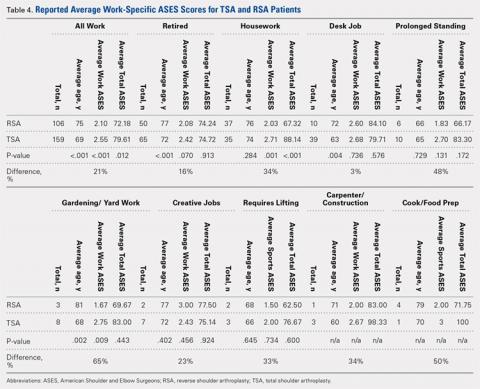
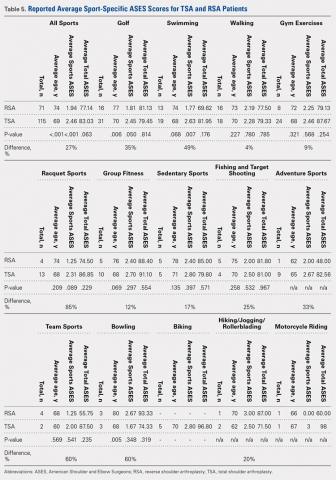
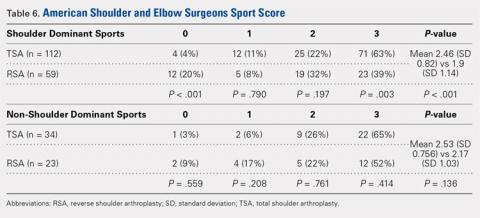
User login
Osteochondritis Dissecans Lesion of the Radial Head
ABSTRACT
This case shows an atypical presentation of an osteochondritis dissecans (OCD) lesion of the radial head with detachment diagnosed on plain radiographs and magnetic resonance imaging (MRI). OCD lesions are rather uncommon in the elbow joint; however, when present, these lesions are typically seen in throwing athletes or gymnasts who engage in activities involving repetitive trauma to the elbow. Involvement of the radial head is extremely rare, accounting for <5% of all elbow OCD lesions. Conventional radiographs have low sensitivity for detecting OCD lesions and may frequently miss these lesions in the early stages. MRI, the imaging modality of choice, can detect these lesions at the earliest stage and provide a clear picture of the involved articular cartilage and underlying bone. Treatment options can vary between nonoperative and operative management depending on several factors, including age and activity level of the patient, size and type of lesion, and clinical presentation. This case represents a radial head OCD lesion managed by arthroscopic débridement alone, resulting in a positive outcome.
Continue to: Case Report...
CASE REPORT
A healthy, 14-year-old, left-hand-dominant adolescent boy presented to the office with a chief complaint of pain localized to the posterolateral aspect of his elbow. He described an injury where he felt a “pop” in his elbow followed by immediate pain in the posterolateral elbow after throwing a pitch during a baseball game. Since the injury, the patient had experienced difficulty extending his elbow and a sharp, throbbing pain during forearm rotation. The patient also reported an intermittent clicking feeling in the elbow. Prior to this injury, he had no elbow pain. He presented in an otherwise normal state of health with no reported past medical or surgical history and no previous trauma to the left upper extremity.
Physical examination demonstrated a mild effusion of the left elbow in the region of the posterolateral corner or “soft spot” with tenderness to palpation over the radial head. The patient had restricted elbow motion with 30° to 135° of flexion. He had 90° of pronation and supination. Ligamentous examination revealed stability of the elbow to both varus and valgus stress at 30° of flexion. No deficits were observed upon upper-extremity neurovascular examination.
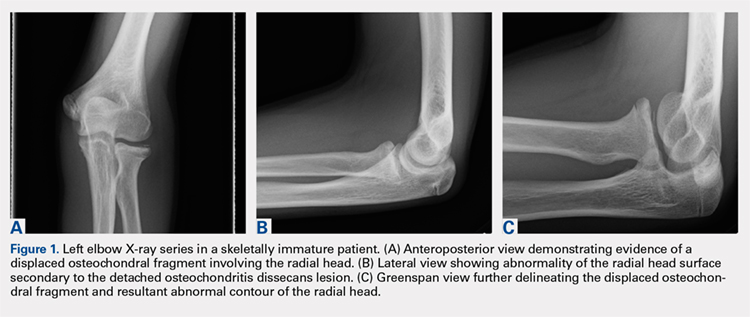
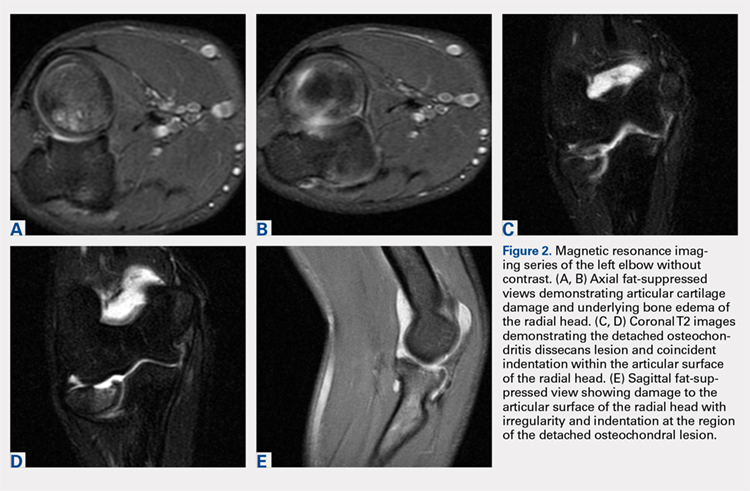
Plain radiographs of the left elbow were initially taken. Anteroposterior, lateral, and Greenspan views revealed evidence of a displaced osteochondral fragment of the radial head in this skeletally immature patient. No involvement of the capitellum was apparent (Figures 1A-1C). Non-contrast magnetic resonance imaging (MRI) of the left elbow was subsequently obtained to evaluate the lesion further, and the images confirmed an unstable osteochondritis dissecans (OCD) lesion of the radial head with a detached fragment entrapped within the elbow joint (Figures 2A-2E).
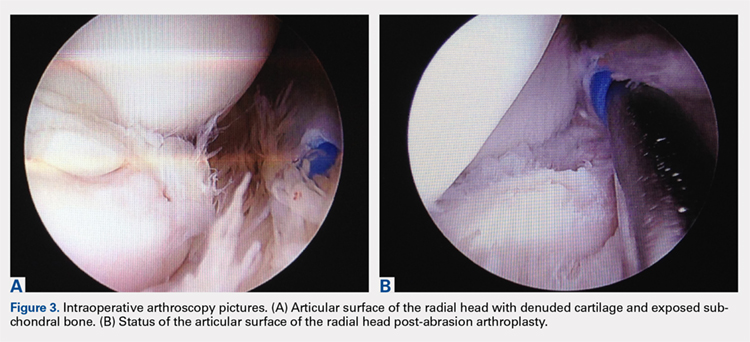
Elbow arthroscopy was performed to evaluate the extent of the OCD lesion to enable determination of the integrity of the cartilaginous surface and remove the loose body entrapped within the elbow joint. Multiple loose bodies (all <5 mm in size) were removed from the elbow joint. Visualization of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. The main chondral defect measured approximately 4 mm in size. Probing of the lesion confirmed no stable edge; thus, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth (Figures 3A, 3B).
The patient was started on physical therapy consisting of active and active-assisted elbow ranges of motion on postoperative day 10. At the 6-week follow up, the patient presented to the office with pain-free motion of the left elbow ranging from −5° to 135° of flexion. He maintained full pronation and supination. At this point, the patient was advised to begin a throwing program. Three months after treatment, the patient resumed baseball activities, including throwing, with pain-free, full range of motion of the elbow. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Continue to: Discussion...
DISCUSSION
Elbow pain is a common complaint among young baseball players. OCD lesions, however, are an uncommon entity associated with elbow pathology.1 The overall incidence of OCD lesions is between 15 to 30 per 100,000 people.2-3 Specifically in patients aged 2 to 19 years, the incidence of elbow OCD lesions is 2.2 per 100,000 patients and 3.8 and 0.6 per 100,000 for males and females, respectively.4 Radial head OCD lesions are extremely rare, occurring in <5% of all elbow OCD cases.1 The majority of these lesions are asymptomatic and typically seen in patients who engage in repetitive overhead and upper-extremity weight-bearing activities. Reports indicate that the incidence of these lesions is on the rise and the age of presentation is decreasing, likely because of increased awareness of the disease and increasing involvement of young athletes in competitive athletics.4-5 Most patients with elbow OCD have a history of repetitive overuse of the elbow, as seen in baseball players, leading to excessive compressive and shear forces across the radiocapitellar joint and progression of the dissecans lesion.6
Patients with OCD lesions of the elbow typically present with inflammatory type symptoms and lateral elbow pain. The pain tends to be mild at rest and becomes more pronounced with activity. Patients often wait until mechanical symptoms ensue (eg, clicking, catching, or locking) before presenting to the office. On physical examination, pain in the region of the OCD lesion is usually accompanied by a mild effusion. Stiffness, particularly a loss of terminal extension, may accompany the mechanical symptoms on range of motion testing.7
Workup of elbow OCD lesions begins with obtaining plain radiographs of the elbow. Plain films are of limited use in evaluating these lesions but can help determine separation and the approximate size of the fragment.8 Further work-up must include MRI sequences, which allow for the best evaluation of the articular cartilage, underlying bone, and, specifically, the size and degree of separation of the OCD lesion.9
Nonoperative treatment of OCD lesions is usually successful if diagnosed early. Such treatment consists of activity modification, rest, anti-inflammatory medications, and a gradual return to athletic activities over the next 3 to 6 months provided the symptoms abate.10-11 During this interval, physical therapy may be employed to preserve or regain range of motion in the elbow. Clinical evidence has demonstrated improved outcomes in younger athletes with open physes.12 Returning to athletic activities is advised only when complete resolution of symptoms has been achieved and full motion about the elbow and shoulder girdle has been regained.6
If symptoms persist despite nonoperative management, or if evidence of an unstable lesion (ie, detached fragment) is obtained, operative intervention is appropriate. Operative management includes diagnostic arthroscopy of the entire elbow, removal of any small, loose bodies, and synovectomy as needed. Thereafter, the OCD lesion must be addressed. In cases of capitellar OCD lesions, if the articular cartilage surface is intact, antegrade or retrograde drilling of the subchondral bone is appropriate and will likely result in a good-to-excellent functional outcome.13-14 If disruption to the articular cartilage fissures is found or the lesion appears to be separating from the native bone, fixation of the fragment can be attempted, provided an adequate portion of the subchondral bone remains attached to the OCD lesion.6,14 Oftentimes, the bony bed must be prepared prior to fixation by removal of any fibrous tissue overlying the subchondral bone and ensuring adequate bleeding across the entire bed. Care should be taken to remove any fibrous tissue underlying the OCD lesion. If the OCD lesion is completely loose and/or the bone stock is insufficient or fragmented, arthroscopic removal of the OCD lesion followed by débridement and abrasion arthroplasty of subchondral bone is recommended.15 Improved functional outcomes from this procedure can be expected in contained lesions.15 If the patient continues to be symptomatic, osteochondral autograft or allograft procedures can be attempted depending on the size of the remaining defect.16-18
Other cases of radial head OCD lesions have been reported in the literature.19-20 In 2009, Dotzis and colleagues19 reported a case of an OCD lesion that was managed nonsurgically with observation alone as the lesion was stable and non-detached. Tatebe and colleagues20 reported 4 cases in which OCD involved the radial head and was accompanied by radial head subluxation. All lesions were located at the posteromedial aspect of the radial head with anterior subluxation of the radial head.20 Three of the cases were managed surgically via ulnar osteotomy (2 cases) and fragment removal (1 case).20 All except the 1 case treated by fragment excision revealed a good outcome.20 The patient in this case presented with a detached lesion, confirmed on MRI, with pain, mechanical symptoms, and of loss of terminal extension. Given the chronicity of the injury and the presence of mechanical symptoms, the decision was made to proceed with operative intervention. During elbow arthroscopy, multiple loose bodies were removed from the elbow joint, and inspection of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. Since the OCD lesion was completely loose and the bone stock was insufficient and too fragmented to attempt fixation, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth. At the 6-week follow up, the patient regained full range of motion of this elbow with no complaints of pain. At the 3-month follow up, the patient reported no pain after returning to throwing and all baseball-related activities.
CONCLUSION
This report presents an extremely rare case of an OCD lesion involving the radial head. Diagnosis and treatment of this lesion followed a protocol similar to that used for the management of capitellar OCD lesions. When dealing with elbow OCD lesions, especially in the skeletally immature patient population, nonsurgical management and a gradual return to activities should be attempted. If symptoms persist despite nonoperative management or evidence of an unstable lesion (as presented in this case) is obtained, operative intervention is appropriate.
- Jans LB, Ditchfield M, Anna G, Jaremko JL, Verstraete KL. MR imaging findings and MR criteria for instability in osteochondritis dissecans of the elbow in children. Eur J Radiol. 2012;81(6):1306-1310. doi:10.1016/j.ejrad.2011.01.007.
- Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg. 1984;66(9):1340-1348. doi:10.2106/00004623-198466090-00003.
- Lindén B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47(6):664-667. doi:10.3109/17453677608988756.
- Kessler JI, Nikizad H, Shea KG, Jacobs JC, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42(2):320-326. doi:10.1177/0363546513510390.
- Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current Concepts Review. Am J Sports Med. 2006;34(7):1181-1191. doi:10.1177/0363546506290127.
- Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205-1214. doi:10.2106/JBJS.F.00622.
- Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteo Chondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216(1):207-212. doi:10.1148/radiology.216.1.r00jl29207.
- Kijowski R, De Smet AA. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skeletal Radiol. 2005;34(5):266-271. doi:10.1007/s00256-005-0899-6.
- Kijowski R, De Smet AA. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. AJR Am J Roentgenol. 2005;185:1453-1459. doi:10.2214/AJR.04.1570.
- Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27(6):728-732. doi:10.1177/03635465990270060701.
- Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363(363):108-115. doi:10.1097/00003086-199906000-00014.
- Pill SG, Ganley TJ, Milam RA, Lou JE, Meyer JS, Flynn JM. Role of magnetic resonance imaging and clinical criteria in predicting successful nonoperative treatment of osteochondritis dissecans in children. J Pediatr Orthop. 2003;23(1):102-108. doi:10.1097/01241398-200301000-00021.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Byrd JWT, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30(4):474-478. doi:10.1177/03635465020300040401.
- Krijnen MR, Lim L, Willems WJ. Arthroscopic treatment of osteochondritis dissecans of the capitellum: report of 5 female athletes. Arthroscopy. 2003;19(2):210-214. doi:10.1053/jars.2003.50052.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34(5):714-720. doi:10.1177/0363546505282620.
- Ahmad CS, ElAttrache NS. Mosaicplasty for capitellar osteochondritis dissecans. In: Yamaguchi K, O'Driscoll S, King G, McKee M, eds. [In press] Advanced Reconstruction Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons.
- Dotzis A, Galissier B, Peyrou P, Longis B, Moulies D. Osteochondritis dissecans of the radial head: a case report. J Shoulder Elbow Surg. 2009;18(1):e18-e21. doi:10.1016/j.jse.2008.04.009.
- Tatebe M, Hirata H, Shinohara T, Yamamoto M, Morita A, Horii E. Pathomechanical significance of radial head subluxation in the onset of osteochondritis dissecans of the radial head. J Orthop Trauma. 2012;26(1):e4-e6. doi:10.1097/BOT.0b013e318214d678.
ABSTRACT
This case shows an atypical presentation of an osteochondritis dissecans (OCD) lesion of the radial head with detachment diagnosed on plain radiographs and magnetic resonance imaging (MRI). OCD lesions are rather uncommon in the elbow joint; however, when present, these lesions are typically seen in throwing athletes or gymnasts who engage in activities involving repetitive trauma to the elbow. Involvement of the radial head is extremely rare, accounting for <5% of all elbow OCD lesions. Conventional radiographs have low sensitivity for detecting OCD lesions and may frequently miss these lesions in the early stages. MRI, the imaging modality of choice, can detect these lesions at the earliest stage and provide a clear picture of the involved articular cartilage and underlying bone. Treatment options can vary between nonoperative and operative management depending on several factors, including age and activity level of the patient, size and type of lesion, and clinical presentation. This case represents a radial head OCD lesion managed by arthroscopic débridement alone, resulting in a positive outcome.
Continue to: Case Report...
CASE REPORT
A healthy, 14-year-old, left-hand-dominant adolescent boy presented to the office with a chief complaint of pain localized to the posterolateral aspect of his elbow. He described an injury where he felt a “pop” in his elbow followed by immediate pain in the posterolateral elbow after throwing a pitch during a baseball game. Since the injury, the patient had experienced difficulty extending his elbow and a sharp, throbbing pain during forearm rotation. The patient also reported an intermittent clicking feeling in the elbow. Prior to this injury, he had no elbow pain. He presented in an otherwise normal state of health with no reported past medical or surgical history and no previous trauma to the left upper extremity.
Physical examination demonstrated a mild effusion of the left elbow in the region of the posterolateral corner or “soft spot” with tenderness to palpation over the radial head. The patient had restricted elbow motion with 30° to 135° of flexion. He had 90° of pronation and supination. Ligamentous examination revealed stability of the elbow to both varus and valgus stress at 30° of flexion. No deficits were observed upon upper-extremity neurovascular examination.
Plain radiographs of the left elbow were initially taken. Anteroposterior, lateral, and Greenspan views revealed evidence of a displaced osteochondral fragment of the radial head in this skeletally immature patient. No involvement of the capitellum was apparent (Figures 1A-1C). Non-contrast magnetic resonance imaging (MRI) of the left elbow was subsequently obtained to evaluate the lesion further, and the images confirmed an unstable osteochondritis dissecans (OCD) lesion of the radial head with a detached fragment entrapped within the elbow joint (Figures 2A-2E).


Elbow arthroscopy was performed to evaluate the extent of the OCD lesion to enable determination of the integrity of the cartilaginous surface and remove the loose body entrapped within the elbow joint. Multiple loose bodies (all <5 mm in size) were removed from the elbow joint. Visualization of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. The main chondral defect measured approximately 4 mm in size. Probing of the lesion confirmed no stable edge; thus, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth (Figures 3A, 3B).

The patient was started on physical therapy consisting of active and active-assisted elbow ranges of motion on postoperative day 10. At the 6-week follow up, the patient presented to the office with pain-free motion of the left elbow ranging from −5° to 135° of flexion. He maintained full pronation and supination. At this point, the patient was advised to begin a throwing program. Three months after treatment, the patient resumed baseball activities, including throwing, with pain-free, full range of motion of the elbow. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Continue to: Discussion...
DISCUSSION
Elbow pain is a common complaint among young baseball players. OCD lesions, however, are an uncommon entity associated with elbow pathology.1 The overall incidence of OCD lesions is between 15 to 30 per 100,000 people.2-3 Specifically in patients aged 2 to 19 years, the incidence of elbow OCD lesions is 2.2 per 100,000 patients and 3.8 and 0.6 per 100,000 for males and females, respectively.4 Radial head OCD lesions are extremely rare, occurring in <5% of all elbow OCD cases.1 The majority of these lesions are asymptomatic and typically seen in patients who engage in repetitive overhead and upper-extremity weight-bearing activities. Reports indicate that the incidence of these lesions is on the rise and the age of presentation is decreasing, likely because of increased awareness of the disease and increasing involvement of young athletes in competitive athletics.4-5 Most patients with elbow OCD have a history of repetitive overuse of the elbow, as seen in baseball players, leading to excessive compressive and shear forces across the radiocapitellar joint and progression of the dissecans lesion.6
Patients with OCD lesions of the elbow typically present with inflammatory type symptoms and lateral elbow pain. The pain tends to be mild at rest and becomes more pronounced with activity. Patients often wait until mechanical symptoms ensue (eg, clicking, catching, or locking) before presenting to the office. On physical examination, pain in the region of the OCD lesion is usually accompanied by a mild effusion. Stiffness, particularly a loss of terminal extension, may accompany the mechanical symptoms on range of motion testing.7
Workup of elbow OCD lesions begins with obtaining plain radiographs of the elbow. Plain films are of limited use in evaluating these lesions but can help determine separation and the approximate size of the fragment.8 Further work-up must include MRI sequences, which allow for the best evaluation of the articular cartilage, underlying bone, and, specifically, the size and degree of separation of the OCD lesion.9
Nonoperative treatment of OCD lesions is usually successful if diagnosed early. Such treatment consists of activity modification, rest, anti-inflammatory medications, and a gradual return to athletic activities over the next 3 to 6 months provided the symptoms abate.10-11 During this interval, physical therapy may be employed to preserve or regain range of motion in the elbow. Clinical evidence has demonstrated improved outcomes in younger athletes with open physes.12 Returning to athletic activities is advised only when complete resolution of symptoms has been achieved and full motion about the elbow and shoulder girdle has been regained.6
If symptoms persist despite nonoperative management, or if evidence of an unstable lesion (ie, detached fragment) is obtained, operative intervention is appropriate. Operative management includes diagnostic arthroscopy of the entire elbow, removal of any small, loose bodies, and synovectomy as needed. Thereafter, the OCD lesion must be addressed. In cases of capitellar OCD lesions, if the articular cartilage surface is intact, antegrade or retrograde drilling of the subchondral bone is appropriate and will likely result in a good-to-excellent functional outcome.13-14 If disruption to the articular cartilage fissures is found or the lesion appears to be separating from the native bone, fixation of the fragment can be attempted, provided an adequate portion of the subchondral bone remains attached to the OCD lesion.6,14 Oftentimes, the bony bed must be prepared prior to fixation by removal of any fibrous tissue overlying the subchondral bone and ensuring adequate bleeding across the entire bed. Care should be taken to remove any fibrous tissue underlying the OCD lesion. If the OCD lesion is completely loose and/or the bone stock is insufficient or fragmented, arthroscopic removal of the OCD lesion followed by débridement and abrasion arthroplasty of subchondral bone is recommended.15 Improved functional outcomes from this procedure can be expected in contained lesions.15 If the patient continues to be symptomatic, osteochondral autograft or allograft procedures can be attempted depending on the size of the remaining defect.16-18
Other cases of radial head OCD lesions have been reported in the literature.19-20 In 2009, Dotzis and colleagues19 reported a case of an OCD lesion that was managed nonsurgically with observation alone as the lesion was stable and non-detached. Tatebe and colleagues20 reported 4 cases in which OCD involved the radial head and was accompanied by radial head subluxation. All lesions were located at the posteromedial aspect of the radial head with anterior subluxation of the radial head.20 Three of the cases were managed surgically via ulnar osteotomy (2 cases) and fragment removal (1 case).20 All except the 1 case treated by fragment excision revealed a good outcome.20 The patient in this case presented with a detached lesion, confirmed on MRI, with pain, mechanical symptoms, and of loss of terminal extension. Given the chronicity of the injury and the presence of mechanical symptoms, the decision was made to proceed with operative intervention. During elbow arthroscopy, multiple loose bodies were removed from the elbow joint, and inspection of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. Since the OCD lesion was completely loose and the bone stock was insufficient and too fragmented to attempt fixation, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth. At the 6-week follow up, the patient regained full range of motion of this elbow with no complaints of pain. At the 3-month follow up, the patient reported no pain after returning to throwing and all baseball-related activities.
CONCLUSION
This report presents an extremely rare case of an OCD lesion involving the radial head. Diagnosis and treatment of this lesion followed a protocol similar to that used for the management of capitellar OCD lesions. When dealing with elbow OCD lesions, especially in the skeletally immature patient population, nonsurgical management and a gradual return to activities should be attempted. If symptoms persist despite nonoperative management or evidence of an unstable lesion (as presented in this case) is obtained, operative intervention is appropriate.
ABSTRACT
This case shows an atypical presentation of an osteochondritis dissecans (OCD) lesion of the radial head with detachment diagnosed on plain radiographs and magnetic resonance imaging (MRI). OCD lesions are rather uncommon in the elbow joint; however, when present, these lesions are typically seen in throwing athletes or gymnasts who engage in activities involving repetitive trauma to the elbow. Involvement of the radial head is extremely rare, accounting for <5% of all elbow OCD lesions. Conventional radiographs have low sensitivity for detecting OCD lesions and may frequently miss these lesions in the early stages. MRI, the imaging modality of choice, can detect these lesions at the earliest stage and provide a clear picture of the involved articular cartilage and underlying bone. Treatment options can vary between nonoperative and operative management depending on several factors, including age and activity level of the patient, size and type of lesion, and clinical presentation. This case represents a radial head OCD lesion managed by arthroscopic débridement alone, resulting in a positive outcome.
Continue to: Case Report...
CASE REPORT
A healthy, 14-year-old, left-hand-dominant adolescent boy presented to the office with a chief complaint of pain localized to the posterolateral aspect of his elbow. He described an injury where he felt a “pop” in his elbow followed by immediate pain in the posterolateral elbow after throwing a pitch during a baseball game. Since the injury, the patient had experienced difficulty extending his elbow and a sharp, throbbing pain during forearm rotation. The patient also reported an intermittent clicking feeling in the elbow. Prior to this injury, he had no elbow pain. He presented in an otherwise normal state of health with no reported past medical or surgical history and no previous trauma to the left upper extremity.
Physical examination demonstrated a mild effusion of the left elbow in the region of the posterolateral corner or “soft spot” with tenderness to palpation over the radial head. The patient had restricted elbow motion with 30° to 135° of flexion. He had 90° of pronation and supination. Ligamentous examination revealed stability of the elbow to both varus and valgus stress at 30° of flexion. No deficits were observed upon upper-extremity neurovascular examination.
Plain radiographs of the left elbow were initially taken. Anteroposterior, lateral, and Greenspan views revealed evidence of a displaced osteochondral fragment of the radial head in this skeletally immature patient. No involvement of the capitellum was apparent (Figures 1A-1C). Non-contrast magnetic resonance imaging (MRI) of the left elbow was subsequently obtained to evaluate the lesion further, and the images confirmed an unstable osteochondritis dissecans (OCD) lesion of the radial head with a detached fragment entrapped within the elbow joint (Figures 2A-2E).


Elbow arthroscopy was performed to evaluate the extent of the OCD lesion to enable determination of the integrity of the cartilaginous surface and remove the loose body entrapped within the elbow joint. Multiple loose bodies (all <5 mm in size) were removed from the elbow joint. Visualization of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. The main chondral defect measured approximately 4 mm in size. Probing of the lesion confirmed no stable edge; thus, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth (Figures 3A, 3B).

The patient was started on physical therapy consisting of active and active-assisted elbow ranges of motion on postoperative day 10. At the 6-week follow up, the patient presented to the office with pain-free motion of the left elbow ranging from −5° to 135° of flexion. He maintained full pronation and supination. At this point, the patient was advised to begin a throwing program. Three months after treatment, the patient resumed baseball activities, including throwing, with pain-free, full range of motion of the elbow. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Continue to: Discussion...
DISCUSSION
Elbow pain is a common complaint among young baseball players. OCD lesions, however, are an uncommon entity associated with elbow pathology.1 The overall incidence of OCD lesions is between 15 to 30 per 100,000 people.2-3 Specifically in patients aged 2 to 19 years, the incidence of elbow OCD lesions is 2.2 per 100,000 patients and 3.8 and 0.6 per 100,000 for males and females, respectively.4 Radial head OCD lesions are extremely rare, occurring in <5% of all elbow OCD cases.1 The majority of these lesions are asymptomatic and typically seen in patients who engage in repetitive overhead and upper-extremity weight-bearing activities. Reports indicate that the incidence of these lesions is on the rise and the age of presentation is decreasing, likely because of increased awareness of the disease and increasing involvement of young athletes in competitive athletics.4-5 Most patients with elbow OCD have a history of repetitive overuse of the elbow, as seen in baseball players, leading to excessive compressive and shear forces across the radiocapitellar joint and progression of the dissecans lesion.6
Patients with OCD lesions of the elbow typically present with inflammatory type symptoms and lateral elbow pain. The pain tends to be mild at rest and becomes more pronounced with activity. Patients often wait until mechanical symptoms ensue (eg, clicking, catching, or locking) before presenting to the office. On physical examination, pain in the region of the OCD lesion is usually accompanied by a mild effusion. Stiffness, particularly a loss of terminal extension, may accompany the mechanical symptoms on range of motion testing.7
Workup of elbow OCD lesions begins with obtaining plain radiographs of the elbow. Plain films are of limited use in evaluating these lesions but can help determine separation and the approximate size of the fragment.8 Further work-up must include MRI sequences, which allow for the best evaluation of the articular cartilage, underlying bone, and, specifically, the size and degree of separation of the OCD lesion.9
Nonoperative treatment of OCD lesions is usually successful if diagnosed early. Such treatment consists of activity modification, rest, anti-inflammatory medications, and a gradual return to athletic activities over the next 3 to 6 months provided the symptoms abate.10-11 During this interval, physical therapy may be employed to preserve or regain range of motion in the elbow. Clinical evidence has demonstrated improved outcomes in younger athletes with open physes.12 Returning to athletic activities is advised only when complete resolution of symptoms has been achieved and full motion about the elbow and shoulder girdle has been regained.6
If symptoms persist despite nonoperative management, or if evidence of an unstable lesion (ie, detached fragment) is obtained, operative intervention is appropriate. Operative management includes diagnostic arthroscopy of the entire elbow, removal of any small, loose bodies, and synovectomy as needed. Thereafter, the OCD lesion must be addressed. In cases of capitellar OCD lesions, if the articular cartilage surface is intact, antegrade or retrograde drilling of the subchondral bone is appropriate and will likely result in a good-to-excellent functional outcome.13-14 If disruption to the articular cartilage fissures is found or the lesion appears to be separating from the native bone, fixation of the fragment can be attempted, provided an adequate portion of the subchondral bone remains attached to the OCD lesion.6,14 Oftentimes, the bony bed must be prepared prior to fixation by removal of any fibrous tissue overlying the subchondral bone and ensuring adequate bleeding across the entire bed. Care should be taken to remove any fibrous tissue underlying the OCD lesion. If the OCD lesion is completely loose and/or the bone stock is insufficient or fragmented, arthroscopic removal of the OCD lesion followed by débridement and abrasion arthroplasty of subchondral bone is recommended.15 Improved functional outcomes from this procedure can be expected in contained lesions.15 If the patient continues to be symptomatic, osteochondral autograft or allograft procedures can be attempted depending on the size of the remaining defect.16-18
Other cases of radial head OCD lesions have been reported in the literature.19-20 In 2009, Dotzis and colleagues19 reported a case of an OCD lesion that was managed nonsurgically with observation alone as the lesion was stable and non-detached. Tatebe and colleagues20 reported 4 cases in which OCD involved the radial head and was accompanied by radial head subluxation. All lesions were located at the posteromedial aspect of the radial head with anterior subluxation of the radial head.20 Three of the cases were managed surgically via ulnar osteotomy (2 cases) and fragment removal (1 case).20 All except the 1 case treated by fragment excision revealed a good outcome.20 The patient in this case presented with a detached lesion, confirmed on MRI, with pain, mechanical symptoms, and of loss of terminal extension. Given the chronicity of the injury and the presence of mechanical symptoms, the decision was made to proceed with operative intervention. During elbow arthroscopy, multiple loose bodies were removed from the elbow joint, and inspection of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. Since the OCD lesion was completely loose and the bone stock was insufficient and too fragmented to attempt fixation, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth. At the 6-week follow up, the patient regained full range of motion of this elbow with no complaints of pain. At the 3-month follow up, the patient reported no pain after returning to throwing and all baseball-related activities.
CONCLUSION
This report presents an extremely rare case of an OCD lesion involving the radial head. Diagnosis and treatment of this lesion followed a protocol similar to that used for the management of capitellar OCD lesions. When dealing with elbow OCD lesions, especially in the skeletally immature patient population, nonsurgical management and a gradual return to activities should be attempted. If symptoms persist despite nonoperative management or evidence of an unstable lesion (as presented in this case) is obtained, operative intervention is appropriate.
- Jans LB, Ditchfield M, Anna G, Jaremko JL, Verstraete KL. MR imaging findings and MR criteria for instability in osteochondritis dissecans of the elbow in children. Eur J Radiol. 2012;81(6):1306-1310. doi:10.1016/j.ejrad.2011.01.007.
- Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg. 1984;66(9):1340-1348. doi:10.2106/00004623-198466090-00003.
- Lindén B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47(6):664-667. doi:10.3109/17453677608988756.
- Kessler JI, Nikizad H, Shea KG, Jacobs JC, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42(2):320-326. doi:10.1177/0363546513510390.
- Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current Concepts Review. Am J Sports Med. 2006;34(7):1181-1191. doi:10.1177/0363546506290127.
- Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205-1214. doi:10.2106/JBJS.F.00622.
- Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteo Chondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216(1):207-212. doi:10.1148/radiology.216.1.r00jl29207.
- Kijowski R, De Smet AA. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skeletal Radiol. 2005;34(5):266-271. doi:10.1007/s00256-005-0899-6.
- Kijowski R, De Smet AA. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. AJR Am J Roentgenol. 2005;185:1453-1459. doi:10.2214/AJR.04.1570.
- Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27(6):728-732. doi:10.1177/03635465990270060701.
- Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363(363):108-115. doi:10.1097/00003086-199906000-00014.
- Pill SG, Ganley TJ, Milam RA, Lou JE, Meyer JS, Flynn JM. Role of magnetic resonance imaging and clinical criteria in predicting successful nonoperative treatment of osteochondritis dissecans in children. J Pediatr Orthop. 2003;23(1):102-108. doi:10.1097/01241398-200301000-00021.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Byrd JWT, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30(4):474-478. doi:10.1177/03635465020300040401.
- Krijnen MR, Lim L, Willems WJ. Arthroscopic treatment of osteochondritis dissecans of the capitellum: report of 5 female athletes. Arthroscopy. 2003;19(2):210-214. doi:10.1053/jars.2003.50052.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34(5):714-720. doi:10.1177/0363546505282620.
- Ahmad CS, ElAttrache NS. Mosaicplasty for capitellar osteochondritis dissecans. In: Yamaguchi K, O'Driscoll S, King G, McKee M, eds. [In press] Advanced Reconstruction Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons.
- Dotzis A, Galissier B, Peyrou P, Longis B, Moulies D. Osteochondritis dissecans of the radial head: a case report. J Shoulder Elbow Surg. 2009;18(1):e18-e21. doi:10.1016/j.jse.2008.04.009.
- Tatebe M, Hirata H, Shinohara T, Yamamoto M, Morita A, Horii E. Pathomechanical significance of radial head subluxation in the onset of osteochondritis dissecans of the radial head. J Orthop Trauma. 2012;26(1):e4-e6. doi:10.1097/BOT.0b013e318214d678.
- Jans LB, Ditchfield M, Anna G, Jaremko JL, Verstraete KL. MR imaging findings and MR criteria for instability in osteochondritis dissecans of the elbow in children. Eur J Radiol. 2012;81(6):1306-1310. doi:10.1016/j.ejrad.2011.01.007.
- Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg. 1984;66(9):1340-1348. doi:10.2106/00004623-198466090-00003.
- Lindén B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47(6):664-667. doi:10.3109/17453677608988756.
- Kessler JI, Nikizad H, Shea KG, Jacobs JC, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42(2):320-326. doi:10.1177/0363546513510390.
- Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current Concepts Review. Am J Sports Med. 2006;34(7):1181-1191. doi:10.1177/0363546506290127.
- Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205-1214. doi:10.2106/JBJS.F.00622.
- Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteo Chondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216(1):207-212. doi:10.1148/radiology.216.1.r00jl29207.
- Kijowski R, De Smet AA. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skeletal Radiol. 2005;34(5):266-271. doi:10.1007/s00256-005-0899-6.
- Kijowski R, De Smet AA. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. AJR Am J Roentgenol. 2005;185:1453-1459. doi:10.2214/AJR.04.1570.
- Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27(6):728-732. doi:10.1177/03635465990270060701.
- Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363(363):108-115. doi:10.1097/00003086-199906000-00014.
- Pill SG, Ganley TJ, Milam RA, Lou JE, Meyer JS, Flynn JM. Role of magnetic resonance imaging and clinical criteria in predicting successful nonoperative treatment of osteochondritis dissecans in children. J Pediatr Orthop. 2003;23(1):102-108. doi:10.1097/01241398-200301000-00021.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Byrd JWT, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30(4):474-478. doi:10.1177/03635465020300040401.
- Krijnen MR, Lim L, Willems WJ. Arthroscopic treatment of osteochondritis dissecans of the capitellum: report of 5 female athletes. Arthroscopy. 2003;19(2):210-214. doi:10.1053/jars.2003.50052.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34(5):714-720. doi:10.1177/0363546505282620.
- Ahmad CS, ElAttrache NS. Mosaicplasty for capitellar osteochondritis dissecans. In: Yamaguchi K, O'Driscoll S, King G, McKee M, eds. [In press] Advanced Reconstruction Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons.
- Dotzis A, Galissier B, Peyrou P, Longis B, Moulies D. Osteochondritis dissecans of the radial head: a case report. J Shoulder Elbow Surg. 2009;18(1):e18-e21. doi:10.1016/j.jse.2008.04.009.
- Tatebe M, Hirata H, Shinohara T, Yamamoto M, Morita A, Horii E. Pathomechanical significance of radial head subluxation in the onset of osteochondritis dissecans of the radial head. J Orthop Trauma. 2012;26(1):e4-e6. doi:10.1097/BOT.0b013e318214d678.
TAKE-HOME POINTS
- Radial Head OCD lesions are uncommon.
- Typically present in athletes that engage in repetitive trauma to elbow (throwers, gymnasts).
- MRI is the best modality for making diagnosis.
- Attempt nonsurgical treatment initially, especially in skeletally immature patients.
- If nonsurgical fails or there is an unstable lesion, consider operative intervention.
Volumetric Considerations for Valving Long-Arm Casts: The Utility of the Cast Spacer
ABSTRACT
Fiberglass casts are frequently valved to accommodate swelling following injury or surgery. The use of cast spacers has been recommended to bridge this gap between pressure reduction and cast strength, but no studies have assessed their effect on cast pressure.
We applied 30 long-arm fiberglass casts to adult volunteers, divided between a univalve group and a bivalve group. A pediatric blood pressure bladder was applied under the cast to simulate soft tissue swelling. Valved casts were secured using an elastic wrap, 10-mm cast spacer, or 15-mm cast spacer. Measurements of cast pressure and circumference were performed at each stage and compared on the basis of type of valve and securement.
Our results indicated that cast univalving resulted in an approximately 60% reduction in cast pressures, with a 75% reduction seen in the bivalve group. The addition of cast spacers resulted in significant pressure reductions for both valving groups. The univalve group secured with a 10-mm cast spacer produced reductions in cast pressure similar to those of the elastic-wrapped bivalve cast, both with the cast padding intact and with it released.
The use of cast spacers results in significant cast pressure reductions, regardless of valving technique. A univalved cast secured with a cast spacer can produce decreases in cast pressures similar to those seen with an elastic-wrapped bivalved cast, and it is a viable option for reducing cast pressure without compromising cast structural integrity with a bivalve technique.
Continue to: Complications following closed reduction...
Complications following closed reduction and casting of pediatric forearm fractures are rare, but they do occur. Arguably the most devastating of these complications is the risk of developing compartment syndrome or Volkmann contracture secondary to injury-associated swelling under a circumferential cast.1-4 The peak in swelling can develop from 4 to 24 hours following the initial cast application,5 and as such, medical providers may not be able to identify it early because most children are discharged following closed reductions. For this reason, many providers implement prophylactic measures to minimize pressure-related complications.
A popular method for reducing pressure accumulation within a cast is to valve, or cut, the cast. Previous investigations have shown that cast valving results in significant reductions in cast pressure.2,6-9 Bivalving a circumferential cast results in significantly greater reductions in cast pressure when compared with univalve techniques;6,7,9 however, bivalving has also been shown to result in significant impairment in the structural integrity of the cast.10 An additional method to facilitate cast pressure reduction without impairing the structural integrity of the cast that accompanies a bivalve is to incorporate a cast spacer with a univalve technique to hold the split cast open.11 Although this method is commonly used in clinical practice, its ability to mitigate cast pressures has not previously been investigated.
The goal of this study is to investigate the influence of incorporating cast spacers with valved long-arm casts. We hypothesized that cast spacers would provide a greater pressure reduction for both univalved and bivalved casts when compared with the use of an elastic wrap. Additionally, we proposed that by incorporating a cast spacer with a univalved cast, we could attain pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap.
MATERIALS AND METHODS
Upon receiving approval from the Institutional Review Board, experimental testing began with the application of 30 total casts performed on uninjured adult human volunteers. Pressure readings were provided with the use of a bladder from a pediatric blood pressure cuff (Welch Allyn Inc), as previously described.6 The bladder was placed on the volar aspect of the volunteer’s forearm, held in place with a 3-in diameter cotton stockinet (3M). Cotton cast padding (Webril-Kendall) was applied, 3 in wide and 2 layers thick, and a long-arm cast was applied, 2 layers thick with 3-in wide fiberglass casting material (Scotchcast Plus Casting Tape; 3M).
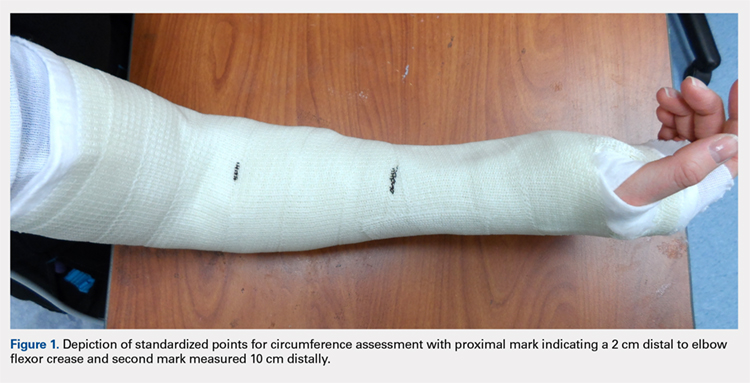
Once the cast was applied and allowed to set, the blood pressure bladder was inflated to 100 mm Hg. After inflation, forearm cast circumference was measured at 2 set points, assessed at points 2 cm distal to the elbow flexor crease and 10 cm distal to the previous point (Figure 1). Using these data, we calculated estimated cast volume using the volumetric equation for a frustum. Following this point, casts were split into 2 experimental groups, univalve or bivalve, with 15 casts comprising each group. The univalve group consisted of a single cut along the dorsum of the extremity, and the bivalve group incorporated a second cut to the volar extremity. Cast valving was performed using an oscillating cast saw (Cast Vac; Stryker Instruments), with care taken to ensure the continuity of the underlying cast padding.
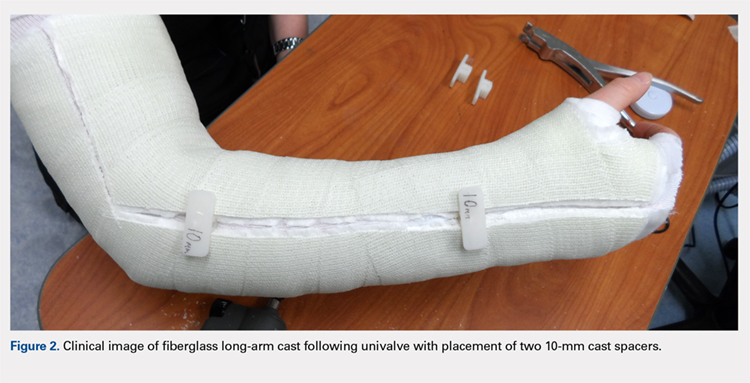
Continue to: Following valving, casts were secured via...
Following valving, casts were secured via 3 separate techniques: overwrap with a 3-in elastic wrap (Econo Wrap; Vitality Medical), application of two 10-mm and 15-mm cast spacers (CastWedge; DM Systems) (Figure 2). After securement, cast pressures were recorded, and circumference measurements were performed at the 2 previously identified points. The cast padding was then cut at the valve site and secured via the 3 listed techniques. Cast pressure and circumference measurements were performed at set time points (Figure 3). Changes in cast pressure were recorded in terms of the amount of change from the initial cast placement to account for differences in the size of volunteers’ forearms. Volumetric calculations were performed only for the spacer subgroups owing to the added material in the elastic wrap group. Estimated cast volume was calculated using the equation for volume of a frustum (Figure 4).
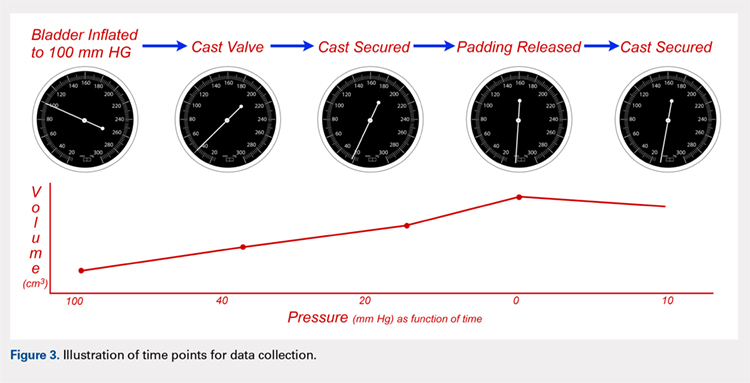
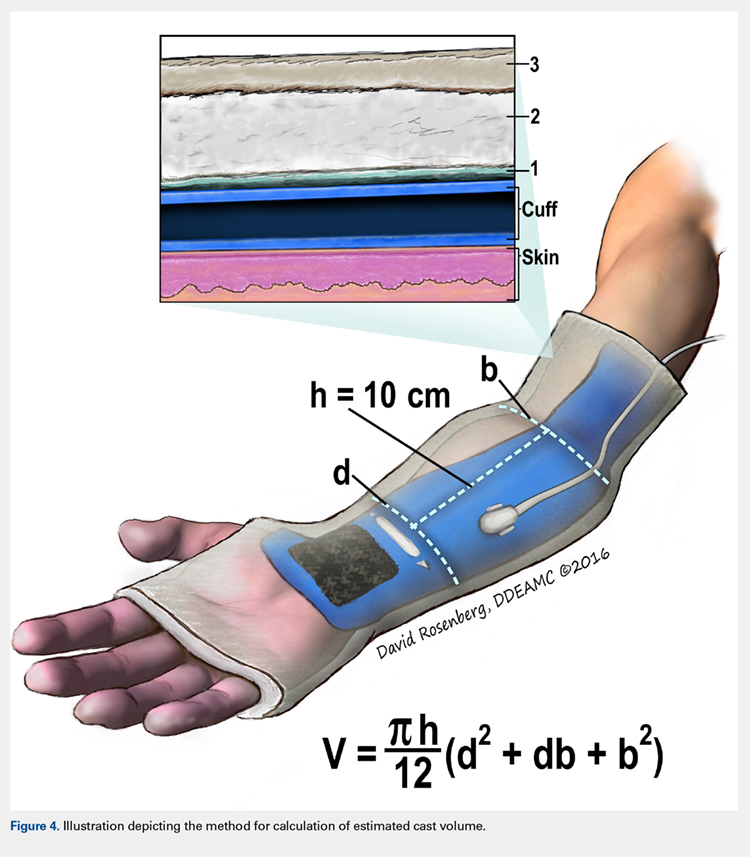
We used a 2-cast type (univalve and bivalve) by 4 securement subgroups (initial, elastic wrap, 10-mm spacer, and 15-mm spacer) design, with cast type serving as a between-subject measure and securement serving as a within-subject variable. An a priori power analysis showed that a minimum sample size of 15 subjects per condition should provide sufficient power of .80 and alpha set at .05, for a total of 30 casts. Statistical analyses were performed using IBM SPSS Statistics software version 21 (IBM). Experimental groups were analyzed using mixed-design analysis of variance (ANOVA). Post hoc comparisons between valving groups and cast securement were performed using Scheffe’s test to control for type II errors. Change in cast volume between the initial cast and cast spacers groups was compared using paired Student’s t tests. Statistical significance was predetermined as P < .05.
RESULTS
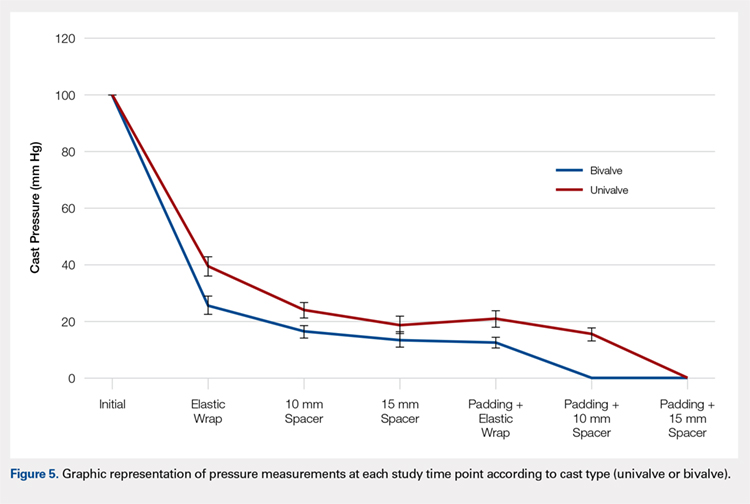
A summary of collected data for cast pressure and volume is detailed in Table 1, subdividing the variables on the basis of cast type and type of securement. Recorded pressures of the different subgroups are depicted in Figures 5 and 6 according to type of securement (initial, elastic wrap, 10-mm spacer, or 15-mm spacer). Results of the mixed-design ANOVA demonstrated significant differences between the initial cast pressure and univalve and bivalve groups (P < .05). There was a main effect for bivalve having lower pressure overall (F [1, 1)] = 3321.51, P < .001). There was also a main effect indicating that pressure was different for each type of securement (elastic wrap, 10-mm spacer, 15-mm spacer) (F [1, 28] = 538.54, P <. 01). Post hoc testing confirmed pressure decreased significantly, in descending order from elastic wrap, to 10-mm spacers, to 15-mm spacers (P < .05).
Table 1. Cumulative Data for Two Casting groups at Each Timepoint
Cast | Pressure | Standard Deviation | Volume |
Univalve |
|
|
|
Initial | 100 | --- | 2654.3 |
Elastic Wrap | 39.47 | 3.33 | --- |
10-mm Spacer | 23.93 | 2.73 | 2708.23 |
15-mm Spacer | 18.87 | 2.94 | 2734.86 |
Padding and Elastic Wrap | 20.93 | 2.91 | --- |
Padding and 10-mm Spacer | 15.46 | 2.19 | 2733.24 |
Padding and 15-mm Spacer | 0 | --- | 2819.27 |
Bivalve |
|
|
|
Initial | 100 | --- | 2839.3 |
Elastic Wrap | 25.9 | 3.17 | --- |
10-mm Spacer | 16.53 | 2.32 | 3203.13 |
15-mm Spacer | 13.6 | 2.74 | 3380.32 |
Padding and Elastic Wrap | 12.67 | 1.95 | --- |
Padding and 10-mm Spacer | 0 | --- | 3296.55 |
Padding and 15- mm Spacer | 0 | --- | 3438.67 |
Continue to: Table 2...
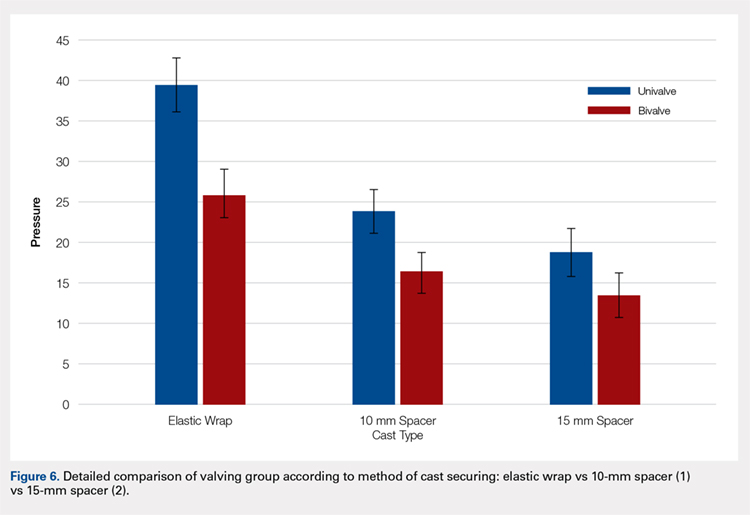
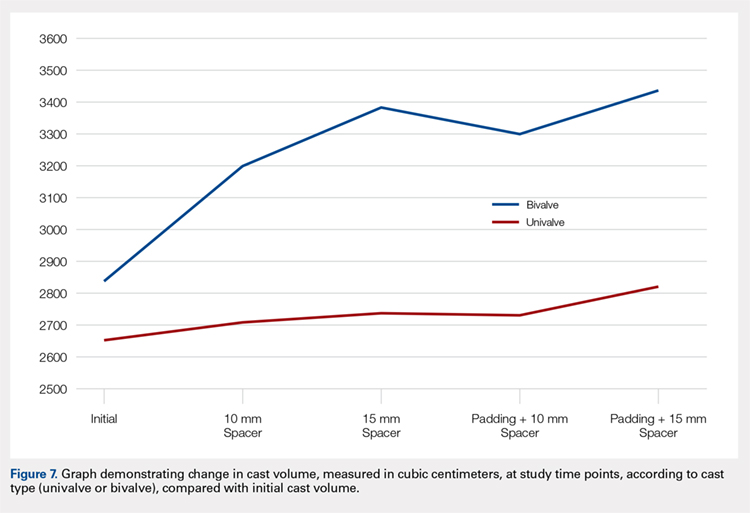
The summary of volumetric changes is listed in Table 2. The decrease in pressure correlated with an associated increase in cast volume, as demonstrated in Figure 7. The degree of increase in cast volume was more pronounced in the bivalve group (P < .001). The volume increased in the 15-mm group compared with the 10-mm group for both groups (P < .001) and increased for each spacer group with the release of the underlying padding (P < .05).
Table 2. Volumetric Data
Cast | Average Volumetric change (cm3) | Standard Deviation |
Univalve |
|
|
10-mm Spacer | 175.6 | 65.4 |
15-mm Spacer | 269.4 | 73.3 |
Padding and 10-mm Spacer | 202.3 | 62.5 |
Padding and 15-mm Spacer | 294.1 | 66.9 |
Bivalve |
|
|
10-mm Spacer | 363.7 | 67.2 |
15-mm Spacer | 540.9 | 85.7 |
Padding and 10-mm Spacer | 457.2 | 97.9 |
Padding and 15-mm Spacer | 599.3 | 84.2 |
Analysis of the planned comparisons demonstrated no significant difference between the bivalve with elastic wrap and univalve with 10-mm spacer subgroups (t [28] = 1.85, P = .075, d = .68). In comparing the bivalve with elastic wrap group with the univalve and 15-mm spacer subgroup, the univalve group showed significantly lower pressures [t [28] = 6.32, P < .001, d = .2.31).
DISCUSSION
Valving of circumferential casting is a well-established technique to minimize potential pressure-related complications. Previous studies have demonstrated that univalving techniques produce a 65% reduction in cast pressure, whereas bivalving produces an 80% decrease.6,7,9 Our results showed comparable pressure reductions of 75% with bivalving and 60% with univalving. The type of cast padding has been shown to have a significant effect on the cast pressure, favoring lower pressures with cotton padding over synthetic and waterproof padding, which, when released, can provide an additional 10% pressure reduction.6,7
Although bivalving techniques are superior in pressure reduction, the reduction comes at the cost of the cast’s structural integrity. Crickard and colleagues10 performed a biomechanical assessment of the structural integrity by 3-point bending of casts following univalve and bivalve compared with an intact cast. The authors found that valving resulted in a significant decrease in the casts’ bending stiffness and load to failure, with bivalved casts demonstrating a significantly lower load to failure than univalved casts. One technique that has been used to enhance the pressure reduction in univalved casting techniques is the application of a cast spacer. Rang and colleagues11 recommended this technique as part of a graded cast-splitting approach for the treatment of children’s fractures. This technique was applied to fractures with only modest anticipated swelling, which accounted for approximately 95% of casts applied in their children’s hospital. Our results support the use of cast spacers, demonstrating significant reduction in cast pressure in both univalve and bivalve techniques. Additionally, we found that a univalved cast with a 10-mm cast spacer provided pressure reduction similar to that of a bivalved cast.
The theory behind the application of cast spacers is that a split fiberglass cast will not remain open unless held in position.11 Holding the cast open is less of a restraint to pressure reduction in bivalving techniques, because the split cast no longer has the contralateral intact hinge point to resist cast opening, demonstrated in the compromise in structural integrity seen with this technique.10 By maintaining the split cast in an opened position, the effective volume of the cast is increased, which allows for the reduction in cast pressure. This is demonstrated in our results indicating an increase in estimated cast volume with an associated incremental reduction in cast pressure with the application of incrementally sized cast spacers. Although this technique does have the potential for skin irritation caused by cast expansion, as well as local swelling at the cast window location, it is a cost-effective treatment method compared with overwrapping a bivalved cast, $1.55 for 1 cast spacer vs an estimated $200 for a forearm cast application.
This study is not without its limitations. Our model does not account for the soft tissue injury associated with forearm fractures. However, by using human volunteers, we were able to include the viscoelastic properties that are omitted with nonliving models, and our results do align with those of previous investigations regarding pressure change following valving. We did not incorporate a 3-point molding technique commonly used with reduction and casting of acute forearm fractures, owing to the lack of a standardized method for applying the mold to healthy volunteers. Although molding is necessary for most fractures in which valving is considered, we believe our data still provide valuable information. Additionally, valving of circumferential casts has not been shown, prospectively, to result in a reduction of cast-related compartment syndrome, maintenance of reduction, or need for surgery.12,13 However, these results are reflective of reliable patients who completed the requisite follow-up care necessary for inclusion in a randomized controlled trial and may be applicable to unreliable patients or patient situations, a setting in which the compromise in cast structural integrity may be unacceptable.
CONCLUSION
We demonstrated that incorporating cast spacers into valved long-arm casts provides pressure reduction comparable to that achieved with the use of an elastic wrap. The addition of a 10-mm cast spacer to a univalved long-arm cast provides pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap. A univalved cast secured with a cast spacer is a viable option for treatment of displaced pediatric forearm fractures, without compromising the cast’s structural integrity as required with bivalved techniques.
This paper will be judged for the Resident Writer’s Award.
- Halanski M, Noonan KJ. Cast and splint immobilization: complications. J Am Acad Orthop Surg. 2008;16(1):30-40.
- Zaino CJ, Patel MR, Arief MS, Pivec R. The effectiveness of bivalving, cast spreading, and webril cutting to reduce cast pressure in a fiberglass short arm cast. J Bone Joint Surg Am. 2015;97(5):374-380. doi:10.2106/JBJS.N.00579.
- Rodriguez-Merchan EC. Pediatric fractures of the forearm. Clin Orthop Relat Res. 2005;(432):65-72.
- von Volkmann R. Ischaemic muscle paralyses and contractures. Clin Orthop Relat Res. 1967;50:5-56. doi:10.1097/BLO.0b013e318032561f.
- Patrick JH, Levack B. A study of pressures beneath forearm plasters. Injury. 1981;13(1):37-41.
- Roberts A, Shaw KA, Boomsma SE, Cameron CD. Effect of casting material on the cast pressure after sequential cast splitting. J Pediatr Orthop. 2017;37(1):74-77. doi:10.1097/BPO.0000000000000574.
- Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
- Capo JT, Renard RL, Moulton MJ, et al. How is forearm compliance affected by various circumferential dressings? Clin Orthop Relat Res. 2014 472(10):3228-3234. doi:10.1007/s11999-014-3747-y.
- Bingold AC. On splitting plasters. A useful analogy. J Bone Joint Surg Br. 1979;61-b(3):294-295.
- Crickard CV, Riccio AI, Carney JR, Anderson TD. Analysis and comparison of the biomechanical properties of univalved and bivalved cast models. J Pediatr Orthop.2011;31(1):39-43. doi:10.1097/BPO.0b013e318202c446.
- Rang M, Wenger DR, Pring ME. Rang's Children's Fractures. 3rd ed. Wenger DR, Rang M, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Schulte D, Habernig S, Zuzak T, et al. Forearm fractures in children: split opinions about splitting the cast. Eur J Pediatr Surg. 2014;24(2):163-167. doi:10.1055/s-0033-1341412.
- Bae DS, Valim C, Connell P, Brustowicz KA, Waters PM. Bivalved versus circumferential cast immobilization for displaced forearm fractures: a randomized clinical trial to assess efficacy and safety. J Pediatr Orthop. 2017;37(4):239-246 doi:10.1097/BPO.0000000000000655.
ABSTRACT
Fiberglass casts are frequently valved to accommodate swelling following injury or surgery. The use of cast spacers has been recommended to bridge this gap between pressure reduction and cast strength, but no studies have assessed their effect on cast pressure.
We applied 30 long-arm fiberglass casts to adult volunteers, divided between a univalve group and a bivalve group. A pediatric blood pressure bladder was applied under the cast to simulate soft tissue swelling. Valved casts were secured using an elastic wrap, 10-mm cast spacer, or 15-mm cast spacer. Measurements of cast pressure and circumference were performed at each stage and compared on the basis of type of valve and securement.
Our results indicated that cast univalving resulted in an approximately 60% reduction in cast pressures, with a 75% reduction seen in the bivalve group. The addition of cast spacers resulted in significant pressure reductions for both valving groups. The univalve group secured with a 10-mm cast spacer produced reductions in cast pressure similar to those of the elastic-wrapped bivalve cast, both with the cast padding intact and with it released.
The use of cast spacers results in significant cast pressure reductions, regardless of valving technique. A univalved cast secured with a cast spacer can produce decreases in cast pressures similar to those seen with an elastic-wrapped bivalved cast, and it is a viable option for reducing cast pressure without compromising cast structural integrity with a bivalve technique.
Continue to: Complications following closed reduction...
Complications following closed reduction and casting of pediatric forearm fractures are rare, but they do occur. Arguably the most devastating of these complications is the risk of developing compartment syndrome or Volkmann contracture secondary to injury-associated swelling under a circumferential cast.1-4 The peak in swelling can develop from 4 to 24 hours following the initial cast application,5 and as such, medical providers may not be able to identify it early because most children are discharged following closed reductions. For this reason, many providers implement prophylactic measures to minimize pressure-related complications.
A popular method for reducing pressure accumulation within a cast is to valve, or cut, the cast. Previous investigations have shown that cast valving results in significant reductions in cast pressure.2,6-9 Bivalving a circumferential cast results in significantly greater reductions in cast pressure when compared with univalve techniques;6,7,9 however, bivalving has also been shown to result in significant impairment in the structural integrity of the cast.10 An additional method to facilitate cast pressure reduction without impairing the structural integrity of the cast that accompanies a bivalve is to incorporate a cast spacer with a univalve technique to hold the split cast open.11 Although this method is commonly used in clinical practice, its ability to mitigate cast pressures has not previously been investigated.
The goal of this study is to investigate the influence of incorporating cast spacers with valved long-arm casts. We hypothesized that cast spacers would provide a greater pressure reduction for both univalved and bivalved casts when compared with the use of an elastic wrap. Additionally, we proposed that by incorporating a cast spacer with a univalved cast, we could attain pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap.
MATERIALS AND METHODS
Upon receiving approval from the Institutional Review Board, experimental testing began with the application of 30 total casts performed on uninjured adult human volunteers. Pressure readings were provided with the use of a bladder from a pediatric blood pressure cuff (Welch Allyn Inc), as previously described.6 The bladder was placed on the volar aspect of the volunteer’s forearm, held in place with a 3-in diameter cotton stockinet (3M). Cotton cast padding (Webril-Kendall) was applied, 3 in wide and 2 layers thick, and a long-arm cast was applied, 2 layers thick with 3-in wide fiberglass casting material (Scotchcast Plus Casting Tape; 3M).
Once the cast was applied and allowed to set, the blood pressure bladder was inflated to 100 mm Hg. After inflation, forearm cast circumference was measured at 2 set points, assessed at points 2 cm distal to the elbow flexor crease and 10 cm distal to the previous point (Figure 1). Using these data, we calculated estimated cast volume using the volumetric equation for a frustum. Following this point, casts were split into 2 experimental groups, univalve or bivalve, with 15 casts comprising each group. The univalve group consisted of a single cut along the dorsum of the extremity, and the bivalve group incorporated a second cut to the volar extremity. Cast valving was performed using an oscillating cast saw (Cast Vac; Stryker Instruments), with care taken to ensure the continuity of the underlying cast padding.

Continue to: Following valving, casts were secured via...
Following valving, casts were secured via 3 separate techniques: overwrap with a 3-in elastic wrap (Econo Wrap; Vitality Medical), application of two 10-mm and 15-mm cast spacers (CastWedge; DM Systems) (Figure 2). After securement, cast pressures were recorded, and circumference measurements were performed at the 2 previously identified points. The cast padding was then cut at the valve site and secured via the 3 listed techniques. Cast pressure and circumference measurements were performed at set time points (Figure 3). Changes in cast pressure were recorded in terms of the amount of change from the initial cast placement to account for differences in the size of volunteers’ forearms. Volumetric calculations were performed only for the spacer subgroups owing to the added material in the elastic wrap group. Estimated cast volume was calculated using the equation for volume of a frustum (Figure 4).



We used a 2-cast type (univalve and bivalve) by 4 securement subgroups (initial, elastic wrap, 10-mm spacer, and 15-mm spacer) design, with cast type serving as a between-subject measure and securement serving as a within-subject variable. An a priori power analysis showed that a minimum sample size of 15 subjects per condition should provide sufficient power of .80 and alpha set at .05, for a total of 30 casts. Statistical analyses were performed using IBM SPSS Statistics software version 21 (IBM). Experimental groups were analyzed using mixed-design analysis of variance (ANOVA). Post hoc comparisons between valving groups and cast securement were performed using Scheffe’s test to control for type II errors. Change in cast volume between the initial cast and cast spacers groups was compared using paired Student’s t tests. Statistical significance was predetermined as P < .05.
RESULTS
A summary of collected data for cast pressure and volume is detailed in Table 1, subdividing the variables on the basis of cast type and type of securement. Recorded pressures of the different subgroups are depicted in Figures 5 and 6 according to type of securement (initial, elastic wrap, 10-mm spacer, or 15-mm spacer). Results of the mixed-design ANOVA demonstrated significant differences between the initial cast pressure and univalve and bivalve groups (P < .05). There was a main effect for bivalve having lower pressure overall (F [1, 1)] = 3321.51, P < .001). There was also a main effect indicating that pressure was different for each type of securement (elastic wrap, 10-mm spacer, 15-mm spacer) (F [1, 28] = 538.54, P <. 01). Post hoc testing confirmed pressure decreased significantly, in descending order from elastic wrap, to 10-mm spacers, to 15-mm spacers (P < .05).
Table 1. Cumulative Data for Two Casting groups at Each Timepoint
Cast | Pressure | Standard Deviation | Volume |
Univalve |
|
|
|
Initial | 100 | --- | 2654.3 |
Elastic Wrap | 39.47 | 3.33 | --- |
10-mm Spacer | 23.93 | 2.73 | 2708.23 |
15-mm Spacer | 18.87 | 2.94 | 2734.86 |
Padding and Elastic Wrap | 20.93 | 2.91 | --- |
Padding and 10-mm Spacer | 15.46 | 2.19 | 2733.24 |
Padding and 15-mm Spacer | 0 | --- | 2819.27 |
Bivalve |
|
|
|
Initial | 100 | --- | 2839.3 |
Elastic Wrap | 25.9 | 3.17 | --- |
10-mm Spacer | 16.53 | 2.32 | 3203.13 |
15-mm Spacer | 13.6 | 2.74 | 3380.32 |
Padding and Elastic Wrap | 12.67 | 1.95 | --- |
Padding and 10-mm Spacer | 0 | --- | 3296.55 |
Padding and 15- mm Spacer | 0 | --- | 3438.67 |


Continue to: Table 2...
The summary of volumetric changes is listed in Table 2. The decrease in pressure correlated with an associated increase in cast volume, as demonstrated in Figure 7. The degree of increase in cast volume was more pronounced in the bivalve group (P < .001). The volume increased in the 15-mm group compared with the 10-mm group for both groups (P < .001) and increased for each spacer group with the release of the underlying padding (P < .05).
Table 2. Volumetric Data
Cast | Average Volumetric change (cm3) | Standard Deviation |
Univalve |
|
|
10-mm Spacer | 175.6 | 65.4 |
15-mm Spacer | 269.4 | 73.3 |
Padding and 10-mm Spacer | 202.3 | 62.5 |
Padding and 15-mm Spacer | 294.1 | 66.9 |
Bivalve |
|
|
10-mm Spacer | 363.7 | 67.2 |
15-mm Spacer | 540.9 | 85.7 |
Padding and 10-mm Spacer | 457.2 | 97.9 |
Padding and 15-mm Spacer | 599.3 | 84.2 |

Analysis of the planned comparisons demonstrated no significant difference between the bivalve with elastic wrap and univalve with 10-mm spacer subgroups (t [28] = 1.85, P = .075, d = .68). In comparing the bivalve with elastic wrap group with the univalve and 15-mm spacer subgroup, the univalve group showed significantly lower pressures [t [28] = 6.32, P < .001, d = .2.31).
DISCUSSION
Valving of circumferential casting is a well-established technique to minimize potential pressure-related complications. Previous studies have demonstrated that univalving techniques produce a 65% reduction in cast pressure, whereas bivalving produces an 80% decrease.6,7,9 Our results showed comparable pressure reductions of 75% with bivalving and 60% with univalving. The type of cast padding has been shown to have a significant effect on the cast pressure, favoring lower pressures with cotton padding over synthetic and waterproof padding, which, when released, can provide an additional 10% pressure reduction.6,7
Although bivalving techniques are superior in pressure reduction, the reduction comes at the cost of the cast’s structural integrity. Crickard and colleagues10 performed a biomechanical assessment of the structural integrity by 3-point bending of casts following univalve and bivalve compared with an intact cast. The authors found that valving resulted in a significant decrease in the casts’ bending stiffness and load to failure, with bivalved casts demonstrating a significantly lower load to failure than univalved casts. One technique that has been used to enhance the pressure reduction in univalved casting techniques is the application of a cast spacer. Rang and colleagues11 recommended this technique as part of a graded cast-splitting approach for the treatment of children’s fractures. This technique was applied to fractures with only modest anticipated swelling, which accounted for approximately 95% of casts applied in their children’s hospital. Our results support the use of cast spacers, demonstrating significant reduction in cast pressure in both univalve and bivalve techniques. Additionally, we found that a univalved cast with a 10-mm cast spacer provided pressure reduction similar to that of a bivalved cast.
The theory behind the application of cast spacers is that a split fiberglass cast will not remain open unless held in position.11 Holding the cast open is less of a restraint to pressure reduction in bivalving techniques, because the split cast no longer has the contralateral intact hinge point to resist cast opening, demonstrated in the compromise in structural integrity seen with this technique.10 By maintaining the split cast in an opened position, the effective volume of the cast is increased, which allows for the reduction in cast pressure. This is demonstrated in our results indicating an increase in estimated cast volume with an associated incremental reduction in cast pressure with the application of incrementally sized cast spacers. Although this technique does have the potential for skin irritation caused by cast expansion, as well as local swelling at the cast window location, it is a cost-effective treatment method compared with overwrapping a bivalved cast, $1.55 for 1 cast spacer vs an estimated $200 for a forearm cast application.
This study is not without its limitations. Our model does not account for the soft tissue injury associated with forearm fractures. However, by using human volunteers, we were able to include the viscoelastic properties that are omitted with nonliving models, and our results do align with those of previous investigations regarding pressure change following valving. We did not incorporate a 3-point molding technique commonly used with reduction and casting of acute forearm fractures, owing to the lack of a standardized method for applying the mold to healthy volunteers. Although molding is necessary for most fractures in which valving is considered, we believe our data still provide valuable information. Additionally, valving of circumferential casts has not been shown, prospectively, to result in a reduction of cast-related compartment syndrome, maintenance of reduction, or need for surgery.12,13 However, these results are reflective of reliable patients who completed the requisite follow-up care necessary for inclusion in a randomized controlled trial and may be applicable to unreliable patients or patient situations, a setting in which the compromise in cast structural integrity may be unacceptable.
CONCLUSION
We demonstrated that incorporating cast spacers into valved long-arm casts provides pressure reduction comparable to that achieved with the use of an elastic wrap. The addition of a 10-mm cast spacer to a univalved long-arm cast provides pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap. A univalved cast secured with a cast spacer is a viable option for treatment of displaced pediatric forearm fractures, without compromising the cast’s structural integrity as required with bivalved techniques.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Fiberglass casts are frequently valved to accommodate swelling following injury or surgery. The use of cast spacers has been recommended to bridge this gap between pressure reduction and cast strength, but no studies have assessed their effect on cast pressure.
We applied 30 long-arm fiberglass casts to adult volunteers, divided between a univalve group and a bivalve group. A pediatric blood pressure bladder was applied under the cast to simulate soft tissue swelling. Valved casts were secured using an elastic wrap, 10-mm cast spacer, or 15-mm cast spacer. Measurements of cast pressure and circumference were performed at each stage and compared on the basis of type of valve and securement.
Our results indicated that cast univalving resulted in an approximately 60% reduction in cast pressures, with a 75% reduction seen in the bivalve group. The addition of cast spacers resulted in significant pressure reductions for both valving groups. The univalve group secured with a 10-mm cast spacer produced reductions in cast pressure similar to those of the elastic-wrapped bivalve cast, both with the cast padding intact and with it released.
The use of cast spacers results in significant cast pressure reductions, regardless of valving technique. A univalved cast secured with a cast spacer can produce decreases in cast pressures similar to those seen with an elastic-wrapped bivalved cast, and it is a viable option for reducing cast pressure without compromising cast structural integrity with a bivalve technique.
Continue to: Complications following closed reduction...
Complications following closed reduction and casting of pediatric forearm fractures are rare, but they do occur. Arguably the most devastating of these complications is the risk of developing compartment syndrome or Volkmann contracture secondary to injury-associated swelling under a circumferential cast.1-4 The peak in swelling can develop from 4 to 24 hours following the initial cast application,5 and as such, medical providers may not be able to identify it early because most children are discharged following closed reductions. For this reason, many providers implement prophylactic measures to minimize pressure-related complications.
A popular method for reducing pressure accumulation within a cast is to valve, or cut, the cast. Previous investigations have shown that cast valving results in significant reductions in cast pressure.2,6-9 Bivalving a circumferential cast results in significantly greater reductions in cast pressure when compared with univalve techniques;6,7,9 however, bivalving has also been shown to result in significant impairment in the structural integrity of the cast.10 An additional method to facilitate cast pressure reduction without impairing the structural integrity of the cast that accompanies a bivalve is to incorporate a cast spacer with a univalve technique to hold the split cast open.11 Although this method is commonly used in clinical practice, its ability to mitigate cast pressures has not previously been investigated.
The goal of this study is to investigate the influence of incorporating cast spacers with valved long-arm casts. We hypothesized that cast spacers would provide a greater pressure reduction for both univalved and bivalved casts when compared with the use of an elastic wrap. Additionally, we proposed that by incorporating a cast spacer with a univalved cast, we could attain pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap.
MATERIALS AND METHODS
Upon receiving approval from the Institutional Review Board, experimental testing began with the application of 30 total casts performed on uninjured adult human volunteers. Pressure readings were provided with the use of a bladder from a pediatric blood pressure cuff (Welch Allyn Inc), as previously described.6 The bladder was placed on the volar aspect of the volunteer’s forearm, held in place with a 3-in diameter cotton stockinet (3M). Cotton cast padding (Webril-Kendall) was applied, 3 in wide and 2 layers thick, and a long-arm cast was applied, 2 layers thick with 3-in wide fiberglass casting material (Scotchcast Plus Casting Tape; 3M).
Once the cast was applied and allowed to set, the blood pressure bladder was inflated to 100 mm Hg. After inflation, forearm cast circumference was measured at 2 set points, assessed at points 2 cm distal to the elbow flexor crease and 10 cm distal to the previous point (Figure 1). Using these data, we calculated estimated cast volume using the volumetric equation for a frustum. Following this point, casts were split into 2 experimental groups, univalve or bivalve, with 15 casts comprising each group. The univalve group consisted of a single cut along the dorsum of the extremity, and the bivalve group incorporated a second cut to the volar extremity. Cast valving was performed using an oscillating cast saw (Cast Vac; Stryker Instruments), with care taken to ensure the continuity of the underlying cast padding.

Continue to: Following valving, casts were secured via...
Following valving, casts were secured via 3 separate techniques: overwrap with a 3-in elastic wrap (Econo Wrap; Vitality Medical), application of two 10-mm and 15-mm cast spacers (CastWedge; DM Systems) (Figure 2). After securement, cast pressures were recorded, and circumference measurements were performed at the 2 previously identified points. The cast padding was then cut at the valve site and secured via the 3 listed techniques. Cast pressure and circumference measurements were performed at set time points (Figure 3). Changes in cast pressure were recorded in terms of the amount of change from the initial cast placement to account for differences in the size of volunteers’ forearms. Volumetric calculations were performed only for the spacer subgroups owing to the added material in the elastic wrap group. Estimated cast volume was calculated using the equation for volume of a frustum (Figure 4).



We used a 2-cast type (univalve and bivalve) by 4 securement subgroups (initial, elastic wrap, 10-mm spacer, and 15-mm spacer) design, with cast type serving as a between-subject measure and securement serving as a within-subject variable. An a priori power analysis showed that a minimum sample size of 15 subjects per condition should provide sufficient power of .80 and alpha set at .05, for a total of 30 casts. Statistical analyses were performed using IBM SPSS Statistics software version 21 (IBM). Experimental groups were analyzed using mixed-design analysis of variance (ANOVA). Post hoc comparisons between valving groups and cast securement were performed using Scheffe’s test to control for type II errors. Change in cast volume between the initial cast and cast spacers groups was compared using paired Student’s t tests. Statistical significance was predetermined as P < .05.
RESULTS
A summary of collected data for cast pressure and volume is detailed in Table 1, subdividing the variables on the basis of cast type and type of securement. Recorded pressures of the different subgroups are depicted in Figures 5 and 6 according to type of securement (initial, elastic wrap, 10-mm spacer, or 15-mm spacer). Results of the mixed-design ANOVA demonstrated significant differences between the initial cast pressure and univalve and bivalve groups (P < .05). There was a main effect for bivalve having lower pressure overall (F [1, 1)] = 3321.51, P < .001). There was also a main effect indicating that pressure was different for each type of securement (elastic wrap, 10-mm spacer, 15-mm spacer) (F [1, 28] = 538.54, P <. 01). Post hoc testing confirmed pressure decreased significantly, in descending order from elastic wrap, to 10-mm spacers, to 15-mm spacers (P < .05).
Table 1. Cumulative Data for Two Casting groups at Each Timepoint
Cast | Pressure | Standard Deviation | Volume |
Univalve |
|
|
|
Initial | 100 | --- | 2654.3 |
Elastic Wrap | 39.47 | 3.33 | --- |
10-mm Spacer | 23.93 | 2.73 | 2708.23 |
15-mm Spacer | 18.87 | 2.94 | 2734.86 |
Padding and Elastic Wrap | 20.93 | 2.91 | --- |
Padding and 10-mm Spacer | 15.46 | 2.19 | 2733.24 |
Padding and 15-mm Spacer | 0 | --- | 2819.27 |
Bivalve |
|
|
|
Initial | 100 | --- | 2839.3 |
Elastic Wrap | 25.9 | 3.17 | --- |
10-mm Spacer | 16.53 | 2.32 | 3203.13 |
15-mm Spacer | 13.6 | 2.74 | 3380.32 |
Padding and Elastic Wrap | 12.67 | 1.95 | --- |
Padding and 10-mm Spacer | 0 | --- | 3296.55 |
Padding and 15- mm Spacer | 0 | --- | 3438.67 |


Continue to: Table 2...
The summary of volumetric changes is listed in Table 2. The decrease in pressure correlated with an associated increase in cast volume, as demonstrated in Figure 7. The degree of increase in cast volume was more pronounced in the bivalve group (P < .001). The volume increased in the 15-mm group compared with the 10-mm group for both groups (P < .001) and increased for each spacer group with the release of the underlying padding (P < .05).
Table 2. Volumetric Data
Cast | Average Volumetric change (cm3) | Standard Deviation |
Univalve |
|
|
10-mm Spacer | 175.6 | 65.4 |
15-mm Spacer | 269.4 | 73.3 |
Padding and 10-mm Spacer | 202.3 | 62.5 |
Padding and 15-mm Spacer | 294.1 | 66.9 |
Bivalve |
|
|
10-mm Spacer | 363.7 | 67.2 |
15-mm Spacer | 540.9 | 85.7 |
Padding and 10-mm Spacer | 457.2 | 97.9 |
Padding and 15-mm Spacer | 599.3 | 84.2 |

Analysis of the planned comparisons demonstrated no significant difference between the bivalve with elastic wrap and univalve with 10-mm spacer subgroups (t [28] = 1.85, P = .075, d = .68). In comparing the bivalve with elastic wrap group with the univalve and 15-mm spacer subgroup, the univalve group showed significantly lower pressures [t [28] = 6.32, P < .001, d = .2.31).
DISCUSSION
Valving of circumferential casting is a well-established technique to minimize potential pressure-related complications. Previous studies have demonstrated that univalving techniques produce a 65% reduction in cast pressure, whereas bivalving produces an 80% decrease.6,7,9 Our results showed comparable pressure reductions of 75% with bivalving and 60% with univalving. The type of cast padding has been shown to have a significant effect on the cast pressure, favoring lower pressures with cotton padding over synthetic and waterproof padding, which, when released, can provide an additional 10% pressure reduction.6,7
Although bivalving techniques are superior in pressure reduction, the reduction comes at the cost of the cast’s structural integrity. Crickard and colleagues10 performed a biomechanical assessment of the structural integrity by 3-point bending of casts following univalve and bivalve compared with an intact cast. The authors found that valving resulted in a significant decrease in the casts’ bending stiffness and load to failure, with bivalved casts demonstrating a significantly lower load to failure than univalved casts. One technique that has been used to enhance the pressure reduction in univalved casting techniques is the application of a cast spacer. Rang and colleagues11 recommended this technique as part of a graded cast-splitting approach for the treatment of children’s fractures. This technique was applied to fractures with only modest anticipated swelling, which accounted for approximately 95% of casts applied in their children’s hospital. Our results support the use of cast spacers, demonstrating significant reduction in cast pressure in both univalve and bivalve techniques. Additionally, we found that a univalved cast with a 10-mm cast spacer provided pressure reduction similar to that of a bivalved cast.
The theory behind the application of cast spacers is that a split fiberglass cast will not remain open unless held in position.11 Holding the cast open is less of a restraint to pressure reduction in bivalving techniques, because the split cast no longer has the contralateral intact hinge point to resist cast opening, demonstrated in the compromise in structural integrity seen with this technique.10 By maintaining the split cast in an opened position, the effective volume of the cast is increased, which allows for the reduction in cast pressure. This is demonstrated in our results indicating an increase in estimated cast volume with an associated incremental reduction in cast pressure with the application of incrementally sized cast spacers. Although this technique does have the potential for skin irritation caused by cast expansion, as well as local swelling at the cast window location, it is a cost-effective treatment method compared with overwrapping a bivalved cast, $1.55 for 1 cast spacer vs an estimated $200 for a forearm cast application.
This study is not without its limitations. Our model does not account for the soft tissue injury associated with forearm fractures. However, by using human volunteers, we were able to include the viscoelastic properties that are omitted with nonliving models, and our results do align with those of previous investigations regarding pressure change following valving. We did not incorporate a 3-point molding technique commonly used with reduction and casting of acute forearm fractures, owing to the lack of a standardized method for applying the mold to healthy volunteers. Although molding is necessary for most fractures in which valving is considered, we believe our data still provide valuable information. Additionally, valving of circumferential casts has not been shown, prospectively, to result in a reduction of cast-related compartment syndrome, maintenance of reduction, or need for surgery.12,13 However, these results are reflective of reliable patients who completed the requisite follow-up care necessary for inclusion in a randomized controlled trial and may be applicable to unreliable patients or patient situations, a setting in which the compromise in cast structural integrity may be unacceptable.
CONCLUSION
We demonstrated that incorporating cast spacers into valved long-arm casts provides pressure reduction comparable to that achieved with the use of an elastic wrap. The addition of a 10-mm cast spacer to a univalved long-arm cast provides pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap. A univalved cast secured with a cast spacer is a viable option for treatment of displaced pediatric forearm fractures, without compromising the cast’s structural integrity as required with bivalved techniques.
This paper will be judged for the Resident Writer’s Award.
- Halanski M, Noonan KJ. Cast and splint immobilization: complications. J Am Acad Orthop Surg. 2008;16(1):30-40.
- Zaino CJ, Patel MR, Arief MS, Pivec R. The effectiveness of bivalving, cast spreading, and webril cutting to reduce cast pressure in a fiberglass short arm cast. J Bone Joint Surg Am. 2015;97(5):374-380. doi:10.2106/JBJS.N.00579.
- Rodriguez-Merchan EC. Pediatric fractures of the forearm. Clin Orthop Relat Res. 2005;(432):65-72.
- von Volkmann R. Ischaemic muscle paralyses and contractures. Clin Orthop Relat Res. 1967;50:5-56. doi:10.1097/BLO.0b013e318032561f.
- Patrick JH, Levack B. A study of pressures beneath forearm plasters. Injury. 1981;13(1):37-41.
- Roberts A, Shaw KA, Boomsma SE, Cameron CD. Effect of casting material on the cast pressure after sequential cast splitting. J Pediatr Orthop. 2017;37(1):74-77. doi:10.1097/BPO.0000000000000574.
- Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
- Capo JT, Renard RL, Moulton MJ, et al. How is forearm compliance affected by various circumferential dressings? Clin Orthop Relat Res. 2014 472(10):3228-3234. doi:10.1007/s11999-014-3747-y.
- Bingold AC. On splitting plasters. A useful analogy. J Bone Joint Surg Br. 1979;61-b(3):294-295.
- Crickard CV, Riccio AI, Carney JR, Anderson TD. Analysis and comparison of the biomechanical properties of univalved and bivalved cast models. J Pediatr Orthop.2011;31(1):39-43. doi:10.1097/BPO.0b013e318202c446.
- Rang M, Wenger DR, Pring ME. Rang's Children's Fractures. 3rd ed. Wenger DR, Rang M, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Schulte D, Habernig S, Zuzak T, et al. Forearm fractures in children: split opinions about splitting the cast. Eur J Pediatr Surg. 2014;24(2):163-167. doi:10.1055/s-0033-1341412.
- Bae DS, Valim C, Connell P, Brustowicz KA, Waters PM. Bivalved versus circumferential cast immobilization for displaced forearm fractures: a randomized clinical trial to assess efficacy and safety. J Pediatr Orthop. 2017;37(4):239-246 doi:10.1097/BPO.0000000000000655.
- Halanski M, Noonan KJ. Cast and splint immobilization: complications. J Am Acad Orthop Surg. 2008;16(1):30-40.
- Zaino CJ, Patel MR, Arief MS, Pivec R. The effectiveness of bivalving, cast spreading, and webril cutting to reduce cast pressure in a fiberglass short arm cast. J Bone Joint Surg Am. 2015;97(5):374-380. doi:10.2106/JBJS.N.00579.
- Rodriguez-Merchan EC. Pediatric fractures of the forearm. Clin Orthop Relat Res. 2005;(432):65-72.
- von Volkmann R. Ischaemic muscle paralyses and contractures. Clin Orthop Relat Res. 1967;50:5-56. doi:10.1097/BLO.0b013e318032561f.
- Patrick JH, Levack B. A study of pressures beneath forearm plasters. Injury. 1981;13(1):37-41.
- Roberts A, Shaw KA, Boomsma SE, Cameron CD. Effect of casting material on the cast pressure after sequential cast splitting. J Pediatr Orthop. 2017;37(1):74-77. doi:10.1097/BPO.0000000000000574.
- Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
- Capo JT, Renard RL, Moulton MJ, et al. How is forearm compliance affected by various circumferential dressings? Clin Orthop Relat Res. 2014 472(10):3228-3234. doi:10.1007/s11999-014-3747-y.
- Bingold AC. On splitting plasters. A useful analogy. J Bone Joint Surg Br. 1979;61-b(3):294-295.
- Crickard CV, Riccio AI, Carney JR, Anderson TD. Analysis and comparison of the biomechanical properties of univalved and bivalved cast models. J Pediatr Orthop.2011;31(1):39-43. doi:10.1097/BPO.0b013e318202c446.
- Rang M, Wenger DR, Pring ME. Rang's Children's Fractures. 3rd ed. Wenger DR, Rang M, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Schulte D, Habernig S, Zuzak T, et al. Forearm fractures in children: split opinions about splitting the cast. Eur J Pediatr Surg. 2014;24(2):163-167. doi:10.1055/s-0033-1341412.
- Bae DS, Valim C, Connell P, Brustowicz KA, Waters PM. Bivalved versus circumferential cast immobilization for displaced forearm fractures: a randomized clinical trial to assess efficacy and safety. J Pediatr Orthop. 2017;37(4):239-246 doi:10.1097/BPO.0000000000000655.
TAKE-HOME POINTS
- Valving a long-arm cast results in decreased cast pressures.
- Univalving can produce a 60% reduction in cast pressure.
- Bivalving produces a 75% reduction in cast pressure.
- Release of the underlying cast padding produces an additional pressure reduction.
- Adding a cast spacer to a univalved cast obtains similar pressure reduction to a bivalved cast.
Nearly one-quarter of presurgery patients already using opioids
at a large academic medical center, a cross-sectional observational study has determined.
Prescription or illegal opioid use can have profound implications for surgical outcomes and continued postoperative medication abuse. “Preoperative opioid use was associated with a greater burden of comorbid disease and multiple risk factors for poor recovery. ... Opioid-tolerant patients are at risk for opioid-associated adverse events and are less likely to discontinue opioid-based therapy after their surgery,” wrote Paul E. Hilliard, MD, and a team of researchers at the University of Michigan Health System. Although the question of preoperative opioid use has been examined and the Michigan findings are consistent with earlier estimates of prevalence (Ann Surg. 2017;265[4]:695-701), this study sought a more detailed profile of both the characteristics of these patients and the types of procedures correlated with opioid use.
Patient data were derived primarily from two ongoing institutional registries, the Michigan Genomics Initiative and the Analgesic Outcomes Study. Each of these projects involved recruiting nonemergency surgery patients to participate and self-report on pain and affect issues. Opioid use data were extracted from the preop anesthesia history and from physical examination. A total of 34,186 patients were recruited for this study; 54.2% were women, 89.1% were white, and the mean age was 53.1 years. Overall, 23.1% of these patients were taking opioids of various kinds, mostly by prescription along with nonprescription opioids and illegal drugs of other kinds.
The most common opioids found in this patient sample were hydrocodone bitartrate (59.4%), tramadol hydrochloride (21.2%) and oxycodone hydrochloride (18.5%), although the duration or frequency of use was not determined.
“In our experience, in surveys like this patients are pretty honest. [The data do not] track to their medical record, but was done privately for research. That having been said, I am sure there is significant underreporting,” study coauthor Michael J. Englesbe, MD, FACS, said in an interview. In addition to some nondisclosure by study participants, the exclusion of patients admitted to surgery from the ED could mean that 23.1% is a conservative estimate, he noted.
Patient characteristics included in the study (tobacco use, alcohol use, sleep apnea, pain, life satisfaction, depression, anxiety) were self-reported and validated using tools such as the Brief Pain Inventory, the Fibromyalgia Survey, and the Hospital Anxiety and Depression Scale. Procedural data were derived from patient records and ICD-10 data and rated via the ASA score and Charlson Comorbidity Index.
A multivariate analysis of patient characteristics found that age between 31 and 40, tobacco use, heavy alcohol use, pain score, depression, comorbidities reflected in a higher ASA score, and Charlson Comorbidity Score were all significant risk factors for presurgical opiate use.
Patients who were scheduled for surgical procedures involving lower extremities (adjusted odds ratio 3.61, 95% confidence interval, 2.81-4.64) were at the highest risk for opioid use, followed by pelvis surgery, excluding hip (aOR, 3.09, 95% CI, 1.88-5.08), upper arm or elbow (aOR, 3.07, 95% CI, 2.12-4.45), and spine surgery (aOR, 2.68, 95% CI, 2.15-3.32).
The study also broke out the data by presurgery opioid usage and surgery service. Of patients having spine neurosurgery, 55.1% were already taking opioids, and among those having orthopedic spine surgery, 65.1% were taking opioids. General surgery patients were not among those mostly likely to be using opioids (gastrointestinal surgery, 19.3% and endocrine surgery 14.3%). “Certain surgical services may be more likely to encounter patients with high comorbidities for opioid use, and more targeted opioid education strategies aimed at those services may help to mitigate risk in the postoperative period,” the authors wrote.
“All surgeons should take a preop pain history. They should ask about current pain and previous pain experiences. They should also ask about a history of substance use disorder. This should lead into a discussion of the pain expectations from the procedure. Patients should expect to be in pain, that is normal. Pain-free surgery is rare. If a patient has a complex pain history or takes chronic opioids, the surgeon should consider referring them to anesthesia for formal preop pain management planning and potentially weaning of opioid dose prior to elective surgery,” noted Dr. Englesbe, the Cyrenus G. Darling Sr., MD and Cyrenus G Darling Jr., MD Professor of Surgery, and faculty at the Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor.
Surgeons are likely to see patients with a past history of opioid dependence or who are recovering from substance abuse. “Every effort should be made to avoid opioids in these patients. We have developed a Pain Optimization Pathway which facilitates no postoperative opioids for these and other patients. These patients are at high risk to relapse and surgeons must know who these patients are so they can provide optimal care,” Dr. Englesbe added.The limitations of this study as reported by the authors include the single-center design, the nondiverse racial makeup of the sample, and the difficulty of ascertaining the dosing and duration of opioid use, both prescription and illegal.
The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
SOURCE: Hilliard PE et al. JAMA Surg. 2018 Jul 11. doi: 10.1001/jamasurg.2018.2102.
at a large academic medical center, a cross-sectional observational study has determined.
Prescription or illegal opioid use can have profound implications for surgical outcomes and continued postoperative medication abuse. “Preoperative opioid use was associated with a greater burden of comorbid disease and multiple risk factors for poor recovery. ... Opioid-tolerant patients are at risk for opioid-associated adverse events and are less likely to discontinue opioid-based therapy after their surgery,” wrote Paul E. Hilliard, MD, and a team of researchers at the University of Michigan Health System. Although the question of preoperative opioid use has been examined and the Michigan findings are consistent with earlier estimates of prevalence (Ann Surg. 2017;265[4]:695-701), this study sought a more detailed profile of both the characteristics of these patients and the types of procedures correlated with opioid use.
Patient data were derived primarily from two ongoing institutional registries, the Michigan Genomics Initiative and the Analgesic Outcomes Study. Each of these projects involved recruiting nonemergency surgery patients to participate and self-report on pain and affect issues. Opioid use data were extracted from the preop anesthesia history and from physical examination. A total of 34,186 patients were recruited for this study; 54.2% were women, 89.1% were white, and the mean age was 53.1 years. Overall, 23.1% of these patients were taking opioids of various kinds, mostly by prescription along with nonprescription opioids and illegal drugs of other kinds.
The most common opioids found in this patient sample were hydrocodone bitartrate (59.4%), tramadol hydrochloride (21.2%) and oxycodone hydrochloride (18.5%), although the duration or frequency of use was not determined.
“In our experience, in surveys like this patients are pretty honest. [The data do not] track to their medical record, but was done privately for research. That having been said, I am sure there is significant underreporting,” study coauthor Michael J. Englesbe, MD, FACS, said in an interview. In addition to some nondisclosure by study participants, the exclusion of patients admitted to surgery from the ED could mean that 23.1% is a conservative estimate, he noted.
Patient characteristics included in the study (tobacco use, alcohol use, sleep apnea, pain, life satisfaction, depression, anxiety) were self-reported and validated using tools such as the Brief Pain Inventory, the Fibromyalgia Survey, and the Hospital Anxiety and Depression Scale. Procedural data were derived from patient records and ICD-10 data and rated via the ASA score and Charlson Comorbidity Index.
A multivariate analysis of patient characteristics found that age between 31 and 40, tobacco use, heavy alcohol use, pain score, depression, comorbidities reflected in a higher ASA score, and Charlson Comorbidity Score were all significant risk factors for presurgical opiate use.
Patients who were scheduled for surgical procedures involving lower extremities (adjusted odds ratio 3.61, 95% confidence interval, 2.81-4.64) were at the highest risk for opioid use, followed by pelvis surgery, excluding hip (aOR, 3.09, 95% CI, 1.88-5.08), upper arm or elbow (aOR, 3.07, 95% CI, 2.12-4.45), and spine surgery (aOR, 2.68, 95% CI, 2.15-3.32).
The study also broke out the data by presurgery opioid usage and surgery service. Of patients having spine neurosurgery, 55.1% were already taking opioids, and among those having orthopedic spine surgery, 65.1% were taking opioids. General surgery patients were not among those mostly likely to be using opioids (gastrointestinal surgery, 19.3% and endocrine surgery 14.3%). “Certain surgical services may be more likely to encounter patients with high comorbidities for opioid use, and more targeted opioid education strategies aimed at those services may help to mitigate risk in the postoperative period,” the authors wrote.
“All surgeons should take a preop pain history. They should ask about current pain and previous pain experiences. They should also ask about a history of substance use disorder. This should lead into a discussion of the pain expectations from the procedure. Patients should expect to be in pain, that is normal. Pain-free surgery is rare. If a patient has a complex pain history or takes chronic opioids, the surgeon should consider referring them to anesthesia for formal preop pain management planning and potentially weaning of opioid dose prior to elective surgery,” noted Dr. Englesbe, the Cyrenus G. Darling Sr., MD and Cyrenus G Darling Jr., MD Professor of Surgery, and faculty at the Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor.
Surgeons are likely to see patients with a past history of opioid dependence or who are recovering from substance abuse. “Every effort should be made to avoid opioids in these patients. We have developed a Pain Optimization Pathway which facilitates no postoperative opioids for these and other patients. These patients are at high risk to relapse and surgeons must know who these patients are so they can provide optimal care,” Dr. Englesbe added.The limitations of this study as reported by the authors include the single-center design, the nondiverse racial makeup of the sample, and the difficulty of ascertaining the dosing and duration of opioid use, both prescription and illegal.
The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
SOURCE: Hilliard PE et al. JAMA Surg. 2018 Jul 11. doi: 10.1001/jamasurg.2018.2102.
at a large academic medical center, a cross-sectional observational study has determined.
Prescription or illegal opioid use can have profound implications for surgical outcomes and continued postoperative medication abuse. “Preoperative opioid use was associated with a greater burden of comorbid disease and multiple risk factors for poor recovery. ... Opioid-tolerant patients are at risk for opioid-associated adverse events and are less likely to discontinue opioid-based therapy after their surgery,” wrote Paul E. Hilliard, MD, and a team of researchers at the University of Michigan Health System. Although the question of preoperative opioid use has been examined and the Michigan findings are consistent with earlier estimates of prevalence (Ann Surg. 2017;265[4]:695-701), this study sought a more detailed profile of both the characteristics of these patients and the types of procedures correlated with opioid use.
Patient data were derived primarily from two ongoing institutional registries, the Michigan Genomics Initiative and the Analgesic Outcomes Study. Each of these projects involved recruiting nonemergency surgery patients to participate and self-report on pain and affect issues. Opioid use data were extracted from the preop anesthesia history and from physical examination. A total of 34,186 patients were recruited for this study; 54.2% were women, 89.1% were white, and the mean age was 53.1 years. Overall, 23.1% of these patients were taking opioids of various kinds, mostly by prescription along with nonprescription opioids and illegal drugs of other kinds.
The most common opioids found in this patient sample were hydrocodone bitartrate (59.4%), tramadol hydrochloride (21.2%) and oxycodone hydrochloride (18.5%), although the duration or frequency of use was not determined.
“In our experience, in surveys like this patients are pretty honest. [The data do not] track to their medical record, but was done privately for research. That having been said, I am sure there is significant underreporting,” study coauthor Michael J. Englesbe, MD, FACS, said in an interview. In addition to some nondisclosure by study participants, the exclusion of patients admitted to surgery from the ED could mean that 23.1% is a conservative estimate, he noted.
Patient characteristics included in the study (tobacco use, alcohol use, sleep apnea, pain, life satisfaction, depression, anxiety) were self-reported and validated using tools such as the Brief Pain Inventory, the Fibromyalgia Survey, and the Hospital Anxiety and Depression Scale. Procedural data were derived from patient records and ICD-10 data and rated via the ASA score and Charlson Comorbidity Index.
A multivariate analysis of patient characteristics found that age between 31 and 40, tobacco use, heavy alcohol use, pain score, depression, comorbidities reflected in a higher ASA score, and Charlson Comorbidity Score were all significant risk factors for presurgical opiate use.
Patients who were scheduled for surgical procedures involving lower extremities (adjusted odds ratio 3.61, 95% confidence interval, 2.81-4.64) were at the highest risk for opioid use, followed by pelvis surgery, excluding hip (aOR, 3.09, 95% CI, 1.88-5.08), upper arm or elbow (aOR, 3.07, 95% CI, 2.12-4.45), and spine surgery (aOR, 2.68, 95% CI, 2.15-3.32).
The study also broke out the data by presurgery opioid usage and surgery service. Of patients having spine neurosurgery, 55.1% were already taking opioids, and among those having orthopedic spine surgery, 65.1% were taking opioids. General surgery patients were not among those mostly likely to be using opioids (gastrointestinal surgery, 19.3% and endocrine surgery 14.3%). “Certain surgical services may be more likely to encounter patients with high comorbidities for opioid use, and more targeted opioid education strategies aimed at those services may help to mitigate risk in the postoperative period,” the authors wrote.
“All surgeons should take a preop pain history. They should ask about current pain and previous pain experiences. They should also ask about a history of substance use disorder. This should lead into a discussion of the pain expectations from the procedure. Patients should expect to be in pain, that is normal. Pain-free surgery is rare. If a patient has a complex pain history or takes chronic opioids, the surgeon should consider referring them to anesthesia for formal preop pain management planning and potentially weaning of opioid dose prior to elective surgery,” noted Dr. Englesbe, the Cyrenus G. Darling Sr., MD and Cyrenus G Darling Jr., MD Professor of Surgery, and faculty at the Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor.
Surgeons are likely to see patients with a past history of opioid dependence or who are recovering from substance abuse. “Every effort should be made to avoid opioids in these patients. We have developed a Pain Optimization Pathway which facilitates no postoperative opioids for these and other patients. These patients are at high risk to relapse and surgeons must know who these patients are so they can provide optimal care,” Dr. Englesbe added.The limitations of this study as reported by the authors include the single-center design, the nondiverse racial makeup of the sample, and the difficulty of ascertaining the dosing and duration of opioid use, both prescription and illegal.
The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
SOURCE: Hilliard PE et al. JAMA Surg. 2018 Jul 11. doi: 10.1001/jamasurg.2018.2102.
FROM JAMA SURGERY
Key clinical point: Preoperative opioid use is prevalent in patients who are having spinal surgery and have depression.
Major finding:
Study details: An observational study of 34,186 surgical patients in the University of Michigan Health system.
Disclosures: The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
Source: Hilliard P E et al. JAMA Surg. 2018 Jul 11;. doi:10.1001/jamasurg.2018.2102.
Treatment of Grade III Acromioclavicular Separations in Professional Baseball Pitchers: A Survey of Major League Baseball Team Physicians
ABSTRACT
Despite advancements in surgical technique and understanding of throwing mechanics, controversy persists regarding the treatment of grade III acromioclavicular (AC) joint separations, particularly in throwing athletes. Twenty-eight major league baseball (MLB) orthopedic team physicians were surveyed to determine their definitive management of a grade III AC separation in the dominant arm of a professional baseball pitcher and their experience treating AC joint separations in starting pitchers and position players. Return-to-play outcomes were also evaluated. Twenty (71.4%) team physicians recommended nonoperative intervention compared to 8 (28.6%) who would have operated acutely. Eighteen (64.3%) team physicians had treated at least 1 professional pitcher with a grade III AC separation; 51 (77.3%) pitchers had been treated nonoperatively compared to 15 (22.7%) operatively. No difference was observed in the proportion of pitchers who returned to the same level of play (P = .54), had full, unrestricted range of motion (P = .23), or had full pain relief (P = .19) between the operatively and nonoperatively treated MLB pitchers. The majority (53.6%) of physicians would not include an injection if the injury was treated nonoperatively. Open coracoclavicular reconstruction (65.2%) was preferred for operative cases; 66.7% of surgeons would also include distal clavicle excision as an adjunct procedure. About 90% of physicians would return pitchers to throwing >12 weeks after surgery compared to after 4 to 6 weeks in nonoperatively treated cases. In conclusion, MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in professional pitchers. If operative intervention is required, ligament reconstruction with adjunct distal clavicle excision were the most commonly performed procedures.
Continue to: Despite advancements in surgucal technique...
Despite advancements in surgical technique and improved understanding of the physiology of throwing mechanics, controversy persists regarding the preferred treatment for grade III acromioclavicular (AC) joint separations.1-6 Nonsurgical management has demonstrated return to prior function with fewer complications.7 However, there is a growing body of evidence demonstrating that surgical intervention is associated with more favorable outcomes8 and should be considered in patients who place high functional demands on their shoulders.9
The reported results on professional athletes in the literature remain ambivalent. Multiple small case reports/series have reported successful nonoperative treatment of elite athletes.10-12 Not surprisingly, McFarland and colleagues13 reported in 1997 that 69% of major league baseball (MLB) team physicians preferred nonoperative treatment for a theoretical starting pitcher sustaining a grade III AC separation 1 week prior to the start of the season. In contrast, reports of an inability to throw at a pre-injury level are equally commonplace.14,15 Nevertheless, all of these studies were limited to small cohorts, as the incidence of grade III AC separations in elite throwing athletes is relatively uncommon.13,16
In this study, we re-evaluated the study performed by McFarland and colleagues13 in 1997 by surveying all active MLB team orthopedic surgeons. We asked them how they would treat a grade III AC separation in a starting professional baseball pitcher. The physicians were also asked about their personal experience evaluating outcomes in these elite athletes. Given our improved understanding of the anatomy, pathophysiology, and surgical techniques for treating grade III AC separations, we hypothesize that more MLB team physicians would favor operative intervention treatment in professional baseball pitchers, as their vocation places higher demands on their shoulders.
MATERIALS AND METHODS
A questionnaire (Appendix A) was distributed to the team physicians of all 30 MLB teams. In addition to surgeon demographics, including age, years in practice, and years of taking care of an MLB team, the initial section of the questionnaire asked orthopedic surgeons how they would treat a theoretical starting pitcher who sustained a grade III AC joint separation of the dominant throwing arm 1 week prior to the start of the season. Physicians who preferred nonoperative treatment were asked whether they would use an injection (and what type), as well as when they would allow the pitcher to start a progressive interval throwing program. Physicians who preferred operative treatment were asked to rank their indications for operating, what procedure they would use (eg, open vs arthroscopic or coracoclavicular ligament repair vs reconstruction), and whether the surgical intervention would include distal clavicle excision. Both groups of physicians were also asked if their preferred treatment would change if the injury were to occur at the end of the season.
The second portion of the questionnaire asked surgeons about their experience treating AC joint separations in both starting pitchers and position players, as well as to describe the long-term outcomes of their preferred treatment, including time to return to full clearance for pitching, whether their patients returned to their prior level of play, and whether these patients had full pain relief. Finally, physicians were asked if any of the nonoperatively treated players ultimately crossed over and required operative intervention.
Continue to: Statistics...
STATISTICS
Descriptive statistics were used for continuous variables, and frequencies were used for categorical variables. Linear regression was performed to determine the correlation between the physician’s training or experience in treating AC joint separations and their recommended treatment. Fischer’s exact test/chi-square analysis was used to compare categorical variables. All tests were conducted using 2-sided hypothesis testing with statistical significance set at P < .05. All statistical analyses were conducted with SPSS 21.0 software (IBM Corporation).
RESULTS
A total of 28 MLB team physicians completed the questionnaires from 18 of the 30 MLB teams. The average age of the responders was 50.5 years (range, 34-60 years), with an average of 18.2 years in practice (range, 2-30 years) and 10.8 years (range, 1-24 years) taking care of their current professional baseball team. About 82% of the team physicians completed a sports medicine fellowship. On average, physicians saw 16.6 (range, 5-50) grade III or higher AC joint separations per year, and operated on 4.6 (range, 0-10) per year.
Nonoperative treatment was the preferred treatment for a grade III AC joint separation in a starting professional baseball pitcher for the majority of team physicians (20/28). No correlation was observed between the physician’s age (P = .881), years in practice (P = .915), years taking care of their professional team (P = .989), percentage of practice focused on shoulders (P = .986), number of AC joint injuries seen (P = .325), or number of surgeries performed per year (P = .807) with the team physician’s preferred treatment. Compared to the proportion reported originally by McFarland and colleagues13 in 1997 (69%), there was no difference in the proportion of team physicians that recommended nonoperative treatment (P = 1).
If treating this injury nonoperatively, 46.4% of physicians would also use an injection, with orthobiologics (eg, platelet-rich plasma) as the most popular choice (Table 1). No consensus was provided on the timeframe to return pitchers back to a progressive interval throwing program; however, 46.67% of physicians would return pitchers 4 to 6 weeks after a nonoperatively treated injury, while 35.7% would return pitchers 7 to 12 weeks after the initial injury.
Table 1. Treatment Preferences of Grade III AC Separation by MLB Team Physicians
Nonoperativea | |
Yes injection | 13 (46.4%) |
Cortisone | 3 (23.1%) |
Orthobiologic | 10 (76.9%) |
Local anesthetic (eg, lidocaine) | 1 (7.7%) |
Intramuscular toradol | 3 (23.1%) |
No injection | 15 (53.6%) |
Operativea | |
Open coracoclavicular ligament repair | 3 (13.0%) |
Open coracoclavicular ligament reconstruction | 15 (65.2%) |
Arthroscopic reconstruction with graft | 6 (26.1%) |
Arthroscopic repair with implant (ie, tight-rope) | 2 (8.7%) |
Distal clavicle excisionb | 16 (66.7%) |
Would not intervene operatively | 5 (17.9%) |
|
|
aRespondents were allowed to choose more than 1 treatment in each category. bChosen as an adjunct treatment.
Abbreviations: AC, acromioclavicular; MLB, major league baseball.
Most physicians (64.3%) cited functional limitations as the most important reason for indicating operative treatment, followed by pain (21.4%), and a deformity (14.3%). About 65% preferred open coracoclavicular ligament reconstruction. No physician recommended the Weaver-Dunn procedure or use of hardware (eg, hook plate). Of those who preferred an operative intervention, 66.7% would also include a distal clavicle excision, which is significantly higher than the proportion reported by McFarland and colleagues13 (23%, P = .0170). About 90% of physicians would return pitchers to play >12 weeks after operative treatment.
Continue to: If the injury occurred at the end ...
If the injury occurred at the end of the season, 7 of the 20 orthopedists (35%) who recommended nonoperative treatment said they would change to an operative intervention. Eighteen of 28 responders would have the same algorithm for MLB position players. Team physicians were less likely to recommend operative intervention in position players due to less demand on the arm and increased ability to accommodate the injury by altering their throwing mechanics.
Eighteen (64%) of the team physicians had treated at least 1 professional pitcher with a grade III AC separation in his dominant arm, and 11 (39.3%) had treated >1. Collectively, team physicians had treated 15 professional pitchers operatively, and 51 nonoperatively; only 3 patients converted to operative intervention after a failed nonoperative treatment.
Of the pitchers treated operatively, 93.3% (14) of pitchers returned to their prior level of pitching. The 1 patient who failed to return to the same level of pitching retired instead of returning to play. About 80% (12) of the pitchers had full pain relief, and 93.3% (14) had full range of motion (ROM). The pitcher who failed to regain full ROM also had a concomitant rotator cuff repair. The only complication reported from an operative intervention was a pitcher who sustained a coracoid fracture 10 months postoperatively while throwing 100 mph. Of the pitchers treated nonoperatively, 96% returned to their prior level of pitching, 92.2% (47) had full complete pain relief when throwing, and 100% had full ROM. No differences were observed between the proportion of pitchers who returned to their prior level of pitching, regained full ROM, or had full pain relief in the operative and nonoperative groups (Table 2).
Table 2. Outcomes of Treatment of Grade III AC Separation in 58 Professional Baseball Players
| Operative | Nonoperative | P-value |
Return to same level of play | 14/15 (93.3%) | 49/51 (96%) | 0.54 |
Full pain relief | 12/15 (80%) | 47/51 (92.2%) | 0.19 |
Full ROM | 14/15 (93.3%) | 51/51 (100%) | 0.23 |
Abbreviations: AC, acromioclavicular; ROM, range of motion.
DISCUSSION
Controversy persists regarding the optimal management of acute grade III AC separations, with the current available evidence potentially suggesting better cosmetic and radiological results but no definite differences in clinical results.1-6,17,18 In the absence of formal clinical practice guidelines, surgeons rely on their own experience or defer to the anecdotal experience of experts in the field. Our initial hypothesis was false in this survey of MLB team physicians taking care of overhead throwing athletes at the highest level. Our results demonstrate that despite improved techniques and an increased understanding of the pathophysiology of AC joint separations, conservative management is still the preferred treatment for acute grade III AC joint separations in professional baseball pitchers. The proportion of team physicians recommending nonoperative treatment in our series was essentially equivalent to the results reported by McFarland and colleagues13 in 1997, suggesting that the pendulum continues to favor conservative management initially. This status quo likely reflects both the dearth of literature suggesting a substantial benefit of acute operative repair, as well as the ability to accommodate with conservative measures after most grade III AC injuries, even at the highest level of athletic competition.
These results are also consistent with trends from the last few decades. In the 1970s, the overwhelming preference for treating an acute complete AC joint separation was surgical repair, with Powers and Bach10 reporting in a 1974 survey of 163 chairmen of orthopedic programs around the country that 91.5% advocated surgical treatment. However, surgical preference had reversed by the 1990s. Of the 187 chairmen and 59 team physicians surveyed by Cox19 in 1992, 72% and 86% respectively preferred nonoperative treatment in a theoretical 21-year-old athlete with a grade III AC separation. Nissen and Chatterjee20 reported in 2007 on a survey of all American Orthopaedic Society for Sports Medicine surgeons (N = 577) and Accreditation Council for Graduate Medical Education orthopedic program residency directors (N = 87) that >80% of responders preferred conservative measures for this acute injury. The reversal of trends has also been corroborated by recent multicenter trials demonstrating no difference in clinical outcomes between operative and nonoperative treatment of high grade AC joint dislocations, albeit these patients were not all high level overhead throwing athletes.17,18
Continue to: The trends in surgical interventions are notable...
The trends in surgical interventions are notable within the smaller subset of patients who are indicated for operative repair. Use of hardware and primary ligament repair, while popular in the surveys conducted in the 1970s10 and 1990s13 and even present in Nissen and Chatterjee’s20 2007 survey, were noticeably absent from our survey results, with the majority of respondents preferring open coracoclavicular ligament reconstruction. The role of distal clavicle excision has also expanded, from 23% of team physicians recommending it in 199713 to 57% to 59% in Nissen and Chatterjee’s20 2007 survey, to 66.7% in our series. This trend is not surprising as several recent cadaveric biomechanical studies have demonstrated that not only do peak graft forces not increase significantly,21 the anterior-posterior and superior-inferior motion at the AC joint following ligament reconstruction is maintained despite resection of the lateral clavicle.22 Additionally, primary distal clavicle excision may prevent the development of post-traumatic arthritis at the AC joint and osteolysis of the distal clavicle as a possible pain generator in the future.23 However, some respondents cautioned against performing a concomitant distal clavicle excision, as some biomechanical data demonstrate that resecting the distal clavicle may lead to increased horizontal translation at the AC joint despite intact superior and posterior AC capsules.24 Professional baseball pitchers may also be more lax and thus prone to more instability. Primary repair or reconstruction may not always lead to complete pre-injury stability in these individuals. This subtle unrecognized instability is hard to diagnosis and may be a persistent source of pain; thus, adding a distal clavicle excision may actually exacerbate the instability.
The nuanced indications for operative intervention, such as the presence of associated lesions were not captured by our survey.25 While most team physicians cited functional limitations as their most common reason for offering surgery, several MLB orthopedic surgeons also commented on evaluating the stability of the AC joint after a grade III injury, akin to the consensus statement from the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS) Upper Extremity Committee26 in 2014 that diversified the Rockwood Grade III AC joint separation into its IIIA and IIIB classifications. The ISAKOS recommendations include initial conservative management and a second evaluation (both clinical and radiographic) for grade III lesions 3 to 6 weeks after the injury. However, as professional baseball is an incredibly profitable sport with an annual revenue approaching $9.5 billion27 and pitching salaries up to $32.5 million in 2015, serious financial considerations must be given to players who wish to avoid undergoing delayed surgery.
This study has shortcomings typical of expert opinion papers. The retrospective nature of this study places the data at risk of recall bias. Objective data (eg, terminal ROM, pain relief, and return to play) were obtained from a retrospective chart review; however, no standard documentation or collection method was used given the number of surgeons involved and, thus, conclusions based on treatment outcomes are imperfect. Another major weakness of this survey is the relatively small number of patients and respondents. An a priori power analysis was not available, as this was a retrospective review. A comparative trial will be necessary to definitively support one treatment over another. Assuming a 95% return to play in the nonoperatively treated group, approximately 300 patients would be needed in a prospective 2-armed study with 80% power to detect a 10% reduction in the incidence of return to play using an alpha level of 0.05 and assuming no loss to follow-up. This sample size would be difficult to achieve in this patient population.
However, compared to past series,13 the number of professional baseball players treated by the collective experience of these MLB team physicians is the largest reported to date. As suggested above, the rarity of this condition in elite athletes precludes the ability to have matched controls to definitively determine the optimal treatment, which may explain the lack of difference in the return to play, ROM, and pain relief outcomes. Instead, we can only extrapolate based on the collective anecdotal experience of the MLB team physicians.
CONCLUSION
Despite advances in surgical technique and understanding of throwing mechanics, the majority of MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in a professional baseball pitcher. Open coracoclavicular ligament reconstruction was preferred for those who preferred operative intervention. An increasing number of orthopedic surgeons now consider a distal clavicle excision as an adjunct procedure.
This paper will be judged for the Resident Writer’s Award.
- Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;455:38-44. doi:10.1097/BLO.0b013e318030df83.
- Ceccarelli E, Bondì R, Alviti F, Garofalo R, Miulli F, Padua R. Treatment of acute grade III acromioclavicular dislocation: A lack of evidence. J Orthop Traumatol. 2008;9(2):105-108. doi:10.1007/s10195-008-0013-7.
- Smith TO, Chester R, Pearse EO, Hing CB. Operative versus non-operative management following rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthop Traumatol. 2011;12(1):19-27. doi:10.1007/s10195-011-0127-1.
- Beitzel K, Cote MP, Apostolakos J, et al. Current concepts in the treatment of acromioclavicular joint dislocations. Arthroscopy. 2013;29(2):387-397. doi:10.1016/j.arthro.2012.11.023.
- Korsten K, Gunning AC, Leenen LP. Operative or conservative treatment in patients with rockwood type III acromioclavicular dislocation: a systematic review and update of current literature. Int Orthop. 2014;38(4):831-838. doi:10.1007/s00264-013-2143-7.
- Modi CS, Beazley J, Zywiel MG, Lawrence TM, Veillette CJ. Controversies relating to the management of acromioclavicular joint dislocations. Bone Joint J. 2013;95-B(12):1595-1602. doi:10.1302/0301-620X.95B12.31802.
- Reid D, Polson K, Johnson L. Acromioclavicular joint separations grades I-III: a review of the literature and development of best practice guidelines. Sports Med. 2012;42(8):681-696. doi:10.2165/11633460-000000000-00000.
- Farber AJ, Cascio BM, Wilckens JH. Type III acromioclavicular separation: rationale for anatomical reconstruction. Am J Orthop. 2008;37(7):349-355.
- Li X, Ma R, Bedi A, Dines DM, Altchek DW, Dines JS. Management of acromioclavicular joint injuries. J Bone Joint Surg Am. 2014;96(1):73-84. doi:10.2106/JBJS.L.00734.
- Powers JA, Bach PJ. Acromioclavicular separations. Closed or open treatment? Clin Orthop Relat Res. 1974;104(104):213-223. doi:10.1097/00003086-197410000-00024.
- Glick JM, Milburn LJ, Haggerty JF, Nishimoto D. Dislocated acromioclavicular joint: follow-up study of 35 unreduced acromioclavicular dislocations. Am J Sports Med. 1977;5(6):264-270. doi:10.1177/036354657700500614.
- Watson ST, Wyland DJ. Return to play after nonoperative management for a severe type III acromioclavicular separation in the throwing shoulder of a collegiate pitcher. Phys Sportsmed. 2015;43(1):99-103. doi:10.1080/00913847.2015.1001937.
- McFarland EG, Blivin SJ, Doehring CB, Curl LA, Silberstein C. Treatment of grade III acromioclavicular separations in professional throwing athletes: results of a survey. Am J Orthop. 1997;26(11):771-774.
- Wojtys EM, Nelson G. Conservative treatment of grade III acromioclavicular dislocations. Clin Orthop Relat Res. 1991;268(268):112-119.
- Galpin RD, Hawkins RJ, Grainger RW. A comparative analysis of operative versus nonoperative treatment of grade III acromioclavicular separations. Clin Orthop Relat Res. 1985;193(193):150-155. doi:10.1097/00003086-198503000-00020.
- Pallis M, Cameron KL, Svoboda SJ, Owens BD. Epidemiology of acromioclavicular joint injury in young athletes. Am J Sports Med. 2012;40(9):2072-2077. doi:10.1177/0363546512450162.
- Canadian Orthopaedic Trauma Society. Multicenter randomized clinical trial of nonoperative versus operative treatment of acute acromio-clavicular joint dislocation. J Orthop Trauma. 2015;29(11):479-487. doi:10.1097/BOT.0000000000000437.
- Joukainen A, Kröger H, Niemitukia L, Mäkelä EA, Väätäinen U. Results of operative and nonoperative treatment of rockwood types III and V acromioclavicular joint dislocation: a prospective, randomized trial with an 18- to 20-year follow-up. Orthop J Sports Med. 2014;2(12):2325967114560130. doi:10.1177/2325967114560130.
- Cox JS. Current method of treatment of acromioclavicular joint dislocations. Orthopedics. 1992;15(9):1041-1044.
- Nissen CW, Chatterjee A. Type III acromioclavicular separation: results of a recent survey on its management. Am J Orthop. 2007;36(2):89-93.
- Kowalsky MS, Kremenic IJ, Orishimo KF, McHugh MP, Nicholas SJ, Lee SJ. The effect of distal clavicle excision on in situ graft forces in coracoclavicular ligament reconstruction. Am J Sports Med. 2010;38(11):2313-2319. doi:10.1177/0363546510374447.
- Beaver AB, Parks BG, Hinton RY. Biomechanical analysis of distal clavicle excision with acromioclavicular joint reconstruction. Am J Sports Med. 2013;41(7):1684-1688. doi:10.1177/0363546513488750.
- Mumford EB. Acromioclavicular dislocation. J Bone Joint Surg Am. 1941;23:799-802.
- Beitzel K, Sablan N, Chowaniec DM, et al. Sequential resection of the distal clavicle and its effects on horizontal acromioclavicular joint translation. Am J Sports Med. 2012;40(3):681-685. doi:10.1177/0363546511428880.
- Arrigoni P, Brady PC, Zottarelli L, et al. Associated lesions requiring additional surgical treatment in grade 3 acromioclavicular joint dislocations. Arthroscopy. 2014;30(1):6-10. doi:10.1016/j.arthro.2013.10.006.
- Beitzel K, Mazzocca AD, Bak K, et al. ISAKOS upper extremity committee consensus statement on the need for diversification of the rockwood classification for acromioclavicular joint injuries. Arthroscopy. 2014;30(2):271-278. doi:10.1016/j.arthro.2013.11.005.
- Brown M. MLB sees record revenues for 2015, up $500 million and approaching $9.5 billion. Forbes Web site. http://www.forbes.com/sites/maurybrown/2015/12/04/mlb-sees-record-revenu.... Published December 4, 2015. Accessed February 4, 2016.
ABSTRACT
Despite advancements in surgical technique and understanding of throwing mechanics, controversy persists regarding the treatment of grade III acromioclavicular (AC) joint separations, particularly in throwing athletes. Twenty-eight major league baseball (MLB) orthopedic team physicians were surveyed to determine their definitive management of a grade III AC separation in the dominant arm of a professional baseball pitcher and their experience treating AC joint separations in starting pitchers and position players. Return-to-play outcomes were also evaluated. Twenty (71.4%) team physicians recommended nonoperative intervention compared to 8 (28.6%) who would have operated acutely. Eighteen (64.3%) team physicians had treated at least 1 professional pitcher with a grade III AC separation; 51 (77.3%) pitchers had been treated nonoperatively compared to 15 (22.7%) operatively. No difference was observed in the proportion of pitchers who returned to the same level of play (P = .54), had full, unrestricted range of motion (P = .23), or had full pain relief (P = .19) between the operatively and nonoperatively treated MLB pitchers. The majority (53.6%) of physicians would not include an injection if the injury was treated nonoperatively. Open coracoclavicular reconstruction (65.2%) was preferred for operative cases; 66.7% of surgeons would also include distal clavicle excision as an adjunct procedure. About 90% of physicians would return pitchers to throwing >12 weeks after surgery compared to after 4 to 6 weeks in nonoperatively treated cases. In conclusion, MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in professional pitchers. If operative intervention is required, ligament reconstruction with adjunct distal clavicle excision were the most commonly performed procedures.
Continue to: Despite advancements in surgucal technique...
Despite advancements in surgical technique and improved understanding of the physiology of throwing mechanics, controversy persists regarding the preferred treatment for grade III acromioclavicular (AC) joint separations.1-6 Nonsurgical management has demonstrated return to prior function with fewer complications.7 However, there is a growing body of evidence demonstrating that surgical intervention is associated with more favorable outcomes8 and should be considered in patients who place high functional demands on their shoulders.9
The reported results on professional athletes in the literature remain ambivalent. Multiple small case reports/series have reported successful nonoperative treatment of elite athletes.10-12 Not surprisingly, McFarland and colleagues13 reported in 1997 that 69% of major league baseball (MLB) team physicians preferred nonoperative treatment for a theoretical starting pitcher sustaining a grade III AC separation 1 week prior to the start of the season. In contrast, reports of an inability to throw at a pre-injury level are equally commonplace.14,15 Nevertheless, all of these studies were limited to small cohorts, as the incidence of grade III AC separations in elite throwing athletes is relatively uncommon.13,16
In this study, we re-evaluated the study performed by McFarland and colleagues13 in 1997 by surveying all active MLB team orthopedic surgeons. We asked them how they would treat a grade III AC separation in a starting professional baseball pitcher. The physicians were also asked about their personal experience evaluating outcomes in these elite athletes. Given our improved understanding of the anatomy, pathophysiology, and surgical techniques for treating grade III AC separations, we hypothesize that more MLB team physicians would favor operative intervention treatment in professional baseball pitchers, as their vocation places higher demands on their shoulders.
MATERIALS AND METHODS
A questionnaire (Appendix A) was distributed to the team physicians of all 30 MLB teams. In addition to surgeon demographics, including age, years in practice, and years of taking care of an MLB team, the initial section of the questionnaire asked orthopedic surgeons how they would treat a theoretical starting pitcher who sustained a grade III AC joint separation of the dominant throwing arm 1 week prior to the start of the season. Physicians who preferred nonoperative treatment were asked whether they would use an injection (and what type), as well as when they would allow the pitcher to start a progressive interval throwing program. Physicians who preferred operative treatment were asked to rank their indications for operating, what procedure they would use (eg, open vs arthroscopic or coracoclavicular ligament repair vs reconstruction), and whether the surgical intervention would include distal clavicle excision. Both groups of physicians were also asked if their preferred treatment would change if the injury were to occur at the end of the season.
The second portion of the questionnaire asked surgeons about their experience treating AC joint separations in both starting pitchers and position players, as well as to describe the long-term outcomes of their preferred treatment, including time to return to full clearance for pitching, whether their patients returned to their prior level of play, and whether these patients had full pain relief. Finally, physicians were asked if any of the nonoperatively treated players ultimately crossed over and required operative intervention.
Continue to: Statistics...
STATISTICS
Descriptive statistics were used for continuous variables, and frequencies were used for categorical variables. Linear regression was performed to determine the correlation between the physician’s training or experience in treating AC joint separations and their recommended treatment. Fischer’s exact test/chi-square analysis was used to compare categorical variables. All tests were conducted using 2-sided hypothesis testing with statistical significance set at P < .05. All statistical analyses were conducted with SPSS 21.0 software (IBM Corporation).
RESULTS
A total of 28 MLB team physicians completed the questionnaires from 18 of the 30 MLB teams. The average age of the responders was 50.5 years (range, 34-60 years), with an average of 18.2 years in practice (range, 2-30 years) and 10.8 years (range, 1-24 years) taking care of their current professional baseball team. About 82% of the team physicians completed a sports medicine fellowship. On average, physicians saw 16.6 (range, 5-50) grade III or higher AC joint separations per year, and operated on 4.6 (range, 0-10) per year.
Nonoperative treatment was the preferred treatment for a grade III AC joint separation in a starting professional baseball pitcher for the majority of team physicians (20/28). No correlation was observed between the physician’s age (P = .881), years in practice (P = .915), years taking care of their professional team (P = .989), percentage of practice focused on shoulders (P = .986), number of AC joint injuries seen (P = .325), or number of surgeries performed per year (P = .807) with the team physician’s preferred treatment. Compared to the proportion reported originally by McFarland and colleagues13 in 1997 (69%), there was no difference in the proportion of team physicians that recommended nonoperative treatment (P = 1).
If treating this injury nonoperatively, 46.4% of physicians would also use an injection, with orthobiologics (eg, platelet-rich plasma) as the most popular choice (Table 1). No consensus was provided on the timeframe to return pitchers back to a progressive interval throwing program; however, 46.67% of physicians would return pitchers 4 to 6 weeks after a nonoperatively treated injury, while 35.7% would return pitchers 7 to 12 weeks after the initial injury.
Table 1. Treatment Preferences of Grade III AC Separation by MLB Team Physicians
Nonoperativea | |
Yes injection | 13 (46.4%) |
Cortisone | 3 (23.1%) |
Orthobiologic | 10 (76.9%) |
Local anesthetic (eg, lidocaine) | 1 (7.7%) |
Intramuscular toradol | 3 (23.1%) |
No injection | 15 (53.6%) |
Operativea | |
Open coracoclavicular ligament repair | 3 (13.0%) |
Open coracoclavicular ligament reconstruction | 15 (65.2%) |
Arthroscopic reconstruction with graft | 6 (26.1%) |
Arthroscopic repair with implant (ie, tight-rope) | 2 (8.7%) |
Distal clavicle excisionb | 16 (66.7%) |
Would not intervene operatively | 5 (17.9%) |
|
|
aRespondents were allowed to choose more than 1 treatment in each category. bChosen as an adjunct treatment.
Abbreviations: AC, acromioclavicular; MLB, major league baseball.
Most physicians (64.3%) cited functional limitations as the most important reason for indicating operative treatment, followed by pain (21.4%), and a deformity (14.3%). About 65% preferred open coracoclavicular ligament reconstruction. No physician recommended the Weaver-Dunn procedure or use of hardware (eg, hook plate). Of those who preferred an operative intervention, 66.7% would also include a distal clavicle excision, which is significantly higher than the proportion reported by McFarland and colleagues13 (23%, P = .0170). About 90% of physicians would return pitchers to play >12 weeks after operative treatment.
Continue to: If the injury occurred at the end ...
If the injury occurred at the end of the season, 7 of the 20 orthopedists (35%) who recommended nonoperative treatment said they would change to an operative intervention. Eighteen of 28 responders would have the same algorithm for MLB position players. Team physicians were less likely to recommend operative intervention in position players due to less demand on the arm and increased ability to accommodate the injury by altering their throwing mechanics.
Eighteen (64%) of the team physicians had treated at least 1 professional pitcher with a grade III AC separation in his dominant arm, and 11 (39.3%) had treated >1. Collectively, team physicians had treated 15 professional pitchers operatively, and 51 nonoperatively; only 3 patients converted to operative intervention after a failed nonoperative treatment.
Of the pitchers treated operatively, 93.3% (14) of pitchers returned to their prior level of pitching. The 1 patient who failed to return to the same level of pitching retired instead of returning to play. About 80% (12) of the pitchers had full pain relief, and 93.3% (14) had full range of motion (ROM). The pitcher who failed to regain full ROM also had a concomitant rotator cuff repair. The only complication reported from an operative intervention was a pitcher who sustained a coracoid fracture 10 months postoperatively while throwing 100 mph. Of the pitchers treated nonoperatively, 96% returned to their prior level of pitching, 92.2% (47) had full complete pain relief when throwing, and 100% had full ROM. No differences were observed between the proportion of pitchers who returned to their prior level of pitching, regained full ROM, or had full pain relief in the operative and nonoperative groups (Table 2).
Table 2. Outcomes of Treatment of Grade III AC Separation in 58 Professional Baseball Players
| Operative | Nonoperative | P-value |
Return to same level of play | 14/15 (93.3%) | 49/51 (96%) | 0.54 |
Full pain relief | 12/15 (80%) | 47/51 (92.2%) | 0.19 |
Full ROM | 14/15 (93.3%) | 51/51 (100%) | 0.23 |
Abbreviations: AC, acromioclavicular; ROM, range of motion.
DISCUSSION
Controversy persists regarding the optimal management of acute grade III AC separations, with the current available evidence potentially suggesting better cosmetic and radiological results but no definite differences in clinical results.1-6,17,18 In the absence of formal clinical practice guidelines, surgeons rely on their own experience or defer to the anecdotal experience of experts in the field. Our initial hypothesis was false in this survey of MLB team physicians taking care of overhead throwing athletes at the highest level. Our results demonstrate that despite improved techniques and an increased understanding of the pathophysiology of AC joint separations, conservative management is still the preferred treatment for acute grade III AC joint separations in professional baseball pitchers. The proportion of team physicians recommending nonoperative treatment in our series was essentially equivalent to the results reported by McFarland and colleagues13 in 1997, suggesting that the pendulum continues to favor conservative management initially. This status quo likely reflects both the dearth of literature suggesting a substantial benefit of acute operative repair, as well as the ability to accommodate with conservative measures after most grade III AC injuries, even at the highest level of athletic competition.
These results are also consistent with trends from the last few decades. In the 1970s, the overwhelming preference for treating an acute complete AC joint separation was surgical repair, with Powers and Bach10 reporting in a 1974 survey of 163 chairmen of orthopedic programs around the country that 91.5% advocated surgical treatment. However, surgical preference had reversed by the 1990s. Of the 187 chairmen and 59 team physicians surveyed by Cox19 in 1992, 72% and 86% respectively preferred nonoperative treatment in a theoretical 21-year-old athlete with a grade III AC separation. Nissen and Chatterjee20 reported in 2007 on a survey of all American Orthopaedic Society for Sports Medicine surgeons (N = 577) and Accreditation Council for Graduate Medical Education orthopedic program residency directors (N = 87) that >80% of responders preferred conservative measures for this acute injury. The reversal of trends has also been corroborated by recent multicenter trials demonstrating no difference in clinical outcomes between operative and nonoperative treatment of high grade AC joint dislocations, albeit these patients were not all high level overhead throwing athletes.17,18
Continue to: The trends in surgical interventions are notable...
The trends in surgical interventions are notable within the smaller subset of patients who are indicated for operative repair. Use of hardware and primary ligament repair, while popular in the surveys conducted in the 1970s10 and 1990s13 and even present in Nissen and Chatterjee’s20 2007 survey, were noticeably absent from our survey results, with the majority of respondents preferring open coracoclavicular ligament reconstruction. The role of distal clavicle excision has also expanded, from 23% of team physicians recommending it in 199713 to 57% to 59% in Nissen and Chatterjee’s20 2007 survey, to 66.7% in our series. This trend is not surprising as several recent cadaveric biomechanical studies have demonstrated that not only do peak graft forces not increase significantly,21 the anterior-posterior and superior-inferior motion at the AC joint following ligament reconstruction is maintained despite resection of the lateral clavicle.22 Additionally, primary distal clavicle excision may prevent the development of post-traumatic arthritis at the AC joint and osteolysis of the distal clavicle as a possible pain generator in the future.23 However, some respondents cautioned against performing a concomitant distal clavicle excision, as some biomechanical data demonstrate that resecting the distal clavicle may lead to increased horizontal translation at the AC joint despite intact superior and posterior AC capsules.24 Professional baseball pitchers may also be more lax and thus prone to more instability. Primary repair or reconstruction may not always lead to complete pre-injury stability in these individuals. This subtle unrecognized instability is hard to diagnosis and may be a persistent source of pain; thus, adding a distal clavicle excision may actually exacerbate the instability.
The nuanced indications for operative intervention, such as the presence of associated lesions were not captured by our survey.25 While most team physicians cited functional limitations as their most common reason for offering surgery, several MLB orthopedic surgeons also commented on evaluating the stability of the AC joint after a grade III injury, akin to the consensus statement from the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS) Upper Extremity Committee26 in 2014 that diversified the Rockwood Grade III AC joint separation into its IIIA and IIIB classifications. The ISAKOS recommendations include initial conservative management and a second evaluation (both clinical and radiographic) for grade III lesions 3 to 6 weeks after the injury. However, as professional baseball is an incredibly profitable sport with an annual revenue approaching $9.5 billion27 and pitching salaries up to $32.5 million in 2015, serious financial considerations must be given to players who wish to avoid undergoing delayed surgery.
This study has shortcomings typical of expert opinion papers. The retrospective nature of this study places the data at risk of recall bias. Objective data (eg, terminal ROM, pain relief, and return to play) were obtained from a retrospective chart review; however, no standard documentation or collection method was used given the number of surgeons involved and, thus, conclusions based on treatment outcomes are imperfect. Another major weakness of this survey is the relatively small number of patients and respondents. An a priori power analysis was not available, as this was a retrospective review. A comparative trial will be necessary to definitively support one treatment over another. Assuming a 95% return to play in the nonoperatively treated group, approximately 300 patients would be needed in a prospective 2-armed study with 80% power to detect a 10% reduction in the incidence of return to play using an alpha level of 0.05 and assuming no loss to follow-up. This sample size would be difficult to achieve in this patient population.
However, compared to past series,13 the number of professional baseball players treated by the collective experience of these MLB team physicians is the largest reported to date. As suggested above, the rarity of this condition in elite athletes precludes the ability to have matched controls to definitively determine the optimal treatment, which may explain the lack of difference in the return to play, ROM, and pain relief outcomes. Instead, we can only extrapolate based on the collective anecdotal experience of the MLB team physicians.
CONCLUSION
Despite advances in surgical technique and understanding of throwing mechanics, the majority of MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in a professional baseball pitcher. Open coracoclavicular ligament reconstruction was preferred for those who preferred operative intervention. An increasing number of orthopedic surgeons now consider a distal clavicle excision as an adjunct procedure.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Despite advancements in surgical technique and understanding of throwing mechanics, controversy persists regarding the treatment of grade III acromioclavicular (AC) joint separations, particularly in throwing athletes. Twenty-eight major league baseball (MLB) orthopedic team physicians were surveyed to determine their definitive management of a grade III AC separation in the dominant arm of a professional baseball pitcher and their experience treating AC joint separations in starting pitchers and position players. Return-to-play outcomes were also evaluated. Twenty (71.4%) team physicians recommended nonoperative intervention compared to 8 (28.6%) who would have operated acutely. Eighteen (64.3%) team physicians had treated at least 1 professional pitcher with a grade III AC separation; 51 (77.3%) pitchers had been treated nonoperatively compared to 15 (22.7%) operatively. No difference was observed in the proportion of pitchers who returned to the same level of play (P = .54), had full, unrestricted range of motion (P = .23), or had full pain relief (P = .19) between the operatively and nonoperatively treated MLB pitchers. The majority (53.6%) of physicians would not include an injection if the injury was treated nonoperatively. Open coracoclavicular reconstruction (65.2%) was preferred for operative cases; 66.7% of surgeons would also include distal clavicle excision as an adjunct procedure. About 90% of physicians would return pitchers to throwing >12 weeks after surgery compared to after 4 to 6 weeks in nonoperatively treated cases. In conclusion, MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in professional pitchers. If operative intervention is required, ligament reconstruction with adjunct distal clavicle excision were the most commonly performed procedures.
Continue to: Despite advancements in surgucal technique...
Despite advancements in surgical technique and improved understanding of the physiology of throwing mechanics, controversy persists regarding the preferred treatment for grade III acromioclavicular (AC) joint separations.1-6 Nonsurgical management has demonstrated return to prior function with fewer complications.7 However, there is a growing body of evidence demonstrating that surgical intervention is associated with more favorable outcomes8 and should be considered in patients who place high functional demands on their shoulders.9
The reported results on professional athletes in the literature remain ambivalent. Multiple small case reports/series have reported successful nonoperative treatment of elite athletes.10-12 Not surprisingly, McFarland and colleagues13 reported in 1997 that 69% of major league baseball (MLB) team physicians preferred nonoperative treatment for a theoretical starting pitcher sustaining a grade III AC separation 1 week prior to the start of the season. In contrast, reports of an inability to throw at a pre-injury level are equally commonplace.14,15 Nevertheless, all of these studies were limited to small cohorts, as the incidence of grade III AC separations in elite throwing athletes is relatively uncommon.13,16
In this study, we re-evaluated the study performed by McFarland and colleagues13 in 1997 by surveying all active MLB team orthopedic surgeons. We asked them how they would treat a grade III AC separation in a starting professional baseball pitcher. The physicians were also asked about their personal experience evaluating outcomes in these elite athletes. Given our improved understanding of the anatomy, pathophysiology, and surgical techniques for treating grade III AC separations, we hypothesize that more MLB team physicians would favor operative intervention treatment in professional baseball pitchers, as their vocation places higher demands on their shoulders.
MATERIALS AND METHODS
A questionnaire (Appendix A) was distributed to the team physicians of all 30 MLB teams. In addition to surgeon demographics, including age, years in practice, and years of taking care of an MLB team, the initial section of the questionnaire asked orthopedic surgeons how they would treat a theoretical starting pitcher who sustained a grade III AC joint separation of the dominant throwing arm 1 week prior to the start of the season. Physicians who preferred nonoperative treatment were asked whether they would use an injection (and what type), as well as when they would allow the pitcher to start a progressive interval throwing program. Physicians who preferred operative treatment were asked to rank their indications for operating, what procedure they would use (eg, open vs arthroscopic or coracoclavicular ligament repair vs reconstruction), and whether the surgical intervention would include distal clavicle excision. Both groups of physicians were also asked if their preferred treatment would change if the injury were to occur at the end of the season.
The second portion of the questionnaire asked surgeons about their experience treating AC joint separations in both starting pitchers and position players, as well as to describe the long-term outcomes of their preferred treatment, including time to return to full clearance for pitching, whether their patients returned to their prior level of play, and whether these patients had full pain relief. Finally, physicians were asked if any of the nonoperatively treated players ultimately crossed over and required operative intervention.
Continue to: Statistics...
STATISTICS
Descriptive statistics were used for continuous variables, and frequencies were used for categorical variables. Linear regression was performed to determine the correlation between the physician’s training or experience in treating AC joint separations and their recommended treatment. Fischer’s exact test/chi-square analysis was used to compare categorical variables. All tests were conducted using 2-sided hypothesis testing with statistical significance set at P < .05. All statistical analyses were conducted with SPSS 21.0 software (IBM Corporation).
RESULTS
A total of 28 MLB team physicians completed the questionnaires from 18 of the 30 MLB teams. The average age of the responders was 50.5 years (range, 34-60 years), with an average of 18.2 years in practice (range, 2-30 years) and 10.8 years (range, 1-24 years) taking care of their current professional baseball team. About 82% of the team physicians completed a sports medicine fellowship. On average, physicians saw 16.6 (range, 5-50) grade III or higher AC joint separations per year, and operated on 4.6 (range, 0-10) per year.
Nonoperative treatment was the preferred treatment for a grade III AC joint separation in a starting professional baseball pitcher for the majority of team physicians (20/28). No correlation was observed between the physician’s age (P = .881), years in practice (P = .915), years taking care of their professional team (P = .989), percentage of practice focused on shoulders (P = .986), number of AC joint injuries seen (P = .325), or number of surgeries performed per year (P = .807) with the team physician’s preferred treatment. Compared to the proportion reported originally by McFarland and colleagues13 in 1997 (69%), there was no difference in the proportion of team physicians that recommended nonoperative treatment (P = 1).
If treating this injury nonoperatively, 46.4% of physicians would also use an injection, with orthobiologics (eg, platelet-rich plasma) as the most popular choice (Table 1). No consensus was provided on the timeframe to return pitchers back to a progressive interval throwing program; however, 46.67% of physicians would return pitchers 4 to 6 weeks after a nonoperatively treated injury, while 35.7% would return pitchers 7 to 12 weeks after the initial injury.
Table 1. Treatment Preferences of Grade III AC Separation by MLB Team Physicians
Nonoperativea | |
Yes injection | 13 (46.4%) |
Cortisone | 3 (23.1%) |
Orthobiologic | 10 (76.9%) |
Local anesthetic (eg, lidocaine) | 1 (7.7%) |
Intramuscular toradol | 3 (23.1%) |
No injection | 15 (53.6%) |
Operativea | |
Open coracoclavicular ligament repair | 3 (13.0%) |
Open coracoclavicular ligament reconstruction | 15 (65.2%) |
Arthroscopic reconstruction with graft | 6 (26.1%) |
Arthroscopic repair with implant (ie, tight-rope) | 2 (8.7%) |
Distal clavicle excisionb | 16 (66.7%) |
Would not intervene operatively | 5 (17.9%) |
|
|
aRespondents were allowed to choose more than 1 treatment in each category. bChosen as an adjunct treatment.
Abbreviations: AC, acromioclavicular; MLB, major league baseball.
Most physicians (64.3%) cited functional limitations as the most important reason for indicating operative treatment, followed by pain (21.4%), and a deformity (14.3%). About 65% preferred open coracoclavicular ligament reconstruction. No physician recommended the Weaver-Dunn procedure or use of hardware (eg, hook plate). Of those who preferred an operative intervention, 66.7% would also include a distal clavicle excision, which is significantly higher than the proportion reported by McFarland and colleagues13 (23%, P = .0170). About 90% of physicians would return pitchers to play >12 weeks after operative treatment.
Continue to: If the injury occurred at the end ...
If the injury occurred at the end of the season, 7 of the 20 orthopedists (35%) who recommended nonoperative treatment said they would change to an operative intervention. Eighteen of 28 responders would have the same algorithm for MLB position players. Team physicians were less likely to recommend operative intervention in position players due to less demand on the arm and increased ability to accommodate the injury by altering their throwing mechanics.
Eighteen (64%) of the team physicians had treated at least 1 professional pitcher with a grade III AC separation in his dominant arm, and 11 (39.3%) had treated >1. Collectively, team physicians had treated 15 professional pitchers operatively, and 51 nonoperatively; only 3 patients converted to operative intervention after a failed nonoperative treatment.
Of the pitchers treated operatively, 93.3% (14) of pitchers returned to their prior level of pitching. The 1 patient who failed to return to the same level of pitching retired instead of returning to play. About 80% (12) of the pitchers had full pain relief, and 93.3% (14) had full range of motion (ROM). The pitcher who failed to regain full ROM also had a concomitant rotator cuff repair. The only complication reported from an operative intervention was a pitcher who sustained a coracoid fracture 10 months postoperatively while throwing 100 mph. Of the pitchers treated nonoperatively, 96% returned to their prior level of pitching, 92.2% (47) had full complete pain relief when throwing, and 100% had full ROM. No differences were observed between the proportion of pitchers who returned to their prior level of pitching, regained full ROM, or had full pain relief in the operative and nonoperative groups (Table 2).
Table 2. Outcomes of Treatment of Grade III AC Separation in 58 Professional Baseball Players
| Operative | Nonoperative | P-value |
Return to same level of play | 14/15 (93.3%) | 49/51 (96%) | 0.54 |
Full pain relief | 12/15 (80%) | 47/51 (92.2%) | 0.19 |
Full ROM | 14/15 (93.3%) | 51/51 (100%) | 0.23 |
Abbreviations: AC, acromioclavicular; ROM, range of motion.
DISCUSSION
Controversy persists regarding the optimal management of acute grade III AC separations, with the current available evidence potentially suggesting better cosmetic and radiological results but no definite differences in clinical results.1-6,17,18 In the absence of formal clinical practice guidelines, surgeons rely on their own experience or defer to the anecdotal experience of experts in the field. Our initial hypothesis was false in this survey of MLB team physicians taking care of overhead throwing athletes at the highest level. Our results demonstrate that despite improved techniques and an increased understanding of the pathophysiology of AC joint separations, conservative management is still the preferred treatment for acute grade III AC joint separations in professional baseball pitchers. The proportion of team physicians recommending nonoperative treatment in our series was essentially equivalent to the results reported by McFarland and colleagues13 in 1997, suggesting that the pendulum continues to favor conservative management initially. This status quo likely reflects both the dearth of literature suggesting a substantial benefit of acute operative repair, as well as the ability to accommodate with conservative measures after most grade III AC injuries, even at the highest level of athletic competition.
These results are also consistent with trends from the last few decades. In the 1970s, the overwhelming preference for treating an acute complete AC joint separation was surgical repair, with Powers and Bach10 reporting in a 1974 survey of 163 chairmen of orthopedic programs around the country that 91.5% advocated surgical treatment. However, surgical preference had reversed by the 1990s. Of the 187 chairmen and 59 team physicians surveyed by Cox19 in 1992, 72% and 86% respectively preferred nonoperative treatment in a theoretical 21-year-old athlete with a grade III AC separation. Nissen and Chatterjee20 reported in 2007 on a survey of all American Orthopaedic Society for Sports Medicine surgeons (N = 577) and Accreditation Council for Graduate Medical Education orthopedic program residency directors (N = 87) that >80% of responders preferred conservative measures for this acute injury. The reversal of trends has also been corroborated by recent multicenter trials demonstrating no difference in clinical outcomes between operative and nonoperative treatment of high grade AC joint dislocations, albeit these patients were not all high level overhead throwing athletes.17,18
Continue to: The trends in surgical interventions are notable...
The trends in surgical interventions are notable within the smaller subset of patients who are indicated for operative repair. Use of hardware and primary ligament repair, while popular in the surveys conducted in the 1970s10 and 1990s13 and even present in Nissen and Chatterjee’s20 2007 survey, were noticeably absent from our survey results, with the majority of respondents preferring open coracoclavicular ligament reconstruction. The role of distal clavicle excision has also expanded, from 23% of team physicians recommending it in 199713 to 57% to 59% in Nissen and Chatterjee’s20 2007 survey, to 66.7% in our series. This trend is not surprising as several recent cadaveric biomechanical studies have demonstrated that not only do peak graft forces not increase significantly,21 the anterior-posterior and superior-inferior motion at the AC joint following ligament reconstruction is maintained despite resection of the lateral clavicle.22 Additionally, primary distal clavicle excision may prevent the development of post-traumatic arthritis at the AC joint and osteolysis of the distal clavicle as a possible pain generator in the future.23 However, some respondents cautioned against performing a concomitant distal clavicle excision, as some biomechanical data demonstrate that resecting the distal clavicle may lead to increased horizontal translation at the AC joint despite intact superior and posterior AC capsules.24 Professional baseball pitchers may also be more lax and thus prone to more instability. Primary repair or reconstruction may not always lead to complete pre-injury stability in these individuals. This subtle unrecognized instability is hard to diagnosis and may be a persistent source of pain; thus, adding a distal clavicle excision may actually exacerbate the instability.
The nuanced indications for operative intervention, such as the presence of associated lesions were not captured by our survey.25 While most team physicians cited functional limitations as their most common reason for offering surgery, several MLB orthopedic surgeons also commented on evaluating the stability of the AC joint after a grade III injury, akin to the consensus statement from the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS) Upper Extremity Committee26 in 2014 that diversified the Rockwood Grade III AC joint separation into its IIIA and IIIB classifications. The ISAKOS recommendations include initial conservative management and a second evaluation (both clinical and radiographic) for grade III lesions 3 to 6 weeks after the injury. However, as professional baseball is an incredibly profitable sport with an annual revenue approaching $9.5 billion27 and pitching salaries up to $32.5 million in 2015, serious financial considerations must be given to players who wish to avoid undergoing delayed surgery.
This study has shortcomings typical of expert opinion papers. The retrospective nature of this study places the data at risk of recall bias. Objective data (eg, terminal ROM, pain relief, and return to play) were obtained from a retrospective chart review; however, no standard documentation or collection method was used given the number of surgeons involved and, thus, conclusions based on treatment outcomes are imperfect. Another major weakness of this survey is the relatively small number of patients and respondents. An a priori power analysis was not available, as this was a retrospective review. A comparative trial will be necessary to definitively support one treatment over another. Assuming a 95% return to play in the nonoperatively treated group, approximately 300 patients would be needed in a prospective 2-armed study with 80% power to detect a 10% reduction in the incidence of return to play using an alpha level of 0.05 and assuming no loss to follow-up. This sample size would be difficult to achieve in this patient population.
However, compared to past series,13 the number of professional baseball players treated by the collective experience of these MLB team physicians is the largest reported to date. As suggested above, the rarity of this condition in elite athletes precludes the ability to have matched controls to definitively determine the optimal treatment, which may explain the lack of difference in the return to play, ROM, and pain relief outcomes. Instead, we can only extrapolate based on the collective anecdotal experience of the MLB team physicians.
CONCLUSION
Despite advances in surgical technique and understanding of throwing mechanics, the majority of MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in a professional baseball pitcher. Open coracoclavicular ligament reconstruction was preferred for those who preferred operative intervention. An increasing number of orthopedic surgeons now consider a distal clavicle excision as an adjunct procedure.
This paper will be judged for the Resident Writer’s Award.
- Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;455:38-44. doi:10.1097/BLO.0b013e318030df83.
- Ceccarelli E, Bondì R, Alviti F, Garofalo R, Miulli F, Padua R. Treatment of acute grade III acromioclavicular dislocation: A lack of evidence. J Orthop Traumatol. 2008;9(2):105-108. doi:10.1007/s10195-008-0013-7.
- Smith TO, Chester R, Pearse EO, Hing CB. Operative versus non-operative management following rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthop Traumatol. 2011;12(1):19-27. doi:10.1007/s10195-011-0127-1.
- Beitzel K, Cote MP, Apostolakos J, et al. Current concepts in the treatment of acromioclavicular joint dislocations. Arthroscopy. 2013;29(2):387-397. doi:10.1016/j.arthro.2012.11.023.
- Korsten K, Gunning AC, Leenen LP. Operative or conservative treatment in patients with rockwood type III acromioclavicular dislocation: a systematic review and update of current literature. Int Orthop. 2014;38(4):831-838. doi:10.1007/s00264-013-2143-7.
- Modi CS, Beazley J, Zywiel MG, Lawrence TM, Veillette CJ. Controversies relating to the management of acromioclavicular joint dislocations. Bone Joint J. 2013;95-B(12):1595-1602. doi:10.1302/0301-620X.95B12.31802.
- Reid D, Polson K, Johnson L. Acromioclavicular joint separations grades I-III: a review of the literature and development of best practice guidelines. Sports Med. 2012;42(8):681-696. doi:10.2165/11633460-000000000-00000.
- Farber AJ, Cascio BM, Wilckens JH. Type III acromioclavicular separation: rationale for anatomical reconstruction. Am J Orthop. 2008;37(7):349-355.
- Li X, Ma R, Bedi A, Dines DM, Altchek DW, Dines JS. Management of acromioclavicular joint injuries. J Bone Joint Surg Am. 2014;96(1):73-84. doi:10.2106/JBJS.L.00734.
- Powers JA, Bach PJ. Acromioclavicular separations. Closed or open treatment? Clin Orthop Relat Res. 1974;104(104):213-223. doi:10.1097/00003086-197410000-00024.
- Glick JM, Milburn LJ, Haggerty JF, Nishimoto D. Dislocated acromioclavicular joint: follow-up study of 35 unreduced acromioclavicular dislocations. Am J Sports Med. 1977;5(6):264-270. doi:10.1177/036354657700500614.
- Watson ST, Wyland DJ. Return to play after nonoperative management for a severe type III acromioclavicular separation in the throwing shoulder of a collegiate pitcher. Phys Sportsmed. 2015;43(1):99-103. doi:10.1080/00913847.2015.1001937.
- McFarland EG, Blivin SJ, Doehring CB, Curl LA, Silberstein C. Treatment of grade III acromioclavicular separations in professional throwing athletes: results of a survey. Am J Orthop. 1997;26(11):771-774.
- Wojtys EM, Nelson G. Conservative treatment of grade III acromioclavicular dislocations. Clin Orthop Relat Res. 1991;268(268):112-119.
- Galpin RD, Hawkins RJ, Grainger RW. A comparative analysis of operative versus nonoperative treatment of grade III acromioclavicular separations. Clin Orthop Relat Res. 1985;193(193):150-155. doi:10.1097/00003086-198503000-00020.
- Pallis M, Cameron KL, Svoboda SJ, Owens BD. Epidemiology of acromioclavicular joint injury in young athletes. Am J Sports Med. 2012;40(9):2072-2077. doi:10.1177/0363546512450162.
- Canadian Orthopaedic Trauma Society. Multicenter randomized clinical trial of nonoperative versus operative treatment of acute acromio-clavicular joint dislocation. J Orthop Trauma. 2015;29(11):479-487. doi:10.1097/BOT.0000000000000437.
- Joukainen A, Kröger H, Niemitukia L, Mäkelä EA, Väätäinen U. Results of operative and nonoperative treatment of rockwood types III and V acromioclavicular joint dislocation: a prospective, randomized trial with an 18- to 20-year follow-up. Orthop J Sports Med. 2014;2(12):2325967114560130. doi:10.1177/2325967114560130.
- Cox JS. Current method of treatment of acromioclavicular joint dislocations. Orthopedics. 1992;15(9):1041-1044.
- Nissen CW, Chatterjee A. Type III acromioclavicular separation: results of a recent survey on its management. Am J Orthop. 2007;36(2):89-93.
- Kowalsky MS, Kremenic IJ, Orishimo KF, McHugh MP, Nicholas SJ, Lee SJ. The effect of distal clavicle excision on in situ graft forces in coracoclavicular ligament reconstruction. Am J Sports Med. 2010;38(11):2313-2319. doi:10.1177/0363546510374447.
- Beaver AB, Parks BG, Hinton RY. Biomechanical analysis of distal clavicle excision with acromioclavicular joint reconstruction. Am J Sports Med. 2013;41(7):1684-1688. doi:10.1177/0363546513488750.
- Mumford EB. Acromioclavicular dislocation. J Bone Joint Surg Am. 1941;23:799-802.
- Beitzel K, Sablan N, Chowaniec DM, et al. Sequential resection of the distal clavicle and its effects on horizontal acromioclavicular joint translation. Am J Sports Med. 2012;40(3):681-685. doi:10.1177/0363546511428880.
- Arrigoni P, Brady PC, Zottarelli L, et al. Associated lesions requiring additional surgical treatment in grade 3 acromioclavicular joint dislocations. Arthroscopy. 2014;30(1):6-10. doi:10.1016/j.arthro.2013.10.006.
- Beitzel K, Mazzocca AD, Bak K, et al. ISAKOS upper extremity committee consensus statement on the need for diversification of the rockwood classification for acromioclavicular joint injuries. Arthroscopy. 2014;30(2):271-278. doi:10.1016/j.arthro.2013.11.005.
- Brown M. MLB sees record revenues for 2015, up $500 million and approaching $9.5 billion. Forbes Web site. http://www.forbes.com/sites/maurybrown/2015/12/04/mlb-sees-record-revenu.... Published December 4, 2015. Accessed February 4, 2016.
- Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;455:38-44. doi:10.1097/BLO.0b013e318030df83.
- Ceccarelli E, Bondì R, Alviti F, Garofalo R, Miulli F, Padua R. Treatment of acute grade III acromioclavicular dislocation: A lack of evidence. J Orthop Traumatol. 2008;9(2):105-108. doi:10.1007/s10195-008-0013-7.
- Smith TO, Chester R, Pearse EO, Hing CB. Operative versus non-operative management following rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthop Traumatol. 2011;12(1):19-27. doi:10.1007/s10195-011-0127-1.
- Beitzel K, Cote MP, Apostolakos J, et al. Current concepts in the treatment of acromioclavicular joint dislocations. Arthroscopy. 2013;29(2):387-397. doi:10.1016/j.arthro.2012.11.023.
- Korsten K, Gunning AC, Leenen LP. Operative or conservative treatment in patients with rockwood type III acromioclavicular dislocation: a systematic review and update of current literature. Int Orthop. 2014;38(4):831-838. doi:10.1007/s00264-013-2143-7.
- Modi CS, Beazley J, Zywiel MG, Lawrence TM, Veillette CJ. Controversies relating to the management of acromioclavicular joint dislocations. Bone Joint J. 2013;95-B(12):1595-1602. doi:10.1302/0301-620X.95B12.31802.
- Reid D, Polson K, Johnson L. Acromioclavicular joint separations grades I-III: a review of the literature and development of best practice guidelines. Sports Med. 2012;42(8):681-696. doi:10.2165/11633460-000000000-00000.
- Farber AJ, Cascio BM, Wilckens JH. Type III acromioclavicular separation: rationale for anatomical reconstruction. Am J Orthop. 2008;37(7):349-355.
- Li X, Ma R, Bedi A, Dines DM, Altchek DW, Dines JS. Management of acromioclavicular joint injuries. J Bone Joint Surg Am. 2014;96(1):73-84. doi:10.2106/JBJS.L.00734.
- Powers JA, Bach PJ. Acromioclavicular separations. Closed or open treatment? Clin Orthop Relat Res. 1974;104(104):213-223. doi:10.1097/00003086-197410000-00024.
- Glick JM, Milburn LJ, Haggerty JF, Nishimoto D. Dislocated acromioclavicular joint: follow-up study of 35 unreduced acromioclavicular dislocations. Am J Sports Med. 1977;5(6):264-270. doi:10.1177/036354657700500614.
- Watson ST, Wyland DJ. Return to play after nonoperative management for a severe type III acromioclavicular separation in the throwing shoulder of a collegiate pitcher. Phys Sportsmed. 2015;43(1):99-103. doi:10.1080/00913847.2015.1001937.
- McFarland EG, Blivin SJ, Doehring CB, Curl LA, Silberstein C. Treatment of grade III acromioclavicular separations in professional throwing athletes: results of a survey. Am J Orthop. 1997;26(11):771-774.
- Wojtys EM, Nelson G. Conservative treatment of grade III acromioclavicular dislocations. Clin Orthop Relat Res. 1991;268(268):112-119.
- Galpin RD, Hawkins RJ, Grainger RW. A comparative analysis of operative versus nonoperative treatment of grade III acromioclavicular separations. Clin Orthop Relat Res. 1985;193(193):150-155. doi:10.1097/00003086-198503000-00020.
- Pallis M, Cameron KL, Svoboda SJ, Owens BD. Epidemiology of acromioclavicular joint injury in young athletes. Am J Sports Med. 2012;40(9):2072-2077. doi:10.1177/0363546512450162.
- Canadian Orthopaedic Trauma Society. Multicenter randomized clinical trial of nonoperative versus operative treatment of acute acromio-clavicular joint dislocation. J Orthop Trauma. 2015;29(11):479-487. doi:10.1097/BOT.0000000000000437.
- Joukainen A, Kröger H, Niemitukia L, Mäkelä EA, Väätäinen U. Results of operative and nonoperative treatment of rockwood types III and V acromioclavicular joint dislocation: a prospective, randomized trial with an 18- to 20-year follow-up. Orthop J Sports Med. 2014;2(12):2325967114560130. doi:10.1177/2325967114560130.
- Cox JS. Current method of treatment of acromioclavicular joint dislocations. Orthopedics. 1992;15(9):1041-1044.
- Nissen CW, Chatterjee A. Type III acromioclavicular separation: results of a recent survey on its management. Am J Orthop. 2007;36(2):89-93.
- Kowalsky MS, Kremenic IJ, Orishimo KF, McHugh MP, Nicholas SJ, Lee SJ. The effect of distal clavicle excision on in situ graft forces in coracoclavicular ligament reconstruction. Am J Sports Med. 2010;38(11):2313-2319. doi:10.1177/0363546510374447.
- Beaver AB, Parks BG, Hinton RY. Biomechanical analysis of distal clavicle excision with acromioclavicular joint reconstruction. Am J Sports Med. 2013;41(7):1684-1688. doi:10.1177/0363546513488750.
- Mumford EB. Acromioclavicular dislocation. J Bone Joint Surg Am. 1941;23:799-802.
- Beitzel K, Sablan N, Chowaniec DM, et al. Sequential resection of the distal clavicle and its effects on horizontal acromioclavicular joint translation. Am J Sports Med. 2012;40(3):681-685. doi:10.1177/0363546511428880.
- Arrigoni P, Brady PC, Zottarelli L, et al. Associated lesions requiring additional surgical treatment in grade 3 acromioclavicular joint dislocations. Arthroscopy. 2014;30(1):6-10. doi:10.1016/j.arthro.2013.10.006.
- Beitzel K, Mazzocca AD, Bak K, et al. ISAKOS upper extremity committee consensus statement on the need for diversification of the rockwood classification for acromioclavicular joint injuries. Arthroscopy. 2014;30(2):271-278. doi:10.1016/j.arthro.2013.11.005.
- Brown M. MLB sees record revenues for 2015, up $500 million and approaching $9.5 billion. Forbes Web site. http://www.forbes.com/sites/maurybrown/2015/12/04/mlb-sees-record-revenu.... Published December 4, 2015. Accessed February 4, 2016.
TAKE-HOME POINTS
- There was no difference in return to previous level of play between professional pitchers treated nonoperatively and operatively for grade III AC separation.
- MLB team physicians prefer nonoperative management for acute grade III AC joint separation in professional pitchers.
- The majority of MLB physicians do not use injections for nonoperative treatment of grade III AC separations; however, use of orthobiologics (eg, PRP) is becoming more commonplace.
- Persistent functional limitations and pain are the most common surgical indications for treatment of grade III AC separation in high level throwing athletes.
- If operative intervention is indicated for grade III AC separation, open coracoclavicular reconstruction and adjunct distal clavicle excision are preferred by most MLB team physicians.
Reasons for Readmission Following Primary Total Shoulder Arthroplasty
ABSTRACT
An increasing interest focuses on the rates and risk factors for hospital readmission. However, little is known regarding the readmission following total shoulder arthroplasty (TSA). This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA. Patients undergoing TSA (anatomic or reverse) as part of the American College of Surgeons National Surgical Quality Improvement Program in 2011 to 2013 were identified. The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. Using multivariate regression, demographic and comorbidity factors were tested for independent association with readmission. Finally, the reasons for readmission were characterized. A total of 3627 patients were identified. Among the admitted patients, 93 (2.56%) were readmitted within 30 days of surgery. The independent risk factors for readmission included old age (for age 60-69 years, relative risk [RR] = 1.6; for age 70-79 years, RR = 2.3; for age ≥80 years, RR = 23.1; P = .042), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission comprised pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%). Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at higher risk for readmission and should be counseled and monitored accordingly.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) is performed with increasing frequency in the United States and is considered to be cost-effective.1-4 Following the procedure, patients generally achieve shoulder function and pain relief.5-8 Despite the success of the procedure, the growing literature on TSA has also reported rates of complications between 3.6% and 25% of the treated patients.9-16
In recent years, an increasing interest has focused on the rates and risk factors for unplanned hospital readmissions; these variables may not only reflect the quality of patient care but also result in considerable costs to the healthcare system. For instance, among Medicare patients, readmissions within 30 days of discharge occur in almost 20% of cases, costing $17.4 billion per year.17 Readmission rates increasingly factor into hospital performance metrics and reimbursement, including the Hospital Readmissions Reduction Program of the Patient Protection and Affordable Care Act that reduces Centers for Medicare and Medicaid Services payments to hospitals with high 30-day readmission rates.18
To date, only a few studies have evaluated readmission following TSA, with 30- to 90-day readmission rates ranging from 4.5% to 7.3%.19-23 These studies comprised single institution series20,22 and analyses of administrative databases.19,21,23 Most studies have shown that readmission occurs more often for medical than surgical reasons, with surgical reasons most commonly including infection and dislocation.19-23 However, only limited analyses have been conducted regarding risk factors for readmission.21,23 To date and to our knowledge, no study has investigated reasons for readmission following TSA using nationwide data.
This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA in the United States using the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database.
METHODS
DATA SOURCE
The NSQIP database was utilized to address the study purpose. NSQIP is a nationwide prospective surgical registry established by the American College of Surgeons and reports data from academic and community hospitals across the United States.24 Patients undertaking surgery at these centers are followed by the surgical clinical reviewers at the participating NSQIP sites prospectively for 30 days following the procedure to record complications including readmission. Preoperative and surgical data, such as demographics, medical comorbid diseases, and operative time, are also included. Previous studies have analyzed the complications of various orthopedic surgeries using the NSQIP data.14,16,25-30
DATA COLLECTION
We retrospectively identified from NSQIP the patients who underwent primary TSA (anatomic or reverse) in 2013 to 2014. The timeframe 2013 to 2014 was used because NSQIP only began recording reasons for readmission in 2013. The inclusion criteria were as follows: Current Procedural Terminology (CPT) code for TSA (23472); preoperative diagnosis according to the International Classification of Diseases, Ninth Revision (ICD-9) codes 714.0, 715.11, 715.31, 715.91, 715.21, 715.89, 716.xx 718.xx, 719.xx, 726.x, 727.xx, and 733.41 (where x is a wild card digit); and no missing demographic, comorbidity, or outcome data. Anatomic and reverse TSA were analyzed together because they share the same CPT code, and the NSQIP database prevents searching by the ICD-9 procedure code.
The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. The reasons for readmission in this 30-day period were only available in 2013 and were determined using the ICD-9 diagnosis codes. Patient demographics were recorded for use in identifying potential risk factors for readmission; the demographic data included sex, age, smoking status, body mass index (BMI), and comorbidities, including end-stage renal disease, dyspnea on exertion, congestive heart failure, diabetes mellitus, hypertension, and chronic obstructive pulmonary disease (COPD).
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were performed using Stata version 13.1 (StataCorp). First, using bivariate and multivariate regression, demographic and comorbidity factors were tested for independent association with readmission to the hospital within 30 days of surgery. Second, among the readmitted patients, the reasons for readmission were tabulated. Of note, the reasons for readmission were only documented for the procedures performed in 2013. All tests were 2-tailed and conducted at an α level of 0.05.
RESTULTS
A total of 3627 TSA patients were identified. The mean age (± standard deviation) was 69.4 ± 9.5 years, 55.8% of patients were female, and mean BMI was 30.1 ± 7.0 years. Table 1 provides the additional demographic data. Of the 3627 included patients, 93 (2.56%) were readmitted within 30 days of surgery. The 95% confidence interval for the estimated rate of readmission reached 2.05% to 3.08%.
Table 1. Patient Population
| Number | Percent |
Total | 3627 | 100.0% |
Age |
|
|
18-59 | 539 | 14.9% |
60-69 | 1235 | 34.1% |
70-79 | 1317 | 36.3% |
≥80 | 536 | 14.8% |
Sex |
|
|
Male | 1603 | 44.2% |
Female | 2024 | 55.8% |
Body mass index |
|
|
Normal (<25 kg/m2) | 650 | 17.9% |
Overweight (25-30 kg/m2) | 1147 | 31.6% |
Obese (≥30 kg/m2) | 1830 | 50.5% |
Functional status |
|
|
Independent | 3544 | 97.7% |
Dependent | 83 | 2.3% |
Diabetes mellitus |
|
|
No | 3022 | 83.3% |
Yes | 605 | 16.7% |
Dyspnea on exertion |
|
|
No | 3393 | 93.6% |
Yes | 234 | 6.5% |
Hypertension |
|
|
No | 1192 | 32.9% |
Yes | 2435 | 67.1% |
COPD |
|
|
No | 3384 | 93.3% |
Yes | 243 | 6.7% |
Current smoker |
|
|
No | 3249 | 89.6% |
Yes | 378 | 10.4% |
Anemia |
|
|
No | 3051 | 84.1% |
Yes | 576 | 15.9% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
In the bivariate analyses (Table 2), the following factors were positively associated readmission: older age (60-69 years, relative risk [RR] = 1.6; 70-79 years, RR = 2.2; ≥80 years, RR = 3.3; P = .011), dependent functional status (RR = 2.9, P = .008), and anemia (RR = 2.2, P < .001).
Table 2. Bivariate Analysis of Risk Factors for Readmission
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.011 |
18-59 | 1.30% | Ref. | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.2 | 1.0-4.9 |
|
≥80 | 4.29% | 3.3 | 1.4-7.6 |
|
Sex |
|
|
| 0.099 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.4 | 0.9-2.1 |
|
Body mass index |
|
|
| 0.764 |
Normal (<25 kg/m2) | 2.92% | Ref. | - |
|
Overweight (25-30 kg/m2) | 2.35% | 0.8 | 0.5-1.4 |
|
Obese (≥30 kg/m2) | 2.57% | 0.9 | 0.5-1.5 |
|
Functional status |
|
|
| 0.008 |
Independent | 2.45% | Ref. | - |
|
Dependent | 7.23% | 2.9 | 1.3-6.5 |
|
Diabetes mellitus |
|
|
| 0.483 |
No | 2.48% | Ref. | - |
|
Yes | 2.98% | 1.2 | 0.7-2.0 |
|
Dyspnea on exertion |
|
|
| 0.393 |
No | 2.51% | Ref. | - |
|
Yes | 3.42% | 1.4 | 0.7-2.8 |
|
Hypertension |
|
|
| 0.145 |
No | 2.01% | Ref. | - |
|
Yes | 2.83% | 1.4 | 0.9-2.2 |
|
COPD |
|
|
| 0.457 |
No | 2.51% | Ref. | - |
|
Yes | 3.29% | 1.3 | 0.6-2.7 |
|
Current smoker |
|
|
| 0.116 |
No | 2.71% | Ref. | - |
|
Yes | 1.32% | 0.5 | 0.2-1.2 |
|
Anemia |
|
|
| <0.001 |
No | 2.16% | Ref. | - |
|
Yes | 4.69% | 2.2 | 1.4-3.4 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
In the multivariate analyses (Table 3), the following factors were independent risk factors for readmission: older age (60-69 years, RR = 1.6; 70-79 years, RR = 2.3; ≥80 years, RR = 3.1; P =.027), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). Interestingly, readmission showed no independent association with diabetes, dyspnea on exertion, BMI, COPD, hypertension, or current smoking status (P > .05 for each).
Table 3. Independent Risk Factors for Readmission on Multivariate Analysis
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.027 |
18-59 | 1.30% | Ref | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.3 | 1.0-5.1 |
|
≥80 | 4.29% | 3.1 | 1.3-7.4 |
|
Sex |
|
|
| 0.025 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.6 | 1.1-2.4 |
|
Anemia |
|
|
| 0.005 |
No | 2.16% | Ref | - |
|
Yes | 4.69% | 1.9 | 1.2-3.0 |
|
Functional status |
|
|
| 0.012 |
Independent | 2.45% | Ref | - |
|
Dependent | 7.23% | 2.8 | 1.3-6.2 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
Continue to: Table 4...
The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission included pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%) (Table 4).
Table 4. Reasons for Readmission
| Number | Percent |
Pneumonia | 14 | 16.7% |
Dislocation | 7 | 8.3% |
Pulmonary embolism | 7 | 8.3% |
Surgical site infection | 6 | 7.1% |
Atrial fibrillation | 4 | 4.8% |
Hematoma | 4 | 4.8% |
Altered mental status | 3 | 3.6% |
Chest pain | 3 | 3.6% |
Renal insufficiency/kidney failure | 3 | 3.6% |
Urinary tract infection | 3 | 3.6% |
Acute gastric or duodenal ulcer | 2 | 2.4% |
Dermatitis/other allergic reaction | 2 | 2.4% |
Orthostatic hypotension/syncope | 2 | 2.4% |
Pain | 2 | 2.4% |
Respiratory distress | 2 | 2.4% |
Sepsis | 2 | 2.4% |
Urinary retention | 2 | 2.4% |
Acute cholecystitis | 1 | 1.2% |
Cerebrovascular accident | 1 | 1.2% |
Constipation | 1 | 1.2% |
Contusion of shoulder | 1 | 1.2% |
Deep venous thrombosis requiring therapy | 1 | 1.2% |
Gastrointestinal hemorrhage | 1 | 1.2% |
Gout | 1 | 1.2% |
Hepatic encephalopathy | 1 | 1.2% |
Intestinal infection | 1 | 1.2% |
Narcotic overdose | 1 | 1.2% |
Nausea/vomiting | 1 | 1.2% |
Proximal humerus fracture | 1 | 1.2% |
Rotator cuff tear | 1 | 1.2% |
Seroma | 1 | 1.2% |
Unspecified disease of pericardium | 1 | 1.2% |
Weakness | 1 | 1.2% |
DISCUSSION
Our analysis of 3042 TSAs from the NSQIP database suggests that unplanned readmission to the hospital occurs following about 1 in 40 cases of TSA. The study also suggests that the most common reasons for readmission encompass pneumonia, dislocation, pulmonary embolism, and surgical site infection. Old age, male sex, anemia, and dependent functional status serve as risk factors for readmission, and patients with such factors should be counseled and monitored accordingly.
In recent years, an increasing emphasis has centered on reducing rates of hospital readmission, with programs such as the Hospital Readmissions Reduction Program of the Affordable Care Act cutting reimbursements for hospitals with high 30-day readmission rates.17,18 To date, only a few studies have evaluated the reasons for readmission and readmission rates for TSA.19-23 Initial reports consisted of single-institution TSA registry reviews. For example, Mahoney and colleagues20 retrospectively evaluated shoulder arthroplasty procedures at their institution to document the readmission rates, finding a 5.9% readmission rate at 30 days. Readmission occurred more frequently in the first 30 days following discharge than in the 30- to 90-day period, with the most common reasons for readmission including medical complications, infection, and dislocation. Streubel and colleagues22 evaluated reoperation rates from their institution’s TSA registry, finding a 0.6% reoperation rate for primary TSA at 30 days and 1.5% for revision TSA. Instability and infection were the most common indications for reoperation. Our findings confirm these single-institution results and demonstrate their application to a nationwide sample of TSA, not just to high-volume academic centers. We similarly observed that dislocation, surgical site infection, and medical complications (mostly pneumonia and pulmonary embolism) were common causes of readmission, and that the 30-day readmission rate was about 1 in 40.
Several authors have since used statewide databases to analyze and determine risk factors for readmission following TSA. Lyman and colleagues19 used the New York State Database to show that higher hospital TSA surgical volume was associated with a lower rate of readmission when age and comorbidities were controlled for in a multivariate model. Old age was also associated with an increased readmission rate in their multivariate analysis, but comorbidities (as measured by the Charlson comorbidity index) presented a nonsignificant associative trend. These authors opted not to determine specific causes of readmission. Schairer and colleagues21 used State Inpatient Databases from 7 states, finding a 90-day readmission rate of 7.3%, 82% of which were due to medical complications and 18% of which were due to surgical complications (mostly infection and dislocation). Their multivariate regression revealed that male sex, reverse TSA, Medicaid insurance, patients discharged to inpatient rehabilitation or nursing facilities, medical comorbidities, and low-volume TSA hospitals were associated with readmission. Zhang and colleagues23 used the same source to show that the 90-day readmission rate reached 14% for surgically treated proximal humerus fractures and higher for patients who underwent open reduction internal fixation, were female, were African American, were discharged to a nursing facility, possessed Medicaid insurance, or experienced medical comorbidities. Most recently, Basques and colleagues31 analyzed 1505 TSA cases from 2011 and 2012 in the NSQIP database, finding a 3.3% rate of readmission, with heart disease and hypertension as risk factors for readmission. Although the limitations of the NSQIP database prevented us from analyzing surgeon and hospital TSA volume or reverse vs anatomic TSA, our results confirm that the findings from statewide database studies apply to the United States nationwide NSQIP database. Old patient age, male sex, and medical comorbidities (anemia and dependent functional status) are independent risk factors for TSA readmission. We identified pneumonia, dislocation, pulmonary embolism, and surgical site infection as the most common reasons for readmission.
This study features several limitations that should be considered when interpreting the results. Anatomic and reverse TSA share a CPT code and were not separated using NSQIP data. A number of studies have reported that reverse TSA may place patients at higher risk for readmission;20,21 however, confounding by other patient factors could play a role in this finding. The 30-day timeframe for readmission is another potential limitation; however, this timeframe is frequently used in other studies and is the relevant timeframe for the reduced reimbursement penalties from the Hospital Readmissions Reduction Program of the Affordable Care Act.18 Furthermore, the NSQIP database contains no information on surgeon or hospital TSA volume, which is a result of safeguards for patient and provider privacy. Additionally, readmission data were only available for 2011 to 2013, with causes of readmission only present in 2013. Although provided with such current information, we cannot analyze readmission trends over time, such as in response to the Affordable Care Act of 2010. Finally, although NSQIP surgical clinical reviewers strive to identify readmissions to other hospitals during their reviews of outpatient medical records, proportions of these readmissions are possibly missed. Therefore, our 30-day readmission rate may slightly underestimate the true rate.
Despite these limitations, the NSQIP database offers a unique opportunity to examine risk factors and reasons for readmission following TSA. The prior literature on readmission following TSA stemmed either from limited samples or administrative data, which feature known limitations.32 By utilizing a large, prospective, non-administrative, nationwide sample, our findings are probably both more reliable and generalizable to the country as a whole.
CONCLUSION
Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at a higher risk for readmission and should be counseled and monitored accordingly.
This paper will be judged for the Resident Writer’s Award.
- Adams JE, Sperling JW, Hoskin TL, Melton LJ, Cofield RH. Shoulder arthroplasty in Olmsted County, Minnesota, 1976-2000: a population-based study. J Shoulder Elbow Surg.2006;15(1):50-55. doi:10.1016/j.jse.2005.04.009.
- Jain NB, Higgins LD, Guller U, Pietrobon R, Katz JN. Trends in the epidemiology of total shoulder arthroplasty in the United States from 1990-2000. Arthritis Rheum.2006;55(4):591-597. doi:10.1002/art.22102.
- Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994. doi:10.2106/JBJS.J.01994.
- Mather RC, Watters TS, Orlando LA, Bolognesi MP, Moorman CT. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg.2010;19(3):325-334. doi:10.1016/j.jse.2009.11.057.
- Carter MJ, Mikuls TR, Nayak S, Fehringer EV, Michaud K. Impact of total shoulder arthroplasty on generic and shoulder-specific health-related quality-of-life measures: a systematic literature review and meta-analysis. J Bone Joint Surg Am. 2012;94(17):e127. doi:10.2106/JBJS.K.00204.
- Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479. doi:10.1016/j.jse.2005.02.009.
- Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635. doi:10.1016/j.jse.2012.07.005.
- Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. J Bone Joint Surg Am.2014;96(3):198-205. doi:10.2106/JBJS.M.00079.
- Bohsali KI, Wirth MA, Rockwood CA. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292. doi:10.2106/JBJS.F.00125.
- Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860. doi:10.1016/j.arth.2013.07.002.
- Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
- Papadonikolakis A, Neradilek MB, Matsen FA. Failure of the glenoid component in anatomic total shoulder arthroplasty: a systematic review of the English-language literature between 2006 and 2012. J Bone Joint Surg Am. 2013;95(24):2205-2212. doi:10.2106/JBJS.L.00552.
- Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg.2014;23(11):1647-1654. doi:10.1016/j.jse.2014.04.015.
- Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453. doi:10.1016/j.jse.2014.01.052.
- Sperling JW, Hawkins RJ, Walch G, Mahoney AP, Zuckerman JD. Complications in total shoulder arthroplasty. Instr Course Lect. 2013;62:135-141.
- Shields E, Thirukumaran C, Thorsness R, Noyes K, Voloshin I. An analysis of adult patient risk factors and complications within 30 days after arthroscopic shoulder surgery. Arthroscopy. 2015;31(5):807-815. doi:10.1016/j.arthro.2014.12.011.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563.
- Centers for Medicare & Medicaid Services. Readmissions reduction program (HRRP). . Updated April 27, 2018. Accessed June 29, 2018.
- Lyman S, Jones EC, Bach PB, Peterson MG, Marx RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;432:132-137. doi:10.1097/01.blo.0000150571.51381.9a.
- Mahoney A, Bosco JA, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381. doi:10.1016/j.jse.2013.08.007.
- Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355. doi:10.1016/j.jse.2013.12.004.
- Streubel PN, Simone JP, Sperling JW, Cofield R. Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(3):e17. doi:10.2106/JBJS.M.00127.
- Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop Relat Res. 2014;472(8):2317-2324. doi:10.1007/s11999-014-3613-y.
- American College of Surgeons. ACS National Surgical Quality Improvement Program. http://www.acsnsqip.org. Accessed July 15, 2015.
- Basques BA, Gardner EC, Varthi AG, et al. Risk factors for short-term adverse events and readmission after arthroscopic meniscectomy: does age matter? Am J Sports Med.2015;43(1):169-175. doi:10.1177/0363546514551923.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Does resident involvement impact post-operative complications following primary total knee arthroplasty? An analysis of 24,529 cases. J Arthroplasty. 2014;29(7):1468-1472.e2. doi:10.1016/j.arth.2014.02.036.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Resident involvement does not influence complication after total hip arthroplasty: an analysis of 13,109 cases. J Arthroplasty. 2014;29(10):1919-1924. doi:10.1016/j.arth.2014.06.003.
- Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.e1. doi:10.1016/j.jse.2013.06.022.
- Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the national surgical quality improvement program database. J Bone Joint Surg Am. 2013;95(14):e98 1-10. doi:10.2106/JBJS.L.01440.
- Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30. doi:10.1016/j.jse.2014.05.016.
- Basques BA, Gardner EC, Toy JO, Golinvaux NS, Bohl DD, Grauer JN. Length of stay and readmission after total shoulder arthroplasty: an analysis of 1505 cases. Am J Orthop.2015;44(8):E268-E271.
- Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/JBJS.M.01490.
ABSTRACT
An increasing interest focuses on the rates and risk factors for hospital readmission. However, little is known regarding the readmission following total shoulder arthroplasty (TSA). This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA. Patients undergoing TSA (anatomic or reverse) as part of the American College of Surgeons National Surgical Quality Improvement Program in 2011 to 2013 were identified. The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. Using multivariate regression, demographic and comorbidity factors were tested for independent association with readmission. Finally, the reasons for readmission were characterized. A total of 3627 patients were identified. Among the admitted patients, 93 (2.56%) were readmitted within 30 days of surgery. The independent risk factors for readmission included old age (for age 60-69 years, relative risk [RR] = 1.6; for age 70-79 years, RR = 2.3; for age ≥80 years, RR = 23.1; P = .042), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission comprised pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%). Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at higher risk for readmission and should be counseled and monitored accordingly.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) is performed with increasing frequency in the United States and is considered to be cost-effective.1-4 Following the procedure, patients generally achieve shoulder function and pain relief.5-8 Despite the success of the procedure, the growing literature on TSA has also reported rates of complications between 3.6% and 25% of the treated patients.9-16
In recent years, an increasing interest has focused on the rates and risk factors for unplanned hospital readmissions; these variables may not only reflect the quality of patient care but also result in considerable costs to the healthcare system. For instance, among Medicare patients, readmissions within 30 days of discharge occur in almost 20% of cases, costing $17.4 billion per year.17 Readmission rates increasingly factor into hospital performance metrics and reimbursement, including the Hospital Readmissions Reduction Program of the Patient Protection and Affordable Care Act that reduces Centers for Medicare and Medicaid Services payments to hospitals with high 30-day readmission rates.18
To date, only a few studies have evaluated readmission following TSA, with 30- to 90-day readmission rates ranging from 4.5% to 7.3%.19-23 These studies comprised single institution series20,22 and analyses of administrative databases.19,21,23 Most studies have shown that readmission occurs more often for medical than surgical reasons, with surgical reasons most commonly including infection and dislocation.19-23 However, only limited analyses have been conducted regarding risk factors for readmission.21,23 To date and to our knowledge, no study has investigated reasons for readmission following TSA using nationwide data.
This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA in the United States using the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database.
METHODS
DATA SOURCE
The NSQIP database was utilized to address the study purpose. NSQIP is a nationwide prospective surgical registry established by the American College of Surgeons and reports data from academic and community hospitals across the United States.24 Patients undertaking surgery at these centers are followed by the surgical clinical reviewers at the participating NSQIP sites prospectively for 30 days following the procedure to record complications including readmission. Preoperative and surgical data, such as demographics, medical comorbid diseases, and operative time, are also included. Previous studies have analyzed the complications of various orthopedic surgeries using the NSQIP data.14,16,25-30
DATA COLLECTION
We retrospectively identified from NSQIP the patients who underwent primary TSA (anatomic or reverse) in 2013 to 2014. The timeframe 2013 to 2014 was used because NSQIP only began recording reasons for readmission in 2013. The inclusion criteria were as follows: Current Procedural Terminology (CPT) code for TSA (23472); preoperative diagnosis according to the International Classification of Diseases, Ninth Revision (ICD-9) codes 714.0, 715.11, 715.31, 715.91, 715.21, 715.89, 716.xx 718.xx, 719.xx, 726.x, 727.xx, and 733.41 (where x is a wild card digit); and no missing demographic, comorbidity, or outcome data. Anatomic and reverse TSA were analyzed together because they share the same CPT code, and the NSQIP database prevents searching by the ICD-9 procedure code.
The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. The reasons for readmission in this 30-day period were only available in 2013 and were determined using the ICD-9 diagnosis codes. Patient demographics were recorded for use in identifying potential risk factors for readmission; the demographic data included sex, age, smoking status, body mass index (BMI), and comorbidities, including end-stage renal disease, dyspnea on exertion, congestive heart failure, diabetes mellitus, hypertension, and chronic obstructive pulmonary disease (COPD).
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were performed using Stata version 13.1 (StataCorp). First, using bivariate and multivariate regression, demographic and comorbidity factors were tested for independent association with readmission to the hospital within 30 days of surgery. Second, among the readmitted patients, the reasons for readmission were tabulated. Of note, the reasons for readmission were only documented for the procedures performed in 2013. All tests were 2-tailed and conducted at an α level of 0.05.
RESTULTS
A total of 3627 TSA patients were identified. The mean age (± standard deviation) was 69.4 ± 9.5 years, 55.8% of patients were female, and mean BMI was 30.1 ± 7.0 years. Table 1 provides the additional demographic data. Of the 3627 included patients, 93 (2.56%) were readmitted within 30 days of surgery. The 95% confidence interval for the estimated rate of readmission reached 2.05% to 3.08%.
Table 1. Patient Population
| Number | Percent |
Total | 3627 | 100.0% |
Age |
|
|
18-59 | 539 | 14.9% |
60-69 | 1235 | 34.1% |
70-79 | 1317 | 36.3% |
≥80 | 536 | 14.8% |
Sex |
|
|
Male | 1603 | 44.2% |
Female | 2024 | 55.8% |
Body mass index |
|
|
Normal (<25 kg/m2) | 650 | 17.9% |
Overweight (25-30 kg/m2) | 1147 | 31.6% |
Obese (≥30 kg/m2) | 1830 | 50.5% |
Functional status |
|
|
Independent | 3544 | 97.7% |
Dependent | 83 | 2.3% |
Diabetes mellitus |
|
|
No | 3022 | 83.3% |
Yes | 605 | 16.7% |
Dyspnea on exertion |
|
|
No | 3393 | 93.6% |
Yes | 234 | 6.5% |
Hypertension |
|
|
No | 1192 | 32.9% |
Yes | 2435 | 67.1% |
COPD |
|
|
No | 3384 | 93.3% |
Yes | 243 | 6.7% |
Current smoker |
|
|
No | 3249 | 89.6% |
Yes | 378 | 10.4% |
Anemia |
|
|
No | 3051 | 84.1% |
Yes | 576 | 15.9% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
In the bivariate analyses (Table 2), the following factors were positively associated readmission: older age (60-69 years, relative risk [RR] = 1.6; 70-79 years, RR = 2.2; ≥80 years, RR = 3.3; P = .011), dependent functional status (RR = 2.9, P = .008), and anemia (RR = 2.2, P < .001).
Table 2. Bivariate Analysis of Risk Factors for Readmission
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.011 |
18-59 | 1.30% | Ref. | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.2 | 1.0-4.9 |
|
≥80 | 4.29% | 3.3 | 1.4-7.6 |
|
Sex |
|
|
| 0.099 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.4 | 0.9-2.1 |
|
Body mass index |
|
|
| 0.764 |
Normal (<25 kg/m2) | 2.92% | Ref. | - |
|
Overweight (25-30 kg/m2) | 2.35% | 0.8 | 0.5-1.4 |
|
Obese (≥30 kg/m2) | 2.57% | 0.9 | 0.5-1.5 |
|
Functional status |
|
|
| 0.008 |
Independent | 2.45% | Ref. | - |
|
Dependent | 7.23% | 2.9 | 1.3-6.5 |
|
Diabetes mellitus |
|
|
| 0.483 |
No | 2.48% | Ref. | - |
|
Yes | 2.98% | 1.2 | 0.7-2.0 |
|
Dyspnea on exertion |
|
|
| 0.393 |
No | 2.51% | Ref. | - |
|
Yes | 3.42% | 1.4 | 0.7-2.8 |
|
Hypertension |
|
|
| 0.145 |
No | 2.01% | Ref. | - |
|
Yes | 2.83% | 1.4 | 0.9-2.2 |
|
COPD |
|
|
| 0.457 |
No | 2.51% | Ref. | - |
|
Yes | 3.29% | 1.3 | 0.6-2.7 |
|
Current smoker |
|
|
| 0.116 |
No | 2.71% | Ref. | - |
|
Yes | 1.32% | 0.5 | 0.2-1.2 |
|
Anemia |
|
|
| <0.001 |
No | 2.16% | Ref. | - |
|
Yes | 4.69% | 2.2 | 1.4-3.4 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
In the multivariate analyses (Table 3), the following factors were independent risk factors for readmission: older age (60-69 years, RR = 1.6; 70-79 years, RR = 2.3; ≥80 years, RR = 3.1; P =.027), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). Interestingly, readmission showed no independent association with diabetes, dyspnea on exertion, BMI, COPD, hypertension, or current smoking status (P > .05 for each).
Table 3. Independent Risk Factors for Readmission on Multivariate Analysis
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.027 |
18-59 | 1.30% | Ref | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.3 | 1.0-5.1 |
|
≥80 | 4.29% | 3.1 | 1.3-7.4 |
|
Sex |
|
|
| 0.025 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.6 | 1.1-2.4 |
|
Anemia |
|
|
| 0.005 |
No | 2.16% | Ref | - |
|
Yes | 4.69% | 1.9 | 1.2-3.0 |
|
Functional status |
|
|
| 0.012 |
Independent | 2.45% | Ref | - |
|
Dependent | 7.23% | 2.8 | 1.3-6.2 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
Continue to: Table 4...
The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission included pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%) (Table 4).
Table 4. Reasons for Readmission
| Number | Percent |
Pneumonia | 14 | 16.7% |
Dislocation | 7 | 8.3% |
Pulmonary embolism | 7 | 8.3% |
Surgical site infection | 6 | 7.1% |
Atrial fibrillation | 4 | 4.8% |
Hematoma | 4 | 4.8% |
Altered mental status | 3 | 3.6% |
Chest pain | 3 | 3.6% |
Renal insufficiency/kidney failure | 3 | 3.6% |
Urinary tract infection | 3 | 3.6% |
Acute gastric or duodenal ulcer | 2 | 2.4% |
Dermatitis/other allergic reaction | 2 | 2.4% |
Orthostatic hypotension/syncope | 2 | 2.4% |
Pain | 2 | 2.4% |
Respiratory distress | 2 | 2.4% |
Sepsis | 2 | 2.4% |
Urinary retention | 2 | 2.4% |
Acute cholecystitis | 1 | 1.2% |
Cerebrovascular accident | 1 | 1.2% |
Constipation | 1 | 1.2% |
Contusion of shoulder | 1 | 1.2% |
Deep venous thrombosis requiring therapy | 1 | 1.2% |
Gastrointestinal hemorrhage | 1 | 1.2% |
Gout | 1 | 1.2% |
Hepatic encephalopathy | 1 | 1.2% |
Intestinal infection | 1 | 1.2% |
Narcotic overdose | 1 | 1.2% |
Nausea/vomiting | 1 | 1.2% |
Proximal humerus fracture | 1 | 1.2% |
Rotator cuff tear | 1 | 1.2% |
Seroma | 1 | 1.2% |
Unspecified disease of pericardium | 1 | 1.2% |
Weakness | 1 | 1.2% |
DISCUSSION
Our analysis of 3042 TSAs from the NSQIP database suggests that unplanned readmission to the hospital occurs following about 1 in 40 cases of TSA. The study also suggests that the most common reasons for readmission encompass pneumonia, dislocation, pulmonary embolism, and surgical site infection. Old age, male sex, anemia, and dependent functional status serve as risk factors for readmission, and patients with such factors should be counseled and monitored accordingly.
In recent years, an increasing emphasis has centered on reducing rates of hospital readmission, with programs such as the Hospital Readmissions Reduction Program of the Affordable Care Act cutting reimbursements for hospitals with high 30-day readmission rates.17,18 To date, only a few studies have evaluated the reasons for readmission and readmission rates for TSA.19-23 Initial reports consisted of single-institution TSA registry reviews. For example, Mahoney and colleagues20 retrospectively evaluated shoulder arthroplasty procedures at their institution to document the readmission rates, finding a 5.9% readmission rate at 30 days. Readmission occurred more frequently in the first 30 days following discharge than in the 30- to 90-day period, with the most common reasons for readmission including medical complications, infection, and dislocation. Streubel and colleagues22 evaluated reoperation rates from their institution’s TSA registry, finding a 0.6% reoperation rate for primary TSA at 30 days and 1.5% for revision TSA. Instability and infection were the most common indications for reoperation. Our findings confirm these single-institution results and demonstrate their application to a nationwide sample of TSA, not just to high-volume academic centers. We similarly observed that dislocation, surgical site infection, and medical complications (mostly pneumonia and pulmonary embolism) were common causes of readmission, and that the 30-day readmission rate was about 1 in 40.
Several authors have since used statewide databases to analyze and determine risk factors for readmission following TSA. Lyman and colleagues19 used the New York State Database to show that higher hospital TSA surgical volume was associated with a lower rate of readmission when age and comorbidities were controlled for in a multivariate model. Old age was also associated with an increased readmission rate in their multivariate analysis, but comorbidities (as measured by the Charlson comorbidity index) presented a nonsignificant associative trend. These authors opted not to determine specific causes of readmission. Schairer and colleagues21 used State Inpatient Databases from 7 states, finding a 90-day readmission rate of 7.3%, 82% of which were due to medical complications and 18% of which were due to surgical complications (mostly infection and dislocation). Their multivariate regression revealed that male sex, reverse TSA, Medicaid insurance, patients discharged to inpatient rehabilitation or nursing facilities, medical comorbidities, and low-volume TSA hospitals were associated with readmission. Zhang and colleagues23 used the same source to show that the 90-day readmission rate reached 14% for surgically treated proximal humerus fractures and higher for patients who underwent open reduction internal fixation, were female, were African American, were discharged to a nursing facility, possessed Medicaid insurance, or experienced medical comorbidities. Most recently, Basques and colleagues31 analyzed 1505 TSA cases from 2011 and 2012 in the NSQIP database, finding a 3.3% rate of readmission, with heart disease and hypertension as risk factors for readmission. Although the limitations of the NSQIP database prevented us from analyzing surgeon and hospital TSA volume or reverse vs anatomic TSA, our results confirm that the findings from statewide database studies apply to the United States nationwide NSQIP database. Old patient age, male sex, and medical comorbidities (anemia and dependent functional status) are independent risk factors for TSA readmission. We identified pneumonia, dislocation, pulmonary embolism, and surgical site infection as the most common reasons for readmission.
This study features several limitations that should be considered when interpreting the results. Anatomic and reverse TSA share a CPT code and were not separated using NSQIP data. A number of studies have reported that reverse TSA may place patients at higher risk for readmission;20,21 however, confounding by other patient factors could play a role in this finding. The 30-day timeframe for readmission is another potential limitation; however, this timeframe is frequently used in other studies and is the relevant timeframe for the reduced reimbursement penalties from the Hospital Readmissions Reduction Program of the Affordable Care Act.18 Furthermore, the NSQIP database contains no information on surgeon or hospital TSA volume, which is a result of safeguards for patient and provider privacy. Additionally, readmission data were only available for 2011 to 2013, with causes of readmission only present in 2013. Although provided with such current information, we cannot analyze readmission trends over time, such as in response to the Affordable Care Act of 2010. Finally, although NSQIP surgical clinical reviewers strive to identify readmissions to other hospitals during their reviews of outpatient medical records, proportions of these readmissions are possibly missed. Therefore, our 30-day readmission rate may slightly underestimate the true rate.
Despite these limitations, the NSQIP database offers a unique opportunity to examine risk factors and reasons for readmission following TSA. The prior literature on readmission following TSA stemmed either from limited samples or administrative data, which feature known limitations.32 By utilizing a large, prospective, non-administrative, nationwide sample, our findings are probably both more reliable and generalizable to the country as a whole.
CONCLUSION
Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at a higher risk for readmission and should be counseled and monitored accordingly.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
An increasing interest focuses on the rates and risk factors for hospital readmission. However, little is known regarding the readmission following total shoulder arthroplasty (TSA). This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA. Patients undergoing TSA (anatomic or reverse) as part of the American College of Surgeons National Surgical Quality Improvement Program in 2011 to 2013 were identified. The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. Using multivariate regression, demographic and comorbidity factors were tested for independent association with readmission. Finally, the reasons for readmission were characterized. A total of 3627 patients were identified. Among the admitted patients, 93 (2.56%) were readmitted within 30 days of surgery. The independent risk factors for readmission included old age (for age 60-69 years, relative risk [RR] = 1.6; for age 70-79 years, RR = 2.3; for age ≥80 years, RR = 23.1; P = .042), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission comprised pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%). Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at higher risk for readmission and should be counseled and monitored accordingly.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) is performed with increasing frequency in the United States and is considered to be cost-effective.1-4 Following the procedure, patients generally achieve shoulder function and pain relief.5-8 Despite the success of the procedure, the growing literature on TSA has also reported rates of complications between 3.6% and 25% of the treated patients.9-16
In recent years, an increasing interest has focused on the rates and risk factors for unplanned hospital readmissions; these variables may not only reflect the quality of patient care but also result in considerable costs to the healthcare system. For instance, among Medicare patients, readmissions within 30 days of discharge occur in almost 20% of cases, costing $17.4 billion per year.17 Readmission rates increasingly factor into hospital performance metrics and reimbursement, including the Hospital Readmissions Reduction Program of the Patient Protection and Affordable Care Act that reduces Centers for Medicare and Medicaid Services payments to hospitals with high 30-day readmission rates.18
To date, only a few studies have evaluated readmission following TSA, with 30- to 90-day readmission rates ranging from 4.5% to 7.3%.19-23 These studies comprised single institution series20,22 and analyses of administrative databases.19,21,23 Most studies have shown that readmission occurs more often for medical than surgical reasons, with surgical reasons most commonly including infection and dislocation.19-23 However, only limited analyses have been conducted regarding risk factors for readmission.21,23 To date and to our knowledge, no study has investigated reasons for readmission following TSA using nationwide data.
This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA in the United States using the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database.
METHODS
DATA SOURCE
The NSQIP database was utilized to address the study purpose. NSQIP is a nationwide prospective surgical registry established by the American College of Surgeons and reports data from academic and community hospitals across the United States.24 Patients undertaking surgery at these centers are followed by the surgical clinical reviewers at the participating NSQIP sites prospectively for 30 days following the procedure to record complications including readmission. Preoperative and surgical data, such as demographics, medical comorbid diseases, and operative time, are also included. Previous studies have analyzed the complications of various orthopedic surgeries using the NSQIP data.14,16,25-30
DATA COLLECTION
We retrospectively identified from NSQIP the patients who underwent primary TSA (anatomic or reverse) in 2013 to 2014. The timeframe 2013 to 2014 was used because NSQIP only began recording reasons for readmission in 2013. The inclusion criteria were as follows: Current Procedural Terminology (CPT) code for TSA (23472); preoperative diagnosis according to the International Classification of Diseases, Ninth Revision (ICD-9) codes 714.0, 715.11, 715.31, 715.91, 715.21, 715.89, 716.xx 718.xx, 719.xx, 726.x, 727.xx, and 733.41 (where x is a wild card digit); and no missing demographic, comorbidity, or outcome data. Anatomic and reverse TSA were analyzed together because they share the same CPT code, and the NSQIP database prevents searching by the ICD-9 procedure code.
The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. The reasons for readmission in this 30-day period were only available in 2013 and were determined using the ICD-9 diagnosis codes. Patient demographics were recorded for use in identifying potential risk factors for readmission; the demographic data included sex, age, smoking status, body mass index (BMI), and comorbidities, including end-stage renal disease, dyspnea on exertion, congestive heart failure, diabetes mellitus, hypertension, and chronic obstructive pulmonary disease (COPD).
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were performed using Stata version 13.1 (StataCorp). First, using bivariate and multivariate regression, demographic and comorbidity factors were tested for independent association with readmission to the hospital within 30 days of surgery. Second, among the readmitted patients, the reasons for readmission were tabulated. Of note, the reasons for readmission were only documented for the procedures performed in 2013. All tests were 2-tailed and conducted at an α level of 0.05.
RESTULTS
A total of 3627 TSA patients were identified. The mean age (± standard deviation) was 69.4 ± 9.5 years, 55.8% of patients were female, and mean BMI was 30.1 ± 7.0 years. Table 1 provides the additional demographic data. Of the 3627 included patients, 93 (2.56%) were readmitted within 30 days of surgery. The 95% confidence interval for the estimated rate of readmission reached 2.05% to 3.08%.
Table 1. Patient Population
| Number | Percent |
Total | 3627 | 100.0% |
Age |
|
|
18-59 | 539 | 14.9% |
60-69 | 1235 | 34.1% |
70-79 | 1317 | 36.3% |
≥80 | 536 | 14.8% |
Sex |
|
|
Male | 1603 | 44.2% |
Female | 2024 | 55.8% |
Body mass index |
|
|
Normal (<25 kg/m2) | 650 | 17.9% |
Overweight (25-30 kg/m2) | 1147 | 31.6% |
Obese (≥30 kg/m2) | 1830 | 50.5% |
Functional status |
|
|
Independent | 3544 | 97.7% |
Dependent | 83 | 2.3% |
Diabetes mellitus |
|
|
No | 3022 | 83.3% |
Yes | 605 | 16.7% |
Dyspnea on exertion |
|
|
No | 3393 | 93.6% |
Yes | 234 | 6.5% |
Hypertension |
|
|
No | 1192 | 32.9% |
Yes | 2435 | 67.1% |
COPD |
|
|
No | 3384 | 93.3% |
Yes | 243 | 6.7% |
Current smoker |
|
|
No | 3249 | 89.6% |
Yes | 378 | 10.4% |
Anemia |
|
|
No | 3051 | 84.1% |
Yes | 576 | 15.9% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
In the bivariate analyses (Table 2), the following factors were positively associated readmission: older age (60-69 years, relative risk [RR] = 1.6; 70-79 years, RR = 2.2; ≥80 years, RR = 3.3; P = .011), dependent functional status (RR = 2.9, P = .008), and anemia (RR = 2.2, P < .001).
Table 2. Bivariate Analysis of Risk Factors for Readmission
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.011 |
18-59 | 1.30% | Ref. | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.2 | 1.0-4.9 |
|
≥80 | 4.29% | 3.3 | 1.4-7.6 |
|
Sex |
|
|
| 0.099 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.4 | 0.9-2.1 |
|
Body mass index |
|
|
| 0.764 |
Normal (<25 kg/m2) | 2.92% | Ref. | - |
|
Overweight (25-30 kg/m2) | 2.35% | 0.8 | 0.5-1.4 |
|
Obese (≥30 kg/m2) | 2.57% | 0.9 | 0.5-1.5 |
|
Functional status |
|
|
| 0.008 |
Independent | 2.45% | Ref. | - |
|
Dependent | 7.23% | 2.9 | 1.3-6.5 |
|
Diabetes mellitus |
|
|
| 0.483 |
No | 2.48% | Ref. | - |
|
Yes | 2.98% | 1.2 | 0.7-2.0 |
|
Dyspnea on exertion |
|
|
| 0.393 |
No | 2.51% | Ref. | - |
|
Yes | 3.42% | 1.4 | 0.7-2.8 |
|
Hypertension |
|
|
| 0.145 |
No | 2.01% | Ref. | - |
|
Yes | 2.83% | 1.4 | 0.9-2.2 |
|
COPD |
|
|
| 0.457 |
No | 2.51% | Ref. | - |
|
Yes | 3.29% | 1.3 | 0.6-2.7 |
|
Current smoker |
|
|
| 0.116 |
No | 2.71% | Ref. | - |
|
Yes | 1.32% | 0.5 | 0.2-1.2 |
|
Anemia |
|
|
| <0.001 |
No | 2.16% | Ref. | - |
|
Yes | 4.69% | 2.2 | 1.4-3.4 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
In the multivariate analyses (Table 3), the following factors were independent risk factors for readmission: older age (60-69 years, RR = 1.6; 70-79 years, RR = 2.3; ≥80 years, RR = 3.1; P =.027), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). Interestingly, readmission showed no independent association with diabetes, dyspnea on exertion, BMI, COPD, hypertension, or current smoking status (P > .05 for each).
Table 3. Independent Risk Factors for Readmission on Multivariate Analysis
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.027 |
18-59 | 1.30% | Ref | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.3 | 1.0-5.1 |
|
≥80 | 4.29% | 3.1 | 1.3-7.4 |
|
Sex |
|
|
| 0.025 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.6 | 1.1-2.4 |
|
Anemia |
|
|
| 0.005 |
No | 2.16% | Ref | - |
|
Yes | 4.69% | 1.9 | 1.2-3.0 |
|
Functional status |
|
|
| 0.012 |
Independent | 2.45% | Ref | - |
|
Dependent | 7.23% | 2.8 | 1.3-6.2 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
Continue to: Table 4...
The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission included pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%) (Table 4).
Table 4. Reasons for Readmission
| Number | Percent |
Pneumonia | 14 | 16.7% |
Dislocation | 7 | 8.3% |
Pulmonary embolism | 7 | 8.3% |
Surgical site infection | 6 | 7.1% |
Atrial fibrillation | 4 | 4.8% |
Hematoma | 4 | 4.8% |
Altered mental status | 3 | 3.6% |
Chest pain | 3 | 3.6% |
Renal insufficiency/kidney failure | 3 | 3.6% |
Urinary tract infection | 3 | 3.6% |
Acute gastric or duodenal ulcer | 2 | 2.4% |
Dermatitis/other allergic reaction | 2 | 2.4% |
Orthostatic hypotension/syncope | 2 | 2.4% |
Pain | 2 | 2.4% |
Respiratory distress | 2 | 2.4% |
Sepsis | 2 | 2.4% |
Urinary retention | 2 | 2.4% |
Acute cholecystitis | 1 | 1.2% |
Cerebrovascular accident | 1 | 1.2% |
Constipation | 1 | 1.2% |
Contusion of shoulder | 1 | 1.2% |
Deep venous thrombosis requiring therapy | 1 | 1.2% |
Gastrointestinal hemorrhage | 1 | 1.2% |
Gout | 1 | 1.2% |
Hepatic encephalopathy | 1 | 1.2% |
Intestinal infection | 1 | 1.2% |
Narcotic overdose | 1 | 1.2% |
Nausea/vomiting | 1 | 1.2% |
Proximal humerus fracture | 1 | 1.2% |
Rotator cuff tear | 1 | 1.2% |
Seroma | 1 | 1.2% |
Unspecified disease of pericardium | 1 | 1.2% |
Weakness | 1 | 1.2% |
DISCUSSION
Our analysis of 3042 TSAs from the NSQIP database suggests that unplanned readmission to the hospital occurs following about 1 in 40 cases of TSA. The study also suggests that the most common reasons for readmission encompass pneumonia, dislocation, pulmonary embolism, and surgical site infection. Old age, male sex, anemia, and dependent functional status serve as risk factors for readmission, and patients with such factors should be counseled and monitored accordingly.
In recent years, an increasing emphasis has centered on reducing rates of hospital readmission, with programs such as the Hospital Readmissions Reduction Program of the Affordable Care Act cutting reimbursements for hospitals with high 30-day readmission rates.17,18 To date, only a few studies have evaluated the reasons for readmission and readmission rates for TSA.19-23 Initial reports consisted of single-institution TSA registry reviews. For example, Mahoney and colleagues20 retrospectively evaluated shoulder arthroplasty procedures at their institution to document the readmission rates, finding a 5.9% readmission rate at 30 days. Readmission occurred more frequently in the first 30 days following discharge than in the 30- to 90-day period, with the most common reasons for readmission including medical complications, infection, and dislocation. Streubel and colleagues22 evaluated reoperation rates from their institution’s TSA registry, finding a 0.6% reoperation rate for primary TSA at 30 days and 1.5% for revision TSA. Instability and infection were the most common indications for reoperation. Our findings confirm these single-institution results and demonstrate their application to a nationwide sample of TSA, not just to high-volume academic centers. We similarly observed that dislocation, surgical site infection, and medical complications (mostly pneumonia and pulmonary embolism) were common causes of readmission, and that the 30-day readmission rate was about 1 in 40.
Several authors have since used statewide databases to analyze and determine risk factors for readmission following TSA. Lyman and colleagues19 used the New York State Database to show that higher hospital TSA surgical volume was associated with a lower rate of readmission when age and comorbidities were controlled for in a multivariate model. Old age was also associated with an increased readmission rate in their multivariate analysis, but comorbidities (as measured by the Charlson comorbidity index) presented a nonsignificant associative trend. These authors opted not to determine specific causes of readmission. Schairer and colleagues21 used State Inpatient Databases from 7 states, finding a 90-day readmission rate of 7.3%, 82% of which were due to medical complications and 18% of which were due to surgical complications (mostly infection and dislocation). Their multivariate regression revealed that male sex, reverse TSA, Medicaid insurance, patients discharged to inpatient rehabilitation or nursing facilities, medical comorbidities, and low-volume TSA hospitals were associated with readmission. Zhang and colleagues23 used the same source to show that the 90-day readmission rate reached 14% for surgically treated proximal humerus fractures and higher for patients who underwent open reduction internal fixation, were female, were African American, were discharged to a nursing facility, possessed Medicaid insurance, or experienced medical comorbidities. Most recently, Basques and colleagues31 analyzed 1505 TSA cases from 2011 and 2012 in the NSQIP database, finding a 3.3% rate of readmission, with heart disease and hypertension as risk factors for readmission. Although the limitations of the NSQIP database prevented us from analyzing surgeon and hospital TSA volume or reverse vs anatomic TSA, our results confirm that the findings from statewide database studies apply to the United States nationwide NSQIP database. Old patient age, male sex, and medical comorbidities (anemia and dependent functional status) are independent risk factors for TSA readmission. We identified pneumonia, dislocation, pulmonary embolism, and surgical site infection as the most common reasons for readmission.
This study features several limitations that should be considered when interpreting the results. Anatomic and reverse TSA share a CPT code and were not separated using NSQIP data. A number of studies have reported that reverse TSA may place patients at higher risk for readmission;20,21 however, confounding by other patient factors could play a role in this finding. The 30-day timeframe for readmission is another potential limitation; however, this timeframe is frequently used in other studies and is the relevant timeframe for the reduced reimbursement penalties from the Hospital Readmissions Reduction Program of the Affordable Care Act.18 Furthermore, the NSQIP database contains no information on surgeon or hospital TSA volume, which is a result of safeguards for patient and provider privacy. Additionally, readmission data were only available for 2011 to 2013, with causes of readmission only present in 2013. Although provided with such current information, we cannot analyze readmission trends over time, such as in response to the Affordable Care Act of 2010. Finally, although NSQIP surgical clinical reviewers strive to identify readmissions to other hospitals during their reviews of outpatient medical records, proportions of these readmissions are possibly missed. Therefore, our 30-day readmission rate may slightly underestimate the true rate.
Despite these limitations, the NSQIP database offers a unique opportunity to examine risk factors and reasons for readmission following TSA. The prior literature on readmission following TSA stemmed either from limited samples or administrative data, which feature known limitations.32 By utilizing a large, prospective, non-administrative, nationwide sample, our findings are probably both more reliable and generalizable to the country as a whole.
CONCLUSION
Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at a higher risk for readmission and should be counseled and monitored accordingly.
This paper will be judged for the Resident Writer’s Award.
- Adams JE, Sperling JW, Hoskin TL, Melton LJ, Cofield RH. Shoulder arthroplasty in Olmsted County, Minnesota, 1976-2000: a population-based study. J Shoulder Elbow Surg.2006;15(1):50-55. doi:10.1016/j.jse.2005.04.009.
- Jain NB, Higgins LD, Guller U, Pietrobon R, Katz JN. Trends in the epidemiology of total shoulder arthroplasty in the United States from 1990-2000. Arthritis Rheum.2006;55(4):591-597. doi:10.1002/art.22102.
- Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994. doi:10.2106/JBJS.J.01994.
- Mather RC, Watters TS, Orlando LA, Bolognesi MP, Moorman CT. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg.2010;19(3):325-334. doi:10.1016/j.jse.2009.11.057.
- Carter MJ, Mikuls TR, Nayak S, Fehringer EV, Michaud K. Impact of total shoulder arthroplasty on generic and shoulder-specific health-related quality-of-life measures: a systematic literature review and meta-analysis. J Bone Joint Surg Am. 2012;94(17):e127. doi:10.2106/JBJS.K.00204.
- Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479. doi:10.1016/j.jse.2005.02.009.
- Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635. doi:10.1016/j.jse.2012.07.005.
- Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. J Bone Joint Surg Am.2014;96(3):198-205. doi:10.2106/JBJS.M.00079.
- Bohsali KI, Wirth MA, Rockwood CA. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292. doi:10.2106/JBJS.F.00125.
- Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860. doi:10.1016/j.arth.2013.07.002.
- Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
- Papadonikolakis A, Neradilek MB, Matsen FA. Failure of the glenoid component in anatomic total shoulder arthroplasty: a systematic review of the English-language literature between 2006 and 2012. J Bone Joint Surg Am. 2013;95(24):2205-2212. doi:10.2106/JBJS.L.00552.
- Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg.2014;23(11):1647-1654. doi:10.1016/j.jse.2014.04.015.
- Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453. doi:10.1016/j.jse.2014.01.052.
- Sperling JW, Hawkins RJ, Walch G, Mahoney AP, Zuckerman JD. Complications in total shoulder arthroplasty. Instr Course Lect. 2013;62:135-141.
- Shields E, Thirukumaran C, Thorsness R, Noyes K, Voloshin I. An analysis of adult patient risk factors and complications within 30 days after arthroscopic shoulder surgery. Arthroscopy. 2015;31(5):807-815. doi:10.1016/j.arthro.2014.12.011.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563.
- Centers for Medicare & Medicaid Services. Readmissions reduction program (HRRP). . Updated April 27, 2018. Accessed June 29, 2018.
- Lyman S, Jones EC, Bach PB, Peterson MG, Marx RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;432:132-137. doi:10.1097/01.blo.0000150571.51381.9a.
- Mahoney A, Bosco JA, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381. doi:10.1016/j.jse.2013.08.007.
- Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355. doi:10.1016/j.jse.2013.12.004.
- Streubel PN, Simone JP, Sperling JW, Cofield R. Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(3):e17. doi:10.2106/JBJS.M.00127.
- Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop Relat Res. 2014;472(8):2317-2324. doi:10.1007/s11999-014-3613-y.
- American College of Surgeons. ACS National Surgical Quality Improvement Program. http://www.acsnsqip.org. Accessed July 15, 2015.
- Basques BA, Gardner EC, Varthi AG, et al. Risk factors for short-term adverse events and readmission after arthroscopic meniscectomy: does age matter? Am J Sports Med.2015;43(1):169-175. doi:10.1177/0363546514551923.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Does resident involvement impact post-operative complications following primary total knee arthroplasty? An analysis of 24,529 cases. J Arthroplasty. 2014;29(7):1468-1472.e2. doi:10.1016/j.arth.2014.02.036.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Resident involvement does not influence complication after total hip arthroplasty: an analysis of 13,109 cases. J Arthroplasty. 2014;29(10):1919-1924. doi:10.1016/j.arth.2014.06.003.
- Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.e1. doi:10.1016/j.jse.2013.06.022.
- Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the national surgical quality improvement program database. J Bone Joint Surg Am. 2013;95(14):e98 1-10. doi:10.2106/JBJS.L.01440.
- Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30. doi:10.1016/j.jse.2014.05.016.
- Basques BA, Gardner EC, Toy JO, Golinvaux NS, Bohl DD, Grauer JN. Length of stay and readmission after total shoulder arthroplasty: an analysis of 1505 cases. Am J Orthop.2015;44(8):E268-E271.
- Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/JBJS.M.01490.
- Adams JE, Sperling JW, Hoskin TL, Melton LJ, Cofield RH. Shoulder arthroplasty in Olmsted County, Minnesota, 1976-2000: a population-based study. J Shoulder Elbow Surg.2006;15(1):50-55. doi:10.1016/j.jse.2005.04.009.
- Jain NB, Higgins LD, Guller U, Pietrobon R, Katz JN. Trends in the epidemiology of total shoulder arthroplasty in the United States from 1990-2000. Arthritis Rheum.2006;55(4):591-597. doi:10.1002/art.22102.
- Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994. doi:10.2106/JBJS.J.01994.
- Mather RC, Watters TS, Orlando LA, Bolognesi MP, Moorman CT. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg.2010;19(3):325-334. doi:10.1016/j.jse.2009.11.057.
- Carter MJ, Mikuls TR, Nayak S, Fehringer EV, Michaud K. Impact of total shoulder arthroplasty on generic and shoulder-specific health-related quality-of-life measures: a systematic literature review and meta-analysis. J Bone Joint Surg Am. 2012;94(17):e127. doi:10.2106/JBJS.K.00204.
- Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479. doi:10.1016/j.jse.2005.02.009.
- Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635. doi:10.1016/j.jse.2012.07.005.
- Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. J Bone Joint Surg Am.2014;96(3):198-205. doi:10.2106/JBJS.M.00079.
- Bohsali KI, Wirth MA, Rockwood CA. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292. doi:10.2106/JBJS.F.00125.
- Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860. doi:10.1016/j.arth.2013.07.002.
- Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
- Papadonikolakis A, Neradilek MB, Matsen FA. Failure of the glenoid component in anatomic total shoulder arthroplasty: a systematic review of the English-language literature between 2006 and 2012. J Bone Joint Surg Am. 2013;95(24):2205-2212. doi:10.2106/JBJS.L.00552.
- Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg.2014;23(11):1647-1654. doi:10.1016/j.jse.2014.04.015.
- Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453. doi:10.1016/j.jse.2014.01.052.
- Sperling JW, Hawkins RJ, Walch G, Mahoney AP, Zuckerman JD. Complications in total shoulder arthroplasty. Instr Course Lect. 2013;62:135-141.
- Shields E, Thirukumaran C, Thorsness R, Noyes K, Voloshin I. An analysis of adult patient risk factors and complications within 30 days after arthroscopic shoulder surgery. Arthroscopy. 2015;31(5):807-815. doi:10.1016/j.arthro.2014.12.011.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563.
- Centers for Medicare & Medicaid Services. Readmissions reduction program (HRRP). . Updated April 27, 2018. Accessed June 29, 2018.
- Lyman S, Jones EC, Bach PB, Peterson MG, Marx RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;432:132-137. doi:10.1097/01.blo.0000150571.51381.9a.
- Mahoney A, Bosco JA, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381. doi:10.1016/j.jse.2013.08.007.
- Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355. doi:10.1016/j.jse.2013.12.004.
- Streubel PN, Simone JP, Sperling JW, Cofield R. Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(3):e17. doi:10.2106/JBJS.M.00127.
- Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop Relat Res. 2014;472(8):2317-2324. doi:10.1007/s11999-014-3613-y.
- American College of Surgeons. ACS National Surgical Quality Improvement Program. http://www.acsnsqip.org. Accessed July 15, 2015.
- Basques BA, Gardner EC, Varthi AG, et al. Risk factors for short-term adverse events and readmission after arthroscopic meniscectomy: does age matter? Am J Sports Med.2015;43(1):169-175. doi:10.1177/0363546514551923.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Does resident involvement impact post-operative complications following primary total knee arthroplasty? An analysis of 24,529 cases. J Arthroplasty. 2014;29(7):1468-1472.e2. doi:10.1016/j.arth.2014.02.036.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Resident involvement does not influence complication after total hip arthroplasty: an analysis of 13,109 cases. J Arthroplasty. 2014;29(10):1919-1924. doi:10.1016/j.arth.2014.06.003.
- Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.e1. doi:10.1016/j.jse.2013.06.022.
- Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the national surgical quality improvement program database. J Bone Joint Surg Am. 2013;95(14):e98 1-10. doi:10.2106/JBJS.L.01440.
- Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30. doi:10.1016/j.jse.2014.05.016.
- Basques BA, Gardner EC, Toy JO, Golinvaux NS, Bohl DD, Grauer JN. Length of stay and readmission after total shoulder arthroplasty: an analysis of 1505 cases. Am J Orthop.2015;44(8):E268-E271.
- Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/JBJS.M.01490.
TAKE-HOME POINTS
- Shoulder arthroplasty is an increasingly commonly performed procedure for shoulder arthritis and other conditions.
- Unplanned readmission in the 30 days after shoulder arthroplasty occurred in about 1 of 40 cases.
- Increasing age was associated with readmission, particularly age >80 years.
- Other risk factors for readmission were male sex, anemia, and dependent functional status.
- The most common reasons for readmission were pneumonia, dislocation, pulmonary embolism, and surgical site infection.
Shoulder Arthroplasty in Patients with Rheumatoid Arthritis: A Population-Based Study Examining Utilization, Adverse Events, Length of Stay, and Cost
ABSTRACT
It has been suggested that the utilization of joint arthroplasty in patients with rheumatoid arthritis (RA) is decreasing; however, this observation is largely based upon evidence pertaining to lower-extremity joint arthroplasty. It remains unknown if these observed trends also hold true for shoulder arthroplasty. The purpose of this study is to utilize a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. Secondarily, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and to compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. Using a large population database in the US, we determined the annual rates of shoulder arthroplasty (overall and individual) in RA patients between 2002 and 2011. Early adverse events, length of stay, and hospitalization costs were determined and compared with those of non-RA patients undergoing shoulder arthroplasty. Overall, we identified 332,593 patients who underwent shoulder arthroplasty between 2002 and 2011, of whom 17,883 patients (5.4%) had a diagnosis of RA. Over the study period, there was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly total shoulder arthroplasty. Over the same period, there was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease. There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients. Non-RA patients had a significantly shorter length of stay; however, the difference did not appear to be clinically significant. In conclusion, the utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Continue to: It has been suggested...
It has been suggested that the utilization of total joint arthroplasty (TJA) in patients with rheumatoid arthritis (RA) is decreasing over time;1 however, this observation is largely based upon evidence pertaining to lower extremity TJA.2 It remains unknown if these observed trends also hold true for shoulder arthroplasty, whereby the utilization of shoulder arthroplasty in RA patients is not limited to the management of end-stage inflammatory arthropathy. In this study, we used a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. As a secondary objective, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. We hypothesize that the utilization of shoulder arthroplasty in RA patients would be decreasing, but adverse events, length of stay, and hospitalization costs would not differ between patients with and without RA undergoing shoulder arthroplasty.
METHODS
We conducted a retrospective cohort study using the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) from 2002 to 2011.3 The NIS comprises a 20% stratified sample of all hospital discharges in the US. The NIS includes information about patient characteristics (age, sex, insurance status, and medical comorbidities) and hospitalization outcomes (adverse events, costs, and length of stay). The NIS allows identification of hospitalizations according to procedures and diagnoses using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Given the anonymity of this study, it was exempt from Institutional Review Board ethics approval.
Hospitalizations were selected for the study based on ICD-9-CM procedural codes for hemiarthroplasty (81.81), anatomic total shoulder arthroplasty (TSA) (81.80), and reverse TSA (81.88). These patients were then stratified by an ICD-9-CM diagnosis of RA (714.X). We also utilized ICD-9-CM diagnosis codes to determine the presence of rotator cuff pathology at the time of shoulder arthroplasty (726.13, 727.61, 840.4) and to exclude patients with a history of trauma (812.X, 716.11, 733.8X). In a separate analysis, all patients in the NIS database with an ICD-9-CM diagnosis of RA were identified for each calendar year of the study, and a national estimate of RA patients was generated annually to assess overall and individual utilization rates of shoulder arthroplasty in this population (the national estimate served as the denominator).
Preoperative patient data withdrawn from the NIS included age, sex, insurance status, and medical comorbidities. An Elixhauser Comorbidity Index (ECI) was generated for each patient based on the presence of 29 comorbid conditions. The ECI was chosen because of its capacity to accurately predict mortality and represent the patient burden of comorbidities in similar administrative database studies.4-6
Early adverse events were also chosen based on ICD-9-CM diagnosis codes (Appendix A), and included the following: death, acute kidney injury, cardiac arrest, thromboembolic event, myocardial infarction, peripheral nerve injury, pneumonia, sepsis, stroke, surgical site infection, urinary tract infection, and wound dehiscence. The overall adverse event rate was defined as the occurrence of ≥1 of the above adverse events in a patient.
Appendix A. ICD-9-CM Codes Corresponding to Postoperative Adverse Events
Event | ICD-9-CM |
Acute kidney injury | 584.5-584.9 |
Cardiac arrest | 427.41, 427.5 |
Thromboembolic event | 453.2-453.4, 453.82-453.86, 415.1 |
Myocardial Infarction | 410.00-410.92 |
Peripheral nerve injury | 953.0-953.9 954.0-954.9, 955.0-955.9, 956.0-956.9 |
Pneumonia | 480.0-480.9, 481, 482.0-482.9, 483.0-483.8, 484.1-484.8, 485, 486 |
Sepsis | 038.0-038.9, 112.5, 785.52, 995.91, 995.92 |
Stroke | 430, 432, 433.01-434.91, 997.02 |
Surgical site infection | 998.51, 998.59, 996.67 |
Urinary tract infection | 599 |
Wound dehiscence | 998.30-998.33 |
Abbreviation: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification
Length of stay and total hospital charges were available for each patient. Length of stay represents the number of calendar days a patient stayed in the hospital. All hospital charges were converted to hospitalization costs using the HCUP Cost-to-Charge Ratio Files. All hospitalization costs were adjusted for inflation using the US Bureau of Labor statistics yearly inflation calculator to represent charges in the year 2011, which was the final and most recent year in this study.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were conducted using Stata version 13.1 (StataCorp, LP). All analyses took into account the complex survey design of the NIS. Discharge weights, strata, and cluster variables were included to correctly estimate variance and to produce national estimates from the stratified sample. Pearson’s chi-squared test was used to compare age, sex, ECI, and insurance status between RA and non-RA patients undergoing shoulder arthroplasty.
Bivariate and multivariate logistic regressions were subsequently used to compare the rates of adverse events between RA and non-RA patients undergoing shoulder arthroplasty (non-RA cases were used as the reference). Multivariate linear regressions were used to compare hospital length of stay and hospitalization costs between RA and non-RA patients undergoing shoulder arthroplasty. The multivariate regressions were adjusted for baseline differences in age, sex, ECI, and insurance status. Cochran-Armitage tests for trend were used to assess trends over time. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
RESULTS
Overall, we identified 332,593 patients who underwent shoulder arthroplasty in the US between 2002 and 2011, of which 17,883 patients (5.4%) had a diagnosis of RA. In comparison with non-RA patients undergoing shoulder arthroplasty, patients with RA at the time of shoulder arthroplasty were significantly younger (65.2 ± 12.5 years vs 68.4 ± 11.0 years, P < .001), included a significantly greater proportion of female patients (76.7% vs 53.8%, P < .001), and included a significantly higher proportion of patients with Medicaid insurance (3.6% vs 2.3%, P < .001). There were no significant differences in the mean ECI between patients with and without a diagnosis of RA (Table 1). As depicted in Table 1, there were significant differences in the utilization of specific shoulder arthroplasty types between patients with and without RA, whereby a significantly greater proportion of RA patients underwent hemiarthroplasty (HA) (31.6% vs 29.3%, P = .002) and reverse TSA (7.7% vs 6.6%, P = .002), whereas a significantly greater proportion of non-RA patients underwent anatomic SA (64.0% vs 60.8%, P = .002).
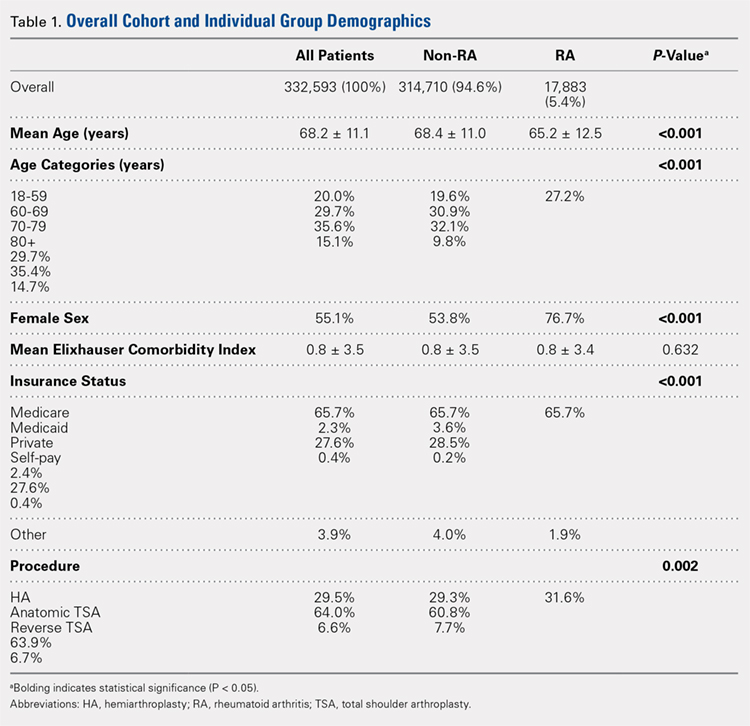
Over the study period from 2002 to 2011, there was a significant increase in the overall utilization of shoulder arthroplasty in RA patients, as indicated by both the absolute number and the proportion of patients with a diagnosis of RA (P < .001) (Table 2, Figure). More specifically, 0.39% of RA patients underwent shoulder arthroplasty in 2002, as compared with 0.58% of RA patients in 2011 (P < .001) (Table 2). With respect to specific arthroplasty types, there was an exponential rise in the utilization of reverse TSA beginning in 2010 and a corresponding decrease in the rates of both HA and anatomic TSA (Table 2, Figure). In addition to changes in shoulder arthroplasty utilization over time among RA patients, we also observed a significant increase in the number of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease (9.7% in 2002 to 15.2% in 2011, P < .001).
Table 2. The Annual Utilization of Shoulder Arthroplasty Among Patients with a Diagnosis of Rheumatoid Arthritis.
Proportion of RA patients |
| ||||
Year | Overall Rate of Shoulder Arthroplastya | HA | Anatomic TSA | Reverse TSA | |
2002 | 0.39 | 0.23 | 0.16 | 0 | |
2003 | 0.37 | 0.19 | 0.18 | 0 | |
2004 | 0.46 | 0.25 | 0.21 | 0 | |
2005 | 0.46 | 0.21 | 0.25 | 0 | |
2006 | 0.47 | 0.20 | 0.27 | 0 | |
2007 | 0.55 | 0.22 | 0.33 | 0 | |
2008 | 0.47 | 0.17 | 0.30 | 0 | |
2009 | 0.50 | 0.15 | 0.35 | 0 | |
2010 | 0.58 | 0.15 | 0.37 | 0.06 | |
2011 | 0.58 | 0.12 | 0.23 | 0.23 | |
Absolute number of RA patients |
| ||||
2002 | 1295 | 768 | 527 | 0 | |
2003 | 1247 | 650 | 597 | 0 | |
2004 | 1667 | 906 | 761 | 0 | |
2005 | 1722 | 776 | 946 | 0 | |
2006 | 1847 | 794 | 1053 | 0 | |
2007 | 2249 | 910 | 1339 | 0 | |
2008 | 2194 | 799 | 1395 | 0 | |
2009 | 2407 | 724 | 1683 | 0 | |
2010 | 2869 | 722 | 1857 | 290 | |
2011 | 3193 | 649 | 1261 | 1283 | |
aRate determined as number of RA patients undergoing shoulder arthroplasty compared to the number of patients with an RA diagnosis in the stated calendar year.
Abbreviations: HA, hemiarthroplasty; RA, rheumatoid arthritis; TSA, total shoulder arthroplasty.
Continue to: Among patients with RA...
Among patients with RA undergoing shoulder arthroplasty, the overall rate of early adverse events was 3.12%, of which the most common early adverse events were urinary tract infections (1.8%), acute kidney injury (0.66%), and pneumonia (0.38%) (Table 3). As compared with patients without a diagnosis of RA undergoing shoulder arthroplasty, there were no significant differences in the overall and individual rates of early adverse events (Table 3).
Table 3. A Comparison of Early Adverse Events, Length of Stay, and Cost Between Patients With and Without Rheumatoid Arthritis (RA) Undergoing Shoulder Arthroplasty
Comparison of Early Adverse Event Rates |
| ||||
| Non-RA Patients | RA Patients | Multivariate Logistic Regression | ||
Odds Ratio | P-Value | ||||
Overall adverse event rate | 3.02% | 3.12% | 1.0 | 0.83 | |
Specific adverse event rate |
|
|
|
| |
Death | 0.08% | 0.05% | 0.9 | 0.91 | |
Acute kidney injury | 0.85% | 0.66% | 0.9 | 0.59 | |
Cardiac arrest | 0.05% | 0.05% | 1.3 | 0.70 | |
Thromboembolic event | 0.01% | 0.00% | - | - | |
Myocardial Infarction | 0.22% | 0.06% | 0.4 | 0.17 | |
Peripheral nerve injury | 0.08% | 0.11% | 1.5 | 0.45 | |
Pneumonia | 0.47% | 0.38% | 0.9 | 0.70 | |
Sepsis | 0.08% | 0.08% | 1.3 | 0.62 | |
Stroke | 0.07% | 0.05% | 0.9 | 0.93 | |
Surgical site infection | 0.09% | 0.13% | 1.4 | 0.52 | |
Urinary tract infection | 1.44% | 1.80% | 1.1 | 0.46 | |
Wound dehiscence | 0.01% | 0.05% | 3.6 | 0.09 | |
Comparison of Length of Stay and Hospital Charges | |||||
| Non-RA Patients (percent) | RA Patients (percent) | Multivariate Linear Regression | ||
Beta | P-Value | ||||
Length of staya | 2.3±2.0 | 2.4±1.6 | +0.1 | 0.002 | |
Hospitalization costb | 14,826±8,336 | 14,787±7,625 | +93 | 0.59 | |
aReported in days. bReported in 2011 US dollars, adjusted for inflation.
The mean length of stay following shoulder arthroplasty in RA patients was 2.4 ± 1.6 days, and the mean hospitalization cost was $14,787 ± $7625 (Table 3). As compared with non-RA patients undergoing shoulder arthroplasty, there were no significant differences in the mean hospitalization costs; however, non-RA patients had a significantly shorter length of stay by 0.1 days (P = .002) (Table 3).
DISCUSSION
In this study, we observed that the utilization of shoulder arthroplasty in patients with RA increased significantly in the decade from 2002 to 2011, largely related to a rise in TSA. Interestingly, we also observed a corresponding rise in the proportion of RA patients undergoing shoulder arthroplasty with a diagnosis of rotator cuff disease, and we believe that this may partly account for the recent increase in the use of the reverse TSA in this patient population. Additionally, we found shoulder arthroplasty in RA patients to be safe in the early postoperative period, with no significant increase in cost as compared with patients undergoing shoulder arthroplasty without a diagnosis of RA. Although we did observe a significant increase in length of stay among RA patients as compared with non-RA patients, the absolute difference was only 0.1 days, and given the aforementioned similarities in cost between RA and non-RA patients, we do not believe this difference to be clinically significant.
It has been theorized that the utilization of TJA in RA patients has been decreasing with improvements in medical management; however, this is largely based upon literature pertaining to lower extremity TJA.2 On the contrary, past research pertaining to the utilization of shoulder arthroplasty in RA patients has been highly variable. For instance, a Swedish study demonstrated a statistically significant decrease in admissions associated with RA-related upper limb surgery and a stable rate of shoulder arthroplasty between 1998 and 2004.7 Similarly, a Finnish study demonstrated that the annual incidence of primary joint arthroplasty in RA patients had declined from 1995 to 2010, with a greater decline for upper-limb arthroplasty as compared with lower-limb arthroplasty.8 Despite these European observations, Jain and colleagues9 reported an increasing rate of TSA among RA patients in the US between the years 1992 and 2005. In this study, we demonstrate a clear increase in the utilization of shoulder arthroplasty among RA patients between 2002 and 2011. What was most striking about our observation was that the rise in utilization appeared to be driven by an increase in TSA, whereas the utilization of HA decreased over time. This change in practice likely reflects several factors, including the multitude of studies that have demonstrated improved outcomes with anatomic TSA as compared with HA in RA patients.10-14
Perhaps the most interesting aspect of our data was the recent exponential rise in the utilization of the reverse TSA. Despite improved outcomes following TSA as compared with HA in RA patients, these outcomes all appear to be highly dependent upon the integrity of the rotator cuff.10 In fact, there is evidence that failure of the rotator cuff could be as high as 75% within 10 years of TSA in patients with RA,15 which ultimately could jeopardize the long-term durability of the TSA implant in this patient population.11 For this reason, interest in the reverse TSA for the RA patient population has increased since its introduction in the US in 2004;16 in fact, in RA patients with end-stage inflammatory arthropathy and a damaged rotator cuff, the reverse TSA has demonstrated excellent results.17-20 Based upon this evidence, it is not surprising that we found an exponential rise in the use of the reverse TSA since 2010, which corresponds to the introduction of an ICD-9 code for this implant.21 Prior to 2010, it is likely that many implanted reverse TSAs were coded as TSA, and for this reason, we believe that the observed rise in the utilization of TSA in RA patients prior to 2010 may have been partly fueled by an increase in the use of the reverse TSA. To further support this theory, there was a dramatic decrease in the use of anatomic TSA following 2010, and we believe this was related to increased awareness of the newly introduced reverse TSA code among surgeons.
Another consideration when examining the utilization of shoulder arthroplasty in RA patients is its versatility in managing different disease states, including rotator cuff disease. As has been documented in the literature, outcomes of rotator cuff repair in RA patients are discouraging.22 For this reason, it is reasonable for surgeons and patients with RA to consider alternatives to rotator cuff repair when nonoperative management has failed to provide adequate improvement in symptoms. One alternative may be shoulder arthroplasty, namely the reverse TSA. In this study, we observed a significant increase in the rate of diagnosis of rotator cuff disease among RA patients undergoing shoulder arthroplasty from 2002 to 2011 (9.7% in 2002 to 15.2% in 2011, P < .001), and it is our belief that the simultaneous increase in the diagnosis of rotator cuff disease and use of TSA is not coincidental. More specifically, there is likely an emerging trend among surgeons toward using the reverse TSA to manage rotator cuff tears in the RA population, rather than undertaking a rotator cuff repair that carries a high rate of failure. Going forward, there is a need to not only identify this trend more clearly but to also compare the outcomes between reverse TSA and rotator cuff repair in the management of rotator cuff tears in RA patients.
Continue to: In this study, we observed...
In this study, we observed that RA patients undergoing shoulder arthroplasty were significantly younger than non-RA patients undergoing shoulder arthroplasty. At first, this observation seems to counter recent literature suggesting that the age of patients with inflammatory arthropathy undergoing TJA is increasing over time;1 however, looking more closely at the data, it becomes clearer that the mean age we report is actually a relative increase as compared with past clinical studies pertaining to RA patients undergoing shoulder arthroplasty (mean ages of 47 years,23 55 years,24 60 years,10 and 62 years25). On the other hand, the continued existence of an age gap between RA and non-RA patients undergoing shoulder arthroplasty may be the result of several possible phenomena. First, this may reflect issues with patient access to and coverage of expensive biologic antirheumatic medication that would otherwise mitigate disease progression. For instance, the out-of-pocket expense for biologic medication through Medicaid and Medicare is substantial,26 which has direct implications on over two-thirds of our RA cohort. Second, it may be skewed by the proportion of RA patients who have previously been or continue to be poorly managed, enabling disease progression to end-stage arthropathy at a younger age. Ultimately, further investigation is needed to determine the reasons for this continued age disparity.
In comparing RA and non-RA patients undergoing shoulder arthroplasty, we did not find a significant difference in the overall nor the individual rates of early adverse events. This finding appears to be unique, as similar studies pertaining to total knee arthroplasty (TKA) demonstrated a significantly higher incidence of postoperative pneumonia and bleeding requiring transfusion among RA patients as compared with non-RA patients.27 In patients with RA being treated with biologic medication and undergoing shoulder arthroplasty, the frequent concern in the postoperative period is the integrity of the wound and the potential for infection.28 In this study, we did not find a significant difference in the rate of early infection, and although the difference in the rate of early wound dehiscence approached significance, it did not meet the threshold of 0.05 (P = .09). This finding is in keeping with the aforementioned NIS study pertaining to TKA, and we believe that it likely reflects the short duration of follow-up for patients in both studies. Given the nature of the database we utilized, we were only privy to complications that arose during the inpatient hospital stay, and it is likely that the clear majority of patients who develop a postoperative infection or wound dehiscence do so in the postoperative setting following discharge. A second concern regarding postoperative wound complications is the management of biologic medication in the perioperative period, which we cannot determine using this database. Despite all these limitations specific to this database, a past systematic review of reverse TSA in RA patients found a low rate of deep infection after reverse TSA in RA patients (3.3%),17 which was not higher than that after shoulder arthroplasty performed in non-RA patients.
A final demonstration from this study is that the hospital length of stay was significantly longer for RA patients than non-RA patients undergoing shoulder arthroplasty; however, given that the difference was only 0.1 days, and there was no significant difference in hospitalization cost, we are inclined to believe that statistical significance may not translate into clinical significance in this scenario. Ultimately, we do believe that length of stay is an important consideration in the current healthcare system, and given our finding that shoulder arthroplasty in the RA patient is safe in the early postoperative period, that a prolonged postoperative hospitalization is not warranted on the sole basis of a patient’s history of RA.
As with all studies using data from a search of an administrative database, such as the NIS database, this study has limitations. First, this type of research is limited by the reliability of both diagnosis and procedural coding. Although the NIS database has demonstrated high reliability,3 it is still possible that events may have been miscoded. Second, the tracking period for adverse events is limited to the inpatient hospital stay, which may be too short to detect certain postoperative complications. As such, the rates we report are likely underestimates of the true incidence of these complications, but this is true for both the RA and non-RA populations. Third, the comparisons we draw between RA and non-RA patients are limited to the scope of the NIS database and the available data; as such, we could not draw comparisons between preoperative disease stage, intraoperative findings, and postoperative course following hospital discharge. Lastly, our data are limited to a distinct period between 2002 and 2011 and may not reflect current practice. Ultimately, our findings may underestimate current trends in shoulder arthroplasty utilization among RA patients, particularly for the reverse TSA.
CONCLUSION
In this study, we found that the utilization of shoulder arthroplasty in patients with RA increased significantly from 2002 to 2011, largely related to a rise in the utilization of TSA. Similarly, we observed a rise in the proportion of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease, and we believe the increased utilization of shoulder arthroplasty among RA patients resulted from management of both end-stage inflammatory arthropathy and rotator cuff disease. Although we did not find a significant difference between RA and non-RA patients in the rates of early adverse events and overall hospitalization costs following shoulder arthroplasty, length of stay was significantly longer among RA patients; however, the absolute difference does not appear to be clinically significant.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol. 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983-2007. Ann Rheum Dis. 2010;69(5):868-871. doi:10.1136/ard.2009.112474.
- HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality; 2002-2011.
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
- Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50(12):1109-1118. doi:10.1097/MLR.0b013e31825f64d0.
- van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. doi:10.1097/MLR.0b013e31819432e5.
- Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatol Oxf. 2008 ;47(4):491-494. doi. 10.1093/rheumatology/ken009.
- Jämsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 through 2010. Acta Orthop. 2013;84(4):331-337. doi:10.3109/17453674.2013.810519.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Barlow JD, Yuan BJ, Schleck CD, Harmsen WS, Cofield RH, Sperling JW. Shoulder arthroplasty for rheumatoid arthritis: 303 consecutive cases with minimum 5-year follow-up. J Shoulder Elbow Surg. 2014;23(6):791-799. doi:10.1016/j.jse.2013.09.016.
- Collins DN, Harryman DT, Wirth MA. Shoulder arthroplasty for the treatment of inflammatory arthritis. J Bone Joint Surg Am. 2004;86–A(11):2489-2496. doi:10.2106/00004623-200411000-00020.
- Rahme H, Mattsson P, Wikblad L, Larsson S. Cement and press-fit humeral stem fixation provides similar results in rheumatoid patients. Clin Orthop Relat Res. 2006;448:28-32. doi:10.1097/01.blo.0000224007.25636.85.
- Rozing PM, Nagels J, Rozing MP. Prognostic factors in arthroplasty in the rheumatoid shoulder. HSS J. 2011;7(1):29-36. doi:10.1007/s11420-010-9172-1.
- Sperling JW, Cofield RH, Schleck CD, Harmsen WS. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16(6):683-690. doi:10.1016/j.jse.2007.02.135.
- Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91(12):1594-1600. doi:10.1302/0301-620X.91B12.22139.
- Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747. doi:10.2106/JBJS.E.00851.
- Gee ECA, Hanson EK, Saithna A. Reverse shoulder arthroplasty in rheumatoid arthritis: A systematic review. Open Orthop J. 2015;9:237-245. doi:10.2174/1874325001509010237.
- Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2010;19(7):1076-1084. doi:10.1016/j.jse.2009.11.049.
- Postacchini R, Carbone S, Canero G, Ripani M, Postacchini F. Reverse shoulder prosthesis in patients with rheumatoid arthritis: a systematic review. Int Orthop. 2016;40(5):965-973. doi:10.1007/s00264-015-2916-2.
- Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22. doi:10.1067/mse.2001.110515.
- American Medical Association. American Medical Association Web site. www.ama-assn.org/ama. Accessed January 15, 2016.
- Smith AM, Sperling JW, Cofield RH. Rotator cuff repair in patients with rheumatoid arthritis. J Bone Joint Surg. 2005;87(8):1782-1787. doi:10.2106/JBJS.D.02452.
- Betts HM, Abu-Rajab R, Nunn T, Brooksbank AJ. Total shoulder replacement in rheumatoid disease: a 16- to 23-year follow-up. J Bone Joint Surg Br. 2009;91(9):1197-1200. doi:10.1302/0301-620X.91B9.22035.
- Geervliet PC, Somford MP, Winia P, van den Bekerom MP. Long-term results of shoulder hemiarthroplasty in patients with rheumatoid arthritis. Orthopedics. 2015;38(1):e38-e42. doi:10.3928/01477447-20150105-58.
- Hettrich CM, Weldon E III, Boorman RS, Parsons M IV, Matsen FA III. Preoperative factors associated with improvements in shoulder function after humeral hemiarthroplasty. J Bone Joint Surg. 2004;86–A(7):1446-1451.
- Yazdany J, Dudley RA, Chen R, Lin GA, Tseng CW. Coverage for high-cost specialty drugs for rheumatoid arthritis in Medicare Part D. Arthritis Rheumatol. 2015;67(6):1474-1480. doi:10.1002/art.39079.
- Jauregui JJ, Kapadia BH, Dixit A, et al. Thirty-day complications in rheumatoid patients following total knee arthroplasty. Clin Rheumatol. 2016;35(3):595-600. doi:10.1007/s10067-015-3037-4.
- Trail IA, Nuttall D. The results of shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Br. 2002;84(8):1121-1125. doi:10.1302/0301-620X.84B8.0841121
ABSTRACT
It has been suggested that the utilization of joint arthroplasty in patients with rheumatoid arthritis (RA) is decreasing; however, this observation is largely based upon evidence pertaining to lower-extremity joint arthroplasty. It remains unknown if these observed trends also hold true for shoulder arthroplasty. The purpose of this study is to utilize a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. Secondarily, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and to compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. Using a large population database in the US, we determined the annual rates of shoulder arthroplasty (overall and individual) in RA patients between 2002 and 2011. Early adverse events, length of stay, and hospitalization costs were determined and compared with those of non-RA patients undergoing shoulder arthroplasty. Overall, we identified 332,593 patients who underwent shoulder arthroplasty between 2002 and 2011, of whom 17,883 patients (5.4%) had a diagnosis of RA. Over the study period, there was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly total shoulder arthroplasty. Over the same period, there was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease. There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients. Non-RA patients had a significantly shorter length of stay; however, the difference did not appear to be clinically significant. In conclusion, the utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Continue to: It has been suggested...
It has been suggested that the utilization of total joint arthroplasty (TJA) in patients with rheumatoid arthritis (RA) is decreasing over time;1 however, this observation is largely based upon evidence pertaining to lower extremity TJA.2 It remains unknown if these observed trends also hold true for shoulder arthroplasty, whereby the utilization of shoulder arthroplasty in RA patients is not limited to the management of end-stage inflammatory arthropathy. In this study, we used a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. As a secondary objective, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. We hypothesize that the utilization of shoulder arthroplasty in RA patients would be decreasing, but adverse events, length of stay, and hospitalization costs would not differ between patients with and without RA undergoing shoulder arthroplasty.
METHODS
We conducted a retrospective cohort study using the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) from 2002 to 2011.3 The NIS comprises a 20% stratified sample of all hospital discharges in the US. The NIS includes information about patient characteristics (age, sex, insurance status, and medical comorbidities) and hospitalization outcomes (adverse events, costs, and length of stay). The NIS allows identification of hospitalizations according to procedures and diagnoses using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Given the anonymity of this study, it was exempt from Institutional Review Board ethics approval.
Hospitalizations were selected for the study based on ICD-9-CM procedural codes for hemiarthroplasty (81.81), anatomic total shoulder arthroplasty (TSA) (81.80), and reverse TSA (81.88). These patients were then stratified by an ICD-9-CM diagnosis of RA (714.X). We also utilized ICD-9-CM diagnosis codes to determine the presence of rotator cuff pathology at the time of shoulder arthroplasty (726.13, 727.61, 840.4) and to exclude patients with a history of trauma (812.X, 716.11, 733.8X). In a separate analysis, all patients in the NIS database with an ICD-9-CM diagnosis of RA were identified for each calendar year of the study, and a national estimate of RA patients was generated annually to assess overall and individual utilization rates of shoulder arthroplasty in this population (the national estimate served as the denominator).
Preoperative patient data withdrawn from the NIS included age, sex, insurance status, and medical comorbidities. An Elixhauser Comorbidity Index (ECI) was generated for each patient based on the presence of 29 comorbid conditions. The ECI was chosen because of its capacity to accurately predict mortality and represent the patient burden of comorbidities in similar administrative database studies.4-6
Early adverse events were also chosen based on ICD-9-CM diagnosis codes (Appendix A), and included the following: death, acute kidney injury, cardiac arrest, thromboembolic event, myocardial infarction, peripheral nerve injury, pneumonia, sepsis, stroke, surgical site infection, urinary tract infection, and wound dehiscence. The overall adverse event rate was defined as the occurrence of ≥1 of the above adverse events in a patient.
Appendix A. ICD-9-CM Codes Corresponding to Postoperative Adverse Events
Event | ICD-9-CM |
Acute kidney injury | 584.5-584.9 |
Cardiac arrest | 427.41, 427.5 |
Thromboembolic event | 453.2-453.4, 453.82-453.86, 415.1 |
Myocardial Infarction | 410.00-410.92 |
Peripheral nerve injury | 953.0-953.9 954.0-954.9, 955.0-955.9, 956.0-956.9 |
Pneumonia | 480.0-480.9, 481, 482.0-482.9, 483.0-483.8, 484.1-484.8, 485, 486 |
Sepsis | 038.0-038.9, 112.5, 785.52, 995.91, 995.92 |
Stroke | 430, 432, 433.01-434.91, 997.02 |
Surgical site infection | 998.51, 998.59, 996.67 |
Urinary tract infection | 599 |
Wound dehiscence | 998.30-998.33 |
Abbreviation: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification
Length of stay and total hospital charges were available for each patient. Length of stay represents the number of calendar days a patient stayed in the hospital. All hospital charges were converted to hospitalization costs using the HCUP Cost-to-Charge Ratio Files. All hospitalization costs were adjusted for inflation using the US Bureau of Labor statistics yearly inflation calculator to represent charges in the year 2011, which was the final and most recent year in this study.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were conducted using Stata version 13.1 (StataCorp, LP). All analyses took into account the complex survey design of the NIS. Discharge weights, strata, and cluster variables were included to correctly estimate variance and to produce national estimates from the stratified sample. Pearson’s chi-squared test was used to compare age, sex, ECI, and insurance status between RA and non-RA patients undergoing shoulder arthroplasty.
Bivariate and multivariate logistic regressions were subsequently used to compare the rates of adverse events between RA and non-RA patients undergoing shoulder arthroplasty (non-RA cases were used as the reference). Multivariate linear regressions were used to compare hospital length of stay and hospitalization costs between RA and non-RA patients undergoing shoulder arthroplasty. The multivariate regressions were adjusted for baseline differences in age, sex, ECI, and insurance status. Cochran-Armitage tests for trend were used to assess trends over time. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
RESULTS
Overall, we identified 332,593 patients who underwent shoulder arthroplasty in the US between 2002 and 2011, of which 17,883 patients (5.4%) had a diagnosis of RA. In comparison with non-RA patients undergoing shoulder arthroplasty, patients with RA at the time of shoulder arthroplasty were significantly younger (65.2 ± 12.5 years vs 68.4 ± 11.0 years, P < .001), included a significantly greater proportion of female patients (76.7% vs 53.8%, P < .001), and included a significantly higher proportion of patients with Medicaid insurance (3.6% vs 2.3%, P < .001). There were no significant differences in the mean ECI between patients with and without a diagnosis of RA (Table 1). As depicted in Table 1, there were significant differences in the utilization of specific shoulder arthroplasty types between patients with and without RA, whereby a significantly greater proportion of RA patients underwent hemiarthroplasty (HA) (31.6% vs 29.3%, P = .002) and reverse TSA (7.7% vs 6.6%, P = .002), whereas a significantly greater proportion of non-RA patients underwent anatomic SA (64.0% vs 60.8%, P = .002).

Over the study period from 2002 to 2011, there was a significant increase in the overall utilization of shoulder arthroplasty in RA patients, as indicated by both the absolute number and the proportion of patients with a diagnosis of RA (P < .001) (Table 2, Figure). More specifically, 0.39% of RA patients underwent shoulder arthroplasty in 2002, as compared with 0.58% of RA patients in 2011 (P < .001) (Table 2). With respect to specific arthroplasty types, there was an exponential rise in the utilization of reverse TSA beginning in 2010 and a corresponding decrease in the rates of both HA and anatomic TSA (Table 2, Figure). In addition to changes in shoulder arthroplasty utilization over time among RA patients, we also observed a significant increase in the number of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease (9.7% in 2002 to 15.2% in 2011, P < .001).
Table 2. The Annual Utilization of Shoulder Arthroplasty Among Patients with a Diagnosis of Rheumatoid Arthritis.
Proportion of RA patients |
| ||||
Year | Overall Rate of Shoulder Arthroplastya | HA | Anatomic TSA | Reverse TSA | |
2002 | 0.39 | 0.23 | 0.16 | 0 | |
2003 | 0.37 | 0.19 | 0.18 | 0 | |
2004 | 0.46 | 0.25 | 0.21 | 0 | |
2005 | 0.46 | 0.21 | 0.25 | 0 | |
2006 | 0.47 | 0.20 | 0.27 | 0 | |
2007 | 0.55 | 0.22 | 0.33 | 0 | |
2008 | 0.47 | 0.17 | 0.30 | 0 | |
2009 | 0.50 | 0.15 | 0.35 | 0 | |
2010 | 0.58 | 0.15 | 0.37 | 0.06 | |
2011 | 0.58 | 0.12 | 0.23 | 0.23 | |
Absolute number of RA patients |
| ||||
2002 | 1295 | 768 | 527 | 0 | |
2003 | 1247 | 650 | 597 | 0 | |
2004 | 1667 | 906 | 761 | 0 | |
2005 | 1722 | 776 | 946 | 0 | |
2006 | 1847 | 794 | 1053 | 0 | |
2007 | 2249 | 910 | 1339 | 0 | |
2008 | 2194 | 799 | 1395 | 0 | |
2009 | 2407 | 724 | 1683 | 0 | |
2010 | 2869 | 722 | 1857 | 290 | |
2011 | 3193 | 649 | 1261 | 1283 | |
aRate determined as number of RA patients undergoing shoulder arthroplasty compared to the number of patients with an RA diagnosis in the stated calendar year.
Abbreviations: HA, hemiarthroplasty; RA, rheumatoid arthritis; TSA, total shoulder arthroplasty.

Continue to: Among patients with RA...
Among patients with RA undergoing shoulder arthroplasty, the overall rate of early adverse events was 3.12%, of which the most common early adverse events were urinary tract infections (1.8%), acute kidney injury (0.66%), and pneumonia (0.38%) (Table 3). As compared with patients without a diagnosis of RA undergoing shoulder arthroplasty, there were no significant differences in the overall and individual rates of early adverse events (Table 3).
Table 3. A Comparison of Early Adverse Events, Length of Stay, and Cost Between Patients With and Without Rheumatoid Arthritis (RA) Undergoing Shoulder Arthroplasty
Comparison of Early Adverse Event Rates |
| ||||
| Non-RA Patients | RA Patients | Multivariate Logistic Regression | ||
Odds Ratio | P-Value | ||||
Overall adverse event rate | 3.02% | 3.12% | 1.0 | 0.83 | |
Specific adverse event rate |
|
|
|
| |
Death | 0.08% | 0.05% | 0.9 | 0.91 | |
Acute kidney injury | 0.85% | 0.66% | 0.9 | 0.59 | |
Cardiac arrest | 0.05% | 0.05% | 1.3 | 0.70 | |
Thromboembolic event | 0.01% | 0.00% | - | - | |
Myocardial Infarction | 0.22% | 0.06% | 0.4 | 0.17 | |
Peripheral nerve injury | 0.08% | 0.11% | 1.5 | 0.45 | |
Pneumonia | 0.47% | 0.38% | 0.9 | 0.70 | |
Sepsis | 0.08% | 0.08% | 1.3 | 0.62 | |
Stroke | 0.07% | 0.05% | 0.9 | 0.93 | |
Surgical site infection | 0.09% | 0.13% | 1.4 | 0.52 | |
Urinary tract infection | 1.44% | 1.80% | 1.1 | 0.46 | |
Wound dehiscence | 0.01% | 0.05% | 3.6 | 0.09 | |
Comparison of Length of Stay and Hospital Charges | |||||
| Non-RA Patients (percent) | RA Patients (percent) | Multivariate Linear Regression | ||
Beta | P-Value | ||||
Length of staya | 2.3±2.0 | 2.4±1.6 | +0.1 | 0.002 | |
Hospitalization costb | 14,826±8,336 | 14,787±7,625 | +93 | 0.59 | |
aReported in days. bReported in 2011 US dollars, adjusted for inflation.
The mean length of stay following shoulder arthroplasty in RA patients was 2.4 ± 1.6 days, and the mean hospitalization cost was $14,787 ± $7625 (Table 3). As compared with non-RA patients undergoing shoulder arthroplasty, there were no significant differences in the mean hospitalization costs; however, non-RA patients had a significantly shorter length of stay by 0.1 days (P = .002) (Table 3).
DISCUSSION
In this study, we observed that the utilization of shoulder arthroplasty in patients with RA increased significantly in the decade from 2002 to 2011, largely related to a rise in TSA. Interestingly, we also observed a corresponding rise in the proportion of RA patients undergoing shoulder arthroplasty with a diagnosis of rotator cuff disease, and we believe that this may partly account for the recent increase in the use of the reverse TSA in this patient population. Additionally, we found shoulder arthroplasty in RA patients to be safe in the early postoperative period, with no significant increase in cost as compared with patients undergoing shoulder arthroplasty without a diagnosis of RA. Although we did observe a significant increase in length of stay among RA patients as compared with non-RA patients, the absolute difference was only 0.1 days, and given the aforementioned similarities in cost between RA and non-RA patients, we do not believe this difference to be clinically significant.
It has been theorized that the utilization of TJA in RA patients has been decreasing with improvements in medical management; however, this is largely based upon literature pertaining to lower extremity TJA.2 On the contrary, past research pertaining to the utilization of shoulder arthroplasty in RA patients has been highly variable. For instance, a Swedish study demonstrated a statistically significant decrease in admissions associated with RA-related upper limb surgery and a stable rate of shoulder arthroplasty between 1998 and 2004.7 Similarly, a Finnish study demonstrated that the annual incidence of primary joint arthroplasty in RA patients had declined from 1995 to 2010, with a greater decline for upper-limb arthroplasty as compared with lower-limb arthroplasty.8 Despite these European observations, Jain and colleagues9 reported an increasing rate of TSA among RA patients in the US between the years 1992 and 2005. In this study, we demonstrate a clear increase in the utilization of shoulder arthroplasty among RA patients between 2002 and 2011. What was most striking about our observation was that the rise in utilization appeared to be driven by an increase in TSA, whereas the utilization of HA decreased over time. This change in practice likely reflects several factors, including the multitude of studies that have demonstrated improved outcomes with anatomic TSA as compared with HA in RA patients.10-14
Perhaps the most interesting aspect of our data was the recent exponential rise in the utilization of the reverse TSA. Despite improved outcomes following TSA as compared with HA in RA patients, these outcomes all appear to be highly dependent upon the integrity of the rotator cuff.10 In fact, there is evidence that failure of the rotator cuff could be as high as 75% within 10 years of TSA in patients with RA,15 which ultimately could jeopardize the long-term durability of the TSA implant in this patient population.11 For this reason, interest in the reverse TSA for the RA patient population has increased since its introduction in the US in 2004;16 in fact, in RA patients with end-stage inflammatory arthropathy and a damaged rotator cuff, the reverse TSA has demonstrated excellent results.17-20 Based upon this evidence, it is not surprising that we found an exponential rise in the use of the reverse TSA since 2010, which corresponds to the introduction of an ICD-9 code for this implant.21 Prior to 2010, it is likely that many implanted reverse TSAs were coded as TSA, and for this reason, we believe that the observed rise in the utilization of TSA in RA patients prior to 2010 may have been partly fueled by an increase in the use of the reverse TSA. To further support this theory, there was a dramatic decrease in the use of anatomic TSA following 2010, and we believe this was related to increased awareness of the newly introduced reverse TSA code among surgeons.
Another consideration when examining the utilization of shoulder arthroplasty in RA patients is its versatility in managing different disease states, including rotator cuff disease. As has been documented in the literature, outcomes of rotator cuff repair in RA patients are discouraging.22 For this reason, it is reasonable for surgeons and patients with RA to consider alternatives to rotator cuff repair when nonoperative management has failed to provide adequate improvement in symptoms. One alternative may be shoulder arthroplasty, namely the reverse TSA. In this study, we observed a significant increase in the rate of diagnosis of rotator cuff disease among RA patients undergoing shoulder arthroplasty from 2002 to 2011 (9.7% in 2002 to 15.2% in 2011, P < .001), and it is our belief that the simultaneous increase in the diagnosis of rotator cuff disease and use of TSA is not coincidental. More specifically, there is likely an emerging trend among surgeons toward using the reverse TSA to manage rotator cuff tears in the RA population, rather than undertaking a rotator cuff repair that carries a high rate of failure. Going forward, there is a need to not only identify this trend more clearly but to also compare the outcomes between reverse TSA and rotator cuff repair in the management of rotator cuff tears in RA patients.
Continue to: In this study, we observed...
In this study, we observed that RA patients undergoing shoulder arthroplasty were significantly younger than non-RA patients undergoing shoulder arthroplasty. At first, this observation seems to counter recent literature suggesting that the age of patients with inflammatory arthropathy undergoing TJA is increasing over time;1 however, looking more closely at the data, it becomes clearer that the mean age we report is actually a relative increase as compared with past clinical studies pertaining to RA patients undergoing shoulder arthroplasty (mean ages of 47 years,23 55 years,24 60 years,10 and 62 years25). On the other hand, the continued existence of an age gap between RA and non-RA patients undergoing shoulder arthroplasty may be the result of several possible phenomena. First, this may reflect issues with patient access to and coverage of expensive biologic antirheumatic medication that would otherwise mitigate disease progression. For instance, the out-of-pocket expense for biologic medication through Medicaid and Medicare is substantial,26 which has direct implications on over two-thirds of our RA cohort. Second, it may be skewed by the proportion of RA patients who have previously been or continue to be poorly managed, enabling disease progression to end-stage arthropathy at a younger age. Ultimately, further investigation is needed to determine the reasons for this continued age disparity.
In comparing RA and non-RA patients undergoing shoulder arthroplasty, we did not find a significant difference in the overall nor the individual rates of early adverse events. This finding appears to be unique, as similar studies pertaining to total knee arthroplasty (TKA) demonstrated a significantly higher incidence of postoperative pneumonia and bleeding requiring transfusion among RA patients as compared with non-RA patients.27 In patients with RA being treated with biologic medication and undergoing shoulder arthroplasty, the frequent concern in the postoperative period is the integrity of the wound and the potential for infection.28 In this study, we did not find a significant difference in the rate of early infection, and although the difference in the rate of early wound dehiscence approached significance, it did not meet the threshold of 0.05 (P = .09). This finding is in keeping with the aforementioned NIS study pertaining to TKA, and we believe that it likely reflects the short duration of follow-up for patients in both studies. Given the nature of the database we utilized, we were only privy to complications that arose during the inpatient hospital stay, and it is likely that the clear majority of patients who develop a postoperative infection or wound dehiscence do so in the postoperative setting following discharge. A second concern regarding postoperative wound complications is the management of biologic medication in the perioperative period, which we cannot determine using this database. Despite all these limitations specific to this database, a past systematic review of reverse TSA in RA patients found a low rate of deep infection after reverse TSA in RA patients (3.3%),17 which was not higher than that after shoulder arthroplasty performed in non-RA patients.
A final demonstration from this study is that the hospital length of stay was significantly longer for RA patients than non-RA patients undergoing shoulder arthroplasty; however, given that the difference was only 0.1 days, and there was no significant difference in hospitalization cost, we are inclined to believe that statistical significance may not translate into clinical significance in this scenario. Ultimately, we do believe that length of stay is an important consideration in the current healthcare system, and given our finding that shoulder arthroplasty in the RA patient is safe in the early postoperative period, that a prolonged postoperative hospitalization is not warranted on the sole basis of a patient’s history of RA.
As with all studies using data from a search of an administrative database, such as the NIS database, this study has limitations. First, this type of research is limited by the reliability of both diagnosis and procedural coding. Although the NIS database has demonstrated high reliability,3 it is still possible that events may have been miscoded. Second, the tracking period for adverse events is limited to the inpatient hospital stay, which may be too short to detect certain postoperative complications. As such, the rates we report are likely underestimates of the true incidence of these complications, but this is true for both the RA and non-RA populations. Third, the comparisons we draw between RA and non-RA patients are limited to the scope of the NIS database and the available data; as such, we could not draw comparisons between preoperative disease stage, intraoperative findings, and postoperative course following hospital discharge. Lastly, our data are limited to a distinct period between 2002 and 2011 and may not reflect current practice. Ultimately, our findings may underestimate current trends in shoulder arthroplasty utilization among RA patients, particularly for the reverse TSA.
CONCLUSION
In this study, we found that the utilization of shoulder arthroplasty in patients with RA increased significantly from 2002 to 2011, largely related to a rise in the utilization of TSA. Similarly, we observed a rise in the proportion of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease, and we believe the increased utilization of shoulder arthroplasty among RA patients resulted from management of both end-stage inflammatory arthropathy and rotator cuff disease. Although we did not find a significant difference between RA and non-RA patients in the rates of early adverse events and overall hospitalization costs following shoulder arthroplasty, length of stay was significantly longer among RA patients; however, the absolute difference does not appear to be clinically significant.
ABSTRACT
It has been suggested that the utilization of joint arthroplasty in patients with rheumatoid arthritis (RA) is decreasing; however, this observation is largely based upon evidence pertaining to lower-extremity joint arthroplasty. It remains unknown if these observed trends also hold true for shoulder arthroplasty. The purpose of this study is to utilize a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. Secondarily, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and to compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. Using a large population database in the US, we determined the annual rates of shoulder arthroplasty (overall and individual) in RA patients between 2002 and 2011. Early adverse events, length of stay, and hospitalization costs were determined and compared with those of non-RA patients undergoing shoulder arthroplasty. Overall, we identified 332,593 patients who underwent shoulder arthroplasty between 2002 and 2011, of whom 17,883 patients (5.4%) had a diagnosis of RA. Over the study period, there was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly total shoulder arthroplasty. Over the same period, there was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease. There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients. Non-RA patients had a significantly shorter length of stay; however, the difference did not appear to be clinically significant. In conclusion, the utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Continue to: It has been suggested...
It has been suggested that the utilization of total joint arthroplasty (TJA) in patients with rheumatoid arthritis (RA) is decreasing over time;1 however, this observation is largely based upon evidence pertaining to lower extremity TJA.2 It remains unknown if these observed trends also hold true for shoulder arthroplasty, whereby the utilization of shoulder arthroplasty in RA patients is not limited to the management of end-stage inflammatory arthropathy. In this study, we used a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. As a secondary objective, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. We hypothesize that the utilization of shoulder arthroplasty in RA patients would be decreasing, but adverse events, length of stay, and hospitalization costs would not differ between patients with and without RA undergoing shoulder arthroplasty.
METHODS
We conducted a retrospective cohort study using the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) from 2002 to 2011.3 The NIS comprises a 20% stratified sample of all hospital discharges in the US. The NIS includes information about patient characteristics (age, sex, insurance status, and medical comorbidities) and hospitalization outcomes (adverse events, costs, and length of stay). The NIS allows identification of hospitalizations according to procedures and diagnoses using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Given the anonymity of this study, it was exempt from Institutional Review Board ethics approval.
Hospitalizations were selected for the study based on ICD-9-CM procedural codes for hemiarthroplasty (81.81), anatomic total shoulder arthroplasty (TSA) (81.80), and reverse TSA (81.88). These patients were then stratified by an ICD-9-CM diagnosis of RA (714.X). We also utilized ICD-9-CM diagnosis codes to determine the presence of rotator cuff pathology at the time of shoulder arthroplasty (726.13, 727.61, 840.4) and to exclude patients with a history of trauma (812.X, 716.11, 733.8X). In a separate analysis, all patients in the NIS database with an ICD-9-CM diagnosis of RA were identified for each calendar year of the study, and a national estimate of RA patients was generated annually to assess overall and individual utilization rates of shoulder arthroplasty in this population (the national estimate served as the denominator).
Preoperative patient data withdrawn from the NIS included age, sex, insurance status, and medical comorbidities. An Elixhauser Comorbidity Index (ECI) was generated for each patient based on the presence of 29 comorbid conditions. The ECI was chosen because of its capacity to accurately predict mortality and represent the patient burden of comorbidities in similar administrative database studies.4-6
Early adverse events were also chosen based on ICD-9-CM diagnosis codes (Appendix A), and included the following: death, acute kidney injury, cardiac arrest, thromboembolic event, myocardial infarction, peripheral nerve injury, pneumonia, sepsis, stroke, surgical site infection, urinary tract infection, and wound dehiscence. The overall adverse event rate was defined as the occurrence of ≥1 of the above adverse events in a patient.
Appendix A. ICD-9-CM Codes Corresponding to Postoperative Adverse Events
Event | ICD-9-CM |
Acute kidney injury | 584.5-584.9 |
Cardiac arrest | 427.41, 427.5 |
Thromboembolic event | 453.2-453.4, 453.82-453.86, 415.1 |
Myocardial Infarction | 410.00-410.92 |
Peripheral nerve injury | 953.0-953.9 954.0-954.9, 955.0-955.9, 956.0-956.9 |
Pneumonia | 480.0-480.9, 481, 482.0-482.9, 483.0-483.8, 484.1-484.8, 485, 486 |
Sepsis | 038.0-038.9, 112.5, 785.52, 995.91, 995.92 |
Stroke | 430, 432, 433.01-434.91, 997.02 |
Surgical site infection | 998.51, 998.59, 996.67 |
Urinary tract infection | 599 |
Wound dehiscence | 998.30-998.33 |
Abbreviation: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification
Length of stay and total hospital charges were available for each patient. Length of stay represents the number of calendar days a patient stayed in the hospital. All hospital charges were converted to hospitalization costs using the HCUP Cost-to-Charge Ratio Files. All hospitalization costs were adjusted for inflation using the US Bureau of Labor statistics yearly inflation calculator to represent charges in the year 2011, which was the final and most recent year in this study.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were conducted using Stata version 13.1 (StataCorp, LP). All analyses took into account the complex survey design of the NIS. Discharge weights, strata, and cluster variables were included to correctly estimate variance and to produce national estimates from the stratified sample. Pearson’s chi-squared test was used to compare age, sex, ECI, and insurance status between RA and non-RA patients undergoing shoulder arthroplasty.
Bivariate and multivariate logistic regressions were subsequently used to compare the rates of adverse events between RA and non-RA patients undergoing shoulder arthroplasty (non-RA cases were used as the reference). Multivariate linear regressions were used to compare hospital length of stay and hospitalization costs between RA and non-RA patients undergoing shoulder arthroplasty. The multivariate regressions were adjusted for baseline differences in age, sex, ECI, and insurance status. Cochran-Armitage tests for trend were used to assess trends over time. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
RESULTS
Overall, we identified 332,593 patients who underwent shoulder arthroplasty in the US between 2002 and 2011, of which 17,883 patients (5.4%) had a diagnosis of RA. In comparison with non-RA patients undergoing shoulder arthroplasty, patients with RA at the time of shoulder arthroplasty were significantly younger (65.2 ± 12.5 years vs 68.4 ± 11.0 years, P < .001), included a significantly greater proportion of female patients (76.7% vs 53.8%, P < .001), and included a significantly higher proportion of patients with Medicaid insurance (3.6% vs 2.3%, P < .001). There were no significant differences in the mean ECI between patients with and without a diagnosis of RA (Table 1). As depicted in Table 1, there were significant differences in the utilization of specific shoulder arthroplasty types between patients with and without RA, whereby a significantly greater proportion of RA patients underwent hemiarthroplasty (HA) (31.6% vs 29.3%, P = .002) and reverse TSA (7.7% vs 6.6%, P = .002), whereas a significantly greater proportion of non-RA patients underwent anatomic SA (64.0% vs 60.8%, P = .002).

Over the study period from 2002 to 2011, there was a significant increase in the overall utilization of shoulder arthroplasty in RA patients, as indicated by both the absolute number and the proportion of patients with a diagnosis of RA (P < .001) (Table 2, Figure). More specifically, 0.39% of RA patients underwent shoulder arthroplasty in 2002, as compared with 0.58% of RA patients in 2011 (P < .001) (Table 2). With respect to specific arthroplasty types, there was an exponential rise in the utilization of reverse TSA beginning in 2010 and a corresponding decrease in the rates of both HA and anatomic TSA (Table 2, Figure). In addition to changes in shoulder arthroplasty utilization over time among RA patients, we also observed a significant increase in the number of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease (9.7% in 2002 to 15.2% in 2011, P < .001).
Table 2. The Annual Utilization of Shoulder Arthroplasty Among Patients with a Diagnosis of Rheumatoid Arthritis.
Proportion of RA patients |
| ||||
Year | Overall Rate of Shoulder Arthroplastya | HA | Anatomic TSA | Reverse TSA | |
2002 | 0.39 | 0.23 | 0.16 | 0 | |
2003 | 0.37 | 0.19 | 0.18 | 0 | |
2004 | 0.46 | 0.25 | 0.21 | 0 | |
2005 | 0.46 | 0.21 | 0.25 | 0 | |
2006 | 0.47 | 0.20 | 0.27 | 0 | |
2007 | 0.55 | 0.22 | 0.33 | 0 | |
2008 | 0.47 | 0.17 | 0.30 | 0 | |
2009 | 0.50 | 0.15 | 0.35 | 0 | |
2010 | 0.58 | 0.15 | 0.37 | 0.06 | |
2011 | 0.58 | 0.12 | 0.23 | 0.23 | |
Absolute number of RA patients |
| ||||
2002 | 1295 | 768 | 527 | 0 | |
2003 | 1247 | 650 | 597 | 0 | |
2004 | 1667 | 906 | 761 | 0 | |
2005 | 1722 | 776 | 946 | 0 | |
2006 | 1847 | 794 | 1053 | 0 | |
2007 | 2249 | 910 | 1339 | 0 | |
2008 | 2194 | 799 | 1395 | 0 | |
2009 | 2407 | 724 | 1683 | 0 | |
2010 | 2869 | 722 | 1857 | 290 | |
2011 | 3193 | 649 | 1261 | 1283 | |
aRate determined as number of RA patients undergoing shoulder arthroplasty compared to the number of patients with an RA diagnosis in the stated calendar year.
Abbreviations: HA, hemiarthroplasty; RA, rheumatoid arthritis; TSA, total shoulder arthroplasty.

Continue to: Among patients with RA...
Among patients with RA undergoing shoulder arthroplasty, the overall rate of early adverse events was 3.12%, of which the most common early adverse events were urinary tract infections (1.8%), acute kidney injury (0.66%), and pneumonia (0.38%) (Table 3). As compared with patients without a diagnosis of RA undergoing shoulder arthroplasty, there were no significant differences in the overall and individual rates of early adverse events (Table 3).
Table 3. A Comparison of Early Adverse Events, Length of Stay, and Cost Between Patients With and Without Rheumatoid Arthritis (RA) Undergoing Shoulder Arthroplasty
Comparison of Early Adverse Event Rates |
| ||||
| Non-RA Patients | RA Patients | Multivariate Logistic Regression | ||
Odds Ratio | P-Value | ||||
Overall adverse event rate | 3.02% | 3.12% | 1.0 | 0.83 | |
Specific adverse event rate |
|
|
|
| |
Death | 0.08% | 0.05% | 0.9 | 0.91 | |
Acute kidney injury | 0.85% | 0.66% | 0.9 | 0.59 | |
Cardiac arrest | 0.05% | 0.05% | 1.3 | 0.70 | |
Thromboembolic event | 0.01% | 0.00% | - | - | |
Myocardial Infarction | 0.22% | 0.06% | 0.4 | 0.17 | |
Peripheral nerve injury | 0.08% | 0.11% | 1.5 | 0.45 | |
Pneumonia | 0.47% | 0.38% | 0.9 | 0.70 | |
Sepsis | 0.08% | 0.08% | 1.3 | 0.62 | |
Stroke | 0.07% | 0.05% | 0.9 | 0.93 | |
Surgical site infection | 0.09% | 0.13% | 1.4 | 0.52 | |
Urinary tract infection | 1.44% | 1.80% | 1.1 | 0.46 | |
Wound dehiscence | 0.01% | 0.05% | 3.6 | 0.09 | |
Comparison of Length of Stay and Hospital Charges | |||||
| Non-RA Patients (percent) | RA Patients (percent) | Multivariate Linear Regression | ||
Beta | P-Value | ||||
Length of staya | 2.3±2.0 | 2.4±1.6 | +0.1 | 0.002 | |
Hospitalization costb | 14,826±8,336 | 14,787±7,625 | +93 | 0.59 | |
aReported in days. bReported in 2011 US dollars, adjusted for inflation.
The mean length of stay following shoulder arthroplasty in RA patients was 2.4 ± 1.6 days, and the mean hospitalization cost was $14,787 ± $7625 (Table 3). As compared with non-RA patients undergoing shoulder arthroplasty, there were no significant differences in the mean hospitalization costs; however, non-RA patients had a significantly shorter length of stay by 0.1 days (P = .002) (Table 3).
DISCUSSION
In this study, we observed that the utilization of shoulder arthroplasty in patients with RA increased significantly in the decade from 2002 to 2011, largely related to a rise in TSA. Interestingly, we also observed a corresponding rise in the proportion of RA patients undergoing shoulder arthroplasty with a diagnosis of rotator cuff disease, and we believe that this may partly account for the recent increase in the use of the reverse TSA in this patient population. Additionally, we found shoulder arthroplasty in RA patients to be safe in the early postoperative period, with no significant increase in cost as compared with patients undergoing shoulder arthroplasty without a diagnosis of RA. Although we did observe a significant increase in length of stay among RA patients as compared with non-RA patients, the absolute difference was only 0.1 days, and given the aforementioned similarities in cost between RA and non-RA patients, we do not believe this difference to be clinically significant.
It has been theorized that the utilization of TJA in RA patients has been decreasing with improvements in medical management; however, this is largely based upon literature pertaining to lower extremity TJA.2 On the contrary, past research pertaining to the utilization of shoulder arthroplasty in RA patients has been highly variable. For instance, a Swedish study demonstrated a statistically significant decrease in admissions associated with RA-related upper limb surgery and a stable rate of shoulder arthroplasty between 1998 and 2004.7 Similarly, a Finnish study demonstrated that the annual incidence of primary joint arthroplasty in RA patients had declined from 1995 to 2010, with a greater decline for upper-limb arthroplasty as compared with lower-limb arthroplasty.8 Despite these European observations, Jain and colleagues9 reported an increasing rate of TSA among RA patients in the US between the years 1992 and 2005. In this study, we demonstrate a clear increase in the utilization of shoulder arthroplasty among RA patients between 2002 and 2011. What was most striking about our observation was that the rise in utilization appeared to be driven by an increase in TSA, whereas the utilization of HA decreased over time. This change in practice likely reflects several factors, including the multitude of studies that have demonstrated improved outcomes with anatomic TSA as compared with HA in RA patients.10-14
Perhaps the most interesting aspect of our data was the recent exponential rise in the utilization of the reverse TSA. Despite improved outcomes following TSA as compared with HA in RA patients, these outcomes all appear to be highly dependent upon the integrity of the rotator cuff.10 In fact, there is evidence that failure of the rotator cuff could be as high as 75% within 10 years of TSA in patients with RA,15 which ultimately could jeopardize the long-term durability of the TSA implant in this patient population.11 For this reason, interest in the reverse TSA for the RA patient population has increased since its introduction in the US in 2004;16 in fact, in RA patients with end-stage inflammatory arthropathy and a damaged rotator cuff, the reverse TSA has demonstrated excellent results.17-20 Based upon this evidence, it is not surprising that we found an exponential rise in the use of the reverse TSA since 2010, which corresponds to the introduction of an ICD-9 code for this implant.21 Prior to 2010, it is likely that many implanted reverse TSAs were coded as TSA, and for this reason, we believe that the observed rise in the utilization of TSA in RA patients prior to 2010 may have been partly fueled by an increase in the use of the reverse TSA. To further support this theory, there was a dramatic decrease in the use of anatomic TSA following 2010, and we believe this was related to increased awareness of the newly introduced reverse TSA code among surgeons.
Another consideration when examining the utilization of shoulder arthroplasty in RA patients is its versatility in managing different disease states, including rotator cuff disease. As has been documented in the literature, outcomes of rotator cuff repair in RA patients are discouraging.22 For this reason, it is reasonable for surgeons and patients with RA to consider alternatives to rotator cuff repair when nonoperative management has failed to provide adequate improvement in symptoms. One alternative may be shoulder arthroplasty, namely the reverse TSA. In this study, we observed a significant increase in the rate of diagnosis of rotator cuff disease among RA patients undergoing shoulder arthroplasty from 2002 to 2011 (9.7% in 2002 to 15.2% in 2011, P < .001), and it is our belief that the simultaneous increase in the diagnosis of rotator cuff disease and use of TSA is not coincidental. More specifically, there is likely an emerging trend among surgeons toward using the reverse TSA to manage rotator cuff tears in the RA population, rather than undertaking a rotator cuff repair that carries a high rate of failure. Going forward, there is a need to not only identify this trend more clearly but to also compare the outcomes between reverse TSA and rotator cuff repair in the management of rotator cuff tears in RA patients.
Continue to: In this study, we observed...
In this study, we observed that RA patients undergoing shoulder arthroplasty were significantly younger than non-RA patients undergoing shoulder arthroplasty. At first, this observation seems to counter recent literature suggesting that the age of patients with inflammatory arthropathy undergoing TJA is increasing over time;1 however, looking more closely at the data, it becomes clearer that the mean age we report is actually a relative increase as compared with past clinical studies pertaining to RA patients undergoing shoulder arthroplasty (mean ages of 47 years,23 55 years,24 60 years,10 and 62 years25). On the other hand, the continued existence of an age gap between RA and non-RA patients undergoing shoulder arthroplasty may be the result of several possible phenomena. First, this may reflect issues with patient access to and coverage of expensive biologic antirheumatic medication that would otherwise mitigate disease progression. For instance, the out-of-pocket expense for biologic medication through Medicaid and Medicare is substantial,26 which has direct implications on over two-thirds of our RA cohort. Second, it may be skewed by the proportion of RA patients who have previously been or continue to be poorly managed, enabling disease progression to end-stage arthropathy at a younger age. Ultimately, further investigation is needed to determine the reasons for this continued age disparity.
In comparing RA and non-RA patients undergoing shoulder arthroplasty, we did not find a significant difference in the overall nor the individual rates of early adverse events. This finding appears to be unique, as similar studies pertaining to total knee arthroplasty (TKA) demonstrated a significantly higher incidence of postoperative pneumonia and bleeding requiring transfusion among RA patients as compared with non-RA patients.27 In patients with RA being treated with biologic medication and undergoing shoulder arthroplasty, the frequent concern in the postoperative period is the integrity of the wound and the potential for infection.28 In this study, we did not find a significant difference in the rate of early infection, and although the difference in the rate of early wound dehiscence approached significance, it did not meet the threshold of 0.05 (P = .09). This finding is in keeping with the aforementioned NIS study pertaining to TKA, and we believe that it likely reflects the short duration of follow-up for patients in both studies. Given the nature of the database we utilized, we were only privy to complications that arose during the inpatient hospital stay, and it is likely that the clear majority of patients who develop a postoperative infection or wound dehiscence do so in the postoperative setting following discharge. A second concern regarding postoperative wound complications is the management of biologic medication in the perioperative period, which we cannot determine using this database. Despite all these limitations specific to this database, a past systematic review of reverse TSA in RA patients found a low rate of deep infection after reverse TSA in RA patients (3.3%),17 which was not higher than that after shoulder arthroplasty performed in non-RA patients.
A final demonstration from this study is that the hospital length of stay was significantly longer for RA patients than non-RA patients undergoing shoulder arthroplasty; however, given that the difference was only 0.1 days, and there was no significant difference in hospitalization cost, we are inclined to believe that statistical significance may not translate into clinical significance in this scenario. Ultimately, we do believe that length of stay is an important consideration in the current healthcare system, and given our finding that shoulder arthroplasty in the RA patient is safe in the early postoperative period, that a prolonged postoperative hospitalization is not warranted on the sole basis of a patient’s history of RA.
As with all studies using data from a search of an administrative database, such as the NIS database, this study has limitations. First, this type of research is limited by the reliability of both diagnosis and procedural coding. Although the NIS database has demonstrated high reliability,3 it is still possible that events may have been miscoded. Second, the tracking period for adverse events is limited to the inpatient hospital stay, which may be too short to detect certain postoperative complications. As such, the rates we report are likely underestimates of the true incidence of these complications, but this is true for both the RA and non-RA populations. Third, the comparisons we draw between RA and non-RA patients are limited to the scope of the NIS database and the available data; as such, we could not draw comparisons between preoperative disease stage, intraoperative findings, and postoperative course following hospital discharge. Lastly, our data are limited to a distinct period between 2002 and 2011 and may not reflect current practice. Ultimately, our findings may underestimate current trends in shoulder arthroplasty utilization among RA patients, particularly for the reverse TSA.
CONCLUSION
In this study, we found that the utilization of shoulder arthroplasty in patients with RA increased significantly from 2002 to 2011, largely related to a rise in the utilization of TSA. Similarly, we observed a rise in the proportion of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease, and we believe the increased utilization of shoulder arthroplasty among RA patients resulted from management of both end-stage inflammatory arthropathy and rotator cuff disease. Although we did not find a significant difference between RA and non-RA patients in the rates of early adverse events and overall hospitalization costs following shoulder arthroplasty, length of stay was significantly longer among RA patients; however, the absolute difference does not appear to be clinically significant.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol. 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983-2007. Ann Rheum Dis. 2010;69(5):868-871. doi:10.1136/ard.2009.112474.
- HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality; 2002-2011.
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
- Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50(12):1109-1118. doi:10.1097/MLR.0b013e31825f64d0.
- van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. doi:10.1097/MLR.0b013e31819432e5.
- Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatol Oxf. 2008 ;47(4):491-494. doi. 10.1093/rheumatology/ken009.
- Jämsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 through 2010. Acta Orthop. 2013;84(4):331-337. doi:10.3109/17453674.2013.810519.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Barlow JD, Yuan BJ, Schleck CD, Harmsen WS, Cofield RH, Sperling JW. Shoulder arthroplasty for rheumatoid arthritis: 303 consecutive cases with minimum 5-year follow-up. J Shoulder Elbow Surg. 2014;23(6):791-799. doi:10.1016/j.jse.2013.09.016.
- Collins DN, Harryman DT, Wirth MA. Shoulder arthroplasty for the treatment of inflammatory arthritis. J Bone Joint Surg Am. 2004;86–A(11):2489-2496. doi:10.2106/00004623-200411000-00020.
- Rahme H, Mattsson P, Wikblad L, Larsson S. Cement and press-fit humeral stem fixation provides similar results in rheumatoid patients. Clin Orthop Relat Res. 2006;448:28-32. doi:10.1097/01.blo.0000224007.25636.85.
- Rozing PM, Nagels J, Rozing MP. Prognostic factors in arthroplasty in the rheumatoid shoulder. HSS J. 2011;7(1):29-36. doi:10.1007/s11420-010-9172-1.
- Sperling JW, Cofield RH, Schleck CD, Harmsen WS. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16(6):683-690. doi:10.1016/j.jse.2007.02.135.
- Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91(12):1594-1600. doi:10.1302/0301-620X.91B12.22139.
- Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747. doi:10.2106/JBJS.E.00851.
- Gee ECA, Hanson EK, Saithna A. Reverse shoulder arthroplasty in rheumatoid arthritis: A systematic review. Open Orthop J. 2015;9:237-245. doi:10.2174/1874325001509010237.
- Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2010;19(7):1076-1084. doi:10.1016/j.jse.2009.11.049.
- Postacchini R, Carbone S, Canero G, Ripani M, Postacchini F. Reverse shoulder prosthesis in patients with rheumatoid arthritis: a systematic review. Int Orthop. 2016;40(5):965-973. doi:10.1007/s00264-015-2916-2.
- Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22. doi:10.1067/mse.2001.110515.
- American Medical Association. American Medical Association Web site. www.ama-assn.org/ama. Accessed January 15, 2016.
- Smith AM, Sperling JW, Cofield RH. Rotator cuff repair in patients with rheumatoid arthritis. J Bone Joint Surg. 2005;87(8):1782-1787. doi:10.2106/JBJS.D.02452.
- Betts HM, Abu-Rajab R, Nunn T, Brooksbank AJ. Total shoulder replacement in rheumatoid disease: a 16- to 23-year follow-up. J Bone Joint Surg Br. 2009;91(9):1197-1200. doi:10.1302/0301-620X.91B9.22035.
- Geervliet PC, Somford MP, Winia P, van den Bekerom MP. Long-term results of shoulder hemiarthroplasty in patients with rheumatoid arthritis. Orthopedics. 2015;38(1):e38-e42. doi:10.3928/01477447-20150105-58.
- Hettrich CM, Weldon E III, Boorman RS, Parsons M IV, Matsen FA III. Preoperative factors associated with improvements in shoulder function after humeral hemiarthroplasty. J Bone Joint Surg. 2004;86–A(7):1446-1451.
- Yazdany J, Dudley RA, Chen R, Lin GA, Tseng CW. Coverage for high-cost specialty drugs for rheumatoid arthritis in Medicare Part D. Arthritis Rheumatol. 2015;67(6):1474-1480. doi:10.1002/art.39079.
- Jauregui JJ, Kapadia BH, Dixit A, et al. Thirty-day complications in rheumatoid patients following total knee arthroplasty. Clin Rheumatol. 2016;35(3):595-600. doi:10.1007/s10067-015-3037-4.
- Trail IA, Nuttall D. The results of shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Br. 2002;84(8):1121-1125. doi:10.1302/0301-620X.84B8.0841121
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol. 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983-2007. Ann Rheum Dis. 2010;69(5):868-871. doi:10.1136/ard.2009.112474.
- HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality; 2002-2011.
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
- Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50(12):1109-1118. doi:10.1097/MLR.0b013e31825f64d0.
- van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. doi:10.1097/MLR.0b013e31819432e5.
- Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatol Oxf. 2008 ;47(4):491-494. doi. 10.1093/rheumatology/ken009.
- Jämsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 through 2010. Acta Orthop. 2013;84(4):331-337. doi:10.3109/17453674.2013.810519.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Barlow JD, Yuan BJ, Schleck CD, Harmsen WS, Cofield RH, Sperling JW. Shoulder arthroplasty for rheumatoid arthritis: 303 consecutive cases with minimum 5-year follow-up. J Shoulder Elbow Surg. 2014;23(6):791-799. doi:10.1016/j.jse.2013.09.016.
- Collins DN, Harryman DT, Wirth MA. Shoulder arthroplasty for the treatment of inflammatory arthritis. J Bone Joint Surg Am. 2004;86–A(11):2489-2496. doi:10.2106/00004623-200411000-00020.
- Rahme H, Mattsson P, Wikblad L, Larsson S. Cement and press-fit humeral stem fixation provides similar results in rheumatoid patients. Clin Orthop Relat Res. 2006;448:28-32. doi:10.1097/01.blo.0000224007.25636.85.
- Rozing PM, Nagels J, Rozing MP. Prognostic factors in arthroplasty in the rheumatoid shoulder. HSS J. 2011;7(1):29-36. doi:10.1007/s11420-010-9172-1.
- Sperling JW, Cofield RH, Schleck CD, Harmsen WS. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16(6):683-690. doi:10.1016/j.jse.2007.02.135.
- Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91(12):1594-1600. doi:10.1302/0301-620X.91B12.22139.
- Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747. doi:10.2106/JBJS.E.00851.
- Gee ECA, Hanson EK, Saithna A. Reverse shoulder arthroplasty in rheumatoid arthritis: A systematic review. Open Orthop J. 2015;9:237-245. doi:10.2174/1874325001509010237.
- Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2010;19(7):1076-1084. doi:10.1016/j.jse.2009.11.049.
- Postacchini R, Carbone S, Canero G, Ripani M, Postacchini F. Reverse shoulder prosthesis in patients with rheumatoid arthritis: a systematic review. Int Orthop. 2016;40(5):965-973. doi:10.1007/s00264-015-2916-2.
- Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22. doi:10.1067/mse.2001.110515.
- American Medical Association. American Medical Association Web site. www.ama-assn.org/ama. Accessed January 15, 2016.
- Smith AM, Sperling JW, Cofield RH. Rotator cuff repair in patients with rheumatoid arthritis. J Bone Joint Surg. 2005;87(8):1782-1787. doi:10.2106/JBJS.D.02452.
- Betts HM, Abu-Rajab R, Nunn T, Brooksbank AJ. Total shoulder replacement in rheumatoid disease: a 16- to 23-year follow-up. J Bone Joint Surg Br. 2009;91(9):1197-1200. doi:10.1302/0301-620X.91B9.22035.
- Geervliet PC, Somford MP, Winia P, van den Bekerom MP. Long-term results of shoulder hemiarthroplasty in patients with rheumatoid arthritis. Orthopedics. 2015;38(1):e38-e42. doi:10.3928/01477447-20150105-58.
- Hettrich CM, Weldon E III, Boorman RS, Parsons M IV, Matsen FA III. Preoperative factors associated with improvements in shoulder function after humeral hemiarthroplasty. J Bone Joint Surg. 2004;86–A(7):1446-1451.
- Yazdany J, Dudley RA, Chen R, Lin GA, Tseng CW. Coverage for high-cost specialty drugs for rheumatoid arthritis in Medicare Part D. Arthritis Rheumatol. 2015;67(6):1474-1480. doi:10.1002/art.39079.
- Jauregui JJ, Kapadia BH, Dixit A, et al. Thirty-day complications in rheumatoid patients following total knee arthroplasty. Clin Rheumatol. 2016;35(3):595-600. doi:10.1007/s10067-015-3037-4.
- Trail IA, Nuttall D. The results of shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Br. 2002;84(8):1121-1125. doi:10.1302/0301-620X.84B8.0841121
TAKE-HOME POINTS
- There was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly TSA.
- There was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease.
- There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients.
- Non-RA patients had a significantly shorter length of stay.
- The utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Continuous Cryotherapy vs Ice Following Total Shoulder Arthroplasty: A Randomized Control Trial
ABSTRACT
Postoperative pain management is an important component of total shoulder arthroplasty (TSA). Continuous cryotherapy (CC) has been proposed as a means of improving postoperative pain control. However, CC represents an increased cost not typically covered by insurance. The purpose of this study is to compare CC to plain ice (ICE) following TSA. The hypothesis was that CC would lead to lower pain scores and decreased narcotic usage during the first 2 weeks postoperatively.
A randomized controlled trial was performed to compare CC to ICE. Forty patients were randomized to receive either CC or ICE following TSA. The rehabilitation and pain control protocols were otherwise standardized. Visual analog scales (VAS) for pain, satisfaction with cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery. Narcotic usage in morphine equivalents was also recorded.
No significant differences in preoperative pain (5.9 vs 6.8; P = .121), or postoperative pain at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593) or 14 days (2.5 vs 2.7; P = .742) were observed between the CC and ICE groups. Similarly, no differences in quality of sleep, satisfaction with the cold therapy, or narcotic usage at any time interval were observed between the 2 groups.
No differences in pain control, quality of sleep, patient satisfaction, or narcotic usage were detected between CC and ICE following TSA. CC may offer convenience as an advantage, but the increased cost associated with this type of treatment may not be justified.
The number of total shoulder arthroplasties (TSAs) performed annually is increasing dramatically.1 At the same time, there has been a push toward decreased length of hospital stay and earlier mobilization following joint replacement surgery. Central to these goals is adequate pain control. Multimodal pain pathways exist, and one of the safest and cheapest methods of pain control is cold therapy, which can be accomplished with continuous cryotherapy (CC) or plain ice (ICE).
Continue to: The mechanism of cryotherapy...
The mechanism of cryotherapy for controlling pain is poorly understood. Cryotherapy reduces leukocyte migration and slows down nerve signal transmission, which reduces inflammation, thereby producing a short-term analgesic effect. Stalman and colleagues2 reported on a randomized control study that evaluated the effects of postoperative cooling after knee arthroscopy. Measurements of metabolic and inflammatory markers in the synovial membrane were used to assess whether cryotherapy provides a temperature-sensitive release of prostaglandin E2. Cryotherapy lowered the temperature in the postoperative knee, and synovial prostaglandin concentrations were correlated with temperature. Because prostaglandin is a marker of inflammation and pain, the conclusion was that postoperative cooling appeared to have an anti-inflammatory effect.
The knee literature contains multiple studies that have examined the benefits of cryotherapy after both arthroscopic and arthroplasty procedures. The clinical benefits on pain have been equivocal with some studies showing improvements using cryotherapy3,4 and others showing no difference in the treatment group.5,6
Few studies have examined cryotherapy for the shoulder. Speer and colleagues7 demonstrated that postoperative use of CC was effective in reducing recovery time after shoulder surgery. However; they did not provide an ICE comparative group and did not focus specifically on TSA. In another study, Kraeutler and colleagues8 examined only arthroscopic shoulder surgery cases in a randomized prospective trial and found no significant different between CC and ICE. They concluded that there did not appear to be a significant benefit in using CC over ICE for arthroscopic shoulder procedures.
The purpose of this study is to prospectively evaluate CC and ICE following TSA. The hypothesis was that CC leads to improved pain control, less narcotic consumption, and improved quality of sleep compared to ICE in the immediate postoperative period following TSA.
MATERIALS AND METHODS
This was a prospective randomized control study of patients undergoing TSA receiving either CC or ICE postoperatively. Institutional Review Board approval was obtained before commencement of the study. Inclusion criteria included patients aged 30 to 90 years old undergoing a primary or revision shoulder arthroplasty procedure between June 2015 and January 2016. Exclusion criteria included hemiarthroplasty procedures.
Continue to: Three patients refused...
Three patients refused to participate in the study. Enrollment was performed until 40 patients were enrolled in the study (20 patients in each group). Randomization was performed with a random number generator, and patients were assigned to a treatment group following consent to participate. Complete follow-up was available for all patients. There were 13 (65%) male patients in the CC group. The average age of the CC group at the time of surgery was 68.7 years (range). There were 11 male patients in the ICE group. The average age of the ICE group at the time of surgery was 73.2 years (range). The dominant extremity was involved in 9 (45%) patients in the CC group and in 11 patients (55%) in the ICE group. Surgical case specifics are summarized in Table 1.
Table 1. Summary of Surgical Cases
| CC group (n = 20) | ICE group (n = 20) |
Primary TSA | 7 (35%) | 9 (45%) |
Primary RSA | 12 (60%) | 9 (45%) |
Revision arthroplasty | 1 (5%) | 2 (10%) |
Abbreviations: CC, continuous cryotherapy; ICE, plain ice; RSA, reverse shoulder arthroplasty; TSA, total shoulder arthroplasty.
All surgeries were performed by Dr. Denard. All patients received a single-shot interscalene nerve block prior to the procedure. A deltopectoral approach was utilized, and the subscapularis was managed with the peel technique.9 All patients were admitted to the hospital following surgery. Standard postoperative pain control consisted of as-needed intravenous morphine (1-2 mg every 2 hours, as needed) or an oral narcotic (hydrocodone/acetaminophen 5/325mg, 1-2 every 4 hours, as needed) which was also provided at discharge. However, total narcotic usage was recorded in morphine equivalents to account for substitutions. No non-steroidal anti-inflammatory drugs were allowed until 3 months postoperatively.
The CC group received treatment from a commercially available cryotherapy unit (Polar Care; Breg). All patients received instructions by a medical professional on how to use the unit. The unit was applied immediately postoperatively and set at a temperature of 45°F to 55°F. Patients were instructed to use the unit continuously during postoperative days 0 to 3. This cryotherapy was administered by a nurse while in the hospital but was left to the responsibility of the patient upon discharge. Patients were instructed to use the unit as needed for pain control during the day and continuously while asleep from days 4 to14.
The ICE group used standard ice packs postoperatively. The patients were instructed to apply an ice pack for 20 min every 2 hours while awake during days 0 to 3. This therapy was administered by a nurse while in the hospital but left to the responsibility of the patient upon discharge. Patients were instructed to use ice packs as needed for pain control during the day at a maximum of 20 minutes per hour on postoperative days 4 to 14. Compliance by both groups was monitored using a patient survey after hospital discharge. The number of hours that patients used either the CC or ICE per 24-hour period was recorded at 24 hours, 3 days, 7 days, and 14 days. The nursing staff recorded the number of hours of use of either cold modality for each patient prior to hospital discharge. The average length of stay as an inpatient was 1.2 days for the CC group and 1.3 days for the ICE group.
Visual analog scales (VAS) for pain, satisfaction with the cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery.
Continue to: The Wilcoxon rank-sum test...
STATISTICAL METHOD
The Wilcoxon rank-sum test was used to assess whether scores changed significantly from the preoperative period to the different postoperative time intervals, as well as to assess the values for pain, quality of sleep, and patient satisfaction. P-values <.05 were considered significant.
RESULTS
No differences were observed in the baseline characteristics between the 2 groups. Both groups showed improvements in pain, quality of sleep, and satisfaction with the cold therapy from the preoperative period to the final follow-up.
The VAS pain scores were not different between the CC and ICE groups preoperatively (5.9 vs 6.8; P = .121) or postoperatively at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593), or 14 days (2.5 vs 2.7; P = .742). Both cohorts demonstrated improved overall pain throughout the study period. These findings are summarized in Table 2.
Table 2. Summary of VAS Pain Scores With Cold Therapy
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
Preoperative | 5.9 ± 4.1 | 6.8 ± 5.3 | .121 | 3.3-8.3 |
24 hours | 4.2 ± 3.0 | 4.3 ± 3.1 | .989 | 2.9-5.7 |
3 days | 4.8 ± 2.7 | 4.7 ± 3.2 | .944 | 3.2-6.3 |
7 days | 2.9 ± 1.8 | 3.3 ± 2.5 | .593 | 2.1-4.4 |
14 days | 2.5 ± 2.1 | 2.7 ± 1.8 | .742 | 1.5-3.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
The number of morphine equivalents of pain medication was not different between the CC and ICE groups postoperatively at 24 hours (43 vs 38 mg; P = .579), 3 days (149 vs 116 mg; P = .201), 7 days (308 vs 228 mg; P = .181), or 14 days (431 vs 348 mg; P = .213). Both groups showed increased narcotic consumption from 24 hours postoperatively until the 2-week follow-up. Narcotic consumption is summarized in Table 3.
Table 3. Summary of Narcotic Consumption in Morphine Equivalents
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 43.0 ± 36.7 | 38.0 ± 42.9 | .579 | 17.9-60.1 |
3 days | 149.0 ± 106.5 | 116.3 ± 108.9 | .201 | 63.4-198.7 |
7 days | 308.1 ± 234.0 | 228 ± 258.3 | .181 | 107.1-348.9 |
14 days | 430.8 ± 384.2 | 347.5 ± 493.4 | .213 | 116.6-610.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice.
VAS for quality of sleep improved in both groups from 24 hours postoperatively until the final follow-up. However, no significant differences in sleep quality were observed between the CC and ICE groups postoperatively at 24 hours (5.1 vs 4.3; P = .382), 3 days (5.1 vs 5.3; P = .601), 7 days (6.0 vs 6.7; P = .319), or 14 days (6.5 vs 7.1; P = .348). The VAS scores for sleep quality are reported in Table 4.
Table 4. Summary of VAS Sleep Quality With Cold Therapya
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 5.1 ± 2.8 | 4.3 ± 2.4 | .382 | 3.2-6.4 |
3 days | 5.1 ± 1.9 | 5.3 ± 2.3 | .601 | 4.2-6.5 |
7 days | 6.0 ± 2.3 | 6.7 ± 2.1 | .319 | 4.9-7.7 |
14 days | 6.5 ± 2.3 | 7.1 ± 2.5 | .348 | 5.3-8.4 |
a0-10 rating with 10 being the highest possible score.
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
Continue to: Finally, VAS patient satisfaction...
Finally, VAS patient satisfaction scores were not different between the CC and ICE groups postoperatively at 24 hours (7.3 vs 6.1; P = .315), 3 days (6.1 vs 6.6; P = .698), 7 days (6.6 vs 6.9; P = .670), or 14 days (7.1 vs 6.3; P = .288).
While compliance within each group utilizing the randomly assigned cold modality was similar, the usage by the CC group was consistently higher at all time points recorded. No complications or reoperations were observed in either group.
DISCUSSION
The optimal method for managing postoperative pain from an arthroplasty procedure is controversial. This prospective randomized study attempted to confirm the hypothesis that CC infers better pain control, improves quality of sleep, and decreases narcotic usage compared to ICE in the first 2 weeks after a TSA procedure. The results of this study refuted our hypothesis, demonstrating no significant difference in pain control, satisfaction, narcotic usage, or sleep quality between the CC and ICE cohorts at all time points studied.
Studies on knees and lower extremities demonstrate equivocal results for the role CC plays in providing improved postoperative pain control. Thienpont10 evaluated CC in a randomized control trial comparing plain ice packs postoperatively in patients who underwent TKA. The author found no significant difference in VAS for pain or narcotic consumption in morphine equivalents. Thienpont10 recommended that CC not be used for outpatient knee arthroplasty as it is an additional cost that does not improve pain significantly. Healy and colleagues5 reported similar results that CC did not demonstrate a difference in narcotic requirement or pain control compared to plain ice packs, as well as no difference in local postoperative swelling or wound drainage. However, a recently published randomized trial by Su and colleagues11 comparing a cryopneumatic device and ICE with static compression in patients who underwent TKA demonstrated significantly lower narcotic consumption and increased ambulation distances in the treatment group. The treatment group consumed approximately 170 mg morphine equivalents less than the control group between discharge and the 2-week postoperative visit. In addition, a significant difference was observed in the satisfaction scores in the treatment group.11 Similarly, a meta-analysis by Raynor and colleagues12 on randomized clinical trials comparing cryotherapy to a placebo group after anterior cruciate ligament reconstruction showed that cryotherapy is associated with significantly lower postoperative pain (P = .02), but demonstrated no difference in postoperative drainage (P = .23) or range of motion (P = .25).
Although multiple studies have been published regarding the efficacy of cryotherapy after knee surgery, very few studies have compared CC to conventional ICE after shoulder surgery. A prospective randomized trial was performed by Singh and colleagues13 to compare CC vs no ICE in open and arthroscopic shoulder surgery patients. Both the open and arthroscopic groups receiving CC demonstrated significant reductions in pain frequency and more restful sleep at the 7-day, 14-day, and 21-day intervals compared to the control group. However, they did not compare the commercial unit to ICE. In contrast, a study by Kraeutler and colleagues8 randomized 46 patients to receive either CC or ICE in the setting of arthroscopic shoulder surgery. Although no significant difference was observed in morphine equivalent dosage between the 2 groups, the CC group used more pain medication on every postoperative day during the first week after surgery. They found no difference between the 2 groups with regards to narcotic consumption or pain scores. The results of this study mirror those by Kraeutler and colleagues,8 demonstrating no difference in pain scores, sleep quality, or narcotic consumption.
Continue to: With rising costs in the US...
With rising costs in the US healthcare system, a great deal of interest has developed in the application of value-based principles to healthcare. Value can be defined as a gain in benefits over the costs expended.14 The average cost for a commercial CC unit used in this study was $260. A pack of ICE is a nominal cost. Based on the results of this study, the cost of the commercial CC device may not be justified when compared to the cost of an ice pack.
The major strengths of this study are the randomized design and multiple data points during the early postoperative period. However, there are several limitations. First, we did not objectively measure compliance of either therapy and relied only on a patient survey. Usage of the commercial CC unit in hours decreased over half between days 3 and 14. This occurred despite training on the application and specific instructions. We believe this reflects “real-world” usage, but it is possible that compliance affected our results. Second, all patients in this study had a single-shot interscalene block. While this is standard at our institution, it is possible that either CC or ICE would have a more significant effect in the absence of an interscalene block. Finally, we did not evaluate final outcomes in this study and therefore cannot determine if the final outcome was different between the 2 groups. Our goal was simply to evaluate the first 2 weeks following surgery, as this is the most painful period following TSA.
CONCLUSION
There was no difference between CC and ICE in terms of pain control, quality of sleep, patient satisfaction, or narcotic consumption following TSA. CC may offer convenience advantages, but the increased cost associated with this type of unit may not be justified.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/jbjs.j.01994.
2. Stalman A, Berglund L, Dungnerc E, Arner P, Fellander-Tsai L. Temperature sensitive release of prostaglandin E2 and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2016/jbjs.j.01790.
3. Levy AS, Marmar E. The role of cold compression dressings in the postoperative treatment of total knee arthroplasty. Clin Orthop Relat Res. 1993;297:174-178. doi:10.1097/00003086-199312000-00029.
4. Webb JM, Williams D, Ivory JP, Day S, Williamson DM. The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopaedics 1998;21(1):59-61.
5. Healy WL, Seidman J, Pfeifer BA, Brown DG. Cold compressive dressing after total knee arthroplasty. Clin Orthop Relat Res. 1994;299:143-146. doi:10.1097/00003086-199402000-00019.
6. Whitelaw GP, DeMuth KA, Demos HA, Schepsis A, Jacques E. The use of Cryo/Cuff versus ice and elastic wrap in the postoperative care of knee arthroscopy patients. Am J Knee Surg. 1995;8(1):28-30.
7. Speer KP, Warren RF, Horowitz L. The efficacy of cryotherapy in the postoperative shoulder. J Shoulder Elbow Surg. 1996;5(1):62-68. doi:10.16/s1058-2746(96)80032-2.
8. Kraeutler MJ, Reynolds KA, Long C, McCarthy EC. Compressive cryotherapy versus ice- a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elbow Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
9. DeFranco MJ, Higgins LD, Warner JP. Subscapularis management in open shoulder surgery. J Am Acad Orthop Surg. 2010;18(12):707-717. doi:10.5435/00124635-201012000-00001.
10. Thienpont E. Does advanced cryotherapy reduce pain and narcotic consumption after knee arthroplasty. Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
11. Su EP, Perna M, Boettner F, Mayman DJ, et al. A prospective, multicenter, randomized trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620x.94B11.30832.
12. Raynor MC, Pietrobon R, Guller U, Higgins LD. Cryotherapy after ACL reconstruction- a meta analysis. J Knee Surg. 2005;18(2):123-129. doi:10.1055/s-0030-1248169.
13. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: a prospective randomized investigation. J Shoulder Elbow Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
14. Black EM, Higgins LD, Warner JP. Value based shoulder surgery: outcomes driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1-10. doi:10.1016/j.se.2013.02.008.
ABSTRACT
Postoperative pain management is an important component of total shoulder arthroplasty (TSA). Continuous cryotherapy (CC) has been proposed as a means of improving postoperative pain control. However, CC represents an increased cost not typically covered by insurance. The purpose of this study is to compare CC to plain ice (ICE) following TSA. The hypothesis was that CC would lead to lower pain scores and decreased narcotic usage during the first 2 weeks postoperatively.
A randomized controlled trial was performed to compare CC to ICE. Forty patients were randomized to receive either CC or ICE following TSA. The rehabilitation and pain control protocols were otherwise standardized. Visual analog scales (VAS) for pain, satisfaction with cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery. Narcotic usage in morphine equivalents was also recorded.
No significant differences in preoperative pain (5.9 vs 6.8; P = .121), or postoperative pain at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593) or 14 days (2.5 vs 2.7; P = .742) were observed between the CC and ICE groups. Similarly, no differences in quality of sleep, satisfaction with the cold therapy, or narcotic usage at any time interval were observed between the 2 groups.
No differences in pain control, quality of sleep, patient satisfaction, or narcotic usage were detected between CC and ICE following TSA. CC may offer convenience as an advantage, but the increased cost associated with this type of treatment may not be justified.
The number of total shoulder arthroplasties (TSAs) performed annually is increasing dramatically.1 At the same time, there has been a push toward decreased length of hospital stay and earlier mobilization following joint replacement surgery. Central to these goals is adequate pain control. Multimodal pain pathways exist, and one of the safest and cheapest methods of pain control is cold therapy, which can be accomplished with continuous cryotherapy (CC) or plain ice (ICE).
Continue to: The mechanism of cryotherapy...
The mechanism of cryotherapy for controlling pain is poorly understood. Cryotherapy reduces leukocyte migration and slows down nerve signal transmission, which reduces inflammation, thereby producing a short-term analgesic effect. Stalman and colleagues2 reported on a randomized control study that evaluated the effects of postoperative cooling after knee arthroscopy. Measurements of metabolic and inflammatory markers in the synovial membrane were used to assess whether cryotherapy provides a temperature-sensitive release of prostaglandin E2. Cryotherapy lowered the temperature in the postoperative knee, and synovial prostaglandin concentrations were correlated with temperature. Because prostaglandin is a marker of inflammation and pain, the conclusion was that postoperative cooling appeared to have an anti-inflammatory effect.
The knee literature contains multiple studies that have examined the benefits of cryotherapy after both arthroscopic and arthroplasty procedures. The clinical benefits on pain have been equivocal with some studies showing improvements using cryotherapy3,4 and others showing no difference in the treatment group.5,6
Few studies have examined cryotherapy for the shoulder. Speer and colleagues7 demonstrated that postoperative use of CC was effective in reducing recovery time after shoulder surgery. However; they did not provide an ICE comparative group and did not focus specifically on TSA. In another study, Kraeutler and colleagues8 examined only arthroscopic shoulder surgery cases in a randomized prospective trial and found no significant different between CC and ICE. They concluded that there did not appear to be a significant benefit in using CC over ICE for arthroscopic shoulder procedures.
The purpose of this study is to prospectively evaluate CC and ICE following TSA. The hypothesis was that CC leads to improved pain control, less narcotic consumption, and improved quality of sleep compared to ICE in the immediate postoperative period following TSA.
MATERIALS AND METHODS
This was a prospective randomized control study of patients undergoing TSA receiving either CC or ICE postoperatively. Institutional Review Board approval was obtained before commencement of the study. Inclusion criteria included patients aged 30 to 90 years old undergoing a primary or revision shoulder arthroplasty procedure between June 2015 and January 2016. Exclusion criteria included hemiarthroplasty procedures.
Continue to: Three patients refused...
Three patients refused to participate in the study. Enrollment was performed until 40 patients were enrolled in the study (20 patients in each group). Randomization was performed with a random number generator, and patients were assigned to a treatment group following consent to participate. Complete follow-up was available for all patients. There were 13 (65%) male patients in the CC group. The average age of the CC group at the time of surgery was 68.7 years (range). There were 11 male patients in the ICE group. The average age of the ICE group at the time of surgery was 73.2 years (range). The dominant extremity was involved in 9 (45%) patients in the CC group and in 11 patients (55%) in the ICE group. Surgical case specifics are summarized in Table 1.
Table 1. Summary of Surgical Cases
| CC group (n = 20) | ICE group (n = 20) |
Primary TSA | 7 (35%) | 9 (45%) |
Primary RSA | 12 (60%) | 9 (45%) |
Revision arthroplasty | 1 (5%) | 2 (10%) |
Abbreviations: CC, continuous cryotherapy; ICE, plain ice; RSA, reverse shoulder arthroplasty; TSA, total shoulder arthroplasty.
All surgeries were performed by Dr. Denard. All patients received a single-shot interscalene nerve block prior to the procedure. A deltopectoral approach was utilized, and the subscapularis was managed with the peel technique.9 All patients were admitted to the hospital following surgery. Standard postoperative pain control consisted of as-needed intravenous morphine (1-2 mg every 2 hours, as needed) or an oral narcotic (hydrocodone/acetaminophen 5/325mg, 1-2 every 4 hours, as needed) which was also provided at discharge. However, total narcotic usage was recorded in morphine equivalents to account for substitutions. No non-steroidal anti-inflammatory drugs were allowed until 3 months postoperatively.
The CC group received treatment from a commercially available cryotherapy unit (Polar Care; Breg). All patients received instructions by a medical professional on how to use the unit. The unit was applied immediately postoperatively and set at a temperature of 45°F to 55°F. Patients were instructed to use the unit continuously during postoperative days 0 to 3. This cryotherapy was administered by a nurse while in the hospital but was left to the responsibility of the patient upon discharge. Patients were instructed to use the unit as needed for pain control during the day and continuously while asleep from days 4 to14.
The ICE group used standard ice packs postoperatively. The patients were instructed to apply an ice pack for 20 min every 2 hours while awake during days 0 to 3. This therapy was administered by a nurse while in the hospital but left to the responsibility of the patient upon discharge. Patients were instructed to use ice packs as needed for pain control during the day at a maximum of 20 minutes per hour on postoperative days 4 to 14. Compliance by both groups was monitored using a patient survey after hospital discharge. The number of hours that patients used either the CC or ICE per 24-hour period was recorded at 24 hours, 3 days, 7 days, and 14 days. The nursing staff recorded the number of hours of use of either cold modality for each patient prior to hospital discharge. The average length of stay as an inpatient was 1.2 days for the CC group and 1.3 days for the ICE group.
Visual analog scales (VAS) for pain, satisfaction with the cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery.
Continue to: The Wilcoxon rank-sum test...
STATISTICAL METHOD
The Wilcoxon rank-sum test was used to assess whether scores changed significantly from the preoperative period to the different postoperative time intervals, as well as to assess the values for pain, quality of sleep, and patient satisfaction. P-values <.05 were considered significant.
RESULTS
No differences were observed in the baseline characteristics between the 2 groups. Both groups showed improvements in pain, quality of sleep, and satisfaction with the cold therapy from the preoperative period to the final follow-up.
The VAS pain scores were not different between the CC and ICE groups preoperatively (5.9 vs 6.8; P = .121) or postoperatively at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593), or 14 days (2.5 vs 2.7; P = .742). Both cohorts demonstrated improved overall pain throughout the study period. These findings are summarized in Table 2.
Table 2. Summary of VAS Pain Scores With Cold Therapy
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
Preoperative | 5.9 ± 4.1 | 6.8 ± 5.3 | .121 | 3.3-8.3 |
24 hours | 4.2 ± 3.0 | 4.3 ± 3.1 | .989 | 2.9-5.7 |
3 days | 4.8 ± 2.7 | 4.7 ± 3.2 | .944 | 3.2-6.3 |
7 days | 2.9 ± 1.8 | 3.3 ± 2.5 | .593 | 2.1-4.4 |
14 days | 2.5 ± 2.1 | 2.7 ± 1.8 | .742 | 1.5-3.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
The number of morphine equivalents of pain medication was not different between the CC and ICE groups postoperatively at 24 hours (43 vs 38 mg; P = .579), 3 days (149 vs 116 mg; P = .201), 7 days (308 vs 228 mg; P = .181), or 14 days (431 vs 348 mg; P = .213). Both groups showed increased narcotic consumption from 24 hours postoperatively until the 2-week follow-up. Narcotic consumption is summarized in Table 3.
Table 3. Summary of Narcotic Consumption in Morphine Equivalents
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 43.0 ± 36.7 | 38.0 ± 42.9 | .579 | 17.9-60.1 |
3 days | 149.0 ± 106.5 | 116.3 ± 108.9 | .201 | 63.4-198.7 |
7 days | 308.1 ± 234.0 | 228 ± 258.3 | .181 | 107.1-348.9 |
14 days | 430.8 ± 384.2 | 347.5 ± 493.4 | .213 | 116.6-610.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice.
VAS for quality of sleep improved in both groups from 24 hours postoperatively until the final follow-up. However, no significant differences in sleep quality were observed between the CC and ICE groups postoperatively at 24 hours (5.1 vs 4.3; P = .382), 3 days (5.1 vs 5.3; P = .601), 7 days (6.0 vs 6.7; P = .319), or 14 days (6.5 vs 7.1; P = .348). The VAS scores for sleep quality are reported in Table 4.
Table 4. Summary of VAS Sleep Quality With Cold Therapya
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 5.1 ± 2.8 | 4.3 ± 2.4 | .382 | 3.2-6.4 |
3 days | 5.1 ± 1.9 | 5.3 ± 2.3 | .601 | 4.2-6.5 |
7 days | 6.0 ± 2.3 | 6.7 ± 2.1 | .319 | 4.9-7.7 |
14 days | 6.5 ± 2.3 | 7.1 ± 2.5 | .348 | 5.3-8.4 |
a0-10 rating with 10 being the highest possible score.
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
Continue to: Finally, VAS patient satisfaction...
Finally, VAS patient satisfaction scores were not different between the CC and ICE groups postoperatively at 24 hours (7.3 vs 6.1; P = .315), 3 days (6.1 vs 6.6; P = .698), 7 days (6.6 vs 6.9; P = .670), or 14 days (7.1 vs 6.3; P = .288).
While compliance within each group utilizing the randomly assigned cold modality was similar, the usage by the CC group was consistently higher at all time points recorded. No complications or reoperations were observed in either group.
DISCUSSION
The optimal method for managing postoperative pain from an arthroplasty procedure is controversial. This prospective randomized study attempted to confirm the hypothesis that CC infers better pain control, improves quality of sleep, and decreases narcotic usage compared to ICE in the first 2 weeks after a TSA procedure. The results of this study refuted our hypothesis, demonstrating no significant difference in pain control, satisfaction, narcotic usage, or sleep quality between the CC and ICE cohorts at all time points studied.
Studies on knees and lower extremities demonstrate equivocal results for the role CC plays in providing improved postoperative pain control. Thienpont10 evaluated CC in a randomized control trial comparing plain ice packs postoperatively in patients who underwent TKA. The author found no significant difference in VAS for pain or narcotic consumption in morphine equivalents. Thienpont10 recommended that CC not be used for outpatient knee arthroplasty as it is an additional cost that does not improve pain significantly. Healy and colleagues5 reported similar results that CC did not demonstrate a difference in narcotic requirement or pain control compared to plain ice packs, as well as no difference in local postoperative swelling or wound drainage. However, a recently published randomized trial by Su and colleagues11 comparing a cryopneumatic device and ICE with static compression in patients who underwent TKA demonstrated significantly lower narcotic consumption and increased ambulation distances in the treatment group. The treatment group consumed approximately 170 mg morphine equivalents less than the control group between discharge and the 2-week postoperative visit. In addition, a significant difference was observed in the satisfaction scores in the treatment group.11 Similarly, a meta-analysis by Raynor and colleagues12 on randomized clinical trials comparing cryotherapy to a placebo group after anterior cruciate ligament reconstruction showed that cryotherapy is associated with significantly lower postoperative pain (P = .02), but demonstrated no difference in postoperative drainage (P = .23) or range of motion (P = .25).
Although multiple studies have been published regarding the efficacy of cryotherapy after knee surgery, very few studies have compared CC to conventional ICE after shoulder surgery. A prospective randomized trial was performed by Singh and colleagues13 to compare CC vs no ICE in open and arthroscopic shoulder surgery patients. Both the open and arthroscopic groups receiving CC demonstrated significant reductions in pain frequency and more restful sleep at the 7-day, 14-day, and 21-day intervals compared to the control group. However, they did not compare the commercial unit to ICE. In contrast, a study by Kraeutler and colleagues8 randomized 46 patients to receive either CC or ICE in the setting of arthroscopic shoulder surgery. Although no significant difference was observed in morphine equivalent dosage between the 2 groups, the CC group used more pain medication on every postoperative day during the first week after surgery. They found no difference between the 2 groups with regards to narcotic consumption or pain scores. The results of this study mirror those by Kraeutler and colleagues,8 demonstrating no difference in pain scores, sleep quality, or narcotic consumption.
Continue to: With rising costs in the US...
With rising costs in the US healthcare system, a great deal of interest has developed in the application of value-based principles to healthcare. Value can be defined as a gain in benefits over the costs expended.14 The average cost for a commercial CC unit used in this study was $260. A pack of ICE is a nominal cost. Based on the results of this study, the cost of the commercial CC device may not be justified when compared to the cost of an ice pack.
The major strengths of this study are the randomized design and multiple data points during the early postoperative period. However, there are several limitations. First, we did not objectively measure compliance of either therapy and relied only on a patient survey. Usage of the commercial CC unit in hours decreased over half between days 3 and 14. This occurred despite training on the application and specific instructions. We believe this reflects “real-world” usage, but it is possible that compliance affected our results. Second, all patients in this study had a single-shot interscalene block. While this is standard at our institution, it is possible that either CC or ICE would have a more significant effect in the absence of an interscalene block. Finally, we did not evaluate final outcomes in this study and therefore cannot determine if the final outcome was different between the 2 groups. Our goal was simply to evaluate the first 2 weeks following surgery, as this is the most painful period following TSA.
CONCLUSION
There was no difference between CC and ICE in terms of pain control, quality of sleep, patient satisfaction, or narcotic consumption following TSA. CC may offer convenience advantages, but the increased cost associated with this type of unit may not be justified.
ABSTRACT
Postoperative pain management is an important component of total shoulder arthroplasty (TSA). Continuous cryotherapy (CC) has been proposed as a means of improving postoperative pain control. However, CC represents an increased cost not typically covered by insurance. The purpose of this study is to compare CC to plain ice (ICE) following TSA. The hypothesis was that CC would lead to lower pain scores and decreased narcotic usage during the first 2 weeks postoperatively.
A randomized controlled trial was performed to compare CC to ICE. Forty patients were randomized to receive either CC or ICE following TSA. The rehabilitation and pain control protocols were otherwise standardized. Visual analog scales (VAS) for pain, satisfaction with cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery. Narcotic usage in morphine equivalents was also recorded.
No significant differences in preoperative pain (5.9 vs 6.8; P = .121), or postoperative pain at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593) or 14 days (2.5 vs 2.7; P = .742) were observed between the CC and ICE groups. Similarly, no differences in quality of sleep, satisfaction with the cold therapy, or narcotic usage at any time interval were observed between the 2 groups.
No differences in pain control, quality of sleep, patient satisfaction, or narcotic usage were detected between CC and ICE following TSA. CC may offer convenience as an advantage, but the increased cost associated with this type of treatment may not be justified.
The number of total shoulder arthroplasties (TSAs) performed annually is increasing dramatically.1 At the same time, there has been a push toward decreased length of hospital stay and earlier mobilization following joint replacement surgery. Central to these goals is adequate pain control. Multimodal pain pathways exist, and one of the safest and cheapest methods of pain control is cold therapy, which can be accomplished with continuous cryotherapy (CC) or plain ice (ICE).
Continue to: The mechanism of cryotherapy...
The mechanism of cryotherapy for controlling pain is poorly understood. Cryotherapy reduces leukocyte migration and slows down nerve signal transmission, which reduces inflammation, thereby producing a short-term analgesic effect. Stalman and colleagues2 reported on a randomized control study that evaluated the effects of postoperative cooling after knee arthroscopy. Measurements of metabolic and inflammatory markers in the synovial membrane were used to assess whether cryotherapy provides a temperature-sensitive release of prostaglandin E2. Cryotherapy lowered the temperature in the postoperative knee, and synovial prostaglandin concentrations were correlated with temperature. Because prostaglandin is a marker of inflammation and pain, the conclusion was that postoperative cooling appeared to have an anti-inflammatory effect.
The knee literature contains multiple studies that have examined the benefits of cryotherapy after both arthroscopic and arthroplasty procedures. The clinical benefits on pain have been equivocal with some studies showing improvements using cryotherapy3,4 and others showing no difference in the treatment group.5,6
Few studies have examined cryotherapy for the shoulder. Speer and colleagues7 demonstrated that postoperative use of CC was effective in reducing recovery time after shoulder surgery. However; they did not provide an ICE comparative group and did not focus specifically on TSA. In another study, Kraeutler and colleagues8 examined only arthroscopic shoulder surgery cases in a randomized prospective trial and found no significant different between CC and ICE. They concluded that there did not appear to be a significant benefit in using CC over ICE for arthroscopic shoulder procedures.
The purpose of this study is to prospectively evaluate CC and ICE following TSA. The hypothesis was that CC leads to improved pain control, less narcotic consumption, and improved quality of sleep compared to ICE in the immediate postoperative period following TSA.
MATERIALS AND METHODS
This was a prospective randomized control study of patients undergoing TSA receiving either CC or ICE postoperatively. Institutional Review Board approval was obtained before commencement of the study. Inclusion criteria included patients aged 30 to 90 years old undergoing a primary or revision shoulder arthroplasty procedure between June 2015 and January 2016. Exclusion criteria included hemiarthroplasty procedures.
Continue to: Three patients refused...
Three patients refused to participate in the study. Enrollment was performed until 40 patients were enrolled in the study (20 patients in each group). Randomization was performed with a random number generator, and patients were assigned to a treatment group following consent to participate. Complete follow-up was available for all patients. There were 13 (65%) male patients in the CC group. The average age of the CC group at the time of surgery was 68.7 years (range). There were 11 male patients in the ICE group. The average age of the ICE group at the time of surgery was 73.2 years (range). The dominant extremity was involved in 9 (45%) patients in the CC group and in 11 patients (55%) in the ICE group. Surgical case specifics are summarized in Table 1.
Table 1. Summary of Surgical Cases
| CC group (n = 20) | ICE group (n = 20) |
Primary TSA | 7 (35%) | 9 (45%) |
Primary RSA | 12 (60%) | 9 (45%) |
Revision arthroplasty | 1 (5%) | 2 (10%) |
Abbreviations: CC, continuous cryotherapy; ICE, plain ice; RSA, reverse shoulder arthroplasty; TSA, total shoulder arthroplasty.
All surgeries were performed by Dr. Denard. All patients received a single-shot interscalene nerve block prior to the procedure. A deltopectoral approach was utilized, and the subscapularis was managed with the peel technique.9 All patients were admitted to the hospital following surgery. Standard postoperative pain control consisted of as-needed intravenous morphine (1-2 mg every 2 hours, as needed) or an oral narcotic (hydrocodone/acetaminophen 5/325mg, 1-2 every 4 hours, as needed) which was also provided at discharge. However, total narcotic usage was recorded in morphine equivalents to account for substitutions. No non-steroidal anti-inflammatory drugs were allowed until 3 months postoperatively.
The CC group received treatment from a commercially available cryotherapy unit (Polar Care; Breg). All patients received instructions by a medical professional on how to use the unit. The unit was applied immediately postoperatively and set at a temperature of 45°F to 55°F. Patients were instructed to use the unit continuously during postoperative days 0 to 3. This cryotherapy was administered by a nurse while in the hospital but was left to the responsibility of the patient upon discharge. Patients were instructed to use the unit as needed for pain control during the day and continuously while asleep from days 4 to14.
The ICE group used standard ice packs postoperatively. The patients were instructed to apply an ice pack for 20 min every 2 hours while awake during days 0 to 3. This therapy was administered by a nurse while in the hospital but left to the responsibility of the patient upon discharge. Patients were instructed to use ice packs as needed for pain control during the day at a maximum of 20 minutes per hour on postoperative days 4 to 14. Compliance by both groups was monitored using a patient survey after hospital discharge. The number of hours that patients used either the CC or ICE per 24-hour period was recorded at 24 hours, 3 days, 7 days, and 14 days. The nursing staff recorded the number of hours of use of either cold modality for each patient prior to hospital discharge. The average length of stay as an inpatient was 1.2 days for the CC group and 1.3 days for the ICE group.
Visual analog scales (VAS) for pain, satisfaction with the cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery.
Continue to: The Wilcoxon rank-sum test...
STATISTICAL METHOD
The Wilcoxon rank-sum test was used to assess whether scores changed significantly from the preoperative period to the different postoperative time intervals, as well as to assess the values for pain, quality of sleep, and patient satisfaction. P-values <.05 were considered significant.
RESULTS
No differences were observed in the baseline characteristics between the 2 groups. Both groups showed improvements in pain, quality of sleep, and satisfaction with the cold therapy from the preoperative period to the final follow-up.
The VAS pain scores were not different between the CC and ICE groups preoperatively (5.9 vs 6.8; P = .121) or postoperatively at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593), or 14 days (2.5 vs 2.7; P = .742). Both cohorts demonstrated improved overall pain throughout the study period. These findings are summarized in Table 2.
Table 2. Summary of VAS Pain Scores With Cold Therapy
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
Preoperative | 5.9 ± 4.1 | 6.8 ± 5.3 | .121 | 3.3-8.3 |
24 hours | 4.2 ± 3.0 | 4.3 ± 3.1 | .989 | 2.9-5.7 |
3 days | 4.8 ± 2.7 | 4.7 ± 3.2 | .944 | 3.2-6.3 |
7 days | 2.9 ± 1.8 | 3.3 ± 2.5 | .593 | 2.1-4.4 |
14 days | 2.5 ± 2.1 | 2.7 ± 1.8 | .742 | 1.5-3.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
The number of morphine equivalents of pain medication was not different between the CC and ICE groups postoperatively at 24 hours (43 vs 38 mg; P = .579), 3 days (149 vs 116 mg; P = .201), 7 days (308 vs 228 mg; P = .181), or 14 days (431 vs 348 mg; P = .213). Both groups showed increased narcotic consumption from 24 hours postoperatively until the 2-week follow-up. Narcotic consumption is summarized in Table 3.
Table 3. Summary of Narcotic Consumption in Morphine Equivalents
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 43.0 ± 36.7 | 38.0 ± 42.9 | .579 | 17.9-60.1 |
3 days | 149.0 ± 106.5 | 116.3 ± 108.9 | .201 | 63.4-198.7 |
7 days | 308.1 ± 234.0 | 228 ± 258.3 | .181 | 107.1-348.9 |
14 days | 430.8 ± 384.2 | 347.5 ± 493.4 | .213 | 116.6-610.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice.
VAS for quality of sleep improved in both groups from 24 hours postoperatively until the final follow-up. However, no significant differences in sleep quality were observed between the CC and ICE groups postoperatively at 24 hours (5.1 vs 4.3; P = .382), 3 days (5.1 vs 5.3; P = .601), 7 days (6.0 vs 6.7; P = .319), or 14 days (6.5 vs 7.1; P = .348). The VAS scores for sleep quality are reported in Table 4.
Table 4. Summary of VAS Sleep Quality With Cold Therapya
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 5.1 ± 2.8 | 4.3 ± 2.4 | .382 | 3.2-6.4 |
3 days | 5.1 ± 1.9 | 5.3 ± 2.3 | .601 | 4.2-6.5 |
7 days | 6.0 ± 2.3 | 6.7 ± 2.1 | .319 | 4.9-7.7 |
14 days | 6.5 ± 2.3 | 7.1 ± 2.5 | .348 | 5.3-8.4 |
a0-10 rating with 10 being the highest possible score.
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
Continue to: Finally, VAS patient satisfaction...
Finally, VAS patient satisfaction scores were not different between the CC and ICE groups postoperatively at 24 hours (7.3 vs 6.1; P = .315), 3 days (6.1 vs 6.6; P = .698), 7 days (6.6 vs 6.9; P = .670), or 14 days (7.1 vs 6.3; P = .288).
While compliance within each group utilizing the randomly assigned cold modality was similar, the usage by the CC group was consistently higher at all time points recorded. No complications or reoperations were observed in either group.
DISCUSSION
The optimal method for managing postoperative pain from an arthroplasty procedure is controversial. This prospective randomized study attempted to confirm the hypothesis that CC infers better pain control, improves quality of sleep, and decreases narcotic usage compared to ICE in the first 2 weeks after a TSA procedure. The results of this study refuted our hypothesis, demonstrating no significant difference in pain control, satisfaction, narcotic usage, or sleep quality between the CC and ICE cohorts at all time points studied.
Studies on knees and lower extremities demonstrate equivocal results for the role CC plays in providing improved postoperative pain control. Thienpont10 evaluated CC in a randomized control trial comparing plain ice packs postoperatively in patients who underwent TKA. The author found no significant difference in VAS for pain or narcotic consumption in morphine equivalents. Thienpont10 recommended that CC not be used for outpatient knee arthroplasty as it is an additional cost that does not improve pain significantly. Healy and colleagues5 reported similar results that CC did not demonstrate a difference in narcotic requirement or pain control compared to plain ice packs, as well as no difference in local postoperative swelling or wound drainage. However, a recently published randomized trial by Su and colleagues11 comparing a cryopneumatic device and ICE with static compression in patients who underwent TKA demonstrated significantly lower narcotic consumption and increased ambulation distances in the treatment group. The treatment group consumed approximately 170 mg morphine equivalents less than the control group between discharge and the 2-week postoperative visit. In addition, a significant difference was observed in the satisfaction scores in the treatment group.11 Similarly, a meta-analysis by Raynor and colleagues12 on randomized clinical trials comparing cryotherapy to a placebo group after anterior cruciate ligament reconstruction showed that cryotherapy is associated with significantly lower postoperative pain (P = .02), but demonstrated no difference in postoperative drainage (P = .23) or range of motion (P = .25).
Although multiple studies have been published regarding the efficacy of cryotherapy after knee surgery, very few studies have compared CC to conventional ICE after shoulder surgery. A prospective randomized trial was performed by Singh and colleagues13 to compare CC vs no ICE in open and arthroscopic shoulder surgery patients. Both the open and arthroscopic groups receiving CC demonstrated significant reductions in pain frequency and more restful sleep at the 7-day, 14-day, and 21-day intervals compared to the control group. However, they did not compare the commercial unit to ICE. In contrast, a study by Kraeutler and colleagues8 randomized 46 patients to receive either CC or ICE in the setting of arthroscopic shoulder surgery. Although no significant difference was observed in morphine equivalent dosage between the 2 groups, the CC group used more pain medication on every postoperative day during the first week after surgery. They found no difference between the 2 groups with regards to narcotic consumption or pain scores. The results of this study mirror those by Kraeutler and colleagues,8 demonstrating no difference in pain scores, sleep quality, or narcotic consumption.
Continue to: With rising costs in the US...
With rising costs in the US healthcare system, a great deal of interest has developed in the application of value-based principles to healthcare. Value can be defined as a gain in benefits over the costs expended.14 The average cost for a commercial CC unit used in this study was $260. A pack of ICE is a nominal cost. Based on the results of this study, the cost of the commercial CC device may not be justified when compared to the cost of an ice pack.
The major strengths of this study are the randomized design and multiple data points during the early postoperative period. However, there are several limitations. First, we did not objectively measure compliance of either therapy and relied only on a patient survey. Usage of the commercial CC unit in hours decreased over half between days 3 and 14. This occurred despite training on the application and specific instructions. We believe this reflects “real-world” usage, but it is possible that compliance affected our results. Second, all patients in this study had a single-shot interscalene block. While this is standard at our institution, it is possible that either CC or ICE would have a more significant effect in the absence of an interscalene block. Finally, we did not evaluate final outcomes in this study and therefore cannot determine if the final outcome was different between the 2 groups. Our goal was simply to evaluate the first 2 weeks following surgery, as this is the most painful period following TSA.
CONCLUSION
There was no difference between CC and ICE in terms of pain control, quality of sleep, patient satisfaction, or narcotic consumption following TSA. CC may offer convenience advantages, but the increased cost associated with this type of unit may not be justified.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/jbjs.j.01994.
2. Stalman A, Berglund L, Dungnerc E, Arner P, Fellander-Tsai L. Temperature sensitive release of prostaglandin E2 and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2016/jbjs.j.01790.
3. Levy AS, Marmar E. The role of cold compression dressings in the postoperative treatment of total knee arthroplasty. Clin Orthop Relat Res. 1993;297:174-178. doi:10.1097/00003086-199312000-00029.
4. Webb JM, Williams D, Ivory JP, Day S, Williamson DM. The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopaedics 1998;21(1):59-61.
5. Healy WL, Seidman J, Pfeifer BA, Brown DG. Cold compressive dressing after total knee arthroplasty. Clin Orthop Relat Res. 1994;299:143-146. doi:10.1097/00003086-199402000-00019.
6. Whitelaw GP, DeMuth KA, Demos HA, Schepsis A, Jacques E. The use of Cryo/Cuff versus ice and elastic wrap in the postoperative care of knee arthroscopy patients. Am J Knee Surg. 1995;8(1):28-30.
7. Speer KP, Warren RF, Horowitz L. The efficacy of cryotherapy in the postoperative shoulder. J Shoulder Elbow Surg. 1996;5(1):62-68. doi:10.16/s1058-2746(96)80032-2.
8. Kraeutler MJ, Reynolds KA, Long C, McCarthy EC. Compressive cryotherapy versus ice- a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elbow Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
9. DeFranco MJ, Higgins LD, Warner JP. Subscapularis management in open shoulder surgery. J Am Acad Orthop Surg. 2010;18(12):707-717. doi:10.5435/00124635-201012000-00001.
10. Thienpont E. Does advanced cryotherapy reduce pain and narcotic consumption after knee arthroplasty. Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
11. Su EP, Perna M, Boettner F, Mayman DJ, et al. A prospective, multicenter, randomized trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620x.94B11.30832.
12. Raynor MC, Pietrobon R, Guller U, Higgins LD. Cryotherapy after ACL reconstruction- a meta analysis. J Knee Surg. 2005;18(2):123-129. doi:10.1055/s-0030-1248169.
13. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: a prospective randomized investigation. J Shoulder Elbow Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
14. Black EM, Higgins LD, Warner JP. Value based shoulder surgery: outcomes driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1-10. doi:10.1016/j.se.2013.02.008.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/jbjs.j.01994.
2. Stalman A, Berglund L, Dungnerc E, Arner P, Fellander-Tsai L. Temperature sensitive release of prostaglandin E2 and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2016/jbjs.j.01790.
3. Levy AS, Marmar E. The role of cold compression dressings in the postoperative treatment of total knee arthroplasty. Clin Orthop Relat Res. 1993;297:174-178. doi:10.1097/00003086-199312000-00029.
4. Webb JM, Williams D, Ivory JP, Day S, Williamson DM. The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopaedics 1998;21(1):59-61.
5. Healy WL, Seidman J, Pfeifer BA, Brown DG. Cold compressive dressing after total knee arthroplasty. Clin Orthop Relat Res. 1994;299:143-146. doi:10.1097/00003086-199402000-00019.
6. Whitelaw GP, DeMuth KA, Demos HA, Schepsis A, Jacques E. The use of Cryo/Cuff versus ice and elastic wrap in the postoperative care of knee arthroscopy patients. Am J Knee Surg. 1995;8(1):28-30.
7. Speer KP, Warren RF, Horowitz L. The efficacy of cryotherapy in the postoperative shoulder. J Shoulder Elbow Surg. 1996;5(1):62-68. doi:10.16/s1058-2746(96)80032-2.
8. Kraeutler MJ, Reynolds KA, Long C, McCarthy EC. Compressive cryotherapy versus ice- a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elbow Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
9. DeFranco MJ, Higgins LD, Warner JP. Subscapularis management in open shoulder surgery. J Am Acad Orthop Surg. 2010;18(12):707-717. doi:10.5435/00124635-201012000-00001.
10. Thienpont E. Does advanced cryotherapy reduce pain and narcotic consumption after knee arthroplasty. Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
11. Su EP, Perna M, Boettner F, Mayman DJ, et al. A prospective, multicenter, randomized trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620x.94B11.30832.
12. Raynor MC, Pietrobon R, Guller U, Higgins LD. Cryotherapy after ACL reconstruction- a meta analysis. J Knee Surg. 2005;18(2):123-129. doi:10.1055/s-0030-1248169.
13. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: a prospective randomized investigation. J Shoulder Elbow Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
14. Black EM, Higgins LD, Warner JP. Value based shoulder surgery: outcomes driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1-10. doi:10.1016/j.se.2013.02.008.
TAKE-HOME POINTS
- CC has been proposed as a means of improving postoperative pain control.
- CC represents a cost typically not covered by insurances.
- No difference was noted between the 2 groups in quality of sleep, satisfaction with the cold therapy, or narcotic usage at any time interval.
- While CC may offer convenience advantages, the increased cost associated with this type of unit may not be justified.
- The mechanism for CC for pain control is poorly understood.
Open vs Percutaneous vs Arthroscopic Surgical Treatment of Lateral Epicondylitis: An Updated Systematic Review
ABSTRACT
This study was performed to compare outcomes of open, arthroscopic, and percutaneous surgical techniques for lateral epicondylitis. We searched PubMed (MEDLINE) for literature published between January 1, 2004 and May 23, 2015 using these key words: lateral epicondylitis AND (surgery OR operative OR surgical OR open OR arthroscopic OR percutaneous). Meta-analyses were performed for outcomes reported in 3 studies using 2-sample and 2-proportion Z-tests. Thirty-five studies including 1640 elbows (1055 open, 401 arthroscopic, 184 percutaneous) met the inclusion criteria. There were no differences between groups regarding duration to return to work, complication rate, or patient satisfaction. A greater proportion of patients were pain free in the open group than in the arthroscopic group (70% vs 60%). Despite the absence of a difference among techniques regarding return to work and subjective function, we recommend open débridement as the technique most likely to achieve a pain-free outcome.
Continue to: Lateral epicondylitis affects...
Lateral epicondylitis affects 1% to 3% of adults each year. Although common, symptoms of lateral epicondylitis resolve spontaneously within a year of symptom onset in 80% of cases, and only 3% of patients who seek medical treatment ultimately require surgical intervention within 2 years of symptom onset.1 Despite a relatively low percentage of patients who require surgery, Sanders and colleagues1 noted a significant increase in the rate of surgical intervention from 1.1% to 3.2% of cases in the last 15 years. Surgical intervention is generally indicated when pain and functional disability persist after 6 to 12 months of nonsurgical treatment. Traditional surgical treatment involves open release/débridement of the extensor carpi radialis (ECRB) origin; however, with the increasing prevalence of surgical intervention, surgeons have demonstrated a rising interest in less invasive techniques like arthroscopic release/débridement and percutaneous tenotomy as alternatives to traditional open débridement. While favorable results have been reported for all 3 techniques, there is no current consensus regarding the optimal surgical technique. In 2007, Lo and Safran2 reported no difference in the results of open, percutaneous, and arthroscopic techniques regarding any outcome measure in a systematic review of 33 papers. We conducted a repeat systematic review of the current literature to update Lo and Safran’s2 review and to ascertain if more recent literature demonstrates superiority of 1 technique regarding pain relief, subjective questionnaire data, subjective satisfaction, restoration of strength, and return to work. We hypothesized that return to work would be accelerated, pain decreased, and function improved in the early postoperative period in the arthroscopic and percutaneous groups, but there would be no difference in ultimate pain, functional outcome, or subjective satisfaction.
METHODS
SEARCH STRATEGY AND STUDY SELECTION
We conducted a systematic review of the literature to update the topic of surgical intervention with lateral epicondylitis since the publication of the most recent review by Lo and Safran2 in 2007, which included all relevant studies published up to 2004. To include all relevant studies published since that time, we searched PubMed (MEDLINE) for all literature published from January 1, 2004 to May 23, 2015 using the following key words: lateral epicondylitis AND (surgery OR operative OR surgical OR open OR arthroscopic OR percutaneous). General search terms were utilized to avoid unintentional exclusion of relevant studies. Two authors reviewed the abstracts of all resultant citations. Table 1 outlines the inclusion and exclusion criteria for the search. References from all included studies were reviewed for applicable articles that were not captured by the initial broad search strategy. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) trial flow chart shows the study selection algorithm (Figure 1).
Table 1. Inclusion and Exclusion Criteria for the Analyzed Studies
Inclusion Criteria | Exclusion Criteria |
|
|
DATA EXTRACTION AND ANALYSIS
Data were extracted from the included studies by 2 reviewers using data abstraction forms. All study, subject, and surgery parameters were collected. The study and subject demographic parameters analyzed included year of publication, level of evidence, presence of study financial conflict of interest, number of subjects and elbows, gender, age, proportion in whom the dominant extremity was involved, proportion who were laborers, proportion who had a workman’s compensation claim, duration of symptoms prior to surgical intervention, and surgical technique employed (open, arthroscopic, or percutaneous). We recorded the following clinical outcomes: proportion of patients with complete pain relief, proportion who were partially or completely satisfied, proportion who were improved, duration to return to work, grip strength, Disabilities of the Arm, Shoulder, and Hand (DASH) score, visual analog scale (VAS) pain score, and complication rate.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Data from all studies were pooled and descriptive statistics were reported as weighted mean ± weighted standard deviation for continuous variables and frequency with percentage for categorical variables. A meta-analysis was performed for all outcome measures that were reported in 3 or more studies within a specific treatment cohort. Data were analyzed using 2-sample and 2-proportion Z-tests. Results were considered statistically significant at P < .05.
RESULTS
LITERATURE RESEARCH
Using the aforementioned search strategy, 154 studies were identified. Following application of the inclusion and exclusion criteria, 35 studies were included in the analysis (Figure 1). One study compared open and percutaneous techniques, and another compared arthroscopic and percutaneous techniques, rendering a total of 19 studies examining open surgical techniques for treatment of lateral epicondylitis,3-21 12 studies examining arthroscopic techniques,14,22-32 and 6 studies reporting percutaneous surgical treatment of lateral epicondylitis29,33-37 (Table 2). There was1 level I study (3%), 6 level III studies (17%), and 28 level IV studies (80%).
Table 2. Study Demographic Data for Open, Arthroscopic, and Percutaneous Lateral Epicondylectomy
| Open | Arthroscopic | Percutaneous | Total |
Number of studies | 19 | 12 | 6 | 35 |
Level of evidence |
|
|
|
|
I | 1 (5%) | 0 | 0 | 1 (3%) |
II | 0 | 0 | 0 | 0 |
III | 3 (16%) | 4 (33%) | 1 (17%) | 6 (17%) |
IV | 15 (79%) | 8 (67%) | 5 (83%) | 28 (80%) |
US: International | 8:12 | 3:9 | 1:5 | 12:24 |
Journals of publication |
|
|
|
|
AJSM | 3 | 1 | 1 | 5 |
JSES | 2 | 2 | 1 | 5 |
Arthroscopy | 2 | 2 | 0 | 3 |
KSSTA | 1 | 2 | 0 | 3 |
CORR | 0 | 2 | 0 | 2 |
JHS | 0 | 1 | 0 | 1 |
JOS | 1 | 1 | 0 | 2 |
AJO | 2 | 0 | 0 | 2 |
Other | 8 | 1 | 4 | 12 |
Abbreviations: AJO, The American Journal of Orthopedics; AJSM, American Journal of Sports Medicine; Arthroscopy, The Journal of Arthroscopy and Related Surgery; CORR, Clinical Orthopaedics & Related Research; JHS, Journal of Hand Surgery; JOS, Journal of Orthopaedic Surgery; JSES, Journal of Shoulder and Elbow Surgery; KSSTA, Knee Surgery, Sports Traumatology, and Arthroscopy.
SUBJECT DEMOGRAPHICS
The 35 included studies comprised 1579 patients and 1640 elbows. Among these, 1055 (64%) elbows underwent open (O), 401 (25%) underwent arthroscopic (A), and 184 (11%) underwent percutaneous (P) treatment. The average age was 45.7 years, 47% of the patients were male, 43% were laborers, 31% had worker’s compensation claims, and the dominant extremity was involved in 62% of patients. The percutaneous cohort was older than the open cohort (P = 46.9, O = 45.4, A = 45.8; P = .036). The duration of symptoms was shorter in the percutaneous cohort than in the other 2 groups and shorter in the arthroscopic cohort than in the open cohort (P = 8 months, O = 23 months, A = 18 months; P < .001). There were no significant differences between groups regarding gender, occupation, worker’s compensation status, or involvement of the dominant extremity (Table 3).
Table 3. Subject Demographics for Open, Arthroscopic, and Percutaneous Groups
| Open | Arthroscopic | Percutaneous |
Subjects (N) | 999 | 397 | 183 |
Elbows (N) | 1055 | 401 | 184 |
Elbows with follow-up (%) | 915 (87%) | 350 (87%) | 181 (98%) |
Males (%) | 427 (47%) | 173 (49%) | 78 (43%) |
Females (%) | 488 (53%) | 177 (51%) | 103 (57%) |
Mean age (years) | 45.4 | 45.8 | 46.9 |
Dominant elbow (%) | 70% | 69% | 53% |
Laborer (%) | 56% | 53% | 48% |
Work comp (%) | 36% | 30% | NR |
Symptoms to operation (months) | 23 | 18 | 8 |
Min. symptoms to operation (months) | 6 | 6 | 3 |
Mean follow-up (months) | 60 | 44 | 11 |
MATA-ANALYSIS CLINICAL OUTCOMES
Clinical outcome results were pooled for all studies reporting the same outcome measure for the same technique (open, arthroscopic, or percutaneous). A meta-analysis was performed for all outcome measures that were reported in a minimum of 3 studies utilizing the same surgical technique (Table 4).
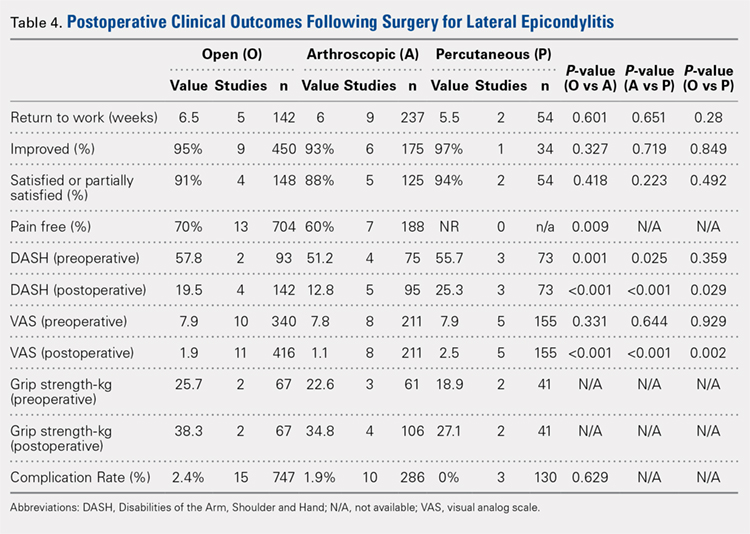
PAIN RELIEF
Thirteen open studies,3,5,7,8,11-16,18,19,21 7 arthroscopic studies14,22-24,26,27,31 and 0 percutaneous studies reported the proportion of patients who were pain free at final follow-up. The proportion of patients who were pain free following open débridement was greater than that in the arthroscopic cohort (O = 70%, A = 60%; P = .009) (Table 4).
Continue to: Subjective improvement and satisfaction...
SUBJECTIVE IMPROVEMENT AND SATISFACTION
Nine open studies, 6 arthroscopic studies, and 1 percutaneous study reported the proportion of patients who felt that their condition had been improved as a result of surgery. There was no difference in the proportion of patients who experienced improvement between the open and arthroscopic cohorts. Four open studies,3,11,12 5 arthroscopic studies,22,26,28,29,32 and 2 percutaneous studies29,36 reported the proportion of patients who were satisfied or partially satisfied with the results of the procedure. There was no difference between the open and arthroscopic groups in the proportion of patients who were satisfied or partially satisfied (Table 4).
RETURN TO WORK
The duration to return to work following surgery was reported in 5 open studies,4,5,10,13,14 9 arthroscopic studies,14,23-29,32 and 2 percutaneous studies.29,36 There was no statistically significant difference between the open and arthroscopic groups with regard to duration to return to work (O = 6.5 weeks, A = 6 weeks; P = .601). The percutaneous technique could not be included in the meta-analysis due to the presence of only 2 studies, but the pooled mean duration to return to work in these 2 studies was 5.5 weeks (Table 4).
GRIP STRENGTH
Postoperative grip strength was reported in 2 open studies,10,19 4 arthroscopic studies,28,30,32 and 2 percutaneous studies.35-36 A meta-analysis could not be performed on all the groups due to the presence of only 2 open and 2 percutaneous studies reporting grip strength. The pooled averages were O = 38.3 kg, A = 34.8 kg, and P = 27.1 kg (Table 4).
DASH SCORE
The postoperative DASH score was reported in 4 open studies,4,15,17,19,20 5 arthroscopic studies,28-31 and 3 percutaneous studies.29,33,36 At final follow-up, the mean DASH score was higher in the arthroscopic group than in the open and percutaneous groups (A = 12.8, O = 19.5, P = 25.3; P < .001 for both comparisons), and the mean DASH score was significantly higher in the open group than in the percutaneous group (P = .029). The reporting of DASH scores in the early postoperative period was not sufficiently consistent to allow us to test our hypothesis that there would be early differences in function between groups (Table 4).
VAS PAIN SCORE
Postoperative VAS pain scores were reported in 11 open studies,6,8-10,12,15,19-21 8 arthroscopic studies,24-26,29-32 and 5 percutaneous studies.29,33,35-37 At final follow-up, there was a lower mean VAS score in the arthroscopic group than in the open and percutaneous groups (A = 1.1, O = 1.9, and P = 2.5; P < .001 for both comparisons) and a lower mean VAS score in the open group than in the percutaneous group (P = .002) (Table 4). Reporting of VAS scores in the early postoperative period in the included studies wan not sufficiently consistent to allow us to test our hypothesis that there would be early differences in pain between groups.
COMPLICATIONS
The complication rate was reported in 15 open studies, 10 arthroscopic studies, and 3 percutaneous studies. There was no difference in the complication rate between the open and arthroscopic techniques (O = 2.4%, A = 1.9%; P = .629) (Table 4). Complications noted in the open cohort included superficial wound infection (6), hematoma (5), synovial fistula (2), seroma (2), and posterior interosseous nerve palsy (1). Complications noted in the arthroscopic cohort included superficial infection (3), hematoma (1), and transient paresthesia (1). Of note, there were no complications in the percutaneous group.
Continue to: Discussion...
DISCUSSION
The primary purpose of this review was to determine if definitive evidence suggests that any 1 of open, percutaneous, or arthroscopic surgical treatment is superior to the other 2 for relieving pain, improving functionality, restoring strength, or accelerating return to work. The most striking finding of this study was a significantly higher proportion of patients who were pain free at final follow-up in the open group than in the arthroscopic group (70% vs 60%, P = .009) (Table 4). At final follow-up, there were no significant differences between groups regarding duration to return to work, proportion who were improved, proportion who were satisfied or partially satisfied, and complication rate. Average VAS and DASH scores at final follow-up were lower in the arthroscopic group than in the open and percutaneous groups (Figure 2). However, although the difference between mean DASH scores in the arthroscopic and open groups (6.7 points) was statistically significant, it is likely not clinically significant, as the minimal clinically important difference (MCID) for the DASH score is 10 points, as demonstrated by Sorensen and colleagues.38 Although it has not been specifically defined for lateral epicondylitis, the MCID for VAS pain has been reported in the literature to range from 1.0 to 1.4.39-40 Therefore, as for the DASH score, the difference witnessed between the open and arthroscopic groups (0.8) is likely not clinically significant. Of note, the differences between values for arthroscopic and percutaneous techniques are greater than the MCID.
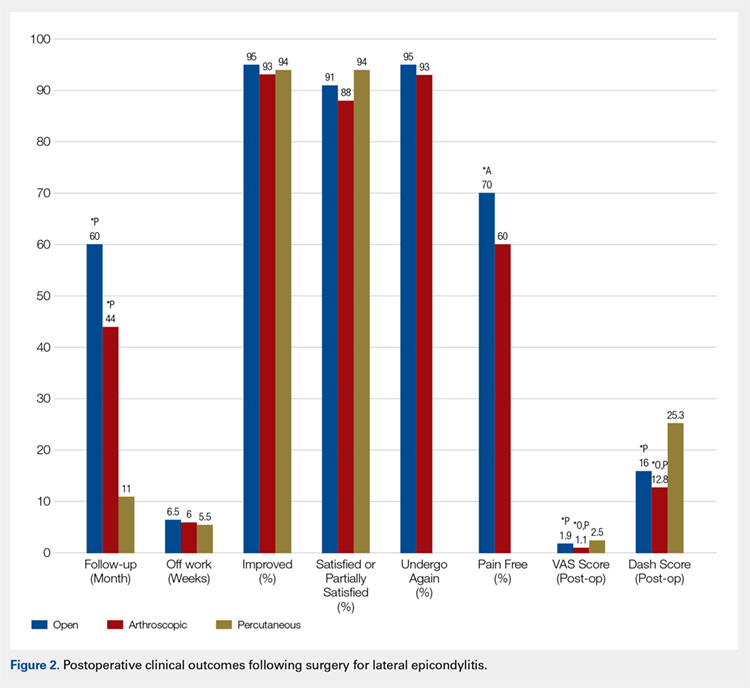
In light of a recent increase in the prevalence of surgical intervention for lateral epicondylitis, many authors have promoted arthroscopic and percutaneous techniques as alternatives to traditional open débridement with the goal of achieving the same results with decreased morbidity and accelerated return to work. Given the increased proportion of patients who were pain free at final follow-up in the open cohort, it is our contention that open release/débridement of the common extensor/ECRB origin allows the surgeon to fully appreciate the extent of tendinotic tissue that is contributing to the patient’s symptoms and to address the pathology in its entirety. Other authors have also questioned whether the full extent of extra-articular tendinosis can be accurately identified arthroscopically. Cummins41 demonstrated, in a series of 18 patients who underwent arthroscopic ECRB débridement, that 6 patients had residual tendinosis upon open evaluation and 10 had residual tendinosis on histologic assessment. Additionally, in the same series, residual tendinopathy was associated with poorer clinical outcomes.
The improved visualization associated with an open technique comes at minimal expense, as the incision was only 1.5 cm to 5 cm in 13 of 15 papers reporting incision length.3,4,6,8-11,13,15,18-20 This increased exposure may not translate into increased morbidity, as there was no increase in the duration to return to work nor the complication rate. As a result of the extensive instrumentation necessary for arthroscopic techniques, open techniques also appear to be less expensive. Analyses in the literature have suggested increased expenditures associated with arthroscopic treatment ranging from 23%42 to 100%43 greater than those of open treatment.
Although obvious, it should be noted that a percutaneous tenotomy does not permit assessment of the extent of pathologic tendinosis. As a result of an inability to visualize and débride pathologic tissue, percutaneous tenotomy rendered inferior outcomes to open and arthroscopic techniques in terms of both postoperative VAS pain score and DASH score. Nonetheless, it is a relatively rapid and simple technique and resulted in zero complications in 184 elbows. Overall, percutaneous tenotomy appears to be an inferior technique to open and arthroscopic techniques in terms of achieving complete pain relief and optimal functional recovery; however, it may be useful in those who wish to avoid a more invasive intervention.
LIMITATIONS
The most significant limitation of this study was the heterogeneity in the techniques utilized in each group. Among the 19 papers in the open cohort, 11 used techniques aimed at lengthening or release of the extensor origin, 7 performed débridement of tendinotic tissue at the ECRB origin, and 1 compared these approaches. Exposures ranged from 1.5 cm to 8 cm in length, 3 techniques added tendon repair following débridement, and 2 utilized a radiofrequency device.
Among the 12 papers in the arthroscopic cohort, 8 performed arthroscopic (inside-out) débridement of the tendinotic tissue at the ECRB origin, 3 performed arthroscopic release of the ECRB tendon, and 1 performed endoscopic ECRB release in an outside-in fashion. Four techniques added posterior synovial plica excision and 4 added decortication of the lateral epicondyle débridement or release. Some authors advocate for arthroscopic intervention on the grounds that it permits evaluation and correction of other intra-articular pathology. With this in mind, some authors have suggested that a synovial fold (plica) adjacent to the radiocapitellar joint may contribute to lateral elbow pain.27,44 Nevertheless, in the only comparative trial in the literature, Rhyou and Kim30 demonstrated that excision of posterior synovial fold failed to enhance pain relief or function in a retrospective cohort study comparing arthroscopic débridement with and without plica excision.
Continue to: Some authors advocate...
Some authors advocate decorticating the non-articular, lateral epicondyle with a shaver to stimulate bleeding and promote a healing response. However, 1 study in our review compared arthroscopic ECRB release with and without decortication and found that decortication significantly increased pain up to 4 weeks postoperatively, increased duration to return to work, and did not improve the ultimate clinical result.25 Of note, others have used a similar rationale to advocate drilling the lateral epicondyle when utilizing an open technique. However, Dunn and colleagues8 note that they have modified the Nirschl technique to eliminate drilling because they feel it increases postoperative pain and may damage the extensor digitorum communis origin.
Among the 6 papers in the percutaneous tenotomy cohort, 2 performed tenotomy with a hypodermic needle, 2 with a scalpel through a limited incision (0.5 cm-1 cm), 1 using a TX1 tissue removal system (Tenex Health), and 1 with a percutaneous radiofrequency probe. In 3 techniques, ultrasound was used to direct the tenotomy.
The quality of this review is also limited by the studies included for analysis, as with any systematic review. Because 28 of the 35 included studies were classified as evidence level IV, the likelihood of methodological bias is increased. The majority of studies contained ≥1 demonstrable biases, including selection, detection, attrition biases, or a combination. Selection bias is prevalent among predominantly level IV studies, in which the authors have selected their preferred surgical technique. There was heterogeneity in the reporting of preoperative variables and the outcome measures that were utilized. Scoring systems, such as the Nirschl Tennis Elbow Score and the Mayo Elbow Performance Index, would have been valuable in comparing the groups had they been more consistently reported. The heterogeneity in clinical outcome tools and the lack of reported outcome variance or standard deviations prevented a formal meta-analysis of some of these outcome measures. Due to inconsistent reporting, we were also unable to test our hypothesis that there would be less pain and improved function in the arthroscopic and/or percutaneous cohorts in the early postoperative period compared to the open cohort due to the less invasive techniques used. Although the differences in DASH and VAS scores at final follow-up likely did not meet the MCID threshold, these differences may have been greater and more clinically relevant in the early postoperative period.
CONCLUSION
We hypothesized that the arthroscopic and percutaneous groups would experience accelerated return to work and reduced pain in the early postoperative period but no difference in ultimate pain, functional outcome, or subjective satisfaction. There is no difference between open, arthroscopic, and percutaneous surgical treatment for lateral epicondylitis regarding return to work and subjective satisfaction; however, open treatment led to a greater percentage of patients being pain free at final follow-up. While arthroscopic treatment led to better pain and functional scores at final follow-up, the absolute differences were quite small and likely not clinically significant. In light of the available evidence, we recommend open débridement as the best means of minimizing cost and achieving a pain-free outcome in the long term. For future investigators, it would be useful to perform a randomized clinical study directly comparing open, arthroscopic, and percutaneous techniques, including assessment of pain and functional scores in the early postoperative period, and to further evaluate differences in cost among the various techniques.
This paper will be judged for the Resident Writer’s Award.
- Sanders TL Jr, Maradit Kremers H, Bryan AJ, Ransom JE, Smith J, Morrey BF. The epidemiology and health care burden of tennis elbow: a population-based study. Am J Sports Med. 2015;43(5):1066-1071. doi:10.1177/0363546514568087.
- Lo MY, Safran MR. Surgical treatment of lateral epicondylitis: a systematic review. Clin Orthop Relat Res. 2007;463:98-106. doi:10.1097/BLO.0b013e3181483dc4.
- Balk ML, Hagberg WC, Buterbaugh GA, Imbriglia JE. Outcome of surgery for lateral epicondylitis (tennis elbow): effect of worker’s compensation. Am J Orthop. 2005;34(3):122-126; discussion 126.
- Barth J, Mahieu P, Hollevoet N. Extensor tendon and fascia sectioning of extensors at the musculotendinous unit in lateral epicondylitis. Acta Orthop Belg. 2013;79(3):266-270.
- Bigorre N, Raimbeau G, Fouque PA, Cast YS, Rabarin F, Cesari B. Lateral epicondylitis treatment by extensor carpi radialis fasciotomy and radial nerve decompression: is outcome influenced by the occupational disease compensation aspect? Orthop Traumatol Surg Res. 2011;97(2):159-163. doi:10.1016/j.otsr.2010.11.007.
- Cho BK, Kim YM, Kim DS, et al. Mini-open muscle resection procedure under local anesthesia for lateral and medial epicondylitis. Clin Orthop Surg. 2009;1(3):123-127. doi:10.4055/cios.2009.1.3.123.
- Coleman B, Quinlan JF, Matheson JA. Surgical treatment for lateral epicondylitis: a long-term follow-up of results. J Shoulder Elbow Surg. 2010;19(3):363-367. doi:10.1016/j.jse.2009.09.008.
- Dunn JH, Kim JJ, Davis L, Nirschl RP. Ten- to 14-year follow-up of the Nirschl surgical technique for lateral epicondylitis. Am J Sports Med. 2008;36(2):261-266. doi:10.1177/0363546507308932.
- Manon-Matos Y, Oron A, Wolff TW. Combined common extensor and supinator aponeurotomy for the treatment of lateral epicondylitis. Tech Hand Up Extrem Surg. 2013;17(3):179-181. doi:10.1097/BTH.0b013e31829e0eeb.
- Meknas K, Odden-Miland A, Mercer JB, Castillejo M, Johansen O. Radiofrequency microtenotomy: a promising method for treatment of recalcitrant lateral epicondylitis. Am J Sports Med. 2008;36(10):1960-1965. doi:10.1177/0363546508318045.
- Pruzansky ME, Gantsoudes GD, Watters N. Late surgical results of reattachment to bone in repair of chronic lateral epicondylitis. Am J Orthop. 2009;38(6):295-299.
- Rayan F, Rao V Sr, Purushothamdas S, Mukundan C, Shafqat SO. Common extensor origin release in recalcitrant lateral epicondylitis – role justified? J Orthop Surg Res. 2010;5:31. doi:10.1186/1749-799X-5-31.
- Reddy VR, Satheesan KS, Bayliss N. Outcome of Boyd-McLeod procedure for recalcitrant lateral epicondylitis of elbow. Rheumatol Int. 2011;31(8):1081-1084. doi:10.1007/s00296-010-1450-1.
- Rubenthaler F, Wiese M, Senge A, Keller L, Wittenberg RH. Long-term follow-up of open and endoscopic Hohmann procedures for lateral epicondylitis. Arthroscopy. 2005;21(6):684-690. doi:10.1016/j.arthro.2005.03.017.
- Ruch DS, Orr SB, Richard MJ, Leversedge FJ, Mithani SK, Laino DK. A comparison of debridement with and without anconeus muscle flap for treatment of refractory lateral epicondylitis. J Shoulder Elbow Surg. 2015;24(2):236-241. doi:10.1016/j.jse.2014.09.035.
- Siddiqui MA, Koh J, Kua J, Cheung T, Chang P. Functional outcome assessment after open tennis elbow release: what are the predictor parameters? Singapore Med J. 2011;52(2):73-76.
- Solheim E, Hegna J, Øyen J. Extensor tendon release in tennis elbow: results and prognostic factors in 80 elbows. Knee Surg Sports Traumatol Arthrosc. 2011;19(6):1023-1027. doi:10.1007/s00167-011-1477-1.
- Svernlöv B, Adolfsson L. Outcome of release of the lateral extensor muscle origin for epicondylitis. Scand J Plast Reconstr Surg Hand Surg. 2006;40(3):161-165. doi:10.1080/02844310500491492.
- Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21(7):851-860. doi:10.1016/j.arthro.2005.03.019.
- Thornton SJ, Rogers JR, Prickett WD, Dunn WR, Allen AA, Hannafin JA. Treatment of recalcitrant lateral epicondylitis with suture anchor repair. Am J Sports Med. 2005;33(10):1558-1564. doi:10.1177/0363546505276758.
- Wang AW, Erak S. Fractional lengthening of forearm extensors for resistant lateral epicondylitis. ANZ J Surg. 2007;77(11):981-984. doi:10.1111/j.1445-2197.2007.04294.x.
- Baker CL Jr, Baker CL 3rd. Long-term follow-up of arthroscopic treatment of lateral epicondylitis. Am J Sports Med. 2008;36(2):254-260. doi:10.1177/0363546507311599.
- Grewal R, MacDermid JC, Shah P, King GJ. Functional outcome of arthroscopic extensor carpi radialis brevis tendon release in chronic lateral epicondylitis. J Hand Surg Am. 2009;34(5):849-857. doi:10.1016/j.jhsa.2009.02.006.
- Jerosch J, Schunck J. Arthroscopic treatment of lateral epicondylitis: indication, technique and early results. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):379-382. doi:10.1007/s00167-005-0662-5.
- Kim JW, Chun CH, Shim DM, et al. Arthroscopic treatment of lateral epicondylitis: comparison of the outcome of ECRB release with and without decortication. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1178-1183. doi:10.1007/s00167-011-1507-z.
- Lattermann C, Romeo AA, Anbari A, et al. Arthroscopic debridement of the extensor carpi radialis brevis for recalcitrant lateral epicondylitis. J Shoulder Elbow Surg. 2010;19(5):651-656. doi:10.1016/j.jse.2010.02.008.
- Mullett H, Sprague M, Brown G, Hausman M. Arthroscopic treatment of lateral epicondylitis: clinical and cadaveric studies. Clin Orthop Relat Res. 2005;439:123-128. doi:10.1097/01.blo.0000176143.08886.fe.
- Oki G, Iba K, Sasaki K, Yamashita T, Wada T. Time to functional recovery after arthroscopic surgery for tennis elbow. J Shoulder Elbow Surg. 2014;23(10):1527-1531. doi:10.1016/j.jse.2014.05.010.
- Othman AM. Arthroscopic versus percutaneous release of common extensor origin for treatment of chronic tennis elbow. Arch Orthop Trauma Surg. 2011;131(3):383-388. doi:10.1007/s00402-011-1260-2.
- Rhyou IH, Kim KW. Is posterior synovial plica excision necessary for refractory lateral epicondylitis of the elbow? Clin Orthop Relat Res. 2013;471(1):284-290. doi:10.1007/s11999-012-2585-z.
- Wada T, Moriya T, Iba K, et al. Functional outcomes after arthroscopic treatment of lateral epicondylitis. J Orthop Sci. 2009;14(2):167-174. doi:10.1007/s00776-008-1304-9.
- Yoon JP, Chung SW, Yi JH, et al. Prognostic factors of arthroscopic extensor carpi radialis brevis release for lateral epicondylitis. Arthroscopy. 2015;31(7):1232-1237. doi:10.1016/j.arthro.2015.02.006.
- Barnes DE, Beckley JM, Smith J. Percutaneous ultrasonic tenotomy for chronic elbow tendinosis: a prospective study. J Shoulder Elbow Surg. 2015;24(1):67-73. doi:10.1016/j.jse.2014.07.017.
- Kaleli T, Ozturk C, Temiz A, Tirelioglu O. Surgical treatment of tennis elbow: percutaneous release of the common extensor origin. Acta Orthop Belg. 2004;70(2):131-133.
- Lin MT, Chou LW, Chen HS, Kao MJ. Percutaneous soft tissue release for treating chronic recurrent myofascial pain associated with lateral epicondylitis: 6 case studies. Evid Based Complement Alternat Med. 2012;2012:142941. doi:10.1155/2012/142941.
- Lin CL, Lee JS, Su WR, Kuo LC, Tai TW, Jou IM. Clinical and ultrasonographic results of ultrasonographically guided percutaneous radiofrequency lesioning in the treatment of recalcitrant lateral epicondylitis. Am J Sports Med. 2011;39(11):2429-2435. doi:10.1177/0363546511417096.
- Zhu J, Hu B, Xing C, Li J. Ultrasound-guided, minimally invasive, percutaneous needle puncture treatment for tennis elbow. Adv Ther. 2008;25(10):1031-1036. doi:10.1007/s12325-008-0099-6.
- Sorensen AA, Howard D, Tan WH, Ketchersid J, Calfee RP. Minimal clinically important differences of 3 patient-related outcomes instruments. J Hand Surg Am. 2013;38(4):641-649. doi:10.1016/j.jhsa.2012.12.032.
- Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;18(3):205-207. doi:10.1136/emj.18.3.205.
- Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elbow Surg. 2009;18(6):927-932. doi:10.1016/j.jse.2009.03.021.
- Cummins CA. Lateral epicondylitis: in vivo assessment of arthroscopic debridement and correlation with patient outcomes. Am J Sports Med. 2006;34(9):1486-1491. doi:10.1177/0363546506288016.
- Stapleton TR, Baker CL. Arthroscopic treatment of lateral epicondylitis: a clinical study. Arthroscopy. 1996;1:365-366.
- Hastings H. Open treatment for lateral tennis elbow good for certain indications. Orthop Today. 2009;2:1-2.
- Duparc F, Putz R, Michot C, Muller JM, Fréger P. The synovial fold of the humeroradial joint: anatomical and histological features, and clinical relevance in lateral epicondylalgia of the elbow. Surg Radiol Anat. 2002;24(5):302-307. doi:10.1007/s00276-002-0055-0.
ABSTRACT
This study was performed to compare outcomes of open, arthroscopic, and percutaneous surgical techniques for lateral epicondylitis. We searched PubMed (MEDLINE) for literature published between January 1, 2004 and May 23, 2015 using these key words: lateral epicondylitis AND (surgery OR operative OR surgical OR open OR arthroscopic OR percutaneous). Meta-analyses were performed for outcomes reported in 3 studies using 2-sample and 2-proportion Z-tests. Thirty-five studies including 1640 elbows (1055 open, 401 arthroscopic, 184 percutaneous) met the inclusion criteria. There were no differences between groups regarding duration to return to work, complication rate, or patient satisfaction. A greater proportion of patients were pain free in the open group than in the arthroscopic group (70% vs 60%). Despite the absence of a difference among techniques regarding return to work and subjective function, we recommend open débridement as the technique most likely to achieve a pain-free outcome.
Continue to: Lateral epicondylitis affects...
Lateral epicondylitis affects 1% to 3% of adults each year. Although common, symptoms of lateral epicondylitis resolve spontaneously within a year of symptom onset in 80% of cases, and only 3% of patients who seek medical treatment ultimately require surgical intervention within 2 years of symptom onset.1 Despite a relatively low percentage of patients who require surgery, Sanders and colleagues1 noted a significant increase in the rate of surgical intervention from 1.1% to 3.2% of cases in the last 15 years. Surgical intervention is generally indicated when pain and functional disability persist after 6 to 12 months of nonsurgical treatment. Traditional surgical treatment involves open release/débridement of the extensor carpi radialis (ECRB) origin; however, with the increasing prevalence of surgical intervention, surgeons have demonstrated a rising interest in less invasive techniques like arthroscopic release/débridement and percutaneous tenotomy as alternatives to traditional open débridement. While favorable results have been reported for all 3 techniques, there is no current consensus regarding the optimal surgical technique. In 2007, Lo and Safran2 reported no difference in the results of open, percutaneous, and arthroscopic techniques regarding any outcome measure in a systematic review of 33 papers. We conducted a repeat systematic review of the current literature to update Lo and Safran’s2 review and to ascertain if more recent literature demonstrates superiority of 1 technique regarding pain relief, subjective questionnaire data, subjective satisfaction, restoration of strength, and return to work. We hypothesized that return to work would be accelerated, pain decreased, and function improved in the early postoperative period in the arthroscopic and percutaneous groups, but there would be no difference in ultimate pain, functional outcome, or subjective satisfaction.
METHODS
SEARCH STRATEGY AND STUDY SELECTION
We conducted a systematic review of the literature to update the topic of surgical intervention with lateral epicondylitis since the publication of the most recent review by Lo and Safran2 in 2007, which included all relevant studies published up to 2004. To include all relevant studies published since that time, we searched PubMed (MEDLINE) for all literature published from January 1, 2004 to May 23, 2015 using the following key words: lateral epicondylitis AND (surgery OR operative OR surgical OR open OR arthroscopic OR percutaneous). General search terms were utilized to avoid unintentional exclusion of relevant studies. Two authors reviewed the abstracts of all resultant citations. Table 1 outlines the inclusion and exclusion criteria for the search. References from all included studies were reviewed for applicable articles that were not captured by the initial broad search strategy. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) trial flow chart shows the study selection algorithm (Figure 1).
Table 1. Inclusion and Exclusion Criteria for the Analyzed Studies
Inclusion Criteria | Exclusion Criteria |
|
|

DATA EXTRACTION AND ANALYSIS
Data were extracted from the included studies by 2 reviewers using data abstraction forms. All study, subject, and surgery parameters were collected. The study and subject demographic parameters analyzed included year of publication, level of evidence, presence of study financial conflict of interest, number of subjects and elbows, gender, age, proportion in whom the dominant extremity was involved, proportion who were laborers, proportion who had a workman’s compensation claim, duration of symptoms prior to surgical intervention, and surgical technique employed (open, arthroscopic, or percutaneous). We recorded the following clinical outcomes: proportion of patients with complete pain relief, proportion who were partially or completely satisfied, proportion who were improved, duration to return to work, grip strength, Disabilities of the Arm, Shoulder, and Hand (DASH) score, visual analog scale (VAS) pain score, and complication rate.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Data from all studies were pooled and descriptive statistics were reported as weighted mean ± weighted standard deviation for continuous variables and frequency with percentage for categorical variables. A meta-analysis was performed for all outcome measures that were reported in 3 or more studies within a specific treatment cohort. Data were analyzed using 2-sample and 2-proportion Z-tests. Results were considered statistically significant at P < .05.
RESULTS
LITERATURE RESEARCH
Using the aforementioned search strategy, 154 studies were identified. Following application of the inclusion and exclusion criteria, 35 studies were included in the analysis (Figure 1). One study compared open and percutaneous techniques, and another compared arthroscopic and percutaneous techniques, rendering a total of 19 studies examining open surgical techniques for treatment of lateral epicondylitis,3-21 12 studies examining arthroscopic techniques,14,22-32 and 6 studies reporting percutaneous surgical treatment of lateral epicondylitis29,33-37 (Table 2). There was1 level I study (3%), 6 level III studies (17%), and 28 level IV studies (80%).
Table 2. Study Demographic Data for Open, Arthroscopic, and Percutaneous Lateral Epicondylectomy
| Open | Arthroscopic | Percutaneous | Total |
Number of studies | 19 | 12 | 6 | 35 |
Level of evidence |
|
|
|
|
I | 1 (5%) | 0 | 0 | 1 (3%) |
II | 0 | 0 | 0 | 0 |
III | 3 (16%) | 4 (33%) | 1 (17%) | 6 (17%) |
IV | 15 (79%) | 8 (67%) | 5 (83%) | 28 (80%) |
US: International | 8:12 | 3:9 | 1:5 | 12:24 |
Journals of publication |
|
|
|
|
AJSM | 3 | 1 | 1 | 5 |
JSES | 2 | 2 | 1 | 5 |
Arthroscopy | 2 | 2 | 0 | 3 |
KSSTA | 1 | 2 | 0 | 3 |
CORR | 0 | 2 | 0 | 2 |
JHS | 0 | 1 | 0 | 1 |
JOS | 1 | 1 | 0 | 2 |
AJO | 2 | 0 | 0 | 2 |
Other | 8 | 1 | 4 | 12 |
Abbreviations: AJO, The American Journal of Orthopedics; AJSM, American Journal of Sports Medicine; Arthroscopy, The Journal of Arthroscopy and Related Surgery; CORR, Clinical Orthopaedics & Related Research; JHS, Journal of Hand Surgery; JOS, Journal of Orthopaedic Surgery; JSES, Journal of Shoulder and Elbow Surgery; KSSTA, Knee Surgery, Sports Traumatology, and Arthroscopy.
SUBJECT DEMOGRAPHICS
The 35 included studies comprised 1579 patients and 1640 elbows. Among these, 1055 (64%) elbows underwent open (O), 401 (25%) underwent arthroscopic (A), and 184 (11%) underwent percutaneous (P) treatment. The average age was 45.7 years, 47% of the patients were male, 43% were laborers, 31% had worker’s compensation claims, and the dominant extremity was involved in 62% of patients. The percutaneous cohort was older than the open cohort (P = 46.9, O = 45.4, A = 45.8; P = .036). The duration of symptoms was shorter in the percutaneous cohort than in the other 2 groups and shorter in the arthroscopic cohort than in the open cohort (P = 8 months, O = 23 months, A = 18 months; P < .001). There were no significant differences between groups regarding gender, occupation, worker’s compensation status, or involvement of the dominant extremity (Table 3).
Table 3. Subject Demographics for Open, Arthroscopic, and Percutaneous Groups
| Open | Arthroscopic | Percutaneous |
Subjects (N) | 999 | 397 | 183 |
Elbows (N) | 1055 | 401 | 184 |
Elbows with follow-up (%) | 915 (87%) | 350 (87%) | 181 (98%) |
Males (%) | 427 (47%) | 173 (49%) | 78 (43%) |
Females (%) | 488 (53%) | 177 (51%) | 103 (57%) |
Mean age (years) | 45.4 | 45.8 | 46.9 |
Dominant elbow (%) | 70% | 69% | 53% |
Laborer (%) | 56% | 53% | 48% |
Work comp (%) | 36% | 30% | NR |
Symptoms to operation (months) | 23 | 18 | 8 |
Min. symptoms to operation (months) | 6 | 6 | 3 |
Mean follow-up (months) | 60 | 44 | 11 |
MATA-ANALYSIS CLINICAL OUTCOMES
Clinical outcome results were pooled for all studies reporting the same outcome measure for the same technique (open, arthroscopic, or percutaneous). A meta-analysis was performed for all outcome measures that were reported in a minimum of 3 studies utilizing the same surgical technique (Table 4).

PAIN RELIEF
Thirteen open studies,3,5,7,8,11-16,18,19,21 7 arthroscopic studies14,22-24,26,27,31 and 0 percutaneous studies reported the proportion of patients who were pain free at final follow-up. The proportion of patients who were pain free following open débridement was greater than that in the arthroscopic cohort (O = 70%, A = 60%; P = .009) (Table 4).
Continue to: Subjective improvement and satisfaction...
SUBJECTIVE IMPROVEMENT AND SATISFACTION
Nine open studies, 6 arthroscopic studies, and 1 percutaneous study reported the proportion of patients who felt that their condition had been improved as a result of surgery. There was no difference in the proportion of patients who experienced improvement between the open and arthroscopic cohorts. Four open studies,3,11,12 5 arthroscopic studies,22,26,28,29,32 and 2 percutaneous studies29,36 reported the proportion of patients who were satisfied or partially satisfied with the results of the procedure. There was no difference between the open and arthroscopic groups in the proportion of patients who were satisfied or partially satisfied (Table 4).
RETURN TO WORK
The duration to return to work following surgery was reported in 5 open studies,4,5,10,13,14 9 arthroscopic studies,14,23-29,32 and 2 percutaneous studies.29,36 There was no statistically significant difference between the open and arthroscopic groups with regard to duration to return to work (O = 6.5 weeks, A = 6 weeks; P = .601). The percutaneous technique could not be included in the meta-analysis due to the presence of only 2 studies, but the pooled mean duration to return to work in these 2 studies was 5.5 weeks (Table 4).
GRIP STRENGTH
Postoperative grip strength was reported in 2 open studies,10,19 4 arthroscopic studies,28,30,32 and 2 percutaneous studies.35-36 A meta-analysis could not be performed on all the groups due to the presence of only 2 open and 2 percutaneous studies reporting grip strength. The pooled averages were O = 38.3 kg, A = 34.8 kg, and P = 27.1 kg (Table 4).
DASH SCORE
The postoperative DASH score was reported in 4 open studies,4,15,17,19,20 5 arthroscopic studies,28-31 and 3 percutaneous studies.29,33,36 At final follow-up, the mean DASH score was higher in the arthroscopic group than in the open and percutaneous groups (A = 12.8, O = 19.5, P = 25.3; P < .001 for both comparisons), and the mean DASH score was significantly higher in the open group than in the percutaneous group (P = .029). The reporting of DASH scores in the early postoperative period was not sufficiently consistent to allow us to test our hypothesis that there would be early differences in function between groups (Table 4).
VAS PAIN SCORE
Postoperative VAS pain scores were reported in 11 open studies,6,8-10,12,15,19-21 8 arthroscopic studies,24-26,29-32 and 5 percutaneous studies.29,33,35-37 At final follow-up, there was a lower mean VAS score in the arthroscopic group than in the open and percutaneous groups (A = 1.1, O = 1.9, and P = 2.5; P < .001 for both comparisons) and a lower mean VAS score in the open group than in the percutaneous group (P = .002) (Table 4). Reporting of VAS scores in the early postoperative period in the included studies wan not sufficiently consistent to allow us to test our hypothesis that there would be early differences in pain between groups.
COMPLICATIONS
The complication rate was reported in 15 open studies, 10 arthroscopic studies, and 3 percutaneous studies. There was no difference in the complication rate between the open and arthroscopic techniques (O = 2.4%, A = 1.9%; P = .629) (Table 4). Complications noted in the open cohort included superficial wound infection (6), hematoma (5), synovial fistula (2), seroma (2), and posterior interosseous nerve palsy (1). Complications noted in the arthroscopic cohort included superficial infection (3), hematoma (1), and transient paresthesia (1). Of note, there were no complications in the percutaneous group.
Continue to: Discussion...
DISCUSSION
The primary purpose of this review was to determine if definitive evidence suggests that any 1 of open, percutaneous, or arthroscopic surgical treatment is superior to the other 2 for relieving pain, improving functionality, restoring strength, or accelerating return to work. The most striking finding of this study was a significantly higher proportion of patients who were pain free at final follow-up in the open group than in the arthroscopic group (70% vs 60%, P = .009) (Table 4). At final follow-up, there were no significant differences between groups regarding duration to return to work, proportion who were improved, proportion who were satisfied or partially satisfied, and complication rate. Average VAS and DASH scores at final follow-up were lower in the arthroscopic group than in the open and percutaneous groups (Figure 2). However, although the difference between mean DASH scores in the arthroscopic and open groups (6.7 points) was statistically significant, it is likely not clinically significant, as the minimal clinically important difference (MCID) for the DASH score is 10 points, as demonstrated by Sorensen and colleagues.38 Although it has not been specifically defined for lateral epicondylitis, the MCID for VAS pain has been reported in the literature to range from 1.0 to 1.4.39-40 Therefore, as for the DASH score, the difference witnessed between the open and arthroscopic groups (0.8) is likely not clinically significant. Of note, the differences between values for arthroscopic and percutaneous techniques are greater than the MCID.

In light of a recent increase in the prevalence of surgical intervention for lateral epicondylitis, many authors have promoted arthroscopic and percutaneous techniques as alternatives to traditional open débridement with the goal of achieving the same results with decreased morbidity and accelerated return to work. Given the increased proportion of patients who were pain free at final follow-up in the open cohort, it is our contention that open release/débridement of the common extensor/ECRB origin allows the surgeon to fully appreciate the extent of tendinotic tissue that is contributing to the patient’s symptoms and to address the pathology in its entirety. Other authors have also questioned whether the full extent of extra-articular tendinosis can be accurately identified arthroscopically. Cummins41 demonstrated, in a series of 18 patients who underwent arthroscopic ECRB débridement, that 6 patients had residual tendinosis upon open evaluation and 10 had residual tendinosis on histologic assessment. Additionally, in the same series, residual tendinopathy was associated with poorer clinical outcomes.
The improved visualization associated with an open technique comes at minimal expense, as the incision was only 1.5 cm to 5 cm in 13 of 15 papers reporting incision length.3,4,6,8-11,13,15,18-20 This increased exposure may not translate into increased morbidity, as there was no increase in the duration to return to work nor the complication rate. As a result of the extensive instrumentation necessary for arthroscopic techniques, open techniques also appear to be less expensive. Analyses in the literature have suggested increased expenditures associated with arthroscopic treatment ranging from 23%42 to 100%43 greater than those of open treatment.
Although obvious, it should be noted that a percutaneous tenotomy does not permit assessment of the extent of pathologic tendinosis. As a result of an inability to visualize and débride pathologic tissue, percutaneous tenotomy rendered inferior outcomes to open and arthroscopic techniques in terms of both postoperative VAS pain score and DASH score. Nonetheless, it is a relatively rapid and simple technique and resulted in zero complications in 184 elbows. Overall, percutaneous tenotomy appears to be an inferior technique to open and arthroscopic techniques in terms of achieving complete pain relief and optimal functional recovery; however, it may be useful in those who wish to avoid a more invasive intervention.
LIMITATIONS
The most significant limitation of this study was the heterogeneity in the techniques utilized in each group. Among the 19 papers in the open cohort, 11 used techniques aimed at lengthening or release of the extensor origin, 7 performed débridement of tendinotic tissue at the ECRB origin, and 1 compared these approaches. Exposures ranged from 1.5 cm to 8 cm in length, 3 techniques added tendon repair following débridement, and 2 utilized a radiofrequency device.
Among the 12 papers in the arthroscopic cohort, 8 performed arthroscopic (inside-out) débridement of the tendinotic tissue at the ECRB origin, 3 performed arthroscopic release of the ECRB tendon, and 1 performed endoscopic ECRB release in an outside-in fashion. Four techniques added posterior synovial plica excision and 4 added decortication of the lateral epicondyle débridement or release. Some authors advocate for arthroscopic intervention on the grounds that it permits evaluation and correction of other intra-articular pathology. With this in mind, some authors have suggested that a synovial fold (plica) adjacent to the radiocapitellar joint may contribute to lateral elbow pain.27,44 Nevertheless, in the only comparative trial in the literature, Rhyou and Kim30 demonstrated that excision of posterior synovial fold failed to enhance pain relief or function in a retrospective cohort study comparing arthroscopic débridement with and without plica excision.
Continue to: Some authors advocate...
Some authors advocate decorticating the non-articular, lateral epicondyle with a shaver to stimulate bleeding and promote a healing response. However, 1 study in our review compared arthroscopic ECRB release with and without decortication and found that decortication significantly increased pain up to 4 weeks postoperatively, increased duration to return to work, and did not improve the ultimate clinical result.25 Of note, others have used a similar rationale to advocate drilling the lateral epicondyle when utilizing an open technique. However, Dunn and colleagues8 note that they have modified the Nirschl technique to eliminate drilling because they feel it increases postoperative pain and may damage the extensor digitorum communis origin.
Among the 6 papers in the percutaneous tenotomy cohort, 2 performed tenotomy with a hypodermic needle, 2 with a scalpel through a limited incision (0.5 cm-1 cm), 1 using a TX1 tissue removal system (Tenex Health), and 1 with a percutaneous radiofrequency probe. In 3 techniques, ultrasound was used to direct the tenotomy.
The quality of this review is also limited by the studies included for analysis, as with any systematic review. Because 28 of the 35 included studies were classified as evidence level IV, the likelihood of methodological bias is increased. The majority of studies contained ≥1 demonstrable biases, including selection, detection, attrition biases, or a combination. Selection bias is prevalent among predominantly level IV studies, in which the authors have selected their preferred surgical technique. There was heterogeneity in the reporting of preoperative variables and the outcome measures that were utilized. Scoring systems, such as the Nirschl Tennis Elbow Score and the Mayo Elbow Performance Index, would have been valuable in comparing the groups had they been more consistently reported. The heterogeneity in clinical outcome tools and the lack of reported outcome variance or standard deviations prevented a formal meta-analysis of some of these outcome measures. Due to inconsistent reporting, we were also unable to test our hypothesis that there would be less pain and improved function in the arthroscopic and/or percutaneous cohorts in the early postoperative period compared to the open cohort due to the less invasive techniques used. Although the differences in DASH and VAS scores at final follow-up likely did not meet the MCID threshold, these differences may have been greater and more clinically relevant in the early postoperative period.
CONCLUSION
We hypothesized that the arthroscopic and percutaneous groups would experience accelerated return to work and reduced pain in the early postoperative period but no difference in ultimate pain, functional outcome, or subjective satisfaction. There is no difference between open, arthroscopic, and percutaneous surgical treatment for lateral epicondylitis regarding return to work and subjective satisfaction; however, open treatment led to a greater percentage of patients being pain free at final follow-up. While arthroscopic treatment led to better pain and functional scores at final follow-up, the absolute differences were quite small and likely not clinically significant. In light of the available evidence, we recommend open débridement as the best means of minimizing cost and achieving a pain-free outcome in the long term. For future investigators, it would be useful to perform a randomized clinical study directly comparing open, arthroscopic, and percutaneous techniques, including assessment of pain and functional scores in the early postoperative period, and to further evaluate differences in cost among the various techniques.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
This study was performed to compare outcomes of open, arthroscopic, and percutaneous surgical techniques for lateral epicondylitis. We searched PubMed (MEDLINE) for literature published between January 1, 2004 and May 23, 2015 using these key words: lateral epicondylitis AND (surgery OR operative OR surgical OR open OR arthroscopic OR percutaneous). Meta-analyses were performed for outcomes reported in 3 studies using 2-sample and 2-proportion Z-tests. Thirty-five studies including 1640 elbows (1055 open, 401 arthroscopic, 184 percutaneous) met the inclusion criteria. There were no differences between groups regarding duration to return to work, complication rate, or patient satisfaction. A greater proportion of patients were pain free in the open group than in the arthroscopic group (70% vs 60%). Despite the absence of a difference among techniques regarding return to work and subjective function, we recommend open débridement as the technique most likely to achieve a pain-free outcome.
Continue to: Lateral epicondylitis affects...
Lateral epicondylitis affects 1% to 3% of adults each year. Although common, symptoms of lateral epicondylitis resolve spontaneously within a year of symptom onset in 80% of cases, and only 3% of patients who seek medical treatment ultimately require surgical intervention within 2 years of symptom onset.1 Despite a relatively low percentage of patients who require surgery, Sanders and colleagues1 noted a significant increase in the rate of surgical intervention from 1.1% to 3.2% of cases in the last 15 years. Surgical intervention is generally indicated when pain and functional disability persist after 6 to 12 months of nonsurgical treatment. Traditional surgical treatment involves open release/débridement of the extensor carpi radialis (ECRB) origin; however, with the increasing prevalence of surgical intervention, surgeons have demonstrated a rising interest in less invasive techniques like arthroscopic release/débridement and percutaneous tenotomy as alternatives to traditional open débridement. While favorable results have been reported for all 3 techniques, there is no current consensus regarding the optimal surgical technique. In 2007, Lo and Safran2 reported no difference in the results of open, percutaneous, and arthroscopic techniques regarding any outcome measure in a systematic review of 33 papers. We conducted a repeat systematic review of the current literature to update Lo and Safran’s2 review and to ascertain if more recent literature demonstrates superiority of 1 technique regarding pain relief, subjective questionnaire data, subjective satisfaction, restoration of strength, and return to work. We hypothesized that return to work would be accelerated, pain decreased, and function improved in the early postoperative period in the arthroscopic and percutaneous groups, but there would be no difference in ultimate pain, functional outcome, or subjective satisfaction.
METHODS
SEARCH STRATEGY AND STUDY SELECTION
We conducted a systematic review of the literature to update the topic of surgical intervention with lateral epicondylitis since the publication of the most recent review by Lo and Safran2 in 2007, which included all relevant studies published up to 2004. To include all relevant studies published since that time, we searched PubMed (MEDLINE) for all literature published from January 1, 2004 to May 23, 2015 using the following key words: lateral epicondylitis AND (surgery OR operative OR surgical OR open OR arthroscopic OR percutaneous). General search terms were utilized to avoid unintentional exclusion of relevant studies. Two authors reviewed the abstracts of all resultant citations. Table 1 outlines the inclusion and exclusion criteria for the search. References from all included studies were reviewed for applicable articles that were not captured by the initial broad search strategy. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) trial flow chart shows the study selection algorithm (Figure 1).
Table 1. Inclusion and Exclusion Criteria for the Analyzed Studies
Inclusion Criteria | Exclusion Criteria |
|
|

DATA EXTRACTION AND ANALYSIS
Data were extracted from the included studies by 2 reviewers using data abstraction forms. All study, subject, and surgery parameters were collected. The study and subject demographic parameters analyzed included year of publication, level of evidence, presence of study financial conflict of interest, number of subjects and elbows, gender, age, proportion in whom the dominant extremity was involved, proportion who were laborers, proportion who had a workman’s compensation claim, duration of symptoms prior to surgical intervention, and surgical technique employed (open, arthroscopic, or percutaneous). We recorded the following clinical outcomes: proportion of patients with complete pain relief, proportion who were partially or completely satisfied, proportion who were improved, duration to return to work, grip strength, Disabilities of the Arm, Shoulder, and Hand (DASH) score, visual analog scale (VAS) pain score, and complication rate.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Data from all studies were pooled and descriptive statistics were reported as weighted mean ± weighted standard deviation for continuous variables and frequency with percentage for categorical variables. A meta-analysis was performed for all outcome measures that were reported in 3 or more studies within a specific treatment cohort. Data were analyzed using 2-sample and 2-proportion Z-tests. Results were considered statistically significant at P < .05.
RESULTS
LITERATURE RESEARCH
Using the aforementioned search strategy, 154 studies were identified. Following application of the inclusion and exclusion criteria, 35 studies were included in the analysis (Figure 1). One study compared open and percutaneous techniques, and another compared arthroscopic and percutaneous techniques, rendering a total of 19 studies examining open surgical techniques for treatment of lateral epicondylitis,3-21 12 studies examining arthroscopic techniques,14,22-32 and 6 studies reporting percutaneous surgical treatment of lateral epicondylitis29,33-37 (Table 2). There was1 level I study (3%), 6 level III studies (17%), and 28 level IV studies (80%).
Table 2. Study Demographic Data for Open, Arthroscopic, and Percutaneous Lateral Epicondylectomy
| Open | Arthroscopic | Percutaneous | Total |
Number of studies | 19 | 12 | 6 | 35 |
Level of evidence |
|
|
|
|
I | 1 (5%) | 0 | 0 | 1 (3%) |
II | 0 | 0 | 0 | 0 |
III | 3 (16%) | 4 (33%) | 1 (17%) | 6 (17%) |
IV | 15 (79%) | 8 (67%) | 5 (83%) | 28 (80%) |
US: International | 8:12 | 3:9 | 1:5 | 12:24 |
Journals of publication |
|
|
|
|
AJSM | 3 | 1 | 1 | 5 |
JSES | 2 | 2 | 1 | 5 |
Arthroscopy | 2 | 2 | 0 | 3 |
KSSTA | 1 | 2 | 0 | 3 |
CORR | 0 | 2 | 0 | 2 |
JHS | 0 | 1 | 0 | 1 |
JOS | 1 | 1 | 0 | 2 |
AJO | 2 | 0 | 0 | 2 |
Other | 8 | 1 | 4 | 12 |
Abbreviations: AJO, The American Journal of Orthopedics; AJSM, American Journal of Sports Medicine; Arthroscopy, The Journal of Arthroscopy and Related Surgery; CORR, Clinical Orthopaedics & Related Research; JHS, Journal of Hand Surgery; JOS, Journal of Orthopaedic Surgery; JSES, Journal of Shoulder and Elbow Surgery; KSSTA, Knee Surgery, Sports Traumatology, and Arthroscopy.
SUBJECT DEMOGRAPHICS
The 35 included studies comprised 1579 patients and 1640 elbows. Among these, 1055 (64%) elbows underwent open (O), 401 (25%) underwent arthroscopic (A), and 184 (11%) underwent percutaneous (P) treatment. The average age was 45.7 years, 47% of the patients were male, 43% were laborers, 31% had worker’s compensation claims, and the dominant extremity was involved in 62% of patients. The percutaneous cohort was older than the open cohort (P = 46.9, O = 45.4, A = 45.8; P = .036). The duration of symptoms was shorter in the percutaneous cohort than in the other 2 groups and shorter in the arthroscopic cohort than in the open cohort (P = 8 months, O = 23 months, A = 18 months; P < .001). There were no significant differences between groups regarding gender, occupation, worker’s compensation status, or involvement of the dominant extremity (Table 3).
Table 3. Subject Demographics for Open, Arthroscopic, and Percutaneous Groups
| Open | Arthroscopic | Percutaneous |
Subjects (N) | 999 | 397 | 183 |
Elbows (N) | 1055 | 401 | 184 |
Elbows with follow-up (%) | 915 (87%) | 350 (87%) | 181 (98%) |
Males (%) | 427 (47%) | 173 (49%) | 78 (43%) |
Females (%) | 488 (53%) | 177 (51%) | 103 (57%) |
Mean age (years) | 45.4 | 45.8 | 46.9 |
Dominant elbow (%) | 70% | 69% | 53% |
Laborer (%) | 56% | 53% | 48% |
Work comp (%) | 36% | 30% | NR |
Symptoms to operation (months) | 23 | 18 | 8 |
Min. symptoms to operation (months) | 6 | 6 | 3 |
Mean follow-up (months) | 60 | 44 | 11 |
MATA-ANALYSIS CLINICAL OUTCOMES
Clinical outcome results were pooled for all studies reporting the same outcome measure for the same technique (open, arthroscopic, or percutaneous). A meta-analysis was performed for all outcome measures that were reported in a minimum of 3 studies utilizing the same surgical technique (Table 4).

PAIN RELIEF
Thirteen open studies,3,5,7,8,11-16,18,19,21 7 arthroscopic studies14,22-24,26,27,31 and 0 percutaneous studies reported the proportion of patients who were pain free at final follow-up. The proportion of patients who were pain free following open débridement was greater than that in the arthroscopic cohort (O = 70%, A = 60%; P = .009) (Table 4).
Continue to: Subjective improvement and satisfaction...
SUBJECTIVE IMPROVEMENT AND SATISFACTION
Nine open studies, 6 arthroscopic studies, and 1 percutaneous study reported the proportion of patients who felt that their condition had been improved as a result of surgery. There was no difference in the proportion of patients who experienced improvement between the open and arthroscopic cohorts. Four open studies,3,11,12 5 arthroscopic studies,22,26,28,29,32 and 2 percutaneous studies29,36 reported the proportion of patients who were satisfied or partially satisfied with the results of the procedure. There was no difference between the open and arthroscopic groups in the proportion of patients who were satisfied or partially satisfied (Table 4).
RETURN TO WORK
The duration to return to work following surgery was reported in 5 open studies,4,5,10,13,14 9 arthroscopic studies,14,23-29,32 and 2 percutaneous studies.29,36 There was no statistically significant difference between the open and arthroscopic groups with regard to duration to return to work (O = 6.5 weeks, A = 6 weeks; P = .601). The percutaneous technique could not be included in the meta-analysis due to the presence of only 2 studies, but the pooled mean duration to return to work in these 2 studies was 5.5 weeks (Table 4).
GRIP STRENGTH
Postoperative grip strength was reported in 2 open studies,10,19 4 arthroscopic studies,28,30,32 and 2 percutaneous studies.35-36 A meta-analysis could not be performed on all the groups due to the presence of only 2 open and 2 percutaneous studies reporting grip strength. The pooled averages were O = 38.3 kg, A = 34.8 kg, and P = 27.1 kg (Table 4).
DASH SCORE
The postoperative DASH score was reported in 4 open studies,4,15,17,19,20 5 arthroscopic studies,28-31 and 3 percutaneous studies.29,33,36 At final follow-up, the mean DASH score was higher in the arthroscopic group than in the open and percutaneous groups (A = 12.8, O = 19.5, P = 25.3; P < .001 for both comparisons), and the mean DASH score was significantly higher in the open group than in the percutaneous group (P = .029). The reporting of DASH scores in the early postoperative period was not sufficiently consistent to allow us to test our hypothesis that there would be early differences in function between groups (Table 4).
VAS PAIN SCORE
Postoperative VAS pain scores were reported in 11 open studies,6,8-10,12,15,19-21 8 arthroscopic studies,24-26,29-32 and 5 percutaneous studies.29,33,35-37 At final follow-up, there was a lower mean VAS score in the arthroscopic group than in the open and percutaneous groups (A = 1.1, O = 1.9, and P = 2.5; P < .001 for both comparisons) and a lower mean VAS score in the open group than in the percutaneous group (P = .002) (Table 4). Reporting of VAS scores in the early postoperative period in the included studies wan not sufficiently consistent to allow us to test our hypothesis that there would be early differences in pain between groups.
COMPLICATIONS
The complication rate was reported in 15 open studies, 10 arthroscopic studies, and 3 percutaneous studies. There was no difference in the complication rate between the open and arthroscopic techniques (O = 2.4%, A = 1.9%; P = .629) (Table 4). Complications noted in the open cohort included superficial wound infection (6), hematoma (5), synovial fistula (2), seroma (2), and posterior interosseous nerve palsy (1). Complications noted in the arthroscopic cohort included superficial infection (3), hematoma (1), and transient paresthesia (1). Of note, there were no complications in the percutaneous group.
Continue to: Discussion...
DISCUSSION
The primary purpose of this review was to determine if definitive evidence suggests that any 1 of open, percutaneous, or arthroscopic surgical treatment is superior to the other 2 for relieving pain, improving functionality, restoring strength, or accelerating return to work. The most striking finding of this study was a significantly higher proportion of patients who were pain free at final follow-up in the open group than in the arthroscopic group (70% vs 60%, P = .009) (Table 4). At final follow-up, there were no significant differences between groups regarding duration to return to work, proportion who were improved, proportion who were satisfied or partially satisfied, and complication rate. Average VAS and DASH scores at final follow-up were lower in the arthroscopic group than in the open and percutaneous groups (Figure 2). However, although the difference between mean DASH scores in the arthroscopic and open groups (6.7 points) was statistically significant, it is likely not clinically significant, as the minimal clinically important difference (MCID) for the DASH score is 10 points, as demonstrated by Sorensen and colleagues.38 Although it has not been specifically defined for lateral epicondylitis, the MCID for VAS pain has been reported in the literature to range from 1.0 to 1.4.39-40 Therefore, as for the DASH score, the difference witnessed between the open and arthroscopic groups (0.8) is likely not clinically significant. Of note, the differences between values for arthroscopic and percutaneous techniques are greater than the MCID.

In light of a recent increase in the prevalence of surgical intervention for lateral epicondylitis, many authors have promoted arthroscopic and percutaneous techniques as alternatives to traditional open débridement with the goal of achieving the same results with decreased morbidity and accelerated return to work. Given the increased proportion of patients who were pain free at final follow-up in the open cohort, it is our contention that open release/débridement of the common extensor/ECRB origin allows the surgeon to fully appreciate the extent of tendinotic tissue that is contributing to the patient’s symptoms and to address the pathology in its entirety. Other authors have also questioned whether the full extent of extra-articular tendinosis can be accurately identified arthroscopically. Cummins41 demonstrated, in a series of 18 patients who underwent arthroscopic ECRB débridement, that 6 patients had residual tendinosis upon open evaluation and 10 had residual tendinosis on histologic assessment. Additionally, in the same series, residual tendinopathy was associated with poorer clinical outcomes.
The improved visualization associated with an open technique comes at minimal expense, as the incision was only 1.5 cm to 5 cm in 13 of 15 papers reporting incision length.3,4,6,8-11,13,15,18-20 This increased exposure may not translate into increased morbidity, as there was no increase in the duration to return to work nor the complication rate. As a result of the extensive instrumentation necessary for arthroscopic techniques, open techniques also appear to be less expensive. Analyses in the literature have suggested increased expenditures associated with arthroscopic treatment ranging from 23%42 to 100%43 greater than those of open treatment.
Although obvious, it should be noted that a percutaneous tenotomy does not permit assessment of the extent of pathologic tendinosis. As a result of an inability to visualize and débride pathologic tissue, percutaneous tenotomy rendered inferior outcomes to open and arthroscopic techniques in terms of both postoperative VAS pain score and DASH score. Nonetheless, it is a relatively rapid and simple technique and resulted in zero complications in 184 elbows. Overall, percutaneous tenotomy appears to be an inferior technique to open and arthroscopic techniques in terms of achieving complete pain relief and optimal functional recovery; however, it may be useful in those who wish to avoid a more invasive intervention.
LIMITATIONS
The most significant limitation of this study was the heterogeneity in the techniques utilized in each group. Among the 19 papers in the open cohort, 11 used techniques aimed at lengthening or release of the extensor origin, 7 performed débridement of tendinotic tissue at the ECRB origin, and 1 compared these approaches. Exposures ranged from 1.5 cm to 8 cm in length, 3 techniques added tendon repair following débridement, and 2 utilized a radiofrequency device.
Among the 12 papers in the arthroscopic cohort, 8 performed arthroscopic (inside-out) débridement of the tendinotic tissue at the ECRB origin, 3 performed arthroscopic release of the ECRB tendon, and 1 performed endoscopic ECRB release in an outside-in fashion. Four techniques added posterior synovial plica excision and 4 added decortication of the lateral epicondyle débridement or release. Some authors advocate for arthroscopic intervention on the grounds that it permits evaluation and correction of other intra-articular pathology. With this in mind, some authors have suggested that a synovial fold (plica) adjacent to the radiocapitellar joint may contribute to lateral elbow pain.27,44 Nevertheless, in the only comparative trial in the literature, Rhyou and Kim30 demonstrated that excision of posterior synovial fold failed to enhance pain relief or function in a retrospective cohort study comparing arthroscopic débridement with and without plica excision.
Continue to: Some authors advocate...
Some authors advocate decorticating the non-articular, lateral epicondyle with a shaver to stimulate bleeding and promote a healing response. However, 1 study in our review compared arthroscopic ECRB release with and without decortication and found that decortication significantly increased pain up to 4 weeks postoperatively, increased duration to return to work, and did not improve the ultimate clinical result.25 Of note, others have used a similar rationale to advocate drilling the lateral epicondyle when utilizing an open technique. However, Dunn and colleagues8 note that they have modified the Nirschl technique to eliminate drilling because they feel it increases postoperative pain and may damage the extensor digitorum communis origin.
Among the 6 papers in the percutaneous tenotomy cohort, 2 performed tenotomy with a hypodermic needle, 2 with a scalpel through a limited incision (0.5 cm-1 cm), 1 using a TX1 tissue removal system (Tenex Health), and 1 with a percutaneous radiofrequency probe. In 3 techniques, ultrasound was used to direct the tenotomy.
The quality of this review is also limited by the studies included for analysis, as with any systematic review. Because 28 of the 35 included studies were classified as evidence level IV, the likelihood of methodological bias is increased. The majority of studies contained ≥1 demonstrable biases, including selection, detection, attrition biases, or a combination. Selection bias is prevalent among predominantly level IV studies, in which the authors have selected their preferred surgical technique. There was heterogeneity in the reporting of preoperative variables and the outcome measures that were utilized. Scoring systems, such as the Nirschl Tennis Elbow Score and the Mayo Elbow Performance Index, would have been valuable in comparing the groups had they been more consistently reported. The heterogeneity in clinical outcome tools and the lack of reported outcome variance or standard deviations prevented a formal meta-analysis of some of these outcome measures. Due to inconsistent reporting, we were also unable to test our hypothesis that there would be less pain and improved function in the arthroscopic and/or percutaneous cohorts in the early postoperative period compared to the open cohort due to the less invasive techniques used. Although the differences in DASH and VAS scores at final follow-up likely did not meet the MCID threshold, these differences may have been greater and more clinically relevant in the early postoperative period.
CONCLUSION
We hypothesized that the arthroscopic and percutaneous groups would experience accelerated return to work and reduced pain in the early postoperative period but no difference in ultimate pain, functional outcome, or subjective satisfaction. There is no difference between open, arthroscopic, and percutaneous surgical treatment for lateral epicondylitis regarding return to work and subjective satisfaction; however, open treatment led to a greater percentage of patients being pain free at final follow-up. While arthroscopic treatment led to better pain and functional scores at final follow-up, the absolute differences were quite small and likely not clinically significant. In light of the available evidence, we recommend open débridement as the best means of minimizing cost and achieving a pain-free outcome in the long term. For future investigators, it would be useful to perform a randomized clinical study directly comparing open, arthroscopic, and percutaneous techniques, including assessment of pain and functional scores in the early postoperative period, and to further evaluate differences in cost among the various techniques.
This paper will be judged for the Resident Writer’s Award.
- Sanders TL Jr, Maradit Kremers H, Bryan AJ, Ransom JE, Smith J, Morrey BF. The epidemiology and health care burden of tennis elbow: a population-based study. Am J Sports Med. 2015;43(5):1066-1071. doi:10.1177/0363546514568087.
- Lo MY, Safran MR. Surgical treatment of lateral epicondylitis: a systematic review. Clin Orthop Relat Res. 2007;463:98-106. doi:10.1097/BLO.0b013e3181483dc4.
- Balk ML, Hagberg WC, Buterbaugh GA, Imbriglia JE. Outcome of surgery for lateral epicondylitis (tennis elbow): effect of worker’s compensation. Am J Orthop. 2005;34(3):122-126; discussion 126.
- Barth J, Mahieu P, Hollevoet N. Extensor tendon and fascia sectioning of extensors at the musculotendinous unit in lateral epicondylitis. Acta Orthop Belg. 2013;79(3):266-270.
- Bigorre N, Raimbeau G, Fouque PA, Cast YS, Rabarin F, Cesari B. Lateral epicondylitis treatment by extensor carpi radialis fasciotomy and radial nerve decompression: is outcome influenced by the occupational disease compensation aspect? Orthop Traumatol Surg Res. 2011;97(2):159-163. doi:10.1016/j.otsr.2010.11.007.
- Cho BK, Kim YM, Kim DS, et al. Mini-open muscle resection procedure under local anesthesia for lateral and medial epicondylitis. Clin Orthop Surg. 2009;1(3):123-127. doi:10.4055/cios.2009.1.3.123.
- Coleman B, Quinlan JF, Matheson JA. Surgical treatment for lateral epicondylitis: a long-term follow-up of results. J Shoulder Elbow Surg. 2010;19(3):363-367. doi:10.1016/j.jse.2009.09.008.
- Dunn JH, Kim JJ, Davis L, Nirschl RP. Ten- to 14-year follow-up of the Nirschl surgical technique for lateral epicondylitis. Am J Sports Med. 2008;36(2):261-266. doi:10.1177/0363546507308932.
- Manon-Matos Y, Oron A, Wolff TW. Combined common extensor and supinator aponeurotomy for the treatment of lateral epicondylitis. Tech Hand Up Extrem Surg. 2013;17(3):179-181. doi:10.1097/BTH.0b013e31829e0eeb.
- Meknas K, Odden-Miland A, Mercer JB, Castillejo M, Johansen O. Radiofrequency microtenotomy: a promising method for treatment of recalcitrant lateral epicondylitis. Am J Sports Med. 2008;36(10):1960-1965. doi:10.1177/0363546508318045.
- Pruzansky ME, Gantsoudes GD, Watters N. Late surgical results of reattachment to bone in repair of chronic lateral epicondylitis. Am J Orthop. 2009;38(6):295-299.
- Rayan F, Rao V Sr, Purushothamdas S, Mukundan C, Shafqat SO. Common extensor origin release in recalcitrant lateral epicondylitis – role justified? J Orthop Surg Res. 2010;5:31. doi:10.1186/1749-799X-5-31.
- Reddy VR, Satheesan KS, Bayliss N. Outcome of Boyd-McLeod procedure for recalcitrant lateral epicondylitis of elbow. Rheumatol Int. 2011;31(8):1081-1084. doi:10.1007/s00296-010-1450-1.
- Rubenthaler F, Wiese M, Senge A, Keller L, Wittenberg RH. Long-term follow-up of open and endoscopic Hohmann procedures for lateral epicondylitis. Arthroscopy. 2005;21(6):684-690. doi:10.1016/j.arthro.2005.03.017.
- Ruch DS, Orr SB, Richard MJ, Leversedge FJ, Mithani SK, Laino DK. A comparison of debridement with and without anconeus muscle flap for treatment of refractory lateral epicondylitis. J Shoulder Elbow Surg. 2015;24(2):236-241. doi:10.1016/j.jse.2014.09.035.
- Siddiqui MA, Koh J, Kua J, Cheung T, Chang P. Functional outcome assessment after open tennis elbow release: what are the predictor parameters? Singapore Med J. 2011;52(2):73-76.
- Solheim E, Hegna J, Øyen J. Extensor tendon release in tennis elbow: results and prognostic factors in 80 elbows. Knee Surg Sports Traumatol Arthrosc. 2011;19(6):1023-1027. doi:10.1007/s00167-011-1477-1.
- Svernlöv B, Adolfsson L. Outcome of release of the lateral extensor muscle origin for epicondylitis. Scand J Plast Reconstr Surg Hand Surg. 2006;40(3):161-165. doi:10.1080/02844310500491492.
- Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21(7):851-860. doi:10.1016/j.arthro.2005.03.019.
- Thornton SJ, Rogers JR, Prickett WD, Dunn WR, Allen AA, Hannafin JA. Treatment of recalcitrant lateral epicondylitis with suture anchor repair. Am J Sports Med. 2005;33(10):1558-1564. doi:10.1177/0363546505276758.
- Wang AW, Erak S. Fractional lengthening of forearm extensors for resistant lateral epicondylitis. ANZ J Surg. 2007;77(11):981-984. doi:10.1111/j.1445-2197.2007.04294.x.
- Baker CL Jr, Baker CL 3rd. Long-term follow-up of arthroscopic treatment of lateral epicondylitis. Am J Sports Med. 2008;36(2):254-260. doi:10.1177/0363546507311599.
- Grewal R, MacDermid JC, Shah P, King GJ. Functional outcome of arthroscopic extensor carpi radialis brevis tendon release in chronic lateral epicondylitis. J Hand Surg Am. 2009;34(5):849-857. doi:10.1016/j.jhsa.2009.02.006.
- Jerosch J, Schunck J. Arthroscopic treatment of lateral epicondylitis: indication, technique and early results. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):379-382. doi:10.1007/s00167-005-0662-5.
- Kim JW, Chun CH, Shim DM, et al. Arthroscopic treatment of lateral epicondylitis: comparison of the outcome of ECRB release with and without decortication. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1178-1183. doi:10.1007/s00167-011-1507-z.
- Lattermann C, Romeo AA, Anbari A, et al. Arthroscopic debridement of the extensor carpi radialis brevis for recalcitrant lateral epicondylitis. J Shoulder Elbow Surg. 2010;19(5):651-656. doi:10.1016/j.jse.2010.02.008.
- Mullett H, Sprague M, Brown G, Hausman M. Arthroscopic treatment of lateral epicondylitis: clinical and cadaveric studies. Clin Orthop Relat Res. 2005;439:123-128. doi:10.1097/01.blo.0000176143.08886.fe.
- Oki G, Iba K, Sasaki K, Yamashita T, Wada T. Time to functional recovery after arthroscopic surgery for tennis elbow. J Shoulder Elbow Surg. 2014;23(10):1527-1531. doi:10.1016/j.jse.2014.05.010.
- Othman AM. Arthroscopic versus percutaneous release of common extensor origin for treatment of chronic tennis elbow. Arch Orthop Trauma Surg. 2011;131(3):383-388. doi:10.1007/s00402-011-1260-2.
- Rhyou IH, Kim KW. Is posterior synovial plica excision necessary for refractory lateral epicondylitis of the elbow? Clin Orthop Relat Res. 2013;471(1):284-290. doi:10.1007/s11999-012-2585-z.
- Wada T, Moriya T, Iba K, et al. Functional outcomes after arthroscopic treatment of lateral epicondylitis. J Orthop Sci. 2009;14(2):167-174. doi:10.1007/s00776-008-1304-9.
- Yoon JP, Chung SW, Yi JH, et al. Prognostic factors of arthroscopic extensor carpi radialis brevis release for lateral epicondylitis. Arthroscopy. 2015;31(7):1232-1237. doi:10.1016/j.arthro.2015.02.006.
- Barnes DE, Beckley JM, Smith J. Percutaneous ultrasonic tenotomy for chronic elbow tendinosis: a prospective study. J Shoulder Elbow Surg. 2015;24(1):67-73. doi:10.1016/j.jse.2014.07.017.
- Kaleli T, Ozturk C, Temiz A, Tirelioglu O. Surgical treatment of tennis elbow: percutaneous release of the common extensor origin. Acta Orthop Belg. 2004;70(2):131-133.
- Lin MT, Chou LW, Chen HS, Kao MJ. Percutaneous soft tissue release for treating chronic recurrent myofascial pain associated with lateral epicondylitis: 6 case studies. Evid Based Complement Alternat Med. 2012;2012:142941. doi:10.1155/2012/142941.
- Lin CL, Lee JS, Su WR, Kuo LC, Tai TW, Jou IM. Clinical and ultrasonographic results of ultrasonographically guided percutaneous radiofrequency lesioning in the treatment of recalcitrant lateral epicondylitis. Am J Sports Med. 2011;39(11):2429-2435. doi:10.1177/0363546511417096.
- Zhu J, Hu B, Xing C, Li J. Ultrasound-guided, minimally invasive, percutaneous needle puncture treatment for tennis elbow. Adv Ther. 2008;25(10):1031-1036. doi:10.1007/s12325-008-0099-6.
- Sorensen AA, Howard D, Tan WH, Ketchersid J, Calfee RP. Minimal clinically important differences of 3 patient-related outcomes instruments. J Hand Surg Am. 2013;38(4):641-649. doi:10.1016/j.jhsa.2012.12.032.
- Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;18(3):205-207. doi:10.1136/emj.18.3.205.
- Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elbow Surg. 2009;18(6):927-932. doi:10.1016/j.jse.2009.03.021.
- Cummins CA. Lateral epicondylitis: in vivo assessment of arthroscopic debridement and correlation with patient outcomes. Am J Sports Med. 2006;34(9):1486-1491. doi:10.1177/0363546506288016.
- Stapleton TR, Baker CL. Arthroscopic treatment of lateral epicondylitis: a clinical study. Arthroscopy. 1996;1:365-366.
- Hastings H. Open treatment for lateral tennis elbow good for certain indications. Orthop Today. 2009;2:1-2.
- Duparc F, Putz R, Michot C, Muller JM, Fréger P. The synovial fold of the humeroradial joint: anatomical and histological features, and clinical relevance in lateral epicondylalgia of the elbow. Surg Radiol Anat. 2002;24(5):302-307. doi:10.1007/s00276-002-0055-0.
- Sanders TL Jr, Maradit Kremers H, Bryan AJ, Ransom JE, Smith J, Morrey BF. The epidemiology and health care burden of tennis elbow: a population-based study. Am J Sports Med. 2015;43(5):1066-1071. doi:10.1177/0363546514568087.
- Lo MY, Safran MR. Surgical treatment of lateral epicondylitis: a systematic review. Clin Orthop Relat Res. 2007;463:98-106. doi:10.1097/BLO.0b013e3181483dc4.
- Balk ML, Hagberg WC, Buterbaugh GA, Imbriglia JE. Outcome of surgery for lateral epicondylitis (tennis elbow): effect of worker’s compensation. Am J Orthop. 2005;34(3):122-126; discussion 126.
- Barth J, Mahieu P, Hollevoet N. Extensor tendon and fascia sectioning of extensors at the musculotendinous unit in lateral epicondylitis. Acta Orthop Belg. 2013;79(3):266-270.
- Bigorre N, Raimbeau G, Fouque PA, Cast YS, Rabarin F, Cesari B. Lateral epicondylitis treatment by extensor carpi radialis fasciotomy and radial nerve decompression: is outcome influenced by the occupational disease compensation aspect? Orthop Traumatol Surg Res. 2011;97(2):159-163. doi:10.1016/j.otsr.2010.11.007.
- Cho BK, Kim YM, Kim DS, et al. Mini-open muscle resection procedure under local anesthesia for lateral and medial epicondylitis. Clin Orthop Surg. 2009;1(3):123-127. doi:10.4055/cios.2009.1.3.123.
- Coleman B, Quinlan JF, Matheson JA. Surgical treatment for lateral epicondylitis: a long-term follow-up of results. J Shoulder Elbow Surg. 2010;19(3):363-367. doi:10.1016/j.jse.2009.09.008.
- Dunn JH, Kim JJ, Davis L, Nirschl RP. Ten- to 14-year follow-up of the Nirschl surgical technique for lateral epicondylitis. Am J Sports Med. 2008;36(2):261-266. doi:10.1177/0363546507308932.
- Manon-Matos Y, Oron A, Wolff TW. Combined common extensor and supinator aponeurotomy for the treatment of lateral epicondylitis. Tech Hand Up Extrem Surg. 2013;17(3):179-181. doi:10.1097/BTH.0b013e31829e0eeb.
- Meknas K, Odden-Miland A, Mercer JB, Castillejo M, Johansen O. Radiofrequency microtenotomy: a promising method for treatment of recalcitrant lateral epicondylitis. Am J Sports Med. 2008;36(10):1960-1965. doi:10.1177/0363546508318045.
- Pruzansky ME, Gantsoudes GD, Watters N. Late surgical results of reattachment to bone in repair of chronic lateral epicondylitis. Am J Orthop. 2009;38(6):295-299.
- Rayan F, Rao V Sr, Purushothamdas S, Mukundan C, Shafqat SO. Common extensor origin release in recalcitrant lateral epicondylitis – role justified? J Orthop Surg Res. 2010;5:31. doi:10.1186/1749-799X-5-31.
- Reddy VR, Satheesan KS, Bayliss N. Outcome of Boyd-McLeod procedure for recalcitrant lateral epicondylitis of elbow. Rheumatol Int. 2011;31(8):1081-1084. doi:10.1007/s00296-010-1450-1.
- Rubenthaler F, Wiese M, Senge A, Keller L, Wittenberg RH. Long-term follow-up of open and endoscopic Hohmann procedures for lateral epicondylitis. Arthroscopy. 2005;21(6):684-690. doi:10.1016/j.arthro.2005.03.017.
- Ruch DS, Orr SB, Richard MJ, Leversedge FJ, Mithani SK, Laino DK. A comparison of debridement with and without anconeus muscle flap for treatment of refractory lateral epicondylitis. J Shoulder Elbow Surg. 2015;24(2):236-241. doi:10.1016/j.jse.2014.09.035.
- Siddiqui MA, Koh J, Kua J, Cheung T, Chang P. Functional outcome assessment after open tennis elbow release: what are the predictor parameters? Singapore Med J. 2011;52(2):73-76.
- Solheim E, Hegna J, Øyen J. Extensor tendon release in tennis elbow: results and prognostic factors in 80 elbows. Knee Surg Sports Traumatol Arthrosc. 2011;19(6):1023-1027. doi:10.1007/s00167-011-1477-1.
- Svernlöv B, Adolfsson L. Outcome of release of the lateral extensor muscle origin for epicondylitis. Scand J Plast Reconstr Surg Hand Surg. 2006;40(3):161-165. doi:10.1080/02844310500491492.
- Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21(7):851-860. doi:10.1016/j.arthro.2005.03.019.
- Thornton SJ, Rogers JR, Prickett WD, Dunn WR, Allen AA, Hannafin JA. Treatment of recalcitrant lateral epicondylitis with suture anchor repair. Am J Sports Med. 2005;33(10):1558-1564. doi:10.1177/0363546505276758.
- Wang AW, Erak S. Fractional lengthening of forearm extensors for resistant lateral epicondylitis. ANZ J Surg. 2007;77(11):981-984. doi:10.1111/j.1445-2197.2007.04294.x.
- Baker CL Jr, Baker CL 3rd. Long-term follow-up of arthroscopic treatment of lateral epicondylitis. Am J Sports Med. 2008;36(2):254-260. doi:10.1177/0363546507311599.
- Grewal R, MacDermid JC, Shah P, King GJ. Functional outcome of arthroscopic extensor carpi radialis brevis tendon release in chronic lateral epicondylitis. J Hand Surg Am. 2009;34(5):849-857. doi:10.1016/j.jhsa.2009.02.006.
- Jerosch J, Schunck J. Arthroscopic treatment of lateral epicondylitis: indication, technique and early results. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):379-382. doi:10.1007/s00167-005-0662-5.
- Kim JW, Chun CH, Shim DM, et al. Arthroscopic treatment of lateral epicondylitis: comparison of the outcome of ECRB release with and without decortication. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1178-1183. doi:10.1007/s00167-011-1507-z.
- Lattermann C, Romeo AA, Anbari A, et al. Arthroscopic debridement of the extensor carpi radialis brevis for recalcitrant lateral epicondylitis. J Shoulder Elbow Surg. 2010;19(5):651-656. doi:10.1016/j.jse.2010.02.008.
- Mullett H, Sprague M, Brown G, Hausman M. Arthroscopic treatment of lateral epicondylitis: clinical and cadaveric studies. Clin Orthop Relat Res. 2005;439:123-128. doi:10.1097/01.blo.0000176143.08886.fe.
- Oki G, Iba K, Sasaki K, Yamashita T, Wada T. Time to functional recovery after arthroscopic surgery for tennis elbow. J Shoulder Elbow Surg. 2014;23(10):1527-1531. doi:10.1016/j.jse.2014.05.010.
- Othman AM. Arthroscopic versus percutaneous release of common extensor origin for treatment of chronic tennis elbow. Arch Orthop Trauma Surg. 2011;131(3):383-388. doi:10.1007/s00402-011-1260-2.
- Rhyou IH, Kim KW. Is posterior synovial plica excision necessary for refractory lateral epicondylitis of the elbow? Clin Orthop Relat Res. 2013;471(1):284-290. doi:10.1007/s11999-012-2585-z.
- Wada T, Moriya T, Iba K, et al. Functional outcomes after arthroscopic treatment of lateral epicondylitis. J Orthop Sci. 2009;14(2):167-174. doi:10.1007/s00776-008-1304-9.
- Yoon JP, Chung SW, Yi JH, et al. Prognostic factors of arthroscopic extensor carpi radialis brevis release for lateral epicondylitis. Arthroscopy. 2015;31(7):1232-1237. doi:10.1016/j.arthro.2015.02.006.
- Barnes DE, Beckley JM, Smith J. Percutaneous ultrasonic tenotomy for chronic elbow tendinosis: a prospective study. J Shoulder Elbow Surg. 2015;24(1):67-73. doi:10.1016/j.jse.2014.07.017.
- Kaleli T, Ozturk C, Temiz A, Tirelioglu O. Surgical treatment of tennis elbow: percutaneous release of the common extensor origin. Acta Orthop Belg. 2004;70(2):131-133.
- Lin MT, Chou LW, Chen HS, Kao MJ. Percutaneous soft tissue release for treating chronic recurrent myofascial pain associated with lateral epicondylitis: 6 case studies. Evid Based Complement Alternat Med. 2012;2012:142941. doi:10.1155/2012/142941.
- Lin CL, Lee JS, Su WR, Kuo LC, Tai TW, Jou IM. Clinical and ultrasonographic results of ultrasonographically guided percutaneous radiofrequency lesioning in the treatment of recalcitrant lateral epicondylitis. Am J Sports Med. 2011;39(11):2429-2435. doi:10.1177/0363546511417096.
- Zhu J, Hu B, Xing C, Li J. Ultrasound-guided, minimally invasive, percutaneous needle puncture treatment for tennis elbow. Adv Ther. 2008;25(10):1031-1036. doi:10.1007/s12325-008-0099-6.
- Sorensen AA, Howard D, Tan WH, Ketchersid J, Calfee RP. Minimal clinically important differences of 3 patient-related outcomes instruments. J Hand Surg Am. 2013;38(4):641-649. doi:10.1016/j.jhsa.2012.12.032.
- Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;18(3):205-207. doi:10.1136/emj.18.3.205.
- Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elbow Surg. 2009;18(6):927-932. doi:10.1016/j.jse.2009.03.021.
- Cummins CA. Lateral epicondylitis: in vivo assessment of arthroscopic debridement and correlation with patient outcomes. Am J Sports Med. 2006;34(9):1486-1491. doi:10.1177/0363546506288016.
- Stapleton TR, Baker CL. Arthroscopic treatment of lateral epicondylitis: a clinical study. Arthroscopy. 1996;1:365-366.
- Hastings H. Open treatment for lateral tennis elbow good for certain indications. Orthop Today. 2009;2:1-2.
- Duparc F, Putz R, Michot C, Muller JM, Fréger P. The synovial fold of the humeroradial joint: anatomical and histological features, and clinical relevance in lateral epicondylalgia of the elbow. Surg Radiol Anat. 2002;24(5):302-307. doi:10.1007/s00276-002-0055-0.
TAKE-HOME POINTS
- While favorable results have been reported for open, arthroscopic, and percutaneous surgical techniques, there is no current consensus regarding the optimal technique for lateral epicondylitis.
- There is no difference between open, arthroscopic, and percutaneous surgical treatment for lateral epicondylitis regarding return to work and subjective satisfaction.
- Open treatment led to a greater percentage of patients being pain free at final follow-up.
- While arthroscopic treatment led to better pain and functional scores at final follow-up, the absolute differences were quite small and likely not clinically significant.
- We recommend open débridement as the best means of minimizing cost and achieving a pain-free outcome in the long-term.
Impact of Sagittal Rotation on Axial Glenoid Width Measurement in the Setting of Glenoid Bone Loss
ABSTRACT
Standard 2-dimensional (2-D) computed tomography (CT) scans of the shoulder are often aligned to the plane of the body as opposed to the plane of the scapula, which may challenge the ability to accurately measure glenoid width and glenoid bone loss (GBL). The purpose of this study is to determine the effect of sagittal rotation of the glenoid on axial anterior-posterior (AP) glenoid width measurements in the setting of anterior GBL.
Forty-three CT scans from consecutive patients with anterior GBL (minimum 10%) were reformatted utilizing open-source DICOM software (OsiriX MD). Patients were grouped according to extent of GBL: I, 10% to 14.9% (N = 12); II, 15% to 19.9% (N = 16); and III, >20% (N = 15). The uncorrected (UNCORR) and corrected (CORR) images were assessed in the axial plane at 5 standardized cuts and measured for AP glenoid width.
For groups I and III, UNCORR scans underestimated axial AP width (and thus overestimated anterior GBL) in cuts 1 and 2, while in cuts 3 to 5, the axial AP width was overestimated (GBL was underestimated). In Group II, axial AP width was underestimated (GBL was overestimated), while in cuts 2 to 5, the axial AP width was overestimated (GBL was underestimated). Overall, AP glenoid width was consistently underestimated in cut 1, the most caudal cut; while AP glenoid width was consistently overestimated in cuts 3 to 5, the more cephalad cuts.
UNCORR 2-D CT scans inaccurately estimated glenoid width and the degree of anterior GBL. This data suggests that corrected 2D CT scans or a 3-dimensional (3-D) reconstruction can help in accurately defining the anterior GBL in patients with shoulder instability.
The treatment of glenohumeral instability has substantially evolved over the past several decades. The understanding of glenoid bone loss (GBL), in particular, has advanced to such a level that we utilize the quantification of GBL for surgical decision-making. Unrecognized and/or untreated GBL is associated with recurrent instability, pain, and disability. Controversy exists, however, regarding the precise amount of anterior GBL that is significant enough to warrant surgical treatment. While historically, 25%1,2 of anterior GBL was thought to be the critical number required to warrant osseous augmentation, studies that are more recent have highlighted the need to perform osseous glenoid reconstruction with lesser degrees of GBL, particularly in the contact athlete.3-9 As small differences in the amount of GBL can change surgical decision-making from an all-soft tissue repair to an osseous reconstruction, it is paramount that we have accurate, valid, and reproducible methods for calculating GBL.
Continue to: Historically, plain radiographs...
Historically, plain radiographs have been the mainstay for evaluating the glenohumeral joint, including Grashey and axillary views, allowing clinicians to evaluate the congruency of the glenohumeral joint and to assess bone loss on both the glenoid and humeral head.1,10 While large, acute fractures of the glenoid are fairly evident on radiographs, including the Grashey view,11 shoulders with chronic and/or attritional anterior GBL are more difficult to evaluate, and often do not provide the information necessary to guide surgical decision-making.
Computed tomography (CT) of the shoulder has become the most commonly utilized imaging modality in the evaluation of patients with shoulder instability associated with GBL. Standard 2-dimensional (2-D) CT scans of the shoulder are often aligned to the plane of the body as opposed to the plane of the scapula/glenoid, as standard protocols often fail to account for the anterior sagittal rotation of the scapula/glenoid, similar to the disadvantage of standard radiographs. While 3-dimensional (3-D) CT reconstructions eliminate the effect of gantry angles, and thus allow for an en face view of the glenoid, 3-D reconstructions are not always available, and cannot always be measured.12-14 Thus, improved methodology for utilizing standard 2D scans is warranted, as the ability to correctly align the axial CT scan to the axis of the glenoid may allow for more accurate GBL measurements, which will ultimately impact surgical decision-making. Recently, Gross and colleagues15 reported the effect of sagittal rotation of the glenoid on axial measurements of anterior-posterior (AP) glenoid width and glenoid version in normal glenoids, without bone loss, and found that the mean angle of correction needed to align the sagittal plane was 20.1° ± 1.2° of rotation. To the authors’ knowledge, this same methodology has not been applied to patients with clinically meaningful anterior GBL. Given that the average glenoid width in human shoulders is 24.4 mm ± 2.9 mm,16 1 mm of glenoid bone loss (GBL) corresponds to approximately 4% of the glenoid width, and thus even subtle differences in the interpretation of GBL may have substantial clinical implications. Therefore, the purpose of this study is to determine the effect of sagittal rotation of the glenoid on axial AP glenoid width measurements in the setting of clinically significant anterior GBL.
METHODS
This study was approved by Massachusetts General Hospital Institutional Review Board. A retrospective review of consecutive patients with a diagnosis of anterior shoulder instability between 2009 and 2013 was conducted. Inclusion criteria comprised patients with a minimum of 10% anterior GBL, an available CT scan of the affected shoulder, and no history of prior ipsilateral surgeries. Exclusion criteria comprised evidence of degenerative changes to the glenoid and/or humeral head, as well as prior ipsilateral shoulder surgery. Sixty consecutive patients were originally identified as having anterior shoulder instability, and 17 were excluded based on the inclusion/exclusion criteria, leaving 43 patients (43 shoulders) available for inclusion. Shoulder CT scans from all 43 patients were reformatted utilizing open-source DICOM software (OsiriX MD, version 2.5.1 65-bit) multi-planar reconstruction (MPR).
CT PROTOCOL
All patients underwent a standard glenohumeral CT scan using a Siemens Sensation 64 Scanner (Siemens), a 64-detector scanner. Scans were acquired with 0.6 mm of collimation, 140 kV, and 300 mA-seconds. Slice thickness was set to 2 mm. All patient information was de-identified for analysis.
The uncorrected (UNCORR) scans were defined as the default orientation on the scanner. In the UNCORR scans, the axial, coronal, and sagittal views were oriented relative to the scanner gantry table, as opposed to the anatomy of the glenoid. The corrected (CORR) CT scans were aligned in all 3 planes relative to the glenoid face, and thus the cuts were perpendicular to the long axis of the glenoid.15 This resulted in sagittal cuts perpendicular to the 12-o’clock to 6-o’clock axis in the sagittal plane (Figure 1).
Continue to: In a de-identified fashion...
IMAGE ANALYSIS AND REFORMATTING
In a de-identified fashion, all CT scans were imported and analyzed using open-source Digital Imaging and Communications in Medicine (DICOM) software (OsiriX MD, version 2.5.1 64-bit). By following a previously developed method, CT scans were reformatted using OsiriX MPR. The OsiriX software has an MPR function that allows simultaneous manipulation of 2-D CT scans in 3 orthogonal planes: axial, sagittal, and coronal. In the MPR mode, the alternation of 1 plane directly affects the orientation of the remaining 2 planes. Thus, by using an MPR, one can analyze the impact that a default CT scan performed relative to the gantry of the table, UNCORR, has on the axial images.
First, the en face view was obtained via a 2-step process: alignment of the axial plane to account for the scapular angle, followed by alignment of the coronal plane to adjust for the glenoid inclination.15 These 2 adjustments provided a true en face sagittal glenoid view. The final adjustment step was a sagittal en face rotation of the glenoid such that the superior and inferior glenoid tubercles were placed on the 12-o’clock to 6-o’clock axis (CORR scan). Previous studies have identified a central longitudinal axis that was used in this method to align the supraglenoid tubercle with the 12-o’clock to 6-o’clock axis on the glenoid face.15,17,18 The standard error of mean was 1.21°. This new CORR view resulted in axial cuts through the glenoid that were oriented perpendicular to the 12-o’clock to 6-o’clock axis. The UNCORR and CORR images were assessed in the axial plane at 5 standardized cuts and measured for AP glenoid width by 2 independent observers in a blinded, randomized fashion. When the measured AP width of the UNCORR scan was less than that measured on the CORR scan, the AP width of the glenoid was considered underestimated, and the degree of GBL was considered overestimated (Figure 2).
SCAPULAR ANGLE
Scapular angle measurements were performed on the axial view as the angle between a line through the long axis of the body of the scapula, and a line parallel to the CT gantry table.15,19 Subsequently, the axial plane was aligned to the glenoid surface.
CORONAL INCLINATION
Coronal inclination measurements were performed on the sagittal view as the angle between a line tangential to the face of the glenoid and a line perpendicular to the CT gantry table. Positive values represented superior inclination, while negative values represented inferior glenoid inclination.15
SAGITTAL ROTATION
Sagittal rotation measurements were performed using the built-in angle measurement tool in OsiriX in the sagittal plane since the degree of rotation required aligning the long axis of the glenoid to the 12-o’clock to 6-o’clock axis. The amount of rotation was defined as the rotation angle.15
Continue to: Similarly, as described by Gross...
GLENOID WIDTH
Similarly, as described by Gross and colleagues,15 the sagittal en face view was divided via 5 cuts, throughout a superimposed best-fit circle that closely represents the glenoid.9,15,20 For both the UNCORR and CORR, glenoid width (AP distance) was measured on the axial image at the widest point from AP cortex across the glenoid face.
PATIENT GROUPS
Utilizing the en face 3-D CT reconstruction view of the glenoid as the gold standard, patients were placed into 1 of 3 groups according to the degree of anterior GBL measured via the surface method.9,20 The groups were as follows:
I. 10% to 14.9% (N = 12)
II. 15% to 19.9% (N = 16)
III. >20% (N = 15)
STATISTICAL METHODS
Paired t-tests were used to compare all measurements between CORR and UNCORR scans for each of the 5 cuts. A P-value of .05 was used as the threshold for statistical significance in 2-tailed comparisons. Mean and standard errors are presented with standard deviations throughout the study. For interobserver reliability, the measurements between the observers, the intraclass correlation coefficient was calculated. All statistics were performed with SPSS (Version 22).
RESULTS
The study cohort was comprised of 19 left shoulders (44%) and 24 right shoulders (56%), including 36 male patients (84%) and 7 female patients (16%). The average age was 27.8 years (range, 21-40 years). The variability in measured difference, with respect to AP width, was 1.05 mm. The UNCORR CT scans required a mean correction for coronal inclination of 7.0° ± 5.8° (range, -8°-6°). The UNCORR CT scans required a mean correction for scapular angle of 30.2° ± 8.0° (range, 15°-49°). The mean angle of sagittal rotation required to align the glenoid face with the 12-o’clock to 6-o’clock axis was 24.2° ± 5.1 ° (range, 13°-30°). These results are summarized in Table 1.
Table 1. Mean Correction Values Required to Correct the Uncorrected Images to the Corrected Images | |||
Anatomic alignment | Mean (degrees) | Range (degrees) | SD (degrees) |
Scapular angle | 30.2 | 15-49 | 8.0 |
Coronal Inclination | 7.0 | -8-6 | 5.8 |
Sagittal rotation | 24.2 | 13-30 | 5.1 |
For all measurements, the intraclass correlation coefficient for independent observers for all cuts within the 3 groups was r >.900 in all cases.
On an optimized CT scan, over 5 standardized cuts across a best-fit circle of the inferior glenoid, there was a statistically significant absolute mean difference of 12.6% in axial AP glenoid width (2.86 mm ± 2.00 mm, P =.016) when compared with the UNCORR scan. This corresponds to a 3% to 21% error in measurement of the AP width of the glenoid.
Continue to: For the entire cohort...
For the entire cohort of 43 patients, the UNCORR scans underestimated the axial AP width (and thus overestimated GBL) in cut 1 (P =.003), and overestimated the axial AP width (and thus underestimated GBL) in cuts 3 to 5 (P < .001 for all) compared with that of the CORR scans. There was no significant difference between the UNCORR and CORR scans in cut 2 (P = .331).
For groups I (10%-14.9% GBL) and III (>20% GBL), the UNCORR scans underestimated the axial AP width (and thus overestimated anterior GBL) in cuts 1 and 2, while in cuts 3 to 5, the axial AP width was overestimated (GBL was underestimated) (Tables 2, 3). In Group II (15%-19.9% GBL), the axial AP width was underestimated (GBL was overestimated), while in cuts 2 to 5, the axial AP width was overestimated (GBL was underestimated). Overall, AP glenoid width was consistently underestimated in cut 1, the most caudal cut, while AP glenoid width was consistently overestimated in cuts 3 to 5, the more cephalad cuts.
Table 2. Absolute Mean Difference in Axial AP Width (mm) Between Corrected and Uncorrected Images (% difference) | |||||
Cut 1 (Caudal) | Cut 2 | Cut 3 (Center) | Cut 4 | Cut 5 (Cephalad) | |
Group I: 10%-14.9% GBL | 2.4 mm (15.3%) | 1.8 mm (9.0%) | 1.8 mm (7.7%) | 3.0 mm (11.7%) | 4.0 mm (16.8%) |
Group II: 15%-19.9% GBL | 1.8 mm (13.1%) | 1.7 mm (7.9%) | 2.8 mm (10.6%) | 4.1 mm (14.4%) | 4.8 mm (16.9%) |
Group III: >20% | 2.8 mm (16.1%) | 1.9 mm (8.0%) | 2.3 mm (10.3) | 4.4 mm (16.6%) | 5.2 mm (17.0%) |
Abbreviations: AP, anterior-posterior; GBL, glenoid bone loss.
Table 3. Mean AP Glenoid Width Based on CORR and UNCORR Images for the Entire Cohort of 43 Patients | |||||
Axial cut | Mean AP width (mm) | Mean AP width (mm) | Absolute mean AP width difference (mm) | Absolute mean AP width difference (%) | P value |
(Caudal) 1 | 16.6208 | 18.4958 | -1.875 | 14.7768 | .0029565 |
2 | 20.6558 | 21.3166 | -0.661 | 3.6137 | .3310965 |
3 | 24.2583 | 22.3125 | 1.946 | 7.8042 | <.0001 |
4 | 26.1291 | 21.8916 | 4.238 | 15.8449 | <.0001 |
(Rostral) 5 | 26.0875 | 20.4875 | 5.6 | 20.9717 | <.0001 |
Abbreviations: AP, anterior-posterior; CORR, corrected; UNCORR, uncorrected.
DISCUSSION
The principle findings of this study demonstrate that UNCORR conventional 2-D CT scans inaccurately estimate glenoid width as well as inaccurately quantify the degree of anterior GBL. Underestimations of GBL may lead to insufficient treatment of clinically meaningful GBL, thereby increasing the risk of instability recurrence; whereas overestimations of GBL may lead to unnecessary treatment, subjecting patients to increased surgical morbidity. Therefore, the authors recommend correcting the orientation of the scapula in cases wherein clinical decisions are entirely based on 2-D CT, or using alternative methods for quantifying GBL, specifically in the form of 3-D reconstructions.
The use of axial imaging, with CT scans and/or magnetic resonance imaging, is growing in popularity for evaluation of both glenoid anatomy and GBL. Nevertheless, despite our improved ability to critically evaluate the glenoid using these advanced imaging modalities, the images themselves require scrutiny by clinicians to determine if the images accurately depict the true anatomy of the glenoid. As demonstrated by Gross and colleagues,15 conventional 2D CT scan protocols are not optimized to the anatomy of the glenohumeral joint, even in patients without GBL. Due to the alignment of the image relative to the plane of the scapula as opposed to the plane of the glenoid, UNCORR scans result in significantly different measurements of glenoid version (2.0° ± 0.1°) and AP glenoid width (1.2 mm ± 0.42 mm) compared with corrected scans, requiring an average 20.1° ± 1.2° of correction to align the sagittal plane. In the present study involving the patients with GBL, we also found that conventional, UNCORR 2-D CT scan protocols inaccurately estimate glenoid width and the degree of anterior GBL. In particular, AP glenoid width was consistently underestimated in the more caudal cuts, while AP glenoid width was consistently overestimated in the more cephalad cuts. Thus, anterior GBL was overestimated (AP glenoid width was underestimated) in the more caudal cuts, whereas anterior GBL was underestimated in the more cranial cuts (AP glenoid width was overestimated). Given that approximately 1 mm of glenoid bone corresponds to approximately 4% of glenoid width,16 even subtle differences in the interpretation of GBL may lead to gross overestimation/underestimation of bone loss, with significant clinical implications.
In the anterior instability patient population, clinical decision-making is often based on the degree of GBL as determined by advanced imaging modalities. In addition to other patient-specific factors, including age, gender, activity level, type of sport, and number of prior dislocations and/or prior surgeries, the quantity of GBL will often determine which surgical procedure needs to be performed. Typically, patients with >20% to 25% anterior GBL are indicated for a glenoid reconstruction procedure, most commonly via the Latarjet procedure (coracoid transfer).21-27 The Latarjet procedure remains an excellent technique for appropriately indicated patients, with historically good clinical outcomes and low recurrence rates. Complications associated with the Latarjet procedure, however, are not uncommon, including devastating neuropraxia of the axillary and musculocutaneous nerves, and occasionally permanent neurologic deficits.28 Thus, it is critical to avoid overtreating patients with recurrent instability and GBL. As demonstrated by this study, depending on the cranial-to-caudal location on the glenoid, current 2-D CT techniques may underestimate AP glenoid width, resulting in an overestimation of GBL, potentially leading to the decision to proceed with glenoid bone reconstruction when such a procedure is not required. On the contrary, overestimation of AP glenoid width, which occurs in the more cephalad cuts of the glenoid, is perhaps more worrisome, as the resulting underestimation of GBL may lead to inadequate treatment of patients with recurrent instability. Certainly, one of the main risk factors for failed soft tissue shoulder stabilization is a failure to address GBL. If clinical decisions are made based on UNCORR 2-D CT scans, which are often inaccurate with respect to AP glenoid width by an average 2.86 mm ± 2.00 mm (equivalent to 12.6% ± 6.9% GBL) as determined in this study, patients who truly require osseous glenoid reconstructions may be indicated for only soft tissue stabilization, based on the underestimation of GBL.
Continue to: The current gold standard...
The current gold standard for GBL measurement is a perfect-fit circle performed on a 3-D CT scan.22 To that end, it would have been useful to measure the glenoids from this study on 3-D CT scans and compare the data with both UNCORR and CORR measurements. This would have provided a better understanding to what extent the CORR measurements on 2-D scans are relatable with the gold standard. As 3-D CT scans provide a better en face view of the glenoid, more accurate GBL measurements, and ease of 3-D manipulation, they have become more widely used across the country.29,30 Nevertheless, in situations where 3-D imaging is more challenging to obtain because of technology or cost limitations, having a strategy for ensuring proper orientation of 2-D scans would have a substantial impact on clinical decision-making. If such corrections are not made, the inaccuracy of current 2-D scanning protocols justifies the cost 3-D reconstruction protocols. The difference in GBL measurements are critical in cases of increasingly large degrees of GBL, as in these instances, the inferior glenoid becomes more of an inverted-pear shape as opposed to a perfect circle, and differences in CORR and UNCORR images are likely to be more profound.
LIMITATIONS
This study has limitations, such as the relatively small sample size and the selection bias by the reviewers with potential differences in interobserver reliability. Further, minor modifications during the reformatting process may be found with each attempt to manipulate the images and may result in minor, insignificant differences in AP width measurements. Performing 1 or more additional CT scans on the same cohort of patients would have been helpful; however, due to the increased risk of radiation exposure, this was not performed. Performing CT scans on cadaveric specimens with GBL and applying the study methodology would also have been helpful to provide independent verification of our clinical findings; however, specimens were not available for this study. Another limitation of this study is that we did not compare our findings with the findings of glenoid width, and bone loss, as determined using the circle method, which is commonly utilized when 3-D reconstructions are available. In this study, the purpose was to utilize only the 2-D reformatted images, with the assumption that 3-D reconstructions are not always available, and cannot always be measured. To minimize selection bias, the investigators measured the correction effects within groups of patients with similar degrees of GBL (10%-14.9%, 15%-19.9%, and >20%). In addition, not all the selected patients showed degenerative glenoid changes or irregular glenoid shape indicating previous bone augmentation.
CONCLUSIONS
UNCORR 2D CT scans inaccurately estimate glenoid width and the degree of anterior GBL. The clinical implications of these findings are profound and suggest corrected 2D CT scans or 3D reconstruction allow measurements to be taken in the axis of the glenoid to accurately define the anatomy and quantity of anterior GBL in patients with shoulder instability.
1. Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: results for a series of 28 shoulders treated with the Latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202. doi:10.1007/s10195-012-0201-3.
2. Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35-46.
3. Bhatia S, Ghodadra NS, Romeo AA, et al. The importance of the recognition and treatment of glenoid bone loss in an athletic population. Sports Health. 2011;3(5):435-440. doi:10.1177/1941738111414126.
4. Lo IK, Parten PM, Burkhart SS. The inverted pear glenoid: an indicator of significant glenoid bone loss. Arthroscopy. 2004;20(2):169-174. doi:10.1016/j.arthro.2003.11.036.
5. Mologne TS, Provencher MT, Menzel KA, Vachon TA, Dewing CB. Arthroscopic stabilization in patients with an inverted pear glenoid: results in patients with bone loss of the anterior glenoid. Am J Sports Med. 2007;35(8):1276-1283. doi:10.1177/0363546507300262.
6. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
7. Provencher MT, Bhatia S, Ghodadra NS, et al. Recurrent shoulder instability: current concepts for evaluation and management of glenoid bone loss. J Bone Joint Surg Am. 2010;92(suppl 2):133-151. doi:10.2106/JBJS.J.00906.
8. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. Apparent causes of failure and treatment. J Bone Joint Surg Am. 1984;66(2):159-168.
9. Sugaya H, Moriishi J, Dohi M, Kon Y, Tsuchiya A. Glenoid rim morphology in recurrent anterior glenohumeral instability. J Bone Joint Surg Am. 2003;85-A(5):878-884.
10. Edwards TB, Boulahia A, Walch G. Radiographic analysis of bone defects in chronic anterior shoulder instability. Arthroscopy. 2003;19(7):732-739.
11. Jankauskas L, Rudiger HA, Pfirrmann CW, Jost B, Gerber C. Loss of the sclerotic line of the glenoid on anteroposterior radiographs of the shoulder: a diagnostic sign for an osseous defect of the anterior glenoid rim. J Shoulder Elbow Surg. 2010;19(1):151-156. doi:10.1016/j.jse.2009.04.013.
12. Altan E, Ozbaydar MU, Tonbul M, Yalcin L. Comparison of two different measurement methods to determine glenoid bone defects: area or width? J Shoulder Elbow Surg. 2014;23(8):1215-1222. doi:10.1016/j.jse.2013.11.029.
13. Bishop JY, Jones GL, Rerko MA, Donaldson C, Group MS. 3-D CT is the most reliable imaging modality when quantifying glenoid bone loss. Clin Orthop Relat Res. 2013;471(4):1251-1256. doi:10.1007/s11999-012-2607-x.
14. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008; 24(4):376-382. doi:10.1016/j.arthro.2007.10.008.
15. Gross DJ, Golijanin P, Dumont GD, et al. The effect of sagittal rotation of the glenoid on axial glenoid width and glenoid version in computed tomography scan imaging. J Shoulder Elbow Surg. 2016;25(1):61-68. doi:10.1016/j.jse.2015.06.017.
16. Lenart BA, Freedman R, Van Thiel GS, et al. Magnetic resonance imaging evaluation of normal glenoid length and width: an anatomic study. Arthroscopy. 2014;30(8):915-920. doi:10.1016/j.arthro.2014.03.006.
17. Bois AJ, Fening SD, Polster J, Jones MH, Miniaci A. Quantifying glenoid bone loss in anterior shoulder instability: reliability and accuracy of 2-dimensional and 3-dimensional computed tomography measurement techniques. Am J Sports Med. 2012;40(11):2569-2577. doi:10.1177/0363546512458247.
18. Griffith JF, Antonio GE, Tong CW, Ming CK. Anterior shoulder dislocation: quantification of glenoid bone loss with CT. AJR Am J Roentgenol. 2003;180(5):1423-1430. doi:10.2214/ajr.180.5.1801423.
19. Hoenecke HR Jr, Hermida JC, Flores-Hernandez C, D'Lima DD. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(2):166-171. doi:10.1016/j.jse.2009.08.009.
20. Huijsmans PE, de Witte PB, de Villiers RV, et al. Recurrent anterior shoulder instability: accuracy of estimations of glenoid bone loss with computed tomography is insufficient for therapeutic decision-making. Skeletal Radiol. 2011;40(10):1329-1334. doi:10.1007/s00256-011-1184-5.
21. Bhatia S, Frank RM, Ghodadra NS, et al. The outcomes and surgical techniques of the latarjet procedure. Arthroscopy. 2014;30(2):227-235. doi:10.1016/j.arthro.2013.10.013.
22. Cunningham G, Benchouk S, Kherad O, Ladermann A. Comparison of arthroscopic and open Latarjet with a learning curve analysis. Knee Surg Sports Traumatol Arthrosc. 2015;24(2):540-545. doi:10.1007/s00167-015-3910-3.
23. Fedorka CJ, Mulcahey MK. Recurrent anterior shoulder instability: a review of the Latarjet procedure and its postoperative rehabilitation. Phys Sportsmed. 2015;43(1):73-79. doi:10.1080/00913847.2015.1005543.
24. Flinkkila T, Sirniö K. Open Latarjet procedure for failed arthroscopic Bankart repair. Orthop Traumatol Surg Res. 2015;101(1):35-38. doi:10.1016/j.otsr.2014.11.005.
25. Hovelius L, Sandström B, Saebö M. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: study II-the evolution of dislocation arthropathy. J Shoulder Elbow Surg. 2006;15(3):279-289. doi:10.1016/j.jse.2005.09.014.
26. Hovelius L, Sandström B, Sundgren K, Saebö M. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: study I--clinical results. J Shoulder Elbow Surg. 2004;13(5):509-516. doi:10.1016/S1058274604000916.
27. Hovelius L, Vikerfors O, Olofsson A, Svensson O, Rahme H. Bristow-Latarjet and Bankart: a comparative study of shoulder stabilization in 185 shoulders during a seventeen-year follow-up. J Shoulder Elbow Surg. 2011;20(7):1095-1101. doi:10.1016/j.jse.2011.02.005.
28. Gupta A, Delaney R, Petkin K, Lafosse L. Complications of the Latarjet procedure. Curr Rev Musculoskelet Med. 2015;8(1):59-66. doi:10.1007/s12178-015-9258-y.
29. Kwon YW, Powell KA, Yum JK, Brems JJ, Iannotti JP. Use of three-dimensional computed tomography for the analysis of the glenoid anatomy. J Shoulder Elbow Surg. 2005;14(1):85-90. doi:10.1016/j.jse.2004.04.011.
30. Saito H, Itoi E, Sugaya H, Minagawa H, Yamamoto N, Tuoheti Y. Location of the glenoid defect in shoulders with recurrent anterior dislocation. Am J Sports Med. 2005;33(6):889-893. doi:10.1177/0363546504271521.
ABSTRACT
Standard 2-dimensional (2-D) computed tomography (CT) scans of the shoulder are often aligned to the plane of the body as opposed to the plane of the scapula, which may challenge the ability to accurately measure glenoid width and glenoid bone loss (GBL). The purpose of this study is to determine the effect of sagittal rotation of the glenoid on axial anterior-posterior (AP) glenoid width measurements in the setting of anterior GBL.
Forty-three CT scans from consecutive patients with anterior GBL (minimum 10%) were reformatted utilizing open-source DICOM software (OsiriX MD). Patients were grouped according to extent of GBL: I, 10% to 14.9% (N = 12); II, 15% to 19.9% (N = 16); and III, >20% (N = 15). The uncorrected (UNCORR) and corrected (CORR) images were assessed in the axial plane at 5 standardized cuts and measured for AP glenoid width.
For groups I and III, UNCORR scans underestimated axial AP width (and thus overestimated anterior GBL) in cuts 1 and 2, while in cuts 3 to 5, the axial AP width was overestimated (GBL was underestimated). In Group II, axial AP width was underestimated (GBL was overestimated), while in cuts 2 to 5, the axial AP width was overestimated (GBL was underestimated). Overall, AP glenoid width was consistently underestimated in cut 1, the most caudal cut; while AP glenoid width was consistently overestimated in cuts 3 to 5, the more cephalad cuts.
UNCORR 2-D CT scans inaccurately estimated glenoid width and the degree of anterior GBL. This data suggests that corrected 2D CT scans or a 3-dimensional (3-D) reconstruction can help in accurately defining the anterior GBL in patients with shoulder instability.
The treatment of glenohumeral instability has substantially evolved over the past several decades. The understanding of glenoid bone loss (GBL), in particular, has advanced to such a level that we utilize the quantification of GBL for surgical decision-making. Unrecognized and/or untreated GBL is associated with recurrent instability, pain, and disability. Controversy exists, however, regarding the precise amount of anterior GBL that is significant enough to warrant surgical treatment. While historically, 25%1,2 of anterior GBL was thought to be the critical number required to warrant osseous augmentation, studies that are more recent have highlighted the need to perform osseous glenoid reconstruction with lesser degrees of GBL, particularly in the contact athlete.3-9 As small differences in the amount of GBL can change surgical decision-making from an all-soft tissue repair to an osseous reconstruction, it is paramount that we have accurate, valid, and reproducible methods for calculating GBL.
Continue to: Historically, plain radiographs...
Historically, plain radiographs have been the mainstay for evaluating the glenohumeral joint, including Grashey and axillary views, allowing clinicians to evaluate the congruency of the glenohumeral joint and to assess bone loss on both the glenoid and humeral head.1,10 While large, acute fractures of the glenoid are fairly evident on radiographs, including the Grashey view,11 shoulders with chronic and/or attritional anterior GBL are more difficult to evaluate, and often do not provide the information necessary to guide surgical decision-making.
Computed tomography (CT) of the shoulder has become the most commonly utilized imaging modality in the evaluation of patients with shoulder instability associated with GBL. Standard 2-dimensional (2-D) CT scans of the shoulder are often aligned to the plane of the body as opposed to the plane of the scapula/glenoid, as standard protocols often fail to account for the anterior sagittal rotation of the scapula/glenoid, similar to the disadvantage of standard radiographs. While 3-dimensional (3-D) CT reconstructions eliminate the effect of gantry angles, and thus allow for an en face view of the glenoid, 3-D reconstructions are not always available, and cannot always be measured.12-14 Thus, improved methodology for utilizing standard 2D scans is warranted, as the ability to correctly align the axial CT scan to the axis of the glenoid may allow for more accurate GBL measurements, which will ultimately impact surgical decision-making. Recently, Gross and colleagues15 reported the effect of sagittal rotation of the glenoid on axial measurements of anterior-posterior (AP) glenoid width and glenoid version in normal glenoids, without bone loss, and found that the mean angle of correction needed to align the sagittal plane was 20.1° ± 1.2° of rotation. To the authors’ knowledge, this same methodology has not been applied to patients with clinically meaningful anterior GBL. Given that the average glenoid width in human shoulders is 24.4 mm ± 2.9 mm,16 1 mm of glenoid bone loss (GBL) corresponds to approximately 4% of the glenoid width, and thus even subtle differences in the interpretation of GBL may have substantial clinical implications. Therefore, the purpose of this study is to determine the effect of sagittal rotation of the glenoid on axial AP glenoid width measurements in the setting of clinically significant anterior GBL.
METHODS
This study was approved by Massachusetts General Hospital Institutional Review Board. A retrospective review of consecutive patients with a diagnosis of anterior shoulder instability between 2009 and 2013 was conducted. Inclusion criteria comprised patients with a minimum of 10% anterior GBL, an available CT scan of the affected shoulder, and no history of prior ipsilateral surgeries. Exclusion criteria comprised evidence of degenerative changes to the glenoid and/or humeral head, as well as prior ipsilateral shoulder surgery. Sixty consecutive patients were originally identified as having anterior shoulder instability, and 17 were excluded based on the inclusion/exclusion criteria, leaving 43 patients (43 shoulders) available for inclusion. Shoulder CT scans from all 43 patients were reformatted utilizing open-source DICOM software (OsiriX MD, version 2.5.1 65-bit) multi-planar reconstruction (MPR).
CT PROTOCOL
All patients underwent a standard glenohumeral CT scan using a Siemens Sensation 64 Scanner (Siemens), a 64-detector scanner. Scans were acquired with 0.6 mm of collimation, 140 kV, and 300 mA-seconds. Slice thickness was set to 2 mm. All patient information was de-identified for analysis.
The uncorrected (UNCORR) scans were defined as the default orientation on the scanner. In the UNCORR scans, the axial, coronal, and sagittal views were oriented relative to the scanner gantry table, as opposed to the anatomy of the glenoid. The corrected (CORR) CT scans were aligned in all 3 planes relative to the glenoid face, and thus the cuts were perpendicular to the long axis of the glenoid.15 This resulted in sagittal cuts perpendicular to the 12-o’clock to 6-o’clock axis in the sagittal plane (Figure 1).
Continue to: In a de-identified fashion...
IMAGE ANALYSIS AND REFORMATTING
In a de-identified fashion, all CT scans were imported and analyzed using open-source Digital Imaging and Communications in Medicine (DICOM) software (OsiriX MD, version 2.5.1 64-bit). By following a previously developed method, CT scans were reformatted using OsiriX MPR. The OsiriX software has an MPR function that allows simultaneous manipulation of 2-D CT scans in 3 orthogonal planes: axial, sagittal, and coronal. In the MPR mode, the alternation of 1 plane directly affects the orientation of the remaining 2 planes. Thus, by using an MPR, one can analyze the impact that a default CT scan performed relative to the gantry of the table, UNCORR, has on the axial images.
First, the en face view was obtained via a 2-step process: alignment of the axial plane to account for the scapular angle, followed by alignment of the coronal plane to adjust for the glenoid inclination.15 These 2 adjustments provided a true en face sagittal glenoid view. The final adjustment step was a sagittal en face rotation of the glenoid such that the superior and inferior glenoid tubercles were placed on the 12-o’clock to 6-o’clock axis (CORR scan). Previous studies have identified a central longitudinal axis that was used in this method to align the supraglenoid tubercle with the 12-o’clock to 6-o’clock axis on the glenoid face.15,17,18 The standard error of mean was 1.21°. This new CORR view resulted in axial cuts through the glenoid that were oriented perpendicular to the 12-o’clock to 6-o’clock axis. The UNCORR and CORR images were assessed in the axial plane at 5 standardized cuts and measured for AP glenoid width by 2 independent observers in a blinded, randomized fashion. When the measured AP width of the UNCORR scan was less than that measured on the CORR scan, the AP width of the glenoid was considered underestimated, and the degree of GBL was considered overestimated (Figure 2).
SCAPULAR ANGLE
Scapular angle measurements were performed on the axial view as the angle between a line through the long axis of the body of the scapula, and a line parallel to the CT gantry table.15,19 Subsequently, the axial plane was aligned to the glenoid surface.
CORONAL INCLINATION
Coronal inclination measurements were performed on the sagittal view as the angle between a line tangential to the face of the glenoid and a line perpendicular to the CT gantry table. Positive values represented superior inclination, while negative values represented inferior glenoid inclination.15
SAGITTAL ROTATION
Sagittal rotation measurements were performed using the built-in angle measurement tool in OsiriX in the sagittal plane since the degree of rotation required aligning the long axis of the glenoid to the 12-o’clock to 6-o’clock axis. The amount of rotation was defined as the rotation angle.15
Continue to: Similarly, as described by Gross...
GLENOID WIDTH
Similarly, as described by Gross and colleagues,15 the sagittal en face view was divided via 5 cuts, throughout a superimposed best-fit circle that closely represents the glenoid.9,15,20 For both the UNCORR and CORR, glenoid width (AP distance) was measured on the axial image at the widest point from AP cortex across the glenoid face.
PATIENT GROUPS
Utilizing the en face 3-D CT reconstruction view of the glenoid as the gold standard, patients were placed into 1 of 3 groups according to the degree of anterior GBL measured via the surface method.9,20 The groups were as follows:
I. 10% to 14.9% (N = 12)
II. 15% to 19.9% (N = 16)
III. >20% (N = 15)
STATISTICAL METHODS
Paired t-tests were used to compare all measurements between CORR and UNCORR scans for each of the 5 cuts. A P-value of .05 was used as the threshold for statistical significance in 2-tailed comparisons. Mean and standard errors are presented with standard deviations throughout the study. For interobserver reliability, the measurements between the observers, the intraclass correlation coefficient was calculated. All statistics were performed with SPSS (Version 22).
RESULTS
The study cohort was comprised of 19 left shoulders (44%) and 24 right shoulders (56%), including 36 male patients (84%) and 7 female patients (16%). The average age was 27.8 years (range, 21-40 years). The variability in measured difference, with respect to AP width, was 1.05 mm. The UNCORR CT scans required a mean correction for coronal inclination of 7.0° ± 5.8° (range, -8°-6°). The UNCORR CT scans required a mean correction for scapular angle of 30.2° ± 8.0° (range, 15°-49°). The mean angle of sagittal rotation required to align the glenoid face with the 12-o’clock to 6-o’clock axis was 24.2° ± 5.1 ° (range, 13°-30°). These results are summarized in Table 1.
Table 1. Mean Correction Values Required to Correct the Uncorrected Images to the Corrected Images | |||
Anatomic alignment | Mean (degrees) | Range (degrees) | SD (degrees) |
Scapular angle | 30.2 | 15-49 | 8.0 |
Coronal Inclination | 7.0 | -8-6 | 5.8 |
Sagittal rotation | 24.2 | 13-30 | 5.1 |
For all measurements, the intraclass correlation coefficient for independent observers for all cuts within the 3 groups was r >.900 in all cases.
On an optimized CT scan, over 5 standardized cuts across a best-fit circle of the inferior glenoid, there was a statistically significant absolute mean difference of 12.6% in axial AP glenoid width (2.86 mm ± 2.00 mm, P =.016) when compared with the UNCORR scan. This corresponds to a 3% to 21% error in measurement of the AP width of the glenoid.
Continue to: For the entire cohort...
For the entire cohort of 43 patients, the UNCORR scans underestimated the axial AP width (and thus overestimated GBL) in cut 1 (P =.003), and overestimated the axial AP width (and thus underestimated GBL) in cuts 3 to 5 (P < .001 for all) compared with that of the CORR scans. There was no significant difference between the UNCORR and CORR scans in cut 2 (P = .331).
For groups I (10%-14.9% GBL) and III (>20% GBL), the UNCORR scans underestimated the axial AP width (and thus overestimated anterior GBL) in cuts 1 and 2, while in cuts 3 to 5, the axial AP width was overestimated (GBL was underestimated) (Tables 2, 3). In Group II (15%-19.9% GBL), the axial AP width was underestimated (GBL was overestimated), while in cuts 2 to 5, the axial AP width was overestimated (GBL was underestimated). Overall, AP glenoid width was consistently underestimated in cut 1, the most caudal cut, while AP glenoid width was consistently overestimated in cuts 3 to 5, the more cephalad cuts.
Table 2. Absolute Mean Difference in Axial AP Width (mm) Between Corrected and Uncorrected Images (% difference) | |||||
Cut 1 (Caudal) | Cut 2 | Cut 3 (Center) | Cut 4 | Cut 5 (Cephalad) | |
Group I: 10%-14.9% GBL | 2.4 mm (15.3%) | 1.8 mm (9.0%) | 1.8 mm (7.7%) | 3.0 mm (11.7%) | 4.0 mm (16.8%) |
Group II: 15%-19.9% GBL | 1.8 mm (13.1%) | 1.7 mm (7.9%) | 2.8 mm (10.6%) | 4.1 mm (14.4%) | 4.8 mm (16.9%) |
Group III: >20% | 2.8 mm (16.1%) | 1.9 mm (8.0%) | 2.3 mm (10.3) | 4.4 mm (16.6%) | 5.2 mm (17.0%) |
Abbreviations: AP, anterior-posterior; GBL, glenoid bone loss.
Table 3. Mean AP Glenoid Width Based on CORR and UNCORR Images for the Entire Cohort of 43 Patients | |||||
Axial cut | Mean AP width (mm) | Mean AP width (mm) | Absolute mean AP width difference (mm) | Absolute mean AP width difference (%) | P value |
(Caudal) 1 | 16.6208 | 18.4958 | -1.875 | 14.7768 | .0029565 |
2 | 20.6558 | 21.3166 | -0.661 | 3.6137 | .3310965 |
3 | 24.2583 | 22.3125 | 1.946 | 7.8042 | <.0001 |
4 | 26.1291 | 21.8916 | 4.238 | 15.8449 | <.0001 |
(Rostral) 5 | 26.0875 | 20.4875 | 5.6 | 20.9717 | <.0001 |
Abbreviations: AP, anterior-posterior; CORR, corrected; UNCORR, uncorrected.
DISCUSSION
The principle findings of this study demonstrate that UNCORR conventional 2-D CT scans inaccurately estimate glenoid width as well as inaccurately quantify the degree of anterior GBL. Underestimations of GBL may lead to insufficient treatment of clinically meaningful GBL, thereby increasing the risk of instability recurrence; whereas overestimations of GBL may lead to unnecessary treatment, subjecting patients to increased surgical morbidity. Therefore, the authors recommend correcting the orientation of the scapula in cases wherein clinical decisions are entirely based on 2-D CT, or using alternative methods for quantifying GBL, specifically in the form of 3-D reconstructions.
The use of axial imaging, with CT scans and/or magnetic resonance imaging, is growing in popularity for evaluation of both glenoid anatomy and GBL. Nevertheless, despite our improved ability to critically evaluate the glenoid using these advanced imaging modalities, the images themselves require scrutiny by clinicians to determine if the images accurately depict the true anatomy of the glenoid. As demonstrated by Gross and colleagues,15 conventional 2D CT scan protocols are not optimized to the anatomy of the glenohumeral joint, even in patients without GBL. Due to the alignment of the image relative to the plane of the scapula as opposed to the plane of the glenoid, UNCORR scans result in significantly different measurements of glenoid version (2.0° ± 0.1°) and AP glenoid width (1.2 mm ± 0.42 mm) compared with corrected scans, requiring an average 20.1° ± 1.2° of correction to align the sagittal plane. In the present study involving the patients with GBL, we also found that conventional, UNCORR 2-D CT scan protocols inaccurately estimate glenoid width and the degree of anterior GBL. In particular, AP glenoid width was consistently underestimated in the more caudal cuts, while AP glenoid width was consistently overestimated in the more cephalad cuts. Thus, anterior GBL was overestimated (AP glenoid width was underestimated) in the more caudal cuts, whereas anterior GBL was underestimated in the more cranial cuts (AP glenoid width was overestimated). Given that approximately 1 mm of glenoid bone corresponds to approximately 4% of glenoid width,16 even subtle differences in the interpretation of GBL may lead to gross overestimation/underestimation of bone loss, with significant clinical implications.
In the anterior instability patient population, clinical decision-making is often based on the degree of GBL as determined by advanced imaging modalities. In addition to other patient-specific factors, including age, gender, activity level, type of sport, and number of prior dislocations and/or prior surgeries, the quantity of GBL will often determine which surgical procedure needs to be performed. Typically, patients with >20% to 25% anterior GBL are indicated for a glenoid reconstruction procedure, most commonly via the Latarjet procedure (coracoid transfer).21-27 The Latarjet procedure remains an excellent technique for appropriately indicated patients, with historically good clinical outcomes and low recurrence rates. Complications associated with the Latarjet procedure, however, are not uncommon, including devastating neuropraxia of the axillary and musculocutaneous nerves, and occasionally permanent neurologic deficits.28 Thus, it is critical to avoid overtreating patients with recurrent instability and GBL. As demonstrated by this study, depending on the cranial-to-caudal location on the glenoid, current 2-D CT techniques may underestimate AP glenoid width, resulting in an overestimation of GBL, potentially leading to the decision to proceed with glenoid bone reconstruction when such a procedure is not required. On the contrary, overestimation of AP glenoid width, which occurs in the more cephalad cuts of the glenoid, is perhaps more worrisome, as the resulting underestimation of GBL may lead to inadequate treatment of patients with recurrent instability. Certainly, one of the main risk factors for failed soft tissue shoulder stabilization is a failure to address GBL. If clinical decisions are made based on UNCORR 2-D CT scans, which are often inaccurate with respect to AP glenoid width by an average 2.86 mm ± 2.00 mm (equivalent to 12.6% ± 6.9% GBL) as determined in this study, patients who truly require osseous glenoid reconstructions may be indicated for only soft tissue stabilization, based on the underestimation of GBL.
Continue to: The current gold standard...
The current gold standard for GBL measurement is a perfect-fit circle performed on a 3-D CT scan.22 To that end, it would have been useful to measure the glenoids from this study on 3-D CT scans and compare the data with both UNCORR and CORR measurements. This would have provided a better understanding to what extent the CORR measurements on 2-D scans are relatable with the gold standard. As 3-D CT scans provide a better en face view of the glenoid, more accurate GBL measurements, and ease of 3-D manipulation, they have become more widely used across the country.29,30 Nevertheless, in situations where 3-D imaging is more challenging to obtain because of technology or cost limitations, having a strategy for ensuring proper orientation of 2-D scans would have a substantial impact on clinical decision-making. If such corrections are not made, the inaccuracy of current 2-D scanning protocols justifies the cost 3-D reconstruction protocols. The difference in GBL measurements are critical in cases of increasingly large degrees of GBL, as in these instances, the inferior glenoid becomes more of an inverted-pear shape as opposed to a perfect circle, and differences in CORR and UNCORR images are likely to be more profound.
LIMITATIONS
This study has limitations, such as the relatively small sample size and the selection bias by the reviewers with potential differences in interobserver reliability. Further, minor modifications during the reformatting process may be found with each attempt to manipulate the images and may result in minor, insignificant differences in AP width measurements. Performing 1 or more additional CT scans on the same cohort of patients would have been helpful; however, due to the increased risk of radiation exposure, this was not performed. Performing CT scans on cadaveric specimens with GBL and applying the study methodology would also have been helpful to provide independent verification of our clinical findings; however, specimens were not available for this study. Another limitation of this study is that we did not compare our findings with the findings of glenoid width, and bone loss, as determined using the circle method, which is commonly utilized when 3-D reconstructions are available. In this study, the purpose was to utilize only the 2-D reformatted images, with the assumption that 3-D reconstructions are not always available, and cannot always be measured. To minimize selection bias, the investigators measured the correction effects within groups of patients with similar degrees of GBL (10%-14.9%, 15%-19.9%, and >20%). In addition, not all the selected patients showed degenerative glenoid changes or irregular glenoid shape indicating previous bone augmentation.
CONCLUSIONS
UNCORR 2D CT scans inaccurately estimate glenoid width and the degree of anterior GBL. The clinical implications of these findings are profound and suggest corrected 2D CT scans or 3D reconstruction allow measurements to be taken in the axis of the glenoid to accurately define the anatomy and quantity of anterior GBL in patients with shoulder instability.
ABSTRACT
Standard 2-dimensional (2-D) computed tomography (CT) scans of the shoulder are often aligned to the plane of the body as opposed to the plane of the scapula, which may challenge the ability to accurately measure glenoid width and glenoid bone loss (GBL). The purpose of this study is to determine the effect of sagittal rotation of the glenoid on axial anterior-posterior (AP) glenoid width measurements in the setting of anterior GBL.
Forty-three CT scans from consecutive patients with anterior GBL (minimum 10%) were reformatted utilizing open-source DICOM software (OsiriX MD). Patients were grouped according to extent of GBL: I, 10% to 14.9% (N = 12); II, 15% to 19.9% (N = 16); and III, >20% (N = 15). The uncorrected (UNCORR) and corrected (CORR) images were assessed in the axial plane at 5 standardized cuts and measured for AP glenoid width.
For groups I and III, UNCORR scans underestimated axial AP width (and thus overestimated anterior GBL) in cuts 1 and 2, while in cuts 3 to 5, the axial AP width was overestimated (GBL was underestimated). In Group II, axial AP width was underestimated (GBL was overestimated), while in cuts 2 to 5, the axial AP width was overestimated (GBL was underestimated). Overall, AP glenoid width was consistently underestimated in cut 1, the most caudal cut; while AP glenoid width was consistently overestimated in cuts 3 to 5, the more cephalad cuts.
UNCORR 2-D CT scans inaccurately estimated glenoid width and the degree of anterior GBL. This data suggests that corrected 2D CT scans or a 3-dimensional (3-D) reconstruction can help in accurately defining the anterior GBL in patients with shoulder instability.
The treatment of glenohumeral instability has substantially evolved over the past several decades. The understanding of glenoid bone loss (GBL), in particular, has advanced to such a level that we utilize the quantification of GBL for surgical decision-making. Unrecognized and/or untreated GBL is associated with recurrent instability, pain, and disability. Controversy exists, however, regarding the precise amount of anterior GBL that is significant enough to warrant surgical treatment. While historically, 25%1,2 of anterior GBL was thought to be the critical number required to warrant osseous augmentation, studies that are more recent have highlighted the need to perform osseous glenoid reconstruction with lesser degrees of GBL, particularly in the contact athlete.3-9 As small differences in the amount of GBL can change surgical decision-making from an all-soft tissue repair to an osseous reconstruction, it is paramount that we have accurate, valid, and reproducible methods for calculating GBL.
Continue to: Historically, plain radiographs...
Historically, plain radiographs have been the mainstay for evaluating the glenohumeral joint, including Grashey and axillary views, allowing clinicians to evaluate the congruency of the glenohumeral joint and to assess bone loss on both the glenoid and humeral head.1,10 While large, acute fractures of the glenoid are fairly evident on radiographs, including the Grashey view,11 shoulders with chronic and/or attritional anterior GBL are more difficult to evaluate, and often do not provide the information necessary to guide surgical decision-making.
Computed tomography (CT) of the shoulder has become the most commonly utilized imaging modality in the evaluation of patients with shoulder instability associated with GBL. Standard 2-dimensional (2-D) CT scans of the shoulder are often aligned to the plane of the body as opposed to the plane of the scapula/glenoid, as standard protocols often fail to account for the anterior sagittal rotation of the scapula/glenoid, similar to the disadvantage of standard radiographs. While 3-dimensional (3-D) CT reconstructions eliminate the effect of gantry angles, and thus allow for an en face view of the glenoid, 3-D reconstructions are not always available, and cannot always be measured.12-14 Thus, improved methodology for utilizing standard 2D scans is warranted, as the ability to correctly align the axial CT scan to the axis of the glenoid may allow for more accurate GBL measurements, which will ultimately impact surgical decision-making. Recently, Gross and colleagues15 reported the effect of sagittal rotation of the glenoid on axial measurements of anterior-posterior (AP) glenoid width and glenoid version in normal glenoids, without bone loss, and found that the mean angle of correction needed to align the sagittal plane was 20.1° ± 1.2° of rotation. To the authors’ knowledge, this same methodology has not been applied to patients with clinically meaningful anterior GBL. Given that the average glenoid width in human shoulders is 24.4 mm ± 2.9 mm,16 1 mm of glenoid bone loss (GBL) corresponds to approximately 4% of the glenoid width, and thus even subtle differences in the interpretation of GBL may have substantial clinical implications. Therefore, the purpose of this study is to determine the effect of sagittal rotation of the glenoid on axial AP glenoid width measurements in the setting of clinically significant anterior GBL.
METHODS
This study was approved by Massachusetts General Hospital Institutional Review Board. A retrospective review of consecutive patients with a diagnosis of anterior shoulder instability between 2009 and 2013 was conducted. Inclusion criteria comprised patients with a minimum of 10% anterior GBL, an available CT scan of the affected shoulder, and no history of prior ipsilateral surgeries. Exclusion criteria comprised evidence of degenerative changes to the glenoid and/or humeral head, as well as prior ipsilateral shoulder surgery. Sixty consecutive patients were originally identified as having anterior shoulder instability, and 17 were excluded based on the inclusion/exclusion criteria, leaving 43 patients (43 shoulders) available for inclusion. Shoulder CT scans from all 43 patients were reformatted utilizing open-source DICOM software (OsiriX MD, version 2.5.1 65-bit) multi-planar reconstruction (MPR).
CT PROTOCOL
All patients underwent a standard glenohumeral CT scan using a Siemens Sensation 64 Scanner (Siemens), a 64-detector scanner. Scans were acquired with 0.6 mm of collimation, 140 kV, and 300 mA-seconds. Slice thickness was set to 2 mm. All patient information was de-identified for analysis.
The uncorrected (UNCORR) scans were defined as the default orientation on the scanner. In the UNCORR scans, the axial, coronal, and sagittal views were oriented relative to the scanner gantry table, as opposed to the anatomy of the glenoid. The corrected (CORR) CT scans were aligned in all 3 planes relative to the glenoid face, and thus the cuts were perpendicular to the long axis of the glenoid.15 This resulted in sagittal cuts perpendicular to the 12-o’clock to 6-o’clock axis in the sagittal plane (Figure 1).
Continue to: In a de-identified fashion...
IMAGE ANALYSIS AND REFORMATTING
In a de-identified fashion, all CT scans were imported and analyzed using open-source Digital Imaging and Communications in Medicine (DICOM) software (OsiriX MD, version 2.5.1 64-bit). By following a previously developed method, CT scans were reformatted using OsiriX MPR. The OsiriX software has an MPR function that allows simultaneous manipulation of 2-D CT scans in 3 orthogonal planes: axial, sagittal, and coronal. In the MPR mode, the alternation of 1 plane directly affects the orientation of the remaining 2 planes. Thus, by using an MPR, one can analyze the impact that a default CT scan performed relative to the gantry of the table, UNCORR, has on the axial images.
First, the en face view was obtained via a 2-step process: alignment of the axial plane to account for the scapular angle, followed by alignment of the coronal plane to adjust for the glenoid inclination.15 These 2 adjustments provided a true en face sagittal glenoid view. The final adjustment step was a sagittal en face rotation of the glenoid such that the superior and inferior glenoid tubercles were placed on the 12-o’clock to 6-o’clock axis (CORR scan). Previous studies have identified a central longitudinal axis that was used in this method to align the supraglenoid tubercle with the 12-o’clock to 6-o’clock axis on the glenoid face.15,17,18 The standard error of mean was 1.21°. This new CORR view resulted in axial cuts through the glenoid that were oriented perpendicular to the 12-o’clock to 6-o’clock axis. The UNCORR and CORR images were assessed in the axial plane at 5 standardized cuts and measured for AP glenoid width by 2 independent observers in a blinded, randomized fashion. When the measured AP width of the UNCORR scan was less than that measured on the CORR scan, the AP width of the glenoid was considered underestimated, and the degree of GBL was considered overestimated (Figure 2).
SCAPULAR ANGLE
Scapular angle measurements were performed on the axial view as the angle between a line through the long axis of the body of the scapula, and a line parallel to the CT gantry table.15,19 Subsequently, the axial plane was aligned to the glenoid surface.
CORONAL INCLINATION
Coronal inclination measurements were performed on the sagittal view as the angle between a line tangential to the face of the glenoid and a line perpendicular to the CT gantry table. Positive values represented superior inclination, while negative values represented inferior glenoid inclination.15
SAGITTAL ROTATION
Sagittal rotation measurements were performed using the built-in angle measurement tool in OsiriX in the sagittal plane since the degree of rotation required aligning the long axis of the glenoid to the 12-o’clock to 6-o’clock axis. The amount of rotation was defined as the rotation angle.15
Continue to: Similarly, as described by Gross...
GLENOID WIDTH
Similarly, as described by Gross and colleagues,15 the sagittal en face view was divided via 5 cuts, throughout a superimposed best-fit circle that closely represents the glenoid.9,15,20 For both the UNCORR and CORR, glenoid width (AP distance) was measured on the axial image at the widest point from AP cortex across the glenoid face.
PATIENT GROUPS
Utilizing the en face 3-D CT reconstruction view of the glenoid as the gold standard, patients were placed into 1 of 3 groups according to the degree of anterior GBL measured via the surface method.9,20 The groups were as follows:
I. 10% to 14.9% (N = 12)
II. 15% to 19.9% (N = 16)
III. >20% (N = 15)
STATISTICAL METHODS
Paired t-tests were used to compare all measurements between CORR and UNCORR scans for each of the 5 cuts. A P-value of .05 was used as the threshold for statistical significance in 2-tailed comparisons. Mean and standard errors are presented with standard deviations throughout the study. For interobserver reliability, the measurements between the observers, the intraclass correlation coefficient was calculated. All statistics were performed with SPSS (Version 22).
RESULTS
The study cohort was comprised of 19 left shoulders (44%) and 24 right shoulders (56%), including 36 male patients (84%) and 7 female patients (16%). The average age was 27.8 years (range, 21-40 years). The variability in measured difference, with respect to AP width, was 1.05 mm. The UNCORR CT scans required a mean correction for coronal inclination of 7.0° ± 5.8° (range, -8°-6°). The UNCORR CT scans required a mean correction for scapular angle of 30.2° ± 8.0° (range, 15°-49°). The mean angle of sagittal rotation required to align the glenoid face with the 12-o’clock to 6-o’clock axis was 24.2° ± 5.1 ° (range, 13°-30°). These results are summarized in Table 1.
Table 1. Mean Correction Values Required to Correct the Uncorrected Images to the Corrected Images | |||
Anatomic alignment | Mean (degrees) | Range (degrees) | SD (degrees) |
Scapular angle | 30.2 | 15-49 | 8.0 |
Coronal Inclination | 7.0 | -8-6 | 5.8 |
Sagittal rotation | 24.2 | 13-30 | 5.1 |
For all measurements, the intraclass correlation coefficient for independent observers for all cuts within the 3 groups was r >.900 in all cases.
On an optimized CT scan, over 5 standardized cuts across a best-fit circle of the inferior glenoid, there was a statistically significant absolute mean difference of 12.6% in axial AP glenoid width (2.86 mm ± 2.00 mm, P =.016) when compared with the UNCORR scan. This corresponds to a 3% to 21% error in measurement of the AP width of the glenoid.
Continue to: For the entire cohort...
For the entire cohort of 43 patients, the UNCORR scans underestimated the axial AP width (and thus overestimated GBL) in cut 1 (P =.003), and overestimated the axial AP width (and thus underestimated GBL) in cuts 3 to 5 (P < .001 for all) compared with that of the CORR scans. There was no significant difference between the UNCORR and CORR scans in cut 2 (P = .331).
For groups I (10%-14.9% GBL) and III (>20% GBL), the UNCORR scans underestimated the axial AP width (and thus overestimated anterior GBL) in cuts 1 and 2, while in cuts 3 to 5, the axial AP width was overestimated (GBL was underestimated) (Tables 2, 3). In Group II (15%-19.9% GBL), the axial AP width was underestimated (GBL was overestimated), while in cuts 2 to 5, the axial AP width was overestimated (GBL was underestimated). Overall, AP glenoid width was consistently underestimated in cut 1, the most caudal cut, while AP glenoid width was consistently overestimated in cuts 3 to 5, the more cephalad cuts.
Table 2. Absolute Mean Difference in Axial AP Width (mm) Between Corrected and Uncorrected Images (% difference) | |||||
Cut 1 (Caudal) | Cut 2 | Cut 3 (Center) | Cut 4 | Cut 5 (Cephalad) | |
Group I: 10%-14.9% GBL | 2.4 mm (15.3%) | 1.8 mm (9.0%) | 1.8 mm (7.7%) | 3.0 mm (11.7%) | 4.0 mm (16.8%) |
Group II: 15%-19.9% GBL | 1.8 mm (13.1%) | 1.7 mm (7.9%) | 2.8 mm (10.6%) | 4.1 mm (14.4%) | 4.8 mm (16.9%) |
Group III: >20% | 2.8 mm (16.1%) | 1.9 mm (8.0%) | 2.3 mm (10.3) | 4.4 mm (16.6%) | 5.2 mm (17.0%) |
Abbreviations: AP, anterior-posterior; GBL, glenoid bone loss.
Table 3. Mean AP Glenoid Width Based on CORR and UNCORR Images for the Entire Cohort of 43 Patients | |||||
Axial cut | Mean AP width (mm) | Mean AP width (mm) | Absolute mean AP width difference (mm) | Absolute mean AP width difference (%) | P value |
(Caudal) 1 | 16.6208 | 18.4958 | -1.875 | 14.7768 | .0029565 |
2 | 20.6558 | 21.3166 | -0.661 | 3.6137 | .3310965 |
3 | 24.2583 | 22.3125 | 1.946 | 7.8042 | <.0001 |
4 | 26.1291 | 21.8916 | 4.238 | 15.8449 | <.0001 |
(Rostral) 5 | 26.0875 | 20.4875 | 5.6 | 20.9717 | <.0001 |
Abbreviations: AP, anterior-posterior; CORR, corrected; UNCORR, uncorrected.
DISCUSSION
The principle findings of this study demonstrate that UNCORR conventional 2-D CT scans inaccurately estimate glenoid width as well as inaccurately quantify the degree of anterior GBL. Underestimations of GBL may lead to insufficient treatment of clinically meaningful GBL, thereby increasing the risk of instability recurrence; whereas overestimations of GBL may lead to unnecessary treatment, subjecting patients to increased surgical morbidity. Therefore, the authors recommend correcting the orientation of the scapula in cases wherein clinical decisions are entirely based on 2-D CT, or using alternative methods for quantifying GBL, specifically in the form of 3-D reconstructions.
The use of axial imaging, with CT scans and/or magnetic resonance imaging, is growing in popularity for evaluation of both glenoid anatomy and GBL. Nevertheless, despite our improved ability to critically evaluate the glenoid using these advanced imaging modalities, the images themselves require scrutiny by clinicians to determine if the images accurately depict the true anatomy of the glenoid. As demonstrated by Gross and colleagues,15 conventional 2D CT scan protocols are not optimized to the anatomy of the glenohumeral joint, even in patients without GBL. Due to the alignment of the image relative to the plane of the scapula as opposed to the plane of the glenoid, UNCORR scans result in significantly different measurements of glenoid version (2.0° ± 0.1°) and AP glenoid width (1.2 mm ± 0.42 mm) compared with corrected scans, requiring an average 20.1° ± 1.2° of correction to align the sagittal plane. In the present study involving the patients with GBL, we also found that conventional, UNCORR 2-D CT scan protocols inaccurately estimate glenoid width and the degree of anterior GBL. In particular, AP glenoid width was consistently underestimated in the more caudal cuts, while AP glenoid width was consistently overestimated in the more cephalad cuts. Thus, anterior GBL was overestimated (AP glenoid width was underestimated) in the more caudal cuts, whereas anterior GBL was underestimated in the more cranial cuts (AP glenoid width was overestimated). Given that approximately 1 mm of glenoid bone corresponds to approximately 4% of glenoid width,16 even subtle differences in the interpretation of GBL may lead to gross overestimation/underestimation of bone loss, with significant clinical implications.
In the anterior instability patient population, clinical decision-making is often based on the degree of GBL as determined by advanced imaging modalities. In addition to other patient-specific factors, including age, gender, activity level, type of sport, and number of prior dislocations and/or prior surgeries, the quantity of GBL will often determine which surgical procedure needs to be performed. Typically, patients with >20% to 25% anterior GBL are indicated for a glenoid reconstruction procedure, most commonly via the Latarjet procedure (coracoid transfer).21-27 The Latarjet procedure remains an excellent technique for appropriately indicated patients, with historically good clinical outcomes and low recurrence rates. Complications associated with the Latarjet procedure, however, are not uncommon, including devastating neuropraxia of the axillary and musculocutaneous nerves, and occasionally permanent neurologic deficits.28 Thus, it is critical to avoid overtreating patients with recurrent instability and GBL. As demonstrated by this study, depending on the cranial-to-caudal location on the glenoid, current 2-D CT techniques may underestimate AP glenoid width, resulting in an overestimation of GBL, potentially leading to the decision to proceed with glenoid bone reconstruction when such a procedure is not required. On the contrary, overestimation of AP glenoid width, which occurs in the more cephalad cuts of the glenoid, is perhaps more worrisome, as the resulting underestimation of GBL may lead to inadequate treatment of patients with recurrent instability. Certainly, one of the main risk factors for failed soft tissue shoulder stabilization is a failure to address GBL. If clinical decisions are made based on UNCORR 2-D CT scans, which are often inaccurate with respect to AP glenoid width by an average 2.86 mm ± 2.00 mm (equivalent to 12.6% ± 6.9% GBL) as determined in this study, patients who truly require osseous glenoid reconstructions may be indicated for only soft tissue stabilization, based on the underestimation of GBL.
Continue to: The current gold standard...
The current gold standard for GBL measurement is a perfect-fit circle performed on a 3-D CT scan.22 To that end, it would have been useful to measure the glenoids from this study on 3-D CT scans and compare the data with both UNCORR and CORR measurements. This would have provided a better understanding to what extent the CORR measurements on 2-D scans are relatable with the gold standard. As 3-D CT scans provide a better en face view of the glenoid, more accurate GBL measurements, and ease of 3-D manipulation, they have become more widely used across the country.29,30 Nevertheless, in situations where 3-D imaging is more challenging to obtain because of technology or cost limitations, having a strategy for ensuring proper orientation of 2-D scans would have a substantial impact on clinical decision-making. If such corrections are not made, the inaccuracy of current 2-D scanning protocols justifies the cost 3-D reconstruction protocols. The difference in GBL measurements are critical in cases of increasingly large degrees of GBL, as in these instances, the inferior glenoid becomes more of an inverted-pear shape as opposed to a perfect circle, and differences in CORR and UNCORR images are likely to be more profound.
LIMITATIONS
This study has limitations, such as the relatively small sample size and the selection bias by the reviewers with potential differences in interobserver reliability. Further, minor modifications during the reformatting process may be found with each attempt to manipulate the images and may result in minor, insignificant differences in AP width measurements. Performing 1 or more additional CT scans on the same cohort of patients would have been helpful; however, due to the increased risk of radiation exposure, this was not performed. Performing CT scans on cadaveric specimens with GBL and applying the study methodology would also have been helpful to provide independent verification of our clinical findings; however, specimens were not available for this study. Another limitation of this study is that we did not compare our findings with the findings of glenoid width, and bone loss, as determined using the circle method, which is commonly utilized when 3-D reconstructions are available. In this study, the purpose was to utilize only the 2-D reformatted images, with the assumption that 3-D reconstructions are not always available, and cannot always be measured. To minimize selection bias, the investigators measured the correction effects within groups of patients with similar degrees of GBL (10%-14.9%, 15%-19.9%, and >20%). In addition, not all the selected patients showed degenerative glenoid changes or irregular glenoid shape indicating previous bone augmentation.
CONCLUSIONS
UNCORR 2D CT scans inaccurately estimate glenoid width and the degree of anterior GBL. The clinical implications of these findings are profound and suggest corrected 2D CT scans or 3D reconstruction allow measurements to be taken in the axis of the glenoid to accurately define the anatomy and quantity of anterior GBL in patients with shoulder instability.
1. Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: results for a series of 28 shoulders treated with the Latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202. doi:10.1007/s10195-012-0201-3.
2. Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35-46.
3. Bhatia S, Ghodadra NS, Romeo AA, et al. The importance of the recognition and treatment of glenoid bone loss in an athletic population. Sports Health. 2011;3(5):435-440. doi:10.1177/1941738111414126.
4. Lo IK, Parten PM, Burkhart SS. The inverted pear glenoid: an indicator of significant glenoid bone loss. Arthroscopy. 2004;20(2):169-174. doi:10.1016/j.arthro.2003.11.036.
5. Mologne TS, Provencher MT, Menzel KA, Vachon TA, Dewing CB. Arthroscopic stabilization in patients with an inverted pear glenoid: results in patients with bone loss of the anterior glenoid. Am J Sports Med. 2007;35(8):1276-1283. doi:10.1177/0363546507300262.
6. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
7. Provencher MT, Bhatia S, Ghodadra NS, et al. Recurrent shoulder instability: current concepts for evaluation and management of glenoid bone loss. J Bone Joint Surg Am. 2010;92(suppl 2):133-151. doi:10.2106/JBJS.J.00906.
8. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. Apparent causes of failure and treatment. J Bone Joint Surg Am. 1984;66(2):159-168.
9. Sugaya H, Moriishi J, Dohi M, Kon Y, Tsuchiya A. Glenoid rim morphology in recurrent anterior glenohumeral instability. J Bone Joint Surg Am. 2003;85-A(5):878-884.
10. Edwards TB, Boulahia A, Walch G. Radiographic analysis of bone defects in chronic anterior shoulder instability. Arthroscopy. 2003;19(7):732-739.
11. Jankauskas L, Rudiger HA, Pfirrmann CW, Jost B, Gerber C. Loss of the sclerotic line of the glenoid on anteroposterior radiographs of the shoulder: a diagnostic sign for an osseous defect of the anterior glenoid rim. J Shoulder Elbow Surg. 2010;19(1):151-156. doi:10.1016/j.jse.2009.04.013.
12. Altan E, Ozbaydar MU, Tonbul M, Yalcin L. Comparison of two different measurement methods to determine glenoid bone defects: area or width? J Shoulder Elbow Surg. 2014;23(8):1215-1222. doi:10.1016/j.jse.2013.11.029.
13. Bishop JY, Jones GL, Rerko MA, Donaldson C, Group MS. 3-D CT is the most reliable imaging modality when quantifying glenoid bone loss. Clin Orthop Relat Res. 2013;471(4):1251-1256. doi:10.1007/s11999-012-2607-x.
14. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008; 24(4):376-382. doi:10.1016/j.arthro.2007.10.008.
15. Gross DJ, Golijanin P, Dumont GD, et al. The effect of sagittal rotation of the glenoid on axial glenoid width and glenoid version in computed tomography scan imaging. J Shoulder Elbow Surg. 2016;25(1):61-68. doi:10.1016/j.jse.2015.06.017.
16. Lenart BA, Freedman R, Van Thiel GS, et al. Magnetic resonance imaging evaluation of normal glenoid length and width: an anatomic study. Arthroscopy. 2014;30(8):915-920. doi:10.1016/j.arthro.2014.03.006.
17. Bois AJ, Fening SD, Polster J, Jones MH, Miniaci A. Quantifying glenoid bone loss in anterior shoulder instability: reliability and accuracy of 2-dimensional and 3-dimensional computed tomography measurement techniques. Am J Sports Med. 2012;40(11):2569-2577. doi:10.1177/0363546512458247.
18. Griffith JF, Antonio GE, Tong CW, Ming CK. Anterior shoulder dislocation: quantification of glenoid bone loss with CT. AJR Am J Roentgenol. 2003;180(5):1423-1430. doi:10.2214/ajr.180.5.1801423.
19. Hoenecke HR Jr, Hermida JC, Flores-Hernandez C, D'Lima DD. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(2):166-171. doi:10.1016/j.jse.2009.08.009.
20. Huijsmans PE, de Witte PB, de Villiers RV, et al. Recurrent anterior shoulder instability: accuracy of estimations of glenoid bone loss with computed tomography is insufficient for therapeutic decision-making. Skeletal Radiol. 2011;40(10):1329-1334. doi:10.1007/s00256-011-1184-5.
21. Bhatia S, Frank RM, Ghodadra NS, et al. The outcomes and surgical techniques of the latarjet procedure. Arthroscopy. 2014;30(2):227-235. doi:10.1016/j.arthro.2013.10.013.
22. Cunningham G, Benchouk S, Kherad O, Ladermann A. Comparison of arthroscopic and open Latarjet with a learning curve analysis. Knee Surg Sports Traumatol Arthrosc. 2015;24(2):540-545. doi:10.1007/s00167-015-3910-3.
23. Fedorka CJ, Mulcahey MK. Recurrent anterior shoulder instability: a review of the Latarjet procedure and its postoperative rehabilitation. Phys Sportsmed. 2015;43(1):73-79. doi:10.1080/00913847.2015.1005543.
24. Flinkkila T, Sirniö K. Open Latarjet procedure for failed arthroscopic Bankart repair. Orthop Traumatol Surg Res. 2015;101(1):35-38. doi:10.1016/j.otsr.2014.11.005.
25. Hovelius L, Sandström B, Saebö M. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: study II-the evolution of dislocation arthropathy. J Shoulder Elbow Surg. 2006;15(3):279-289. doi:10.1016/j.jse.2005.09.014.
26. Hovelius L, Sandström B, Sundgren K, Saebö M. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: study I--clinical results. J Shoulder Elbow Surg. 2004;13(5):509-516. doi:10.1016/S1058274604000916.
27. Hovelius L, Vikerfors O, Olofsson A, Svensson O, Rahme H. Bristow-Latarjet and Bankart: a comparative study of shoulder stabilization in 185 shoulders during a seventeen-year follow-up. J Shoulder Elbow Surg. 2011;20(7):1095-1101. doi:10.1016/j.jse.2011.02.005.
28. Gupta A, Delaney R, Petkin K, Lafosse L. Complications of the Latarjet procedure. Curr Rev Musculoskelet Med. 2015;8(1):59-66. doi:10.1007/s12178-015-9258-y.
29. Kwon YW, Powell KA, Yum JK, Brems JJ, Iannotti JP. Use of three-dimensional computed tomography for the analysis of the glenoid anatomy. J Shoulder Elbow Surg. 2005;14(1):85-90. doi:10.1016/j.jse.2004.04.011.
30. Saito H, Itoi E, Sugaya H, Minagawa H, Yamamoto N, Tuoheti Y. Location of the glenoid defect in shoulders with recurrent anterior dislocation. Am J Sports Med. 2005;33(6):889-893. doi:10.1177/0363546504271521.
1. Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: results for a series of 28 shoulders treated with the Latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202. doi:10.1007/s10195-012-0201-3.
2. Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35-46.
3. Bhatia S, Ghodadra NS, Romeo AA, et al. The importance of the recognition and treatment of glenoid bone loss in an athletic population. Sports Health. 2011;3(5):435-440. doi:10.1177/1941738111414126.
4. Lo IK, Parten PM, Burkhart SS. The inverted pear glenoid: an indicator of significant glenoid bone loss. Arthroscopy. 2004;20(2):169-174. doi:10.1016/j.arthro.2003.11.036.
5. Mologne TS, Provencher MT, Menzel KA, Vachon TA, Dewing CB. Arthroscopic stabilization in patients with an inverted pear glenoid: results in patients with bone loss of the anterior glenoid. Am J Sports Med. 2007;35(8):1276-1283. doi:10.1177/0363546507300262.
6. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
7. Provencher MT, Bhatia S, Ghodadra NS, et al. Recurrent shoulder instability: current concepts for evaluation and management of glenoid bone loss. J Bone Joint Surg Am. 2010;92(suppl 2):133-151. doi:10.2106/JBJS.J.00906.
8. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. Apparent causes of failure and treatment. J Bone Joint Surg Am. 1984;66(2):159-168.
9. Sugaya H, Moriishi J, Dohi M, Kon Y, Tsuchiya A. Glenoid rim morphology in recurrent anterior glenohumeral instability. J Bone Joint Surg Am. 2003;85-A(5):878-884.
10. Edwards TB, Boulahia A, Walch G. Radiographic analysis of bone defects in chronic anterior shoulder instability. Arthroscopy. 2003;19(7):732-739.
11. Jankauskas L, Rudiger HA, Pfirrmann CW, Jost B, Gerber C. Loss of the sclerotic line of the glenoid on anteroposterior radiographs of the shoulder: a diagnostic sign for an osseous defect of the anterior glenoid rim. J Shoulder Elbow Surg. 2010;19(1):151-156. doi:10.1016/j.jse.2009.04.013.
12. Altan E, Ozbaydar MU, Tonbul M, Yalcin L. Comparison of two different measurement methods to determine glenoid bone defects: area or width? J Shoulder Elbow Surg. 2014;23(8):1215-1222. doi:10.1016/j.jse.2013.11.029.
13. Bishop JY, Jones GL, Rerko MA, Donaldson C, Group MS. 3-D CT is the most reliable imaging modality when quantifying glenoid bone loss. Clin Orthop Relat Res. 2013;471(4):1251-1256. doi:10.1007/s11999-012-2607-x.
14. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008; 24(4):376-382. doi:10.1016/j.arthro.2007.10.008.
15. Gross DJ, Golijanin P, Dumont GD, et al. The effect of sagittal rotation of the glenoid on axial glenoid width and glenoid version in computed tomography scan imaging. J Shoulder Elbow Surg. 2016;25(1):61-68. doi:10.1016/j.jse.2015.06.017.
16. Lenart BA, Freedman R, Van Thiel GS, et al. Magnetic resonance imaging evaluation of normal glenoid length and width: an anatomic study. Arthroscopy. 2014;30(8):915-920. doi:10.1016/j.arthro.2014.03.006.
17. Bois AJ, Fening SD, Polster J, Jones MH, Miniaci A. Quantifying glenoid bone loss in anterior shoulder instability: reliability and accuracy of 2-dimensional and 3-dimensional computed tomography measurement techniques. Am J Sports Med. 2012;40(11):2569-2577. doi:10.1177/0363546512458247.
18. Griffith JF, Antonio GE, Tong CW, Ming CK. Anterior shoulder dislocation: quantification of glenoid bone loss with CT. AJR Am J Roentgenol. 2003;180(5):1423-1430. doi:10.2214/ajr.180.5.1801423.
19. Hoenecke HR Jr, Hermida JC, Flores-Hernandez C, D'Lima DD. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(2):166-171. doi:10.1016/j.jse.2009.08.009.
20. Huijsmans PE, de Witte PB, de Villiers RV, et al. Recurrent anterior shoulder instability: accuracy of estimations of glenoid bone loss with computed tomography is insufficient for therapeutic decision-making. Skeletal Radiol. 2011;40(10):1329-1334. doi:10.1007/s00256-011-1184-5.
21. Bhatia S, Frank RM, Ghodadra NS, et al. The outcomes and surgical techniques of the latarjet procedure. Arthroscopy. 2014;30(2):227-235. doi:10.1016/j.arthro.2013.10.013.
22. Cunningham G, Benchouk S, Kherad O, Ladermann A. Comparison of arthroscopic and open Latarjet with a learning curve analysis. Knee Surg Sports Traumatol Arthrosc. 2015;24(2):540-545. doi:10.1007/s00167-015-3910-3.
23. Fedorka CJ, Mulcahey MK. Recurrent anterior shoulder instability: a review of the Latarjet procedure and its postoperative rehabilitation. Phys Sportsmed. 2015;43(1):73-79. doi:10.1080/00913847.2015.1005543.
24. Flinkkila T, Sirniö K. Open Latarjet procedure for failed arthroscopic Bankart repair. Orthop Traumatol Surg Res. 2015;101(1):35-38. doi:10.1016/j.otsr.2014.11.005.
25. Hovelius L, Sandström B, Saebö M. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: study II-the evolution of dislocation arthropathy. J Shoulder Elbow Surg. 2006;15(3):279-289. doi:10.1016/j.jse.2005.09.014.
26. Hovelius L, Sandström B, Sundgren K, Saebö M. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: study I--clinical results. J Shoulder Elbow Surg. 2004;13(5):509-516. doi:10.1016/S1058274604000916.
27. Hovelius L, Vikerfors O, Olofsson A, Svensson O, Rahme H. Bristow-Latarjet and Bankart: a comparative study of shoulder stabilization in 185 shoulders during a seventeen-year follow-up. J Shoulder Elbow Surg. 2011;20(7):1095-1101. doi:10.1016/j.jse.2011.02.005.
28. Gupta A, Delaney R, Petkin K, Lafosse L. Complications of the Latarjet procedure. Curr Rev Musculoskelet Med. 2015;8(1):59-66. doi:10.1007/s12178-015-9258-y.
29. Kwon YW, Powell KA, Yum JK, Brems JJ, Iannotti JP. Use of three-dimensional computed tomography for the analysis of the glenoid anatomy. J Shoulder Elbow Surg. 2005;14(1):85-90. doi:10.1016/j.jse.2004.04.011.
30. Saito H, Itoi E, Sugaya H, Minagawa H, Yamamoto N, Tuoheti Y. Location of the glenoid defect in shoulders with recurrent anterior dislocation. Am J Sports Med. 2005;33(6):889-893. doi:10.1177/0363546504271521.
TAKE-HOME POINTS
- Standard 2-D CT scans of the shoulder are often aligned to the plane of the body as opposed to the plane of the scapula, which may challenge the ability to accurately measure glenoid width and GBL.
- Underestimations of GBL may lead to insufficient treatment of clinically meaningful GBL, thereby increasing the risk of instability recurrence; whereas overestimations of GBL may lead to unnecessary treatment, subjecting patients to increased surgical morbidity.
- AP glenoid width was consistently underestimated in uncorrected axial cut 1, the most caudal cut.
- AP glenoid width was consistently overestimated in uncorrected axial cuts 3 to 5, the more cephalad cuts.
- CORR 2-D CT scans or a 3-D reconstruction can help in accurately defining the anterior GBL in patients with shoulder instability.
Participation in Work and Sport Following Reverse and Total Shoulder Arthroplasty
ABSTRACT
Both anatomical total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA) are routinely performed for patients who desire to continuously work or participate in sports. This study analyzes and compares the ability of patients to work and partake in sports following shoulder arthroplasty based on responses to clinical outcome surveys.
A retrospective review of the shoulder surgery repository was performed for all patients treated with TSA and RSA and who completed questions 9 and 10 on the activity patient self-evaluation portion of the American Shoulder and Elbow Surgeons (ASES) Assessment Form. Patients with a minimum of 1-year follow-up were included if a sport or work was identified. The analysis included 162 patients with TSA and 114 patients with RSA. Comparisons were made between TSA and RSA in terms of the specific ASES scores (rated 0-3) reported for ability to work and participate in sports and total ASES scores, and scores based on specific sports or line of work reported. Comparisons were also made between sports predominantly using shoulder function and those that do not.
TSA patients had a 27% higher ability to participate in sports (average specific ASES score: 2.5 vs 1.9, P < .001) than RSA patients and presented significantly higher scores for swimming and golf. Compared with RSA patients, TSA patients demonstrated more ability to participate in sports requiring shoulder function without difficulty, as 63% reported maximal scores (P = .003). Total shoulder arthroplasty patients also demonstrated a 21% higher ability to work than RSA patients (average specific ASES scores: 2.6 vs 2.1, P < .001), yielding significantly higher scores for housework and gardening.
Both TSA and RSA allow for participation in work and sports, with TSA patients reporting better overall ability to participate. For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
End-stage shoulder arthritis has been successfully treated with anatomical total shoulder arthroplasty (TSA) with high rates of functional recovery.1 With the introduction of reverse shoulder arthroplasty (RSA), indications for TSA have expanded.2-6 With continuing expansion of surgical indications, a more diverse and potentially active patient population is now being treated. As patients exhibit increased awareness of health and wellness, they demonstrate significant interest in understanding their ability to work or participate in sports after surgery.7 Patients no longer focus on pain relief as the only goal of surgery. A recent study of patients aged 65 years and undergoing shoulder arthroplasty revealed that 64% of the patients listed the ability to return to sports as the main reason for undergoing surgery,8 highlighting the significance of sports play in a patient’s life. Prior to surgery, shoulder pathologies lead to impairment in function, range of motion, and pain,9 hindering a patient to participate in both work and sports. With the intervention yielding improvement to these areas6,9-13 with increased patient satisfaction,10,13 accurately tailoring patient expectations for participation in sports and work postoperatively becomes increasingly important.
Continue to: Although several studies...
Although several studies have demonstrated the ability of patients to return to sports following TSA,8,14-18 a limited number of studies discuss the return to sports following RSA.19-21 Despite known postoperative improvements, no clear consensus is reached as to which specific sports patients can return to and at what level of participation is to be expected. Surveyed members of the American Shoulder and Elbow Surgeons (ASES) universally favored full return to sports, except for contact sports for TSA patients, whereas other surgeons are more conservative to allow RSA patients to return to activities.22 To our knowledge, no other study has investigated the ability to work following RSA. Furthermore, no other study has used patient-reported outcomes to compare the quality of participation in sports or work between TSA and RSA patients following surgery. This study reports the ability of patients treated with TSA and RSA to work and participate in sports based on clinical outcome surveys. We hypothesize that TSA patients will be allowed to work and participate in sports with less difficulty than RSA patients.
MATERIALS AND METHODS
Following Institutional Review Board approval, a retrospective review was performed on all patients treated with TSA or RSA and who completed questions 9 and/or 10 (by score and named usual sport and/or work) on the activity patient self-evaluation portion of the ASES23 Assessment Form between 2007 to 2014; queries were made via the Shoulder Outcomes Repository. A minimum of 12-month follow-up was required, as functional recovery has been shown to plateau or nearly plateau by 12 months.11 Patients were excluded if <12 months of follow-up was available, if they failed to provide a written answer for questions 9 or 10 on the activity patient self-evaluation portion of the ASES Assessment Form, or if they required a revision shoulder arthroplasty. A single fellowship-trained shoulder and elbow surgeon performed all procedures via the same deltopectoral approach and prescribed identical postoperative rehabilitation for both TSA and RSA patients. The database query yielded 162 TSA and 114 RSA patients, for a total of 276 patients eligible for the study.
For all patients, the most recent follow-up ASES score was used. Comparisons were made between TSA and RSA for total ASES scores and response groups for usual sport (ASES question 9) and usual work (ASES question 10). The ASES questionnaire provides patients with 4 choices for each question based on the ability to perform each activity: 0, unable to do; 1, very difficult; 2, somewhat difficult; and 3, not difficult. The questionnaire also allows the patients to identify their usual work and sports. If patients noted >1 sport or work activity, they were included within multiple subgroups. Patients were further compared by age and gender.
Work was subdivided to include retired, housework, desk jobs, prolonged standing, gardening/yard work, jobs requiring lifting, carpenter/construction, cook/food preparation, and creative jobs (Table 1).
Statistical analysis was performed with SPSS Version 21 (IBM). Unpaired t tests were used to determine differences between groups. A P-value of <.05 was deemed significant.
Continue to: A total of 276 patients...
RESULTS
A total of 276 patients that met the inclusion criteria were eligible for the study, with 162 having undergone TSA and 114 with RSA. Overall average follow-up totaled 29 months (range, 12-91 months). RSA patients (average age, 75 years old; range, 46-88 years) were significantly older than TSA patients (average age, 69 years old; range, 32-89 years; P = .001). Significantly more women were treated with TSA (52% TSA; 48% RSA; P = .012), whereas significantly more men were treated with TSA (67% TSA; 33% RSA, P = .012). Total ASES scores were significantly higher for TSA patients than RSA patients in work (P = .012) (Table 4) but not in sports (P = .063) (Table 5) categories.
SPORTS
A total of 186 patients, comprising of 71 RSA and 115 TSA individuals, responded to question 9 of the ASES questionnaire (Table 5). Among usually reported sports, golf (25%), swimming (17%), and walking (18%) were the most commonly cited. RSA patients indicating a sport were significantly older than TSA patients (74 years vs 69 years, P < .001). TSA patients reported a 27% higher difference in overall ability to participate in sports, with an average ASES sport-specific score of 2.5 compared with the 1.9 for RSA patients (P < .001).
Among specific sports, TSA patients reported significantly higher scores for swimming (2.6 vs 1.8, P = .007) and golf (2.5 vs 1.8, P = .050). However, no significant differences were observed for walking, gym exercises, and racquet sports (Table 5). Among sport subsets, RSA patients were significantly older for golf (77 years vs 70 years, P = .006) and bowling (80 years vs 68 years, P = .005). Five TSA patients reported biking as their sport, whereas no RSA patient reported such activity. Within each subset of sports, no significant differences were noted in average ASES total scores.
TSA patients demonstrated a more significant ability to perform usual sports that involve shoulder function without difficulty (score of 3). In shoulder dominant sports, a total of 63% of TSA patients reported a score of 3 compared with the 39% of RSA patients (P = .003). RSA patients more often reported an inability to perform shoulder specific sports, as proven by 20% of RSA patients reporting a score of 0 compared with 4% of TSA patients (P < .001) (Table 6).
WORK
A total of 265 patients, including 106 RSA and 159 TSA patients, responded to question 10 of the ASES questionnaire. Among usually reported work, retirement (43%), housework (27%), and desk jobs (18%) were the most commonly cited. RSA patients denoting a work were significantly older than TSA patients (75 years vs 69 years, P < .001). Patients with TSA presented a 21% higher difference in the overall ability to work, featuring an average ASES work-specific score of 2.6 compared with the 2.1 for RSA patients (P < .001) (Table 4).
Continue to: Among specific work activities...
Among specific work activities, TSA patients reported significantly higher scores for housework (2.7 vs 2; 34% difference; P = .001) and gardening (2.8 vs 1.7; 65% difference; P = .009) in comparison with RSA patients. However, no significant differences were observed for other work activities, including retirement, desk job, prolonged standing, creative jobs, lifting jobs, or construction (Table 4). Among the work subgroups, RSA patients were older than TSA patients for the retired group (77 years vs 72 years; P < .001) and gardening (81 years vs 68 years; P = .002).
DISCUSSION
The ability to participate in sports and work is a common goal for shoulder arthroplasty patients. However, the ability at which participation occurs has not been examined. This study illustrates not only the ability to engage in usual work or sport, but provides some insights into patient-reported quality of participation. Overall, TSA patients featured 27% higher sport-specific ASES scores and 21% higher work-specific ASES scores than RSA patients, confirming our hypothesis that TSA patients can participate in work or sports with less difficulty in general. This study is the first to stratify the difficulty of participating in sports in general and in specific sports identified by patients. Although statistical analysis was performed for individual sports and work reported, the use of small cohorts possibly affected the ability to detect significant differences. The data presented in this study can thus be used as descriptive evidence of what a patient may expect to be able to do following surgery, helping to define patient expectations prior to electing to undergo shoulder arthroplasty.
Among specific sports identified by patients, a few significant differences were observed between RSA and TSA patients. However, ASES-specific scores almost universally favored TSA. Of the sport subgroups, swimming and golf showed significant differences. For swimming, this difference was fairly significant, as TSA patients demonstrated a 49% higher score than their RSA counterparts, but without differences in age or total ASES score (Table 5). Alteration in shoulder mechanics after RSA may be used to explain the difficulty in returning to swimming, as additional time may be needed to adapt to new mechanics.24 McCarty and colleagues8 demonstrated that 90% of patients following TSA fully resumed participation in swimming within 6 months of surgery, and further stated that repetitive motions of swimming caused no effects on short-term outcomes. No similar analysis of swimming has been reported for RSA patients. Based upon our findings, the average RSA patient can experience some difficulties when returning to swimming after surgery (average specific ASES score, 1.8).
Jensen and Rockwood16 were among the first to demonstrate successful return to golf of 24 patients who had undergone either TSA or hemiarthroplasty (HA), showing a 5-stroke improvement in their game. A recent study investigating patient-reported activity in patients aged 75 years and undergoing RSA showed that 23% of patients returned to high-level activity sports, such as golf, motorcycle riding, or free weights.19 All patients who participated in golf before surgery resumed playing following surgery; however, golf was listed among the top activities that patients wanted to participate in but could not for any reason.19 Our data suggest that golfers with TSA will face less difficulty returning to sports compared with their RSA counterparts (average specific ASES score, 2.5 vs 1.8, who might find golf somewhat difficult.
Although no study has provided a clear consensus as to which activities are safe to perform following shoulder arthroplasty, experts have suggested that activities that impart high loads on the glenohumeral joint should be avoided.15 Among TSA patients, McCarty and colleagues8 reported high rates of return for swimmers, golfers, and tennis players; however, relatively low rates were reported for weight lifting, bowling, and softball (20%). Within our study group, golf, swimming, and walking were listed among the most popular sports performed. Although weight lifting, bowling, and softball were less commonly identified as usual sports within our study, patients treated with TSA demonstrated more ease to participate than RSA patients. This result was observed with ASES-specific scores noted for weight lifting and gym exercises (TSA, 2.5; RSA, 2.3) and team sports, such as softball (TSA, 2; RSA, 1.3). However, for bowling, RSA patients showed a trend toward more ability (RSA, 2.7; TSA, 1.7).
Continue to: Among specific work activities...
Successful return to sports that involve shoulder function, such as golf and swimming, has been demonstrated for TSA.8,14,16,17 However, studies have reported that return to these sports can be difficult for RSA patients.20 Fink and colleagues19 reported that following RSA, 48.7% of patients returned to moderate-intensity sports, such as swimming and golf. Consistent with these findings, in our study, TSA patients demonstrated a significantly higher ability to participate in their usual sports without difficulty (ASES-specific score of 3). This observation may relate to lower ultimate achievements in range of motion and strength in patients treated with RSA, when compared with TSA patients,24,25 and the generalized practice of utilizing RSA for lower-demand patients (RSA patients in this study were older).
Overall, participation in work was 21% easier for TSA patients than RSA patients. Although the majority of our patients cited retirement as their primary work, which is consistent with what one would expect with the mean age of this study’s cohorts (RSA, 75 years; TSA, 69 years), housework and gardening were the only specifically identified forms of work that demonstrated significant differences between RSA and TSA patients. A few reports in the literature documented the ability to return to work after shoulder arthroplasty. In a recent report on 13 workers’ compensation patients treated with TSA, only 1 patient returned to the same job, and 54% did not return to work.26 In a study comparing 14 workers’ compensation to a matched group of controls with all members treated with RSA, the workers’ compensation group yielded a lower return-to-work rate (14.2%) than the controls (41.7%).27 In a large study of 154 TSA patients, 14% returned to work, but specific jobs were not described in this analysis.14
The results of this study suggest that more TSA patients successfully participate in low-demand activities, such as gardening or housework. Zarkadas and colleagues18 reported that 65% of TSA and 47% of HA patients successfully returned to gardening compared with 42% of RSA patients observed in a continuation study.20 This study showed that TSA patients yielded a 65% difference in ability to work in gardening and 34% difference in ability to perform housework compared with RSA patients. Based on these findings, TSA patients can expect to experience no difficulty in performing housework or gardening, whereas RSA patients may find these tasks difficult to a certain degree.
The main limitation of this study is the reporting bias that results from survey-based studies. Possibly, more people engage in specific sports or work than what were reported. This type of study also features an inherent selection bias, as patients with highly and physically demanding jobs or usual sports were less likely to have been offered either TSA or RSA. An additional important limitation is the relatively small cohorts within sport and work subgroups; the small cohorts probably underpowered the statistical results of this study and made these findings valuable mostly as descriptive observations. Larger studies focusing on each subgroup will further clarify the ability of shoulder arthroplasty to perform individual sports or work. Further studies evaluating preoperative to postoperative sports- and work-specific ASES scores would provide notable insights into the functional improvements observed within each sport or work following surgery. The relatively large study population of 276 patients strengthened the findings, which relate to the overall ability to participate in sports and work for TSA and RSA patients. Finally, the evaluated TSA and RSA patients possibly represent different groups (significant difference in age and gender) with different underlying pathologies and potentially different demands and expectations. However, comparisons among these groups of patients bear importance in defining patient expectations related to surgery. Still, the ability to participate in sport or work possibly relates more to the limitations of the implant used than patient pathology. This possibility warrants further investigation.
CONCLUSION
Both TSA and RSA allow for participation in work and sports, with TSA patients reporting easier overall ability to participate. For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
1. Fehringer EV, Kopjar B, Boorman RS, Churchill RS, Smith KL, Matsen FA 3rd. Characterizing the functional improvement after total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Am. 2002;84-A(8):1349-1353.
2. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055. doi:10.2106/JBJS.L.01637.
3. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
4. Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189-195.
5. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1478-1483. doi:10.1016/j.jse.2011.11.004.
6. Sebastia-Forcada E, Cebrian-Gomez R, Lizaur-Utrilla A, Gil-Guillen V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419-1426. doi:10.1016/j.jse.2014.06.035.
7. Henn RF 3rd, Ghomrawi H, Rutledge JR, Mazumdar M, Mancuso CA, Marx RG. Preoperative patient expectations of total shoulder arthroplasty. J Bone Joint Surg Am. 2011;93(22):2110-2115. doi:10.2106/JBJS.J.01114.
8. McCarty EC, Marx RG, Maerz D, Altchek D, Warren RF. Sports participation after shoulder replacement surgery. Am J Sports Med. 2008;36(8):1577-1581. doi:10.1177/0363546508317126.
9. Puskas B, Harreld K, Clark R, Downes K, Virani NA, Frankle M. Isometric strength, range of motion, and impairment before and after total and reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(7):869-876. doi:10.1016/j.jse.2012.09.004.
10. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479.
11. Levy JC, Everding NG, Gil CC Jr., Stephens S, Giveans MR. Speed of recovery after shoulder arthroplasty: a comparison of reverse and anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(12):1872-1881. doi:10.1016/j.jse.2014.04.014.
12. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482. doi:10.1007/s11999-010-1683-z.
13. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
14. Bulhoff M, Sattler P, Bruckner T, Loew M, Zeifang F, Raiss P. Do patients return to sports and work after total shoulder replacement surgery? Am J Sports Med. 2015;43(2):423-427. doi:10.1177/0363546514557940.
15. Healy WL, Iorio R, Lemos MJ. Athletic activity after joint replacement. Am J Sports Med. 2001;29(3):377-388.
16. Jensen KL, Rockwood CA Jr. Shoulder arthroplasty in recreational golfers. J Shoulder Elbow Surg. 1998;7(4):362-367.
17. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105. doi:10.1177/0363546510371368.
18. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280. doi:10.1016/j.jse.2010.06.007.
19. Fink Barnes LA, Grantham WJ, Meadows MC, Bigliani LU, Levine WN, Ahmad CS. Sports activity after reverse total shoulder arthroplasty with minimum 2-year follow-up. Am J Orthop. 2015;44(2):68-72.
20. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469. doi:10.1016/j.jse.2011.11.012.
21. Simovitch RW, Gerard BK, Brees JA, Fullick R, Kearse JC. Outcomes of reverse total shoulder arthroplasty in a senior athletic population. J Shoulder Elbow Surg. 2015;24(9):1481-1485. doi:10.1016/j.jse.2015.03.011.
22. Golant A, Christoforou D, Zuckerman JD, Kwon YW. Return to sports after shoulder arthroplasty: a survey of surgeons' preferences. J Shoulder Elbow Surg. 2012;21(4):554-560. doi:10.1016/j.jse.2010.11.021.
23. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
24. Alta TD, de Toledo JM, Veeger HE, Janssen TW, Willems WJ. The active and passive kinematic difference between primary reverse and total shoulder prostheses. J Shoulder Elbow Surg. 2014;23(9):1395-1402. doi:10.1016/j.jse.2014.01.040.
25. Alta TD, Veeger DH, de Toledo JM, Janssen TW, Willems WJ. Isokinetic strength differences between patients with primary reverse and total shoulder prostheses: muscle strength quantified with a dynamometer. Clin Biomech (Bristol, Avon). 2014;29(9):965-970. doi:10.1016/j.clinbiomech.2014.08.018.
26. Jawa A, Dasti UR, Fasulo SM, Vaickus MH, Curtis AS, Miller SL. Anatomic total shoulder arthroplasty for patients receiving workers' compensation. J Shoulder Elbow Surg. 2015;24(11):1694-1697. doi:10.1016/j.jse.2015.04.017.
27. Morris BJ, Haigler RE, Laughlin MS, Elkousy HA, Gartsman GM, Edwards TB. Workers' compensation claims and outcomes after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(3):453-459. doi:10.1016/j.jse.2014.07.009.
ABSTRACT
Both anatomical total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA) are routinely performed for patients who desire to continuously work or participate in sports. This study analyzes and compares the ability of patients to work and partake in sports following shoulder arthroplasty based on responses to clinical outcome surveys.
A retrospective review of the shoulder surgery repository was performed for all patients treated with TSA and RSA and who completed questions 9 and 10 on the activity patient self-evaluation portion of the American Shoulder and Elbow Surgeons (ASES) Assessment Form. Patients with a minimum of 1-year follow-up were included if a sport or work was identified. The analysis included 162 patients with TSA and 114 patients with RSA. Comparisons were made between TSA and RSA in terms of the specific ASES scores (rated 0-3) reported for ability to work and participate in sports and total ASES scores, and scores based on specific sports or line of work reported. Comparisons were also made between sports predominantly using shoulder function and those that do not.
TSA patients had a 27% higher ability to participate in sports (average specific ASES score: 2.5 vs 1.9, P < .001) than RSA patients and presented significantly higher scores for swimming and golf. Compared with RSA patients, TSA patients demonstrated more ability to participate in sports requiring shoulder function without difficulty, as 63% reported maximal scores (P = .003). Total shoulder arthroplasty patients also demonstrated a 21% higher ability to work than RSA patients (average specific ASES scores: 2.6 vs 2.1, P < .001), yielding significantly higher scores for housework and gardening.
Both TSA and RSA allow for participation in work and sports, with TSA patients reporting better overall ability to participate. For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
End-stage shoulder arthritis has been successfully treated with anatomical total shoulder arthroplasty (TSA) with high rates of functional recovery.1 With the introduction of reverse shoulder arthroplasty (RSA), indications for TSA have expanded.2-6 With continuing expansion of surgical indications, a more diverse and potentially active patient population is now being treated. As patients exhibit increased awareness of health and wellness, they demonstrate significant interest in understanding their ability to work or participate in sports after surgery.7 Patients no longer focus on pain relief as the only goal of surgery. A recent study of patients aged 65 years and undergoing shoulder arthroplasty revealed that 64% of the patients listed the ability to return to sports as the main reason for undergoing surgery,8 highlighting the significance of sports play in a patient’s life. Prior to surgery, shoulder pathologies lead to impairment in function, range of motion, and pain,9 hindering a patient to participate in both work and sports. With the intervention yielding improvement to these areas6,9-13 with increased patient satisfaction,10,13 accurately tailoring patient expectations for participation in sports and work postoperatively becomes increasingly important.
Continue to: Although several studies...
Although several studies have demonstrated the ability of patients to return to sports following TSA,8,14-18 a limited number of studies discuss the return to sports following RSA.19-21 Despite known postoperative improvements, no clear consensus is reached as to which specific sports patients can return to and at what level of participation is to be expected. Surveyed members of the American Shoulder and Elbow Surgeons (ASES) universally favored full return to sports, except for contact sports for TSA patients, whereas other surgeons are more conservative to allow RSA patients to return to activities.22 To our knowledge, no other study has investigated the ability to work following RSA. Furthermore, no other study has used patient-reported outcomes to compare the quality of participation in sports or work between TSA and RSA patients following surgery. This study reports the ability of patients treated with TSA and RSA to work and participate in sports based on clinical outcome surveys. We hypothesize that TSA patients will be allowed to work and participate in sports with less difficulty than RSA patients.
MATERIALS AND METHODS
Following Institutional Review Board approval, a retrospective review was performed on all patients treated with TSA or RSA and who completed questions 9 and/or 10 (by score and named usual sport and/or work) on the activity patient self-evaluation portion of the ASES23 Assessment Form between 2007 to 2014; queries were made via the Shoulder Outcomes Repository. A minimum of 12-month follow-up was required, as functional recovery has been shown to plateau or nearly plateau by 12 months.11 Patients were excluded if <12 months of follow-up was available, if they failed to provide a written answer for questions 9 or 10 on the activity patient self-evaluation portion of the ASES Assessment Form, or if they required a revision shoulder arthroplasty. A single fellowship-trained shoulder and elbow surgeon performed all procedures via the same deltopectoral approach and prescribed identical postoperative rehabilitation for both TSA and RSA patients. The database query yielded 162 TSA and 114 RSA patients, for a total of 276 patients eligible for the study.
For all patients, the most recent follow-up ASES score was used. Comparisons were made between TSA and RSA for total ASES scores and response groups for usual sport (ASES question 9) and usual work (ASES question 10). The ASES questionnaire provides patients with 4 choices for each question based on the ability to perform each activity: 0, unable to do; 1, very difficult; 2, somewhat difficult; and 3, not difficult. The questionnaire also allows the patients to identify their usual work and sports. If patients noted >1 sport or work activity, they were included within multiple subgroups. Patients were further compared by age and gender.
Work was subdivided to include retired, housework, desk jobs, prolonged standing, gardening/yard work, jobs requiring lifting, carpenter/construction, cook/food preparation, and creative jobs (Table 1).
Statistical analysis was performed with SPSS Version 21 (IBM). Unpaired t tests were used to determine differences between groups. A P-value of <.05 was deemed significant.
Continue to: A total of 276 patients...
RESULTS
A total of 276 patients that met the inclusion criteria were eligible for the study, with 162 having undergone TSA and 114 with RSA. Overall average follow-up totaled 29 months (range, 12-91 months). RSA patients (average age, 75 years old; range, 46-88 years) were significantly older than TSA patients (average age, 69 years old; range, 32-89 years; P = .001). Significantly more women were treated with TSA (52% TSA; 48% RSA; P = .012), whereas significantly more men were treated with TSA (67% TSA; 33% RSA, P = .012). Total ASES scores were significantly higher for TSA patients than RSA patients in work (P = .012) (Table 4) but not in sports (P = .063) (Table 5) categories.
SPORTS
A total of 186 patients, comprising of 71 RSA and 115 TSA individuals, responded to question 9 of the ASES questionnaire (Table 5). Among usually reported sports, golf (25%), swimming (17%), and walking (18%) were the most commonly cited. RSA patients indicating a sport were significantly older than TSA patients (74 years vs 69 years, P < .001). TSA patients reported a 27% higher difference in overall ability to participate in sports, with an average ASES sport-specific score of 2.5 compared with the 1.9 for RSA patients (P < .001).
Among specific sports, TSA patients reported significantly higher scores for swimming (2.6 vs 1.8, P = .007) and golf (2.5 vs 1.8, P = .050). However, no significant differences were observed for walking, gym exercises, and racquet sports (Table 5). Among sport subsets, RSA patients were significantly older for golf (77 years vs 70 years, P = .006) and bowling (80 years vs 68 years, P = .005). Five TSA patients reported biking as their sport, whereas no RSA patient reported such activity. Within each subset of sports, no significant differences were noted in average ASES total scores.
TSA patients demonstrated a more significant ability to perform usual sports that involve shoulder function without difficulty (score of 3). In shoulder dominant sports, a total of 63% of TSA patients reported a score of 3 compared with the 39% of RSA patients (P = .003). RSA patients more often reported an inability to perform shoulder specific sports, as proven by 20% of RSA patients reporting a score of 0 compared with 4% of TSA patients (P < .001) (Table 6).
WORK
A total of 265 patients, including 106 RSA and 159 TSA patients, responded to question 10 of the ASES questionnaire. Among usually reported work, retirement (43%), housework (27%), and desk jobs (18%) were the most commonly cited. RSA patients denoting a work were significantly older than TSA patients (75 years vs 69 years, P < .001). Patients with TSA presented a 21% higher difference in the overall ability to work, featuring an average ASES work-specific score of 2.6 compared with the 2.1 for RSA patients (P < .001) (Table 4).
Continue to: Among specific work activities...
Among specific work activities, TSA patients reported significantly higher scores for housework (2.7 vs 2; 34% difference; P = .001) and gardening (2.8 vs 1.7; 65% difference; P = .009) in comparison with RSA patients. However, no significant differences were observed for other work activities, including retirement, desk job, prolonged standing, creative jobs, lifting jobs, or construction (Table 4). Among the work subgroups, RSA patients were older than TSA patients for the retired group (77 years vs 72 years; P < .001) and gardening (81 years vs 68 years; P = .002).
DISCUSSION
The ability to participate in sports and work is a common goal for shoulder arthroplasty patients. However, the ability at which participation occurs has not been examined. This study illustrates not only the ability to engage in usual work or sport, but provides some insights into patient-reported quality of participation. Overall, TSA patients featured 27% higher sport-specific ASES scores and 21% higher work-specific ASES scores than RSA patients, confirming our hypothesis that TSA patients can participate in work or sports with less difficulty in general. This study is the first to stratify the difficulty of participating in sports in general and in specific sports identified by patients. Although statistical analysis was performed for individual sports and work reported, the use of small cohorts possibly affected the ability to detect significant differences. The data presented in this study can thus be used as descriptive evidence of what a patient may expect to be able to do following surgery, helping to define patient expectations prior to electing to undergo shoulder arthroplasty.
Among specific sports identified by patients, a few significant differences were observed between RSA and TSA patients. However, ASES-specific scores almost universally favored TSA. Of the sport subgroups, swimming and golf showed significant differences. For swimming, this difference was fairly significant, as TSA patients demonstrated a 49% higher score than their RSA counterparts, but without differences in age or total ASES score (Table 5). Alteration in shoulder mechanics after RSA may be used to explain the difficulty in returning to swimming, as additional time may be needed to adapt to new mechanics.24 McCarty and colleagues8 demonstrated that 90% of patients following TSA fully resumed participation in swimming within 6 months of surgery, and further stated that repetitive motions of swimming caused no effects on short-term outcomes. No similar analysis of swimming has been reported for RSA patients. Based upon our findings, the average RSA patient can experience some difficulties when returning to swimming after surgery (average specific ASES score, 1.8).
Jensen and Rockwood16 were among the first to demonstrate successful return to golf of 24 patients who had undergone either TSA or hemiarthroplasty (HA), showing a 5-stroke improvement in their game. A recent study investigating patient-reported activity in patients aged 75 years and undergoing RSA showed that 23% of patients returned to high-level activity sports, such as golf, motorcycle riding, or free weights.19 All patients who participated in golf before surgery resumed playing following surgery; however, golf was listed among the top activities that patients wanted to participate in but could not for any reason.19 Our data suggest that golfers with TSA will face less difficulty returning to sports compared with their RSA counterparts (average specific ASES score, 2.5 vs 1.8, who might find golf somewhat difficult.
Although no study has provided a clear consensus as to which activities are safe to perform following shoulder arthroplasty, experts have suggested that activities that impart high loads on the glenohumeral joint should be avoided.15 Among TSA patients, McCarty and colleagues8 reported high rates of return for swimmers, golfers, and tennis players; however, relatively low rates were reported for weight lifting, bowling, and softball (20%). Within our study group, golf, swimming, and walking were listed among the most popular sports performed. Although weight lifting, bowling, and softball were less commonly identified as usual sports within our study, patients treated with TSA demonstrated more ease to participate than RSA patients. This result was observed with ASES-specific scores noted for weight lifting and gym exercises (TSA, 2.5; RSA, 2.3) and team sports, such as softball (TSA, 2; RSA, 1.3). However, for bowling, RSA patients showed a trend toward more ability (RSA, 2.7; TSA, 1.7).
Continue to: Among specific work activities...
Successful return to sports that involve shoulder function, such as golf and swimming, has been demonstrated for TSA.8,14,16,17 However, studies have reported that return to these sports can be difficult for RSA patients.20 Fink and colleagues19 reported that following RSA, 48.7% of patients returned to moderate-intensity sports, such as swimming and golf. Consistent with these findings, in our study, TSA patients demonstrated a significantly higher ability to participate in their usual sports without difficulty (ASES-specific score of 3). This observation may relate to lower ultimate achievements in range of motion and strength in patients treated with RSA, when compared with TSA patients,24,25 and the generalized practice of utilizing RSA for lower-demand patients (RSA patients in this study were older).
Overall, participation in work was 21% easier for TSA patients than RSA patients. Although the majority of our patients cited retirement as their primary work, which is consistent with what one would expect with the mean age of this study’s cohorts (RSA, 75 years; TSA, 69 years), housework and gardening were the only specifically identified forms of work that demonstrated significant differences between RSA and TSA patients. A few reports in the literature documented the ability to return to work after shoulder arthroplasty. In a recent report on 13 workers’ compensation patients treated with TSA, only 1 patient returned to the same job, and 54% did not return to work.26 In a study comparing 14 workers’ compensation to a matched group of controls with all members treated with RSA, the workers’ compensation group yielded a lower return-to-work rate (14.2%) than the controls (41.7%).27 In a large study of 154 TSA patients, 14% returned to work, but specific jobs were not described in this analysis.14
The results of this study suggest that more TSA patients successfully participate in low-demand activities, such as gardening or housework. Zarkadas and colleagues18 reported that 65% of TSA and 47% of HA patients successfully returned to gardening compared with 42% of RSA patients observed in a continuation study.20 This study showed that TSA patients yielded a 65% difference in ability to work in gardening and 34% difference in ability to perform housework compared with RSA patients. Based on these findings, TSA patients can expect to experience no difficulty in performing housework or gardening, whereas RSA patients may find these tasks difficult to a certain degree.
The main limitation of this study is the reporting bias that results from survey-based studies. Possibly, more people engage in specific sports or work than what were reported. This type of study also features an inherent selection bias, as patients with highly and physically demanding jobs or usual sports were less likely to have been offered either TSA or RSA. An additional important limitation is the relatively small cohorts within sport and work subgroups; the small cohorts probably underpowered the statistical results of this study and made these findings valuable mostly as descriptive observations. Larger studies focusing on each subgroup will further clarify the ability of shoulder arthroplasty to perform individual sports or work. Further studies evaluating preoperative to postoperative sports- and work-specific ASES scores would provide notable insights into the functional improvements observed within each sport or work following surgery. The relatively large study population of 276 patients strengthened the findings, which relate to the overall ability to participate in sports and work for TSA and RSA patients. Finally, the evaluated TSA and RSA patients possibly represent different groups (significant difference in age and gender) with different underlying pathologies and potentially different demands and expectations. However, comparisons among these groups of patients bear importance in defining patient expectations related to surgery. Still, the ability to participate in sport or work possibly relates more to the limitations of the implant used than patient pathology. This possibility warrants further investigation.
CONCLUSION
Both TSA and RSA allow for participation in work and sports, with TSA patients reporting easier overall ability to participate. For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
ABSTRACT
Both anatomical total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA) are routinely performed for patients who desire to continuously work or participate in sports. This study analyzes and compares the ability of patients to work and partake in sports following shoulder arthroplasty based on responses to clinical outcome surveys.
A retrospective review of the shoulder surgery repository was performed for all patients treated with TSA and RSA and who completed questions 9 and 10 on the activity patient self-evaluation portion of the American Shoulder and Elbow Surgeons (ASES) Assessment Form. Patients with a minimum of 1-year follow-up were included if a sport or work was identified. The analysis included 162 patients with TSA and 114 patients with RSA. Comparisons were made between TSA and RSA in terms of the specific ASES scores (rated 0-3) reported for ability to work and participate in sports and total ASES scores, and scores based on specific sports or line of work reported. Comparisons were also made between sports predominantly using shoulder function and those that do not.
TSA patients had a 27% higher ability to participate in sports (average specific ASES score: 2.5 vs 1.9, P < .001) than RSA patients and presented significantly higher scores for swimming and golf. Compared with RSA patients, TSA patients demonstrated more ability to participate in sports requiring shoulder function without difficulty, as 63% reported maximal scores (P = .003). Total shoulder arthroplasty patients also demonstrated a 21% higher ability to work than RSA patients (average specific ASES scores: 2.6 vs 2.1, P < .001), yielding significantly higher scores for housework and gardening.
Both TSA and RSA allow for participation in work and sports, with TSA patients reporting better overall ability to participate. For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
End-stage shoulder arthritis has been successfully treated with anatomical total shoulder arthroplasty (TSA) with high rates of functional recovery.1 With the introduction of reverse shoulder arthroplasty (RSA), indications for TSA have expanded.2-6 With continuing expansion of surgical indications, a more diverse and potentially active patient population is now being treated. As patients exhibit increased awareness of health and wellness, they demonstrate significant interest in understanding their ability to work or participate in sports after surgery.7 Patients no longer focus on pain relief as the only goal of surgery. A recent study of patients aged 65 years and undergoing shoulder arthroplasty revealed that 64% of the patients listed the ability to return to sports as the main reason for undergoing surgery,8 highlighting the significance of sports play in a patient’s life. Prior to surgery, shoulder pathologies lead to impairment in function, range of motion, and pain,9 hindering a patient to participate in both work and sports. With the intervention yielding improvement to these areas6,9-13 with increased patient satisfaction,10,13 accurately tailoring patient expectations for participation in sports and work postoperatively becomes increasingly important.
Continue to: Although several studies...
Although several studies have demonstrated the ability of patients to return to sports following TSA,8,14-18 a limited number of studies discuss the return to sports following RSA.19-21 Despite known postoperative improvements, no clear consensus is reached as to which specific sports patients can return to and at what level of participation is to be expected. Surveyed members of the American Shoulder and Elbow Surgeons (ASES) universally favored full return to sports, except for contact sports for TSA patients, whereas other surgeons are more conservative to allow RSA patients to return to activities.22 To our knowledge, no other study has investigated the ability to work following RSA. Furthermore, no other study has used patient-reported outcomes to compare the quality of participation in sports or work between TSA and RSA patients following surgery. This study reports the ability of patients treated with TSA and RSA to work and participate in sports based on clinical outcome surveys. We hypothesize that TSA patients will be allowed to work and participate in sports with less difficulty than RSA patients.
MATERIALS AND METHODS
Following Institutional Review Board approval, a retrospective review was performed on all patients treated with TSA or RSA and who completed questions 9 and/or 10 (by score and named usual sport and/or work) on the activity patient self-evaluation portion of the ASES23 Assessment Form between 2007 to 2014; queries were made via the Shoulder Outcomes Repository. A minimum of 12-month follow-up was required, as functional recovery has been shown to plateau or nearly plateau by 12 months.11 Patients were excluded if <12 months of follow-up was available, if they failed to provide a written answer for questions 9 or 10 on the activity patient self-evaluation portion of the ASES Assessment Form, or if they required a revision shoulder arthroplasty. A single fellowship-trained shoulder and elbow surgeon performed all procedures via the same deltopectoral approach and prescribed identical postoperative rehabilitation for both TSA and RSA patients. The database query yielded 162 TSA and 114 RSA patients, for a total of 276 patients eligible for the study.
For all patients, the most recent follow-up ASES score was used. Comparisons were made between TSA and RSA for total ASES scores and response groups for usual sport (ASES question 9) and usual work (ASES question 10). The ASES questionnaire provides patients with 4 choices for each question based on the ability to perform each activity: 0, unable to do; 1, very difficult; 2, somewhat difficult; and 3, not difficult. The questionnaire also allows the patients to identify their usual work and sports. If patients noted >1 sport or work activity, they were included within multiple subgroups. Patients were further compared by age and gender.
Work was subdivided to include retired, housework, desk jobs, prolonged standing, gardening/yard work, jobs requiring lifting, carpenter/construction, cook/food preparation, and creative jobs (Table 1).
Statistical analysis was performed with SPSS Version 21 (IBM). Unpaired t tests were used to determine differences between groups. A P-value of <.05 was deemed significant.
Continue to: A total of 276 patients...
RESULTS
A total of 276 patients that met the inclusion criteria were eligible for the study, with 162 having undergone TSA and 114 with RSA. Overall average follow-up totaled 29 months (range, 12-91 months). RSA patients (average age, 75 years old; range, 46-88 years) were significantly older than TSA patients (average age, 69 years old; range, 32-89 years; P = .001). Significantly more women were treated with TSA (52% TSA; 48% RSA; P = .012), whereas significantly more men were treated with TSA (67% TSA; 33% RSA, P = .012). Total ASES scores were significantly higher for TSA patients than RSA patients in work (P = .012) (Table 4) but not in sports (P = .063) (Table 5) categories.
SPORTS
A total of 186 patients, comprising of 71 RSA and 115 TSA individuals, responded to question 9 of the ASES questionnaire (Table 5). Among usually reported sports, golf (25%), swimming (17%), and walking (18%) were the most commonly cited. RSA patients indicating a sport were significantly older than TSA patients (74 years vs 69 years, P < .001). TSA patients reported a 27% higher difference in overall ability to participate in sports, with an average ASES sport-specific score of 2.5 compared with the 1.9 for RSA patients (P < .001).
Among specific sports, TSA patients reported significantly higher scores for swimming (2.6 vs 1.8, P = .007) and golf (2.5 vs 1.8, P = .050). However, no significant differences were observed for walking, gym exercises, and racquet sports (Table 5). Among sport subsets, RSA patients were significantly older for golf (77 years vs 70 years, P = .006) and bowling (80 years vs 68 years, P = .005). Five TSA patients reported biking as their sport, whereas no RSA patient reported such activity. Within each subset of sports, no significant differences were noted in average ASES total scores.
TSA patients demonstrated a more significant ability to perform usual sports that involve shoulder function without difficulty (score of 3). In shoulder dominant sports, a total of 63% of TSA patients reported a score of 3 compared with the 39% of RSA patients (P = .003). RSA patients more often reported an inability to perform shoulder specific sports, as proven by 20% of RSA patients reporting a score of 0 compared with 4% of TSA patients (P < .001) (Table 6).
WORK
A total of 265 patients, including 106 RSA and 159 TSA patients, responded to question 10 of the ASES questionnaire. Among usually reported work, retirement (43%), housework (27%), and desk jobs (18%) were the most commonly cited. RSA patients denoting a work were significantly older than TSA patients (75 years vs 69 years, P < .001). Patients with TSA presented a 21% higher difference in the overall ability to work, featuring an average ASES work-specific score of 2.6 compared with the 2.1 for RSA patients (P < .001) (Table 4).
Continue to: Among specific work activities...
Among specific work activities, TSA patients reported significantly higher scores for housework (2.7 vs 2; 34% difference; P = .001) and gardening (2.8 vs 1.7; 65% difference; P = .009) in comparison with RSA patients. However, no significant differences were observed for other work activities, including retirement, desk job, prolonged standing, creative jobs, lifting jobs, or construction (Table 4). Among the work subgroups, RSA patients were older than TSA patients for the retired group (77 years vs 72 years; P < .001) and gardening (81 years vs 68 years; P = .002).
DISCUSSION
The ability to participate in sports and work is a common goal for shoulder arthroplasty patients. However, the ability at which participation occurs has not been examined. This study illustrates not only the ability to engage in usual work or sport, but provides some insights into patient-reported quality of participation. Overall, TSA patients featured 27% higher sport-specific ASES scores and 21% higher work-specific ASES scores than RSA patients, confirming our hypothesis that TSA patients can participate in work or sports with less difficulty in general. This study is the first to stratify the difficulty of participating in sports in general and in specific sports identified by patients. Although statistical analysis was performed for individual sports and work reported, the use of small cohorts possibly affected the ability to detect significant differences. The data presented in this study can thus be used as descriptive evidence of what a patient may expect to be able to do following surgery, helping to define patient expectations prior to electing to undergo shoulder arthroplasty.
Among specific sports identified by patients, a few significant differences were observed between RSA and TSA patients. However, ASES-specific scores almost universally favored TSA. Of the sport subgroups, swimming and golf showed significant differences. For swimming, this difference was fairly significant, as TSA patients demonstrated a 49% higher score than their RSA counterparts, but without differences in age or total ASES score (Table 5). Alteration in shoulder mechanics after RSA may be used to explain the difficulty in returning to swimming, as additional time may be needed to adapt to new mechanics.24 McCarty and colleagues8 demonstrated that 90% of patients following TSA fully resumed participation in swimming within 6 months of surgery, and further stated that repetitive motions of swimming caused no effects on short-term outcomes. No similar analysis of swimming has been reported for RSA patients. Based upon our findings, the average RSA patient can experience some difficulties when returning to swimming after surgery (average specific ASES score, 1.8).
Jensen and Rockwood16 were among the first to demonstrate successful return to golf of 24 patients who had undergone either TSA or hemiarthroplasty (HA), showing a 5-stroke improvement in their game. A recent study investigating patient-reported activity in patients aged 75 years and undergoing RSA showed that 23% of patients returned to high-level activity sports, such as golf, motorcycle riding, or free weights.19 All patients who participated in golf before surgery resumed playing following surgery; however, golf was listed among the top activities that patients wanted to participate in but could not for any reason.19 Our data suggest that golfers with TSA will face less difficulty returning to sports compared with their RSA counterparts (average specific ASES score, 2.5 vs 1.8, who might find golf somewhat difficult.
Although no study has provided a clear consensus as to which activities are safe to perform following shoulder arthroplasty, experts have suggested that activities that impart high loads on the glenohumeral joint should be avoided.15 Among TSA patients, McCarty and colleagues8 reported high rates of return for swimmers, golfers, and tennis players; however, relatively low rates were reported for weight lifting, bowling, and softball (20%). Within our study group, golf, swimming, and walking were listed among the most popular sports performed. Although weight lifting, bowling, and softball were less commonly identified as usual sports within our study, patients treated with TSA demonstrated more ease to participate than RSA patients. This result was observed with ASES-specific scores noted for weight lifting and gym exercises (TSA, 2.5; RSA, 2.3) and team sports, such as softball (TSA, 2; RSA, 1.3). However, for bowling, RSA patients showed a trend toward more ability (RSA, 2.7; TSA, 1.7).
Continue to: Among specific work activities...
Successful return to sports that involve shoulder function, such as golf and swimming, has been demonstrated for TSA.8,14,16,17 However, studies have reported that return to these sports can be difficult for RSA patients.20 Fink and colleagues19 reported that following RSA, 48.7% of patients returned to moderate-intensity sports, such as swimming and golf. Consistent with these findings, in our study, TSA patients demonstrated a significantly higher ability to participate in their usual sports without difficulty (ASES-specific score of 3). This observation may relate to lower ultimate achievements in range of motion and strength in patients treated with RSA, when compared with TSA patients,24,25 and the generalized practice of utilizing RSA for lower-demand patients (RSA patients in this study were older).
Overall, participation in work was 21% easier for TSA patients than RSA patients. Although the majority of our patients cited retirement as their primary work, which is consistent with what one would expect with the mean age of this study’s cohorts (RSA, 75 years; TSA, 69 years), housework and gardening were the only specifically identified forms of work that demonstrated significant differences between RSA and TSA patients. A few reports in the literature documented the ability to return to work after shoulder arthroplasty. In a recent report on 13 workers’ compensation patients treated with TSA, only 1 patient returned to the same job, and 54% did not return to work.26 In a study comparing 14 workers’ compensation to a matched group of controls with all members treated with RSA, the workers’ compensation group yielded a lower return-to-work rate (14.2%) than the controls (41.7%).27 In a large study of 154 TSA patients, 14% returned to work, but specific jobs were not described in this analysis.14
The results of this study suggest that more TSA patients successfully participate in low-demand activities, such as gardening or housework. Zarkadas and colleagues18 reported that 65% of TSA and 47% of HA patients successfully returned to gardening compared with 42% of RSA patients observed in a continuation study.20 This study showed that TSA patients yielded a 65% difference in ability to work in gardening and 34% difference in ability to perform housework compared with RSA patients. Based on these findings, TSA patients can expect to experience no difficulty in performing housework or gardening, whereas RSA patients may find these tasks difficult to a certain degree.
The main limitation of this study is the reporting bias that results from survey-based studies. Possibly, more people engage in specific sports or work than what were reported. This type of study also features an inherent selection bias, as patients with highly and physically demanding jobs or usual sports were less likely to have been offered either TSA or RSA. An additional important limitation is the relatively small cohorts within sport and work subgroups; the small cohorts probably underpowered the statistical results of this study and made these findings valuable mostly as descriptive observations. Larger studies focusing on each subgroup will further clarify the ability of shoulder arthroplasty to perform individual sports or work. Further studies evaluating preoperative to postoperative sports- and work-specific ASES scores would provide notable insights into the functional improvements observed within each sport or work following surgery. The relatively large study population of 276 patients strengthened the findings, which relate to the overall ability to participate in sports and work for TSA and RSA patients. Finally, the evaluated TSA and RSA patients possibly represent different groups (significant difference in age and gender) with different underlying pathologies and potentially different demands and expectations. However, comparisons among these groups of patients bear importance in defining patient expectations related to surgery. Still, the ability to participate in sport or work possibly relates more to the limitations of the implant used than patient pathology. This possibility warrants further investigation.
CONCLUSION
Both TSA and RSA allow for participation in work and sports, with TSA patients reporting easier overall ability to participate. For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
1. Fehringer EV, Kopjar B, Boorman RS, Churchill RS, Smith KL, Matsen FA 3rd. Characterizing the functional improvement after total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Am. 2002;84-A(8):1349-1353.
2. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055. doi:10.2106/JBJS.L.01637.
3. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
4. Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189-195.
5. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1478-1483. doi:10.1016/j.jse.2011.11.004.
6. Sebastia-Forcada E, Cebrian-Gomez R, Lizaur-Utrilla A, Gil-Guillen V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419-1426. doi:10.1016/j.jse.2014.06.035.
7. Henn RF 3rd, Ghomrawi H, Rutledge JR, Mazumdar M, Mancuso CA, Marx RG. Preoperative patient expectations of total shoulder arthroplasty. J Bone Joint Surg Am. 2011;93(22):2110-2115. doi:10.2106/JBJS.J.01114.
8. McCarty EC, Marx RG, Maerz D, Altchek D, Warren RF. Sports participation after shoulder replacement surgery. Am J Sports Med. 2008;36(8):1577-1581. doi:10.1177/0363546508317126.
9. Puskas B, Harreld K, Clark R, Downes K, Virani NA, Frankle M. Isometric strength, range of motion, and impairment before and after total and reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(7):869-876. doi:10.1016/j.jse.2012.09.004.
10. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479.
11. Levy JC, Everding NG, Gil CC Jr., Stephens S, Giveans MR. Speed of recovery after shoulder arthroplasty: a comparison of reverse and anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(12):1872-1881. doi:10.1016/j.jse.2014.04.014.
12. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482. doi:10.1007/s11999-010-1683-z.
13. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
14. Bulhoff M, Sattler P, Bruckner T, Loew M, Zeifang F, Raiss P. Do patients return to sports and work after total shoulder replacement surgery? Am J Sports Med. 2015;43(2):423-427. doi:10.1177/0363546514557940.
15. Healy WL, Iorio R, Lemos MJ. Athletic activity after joint replacement. Am J Sports Med. 2001;29(3):377-388.
16. Jensen KL, Rockwood CA Jr. Shoulder arthroplasty in recreational golfers. J Shoulder Elbow Surg. 1998;7(4):362-367.
17. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105. doi:10.1177/0363546510371368.
18. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280. doi:10.1016/j.jse.2010.06.007.
19. Fink Barnes LA, Grantham WJ, Meadows MC, Bigliani LU, Levine WN, Ahmad CS. Sports activity after reverse total shoulder arthroplasty with minimum 2-year follow-up. Am J Orthop. 2015;44(2):68-72.
20. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469. doi:10.1016/j.jse.2011.11.012.
21. Simovitch RW, Gerard BK, Brees JA, Fullick R, Kearse JC. Outcomes of reverse total shoulder arthroplasty in a senior athletic population. J Shoulder Elbow Surg. 2015;24(9):1481-1485. doi:10.1016/j.jse.2015.03.011.
22. Golant A, Christoforou D, Zuckerman JD, Kwon YW. Return to sports after shoulder arthroplasty: a survey of surgeons' preferences. J Shoulder Elbow Surg. 2012;21(4):554-560. doi:10.1016/j.jse.2010.11.021.
23. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
24. Alta TD, de Toledo JM, Veeger HE, Janssen TW, Willems WJ. The active and passive kinematic difference between primary reverse and total shoulder prostheses. J Shoulder Elbow Surg. 2014;23(9):1395-1402. doi:10.1016/j.jse.2014.01.040.
25. Alta TD, Veeger DH, de Toledo JM, Janssen TW, Willems WJ. Isokinetic strength differences between patients with primary reverse and total shoulder prostheses: muscle strength quantified with a dynamometer. Clin Biomech (Bristol, Avon). 2014;29(9):965-970. doi:10.1016/j.clinbiomech.2014.08.018.
26. Jawa A, Dasti UR, Fasulo SM, Vaickus MH, Curtis AS, Miller SL. Anatomic total shoulder arthroplasty for patients receiving workers' compensation. J Shoulder Elbow Surg. 2015;24(11):1694-1697. doi:10.1016/j.jse.2015.04.017.
27. Morris BJ, Haigler RE, Laughlin MS, Elkousy HA, Gartsman GM, Edwards TB. Workers' compensation claims and outcomes after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(3):453-459. doi:10.1016/j.jse.2014.07.009.
1. Fehringer EV, Kopjar B, Boorman RS, Churchill RS, Smith KL, Matsen FA 3rd. Characterizing the functional improvement after total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Am. 2002;84-A(8):1349-1353.
2. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055. doi:10.2106/JBJS.L.01637.
3. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
4. Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189-195.
5. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1478-1483. doi:10.1016/j.jse.2011.11.004.
6. Sebastia-Forcada E, Cebrian-Gomez R, Lizaur-Utrilla A, Gil-Guillen V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419-1426. doi:10.1016/j.jse.2014.06.035.
7. Henn RF 3rd, Ghomrawi H, Rutledge JR, Mazumdar M, Mancuso CA, Marx RG. Preoperative patient expectations of total shoulder arthroplasty. J Bone Joint Surg Am. 2011;93(22):2110-2115. doi:10.2106/JBJS.J.01114.
8. McCarty EC, Marx RG, Maerz D, Altchek D, Warren RF. Sports participation after shoulder replacement surgery. Am J Sports Med. 2008;36(8):1577-1581. doi:10.1177/0363546508317126.
9. Puskas B, Harreld K, Clark R, Downes K, Virani NA, Frankle M. Isometric strength, range of motion, and impairment before and after total and reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(7):869-876. doi:10.1016/j.jse.2012.09.004.
10. Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479.
11. Levy JC, Everding NG, Gil CC Jr., Stephens S, Giveans MR. Speed of recovery after shoulder arthroplasty: a comparison of reverse and anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(12):1872-1881. doi:10.1016/j.jse.2014.04.014.
12. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482. doi:10.1007/s11999-010-1683-z.
13. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
14. Bulhoff M, Sattler P, Bruckner T, Loew M, Zeifang F, Raiss P. Do patients return to sports and work after total shoulder replacement surgery? Am J Sports Med. 2015;43(2):423-427. doi:10.1177/0363546514557940.
15. Healy WL, Iorio R, Lemos MJ. Athletic activity after joint replacement. Am J Sports Med. 2001;29(3):377-388.
16. Jensen KL, Rockwood CA Jr. Shoulder arthroplasty in recreational golfers. J Shoulder Elbow Surg. 1998;7(4):362-367.
17. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105. doi:10.1177/0363546510371368.
18. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280. doi:10.1016/j.jse.2010.06.007.
19. Fink Barnes LA, Grantham WJ, Meadows MC, Bigliani LU, Levine WN, Ahmad CS. Sports activity after reverse total shoulder arthroplasty with minimum 2-year follow-up. Am J Orthop. 2015;44(2):68-72.
20. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469. doi:10.1016/j.jse.2011.11.012.
21. Simovitch RW, Gerard BK, Brees JA, Fullick R, Kearse JC. Outcomes of reverse total shoulder arthroplasty in a senior athletic population. J Shoulder Elbow Surg. 2015;24(9):1481-1485. doi:10.1016/j.jse.2015.03.011.
22. Golant A, Christoforou D, Zuckerman JD, Kwon YW. Return to sports after shoulder arthroplasty: a survey of surgeons' preferences. J Shoulder Elbow Surg. 2012;21(4):554-560. doi:10.1016/j.jse.2010.11.021.
23. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
24. Alta TD, de Toledo JM, Veeger HE, Janssen TW, Willems WJ. The active and passive kinematic difference between primary reverse and total shoulder prostheses. J Shoulder Elbow Surg. 2014;23(9):1395-1402. doi:10.1016/j.jse.2014.01.040.
25. Alta TD, Veeger DH, de Toledo JM, Janssen TW, Willems WJ. Isokinetic strength differences between patients with primary reverse and total shoulder prostheses: muscle strength quantified with a dynamometer. Clin Biomech (Bristol, Avon). 2014;29(9):965-970. doi:10.1016/j.clinbiomech.2014.08.018.
26. Jawa A, Dasti UR, Fasulo SM, Vaickus MH, Curtis AS, Miller SL. Anatomic total shoulder arthroplasty for patients receiving workers' compensation. J Shoulder Elbow Surg. 2015;24(11):1694-1697. doi:10.1016/j.jse.2015.04.017.
27. Morris BJ, Haigler RE, Laughlin MS, Elkousy HA, Gartsman GM, Edwards TB. Workers' compensation claims and outcomes after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(3):453-459. doi:10.1016/j.jse.2014.07.009.
TAKE-HOME POINTS
- Both anatomic (TSA) and reverse shoulder arthroplasty (RSA) allow for the participation in work and sports.
- TSA patients report easier overall ability to participate in sports, specifically golf and swimming.
- For sports involving shoulder function, TSA patients more commonly report maximal ability to participate than RSA patients.
- TSA patients report easier overall ability to return to work-related activities, specifically housework and gardening.
- TSA patients featured 27% higher sport-specific ASES scores and 21% higher work-specific ASES scores than RSA patients.