User login
‘Fascinating’ link between Alzheimer’s and COVID-19
The findings could lead to new treatment targets to slow progression and severity of both diseases.
Investigators found that a single genetic variant in the oligoadenylate synthetase 1 (OAS1) gene increases the risk for AD and that related variants in the same gene increase the likelihood of severe COVID-19 outcomes.
“These findings may allow us to identify new drug targets to slow progression of both diseases and reduce their severity,” Dervis Salih, PhD, senior research associate, UK Dementia Research Institute, University College London, said in an interview.
“Our work also suggests new approaches to treat both diseases with the same drugs,” Dr. Salih added.
The study was published online Oct. 7 in Brain.
Shared genetic network
The OAS1 gene is expressed in microglia, a type of immune cell that makes up around 10% of all cells in the brain.
In earlier work, investigators found evidence suggesting a link between the OAS1 gene and AD, but the function of the gene in microglia was unknown.
To further investigate the gene’s link to AD, they sequenced genetic data from 2,547 people – half with AD, and half without.
The genotyping analysis confirmed that the single-nucleotide polymorphism (SNP) rs1131454 within OAS1 is significantly associated with AD.
Given that the same OAS1 locus has recently been linked with severe COVID-19 outcomes, the researchers investigated four variants on the OAS1 gene.
Results indicate that SNPs within OAS1 associated with AD also show linkage to SNP variants associated with critical illness in COVID-19.
The rs1131454 (risk allele A) and rs4766676 (risk allele T) are associated with AD, and rs10735079 (risk allele A) and rs6489867 (risk allele T) are associated with critical illness with COVID-19, the investigators reported. All of these risk alleles dampen expression of OAS1.
“This study also provides strong new evidence that interferon signaling by the innate immune system plays a substantial role in the progression of Alzheimer’s,” said Dr. Salih.
“Identifying this shared genetic network in innate immune cells will allow us with future work to identify new biomarkers to track disease progression and also predict disease risk better for both disorders,” he added.
‘Fascinating’ link
In a statement from the UK nonprofit organization, Science Media Center, Kenneth Baillie, MBChB, with the University of Edinburgh, said this study builds on a discovery he and his colleagues made last year that OAS1 variants are associated with severe COVID-19.
“In the ISARIC4C study, we recently found that this is probably due to a change in the way cell membranes detect viruses, but this mechanism doesn’t explain the fascinating association with Alzheimer’s disease reported in this new work,” Dr. Baillie said.
“It is often the case that the same gene can have different roles in different parts of the body. Importantly, it doesn’t mean that having COVID-19 has any effect on your risk of Alzheimer’s,” he added.
Also weighing in on the new study, Jonathan Schott, MD, professor of neurology, University College London, noted that dementia is the “main preexisting health condition associated with COVID-19 mortality, accounting for about one in four deaths from COVID-19 between March and June 2020.
“While some of this excessive mortality may relate to people with dementia being overrepresented in care homes, which were particularly hard hit by the pandemic, or due to general increased vulnerability to infections, there have been questions as to whether there are common factors that might increase susceptibility both to developing dementia and to dying from COVID-19,” Dr. Schott explained.
This “elegant paper” provides evidence for the latter, “suggesting a common genetic mechanism both for Alzheimer’s disease and for severe COVID-19 infection,” Dr. Schott said.
“The identification of a genetic risk factor and elucidation of inflammatory pathways through which it may increase risk has important implications for our understanding of both diseases, with potential implications for novel treatments,” he added.
The study was funded by the UK Dementia Research Institute. The authors have disclosed no relevant financial relationships. Dr. Schott serves as chief medical officer for Alzheimer’s Research UK and is clinical adviser to the UK Dementia Research Institute. Dr. Baillie has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings could lead to new treatment targets to slow progression and severity of both diseases.
Investigators found that a single genetic variant in the oligoadenylate synthetase 1 (OAS1) gene increases the risk for AD and that related variants in the same gene increase the likelihood of severe COVID-19 outcomes.
“These findings may allow us to identify new drug targets to slow progression of both diseases and reduce their severity,” Dervis Salih, PhD, senior research associate, UK Dementia Research Institute, University College London, said in an interview.
“Our work also suggests new approaches to treat both diseases with the same drugs,” Dr. Salih added.
The study was published online Oct. 7 in Brain.
Shared genetic network
The OAS1 gene is expressed in microglia, a type of immune cell that makes up around 10% of all cells in the brain.
In earlier work, investigators found evidence suggesting a link between the OAS1 gene and AD, but the function of the gene in microglia was unknown.
To further investigate the gene’s link to AD, they sequenced genetic data from 2,547 people – half with AD, and half without.
The genotyping analysis confirmed that the single-nucleotide polymorphism (SNP) rs1131454 within OAS1 is significantly associated with AD.
Given that the same OAS1 locus has recently been linked with severe COVID-19 outcomes, the researchers investigated four variants on the OAS1 gene.
Results indicate that SNPs within OAS1 associated with AD also show linkage to SNP variants associated with critical illness in COVID-19.
The rs1131454 (risk allele A) and rs4766676 (risk allele T) are associated with AD, and rs10735079 (risk allele A) and rs6489867 (risk allele T) are associated with critical illness with COVID-19, the investigators reported. All of these risk alleles dampen expression of OAS1.
“This study also provides strong new evidence that interferon signaling by the innate immune system plays a substantial role in the progression of Alzheimer’s,” said Dr. Salih.
“Identifying this shared genetic network in innate immune cells will allow us with future work to identify new biomarkers to track disease progression and also predict disease risk better for both disorders,” he added.
‘Fascinating’ link
In a statement from the UK nonprofit organization, Science Media Center, Kenneth Baillie, MBChB, with the University of Edinburgh, said this study builds on a discovery he and his colleagues made last year that OAS1 variants are associated with severe COVID-19.
“In the ISARIC4C study, we recently found that this is probably due to a change in the way cell membranes detect viruses, but this mechanism doesn’t explain the fascinating association with Alzheimer’s disease reported in this new work,” Dr. Baillie said.
“It is often the case that the same gene can have different roles in different parts of the body. Importantly, it doesn’t mean that having COVID-19 has any effect on your risk of Alzheimer’s,” he added.
Also weighing in on the new study, Jonathan Schott, MD, professor of neurology, University College London, noted that dementia is the “main preexisting health condition associated with COVID-19 mortality, accounting for about one in four deaths from COVID-19 between March and June 2020.
“While some of this excessive mortality may relate to people with dementia being overrepresented in care homes, which were particularly hard hit by the pandemic, or due to general increased vulnerability to infections, there have been questions as to whether there are common factors that might increase susceptibility both to developing dementia and to dying from COVID-19,” Dr. Schott explained.
This “elegant paper” provides evidence for the latter, “suggesting a common genetic mechanism both for Alzheimer’s disease and for severe COVID-19 infection,” Dr. Schott said.
“The identification of a genetic risk factor and elucidation of inflammatory pathways through which it may increase risk has important implications for our understanding of both diseases, with potential implications for novel treatments,” he added.
The study was funded by the UK Dementia Research Institute. The authors have disclosed no relevant financial relationships. Dr. Schott serves as chief medical officer for Alzheimer’s Research UK and is clinical adviser to the UK Dementia Research Institute. Dr. Baillie has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings could lead to new treatment targets to slow progression and severity of both diseases.
Investigators found that a single genetic variant in the oligoadenylate synthetase 1 (OAS1) gene increases the risk for AD and that related variants in the same gene increase the likelihood of severe COVID-19 outcomes.
“These findings may allow us to identify new drug targets to slow progression of both diseases and reduce their severity,” Dervis Salih, PhD, senior research associate, UK Dementia Research Institute, University College London, said in an interview.
“Our work also suggests new approaches to treat both diseases with the same drugs,” Dr. Salih added.
The study was published online Oct. 7 in Brain.
Shared genetic network
The OAS1 gene is expressed in microglia, a type of immune cell that makes up around 10% of all cells in the brain.
In earlier work, investigators found evidence suggesting a link between the OAS1 gene and AD, but the function of the gene in microglia was unknown.
To further investigate the gene’s link to AD, they sequenced genetic data from 2,547 people – half with AD, and half without.
The genotyping analysis confirmed that the single-nucleotide polymorphism (SNP) rs1131454 within OAS1 is significantly associated with AD.
Given that the same OAS1 locus has recently been linked with severe COVID-19 outcomes, the researchers investigated four variants on the OAS1 gene.
Results indicate that SNPs within OAS1 associated with AD also show linkage to SNP variants associated with critical illness in COVID-19.
The rs1131454 (risk allele A) and rs4766676 (risk allele T) are associated with AD, and rs10735079 (risk allele A) and rs6489867 (risk allele T) are associated with critical illness with COVID-19, the investigators reported. All of these risk alleles dampen expression of OAS1.
“This study also provides strong new evidence that interferon signaling by the innate immune system plays a substantial role in the progression of Alzheimer’s,” said Dr. Salih.
“Identifying this shared genetic network in innate immune cells will allow us with future work to identify new biomarkers to track disease progression and also predict disease risk better for both disorders,” he added.
‘Fascinating’ link
In a statement from the UK nonprofit organization, Science Media Center, Kenneth Baillie, MBChB, with the University of Edinburgh, said this study builds on a discovery he and his colleagues made last year that OAS1 variants are associated with severe COVID-19.
“In the ISARIC4C study, we recently found that this is probably due to a change in the way cell membranes detect viruses, but this mechanism doesn’t explain the fascinating association with Alzheimer’s disease reported in this new work,” Dr. Baillie said.
“It is often the case that the same gene can have different roles in different parts of the body. Importantly, it doesn’t mean that having COVID-19 has any effect on your risk of Alzheimer’s,” he added.
Also weighing in on the new study, Jonathan Schott, MD, professor of neurology, University College London, noted that dementia is the “main preexisting health condition associated with COVID-19 mortality, accounting for about one in four deaths from COVID-19 between March and June 2020.
“While some of this excessive mortality may relate to people with dementia being overrepresented in care homes, which were particularly hard hit by the pandemic, or due to general increased vulnerability to infections, there have been questions as to whether there are common factors that might increase susceptibility both to developing dementia and to dying from COVID-19,” Dr. Schott explained.
This “elegant paper” provides evidence for the latter, “suggesting a common genetic mechanism both for Alzheimer’s disease and for severe COVID-19 infection,” Dr. Schott said.
“The identification of a genetic risk factor and elucidation of inflammatory pathways through which it may increase risk has important implications for our understanding of both diseases, with potential implications for novel treatments,” he added.
The study was funded by the UK Dementia Research Institute. The authors have disclosed no relevant financial relationships. Dr. Schott serves as chief medical officer for Alzheimer’s Research UK and is clinical adviser to the UK Dementia Research Institute. Dr. Baillie has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Even one vaccinated member can cut family’s COVID risk
The chances are reduced even further with each additional vaccinated or otherwise immune family member, according to new data.
Lead author Peter Nordström, MD, PhD, with the unit of geriatric medicine, Umeå (Sweden) University, said in an interview the message is important for public health: “When you vaccinate, you do not just protect yourself but also your relatives.”
The findings were published online on Oct. 11, 2021, in JAMA Internal Medicine.
Researchers analyzed data from 1,789,728 individuals from 814,806 families from nationwide registries in Sweden. All individuals had acquired immunity either from previously being infected with SARS-CoV-2 or by being fully vaccinated (that is, having received two doses of the Moderna, Pfizer, or Oxford/AstraZeneca vaccines). Persons were considered for inclusion until May 26, 2021.
Each person with immunity was matched in a 1:1 ratio to a person without immunity from a cohort of individuals with families that had from two to five members. Families with more than five members were excluded because of small sample sizes.
Primarily nonimmune families in which there was one immune family member had a 45%-61% lower risk of contracting COVID-19 (hazard ratio, 0.39-0.55; 95% confidence interval, 0.37-0.61; P < .001).
The risk reduction increased to 75%-86% when two family members were immune (HR, 0.14-0.25; 95% CI, 0.11-0.27; P < .001).
It increased to 91%-94% when three family members were immune (HR, 0.06-0.09; 95% CI, 0.04-0.10; P < .001) and to 97% with four immune family members (HR, 0.03; 95% CI, 0.02-0.05; P < .001).
“The results were similar for the outcome of COVID-19 infection that was severe enough to warrant a hospital stay,” the authors wrote. They listed as an example that, in three-member families in which two members were immune, the remaining nonimmune family member had an 80% lower risk for hospitalization (HR, 0.20; 95% CI, 0.10-0.43; P < .001).
Global implications
Dr. Nordström said the team used the family setting because it was more easily identifiable as a cohort with the national registries and because COVID-19 is spread among people in close contact with each other. The findings have implications for other groups that spend large amounts of time together and for herd immunity, he added.
The findings may be particularly welcome in regions of the world where vaccination rates are very low. The authors noted that most of the global population has not yet been vaccinated and that “it is anticipated that most of the population in low-income countries will be unable to receive a vaccine in 2021, with current vaccination rates suggesting that completely inoculating 70%-85% of the global population may take up to 5 years.”
Jill Foster, MD, a pediatric infectious disease specialist at the University of Minnesota, Minneapolis, said in an interview she agrees that the news could encourage countries that have very low vaccination rates.
This study may help motivate areas with few resources to start small, she said: “Even one is better than zero.”
She added that this news could also help ease the minds of families that have immunocompromised members or in which there are children who are too young to be vaccinated.
With these data, she said, people can see there’s something they can do to help protect a family member.
Dr. Foster said that although it’s intuitive to think that the more vaccinated people there are in a family, the safer people are, “it’s really nice to see the data coming out of such a large dataset.”
The authors acknowledged that a limitation of the study is that, at the time the study was conducted, the Delta variant was uncommon in Sweden. It is therefore unclear whether the findings regarding immunity are still relevant in Sweden and elsewhere now that the Delta strain is dominant.
The authors reported no relevant financial relationships. Dr. Foster has received grant support from Moderna.
A version of this article first appeared on Medscape.com.
The chances are reduced even further with each additional vaccinated or otherwise immune family member, according to new data.
Lead author Peter Nordström, MD, PhD, with the unit of geriatric medicine, Umeå (Sweden) University, said in an interview the message is important for public health: “When you vaccinate, you do not just protect yourself but also your relatives.”
The findings were published online on Oct. 11, 2021, in JAMA Internal Medicine.
Researchers analyzed data from 1,789,728 individuals from 814,806 families from nationwide registries in Sweden. All individuals had acquired immunity either from previously being infected with SARS-CoV-2 or by being fully vaccinated (that is, having received two doses of the Moderna, Pfizer, or Oxford/AstraZeneca vaccines). Persons were considered for inclusion until May 26, 2021.
Each person with immunity was matched in a 1:1 ratio to a person without immunity from a cohort of individuals with families that had from two to five members. Families with more than five members were excluded because of small sample sizes.
Primarily nonimmune families in which there was one immune family member had a 45%-61% lower risk of contracting COVID-19 (hazard ratio, 0.39-0.55; 95% confidence interval, 0.37-0.61; P < .001).
The risk reduction increased to 75%-86% when two family members were immune (HR, 0.14-0.25; 95% CI, 0.11-0.27; P < .001).
It increased to 91%-94% when three family members were immune (HR, 0.06-0.09; 95% CI, 0.04-0.10; P < .001) and to 97% with four immune family members (HR, 0.03; 95% CI, 0.02-0.05; P < .001).
“The results were similar for the outcome of COVID-19 infection that was severe enough to warrant a hospital stay,” the authors wrote. They listed as an example that, in three-member families in which two members were immune, the remaining nonimmune family member had an 80% lower risk for hospitalization (HR, 0.20; 95% CI, 0.10-0.43; P < .001).
Global implications
Dr. Nordström said the team used the family setting because it was more easily identifiable as a cohort with the national registries and because COVID-19 is spread among people in close contact with each other. The findings have implications for other groups that spend large amounts of time together and for herd immunity, he added.
The findings may be particularly welcome in regions of the world where vaccination rates are very low. The authors noted that most of the global population has not yet been vaccinated and that “it is anticipated that most of the population in low-income countries will be unable to receive a vaccine in 2021, with current vaccination rates suggesting that completely inoculating 70%-85% of the global population may take up to 5 years.”
Jill Foster, MD, a pediatric infectious disease specialist at the University of Minnesota, Minneapolis, said in an interview she agrees that the news could encourage countries that have very low vaccination rates.
This study may help motivate areas with few resources to start small, she said: “Even one is better than zero.”
She added that this news could also help ease the minds of families that have immunocompromised members or in which there are children who are too young to be vaccinated.
With these data, she said, people can see there’s something they can do to help protect a family member.
Dr. Foster said that although it’s intuitive to think that the more vaccinated people there are in a family, the safer people are, “it’s really nice to see the data coming out of such a large dataset.”
The authors acknowledged that a limitation of the study is that, at the time the study was conducted, the Delta variant was uncommon in Sweden. It is therefore unclear whether the findings regarding immunity are still relevant in Sweden and elsewhere now that the Delta strain is dominant.
The authors reported no relevant financial relationships. Dr. Foster has received grant support from Moderna.
A version of this article first appeared on Medscape.com.
The chances are reduced even further with each additional vaccinated or otherwise immune family member, according to new data.
Lead author Peter Nordström, MD, PhD, with the unit of geriatric medicine, Umeå (Sweden) University, said in an interview the message is important for public health: “When you vaccinate, you do not just protect yourself but also your relatives.”
The findings were published online on Oct. 11, 2021, in JAMA Internal Medicine.
Researchers analyzed data from 1,789,728 individuals from 814,806 families from nationwide registries in Sweden. All individuals had acquired immunity either from previously being infected with SARS-CoV-2 or by being fully vaccinated (that is, having received two doses of the Moderna, Pfizer, or Oxford/AstraZeneca vaccines). Persons were considered for inclusion until May 26, 2021.
Each person with immunity was matched in a 1:1 ratio to a person without immunity from a cohort of individuals with families that had from two to five members. Families with more than five members were excluded because of small sample sizes.
Primarily nonimmune families in which there was one immune family member had a 45%-61% lower risk of contracting COVID-19 (hazard ratio, 0.39-0.55; 95% confidence interval, 0.37-0.61; P < .001).
The risk reduction increased to 75%-86% when two family members were immune (HR, 0.14-0.25; 95% CI, 0.11-0.27; P < .001).
It increased to 91%-94% when three family members were immune (HR, 0.06-0.09; 95% CI, 0.04-0.10; P < .001) and to 97% with four immune family members (HR, 0.03; 95% CI, 0.02-0.05; P < .001).
“The results were similar for the outcome of COVID-19 infection that was severe enough to warrant a hospital stay,” the authors wrote. They listed as an example that, in three-member families in which two members were immune, the remaining nonimmune family member had an 80% lower risk for hospitalization (HR, 0.20; 95% CI, 0.10-0.43; P < .001).
Global implications
Dr. Nordström said the team used the family setting because it was more easily identifiable as a cohort with the national registries and because COVID-19 is spread among people in close contact with each other. The findings have implications for other groups that spend large amounts of time together and for herd immunity, he added.
The findings may be particularly welcome in regions of the world where vaccination rates are very low. The authors noted that most of the global population has not yet been vaccinated and that “it is anticipated that most of the population in low-income countries will be unable to receive a vaccine in 2021, with current vaccination rates suggesting that completely inoculating 70%-85% of the global population may take up to 5 years.”
Jill Foster, MD, a pediatric infectious disease specialist at the University of Minnesota, Minneapolis, said in an interview she agrees that the news could encourage countries that have very low vaccination rates.
This study may help motivate areas with few resources to start small, she said: “Even one is better than zero.”
She added that this news could also help ease the minds of families that have immunocompromised members or in which there are children who are too young to be vaccinated.
With these data, she said, people can see there’s something they can do to help protect a family member.
Dr. Foster said that although it’s intuitive to think that the more vaccinated people there are in a family, the safer people are, “it’s really nice to see the data coming out of such a large dataset.”
The authors acknowledged that a limitation of the study is that, at the time the study was conducted, the Delta variant was uncommon in Sweden. It is therefore unclear whether the findings regarding immunity are still relevant in Sweden and elsewhere now that the Delta strain is dominant.
The authors reported no relevant financial relationships. Dr. Foster has received grant support from Moderna.
A version of this article first appeared on Medscape.com.
CDC: Children just as vulnerable to COVID as adults
The study, which focused on 1,000 schools in Arizona’s Maricopa and Pima counties, found that there were 113 COVID-19 outbreaks in schools without mask requirements in the first month of in-person learning. There were 16 outbreaks in schools with mask requirements.
“Masks in schools work to protect our children, to keep them and their school communities safe, and to keep them in school for in-person learning,” CDC Director Rochelle Walensky, MD, said at an Oct. 13 White House briefing.
But, she said, more than 95% of schools across the country had remained open through the end of September, despite 1,800 school closures affecting nearly 1 million students.
Protection for children in school is just one piece of the puzzle, Dr. Walensky said – there must also be COVID-safe practices at home to limit transmission. A CDC study published in October found that children had similar infection rates, compared with adults, confirming there is risk to people of all ages.
“For those children not yet eligible for vaccination, the best protection we can provide them is to make sure everyone around them in the household is vaccinated and to make sure they’re wearing a mask in school and during indoor extracurricular activities,” Dr. Walensky said.
Meanwhile, Pfizer’s vaccine for children ages 5-11 may be approved by early November. The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee will meet Oct. 26 to discuss available data, and the CDC’s Advisory Committee on Immunization Practices will meet Nov. 2. A decision is expected soon after.
A version of this article first appeared on WebMD.com.
The study, which focused on 1,000 schools in Arizona’s Maricopa and Pima counties, found that there were 113 COVID-19 outbreaks in schools without mask requirements in the first month of in-person learning. There were 16 outbreaks in schools with mask requirements.
“Masks in schools work to protect our children, to keep them and their school communities safe, and to keep them in school for in-person learning,” CDC Director Rochelle Walensky, MD, said at an Oct. 13 White House briefing.
But, she said, more than 95% of schools across the country had remained open through the end of September, despite 1,800 school closures affecting nearly 1 million students.
Protection for children in school is just one piece of the puzzle, Dr. Walensky said – there must also be COVID-safe practices at home to limit transmission. A CDC study published in October found that children had similar infection rates, compared with adults, confirming there is risk to people of all ages.
“For those children not yet eligible for vaccination, the best protection we can provide them is to make sure everyone around them in the household is vaccinated and to make sure they’re wearing a mask in school and during indoor extracurricular activities,” Dr. Walensky said.
Meanwhile, Pfizer’s vaccine for children ages 5-11 may be approved by early November. The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee will meet Oct. 26 to discuss available data, and the CDC’s Advisory Committee on Immunization Practices will meet Nov. 2. A decision is expected soon after.
A version of this article first appeared on WebMD.com.
The study, which focused on 1,000 schools in Arizona’s Maricopa and Pima counties, found that there were 113 COVID-19 outbreaks in schools without mask requirements in the first month of in-person learning. There were 16 outbreaks in schools with mask requirements.
“Masks in schools work to protect our children, to keep them and their school communities safe, and to keep them in school for in-person learning,” CDC Director Rochelle Walensky, MD, said at an Oct. 13 White House briefing.
But, she said, more than 95% of schools across the country had remained open through the end of September, despite 1,800 school closures affecting nearly 1 million students.
Protection for children in school is just one piece of the puzzle, Dr. Walensky said – there must also be COVID-safe practices at home to limit transmission. A CDC study published in October found that children had similar infection rates, compared with adults, confirming there is risk to people of all ages.
“For those children not yet eligible for vaccination, the best protection we can provide them is to make sure everyone around them in the household is vaccinated and to make sure they’re wearing a mask in school and during indoor extracurricular activities,” Dr. Walensky said.
Meanwhile, Pfizer’s vaccine for children ages 5-11 may be approved by early November. The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee will meet Oct. 26 to discuss available data, and the CDC’s Advisory Committee on Immunization Practices will meet Nov. 2. A decision is expected soon after.
A version of this article first appeared on WebMD.com.
COPD: Higher mortality with low baseline CO diffusing capacity
Patients with a baseline DLCO (diffusing capacity for carbon monoxide) of < 60% of predicted have more severe disease clinical expression with higher mortality risk, according to a long-term observational study of Global Initiative for Obstructive Lung Disease (GOLD) I chronic obstructive pulmonary disease (COPD) patients. Clarifying mechanisms of low DLCO may help clinicians direct interventions toward ameliorating the low capacity, Juan Pablo de Torres, MD, and colleagues wrote in the journal CHEST®.
Defining increased risk
“Can a DLCO threshold help define an increased risk of death and a different clinical presentation in GOLD I patients?” the researchers questioned. For evaluation of COPD, the GOLD does not currently promote the use of DLCO, and the clinical and prognostic utility of a low DLCO has not been studied, the authors noted.
Several COPD studies, however, have shown associations between low DLCO values and reduced exercise capacity, increased symptoms, risk of severe exacerbations, and mortality. The patients included in these studies, however, have generally had moderate to severe airflow limitation, and have not had postbronchodilator forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) < 0.70 and an FEV1 ≥ 80%, defined by GOLD as COPD spirometric stage I. These mild obstruction GOLD I patients, in large epidemiological studies, do have increased risk of death. But it is often assumed, Dr. de Torres and colleagues noted, that “mild” suggests a good prognosis. They propose that a simple DLCO measurement could help identify those GOLD I patients with “worse overall COPD compromise and an increased risk of death.” Importantly, GOLD I represents the largest percentage of patients with airflow limitation that epidemiological studies have identified.
The researchers enrolled 360 GOLD stage I COPD patients, recording their age, sex, pack-years’ history, body mass index, dyspnea, lung function measurements, exercise capacity, BODE (body mass index, airflow obstruction, dyspnea, and exercise capacity) index, and history of exacerbations, and followed them for a mean of 109 months. They identified a cutoff DLCO value for all-cause mortality, compared the clinical and physiological characteristics of patients above and below the threshold, and explored the predictive power of that cutoff value.
All-cause mortality difference
The mean age in the overall population studied was 63 years (31% were women), with 43% active smokers, and pack-years history of 45. Overall mortality was 11% during the follow-up period. The predominantly male population was mildly overweight, had few comorbidities, normal FEV1 values, mild dyspnea, normal 6-minute walk distance, and very few exacerbations.
Analysis showed a DLCO cutoff value of < 60% was associated with a significant all-cause mortality differential (DLCO ≥ 60%: 9% vs. DLCO < 60%: 23%, P = .01). At a same FEV1% predicted and Charlson score, patients with DLCO < 60% had lower BMI, more dyspnea, lower inspiratory capacity (IC)/total lung capacity (TLC) ratio, lower 6-minute walk distance, and higher BODE index. Adjusted Cox multiple regression analysis confirmed that a DLCO < 60% was associated with an all-cause mortality hazard ratio [HR] of 3.37, (95% confidence interval, 1.35-8.39; P = .009).
Multiorgan loss of tissue
The researchers found that patients with baseline DLCO < 60% were more likely to be women (46% versus 28%), and to have a lower BMI (25 vs. 27), higher pack-year history (54 vs. 43), the same spirometric values but lower IC/TLC ratio (.37 vs. .40), a lower walk distance (443 vs. 485 meters), higher dyspnea (MRC score 1.1 vs. .7), similar exacerbation rate, higher BODE index (.5 vs. .2) and higher mortality than patients with higher DLCO % predicted values. This group, Dr. de Torres and colleagues suggest, represents a multiorgan loss of tissue, a phenotype associated with worse clinical outcomes and prognosis.
“Low DLCO in these patients,” Dr. de Torres said in an interview, “could mainly be secondary to coexistent emphysema, which is the most common cause of low DLCO in this population. Also possible, but less likely, is coexistent pulmonary hypertension.” He noted further that “This study opens the door to research specifically testing if such is the case, and if it is, for clinicians to use available therapies to prevent adverse outcomes.”
Comorbidity burden
Patients with GOLD I COPD die more often of cardiovascular disease instead of underlying lung disease, according to Richard H. Zou, MD, and Jessica Bon, MD, of the University of Pittsburgh, in an accompanying editorial in the journal CHEST.
Increased mortality rates, they suggest, may be related to higher comorbidity burden, particularly comorbidities associated with cardiovascular-related health. Subclinical cardiovascular disease is a common comorbidity in COPD, and concomitant endothelial dysfunction has been associated with both cardiovascular disease and early emphysema in smokers. They may have disproportionately reduced DLCO levels because of parenchymal destruction.
“This study suggests that DLCO can be used to identify patients with GOLD I COPD at increased death risk and that individuals with mild airflow obstruction with DLCO <60% predicted are a clinical phenotype distinct from those with higher DLCO levels,” Dr. Zhou and Dr. Bon concluded.
The researchers and the editorialists declared that they had no disclosures. One of the three cohorts assessed in the current study (CHAIN cohort in Spain) received funding from AstraZeneca.
Patients with a baseline DLCO (diffusing capacity for carbon monoxide) of < 60% of predicted have more severe disease clinical expression with higher mortality risk, according to a long-term observational study of Global Initiative for Obstructive Lung Disease (GOLD) I chronic obstructive pulmonary disease (COPD) patients. Clarifying mechanisms of low DLCO may help clinicians direct interventions toward ameliorating the low capacity, Juan Pablo de Torres, MD, and colleagues wrote in the journal CHEST®.
Defining increased risk
“Can a DLCO threshold help define an increased risk of death and a different clinical presentation in GOLD I patients?” the researchers questioned. For evaluation of COPD, the GOLD does not currently promote the use of DLCO, and the clinical and prognostic utility of a low DLCO has not been studied, the authors noted.
Several COPD studies, however, have shown associations between low DLCO values and reduced exercise capacity, increased symptoms, risk of severe exacerbations, and mortality. The patients included in these studies, however, have generally had moderate to severe airflow limitation, and have not had postbronchodilator forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) < 0.70 and an FEV1 ≥ 80%, defined by GOLD as COPD spirometric stage I. These mild obstruction GOLD I patients, in large epidemiological studies, do have increased risk of death. But it is often assumed, Dr. de Torres and colleagues noted, that “mild” suggests a good prognosis. They propose that a simple DLCO measurement could help identify those GOLD I patients with “worse overall COPD compromise and an increased risk of death.” Importantly, GOLD I represents the largest percentage of patients with airflow limitation that epidemiological studies have identified.
The researchers enrolled 360 GOLD stage I COPD patients, recording their age, sex, pack-years’ history, body mass index, dyspnea, lung function measurements, exercise capacity, BODE (body mass index, airflow obstruction, dyspnea, and exercise capacity) index, and history of exacerbations, and followed them for a mean of 109 months. They identified a cutoff DLCO value for all-cause mortality, compared the clinical and physiological characteristics of patients above and below the threshold, and explored the predictive power of that cutoff value.
All-cause mortality difference
The mean age in the overall population studied was 63 years (31% were women), with 43% active smokers, and pack-years history of 45. Overall mortality was 11% during the follow-up period. The predominantly male population was mildly overweight, had few comorbidities, normal FEV1 values, mild dyspnea, normal 6-minute walk distance, and very few exacerbations.
Analysis showed a DLCO cutoff value of < 60% was associated with a significant all-cause mortality differential (DLCO ≥ 60%: 9% vs. DLCO < 60%: 23%, P = .01). At a same FEV1% predicted and Charlson score, patients with DLCO < 60% had lower BMI, more dyspnea, lower inspiratory capacity (IC)/total lung capacity (TLC) ratio, lower 6-minute walk distance, and higher BODE index. Adjusted Cox multiple regression analysis confirmed that a DLCO < 60% was associated with an all-cause mortality hazard ratio [HR] of 3.37, (95% confidence interval, 1.35-8.39; P = .009).
Multiorgan loss of tissue
The researchers found that patients with baseline DLCO < 60% were more likely to be women (46% versus 28%), and to have a lower BMI (25 vs. 27), higher pack-year history (54 vs. 43), the same spirometric values but lower IC/TLC ratio (.37 vs. .40), a lower walk distance (443 vs. 485 meters), higher dyspnea (MRC score 1.1 vs. .7), similar exacerbation rate, higher BODE index (.5 vs. .2) and higher mortality than patients with higher DLCO % predicted values. This group, Dr. de Torres and colleagues suggest, represents a multiorgan loss of tissue, a phenotype associated with worse clinical outcomes and prognosis.
“Low DLCO in these patients,” Dr. de Torres said in an interview, “could mainly be secondary to coexistent emphysema, which is the most common cause of low DLCO in this population. Also possible, but less likely, is coexistent pulmonary hypertension.” He noted further that “This study opens the door to research specifically testing if such is the case, and if it is, for clinicians to use available therapies to prevent adverse outcomes.”
Comorbidity burden
Patients with GOLD I COPD die more often of cardiovascular disease instead of underlying lung disease, according to Richard H. Zou, MD, and Jessica Bon, MD, of the University of Pittsburgh, in an accompanying editorial in the journal CHEST.
Increased mortality rates, they suggest, may be related to higher comorbidity burden, particularly comorbidities associated with cardiovascular-related health. Subclinical cardiovascular disease is a common comorbidity in COPD, and concomitant endothelial dysfunction has been associated with both cardiovascular disease and early emphysema in smokers. They may have disproportionately reduced DLCO levels because of parenchymal destruction.
“This study suggests that DLCO can be used to identify patients with GOLD I COPD at increased death risk and that individuals with mild airflow obstruction with DLCO <60% predicted are a clinical phenotype distinct from those with higher DLCO levels,” Dr. Zhou and Dr. Bon concluded.
The researchers and the editorialists declared that they had no disclosures. One of the three cohorts assessed in the current study (CHAIN cohort in Spain) received funding from AstraZeneca.
Patients with a baseline DLCO (diffusing capacity for carbon monoxide) of < 60% of predicted have more severe disease clinical expression with higher mortality risk, according to a long-term observational study of Global Initiative for Obstructive Lung Disease (GOLD) I chronic obstructive pulmonary disease (COPD) patients. Clarifying mechanisms of low DLCO may help clinicians direct interventions toward ameliorating the low capacity, Juan Pablo de Torres, MD, and colleagues wrote in the journal CHEST®.
Defining increased risk
“Can a DLCO threshold help define an increased risk of death and a different clinical presentation in GOLD I patients?” the researchers questioned. For evaluation of COPD, the GOLD does not currently promote the use of DLCO, and the clinical and prognostic utility of a low DLCO has not been studied, the authors noted.
Several COPD studies, however, have shown associations between low DLCO values and reduced exercise capacity, increased symptoms, risk of severe exacerbations, and mortality. The patients included in these studies, however, have generally had moderate to severe airflow limitation, and have not had postbronchodilator forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) < 0.70 and an FEV1 ≥ 80%, defined by GOLD as COPD spirometric stage I. These mild obstruction GOLD I patients, in large epidemiological studies, do have increased risk of death. But it is often assumed, Dr. de Torres and colleagues noted, that “mild” suggests a good prognosis. They propose that a simple DLCO measurement could help identify those GOLD I patients with “worse overall COPD compromise and an increased risk of death.” Importantly, GOLD I represents the largest percentage of patients with airflow limitation that epidemiological studies have identified.
The researchers enrolled 360 GOLD stage I COPD patients, recording their age, sex, pack-years’ history, body mass index, dyspnea, lung function measurements, exercise capacity, BODE (body mass index, airflow obstruction, dyspnea, and exercise capacity) index, and history of exacerbations, and followed them for a mean of 109 months. They identified a cutoff DLCO value for all-cause mortality, compared the clinical and physiological characteristics of patients above and below the threshold, and explored the predictive power of that cutoff value.
All-cause mortality difference
The mean age in the overall population studied was 63 years (31% were women), with 43% active smokers, and pack-years history of 45. Overall mortality was 11% during the follow-up period. The predominantly male population was mildly overweight, had few comorbidities, normal FEV1 values, mild dyspnea, normal 6-minute walk distance, and very few exacerbations.
Analysis showed a DLCO cutoff value of < 60% was associated with a significant all-cause mortality differential (DLCO ≥ 60%: 9% vs. DLCO < 60%: 23%, P = .01). At a same FEV1% predicted and Charlson score, patients with DLCO < 60% had lower BMI, more dyspnea, lower inspiratory capacity (IC)/total lung capacity (TLC) ratio, lower 6-minute walk distance, and higher BODE index. Adjusted Cox multiple regression analysis confirmed that a DLCO < 60% was associated with an all-cause mortality hazard ratio [HR] of 3.37, (95% confidence interval, 1.35-8.39; P = .009).
Multiorgan loss of tissue
The researchers found that patients with baseline DLCO < 60% were more likely to be women (46% versus 28%), and to have a lower BMI (25 vs. 27), higher pack-year history (54 vs. 43), the same spirometric values but lower IC/TLC ratio (.37 vs. .40), a lower walk distance (443 vs. 485 meters), higher dyspnea (MRC score 1.1 vs. .7), similar exacerbation rate, higher BODE index (.5 vs. .2) and higher mortality than patients with higher DLCO % predicted values. This group, Dr. de Torres and colleagues suggest, represents a multiorgan loss of tissue, a phenotype associated with worse clinical outcomes and prognosis.
“Low DLCO in these patients,” Dr. de Torres said in an interview, “could mainly be secondary to coexistent emphysema, which is the most common cause of low DLCO in this population. Also possible, but less likely, is coexistent pulmonary hypertension.” He noted further that “This study opens the door to research specifically testing if such is the case, and if it is, for clinicians to use available therapies to prevent adverse outcomes.”
Comorbidity burden
Patients with GOLD I COPD die more often of cardiovascular disease instead of underlying lung disease, according to Richard H. Zou, MD, and Jessica Bon, MD, of the University of Pittsburgh, in an accompanying editorial in the journal CHEST.
Increased mortality rates, they suggest, may be related to higher comorbidity burden, particularly comorbidities associated with cardiovascular-related health. Subclinical cardiovascular disease is a common comorbidity in COPD, and concomitant endothelial dysfunction has been associated with both cardiovascular disease and early emphysema in smokers. They may have disproportionately reduced DLCO levels because of parenchymal destruction.
“This study suggests that DLCO can be used to identify patients with GOLD I COPD at increased death risk and that individuals with mild airflow obstruction with DLCO <60% predicted are a clinical phenotype distinct from those with higher DLCO levels,” Dr. Zhou and Dr. Bon concluded.
The researchers and the editorialists declared that they had no disclosures. One of the three cohorts assessed in the current study (CHAIN cohort in Spain) received funding from AstraZeneca.
FROM THE JOURNAL CHEST®
No short-term death risk in elderly after COVID-19 vaccines
and launched an investigation into the safety of the BNT162b2 vaccine (Comirnaty; Pfizer-BioNTech).
Now, the results of that investigation and of a subsequent larger study of nursing home residents in Norway have shown no increased risk for short-term mortality following COVID-19 vaccination in the overall population of elderly patients. The new research also showed clear evidence of a survival benefit compared with the unvaccinated population, Anette Hylen Ranhoff, MD, PhD, said at the annual meeting of the European Geriatric Medicine Society, held in a hybrid format in Athens, Greece, and online.
“We found no evidence of increased short-term mortality among vaccinated older individuals, and particularly not among the nursing home patients,” said Dr. Ranhoff, a senior researcher at the Norwegian Institute of Public Health and professor at University of Bergen, Norway. “But we think that this [lower] mortality risk was most likely a sort of ‘healthy-vaccinee’ effect, which means that people who were a bit more healthy were vaccinated, and not those who were the very, very most frail.”
“We have more or less the same data in France about events, with very high rates of vaccination,” said session moderator Athanase Benetos MD, PhD, professor and chairman of geriatric medicine at the University Hospital of Nancy in France, who was not involved in the study.
“In my department, a month after the end of the vaccination and at the same time while the pandemic in the city was going up, we had a 90% decrease in mortality from COVID in the nursing homes,” he told Dr. Ranhoff.
Potential risks
Frail elderly patients were not included in clinical trials of COVID-19 vaccines, and although previous studies have shown a low incidence of local or systemic reactions to vaccination among older people, “we think that quite mild adverse events following vaccination could trigger and destabilize a frail person,” Dr. Ranhoff said.
As reported Jan. 15, 2021, in BMJ, investigation by the Norwegian Medicines Agency (NOMA) into 13 of the 23 reported cases concluded that common adverse reactions associated with mRNA vaccines could have contributed to the deaths of some of the frail elderly patients
Steinar Madsen, MD, NOMA medical director, told BMJ “we are not alarmed or worried about this, because these are very rare occurrences and they occurred in very frail patients with very serious disease.”
Health authorities investigate
In response to the report and at the request of the Norwegian Public Health Institute and NOMA, Dr. Ranhoff and colleagues investigated the first 100 deaths among nursing-home residents who received the vaccine. The team consisted of three geriatricians and an infectious disease specialist who sees patients in nursing homes.
They looked at each patient’s clinical course before and after vaccination, their health trajectory and life expectancy at the time of vaccination, new symptoms following vaccination, and the time from vaccination to new symptoms and to death.
In addition, the investigators evaluated Clinical Frailty Scale (CFS) scores for each patient. CFS scores range from 1 (very fit) to 9 (terminally ill, with a life expectancy of less than 6 months who are otherwise evidently frail).
The initial investigation found that among 95 evaluable patients, the association between vaccination and death was “probable” in 10, “possible” in 26, and “unlikely” in 59.
The mean time from vaccination to symptoms was 1.4 days in the probable cases, 2.5 days in the possible cases, and 4.7 days in the unlikely cases.
The mean time from vaccination to death was 3.1, 8.3, and 8.2 days, respectively.
In all three categories, the patients had mean CFS scores ranging from 7.6 to 7.9, putting them in the “severely frail” category, defined as people who are completely dependent for personal care but seem stable and not at high risk for dying.
“We have quite many nursing home residents in Norway, 35,000; more than 80% have dementia, and the mean age is 85 years. We know that approximately 45 people die every day in these nursing homes, and their mean age of death is 87.5 years,” Dr. Ranhoff said.
Population-wide study
Dr. Ranhoff and colleagues also looked more broadly into the question of potential vaccine-related mortality in the total population of older people in Norway from the day of vaccination to follow-up at 3 weeks.
They conducted a matched cohort study to investigate the relationship between the mRNA SARS-CoV-2 vaccine and overall death among persons aged 65 and older in the general population, and across four groups: patients receiving home-based care, long-term nursing home patients, short-term nursing home patients, and those not receiving health services.
The researchers identified a total of 967,786 residents of Norway aged 65 and over at the start of the country’s vaccination campaign at the end of December, 2020, and they matched vaccinated individuals with unvaccinated persons based on demographic, geographic, and clinical risk group factors.
Dr. Ranhoff showed Kaplan-Meier survival curves for the total population and for each of the health-service states. In all cases there was a clear survival benefit for vaccinated vs. unvaccinated patients. She did not, however, provide specific numbers or hazard ratios for the differences between vaccinated and unvaccinated individuals in each of the comparisons.
The study was supported by the Norwegian Institute of Public Health. Dr. Ranhoff and Dr. Benetos reported no conflicts of interest.
and launched an investigation into the safety of the BNT162b2 vaccine (Comirnaty; Pfizer-BioNTech).
Now, the results of that investigation and of a subsequent larger study of nursing home residents in Norway have shown no increased risk for short-term mortality following COVID-19 vaccination in the overall population of elderly patients. The new research also showed clear evidence of a survival benefit compared with the unvaccinated population, Anette Hylen Ranhoff, MD, PhD, said at the annual meeting of the European Geriatric Medicine Society, held in a hybrid format in Athens, Greece, and online.
“We found no evidence of increased short-term mortality among vaccinated older individuals, and particularly not among the nursing home patients,” said Dr. Ranhoff, a senior researcher at the Norwegian Institute of Public Health and professor at University of Bergen, Norway. “But we think that this [lower] mortality risk was most likely a sort of ‘healthy-vaccinee’ effect, which means that people who were a bit more healthy were vaccinated, and not those who were the very, very most frail.”
“We have more or less the same data in France about events, with very high rates of vaccination,” said session moderator Athanase Benetos MD, PhD, professor and chairman of geriatric medicine at the University Hospital of Nancy in France, who was not involved in the study.
“In my department, a month after the end of the vaccination and at the same time while the pandemic in the city was going up, we had a 90% decrease in mortality from COVID in the nursing homes,” he told Dr. Ranhoff.
Potential risks
Frail elderly patients were not included in clinical trials of COVID-19 vaccines, and although previous studies have shown a low incidence of local or systemic reactions to vaccination among older people, “we think that quite mild adverse events following vaccination could trigger and destabilize a frail person,” Dr. Ranhoff said.
As reported Jan. 15, 2021, in BMJ, investigation by the Norwegian Medicines Agency (NOMA) into 13 of the 23 reported cases concluded that common adverse reactions associated with mRNA vaccines could have contributed to the deaths of some of the frail elderly patients
Steinar Madsen, MD, NOMA medical director, told BMJ “we are not alarmed or worried about this, because these are very rare occurrences and they occurred in very frail patients with very serious disease.”
Health authorities investigate
In response to the report and at the request of the Norwegian Public Health Institute and NOMA, Dr. Ranhoff and colleagues investigated the first 100 deaths among nursing-home residents who received the vaccine. The team consisted of three geriatricians and an infectious disease specialist who sees patients in nursing homes.
They looked at each patient’s clinical course before and after vaccination, their health trajectory and life expectancy at the time of vaccination, new symptoms following vaccination, and the time from vaccination to new symptoms and to death.
In addition, the investigators evaluated Clinical Frailty Scale (CFS) scores for each patient. CFS scores range from 1 (very fit) to 9 (terminally ill, with a life expectancy of less than 6 months who are otherwise evidently frail).
The initial investigation found that among 95 evaluable patients, the association between vaccination and death was “probable” in 10, “possible” in 26, and “unlikely” in 59.
The mean time from vaccination to symptoms was 1.4 days in the probable cases, 2.5 days in the possible cases, and 4.7 days in the unlikely cases.
The mean time from vaccination to death was 3.1, 8.3, and 8.2 days, respectively.
In all three categories, the patients had mean CFS scores ranging from 7.6 to 7.9, putting them in the “severely frail” category, defined as people who are completely dependent for personal care but seem stable and not at high risk for dying.
“We have quite many nursing home residents in Norway, 35,000; more than 80% have dementia, and the mean age is 85 years. We know that approximately 45 people die every day in these nursing homes, and their mean age of death is 87.5 years,” Dr. Ranhoff said.
Population-wide study
Dr. Ranhoff and colleagues also looked more broadly into the question of potential vaccine-related mortality in the total population of older people in Norway from the day of vaccination to follow-up at 3 weeks.
They conducted a matched cohort study to investigate the relationship between the mRNA SARS-CoV-2 vaccine and overall death among persons aged 65 and older in the general population, and across four groups: patients receiving home-based care, long-term nursing home patients, short-term nursing home patients, and those not receiving health services.
The researchers identified a total of 967,786 residents of Norway aged 65 and over at the start of the country’s vaccination campaign at the end of December, 2020, and they matched vaccinated individuals with unvaccinated persons based on demographic, geographic, and clinical risk group factors.
Dr. Ranhoff showed Kaplan-Meier survival curves for the total population and for each of the health-service states. In all cases there was a clear survival benefit for vaccinated vs. unvaccinated patients. She did not, however, provide specific numbers or hazard ratios for the differences between vaccinated and unvaccinated individuals in each of the comparisons.
The study was supported by the Norwegian Institute of Public Health. Dr. Ranhoff and Dr. Benetos reported no conflicts of interest.
and launched an investigation into the safety of the BNT162b2 vaccine (Comirnaty; Pfizer-BioNTech).
Now, the results of that investigation and of a subsequent larger study of nursing home residents in Norway have shown no increased risk for short-term mortality following COVID-19 vaccination in the overall population of elderly patients. The new research also showed clear evidence of a survival benefit compared with the unvaccinated population, Anette Hylen Ranhoff, MD, PhD, said at the annual meeting of the European Geriatric Medicine Society, held in a hybrid format in Athens, Greece, and online.
“We found no evidence of increased short-term mortality among vaccinated older individuals, and particularly not among the nursing home patients,” said Dr. Ranhoff, a senior researcher at the Norwegian Institute of Public Health and professor at University of Bergen, Norway. “But we think that this [lower] mortality risk was most likely a sort of ‘healthy-vaccinee’ effect, which means that people who were a bit more healthy were vaccinated, and not those who were the very, very most frail.”
“We have more or less the same data in France about events, with very high rates of vaccination,” said session moderator Athanase Benetos MD, PhD, professor and chairman of geriatric medicine at the University Hospital of Nancy in France, who was not involved in the study.
“In my department, a month after the end of the vaccination and at the same time while the pandemic in the city was going up, we had a 90% decrease in mortality from COVID in the nursing homes,” he told Dr. Ranhoff.
Potential risks
Frail elderly patients were not included in clinical trials of COVID-19 vaccines, and although previous studies have shown a low incidence of local or systemic reactions to vaccination among older people, “we think that quite mild adverse events following vaccination could trigger and destabilize a frail person,” Dr. Ranhoff said.
As reported Jan. 15, 2021, in BMJ, investigation by the Norwegian Medicines Agency (NOMA) into 13 of the 23 reported cases concluded that common adverse reactions associated with mRNA vaccines could have contributed to the deaths of some of the frail elderly patients
Steinar Madsen, MD, NOMA medical director, told BMJ “we are not alarmed or worried about this, because these are very rare occurrences and they occurred in very frail patients with very serious disease.”
Health authorities investigate
In response to the report and at the request of the Norwegian Public Health Institute and NOMA, Dr. Ranhoff and colleagues investigated the first 100 deaths among nursing-home residents who received the vaccine. The team consisted of three geriatricians and an infectious disease specialist who sees patients in nursing homes.
They looked at each patient’s clinical course before and after vaccination, their health trajectory and life expectancy at the time of vaccination, new symptoms following vaccination, and the time from vaccination to new symptoms and to death.
In addition, the investigators evaluated Clinical Frailty Scale (CFS) scores for each patient. CFS scores range from 1 (very fit) to 9 (terminally ill, with a life expectancy of less than 6 months who are otherwise evidently frail).
The initial investigation found that among 95 evaluable patients, the association between vaccination and death was “probable” in 10, “possible” in 26, and “unlikely” in 59.
The mean time from vaccination to symptoms was 1.4 days in the probable cases, 2.5 days in the possible cases, and 4.7 days in the unlikely cases.
The mean time from vaccination to death was 3.1, 8.3, and 8.2 days, respectively.
In all three categories, the patients had mean CFS scores ranging from 7.6 to 7.9, putting them in the “severely frail” category, defined as people who are completely dependent for personal care but seem stable and not at high risk for dying.
“We have quite many nursing home residents in Norway, 35,000; more than 80% have dementia, and the mean age is 85 years. We know that approximately 45 people die every day in these nursing homes, and their mean age of death is 87.5 years,” Dr. Ranhoff said.
Population-wide study
Dr. Ranhoff and colleagues also looked more broadly into the question of potential vaccine-related mortality in the total population of older people in Norway from the day of vaccination to follow-up at 3 weeks.
They conducted a matched cohort study to investigate the relationship between the mRNA SARS-CoV-2 vaccine and overall death among persons aged 65 and older in the general population, and across four groups: patients receiving home-based care, long-term nursing home patients, short-term nursing home patients, and those not receiving health services.
The researchers identified a total of 967,786 residents of Norway aged 65 and over at the start of the country’s vaccination campaign at the end of December, 2020, and they matched vaccinated individuals with unvaccinated persons based on demographic, geographic, and clinical risk group factors.
Dr. Ranhoff showed Kaplan-Meier survival curves for the total population and for each of the health-service states. In all cases there was a clear survival benefit for vaccinated vs. unvaccinated patients. She did not, however, provide specific numbers or hazard ratios for the differences between vaccinated and unvaccinated individuals in each of the comparisons.
The study was supported by the Norwegian Institute of Public Health. Dr. Ranhoff and Dr. Benetos reported no conflicts of interest.
FROM EUGMS 2021
Omega-3s tame inflammation in elderly COVID-19 patients
results of a small randomized controlled trial suggest.
Results of the study, which included 22 patients with multiple comorbidities, were presented at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
The patients, who had a median age of 81 years, were randomized to receive an intravenous infusion of an omega-3 polyunsaturated fatty acid (PUFA) emulsion containing 10 g of fish oil per 100 mL or a saline placebo.
Those who received the intravenous infusion had significant decreases from baseline to end of treatment in the neutrophil-to-lymphocyte ratio (NLR), indicating marked reductions in systemic inflammation.
In contrast, patients randomized to a saline placebo had no significant improvements in NLR, Magnus Bäck, MD, PhD, from the Karolinska Institute in Stockholm reported at the meeting.
“Our lipidomic analysis also showed that omega-3 treatment skewed the lipid response, with reduced levels of proinflammatory lipid mediators, and increased levels of proresolving mediators,” according to a late-breaking abstract, which Dr. Bäck presented during the session.
Omega-3 treatment was not significantly associated with reduction in either C-reactive protein (CRP) or the proinflammatory cytokine interleukin-6, however.
‘Eicosanoid storm’
In a review article published in January 2021 in the open-access journal Frontiers in Physiology, Dr. Bäck and colleagues outlined the rationale for their randomized trial.
“Excessive inflammation has been reported in severe cases with respiratory failure and cardiovascular complications,” they wrote. “In addition to the release of cytokines, referred to as cytokine release syndrome or ‘cytokine storm,’ increased proinflammatory lipid mediators derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid may cause an ‘eicosanoid storm,’ which contributes to the uncontrolled systemic inflammation.”
Omega-3 PUFA contains proresolving mediators that can limit inflammatory reactions, suggesting the possibility of an inflammation-resolving benefit in patients with COVID-19 without concerns about immunosuppression, the authors hypothesized.
Trial details
In the trial, COVID-Omega-F, they enrolled patients with a COVID-19 diagnosis requiring hospitalization. Patients with an allergy to fish oil or who had contraindications to intravenous PUFA administration (for example, risk for bleeding, shock, or emboli) were excluded.
Ten patients were randomly assigned to receive infusions of the omega-3 PUFA and 12 were assigned to receive infusions of the placebo, once daily for 5 days. The primary outcome measure was change in inflammatory biomarkers, including white blood cell counts, CRP, cytokines, and lipid mediators.
Baseline demographic and clinical characteristics were similar between the two study arms, with a median of about 7 days since the onset of symptoms, and 3.5 days since a diagnosis of COVID-19.
All patients had low lymphocyte responses reflected by a high NLR, a prognostic measure for worse outcomes in patients with COVID-19 infections, Dr. Bäck said.
Inflammation was moderate, with a CRP of 65 mg/L in the placebo group and 62 mg/L in the omega-3 group.
Seven patients in each study arm received concomitant corticoid treatment. Two patients in each arm died in hospital, but there were no serious treatment-related adverse events.
Inflammatory markers improve
As noted before, there was a significant decline in NLR from baseline among patients randomized to omega-3 (P = .02) but no corresponding decrease in patients assigned to placebo infusions.
“The significant decrease was largely driven by an increase in the lymphocyte count in the omega-3 treated group (P = .004), whereas lymphocytes did not significantly change,” Dr. Bäck said.
As expected, patients in the omega-3 group had pronounced increases in omega-3 fatty acids, including eicosapentaenoic acid and docosahexaenoic acid.
The metabolism of fatty acids also differed markedly between the groups, with a significant decrease in the omega-3 group but not the placebo group in proinflammatory mediators, and an increase in precursors to proresolving mediators, Dr. Bäck noted.
AFib concerns
In a question-and-answer part of the session, a physician who identified herself as “Senya from Russia” questioned the safety of omega-3 treatment in this population, “because recently there was a meta-analysis which showed that omega-3 fatty acids will increase the risk of atrial fibrillation in older adults especially.”
The systematic review and meta-analysis she referred to, published in Circulation and reported on by this news organization, showed that, among 81,210 patients with a mean age of 65 enrolled in seven randomized controlled trials, omega-3 fatty acid supplementation was associated with a 25% increase in risk for atrial fibrillation. This risk appeared to be higher in trials testing doses greater than 1 g/day, according to the paper.
“This was not monitored in this study,” Dr. Bäck replied. “It is true that the meta-analysis showed an increased incidence of atrial fibrillation, so it would be something to monitor in case this trial would be expanded to a larger population.”
The study was supported by the Karolinska Institute. Dr. Bäck disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results of a small randomized controlled trial suggest.
Results of the study, which included 22 patients with multiple comorbidities, were presented at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
The patients, who had a median age of 81 years, were randomized to receive an intravenous infusion of an omega-3 polyunsaturated fatty acid (PUFA) emulsion containing 10 g of fish oil per 100 mL or a saline placebo.
Those who received the intravenous infusion had significant decreases from baseline to end of treatment in the neutrophil-to-lymphocyte ratio (NLR), indicating marked reductions in systemic inflammation.
In contrast, patients randomized to a saline placebo had no significant improvements in NLR, Magnus Bäck, MD, PhD, from the Karolinska Institute in Stockholm reported at the meeting.
“Our lipidomic analysis also showed that omega-3 treatment skewed the lipid response, with reduced levels of proinflammatory lipid mediators, and increased levels of proresolving mediators,” according to a late-breaking abstract, which Dr. Bäck presented during the session.
Omega-3 treatment was not significantly associated with reduction in either C-reactive protein (CRP) or the proinflammatory cytokine interleukin-6, however.
‘Eicosanoid storm’
In a review article published in January 2021 in the open-access journal Frontiers in Physiology, Dr. Bäck and colleagues outlined the rationale for their randomized trial.
“Excessive inflammation has been reported in severe cases with respiratory failure and cardiovascular complications,” they wrote. “In addition to the release of cytokines, referred to as cytokine release syndrome or ‘cytokine storm,’ increased proinflammatory lipid mediators derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid may cause an ‘eicosanoid storm,’ which contributes to the uncontrolled systemic inflammation.”
Omega-3 PUFA contains proresolving mediators that can limit inflammatory reactions, suggesting the possibility of an inflammation-resolving benefit in patients with COVID-19 without concerns about immunosuppression, the authors hypothesized.
Trial details
In the trial, COVID-Omega-F, they enrolled patients with a COVID-19 diagnosis requiring hospitalization. Patients with an allergy to fish oil or who had contraindications to intravenous PUFA administration (for example, risk for bleeding, shock, or emboli) were excluded.
Ten patients were randomly assigned to receive infusions of the omega-3 PUFA and 12 were assigned to receive infusions of the placebo, once daily for 5 days. The primary outcome measure was change in inflammatory biomarkers, including white blood cell counts, CRP, cytokines, and lipid mediators.
Baseline demographic and clinical characteristics were similar between the two study arms, with a median of about 7 days since the onset of symptoms, and 3.5 days since a diagnosis of COVID-19.
All patients had low lymphocyte responses reflected by a high NLR, a prognostic measure for worse outcomes in patients with COVID-19 infections, Dr. Bäck said.
Inflammation was moderate, with a CRP of 65 mg/L in the placebo group and 62 mg/L in the omega-3 group.
Seven patients in each study arm received concomitant corticoid treatment. Two patients in each arm died in hospital, but there were no serious treatment-related adverse events.
Inflammatory markers improve
As noted before, there was a significant decline in NLR from baseline among patients randomized to omega-3 (P = .02) but no corresponding decrease in patients assigned to placebo infusions.
“The significant decrease was largely driven by an increase in the lymphocyte count in the omega-3 treated group (P = .004), whereas lymphocytes did not significantly change,” Dr. Bäck said.
As expected, patients in the omega-3 group had pronounced increases in omega-3 fatty acids, including eicosapentaenoic acid and docosahexaenoic acid.
The metabolism of fatty acids also differed markedly between the groups, with a significant decrease in the omega-3 group but not the placebo group in proinflammatory mediators, and an increase in precursors to proresolving mediators, Dr. Bäck noted.
AFib concerns
In a question-and-answer part of the session, a physician who identified herself as “Senya from Russia” questioned the safety of omega-3 treatment in this population, “because recently there was a meta-analysis which showed that omega-3 fatty acids will increase the risk of atrial fibrillation in older adults especially.”
The systematic review and meta-analysis she referred to, published in Circulation and reported on by this news organization, showed that, among 81,210 patients with a mean age of 65 enrolled in seven randomized controlled trials, omega-3 fatty acid supplementation was associated with a 25% increase in risk for atrial fibrillation. This risk appeared to be higher in trials testing doses greater than 1 g/day, according to the paper.
“This was not monitored in this study,” Dr. Bäck replied. “It is true that the meta-analysis showed an increased incidence of atrial fibrillation, so it would be something to monitor in case this trial would be expanded to a larger population.”
The study was supported by the Karolinska Institute. Dr. Bäck disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results of a small randomized controlled trial suggest.
Results of the study, which included 22 patients with multiple comorbidities, were presented at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
The patients, who had a median age of 81 years, were randomized to receive an intravenous infusion of an omega-3 polyunsaturated fatty acid (PUFA) emulsion containing 10 g of fish oil per 100 mL or a saline placebo.
Those who received the intravenous infusion had significant decreases from baseline to end of treatment in the neutrophil-to-lymphocyte ratio (NLR), indicating marked reductions in systemic inflammation.
In contrast, patients randomized to a saline placebo had no significant improvements in NLR, Magnus Bäck, MD, PhD, from the Karolinska Institute in Stockholm reported at the meeting.
“Our lipidomic analysis also showed that omega-3 treatment skewed the lipid response, with reduced levels of proinflammatory lipid mediators, and increased levels of proresolving mediators,” according to a late-breaking abstract, which Dr. Bäck presented during the session.
Omega-3 treatment was not significantly associated with reduction in either C-reactive protein (CRP) or the proinflammatory cytokine interleukin-6, however.
‘Eicosanoid storm’
In a review article published in January 2021 in the open-access journal Frontiers in Physiology, Dr. Bäck and colleagues outlined the rationale for their randomized trial.
“Excessive inflammation has been reported in severe cases with respiratory failure and cardiovascular complications,” they wrote. “In addition to the release of cytokines, referred to as cytokine release syndrome or ‘cytokine storm,’ increased proinflammatory lipid mediators derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid may cause an ‘eicosanoid storm,’ which contributes to the uncontrolled systemic inflammation.”
Omega-3 PUFA contains proresolving mediators that can limit inflammatory reactions, suggesting the possibility of an inflammation-resolving benefit in patients with COVID-19 without concerns about immunosuppression, the authors hypothesized.
Trial details
In the trial, COVID-Omega-F, they enrolled patients with a COVID-19 diagnosis requiring hospitalization. Patients with an allergy to fish oil or who had contraindications to intravenous PUFA administration (for example, risk for bleeding, shock, or emboli) were excluded.
Ten patients were randomly assigned to receive infusions of the omega-3 PUFA and 12 were assigned to receive infusions of the placebo, once daily for 5 days. The primary outcome measure was change in inflammatory biomarkers, including white blood cell counts, CRP, cytokines, and lipid mediators.
Baseline demographic and clinical characteristics were similar between the two study arms, with a median of about 7 days since the onset of symptoms, and 3.5 days since a diagnosis of COVID-19.
All patients had low lymphocyte responses reflected by a high NLR, a prognostic measure for worse outcomes in patients with COVID-19 infections, Dr. Bäck said.
Inflammation was moderate, with a CRP of 65 mg/L in the placebo group and 62 mg/L in the omega-3 group.
Seven patients in each study arm received concomitant corticoid treatment. Two patients in each arm died in hospital, but there were no serious treatment-related adverse events.
Inflammatory markers improve
As noted before, there was a significant decline in NLR from baseline among patients randomized to omega-3 (P = .02) but no corresponding decrease in patients assigned to placebo infusions.
“The significant decrease was largely driven by an increase in the lymphocyte count in the omega-3 treated group (P = .004), whereas lymphocytes did not significantly change,” Dr. Bäck said.
As expected, patients in the omega-3 group had pronounced increases in omega-3 fatty acids, including eicosapentaenoic acid and docosahexaenoic acid.
The metabolism of fatty acids also differed markedly between the groups, with a significant decrease in the omega-3 group but not the placebo group in proinflammatory mediators, and an increase in precursors to proresolving mediators, Dr. Bäck noted.
AFib concerns
In a question-and-answer part of the session, a physician who identified herself as “Senya from Russia” questioned the safety of omega-3 treatment in this population, “because recently there was a meta-analysis which showed that omega-3 fatty acids will increase the risk of atrial fibrillation in older adults especially.”
The systematic review and meta-analysis she referred to, published in Circulation and reported on by this news organization, showed that, among 81,210 patients with a mean age of 65 enrolled in seven randomized controlled trials, omega-3 fatty acid supplementation was associated with a 25% increase in risk for atrial fibrillation. This risk appeared to be higher in trials testing doses greater than 1 g/day, according to the paper.
“This was not monitored in this study,” Dr. Bäck replied. “It is true that the meta-analysis showed an increased incidence of atrial fibrillation, so it would be something to monitor in case this trial would be expanded to a larger population.”
The study was supported by the Karolinska Institute. Dr. Bäck disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EUGMS
Bystander actions can reduce children’s risk of drowning
The likelihood that a child will survive a near-drowning without long-term damage is substantially greater if a bystander attempts a rescue, even if that person doesn’t perform cardiopulmonary resuscitation (CPR), according to new research presented October 10 at the American Academy of Pediatrics (AAP) 2021 National Conference.
“The extent to which bystander rescue is associated with reduced odds of unfavorable drowning outcomes was surprising,” said lead investigator Rohit P. Shenoi, MD, professor of pediatrics at Baylor College of Medicine and attending physician at Texas Children’s Hospital, Houston.
“While we do know that early rescue and resuscitation is helpful in preventing severe drowning injury, the degree of benefit from bystander rescue in all cases of pediatric drowning has not been described so far,” he told this news organization.
The fact that a bystander’s rescue attempt improves a child’s odds of a good outcome is not surprising on its own, but the magnitude of the finding really affirms the importance of bystander intervention, said Benjamin Hoffman, MD, professor of pediatrics at the Oregon Health & Science University School of Medicine and medical director of the Tom Sargent Safety Center at the Doernbecher Children’s Hospital, Portland.
“If an adult finds a child in the water, even if they don’t administer formal CPR, they’re going to be doing things” to try to help, Dr. Hoffman, who was not involved in this research but who specializes in child injury prevention, said in an interview. The act of intervening – whether it’s formal CPR or a CPR attempt or even just calling appropriate first responders – “likely impacts the duration of the submersion” and “clearly makes a difference.”
Drowning is the leading cause of death for children younger than 4 years, Dr. Hoffman noted, adding that the AAP recommends swimming lessons for children older than 1 year to reduce that risk.
In their cross-sectional study, Dr. Shenoi and his colleagues analyzed data on drownings and near-drownings in children and adolescents younger than 18 years using hospital, emergency medical services, and child fatality records from Harris County, Texas.
They analyzed 237 incidents from 2010 to 2013 in which the young person was submerged. Median age of the victims was 3.2 years, 60% were male, 64% were Black, Hispanic, or Native American, and 78% occurred in a swimming pool.
Unfavorable outcomes – defined as death or severe impairment after hospital discharge – were experienced by 38 victims (16%) and were significantly associated with being submerged for longer than 5 minutes (P < .001).
The odds of an unfavorable outcome dropped by 80% if a bystander attempted a rescue, whether or not they performed CPR (adjusted odds ratio, 0.2; P = .004). If the bystander performed CPR, the odds of an unfavorable outcome dropped by a similar amount, but the difference was not statistically significant (aOR, 0.22; P = .07).
However, previous research has shown a significant reduction in poor outcomes when CPR is administered to children who have been submerged, Dr. Hoffman explained.
The most important thing a bystander can do is simply get a submerged child out of the water. “Early rescue in drowning terminates what is initially a respiratory arrest from progressing to a full cardiopulmonary arrest with severe hypoxic brain injury and death,” Dr. Shenoi said.
“CPR is also very important, and rescue and resuscitation go hand in hand. We encourage all laypersons to be trained in CPR so that they can administer correct CPR techniques,” he added.
Both Dr. Shenoi and Dr. Hoffman emphasized the value of CPR training for adults, as the AAP recommends, and the importance of other precautions that reduce the risk of drowning.
“Drowning prevention should consist of multiple layers of prevention,” Dr. Shenoi said. These consist of “close, constant, and attentive supervision; isolation fencing for swimming pools; and water competency, including water-safety knowledge, basic swim skills, and the ability to recognize and respond to a swimmer in trouble, use of life jackets, and early bystander CPR.”
The relative importance of each of those layers depends on geography and circumstances, Dr. Hoffman said. Pools are the most common drowning sites in the United States overall, but they’re much more common in warmer states, such as California, Florida, and Texas, which have more pools. In contrast, drownings in Oregon are more likely to occur in rivers, so prevention is more about access to life jackets and increasing access to swim lessons.
The findings from this study drive home how important it is for physicians to provide anticipatory guidance to families on reducing the risk of drowning. Pediatricians should convey to families the need for different layers of protection, he added.
“If your family spends a lot of time around water, whether open water or swimming pools, the more layers you can provide, the better off you’re going to be,” Dr. Hoffman said.
Dr. Shenoi echoed this sentiment.
“The take-home message is to be observant if you are entrusted with the care of a child around water,” Dr. Shenoi said. “If you notice the child to be drowning, either attempt rescue yourself if it is safe to do so or enlist the help of others to save the victim as soon as possible. However, the rescuer should not place himself or herself in danger when attempting rescue.”
The five steps in the “drowning chain of survival” – preventing drowning, recognizing distress, providing flotation, removing the victim from the water, and providing care and CPR as needed – are key to reducing drowning deaths and injury, Dr. Shenoi emphasized.
Dr. Shenoi has disclosed no relevant financial relationships. Dr. Hoffman is a paid consultant on child drowning prevention for the nonprofit Anonymous Philanthropy.
A version of this article first appeared on Medscape.com.
The likelihood that a child will survive a near-drowning without long-term damage is substantially greater if a bystander attempts a rescue, even if that person doesn’t perform cardiopulmonary resuscitation (CPR), according to new research presented October 10 at the American Academy of Pediatrics (AAP) 2021 National Conference.
“The extent to which bystander rescue is associated with reduced odds of unfavorable drowning outcomes was surprising,” said lead investigator Rohit P. Shenoi, MD, professor of pediatrics at Baylor College of Medicine and attending physician at Texas Children’s Hospital, Houston.
“While we do know that early rescue and resuscitation is helpful in preventing severe drowning injury, the degree of benefit from bystander rescue in all cases of pediatric drowning has not been described so far,” he told this news organization.
The fact that a bystander’s rescue attempt improves a child’s odds of a good outcome is not surprising on its own, but the magnitude of the finding really affirms the importance of bystander intervention, said Benjamin Hoffman, MD, professor of pediatrics at the Oregon Health & Science University School of Medicine and medical director of the Tom Sargent Safety Center at the Doernbecher Children’s Hospital, Portland.
“If an adult finds a child in the water, even if they don’t administer formal CPR, they’re going to be doing things” to try to help, Dr. Hoffman, who was not involved in this research but who specializes in child injury prevention, said in an interview. The act of intervening – whether it’s formal CPR or a CPR attempt or even just calling appropriate first responders – “likely impacts the duration of the submersion” and “clearly makes a difference.”
Drowning is the leading cause of death for children younger than 4 years, Dr. Hoffman noted, adding that the AAP recommends swimming lessons for children older than 1 year to reduce that risk.
In their cross-sectional study, Dr. Shenoi and his colleagues analyzed data on drownings and near-drownings in children and adolescents younger than 18 years using hospital, emergency medical services, and child fatality records from Harris County, Texas.
They analyzed 237 incidents from 2010 to 2013 in which the young person was submerged. Median age of the victims was 3.2 years, 60% were male, 64% were Black, Hispanic, or Native American, and 78% occurred in a swimming pool.
Unfavorable outcomes – defined as death or severe impairment after hospital discharge – were experienced by 38 victims (16%) and were significantly associated with being submerged for longer than 5 minutes (P < .001).
The odds of an unfavorable outcome dropped by 80% if a bystander attempted a rescue, whether or not they performed CPR (adjusted odds ratio, 0.2; P = .004). If the bystander performed CPR, the odds of an unfavorable outcome dropped by a similar amount, but the difference was not statistically significant (aOR, 0.22; P = .07).
However, previous research has shown a significant reduction in poor outcomes when CPR is administered to children who have been submerged, Dr. Hoffman explained.
The most important thing a bystander can do is simply get a submerged child out of the water. “Early rescue in drowning terminates what is initially a respiratory arrest from progressing to a full cardiopulmonary arrest with severe hypoxic brain injury and death,” Dr. Shenoi said.
“CPR is also very important, and rescue and resuscitation go hand in hand. We encourage all laypersons to be trained in CPR so that they can administer correct CPR techniques,” he added.
Both Dr. Shenoi and Dr. Hoffman emphasized the value of CPR training for adults, as the AAP recommends, and the importance of other precautions that reduce the risk of drowning.
“Drowning prevention should consist of multiple layers of prevention,” Dr. Shenoi said. These consist of “close, constant, and attentive supervision; isolation fencing for swimming pools; and water competency, including water-safety knowledge, basic swim skills, and the ability to recognize and respond to a swimmer in trouble, use of life jackets, and early bystander CPR.”
The relative importance of each of those layers depends on geography and circumstances, Dr. Hoffman said. Pools are the most common drowning sites in the United States overall, but they’re much more common in warmer states, such as California, Florida, and Texas, which have more pools. In contrast, drownings in Oregon are more likely to occur in rivers, so prevention is more about access to life jackets and increasing access to swim lessons.
The findings from this study drive home how important it is for physicians to provide anticipatory guidance to families on reducing the risk of drowning. Pediatricians should convey to families the need for different layers of protection, he added.
“If your family spends a lot of time around water, whether open water or swimming pools, the more layers you can provide, the better off you’re going to be,” Dr. Hoffman said.
Dr. Shenoi echoed this sentiment.
“The take-home message is to be observant if you are entrusted with the care of a child around water,” Dr. Shenoi said. “If you notice the child to be drowning, either attempt rescue yourself if it is safe to do so or enlist the help of others to save the victim as soon as possible. However, the rescuer should not place himself or herself in danger when attempting rescue.”
The five steps in the “drowning chain of survival” – preventing drowning, recognizing distress, providing flotation, removing the victim from the water, and providing care and CPR as needed – are key to reducing drowning deaths and injury, Dr. Shenoi emphasized.
Dr. Shenoi has disclosed no relevant financial relationships. Dr. Hoffman is a paid consultant on child drowning prevention for the nonprofit Anonymous Philanthropy.
A version of this article first appeared on Medscape.com.
The likelihood that a child will survive a near-drowning without long-term damage is substantially greater if a bystander attempts a rescue, even if that person doesn’t perform cardiopulmonary resuscitation (CPR), according to new research presented October 10 at the American Academy of Pediatrics (AAP) 2021 National Conference.
“The extent to which bystander rescue is associated with reduced odds of unfavorable drowning outcomes was surprising,” said lead investigator Rohit P. Shenoi, MD, professor of pediatrics at Baylor College of Medicine and attending physician at Texas Children’s Hospital, Houston.
“While we do know that early rescue and resuscitation is helpful in preventing severe drowning injury, the degree of benefit from bystander rescue in all cases of pediatric drowning has not been described so far,” he told this news organization.
The fact that a bystander’s rescue attempt improves a child’s odds of a good outcome is not surprising on its own, but the magnitude of the finding really affirms the importance of bystander intervention, said Benjamin Hoffman, MD, professor of pediatrics at the Oregon Health & Science University School of Medicine and medical director of the Tom Sargent Safety Center at the Doernbecher Children’s Hospital, Portland.
“If an adult finds a child in the water, even if they don’t administer formal CPR, they’re going to be doing things” to try to help, Dr. Hoffman, who was not involved in this research but who specializes in child injury prevention, said in an interview. The act of intervening – whether it’s formal CPR or a CPR attempt or even just calling appropriate first responders – “likely impacts the duration of the submersion” and “clearly makes a difference.”
Drowning is the leading cause of death for children younger than 4 years, Dr. Hoffman noted, adding that the AAP recommends swimming lessons for children older than 1 year to reduce that risk.
In their cross-sectional study, Dr. Shenoi and his colleagues analyzed data on drownings and near-drownings in children and adolescents younger than 18 years using hospital, emergency medical services, and child fatality records from Harris County, Texas.
They analyzed 237 incidents from 2010 to 2013 in which the young person was submerged. Median age of the victims was 3.2 years, 60% were male, 64% were Black, Hispanic, or Native American, and 78% occurred in a swimming pool.
Unfavorable outcomes – defined as death or severe impairment after hospital discharge – were experienced by 38 victims (16%) and were significantly associated with being submerged for longer than 5 minutes (P < .001).
The odds of an unfavorable outcome dropped by 80% if a bystander attempted a rescue, whether or not they performed CPR (adjusted odds ratio, 0.2; P = .004). If the bystander performed CPR, the odds of an unfavorable outcome dropped by a similar amount, but the difference was not statistically significant (aOR, 0.22; P = .07).
However, previous research has shown a significant reduction in poor outcomes when CPR is administered to children who have been submerged, Dr. Hoffman explained.
The most important thing a bystander can do is simply get a submerged child out of the water. “Early rescue in drowning terminates what is initially a respiratory arrest from progressing to a full cardiopulmonary arrest with severe hypoxic brain injury and death,” Dr. Shenoi said.
“CPR is also very important, and rescue and resuscitation go hand in hand. We encourage all laypersons to be trained in CPR so that they can administer correct CPR techniques,” he added.
Both Dr. Shenoi and Dr. Hoffman emphasized the value of CPR training for adults, as the AAP recommends, and the importance of other precautions that reduce the risk of drowning.
“Drowning prevention should consist of multiple layers of prevention,” Dr. Shenoi said. These consist of “close, constant, and attentive supervision; isolation fencing for swimming pools; and water competency, including water-safety knowledge, basic swim skills, and the ability to recognize and respond to a swimmer in trouble, use of life jackets, and early bystander CPR.”
The relative importance of each of those layers depends on geography and circumstances, Dr. Hoffman said. Pools are the most common drowning sites in the United States overall, but they’re much more common in warmer states, such as California, Florida, and Texas, which have more pools. In contrast, drownings in Oregon are more likely to occur in rivers, so prevention is more about access to life jackets and increasing access to swim lessons.
The findings from this study drive home how important it is for physicians to provide anticipatory guidance to families on reducing the risk of drowning. Pediatricians should convey to families the need for different layers of protection, he added.
“If your family spends a lot of time around water, whether open water or swimming pools, the more layers you can provide, the better off you’re going to be,” Dr. Hoffman said.
Dr. Shenoi echoed this sentiment.
“The take-home message is to be observant if you are entrusted with the care of a child around water,” Dr. Shenoi said. “If you notice the child to be drowning, either attempt rescue yourself if it is safe to do so or enlist the help of others to save the victim as soon as possible. However, the rescuer should not place himself or herself in danger when attempting rescue.”
The five steps in the “drowning chain of survival” – preventing drowning, recognizing distress, providing flotation, removing the victim from the water, and providing care and CPR as needed – are key to reducing drowning deaths and injury, Dr. Shenoi emphasized.
Dr. Shenoi has disclosed no relevant financial relationships. Dr. Hoffman is a paid consultant on child drowning prevention for the nonprofit Anonymous Philanthropy.
A version of this article first appeared on Medscape.com.
Effect of COVID-19 pandemic on respiratory infectious diseases in primary care practice
A secondary consequence of public health measures to prevent the spread of SARS-CoV-2 included a concurrent reduction in risk for children to acquire and spread other respiratory viral infectious diseases. In the Rochester, N.Y., area, we had an ongoing prospective study in primary care pediatric practices that afforded an opportunity to assess the effect of the pandemic control measures on all infectious disease illness visits in young children. Specifically, in children aged 6-36 months old, our study was in place when the pandemic began with a primary objective to evaluate the changing epidemiology of acute otitis media (AOM) and nasopharyngeal colonization by potential bacterial respiratory pathogens in community-based primary care pediatric practices. As the public health measures mandated by New York State Department of Health were implemented, we prospectively quantified their effect on physician-diagnosed infectious disease illness visits. The incidence of infectious disease visits by a cohort of young children during the COVID-19 pandemic period March 15, 2020, through Dec. 31, 2020, was compared with the same time frame in the preceding year, 2019.1
Recommendations of the New York State Department of Health for public health, changes in school and day care attendance, and clinical practice during the study time frame
On March 7, 2020, a state of emergency was declared in New York because of the COVID-19 pandemic. All schools were required to close. A mandated order for public use of masks in adults and children more than 2 years of age was enacted. In the Finger Lakes region of Upstate New York, where the two primary care pediatric practices reside, complete lockdown was partially lifted on May 15, 2020, and further lifted on June 26, 2020. Almost all regional school districts opened to at least hybrid learning models for all students starting Sept. 8, 2020. On March 6, 2020, video telehealth and telephone call visits were introduced as routine practice. Well-child visits were limited to those less than 2 years of age, then gradually expanded to all ages by late May 2020. During the “stay at home” phase of the New York State lockdown, day care services were considered an essential business. Day care child density was limited. All children less than 2 years old were required to wear a mask while in the facility. Upon arrival, children with any respiratory symptoms or fever were excluded. For the school year commencing September 2020, almost all regional school districts opened to virtual, hybrid, or in-person learning models. Exclusion occurred similar to that of the day care facilities.
Incidence of respiratory infectious disease illnesses
Clinical diagnoses and healthy visits of 144 children from March 15 to Dec. 31, 2020 (beginning of the pandemic) were compared to 215 children during the same months in 2019 (prepandemic). Pediatric SARS-CoV-2 positivity rates trended up alongside community spread. Pediatric practice positivity rates rose from 1.9% in October 2020 to 19% in December 2020.
The table shows the incidence of significantly different infectious disease illness visits in the two study cohorts.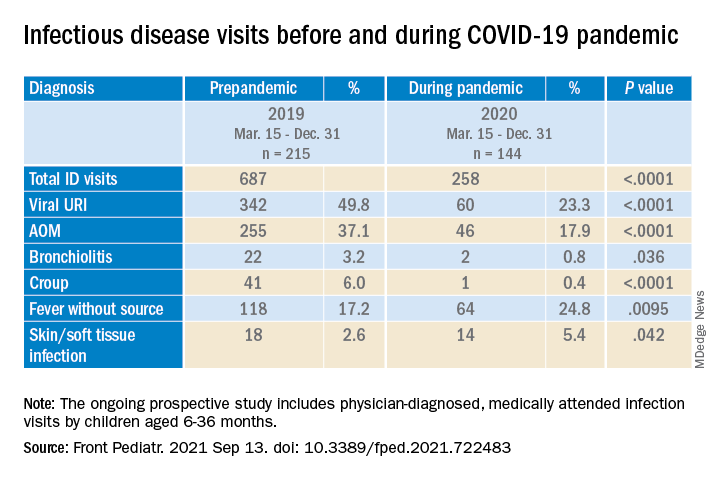
During the pandemic, 258 infection visits occurred among 144 pandemic cohort children, compared with 687 visits among 215 prepandemic cohort children, a 1.8-fold decrease (P < .0001). The proportion of children with visits for AOM (3.7-fold; P < .0001), bronchiolitis (7.4-fold; P = .036), croup (27.5-fold; P < .0001), and viral upper respiratory infection (3.8-fold; P < .0001) decreased significantly. Fever without a source (1.4-fold decrease; P = .009) and skin/soft tissue infection (2.1-fold decrease; P = .042) represented a higher proportion of visits during the pandemic.
Prescription of antibiotics significantly decreased (P < .001) during the pandemic.
Change in care practices
In the prepandemic period, virtual visits, leading to a diagnosis and treatment and referring children to an urgent care or hospital emergency department during regular office hours were rare. During the pandemic, this changed. Significantly increased use of telemedicine visits (P < .0001) and significantly decreased office and urgent care visits (P < .0001) occurred during the pandemic. Telehealth visits peaked the week of April 12, 2020, at 45% of all pediatric visits. In-person illness visits gradually returned to year-to-year volumes in August-September 2020 with school opening. Early in the pandemic, both pediatric offices limited patient encounters to well-child visits in the first 2 years of life to not miss opportunities for childhood vaccinations. However, some parents were reluctant to bring their children to those visits. There was no significant change in frequency of healthy child visits during the pandemic.
To our knowledge, this was the first study from primary care pediatric practices in the United States to analyze the effect on infectious diseases during the first 9 months of the pandemic, including the 6-month time period after the reopening from the first 3 months of lockdown. One prior study from a primary care network in Massachusetts reported significant decreases in respiratory infectious diseases for children aged 0-17 years during the first months of the pandemic during lockdown.2 A study in Tennessee that included hospital emergency department, urgent care, primary care, and retail health clinics also reported respiratory infection diagnoses as well as antibiotic prescription were reduced in the early months of the pandemic.3
Our study shows an overall reduction in frequency of respiratory illness visits in children 6-36 months old during the first 9 months of the COVID-19 pandemic. We learned the value of using technology in the form of virtual visits to render care. Perhaps as the pandemic subsides, many of the hand-washing and sanitizing practices will remain in place and lead to less frequent illness in children in the future. However, there may be temporary negative consequences from the “immune debt” that has occurred from a prolonged time span when children were not becoming infected with respiratory pathogens.4 We will see what unfolds in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz is pediatric medical director at Rochester (N.Y.) Regional Health. Dr. Pichichero and Dr. Schulz have no conflicts of interest to disclose. This study was funded in part by the Centers for Disease Control and Prevention.
References
1. Kaur R et al. Front Pediatr. 2021;(9)722483:1-8.
2. Hatoun J et al. Pediatrics. 2020;146(4):e2020006460.
3. Katz SE et al. J Pediatric Infect Dis Soc. 2021;10(1):62-4.
4. Cohen R et al. Infect. Dis Now. 2021; 51(5)418-23.
A secondary consequence of public health measures to prevent the spread of SARS-CoV-2 included a concurrent reduction in risk for children to acquire and spread other respiratory viral infectious diseases. In the Rochester, N.Y., area, we had an ongoing prospective study in primary care pediatric practices that afforded an opportunity to assess the effect of the pandemic control measures on all infectious disease illness visits in young children. Specifically, in children aged 6-36 months old, our study was in place when the pandemic began with a primary objective to evaluate the changing epidemiology of acute otitis media (AOM) and nasopharyngeal colonization by potential bacterial respiratory pathogens in community-based primary care pediatric practices. As the public health measures mandated by New York State Department of Health were implemented, we prospectively quantified their effect on physician-diagnosed infectious disease illness visits. The incidence of infectious disease visits by a cohort of young children during the COVID-19 pandemic period March 15, 2020, through Dec. 31, 2020, was compared with the same time frame in the preceding year, 2019.1
Recommendations of the New York State Department of Health for public health, changes in school and day care attendance, and clinical practice during the study time frame
On March 7, 2020, a state of emergency was declared in New York because of the COVID-19 pandemic. All schools were required to close. A mandated order for public use of masks in adults and children more than 2 years of age was enacted. In the Finger Lakes region of Upstate New York, where the two primary care pediatric practices reside, complete lockdown was partially lifted on May 15, 2020, and further lifted on June 26, 2020. Almost all regional school districts opened to at least hybrid learning models for all students starting Sept. 8, 2020. On March 6, 2020, video telehealth and telephone call visits were introduced as routine practice. Well-child visits were limited to those less than 2 years of age, then gradually expanded to all ages by late May 2020. During the “stay at home” phase of the New York State lockdown, day care services were considered an essential business. Day care child density was limited. All children less than 2 years old were required to wear a mask while in the facility. Upon arrival, children with any respiratory symptoms or fever were excluded. For the school year commencing September 2020, almost all regional school districts opened to virtual, hybrid, or in-person learning models. Exclusion occurred similar to that of the day care facilities.
Incidence of respiratory infectious disease illnesses
Clinical diagnoses and healthy visits of 144 children from March 15 to Dec. 31, 2020 (beginning of the pandemic) were compared to 215 children during the same months in 2019 (prepandemic). Pediatric SARS-CoV-2 positivity rates trended up alongside community spread. Pediatric practice positivity rates rose from 1.9% in October 2020 to 19% in December 2020.
The table shows the incidence of significantly different infectious disease illness visits in the two study cohorts.
During the pandemic, 258 infection visits occurred among 144 pandemic cohort children, compared with 687 visits among 215 prepandemic cohort children, a 1.8-fold decrease (P < .0001). The proportion of children with visits for AOM (3.7-fold; P < .0001), bronchiolitis (7.4-fold; P = .036), croup (27.5-fold; P < .0001), and viral upper respiratory infection (3.8-fold; P < .0001) decreased significantly. Fever without a source (1.4-fold decrease; P = .009) and skin/soft tissue infection (2.1-fold decrease; P = .042) represented a higher proportion of visits during the pandemic.
Prescription of antibiotics significantly decreased (P < .001) during the pandemic.
Change in care practices
In the prepandemic period, virtual visits, leading to a diagnosis and treatment and referring children to an urgent care or hospital emergency department during regular office hours were rare. During the pandemic, this changed. Significantly increased use of telemedicine visits (P < .0001) and significantly decreased office and urgent care visits (P < .0001) occurred during the pandemic. Telehealth visits peaked the week of April 12, 2020, at 45% of all pediatric visits. In-person illness visits gradually returned to year-to-year volumes in August-September 2020 with school opening. Early in the pandemic, both pediatric offices limited patient encounters to well-child visits in the first 2 years of life to not miss opportunities for childhood vaccinations. However, some parents were reluctant to bring their children to those visits. There was no significant change in frequency of healthy child visits during the pandemic.
To our knowledge, this was the first study from primary care pediatric practices in the United States to analyze the effect on infectious diseases during the first 9 months of the pandemic, including the 6-month time period after the reopening from the first 3 months of lockdown. One prior study from a primary care network in Massachusetts reported significant decreases in respiratory infectious diseases for children aged 0-17 years during the first months of the pandemic during lockdown.2 A study in Tennessee that included hospital emergency department, urgent care, primary care, and retail health clinics also reported respiratory infection diagnoses as well as antibiotic prescription were reduced in the early months of the pandemic.3
Our study shows an overall reduction in frequency of respiratory illness visits in children 6-36 months old during the first 9 months of the COVID-19 pandemic. We learned the value of using technology in the form of virtual visits to render care. Perhaps as the pandemic subsides, many of the hand-washing and sanitizing practices will remain in place and lead to less frequent illness in children in the future. However, there may be temporary negative consequences from the “immune debt” that has occurred from a prolonged time span when children were not becoming infected with respiratory pathogens.4 We will see what unfolds in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz is pediatric medical director at Rochester (N.Y.) Regional Health. Dr. Pichichero and Dr. Schulz have no conflicts of interest to disclose. This study was funded in part by the Centers for Disease Control and Prevention.
References
1. Kaur R et al. Front Pediatr. 2021;(9)722483:1-8.
2. Hatoun J et al. Pediatrics. 2020;146(4):e2020006460.
3. Katz SE et al. J Pediatric Infect Dis Soc. 2021;10(1):62-4.
4. Cohen R et al. Infect. Dis Now. 2021; 51(5)418-23.
A secondary consequence of public health measures to prevent the spread of SARS-CoV-2 included a concurrent reduction in risk for children to acquire and spread other respiratory viral infectious diseases. In the Rochester, N.Y., area, we had an ongoing prospective study in primary care pediatric practices that afforded an opportunity to assess the effect of the pandemic control measures on all infectious disease illness visits in young children. Specifically, in children aged 6-36 months old, our study was in place when the pandemic began with a primary objective to evaluate the changing epidemiology of acute otitis media (AOM) and nasopharyngeal colonization by potential bacterial respiratory pathogens in community-based primary care pediatric practices. As the public health measures mandated by New York State Department of Health were implemented, we prospectively quantified their effect on physician-diagnosed infectious disease illness visits. The incidence of infectious disease visits by a cohort of young children during the COVID-19 pandemic period March 15, 2020, through Dec. 31, 2020, was compared with the same time frame in the preceding year, 2019.1
Recommendations of the New York State Department of Health for public health, changes in school and day care attendance, and clinical practice during the study time frame
On March 7, 2020, a state of emergency was declared in New York because of the COVID-19 pandemic. All schools were required to close. A mandated order for public use of masks in adults and children more than 2 years of age was enacted. In the Finger Lakes region of Upstate New York, where the two primary care pediatric practices reside, complete lockdown was partially lifted on May 15, 2020, and further lifted on June 26, 2020. Almost all regional school districts opened to at least hybrid learning models for all students starting Sept. 8, 2020. On March 6, 2020, video telehealth and telephone call visits were introduced as routine practice. Well-child visits were limited to those less than 2 years of age, then gradually expanded to all ages by late May 2020. During the “stay at home” phase of the New York State lockdown, day care services were considered an essential business. Day care child density was limited. All children less than 2 years old were required to wear a mask while in the facility. Upon arrival, children with any respiratory symptoms or fever were excluded. For the school year commencing September 2020, almost all regional school districts opened to virtual, hybrid, or in-person learning models. Exclusion occurred similar to that of the day care facilities.
Incidence of respiratory infectious disease illnesses
Clinical diagnoses and healthy visits of 144 children from March 15 to Dec. 31, 2020 (beginning of the pandemic) were compared to 215 children during the same months in 2019 (prepandemic). Pediatric SARS-CoV-2 positivity rates trended up alongside community spread. Pediatric practice positivity rates rose from 1.9% in October 2020 to 19% in December 2020.
The table shows the incidence of significantly different infectious disease illness visits in the two study cohorts.
During the pandemic, 258 infection visits occurred among 144 pandemic cohort children, compared with 687 visits among 215 prepandemic cohort children, a 1.8-fold decrease (P < .0001). The proportion of children with visits for AOM (3.7-fold; P < .0001), bronchiolitis (7.4-fold; P = .036), croup (27.5-fold; P < .0001), and viral upper respiratory infection (3.8-fold; P < .0001) decreased significantly. Fever without a source (1.4-fold decrease; P = .009) and skin/soft tissue infection (2.1-fold decrease; P = .042) represented a higher proportion of visits during the pandemic.
Prescription of antibiotics significantly decreased (P < .001) during the pandemic.
Change in care practices
In the prepandemic period, virtual visits, leading to a diagnosis and treatment and referring children to an urgent care or hospital emergency department during regular office hours were rare. During the pandemic, this changed. Significantly increased use of telemedicine visits (P < .0001) and significantly decreased office and urgent care visits (P < .0001) occurred during the pandemic. Telehealth visits peaked the week of April 12, 2020, at 45% of all pediatric visits. In-person illness visits gradually returned to year-to-year volumes in August-September 2020 with school opening. Early in the pandemic, both pediatric offices limited patient encounters to well-child visits in the first 2 years of life to not miss opportunities for childhood vaccinations. However, some parents were reluctant to bring their children to those visits. There was no significant change in frequency of healthy child visits during the pandemic.
To our knowledge, this was the first study from primary care pediatric practices in the United States to analyze the effect on infectious diseases during the first 9 months of the pandemic, including the 6-month time period after the reopening from the first 3 months of lockdown. One prior study from a primary care network in Massachusetts reported significant decreases in respiratory infectious diseases for children aged 0-17 years during the first months of the pandemic during lockdown.2 A study in Tennessee that included hospital emergency department, urgent care, primary care, and retail health clinics also reported respiratory infection diagnoses as well as antibiotic prescription were reduced in the early months of the pandemic.3
Our study shows an overall reduction in frequency of respiratory illness visits in children 6-36 months old during the first 9 months of the COVID-19 pandemic. We learned the value of using technology in the form of virtual visits to render care. Perhaps as the pandemic subsides, many of the hand-washing and sanitizing practices will remain in place and lead to less frequent illness in children in the future. However, there may be temporary negative consequences from the “immune debt” that has occurred from a prolonged time span when children were not becoming infected with respiratory pathogens.4 We will see what unfolds in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz is pediatric medical director at Rochester (N.Y.) Regional Health. Dr. Pichichero and Dr. Schulz have no conflicts of interest to disclose. This study was funded in part by the Centers for Disease Control and Prevention.
References
1. Kaur R et al. Front Pediatr. 2021;(9)722483:1-8.
2. Hatoun J et al. Pediatrics. 2020;146(4):e2020006460.
3. Katz SE et al. J Pediatric Infect Dis Soc. 2021;10(1):62-4.
4. Cohen R et al. Infect. Dis Now. 2021; 51(5)418-23.
Lung transplantation for patients with severe COVID-19
As of September 2021, over 222 million people worldwide (WHO, 2021) and 40 million Americans (CDC, 2021) have been infected with the novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). The total number of infections in the United States began climbing again this summer with the persistence of vaccine reluctance among a significant proportion of the population and the emergence of the much more infectious B.1.617.2 (Delta) variant. While the clinical illness caused by the SARS-CoV-2 virus, referred to as the Coronavirus disease 2019 (COVID-19), is mostly mild, approximately 10% of cases develop acute respiratory distress syndrome (ARDS) (Remuzzi A, et al. Lancet. 2020;395[10231]:1225-8). A small but substantial proportion of patients with COVID-19 ARDS fails to respond to the various supportive measures and requires extracorporeal membrane oxygenation (ECMO) support. The overarching goal of the different support strategies, including ECMO, is to provide time for the lungs to recover from ARDS. ECMO has the theoretical advantage over other strategies in facilitating recovery by allowing the injured lungs to ‘rest’ as the oxygenation and ventilation needs are met in an extracorporeal fashion. Regardless, a small number of patients with COVID-19 ARDS will not recover enough pulmonary function to allow them to be weaned from the various respiratory support strategies.
For patients with irreversible lung injury, lung transplantation (LT) is a potential consideration. Earlier in the pandemic, older patients with significant comorbid illnesses were more vulnerable to severe COVID-19, often precluding consideration for transplantation. However, the emergence of the Delta variant may have altered this dynamic via a substantial increase in the incidence of COVID-19 ARDS among younger and healthier patients. A handful of patients with COVID-19 ARDS have already had successful transplantation. However, the overall number is still small (Bharat A, et al. Sci Translat Med. 2020 Dec 16;12[574]:eabe4282. doi: 10.1126/scitranslmed.abe4282. Epub 2020 Nov 30; and Hawkins R, et al. Transplantation. 2021;6:1381-7), and there is a lack of long-term outcomes data among these patients.
There is currently little guidance regarding criteria for patient selection and consideration for LT among patients with COVID-19 ARDS. Given that the SARS-CoV-2 virus is a novel pathogen that leads to an illness that is unique from other forms of viral pneumonia, specific considerations regarding LT should be made among these patients. In the current article, we discuss some of the pertinent issues related to the consideration of LT among patients with COVID-19 ARDS.
The timing for considering LT is one of the most important aspects. First, patients with COVID-19 ARDS must not be actively infected at the time of transplantation consideration. It has been suggested that LT should only be considered in patients with two separate negative polymerase chain reaction (PCR) test results for SARS-CoV-2 from bronchoalveolar lavage fluid 24 hours apart and at least 4 weeks after the onset of COVID-19 symptoms (Bharat A, et al. Sci Translat Med. 2020 Dec 16;12[574]:eabe4282. doi: 10.1126/scitranslmed.abe4282. Epub 2020 Nov 30). Among patients with persistently positive SARS-CoV-2 PCR 4 to 6 weeks after symptom onset, a negative viral culture from a bronchoalveolar lavage (BAL) can be used to confirm viral inactivity (Lang C, et al. Lancet Respir Med. 2020;8[10]:1057-60).
Despite the sparse data in this domain, there seems to be a consensus in the literature that LT could be considered once 4 to 6 weeks have elapsed since the onset of the respiratory failure (Cypel M, et al. Lancet Respir Med. 2020;8[10]:944-6). This timeline is felt to be long enough to alleviate the concerns regarding ongoing inflammatory processes that may be reversible while not so long to risk the development of non-pulmonary complications or severe debility that may become significant barriers to transplant candidacy. An exception may be made in patients with medically unmanageable complications such as recalcitrant bronchopleural fistulae in the background of fibrotic changes or right ventricular failure from severe pulmonary hypertension. Regardless, this timeline is borrowed from the approach to irreversible ARDS from other forms of viral pneumonia. It is not clear if it is appropriate to extrapolate past experience to COVID-19, which is a disease unlike any other seen during the LT era: a profound inflammatory phase mediated by a cytokine storm as the etiologic basis for the organ dysfunction, activation of coagulation pathways in pulmonary circulation leading to immunothrombosis contributing to the refractory hypoxemia, favorable effects of anticoagulants, diverse pulmonary physiologic phenotypes of ARDS, an increased risk of pleural complications, and utilization of novel anti-inflammatory therapies with consequent risks ofsecondary infections are all unique to COVID-19. A recent study found that patients requiring ECMO for COVID-19 ARDS took longer to recover lung function but had similar survival rates to patients on ECMO with other virus-induced ARDS (Raff LA, et al. Am J Surg. 2021;S0002-9610[21]00233-6. doi: 10.1016/j.amjsurg.2021.04.004. Online ahead of print).These data support pursuing a more conservative timeline for consideration of LT.
Determining the reversibility of pulmonary impairment in COVID-19 ARDS is another challenge. The nature of the pulmonary opacities should be assessed on CT scan imaging as close as possible to the time of LT consideration. Differentiating the extent of irreversible parenchymal scarring vs salvageability during acute illness can be challenging. The presence of extensive architectural distortion with or without bullous changes, while being the best indicator of irreversibility, may not be sensitive enough. The standard of care in such situations remains serial assessments, often weekly, by a dedicated multidisciplinary group. We have found it useful to augment the imaging data with pulmonary physiologic assessments, including the extent of ventilator and ECMO support as well as dynamic and static compliance trends. Improvement in physiologic data often precedes radiologic improvement. Nonetheless, an important area of future research is to identify objective markers for determining reversibility, which could include novel biomarkers in serum or bronchoalveolar lavage fluid.
When a determination is made regarding the irreversibility of pulmonary impairment, the LT evaluation should begin promptly. Pre-transplant deconditioning and debility is associated with worse post-transplant outcomes. In this regard, patients managed using an ambulatory ECMO strategy may have superior rehabilitation potential. Furthermore, an attempt should be made during the evaluation to wean sedation in order to facilitate discussions regarding the rigors of LT with the patient alongside present family members. An additional consideration, given the use of immunomodulatory medications for COVID-19 and prolonged intubation, is the dramatically increased risk of multi-drug resistant infections in this population; these must be aggressively managed for patients to remain eligible for LT.
The degree of pulmonary impairment and frequent colonization of the airways will likely dictate bilateral LT as the preferred strategy, although surgical feasibility may, at times, be the overriding determinant. Regardless of the type of transplant, certain unique aspects should be anticipated. The inflammatory responses during COVID-19 that often spill outside the confines of the pulmonary parenchyma, along with potentially frequent thoracic interventions prior to transplant, create significant technical challenges during the operation. Native pneumonectomy can take longer than usual leading to prolonged ischemic time, increased need for intra-operative blood products, and raised risk for primary graft dysfunction. All of these factors have a significant impact on early and late outcomes. Finally, the long-term immunologic consequences of severe infection from a novel virus remain unknown, and it is unclear if COVID-19 ARDS patients bridged to transplant will enjoy comparable survival. It is pertinent to acknowledge that the high-risk nature of such transplants is substantially accentuated due to several unique characteristics of the illness related to COVID-19.
The emergence of the COVID-19 pandemic has led to an increase in the number of urgent inpatient lung transplant consultations for refractory ARDS. While the basic principles of LT candidate selection should continue to guide us, the unique characteristics of this illness merit using a customized approach. There are few validated predictors to guide decision-making, and longitudinal assessments by a dedicated multidisciplinary group remain the best strategy. Finally, in the absence of systemic studies and lack of longitudinal outcomes data, there is an emergent need to establish consensus guidelines regarding the approach to LT consideration in these patients.
Dr. Quinn and Dr. Banga are with the Lung Transplant Program, Divisions of Pulmonary and Critical Care Medicine, University of Texas Southwestern Medical Center, Dallas.
As of September 2021, over 222 million people worldwide (WHO, 2021) and 40 million Americans (CDC, 2021) have been infected with the novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). The total number of infections in the United States began climbing again this summer with the persistence of vaccine reluctance among a significant proportion of the population and the emergence of the much more infectious B.1.617.2 (Delta) variant. While the clinical illness caused by the SARS-CoV-2 virus, referred to as the Coronavirus disease 2019 (COVID-19), is mostly mild, approximately 10% of cases develop acute respiratory distress syndrome (ARDS) (Remuzzi A, et al. Lancet. 2020;395[10231]:1225-8). A small but substantial proportion of patients with COVID-19 ARDS fails to respond to the various supportive measures and requires extracorporeal membrane oxygenation (ECMO) support. The overarching goal of the different support strategies, including ECMO, is to provide time for the lungs to recover from ARDS. ECMO has the theoretical advantage over other strategies in facilitating recovery by allowing the injured lungs to ‘rest’ as the oxygenation and ventilation needs are met in an extracorporeal fashion. Regardless, a small number of patients with COVID-19 ARDS will not recover enough pulmonary function to allow them to be weaned from the various respiratory support strategies.
For patients with irreversible lung injury, lung transplantation (LT) is a potential consideration. Earlier in the pandemic, older patients with significant comorbid illnesses were more vulnerable to severe COVID-19, often precluding consideration for transplantation. However, the emergence of the Delta variant may have altered this dynamic via a substantial increase in the incidence of COVID-19 ARDS among younger and healthier patients. A handful of patients with COVID-19 ARDS have already had successful transplantation. However, the overall number is still small (Bharat A, et al. Sci Translat Med. 2020 Dec 16;12[574]:eabe4282. doi: 10.1126/scitranslmed.abe4282. Epub 2020 Nov 30; and Hawkins R, et al. Transplantation. 2021;6:1381-7), and there is a lack of long-term outcomes data among these patients.
There is currently little guidance regarding criteria for patient selection and consideration for LT among patients with COVID-19 ARDS. Given that the SARS-CoV-2 virus is a novel pathogen that leads to an illness that is unique from other forms of viral pneumonia, specific considerations regarding LT should be made among these patients. In the current article, we discuss some of the pertinent issues related to the consideration of LT among patients with COVID-19 ARDS.
The timing for considering LT is one of the most important aspects. First, patients with COVID-19 ARDS must not be actively infected at the time of transplantation consideration. It has been suggested that LT should only be considered in patients with two separate negative polymerase chain reaction (PCR) test results for SARS-CoV-2 from bronchoalveolar lavage fluid 24 hours apart and at least 4 weeks after the onset of COVID-19 symptoms (Bharat A, et al. Sci Translat Med. 2020 Dec 16;12[574]:eabe4282. doi: 10.1126/scitranslmed.abe4282. Epub 2020 Nov 30). Among patients with persistently positive SARS-CoV-2 PCR 4 to 6 weeks after symptom onset, a negative viral culture from a bronchoalveolar lavage (BAL) can be used to confirm viral inactivity (Lang C, et al. Lancet Respir Med. 2020;8[10]:1057-60).
Despite the sparse data in this domain, there seems to be a consensus in the literature that LT could be considered once 4 to 6 weeks have elapsed since the onset of the respiratory failure (Cypel M, et al. Lancet Respir Med. 2020;8[10]:944-6). This timeline is felt to be long enough to alleviate the concerns regarding ongoing inflammatory processes that may be reversible while not so long to risk the development of non-pulmonary complications or severe debility that may become significant barriers to transplant candidacy. An exception may be made in patients with medically unmanageable complications such as recalcitrant bronchopleural fistulae in the background of fibrotic changes or right ventricular failure from severe pulmonary hypertension. Regardless, this timeline is borrowed from the approach to irreversible ARDS from other forms of viral pneumonia. It is not clear if it is appropriate to extrapolate past experience to COVID-19, which is a disease unlike any other seen during the LT era: a profound inflammatory phase mediated by a cytokine storm as the etiologic basis for the organ dysfunction, activation of coagulation pathways in pulmonary circulation leading to immunothrombosis contributing to the refractory hypoxemia, favorable effects of anticoagulants, diverse pulmonary physiologic phenotypes of ARDS, an increased risk of pleural complications, and utilization of novel anti-inflammatory therapies with consequent risks ofsecondary infections are all unique to COVID-19. A recent study found that patients requiring ECMO for COVID-19 ARDS took longer to recover lung function but had similar survival rates to patients on ECMO with other virus-induced ARDS (Raff LA, et al. Am J Surg. 2021;S0002-9610[21]00233-6. doi: 10.1016/j.amjsurg.2021.04.004. Online ahead of print).These data support pursuing a more conservative timeline for consideration of LT.
Determining the reversibility of pulmonary impairment in COVID-19 ARDS is another challenge. The nature of the pulmonary opacities should be assessed on CT scan imaging as close as possible to the time of LT consideration. Differentiating the extent of irreversible parenchymal scarring vs salvageability during acute illness can be challenging. The presence of extensive architectural distortion with or without bullous changes, while being the best indicator of irreversibility, may not be sensitive enough. The standard of care in such situations remains serial assessments, often weekly, by a dedicated multidisciplinary group. We have found it useful to augment the imaging data with pulmonary physiologic assessments, including the extent of ventilator and ECMO support as well as dynamic and static compliance trends. Improvement in physiologic data often precedes radiologic improvement. Nonetheless, an important area of future research is to identify objective markers for determining reversibility, which could include novel biomarkers in serum or bronchoalveolar lavage fluid.
When a determination is made regarding the irreversibility of pulmonary impairment, the LT evaluation should begin promptly. Pre-transplant deconditioning and debility is associated with worse post-transplant outcomes. In this regard, patients managed using an ambulatory ECMO strategy may have superior rehabilitation potential. Furthermore, an attempt should be made during the evaluation to wean sedation in order to facilitate discussions regarding the rigors of LT with the patient alongside present family members. An additional consideration, given the use of immunomodulatory medications for COVID-19 and prolonged intubation, is the dramatically increased risk of multi-drug resistant infections in this population; these must be aggressively managed for patients to remain eligible for LT.
The degree of pulmonary impairment and frequent colonization of the airways will likely dictate bilateral LT as the preferred strategy, although surgical feasibility may, at times, be the overriding determinant. Regardless of the type of transplant, certain unique aspects should be anticipated. The inflammatory responses during COVID-19 that often spill outside the confines of the pulmonary parenchyma, along with potentially frequent thoracic interventions prior to transplant, create significant technical challenges during the operation. Native pneumonectomy can take longer than usual leading to prolonged ischemic time, increased need for intra-operative blood products, and raised risk for primary graft dysfunction. All of these factors have a significant impact on early and late outcomes. Finally, the long-term immunologic consequences of severe infection from a novel virus remain unknown, and it is unclear if COVID-19 ARDS patients bridged to transplant will enjoy comparable survival. It is pertinent to acknowledge that the high-risk nature of such transplants is substantially accentuated due to several unique characteristics of the illness related to COVID-19.
The emergence of the COVID-19 pandemic has led to an increase in the number of urgent inpatient lung transplant consultations for refractory ARDS. While the basic principles of LT candidate selection should continue to guide us, the unique characteristics of this illness merit using a customized approach. There are few validated predictors to guide decision-making, and longitudinal assessments by a dedicated multidisciplinary group remain the best strategy. Finally, in the absence of systemic studies and lack of longitudinal outcomes data, there is an emergent need to establish consensus guidelines regarding the approach to LT consideration in these patients.
Dr. Quinn and Dr. Banga are with the Lung Transplant Program, Divisions of Pulmonary and Critical Care Medicine, University of Texas Southwestern Medical Center, Dallas.
As of September 2021, over 222 million people worldwide (WHO, 2021) and 40 million Americans (CDC, 2021) have been infected with the novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). The total number of infections in the United States began climbing again this summer with the persistence of vaccine reluctance among a significant proportion of the population and the emergence of the much more infectious B.1.617.2 (Delta) variant. While the clinical illness caused by the SARS-CoV-2 virus, referred to as the Coronavirus disease 2019 (COVID-19), is mostly mild, approximately 10% of cases develop acute respiratory distress syndrome (ARDS) (Remuzzi A, et al. Lancet. 2020;395[10231]:1225-8). A small but substantial proportion of patients with COVID-19 ARDS fails to respond to the various supportive measures and requires extracorporeal membrane oxygenation (ECMO) support. The overarching goal of the different support strategies, including ECMO, is to provide time for the lungs to recover from ARDS. ECMO has the theoretical advantage over other strategies in facilitating recovery by allowing the injured lungs to ‘rest’ as the oxygenation and ventilation needs are met in an extracorporeal fashion. Regardless, a small number of patients with COVID-19 ARDS will not recover enough pulmonary function to allow them to be weaned from the various respiratory support strategies.
For patients with irreversible lung injury, lung transplantation (LT) is a potential consideration. Earlier in the pandemic, older patients with significant comorbid illnesses were more vulnerable to severe COVID-19, often precluding consideration for transplantation. However, the emergence of the Delta variant may have altered this dynamic via a substantial increase in the incidence of COVID-19 ARDS among younger and healthier patients. A handful of patients with COVID-19 ARDS have already had successful transplantation. However, the overall number is still small (Bharat A, et al. Sci Translat Med. 2020 Dec 16;12[574]:eabe4282. doi: 10.1126/scitranslmed.abe4282. Epub 2020 Nov 30; and Hawkins R, et al. Transplantation. 2021;6:1381-7), and there is a lack of long-term outcomes data among these patients.
There is currently little guidance regarding criteria for patient selection and consideration for LT among patients with COVID-19 ARDS. Given that the SARS-CoV-2 virus is a novel pathogen that leads to an illness that is unique from other forms of viral pneumonia, specific considerations regarding LT should be made among these patients. In the current article, we discuss some of the pertinent issues related to the consideration of LT among patients with COVID-19 ARDS.
The timing for considering LT is one of the most important aspects. First, patients with COVID-19 ARDS must not be actively infected at the time of transplantation consideration. It has been suggested that LT should only be considered in patients with two separate negative polymerase chain reaction (PCR) test results for SARS-CoV-2 from bronchoalveolar lavage fluid 24 hours apart and at least 4 weeks after the onset of COVID-19 symptoms (Bharat A, et al. Sci Translat Med. 2020 Dec 16;12[574]:eabe4282. doi: 10.1126/scitranslmed.abe4282. Epub 2020 Nov 30). Among patients with persistently positive SARS-CoV-2 PCR 4 to 6 weeks after symptom onset, a negative viral culture from a bronchoalveolar lavage (BAL) can be used to confirm viral inactivity (Lang C, et al. Lancet Respir Med. 2020;8[10]:1057-60).
Despite the sparse data in this domain, there seems to be a consensus in the literature that LT could be considered once 4 to 6 weeks have elapsed since the onset of the respiratory failure (Cypel M, et al. Lancet Respir Med. 2020;8[10]:944-6). This timeline is felt to be long enough to alleviate the concerns regarding ongoing inflammatory processes that may be reversible while not so long to risk the development of non-pulmonary complications or severe debility that may become significant barriers to transplant candidacy. An exception may be made in patients with medically unmanageable complications such as recalcitrant bronchopleural fistulae in the background of fibrotic changes or right ventricular failure from severe pulmonary hypertension. Regardless, this timeline is borrowed from the approach to irreversible ARDS from other forms of viral pneumonia. It is not clear if it is appropriate to extrapolate past experience to COVID-19, which is a disease unlike any other seen during the LT era: a profound inflammatory phase mediated by a cytokine storm as the etiologic basis for the organ dysfunction, activation of coagulation pathways in pulmonary circulation leading to immunothrombosis contributing to the refractory hypoxemia, favorable effects of anticoagulants, diverse pulmonary physiologic phenotypes of ARDS, an increased risk of pleural complications, and utilization of novel anti-inflammatory therapies with consequent risks ofsecondary infections are all unique to COVID-19. A recent study found that patients requiring ECMO for COVID-19 ARDS took longer to recover lung function but had similar survival rates to patients on ECMO with other virus-induced ARDS (Raff LA, et al. Am J Surg. 2021;S0002-9610[21]00233-6. doi: 10.1016/j.amjsurg.2021.04.004. Online ahead of print).These data support pursuing a more conservative timeline for consideration of LT.
Determining the reversibility of pulmonary impairment in COVID-19 ARDS is another challenge. The nature of the pulmonary opacities should be assessed on CT scan imaging as close as possible to the time of LT consideration. Differentiating the extent of irreversible parenchymal scarring vs salvageability during acute illness can be challenging. The presence of extensive architectural distortion with or without bullous changes, while being the best indicator of irreversibility, may not be sensitive enough. The standard of care in such situations remains serial assessments, often weekly, by a dedicated multidisciplinary group. We have found it useful to augment the imaging data with pulmonary physiologic assessments, including the extent of ventilator and ECMO support as well as dynamic and static compliance trends. Improvement in physiologic data often precedes radiologic improvement. Nonetheless, an important area of future research is to identify objective markers for determining reversibility, which could include novel biomarkers in serum or bronchoalveolar lavage fluid.
When a determination is made regarding the irreversibility of pulmonary impairment, the LT evaluation should begin promptly. Pre-transplant deconditioning and debility is associated with worse post-transplant outcomes. In this regard, patients managed using an ambulatory ECMO strategy may have superior rehabilitation potential. Furthermore, an attempt should be made during the evaluation to wean sedation in order to facilitate discussions regarding the rigors of LT with the patient alongside present family members. An additional consideration, given the use of immunomodulatory medications for COVID-19 and prolonged intubation, is the dramatically increased risk of multi-drug resistant infections in this population; these must be aggressively managed for patients to remain eligible for LT.
The degree of pulmonary impairment and frequent colonization of the airways will likely dictate bilateral LT as the preferred strategy, although surgical feasibility may, at times, be the overriding determinant. Regardless of the type of transplant, certain unique aspects should be anticipated. The inflammatory responses during COVID-19 that often spill outside the confines of the pulmonary parenchyma, along with potentially frequent thoracic interventions prior to transplant, create significant technical challenges during the operation. Native pneumonectomy can take longer than usual leading to prolonged ischemic time, increased need for intra-operative blood products, and raised risk for primary graft dysfunction. All of these factors have a significant impact on early and late outcomes. Finally, the long-term immunologic consequences of severe infection from a novel virus remain unknown, and it is unclear if COVID-19 ARDS patients bridged to transplant will enjoy comparable survival. It is pertinent to acknowledge that the high-risk nature of such transplants is substantially accentuated due to several unique characteristics of the illness related to COVID-19.
The emergence of the COVID-19 pandemic has led to an increase in the number of urgent inpatient lung transplant consultations for refractory ARDS. While the basic principles of LT candidate selection should continue to guide us, the unique characteristics of this illness merit using a customized approach. There are few validated predictors to guide decision-making, and longitudinal assessments by a dedicated multidisciplinary group remain the best strategy. Finally, in the absence of systemic studies and lack of longitudinal outcomes data, there is an emergent need to establish consensus guidelines regarding the approach to LT consideration in these patients.
Dr. Quinn and Dr. Banga are with the Lung Transplant Program, Divisions of Pulmonary and Critical Care Medicine, University of Texas Southwestern Medical Center, Dallas.
Cavitary Lung Lesion in a Tuberculosis-Negative Patient
A patient with worsening chronic cough, shortness of breath, and hemoptysis tested negative for tuberculosis; but a chest computed tomography scan showed an upper left lobe cavitary lesion.
A 71-year-old, currently homeless male veteran with a 29 pack-year history of smoking and history of alcohol abuse presented to the emergency department at Washington DC Veterans Affairs Medical Center with worsening chronic cough and shortness of breath. He had no history of HIV or immunosuppressant medications. Four weeks prior, he was treated at an outpatient urgent care for community acquired pneumonia with a 10-day course of oral amoxicillin/clavulanic acid 875 mg twice daily and azithromycin 500 mg day 1, then 250 mg days 2 through 5. Despite antibiotic therapy, his symptoms continued to worsen, and he developed hemoptysis. He also reported weight loss of 20 lb in the past 3 months despite a strong appetite and adequate oral intake. He reported no fevers and night sweats. A review of the patient’s systems was otherwise unremarkable.
On examination, the patient was afebrile at 37.2 °C but tachycardic at 108 beats/min. He also was tachypneic at 22 breaths/min with an oxygen saturation of 89% on room air. Decreased breath sounds in the left upper lobe were noted on auscultation of the lung fields. Laboratory test results were notable for a leukocytosis of 14.3 k/μL (reference range, 4-11k/μL) and an elevated erythrocyte sedimentation rate (ESR) of 25.08 mm/h (reference range, 0-16 mm/h) and C-reactive protein (CRP) of 4.75 mg/L (reference range, 0.00-3.00 mg/L). Liver-associated enzymes and a coagulation panel were within normal limits. His QuantiFERON-TB Gold tuberculosis (TB) blood test was negative. A computed tomography (CT) scan of the chest was obtained, which showed an interval increase of a known upper left lobe cavitary lesion compared with that of prior imaging and the presence of a ball-shaped lesion in the cavity (Figures 1 and 2).
In addition to the imaging, the patient underwent bronchoscopy with bronchoalveolar lavage (BAL) to further evaluate the upper left lobe cavitary lesion. The differential diagnosis for pulmonary cavities is described in the Table. The BAL aspirates were negative for acid-fast bacteria; however, periodic acid–Schiff stain and Grocott methenamine silver stain showed fungal elements. He was diagnosed with chronic cavitary pulmonary aspergillosis (CCPA), confirmed with serum antigen (galactomannan assay) and serum immunoglobulin G (IgG) positive for Aspergillus fumigatus (A fumigatus). Mycologic cultures were positive for A fumigatus.
Discussion
Aspergillomas are accumulations of Aspergillus spp hyphae, fibrin, and other inflammatory components that typically occur in preexisting pulmonary cavities.1 They are most frequently caused by A fumigatus, which is ubiquitous in the environment and acquired via inhalation of airborne spores in 90% of cases.2 The typical ball-shaped appearance forms when hyphae growing along the inside walls of the cavity ultimately fall inward, usually leaving a surrounding pocket of air that can be seen on diagnostic imaging. CCPA falls within the chronic pulmonary aspergillosis (CPA) category, which includes a spectrum of other subtypes to include single aspergillomas, Aspergillus nodules, and chronic fibrosing pulmonary aspergillosis (CFPA). The prevalence of CPA and its subtypes are limited to case reports and case series in the literature, with reported rates differing up to 40-fold based on region, treatment, and diagnosis criteria.3,4 Models developed by Denning and colleagues mirror those used by The World Health Organization and estimate 1.2 million people have CPA as a sequela to pulmonary TB globally.5
A single aspergilloma (simple aspergilloma) is typically not invasive, whereas CCPA (complex aspergilloma) is the most common CPA and can behave more invasively.6,7 Both can occur in immunocompetent hosts. One study followed 140 individuals with aspergillomas for more than 7 years and found that 60.8% of aspergillomas remained stable in size, while 25.9% increased and 13.3% decreased in size. Half of cases were complicated by hemoptysis, but only 4.2% of cases became invasive.8 Roughly 70% of aspergillomas occur in individuals with a previous history of TB, but any pulmonary cavity can put a patient at increased risk.
Cases have been observed in patients with pulmonary cysts, emphysema/chronic obstructive pulmonary disease, bullae, lung cancer, sarcoidosis, other fungal cavities, and previous lung surgeries.9 Because of its association with CPA, TB testing should be completed as part of the workup as was the case in our patient. Although QuantiFERON-TB Gold has an estimated sensitivity of 92% per the manufacturer’s package insert, results can vary depending on the setting and extent of the TB.10
Clinical features of Aspergillus infection in immunocompetent individuals include weight loss, chronic nonproductive cough, hemoptysis of variable severity, fatigue, and/or shortness of breath.11 CT is the imaging modality of choice and will typically show an upper-lobe cavitation with or without a fungal ball. For patients with suspicious imaging, laboratory testing with serum Aspergillus IgG antibodies should be performed. Aspergillus antigen testing is performed with galactomannan enzyme immunoassay, which detects galactomannan, a polysaccharide antigen that exists primarily in the cell walls of Aspergillus spp. This should be performed on BAL washings rather than serum, however, as serum testing has poor sensitivity.11 Sputum culture is not very sensitive, and although the polymerase chain reaction of sputum and BAL fluid are more sensitive than culture, false-positive results can occur with transient colonization or contamination of samples.11,12 Elevations of inflammatory markers, namely ESR and CRP, are commonly present but not specific for CPA.
Denning and colleagues propose the following criteria for diagnosing CCPA: one large cavity or 2 or more cavities on chest imaging with or without a fungal ball (aspergilloma) in one or more of the cavities (exclude patients with other chronic fungal cavitary lesions, eg, pulmonary histoplasmosis, coccidioidomycosis, and paracoccidioidomycosis); and at least one of the following symptoms for at least 3 months: fever, weight loss, fatigue, cough, sputum production, hemoptysis, or shortness of breath; and a positive Aspergillus IgG with or without culture of Aspergillus spp from the lungs.11Our case fulfills the diagnostic criteria for CCPA. The ≥ 3 months of weight loss was useful in differentiating this case from a single aspergilloma in which the role of antifungal treatment remains unclear especially in those who are asymptomatic.2 In those with single aspergillomas with significant hemoptysis, embolization may be required. In the management of localized CCPA, surgical excision is recommended and curative in many cases.6,11 If left untreated, CCPA carries a 5-year mortality rate as high as 80% and often is accompanied with progression to CFPA, the terminal fibrosing evolution of CCPA, resulting in major fibrotic lung destruction.6 Oral azoles with or without surgical management also are useful in preventing clinical and radiologic progression.6
A multidisciplinary team, including infectious disease and surgery carefully discussed treatment options with the patient. Surgery was offered and the patient declined. We then decided on a trial of medical management alone based on shared decision making. In accordance with the recommendations from our infectious disease colleagues, the patient was started on a voriconazole 200 mg orally twice daily. Duration of therapy was planned for 6 months, with close monitoring of hepatic function, serum electrolytes, and visual function.13
Conclusions
This case highlights important differences among the CPA subtypes and how management differs based on etiology. Diagnostic criteria for CCPA were discussed, and in any patient with the constellation of the symptoms described with one or more cavitary lesions noted on imaging, CCPA should be considered regardless of immunocompetence. A multidisciplinary treatment approach with medical and surgical considerations is crucial to prevent progression to CFPA.
1. Kon K, Rai M, eds. The Microbiology of Respiratory System Infections. Academic Press; 2016.
2. Alguire P, Chick D, eds. ACP MKSAP 18: Medical Knowledge Self-Assessment Program. American College of Physicians; 2018.
3. Tuberculosis Association. Aspergilloma and residual tuberculous cavities. The results of a resurvey. Tubercle. 1970;51(3):227-245.
4. Tuberculosis Association. Aspergillus in persistent lung cavities after tuberculosis. A report from the Research Committee of the British Tuberculosis Association. Tubercle. 968;49(1):1-11.
5. Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ. 2011;89(12):864-872. doi:10.2471/BLT.11.089441
6. Page ID, Byanyima R, Hosmane S, et al. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J. 2019;53(3):1801184. doi:10.1183/13993003.01184-2018
7. Kousha, M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20(121):156-174. doi:10.1183/09059180.00001011
8. Lee JK, Lee Y, Park SS, et al. Clinical course and prognostic factors of pulmonary aspergilloma. Respirology. 2014;19(7):1066-1072. doi:10.1111/resp.12344
9. Kawamura S, Maesaki S, Tomono K, Tashiro T, Kohno S. Clinical evaluation of 61 patients with pulmonary aspergilloma. Intern Med. 2000;39(3):209-212. doi:10.2169/internalmedicine.39.209
10. QuantiFERON-TB Gold ELISA. Package insert. Qiagen; November 2019.
11. Denning DW, Cadranel J, Beigelman-Aubry C, et al; European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47(1):45-68. doi:10.1183/13993003.00583-2015. PMID: 26699723.
12. Denning DW, Park S, Lass-Florl C, et al. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis. 2011;52(9):1123-9. doi:10.1093/cid/cir179
13. Patterson TF, Thompson GR III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1-e60. doi:10.1093/cid/ciw326
A patient with worsening chronic cough, shortness of breath, and hemoptysis tested negative for tuberculosis; but a chest computed tomography scan showed an upper left lobe cavitary lesion.
A 71-year-old, currently homeless male veteran with a 29 pack-year history of smoking and history of alcohol abuse presented to the emergency department at Washington DC Veterans Affairs Medical Center with worsening chronic cough and shortness of breath. He had no history of HIV or immunosuppressant medications. Four weeks prior, he was treated at an outpatient urgent care for community acquired pneumonia with a 10-day course of oral amoxicillin/clavulanic acid 875 mg twice daily and azithromycin 500 mg day 1, then 250 mg days 2 through 5. Despite antibiotic therapy, his symptoms continued to worsen, and he developed hemoptysis. He also reported weight loss of 20 lb in the past 3 months despite a strong appetite and adequate oral intake. He reported no fevers and night sweats. A review of the patient’s systems was otherwise unremarkable.
On examination, the patient was afebrile at 37.2 °C but tachycardic at 108 beats/min. He also was tachypneic at 22 breaths/min with an oxygen saturation of 89% on room air. Decreased breath sounds in the left upper lobe were noted on auscultation of the lung fields. Laboratory test results were notable for a leukocytosis of 14.3 k/μL (reference range, 4-11k/μL) and an elevated erythrocyte sedimentation rate (ESR) of 25.08 mm/h (reference range, 0-16 mm/h) and C-reactive protein (CRP) of 4.75 mg/L (reference range, 0.00-3.00 mg/L). Liver-associated enzymes and a coagulation panel were within normal limits. His QuantiFERON-TB Gold tuberculosis (TB) blood test was negative. A computed tomography (CT) scan of the chest was obtained, which showed an interval increase of a known upper left lobe cavitary lesion compared with that of prior imaging and the presence of a ball-shaped lesion in the cavity (Figures 1 and 2).
In addition to the imaging, the patient underwent bronchoscopy with bronchoalveolar lavage (BAL) to further evaluate the upper left lobe cavitary lesion. The differential diagnosis for pulmonary cavities is described in the Table. The BAL aspirates were negative for acid-fast bacteria; however, periodic acid–Schiff stain and Grocott methenamine silver stain showed fungal elements. He was diagnosed with chronic cavitary pulmonary aspergillosis (CCPA), confirmed with serum antigen (galactomannan assay) and serum immunoglobulin G (IgG) positive for Aspergillus fumigatus (A fumigatus). Mycologic cultures were positive for A fumigatus.
Discussion
Aspergillomas are accumulations of Aspergillus spp hyphae, fibrin, and other inflammatory components that typically occur in preexisting pulmonary cavities.1 They are most frequently caused by A fumigatus, which is ubiquitous in the environment and acquired via inhalation of airborne spores in 90% of cases.2 The typical ball-shaped appearance forms when hyphae growing along the inside walls of the cavity ultimately fall inward, usually leaving a surrounding pocket of air that can be seen on diagnostic imaging. CCPA falls within the chronic pulmonary aspergillosis (CPA) category, which includes a spectrum of other subtypes to include single aspergillomas, Aspergillus nodules, and chronic fibrosing pulmonary aspergillosis (CFPA). The prevalence of CPA and its subtypes are limited to case reports and case series in the literature, with reported rates differing up to 40-fold based on region, treatment, and diagnosis criteria.3,4 Models developed by Denning and colleagues mirror those used by The World Health Organization and estimate 1.2 million people have CPA as a sequela to pulmonary TB globally.5
A single aspergilloma (simple aspergilloma) is typically not invasive, whereas CCPA (complex aspergilloma) is the most common CPA and can behave more invasively.6,7 Both can occur in immunocompetent hosts. One study followed 140 individuals with aspergillomas for more than 7 years and found that 60.8% of aspergillomas remained stable in size, while 25.9% increased and 13.3% decreased in size. Half of cases were complicated by hemoptysis, but only 4.2% of cases became invasive.8 Roughly 70% of aspergillomas occur in individuals with a previous history of TB, but any pulmonary cavity can put a patient at increased risk.
Cases have been observed in patients with pulmonary cysts, emphysema/chronic obstructive pulmonary disease, bullae, lung cancer, sarcoidosis, other fungal cavities, and previous lung surgeries.9 Because of its association with CPA, TB testing should be completed as part of the workup as was the case in our patient. Although QuantiFERON-TB Gold has an estimated sensitivity of 92% per the manufacturer’s package insert, results can vary depending on the setting and extent of the TB.10
Clinical features of Aspergillus infection in immunocompetent individuals include weight loss, chronic nonproductive cough, hemoptysis of variable severity, fatigue, and/or shortness of breath.11 CT is the imaging modality of choice and will typically show an upper-lobe cavitation with or without a fungal ball. For patients with suspicious imaging, laboratory testing with serum Aspergillus IgG antibodies should be performed. Aspergillus antigen testing is performed with galactomannan enzyme immunoassay, which detects galactomannan, a polysaccharide antigen that exists primarily in the cell walls of Aspergillus spp. This should be performed on BAL washings rather than serum, however, as serum testing has poor sensitivity.11 Sputum culture is not very sensitive, and although the polymerase chain reaction of sputum and BAL fluid are more sensitive than culture, false-positive results can occur with transient colonization or contamination of samples.11,12 Elevations of inflammatory markers, namely ESR and CRP, are commonly present but not specific for CPA.
Denning and colleagues propose the following criteria for diagnosing CCPA: one large cavity or 2 or more cavities on chest imaging with or without a fungal ball (aspergilloma) in one or more of the cavities (exclude patients with other chronic fungal cavitary lesions, eg, pulmonary histoplasmosis, coccidioidomycosis, and paracoccidioidomycosis); and at least one of the following symptoms for at least 3 months: fever, weight loss, fatigue, cough, sputum production, hemoptysis, or shortness of breath; and a positive Aspergillus IgG with or without culture of Aspergillus spp from the lungs.11Our case fulfills the diagnostic criteria for CCPA. The ≥ 3 months of weight loss was useful in differentiating this case from a single aspergilloma in which the role of antifungal treatment remains unclear especially in those who are asymptomatic.2 In those with single aspergillomas with significant hemoptysis, embolization may be required. In the management of localized CCPA, surgical excision is recommended and curative in many cases.6,11 If left untreated, CCPA carries a 5-year mortality rate as high as 80% and often is accompanied with progression to CFPA, the terminal fibrosing evolution of CCPA, resulting in major fibrotic lung destruction.6 Oral azoles with or without surgical management also are useful in preventing clinical and radiologic progression.6
A multidisciplinary team, including infectious disease and surgery carefully discussed treatment options with the patient. Surgery was offered and the patient declined. We then decided on a trial of medical management alone based on shared decision making. In accordance with the recommendations from our infectious disease colleagues, the patient was started on a voriconazole 200 mg orally twice daily. Duration of therapy was planned for 6 months, with close monitoring of hepatic function, serum electrolytes, and visual function.13
Conclusions
This case highlights important differences among the CPA subtypes and how management differs based on etiology. Diagnostic criteria for CCPA were discussed, and in any patient with the constellation of the symptoms described with one or more cavitary lesions noted on imaging, CCPA should be considered regardless of immunocompetence. A multidisciplinary treatment approach with medical and surgical considerations is crucial to prevent progression to CFPA.
A patient with worsening chronic cough, shortness of breath, and hemoptysis tested negative for tuberculosis; but a chest computed tomography scan showed an upper left lobe cavitary lesion.
A 71-year-old, currently homeless male veteran with a 29 pack-year history of smoking and history of alcohol abuse presented to the emergency department at Washington DC Veterans Affairs Medical Center with worsening chronic cough and shortness of breath. He had no history of HIV or immunosuppressant medications. Four weeks prior, he was treated at an outpatient urgent care for community acquired pneumonia with a 10-day course of oral amoxicillin/clavulanic acid 875 mg twice daily and azithromycin 500 mg day 1, then 250 mg days 2 through 5. Despite antibiotic therapy, his symptoms continued to worsen, and he developed hemoptysis. He also reported weight loss of 20 lb in the past 3 months despite a strong appetite and adequate oral intake. He reported no fevers and night sweats. A review of the patient’s systems was otherwise unremarkable.
On examination, the patient was afebrile at 37.2 °C but tachycardic at 108 beats/min. He also was tachypneic at 22 breaths/min with an oxygen saturation of 89% on room air. Decreased breath sounds in the left upper lobe were noted on auscultation of the lung fields. Laboratory test results were notable for a leukocytosis of 14.3 k/μL (reference range, 4-11k/μL) and an elevated erythrocyte sedimentation rate (ESR) of 25.08 mm/h (reference range, 0-16 mm/h) and C-reactive protein (CRP) of 4.75 mg/L (reference range, 0.00-3.00 mg/L). Liver-associated enzymes and a coagulation panel were within normal limits. His QuantiFERON-TB Gold tuberculosis (TB) blood test was negative. A computed tomography (CT) scan of the chest was obtained, which showed an interval increase of a known upper left lobe cavitary lesion compared with that of prior imaging and the presence of a ball-shaped lesion in the cavity (Figures 1 and 2).
In addition to the imaging, the patient underwent bronchoscopy with bronchoalveolar lavage (BAL) to further evaluate the upper left lobe cavitary lesion. The differential diagnosis for pulmonary cavities is described in the Table. The BAL aspirates were negative for acid-fast bacteria; however, periodic acid–Schiff stain and Grocott methenamine silver stain showed fungal elements. He was diagnosed with chronic cavitary pulmonary aspergillosis (CCPA), confirmed with serum antigen (galactomannan assay) and serum immunoglobulin G (IgG) positive for Aspergillus fumigatus (A fumigatus). Mycologic cultures were positive for A fumigatus.
Discussion
Aspergillomas are accumulations of Aspergillus spp hyphae, fibrin, and other inflammatory components that typically occur in preexisting pulmonary cavities.1 They are most frequently caused by A fumigatus, which is ubiquitous in the environment and acquired via inhalation of airborne spores in 90% of cases.2 The typical ball-shaped appearance forms when hyphae growing along the inside walls of the cavity ultimately fall inward, usually leaving a surrounding pocket of air that can be seen on diagnostic imaging. CCPA falls within the chronic pulmonary aspergillosis (CPA) category, which includes a spectrum of other subtypes to include single aspergillomas, Aspergillus nodules, and chronic fibrosing pulmonary aspergillosis (CFPA). The prevalence of CPA and its subtypes are limited to case reports and case series in the literature, with reported rates differing up to 40-fold based on region, treatment, and diagnosis criteria.3,4 Models developed by Denning and colleagues mirror those used by The World Health Organization and estimate 1.2 million people have CPA as a sequela to pulmonary TB globally.5
A single aspergilloma (simple aspergilloma) is typically not invasive, whereas CCPA (complex aspergilloma) is the most common CPA and can behave more invasively.6,7 Both can occur in immunocompetent hosts. One study followed 140 individuals with aspergillomas for more than 7 years and found that 60.8% of aspergillomas remained stable in size, while 25.9% increased and 13.3% decreased in size. Half of cases were complicated by hemoptysis, but only 4.2% of cases became invasive.8 Roughly 70% of aspergillomas occur in individuals with a previous history of TB, but any pulmonary cavity can put a patient at increased risk.
Cases have been observed in patients with pulmonary cysts, emphysema/chronic obstructive pulmonary disease, bullae, lung cancer, sarcoidosis, other fungal cavities, and previous lung surgeries.9 Because of its association with CPA, TB testing should be completed as part of the workup as was the case in our patient. Although QuantiFERON-TB Gold has an estimated sensitivity of 92% per the manufacturer’s package insert, results can vary depending on the setting and extent of the TB.10
Clinical features of Aspergillus infection in immunocompetent individuals include weight loss, chronic nonproductive cough, hemoptysis of variable severity, fatigue, and/or shortness of breath.11 CT is the imaging modality of choice and will typically show an upper-lobe cavitation with or without a fungal ball. For patients with suspicious imaging, laboratory testing with serum Aspergillus IgG antibodies should be performed. Aspergillus antigen testing is performed with galactomannan enzyme immunoassay, which detects galactomannan, a polysaccharide antigen that exists primarily in the cell walls of Aspergillus spp. This should be performed on BAL washings rather than serum, however, as serum testing has poor sensitivity.11 Sputum culture is not very sensitive, and although the polymerase chain reaction of sputum and BAL fluid are more sensitive than culture, false-positive results can occur with transient colonization or contamination of samples.11,12 Elevations of inflammatory markers, namely ESR and CRP, are commonly present but not specific for CPA.
Denning and colleagues propose the following criteria for diagnosing CCPA: one large cavity or 2 or more cavities on chest imaging with or without a fungal ball (aspergilloma) in one or more of the cavities (exclude patients with other chronic fungal cavitary lesions, eg, pulmonary histoplasmosis, coccidioidomycosis, and paracoccidioidomycosis); and at least one of the following symptoms for at least 3 months: fever, weight loss, fatigue, cough, sputum production, hemoptysis, or shortness of breath; and a positive Aspergillus IgG with or without culture of Aspergillus spp from the lungs.11Our case fulfills the diagnostic criteria for CCPA. The ≥ 3 months of weight loss was useful in differentiating this case from a single aspergilloma in which the role of antifungal treatment remains unclear especially in those who are asymptomatic.2 In those with single aspergillomas with significant hemoptysis, embolization may be required. In the management of localized CCPA, surgical excision is recommended and curative in many cases.6,11 If left untreated, CCPA carries a 5-year mortality rate as high as 80% and often is accompanied with progression to CFPA, the terminal fibrosing evolution of CCPA, resulting in major fibrotic lung destruction.6 Oral azoles with or without surgical management also are useful in preventing clinical and radiologic progression.6
A multidisciplinary team, including infectious disease and surgery carefully discussed treatment options with the patient. Surgery was offered and the patient declined. We then decided on a trial of medical management alone based on shared decision making. In accordance with the recommendations from our infectious disease colleagues, the patient was started on a voriconazole 200 mg orally twice daily. Duration of therapy was planned for 6 months, with close monitoring of hepatic function, serum electrolytes, and visual function.13
Conclusions
This case highlights important differences among the CPA subtypes and how management differs based on etiology. Diagnostic criteria for CCPA were discussed, and in any patient with the constellation of the symptoms described with one or more cavitary lesions noted on imaging, CCPA should be considered regardless of immunocompetence. A multidisciplinary treatment approach with medical and surgical considerations is crucial to prevent progression to CFPA.
1. Kon K, Rai M, eds. The Microbiology of Respiratory System Infections. Academic Press; 2016.
2. Alguire P, Chick D, eds. ACP MKSAP 18: Medical Knowledge Self-Assessment Program. American College of Physicians; 2018.
3. Tuberculosis Association. Aspergilloma and residual tuberculous cavities. The results of a resurvey. Tubercle. 1970;51(3):227-245.
4. Tuberculosis Association. Aspergillus in persistent lung cavities after tuberculosis. A report from the Research Committee of the British Tuberculosis Association. Tubercle. 968;49(1):1-11.
5. Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ. 2011;89(12):864-872. doi:10.2471/BLT.11.089441
6. Page ID, Byanyima R, Hosmane S, et al. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J. 2019;53(3):1801184. doi:10.1183/13993003.01184-2018
7. Kousha, M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20(121):156-174. doi:10.1183/09059180.00001011
8. Lee JK, Lee Y, Park SS, et al. Clinical course and prognostic factors of pulmonary aspergilloma. Respirology. 2014;19(7):1066-1072. doi:10.1111/resp.12344
9. Kawamura S, Maesaki S, Tomono K, Tashiro T, Kohno S. Clinical evaluation of 61 patients with pulmonary aspergilloma. Intern Med. 2000;39(3):209-212. doi:10.2169/internalmedicine.39.209
10. QuantiFERON-TB Gold ELISA. Package insert. Qiagen; November 2019.
11. Denning DW, Cadranel J, Beigelman-Aubry C, et al; European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47(1):45-68. doi:10.1183/13993003.00583-2015. PMID: 26699723.
12. Denning DW, Park S, Lass-Florl C, et al. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis. 2011;52(9):1123-9. doi:10.1093/cid/cir179
13. Patterson TF, Thompson GR III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1-e60. doi:10.1093/cid/ciw326
1. Kon K, Rai M, eds. The Microbiology of Respiratory System Infections. Academic Press; 2016.
2. Alguire P, Chick D, eds. ACP MKSAP 18: Medical Knowledge Self-Assessment Program. American College of Physicians; 2018.
3. Tuberculosis Association. Aspergilloma and residual tuberculous cavities. The results of a resurvey. Tubercle. 1970;51(3):227-245.
4. Tuberculosis Association. Aspergillus in persistent lung cavities after tuberculosis. A report from the Research Committee of the British Tuberculosis Association. Tubercle. 968;49(1):1-11.
5. Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ. 2011;89(12):864-872. doi:10.2471/BLT.11.089441
6. Page ID, Byanyima R, Hosmane S, et al. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J. 2019;53(3):1801184. doi:10.1183/13993003.01184-2018
7. Kousha, M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20(121):156-174. doi:10.1183/09059180.00001011
8. Lee JK, Lee Y, Park SS, et al. Clinical course and prognostic factors of pulmonary aspergilloma. Respirology. 2014;19(7):1066-1072. doi:10.1111/resp.12344
9. Kawamura S, Maesaki S, Tomono K, Tashiro T, Kohno S. Clinical evaluation of 61 patients with pulmonary aspergilloma. Intern Med. 2000;39(3):209-212. doi:10.2169/internalmedicine.39.209
10. QuantiFERON-TB Gold ELISA. Package insert. Qiagen; November 2019.
11. Denning DW, Cadranel J, Beigelman-Aubry C, et al; European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47(1):45-68. doi:10.1183/13993003.00583-2015. PMID: 26699723.
12. Denning DW, Park S, Lass-Florl C, et al. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis. 2011;52(9):1123-9. doi:10.1093/cid/cir179
13. Patterson TF, Thompson GR III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1-e60. doi:10.1093/cid/ciw326