User login
Serum urate level governs management of difficult-to-treat gout
LAS VEGAS – Management of difficult-to-treat gout calls for a familiar therapeutic goal: lowering the serum urate level to less than 6 mg/dL. Underused treatment approaches, such as escalating the dose of allopurinol or adding probenecid, can help almost all patients reach this target, said Brian F. Mandell, MD, PhD, professor of rheumatic and immunologic disease at the Cleveland Clinic.
“The major reason for treatment resistance has nothing to do with the drugs not working,” Dr. Mandell said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “And it does not even have to do ... with patient compliance. It is actually due to us and lack of appropriate monitoring and dosing of the medicines. We do not push the dose up.”
The urate saturation point in physiologic fluids with protein is about 6.8 mg/dL. Physicians and investigators have used 6 mg/dL as a target serum urate level in patients with gout for decades. “The bottom line is lowering the serum urate for 12 months reduces gout flares. There is absolutely no reason to question the physicochemical effect of lowering serum urate and dissolving the deposits and ultimately reducing attacks,” Dr. Mandell said. Urate lowering therapy takes time to reduce flare frequency and tophi, however. “It does not happen in 6 months in everyone,” he said.
Addressing intolerance and undertreatment
Clinicians may encounter various challenges when managing patients with gout. In cases of resistant gout, the target serum urate level may not be reached easily. At first, gout attacks and tophi may persist after levels decrease to less than 6 mg/dL. Complicated gout may occur when comorbidities limit treatment options or when tophi cause dramatic mechanical dysfunction.
“There is one way to manage all of these [scenarios], and that is to lower the serum urate,” Dr. Mandell said. “That is the management approach for chronic gout.”
Because this approach does not produce quick results, patients with limited life expectancy may not be appropriate candidates, although they still may benefit from prophylaxis against gout attacks, treatment of attacks, and surgery, he said.
Intolerance to a xanthine oxidase inhibitor is one potential treatment obstacle. If allopurinol causes gastrointestinal adverse effects or hypersensitivity reactions, switching to febuxostat (Uloric) may overcome this problem. Desensitizing patients with a mild allergy to allopurinol is another possible tactic. In addition, treating patients with a uricosuric such as probenecid as monotherapy or in combination with a xanthine oxidase inhibitor may help, Dr. Mandell said.
Increasing the dose of the xanthine oxidase inhibitor beyond the maximal dose listed by the Food and Drug Administration – 800 mg for allopurinol or 80 mg for febuxostat – is an option, Dr. Mandell said. In Europe, the maximal dose for allopurinol is 900 mg, and physicians have clinical experience pushing the dose of allopurinol to greater than 1,000 mg in rare instances, he noted. “There is not a dose-limiting toxicity to allopurinol,” he said. There is a bioavailability issue, however, and splitting the dose at doses greater than 300 mg probably is warranted, he added.
If these approaches fail to lower the serum urate level to below 6 mg/dL, rigid dietary changes may be a next step. Adjusting other medications also may be an option. For example, physicians might weigh using losartan as a blood pressure medicine instead of a thiazide.
Finally, physicians can debulk urate deposits with pegloticase. “Dramatically lower the body load of serum urate, and then come back and use your traditional drugs,” he said. After treatment with enzyme replacement therapy, patients almost invariably require lower doses of allopurinol or febuxostat, he said.
Also, in severe cases when the time necessary for traditional urate-lowering therapy to work may not make it the most appropriate route, aggressive therapy with pegloticase may be warranted, Dr. Mandell said.
The FAST trial
The ongoing Febuxostat versus Allopurinol Streamlined Trial (FAST) has provided data about undertreatment with allopurinol and the effects of increasing the dose. The prospective, randomized, open-label study is comparing the cardiovascular safety of allopurinol and febuxostat in patients with symptomatic hyperuricemia. It enrolled patients who were on allopurinol in normal clinical practice. To enter, patients had to have a serum urate level below 6 mg/dL. If patients’ levels were not below 6 mg/dL, investigators increased the dose of allopurinol to try to reach that target (Semin Arthritis Rheum. 2014 Aug;44[1]:25-30).
“Basically, this part of the study is a dose-escalation trial for efficacy,” Dr. Mandell said. “Of 400 patients taking allopurinol, 36% still had a urate above 6 [mg/dL]. ... If you uptitrated the dose, 97% of people were able to get to 6. Uptitration works. You just actually need to do it.” The results indicate that a 100-mg increase in allopurinol dose decreases serum urate by about 1 mg/dL.
Allopurinol hypersensitivity and chronic kidney disease
Patients with chronic kidney disease may have increased risk of allopurinol hypersensitivity. For a while, researchers postulated that oxypurinol, the active component of allopurinol, built up and caused toxicity in some patients with chronic kidney disease. As a result, researchers suggested adjusting the dose for patients with chronic kidney disease.
One problem with this approach is that only about 20% of patients with chronic kidney disease would reach the treatment target with the suggested doses, Dr. Mandell said. “You are exposing them to some potential risk with a very low chance of actually getting any efficacy at all,” he said.
Furthermore, allopurinol hypersensitivity behaves like an allergic reaction, not a toxicity reaction. Small studies suggest that starting allopurinol at a low dose and slowly increasing the dose may be safe in patients with chronic kidney disease. Allopurinol is not nephrotoxic, and some data indicate that it may be nephroprotective, he said.
Dr. Mandell reported that in recent years he was a clinical investigator and consultant for Horizon and a consultant for Takeda and Ardea/AstraZeneca/Ironwood.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Management of difficult-to-treat gout calls for a familiar therapeutic goal: lowering the serum urate level to less than 6 mg/dL. Underused treatment approaches, such as escalating the dose of allopurinol or adding probenecid, can help almost all patients reach this target, said Brian F. Mandell, MD, PhD, professor of rheumatic and immunologic disease at the Cleveland Clinic.
“The major reason for treatment resistance has nothing to do with the drugs not working,” Dr. Mandell said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “And it does not even have to do ... with patient compliance. It is actually due to us and lack of appropriate monitoring and dosing of the medicines. We do not push the dose up.”
The urate saturation point in physiologic fluids with protein is about 6.8 mg/dL. Physicians and investigators have used 6 mg/dL as a target serum urate level in patients with gout for decades. “The bottom line is lowering the serum urate for 12 months reduces gout flares. There is absolutely no reason to question the physicochemical effect of lowering serum urate and dissolving the deposits and ultimately reducing attacks,” Dr. Mandell said. Urate lowering therapy takes time to reduce flare frequency and tophi, however. “It does not happen in 6 months in everyone,” he said.
Addressing intolerance and undertreatment
Clinicians may encounter various challenges when managing patients with gout. In cases of resistant gout, the target serum urate level may not be reached easily. At first, gout attacks and tophi may persist after levels decrease to less than 6 mg/dL. Complicated gout may occur when comorbidities limit treatment options or when tophi cause dramatic mechanical dysfunction.
“There is one way to manage all of these [scenarios], and that is to lower the serum urate,” Dr. Mandell said. “That is the management approach for chronic gout.”
Because this approach does not produce quick results, patients with limited life expectancy may not be appropriate candidates, although they still may benefit from prophylaxis against gout attacks, treatment of attacks, and surgery, he said.
Intolerance to a xanthine oxidase inhibitor is one potential treatment obstacle. If allopurinol causes gastrointestinal adverse effects or hypersensitivity reactions, switching to febuxostat (Uloric) may overcome this problem. Desensitizing patients with a mild allergy to allopurinol is another possible tactic. In addition, treating patients with a uricosuric such as probenecid as monotherapy or in combination with a xanthine oxidase inhibitor may help, Dr. Mandell said.
Increasing the dose of the xanthine oxidase inhibitor beyond the maximal dose listed by the Food and Drug Administration – 800 mg for allopurinol or 80 mg for febuxostat – is an option, Dr. Mandell said. In Europe, the maximal dose for allopurinol is 900 mg, and physicians have clinical experience pushing the dose of allopurinol to greater than 1,000 mg in rare instances, he noted. “There is not a dose-limiting toxicity to allopurinol,” he said. There is a bioavailability issue, however, and splitting the dose at doses greater than 300 mg probably is warranted, he added.
If these approaches fail to lower the serum urate level to below 6 mg/dL, rigid dietary changes may be a next step. Adjusting other medications also may be an option. For example, physicians might weigh using losartan as a blood pressure medicine instead of a thiazide.
Finally, physicians can debulk urate deposits with pegloticase. “Dramatically lower the body load of serum urate, and then come back and use your traditional drugs,” he said. After treatment with enzyme replacement therapy, patients almost invariably require lower doses of allopurinol or febuxostat, he said.
Also, in severe cases when the time necessary for traditional urate-lowering therapy to work may not make it the most appropriate route, aggressive therapy with pegloticase may be warranted, Dr. Mandell said.
The FAST trial
The ongoing Febuxostat versus Allopurinol Streamlined Trial (FAST) has provided data about undertreatment with allopurinol and the effects of increasing the dose. The prospective, randomized, open-label study is comparing the cardiovascular safety of allopurinol and febuxostat in patients with symptomatic hyperuricemia. It enrolled patients who were on allopurinol in normal clinical practice. To enter, patients had to have a serum urate level below 6 mg/dL. If patients’ levels were not below 6 mg/dL, investigators increased the dose of allopurinol to try to reach that target (Semin Arthritis Rheum. 2014 Aug;44[1]:25-30).
“Basically, this part of the study is a dose-escalation trial for efficacy,” Dr. Mandell said. “Of 400 patients taking allopurinol, 36% still had a urate above 6 [mg/dL]. ... If you uptitrated the dose, 97% of people were able to get to 6. Uptitration works. You just actually need to do it.” The results indicate that a 100-mg increase in allopurinol dose decreases serum urate by about 1 mg/dL.
Allopurinol hypersensitivity and chronic kidney disease
Patients with chronic kidney disease may have increased risk of allopurinol hypersensitivity. For a while, researchers postulated that oxypurinol, the active component of allopurinol, built up and caused toxicity in some patients with chronic kidney disease. As a result, researchers suggested adjusting the dose for patients with chronic kidney disease.
One problem with this approach is that only about 20% of patients with chronic kidney disease would reach the treatment target with the suggested doses, Dr. Mandell said. “You are exposing them to some potential risk with a very low chance of actually getting any efficacy at all,” he said.
Furthermore, allopurinol hypersensitivity behaves like an allergic reaction, not a toxicity reaction. Small studies suggest that starting allopurinol at a low dose and slowly increasing the dose may be safe in patients with chronic kidney disease. Allopurinol is not nephrotoxic, and some data indicate that it may be nephroprotective, he said.
Dr. Mandell reported that in recent years he was a clinical investigator and consultant for Horizon and a consultant for Takeda and Ardea/AstraZeneca/Ironwood.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Management of difficult-to-treat gout calls for a familiar therapeutic goal: lowering the serum urate level to less than 6 mg/dL. Underused treatment approaches, such as escalating the dose of allopurinol or adding probenecid, can help almost all patients reach this target, said Brian F. Mandell, MD, PhD, professor of rheumatic and immunologic disease at the Cleveland Clinic.
“The major reason for treatment resistance has nothing to do with the drugs not working,” Dr. Mandell said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “And it does not even have to do ... with patient compliance. It is actually due to us and lack of appropriate monitoring and dosing of the medicines. We do not push the dose up.”
The urate saturation point in physiologic fluids with protein is about 6.8 mg/dL. Physicians and investigators have used 6 mg/dL as a target serum urate level in patients with gout for decades. “The bottom line is lowering the serum urate for 12 months reduces gout flares. There is absolutely no reason to question the physicochemical effect of lowering serum urate and dissolving the deposits and ultimately reducing attacks,” Dr. Mandell said. Urate lowering therapy takes time to reduce flare frequency and tophi, however. “It does not happen in 6 months in everyone,” he said.
Addressing intolerance and undertreatment
Clinicians may encounter various challenges when managing patients with gout. In cases of resistant gout, the target serum urate level may not be reached easily. At first, gout attacks and tophi may persist after levels decrease to less than 6 mg/dL. Complicated gout may occur when comorbidities limit treatment options or when tophi cause dramatic mechanical dysfunction.
“There is one way to manage all of these [scenarios], and that is to lower the serum urate,” Dr. Mandell said. “That is the management approach for chronic gout.”
Because this approach does not produce quick results, patients with limited life expectancy may not be appropriate candidates, although they still may benefit from prophylaxis against gout attacks, treatment of attacks, and surgery, he said.
Intolerance to a xanthine oxidase inhibitor is one potential treatment obstacle. If allopurinol causes gastrointestinal adverse effects or hypersensitivity reactions, switching to febuxostat (Uloric) may overcome this problem. Desensitizing patients with a mild allergy to allopurinol is another possible tactic. In addition, treating patients with a uricosuric such as probenecid as monotherapy or in combination with a xanthine oxidase inhibitor may help, Dr. Mandell said.
Increasing the dose of the xanthine oxidase inhibitor beyond the maximal dose listed by the Food and Drug Administration – 800 mg for allopurinol or 80 mg for febuxostat – is an option, Dr. Mandell said. In Europe, the maximal dose for allopurinol is 900 mg, and physicians have clinical experience pushing the dose of allopurinol to greater than 1,000 mg in rare instances, he noted. “There is not a dose-limiting toxicity to allopurinol,” he said. There is a bioavailability issue, however, and splitting the dose at doses greater than 300 mg probably is warranted, he added.
If these approaches fail to lower the serum urate level to below 6 mg/dL, rigid dietary changes may be a next step. Adjusting other medications also may be an option. For example, physicians might weigh using losartan as a blood pressure medicine instead of a thiazide.
Finally, physicians can debulk urate deposits with pegloticase. “Dramatically lower the body load of serum urate, and then come back and use your traditional drugs,” he said. After treatment with enzyme replacement therapy, patients almost invariably require lower doses of allopurinol or febuxostat, he said.
Also, in severe cases when the time necessary for traditional urate-lowering therapy to work may not make it the most appropriate route, aggressive therapy with pegloticase may be warranted, Dr. Mandell said.
The FAST trial
The ongoing Febuxostat versus Allopurinol Streamlined Trial (FAST) has provided data about undertreatment with allopurinol and the effects of increasing the dose. The prospective, randomized, open-label study is comparing the cardiovascular safety of allopurinol and febuxostat in patients with symptomatic hyperuricemia. It enrolled patients who were on allopurinol in normal clinical practice. To enter, patients had to have a serum urate level below 6 mg/dL. If patients’ levels were not below 6 mg/dL, investigators increased the dose of allopurinol to try to reach that target (Semin Arthritis Rheum. 2014 Aug;44[1]:25-30).
“Basically, this part of the study is a dose-escalation trial for efficacy,” Dr. Mandell said. “Of 400 patients taking allopurinol, 36% still had a urate above 6 [mg/dL]. ... If you uptitrated the dose, 97% of people were able to get to 6. Uptitration works. You just actually need to do it.” The results indicate that a 100-mg increase in allopurinol dose decreases serum urate by about 1 mg/dL.
Allopurinol hypersensitivity and chronic kidney disease
Patients with chronic kidney disease may have increased risk of allopurinol hypersensitivity. For a while, researchers postulated that oxypurinol, the active component of allopurinol, built up and caused toxicity in some patients with chronic kidney disease. As a result, researchers suggested adjusting the dose for patients with chronic kidney disease.
One problem with this approach is that only about 20% of patients with chronic kidney disease would reach the treatment target with the suggested doses, Dr. Mandell said. “You are exposing them to some potential risk with a very low chance of actually getting any efficacy at all,” he said.
Furthermore, allopurinol hypersensitivity behaves like an allergic reaction, not a toxicity reaction. Small studies suggest that starting allopurinol at a low dose and slowly increasing the dose may be safe in patients with chronic kidney disease. Allopurinol is not nephrotoxic, and some data indicate that it may be nephroprotective, he said.
Dr. Mandell reported that in recent years he was a clinical investigator and consultant for Horizon and a consultant for Takeda and Ardea/AstraZeneca/Ironwood.
Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM PRD 2019
Click for Credit: Long-term antibiotics & stroke, CHD; Postvaccination seizures; more
Here are 5 articles from the November issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Poor response to statins hikes risk of cardiovascular events
To take the posttest, go to: https://bit.ly/2MVHlDR
Expires April 17, 2020
2. Postvaccination febrile seizures are no more severe than other febrile seizures
To take the posttest, go to: https://bit.ly/2VUJzaE
Expires April 19, 2020
3. Hydroxychloroquine adherence in SLE: worse than you thought
To take the posttest, go to: https://bit.ly/2oT00Z9
Expires April 22, 2020
4. Long-term antibiotic use may heighten stroke, CHD risk
To take the posttest, go to: https://bit.ly/2OUUVu5
Expires April 28, 2020
5. Knowledge gaps about long-term osteoporosis drug therapy benefits, risks remain large
To take the posttest, go to: https://bit.ly/2Msgqkb
Expires May 1, 2020
Here are 5 articles from the November issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Poor response to statins hikes risk of cardiovascular events
To take the posttest, go to: https://bit.ly/2MVHlDR
Expires April 17, 2020
2. Postvaccination febrile seizures are no more severe than other febrile seizures
To take the posttest, go to: https://bit.ly/2VUJzaE
Expires April 19, 2020
3. Hydroxychloroquine adherence in SLE: worse than you thought
To take the posttest, go to: https://bit.ly/2oT00Z9
Expires April 22, 2020
4. Long-term antibiotic use may heighten stroke, CHD risk
To take the posttest, go to: https://bit.ly/2OUUVu5
Expires April 28, 2020
5. Knowledge gaps about long-term osteoporosis drug therapy benefits, risks remain large
To take the posttest, go to: https://bit.ly/2Msgqkb
Expires May 1, 2020
Here are 5 articles from the November issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Poor response to statins hikes risk of cardiovascular events
To take the posttest, go to: https://bit.ly/2MVHlDR
Expires April 17, 2020
2. Postvaccination febrile seizures are no more severe than other febrile seizures
To take the posttest, go to: https://bit.ly/2VUJzaE
Expires April 19, 2020
3. Hydroxychloroquine adherence in SLE: worse than you thought
To take the posttest, go to: https://bit.ly/2oT00Z9
Expires April 22, 2020
4. Long-term antibiotic use may heighten stroke, CHD risk
To take the posttest, go to: https://bit.ly/2OUUVu5
Expires April 28, 2020
5. Knowledge gaps about long-term osteoporosis drug therapy benefits, risks remain large
To take the posttest, go to: https://bit.ly/2Msgqkb
Expires May 1, 2020
Appropriate laboratory testing in Lyme disease
Lyme disease is a complex multisystem bacterial infection affecting the skin, joints, heart, and nervous system. The full spectrum of disease was first recognized and the disease was named in the 1970s during an outbreak of arthritis in children in the town of Lyme, Connecticut.1
This review describes the epidemiology and pathogenesis of Lyme disease, the advantages and disadvantages of current diagnostic methods, and diagnostic algorithms.
THE MOST COMMON TICK-BORNE INFECTION IN NORTH AMERICA
Lyme disease is the most common tick-borne infection in North America.2,3 In the United States, more than 30,000 cases are reported annually. In fact, in 2017, the number of cases was about 42,000, a 16% increase from the previous year, according to the US Centers for Disease Control and Prevention (CDC).
Infected nymphs account for most cases.
The infection is caused by Borrelia burgdorferi, a particularly arthritogenic spirochete transmitted by Ixodes scapularis (the black-legged deer tick, (Figure 1) and Ixodes pacificus (the Western black-legged tick). Although the infection can occur at any time of the year, its peak incidence is in May to late September, coinciding with increased outdoor recreational activity in areas where ticks live.3,4 The typical tick habitat consists of deciduous woodland with sufficient humidity provided by a good layer of decaying vegetation. However, people can contract Lyme disease in their own backyard.3
Most cases of Lyme disease are seen in the northeastern United States, mainly in suburban and rural areas.2,3 Other areas affected include the midwestern states of Minnesota, Wisconsin, and Michigan, as well as northern California.4 Fourteen states and the District of Columbia report a high average incidence (> 10 cases per 100,000 persons) (Table 1).2
FIRST COMES IgM, THEN IgG
The pathogenesis and the different stages of infection should inform laboratory testing in Lyme disease.
It is estimated that only 5% of infected ticks that bite people actually transmit their spirochetes to the human host.5 However, once infected, the patient’s innate immune system mounts a response that results in the classic erythema migrans rash at the bite site. A rash develops in only about 85% of patients who are infected and can appear at any time between 3 and 30 days, but most commonly after 7 days. Hence, a rash occurring within the first few hours of tick contact is not erythema migrans and does not indicate infection, but rather an early reaction to tick salivary antigens.5
Antibody levels remain below the detection limits of currently available serologic tests in the first 7 days after exposure. Immunoglobulin M (IgM) antibody titers peak between 8 and 14 days after tick contact, but IgM antibodies may never develop if the patient is started on early appropriate antimicrobial therapy.5
If the infection is not treated, the spirochete may disseminate through the blood from the bite site to different tissues.3 Both cell-mediated and antibody-mediated immunity swing into action to kill the spirochetes at this stage. The IgM antibody response occurs in 1 to 2 weeks, followed by a robust IgG response in 2 to 4 weeks.6
Because IgM can also cross-react with antigens other than those associated with B burgdorferi, the IgM test is less specific than the IgG test for Lyme disease.
Once a patient is exposed and mounts an antibody-mediated response to the spirochete, the antibody profile may persist for months to years, even after successful antibiotic treatment and cure of the disease.5
Despite the immune system’s robust series of defenses, untreated B burgdorferi infection can persist, as the organism has a bag of tricks to evade destruction. It can decrease its expression of specific immunogenic surface-exposed proteins, change its antigenic properties through recombination, and bind to the patient’s extracellular matrix proteins to facilitate further dissemination.3
Certain host-genetic factors also play a role in the pathogenesis of Lyme disease, such as the HLA-DR4 allele, which has been associated with antibiotic-refractory Lyme-related arthritis.3
LYME DISEASE EVOLVES THROUGH STAGES
Lyme disease evolves through stages broadly classified as early and late infection, with significant variability in its presentation.7
Early infection
Early disease is further subdivided into “localized” infection (stage 1), characterized by a single erythema migrans lesion and local lymphadenopathy, and “disseminated” infection (stage 2), associated with multiple erythema migrans lesions distant from the bite site, facial nerve palsy, radiculoneuritis, meningitis, carditis, or migratory arthritis or arthralgia.8
Highly specific physical findings include erythema migrans, cranial nerve palsy, high-grade or progressive conduction block, and recurrent migratory polyarthritis. Less specific symptoms and signs of Lyme disease include arthralgia, myalgia, neck stiffness, palpitations, and myocarditis.5
Erythema migrans lesions are evident in at least 85% of patients with early disease.9 If they are not apparent on physical examination, they may be located at hidden sites and may be atypical in appearance or transient.5
If treatment is not started in the initial stage of the disease, 60% of infected patients may develop disseminated infection.5 Progressive, untreated infection can manifest with Lyme arthritis and neuroborreliosis.7
Noncutaneous manifestations are less common now than in the past due to increased awareness of the disease and early initiation of treatment.10
Late infection
Manifestations of late (stage 3) infection include oligoarthritis (affecting any joint but often the knee) and neuroborreliosis. Clinical signs and symptoms of Lyme disease may take months to resolve even after appropriate antimicrobial therapy is completed. This should not be interpreted as ongoing, persistent infection, but as related to host immune-mediated activity.5
INTERPRET LABORATORY RESULTS BASED ON PRETEST PROBABILITY
The usefulness of a laboratory test depends on the individual patient’s pretest probability of infection, which in turn depends on the patient’s epidemiologic risk of exposure and clinical features of Lyme disease. Patients with a high pretest probability—eg, a history of a tick bite followed by the classic erythema migrans rash—do not need testing and can start antimicrobial therapy right away.11
Serologic tests are the gold standard
Prompt diagnosis is important, as early Lyme disease is easily treatable without any future sequelae.11
Tests for Lyme disease can be divided into direct methods, which detect the spirochete itself by culture or by polymerase chain reaction (PCR), and indirect methods, which detect antibodies (Table 2). Direct tests lack sensitivity for Lyme disease; hence, serologic tests remain the gold standard. Currently recommended is a standard 2-tier testing strategy using an enzyme-linked immunosorbent assay (ELISA) followed by Western blot for confirmation.
DIRECT METHODS
Culture lacks sensitivity
A number of factors limit the sensitivity of direct culture for diagnosing Lyme disease. B burgdorferi does not grow easily in culture, requiring special media, low temperatures, and long periods of incubation. Only a relatively few spirochetes are present in human tissues and body fluids to begin with, and bacterial counts are further reduced with duration and dissemination of infection.5 All of these limit the possibility of detecting this organism.
Polymerase chain reaction may help in some situations
Molecular assays are not part of the standard evaluation and should be used only in conjunction with serologic testing.7 These tests have high specificity but lack consistent sensitivity.
That said, PCR testing may be useful:
- In early infection, before antibody responses develop
- In reinfection, when serologic tests are not reliable because the antibodies persist for many years after an infection in many patients
- In endemic areas where serologic testing has high false-positive rates due to high baseline population seropositivity for anti-Borrelia antibodies caused by subclinical infection.3
PCR assays that target plasmid-borne genes encoding outer surface proteins A and C (OspA and OspC) and VisE (variable major protein-like sequence, expressed) are more sensitive than those that detect chromosomal 16s ribosomal ribonucleic acid (rRNA) genes, as plasmid-rich “blebs” are shed in larger concentrations than chromosomal DNA during active infection.7 However, these plasmid-contained genes persist in body tissues and fluids even after the infection is cleared, and their detection may not necessarily correlate with ongoing disease.8 Detection of chromosomal 16s rRNA genes is a better predictor of true organism viability.
The sensitivity of PCR for borrelial DNA depends on the type of sample. If a skin biopsy sample is taken of the leading edge of an erythema migrans lesion, the sensitivity is 69% and the specificity is 100%. In patients with Lyme arthritis, PCR of the synovial fluid has a sensitivity of up to 80%. However, the sensitivity of PCR of the cerebrospinal fluid of patients with neurologic manifestations of Lyme disease is only 19%.7 PCR of other clinical samples, including blood and urine, is not recommended, as spirochetes are primarily confined to tissues, and very few are present in these body fluids.3,12
The disadvantage of PCR is that a positive result does not always mean active infection, as the DNA of the dead microbe persists for several months even after successful treatment.8
INDIRECT METHODS
Enzyme-linked immunosorbent assay
ELISAs detect anti-Borrelia antibodies. Early-generation ELISAs, still used in many laboratories, use whole-cell extracts of B burgdorferi. Examples are the Vidas Lyme screen (Biomérieux, biomerieux-usa.com) and the Wampole B burgdorferi IgG/M EIA II assay (Alere, www.alere.com). Newer ELISAs use recombinant proteins.13
Three major targets for ELISA antibodies are flagellin (Fla), outer surface protein C (OspC), and VisE, especially the invariable region 6 (IR6). Among these, VisE-IR6 is the most conserved region in B burgdorferi.
Early-generation assays have a sensitivity of 89% and specificity of 72%.11 However, the patient’s serum may have antibodies that cross-react with unrelated bacterial antigens, leading to false-positive results (Table 3). Whole-cell sonicate assays are not recommended as an independent test and must be confirmed with Western blot testing when assay results are indeterminate or positive.11
Newer-generation ELISAs detect antibodies targeting recombinant proteins of VisE, especially a synthetic peptide C6, within IR6.13 VisE-IR6 is the most conserved region of the B burgdorferi complex, and its detection is a highly specific finding, supporting the diagnosis of Lyme disease. Antibodies against VisE-IR6 antigen are the earliest to develop.5 An example of a newer-generation serologic test is the VisE C6 Lyme EIA kit, approved as a first-tier test by the US Food and Drug Administration in 2001. This test has a specificity of 99%,14,15 and its specificity is further increased when used in conjunction with Western blot (99.5%).15 The advantage of the C6 antibody test is that it is more sensitive than 2-tier testing during early infection (sensitivity 29%–74% vs 17%–40% in early localized infection, and 56%–90% vs 27%–78% in early disseminated infection).6
During early infection, older and newer ELISAs are less sensitive because of the limited number of antigens expressed at this stage.13 All patients suspected of having early Lyme disease who are seronegative at initial testing should have follow-up testing to look for seroconversion.13
Western blot
Western blot (immunoblot) testing identifies IgM and IgG antibodies against specific B burgdorferi antigens. It is considered positive if it detects at least 2 of a possible 3 specific IgM bands in the first 4 weeks of disease or at least 5 of 10 specific IgG bands after 4 weeks of disease (Table 4 and Figure 2).16
The nature of the bands indicates the duration of infection: Western blot bands against 23-kD OspC and 41-kD FlaB are seen in early localized infection, whereas bands against all 3 B burgdorferi proteins will be seen after several weeks of disease.17 The IgM result should be interpreted carefully, as only 2 bands are required for the test to be positive, and IgM binds to antigen less specifically than IgG.12
Interpreting the IgM Western blot test: The ‘1-month rule’
If clinical symptoms and signs of Lyme disease have been present for more than 1 month, IgM reactivity alone should not be used to support the diagnosis, in view of the likelihood of a false-positive test result in this situation.18 This is called the “1-month rule” in the diagnosis of Lyme disease.13
In early localized infection, Western blot is only half as sensitive as ELISA testing. Since the overall sensitivity of a 2-step algorithm is equal to that of its least sensitive component, 2-tiered testing is not useful in early disease.13
Although currently considered the most specific test for confirmation of Lyme disease, Western blot has limitations. It is technically and interpretively complex and is thus not universally available.13 The blots are scored by visual examination, compromising the reproducibility of the test, although densitometric blot analysis techniques and automated scanning and scoring attempt to address some of these limitations.13 Like the ELISA, Western blot can have false-positive results in healthy individuals without tick exposure, as nonspecific IgM immunoblots develop faint bands. This is because of cross-reaction between B burgdorferi antigens and antigens from other microorganisms. Around 50% of healthy adults show low-level serum IgG reactivity against the FlaB antigen, leading to false-positive results as well. In cases in which the Western blot result is indeterminate, other etiologies must be considered.
False-positive IgM Western blots are a significant problem. In a 5-year retrospective study done at 63 US Air Force healthcare facilities, 113 (53.3%) of 212 IgM Western blots were falsely positive.19 A false-positive test was defined as one that failed to meet seropositivity (a first-tier test omitted or negative, > 30 days of symptoms with negative IgG blot), lack of exposure including residing in areas without documented tick habitats, patients having atypical or no symptoms, and negative serology within 30 days of a positive test.
In a similar study done in a highly endemic area, 50 (27.5%) of 182 patients had a false-positive test.20 Physicians need to be careful when interpreting IgM Western blots. It is always important to consider locale, epidemiology, and symptoms when interpreting the test.
Limitations of serologic tests for Lyme disease
Currently available serologic tests have inherent limitations:
- Antibodies against B burgdorferi take at least 1 week to develop
- The background rate of seropositivity in endemic areas can be up to 4%, affecting the utility of a positive test result
- Serologic tests cannot be used as tests of cure because antibodies can persist for months to years even after appropriate antimicrobial therapy and cure of disease; thus, a positive serologic result could represent active infection or remote exposure21
- Antibodies can cross-react with related bacteria, including other borrelial or treponemal spirochetes
- False-positive serologic test results can also occur in association with other medical conditions such as polyclonal gammopathies and systemic lupus erythematosus.12
RECOMMENDATIONS FOR TESTING
Standard 2-tier testing
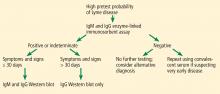
The CDC released recommendations for diagnosing Lyme disease after a second national conference of serologic diagnosis of Lyme disease in October 1994.18 The 2-tiered testing method, involving a sensitive ELISA followed by the Western blot to confirm positive and indeterminate ELISA results, was suggested as the gold standard for diagnosis (Figure 3). Of note, negative ELISA results do not require further testing.11
The sensitivity of 2-tiered testing depends on the stage of the disease. Unfortunately, this method has a wide range of sensitivity (17% to 78%) in stage 1 disease. In the same stage, the sensitivity increases from 14.1% in patients with a single erythema migrans lesion and early localized infection to 65.4% in those with multiple lesions. The algorithm has excellent sensitivity in late stage 3 infection (96% to 100%).5
A 2-step ELISA algorithm
A 2-step ELISA algorithm (without the Western blot) that includes the whole-cell sonicate assay followed by the VisE C6 peptide assay actually showed higher sensitivity and comparable specificity compared with 2-tiered testing in early localized disease (sensitivity 61%–74% vs 29%–48%, respectively; specificity 99.5% for both methods).22 This higher sensitivity was even more pronounced in early disseminated infection (sensitivity 100% vs 40%, respectively). By late infection, the sensitivities of both testing strategies reached 100%. Compared with the Western blot, the 2-step ELISA algorithm was simpler to execute in a reproducible fashion.5
The Infectious Diseases Society of America is revising its current guidelines, with an update expected late this year, which may shift the recommendation from 2-tiered testing to the 2-step ELISA algorithm.
Multiplex testing
To overcome the intrinsic problems of protein-based assays, a multiplexed, array-based assay for the diagnosis of tick-borne infections called Tick-Borne Disease Serochip (TBD-Serochip) was established using recombinant antigens that identify key immunodominant epitopes.8 More studies are needed to establish the validity and usefulness of these tests in clinical practice.
Who should not be tested?
The American College of Physicians6 recommends against testing in patients:
- Presenting with nonspecific symptoms (eg, headache, myalgia, fatigue, arthralgia) without objective signs of Lyme disease
- With low pretest probability of infection based on epidemiologic exposures and clinical features
- Living in Lyme-endemic areas with no history of tick exposure6
- Presenting less than 1 week after tick exposure5
- Seeking a test of cure for treated Lyme disease.
DIAGNOSIS IN SPECIAL SITUATIONS
Early Lyme disease
The classic erythema migrans lesion on physical examination of a patient with suspected Lyme disease is diagnostic and does not require laboratory confirmation.10
In ambiguous cases, 2-tiered testing of a serum sample during the acute presentation and again 4 to 6 weeks later can be useful. In patients who remain seronegative on paired serum samples despite symptoms lasting longer than 6 weeks and no antibiotic treatment in the interim, the diagnosis of Lyme disease is unlikely, and another diagnosis should be sought.3
Antimicrobial therapy may block the serologic response; hence, negative serologic testing in patients started on empiric antibiotics should not rule out Lyme disease.6
PCR or bacterial culture testing is not recommended in the evaluation of suspected early Lyme disease.
Central nervous system Lyme disease
Central nervous system Lyme disease is diagnosed by 2-tiered testing using peripheral blood samples because all patients with this infectious manifestation should have mounted an adequate IgG response in the blood.11
B cells migrate to and proliferate inside the central nervous system, leading to intrathecal production of anti-Borrelia antibodies. An index of cerebrospinal fluid to serum antibody greater than 1 is thus also indicative of neuroborreliosis.12 Thus, performing lumbar puncture to detect intrathecal production of antibodies may support the diagnosis of central nervous system Lyme disease; however, it is not necessary.11
Antibodies persist in the central nervous system for many years after appropriate antimicrobial treatment.
Lyme arthritis
Articular involvement in Lyme disease is characterized by a robust humoral response such that a negative IgG serologic test virtually rules out Lyme arthritis.23 PCR testing of synovial fluid for borrelial DNA has a sensitivity of 80% but may become falsely negative after 1 to 2 months of antibiotic treatment.24,25 In an algorithm suggested by Puius et al,23 PCR testing of synovial fluid should be done in patients who have minimal to no response after 2 months of appropriate oral antimicrobial therapy to determine whether intravenous antibiotics are merited.
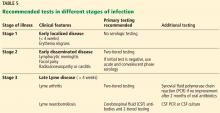
Table 5 summarizes the tests of choice in different clinical stages of infection.
Acknowledgment: The authors would like to acknowledge Anita Modi, MD, and Ceena N. Jacob, MD, for reviewing the manuscript and providing valuable suggestions, and Belinda Yen-Lieberman, PhD, for contributing pictures of the Western blot test results.
- Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 1977; 20(1):7–17. doi:10.1002/art.1780200102
- Centers for Disease Control and Prevention (CDC). Lyme disease: recent surveillance data. https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html. Accessed August 12, 2019.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet 2012; 379(9814):461–473. doi:10.1016/S0140-6736(11)60103-7
- Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015; 29(2):269–280. doi:10.1016/j.idc.2015.02.004
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med 2015; 35(4):797–814. doi:10.1016/j.cll.2015.08.001
- Hu LT. Lyme disease. Ann Intern Med 2016; 164(9):ITC65–ITC80. doi:10.7326/AITC201605030
- Alby K, Capraro GA. Alternatives to serologic testing for diagnosis of Lyme disease. Clin Lab Med 2015; 35(4):815–825. doi:10.1016/j.cll.2015.07.005
- Dumler JS. Molecular diagnosis of Lyme disease: review and meta-analysis. Mol Diagn 2001; 6(1):1–11. doi:10.1054/modi.2001.21898
- Wormser GP, McKenna D, Carlin J, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med 2005; 142(9):751–755. doi:10.7326/0003-4819-142-9-200505030-00011
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43(9):1089–1134. doi:10.1086/508667
- Guidelines for laboratory evaluation in the diagnosis of Lyme disease. American College of Physicians. Ann Intern Med 1997; 127(12):1106–1108. doi:10.7326/0003-4819-127-12-199712150-00010
- Halperin JJ. Lyme disease: a multisystem infection that affects the nervous system. Continuum (Minneap Minn) 2012; 18(6 Infectious Disease):1338–1350. doi:10.1212/01.CON.0000423850.24900.3a
- Branda JA, Body BA, Boyle J, et al. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 2018; 66(7):1133–1139. doi:10.1093/cid/cix943
- Immunetics. Immunetics® C6 Lyme ELISA™ Kit. http://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/CF-E601-096A-C6-Pkg-Insrt.pdf. Accessed August 12, 2019.
- Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet 2014; 15(1):34–48. doi:10.1038/nrg3575
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44(31):590–591. pmid:7623762
- Steere AC, Mchugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 2008; 47(2):188–195. doi:10.1086/589242
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA 1995; 274(12):937. pmid:7674514
- Webber BJ, Burganowski RP, Colton L, Escobar JD, Pathak SR, Gambino-Shirley KJ. Lyme disease overdiagnosis in a large healthcare system: a population-based, retrospective study. Clin Microbiol Infect 2019. doi:10.1016/j.cmi.2019.02.020. Epub ahead of print.
- Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18(12):1236–1240. doi:10.1111/j.1469-0691.2011.03749.x
- Hilton E, DeVoti J, Benach JL, et al. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am J Med 1999; 106(4):404–409. doi:10.1016/s0002-9343(99)00046-7
- Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 2011; 53(6):541–547. doi:10.1093/cid/cir464
- Puius YA, Kalish RA. Lyme arthritis: pathogenesis, clinical presentation, and management. Infect Dis Clin North Am 2008; 22(2):289–300. doi:10.1016/j.idc.2007.12.014
- Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 1994; 330(4):229–234. doi:10.1056/NEJM199401273300401
- Liebling MR, Nishio MJ, Rodriguez A, Sigal LH, Jin T, Louie JS. The polymerase chain reaction for the detection of Borrelia burgdorferi in human body fluids. Arthritis Rheum 1993; 36(5):665–975. doi:10.1002/art.1780360514
Lyme disease is a complex multisystem bacterial infection affecting the skin, joints, heart, and nervous system. The full spectrum of disease was first recognized and the disease was named in the 1970s during an outbreak of arthritis in children in the town of Lyme, Connecticut.1
This review describes the epidemiology and pathogenesis of Lyme disease, the advantages and disadvantages of current diagnostic methods, and diagnostic algorithms.
THE MOST COMMON TICK-BORNE INFECTION IN NORTH AMERICA
Lyme disease is the most common tick-borne infection in North America.2,3 In the United States, more than 30,000 cases are reported annually. In fact, in 2017, the number of cases was about 42,000, a 16% increase from the previous year, according to the US Centers for Disease Control and Prevention (CDC).
Infected nymphs account for most cases.
The infection is caused by Borrelia burgdorferi, a particularly arthritogenic spirochete transmitted by Ixodes scapularis (the black-legged deer tick, (Figure 1) and Ixodes pacificus (the Western black-legged tick). Although the infection can occur at any time of the year, its peak incidence is in May to late September, coinciding with increased outdoor recreational activity in areas where ticks live.3,4 The typical tick habitat consists of deciduous woodland with sufficient humidity provided by a good layer of decaying vegetation. However, people can contract Lyme disease in their own backyard.3
Most cases of Lyme disease are seen in the northeastern United States, mainly in suburban and rural areas.2,3 Other areas affected include the midwestern states of Minnesota, Wisconsin, and Michigan, as well as northern California.4 Fourteen states and the District of Columbia report a high average incidence (> 10 cases per 100,000 persons) (Table 1).2
FIRST COMES IgM, THEN IgG
The pathogenesis and the different stages of infection should inform laboratory testing in Lyme disease.
It is estimated that only 5% of infected ticks that bite people actually transmit their spirochetes to the human host.5 However, once infected, the patient’s innate immune system mounts a response that results in the classic erythema migrans rash at the bite site. A rash develops in only about 85% of patients who are infected and can appear at any time between 3 and 30 days, but most commonly after 7 days. Hence, a rash occurring within the first few hours of tick contact is not erythema migrans and does not indicate infection, but rather an early reaction to tick salivary antigens.5
Antibody levels remain below the detection limits of currently available serologic tests in the first 7 days after exposure. Immunoglobulin M (IgM) antibody titers peak between 8 and 14 days after tick contact, but IgM antibodies may never develop if the patient is started on early appropriate antimicrobial therapy.5
If the infection is not treated, the spirochete may disseminate through the blood from the bite site to different tissues.3 Both cell-mediated and antibody-mediated immunity swing into action to kill the spirochetes at this stage. The IgM antibody response occurs in 1 to 2 weeks, followed by a robust IgG response in 2 to 4 weeks.6
Because IgM can also cross-react with antigens other than those associated with B burgdorferi, the IgM test is less specific than the IgG test for Lyme disease.
Once a patient is exposed and mounts an antibody-mediated response to the spirochete, the antibody profile may persist for months to years, even after successful antibiotic treatment and cure of the disease.5
Despite the immune system’s robust series of defenses, untreated B burgdorferi infection can persist, as the organism has a bag of tricks to evade destruction. It can decrease its expression of specific immunogenic surface-exposed proteins, change its antigenic properties through recombination, and bind to the patient’s extracellular matrix proteins to facilitate further dissemination.3
Certain host-genetic factors also play a role in the pathogenesis of Lyme disease, such as the HLA-DR4 allele, which has been associated with antibiotic-refractory Lyme-related arthritis.3
LYME DISEASE EVOLVES THROUGH STAGES
Lyme disease evolves through stages broadly classified as early and late infection, with significant variability in its presentation.7
Early infection
Early disease is further subdivided into “localized” infection (stage 1), characterized by a single erythema migrans lesion and local lymphadenopathy, and “disseminated” infection (stage 2), associated with multiple erythema migrans lesions distant from the bite site, facial nerve palsy, radiculoneuritis, meningitis, carditis, or migratory arthritis or arthralgia.8
Highly specific physical findings include erythema migrans, cranial nerve palsy, high-grade or progressive conduction block, and recurrent migratory polyarthritis. Less specific symptoms and signs of Lyme disease include arthralgia, myalgia, neck stiffness, palpitations, and myocarditis.5
Erythema migrans lesions are evident in at least 85% of patients with early disease.9 If they are not apparent on physical examination, they may be located at hidden sites and may be atypical in appearance or transient.5
If treatment is not started in the initial stage of the disease, 60% of infected patients may develop disseminated infection.5 Progressive, untreated infection can manifest with Lyme arthritis and neuroborreliosis.7
Noncutaneous manifestations are less common now than in the past due to increased awareness of the disease and early initiation of treatment.10
Late infection
Manifestations of late (stage 3) infection include oligoarthritis (affecting any joint but often the knee) and neuroborreliosis. Clinical signs and symptoms of Lyme disease may take months to resolve even after appropriate antimicrobial therapy is completed. This should not be interpreted as ongoing, persistent infection, but as related to host immune-mediated activity.5
INTERPRET LABORATORY RESULTS BASED ON PRETEST PROBABILITY
The usefulness of a laboratory test depends on the individual patient’s pretest probability of infection, which in turn depends on the patient’s epidemiologic risk of exposure and clinical features of Lyme disease. Patients with a high pretest probability—eg, a history of a tick bite followed by the classic erythema migrans rash—do not need testing and can start antimicrobial therapy right away.11
Serologic tests are the gold standard
Prompt diagnosis is important, as early Lyme disease is easily treatable without any future sequelae.11
Tests for Lyme disease can be divided into direct methods, which detect the spirochete itself by culture or by polymerase chain reaction (PCR), and indirect methods, which detect antibodies (Table 2). Direct tests lack sensitivity for Lyme disease; hence, serologic tests remain the gold standard. Currently recommended is a standard 2-tier testing strategy using an enzyme-linked immunosorbent assay (ELISA) followed by Western blot for confirmation.
DIRECT METHODS
Culture lacks sensitivity
A number of factors limit the sensitivity of direct culture for diagnosing Lyme disease. B burgdorferi does not grow easily in culture, requiring special media, low temperatures, and long periods of incubation. Only a relatively few spirochetes are present in human tissues and body fluids to begin with, and bacterial counts are further reduced with duration and dissemination of infection.5 All of these limit the possibility of detecting this organism.
Polymerase chain reaction may help in some situations
Molecular assays are not part of the standard evaluation and should be used only in conjunction with serologic testing.7 These tests have high specificity but lack consistent sensitivity.
That said, PCR testing may be useful:
- In early infection, before antibody responses develop
- In reinfection, when serologic tests are not reliable because the antibodies persist for many years after an infection in many patients
- In endemic areas where serologic testing has high false-positive rates due to high baseline population seropositivity for anti-Borrelia antibodies caused by subclinical infection.3
PCR assays that target plasmid-borne genes encoding outer surface proteins A and C (OspA and OspC) and VisE (variable major protein-like sequence, expressed) are more sensitive than those that detect chromosomal 16s ribosomal ribonucleic acid (rRNA) genes, as plasmid-rich “blebs” are shed in larger concentrations than chromosomal DNA during active infection.7 However, these plasmid-contained genes persist in body tissues and fluids even after the infection is cleared, and their detection may not necessarily correlate with ongoing disease.8 Detection of chromosomal 16s rRNA genes is a better predictor of true organism viability.
The sensitivity of PCR for borrelial DNA depends on the type of sample. If a skin biopsy sample is taken of the leading edge of an erythema migrans lesion, the sensitivity is 69% and the specificity is 100%. In patients with Lyme arthritis, PCR of the synovial fluid has a sensitivity of up to 80%. However, the sensitivity of PCR of the cerebrospinal fluid of patients with neurologic manifestations of Lyme disease is only 19%.7 PCR of other clinical samples, including blood and urine, is not recommended, as spirochetes are primarily confined to tissues, and very few are present in these body fluids.3,12
The disadvantage of PCR is that a positive result does not always mean active infection, as the DNA of the dead microbe persists for several months even after successful treatment.8
INDIRECT METHODS
Enzyme-linked immunosorbent assay
ELISAs detect anti-Borrelia antibodies. Early-generation ELISAs, still used in many laboratories, use whole-cell extracts of B burgdorferi. Examples are the Vidas Lyme screen (Biomérieux, biomerieux-usa.com) and the Wampole B burgdorferi IgG/M EIA II assay (Alere, www.alere.com). Newer ELISAs use recombinant proteins.13
Three major targets for ELISA antibodies are flagellin (Fla), outer surface protein C (OspC), and VisE, especially the invariable region 6 (IR6). Among these, VisE-IR6 is the most conserved region in B burgdorferi.
Early-generation assays have a sensitivity of 89% and specificity of 72%.11 However, the patient’s serum may have antibodies that cross-react with unrelated bacterial antigens, leading to false-positive results (Table 3). Whole-cell sonicate assays are not recommended as an independent test and must be confirmed with Western blot testing when assay results are indeterminate or positive.11
Newer-generation ELISAs detect antibodies targeting recombinant proteins of VisE, especially a synthetic peptide C6, within IR6.13 VisE-IR6 is the most conserved region of the B burgdorferi complex, and its detection is a highly specific finding, supporting the diagnosis of Lyme disease. Antibodies against VisE-IR6 antigen are the earliest to develop.5 An example of a newer-generation serologic test is the VisE C6 Lyme EIA kit, approved as a first-tier test by the US Food and Drug Administration in 2001. This test has a specificity of 99%,14,15 and its specificity is further increased when used in conjunction with Western blot (99.5%).15 The advantage of the C6 antibody test is that it is more sensitive than 2-tier testing during early infection (sensitivity 29%–74% vs 17%–40% in early localized infection, and 56%–90% vs 27%–78% in early disseminated infection).6
During early infection, older and newer ELISAs are less sensitive because of the limited number of antigens expressed at this stage.13 All patients suspected of having early Lyme disease who are seronegative at initial testing should have follow-up testing to look for seroconversion.13
Western blot
Western blot (immunoblot) testing identifies IgM and IgG antibodies against specific B burgdorferi antigens. It is considered positive if it detects at least 2 of a possible 3 specific IgM bands in the first 4 weeks of disease or at least 5 of 10 specific IgG bands after 4 weeks of disease (Table 4 and Figure 2).16
The nature of the bands indicates the duration of infection: Western blot bands against 23-kD OspC and 41-kD FlaB are seen in early localized infection, whereas bands against all 3 B burgdorferi proteins will be seen after several weeks of disease.17 The IgM result should be interpreted carefully, as only 2 bands are required for the test to be positive, and IgM binds to antigen less specifically than IgG.12
Interpreting the IgM Western blot test: The ‘1-month rule’
If clinical symptoms and signs of Lyme disease have been present for more than 1 month, IgM reactivity alone should not be used to support the diagnosis, in view of the likelihood of a false-positive test result in this situation.18 This is called the “1-month rule” in the diagnosis of Lyme disease.13
In early localized infection, Western blot is only half as sensitive as ELISA testing. Since the overall sensitivity of a 2-step algorithm is equal to that of its least sensitive component, 2-tiered testing is not useful in early disease.13
Although currently considered the most specific test for confirmation of Lyme disease, Western blot has limitations. It is technically and interpretively complex and is thus not universally available.13 The blots are scored by visual examination, compromising the reproducibility of the test, although densitometric blot analysis techniques and automated scanning and scoring attempt to address some of these limitations.13 Like the ELISA, Western blot can have false-positive results in healthy individuals without tick exposure, as nonspecific IgM immunoblots develop faint bands. This is because of cross-reaction between B burgdorferi antigens and antigens from other microorganisms. Around 50% of healthy adults show low-level serum IgG reactivity against the FlaB antigen, leading to false-positive results as well. In cases in which the Western blot result is indeterminate, other etiologies must be considered.
False-positive IgM Western blots are a significant problem. In a 5-year retrospective study done at 63 US Air Force healthcare facilities, 113 (53.3%) of 212 IgM Western blots were falsely positive.19 A false-positive test was defined as one that failed to meet seropositivity (a first-tier test omitted or negative, > 30 days of symptoms with negative IgG blot), lack of exposure including residing in areas without documented tick habitats, patients having atypical or no symptoms, and negative serology within 30 days of a positive test.
In a similar study done in a highly endemic area, 50 (27.5%) of 182 patients had a false-positive test.20 Physicians need to be careful when interpreting IgM Western blots. It is always important to consider locale, epidemiology, and symptoms when interpreting the test.
Limitations of serologic tests for Lyme disease
Currently available serologic tests have inherent limitations:
- Antibodies against B burgdorferi take at least 1 week to develop
- The background rate of seropositivity in endemic areas can be up to 4%, affecting the utility of a positive test result
- Serologic tests cannot be used as tests of cure because antibodies can persist for months to years even after appropriate antimicrobial therapy and cure of disease; thus, a positive serologic result could represent active infection or remote exposure21
- Antibodies can cross-react with related bacteria, including other borrelial or treponemal spirochetes
- False-positive serologic test results can also occur in association with other medical conditions such as polyclonal gammopathies and systemic lupus erythematosus.12
RECOMMENDATIONS FOR TESTING
Standard 2-tier testing
The CDC released recommendations for diagnosing Lyme disease after a second national conference of serologic diagnosis of Lyme disease in October 1994.18 The 2-tiered testing method, involving a sensitive ELISA followed by the Western blot to confirm positive and indeterminate ELISA results, was suggested as the gold standard for diagnosis (Figure 3). Of note, negative ELISA results do not require further testing.11
The sensitivity of 2-tiered testing depends on the stage of the disease. Unfortunately, this method has a wide range of sensitivity (17% to 78%) in stage 1 disease. In the same stage, the sensitivity increases from 14.1% in patients with a single erythema migrans lesion and early localized infection to 65.4% in those with multiple lesions. The algorithm has excellent sensitivity in late stage 3 infection (96% to 100%).5
A 2-step ELISA algorithm
A 2-step ELISA algorithm (without the Western blot) that includes the whole-cell sonicate assay followed by the VisE C6 peptide assay actually showed higher sensitivity and comparable specificity compared with 2-tiered testing in early localized disease (sensitivity 61%–74% vs 29%–48%, respectively; specificity 99.5% for both methods).22 This higher sensitivity was even more pronounced in early disseminated infection (sensitivity 100% vs 40%, respectively). By late infection, the sensitivities of both testing strategies reached 100%. Compared with the Western blot, the 2-step ELISA algorithm was simpler to execute in a reproducible fashion.5
The Infectious Diseases Society of America is revising its current guidelines, with an update expected late this year, which may shift the recommendation from 2-tiered testing to the 2-step ELISA algorithm.
Multiplex testing
To overcome the intrinsic problems of protein-based assays, a multiplexed, array-based assay for the diagnosis of tick-borne infections called Tick-Borne Disease Serochip (TBD-Serochip) was established using recombinant antigens that identify key immunodominant epitopes.8 More studies are needed to establish the validity and usefulness of these tests in clinical practice.
Who should not be tested?
The American College of Physicians6 recommends against testing in patients:
- Presenting with nonspecific symptoms (eg, headache, myalgia, fatigue, arthralgia) without objective signs of Lyme disease
- With low pretest probability of infection based on epidemiologic exposures and clinical features
- Living in Lyme-endemic areas with no history of tick exposure6
- Presenting less than 1 week after tick exposure5
- Seeking a test of cure for treated Lyme disease.
DIAGNOSIS IN SPECIAL SITUATIONS
Early Lyme disease
The classic erythema migrans lesion on physical examination of a patient with suspected Lyme disease is diagnostic and does not require laboratory confirmation.10
In ambiguous cases, 2-tiered testing of a serum sample during the acute presentation and again 4 to 6 weeks later can be useful. In patients who remain seronegative on paired serum samples despite symptoms lasting longer than 6 weeks and no antibiotic treatment in the interim, the diagnosis of Lyme disease is unlikely, and another diagnosis should be sought.3
Antimicrobial therapy may block the serologic response; hence, negative serologic testing in patients started on empiric antibiotics should not rule out Lyme disease.6
PCR or bacterial culture testing is not recommended in the evaluation of suspected early Lyme disease.
Central nervous system Lyme disease
Central nervous system Lyme disease is diagnosed by 2-tiered testing using peripheral blood samples because all patients with this infectious manifestation should have mounted an adequate IgG response in the blood.11
B cells migrate to and proliferate inside the central nervous system, leading to intrathecal production of anti-Borrelia antibodies. An index of cerebrospinal fluid to serum antibody greater than 1 is thus also indicative of neuroborreliosis.12 Thus, performing lumbar puncture to detect intrathecal production of antibodies may support the diagnosis of central nervous system Lyme disease; however, it is not necessary.11
Antibodies persist in the central nervous system for many years after appropriate antimicrobial treatment.
Lyme arthritis
Articular involvement in Lyme disease is characterized by a robust humoral response such that a negative IgG serologic test virtually rules out Lyme arthritis.23 PCR testing of synovial fluid for borrelial DNA has a sensitivity of 80% but may become falsely negative after 1 to 2 months of antibiotic treatment.24,25 In an algorithm suggested by Puius et al,23 PCR testing of synovial fluid should be done in patients who have minimal to no response after 2 months of appropriate oral antimicrobial therapy to determine whether intravenous antibiotics are merited.
Table 5 summarizes the tests of choice in different clinical stages of infection.
Acknowledgment: The authors would like to acknowledge Anita Modi, MD, and Ceena N. Jacob, MD, for reviewing the manuscript and providing valuable suggestions, and Belinda Yen-Lieberman, PhD, for contributing pictures of the Western blot test results.
Lyme disease is a complex multisystem bacterial infection affecting the skin, joints, heart, and nervous system. The full spectrum of disease was first recognized and the disease was named in the 1970s during an outbreak of arthritis in children in the town of Lyme, Connecticut.1
This review describes the epidemiology and pathogenesis of Lyme disease, the advantages and disadvantages of current diagnostic methods, and diagnostic algorithms.
THE MOST COMMON TICK-BORNE INFECTION IN NORTH AMERICA
Lyme disease is the most common tick-borne infection in North America.2,3 In the United States, more than 30,000 cases are reported annually. In fact, in 2017, the number of cases was about 42,000, a 16% increase from the previous year, according to the US Centers for Disease Control and Prevention (CDC).
Infected nymphs account for most cases.
The infection is caused by Borrelia burgdorferi, a particularly arthritogenic spirochete transmitted by Ixodes scapularis (the black-legged deer tick, (Figure 1) and Ixodes pacificus (the Western black-legged tick). Although the infection can occur at any time of the year, its peak incidence is in May to late September, coinciding with increased outdoor recreational activity in areas where ticks live.3,4 The typical tick habitat consists of deciduous woodland with sufficient humidity provided by a good layer of decaying vegetation. However, people can contract Lyme disease in their own backyard.3
Most cases of Lyme disease are seen in the northeastern United States, mainly in suburban and rural areas.2,3 Other areas affected include the midwestern states of Minnesota, Wisconsin, and Michigan, as well as northern California.4 Fourteen states and the District of Columbia report a high average incidence (> 10 cases per 100,000 persons) (Table 1).2
FIRST COMES IgM, THEN IgG
The pathogenesis and the different stages of infection should inform laboratory testing in Lyme disease.
It is estimated that only 5% of infected ticks that bite people actually transmit their spirochetes to the human host.5 However, once infected, the patient’s innate immune system mounts a response that results in the classic erythema migrans rash at the bite site. A rash develops in only about 85% of patients who are infected and can appear at any time between 3 and 30 days, but most commonly after 7 days. Hence, a rash occurring within the first few hours of tick contact is not erythema migrans and does not indicate infection, but rather an early reaction to tick salivary antigens.5
Antibody levels remain below the detection limits of currently available serologic tests in the first 7 days after exposure. Immunoglobulin M (IgM) antibody titers peak between 8 and 14 days after tick contact, but IgM antibodies may never develop if the patient is started on early appropriate antimicrobial therapy.5
If the infection is not treated, the spirochete may disseminate through the blood from the bite site to different tissues.3 Both cell-mediated and antibody-mediated immunity swing into action to kill the spirochetes at this stage. The IgM antibody response occurs in 1 to 2 weeks, followed by a robust IgG response in 2 to 4 weeks.6
Because IgM can also cross-react with antigens other than those associated with B burgdorferi, the IgM test is less specific than the IgG test for Lyme disease.
Once a patient is exposed and mounts an antibody-mediated response to the spirochete, the antibody profile may persist for months to years, even after successful antibiotic treatment and cure of the disease.5
Despite the immune system’s robust series of defenses, untreated B burgdorferi infection can persist, as the organism has a bag of tricks to evade destruction. It can decrease its expression of specific immunogenic surface-exposed proteins, change its antigenic properties through recombination, and bind to the patient’s extracellular matrix proteins to facilitate further dissemination.3
Certain host-genetic factors also play a role in the pathogenesis of Lyme disease, such as the HLA-DR4 allele, which has been associated with antibiotic-refractory Lyme-related arthritis.3
LYME DISEASE EVOLVES THROUGH STAGES
Lyme disease evolves through stages broadly classified as early and late infection, with significant variability in its presentation.7
Early infection
Early disease is further subdivided into “localized” infection (stage 1), characterized by a single erythema migrans lesion and local lymphadenopathy, and “disseminated” infection (stage 2), associated with multiple erythema migrans lesions distant from the bite site, facial nerve palsy, radiculoneuritis, meningitis, carditis, or migratory arthritis or arthralgia.8
Highly specific physical findings include erythema migrans, cranial nerve palsy, high-grade or progressive conduction block, and recurrent migratory polyarthritis. Less specific symptoms and signs of Lyme disease include arthralgia, myalgia, neck stiffness, palpitations, and myocarditis.5
Erythema migrans lesions are evident in at least 85% of patients with early disease.9 If they are not apparent on physical examination, they may be located at hidden sites and may be atypical in appearance or transient.5
If treatment is not started in the initial stage of the disease, 60% of infected patients may develop disseminated infection.5 Progressive, untreated infection can manifest with Lyme arthritis and neuroborreliosis.7
Noncutaneous manifestations are less common now than in the past due to increased awareness of the disease and early initiation of treatment.10
Late infection
Manifestations of late (stage 3) infection include oligoarthritis (affecting any joint but often the knee) and neuroborreliosis. Clinical signs and symptoms of Lyme disease may take months to resolve even after appropriate antimicrobial therapy is completed. This should not be interpreted as ongoing, persistent infection, but as related to host immune-mediated activity.5
INTERPRET LABORATORY RESULTS BASED ON PRETEST PROBABILITY
The usefulness of a laboratory test depends on the individual patient’s pretest probability of infection, which in turn depends on the patient’s epidemiologic risk of exposure and clinical features of Lyme disease. Patients with a high pretest probability—eg, a history of a tick bite followed by the classic erythema migrans rash—do not need testing and can start antimicrobial therapy right away.11
Serologic tests are the gold standard
Prompt diagnosis is important, as early Lyme disease is easily treatable without any future sequelae.11
Tests for Lyme disease can be divided into direct methods, which detect the spirochete itself by culture or by polymerase chain reaction (PCR), and indirect methods, which detect antibodies (Table 2). Direct tests lack sensitivity for Lyme disease; hence, serologic tests remain the gold standard. Currently recommended is a standard 2-tier testing strategy using an enzyme-linked immunosorbent assay (ELISA) followed by Western blot for confirmation.
DIRECT METHODS
Culture lacks sensitivity
A number of factors limit the sensitivity of direct culture for diagnosing Lyme disease. B burgdorferi does not grow easily in culture, requiring special media, low temperatures, and long periods of incubation. Only a relatively few spirochetes are present in human tissues and body fluids to begin with, and bacterial counts are further reduced with duration and dissemination of infection.5 All of these limit the possibility of detecting this organism.
Polymerase chain reaction may help in some situations
Molecular assays are not part of the standard evaluation and should be used only in conjunction with serologic testing.7 These tests have high specificity but lack consistent sensitivity.
That said, PCR testing may be useful:
- In early infection, before antibody responses develop
- In reinfection, when serologic tests are not reliable because the antibodies persist for many years after an infection in many patients
- In endemic areas where serologic testing has high false-positive rates due to high baseline population seropositivity for anti-Borrelia antibodies caused by subclinical infection.3
PCR assays that target plasmid-borne genes encoding outer surface proteins A and C (OspA and OspC) and VisE (variable major protein-like sequence, expressed) are more sensitive than those that detect chromosomal 16s ribosomal ribonucleic acid (rRNA) genes, as plasmid-rich “blebs” are shed in larger concentrations than chromosomal DNA during active infection.7 However, these plasmid-contained genes persist in body tissues and fluids even after the infection is cleared, and their detection may not necessarily correlate with ongoing disease.8 Detection of chromosomal 16s rRNA genes is a better predictor of true organism viability.
The sensitivity of PCR for borrelial DNA depends on the type of sample. If a skin biopsy sample is taken of the leading edge of an erythema migrans lesion, the sensitivity is 69% and the specificity is 100%. In patients with Lyme arthritis, PCR of the synovial fluid has a sensitivity of up to 80%. However, the sensitivity of PCR of the cerebrospinal fluid of patients with neurologic manifestations of Lyme disease is only 19%.7 PCR of other clinical samples, including blood and urine, is not recommended, as spirochetes are primarily confined to tissues, and very few are present in these body fluids.3,12
The disadvantage of PCR is that a positive result does not always mean active infection, as the DNA of the dead microbe persists for several months even after successful treatment.8
INDIRECT METHODS
Enzyme-linked immunosorbent assay
ELISAs detect anti-Borrelia antibodies. Early-generation ELISAs, still used in many laboratories, use whole-cell extracts of B burgdorferi. Examples are the Vidas Lyme screen (Biomérieux, biomerieux-usa.com) and the Wampole B burgdorferi IgG/M EIA II assay (Alere, www.alere.com). Newer ELISAs use recombinant proteins.13
Three major targets for ELISA antibodies are flagellin (Fla), outer surface protein C (OspC), and VisE, especially the invariable region 6 (IR6). Among these, VisE-IR6 is the most conserved region in B burgdorferi.
Early-generation assays have a sensitivity of 89% and specificity of 72%.11 However, the patient’s serum may have antibodies that cross-react with unrelated bacterial antigens, leading to false-positive results (Table 3). Whole-cell sonicate assays are not recommended as an independent test and must be confirmed with Western blot testing when assay results are indeterminate or positive.11
Newer-generation ELISAs detect antibodies targeting recombinant proteins of VisE, especially a synthetic peptide C6, within IR6.13 VisE-IR6 is the most conserved region of the B burgdorferi complex, and its detection is a highly specific finding, supporting the diagnosis of Lyme disease. Antibodies against VisE-IR6 antigen are the earliest to develop.5 An example of a newer-generation serologic test is the VisE C6 Lyme EIA kit, approved as a first-tier test by the US Food and Drug Administration in 2001. This test has a specificity of 99%,14,15 and its specificity is further increased when used in conjunction with Western blot (99.5%).15 The advantage of the C6 antibody test is that it is more sensitive than 2-tier testing during early infection (sensitivity 29%–74% vs 17%–40% in early localized infection, and 56%–90% vs 27%–78% in early disseminated infection).6
During early infection, older and newer ELISAs are less sensitive because of the limited number of antigens expressed at this stage.13 All patients suspected of having early Lyme disease who are seronegative at initial testing should have follow-up testing to look for seroconversion.13
Western blot
Western blot (immunoblot) testing identifies IgM and IgG antibodies against specific B burgdorferi antigens. It is considered positive if it detects at least 2 of a possible 3 specific IgM bands in the first 4 weeks of disease or at least 5 of 10 specific IgG bands after 4 weeks of disease (Table 4 and Figure 2).16
The nature of the bands indicates the duration of infection: Western blot bands against 23-kD OspC and 41-kD FlaB are seen in early localized infection, whereas bands against all 3 B burgdorferi proteins will be seen after several weeks of disease.17 The IgM result should be interpreted carefully, as only 2 bands are required for the test to be positive, and IgM binds to antigen less specifically than IgG.12
Interpreting the IgM Western blot test: The ‘1-month rule’
If clinical symptoms and signs of Lyme disease have been present for more than 1 month, IgM reactivity alone should not be used to support the diagnosis, in view of the likelihood of a false-positive test result in this situation.18 This is called the “1-month rule” in the diagnosis of Lyme disease.13
In early localized infection, Western blot is only half as sensitive as ELISA testing. Since the overall sensitivity of a 2-step algorithm is equal to that of its least sensitive component, 2-tiered testing is not useful in early disease.13
Although currently considered the most specific test for confirmation of Lyme disease, Western blot has limitations. It is technically and interpretively complex and is thus not universally available.13 The blots are scored by visual examination, compromising the reproducibility of the test, although densitometric blot analysis techniques and automated scanning and scoring attempt to address some of these limitations.13 Like the ELISA, Western blot can have false-positive results in healthy individuals without tick exposure, as nonspecific IgM immunoblots develop faint bands. This is because of cross-reaction between B burgdorferi antigens and antigens from other microorganisms. Around 50% of healthy adults show low-level serum IgG reactivity against the FlaB antigen, leading to false-positive results as well. In cases in which the Western blot result is indeterminate, other etiologies must be considered.
False-positive IgM Western blots are a significant problem. In a 5-year retrospective study done at 63 US Air Force healthcare facilities, 113 (53.3%) of 212 IgM Western blots were falsely positive.19 A false-positive test was defined as one that failed to meet seropositivity (a first-tier test omitted or negative, > 30 days of symptoms with negative IgG blot), lack of exposure including residing in areas without documented tick habitats, patients having atypical or no symptoms, and negative serology within 30 days of a positive test.
In a similar study done in a highly endemic area, 50 (27.5%) of 182 patients had a false-positive test.20 Physicians need to be careful when interpreting IgM Western blots. It is always important to consider locale, epidemiology, and symptoms when interpreting the test.
Limitations of serologic tests for Lyme disease
Currently available serologic tests have inherent limitations:
- Antibodies against B burgdorferi take at least 1 week to develop
- The background rate of seropositivity in endemic areas can be up to 4%, affecting the utility of a positive test result
- Serologic tests cannot be used as tests of cure because antibodies can persist for months to years even after appropriate antimicrobial therapy and cure of disease; thus, a positive serologic result could represent active infection or remote exposure21
- Antibodies can cross-react with related bacteria, including other borrelial or treponemal spirochetes
- False-positive serologic test results can also occur in association with other medical conditions such as polyclonal gammopathies and systemic lupus erythematosus.12
RECOMMENDATIONS FOR TESTING
Standard 2-tier testing
The CDC released recommendations for diagnosing Lyme disease after a second national conference of serologic diagnosis of Lyme disease in October 1994.18 The 2-tiered testing method, involving a sensitive ELISA followed by the Western blot to confirm positive and indeterminate ELISA results, was suggested as the gold standard for diagnosis (Figure 3). Of note, negative ELISA results do not require further testing.11
The sensitivity of 2-tiered testing depends on the stage of the disease. Unfortunately, this method has a wide range of sensitivity (17% to 78%) in stage 1 disease. In the same stage, the sensitivity increases from 14.1% in patients with a single erythema migrans lesion and early localized infection to 65.4% in those with multiple lesions. The algorithm has excellent sensitivity in late stage 3 infection (96% to 100%).5
A 2-step ELISA algorithm
A 2-step ELISA algorithm (without the Western blot) that includes the whole-cell sonicate assay followed by the VisE C6 peptide assay actually showed higher sensitivity and comparable specificity compared with 2-tiered testing in early localized disease (sensitivity 61%–74% vs 29%–48%, respectively; specificity 99.5% for both methods).22 This higher sensitivity was even more pronounced in early disseminated infection (sensitivity 100% vs 40%, respectively). By late infection, the sensitivities of both testing strategies reached 100%. Compared with the Western blot, the 2-step ELISA algorithm was simpler to execute in a reproducible fashion.5
The Infectious Diseases Society of America is revising its current guidelines, with an update expected late this year, which may shift the recommendation from 2-tiered testing to the 2-step ELISA algorithm.
Multiplex testing
To overcome the intrinsic problems of protein-based assays, a multiplexed, array-based assay for the diagnosis of tick-borne infections called Tick-Borne Disease Serochip (TBD-Serochip) was established using recombinant antigens that identify key immunodominant epitopes.8 More studies are needed to establish the validity and usefulness of these tests in clinical practice.
Who should not be tested?
The American College of Physicians6 recommends against testing in patients:
- Presenting with nonspecific symptoms (eg, headache, myalgia, fatigue, arthralgia) without objective signs of Lyme disease
- With low pretest probability of infection based on epidemiologic exposures and clinical features
- Living in Lyme-endemic areas with no history of tick exposure6
- Presenting less than 1 week after tick exposure5
- Seeking a test of cure for treated Lyme disease.
DIAGNOSIS IN SPECIAL SITUATIONS
Early Lyme disease
The classic erythema migrans lesion on physical examination of a patient with suspected Lyme disease is diagnostic and does not require laboratory confirmation.10
In ambiguous cases, 2-tiered testing of a serum sample during the acute presentation and again 4 to 6 weeks later can be useful. In patients who remain seronegative on paired serum samples despite symptoms lasting longer than 6 weeks and no antibiotic treatment in the interim, the diagnosis of Lyme disease is unlikely, and another diagnosis should be sought.3
Antimicrobial therapy may block the serologic response; hence, negative serologic testing in patients started on empiric antibiotics should not rule out Lyme disease.6
PCR or bacterial culture testing is not recommended in the evaluation of suspected early Lyme disease.
Central nervous system Lyme disease
Central nervous system Lyme disease is diagnosed by 2-tiered testing using peripheral blood samples because all patients with this infectious manifestation should have mounted an adequate IgG response in the blood.11
B cells migrate to and proliferate inside the central nervous system, leading to intrathecal production of anti-Borrelia antibodies. An index of cerebrospinal fluid to serum antibody greater than 1 is thus also indicative of neuroborreliosis.12 Thus, performing lumbar puncture to detect intrathecal production of antibodies may support the diagnosis of central nervous system Lyme disease; however, it is not necessary.11
Antibodies persist in the central nervous system for many years after appropriate antimicrobial treatment.
Lyme arthritis
Articular involvement in Lyme disease is characterized by a robust humoral response such that a negative IgG serologic test virtually rules out Lyme arthritis.23 PCR testing of synovial fluid for borrelial DNA has a sensitivity of 80% but may become falsely negative after 1 to 2 months of antibiotic treatment.24,25 In an algorithm suggested by Puius et al,23 PCR testing of synovial fluid should be done in patients who have minimal to no response after 2 months of appropriate oral antimicrobial therapy to determine whether intravenous antibiotics are merited.
Table 5 summarizes the tests of choice in different clinical stages of infection.
Acknowledgment: The authors would like to acknowledge Anita Modi, MD, and Ceena N. Jacob, MD, for reviewing the manuscript and providing valuable suggestions, and Belinda Yen-Lieberman, PhD, for contributing pictures of the Western blot test results.
- Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 1977; 20(1):7–17. doi:10.1002/art.1780200102
- Centers for Disease Control and Prevention (CDC). Lyme disease: recent surveillance data. https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html. Accessed August 12, 2019.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet 2012; 379(9814):461–473. doi:10.1016/S0140-6736(11)60103-7
- Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015; 29(2):269–280. doi:10.1016/j.idc.2015.02.004
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med 2015; 35(4):797–814. doi:10.1016/j.cll.2015.08.001
- Hu LT. Lyme disease. Ann Intern Med 2016; 164(9):ITC65–ITC80. doi:10.7326/AITC201605030
- Alby K, Capraro GA. Alternatives to serologic testing for diagnosis of Lyme disease. Clin Lab Med 2015; 35(4):815–825. doi:10.1016/j.cll.2015.07.005
- Dumler JS. Molecular diagnosis of Lyme disease: review and meta-analysis. Mol Diagn 2001; 6(1):1–11. doi:10.1054/modi.2001.21898
- Wormser GP, McKenna D, Carlin J, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med 2005; 142(9):751–755. doi:10.7326/0003-4819-142-9-200505030-00011
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43(9):1089–1134. doi:10.1086/508667
- Guidelines for laboratory evaluation in the diagnosis of Lyme disease. American College of Physicians. Ann Intern Med 1997; 127(12):1106–1108. doi:10.7326/0003-4819-127-12-199712150-00010
- Halperin JJ. Lyme disease: a multisystem infection that affects the nervous system. Continuum (Minneap Minn) 2012; 18(6 Infectious Disease):1338–1350. doi:10.1212/01.CON.0000423850.24900.3a
- Branda JA, Body BA, Boyle J, et al. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 2018; 66(7):1133–1139. doi:10.1093/cid/cix943
- Immunetics. Immunetics® C6 Lyme ELISA™ Kit. http://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/CF-E601-096A-C6-Pkg-Insrt.pdf. Accessed August 12, 2019.
- Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet 2014; 15(1):34–48. doi:10.1038/nrg3575
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44(31):590–591. pmid:7623762
- Steere AC, Mchugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 2008; 47(2):188–195. doi:10.1086/589242
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA 1995; 274(12):937. pmid:7674514
- Webber BJ, Burganowski RP, Colton L, Escobar JD, Pathak SR, Gambino-Shirley KJ. Lyme disease overdiagnosis in a large healthcare system: a population-based, retrospective study. Clin Microbiol Infect 2019. doi:10.1016/j.cmi.2019.02.020. Epub ahead of print.
- Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18(12):1236–1240. doi:10.1111/j.1469-0691.2011.03749.x
- Hilton E, DeVoti J, Benach JL, et al. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am J Med 1999; 106(4):404–409. doi:10.1016/s0002-9343(99)00046-7
- Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 2011; 53(6):541–547. doi:10.1093/cid/cir464
- Puius YA, Kalish RA. Lyme arthritis: pathogenesis, clinical presentation, and management. Infect Dis Clin North Am 2008; 22(2):289–300. doi:10.1016/j.idc.2007.12.014
- Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 1994; 330(4):229–234. doi:10.1056/NEJM199401273300401
- Liebling MR, Nishio MJ, Rodriguez A, Sigal LH, Jin T, Louie JS. The polymerase chain reaction for the detection of Borrelia burgdorferi in human body fluids. Arthritis Rheum 1993; 36(5):665–975. doi:10.1002/art.1780360514
- Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 1977; 20(1):7–17. doi:10.1002/art.1780200102
- Centers for Disease Control and Prevention (CDC). Lyme disease: recent surveillance data. https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html. Accessed August 12, 2019.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet 2012; 379(9814):461–473. doi:10.1016/S0140-6736(11)60103-7
- Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015; 29(2):269–280. doi:10.1016/j.idc.2015.02.004
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med 2015; 35(4):797–814. doi:10.1016/j.cll.2015.08.001
- Hu LT. Lyme disease. Ann Intern Med 2016; 164(9):ITC65–ITC80. doi:10.7326/AITC201605030
- Alby K, Capraro GA. Alternatives to serologic testing for diagnosis of Lyme disease. Clin Lab Med 2015; 35(4):815–825. doi:10.1016/j.cll.2015.07.005
- Dumler JS. Molecular diagnosis of Lyme disease: review and meta-analysis. Mol Diagn 2001; 6(1):1–11. doi:10.1054/modi.2001.21898
- Wormser GP, McKenna D, Carlin J, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med 2005; 142(9):751–755. doi:10.7326/0003-4819-142-9-200505030-00011
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43(9):1089–1134. doi:10.1086/508667
- Guidelines for laboratory evaluation in the diagnosis of Lyme disease. American College of Physicians. Ann Intern Med 1997; 127(12):1106–1108. doi:10.7326/0003-4819-127-12-199712150-00010
- Halperin JJ. Lyme disease: a multisystem infection that affects the nervous system. Continuum (Minneap Minn) 2012; 18(6 Infectious Disease):1338–1350. doi:10.1212/01.CON.0000423850.24900.3a
- Branda JA, Body BA, Boyle J, et al. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 2018; 66(7):1133–1139. doi:10.1093/cid/cix943
- Immunetics. Immunetics® C6 Lyme ELISA™ Kit. http://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/CF-E601-096A-C6-Pkg-Insrt.pdf. Accessed August 12, 2019.
- Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet 2014; 15(1):34–48. doi:10.1038/nrg3575
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44(31):590–591. pmid:7623762
- Steere AC, Mchugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 2008; 47(2):188–195. doi:10.1086/589242
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA 1995; 274(12):937. pmid:7674514
- Webber BJ, Burganowski RP, Colton L, Escobar JD, Pathak SR, Gambino-Shirley KJ. Lyme disease overdiagnosis in a large healthcare system: a population-based, retrospective study. Clin Microbiol Infect 2019. doi:10.1016/j.cmi.2019.02.020. Epub ahead of print.
- Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18(12):1236–1240. doi:10.1111/j.1469-0691.2011.03749.x
- Hilton E, DeVoti J, Benach JL, et al. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am J Med 1999; 106(4):404–409. doi:10.1016/s0002-9343(99)00046-7
- Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 2011; 53(6):541–547. doi:10.1093/cid/cir464
- Puius YA, Kalish RA. Lyme arthritis: pathogenesis, clinical presentation, and management. Infect Dis Clin North Am 2008; 22(2):289–300. doi:10.1016/j.idc.2007.12.014
- Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 1994; 330(4):229–234. doi:10.1056/NEJM199401273300401
- Liebling MR, Nishio MJ, Rodriguez A, Sigal LH, Jin T, Louie JS. The polymerase chain reaction for the detection of Borrelia burgdorferi in human body fluids. Arthritis Rheum 1993; 36(5):665–975. doi:10.1002/art.1780360514
KEY POINTS
- Lyme disease, the most common tick-borne infection in North America, is a complex multisystem bacterial disease caused by Borrelia burgdorferi.
- Lyme disease preferably affects the skin, joints, and nervous system and presents with typical and atypical features. Certain clinical features are diagnostic. Its diagnosis is mainly clinical and epidemiologic and, when doubtful, is supported by serologic testing.
- Standard 2-tiered testing is the diagnostic testing method of choice—enzyme-linked immunoassay followed by Western blot. Interpretation of the bands depends on the duration of infection.
- When interpreting the test results, be aware of false-positives and the reasons for them.
A link between A-fib and sleep apnea is no surprise, but why?
Is the relationship between A-fib and sleep apnea more than a coincidence stemming from the number of shared associated comorbidities? Significantly, the treatment of obstructive sleep apnea with continuous positive airway pressure (CPAP) has been shown to decrease the recurrence of A-fib after pharmacologic or electrical conversion and after interventional pulmonary vein interruption.1 This suggests that at least in some cases, sleep apnea plays an active role in initiating and possibly also maintaining A-fib. The immediate culprit mediators that come to mind are hypoxia and hypercapnea; both are at least partially ameliorated by the successful use of CPAP, and both are reasonable physiologic candidates for induction of A-fib. Hypoxia is supported by clinical observation, and hypercapnea by experimental modeling.2
It is easy for clinicians to conceptualize the organ effects of hypoxia and hypercapnea. We are accustomed to seeing clinical ramifications of these in the emergency department and intensive care unit, particularly those affecting the brain and heart, organs critically dependent on transmembrane ion flow. We may recall from biochemistry classes the effects of hypoxia on intracellular metabolism and the implications on energy stores, mitochondrial function, and ion translocation. Recent work on the cellular effects of hypoxia, including research that resulted in a Nobel prize, has drawn major attention to patterned cellular responses to intermittent and persistent hypoxia. This includes recognition of epigenetic changes resulting in localized cardiac remodeling and fibrosis,3 factors that clearly affect the expression of arrhythmias, including A-fib.
But the interrelationship between A-fib and sleep apnea may be even more convoluted and intriguing. It now seems that most things cardiac are associated with inflammation in some guise, and the A-fib connection with sleep apnea may not be an exception. Almost 20 years ago, it was recognized that A-fib is associated with an elevation in circulating C-reactive protein (CRP),4 a biomarker of “inflammation,” although not necessarily an active participant. Recent reviews of this connection have been published,5 and successful anti-inflammatory approaches to preventing A-fib using colchicine have been described.6 So how does this tie in with sleep apnea?
A number of papers have now demonstrated that sleep apnea is also associated with an elevation in CRP,7 perhaps due to increases in tumor necrosis factor (TNF)-alpha in response to the intermittent hypoxia of sleep apnea. TNF can drive the inflammatory response through increased expression of genes regulated by nuclear factor kappa-B.8 While it certainly warrants consideration that the elevated biomarkers of inflammation in patients with sleep apnea actually reflect the presence of the frequent comorbidities, including visceral obesity, treating sleep apnea with CPAP (comparable to what I noted above in patients with A-fib) has been shown to reduce circulating CRP levels.9
As our understanding of the biologic underpinnings of A-fib and sleep apnea continue to grow, the practical clinical implications of the relationship between them, as described by Ayache et al, may achieve greater clarity. The two conditions commonly coexist, and treating the sleep apnea results in better rhythm-directed outcomes in the A-fib.
Stay tuned, there is certainly more to learn about this.
- Shukla A, Aizer A, Holmes D, et al. Effect of sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC Clin Electropysiol 2015; 1(1–2):41–51. doi:10.1016/j.jacep.2015.02.014
- Stevenson IH, Roberts-Thomson KC, Kistler PM, et al. Atrial electrophysiology is altered by acute hypercapnea but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010; 7(9):1263–1270. doi:10.1016/j.hrthm.2010.03.020
- Zhang W, Song M, Qu J, Liu G. Epigenetic modifications in cardiovascular aging and diseases. Circ Res 2018; 123(7):773–786. doi:10.1161/CIRCRESAHA.118.312497
- Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001; 104(24):2886–2891. doi:10.1161/hc4901.101760
- Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol 2012; 60(22):2263–2270. doi:10.1016/j.jacc.2012.04.063
- Lee JZ, Singh N, Howe CL, et al. Colchicine for prevention of post-operative atrial fibrillation: a meta-analysis. JACC Clin Electrophysiol 2016; 2(1):78–85. doi:10.1016/j.jacep.2015.09.016
- Van der Touw T, Andronicos NM, Smart N. Is C-reactive protein elevated in obstructive sleep apnea? A systematic review and meta-analysis. Biomarkers 2019; 24(5):429–435. doi:10.1080/1354750X.2019.1600025
- Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnea syndrome? Thorax 2009; 64(7):631–636. doi:10.1136/thx.2008.105577
- Ishida K, Kato M, Kato Y, et al. Appropriate use of nasal continuous positive airway pressure decreases elevated C-reactive protein in patients with obstructive sleep apnea. Chest 2009; 136(1):125–129. doi:10.1378/chest.08-1431
Is the relationship between A-fib and sleep apnea more than a coincidence stemming from the number of shared associated comorbidities? Significantly, the treatment of obstructive sleep apnea with continuous positive airway pressure (CPAP) has been shown to decrease the recurrence of A-fib after pharmacologic or electrical conversion and after interventional pulmonary vein interruption.1 This suggests that at least in some cases, sleep apnea plays an active role in initiating and possibly also maintaining A-fib. The immediate culprit mediators that come to mind are hypoxia and hypercapnea; both are at least partially ameliorated by the successful use of CPAP, and both are reasonable physiologic candidates for induction of A-fib. Hypoxia is supported by clinical observation, and hypercapnea by experimental modeling.2
It is easy for clinicians to conceptualize the organ effects of hypoxia and hypercapnea. We are accustomed to seeing clinical ramifications of these in the emergency department and intensive care unit, particularly those affecting the brain and heart, organs critically dependent on transmembrane ion flow. We may recall from biochemistry classes the effects of hypoxia on intracellular metabolism and the implications on energy stores, mitochondrial function, and ion translocation. Recent work on the cellular effects of hypoxia, including research that resulted in a Nobel prize, has drawn major attention to patterned cellular responses to intermittent and persistent hypoxia. This includes recognition of epigenetic changes resulting in localized cardiac remodeling and fibrosis,3 factors that clearly affect the expression of arrhythmias, including A-fib.
But the interrelationship between A-fib and sleep apnea may be even more convoluted and intriguing. It now seems that most things cardiac are associated with inflammation in some guise, and the A-fib connection with sleep apnea may not be an exception. Almost 20 years ago, it was recognized that A-fib is associated with an elevation in circulating C-reactive protein (CRP),4 a biomarker of “inflammation,” although not necessarily an active participant. Recent reviews of this connection have been published,5 and successful anti-inflammatory approaches to preventing A-fib using colchicine have been described.6 So how does this tie in with sleep apnea?
A number of papers have now demonstrated that sleep apnea is also associated with an elevation in CRP,7 perhaps due to increases in tumor necrosis factor (TNF)-alpha in response to the intermittent hypoxia of sleep apnea. TNF can drive the inflammatory response through increased expression of genes regulated by nuclear factor kappa-B.8 While it certainly warrants consideration that the elevated biomarkers of inflammation in patients with sleep apnea actually reflect the presence of the frequent comorbidities, including visceral obesity, treating sleep apnea with CPAP (comparable to what I noted above in patients with A-fib) has been shown to reduce circulating CRP levels.9
As our understanding of the biologic underpinnings of A-fib and sleep apnea continue to grow, the practical clinical implications of the relationship between them, as described by Ayache et al, may achieve greater clarity. The two conditions commonly coexist, and treating the sleep apnea results in better rhythm-directed outcomes in the A-fib.
Stay tuned, there is certainly more to learn about this.
Is the relationship between A-fib and sleep apnea more than a coincidence stemming from the number of shared associated comorbidities? Significantly, the treatment of obstructive sleep apnea with continuous positive airway pressure (CPAP) has been shown to decrease the recurrence of A-fib after pharmacologic or electrical conversion and after interventional pulmonary vein interruption.1 This suggests that at least in some cases, sleep apnea plays an active role in initiating and possibly also maintaining A-fib. The immediate culprit mediators that come to mind are hypoxia and hypercapnea; both are at least partially ameliorated by the successful use of CPAP, and both are reasonable physiologic candidates for induction of A-fib. Hypoxia is supported by clinical observation, and hypercapnea by experimental modeling.2
It is easy for clinicians to conceptualize the organ effects of hypoxia and hypercapnea. We are accustomed to seeing clinical ramifications of these in the emergency department and intensive care unit, particularly those affecting the brain and heart, organs critically dependent on transmembrane ion flow. We may recall from biochemistry classes the effects of hypoxia on intracellular metabolism and the implications on energy stores, mitochondrial function, and ion translocation. Recent work on the cellular effects of hypoxia, including research that resulted in a Nobel prize, has drawn major attention to patterned cellular responses to intermittent and persistent hypoxia. This includes recognition of epigenetic changes resulting in localized cardiac remodeling and fibrosis,3 factors that clearly affect the expression of arrhythmias, including A-fib.
But the interrelationship between A-fib and sleep apnea may be even more convoluted and intriguing. It now seems that most things cardiac are associated with inflammation in some guise, and the A-fib connection with sleep apnea may not be an exception. Almost 20 years ago, it was recognized that A-fib is associated with an elevation in circulating C-reactive protein (CRP),4 a biomarker of “inflammation,” although not necessarily an active participant. Recent reviews of this connection have been published,5 and successful anti-inflammatory approaches to preventing A-fib using colchicine have been described.6 So how does this tie in with sleep apnea?
A number of papers have now demonstrated that sleep apnea is also associated with an elevation in CRP,7 perhaps due to increases in tumor necrosis factor (TNF)-alpha in response to the intermittent hypoxia of sleep apnea. TNF can drive the inflammatory response through increased expression of genes regulated by nuclear factor kappa-B.8 While it certainly warrants consideration that the elevated biomarkers of inflammation in patients with sleep apnea actually reflect the presence of the frequent comorbidities, including visceral obesity, treating sleep apnea with CPAP (comparable to what I noted above in patients with A-fib) has been shown to reduce circulating CRP levels.9
As our understanding of the biologic underpinnings of A-fib and sleep apnea continue to grow, the practical clinical implications of the relationship between them, as described by Ayache et al, may achieve greater clarity. The two conditions commonly coexist, and treating the sleep apnea results in better rhythm-directed outcomes in the A-fib.
Stay tuned, there is certainly more to learn about this.
- Shukla A, Aizer A, Holmes D, et al. Effect of sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC Clin Electropysiol 2015; 1(1–2):41–51. doi:10.1016/j.jacep.2015.02.014
- Stevenson IH, Roberts-Thomson KC, Kistler PM, et al. Atrial electrophysiology is altered by acute hypercapnea but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010; 7(9):1263–1270. doi:10.1016/j.hrthm.2010.03.020
- Zhang W, Song M, Qu J, Liu G. Epigenetic modifications in cardiovascular aging and diseases. Circ Res 2018; 123(7):773–786. doi:10.1161/CIRCRESAHA.118.312497
- Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001; 104(24):2886–2891. doi:10.1161/hc4901.101760
- Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol 2012; 60(22):2263–2270. doi:10.1016/j.jacc.2012.04.063
- Lee JZ, Singh N, Howe CL, et al. Colchicine for prevention of post-operative atrial fibrillation: a meta-analysis. JACC Clin Electrophysiol 2016; 2(1):78–85. doi:10.1016/j.jacep.2015.09.016
- Van der Touw T, Andronicos NM, Smart N. Is C-reactive protein elevated in obstructive sleep apnea? A systematic review and meta-analysis. Biomarkers 2019; 24(5):429–435. doi:10.1080/1354750X.2019.1600025
- Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnea syndrome? Thorax 2009; 64(7):631–636. doi:10.1136/thx.2008.105577
- Ishida K, Kato M, Kato Y, et al. Appropriate use of nasal continuous positive airway pressure decreases elevated C-reactive protein in patients with obstructive sleep apnea. Chest 2009; 136(1):125–129. doi:10.1378/chest.08-1431
- Shukla A, Aizer A, Holmes D, et al. Effect of sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC Clin Electropysiol 2015; 1(1–2):41–51. doi:10.1016/j.jacep.2015.02.014
- Stevenson IH, Roberts-Thomson KC, Kistler PM, et al. Atrial electrophysiology is altered by acute hypercapnea but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010; 7(9):1263–1270. doi:10.1016/j.hrthm.2010.03.020
- Zhang W, Song M, Qu J, Liu G. Epigenetic modifications in cardiovascular aging and diseases. Circ Res 2018; 123(7):773–786. doi:10.1161/CIRCRESAHA.118.312497
- Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001; 104(24):2886–2891. doi:10.1161/hc4901.101760
- Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol 2012; 60(22):2263–2270. doi:10.1016/j.jacc.2012.04.063
- Lee JZ, Singh N, Howe CL, et al. Colchicine for prevention of post-operative atrial fibrillation: a meta-analysis. JACC Clin Electrophysiol 2016; 2(1):78–85. doi:10.1016/j.jacep.2015.09.016
- Van der Touw T, Andronicos NM, Smart N. Is C-reactive protein elevated in obstructive sleep apnea? A systematic review and meta-analysis. Biomarkers 2019; 24(5):429–435. doi:10.1080/1354750X.2019.1600025
- Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnea syndrome? Thorax 2009; 64(7):631–636. doi:10.1136/thx.2008.105577
- Ishida K, Kato M, Kato Y, et al. Appropriate use of nasal continuous positive airway pressure decreases elevated C-reactive protein in patients with obstructive sleep apnea. Chest 2009; 136(1):125–129. doi:10.1378/chest.08-1431
Imaging reveals different clinico-pathologic patterns in Takayasu’s, giant cell arteritis
a retrospective cohort study has found.
“Clinical symptoms were not sensitive markers of underlying vascular pathology but were specific when present,” Despina Michailidou, MD, PhD, and colleagues wrote in Annals of the Rheumatic Diseases. “Vascular imaging should be considered in the management of these patients since reliance on the presence of clinical symptoms may not be sensitive to detect vascular pathology within an acceptable window to prevent or minimize damage.”
Dr. Michailidou and coauthors in the Systemic Autoimmunity Branch of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) examined the relationships between clinical presentation and imaging findings in 110 patients involved in an ongoing observational cohort study at the National Institutes of Health, including 56 with Takayasu’s arteritis (TAK) and 54 with giant cell arteritis (GCA). The study included data from 270 visits. Dr. Michailidou conducted the study while she was a research fellow at NIAMS, and she is now a rheumatology fellow at the University of Washington, Seattle.
The team looked at 11 symptoms (lightheadedness, positional lightheadedness, carotidynia, arm claudication vertigo, frontotemporal and posterior headache, posterior neck pain, blurred vision, vision loss, and major CNS events, including stroke, transient ischemic attack, or syncope). These were related to findings on MR angiography (MRA) and 18F-fluorodeoxyglucose PET (FDG-PET).
There were no significant between-group differences in six of the symptoms. However, those with TAK had significantly higher rates of carotidynia (21% vs. 0%), lightheadedness (30% vs. 9%), positional lightheadedness (29% vs. 5%), major CNS events (25% vs. 9%), and arm claudication (52% vs. 28%). Arm claudication was the most common symptom in those with TAK (52%), and blurred vision the most common in patients with GCA (37%).
On the day of evaluation, 8% of patients with TAK reported carotidynia; none of the GCA patients reported this. On FDG-PET, carotidynia was more strongly associated with inflammation of the carotid artery than with damage of the carotid artery on MRA.
The sensitivity of this association was low, which indicates “that an absence of carotidynia could still be associated with imaging abnormalities in the carotid artery, particularly on MRA compared with FDG-PET,” the authors wrote. But specificity was high for both FDG-PET and MRA, suggesting that carotidynia was strongly associated with corresponding carotid artery abnormalities on both FDG-PET and MRA.
More of those with GCA than those with TAK reported posterior neck pain (18% vs. 7%). It was significantly associated with vertebral artery inflammation in those with GCA, but not in those with TAK. There was no significant association with vertebral artery damage in either group.
While sensitivity was low for posterior neck pain and imaging abnormalities, specificity was very high in both groups, which indicates “the presence of posterior neck pain was strongly associated with corresponding vertebral artery abnormalities on both FDG-PET and MRA.”
Posterior headache was present in 5% of GCA patients and was significantly associated with vertebral artery damage, but it was not associated with such damage in patients with TAK.
“While posterior headaches in the occipital region are uncommon in patients with GCA, this study emphasizes that presence of a posterior headache should alert the clinician to the likelihood of associated vascular inflammation and damage in the corresponding vertebral artery,” the researchers wrote.
About 6% of patients with TAK and 10% of those with GCA reported frontotemporal headache. The headache was not associated with carotid PET activity or damage in either group of patients.
“While frontotemporal headaches frequently occur in patients with TAK, and are a cardinal feature of GCA, headaches in this region may reflect inflammation in smaller branches of cranial arteries, rather than the corresponding larger arteries of the neck,” the investigators wrote.
Arm claudication was the most commonly reported symptom overall, present in 52% of those with TAK and 28% of those with GCA. It was more strongly associated with subclavian artery damage on MRA than with inflammation on FDG-PET.
The investigators also assessed the association between specific clinical symptoms and the number of affected neck arteries. Patients with large vessel vasculitis and an increased number of damaged neck arteries on MRA were significantly more likely to experience lightheadedness (odds ratio, 2.61), positional lightheadedness (OR, 3.51), or a major CNS event (OR, 3.23). But those with large vessel vasculitis and inflamed neck arteries on FDG-PET were more likely to experience posterior headache (OR, 2.84).
The study isn’t intended to dictate how MRA and FDG-PET should be employed with these patients, the authors noted.
“Rather, these findings may help clinicians predict imaging pathology in specific vascular territories based on patient-reported symptoms and may inform which type of imaging modality would be the most useful to obtain in certain clinical scenarios, recognizing that additional sequences to detect wall morphology may augment the ability of MR-based assessments to detect vascular inflammation in addition to luminal damage.”
The Division of Intramural Research at NIAMS funded the research. The authors had no financial disclosures.
SOURCE: Michailidou D et al. Ann Rheum Dis. 2019 Oct 24. doi: 10.1136/annrheumdis-2019-216145.
a retrospective cohort study has found.
“Clinical symptoms were not sensitive markers of underlying vascular pathology but were specific when present,” Despina Michailidou, MD, PhD, and colleagues wrote in Annals of the Rheumatic Diseases. “Vascular imaging should be considered in the management of these patients since reliance on the presence of clinical symptoms may not be sensitive to detect vascular pathology within an acceptable window to prevent or minimize damage.”
Dr. Michailidou and coauthors in the Systemic Autoimmunity Branch of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) examined the relationships between clinical presentation and imaging findings in 110 patients involved in an ongoing observational cohort study at the National Institutes of Health, including 56 with Takayasu’s arteritis (TAK) and 54 with giant cell arteritis (GCA). The study included data from 270 visits. Dr. Michailidou conducted the study while she was a research fellow at NIAMS, and she is now a rheumatology fellow at the University of Washington, Seattle.
The team looked at 11 symptoms (lightheadedness, positional lightheadedness, carotidynia, arm claudication vertigo, frontotemporal and posterior headache, posterior neck pain, blurred vision, vision loss, and major CNS events, including stroke, transient ischemic attack, or syncope). These were related to findings on MR angiography (MRA) and 18F-fluorodeoxyglucose PET (FDG-PET).
There were no significant between-group differences in six of the symptoms. However, those with TAK had significantly higher rates of carotidynia (21% vs. 0%), lightheadedness (30% vs. 9%), positional lightheadedness (29% vs. 5%), major CNS events (25% vs. 9%), and arm claudication (52% vs. 28%). Arm claudication was the most common symptom in those with TAK (52%), and blurred vision the most common in patients with GCA (37%).
On the day of evaluation, 8% of patients with TAK reported carotidynia; none of the GCA patients reported this. On FDG-PET, carotidynia was more strongly associated with inflammation of the carotid artery than with damage of the carotid artery on MRA.
The sensitivity of this association was low, which indicates “that an absence of carotidynia could still be associated with imaging abnormalities in the carotid artery, particularly on MRA compared with FDG-PET,” the authors wrote. But specificity was high for both FDG-PET and MRA, suggesting that carotidynia was strongly associated with corresponding carotid artery abnormalities on both FDG-PET and MRA.
More of those with GCA than those with TAK reported posterior neck pain (18% vs. 7%). It was significantly associated with vertebral artery inflammation in those with GCA, but not in those with TAK. There was no significant association with vertebral artery damage in either group.
While sensitivity was low for posterior neck pain and imaging abnormalities, specificity was very high in both groups, which indicates “the presence of posterior neck pain was strongly associated with corresponding vertebral artery abnormalities on both FDG-PET and MRA.”
Posterior headache was present in 5% of GCA patients and was significantly associated with vertebral artery damage, but it was not associated with such damage in patients with TAK.
“While posterior headaches in the occipital region are uncommon in patients with GCA, this study emphasizes that presence of a posterior headache should alert the clinician to the likelihood of associated vascular inflammation and damage in the corresponding vertebral artery,” the researchers wrote.
About 6% of patients with TAK and 10% of those with GCA reported frontotemporal headache. The headache was not associated with carotid PET activity or damage in either group of patients.
“While frontotemporal headaches frequently occur in patients with TAK, and are a cardinal feature of GCA, headaches in this region may reflect inflammation in smaller branches of cranial arteries, rather than the corresponding larger arteries of the neck,” the investigators wrote.
Arm claudication was the most commonly reported symptom overall, present in 52% of those with TAK and 28% of those with GCA. It was more strongly associated with subclavian artery damage on MRA than with inflammation on FDG-PET.
The investigators also assessed the association between specific clinical symptoms and the number of affected neck arteries. Patients with large vessel vasculitis and an increased number of damaged neck arteries on MRA were significantly more likely to experience lightheadedness (odds ratio, 2.61), positional lightheadedness (OR, 3.51), or a major CNS event (OR, 3.23). But those with large vessel vasculitis and inflamed neck arteries on FDG-PET were more likely to experience posterior headache (OR, 2.84).
The study isn’t intended to dictate how MRA and FDG-PET should be employed with these patients, the authors noted.
“Rather, these findings may help clinicians predict imaging pathology in specific vascular territories based on patient-reported symptoms and may inform which type of imaging modality would be the most useful to obtain in certain clinical scenarios, recognizing that additional sequences to detect wall morphology may augment the ability of MR-based assessments to detect vascular inflammation in addition to luminal damage.”
The Division of Intramural Research at NIAMS funded the research. The authors had no financial disclosures.
SOURCE: Michailidou D et al. Ann Rheum Dis. 2019 Oct 24. doi: 10.1136/annrheumdis-2019-216145.
a retrospective cohort study has found.
“Clinical symptoms were not sensitive markers of underlying vascular pathology but were specific when present,” Despina Michailidou, MD, PhD, and colleagues wrote in Annals of the Rheumatic Diseases. “Vascular imaging should be considered in the management of these patients since reliance on the presence of clinical symptoms may not be sensitive to detect vascular pathology within an acceptable window to prevent or minimize damage.”
Dr. Michailidou and coauthors in the Systemic Autoimmunity Branch of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) examined the relationships between clinical presentation and imaging findings in 110 patients involved in an ongoing observational cohort study at the National Institutes of Health, including 56 with Takayasu’s arteritis (TAK) and 54 with giant cell arteritis (GCA). The study included data from 270 visits. Dr. Michailidou conducted the study while she was a research fellow at NIAMS, and she is now a rheumatology fellow at the University of Washington, Seattle.
The team looked at 11 symptoms (lightheadedness, positional lightheadedness, carotidynia, arm claudication vertigo, frontotemporal and posterior headache, posterior neck pain, blurred vision, vision loss, and major CNS events, including stroke, transient ischemic attack, or syncope). These were related to findings on MR angiography (MRA) and 18F-fluorodeoxyglucose PET (FDG-PET).
There were no significant between-group differences in six of the symptoms. However, those with TAK had significantly higher rates of carotidynia (21% vs. 0%), lightheadedness (30% vs. 9%), positional lightheadedness (29% vs. 5%), major CNS events (25% vs. 9%), and arm claudication (52% vs. 28%). Arm claudication was the most common symptom in those with TAK (52%), and blurred vision the most common in patients with GCA (37%).
On the day of evaluation, 8% of patients with TAK reported carotidynia; none of the GCA patients reported this. On FDG-PET, carotidynia was more strongly associated with inflammation of the carotid artery than with damage of the carotid artery on MRA.
The sensitivity of this association was low, which indicates “that an absence of carotidynia could still be associated with imaging abnormalities in the carotid artery, particularly on MRA compared with FDG-PET,” the authors wrote. But specificity was high for both FDG-PET and MRA, suggesting that carotidynia was strongly associated with corresponding carotid artery abnormalities on both FDG-PET and MRA.
More of those with GCA than those with TAK reported posterior neck pain (18% vs. 7%). It was significantly associated with vertebral artery inflammation in those with GCA, but not in those with TAK. There was no significant association with vertebral artery damage in either group.
While sensitivity was low for posterior neck pain and imaging abnormalities, specificity was very high in both groups, which indicates “the presence of posterior neck pain was strongly associated with corresponding vertebral artery abnormalities on both FDG-PET and MRA.”
Posterior headache was present in 5% of GCA patients and was significantly associated with vertebral artery damage, but it was not associated with such damage in patients with TAK.
“While posterior headaches in the occipital region are uncommon in patients with GCA, this study emphasizes that presence of a posterior headache should alert the clinician to the likelihood of associated vascular inflammation and damage in the corresponding vertebral artery,” the researchers wrote.
About 6% of patients with TAK and 10% of those with GCA reported frontotemporal headache. The headache was not associated with carotid PET activity or damage in either group of patients.
“While frontotemporal headaches frequently occur in patients with TAK, and are a cardinal feature of GCA, headaches in this region may reflect inflammation in smaller branches of cranial arteries, rather than the corresponding larger arteries of the neck,” the investigators wrote.
Arm claudication was the most commonly reported symptom overall, present in 52% of those with TAK and 28% of those with GCA. It was more strongly associated with subclavian artery damage on MRA than with inflammation on FDG-PET.
The investigators also assessed the association between specific clinical symptoms and the number of affected neck arteries. Patients with large vessel vasculitis and an increased number of damaged neck arteries on MRA were significantly more likely to experience lightheadedness (odds ratio, 2.61), positional lightheadedness (OR, 3.51), or a major CNS event (OR, 3.23). But those with large vessel vasculitis and inflamed neck arteries on FDG-PET were more likely to experience posterior headache (OR, 2.84).
The study isn’t intended to dictate how MRA and FDG-PET should be employed with these patients, the authors noted.
“Rather, these findings may help clinicians predict imaging pathology in specific vascular territories based on patient-reported symptoms and may inform which type of imaging modality would be the most useful to obtain in certain clinical scenarios, recognizing that additional sequences to detect wall morphology may augment the ability of MR-based assessments to detect vascular inflammation in addition to luminal damage.”
The Division of Intramural Research at NIAMS funded the research. The authors had no financial disclosures.
SOURCE: Michailidou D et al. Ann Rheum Dis. 2019 Oct 24. doi: 10.1136/annrheumdis-2019-216145.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: The associations between findings on MR angiography (MRA) and 18F-fluorodeoxyglucose (FDG) PET and differing clinical symptom presentations in patients with Takayasu’s arteritis and those with giant cell arteritis may be used to predict imaging pathology.
Major finding: Arm claudication was the most commonly reported symptom overall, present in 52% of those with Takayasu’s arteritis and 28% of those with giant cell arteritis. It was more strongly associated with subclavian artery damage on MRA than with inflammation on FDG-PET.
Study details: The cohort comprised 56 patients with Takayasu’s arteritis and 54 with giant cell arteritis who together had made 270 visits.
Disclosures: The Division of Intramural Research at the National Institute of Arthritis and Musculoskeletal and Skin Diseases funded the research. The investigators had no financial disclosures.
Source: Michailidou D et al. Ann Rheum Dis. 2019 Oct 24. doi: 10.1136/annrheumdis-2019-216145.
Psoriasis risk rises with TNF inhibitor use in children with inflammatory disorders
, a retrospective cohort study has determined.
“The incidence rate and risk factors of psoriasis in children with IBD [inflammatory bowel disease], JIA [juvenile idiopathic arthritis], or CNO [chronic nonbacterial osteomyelitis] who are exposed to TNFi [tumor necrosis factor inhibitors] are unknown. Additionally, there is a well-established association between these inflammatory conditions and psoriasis development. Yet, as TNFi can both treat and trigger psoriasis, it is not clear how TNFi exposure affects this relationship,” wrote Lisa H. Buckley, MD, of Children’s Hospital at Vanderbilt, Nashville, Tenn., and colleagues. Their report is in Arthritis Care & Research.
The team examined the relationship in children who were treated for an inflammatory disorder at Children’s Hospital of Philadelphia during 2008-2018. IBD was most common at 74%, followed by JIA at 24% and CNO at 2%.
Among 4,111 children with those inflammatory disorders, the psoriasis incidence was 12.3 per 1,000 person-years in exposed children and 3.8 per 1,000 person-years in unexposed. This significant difference equated to a hazard ratio of 3.84 for developing psoriasis after TNFi exposure.
“These data reflect the established association between inflammatory conditions and psoriasis development and suggest that TNFi exposure further increases the risk of psoriasis,” Dr. Buckley and coauthors wrote.
The median duration of follow-up in this study was about 2.5 years for patients exposed to TNFi and 2 years for those unexposed. Among the entire cohort, 39% had been exposed to a TNFi, with 4,705 person-years of follow-up. Among the unexposed children (61%), there were 6,604 person-years of follow-up.
In all, 83 cases of psoriasis developed: 58 in the exposed group and 25 in the unexposed group. Psoriasis incidence varied by disorder. Exposed children with IBD had a higher incidence than did unexposed children (10.9 vs. 2.6 per 1,000 person-years; HR = 4.52). Exposed children with JIA also had a higher incidence than did unexposed children (14.7 vs. 5.5 per 1,000 person-years; HR = 2.90). Among those with CNO, incidences were similar for exposed and unexposed children (33.5 and 38.9 per 1,000 person-years).
A family history of psoriasis significantly increased the risk of psoriasis with a hazard ratio of 3.11, the authors noted. But none of the other covariates (age, sex, race, obesity, methotrexate exposure, and underlying diagnosis) exerted a significant additional risk.
The study had no outside funding source. The authors had no financial disclosures. Dr. Buckley conducted the research when she was a pediatric rheumatology fellow at Children’s Hospital of Philadelphia.
SOURCE: Buckley LH et al. Arthritis Care Res. 2019 Oct 23. doi: 10.1002/ACR.24100
, a retrospective cohort study has determined.
“The incidence rate and risk factors of psoriasis in children with IBD [inflammatory bowel disease], JIA [juvenile idiopathic arthritis], or CNO [chronic nonbacterial osteomyelitis] who are exposed to TNFi [tumor necrosis factor inhibitors] are unknown. Additionally, there is a well-established association between these inflammatory conditions and psoriasis development. Yet, as TNFi can both treat and trigger psoriasis, it is not clear how TNFi exposure affects this relationship,” wrote Lisa H. Buckley, MD, of Children’s Hospital at Vanderbilt, Nashville, Tenn., and colleagues. Their report is in Arthritis Care & Research.
The team examined the relationship in children who were treated for an inflammatory disorder at Children’s Hospital of Philadelphia during 2008-2018. IBD was most common at 74%, followed by JIA at 24% and CNO at 2%.
Among 4,111 children with those inflammatory disorders, the psoriasis incidence was 12.3 per 1,000 person-years in exposed children and 3.8 per 1,000 person-years in unexposed. This significant difference equated to a hazard ratio of 3.84 for developing psoriasis after TNFi exposure.
“These data reflect the established association between inflammatory conditions and psoriasis development and suggest that TNFi exposure further increases the risk of psoriasis,” Dr. Buckley and coauthors wrote.
The median duration of follow-up in this study was about 2.5 years for patients exposed to TNFi and 2 years for those unexposed. Among the entire cohort, 39% had been exposed to a TNFi, with 4,705 person-years of follow-up. Among the unexposed children (61%), there were 6,604 person-years of follow-up.
In all, 83 cases of psoriasis developed: 58 in the exposed group and 25 in the unexposed group. Psoriasis incidence varied by disorder. Exposed children with IBD had a higher incidence than did unexposed children (10.9 vs. 2.6 per 1,000 person-years; HR = 4.52). Exposed children with JIA also had a higher incidence than did unexposed children (14.7 vs. 5.5 per 1,000 person-years; HR = 2.90). Among those with CNO, incidences were similar for exposed and unexposed children (33.5 and 38.9 per 1,000 person-years).
A family history of psoriasis significantly increased the risk of psoriasis with a hazard ratio of 3.11, the authors noted. But none of the other covariates (age, sex, race, obesity, methotrexate exposure, and underlying diagnosis) exerted a significant additional risk.
The study had no outside funding source. The authors had no financial disclosures. Dr. Buckley conducted the research when she was a pediatric rheumatology fellow at Children’s Hospital of Philadelphia.
SOURCE: Buckley LH et al. Arthritis Care Res. 2019 Oct 23. doi: 10.1002/ACR.24100
, a retrospective cohort study has determined.
“The incidence rate and risk factors of psoriasis in children with IBD [inflammatory bowel disease], JIA [juvenile idiopathic arthritis], or CNO [chronic nonbacterial osteomyelitis] who are exposed to TNFi [tumor necrosis factor inhibitors] are unknown. Additionally, there is a well-established association between these inflammatory conditions and psoriasis development. Yet, as TNFi can both treat and trigger psoriasis, it is not clear how TNFi exposure affects this relationship,” wrote Lisa H. Buckley, MD, of Children’s Hospital at Vanderbilt, Nashville, Tenn., and colleagues. Their report is in Arthritis Care & Research.
The team examined the relationship in children who were treated for an inflammatory disorder at Children’s Hospital of Philadelphia during 2008-2018. IBD was most common at 74%, followed by JIA at 24% and CNO at 2%.
Among 4,111 children with those inflammatory disorders, the psoriasis incidence was 12.3 per 1,000 person-years in exposed children and 3.8 per 1,000 person-years in unexposed. This significant difference equated to a hazard ratio of 3.84 for developing psoriasis after TNFi exposure.
“These data reflect the established association between inflammatory conditions and psoriasis development and suggest that TNFi exposure further increases the risk of psoriasis,” Dr. Buckley and coauthors wrote.
The median duration of follow-up in this study was about 2.5 years for patients exposed to TNFi and 2 years for those unexposed. Among the entire cohort, 39% had been exposed to a TNFi, with 4,705 person-years of follow-up. Among the unexposed children (61%), there were 6,604 person-years of follow-up.
In all, 83 cases of psoriasis developed: 58 in the exposed group and 25 in the unexposed group. Psoriasis incidence varied by disorder. Exposed children with IBD had a higher incidence than did unexposed children (10.9 vs. 2.6 per 1,000 person-years; HR = 4.52). Exposed children with JIA also had a higher incidence than did unexposed children (14.7 vs. 5.5 per 1,000 person-years; HR = 2.90). Among those with CNO, incidences were similar for exposed and unexposed children (33.5 and 38.9 per 1,000 person-years).
A family history of psoriasis significantly increased the risk of psoriasis with a hazard ratio of 3.11, the authors noted. But none of the other covariates (age, sex, race, obesity, methotrexate exposure, and underlying diagnosis) exerted a significant additional risk.
The study had no outside funding source. The authors had no financial disclosures. Dr. Buckley conducted the research when she was a pediatric rheumatology fellow at Children’s Hospital of Philadelphia.
SOURCE: Buckley LH et al. Arthritis Care Res. 2019 Oct 23. doi: 10.1002/ACR.24100
FROM ARTHRITIS RESEARCH & CARE
Severe hypoglycemia, poor glycemic control fuels fracture risk in older diabetic patients
Patients with type 2 diabetes and poor glycemic control or severe hypoglycemia may be at greater risk for fracture, according to recent research from a Japanese cohort of older men and postmenopausal women.
“The impacts of severe hypoglycemia and poor glycemic control on fractures appeared to be independent,” noted Yuji Komorita, MD, PhD, of the department of medicine and clinical science, Graduate School of Medical Sciences at Kyushu University, and colleagues. “This study suggests that the glycemic target to prevent fractures may be HbA1c <75 mmol/mol, which is far higher than that used to prevent microvascular complications, and higher than that for older adults with type 2 diabetes.”
Dr. Komorita and colleagues performed a prospective analysis of fracture incidence for 2,755 men and 1,951 postmenopausal women with type 2 diabetes in the Fukuoka Diabetes Registry who were mean 66 years old between April 2008 and October 2010. At the start of the study, the researchers assessed patient diabetes duration, previous fracture history, physical activity, alcohol and smoking status, whether patients were treated for diabetic retinopathy with laser photocoagulation, and their history of coronary artery disease or stroke. Patients were followed for a median 5.3 years, during which fractures were assessed through an annual self-administered questionnaire, with the results stratified by glycemic control and hypoglycemia.
Overall, there were 249 men and 413 women who experienced fractures during the study period, with a follow-up rate of 97.6%. In a multivariate analysis, patients with a higher risk of fracture included those with two or more episodes of severe hypoglycemia (hazard ratio, 2.25; 95% confidence interval, 1.57-3.22) and one episode of severe hypoglycemia (HR, 1.57; 95% CI, 1.11-2.20). In patients without severe hypoglycemic episodes, there was an increased risk of fracture in those with baseline hemoglobin A1c (HbA1c) level of 53 to less than 64 mmol/mol (7% to less than 8%; HR, 1.14; 0.94-1.39), 64 to less than 75 mmol/mol (8% to less than 9%; HR, 1.11; 95% CI, 0.86-1.43), and at least 75 mmol/mol (at least 9%; HR, 1.45; 95% CI, 1.06-1.98).
Compared with postmenopausal women, the unadjusted risk of fracture was higher in men with multiple severe hypoglycemic episodes (HR, 3.46; 95% CI, 2.05-5.85) and one episode of hypoglycemia (HR, 2.81; 95% CI, 1.74-4.56). These higher risks in older men persisted after adjustment for age, multivariate factors, and HbA1c.
“The association between severe hypoglycemia, poor glycemic control, and fracture risk at any anatomic site seems to be stronger in men than in postmenopausal women, although the interaction between men and postmenopausal women for fracture risk was not significant,” the researchers said. “The higher incidence rate of fractures in postmenopausal women, compared with men, was attributed to drastic changes in sex hormones after menopause, which may reduce the apparent impacts of hyperglycemia and severe hypoglycemia on postmenopausal women.”
Researchers said they did not consider potential external factors for fracture incidence, nor did they measure incident falls or other markers of bone health, such as bone mineral density and 25-hydroxyvitamin D levels. They also noted among the limitations of the study the self-reported nature of fracture reporting, and the lack of generalizability of the results.
This study was funded in part by grants from The Japan Society for the Promotion of Science KAKENHI from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Junior Scientist Development Grant supported by the Japan Diabetes Society; and the Lilly Research Grant Program for Bone & Mineral Research. The authors reported no relevant conflicts of interest.
SOURCE: Komorita Y et al. Diabet Med. 2019 Sep 25. doi: 10.1111/dme.14142.
Patients with type 2 diabetes and poor glycemic control or severe hypoglycemia may be at greater risk for fracture, according to recent research from a Japanese cohort of older men and postmenopausal women.
“The impacts of severe hypoglycemia and poor glycemic control on fractures appeared to be independent,” noted Yuji Komorita, MD, PhD, of the department of medicine and clinical science, Graduate School of Medical Sciences at Kyushu University, and colleagues. “This study suggests that the glycemic target to prevent fractures may be HbA1c <75 mmol/mol, which is far higher than that used to prevent microvascular complications, and higher than that for older adults with type 2 diabetes.”
Dr. Komorita and colleagues performed a prospective analysis of fracture incidence for 2,755 men and 1,951 postmenopausal women with type 2 diabetes in the Fukuoka Diabetes Registry who were mean 66 years old between April 2008 and October 2010. At the start of the study, the researchers assessed patient diabetes duration, previous fracture history, physical activity, alcohol and smoking status, whether patients were treated for diabetic retinopathy with laser photocoagulation, and their history of coronary artery disease or stroke. Patients were followed for a median 5.3 years, during which fractures were assessed through an annual self-administered questionnaire, with the results stratified by glycemic control and hypoglycemia.
Overall, there were 249 men and 413 women who experienced fractures during the study period, with a follow-up rate of 97.6%. In a multivariate analysis, patients with a higher risk of fracture included those with two or more episodes of severe hypoglycemia (hazard ratio, 2.25; 95% confidence interval, 1.57-3.22) and one episode of severe hypoglycemia (HR, 1.57; 95% CI, 1.11-2.20). In patients without severe hypoglycemic episodes, there was an increased risk of fracture in those with baseline hemoglobin A1c (HbA1c) level of 53 to less than 64 mmol/mol (7% to less than 8%; HR, 1.14; 0.94-1.39), 64 to less than 75 mmol/mol (8% to less than 9%; HR, 1.11; 95% CI, 0.86-1.43), and at least 75 mmol/mol (at least 9%; HR, 1.45; 95% CI, 1.06-1.98).
Compared with postmenopausal women, the unadjusted risk of fracture was higher in men with multiple severe hypoglycemic episodes (HR, 3.46; 95% CI, 2.05-5.85) and one episode of hypoglycemia (HR, 2.81; 95% CI, 1.74-4.56). These higher risks in older men persisted after adjustment for age, multivariate factors, and HbA1c.
“The association between severe hypoglycemia, poor glycemic control, and fracture risk at any anatomic site seems to be stronger in men than in postmenopausal women, although the interaction between men and postmenopausal women for fracture risk was not significant,” the researchers said. “The higher incidence rate of fractures in postmenopausal women, compared with men, was attributed to drastic changes in sex hormones after menopause, which may reduce the apparent impacts of hyperglycemia and severe hypoglycemia on postmenopausal women.”
Researchers said they did not consider potential external factors for fracture incidence, nor did they measure incident falls or other markers of bone health, such as bone mineral density and 25-hydroxyvitamin D levels. They also noted among the limitations of the study the self-reported nature of fracture reporting, and the lack of generalizability of the results.
This study was funded in part by grants from The Japan Society for the Promotion of Science KAKENHI from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Junior Scientist Development Grant supported by the Japan Diabetes Society; and the Lilly Research Grant Program for Bone & Mineral Research. The authors reported no relevant conflicts of interest.
SOURCE: Komorita Y et al. Diabet Med. 2019 Sep 25. doi: 10.1111/dme.14142.
Patients with type 2 diabetes and poor glycemic control or severe hypoglycemia may be at greater risk for fracture, according to recent research from a Japanese cohort of older men and postmenopausal women.
“The impacts of severe hypoglycemia and poor glycemic control on fractures appeared to be independent,” noted Yuji Komorita, MD, PhD, of the department of medicine and clinical science, Graduate School of Medical Sciences at Kyushu University, and colleagues. “This study suggests that the glycemic target to prevent fractures may be HbA1c <75 mmol/mol, which is far higher than that used to prevent microvascular complications, and higher than that for older adults with type 2 diabetes.”
Dr. Komorita and colleagues performed a prospective analysis of fracture incidence for 2,755 men and 1,951 postmenopausal women with type 2 diabetes in the Fukuoka Diabetes Registry who were mean 66 years old between April 2008 and October 2010. At the start of the study, the researchers assessed patient diabetes duration, previous fracture history, physical activity, alcohol and smoking status, whether patients were treated for diabetic retinopathy with laser photocoagulation, and their history of coronary artery disease or stroke. Patients were followed for a median 5.3 years, during which fractures were assessed through an annual self-administered questionnaire, with the results stratified by glycemic control and hypoglycemia.
Overall, there were 249 men and 413 women who experienced fractures during the study period, with a follow-up rate of 97.6%. In a multivariate analysis, patients with a higher risk of fracture included those with two or more episodes of severe hypoglycemia (hazard ratio, 2.25; 95% confidence interval, 1.57-3.22) and one episode of severe hypoglycemia (HR, 1.57; 95% CI, 1.11-2.20). In patients without severe hypoglycemic episodes, there was an increased risk of fracture in those with baseline hemoglobin A1c (HbA1c) level of 53 to less than 64 mmol/mol (7% to less than 8%; HR, 1.14; 0.94-1.39), 64 to less than 75 mmol/mol (8% to less than 9%; HR, 1.11; 95% CI, 0.86-1.43), and at least 75 mmol/mol (at least 9%; HR, 1.45; 95% CI, 1.06-1.98).
Compared with postmenopausal women, the unadjusted risk of fracture was higher in men with multiple severe hypoglycemic episodes (HR, 3.46; 95% CI, 2.05-5.85) and one episode of hypoglycemia (HR, 2.81; 95% CI, 1.74-4.56). These higher risks in older men persisted after adjustment for age, multivariate factors, and HbA1c.
“The association between severe hypoglycemia, poor glycemic control, and fracture risk at any anatomic site seems to be stronger in men than in postmenopausal women, although the interaction between men and postmenopausal women for fracture risk was not significant,” the researchers said. “The higher incidence rate of fractures in postmenopausal women, compared with men, was attributed to drastic changes in sex hormones after menopause, which may reduce the apparent impacts of hyperglycemia and severe hypoglycemia on postmenopausal women.”
Researchers said they did not consider potential external factors for fracture incidence, nor did they measure incident falls or other markers of bone health, such as bone mineral density and 25-hydroxyvitamin D levels. They also noted among the limitations of the study the self-reported nature of fracture reporting, and the lack of generalizability of the results.
This study was funded in part by grants from The Japan Society for the Promotion of Science KAKENHI from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Junior Scientist Development Grant supported by the Japan Diabetes Society; and the Lilly Research Grant Program for Bone & Mineral Research. The authors reported no relevant conflicts of interest.
SOURCE: Komorita Y et al. Diabet Med. 2019 Sep 25. doi: 10.1111/dme.14142.
FROM DIABETIC MEDICINE
TNF level–based dosing of infliximab does not increase RA remission rate
A new study has indicated that tailoring dosage of infliximab based on serum levels of tumor necrosis factor–alpha did not increase the sustained remission rate in rheumatoid arthritis patients.
“The results did not support our initial hypothesis that deep remission and subsequent sustained discontinuation of infliximab can be achieved by intensive and finely tuned treatments with appropriate doses of TNF [tumor necrosis factor] inhibitors,” wrote Yoshiya Tanaka, MD, PhD, of the University of Occupational and Environmental Health, Kitakyushu, Japan. The study was published in Annals of the Rheumatic Diseases.
To determine if levels of TNF-alpha should initiate an increase in infliximab dosage, the researchers launched a multicenter, randomized trial of 337 patients with infliximab-naive RA. Patients were assigned to two groups: standard infliximab treatment of 3 mg/kg at weeks 0, 2, 6, and then every 8 weeks (n = 167) or programmed infliximab treatment (n = 170) in which the dose was adjusted at week 14 based on low, intermediate, or high levels of baseline TNF-alpha.
Patients with low levels (below 0.55 pg/mL) continued receiving 3 mg/kg every 8 weeks. Intermediate levels (between 0.55 and 1.65 pg/mL) meant an increase to 6 mg/kg every 8 weeks. High levels (1.65 pg/mL or higher) meant an increase to 6 mg/kg at week 14 and to 10 mg/kg at week 22 and every 8 weeks afterward. The goal was to discontinue infliximab at 54 weeks and reassess 1 year later.
After 54 weeks, 39.4% of patients in the programmed group and 32.3% of patients in the standard group achieved remission, defined as a Simplified Disease Activity Index (SDAI) score of 3.3 or lower. After 106 weeks, 23.5% of the programmed group and 21.6% of the standard group had maintained discontinuation of infliximab (–2.2% difference; 95% confidence interval, –6.6% to 11.0%; P = .631). After analysis, the most significant predictor of sustained discontinuation was a baseline SDAI less than 26.
The authors acknowledged their study’s limitations, including the initial version of the study not being double blinded. In addition, they noted that the short length of treatment and the low dosage overall “might not be intense enough to achieve differences in disease control between the two groups.”
The study was supported by a research grant from the Ministry of Health, Labor and Welfare of Japan. The authors reported numerous potential conflicts of interest, including receiving grants, consulting fees, speaking fees, and/or honoraria from various medical and pharmaceutical companies.
SOURCE: Tanaka Y et al. Ann Rheum Dis. 2019 Oct 19. doi: 10.1136/annrheumdis-2019-216169.
A new study has indicated that tailoring dosage of infliximab based on serum levels of tumor necrosis factor–alpha did not increase the sustained remission rate in rheumatoid arthritis patients.
“The results did not support our initial hypothesis that deep remission and subsequent sustained discontinuation of infliximab can be achieved by intensive and finely tuned treatments with appropriate doses of TNF [tumor necrosis factor] inhibitors,” wrote Yoshiya Tanaka, MD, PhD, of the University of Occupational and Environmental Health, Kitakyushu, Japan. The study was published in Annals of the Rheumatic Diseases.
To determine if levels of TNF-alpha should initiate an increase in infliximab dosage, the researchers launched a multicenter, randomized trial of 337 patients with infliximab-naive RA. Patients were assigned to two groups: standard infliximab treatment of 3 mg/kg at weeks 0, 2, 6, and then every 8 weeks (n = 167) or programmed infliximab treatment (n = 170) in which the dose was adjusted at week 14 based on low, intermediate, or high levels of baseline TNF-alpha.
Patients with low levels (below 0.55 pg/mL) continued receiving 3 mg/kg every 8 weeks. Intermediate levels (between 0.55 and 1.65 pg/mL) meant an increase to 6 mg/kg every 8 weeks. High levels (1.65 pg/mL or higher) meant an increase to 6 mg/kg at week 14 and to 10 mg/kg at week 22 and every 8 weeks afterward. The goal was to discontinue infliximab at 54 weeks and reassess 1 year later.
After 54 weeks, 39.4% of patients in the programmed group and 32.3% of patients in the standard group achieved remission, defined as a Simplified Disease Activity Index (SDAI) score of 3.3 or lower. After 106 weeks, 23.5% of the programmed group and 21.6% of the standard group had maintained discontinuation of infliximab (–2.2% difference; 95% confidence interval, –6.6% to 11.0%; P = .631). After analysis, the most significant predictor of sustained discontinuation was a baseline SDAI less than 26.
The authors acknowledged their study’s limitations, including the initial version of the study not being double blinded. In addition, they noted that the short length of treatment and the low dosage overall “might not be intense enough to achieve differences in disease control between the two groups.”
The study was supported by a research grant from the Ministry of Health, Labor and Welfare of Japan. The authors reported numerous potential conflicts of interest, including receiving grants, consulting fees, speaking fees, and/or honoraria from various medical and pharmaceutical companies.
SOURCE: Tanaka Y et al. Ann Rheum Dis. 2019 Oct 19. doi: 10.1136/annrheumdis-2019-216169.
A new study has indicated that tailoring dosage of infliximab based on serum levels of tumor necrosis factor–alpha did not increase the sustained remission rate in rheumatoid arthritis patients.
“The results did not support our initial hypothesis that deep remission and subsequent sustained discontinuation of infliximab can be achieved by intensive and finely tuned treatments with appropriate doses of TNF [tumor necrosis factor] inhibitors,” wrote Yoshiya Tanaka, MD, PhD, of the University of Occupational and Environmental Health, Kitakyushu, Japan. The study was published in Annals of the Rheumatic Diseases.
To determine if levels of TNF-alpha should initiate an increase in infliximab dosage, the researchers launched a multicenter, randomized trial of 337 patients with infliximab-naive RA. Patients were assigned to two groups: standard infliximab treatment of 3 mg/kg at weeks 0, 2, 6, and then every 8 weeks (n = 167) or programmed infliximab treatment (n = 170) in which the dose was adjusted at week 14 based on low, intermediate, or high levels of baseline TNF-alpha.
Patients with low levels (below 0.55 pg/mL) continued receiving 3 mg/kg every 8 weeks. Intermediate levels (between 0.55 and 1.65 pg/mL) meant an increase to 6 mg/kg every 8 weeks. High levels (1.65 pg/mL or higher) meant an increase to 6 mg/kg at week 14 and to 10 mg/kg at week 22 and every 8 weeks afterward. The goal was to discontinue infliximab at 54 weeks and reassess 1 year later.
After 54 weeks, 39.4% of patients in the programmed group and 32.3% of patients in the standard group achieved remission, defined as a Simplified Disease Activity Index (SDAI) score of 3.3 or lower. After 106 weeks, 23.5% of the programmed group and 21.6% of the standard group had maintained discontinuation of infliximab (–2.2% difference; 95% confidence interval, –6.6% to 11.0%; P = .631). After analysis, the most significant predictor of sustained discontinuation was a baseline SDAI less than 26.
The authors acknowledged their study’s limitations, including the initial version of the study not being double blinded. In addition, they noted that the short length of treatment and the low dosage overall “might not be intense enough to achieve differences in disease control between the two groups.”
The study was supported by a research grant from the Ministry of Health, Labor and Welfare of Japan. The authors reported numerous potential conflicts of interest, including receiving grants, consulting fees, speaking fees, and/or honoraria from various medical and pharmaceutical companies.
SOURCE: Tanaka Y et al. Ann Rheum Dis. 2019 Oct 19. doi: 10.1136/annrheumdis-2019-216169.
FROM ANNALS OF THE RHEUMATIC DISEASES
Liver abnormalities, disease common in patients with psoriatic arthritis
Liver abnormalities in patients with psoriatic arthritis are common and are associated with higher body mass index, more severe disease, and certain therapies, new research suggests.
Patients with psoriatic arthritis (PsA) often have comorbidities such as cardiovascular disease, metabolic syndrome, inflammatory bowel disease, osteoporosis, malignancy, and ophthalmic disease, and liver disease is no exception, wrote Rattapol Pakchotanon, MD, of the department of internal medicine at Phramongkutlao Hospital and College of Medicine, Bangkok, and associates. Their report is in the Journal of Rheumatology.
In psoriasis patients, the prevalence of liver abnormalities has been 24%-36% in previous research, but research regarding liver disease in PsA has been limited.
Of 1,061 patients from the University of Toronto Psoriatic Arthritis Clinic who were included in the study, 343 (32%) had liver abnormalities, including 256 who developed a liver abnormality or disease after their first evaluation at the clinic. Liver abnormality was defined as having aspartate transaminase, alanine transaminase, or alkaline phosphatase levels 1.5 times the upper limit of normal or greater, and liver diseases included drug-induced liver injury, fatty liver, viral hepatitis, autoimmune liver disease, alcoholic liver disease, liver fibrosis, and cirrhosis.
Among the patients with PsA who developed liver abnormalities or disease after their first visit, liver abnormalities occurred after an average of 8.3 years of follow-up and at a mean age of 50.5 years. The average BMI in this group was 29.7 kg/m2, and 11% of patients consumed alcohol daily. A total of 105 patients had recurrent liver abnormalities, and the rest had only one visit with an abnormality; those with transient abnormalities were significantly less likely to have evidence of liver disease (P less than .001).
The most common cause of liver disease was drug-induced hepatitis (14%) and fatty liver (13%). Alcohol-induced hepatitis occurred in 10 patients, and cirrhosis was reported in 2 patients.
In a multivariable analysis, factors found to be independently associated with liver abnormalities in PsA included BMI (odds ratio, 1.07; 95% confidence interval, 1.02-1.12; P = .007), daily alcohol intake (OR, 4.46; 95% CI, 1.30-15.28; P = .02), damaged joint count (OR, 1.04; 95% CI, 1.01-1.08; P = .01), elevated C-reactive protein (OR, 2.00; 95% CI, 1.04-3.85; P = .04), use of methotrexate or leflunomide (OR, 4.39; 95% CI, 1.67-11.54; P = .003), and use of tumor necrosis factor inhibitors (OR, 10.56; 95% CI, 3.63-30.69; P less than .0001).
“We recommend monitoring liver function tests in these high risk PsA patients,” the researchers concluded. “This is important in the management of patients with PsA as many of the therapeutic options may aggravate or even lead to liver abnormalities in this patient population.”
The study was funded in part by the Arthritis Society, the Canadian Institutes of Health Research, and the Krembil Foundation. The investigators reported that they had no conflicts of interest. Dr. Pakchotanon conducted the research while he was at the Centre for Prognosis Studies in the Rheumatic Diseases at Toronto Western Hospital.
SOURCE: Gladman DD et al. J Rheumatol. 2019 Oct 15. doi: 10.3899/jrheum.181312
Liver abnormalities in patients with psoriatic arthritis are common and are associated with higher body mass index, more severe disease, and certain therapies, new research suggests.
Patients with psoriatic arthritis (PsA) often have comorbidities such as cardiovascular disease, metabolic syndrome, inflammatory bowel disease, osteoporosis, malignancy, and ophthalmic disease, and liver disease is no exception, wrote Rattapol Pakchotanon, MD, of the department of internal medicine at Phramongkutlao Hospital and College of Medicine, Bangkok, and associates. Their report is in the Journal of Rheumatology.
In psoriasis patients, the prevalence of liver abnormalities has been 24%-36% in previous research, but research regarding liver disease in PsA has been limited.
Of 1,061 patients from the University of Toronto Psoriatic Arthritis Clinic who were included in the study, 343 (32%) had liver abnormalities, including 256 who developed a liver abnormality or disease after their first evaluation at the clinic. Liver abnormality was defined as having aspartate transaminase, alanine transaminase, or alkaline phosphatase levels 1.5 times the upper limit of normal or greater, and liver diseases included drug-induced liver injury, fatty liver, viral hepatitis, autoimmune liver disease, alcoholic liver disease, liver fibrosis, and cirrhosis.
Among the patients with PsA who developed liver abnormalities or disease after their first visit, liver abnormalities occurred after an average of 8.3 years of follow-up and at a mean age of 50.5 years. The average BMI in this group was 29.7 kg/m2, and 11% of patients consumed alcohol daily. A total of 105 patients had recurrent liver abnormalities, and the rest had only one visit with an abnormality; those with transient abnormalities were significantly less likely to have evidence of liver disease (P less than .001).
The most common cause of liver disease was drug-induced hepatitis (14%) and fatty liver (13%). Alcohol-induced hepatitis occurred in 10 patients, and cirrhosis was reported in 2 patients.
In a multivariable analysis, factors found to be independently associated with liver abnormalities in PsA included BMI (odds ratio, 1.07; 95% confidence interval, 1.02-1.12; P = .007), daily alcohol intake (OR, 4.46; 95% CI, 1.30-15.28; P = .02), damaged joint count (OR, 1.04; 95% CI, 1.01-1.08; P = .01), elevated C-reactive protein (OR, 2.00; 95% CI, 1.04-3.85; P = .04), use of methotrexate or leflunomide (OR, 4.39; 95% CI, 1.67-11.54; P = .003), and use of tumor necrosis factor inhibitors (OR, 10.56; 95% CI, 3.63-30.69; P less than .0001).
“We recommend monitoring liver function tests in these high risk PsA patients,” the researchers concluded. “This is important in the management of patients with PsA as many of the therapeutic options may aggravate or even lead to liver abnormalities in this patient population.”
The study was funded in part by the Arthritis Society, the Canadian Institutes of Health Research, and the Krembil Foundation. The investigators reported that they had no conflicts of interest. Dr. Pakchotanon conducted the research while he was at the Centre for Prognosis Studies in the Rheumatic Diseases at Toronto Western Hospital.
SOURCE: Gladman DD et al. J Rheumatol. 2019 Oct 15. doi: 10.3899/jrheum.181312
Liver abnormalities in patients with psoriatic arthritis are common and are associated with higher body mass index, more severe disease, and certain therapies, new research suggests.
Patients with psoriatic arthritis (PsA) often have comorbidities such as cardiovascular disease, metabolic syndrome, inflammatory bowel disease, osteoporosis, malignancy, and ophthalmic disease, and liver disease is no exception, wrote Rattapol Pakchotanon, MD, of the department of internal medicine at Phramongkutlao Hospital and College of Medicine, Bangkok, and associates. Their report is in the Journal of Rheumatology.
In psoriasis patients, the prevalence of liver abnormalities has been 24%-36% in previous research, but research regarding liver disease in PsA has been limited.
Of 1,061 patients from the University of Toronto Psoriatic Arthritis Clinic who were included in the study, 343 (32%) had liver abnormalities, including 256 who developed a liver abnormality or disease after their first evaluation at the clinic. Liver abnormality was defined as having aspartate transaminase, alanine transaminase, or alkaline phosphatase levels 1.5 times the upper limit of normal or greater, and liver diseases included drug-induced liver injury, fatty liver, viral hepatitis, autoimmune liver disease, alcoholic liver disease, liver fibrosis, and cirrhosis.
Among the patients with PsA who developed liver abnormalities or disease after their first visit, liver abnormalities occurred after an average of 8.3 years of follow-up and at a mean age of 50.5 years. The average BMI in this group was 29.7 kg/m2, and 11% of patients consumed alcohol daily. A total of 105 patients had recurrent liver abnormalities, and the rest had only one visit with an abnormality; those with transient abnormalities were significantly less likely to have evidence of liver disease (P less than .001).
The most common cause of liver disease was drug-induced hepatitis (14%) and fatty liver (13%). Alcohol-induced hepatitis occurred in 10 patients, and cirrhosis was reported in 2 patients.
In a multivariable analysis, factors found to be independently associated with liver abnormalities in PsA included BMI (odds ratio, 1.07; 95% confidence interval, 1.02-1.12; P = .007), daily alcohol intake (OR, 4.46; 95% CI, 1.30-15.28; P = .02), damaged joint count (OR, 1.04; 95% CI, 1.01-1.08; P = .01), elevated C-reactive protein (OR, 2.00; 95% CI, 1.04-3.85; P = .04), use of methotrexate or leflunomide (OR, 4.39; 95% CI, 1.67-11.54; P = .003), and use of tumor necrosis factor inhibitors (OR, 10.56; 95% CI, 3.63-30.69; P less than .0001).
“We recommend monitoring liver function tests in these high risk PsA patients,” the researchers concluded. “This is important in the management of patients with PsA as many of the therapeutic options may aggravate or even lead to liver abnormalities in this patient population.”
The study was funded in part by the Arthritis Society, the Canadian Institutes of Health Research, and the Krembil Foundation. The investigators reported that they had no conflicts of interest. Dr. Pakchotanon conducted the research while he was at the Centre for Prognosis Studies in the Rheumatic Diseases at Toronto Western Hospital.
SOURCE: Gladman DD et al. J Rheumatol. 2019 Oct 15. doi: 10.3899/jrheum.181312
FROM THE JOURNAL OF RHEUMATOLOGY
DNA methylation changes: An early biomarker for methotrexate response?
An investigation into a potential biomarker for response to methotrexate found that changes in DNA methylation at 4 weeks were associated with improvements in rheumatoid arthritis (RA) patients at 6 months.
“The findings in the current study are promising and appear to identify methylation patterns that are predictive of improvement of SJC [swollen joint count] and CRP [C-reactive protein],” wrote Nisha Nair, PhD, of the University of Manchester (England) and her coauthors. The study was published in Rheumatology.
The investigators analyzed DNA samples taken from patients recruited into the Rheumatoid Arthritis Medication Study (RAMS) who had RA or inflammatory polyarthritis and were beginning methotrexate for the first time. The samples were collected at baseline and at 4 weeks from patients who were classified as having good (n = 34) or poor (n = 34) responses to methotrexate after 6 months according to European League Against Rheumatism response criteria, in which good response was defined as having a 28-joint disease activity score (DAS28) of 3.2 or less and a 1.2-point improvement in DAS28 at 6 months, and poor response was defined as having DAS28 higher than 5.1 and improvement of 0.6 points or less at 6 months.
After analysis, two differentially methylated positions that differed between good and poor responders were identified in samples taken at the 4-week mark (P less than 1 × 10–6). Four CpG (cytosine-phosphate-guanine) sites also predicted improvements in RA patients at 6 months: Two sites associated increased methylation in good responders at 4 weeks with long-term SJC improvement, while two others associated increased methylation at baseline and in good responders at 4 weeks with improvement of CRP levels.
The authors acknowledged their study’s limitations, including the fact that the relapsing nature of RA could have contributed to natural variance in DAS28. In addition, the study’s lack of a control group does not allow for separating prognostic from theranostic biomarkers, although they added that “in terms of selecting treatments that will be effective, that may not necessarily matter in the clinical setting.”
The study was funded by the Medical Research Council and Versus Arthritis. The authors reported no conflicts of interest.
SOURCE: Nair N et al. Rheumatology. 2019 Oct 10. doi: 10.1093/rheumatology/kez411
An investigation into a potential biomarker for response to methotrexate found that changes in DNA methylation at 4 weeks were associated with improvements in rheumatoid arthritis (RA) patients at 6 months.
“The findings in the current study are promising and appear to identify methylation patterns that are predictive of improvement of SJC [swollen joint count] and CRP [C-reactive protein],” wrote Nisha Nair, PhD, of the University of Manchester (England) and her coauthors. The study was published in Rheumatology.
The investigators analyzed DNA samples taken from patients recruited into the Rheumatoid Arthritis Medication Study (RAMS) who had RA or inflammatory polyarthritis and were beginning methotrexate for the first time. The samples were collected at baseline and at 4 weeks from patients who were classified as having good (n = 34) or poor (n = 34) responses to methotrexate after 6 months according to European League Against Rheumatism response criteria, in which good response was defined as having a 28-joint disease activity score (DAS28) of 3.2 or less and a 1.2-point improvement in DAS28 at 6 months, and poor response was defined as having DAS28 higher than 5.1 and improvement of 0.6 points or less at 6 months.
After analysis, two differentially methylated positions that differed between good and poor responders were identified in samples taken at the 4-week mark (P less than 1 × 10–6). Four CpG (cytosine-phosphate-guanine) sites also predicted improvements in RA patients at 6 months: Two sites associated increased methylation in good responders at 4 weeks with long-term SJC improvement, while two others associated increased methylation at baseline and in good responders at 4 weeks with improvement of CRP levels.
The authors acknowledged their study’s limitations, including the fact that the relapsing nature of RA could have contributed to natural variance in DAS28. In addition, the study’s lack of a control group does not allow for separating prognostic from theranostic biomarkers, although they added that “in terms of selecting treatments that will be effective, that may not necessarily matter in the clinical setting.”
The study was funded by the Medical Research Council and Versus Arthritis. The authors reported no conflicts of interest.
SOURCE: Nair N et al. Rheumatology. 2019 Oct 10. doi: 10.1093/rheumatology/kez411
An investigation into a potential biomarker for response to methotrexate found that changes in DNA methylation at 4 weeks were associated with improvements in rheumatoid arthritis (RA) patients at 6 months.
“The findings in the current study are promising and appear to identify methylation patterns that are predictive of improvement of SJC [swollen joint count] and CRP [C-reactive protein],” wrote Nisha Nair, PhD, of the University of Manchester (England) and her coauthors. The study was published in Rheumatology.
The investigators analyzed DNA samples taken from patients recruited into the Rheumatoid Arthritis Medication Study (RAMS) who had RA or inflammatory polyarthritis and were beginning methotrexate for the first time. The samples were collected at baseline and at 4 weeks from patients who were classified as having good (n = 34) or poor (n = 34) responses to methotrexate after 6 months according to European League Against Rheumatism response criteria, in which good response was defined as having a 28-joint disease activity score (DAS28) of 3.2 or less and a 1.2-point improvement in DAS28 at 6 months, and poor response was defined as having DAS28 higher than 5.1 and improvement of 0.6 points or less at 6 months.
After analysis, two differentially methylated positions that differed between good and poor responders were identified in samples taken at the 4-week mark (P less than 1 × 10–6). Four CpG (cytosine-phosphate-guanine) sites also predicted improvements in RA patients at 6 months: Two sites associated increased methylation in good responders at 4 weeks with long-term SJC improvement, while two others associated increased methylation at baseline and in good responders at 4 weeks with improvement of CRP levels.
The authors acknowledged their study’s limitations, including the fact that the relapsing nature of RA could have contributed to natural variance in DAS28. In addition, the study’s lack of a control group does not allow for separating prognostic from theranostic biomarkers, although they added that “in terms of selecting treatments that will be effective, that may not necessarily matter in the clinical setting.”
The study was funded by the Medical Research Council and Versus Arthritis. The authors reported no conflicts of interest.
SOURCE: Nair N et al. Rheumatology. 2019 Oct 10. doi: 10.1093/rheumatology/kez411
FROM RHEUMATOLOGY


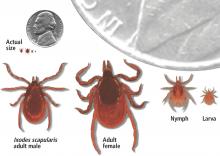



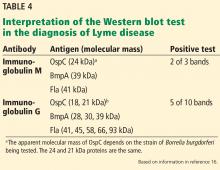
![Positive Western blot test (Borrelia B31 ViraStripe [Viramed Diagnostics]) in a patient who presented with rash and arthritis. This test uses purified specific antigens of strain B31 of Borrelia burgdorferi sensu stricto. Positive Western blot test (Borrelia B31 ViraStripe [Viramed Diagnostics]) in a patient who presented with rash and arthritis. This test uses purified specific antigens of strain B31 of Borrelia burgdorferi sensu stricto.](https://cdn.mdedge.com/files/s3fs-public/styles/medium/public/756fig2.jpg?itok=fMD7ruHJ)