User login
IV gentamicin improves junctional epidermolysis bullosa in children
Intravenous (JEB) caused by nonsense variants.
The newly generated structural protein persisted during the 3-month randomized clinical trial and was associated with significant wound closure – with no signs of ototoxic effects, nephrotoxic effects, or anti–laminin 332 autoantibody induction, investigators recently reported in JAMA Dermatology.
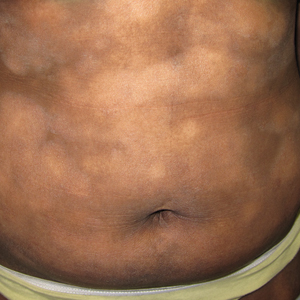
JEB is a rare, autosomal recessive disorder caused mainly by nonsense variants (i.e., mutations) in the LAMA3, LAMB3, or LAMC2 genes that encode laminin, resulting in widespread blisters and erosions of the skin. Current treatment is limited to supportive management and palliative care, and children with its severe subtype are likely to die within the first year of life.
“With data indicating a robust response to short-term gentamicin treatment and the marked stability of laminin 332, we envision that gentamicin could be delivered as a short-term pulse therapy every 2-3 months for patients with JEB caused by nonsense variants,” the researchers wrote.
Of the five patients, ages 3 months to 10 years, three received 7.5 mg/kg IV gentamicin daily for 14 days, and two received 10 mg/kg daily for 24 days at the University of Southern California, Los Angeles.
All had confirmed nonsense variants in LAMA3 or LAMB3 in one or two alleles, and all had minimal laminin 332 expression at baseline as determined by immunofluorescence. After treatment, each of the children had increased, sustained expression of laminin 332.
The researchers monitored three open wounds in each patient. By 1 month, seven of nine wounds in those receiving the lower-dose therapy and all of the wounds in those receiving the higher-dose therapy showed at least 50% closure. By 3 months, eight of nine wounds in the lower-dose group, and all wounds in the higher-dose group showed greater than 85% closure.
In an interview, senior investigators Mei Chen, PhD, professor of dermatology, and David T. Woodley, MD, professor and chair of dermatology, both at USC, emphasized laminin’s long half-life.“Once these skin structural proteins are generated at the dermal-epidermal junction, they are long-lasting structures, which means the therapy can be pulsed rather than continuously delivered, which can obviate some of the known side effects of the medication,” Dr. Woodley said.
Gentamicin, an aminoglycoside, works as a “read-through therapy,” inducing ribosomal read-through of premature termination codons (PTCs) caused by nonsense mutations. The read-through allows translation to proceed and full-length proteins to be generated.
Gentamicin read-through therapy is also being investigated for recessive dystrophic epidermolysis bullosa (RDEB) attributable to nonsense mutations. The culprit mutations in this form of EB occur in a gene that encodes collagen type VII alpha 1, which, like laminin, is responsible for dermal-epidermal adherence. A clinical trial of intravenous gentamicin for RDEB is ongoing at USC, Dr. Chen said.
EBS-MD case report
It may also have a role in treating epidermolysis bullosa simplex with muscular dystrophy (EBS-MD), according to investigators in Madrid. Their case report, published in JAMA Dermatology, details how two 14-day courses of infused gentamicin therapy were followed by re-expression of plectin in the skin for 4-5 months and mild improvement in symptoms in one patient, a woman in her 30s, with a homozygous nonsense variant in PLEC1.
In an editorial accompanying the two reports, Anna L. Bruckner, MD, MSCS, professor of dermatology, University of Colorado at Denver, Aurora, and colleagues expressed cautious optimism and said that additional research on the feasibility, possible cumulative toxic effects, risk of microbial resistance, and overall clinical relevance is needed.
Still, the “investigators should be applauded for taking advantage of a readily available systemic treatment to target cutaneous and extracutaneous symptoms of patients who have very limited treatment options at this time,” they wrote. While all forms of EB are considered orphan disorders, JEB and EBS-MD have received less research attention than RDEB.
The JEB study evaluated patients with clinical assessments/quality of life surveys and with a validated clinical score that considers skin and mucosae – the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI). There were small positive changes in EBDASI scores, but data were incomplete and therefore difficult to interpret.
A “noteworthy” finding, the authors wrote, were improvements in emotions and functioning in two of the children who were eligible given their older ages for assessment with the Skindex-16 quality-of-life survey. The improvements suggest “potential psychosocial benefits” of the gentamicin therapy.
The JEB study was supported in part by grants from the EB Research Partnership and EB Medical Research Foundation and an award from the Congressionally Directed Medical Research Program. In addition to the grants, Dr. Woodley and Dr. Chen reported receiving personal fees from Phoenix Tissue Repair outside of the submitted work. For the EBS-MD case report, the authors reported no disclosures. Dr. Bruckner, corresponding author of the editorial, reported grants from several companies outside the submitted work.
Intravenous (JEB) caused by nonsense variants.
The newly generated structural protein persisted during the 3-month randomized clinical trial and was associated with significant wound closure – with no signs of ototoxic effects, nephrotoxic effects, or anti–laminin 332 autoantibody induction, investigators recently reported in JAMA Dermatology.
JEB is a rare, autosomal recessive disorder caused mainly by nonsense variants (i.e., mutations) in the LAMA3, LAMB3, or LAMC2 genes that encode laminin, resulting in widespread blisters and erosions of the skin. Current treatment is limited to supportive management and palliative care, and children with its severe subtype are likely to die within the first year of life.
“With data indicating a robust response to short-term gentamicin treatment and the marked stability of laminin 332, we envision that gentamicin could be delivered as a short-term pulse therapy every 2-3 months for patients with JEB caused by nonsense variants,” the researchers wrote.
Of the five patients, ages 3 months to 10 years, three received 7.5 mg/kg IV gentamicin daily for 14 days, and two received 10 mg/kg daily for 24 days at the University of Southern California, Los Angeles.
All had confirmed nonsense variants in LAMA3 or LAMB3 in one or two alleles, and all had minimal laminin 332 expression at baseline as determined by immunofluorescence. After treatment, each of the children had increased, sustained expression of laminin 332.
The researchers monitored three open wounds in each patient. By 1 month, seven of nine wounds in those receiving the lower-dose therapy and all of the wounds in those receiving the higher-dose therapy showed at least 50% closure. By 3 months, eight of nine wounds in the lower-dose group, and all wounds in the higher-dose group showed greater than 85% closure.
In an interview, senior investigators Mei Chen, PhD, professor of dermatology, and David T. Woodley, MD, professor and chair of dermatology, both at USC, emphasized laminin’s long half-life.“Once these skin structural proteins are generated at the dermal-epidermal junction, they are long-lasting structures, which means the therapy can be pulsed rather than continuously delivered, which can obviate some of the known side effects of the medication,” Dr. Woodley said.
Gentamicin, an aminoglycoside, works as a “read-through therapy,” inducing ribosomal read-through of premature termination codons (PTCs) caused by nonsense mutations. The read-through allows translation to proceed and full-length proteins to be generated.
Gentamicin read-through therapy is also being investigated for recessive dystrophic epidermolysis bullosa (RDEB) attributable to nonsense mutations. The culprit mutations in this form of EB occur in a gene that encodes collagen type VII alpha 1, which, like laminin, is responsible for dermal-epidermal adherence. A clinical trial of intravenous gentamicin for RDEB is ongoing at USC, Dr. Chen said.
EBS-MD case report
It may also have a role in treating epidermolysis bullosa simplex with muscular dystrophy (EBS-MD), according to investigators in Madrid. Their case report, published in JAMA Dermatology, details how two 14-day courses of infused gentamicin therapy were followed by re-expression of plectin in the skin for 4-5 months and mild improvement in symptoms in one patient, a woman in her 30s, with a homozygous nonsense variant in PLEC1.
In an editorial accompanying the two reports, Anna L. Bruckner, MD, MSCS, professor of dermatology, University of Colorado at Denver, Aurora, and colleagues expressed cautious optimism and said that additional research on the feasibility, possible cumulative toxic effects, risk of microbial resistance, and overall clinical relevance is needed.
Still, the “investigators should be applauded for taking advantage of a readily available systemic treatment to target cutaneous and extracutaneous symptoms of patients who have very limited treatment options at this time,” they wrote. While all forms of EB are considered orphan disorders, JEB and EBS-MD have received less research attention than RDEB.
The JEB study evaluated patients with clinical assessments/quality of life surveys and with a validated clinical score that considers skin and mucosae – the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI). There were small positive changes in EBDASI scores, but data were incomplete and therefore difficult to interpret.
A “noteworthy” finding, the authors wrote, were improvements in emotions and functioning in two of the children who were eligible given their older ages for assessment with the Skindex-16 quality-of-life survey. The improvements suggest “potential psychosocial benefits” of the gentamicin therapy.
The JEB study was supported in part by grants from the EB Research Partnership and EB Medical Research Foundation and an award from the Congressionally Directed Medical Research Program. In addition to the grants, Dr. Woodley and Dr. Chen reported receiving personal fees from Phoenix Tissue Repair outside of the submitted work. For the EBS-MD case report, the authors reported no disclosures. Dr. Bruckner, corresponding author of the editorial, reported grants from several companies outside the submitted work.
Intravenous (JEB) caused by nonsense variants.
The newly generated structural protein persisted during the 3-month randomized clinical trial and was associated with significant wound closure – with no signs of ototoxic effects, nephrotoxic effects, or anti–laminin 332 autoantibody induction, investigators recently reported in JAMA Dermatology.
JEB is a rare, autosomal recessive disorder caused mainly by nonsense variants (i.e., mutations) in the LAMA3, LAMB3, or LAMC2 genes that encode laminin, resulting in widespread blisters and erosions of the skin. Current treatment is limited to supportive management and palliative care, and children with its severe subtype are likely to die within the first year of life.
“With data indicating a robust response to short-term gentamicin treatment and the marked stability of laminin 332, we envision that gentamicin could be delivered as a short-term pulse therapy every 2-3 months for patients with JEB caused by nonsense variants,” the researchers wrote.
Of the five patients, ages 3 months to 10 years, three received 7.5 mg/kg IV gentamicin daily for 14 days, and two received 10 mg/kg daily for 24 days at the University of Southern California, Los Angeles.
All had confirmed nonsense variants in LAMA3 or LAMB3 in one or two alleles, and all had minimal laminin 332 expression at baseline as determined by immunofluorescence. After treatment, each of the children had increased, sustained expression of laminin 332.
The researchers monitored three open wounds in each patient. By 1 month, seven of nine wounds in those receiving the lower-dose therapy and all of the wounds in those receiving the higher-dose therapy showed at least 50% closure. By 3 months, eight of nine wounds in the lower-dose group, and all wounds in the higher-dose group showed greater than 85% closure.
In an interview, senior investigators Mei Chen, PhD, professor of dermatology, and David T. Woodley, MD, professor and chair of dermatology, both at USC, emphasized laminin’s long half-life.“Once these skin structural proteins are generated at the dermal-epidermal junction, they are long-lasting structures, which means the therapy can be pulsed rather than continuously delivered, which can obviate some of the known side effects of the medication,” Dr. Woodley said.
Gentamicin, an aminoglycoside, works as a “read-through therapy,” inducing ribosomal read-through of premature termination codons (PTCs) caused by nonsense mutations. The read-through allows translation to proceed and full-length proteins to be generated.
Gentamicin read-through therapy is also being investigated for recessive dystrophic epidermolysis bullosa (RDEB) attributable to nonsense mutations. The culprit mutations in this form of EB occur in a gene that encodes collagen type VII alpha 1, which, like laminin, is responsible for dermal-epidermal adherence. A clinical trial of intravenous gentamicin for RDEB is ongoing at USC, Dr. Chen said.
EBS-MD case report
It may also have a role in treating epidermolysis bullosa simplex with muscular dystrophy (EBS-MD), according to investigators in Madrid. Their case report, published in JAMA Dermatology, details how two 14-day courses of infused gentamicin therapy were followed by re-expression of plectin in the skin for 4-5 months and mild improvement in symptoms in one patient, a woman in her 30s, with a homozygous nonsense variant in PLEC1.
In an editorial accompanying the two reports, Anna L. Bruckner, MD, MSCS, professor of dermatology, University of Colorado at Denver, Aurora, and colleagues expressed cautious optimism and said that additional research on the feasibility, possible cumulative toxic effects, risk of microbial resistance, and overall clinical relevance is needed.
Still, the “investigators should be applauded for taking advantage of a readily available systemic treatment to target cutaneous and extracutaneous symptoms of patients who have very limited treatment options at this time,” they wrote. While all forms of EB are considered orphan disorders, JEB and EBS-MD have received less research attention than RDEB.
The JEB study evaluated patients with clinical assessments/quality of life surveys and with a validated clinical score that considers skin and mucosae – the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI). There were small positive changes in EBDASI scores, but data were incomplete and therefore difficult to interpret.
A “noteworthy” finding, the authors wrote, were improvements in emotions and functioning in two of the children who were eligible given their older ages for assessment with the Skindex-16 quality-of-life survey. The improvements suggest “potential psychosocial benefits” of the gentamicin therapy.
The JEB study was supported in part by grants from the EB Research Partnership and EB Medical Research Foundation and an award from the Congressionally Directed Medical Research Program. In addition to the grants, Dr. Woodley and Dr. Chen reported receiving personal fees from Phoenix Tissue Repair outside of the submitted work. For the EBS-MD case report, the authors reported no disclosures. Dr. Bruckner, corresponding author of the editorial, reported grants from several companies outside the submitted work.
FROM JAMA DERMATOLOGY
FDA advisory panel rejects new ALS drug
Six of 10 members of the FDA Peripheral and Central Nervous System Drugs Advisory Committee decided there is not enough evidence to support approval of the drug from Amylyx Pharmaceuticals. The evidence from a single phase 2 trial is insufficient, the panel said.
The fate of the drug, known as AMX0035, and the panel’s vote, has been closely watched as new treatments for this devastating disease are greatly needed. Committee members said they were moved by passionate testimony from patients, caregivers, and others. But, they believe the evidence does not meet the required standard for FDA approval.
“We were asked to look for substantial evidence of persuasiveness and robustness, and I think this one trial doesn’t quite meet that bar and was problematic,” said Kenneth Fischbeck, MD, investigator with the National Institute of Neurological Disorders and Stroke. “It would be a disservice to patients and their families to move ahead and approve a treatment that is of uncertain benefit,” said Dr. Fischbeck.
The committee’s vote is not binding. While the FDA often follows its advisors’ decisions, the agency last year approved a controversial new drug for Alzheimer’s disease after a similar advisory committee voted against it.
Phase 3 study in the works
This new ALS drug was shown to slow the decline caused by ALS, sometimes known as Lou Gehrig’s disease, Jamie Timmons, MD, head of scientific communications at Amylyx Pharmaceuticals, said. The study found the drug slowed decline by 25%, compared with patients taking a placebo. That change is considered clinically meaningful.
This is the first time a treatment has shown a benefit on both function and survival in ALS, the two key measures in a relentlessly progressive, fatal disease, said Joshua Cohen, co-CEO and co-founder of Amylyx.
During the meeting, patients with ALS said they were willing to accept greater risk for the possibility of having even a little more time with their loved ones and argued that the drug contains two compounds that are already available. They pleaded for the FDA to exercise its regulatory flexibility in approving this experimental drug.
However, the FDA panel raised a number of issues with the trial. These concerns included the study’s small sample size and no survival benefit at 24 weeks.
Many panel members said they hope an ongoing phase III trial will be more definitive because it’s so much larger. The results of that trial are expected by early 2024.
A version of this article first appeared on WebMD.com.
Six of 10 members of the FDA Peripheral and Central Nervous System Drugs Advisory Committee decided there is not enough evidence to support approval of the drug from Amylyx Pharmaceuticals. The evidence from a single phase 2 trial is insufficient, the panel said.
The fate of the drug, known as AMX0035, and the panel’s vote, has been closely watched as new treatments for this devastating disease are greatly needed. Committee members said they were moved by passionate testimony from patients, caregivers, and others. But, they believe the evidence does not meet the required standard for FDA approval.
“We were asked to look for substantial evidence of persuasiveness and robustness, and I think this one trial doesn’t quite meet that bar and was problematic,” said Kenneth Fischbeck, MD, investigator with the National Institute of Neurological Disorders and Stroke. “It would be a disservice to patients and their families to move ahead and approve a treatment that is of uncertain benefit,” said Dr. Fischbeck.
The committee’s vote is not binding. While the FDA often follows its advisors’ decisions, the agency last year approved a controversial new drug for Alzheimer’s disease after a similar advisory committee voted against it.
Phase 3 study in the works
This new ALS drug was shown to slow the decline caused by ALS, sometimes known as Lou Gehrig’s disease, Jamie Timmons, MD, head of scientific communications at Amylyx Pharmaceuticals, said. The study found the drug slowed decline by 25%, compared with patients taking a placebo. That change is considered clinically meaningful.
This is the first time a treatment has shown a benefit on both function and survival in ALS, the two key measures in a relentlessly progressive, fatal disease, said Joshua Cohen, co-CEO and co-founder of Amylyx.
During the meeting, patients with ALS said they were willing to accept greater risk for the possibility of having even a little more time with their loved ones and argued that the drug contains two compounds that are already available. They pleaded for the FDA to exercise its regulatory flexibility in approving this experimental drug.
However, the FDA panel raised a number of issues with the trial. These concerns included the study’s small sample size and no survival benefit at 24 weeks.
Many panel members said they hope an ongoing phase III trial will be more definitive because it’s so much larger. The results of that trial are expected by early 2024.
A version of this article first appeared on WebMD.com.
Six of 10 members of the FDA Peripheral and Central Nervous System Drugs Advisory Committee decided there is not enough evidence to support approval of the drug from Amylyx Pharmaceuticals. The evidence from a single phase 2 trial is insufficient, the panel said.
The fate of the drug, known as AMX0035, and the panel’s vote, has been closely watched as new treatments for this devastating disease are greatly needed. Committee members said they were moved by passionate testimony from patients, caregivers, and others. But, they believe the evidence does not meet the required standard for FDA approval.
“We were asked to look for substantial evidence of persuasiveness and robustness, and I think this one trial doesn’t quite meet that bar and was problematic,” said Kenneth Fischbeck, MD, investigator with the National Institute of Neurological Disorders and Stroke. “It would be a disservice to patients and their families to move ahead and approve a treatment that is of uncertain benefit,” said Dr. Fischbeck.
The committee’s vote is not binding. While the FDA often follows its advisors’ decisions, the agency last year approved a controversial new drug for Alzheimer’s disease after a similar advisory committee voted against it.
Phase 3 study in the works
This new ALS drug was shown to slow the decline caused by ALS, sometimes known as Lou Gehrig’s disease, Jamie Timmons, MD, head of scientific communications at Amylyx Pharmaceuticals, said. The study found the drug slowed decline by 25%, compared with patients taking a placebo. That change is considered clinically meaningful.
This is the first time a treatment has shown a benefit on both function and survival in ALS, the two key measures in a relentlessly progressive, fatal disease, said Joshua Cohen, co-CEO and co-founder of Amylyx.
During the meeting, patients with ALS said they were willing to accept greater risk for the possibility of having even a little more time with their loved ones and argued that the drug contains two compounds that are already available. They pleaded for the FDA to exercise its regulatory flexibility in approving this experimental drug.
However, the FDA panel raised a number of issues with the trial. These concerns included the study’s small sample size and no survival benefit at 24 weeks.
Many panel members said they hope an ongoing phase III trial will be more definitive because it’s so much larger. The results of that trial are expected by early 2024.
A version of this article first appeared on WebMD.com.
Dupilumab treats itch and clears lesions in prurigo nodularis patients
BOSTON – of treatment, in a phase 3 trial presented at the American Academy of Dermatology 2022 Annual Meeting.
There are currently no Food and Drug Administration–approved systemic therapies for PN. Although several treatments for the disease are used off label for the condition, such as ultraviolet light therapy and immunosuppressive agents, moderate to severe PN is usually difficult to control, noted Gil Yosipovitch, MD, director of the Miami Itch Center at the University of Miami Miller School of Medicine, Florida. He led the research and presented the findings at the conference.
“Many dermatologists feel very uncomfortable dealing with these patients because they suffer from chronicity, they are miserable, and previously, the drugs didn’t work well,” Dr. Yosipovitch told this news organization. The results from this trial “are very promising,” he said. “It opens a new field of treatment for itchy conditions.”
The trial, named LIBERTY-PN PRIME2, enrolled patients aged 18-80 who had been living with PN for at least 3 months. Patients had at least 20 lesions at baseline as well as severe itch, defined as a score of 7 or greater on the Worst Itch Numerical Rating Scale (WI-NRS). The scale ranges from 0 (no itch) to 10 (worst itch imaginable). Participants also had a history of treatment failure with medium to super-potent topical corticosteroids (TCSs), or treatment with TCSs was not medically advisable for them.
The randomized, double-blinded study enrolled 160 adults with PN. Of those, 78 were assigned to the treatment arm and received a 600-mg loading dose of dupilumab, administered subcutaneously, followed by 300-mg doses every 2 weeks for 24 weeks; 82 patients were allocated to receive placebo.
During the study, 25 patients in the placebo arm discontinued treatment. In the treatment arm, one patient was not treated and two discontinued treatment due to lack of efficacy.
The primary endpoint of the study was a reduction of at least 4 points on the WI-NRS at 12 weeks. Secondary endpoints included at least a 4-point WI-NRS reduction at 24 weeks and clear to nearly clear skin, defined as having a score of 0 or 1 on the Investigator’s Global Assessment PN-Stage (IGN PN-S). The scale ranges from 0 (clear) to 4 (severe).
At 12 weeks, 37.2% of patients given dupilumab reported a reduction of at least 4 points in WI-NRS, compared with 22.0% of patients given placebo (P = .0216). By 24 weeks, 57.7% of adults who received dupilumab achieved a greater than or equal to 4-point reduction in WI-NRS, compared with 19.5% of those who received placebo (P < .0001). Additionally, 44.9% of participants in the treatment arm achieved a score of 0 or 1 on the IGA PN-S, compared with 15.9% of those in the placebo arm (P < .0001).
Forty-four participants who received dupilumab (57.1%) and 42 participants who received placebo (51.2%) reported at least one treatment-emergent adverse event (TEAE) during the study, though none of these events were serious. The most common TEAE in the study was headache, occurring in five patients taking placebo and four patients receiving dupilumab. In the dupilumab group, there were five cases of herpes virus infection, four non-herpes skin infections, and three cases of conjunctivitis. In the placebo group, seven non-herpes skin infections were reported.
Sanofi and Regeneron, who jointly developed dupilumab, plan to file for regulatory approval for dupilumab for PN “around the world” in the first half of this year, according to a press release.
“It’s great news and a step in the right direction,” Sarina Elmariah, MD, PhD, a dermatologist at Massachusetts General Hospital and instructor of dermatology at Harvard Medical School, both in Boston, told this news organization. She was not involved with the research.
“We’re finally starting to shed light on this condition and its pathogenesis,” she said. She noted that other potential therapeutics for PN are also in development. “It’s reflective of the fact that we are making strides in this area.”
Sanofi and Regeneron Pharmaceuticals sponsored the LIBERTY-PN PRIME2 trial. Dr. Yosipovitch has reported financial relationships with Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron, Sanofi, and Trevi Therapeutics. Dr. Elmariah is on the advisory boards of Sanofi, Galderma, and Trevi Therapeutics.
A version of this article first appeared on Medscape.com.
BOSTON – of treatment, in a phase 3 trial presented at the American Academy of Dermatology 2022 Annual Meeting.
There are currently no Food and Drug Administration–approved systemic therapies for PN. Although several treatments for the disease are used off label for the condition, such as ultraviolet light therapy and immunosuppressive agents, moderate to severe PN is usually difficult to control, noted Gil Yosipovitch, MD, director of the Miami Itch Center at the University of Miami Miller School of Medicine, Florida. He led the research and presented the findings at the conference.
“Many dermatologists feel very uncomfortable dealing with these patients because they suffer from chronicity, they are miserable, and previously, the drugs didn’t work well,” Dr. Yosipovitch told this news organization. The results from this trial “are very promising,” he said. “It opens a new field of treatment for itchy conditions.”
The trial, named LIBERTY-PN PRIME2, enrolled patients aged 18-80 who had been living with PN for at least 3 months. Patients had at least 20 lesions at baseline as well as severe itch, defined as a score of 7 or greater on the Worst Itch Numerical Rating Scale (WI-NRS). The scale ranges from 0 (no itch) to 10 (worst itch imaginable). Participants also had a history of treatment failure with medium to super-potent topical corticosteroids (TCSs), or treatment with TCSs was not medically advisable for them.
The randomized, double-blinded study enrolled 160 adults with PN. Of those, 78 were assigned to the treatment arm and received a 600-mg loading dose of dupilumab, administered subcutaneously, followed by 300-mg doses every 2 weeks for 24 weeks; 82 patients were allocated to receive placebo.
During the study, 25 patients in the placebo arm discontinued treatment. In the treatment arm, one patient was not treated and two discontinued treatment due to lack of efficacy.
The primary endpoint of the study was a reduction of at least 4 points on the WI-NRS at 12 weeks. Secondary endpoints included at least a 4-point WI-NRS reduction at 24 weeks and clear to nearly clear skin, defined as having a score of 0 or 1 on the Investigator’s Global Assessment PN-Stage (IGN PN-S). The scale ranges from 0 (clear) to 4 (severe).
At 12 weeks, 37.2% of patients given dupilumab reported a reduction of at least 4 points in WI-NRS, compared with 22.0% of patients given placebo (P = .0216). By 24 weeks, 57.7% of adults who received dupilumab achieved a greater than or equal to 4-point reduction in WI-NRS, compared with 19.5% of those who received placebo (P < .0001). Additionally, 44.9% of participants in the treatment arm achieved a score of 0 or 1 on the IGA PN-S, compared with 15.9% of those in the placebo arm (P < .0001).
Forty-four participants who received dupilumab (57.1%) and 42 participants who received placebo (51.2%) reported at least one treatment-emergent adverse event (TEAE) during the study, though none of these events were serious. The most common TEAE in the study was headache, occurring in five patients taking placebo and four patients receiving dupilumab. In the dupilumab group, there were five cases of herpes virus infection, four non-herpes skin infections, and three cases of conjunctivitis. In the placebo group, seven non-herpes skin infections were reported.
Sanofi and Regeneron, who jointly developed dupilumab, plan to file for regulatory approval for dupilumab for PN “around the world” in the first half of this year, according to a press release.
“It’s great news and a step in the right direction,” Sarina Elmariah, MD, PhD, a dermatologist at Massachusetts General Hospital and instructor of dermatology at Harvard Medical School, both in Boston, told this news organization. She was not involved with the research.
“We’re finally starting to shed light on this condition and its pathogenesis,” she said. She noted that other potential therapeutics for PN are also in development. “It’s reflective of the fact that we are making strides in this area.”
Sanofi and Regeneron Pharmaceuticals sponsored the LIBERTY-PN PRIME2 trial. Dr. Yosipovitch has reported financial relationships with Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron, Sanofi, and Trevi Therapeutics. Dr. Elmariah is on the advisory boards of Sanofi, Galderma, and Trevi Therapeutics.
A version of this article first appeared on Medscape.com.
BOSTON – of treatment, in a phase 3 trial presented at the American Academy of Dermatology 2022 Annual Meeting.
There are currently no Food and Drug Administration–approved systemic therapies for PN. Although several treatments for the disease are used off label for the condition, such as ultraviolet light therapy and immunosuppressive agents, moderate to severe PN is usually difficult to control, noted Gil Yosipovitch, MD, director of the Miami Itch Center at the University of Miami Miller School of Medicine, Florida. He led the research and presented the findings at the conference.
“Many dermatologists feel very uncomfortable dealing with these patients because they suffer from chronicity, they are miserable, and previously, the drugs didn’t work well,” Dr. Yosipovitch told this news organization. The results from this trial “are very promising,” he said. “It opens a new field of treatment for itchy conditions.”
The trial, named LIBERTY-PN PRIME2, enrolled patients aged 18-80 who had been living with PN for at least 3 months. Patients had at least 20 lesions at baseline as well as severe itch, defined as a score of 7 or greater on the Worst Itch Numerical Rating Scale (WI-NRS). The scale ranges from 0 (no itch) to 10 (worst itch imaginable). Participants also had a history of treatment failure with medium to super-potent topical corticosteroids (TCSs), or treatment with TCSs was not medically advisable for them.
The randomized, double-blinded study enrolled 160 adults with PN. Of those, 78 were assigned to the treatment arm and received a 600-mg loading dose of dupilumab, administered subcutaneously, followed by 300-mg doses every 2 weeks for 24 weeks; 82 patients were allocated to receive placebo.
During the study, 25 patients in the placebo arm discontinued treatment. In the treatment arm, one patient was not treated and two discontinued treatment due to lack of efficacy.
The primary endpoint of the study was a reduction of at least 4 points on the WI-NRS at 12 weeks. Secondary endpoints included at least a 4-point WI-NRS reduction at 24 weeks and clear to nearly clear skin, defined as having a score of 0 or 1 on the Investigator’s Global Assessment PN-Stage (IGN PN-S). The scale ranges from 0 (clear) to 4 (severe).
At 12 weeks, 37.2% of patients given dupilumab reported a reduction of at least 4 points in WI-NRS, compared with 22.0% of patients given placebo (P = .0216). By 24 weeks, 57.7% of adults who received dupilumab achieved a greater than or equal to 4-point reduction in WI-NRS, compared with 19.5% of those who received placebo (P < .0001). Additionally, 44.9% of participants in the treatment arm achieved a score of 0 or 1 on the IGA PN-S, compared with 15.9% of those in the placebo arm (P < .0001).
Forty-four participants who received dupilumab (57.1%) and 42 participants who received placebo (51.2%) reported at least one treatment-emergent adverse event (TEAE) during the study, though none of these events were serious. The most common TEAE in the study was headache, occurring in five patients taking placebo and four patients receiving dupilumab. In the dupilumab group, there were five cases of herpes virus infection, four non-herpes skin infections, and three cases of conjunctivitis. In the placebo group, seven non-herpes skin infections were reported.
Sanofi and Regeneron, who jointly developed dupilumab, plan to file for regulatory approval for dupilumab for PN “around the world” in the first half of this year, according to a press release.
“It’s great news and a step in the right direction,” Sarina Elmariah, MD, PhD, a dermatologist at Massachusetts General Hospital and instructor of dermatology at Harvard Medical School, both in Boston, told this news organization. She was not involved with the research.
“We’re finally starting to shed light on this condition and its pathogenesis,” she said. She noted that other potential therapeutics for PN are also in development. “It’s reflective of the fact that we are making strides in this area.”
Sanofi and Regeneron Pharmaceuticals sponsored the LIBERTY-PN PRIME2 trial. Dr. Yosipovitch has reported financial relationships with Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron, Sanofi, and Trevi Therapeutics. Dr. Elmariah is on the advisory boards of Sanofi, Galderma, and Trevi Therapeutics.
A version of this article first appeared on Medscape.com.
AT AAD 2022
Surgery in CJD patients a potential risk factor for transmission
About one in six patients with Creutzfeldt-Jakob disease (CJD) undergo surgery, raising the risk of iatrogenic transmission of this rare but universally fatal prion disease.
In a retrospective analysis, researchers found that 26 of 121 (21%) patients with probable or definite CJD at four U.S. academic medical centers underwent a total of 55 procedures.
These included high-risk procedures for two patients with neuropathologically proven CJD. One underwent ophthalmic artery aneurysm clipping for unruptured aneurysm, and the other underwent diagnostic brain biopsy.
“The findings were definitely surprising to me and my team – particularly the high frequency with which patients with an irreversible and particularly transmissible neurologic disease underwent invasive medical procedures either just before or shortly after the emergence of symptoms later attributed to CJD,” study investigator Gregory Day, MD, with the Mayo Clinic, Jacksonville, Fla., said in an interview.
The study was published online March 9, 2022, in JAMA Network Open.
Poor infection control
The investigators noted that the majority of CJD cases are sporadic or are inherited, but research shows that prion transmission can occur via contaminated tissues or reusable medical equipment.
While the risk of iatrogenic transmission is highest following procedures involving the central nervous system, where prion burden is highest, experimental models suggest CJD transmission can occur after contact with other tissues, including nasal mucosa, lung, lymph nodes, and spleen, the researchers noted.
“If these models are accurate, surgical procedures involving these tissues may pose a risk to patients,” the investigators wrote.
To determine the potential scope of this problem, the researchers examined the frequency of invasive procedures performed in patients with CJD at four tertiary care centers.
“In several cases, these procedures were done with clear indications [such as] fixation or joint replacement following a fracture. In several others, however, the procedures were unlikely to help the patient. For instance, a hip replacement for walking difficulties that were actually due to changes in the brain due to CJD,” Dr. Day said.
“Even more surprising was the low frequency with which appropriate surgical precautions/infection control procedures were used in patients with established diagnoses of CJD,” he noted.
Only one procedure was performed with sterilization techniques adequate to prevent CJD.
Dr. Day said the findings aren’t necessarily cause for immediate alarm, but they do highlight an area for potential improvement, including better screening of patients who have new and unexplained symptoms before proceeding with surgery, especially surgery of the central nervous system, where prion burden is high.
Another potential solution is to develop and support program surveillance and to work with public health organizations such the Centers for Disease Control and Prevention and the National Prion Disease Pathology Surveillance Center to elicit a surgical history in patients diagnosed with prion disease.
“Active nationwide surveillance is needed to determine the true scope of this potential problem and to develop strategies to mitigate the potential risk of iatrogenic prion transmission to future patients,” Dr. Day said.
True prevalence unknown
The authors of an invited commentary noted that, while most CJD infections occur sporadically, iatrogenic transmission is possible. Approximately 500 such cases have been reported worldwide to date.
“Yet, reported transmission from surgical procedures remains rare, with fewer than 10 confirmed CJD cases described in the literature, although the true prevalence is difficult to quantify as confirmed diagnosis requires autopsy,” wrote Beatrice Sun, MD, and Joseph Forrester, MD, with the department of surgery, Stanford (Calif.) University.
They noted that, over a 15-year period, 19 suspected iatrogenic CJD exposures were reported to the CDC – two from ophthalmology procedures, and 17 from neurosurgical procedures.
In all 19 cases, the diagnosis of CJD was unknown before the intervention, and all surgical instruments underwent normal decontamination protocols, which are inadequate to eradicate prion disease.
For patients with suspected or confirmed CJD, the World Health Organization has published infection control guidelines to prevent transmission of spongiform encephalopathies.
The guidelines recommend proper communication with all staff involved in the surgical procedure and the sterilization of supplies to be aware of potential exposure; minimizing the number of staff in the operating room; using single-use equipment whenever possible and disposing of it by incineration; using protective coverings for all nondisposable equipment; and scheduling such procedures at the end of the day to allow adequate time for decontamination.
Funding for the study was provided by the National Institutes of Health. Dr. Day owns stock in ANI Pharmaceuticals; serves as a consultant for Parabon Nanolabs, as a topic editor (dementia) for DynaMed, and as the clinical director of the Anti-NMDA Receptor Encephalitis Foundation (uncompensated). Dr. Forrester reported receiving unrestricted research funding from Varian and has received grant funding from the Surgical Infections Society.
A version of this article first appeared on Medscape.com.
About one in six patients with Creutzfeldt-Jakob disease (CJD) undergo surgery, raising the risk of iatrogenic transmission of this rare but universally fatal prion disease.
In a retrospective analysis, researchers found that 26 of 121 (21%) patients with probable or definite CJD at four U.S. academic medical centers underwent a total of 55 procedures.
These included high-risk procedures for two patients with neuropathologically proven CJD. One underwent ophthalmic artery aneurysm clipping for unruptured aneurysm, and the other underwent diagnostic brain biopsy.
“The findings were definitely surprising to me and my team – particularly the high frequency with which patients with an irreversible and particularly transmissible neurologic disease underwent invasive medical procedures either just before or shortly after the emergence of symptoms later attributed to CJD,” study investigator Gregory Day, MD, with the Mayo Clinic, Jacksonville, Fla., said in an interview.
The study was published online March 9, 2022, in JAMA Network Open.
Poor infection control
The investigators noted that the majority of CJD cases are sporadic or are inherited, but research shows that prion transmission can occur via contaminated tissues or reusable medical equipment.
While the risk of iatrogenic transmission is highest following procedures involving the central nervous system, where prion burden is highest, experimental models suggest CJD transmission can occur after contact with other tissues, including nasal mucosa, lung, lymph nodes, and spleen, the researchers noted.
“If these models are accurate, surgical procedures involving these tissues may pose a risk to patients,” the investigators wrote.
To determine the potential scope of this problem, the researchers examined the frequency of invasive procedures performed in patients with CJD at four tertiary care centers.
“In several cases, these procedures were done with clear indications [such as] fixation or joint replacement following a fracture. In several others, however, the procedures were unlikely to help the patient. For instance, a hip replacement for walking difficulties that were actually due to changes in the brain due to CJD,” Dr. Day said.
“Even more surprising was the low frequency with which appropriate surgical precautions/infection control procedures were used in patients with established diagnoses of CJD,” he noted.
Only one procedure was performed with sterilization techniques adequate to prevent CJD.
Dr. Day said the findings aren’t necessarily cause for immediate alarm, but they do highlight an area for potential improvement, including better screening of patients who have new and unexplained symptoms before proceeding with surgery, especially surgery of the central nervous system, where prion burden is high.
Another potential solution is to develop and support program surveillance and to work with public health organizations such the Centers for Disease Control and Prevention and the National Prion Disease Pathology Surveillance Center to elicit a surgical history in patients diagnosed with prion disease.
“Active nationwide surveillance is needed to determine the true scope of this potential problem and to develop strategies to mitigate the potential risk of iatrogenic prion transmission to future patients,” Dr. Day said.
True prevalence unknown
The authors of an invited commentary noted that, while most CJD infections occur sporadically, iatrogenic transmission is possible. Approximately 500 such cases have been reported worldwide to date.
“Yet, reported transmission from surgical procedures remains rare, with fewer than 10 confirmed CJD cases described in the literature, although the true prevalence is difficult to quantify as confirmed diagnosis requires autopsy,” wrote Beatrice Sun, MD, and Joseph Forrester, MD, with the department of surgery, Stanford (Calif.) University.
They noted that, over a 15-year period, 19 suspected iatrogenic CJD exposures were reported to the CDC – two from ophthalmology procedures, and 17 from neurosurgical procedures.
In all 19 cases, the diagnosis of CJD was unknown before the intervention, and all surgical instruments underwent normal decontamination protocols, which are inadequate to eradicate prion disease.
For patients with suspected or confirmed CJD, the World Health Organization has published infection control guidelines to prevent transmission of spongiform encephalopathies.
The guidelines recommend proper communication with all staff involved in the surgical procedure and the sterilization of supplies to be aware of potential exposure; minimizing the number of staff in the operating room; using single-use equipment whenever possible and disposing of it by incineration; using protective coverings for all nondisposable equipment; and scheduling such procedures at the end of the day to allow adequate time for decontamination.
Funding for the study was provided by the National Institutes of Health. Dr. Day owns stock in ANI Pharmaceuticals; serves as a consultant for Parabon Nanolabs, as a topic editor (dementia) for DynaMed, and as the clinical director of the Anti-NMDA Receptor Encephalitis Foundation (uncompensated). Dr. Forrester reported receiving unrestricted research funding from Varian and has received grant funding from the Surgical Infections Society.
A version of this article first appeared on Medscape.com.
About one in six patients with Creutzfeldt-Jakob disease (CJD) undergo surgery, raising the risk of iatrogenic transmission of this rare but universally fatal prion disease.
In a retrospective analysis, researchers found that 26 of 121 (21%) patients with probable or definite CJD at four U.S. academic medical centers underwent a total of 55 procedures.
These included high-risk procedures for two patients with neuropathologically proven CJD. One underwent ophthalmic artery aneurysm clipping for unruptured aneurysm, and the other underwent diagnostic brain biopsy.
“The findings were definitely surprising to me and my team – particularly the high frequency with which patients with an irreversible and particularly transmissible neurologic disease underwent invasive medical procedures either just before or shortly after the emergence of symptoms later attributed to CJD,” study investigator Gregory Day, MD, with the Mayo Clinic, Jacksonville, Fla., said in an interview.
The study was published online March 9, 2022, in JAMA Network Open.
Poor infection control
The investigators noted that the majority of CJD cases are sporadic or are inherited, but research shows that prion transmission can occur via contaminated tissues or reusable medical equipment.
While the risk of iatrogenic transmission is highest following procedures involving the central nervous system, where prion burden is highest, experimental models suggest CJD transmission can occur after contact with other tissues, including nasal mucosa, lung, lymph nodes, and spleen, the researchers noted.
“If these models are accurate, surgical procedures involving these tissues may pose a risk to patients,” the investigators wrote.
To determine the potential scope of this problem, the researchers examined the frequency of invasive procedures performed in patients with CJD at four tertiary care centers.
“In several cases, these procedures were done with clear indications [such as] fixation or joint replacement following a fracture. In several others, however, the procedures were unlikely to help the patient. For instance, a hip replacement for walking difficulties that were actually due to changes in the brain due to CJD,” Dr. Day said.
“Even more surprising was the low frequency with which appropriate surgical precautions/infection control procedures were used in patients with established diagnoses of CJD,” he noted.
Only one procedure was performed with sterilization techniques adequate to prevent CJD.
Dr. Day said the findings aren’t necessarily cause for immediate alarm, but they do highlight an area for potential improvement, including better screening of patients who have new and unexplained symptoms before proceeding with surgery, especially surgery of the central nervous system, where prion burden is high.
Another potential solution is to develop and support program surveillance and to work with public health organizations such the Centers for Disease Control and Prevention and the National Prion Disease Pathology Surveillance Center to elicit a surgical history in patients diagnosed with prion disease.
“Active nationwide surveillance is needed to determine the true scope of this potential problem and to develop strategies to mitigate the potential risk of iatrogenic prion transmission to future patients,” Dr. Day said.
True prevalence unknown
The authors of an invited commentary noted that, while most CJD infections occur sporadically, iatrogenic transmission is possible. Approximately 500 such cases have been reported worldwide to date.
“Yet, reported transmission from surgical procedures remains rare, with fewer than 10 confirmed CJD cases described in the literature, although the true prevalence is difficult to quantify as confirmed diagnosis requires autopsy,” wrote Beatrice Sun, MD, and Joseph Forrester, MD, with the department of surgery, Stanford (Calif.) University.
They noted that, over a 15-year period, 19 suspected iatrogenic CJD exposures were reported to the CDC – two from ophthalmology procedures, and 17 from neurosurgical procedures.
In all 19 cases, the diagnosis of CJD was unknown before the intervention, and all surgical instruments underwent normal decontamination protocols, which are inadequate to eradicate prion disease.
For patients with suspected or confirmed CJD, the World Health Organization has published infection control guidelines to prevent transmission of spongiform encephalopathies.
The guidelines recommend proper communication with all staff involved in the surgical procedure and the sterilization of supplies to be aware of potential exposure; minimizing the number of staff in the operating room; using single-use equipment whenever possible and disposing of it by incineration; using protective coverings for all nondisposable equipment; and scheduling such procedures at the end of the day to allow adequate time for decontamination.
Funding for the study was provided by the National Institutes of Health. Dr. Day owns stock in ANI Pharmaceuticals; serves as a consultant for Parabon Nanolabs, as a topic editor (dementia) for DynaMed, and as the clinical director of the Anti-NMDA Receptor Encephalitis Foundation (uncompensated). Dr. Forrester reported receiving unrestricted research funding from Varian and has received grant funding from the Surgical Infections Society.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Novel isotretinoin ointment for congenital ichthyosis shows promise
BOSTON – , results from a phase 2b study demonstrated.
“Patients with these deficiencies have generally had very limited treatment options, including lifelong use of emollients and keratolytics, and in severe cases, systemic retinoids,” Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said at a late-breaking abstract session at the annual meeting of the American Academy of Dermatology. “There is currently no [Food and Drug Administration]-approved drug for CI. So, imagine your patients and their parents, and the frustration they must feel.”
In a study known as CONTROL, he and his colleagues evaluated the effect of TMB-001 on two subtypes of congenital ichthyosis: X-linked recessive ichthyosis (XLRI) and autosomal recessive congenital ichthyosis–lamellar ichthyosis (ARCI-LI). Of the two, the most common is XLRI, which has an estimated incidence of 1:3,000 and is caused by a deficiency of steroid sulfatase, resulting in cholesterol sulfate accumulation in the stratum corneum, retained corneodesmosomes, and reduced corneocyte desquamation, Dr. Bunick said.
ARCI-LI is rarer, with a prevalence of 1:100,000, and has been linked to mutations in six genes, most commonly TGM1, resulting in enzyme inactivation and deficient cross-linking of cornified cell envelope proteins.
TMB-001 is a proprietary, novel, topical isotretinoin formulation to treat CI that is being developed by Timber Pharmaceuticals. It uses a patented “IPEG” technology isotretinoin delivery system designed specifically for patients with CI. In a prior phase 2a study, TMB-001 0.1% and 0.2% ointment twice a day demonstrated greater improvement in ≥ 1 and ≥ 2 Investigator Global Assessment (IGA) scores compared with vehicle. Scaling in all patients treated with TMB-001 was considered clear, almost clear, or mild at 8 weeks, and no concerning safety signals were observed.
For the current trial, 33 patients with genetically confirmed XLRI/ARCI-LI and ≥ 2 (out of 4) Visual Index for Ichthyosis Severity (VIIS) assessment areas with a ≥ 3 scaling score were randomized 1:1:1 to TMB-001 0.05%, TMB-001 0.1%, or vehicle twice daily for 12 weeks. Primary and secondary efficacy endpoints were reduction of ≥ 50% compared with baseline in VIIS-scaling (VIIS-50) and a ≥ 2-grade reduction in the Investigator Global Assessment (IGA)–scaling score compared with baseline. The patients ranged in age from 9 to 80 years, the majority were White, and their baseline body surface area (BSA) affected ranged from 28% to 38%.
Of the 33 patients, 11 patients received TMB-001 0.05%, 10 received TMB-001 0.1%, and 12 received the vehicle.
Among all patients, 55% had ARCI-LI and 45% had XLRI subtypes, and those with ARCI-LI had greater prior use of corticosteroid, emollient, and oral/topical retinoids. Overall, 100%, 50%, and 75% of patients with XLRI and 100%, 33%, and 17% of patients with ARCI-LI achieved VIIS-50 after receiving TMB-001 0.05%, TMB-001 0.1%, and vehicle, respectively.
An improvement of a ≥ 2-grade IGA score was observed in 100%, 50%, and 25% of patients with XLRI and 100%, 67%, and none of patients with ARCI-LI who received TMB-001 0.05%, TMB-001 0.1%, and vehicle, respectively.
Dr. Bunick reported that there were no serious adverse events, no hospitalizations, and no patient deaths. Six patients discontinued treatment, five because of participant withdrawal and one because of physician withdrawal. The four most common treatment-emergent adverse events were erythema (21%), pruritus (21%), pain (15%) and dermatitis (12%).
“These results support ongoing investigation of TMB-001 as a promising alternative to systemic retinoids for participants with CI,” Dr. Bunick concluded. He noted that while he is not privy to details of TMB-001’s IPEG delivery system, “the way they have used polyethylene glycol to encapsulate the isotretinoin allows for greater barrier penetration and reduces a lot of the tolerability issues that are seen with other topical retinoids.” In his view, “that is providing this retinoid a greater chance of success. The patented delivery system is not only designed to help the isotretinoin do its job, but also to provide that stability and the ability to compound it, which have been barriers to success in the past.”
Phase 3 trials of the agent are scheduled to begin in June of 2022.
Amy S. Paller, MD, professor and chair of the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that she was impressed that no significant changes from baseline laboratory clinical assessments were observed. “If that’s true, then we don’t have to be monitoring these patients in the same way as with systemic agents,” said Dr. Paller, who was involved in the phase 2a proof-of-concept trial of TMB-001. “I think that deserves more investigation. Hopefully that will be looked at in the phase 3 trial.”
Dr. Bunick reported having no disclosures related to his presentation. Dr. Paller disclosed that she is consultant to and/or an investigator for numerous pharmaceutical companies.
*A change correcting the age range of the patients in the study was made on 3/29/22.
BOSTON – , results from a phase 2b study demonstrated.
“Patients with these deficiencies have generally had very limited treatment options, including lifelong use of emollients and keratolytics, and in severe cases, systemic retinoids,” Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said at a late-breaking abstract session at the annual meeting of the American Academy of Dermatology. “There is currently no [Food and Drug Administration]-approved drug for CI. So, imagine your patients and their parents, and the frustration they must feel.”
In a study known as CONTROL, he and his colleagues evaluated the effect of TMB-001 on two subtypes of congenital ichthyosis: X-linked recessive ichthyosis (XLRI) and autosomal recessive congenital ichthyosis–lamellar ichthyosis (ARCI-LI). Of the two, the most common is XLRI, which has an estimated incidence of 1:3,000 and is caused by a deficiency of steroid sulfatase, resulting in cholesterol sulfate accumulation in the stratum corneum, retained corneodesmosomes, and reduced corneocyte desquamation, Dr. Bunick said.
ARCI-LI is rarer, with a prevalence of 1:100,000, and has been linked to mutations in six genes, most commonly TGM1, resulting in enzyme inactivation and deficient cross-linking of cornified cell envelope proteins.
TMB-001 is a proprietary, novel, topical isotretinoin formulation to treat CI that is being developed by Timber Pharmaceuticals. It uses a patented “IPEG” technology isotretinoin delivery system designed specifically for patients with CI. In a prior phase 2a study, TMB-001 0.1% and 0.2% ointment twice a day demonstrated greater improvement in ≥ 1 and ≥ 2 Investigator Global Assessment (IGA) scores compared with vehicle. Scaling in all patients treated with TMB-001 was considered clear, almost clear, or mild at 8 weeks, and no concerning safety signals were observed.
For the current trial, 33 patients with genetically confirmed XLRI/ARCI-LI and ≥ 2 (out of 4) Visual Index for Ichthyosis Severity (VIIS) assessment areas with a ≥ 3 scaling score were randomized 1:1:1 to TMB-001 0.05%, TMB-001 0.1%, or vehicle twice daily for 12 weeks. Primary and secondary efficacy endpoints were reduction of ≥ 50% compared with baseline in VIIS-scaling (VIIS-50) and a ≥ 2-grade reduction in the Investigator Global Assessment (IGA)–scaling score compared with baseline. The patients ranged in age from 9 to 80 years, the majority were White, and their baseline body surface area (BSA) affected ranged from 28% to 38%.
Of the 33 patients, 11 patients received TMB-001 0.05%, 10 received TMB-001 0.1%, and 12 received the vehicle.
Among all patients, 55% had ARCI-LI and 45% had XLRI subtypes, and those with ARCI-LI had greater prior use of corticosteroid, emollient, and oral/topical retinoids. Overall, 100%, 50%, and 75% of patients with XLRI and 100%, 33%, and 17% of patients with ARCI-LI achieved VIIS-50 after receiving TMB-001 0.05%, TMB-001 0.1%, and vehicle, respectively.
An improvement of a ≥ 2-grade IGA score was observed in 100%, 50%, and 25% of patients with XLRI and 100%, 67%, and none of patients with ARCI-LI who received TMB-001 0.05%, TMB-001 0.1%, and vehicle, respectively.
Dr. Bunick reported that there were no serious adverse events, no hospitalizations, and no patient deaths. Six patients discontinued treatment, five because of participant withdrawal and one because of physician withdrawal. The four most common treatment-emergent adverse events were erythema (21%), pruritus (21%), pain (15%) and dermatitis (12%).
“These results support ongoing investigation of TMB-001 as a promising alternative to systemic retinoids for participants with CI,” Dr. Bunick concluded. He noted that while he is not privy to details of TMB-001’s IPEG delivery system, “the way they have used polyethylene glycol to encapsulate the isotretinoin allows for greater barrier penetration and reduces a lot of the tolerability issues that are seen with other topical retinoids.” In his view, “that is providing this retinoid a greater chance of success. The patented delivery system is not only designed to help the isotretinoin do its job, but also to provide that stability and the ability to compound it, which have been barriers to success in the past.”
Phase 3 trials of the agent are scheduled to begin in June of 2022.
Amy S. Paller, MD, professor and chair of the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that she was impressed that no significant changes from baseline laboratory clinical assessments were observed. “If that’s true, then we don’t have to be monitoring these patients in the same way as with systemic agents,” said Dr. Paller, who was involved in the phase 2a proof-of-concept trial of TMB-001. “I think that deserves more investigation. Hopefully that will be looked at in the phase 3 trial.”
Dr. Bunick reported having no disclosures related to his presentation. Dr. Paller disclosed that she is consultant to and/or an investigator for numerous pharmaceutical companies.
*A change correcting the age range of the patients in the study was made on 3/29/22.
BOSTON – , results from a phase 2b study demonstrated.
“Patients with these deficiencies have generally had very limited treatment options, including lifelong use of emollients and keratolytics, and in severe cases, systemic retinoids,” Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Conn., said at a late-breaking abstract session at the annual meeting of the American Academy of Dermatology. “There is currently no [Food and Drug Administration]-approved drug for CI. So, imagine your patients and their parents, and the frustration they must feel.”
In a study known as CONTROL, he and his colleagues evaluated the effect of TMB-001 on two subtypes of congenital ichthyosis: X-linked recessive ichthyosis (XLRI) and autosomal recessive congenital ichthyosis–lamellar ichthyosis (ARCI-LI). Of the two, the most common is XLRI, which has an estimated incidence of 1:3,000 and is caused by a deficiency of steroid sulfatase, resulting in cholesterol sulfate accumulation in the stratum corneum, retained corneodesmosomes, and reduced corneocyte desquamation, Dr. Bunick said.
ARCI-LI is rarer, with a prevalence of 1:100,000, and has been linked to mutations in six genes, most commonly TGM1, resulting in enzyme inactivation and deficient cross-linking of cornified cell envelope proteins.
TMB-001 is a proprietary, novel, topical isotretinoin formulation to treat CI that is being developed by Timber Pharmaceuticals. It uses a patented “IPEG” technology isotretinoin delivery system designed specifically for patients with CI. In a prior phase 2a study, TMB-001 0.1% and 0.2% ointment twice a day demonstrated greater improvement in ≥ 1 and ≥ 2 Investigator Global Assessment (IGA) scores compared with vehicle. Scaling in all patients treated with TMB-001 was considered clear, almost clear, or mild at 8 weeks, and no concerning safety signals were observed.
For the current trial, 33 patients with genetically confirmed XLRI/ARCI-LI and ≥ 2 (out of 4) Visual Index for Ichthyosis Severity (VIIS) assessment areas with a ≥ 3 scaling score were randomized 1:1:1 to TMB-001 0.05%, TMB-001 0.1%, or vehicle twice daily for 12 weeks. Primary and secondary efficacy endpoints were reduction of ≥ 50% compared with baseline in VIIS-scaling (VIIS-50) and a ≥ 2-grade reduction in the Investigator Global Assessment (IGA)–scaling score compared with baseline. The patients ranged in age from 9 to 80 years, the majority were White, and their baseline body surface area (BSA) affected ranged from 28% to 38%.
Of the 33 patients, 11 patients received TMB-001 0.05%, 10 received TMB-001 0.1%, and 12 received the vehicle.
Among all patients, 55% had ARCI-LI and 45% had XLRI subtypes, and those with ARCI-LI had greater prior use of corticosteroid, emollient, and oral/topical retinoids. Overall, 100%, 50%, and 75% of patients with XLRI and 100%, 33%, and 17% of patients with ARCI-LI achieved VIIS-50 after receiving TMB-001 0.05%, TMB-001 0.1%, and vehicle, respectively.
An improvement of a ≥ 2-grade IGA score was observed in 100%, 50%, and 25% of patients with XLRI and 100%, 67%, and none of patients with ARCI-LI who received TMB-001 0.05%, TMB-001 0.1%, and vehicle, respectively.
Dr. Bunick reported that there were no serious adverse events, no hospitalizations, and no patient deaths. Six patients discontinued treatment, five because of participant withdrawal and one because of physician withdrawal. The four most common treatment-emergent adverse events were erythema (21%), pruritus (21%), pain (15%) and dermatitis (12%).
“These results support ongoing investigation of TMB-001 as a promising alternative to systemic retinoids for participants with CI,” Dr. Bunick concluded. He noted that while he is not privy to details of TMB-001’s IPEG delivery system, “the way they have used polyethylene glycol to encapsulate the isotretinoin allows for greater barrier penetration and reduces a lot of the tolerability issues that are seen with other topical retinoids.” In his view, “that is providing this retinoid a greater chance of success. The patented delivery system is not only designed to help the isotretinoin do its job, but also to provide that stability and the ability to compound it, which have been barriers to success in the past.”
Phase 3 trials of the agent are scheduled to begin in June of 2022.
Amy S. Paller, MD, professor and chair of the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that she was impressed that no significant changes from baseline laboratory clinical assessments were observed. “If that’s true, then we don’t have to be monitoring these patients in the same way as with systemic agents,” said Dr. Paller, who was involved in the phase 2a proof-of-concept trial of TMB-001. “I think that deserves more investigation. Hopefully that will be looked at in the phase 3 trial.”
Dr. Bunick reported having no disclosures related to his presentation. Dr. Paller disclosed that she is consultant to and/or an investigator for numerous pharmaceutical companies.
*A change correcting the age range of the patients in the study was made on 3/29/22.
AT AAD 2022
Brain implant allows fully paralyzed patient to communicate
Using a commercially available implant and newly designed software, the patient, who was in the advanced stages of Lou Gehrig’s disease and unable to move his eyes, was able to interact with researchers and caregivers, requesting goulash, beer, and music from the band Tool, thanking the researchers who developed the technology and inviting his 4-year-old son to watch a Disney film.
The investigators note the study shows for the first time that communication is possible in patients in a completely locked-in state (CLIS) and offers hope for a better quality of life in this population.
“It should encourage them to live after artificial respiration and to ask for brain-computer interfaces before they become CLIS,” study investigator Niels Birbaumer, PhD, a professor emeritus of the University of Tübingen, Germany, said in an interview. The study was published online March 22 in Nature Communications.
Although the findings appear promising, they build on previous research that was the subject of a 2019 investigation by the largest grant-funding agency in Germany. This controversy prompted the institute that led the current research to appoint an independent expert to audit and monitor the new study.
Mechanism a ‘mystery’
Use of brain-computer interface (BCI) technology to allow ALS patients to communicate has increased in recent years. BCIs capture brain signals, transmit them to a computer, and convert them into a command that the computer carries out.
Previous research shows patients with ALS who retain eye movement and control have been able to use BCIs to communicate. However, until now, the technology has not worked as well in CLIS patients, who have full-body paralysis.
In 2019, German and Swiss researchers implanted two 64-microde arrays in the brain of a 34-year-old patient who was diagnosed with ALS in 2015.
The electrodes measure neuronal activity while an amplifier located on the outside of the patient’s skull amplifies the signals to a computer. Software created by the research team decodes the signals and translates them into commands.
Using an auditory feedback system, the patient was able to use his mind to modulate the pitch of a tone to either high (meaning “yes”) or low (meaning “no.”) Just how the brain does this is a mystery, Dr. Birbaumer said.
A speller program reads letters aloud, first in groups and then individually. When a group contained letters the patient needed to spell a word, he used auditory feedback to select the high-pitch tone.
Initially, the patient was able to correctly spell his name. Ultimately, he was able to form complete sentences. The patient correctly spelled words on 44 of the 107 days in that phase of the experiment, spelling an average of just one character per minute.
Still, the researchers note he was able to interact with his caretakers, family, and researchers, even offering input on changes to make the device more effective.
Controversial history
In 2017, Dr. Birbaumer and Ujwal Chaudhary, PhD, who is the lead author on this current study, published a study in PLOS Biology. That research analyzed a brain-monitoring technique that the scientists claimed enabled patients with ALS who were completely locked in to answer yes or no questions correctly.
Allegations from a whistleblower at the University of Tübingen, where Dr. Birbaumer was a senior professor and Dr. Chaudhary was a postdoctoral researcher, prompted an investigation by the Deutsche Forschungsgemeinschaft, or German Research Foundation (DFG).
The whistleblower claimed that the 2017 paper and a second study published in 2019 contained incomplete data and misrepresented the findings. The DFG investigation found evidence of scientific misconduct and required that Dr. Birbaumer return the grant he had received for the research. The agency also banned Dr. Birbaumer from applying for grants or serving as a grant reviewer for 5 years. Dr. Chaudhary was banned for 3 years. PLOS Biology later retracted the papers.
Both researchers have refuted the allegations and have reportedly sued the German Research Foundation.
“We have no information about the status of our lawsuit against the DFG; it’s still pending,” Dr. Birbaumer told this news organization. “I hope they investigate our present study because the study of 2017 they did not investigate carefully enough.”
Results ‘not stunningly good’
The controversial history prompted the Wyss Center, Geneva, which led this new study, to seek out at an independent BCI expert to audit and monitor the study.
Nick Ramsey, PhD, a professor of cognitive neuroscience at the Brain Center of the University Medical Center Utrecht, the Netherlands, agreed to take on the assignment in March 2020.
Dr. Ramsey has also conducted research on BCI in patients with ALS, but his work has not included patients in CLIS.
“I judged the study to be compliant with universal standards of scientific integrity,” Dr. Ramsey told this news organization. “I am confident that the data and results presented in the paper are valid and will withstand academic and medical scrutiny.”
Commenting on the new findings, Dr. Ramsey noted that the results of the study are “not stunningly good, as the user could only communicate during a limited number of days, and even then with considerable slowness,” Dr. Ramsey said. However, he added that the study does provide proof of principle that communication is possible in CLIS patients.
“The question remains whether a BCI implant continues to work well in these patients, as there are some indications that people in such a state may lose their mental capabilities within months or a few years as a result of the disease and can thus no longer generate a wish to communicate,” Dr. Ramsey said.
Responding to a query from this news organization, a spokesperson for Nature Communications declined to comment on the new study but said that journal editors are “are alert to controversies within each field and take care when considering submissions during the peer-review process.”
“We have rigorous policies to safeguard the integrity of the research we publish,” the spokesperson continued, “including to ensure that research has been conducted to a high ethical standard and is reported transparently.”
The research was funded by Wyss Center for Bio and Neuroengineering, Geneva and Deutsche Forschungsgemeinschaft. The authors have disclosed no relevant financial relationships. Dr. Ramsey received payment from the Wyss Center for his advisory role in this project.
A version of this article first appeared on Medscape.com.
Using a commercially available implant and newly designed software, the patient, who was in the advanced stages of Lou Gehrig’s disease and unable to move his eyes, was able to interact with researchers and caregivers, requesting goulash, beer, and music from the band Tool, thanking the researchers who developed the technology and inviting his 4-year-old son to watch a Disney film.
The investigators note the study shows for the first time that communication is possible in patients in a completely locked-in state (CLIS) and offers hope for a better quality of life in this population.
“It should encourage them to live after artificial respiration and to ask for brain-computer interfaces before they become CLIS,” study investigator Niels Birbaumer, PhD, a professor emeritus of the University of Tübingen, Germany, said in an interview. The study was published online March 22 in Nature Communications.
Although the findings appear promising, they build on previous research that was the subject of a 2019 investigation by the largest grant-funding agency in Germany. This controversy prompted the institute that led the current research to appoint an independent expert to audit and monitor the new study.
Mechanism a ‘mystery’
Use of brain-computer interface (BCI) technology to allow ALS patients to communicate has increased in recent years. BCIs capture brain signals, transmit them to a computer, and convert them into a command that the computer carries out.
Previous research shows patients with ALS who retain eye movement and control have been able to use BCIs to communicate. However, until now, the technology has not worked as well in CLIS patients, who have full-body paralysis.
In 2019, German and Swiss researchers implanted two 64-microde arrays in the brain of a 34-year-old patient who was diagnosed with ALS in 2015.
The electrodes measure neuronal activity while an amplifier located on the outside of the patient’s skull amplifies the signals to a computer. Software created by the research team decodes the signals and translates them into commands.
Using an auditory feedback system, the patient was able to use his mind to modulate the pitch of a tone to either high (meaning “yes”) or low (meaning “no.”) Just how the brain does this is a mystery, Dr. Birbaumer said.
A speller program reads letters aloud, first in groups and then individually. When a group contained letters the patient needed to spell a word, he used auditory feedback to select the high-pitch tone.
Initially, the patient was able to correctly spell his name. Ultimately, he was able to form complete sentences. The patient correctly spelled words on 44 of the 107 days in that phase of the experiment, spelling an average of just one character per minute.
Still, the researchers note he was able to interact with his caretakers, family, and researchers, even offering input on changes to make the device more effective.
Controversial history
In 2017, Dr. Birbaumer and Ujwal Chaudhary, PhD, who is the lead author on this current study, published a study in PLOS Biology. That research analyzed a brain-monitoring technique that the scientists claimed enabled patients with ALS who were completely locked in to answer yes or no questions correctly.
Allegations from a whistleblower at the University of Tübingen, where Dr. Birbaumer was a senior professor and Dr. Chaudhary was a postdoctoral researcher, prompted an investigation by the Deutsche Forschungsgemeinschaft, or German Research Foundation (DFG).
The whistleblower claimed that the 2017 paper and a second study published in 2019 contained incomplete data and misrepresented the findings. The DFG investigation found evidence of scientific misconduct and required that Dr. Birbaumer return the grant he had received for the research. The agency also banned Dr. Birbaumer from applying for grants or serving as a grant reviewer for 5 years. Dr. Chaudhary was banned for 3 years. PLOS Biology later retracted the papers.
Both researchers have refuted the allegations and have reportedly sued the German Research Foundation.
“We have no information about the status of our lawsuit against the DFG; it’s still pending,” Dr. Birbaumer told this news organization. “I hope they investigate our present study because the study of 2017 they did not investigate carefully enough.”
Results ‘not stunningly good’
The controversial history prompted the Wyss Center, Geneva, which led this new study, to seek out at an independent BCI expert to audit and monitor the study.
Nick Ramsey, PhD, a professor of cognitive neuroscience at the Brain Center of the University Medical Center Utrecht, the Netherlands, agreed to take on the assignment in March 2020.
Dr. Ramsey has also conducted research on BCI in patients with ALS, but his work has not included patients in CLIS.
“I judged the study to be compliant with universal standards of scientific integrity,” Dr. Ramsey told this news organization. “I am confident that the data and results presented in the paper are valid and will withstand academic and medical scrutiny.”
Commenting on the new findings, Dr. Ramsey noted that the results of the study are “not stunningly good, as the user could only communicate during a limited number of days, and even then with considerable slowness,” Dr. Ramsey said. However, he added that the study does provide proof of principle that communication is possible in CLIS patients.
“The question remains whether a BCI implant continues to work well in these patients, as there are some indications that people in such a state may lose their mental capabilities within months or a few years as a result of the disease and can thus no longer generate a wish to communicate,” Dr. Ramsey said.
Responding to a query from this news organization, a spokesperson for Nature Communications declined to comment on the new study but said that journal editors are “are alert to controversies within each field and take care when considering submissions during the peer-review process.”
“We have rigorous policies to safeguard the integrity of the research we publish,” the spokesperson continued, “including to ensure that research has been conducted to a high ethical standard and is reported transparently.”
The research was funded by Wyss Center for Bio and Neuroengineering, Geneva and Deutsche Forschungsgemeinschaft. The authors have disclosed no relevant financial relationships. Dr. Ramsey received payment from the Wyss Center for his advisory role in this project.
A version of this article first appeared on Medscape.com.
Using a commercially available implant and newly designed software, the patient, who was in the advanced stages of Lou Gehrig’s disease and unable to move his eyes, was able to interact with researchers and caregivers, requesting goulash, beer, and music from the band Tool, thanking the researchers who developed the technology and inviting his 4-year-old son to watch a Disney film.
The investigators note the study shows for the first time that communication is possible in patients in a completely locked-in state (CLIS) and offers hope for a better quality of life in this population.
“It should encourage them to live after artificial respiration and to ask for brain-computer interfaces before they become CLIS,” study investigator Niels Birbaumer, PhD, a professor emeritus of the University of Tübingen, Germany, said in an interview. The study was published online March 22 in Nature Communications.
Although the findings appear promising, they build on previous research that was the subject of a 2019 investigation by the largest grant-funding agency in Germany. This controversy prompted the institute that led the current research to appoint an independent expert to audit and monitor the new study.
Mechanism a ‘mystery’
Use of brain-computer interface (BCI) technology to allow ALS patients to communicate has increased in recent years. BCIs capture brain signals, transmit them to a computer, and convert them into a command that the computer carries out.
Previous research shows patients with ALS who retain eye movement and control have been able to use BCIs to communicate. However, until now, the technology has not worked as well in CLIS patients, who have full-body paralysis.
In 2019, German and Swiss researchers implanted two 64-microde arrays in the brain of a 34-year-old patient who was diagnosed with ALS in 2015.
The electrodes measure neuronal activity while an amplifier located on the outside of the patient’s skull amplifies the signals to a computer. Software created by the research team decodes the signals and translates them into commands.
Using an auditory feedback system, the patient was able to use his mind to modulate the pitch of a tone to either high (meaning “yes”) or low (meaning “no.”) Just how the brain does this is a mystery, Dr. Birbaumer said.
A speller program reads letters aloud, first in groups and then individually. When a group contained letters the patient needed to spell a word, he used auditory feedback to select the high-pitch tone.
Initially, the patient was able to correctly spell his name. Ultimately, he was able to form complete sentences. The patient correctly spelled words on 44 of the 107 days in that phase of the experiment, spelling an average of just one character per minute.
Still, the researchers note he was able to interact with his caretakers, family, and researchers, even offering input on changes to make the device more effective.
Controversial history
In 2017, Dr. Birbaumer and Ujwal Chaudhary, PhD, who is the lead author on this current study, published a study in PLOS Biology. That research analyzed a brain-monitoring technique that the scientists claimed enabled patients with ALS who were completely locked in to answer yes or no questions correctly.
Allegations from a whistleblower at the University of Tübingen, where Dr. Birbaumer was a senior professor and Dr. Chaudhary was a postdoctoral researcher, prompted an investigation by the Deutsche Forschungsgemeinschaft, or German Research Foundation (DFG).
The whistleblower claimed that the 2017 paper and a second study published in 2019 contained incomplete data and misrepresented the findings. The DFG investigation found evidence of scientific misconduct and required that Dr. Birbaumer return the grant he had received for the research. The agency also banned Dr. Birbaumer from applying for grants or serving as a grant reviewer for 5 years. Dr. Chaudhary was banned for 3 years. PLOS Biology later retracted the papers.
Both researchers have refuted the allegations and have reportedly sued the German Research Foundation.
“We have no information about the status of our lawsuit against the DFG; it’s still pending,” Dr. Birbaumer told this news organization. “I hope they investigate our present study because the study of 2017 they did not investigate carefully enough.”
Results ‘not stunningly good’
The controversial history prompted the Wyss Center, Geneva, which led this new study, to seek out at an independent BCI expert to audit and monitor the study.
Nick Ramsey, PhD, a professor of cognitive neuroscience at the Brain Center of the University Medical Center Utrecht, the Netherlands, agreed to take on the assignment in March 2020.
Dr. Ramsey has also conducted research on BCI in patients with ALS, but his work has not included patients in CLIS.
“I judged the study to be compliant with universal standards of scientific integrity,” Dr. Ramsey told this news organization. “I am confident that the data and results presented in the paper are valid and will withstand academic and medical scrutiny.”
Commenting on the new findings, Dr. Ramsey noted that the results of the study are “not stunningly good, as the user could only communicate during a limited number of days, and even then with considerable slowness,” Dr. Ramsey said. However, he added that the study does provide proof of principle that communication is possible in CLIS patients.
“The question remains whether a BCI implant continues to work well in these patients, as there are some indications that people in such a state may lose their mental capabilities within months or a few years as a result of the disease and can thus no longer generate a wish to communicate,” Dr. Ramsey said.
Responding to a query from this news organization, a spokesperson for Nature Communications declined to comment on the new study but said that journal editors are “are alert to controversies within each field and take care when considering submissions during the peer-review process.”
“We have rigorous policies to safeguard the integrity of the research we publish,” the spokesperson continued, “including to ensure that research has been conducted to a high ethical standard and is reported transparently.”
The research was funded by Wyss Center for Bio and Neuroengineering, Geneva and Deutsche Forschungsgemeinschaft. The authors have disclosed no relevant financial relationships. Dr. Ramsey received payment from the Wyss Center for his advisory role in this project.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Kawasaki disease guideline highlights rheumatology angles
All Kawasaki disease (KD) patients should be treated first with intravenous immunoglobulin, according to an updated guideline issued jointly by the American College of Rheumatology and the Vasculitis Foundation.
KD has low mortality when treated appropriately, guideline first author Mark Gorelik, MD, assistant professor of pediatrics at Columbia University, New York, and colleagues wrote.
The update is important at this time because new evidence continues to emerge in the clinical management of KD, Dr. Gorelik said in an interview.
“In addition, this guideline approaches Kawasaki disease from a perspective of acting as an adjunct to the already existing and excellent American Heart Association guidelines by adding information in areas that rheumatologists may play a role,” Dr. Gorelik said. “This is specifically regarding patients who may require additional therapy beyond standard IVIg, such as patients who may be at higher risk of morbidity from disease and patients who have refractory disease,” he explained.
The guideline, published in Arthritis & Rheumatology, includes 11 recommendations, 1 good practice statement, and 1 ungraded position statement. The good practice statement emphasizes that all patients with KD should be initially treated with IVIg.
The position statement advises that either nonglucocorticoid immunosuppressive therapy or glucocorticoids may be used for patients with acute KD whose fever persists despite repeated IVIg treatment. No clinical evidence currently supports the superiority of either nonglucocorticoid immunosuppressive therapy or glucocorticoids; therefore, the authors support the use of either based on what is appropriate in any given clinical situation. Although optimal dosage and duration of glucocorticoids have yet to be determined in a U.S. population, the authors described a typical glucocorticoid dosage as starting prednisone at 2 mg/kg per day, with a maximum of 60 mg/day, and dose tapering over 15 days.
The 11 recommendations consist of 7 strong and 4 conditional recommendations. The strong recommendations focus on prompt treatment of incomplete KD, treatment with aspirin, and obtaining an echocardiogram in patients with unexplained macrophage activation syndrome or shock. The conditional recommendations support using established therapy promptly at disease onset, then identifying cases in which additional therapy is needed.
Dr. Gorelik highlighted four clinical takeaways from the guideline. First, “patients with higher risk for complications do exist in Kawasaki disease, and that these patients can be treated more aggressively,” he said. “Specifically, patients with aneurysms seen at first ultrasound, and patients who are under 6 months, are more likely to have progressive and/or refractory disease; these patients can be treated with an adjunctive short course of corticosteroids.”
Second, “the use of high-dose aspirin for patients with Kawasaki disease does not have strong basis in evidence. While aspirin itself of some dose is necessary for patients with Kawasaki disease, use of either high- or low-dose aspirin has the same outcome for patients, and a physician may choose either of these in practice,” he said.
Third, “we continue to recommend that refractory patients with Kawasaki disease be treated with a second dose of IVIg; however, there are many scenarios in which a physician may choose either corticosteroids [either a single high dose of >10 mg/kg, or a short moderate-dose course of 2 mg/kg per day for 5-7 days] or a biologic agent such as infliximab. ... These are valid choices for therapy in patients with refractory Kawasaki disease,” he emphasized.
Fourth, “physicians should discard the idea of treating before [and conversely, not treating after] 10 days of fever,” Dr. Gorelik said. “Patients with Kawasaki disease should be treated as soon as the diagnosis is made, regardless of whether this patient is on day 5, day 12, or day 20 of symptoms.”
Update incorporates emerging evidence
Potential barriers to implementing the guideline in practice include the challenge of weaning doctors from practices that are habitual in medicine, Dr. Gorelik said. “One of these is the use of high-dose aspirin for Kawasaki disease; a number of studies have shown over the past decade or more that high-dose aspirin has no greater effect than lower-dose aspirin for Kawasaki disease. Despite all of these studies, the use of high-dose aspirin continued. High-dose aspirin for Kawasaki disease was used in the era prior to use of IVIg as an anti-inflammatory agent. However, it has poor efficacy in this regard, and the true benefit for aspirin is for anticoagulation for patients at risk of a clot, and this is just as effective in lower doses. Expressing this in a guideline could help to change practices by helping physicians understand not only what they are guided to do, but why.”
Additional research is needed to better identify high-risk patients in non-Japanese populations, he noted. “While studies from Japan suggest that higher-risk patients can be identified based on various parameters, these have not been well replicated in non-Japanese populations. Good research that identifies which patients may be more at risk in other populations would be helpful to more precisely target high-risk therapy.”
Other research needs include a clearer understanding of the best therapies for refractory patients, Dr. Gorelik said. “One area of the most difficulty was determining whether patients with refractory disease should have repeated IVIg or a switch to glucocorticoids and biologic agents. Some of this research is underway, and some was published just as these guidelines were being drawn, and this particular area is one that is likely to change significantly. While currently we recommend a repeated dose of IVIg, it is likely that over the very near term, the use of repeated IVIg in KD will be curtailed” because of concerns such as the relatively high rate of hemolysis. Research to identify which therapy has a noninferior effect with a superior risk profile is needed; such research “will likely result in a future iteration of these guidelines specifically related to this question,” he concluded.
The KD guideline is the final companion to three additional ACR/VF vasculitis guidelines that were released in July 2021. The guideline research received no outside funding. The researchers had no financial conflicts to disclose.
All Kawasaki disease (KD) patients should be treated first with intravenous immunoglobulin, according to an updated guideline issued jointly by the American College of Rheumatology and the Vasculitis Foundation.
KD has low mortality when treated appropriately, guideline first author Mark Gorelik, MD, assistant professor of pediatrics at Columbia University, New York, and colleagues wrote.
The update is important at this time because new evidence continues to emerge in the clinical management of KD, Dr. Gorelik said in an interview.
“In addition, this guideline approaches Kawasaki disease from a perspective of acting as an adjunct to the already existing and excellent American Heart Association guidelines by adding information in areas that rheumatologists may play a role,” Dr. Gorelik said. “This is specifically regarding patients who may require additional therapy beyond standard IVIg, such as patients who may be at higher risk of morbidity from disease and patients who have refractory disease,” he explained.
The guideline, published in Arthritis & Rheumatology, includes 11 recommendations, 1 good practice statement, and 1 ungraded position statement. The good practice statement emphasizes that all patients with KD should be initially treated with IVIg.
The position statement advises that either nonglucocorticoid immunosuppressive therapy or glucocorticoids may be used for patients with acute KD whose fever persists despite repeated IVIg treatment. No clinical evidence currently supports the superiority of either nonglucocorticoid immunosuppressive therapy or glucocorticoids; therefore, the authors support the use of either based on what is appropriate in any given clinical situation. Although optimal dosage and duration of glucocorticoids have yet to be determined in a U.S. population, the authors described a typical glucocorticoid dosage as starting prednisone at 2 mg/kg per day, with a maximum of 60 mg/day, and dose tapering over 15 days.
The 11 recommendations consist of 7 strong and 4 conditional recommendations. The strong recommendations focus on prompt treatment of incomplete KD, treatment with aspirin, and obtaining an echocardiogram in patients with unexplained macrophage activation syndrome or shock. The conditional recommendations support using established therapy promptly at disease onset, then identifying cases in which additional therapy is needed.
Dr. Gorelik highlighted four clinical takeaways from the guideline. First, “patients with higher risk for complications do exist in Kawasaki disease, and that these patients can be treated more aggressively,” he said. “Specifically, patients with aneurysms seen at first ultrasound, and patients who are under 6 months, are more likely to have progressive and/or refractory disease; these patients can be treated with an adjunctive short course of corticosteroids.”
Second, “the use of high-dose aspirin for patients with Kawasaki disease does not have strong basis in evidence. While aspirin itself of some dose is necessary for patients with Kawasaki disease, use of either high- or low-dose aspirin has the same outcome for patients, and a physician may choose either of these in practice,” he said.
Third, “we continue to recommend that refractory patients with Kawasaki disease be treated with a second dose of IVIg; however, there are many scenarios in which a physician may choose either corticosteroids [either a single high dose of >10 mg/kg, or a short moderate-dose course of 2 mg/kg per day for 5-7 days] or a biologic agent such as infliximab. ... These are valid choices for therapy in patients with refractory Kawasaki disease,” he emphasized.
Fourth, “physicians should discard the idea of treating before [and conversely, not treating after] 10 days of fever,” Dr. Gorelik said. “Patients with Kawasaki disease should be treated as soon as the diagnosis is made, regardless of whether this patient is on day 5, day 12, or day 20 of symptoms.”
Update incorporates emerging evidence
Potential barriers to implementing the guideline in practice include the challenge of weaning doctors from practices that are habitual in medicine, Dr. Gorelik said. “One of these is the use of high-dose aspirin for Kawasaki disease; a number of studies have shown over the past decade or more that high-dose aspirin has no greater effect than lower-dose aspirin for Kawasaki disease. Despite all of these studies, the use of high-dose aspirin continued. High-dose aspirin for Kawasaki disease was used in the era prior to use of IVIg as an anti-inflammatory agent. However, it has poor efficacy in this regard, and the true benefit for aspirin is for anticoagulation for patients at risk of a clot, and this is just as effective in lower doses. Expressing this in a guideline could help to change practices by helping physicians understand not only what they are guided to do, but why.”
Additional research is needed to better identify high-risk patients in non-Japanese populations, he noted. “While studies from Japan suggest that higher-risk patients can be identified based on various parameters, these have not been well replicated in non-Japanese populations. Good research that identifies which patients may be more at risk in other populations would be helpful to more precisely target high-risk therapy.”
Other research needs include a clearer understanding of the best therapies for refractory patients, Dr. Gorelik said. “One area of the most difficulty was determining whether patients with refractory disease should have repeated IVIg or a switch to glucocorticoids and biologic agents. Some of this research is underway, and some was published just as these guidelines were being drawn, and this particular area is one that is likely to change significantly. While currently we recommend a repeated dose of IVIg, it is likely that over the very near term, the use of repeated IVIg in KD will be curtailed” because of concerns such as the relatively high rate of hemolysis. Research to identify which therapy has a noninferior effect with a superior risk profile is needed; such research “will likely result in a future iteration of these guidelines specifically related to this question,” he concluded.
The KD guideline is the final companion to three additional ACR/VF vasculitis guidelines that were released in July 2021. The guideline research received no outside funding. The researchers had no financial conflicts to disclose.
All Kawasaki disease (KD) patients should be treated first with intravenous immunoglobulin, according to an updated guideline issued jointly by the American College of Rheumatology and the Vasculitis Foundation.
KD has low mortality when treated appropriately, guideline first author Mark Gorelik, MD, assistant professor of pediatrics at Columbia University, New York, and colleagues wrote.
The update is important at this time because new evidence continues to emerge in the clinical management of KD, Dr. Gorelik said in an interview.
“In addition, this guideline approaches Kawasaki disease from a perspective of acting as an adjunct to the already existing and excellent American Heart Association guidelines by adding information in areas that rheumatologists may play a role,” Dr. Gorelik said. “This is specifically regarding patients who may require additional therapy beyond standard IVIg, such as patients who may be at higher risk of morbidity from disease and patients who have refractory disease,” he explained.
The guideline, published in Arthritis & Rheumatology, includes 11 recommendations, 1 good practice statement, and 1 ungraded position statement. The good practice statement emphasizes that all patients with KD should be initially treated with IVIg.
The position statement advises that either nonglucocorticoid immunosuppressive therapy or glucocorticoids may be used for patients with acute KD whose fever persists despite repeated IVIg treatment. No clinical evidence currently supports the superiority of either nonglucocorticoid immunosuppressive therapy or glucocorticoids; therefore, the authors support the use of either based on what is appropriate in any given clinical situation. Although optimal dosage and duration of glucocorticoids have yet to be determined in a U.S. population, the authors described a typical glucocorticoid dosage as starting prednisone at 2 mg/kg per day, with a maximum of 60 mg/day, and dose tapering over 15 days.
The 11 recommendations consist of 7 strong and 4 conditional recommendations. The strong recommendations focus on prompt treatment of incomplete KD, treatment with aspirin, and obtaining an echocardiogram in patients with unexplained macrophage activation syndrome or shock. The conditional recommendations support using established therapy promptly at disease onset, then identifying cases in which additional therapy is needed.
Dr. Gorelik highlighted four clinical takeaways from the guideline. First, “patients with higher risk for complications do exist in Kawasaki disease, and that these patients can be treated more aggressively,” he said. “Specifically, patients with aneurysms seen at first ultrasound, and patients who are under 6 months, are more likely to have progressive and/or refractory disease; these patients can be treated with an adjunctive short course of corticosteroids.”
Second, “the use of high-dose aspirin for patients with Kawasaki disease does not have strong basis in evidence. While aspirin itself of some dose is necessary for patients with Kawasaki disease, use of either high- or low-dose aspirin has the same outcome for patients, and a physician may choose either of these in practice,” he said.
Third, “we continue to recommend that refractory patients with Kawasaki disease be treated with a second dose of IVIg; however, there are many scenarios in which a physician may choose either corticosteroids [either a single high dose of >10 mg/kg, or a short moderate-dose course of 2 mg/kg per day for 5-7 days] or a biologic agent such as infliximab. ... These are valid choices for therapy in patients with refractory Kawasaki disease,” he emphasized.
Fourth, “physicians should discard the idea of treating before [and conversely, not treating after] 10 days of fever,” Dr. Gorelik said. “Patients with Kawasaki disease should be treated as soon as the diagnosis is made, regardless of whether this patient is on day 5, day 12, or day 20 of symptoms.”
Update incorporates emerging evidence
Potential barriers to implementing the guideline in practice include the challenge of weaning doctors from practices that are habitual in medicine, Dr. Gorelik said. “One of these is the use of high-dose aspirin for Kawasaki disease; a number of studies have shown over the past decade or more that high-dose aspirin has no greater effect than lower-dose aspirin for Kawasaki disease. Despite all of these studies, the use of high-dose aspirin continued. High-dose aspirin for Kawasaki disease was used in the era prior to use of IVIg as an anti-inflammatory agent. However, it has poor efficacy in this regard, and the true benefit for aspirin is for anticoagulation for patients at risk of a clot, and this is just as effective in lower doses. Expressing this in a guideline could help to change practices by helping physicians understand not only what they are guided to do, but why.”
Additional research is needed to better identify high-risk patients in non-Japanese populations, he noted. “While studies from Japan suggest that higher-risk patients can be identified based on various parameters, these have not been well replicated in non-Japanese populations. Good research that identifies which patients may be more at risk in other populations would be helpful to more precisely target high-risk therapy.”
Other research needs include a clearer understanding of the best therapies for refractory patients, Dr. Gorelik said. “One area of the most difficulty was determining whether patients with refractory disease should have repeated IVIg or a switch to glucocorticoids and biologic agents. Some of this research is underway, and some was published just as these guidelines were being drawn, and this particular area is one that is likely to change significantly. While currently we recommend a repeated dose of IVIg, it is likely that over the very near term, the use of repeated IVIg in KD will be curtailed” because of concerns such as the relatively high rate of hemolysis. Research to identify which therapy has a noninferior effect with a superior risk profile is needed; such research “will likely result in a future iteration of these guidelines specifically related to this question,” he concluded.
The KD guideline is the final companion to three additional ACR/VF vasculitis guidelines that were released in July 2021. The guideline research received no outside funding. The researchers had no financial conflicts to disclose.
FROM ARTHRITIS & RHEUMATOLOGY
Morphology of Mycosis Fungoides and Sézary Syndrome in Skin of Color
Mycosis fungoides (MF) and Sézary syndrome (SS) are non-Hodgkin T-cell lymphomas that make up the majority of cutaneous T-cell lymphomas. These conditions commonly affect Black patients, with an incidence rate of 12.6 cases of cutaneous T-cell lymphomas per million individuals vs 9.8 per million individuals in non–skin of color (SoC) patients.1 However, educational resources tend to focus on the clinical manifestations of MF/SS in lighter skin types, describing MF as erythematous patches, plaques, or tumors presenting in non–sun-exposed areas of the skin and SS as generalized erythroderma.2 Skin of color, comprised of Fitzpatrick skin types (FSTs) IV to VI,3 is poorly represented across dermatology textbooks,4,5 medical student resources,6 and peer-reviewed publications,7 raising awareness for the need to address this disparity.
Skin of color patients with MF/SS display variable morphologies, including features such as hyperpigmentation and hypopigmentation,8 the latter being exceedingly rare in non-SoC patients.9 Familiarity with these differences among providers is essential to allow for equitable diagnosis and treatment across all skin types, especially in light of data predicting that by 2044 more than 50% of the US population will be people of color.10 Patients with SoC are of many ethnic and racial backgrounds, including Black, Hispanic, American Indian, Pacific Islander, and Asian.11
Along with morphologic differences, there also are several racial disparities in the prognosis and survival of patients with MF/SS. Black patients diagnosed with MF present with greater body surface area affected, and Black women with MF have reduced survival rates compared to their White counterparts.12 Given these racial disparities in survival and representation in educational resources, we aimed to quantify the frequency of various morphologic characteristics of MF/SS in patients with SoC vs non-SoC patients to facilitate better recognition of early MF/SS in SoC patients by medical providers.
Methods
We performed a retrospective chart review following approval from the institutional review board at Northwestern University (Chicago, Illinois). We identified all patients with FSTs IV to VI and biopsy-proven MF/SS who had been clinically photographed in our clinic from January 1998 to December 2019. Only photographs that were high quality enough to review morphologic features were included in our review. Fitzpatrick skin type was determined based on electronic medical record documentation. If photographs were available from multiple visits for the same patient, only those showing posttreatment nonactive lesions were included. Additionally, 36 patients with FSTs I to III (non-SoC) and biopsy-proven MF/SS were included in our review as a comparison with the SoC cohort. The primary outcomes for this study included the presence of scale, erythema, hyperpigmentation, hypopigmentation, violaceous color, lichenification, silver hue, dyschromia, alopecia, poikiloderma, atrophy, and ulceration in active lesions. Dyschromia was defined by the presence of both hypopigmentation and hyperpigmentation. Poikiloderma was defined by hypopigmentation and hyperpigmentation, telangiectasia, and atrophy. Secondary outcomes included evaluation of those same characteristics in posttreatment nonactive lesions. All photographs were independently assessed by 3 authors (M.L.E., C.J.W., J.M.M.), and discrepancies were resolved by further review of the photograph in question and discussion.
Statistical Analysis—Summary statistics were applied to describe demographic and clinical characteristics. The χ2 test was used for categorical variables. Results achieving P<.05 were considered statistically significant.
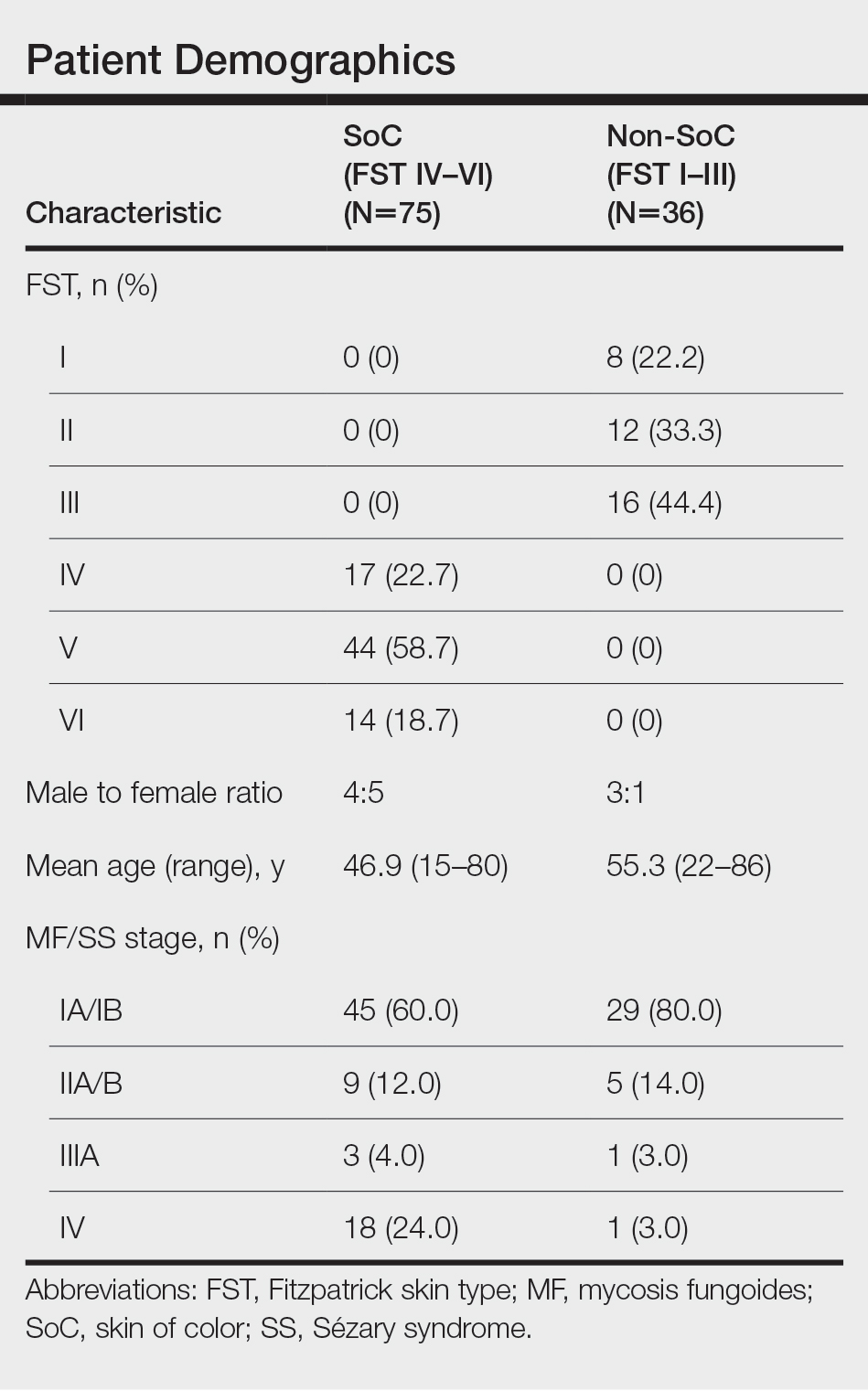
Results
We reviewed photographs of 111 patients across all skin types (8, FST I; 12, FST II; 16, FST III; 17, FST IV; 44, FST V; 14, FST VI). The cohort was 47% female, and the mean age was 49.7 years (range, 15–86 years). The majority of the cohort had early-stage MF (stage IA or IB). There were more cases of SS in the SoC cohort than the non-SoC cohort (Table). Only 5 photographs had discrepancies and required discussion among the reviewers to achieve consensus.
![Frequency of morphologic features found in skin of color (SoC [Fitzpatrick skin types IV–VI]) vs non-SoC (Fitzpatrick skin types I–III) patients with mycosis fungoides/Sézary syndrome Frequency of morphologic features found in skin of color (SoC [Fitzpatrick skin types IV–VI]) vs non-SoC (Fitzpatrick skin types I–III) patients with mycosis fungoides/Sézary syndrome](https://cdn.mdedge.com/files/s3fs-public/Espinosa_1.JPG)
Regarding morphologic characteristics in active lesions (Figure 1), scale was present in almost all patients (99% in SoC, 94% in non-SoC). Erythema was present in nearly all non-SoC patients (94%) but only in 69% of SoC patients (P=.003). Poikiloderma also was found to be present at higher frequencies in non-SoC patients compared with SoC patients (19% and 4%, respectively [P=.008]). However, hyperpigmentation (80% vs 39%), lichenification (43% vs 17%), and silver hue (25% vs 3%) were more common in SoC patients than non-SoC patients (P<.05). There were no significant differences in the remaining features, including hypopigmentation (39% vs 25%), dyschromia (24% vs 19%), violaceous color (44% vs 25%), atrophy (11% vs 22%), alopecia (23% vs 31%), and ulceration (16% vs 8%) between SoC and non-SoC patients (P>.05). Photographs of MF in patients with SoC can be seen in Figure 2.
![Representative photographs of mycosis fungoides (MF) in skin of color (Fitzpatrick skin types [FSTs] IV–VI) Representative photographs of mycosis fungoides (MF) in skin of color (Fitzpatrick skin types [FSTs] IV–VI)](https://cdn.mdedge.com/files/s3fs-public/CT109003003_e_Fig2_ABCDE.JPG)
Posttreatment (nonactive) photographs were available for 26 patients (6 non-SoC, 20 SoC). We found that across all FST groups, hyperpigmentation was more common than hypopigmentation in areas of previously active disease. Statistical analysis was not completed given that few non-SoC photographs were available for comparison.
Comment
This qualitative review demonstrates the heterogeneity of MF/SS in SoC patients and that these conditions do not present in this population with the classic erythematous patches and plaques found in non-SoC patients. We found that hyperpigmentation, lichenification, and silver hue were present at higher rates in patients with FSTs IV to VI compared to those with FSTs I to III, which had higher rates of erythema and poikiloderma. Familiarity with these morphologic features along with increased exposure to clinical photographs of MF/SS in SoC patients will aid in the visual recognition required for this diagnosis, since erythema is harder to identify in darker skin types. Recognizing the unique findings of MF in patients with SoC as well as in patients with lighter skin types will enable earlier diagnosis and treatment of MF/SS across all skin types. If MF is diagnosed and treated early, life expectancy is similar to that of patients without MF.13 However, the 5-year survival rate for advanced-stage MF/SS is 52% across all skin types, and studies have found that Black patients with advanced-stage disease have worse outcomes despite accounting for demographic factors and tumor stage.14,15 Given the worse outcomes in SoC patients with advanced-stage MF/SS, earlier diagnosis could help address this disparity.8,13,14 Similar morphologic features could be used in diagnosing other inflammatory conditions; studies have shown that the lack of recognition of erythema in Black children has led to delayed diagnosis of atopic dermatitis and subsequent inadequate treatment.16,17
The morphologic presentation of MF/SS in SoC patients also can influence an optimal treatment plan for this population. Hypopigmented MF responds better to phototherapy than hyperpigmented MF, as phototherapy has been shown to have decreased efficacy with increasing FST.18 Therefore, for patients with FSTs IV to VI, topical agents such as nitrogen mustard or bexarotene may be more suitable treatment options, as the efficacy of these treatments is independent of skin color.8 However, nitrogen mustard commonly leads to postinflammatory hyperpigmentation, and topical bexarotene may lead to erythema or irritation; therefore, providers must counsel patients on these possible side effects. For refractory disease, adjunct systemic treatments such as oral bexarotene, subcutaneous interferon, methotrexate, or radiation therapy may be considered.8
In addition to aiding in the prompt diagnosis and treatment of MF/SS in SoC patients, our findings may be used to better assess the extent of disease and distinguish between active MF/SS lesions vs xerosis cutis or residual dyschromia from previously treated lesions. It is important to note that these morphologic features must be taken into account with a complete history and work-up. The differential diagnosis of MF/SS includes conditions such as atopic dermatitis, psoriasis, tinea corporis, and drug reactions, which may have similar morphology in SoC.19
Limitations of our study include the single-center design and the use of photographs instead of in-person examination; however, our cutaneous lymphoma clinic serves a diverse patient population, and our 3 reviewers rated the photographs independently. Discussion amongst the reviewers to address discrepancies was only required for 5 photographs, indicating the high inter-reviewer reliability. Additionally, the original purpose of FST was to assess for the propensity of the skin to burn when undergoing phototherapy, not to serve as a marker for skin color. We recommend trainees and clinicians be mindful about the purpose of FST and to use inclusive language (eg, using the terms skin irritation, skin tenderness, or skin becoming darker from the sun instead of tanning) when determining FST in darker-skinned individuals.20 Future directions include examining if certain treatments are associated with prolonged dyschromia.
Conclusion
In our single-institution retrospective study, we found differences in the morphologic presentation of MF/SS in SoC patients vs non-SoC patients. While erythema is a common feature in non-SoC patients, clinical features of hyperpigmentation, lichenification, and silver hue should be carefully evaluated in the diagnosis of MF/SS in SoC patients. Knowledge of the heterogenous presentation of MF/SS in patients with SoC allows for expedited diagnosis and treatment, leading to better clinical outcomes. Valuable resources, including Taylor and Kelly’s Dermatology for Skin of Color, the Skin of Color Society, and VisualDx educate providers on how dermatologic conditions present in darker skin types. However, there is still work to be done to enhance diversity in educational resources in order to provide equitable care to patients of all skin types.
- Korgavkar K, Xiong M, Weinstock M. Changing incidence trends of cutaneous T-cell lymphoma. JAMA Dermatol. 2013;149:1295-1299. doi:10.1001/jamadermatol.2013.5526
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.E1-E16; quiz 221-222. doi:10.1016/j.jaad.2013.07.049
- Tull RZ, Kerby E, Subash JJ, et al. Ethnic skin centers in the United States: where are we in 2020?. J Am Acad Dermatol. 2020;83:1757-1759. doi:10.1016/j.jaad.2020.03.054
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196. doi:10.1016/j.jaad.2020.04.084
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Jones VA, Clark KA, Shobajo MT, et al. Skin of color representation in medical education: an analysis of popular preparatory materials used for United States medical licensing examinations. J Am Acad Dermatol. 2021;85:773-775. doi:10.1016/j.jaad.2020.07.112
- Montgomery SN, Elbuluk N. A quantitative analysis of research publications focused on the top chief complaints in skin of color patients. J Am Acad Dermatol. 2021;85:241-242. doi:10.1016/j.jaad.2020.08.031
- Hinds GA, Heald P. Cutaneous T-cell lymphoma in skin of color. J Am Acad Dermatol. 2009;60:359-375; quiz 376-378. doi:10.1016/j.jaad.2008.10.031
- Ardigó M, Borroni G, Muscardin L, et al. Hypopigmented mycosis fungoides in Caucasian patients: a clinicopathologic study of 7 cases. J Am Acad Dermatol. 2003;49:264-270. doi:10.1067/s0190-9622(03)00907-1
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. Updated October 8, 2021. Accessed February 28, 2022. https://www.census.gov/library/publications/2015/demo/p25-1143.html
- Taylor SC, Kyei A. Defining skin of color. In: Kelly AP, Taylor SC, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Huang AH, Kwatra SG, Khanna R, et al. Racial disparities in the clinical presentation and prognosis of patients with mycosis fungoides. J Natl Med Assoc. 2019;111:633-639. doi:10.1016/j.jnma.2019.08.006
- Kim YH, Jensen RA, Watanabe GL, et al. Clinical stage IA (limited patch and plaque) mycosis fungoides. a long-term outcome analysis. Arch Dermatol. 1996;132:1309-1313.
- Scarisbrick JJ, Prince HM, Vermeer MH, et al. Cutaneous lymphoma international consortium study of outcome in advanced stages of mycosis fungoides and Sézary syndrome: effect of specific prognostic markers on survival and development of a prognostic model. J Clin Oncol. 2015;33:3766-3773. doi:10.1200/JCO.2015.61.7142
- Nath SK, Yu JB, Wilson LD. Poorer prognosis of African-American patients with mycosis fungoides: an analysis of the SEER dataset, 1988 to 2008. Clin Lymphoma Myeloma Leuk. 2014;14:419-423. doi:10.1016/j.clml.2013.12.018
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925. doi:10.1046/j.1365-2133.2002.04965.x
- Poladian K, De Souza B, McMichael AJ. Atopic dermatitis in adolescents with skin of color. Cutis. 2019;104:164-168.
- Yones SS, Palmer RA, Garibaldinos TT, et al. Randomized double-blind trial of the treatment of chronic plaque psoriasis: efficacy of psoralen-UV-A therapy vs narrowband UV-B therapy. Arch Dermatol. 2006;142:836-842. doi:10.1001/archderm.142.7.836
- Currimbhoy S, Pandya AG. Cutaneous T-cell lymphoma. In: Kelly AP, Taylor SC, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
Mycosis fungoides (MF) and Sézary syndrome (SS) are non-Hodgkin T-cell lymphomas that make up the majority of cutaneous T-cell lymphomas. These conditions commonly affect Black patients, with an incidence rate of 12.6 cases of cutaneous T-cell lymphomas per million individuals vs 9.8 per million individuals in non–skin of color (SoC) patients.1 However, educational resources tend to focus on the clinical manifestations of MF/SS in lighter skin types, describing MF as erythematous patches, plaques, or tumors presenting in non–sun-exposed areas of the skin and SS as generalized erythroderma.2 Skin of color, comprised of Fitzpatrick skin types (FSTs) IV to VI,3 is poorly represented across dermatology textbooks,4,5 medical student resources,6 and peer-reviewed publications,7 raising awareness for the need to address this disparity.
Skin of color patients with MF/SS display variable morphologies, including features such as hyperpigmentation and hypopigmentation,8 the latter being exceedingly rare in non-SoC patients.9 Familiarity with these differences among providers is essential to allow for equitable diagnosis and treatment across all skin types, especially in light of data predicting that by 2044 more than 50% of the US population will be people of color.10 Patients with SoC are of many ethnic and racial backgrounds, including Black, Hispanic, American Indian, Pacific Islander, and Asian.11
Along with morphologic differences, there also are several racial disparities in the prognosis and survival of patients with MF/SS. Black patients diagnosed with MF present with greater body surface area affected, and Black women with MF have reduced survival rates compared to their White counterparts.12 Given these racial disparities in survival and representation in educational resources, we aimed to quantify the frequency of various morphologic characteristics of MF/SS in patients with SoC vs non-SoC patients to facilitate better recognition of early MF/SS in SoC patients by medical providers.
Methods
We performed a retrospective chart review following approval from the institutional review board at Northwestern University (Chicago, Illinois). We identified all patients with FSTs IV to VI and biopsy-proven MF/SS who had been clinically photographed in our clinic from January 1998 to December 2019. Only photographs that were high quality enough to review morphologic features were included in our review. Fitzpatrick skin type was determined based on electronic medical record documentation. If photographs were available from multiple visits for the same patient, only those showing posttreatment nonactive lesions were included. Additionally, 36 patients with FSTs I to III (non-SoC) and biopsy-proven MF/SS were included in our review as a comparison with the SoC cohort. The primary outcomes for this study included the presence of scale, erythema, hyperpigmentation, hypopigmentation, violaceous color, lichenification, silver hue, dyschromia, alopecia, poikiloderma, atrophy, and ulceration in active lesions. Dyschromia was defined by the presence of both hypopigmentation and hyperpigmentation. Poikiloderma was defined by hypopigmentation and hyperpigmentation, telangiectasia, and atrophy. Secondary outcomes included evaluation of those same characteristics in posttreatment nonactive lesions. All photographs were independently assessed by 3 authors (M.L.E., C.J.W., J.M.M.), and discrepancies were resolved by further review of the photograph in question and discussion.
Statistical Analysis—Summary statistics were applied to describe demographic and clinical characteristics. The χ2 test was used for categorical variables. Results achieving P<.05 were considered statistically significant.

Results
We reviewed photographs of 111 patients across all skin types (8, FST I; 12, FST II; 16, FST III; 17, FST IV; 44, FST V; 14, FST VI). The cohort was 47% female, and the mean age was 49.7 years (range, 15–86 years). The majority of the cohort had early-stage MF (stage IA or IB). There were more cases of SS in the SoC cohort than the non-SoC cohort (Table). Only 5 photographs had discrepancies and required discussion among the reviewers to achieve consensus.
![Frequency of morphologic features found in skin of color (SoC [Fitzpatrick skin types IV–VI]) vs non-SoC (Fitzpatrick skin types I–III) patients with mycosis fungoides/Sézary syndrome Frequency of morphologic features found in skin of color (SoC [Fitzpatrick skin types IV–VI]) vs non-SoC (Fitzpatrick skin types I–III) patients with mycosis fungoides/Sézary syndrome](https://cdn.mdedge.com/files/s3fs-public/Espinosa_1.JPG)
Regarding morphologic characteristics in active lesions (Figure 1), scale was present in almost all patients (99% in SoC, 94% in non-SoC). Erythema was present in nearly all non-SoC patients (94%) but only in 69% of SoC patients (P=.003). Poikiloderma also was found to be present at higher frequencies in non-SoC patients compared with SoC patients (19% and 4%, respectively [P=.008]). However, hyperpigmentation (80% vs 39%), lichenification (43% vs 17%), and silver hue (25% vs 3%) were more common in SoC patients than non-SoC patients (P<.05). There were no significant differences in the remaining features, including hypopigmentation (39% vs 25%), dyschromia (24% vs 19%), violaceous color (44% vs 25%), atrophy (11% vs 22%), alopecia (23% vs 31%), and ulceration (16% vs 8%) between SoC and non-SoC patients (P>.05). Photographs of MF in patients with SoC can be seen in Figure 2.
![Representative photographs of mycosis fungoides (MF) in skin of color (Fitzpatrick skin types [FSTs] IV–VI) Representative photographs of mycosis fungoides (MF) in skin of color (Fitzpatrick skin types [FSTs] IV–VI)](https://cdn.mdedge.com/files/s3fs-public/CT109003003_e_Fig2_ABCDE.JPG)
Posttreatment (nonactive) photographs were available for 26 patients (6 non-SoC, 20 SoC). We found that across all FST groups, hyperpigmentation was more common than hypopigmentation in areas of previously active disease. Statistical analysis was not completed given that few non-SoC photographs were available for comparison.
Comment
This qualitative review demonstrates the heterogeneity of MF/SS in SoC patients and that these conditions do not present in this population with the classic erythematous patches and plaques found in non-SoC patients. We found that hyperpigmentation, lichenification, and silver hue were present at higher rates in patients with FSTs IV to VI compared to those with FSTs I to III, which had higher rates of erythema and poikiloderma. Familiarity with these morphologic features along with increased exposure to clinical photographs of MF/SS in SoC patients will aid in the visual recognition required for this diagnosis, since erythema is harder to identify in darker skin types. Recognizing the unique findings of MF in patients with SoC as well as in patients with lighter skin types will enable earlier diagnosis and treatment of MF/SS across all skin types. If MF is diagnosed and treated early, life expectancy is similar to that of patients without MF.13 However, the 5-year survival rate for advanced-stage MF/SS is 52% across all skin types, and studies have found that Black patients with advanced-stage disease have worse outcomes despite accounting for demographic factors and tumor stage.14,15 Given the worse outcomes in SoC patients with advanced-stage MF/SS, earlier diagnosis could help address this disparity.8,13,14 Similar morphologic features could be used in diagnosing other inflammatory conditions; studies have shown that the lack of recognition of erythema in Black children has led to delayed diagnosis of atopic dermatitis and subsequent inadequate treatment.16,17
The morphologic presentation of MF/SS in SoC patients also can influence an optimal treatment plan for this population. Hypopigmented MF responds better to phototherapy than hyperpigmented MF, as phototherapy has been shown to have decreased efficacy with increasing FST.18 Therefore, for patients with FSTs IV to VI, topical agents such as nitrogen mustard or bexarotene may be more suitable treatment options, as the efficacy of these treatments is independent of skin color.8 However, nitrogen mustard commonly leads to postinflammatory hyperpigmentation, and topical bexarotene may lead to erythema or irritation; therefore, providers must counsel patients on these possible side effects. For refractory disease, adjunct systemic treatments such as oral bexarotene, subcutaneous interferon, methotrexate, or radiation therapy may be considered.8
In addition to aiding in the prompt diagnosis and treatment of MF/SS in SoC patients, our findings may be used to better assess the extent of disease and distinguish between active MF/SS lesions vs xerosis cutis or residual dyschromia from previously treated lesions. It is important to note that these morphologic features must be taken into account with a complete history and work-up. The differential diagnosis of MF/SS includes conditions such as atopic dermatitis, psoriasis, tinea corporis, and drug reactions, which may have similar morphology in SoC.19
Limitations of our study include the single-center design and the use of photographs instead of in-person examination; however, our cutaneous lymphoma clinic serves a diverse patient population, and our 3 reviewers rated the photographs independently. Discussion amongst the reviewers to address discrepancies was only required for 5 photographs, indicating the high inter-reviewer reliability. Additionally, the original purpose of FST was to assess for the propensity of the skin to burn when undergoing phototherapy, not to serve as a marker for skin color. We recommend trainees and clinicians be mindful about the purpose of FST and to use inclusive language (eg, using the terms skin irritation, skin tenderness, or skin becoming darker from the sun instead of tanning) when determining FST in darker-skinned individuals.20 Future directions include examining if certain treatments are associated with prolonged dyschromia.
Conclusion
In our single-institution retrospective study, we found differences in the morphologic presentation of MF/SS in SoC patients vs non-SoC patients. While erythema is a common feature in non-SoC patients, clinical features of hyperpigmentation, lichenification, and silver hue should be carefully evaluated in the diagnosis of MF/SS in SoC patients. Knowledge of the heterogenous presentation of MF/SS in patients with SoC allows for expedited diagnosis and treatment, leading to better clinical outcomes. Valuable resources, including Taylor and Kelly’s Dermatology for Skin of Color, the Skin of Color Society, and VisualDx educate providers on how dermatologic conditions present in darker skin types. However, there is still work to be done to enhance diversity in educational resources in order to provide equitable care to patients of all skin types.
Mycosis fungoides (MF) and Sézary syndrome (SS) are non-Hodgkin T-cell lymphomas that make up the majority of cutaneous T-cell lymphomas. These conditions commonly affect Black patients, with an incidence rate of 12.6 cases of cutaneous T-cell lymphomas per million individuals vs 9.8 per million individuals in non–skin of color (SoC) patients.1 However, educational resources tend to focus on the clinical manifestations of MF/SS in lighter skin types, describing MF as erythematous patches, plaques, or tumors presenting in non–sun-exposed areas of the skin and SS as generalized erythroderma.2 Skin of color, comprised of Fitzpatrick skin types (FSTs) IV to VI,3 is poorly represented across dermatology textbooks,4,5 medical student resources,6 and peer-reviewed publications,7 raising awareness for the need to address this disparity.
Skin of color patients with MF/SS display variable morphologies, including features such as hyperpigmentation and hypopigmentation,8 the latter being exceedingly rare in non-SoC patients.9 Familiarity with these differences among providers is essential to allow for equitable diagnosis and treatment across all skin types, especially in light of data predicting that by 2044 more than 50% of the US population will be people of color.10 Patients with SoC are of many ethnic and racial backgrounds, including Black, Hispanic, American Indian, Pacific Islander, and Asian.11
Along with morphologic differences, there also are several racial disparities in the prognosis and survival of patients with MF/SS. Black patients diagnosed with MF present with greater body surface area affected, and Black women with MF have reduced survival rates compared to their White counterparts.12 Given these racial disparities in survival and representation in educational resources, we aimed to quantify the frequency of various morphologic characteristics of MF/SS in patients with SoC vs non-SoC patients to facilitate better recognition of early MF/SS in SoC patients by medical providers.
Methods
We performed a retrospective chart review following approval from the institutional review board at Northwestern University (Chicago, Illinois). We identified all patients with FSTs IV to VI and biopsy-proven MF/SS who had been clinically photographed in our clinic from January 1998 to December 2019. Only photographs that were high quality enough to review morphologic features were included in our review. Fitzpatrick skin type was determined based on electronic medical record documentation. If photographs were available from multiple visits for the same patient, only those showing posttreatment nonactive lesions were included. Additionally, 36 patients with FSTs I to III (non-SoC) and biopsy-proven MF/SS were included in our review as a comparison with the SoC cohort. The primary outcomes for this study included the presence of scale, erythema, hyperpigmentation, hypopigmentation, violaceous color, lichenification, silver hue, dyschromia, alopecia, poikiloderma, atrophy, and ulceration in active lesions. Dyschromia was defined by the presence of both hypopigmentation and hyperpigmentation. Poikiloderma was defined by hypopigmentation and hyperpigmentation, telangiectasia, and atrophy. Secondary outcomes included evaluation of those same characteristics in posttreatment nonactive lesions. All photographs were independently assessed by 3 authors (M.L.E., C.J.W., J.M.M.), and discrepancies were resolved by further review of the photograph in question and discussion.
Statistical Analysis—Summary statistics were applied to describe demographic and clinical characteristics. The χ2 test was used for categorical variables. Results achieving P<.05 were considered statistically significant.

Results
We reviewed photographs of 111 patients across all skin types (8, FST I; 12, FST II; 16, FST III; 17, FST IV; 44, FST V; 14, FST VI). The cohort was 47% female, and the mean age was 49.7 years (range, 15–86 years). The majority of the cohort had early-stage MF (stage IA or IB). There were more cases of SS in the SoC cohort than the non-SoC cohort (Table). Only 5 photographs had discrepancies and required discussion among the reviewers to achieve consensus.
![Frequency of morphologic features found in skin of color (SoC [Fitzpatrick skin types IV–VI]) vs non-SoC (Fitzpatrick skin types I–III) patients with mycosis fungoides/Sézary syndrome Frequency of morphologic features found in skin of color (SoC [Fitzpatrick skin types IV–VI]) vs non-SoC (Fitzpatrick skin types I–III) patients with mycosis fungoides/Sézary syndrome](https://cdn.mdedge.com/files/s3fs-public/Espinosa_1.JPG)
Regarding morphologic characteristics in active lesions (Figure 1), scale was present in almost all patients (99% in SoC, 94% in non-SoC). Erythema was present in nearly all non-SoC patients (94%) but only in 69% of SoC patients (P=.003). Poikiloderma also was found to be present at higher frequencies in non-SoC patients compared with SoC patients (19% and 4%, respectively [P=.008]). However, hyperpigmentation (80% vs 39%), lichenification (43% vs 17%), and silver hue (25% vs 3%) were more common in SoC patients than non-SoC patients (P<.05). There were no significant differences in the remaining features, including hypopigmentation (39% vs 25%), dyschromia (24% vs 19%), violaceous color (44% vs 25%), atrophy (11% vs 22%), alopecia (23% vs 31%), and ulceration (16% vs 8%) between SoC and non-SoC patients (P>.05). Photographs of MF in patients with SoC can be seen in Figure 2.
![Representative photographs of mycosis fungoides (MF) in skin of color (Fitzpatrick skin types [FSTs] IV–VI) Representative photographs of mycosis fungoides (MF) in skin of color (Fitzpatrick skin types [FSTs] IV–VI)](https://cdn.mdedge.com/files/s3fs-public/CT109003003_e_Fig2_ABCDE.JPG)
Posttreatment (nonactive) photographs were available for 26 patients (6 non-SoC, 20 SoC). We found that across all FST groups, hyperpigmentation was more common than hypopigmentation in areas of previously active disease. Statistical analysis was not completed given that few non-SoC photographs were available for comparison.
Comment
This qualitative review demonstrates the heterogeneity of MF/SS in SoC patients and that these conditions do not present in this population with the classic erythematous patches and plaques found in non-SoC patients. We found that hyperpigmentation, lichenification, and silver hue were present at higher rates in patients with FSTs IV to VI compared to those with FSTs I to III, which had higher rates of erythema and poikiloderma. Familiarity with these morphologic features along with increased exposure to clinical photographs of MF/SS in SoC patients will aid in the visual recognition required for this diagnosis, since erythema is harder to identify in darker skin types. Recognizing the unique findings of MF in patients with SoC as well as in patients with lighter skin types will enable earlier diagnosis and treatment of MF/SS across all skin types. If MF is diagnosed and treated early, life expectancy is similar to that of patients without MF.13 However, the 5-year survival rate for advanced-stage MF/SS is 52% across all skin types, and studies have found that Black patients with advanced-stage disease have worse outcomes despite accounting for demographic factors and tumor stage.14,15 Given the worse outcomes in SoC patients with advanced-stage MF/SS, earlier diagnosis could help address this disparity.8,13,14 Similar morphologic features could be used in diagnosing other inflammatory conditions; studies have shown that the lack of recognition of erythema in Black children has led to delayed diagnosis of atopic dermatitis and subsequent inadequate treatment.16,17
The morphologic presentation of MF/SS in SoC patients also can influence an optimal treatment plan for this population. Hypopigmented MF responds better to phototherapy than hyperpigmented MF, as phototherapy has been shown to have decreased efficacy with increasing FST.18 Therefore, for patients with FSTs IV to VI, topical agents such as nitrogen mustard or bexarotene may be more suitable treatment options, as the efficacy of these treatments is independent of skin color.8 However, nitrogen mustard commonly leads to postinflammatory hyperpigmentation, and topical bexarotene may lead to erythema or irritation; therefore, providers must counsel patients on these possible side effects. For refractory disease, adjunct systemic treatments such as oral bexarotene, subcutaneous interferon, methotrexate, or radiation therapy may be considered.8
In addition to aiding in the prompt diagnosis and treatment of MF/SS in SoC patients, our findings may be used to better assess the extent of disease and distinguish between active MF/SS lesions vs xerosis cutis or residual dyschromia from previously treated lesions. It is important to note that these morphologic features must be taken into account with a complete history and work-up. The differential diagnosis of MF/SS includes conditions such as atopic dermatitis, psoriasis, tinea corporis, and drug reactions, which may have similar morphology in SoC.19
Limitations of our study include the single-center design and the use of photographs instead of in-person examination; however, our cutaneous lymphoma clinic serves a diverse patient population, and our 3 reviewers rated the photographs independently. Discussion amongst the reviewers to address discrepancies was only required for 5 photographs, indicating the high inter-reviewer reliability. Additionally, the original purpose of FST was to assess for the propensity of the skin to burn when undergoing phototherapy, not to serve as a marker for skin color. We recommend trainees and clinicians be mindful about the purpose of FST and to use inclusive language (eg, using the terms skin irritation, skin tenderness, or skin becoming darker from the sun instead of tanning) when determining FST in darker-skinned individuals.20 Future directions include examining if certain treatments are associated with prolonged dyschromia.
Conclusion
In our single-institution retrospective study, we found differences in the morphologic presentation of MF/SS in SoC patients vs non-SoC patients. While erythema is a common feature in non-SoC patients, clinical features of hyperpigmentation, lichenification, and silver hue should be carefully evaluated in the diagnosis of MF/SS in SoC patients. Knowledge of the heterogenous presentation of MF/SS in patients with SoC allows for expedited diagnosis and treatment, leading to better clinical outcomes. Valuable resources, including Taylor and Kelly’s Dermatology for Skin of Color, the Skin of Color Society, and VisualDx educate providers on how dermatologic conditions present in darker skin types. However, there is still work to be done to enhance diversity in educational resources in order to provide equitable care to patients of all skin types.
- Korgavkar K, Xiong M, Weinstock M. Changing incidence trends of cutaneous T-cell lymphoma. JAMA Dermatol. 2013;149:1295-1299. doi:10.1001/jamadermatol.2013.5526
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.E1-E16; quiz 221-222. doi:10.1016/j.jaad.2013.07.049
- Tull RZ, Kerby E, Subash JJ, et al. Ethnic skin centers in the United States: where are we in 2020?. J Am Acad Dermatol. 2020;83:1757-1759. doi:10.1016/j.jaad.2020.03.054
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196. doi:10.1016/j.jaad.2020.04.084
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Jones VA, Clark KA, Shobajo MT, et al. Skin of color representation in medical education: an analysis of popular preparatory materials used for United States medical licensing examinations. J Am Acad Dermatol. 2021;85:773-775. doi:10.1016/j.jaad.2020.07.112
- Montgomery SN, Elbuluk N. A quantitative analysis of research publications focused on the top chief complaints in skin of color patients. J Am Acad Dermatol. 2021;85:241-242. doi:10.1016/j.jaad.2020.08.031
- Hinds GA, Heald P. Cutaneous T-cell lymphoma in skin of color. J Am Acad Dermatol. 2009;60:359-375; quiz 376-378. doi:10.1016/j.jaad.2008.10.031
- Ardigó M, Borroni G, Muscardin L, et al. Hypopigmented mycosis fungoides in Caucasian patients: a clinicopathologic study of 7 cases. J Am Acad Dermatol. 2003;49:264-270. doi:10.1067/s0190-9622(03)00907-1
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. Updated October 8, 2021. Accessed February 28, 2022. https://www.census.gov/library/publications/2015/demo/p25-1143.html
- Taylor SC, Kyei A. Defining skin of color. In: Kelly AP, Taylor SC, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Huang AH, Kwatra SG, Khanna R, et al. Racial disparities in the clinical presentation and prognosis of patients with mycosis fungoides. J Natl Med Assoc. 2019;111:633-639. doi:10.1016/j.jnma.2019.08.006
- Kim YH, Jensen RA, Watanabe GL, et al. Clinical stage IA (limited patch and plaque) mycosis fungoides. a long-term outcome analysis. Arch Dermatol. 1996;132:1309-1313.
- Scarisbrick JJ, Prince HM, Vermeer MH, et al. Cutaneous lymphoma international consortium study of outcome in advanced stages of mycosis fungoides and Sézary syndrome: effect of specific prognostic markers on survival and development of a prognostic model. J Clin Oncol. 2015;33:3766-3773. doi:10.1200/JCO.2015.61.7142
- Nath SK, Yu JB, Wilson LD. Poorer prognosis of African-American patients with mycosis fungoides: an analysis of the SEER dataset, 1988 to 2008. Clin Lymphoma Myeloma Leuk. 2014;14:419-423. doi:10.1016/j.clml.2013.12.018
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925. doi:10.1046/j.1365-2133.2002.04965.x
- Poladian K, De Souza B, McMichael AJ. Atopic dermatitis in adolescents with skin of color. Cutis. 2019;104:164-168.
- Yones SS, Palmer RA, Garibaldinos TT, et al. Randomized double-blind trial of the treatment of chronic plaque psoriasis: efficacy of psoralen-UV-A therapy vs narrowband UV-B therapy. Arch Dermatol. 2006;142:836-842. doi:10.1001/archderm.142.7.836
- Currimbhoy S, Pandya AG. Cutaneous T-cell lymphoma. In: Kelly AP, Taylor SC, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
- Korgavkar K, Xiong M, Weinstock M. Changing incidence trends of cutaneous T-cell lymphoma. JAMA Dermatol. 2013;149:1295-1299. doi:10.1001/jamadermatol.2013.5526
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.E1-E16; quiz 221-222. doi:10.1016/j.jaad.2013.07.049
- Tull RZ, Kerby E, Subash JJ, et al. Ethnic skin centers in the United States: where are we in 2020?. J Am Acad Dermatol. 2020;83:1757-1759. doi:10.1016/j.jaad.2020.03.054
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196. doi:10.1016/j.jaad.2020.04.084
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Jones VA, Clark KA, Shobajo MT, et al. Skin of color representation in medical education: an analysis of popular preparatory materials used for United States medical licensing examinations. J Am Acad Dermatol. 2021;85:773-775. doi:10.1016/j.jaad.2020.07.112
- Montgomery SN, Elbuluk N. A quantitative analysis of research publications focused on the top chief complaints in skin of color patients. J Am Acad Dermatol. 2021;85:241-242. doi:10.1016/j.jaad.2020.08.031
- Hinds GA, Heald P. Cutaneous T-cell lymphoma in skin of color. J Am Acad Dermatol. 2009;60:359-375; quiz 376-378. doi:10.1016/j.jaad.2008.10.031
- Ardigó M, Borroni G, Muscardin L, et al. Hypopigmented mycosis fungoides in Caucasian patients: a clinicopathologic study of 7 cases. J Am Acad Dermatol. 2003;49:264-270. doi:10.1067/s0190-9622(03)00907-1
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. Updated October 8, 2021. Accessed February 28, 2022. https://www.census.gov/library/publications/2015/demo/p25-1143.html
- Taylor SC, Kyei A. Defining skin of color. In: Kelly AP, Taylor SC, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Huang AH, Kwatra SG, Khanna R, et al. Racial disparities in the clinical presentation and prognosis of patients with mycosis fungoides. J Natl Med Assoc. 2019;111:633-639. doi:10.1016/j.jnma.2019.08.006
- Kim YH, Jensen RA, Watanabe GL, et al. Clinical stage IA (limited patch and plaque) mycosis fungoides. a long-term outcome analysis. Arch Dermatol. 1996;132:1309-1313.
- Scarisbrick JJ, Prince HM, Vermeer MH, et al. Cutaneous lymphoma international consortium study of outcome in advanced stages of mycosis fungoides and Sézary syndrome: effect of specific prognostic markers on survival and development of a prognostic model. J Clin Oncol. 2015;33:3766-3773. doi:10.1200/JCO.2015.61.7142
- Nath SK, Yu JB, Wilson LD. Poorer prognosis of African-American patients with mycosis fungoides: an analysis of the SEER dataset, 1988 to 2008. Clin Lymphoma Myeloma Leuk. 2014;14:419-423. doi:10.1016/j.clml.2013.12.018
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925. doi:10.1046/j.1365-2133.2002.04965.x
- Poladian K, De Souza B, McMichael AJ. Atopic dermatitis in adolescents with skin of color. Cutis. 2019;104:164-168.
- Yones SS, Palmer RA, Garibaldinos TT, et al. Randomized double-blind trial of the treatment of chronic plaque psoriasis: efficacy of psoralen-UV-A therapy vs narrowband UV-B therapy. Arch Dermatol. 2006;142:836-842. doi:10.1001/archderm.142.7.836
- Currimbhoy S, Pandya AG. Cutaneous T-cell lymphoma. In: Kelly AP, Taylor SC, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
Practice Points
- Dermatologists should be familiar with the variable morphology of mycosis fungoides (MF)/Sézary syndrome (SS) exhibited by patients of all skin types to ensure prompt diagnosis and treatment.
- Patients with skin of color (SoC)(Fitzpatrick skin types IV–VI) with MF/SS are more likely than non-SoC patients (Fitzpatrick skin types I–III) to present with hyperpigmentation, a silver hue, and lichenification, whereas non-SoC patients commonly present with erythema and poikiloderma.
New JIA guidelines emphasize earlier DMARD use
Treatment of systemic juvenile idiopathic arthritis (sJIA) should emphasize early use of conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs), compared with the previous reliance on NSAIDs and glucocorticoids, according to new guidelines from the American College of Rheumatology. The recently published 2021 guidelines focus on therapeutic approaches for oligoarthritis, temporomandibular joint (TMJ) arthritis, and sJIA.
“Systemic JIA should be treated early with biologics to rapidly bring disease under control and to avoid long-term use of glucocorticoids,” Karen Onel, MD, chief of the division of pediatric rheumatology at Weill Cornell Medicine, New York, and lead author of the guidelines, told this news organization. “Unfortunately, biologics can and are frequently denied for first-line use. For this reason, the guidelines are critically important as they demonstrate that first-line use of biologics are standard of care for the treatment of sJIA.”
The new publication is the second part of the ACR’s process to update JIA guidelines that began in 2017 and complements the release in 2019 of guidelines on the management of nonsystemic polyarthritis, sacroiliitis, and enthesitis, as well as a separate guidance on JIA-associated uveitis. The new guidelines include a second publication focused on nonpharmacologic therapies, medication monitoring, immunizations, and imaging. Both sets of guidelines grew out of a 15-member panel that included young adults with JIA and caregivers of children with JIA, and which required at least 70% agreement on recommendations.
“Though the scope of the two guidelines differed, one thing they had in common is the recognition of the importance of shared decision-making with the patient/caregiver,” Dr. Onel said. “Not every decision will be appropriate for every patient, which is why it was so instrumental to receive input from both patients and caregivers when creating these recommendations.”
Oligoarticular and TMJ arthritis
Oligoarticular and TMJ arthritis have similar recommendations, beginning with NSAIDs conditionally recommended and intra-articular glucocorticoids (IAGCs) strongly recommended as part of initial therapy. For oligoarticular arthritis, the guidelines specifically include a strong recommendation of triamcinolone hexacetonide as the preferred agent; no preferred agent is recommended for TMJ arthritis.
“The one thing that the panel was unanimous about was the use of triamcinolone hexacetonide for intra-articular steroid injections in oligoarticular kids,” Susan Shenoi, MBBS, MS, an associate professor and clinical director of pediatric rheumatology at Seattle Children’s Hospital and Research Center, said in an interview. “Triamcinolone hexacetonide has not been available recently, and through advocacy efforts, there is now a pathway to get that medication,” added Dr. Shenoi, a coauthor on the guidelines.
Dr. Onel said that “triamcinolone hexacetonide has been shown to be superior to alternative injectable glucocorticoids in achieving and maintaining remission in children with JIA,” but its unavailability meant physicians had to consider less effective, more potent, or more costly alternatives.” To address the shortage, “the FDA allowed the importation of one particular formulation of triamcinolone hexacetonide [Hexatrione 2%] specifically for joint injections in patients with JIA.”
The guidelines conditionally recommend against oral glucocorticoids for initial therapy for both oligoarticular and TMJ arthritis. In fact, throughout the guidelines it’s clear that the authors emphasize using steroids as little as possible, Dr. Shenoi said.
“Steroids are great anti-inflammatories, but in kids we worry about the long-term effects on growth and metabolism, and now we have many more DMARDs available,” Dr. Shenoi said.
The guidelines strongly recommend conventional synthetic DMARDs for patients with either of these diseases who cannot tolerate or do not respond to NSAIDs or IAGCs, with methotrexate conditionally recommended over leflunomide (Arava) for TMJ and over leflunomide, sulfasalazine (Azulfidine, Sulfazine), and hydroxychloroquine, respectively, for oligoarticular arthritis.
“NSAIDs remain widely used despite evidence supporting early use of DMARDs,” Dr. Onel said. “NSAIDs are readily available and familiar; however, they will not prevent disease progression. These guidelines should encourage short courses of NSAIDs only.”
If patients do not respond to or cannot tolerate NSAIDs, IAGCs, and at least one conventional DMARD, the guidelines strongly recommend a biologic DMARD for oligoarticular arthritis and conditionally recommend one for TMJ arthritis, without any preferences to the specific agent.
The guidelines also advise using validated disease activity measures to guide treatment decisions.
“The most important thing when you’re looking at these patients is to determine, do they have active disease or not?” Dr. Shenoi said. “If they have active disease, then you really want to step up therapy.” Using the relatively new concept of treat-to-target, Dr. Shenoi added that a crucial part of shared decision-making with the family is identifying the most appropriate target for that family “and then really trying hard to achieve that target.”
The guidelines also list risk factors for poor outcome that can be used to guide treatment decisions.
“Specific involvement of key joints, such as TMJ, wrist, sacroiliac, hip, and ankle, and other features were considered reasonable justification for early escalation of therapy,” Dr. Onel said. Other features included presence of erosive disease or enthesitis, delay in diagnosis, elevated levels of inflammation markers, and symmetric disease. “Moving quickly may be needed for a patient who is rapidly worsening, while moving slower may be appropriate for somebody who has improved substantially, but not fully.”
Systemic JIA with and without macrophage activation syndrome
For systemic JIA without macrophage activation syndrome (MAS), the guidelines similarly advise against oral glucocorticoids as initial monotherapy while conditionally recommending NSAIDs for initial monotherapy. Where the guidelines differ most from those for oligoarticular and TMJ arthritis is in progression of DMARD use, with a strong recommendation against conventional synthetic DMARDs as an initial monotherapy and interleukin-1 and IL-6 inhibitors conditionally recommended for initial monotherapy.
For patients who don’t adequately respond to NSAIDs or glucocorticoids, IL-1 and IL-6 inhibitors are strongly recommended over a single or combination of conventional DMARDs. Residual arthritis or an incomplete response to IL-1 or IL-6 inhibitors should lead next to biologic or conventional DMARDs instead of long-term glucocorticoids.
For patients with MAS, the guidelines conditionally recommend IL-1 and IL-6 inhibitors over calcineurin inhibitor monotherapy to reach inactive disease and MAS resolution, with glucocorticoids conditionally recommended in initial treatment. Again, however, for patients with incomplete responses to IL-1 or IL-6 inhibitors or with residual arthritis, the guidelines advise biologic or conventional DMARDs over long-term glucocorticoids.
In patients with sJIA with or without a history of MAS who have inactive disease, practitioners should taper and discontinue glucocorticoids (a strong recommendation). A conditional recommendation for tapering and discontinuing biologic DMARDs follows attainment of inactive disease.
Beyond pharmacology
Although many of the nonpharmacologic recommendations did not have strong evidence based on assessment with Grading of Recommendations Assessment, Development, and Evaluation methodology, consensus was more often the case than not, Dr. Onel said, such as with vaccination.
“There was strong support for the use of immunizations in children with JIA and specific guidance for children with JIA receiving immunosuppression, not on immunosuppression, and children who are underimmunized or unimmunized,” she said. “Although the supportive evidence was very low as per GRADE, panel members were strongly in favor [of immunizations], given risk of infection for immunosuppressed children as well as the preponderance of evidence in similar disease states, such as IBD [inflammatory bowel disease].”
An area with less consensus was whether to check antibody titers for vaccine-preventable childhood infections before beginning immunosuppressive medication, but more panelists opposed the practice than supported it, Dr. Onel said.
“Some panelists felt that the information might be useful for risk management in case of an outbreak or exposure,” she said. “Most believed that screening a fully immunized child was of low benefit and might delay treatment and incur unnecessary cost.”
The process of developing the documents also reveals where the biggest gaps are in research.
“One of the things that we should strive for in the future is really to do more systematic studies so we have better quality of evidence going forward,” Dr. Shenoi said. Overall, however, the guidelines also reveal the progress made in treatment of JIA.
“We now know some of the key cytokines that are involved in the disease pathogenesis, and we have effective therapies for some of these pathways,” Dr. Shenoi said. “We used to use a lot more toxic medication for systemic JIA, and in past decades, these patients used to be on steroids forever. Now we have targeted therapies, and we have some patients who don’t ever need steroids because people are moving toward targeted therapies and having good results. That’s a huge step forward in the field.”
The research was funded by the ACR. Dr. Shenoi has been a consultant for Pfizer. Dr. Onel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment of systemic juvenile idiopathic arthritis (sJIA) should emphasize early use of conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs), compared with the previous reliance on NSAIDs and glucocorticoids, according to new guidelines from the American College of Rheumatology. The recently published 2021 guidelines focus on therapeutic approaches for oligoarthritis, temporomandibular joint (TMJ) arthritis, and sJIA.
“Systemic JIA should be treated early with biologics to rapidly bring disease under control and to avoid long-term use of glucocorticoids,” Karen Onel, MD, chief of the division of pediatric rheumatology at Weill Cornell Medicine, New York, and lead author of the guidelines, told this news organization. “Unfortunately, biologics can and are frequently denied for first-line use. For this reason, the guidelines are critically important as they demonstrate that first-line use of biologics are standard of care for the treatment of sJIA.”
The new publication is the second part of the ACR’s process to update JIA guidelines that began in 2017 and complements the release in 2019 of guidelines on the management of nonsystemic polyarthritis, sacroiliitis, and enthesitis, as well as a separate guidance on JIA-associated uveitis. The new guidelines include a second publication focused on nonpharmacologic therapies, medication monitoring, immunizations, and imaging. Both sets of guidelines grew out of a 15-member panel that included young adults with JIA and caregivers of children with JIA, and which required at least 70% agreement on recommendations.
“Though the scope of the two guidelines differed, one thing they had in common is the recognition of the importance of shared decision-making with the patient/caregiver,” Dr. Onel said. “Not every decision will be appropriate for every patient, which is why it was so instrumental to receive input from both patients and caregivers when creating these recommendations.”
Oligoarticular and TMJ arthritis
Oligoarticular and TMJ arthritis have similar recommendations, beginning with NSAIDs conditionally recommended and intra-articular glucocorticoids (IAGCs) strongly recommended as part of initial therapy. For oligoarticular arthritis, the guidelines specifically include a strong recommendation of triamcinolone hexacetonide as the preferred agent; no preferred agent is recommended for TMJ arthritis.
“The one thing that the panel was unanimous about was the use of triamcinolone hexacetonide for intra-articular steroid injections in oligoarticular kids,” Susan Shenoi, MBBS, MS, an associate professor and clinical director of pediatric rheumatology at Seattle Children’s Hospital and Research Center, said in an interview. “Triamcinolone hexacetonide has not been available recently, and through advocacy efforts, there is now a pathway to get that medication,” added Dr. Shenoi, a coauthor on the guidelines.
Dr. Onel said that “triamcinolone hexacetonide has been shown to be superior to alternative injectable glucocorticoids in achieving and maintaining remission in children with JIA,” but its unavailability meant physicians had to consider less effective, more potent, or more costly alternatives.” To address the shortage, “the FDA allowed the importation of one particular formulation of triamcinolone hexacetonide [Hexatrione 2%] specifically for joint injections in patients with JIA.”
The guidelines conditionally recommend against oral glucocorticoids for initial therapy for both oligoarticular and TMJ arthritis. In fact, throughout the guidelines it’s clear that the authors emphasize using steroids as little as possible, Dr. Shenoi said.
“Steroids are great anti-inflammatories, but in kids we worry about the long-term effects on growth and metabolism, and now we have many more DMARDs available,” Dr. Shenoi said.
The guidelines strongly recommend conventional synthetic DMARDs for patients with either of these diseases who cannot tolerate or do not respond to NSAIDs or IAGCs, with methotrexate conditionally recommended over leflunomide (Arava) for TMJ and over leflunomide, sulfasalazine (Azulfidine, Sulfazine), and hydroxychloroquine, respectively, for oligoarticular arthritis.
“NSAIDs remain widely used despite evidence supporting early use of DMARDs,” Dr. Onel said. “NSAIDs are readily available and familiar; however, they will not prevent disease progression. These guidelines should encourage short courses of NSAIDs only.”
If patients do not respond to or cannot tolerate NSAIDs, IAGCs, and at least one conventional DMARD, the guidelines strongly recommend a biologic DMARD for oligoarticular arthritis and conditionally recommend one for TMJ arthritis, without any preferences to the specific agent.
The guidelines also advise using validated disease activity measures to guide treatment decisions.
“The most important thing when you’re looking at these patients is to determine, do they have active disease or not?” Dr. Shenoi said. “If they have active disease, then you really want to step up therapy.” Using the relatively new concept of treat-to-target, Dr. Shenoi added that a crucial part of shared decision-making with the family is identifying the most appropriate target for that family “and then really trying hard to achieve that target.”
The guidelines also list risk factors for poor outcome that can be used to guide treatment decisions.
“Specific involvement of key joints, such as TMJ, wrist, sacroiliac, hip, and ankle, and other features were considered reasonable justification for early escalation of therapy,” Dr. Onel said. Other features included presence of erosive disease or enthesitis, delay in diagnosis, elevated levels of inflammation markers, and symmetric disease. “Moving quickly may be needed for a patient who is rapidly worsening, while moving slower may be appropriate for somebody who has improved substantially, but not fully.”
Systemic JIA with and without macrophage activation syndrome
For systemic JIA without macrophage activation syndrome (MAS), the guidelines similarly advise against oral glucocorticoids as initial monotherapy while conditionally recommending NSAIDs for initial monotherapy. Where the guidelines differ most from those for oligoarticular and TMJ arthritis is in progression of DMARD use, with a strong recommendation against conventional synthetic DMARDs as an initial monotherapy and interleukin-1 and IL-6 inhibitors conditionally recommended for initial monotherapy.
For patients who don’t adequately respond to NSAIDs or glucocorticoids, IL-1 and IL-6 inhibitors are strongly recommended over a single or combination of conventional DMARDs. Residual arthritis or an incomplete response to IL-1 or IL-6 inhibitors should lead next to biologic or conventional DMARDs instead of long-term glucocorticoids.
For patients with MAS, the guidelines conditionally recommend IL-1 and IL-6 inhibitors over calcineurin inhibitor monotherapy to reach inactive disease and MAS resolution, with glucocorticoids conditionally recommended in initial treatment. Again, however, for patients with incomplete responses to IL-1 or IL-6 inhibitors or with residual arthritis, the guidelines advise biologic or conventional DMARDs over long-term glucocorticoids.
In patients with sJIA with or without a history of MAS who have inactive disease, practitioners should taper and discontinue glucocorticoids (a strong recommendation). A conditional recommendation for tapering and discontinuing biologic DMARDs follows attainment of inactive disease.
Beyond pharmacology
Although many of the nonpharmacologic recommendations did not have strong evidence based on assessment with Grading of Recommendations Assessment, Development, and Evaluation methodology, consensus was more often the case than not, Dr. Onel said, such as with vaccination.
“There was strong support for the use of immunizations in children with JIA and specific guidance for children with JIA receiving immunosuppression, not on immunosuppression, and children who are underimmunized or unimmunized,” she said. “Although the supportive evidence was very low as per GRADE, panel members were strongly in favor [of immunizations], given risk of infection for immunosuppressed children as well as the preponderance of evidence in similar disease states, such as IBD [inflammatory bowel disease].”
An area with less consensus was whether to check antibody titers for vaccine-preventable childhood infections before beginning immunosuppressive medication, but more panelists opposed the practice than supported it, Dr. Onel said.
“Some panelists felt that the information might be useful for risk management in case of an outbreak or exposure,” she said. “Most believed that screening a fully immunized child was of low benefit and might delay treatment and incur unnecessary cost.”
The process of developing the documents also reveals where the biggest gaps are in research.
“One of the things that we should strive for in the future is really to do more systematic studies so we have better quality of evidence going forward,” Dr. Shenoi said. Overall, however, the guidelines also reveal the progress made in treatment of JIA.
“We now know some of the key cytokines that are involved in the disease pathogenesis, and we have effective therapies for some of these pathways,” Dr. Shenoi said. “We used to use a lot more toxic medication for systemic JIA, and in past decades, these patients used to be on steroids forever. Now we have targeted therapies, and we have some patients who don’t ever need steroids because people are moving toward targeted therapies and having good results. That’s a huge step forward in the field.”
The research was funded by the ACR. Dr. Shenoi has been a consultant for Pfizer. Dr. Onel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment of systemic juvenile idiopathic arthritis (sJIA) should emphasize early use of conventional synthetic and biologic disease-modifying antirheumatic drugs (DMARDs), compared with the previous reliance on NSAIDs and glucocorticoids, according to new guidelines from the American College of Rheumatology. The recently published 2021 guidelines focus on therapeutic approaches for oligoarthritis, temporomandibular joint (TMJ) arthritis, and sJIA.
“Systemic JIA should be treated early with biologics to rapidly bring disease under control and to avoid long-term use of glucocorticoids,” Karen Onel, MD, chief of the division of pediatric rheumatology at Weill Cornell Medicine, New York, and lead author of the guidelines, told this news organization. “Unfortunately, biologics can and are frequently denied for first-line use. For this reason, the guidelines are critically important as they demonstrate that first-line use of biologics are standard of care for the treatment of sJIA.”
The new publication is the second part of the ACR’s process to update JIA guidelines that began in 2017 and complements the release in 2019 of guidelines on the management of nonsystemic polyarthritis, sacroiliitis, and enthesitis, as well as a separate guidance on JIA-associated uveitis. The new guidelines include a second publication focused on nonpharmacologic therapies, medication monitoring, immunizations, and imaging. Both sets of guidelines grew out of a 15-member panel that included young adults with JIA and caregivers of children with JIA, and which required at least 70% agreement on recommendations.
“Though the scope of the two guidelines differed, one thing they had in common is the recognition of the importance of shared decision-making with the patient/caregiver,” Dr. Onel said. “Not every decision will be appropriate for every patient, which is why it was so instrumental to receive input from both patients and caregivers when creating these recommendations.”
Oligoarticular and TMJ arthritis
Oligoarticular and TMJ arthritis have similar recommendations, beginning with NSAIDs conditionally recommended and intra-articular glucocorticoids (IAGCs) strongly recommended as part of initial therapy. For oligoarticular arthritis, the guidelines specifically include a strong recommendation of triamcinolone hexacetonide as the preferred agent; no preferred agent is recommended for TMJ arthritis.
“The one thing that the panel was unanimous about was the use of triamcinolone hexacetonide for intra-articular steroid injections in oligoarticular kids,” Susan Shenoi, MBBS, MS, an associate professor and clinical director of pediatric rheumatology at Seattle Children’s Hospital and Research Center, said in an interview. “Triamcinolone hexacetonide has not been available recently, and through advocacy efforts, there is now a pathway to get that medication,” added Dr. Shenoi, a coauthor on the guidelines.
Dr. Onel said that “triamcinolone hexacetonide has been shown to be superior to alternative injectable glucocorticoids in achieving and maintaining remission in children with JIA,” but its unavailability meant physicians had to consider less effective, more potent, or more costly alternatives.” To address the shortage, “the FDA allowed the importation of one particular formulation of triamcinolone hexacetonide [Hexatrione 2%] specifically for joint injections in patients with JIA.”
The guidelines conditionally recommend against oral glucocorticoids for initial therapy for both oligoarticular and TMJ arthritis. In fact, throughout the guidelines it’s clear that the authors emphasize using steroids as little as possible, Dr. Shenoi said.
“Steroids are great anti-inflammatories, but in kids we worry about the long-term effects on growth and metabolism, and now we have many more DMARDs available,” Dr. Shenoi said.
The guidelines strongly recommend conventional synthetic DMARDs for patients with either of these diseases who cannot tolerate or do not respond to NSAIDs or IAGCs, with methotrexate conditionally recommended over leflunomide (Arava) for TMJ and over leflunomide, sulfasalazine (Azulfidine, Sulfazine), and hydroxychloroquine, respectively, for oligoarticular arthritis.
“NSAIDs remain widely used despite evidence supporting early use of DMARDs,” Dr. Onel said. “NSAIDs are readily available and familiar; however, they will not prevent disease progression. These guidelines should encourage short courses of NSAIDs only.”
If patients do not respond to or cannot tolerate NSAIDs, IAGCs, and at least one conventional DMARD, the guidelines strongly recommend a biologic DMARD for oligoarticular arthritis and conditionally recommend one for TMJ arthritis, without any preferences to the specific agent.
The guidelines also advise using validated disease activity measures to guide treatment decisions.
“The most important thing when you’re looking at these patients is to determine, do they have active disease or not?” Dr. Shenoi said. “If they have active disease, then you really want to step up therapy.” Using the relatively new concept of treat-to-target, Dr. Shenoi added that a crucial part of shared decision-making with the family is identifying the most appropriate target for that family “and then really trying hard to achieve that target.”
The guidelines also list risk factors for poor outcome that can be used to guide treatment decisions.
“Specific involvement of key joints, such as TMJ, wrist, sacroiliac, hip, and ankle, and other features were considered reasonable justification for early escalation of therapy,” Dr. Onel said. Other features included presence of erosive disease or enthesitis, delay in diagnosis, elevated levels of inflammation markers, and symmetric disease. “Moving quickly may be needed for a patient who is rapidly worsening, while moving slower may be appropriate for somebody who has improved substantially, but not fully.”
Systemic JIA with and without macrophage activation syndrome
For systemic JIA without macrophage activation syndrome (MAS), the guidelines similarly advise against oral glucocorticoids as initial monotherapy while conditionally recommending NSAIDs for initial monotherapy. Where the guidelines differ most from those for oligoarticular and TMJ arthritis is in progression of DMARD use, with a strong recommendation against conventional synthetic DMARDs as an initial monotherapy and interleukin-1 and IL-6 inhibitors conditionally recommended for initial monotherapy.
For patients who don’t adequately respond to NSAIDs or glucocorticoids, IL-1 and IL-6 inhibitors are strongly recommended over a single or combination of conventional DMARDs. Residual arthritis or an incomplete response to IL-1 or IL-6 inhibitors should lead next to biologic or conventional DMARDs instead of long-term glucocorticoids.
For patients with MAS, the guidelines conditionally recommend IL-1 and IL-6 inhibitors over calcineurin inhibitor monotherapy to reach inactive disease and MAS resolution, with glucocorticoids conditionally recommended in initial treatment. Again, however, for patients with incomplete responses to IL-1 or IL-6 inhibitors or with residual arthritis, the guidelines advise biologic or conventional DMARDs over long-term glucocorticoids.
In patients with sJIA with or without a history of MAS who have inactive disease, practitioners should taper and discontinue glucocorticoids (a strong recommendation). A conditional recommendation for tapering and discontinuing biologic DMARDs follows attainment of inactive disease.
Beyond pharmacology
Although many of the nonpharmacologic recommendations did not have strong evidence based on assessment with Grading of Recommendations Assessment, Development, and Evaluation methodology, consensus was more often the case than not, Dr. Onel said, such as with vaccination.
“There was strong support for the use of immunizations in children with JIA and specific guidance for children with JIA receiving immunosuppression, not on immunosuppression, and children who are underimmunized or unimmunized,” she said. “Although the supportive evidence was very low as per GRADE, panel members were strongly in favor [of immunizations], given risk of infection for immunosuppressed children as well as the preponderance of evidence in similar disease states, such as IBD [inflammatory bowel disease].”
An area with less consensus was whether to check antibody titers for vaccine-preventable childhood infections before beginning immunosuppressive medication, but more panelists opposed the practice than supported it, Dr. Onel said.
“Some panelists felt that the information might be useful for risk management in case of an outbreak or exposure,” she said. “Most believed that screening a fully immunized child was of low benefit and might delay treatment and incur unnecessary cost.”
The process of developing the documents also reveals where the biggest gaps are in research.
“One of the things that we should strive for in the future is really to do more systematic studies so we have better quality of evidence going forward,” Dr. Shenoi said. Overall, however, the guidelines also reveal the progress made in treatment of JIA.
“We now know some of the key cytokines that are involved in the disease pathogenesis, and we have effective therapies for some of these pathways,” Dr. Shenoi said. “We used to use a lot more toxic medication for systemic JIA, and in past decades, these patients used to be on steroids forever. Now we have targeted therapies, and we have some patients who don’t ever need steroids because people are moving toward targeted therapies and having good results. That’s a huge step forward in the field.”
The research was funded by the ACR. Dr. Shenoi has been a consultant for Pfizer. Dr. Onel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
High early recurrence rates with Merkel cell carcinoma
, and more than half of all patients with stage IV disease will have a recurrence within 1 year of definitive therapy, results of a new study show.
A study of 618 patients with MCC who were enrolled in a Seattle-based data repository shows that among all patients the 5-year recurrence rate was 40%. The risk of recurrence within the first year was 11% for patients with pathologic stage I disease, 33% for those with stage IIA/IIB disease, 45% for those with stage IIIB disease, and 58% for patients with pathologic stage IV MCC.
Approximately 95% of all recurrences happened within 3 years of the initial diagnosis, report Aubriana McEvoy, MD, from the University of Washington in Seattle, and colleagues.
“This cohort study indicates that the highest yield (and likely most cost-effective) time period for detecting MCC recurrence is 1-3 years after diagnosis,” they write in a study published online in JAMA Dermatology.
The estimated annual incidence of MCC in the United States in 2018 was 2,000 according to the American Cancer Society. The annual incidence rate is rising rapidly, however, and is estimated to reach 3,284 by 2025, McEvoy and colleagues write.
Although MCC is known to have high recurrence rates and is associated with a higher mortality rate than malignant melanoma, recurrence rate data are not captured by either the Surveillance, Epidemiology, and End Results (SEER) database or by the National Cancer Database. As a result, estimates of recurrence rates with MCC have been all over the map, ranging from 27% to 77%, depending on the population studied.
But as senior author Paul Nghiem, MD, PhD, professor and chair of dermatology at the University of Washington, Seattle, told this news organization, recurrence rates over time in their study were remarkably consistent.
“The biggest surprise to me was that, when we broke our nearly 20-year cohort into three 5- or 6-year chunks, every one of the groups had a 40% recurrence rate, within 1%. So we feel really confident that’s the right number,” he said.
Dr. Nghiem and colleagues report that, in contrast to patients with MCC, approximately 19% of patients with melanoma will have a recurrence, as will an estimated 5%-9% of patients with squamous cell carcinoma and 1%-10% of patients with basal cell carcinoma.
The fact that recurrence rates of MCC have remained stable over time despite presumed improvements in definitive therapy is disappointing, Dr. Nghiem acknowledged. He noted that it’s still unclear whether immunotherapy will have the same dramatic effect on survival rates for patients with MCC as it has for patients with malignant melanoma.
The high recurrence rates following definitive therapy for patients with early-stage disease was a novel finding, commented Shawn Demehri, MD, PhD, director of the high-risk skin cancer clinic at Massachusetts General Hospital in Boston.
“When you’re looking at patients with stage I or stage II, and they have definitive surgery but still have recurrences at a higher rate than melanoma, it brings home the point that these are among the most aggressive tumors of the skin,” he said in an interview.
The high recurrence rates seen with MCC are attributable to a variety of factors.
“This is a rare cancer of mostly older individuals with a lot of comorbidities, and also a cancer that, even though it is a primary cancer, might be detected a little later than even a melanoma primary tumor, just because of the nature of the neuroendocrine tumor cells,” he said.
Dr. Demehri was not involved in the study.
Prospective cohort
The study cohort consisted of 618 patients with MCC. The median age of the patients was 69, and 227 (37%) were women. The patients were enrolled within 6 months of their diagnosis in the prospective data repository from 2003 through 2019. Of this group, 223 had a recurrence of MCC.
As noted, there was a high risk of recurrence within 1 year, ranging from 11% for patients with pathologic stage I tumors to 58% for those with stage IV disease, and 95% of all recurrences occurred within 3 years of definitive therapy.
To get a better picture of the natural history of MCC recurrence, the investigators studied a cohort of patients with pathologically confirmed MCC who were prospectively enrolled from January 2003 through April 2019 in a data repository maintained at the University of Washington.
In addition to disease stage, factors associated with increased recurrence risk in univariable analyses include immunosuppression (hazard ratio, 2.4; P < .001), male sex (HR, 1.9; P < .001), known primary lesion among patients with clinically detectable nodal disease (HR, 2.3; P = .001), and older age (HR, 1.1, P = .06 for each 10-year increase).
Of the 187 patients in the cohort who died during the study, 121 died from MCC. At 4 years after diagnosis, MCC-specific survival rates were 95% for patients with pathologic stage I, 84% with stage IIA/IIB, 80% with stage IIIA, 58% with stage IIIB, and 41% with stage IV.
Evidence supports close monitoring within the first 3 years for patients with stage I-II MCC. Local recurrence within or adjacent to the primary tumor scar was associated with a 5-year MCC-specific survival rate of 85%, compared with 88% of patients with stage I or II disease who did not have recurrences.
“Because more than 90% of MCC recurrences arise within 3 years, it is appropriate to adjust surveillance intensity accordingly. Stage- and time-specific recurrence data can assist in appropriately focusing surveillance resources on patients and time intervals in which recurrence risk is highest,” the authors wrote.
“If you’re a patient who has not had your cancer come back for 3, 4, or 5 years, you can really cut down on the intensity of your follow-up and scans,” Dr. Nghiem said.
“We do now have two excellent blood tests that are working very well, and we have really good ways to detect the cancer coming back early, and that’s important, because we have potentially curative therapies that tend to work better if you catch the cancer early,” he said.
The study was supported by the National Institutes of Health. Dr. Nghiem reported personal fees and institutional support outside the study from several companies and patents for Merkel cell therapies with the University of Washington and University of Denmark. Dr. Demehri has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, and more than half of all patients with stage IV disease will have a recurrence within 1 year of definitive therapy, results of a new study show.
A study of 618 patients with MCC who were enrolled in a Seattle-based data repository shows that among all patients the 5-year recurrence rate was 40%. The risk of recurrence within the first year was 11% for patients with pathologic stage I disease, 33% for those with stage IIA/IIB disease, 45% for those with stage IIIB disease, and 58% for patients with pathologic stage IV MCC.
Approximately 95% of all recurrences happened within 3 years of the initial diagnosis, report Aubriana McEvoy, MD, from the University of Washington in Seattle, and colleagues.
“This cohort study indicates that the highest yield (and likely most cost-effective) time period for detecting MCC recurrence is 1-3 years after diagnosis,” they write in a study published online in JAMA Dermatology.
The estimated annual incidence of MCC in the United States in 2018 was 2,000 according to the American Cancer Society. The annual incidence rate is rising rapidly, however, and is estimated to reach 3,284 by 2025, McEvoy and colleagues write.
Although MCC is known to have high recurrence rates and is associated with a higher mortality rate than malignant melanoma, recurrence rate data are not captured by either the Surveillance, Epidemiology, and End Results (SEER) database or by the National Cancer Database. As a result, estimates of recurrence rates with MCC have been all over the map, ranging from 27% to 77%, depending on the population studied.
But as senior author Paul Nghiem, MD, PhD, professor and chair of dermatology at the University of Washington, Seattle, told this news organization, recurrence rates over time in their study were remarkably consistent.
“The biggest surprise to me was that, when we broke our nearly 20-year cohort into three 5- or 6-year chunks, every one of the groups had a 40% recurrence rate, within 1%. So we feel really confident that’s the right number,” he said.
Dr. Nghiem and colleagues report that, in contrast to patients with MCC, approximately 19% of patients with melanoma will have a recurrence, as will an estimated 5%-9% of patients with squamous cell carcinoma and 1%-10% of patients with basal cell carcinoma.
The fact that recurrence rates of MCC have remained stable over time despite presumed improvements in definitive therapy is disappointing, Dr. Nghiem acknowledged. He noted that it’s still unclear whether immunotherapy will have the same dramatic effect on survival rates for patients with MCC as it has for patients with malignant melanoma.
The high recurrence rates following definitive therapy for patients with early-stage disease was a novel finding, commented Shawn Demehri, MD, PhD, director of the high-risk skin cancer clinic at Massachusetts General Hospital in Boston.
“When you’re looking at patients with stage I or stage II, and they have definitive surgery but still have recurrences at a higher rate than melanoma, it brings home the point that these are among the most aggressive tumors of the skin,” he said in an interview.
The high recurrence rates seen with MCC are attributable to a variety of factors.
“This is a rare cancer of mostly older individuals with a lot of comorbidities, and also a cancer that, even though it is a primary cancer, might be detected a little later than even a melanoma primary tumor, just because of the nature of the neuroendocrine tumor cells,” he said.
Dr. Demehri was not involved in the study.
Prospective cohort
The study cohort consisted of 618 patients with MCC. The median age of the patients was 69, and 227 (37%) were women. The patients were enrolled within 6 months of their diagnosis in the prospective data repository from 2003 through 2019. Of this group, 223 had a recurrence of MCC.
As noted, there was a high risk of recurrence within 1 year, ranging from 11% for patients with pathologic stage I tumors to 58% for those with stage IV disease, and 95% of all recurrences occurred within 3 years of definitive therapy.
To get a better picture of the natural history of MCC recurrence, the investigators studied a cohort of patients with pathologically confirmed MCC who were prospectively enrolled from January 2003 through April 2019 in a data repository maintained at the University of Washington.
In addition to disease stage, factors associated with increased recurrence risk in univariable analyses include immunosuppression (hazard ratio, 2.4; P < .001), male sex (HR, 1.9; P < .001), known primary lesion among patients with clinically detectable nodal disease (HR, 2.3; P = .001), and older age (HR, 1.1, P = .06 for each 10-year increase).
Of the 187 patients in the cohort who died during the study, 121 died from MCC. At 4 years after diagnosis, MCC-specific survival rates were 95% for patients with pathologic stage I, 84% with stage IIA/IIB, 80% with stage IIIA, 58% with stage IIIB, and 41% with stage IV.
Evidence supports close monitoring within the first 3 years for patients with stage I-II MCC. Local recurrence within or adjacent to the primary tumor scar was associated with a 5-year MCC-specific survival rate of 85%, compared with 88% of patients with stage I or II disease who did not have recurrences.
“Because more than 90% of MCC recurrences arise within 3 years, it is appropriate to adjust surveillance intensity accordingly. Stage- and time-specific recurrence data can assist in appropriately focusing surveillance resources on patients and time intervals in which recurrence risk is highest,” the authors wrote.
“If you’re a patient who has not had your cancer come back for 3, 4, or 5 years, you can really cut down on the intensity of your follow-up and scans,” Dr. Nghiem said.
“We do now have two excellent blood tests that are working very well, and we have really good ways to detect the cancer coming back early, and that’s important, because we have potentially curative therapies that tend to work better if you catch the cancer early,” he said.
The study was supported by the National Institutes of Health. Dr. Nghiem reported personal fees and institutional support outside the study from several companies and patents for Merkel cell therapies with the University of Washington and University of Denmark. Dr. Demehri has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, and more than half of all patients with stage IV disease will have a recurrence within 1 year of definitive therapy, results of a new study show.
A study of 618 patients with MCC who were enrolled in a Seattle-based data repository shows that among all patients the 5-year recurrence rate was 40%. The risk of recurrence within the first year was 11% for patients with pathologic stage I disease, 33% for those with stage IIA/IIB disease, 45% for those with stage IIIB disease, and 58% for patients with pathologic stage IV MCC.
Approximately 95% of all recurrences happened within 3 years of the initial diagnosis, report Aubriana McEvoy, MD, from the University of Washington in Seattle, and colleagues.
“This cohort study indicates that the highest yield (and likely most cost-effective) time period for detecting MCC recurrence is 1-3 years after diagnosis,” they write in a study published online in JAMA Dermatology.
The estimated annual incidence of MCC in the United States in 2018 was 2,000 according to the American Cancer Society. The annual incidence rate is rising rapidly, however, and is estimated to reach 3,284 by 2025, McEvoy and colleagues write.
Although MCC is known to have high recurrence rates and is associated with a higher mortality rate than malignant melanoma, recurrence rate data are not captured by either the Surveillance, Epidemiology, and End Results (SEER) database or by the National Cancer Database. As a result, estimates of recurrence rates with MCC have been all over the map, ranging from 27% to 77%, depending on the population studied.
But as senior author Paul Nghiem, MD, PhD, professor and chair of dermatology at the University of Washington, Seattle, told this news organization, recurrence rates over time in their study were remarkably consistent.
“The biggest surprise to me was that, when we broke our nearly 20-year cohort into three 5- or 6-year chunks, every one of the groups had a 40% recurrence rate, within 1%. So we feel really confident that’s the right number,” he said.
Dr. Nghiem and colleagues report that, in contrast to patients with MCC, approximately 19% of patients with melanoma will have a recurrence, as will an estimated 5%-9% of patients with squamous cell carcinoma and 1%-10% of patients with basal cell carcinoma.
The fact that recurrence rates of MCC have remained stable over time despite presumed improvements in definitive therapy is disappointing, Dr. Nghiem acknowledged. He noted that it’s still unclear whether immunotherapy will have the same dramatic effect on survival rates for patients with MCC as it has for patients with malignant melanoma.
The high recurrence rates following definitive therapy for patients with early-stage disease was a novel finding, commented Shawn Demehri, MD, PhD, director of the high-risk skin cancer clinic at Massachusetts General Hospital in Boston.
“When you’re looking at patients with stage I or stage II, and they have definitive surgery but still have recurrences at a higher rate than melanoma, it brings home the point that these are among the most aggressive tumors of the skin,” he said in an interview.
The high recurrence rates seen with MCC are attributable to a variety of factors.
“This is a rare cancer of mostly older individuals with a lot of comorbidities, and also a cancer that, even though it is a primary cancer, might be detected a little later than even a melanoma primary tumor, just because of the nature of the neuroendocrine tumor cells,” he said.
Dr. Demehri was not involved in the study.
Prospective cohort
The study cohort consisted of 618 patients with MCC. The median age of the patients was 69, and 227 (37%) were women. The patients were enrolled within 6 months of their diagnosis in the prospective data repository from 2003 through 2019. Of this group, 223 had a recurrence of MCC.
As noted, there was a high risk of recurrence within 1 year, ranging from 11% for patients with pathologic stage I tumors to 58% for those with stage IV disease, and 95% of all recurrences occurred within 3 years of definitive therapy.
To get a better picture of the natural history of MCC recurrence, the investigators studied a cohort of patients with pathologically confirmed MCC who were prospectively enrolled from January 2003 through April 2019 in a data repository maintained at the University of Washington.
In addition to disease stage, factors associated with increased recurrence risk in univariable analyses include immunosuppression (hazard ratio, 2.4; P < .001), male sex (HR, 1.9; P < .001), known primary lesion among patients with clinically detectable nodal disease (HR, 2.3; P = .001), and older age (HR, 1.1, P = .06 for each 10-year increase).
Of the 187 patients in the cohort who died during the study, 121 died from MCC. At 4 years after diagnosis, MCC-specific survival rates were 95% for patients with pathologic stage I, 84% with stage IIA/IIB, 80% with stage IIIA, 58% with stage IIIB, and 41% with stage IV.
Evidence supports close monitoring within the first 3 years for patients with stage I-II MCC. Local recurrence within or adjacent to the primary tumor scar was associated with a 5-year MCC-specific survival rate of 85%, compared with 88% of patients with stage I or II disease who did not have recurrences.
“Because more than 90% of MCC recurrences arise within 3 years, it is appropriate to adjust surveillance intensity accordingly. Stage- and time-specific recurrence data can assist in appropriately focusing surveillance resources on patients and time intervals in which recurrence risk is highest,” the authors wrote.
“If you’re a patient who has not had your cancer come back for 3, 4, or 5 years, you can really cut down on the intensity of your follow-up and scans,” Dr. Nghiem said.
“We do now have two excellent blood tests that are working very well, and we have really good ways to detect the cancer coming back early, and that’s important, because we have potentially curative therapies that tend to work better if you catch the cancer early,” he said.
The study was supported by the National Institutes of Health. Dr. Nghiem reported personal fees and institutional support outside the study from several companies and patents for Merkel cell therapies with the University of Washington and University of Denmark. Dr. Demehri has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY