”
Other research suggests or is investigating possible chronotherapy benefits for depression, glaucoma, respiratory diseases, stroke treatment, epilepsy, and sedatives used in surgery. So why aren’t healthcare providers adding time of day to more prescriptions? “What’s missing is more reliable data,” Dr. Dyar said.
Should You Use Chronotherapy Now?
Experts emphasize that more research is needed before doctors use chronotherapy and before medical organizations include it in treatment recommendations. But for some patients, circadian dosing may be worth a try:
Night owls whose blood pressure isn’t well controlled. Dr. Dyar and Dr. Pigazzani said night-time blood pressure drugs may be helpful for people with a “late chronotype.” Of course, patients shouldn’t change their medication schedule on their own, they said. And doctors may want to consider other concerns, like more overnight bathroom visits with evening diuretics.
In their study, the researchers determined participants’ chronotype with a few questions from the Munich Chronotype Questionnaire about what time they fell asleep and woke up on workdays and days off and whether they considered themselves “morning types” or “evening types.” (The questions can be found in supplementary data for the study.)
If a physician thinks matching the timing of a dose with chronotype would help, they can consider it, Dr. Pigazzani said. “However, I must add that this was an observational study, so I would advise healthcare practitioners to wait for our data to be confirmed in new RCTs of personalized chronotherapy of hypertension.”
Children and older adults getting vaccines. Timing COVID shots and possibly other vaccines from late morning to mid-afternoon could have a small benefit for individuals and a bigger public-health benefit, Dr. Haspel said. But the most important thing is getting vaccinated. “If you can only get one in the evening, it’s still worthwhile. Timing may add oomph at a public-health level for more vulnerable groups.”
A version of this article appeared on Medscape.com.
Do drugs work better if taken by the clock?
A new analysis published in The Lancet journal’s eClinicalMedicine suggests: Yes, they do — if you consider the patient’s individual body clock. The study is the first to find that timing blood pressure drugs to a person’s personal “chronotype” — that is, whether they are a night owl or an early bird — may reduce the risk for a heart attack.
The findings represent a significant advance in the field of circadian medicine or “chronotherapy” — timing drug administration to circadian rhythms. A growing stack of research suggests this approach could reduce side effects and improve the effectiveness of a wide range of therapies, including vaccines, cancer treatments, and drugs for depression, glaucoma, pain, seizures, and other conditions. Still, despite decades of research, time of day is rarely considered in writing prescriptions.
“We are really just at the beginning of an exciting new way of looking at patient care,” said Kenneth A. Dyar, PhD, whose lab at Helmholtz Zentrum München’s Institute for Diabetes and Cancer focuses on metabolic physiology. Dr. Dyar is co-lead author of the new blood pressure analysis.
“Chronotherapy is a rapidly growing field,” he said, “and I suspect we are soon going to see more and more studies focused on ‘personalized chronotherapy,’ not only in hypertension but also potentially in other clinical areas.”
The ‘Missing Piece’ in Chronotherapy Research
Blood pressure drugs have long been chronotherapy’s battleground. After all, blood pressure follows a circadian rhythm, peaking in the morning and dropping at night.
That healthy overnight dip can disappear in people with diabetes, kidney disease, and obstructive sleep apnea. Some physicians have suggested a bed-time dose to restore that dip. But studies have had mixed results, so “take at bedtime” has become a less common recommendation in recent years.
But the debate continued. After a large 2019 Spanish study found that bedtime doses had benefits so big that the results drew questions, an even larger, 2022 randomized, controlled trial from the University of Dundee in Dundee, Scotland — called the TIME study — aimed to settle the question.
Researchers assigned over 21,000 people to take morning or night hypertension drugs for several years and found no difference in cardiovascular outcomes.
“We did this study thinking nocturnal blood pressure tablets might be better,” said Thomas MacDonald, MD, professor emeritus of clinical pharmacology and pharmacoepidemiology at the University of Dundee and principal investigator for the TIME study and the recent chronotype analysis. “But there was no difference for heart attacks, strokes, or vascular death.”
So, the researchers then looked at participants’ chronotypes, sorting outcomes based on whether the participants were late-to-bed, late-to-rise “night owls” or early-to-bed, early-to-rise “morning larks.”
Their analysis of these 5358 TIME participants found the following results: Risk for hospitalization for a heart attack was at least 34% lower for “owls” who took their drugs at bedtime. By contrast, owls’ heart attack risk was at least 62% higher with morning doses. For “larks,” the opposite was true. Morning doses were associated with an 11% lower heart attack risk and night doses with an 11% higher risk, according to supplemental data.
The personalized approach could explain why some previous chronotherapy studies have failed to show a benefit. Those studies did not individualize drug timing as this one did. But personalization could be key to circadian medicine’s success.
“Our ‘internal personal time’ appears to be an important variable to consider when dosing antihypertensives,” said co-lead author Filippo Pigazzani, MD, PhD, clinical senior lecturer and honorary consultant cardiologist at the University of Dundee School of Medicine. “Chronotherapy research has been going on for decades. We knew there was something important with time of day. But researchers haven’t considered the internal time of individual people. I think that is the missing piece.”
The analysis has several important limitations, the researchers said. A total of 95% of participants were White. And it was an observational study, not a true randomized comparison. “We started it late in the original TIME study,” Dr. MacDonald said. “You could argue we were reporting on those who survived long enough to get into the analysis.” More research is needed, they concluded.
Looking Beyond Blood Pressure
What about the rest of the body? “Almost all the cells of our body contain ‘circadian clocks’ that are synchronized by daily environmental cues, including light-dark, activity-rest, and feeding-fasting cycles,” said Dr. Dyar.
An estimated 50% of prescription drugs hit targets in the body that have circadian patterns. So, experts suspect that syncing a drug with a person’s body clock might increase effectiveness of many drugs.
A handful of US Food and Drug Administration–approved drugs already have time-of-day recommendations on the label for effectiveness or to limit side effects, including bedtime or evening for the insomnia drug Ambien, the HIV antiviral Atripla, and cholesterol-lowering Zocor. Others are intended to be taken with or after your last meal of the day, such as the long-acting insulin Levemir and the cardiovascular drug Xarelto. A morning recommendation comes with the proton pump inhibitor Nexium and the attention-deficit/hyperactivity disorder drug Ritalin.
Interest is expanding. About one third of the papers published about chronotherapy in the past 25 years have come out in the past 5 years. The May 2024 meeting of the Society for Research on Biological Rhythms featured a day-long session aimed at bringing clinicians up to speed. An organization called the International Association of Circadian Health Clinics is trying to bring circadian medicine findings to clinicians and their patients and to support research.
Moreover, while recent research suggests minding the clock could have benefits for a wide range of treatments, ignoring it could cause problems.
In a Massachusetts Institute of Technology study published in April in Science Advances, researchers looked at engineered livers made from human donor cells and found more than 300 genes that operate on a circadian schedule, many with roles in drug metabolism. They also found that circadian patterns affected the toxicity of acetaminophen and atorvastatin. Identifying the time of day to take these drugs could maximize effectiveness and minimize adverse effects, the researchers said.
Timing and the Immune System
Circadian rhythms are also seen in immune processes. In a 2023 study in The Journal of Clinical Investigation of vaccine data from 1.5 million people in Israel, researchers found that children and older adults who got their second dose of the Pfizer mRNA COVID vaccine earlier in the day were about 36% less likely to be hospitalized with SARS-CoV-2 infection than those who got an evening shot.
“The sweet spot in our data was somewhere around late morning to late afternoon,” said lead researcher Jeffrey Haspel, MD, PhD, associate professor of medicine in the division of pulmonary and critical care medicine at Washington University School of Medicine in St. Louis.
In a multicenter, 2024 analysis of 13 studies of immunotherapy for advanced cancers in 1663 people, researchers found treatment earlier in the day was associated with longer survival time and longer survival without cancer progression.
“Patients with selected metastatic cancers seemed to largely benefit from early [time of day] infusions, which is consistent with circadian mechanisms in immune-cell functions and trafficking,” the researchers noted. But “retrospective randomized trials are needed to establish recommendations for optimal circadian timing.”
Other research suggests or is investigating possible chronotherapy benefits for depression, glaucoma, respiratory diseases, stroke treatment, epilepsy, and sedatives used in surgery. So why aren’t healthcare providers adding time of day to more prescriptions? “What’s missing is more reliable data,” Dr. Dyar said.
Should You Use Chronotherapy Now?
Experts emphasize that more research is needed before doctors use chronotherapy and before medical organizations include it in treatment recommendations. But for some patients, circadian dosing may be worth a try:
Night owls whose blood pressure isn’t well controlled. Dr. Dyar and Dr. Pigazzani said night-time blood pressure drugs may be helpful for people with a “late chronotype.” Of course, patients shouldn’t change their medication schedule on their own, they said. And doctors may want to consider other concerns, like more overnight bathroom visits with evening diuretics.
In their study, the researchers determined participants’ chronotype with a few questions from the Munich Chronotype Questionnaire about what time they fell asleep and woke up on workdays and days off and whether they considered themselves “morning types” or “evening types.” (The questions can be found in supplementary data for the study.)
If a physician thinks matching the timing of a dose with chronotype would help, they can consider it, Dr. Pigazzani said. “However, I must add that this was an observational study, so I would advise healthcare practitioners to wait for our data to be confirmed in new RCTs of personalized chronotherapy of hypertension.”
Children and older adults getting vaccines. Timing COVID shots and possibly other vaccines from late morning to mid-afternoon could have a small benefit for individuals and a bigger public-health benefit, Dr. Haspel said. But the most important thing is getting vaccinated. “If you can only get one in the evening, it’s still worthwhile. Timing may add oomph at a public-health level for more vulnerable groups.”
A version of this article appeared on Medscape.com.
Seniors in Households with Children Have Sixfold Higher Risk for Pneumococcal Disease
BARCELONA, SPAIN — Streptococcus pneumoniae, the bacteria that causes pneumococcal disease, is sixfold more likely to colonize adults older than 60 years who have regular contact with children than those who do not, data from a community-based study showed.
However, there is “no clear evidence of adult-to-adult transmission,” and the researchers, led by Anne L. Wyllie, PhD, from the Yale School of Public Health, New Haven, Connecticut, noted that the study results suggest “the main benefit of adult pneumococcal conjugate vaccine (PCV) immunization is to directly protect adults who are exposed to children, who still carry and transmit some vaccine-type pneumococci despite successful pediatric national immunization programs.”
The data show that relatively high pneumococcus carriage rates are seen in people who have regular contact with children, who have had contact in the previous 2 weeks, and who have had contact for extended periods, Dr. Wyllie explained.
Preschoolers in particular were found to be most likely to transmit pneumococcus to older adults. “It is the 24- to 59-month-olds who are most associated with pneumococcal carriage, more than 1- to 2-year-olds,” she reported. However, transmission rates from children younger than 1 year are higher than those from children aged 1-2 years, she added.
The findings were presented at the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) 2024 global conference, formerly known as the ECCMID conference.
Originally Designed to Investigate Adult-to-Adult Transmission
The researchers wanted to understand the sources and dynamics of transmission, as well as the risk factors for pneumococcal disease in older adults, to help predict the effect of PCVs in people older than 60 years.
Although “we designed the study to specifically look at transmission between adults, in the end, we were presented with a very unique scenario” — restricted social mixing as a result of the COVID pandemic — during which “no community activities were happening,” Dr. Wyllie said. Because of this, the team was able to determine “the source of acquisition or transmission to the older adults was, very likely, coming from contact with children.”
Pneumococci are commonly found in respiratory tracts of healthy people. The US Centers for Disease Control and Prevention estimated that 20%-60% of school-aged children may be colonized compared with only 5%-10% of adults without children.
The longitudinal study was conducted among household pairs, such as married couples who were both aged at least 60 years and who did not have people younger than 60 years living in the household, in New Haven over two winter seasons: 2020-2021 and 2021-2022.
Self-collected saliva samples were assessed, and surveys on social behaviors and health were completed every 2 weeks for a 10-week period (with six study visits). The saliva sampling method was used because the researchers considered it to be more effective than samples from nasopharyngeal swabs. Quantitative polymerase chain reaction assays were used to test the saliva samples for the presence of pneumococcal DNA (pneumococcus genes piaB and lytA) and the diversity of pneumococcal strains (36 serotypes were targeted).
Strongly Suggestive of Transmission From Children to Older Adults
Of the 121 adults living in 61 households who were enrolled in the study, 62 adults participated in both seasons. Mean age was 70.9 years (range, 60-86 years), 51% of participants were women, and 85% were White.
Overall, 52 of 1088 (4.8%) samples tested positive for pneumococcus, and 27 of 121 (22.3%) adults were colonized on at least one sampling visit. Some were colonized at multiple timepoints, and two were colonized throughout the 10-week sampling period. Of the two participants who were colonized at five of six timepoints, one reported daily contact with children younger than 5 years and children aged 5-9 years in the two study seasons. This person was also positive at three of six sampling points during the first study season.
There were five instances in which both members of the household were carriers in the same season, although not necessarily at the same timepoint. Numbers were too small to determine whether transmission had occurred between the household pairs.
Contact with a 24- to 59-month-old child (older than 2 years but younger than 5 years) had the strongest association with elevated odds of carrying pneumococcus, the authors reported in their preprint, although the frequency and intensity of contact also mattered.
At any sampled time (point prevalence), pneumococcal carriage was substantially — just over sixfold — higher among older adults who had contact with children daily or every few days (10%) than among those who had no contact with children (1.6%).
In particular, contact between adults and children younger than 5 years and children aged 5-9 years was found to lead to elevated point prevalences of 13.8% and 14.1%, respectively. Pneumococcal carriage in children older than 10 years was lower, with a point prevalence of 8.3%.
The younger the child, the greater the point prevalence; point prevalences were 13.8% for samples from children aged 1 year and younger, 10.5% for samples from children aged 1-2 years, and 17.8% for children aged 2-5 years.
Carriage prevalence was higher in older adults who reported daily contact with children (15.7%) or contact every few days (14.0%) than in those who reported contact with children only once or twice a month (4.5%) or never (1.8%), they wrote.
“Older people who have a lot of contact with kids and are more susceptible to respiratory viruses can get a secondary infection from pneumococcus, especially during the cold and flu seasons. Vaccination can help to protect them or lessen severity of the illness,” Wyllie pointed out.
However, adult PCV immunization may not have a major impact on onward transmission to other adults, the authors wrote in their preprint.
This study supports prior work demonstrating that pneumococcal colonization is greater in households with children than in those without, said Stephen Pelton, MD, a pediatric infectious disease specialist from Boston University schools of medicine and public health. “The unique aspect is that Dr. Wyllie’s group has looked at individuals over age 60 and used the most sensitive methods currently available to detect pneumococcal carriage.”
“At the most recent ISPPD [International Society of Pneumonia and Pneumococcal Diseases conference], the role of adult-to-adult transmission in the community was discussed. This study confirms the critical role children play in community transmission of the pneumococcus,” Dr. Pelton noted.
Dr. Wyllie received consulting and/or advisory board fees from Pfizer, Merck, Diasorin, PPS Health, Primary Health, Co-Diagnostics, and Global Diagnostic Systems for work unrelated to this project and is the principal investigator on research grants from Pfizer, Merck, NIH RADx-UP, and SalivaDirect, Inc. to Yale University and from NIH RADx, Balvi.io, and Shield T3 to SalivaDirect, Inc. Dr. Pelton received honoraria from Merck, Pfizer, Sanofi, and GSK for participation in Pneumococcal Advisory Boards and DSMB (Sanofi). Boston Medical Center received grant funding for investigator-initiated research from Merck and Pfizer.
A version of this article appeared on Medscape.com.
BARCELONA, SPAIN — Streptococcus pneumoniae, the bacteria that causes pneumococcal disease, is sixfold more likely to colonize adults older than 60 years who have regular contact with children than those who do not, data from a community-based study showed.
However, there is “no clear evidence of adult-to-adult transmission,” and the researchers, led by Anne L. Wyllie, PhD, from the Yale School of Public Health, New Haven, Connecticut, noted that the study results suggest “the main benefit of adult pneumococcal conjugate vaccine (PCV) immunization is to directly protect adults who are exposed to children, who still carry and transmit some vaccine-type pneumococci despite successful pediatric national immunization programs.”
The data show that relatively high pneumococcus carriage rates are seen in people who have regular contact with children, who have had contact in the previous 2 weeks, and who have had contact for extended periods, Dr. Wyllie explained.
Preschoolers in particular were found to be most likely to transmit pneumococcus to older adults. “It is the 24- to 59-month-olds who are most associated with pneumococcal carriage, more than 1- to 2-year-olds,” she reported. However, transmission rates from children younger than 1 year are higher than those from children aged 1-2 years, she added.
The findings were presented at the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) 2024 global conference, formerly known as the ECCMID conference.
Originally Designed to Investigate Adult-to-Adult Transmission
The researchers wanted to understand the sources and dynamics of transmission, as well as the risk factors for pneumococcal disease in older adults, to help predict the effect of PCVs in people older than 60 years.
Although “we designed the study to specifically look at transmission between adults, in the end, we were presented with a very unique scenario” — restricted social mixing as a result of the COVID pandemic — during which “no community activities were happening,” Dr. Wyllie said. Because of this, the team was able to determine “the source of acquisition or transmission to the older adults was, very likely, coming from contact with children.”
Pneumococci are commonly found in respiratory tracts of healthy people. The US Centers for Disease Control and Prevention estimated that 20%-60% of school-aged children may be colonized compared with only 5%-10% of adults without children.
The longitudinal study was conducted among household pairs, such as married couples who were both aged at least 60 years and who did not have people younger than 60 years living in the household, in New Haven over two winter seasons: 2020-2021 and 2021-2022.
Self-collected saliva samples were assessed, and surveys on social behaviors and health were completed every 2 weeks for a 10-week period (with six study visits). The saliva sampling method was used because the researchers considered it to be more effective than samples from nasopharyngeal swabs. Quantitative polymerase chain reaction assays were used to test the saliva samples for the presence of pneumococcal DNA (pneumococcus genes piaB and lytA) and the diversity of pneumococcal strains (36 serotypes were targeted).
Strongly Suggestive of Transmission From Children to Older Adults
Of the 121 adults living in 61 households who were enrolled in the study, 62 adults participated in both seasons. Mean age was 70.9 years (range, 60-86 years), 51% of participants were women, and 85% were White.
Overall, 52 of 1088 (4.8%) samples tested positive for pneumococcus, and 27 of 121 (22.3%) adults were colonized on at least one sampling visit. Some were colonized at multiple timepoints, and two were colonized throughout the 10-week sampling period. Of the two participants who were colonized at five of six timepoints, one reported daily contact with children younger than 5 years and children aged 5-9 years in the two study seasons. This person was also positive at three of six sampling points during the first study season.
There were five instances in which both members of the household were carriers in the same season, although not necessarily at the same timepoint. Numbers were too small to determine whether transmission had occurred between the household pairs.
Contact with a 24- to 59-month-old child (older than 2 years but younger than 5 years) had the strongest association with elevated odds of carrying pneumococcus, the authors reported in their preprint, although the frequency and intensity of contact also mattered.
At any sampled time (point prevalence), pneumococcal carriage was substantially — just over sixfold — higher among older adults who had contact with children daily or every few days (10%) than among those who had no contact with children (1.6%).
In particular, contact between adults and children younger than 5 years and children aged 5-9 years was found to lead to elevated point prevalences of 13.8% and 14.1%, respectively. Pneumococcal carriage in children older than 10 years was lower, with a point prevalence of 8.3%.
The younger the child, the greater the point prevalence; point prevalences were 13.8% for samples from children aged 1 year and younger, 10.5% for samples from children aged 1-2 years, and 17.8% for children aged 2-5 years.
Carriage prevalence was higher in older adults who reported daily contact with children (15.7%) or contact every few days (14.0%) than in those who reported contact with children only once or twice a month (4.5%) or never (1.8%), they wrote.
“Older people who have a lot of contact with kids and are more susceptible to respiratory viruses can get a secondary infection from pneumococcus, especially during the cold and flu seasons. Vaccination can help to protect them or lessen severity of the illness,” Wyllie pointed out.
However, adult PCV immunization may not have a major impact on onward transmission to other adults, the authors wrote in their preprint.
This study supports prior work demonstrating that pneumococcal colonization is greater in households with children than in those without, said Stephen Pelton, MD, a pediatric infectious disease specialist from Boston University schools of medicine and public health. “The unique aspect is that Dr. Wyllie’s group has looked at individuals over age 60 and used the most sensitive methods currently available to detect pneumococcal carriage.”
“At the most recent ISPPD [International Society of Pneumonia and Pneumococcal Diseases conference], the role of adult-to-adult transmission in the community was discussed. This study confirms the critical role children play in community transmission of the pneumococcus,” Dr. Pelton noted.
Dr. Wyllie received consulting and/or advisory board fees from Pfizer, Merck, Diasorin, PPS Health, Primary Health, Co-Diagnostics, and Global Diagnostic Systems for work unrelated to this project and is the principal investigator on research grants from Pfizer, Merck, NIH RADx-UP, and SalivaDirect, Inc. to Yale University and from NIH RADx, Balvi.io, and Shield T3 to SalivaDirect, Inc. Dr. Pelton received honoraria from Merck, Pfizer, Sanofi, and GSK for participation in Pneumococcal Advisory Boards and DSMB (Sanofi). Boston Medical Center received grant funding for investigator-initiated research from Merck and Pfizer.
A version of this article appeared on Medscape.com.
BARCELONA, SPAIN — Streptococcus pneumoniae, the bacteria that causes pneumococcal disease, is sixfold more likely to colonize adults older than 60 years who have regular contact with children than those who do not, data from a community-based study showed.
However, there is “no clear evidence of adult-to-adult transmission,” and the researchers, led by Anne L. Wyllie, PhD, from the Yale School of Public Health, New Haven, Connecticut, noted that the study results suggest “the main benefit of adult pneumococcal conjugate vaccine (PCV) immunization is to directly protect adults who are exposed to children, who still carry and transmit some vaccine-type pneumococci despite successful pediatric national immunization programs.”
The data show that relatively high pneumococcus carriage rates are seen in people who have regular contact with children, who have had contact in the previous 2 weeks, and who have had contact for extended periods, Dr. Wyllie explained.
Preschoolers in particular were found to be most likely to transmit pneumococcus to older adults. “It is the 24- to 59-month-olds who are most associated with pneumococcal carriage, more than 1- to 2-year-olds,” she reported. However, transmission rates from children younger than 1 year are higher than those from children aged 1-2 years, she added.
The findings were presented at the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) 2024 global conference, formerly known as the ECCMID conference.
Originally Designed to Investigate Adult-to-Adult Transmission
The researchers wanted to understand the sources and dynamics of transmission, as well as the risk factors for pneumococcal disease in older adults, to help predict the effect of PCVs in people older than 60 years.
Although “we designed the study to specifically look at transmission between adults, in the end, we were presented with a very unique scenario” — restricted social mixing as a result of the COVID pandemic — during which “no community activities were happening,” Dr. Wyllie said. Because of this, the team was able to determine “the source of acquisition or transmission to the older adults was, very likely, coming from contact with children.”
Pneumococci are commonly found in respiratory tracts of healthy people. The US Centers for Disease Control and Prevention estimated that 20%-60% of school-aged children may be colonized compared with only 5%-10% of adults without children.
The longitudinal study was conducted among household pairs, such as married couples who were both aged at least 60 years and who did not have people younger than 60 years living in the household, in New Haven over two winter seasons: 2020-2021 and 2021-2022.
Self-collected saliva samples were assessed, and surveys on social behaviors and health were completed every 2 weeks for a 10-week period (with six study visits). The saliva sampling method was used because the researchers considered it to be more effective than samples from nasopharyngeal swabs. Quantitative polymerase chain reaction assays were used to test the saliva samples for the presence of pneumococcal DNA (pneumococcus genes piaB and lytA) and the diversity of pneumococcal strains (36 serotypes were targeted).
Strongly Suggestive of Transmission From Children to Older Adults
Of the 121 adults living in 61 households who were enrolled in the study, 62 adults participated in both seasons. Mean age was 70.9 years (range, 60-86 years), 51% of participants were women, and 85% were White.
Overall, 52 of 1088 (4.8%) samples tested positive for pneumococcus, and 27 of 121 (22.3%) adults were colonized on at least one sampling visit. Some were colonized at multiple timepoints, and two were colonized throughout the 10-week sampling period. Of the two participants who were colonized at five of six timepoints, one reported daily contact with children younger than 5 years and children aged 5-9 years in the two study seasons. This person was also positive at three of six sampling points during the first study season.
There were five instances in which both members of the household were carriers in the same season, although not necessarily at the same timepoint. Numbers were too small to determine whether transmission had occurred between the household pairs.
Contact with a 24- to 59-month-old child (older than 2 years but younger than 5 years) had the strongest association with elevated odds of carrying pneumococcus, the authors reported in their preprint, although the frequency and intensity of contact also mattered.
At any sampled time (point prevalence), pneumococcal carriage was substantially — just over sixfold — higher among older adults who had contact with children daily or every few days (10%) than among those who had no contact with children (1.6%).
In particular, contact between adults and children younger than 5 years and children aged 5-9 years was found to lead to elevated point prevalences of 13.8% and 14.1%, respectively. Pneumococcal carriage in children older than 10 years was lower, with a point prevalence of 8.3%.
The younger the child, the greater the point prevalence; point prevalences were 13.8% for samples from children aged 1 year and younger, 10.5% for samples from children aged 1-2 years, and 17.8% for children aged 2-5 years.
Carriage prevalence was higher in older adults who reported daily contact with children (15.7%) or contact every few days (14.0%) than in those who reported contact with children only once or twice a month (4.5%) or never (1.8%), they wrote.
“Older people who have a lot of contact with kids and are more susceptible to respiratory viruses can get a secondary infection from pneumococcus, especially during the cold and flu seasons. Vaccination can help to protect them or lessen severity of the illness,” Wyllie pointed out.
However, adult PCV immunization may not have a major impact on onward transmission to other adults, the authors wrote in their preprint.
This study supports prior work demonstrating that pneumococcal colonization is greater in households with children than in those without, said Stephen Pelton, MD, a pediatric infectious disease specialist from Boston University schools of medicine and public health. “The unique aspect is that Dr. Wyllie’s group has looked at individuals over age 60 and used the most sensitive methods currently available to detect pneumococcal carriage.”
“At the most recent ISPPD [International Society of Pneumonia and Pneumococcal Diseases conference], the role of adult-to-adult transmission in the community was discussed. This study confirms the critical role children play in community transmission of the pneumococcus,” Dr. Pelton noted.
Dr. Wyllie received consulting and/or advisory board fees from Pfizer, Merck, Diasorin, PPS Health, Primary Health, Co-Diagnostics, and Global Diagnostic Systems for work unrelated to this project and is the principal investigator on research grants from Pfizer, Merck, NIH RADx-UP, and SalivaDirect, Inc. to Yale University and from NIH RADx, Balvi.io, and Shield T3 to SalivaDirect, Inc. Dr. Pelton received honoraria from Merck, Pfizer, Sanofi, and GSK for participation in Pneumococcal Advisory Boards and DSMB (Sanofi). Boston Medical Center received grant funding for investigator-initiated research from Merck and Pfizer.
A version of this article appeared on Medscape.com.
FROM ESCMID GLOBAL 2024


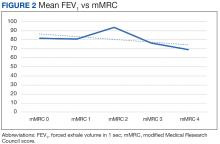
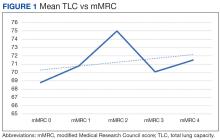
 Author disclosures
Author disclosures


