User login
Worried parents scramble to vaccinate kids despite FDA guidance
One week after reporting promising results from the trial of their COVID-19 vaccine in children ages 5-11, Pfizer and BioNTech announced they’d submitted the data to the Food and Drug Administration. But that hasn’t stopped some parents from discreetly getting their children under age 12 vaccinated.
“The FDA, you never want to get ahead of their judgment,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told MSNBC on Sept. 28. “But I would imagine in the next few weeks, they will examine that data and hopefully they’ll give the okay so that we can start vaccinating children, hopefully before the end of October.”
Lying to vaccinate now
More than half of all parents with children under 12 say they plan to get their kids vaccinated, according to a Gallup poll.
And although the FDA and the American Academy of Pediatrics have warned against it, some parents whose children can pass for 12 have lied to get them vaccinated already.
Dawn G. is a mom of two in southwest Missouri, where less than 45% of the population has been fully vaccinated. Her son turns 12 in early October, but in-person school started in mid-August.
“It was scary, thinking of him going to school for even 2 months,” she said. “Some parents thought their kid had a low chance of getting COVID, and their kid died. Nobody expects it to be them.”
In July, she and her husband took their son to a walk-in clinic and lied about his age.
“So many things can happen, from bullying to school shootings, and now this added pandemic risk,” she said. “I’ll do anything I can to protect my child, and a birthdate seems so arbitrary. He’ll be 12 in a matter of weeks. It seems ridiculous that that date would stop me from protecting him.”
In northern California, Carrie S. had a similar thought. When the vaccine was authorized for children ages 12-15 in May, the older of her two children got the shot right away. But her youngest doesn’t turn 12 until November.
“We were tempted to get the younger one vaccinated in May, but it didn’t seem like a rush. We were willing to wait to get the dosage right,” she ssaid. “But as Delta came through, there were no options for online school, the CDC was dropping mask expectations –it seemed like the world was ready to forget the pandemic was happening. It seemed like the least-bad option to get her vaccinated so she could go back to school, and we could find some balance of risk in our lives.”
Adult vs. pediatric doses
For now, experts advise against getting younger children vaccinated, even those who are the size of an adult, because of the way the human immune system develops.
“It’s not really about size,” said Anne Liu, MD, an immunologist and pediatrics professor at Stanford (Calif.) University. “The immune system behaves differently at different ages. Younger kids tend to have a more exuberant innate immune system, which is the part of the immune system that senses danger, even before it has developed a memory response.”
The adult Pfizer-BioNTech vaccine contains 30 mcg of mRNA, while the pediatric dose is just 10 mcg. That smaller dose produces an immune response similar to what’s seen in adults who receive 30 mcg, according to Pfizer.
“We were one of the sites that was involved in the phase 1 trial, a lot of times that’s called a dose-finding trial,” said Michael Smith, MD, a coinvestigator for the COVID vaccine trials done at Duke University. “And basically, if younger kids got a higher dose, they had more of a reaction, so it hurt more. They had fever, they had more redness and swelling at the site of the injection, and they just felt lousy, more than at the lower doses.”
At this point, with Pfizer’s data showing that younger children need a smaller dose, it doesn’t make sense to lie about your child’s age, said Dr. Smith.
“If my two options were having my child get the infection versus getting the vaccine, I’d get the vaccine. But we’re a few weeks away from getting the lower dose approved in kids,” he said. “It’s certainly safer. I don’t expect major, lifelong side effects from the higher dose, but it’s going to hurt, your kid’s going to have a fever, they’re going to feel lousy for a couple days, and they just don’t need that much antigen.”
A version of this article first appeared on WebMD.com.
One week after reporting promising results from the trial of their COVID-19 vaccine in children ages 5-11, Pfizer and BioNTech announced they’d submitted the data to the Food and Drug Administration. But that hasn’t stopped some parents from discreetly getting their children under age 12 vaccinated.
“The FDA, you never want to get ahead of their judgment,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told MSNBC on Sept. 28. “But I would imagine in the next few weeks, they will examine that data and hopefully they’ll give the okay so that we can start vaccinating children, hopefully before the end of October.”
Lying to vaccinate now
More than half of all parents with children under 12 say they plan to get their kids vaccinated, according to a Gallup poll.
And although the FDA and the American Academy of Pediatrics have warned against it, some parents whose children can pass for 12 have lied to get them vaccinated already.
Dawn G. is a mom of two in southwest Missouri, where less than 45% of the population has been fully vaccinated. Her son turns 12 in early October, but in-person school started in mid-August.
“It was scary, thinking of him going to school for even 2 months,” she said. “Some parents thought their kid had a low chance of getting COVID, and their kid died. Nobody expects it to be them.”
In July, she and her husband took their son to a walk-in clinic and lied about his age.
“So many things can happen, from bullying to school shootings, and now this added pandemic risk,” she said. “I’ll do anything I can to protect my child, and a birthdate seems so arbitrary. He’ll be 12 in a matter of weeks. It seems ridiculous that that date would stop me from protecting him.”
In northern California, Carrie S. had a similar thought. When the vaccine was authorized for children ages 12-15 in May, the older of her two children got the shot right away. But her youngest doesn’t turn 12 until November.
“We were tempted to get the younger one vaccinated in May, but it didn’t seem like a rush. We were willing to wait to get the dosage right,” she ssaid. “But as Delta came through, there were no options for online school, the CDC was dropping mask expectations –it seemed like the world was ready to forget the pandemic was happening. It seemed like the least-bad option to get her vaccinated so she could go back to school, and we could find some balance of risk in our lives.”
Adult vs. pediatric doses
For now, experts advise against getting younger children vaccinated, even those who are the size of an adult, because of the way the human immune system develops.
“It’s not really about size,” said Anne Liu, MD, an immunologist and pediatrics professor at Stanford (Calif.) University. “The immune system behaves differently at different ages. Younger kids tend to have a more exuberant innate immune system, which is the part of the immune system that senses danger, even before it has developed a memory response.”
The adult Pfizer-BioNTech vaccine contains 30 mcg of mRNA, while the pediatric dose is just 10 mcg. That smaller dose produces an immune response similar to what’s seen in adults who receive 30 mcg, according to Pfizer.
“We were one of the sites that was involved in the phase 1 trial, a lot of times that’s called a dose-finding trial,” said Michael Smith, MD, a coinvestigator for the COVID vaccine trials done at Duke University. “And basically, if younger kids got a higher dose, they had more of a reaction, so it hurt more. They had fever, they had more redness and swelling at the site of the injection, and they just felt lousy, more than at the lower doses.”
At this point, with Pfizer’s data showing that younger children need a smaller dose, it doesn’t make sense to lie about your child’s age, said Dr. Smith.
“If my two options were having my child get the infection versus getting the vaccine, I’d get the vaccine. But we’re a few weeks away from getting the lower dose approved in kids,” he said. “It’s certainly safer. I don’t expect major, lifelong side effects from the higher dose, but it’s going to hurt, your kid’s going to have a fever, they’re going to feel lousy for a couple days, and they just don’t need that much antigen.”
A version of this article first appeared on WebMD.com.
One week after reporting promising results from the trial of their COVID-19 vaccine in children ages 5-11, Pfizer and BioNTech announced they’d submitted the data to the Food and Drug Administration. But that hasn’t stopped some parents from discreetly getting their children under age 12 vaccinated.
“The FDA, you never want to get ahead of their judgment,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told MSNBC on Sept. 28. “But I would imagine in the next few weeks, they will examine that data and hopefully they’ll give the okay so that we can start vaccinating children, hopefully before the end of October.”
Lying to vaccinate now
More than half of all parents with children under 12 say they plan to get their kids vaccinated, according to a Gallup poll.
And although the FDA and the American Academy of Pediatrics have warned against it, some parents whose children can pass for 12 have lied to get them vaccinated already.
Dawn G. is a mom of two in southwest Missouri, where less than 45% of the population has been fully vaccinated. Her son turns 12 in early October, but in-person school started in mid-August.
“It was scary, thinking of him going to school for even 2 months,” she said. “Some parents thought their kid had a low chance of getting COVID, and their kid died. Nobody expects it to be them.”
In July, she and her husband took their son to a walk-in clinic and lied about his age.
“So many things can happen, from bullying to school shootings, and now this added pandemic risk,” she said. “I’ll do anything I can to protect my child, and a birthdate seems so arbitrary. He’ll be 12 in a matter of weeks. It seems ridiculous that that date would stop me from protecting him.”
In northern California, Carrie S. had a similar thought. When the vaccine was authorized for children ages 12-15 in May, the older of her two children got the shot right away. But her youngest doesn’t turn 12 until November.
“We were tempted to get the younger one vaccinated in May, but it didn’t seem like a rush. We were willing to wait to get the dosage right,” she ssaid. “But as Delta came through, there were no options for online school, the CDC was dropping mask expectations –it seemed like the world was ready to forget the pandemic was happening. It seemed like the least-bad option to get her vaccinated so she could go back to school, and we could find some balance of risk in our lives.”
Adult vs. pediatric doses
For now, experts advise against getting younger children vaccinated, even those who are the size of an adult, because of the way the human immune system develops.
“It’s not really about size,” said Anne Liu, MD, an immunologist and pediatrics professor at Stanford (Calif.) University. “The immune system behaves differently at different ages. Younger kids tend to have a more exuberant innate immune system, which is the part of the immune system that senses danger, even before it has developed a memory response.”
The adult Pfizer-BioNTech vaccine contains 30 mcg of mRNA, while the pediatric dose is just 10 mcg. That smaller dose produces an immune response similar to what’s seen in adults who receive 30 mcg, according to Pfizer.
“We were one of the sites that was involved in the phase 1 trial, a lot of times that’s called a dose-finding trial,” said Michael Smith, MD, a coinvestigator for the COVID vaccine trials done at Duke University. “And basically, if younger kids got a higher dose, they had more of a reaction, so it hurt more. They had fever, they had more redness and swelling at the site of the injection, and they just felt lousy, more than at the lower doses.”
At this point, with Pfizer’s data showing that younger children need a smaller dose, it doesn’t make sense to lie about your child’s age, said Dr. Smith.
“If my two options were having my child get the infection versus getting the vaccine, I’d get the vaccine. But we’re a few weeks away from getting the lower dose approved in kids,” he said. “It’s certainly safer. I don’t expect major, lifelong side effects from the higher dose, but it’s going to hurt, your kid’s going to have a fever, they’re going to feel lousy for a couple days, and they just don’t need that much antigen.”
A version of this article first appeared on WebMD.com.
Mobile Integrated Health: Reducing Chronic Obstructive Pulmonary Disease Hospitalizations Through Novel Outpatient Care Initiatives
From the Mobile Integrated Health and Emergency Medicine Department, South Shore Health, Weymouth, MA.
Objective: To develop a process through which Mobile Integrated Health (MIH) can treat patients with chronic obstructive pulmonary disease (COPD) at high risk for readmission in an outpatient setting. In turn, South Shore Hospital (SSH) looks to leverage MIH to improve hospital flow, decrease costs, and improve patient quality of life.
Methods: With the recent approval of hospital-based MIH programs in Massachusetts, SSH used MIH to target specific patient demographics in an at-home setting. Here, we describe the planning and implementation of this program for patients with COPD. Key components to success include collaboration among providers, early follow-up visits, patient education, and in-depth medical reconciliations. Analysis includes a retrospective examination of a structured COPD outpatient pathway.
Results: A total of 214 patients with COPD were treated with MIH from March 2, 2020, to August 1, 2021. Eighty-seven emergent visits were conducted, and more than 650 total visits were made. A more intensive outpatient pathway was implemented for patients deemed to be at the highest risk for readmission by pulmonary specialists.
Conclusion: This process can serve as a template for future institutions to treat patients with COPD using MIH or similar hospital-at-home services.
Keywords: Mobile Integrated Health; MIH; COPD; population health.
It is estimated that chronic obstructive pulmonary disease (COPD) affects more than 16 million Americans1 and accounts for more than 700 000 hospitalizations each year in the US.2 Thirty-day COPD readmission rates hover around 22.6%,3 and readmission within 90 days of initial discharge can jump to between 31% and 35%.4 This is the highest of any patient demographic, and more than half of these readmissions are due to COPD. To counter this, government and state entities have made nationwide efforts to encourage health systems to focus on preventing readmissions. In October 2014, the US added COPD to the active list of diseases in Medicare’s Hospital Readmissions Reduction Program (HRRP), later adding COPD to various risk-based bundle programs that hospitals may choose to opt into. These programs are designed to reduce all-cause readmissions after an acute exacerbation of COPD, as the HRRP penalizes hospitals for all-cause 30-day readmissions.3 However, what is most troubling is that, despite these efforts, readmission rates have not dropped in the past decade.5 COPD remains the third leading cause of death in America and still poses a significant burden both clinically and economically to hospitals across the country.3
A solution that is gaining traction is to encourage outpatient care initiatives and discharge pathways. Early follow-up is proven to decrease chances of readmission, and studies have shown that more than half of readmitted patients did not follow up with a primary care physician (PCP) within 30 days of their initial discharge.6 Additionally, large meta-analyses show hospital-at-home–type programs can lead to reductions in mortality, decrease costs, decrease readmissions, and increase patient satisfaction.7-9 Therefore, for more challenging patient populations with regard to readmissions and mortality, Mobile Integrated Health (MIH) may be the solution that we are looking for.
This article presents a viable process to treat patients with COPD in an outpatient setting with MIH Services. It includes an examination of what makes MIH successful as well as a closer look at a structured COPD outpatient pathway.
Methods
South Shore Hospital (SSH) is an independent, not-for-profit hospital located in Weymouth, Massachusetts. It is host to 400 beds, 100 000 annual visits to the emergency department (ED), and its own emergency medical services program. In March 2020, SSH became the first Massachusetts hospital-based program to acquire an MIH license. MIH paramedics receive 300 hours of specialized training, including time in clinical clerkships shadowing pulmonary specialists, cardiology/congestive heart failure (CHF) providers, addiction medicine specialists, home care and care progression colleagues, and wound center providers. Specialist providers become more comfortable with paramedic capabilities as a result of these clerkships, improving interactions and relationships going forward. At the time of writing, SSH MIH is staffed by 12 paramedics, 4 of whom are full time; 2 medical directors; 2 internal coordinators; and 1 registered nurse (RN). A minimum of 2 paramedics are on call each day, each with twice-daily intravenous (IV) capabilities. The first shift slot is 16 hours, from 7:00 AM to 11:00
The goal of developing MIH is to improve upon the current standard of care. For hospitals without MIH capabilities, there are limited options to treat acute exacerbations of chronic obstructive pulmonary disease (AECOPD) patients postdischarge. It is common for the only outpatient referral to be a lone PCP visit, and many patients who need more extensive treatment options don’t have access to a timely PCP follow-up or resources for alternative care. This is part of why there has been little improvement in the 21st century with regard to reducing COPD hospitalizations. As it stands, approximately 10% to 55% of all AECOPD readmissions are preventable, and more than one-fifth of patients with COPD are rehospitalized within 30 days of discharge.3 In response, MIH has been designed to provide robust care options postdischarge in the patient home, with the eventual goal of reducing preventable hospitalizations and readmissions for all patients with COPD.
Patient selection
Patients with COPD are admitted to the MIH program in 1 of 3 ways: (1) directly from the ED; (2) at discharge from inpatient care; or (3) from a SSH affiliate referral.
With option 1, the ED physician assesses patient need for MIH services and places a referral to MIH in the electronic medical record (EMR). The ED provider also specifies whether follow-up is “urgent” and sets an alternative level of priority if not. With option 2, the inpatient provider and case manager follow a similar process, first determining whether a patient is stable enough to go home with outpatient services and then if MIH would be beneficial to the patient. If the patient is discharged home, a follow-up visit by an MIH paramedic is scheduled within 48 hours. With option 3, the patient is referred to MIH by an affiliate of SSH. This can be through the patient’s PCP, their visiting nurse association (VNA) service provider, or through any SSH urgent care center. In all 3 referral processes, the patient has the option to consent into the program or refuse services. Once referred, MIH coordinators review patients on a case-by-case basis. Patients with a history of prior admissions are given preference, with the goal being to keep the frailer, older, and comorbid patients at home. Other considerations include recent admission(s), length of stay, and overall stability. Social factors considered by the team include whether the patient lives alone and has alternative home services and the patient’s total distance from the hospital. Patients with a history of violence, mental health concerns, or substance abuse go through a more extensive screening process to ensure paramedic safety.
Given their patient profile and high hospital usage rates, MIH is sometimes requested for patients with end-stage COPD. Many of these patients benefit from MIH goals-of-care conversations to ensure they understand all their options and choose an approach that fits their preferences. In these cases, MIH has been instrumental in assisting patients and families with completing Medical Orders for Life-Sustaining Treatment and health care proxy forms and transitioning patients to palliative care, hospice, advanced-illness care management programs, or other long-term care options to prevent the need for rehospitalization. The MIH team focuses heavily on providing quality end-of-life care for patients and aligning care models with patient and family goals, often finding that having these sensitive conversations in the comfort of home enables transparency and comfort not otherwise experienced by hospitalized patients.
Initial patient follow-up
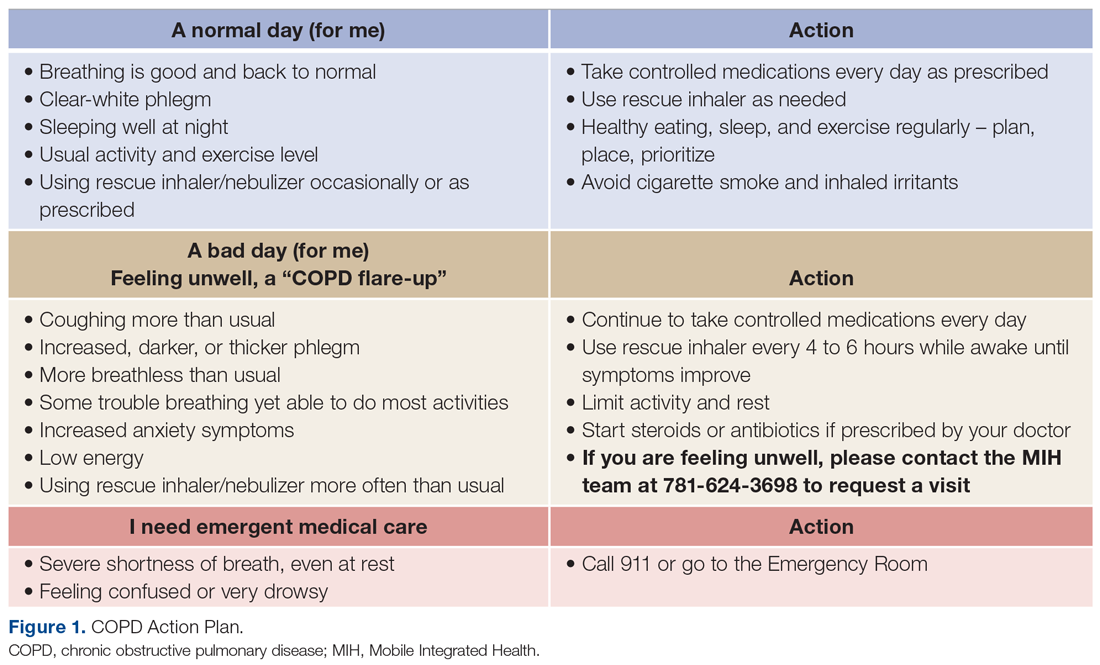
For patients with COPD enrolled in the MIH program, their first patient visit is scheduled within 48 hours of discharge from the ED or inpatient hospital. In many cases, this visit can be conducted within 24 hours of returning home. Once at the patient’s home, the paramedic begins with general introductions, vital signs, and a basic physical examination. The remainder of the visit focuses on patient education and symptom recognition. The paramedic reviews the COPD action plan (Figure 1), including how to recognize the onset of a “COPD flare-up” and the appropriate response. Patients are provided with a paper copy of the action plan for future reference.
The next point of educational emphasis is the patient’s individual medication regimen. This involves differentiating between control (daily) and rescue medications, how to use oxygen tanks, and how to safely wean off of oxygen. Specific attention is given to how to use a metered-dose inhaler, as studies have found that more than half of all patients use their inhaler devices incorrectly.10
Paramedics also complete a home safety evaluation of the patient’s residence, which involves checking for tripping hazards, lighting, handrails, slippery surfaces, and general access to patient medication. If an issue cannot be resolved by the paramedic on site and is considered a safety hazard, it is reported back to the hospital team for assistance.
Finally, patients are educated on the capabilities of MIH as a program and what to expect when they reach out over the phone. Patients are given a phone number to call for both “urgent” and “nonemergent” situations. In both cases, they will be greeted by one of the MIH coordinators or nurses who assist with triaging patient symptoms, scheduling a visit, or providing other guidance. It is a point of emphasis that the patient can use MIH for more than just COPD and should call in the event of any illness or discomfort (eg, dehydration, fever) in an effort to prevent unnecessary ED visits.
Medication reconciliation
Patients with COPD often have complex medication regimens. To help alleviate any confusion, medication reconciliations are done in conjunction with every COPD patient’s initial visit. During this process, the paramedic first takes an inventory of all medications in the patient home. Common reasons for nonadherence include confusing packaging, inability to reach the pharmacy, or medication not being covered by insurance. The paramedic reconciles the updated medication regimen against the medications that are physically in the home. Once the initial review is complete, the paramedic teleconferences with a registered hospitalist pharmacist (RHP) for a more in-depth review. Over video chat, the RHP reviews each medication individually to make sure the patient understands how many times per day they take each medication, whether it is a control or rescue medication, and what times of the day to take them. The RHP will then clarify any other medication questions the patient has, assure all recent medications have been picked up from the pharmacy, and determine any barriers, such as cost or transportation.
Follow-ups and PCP involvement
At each in-person visit, paramedics coordinate with an advanced practice clinician (APC) through telehealth communication. On these video calls with a provider, the paramedic relays relevant information pertaining to patient history, vital signs, and current status. Any concerning findings, symptoms of COPD flare-ups, or recent changes in status will be discussed. The APC then speaks directly to the patient to gather additional details about their condition and any recent hospitalizations, with their primary role being to make clinical decisions on further treatment. For the COPD population, this often includes orders for the MIH paramedic to administer IV medication (ie, IV methylprednisolone or other corticosteroids), antibiotics, home nebulizers, and at-home oxygen.
Second and third follow-up paramedic visits are often less intensive. Although these visits often still involve telehealth calls to the APC, the overall focus shifts toward medication adherence, ED avoidance, and readmission avoidance. On these visits, the paramedic also checks vitals, conducts a physical examination, and completes follow-up testing or orders per the APC.
PCP involvement is critical to streamlining and transitioning patient care. Patients who are admitted to MIH without insurance or a PCP are assisted in the process of finding one. PCPs automatically receive a patient enrollment letter when their patient is seen by an MIH paramedic. Following each individual visit, paramedic and APC notes are sent to the PCP through the EMR or via fax, at which time the PCP may be consulted on patient history and/or future care decisions. After the transition back to care by their PCP, patients are still encouraged to utilize MIH if acute changes arise. If a patient is readmitted back to the hospital, MIH is automatically notified, and coordinators will assess whether there is continued need for outpatient services or areas for potential improvement.
Emergent MIH visits
While MIH visits with patients with COPD are often scheduled, MIH can also be leveraged in urgent situations to prevent the need for a patient to come to the ED or hospital. Patients with COPD are told to call MIH if they have worsening symptoms or have exhausted all methods of self-treatment without an improvement in status. In this case, a paramedic is notified and sent to the patient’s home at the earliest time possible. The paramedic then completes an assessment of the patient’s status and relays information to the MIH APC or medical director. From there, treatment decisions, such as starting the patient on an IV, using nebulizers, or doing an electrocardiogram for diagnostic purposes, are guided by the provider team with the ultimate goal of caring for the patient in the home. For our population, providing urgent care in the home has proven to be an effective way to avoid unnecessary readmissions while still ensuring high-quality patient care.
Outpatient pathway
In May 2021, select patients with COPD were given the option to participate in a more intensive MIH outpatient pathway. Pilot patients were chosen by 2 pulmonary specialists, with a focus on enrolling patients with COPD at the highest risk for readmission. Patients who opted in were followed by MIH for a total of 30 days.
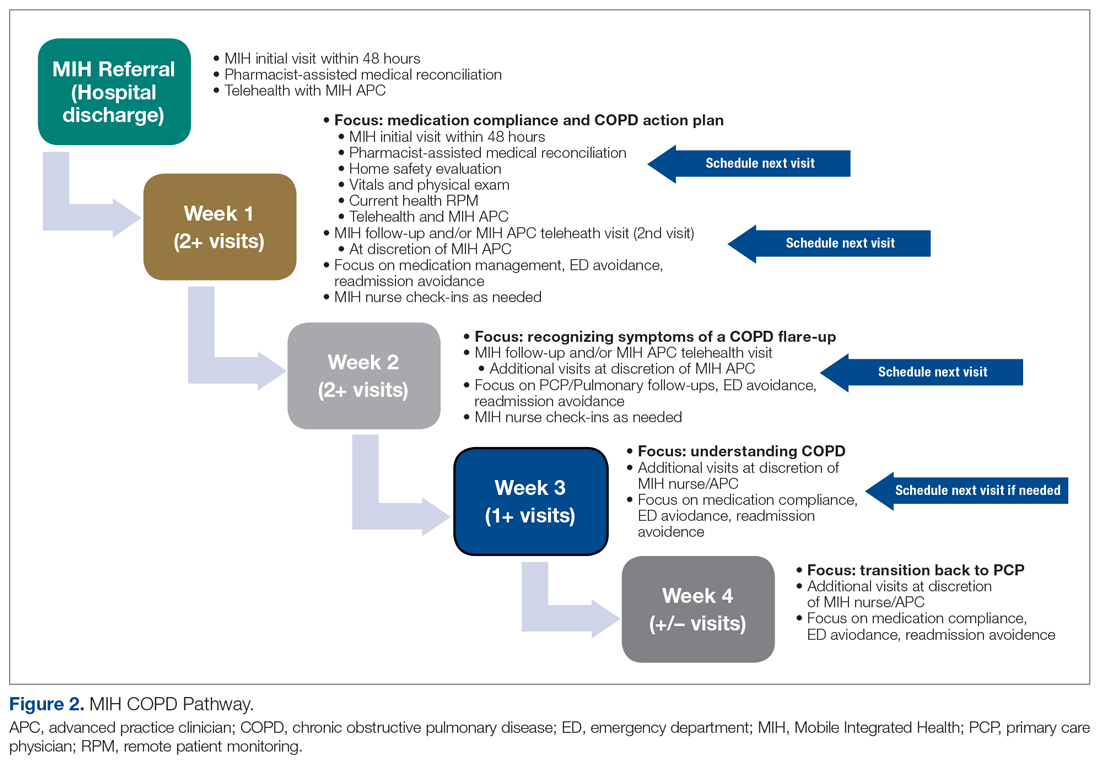
The first visit was made as usual within 48 hours of discharge. Patients received education, medication reconciliation, vitals examination, home safety evaluation, and a facilitated telehealth evaluation with the APC. What differentiates the pathway from standard MIH services is that after the first visit, the follow-ups are prescheduled and more numerous. This is outlined best in Figure 2, which serves as a guideline for coordinators and paramedics in the cadence and focus of visits for each patient on the pathway. The initial 2 weeks are designed to check in on the patient in person and ensure active recovery. The latter 2 weeks are designed to ensure that the patient follows up with their care team and understands their medications and action plan going forward. Pathway patients were also monitored using a remote patient monitoring (RPM) kit. On the initial visit, paramedics set up the RPM equipment and provided a demonstration on how to use each device. Patients were issued a Bluetooth-enabled scale, blood pressure cuff, video-enabled tablet, and wearable device. The wearable device continuously recorded respiration rate, heart rate, and oxygen saturation and had fall-detection enabled. Over the course of a month, an experienced MIH nurse monitored the vitals transmitted by the wearable device and checked patient weight and blood pressure 1 to 2 times per day, utilizing these data to proactively outreach to patients if abnormalities occurred. Prior to the start of the program, the MIH nurse contacted each patient to introduce herself and notify them that they would receive a call if any vitals were unusual.
Results
MIH treated 214 patients with COPD from March 2, 2020, to August 2, 2021. In total, paramedics made more than 650 visits. Eighty-seven of these were documented as urgent visits with AECOPD, shortness of breath, cough, or wheezing as the primary concern.
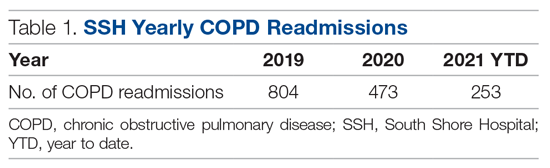
In the calendar year of 2019, our institution admitted 804 patients with a primary diagnosis of COPD. In 2020, the first year with MIH, total COPD admissions decreased to 473; however, the effect of the COVID-19 pandemic cannot be discounted. At of the time of writing—219 days into 2021—253 patients with COPD have been admitted thus far (Table 1).
Pathway results
Sixteen patients were referred to the MIH COPD Discharge Pathway Pilot during May 2021. Ten patients went on to complete the entire 30-day pathway. Six did not finish the program. Three of these 6 patients were referred by a pulmonary specialist for enrollment but not ultimately referred to the pilot program by case management and therefore not enrolled. The other 3 of the 6 patients who did not complete the pilot program were enrolled but discontinued owing to noncompliance.
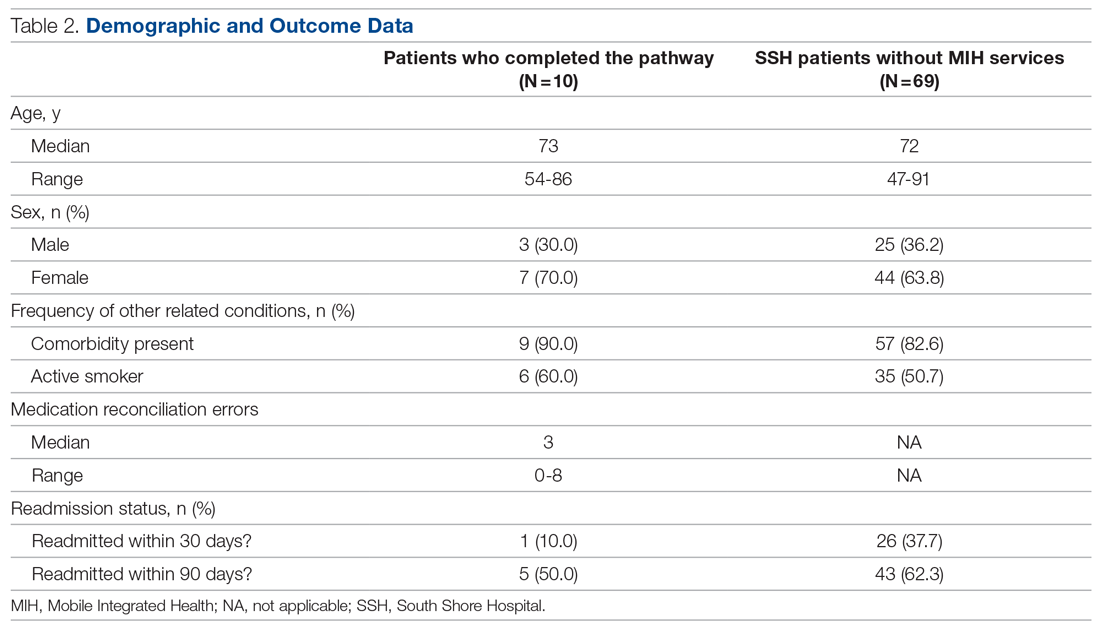
Of the 10 patients who completed the pathway, 3 patients were male, and 7 were female. Ages ranged from 55 to 84 years. On average, the RHP found 3.6 medication reconciliation errors per patient. One patient was readmitted within 30 days (only 3 days after the initial discharge), and 5 were readmitted within 90 days.
A retrospective analysis was conducted on patients with COPD who were not provided with MIH services and were admitted to our hospital between September 1, 2020, and March 1, 2021, for comparison. Age, sex, and other related conditions are shown in Table 2. Medication reconciliation error data were not tracked for this demographic, as they did not have an in-home medication reconciliation completed.
Discussion
MIH has treated 214 patients with COPD from March 2, 2020, to August 2, 2021, a 17-month period. In that same timeframe, the hospital experienced a 42% decrease in COPD admissions. Although this effect is not the sole product of MIH (specifically, COVID-19 caused a drop in all-cause hospital admissions), we believe MIH did play a small role in this reduction. Eighty-seven emergent visits were conducted for patients with a primary complaint of AECOPD, shortness of breath, cough, or wheezing. On these visits, MIH provided urgent treatment to prevent the patient returning to the ED and potentially leading to readmission.
The program’s impact extends beyond the numbers. With more than 200 patients with COPD treated at home, we improved hospital flow, shortened patients’ overall length of stay, and increased capacity in the ED and inpatient units. In addition, MIH has been able to fill in care gaps present in the current health care system by providing acute care in the home to patients who otherwise have access-to-care and transportation issues.
What made the program successful
With the COPD population prone to having complex medication regimens, medication reconciliations were critical to improving patient outcomes. During the documented medication reconciliations for pathway patients, 8 of 10 patients had medication errors identified. Some of the more common errors included incorrect inhaler usage, patient medication not arriving to the pharmacy for a week or more after discharge, prescribed medication dosages that were too high or too low, and a lack of transportation to pick up the patient’s prescription. Even more problematic is that 7 of these 8 patients required multiple interventions to correct their regimen. What was cited as most beneficial by both the paramedic and the RHP was taking time to walk through each medication individually and ensuring that the patient could recite back how often and when they should be using it. What also proved to be helpful was spending extra time on the inhalers and nebulizers. Multiple patients did not know how to use them properly and/or cited a history of struggling with them.
The MIH COPD pathway patients showed encouraging preliminary results. In the initial 30-day window, only 1 of 10 (10%) patients was readmitted, which is lower than the 37.7% rate for comparable patients who did not have MIH services. This could imply that patients with COPD respond positively to active and consistent management with predetermined points of contact. Ninety-day readmission rates jumped to 5 of 10, with 4 of these patients being readmitted multiple times. Approximately half of these readmissions were COPD related. It is important to remember that the patients being targeted by the pathway are deemed to be at very high risk of readmission. As such, one could expect that even with a successful reduction in rates, pathway patient readmission rates may be slightly elevated compared with national COPD averages.
Given the more personalized and at-home care, patients also expressed higher levels of care satisfaction. Most patients want to avoid the hospital at all costs, and MIH provides a safe and effective alternative. Patients with COPD have also relayed that the education they receive on their medication, disease, and how to use MIH has been useful. This is reflected in the volume of urgent calls that MIH receives. A patient calling MIH in place of 911 shows not only that the patient has a level of trust in the MIH team, but also that they have learned how to recognize symptoms earlier to prevent major flare-ups.
This study had several limitations. On the pilot pathway, 3 patients were removed from MIH services because of repeated noncompliance. These instances primarily involved aggression toward the paramedics, both verbal and physical, as well as refusal to allow the MIH paramedics into the home. Going forward, it will be valuable to have a screening process for pathway patients to determine likelihood of compliance. This could include speaking to the patient’s PCP or other in-hospital providers before accepting them into the program.
Remote patient monitoring also presented its challenges. Despite extensive equipment demonstrations, some patients struggled to grasp the technology. Some of the biggest problems cited were confusion operating the tablet, inability to charge the devices, and issues with connectivity. In the future, it may be useful to simplify the devices even more. Further work should also be done to evaluate the efficacy of remote patient technology in this specific setting, as studies have shown varied results with regard to RPM success. In 1 meta-analysis of 91 different published studies that took place between 2015 and 2020, approximately half of the RPM studies resulted in no change in hospital readmissions, length of stay, or ED presentations, while the other half saw improvement in these categories.11 We suspect that the greatest benefits of our work came from the patient education, trust built over time, in-home urgent evaluations, and 1-on-1 time with the paramedic.
With many people forgoing care during the pandemic, COVID-19 has also caused a downward trend in overall and non-COVID-19 admissions. In a review of more than 500 000 ED visits in Massachusetts between March 11, 2020, and September 8, 2021, there was a 32% decrease in admissions when compared with those same weeks in 2019.10 There was an even greater drop-off when it came to COPD and other respiratory-related admissions. In evaluating the impact SSH MIH has made, it is important to recognize that the pandemic contributed to reducing total COPD admissions. Adding merit to the success of MIH in contributing to the reduction in admissions is the continued downward trend in total COPD admissions year-to-date in 2021. Despite total hospital usage rates increasing at our institution over the course of this year, the overall COPD usage rates have remained lower than before.
Another limitation is that in the selection of patients, both for general MIH care and for the COPD pathway, there was room for bias. The pilot pathway was offered specifically to patients at the highest risk for readmission; however, patients were referred at the discretion of our pulmonologist care team and not selected by any standardized rubric. Additionally, MIH only operates on a 16-hour schedule. This means that patients admitted to the ED or inpatient at night may sometimes be missed and not referred to MIH for care.
The biggest caveat to the pathway results is, of course, the small sample size. With only 10 patients completing the pilot, it is impossible to come to any concrete conclusions. Such an intensive pathway requires dedicating large amounts of time and resources, which is why the pilot was small. However, considering the preliminary results, the outline given could provide a starting point for future work to evaluate a similar COPD pathway on a larger scale.
Future considerations
Risk stratification of patients is critical to achieving even further reductions in readmissions and mortality. Hospitals can get the most value from MIH by focusing on patients with COPD at the highest risk for return, and it would be valuable to explicitly define who fits into this criterion. Utilizing a tool similar to the LACE index for readmission but tailoring it to patients with COPD when admitting patients into the program would be a logical next step.
Reducing the points of patient contact could also prove valuable. Over the course of a patient’s time with MIH, they interact with an RHP, APC, paramedic, RN, and discharging hospitalist. Additionally, we found many patients had VNA services, home health aides, care managers, and/or social workers involved in their care. Some patients found this to be stressful and overwhelming, especially regarding the number of outreach calls soon after discharge.
It would also be useful to look at the impact of MIH on total COPD admissions independent of the artificial variation created by COVID-19. This may require waiting until there are higher levels of vaccination and/or finding ways to control for the potential variation. In doing so, one could look at the direct effect MIH has on COPD readmissions when compared with a control group without MIH services, which could then serve as a comparison point to the results of this study. As it stands, given the relative novelty of MIH, there are primarily only broad reviews of MIH’s effectiveness and/or impact on patient populations that have been published. Of these, only a few directly mentioned MIH in relation to COPD, and none have comparable designs that look at overall COPD hospitalization reductions post-MIH implementation. There is also little to no literature looking at the utilization of MIH in a more intensive COPD outpatient pathway.
Finally, MIH has proven to be a useful tool for our institution in many areas outside of COPD management. Specifically, MIH has been utilized as a mobile influenza and COVID-19 vaccination unit and in-home testing service and now operates both a hospital-at-home and skilled nursing facility-at-home program. Analysis of the overall needs of the system and where this valuable MIH resource would have the biggest impact will be key in future growth opportunities.
Conclusion
MIH has been an invaluable tool for our hospital, especially in light of the recent shift toward more in-home and virtual care. MIH cared for 214 patients with COPD with more than 650 visits between March 2020 and August 2021. Eighty-seven emergent COPD visits were conducted, and COPD admissions were reduced dramatically from 2019 to 2020. MIH services have improved hospital flow, allowed for earlier discharge from the ED and inpatient care, and helped improve all-cause COPD readmission rates. The importance of postdischarge care and follow-up visits for patients with COPD, especially those at higher risk for readmission, cannot be understated. We hope our experience working to improve COPD patient outcomes serves as valuable a reference point for future MIH programs.
Corresponding author: Kelly Lannutti, DO, Mobile Integrated Health and Emergency Medicine Department, South Shore Health, 55 Fogg Rd, South Weymouth, MA 02190; [email protected].
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease (COPD). Accessed September 10, 2011. https://www.cdc.gov/copd/index.html
2. Wier LM, Elixhauser A, Pfuntner A, AuDH. Overview of Hospitalizations among Patients with COPD, 2008. Statistical Brief #106. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2011.
3. Shah T, Press,VG, Huisingh-Scheetz M, White SR. COPD Readmissions: Addressing COPD in the Era of Value-Based Health Care. Chest. 2016;150(4):916-926. doi:10.1016/j.chest.2016.05.002
4. Harries TH, Thornton H, Crichton S, et al. Hospital readmissions for COPD: a retrospective longitudinal study. NPJ Prim Care Respir Med. 2017;27(1):31. doi:10.1038/s41533-017-0028-8
5. Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001-2012 and Nationwide Emergency Department Sample 2006-2011. Chest. 2015;147(4):989-998. doi:10.1378/chest.14-2146
6. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563
7. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi:10.1503/cmaj.081491
8. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital in the home.” Med J Aust. 2012;197(9):512-519. doi:10.5694/mja12.10480
9. Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
10. Nourazari S, Davis SR, Granovsky R, et al. Decreased hospital admissions through emergency departments during the COVID-19 pandemic. Am J Emerg Med. 2021;42:203-210. doi:10.1016/j.ajem.2020.11.029
11. Taylor ML, Thomas EE, Snoswell CL, et al. Does remote patient monitoring reduce acute care use? A systematic review. BMJ Open. 2021;11(3):e040232. doi:10.1136/bmj/open-2020-040232
From the Mobile Integrated Health and Emergency Medicine Department, South Shore Health, Weymouth, MA.
Objective: To develop a process through which Mobile Integrated Health (MIH) can treat patients with chronic obstructive pulmonary disease (COPD) at high risk for readmission in an outpatient setting. In turn, South Shore Hospital (SSH) looks to leverage MIH to improve hospital flow, decrease costs, and improve patient quality of life.
Methods: With the recent approval of hospital-based MIH programs in Massachusetts, SSH used MIH to target specific patient demographics in an at-home setting. Here, we describe the planning and implementation of this program for patients with COPD. Key components to success include collaboration among providers, early follow-up visits, patient education, and in-depth medical reconciliations. Analysis includes a retrospective examination of a structured COPD outpatient pathway.
Results: A total of 214 patients with COPD were treated with MIH from March 2, 2020, to August 1, 2021. Eighty-seven emergent visits were conducted, and more than 650 total visits were made. A more intensive outpatient pathway was implemented for patients deemed to be at the highest risk for readmission by pulmonary specialists.
Conclusion: This process can serve as a template for future institutions to treat patients with COPD using MIH or similar hospital-at-home services.
Keywords: Mobile Integrated Health; MIH; COPD; population health.
It is estimated that chronic obstructive pulmonary disease (COPD) affects more than 16 million Americans1 and accounts for more than 700 000 hospitalizations each year in the US.2 Thirty-day COPD readmission rates hover around 22.6%,3 and readmission within 90 days of initial discharge can jump to between 31% and 35%.4 This is the highest of any patient demographic, and more than half of these readmissions are due to COPD. To counter this, government and state entities have made nationwide efforts to encourage health systems to focus on preventing readmissions. In October 2014, the US added COPD to the active list of diseases in Medicare’s Hospital Readmissions Reduction Program (HRRP), later adding COPD to various risk-based bundle programs that hospitals may choose to opt into. These programs are designed to reduce all-cause readmissions after an acute exacerbation of COPD, as the HRRP penalizes hospitals for all-cause 30-day readmissions.3 However, what is most troubling is that, despite these efforts, readmission rates have not dropped in the past decade.5 COPD remains the third leading cause of death in America and still poses a significant burden both clinically and economically to hospitals across the country.3
A solution that is gaining traction is to encourage outpatient care initiatives and discharge pathways. Early follow-up is proven to decrease chances of readmission, and studies have shown that more than half of readmitted patients did not follow up with a primary care physician (PCP) within 30 days of their initial discharge.6 Additionally, large meta-analyses show hospital-at-home–type programs can lead to reductions in mortality, decrease costs, decrease readmissions, and increase patient satisfaction.7-9 Therefore, for more challenging patient populations with regard to readmissions and mortality, Mobile Integrated Health (MIH) may be the solution that we are looking for.
This article presents a viable process to treat patients with COPD in an outpatient setting with MIH Services. It includes an examination of what makes MIH successful as well as a closer look at a structured COPD outpatient pathway.
Methods
South Shore Hospital (SSH) is an independent, not-for-profit hospital located in Weymouth, Massachusetts. It is host to 400 beds, 100 000 annual visits to the emergency department (ED), and its own emergency medical services program. In March 2020, SSH became the first Massachusetts hospital-based program to acquire an MIH license. MIH paramedics receive 300 hours of specialized training, including time in clinical clerkships shadowing pulmonary specialists, cardiology/congestive heart failure (CHF) providers, addiction medicine specialists, home care and care progression colleagues, and wound center providers. Specialist providers become more comfortable with paramedic capabilities as a result of these clerkships, improving interactions and relationships going forward. At the time of writing, SSH MIH is staffed by 12 paramedics, 4 of whom are full time; 2 medical directors; 2 internal coordinators; and 1 registered nurse (RN). A minimum of 2 paramedics are on call each day, each with twice-daily intravenous (IV) capabilities. The first shift slot is 16 hours, from 7:00 AM to 11:00
The goal of developing MIH is to improve upon the current standard of care. For hospitals without MIH capabilities, there are limited options to treat acute exacerbations of chronic obstructive pulmonary disease (AECOPD) patients postdischarge. It is common for the only outpatient referral to be a lone PCP visit, and many patients who need more extensive treatment options don’t have access to a timely PCP follow-up or resources for alternative care. This is part of why there has been little improvement in the 21st century with regard to reducing COPD hospitalizations. As it stands, approximately 10% to 55% of all AECOPD readmissions are preventable, and more than one-fifth of patients with COPD are rehospitalized within 30 days of discharge.3 In response, MIH has been designed to provide robust care options postdischarge in the patient home, with the eventual goal of reducing preventable hospitalizations and readmissions for all patients with COPD.
Patient selection
Patients with COPD are admitted to the MIH program in 1 of 3 ways: (1) directly from the ED; (2) at discharge from inpatient care; or (3) from a SSH affiliate referral.
With option 1, the ED physician assesses patient need for MIH services and places a referral to MIH in the electronic medical record (EMR). The ED provider also specifies whether follow-up is “urgent” and sets an alternative level of priority if not. With option 2, the inpatient provider and case manager follow a similar process, first determining whether a patient is stable enough to go home with outpatient services and then if MIH would be beneficial to the patient. If the patient is discharged home, a follow-up visit by an MIH paramedic is scheduled within 48 hours. With option 3, the patient is referred to MIH by an affiliate of SSH. This can be through the patient’s PCP, their visiting nurse association (VNA) service provider, or through any SSH urgent care center. In all 3 referral processes, the patient has the option to consent into the program or refuse services. Once referred, MIH coordinators review patients on a case-by-case basis. Patients with a history of prior admissions are given preference, with the goal being to keep the frailer, older, and comorbid patients at home. Other considerations include recent admission(s), length of stay, and overall stability. Social factors considered by the team include whether the patient lives alone and has alternative home services and the patient’s total distance from the hospital. Patients with a history of violence, mental health concerns, or substance abuse go through a more extensive screening process to ensure paramedic safety.
Given their patient profile and high hospital usage rates, MIH is sometimes requested for patients with end-stage COPD. Many of these patients benefit from MIH goals-of-care conversations to ensure they understand all their options and choose an approach that fits their preferences. In these cases, MIH has been instrumental in assisting patients and families with completing Medical Orders for Life-Sustaining Treatment and health care proxy forms and transitioning patients to palliative care, hospice, advanced-illness care management programs, or other long-term care options to prevent the need for rehospitalization. The MIH team focuses heavily on providing quality end-of-life care for patients and aligning care models with patient and family goals, often finding that having these sensitive conversations in the comfort of home enables transparency and comfort not otherwise experienced by hospitalized patients.
Initial patient follow-up
For patients with COPD enrolled in the MIH program, their first patient visit is scheduled within 48 hours of discharge from the ED or inpatient hospital. In many cases, this visit can be conducted within 24 hours of returning home. Once at the patient’s home, the paramedic begins with general introductions, vital signs, and a basic physical examination. The remainder of the visit focuses on patient education and symptom recognition. The paramedic reviews the COPD action plan (Figure 1), including how to recognize the onset of a “COPD flare-up” and the appropriate response. Patients are provided with a paper copy of the action plan for future reference.

The next point of educational emphasis is the patient’s individual medication regimen. This involves differentiating between control (daily) and rescue medications, how to use oxygen tanks, and how to safely wean off of oxygen. Specific attention is given to how to use a metered-dose inhaler, as studies have found that more than half of all patients use their inhaler devices incorrectly.10
Paramedics also complete a home safety evaluation of the patient’s residence, which involves checking for tripping hazards, lighting, handrails, slippery surfaces, and general access to patient medication. If an issue cannot be resolved by the paramedic on site and is considered a safety hazard, it is reported back to the hospital team for assistance.
Finally, patients are educated on the capabilities of MIH as a program and what to expect when they reach out over the phone. Patients are given a phone number to call for both “urgent” and “nonemergent” situations. In both cases, they will be greeted by one of the MIH coordinators or nurses who assist with triaging patient symptoms, scheduling a visit, or providing other guidance. It is a point of emphasis that the patient can use MIH for more than just COPD and should call in the event of any illness or discomfort (eg, dehydration, fever) in an effort to prevent unnecessary ED visits.
Medication reconciliation
Patients with COPD often have complex medication regimens. To help alleviate any confusion, medication reconciliations are done in conjunction with every COPD patient’s initial visit. During this process, the paramedic first takes an inventory of all medications in the patient home. Common reasons for nonadherence include confusing packaging, inability to reach the pharmacy, or medication not being covered by insurance. The paramedic reconciles the updated medication regimen against the medications that are physically in the home. Once the initial review is complete, the paramedic teleconferences with a registered hospitalist pharmacist (RHP) for a more in-depth review. Over video chat, the RHP reviews each medication individually to make sure the patient understands how many times per day they take each medication, whether it is a control or rescue medication, and what times of the day to take them. The RHP will then clarify any other medication questions the patient has, assure all recent medications have been picked up from the pharmacy, and determine any barriers, such as cost or transportation.
Follow-ups and PCP involvement
At each in-person visit, paramedics coordinate with an advanced practice clinician (APC) through telehealth communication. On these video calls with a provider, the paramedic relays relevant information pertaining to patient history, vital signs, and current status. Any concerning findings, symptoms of COPD flare-ups, or recent changes in status will be discussed. The APC then speaks directly to the patient to gather additional details about their condition and any recent hospitalizations, with their primary role being to make clinical decisions on further treatment. For the COPD population, this often includes orders for the MIH paramedic to administer IV medication (ie, IV methylprednisolone or other corticosteroids), antibiotics, home nebulizers, and at-home oxygen.
Second and third follow-up paramedic visits are often less intensive. Although these visits often still involve telehealth calls to the APC, the overall focus shifts toward medication adherence, ED avoidance, and readmission avoidance. On these visits, the paramedic also checks vitals, conducts a physical examination, and completes follow-up testing or orders per the APC.
PCP involvement is critical to streamlining and transitioning patient care. Patients who are admitted to MIH without insurance or a PCP are assisted in the process of finding one. PCPs automatically receive a patient enrollment letter when their patient is seen by an MIH paramedic. Following each individual visit, paramedic and APC notes are sent to the PCP through the EMR or via fax, at which time the PCP may be consulted on patient history and/or future care decisions. After the transition back to care by their PCP, patients are still encouraged to utilize MIH if acute changes arise. If a patient is readmitted back to the hospital, MIH is automatically notified, and coordinators will assess whether there is continued need for outpatient services or areas for potential improvement.
Emergent MIH visits
While MIH visits with patients with COPD are often scheduled, MIH can also be leveraged in urgent situations to prevent the need for a patient to come to the ED or hospital. Patients with COPD are told to call MIH if they have worsening symptoms or have exhausted all methods of self-treatment without an improvement in status. In this case, a paramedic is notified and sent to the patient’s home at the earliest time possible. The paramedic then completes an assessment of the patient’s status and relays information to the MIH APC or medical director. From there, treatment decisions, such as starting the patient on an IV, using nebulizers, or doing an electrocardiogram for diagnostic purposes, are guided by the provider team with the ultimate goal of caring for the patient in the home. For our population, providing urgent care in the home has proven to be an effective way to avoid unnecessary readmissions while still ensuring high-quality patient care.
Outpatient pathway
In May 2021, select patients with COPD were given the option to participate in a more intensive MIH outpatient pathway. Pilot patients were chosen by 2 pulmonary specialists, with a focus on enrolling patients with COPD at the highest risk for readmission. Patients who opted in were followed by MIH for a total of 30 days.
The first visit was made as usual within 48 hours of discharge. Patients received education, medication reconciliation, vitals examination, home safety evaluation, and a facilitated telehealth evaluation with the APC. What differentiates the pathway from standard MIH services is that after the first visit, the follow-ups are prescheduled and more numerous. This is outlined best in Figure 2, which serves as a guideline for coordinators and paramedics in the cadence and focus of visits for each patient on the pathway. The initial 2 weeks are designed to check in on the patient in person and ensure active recovery. The latter 2 weeks are designed to ensure that the patient follows up with their care team and understands their medications and action plan going forward. Pathway patients were also monitored using a remote patient monitoring (RPM) kit. On the initial visit, paramedics set up the RPM equipment and provided a demonstration on how to use each device. Patients were issued a Bluetooth-enabled scale, blood pressure cuff, video-enabled tablet, and wearable device. The wearable device continuously recorded respiration rate, heart rate, and oxygen saturation and had fall-detection enabled. Over the course of a month, an experienced MIH nurse monitored the vitals transmitted by the wearable device and checked patient weight and blood pressure 1 to 2 times per day, utilizing these data to proactively outreach to patients if abnormalities occurred. Prior to the start of the program, the MIH nurse contacted each patient to introduce herself and notify them that they would receive a call if any vitals were unusual.

Results
MIH treated 214 patients with COPD from March 2, 2020, to August 2, 2021. In total, paramedics made more than 650 visits. Eighty-seven of these were documented as urgent visits with AECOPD, shortness of breath, cough, or wheezing as the primary concern.
In the calendar year of 2019, our institution admitted 804 patients with a primary diagnosis of COPD. In 2020, the first year with MIH, total COPD admissions decreased to 473; however, the effect of the COVID-19 pandemic cannot be discounted. At of the time of writing—219 days into 2021—253 patients with COPD have been admitted thus far (Table 1).

Pathway results
Sixteen patients were referred to the MIH COPD Discharge Pathway Pilot during May 2021. Ten patients went on to complete the entire 30-day pathway. Six did not finish the program. Three of these 6 patients were referred by a pulmonary specialist for enrollment but not ultimately referred to the pilot program by case management and therefore not enrolled. The other 3 of the 6 patients who did not complete the pilot program were enrolled but discontinued owing to noncompliance.
Of the 10 patients who completed the pathway, 3 patients were male, and 7 were female. Ages ranged from 55 to 84 years. On average, the RHP found 3.6 medication reconciliation errors per patient. One patient was readmitted within 30 days (only 3 days after the initial discharge), and 5 were readmitted within 90 days.
A retrospective analysis was conducted on patients with COPD who were not provided with MIH services and were admitted to our hospital between September 1, 2020, and March 1, 2021, for comparison. Age, sex, and other related conditions are shown in Table 2. Medication reconciliation error data were not tracked for this demographic, as they did not have an in-home medication reconciliation completed.

Discussion
MIH has treated 214 patients with COPD from March 2, 2020, to August 2, 2021, a 17-month period. In that same timeframe, the hospital experienced a 42% decrease in COPD admissions. Although this effect is not the sole product of MIH (specifically, COVID-19 caused a drop in all-cause hospital admissions), we believe MIH did play a small role in this reduction. Eighty-seven emergent visits were conducted for patients with a primary complaint of AECOPD, shortness of breath, cough, or wheezing. On these visits, MIH provided urgent treatment to prevent the patient returning to the ED and potentially leading to readmission.
The program’s impact extends beyond the numbers. With more than 200 patients with COPD treated at home, we improved hospital flow, shortened patients’ overall length of stay, and increased capacity in the ED and inpatient units. In addition, MIH has been able to fill in care gaps present in the current health care system by providing acute care in the home to patients who otherwise have access-to-care and transportation issues.
What made the program successful
With the COPD population prone to having complex medication regimens, medication reconciliations were critical to improving patient outcomes. During the documented medication reconciliations for pathway patients, 8 of 10 patients had medication errors identified. Some of the more common errors included incorrect inhaler usage, patient medication not arriving to the pharmacy for a week or more after discharge, prescribed medication dosages that were too high or too low, and a lack of transportation to pick up the patient’s prescription. Even more problematic is that 7 of these 8 patients required multiple interventions to correct their regimen. What was cited as most beneficial by both the paramedic and the RHP was taking time to walk through each medication individually and ensuring that the patient could recite back how often and when they should be using it. What also proved to be helpful was spending extra time on the inhalers and nebulizers. Multiple patients did not know how to use them properly and/or cited a history of struggling with them.
The MIH COPD pathway patients showed encouraging preliminary results. In the initial 30-day window, only 1 of 10 (10%) patients was readmitted, which is lower than the 37.7% rate for comparable patients who did not have MIH services. This could imply that patients with COPD respond positively to active and consistent management with predetermined points of contact. Ninety-day readmission rates jumped to 5 of 10, with 4 of these patients being readmitted multiple times. Approximately half of these readmissions were COPD related. It is important to remember that the patients being targeted by the pathway are deemed to be at very high risk of readmission. As such, one could expect that even with a successful reduction in rates, pathway patient readmission rates may be slightly elevated compared with national COPD averages.
Given the more personalized and at-home care, patients also expressed higher levels of care satisfaction. Most patients want to avoid the hospital at all costs, and MIH provides a safe and effective alternative. Patients with COPD have also relayed that the education they receive on their medication, disease, and how to use MIH has been useful. This is reflected in the volume of urgent calls that MIH receives. A patient calling MIH in place of 911 shows not only that the patient has a level of trust in the MIH team, but also that they have learned how to recognize symptoms earlier to prevent major flare-ups.
This study had several limitations. On the pilot pathway, 3 patients were removed from MIH services because of repeated noncompliance. These instances primarily involved aggression toward the paramedics, both verbal and physical, as well as refusal to allow the MIH paramedics into the home. Going forward, it will be valuable to have a screening process for pathway patients to determine likelihood of compliance. This could include speaking to the patient’s PCP or other in-hospital providers before accepting them into the program.
Remote patient monitoring also presented its challenges. Despite extensive equipment demonstrations, some patients struggled to grasp the technology. Some of the biggest problems cited were confusion operating the tablet, inability to charge the devices, and issues with connectivity. In the future, it may be useful to simplify the devices even more. Further work should also be done to evaluate the efficacy of remote patient technology in this specific setting, as studies have shown varied results with regard to RPM success. In 1 meta-analysis of 91 different published studies that took place between 2015 and 2020, approximately half of the RPM studies resulted in no change in hospital readmissions, length of stay, or ED presentations, while the other half saw improvement in these categories.11 We suspect that the greatest benefits of our work came from the patient education, trust built over time, in-home urgent evaluations, and 1-on-1 time with the paramedic.
With many people forgoing care during the pandemic, COVID-19 has also caused a downward trend in overall and non-COVID-19 admissions. In a review of more than 500 000 ED visits in Massachusetts between March 11, 2020, and September 8, 2021, there was a 32% decrease in admissions when compared with those same weeks in 2019.10 There was an even greater drop-off when it came to COPD and other respiratory-related admissions. In evaluating the impact SSH MIH has made, it is important to recognize that the pandemic contributed to reducing total COPD admissions. Adding merit to the success of MIH in contributing to the reduction in admissions is the continued downward trend in total COPD admissions year-to-date in 2021. Despite total hospital usage rates increasing at our institution over the course of this year, the overall COPD usage rates have remained lower than before.
Another limitation is that in the selection of patients, both for general MIH care and for the COPD pathway, there was room for bias. The pilot pathway was offered specifically to patients at the highest risk for readmission; however, patients were referred at the discretion of our pulmonologist care team and not selected by any standardized rubric. Additionally, MIH only operates on a 16-hour schedule. This means that patients admitted to the ED or inpatient at night may sometimes be missed and not referred to MIH for care.
The biggest caveat to the pathway results is, of course, the small sample size. With only 10 patients completing the pilot, it is impossible to come to any concrete conclusions. Such an intensive pathway requires dedicating large amounts of time and resources, which is why the pilot was small. However, considering the preliminary results, the outline given could provide a starting point for future work to evaluate a similar COPD pathway on a larger scale.
Future considerations
Risk stratification of patients is critical to achieving even further reductions in readmissions and mortality. Hospitals can get the most value from MIH by focusing on patients with COPD at the highest risk for return, and it would be valuable to explicitly define who fits into this criterion. Utilizing a tool similar to the LACE index for readmission but tailoring it to patients with COPD when admitting patients into the program would be a logical next step.
Reducing the points of patient contact could also prove valuable. Over the course of a patient’s time with MIH, they interact with an RHP, APC, paramedic, RN, and discharging hospitalist. Additionally, we found many patients had VNA services, home health aides, care managers, and/or social workers involved in their care. Some patients found this to be stressful and overwhelming, especially regarding the number of outreach calls soon after discharge.
It would also be useful to look at the impact of MIH on total COPD admissions independent of the artificial variation created by COVID-19. This may require waiting until there are higher levels of vaccination and/or finding ways to control for the potential variation. In doing so, one could look at the direct effect MIH has on COPD readmissions when compared with a control group without MIH services, which could then serve as a comparison point to the results of this study. As it stands, given the relative novelty of MIH, there are primarily only broad reviews of MIH’s effectiveness and/or impact on patient populations that have been published. Of these, only a few directly mentioned MIH in relation to COPD, and none have comparable designs that look at overall COPD hospitalization reductions post-MIH implementation. There is also little to no literature looking at the utilization of MIH in a more intensive COPD outpatient pathway.
Finally, MIH has proven to be a useful tool for our institution in many areas outside of COPD management. Specifically, MIH has been utilized as a mobile influenza and COVID-19 vaccination unit and in-home testing service and now operates both a hospital-at-home and skilled nursing facility-at-home program. Analysis of the overall needs of the system and where this valuable MIH resource would have the biggest impact will be key in future growth opportunities.
Conclusion
MIH has been an invaluable tool for our hospital, especially in light of the recent shift toward more in-home and virtual care. MIH cared for 214 patients with COPD with more than 650 visits between March 2020 and August 2021. Eighty-seven emergent COPD visits were conducted, and COPD admissions were reduced dramatically from 2019 to 2020. MIH services have improved hospital flow, allowed for earlier discharge from the ED and inpatient care, and helped improve all-cause COPD readmission rates. The importance of postdischarge care and follow-up visits for patients with COPD, especially those at higher risk for readmission, cannot be understated. We hope our experience working to improve COPD patient outcomes serves as valuable a reference point for future MIH programs.
Corresponding author: Kelly Lannutti, DO, Mobile Integrated Health and Emergency Medicine Department, South Shore Health, 55 Fogg Rd, South Weymouth, MA 02190; [email protected].
Financial disclosures: None.
From the Mobile Integrated Health and Emergency Medicine Department, South Shore Health, Weymouth, MA.
Objective: To develop a process through which Mobile Integrated Health (MIH) can treat patients with chronic obstructive pulmonary disease (COPD) at high risk for readmission in an outpatient setting. In turn, South Shore Hospital (SSH) looks to leverage MIH to improve hospital flow, decrease costs, and improve patient quality of life.
Methods: With the recent approval of hospital-based MIH programs in Massachusetts, SSH used MIH to target specific patient demographics in an at-home setting. Here, we describe the planning and implementation of this program for patients with COPD. Key components to success include collaboration among providers, early follow-up visits, patient education, and in-depth medical reconciliations. Analysis includes a retrospective examination of a structured COPD outpatient pathway.
Results: A total of 214 patients with COPD were treated with MIH from March 2, 2020, to August 1, 2021. Eighty-seven emergent visits were conducted, and more than 650 total visits were made. A more intensive outpatient pathway was implemented for patients deemed to be at the highest risk for readmission by pulmonary specialists.
Conclusion: This process can serve as a template for future institutions to treat patients with COPD using MIH or similar hospital-at-home services.
Keywords: Mobile Integrated Health; MIH; COPD; population health.
It is estimated that chronic obstructive pulmonary disease (COPD) affects more than 16 million Americans1 and accounts for more than 700 000 hospitalizations each year in the US.2 Thirty-day COPD readmission rates hover around 22.6%,3 and readmission within 90 days of initial discharge can jump to between 31% and 35%.4 This is the highest of any patient demographic, and more than half of these readmissions are due to COPD. To counter this, government and state entities have made nationwide efforts to encourage health systems to focus on preventing readmissions. In October 2014, the US added COPD to the active list of diseases in Medicare’s Hospital Readmissions Reduction Program (HRRP), later adding COPD to various risk-based bundle programs that hospitals may choose to opt into. These programs are designed to reduce all-cause readmissions after an acute exacerbation of COPD, as the HRRP penalizes hospitals for all-cause 30-day readmissions.3 However, what is most troubling is that, despite these efforts, readmission rates have not dropped in the past decade.5 COPD remains the third leading cause of death in America and still poses a significant burden both clinically and economically to hospitals across the country.3
A solution that is gaining traction is to encourage outpatient care initiatives and discharge pathways. Early follow-up is proven to decrease chances of readmission, and studies have shown that more than half of readmitted patients did not follow up with a primary care physician (PCP) within 30 days of their initial discharge.6 Additionally, large meta-analyses show hospital-at-home–type programs can lead to reductions in mortality, decrease costs, decrease readmissions, and increase patient satisfaction.7-9 Therefore, for more challenging patient populations with regard to readmissions and mortality, Mobile Integrated Health (MIH) may be the solution that we are looking for.
This article presents a viable process to treat patients with COPD in an outpatient setting with MIH Services. It includes an examination of what makes MIH successful as well as a closer look at a structured COPD outpatient pathway.
Methods
South Shore Hospital (SSH) is an independent, not-for-profit hospital located in Weymouth, Massachusetts. It is host to 400 beds, 100 000 annual visits to the emergency department (ED), and its own emergency medical services program. In March 2020, SSH became the first Massachusetts hospital-based program to acquire an MIH license. MIH paramedics receive 300 hours of specialized training, including time in clinical clerkships shadowing pulmonary specialists, cardiology/congestive heart failure (CHF) providers, addiction medicine specialists, home care and care progression colleagues, and wound center providers. Specialist providers become more comfortable with paramedic capabilities as a result of these clerkships, improving interactions and relationships going forward. At the time of writing, SSH MIH is staffed by 12 paramedics, 4 of whom are full time; 2 medical directors; 2 internal coordinators; and 1 registered nurse (RN). A minimum of 2 paramedics are on call each day, each with twice-daily intravenous (IV) capabilities. The first shift slot is 16 hours, from 7:00 AM to 11:00
The goal of developing MIH is to improve upon the current standard of care. For hospitals without MIH capabilities, there are limited options to treat acute exacerbations of chronic obstructive pulmonary disease (AECOPD) patients postdischarge. It is common for the only outpatient referral to be a lone PCP visit, and many patients who need more extensive treatment options don’t have access to a timely PCP follow-up or resources for alternative care. This is part of why there has been little improvement in the 21st century with regard to reducing COPD hospitalizations. As it stands, approximately 10% to 55% of all AECOPD readmissions are preventable, and more than one-fifth of patients with COPD are rehospitalized within 30 days of discharge.3 In response, MIH has been designed to provide robust care options postdischarge in the patient home, with the eventual goal of reducing preventable hospitalizations and readmissions for all patients with COPD.
Patient selection
Patients with COPD are admitted to the MIH program in 1 of 3 ways: (1) directly from the ED; (2) at discharge from inpatient care; or (3) from a SSH affiliate referral.
With option 1, the ED physician assesses patient need for MIH services and places a referral to MIH in the electronic medical record (EMR). The ED provider also specifies whether follow-up is “urgent” and sets an alternative level of priority if not. With option 2, the inpatient provider and case manager follow a similar process, first determining whether a patient is stable enough to go home with outpatient services and then if MIH would be beneficial to the patient. If the patient is discharged home, a follow-up visit by an MIH paramedic is scheduled within 48 hours. With option 3, the patient is referred to MIH by an affiliate of SSH. This can be through the patient’s PCP, their visiting nurse association (VNA) service provider, or through any SSH urgent care center. In all 3 referral processes, the patient has the option to consent into the program or refuse services. Once referred, MIH coordinators review patients on a case-by-case basis. Patients with a history of prior admissions are given preference, with the goal being to keep the frailer, older, and comorbid patients at home. Other considerations include recent admission(s), length of stay, and overall stability. Social factors considered by the team include whether the patient lives alone and has alternative home services and the patient’s total distance from the hospital. Patients with a history of violence, mental health concerns, or substance abuse go through a more extensive screening process to ensure paramedic safety.
Given their patient profile and high hospital usage rates, MIH is sometimes requested for patients with end-stage COPD. Many of these patients benefit from MIH goals-of-care conversations to ensure they understand all their options and choose an approach that fits their preferences. In these cases, MIH has been instrumental in assisting patients and families with completing Medical Orders for Life-Sustaining Treatment and health care proxy forms and transitioning patients to palliative care, hospice, advanced-illness care management programs, or other long-term care options to prevent the need for rehospitalization. The MIH team focuses heavily on providing quality end-of-life care for patients and aligning care models with patient and family goals, often finding that having these sensitive conversations in the comfort of home enables transparency and comfort not otherwise experienced by hospitalized patients.
Initial patient follow-up
For patients with COPD enrolled in the MIH program, their first patient visit is scheduled within 48 hours of discharge from the ED or inpatient hospital. In many cases, this visit can be conducted within 24 hours of returning home. Once at the patient’s home, the paramedic begins with general introductions, vital signs, and a basic physical examination. The remainder of the visit focuses on patient education and symptom recognition. The paramedic reviews the COPD action plan (Figure 1), including how to recognize the onset of a “COPD flare-up” and the appropriate response. Patients are provided with a paper copy of the action plan for future reference.

The next point of educational emphasis is the patient’s individual medication regimen. This involves differentiating between control (daily) and rescue medications, how to use oxygen tanks, and how to safely wean off of oxygen. Specific attention is given to how to use a metered-dose inhaler, as studies have found that more than half of all patients use their inhaler devices incorrectly.10
Paramedics also complete a home safety evaluation of the patient’s residence, which involves checking for tripping hazards, lighting, handrails, slippery surfaces, and general access to patient medication. If an issue cannot be resolved by the paramedic on site and is considered a safety hazard, it is reported back to the hospital team for assistance.
Finally, patients are educated on the capabilities of MIH as a program and what to expect when they reach out over the phone. Patients are given a phone number to call for both “urgent” and “nonemergent” situations. In both cases, they will be greeted by one of the MIH coordinators or nurses who assist with triaging patient symptoms, scheduling a visit, or providing other guidance. It is a point of emphasis that the patient can use MIH for more than just COPD and should call in the event of any illness or discomfort (eg, dehydration, fever) in an effort to prevent unnecessary ED visits.
Medication reconciliation
Patients with COPD often have complex medication regimens. To help alleviate any confusion, medication reconciliations are done in conjunction with every COPD patient’s initial visit. During this process, the paramedic first takes an inventory of all medications in the patient home. Common reasons for nonadherence include confusing packaging, inability to reach the pharmacy, or medication not being covered by insurance. The paramedic reconciles the updated medication regimen against the medications that are physically in the home. Once the initial review is complete, the paramedic teleconferences with a registered hospitalist pharmacist (RHP) for a more in-depth review. Over video chat, the RHP reviews each medication individually to make sure the patient understands how many times per day they take each medication, whether it is a control or rescue medication, and what times of the day to take them. The RHP will then clarify any other medication questions the patient has, assure all recent medications have been picked up from the pharmacy, and determine any barriers, such as cost or transportation.
Follow-ups and PCP involvement
At each in-person visit, paramedics coordinate with an advanced practice clinician (APC) through telehealth communication. On these video calls with a provider, the paramedic relays relevant information pertaining to patient history, vital signs, and current status. Any concerning findings, symptoms of COPD flare-ups, or recent changes in status will be discussed. The APC then speaks directly to the patient to gather additional details about their condition and any recent hospitalizations, with their primary role being to make clinical decisions on further treatment. For the COPD population, this often includes orders for the MIH paramedic to administer IV medication (ie, IV methylprednisolone or other corticosteroids), antibiotics, home nebulizers, and at-home oxygen.
Second and third follow-up paramedic visits are often less intensive. Although these visits often still involve telehealth calls to the APC, the overall focus shifts toward medication adherence, ED avoidance, and readmission avoidance. On these visits, the paramedic also checks vitals, conducts a physical examination, and completes follow-up testing or orders per the APC.
PCP involvement is critical to streamlining and transitioning patient care. Patients who are admitted to MIH without insurance or a PCP are assisted in the process of finding one. PCPs automatically receive a patient enrollment letter when their patient is seen by an MIH paramedic. Following each individual visit, paramedic and APC notes are sent to the PCP through the EMR or via fax, at which time the PCP may be consulted on patient history and/or future care decisions. After the transition back to care by their PCP, patients are still encouraged to utilize MIH if acute changes arise. If a patient is readmitted back to the hospital, MIH is automatically notified, and coordinators will assess whether there is continued need for outpatient services or areas for potential improvement.
Emergent MIH visits
While MIH visits with patients with COPD are often scheduled, MIH can also be leveraged in urgent situations to prevent the need for a patient to come to the ED or hospital. Patients with COPD are told to call MIH if they have worsening symptoms or have exhausted all methods of self-treatment without an improvement in status. In this case, a paramedic is notified and sent to the patient’s home at the earliest time possible. The paramedic then completes an assessment of the patient’s status and relays information to the MIH APC or medical director. From there, treatment decisions, such as starting the patient on an IV, using nebulizers, or doing an electrocardiogram for diagnostic purposes, are guided by the provider team with the ultimate goal of caring for the patient in the home. For our population, providing urgent care in the home has proven to be an effective way to avoid unnecessary readmissions while still ensuring high-quality patient care.
Outpatient pathway
In May 2021, select patients with COPD were given the option to participate in a more intensive MIH outpatient pathway. Pilot patients were chosen by 2 pulmonary specialists, with a focus on enrolling patients with COPD at the highest risk for readmission. Patients who opted in were followed by MIH for a total of 30 days.
The first visit was made as usual within 48 hours of discharge. Patients received education, medication reconciliation, vitals examination, home safety evaluation, and a facilitated telehealth evaluation with the APC. What differentiates the pathway from standard MIH services is that after the first visit, the follow-ups are prescheduled and more numerous. This is outlined best in Figure 2, which serves as a guideline for coordinators and paramedics in the cadence and focus of visits for each patient on the pathway. The initial 2 weeks are designed to check in on the patient in person and ensure active recovery. The latter 2 weeks are designed to ensure that the patient follows up with their care team and understands their medications and action plan going forward. Pathway patients were also monitored using a remote patient monitoring (RPM) kit. On the initial visit, paramedics set up the RPM equipment and provided a demonstration on how to use each device. Patients were issued a Bluetooth-enabled scale, blood pressure cuff, video-enabled tablet, and wearable device. The wearable device continuously recorded respiration rate, heart rate, and oxygen saturation and had fall-detection enabled. Over the course of a month, an experienced MIH nurse monitored the vitals transmitted by the wearable device and checked patient weight and blood pressure 1 to 2 times per day, utilizing these data to proactively outreach to patients if abnormalities occurred. Prior to the start of the program, the MIH nurse contacted each patient to introduce herself and notify them that they would receive a call if any vitals were unusual.

Results
MIH treated 214 patients with COPD from March 2, 2020, to August 2, 2021. In total, paramedics made more than 650 visits. Eighty-seven of these were documented as urgent visits with AECOPD, shortness of breath, cough, or wheezing as the primary concern.
In the calendar year of 2019, our institution admitted 804 patients with a primary diagnosis of COPD. In 2020, the first year with MIH, total COPD admissions decreased to 473; however, the effect of the COVID-19 pandemic cannot be discounted. At of the time of writing—219 days into 2021—253 patients with COPD have been admitted thus far (Table 1).

Pathway results
Sixteen patients were referred to the MIH COPD Discharge Pathway Pilot during May 2021. Ten patients went on to complete the entire 30-day pathway. Six did not finish the program. Three of these 6 patients were referred by a pulmonary specialist for enrollment but not ultimately referred to the pilot program by case management and therefore not enrolled. The other 3 of the 6 patients who did not complete the pilot program were enrolled but discontinued owing to noncompliance.
Of the 10 patients who completed the pathway, 3 patients were male, and 7 were female. Ages ranged from 55 to 84 years. On average, the RHP found 3.6 medication reconciliation errors per patient. One patient was readmitted within 30 days (only 3 days after the initial discharge), and 5 were readmitted within 90 days.
A retrospective analysis was conducted on patients with COPD who were not provided with MIH services and were admitted to our hospital between September 1, 2020, and March 1, 2021, for comparison. Age, sex, and other related conditions are shown in Table 2. Medication reconciliation error data were not tracked for this demographic, as they did not have an in-home medication reconciliation completed.

Discussion
MIH has treated 214 patients with COPD from March 2, 2020, to August 2, 2021, a 17-month period. In that same timeframe, the hospital experienced a 42% decrease in COPD admissions. Although this effect is not the sole product of MIH (specifically, COVID-19 caused a drop in all-cause hospital admissions), we believe MIH did play a small role in this reduction. Eighty-seven emergent visits were conducted for patients with a primary complaint of AECOPD, shortness of breath, cough, or wheezing. On these visits, MIH provided urgent treatment to prevent the patient returning to the ED and potentially leading to readmission.
The program’s impact extends beyond the numbers. With more than 200 patients with COPD treated at home, we improved hospital flow, shortened patients’ overall length of stay, and increased capacity in the ED and inpatient units. In addition, MIH has been able to fill in care gaps present in the current health care system by providing acute care in the home to patients who otherwise have access-to-care and transportation issues.
What made the program successful
With the COPD population prone to having complex medication regimens, medication reconciliations were critical to improving patient outcomes. During the documented medication reconciliations for pathway patients, 8 of 10 patients had medication errors identified. Some of the more common errors included incorrect inhaler usage, patient medication not arriving to the pharmacy for a week or more after discharge, prescribed medication dosages that were too high or too low, and a lack of transportation to pick up the patient’s prescription. Even more problematic is that 7 of these 8 patients required multiple interventions to correct their regimen. What was cited as most beneficial by both the paramedic and the RHP was taking time to walk through each medication individually and ensuring that the patient could recite back how often and when they should be using it. What also proved to be helpful was spending extra time on the inhalers and nebulizers. Multiple patients did not know how to use them properly and/or cited a history of struggling with them.
The MIH COPD pathway patients showed encouraging preliminary results. In the initial 30-day window, only 1 of 10 (10%) patients was readmitted, which is lower than the 37.7% rate for comparable patients who did not have MIH services. This could imply that patients with COPD respond positively to active and consistent management with predetermined points of contact. Ninety-day readmission rates jumped to 5 of 10, with 4 of these patients being readmitted multiple times. Approximately half of these readmissions were COPD related. It is important to remember that the patients being targeted by the pathway are deemed to be at very high risk of readmission. As such, one could expect that even with a successful reduction in rates, pathway patient readmission rates may be slightly elevated compared with national COPD averages.
Given the more personalized and at-home care, patients also expressed higher levels of care satisfaction. Most patients want to avoid the hospital at all costs, and MIH provides a safe and effective alternative. Patients with COPD have also relayed that the education they receive on their medication, disease, and how to use MIH has been useful. This is reflected in the volume of urgent calls that MIH receives. A patient calling MIH in place of 911 shows not only that the patient has a level of trust in the MIH team, but also that they have learned how to recognize symptoms earlier to prevent major flare-ups.
This study had several limitations. On the pilot pathway, 3 patients were removed from MIH services because of repeated noncompliance. These instances primarily involved aggression toward the paramedics, both verbal and physical, as well as refusal to allow the MIH paramedics into the home. Going forward, it will be valuable to have a screening process for pathway patients to determine likelihood of compliance. This could include speaking to the patient’s PCP or other in-hospital providers before accepting them into the program.
Remote patient monitoring also presented its challenges. Despite extensive equipment demonstrations, some patients struggled to grasp the technology. Some of the biggest problems cited were confusion operating the tablet, inability to charge the devices, and issues with connectivity. In the future, it may be useful to simplify the devices even more. Further work should also be done to evaluate the efficacy of remote patient technology in this specific setting, as studies have shown varied results with regard to RPM success. In 1 meta-analysis of 91 different published studies that took place between 2015 and 2020, approximately half of the RPM studies resulted in no change in hospital readmissions, length of stay, or ED presentations, while the other half saw improvement in these categories.11 We suspect that the greatest benefits of our work came from the patient education, trust built over time, in-home urgent evaluations, and 1-on-1 time with the paramedic.
With many people forgoing care during the pandemic, COVID-19 has also caused a downward trend in overall and non-COVID-19 admissions. In a review of more than 500 000 ED visits in Massachusetts between March 11, 2020, and September 8, 2021, there was a 32% decrease in admissions when compared with those same weeks in 2019.10 There was an even greater drop-off when it came to COPD and other respiratory-related admissions. In evaluating the impact SSH MIH has made, it is important to recognize that the pandemic contributed to reducing total COPD admissions. Adding merit to the success of MIH in contributing to the reduction in admissions is the continued downward trend in total COPD admissions year-to-date in 2021. Despite total hospital usage rates increasing at our institution over the course of this year, the overall COPD usage rates have remained lower than before.
Another limitation is that in the selection of patients, both for general MIH care and for the COPD pathway, there was room for bias. The pilot pathway was offered specifically to patients at the highest risk for readmission; however, patients were referred at the discretion of our pulmonologist care team and not selected by any standardized rubric. Additionally, MIH only operates on a 16-hour schedule. This means that patients admitted to the ED or inpatient at night may sometimes be missed and not referred to MIH for care.
The biggest caveat to the pathway results is, of course, the small sample size. With only 10 patients completing the pilot, it is impossible to come to any concrete conclusions. Such an intensive pathway requires dedicating large amounts of time and resources, which is why the pilot was small. However, considering the preliminary results, the outline given could provide a starting point for future work to evaluate a similar COPD pathway on a larger scale.
Future considerations
Risk stratification of patients is critical to achieving even further reductions in readmissions and mortality. Hospitals can get the most value from MIH by focusing on patients with COPD at the highest risk for return, and it would be valuable to explicitly define who fits into this criterion. Utilizing a tool similar to the LACE index for readmission but tailoring it to patients with COPD when admitting patients into the program would be a logical next step.
Reducing the points of patient contact could also prove valuable. Over the course of a patient’s time with MIH, they interact with an RHP, APC, paramedic, RN, and discharging hospitalist. Additionally, we found many patients had VNA services, home health aides, care managers, and/or social workers involved in their care. Some patients found this to be stressful and overwhelming, especially regarding the number of outreach calls soon after discharge.
It would also be useful to look at the impact of MIH on total COPD admissions independent of the artificial variation created by COVID-19. This may require waiting until there are higher levels of vaccination and/or finding ways to control for the potential variation. In doing so, one could look at the direct effect MIH has on COPD readmissions when compared with a control group without MIH services, which could then serve as a comparison point to the results of this study. As it stands, given the relative novelty of MIH, there are primarily only broad reviews of MIH’s effectiveness and/or impact on patient populations that have been published. Of these, only a few directly mentioned MIH in relation to COPD, and none have comparable designs that look at overall COPD hospitalization reductions post-MIH implementation. There is also little to no literature looking at the utilization of MIH in a more intensive COPD outpatient pathway.
Finally, MIH has proven to be a useful tool for our institution in many areas outside of COPD management. Specifically, MIH has been utilized as a mobile influenza and COVID-19 vaccination unit and in-home testing service and now operates both a hospital-at-home and skilled nursing facility-at-home program. Analysis of the overall needs of the system and where this valuable MIH resource would have the biggest impact will be key in future growth opportunities.
Conclusion
MIH has been an invaluable tool for our hospital, especially in light of the recent shift toward more in-home and virtual care. MIH cared for 214 patients with COPD with more than 650 visits between March 2020 and August 2021. Eighty-seven emergent COPD visits were conducted, and COPD admissions were reduced dramatically from 2019 to 2020. MIH services have improved hospital flow, allowed for earlier discharge from the ED and inpatient care, and helped improve all-cause COPD readmission rates. The importance of postdischarge care and follow-up visits for patients with COPD, especially those at higher risk for readmission, cannot be understated. We hope our experience working to improve COPD patient outcomes serves as valuable a reference point for future MIH programs.
Corresponding author: Kelly Lannutti, DO, Mobile Integrated Health and Emergency Medicine Department, South Shore Health, 55 Fogg Rd, South Weymouth, MA 02190; [email protected].
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease (COPD). Accessed September 10, 2011. https://www.cdc.gov/copd/index.html
2. Wier LM, Elixhauser A, Pfuntner A, AuDH. Overview of Hospitalizations among Patients with COPD, 2008. Statistical Brief #106. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2011.
3. Shah T, Press,VG, Huisingh-Scheetz M, White SR. COPD Readmissions: Addressing COPD in the Era of Value-Based Health Care. Chest. 2016;150(4):916-926. doi:10.1016/j.chest.2016.05.002
4. Harries TH, Thornton H, Crichton S, et al. Hospital readmissions for COPD: a retrospective longitudinal study. NPJ Prim Care Respir Med. 2017;27(1):31. doi:10.1038/s41533-017-0028-8
5. Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001-2012 and Nationwide Emergency Department Sample 2006-2011. Chest. 2015;147(4):989-998. doi:10.1378/chest.14-2146
6. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563
7. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi:10.1503/cmaj.081491
8. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital in the home.” Med J Aust. 2012;197(9):512-519. doi:10.5694/mja12.10480
9. Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
10. Nourazari S, Davis SR, Granovsky R, et al. Decreased hospital admissions through emergency departments during the COVID-19 pandemic. Am J Emerg Med. 2021;42:203-210. doi:10.1016/j.ajem.2020.11.029
11. Taylor ML, Thomas EE, Snoswell CL, et al. Does remote patient monitoring reduce acute care use? A systematic review. BMJ Open. 2021;11(3):e040232. doi:10.1136/bmj/open-2020-040232
1. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease (COPD). Accessed September 10, 2011. https://www.cdc.gov/copd/index.html
2. Wier LM, Elixhauser A, Pfuntner A, AuDH. Overview of Hospitalizations among Patients with COPD, 2008. Statistical Brief #106. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2011.
3. Shah T, Press,VG, Huisingh-Scheetz M, White SR. COPD Readmissions: Addressing COPD in the Era of Value-Based Health Care. Chest. 2016;150(4):916-926. doi:10.1016/j.chest.2016.05.002
4. Harries TH, Thornton H, Crichton S, et al. Hospital readmissions for COPD: a retrospective longitudinal study. NPJ Prim Care Respir Med. 2017;27(1):31. doi:10.1038/s41533-017-0028-8
5. Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001-2012 and Nationwide Emergency Department Sample 2006-2011. Chest. 2015;147(4):989-998. doi:10.1378/chest.14-2146
6. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563
7. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi:10.1503/cmaj.081491
8. Caplan GA, Sulaiman NS, Mangin DA, et al. A meta-analysis of “hospital in the home.” Med J Aust. 2012;197(9):512-519. doi:10.5694/mja12.10480
9. Portillo EC, Wilcox A, Seckel E, et al. Reducing COPD readmission rates: using a COPD care service during care transitions. Fed Pract. 2018;35(11):30-36.
10. Nourazari S, Davis SR, Granovsky R, et al. Decreased hospital admissions through emergency departments during the COVID-19 pandemic. Am J Emerg Med. 2021;42:203-210. doi:10.1016/j.ajem.2020.11.029
11. Taylor ML, Thomas EE, Snoswell CL, et al. Does remote patient monitoring reduce acute care use? A systematic review. BMJ Open. 2021;11(3):e040232. doi:10.1136/bmj/open-2020-040232
Cardiogenic shock teams again tied to lower mortality
A large multicenter study provides further evidence supporting the rationale for multidisciplinary teams for cardiogenic shock, one of the most lethal diseases in cardiovascular medicine.
The analysis of 24 critical care ICUs in the Critical Care Cardiology Trials Network showed that the presence of a shock team was independently associated with a 28% lower risk for CICU mortality (23% vs. 29%; odds ratio, 0.72; P = .016).
Patients treated by a shock team also had significantly shorter CICU stays and less need for mechanical ventilation or renal replacement therapy, as reported in the Journal of the American College of Cardiology.
“It’s observational, but the association that we’re seeing here, just because of our sample size, is the strongest that’s been published yet,” lead author Alexander Papolos, MD, MedStar Washington Hospital Center, said in an interview.
Although a causal relationship cannot be drawn, the authors suggest several factors that could explain the findings, including a shock team’s ability to rapidly diagnose and treat cardiogenic shock before multiorgan dysfunction occurs.
Centers with shock teams also used significantly more pulmonary artery catheters (60% vs. 49%; adjusted OR, 1.86; P < .001) and placed them earlier (0.3 vs. 0.66 days; P = .019).
Pulmonary artery catheter (PAC) use has declined after earlier trials like ESCAPE showed little or no benefit in other acutely ill patient groups, but positive results have been reported recently in cardiogenic shock, where a PAC is needed to determine the severity of the lesion and the phenotype, Dr. Papolos observed.
A 2018 study showed PAC use was tied to increased survival among patients with acute myocardial infarction cardiogenic shock (AMI-CS) supported with the Impella (Abiomed) device. Additionally, a 2021 study by the Cardiogenic Shock Working Group demonstrated a dose-dependent survival response based on the completeness of hemodynamic assessment by PAC prior to initiating mechanical circulatory support (MCS).
A third factor might be that a structured, team-based evaluation can facilitate timely and optimal MCS device selection, deployment, and management, suggested Dr. Papolos.
Centers with shock teams used more advanced types of MCS – defined as Impella, TandemHeart (LivaNova), extracorporeal membrane oxygenation, and temporary or durable surgical ventricular assist devices – than those without a shock team (53% vs. 43%; adjusted OR, 1.73; P = .005) and did so more often as the initial device (42% vs. 28%; P = .002).
Overall MCS use was lower at shock team centers (35% vs. 43%), driven by less frequent use of intra-aortic balloon pumps (58% vs. 72%).
“The standard, basic MCS has always been the balloon pump because it’s something that’s easy to put in at the cath lab or at the bedside,” Dr. Papolos said. “So, if you take away having all of the information and having the right people at the table to discuss what the best level of support is, then you’re going to end up with balloon pumps, and that’s what we saw here.”
The study involved 6,872 consecutive medical admissions at 24 level 1 CICU centers during an annual 2-month period from 2017 to 2019. Of these, 1,242 admissions were for cardiogenic shock and 546 (44%) were treated at one of 10 centers with a shock team.
Shock team centers had higher-acuity patients than centers without a shock team (Sequential Organ Failure Assessment score, 4 vs. 3) but a similar proportion of patients with AMI-CS (27% vs. 28%).
Among all admissions, CICU mortality was not significantly different between centers with and without a shock team.
For cardiogenic shock patients treated at centers with and without a shock team, the median CICU stay was 4.0 and 5.1 days, respectively, mechanical ventilation was used in 41% and 52%, respectively, and new renal replacement therapy in 11% and 19%, respectively (P < .001 for all).
Shock team centers used significantly more PACs for AMI-CS and non–AMI-CS admissions; advanced MCS therapy was also greater in the AMI-CS subgroup.
Lower CICU mortality at shock team centers persisted among patients with non-AMI-CS (adjusted OR, 0.67; P = .017) and AMI-CS (adjusted OR, 0.79; P = .344).
“This analysis supports that all AHA level 1 cardiac ICUs should strongly consider having a shock team,” Dr. Papolos said.
Evidence from single centers and the National Cardiogenic Shock Initiative has shown improved survival with a cardiogenic shock algorithm, but this is the first report specifically comparing no shock teams with shock teams, Perwaiz Meraj, MD, Northwell Health, Manhansett, N.Y., told this news organization.
“People may say that it’s just another paper that’s saying, ‘shock teams, shock teams, rah, rah, rah,’ but it’s important for all of us to really take a close look under the covers and see how are we best managing these patients, what teams are we putting together, and to create systems of care, where if you’re at a center that really doesn’t have the capabilities of doing this, then you should partner up with a center that does,” he said.
Notably, the 10 shock teams were present only in medium or large urban, academic medical centers with more than 500 beds. Although they followed individual protocols, survey results show service-line representation, structure, and operations were similar across centers.
They all had a centralized way to activate the shock team, the service was 24/7, and members came from areas such as critical care cardiology (100%), cardiac surgery (100%), interventional cardiology (90%), advanced heart failure (80%), and extracorporeal membrane oxygenation service (70%).
Limitations of the study include the possibility of residual confounding, the fact that the registry did not capture patients with cardiogenic shock managed outside the CICU or the time of onset of cardiogenic shock, and data were limited on inotropic strategies, sedation practices, and ventilator management, the authors wrote.
“Although many critics will continue to discuss the lack of randomized controlled trials in cardiogenic shock, this paper supports the process previously outlined of a multidisciplinary team-based approach improving survival,” Dr. Meraj and William W. O’Neill, MD, director of the Center for Structural Heart Disease and Henry Ford Health System, Detroit, and the force behind the National Cardiogenic Shock Initiative, wrote in an accompanying editorial.
They point out that the report doesn’t address the escalation of care based on invasive hemodynamics in the CICU and the protocols to prevent acute vascular/limb complications (ALI) that can arise from the use of MCS.
“Many procedural techniques and novel CICU models exist to mitigate the risk of ALI in CS patients with MCS,” they wrote. “Finally, escalation of care and support is vital to the continued success of any shock team and center.”
One coauthor has served as a consultant to Abbott. Another has served as a consultant to the Abiomed critical care advisory board. All other authors reported having no relevant financial relationships. Dr. Meraj has received research and grant funding from Abiomed, Medtronic, CSI, and Boston Scientific. Dr. O’Neill has received consulting/speaker honoraria from Abiomed, Boston Scientific, and Abbott.
A version of this article first appeared on Medscape.com.
A large multicenter study provides further evidence supporting the rationale for multidisciplinary teams for cardiogenic shock, one of the most lethal diseases in cardiovascular medicine.
The analysis of 24 critical care ICUs in the Critical Care Cardiology Trials Network showed that the presence of a shock team was independently associated with a 28% lower risk for CICU mortality (23% vs. 29%; odds ratio, 0.72; P = .016).
Patients treated by a shock team also had significantly shorter CICU stays and less need for mechanical ventilation or renal replacement therapy, as reported in the Journal of the American College of Cardiology.
“It’s observational, but the association that we’re seeing here, just because of our sample size, is the strongest that’s been published yet,” lead author Alexander Papolos, MD, MedStar Washington Hospital Center, said in an interview.
Although a causal relationship cannot be drawn, the authors suggest several factors that could explain the findings, including a shock team’s ability to rapidly diagnose and treat cardiogenic shock before multiorgan dysfunction occurs.
Centers with shock teams also used significantly more pulmonary artery catheters (60% vs. 49%; adjusted OR, 1.86; P < .001) and placed them earlier (0.3 vs. 0.66 days; P = .019).
Pulmonary artery catheter (PAC) use has declined after earlier trials like ESCAPE showed little or no benefit in other acutely ill patient groups, but positive results have been reported recently in cardiogenic shock, where a PAC is needed to determine the severity of the lesion and the phenotype, Dr. Papolos observed.
A 2018 study showed PAC use was tied to increased survival among patients with acute myocardial infarction cardiogenic shock (AMI-CS) supported with the Impella (Abiomed) device. Additionally, a 2021 study by the Cardiogenic Shock Working Group demonstrated a dose-dependent survival response based on the completeness of hemodynamic assessment by PAC prior to initiating mechanical circulatory support (MCS).
A third factor might be that a structured, team-based evaluation can facilitate timely and optimal MCS device selection, deployment, and management, suggested Dr. Papolos.
Centers with shock teams used more advanced types of MCS – defined as Impella, TandemHeart (LivaNova), extracorporeal membrane oxygenation, and temporary or durable surgical ventricular assist devices – than those without a shock team (53% vs. 43%; adjusted OR, 1.73; P = .005) and did so more often as the initial device (42% vs. 28%; P = .002).
Overall MCS use was lower at shock team centers (35% vs. 43%), driven by less frequent use of intra-aortic balloon pumps (58% vs. 72%).
“The standard, basic MCS has always been the balloon pump because it’s something that’s easy to put in at the cath lab or at the bedside,” Dr. Papolos said. “So, if you take away having all of the information and having the right people at the table to discuss what the best level of support is, then you’re going to end up with balloon pumps, and that’s what we saw here.”
The study involved 6,872 consecutive medical admissions at 24 level 1 CICU centers during an annual 2-month period from 2017 to 2019. Of these, 1,242 admissions were for cardiogenic shock and 546 (44%) were treated at one of 10 centers with a shock team.
Shock team centers had higher-acuity patients than centers without a shock team (Sequential Organ Failure Assessment score, 4 vs. 3) but a similar proportion of patients with AMI-CS (27% vs. 28%).
Among all admissions, CICU mortality was not significantly different between centers with and without a shock team.
For cardiogenic shock patients treated at centers with and without a shock team, the median CICU stay was 4.0 and 5.1 days, respectively, mechanical ventilation was used in 41% and 52%, respectively, and new renal replacement therapy in 11% and 19%, respectively (P < .001 for all).
Shock team centers used significantly more PACs for AMI-CS and non–AMI-CS admissions; advanced MCS therapy was also greater in the AMI-CS subgroup.
Lower CICU mortality at shock team centers persisted among patients with non-AMI-CS (adjusted OR, 0.67; P = .017) and AMI-CS (adjusted OR, 0.79; P = .344).
“This analysis supports that all AHA level 1 cardiac ICUs should strongly consider having a shock team,” Dr. Papolos said.
Evidence from single centers and the National Cardiogenic Shock Initiative has shown improved survival with a cardiogenic shock algorithm, but this is the first report specifically comparing no shock teams with shock teams, Perwaiz Meraj, MD, Northwell Health, Manhansett, N.Y., told this news organization.
“People may say that it’s just another paper that’s saying, ‘shock teams, shock teams, rah, rah, rah,’ but it’s important for all of us to really take a close look under the covers and see how are we best managing these patients, what teams are we putting together, and to create systems of care, where if you’re at a center that really doesn’t have the capabilities of doing this, then you should partner up with a center that does,” he said.
Notably, the 10 shock teams were present only in medium or large urban, academic medical centers with more than 500 beds. Although they followed individual protocols, survey results show service-line representation, structure, and operations were similar across centers.
They all had a centralized way to activate the shock team, the service was 24/7, and members came from areas such as critical care cardiology (100%), cardiac surgery (100%), interventional cardiology (90%), advanced heart failure (80%), and extracorporeal membrane oxygenation service (70%).
Limitations of the study include the possibility of residual confounding, the fact that the registry did not capture patients with cardiogenic shock managed outside the CICU or the time of onset of cardiogenic shock, and data were limited on inotropic strategies, sedation practices, and ventilator management, the authors wrote.
“Although many critics will continue to discuss the lack of randomized controlled trials in cardiogenic shock, this paper supports the process previously outlined of a multidisciplinary team-based approach improving survival,” Dr. Meraj and William W. O’Neill, MD, director of the Center for Structural Heart Disease and Henry Ford Health System, Detroit, and the force behind the National Cardiogenic Shock Initiative, wrote in an accompanying editorial.
They point out that the report doesn’t address the escalation of care based on invasive hemodynamics in the CICU and the protocols to prevent acute vascular/limb complications (ALI) that can arise from the use of MCS.
“Many procedural techniques and novel CICU models exist to mitigate the risk of ALI in CS patients with MCS,” they wrote. “Finally, escalation of care and support is vital to the continued success of any shock team and center.”
One coauthor has served as a consultant to Abbott. Another has served as a consultant to the Abiomed critical care advisory board. All other authors reported having no relevant financial relationships. Dr. Meraj has received research and grant funding from Abiomed, Medtronic, CSI, and Boston Scientific. Dr. O’Neill has received consulting/speaker honoraria from Abiomed, Boston Scientific, and Abbott.
A version of this article first appeared on Medscape.com.
A large multicenter study provides further evidence supporting the rationale for multidisciplinary teams for cardiogenic shock, one of the most lethal diseases in cardiovascular medicine.
The analysis of 24 critical care ICUs in the Critical Care Cardiology Trials Network showed that the presence of a shock team was independently associated with a 28% lower risk for CICU mortality (23% vs. 29%; odds ratio, 0.72; P = .016).
Patients treated by a shock team also had significantly shorter CICU stays and less need for mechanical ventilation or renal replacement therapy, as reported in the Journal of the American College of Cardiology.
“It’s observational, but the association that we’re seeing here, just because of our sample size, is the strongest that’s been published yet,” lead author Alexander Papolos, MD, MedStar Washington Hospital Center, said in an interview.
Although a causal relationship cannot be drawn, the authors suggest several factors that could explain the findings, including a shock team’s ability to rapidly diagnose and treat cardiogenic shock before multiorgan dysfunction occurs.
Centers with shock teams also used significantly more pulmonary artery catheters (60% vs. 49%; adjusted OR, 1.86; P < .001) and placed them earlier (0.3 vs. 0.66 days; P = .019).
Pulmonary artery catheter (PAC) use has declined after earlier trials like ESCAPE showed little or no benefit in other acutely ill patient groups, but positive results have been reported recently in cardiogenic shock, where a PAC is needed to determine the severity of the lesion and the phenotype, Dr. Papolos observed.
A 2018 study showed PAC use was tied to increased survival among patients with acute myocardial infarction cardiogenic shock (AMI-CS) supported with the Impella (Abiomed) device. Additionally, a 2021 study by the Cardiogenic Shock Working Group demonstrated a dose-dependent survival response based on the completeness of hemodynamic assessment by PAC prior to initiating mechanical circulatory support (MCS).
A third factor might be that a structured, team-based evaluation can facilitate timely and optimal MCS device selection, deployment, and management, suggested Dr. Papolos.
Centers with shock teams used more advanced types of MCS – defined as Impella, TandemHeart (LivaNova), extracorporeal membrane oxygenation, and temporary or durable surgical ventricular assist devices – than those without a shock team (53% vs. 43%; adjusted OR, 1.73; P = .005) and did so more often as the initial device (42% vs. 28%; P = .002).
Overall MCS use was lower at shock team centers (35% vs. 43%), driven by less frequent use of intra-aortic balloon pumps (58% vs. 72%).
“The standard, basic MCS has always been the balloon pump because it’s something that’s easy to put in at the cath lab or at the bedside,” Dr. Papolos said. “So, if you take away having all of the information and having the right people at the table to discuss what the best level of support is, then you’re going to end up with balloon pumps, and that’s what we saw here.”
The study involved 6,872 consecutive medical admissions at 24 level 1 CICU centers during an annual 2-month period from 2017 to 2019. Of these, 1,242 admissions were for cardiogenic shock and 546 (44%) were treated at one of 10 centers with a shock team.
Shock team centers had higher-acuity patients than centers without a shock team (Sequential Organ Failure Assessment score, 4 vs. 3) but a similar proportion of patients with AMI-CS (27% vs. 28%).
Among all admissions, CICU mortality was not significantly different between centers with and without a shock team.
For cardiogenic shock patients treated at centers with and without a shock team, the median CICU stay was 4.0 and 5.1 days, respectively, mechanical ventilation was used in 41% and 52%, respectively, and new renal replacement therapy in 11% and 19%, respectively (P < .001 for all).
Shock team centers used significantly more PACs for AMI-CS and non–AMI-CS admissions; advanced MCS therapy was also greater in the AMI-CS subgroup.
Lower CICU mortality at shock team centers persisted among patients with non-AMI-CS (adjusted OR, 0.67; P = .017) and AMI-CS (adjusted OR, 0.79; P = .344).
“This analysis supports that all AHA level 1 cardiac ICUs should strongly consider having a shock team,” Dr. Papolos said.
Evidence from single centers and the National Cardiogenic Shock Initiative has shown improved survival with a cardiogenic shock algorithm, but this is the first report specifically comparing no shock teams with shock teams, Perwaiz Meraj, MD, Northwell Health, Manhansett, N.Y., told this news organization.
“People may say that it’s just another paper that’s saying, ‘shock teams, shock teams, rah, rah, rah,’ but it’s important for all of us to really take a close look under the covers and see how are we best managing these patients, what teams are we putting together, and to create systems of care, where if you’re at a center that really doesn’t have the capabilities of doing this, then you should partner up with a center that does,” he said.
Notably, the 10 shock teams were present only in medium or large urban, academic medical centers with more than 500 beds. Although they followed individual protocols, survey results show service-line representation, structure, and operations were similar across centers.
They all had a centralized way to activate the shock team, the service was 24/7, and members came from areas such as critical care cardiology (100%), cardiac surgery (100%), interventional cardiology (90%), advanced heart failure (80%), and extracorporeal membrane oxygenation service (70%).
Limitations of the study include the possibility of residual confounding, the fact that the registry did not capture patients with cardiogenic shock managed outside the CICU or the time of onset of cardiogenic shock, and data were limited on inotropic strategies, sedation practices, and ventilator management, the authors wrote.
“Although many critics will continue to discuss the lack of randomized controlled trials in cardiogenic shock, this paper supports the process previously outlined of a multidisciplinary team-based approach improving survival,” Dr. Meraj and William W. O’Neill, MD, director of the Center for Structural Heart Disease and Henry Ford Health System, Detroit, and the force behind the National Cardiogenic Shock Initiative, wrote in an accompanying editorial.
They point out that the report doesn’t address the escalation of care based on invasive hemodynamics in the CICU and the protocols to prevent acute vascular/limb complications (ALI) that can arise from the use of MCS.
“Many procedural techniques and novel CICU models exist to mitigate the risk of ALI in CS patients with MCS,” they wrote. “Finally, escalation of care and support is vital to the continued success of any shock team and center.”
One coauthor has served as a consultant to Abbott. Another has served as a consultant to the Abiomed critical care advisory board. All other authors reported having no relevant financial relationships. Dr. Meraj has received research and grant funding from Abiomed, Medtronic, CSI, and Boston Scientific. Dr. O’Neill has received consulting/speaker honoraria from Abiomed, Boston Scientific, and Abbott.
A version of this article first appeared on Medscape.com.
COVID-19 causes major interruption in global HIV progress
“We’ve been set back by COVID but we’ve seen remarkable resilience, a lot of innovation and creativity,” Siobhan Crowley MD, head of HIV at the Global Fund, said in an interview.
“If you consider that 21.9 million people are getting antiretrovirals at this point through the Global Fund, I think that needs to be appreciated. Ten years ago, that wouldn’t have been the case; all of those people would have disappeared into the ethers,” she said.
Through close partnerships with the U.S. Agency for International Development, the U.S. President’s Emergency Plan for AIDS Relief, and other Western countries and organizations, the Global Fund has invested $22.7 billion in programs to prevent and treat HIV and AIDS, and $3.8 billion in tuberculosis (TB)/HIV programs, according to the organization’s 2021 Results Report.
But the report also underscores the significant effect that the COVID-19 pandemic has had on funded countries’ progress toward achieving renewed 90-90-90 targets for HIV testing/diagnosis, treatment, and viral suppression by 2030.
The setbacks have been challenging and have touched nearly every service from prevention to treatment. According to the report, between 2019 and 2020:
- Voluntary male circumcision declined by 27%.
- Numbers reached by HIV prevention programs fell by 11%.
- 4.5% fewer mothers received medications to prevent HIV transmission to their babies.
- HIV testing services, including initiation, decreased by 22%.
The numbers tell only a part of the story, according to Dr. Crowley.
“We put in place an emergency mechanism to make funds available for countries to do everything except vaccines in support of COVID,” Dr. Crowley explained. (As of August 2021, these funds had been allocated to 107 countries and 16 multicountry programs.)
Countries were advised that they could use the emergency funds three different ways: 1) for COVID-specific purposes (e.g., diagnostics, oxygen, personal protective equipment; 2) to support mitigation strategies geared toward protecting existing HIV, tuberculosis, and malaria programs and getting them back on track; and 3) for so-called “health system fixes,” such as investing in data systems to track COVID, HIV, and other core diseases, as well as the community workforce.
With regard to HIV, each country supported by the Global Fund was asked to ensure that multimonth (3-6 months) dispensing was implemented and/or accelerated so that patients could avoid congested facilities, and, wherever possible, that drugs were delivered or accessed outside the facility. One example of the success of this effort was found in South Africa, where the number of people on antiretrovirals increased almost threefold, from 1.2 million to 4.2 million people.
Countries also were asked to adapt HIV testing procedures by, for example, moving organized testing out of the facilities and into neighborhoods to meet people where they are. Rapid diagnostic testing and triage care linkage using technologies such as WhatsApp were the result, as were opportunities for home testing which, Dr. Crowley noted, remains a critical component of the overall strategy.
“The self-test is important for two reasons, not just because you are trying to find people with HIV, but also, when people know that they’re negative, they know what they can or should do to stay negative,” she said. “It’s quite a powerful motivator.”
Self-testing might also help countries motivate the 6 million people who know that they have HIV but are not on treatment. But there are still 4.1 million residing in these countries who aren’t aware that they are infected, according to the report. This figure is especially troubling, considering that some may also be harboring TB coinfections, including multidrug-resistant TB (MDR-TB).
The imperfect storm globally and in the U.S.
“One of the things that was striking in the report was the decline in the number of people reached with testing and prevention services,” Chris Beyrer, MD, MPH, the Desmond M. Tutu Professor of Public Health and Human Rights at the Johns Hopkins Bloomberg School of Public Health in Baltimore, said in an interview. Dr. Beyrer was not involved in the report’s development.
“You know, a 10% decline in 1 year to reach people in need is substantial,” he said. “Let’s say it continues; many people are predicting that we won’t have reasonable coverage for low-income countries with COVID until 2023. That adds up to a substantial decline in people reached with these services.”
Dr. Beyrer also expressed concern about the convergence of HIV and TB in already overburdened, fragile health care systems. “Globally, the No. 1 cause of death for people living with HIV is TB, and of course, it’s highly transmissible. So, in many high-burden countries, children are exposed, typically from household members early on, and so the number of people with latent TB infection is just enormous.
“If you look at the report, the worst outcomes are MDR-TB. Those multidrug-resistant and extensively-drug-resistant strains are really a threat to everybody,” Dr. Beyrer said.
But it’s not time for U.S. providers to rest on their laurels either. Dr. Beyrer noted that the 22% decline in HIV testing reported by the Global Fund is similar to what has been happening in the United States with elective procedures such as HIV testing and even preventive procedures like medical male circumcision.
“It’s very clear here in the Global Fund data that the majority of new infections worldwide are in key populations [that] include gay and bisexual men, men who have sex with men, transgender women who have sex with men, people who inject drugs, and sex workers of all genders. Those are people who already faced barriers to health care access and were made worse by COVID.”
Dr. Beyrer noted that, according to the Centers for Disease Control and Prevention, in 2019 in the United States, 68% of new HIV infections occurred in gay and bisexual men, and the effect that COVID-19 will have is still unknown. He also noted the similarity between the most marginalized populations in the Global Fund report and African American men, who have not realized the same increase in the use of preexposure prophylaxis or the same decline in new infections as have their White counterparts.
“It’s also where we are seeing the worst of COVID, low immunization coverage, and high rates of hospitalization and death. ... It’s a dark, dark time for many,” Dr. Crowley said. “And there has also been some amazing resilience and adaptation. The weird thing is, the HIV platform is a natural platform; I mean, if we can keep 21.9 million people on treatment, we can probably deliver them a COVID test and a vaccine.”
Dr. Crowley and Dr. Beyrer report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“We’ve been set back by COVID but we’ve seen remarkable resilience, a lot of innovation and creativity,” Siobhan Crowley MD, head of HIV at the Global Fund, said in an interview.
“If you consider that 21.9 million people are getting antiretrovirals at this point through the Global Fund, I think that needs to be appreciated. Ten years ago, that wouldn’t have been the case; all of those people would have disappeared into the ethers,” she said.
Through close partnerships with the U.S. Agency for International Development, the U.S. President’s Emergency Plan for AIDS Relief, and other Western countries and organizations, the Global Fund has invested $22.7 billion in programs to prevent and treat HIV and AIDS, and $3.8 billion in tuberculosis (TB)/HIV programs, according to the organization’s 2021 Results Report.
But the report also underscores the significant effect that the COVID-19 pandemic has had on funded countries’ progress toward achieving renewed 90-90-90 targets for HIV testing/diagnosis, treatment, and viral suppression by 2030.
The setbacks have been challenging and have touched nearly every service from prevention to treatment. According to the report, between 2019 and 2020:
- Voluntary male circumcision declined by 27%.
- Numbers reached by HIV prevention programs fell by 11%.
- 4.5% fewer mothers received medications to prevent HIV transmission to their babies.
- HIV testing services, including initiation, decreased by 22%.
The numbers tell only a part of the story, according to Dr. Crowley.
“We put in place an emergency mechanism to make funds available for countries to do everything except vaccines in support of COVID,” Dr. Crowley explained. (As of August 2021, these funds had been allocated to 107 countries and 16 multicountry programs.)
Countries were advised that they could use the emergency funds three different ways: 1) for COVID-specific purposes (e.g., diagnostics, oxygen, personal protective equipment; 2) to support mitigation strategies geared toward protecting existing HIV, tuberculosis, and malaria programs and getting them back on track; and 3) for so-called “health system fixes,” such as investing in data systems to track COVID, HIV, and other core diseases, as well as the community workforce.
With regard to HIV, each country supported by the Global Fund was asked to ensure that multimonth (3-6 months) dispensing was implemented and/or accelerated so that patients could avoid congested facilities, and, wherever possible, that drugs were delivered or accessed outside the facility. One example of the success of this effort was found in South Africa, where the number of people on antiretrovirals increased almost threefold, from 1.2 million to 4.2 million people.
Countries also were asked to adapt HIV testing procedures by, for example, moving organized testing out of the facilities and into neighborhoods to meet people where they are. Rapid diagnostic testing and triage care linkage using technologies such as WhatsApp were the result, as were opportunities for home testing which, Dr. Crowley noted, remains a critical component of the overall strategy.
“The self-test is important for two reasons, not just because you are trying to find people with HIV, but also, when people know that they’re negative, they know what they can or should do to stay negative,” she said. “It’s quite a powerful motivator.”
Self-testing might also help countries motivate the 6 million people who know that they have HIV but are not on treatment. But there are still 4.1 million residing in these countries who aren’t aware that they are infected, according to the report. This figure is especially troubling, considering that some may also be harboring TB coinfections, including multidrug-resistant TB (MDR-TB).
The imperfect storm globally and in the U.S.
“One of the things that was striking in the report was the decline in the number of people reached with testing and prevention services,” Chris Beyrer, MD, MPH, the Desmond M. Tutu Professor of Public Health and Human Rights at the Johns Hopkins Bloomberg School of Public Health in Baltimore, said in an interview. Dr. Beyrer was not involved in the report’s development.
“You know, a 10% decline in 1 year to reach people in need is substantial,” he said. “Let’s say it continues; many people are predicting that we won’t have reasonable coverage for low-income countries with COVID until 2023. That adds up to a substantial decline in people reached with these services.”
Dr. Beyrer also expressed concern about the convergence of HIV and TB in already overburdened, fragile health care systems. “Globally, the No. 1 cause of death for people living with HIV is TB, and of course, it’s highly transmissible. So, in many high-burden countries, children are exposed, typically from household members early on, and so the number of people with latent TB infection is just enormous.
“If you look at the report, the worst outcomes are MDR-TB. Those multidrug-resistant and extensively-drug-resistant strains are really a threat to everybody,” Dr. Beyrer said.
But it’s not time for U.S. providers to rest on their laurels either. Dr. Beyrer noted that the 22% decline in HIV testing reported by the Global Fund is similar to what has been happening in the United States with elective procedures such as HIV testing and even preventive procedures like medical male circumcision.
“It’s very clear here in the Global Fund data that the majority of new infections worldwide are in key populations [that] include gay and bisexual men, men who have sex with men, transgender women who have sex with men, people who inject drugs, and sex workers of all genders. Those are people who already faced barriers to health care access and were made worse by COVID.”
Dr. Beyrer noted that, according to the Centers for Disease Control and Prevention, in 2019 in the United States, 68% of new HIV infections occurred in gay and bisexual men, and the effect that COVID-19 will have is still unknown. He also noted the similarity between the most marginalized populations in the Global Fund report and African American men, who have not realized the same increase in the use of preexposure prophylaxis or the same decline in new infections as have their White counterparts.
“It’s also where we are seeing the worst of COVID, low immunization coverage, and high rates of hospitalization and death. ... It’s a dark, dark time for many,” Dr. Crowley said. “And there has also been some amazing resilience and adaptation. The weird thing is, the HIV platform is a natural platform; I mean, if we can keep 21.9 million people on treatment, we can probably deliver them a COVID test and a vaccine.”
Dr. Crowley and Dr. Beyrer report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“We’ve been set back by COVID but we’ve seen remarkable resilience, a lot of innovation and creativity,” Siobhan Crowley MD, head of HIV at the Global Fund, said in an interview.
“If you consider that 21.9 million people are getting antiretrovirals at this point through the Global Fund, I think that needs to be appreciated. Ten years ago, that wouldn’t have been the case; all of those people would have disappeared into the ethers,” she said.
Through close partnerships with the U.S. Agency for International Development, the U.S. President’s Emergency Plan for AIDS Relief, and other Western countries and organizations, the Global Fund has invested $22.7 billion in programs to prevent and treat HIV and AIDS, and $3.8 billion in tuberculosis (TB)/HIV programs, according to the organization’s 2021 Results Report.
But the report also underscores the significant effect that the COVID-19 pandemic has had on funded countries’ progress toward achieving renewed 90-90-90 targets for HIV testing/diagnosis, treatment, and viral suppression by 2030.
The setbacks have been challenging and have touched nearly every service from prevention to treatment. According to the report, between 2019 and 2020:
- Voluntary male circumcision declined by 27%.
- Numbers reached by HIV prevention programs fell by 11%.
- 4.5% fewer mothers received medications to prevent HIV transmission to their babies.
- HIV testing services, including initiation, decreased by 22%.
The numbers tell only a part of the story, according to Dr. Crowley.
“We put in place an emergency mechanism to make funds available for countries to do everything except vaccines in support of COVID,” Dr. Crowley explained. (As of August 2021, these funds had been allocated to 107 countries and 16 multicountry programs.)
Countries were advised that they could use the emergency funds three different ways: 1) for COVID-specific purposes (e.g., diagnostics, oxygen, personal protective equipment; 2) to support mitigation strategies geared toward protecting existing HIV, tuberculosis, and malaria programs and getting them back on track; and 3) for so-called “health system fixes,” such as investing in data systems to track COVID, HIV, and other core diseases, as well as the community workforce.
With regard to HIV, each country supported by the Global Fund was asked to ensure that multimonth (3-6 months) dispensing was implemented and/or accelerated so that patients could avoid congested facilities, and, wherever possible, that drugs were delivered or accessed outside the facility. One example of the success of this effort was found in South Africa, where the number of people on antiretrovirals increased almost threefold, from 1.2 million to 4.2 million people.
Countries also were asked to adapt HIV testing procedures by, for example, moving organized testing out of the facilities and into neighborhoods to meet people where they are. Rapid diagnostic testing and triage care linkage using technologies such as WhatsApp were the result, as were opportunities for home testing which, Dr. Crowley noted, remains a critical component of the overall strategy.
“The self-test is important for two reasons, not just because you are trying to find people with HIV, but also, when people know that they’re negative, they know what they can or should do to stay negative,” she said. “It’s quite a powerful motivator.”
Self-testing might also help countries motivate the 6 million people who know that they have HIV but are not on treatment. But there are still 4.1 million residing in these countries who aren’t aware that they are infected, according to the report. This figure is especially troubling, considering that some may also be harboring TB coinfections, including multidrug-resistant TB (MDR-TB).
The imperfect storm globally and in the U.S.
“One of the things that was striking in the report was the decline in the number of people reached with testing and prevention services,” Chris Beyrer, MD, MPH, the Desmond M. Tutu Professor of Public Health and Human Rights at the Johns Hopkins Bloomberg School of Public Health in Baltimore, said in an interview. Dr. Beyrer was not involved in the report’s development.
“You know, a 10% decline in 1 year to reach people in need is substantial,” he said. “Let’s say it continues; many people are predicting that we won’t have reasonable coverage for low-income countries with COVID until 2023. That adds up to a substantial decline in people reached with these services.”
Dr. Beyrer also expressed concern about the convergence of HIV and TB in already overburdened, fragile health care systems. “Globally, the No. 1 cause of death for people living with HIV is TB, and of course, it’s highly transmissible. So, in many high-burden countries, children are exposed, typically from household members early on, and so the number of people with latent TB infection is just enormous.
“If you look at the report, the worst outcomes are MDR-TB. Those multidrug-resistant and extensively-drug-resistant strains are really a threat to everybody,” Dr. Beyrer said.
But it’s not time for U.S. providers to rest on their laurels either. Dr. Beyrer noted that the 22% decline in HIV testing reported by the Global Fund is similar to what has been happening in the United States with elective procedures such as HIV testing and even preventive procedures like medical male circumcision.
“It’s very clear here in the Global Fund data that the majority of new infections worldwide are in key populations [that] include gay and bisexual men, men who have sex with men, transgender women who have sex with men, people who inject drugs, and sex workers of all genders. Those are people who already faced barriers to health care access and were made worse by COVID.”
Dr. Beyrer noted that, according to the Centers for Disease Control and Prevention, in 2019 in the United States, 68% of new HIV infections occurred in gay and bisexual men, and the effect that COVID-19 will have is still unknown. He also noted the similarity between the most marginalized populations in the Global Fund report and African American men, who have not realized the same increase in the use of preexposure prophylaxis or the same decline in new infections as have their White counterparts.
“It’s also where we are seeing the worst of COVID, low immunization coverage, and high rates of hospitalization and death. ... It’s a dark, dark time for many,” Dr. Crowley said. “And there has also been some amazing resilience and adaptation. The weird thing is, the HIV platform is a natural platform; I mean, if we can keep 21.9 million people on treatment, we can probably deliver them a COVID test and a vaccine.”
Dr. Crowley and Dr. Beyrer report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Updated USPSTF screening guidelines may reduce lung cancer deaths
ILLUSTRATIVE CASE
A 50-year-old woman presents to your office for a well-woman exam. Her past medical history includes a 22-pack-year smoking history (she quit 5 years ago), well-controlled hypertension, and mild obesity. She has no family history of cancer, but she does have a family history of type 2 diabetes and heart disease. Besides age- and risk-appropriate laboratory tests, cervical cancer screening, breast cancer screening, and initial colon cancer screening, are there any other preventive services you would offer her?
Lung cancer is the second most common cancer in both men and women, and it is the leading cause of cancer death in the United States—regardless of gender. The American Cancer Society estimates that 235,760 people will be diagnosed with lung cancer and 131,880 people will die of the disease in 2021.2
In the 2015 National Cancer Institute report on the economic costs of cancer, direct and indirect costs of lung cancer totaled $21.1 billion annually. Lost productivity from lung cancer added another $36.1 billion in annual costs.3 The economic costs increased to $23.8 billion in 2020, with no data on lost productivity.4
Smoking tobacco is by far the primary risk factor for lung cancer, and it is estimated to account for 90% of all lung cancer cases. Compared with nonsmokers, the relative risk of lung cancer is approximately 20 times higher for smokers.5,6
Because the median age of lung cancer diagnosis is 70 years, increasing age is also considered a risk factor for lung cancer.2,7
Although lung cancer has a relatively poor prognosis—with an average 5-year survival rate of 20.5%—early-stage lung cancer is more amenable to treatment and has a better prognosis (as is true with many cancers).1
LDCT has a high sensitivity, as well as a reasonable specificity, for lung cancer detection. There is demonstrated benefit in screening patients who are at high risk for lung cancer.8-11 In 2013, the USPSTF recommended annual lung cancer screening (B recommendation) with LDCT in adults 55 to 80 years of age who have a 30-pack-year smoking history, and who currently smoke or quit within the past 15 years.1
Continue to: STUDY SUMMARY
STUDY SUMMARY
Broader eligibility for screening supports mortality benefit
This is an update to the 2013 clinical practice guideline on lung cancer screening. The USPSTF used 2 methods to provide the best possible evidence for the recommendations. The first method was a systematic review of the accuracy of screening for lung cancer with LDCT, evaluating both the benefits and harms of lung cancer screening. The systematic review examined various subgroups, the number and/or frequency of LDCT scans, and various approaches to reducing false-positive results. In addition to the systematic review, they used collaborative modeling studies to determine the optimal age for beginning and ending screening, the optimal screening interval, and the relative benefits and harms of various screening strategies. These modeling studies complemented the evidence review.
The review included 7 randomized controlled trials (RCTs), plus the modeling studies. Only the National Lung Screening Trial (NLST; N = 53,454) and the Nederlands-Leuvens Longkanker Screenings Onderzoek (NELSON) trial (N = 15,792) had adequate power to detect a mortality benefit from screening (NLST: relative risk reduction = 16%; 95% CI, 5%-25%; NELSON: incidence rate ratio = 0.75; 95% CI, 0.61-0.90) compared with no screening.
Screening intervals, from the NLST and NELSON trials as well as the modeling studies, revealed the greatest benefit from annual screening (statistics not shared). Evidence also showed that screening those with lighter smoking histories (< 30 pack-years) and at an earlier age (age 50) provided increased mortality benefit. No evidence was found for a benefit of screening past 80 years of age. The modeling studies concluded that the 2013 USPSTF screening program, using a starting age of 55 and a 30-pack-year smoking history, would reduce mortality by 9.8%, but by changing to a starting age of 50, a 20-pack-year smoking history, and annual screening, the mortality benefit was increased to 13%.1,11
Comparison with computer-based risk prediction models from the Cancer Intervention and Surveillance Modeling Network (CISNET) revealed insufficient evidence at this time to show that prediction model–based screening offered any benefit beyond that of the age and smoking history risk factor model.
The incidence of false-positive results was > 25% in the NLST at baseline and at 1 year. Use of a classification system such as the Lung Imaging Reporting and Data System (Lung-RADS) could reduce that from 26.6% to 12.8%.2 Another potential harm from LDCT screening is radiation exposure. Evidence from several RCTs and cohort studies showed the exposure from 1 LDCT scan to be 0.65 to 2.36 mSv, whereas the annual background radiation in the United States is 2.4 mSv. The modeling studies estimated that there would be 1 death caused by LDCT for every 18.5 cancer deaths avoided.1,11
Continue to: WHAT'S NEW
WHAT’S NEW
Expanded age range, reduced pack-year history
Annual lung cancer screening is now recommended to begin for patients at age 50 years with a 20-pack-year history instead of age 55 years with a 30-pack-year history. This would nearly double (87% overall) the number of people eligible for screening, and it would include more Black patients and women, who tend to smoke fewer cigarettes than their White male counterparts. The American College of Radiology estimates that the expanded screening criteria could save between 30,000 and 60,000 lives per year.12
CAVEATS
Screening criteria for upper age limit, years since smoking remain unchanged
For those patients who quit smoking, the guidelines apply only to those who have stopped smoking within the past 15 years. Furthermore, the benefit does not extend beyond age 80 or where other conditions reduce life expectancy. And, as noted earlier, modeling studies estimate that there would be 1 death caused by LDCT for every 18.5 cancer deaths avoided.1,11
CHALLENGES TO IMPLEMENTATION
Concerns about false-positives, radiation exposure may limit acceptance
Challenges would be based mostly on the need for greater, more detailed dialogue between physicians and patients at higher risk for lung cancer in a time-constrained environment. Also, LDCT may not be available in some areas, and patients and physicians may have concerns regarding repeated CT exposure. In addition, false-positive results increase patient stress and may adversely affect both patient and physician acceptance.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. US Preventive Services Task Force. Lung cancer: screening. Final recommendation statement. March 9, 2021. Accessed August 19, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
2. American Cancer Society. Key statistics for lung cancer. Updated January 12, 2021. Accessed August 19, 2021. www.cancer.org/cancer/lung-cancer/about/key-statistics.html
3. National Cancer Institute. Cancer Trends Progress Report—Financial Burden of Cancer Care. National Institutes of Health; 2015.
4. National Cancer Institute. Cancer Trends Progress Report—Financial Burden of Cancer Care. National Institutes of Health. Updated July 2021. Accessed August 19, 2021. https://progressreport.cancer.gov/after/economic_burden
5. Alberg AJ, Brock MV, Ford JG, et al. Epidemiology of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 suppl):e1S-e29S. doi: 10.1378/chest.12-2345
6. Samet JM. Health benefits of smoking cessation. Clin Chest Med. 1991;12:669-679.
7. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. doi: 10.3322/caac.21254
8. National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
9. Pinsky PF, Church TR, Izmirlian G, et al. The National Lung Screening Trial: results stratified by demographics, smoking history, and lung cancer histology. Cancer. 2013;119:3976-3983. doi: 10.1002/cncr.28326
10. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503-513. doi: 10.1056/NEJMoa1911793
11. Meza R, Jeon J, Toumazis I, et al. Evaluation of the Benefits and Harms of Lung Cancer Screening With Low-Dose Computed Tomography: A Collaborative Modeling Study for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality; 2021.
12. American College of Radiology. Updated USPSTF lung cancer screening guidelines would help save lives. July 7, 2020. Accessed August 19, 2021. www.acr.org/Media-Center/ACR-News-Releases/2020/Updated-USPSTF-Lung-Cancer-Screening-Guidelines-Would-Help-Save-Lives
ILLUSTRATIVE CASE
A 50-year-old woman presents to your office for a well-woman exam. Her past medical history includes a 22-pack-year smoking history (she quit 5 years ago), well-controlled hypertension, and mild obesity. She has no family history of cancer, but she does have a family history of type 2 diabetes and heart disease. Besides age- and risk-appropriate laboratory tests, cervical cancer screening, breast cancer screening, and initial colon cancer screening, are there any other preventive services you would offer her?
Lung cancer is the second most common cancer in both men and women, and it is the leading cause of cancer death in the United States—regardless of gender. The American Cancer Society estimates that 235,760 people will be diagnosed with lung cancer and 131,880 people will die of the disease in 2021.2
In the 2015 National Cancer Institute report on the economic costs of cancer, direct and indirect costs of lung cancer totaled $21.1 billion annually. Lost productivity from lung cancer added another $36.1 billion in annual costs.3 The economic costs increased to $23.8 billion in 2020, with no data on lost productivity.4
Smoking tobacco is by far the primary risk factor for lung cancer, and it is estimated to account for 90% of all lung cancer cases. Compared with nonsmokers, the relative risk of lung cancer is approximately 20 times higher for smokers.5,6
Because the median age of lung cancer diagnosis is 70 years, increasing age is also considered a risk factor for lung cancer.2,7
Although lung cancer has a relatively poor prognosis—with an average 5-year survival rate of 20.5%—early-stage lung cancer is more amenable to treatment and has a better prognosis (as is true with many cancers).1
LDCT has a high sensitivity, as well as a reasonable specificity, for lung cancer detection. There is demonstrated benefit in screening patients who are at high risk for lung cancer.8-11 In 2013, the USPSTF recommended annual lung cancer screening (B recommendation) with LDCT in adults 55 to 80 years of age who have a 30-pack-year smoking history, and who currently smoke or quit within the past 15 years.1
Continue to: STUDY SUMMARY
STUDY SUMMARY
Broader eligibility for screening supports mortality benefit
This is an update to the 2013 clinical practice guideline on lung cancer screening. The USPSTF used 2 methods to provide the best possible evidence for the recommendations. The first method was a systematic review of the accuracy of screening for lung cancer with LDCT, evaluating both the benefits and harms of lung cancer screening. The systematic review examined various subgroups, the number and/or frequency of LDCT scans, and various approaches to reducing false-positive results. In addition to the systematic review, they used collaborative modeling studies to determine the optimal age for beginning and ending screening, the optimal screening interval, and the relative benefits and harms of various screening strategies. These modeling studies complemented the evidence review.
The review included 7 randomized controlled trials (RCTs), plus the modeling studies. Only the National Lung Screening Trial (NLST; N = 53,454) and the Nederlands-Leuvens Longkanker Screenings Onderzoek (NELSON) trial (N = 15,792) had adequate power to detect a mortality benefit from screening (NLST: relative risk reduction = 16%; 95% CI, 5%-25%; NELSON: incidence rate ratio = 0.75; 95% CI, 0.61-0.90) compared with no screening.
Screening intervals, from the NLST and NELSON trials as well as the modeling studies, revealed the greatest benefit from annual screening (statistics not shared). Evidence also showed that screening those with lighter smoking histories (< 30 pack-years) and at an earlier age (age 50) provided increased mortality benefit. No evidence was found for a benefit of screening past 80 years of age. The modeling studies concluded that the 2013 USPSTF screening program, using a starting age of 55 and a 30-pack-year smoking history, would reduce mortality by 9.8%, but by changing to a starting age of 50, a 20-pack-year smoking history, and annual screening, the mortality benefit was increased to 13%.1,11
Comparison with computer-based risk prediction models from the Cancer Intervention and Surveillance Modeling Network (CISNET) revealed insufficient evidence at this time to show that prediction model–based screening offered any benefit beyond that of the age and smoking history risk factor model.
The incidence of false-positive results was > 25% in the NLST at baseline and at 1 year. Use of a classification system such as the Lung Imaging Reporting and Data System (Lung-RADS) could reduce that from 26.6% to 12.8%.2 Another potential harm from LDCT screening is radiation exposure. Evidence from several RCTs and cohort studies showed the exposure from 1 LDCT scan to be 0.65 to 2.36 mSv, whereas the annual background radiation in the United States is 2.4 mSv. The modeling studies estimated that there would be 1 death caused by LDCT for every 18.5 cancer deaths avoided.1,11
Continue to: WHAT'S NEW
WHAT’S NEW
Expanded age range, reduced pack-year history
Annual lung cancer screening is now recommended to begin for patients at age 50 years with a 20-pack-year history instead of age 55 years with a 30-pack-year history. This would nearly double (87% overall) the number of people eligible for screening, and it would include more Black patients and women, who tend to smoke fewer cigarettes than their White male counterparts. The American College of Radiology estimates that the expanded screening criteria could save between 30,000 and 60,000 lives per year.12
CAVEATS
Screening criteria for upper age limit, years since smoking remain unchanged
For those patients who quit smoking, the guidelines apply only to those who have stopped smoking within the past 15 years. Furthermore, the benefit does not extend beyond age 80 or where other conditions reduce life expectancy. And, as noted earlier, modeling studies estimate that there would be 1 death caused by LDCT for every 18.5 cancer deaths avoided.1,11
CHALLENGES TO IMPLEMENTATION
Concerns about false-positives, radiation exposure may limit acceptance
Challenges would be based mostly on the need for greater, more detailed dialogue between physicians and patients at higher risk for lung cancer in a time-constrained environment. Also, LDCT may not be available in some areas, and patients and physicians may have concerns regarding repeated CT exposure. In addition, false-positive results increase patient stress and may adversely affect both patient and physician acceptance.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 50-year-old woman presents to your office for a well-woman exam. Her past medical history includes a 22-pack-year smoking history (she quit 5 years ago), well-controlled hypertension, and mild obesity. She has no family history of cancer, but she does have a family history of type 2 diabetes and heart disease. Besides age- and risk-appropriate laboratory tests, cervical cancer screening, breast cancer screening, and initial colon cancer screening, are there any other preventive services you would offer her?
Lung cancer is the second most common cancer in both men and women, and it is the leading cause of cancer death in the United States—regardless of gender. The American Cancer Society estimates that 235,760 people will be diagnosed with lung cancer and 131,880 people will die of the disease in 2021.2
In the 2015 National Cancer Institute report on the economic costs of cancer, direct and indirect costs of lung cancer totaled $21.1 billion annually. Lost productivity from lung cancer added another $36.1 billion in annual costs.3 The economic costs increased to $23.8 billion in 2020, with no data on lost productivity.4
Smoking tobacco is by far the primary risk factor for lung cancer, and it is estimated to account for 90% of all lung cancer cases. Compared with nonsmokers, the relative risk of lung cancer is approximately 20 times higher for smokers.5,6
Because the median age of lung cancer diagnosis is 70 years, increasing age is also considered a risk factor for lung cancer.2,7
Although lung cancer has a relatively poor prognosis—with an average 5-year survival rate of 20.5%—early-stage lung cancer is more amenable to treatment and has a better prognosis (as is true with many cancers).1
LDCT has a high sensitivity, as well as a reasonable specificity, for lung cancer detection. There is demonstrated benefit in screening patients who are at high risk for lung cancer.8-11 In 2013, the USPSTF recommended annual lung cancer screening (B recommendation) with LDCT in adults 55 to 80 years of age who have a 30-pack-year smoking history, and who currently smoke or quit within the past 15 years.1
Continue to: STUDY SUMMARY
STUDY SUMMARY
Broader eligibility for screening supports mortality benefit
This is an update to the 2013 clinical practice guideline on lung cancer screening. The USPSTF used 2 methods to provide the best possible evidence for the recommendations. The first method was a systematic review of the accuracy of screening for lung cancer with LDCT, evaluating both the benefits and harms of lung cancer screening. The systematic review examined various subgroups, the number and/or frequency of LDCT scans, and various approaches to reducing false-positive results. In addition to the systematic review, they used collaborative modeling studies to determine the optimal age for beginning and ending screening, the optimal screening interval, and the relative benefits and harms of various screening strategies. These modeling studies complemented the evidence review.
The review included 7 randomized controlled trials (RCTs), plus the modeling studies. Only the National Lung Screening Trial (NLST; N = 53,454) and the Nederlands-Leuvens Longkanker Screenings Onderzoek (NELSON) trial (N = 15,792) had adequate power to detect a mortality benefit from screening (NLST: relative risk reduction = 16%; 95% CI, 5%-25%; NELSON: incidence rate ratio = 0.75; 95% CI, 0.61-0.90) compared with no screening.
Screening intervals, from the NLST and NELSON trials as well as the modeling studies, revealed the greatest benefit from annual screening (statistics not shared). Evidence also showed that screening those with lighter smoking histories (< 30 pack-years) and at an earlier age (age 50) provided increased mortality benefit. No evidence was found for a benefit of screening past 80 years of age. The modeling studies concluded that the 2013 USPSTF screening program, using a starting age of 55 and a 30-pack-year smoking history, would reduce mortality by 9.8%, but by changing to a starting age of 50, a 20-pack-year smoking history, and annual screening, the mortality benefit was increased to 13%.1,11
Comparison with computer-based risk prediction models from the Cancer Intervention and Surveillance Modeling Network (CISNET) revealed insufficient evidence at this time to show that prediction model–based screening offered any benefit beyond that of the age and smoking history risk factor model.
The incidence of false-positive results was > 25% in the NLST at baseline and at 1 year. Use of a classification system such as the Lung Imaging Reporting and Data System (Lung-RADS) could reduce that from 26.6% to 12.8%.2 Another potential harm from LDCT screening is radiation exposure. Evidence from several RCTs and cohort studies showed the exposure from 1 LDCT scan to be 0.65 to 2.36 mSv, whereas the annual background radiation in the United States is 2.4 mSv. The modeling studies estimated that there would be 1 death caused by LDCT for every 18.5 cancer deaths avoided.1,11
Continue to: WHAT'S NEW
WHAT’S NEW
Expanded age range, reduced pack-year history
Annual lung cancer screening is now recommended to begin for patients at age 50 years with a 20-pack-year history instead of age 55 years with a 30-pack-year history. This would nearly double (87% overall) the number of people eligible for screening, and it would include more Black patients and women, who tend to smoke fewer cigarettes than their White male counterparts. The American College of Radiology estimates that the expanded screening criteria could save between 30,000 and 60,000 lives per year.12
CAVEATS
Screening criteria for upper age limit, years since smoking remain unchanged
For those patients who quit smoking, the guidelines apply only to those who have stopped smoking within the past 15 years. Furthermore, the benefit does not extend beyond age 80 or where other conditions reduce life expectancy. And, as noted earlier, modeling studies estimate that there would be 1 death caused by LDCT for every 18.5 cancer deaths avoided.1,11
CHALLENGES TO IMPLEMENTATION
Concerns about false-positives, radiation exposure may limit acceptance
Challenges would be based mostly on the need for greater, more detailed dialogue between physicians and patients at higher risk for lung cancer in a time-constrained environment. Also, LDCT may not be available in some areas, and patients and physicians may have concerns regarding repeated CT exposure. In addition, false-positive results increase patient stress and may adversely affect both patient and physician acceptance.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. US Preventive Services Task Force. Lung cancer: screening. Final recommendation statement. March 9, 2021. Accessed August 19, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
2. American Cancer Society. Key statistics for lung cancer. Updated January 12, 2021. Accessed August 19, 2021. www.cancer.org/cancer/lung-cancer/about/key-statistics.html
3. National Cancer Institute. Cancer Trends Progress Report—Financial Burden of Cancer Care. National Institutes of Health; 2015.
4. National Cancer Institute. Cancer Trends Progress Report—Financial Burden of Cancer Care. National Institutes of Health. Updated July 2021. Accessed August 19, 2021. https://progressreport.cancer.gov/after/economic_burden
5. Alberg AJ, Brock MV, Ford JG, et al. Epidemiology of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 suppl):e1S-e29S. doi: 10.1378/chest.12-2345
6. Samet JM. Health benefits of smoking cessation. Clin Chest Med. 1991;12:669-679.
7. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. doi: 10.3322/caac.21254
8. National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
9. Pinsky PF, Church TR, Izmirlian G, et al. The National Lung Screening Trial: results stratified by demographics, smoking history, and lung cancer histology. Cancer. 2013;119:3976-3983. doi: 10.1002/cncr.28326
10. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503-513. doi: 10.1056/NEJMoa1911793
11. Meza R, Jeon J, Toumazis I, et al. Evaluation of the Benefits and Harms of Lung Cancer Screening With Low-Dose Computed Tomography: A Collaborative Modeling Study for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality; 2021.
12. American College of Radiology. Updated USPSTF lung cancer screening guidelines would help save lives. July 7, 2020. Accessed August 19, 2021. www.acr.org/Media-Center/ACR-News-Releases/2020/Updated-USPSTF-Lung-Cancer-Screening-Guidelines-Would-Help-Save-Lives
1. US Preventive Services Task Force. Lung cancer: screening. Final recommendation statement. March 9, 2021. Accessed August 19, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
2. American Cancer Society. Key statistics for lung cancer. Updated January 12, 2021. Accessed August 19, 2021. www.cancer.org/cancer/lung-cancer/about/key-statistics.html
3. National Cancer Institute. Cancer Trends Progress Report—Financial Burden of Cancer Care. National Institutes of Health; 2015.
4. National Cancer Institute. Cancer Trends Progress Report—Financial Burden of Cancer Care. National Institutes of Health. Updated July 2021. Accessed August 19, 2021. https://progressreport.cancer.gov/after/economic_burden
5. Alberg AJ, Brock MV, Ford JG, et al. Epidemiology of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 suppl):e1S-e29S. doi: 10.1378/chest.12-2345
6. Samet JM. Health benefits of smoking cessation. Clin Chest Med. 1991;12:669-679.
7. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. doi: 10.3322/caac.21254
8. National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
9. Pinsky PF, Church TR, Izmirlian G, et al. The National Lung Screening Trial: results stratified by demographics, smoking history, and lung cancer histology. Cancer. 2013;119:3976-3983. doi: 10.1002/cncr.28326
10. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503-513. doi: 10.1056/NEJMoa1911793
11. Meza R, Jeon J, Toumazis I, et al. Evaluation of the Benefits and Harms of Lung Cancer Screening With Low-Dose Computed Tomography: A Collaborative Modeling Study for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality; 2021.
12. American College of Radiology. Updated USPSTF lung cancer screening guidelines would help save lives. July 7, 2020. Accessed August 19, 2021. www.acr.org/Media-Center/ACR-News-Releases/2020/Updated-USPSTF-Lung-Cancer-Screening-Guidelines-Would-Help-Save-Lives
PRACTICE CHANGER
Start assessing risk and screening for lung cancer at age 50 in patients who have a 20-pack-year history of smoking, using low-dose computed tomography (LDCT) scanning. This practice, based on a 2020 US Preventive Services Task Force (USPSTF) guideline update, is expected to reduce annual mortality from lung cancer by an additional 3% or more (from 9.8% to 13%).
STRENGTH OF RECOMMENDATION
A: Evidence-based clinical practice guideline1
US Preventive Services Task Force. Lung cancer: screening. Final recommendation statement. March 9, 2021. Accessed August 19, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
Youth e-cigarette use: Assessing for, and halting, the hidden habit
THE CASE
Joe, an 18-year-old, has been your patient for many years and has an uncomplicated medical history. He presents for his preparticipation sports examination for the upcoming high school baseball season. Joe’s mother, who arrives at the office with him, tells you she’s worried because she found an e-cigarette in his backpack last week. Joe says that many of the kids at his school vape and he tried it a while back and now vapes “a lot.”
After talking further with Joe, you realize that he is vaping every day, using a 5% nicotine pod. Based on previous consults with the behavioral health counselor in your clinic, you know that this level of vaping is about the same as smoking 1 pack of cigarettes per day in terms of nicotine exposure. Joe states that he often vapes in the bathroom at school because he cannot concentrate in class if he doesn’t vape. He also reports that he had previously used 1 pod per week but had recently started vaping more to help with his cravings.
You assess his withdrawal symptoms and learn that he feels on edge when he is not able to vape and that he vapes heavily before going into school because he knows he will not be able to vape again until his third passing period.
●
Electronic cigarettes (e-cigarettes; also called “vapes”) are electronic nicotine delivery systems that heat and aerosolize e-liquid or “e-juice” that is inhaled by the user. The e-liquid is made up primarily of propylene glycol, vegetable glycerin, and flavorings, and often includes nicotine. Nicotine levels in e-cigarettes can range from 0 mg/mL to 60 mg/mL (regular cigarettes contain ~12 mg of nicotine). The nicotine level of the pod available from e-cigarette company JUUL (50 mg/mL e-liquid) is equivalent to about 1 pack of cigarettes.1 E-cigarette devices are relatively affordable; popular brands cost $10 to $20, while the replacement pods or e-liquid are typically about $4 each.
The e-cigarette market is quickly evolving and diversifying. Originally, e-cigarettes looked similar to cigarettes (cig-a-likes) but did not efficiently deliver nicotine to the user.2 E-cigarettes have evolved and some now deliver cigarette-like levels of nicotine to the user.3,4 Youth and young adults primarily use pod-mod e-cigarettes, which have a sleek design and produce less vapor than older e-cigarettes, making them easier to conceal. They can look like a USB flash-drive or have a teardrop shape. Pod-mod e-cigarettes dominate the current market, led by companies such as JUUL, NJOY, and Vuse.5
E-cigarette use is proliferating in the United States, particularly among young people and facilitated by the introduction of pod-based e-cigarettes in appealing flavors.6,7 While rates of current e-cigarette use by US adults is around 5.5%,8 recent data show that 32.7% of US high school students say they’ve used an e-cigarette in the past 30 days.9
Continue to: A double-edged sword
A double-edged sword. E-cigarettes are less harmful than traditional cigarettes in the short term and likely benefit adult smokers who completely substitute e-cigarettes for their tobacco cigarettes.10 In randomized trials of adult smokers, e-cigarette use resulted in moderate combustible-cigarette cessation rates that rival or exceed rates achieved with traditional nicotine replacement therapy (NRT).11-13 However, most e-cigarettes contain addictive nicotine, can facilitate transitions to more harmful forms of tobacco use,10,14,15 and have unknown long-term health effects. Therefore, youth, young adults, and those who are otherwise tobacco naïve should not initiate e-cigarette use.
Moreover, cases of e-cigarette or vaping product use–associated lung injury (EVALI)—a disease linked to vaping that causes cough, fever, shortness of breath, and death—were first identified in August 2019 and peaked in September 2019 before new cases decreased dramatically through January 2020.16 Since the initial cases of EVALI arose, product testing has shown that tetrahydrocannabinol (THC) and vitamin E acetate are the main ingredients linked to EVALI cases.17 For this reason, the Centers for Disease Control and Prevention and others strongly recommend against use of THC-containing e-cigarettes.18
Given the high rates of e-cigarette use among youth and young adults and its potential health harms, it is critical to inquire about e-cigarette use at primary care visits, and, as appropriate, to assess frequency and quantity of use. Patients who require intervention will be more likely to succeed in quitting if they are connected with behavioral health counseling and prescribed medication. This article offers evidence-based guidance to assess and advise teens and young adults regarding the potential health impact of e-cigarettes.
A NEW ICD-10-CM CODE AND A BRIEF ASSESSMENT TOOL
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5)19 and the International Classification of Diseases, 10th Revision (ICD-10-CM),20 a tobacco use disorder is a problematic pattern of use leading to clinically significant impairment or distress. Associated features and behavioral markers of frequency and quantity include use within 30 minutes of waking, daily use, and increasing use. However, with youth, consider intervention for use of any nicotine or tobacco product, including e-cigarettes, regardless of whether it meets the threshold for diagnosis.21
The new code.
Continue to: As with other tobacco use...
As with other tobacco use, assess e-cigarette use patterns by asking questions about the frequency, duration, and quantity of use. Additionally, determine the level of nicotine in the e-liquid (discussed earlier) and evaluate whether the individual displays signs of physiologic dependence (eg, failed attempts to reduce or quit e-cigarette use, increased use, nicotine withdrawal symptoms).
A useful assessment tool. While e-cigarette use is not often included on current substance use screening measures, the above questions can be added to the end of measures such as the CRAFFT (Car-Relax-Alone-Forget-Family and Friends-Trouble) test.22 Additionally, if an adolescent reports vaping, the American Academy of Pediatrics (AAP) recommends using a brief screening tool such as the Hooked on Nicotine Checklist (HONC) to establish his or her level of dependence (TABLE 1).23
The HONC is ideal for a primary care setting because it is brief and has a high level of sensitivity, minimizing false-negative reports24; a patient’s acknowledgement of any item indicates a loss of autonomy over nicotine. Establishing the level of nicotine dependence is particularly pertinent when making decisions regarding the course of treatment and whether to prescribe NRT (eg, nicotine patch, gum, lozenge). Alternatively, you can quickly assess level of dependence by determining the time to first e-cigarette use in the morning. Tobacco guidelines suggest that if time to first use is > 30 minutes, the individual is “moderately dependent”; if time to first use is < 30 minutes after waking, the individual is “severely dependent.”25
COMBINATION TREATMENT IS MOST SUCCESSFUL
Studies have shown that the most effective treatment for tobacco cessation is pairing behavioral treatment with combination NRT (eg, nicotine gum + patch).25,26 The literature on e-cigarette cessation remains in its infancy, but techniques from traditional smoking cessation can be applied because the behaviors differ only in their mode of nicotine delivery.
Behavioral treatment. There are several options for behavioral treatment for tobacco cessation—and thus, e-cigarette cessation. The first step will depend on the patient’s level of motivation. If the patient is not yet ready to quit, consider using brief motivational interviewing. Once the patient is willing to engage in treatment, options include setting a mutually agreed upon quit date or planning for a reduction in the frequency and duration of vaping.
Continue to: Referrals to the Quitline...
Referrals to the Quitline (800-QUIT-NOW) have long been standard practice and can be used to extend primary care treatment.25 Studies show that it is more effective to connect patients directly to the Quitline at their primary care appointment27 than asking them to call after the visit.28,29 We suggest providing direct assistance in the office to patients as they initiate treatment with the Quitline.
Finally, if the level of dependence is severe or the patient is not motivated to quit, connect them with a behavioral health provider in your clinic or with an outside therapist skilled in cognitive behavioral techniques related to tobacco cessation. Discuss with the patient that quitting nicotine use is difficult for many people and that the best option for success is the combination of counseling and medication.25
Nicotine replacement therapy for e-cigarette use. While over-the-counter NRT (nicotine gum, patches, lozenges) is approved by the US Food and Drug Administration only for sale to adults ≥ 18 years, the AAP issued guidance on prescribing NRT for those < 18 years who use e-cigarettes.30 While the AAP does not suggest a lower age limit for prescribing NRT, national data show that < 6% of middle schoolers report e-cigarette use and that e-cigarette use does not become common (~20% current use) until high school.31 It is therefore unlikely that a child < 14 years would require pharmacotherapy. On their fact sheet, the AAP includes the following guidance:
“Patients who are motivated to quit should use as much safe, FDA-approved NRT as needed to avoid smoking or vaping. When assessing a patient’s current level of nicotine use, it may be helpful to understand that using one JUUL pod per day is equivalent to one pack of cigarettes per day …. Pediatricians and other healthcare providers should work with each patient to determine a starting dosage of NRT that is most likely to help them quit successfully. Dosing is based on the patient’s level of nicotine dependence, which can be measured using a screening tool” (TABLE 123).32
The AAP NRT dosing guidelines can be found at downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf.32 Of note, the dosing guidelines for adolescents are the same as those for adults and are based on level of use and dependence. Moreover, the clinician and patient should work together to choose the initial dose and the plan for weaning NRT over time.
Continue to: THE CASE
Based on your conversation with Joe, you administer the HONC screening tool. He scores 9 out of 10, indicating significant loss of autonomy over nicotine. You consult with a behavioral health counselor, who believes that Joe would benefit from counseling and NRT. You discuss this treatment plan with Joe, who says he is ready to quit because he does not like feeling as if he depends on vaping. Your shared decision is to start the 21-mg patch and 4-mg gum with plans to step down from there.
Joe agrees to set a quit date in the following week. The behavioral health counselor then meets with Joe and they develop a quit plan, which is shared with you so you can follow up at the next visit. Joe also agrees to talk with his parents, who are unaware of his level of use and dependence. Everyone agrees on the quit plan, and a follow-up visit is scheduled.
At the follow-up visit 1 month later, Joe and his parents report that he has quit vaping but is still using the patch and gum. You instruct Joe to reduce his NRT use to the 14-mg patch and 2-mg gum and to stop using them over the next 2 to 3 weeks. Everyone is in agreement with the treatment plan. You also re-administer the HONC screening tool and see that Joe’s score has reduced by 7 points to just 2 out of 10. You recommend that Joe continue to see the behavioral health counselor and follow up as needed. (A noted benefit of having a behavioral health counselor in your clinic is the opportunity for informal briefings on patient progress.33,34)
Following each visit with Joe, you make sure to complete documentation on (1) tobacco/e-cigarette use assessment, (2) diagnoses, (3) discussion of benefits of quitting,(4) assessment of readiness to quit, (5) creation and support of a quit plan, and (6) connection with a behavioral health counselor and planned follow-up. (See TABLE 235 for details onbilling codes.)

CORRESPONDENCE
Eleanor L. S. Leavens, PhD, 3901 Rainbow Boulevard, Mail Stop 1008, Kansas City, KS 66160; [email protected]
1. Prochaska JJ, Vogel EA, Benowitz N. Nicotine delivery and cigarette equivalents from vaping a JUULpod. Tob Control. Published online March 24, 2021. doi: 10.1136/tobaccocontrol- 2020-056367
2. Rüther T, Hagedorn D, Schiela K, et al. Nicotine delivery efficiency of first-and second-generation e-cigarettes and its impact on relief of craving during the acute phase of use. Int J Hyg Environ Health. 2018;221:191-198. doi: 10.1016/j.ijheh.2017.10.012
3. Hajek P, Pittaccio K, Pesola F, et al. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e‐cigarette products. Addiction. 2020;115:1141-1148. doi: 10.1111/add.14936
4. Wagener TL, Floyd EL, Stepanov I, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob control. 2017;26:e23-e28. doi: 10.1136/tobaccocontrol-2016-053041
5. Herzog B, Kanada P. Nielsen: Tobacco all channel data thru 8/11 - cig vol decelerates. Published August 21, 2018. Accessed August 19, 2021. https://athra.org.au/wp-content/uploads/2018/09/Wells-Fargo-Nielsen-Tobacco-All-Channel-Report-Period-Ending-8.11.18.pdf
6. Harrell MB, Weaver SR, Loukas A, et al. Flavored e-cigarette use: characterizing youth, young adult, and adult users. Prev Med Rep. 2017;5:33-40. doi: 10.1016/j.pmedr.2016.11.001
7. Morean ME, Butler ER, Bold KW, et al. Preferring more e-cigarette flavors is associated with e-cigarette use frequency among adolescents but not adults. PloS One. 2018;13:e0189015. doi: 10.1371/journal.pone.0189015
8. Obisesan OH, Osei AD, Iftekhar Uddin SM, et al. Trends in e-cigarette use in adults in the United States, 2016-2018. JAMA Intern Med. 2020;180:1394-1398. doi: 10.1001/jamainternmed.2020.2817
9. Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:1013-1019. doi: 10.15585/mmwr.mm6845a2
10. NASEM. Public health consequences of e-cigarettes. National Academies Press; 2018. Accessed August 19, 2021. www.ncbi.nlm.nih.gov/books/NBK507171/
11. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380:629-637. doi: 10.1056/NEJMoa1808779
12. Pulvers K, Nollen NL, Rice M, et al. Effect of pod e-cigarettes vs cigarettes on carcinogen exposure among African American and Latinx smokers: a randomized clinical trial. JAMA Netw Open. 2020;3:e2026324. doi: 10.1001/jamanetworkopen.2020.26324
13. Wang RJ, Bhadriraju S, Glantz SA. E-cigarette use and adult cigarette smoking cessation: a meta-analysis. Am J Public Health. 2021;111:230-246. doi: 10.2105/AJPH.2020.305999
14. Barrington-Trimis JL, Urman R, Berhane K, et al. E-cigarettes and future cigarette use. Pediatrics. 2016;138:e20160379. doi: 10.1542/peds.2016-0379
15. Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797. doi: 10.1001/jamapediatrics.2017.1488
16. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use–associated lung injury—United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. 2020;69:90-94. doi: 10.15585/mmwr.mm6903e2
17. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382:697-705. doi: 10.1056/NEJMoa1916433
18. CDC. Outbreak of lung injury associated with use of e-cigarette, or vaping, products. Updated February 25, 2020. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
20. CDC. International Classification of Diseases, 10th Revision. Updated July 30, 2021. Accessed August 31, 2021. www.cdc.gov/nchs/icd/icd10cm.htm
21. CDC. Surgeon General’s advisory on e-cigarette use among youth. Reviewed April 9, 2019. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/surgeon-general-advisory/index.html
22. Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614. doi: 10.1001/archpedi.156.6.607
23. DiFranza JR, Savageau JA, Fletcher K, et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med. 2002;156:397-403. doi: 10.1001/archpedi.156.4.397
24. Wellman RJ, Savageau JA, Godiwala S, et al. A comparison of the Hooked on Nicotine Checklist and the Fagerström Test for Nicotine Dependence in adult smokers. Nicotine Tob Res. 2006;8:575-580. doi: 10.1080/14622200600789965
25. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Published May 2008. Accessed August 19, 2021. www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/TreatingTobaccoUseandDependence-2008Update.pdf
26. Shah SD, Wilken LA, Winkler SR, et al. Systematic review and meta-analysis of combination therapy for smoking cessation. J Am Pharm Assoc. 2008;48:659-665. doi: 10.1331/JAPhA.2008.07063
27. Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173:458-464. doi: 10.1001/jamainternmed.2013.3751
28. Bentz CJ, Bayley KB, Bonin KE, et al. The feasibility of connecting physician offices to a state-level tobacco quit line. Am J Prev Med. 2006;30:31-37. doi: 10.1016/j.amepre.2005.08.043
29. Borland R, Segan CJ. The potential of quitlines to increase smoking cessation. Drug Alcohol Rev. 2006;25:73-78. doi: 10.1080/09595230500459537
30. Farber HJ, Walley SC, Groner JA, et al. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017. doi: 10.1542/peds.2015-3108
31. Gentzke AS, Wang TW, Jamal A, et al. Tobacco product use among middle and high school students—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1881-1888. doi: 10.15585/mmwr.mm6950a1
32. AAP. Nicotine replacement therapy and adolescent patients: information for pediatricians. Updated November 2019. Accessed August 19, 2021. https://downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf
33. Blasi PR, Cromp D, McDonald S, et al. Approaches to behavioral health integration at high performing primary care practices. J Am Board Fam Med. 2018;31:691-701. doi: 10.3122/jabfm.2018.05.170468
34. Jacobs C, Brieler JA, Salas J, et al. Integrated behavioral health care in family medicine residencies a CERA survey. Fam Med. 2018;50:380-384. doi: 10.22454/FamMed.2018.639260
35. Oliverez M. Quick guide: billing for smoking cessation services. Capture Billing. Accessed August 26, 2021. https://capturebilling.com/how-bill-smoking-cessation-counseling-99406-99407/
THE CASE
Joe, an 18-year-old, has been your patient for many years and has an uncomplicated medical history. He presents for his preparticipation sports examination for the upcoming high school baseball season. Joe’s mother, who arrives at the office with him, tells you she’s worried because she found an e-cigarette in his backpack last week. Joe says that many of the kids at his school vape and he tried it a while back and now vapes “a lot.”
After talking further with Joe, you realize that he is vaping every day, using a 5% nicotine pod. Based on previous consults with the behavioral health counselor in your clinic, you know that this level of vaping is about the same as smoking 1 pack of cigarettes per day in terms of nicotine exposure. Joe states that he often vapes in the bathroom at school because he cannot concentrate in class if he doesn’t vape. He also reports that he had previously used 1 pod per week but had recently started vaping more to help with his cravings.
You assess his withdrawal symptoms and learn that he feels on edge when he is not able to vape and that he vapes heavily before going into school because he knows he will not be able to vape again until his third passing period.
●
Electronic cigarettes (e-cigarettes; also called “vapes”) are electronic nicotine delivery systems that heat and aerosolize e-liquid or “e-juice” that is inhaled by the user. The e-liquid is made up primarily of propylene glycol, vegetable glycerin, and flavorings, and often includes nicotine. Nicotine levels in e-cigarettes can range from 0 mg/mL to 60 mg/mL (regular cigarettes contain ~12 mg of nicotine). The nicotine level of the pod available from e-cigarette company JUUL (50 mg/mL e-liquid) is equivalent to about 1 pack of cigarettes.1 E-cigarette devices are relatively affordable; popular brands cost $10 to $20, while the replacement pods or e-liquid are typically about $4 each.
The e-cigarette market is quickly evolving and diversifying. Originally, e-cigarettes looked similar to cigarettes (cig-a-likes) but did not efficiently deliver nicotine to the user.2 E-cigarettes have evolved and some now deliver cigarette-like levels of nicotine to the user.3,4 Youth and young adults primarily use pod-mod e-cigarettes, which have a sleek design and produce less vapor than older e-cigarettes, making them easier to conceal. They can look like a USB flash-drive or have a teardrop shape. Pod-mod e-cigarettes dominate the current market, led by companies such as JUUL, NJOY, and Vuse.5
E-cigarette use is proliferating in the United States, particularly among young people and facilitated by the introduction of pod-based e-cigarettes in appealing flavors.6,7 While rates of current e-cigarette use by US adults is around 5.5%,8 recent data show that 32.7% of US high school students say they’ve used an e-cigarette in the past 30 days.9
Continue to: A double-edged sword
A double-edged sword. E-cigarettes are less harmful than traditional cigarettes in the short term and likely benefit adult smokers who completely substitute e-cigarettes for their tobacco cigarettes.10 In randomized trials of adult smokers, e-cigarette use resulted in moderate combustible-cigarette cessation rates that rival or exceed rates achieved with traditional nicotine replacement therapy (NRT).11-13 However, most e-cigarettes contain addictive nicotine, can facilitate transitions to more harmful forms of tobacco use,10,14,15 and have unknown long-term health effects. Therefore, youth, young adults, and those who are otherwise tobacco naïve should not initiate e-cigarette use.
Moreover, cases of e-cigarette or vaping product use–associated lung injury (EVALI)—a disease linked to vaping that causes cough, fever, shortness of breath, and death—were first identified in August 2019 and peaked in September 2019 before new cases decreased dramatically through January 2020.16 Since the initial cases of EVALI arose, product testing has shown that tetrahydrocannabinol (THC) and vitamin E acetate are the main ingredients linked to EVALI cases.17 For this reason, the Centers for Disease Control and Prevention and others strongly recommend against use of THC-containing e-cigarettes.18
Given the high rates of e-cigarette use among youth and young adults and its potential health harms, it is critical to inquire about e-cigarette use at primary care visits, and, as appropriate, to assess frequency and quantity of use. Patients who require intervention will be more likely to succeed in quitting if they are connected with behavioral health counseling and prescribed medication. This article offers evidence-based guidance to assess and advise teens and young adults regarding the potential health impact of e-cigarettes.
A NEW ICD-10-CM CODE AND A BRIEF ASSESSMENT TOOL
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5)19 and the International Classification of Diseases, 10th Revision (ICD-10-CM),20 a tobacco use disorder is a problematic pattern of use leading to clinically significant impairment or distress. Associated features and behavioral markers of frequency and quantity include use within 30 minutes of waking, daily use, and increasing use. However, with youth, consider intervention for use of any nicotine or tobacco product, including e-cigarettes, regardless of whether it meets the threshold for diagnosis.21
The new code.
Continue to: As with other tobacco use...
As with other tobacco use, assess e-cigarette use patterns by asking questions about the frequency, duration, and quantity of use. Additionally, determine the level of nicotine in the e-liquid (discussed earlier) and evaluate whether the individual displays signs of physiologic dependence (eg, failed attempts to reduce or quit e-cigarette use, increased use, nicotine withdrawal symptoms).
A useful assessment tool. While e-cigarette use is not often included on current substance use screening measures, the above questions can be added to the end of measures such as the CRAFFT (Car-Relax-Alone-Forget-Family and Friends-Trouble) test.22 Additionally, if an adolescent reports vaping, the American Academy of Pediatrics (AAP) recommends using a brief screening tool such as the Hooked on Nicotine Checklist (HONC) to establish his or her level of dependence (TABLE 1).23

The HONC is ideal for a primary care setting because it is brief and has a high level of sensitivity, minimizing false-negative reports24; a patient’s acknowledgement of any item indicates a loss of autonomy over nicotine. Establishing the level of nicotine dependence is particularly pertinent when making decisions regarding the course of treatment and whether to prescribe NRT (eg, nicotine patch, gum, lozenge). Alternatively, you can quickly assess level of dependence by determining the time to first e-cigarette use in the morning. Tobacco guidelines suggest that if time to first use is > 30 minutes, the individual is “moderately dependent”; if time to first use is < 30 minutes after waking, the individual is “severely dependent.”25
COMBINATION TREATMENT IS MOST SUCCESSFUL
Studies have shown that the most effective treatment for tobacco cessation is pairing behavioral treatment with combination NRT (eg, nicotine gum + patch).25,26 The literature on e-cigarette cessation remains in its infancy, but techniques from traditional smoking cessation can be applied because the behaviors differ only in their mode of nicotine delivery.
Behavioral treatment. There are several options for behavioral treatment for tobacco cessation—and thus, e-cigarette cessation. The first step will depend on the patient’s level of motivation. If the patient is not yet ready to quit, consider using brief motivational interviewing. Once the patient is willing to engage in treatment, options include setting a mutually agreed upon quit date or planning for a reduction in the frequency and duration of vaping.
Continue to: Referrals to the Quitline...
Referrals to the Quitline (800-QUIT-NOW) have long been standard practice and can be used to extend primary care treatment.25 Studies show that it is more effective to connect patients directly to the Quitline at their primary care appointment27 than asking them to call after the visit.28,29 We suggest providing direct assistance in the office to patients as they initiate treatment with the Quitline.
Finally, if the level of dependence is severe or the patient is not motivated to quit, connect them with a behavioral health provider in your clinic or with an outside therapist skilled in cognitive behavioral techniques related to tobacco cessation. Discuss with the patient that quitting nicotine use is difficult for many people and that the best option for success is the combination of counseling and medication.25
Nicotine replacement therapy for e-cigarette use. While over-the-counter NRT (nicotine gum, patches, lozenges) is approved by the US Food and Drug Administration only for sale to adults ≥ 18 years, the AAP issued guidance on prescribing NRT for those < 18 years who use e-cigarettes.30 While the AAP does not suggest a lower age limit for prescribing NRT, national data show that < 6% of middle schoolers report e-cigarette use and that e-cigarette use does not become common (~20% current use) until high school.31 It is therefore unlikely that a child < 14 years would require pharmacotherapy. On their fact sheet, the AAP includes the following guidance:
“Patients who are motivated to quit should use as much safe, FDA-approved NRT as needed to avoid smoking or vaping. When assessing a patient’s current level of nicotine use, it may be helpful to understand that using one JUUL pod per day is equivalent to one pack of cigarettes per day …. Pediatricians and other healthcare providers should work with each patient to determine a starting dosage of NRT that is most likely to help them quit successfully. Dosing is based on the patient’s level of nicotine dependence, which can be measured using a screening tool” (TABLE 123).32
The AAP NRT dosing guidelines can be found at downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf.32 Of note, the dosing guidelines for adolescents are the same as those for adults and are based on level of use and dependence. Moreover, the clinician and patient should work together to choose the initial dose and the plan for weaning NRT over time.
Continue to: THE CASE
Based on your conversation with Joe, you administer the HONC screening tool. He scores 9 out of 10, indicating significant loss of autonomy over nicotine. You consult with a behavioral health counselor, who believes that Joe would benefit from counseling and NRT. You discuss this treatment plan with Joe, who says he is ready to quit because he does not like feeling as if he depends on vaping. Your shared decision is to start the 21-mg patch and 4-mg gum with plans to step down from there.
Joe agrees to set a quit date in the following week. The behavioral health counselor then meets with Joe and they develop a quit plan, which is shared with you so you can follow up at the next visit. Joe also agrees to talk with his parents, who are unaware of his level of use and dependence. Everyone agrees on the quit plan, and a follow-up visit is scheduled.
At the follow-up visit 1 month later, Joe and his parents report that he has quit vaping but is still using the patch and gum. You instruct Joe to reduce his NRT use to the 14-mg patch and 2-mg gum and to stop using them over the next 2 to 3 weeks. Everyone is in agreement with the treatment plan. You also re-administer the HONC screening tool and see that Joe’s score has reduced by 7 points to just 2 out of 10. You recommend that Joe continue to see the behavioral health counselor and follow up as needed. (A noted benefit of having a behavioral health counselor in your clinic is the opportunity for informal briefings on patient progress.33,34)
Following each visit with Joe, you make sure to complete documentation on (1) tobacco/e-cigarette use assessment, (2) diagnoses, (3) discussion of benefits of quitting,(4) assessment of readiness to quit, (5) creation and support of a quit plan, and (6) connection with a behavioral health counselor and planned follow-up. (See TABLE 235 for details onbilling codes.)

CORRESPONDENCE
Eleanor L. S. Leavens, PhD, 3901 Rainbow Boulevard, Mail Stop 1008, Kansas City, KS 66160; [email protected]
THE CASE
Joe, an 18-year-old, has been your patient for many years and has an uncomplicated medical history. He presents for his preparticipation sports examination for the upcoming high school baseball season. Joe’s mother, who arrives at the office with him, tells you she’s worried because she found an e-cigarette in his backpack last week. Joe says that many of the kids at his school vape and he tried it a while back and now vapes “a lot.”
After talking further with Joe, you realize that he is vaping every day, using a 5% nicotine pod. Based on previous consults with the behavioral health counselor in your clinic, you know that this level of vaping is about the same as smoking 1 pack of cigarettes per day in terms of nicotine exposure. Joe states that he often vapes in the bathroom at school because he cannot concentrate in class if he doesn’t vape. He also reports that he had previously used 1 pod per week but had recently started vaping more to help with his cravings.
You assess his withdrawal symptoms and learn that he feels on edge when he is not able to vape and that he vapes heavily before going into school because he knows he will not be able to vape again until his third passing period.
●
Electronic cigarettes (e-cigarettes; also called “vapes”) are electronic nicotine delivery systems that heat and aerosolize e-liquid or “e-juice” that is inhaled by the user. The e-liquid is made up primarily of propylene glycol, vegetable glycerin, and flavorings, and often includes nicotine. Nicotine levels in e-cigarettes can range from 0 mg/mL to 60 mg/mL (regular cigarettes contain ~12 mg of nicotine). The nicotine level of the pod available from e-cigarette company JUUL (50 mg/mL e-liquid) is equivalent to about 1 pack of cigarettes.1 E-cigarette devices are relatively affordable; popular brands cost $10 to $20, while the replacement pods or e-liquid are typically about $4 each.
The e-cigarette market is quickly evolving and diversifying. Originally, e-cigarettes looked similar to cigarettes (cig-a-likes) but did not efficiently deliver nicotine to the user.2 E-cigarettes have evolved and some now deliver cigarette-like levels of nicotine to the user.3,4 Youth and young adults primarily use pod-mod e-cigarettes, which have a sleek design and produce less vapor than older e-cigarettes, making them easier to conceal. They can look like a USB flash-drive or have a teardrop shape. Pod-mod e-cigarettes dominate the current market, led by companies such as JUUL, NJOY, and Vuse.5
E-cigarette use is proliferating in the United States, particularly among young people and facilitated by the introduction of pod-based e-cigarettes in appealing flavors.6,7 While rates of current e-cigarette use by US adults is around 5.5%,8 recent data show that 32.7% of US high school students say they’ve used an e-cigarette in the past 30 days.9
Continue to: A double-edged sword
A double-edged sword. E-cigarettes are less harmful than traditional cigarettes in the short term and likely benefit adult smokers who completely substitute e-cigarettes for their tobacco cigarettes.10 In randomized trials of adult smokers, e-cigarette use resulted in moderate combustible-cigarette cessation rates that rival or exceed rates achieved with traditional nicotine replacement therapy (NRT).11-13 However, most e-cigarettes contain addictive nicotine, can facilitate transitions to more harmful forms of tobacco use,10,14,15 and have unknown long-term health effects. Therefore, youth, young adults, and those who are otherwise tobacco naïve should not initiate e-cigarette use.
Moreover, cases of e-cigarette or vaping product use–associated lung injury (EVALI)—a disease linked to vaping that causes cough, fever, shortness of breath, and death—were first identified in August 2019 and peaked in September 2019 before new cases decreased dramatically through January 2020.16 Since the initial cases of EVALI arose, product testing has shown that tetrahydrocannabinol (THC) and vitamin E acetate are the main ingredients linked to EVALI cases.17 For this reason, the Centers for Disease Control and Prevention and others strongly recommend against use of THC-containing e-cigarettes.18
Given the high rates of e-cigarette use among youth and young adults and its potential health harms, it is critical to inquire about e-cigarette use at primary care visits, and, as appropriate, to assess frequency and quantity of use. Patients who require intervention will be more likely to succeed in quitting if they are connected with behavioral health counseling and prescribed medication. This article offers evidence-based guidance to assess and advise teens and young adults regarding the potential health impact of e-cigarettes.
A NEW ICD-10-CM CODE AND A BRIEF ASSESSMENT TOOL
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5)19 and the International Classification of Diseases, 10th Revision (ICD-10-CM),20 a tobacco use disorder is a problematic pattern of use leading to clinically significant impairment or distress. Associated features and behavioral markers of frequency and quantity include use within 30 minutes of waking, daily use, and increasing use. However, with youth, consider intervention for use of any nicotine or tobacco product, including e-cigarettes, regardless of whether it meets the threshold for diagnosis.21
The new code.
Continue to: As with other tobacco use...
As with other tobacco use, assess e-cigarette use patterns by asking questions about the frequency, duration, and quantity of use. Additionally, determine the level of nicotine in the e-liquid (discussed earlier) and evaluate whether the individual displays signs of physiologic dependence (eg, failed attempts to reduce or quit e-cigarette use, increased use, nicotine withdrawal symptoms).
A useful assessment tool. While e-cigarette use is not often included on current substance use screening measures, the above questions can be added to the end of measures such as the CRAFFT (Car-Relax-Alone-Forget-Family and Friends-Trouble) test.22 Additionally, if an adolescent reports vaping, the American Academy of Pediatrics (AAP) recommends using a brief screening tool such as the Hooked on Nicotine Checklist (HONC) to establish his or her level of dependence (TABLE 1).23

The HONC is ideal for a primary care setting because it is brief and has a high level of sensitivity, minimizing false-negative reports24; a patient’s acknowledgement of any item indicates a loss of autonomy over nicotine. Establishing the level of nicotine dependence is particularly pertinent when making decisions regarding the course of treatment and whether to prescribe NRT (eg, nicotine patch, gum, lozenge). Alternatively, you can quickly assess level of dependence by determining the time to first e-cigarette use in the morning. Tobacco guidelines suggest that if time to first use is > 30 minutes, the individual is “moderately dependent”; if time to first use is < 30 minutes after waking, the individual is “severely dependent.”25
COMBINATION TREATMENT IS MOST SUCCESSFUL
Studies have shown that the most effective treatment for tobacco cessation is pairing behavioral treatment with combination NRT (eg, nicotine gum + patch).25,26 The literature on e-cigarette cessation remains in its infancy, but techniques from traditional smoking cessation can be applied because the behaviors differ only in their mode of nicotine delivery.
Behavioral treatment. There are several options for behavioral treatment for tobacco cessation—and thus, e-cigarette cessation. The first step will depend on the patient’s level of motivation. If the patient is not yet ready to quit, consider using brief motivational interviewing. Once the patient is willing to engage in treatment, options include setting a mutually agreed upon quit date or planning for a reduction in the frequency and duration of vaping.
Continue to: Referrals to the Quitline...
Referrals to the Quitline (800-QUIT-NOW) have long been standard practice and can be used to extend primary care treatment.25 Studies show that it is more effective to connect patients directly to the Quitline at their primary care appointment27 than asking them to call after the visit.28,29 We suggest providing direct assistance in the office to patients as they initiate treatment with the Quitline.
Finally, if the level of dependence is severe or the patient is not motivated to quit, connect them with a behavioral health provider in your clinic or with an outside therapist skilled in cognitive behavioral techniques related to tobacco cessation. Discuss with the patient that quitting nicotine use is difficult for many people and that the best option for success is the combination of counseling and medication.25
Nicotine replacement therapy for e-cigarette use. While over-the-counter NRT (nicotine gum, patches, lozenges) is approved by the US Food and Drug Administration only for sale to adults ≥ 18 years, the AAP issued guidance on prescribing NRT for those < 18 years who use e-cigarettes.30 While the AAP does not suggest a lower age limit for prescribing NRT, national data show that < 6% of middle schoolers report e-cigarette use and that e-cigarette use does not become common (~20% current use) until high school.31 It is therefore unlikely that a child < 14 years would require pharmacotherapy. On their fact sheet, the AAP includes the following guidance:
“Patients who are motivated to quit should use as much safe, FDA-approved NRT as needed to avoid smoking or vaping. When assessing a patient’s current level of nicotine use, it may be helpful to understand that using one JUUL pod per day is equivalent to one pack of cigarettes per day …. Pediatricians and other healthcare providers should work with each patient to determine a starting dosage of NRT that is most likely to help them quit successfully. Dosing is based on the patient’s level of nicotine dependence, which can be measured using a screening tool” (TABLE 123).32
The AAP NRT dosing guidelines can be found at downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf.32 Of note, the dosing guidelines for adolescents are the same as those for adults and are based on level of use and dependence. Moreover, the clinician and patient should work together to choose the initial dose and the plan for weaning NRT over time.
Continue to: THE CASE
Based on your conversation with Joe, you administer the HONC screening tool. He scores 9 out of 10, indicating significant loss of autonomy over nicotine. You consult with a behavioral health counselor, who believes that Joe would benefit from counseling and NRT. You discuss this treatment plan with Joe, who says he is ready to quit because he does not like feeling as if he depends on vaping. Your shared decision is to start the 21-mg patch and 4-mg gum with plans to step down from there.
Joe agrees to set a quit date in the following week. The behavioral health counselor then meets with Joe and they develop a quit plan, which is shared with you so you can follow up at the next visit. Joe also agrees to talk with his parents, who are unaware of his level of use and dependence. Everyone agrees on the quit plan, and a follow-up visit is scheduled.
At the follow-up visit 1 month later, Joe and his parents report that he has quit vaping but is still using the patch and gum. You instruct Joe to reduce his NRT use to the 14-mg patch and 2-mg gum and to stop using them over the next 2 to 3 weeks. Everyone is in agreement with the treatment plan. You also re-administer the HONC screening tool and see that Joe’s score has reduced by 7 points to just 2 out of 10. You recommend that Joe continue to see the behavioral health counselor and follow up as needed. (A noted benefit of having a behavioral health counselor in your clinic is the opportunity for informal briefings on patient progress.33,34)
Following each visit with Joe, you make sure to complete documentation on (1) tobacco/e-cigarette use assessment, (2) diagnoses, (3) discussion of benefits of quitting,(4) assessment of readiness to quit, (5) creation and support of a quit plan, and (6) connection with a behavioral health counselor and planned follow-up. (See TABLE 235 for details onbilling codes.)

CORRESPONDENCE
Eleanor L. S. Leavens, PhD, 3901 Rainbow Boulevard, Mail Stop 1008, Kansas City, KS 66160; [email protected]
1. Prochaska JJ, Vogel EA, Benowitz N. Nicotine delivery and cigarette equivalents from vaping a JUULpod. Tob Control. Published online March 24, 2021. doi: 10.1136/tobaccocontrol- 2020-056367
2. Rüther T, Hagedorn D, Schiela K, et al. Nicotine delivery efficiency of first-and second-generation e-cigarettes and its impact on relief of craving during the acute phase of use. Int J Hyg Environ Health. 2018;221:191-198. doi: 10.1016/j.ijheh.2017.10.012
3. Hajek P, Pittaccio K, Pesola F, et al. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e‐cigarette products. Addiction. 2020;115:1141-1148. doi: 10.1111/add.14936
4. Wagener TL, Floyd EL, Stepanov I, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob control. 2017;26:e23-e28. doi: 10.1136/tobaccocontrol-2016-053041
5. Herzog B, Kanada P. Nielsen: Tobacco all channel data thru 8/11 - cig vol decelerates. Published August 21, 2018. Accessed August 19, 2021. https://athra.org.au/wp-content/uploads/2018/09/Wells-Fargo-Nielsen-Tobacco-All-Channel-Report-Period-Ending-8.11.18.pdf
6. Harrell MB, Weaver SR, Loukas A, et al. Flavored e-cigarette use: characterizing youth, young adult, and adult users. Prev Med Rep. 2017;5:33-40. doi: 10.1016/j.pmedr.2016.11.001
7. Morean ME, Butler ER, Bold KW, et al. Preferring more e-cigarette flavors is associated with e-cigarette use frequency among adolescents but not adults. PloS One. 2018;13:e0189015. doi: 10.1371/journal.pone.0189015
8. Obisesan OH, Osei AD, Iftekhar Uddin SM, et al. Trends in e-cigarette use in adults in the United States, 2016-2018. JAMA Intern Med. 2020;180:1394-1398. doi: 10.1001/jamainternmed.2020.2817
9. Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:1013-1019. doi: 10.15585/mmwr.mm6845a2
10. NASEM. Public health consequences of e-cigarettes. National Academies Press; 2018. Accessed August 19, 2021. www.ncbi.nlm.nih.gov/books/NBK507171/
11. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380:629-637. doi: 10.1056/NEJMoa1808779
12. Pulvers K, Nollen NL, Rice M, et al. Effect of pod e-cigarettes vs cigarettes on carcinogen exposure among African American and Latinx smokers: a randomized clinical trial. JAMA Netw Open. 2020;3:e2026324. doi: 10.1001/jamanetworkopen.2020.26324
13. Wang RJ, Bhadriraju S, Glantz SA. E-cigarette use and adult cigarette smoking cessation: a meta-analysis. Am J Public Health. 2021;111:230-246. doi: 10.2105/AJPH.2020.305999
14. Barrington-Trimis JL, Urman R, Berhane K, et al. E-cigarettes and future cigarette use. Pediatrics. 2016;138:e20160379. doi: 10.1542/peds.2016-0379
15. Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797. doi: 10.1001/jamapediatrics.2017.1488
16. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use–associated lung injury—United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. 2020;69:90-94. doi: 10.15585/mmwr.mm6903e2
17. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382:697-705. doi: 10.1056/NEJMoa1916433
18. CDC. Outbreak of lung injury associated with use of e-cigarette, or vaping, products. Updated February 25, 2020. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
20. CDC. International Classification of Diseases, 10th Revision. Updated July 30, 2021. Accessed August 31, 2021. www.cdc.gov/nchs/icd/icd10cm.htm
21. CDC. Surgeon General’s advisory on e-cigarette use among youth. Reviewed April 9, 2019. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/surgeon-general-advisory/index.html
22. Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614. doi: 10.1001/archpedi.156.6.607
23. DiFranza JR, Savageau JA, Fletcher K, et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med. 2002;156:397-403. doi: 10.1001/archpedi.156.4.397
24. Wellman RJ, Savageau JA, Godiwala S, et al. A comparison of the Hooked on Nicotine Checklist and the Fagerström Test for Nicotine Dependence in adult smokers. Nicotine Tob Res. 2006;8:575-580. doi: 10.1080/14622200600789965
25. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Published May 2008. Accessed August 19, 2021. www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/TreatingTobaccoUseandDependence-2008Update.pdf
26. Shah SD, Wilken LA, Winkler SR, et al. Systematic review and meta-analysis of combination therapy for smoking cessation. J Am Pharm Assoc. 2008;48:659-665. doi: 10.1331/JAPhA.2008.07063
27. Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173:458-464. doi: 10.1001/jamainternmed.2013.3751
28. Bentz CJ, Bayley KB, Bonin KE, et al. The feasibility of connecting physician offices to a state-level tobacco quit line. Am J Prev Med. 2006;30:31-37. doi: 10.1016/j.amepre.2005.08.043
29. Borland R, Segan CJ. The potential of quitlines to increase smoking cessation. Drug Alcohol Rev. 2006;25:73-78. doi: 10.1080/09595230500459537
30. Farber HJ, Walley SC, Groner JA, et al. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017. doi: 10.1542/peds.2015-3108
31. Gentzke AS, Wang TW, Jamal A, et al. Tobacco product use among middle and high school students—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1881-1888. doi: 10.15585/mmwr.mm6950a1
32. AAP. Nicotine replacement therapy and adolescent patients: information for pediatricians. Updated November 2019. Accessed August 19, 2021. https://downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf
33. Blasi PR, Cromp D, McDonald S, et al. Approaches to behavioral health integration at high performing primary care practices. J Am Board Fam Med. 2018;31:691-701. doi: 10.3122/jabfm.2018.05.170468
34. Jacobs C, Brieler JA, Salas J, et al. Integrated behavioral health care in family medicine residencies a CERA survey. Fam Med. 2018;50:380-384. doi: 10.22454/FamMed.2018.639260
35. Oliverez M. Quick guide: billing for smoking cessation services. Capture Billing. Accessed August 26, 2021. https://capturebilling.com/how-bill-smoking-cessation-counseling-99406-99407/
1. Prochaska JJ, Vogel EA, Benowitz N. Nicotine delivery and cigarette equivalents from vaping a JUULpod. Tob Control. Published online March 24, 2021. doi: 10.1136/tobaccocontrol- 2020-056367
2. Rüther T, Hagedorn D, Schiela K, et al. Nicotine delivery efficiency of first-and second-generation e-cigarettes and its impact on relief of craving during the acute phase of use. Int J Hyg Environ Health. 2018;221:191-198. doi: 10.1016/j.ijheh.2017.10.012
3. Hajek P, Pittaccio K, Pesola F, et al. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e‐cigarette products. Addiction. 2020;115:1141-1148. doi: 10.1111/add.14936
4. Wagener TL, Floyd EL, Stepanov I, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob control. 2017;26:e23-e28. doi: 10.1136/tobaccocontrol-2016-053041
5. Herzog B, Kanada P. Nielsen: Tobacco all channel data thru 8/11 - cig vol decelerates. Published August 21, 2018. Accessed August 19, 2021. https://athra.org.au/wp-content/uploads/2018/09/Wells-Fargo-Nielsen-Tobacco-All-Channel-Report-Period-Ending-8.11.18.pdf
6. Harrell MB, Weaver SR, Loukas A, et al. Flavored e-cigarette use: characterizing youth, young adult, and adult users. Prev Med Rep. 2017;5:33-40. doi: 10.1016/j.pmedr.2016.11.001
7. Morean ME, Butler ER, Bold KW, et al. Preferring more e-cigarette flavors is associated with e-cigarette use frequency among adolescents but not adults. PloS One. 2018;13:e0189015. doi: 10.1371/journal.pone.0189015
8. Obisesan OH, Osei AD, Iftekhar Uddin SM, et al. Trends in e-cigarette use in adults in the United States, 2016-2018. JAMA Intern Med. 2020;180:1394-1398. doi: 10.1001/jamainternmed.2020.2817
9. Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:1013-1019. doi: 10.15585/mmwr.mm6845a2
10. NASEM. Public health consequences of e-cigarettes. National Academies Press; 2018. Accessed August 19, 2021. www.ncbi.nlm.nih.gov/books/NBK507171/
11. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380:629-637. doi: 10.1056/NEJMoa1808779
12. Pulvers K, Nollen NL, Rice M, et al. Effect of pod e-cigarettes vs cigarettes on carcinogen exposure among African American and Latinx smokers: a randomized clinical trial. JAMA Netw Open. 2020;3:e2026324. doi: 10.1001/jamanetworkopen.2020.26324
13. Wang RJ, Bhadriraju S, Glantz SA. E-cigarette use and adult cigarette smoking cessation: a meta-analysis. Am J Public Health. 2021;111:230-246. doi: 10.2105/AJPH.2020.305999
14. Barrington-Trimis JL, Urman R, Berhane K, et al. E-cigarettes and future cigarette use. Pediatrics. 2016;138:e20160379. doi: 10.1542/peds.2016-0379
15. Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797. doi: 10.1001/jamapediatrics.2017.1488
16. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use–associated lung injury—United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. 2020;69:90-94. doi: 10.15585/mmwr.mm6903e2
17. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382:697-705. doi: 10.1056/NEJMoa1916433
18. CDC. Outbreak of lung injury associated with use of e-cigarette, or vaping, products. Updated February 25, 2020. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
20. CDC. International Classification of Diseases, 10th Revision. Updated July 30, 2021. Accessed August 31, 2021. www.cdc.gov/nchs/icd/icd10cm.htm
21. CDC. Surgeon General’s advisory on e-cigarette use among youth. Reviewed April 9, 2019. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/surgeon-general-advisory/index.html
22. Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614. doi: 10.1001/archpedi.156.6.607
23. DiFranza JR, Savageau JA, Fletcher K, et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med. 2002;156:397-403. doi: 10.1001/archpedi.156.4.397
24. Wellman RJ, Savageau JA, Godiwala S, et al. A comparison of the Hooked on Nicotine Checklist and the Fagerström Test for Nicotine Dependence in adult smokers. Nicotine Tob Res. 2006;8:575-580. doi: 10.1080/14622200600789965
25. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Published May 2008. Accessed August 19, 2021. www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/TreatingTobaccoUseandDependence-2008Update.pdf
26. Shah SD, Wilken LA, Winkler SR, et al. Systematic review and meta-analysis of combination therapy for smoking cessation. J Am Pharm Assoc. 2008;48:659-665. doi: 10.1331/JAPhA.2008.07063
27. Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173:458-464. doi: 10.1001/jamainternmed.2013.3751
28. Bentz CJ, Bayley KB, Bonin KE, et al. The feasibility of connecting physician offices to a state-level tobacco quit line. Am J Prev Med. 2006;30:31-37. doi: 10.1016/j.amepre.2005.08.043
29. Borland R, Segan CJ. The potential of quitlines to increase smoking cessation. Drug Alcohol Rev. 2006;25:73-78. doi: 10.1080/09595230500459537
30. Farber HJ, Walley SC, Groner JA, et al. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017. doi: 10.1542/peds.2015-3108
31. Gentzke AS, Wang TW, Jamal A, et al. Tobacco product use among middle and high school students—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1881-1888. doi: 10.15585/mmwr.mm6950a1
32. AAP. Nicotine replacement therapy and adolescent patients: information for pediatricians. Updated November 2019. Accessed August 19, 2021. https://downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf
33. Blasi PR, Cromp D, McDonald S, et al. Approaches to behavioral health integration at high performing primary care practices. J Am Board Fam Med. 2018;31:691-701. doi: 10.3122/jabfm.2018.05.170468
34. Jacobs C, Brieler JA, Salas J, et al. Integrated behavioral health care in family medicine residencies a CERA survey. Fam Med. 2018;50:380-384. doi: 10.22454/FamMed.2018.639260
35. Oliverez M. Quick guide: billing for smoking cessation services. Capture Billing. Accessed August 26, 2021. https://capturebilling.com/how-bill-smoking-cessation-counseling-99406-99407/
Refined heart rate cutoffs may improve prognostic value of acute PE scoring systems
In patients with acute pulmonary embolism, using cutoff values other than 110 beats per minute might improve the prognostic value of heart rate at admission, a recent observational study suggests.
For identifying low-risk patients, a cutoff of 80 bpm increased the sensitivity of the simplified Pulmonary Embolism Severity Index (sPESI) from about 94% to nearly 99% among nonhypotensive patients with acute symptomatic pulmonary embolism (PE), according to results of the large, registry-based study.
Similarly, using a 140-bpm cutoff increased the specificity of the Bova score for identifying intermediate-high–risk patients from about 93% to 98% in the study, which was recently published in the journal CHEST.
“Although standard dichotomization of HR [i.e., HR less than 110 vs. greater than 110 bpm] may be useful for guideline recommendations, our results will allow for more accuracy regarding clinical decision-making,” wrote lead author Ana Jaureguízar, MD, of the University of Alcalá in Madrid, on behalf of the RIETE (Registro Informatizado de la Enfermedad TromboEmbólica) investigators.
Intuitive findings inform future research
These observational findings are intuitive and do at least have the potential to inform the design of future randomized clinical trials, according to Albert J. Polito, MD, chief of the division of pulmonary medicine and medical director for the lung center at Mercy Medical Center in Baltimore.
“In medicine, there is a spectrum of risk,” Dr. Polito said in an interview. “While we love our cutoffs, which in this case has traditionally always been that 110 beats per minute for heart rate, it makes sense that there would be some range of risks of bad outcomes.”
Building on the observations of the present study, subsequent prospective randomized studies could potentially aim to determine, for example, when thrombolytic therapy should be considered in nonhypotensive patients with acute PE and higher heart rates.
“It would not be easy to design, but it’s a straightforward question to ask whether patients with the highest heart rates are the ones who potentially might benefit the most from thrombolytic therapy,” Dr. Polito said.
Value of alternative HR cutoffs
Heart rate is a simple and easily available vital sign that is clearly linked to prognosis in patients with pulmonary embolism, authors of the RIETE registry study say in their report. Accordingly, a heart rate threshold of 110 bpm has made its way into scoring systems that seek to identify low-risk patients, such as the sPESI, and those focused on identifying higher-risk patients, such as the Bova score.
However, it has not been clear whether alternative HR cutoffs would improve upon the 110-bpm threshold, they added. At the low-risk end, more accurate scoring systems could optimize the selection of patients for home treatment, while at the intermediate-high–risk end, they could better select patients for close monitoring or advanced PE treatments.
Better granularity on heart rate risks?
To better define the prognostic value of different heart rate thresholds, investigators analyzed data from RIETE, a large, ongoing, multinational prospective registry including patients with objectively confirmed acute venous thromboembolism.
For 44,331 consecutive nonhypotensive symptomatic PEs, the overall rate of 30-day all-cause mortality was 5.1%, and the 30-day PE-related mortality was 1.9%, the authors report.
Significantly poorer outcomes were seen in patients with higher heart rates as compared to patients in the 80-99 bpm range, they also found. As compared to that reference range, odds ratios for 30-day all-cause death ranged from 1.5 for heart rates of 100-109, up to 2.4 for those with heart rates of 140 bpm or greater.
Likewise, patients with higher heart rates had a 1.7- to 2.4-fold greater risk of 30-day PE-related death as compared to the 80- to 99-bpm reference range, while patients with lower heart rates had lesser risk, the data published in CHEST show.
Toward refinement of prognostic scoring
Next, investigators sought to refine the prognostic scoring systems for low-risk PE (sPESI) and intermediate-high–risk PE (Bova).
For sPESI, they found that dropping the cutoff value from 110 to 100 bpm increased the sensitivity of the score from 93.4% to 95.3%. Going down even further to 80 bpm increased sensitivity to 98.8%, according to the report.
By going down from 110 to 80 bpm, the proportion of patients defined as low-risk dropped from 35% to 12%, according to the investigators.
For the Bova score, increasing the cutoff value from 110 to 120 bpm likewise increased specificity from 93.2% to 95%, while going up even further to 140 bpm increased specificity to 98.0%, the report shows.
In sensitivity analyses, the findings were not impacted by excluding younger patients, those who received reperfusion therapies, or those with atrial fibrillation, according to the study findings.
Potential implications for clinical practice
Taken together, these findings could serve as a resource to inform discussions regarding PE management that include whether home therapy or use of thrombolytic therapy is appropriate, investigators said in their report.
“For instance, among low-risk sPESI patients, those with borderline tachycardia [i.e., a heart rate between 100-109 bpm] might benefit from initial hospital observation for trending,” they wrote.
Dr. Jaureguízar reported no disclosures. One coinvestigator reported funding support from the Institute of Health Carlos III (ISCIII) and the European Development Regional Fund (ERDF). One coinvestigator reported consulting in litigation involving two models of inferior vena cava filters.
Dr. Polito reported no disclosures.
In patients with acute pulmonary embolism, using cutoff values other than 110 beats per minute might improve the prognostic value of heart rate at admission, a recent observational study suggests.
For identifying low-risk patients, a cutoff of 80 bpm increased the sensitivity of the simplified Pulmonary Embolism Severity Index (sPESI) from about 94% to nearly 99% among nonhypotensive patients with acute symptomatic pulmonary embolism (PE), according to results of the large, registry-based study.
Similarly, using a 140-bpm cutoff increased the specificity of the Bova score for identifying intermediate-high–risk patients from about 93% to 98% in the study, which was recently published in the journal CHEST.
“Although standard dichotomization of HR [i.e., HR less than 110 vs. greater than 110 bpm] may be useful for guideline recommendations, our results will allow for more accuracy regarding clinical decision-making,” wrote lead author Ana Jaureguízar, MD, of the University of Alcalá in Madrid, on behalf of the RIETE (Registro Informatizado de la Enfermedad TromboEmbólica) investigators.
Intuitive findings inform future research
These observational findings are intuitive and do at least have the potential to inform the design of future randomized clinical trials, according to Albert J. Polito, MD, chief of the division of pulmonary medicine and medical director for the lung center at Mercy Medical Center in Baltimore.
“In medicine, there is a spectrum of risk,” Dr. Polito said in an interview. “While we love our cutoffs, which in this case has traditionally always been that 110 beats per minute for heart rate, it makes sense that there would be some range of risks of bad outcomes.”
Building on the observations of the present study, subsequent prospective randomized studies could potentially aim to determine, for example, when thrombolytic therapy should be considered in nonhypotensive patients with acute PE and higher heart rates.
“It would not be easy to design, but it’s a straightforward question to ask whether patients with the highest heart rates are the ones who potentially might benefit the most from thrombolytic therapy,” Dr. Polito said.
Value of alternative HR cutoffs
Heart rate is a simple and easily available vital sign that is clearly linked to prognosis in patients with pulmonary embolism, authors of the RIETE registry study say in their report. Accordingly, a heart rate threshold of 110 bpm has made its way into scoring systems that seek to identify low-risk patients, such as the sPESI, and those focused on identifying higher-risk patients, such as the Bova score.
However, it has not been clear whether alternative HR cutoffs would improve upon the 110-bpm threshold, they added. At the low-risk end, more accurate scoring systems could optimize the selection of patients for home treatment, while at the intermediate-high–risk end, they could better select patients for close monitoring or advanced PE treatments.
Better granularity on heart rate risks?
To better define the prognostic value of different heart rate thresholds, investigators analyzed data from RIETE, a large, ongoing, multinational prospective registry including patients with objectively confirmed acute venous thromboembolism.
For 44,331 consecutive nonhypotensive symptomatic PEs, the overall rate of 30-day all-cause mortality was 5.1%, and the 30-day PE-related mortality was 1.9%, the authors report.
Significantly poorer outcomes were seen in patients with higher heart rates as compared to patients in the 80-99 bpm range, they also found. As compared to that reference range, odds ratios for 30-day all-cause death ranged from 1.5 for heart rates of 100-109, up to 2.4 for those with heart rates of 140 bpm or greater.
Likewise, patients with higher heart rates had a 1.7- to 2.4-fold greater risk of 30-day PE-related death as compared to the 80- to 99-bpm reference range, while patients with lower heart rates had lesser risk, the data published in CHEST show.
Toward refinement of prognostic scoring
Next, investigators sought to refine the prognostic scoring systems for low-risk PE (sPESI) and intermediate-high–risk PE (Bova).
For sPESI, they found that dropping the cutoff value from 110 to 100 bpm increased the sensitivity of the score from 93.4% to 95.3%. Going down even further to 80 bpm increased sensitivity to 98.8%, according to the report.
By going down from 110 to 80 bpm, the proportion of patients defined as low-risk dropped from 35% to 12%, according to the investigators.
For the Bova score, increasing the cutoff value from 110 to 120 bpm likewise increased specificity from 93.2% to 95%, while going up even further to 140 bpm increased specificity to 98.0%, the report shows.
In sensitivity analyses, the findings were not impacted by excluding younger patients, those who received reperfusion therapies, or those with atrial fibrillation, according to the study findings.
Potential implications for clinical practice
Taken together, these findings could serve as a resource to inform discussions regarding PE management that include whether home therapy or use of thrombolytic therapy is appropriate, investigators said in their report.
“For instance, among low-risk sPESI patients, those with borderline tachycardia [i.e., a heart rate between 100-109 bpm] might benefit from initial hospital observation for trending,” they wrote.
Dr. Jaureguízar reported no disclosures. One coinvestigator reported funding support from the Institute of Health Carlos III (ISCIII) and the European Development Regional Fund (ERDF). One coinvestigator reported consulting in litigation involving two models of inferior vena cava filters.
Dr. Polito reported no disclosures.
In patients with acute pulmonary embolism, using cutoff values other than 110 beats per minute might improve the prognostic value of heart rate at admission, a recent observational study suggests.
For identifying low-risk patients, a cutoff of 80 bpm increased the sensitivity of the simplified Pulmonary Embolism Severity Index (sPESI) from about 94% to nearly 99% among nonhypotensive patients with acute symptomatic pulmonary embolism (PE), according to results of the large, registry-based study.
Similarly, using a 140-bpm cutoff increased the specificity of the Bova score for identifying intermediate-high–risk patients from about 93% to 98% in the study, which was recently published in the journal CHEST.
“Although standard dichotomization of HR [i.e., HR less than 110 vs. greater than 110 bpm] may be useful for guideline recommendations, our results will allow for more accuracy regarding clinical decision-making,” wrote lead author Ana Jaureguízar, MD, of the University of Alcalá in Madrid, on behalf of the RIETE (Registro Informatizado de la Enfermedad TromboEmbólica) investigators.
Intuitive findings inform future research
These observational findings are intuitive and do at least have the potential to inform the design of future randomized clinical trials, according to Albert J. Polito, MD, chief of the division of pulmonary medicine and medical director for the lung center at Mercy Medical Center in Baltimore.
“In medicine, there is a spectrum of risk,” Dr. Polito said in an interview. “While we love our cutoffs, which in this case has traditionally always been that 110 beats per minute for heart rate, it makes sense that there would be some range of risks of bad outcomes.”
Building on the observations of the present study, subsequent prospective randomized studies could potentially aim to determine, for example, when thrombolytic therapy should be considered in nonhypotensive patients with acute PE and higher heart rates.
“It would not be easy to design, but it’s a straightforward question to ask whether patients with the highest heart rates are the ones who potentially might benefit the most from thrombolytic therapy,” Dr. Polito said.
Value of alternative HR cutoffs
Heart rate is a simple and easily available vital sign that is clearly linked to prognosis in patients with pulmonary embolism, authors of the RIETE registry study say in their report. Accordingly, a heart rate threshold of 110 bpm has made its way into scoring systems that seek to identify low-risk patients, such as the sPESI, and those focused on identifying higher-risk patients, such as the Bova score.
However, it has not been clear whether alternative HR cutoffs would improve upon the 110-bpm threshold, they added. At the low-risk end, more accurate scoring systems could optimize the selection of patients for home treatment, while at the intermediate-high–risk end, they could better select patients for close monitoring or advanced PE treatments.
Better granularity on heart rate risks?
To better define the prognostic value of different heart rate thresholds, investigators analyzed data from RIETE, a large, ongoing, multinational prospective registry including patients with objectively confirmed acute venous thromboembolism.
For 44,331 consecutive nonhypotensive symptomatic PEs, the overall rate of 30-day all-cause mortality was 5.1%, and the 30-day PE-related mortality was 1.9%, the authors report.
Significantly poorer outcomes were seen in patients with higher heart rates as compared to patients in the 80-99 bpm range, they also found. As compared to that reference range, odds ratios for 30-day all-cause death ranged from 1.5 for heart rates of 100-109, up to 2.4 for those with heart rates of 140 bpm or greater.
Likewise, patients with higher heart rates had a 1.7- to 2.4-fold greater risk of 30-day PE-related death as compared to the 80- to 99-bpm reference range, while patients with lower heart rates had lesser risk, the data published in CHEST show.
Toward refinement of prognostic scoring
Next, investigators sought to refine the prognostic scoring systems for low-risk PE (sPESI) and intermediate-high–risk PE (Bova).
For sPESI, they found that dropping the cutoff value from 110 to 100 bpm increased the sensitivity of the score from 93.4% to 95.3%. Going down even further to 80 bpm increased sensitivity to 98.8%, according to the report.
By going down from 110 to 80 bpm, the proportion of patients defined as low-risk dropped from 35% to 12%, according to the investigators.
For the Bova score, increasing the cutoff value from 110 to 120 bpm likewise increased specificity from 93.2% to 95%, while going up even further to 140 bpm increased specificity to 98.0%, the report shows.
In sensitivity analyses, the findings were not impacted by excluding younger patients, those who received reperfusion therapies, or those with atrial fibrillation, according to the study findings.
Potential implications for clinical practice
Taken together, these findings could serve as a resource to inform discussions regarding PE management that include whether home therapy or use of thrombolytic therapy is appropriate, investigators said in their report.
“For instance, among low-risk sPESI patients, those with borderline tachycardia [i.e., a heart rate between 100-109 bpm] might benefit from initial hospital observation for trending,” they wrote.
Dr. Jaureguízar reported no disclosures. One coinvestigator reported funding support from the Institute of Health Carlos III (ISCIII) and the European Development Regional Fund (ERDF). One coinvestigator reported consulting in litigation involving two models of inferior vena cava filters.
Dr. Polito reported no disclosures.
FROM CHEST
New sarcoidosis treatment guideline bringing light to the darkness
Nothing about sarcoidosis is easy. First identified in 1877, it is quite common. In the United States, lifetime risk is 2.4% and 0.85% for African American persons and White persons, respectively. Still, it remains an enigma. Despite study of its genetics and immunopathology, we don’t know its cause. Diagnosis is challenging because noncaseating granulomas, the tissue finding associated with sarcoidosis, aren’t specific for the disease. With the exception of Löfgren syndrome, a well-described sarcoid presentation that portends an excellent prognosis, initial signs and symptoms are variable and disease course is unpredictable.
The inherent heterogeneity of sarcoid makes it challenging to study. In the modern era of evidence-based medicine, it’s hard to say much about it with certainty. The American Thoracic Society (ATS) is one of just a few, premier organizations that creates respiratory medicine guidelines. In 1999, they published a sarcoid consensus statement with the European Respiratory Society (ERS), another outstanding and influential respiratory medicine organization, and the World Association of Sarcoidosis and other Granulomatous Disorders (WASOG). For the past 20 years, I’ve been referring trainees to this document for guidance on managing their patients with sarcoid.
Twenty years later, sarcoid remains frustrating and mysterious, but much has changed. Our methods for evaluating evidence and creating guidelines are now based on the GRADE criteria. Now that we have easy access to advanced technologies such as endobronchial ultrasound, obtaining tissue for diagnosis is easier. Our study of sarcoid itself has advanced, with large cohorts providing data on phenotyping, new immunosuppressants being used for treatment, and an improved understanding of cardiac sarcoidosis. In short, we’re in need of a sarcoidosis guideline for the 21st century.
Within in the past 18 months, the ATS and ERS have delivered updated guidelines for diagnosis and treatment. Despite the advancements cited above, sarcoid remains difficult to study. So predictably, neither document issues earth-shattering conclusions. Truth be told, well-done guidelines rarely do. They do provide several important updates that physicians managing patients with sarcoid should note.
The guideline on diagnosis provides recommendations for routine monitoring after diagnosis. Many practicing clinicians took from the 1999 ATS/ERS/WASOG consensus statement that all patients with sarcoid needed to be seen annually. At pulmonary clinics where I’ve worked, we’ve defaulted to annual follow-up for everyone, usually with chest radiography, lab testing, electrocardiography, and referral to ophthalmology. Because a majority of patients with sarcoid will remain asymptomatic or experience spontaneous remission, this practice never really seemed cost-effective or clinically efficient. The new guidelines are far more proscriptive on what monitoring is required and grade requirements at specific levels of certainty and often advise symptom-based assessments in lieu of reflexive annual testing.
The ERS guideline on treatment provides a thoughtful discussion of corticosteroid indications and dosing, broken down by underlying disease severity (assessed by lung function abnormalities and imaging). It also recognizes that two of the most common sarcoid symptoms are fatigue and dyspnea, which are both inherently nonspecific. In practice, proving these symptoms are directly attributable to sarcoid is challenging. The treatment guideline allows for flexibility in these cases, with shared decision-making and trials of low-dose steroids recommended. This seems an excellent hedge against overtreatment with immunosuppressive medications that have harmful side effects.
The ATS and ERS guidelines are not without controversy. Their approach to cardiac sarcoid differs slightly from that recommended by a commonly cited Heart Rhythm Society consensus statement, and despite discussing treatment options, the section on fatigue is quite limited. These two facts and other limitations largely reflect differing interpretations of the limited data; they do not detract from the overall importance of the ATS and ERS guidelines. Sarcoid remains an enigma, but little by little the outstanding academic physicians at the ATS and ERS are providing clarity.
Dr. Holley is an associate professor, department of medicine, Uniformed Services University (USU); program director, pulmonary and critical care medical fellowship, department of medicine, Walter Reed National Military Medical Center, Bethesda, Maryland. He has received a research grant from Fisher-Paykel and income from the American College of Chest Physicians.
A version of this article first appeared on Medscape.com.
Nothing about sarcoidosis is easy. First identified in 1877, it is quite common. In the United States, lifetime risk is 2.4% and 0.85% for African American persons and White persons, respectively. Still, it remains an enigma. Despite study of its genetics and immunopathology, we don’t know its cause. Diagnosis is challenging because noncaseating granulomas, the tissue finding associated with sarcoidosis, aren’t specific for the disease. With the exception of Löfgren syndrome, a well-described sarcoid presentation that portends an excellent prognosis, initial signs and symptoms are variable and disease course is unpredictable.
The inherent heterogeneity of sarcoid makes it challenging to study. In the modern era of evidence-based medicine, it’s hard to say much about it with certainty. The American Thoracic Society (ATS) is one of just a few, premier organizations that creates respiratory medicine guidelines. In 1999, they published a sarcoid consensus statement with the European Respiratory Society (ERS), another outstanding and influential respiratory medicine organization, and the World Association of Sarcoidosis and other Granulomatous Disorders (WASOG). For the past 20 years, I’ve been referring trainees to this document for guidance on managing their patients with sarcoid.
Twenty years later, sarcoid remains frustrating and mysterious, but much has changed. Our methods for evaluating evidence and creating guidelines are now based on the GRADE criteria. Now that we have easy access to advanced technologies such as endobronchial ultrasound, obtaining tissue for diagnosis is easier. Our study of sarcoid itself has advanced, with large cohorts providing data on phenotyping, new immunosuppressants being used for treatment, and an improved understanding of cardiac sarcoidosis. In short, we’re in need of a sarcoidosis guideline for the 21st century.
Within in the past 18 months, the ATS and ERS have delivered updated guidelines for diagnosis and treatment. Despite the advancements cited above, sarcoid remains difficult to study. So predictably, neither document issues earth-shattering conclusions. Truth be told, well-done guidelines rarely do. They do provide several important updates that physicians managing patients with sarcoid should note.
The guideline on diagnosis provides recommendations for routine monitoring after diagnosis. Many practicing clinicians took from the 1999 ATS/ERS/WASOG consensus statement that all patients with sarcoid needed to be seen annually. At pulmonary clinics where I’ve worked, we’ve defaulted to annual follow-up for everyone, usually with chest radiography, lab testing, electrocardiography, and referral to ophthalmology. Because a majority of patients with sarcoid will remain asymptomatic or experience spontaneous remission, this practice never really seemed cost-effective or clinically efficient. The new guidelines are far more proscriptive on what monitoring is required and grade requirements at specific levels of certainty and often advise symptom-based assessments in lieu of reflexive annual testing.
The ERS guideline on treatment provides a thoughtful discussion of corticosteroid indications and dosing, broken down by underlying disease severity (assessed by lung function abnormalities and imaging). It also recognizes that two of the most common sarcoid symptoms are fatigue and dyspnea, which are both inherently nonspecific. In practice, proving these symptoms are directly attributable to sarcoid is challenging. The treatment guideline allows for flexibility in these cases, with shared decision-making and trials of low-dose steroids recommended. This seems an excellent hedge against overtreatment with immunosuppressive medications that have harmful side effects.
The ATS and ERS guidelines are not without controversy. Their approach to cardiac sarcoid differs slightly from that recommended by a commonly cited Heart Rhythm Society consensus statement, and despite discussing treatment options, the section on fatigue is quite limited. These two facts and other limitations largely reflect differing interpretations of the limited data; they do not detract from the overall importance of the ATS and ERS guidelines. Sarcoid remains an enigma, but little by little the outstanding academic physicians at the ATS and ERS are providing clarity.
Dr. Holley is an associate professor, department of medicine, Uniformed Services University (USU); program director, pulmonary and critical care medical fellowship, department of medicine, Walter Reed National Military Medical Center, Bethesda, Maryland. He has received a research grant from Fisher-Paykel and income from the American College of Chest Physicians.
A version of this article first appeared on Medscape.com.
Nothing about sarcoidosis is easy. First identified in 1877, it is quite common. In the United States, lifetime risk is 2.4% and 0.85% for African American persons and White persons, respectively. Still, it remains an enigma. Despite study of its genetics and immunopathology, we don’t know its cause. Diagnosis is challenging because noncaseating granulomas, the tissue finding associated with sarcoidosis, aren’t specific for the disease. With the exception of Löfgren syndrome, a well-described sarcoid presentation that portends an excellent prognosis, initial signs and symptoms are variable and disease course is unpredictable.
The inherent heterogeneity of sarcoid makes it challenging to study. In the modern era of evidence-based medicine, it’s hard to say much about it with certainty. The American Thoracic Society (ATS) is one of just a few, premier organizations that creates respiratory medicine guidelines. In 1999, they published a sarcoid consensus statement with the European Respiratory Society (ERS), another outstanding and influential respiratory medicine organization, and the World Association of Sarcoidosis and other Granulomatous Disorders (WASOG). For the past 20 years, I’ve been referring trainees to this document for guidance on managing their patients with sarcoid.
Twenty years later, sarcoid remains frustrating and mysterious, but much has changed. Our methods for evaluating evidence and creating guidelines are now based on the GRADE criteria. Now that we have easy access to advanced technologies such as endobronchial ultrasound, obtaining tissue for diagnosis is easier. Our study of sarcoid itself has advanced, with large cohorts providing data on phenotyping, new immunosuppressants being used for treatment, and an improved understanding of cardiac sarcoidosis. In short, we’re in need of a sarcoidosis guideline for the 21st century.
Within in the past 18 months, the ATS and ERS have delivered updated guidelines for diagnosis and treatment. Despite the advancements cited above, sarcoid remains difficult to study. So predictably, neither document issues earth-shattering conclusions. Truth be told, well-done guidelines rarely do. They do provide several important updates that physicians managing patients with sarcoid should note.
The guideline on diagnosis provides recommendations for routine monitoring after diagnosis. Many practicing clinicians took from the 1999 ATS/ERS/WASOG consensus statement that all patients with sarcoid needed to be seen annually. At pulmonary clinics where I’ve worked, we’ve defaulted to annual follow-up for everyone, usually with chest radiography, lab testing, electrocardiography, and referral to ophthalmology. Because a majority of patients with sarcoid will remain asymptomatic or experience spontaneous remission, this practice never really seemed cost-effective or clinically efficient. The new guidelines are far more proscriptive on what monitoring is required and grade requirements at specific levels of certainty and often advise symptom-based assessments in lieu of reflexive annual testing.
The ERS guideline on treatment provides a thoughtful discussion of corticosteroid indications and dosing, broken down by underlying disease severity (assessed by lung function abnormalities and imaging). It also recognizes that two of the most common sarcoid symptoms are fatigue and dyspnea, which are both inherently nonspecific. In practice, proving these symptoms are directly attributable to sarcoid is challenging. The treatment guideline allows for flexibility in these cases, with shared decision-making and trials of low-dose steroids recommended. This seems an excellent hedge against overtreatment with immunosuppressive medications that have harmful side effects.
The ATS and ERS guidelines are not without controversy. Their approach to cardiac sarcoid differs slightly from that recommended by a commonly cited Heart Rhythm Society consensus statement, and despite discussing treatment options, the section on fatigue is quite limited. These two facts and other limitations largely reflect differing interpretations of the limited data; they do not detract from the overall importance of the ATS and ERS guidelines. Sarcoid remains an enigma, but little by little the outstanding academic physicians at the ATS and ERS are providing clarity.
Dr. Holley is an associate professor, department of medicine, Uniformed Services University (USU); program director, pulmonary and critical care medical fellowship, department of medicine, Walter Reed National Military Medical Center, Bethesda, Maryland. He has received a research grant from Fisher-Paykel and income from the American College of Chest Physicians.
A version of this article first appeared on Medscape.com.
‘Empathy fatigue’ in clinicians rises with latest COVID-19 surge
Heidi Erickson, MD, is tired. As a pulmonary and critical care physician at Hennepin Healthcare in Minneapolis, she has been providing care for patients with COVID-19 since the start of the pandemic.
It was exhausting from the beginning, as she and her colleagues scrambled to understand how to deal with this new disease. But lately, she has noticed a different kind of exhaustion arising from the knowledge that with vaccines widely available, the latest surge was preventable.
Her intensive care unit is currently as full as it has ever been with COVID-19 patients, many of them young adults and most of them unvaccinated. After the recent death of one patient, an unvaccinated man with teenage children, she had to face his family’s questions about why ivermectin, an antiparasitic medication that was falsely promoted as a COVID-19 treatment, was not administered.
“I’m fatigued because I’m working more than ever, but more people don’t have to die,” Dr. Erickson said in an interview . “It’s been very hard physically, mentally, emotionally.”
Amid yet another surge in COVID-19 cases around the United States, clinicians are speaking out about their growing frustration with this preventable crisis.
Some are using the terms “empathy fatigue” and “compassion fatigue” – a sense that they are losing empathy for unvaccinated individuals who are fueling the pandemic.
Dr. Erickson says she is frustrated not by individual patients but by a system that has allowed disinformation to proliferate. Experts say these types of feelings fit into a widespread pattern of physician burnout that has taken a new turn at this stage of the pandemic.
Paradoxical choices
Empathy is a cornerstone of what clinicians do, and the ability to understand and share a patient’s feelings is an essential skill for providing effective care, says Kaz Nelson, MD, a psychiatrist at the University of Minnesota, Minneapolis.
Practitioners face paradoxical situations all the time, she notes. These include individuals who break bones and go skydiving again, people who have high cholesterol but continue to eat fried foods, and those with advanced lung cancer who continue to smoke.
To treat patients with compassion, practitioners learn to set aside judgment by acknowledging the complexity of human behavior. They may lament the addictive nature of nicotine and advertising that targets children, for example, while still listening and caring.
Empathy requires high-level brain function, but as stress levels rise, brain function that drives empathy tends to shut down. It’s a survival mechanism, Dr. Nelson says.
When health care workers feel overwhelmed, trapped, or threatened by patients demanding unproven treatments or by ICUs with more patients than ventilators, they may experience a fight-or-flight response that makes them defensive, frustrated, angry, or uncaring, notes Mona Masood, DO, a Philadelphia-area psychiatrist and founder of Physician Support Line, a free mental health hotline for doctors.
Some clinicians have taken to Twitter and other social media platforms to post about these types of experiences.
These feelings, which have been brewing for months, have been exacerbated by the complexity of the current situation. Clinicians see a disconnect between what is and what could be, Dr. Nelson notes.
“Prior to vaccines, there weren’t other options, and so we had toxic stress and we had fatigue, but we could still maintain little bits of empathy by saying, ‘You know, people didn’t choose to get infected, and we are in a pandemic.’ We could kind of hate the virus. Now with access to vaccines, that last connection to empathy is removed for many people,” she says.
Self-preservation vs. empathy
Compassion fatigue or empathy fatigue is just one reaction to feeling completely maxed out and overstressed, Dr. Nelson says. Anger at society, such as what Dr. Erickson experienced, is another response.
Practitioners may also feel as if they are just going through the motions of their job, or they might disassociate, ceasing to feel that their patients are human. Plenty of doctors and nurses have cried in their cars after shifts and have posted tearful videos on social media.
Early in the pandemic, Dr. Masood says, physicians who called the support hotline expressed sadness and grief. Now, she had her colleagues hear frustration and anger, along with guilt and shame for having feelings they believe they shouldn’t be having, especially toward patients. They may feel unprofessional or worse – unworthy of being physicians, she says.
One recent caller to the hotline was a long-time ICU physician who had been told so many times by patients that ivermectin was the only medicine that would cure them that he began to doubt himself, says Dr. Masood. This caller needed to be reassured by another physician that he was doing the right thing.
Another emergency department physician told Dr. Masood about a young child who had arrived at the hospital with COVID-19 symptoms. When asked whether the family had been exposed to anyone with COVID-19, the child’s parent lied so that they could be triaged faster.
The physician, who needed to step away from the situation, reached out to Dr. Masood to express her frustration so that she wouldn’t “let it out” on the patient.
“It’s hard to have empathy for people who, for all intents and purposes, are very self-centered,” Dr. Masood says. “We’re at a place where we’re having to choose between self-preservation and empathy.”
How to cope
To help practitioners cope, Dr. Masood offers words that describe what they’re experiencing. She often hears clinicians say things such as, “This is a type of burnout that I feel to my bones,” or “This makes me want to quit,” or “I feel like I’m at the end of my rope.”
She encourages them to consider the terms “empathy fatigue,” and “moral injury” in order to reconcile how their sense of responsibility to take care of people is compromised by factors outside of their control.
It is not shameful to acknowledge that they experience emotions, including difficult ones such as frustration, anger, sadness, and anxiety, Dr. Masood adds.
Being frustrated with a patient doesn’t make someone a bad doctor, and admitting those emotions is the first step toward dealing with them, she says.
before they cause a sense of callousness or other consequences that become harder to heal from as time goes on.
“We’re trained to just go, go, go and sometimes not pause and check in,” she says. Clinicians who open up are likely to find they are not the only ones feeling tired or frustrated right now, she adds.
“Connect with peers and colleagues, because chances are, they can relate,” Dr. Nelson says.
A version of this article first appeared on Medscape.com.
Heidi Erickson, MD, is tired. As a pulmonary and critical care physician at Hennepin Healthcare in Minneapolis, she has been providing care for patients with COVID-19 since the start of the pandemic.
It was exhausting from the beginning, as she and her colleagues scrambled to understand how to deal with this new disease. But lately, she has noticed a different kind of exhaustion arising from the knowledge that with vaccines widely available, the latest surge was preventable.
Her intensive care unit is currently as full as it has ever been with COVID-19 patients, many of them young adults and most of them unvaccinated. After the recent death of one patient, an unvaccinated man with teenage children, she had to face his family’s questions about why ivermectin, an antiparasitic medication that was falsely promoted as a COVID-19 treatment, was not administered.
“I’m fatigued because I’m working more than ever, but more people don’t have to die,” Dr. Erickson said in an interview . “It’s been very hard physically, mentally, emotionally.”
Amid yet another surge in COVID-19 cases around the United States, clinicians are speaking out about their growing frustration with this preventable crisis.
Some are using the terms “empathy fatigue” and “compassion fatigue” – a sense that they are losing empathy for unvaccinated individuals who are fueling the pandemic.
Dr. Erickson says she is frustrated not by individual patients but by a system that has allowed disinformation to proliferate. Experts say these types of feelings fit into a widespread pattern of physician burnout that has taken a new turn at this stage of the pandemic.
Paradoxical choices
Empathy is a cornerstone of what clinicians do, and the ability to understand and share a patient’s feelings is an essential skill for providing effective care, says Kaz Nelson, MD, a psychiatrist at the University of Minnesota, Minneapolis.
Practitioners face paradoxical situations all the time, she notes. These include individuals who break bones and go skydiving again, people who have high cholesterol but continue to eat fried foods, and those with advanced lung cancer who continue to smoke.
To treat patients with compassion, practitioners learn to set aside judgment by acknowledging the complexity of human behavior. They may lament the addictive nature of nicotine and advertising that targets children, for example, while still listening and caring.
Empathy requires high-level brain function, but as stress levels rise, brain function that drives empathy tends to shut down. It’s a survival mechanism, Dr. Nelson says.
When health care workers feel overwhelmed, trapped, or threatened by patients demanding unproven treatments or by ICUs with more patients than ventilators, they may experience a fight-or-flight response that makes them defensive, frustrated, angry, or uncaring, notes Mona Masood, DO, a Philadelphia-area psychiatrist and founder of Physician Support Line, a free mental health hotline for doctors.
Some clinicians have taken to Twitter and other social media platforms to post about these types of experiences.
These feelings, which have been brewing for months, have been exacerbated by the complexity of the current situation. Clinicians see a disconnect between what is and what could be, Dr. Nelson notes.
“Prior to vaccines, there weren’t other options, and so we had toxic stress and we had fatigue, but we could still maintain little bits of empathy by saying, ‘You know, people didn’t choose to get infected, and we are in a pandemic.’ We could kind of hate the virus. Now with access to vaccines, that last connection to empathy is removed for many people,” she says.
Self-preservation vs. empathy
Compassion fatigue or empathy fatigue is just one reaction to feeling completely maxed out and overstressed, Dr. Nelson says. Anger at society, such as what Dr. Erickson experienced, is another response.
Practitioners may also feel as if they are just going through the motions of their job, or they might disassociate, ceasing to feel that their patients are human. Plenty of doctors and nurses have cried in their cars after shifts and have posted tearful videos on social media.
Early in the pandemic, Dr. Masood says, physicians who called the support hotline expressed sadness and grief. Now, she had her colleagues hear frustration and anger, along with guilt and shame for having feelings they believe they shouldn’t be having, especially toward patients. They may feel unprofessional or worse – unworthy of being physicians, she says.
One recent caller to the hotline was a long-time ICU physician who had been told so many times by patients that ivermectin was the only medicine that would cure them that he began to doubt himself, says Dr. Masood. This caller needed to be reassured by another physician that he was doing the right thing.
Another emergency department physician told Dr. Masood about a young child who had arrived at the hospital with COVID-19 symptoms. When asked whether the family had been exposed to anyone with COVID-19, the child’s parent lied so that they could be triaged faster.
The physician, who needed to step away from the situation, reached out to Dr. Masood to express her frustration so that she wouldn’t “let it out” on the patient.
“It’s hard to have empathy for people who, for all intents and purposes, are very self-centered,” Dr. Masood says. “We’re at a place where we’re having to choose between self-preservation and empathy.”
How to cope
To help practitioners cope, Dr. Masood offers words that describe what they’re experiencing. She often hears clinicians say things such as, “This is a type of burnout that I feel to my bones,” or “This makes me want to quit,” or “I feel like I’m at the end of my rope.”
She encourages them to consider the terms “empathy fatigue,” and “moral injury” in order to reconcile how their sense of responsibility to take care of people is compromised by factors outside of their control.
It is not shameful to acknowledge that they experience emotions, including difficult ones such as frustration, anger, sadness, and anxiety, Dr. Masood adds.
Being frustrated with a patient doesn’t make someone a bad doctor, and admitting those emotions is the first step toward dealing with them, she says.
before they cause a sense of callousness or other consequences that become harder to heal from as time goes on.
“We’re trained to just go, go, go and sometimes not pause and check in,” she says. Clinicians who open up are likely to find they are not the only ones feeling tired or frustrated right now, she adds.
“Connect with peers and colleagues, because chances are, they can relate,” Dr. Nelson says.
A version of this article first appeared on Medscape.com.
Heidi Erickson, MD, is tired. As a pulmonary and critical care physician at Hennepin Healthcare in Minneapolis, she has been providing care for patients with COVID-19 since the start of the pandemic.
It was exhausting from the beginning, as she and her colleagues scrambled to understand how to deal with this new disease. But lately, she has noticed a different kind of exhaustion arising from the knowledge that with vaccines widely available, the latest surge was preventable.
Her intensive care unit is currently as full as it has ever been with COVID-19 patients, many of them young adults and most of them unvaccinated. After the recent death of one patient, an unvaccinated man with teenage children, she had to face his family’s questions about why ivermectin, an antiparasitic medication that was falsely promoted as a COVID-19 treatment, was not administered.
“I’m fatigued because I’m working more than ever, but more people don’t have to die,” Dr. Erickson said in an interview . “It’s been very hard physically, mentally, emotionally.”
Amid yet another surge in COVID-19 cases around the United States, clinicians are speaking out about their growing frustration with this preventable crisis.
Some are using the terms “empathy fatigue” and “compassion fatigue” – a sense that they are losing empathy for unvaccinated individuals who are fueling the pandemic.
Dr. Erickson says she is frustrated not by individual patients but by a system that has allowed disinformation to proliferate. Experts say these types of feelings fit into a widespread pattern of physician burnout that has taken a new turn at this stage of the pandemic.
Paradoxical choices
Empathy is a cornerstone of what clinicians do, and the ability to understand and share a patient’s feelings is an essential skill for providing effective care, says Kaz Nelson, MD, a psychiatrist at the University of Minnesota, Minneapolis.
Practitioners face paradoxical situations all the time, she notes. These include individuals who break bones and go skydiving again, people who have high cholesterol but continue to eat fried foods, and those with advanced lung cancer who continue to smoke.
To treat patients with compassion, practitioners learn to set aside judgment by acknowledging the complexity of human behavior. They may lament the addictive nature of nicotine and advertising that targets children, for example, while still listening and caring.
Empathy requires high-level brain function, but as stress levels rise, brain function that drives empathy tends to shut down. It’s a survival mechanism, Dr. Nelson says.
When health care workers feel overwhelmed, trapped, or threatened by patients demanding unproven treatments or by ICUs with more patients than ventilators, they may experience a fight-or-flight response that makes them defensive, frustrated, angry, or uncaring, notes Mona Masood, DO, a Philadelphia-area psychiatrist and founder of Physician Support Line, a free mental health hotline for doctors.
Some clinicians have taken to Twitter and other social media platforms to post about these types of experiences.
These feelings, which have been brewing for months, have been exacerbated by the complexity of the current situation. Clinicians see a disconnect between what is and what could be, Dr. Nelson notes.
“Prior to vaccines, there weren’t other options, and so we had toxic stress and we had fatigue, but we could still maintain little bits of empathy by saying, ‘You know, people didn’t choose to get infected, and we are in a pandemic.’ We could kind of hate the virus. Now with access to vaccines, that last connection to empathy is removed for many people,” she says.
Self-preservation vs. empathy
Compassion fatigue or empathy fatigue is just one reaction to feeling completely maxed out and overstressed, Dr. Nelson says. Anger at society, such as what Dr. Erickson experienced, is another response.
Practitioners may also feel as if they are just going through the motions of their job, or they might disassociate, ceasing to feel that their patients are human. Plenty of doctors and nurses have cried in their cars after shifts and have posted tearful videos on social media.
Early in the pandemic, Dr. Masood says, physicians who called the support hotline expressed sadness and grief. Now, she had her colleagues hear frustration and anger, along with guilt and shame for having feelings they believe they shouldn’t be having, especially toward patients. They may feel unprofessional or worse – unworthy of being physicians, she says.
One recent caller to the hotline was a long-time ICU physician who had been told so many times by patients that ivermectin was the only medicine that would cure them that he began to doubt himself, says Dr. Masood. This caller needed to be reassured by another physician that he was doing the right thing.
Another emergency department physician told Dr. Masood about a young child who had arrived at the hospital with COVID-19 symptoms. When asked whether the family had been exposed to anyone with COVID-19, the child’s parent lied so that they could be triaged faster.
The physician, who needed to step away from the situation, reached out to Dr. Masood to express her frustration so that she wouldn’t “let it out” on the patient.
“It’s hard to have empathy for people who, for all intents and purposes, are very self-centered,” Dr. Masood says. “We’re at a place where we’re having to choose between self-preservation and empathy.”
How to cope
To help practitioners cope, Dr. Masood offers words that describe what they’re experiencing. She often hears clinicians say things such as, “This is a type of burnout that I feel to my bones,” or “This makes me want to quit,” or “I feel like I’m at the end of my rope.”
She encourages them to consider the terms “empathy fatigue,” and “moral injury” in order to reconcile how their sense of responsibility to take care of people is compromised by factors outside of their control.
It is not shameful to acknowledge that they experience emotions, including difficult ones such as frustration, anger, sadness, and anxiety, Dr. Masood adds.
Being frustrated with a patient doesn’t make someone a bad doctor, and admitting those emotions is the first step toward dealing with them, she says.
before they cause a sense of callousness or other consequences that become harder to heal from as time goes on.
“We’re trained to just go, go, go and sometimes not pause and check in,” she says. Clinicians who open up are likely to find they are not the only ones feeling tired or frustrated right now, she adds.
“Connect with peers and colleagues, because chances are, they can relate,” Dr. Nelson says.
A version of this article first appeared on Medscape.com.
Earliest 9/11 responders have higher COPD rates
The earliest responders to reach the site of the destroyed twin towers of the World Trade Center on Sept. 11, 2001, are at highest risk for chronic obstructive pulmonary disease and asthma/COPD overlap (ACO) among all those who worked at the site. The 9/11 attack was the deadliest terrorist attack on American soil.
The findings come from a case-control study that included nearly 18,000 emergency responders and volunteers. The investigators found that those who arrived at the site within 48 hours had an approximately 30% higher risk of developing COPD than those who arrived later, after adjustment for smoking and obesity, reported Rafael E de la Hoz, MD, a professor of environmental medicine, public health, and medicine at Mount Sinai Medical Center, New York, and colleagues.
“In this largest World Trade Center occupational cohort, spirometrically defined COPD and ACO were both modestly but significantly associated with World Trade Center exposure intensity, but the association seemed driven by the overlap,” he said in a narrated poster presentation during the European Respiratory Society 2021 International Congress.
“Around the world, we rely on our emergency workers to help when disasters occur,” commented Arzu Yorgancıoğlu, MD, professor and head of the department of pulmonology at Celal Bayar University, Manisa, Turkey, who was not involved in the study.
“This study shows how important it is to keep monitoring the health of workers, like those who attended the World Trade Center site 20 years ago, as occupational exposure to pollutants can lead to COPD. What we can learn from research like this is not only how best to care for emergency workers who operate in dangerous conditions but also how we can protect them in their work in the future,” she said.
Inconsistent findings
Fire and police personnel, emergency medical workers, construction workers, and others who labored amid the lingering pall of toxic dust and smoke at the World Trade Center site have developed asthma and other lower respiratory tract diseases over the ensuing decades.
“As the occupational cohorts age, there are concerns about chronic, longer latency, and disabling respiratory disease,” Dr. de la Hoz and colleagues wrote.
There have been inconsistent reports about the potential associations between COPD and ACO and the intensity of occupational exposure at the World Trade Center site. This prompted the investigators to further explore these associations using spirometry-defined disease.
They assessed data on 17,996 former World Trade Center site workers who had undergone at least two good-quality spirometric evaluations from 2002 to 2018.
To be classified as having COPD, workers had to have fixed airway obstruction. Those in the ACO subgroup were also required to have prebronchodilator obstruction with forced expiratory volume in 1 second of more than 400 mL in response to bronchodilation.
The patients were matched for sex and height within 5 cm using a 1:4 nested case-control design. Missing data were imputed.
Earliest arrivals paid the highest penalty
Of the total cohort, 85.4% were men, and 85.6% were overweight or obese. A total of 586 workers (3.3%) met the case definition for having COPD; 258 (1.4%) met the definition for having ACO.
The investigators found that the prevalence of self-reported ACO was six times higher than the prevalence of spirometry-confirmed disease. Among those who reported an onset date, 56.7% reported having asthma before COPD; the remainder reported having COPD first.
In analyses adjusted for age, sex, cohort entry period, smoking status, body mass index, metabolic syndrome parameters, and eosinophil levels, both COPD and ACO were significantly associated with early arrival at the World Trade Center site, with an adjusted odds ratio for COPD of 1.3 (95% confidence interval, 1.03-1.64), and an OR for ACO of 1.66 (95% CI, 1.1-2.49).
There was no significant interaction between early site arrival and smoking status.
The association between early exposure and COPD was no longer significant when those with ACO were excluded, the authors noted.
“We also observed that COPD more often followed asthma in these workers than the reverse, suggesting that asthma may have been on the path to COPD in most workers affected by the inhaled toxicants at the disaster site,” Dr. de la Hoz and colleagues wrote.
In addition, “our data suggest that self-reported physician diagnoses of COPD, asthma, and ACO are poorly correlated with objective data in this cohort,” they concluded.
The study was supported by the Centers for Disease Control and Prevention and the National Institute for Occupational Safety and Health. The authors and Dr. Yorgancıoğlu disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The earliest responders to reach the site of the destroyed twin towers of the World Trade Center on Sept. 11, 2001, are at highest risk for chronic obstructive pulmonary disease and asthma/COPD overlap (ACO) among all those who worked at the site. The 9/11 attack was the deadliest terrorist attack on American soil.
The findings come from a case-control study that included nearly 18,000 emergency responders and volunteers. The investigators found that those who arrived at the site within 48 hours had an approximately 30% higher risk of developing COPD than those who arrived later, after adjustment for smoking and obesity, reported Rafael E de la Hoz, MD, a professor of environmental medicine, public health, and medicine at Mount Sinai Medical Center, New York, and colleagues.
“In this largest World Trade Center occupational cohort, spirometrically defined COPD and ACO were both modestly but significantly associated with World Trade Center exposure intensity, but the association seemed driven by the overlap,” he said in a narrated poster presentation during the European Respiratory Society 2021 International Congress.
“Around the world, we rely on our emergency workers to help when disasters occur,” commented Arzu Yorgancıoğlu, MD, professor and head of the department of pulmonology at Celal Bayar University, Manisa, Turkey, who was not involved in the study.
“This study shows how important it is to keep monitoring the health of workers, like those who attended the World Trade Center site 20 years ago, as occupational exposure to pollutants can lead to COPD. What we can learn from research like this is not only how best to care for emergency workers who operate in dangerous conditions but also how we can protect them in their work in the future,” she said.
Inconsistent findings
Fire and police personnel, emergency medical workers, construction workers, and others who labored amid the lingering pall of toxic dust and smoke at the World Trade Center site have developed asthma and other lower respiratory tract diseases over the ensuing decades.
“As the occupational cohorts age, there are concerns about chronic, longer latency, and disabling respiratory disease,” Dr. de la Hoz and colleagues wrote.
There have been inconsistent reports about the potential associations between COPD and ACO and the intensity of occupational exposure at the World Trade Center site. This prompted the investigators to further explore these associations using spirometry-defined disease.
They assessed data on 17,996 former World Trade Center site workers who had undergone at least two good-quality spirometric evaluations from 2002 to 2018.
To be classified as having COPD, workers had to have fixed airway obstruction. Those in the ACO subgroup were also required to have prebronchodilator obstruction with forced expiratory volume in 1 second of more than 400 mL in response to bronchodilation.
The patients were matched for sex and height within 5 cm using a 1:4 nested case-control design. Missing data were imputed.
Earliest arrivals paid the highest penalty
Of the total cohort, 85.4% were men, and 85.6% were overweight or obese. A total of 586 workers (3.3%) met the case definition for having COPD; 258 (1.4%) met the definition for having ACO.
The investigators found that the prevalence of self-reported ACO was six times higher than the prevalence of spirometry-confirmed disease. Among those who reported an onset date, 56.7% reported having asthma before COPD; the remainder reported having COPD first.
In analyses adjusted for age, sex, cohort entry period, smoking status, body mass index, metabolic syndrome parameters, and eosinophil levels, both COPD and ACO were significantly associated with early arrival at the World Trade Center site, with an adjusted odds ratio for COPD of 1.3 (95% confidence interval, 1.03-1.64), and an OR for ACO of 1.66 (95% CI, 1.1-2.49).
There was no significant interaction between early site arrival and smoking status.
The association between early exposure and COPD was no longer significant when those with ACO were excluded, the authors noted.
“We also observed that COPD more often followed asthma in these workers than the reverse, suggesting that asthma may have been on the path to COPD in most workers affected by the inhaled toxicants at the disaster site,” Dr. de la Hoz and colleagues wrote.
In addition, “our data suggest that self-reported physician diagnoses of COPD, asthma, and ACO are poorly correlated with objective data in this cohort,” they concluded.
The study was supported by the Centers for Disease Control and Prevention and the National Institute for Occupational Safety and Health. The authors and Dr. Yorgancıoğlu disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The earliest responders to reach the site of the destroyed twin towers of the World Trade Center on Sept. 11, 2001, are at highest risk for chronic obstructive pulmonary disease and asthma/COPD overlap (ACO) among all those who worked at the site. The 9/11 attack was the deadliest terrorist attack on American soil.
The findings come from a case-control study that included nearly 18,000 emergency responders and volunteers. The investigators found that those who arrived at the site within 48 hours had an approximately 30% higher risk of developing COPD than those who arrived later, after adjustment for smoking and obesity, reported Rafael E de la Hoz, MD, a professor of environmental medicine, public health, and medicine at Mount Sinai Medical Center, New York, and colleagues.
“In this largest World Trade Center occupational cohort, spirometrically defined COPD and ACO were both modestly but significantly associated with World Trade Center exposure intensity, but the association seemed driven by the overlap,” he said in a narrated poster presentation during the European Respiratory Society 2021 International Congress.
“Around the world, we rely on our emergency workers to help when disasters occur,” commented Arzu Yorgancıoğlu, MD, professor and head of the department of pulmonology at Celal Bayar University, Manisa, Turkey, who was not involved in the study.
“This study shows how important it is to keep monitoring the health of workers, like those who attended the World Trade Center site 20 years ago, as occupational exposure to pollutants can lead to COPD. What we can learn from research like this is not only how best to care for emergency workers who operate in dangerous conditions but also how we can protect them in their work in the future,” she said.
Inconsistent findings
Fire and police personnel, emergency medical workers, construction workers, and others who labored amid the lingering pall of toxic dust and smoke at the World Trade Center site have developed asthma and other lower respiratory tract diseases over the ensuing decades.
“As the occupational cohorts age, there are concerns about chronic, longer latency, and disabling respiratory disease,” Dr. de la Hoz and colleagues wrote.
There have been inconsistent reports about the potential associations between COPD and ACO and the intensity of occupational exposure at the World Trade Center site. This prompted the investigators to further explore these associations using spirometry-defined disease.
They assessed data on 17,996 former World Trade Center site workers who had undergone at least two good-quality spirometric evaluations from 2002 to 2018.
To be classified as having COPD, workers had to have fixed airway obstruction. Those in the ACO subgroup were also required to have prebronchodilator obstruction with forced expiratory volume in 1 second of more than 400 mL in response to bronchodilation.
The patients were matched for sex and height within 5 cm using a 1:4 nested case-control design. Missing data were imputed.
Earliest arrivals paid the highest penalty
Of the total cohort, 85.4% were men, and 85.6% were overweight or obese. A total of 586 workers (3.3%) met the case definition for having COPD; 258 (1.4%) met the definition for having ACO.
The investigators found that the prevalence of self-reported ACO was six times higher than the prevalence of spirometry-confirmed disease. Among those who reported an onset date, 56.7% reported having asthma before COPD; the remainder reported having COPD first.
In analyses adjusted for age, sex, cohort entry period, smoking status, body mass index, metabolic syndrome parameters, and eosinophil levels, both COPD and ACO were significantly associated with early arrival at the World Trade Center site, with an adjusted odds ratio for COPD of 1.3 (95% confidence interval, 1.03-1.64), and an OR for ACO of 1.66 (95% CI, 1.1-2.49).
There was no significant interaction between early site arrival and smoking status.
The association between early exposure and COPD was no longer significant when those with ACO were excluded, the authors noted.
“We also observed that COPD more often followed asthma in these workers than the reverse, suggesting that asthma may have been on the path to COPD in most workers affected by the inhaled toxicants at the disaster site,” Dr. de la Hoz and colleagues wrote.
In addition, “our data suggest that self-reported physician diagnoses of COPD, asthma, and ACO are poorly correlated with objective data in this cohort,” they concluded.
The study was supported by the Centers for Disease Control and Prevention and the National Institute for Occupational Safety and Health. The authors and Dr. Yorgancıoğlu disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.