User login
Anticoagulants may have advantage over aspirin for low-risk TAVR
NATIONAL HARBOR, MD. – Anticoagulation reduces the risk of leaflet thrombosis at 30 days relative to antiplatelet therapy in low-risk patients undergoing transcatheter aortic valve replacement (TAVR), according to a randomized feasibility study presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute..
At 30 days, oral anticoagulation with warfarin did not appear to be associated with any increased risk of adverse outcomes, including bleeding events, relative to aspirin, according to Toby Rogers, MD, PhD, the scientific lead for the Structural Heart Disease Program at MedStar Heart & Vascular Institute, Washington.
The rationale for this feasibility study, called LRT 2.0, was to evaluate whether anticoagulation after low-risk TAVR reduces the risk of early subclinical leaflet thrombosis, a potential threat to long-term valve survival.
“In the first LRT trial, HALT [hypoattenuated leaflet thickening] was observed in 13.5% of patients on antiplatelet therapy but only 4.8% of those on oral anticoagulation,” Dr. Rogers said.
The two strategies have not been adequately compared, particularly in low-risk patients, according to Dr. Rogers. He noted that current guidelines recommend dual-antiplatelet therapy after TAVR but the oral anticoagulant warfarin after surgical valve replacement, a situation he characterized as a “discrepancy.”
In the multicenter, randomized LRT 2.0 trial, 94 patients undergoing TAVR and meeting prespecified low-risk criteria, such as a Society of Thoracic Surgeons score of 3 or lower, were randomized to warfarin or to aspirin. The study called for an enrollment of 200 patients but was closed early when the Food and Drug Administration approved TAVR for low-risk patients in 2019, causing “enrollment to dry up over night.”
However, an additional registry cohort was included in a separate analysis. This registry cohort consisted of 30 patients who were evaluated for trial inclusion but were found to be inappropriate for randomization because they already had an indication for anticoagulation or had an elevated risk of bleeding. These low-risk TAVR patients were assigned to anticoagulation or antiplatelet therapy as appropriate.
When the randomized groups were compared, the incidence of HALT at 30 days on CT scan was 4.7% among those on warfarin and 16.3% (P = .07) among those taking aspirin. Dr. Rogers believes the near miss for statistical significance was a problem of power, a position supported by the pooled analysis of randomized and registry patients. With the added patients, the difference in HALT did reach significance (3.1% vs. 16.4%; P = .01).
The numerical differences in reduced leaflet motion and hypoattenuated motion favoring anticoagulation trended for significance in the randomized cohort (P = .12) but reached the cusp of significance in the pooled cohort (1.5% vs. 9.4%; P = .052) for both reduced leaflet motion and hypoattenuated motion).
There were no deaths recorded in any treatment arm, whether restricted to the randomized trial or within the pooled cohort. For the pooled cohort, there were more strokes in the aspirin arm (5.4% vs. 1.5%) but Dr. Rogers said that no conclusions could be drawn about relative risk because of the study size and small number of events.
For anticoagulation relative to antiplatelet therapy, respectively, the incidence of new-onset atrial fibrillation (1.5% vs. 1.8%), pacemaker implantation (11.8% vs. 7.1%), major bleeding (1.5% vs. 5.4%), and median length of stay (2.2 vs. 2.4 days) were all similar. The improvements in hemodynamics 30 days after TAVR were substantial and similar in the two groups, according to Dr. Rogers.
Emphasizing that this is a feasibility study, Dr. Rogers cautioned that these data do not necessarily demonstrate that anticoagulation is a better strategy than antiplatelet therapy in low-risk patients after TAVR, but they do associate anticoagulation with a reduced risk of early leaflet thrombosis.
“We fear leaflet thrombosis for the potential that it will negatively impact valve durability, which is particularly important in younger lower-risk patients who might outlive their first valve prosthesis,” Dr. Rogers said.
Panelists at the late-breaking clinical trial session expressed interest in this concept but generally agreed that longer follow-up is needed. This additional follow-up is important for monitoring effect on leaflet thrombosis as well as on the overall impact of these strategies on adverse events.
“We need to see CT scans at later time points because we do not know where this complication comes from. The trigger for leaflet thrombosis might still be there after 30 days,” said Andreas Baumbach, MD, professor of interventional cardiology at the University of Bristol (England). However, he agreed that this is an important line of research, because the potential risk of leaflet thrombosis is “a very important question for us.”
Dr. Rogers reported financial relationships with Edwards Lifesciences and Medtronic.
NATIONAL HARBOR, MD. – Anticoagulation reduces the risk of leaflet thrombosis at 30 days relative to antiplatelet therapy in low-risk patients undergoing transcatheter aortic valve replacement (TAVR), according to a randomized feasibility study presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute..
At 30 days, oral anticoagulation with warfarin did not appear to be associated with any increased risk of adverse outcomes, including bleeding events, relative to aspirin, according to Toby Rogers, MD, PhD, the scientific lead for the Structural Heart Disease Program at MedStar Heart & Vascular Institute, Washington.
The rationale for this feasibility study, called LRT 2.0, was to evaluate whether anticoagulation after low-risk TAVR reduces the risk of early subclinical leaflet thrombosis, a potential threat to long-term valve survival.
“In the first LRT trial, HALT [hypoattenuated leaflet thickening] was observed in 13.5% of patients on antiplatelet therapy but only 4.8% of those on oral anticoagulation,” Dr. Rogers said.
The two strategies have not been adequately compared, particularly in low-risk patients, according to Dr. Rogers. He noted that current guidelines recommend dual-antiplatelet therapy after TAVR but the oral anticoagulant warfarin after surgical valve replacement, a situation he characterized as a “discrepancy.”
In the multicenter, randomized LRT 2.0 trial, 94 patients undergoing TAVR and meeting prespecified low-risk criteria, such as a Society of Thoracic Surgeons score of 3 or lower, were randomized to warfarin or to aspirin. The study called for an enrollment of 200 patients but was closed early when the Food and Drug Administration approved TAVR for low-risk patients in 2019, causing “enrollment to dry up over night.”
However, an additional registry cohort was included in a separate analysis. This registry cohort consisted of 30 patients who were evaluated for trial inclusion but were found to be inappropriate for randomization because they already had an indication for anticoagulation or had an elevated risk of bleeding. These low-risk TAVR patients were assigned to anticoagulation or antiplatelet therapy as appropriate.
When the randomized groups were compared, the incidence of HALT at 30 days on CT scan was 4.7% among those on warfarin and 16.3% (P = .07) among those taking aspirin. Dr. Rogers believes the near miss for statistical significance was a problem of power, a position supported by the pooled analysis of randomized and registry patients. With the added patients, the difference in HALT did reach significance (3.1% vs. 16.4%; P = .01).
The numerical differences in reduced leaflet motion and hypoattenuated motion favoring anticoagulation trended for significance in the randomized cohort (P = .12) but reached the cusp of significance in the pooled cohort (1.5% vs. 9.4%; P = .052) for both reduced leaflet motion and hypoattenuated motion).
There were no deaths recorded in any treatment arm, whether restricted to the randomized trial or within the pooled cohort. For the pooled cohort, there were more strokes in the aspirin arm (5.4% vs. 1.5%) but Dr. Rogers said that no conclusions could be drawn about relative risk because of the study size and small number of events.
For anticoagulation relative to antiplatelet therapy, respectively, the incidence of new-onset atrial fibrillation (1.5% vs. 1.8%), pacemaker implantation (11.8% vs. 7.1%), major bleeding (1.5% vs. 5.4%), and median length of stay (2.2 vs. 2.4 days) were all similar. The improvements in hemodynamics 30 days after TAVR were substantial and similar in the two groups, according to Dr. Rogers.
Emphasizing that this is a feasibility study, Dr. Rogers cautioned that these data do not necessarily demonstrate that anticoagulation is a better strategy than antiplatelet therapy in low-risk patients after TAVR, but they do associate anticoagulation with a reduced risk of early leaflet thrombosis.
“We fear leaflet thrombosis for the potential that it will negatively impact valve durability, which is particularly important in younger lower-risk patients who might outlive their first valve prosthesis,” Dr. Rogers said.
Panelists at the late-breaking clinical trial session expressed interest in this concept but generally agreed that longer follow-up is needed. This additional follow-up is important for monitoring effect on leaflet thrombosis as well as on the overall impact of these strategies on adverse events.
“We need to see CT scans at later time points because we do not know where this complication comes from. The trigger for leaflet thrombosis might still be there after 30 days,” said Andreas Baumbach, MD, professor of interventional cardiology at the University of Bristol (England). However, he agreed that this is an important line of research, because the potential risk of leaflet thrombosis is “a very important question for us.”
Dr. Rogers reported financial relationships with Edwards Lifesciences and Medtronic.
NATIONAL HARBOR, MD. – Anticoagulation reduces the risk of leaflet thrombosis at 30 days relative to antiplatelet therapy in low-risk patients undergoing transcatheter aortic valve replacement (TAVR), according to a randomized feasibility study presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute..
At 30 days, oral anticoagulation with warfarin did not appear to be associated with any increased risk of adverse outcomes, including bleeding events, relative to aspirin, according to Toby Rogers, MD, PhD, the scientific lead for the Structural Heart Disease Program at MedStar Heart & Vascular Institute, Washington.
The rationale for this feasibility study, called LRT 2.0, was to evaluate whether anticoagulation after low-risk TAVR reduces the risk of early subclinical leaflet thrombosis, a potential threat to long-term valve survival.
“In the first LRT trial, HALT [hypoattenuated leaflet thickening] was observed in 13.5% of patients on antiplatelet therapy but only 4.8% of those on oral anticoagulation,” Dr. Rogers said.
The two strategies have not been adequately compared, particularly in low-risk patients, according to Dr. Rogers. He noted that current guidelines recommend dual-antiplatelet therapy after TAVR but the oral anticoagulant warfarin after surgical valve replacement, a situation he characterized as a “discrepancy.”
In the multicenter, randomized LRT 2.0 trial, 94 patients undergoing TAVR and meeting prespecified low-risk criteria, such as a Society of Thoracic Surgeons score of 3 or lower, were randomized to warfarin or to aspirin. The study called for an enrollment of 200 patients but was closed early when the Food and Drug Administration approved TAVR for low-risk patients in 2019, causing “enrollment to dry up over night.”
However, an additional registry cohort was included in a separate analysis. This registry cohort consisted of 30 patients who were evaluated for trial inclusion but were found to be inappropriate for randomization because they already had an indication for anticoagulation or had an elevated risk of bleeding. These low-risk TAVR patients were assigned to anticoagulation or antiplatelet therapy as appropriate.
When the randomized groups were compared, the incidence of HALT at 30 days on CT scan was 4.7% among those on warfarin and 16.3% (P = .07) among those taking aspirin. Dr. Rogers believes the near miss for statistical significance was a problem of power, a position supported by the pooled analysis of randomized and registry patients. With the added patients, the difference in HALT did reach significance (3.1% vs. 16.4%; P = .01).
The numerical differences in reduced leaflet motion and hypoattenuated motion favoring anticoagulation trended for significance in the randomized cohort (P = .12) but reached the cusp of significance in the pooled cohort (1.5% vs. 9.4%; P = .052) for both reduced leaflet motion and hypoattenuated motion).
There were no deaths recorded in any treatment arm, whether restricted to the randomized trial or within the pooled cohort. For the pooled cohort, there were more strokes in the aspirin arm (5.4% vs. 1.5%) but Dr. Rogers said that no conclusions could be drawn about relative risk because of the study size and small number of events.
For anticoagulation relative to antiplatelet therapy, respectively, the incidence of new-onset atrial fibrillation (1.5% vs. 1.8%), pacemaker implantation (11.8% vs. 7.1%), major bleeding (1.5% vs. 5.4%), and median length of stay (2.2 vs. 2.4 days) were all similar. The improvements in hemodynamics 30 days after TAVR were substantial and similar in the two groups, according to Dr. Rogers.
Emphasizing that this is a feasibility study, Dr. Rogers cautioned that these data do not necessarily demonstrate that anticoagulation is a better strategy than antiplatelet therapy in low-risk patients after TAVR, but they do associate anticoagulation with a reduced risk of early leaflet thrombosis.
“We fear leaflet thrombosis for the potential that it will negatively impact valve durability, which is particularly important in younger lower-risk patients who might outlive their first valve prosthesis,” Dr. Rogers said.
Panelists at the late-breaking clinical trial session expressed interest in this concept but generally agreed that longer follow-up is needed. This additional follow-up is important for monitoring effect on leaflet thrombosis as well as on the overall impact of these strategies on adverse events.
“We need to see CT scans at later time points because we do not know where this complication comes from. The trigger for leaflet thrombosis might still be there after 30 days,” said Andreas Baumbach, MD, professor of interventional cardiology at the University of Bristol (England). However, he agreed that this is an important line of research, because the potential risk of leaflet thrombosis is “a very important question for us.”
Dr. Rogers reported financial relationships with Edwards Lifesciences and Medtronic.
REPORTING FROM CRT 2020
AUGUSTUS: Apixaban surpassed warfarin despite prior stroke or thromboembolism
LOS ANGELES – The edge that the direct-acting oral anticoagulant apixaban (Eliquis) has over warfarin for safely preventing ischemic events in patients with atrial fibrillation and either a recent acute coronary syndrome event or a recent percutaneous coronary intervention held up even in patients with a history of stroke, transient ischemic attack, or thromboembolic event, according to a prespecified secondary analysis of data collected in the AUGUSTUS trial.
The treatment advantages of apixaban, compared with warfarin, seen in the overall AUGUSTUS results, first reported in March 2019, “were consistent” with the benefits seen in the subgroup of enrolled patients with a prior stroke, transient ischemic attack (TIA), or thromboembolic (TE) event, M. Cecilia Bahit, MD, said at the International Stroke Conference sponsored by the American Heart Association.
All patients in AUGUSTUS received a P2Y12 inhibitor antiplatelet drug, which was clopidogrel for more than 90% of patients. The two-by-two factorial design of AUGUSTUS also assessed the safety and efficacy of either adding or withholding aspirin from the two-drug regimen that all patients in the study received with a P2Y12 inhibitor plus an anticoagulant (apixaban or warfarin). The most notable finding of the aspirin versus placebo analysis was that patients without a prior stroke, TIA, or TE event had a “more profound” increase in their rate of major or clinically relevant minor bleeds when also treated with aspirin, compared with patients who received aspirin and had a history of stroke, TIA, or TE event, reported Dr. Bahit, a chief of cardiology and director of clinical research at the INECO Foundation in Rosario, Argentina.
In general, the findings of the secondary analysis that took into account stroke, TIA, or TE history “confirmed” the main AUGUSTUS findings, Dr. Bahit said; an antithrombotic regimen of apixaban plus clopidogrel (or other P2Y12 inhibitor) without aspirin was superior for both efficacy and safety, compared with the alternative regimens that either substituted warfarin for apixaban or that added aspirin.
AUGUSTUS enrolled 4,614 atrial fibrillation (AFib) patients who either had a recent acute coronary syndrome (ACS) event or had recently undergone percutaneous coronary intervention (PCI) at any of 492 sites in 33 countries during 2015-2018. The study’s primary endpoint was the incidence of major or clinically relevant minor bleeds after 6 months, which was significantly lower in the subgroups that received apixaban instead of warfarin and in patients who received placebo instead of aspirin. The secondary endpoint of death or hospitalization after 6 months was also significantly lower in the apixaban-treated patients, compared with those on warfarin, while the aspirin and placebo subgroups showed no difference in the incidence of these events (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
The results reported by Dr. Bahit also highlighted both the high risk faced by patients with AFib who also have had an ACS event or PCI, as well as a prior stroke, TIA, or TE event, noted Larry B. Goldstein, MD, professor and chairman of neurology at the University of Kentucky, Lexington. “It’s difficult, because these patients had an ACS event or PCI, and you don’t want a coronary too close up, but do these patients really need a P2Y12 inhibitor plus an anticoagulant? Could these patients do as well on apixaban only? I would have liked to see that treatment arm in the study,” Dr. Goldstein commented in an interview.
“These are challenging patients because they often require anticoagulation for the AFib as well as antiplatelet agents” for the recent PCI or ACS event, commented Mitchell S.V. Elkind, MD, professor of neurology at Columbia University, New York. “The question has always been: How many blood thinners should these patients be on? Potentially they could be on three different agents [an anticoagulant and two antiplatelet drugs], and we know that all of those drugs together pretty dramatically increase the risk of bleeding. About 15% of the patients in the overall AUGUSTUS trial had either cerebrovascular disease or systemic thromboembolism, so this was a small subgroup of the overall trial, but the overall trial was large so it’s a significant number of patients who met this criteria. The results confirmed that even in a group of patients who may be considered at high risk because they have a prior history of cerebrovascular disease use of apixaban instead of warfarin seemed safer, and that those patients did not need to be on aspirin as well as their other antiplatelet agent. Patients with a history of stroke, in fact, had a lower risk of bleeding than the other patients in this trial, so one could argue that they should be on an agent like apixaban as well as an antiplatelet agent like clopidogrel without addition of aspirin,” he said in a recorded statement.
In addition to implications for using prescription drugs like apixaban and clopidogrel, the findings also send a message about the need for very aggressive implementation of lifestyle measures that can reduce cardiovascular disease risk in these patients, added Dr. Goldstein. The AUGUSTUS outcome analyses that subdivided the study population into those with a prior stroke, TIA, or TE event – 633 patients or about 14% of the 4,581 patients eligible for this analysis – and those who did not have this history showed the extremely high, incrementally elevated risk faced by patients with these prior events.
A history of stroke, TIA, or TE event linked with a jump in the 90-day rate of major or clinically relevant minor bleeds from 13% without this history to 17%, which is a 31% relative increase; it boosted the 90-day rate of death or hospitalization from 25% to 31%, a 24% relative increase; and it jacked up the rate of death or ischemic events from 6% to 9%, a 50% relative increase, Dr. Bahit reported.
These substantial increases “suggest we need to be very aggressive” in managing these high-risk patients who combine a background of AFib, a prior stroke, TIA, or TE events, and a recent ACS event or PCI, Dr. Goldstein observed. In these patients, he suggested that clinicians make sure to address smoking cessation, obesity, exercise, diet, and statin use, and get each of these to an optimal level to further cut risk. If all five of these basic interventions were successfully administered to a patient they could collectively cut the patient’s event risk by about 80%, he added.
AUGUSTUS was funded by Bristol-Myers Squibb and Pfizer, the companies that jointly market apixaban. Dr. Bahit has received honoraria from Pfizer, and from CSL Behring and Merck. Dr. Elkind and Dr. Goldstein had no relevant disclosures.
SOURCE: Bahit MC et al. ISC 2020, Abstract LB22.
LOS ANGELES – The edge that the direct-acting oral anticoagulant apixaban (Eliquis) has over warfarin for safely preventing ischemic events in patients with atrial fibrillation and either a recent acute coronary syndrome event or a recent percutaneous coronary intervention held up even in patients with a history of stroke, transient ischemic attack, or thromboembolic event, according to a prespecified secondary analysis of data collected in the AUGUSTUS trial.
The treatment advantages of apixaban, compared with warfarin, seen in the overall AUGUSTUS results, first reported in March 2019, “were consistent” with the benefits seen in the subgroup of enrolled patients with a prior stroke, transient ischemic attack (TIA), or thromboembolic (TE) event, M. Cecilia Bahit, MD, said at the International Stroke Conference sponsored by the American Heart Association.
All patients in AUGUSTUS received a P2Y12 inhibitor antiplatelet drug, which was clopidogrel for more than 90% of patients. The two-by-two factorial design of AUGUSTUS also assessed the safety and efficacy of either adding or withholding aspirin from the two-drug regimen that all patients in the study received with a P2Y12 inhibitor plus an anticoagulant (apixaban or warfarin). The most notable finding of the aspirin versus placebo analysis was that patients without a prior stroke, TIA, or TE event had a “more profound” increase in their rate of major or clinically relevant minor bleeds when also treated with aspirin, compared with patients who received aspirin and had a history of stroke, TIA, or TE event, reported Dr. Bahit, a chief of cardiology and director of clinical research at the INECO Foundation in Rosario, Argentina.
In general, the findings of the secondary analysis that took into account stroke, TIA, or TE history “confirmed” the main AUGUSTUS findings, Dr. Bahit said; an antithrombotic regimen of apixaban plus clopidogrel (or other P2Y12 inhibitor) without aspirin was superior for both efficacy and safety, compared with the alternative regimens that either substituted warfarin for apixaban or that added aspirin.
AUGUSTUS enrolled 4,614 atrial fibrillation (AFib) patients who either had a recent acute coronary syndrome (ACS) event or had recently undergone percutaneous coronary intervention (PCI) at any of 492 sites in 33 countries during 2015-2018. The study’s primary endpoint was the incidence of major or clinically relevant minor bleeds after 6 months, which was significantly lower in the subgroups that received apixaban instead of warfarin and in patients who received placebo instead of aspirin. The secondary endpoint of death or hospitalization after 6 months was also significantly lower in the apixaban-treated patients, compared with those on warfarin, while the aspirin and placebo subgroups showed no difference in the incidence of these events (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
The results reported by Dr. Bahit also highlighted both the high risk faced by patients with AFib who also have had an ACS event or PCI, as well as a prior stroke, TIA, or TE event, noted Larry B. Goldstein, MD, professor and chairman of neurology at the University of Kentucky, Lexington. “It’s difficult, because these patients had an ACS event or PCI, and you don’t want a coronary too close up, but do these patients really need a P2Y12 inhibitor plus an anticoagulant? Could these patients do as well on apixaban only? I would have liked to see that treatment arm in the study,” Dr. Goldstein commented in an interview.
“These are challenging patients because they often require anticoagulation for the AFib as well as antiplatelet agents” for the recent PCI or ACS event, commented Mitchell S.V. Elkind, MD, professor of neurology at Columbia University, New York. “The question has always been: How many blood thinners should these patients be on? Potentially they could be on three different agents [an anticoagulant and two antiplatelet drugs], and we know that all of those drugs together pretty dramatically increase the risk of bleeding. About 15% of the patients in the overall AUGUSTUS trial had either cerebrovascular disease or systemic thromboembolism, so this was a small subgroup of the overall trial, but the overall trial was large so it’s a significant number of patients who met this criteria. The results confirmed that even in a group of patients who may be considered at high risk because they have a prior history of cerebrovascular disease use of apixaban instead of warfarin seemed safer, and that those patients did not need to be on aspirin as well as their other antiplatelet agent. Patients with a history of stroke, in fact, had a lower risk of bleeding than the other patients in this trial, so one could argue that they should be on an agent like apixaban as well as an antiplatelet agent like clopidogrel without addition of aspirin,” he said in a recorded statement.
In addition to implications for using prescription drugs like apixaban and clopidogrel, the findings also send a message about the need for very aggressive implementation of lifestyle measures that can reduce cardiovascular disease risk in these patients, added Dr. Goldstein. The AUGUSTUS outcome analyses that subdivided the study population into those with a prior stroke, TIA, or TE event – 633 patients or about 14% of the 4,581 patients eligible for this analysis – and those who did not have this history showed the extremely high, incrementally elevated risk faced by patients with these prior events.
A history of stroke, TIA, or TE event linked with a jump in the 90-day rate of major or clinically relevant minor bleeds from 13% without this history to 17%, which is a 31% relative increase; it boosted the 90-day rate of death or hospitalization from 25% to 31%, a 24% relative increase; and it jacked up the rate of death or ischemic events from 6% to 9%, a 50% relative increase, Dr. Bahit reported.
These substantial increases “suggest we need to be very aggressive” in managing these high-risk patients who combine a background of AFib, a prior stroke, TIA, or TE events, and a recent ACS event or PCI, Dr. Goldstein observed. In these patients, he suggested that clinicians make sure to address smoking cessation, obesity, exercise, diet, and statin use, and get each of these to an optimal level to further cut risk. If all five of these basic interventions were successfully administered to a patient they could collectively cut the patient’s event risk by about 80%, he added.
AUGUSTUS was funded by Bristol-Myers Squibb and Pfizer, the companies that jointly market apixaban. Dr. Bahit has received honoraria from Pfizer, and from CSL Behring and Merck. Dr. Elkind and Dr. Goldstein had no relevant disclosures.
SOURCE: Bahit MC et al. ISC 2020, Abstract LB22.
LOS ANGELES – The edge that the direct-acting oral anticoagulant apixaban (Eliquis) has over warfarin for safely preventing ischemic events in patients with atrial fibrillation and either a recent acute coronary syndrome event or a recent percutaneous coronary intervention held up even in patients with a history of stroke, transient ischemic attack, or thromboembolic event, according to a prespecified secondary analysis of data collected in the AUGUSTUS trial.
The treatment advantages of apixaban, compared with warfarin, seen in the overall AUGUSTUS results, first reported in March 2019, “were consistent” with the benefits seen in the subgroup of enrolled patients with a prior stroke, transient ischemic attack (TIA), or thromboembolic (TE) event, M. Cecilia Bahit, MD, said at the International Stroke Conference sponsored by the American Heart Association.
All patients in AUGUSTUS received a P2Y12 inhibitor antiplatelet drug, which was clopidogrel for more than 90% of patients. The two-by-two factorial design of AUGUSTUS also assessed the safety and efficacy of either adding or withholding aspirin from the two-drug regimen that all patients in the study received with a P2Y12 inhibitor plus an anticoagulant (apixaban or warfarin). The most notable finding of the aspirin versus placebo analysis was that patients without a prior stroke, TIA, or TE event had a “more profound” increase in their rate of major or clinically relevant minor bleeds when also treated with aspirin, compared with patients who received aspirin and had a history of stroke, TIA, or TE event, reported Dr. Bahit, a chief of cardiology and director of clinical research at the INECO Foundation in Rosario, Argentina.
In general, the findings of the secondary analysis that took into account stroke, TIA, or TE history “confirmed” the main AUGUSTUS findings, Dr. Bahit said; an antithrombotic regimen of apixaban plus clopidogrel (or other P2Y12 inhibitor) without aspirin was superior for both efficacy and safety, compared with the alternative regimens that either substituted warfarin for apixaban or that added aspirin.
AUGUSTUS enrolled 4,614 atrial fibrillation (AFib) patients who either had a recent acute coronary syndrome (ACS) event or had recently undergone percutaneous coronary intervention (PCI) at any of 492 sites in 33 countries during 2015-2018. The study’s primary endpoint was the incidence of major or clinically relevant minor bleeds after 6 months, which was significantly lower in the subgroups that received apixaban instead of warfarin and in patients who received placebo instead of aspirin. The secondary endpoint of death or hospitalization after 6 months was also significantly lower in the apixaban-treated patients, compared with those on warfarin, while the aspirin and placebo subgroups showed no difference in the incidence of these events (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
The results reported by Dr. Bahit also highlighted both the high risk faced by patients with AFib who also have had an ACS event or PCI, as well as a prior stroke, TIA, or TE event, noted Larry B. Goldstein, MD, professor and chairman of neurology at the University of Kentucky, Lexington. “It’s difficult, because these patients had an ACS event or PCI, and you don’t want a coronary too close up, but do these patients really need a P2Y12 inhibitor plus an anticoagulant? Could these patients do as well on apixaban only? I would have liked to see that treatment arm in the study,” Dr. Goldstein commented in an interview.
“These are challenging patients because they often require anticoagulation for the AFib as well as antiplatelet agents” for the recent PCI or ACS event, commented Mitchell S.V. Elkind, MD, professor of neurology at Columbia University, New York. “The question has always been: How many blood thinners should these patients be on? Potentially they could be on three different agents [an anticoagulant and two antiplatelet drugs], and we know that all of those drugs together pretty dramatically increase the risk of bleeding. About 15% of the patients in the overall AUGUSTUS trial had either cerebrovascular disease or systemic thromboembolism, so this was a small subgroup of the overall trial, but the overall trial was large so it’s a significant number of patients who met this criteria. The results confirmed that even in a group of patients who may be considered at high risk because they have a prior history of cerebrovascular disease use of apixaban instead of warfarin seemed safer, and that those patients did not need to be on aspirin as well as their other antiplatelet agent. Patients with a history of stroke, in fact, had a lower risk of bleeding than the other patients in this trial, so one could argue that they should be on an agent like apixaban as well as an antiplatelet agent like clopidogrel without addition of aspirin,” he said in a recorded statement.
In addition to implications for using prescription drugs like apixaban and clopidogrel, the findings also send a message about the need for very aggressive implementation of lifestyle measures that can reduce cardiovascular disease risk in these patients, added Dr. Goldstein. The AUGUSTUS outcome analyses that subdivided the study population into those with a prior stroke, TIA, or TE event – 633 patients or about 14% of the 4,581 patients eligible for this analysis – and those who did not have this history showed the extremely high, incrementally elevated risk faced by patients with these prior events.
A history of stroke, TIA, or TE event linked with a jump in the 90-day rate of major or clinically relevant minor bleeds from 13% without this history to 17%, which is a 31% relative increase; it boosted the 90-day rate of death or hospitalization from 25% to 31%, a 24% relative increase; and it jacked up the rate of death or ischemic events from 6% to 9%, a 50% relative increase, Dr. Bahit reported.
These substantial increases “suggest we need to be very aggressive” in managing these high-risk patients who combine a background of AFib, a prior stroke, TIA, or TE events, and a recent ACS event or PCI, Dr. Goldstein observed. In these patients, he suggested that clinicians make sure to address smoking cessation, obesity, exercise, diet, and statin use, and get each of these to an optimal level to further cut risk. If all five of these basic interventions were successfully administered to a patient they could collectively cut the patient’s event risk by about 80%, he added.
AUGUSTUS was funded by Bristol-Myers Squibb and Pfizer, the companies that jointly market apixaban. Dr. Bahit has received honoraria from Pfizer, and from CSL Behring and Merck. Dr. Elkind and Dr. Goldstein had no relevant disclosures.
SOURCE: Bahit MC et al. ISC 2020, Abstract LB22.
REPORTING FROM ISC 2020
AFib patients do best on a DOAC started 7-10 days post stroke
LOS ANGELES – When a patient with atrial fibrillation (AFib) has a cardioembolic stroke, the best blood thinner to start may be a direct-acting oral anticoagulant (DOAC), possibly beginning 7-10 days after the index stroke, according to an analysis of 90-day, observational outcomes data from nearly 1,300 patients.
The analysis also suggested that the use of “bridging” anticoagulant treatment by injection before a patient with atrial fibrillation (AFib) starts a daily oral anticoagulant regimen following a cardioembolic stroke is not a good idea. Patients who received bridging anticoagulation had a nearly threefold higher rate of symptomatic intracranial hemorrhage than did patients who did not, and their bridging treatment failed to protect them from recurrent ischemic events, Shadi Yaghi, MD, said at the International Stroke Conference, sponsored by the American Heart Association. The bridging regimens delivered either heparin or low-molecular-weight heparin.
Based on the findings, “it seems reasonable to avoid bridging unless absolutely necessary, to initiate a DOAC unless it’s contraindicated, and to start the DOAC on day 7-10 following the stroke in most patients,” said Dr. Yaghi, a vascular neurologist and director of stroke research at NYU Langone Health in New York.
“It’s been hard to develop a broad guideline on when to start oral anticoagulation” after a cardioembolic stroke in AFib patients. The best time “depends on a lot of variables and how the patient responded to acute treatment,” commented Alexis Simpkins, MD, a vascular and stroke neurologist at the University of Florida in Gainesville. “You want to start treatment before the patient has another stroke, but not so soon that the treatment causes symptomatic hemorrhagic transformation.”
Dr. Yaghi’s suggestion, based on his findings, to start treatment for most patients with a DOAC 7-10 days after their index stroke “shows consistency” with the prevailing guideline recommendation from the AHA/American Stroke Association to start oral anticoagulation in this patient population 4-14 days after the index stroke (Stroke. 2018 March;49[3]:e46-e99), she noted.
A recent article reviewed the uncertainty about the best time to start oral anticoagulation in AFib patients after a cardioembolic stroke and the subtle differences that distinguish various international medical groups that, like the ASA, have made recommendations (Lancet Neurol. 2019 Jan 1;18[1]:117-26). According to this review, a major limitation of these various recommendations has been the lack of actual evidence collected from AFib patients who began receiving a DOAC shortly after a cardioembolic stroke, although the article added that several studies in progress are collecting these data.
The study reported by Dr. Yaghi pooled data collected from 2,084 recent AFib patients with a cardioembolic stroke treated at any of eight comprehensive U.S. stroke centers. They excluded patients who died from causes unrelated to the primary endpoint, those who did not receive an anticoagulant or had incomplete data, and patients lost to follow-up, leaving 1,289 evaluable patients. During their 90-day follow-up, 10% of the patients had an ischemic event, a symptomatic intracranial hemorrhage, or an extracranial hemorrhage.
The study’s primary analysis showed no statistically significant difference in the incidence of recurrent ischemic events, symptomatic intracranial hemorrhage, or both based on when oral anticoagulant treatment began: 0-3 days, 4-14 days, or more than 14 days after the index stroke.
The investigators then subdivided patients into the subgroup that started treatment with a DOAC and the subgroup that started treatment with warfarin and also further subdivided the 4-14 day time window for starting treatment. Results of this analysis showed that patients who received a DOAC and began this treatment 7-10 days after their stroke had a 50% cut in their 90-day events compared with other patients, a difference that fell just short of statistical significance at P = .07. All the other combinations of oral anticoagulant and time of treatment initiation analyzed showed neutral effects that never came near statistical significance.
Secondary data analyses also showed that both patients with a history of a stroke prior to their index stroke and patients with ipsilateral atherosclerosis came close to having a statistically significant increased rate of a subsequent ischemic event during 90-day follow-up. Furthermore, women, patients with a history of hyperlipidemia, and patients who developed hemorrhagic transformation of their index stroke all had significantly increased rates of developing a symptomatic intracranial hemorrhage during 90-day follow-up. When the endpoint was limited to recurrent ischemic events only, patients who received a DOAC were 50% less likely to have an event than were patients treated with warfarin, a statistically significant difference.
Although starting a DOAC 7-10 days after the index stroke seems reasonable based on this analysis, the question needs a prospective, randomized study to create an appropriate evidence base, Dr. Yaghi said.
Dr. Yaghi disclosed a financial relationship with Medtronic. Dr. Simpkins had no disclosures.
SOURCE: Yaghi S et al. Stroke. 2020 Feb;51(suppl 1):A119.
LOS ANGELES – When a patient with atrial fibrillation (AFib) has a cardioembolic stroke, the best blood thinner to start may be a direct-acting oral anticoagulant (DOAC), possibly beginning 7-10 days after the index stroke, according to an analysis of 90-day, observational outcomes data from nearly 1,300 patients.
The analysis also suggested that the use of “bridging” anticoagulant treatment by injection before a patient with atrial fibrillation (AFib) starts a daily oral anticoagulant regimen following a cardioembolic stroke is not a good idea. Patients who received bridging anticoagulation had a nearly threefold higher rate of symptomatic intracranial hemorrhage than did patients who did not, and their bridging treatment failed to protect them from recurrent ischemic events, Shadi Yaghi, MD, said at the International Stroke Conference, sponsored by the American Heart Association. The bridging regimens delivered either heparin or low-molecular-weight heparin.
Based on the findings, “it seems reasonable to avoid bridging unless absolutely necessary, to initiate a DOAC unless it’s contraindicated, and to start the DOAC on day 7-10 following the stroke in most patients,” said Dr. Yaghi, a vascular neurologist and director of stroke research at NYU Langone Health in New York.
“It’s been hard to develop a broad guideline on when to start oral anticoagulation” after a cardioembolic stroke in AFib patients. The best time “depends on a lot of variables and how the patient responded to acute treatment,” commented Alexis Simpkins, MD, a vascular and stroke neurologist at the University of Florida in Gainesville. “You want to start treatment before the patient has another stroke, but not so soon that the treatment causes symptomatic hemorrhagic transformation.”
Dr. Yaghi’s suggestion, based on his findings, to start treatment for most patients with a DOAC 7-10 days after their index stroke “shows consistency” with the prevailing guideline recommendation from the AHA/American Stroke Association to start oral anticoagulation in this patient population 4-14 days after the index stroke (Stroke. 2018 March;49[3]:e46-e99), she noted.
A recent article reviewed the uncertainty about the best time to start oral anticoagulation in AFib patients after a cardioembolic stroke and the subtle differences that distinguish various international medical groups that, like the ASA, have made recommendations (Lancet Neurol. 2019 Jan 1;18[1]:117-26). According to this review, a major limitation of these various recommendations has been the lack of actual evidence collected from AFib patients who began receiving a DOAC shortly after a cardioembolic stroke, although the article added that several studies in progress are collecting these data.
The study reported by Dr. Yaghi pooled data collected from 2,084 recent AFib patients with a cardioembolic stroke treated at any of eight comprehensive U.S. stroke centers. They excluded patients who died from causes unrelated to the primary endpoint, those who did not receive an anticoagulant or had incomplete data, and patients lost to follow-up, leaving 1,289 evaluable patients. During their 90-day follow-up, 10% of the patients had an ischemic event, a symptomatic intracranial hemorrhage, or an extracranial hemorrhage.
The study’s primary analysis showed no statistically significant difference in the incidence of recurrent ischemic events, symptomatic intracranial hemorrhage, or both based on when oral anticoagulant treatment began: 0-3 days, 4-14 days, or more than 14 days after the index stroke.
The investigators then subdivided patients into the subgroup that started treatment with a DOAC and the subgroup that started treatment with warfarin and also further subdivided the 4-14 day time window for starting treatment. Results of this analysis showed that patients who received a DOAC and began this treatment 7-10 days after their stroke had a 50% cut in their 90-day events compared with other patients, a difference that fell just short of statistical significance at P = .07. All the other combinations of oral anticoagulant and time of treatment initiation analyzed showed neutral effects that never came near statistical significance.
Secondary data analyses also showed that both patients with a history of a stroke prior to their index stroke and patients with ipsilateral atherosclerosis came close to having a statistically significant increased rate of a subsequent ischemic event during 90-day follow-up. Furthermore, women, patients with a history of hyperlipidemia, and patients who developed hemorrhagic transformation of their index stroke all had significantly increased rates of developing a symptomatic intracranial hemorrhage during 90-day follow-up. When the endpoint was limited to recurrent ischemic events only, patients who received a DOAC were 50% less likely to have an event than were patients treated with warfarin, a statistically significant difference.
Although starting a DOAC 7-10 days after the index stroke seems reasonable based on this analysis, the question needs a prospective, randomized study to create an appropriate evidence base, Dr. Yaghi said.
Dr. Yaghi disclosed a financial relationship with Medtronic. Dr. Simpkins had no disclosures.
SOURCE: Yaghi S et al. Stroke. 2020 Feb;51(suppl 1):A119.
LOS ANGELES – When a patient with atrial fibrillation (AFib) has a cardioembolic stroke, the best blood thinner to start may be a direct-acting oral anticoagulant (DOAC), possibly beginning 7-10 days after the index stroke, according to an analysis of 90-day, observational outcomes data from nearly 1,300 patients.
The analysis also suggested that the use of “bridging” anticoagulant treatment by injection before a patient with atrial fibrillation (AFib) starts a daily oral anticoagulant regimen following a cardioembolic stroke is not a good idea. Patients who received bridging anticoagulation had a nearly threefold higher rate of symptomatic intracranial hemorrhage than did patients who did not, and their bridging treatment failed to protect them from recurrent ischemic events, Shadi Yaghi, MD, said at the International Stroke Conference, sponsored by the American Heart Association. The bridging regimens delivered either heparin or low-molecular-weight heparin.
Based on the findings, “it seems reasonable to avoid bridging unless absolutely necessary, to initiate a DOAC unless it’s contraindicated, and to start the DOAC on day 7-10 following the stroke in most patients,” said Dr. Yaghi, a vascular neurologist and director of stroke research at NYU Langone Health in New York.
“It’s been hard to develop a broad guideline on when to start oral anticoagulation” after a cardioembolic stroke in AFib patients. The best time “depends on a lot of variables and how the patient responded to acute treatment,” commented Alexis Simpkins, MD, a vascular and stroke neurologist at the University of Florida in Gainesville. “You want to start treatment before the patient has another stroke, but not so soon that the treatment causes symptomatic hemorrhagic transformation.”
Dr. Yaghi’s suggestion, based on his findings, to start treatment for most patients with a DOAC 7-10 days after their index stroke “shows consistency” with the prevailing guideline recommendation from the AHA/American Stroke Association to start oral anticoagulation in this patient population 4-14 days after the index stroke (Stroke. 2018 March;49[3]:e46-e99), she noted.
A recent article reviewed the uncertainty about the best time to start oral anticoagulation in AFib patients after a cardioembolic stroke and the subtle differences that distinguish various international medical groups that, like the ASA, have made recommendations (Lancet Neurol. 2019 Jan 1;18[1]:117-26). According to this review, a major limitation of these various recommendations has been the lack of actual evidence collected from AFib patients who began receiving a DOAC shortly after a cardioembolic stroke, although the article added that several studies in progress are collecting these data.
The study reported by Dr. Yaghi pooled data collected from 2,084 recent AFib patients with a cardioembolic stroke treated at any of eight comprehensive U.S. stroke centers. They excluded patients who died from causes unrelated to the primary endpoint, those who did not receive an anticoagulant or had incomplete data, and patients lost to follow-up, leaving 1,289 evaluable patients. During their 90-day follow-up, 10% of the patients had an ischemic event, a symptomatic intracranial hemorrhage, or an extracranial hemorrhage.
The study’s primary analysis showed no statistically significant difference in the incidence of recurrent ischemic events, symptomatic intracranial hemorrhage, or both based on when oral anticoagulant treatment began: 0-3 days, 4-14 days, or more than 14 days after the index stroke.
The investigators then subdivided patients into the subgroup that started treatment with a DOAC and the subgroup that started treatment with warfarin and also further subdivided the 4-14 day time window for starting treatment. Results of this analysis showed that patients who received a DOAC and began this treatment 7-10 days after their stroke had a 50% cut in their 90-day events compared with other patients, a difference that fell just short of statistical significance at P = .07. All the other combinations of oral anticoagulant and time of treatment initiation analyzed showed neutral effects that never came near statistical significance.
Secondary data analyses also showed that both patients with a history of a stroke prior to their index stroke and patients with ipsilateral atherosclerosis came close to having a statistically significant increased rate of a subsequent ischemic event during 90-day follow-up. Furthermore, women, patients with a history of hyperlipidemia, and patients who developed hemorrhagic transformation of their index stroke all had significantly increased rates of developing a symptomatic intracranial hemorrhage during 90-day follow-up. When the endpoint was limited to recurrent ischemic events only, patients who received a DOAC were 50% less likely to have an event than were patients treated with warfarin, a statistically significant difference.
Although starting a DOAC 7-10 days after the index stroke seems reasonable based on this analysis, the question needs a prospective, randomized study to create an appropriate evidence base, Dr. Yaghi said.
Dr. Yaghi disclosed a financial relationship with Medtronic. Dr. Simpkins had no disclosures.
SOURCE: Yaghi S et al. Stroke. 2020 Feb;51(suppl 1):A119.
REPORTING FROM ISC 2020
After PCI, stopping antiplatelet therapy for surgery appears safe
NATIONAL HARBOR, MD. – Following a percutaneous intervention with a second-generation drug-eluting stent, a judicious interruption of antiplatelet therapy for noncardiac surgery does not increase risk of net adverse clinical events, according to a large dataset presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
Drawn from a multicenter registry in South Korea, it is likely that those in whom antiplatelet therapy was stopped during the perioperative period were at a lower relative risk, but the data remain reassuring, according to Jung-Sun Kim, MD, PhD, professor of medicine at Yonsei University, Seoul, South Korea.
In the registry of patients with a second-generation drug-eluting stent (DES) undergoing noncardiac surgery, “antiplatelet therapy was discontinued in almost half of the patients,” Dr. Kim reported. When these patients were compared with those who did not discontinue antiplatelet therapy, the data, called an “exploratory analysis,” suggested “no increased risk” of a composite of major adverse cardiac events (MACE) or major bleeding.
The retrospective analysis involved 3,582 percutaneous intervention (PCI) patients who had received a second-generation DES and subsequently underwent noncardiac surgery. In 1,750 of these patients, antiplatelet therapy was temporarily discontinued. The remaining 1,832 remained on some form of antiplatelet treatment, whether aspirin, a P2Y12 inhibitor, or dual-antiplatelet therapy.
There were no significant differences in crude rates between groups in rates at 30 days of a composite endpoint of MACE, major bleeding as defined by the International Society on Thrombosis and Haemostasis, or net adverse clinical events (NACE), a composite of adverse events that included MACE and major bleeding.
Relative risks for antiplatelet discontinuation remained generally low even after multiple stratifications performed to explore different variables, including the types of antiplatelet therapy being taken at the time of discontinuation, the types of noncardiac surgery performed, and the duration of discontinuation.
Of these variables, the interval of discontinuation appeared to be most relevant. Antiplatelet discontinuation of 3 days or less appeared to be associated with a higher risk of bleeding, although the difference did not reach significance. Discontinuations of 9 days or more were associated with increased risk of MACE, and this difference did reach statistical significance (hazard ratio, 3.38; 95% confidence interval, 1.36-8.38).
“Discontinuation of antiplatelet therapy for a period of 4-8 days appears to be optimal,” Dr. Kim said.
In general, risk of MACE, major bleeding, or NACE could not be linked to type of surgery, with the exception of intra-abdominal surgery. For this procedure, there appeared to be a lower risk of MACE in those who discontinued relative to those who remained on antiplatelet therapy, Dr. Kim reported.
Importantly, because of the fact that the decision to stop antiplatelet treatment was made by treating physicians, the characteristics of those who discontinued or remained on antiplatelet therapy differed meaningfully. Specifically, those in the discontinuation group were younger and were less likely to have additional risks for thrombotic events such as diabetes or chronic kidney disease. In those who discontinued antiplatelets, the average time since PCI was 23 months versus 16 months in the continuation group.
In addition, “more of the patients underwent higher-risk surgeries in the discontinuation group,” Dr. Kim added.
Relative rates of MACE and NACE remained similar even after risk adjustment, but Dr. Kim advised that the data should be “interpreted cautiously” because of the retrospective nature of the analysis.
A panel of experts invited to comment on the presentation agreed. These data were considered reassuring for clinicians considering an interruption of antiplatelet therapy following PCI with a second-generation DES, but there was uncertainty about their value for defining which patients are the best candidates.
The decision to discontinue antiplatelet drugs for noncardiac surgery is an important and common dilemma, but these data might be best characterized as “a testament to Korean cardiologists making good decisions,” said David J. Moliterno, MD, chairman of the department of medicine at University of Kentucky Health Care, Lexington.
Dr. Kim reported no potential financial conflicts of interest.
NATIONAL HARBOR, MD. – Following a percutaneous intervention with a second-generation drug-eluting stent, a judicious interruption of antiplatelet therapy for noncardiac surgery does not increase risk of net adverse clinical events, according to a large dataset presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
Drawn from a multicenter registry in South Korea, it is likely that those in whom antiplatelet therapy was stopped during the perioperative period were at a lower relative risk, but the data remain reassuring, according to Jung-Sun Kim, MD, PhD, professor of medicine at Yonsei University, Seoul, South Korea.
In the registry of patients with a second-generation drug-eluting stent (DES) undergoing noncardiac surgery, “antiplatelet therapy was discontinued in almost half of the patients,” Dr. Kim reported. When these patients were compared with those who did not discontinue antiplatelet therapy, the data, called an “exploratory analysis,” suggested “no increased risk” of a composite of major adverse cardiac events (MACE) or major bleeding.
The retrospective analysis involved 3,582 percutaneous intervention (PCI) patients who had received a second-generation DES and subsequently underwent noncardiac surgery. In 1,750 of these patients, antiplatelet therapy was temporarily discontinued. The remaining 1,832 remained on some form of antiplatelet treatment, whether aspirin, a P2Y12 inhibitor, or dual-antiplatelet therapy.
There were no significant differences in crude rates between groups in rates at 30 days of a composite endpoint of MACE, major bleeding as defined by the International Society on Thrombosis and Haemostasis, or net adverse clinical events (NACE), a composite of adverse events that included MACE and major bleeding.
Relative risks for antiplatelet discontinuation remained generally low even after multiple stratifications performed to explore different variables, including the types of antiplatelet therapy being taken at the time of discontinuation, the types of noncardiac surgery performed, and the duration of discontinuation.
Of these variables, the interval of discontinuation appeared to be most relevant. Antiplatelet discontinuation of 3 days or less appeared to be associated with a higher risk of bleeding, although the difference did not reach significance. Discontinuations of 9 days or more were associated with increased risk of MACE, and this difference did reach statistical significance (hazard ratio, 3.38; 95% confidence interval, 1.36-8.38).
“Discontinuation of antiplatelet therapy for a period of 4-8 days appears to be optimal,” Dr. Kim said.
In general, risk of MACE, major bleeding, or NACE could not be linked to type of surgery, with the exception of intra-abdominal surgery. For this procedure, there appeared to be a lower risk of MACE in those who discontinued relative to those who remained on antiplatelet therapy, Dr. Kim reported.
Importantly, because of the fact that the decision to stop antiplatelet treatment was made by treating physicians, the characteristics of those who discontinued or remained on antiplatelet therapy differed meaningfully. Specifically, those in the discontinuation group were younger and were less likely to have additional risks for thrombotic events such as diabetes or chronic kidney disease. In those who discontinued antiplatelets, the average time since PCI was 23 months versus 16 months in the continuation group.
In addition, “more of the patients underwent higher-risk surgeries in the discontinuation group,” Dr. Kim added.
Relative rates of MACE and NACE remained similar even after risk adjustment, but Dr. Kim advised that the data should be “interpreted cautiously” because of the retrospective nature of the analysis.
A panel of experts invited to comment on the presentation agreed. These data were considered reassuring for clinicians considering an interruption of antiplatelet therapy following PCI with a second-generation DES, but there was uncertainty about their value for defining which patients are the best candidates.
The decision to discontinue antiplatelet drugs for noncardiac surgery is an important and common dilemma, but these data might be best characterized as “a testament to Korean cardiologists making good decisions,” said David J. Moliterno, MD, chairman of the department of medicine at University of Kentucky Health Care, Lexington.
Dr. Kim reported no potential financial conflicts of interest.
NATIONAL HARBOR, MD. – Following a percutaneous intervention with a second-generation drug-eluting stent, a judicious interruption of antiplatelet therapy for noncardiac surgery does not increase risk of net adverse clinical events, according to a large dataset presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
Drawn from a multicenter registry in South Korea, it is likely that those in whom antiplatelet therapy was stopped during the perioperative period were at a lower relative risk, but the data remain reassuring, according to Jung-Sun Kim, MD, PhD, professor of medicine at Yonsei University, Seoul, South Korea.
In the registry of patients with a second-generation drug-eluting stent (DES) undergoing noncardiac surgery, “antiplatelet therapy was discontinued in almost half of the patients,” Dr. Kim reported. When these patients were compared with those who did not discontinue antiplatelet therapy, the data, called an “exploratory analysis,” suggested “no increased risk” of a composite of major adverse cardiac events (MACE) or major bleeding.
The retrospective analysis involved 3,582 percutaneous intervention (PCI) patients who had received a second-generation DES and subsequently underwent noncardiac surgery. In 1,750 of these patients, antiplatelet therapy was temporarily discontinued. The remaining 1,832 remained on some form of antiplatelet treatment, whether aspirin, a P2Y12 inhibitor, or dual-antiplatelet therapy.
There were no significant differences in crude rates between groups in rates at 30 days of a composite endpoint of MACE, major bleeding as defined by the International Society on Thrombosis and Haemostasis, or net adverse clinical events (NACE), a composite of adverse events that included MACE and major bleeding.
Relative risks for antiplatelet discontinuation remained generally low even after multiple stratifications performed to explore different variables, including the types of antiplatelet therapy being taken at the time of discontinuation, the types of noncardiac surgery performed, and the duration of discontinuation.
Of these variables, the interval of discontinuation appeared to be most relevant. Antiplatelet discontinuation of 3 days or less appeared to be associated with a higher risk of bleeding, although the difference did not reach significance. Discontinuations of 9 days or more were associated with increased risk of MACE, and this difference did reach statistical significance (hazard ratio, 3.38; 95% confidence interval, 1.36-8.38).
“Discontinuation of antiplatelet therapy for a period of 4-8 days appears to be optimal,” Dr. Kim said.
In general, risk of MACE, major bleeding, or NACE could not be linked to type of surgery, with the exception of intra-abdominal surgery. For this procedure, there appeared to be a lower risk of MACE in those who discontinued relative to those who remained on antiplatelet therapy, Dr. Kim reported.
Importantly, because of the fact that the decision to stop antiplatelet treatment was made by treating physicians, the characteristics of those who discontinued or remained on antiplatelet therapy differed meaningfully. Specifically, those in the discontinuation group were younger and were less likely to have additional risks for thrombotic events such as diabetes or chronic kidney disease. In those who discontinued antiplatelets, the average time since PCI was 23 months versus 16 months in the continuation group.
In addition, “more of the patients underwent higher-risk surgeries in the discontinuation group,” Dr. Kim added.
Relative rates of MACE and NACE remained similar even after risk adjustment, but Dr. Kim advised that the data should be “interpreted cautiously” because of the retrospective nature of the analysis.
A panel of experts invited to comment on the presentation agreed. These data were considered reassuring for clinicians considering an interruption of antiplatelet therapy following PCI with a second-generation DES, but there was uncertainty about their value for defining which patients are the best candidates.
The decision to discontinue antiplatelet drugs for noncardiac surgery is an important and common dilemma, but these data might be best characterized as “a testament to Korean cardiologists making good decisions,” said David J. Moliterno, MD, chairman of the department of medicine at University of Kentucky Health Care, Lexington.
Dr. Kim reported no potential financial conflicts of interest.
REPORTING FROM CRT 2020
TNK dose in large-vessel stroke: 0.25 mg/kg is sufficient
A new study suggests that the 0.25-mg/kg dose of the thrombolytic tenecteplase (TNK) is just as good at facilitating reperfusion of the blocked artery in patients with ischemic large-vessel stroke prior to planned thrombectomy as the higher 0.4-mg/kg dose.
The EXTEND-IA TNK Part 2 trial was presented today at the American Stroke Association’s International Stroke Conference (ISC) 2020 in Los Angeles and was published online simultaneously (JAMA. 2020 Feb 20. doi: 10.1001/jama.2020.1511).
“We found the 0.4-mg/kg dose was no better than 0.25 mg/kg. There was absolutely no perceptible difference, so it appears that 0.25 mg/kg is enough,” lead investigator Bruce Campbell, MBBS, PhD, said in an interview.
“Our study was conducted in patients with large-vessel occlusions heading for thrombectomy, but I think the results can be extrapolated to patients with smaller occlusions too,” he added.
The study also showed that one-fifth of patients given tenecteplase experienced reperfusion before thrombectomy was performed. The percentage rose to one-third among patients from rural areas, whose longer times in transport led to an increase in the time between thrombolysis and thrombectomy.
“I think these data are as good as we’re going to get on the optimal dose of TNK. Our endpoint was reperfusion rates – a good, solid biological marker of benefit – but if a difference in clinical outcomes is wanted, that would take a trial of several thousand patients, which is never likely to be done,” said Dr. Campbell, who is from the Department of Neurology at the Royal Melbourne Hospital, Australia.
The researchers note that tenecteplase has a practical advantage over alteplase in that it is given as a bolus injection, whereas alteplase is given as bolus followed by a 1-hour infusion.
Results from the first EXTEND-IA TNK study suggested that tenecteplase 0.25 mg/kg produced higher reperfusion rates than alteplase (N Engl J Med. 2018;378:1573-82). However, the larger NOR-TEST study found no difference in efficacy or safety between a 0.4-mg/kg dose of tenecteplase and alteplase in patients with mild stroke (Lancet Neurol. 2017 Oct;16[10]:781-8).
TNK use in stroke varies around the world. The drug is not licensed for use in stroke anywhere, which Dr. Campbell attributes to a lack of incentive for the manufacturer, Genentech/Boehringer Ingelheim. That company also markets alteplase, the main thrombolytic used in stroke.
But many countries have now included TNK in their stroke guidelines, Dr. Campbell noted. “This has only recently occurred in the U.S., where it has a 2b recommendation, and the dose recommendations are somewhat confusing, advocating 0.25 mg/kg in large-vessel occlusions [as was used in the first EXTEND IA study] and 0.4 mg/kg in non–large vessel occlusions [from the NOR-TEST trial].
“This makes no biological sense whatsoever, recommending a higher dose for smaller occlusions, but that is just a literal translation of the design of the two major studies. I’m hoping our current results will help clarify the dosage issue and that might encourage more use of TNK altogether,” he commented.
For the current study, conducted in Australia and New Zealand, 300 patients who had experienced ischemic large-vessel stroke within 4.5 hours of symptom onset and who were scheduled for endovascular thrombectomy were randomly assigned to receive open-label thrombolysis with tenecteplase 0.4 mg/kg or 0.25 mg/kg.
The primary outcome, reperfusion of greater than 50% of the involved ischemic territory prior to thrombectomy, occurred in 19.3% of both groups. There was also no difference in any of the functional-outcome secondary endpoints or all-cause mortality between the two doses.
“While we didn’t find any extra benefit of the 0.4-mg/kg dose over the 0.25-mg/kg dose, we also didn’t find any extra harm, and this gives us reassurance in the emergency situation if the weight of the patient is overestimated; then we have a window of safety,” Dr. Campbell commented. “While there was a nonsignificant numerical increase in intracranial hemorrhage in the 0.4-mg/kg group, the excess bleeds were caused by puncturing of the vessels during thrombectomy, so I don’t think we can blame the TNK dose for that.
Better reperfusion than with alteplase?
Noting that the original EXTEND-IA TNK study showed higher reperfusion rates with tenecteplase vs alteplase and a trend toward better outcomes on the mRS scale, Campbell reported that a pooled analysis of the TNK results from the current study with those from the first study confirmed these findings.
“We found a doubling in the rate of reperfusion with TNK vs. alteplase, and the [modified Rankin Scale] shift analysis remained positive,” he said.
“I think we say with confidence that TNK is at least as good as alteplase and probably better, but further studies comparing the two agents are ongoing,” he added.
Of note, for the 41 patients from rural areas in the current study, in whom the time from thrombolysis to thrombectomy was longer (152 min vs. 41 min for patients from urban areas), reperfusion rates were higher (34% vs 17%), and there was no difference in dosage between the two groups.
Commenting on these latest results in an interview, Nicola Logallo, MD, of Haukeland University Hospital, Bergen, Norway, who was part of the NOR-TEST trial, said: “There is some evidence supporting the use of TNK 0.4 mg/kg in mild stroke patients, based mainly on the results from the NOR-TEST trial, and the use of TNK 0.25 mg/kg in patients undergoing thrombectomy, based on Dr. Campbell’s previous EXTEND-TNK trial. Dr. Campbell’s new study confirms that probably the higher dose of TNK does not add any advantages in terms of clinical outcome.”
Hemorrhagic complications appear to be similar in the two groups, Dr. Logallo said. “Overall, the 0.25-mg/kg TNK dose could therefore be considered as the most convenient and sensible, at least in patients undergoing thrombectomy. When it comes to the remaining stroke patients receiving thrombolysis, it remains unclear which is the best dose, but studies such as TASTE, NOR-TEST 2, AcT, and ATTEST-2 will hopefully answer this question within the next years.”
Also commenting on the study, Michael Hill, MD, professor of neurology at University of Calgary, Alberta, Canada, said the results “confirm that a good proportion of patients given TNK reperfuse before the angiogram and clarifies the dose. This is useful information.”
Dr. Hill said TNK is used routinely in some countries – mainly in Australia and Norway, where the studies have been conducted – but there is now a movement toward use of TNK in North America, too.
“Studies so far suggest that it could be more effective than alteplase, and as it is more fibrin specific, it could be safer. It is also easier to give with a bolus dose, but perhaps the biggest driver might be that it is cheaper than alteplase. Momentum is building, and many leading investigators are now conducting new studies with TNK with several more studies coming out in the next year or so,” Dr. Hill added.
The EXTEND-IA TNK Part 2 trial was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Campbell reports receiving grants from both institutions during the conduct of the study.
This article first appeared on Medscape.com.
A new study suggests that the 0.25-mg/kg dose of the thrombolytic tenecteplase (TNK) is just as good at facilitating reperfusion of the blocked artery in patients with ischemic large-vessel stroke prior to planned thrombectomy as the higher 0.4-mg/kg dose.
The EXTEND-IA TNK Part 2 trial was presented today at the American Stroke Association’s International Stroke Conference (ISC) 2020 in Los Angeles and was published online simultaneously (JAMA. 2020 Feb 20. doi: 10.1001/jama.2020.1511).
“We found the 0.4-mg/kg dose was no better than 0.25 mg/kg. There was absolutely no perceptible difference, so it appears that 0.25 mg/kg is enough,” lead investigator Bruce Campbell, MBBS, PhD, said in an interview.
“Our study was conducted in patients with large-vessel occlusions heading for thrombectomy, but I think the results can be extrapolated to patients with smaller occlusions too,” he added.
The study also showed that one-fifth of patients given tenecteplase experienced reperfusion before thrombectomy was performed. The percentage rose to one-third among patients from rural areas, whose longer times in transport led to an increase in the time between thrombolysis and thrombectomy.
“I think these data are as good as we’re going to get on the optimal dose of TNK. Our endpoint was reperfusion rates – a good, solid biological marker of benefit – but if a difference in clinical outcomes is wanted, that would take a trial of several thousand patients, which is never likely to be done,” said Dr. Campbell, who is from the Department of Neurology at the Royal Melbourne Hospital, Australia.
The researchers note that tenecteplase has a practical advantage over alteplase in that it is given as a bolus injection, whereas alteplase is given as bolus followed by a 1-hour infusion.
Results from the first EXTEND-IA TNK study suggested that tenecteplase 0.25 mg/kg produced higher reperfusion rates than alteplase (N Engl J Med. 2018;378:1573-82). However, the larger NOR-TEST study found no difference in efficacy or safety between a 0.4-mg/kg dose of tenecteplase and alteplase in patients with mild stroke (Lancet Neurol. 2017 Oct;16[10]:781-8).
TNK use in stroke varies around the world. The drug is not licensed for use in stroke anywhere, which Dr. Campbell attributes to a lack of incentive for the manufacturer, Genentech/Boehringer Ingelheim. That company also markets alteplase, the main thrombolytic used in stroke.
But many countries have now included TNK in their stroke guidelines, Dr. Campbell noted. “This has only recently occurred in the U.S., where it has a 2b recommendation, and the dose recommendations are somewhat confusing, advocating 0.25 mg/kg in large-vessel occlusions [as was used in the first EXTEND IA study] and 0.4 mg/kg in non–large vessel occlusions [from the NOR-TEST trial].
“This makes no biological sense whatsoever, recommending a higher dose for smaller occlusions, but that is just a literal translation of the design of the two major studies. I’m hoping our current results will help clarify the dosage issue and that might encourage more use of TNK altogether,” he commented.
For the current study, conducted in Australia and New Zealand, 300 patients who had experienced ischemic large-vessel stroke within 4.5 hours of symptom onset and who were scheduled for endovascular thrombectomy were randomly assigned to receive open-label thrombolysis with tenecteplase 0.4 mg/kg or 0.25 mg/kg.
The primary outcome, reperfusion of greater than 50% of the involved ischemic territory prior to thrombectomy, occurred in 19.3% of both groups. There was also no difference in any of the functional-outcome secondary endpoints or all-cause mortality between the two doses.
“While we didn’t find any extra benefit of the 0.4-mg/kg dose over the 0.25-mg/kg dose, we also didn’t find any extra harm, and this gives us reassurance in the emergency situation if the weight of the patient is overestimated; then we have a window of safety,” Dr. Campbell commented. “While there was a nonsignificant numerical increase in intracranial hemorrhage in the 0.4-mg/kg group, the excess bleeds were caused by puncturing of the vessels during thrombectomy, so I don’t think we can blame the TNK dose for that.
Better reperfusion than with alteplase?
Noting that the original EXTEND-IA TNK study showed higher reperfusion rates with tenecteplase vs alteplase and a trend toward better outcomes on the mRS scale, Campbell reported that a pooled analysis of the TNK results from the current study with those from the first study confirmed these findings.
“We found a doubling in the rate of reperfusion with TNK vs. alteplase, and the [modified Rankin Scale] shift analysis remained positive,” he said.
“I think we say with confidence that TNK is at least as good as alteplase and probably better, but further studies comparing the two agents are ongoing,” he added.
Of note, for the 41 patients from rural areas in the current study, in whom the time from thrombolysis to thrombectomy was longer (152 min vs. 41 min for patients from urban areas), reperfusion rates were higher (34% vs 17%), and there was no difference in dosage between the two groups.
Commenting on these latest results in an interview, Nicola Logallo, MD, of Haukeland University Hospital, Bergen, Norway, who was part of the NOR-TEST trial, said: “There is some evidence supporting the use of TNK 0.4 mg/kg in mild stroke patients, based mainly on the results from the NOR-TEST trial, and the use of TNK 0.25 mg/kg in patients undergoing thrombectomy, based on Dr. Campbell’s previous EXTEND-TNK trial. Dr. Campbell’s new study confirms that probably the higher dose of TNK does not add any advantages in terms of clinical outcome.”
Hemorrhagic complications appear to be similar in the two groups, Dr. Logallo said. “Overall, the 0.25-mg/kg TNK dose could therefore be considered as the most convenient and sensible, at least in patients undergoing thrombectomy. When it comes to the remaining stroke patients receiving thrombolysis, it remains unclear which is the best dose, but studies such as TASTE, NOR-TEST 2, AcT, and ATTEST-2 will hopefully answer this question within the next years.”
Also commenting on the study, Michael Hill, MD, professor of neurology at University of Calgary, Alberta, Canada, said the results “confirm that a good proportion of patients given TNK reperfuse before the angiogram and clarifies the dose. This is useful information.”
Dr. Hill said TNK is used routinely in some countries – mainly in Australia and Norway, where the studies have been conducted – but there is now a movement toward use of TNK in North America, too.
“Studies so far suggest that it could be more effective than alteplase, and as it is more fibrin specific, it could be safer. It is also easier to give with a bolus dose, but perhaps the biggest driver might be that it is cheaper than alteplase. Momentum is building, and many leading investigators are now conducting new studies with TNK with several more studies coming out in the next year or so,” Dr. Hill added.
The EXTEND-IA TNK Part 2 trial was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Campbell reports receiving grants from both institutions during the conduct of the study.
This article first appeared on Medscape.com.
A new study suggests that the 0.25-mg/kg dose of the thrombolytic tenecteplase (TNK) is just as good at facilitating reperfusion of the blocked artery in patients with ischemic large-vessel stroke prior to planned thrombectomy as the higher 0.4-mg/kg dose.
The EXTEND-IA TNK Part 2 trial was presented today at the American Stroke Association’s International Stroke Conference (ISC) 2020 in Los Angeles and was published online simultaneously (JAMA. 2020 Feb 20. doi: 10.1001/jama.2020.1511).
“We found the 0.4-mg/kg dose was no better than 0.25 mg/kg. There was absolutely no perceptible difference, so it appears that 0.25 mg/kg is enough,” lead investigator Bruce Campbell, MBBS, PhD, said in an interview.
“Our study was conducted in patients with large-vessel occlusions heading for thrombectomy, but I think the results can be extrapolated to patients with smaller occlusions too,” he added.
The study also showed that one-fifth of patients given tenecteplase experienced reperfusion before thrombectomy was performed. The percentage rose to one-third among patients from rural areas, whose longer times in transport led to an increase in the time between thrombolysis and thrombectomy.
“I think these data are as good as we’re going to get on the optimal dose of TNK. Our endpoint was reperfusion rates – a good, solid biological marker of benefit – but if a difference in clinical outcomes is wanted, that would take a trial of several thousand patients, which is never likely to be done,” said Dr. Campbell, who is from the Department of Neurology at the Royal Melbourne Hospital, Australia.
The researchers note that tenecteplase has a practical advantage over alteplase in that it is given as a bolus injection, whereas alteplase is given as bolus followed by a 1-hour infusion.
Results from the first EXTEND-IA TNK study suggested that tenecteplase 0.25 mg/kg produced higher reperfusion rates than alteplase (N Engl J Med. 2018;378:1573-82). However, the larger NOR-TEST study found no difference in efficacy or safety between a 0.4-mg/kg dose of tenecteplase and alteplase in patients with mild stroke (Lancet Neurol. 2017 Oct;16[10]:781-8).
TNK use in stroke varies around the world. The drug is not licensed for use in stroke anywhere, which Dr. Campbell attributes to a lack of incentive for the manufacturer, Genentech/Boehringer Ingelheim. That company also markets alteplase, the main thrombolytic used in stroke.
But many countries have now included TNK in their stroke guidelines, Dr. Campbell noted. “This has only recently occurred in the U.S., where it has a 2b recommendation, and the dose recommendations are somewhat confusing, advocating 0.25 mg/kg in large-vessel occlusions [as was used in the first EXTEND IA study] and 0.4 mg/kg in non–large vessel occlusions [from the NOR-TEST trial].
“This makes no biological sense whatsoever, recommending a higher dose for smaller occlusions, but that is just a literal translation of the design of the two major studies. I’m hoping our current results will help clarify the dosage issue and that might encourage more use of TNK altogether,” he commented.
For the current study, conducted in Australia and New Zealand, 300 patients who had experienced ischemic large-vessel stroke within 4.5 hours of symptom onset and who were scheduled for endovascular thrombectomy were randomly assigned to receive open-label thrombolysis with tenecteplase 0.4 mg/kg or 0.25 mg/kg.
The primary outcome, reperfusion of greater than 50% of the involved ischemic territory prior to thrombectomy, occurred in 19.3% of both groups. There was also no difference in any of the functional-outcome secondary endpoints or all-cause mortality between the two doses.
“While we didn’t find any extra benefit of the 0.4-mg/kg dose over the 0.25-mg/kg dose, we also didn’t find any extra harm, and this gives us reassurance in the emergency situation if the weight of the patient is overestimated; then we have a window of safety,” Dr. Campbell commented. “While there was a nonsignificant numerical increase in intracranial hemorrhage in the 0.4-mg/kg group, the excess bleeds were caused by puncturing of the vessels during thrombectomy, so I don’t think we can blame the TNK dose for that.
Better reperfusion than with alteplase?
Noting that the original EXTEND-IA TNK study showed higher reperfusion rates with tenecteplase vs alteplase and a trend toward better outcomes on the mRS scale, Campbell reported that a pooled analysis of the TNK results from the current study with those from the first study confirmed these findings.
“We found a doubling in the rate of reperfusion with TNK vs. alteplase, and the [modified Rankin Scale] shift analysis remained positive,” he said.
“I think we say with confidence that TNK is at least as good as alteplase and probably better, but further studies comparing the two agents are ongoing,” he added.
Of note, for the 41 patients from rural areas in the current study, in whom the time from thrombolysis to thrombectomy was longer (152 min vs. 41 min for patients from urban areas), reperfusion rates were higher (34% vs 17%), and there was no difference in dosage between the two groups.
Commenting on these latest results in an interview, Nicola Logallo, MD, of Haukeland University Hospital, Bergen, Norway, who was part of the NOR-TEST trial, said: “There is some evidence supporting the use of TNK 0.4 mg/kg in mild stroke patients, based mainly on the results from the NOR-TEST trial, and the use of TNK 0.25 mg/kg in patients undergoing thrombectomy, based on Dr. Campbell’s previous EXTEND-TNK trial. Dr. Campbell’s new study confirms that probably the higher dose of TNK does not add any advantages in terms of clinical outcome.”
Hemorrhagic complications appear to be similar in the two groups, Dr. Logallo said. “Overall, the 0.25-mg/kg TNK dose could therefore be considered as the most convenient and sensible, at least in patients undergoing thrombectomy. When it comes to the remaining stroke patients receiving thrombolysis, it remains unclear which is the best dose, but studies such as TASTE, NOR-TEST 2, AcT, and ATTEST-2 will hopefully answer this question within the next years.”
Also commenting on the study, Michael Hill, MD, professor of neurology at University of Calgary, Alberta, Canada, said the results “confirm that a good proportion of patients given TNK reperfuse before the angiogram and clarifies the dose. This is useful information.”
Dr. Hill said TNK is used routinely in some countries – mainly in Australia and Norway, where the studies have been conducted – but there is now a movement toward use of TNK in North America, too.
“Studies so far suggest that it could be more effective than alteplase, and as it is more fibrin specific, it could be safer. It is also easier to give with a bolus dose, but perhaps the biggest driver might be that it is cheaper than alteplase. Momentum is building, and many leading investigators are now conducting new studies with TNK with several more studies coming out in the next year or so,” Dr. Hill added.
The EXTEND-IA TNK Part 2 trial was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Campbell reports receiving grants from both institutions during the conduct of the study.
This article first appeared on Medscape.com.
What 2019’s top five CAD trials tell us
SNOWMASS, COLO. – A repeated theme threading through much of one prominent interventional cardiologist’s personal list of the top five coronary artery disease (CAD) trials of the past year is that aspirin is very often more trouble than it’s worth.
“For some years I’ve been concerned that the only thing that aspirin does [in patients after percutaneous coronary intervention] is increase your risk of bleeding. It doesn’t really provide any additional ischemic protection,” Malcolm R. Bell, MBBS, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“I’ll remind you that, when we go back to the early stent days, observed Dr. Bell, professor of medicine and vice chair of the department of cardiovascular medicine at the Mayo Clinic in Rochester, Minn.
Here are the key takeaway messages from his five most important randomized trials in CAD during the last year.
AUGUSTUS
For years, cardiologists have grappled with how to best manage high-cardiovascular-risk patients with atrial fibrillation who seem like they might benefit from triple-antithrombotic therapy. AUGUSTUS supplied the answer: Don’t do it. Skip the aspirin and turn instead to a P2Y12 inhibitor plus a non–vitamin K antagonist oral anticoagulant (NOAC), rather than warfarin.
“I would like you to think of triple therapy as a triple threat. That’s really what triple therapy is all about”– a three-pronged threat to patient safety, Dr. Bell commented.
In AUGUSTUS, 4,614 patients with atrial fibrillation and CAD with an acute coronary syndrome (ACS) and/or percutaneous coronary intervention (PCI) in 33 countries were placed on a P2Y12 inhibitor – most often clopidogrel – and randomized double blind to either apixaban (Eliquis) or warfarin, and further to aspirin or placebo, for 6 months of antithrombotic therapy. The strategy of a P2Y12 inhibitor and apixaban without aspirin was the clear winner, resulting in significantly less major bleeding, mortality, and hospitalizations than treatment with a P2Y12 inhibitor and warfarin, with or without aspirin. Most importantly, ischemic event rates didn’t differ between the apixaban and warfarin groups. And patients randomized to aspirin had rates of ischemic events and death or hospitalization similar to placebo-treated controls, meaning aspirin accomplished nothing (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
Dr. Bell noted that a meta-analysis of AUGUSTUS and three smaller randomized trials including more than 10,000 AUGUSTUS-type patients with atrial fibrillation concluded that a treatment strategy utilizing a NOAC and a P2Y12 inhibitor resulted in less bleeding than warfarin plus DAPT, and at no cost in terms of excess ischemic events. Moreover, regimens without aspirin resulted in less intracranial and other major bleeding without any difference in major adverse cardiovascular events (JAMA Cardiol. 2019 Jun 19. doi: 10.1001/jamacardio.2019.1880).
A key message of these four trials is that a NOAC is preferable to warfarin, so much so that, in high-risk patients who are already on warfarin, it’s worth considering a switch to a NOAC.
“And we should really be avoiding DAPT,” Dr. Bell added.
How soon after an ACS and/or PCI should patients with atrial fibrillation stop taking aspirin?
“In AUGUSTUS, randomization occurred at a median of 6 days, so we know that half the patients stopped their aspirin by then. In our own practice, we’re just dropping the aspirin for the most part before the patient leaves the hospital. I think if you leave them with instructions to stop the aspirin in a week’s time or a month’s time it just leads to confusion. And we should also remember that half of the major bleeding after PCI or ACS happens in the first 30 days, so it doesn’t make a lot of sense to say that we should continue it for a month and then drop it,” according to the cardiologist.
SMART-CHOICE and STOPDAPT-2
These two large multicenter studies demonstrate that DAPT can safely be stopped early if needed. SMART-CHOICE from South Korea and STOPDAPT-2 from Japan each randomized roughly 3,000 patients undergoing PCI to 12 months of DAPT or to DAPT for only 3 months or 1 month, respectively, at which point the aspirin was dropped and patients in the abbreviated DAPT arm continued on P2Y12 inhibitor monotherapy, mostly clopidogrel, for the remainder of the 12 months. In the Japanese STOPDAPT-2 trial, 1 month of DAPT proved superior to 12 months of DAPT for the primary composite endpoint of cardiovascular death, MI, stroke, definite stent thrombosis, or major or minor bleeding at 12 months (JAMA. 2019 Jun 25;321[24]:2414-27). In the South Korean SMART-CHOICE trial, 3 months of DAPT was noninferior to 12 months for major adverse cardiac and cerebrovascular events, and superior in terms of bleeding risk (JAMA. 2019 Jun 25;321[24]:2428-37). Of note, roughly half of patients in the two trials were lower-risk individuals undergoing PCI for stable angina.
Dr. Bell noted that, while the TWILIGHT trial (Ticagrelor With or Without Aspirin in High-Risk Patients After PCI) didn’t make his top-five list, it certainly fits well with the two East Asian studies. The TWILIGHT investigators randomized more than 7,000 patients to 12 months of DAPT or discontinuation of aspirin after 3 months. The result: a lower incidence of clinically relevant bleeding with ticagrelor monotherapy, and with no increased risk of death, MI, or stroke, compared with 12 months of DAPT (N Engl J Med. 2019 Nov 21;381[21]:2032-42).
“Again, I would just question what the added value of aspirin is here,” Dr. Bell commented. “Many interventional cardiologists are absolutely terrified of their patients having stent thrombosis, but with second-generation drug-eluting stents – the stents we’re putting in day in and day out – the risk of stent thrombosis is less than 1%. And in these two trials it was less than 0.5%. There’s more risk of having major bleeding events than there is of ischemia, so I think the balance is in favor of preventing bleeding. We know that major bleeding predicts short- and long-term mortality.”
COLCOT
This double-blind trial randomized 4,745 patients within 30 days post MI to low-dose colchicine or placebo on top of excellent rates of background guideline-directed medical therapy. The goal was to see if this anti-inflammatory agent could reduce cardiovascular events independent of any lipid-lowering effect, as was earlier seen with canakinumab in the CANTOS trial. It did so to a statistically significant but relatively modest degree, with a 5.5% rate of the composite cardiovascular events endpoint in the colchicine group and 7.1% in placebo-treated controls (N Engl J Med. 2019 Dec 26;381[26]:2497-505). But Dr. Bell was unimpressed.
“All-cause mortality was identical at 1.8% in both groups. So colchicine is not saving lives. In fact, the only real differences were in stroke – but the study wasn’t powered to look at stroke – and in urgent hospitalization for angina leading to revascularization, which is a soft endpoint,” he observed.
Plus, 2.5% of patients were lost to follow-up, which Dr. Bell considers “a little concerning” in a trial conducted in the current era.
“In my opinion, the evidence that colchicine is effective is weak, and I don’t think really supports the drug’s routine use post MI. We already send these patients out on numerous medications. We have to think about cost/benefit, and if a patient asks me: ‘Is this going to prevent another heart attack or make me live longer?’ I think the unequivocal answer is no,” he said.
These days colchicine is no longer an inexpensive drug, either, at an average cost of $300-$400 per month, the cardiologist added.
COMPLETE
This study randomized more than 4,000 patients with ST-segment elevation MI (STEMI) and multivessel disease to primary PCI of the culprit lesion only or to staged complete revascularization via PCI of all angiographically significant nonculprit lesions. Complete revascularization proved to be the superior strategy, with a 26% reduction in the risk of the composite of cardiovascular death or MI at a median of 3 years (N Engl J Med. 2019 Oct 10;381[15]:1411-21).
The optimal timing of the staged procedure remains unclear, since the study didn’t specify a protocol.
“I’m still a bit uncomfortable doing multivessel PCI at 2 o’clock in the morning in the setting of STEMI in someone I’ve never met before. I don’t think there’s a rush to do anything then. Often in this middle-of-the-night stuff, we miss things or we overinterpret things. I think it’s better to let the patient cool down, get to know them,” according to Dr. Bell.
EXCEL
Publication of the 5-year outcomes of the largest-ever randomized trial of PCI versus coronary artery bypass grafting (CABG) for left main coronary disease has led to furious controversy, with a few of the surgeons involved in the study opting to publically broadcast allegations of misbehavior on the part of the interventional cardiologist study leadership, charges that have been strongly denied.
The actual results are in line with findings reported from smaller randomized trials. At 5 years in EXCEL, there was no significant difference between the PCI and CABG groups in the primary composite endpoint of death, cerebrovascular accident, or MI (N Engl J Med. 2019 Nov 7;381[19]:1820-30). The all-cause mortality rate was 13% in the PCI arm and 9.9% with CABG, but this finding comes with a caveat.
“I’ll emphasize this trial was never powered to look at mortality. Neither were any of the other randomized trials. On the other hand, I don’t think you can necessarily ignore the finding of an absolute 3.1% difference,” Dr. Bell said.
PCI and CABG are both very good, mature therapies for left main disease, in his view. In the setting of more-complex coronary disease in younger patients, he often views the complete revascularization offered by surgery as the preferred option. On the other hand, in an 80-year-old with severe comorbidities, clearly PCI is attractive.
He considers the highly public nature of this interspecialty spat a regrettable black eye for the entire field of cardiovascular medicine. And he predicted that an ongoing outside neutral-party review of the study data and procedures will conclude, as he has, “there was no malfeasance at all in the trial.”
Dr. Bell reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – A repeated theme threading through much of one prominent interventional cardiologist’s personal list of the top five coronary artery disease (CAD) trials of the past year is that aspirin is very often more trouble than it’s worth.
“For some years I’ve been concerned that the only thing that aspirin does [in patients after percutaneous coronary intervention] is increase your risk of bleeding. It doesn’t really provide any additional ischemic protection,” Malcolm R. Bell, MBBS, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“I’ll remind you that, when we go back to the early stent days, observed Dr. Bell, professor of medicine and vice chair of the department of cardiovascular medicine at the Mayo Clinic in Rochester, Minn.
Here are the key takeaway messages from his five most important randomized trials in CAD during the last year.
AUGUSTUS
For years, cardiologists have grappled with how to best manage high-cardiovascular-risk patients with atrial fibrillation who seem like they might benefit from triple-antithrombotic therapy. AUGUSTUS supplied the answer: Don’t do it. Skip the aspirin and turn instead to a P2Y12 inhibitor plus a non–vitamin K antagonist oral anticoagulant (NOAC), rather than warfarin.
“I would like you to think of triple therapy as a triple threat. That’s really what triple therapy is all about”– a three-pronged threat to patient safety, Dr. Bell commented.
In AUGUSTUS, 4,614 patients with atrial fibrillation and CAD with an acute coronary syndrome (ACS) and/or percutaneous coronary intervention (PCI) in 33 countries were placed on a P2Y12 inhibitor – most often clopidogrel – and randomized double blind to either apixaban (Eliquis) or warfarin, and further to aspirin or placebo, for 6 months of antithrombotic therapy. The strategy of a P2Y12 inhibitor and apixaban without aspirin was the clear winner, resulting in significantly less major bleeding, mortality, and hospitalizations than treatment with a P2Y12 inhibitor and warfarin, with or without aspirin. Most importantly, ischemic event rates didn’t differ between the apixaban and warfarin groups. And patients randomized to aspirin had rates of ischemic events and death or hospitalization similar to placebo-treated controls, meaning aspirin accomplished nothing (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
Dr. Bell noted that a meta-analysis of AUGUSTUS and three smaller randomized trials including more than 10,000 AUGUSTUS-type patients with atrial fibrillation concluded that a treatment strategy utilizing a NOAC and a P2Y12 inhibitor resulted in less bleeding than warfarin plus DAPT, and at no cost in terms of excess ischemic events. Moreover, regimens without aspirin resulted in less intracranial and other major bleeding without any difference in major adverse cardiovascular events (JAMA Cardiol. 2019 Jun 19. doi: 10.1001/jamacardio.2019.1880).
A key message of these four trials is that a NOAC is preferable to warfarin, so much so that, in high-risk patients who are already on warfarin, it’s worth considering a switch to a NOAC.
“And we should really be avoiding DAPT,” Dr. Bell added.
How soon after an ACS and/or PCI should patients with atrial fibrillation stop taking aspirin?
“In AUGUSTUS, randomization occurred at a median of 6 days, so we know that half the patients stopped their aspirin by then. In our own practice, we’re just dropping the aspirin for the most part before the patient leaves the hospital. I think if you leave them with instructions to stop the aspirin in a week’s time or a month’s time it just leads to confusion. And we should also remember that half of the major bleeding after PCI or ACS happens in the first 30 days, so it doesn’t make a lot of sense to say that we should continue it for a month and then drop it,” according to the cardiologist.
SMART-CHOICE and STOPDAPT-2
These two large multicenter studies demonstrate that DAPT can safely be stopped early if needed. SMART-CHOICE from South Korea and STOPDAPT-2 from Japan each randomized roughly 3,000 patients undergoing PCI to 12 months of DAPT or to DAPT for only 3 months or 1 month, respectively, at which point the aspirin was dropped and patients in the abbreviated DAPT arm continued on P2Y12 inhibitor monotherapy, mostly clopidogrel, for the remainder of the 12 months. In the Japanese STOPDAPT-2 trial, 1 month of DAPT proved superior to 12 months of DAPT for the primary composite endpoint of cardiovascular death, MI, stroke, definite stent thrombosis, or major or minor bleeding at 12 months (JAMA. 2019 Jun 25;321[24]:2414-27). In the South Korean SMART-CHOICE trial, 3 months of DAPT was noninferior to 12 months for major adverse cardiac and cerebrovascular events, and superior in terms of bleeding risk (JAMA. 2019 Jun 25;321[24]:2428-37). Of note, roughly half of patients in the two trials were lower-risk individuals undergoing PCI for stable angina.
Dr. Bell noted that, while the TWILIGHT trial (Ticagrelor With or Without Aspirin in High-Risk Patients After PCI) didn’t make his top-five list, it certainly fits well with the two East Asian studies. The TWILIGHT investigators randomized more than 7,000 patients to 12 months of DAPT or discontinuation of aspirin after 3 months. The result: a lower incidence of clinically relevant bleeding with ticagrelor monotherapy, and with no increased risk of death, MI, or stroke, compared with 12 months of DAPT (N Engl J Med. 2019 Nov 21;381[21]:2032-42).
“Again, I would just question what the added value of aspirin is here,” Dr. Bell commented. “Many interventional cardiologists are absolutely terrified of their patients having stent thrombosis, but with second-generation drug-eluting stents – the stents we’re putting in day in and day out – the risk of stent thrombosis is less than 1%. And in these two trials it was less than 0.5%. There’s more risk of having major bleeding events than there is of ischemia, so I think the balance is in favor of preventing bleeding. We know that major bleeding predicts short- and long-term mortality.”
COLCOT
This double-blind trial randomized 4,745 patients within 30 days post MI to low-dose colchicine or placebo on top of excellent rates of background guideline-directed medical therapy. The goal was to see if this anti-inflammatory agent could reduce cardiovascular events independent of any lipid-lowering effect, as was earlier seen with canakinumab in the CANTOS trial. It did so to a statistically significant but relatively modest degree, with a 5.5% rate of the composite cardiovascular events endpoint in the colchicine group and 7.1% in placebo-treated controls (N Engl J Med. 2019 Dec 26;381[26]:2497-505). But Dr. Bell was unimpressed.
“All-cause mortality was identical at 1.8% in both groups. So colchicine is not saving lives. In fact, the only real differences were in stroke – but the study wasn’t powered to look at stroke – and in urgent hospitalization for angina leading to revascularization, which is a soft endpoint,” he observed.
Plus, 2.5% of patients were lost to follow-up, which Dr. Bell considers “a little concerning” in a trial conducted in the current era.
“In my opinion, the evidence that colchicine is effective is weak, and I don’t think really supports the drug’s routine use post MI. We already send these patients out on numerous medications. We have to think about cost/benefit, and if a patient asks me: ‘Is this going to prevent another heart attack or make me live longer?’ I think the unequivocal answer is no,” he said.
These days colchicine is no longer an inexpensive drug, either, at an average cost of $300-$400 per month, the cardiologist added.
COMPLETE
This study randomized more than 4,000 patients with ST-segment elevation MI (STEMI) and multivessel disease to primary PCI of the culprit lesion only or to staged complete revascularization via PCI of all angiographically significant nonculprit lesions. Complete revascularization proved to be the superior strategy, with a 26% reduction in the risk of the composite of cardiovascular death or MI at a median of 3 years (N Engl J Med. 2019 Oct 10;381[15]:1411-21).
The optimal timing of the staged procedure remains unclear, since the study didn’t specify a protocol.
“I’m still a bit uncomfortable doing multivessel PCI at 2 o’clock in the morning in the setting of STEMI in someone I’ve never met before. I don’t think there’s a rush to do anything then. Often in this middle-of-the-night stuff, we miss things or we overinterpret things. I think it’s better to let the patient cool down, get to know them,” according to Dr. Bell.
EXCEL
Publication of the 5-year outcomes of the largest-ever randomized trial of PCI versus coronary artery bypass grafting (CABG) for left main coronary disease has led to furious controversy, with a few of the surgeons involved in the study opting to publically broadcast allegations of misbehavior on the part of the interventional cardiologist study leadership, charges that have been strongly denied.
The actual results are in line with findings reported from smaller randomized trials. At 5 years in EXCEL, there was no significant difference between the PCI and CABG groups in the primary composite endpoint of death, cerebrovascular accident, or MI (N Engl J Med. 2019 Nov 7;381[19]:1820-30). The all-cause mortality rate was 13% in the PCI arm and 9.9% with CABG, but this finding comes with a caveat.
“I’ll emphasize this trial was never powered to look at mortality. Neither were any of the other randomized trials. On the other hand, I don’t think you can necessarily ignore the finding of an absolute 3.1% difference,” Dr. Bell said.
PCI and CABG are both very good, mature therapies for left main disease, in his view. In the setting of more-complex coronary disease in younger patients, he often views the complete revascularization offered by surgery as the preferred option. On the other hand, in an 80-year-old with severe comorbidities, clearly PCI is attractive.
He considers the highly public nature of this interspecialty spat a regrettable black eye for the entire field of cardiovascular medicine. And he predicted that an ongoing outside neutral-party review of the study data and procedures will conclude, as he has, “there was no malfeasance at all in the trial.”
Dr. Bell reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – A repeated theme threading through much of one prominent interventional cardiologist’s personal list of the top five coronary artery disease (CAD) trials of the past year is that aspirin is very often more trouble than it’s worth.
“For some years I’ve been concerned that the only thing that aspirin does [in patients after percutaneous coronary intervention] is increase your risk of bleeding. It doesn’t really provide any additional ischemic protection,” Malcolm R. Bell, MBBS, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“I’ll remind you that, when we go back to the early stent days, observed Dr. Bell, professor of medicine and vice chair of the department of cardiovascular medicine at the Mayo Clinic in Rochester, Minn.
Here are the key takeaway messages from his five most important randomized trials in CAD during the last year.
AUGUSTUS
For years, cardiologists have grappled with how to best manage high-cardiovascular-risk patients with atrial fibrillation who seem like they might benefit from triple-antithrombotic therapy. AUGUSTUS supplied the answer: Don’t do it. Skip the aspirin and turn instead to a P2Y12 inhibitor plus a non–vitamin K antagonist oral anticoagulant (NOAC), rather than warfarin.
“I would like you to think of triple therapy as a triple threat. That’s really what triple therapy is all about”– a three-pronged threat to patient safety, Dr. Bell commented.
In AUGUSTUS, 4,614 patients with atrial fibrillation and CAD with an acute coronary syndrome (ACS) and/or percutaneous coronary intervention (PCI) in 33 countries were placed on a P2Y12 inhibitor – most often clopidogrel – and randomized double blind to either apixaban (Eliquis) or warfarin, and further to aspirin or placebo, for 6 months of antithrombotic therapy. The strategy of a P2Y12 inhibitor and apixaban without aspirin was the clear winner, resulting in significantly less major bleeding, mortality, and hospitalizations than treatment with a P2Y12 inhibitor and warfarin, with or without aspirin. Most importantly, ischemic event rates didn’t differ between the apixaban and warfarin groups. And patients randomized to aspirin had rates of ischemic events and death or hospitalization similar to placebo-treated controls, meaning aspirin accomplished nothing (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
Dr. Bell noted that a meta-analysis of AUGUSTUS and three smaller randomized trials including more than 10,000 AUGUSTUS-type patients with atrial fibrillation concluded that a treatment strategy utilizing a NOAC and a P2Y12 inhibitor resulted in less bleeding than warfarin plus DAPT, and at no cost in terms of excess ischemic events. Moreover, regimens without aspirin resulted in less intracranial and other major bleeding without any difference in major adverse cardiovascular events (JAMA Cardiol. 2019 Jun 19. doi: 10.1001/jamacardio.2019.1880).
A key message of these four trials is that a NOAC is preferable to warfarin, so much so that, in high-risk patients who are already on warfarin, it’s worth considering a switch to a NOAC.
“And we should really be avoiding DAPT,” Dr. Bell added.
How soon after an ACS and/or PCI should patients with atrial fibrillation stop taking aspirin?
“In AUGUSTUS, randomization occurred at a median of 6 days, so we know that half the patients stopped their aspirin by then. In our own practice, we’re just dropping the aspirin for the most part before the patient leaves the hospital. I think if you leave them with instructions to stop the aspirin in a week’s time or a month’s time it just leads to confusion. And we should also remember that half of the major bleeding after PCI or ACS happens in the first 30 days, so it doesn’t make a lot of sense to say that we should continue it for a month and then drop it,” according to the cardiologist.
SMART-CHOICE and STOPDAPT-2
These two large multicenter studies demonstrate that DAPT can safely be stopped early if needed. SMART-CHOICE from South Korea and STOPDAPT-2 from Japan each randomized roughly 3,000 patients undergoing PCI to 12 months of DAPT or to DAPT for only 3 months or 1 month, respectively, at which point the aspirin was dropped and patients in the abbreviated DAPT arm continued on P2Y12 inhibitor monotherapy, mostly clopidogrel, for the remainder of the 12 months. In the Japanese STOPDAPT-2 trial, 1 month of DAPT proved superior to 12 months of DAPT for the primary composite endpoint of cardiovascular death, MI, stroke, definite stent thrombosis, or major or minor bleeding at 12 months (JAMA. 2019 Jun 25;321[24]:2414-27). In the South Korean SMART-CHOICE trial, 3 months of DAPT was noninferior to 12 months for major adverse cardiac and cerebrovascular events, and superior in terms of bleeding risk (JAMA. 2019 Jun 25;321[24]:2428-37). Of note, roughly half of patients in the two trials were lower-risk individuals undergoing PCI for stable angina.
Dr. Bell noted that, while the TWILIGHT trial (Ticagrelor With or Without Aspirin in High-Risk Patients After PCI) didn’t make his top-five list, it certainly fits well with the two East Asian studies. The TWILIGHT investigators randomized more than 7,000 patients to 12 months of DAPT or discontinuation of aspirin after 3 months. The result: a lower incidence of clinically relevant bleeding with ticagrelor monotherapy, and with no increased risk of death, MI, or stroke, compared with 12 months of DAPT (N Engl J Med. 2019 Nov 21;381[21]:2032-42).
“Again, I would just question what the added value of aspirin is here,” Dr. Bell commented. “Many interventional cardiologists are absolutely terrified of their patients having stent thrombosis, but with second-generation drug-eluting stents – the stents we’re putting in day in and day out – the risk of stent thrombosis is less than 1%. And in these two trials it was less than 0.5%. There’s more risk of having major bleeding events than there is of ischemia, so I think the balance is in favor of preventing bleeding. We know that major bleeding predicts short- and long-term mortality.”
COLCOT
This double-blind trial randomized 4,745 patients within 30 days post MI to low-dose colchicine or placebo on top of excellent rates of background guideline-directed medical therapy. The goal was to see if this anti-inflammatory agent could reduce cardiovascular events independent of any lipid-lowering effect, as was earlier seen with canakinumab in the CANTOS trial. It did so to a statistically significant but relatively modest degree, with a 5.5% rate of the composite cardiovascular events endpoint in the colchicine group and 7.1% in placebo-treated controls (N Engl J Med. 2019 Dec 26;381[26]:2497-505). But Dr. Bell was unimpressed.
“All-cause mortality was identical at 1.8% in both groups. So colchicine is not saving lives. In fact, the only real differences were in stroke – but the study wasn’t powered to look at stroke – and in urgent hospitalization for angina leading to revascularization, which is a soft endpoint,” he observed.
Plus, 2.5% of patients were lost to follow-up, which Dr. Bell considers “a little concerning” in a trial conducted in the current era.
“In my opinion, the evidence that colchicine is effective is weak, and I don’t think really supports the drug’s routine use post MI. We already send these patients out on numerous medications. We have to think about cost/benefit, and if a patient asks me: ‘Is this going to prevent another heart attack or make me live longer?’ I think the unequivocal answer is no,” he said.
These days colchicine is no longer an inexpensive drug, either, at an average cost of $300-$400 per month, the cardiologist added.
COMPLETE
This study randomized more than 4,000 patients with ST-segment elevation MI (STEMI) and multivessel disease to primary PCI of the culprit lesion only or to staged complete revascularization via PCI of all angiographically significant nonculprit lesions. Complete revascularization proved to be the superior strategy, with a 26% reduction in the risk of the composite of cardiovascular death or MI at a median of 3 years (N Engl J Med. 2019 Oct 10;381[15]:1411-21).
The optimal timing of the staged procedure remains unclear, since the study didn’t specify a protocol.
“I’m still a bit uncomfortable doing multivessel PCI at 2 o’clock in the morning in the setting of STEMI in someone I’ve never met before. I don’t think there’s a rush to do anything then. Often in this middle-of-the-night stuff, we miss things or we overinterpret things. I think it’s better to let the patient cool down, get to know them,” according to Dr. Bell.
EXCEL
Publication of the 5-year outcomes of the largest-ever randomized trial of PCI versus coronary artery bypass grafting (CABG) for left main coronary disease has led to furious controversy, with a few of the surgeons involved in the study opting to publically broadcast allegations of misbehavior on the part of the interventional cardiologist study leadership, charges that have been strongly denied.
The actual results are in line with findings reported from smaller randomized trials. At 5 years in EXCEL, there was no significant difference between the PCI and CABG groups in the primary composite endpoint of death, cerebrovascular accident, or MI (N Engl J Med. 2019 Nov 7;381[19]:1820-30). The all-cause mortality rate was 13% in the PCI arm and 9.9% with CABG, but this finding comes with a caveat.
“I’ll emphasize this trial was never powered to look at mortality. Neither were any of the other randomized trials. On the other hand, I don’t think you can necessarily ignore the finding of an absolute 3.1% difference,” Dr. Bell said.
PCI and CABG are both very good, mature therapies for left main disease, in his view. In the setting of more-complex coronary disease in younger patients, he often views the complete revascularization offered by surgery as the preferred option. On the other hand, in an 80-year-old with severe comorbidities, clearly PCI is attractive.
He considers the highly public nature of this interspecialty spat a regrettable black eye for the entire field of cardiovascular medicine. And he predicted that an ongoing outside neutral-party review of the study data and procedures will conclude, as he has, “there was no malfeasance at all in the trial.”
Dr. Bell reported having no financial conflicts regarding his presentation.
REPORTING FROM ACC SNOWMASS 2020
Vigilance safely keeps AFib patients off anticoagulants post ablation
NATIONAL HARBOR, MD. – A pilot program of daily arrhythmia self-vigilance has allowed selected patients with no atrial fibrillation following a catheter ablation procedure to safely come off a regimen of daily oral anticoagulation despite having residual risk factors for ischemic stroke.
This program, which started several years ago at the University of Pennsylvania in Philadelphia, has now managed 190 patients and followed them for a median of just over 3 years, and during 576 patient-years of follow-up, just a single patient had an ischemic cerebrovascular event that occurred with no atrial fibrillation (AFib) recurrence and appeared to be caused by an atherosclerotic embolism, Francis E. Marchlinski, MD, said at the annual International AF Symposium.
Although this strategy has not yet been tested in a prospective, randomized trial, this anecdotal, single-center experience suggests that the approach is “safe and effective” for selected patients who are eager to come off of their anticoagulation regimen when they remain arrhythmia free following catheter ablation of their AFib, said Dr. Marchlinski, professor of medicine and director of electrophysiology at the University of Pennsylvania. He and his associates developed this strategy as a way to more safely allow these patients to stop taking a daily oral anticoagulant because he found that many patients were stopping on their own, with no safety strategy in place.
“Patients tell me they don’t want to be on an oral anticoagulant because a parent had a hemorrhagic stroke, and they say they’re willing to accept the risk” of having an ischemic stroke by coming off anticoagulation. “This is a way for them to do it safely,” Dr. Marchlinski said in an interview. He stressed that he only allows his patients to go this route if they understand the risk and accept their shared responsibility for vigilant, twice-daily pulse monitoring to detect resumption of an irregular heart beat.
Since 2011, Dr. Marchlinski’s program ablated 1,216 patients with AFib who then remained arrhythmia free during 3 weeks of continuous ECG monitoring following their procedure. Among these patients, 443 had a CHA2DS2-VAScscore of either 0 (men) or 1 (women) that indicated no ongoing need for oral anticoagulation according to current guidelines. Of the remaining 773 patients with a CHA2DS2-VASc score of at least 1 in men and 2 in women, the clinicians determined 583 to be ineligible for the program because of their unwillingness to accept the risk, unwillingness to comply with daily pulse checks, a history of asymptomatic AFib, a CHA2DS2-VASc score greater than 4, or a resting pulse above 90 beats per minute, leaving 190 patients eligible to participate. Among these patients, 105 (55%) had a CHA2DS2-VASc score of 2-4, which should prompt anticoagulation according to current guidelines.Participating patients committed to check their resting pulse by palpation at least twice daily and to contacting the program immediately if their resting rate spiked by more than 20 beats per minutes or in another way seemed irregular. Patients were also instructed to restart their oral anticoagulation immediately if they experienced AFib symptoms that persisted for more than 5 minutes. Many patients in the program also use a wearable device (usually a watch) to monitor their resting pulse and to generate a 30-second ECG recording that they can send as an electronic file to the University of Pennsylvania staff. “We embrace wearables,” Dr. Marchlinski said. Those without a wearable can undergo transtelephonic EEG monitoring to document a suspected arrhythmia recurrence, and all patients undergo annual monitoring by continuous ECG for at least 2 weeks.During follow-up, in addition to the 1 patient free from recurrent AFib who had an atherosclerotic embolism, 34 patients resumed anticoagulant treatment because of AFib recurrence; 12 withdrew from the program because of noncompliance or preference, or because an exclusion appeared; 29 resumed oral anticoagulation transiently but then discontinued the drug a second time when their AFib recurrence resolved; and 114 patients (60% of the starting cohort of 190) remained completely off anticoagulation during a median of 37 months. These data updated a published report from Dr. Marchlinski and his associates on their first 99 patients followed for a median of 30 months (J Cardiovasc Electrophysiol. 2019 May;30[5]:631-8).
This experience underscored the need for ongoing rhythm monitoring even in the absence of AFib symptoms, as six patients developed asymptomatic AFib detected by monitoring, including one patient whose recurrence occurred 30 months after the ablation procedure.
Dr. Marchlinski stressed the stringent selection process he applies to limit this approach to patients who are willing to faithfully monitor their pulse and symptoms daily, and who accept the risk that this approach may pose and their responsibility to stay in contact with the clinical team. The program calls patients at the 6-month mark between annual monitoring to remind them of their need for daily attention.
“Being off anticoagulants is very important to these patients,” he explained, and he highlighted the added workload this strategy places on his staff. “I think this has legs” for adoption by other cardiac arrhythmia programs, “but it depends on the time the staff is willing to spend” monitoring and following these patients, some of whom regularly send in ECG traces from their wearable devices for assessment. “It takes a village” to make this program work, he said.
Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
NATIONAL HARBOR, MD. – A pilot program of daily arrhythmia self-vigilance has allowed selected patients with no atrial fibrillation following a catheter ablation procedure to safely come off a regimen of daily oral anticoagulation despite having residual risk factors for ischemic stroke.
This program, which started several years ago at the University of Pennsylvania in Philadelphia, has now managed 190 patients and followed them for a median of just over 3 years, and during 576 patient-years of follow-up, just a single patient had an ischemic cerebrovascular event that occurred with no atrial fibrillation (AFib) recurrence and appeared to be caused by an atherosclerotic embolism, Francis E. Marchlinski, MD, said at the annual International AF Symposium.
Although this strategy has not yet been tested in a prospective, randomized trial, this anecdotal, single-center experience suggests that the approach is “safe and effective” for selected patients who are eager to come off of their anticoagulation regimen when they remain arrhythmia free following catheter ablation of their AFib, said Dr. Marchlinski, professor of medicine and director of electrophysiology at the University of Pennsylvania. He and his associates developed this strategy as a way to more safely allow these patients to stop taking a daily oral anticoagulant because he found that many patients were stopping on their own, with no safety strategy in place.
“Patients tell me they don’t want to be on an oral anticoagulant because a parent had a hemorrhagic stroke, and they say they’re willing to accept the risk” of having an ischemic stroke by coming off anticoagulation. “This is a way for them to do it safely,” Dr. Marchlinski said in an interview. He stressed that he only allows his patients to go this route if they understand the risk and accept their shared responsibility for vigilant, twice-daily pulse monitoring to detect resumption of an irregular heart beat.
Since 2011, Dr. Marchlinski’s program ablated 1,216 patients with AFib who then remained arrhythmia free during 3 weeks of continuous ECG monitoring following their procedure. Among these patients, 443 had a CHA2DS2-VAScscore of either 0 (men) or 1 (women) that indicated no ongoing need for oral anticoagulation according to current guidelines. Of the remaining 773 patients with a CHA2DS2-VASc score of at least 1 in men and 2 in women, the clinicians determined 583 to be ineligible for the program because of their unwillingness to accept the risk, unwillingness to comply with daily pulse checks, a history of asymptomatic AFib, a CHA2DS2-VASc score greater than 4, or a resting pulse above 90 beats per minute, leaving 190 patients eligible to participate. Among these patients, 105 (55%) had a CHA2DS2-VASc score of 2-4, which should prompt anticoagulation according to current guidelines.Participating patients committed to check their resting pulse by palpation at least twice daily and to contacting the program immediately if their resting rate spiked by more than 20 beats per minutes or in another way seemed irregular. Patients were also instructed to restart their oral anticoagulation immediately if they experienced AFib symptoms that persisted for more than 5 minutes. Many patients in the program also use a wearable device (usually a watch) to monitor their resting pulse and to generate a 30-second ECG recording that they can send as an electronic file to the University of Pennsylvania staff. “We embrace wearables,” Dr. Marchlinski said. Those without a wearable can undergo transtelephonic EEG monitoring to document a suspected arrhythmia recurrence, and all patients undergo annual monitoring by continuous ECG for at least 2 weeks.During follow-up, in addition to the 1 patient free from recurrent AFib who had an atherosclerotic embolism, 34 patients resumed anticoagulant treatment because of AFib recurrence; 12 withdrew from the program because of noncompliance or preference, or because an exclusion appeared; 29 resumed oral anticoagulation transiently but then discontinued the drug a second time when their AFib recurrence resolved; and 114 patients (60% of the starting cohort of 190) remained completely off anticoagulation during a median of 37 months. These data updated a published report from Dr. Marchlinski and his associates on their first 99 patients followed for a median of 30 months (J Cardiovasc Electrophysiol. 2019 May;30[5]:631-8).
This experience underscored the need for ongoing rhythm monitoring even in the absence of AFib symptoms, as six patients developed asymptomatic AFib detected by monitoring, including one patient whose recurrence occurred 30 months after the ablation procedure.
Dr. Marchlinski stressed the stringent selection process he applies to limit this approach to patients who are willing to faithfully monitor their pulse and symptoms daily, and who accept the risk that this approach may pose and their responsibility to stay in contact with the clinical team. The program calls patients at the 6-month mark between annual monitoring to remind them of their need for daily attention.
“Being off anticoagulants is very important to these patients,” he explained, and he highlighted the added workload this strategy places on his staff. “I think this has legs” for adoption by other cardiac arrhythmia programs, “but it depends on the time the staff is willing to spend” monitoring and following these patients, some of whom regularly send in ECG traces from their wearable devices for assessment. “It takes a village” to make this program work, he said.
Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
NATIONAL HARBOR, MD. – A pilot program of daily arrhythmia self-vigilance has allowed selected patients with no atrial fibrillation following a catheter ablation procedure to safely come off a regimen of daily oral anticoagulation despite having residual risk factors for ischemic stroke.
This program, which started several years ago at the University of Pennsylvania in Philadelphia, has now managed 190 patients and followed them for a median of just over 3 years, and during 576 patient-years of follow-up, just a single patient had an ischemic cerebrovascular event that occurred with no atrial fibrillation (AFib) recurrence and appeared to be caused by an atherosclerotic embolism, Francis E. Marchlinski, MD, said at the annual International AF Symposium.
Although this strategy has not yet been tested in a prospective, randomized trial, this anecdotal, single-center experience suggests that the approach is “safe and effective” for selected patients who are eager to come off of their anticoagulation regimen when they remain arrhythmia free following catheter ablation of their AFib, said Dr. Marchlinski, professor of medicine and director of electrophysiology at the University of Pennsylvania. He and his associates developed this strategy as a way to more safely allow these patients to stop taking a daily oral anticoagulant because he found that many patients were stopping on their own, with no safety strategy in place.
“Patients tell me they don’t want to be on an oral anticoagulant because a parent had a hemorrhagic stroke, and they say they’re willing to accept the risk” of having an ischemic stroke by coming off anticoagulation. “This is a way for them to do it safely,” Dr. Marchlinski said in an interview. He stressed that he only allows his patients to go this route if they understand the risk and accept their shared responsibility for vigilant, twice-daily pulse monitoring to detect resumption of an irregular heart beat.
Since 2011, Dr. Marchlinski’s program ablated 1,216 patients with AFib who then remained arrhythmia free during 3 weeks of continuous ECG monitoring following their procedure. Among these patients, 443 had a CHA2DS2-VAScscore of either 0 (men) or 1 (women) that indicated no ongoing need for oral anticoagulation according to current guidelines. Of the remaining 773 patients with a CHA2DS2-VASc score of at least 1 in men and 2 in women, the clinicians determined 583 to be ineligible for the program because of their unwillingness to accept the risk, unwillingness to comply with daily pulse checks, a history of asymptomatic AFib, a CHA2DS2-VASc score greater than 4, or a resting pulse above 90 beats per minute, leaving 190 patients eligible to participate. Among these patients, 105 (55%) had a CHA2DS2-VASc score of 2-4, which should prompt anticoagulation according to current guidelines.Participating patients committed to check their resting pulse by palpation at least twice daily and to contacting the program immediately if their resting rate spiked by more than 20 beats per minutes or in another way seemed irregular. Patients were also instructed to restart their oral anticoagulation immediately if they experienced AFib symptoms that persisted for more than 5 minutes. Many patients in the program also use a wearable device (usually a watch) to monitor their resting pulse and to generate a 30-second ECG recording that they can send as an electronic file to the University of Pennsylvania staff. “We embrace wearables,” Dr. Marchlinski said. Those without a wearable can undergo transtelephonic EEG monitoring to document a suspected arrhythmia recurrence, and all patients undergo annual monitoring by continuous ECG for at least 2 weeks.During follow-up, in addition to the 1 patient free from recurrent AFib who had an atherosclerotic embolism, 34 patients resumed anticoagulant treatment because of AFib recurrence; 12 withdrew from the program because of noncompliance or preference, or because an exclusion appeared; 29 resumed oral anticoagulation transiently but then discontinued the drug a second time when their AFib recurrence resolved; and 114 patients (60% of the starting cohort of 190) remained completely off anticoagulation during a median of 37 months. These data updated a published report from Dr. Marchlinski and his associates on their first 99 patients followed for a median of 30 months (J Cardiovasc Electrophysiol. 2019 May;30[5]:631-8).
This experience underscored the need for ongoing rhythm monitoring even in the absence of AFib symptoms, as six patients developed asymptomatic AFib detected by monitoring, including one patient whose recurrence occurred 30 months after the ablation procedure.
Dr. Marchlinski stressed the stringent selection process he applies to limit this approach to patients who are willing to faithfully monitor their pulse and symptoms daily, and who accept the risk that this approach may pose and their responsibility to stay in contact with the clinical team. The program calls patients at the 6-month mark between annual monitoring to remind them of their need for daily attention.
“Being off anticoagulants is very important to these patients,” he explained, and he highlighted the added workload this strategy places on his staff. “I think this has legs” for adoption by other cardiac arrhythmia programs, “but it depends on the time the staff is willing to spend” monitoring and following these patients, some of whom regularly send in ECG traces from their wearable devices for assessment. “It takes a village” to make this program work, he said.
Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
REPORTING FROM THE AF SYMPOSIUM 2020
DOACs for treatment of cancer-associated venous thromboembolism
Bleeding risk may determine best option
Case
A 52-year-old female with past medical history of diabetes, hypertension, and stage 4 lung cancer on palliative chemotherapy presents with acute-onset dyspnea, pleuritic chest pain, and cough. Her exam is notable for tachycardia, hypoxemia, and diminished breath sounds. A CT pulmonary embolism study shows new left segmental thrombus. What is her preferred method of anticoagulation?
Brief overview of the issue
Venous thromboembolism (VTE) including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a significant concern in the context of malignancy and is associated with higher rates of mortality at 1 year.
The standard of care in the recent past has relied on low-molecular-weight heparin (LMWH) after several trials showed decreased VTE recurrence in cancer patients, compared with vitamin K antagonist (VKA) treatment.1,2 LMWH has been recommended as a first-line treatment by clinical guidelines for cancer-related VTE given lower drug-drug interactions between LMWH and chemotherapy regimens, as compared with traditional VKAs, and it does not rely on intestinal absorption.3
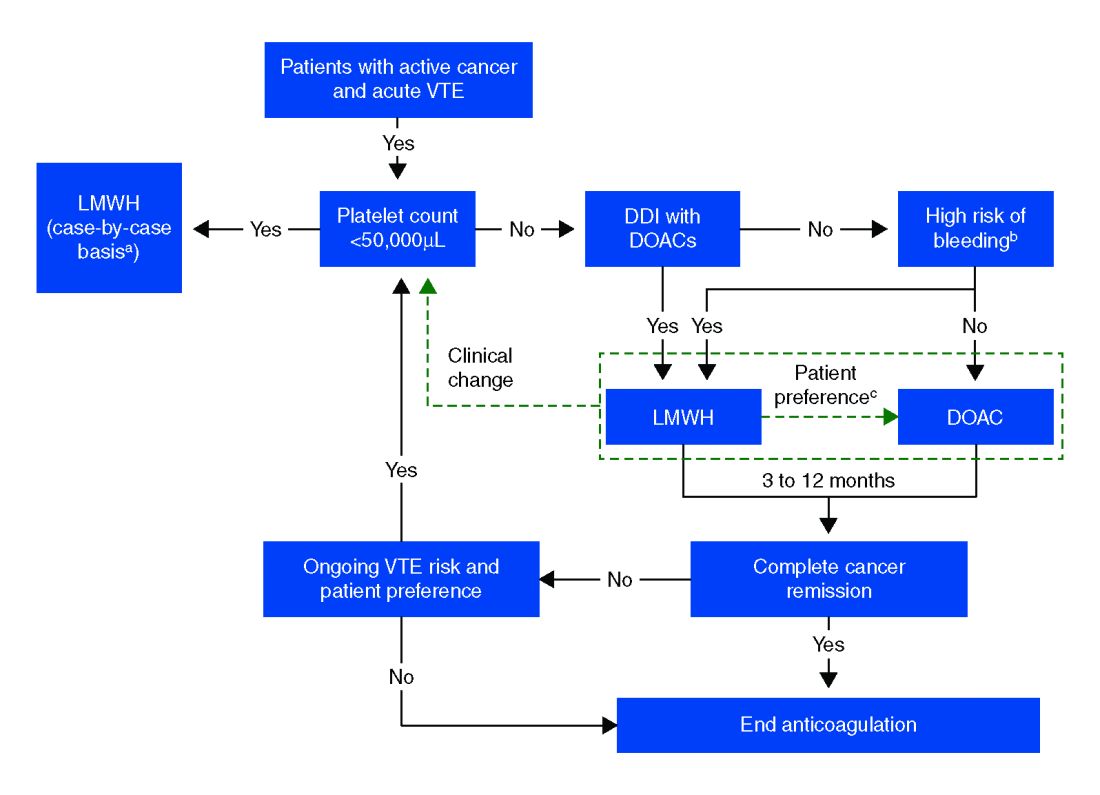
In more recent years, the focus has shifted to direct oral anticoagulants (DOACs) as potential treatment options for cancer-related VTE given their ease of administration, low side-effect profile, and decreased cost. Until recently, studies have mainly been small and largely retrospective, however, several larger randomized control studies have recently been published.
Overview of the data
Several retrospective trials have investigated the use of DOACs in cancer-associated VTE. One study looking at VTE recurrence rates showed a trend towards lower rates with rivaroxaban, compared with LMWH at 6 months (13% vs. 17%) that was significantly lower at 12 months (16.5 % vs. 22%). Similar results were found when comparing rivaroxaban to warfarin. Major bleeding rates were similar among cohorts.4
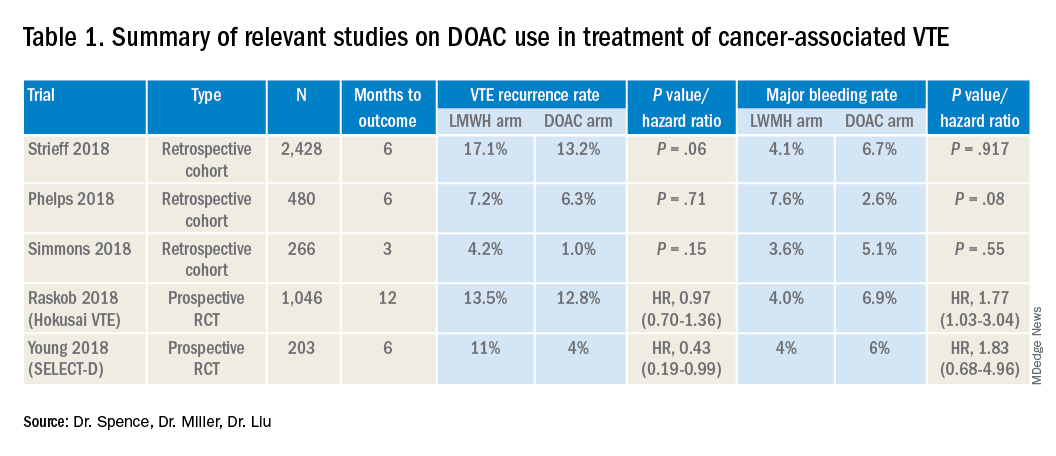
Several other retrospective cohort studies looking at treatment of cancer-associated VTE treated with LMWH vs. DOACs found that overall patients treated with DOACs had cancers with lower risk for VTE and had lower burden of metastatic disease. When this was adjusted for, there was no significant difference in the rate of recurrent cancer-associated thrombosis or major bleeding.5,6
Recently several prospective studies have corroborated the noninferiority or slight superiority of DOACs when compared with LMWH in treatment of cancer-associated VTE, while showing similar rates of bleeding. These are summarized as follows: a prospective, open-label, randomized controlled (RCT), noninferiority trial of 1,046 patients with malignancy-related VTE assigned to either LMWH for at least 5 days, followed by oral edoxaban vs. subcutaneous dalteparin for at least 6 months and up to 12 months. Investigators found no significant difference in the rate of recurrent VTE in the edoxaban group (12.8%), as compared to the dalteparin group (13.5%, P = .006 for noninferiority). Risk of major bleeding was not significantly different between the groups.7
A small RCT of 203 patients comparing recurrent VTE rates with rivaroxaban vs. dalteparin found significantly fewer recurrent clots in the rivaroxaban group compared to the dalteparin group (11% vs 4%) with no significant difference in the 6-month cumulative rate of major bleeding, 4% in the dalteparin group and 6% for the rivaroxaban group.8 Preliminary results from the ADAM VTE trial comparing apixaban to dalteparin found significantly fewer recurrent VTE in the apixaban group (3.4% vs. 14.1%) with no significant difference in major bleeding events (0% vs 2.1%).9 The Caravaggio study is a large multinational randomized, controlled, open-label, noninferiority trial looking at apixaban vs. dalteparin with endpoints being 6-month recurrent VTE and bleeding risk that will likely report results soon.
Risk of bleeding is also a major consideration in VTE treatment as studies suggest that patients with metastatic cancer are at sixfold higher risk for anticoagulant-associated bleeding.3 Subgroup analysis of Hokusai VTE cancer study found that major bleeding occurred in 32 of 522 patients given edoxaban and 16 of 524 patients treated with dalteparin. Excess of major bleeding with edoxaban was confined to patients with GI cancer. However, rates of severe major bleeding at presentation were similar.10
Overall, the existing data suggests that DOACs may be a viable option in the treatment of malignancy-associated VTE given its similar efficacy in preventing recurrent VTE without significant increased risk of major bleeding. The 2018 International Society on Thrombosis and Haemostasis VTE in cancer guidelines have been updated to include rivaroxaban and edoxaban for use in patients at low risk of bleeding, but recommend an informed discussion between patients and clinicians in deciding between DOAC and LMWH.11 The Chest VTE guidelines have not been updated since 2016, prior to when the above mentioned DOAC studies were published.
Application of data to our patient
Compared with patients without cancer, anticoagulation in cancer patients with acute VTE is challenging because of higher rates of VTE recurrence and bleeding, as well as the potential for drug interactions with anticancer agents. Our patient is not at increased risk for gastrointestinal bleeding and no drug interactions exist between her current chemotherapy regimen and the available DOACs, therefore she is a candidate for treatment with a DOAC.
After an informed discussion, she chose to start rivaroxaban for treatment of her pulmonary embolism. While more studies are needed to definitively determine the best treatment for cancer-associated VTE, DOACs appear to be an attractive alternative to LMWH. Patient preferences of taking oral medications over injections as well as the significant cost savings of DOACs over LMWH will likely play into many patients’ and providers’ anticoagulant choices.
Bottom line
Direct oral anticoagulants are a treatment option for cancer-associated VTE in patients at low risk of bleeding complications. Patients at increased risk of bleeding (especially patients with GI malignancies) should continue to be treated with LMWH.
Dr. Spence is a hospitalist and palliative care physician at Denver Health, and an assistant professor of medicine at the University of Colorado at Denver, Aurora. Dr. Miller and Dr. Liu are hospitalists at Denver Health, and assistant professors of medicine at the University of Colorado at Denver.
References
1. Hull RD et al. Long term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patient with cancer. Am J Med. 2006;19(12):1062-72.
2. Lee AY et al. Low-molecular-weight heparin versus Coumadin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-53.
3. Ay C et al. Treatment of cancer-associated venous thromboembolism in the age of direct oral anticoagulants. Ann Oncol. 2019 Mar 27 [epub].
4. Streiff MB et al. Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am J Hematol. 2018 May;93(5):664-71.
5. Phelps MK et al. A single center retrospective cohort study comparing low-molecular-weight heparins to direct oral anticoagulants for the treatment of venous thromboembolism in patients with cancer – A real-world experience. J Oncol Pharm Pract. 2019 Jun;25(4):793-800.
6. Simmons B et al. Efficacy and safety of rivaroxaban compared to enoxaparin in treatment of cancer-associated venous thromboembolism. Eur J Haematol. 2018 Apr 4. (Epub).
7. Raskob GE et al.; Hokusai VTE Cancer Investigators. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018 Feb 15;378(7):615-24.
8. Young AM et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT-D). J Clin Oncol. 2018 Jul 10;36(20):2017-23.
9. McBane, RD et al. Apixaban, dalteparin, in active cancer associated venous thromboembolism, the ADAM VTE trial. Blood. 2018 Nov 29;132(suppl 1):421.
10. Kraaijpoel N et al. Clinical impact of bleeding in cancer-associated venous thromboembolism: Results from the Hokusai VTE cancer study. Thromb Haemost. 2018 Aug;118(8):1439-49.
11. Khorana AA et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J Thromb Haemost. 2018 Sep;16(9):1891-94.
Key points
- DOACs are a reasonable treatment option for malignancy-associated VTE in patients without GI tract malignancies and at low risk for bleeding complications.
- In patients with gastrointestinal malignancies or increased risk of bleeding, DOACs may have an increased bleeding risk and therefore LMWH is recommended.
- An informed discussion should occur between providers and patients to determine the best treatment option for cancer patients with VTE.
Additional reading
Dong Y et al. Efficacy and safety of direct oral anticoagulants versus low-molecular-weight heparin in patients with cancer: A systematic review and meta-analysis. J Thromb Thrombolysis. 2019 May 6.
Khorana AA et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost. 2018 Sep;16(9):1891-94.
Tritschler T et al. Venous thromboembolism advances in diagnosis and treatment. JAMA. 2018 Oct;320(15):1583-94.
Quiz
Which of the following is the recommended treatment of VTE in a patient with brain metastases?
A. Unfractionated heparin
B. Low molecular weight heparin
C. Direct oral anticoagulant
D. Vitamin K antagonist
The answer is B. Although there are very few data, LMWH is the recommended agent in patients with VTE and brain metastases.
A. LMWH has been shown to decrease mortality in patients with VTE and cancer, compared with unfractionated heparin (risk ratio, 0.66).
C. The safety of DOACs is not yet well established in patients with brain tumors. Antidotes and/or specific reversal agents for some DOACs are not available.
D. Vitamin K antagonists such as warfarin are not recommended in cancer patients because LMWH has a reduced risk of recurrent VTE without increased risk of bleeding.
Bleeding risk may determine best option
Bleeding risk may determine best option
Case
A 52-year-old female with past medical history of diabetes, hypertension, and stage 4 lung cancer on palliative chemotherapy presents with acute-onset dyspnea, pleuritic chest pain, and cough. Her exam is notable for tachycardia, hypoxemia, and diminished breath sounds. A CT pulmonary embolism study shows new left segmental thrombus. What is her preferred method of anticoagulation?
Brief overview of the issue
Venous thromboembolism (VTE) including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a significant concern in the context of malignancy and is associated with higher rates of mortality at 1 year.
The standard of care in the recent past has relied on low-molecular-weight heparin (LMWH) after several trials showed decreased VTE recurrence in cancer patients, compared with vitamin K antagonist (VKA) treatment.1,2 LMWH has been recommended as a first-line treatment by clinical guidelines for cancer-related VTE given lower drug-drug interactions between LMWH and chemotherapy regimens, as compared with traditional VKAs, and it does not rely on intestinal absorption.3

In more recent years, the focus has shifted to direct oral anticoagulants (DOACs) as potential treatment options for cancer-related VTE given their ease of administration, low side-effect profile, and decreased cost. Until recently, studies have mainly been small and largely retrospective, however, several larger randomized control studies have recently been published.
Overview of the data
Several retrospective trials have investigated the use of DOACs in cancer-associated VTE. One study looking at VTE recurrence rates showed a trend towards lower rates with rivaroxaban, compared with LMWH at 6 months (13% vs. 17%) that was significantly lower at 12 months (16.5 % vs. 22%). Similar results were found when comparing rivaroxaban to warfarin. Major bleeding rates were similar among cohorts.4

Several other retrospective cohort studies looking at treatment of cancer-associated VTE treated with LMWH vs. DOACs found that overall patients treated with DOACs had cancers with lower risk for VTE and had lower burden of metastatic disease. When this was adjusted for, there was no significant difference in the rate of recurrent cancer-associated thrombosis or major bleeding.5,6
Recently several prospective studies have corroborated the noninferiority or slight superiority of DOACs when compared with LMWH in treatment of cancer-associated VTE, while showing similar rates of bleeding. These are summarized as follows: a prospective, open-label, randomized controlled (RCT), noninferiority trial of 1,046 patients with malignancy-related VTE assigned to either LMWH for at least 5 days, followed by oral edoxaban vs. subcutaneous dalteparin for at least 6 months and up to 12 months. Investigators found no significant difference in the rate of recurrent VTE in the edoxaban group (12.8%), as compared to the dalteparin group (13.5%, P = .006 for noninferiority). Risk of major bleeding was not significantly different between the groups.7
A small RCT of 203 patients comparing recurrent VTE rates with rivaroxaban vs. dalteparin found significantly fewer recurrent clots in the rivaroxaban group compared to the dalteparin group (11% vs 4%) with no significant difference in the 6-month cumulative rate of major bleeding, 4% in the dalteparin group and 6% for the rivaroxaban group.8 Preliminary results from the ADAM VTE trial comparing apixaban to dalteparin found significantly fewer recurrent VTE in the apixaban group (3.4% vs. 14.1%) with no significant difference in major bleeding events (0% vs 2.1%).9 The Caravaggio study is a large multinational randomized, controlled, open-label, noninferiority trial looking at apixaban vs. dalteparin with endpoints being 6-month recurrent VTE and bleeding risk that will likely report results soon.
Risk of bleeding is also a major consideration in VTE treatment as studies suggest that patients with metastatic cancer are at sixfold higher risk for anticoagulant-associated bleeding.3 Subgroup analysis of Hokusai VTE cancer study found that major bleeding occurred in 32 of 522 patients given edoxaban and 16 of 524 patients treated with dalteparin. Excess of major bleeding with edoxaban was confined to patients with GI cancer. However, rates of severe major bleeding at presentation were similar.10
Overall, the existing data suggests that DOACs may be a viable option in the treatment of malignancy-associated VTE given its similar efficacy in preventing recurrent VTE without significant increased risk of major bleeding. The 2018 International Society on Thrombosis and Haemostasis VTE in cancer guidelines have been updated to include rivaroxaban and edoxaban for use in patients at low risk of bleeding, but recommend an informed discussion between patients and clinicians in deciding between DOAC and LMWH.11 The Chest VTE guidelines have not been updated since 2016, prior to when the above mentioned DOAC studies were published.
Application of data to our patient
Compared with patients without cancer, anticoagulation in cancer patients with acute VTE is challenging because of higher rates of VTE recurrence and bleeding, as well as the potential for drug interactions with anticancer agents. Our patient is not at increased risk for gastrointestinal bleeding and no drug interactions exist between her current chemotherapy regimen and the available DOACs, therefore she is a candidate for treatment with a DOAC.
After an informed discussion, she chose to start rivaroxaban for treatment of her pulmonary embolism. While more studies are needed to definitively determine the best treatment for cancer-associated VTE, DOACs appear to be an attractive alternative to LMWH. Patient preferences of taking oral medications over injections as well as the significant cost savings of DOACs over LMWH will likely play into many patients’ and providers’ anticoagulant choices.
Bottom line
Direct oral anticoagulants are a treatment option for cancer-associated VTE in patients at low risk of bleeding complications. Patients at increased risk of bleeding (especially patients with GI malignancies) should continue to be treated with LMWH.
Dr. Spence is a hospitalist and palliative care physician at Denver Health, and an assistant professor of medicine at the University of Colorado at Denver, Aurora. Dr. Miller and Dr. Liu are hospitalists at Denver Health, and assistant professors of medicine at the University of Colorado at Denver.
References
1. Hull RD et al. Long term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patient with cancer. Am J Med. 2006;19(12):1062-72.
2. Lee AY et al. Low-molecular-weight heparin versus Coumadin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-53.
3. Ay C et al. Treatment of cancer-associated venous thromboembolism in the age of direct oral anticoagulants. Ann Oncol. 2019 Mar 27 [epub].
4. Streiff MB et al. Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am J Hematol. 2018 May;93(5):664-71.
5. Phelps MK et al. A single center retrospective cohort study comparing low-molecular-weight heparins to direct oral anticoagulants for the treatment of venous thromboembolism in patients with cancer – A real-world experience. J Oncol Pharm Pract. 2019 Jun;25(4):793-800.
6. Simmons B et al. Efficacy and safety of rivaroxaban compared to enoxaparin in treatment of cancer-associated venous thromboembolism. Eur J Haematol. 2018 Apr 4. (Epub).
7. Raskob GE et al.; Hokusai VTE Cancer Investigators. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018 Feb 15;378(7):615-24.
8. Young AM et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT-D). J Clin Oncol. 2018 Jul 10;36(20):2017-23.
9. McBane, RD et al. Apixaban, dalteparin, in active cancer associated venous thromboembolism, the ADAM VTE trial. Blood. 2018 Nov 29;132(suppl 1):421.
10. Kraaijpoel N et al. Clinical impact of bleeding in cancer-associated venous thromboembolism: Results from the Hokusai VTE cancer study. Thromb Haemost. 2018 Aug;118(8):1439-49.
11. Khorana AA et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J Thromb Haemost. 2018 Sep;16(9):1891-94.
Key points
- DOACs are a reasonable treatment option for malignancy-associated VTE in patients without GI tract malignancies and at low risk for bleeding complications.
- In patients with gastrointestinal malignancies or increased risk of bleeding, DOACs may have an increased bleeding risk and therefore LMWH is recommended.
- An informed discussion should occur between providers and patients to determine the best treatment option for cancer patients with VTE.
Additional reading
Dong Y et al. Efficacy and safety of direct oral anticoagulants versus low-molecular-weight heparin in patients with cancer: A systematic review and meta-analysis. J Thromb Thrombolysis. 2019 May 6.
Khorana AA et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost. 2018 Sep;16(9):1891-94.
Tritschler T et al. Venous thromboembolism advances in diagnosis and treatment. JAMA. 2018 Oct;320(15):1583-94.
Quiz
Which of the following is the recommended treatment of VTE in a patient with brain metastases?
A. Unfractionated heparin
B. Low molecular weight heparin
C. Direct oral anticoagulant
D. Vitamin K antagonist
The answer is B. Although there are very few data, LMWH is the recommended agent in patients with VTE and brain metastases.
A. LMWH has been shown to decrease mortality in patients with VTE and cancer, compared with unfractionated heparin (risk ratio, 0.66).
C. The safety of DOACs is not yet well established in patients with brain tumors. Antidotes and/or specific reversal agents for some DOACs are not available.
D. Vitamin K antagonists such as warfarin are not recommended in cancer patients because LMWH has a reduced risk of recurrent VTE without increased risk of bleeding.
Case
A 52-year-old female with past medical history of diabetes, hypertension, and stage 4 lung cancer on palliative chemotherapy presents with acute-onset dyspnea, pleuritic chest pain, and cough. Her exam is notable for tachycardia, hypoxemia, and diminished breath sounds. A CT pulmonary embolism study shows new left segmental thrombus. What is her preferred method of anticoagulation?
Brief overview of the issue
Venous thromboembolism (VTE) including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a significant concern in the context of malignancy and is associated with higher rates of mortality at 1 year.
The standard of care in the recent past has relied on low-molecular-weight heparin (LMWH) after several trials showed decreased VTE recurrence in cancer patients, compared with vitamin K antagonist (VKA) treatment.1,2 LMWH has been recommended as a first-line treatment by clinical guidelines for cancer-related VTE given lower drug-drug interactions between LMWH and chemotherapy regimens, as compared with traditional VKAs, and it does not rely on intestinal absorption.3

In more recent years, the focus has shifted to direct oral anticoagulants (DOACs) as potential treatment options for cancer-related VTE given their ease of administration, low side-effect profile, and decreased cost. Until recently, studies have mainly been small and largely retrospective, however, several larger randomized control studies have recently been published.
Overview of the data
Several retrospective trials have investigated the use of DOACs in cancer-associated VTE. One study looking at VTE recurrence rates showed a trend towards lower rates with rivaroxaban, compared with LMWH at 6 months (13% vs. 17%) that was significantly lower at 12 months (16.5 % vs. 22%). Similar results were found when comparing rivaroxaban to warfarin. Major bleeding rates were similar among cohorts.4

Several other retrospective cohort studies looking at treatment of cancer-associated VTE treated with LMWH vs. DOACs found that overall patients treated with DOACs had cancers with lower risk for VTE and had lower burden of metastatic disease. When this was adjusted for, there was no significant difference in the rate of recurrent cancer-associated thrombosis or major bleeding.5,6
Recently several prospective studies have corroborated the noninferiority or slight superiority of DOACs when compared with LMWH in treatment of cancer-associated VTE, while showing similar rates of bleeding. These are summarized as follows: a prospective, open-label, randomized controlled (RCT), noninferiority trial of 1,046 patients with malignancy-related VTE assigned to either LMWH for at least 5 days, followed by oral edoxaban vs. subcutaneous dalteparin for at least 6 months and up to 12 months. Investigators found no significant difference in the rate of recurrent VTE in the edoxaban group (12.8%), as compared to the dalteparin group (13.5%, P = .006 for noninferiority). Risk of major bleeding was not significantly different between the groups.7
A small RCT of 203 patients comparing recurrent VTE rates with rivaroxaban vs. dalteparin found significantly fewer recurrent clots in the rivaroxaban group compared to the dalteparin group (11% vs 4%) with no significant difference in the 6-month cumulative rate of major bleeding, 4% in the dalteparin group and 6% for the rivaroxaban group.8 Preliminary results from the ADAM VTE trial comparing apixaban to dalteparin found significantly fewer recurrent VTE in the apixaban group (3.4% vs. 14.1%) with no significant difference in major bleeding events (0% vs 2.1%).9 The Caravaggio study is a large multinational randomized, controlled, open-label, noninferiority trial looking at apixaban vs. dalteparin with endpoints being 6-month recurrent VTE and bleeding risk that will likely report results soon.
Risk of bleeding is also a major consideration in VTE treatment as studies suggest that patients with metastatic cancer are at sixfold higher risk for anticoagulant-associated bleeding.3 Subgroup analysis of Hokusai VTE cancer study found that major bleeding occurred in 32 of 522 patients given edoxaban and 16 of 524 patients treated with dalteparin. Excess of major bleeding with edoxaban was confined to patients with GI cancer. However, rates of severe major bleeding at presentation were similar.10
Overall, the existing data suggests that DOACs may be a viable option in the treatment of malignancy-associated VTE given its similar efficacy in preventing recurrent VTE without significant increased risk of major bleeding. The 2018 International Society on Thrombosis and Haemostasis VTE in cancer guidelines have been updated to include rivaroxaban and edoxaban for use in patients at low risk of bleeding, but recommend an informed discussion between patients and clinicians in deciding between DOAC and LMWH.11 The Chest VTE guidelines have not been updated since 2016, prior to when the above mentioned DOAC studies were published.
Application of data to our patient
Compared with patients without cancer, anticoagulation in cancer patients with acute VTE is challenging because of higher rates of VTE recurrence and bleeding, as well as the potential for drug interactions with anticancer agents. Our patient is not at increased risk for gastrointestinal bleeding and no drug interactions exist between her current chemotherapy regimen and the available DOACs, therefore she is a candidate for treatment with a DOAC.
After an informed discussion, she chose to start rivaroxaban for treatment of her pulmonary embolism. While more studies are needed to definitively determine the best treatment for cancer-associated VTE, DOACs appear to be an attractive alternative to LMWH. Patient preferences of taking oral medications over injections as well as the significant cost savings of DOACs over LMWH will likely play into many patients’ and providers’ anticoagulant choices.
Bottom line
Direct oral anticoagulants are a treatment option for cancer-associated VTE in patients at low risk of bleeding complications. Patients at increased risk of bleeding (especially patients with GI malignancies) should continue to be treated with LMWH.
Dr. Spence is a hospitalist and palliative care physician at Denver Health, and an assistant professor of medicine at the University of Colorado at Denver, Aurora. Dr. Miller and Dr. Liu are hospitalists at Denver Health, and assistant professors of medicine at the University of Colorado at Denver.
References
1. Hull RD et al. Long term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patient with cancer. Am J Med. 2006;19(12):1062-72.
2. Lee AY et al. Low-molecular-weight heparin versus Coumadin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-53.
3. Ay C et al. Treatment of cancer-associated venous thromboembolism in the age of direct oral anticoagulants. Ann Oncol. 2019 Mar 27 [epub].
4. Streiff MB et al. Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am J Hematol. 2018 May;93(5):664-71.
5. Phelps MK et al. A single center retrospective cohort study comparing low-molecular-weight heparins to direct oral anticoagulants for the treatment of venous thromboembolism in patients with cancer – A real-world experience. J Oncol Pharm Pract. 2019 Jun;25(4):793-800.
6. Simmons B et al. Efficacy and safety of rivaroxaban compared to enoxaparin in treatment of cancer-associated venous thromboembolism. Eur J Haematol. 2018 Apr 4. (Epub).
7. Raskob GE et al.; Hokusai VTE Cancer Investigators. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018 Feb 15;378(7):615-24.
8. Young AM et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT-D). J Clin Oncol. 2018 Jul 10;36(20):2017-23.
9. McBane, RD et al. Apixaban, dalteparin, in active cancer associated venous thromboembolism, the ADAM VTE trial. Blood. 2018 Nov 29;132(suppl 1):421.
10. Kraaijpoel N et al. Clinical impact of bleeding in cancer-associated venous thromboembolism: Results from the Hokusai VTE cancer study. Thromb Haemost. 2018 Aug;118(8):1439-49.
11. Khorana AA et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J Thromb Haemost. 2018 Sep;16(9):1891-94.
Key points
- DOACs are a reasonable treatment option for malignancy-associated VTE in patients without GI tract malignancies and at low risk for bleeding complications.
- In patients with gastrointestinal malignancies or increased risk of bleeding, DOACs may have an increased bleeding risk and therefore LMWH is recommended.
- An informed discussion should occur between providers and patients to determine the best treatment option for cancer patients with VTE.
Additional reading
Dong Y et al. Efficacy and safety of direct oral anticoagulants versus low-molecular-weight heparin in patients with cancer: A systematic review and meta-analysis. J Thromb Thrombolysis. 2019 May 6.
Khorana AA et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost. 2018 Sep;16(9):1891-94.
Tritschler T et al. Venous thromboembolism advances in diagnosis and treatment. JAMA. 2018 Oct;320(15):1583-94.
Quiz
Which of the following is the recommended treatment of VTE in a patient with brain metastases?
A. Unfractionated heparin
B. Low molecular weight heparin
C. Direct oral anticoagulant
D. Vitamin K antagonist
The answer is B. Although there are very few data, LMWH is the recommended agent in patients with VTE and brain metastases.
A. LMWH has been shown to decrease mortality in patients with VTE and cancer, compared with unfractionated heparin (risk ratio, 0.66).
C. The safety of DOACs is not yet well established in patients with brain tumors. Antidotes and/or specific reversal agents for some DOACs are not available.
D. Vitamin K antagonists such as warfarin are not recommended in cancer patients because LMWH has a reduced risk of recurrent VTE without increased risk of bleeding.
The evolving landscape of complement inhibition therapy
The introduction of eculizumab, a monoclonal antibody targeting C5 of the complement cascade, revolutionized the treatment of paroxysmal nocturnal hemoglobinuria (PNH), a rare hematologic disorder characterized by complement-mediated intravascular hemolysis, bone marrow failure, and thrombophilia. Treatment options for PNH were limited before eculizumab was approved by the Food and Drug Administration in 2007.
Its use resulted in the inhibition of intravascular hemolysis, hemoglobin stabilization, and substantial reductions in transfusion requirements. Moreover, eculizumab had the unexpected effect of reducing the risk of thromboembolic complications, the most severe complication of PNH. Patients treated with eculizumab experienced fewer thrombotic events (4%), compared with historical cohorts (27%). Importantly, 5-year overall survival rates for patients with PNH taking eculizumab improved more than 90%, compared wity the 80% reported historically.
More than 10 years later, we are tasked with assessing the impact of this drug. Unquestionably, eculizumab has done more for PNH than we could have hoped for. However, 10 years of additional data reveal the limitations of this groundbreaking therapy. Despite the overall sustained response and survival benefit, hematologic response remains variable. Complete normalization of hemoglobin occurs in less than one-third of patients. Transfusion requirements persist in many patients. Residual anemia during eculizumab therapy is at least partly attributed to bone marrow failure, a feature the complement inhibition does not address. Still, pharmacokinetic limitations of the drug also contribute to the lack of complete responses. There is residual intravascular hemolysis because of insufficient inhibition of C5 and the emergence of C3-mediated extravascular hemolysis constitutes an unanticipated mechanistic complication of all C5-mediated therapies.
The last few years have seen a surge in novel anticomplement agents, which improve upon the already well-established inhibition of C5 but also explore the efficacy of targeting earlier aspects of the complement pathway. During the American Society of Hematology (ASH) annual meeting, we had exciting updates on some of the promising new kids on the block.
Ravulizumab, the newest C5 monoclonal antibody approved by the FDA for PNH, displays more robust C5 inhibition, thereby reducing the breakthrough hemolysis still seen with eculizumab use. Crovalimab, also an anti-C5 humanized antibody, is engineered with Sequential Monoclonal Antibody Recycling Technology that improves the half-life of the drug and facilitates subcutaneous dosing while still achieving complete C5 inhibition. Some of the most exciting data is on danicopan, a small-molecule factor D inhibitor that targets the alternative pathway thereby inhibiting C3 convertase and blocking extravascular hemolysis. It has shown promise as a stand-alone agent, as well as with combined C5 inhibition, while promising safety, a reasonable concern as we explore the long-term risks of targeting the proximal complement pathway.
I was recently asked to comment on how the new complement inhibitors are addressing unmet needs in PNH. While the recent presentations at ASH demonstrate an improvement on the efficacy of C5 inhibition, pharmacokinetics, and drug delivery – all which translate to improved hemoglobin and reduced breakthrough hemolysis for PNH patients – I am most excited at the promise this new generation of drugs holds for other diseases. Since its approval for PNH, eculizumab has also been approved for use in atypical hemolytic uremic syndrome (aHUS), myasthenia gravis, and neuromyelitis optica spectrum disorder.
Perhaps the greatest potential I envision for the new generation of drugs is in aHUS, a chronic disease characterized by hemolytic anemia, thrombocytopenia, and end-stage renal disease that cannot be cured with renal transplantation. The pathophysiology involves dysregulation of complement activation because of genetic mutations or autoantibodies to key proteins in the complement cascade. Though we have experienced some success with eculizumab, responses can be incomplete, particularly in patients with C5 mutations. The newer drugs offer the opportunity to inhibit complement activation at both proximal and distal aspects of the cascade, which may prove ideal in a disease in which the affected protein is not consistent. Moreover, preclinical and clinical trials have shown promise for these novel complement inhibitors in other autoimmune diseases: antibody-mediated vasculitis, C3 glomerulopathy, catastrophic antibody syndrome, membranous nephropathy, and lupus nephritis.
The surge of new complement inhibitors could revolutionize our strategy for treatment of autoimmune-mediated diseases, in which downstream complement activation can manifest with life-threatening tissue injury. Inhibition of complement offers a promising strategy for blocking downstream immune-mediated effector mechanisms of injury common in several autoimmune diseases.
As the results from various clinical trials come to fruition, it will be exciting to determine how to best use this new generation of drugs to target new diseases and whether the next decade is poised to eclipse the progress in complement therapy already established by eculizumab.
Dr. Sosa is a benign hematologist at Fox Chase Cancer Center in Philadelphia. Her research interests are in thromboembolic disease, with a focus in racial and gender disparities.
The introduction of eculizumab, a monoclonal antibody targeting C5 of the complement cascade, revolutionized the treatment of paroxysmal nocturnal hemoglobinuria (PNH), a rare hematologic disorder characterized by complement-mediated intravascular hemolysis, bone marrow failure, and thrombophilia. Treatment options for PNH were limited before eculizumab was approved by the Food and Drug Administration in 2007.
Its use resulted in the inhibition of intravascular hemolysis, hemoglobin stabilization, and substantial reductions in transfusion requirements. Moreover, eculizumab had the unexpected effect of reducing the risk of thromboembolic complications, the most severe complication of PNH. Patients treated with eculizumab experienced fewer thrombotic events (4%), compared with historical cohorts (27%). Importantly, 5-year overall survival rates for patients with PNH taking eculizumab improved more than 90%, compared wity the 80% reported historically.
More than 10 years later, we are tasked with assessing the impact of this drug. Unquestionably, eculizumab has done more for PNH than we could have hoped for. However, 10 years of additional data reveal the limitations of this groundbreaking therapy. Despite the overall sustained response and survival benefit, hematologic response remains variable. Complete normalization of hemoglobin occurs in less than one-third of patients. Transfusion requirements persist in many patients. Residual anemia during eculizumab therapy is at least partly attributed to bone marrow failure, a feature the complement inhibition does not address. Still, pharmacokinetic limitations of the drug also contribute to the lack of complete responses. There is residual intravascular hemolysis because of insufficient inhibition of C5 and the emergence of C3-mediated extravascular hemolysis constitutes an unanticipated mechanistic complication of all C5-mediated therapies.
The last few years have seen a surge in novel anticomplement agents, which improve upon the already well-established inhibition of C5 but also explore the efficacy of targeting earlier aspects of the complement pathway. During the American Society of Hematology (ASH) annual meeting, we had exciting updates on some of the promising new kids on the block.
Ravulizumab, the newest C5 monoclonal antibody approved by the FDA for PNH, displays more robust C5 inhibition, thereby reducing the breakthrough hemolysis still seen with eculizumab use. Crovalimab, also an anti-C5 humanized antibody, is engineered with Sequential Monoclonal Antibody Recycling Technology that improves the half-life of the drug and facilitates subcutaneous dosing while still achieving complete C5 inhibition. Some of the most exciting data is on danicopan, a small-molecule factor D inhibitor that targets the alternative pathway thereby inhibiting C3 convertase and blocking extravascular hemolysis. It has shown promise as a stand-alone agent, as well as with combined C5 inhibition, while promising safety, a reasonable concern as we explore the long-term risks of targeting the proximal complement pathway.
I was recently asked to comment on how the new complement inhibitors are addressing unmet needs in PNH. While the recent presentations at ASH demonstrate an improvement on the efficacy of C5 inhibition, pharmacokinetics, and drug delivery – all which translate to improved hemoglobin and reduced breakthrough hemolysis for PNH patients – I am most excited at the promise this new generation of drugs holds for other diseases. Since its approval for PNH, eculizumab has also been approved for use in atypical hemolytic uremic syndrome (aHUS), myasthenia gravis, and neuromyelitis optica spectrum disorder.
Perhaps the greatest potential I envision for the new generation of drugs is in aHUS, a chronic disease characterized by hemolytic anemia, thrombocytopenia, and end-stage renal disease that cannot be cured with renal transplantation. The pathophysiology involves dysregulation of complement activation because of genetic mutations or autoantibodies to key proteins in the complement cascade. Though we have experienced some success with eculizumab, responses can be incomplete, particularly in patients with C5 mutations. The newer drugs offer the opportunity to inhibit complement activation at both proximal and distal aspects of the cascade, which may prove ideal in a disease in which the affected protein is not consistent. Moreover, preclinical and clinical trials have shown promise for these novel complement inhibitors in other autoimmune diseases: antibody-mediated vasculitis, C3 glomerulopathy, catastrophic antibody syndrome, membranous nephropathy, and lupus nephritis.
The surge of new complement inhibitors could revolutionize our strategy for treatment of autoimmune-mediated diseases, in which downstream complement activation can manifest with life-threatening tissue injury. Inhibition of complement offers a promising strategy for blocking downstream immune-mediated effector mechanisms of injury common in several autoimmune diseases.
As the results from various clinical trials come to fruition, it will be exciting to determine how to best use this new generation of drugs to target new diseases and whether the next decade is poised to eclipse the progress in complement therapy already established by eculizumab.
Dr. Sosa is a benign hematologist at Fox Chase Cancer Center in Philadelphia. Her research interests are in thromboembolic disease, with a focus in racial and gender disparities.
The introduction of eculizumab, a monoclonal antibody targeting C5 of the complement cascade, revolutionized the treatment of paroxysmal nocturnal hemoglobinuria (PNH), a rare hematologic disorder characterized by complement-mediated intravascular hemolysis, bone marrow failure, and thrombophilia. Treatment options for PNH were limited before eculizumab was approved by the Food and Drug Administration in 2007.
Its use resulted in the inhibition of intravascular hemolysis, hemoglobin stabilization, and substantial reductions in transfusion requirements. Moreover, eculizumab had the unexpected effect of reducing the risk of thromboembolic complications, the most severe complication of PNH. Patients treated with eculizumab experienced fewer thrombotic events (4%), compared with historical cohorts (27%). Importantly, 5-year overall survival rates for patients with PNH taking eculizumab improved more than 90%, compared wity the 80% reported historically.
More than 10 years later, we are tasked with assessing the impact of this drug. Unquestionably, eculizumab has done more for PNH than we could have hoped for. However, 10 years of additional data reveal the limitations of this groundbreaking therapy. Despite the overall sustained response and survival benefit, hematologic response remains variable. Complete normalization of hemoglobin occurs in less than one-third of patients. Transfusion requirements persist in many patients. Residual anemia during eculizumab therapy is at least partly attributed to bone marrow failure, a feature the complement inhibition does not address. Still, pharmacokinetic limitations of the drug also contribute to the lack of complete responses. There is residual intravascular hemolysis because of insufficient inhibition of C5 and the emergence of C3-mediated extravascular hemolysis constitutes an unanticipated mechanistic complication of all C5-mediated therapies.
The last few years have seen a surge in novel anticomplement agents, which improve upon the already well-established inhibition of C5 but also explore the efficacy of targeting earlier aspects of the complement pathway. During the American Society of Hematology (ASH) annual meeting, we had exciting updates on some of the promising new kids on the block.
Ravulizumab, the newest C5 monoclonal antibody approved by the FDA for PNH, displays more robust C5 inhibition, thereby reducing the breakthrough hemolysis still seen with eculizumab use. Crovalimab, also an anti-C5 humanized antibody, is engineered with Sequential Monoclonal Antibody Recycling Technology that improves the half-life of the drug and facilitates subcutaneous dosing while still achieving complete C5 inhibition. Some of the most exciting data is on danicopan, a small-molecule factor D inhibitor that targets the alternative pathway thereby inhibiting C3 convertase and blocking extravascular hemolysis. It has shown promise as a stand-alone agent, as well as with combined C5 inhibition, while promising safety, a reasonable concern as we explore the long-term risks of targeting the proximal complement pathway.
I was recently asked to comment on how the new complement inhibitors are addressing unmet needs in PNH. While the recent presentations at ASH demonstrate an improvement on the efficacy of C5 inhibition, pharmacokinetics, and drug delivery – all which translate to improved hemoglobin and reduced breakthrough hemolysis for PNH patients – I am most excited at the promise this new generation of drugs holds for other diseases. Since its approval for PNH, eculizumab has also been approved for use in atypical hemolytic uremic syndrome (aHUS), myasthenia gravis, and neuromyelitis optica spectrum disorder.
Perhaps the greatest potential I envision for the new generation of drugs is in aHUS, a chronic disease characterized by hemolytic anemia, thrombocytopenia, and end-stage renal disease that cannot be cured with renal transplantation. The pathophysiology involves dysregulation of complement activation because of genetic mutations or autoantibodies to key proteins in the complement cascade. Though we have experienced some success with eculizumab, responses can be incomplete, particularly in patients with C5 mutations. The newer drugs offer the opportunity to inhibit complement activation at both proximal and distal aspects of the cascade, which may prove ideal in a disease in which the affected protein is not consistent. Moreover, preclinical and clinical trials have shown promise for these novel complement inhibitors in other autoimmune diseases: antibody-mediated vasculitis, C3 glomerulopathy, catastrophic antibody syndrome, membranous nephropathy, and lupus nephritis.
The surge of new complement inhibitors could revolutionize our strategy for treatment of autoimmune-mediated diseases, in which downstream complement activation can manifest with life-threatening tissue injury. Inhibition of complement offers a promising strategy for blocking downstream immune-mediated effector mechanisms of injury common in several autoimmune diseases.
As the results from various clinical trials come to fruition, it will be exciting to determine how to best use this new generation of drugs to target new diseases and whether the next decade is poised to eclipse the progress in complement therapy already established by eculizumab.
Dr. Sosa is a benign hematologist at Fox Chase Cancer Center in Philadelphia. Her research interests are in thromboembolic disease, with a focus in racial and gender disparities.
Are providers asking about menstrual bleeding before/during anticoagulant therapy?
ORLANDO – A small study suggests health care providers may fail to ask patients about heavy menstrual bleeding before or during treatment with oral anticoagulants.
Researchers performed a chart review at a single center, which indicated that 60% of women were not asked about heavy menstrual bleeding before they were prescribed an oral anticoagulant.
Six months after the women started anticoagulant therapy, 29% required treatment for heavy menstrual bleeding. Charts for the remaining 71% of women contained no information about heavy menstrual bleeding.
“We were unable to distinguish between true absence of heavy menstrual bleeding and absence of reporting,” said Bethany T. Samuelson Bannow, MD, of Oregon Health & Science University, Portland.
Dr. Samuelson Bannow presented these findings at the annual meeting of the American Society of Hematology.
She explained that heavy menstrual bleeding is defined as more than 80 mL of blood loss per cycle. It affects 10%-15% of women in their lifetime, and anticoagulants increase the risk of heavy menstrual bleeding.
Studies have shown that heavy menstrual bleeding occurs in 22%-65% of women treated with vitamin K agonists and 20%-27% of women treated with rivaroxaban (Blood. 2017;130[24]:2603-9). However, many anticoagulant studies don’t include heavy menstrual bleeding as an outcome.
To gain more insight, Dr. Samuelson Bannow and colleagues conducted a chart review. Their study included 236 women of reproductive age treated at Oregon Health & Science University between Jan. 1, 2012, and Dec. 31, 2018.
The patients’ median age was 37 years (range, 18-50 years). Most patients (67%) were receiving an oral anticoagulant for venous thromboembolism. The rest were on anticoagulant therapy for arterial thrombosis (6%), atrial fibrillation (6%), a mechanical valve (1%), or “other” reasons (20%).
Dr. Samuelson Bannow said the other group was “almost exclusively women who were receiving prophylaxis” postoperatively or for travel. Most women in this group were receiving rivaroxaban.
Rivaroxaban was the most commonly prescribed anticoagulant in the entire cohort (41%), followed by warfarin (34%) and apixaban (25%).
At the time of anticoagulant prescription, 12% of women reported a history of heavy menstrual bleeding, and 28% did not. For most patients – 60% – there was no discussion of menstrual history documented.
Six months after starting oral anticoagulant therapy, 29% of patients required treatment for heavy menstrual bleeding. For 71% of patients, there was no documentation on the treatment of heavy menstrual bleeding.
Treatment for heavy menstrual bleeding was required in 33% of patients on rivaroxaban, 24% of those on apixaban, and 29% of those on warfarin, a significant difference (P less than .001).
“Rates of heavy menstrual bleeding … are higher in rivaroxaban users,” Dr. Samuelson Bannow said. “This is not the first study to demonstrate this. However, [the rate of heavy menstrual bleeding in this study] is still a lot lower than we would expect based on past levels with warfarin. This tells us we’re probably missing a lot of heavy menstrual bleeding. That’s not too surprising considering how few providers are actually asking about the menses.”
Dr. Samuelson Bannow and colleagues disclosed no conflicts of interest.
SOURCE: Samuelson Bannow BT et al. ASH 2019, Abstract 60.
ORLANDO – A small study suggests health care providers may fail to ask patients about heavy menstrual bleeding before or during treatment with oral anticoagulants.
Researchers performed a chart review at a single center, which indicated that 60% of women were not asked about heavy menstrual bleeding before they were prescribed an oral anticoagulant.
Six months after the women started anticoagulant therapy, 29% required treatment for heavy menstrual bleeding. Charts for the remaining 71% of women contained no information about heavy menstrual bleeding.
“We were unable to distinguish between true absence of heavy menstrual bleeding and absence of reporting,” said Bethany T. Samuelson Bannow, MD, of Oregon Health & Science University, Portland.
Dr. Samuelson Bannow presented these findings at the annual meeting of the American Society of Hematology.
She explained that heavy menstrual bleeding is defined as more than 80 mL of blood loss per cycle. It affects 10%-15% of women in their lifetime, and anticoagulants increase the risk of heavy menstrual bleeding.
Studies have shown that heavy menstrual bleeding occurs in 22%-65% of women treated with vitamin K agonists and 20%-27% of women treated with rivaroxaban (Blood. 2017;130[24]:2603-9). However, many anticoagulant studies don’t include heavy menstrual bleeding as an outcome.
To gain more insight, Dr. Samuelson Bannow and colleagues conducted a chart review. Their study included 236 women of reproductive age treated at Oregon Health & Science University between Jan. 1, 2012, and Dec. 31, 2018.
The patients’ median age was 37 years (range, 18-50 years). Most patients (67%) were receiving an oral anticoagulant for venous thromboembolism. The rest were on anticoagulant therapy for arterial thrombosis (6%), atrial fibrillation (6%), a mechanical valve (1%), or “other” reasons (20%).
Dr. Samuelson Bannow said the other group was “almost exclusively women who were receiving prophylaxis” postoperatively or for travel. Most women in this group were receiving rivaroxaban.
Rivaroxaban was the most commonly prescribed anticoagulant in the entire cohort (41%), followed by warfarin (34%) and apixaban (25%).
At the time of anticoagulant prescription, 12% of women reported a history of heavy menstrual bleeding, and 28% did not. For most patients – 60% – there was no discussion of menstrual history documented.
Six months after starting oral anticoagulant therapy, 29% of patients required treatment for heavy menstrual bleeding. For 71% of patients, there was no documentation on the treatment of heavy menstrual bleeding.
Treatment for heavy menstrual bleeding was required in 33% of patients on rivaroxaban, 24% of those on apixaban, and 29% of those on warfarin, a significant difference (P less than .001).
“Rates of heavy menstrual bleeding … are higher in rivaroxaban users,” Dr. Samuelson Bannow said. “This is not the first study to demonstrate this. However, [the rate of heavy menstrual bleeding in this study] is still a lot lower than we would expect based on past levels with warfarin. This tells us we’re probably missing a lot of heavy menstrual bleeding. That’s not too surprising considering how few providers are actually asking about the menses.”
Dr. Samuelson Bannow and colleagues disclosed no conflicts of interest.
SOURCE: Samuelson Bannow BT et al. ASH 2019, Abstract 60.
ORLANDO – A small study suggests health care providers may fail to ask patients about heavy menstrual bleeding before or during treatment with oral anticoagulants.
Researchers performed a chart review at a single center, which indicated that 60% of women were not asked about heavy menstrual bleeding before they were prescribed an oral anticoagulant.
Six months after the women started anticoagulant therapy, 29% required treatment for heavy menstrual bleeding. Charts for the remaining 71% of women contained no information about heavy menstrual bleeding.
“We were unable to distinguish between true absence of heavy menstrual bleeding and absence of reporting,” said Bethany T. Samuelson Bannow, MD, of Oregon Health & Science University, Portland.
Dr. Samuelson Bannow presented these findings at the annual meeting of the American Society of Hematology.
She explained that heavy menstrual bleeding is defined as more than 80 mL of blood loss per cycle. It affects 10%-15% of women in their lifetime, and anticoagulants increase the risk of heavy menstrual bleeding.
Studies have shown that heavy menstrual bleeding occurs in 22%-65% of women treated with vitamin K agonists and 20%-27% of women treated with rivaroxaban (Blood. 2017;130[24]:2603-9). However, many anticoagulant studies don’t include heavy menstrual bleeding as an outcome.
To gain more insight, Dr. Samuelson Bannow and colleagues conducted a chart review. Their study included 236 women of reproductive age treated at Oregon Health & Science University between Jan. 1, 2012, and Dec. 31, 2018.
The patients’ median age was 37 years (range, 18-50 years). Most patients (67%) were receiving an oral anticoagulant for venous thromboembolism. The rest were on anticoagulant therapy for arterial thrombosis (6%), atrial fibrillation (6%), a mechanical valve (1%), or “other” reasons (20%).
Dr. Samuelson Bannow said the other group was “almost exclusively women who were receiving prophylaxis” postoperatively or for travel. Most women in this group were receiving rivaroxaban.
Rivaroxaban was the most commonly prescribed anticoagulant in the entire cohort (41%), followed by warfarin (34%) and apixaban (25%).
At the time of anticoagulant prescription, 12% of women reported a history of heavy menstrual bleeding, and 28% did not. For most patients – 60% – there was no discussion of menstrual history documented.
Six months after starting oral anticoagulant therapy, 29% of patients required treatment for heavy menstrual bleeding. For 71% of patients, there was no documentation on the treatment of heavy menstrual bleeding.
Treatment for heavy menstrual bleeding was required in 33% of patients on rivaroxaban, 24% of those on apixaban, and 29% of those on warfarin, a significant difference (P less than .001).
“Rates of heavy menstrual bleeding … are higher in rivaroxaban users,” Dr. Samuelson Bannow said. “This is not the first study to demonstrate this. However, [the rate of heavy menstrual bleeding in this study] is still a lot lower than we would expect based on past levels with warfarin. This tells us we’re probably missing a lot of heavy menstrual bleeding. That’s not too surprising considering how few providers are actually asking about the menses.”
Dr. Samuelson Bannow and colleagues disclosed no conflicts of interest.
SOURCE: Samuelson Bannow BT et al. ASH 2019, Abstract 60.
REPORTING FROM ASH 2019























