User login
An Unusual Presentation of Calciphylaxis
To the Editor:
Calciphylaxis (also known as calcific uremic arteriolopathy and calcifying panniculitis) is a rare vasculopathy affecting the small vessels.1 It is characterized by cutaneous ischemia and necrosis secondary to calcification. It is most commonly seen in patients with end-stage renal disease (ESRD) and hyperparathyroidism.1-3 Histopathologic features that are consistent with the diagnosis of calciphylaxis include calcification of medium-sized vessels in the deep dermis or subcutaneous fat as well as smaller distal vessels that supply the papillary dermis and epidermis.4,5 Although it commonly presents as well-demarcated, painful, purplish lesions that evolve into necrotic eschars, calciphylaxis rarely can present with hemorrhagic or serous bullous lesions followed by ulceration, as was seen in our patient.1,5,6 We report this uncommon presentation to highlight the variety in clinical appearance of calciphylaxis and the importance of early diagnosis.
A 43-year-old woman presented to the emergency department for evaluation of chest and abdominal pain that began 1 day prior to presentation. She had a history of systemic lupus erythematosus and ESRD secondary to poststreptococcal glomerulonephritis and was currently on peritoneal dialysis. The patient was admitted for peritonitis and treated with broad-spectrum antibiotics. At the time of admission, the patient also was noted to have several painful bullae on the legs. Her medical history also was remarkable for cerebral infarction, fibromyalgia, cerebral artery occlusion with cerebral infarction, sciatica, hyperlipidemia, deep vein thrombosis, and seizures. She had no history of herpes simplex virus. Surgical history was remarkable for tubal ligation, nephrectomy and kidney transplant, parathyroidectomy, and cholecystectomy. The patient’s medications included sevelamer carbonate, prednisone, epogen, calcium carbonate, esomeprazole, ondansetron, topical gentamicin, and atorvastatin.
Skin examination was performed by the inpatient dermatology service and revealed several tense, 1- to 5-cm, nonhemorrhagic bullae on the thighs and lower legs, some that had ruptured. The lesions were notably tender to palpation. No surrounding erythema, ecchymosis, or warmth was appreciated. The Nikolsky sign was negative. The patient also was noted to have at least grade 2 to 3+ pitting edema of the bilateral legs. The oral and conjunctival mucosae were unremarkable.
Antinuclear antibody, double-stranded DNA, and anti-Smith antibody levels were negative. A punch biopsy of the left lateral thigh revealed intraepidermal vesicular dermatitis with dermal edema suggestive of edema bullae and direct immunofluorescence was negative for immune complex and complement deposition.
Conservative therapy with wound care was recommended. The patient continued to report persistent severe skin pain and developed a subcutaneous nodule on the right inner thigh 1 week later, prompting a second biopsy. Results of the excisional biopsy were nondiagnostic but were suggestive of calciphylaxis, revealing subepidermal bullae with epidermal necrosis, a scant perivascular lymphocytic infiltrate, and extravasated erythrocytes. No evidence of calcification was seen within the vessels. The patient was then started on sodium thiosulfate with hemodialysis for treatment of presumed calciphylaxis.
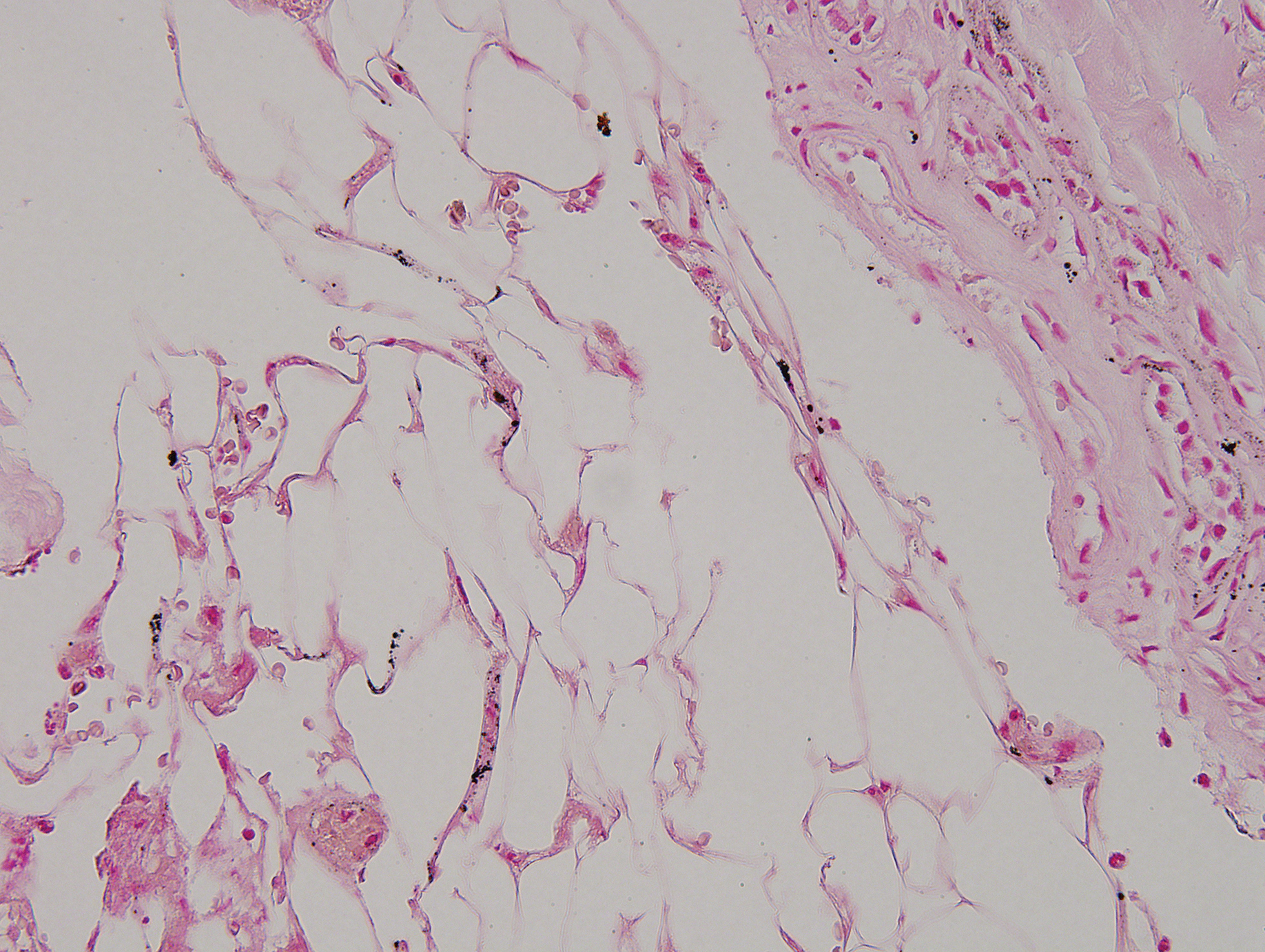
Despite meticulous wound care and treatment with sodium thiosulfate, the patient developed ulcerations with necrotic eschars on the bilateral buttocks, hips, and thighs 1 month later (Figure 1). She subsequently worsened over the next few weeks. She developed sepsis and was transferred to the intensive care unit. A third biopsy was performed, finally confirming the diagnosis of calciphylaxis. Histopathology revealed small blood vessels with basophilic granular deposits in the walls consistent with calcium in the subcutaneous tissue (highlighted with the von Kossa stain), as well as thrombi in the lumens of some vessels; early fat necrosis; focal epidermal necrosis with underlying congested blood vessels with deposits in their walls; a perivascular infiltrate predominately of lymphocytes and neutrophils with scattered nuclear dust; and thick, hyalinized, closely crowded collagen bundles in the reticular dermis and in a widened subcutaneous septum (Figures 2 and 3).


Supportive care and pain control were continued, but the overall prognosis was determined to be very poor, and the patient eventually was discharged to hospice and died.
Although calciphylaxis is commonly seen in patients with ESRD and hyperparathyroidism, patients without renal disease also may develop the condition.2,3 Prior epidemiologic studies have shown a prevalence of 1% in patients with chronic kidney disease and up to 4% in those receiving dialysis.2-5 The average age at presentation is 48 years.6,7 Although calciphylaxis has been noted to affect males and females equally, some studies have suggested a female predominance.5-8
The etiology of calciphylaxis is unknown, but ESRD requiring dialysis, primary or secondary hyperparathyroidism, obesity, diabetes mellitus, skin trauma, and/or a hypercoagulable state may put patients at increased risk for developing this disease.2,3 Other risk factors include systemic corticosteroids, liver disease, increased serum aluminum, and increased erythrocyte sedimentation rate. Although high calcium-phosphate product has been noted as a risk factor in prior studies, one retrospective study found that it does not reliably confirm or exclude a diagnosis of calciphylaxis.8
The pathogenesis of calciphylaxis is not well understood; however, some researchers suggest that an imbalance in calcium-phosphate homeostasis may lead to calciphylaxis; that is, elevated calcium and phosphate levels exceed their solubility and deposit in the walls of small- and medium-sized arteries, which consequently leads to ischemic necrosis and gangrene of the surrounding tissue.9
Clinically, calciphylaxis has an indolent onset and usually presents as well-demarcated, painful, purplish, mottled lesions that evolve into necrotic gray-black eschars and gangrene in adjacent tissues.1,5,6 The ischemic process may even extend to the muscle layer.5 Other common presentations include mild erythematous patches; livedo reticularis; painful nodules; necrotic ulcerating lesions; and more rarely flaccid, hemorrhagic, or serous bullous lesions followed by ulceration, as was seen in our patient.6,9,10 Lesions usually begin at sites of trauma and seem to be distributed symmetrically.5,6 The most commonly affected locations are the legs, specifically the medial thighs, as well as the abdomen and buttocks, but lesions also can be found at more distal sites such as the breasts, tongue, vulva, penis, fingers, and toes.5,6,10 The head and neck region rarely is affected. Although uncommon, calciphylaxis may affect other organs, including the lungs, stomach, kidneys, and adrenal glands.5 The accompanying systemic symptoms and findings may include muscle weakness, tenderness, or myositis with rhabdomyolysis; calcific cerebral embolism; dementia and infarction of the central nervous system; acute respiratory failure; heart disease; atrioventricular block; and calcification of the cardiac conduction system.6 Unlike other forms of peripheral vascular disease, distal pulses are present in calciphylaxis, as blood flow usually is preserved distal and deep to the areas of necrosis.5,6
A careful history and thorough physical examination are important first steps in the diagnosis of this condition.2,10 Although there are no definitive laboratory tests, elevated serum calcium, phosphorous, and calcium-phosphate product levels, as well as parathyroid hormone level, may be suggestive of calciphylaxis.2,5 Leukocytosis may occur if an infection is present.5
The most accurate method to confirm the diagnosis is a deep incisional biopsy from an erythematous, slightly purpuric area adjacent to the necrotic lesion.2,10,11 The histopathologic features used to make the diagnosis include calcification of medium-sized vessels, particularly the intimal or medial layers, in the deep dermis and subcutaneous fat in addition to lobular capillaries of the subcutaneous fat.5,10 These vessels, including the smaller distal vessels that supply the papillary dermis and epidermis, also may be thrombosed due to calcification, leading to vascular occlusion and subsequently ischemic necrosis of the overlying epidermis.10 Other findings may include pseudoxanthoma elasticum changes, panniculitis, and subcutaneous fat necrosis.4,10
The differential diagnosis for calciphylaxis includes peripheral vascular disease, vasculitis, juvenile dermatomyositis, proteins C and S deficiencies, cryofibrinogenemia, calcinosis cutis, and tumoral calcinosis.2 Polyarteritis nodosa, Sjögren syndrome, atherosclerotic peripheral vascular disease, pyoderma gangrenosum, systemic lupus erythematosus, necrotizing fasciitis, septic embolism, and necrosis secondary to warfarin and heparin may mimic calciphylaxis.5
Treatment of calciphylaxis is multidimensional but primarily is supportive.6,11 Controlling calcium and phosphate levels and secondary hyperparathyroidism through diet and phosphate binders (eg, sevelamer hydrochloride) has been shown to be effective.6 Pamidronate, a bisphosphonate, inhibits arterial calcification in animal models and has been reported to treat calciphylaxis, resulting in marked pain reduction and ulcer healing.4,6 Cinacalcet, which functions as a calcimimetic, has been implicated in the treatment of calciphylaxis. It has been used to treat primary and secondary hyperparathyroidism and to normalize serum calcium levels; it also may be used as an alternative to parathyroidectomy.4,6 Intravenous administration of sodium thiosulfate, a potent antioxidant and chelator of calcium, has been helpful in reversing signs and symptoms of calciphylaxis.6,12 It also has been shown to effectively remove extra calcium during peritoneal dialysis.6 Parathyroidectomy has been useful in patients with markedly elevated parathyroid hormone levels, as it suppresses or eliminates the sensitizing agent causing hypercalcemia, elevated calcium-phosphate product, and hyperparathyroidism.1,2,6,13
Wound care and prevention of sepsis are essential in the treatment of calciphylaxis. Management options include surgical debridement, hydrocolloid and biologic dressings, skin grafts, systemic antibiotics, oral pentoxifylline combined with maggot therapy, nutritional support, hyperbaric oxygen therapy, and revascularization and amputation when other interventions have failed. Pain control with analgesics and correction of thrombosis in the skin and blood vessels via anticoagulation therapy also are important complementary treatments.6
The clinical outcome of calciphylaxis is dependent on early diagnosis, antimicrobial therapy, and wound management,9 but overall, the prognosis usually is poor and has a high mortality rate. The most common causes of death are infection and sepsis.1,9 A study of 7 cases reported 100% mortality,14 but other studies have suggested a mortality rate of 60% to 80%.4,10 Female sex and obesity are poor prognostic indicators.2 A better prognosis has been appreciated in cases in which lesions occur at distal sites (eg, lower legs, hands) compared to more proximal sites (eg, abdomen), where 25% and 75% mortalities have been noted, respectively.10,14,15 In one study, the overall mortality rate was 45% in patients with calciphylaxis at 1 year.6 The rate was 41% in patients with plaques only and 67% in those who presented with ulceration. Patients who survive often experience a high degree of morbidity and prolonged hospitalization; these patients often are severely debilitated, especially in the case of limb amputation.6
Our report of calciphylaxis demonstrates the diversity in clinical presentation and emphasizes the importance of early and accurate diagnosis in reducing morbidity and mortality. In our case, the patient presented with skin pain and tense nonhemorrhagic bullae without underlying ecchymotic or erythematous lesions as the earliest sign of calciphylaxis. Physicians should have a high degree of suspicion in the setting of dialysis-dependent ESRD patients with bullae, extreme pain, and continuous decline. We hope that this case will help increase awareness of the varying presentations of this condition.
- Hanafusa T, Yamaguchi Y, Tani M, et al. Intractable wounds caused by calcific uremic arteriolopathy treated with bisphosphonates. J Am Acad Dermatol. 2001;57:1021-1025.
- Somorin AO, Harbi AA, Subaity Y, et al. Calciphylaxis: case report and literature review. Afr J Med Sci. 2002;31:175-178.
- Barreiros HM, Goulão J, Cunha H, et al. Calciphylaxis: a diagnostic and therapeutic challenge. J Dermatol Case Rep. 2013;2:69-70.
- Vedvyas C, Winterfield LS, Vleugels RA. Calciphylaxis: a systematic review of existing and emerging therapies. J Am Acad Dermatol. 2012;67:E253-E260.
- Beitz JM. Calciphylaxis: a case study with differential diagnosis. Ostomy Wound Manag. 2003;49:28-38.
- Daudén E, Oñate M. Calciphylaxis. Dermatol Clin. 2008;26:557-568.
- Oh DH, Eulau D, Tokugawa DA, et al. Five cases of calciphylaxis and a review of the literature. J Am Acad Dermatol. 1999;40:979-987.
- Weenig RH, Sewell LD, Davis MDP, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-578.
- Hanvesakul R, Silva MA, Hejmadi R, et al. Calciphylaxis following kidney transplantation: a case report. J Med Cases. 2009;3:9297.
- Kouba DJ, Owens NM, Barrett TL, et al. An unusual case of calciphylaxis. J Cutan Med Surg. 2004;8:19-22.
- Arch-Ferrer JE, Beenken SW, Rue LW, et al. Therapy for calciphylaxis: an outcome analysis. Surgery. 2003;134:941-945.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Mirza I, Chaubay D, Gunderia H, et al. An unusual presentation of calciphylaxis due to primary hyperparathyroidism. Arch Pathol Lab Med. 2001;125:1351-1353.
- Alain J, Poulin YP, Cloutier RA, et al. Calciphylaxis: seven new cases. J Cutan Med Surg. 2000;4:213-218.
- Hafner J, Keusch G, Wahl C, et al. Calciphylaxis: a syndrome of skin necrosis and acral gangrene in chronic renal failure. Vasa. 1998;27:137-143.
To the Editor:
Calciphylaxis (also known as calcific uremic arteriolopathy and calcifying panniculitis) is a rare vasculopathy affecting the small vessels.1 It is characterized by cutaneous ischemia and necrosis secondary to calcification. It is most commonly seen in patients with end-stage renal disease (ESRD) and hyperparathyroidism.1-3 Histopathologic features that are consistent with the diagnosis of calciphylaxis include calcification of medium-sized vessels in the deep dermis or subcutaneous fat as well as smaller distal vessels that supply the papillary dermis and epidermis.4,5 Although it commonly presents as well-demarcated, painful, purplish lesions that evolve into necrotic eschars, calciphylaxis rarely can present with hemorrhagic or serous bullous lesions followed by ulceration, as was seen in our patient.1,5,6 We report this uncommon presentation to highlight the variety in clinical appearance of calciphylaxis and the importance of early diagnosis.
A 43-year-old woman presented to the emergency department for evaluation of chest and abdominal pain that began 1 day prior to presentation. She had a history of systemic lupus erythematosus and ESRD secondary to poststreptococcal glomerulonephritis and was currently on peritoneal dialysis. The patient was admitted for peritonitis and treated with broad-spectrum antibiotics. At the time of admission, the patient also was noted to have several painful bullae on the legs. Her medical history also was remarkable for cerebral infarction, fibromyalgia, cerebral artery occlusion with cerebral infarction, sciatica, hyperlipidemia, deep vein thrombosis, and seizures. She had no history of herpes simplex virus. Surgical history was remarkable for tubal ligation, nephrectomy and kidney transplant, parathyroidectomy, and cholecystectomy. The patient’s medications included sevelamer carbonate, prednisone, epogen, calcium carbonate, esomeprazole, ondansetron, topical gentamicin, and atorvastatin.
Skin examination was performed by the inpatient dermatology service and revealed several tense, 1- to 5-cm, nonhemorrhagic bullae on the thighs and lower legs, some that had ruptured. The lesions were notably tender to palpation. No surrounding erythema, ecchymosis, or warmth was appreciated. The Nikolsky sign was negative. The patient also was noted to have at least grade 2 to 3+ pitting edema of the bilateral legs. The oral and conjunctival mucosae were unremarkable.
Antinuclear antibody, double-stranded DNA, and anti-Smith antibody levels were negative. A punch biopsy of the left lateral thigh revealed intraepidermal vesicular dermatitis with dermal edema suggestive of edema bullae and direct immunofluorescence was negative for immune complex and complement deposition.
Conservative therapy with wound care was recommended. The patient continued to report persistent severe skin pain and developed a subcutaneous nodule on the right inner thigh 1 week later, prompting a second biopsy. Results of the excisional biopsy were nondiagnostic but were suggestive of calciphylaxis, revealing subepidermal bullae with epidermal necrosis, a scant perivascular lymphocytic infiltrate, and extravasated erythrocytes. No evidence of calcification was seen within the vessels. The patient was then started on sodium thiosulfate with hemodialysis for treatment of presumed calciphylaxis.
Despite meticulous wound care and treatment with sodium thiosulfate, the patient developed ulcerations with necrotic eschars on the bilateral buttocks, hips, and thighs 1 month later (Figure 1). She subsequently worsened over the next few weeks. She developed sepsis and was transferred to the intensive care unit. A third biopsy was performed, finally confirming the diagnosis of calciphylaxis. Histopathology revealed small blood vessels with basophilic granular deposits in the walls consistent with calcium in the subcutaneous tissue (highlighted with the von Kossa stain), as well as thrombi in the lumens of some vessels; early fat necrosis; focal epidermal necrosis with underlying congested blood vessels with deposits in their walls; a perivascular infiltrate predominately of lymphocytes and neutrophils with scattered nuclear dust; and thick, hyalinized, closely crowded collagen bundles in the reticular dermis and in a widened subcutaneous septum (Figures 2 and 3).



Supportive care and pain control were continued, but the overall prognosis was determined to be very poor, and the patient eventually was discharged to hospice and died.
Although calciphylaxis is commonly seen in patients with ESRD and hyperparathyroidism, patients without renal disease also may develop the condition.2,3 Prior epidemiologic studies have shown a prevalence of 1% in patients with chronic kidney disease and up to 4% in those receiving dialysis.2-5 The average age at presentation is 48 years.6,7 Although calciphylaxis has been noted to affect males and females equally, some studies have suggested a female predominance.5-8
The etiology of calciphylaxis is unknown, but ESRD requiring dialysis, primary or secondary hyperparathyroidism, obesity, diabetes mellitus, skin trauma, and/or a hypercoagulable state may put patients at increased risk for developing this disease.2,3 Other risk factors include systemic corticosteroids, liver disease, increased serum aluminum, and increased erythrocyte sedimentation rate. Although high calcium-phosphate product has been noted as a risk factor in prior studies, one retrospective study found that it does not reliably confirm or exclude a diagnosis of calciphylaxis.8
The pathogenesis of calciphylaxis is not well understood; however, some researchers suggest that an imbalance in calcium-phosphate homeostasis may lead to calciphylaxis; that is, elevated calcium and phosphate levels exceed their solubility and deposit in the walls of small- and medium-sized arteries, which consequently leads to ischemic necrosis and gangrene of the surrounding tissue.9
Clinically, calciphylaxis has an indolent onset and usually presents as well-demarcated, painful, purplish, mottled lesions that evolve into necrotic gray-black eschars and gangrene in adjacent tissues.1,5,6 The ischemic process may even extend to the muscle layer.5 Other common presentations include mild erythematous patches; livedo reticularis; painful nodules; necrotic ulcerating lesions; and more rarely flaccid, hemorrhagic, or serous bullous lesions followed by ulceration, as was seen in our patient.6,9,10 Lesions usually begin at sites of trauma and seem to be distributed symmetrically.5,6 The most commonly affected locations are the legs, specifically the medial thighs, as well as the abdomen and buttocks, but lesions also can be found at more distal sites such as the breasts, tongue, vulva, penis, fingers, and toes.5,6,10 The head and neck region rarely is affected. Although uncommon, calciphylaxis may affect other organs, including the lungs, stomach, kidneys, and adrenal glands.5 The accompanying systemic symptoms and findings may include muscle weakness, tenderness, or myositis with rhabdomyolysis; calcific cerebral embolism; dementia and infarction of the central nervous system; acute respiratory failure; heart disease; atrioventricular block; and calcification of the cardiac conduction system.6 Unlike other forms of peripheral vascular disease, distal pulses are present in calciphylaxis, as blood flow usually is preserved distal and deep to the areas of necrosis.5,6
A careful history and thorough physical examination are important first steps in the diagnosis of this condition.2,10 Although there are no definitive laboratory tests, elevated serum calcium, phosphorous, and calcium-phosphate product levels, as well as parathyroid hormone level, may be suggestive of calciphylaxis.2,5 Leukocytosis may occur if an infection is present.5
The most accurate method to confirm the diagnosis is a deep incisional biopsy from an erythematous, slightly purpuric area adjacent to the necrotic lesion.2,10,11 The histopathologic features used to make the diagnosis include calcification of medium-sized vessels, particularly the intimal or medial layers, in the deep dermis and subcutaneous fat in addition to lobular capillaries of the subcutaneous fat.5,10 These vessels, including the smaller distal vessels that supply the papillary dermis and epidermis, also may be thrombosed due to calcification, leading to vascular occlusion and subsequently ischemic necrosis of the overlying epidermis.10 Other findings may include pseudoxanthoma elasticum changes, panniculitis, and subcutaneous fat necrosis.4,10
The differential diagnosis for calciphylaxis includes peripheral vascular disease, vasculitis, juvenile dermatomyositis, proteins C and S deficiencies, cryofibrinogenemia, calcinosis cutis, and tumoral calcinosis.2 Polyarteritis nodosa, Sjögren syndrome, atherosclerotic peripheral vascular disease, pyoderma gangrenosum, systemic lupus erythematosus, necrotizing fasciitis, septic embolism, and necrosis secondary to warfarin and heparin may mimic calciphylaxis.5
Treatment of calciphylaxis is multidimensional but primarily is supportive.6,11 Controlling calcium and phosphate levels and secondary hyperparathyroidism through diet and phosphate binders (eg, sevelamer hydrochloride) has been shown to be effective.6 Pamidronate, a bisphosphonate, inhibits arterial calcification in animal models and has been reported to treat calciphylaxis, resulting in marked pain reduction and ulcer healing.4,6 Cinacalcet, which functions as a calcimimetic, has been implicated in the treatment of calciphylaxis. It has been used to treat primary and secondary hyperparathyroidism and to normalize serum calcium levels; it also may be used as an alternative to parathyroidectomy.4,6 Intravenous administration of sodium thiosulfate, a potent antioxidant and chelator of calcium, has been helpful in reversing signs and symptoms of calciphylaxis.6,12 It also has been shown to effectively remove extra calcium during peritoneal dialysis.6 Parathyroidectomy has been useful in patients with markedly elevated parathyroid hormone levels, as it suppresses or eliminates the sensitizing agent causing hypercalcemia, elevated calcium-phosphate product, and hyperparathyroidism.1,2,6,13
Wound care and prevention of sepsis are essential in the treatment of calciphylaxis. Management options include surgical debridement, hydrocolloid and biologic dressings, skin grafts, systemic antibiotics, oral pentoxifylline combined with maggot therapy, nutritional support, hyperbaric oxygen therapy, and revascularization and amputation when other interventions have failed. Pain control with analgesics and correction of thrombosis in the skin and blood vessels via anticoagulation therapy also are important complementary treatments.6
The clinical outcome of calciphylaxis is dependent on early diagnosis, antimicrobial therapy, and wound management,9 but overall, the prognosis usually is poor and has a high mortality rate. The most common causes of death are infection and sepsis.1,9 A study of 7 cases reported 100% mortality,14 but other studies have suggested a mortality rate of 60% to 80%.4,10 Female sex and obesity are poor prognostic indicators.2 A better prognosis has been appreciated in cases in which lesions occur at distal sites (eg, lower legs, hands) compared to more proximal sites (eg, abdomen), where 25% and 75% mortalities have been noted, respectively.10,14,15 In one study, the overall mortality rate was 45% in patients with calciphylaxis at 1 year.6 The rate was 41% in patients with plaques only and 67% in those who presented with ulceration. Patients who survive often experience a high degree of morbidity and prolonged hospitalization; these patients often are severely debilitated, especially in the case of limb amputation.6
Our report of calciphylaxis demonstrates the diversity in clinical presentation and emphasizes the importance of early and accurate diagnosis in reducing morbidity and mortality. In our case, the patient presented with skin pain and tense nonhemorrhagic bullae without underlying ecchymotic or erythematous lesions as the earliest sign of calciphylaxis. Physicians should have a high degree of suspicion in the setting of dialysis-dependent ESRD patients with bullae, extreme pain, and continuous decline. We hope that this case will help increase awareness of the varying presentations of this condition.
To the Editor:
Calciphylaxis (also known as calcific uremic arteriolopathy and calcifying panniculitis) is a rare vasculopathy affecting the small vessels.1 It is characterized by cutaneous ischemia and necrosis secondary to calcification. It is most commonly seen in patients with end-stage renal disease (ESRD) and hyperparathyroidism.1-3 Histopathologic features that are consistent with the diagnosis of calciphylaxis include calcification of medium-sized vessels in the deep dermis or subcutaneous fat as well as smaller distal vessels that supply the papillary dermis and epidermis.4,5 Although it commonly presents as well-demarcated, painful, purplish lesions that evolve into necrotic eschars, calciphylaxis rarely can present with hemorrhagic or serous bullous lesions followed by ulceration, as was seen in our patient.1,5,6 We report this uncommon presentation to highlight the variety in clinical appearance of calciphylaxis and the importance of early diagnosis.
A 43-year-old woman presented to the emergency department for evaluation of chest and abdominal pain that began 1 day prior to presentation. She had a history of systemic lupus erythematosus and ESRD secondary to poststreptococcal glomerulonephritis and was currently on peritoneal dialysis. The patient was admitted for peritonitis and treated with broad-spectrum antibiotics. At the time of admission, the patient also was noted to have several painful bullae on the legs. Her medical history also was remarkable for cerebral infarction, fibromyalgia, cerebral artery occlusion with cerebral infarction, sciatica, hyperlipidemia, deep vein thrombosis, and seizures. She had no history of herpes simplex virus. Surgical history was remarkable for tubal ligation, nephrectomy and kidney transplant, parathyroidectomy, and cholecystectomy. The patient’s medications included sevelamer carbonate, prednisone, epogen, calcium carbonate, esomeprazole, ondansetron, topical gentamicin, and atorvastatin.
Skin examination was performed by the inpatient dermatology service and revealed several tense, 1- to 5-cm, nonhemorrhagic bullae on the thighs and lower legs, some that had ruptured. The lesions were notably tender to palpation. No surrounding erythema, ecchymosis, or warmth was appreciated. The Nikolsky sign was negative. The patient also was noted to have at least grade 2 to 3+ pitting edema of the bilateral legs. The oral and conjunctival mucosae were unremarkable.
Antinuclear antibody, double-stranded DNA, and anti-Smith antibody levels were negative. A punch biopsy of the left lateral thigh revealed intraepidermal vesicular dermatitis with dermal edema suggestive of edema bullae and direct immunofluorescence was negative for immune complex and complement deposition.
Conservative therapy with wound care was recommended. The patient continued to report persistent severe skin pain and developed a subcutaneous nodule on the right inner thigh 1 week later, prompting a second biopsy. Results of the excisional biopsy were nondiagnostic but were suggestive of calciphylaxis, revealing subepidermal bullae with epidermal necrosis, a scant perivascular lymphocytic infiltrate, and extravasated erythrocytes. No evidence of calcification was seen within the vessels. The patient was then started on sodium thiosulfate with hemodialysis for treatment of presumed calciphylaxis.
Despite meticulous wound care and treatment with sodium thiosulfate, the patient developed ulcerations with necrotic eschars on the bilateral buttocks, hips, and thighs 1 month later (Figure 1). She subsequently worsened over the next few weeks. She developed sepsis and was transferred to the intensive care unit. A third biopsy was performed, finally confirming the diagnosis of calciphylaxis. Histopathology revealed small blood vessels with basophilic granular deposits in the walls consistent with calcium in the subcutaneous tissue (highlighted with the von Kossa stain), as well as thrombi in the lumens of some vessels; early fat necrosis; focal epidermal necrosis with underlying congested blood vessels with deposits in their walls; a perivascular infiltrate predominately of lymphocytes and neutrophils with scattered nuclear dust; and thick, hyalinized, closely crowded collagen bundles in the reticular dermis and in a widened subcutaneous septum (Figures 2 and 3).



Supportive care and pain control were continued, but the overall prognosis was determined to be very poor, and the patient eventually was discharged to hospice and died.
Although calciphylaxis is commonly seen in patients with ESRD and hyperparathyroidism, patients without renal disease also may develop the condition.2,3 Prior epidemiologic studies have shown a prevalence of 1% in patients with chronic kidney disease and up to 4% in those receiving dialysis.2-5 The average age at presentation is 48 years.6,7 Although calciphylaxis has been noted to affect males and females equally, some studies have suggested a female predominance.5-8
The etiology of calciphylaxis is unknown, but ESRD requiring dialysis, primary or secondary hyperparathyroidism, obesity, diabetes mellitus, skin trauma, and/or a hypercoagulable state may put patients at increased risk for developing this disease.2,3 Other risk factors include systemic corticosteroids, liver disease, increased serum aluminum, and increased erythrocyte sedimentation rate. Although high calcium-phosphate product has been noted as a risk factor in prior studies, one retrospective study found that it does not reliably confirm or exclude a diagnosis of calciphylaxis.8
The pathogenesis of calciphylaxis is not well understood; however, some researchers suggest that an imbalance in calcium-phosphate homeostasis may lead to calciphylaxis; that is, elevated calcium and phosphate levels exceed their solubility and deposit in the walls of small- and medium-sized arteries, which consequently leads to ischemic necrosis and gangrene of the surrounding tissue.9
Clinically, calciphylaxis has an indolent onset and usually presents as well-demarcated, painful, purplish, mottled lesions that evolve into necrotic gray-black eschars and gangrene in adjacent tissues.1,5,6 The ischemic process may even extend to the muscle layer.5 Other common presentations include mild erythematous patches; livedo reticularis; painful nodules; necrotic ulcerating lesions; and more rarely flaccid, hemorrhagic, or serous bullous lesions followed by ulceration, as was seen in our patient.6,9,10 Lesions usually begin at sites of trauma and seem to be distributed symmetrically.5,6 The most commonly affected locations are the legs, specifically the medial thighs, as well as the abdomen and buttocks, but lesions also can be found at more distal sites such as the breasts, tongue, vulva, penis, fingers, and toes.5,6,10 The head and neck region rarely is affected. Although uncommon, calciphylaxis may affect other organs, including the lungs, stomach, kidneys, and adrenal glands.5 The accompanying systemic symptoms and findings may include muscle weakness, tenderness, or myositis with rhabdomyolysis; calcific cerebral embolism; dementia and infarction of the central nervous system; acute respiratory failure; heart disease; atrioventricular block; and calcification of the cardiac conduction system.6 Unlike other forms of peripheral vascular disease, distal pulses are present in calciphylaxis, as blood flow usually is preserved distal and deep to the areas of necrosis.5,6
A careful history and thorough physical examination are important first steps in the diagnosis of this condition.2,10 Although there are no definitive laboratory tests, elevated serum calcium, phosphorous, and calcium-phosphate product levels, as well as parathyroid hormone level, may be suggestive of calciphylaxis.2,5 Leukocytosis may occur if an infection is present.5
The most accurate method to confirm the diagnosis is a deep incisional biopsy from an erythematous, slightly purpuric area adjacent to the necrotic lesion.2,10,11 The histopathologic features used to make the diagnosis include calcification of medium-sized vessels, particularly the intimal or medial layers, in the deep dermis and subcutaneous fat in addition to lobular capillaries of the subcutaneous fat.5,10 These vessels, including the smaller distal vessels that supply the papillary dermis and epidermis, also may be thrombosed due to calcification, leading to vascular occlusion and subsequently ischemic necrosis of the overlying epidermis.10 Other findings may include pseudoxanthoma elasticum changes, panniculitis, and subcutaneous fat necrosis.4,10
The differential diagnosis for calciphylaxis includes peripheral vascular disease, vasculitis, juvenile dermatomyositis, proteins C and S deficiencies, cryofibrinogenemia, calcinosis cutis, and tumoral calcinosis.2 Polyarteritis nodosa, Sjögren syndrome, atherosclerotic peripheral vascular disease, pyoderma gangrenosum, systemic lupus erythematosus, necrotizing fasciitis, septic embolism, and necrosis secondary to warfarin and heparin may mimic calciphylaxis.5
Treatment of calciphylaxis is multidimensional but primarily is supportive.6,11 Controlling calcium and phosphate levels and secondary hyperparathyroidism through diet and phosphate binders (eg, sevelamer hydrochloride) has been shown to be effective.6 Pamidronate, a bisphosphonate, inhibits arterial calcification in animal models and has been reported to treat calciphylaxis, resulting in marked pain reduction and ulcer healing.4,6 Cinacalcet, which functions as a calcimimetic, has been implicated in the treatment of calciphylaxis. It has been used to treat primary and secondary hyperparathyroidism and to normalize serum calcium levels; it also may be used as an alternative to parathyroidectomy.4,6 Intravenous administration of sodium thiosulfate, a potent antioxidant and chelator of calcium, has been helpful in reversing signs and symptoms of calciphylaxis.6,12 It also has been shown to effectively remove extra calcium during peritoneal dialysis.6 Parathyroidectomy has been useful in patients with markedly elevated parathyroid hormone levels, as it suppresses or eliminates the sensitizing agent causing hypercalcemia, elevated calcium-phosphate product, and hyperparathyroidism.1,2,6,13
Wound care and prevention of sepsis are essential in the treatment of calciphylaxis. Management options include surgical debridement, hydrocolloid and biologic dressings, skin grafts, systemic antibiotics, oral pentoxifylline combined with maggot therapy, nutritional support, hyperbaric oxygen therapy, and revascularization and amputation when other interventions have failed. Pain control with analgesics and correction of thrombosis in the skin and blood vessels via anticoagulation therapy also are important complementary treatments.6
The clinical outcome of calciphylaxis is dependent on early diagnosis, antimicrobial therapy, and wound management,9 but overall, the prognosis usually is poor and has a high mortality rate. The most common causes of death are infection and sepsis.1,9 A study of 7 cases reported 100% mortality,14 but other studies have suggested a mortality rate of 60% to 80%.4,10 Female sex and obesity are poor prognostic indicators.2 A better prognosis has been appreciated in cases in which lesions occur at distal sites (eg, lower legs, hands) compared to more proximal sites (eg, abdomen), where 25% and 75% mortalities have been noted, respectively.10,14,15 In one study, the overall mortality rate was 45% in patients with calciphylaxis at 1 year.6 The rate was 41% in patients with plaques only and 67% in those who presented with ulceration. Patients who survive often experience a high degree of morbidity and prolonged hospitalization; these patients often are severely debilitated, especially in the case of limb amputation.6
Our report of calciphylaxis demonstrates the diversity in clinical presentation and emphasizes the importance of early and accurate diagnosis in reducing morbidity and mortality. In our case, the patient presented with skin pain and tense nonhemorrhagic bullae without underlying ecchymotic or erythematous lesions as the earliest sign of calciphylaxis. Physicians should have a high degree of suspicion in the setting of dialysis-dependent ESRD patients with bullae, extreme pain, and continuous decline. We hope that this case will help increase awareness of the varying presentations of this condition.
- Hanafusa T, Yamaguchi Y, Tani M, et al. Intractable wounds caused by calcific uremic arteriolopathy treated with bisphosphonates. J Am Acad Dermatol. 2001;57:1021-1025.
- Somorin AO, Harbi AA, Subaity Y, et al. Calciphylaxis: case report and literature review. Afr J Med Sci. 2002;31:175-178.
- Barreiros HM, Goulão J, Cunha H, et al. Calciphylaxis: a diagnostic and therapeutic challenge. J Dermatol Case Rep. 2013;2:69-70.
- Vedvyas C, Winterfield LS, Vleugels RA. Calciphylaxis: a systematic review of existing and emerging therapies. J Am Acad Dermatol. 2012;67:E253-E260.
- Beitz JM. Calciphylaxis: a case study with differential diagnosis. Ostomy Wound Manag. 2003;49:28-38.
- Daudén E, Oñate M. Calciphylaxis. Dermatol Clin. 2008;26:557-568.
- Oh DH, Eulau D, Tokugawa DA, et al. Five cases of calciphylaxis and a review of the literature. J Am Acad Dermatol. 1999;40:979-987.
- Weenig RH, Sewell LD, Davis MDP, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-578.
- Hanvesakul R, Silva MA, Hejmadi R, et al. Calciphylaxis following kidney transplantation: a case report. J Med Cases. 2009;3:9297.
- Kouba DJ, Owens NM, Barrett TL, et al. An unusual case of calciphylaxis. J Cutan Med Surg. 2004;8:19-22.
- Arch-Ferrer JE, Beenken SW, Rue LW, et al. Therapy for calciphylaxis: an outcome analysis. Surgery. 2003;134:941-945.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Mirza I, Chaubay D, Gunderia H, et al. An unusual presentation of calciphylaxis due to primary hyperparathyroidism. Arch Pathol Lab Med. 2001;125:1351-1353.
- Alain J, Poulin YP, Cloutier RA, et al. Calciphylaxis: seven new cases. J Cutan Med Surg. 2000;4:213-218.
- Hafner J, Keusch G, Wahl C, et al. Calciphylaxis: a syndrome of skin necrosis and acral gangrene in chronic renal failure. Vasa. 1998;27:137-143.
- Hanafusa T, Yamaguchi Y, Tani M, et al. Intractable wounds caused by calcific uremic arteriolopathy treated with bisphosphonates. J Am Acad Dermatol. 2001;57:1021-1025.
- Somorin AO, Harbi AA, Subaity Y, et al. Calciphylaxis: case report and literature review. Afr J Med Sci. 2002;31:175-178.
- Barreiros HM, Goulão J, Cunha H, et al. Calciphylaxis: a diagnostic and therapeutic challenge. J Dermatol Case Rep. 2013;2:69-70.
- Vedvyas C, Winterfield LS, Vleugels RA. Calciphylaxis: a systematic review of existing and emerging therapies. J Am Acad Dermatol. 2012;67:E253-E260.
- Beitz JM. Calciphylaxis: a case study with differential diagnosis. Ostomy Wound Manag. 2003;49:28-38.
- Daudén E, Oñate M. Calciphylaxis. Dermatol Clin. 2008;26:557-568.
- Oh DH, Eulau D, Tokugawa DA, et al. Five cases of calciphylaxis and a review of the literature. J Am Acad Dermatol. 1999;40:979-987.
- Weenig RH, Sewell LD, Davis MDP, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-578.
- Hanvesakul R, Silva MA, Hejmadi R, et al. Calciphylaxis following kidney transplantation: a case report. J Med Cases. 2009;3:9297.
- Kouba DJ, Owens NM, Barrett TL, et al. An unusual case of calciphylaxis. J Cutan Med Surg. 2004;8:19-22.
- Arch-Ferrer JE, Beenken SW, Rue LW, et al. Therapy for calciphylaxis: an outcome analysis. Surgery. 2003;134:941-945.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Mirza I, Chaubay D, Gunderia H, et al. An unusual presentation of calciphylaxis due to primary hyperparathyroidism. Arch Pathol Lab Med. 2001;125:1351-1353.
- Alain J, Poulin YP, Cloutier RA, et al. Calciphylaxis: seven new cases. J Cutan Med Surg. 2000;4:213-218.
- Hafner J, Keusch G, Wahl C, et al. Calciphylaxis: a syndrome of skin necrosis and acral gangrene in chronic renal failure. Vasa. 1998;27:137-143.
Practice Points
- Calciphylaxis is a rare microvascular occlusion syndrome characterized by cutaneous ischemia and necrosis secondary to calcification.
- Clinically, lesions present with severely painful, violaceous, retiform patches and plaques, and less commonly bullae that progress to necrotic ulcers on the buttocks, legs, or abdomen, which is most often associated with end-stage renal disease and hyperparathyroidism.
- The diagnosis is made through deep wedge or excisional biopsy and shows calcification of medium-sized vessels in the deep dermis and subcutaneous fat. Treatment requires a multidisciplinary approach, but morbidity and mortality remain high.
Are CRMO and SAPHO syndrome one and the same?
MAUI, HAWAII – Chronic recurrent multifocal osteomyelitis (CRMO) in children and SAPHO syndrome in adults may well be a single clinical syndrome.
That contention, recently put forth by Austrian investigators, resonates with Anne M. Stevens, MD, PhD, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
“Is CRMO just for kids? No,” she asserted at the 2020 Rheumatology Winter Clinical Symposium.
First off, she noted that the nomenclature is shifting: The more familiar acronym CRMO is giving way to CNO (chronic nonbacterial osteomyelitis) in light of evidence that roughly 30% of patients with CRMO start out with a single characteristic bone lesion, with the disease turning multifocal in the subsequent 4 years in the great majority of cases.
SAPHO syndrome – an acronym for synovitis, acne, pustulosis, hyperostosis, and osteitis – a formerly obscure disease entity first described in 1987 in France, has suddenly become a trendy research topic, with three small studies presented at the 2019 annual meeting of the American College of Rheumatology.
CNO is a pediatric autoinflammatory bone disease characterized by sterile bone lesions, most often on the clavicle, spine, mandible, and lower extremities. It is marked by prominent focal bone and/or joint pain, worse at night, with or without swelling. With no agreed-upon diagnostic criteria or biomarkers, CNO is a diagnosis of exclusion. Two-thirds of the time the condition is initially misdiagnosed as bacterial osteomyelitis or a malignant tumor.
Austrian investigators at the University of Graz recently conducted a retrospective comparison of 24 pediatric patients diagnosed with CNO and 10 adults with SAPHO syndrome. The median age at diagnosis of CNO was 12.3 years versus 32.5 years for SAPHO syndrome. The two groups shared compelling similarities in mean number of bone lesions, prevalence of skin involvement, and other aspects of initial clinical presentation, as well as laboratory and histopathologic findings on bone biopsy.
There were, however, several notable clinical differences in this small dataset: CNO bone lesions affected mainly the lower extremities, clavicle, spine, and mandible, while SAPHO syndrome more commonly involved the sternum (50% vs. 8%) and vertebrae (50% vs. 21%). Also, the most frequent cutaneous manifestation was palmoplantar pustulosis in adults with SAPHO syndrome, while severe acne predominated in children with CNO. In both children and adults, the skin lesions most often arose after the bone symptoms, making early diagnosis a challenge.
Another similarity: Although there have been no randomized treatment trials in either CNO or SAPHO syndrome, case series suggest the same treatments are effective for both, with NSAIDs as first line, followed by nonbiologic disease-modifying antirheumatic drugs, tumor necrosis factor (TNF) inhibitors, or bisphosphonates.
CNO diagnosis, treatment, and follow-up
Various investigators have pegged the sensitivity of physical examination for diagnosis of CNO at 31%, radiographs at a lowly 13%, and bone scintigraphy at 74%, all in comparison with MRI.
“Our go-to now is MRI with STIR [short tau inversion recovery],” according to Dr. Stevens. “There’s no contrast – so no IV – no radiation, and it’s fast, 20 minutes for a whole body MRI in a little kid, 45 minutes in a big one.”
Insurers are reluctant to pay for serial whole-body MRIs for patient follow-up, so it’s often necessary to order a series of images covering different body parts.
Her University of Washington colleague Dan Zhao, MD, PhD, is developing infrared thermal imaging as an inexpensive, convenient alternative to MRI which could theoretically be done at home. In a pilot study in 30 children with CNO and 31 controls, inflamed leg segments showed significantly higher temperatures. Larger studies are planned.
Dr. Stevens advised leaning towards a diagnosis of CNO with avoidance of bone biopsy in a patient with multifocal osteomyelitis at the typical sites, a normal CBC, the typical extraosseous manifestations, and normal or only mildly elevated erythrocyte sedimentation rate and C-reactive protein in an otherwise well-appearing child. In contrast, strongly consider a bone biopsy to rule out malignancy or infection if the child has unexplained highly elevated C-reactive protein and erythrocyte sedimentation rate, cytopenia, high fever, excessive pain, lymphadenopathy, hepatosplenomegaly, or suspicious imaging findings.
German rheumatologists have developed a clinical score for diagnosis of CNO. A normal blood cell count gets 13 points; symmetric bone lesions 10; lesions with marginal sclerosis 10; a normal body temperature 9; two or more radiologically proven lesions 7; a C-reactive protein of 1 mg/dL or greater 6; and vertebral, clavicular, or sternal lesions 8. A score of 39 points or more out of a possible 63 had a 97% positive predictive value for CNO in a retrospective study of 224 children with CNO, proven bacterial osteomyelitis, or malignant bone tumors. A score of 28 points or less had a 97% negative predictive value for CNO. An indeterminate score of 29-38 warrants close monitoring.
The scoring system hasn’t been validated, but most pediatric rheumatologists agree that it’s useful, according to Dr. Stevens.
The Childhood Arthritis and Rheumatology Research Alliance (CARRA) is in the process of developing standardized diagnostic and classification criteria and treatment plans for CNO. Dr. Zhao was first author of a CARRA consensus treatment plan for CNO refractory to NSAID monotherapy. The plan for the first 12 months includes three options: methotrexate or sulfasalazine, TNF inhibitors with or without methotrexate, and bisphosphonates.
“The main point of this is you try a medicine and then wait 3 months. If they’re not responding then, switch medicines or add another drug. Monitor every 3 months based upon pain,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
MAUI, HAWAII – Chronic recurrent multifocal osteomyelitis (CRMO) in children and SAPHO syndrome in adults may well be a single clinical syndrome.
That contention, recently put forth by Austrian investigators, resonates with Anne M. Stevens, MD, PhD, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
“Is CRMO just for kids? No,” she asserted at the 2020 Rheumatology Winter Clinical Symposium.
First off, she noted that the nomenclature is shifting: The more familiar acronym CRMO is giving way to CNO (chronic nonbacterial osteomyelitis) in light of evidence that roughly 30% of patients with CRMO start out with a single characteristic bone lesion, with the disease turning multifocal in the subsequent 4 years in the great majority of cases.
SAPHO syndrome – an acronym for synovitis, acne, pustulosis, hyperostosis, and osteitis – a formerly obscure disease entity first described in 1987 in France, has suddenly become a trendy research topic, with three small studies presented at the 2019 annual meeting of the American College of Rheumatology.
CNO is a pediatric autoinflammatory bone disease characterized by sterile bone lesions, most often on the clavicle, spine, mandible, and lower extremities. It is marked by prominent focal bone and/or joint pain, worse at night, with or without swelling. With no agreed-upon diagnostic criteria or biomarkers, CNO is a diagnosis of exclusion. Two-thirds of the time the condition is initially misdiagnosed as bacterial osteomyelitis or a malignant tumor.
Austrian investigators at the University of Graz recently conducted a retrospective comparison of 24 pediatric patients diagnosed with CNO and 10 adults with SAPHO syndrome. The median age at diagnosis of CNO was 12.3 years versus 32.5 years for SAPHO syndrome. The two groups shared compelling similarities in mean number of bone lesions, prevalence of skin involvement, and other aspects of initial clinical presentation, as well as laboratory and histopathologic findings on bone biopsy.
There were, however, several notable clinical differences in this small dataset: CNO bone lesions affected mainly the lower extremities, clavicle, spine, and mandible, while SAPHO syndrome more commonly involved the sternum (50% vs. 8%) and vertebrae (50% vs. 21%). Also, the most frequent cutaneous manifestation was palmoplantar pustulosis in adults with SAPHO syndrome, while severe acne predominated in children with CNO. In both children and adults, the skin lesions most often arose after the bone symptoms, making early diagnosis a challenge.
Another similarity: Although there have been no randomized treatment trials in either CNO or SAPHO syndrome, case series suggest the same treatments are effective for both, with NSAIDs as first line, followed by nonbiologic disease-modifying antirheumatic drugs, tumor necrosis factor (TNF) inhibitors, or bisphosphonates.
CNO diagnosis, treatment, and follow-up
Various investigators have pegged the sensitivity of physical examination for diagnosis of CNO at 31%, radiographs at a lowly 13%, and bone scintigraphy at 74%, all in comparison with MRI.
“Our go-to now is MRI with STIR [short tau inversion recovery],” according to Dr. Stevens. “There’s no contrast – so no IV – no radiation, and it’s fast, 20 minutes for a whole body MRI in a little kid, 45 minutes in a big one.”
Insurers are reluctant to pay for serial whole-body MRIs for patient follow-up, so it’s often necessary to order a series of images covering different body parts.
Her University of Washington colleague Dan Zhao, MD, PhD, is developing infrared thermal imaging as an inexpensive, convenient alternative to MRI which could theoretically be done at home. In a pilot study in 30 children with CNO and 31 controls, inflamed leg segments showed significantly higher temperatures. Larger studies are planned.
Dr. Stevens advised leaning towards a diagnosis of CNO with avoidance of bone biopsy in a patient with multifocal osteomyelitis at the typical sites, a normal CBC, the typical extraosseous manifestations, and normal or only mildly elevated erythrocyte sedimentation rate and C-reactive protein in an otherwise well-appearing child. In contrast, strongly consider a bone biopsy to rule out malignancy or infection if the child has unexplained highly elevated C-reactive protein and erythrocyte sedimentation rate, cytopenia, high fever, excessive pain, lymphadenopathy, hepatosplenomegaly, or suspicious imaging findings.
German rheumatologists have developed a clinical score for diagnosis of CNO. A normal blood cell count gets 13 points; symmetric bone lesions 10; lesions with marginal sclerosis 10; a normal body temperature 9; two or more radiologically proven lesions 7; a C-reactive protein of 1 mg/dL or greater 6; and vertebral, clavicular, or sternal lesions 8. A score of 39 points or more out of a possible 63 had a 97% positive predictive value for CNO in a retrospective study of 224 children with CNO, proven bacterial osteomyelitis, or malignant bone tumors. A score of 28 points or less had a 97% negative predictive value for CNO. An indeterminate score of 29-38 warrants close monitoring.
The scoring system hasn’t been validated, but most pediatric rheumatologists agree that it’s useful, according to Dr. Stevens.
The Childhood Arthritis and Rheumatology Research Alliance (CARRA) is in the process of developing standardized diagnostic and classification criteria and treatment plans for CNO. Dr. Zhao was first author of a CARRA consensus treatment plan for CNO refractory to NSAID monotherapy. The plan for the first 12 months includes three options: methotrexate or sulfasalazine, TNF inhibitors with or without methotrexate, and bisphosphonates.
“The main point of this is you try a medicine and then wait 3 months. If they’re not responding then, switch medicines or add another drug. Monitor every 3 months based upon pain,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
MAUI, HAWAII – Chronic recurrent multifocal osteomyelitis (CRMO) in children and SAPHO syndrome in adults may well be a single clinical syndrome.
That contention, recently put forth by Austrian investigators, resonates with Anne M. Stevens, MD, PhD, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
“Is CRMO just for kids? No,” she asserted at the 2020 Rheumatology Winter Clinical Symposium.
First off, she noted that the nomenclature is shifting: The more familiar acronym CRMO is giving way to CNO (chronic nonbacterial osteomyelitis) in light of evidence that roughly 30% of patients with CRMO start out with a single characteristic bone lesion, with the disease turning multifocal in the subsequent 4 years in the great majority of cases.
SAPHO syndrome – an acronym for synovitis, acne, pustulosis, hyperostosis, and osteitis – a formerly obscure disease entity first described in 1987 in France, has suddenly become a trendy research topic, with three small studies presented at the 2019 annual meeting of the American College of Rheumatology.
CNO is a pediatric autoinflammatory bone disease characterized by sterile bone lesions, most often on the clavicle, spine, mandible, and lower extremities. It is marked by prominent focal bone and/or joint pain, worse at night, with or without swelling. With no agreed-upon diagnostic criteria or biomarkers, CNO is a diagnosis of exclusion. Two-thirds of the time the condition is initially misdiagnosed as bacterial osteomyelitis or a malignant tumor.
Austrian investigators at the University of Graz recently conducted a retrospective comparison of 24 pediatric patients diagnosed with CNO and 10 adults with SAPHO syndrome. The median age at diagnosis of CNO was 12.3 years versus 32.5 years for SAPHO syndrome. The two groups shared compelling similarities in mean number of bone lesions, prevalence of skin involvement, and other aspects of initial clinical presentation, as well as laboratory and histopathologic findings on bone biopsy.
There were, however, several notable clinical differences in this small dataset: CNO bone lesions affected mainly the lower extremities, clavicle, spine, and mandible, while SAPHO syndrome more commonly involved the sternum (50% vs. 8%) and vertebrae (50% vs. 21%). Also, the most frequent cutaneous manifestation was palmoplantar pustulosis in adults with SAPHO syndrome, while severe acne predominated in children with CNO. In both children and adults, the skin lesions most often arose after the bone symptoms, making early diagnosis a challenge.
Another similarity: Although there have been no randomized treatment trials in either CNO or SAPHO syndrome, case series suggest the same treatments are effective for both, with NSAIDs as first line, followed by nonbiologic disease-modifying antirheumatic drugs, tumor necrosis factor (TNF) inhibitors, or bisphosphonates.
CNO diagnosis, treatment, and follow-up
Various investigators have pegged the sensitivity of physical examination for diagnosis of CNO at 31%, radiographs at a lowly 13%, and bone scintigraphy at 74%, all in comparison with MRI.
“Our go-to now is MRI with STIR [short tau inversion recovery],” according to Dr. Stevens. “There’s no contrast – so no IV – no radiation, and it’s fast, 20 minutes for a whole body MRI in a little kid, 45 minutes in a big one.”
Insurers are reluctant to pay for serial whole-body MRIs for patient follow-up, so it’s often necessary to order a series of images covering different body parts.
Her University of Washington colleague Dan Zhao, MD, PhD, is developing infrared thermal imaging as an inexpensive, convenient alternative to MRI which could theoretically be done at home. In a pilot study in 30 children with CNO and 31 controls, inflamed leg segments showed significantly higher temperatures. Larger studies are planned.
Dr. Stevens advised leaning towards a diagnosis of CNO with avoidance of bone biopsy in a patient with multifocal osteomyelitis at the typical sites, a normal CBC, the typical extraosseous manifestations, and normal or only mildly elevated erythrocyte sedimentation rate and C-reactive protein in an otherwise well-appearing child. In contrast, strongly consider a bone biopsy to rule out malignancy or infection if the child has unexplained highly elevated C-reactive protein and erythrocyte sedimentation rate, cytopenia, high fever, excessive pain, lymphadenopathy, hepatosplenomegaly, or suspicious imaging findings.
German rheumatologists have developed a clinical score for diagnosis of CNO. A normal blood cell count gets 13 points; symmetric bone lesions 10; lesions with marginal sclerosis 10; a normal body temperature 9; two or more radiologically proven lesions 7; a C-reactive protein of 1 mg/dL or greater 6; and vertebral, clavicular, or sternal lesions 8. A score of 39 points or more out of a possible 63 had a 97% positive predictive value for CNO in a retrospective study of 224 children with CNO, proven bacterial osteomyelitis, or malignant bone tumors. A score of 28 points or less had a 97% negative predictive value for CNO. An indeterminate score of 29-38 warrants close monitoring.
The scoring system hasn’t been validated, but most pediatric rheumatologists agree that it’s useful, according to Dr. Stevens.
The Childhood Arthritis and Rheumatology Research Alliance (CARRA) is in the process of developing standardized diagnostic and classification criteria and treatment plans for CNO. Dr. Zhao was first author of a CARRA consensus treatment plan for CNO refractory to NSAID monotherapy. The plan for the first 12 months includes three options: methotrexate or sulfasalazine, TNF inhibitors with or without methotrexate, and bisphosphonates.
“The main point of this is you try a medicine and then wait 3 months. If they’re not responding then, switch medicines or add another drug. Monitor every 3 months based upon pain,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
EXPERT ANALYSIS FROM RWCS 2020
Spotlight on SMA, Part 2: The Spinal Muscular Atrophy Treatment Landscape
With newly available disease-modifying therapies, the phenotype of spinal muscular atrophy (SMA) is rapidly changing, and affected individuals are living longer, healthier lives.1-4 This supplement discusses therapeutic strategies, FDA-approved treatment options, and the SMA drug pipeline.
To access Part 1 of the SMA Spotlight series, The Urgent Need for Early Diagnosis in Spinal Muscular Atrophy, visit www.mdedge.com/DiagnosisInSMA.
References
- Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723-1732.
- Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378(7):625-635.
- Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose genereplacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713-1722.
- De Vivo DC, Bertini E, Swoboda KJ, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul Disord. 2019;29(11):842-856.
With newly available disease-modifying therapies, the phenotype of spinal muscular atrophy (SMA) is rapidly changing, and affected individuals are living longer, healthier lives.1-4 This supplement discusses therapeutic strategies, FDA-approved treatment options, and the SMA drug pipeline.
To access Part 1 of the SMA Spotlight series, The Urgent Need for Early Diagnosis in Spinal Muscular Atrophy, visit www.mdedge.com/DiagnosisInSMA.
References
- Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723-1732.
- Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378(7):625-635.
- Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose genereplacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713-1722.
- De Vivo DC, Bertini E, Swoboda KJ, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul Disord. 2019;29(11):842-856.
With newly available disease-modifying therapies, the phenotype of spinal muscular atrophy (SMA) is rapidly changing, and affected individuals are living longer, healthier lives.1-4 This supplement discusses therapeutic strategies, FDA-approved treatment options, and the SMA drug pipeline.
To access Part 1 of the SMA Spotlight series, The Urgent Need for Early Diagnosis in Spinal Muscular Atrophy, visit www.mdedge.com/DiagnosisInSMA.
References
- Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723-1732.
- Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378(7):625-635.
- Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose genereplacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713-1722.
- De Vivo DC, Bertini E, Swoboda KJ, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul Disord. 2019;29(11):842-856.
Dermatology therapies evolve as disease knowledge and investment grow
For much of the past 50 years, many of the drugs used in dermatology have been adopted – and often adapted – from other specialties and used for dermatologic conditions.
“Almost every drug was more or less a hand-me-down” developed first for cancer or other diseases and found later, often serendipitously, to be useful for the skin, said William Eaglstein, MD, thinking back to the 1970s and recalling steroids, tetracyclines, methotrexate, and 5-flourouracil. “The perception always was that skin diseases weren’t serious, that the market was small.”
Much has changed. by dermatologist investigators, and “more and more companies are recognizing the importance of our diseases and the ability to get a return on investment,” said Dr. Eaglstein, past professor and chair of the departments of dermatology at the University of Miami and the University of Pittsburgh, who worked in industry after his academic career.
Psoriasis was a game changer, he and other dermatologists said in interviews. The tumor necrosis factor (TNF)–alpha blockers were first used for other indications, but their marked follow-on success in psoriasis “offered proof of concept clinically – showing that by targeting immune pathways in the skin we could achieve a clinical effect – and proof of concept commercially” that dermatology drugs are worth pursuing by pharmaceutical companies, said William Ju, MD, a cofounder and president of Advancing Innovation in Dermatology, a nonprofit organization that brings together stakeholders to develop novel dermatologic drugs and products.
This resulted in the approval of subsequent biologics, such as ustekinumab (Stelara) which inhibits the signaling of interleukin (IL)–12/IL-23, for psoriasis as their initial indication. Then, biologics targeting IL-17 followed this dermatology-first approach. “Researchers have continued further dissecting out the immunopathological pathways, and antibody drugs targeting IL-23p19 have been approved for psoriasis as the lead indication,” said Dr. Ju, a dermatologist who has worked in industry.
Seth Orlow, MD, PhD, who chairs the department of dermatology at NYU Langone Health, remembers the 1970s through the 1990s as the “era of topicals” developed for dermatologic conditions – topical antifungals, topical corticosteroids, and topical retinoids. The next decade was characterized by formulation tweaks and few novel treatments for dermatology, said Dr. Orlow, who is also professor of pediatric dermatology and director of the program in cutaneous biology at New York University.
Now, given the succession of psoriasis discoveries in the last decade, “large companies are interested in dermatology,” he said in an interview. “There’s an explosion of interest in atopic dermatitis. … and companies are dipping their toes in the water for alopecia areata and vitiligo. That’s amazing.”
Rare diseases like epidermolysis bullosa, ichthyosis, and basal cell nevus syndrome are getting attention as well, boosted by the Orphan Drug Act of 1983, in addition to increased research on disease pathways and growing appreciation of skin diseases. “There’s a lot under development, from small molecules to biologics to gene-based therapies,” Dr. Orlow commented.
The new frontier of atopic dermatitis
The approval in 2017 of dupilumab (Dupixent), a monoclonal antibody that inhibits the signaling of both IL-4 and IL-13) for moderate-severe atopic dermatitis (AD) in adults illustrates the new standing of dermatologic diseases in the field of drug development and commercialization. “Atopic dermatitis had always been the forgotten chronic disease in dermatology. … We’ve had no good treatments,” said Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland. “Dupilumab coming to the forefront [as a dermatology-first indication] has changed the entire perspective of the field. … Everyone is now trying to find the next best drug.”
As with psoriasis, a targeted therapy for AD was made possible by the development in the 1990s of monoclonal antibody technology and the ensuing ability to create biologics that target specific molecules in the body – as well as bedside-to-bench research that homed in on the involvement of particular cytokines.
But there also is a “new understanding of the burden of the disease,” Dr. Simpson observed. In the last 5 years, he said, research funded by the National Eczema Association documented that AD “not only causes inflammation of the skin … but that it affects people at school and in the workplace, that people have multiple mental health comorbidities and skin infections, and that the disease profoundly affects the entire patient in ways that weren’t really recognized or appreciated.”
Having evolved in the footsteps of psoriasis, AD is at a higher starting point in terms of the safety and efficacy of its first biologic, sources said. On the other hand, AD is a much more complex and heterogeneous disease, and researchers are trying to determine which immune pathways and cytokines are most important – and in which populations.
“We’re at the beginning. We’re trying to figure out how to get 80% of patients clear or almost clear [as we can now with psoriasis biologics] rather than almost 40% [as in the dupilumab pivotal trials],” said Dr. Simpson, former cochair of the National Eczema Association’s scientific committee. Public data from ongoing phase 2 and 3 trials of other Th2 cytokine inhibitors suggest that 25%-45% of enrolled patients achieve high levels of clearance, he noted.
Emma Guttman-Yassky, MD, PhD, Sol and Clara Kest Professor and vice-chair for research in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said that AD’s heterogeneity involves “many factors, like ethnicity, age … and whether they have an atopic background such as asthma.”
Her research is showing, for instance, that AD in Asian and black patients is different than AD in European-American patients, and that the presence of comorbidities may well have treatment implications. She has also shown that children may have a different phenotype than adults, with greater activation of the Th17 axis that typifies psoriasis.
“For certain patients, we may need to target more than one pathway, or target a different pathway than the Th2 pathway. And treatment may be different in the setting of comorbidities,” said Dr. Guttman-Yassky, who is also director of the laboratory of inflammatory skin diseases at Mount Sinai. “We may think of one treatment – dupilumab, for example – for someone who has asthma and AD. But for patients who don’t have asthma and are Asian, for instance, or for children, we may need additional agents.”
Her research over the years on AD has taught her the importance of human studies over mouse model studies; it was in humans, she noted, that she and other investigators demonstrated “without doubt” that AD is an immune disease and not simply a barrier disease. The Th2 cytokine pathway appears to play the predominant role in AD, though “there still is a strong Th1 component,” she said.
“We’re in a better position to figure this out today [than in the past 20 or even 10 years],” said Dr. Guttman-Yassky, who recalls being told years ago that AD was a “dead end,” that it “would kill [her] career.” Given the evolution of science and the recognition of comorbidities and seriousness of dermatologic diseases, “the stars are aligned to get more [therapies] to these patients.”
Janus kinase (JAK) inhibitors are among these therapies. Three JAK inhibitors are in or have recently completed phase 3 studies for AD; two are currently approved for rheumatoid arthritis, and the other has been designed specifically for AD, Dr. Simpson pointed out. The drugs are oral small molecule drugs that block the JAK signaling pathways for certain proinflammatory cytokines.
“The JAK inhibitors are a real exciting story for dermatology,” he said. “Theoretically, by blocking more cytokines than biologics do, there could be some safety issues – that’s why we’re awaiting big phase 3 study results so we can figure out the risk-benefit balance and guide our patients as to which drug is best.”
Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center in Portland – a stand-alone dermatology clinical trial center founded in 1998 – likes to envision the evolution of drugs for dermatologic conditions as a funnel, with the most broad-acting drugs at the wide top of the funnel and the most targeted drugs at the bottom tip.
JAK inhibitors, he said, sit near the middle – more targeted and safer than cyclosporine and methotrexate, for instance, but not as targeted as the biologics now available for psoriasis and being developed for AD. “The oral medications that have been developed for psoriasis and those coming for AD are not quite as targeted to the disease,” he noted. “JAK inhibitors have great efficacy – it’s more a question of safety and being able to treat without causing collateral damage.”
Dr. Blauvelt expects the armamentarium of new drugs approved for AD to go from one (dupilumab) to seven within the next 2 years. This will include three new biologics and three new oral JAK inhibitors, he predicts. As the specialty sorts through and integrates these new drugs into practice, dermatologists will increasingly personalize treatment and will face the “nonscientific” challenge of the cost of new therapies and patient access to them, he noted.
In the meantime, said Dr. Simpson, recent drug discoveries have driven more non–pharmaceutical-funded translational research aimed at understanding the underlying biology of AD. The National Institutes of Health, for instance, “is interested in dupilumab and its impact on the skin barrier and skin defense mechanisms,” he said. “We’ll learn a lot more [in coming years].”
Spillover to other diseases
JAK inhibitors – some in oral and some in topical form – are showing efficacy in ongoing research for alopecia areata (AA) and vitiligo as well, Dr. Blauvelt said.
“We’re understanding more about the pathophysiology of these diseases, which historically have been tough diseases for dermatologists to treat,” he said. “The successes in alopecia areata and vitiligo are incredibly exciting actually – it’s very exciting to see hair and pigment coming back. And as we learn more, we should be able to develop [additional] drugs that are more disease targeted than the JAK inhibitors.”
Already, some of the biologics used to treat psoriasis have been studied in patients with hidradenitis suppurativa (HS), a disease in which painful lumps and sometimes tunnels form under the skin, with some success; adalimumab (Humira), a TNF-inhibitor, is now FDA approved for the treatment of moderate-severe HS, and studies are ongoing of IL-17 and IL-23 blockers for the disease.
“The pathophysiology [of HS] is very complex; it’s not nearly as straightforward as psoriasis, and there haven’t been any major breakthroughs yet,” Dr. Blauvelt said. “But the drugs seem to be working better than historical alternatives.”
Regarding AA, Dr. Guttman-Yassky, who is participating in a study of dupilumab for AA, recently found in a retrospective cross-sectional study that patients with the condition are more likely to have atopic comorbidities – asthma, allergic rhinitis, and AD, for instance. “The more comorbid conditions, the greater the risk of developing alopecia areata,” she said. “That could point to a potential pathogenic role of the Th2 axis in the disorder [challenging the traditional view of AA as a singularly Th1-centered disease.] The future will tell.”
Action on rare skin diseases
Both large and small companies have moved into the orphan drug space, investing in research and pursuing orphan drug indications for dermatologic conditions, because “it’s clear now in the marketplace that companies can develop effective drugs for rare disorders and be quite successful,” Dr. Orlow said.
According to a recent analysis, as a result of incentives for rare disease drug development contained in the Orphan Drug Act, 72 indications have been approved for rare skin disease, skin-related cancers, and hereditary disorders with prominent dermatologic manifestations since the law was passed in 1983 (J. Am. Acad. Dermatol. 2019;81[3]:867-77).
Epidermolysis bullosa (EB) is a good example, he and other sources said, of commercial interests merging with growing knowledge of disease pathogenesis as well as the tools needed to develop new treatments.
Research by dermatology scientists and others over the past 40 years, Dr. Ju explained, shed light on the molecular basis underlying the structure and function of the junction between the epidermis and dermis, including the pivotal role that type VII collagen plays in the normal adhesion of these two layers. Researchers then learned that, in EB, the family of genetic diseases characterized by skin fragility, “dystrophic types are caused by mutations in the gene encoding type VII collagen,” he said.
“Just as the advent of monoclonal antibodies allowed us to start attacking psoriasis and atopic dermatitis in unprecedented ways, the advent of gene therapy allows us to potentially address the fundamental molecular genetic defect of various types of EB,” Dr. Ju said.
While gene therapy is “still in its infancy,” companies have begun using the tools to address EB. One gene therapy in the pipeline – in phase 3 clinical trial testing – involves grafting back into patients with recessive dystrophic EB their skin cells that have been genetically modified to produce a correct (nonmutated) type VII collagen, he said.
Basal cell nevus syndrome, or Gorlin syndrome, a rare disease in which patients develop a multitude of basal cell carcinoma tumors, is another example of a “dermatology first” approach, Dr. Ju said. Research identified a genetic mutation that causes the hedgehog signaling pathway to be inappropriately activated in the disease, and a drug, vismodegib, was developed to inhibit this pathway. The drug was initially approved for patients with metastatic basal cell cancer and types of advanced basal cell cancer, and is now being tested in cancers affecting other organs, he said.
Basal cell cancer “is a huge market, but it was really unrecognized in the past,” Dr. Eaglstein said. “Seeing drugs come to market for basal cell cancer – this wouldn’t have happened [decades ago].”
Dr. Ju has worked in the pharmaceutical industry; all other sources in this story have worked with pharmaceutical manufacturers of treatments that are being developed or have been approved to treat dermatologic diseases mentioned in this story. In addition to Dr. Ju, Dr. Eaglstein and Dr. Orlow are cofounders of the Advancing Innovation in Dermatology group; Dr. Orlow is a member of the program committee for the organization’s dermatology summit conference.
For much of the past 50 years, many of the drugs used in dermatology have been adopted – and often adapted – from other specialties and used for dermatologic conditions.
“Almost every drug was more or less a hand-me-down” developed first for cancer or other diseases and found later, often serendipitously, to be useful for the skin, said William Eaglstein, MD, thinking back to the 1970s and recalling steroids, tetracyclines, methotrexate, and 5-flourouracil. “The perception always was that skin diseases weren’t serious, that the market was small.”
Much has changed. by dermatologist investigators, and “more and more companies are recognizing the importance of our diseases and the ability to get a return on investment,” said Dr. Eaglstein, past professor and chair of the departments of dermatology at the University of Miami and the University of Pittsburgh, who worked in industry after his academic career.
Psoriasis was a game changer, he and other dermatologists said in interviews. The tumor necrosis factor (TNF)–alpha blockers were first used for other indications, but their marked follow-on success in psoriasis “offered proof of concept clinically – showing that by targeting immune pathways in the skin we could achieve a clinical effect – and proof of concept commercially” that dermatology drugs are worth pursuing by pharmaceutical companies, said William Ju, MD, a cofounder and president of Advancing Innovation in Dermatology, a nonprofit organization that brings together stakeholders to develop novel dermatologic drugs and products.
This resulted in the approval of subsequent biologics, such as ustekinumab (Stelara) which inhibits the signaling of interleukin (IL)–12/IL-23, for psoriasis as their initial indication. Then, biologics targeting IL-17 followed this dermatology-first approach. “Researchers have continued further dissecting out the immunopathological pathways, and antibody drugs targeting IL-23p19 have been approved for psoriasis as the lead indication,” said Dr. Ju, a dermatologist who has worked in industry.
Seth Orlow, MD, PhD, who chairs the department of dermatology at NYU Langone Health, remembers the 1970s through the 1990s as the “era of topicals” developed for dermatologic conditions – topical antifungals, topical corticosteroids, and topical retinoids. The next decade was characterized by formulation tweaks and few novel treatments for dermatology, said Dr. Orlow, who is also professor of pediatric dermatology and director of the program in cutaneous biology at New York University.
Now, given the succession of psoriasis discoveries in the last decade, “large companies are interested in dermatology,” he said in an interview. “There’s an explosion of interest in atopic dermatitis. … and companies are dipping their toes in the water for alopecia areata and vitiligo. That’s amazing.”
Rare diseases like epidermolysis bullosa, ichthyosis, and basal cell nevus syndrome are getting attention as well, boosted by the Orphan Drug Act of 1983, in addition to increased research on disease pathways and growing appreciation of skin diseases. “There’s a lot under development, from small molecules to biologics to gene-based therapies,” Dr. Orlow commented.
The new frontier of atopic dermatitis
The approval in 2017 of dupilumab (Dupixent), a monoclonal antibody that inhibits the signaling of both IL-4 and IL-13) for moderate-severe atopic dermatitis (AD) in adults illustrates the new standing of dermatologic diseases in the field of drug development and commercialization. “Atopic dermatitis had always been the forgotten chronic disease in dermatology. … We’ve had no good treatments,” said Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland. “Dupilumab coming to the forefront [as a dermatology-first indication] has changed the entire perspective of the field. … Everyone is now trying to find the next best drug.”
As with psoriasis, a targeted therapy for AD was made possible by the development in the 1990s of monoclonal antibody technology and the ensuing ability to create biologics that target specific molecules in the body – as well as bedside-to-bench research that homed in on the involvement of particular cytokines.
But there also is a “new understanding of the burden of the disease,” Dr. Simpson observed. In the last 5 years, he said, research funded by the National Eczema Association documented that AD “not only causes inflammation of the skin … but that it affects people at school and in the workplace, that people have multiple mental health comorbidities and skin infections, and that the disease profoundly affects the entire patient in ways that weren’t really recognized or appreciated.”
Having evolved in the footsteps of psoriasis, AD is at a higher starting point in terms of the safety and efficacy of its first biologic, sources said. On the other hand, AD is a much more complex and heterogeneous disease, and researchers are trying to determine which immune pathways and cytokines are most important – and in which populations.
“We’re at the beginning. We’re trying to figure out how to get 80% of patients clear or almost clear [as we can now with psoriasis biologics] rather than almost 40% [as in the dupilumab pivotal trials],” said Dr. Simpson, former cochair of the National Eczema Association’s scientific committee. Public data from ongoing phase 2 and 3 trials of other Th2 cytokine inhibitors suggest that 25%-45% of enrolled patients achieve high levels of clearance, he noted.
Emma Guttman-Yassky, MD, PhD, Sol and Clara Kest Professor and vice-chair for research in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said that AD’s heterogeneity involves “many factors, like ethnicity, age … and whether they have an atopic background such as asthma.”
Her research is showing, for instance, that AD in Asian and black patients is different than AD in European-American patients, and that the presence of comorbidities may well have treatment implications. She has also shown that children may have a different phenotype than adults, with greater activation of the Th17 axis that typifies psoriasis.
“For certain patients, we may need to target more than one pathway, or target a different pathway than the Th2 pathway. And treatment may be different in the setting of comorbidities,” said Dr. Guttman-Yassky, who is also director of the laboratory of inflammatory skin diseases at Mount Sinai. “We may think of one treatment – dupilumab, for example – for someone who has asthma and AD. But for patients who don’t have asthma and are Asian, for instance, or for children, we may need additional agents.”
Her research over the years on AD has taught her the importance of human studies over mouse model studies; it was in humans, she noted, that she and other investigators demonstrated “without doubt” that AD is an immune disease and not simply a barrier disease. The Th2 cytokine pathway appears to play the predominant role in AD, though “there still is a strong Th1 component,” she said.
“We’re in a better position to figure this out today [than in the past 20 or even 10 years],” said Dr. Guttman-Yassky, who recalls being told years ago that AD was a “dead end,” that it “would kill [her] career.” Given the evolution of science and the recognition of comorbidities and seriousness of dermatologic diseases, “the stars are aligned to get more [therapies] to these patients.”
Janus kinase (JAK) inhibitors are among these therapies. Three JAK inhibitors are in or have recently completed phase 3 studies for AD; two are currently approved for rheumatoid arthritis, and the other has been designed specifically for AD, Dr. Simpson pointed out. The drugs are oral small molecule drugs that block the JAK signaling pathways for certain proinflammatory cytokines.
“The JAK inhibitors are a real exciting story for dermatology,” he said. “Theoretically, by blocking more cytokines than biologics do, there could be some safety issues – that’s why we’re awaiting big phase 3 study results so we can figure out the risk-benefit balance and guide our patients as to which drug is best.”
Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center in Portland – a stand-alone dermatology clinical trial center founded in 1998 – likes to envision the evolution of drugs for dermatologic conditions as a funnel, with the most broad-acting drugs at the wide top of the funnel and the most targeted drugs at the bottom tip.
JAK inhibitors, he said, sit near the middle – more targeted and safer than cyclosporine and methotrexate, for instance, but not as targeted as the biologics now available for psoriasis and being developed for AD. “The oral medications that have been developed for psoriasis and those coming for AD are not quite as targeted to the disease,” he noted. “JAK inhibitors have great efficacy – it’s more a question of safety and being able to treat without causing collateral damage.”
Dr. Blauvelt expects the armamentarium of new drugs approved for AD to go from one (dupilumab) to seven within the next 2 years. This will include three new biologics and three new oral JAK inhibitors, he predicts. As the specialty sorts through and integrates these new drugs into practice, dermatologists will increasingly personalize treatment and will face the “nonscientific” challenge of the cost of new therapies and patient access to them, he noted.
In the meantime, said Dr. Simpson, recent drug discoveries have driven more non–pharmaceutical-funded translational research aimed at understanding the underlying biology of AD. The National Institutes of Health, for instance, “is interested in dupilumab and its impact on the skin barrier and skin defense mechanisms,” he said. “We’ll learn a lot more [in coming years].”
Spillover to other diseases
JAK inhibitors – some in oral and some in topical form – are showing efficacy in ongoing research for alopecia areata (AA) and vitiligo as well, Dr. Blauvelt said.
“We’re understanding more about the pathophysiology of these diseases, which historically have been tough diseases for dermatologists to treat,” he said. “The successes in alopecia areata and vitiligo are incredibly exciting actually – it’s very exciting to see hair and pigment coming back. And as we learn more, we should be able to develop [additional] drugs that are more disease targeted than the JAK inhibitors.”
Already, some of the biologics used to treat psoriasis have been studied in patients with hidradenitis suppurativa (HS), a disease in which painful lumps and sometimes tunnels form under the skin, with some success; adalimumab (Humira), a TNF-inhibitor, is now FDA approved for the treatment of moderate-severe HS, and studies are ongoing of IL-17 and IL-23 blockers for the disease.
“The pathophysiology [of HS] is very complex; it’s not nearly as straightforward as psoriasis, and there haven’t been any major breakthroughs yet,” Dr. Blauvelt said. “But the drugs seem to be working better than historical alternatives.”
Regarding AA, Dr. Guttman-Yassky, who is participating in a study of dupilumab for AA, recently found in a retrospective cross-sectional study that patients with the condition are more likely to have atopic comorbidities – asthma, allergic rhinitis, and AD, for instance. “The more comorbid conditions, the greater the risk of developing alopecia areata,” she said. “That could point to a potential pathogenic role of the Th2 axis in the disorder [challenging the traditional view of AA as a singularly Th1-centered disease.] The future will tell.”
Action on rare skin diseases
Both large and small companies have moved into the orphan drug space, investing in research and pursuing orphan drug indications for dermatologic conditions, because “it’s clear now in the marketplace that companies can develop effective drugs for rare disorders and be quite successful,” Dr. Orlow said.
According to a recent analysis, as a result of incentives for rare disease drug development contained in the Orphan Drug Act, 72 indications have been approved for rare skin disease, skin-related cancers, and hereditary disorders with prominent dermatologic manifestations since the law was passed in 1983 (J. Am. Acad. Dermatol. 2019;81[3]:867-77).
Epidermolysis bullosa (EB) is a good example, he and other sources said, of commercial interests merging with growing knowledge of disease pathogenesis as well as the tools needed to develop new treatments.
Research by dermatology scientists and others over the past 40 years, Dr. Ju explained, shed light on the molecular basis underlying the structure and function of the junction between the epidermis and dermis, including the pivotal role that type VII collagen plays in the normal adhesion of these two layers. Researchers then learned that, in EB, the family of genetic diseases characterized by skin fragility, “dystrophic types are caused by mutations in the gene encoding type VII collagen,” he said.
“Just as the advent of monoclonal antibodies allowed us to start attacking psoriasis and atopic dermatitis in unprecedented ways, the advent of gene therapy allows us to potentially address the fundamental molecular genetic defect of various types of EB,” Dr. Ju said.
While gene therapy is “still in its infancy,” companies have begun using the tools to address EB. One gene therapy in the pipeline – in phase 3 clinical trial testing – involves grafting back into patients with recessive dystrophic EB their skin cells that have been genetically modified to produce a correct (nonmutated) type VII collagen, he said.
Basal cell nevus syndrome, or Gorlin syndrome, a rare disease in which patients develop a multitude of basal cell carcinoma tumors, is another example of a “dermatology first” approach, Dr. Ju said. Research identified a genetic mutation that causes the hedgehog signaling pathway to be inappropriately activated in the disease, and a drug, vismodegib, was developed to inhibit this pathway. The drug was initially approved for patients with metastatic basal cell cancer and types of advanced basal cell cancer, and is now being tested in cancers affecting other organs, he said.
Basal cell cancer “is a huge market, but it was really unrecognized in the past,” Dr. Eaglstein said. “Seeing drugs come to market for basal cell cancer – this wouldn’t have happened [decades ago].”
Dr. Ju has worked in the pharmaceutical industry; all other sources in this story have worked with pharmaceutical manufacturers of treatments that are being developed or have been approved to treat dermatologic diseases mentioned in this story. In addition to Dr. Ju, Dr. Eaglstein and Dr. Orlow are cofounders of the Advancing Innovation in Dermatology group; Dr. Orlow is a member of the program committee for the organization’s dermatology summit conference.
For much of the past 50 years, many of the drugs used in dermatology have been adopted – and often adapted – from other specialties and used for dermatologic conditions.
“Almost every drug was more or less a hand-me-down” developed first for cancer or other diseases and found later, often serendipitously, to be useful for the skin, said William Eaglstein, MD, thinking back to the 1970s and recalling steroids, tetracyclines, methotrexate, and 5-flourouracil. “The perception always was that skin diseases weren’t serious, that the market was small.”
Much has changed. by dermatologist investigators, and “more and more companies are recognizing the importance of our diseases and the ability to get a return on investment,” said Dr. Eaglstein, past professor and chair of the departments of dermatology at the University of Miami and the University of Pittsburgh, who worked in industry after his academic career.
Psoriasis was a game changer, he and other dermatologists said in interviews. The tumor necrosis factor (TNF)–alpha blockers were first used for other indications, but their marked follow-on success in psoriasis “offered proof of concept clinically – showing that by targeting immune pathways in the skin we could achieve a clinical effect – and proof of concept commercially” that dermatology drugs are worth pursuing by pharmaceutical companies, said William Ju, MD, a cofounder and president of Advancing Innovation in Dermatology, a nonprofit organization that brings together stakeholders to develop novel dermatologic drugs and products.
This resulted in the approval of subsequent biologics, such as ustekinumab (Stelara) which inhibits the signaling of interleukin (IL)–12/IL-23, for psoriasis as their initial indication. Then, biologics targeting IL-17 followed this dermatology-first approach. “Researchers have continued further dissecting out the immunopathological pathways, and antibody drugs targeting IL-23p19 have been approved for psoriasis as the lead indication,” said Dr. Ju, a dermatologist who has worked in industry.
Seth Orlow, MD, PhD, who chairs the department of dermatology at NYU Langone Health, remembers the 1970s through the 1990s as the “era of topicals” developed for dermatologic conditions – topical antifungals, topical corticosteroids, and topical retinoids. The next decade was characterized by formulation tweaks and few novel treatments for dermatology, said Dr. Orlow, who is also professor of pediatric dermatology and director of the program in cutaneous biology at New York University.
Now, given the succession of psoriasis discoveries in the last decade, “large companies are interested in dermatology,” he said in an interview. “There’s an explosion of interest in atopic dermatitis. … and companies are dipping their toes in the water for alopecia areata and vitiligo. That’s amazing.”
Rare diseases like epidermolysis bullosa, ichthyosis, and basal cell nevus syndrome are getting attention as well, boosted by the Orphan Drug Act of 1983, in addition to increased research on disease pathways and growing appreciation of skin diseases. “There’s a lot under development, from small molecules to biologics to gene-based therapies,” Dr. Orlow commented.
The new frontier of atopic dermatitis
The approval in 2017 of dupilumab (Dupixent), a monoclonal antibody that inhibits the signaling of both IL-4 and IL-13) for moderate-severe atopic dermatitis (AD) in adults illustrates the new standing of dermatologic diseases in the field of drug development and commercialization. “Atopic dermatitis had always been the forgotten chronic disease in dermatology. … We’ve had no good treatments,” said Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland. “Dupilumab coming to the forefront [as a dermatology-first indication] has changed the entire perspective of the field. … Everyone is now trying to find the next best drug.”
As with psoriasis, a targeted therapy for AD was made possible by the development in the 1990s of monoclonal antibody technology and the ensuing ability to create biologics that target specific molecules in the body – as well as bedside-to-bench research that homed in on the involvement of particular cytokines.
But there also is a “new understanding of the burden of the disease,” Dr. Simpson observed. In the last 5 years, he said, research funded by the National Eczema Association documented that AD “not only causes inflammation of the skin … but that it affects people at school and in the workplace, that people have multiple mental health comorbidities and skin infections, and that the disease profoundly affects the entire patient in ways that weren’t really recognized or appreciated.”
Having evolved in the footsteps of psoriasis, AD is at a higher starting point in terms of the safety and efficacy of its first biologic, sources said. On the other hand, AD is a much more complex and heterogeneous disease, and researchers are trying to determine which immune pathways and cytokines are most important – and in which populations.
“We’re at the beginning. We’re trying to figure out how to get 80% of patients clear or almost clear [as we can now with psoriasis biologics] rather than almost 40% [as in the dupilumab pivotal trials],” said Dr. Simpson, former cochair of the National Eczema Association’s scientific committee. Public data from ongoing phase 2 and 3 trials of other Th2 cytokine inhibitors suggest that 25%-45% of enrolled patients achieve high levels of clearance, he noted.
Emma Guttman-Yassky, MD, PhD, Sol and Clara Kest Professor and vice-chair for research in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said that AD’s heterogeneity involves “many factors, like ethnicity, age … and whether they have an atopic background such as asthma.”
Her research is showing, for instance, that AD in Asian and black patients is different than AD in European-American patients, and that the presence of comorbidities may well have treatment implications. She has also shown that children may have a different phenotype than adults, with greater activation of the Th17 axis that typifies psoriasis.
“For certain patients, we may need to target more than one pathway, or target a different pathway than the Th2 pathway. And treatment may be different in the setting of comorbidities,” said Dr. Guttman-Yassky, who is also director of the laboratory of inflammatory skin diseases at Mount Sinai. “We may think of one treatment – dupilumab, for example – for someone who has asthma and AD. But for patients who don’t have asthma and are Asian, for instance, or for children, we may need additional agents.”
Her research over the years on AD has taught her the importance of human studies over mouse model studies; it was in humans, she noted, that she and other investigators demonstrated “without doubt” that AD is an immune disease and not simply a barrier disease. The Th2 cytokine pathway appears to play the predominant role in AD, though “there still is a strong Th1 component,” she said.
“We’re in a better position to figure this out today [than in the past 20 or even 10 years],” said Dr. Guttman-Yassky, who recalls being told years ago that AD was a “dead end,” that it “would kill [her] career.” Given the evolution of science and the recognition of comorbidities and seriousness of dermatologic diseases, “the stars are aligned to get more [therapies] to these patients.”
Janus kinase (JAK) inhibitors are among these therapies. Three JAK inhibitors are in or have recently completed phase 3 studies for AD; two are currently approved for rheumatoid arthritis, and the other has been designed specifically for AD, Dr. Simpson pointed out. The drugs are oral small molecule drugs that block the JAK signaling pathways for certain proinflammatory cytokines.
“The JAK inhibitors are a real exciting story for dermatology,” he said. “Theoretically, by blocking more cytokines than biologics do, there could be some safety issues – that’s why we’re awaiting big phase 3 study results so we can figure out the risk-benefit balance and guide our patients as to which drug is best.”
Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center in Portland – a stand-alone dermatology clinical trial center founded in 1998 – likes to envision the evolution of drugs for dermatologic conditions as a funnel, with the most broad-acting drugs at the wide top of the funnel and the most targeted drugs at the bottom tip.
JAK inhibitors, he said, sit near the middle – more targeted and safer than cyclosporine and methotrexate, for instance, but not as targeted as the biologics now available for psoriasis and being developed for AD. “The oral medications that have been developed for psoriasis and those coming for AD are not quite as targeted to the disease,” he noted. “JAK inhibitors have great efficacy – it’s more a question of safety and being able to treat without causing collateral damage.”
Dr. Blauvelt expects the armamentarium of new drugs approved for AD to go from one (dupilumab) to seven within the next 2 years. This will include three new biologics and three new oral JAK inhibitors, he predicts. As the specialty sorts through and integrates these new drugs into practice, dermatologists will increasingly personalize treatment and will face the “nonscientific” challenge of the cost of new therapies and patient access to them, he noted.
In the meantime, said Dr. Simpson, recent drug discoveries have driven more non–pharmaceutical-funded translational research aimed at understanding the underlying biology of AD. The National Institutes of Health, for instance, “is interested in dupilumab and its impact on the skin barrier and skin defense mechanisms,” he said. “We’ll learn a lot more [in coming years].”
Spillover to other diseases
JAK inhibitors – some in oral and some in topical form – are showing efficacy in ongoing research for alopecia areata (AA) and vitiligo as well, Dr. Blauvelt said.
“We’re understanding more about the pathophysiology of these diseases, which historically have been tough diseases for dermatologists to treat,” he said. “The successes in alopecia areata and vitiligo are incredibly exciting actually – it’s very exciting to see hair and pigment coming back. And as we learn more, we should be able to develop [additional] drugs that are more disease targeted than the JAK inhibitors.”
Already, some of the biologics used to treat psoriasis have been studied in patients with hidradenitis suppurativa (HS), a disease in which painful lumps and sometimes tunnels form under the skin, with some success; adalimumab (Humira), a TNF-inhibitor, is now FDA approved for the treatment of moderate-severe HS, and studies are ongoing of IL-17 and IL-23 blockers for the disease.
“The pathophysiology [of HS] is very complex; it’s not nearly as straightforward as psoriasis, and there haven’t been any major breakthroughs yet,” Dr. Blauvelt said. “But the drugs seem to be working better than historical alternatives.”
Regarding AA, Dr. Guttman-Yassky, who is participating in a study of dupilumab for AA, recently found in a retrospective cross-sectional study that patients with the condition are more likely to have atopic comorbidities – asthma, allergic rhinitis, and AD, for instance. “The more comorbid conditions, the greater the risk of developing alopecia areata,” she said. “That could point to a potential pathogenic role of the Th2 axis in the disorder [challenging the traditional view of AA as a singularly Th1-centered disease.] The future will tell.”
Action on rare skin diseases
Both large and small companies have moved into the orphan drug space, investing in research and pursuing orphan drug indications for dermatologic conditions, because “it’s clear now in the marketplace that companies can develop effective drugs for rare disorders and be quite successful,” Dr. Orlow said.
According to a recent analysis, as a result of incentives for rare disease drug development contained in the Orphan Drug Act, 72 indications have been approved for rare skin disease, skin-related cancers, and hereditary disorders with prominent dermatologic manifestations since the law was passed in 1983 (J. Am. Acad. Dermatol. 2019;81[3]:867-77).
Epidermolysis bullosa (EB) is a good example, he and other sources said, of commercial interests merging with growing knowledge of disease pathogenesis as well as the tools needed to develop new treatments.
Research by dermatology scientists and others over the past 40 years, Dr. Ju explained, shed light on the molecular basis underlying the structure and function of the junction between the epidermis and dermis, including the pivotal role that type VII collagen plays in the normal adhesion of these two layers. Researchers then learned that, in EB, the family of genetic diseases characterized by skin fragility, “dystrophic types are caused by mutations in the gene encoding type VII collagen,” he said.
“Just as the advent of monoclonal antibodies allowed us to start attacking psoriasis and atopic dermatitis in unprecedented ways, the advent of gene therapy allows us to potentially address the fundamental molecular genetic defect of various types of EB,” Dr. Ju said.
While gene therapy is “still in its infancy,” companies have begun using the tools to address EB. One gene therapy in the pipeline – in phase 3 clinical trial testing – involves grafting back into patients with recessive dystrophic EB their skin cells that have been genetically modified to produce a correct (nonmutated) type VII collagen, he said.
Basal cell nevus syndrome, or Gorlin syndrome, a rare disease in which patients develop a multitude of basal cell carcinoma tumors, is another example of a “dermatology first” approach, Dr. Ju said. Research identified a genetic mutation that causes the hedgehog signaling pathway to be inappropriately activated in the disease, and a drug, vismodegib, was developed to inhibit this pathway. The drug was initially approved for patients with metastatic basal cell cancer and types of advanced basal cell cancer, and is now being tested in cancers affecting other organs, he said.
Basal cell cancer “is a huge market, but it was really unrecognized in the past,” Dr. Eaglstein said. “Seeing drugs come to market for basal cell cancer – this wouldn’t have happened [decades ago].”
Dr. Ju has worked in the pharmaceutical industry; all other sources in this story have worked with pharmaceutical manufacturers of treatments that are being developed or have been approved to treat dermatologic diseases mentioned in this story. In addition to Dr. Ju, Dr. Eaglstein and Dr. Orlow are cofounders of the Advancing Innovation in Dermatology group; Dr. Orlow is a member of the program committee for the organization’s dermatology summit conference.
Accelerated fetal growth in boys associated with development of AML
Accelerated fetal growth was associated with acute myeloid leukemia (AML), especially in infant boys and those with minimally differentiated leukemia, according to researchers from the Childhood Leukemia International Consortium (CLIC).
They assessed data from 22 studies involving a total of 3,564 cases to determine if there was an association between fetal growth and AML. The researchers also examined whether this association might vary by age, sex and disease subtype, according to their report published in the European Journal of Cancer.
The researchers calculated pooled estimates by age, sex and overall for harmonized fetal growth markers in association with AML. They used data from 17 International Fetal and Newborn Growth Consortium for the 21st Century Project studies and performed meta-analyses on 5 more studies. They also did subanalyses based on AML subtype.
They found a nearly 50% increased risk of AML among large-for-gestational-age infant boys (odds ratio [OR]: 1.49, 95% confidence interval [CI]: 1.03-2.14), reduced to 34% in boys aged less than 2 years (OR: 1.34, 95% CI: 1.05-1.71) and 25% in boys aged 0-14 years (OR: 1.25, 95% CI: 1.06-1.46). The association of large for gestational age was stronger in boys with the M0/M1 subtype (OR: 1.80, 95% CI: 1.15-2.83). In addition, large birth length for gestational age was also positively associated with AML (OR: 1.38, 95% CI: 1.00-1.92) in boys. By contrast, there, none of these factors were associated with AML in girls, nor were there associates for girls with respect to decelerated fetal growth markers.
“Although the absolute risk seems to be low at a population level, given the rarity of childhood AML, it would be worth exploring whether modifiable factors leading to macrosomia may also affect AML risk to stimulate future monitoring and preventive interventions before and during pregnancy,” the researchers suggested.
The authors reported that they had no conflicts of interest.
SOURCE: Karalexi MA et al. Eur J Canc. 2020;130:1-11.
Accelerated fetal growth was associated with acute myeloid leukemia (AML), especially in infant boys and those with minimally differentiated leukemia, according to researchers from the Childhood Leukemia International Consortium (CLIC).
They assessed data from 22 studies involving a total of 3,564 cases to determine if there was an association between fetal growth and AML. The researchers also examined whether this association might vary by age, sex and disease subtype, according to their report published in the European Journal of Cancer.
The researchers calculated pooled estimates by age, sex and overall for harmonized fetal growth markers in association with AML. They used data from 17 International Fetal and Newborn Growth Consortium for the 21st Century Project studies and performed meta-analyses on 5 more studies. They also did subanalyses based on AML subtype.
They found a nearly 50% increased risk of AML among large-for-gestational-age infant boys (odds ratio [OR]: 1.49, 95% confidence interval [CI]: 1.03-2.14), reduced to 34% in boys aged less than 2 years (OR: 1.34, 95% CI: 1.05-1.71) and 25% in boys aged 0-14 years (OR: 1.25, 95% CI: 1.06-1.46). The association of large for gestational age was stronger in boys with the M0/M1 subtype (OR: 1.80, 95% CI: 1.15-2.83). In addition, large birth length for gestational age was also positively associated with AML (OR: 1.38, 95% CI: 1.00-1.92) in boys. By contrast, there, none of these factors were associated with AML in girls, nor were there associates for girls with respect to decelerated fetal growth markers.
“Although the absolute risk seems to be low at a population level, given the rarity of childhood AML, it would be worth exploring whether modifiable factors leading to macrosomia may also affect AML risk to stimulate future monitoring and preventive interventions before and during pregnancy,” the researchers suggested.
The authors reported that they had no conflicts of interest.
SOURCE: Karalexi MA et al. Eur J Canc. 2020;130:1-11.
Accelerated fetal growth was associated with acute myeloid leukemia (AML), especially in infant boys and those with minimally differentiated leukemia, according to researchers from the Childhood Leukemia International Consortium (CLIC).
They assessed data from 22 studies involving a total of 3,564 cases to determine if there was an association between fetal growth and AML. The researchers also examined whether this association might vary by age, sex and disease subtype, according to their report published in the European Journal of Cancer.
The researchers calculated pooled estimates by age, sex and overall for harmonized fetal growth markers in association with AML. They used data from 17 International Fetal and Newborn Growth Consortium for the 21st Century Project studies and performed meta-analyses on 5 more studies. They also did subanalyses based on AML subtype.
They found a nearly 50% increased risk of AML among large-for-gestational-age infant boys (odds ratio [OR]: 1.49, 95% confidence interval [CI]: 1.03-2.14), reduced to 34% in boys aged less than 2 years (OR: 1.34, 95% CI: 1.05-1.71) and 25% in boys aged 0-14 years (OR: 1.25, 95% CI: 1.06-1.46). The association of large for gestational age was stronger in boys with the M0/M1 subtype (OR: 1.80, 95% CI: 1.15-2.83). In addition, large birth length for gestational age was also positively associated with AML (OR: 1.38, 95% CI: 1.00-1.92) in boys. By contrast, there, none of these factors were associated with AML in girls, nor were there associates for girls with respect to decelerated fetal growth markers.
“Although the absolute risk seems to be low at a population level, given the rarity of childhood AML, it would be worth exploring whether modifiable factors leading to macrosomia may also affect AML risk to stimulate future monitoring and preventive interventions before and during pregnancy,” the researchers suggested.
The authors reported that they had no conflicts of interest.
SOURCE: Karalexi MA et al. Eur J Canc. 2020;130:1-11.
FROM THE EUROPEAN JOURNAL OF CANCER
Testing times for epidermolysis bullosa topical therapies
LONDON –
Results from trials such as ESSENCE, with allantoin, and DELIVERS, with diacerein, were “disappointing,” Dédée Murrell, BMBCh, MD, pointed out at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Those two topical agents were most likely let down by the trials’ design, said Dr. Murrell, of St. George Hospital, University of New South Wales, Sydney, but she noted that there were still some promising trials that were either ongoing, such as EASE, with Oleogel-S10, or that were about to be unblinded, such as SISTERS, with sirolimus.
Epidermolysis bullosa (EB) is a group of rare genetic diseases that can cause the skin to blister and peel away to varying degrees, causing itchy and painful skin, as well as recurrent wounds, some of which may seem never to heal and that increase the risk for squamous cell carcinoma. Although finding a cure for the disease is high on the research agenda, finding a reliable therapy that can soothe and protect the skin is of equal importance.
Trials and tribulations
Conducting trials in rare diseases can be difficult because the studies are often small and poorly controlled, Dr. Murrell said during an oral presentation at the meeting. To gain regulatory approval, trials need to have an active and a placebo arm, because “even though we’re dealing with a rare disease, we still have to show statistical significance between the two arms.”
However, it is not just about finding enough participants who meet the inclusion criteria and adequately controlling the study, as finding funding can also be a significant hurdle. That is the case particularly when an existing drug with no patent protection is proposed to be repurposed. As an example, Dr. Murrell said that many patients with EB may use gentian violet to treat their condition, but it has been around for so long and is so widely used, that funding a trial to formally prove its merit is unlikely. In addition, “there are special caveats that occur in dermatology clinical trials with topical drugs that don’t exist [in trials] with systemic treatments, one of which is that it is very important to keep other variables the same,” Dr. Murrell said. “So, for example, the dressings need to stay the same throughout a trial with a topical therapy, because if you improve the dressings [during the course of the trial], you could mask the effect of the treatment.” Similarly, the bathing and cleansing routines of the participants need to remain the same throughout the trial.
“We also need to have validated instruments to prove whether these treatments are working, and the instruments need to be objective as well as subjective,” Dr. Murrell advised. For example, inflammation and blistering need to be scored separately from scarring and skin damage. “You have to conduct a clinical trial to be able to verify that there is diminished scarring or damage, because those are the longer-term complications.” Inflammation and blistering are valid endpoints to use in shorter-term studies.
Dr. Murrell also cautioned on getting too enthused about the results of case reports. “We do get excited when we see a patient using something new and they seem to be getting much better,” but such reports do not have a placebo arm, or, if there is one, then there is no vehicle control, she said. It’s important to include a run-in period in a trial to establish a new baseline and to ensure that any effects seen with a topical agent are independent of the carrier substance or any altered bathing behavior or dressing habits, which could skew the results.
ESSENCE and allantoin
So what went wrong in the phase 3 ESSENCE trial with allantoin, which was halted early in September 2017? The trial had included 169 patients with any type of EB – simplex, recessive dystrophic, and junctional non-Herlitz – who were randomized to treatment with the allantoin-containing cream SD-101 or a placebo cream containing only the vehicle. The creams were applied daily to the entire body for 3 months, with the primary endpoint being total wound closure at the end of the treatment period. Total wound closure was a requirement of the Food and Drug Administration, Dr. Murrell said, but it is now known that 100% closure is not always likely, which the agency itself now concedes.
“Most disappointingly, no significant difference was found [between the study drug and placebo], therefore it didn’t meet the primary endpoint, and you’re not even allowed to consider secondary endpoints – those are the rules of the game,” she said. As a result, the trial was stopped in 2017.
For inclusion in the study, patients had to have at least one target wound that had been present for at least 3 weeks, but there was no stratification on the duration of wounds in the randomization process. That meant that some individuals with wounds of shorter duration had unintentionally ended up in the placebo arm – favoring healing – and those with more chronic wounds had been in the allantoin arm. So, because the study arms might not have been equally balanced at baseline, it would have been harder for the actual treatment to demonstrate a benefit, Dr. Murrell suggested.
Another problem with the trial was that the vehicle cream contained elements, such as lanolin, already associated with wound healing. That would have given patients in the placebo arm an advantage because anyone applying the cream every day would probably get better or improve to some degree.
The patients were also required to have daily dressing changes and baths and, “if you give any patient that advice and they comply with it for a period of time, they are going to improve,” whether or not they are applying the study drug. Dr. Murrell said that the researchers likely should have done a run-in period first and then established a new baseline to randomize the patients.
“Lastly, no one had ever done a study of what we essentially tell eczema patients to do every day … to moisturize, because that will provide extra protection and barrier to their skin. So, if anything, the ESSENCE study shows that moisturizing has a protective effect of the vehicle for patients with EB,” she said.
DELIVERS and diacerein
Another trial that was stopped prematurely was the phase 2 DELIVERS study, which was set up to assess the benefits of topical diacerein in people with EB simplex. Diacerein, an extract of rhubarb root, was tested in 54 patients, who were randomized to apply either diacerein or vehicle ointment for 8 weeks.
Initially, the results “looked very promising,” Dr. Murrell said, because there was a trend toward improved EB simplex lesions, with the primary endpoint of at least a 60% reduction in lesions met by 57.1% of diacerein-treated and 53.8% of vehicle-treated patients.
However, the trial included use of the Investigator’s Global Assessment Scale at the FDA’s behest, but the tool had not been validated in previous EB trials, and which didn’t seem to show any benefit of the active over the placebo ointment. (The Investigator’s Global Assessment is a 5-point scale used for overall clinical assessment of severity of disease, ranging from 0 to 4, where a higher score denotes worse outcome.)In a poster presented separately at the meeting, the DELIVERS researchers noted that “the lack of statistical significance in the primary endpoint could be explained in part by milder disease in the diacerein group.” The mean body surface area of EB simplex lesions within the assessment area at baseline was 5.76% in the diacerein group and 7.13% in the vehicle group. The researchers proposed that perhaps a higher concentration of diacerein than the 1% used in the trial might have been needed.
Sirolimus and EB simplex
Dr. Murrell noted that a pilot study, known as the SISTERS trial, had been conducted with a 2% sirolimus topical ointment at her institution and at Stanford (Calif.) University. This prospective, double-blind study had involved 16 patients with EB simplex, in which blisters tend to be confined to the palms of the hands and soles of the feet. The patients were assigned to treat both feet with either topical sirolimus or a placebo cream for 12 weeks. After a 4-week wash-out period, the patients switched to using the opposite cream for an additional 12 weeks.
Sirolimus is an inhibitor of the mTOR pathway, and, according to a description of the study on ClinicalTrials.gov, the researchers’ aim was to inhibit “the mTOR pathway to down-regulate the translation of defective keratin proteins.” That would allow a transition from supportive care, which is the current practice for EB simplex, to using a targeted molecular therapy to improve patient mobility and quality of life, they note on the site.
“We look forward to having that study unblinded,” Dr. Murrell said, adding that “data should be ready in a few months.”
EASE and Oleogel-S10
Oleogel-S10 is a gel that contains a birch bark extract dissolved in sunflower oil. It is already approved in Europe (Episalvan) for the treatment of partial-thickness skin wounds, but its use in EB remains investigational.
In a poster presentation at the meeting, Stella Gewert, MD, of the University of Freiburg (Germany) and colleagues discussed their experience using Oleogel-S10 in the treatment of four patients – each with a different type of EB – who applied the gel for between 6 days and 3 months.
Promising effects were seen, including reduced pruritus and pain, wounds healing more quickly, and reductions in lesion size. “During treatment, dressing requirements were reduced, and patient quality of life improved,” the researchers observed.
Mark Sumeray, MD, the chief medical officer of Amryt Pharmaceuticals, which is developing Oleogel-S10, said it was important to emphasize that Oleogel-S10 is a gel and not a cream. Gels are mixed with oil and are easier to apply – an important consideration for those with EB, he explained, whereas creams tend to be mixed with water and are stickier.
The phase 3 EASE trial is looking at the efficacy and safety of the gel in patients with junctional and dystrophic EB, and recruitment is ongoing, Dr. Murrell said. The primary endpoint is the proportion of patients with the first complete closure of a target wound within 45 days of treatment initiation. The estimated primary completion date for the trial is June 2020, and it is projected to end by 2022.
Scioderm, in collaboration with Amicus, funded the ESSENCE trial; Castle Creek financed the DELIVERS study; Amryt is supporting the EASE study; and Stanford University is sponsor of the SISTERS study. Dr. Murrell has been the principal investigator for trials run by Amicus, Amryt, Castle Creek, and Shire, and she acknowledged receipt of honoraria or consultation fees from those companies and others. Dr. Gewert did not report any financial disclosures. Dr. Sumeray is an employee and shareholder of Amryt.
LONDON –
Results from trials such as ESSENCE, with allantoin, and DELIVERS, with diacerein, were “disappointing,” Dédée Murrell, BMBCh, MD, pointed out at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Those two topical agents were most likely let down by the trials’ design, said Dr. Murrell, of St. George Hospital, University of New South Wales, Sydney, but she noted that there were still some promising trials that were either ongoing, such as EASE, with Oleogel-S10, or that were about to be unblinded, such as SISTERS, with sirolimus.
Epidermolysis bullosa (EB) is a group of rare genetic diseases that can cause the skin to blister and peel away to varying degrees, causing itchy and painful skin, as well as recurrent wounds, some of which may seem never to heal and that increase the risk for squamous cell carcinoma. Although finding a cure for the disease is high on the research agenda, finding a reliable therapy that can soothe and protect the skin is of equal importance.
Trials and tribulations
Conducting trials in rare diseases can be difficult because the studies are often small and poorly controlled, Dr. Murrell said during an oral presentation at the meeting. To gain regulatory approval, trials need to have an active and a placebo arm, because “even though we’re dealing with a rare disease, we still have to show statistical significance between the two arms.”
However, it is not just about finding enough participants who meet the inclusion criteria and adequately controlling the study, as finding funding can also be a significant hurdle. That is the case particularly when an existing drug with no patent protection is proposed to be repurposed. As an example, Dr. Murrell said that many patients with EB may use gentian violet to treat their condition, but it has been around for so long and is so widely used, that funding a trial to formally prove its merit is unlikely. In addition, “there are special caveats that occur in dermatology clinical trials with topical drugs that don’t exist [in trials] with systemic treatments, one of which is that it is very important to keep other variables the same,” Dr. Murrell said. “So, for example, the dressings need to stay the same throughout a trial with a topical therapy, because if you improve the dressings [during the course of the trial], you could mask the effect of the treatment.” Similarly, the bathing and cleansing routines of the participants need to remain the same throughout the trial.
“We also need to have validated instruments to prove whether these treatments are working, and the instruments need to be objective as well as subjective,” Dr. Murrell advised. For example, inflammation and blistering need to be scored separately from scarring and skin damage. “You have to conduct a clinical trial to be able to verify that there is diminished scarring or damage, because those are the longer-term complications.” Inflammation and blistering are valid endpoints to use in shorter-term studies.
Dr. Murrell also cautioned on getting too enthused about the results of case reports. “We do get excited when we see a patient using something new and they seem to be getting much better,” but such reports do not have a placebo arm, or, if there is one, then there is no vehicle control, she said. It’s important to include a run-in period in a trial to establish a new baseline and to ensure that any effects seen with a topical agent are independent of the carrier substance or any altered bathing behavior or dressing habits, which could skew the results.
ESSENCE and allantoin
So what went wrong in the phase 3 ESSENCE trial with allantoin, which was halted early in September 2017? The trial had included 169 patients with any type of EB – simplex, recessive dystrophic, and junctional non-Herlitz – who were randomized to treatment with the allantoin-containing cream SD-101 or a placebo cream containing only the vehicle. The creams were applied daily to the entire body for 3 months, with the primary endpoint being total wound closure at the end of the treatment period. Total wound closure was a requirement of the Food and Drug Administration, Dr. Murrell said, but it is now known that 100% closure is not always likely, which the agency itself now concedes.
“Most disappointingly, no significant difference was found [between the study drug and placebo], therefore it didn’t meet the primary endpoint, and you’re not even allowed to consider secondary endpoints – those are the rules of the game,” she said. As a result, the trial was stopped in 2017.
For inclusion in the study, patients had to have at least one target wound that had been present for at least 3 weeks, but there was no stratification on the duration of wounds in the randomization process. That meant that some individuals with wounds of shorter duration had unintentionally ended up in the placebo arm – favoring healing – and those with more chronic wounds had been in the allantoin arm. So, because the study arms might not have been equally balanced at baseline, it would have been harder for the actual treatment to demonstrate a benefit, Dr. Murrell suggested.
Another problem with the trial was that the vehicle cream contained elements, such as lanolin, already associated with wound healing. That would have given patients in the placebo arm an advantage because anyone applying the cream every day would probably get better or improve to some degree.
The patients were also required to have daily dressing changes and baths and, “if you give any patient that advice and they comply with it for a period of time, they are going to improve,” whether or not they are applying the study drug. Dr. Murrell said that the researchers likely should have done a run-in period first and then established a new baseline to randomize the patients.
“Lastly, no one had ever done a study of what we essentially tell eczema patients to do every day … to moisturize, because that will provide extra protection and barrier to their skin. So, if anything, the ESSENCE study shows that moisturizing has a protective effect of the vehicle for patients with EB,” she said.
DELIVERS and diacerein
Another trial that was stopped prematurely was the phase 2 DELIVERS study, which was set up to assess the benefits of topical diacerein in people with EB simplex. Diacerein, an extract of rhubarb root, was tested in 54 patients, who were randomized to apply either diacerein or vehicle ointment for 8 weeks.
Initially, the results “looked very promising,” Dr. Murrell said, because there was a trend toward improved EB simplex lesions, with the primary endpoint of at least a 60% reduction in lesions met by 57.1% of diacerein-treated and 53.8% of vehicle-treated patients.
However, the trial included use of the Investigator’s Global Assessment Scale at the FDA’s behest, but the tool had not been validated in previous EB trials, and which didn’t seem to show any benefit of the active over the placebo ointment. (The Investigator’s Global Assessment is a 5-point scale used for overall clinical assessment of severity of disease, ranging from 0 to 4, where a higher score denotes worse outcome.)In a poster presented separately at the meeting, the DELIVERS researchers noted that “the lack of statistical significance in the primary endpoint could be explained in part by milder disease in the diacerein group.” The mean body surface area of EB simplex lesions within the assessment area at baseline was 5.76% in the diacerein group and 7.13% in the vehicle group. The researchers proposed that perhaps a higher concentration of diacerein than the 1% used in the trial might have been needed.
Sirolimus and EB simplex
Dr. Murrell noted that a pilot study, known as the SISTERS trial, had been conducted with a 2% sirolimus topical ointment at her institution and at Stanford (Calif.) University. This prospective, double-blind study had involved 16 patients with EB simplex, in which blisters tend to be confined to the palms of the hands and soles of the feet. The patients were assigned to treat both feet with either topical sirolimus or a placebo cream for 12 weeks. After a 4-week wash-out period, the patients switched to using the opposite cream for an additional 12 weeks.
Sirolimus is an inhibitor of the mTOR pathway, and, according to a description of the study on ClinicalTrials.gov, the researchers’ aim was to inhibit “the mTOR pathway to down-regulate the translation of defective keratin proteins.” That would allow a transition from supportive care, which is the current practice for EB simplex, to using a targeted molecular therapy to improve patient mobility and quality of life, they note on the site.
“We look forward to having that study unblinded,” Dr. Murrell said, adding that “data should be ready in a few months.”
EASE and Oleogel-S10
Oleogel-S10 is a gel that contains a birch bark extract dissolved in sunflower oil. It is already approved in Europe (Episalvan) for the treatment of partial-thickness skin wounds, but its use in EB remains investigational.
In a poster presentation at the meeting, Stella Gewert, MD, of the University of Freiburg (Germany) and colleagues discussed their experience using Oleogel-S10 in the treatment of four patients – each with a different type of EB – who applied the gel for between 6 days and 3 months.
Promising effects were seen, including reduced pruritus and pain, wounds healing more quickly, and reductions in lesion size. “During treatment, dressing requirements were reduced, and patient quality of life improved,” the researchers observed.
Mark Sumeray, MD, the chief medical officer of Amryt Pharmaceuticals, which is developing Oleogel-S10, said it was important to emphasize that Oleogel-S10 is a gel and not a cream. Gels are mixed with oil and are easier to apply – an important consideration for those with EB, he explained, whereas creams tend to be mixed with water and are stickier.
The phase 3 EASE trial is looking at the efficacy and safety of the gel in patients with junctional and dystrophic EB, and recruitment is ongoing, Dr. Murrell said. The primary endpoint is the proportion of patients with the first complete closure of a target wound within 45 days of treatment initiation. The estimated primary completion date for the trial is June 2020, and it is projected to end by 2022.
Scioderm, in collaboration with Amicus, funded the ESSENCE trial; Castle Creek financed the DELIVERS study; Amryt is supporting the EASE study; and Stanford University is sponsor of the SISTERS study. Dr. Murrell has been the principal investigator for trials run by Amicus, Amryt, Castle Creek, and Shire, and she acknowledged receipt of honoraria or consultation fees from those companies and others. Dr. Gewert did not report any financial disclosures. Dr. Sumeray is an employee and shareholder of Amryt.
LONDON –
Results from trials such as ESSENCE, with allantoin, and DELIVERS, with diacerein, were “disappointing,” Dédée Murrell, BMBCh, MD, pointed out at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Those two topical agents were most likely let down by the trials’ design, said Dr. Murrell, of St. George Hospital, University of New South Wales, Sydney, but she noted that there were still some promising trials that were either ongoing, such as EASE, with Oleogel-S10, or that were about to be unblinded, such as SISTERS, with sirolimus.
Epidermolysis bullosa (EB) is a group of rare genetic diseases that can cause the skin to blister and peel away to varying degrees, causing itchy and painful skin, as well as recurrent wounds, some of which may seem never to heal and that increase the risk for squamous cell carcinoma. Although finding a cure for the disease is high on the research agenda, finding a reliable therapy that can soothe and protect the skin is of equal importance.
Trials and tribulations
Conducting trials in rare diseases can be difficult because the studies are often small and poorly controlled, Dr. Murrell said during an oral presentation at the meeting. To gain regulatory approval, trials need to have an active and a placebo arm, because “even though we’re dealing with a rare disease, we still have to show statistical significance between the two arms.”
However, it is not just about finding enough participants who meet the inclusion criteria and adequately controlling the study, as finding funding can also be a significant hurdle. That is the case particularly when an existing drug with no patent protection is proposed to be repurposed. As an example, Dr. Murrell said that many patients with EB may use gentian violet to treat their condition, but it has been around for so long and is so widely used, that funding a trial to formally prove its merit is unlikely. In addition, “there are special caveats that occur in dermatology clinical trials with topical drugs that don’t exist [in trials] with systemic treatments, one of which is that it is very important to keep other variables the same,” Dr. Murrell said. “So, for example, the dressings need to stay the same throughout a trial with a topical therapy, because if you improve the dressings [during the course of the trial], you could mask the effect of the treatment.” Similarly, the bathing and cleansing routines of the participants need to remain the same throughout the trial.
“We also need to have validated instruments to prove whether these treatments are working, and the instruments need to be objective as well as subjective,” Dr. Murrell advised. For example, inflammation and blistering need to be scored separately from scarring and skin damage. “You have to conduct a clinical trial to be able to verify that there is diminished scarring or damage, because those are the longer-term complications.” Inflammation and blistering are valid endpoints to use in shorter-term studies.
Dr. Murrell also cautioned on getting too enthused about the results of case reports. “We do get excited when we see a patient using something new and they seem to be getting much better,” but such reports do not have a placebo arm, or, if there is one, then there is no vehicle control, she said. It’s important to include a run-in period in a trial to establish a new baseline and to ensure that any effects seen with a topical agent are independent of the carrier substance or any altered bathing behavior or dressing habits, which could skew the results.
ESSENCE and allantoin
So what went wrong in the phase 3 ESSENCE trial with allantoin, which was halted early in September 2017? The trial had included 169 patients with any type of EB – simplex, recessive dystrophic, and junctional non-Herlitz – who were randomized to treatment with the allantoin-containing cream SD-101 or a placebo cream containing only the vehicle. The creams were applied daily to the entire body for 3 months, with the primary endpoint being total wound closure at the end of the treatment period. Total wound closure was a requirement of the Food and Drug Administration, Dr. Murrell said, but it is now known that 100% closure is not always likely, which the agency itself now concedes.
“Most disappointingly, no significant difference was found [between the study drug and placebo], therefore it didn’t meet the primary endpoint, and you’re not even allowed to consider secondary endpoints – those are the rules of the game,” she said. As a result, the trial was stopped in 2017.
For inclusion in the study, patients had to have at least one target wound that had been present for at least 3 weeks, but there was no stratification on the duration of wounds in the randomization process. That meant that some individuals with wounds of shorter duration had unintentionally ended up in the placebo arm – favoring healing – and those with more chronic wounds had been in the allantoin arm. So, because the study arms might not have been equally balanced at baseline, it would have been harder for the actual treatment to demonstrate a benefit, Dr. Murrell suggested.
Another problem with the trial was that the vehicle cream contained elements, such as lanolin, already associated with wound healing. That would have given patients in the placebo arm an advantage because anyone applying the cream every day would probably get better or improve to some degree.
The patients were also required to have daily dressing changes and baths and, “if you give any patient that advice and they comply with it for a period of time, they are going to improve,” whether or not they are applying the study drug. Dr. Murrell said that the researchers likely should have done a run-in period first and then established a new baseline to randomize the patients.
“Lastly, no one had ever done a study of what we essentially tell eczema patients to do every day … to moisturize, because that will provide extra protection and barrier to their skin. So, if anything, the ESSENCE study shows that moisturizing has a protective effect of the vehicle for patients with EB,” she said.
DELIVERS and diacerein
Another trial that was stopped prematurely was the phase 2 DELIVERS study, which was set up to assess the benefits of topical diacerein in people with EB simplex. Diacerein, an extract of rhubarb root, was tested in 54 patients, who were randomized to apply either diacerein or vehicle ointment for 8 weeks.
Initially, the results “looked very promising,” Dr. Murrell said, because there was a trend toward improved EB simplex lesions, with the primary endpoint of at least a 60% reduction in lesions met by 57.1% of diacerein-treated and 53.8% of vehicle-treated patients.
However, the trial included use of the Investigator’s Global Assessment Scale at the FDA’s behest, but the tool had not been validated in previous EB trials, and which didn’t seem to show any benefit of the active over the placebo ointment. (The Investigator’s Global Assessment is a 5-point scale used for overall clinical assessment of severity of disease, ranging from 0 to 4, where a higher score denotes worse outcome.)In a poster presented separately at the meeting, the DELIVERS researchers noted that “the lack of statistical significance in the primary endpoint could be explained in part by milder disease in the diacerein group.” The mean body surface area of EB simplex lesions within the assessment area at baseline was 5.76% in the diacerein group and 7.13% in the vehicle group. The researchers proposed that perhaps a higher concentration of diacerein than the 1% used in the trial might have been needed.
Sirolimus and EB simplex
Dr. Murrell noted that a pilot study, known as the SISTERS trial, had been conducted with a 2% sirolimus topical ointment at her institution and at Stanford (Calif.) University. This prospective, double-blind study had involved 16 patients with EB simplex, in which blisters tend to be confined to the palms of the hands and soles of the feet. The patients were assigned to treat both feet with either topical sirolimus or a placebo cream for 12 weeks. After a 4-week wash-out period, the patients switched to using the opposite cream for an additional 12 weeks.
Sirolimus is an inhibitor of the mTOR pathway, and, according to a description of the study on ClinicalTrials.gov, the researchers’ aim was to inhibit “the mTOR pathway to down-regulate the translation of defective keratin proteins.” That would allow a transition from supportive care, which is the current practice for EB simplex, to using a targeted molecular therapy to improve patient mobility and quality of life, they note on the site.
“We look forward to having that study unblinded,” Dr. Murrell said, adding that “data should be ready in a few months.”
EASE and Oleogel-S10
Oleogel-S10 is a gel that contains a birch bark extract dissolved in sunflower oil. It is already approved in Europe (Episalvan) for the treatment of partial-thickness skin wounds, but its use in EB remains investigational.
In a poster presentation at the meeting, Stella Gewert, MD, of the University of Freiburg (Germany) and colleagues discussed their experience using Oleogel-S10 in the treatment of four patients – each with a different type of EB – who applied the gel for between 6 days and 3 months.
Promising effects were seen, including reduced pruritus and pain, wounds healing more quickly, and reductions in lesion size. “During treatment, dressing requirements were reduced, and patient quality of life improved,” the researchers observed.
Mark Sumeray, MD, the chief medical officer of Amryt Pharmaceuticals, which is developing Oleogel-S10, said it was important to emphasize that Oleogel-S10 is a gel and not a cream. Gels are mixed with oil and are easier to apply – an important consideration for those with EB, he explained, whereas creams tend to be mixed with water and are stickier.
The phase 3 EASE trial is looking at the efficacy and safety of the gel in patients with junctional and dystrophic EB, and recruitment is ongoing, Dr. Murrell said. The primary endpoint is the proportion of patients with the first complete closure of a target wound within 45 days of treatment initiation. The estimated primary completion date for the trial is June 2020, and it is projected to end by 2022.
Scioderm, in collaboration with Amicus, funded the ESSENCE trial; Castle Creek financed the DELIVERS study; Amryt is supporting the EASE study; and Stanford University is sponsor of the SISTERS study. Dr. Murrell has been the principal investigator for trials run by Amicus, Amryt, Castle Creek, and Shire, and she acknowledged receipt of honoraria or consultation fees from those companies and others. Dr. Gewert did not report any financial disclosures. Dr. Sumeray is an employee and shareholder of Amryt.
EXPERT ANALYSIS FROM EB 2020
Merkel cell carcinoma management undergoes revolution
LAHAINA, HAWAII – The , Paul Nghiem, MD, PhD, declared at the SDEF Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
That’s because dermatologists are typically the physicians who make the diagnosis of Merkel cell carcinoma (MCC), so they’re on the scene from the outset and well positioned to help direct early management of this particularly aggressive malignancy, explained Dr. Nghiem, professor and head of dermatology at the University of Washington, Seattle.
“The management of Merkel is pretty high stakes, and if you get it right at the beginning it makes a huge difference in the side effects, as well as the chances that the patient will have the disease under control,” said Dr. Nghiem, who is sometimes called “the Merkel man” because of his many pioneering contributions to the field.
Better early management
Getting early management right, he added, hinges upon ordering a baseline PET-CT scan to search for metastases before performing definitive surgical excision of the primary tumor.
“There are really important prognostic and therapeutic implications for a baseline scan in almost any patient with early Merkel – and that’s a very different situation than with melanoma,” the dermatologist said. “There’s at least a threefold higher likelihood that the cancer has spread asymptomatically at baseline with Merkel cell carcinoma than with melanoma.”
In a soon-to-be-published study by Dr. Nghiem and coworkers, baseline imaging resulted in prognostically important upstaging that led to an altered management strategy in 12% of 584 patients with MCC, or 1 in 8.
“You don’t want to overtreat locally a lesion that has already spread distantly; you want to start focusing on the distant disease. The local disease is secondary,” he said.
The surgical excision of the primary lesion should be thoughtfully wide without being aggressive or mutilating, and it should involve primary closure. “Definitely avoid flaps and grafts, which delay your further management with radiotherapy by months and months,” Dr. Nghiem advised.
Adjuvant radiotherapy of the primary tumor site is extremely effective at preventing recurrent MCC. In Dr. Nghiem’s view, almost everyone is a candidate: In a series of 803 patients in the Seattle MCC cohort, 92% received local adjuvant radiotherapy. The national rate, in contrast, is only about 50%, highlighting the need for additional physician education.
“A little bit of radiation – one dose – appears to be just as effective as 6 weeks in controlling microscopic disease. That’s probably something we’re going to be moving towards as a field,” he predicted.
Indeed, local adjuvant radiotherapy is so effective in MCC that the surgical margins make no difference. This was demonstrated in a study by Dr. Nghiem and his coinvestigators involving 70 patients with margins greater than 1 cm who received radiotherapy, 70 others with smaller or even positive margins who received radiotherapy, and 35 patients with margins of 1 cm or less who did not receive radiotherapy. There were no MCC recurrences in any of the radiotherapy recipients, regardless of their margin status. In contrast, 7 of the 35 patients who didn’t receive radiation therapy developed a cancer recurrence. Of note, the recurrence rate of MCC is historically about 40% – far greater than for any other skin cancer. Most recurrences happen within the first 2-3 years, Dr. Nghiem observed.
Immune therapy takes center stage
Another major transformation in MCC management has been the emergence of immune therapy as first-line systemic therapy. It has replaced chemotherapy, which is more toxic and has a much shorter average duration of response. Avelumab (Bavencio) and pembrolizumab (Keytruda), the two monoclonal antibodies directed against the protein programmed death–ligand 1 (PD-L1) receptor which are approved for MCC and have been incorporated into the National Comprehensive Cancer Network (NCCN) guidelines, provide a sixfold improvement in survival, compared with chemotherapy. For example, Dr. Nghiem was first author of a multicenter phase 2 study of pembrolizumab in which the 12- and 24-month overall survival rates in pembrolizumab responders were 85% and 79%, compared with just 12% and 6%, respectively, in historical controls on first-line chemotherapy (J Clin Oncol. 2019 Mar 20;37[9]:693-702).
“Merkel cell carcinoma is the most responsive solid tumor to immune therapy,” Dr. Nghiem commented.
Why MCC matters
Although rare, MCC is important because it’s five times more lethal than melanoma. Moreover, its incidence has been rising at a rate roughly twice that of the increase in melanoma since the turn of the century. There are now more than 3,000 new cases of MCC annually, about the same as for cutaneous T-cell lymphoma (CTCL).
“It’s just that you live a long time with CTCL and you don’t with Merkel cell carcinoma. You either get rid of Merkel fast or it gets rid of you,” the dermatologist observed.
It’s a fascinating malignancy, he continued. Eight of 10 cases are caused by Merkel cell polyomavirus, discovered in 2008. The virus is ubiquitously acquired in childhood and then lies dormant on the skin for the next 6 or 7 decades, at which point MCC rates shoot up dramatically, probably due to immunosenescence. Immunosuppressed patients are at 10-fold increased risk for MCC.
Given the rarity of MCC, it doesn’t make sense to actively hunt for it. But Dr. Nghiem and coworkers have developed a handy vowel-based mnemonic that serves to raise the index of suspicion: the “AEIOU” features.
- A = asymptomatic.
- E = expanding rapidly within past 3 months.
- I = immune-mediated.
- O = older than age 50.
- U = UV-exposed skin.
The investigators found in a series of 195 MCC patients that 89% of them possessed three or more of these features (J Am Acad Dermatol. 2008 Mar;58[3]:375-81). But while the AEIOU guide is quite sensitive, it’s not specific.
“If you have any three or more of these features, that lesion probably deserves a biopsy if it’s not readily explained. Even if it’s not a Merkel, it may turn out to be a different nonmelanoma skin cancer, something you want to know about,” Dr. Nghiem said.
A shift in surveillance strategy
Dr. Nghiem was senior author of a major study that validated the clinical utility of a Merkel polyomavirus serology test for monitoring the disease status of patients treated for MCC (Cancer. 2017 Apr 15;123[8]:1464-74). The test, which measures antibodies to Merkel cell polyomavirus oncoproteins, has been incorporated in NCCN guidelines. The blood test is used initially in newly diagnosed MCC to stratify patients into two subgroups: the half who are seropositive at baseline, and the other half who are seronegative. The seropositive group undergoes surveillance via repeat blood testing every 3 months. If antibody levels are low, there is a high degree of certainty that immune therapy is working and remission is present. Thus, the blood test spares patients in this group the expense and radiation exposure entailed in repeated surveillance scans. However, rising antibody levels indicate the cancer has already recurred or will do so within the next several months.
Unfortunately, the blood test cannot be used serially to track disease status in patients who are seronegative at baseline. That group is at 42% increased risk of MCC recurrence.
Immune therapy works in only about two-thirds of MCC patients with distant disease. Leaving the visible primary tumor in place to serve as a real-time window into immune treatment effectiveness is a useful contemporary surveillance strategy.
“By leaving the visible primary there, you will rapidly know if that patient is in the favorable two-thirds group or not,” he explained.
Historically, surgery and surveillance of MCC were based upon the melanoma model, and medical oncologists were trained to treat the malignancy as they would small cell lung cancer. These are now outmoded approaches, Dr. Nghiem said. That’s why a multidisciplinary approach is highly desirable for management of MCC, including dermatologists, pathologists, surgeons, radiation oncologists, medical oncologists, and imaging experts.
Dr. Nghiem and his colleagues have created a comprehensive source of information about Merkel cell carcinoma for physicians and patients at merkelcell.org.
He reported receiving research grants from Bristol-Myers Squibb and serving as a consultant to EMD Serono, Merck, Sanofi/Regeneron, and 4SC.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – The , Paul Nghiem, MD, PhD, declared at the SDEF Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
That’s because dermatologists are typically the physicians who make the diagnosis of Merkel cell carcinoma (MCC), so they’re on the scene from the outset and well positioned to help direct early management of this particularly aggressive malignancy, explained Dr. Nghiem, professor and head of dermatology at the University of Washington, Seattle.
“The management of Merkel is pretty high stakes, and if you get it right at the beginning it makes a huge difference in the side effects, as well as the chances that the patient will have the disease under control,” said Dr. Nghiem, who is sometimes called “the Merkel man” because of his many pioneering contributions to the field.
Better early management
Getting early management right, he added, hinges upon ordering a baseline PET-CT scan to search for metastases before performing definitive surgical excision of the primary tumor.
“There are really important prognostic and therapeutic implications for a baseline scan in almost any patient with early Merkel – and that’s a very different situation than with melanoma,” the dermatologist said. “There’s at least a threefold higher likelihood that the cancer has spread asymptomatically at baseline with Merkel cell carcinoma than with melanoma.”
In a soon-to-be-published study by Dr. Nghiem and coworkers, baseline imaging resulted in prognostically important upstaging that led to an altered management strategy in 12% of 584 patients with MCC, or 1 in 8.
“You don’t want to overtreat locally a lesion that has already spread distantly; you want to start focusing on the distant disease. The local disease is secondary,” he said.
The surgical excision of the primary lesion should be thoughtfully wide without being aggressive or mutilating, and it should involve primary closure. “Definitely avoid flaps and grafts, which delay your further management with radiotherapy by months and months,” Dr. Nghiem advised.
Adjuvant radiotherapy of the primary tumor site is extremely effective at preventing recurrent MCC. In Dr. Nghiem’s view, almost everyone is a candidate: In a series of 803 patients in the Seattle MCC cohort, 92% received local adjuvant radiotherapy. The national rate, in contrast, is only about 50%, highlighting the need for additional physician education.
“A little bit of radiation – one dose – appears to be just as effective as 6 weeks in controlling microscopic disease. That’s probably something we’re going to be moving towards as a field,” he predicted.
Indeed, local adjuvant radiotherapy is so effective in MCC that the surgical margins make no difference. This was demonstrated in a study by Dr. Nghiem and his coinvestigators involving 70 patients with margins greater than 1 cm who received radiotherapy, 70 others with smaller or even positive margins who received radiotherapy, and 35 patients with margins of 1 cm or less who did not receive radiotherapy. There were no MCC recurrences in any of the radiotherapy recipients, regardless of their margin status. In contrast, 7 of the 35 patients who didn’t receive radiation therapy developed a cancer recurrence. Of note, the recurrence rate of MCC is historically about 40% – far greater than for any other skin cancer. Most recurrences happen within the first 2-3 years, Dr. Nghiem observed.
Immune therapy takes center stage
Another major transformation in MCC management has been the emergence of immune therapy as first-line systemic therapy. It has replaced chemotherapy, which is more toxic and has a much shorter average duration of response. Avelumab (Bavencio) and pembrolizumab (Keytruda), the two monoclonal antibodies directed against the protein programmed death–ligand 1 (PD-L1) receptor which are approved for MCC and have been incorporated into the National Comprehensive Cancer Network (NCCN) guidelines, provide a sixfold improvement in survival, compared with chemotherapy. For example, Dr. Nghiem was first author of a multicenter phase 2 study of pembrolizumab in which the 12- and 24-month overall survival rates in pembrolizumab responders were 85% and 79%, compared with just 12% and 6%, respectively, in historical controls on first-line chemotherapy (J Clin Oncol. 2019 Mar 20;37[9]:693-702).
“Merkel cell carcinoma is the most responsive solid tumor to immune therapy,” Dr. Nghiem commented.
Why MCC matters
Although rare, MCC is important because it’s five times more lethal than melanoma. Moreover, its incidence has been rising at a rate roughly twice that of the increase in melanoma since the turn of the century. There are now more than 3,000 new cases of MCC annually, about the same as for cutaneous T-cell lymphoma (CTCL).
“It’s just that you live a long time with CTCL and you don’t with Merkel cell carcinoma. You either get rid of Merkel fast or it gets rid of you,” the dermatologist observed.
It’s a fascinating malignancy, he continued. Eight of 10 cases are caused by Merkel cell polyomavirus, discovered in 2008. The virus is ubiquitously acquired in childhood and then lies dormant on the skin for the next 6 or 7 decades, at which point MCC rates shoot up dramatically, probably due to immunosenescence. Immunosuppressed patients are at 10-fold increased risk for MCC.
Given the rarity of MCC, it doesn’t make sense to actively hunt for it. But Dr. Nghiem and coworkers have developed a handy vowel-based mnemonic that serves to raise the index of suspicion: the “AEIOU” features.
- A = asymptomatic.
- E = expanding rapidly within past 3 months.
- I = immune-mediated.
- O = older than age 50.
- U = UV-exposed skin.
The investigators found in a series of 195 MCC patients that 89% of them possessed three or more of these features (J Am Acad Dermatol. 2008 Mar;58[3]:375-81). But while the AEIOU guide is quite sensitive, it’s not specific.
“If you have any three or more of these features, that lesion probably deserves a biopsy if it’s not readily explained. Even if it’s not a Merkel, it may turn out to be a different nonmelanoma skin cancer, something you want to know about,” Dr. Nghiem said.
A shift in surveillance strategy
Dr. Nghiem was senior author of a major study that validated the clinical utility of a Merkel polyomavirus serology test for monitoring the disease status of patients treated for MCC (Cancer. 2017 Apr 15;123[8]:1464-74). The test, which measures antibodies to Merkel cell polyomavirus oncoproteins, has been incorporated in NCCN guidelines. The blood test is used initially in newly diagnosed MCC to stratify patients into two subgroups: the half who are seropositive at baseline, and the other half who are seronegative. The seropositive group undergoes surveillance via repeat blood testing every 3 months. If antibody levels are low, there is a high degree of certainty that immune therapy is working and remission is present. Thus, the blood test spares patients in this group the expense and radiation exposure entailed in repeated surveillance scans. However, rising antibody levels indicate the cancer has already recurred or will do so within the next several months.
Unfortunately, the blood test cannot be used serially to track disease status in patients who are seronegative at baseline. That group is at 42% increased risk of MCC recurrence.
Immune therapy works in only about two-thirds of MCC patients with distant disease. Leaving the visible primary tumor in place to serve as a real-time window into immune treatment effectiveness is a useful contemporary surveillance strategy.
“By leaving the visible primary there, you will rapidly know if that patient is in the favorable two-thirds group or not,” he explained.
Historically, surgery and surveillance of MCC were based upon the melanoma model, and medical oncologists were trained to treat the malignancy as they would small cell lung cancer. These are now outmoded approaches, Dr. Nghiem said. That’s why a multidisciplinary approach is highly desirable for management of MCC, including dermatologists, pathologists, surgeons, radiation oncologists, medical oncologists, and imaging experts.
Dr. Nghiem and his colleagues have created a comprehensive source of information about Merkel cell carcinoma for physicians and patients at merkelcell.org.
He reported receiving research grants from Bristol-Myers Squibb and serving as a consultant to EMD Serono, Merck, Sanofi/Regeneron, and 4SC.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – The , Paul Nghiem, MD, PhD, declared at the SDEF Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
That’s because dermatologists are typically the physicians who make the diagnosis of Merkel cell carcinoma (MCC), so they’re on the scene from the outset and well positioned to help direct early management of this particularly aggressive malignancy, explained Dr. Nghiem, professor and head of dermatology at the University of Washington, Seattle.
“The management of Merkel is pretty high stakes, and if you get it right at the beginning it makes a huge difference in the side effects, as well as the chances that the patient will have the disease under control,” said Dr. Nghiem, who is sometimes called “the Merkel man” because of his many pioneering contributions to the field.
Better early management
Getting early management right, he added, hinges upon ordering a baseline PET-CT scan to search for metastases before performing definitive surgical excision of the primary tumor.
“There are really important prognostic and therapeutic implications for a baseline scan in almost any patient with early Merkel – and that’s a very different situation than with melanoma,” the dermatologist said. “There’s at least a threefold higher likelihood that the cancer has spread asymptomatically at baseline with Merkel cell carcinoma than with melanoma.”
In a soon-to-be-published study by Dr. Nghiem and coworkers, baseline imaging resulted in prognostically important upstaging that led to an altered management strategy in 12% of 584 patients with MCC, or 1 in 8.
“You don’t want to overtreat locally a lesion that has already spread distantly; you want to start focusing on the distant disease. The local disease is secondary,” he said.
The surgical excision of the primary lesion should be thoughtfully wide without being aggressive or mutilating, and it should involve primary closure. “Definitely avoid flaps and grafts, which delay your further management with radiotherapy by months and months,” Dr. Nghiem advised.
Adjuvant radiotherapy of the primary tumor site is extremely effective at preventing recurrent MCC. In Dr. Nghiem’s view, almost everyone is a candidate: In a series of 803 patients in the Seattle MCC cohort, 92% received local adjuvant radiotherapy. The national rate, in contrast, is only about 50%, highlighting the need for additional physician education.
“A little bit of radiation – one dose – appears to be just as effective as 6 weeks in controlling microscopic disease. That’s probably something we’re going to be moving towards as a field,” he predicted.
Indeed, local adjuvant radiotherapy is so effective in MCC that the surgical margins make no difference. This was demonstrated in a study by Dr. Nghiem and his coinvestigators involving 70 patients with margins greater than 1 cm who received radiotherapy, 70 others with smaller or even positive margins who received radiotherapy, and 35 patients with margins of 1 cm or less who did not receive radiotherapy. There were no MCC recurrences in any of the radiotherapy recipients, regardless of their margin status. In contrast, 7 of the 35 patients who didn’t receive radiation therapy developed a cancer recurrence. Of note, the recurrence rate of MCC is historically about 40% – far greater than for any other skin cancer. Most recurrences happen within the first 2-3 years, Dr. Nghiem observed.
Immune therapy takes center stage
Another major transformation in MCC management has been the emergence of immune therapy as first-line systemic therapy. It has replaced chemotherapy, which is more toxic and has a much shorter average duration of response. Avelumab (Bavencio) and pembrolizumab (Keytruda), the two monoclonal antibodies directed against the protein programmed death–ligand 1 (PD-L1) receptor which are approved for MCC and have been incorporated into the National Comprehensive Cancer Network (NCCN) guidelines, provide a sixfold improvement in survival, compared with chemotherapy. For example, Dr. Nghiem was first author of a multicenter phase 2 study of pembrolizumab in which the 12- and 24-month overall survival rates in pembrolizumab responders were 85% and 79%, compared with just 12% and 6%, respectively, in historical controls on first-line chemotherapy (J Clin Oncol. 2019 Mar 20;37[9]:693-702).
“Merkel cell carcinoma is the most responsive solid tumor to immune therapy,” Dr. Nghiem commented.
Why MCC matters
Although rare, MCC is important because it’s five times more lethal than melanoma. Moreover, its incidence has been rising at a rate roughly twice that of the increase in melanoma since the turn of the century. There are now more than 3,000 new cases of MCC annually, about the same as for cutaneous T-cell lymphoma (CTCL).
“It’s just that you live a long time with CTCL and you don’t with Merkel cell carcinoma. You either get rid of Merkel fast or it gets rid of you,” the dermatologist observed.
It’s a fascinating malignancy, he continued. Eight of 10 cases are caused by Merkel cell polyomavirus, discovered in 2008. The virus is ubiquitously acquired in childhood and then lies dormant on the skin for the next 6 or 7 decades, at which point MCC rates shoot up dramatically, probably due to immunosenescence. Immunosuppressed patients are at 10-fold increased risk for MCC.
Given the rarity of MCC, it doesn’t make sense to actively hunt for it. But Dr. Nghiem and coworkers have developed a handy vowel-based mnemonic that serves to raise the index of suspicion: the “AEIOU” features.
- A = asymptomatic.
- E = expanding rapidly within past 3 months.
- I = immune-mediated.
- O = older than age 50.
- U = UV-exposed skin.
The investigators found in a series of 195 MCC patients that 89% of them possessed three or more of these features (J Am Acad Dermatol. 2008 Mar;58[3]:375-81). But while the AEIOU guide is quite sensitive, it’s not specific.
“If you have any three or more of these features, that lesion probably deserves a biopsy if it’s not readily explained. Even if it’s not a Merkel, it may turn out to be a different nonmelanoma skin cancer, something you want to know about,” Dr. Nghiem said.
A shift in surveillance strategy
Dr. Nghiem was senior author of a major study that validated the clinical utility of a Merkel polyomavirus serology test for monitoring the disease status of patients treated for MCC (Cancer. 2017 Apr 15;123[8]:1464-74). The test, which measures antibodies to Merkel cell polyomavirus oncoproteins, has been incorporated in NCCN guidelines. The blood test is used initially in newly diagnosed MCC to stratify patients into two subgroups: the half who are seropositive at baseline, and the other half who are seronegative. The seropositive group undergoes surveillance via repeat blood testing every 3 months. If antibody levels are low, there is a high degree of certainty that immune therapy is working and remission is present. Thus, the blood test spares patients in this group the expense and radiation exposure entailed in repeated surveillance scans. However, rising antibody levels indicate the cancer has already recurred or will do so within the next several months.
Unfortunately, the blood test cannot be used serially to track disease status in patients who are seronegative at baseline. That group is at 42% increased risk of MCC recurrence.
Immune therapy works in only about two-thirds of MCC patients with distant disease. Leaving the visible primary tumor in place to serve as a real-time window into immune treatment effectiveness is a useful contemporary surveillance strategy.
“By leaving the visible primary there, you will rapidly know if that patient is in the favorable two-thirds group or not,” he explained.
Historically, surgery and surveillance of MCC were based upon the melanoma model, and medical oncologists were trained to treat the malignancy as they would small cell lung cancer. These are now outmoded approaches, Dr. Nghiem said. That’s why a multidisciplinary approach is highly desirable for management of MCC, including dermatologists, pathologists, surgeons, radiation oncologists, medical oncologists, and imaging experts.
Dr. Nghiem and his colleagues have created a comprehensive source of information about Merkel cell carcinoma for physicians and patients at merkelcell.org.
He reported receiving research grants from Bristol-Myers Squibb and serving as a consultant to EMD Serono, Merck, Sanofi/Regeneron, and 4SC.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Rare Neurological Disease Special Report
Our sixth annual Rare Neurological Disease Special Report is our biggest issue yet. It is very gratifying to know we are part of the rare disease community and witness to some of the exciting developments that are transforming this field. There are many newly approved therapies highlighted in the articles in this issue, as well as brief profiles of a number of research groups within the NIH’s Rare Diseases Clinical Research Network and an intriguing profile of how the Netflix show Diagnosis used crowdsourcing to solve medical mysteries, many of which involved rare neurologic conditions. That’s just a sampling of what this issue has to offer. There are too many articles to mention each one, but I hope you take the time to read the entire issue.
—Glenn S. Williams, vice president, group editor, Neurology Reviews
Our sixth annual Rare Neurological Disease Special Report is our biggest issue yet. It is very gratifying to know we are part of the rare disease community and witness to some of the exciting developments that are transforming this field. There are many newly approved therapies highlighted in the articles in this issue, as well as brief profiles of a number of research groups within the NIH’s Rare Diseases Clinical Research Network and an intriguing profile of how the Netflix show Diagnosis used crowdsourcing to solve medical mysteries, many of which involved rare neurologic conditions. That’s just a sampling of what this issue has to offer. There are too many articles to mention each one, but I hope you take the time to read the entire issue.
—Glenn S. Williams, vice president, group editor, Neurology Reviews
Our sixth annual Rare Neurological Disease Special Report is our biggest issue yet. It is very gratifying to know we are part of the rare disease community and witness to some of the exciting developments that are transforming this field. There are many newly approved therapies highlighted in the articles in this issue, as well as brief profiles of a number of research groups within the NIH’s Rare Diseases Clinical Research Network and an intriguing profile of how the Netflix show Diagnosis used crowdsourcing to solve medical mysteries, many of which involved rare neurologic conditions. That’s just a sampling of what this issue has to offer. There are too many articles to mention each one, but I hope you take the time to read the entire issue.
—Glenn S. Williams, vice president, group editor, Neurology Reviews
Breaking bacterial communication may heal EB wounds
LONDON – Disrupting how microorganisms communicate with each other could be a way to overcome antibiotic resistance and to help heal chronic wounds in patients with epidermolysis bullosa (EB), according to presenters at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
The majority of chronic wounds in patients with EB are colonized with microorganisms, with a predominance of Staphylococcus species, said Erik Gerner, an industrial PhD student at Mölnlycke Health Care in Gothenburg, Sweden, and Gothenburg University.
Because of the growing problem of antibiotic resistance, alternative treatments are needed, and one possible alternative for treating infected wounds could be interfering with quorum sensing, the cell-to-cell communication used by bacteria, he said. He is hoping to explore this possibility as a novel treatment strategy for infected wounds.
“Quorum sensing is defined as the ability to detect and respond to population density,” Mr. Gerner said, noting that, when there is a sufficient density of bacteria, “they start to communicate with each other.” This enables them to act as a community and perform actions that they could not do as individual cells. Such actions include forming biofilms, which helps protect bacteria from their environment, such as the immune system. Other actions include collectively switching on the production of virulence factors and becoming resistant to treatments.
“Bacteria use quorum sensing to act collectively,” Mr. Gerner said. “If we could shut down this quorum sensing system, it would be very beneficial … and increase the chances to heal the wound.”
The quorum sensing system is based on the production of signaling molecules called AHL (N-acyl homoserine lactones), which are constantly produced at a low rate. This isn’t a problem until the level of bacteria increases and the level of quorum sensing breaches a threshold, he explained.
There are several benefits of inhibiting bacterial communication through disrupting quorum sensing, namely, “a low risk of resistance,” Mr. Gerner said. There is also potentially less toxin production by bacteria, and this could help the immune system in killing the invading bacteria.
One approach to disrupting quorum testing that Mr. Gerner has been investigating is the use of sodium salicylate (NaSa). So far, preclinical work shows that NaSa can reduce toxin production but not the growth rate of bacteria. The advantage of using NaSa is that it is nontoxic to human dermal fibroblasts, with similar results seen in human keratinocytes and immune cells. His work to date has shown that NaSa reduced activity of NF-kB (a proinflammatory signaling pathway) in differentiated and lipopolysaccharide-stimulated monocytes; NF-kB activated production of proinflammatory cytokines (such as interleukin-1 beta and IL-6) are elevated in EB wounds. “My studies support the bodies of evidence that bacteria use quorum sensing to coordinate … and to produce a large number of toxic factors,” Mr. Gerner concluded. Future studies will look at the potential of NaSa to disrupt this activity.
Skin microbiome of EB wounds
Liat Samuelov, MD, of the department of molecular dermatology at Tel Aviv (Israel) Sourasky Medical Center, presented data on skin microbiome characteristics in eight patients with recessive dystrophic EB (RDEB). This showed that there was reduced bacterial diversity in wounds, and a “progressive development of dysbiosis across different stages of DEB wound formation.”
The skin microbiome has been implicated in several skin diseases, Dr. Samuelov and associates observed in a poster presentation. That includes the autoimmune blistering disease bullous pemphigoid (Exp Dermatol. 2017 Dec;26[12]:1221-7). “Colonization of DEB chronic wounds may lead to systemic infections, result in delayed healing, and possibly be involved in the development of squamous cell carcinoma,” they noted in the poster, “thus accurate delineation of the dysbiotic profile … may point to corrective measures of great therapeutic potential.”
The aim was to see what microorganisms were present in the chronic wounds of the patients. To be included in the study, patients must not have had any antibiotic treatment – oral or topical – in the past 6 months. Samples were taken from an untreated wound, around the wound, and from uninvolved skin, which were compared with samples taken from similar areas in age-matched controls.
Reduced bacterial diversity was observed in RDEB wounds, compared with uninvolved or perilesional areas and the skin of control subjects, Dr. Samuelov said in an oral presentation of the study results. There was increased abundance of Staphylococcus epidermidis and decreased Cutibacterium acnes, which she noted was in contrast to other studies where S. aureus was the most common colonizer in RDEB wounds.
Bacterial composition in each group was calculated using the beta-diversity score, while control samples showed similar microbial composition, the DEB samples had no microbial similarities among different samples. These data “suggest the need to ascertain the potential therapeutic benefit of interventions aimed at restoring normal microbiome composition in DEB,” Dr. Samuelov concluded.
Wound colonization and squamous cell carcinoma
Other research on wound microbiology was presented by Laura E. Levin, MD, a dermatologist at New York–Presbyterian, and associates. “Given the potential role of bacteria-induced inflammation in the development of wound-associated SCC [squamous cell carcinoma] in a subset of patients, we sought to improve our understanding of what microbes colonize and infect the wounds of patients with epidermolysis bullosa,” they explained in their poster.
The researchers, from New York–Presbyterian Morgan Stanley Children’s Hospital and Columbia University Irvine Medical Center, New York, presented data from a retrospective analysis of 739 wound cultures taken between 2001 and 2017 from 158 patients enrolled in the Epidermolysis Bullosa Clinical Characterization and Outcomes Database. In the analysis, just under 70% of patients had DEB, of which 90% were of the RDEB subtype; 13% had EB simplex, 14% had junctional EB, and 3% had an unknown EB subtype.
At least one organism grew in 87% of cultures, with the most common microorganism isolated being Staphylococcus aureus (84% of cultures). Other commonly isolated microbes were Pseudomonas aeruginosa in 35% of cultures, Streptococcus group A in 34% of cultures (of which 22% were Streptococcus pyogenes), Corynebacterium species in 31% of cultures, and Proteus species in 18% of cultures.
“Improved understanding of what microbes are colonizing the wounds of our patients may help improve antibiotic stewardship,” the researchers stated.
Looking at the antibiotic susceptibilities, Dr. Levin and associates found that 68% of 115 cultures were sensitive to methicillin and 60% of 15 cultures were sensitive to mupirocin. “Resistance to many systemic and topical antibiotic agents in EB patients supports surveillance cultures with routine testing for mupirocin susceptibility,” they suggested.
A total of 23 patients developed SCC of whom 10 had cultures that grew S. aureus (90%) and P. aeruginosa (50%), and Proteus species (20%). Among the patients who did not develop SCC, the respective cultures positive for each of those microorganisms were 83%, 34%, and 11%. Perhaps “gram-negative and flagellated organisms may be more common in wounds of patients at risk for SCC,” they observed, adding that further studies were needed to determine if “wound microbiome interventions inhibit the risk of development of SCC and improve outcomes.”
Mr. Gerner’s research is supported by Mölnlycke Health Care. Dr. Samuelov had no disclosures. The work by Dr. Levin and associates is supported by the Pediatric Dermatology Research Alliance, EB Research Partnership, and the Epidermolysis Bullosa Medical Research Foundation.
LONDON – Disrupting how microorganisms communicate with each other could be a way to overcome antibiotic resistance and to help heal chronic wounds in patients with epidermolysis bullosa (EB), according to presenters at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
The majority of chronic wounds in patients with EB are colonized with microorganisms, with a predominance of Staphylococcus species, said Erik Gerner, an industrial PhD student at Mölnlycke Health Care in Gothenburg, Sweden, and Gothenburg University.
Because of the growing problem of antibiotic resistance, alternative treatments are needed, and one possible alternative for treating infected wounds could be interfering with quorum sensing, the cell-to-cell communication used by bacteria, he said. He is hoping to explore this possibility as a novel treatment strategy for infected wounds.
“Quorum sensing is defined as the ability to detect and respond to population density,” Mr. Gerner said, noting that, when there is a sufficient density of bacteria, “they start to communicate with each other.” This enables them to act as a community and perform actions that they could not do as individual cells. Such actions include forming biofilms, which helps protect bacteria from their environment, such as the immune system. Other actions include collectively switching on the production of virulence factors and becoming resistant to treatments.
“Bacteria use quorum sensing to act collectively,” Mr. Gerner said. “If we could shut down this quorum sensing system, it would be very beneficial … and increase the chances to heal the wound.”
The quorum sensing system is based on the production of signaling molecules called AHL (N-acyl homoserine lactones), which are constantly produced at a low rate. This isn’t a problem until the level of bacteria increases and the level of quorum sensing breaches a threshold, he explained.
There are several benefits of inhibiting bacterial communication through disrupting quorum sensing, namely, “a low risk of resistance,” Mr. Gerner said. There is also potentially less toxin production by bacteria, and this could help the immune system in killing the invading bacteria.
One approach to disrupting quorum testing that Mr. Gerner has been investigating is the use of sodium salicylate (NaSa). So far, preclinical work shows that NaSa can reduce toxin production but not the growth rate of bacteria. The advantage of using NaSa is that it is nontoxic to human dermal fibroblasts, with similar results seen in human keratinocytes and immune cells. His work to date has shown that NaSa reduced activity of NF-kB (a proinflammatory signaling pathway) in differentiated and lipopolysaccharide-stimulated monocytes; NF-kB activated production of proinflammatory cytokines (such as interleukin-1 beta and IL-6) are elevated in EB wounds. “My studies support the bodies of evidence that bacteria use quorum sensing to coordinate … and to produce a large number of toxic factors,” Mr. Gerner concluded. Future studies will look at the potential of NaSa to disrupt this activity.
Skin microbiome of EB wounds
Liat Samuelov, MD, of the department of molecular dermatology at Tel Aviv (Israel) Sourasky Medical Center, presented data on skin microbiome characteristics in eight patients with recessive dystrophic EB (RDEB). This showed that there was reduced bacterial diversity in wounds, and a “progressive development of dysbiosis across different stages of DEB wound formation.”
The skin microbiome has been implicated in several skin diseases, Dr. Samuelov and associates observed in a poster presentation. That includes the autoimmune blistering disease bullous pemphigoid (Exp Dermatol. 2017 Dec;26[12]:1221-7). “Colonization of DEB chronic wounds may lead to systemic infections, result in delayed healing, and possibly be involved in the development of squamous cell carcinoma,” they noted in the poster, “thus accurate delineation of the dysbiotic profile … may point to corrective measures of great therapeutic potential.”
The aim was to see what microorganisms were present in the chronic wounds of the patients. To be included in the study, patients must not have had any antibiotic treatment – oral or topical – in the past 6 months. Samples were taken from an untreated wound, around the wound, and from uninvolved skin, which were compared with samples taken from similar areas in age-matched controls.
Reduced bacterial diversity was observed in RDEB wounds, compared with uninvolved or perilesional areas and the skin of control subjects, Dr. Samuelov said in an oral presentation of the study results. There was increased abundance of Staphylococcus epidermidis and decreased Cutibacterium acnes, which she noted was in contrast to other studies where S. aureus was the most common colonizer in RDEB wounds.
Bacterial composition in each group was calculated using the beta-diversity score, while control samples showed similar microbial composition, the DEB samples had no microbial similarities among different samples. These data “suggest the need to ascertain the potential therapeutic benefit of interventions aimed at restoring normal microbiome composition in DEB,” Dr. Samuelov concluded.
Wound colonization and squamous cell carcinoma
Other research on wound microbiology was presented by Laura E. Levin, MD, a dermatologist at New York–Presbyterian, and associates. “Given the potential role of bacteria-induced inflammation in the development of wound-associated SCC [squamous cell carcinoma] in a subset of patients, we sought to improve our understanding of what microbes colonize and infect the wounds of patients with epidermolysis bullosa,” they explained in their poster.
The researchers, from New York–Presbyterian Morgan Stanley Children’s Hospital and Columbia University Irvine Medical Center, New York, presented data from a retrospective analysis of 739 wound cultures taken between 2001 and 2017 from 158 patients enrolled in the Epidermolysis Bullosa Clinical Characterization and Outcomes Database. In the analysis, just under 70% of patients had DEB, of which 90% were of the RDEB subtype; 13% had EB simplex, 14% had junctional EB, and 3% had an unknown EB subtype.
At least one organism grew in 87% of cultures, with the most common microorganism isolated being Staphylococcus aureus (84% of cultures). Other commonly isolated microbes were Pseudomonas aeruginosa in 35% of cultures, Streptococcus group A in 34% of cultures (of which 22% were Streptococcus pyogenes), Corynebacterium species in 31% of cultures, and Proteus species in 18% of cultures.
“Improved understanding of what microbes are colonizing the wounds of our patients may help improve antibiotic stewardship,” the researchers stated.
Looking at the antibiotic susceptibilities, Dr. Levin and associates found that 68% of 115 cultures were sensitive to methicillin and 60% of 15 cultures were sensitive to mupirocin. “Resistance to many systemic and topical antibiotic agents in EB patients supports surveillance cultures with routine testing for mupirocin susceptibility,” they suggested.
A total of 23 patients developed SCC of whom 10 had cultures that grew S. aureus (90%) and P. aeruginosa (50%), and Proteus species (20%). Among the patients who did not develop SCC, the respective cultures positive for each of those microorganisms were 83%, 34%, and 11%. Perhaps “gram-negative and flagellated organisms may be more common in wounds of patients at risk for SCC,” they observed, adding that further studies were needed to determine if “wound microbiome interventions inhibit the risk of development of SCC and improve outcomes.”
Mr. Gerner’s research is supported by Mölnlycke Health Care. Dr. Samuelov had no disclosures. The work by Dr. Levin and associates is supported by the Pediatric Dermatology Research Alliance, EB Research Partnership, and the Epidermolysis Bullosa Medical Research Foundation.
LONDON – Disrupting how microorganisms communicate with each other could be a way to overcome antibiotic resistance and to help heal chronic wounds in patients with epidermolysis bullosa (EB), according to presenters at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
The majority of chronic wounds in patients with EB are colonized with microorganisms, with a predominance of Staphylococcus species, said Erik Gerner, an industrial PhD student at Mölnlycke Health Care in Gothenburg, Sweden, and Gothenburg University.
Because of the growing problem of antibiotic resistance, alternative treatments are needed, and one possible alternative for treating infected wounds could be interfering with quorum sensing, the cell-to-cell communication used by bacteria, he said. He is hoping to explore this possibility as a novel treatment strategy for infected wounds.
“Quorum sensing is defined as the ability to detect and respond to population density,” Mr. Gerner said, noting that, when there is a sufficient density of bacteria, “they start to communicate with each other.” This enables them to act as a community and perform actions that they could not do as individual cells. Such actions include forming biofilms, which helps protect bacteria from their environment, such as the immune system. Other actions include collectively switching on the production of virulence factors and becoming resistant to treatments.
“Bacteria use quorum sensing to act collectively,” Mr. Gerner said. “If we could shut down this quorum sensing system, it would be very beneficial … and increase the chances to heal the wound.”
The quorum sensing system is based on the production of signaling molecules called AHL (N-acyl homoserine lactones), which are constantly produced at a low rate. This isn’t a problem until the level of bacteria increases and the level of quorum sensing breaches a threshold, he explained.
There are several benefits of inhibiting bacterial communication through disrupting quorum sensing, namely, “a low risk of resistance,” Mr. Gerner said. There is also potentially less toxin production by bacteria, and this could help the immune system in killing the invading bacteria.
One approach to disrupting quorum testing that Mr. Gerner has been investigating is the use of sodium salicylate (NaSa). So far, preclinical work shows that NaSa can reduce toxin production but not the growth rate of bacteria. The advantage of using NaSa is that it is nontoxic to human dermal fibroblasts, with similar results seen in human keratinocytes and immune cells. His work to date has shown that NaSa reduced activity of NF-kB (a proinflammatory signaling pathway) in differentiated and lipopolysaccharide-stimulated monocytes; NF-kB activated production of proinflammatory cytokines (such as interleukin-1 beta and IL-6) are elevated in EB wounds. “My studies support the bodies of evidence that bacteria use quorum sensing to coordinate … and to produce a large number of toxic factors,” Mr. Gerner concluded. Future studies will look at the potential of NaSa to disrupt this activity.
Skin microbiome of EB wounds
Liat Samuelov, MD, of the department of molecular dermatology at Tel Aviv (Israel) Sourasky Medical Center, presented data on skin microbiome characteristics in eight patients with recessive dystrophic EB (RDEB). This showed that there was reduced bacterial diversity in wounds, and a “progressive development of dysbiosis across different stages of DEB wound formation.”
The skin microbiome has been implicated in several skin diseases, Dr. Samuelov and associates observed in a poster presentation. That includes the autoimmune blistering disease bullous pemphigoid (Exp Dermatol. 2017 Dec;26[12]:1221-7). “Colonization of DEB chronic wounds may lead to systemic infections, result in delayed healing, and possibly be involved in the development of squamous cell carcinoma,” they noted in the poster, “thus accurate delineation of the dysbiotic profile … may point to corrective measures of great therapeutic potential.”
The aim was to see what microorganisms were present in the chronic wounds of the patients. To be included in the study, patients must not have had any antibiotic treatment – oral or topical – in the past 6 months. Samples were taken from an untreated wound, around the wound, and from uninvolved skin, which were compared with samples taken from similar areas in age-matched controls.
Reduced bacterial diversity was observed in RDEB wounds, compared with uninvolved or perilesional areas and the skin of control subjects, Dr. Samuelov said in an oral presentation of the study results. There was increased abundance of Staphylococcus epidermidis and decreased Cutibacterium acnes, which she noted was in contrast to other studies where S. aureus was the most common colonizer in RDEB wounds.
Bacterial composition in each group was calculated using the beta-diversity score, while control samples showed similar microbial composition, the DEB samples had no microbial similarities among different samples. These data “suggest the need to ascertain the potential therapeutic benefit of interventions aimed at restoring normal microbiome composition in DEB,” Dr. Samuelov concluded.
Wound colonization and squamous cell carcinoma
Other research on wound microbiology was presented by Laura E. Levin, MD, a dermatologist at New York–Presbyterian, and associates. “Given the potential role of bacteria-induced inflammation in the development of wound-associated SCC [squamous cell carcinoma] in a subset of patients, we sought to improve our understanding of what microbes colonize and infect the wounds of patients with epidermolysis bullosa,” they explained in their poster.
The researchers, from New York–Presbyterian Morgan Stanley Children’s Hospital and Columbia University Irvine Medical Center, New York, presented data from a retrospective analysis of 739 wound cultures taken between 2001 and 2017 from 158 patients enrolled in the Epidermolysis Bullosa Clinical Characterization and Outcomes Database. In the analysis, just under 70% of patients had DEB, of which 90% were of the RDEB subtype; 13% had EB simplex, 14% had junctional EB, and 3% had an unknown EB subtype.
At least one organism grew in 87% of cultures, with the most common microorganism isolated being Staphylococcus aureus (84% of cultures). Other commonly isolated microbes were Pseudomonas aeruginosa in 35% of cultures, Streptococcus group A in 34% of cultures (of which 22% were Streptococcus pyogenes), Corynebacterium species in 31% of cultures, and Proteus species in 18% of cultures.
“Improved understanding of what microbes are colonizing the wounds of our patients may help improve antibiotic stewardship,” the researchers stated.
Looking at the antibiotic susceptibilities, Dr. Levin and associates found that 68% of 115 cultures were sensitive to methicillin and 60% of 15 cultures were sensitive to mupirocin. “Resistance to many systemic and topical antibiotic agents in EB patients supports surveillance cultures with routine testing for mupirocin susceptibility,” they suggested.
A total of 23 patients developed SCC of whom 10 had cultures that grew S. aureus (90%) and P. aeruginosa (50%), and Proteus species (20%). Among the patients who did not develop SCC, the respective cultures positive for each of those microorganisms were 83%, 34%, and 11%. Perhaps “gram-negative and flagellated organisms may be more common in wounds of patients at risk for SCC,” they observed, adding that further studies were needed to determine if “wound microbiome interventions inhibit the risk of development of SCC and improve outcomes.”
Mr. Gerner’s research is supported by Mölnlycke Health Care. Dr. Samuelov had no disclosures. The work by Dr. Levin and associates is supported by the Pediatric Dermatology Research Alliance, EB Research Partnership, and the Epidermolysis Bullosa Medical Research Foundation.
REPORTING FROM EB 2020
Osilodrostat gets FDA go-ahead for Cushing’s disease in adults
who either are not good candidates for pituitary gland surgery – the recommended first-line therapy – or in whom the disease persists after surgery.
Cushing’s disease is a rare condition caused when a pituitary tumor releases too much of the hormone adrenocorticotropin, which in turn, triggers the adrenal gland to overproduce cortisol. The condition is associated with serious health complications, including high blood pressure, obesity, type 2 diabetes, and compromised immunity.
Osilodrostat is the first therapy approved by the FDA to tackle the overproduction of cortisol, which it does by blocking the 11-beta-hydroxylase enzyme and thus preventing cortisol synthesis, the agency said in a press release.
In November 2019, the European Medicines Agency recommended the granting of a marketing authorization for osilodrostat, also for treating adults with Cushing’s disease.
The U.S. approval was based on outcomes from a study that evaluated the drug’s safety and efficacy in 137 adults with Cushing’s disease who had undergone pituitary surgery but were not cured, or who were not surgical candidates, according the release. About three-quarters of the patients were women, and the mean age was 41 years.
All of the patients started a 24-week, single-arm, open-label period at a dose of 2 mg of osilodrostat twice daily that could be increased every 2 weeks to 30 mg twice daily.
By week 24, cortisol levels in roughly half the patients were within the normal range, and 71 patients who did not need any more dose increases and who tolerated the drug were randomized to either osilodrostat or placebo for an 8-week withdrawal study. At the end of that time, 86% of the osilodrostat patients maintained their normal-range cortisol levels, compared with 30% of those taking placebo.
Osilodrostat is taken as an oral tablet twice a day, in the morning and evening. Among the common side effects reported in the study were adrenal insufficiency, headache, vomiting, nausea, fatigue, and edema, although hypocortisolism, QTc prolongation, and elevations in adrenal hormone precursors, and androgens may also occur, according to the release.
The drug had been given an Orphan Drug Designation in recognition of its intended use in the treatment of a rare disease. The approval was granted to Novartis.
who either are not good candidates for pituitary gland surgery – the recommended first-line therapy – or in whom the disease persists after surgery.
Cushing’s disease is a rare condition caused when a pituitary tumor releases too much of the hormone adrenocorticotropin, which in turn, triggers the adrenal gland to overproduce cortisol. The condition is associated with serious health complications, including high blood pressure, obesity, type 2 diabetes, and compromised immunity.
Osilodrostat is the first therapy approved by the FDA to tackle the overproduction of cortisol, which it does by blocking the 11-beta-hydroxylase enzyme and thus preventing cortisol synthesis, the agency said in a press release.
In November 2019, the European Medicines Agency recommended the granting of a marketing authorization for osilodrostat, also for treating adults with Cushing’s disease.
The U.S. approval was based on outcomes from a study that evaluated the drug’s safety and efficacy in 137 adults with Cushing’s disease who had undergone pituitary surgery but were not cured, or who were not surgical candidates, according the release. About three-quarters of the patients were women, and the mean age was 41 years.
All of the patients started a 24-week, single-arm, open-label period at a dose of 2 mg of osilodrostat twice daily that could be increased every 2 weeks to 30 mg twice daily.
By week 24, cortisol levels in roughly half the patients were within the normal range, and 71 patients who did not need any more dose increases and who tolerated the drug were randomized to either osilodrostat or placebo for an 8-week withdrawal study. At the end of that time, 86% of the osilodrostat patients maintained their normal-range cortisol levels, compared with 30% of those taking placebo.
Osilodrostat is taken as an oral tablet twice a day, in the morning and evening. Among the common side effects reported in the study were adrenal insufficiency, headache, vomiting, nausea, fatigue, and edema, although hypocortisolism, QTc prolongation, and elevations in adrenal hormone precursors, and androgens may also occur, according to the release.
The drug had been given an Orphan Drug Designation in recognition of its intended use in the treatment of a rare disease. The approval was granted to Novartis.
who either are not good candidates for pituitary gland surgery – the recommended first-line therapy – or in whom the disease persists after surgery.
Cushing’s disease is a rare condition caused when a pituitary tumor releases too much of the hormone adrenocorticotropin, which in turn, triggers the adrenal gland to overproduce cortisol. The condition is associated with serious health complications, including high blood pressure, obesity, type 2 diabetes, and compromised immunity.
Osilodrostat is the first therapy approved by the FDA to tackle the overproduction of cortisol, which it does by blocking the 11-beta-hydroxylase enzyme and thus preventing cortisol synthesis, the agency said in a press release.
In November 2019, the European Medicines Agency recommended the granting of a marketing authorization for osilodrostat, also for treating adults with Cushing’s disease.
The U.S. approval was based on outcomes from a study that evaluated the drug’s safety and efficacy in 137 adults with Cushing’s disease who had undergone pituitary surgery but were not cured, or who were not surgical candidates, according the release. About three-quarters of the patients were women, and the mean age was 41 years.
All of the patients started a 24-week, single-arm, open-label period at a dose of 2 mg of osilodrostat twice daily that could be increased every 2 weeks to 30 mg twice daily.
By week 24, cortisol levels in roughly half the patients were within the normal range, and 71 patients who did not need any more dose increases and who tolerated the drug were randomized to either osilodrostat or placebo for an 8-week withdrawal study. At the end of that time, 86% of the osilodrostat patients maintained their normal-range cortisol levels, compared with 30% of those taking placebo.
Osilodrostat is taken as an oral tablet twice a day, in the morning and evening. Among the common side effects reported in the study were adrenal insufficiency, headache, vomiting, nausea, fatigue, and edema, although hypocortisolism, QTc prolongation, and elevations in adrenal hormone precursors, and androgens may also occur, according to the release.
The drug had been given an Orphan Drug Designation in recognition of its intended use in the treatment of a rare disease. The approval was granted to Novartis.














