User login
Developing an HCV vaccine faces significant challenges
The development of a prophylactic hepatitis C vaccine faces significant challenges, according to a Justin R. Bailey, MD, of Johns Hopkins University, Baltimore, and his colleagues.
Barriers to developing a prophylactic HCV vaccine include the great diversity of the virus, the limited models that are available for vaccine testing, and the currently incomplete understanding of protective immune responses, according to their review published in Gastroenterology.
Functionally, the inability to culture HCV, until recently, and continuing limitations of HCV culture systems pose challenges to standard production of a live-attenuated or inactivated whole HCV vaccine. In addition, there is the risk of causing HCV infection with live-attenuated vaccines.
On a practical level for all forms of vaccine development, a principal challenge “is the extraordinary genetic diversity of the virus. With 7 known genotypes and more than 80 subtypes, the genetic diversity of HCV exceeds that of human immunodeficiency virus-1,” according to the authors (Gastroenterology 2019;156[2]:418-30).
With regard to vaccine testing, there are also significant difficulties: There is a lack of in vitro systems and immunocompetent small-animal models useful for determining whether vaccination induces protective immunity. Although a use of an HCV-like virus, the rat Hepacivirus, provides a new small-animal model for vaccine testing, this virus has limited sequence analogy to HCV.
The development of immunity to HCV in humans is complex and under broad investigation. However, decades of research have revealed that HCV-specific CD4+ helper T cells, CD8+ cytotoxic T cells, and antibodies all play a role in protection against persistent HCV infection, according to the authors, and vaccine strategies to induce all three adaptive immune responses are in development.
“A prophylactic HCV vaccine is an important part of a successful strategy for global control. Although development is not easy, the quest is a worthy challenge,” the authors concluded.
Dr. Bailey and his colleagues reported that they had no conflicts.
SOURCE: Bailey JR et al. Gastroenterology 2019(2);156:418-30.
The development of a prophylactic hepatitis C vaccine faces significant challenges, according to a Justin R. Bailey, MD, of Johns Hopkins University, Baltimore, and his colleagues.
Barriers to developing a prophylactic HCV vaccine include the great diversity of the virus, the limited models that are available for vaccine testing, and the currently incomplete understanding of protective immune responses, according to their review published in Gastroenterology.
Functionally, the inability to culture HCV, until recently, and continuing limitations of HCV culture systems pose challenges to standard production of a live-attenuated or inactivated whole HCV vaccine. In addition, there is the risk of causing HCV infection with live-attenuated vaccines.
On a practical level for all forms of vaccine development, a principal challenge “is the extraordinary genetic diversity of the virus. With 7 known genotypes and more than 80 subtypes, the genetic diversity of HCV exceeds that of human immunodeficiency virus-1,” according to the authors (Gastroenterology 2019;156[2]:418-30).
With regard to vaccine testing, there are also significant difficulties: There is a lack of in vitro systems and immunocompetent small-animal models useful for determining whether vaccination induces protective immunity. Although a use of an HCV-like virus, the rat Hepacivirus, provides a new small-animal model for vaccine testing, this virus has limited sequence analogy to HCV.
The development of immunity to HCV in humans is complex and under broad investigation. However, decades of research have revealed that HCV-specific CD4+ helper T cells, CD8+ cytotoxic T cells, and antibodies all play a role in protection against persistent HCV infection, according to the authors, and vaccine strategies to induce all three adaptive immune responses are in development.
“A prophylactic HCV vaccine is an important part of a successful strategy for global control. Although development is not easy, the quest is a worthy challenge,” the authors concluded.
Dr. Bailey and his colleagues reported that they had no conflicts.
SOURCE: Bailey JR et al. Gastroenterology 2019(2);156:418-30.
The development of a prophylactic hepatitis C vaccine faces significant challenges, according to a Justin R. Bailey, MD, of Johns Hopkins University, Baltimore, and his colleagues.
Barriers to developing a prophylactic HCV vaccine include the great diversity of the virus, the limited models that are available for vaccine testing, and the currently incomplete understanding of protective immune responses, according to their review published in Gastroenterology.
Functionally, the inability to culture HCV, until recently, and continuing limitations of HCV culture systems pose challenges to standard production of a live-attenuated or inactivated whole HCV vaccine. In addition, there is the risk of causing HCV infection with live-attenuated vaccines.
On a practical level for all forms of vaccine development, a principal challenge “is the extraordinary genetic diversity of the virus. With 7 known genotypes and more than 80 subtypes, the genetic diversity of HCV exceeds that of human immunodeficiency virus-1,” according to the authors (Gastroenterology 2019;156[2]:418-30).
With regard to vaccine testing, there are also significant difficulties: There is a lack of in vitro systems and immunocompetent small-animal models useful for determining whether vaccination induces protective immunity. Although a use of an HCV-like virus, the rat Hepacivirus, provides a new small-animal model for vaccine testing, this virus has limited sequence analogy to HCV.
The development of immunity to HCV in humans is complex and under broad investigation. However, decades of research have revealed that HCV-specific CD4+ helper T cells, CD8+ cytotoxic T cells, and antibodies all play a role in protection against persistent HCV infection, according to the authors, and vaccine strategies to induce all three adaptive immune responses are in development.
“A prophylactic HCV vaccine is an important part of a successful strategy for global control. Although development is not easy, the quest is a worthy challenge,” the authors concluded.
Dr. Bailey and his colleagues reported that they had no conflicts.
SOURCE: Bailey JR et al. Gastroenterology 2019(2);156:418-30.
FROM GASTROENTEROLOGY
Flu season shows signs of peaking
The 2018-2019 flu season may have peaked as the major nationwide measure of influenza activity held steady for the week ending Feb. 23, according to the Centers for Disease Control and Prevention. The proportion of outpatient visits for influenza-like illness (ILI) was 5.0% for the most recent reporting week, the CDC’s influenza division said in its March 1 report. The previous week’s outpatient visit rate, originally reported as 5.1%, was revised this week to 5.0% as well, suggesting that flu activity is no longer increasing.
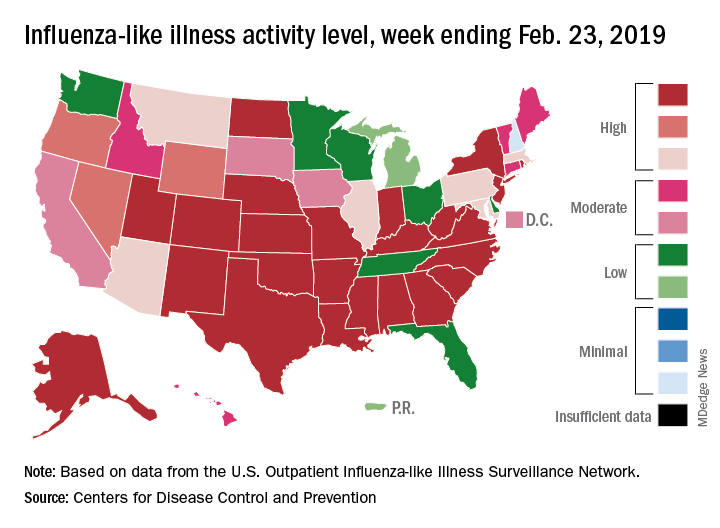
Activity at the state level was more mixed. The number of states at level 10 on the CDC’s 1-10 scale of ILI activity stayed at 24 as Indiana and North Dakota replaced Tennessee and Wyoming, but the number of states in the high range (8-10) of the activity scale increased from 30 to 33, CDC data show.
The signs of plateauing ILI activity did not, however, extend to flu-related deaths, with 15 reported among children – the highest weekly number for the 2018-2019 season, although 11 actually occurred in previous weeks – during the week ending Feb. 23 and 289 deaths among all ages for the week ending Feb. 16, which is already more than the 268 listed the week before despite less complete reporting (82% vs. 97%), the CDC reported. Total flu-related deaths in children are now up to 56, compared with 138 at the corresponding point in the 2017-2018 season.
The 2018-2019 flu season may have peaked as the major nationwide measure of influenza activity held steady for the week ending Feb. 23, according to the Centers for Disease Control and Prevention. The proportion of outpatient visits for influenza-like illness (ILI) was 5.0% for the most recent reporting week, the CDC’s influenza division said in its March 1 report. The previous week’s outpatient visit rate, originally reported as 5.1%, was revised this week to 5.0% as well, suggesting that flu activity is no longer increasing.

Activity at the state level was more mixed. The number of states at level 10 on the CDC’s 1-10 scale of ILI activity stayed at 24 as Indiana and North Dakota replaced Tennessee and Wyoming, but the number of states in the high range (8-10) of the activity scale increased from 30 to 33, CDC data show.
The signs of plateauing ILI activity did not, however, extend to flu-related deaths, with 15 reported among children – the highest weekly number for the 2018-2019 season, although 11 actually occurred in previous weeks – during the week ending Feb. 23 and 289 deaths among all ages for the week ending Feb. 16, which is already more than the 268 listed the week before despite less complete reporting (82% vs. 97%), the CDC reported. Total flu-related deaths in children are now up to 56, compared with 138 at the corresponding point in the 2017-2018 season.
The 2018-2019 flu season may have peaked as the major nationwide measure of influenza activity held steady for the week ending Feb. 23, according to the Centers for Disease Control and Prevention. The proportion of outpatient visits for influenza-like illness (ILI) was 5.0% for the most recent reporting week, the CDC’s influenza division said in its March 1 report. The previous week’s outpatient visit rate, originally reported as 5.1%, was revised this week to 5.0% as well, suggesting that flu activity is no longer increasing.

Activity at the state level was more mixed. The number of states at level 10 on the CDC’s 1-10 scale of ILI activity stayed at 24 as Indiana and North Dakota replaced Tennessee and Wyoming, but the number of states in the high range (8-10) of the activity scale increased from 30 to 33, CDC data show.
The signs of plateauing ILI activity did not, however, extend to flu-related deaths, with 15 reported among children – the highest weekly number for the 2018-2019 season, although 11 actually occurred in previous weeks – during the week ending Feb. 23 and 289 deaths among all ages for the week ending Feb. 16, which is already more than the 268 listed the week before despite less complete reporting (82% vs. 97%), the CDC reported. Total flu-related deaths in children are now up to 56, compared with 138 at the corresponding point in the 2017-2018 season.
Human papillomavirus in 2019: An update on cervical cancer prevention and screening guidelines
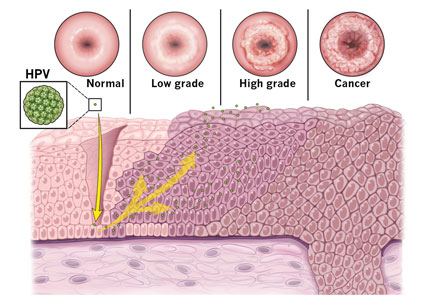
About 12% of women worldwide are infected with human papillomavirus (HPV).1 Persistent HPV infection with high-risk strains such as HPV 6, 11, 16, and 18 cause nearly all cases of cervical cancer and some anal, vaginal, penile, and oropharyngeal cancers.2 An estimated 13,000 cases of invasive cervical cancer will be diagnosed this year in the United States alone.3
Up to 70% of HPV-related cervical cancer cases can be prevented with vaccination. A number of changes have been made to the vaccination schedule within the past few years—patients younger than 15 need only 2 rather than 3 doses, and the vaccine itself can be used in adults up to age 45.
Vaccination and routine cervical cancer screening are both necessary to prevent this disease3 along with effective family and patient counseling. Here, we discuss the most up-to-date HPV vaccination recommendations, current cervical cancer screening guidelines, counseling techniques that increase vaccination acceptance rates, and follow-up protocols for abnormal cervical cancer screening results.
TYPES OF HPV VACCINES
HPV immunization can prevent up to 70% of cases of cervical cancer due to HPV as well as 90% of genital warts.4 The US Food and Drug Administration (FDA) has approved 3 HPV vaccines:
- Gardasil 9 targets HPV types 6, 11, 16, and 18 along with 31, 33, 45, 52, 58—these cause 90% of cervical cancer cases and most cases of genital warts5—making it the most effective vaccine available; Gardasil 9 is the only HPV vaccine currently available in the United States
- The bivalent vaccine (Cervarix) targeted HPV 16 and 18 only, and was discontinued in the United States in 2016
- The quadrivalent HPV vaccine (Gardasil) targeted HPV 16 and 18 as well as 6 and 11, which cause most cases of genital warts; the last available doses in the United States expired in May 2017; it has been replaced by Gardasil 9.
The incidence of cervical cancer in the United States dropped 29% among 15- to 24-year-olds from 2003–2006 when HPV vaccination first started to 2011–2014.6
VACCINE DOSING RECOMMENDATIONS FOR PRIMARY PREVENTION
The Advisory Committee on Immunization Practices (ACIP) revised its HPV vaccine schedule in 2016, when it decreased the necessary doses from 3 to 2 for patients under age 15 and addressed the needs of special patient populations.7 In late 2018, the FDA approved the use of the vaccine in men and women up to age 45. However, no change in guidelines have yet been made (Table 1).
In females, the ACIP recommends starting HPV vaccination at age 11 or 12, but it can be given as early as age 9. A 2-dose schedule is recommended for the 9-valent vaccine before the patient’s 15th birthday (the second dose 6 to 12 months after the first).7 For females who initiate HPV vaccination between ages 15 and 45, a 3-dose schedule is necessary (at 0, 1 to 2, and 6 months).7,8
The change to a 2-dose schedule was prompted by an evaluation of girls ages 9 to 13 randomized to receive either a 2- or 3-dose schedule. Antibody responses with a 2-dose schedule were not inferior to those of young women (ages 16 to 26) who received all 3 doses.9 The geometric mean titer ratios remained noninferior throughout the study period of 36 months.
However, a loss of noninferiority was noted for HPV-18 by 24 months and for HPV-6 by 36 months.9 Thus, further studies are needed to understand the duration of protection with a 2-dose schedule. Nevertheless, decreasing the number of doses makes it a more convenient and cost-effective option for many families.
The recommendations are the same for males except for one notable difference: in males ages 21 to 26, vaccination is not routinely recommended by the ACIP, but rather it is considered a “permissive use” recommendation: ie, the vaccine should be offered and final decisions on administration be made after individualized discussion with the patient.10 Permissive-use status also means the vaccine may not be covered by health insurance. Even though the vaccine is now available to men and women until age 45, many insurance plans do not cover it after age 26.
Children of either sex with a history of sexual abuse should receive their first vaccine dose beginning at age 9.7
Immunocompromised patients should follow the 3-dose schedule regardless of their sex or the age when vaccination was initiated.10
For transgender patients and for men not previously vaccinated who have sex with men, the 3-dose schedule vaccine should be given by the age of 26 (this is a routine recommendation, not a permissive one).8
CHALLENGES OF VACCINATION
Effective patient and family counseling is important. Even though the first HPV vaccine was approved in 2006, only 34.9% of US adolescents were fully vaccinated by 2015. This was in part because providers did not recommend it, were unfamiliar with it, or had concerns about its safety,11,12 and in part because some parents refused it.
The physician must address any myths regarding HPV vaccination and ensure that parents and patients understand that HPV vaccine is safe and effective. Studies have shown that with high-quality recommendations (ie, the care provider strongly endorses the HPV vaccine, encourages same-day vaccination, and discusses cancer prevention), patients are 9 times more likely to start the HPV vaccination schedule and 3 times more likely to follow through with subsequent doses.13
Providing good family and patient education does not necessarily require spending more counseling time. A recent study showed that spending less time discussing the HPV vaccine can lead to better vaccine coverage.14 The study compared parent HPV vaccine counseling techniques and found that simply informing patients and their families that the HPV vaccine was due was associated with a higher vaccine acceptance rate than inviting conversations about it.14 When providers announced that the vaccine was due, assuming the parents were ready to vaccinate, there was a 5.4% increase in HPV vaccination coverage.14
Conversely, physicians who engaged parents in open-ended discussions about the HPV vaccine did not improve HPV vaccination coverage.14 The authors suggested that providers approach HPV vaccination as if they were counseling patients and families about the need to avoid second-hand smoke or the need to use car seats. If parents or patients resist the presumptive announcement approach, expanded counseling and shared decision-making are appropriate. This includes addressing misconceptions that parents and patients may have about the HPV vaccine. The American Cancer Society lists 8 facts to reference (Table 2).15
SECONDARY PREVENTION: CERVICAL CANCER SCREENING
Since the introduction of the Papanicolaou (Pap) test, US cervical cancer incidence rates have decreased by more than 60%.16 Because almost all cervical cancer is preventable with proper screening, all women ages 21 to 65 should be screened.
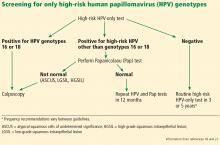
Currently, there are 3 options available for cervical cancer screening: the Pap-only test, the Pap-HPV cotest, and the high-risk HPV-only test (Table 3). The latter 2 options detect high-risk HPV genotypes.
Several organizations have screening algorithms that recommend when to use these tests, but the 3 that shape today’s standard of care in cervical cancer screening come from the American College of Obstetricians and Gynecologists (ACOG), the American Society for Colposcopy and Cervical Pathology (ASCCP), and US Preventive Services Task Force (USPSTF).17–19
Pap-only testing is performed every 3 years to screen for cervical neoplasia that might indicate premalignancy.
Pap-HPV cotesting is performed every 5 years in women older than 30 with past normal screening. Until 2018, all 3 organizations recommended cotesting as the preferred screening algorithm for women ages 30 to 65.17–19 Patients with a history of abnormal test results require more frequent testing as recommended by the ASCCP.18
The high-risk HPV-only test utilizes real-time polymerase chain reaction to detect HPV 16, HPV 18, and 12 other HPV genotypes. Only 2 tests are approved by the FDA as stand-alone cervical cancer screening tests—the Roche Cobas HPV test approved in 2014 and the Becton Dickinson Onclarity HPV assay approved in 2018. Other HPV tests that are used in a cotesting strategy should not be used for high-risk HPV-only testing because their performance characteristics may differ.
In 2015, the Addressing the Need for Advanced HPV Diagnostics (ATHENA) study showed that 1 round of high-risk HPV-only screening for women older than 25 was more sensitive than Pap-only or cotesting for stage 3 cervical intraepithelial neoplasia or more severe disease (after 3 years of follow-up).20 Current guidelines from ASCCP18 and ACOG17 state that the high-risk HPV test can be repeated every 3 years (when used to screen by itself) if the woman is older than 25 and has had a normal test result.
If the HPV test result is positive for high-risk HPV 16 or 18 genotypes, then immediate colposcopy is indicated; women who test positive for one of the other 12 high-risk subtypes will need to undergo a Pap test to determine the appropriate follow-up (Figure 1).18,21
In 2018, the USPSTF updated its recommendations, noting that for women age 30 to 65, Pap-only testing every 3 years, cotesting every 5 years, or high-risk HPV-only testing every 5 years are all appropriate screening strategies, with the Pap-only or high-risk HPV-only screenings being preferred.19 This is in contrast to ACOG and ASCCP recommendations for cotesting every 5 years, with alternative options of Pap-only or HPV-only testing being done every 3 years.17,18
Is there a best screening protocol?
The USPSTF reviewed large randomized and observational studies to summarize the effectiveness of the 3 screening strategies and commissioned a decision analysis model to compare the risks, benefits, and costs of the 3 screening algorithms. The guideline statement notes both cotesting and high-risk HPV testing offer similar cancer detection rates: each prevents 1 additional cancer per 1,000 women screened as opposed to Pap-only testing.19
Also, tests that incorporate high-risk HPV screening may offer better detection of cervical adenocarcinoma (which has a worse prognosis than the more common squamous cell carcinoma type). However, both HPV-based screening strategies are more likely to require additional colposcopies for follow-up than Pap-only screening (1,630 colposcopies required for each cancer prevented with high-risk HPV alone, 1,635 with cotesting). Colposcopy is a simple office procedure that causes minimal discomfort to the patient.
The USPSTF guideline also differs in the recommended frequency of high-risk HPV-only testing; a high-risk HPV result should be repeated every 5 years if normal (as opposed to every 3 years as recommended by ACOG and ASCCP).19 The 5-year recommendation is based on analysis modeling, which suggests that performing high-risk HPV-only testing more frequently is unlikely to improve detection rates but will increase the number of screening tests and colposcopies.19
No trial has directly compared cotesting with high-risk HPV testing for more than 2 rounds of screening. The updated USPSTF recommendations are based on modeling estimates and expert opinion, which assesses cost and benefit vs harm in the long term. Also, no high-risk HPV test is currently FDA-approved for every-5-year screening when used by itself.
All 3 cervical cancer screening methods provide highly effective cancer prevention, so it is important for providers to choose the strategy that best fits their practice. The most critical aspect of screening is getting all women screened, no matter which method is used.
It is critical to remember that the screening intervals are intended for patients without symptoms. Those who have new concerns such as bleeding should have a diagnostic Pap done to evaluate their symptoms.
Follow-up of abnormal results
Regardless of the pathway chosen, appropriate follow-up of any abnormal test result is critical to the early detection of cancer. Established follow-up guidelines exist,22,23 but accessing this information can be difficult for the busy clinician. The ASCCP has a mobile phone application that outlines the action steps corresponding to the patient’s age and results of any combination of Pap or HPV testing. The app also includes the best screening algorithms for a particular patient.24
All guidelines agree that cervical cancer screening should start at age 21, regardless of HPV vaccination status or age of sexual initiation.17,18,25 Screening can be discontinued at age 65 for women with normal screening results in the prior decade (3 consecutive negative Pap results or 2 consecutive negative cotest results).23
For women who have had a total hysterectomy and no history of cervical neoplasia, screening should be stopped immediately after the procedure. However, several high-risk groups of women will need continued screening past the age of 65, or after a hysterectomy.
For a woman with a history of stage 2 cervical intraepithelial neoplasia or higher grade lesions, routine screening is continued for an additional 20 years, even if she is over age 65. Pap-only testing every 3 years is acceptable, because the role of HPV testing is unclear after hysterectomy.23 Prior guidelines suggested annual screening in these patients, so the change to every 3 years is notable. Many gynecologic oncologists will recommend that women with a history of cervical cancer continue annual screening indefinitely.
Within the first 2 to 3 years after treatment for high-grade dysplastic changes, annual follow-up is done by the gynecologic oncology team. Providers who offer follow-up during this time frame should keep in communication with the oncology team to ensure appropriate, individualized care. These recommendations are based on expert opinion, so variations in clinical practice may be seen.
Women infected with the human immunodeficiency virus can have Pap-only testing every 3 years, after a series of 3 normal annual Pap results.26 But screening does not stop at age 65.23,26 For patients who are immunosuppressed or have a history of diethylstilbestrol exposure, screening should be done annually indefinitely.23
- Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 2010; 202(12):1789–1799. doi:10.1086/657321
- de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancer attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012; 13(6):607–615. doi:10.1016/S1470-2045(12)70137-7
- American Cancer Society. Key statistics for cervical cancer. www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed February 14, 2019.
- Thaxton L, Waxman AG. Cervical cancer prevention: immunization and screening 2015. Med Clin North Am 2015; 99(3):469–477. doi:10.1016/j.mcna.2015.01.003
- McNamara M, Batur P, Walsh JME, Johnson KM. HPV update: vaccination, screening, and associated disease. J Gen Intern Med 2016; 31(11):1360–1366. doi:10.1007/s11606-016-3725-z
- Guo F, Cofie LE, Berenson AB. Cervical cancer incidence in young US females after human papillomavirus vaccine introduction. Am J Prev Med 2018; 55(2):197–204. doi:10.1016/j.amepre.2018.03.013
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016; 65(49):1405–1408. doi:10.15585/mmwr.mm6549a5
- Centers for Disease Control and Prevention (CDC). Supplemental information and guidance for vaccination providers regarding use of 9-valent HPV vaccine Information for persons who started an HPV vaccination series with quadrivalent or bivalent HPV vaccine. www.cdc.gov/hpv/downloads/9vhpv-guidance.pdf. Accessed February 14, 2019.
- Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309(17):1793–1802. doi:10.1001/jama.2013.1625
- Markowitz LE, Dunne EF, Saraiya M, et al; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63(RR-05):1–30. pmid:25167164
- Thompson EL, Rosen BL, Vamos CA, Kadono M, Daley EM. Human papillomavirus vaccination: what are the reasons for nonvaccination among US adolescents? J Adolesc Health 2017; 61(3):288–293. doi:10.1016/j.jadohealth.2017.05.015
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2015. MMWR Morb Mortal Wkly Rep 2016; 65(33):850–858. doi:10.15585/mmwr.mm6533a4
- Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: The impact of recommendation quality. Vaccine 2016; 34(9):1187–1192. doi:10.1016/j.vaccine.2016.01.023
- Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements versus conversations to improve HPV vaccination coverage: a randomized trial. Pediatrics 2017; 139(1):e20161764. doi:10.1542/peds.2016-1764
- American Cancer Society. HPV vaccine facts. www.cancer.org/cancer/cancer-causes/infectious-agents/hpv/hpv-vaccine-facts-and-fears.html. Accessed February 14, 2019.
- National Cancer Institute; Chasan R, Manrow R. Cervical cancer. https://report.nih.gov/nihfactsheets/viewfactsheet.aspx?csid=76. Accessed February 14, 2019.
- The American College of Obstetricians and Gynecologists (ACOG). Frequently asked questions. Cervical cancer screening. www.acog.org/Patients/FAQs/Cervical-Cancer-Screening. Accessed February 14, 2019.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137(4):516–542. doi:10.1309/AJCPTGD94EVRSJCG
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol 2015; 136(2):189–197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol 2015; 125(2):330–337. doi:10.1097/AOG.0000000000000669
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 2013; 121(4):829–846. doi:10.1097/AOG.0b013e3182883a34
- Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol 2016; 128(4):e111–e130. doi:10.1097/AOG.0000000000001708
- ASCCP. Mobile app. http://www.asccp.org/store-detail2/asccp-mobile-app. Accessed February 14, 2019.
- USPSTF. Draft recommendation: cervical cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Accessed February 14, 2019.
- Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE; National Institutes of Health; Centers for Disease Control and Prevention; HIV Medicine Association of the Infectious Diseases Society of America. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58(9):1308–1311. doi:10.1093/cid/ciu094
About 12% of women worldwide are infected with human papillomavirus (HPV).1 Persistent HPV infection with high-risk strains such as HPV 6, 11, 16, and 18 cause nearly all cases of cervical cancer and some anal, vaginal, penile, and oropharyngeal cancers.2 An estimated 13,000 cases of invasive cervical cancer will be diagnosed this year in the United States alone.3
Up to 70% of HPV-related cervical cancer cases can be prevented with vaccination. A number of changes have been made to the vaccination schedule within the past few years—patients younger than 15 need only 2 rather than 3 doses, and the vaccine itself can be used in adults up to age 45.
Vaccination and routine cervical cancer screening are both necessary to prevent this disease3 along with effective family and patient counseling. Here, we discuss the most up-to-date HPV vaccination recommendations, current cervical cancer screening guidelines, counseling techniques that increase vaccination acceptance rates, and follow-up protocols for abnormal cervical cancer screening results.
TYPES OF HPV VACCINES
HPV immunization can prevent up to 70% of cases of cervical cancer due to HPV as well as 90% of genital warts.4 The US Food and Drug Administration (FDA) has approved 3 HPV vaccines:
- Gardasil 9 targets HPV types 6, 11, 16, and 18 along with 31, 33, 45, 52, 58—these cause 90% of cervical cancer cases and most cases of genital warts5—making it the most effective vaccine available; Gardasil 9 is the only HPV vaccine currently available in the United States
- The bivalent vaccine (Cervarix) targeted HPV 16 and 18 only, and was discontinued in the United States in 2016
- The quadrivalent HPV vaccine (Gardasil) targeted HPV 16 and 18 as well as 6 and 11, which cause most cases of genital warts; the last available doses in the United States expired in May 2017; it has been replaced by Gardasil 9.
The incidence of cervical cancer in the United States dropped 29% among 15- to 24-year-olds from 2003–2006 when HPV vaccination first started to 2011–2014.6
VACCINE DOSING RECOMMENDATIONS FOR PRIMARY PREVENTION
The Advisory Committee on Immunization Practices (ACIP) revised its HPV vaccine schedule in 2016, when it decreased the necessary doses from 3 to 2 for patients under age 15 and addressed the needs of special patient populations.7 In late 2018, the FDA approved the use of the vaccine in men and women up to age 45. However, no change in guidelines have yet been made (Table 1).
In females, the ACIP recommends starting HPV vaccination at age 11 or 12, but it can be given as early as age 9. A 2-dose schedule is recommended for the 9-valent vaccine before the patient’s 15th birthday (the second dose 6 to 12 months after the first).7 For females who initiate HPV vaccination between ages 15 and 45, a 3-dose schedule is necessary (at 0, 1 to 2, and 6 months).7,8
The change to a 2-dose schedule was prompted by an evaluation of girls ages 9 to 13 randomized to receive either a 2- or 3-dose schedule. Antibody responses with a 2-dose schedule were not inferior to those of young women (ages 16 to 26) who received all 3 doses.9 The geometric mean titer ratios remained noninferior throughout the study period of 36 months.
However, a loss of noninferiority was noted for HPV-18 by 24 months and for HPV-6 by 36 months.9 Thus, further studies are needed to understand the duration of protection with a 2-dose schedule. Nevertheless, decreasing the number of doses makes it a more convenient and cost-effective option for many families.
The recommendations are the same for males except for one notable difference: in males ages 21 to 26, vaccination is not routinely recommended by the ACIP, but rather it is considered a “permissive use” recommendation: ie, the vaccine should be offered and final decisions on administration be made after individualized discussion with the patient.10 Permissive-use status also means the vaccine may not be covered by health insurance. Even though the vaccine is now available to men and women until age 45, many insurance plans do not cover it after age 26.
Children of either sex with a history of sexual abuse should receive their first vaccine dose beginning at age 9.7
Immunocompromised patients should follow the 3-dose schedule regardless of their sex or the age when vaccination was initiated.10
For transgender patients and for men not previously vaccinated who have sex with men, the 3-dose schedule vaccine should be given by the age of 26 (this is a routine recommendation, not a permissive one).8
CHALLENGES OF VACCINATION
Effective patient and family counseling is important. Even though the first HPV vaccine was approved in 2006, only 34.9% of US adolescents were fully vaccinated by 2015. This was in part because providers did not recommend it, were unfamiliar with it, or had concerns about its safety,11,12 and in part because some parents refused it.
The physician must address any myths regarding HPV vaccination and ensure that parents and patients understand that HPV vaccine is safe and effective. Studies have shown that with high-quality recommendations (ie, the care provider strongly endorses the HPV vaccine, encourages same-day vaccination, and discusses cancer prevention), patients are 9 times more likely to start the HPV vaccination schedule and 3 times more likely to follow through with subsequent doses.13
Providing good family and patient education does not necessarily require spending more counseling time. A recent study showed that spending less time discussing the HPV vaccine can lead to better vaccine coverage.14 The study compared parent HPV vaccine counseling techniques and found that simply informing patients and their families that the HPV vaccine was due was associated with a higher vaccine acceptance rate than inviting conversations about it.14 When providers announced that the vaccine was due, assuming the parents were ready to vaccinate, there was a 5.4% increase in HPV vaccination coverage.14
Conversely, physicians who engaged parents in open-ended discussions about the HPV vaccine did not improve HPV vaccination coverage.14 The authors suggested that providers approach HPV vaccination as if they were counseling patients and families about the need to avoid second-hand smoke or the need to use car seats. If parents or patients resist the presumptive announcement approach, expanded counseling and shared decision-making are appropriate. This includes addressing misconceptions that parents and patients may have about the HPV vaccine. The American Cancer Society lists 8 facts to reference (Table 2).15
SECONDARY PREVENTION: CERVICAL CANCER SCREENING
Since the introduction of the Papanicolaou (Pap) test, US cervical cancer incidence rates have decreased by more than 60%.16 Because almost all cervical cancer is preventable with proper screening, all women ages 21 to 65 should be screened.
Currently, there are 3 options available for cervical cancer screening: the Pap-only test, the Pap-HPV cotest, and the high-risk HPV-only test (Table 3). The latter 2 options detect high-risk HPV genotypes.
Several organizations have screening algorithms that recommend when to use these tests, but the 3 that shape today’s standard of care in cervical cancer screening come from the American College of Obstetricians and Gynecologists (ACOG), the American Society for Colposcopy and Cervical Pathology (ASCCP), and US Preventive Services Task Force (USPSTF).17–19
Pap-only testing is performed every 3 years to screen for cervical neoplasia that might indicate premalignancy.
Pap-HPV cotesting is performed every 5 years in women older than 30 with past normal screening. Until 2018, all 3 organizations recommended cotesting as the preferred screening algorithm for women ages 30 to 65.17–19 Patients with a history of abnormal test results require more frequent testing as recommended by the ASCCP.18
The high-risk HPV-only test utilizes real-time polymerase chain reaction to detect HPV 16, HPV 18, and 12 other HPV genotypes. Only 2 tests are approved by the FDA as stand-alone cervical cancer screening tests—the Roche Cobas HPV test approved in 2014 and the Becton Dickinson Onclarity HPV assay approved in 2018. Other HPV tests that are used in a cotesting strategy should not be used for high-risk HPV-only testing because their performance characteristics may differ.
In 2015, the Addressing the Need for Advanced HPV Diagnostics (ATHENA) study showed that 1 round of high-risk HPV-only screening for women older than 25 was more sensitive than Pap-only or cotesting for stage 3 cervical intraepithelial neoplasia or more severe disease (after 3 years of follow-up).20 Current guidelines from ASCCP18 and ACOG17 state that the high-risk HPV test can be repeated every 3 years (when used to screen by itself) if the woman is older than 25 and has had a normal test result.
If the HPV test result is positive for high-risk HPV 16 or 18 genotypes, then immediate colposcopy is indicated; women who test positive for one of the other 12 high-risk subtypes will need to undergo a Pap test to determine the appropriate follow-up (Figure 1).18,21
In 2018, the USPSTF updated its recommendations, noting that for women age 30 to 65, Pap-only testing every 3 years, cotesting every 5 years, or high-risk HPV-only testing every 5 years are all appropriate screening strategies, with the Pap-only or high-risk HPV-only screenings being preferred.19 This is in contrast to ACOG and ASCCP recommendations for cotesting every 5 years, with alternative options of Pap-only or HPV-only testing being done every 3 years.17,18
Is there a best screening protocol?
The USPSTF reviewed large randomized and observational studies to summarize the effectiveness of the 3 screening strategies and commissioned a decision analysis model to compare the risks, benefits, and costs of the 3 screening algorithms. The guideline statement notes both cotesting and high-risk HPV testing offer similar cancer detection rates: each prevents 1 additional cancer per 1,000 women screened as opposed to Pap-only testing.19
Also, tests that incorporate high-risk HPV screening may offer better detection of cervical adenocarcinoma (which has a worse prognosis than the more common squamous cell carcinoma type). However, both HPV-based screening strategies are more likely to require additional colposcopies for follow-up than Pap-only screening (1,630 colposcopies required for each cancer prevented with high-risk HPV alone, 1,635 with cotesting). Colposcopy is a simple office procedure that causes minimal discomfort to the patient.
The USPSTF guideline also differs in the recommended frequency of high-risk HPV-only testing; a high-risk HPV result should be repeated every 5 years if normal (as opposed to every 3 years as recommended by ACOG and ASCCP).19 The 5-year recommendation is based on analysis modeling, which suggests that performing high-risk HPV-only testing more frequently is unlikely to improve detection rates but will increase the number of screening tests and colposcopies.19
No trial has directly compared cotesting with high-risk HPV testing for more than 2 rounds of screening. The updated USPSTF recommendations are based on modeling estimates and expert opinion, which assesses cost and benefit vs harm in the long term. Also, no high-risk HPV test is currently FDA-approved for every-5-year screening when used by itself.
All 3 cervical cancer screening methods provide highly effective cancer prevention, so it is important for providers to choose the strategy that best fits their practice. The most critical aspect of screening is getting all women screened, no matter which method is used.
It is critical to remember that the screening intervals are intended for patients without symptoms. Those who have new concerns such as bleeding should have a diagnostic Pap done to evaluate their symptoms.
Follow-up of abnormal results
Regardless of the pathway chosen, appropriate follow-up of any abnormal test result is critical to the early detection of cancer. Established follow-up guidelines exist,22,23 but accessing this information can be difficult for the busy clinician. The ASCCP has a mobile phone application that outlines the action steps corresponding to the patient’s age and results of any combination of Pap or HPV testing. The app also includes the best screening algorithms for a particular patient.24
All guidelines agree that cervical cancer screening should start at age 21, regardless of HPV vaccination status or age of sexual initiation.17,18,25 Screening can be discontinued at age 65 for women with normal screening results in the prior decade (3 consecutive negative Pap results or 2 consecutive negative cotest results).23
For women who have had a total hysterectomy and no history of cervical neoplasia, screening should be stopped immediately after the procedure. However, several high-risk groups of women will need continued screening past the age of 65, or after a hysterectomy.
For a woman with a history of stage 2 cervical intraepithelial neoplasia or higher grade lesions, routine screening is continued for an additional 20 years, even if she is over age 65. Pap-only testing every 3 years is acceptable, because the role of HPV testing is unclear after hysterectomy.23 Prior guidelines suggested annual screening in these patients, so the change to every 3 years is notable. Many gynecologic oncologists will recommend that women with a history of cervical cancer continue annual screening indefinitely.
Within the first 2 to 3 years after treatment for high-grade dysplastic changes, annual follow-up is done by the gynecologic oncology team. Providers who offer follow-up during this time frame should keep in communication with the oncology team to ensure appropriate, individualized care. These recommendations are based on expert opinion, so variations in clinical practice may be seen.
Women infected with the human immunodeficiency virus can have Pap-only testing every 3 years, after a series of 3 normal annual Pap results.26 But screening does not stop at age 65.23,26 For patients who are immunosuppressed or have a history of diethylstilbestrol exposure, screening should be done annually indefinitely.23
About 12% of women worldwide are infected with human papillomavirus (HPV).1 Persistent HPV infection with high-risk strains such as HPV 6, 11, 16, and 18 cause nearly all cases of cervical cancer and some anal, vaginal, penile, and oropharyngeal cancers.2 An estimated 13,000 cases of invasive cervical cancer will be diagnosed this year in the United States alone.3
Up to 70% of HPV-related cervical cancer cases can be prevented with vaccination. A number of changes have been made to the vaccination schedule within the past few years—patients younger than 15 need only 2 rather than 3 doses, and the vaccine itself can be used in adults up to age 45.
Vaccination and routine cervical cancer screening are both necessary to prevent this disease3 along with effective family and patient counseling. Here, we discuss the most up-to-date HPV vaccination recommendations, current cervical cancer screening guidelines, counseling techniques that increase vaccination acceptance rates, and follow-up protocols for abnormal cervical cancer screening results.
TYPES OF HPV VACCINES
HPV immunization can prevent up to 70% of cases of cervical cancer due to HPV as well as 90% of genital warts.4 The US Food and Drug Administration (FDA) has approved 3 HPV vaccines:
- Gardasil 9 targets HPV types 6, 11, 16, and 18 along with 31, 33, 45, 52, 58—these cause 90% of cervical cancer cases and most cases of genital warts5—making it the most effective vaccine available; Gardasil 9 is the only HPV vaccine currently available in the United States
- The bivalent vaccine (Cervarix) targeted HPV 16 and 18 only, and was discontinued in the United States in 2016
- The quadrivalent HPV vaccine (Gardasil) targeted HPV 16 and 18 as well as 6 and 11, which cause most cases of genital warts; the last available doses in the United States expired in May 2017; it has been replaced by Gardasil 9.
The incidence of cervical cancer in the United States dropped 29% among 15- to 24-year-olds from 2003–2006 when HPV vaccination first started to 2011–2014.6
VACCINE DOSING RECOMMENDATIONS FOR PRIMARY PREVENTION
The Advisory Committee on Immunization Practices (ACIP) revised its HPV vaccine schedule in 2016, when it decreased the necessary doses from 3 to 2 for patients under age 15 and addressed the needs of special patient populations.7 In late 2018, the FDA approved the use of the vaccine in men and women up to age 45. However, no change in guidelines have yet been made (Table 1).
In females, the ACIP recommends starting HPV vaccination at age 11 or 12, but it can be given as early as age 9. A 2-dose schedule is recommended for the 9-valent vaccine before the patient’s 15th birthday (the second dose 6 to 12 months after the first).7 For females who initiate HPV vaccination between ages 15 and 45, a 3-dose schedule is necessary (at 0, 1 to 2, and 6 months).7,8
The change to a 2-dose schedule was prompted by an evaluation of girls ages 9 to 13 randomized to receive either a 2- or 3-dose schedule. Antibody responses with a 2-dose schedule were not inferior to those of young women (ages 16 to 26) who received all 3 doses.9 The geometric mean titer ratios remained noninferior throughout the study period of 36 months.
However, a loss of noninferiority was noted for HPV-18 by 24 months and for HPV-6 by 36 months.9 Thus, further studies are needed to understand the duration of protection with a 2-dose schedule. Nevertheless, decreasing the number of doses makes it a more convenient and cost-effective option for many families.
The recommendations are the same for males except for one notable difference: in males ages 21 to 26, vaccination is not routinely recommended by the ACIP, but rather it is considered a “permissive use” recommendation: ie, the vaccine should be offered and final decisions on administration be made after individualized discussion with the patient.10 Permissive-use status also means the vaccine may not be covered by health insurance. Even though the vaccine is now available to men and women until age 45, many insurance plans do not cover it after age 26.
Children of either sex with a history of sexual abuse should receive their first vaccine dose beginning at age 9.7
Immunocompromised patients should follow the 3-dose schedule regardless of their sex or the age when vaccination was initiated.10
For transgender patients and for men not previously vaccinated who have sex with men, the 3-dose schedule vaccine should be given by the age of 26 (this is a routine recommendation, not a permissive one).8
CHALLENGES OF VACCINATION
Effective patient and family counseling is important. Even though the first HPV vaccine was approved in 2006, only 34.9% of US adolescents were fully vaccinated by 2015. This was in part because providers did not recommend it, were unfamiliar with it, or had concerns about its safety,11,12 and in part because some parents refused it.
The physician must address any myths regarding HPV vaccination and ensure that parents and patients understand that HPV vaccine is safe and effective. Studies have shown that with high-quality recommendations (ie, the care provider strongly endorses the HPV vaccine, encourages same-day vaccination, and discusses cancer prevention), patients are 9 times more likely to start the HPV vaccination schedule and 3 times more likely to follow through with subsequent doses.13
Providing good family and patient education does not necessarily require spending more counseling time. A recent study showed that spending less time discussing the HPV vaccine can lead to better vaccine coverage.14 The study compared parent HPV vaccine counseling techniques and found that simply informing patients and their families that the HPV vaccine was due was associated with a higher vaccine acceptance rate than inviting conversations about it.14 When providers announced that the vaccine was due, assuming the parents were ready to vaccinate, there was a 5.4% increase in HPV vaccination coverage.14
Conversely, physicians who engaged parents in open-ended discussions about the HPV vaccine did not improve HPV vaccination coverage.14 The authors suggested that providers approach HPV vaccination as if they were counseling patients and families about the need to avoid second-hand smoke or the need to use car seats. If parents or patients resist the presumptive announcement approach, expanded counseling and shared decision-making are appropriate. This includes addressing misconceptions that parents and patients may have about the HPV vaccine. The American Cancer Society lists 8 facts to reference (Table 2).15
SECONDARY PREVENTION: CERVICAL CANCER SCREENING
Since the introduction of the Papanicolaou (Pap) test, US cervical cancer incidence rates have decreased by more than 60%.16 Because almost all cervical cancer is preventable with proper screening, all women ages 21 to 65 should be screened.
Currently, there are 3 options available for cervical cancer screening: the Pap-only test, the Pap-HPV cotest, and the high-risk HPV-only test (Table 3). The latter 2 options detect high-risk HPV genotypes.
Several organizations have screening algorithms that recommend when to use these tests, but the 3 that shape today’s standard of care in cervical cancer screening come from the American College of Obstetricians and Gynecologists (ACOG), the American Society for Colposcopy and Cervical Pathology (ASCCP), and US Preventive Services Task Force (USPSTF).17–19
Pap-only testing is performed every 3 years to screen for cervical neoplasia that might indicate premalignancy.
Pap-HPV cotesting is performed every 5 years in women older than 30 with past normal screening. Until 2018, all 3 organizations recommended cotesting as the preferred screening algorithm for women ages 30 to 65.17–19 Patients with a history of abnormal test results require more frequent testing as recommended by the ASCCP.18
The high-risk HPV-only test utilizes real-time polymerase chain reaction to detect HPV 16, HPV 18, and 12 other HPV genotypes. Only 2 tests are approved by the FDA as stand-alone cervical cancer screening tests—the Roche Cobas HPV test approved in 2014 and the Becton Dickinson Onclarity HPV assay approved in 2018. Other HPV tests that are used in a cotesting strategy should not be used for high-risk HPV-only testing because their performance characteristics may differ.
In 2015, the Addressing the Need for Advanced HPV Diagnostics (ATHENA) study showed that 1 round of high-risk HPV-only screening for women older than 25 was more sensitive than Pap-only or cotesting for stage 3 cervical intraepithelial neoplasia or more severe disease (after 3 years of follow-up).20 Current guidelines from ASCCP18 and ACOG17 state that the high-risk HPV test can be repeated every 3 years (when used to screen by itself) if the woman is older than 25 and has had a normal test result.
If the HPV test result is positive for high-risk HPV 16 or 18 genotypes, then immediate colposcopy is indicated; women who test positive for one of the other 12 high-risk subtypes will need to undergo a Pap test to determine the appropriate follow-up (Figure 1).18,21
In 2018, the USPSTF updated its recommendations, noting that for women age 30 to 65, Pap-only testing every 3 years, cotesting every 5 years, or high-risk HPV-only testing every 5 years are all appropriate screening strategies, with the Pap-only or high-risk HPV-only screenings being preferred.19 This is in contrast to ACOG and ASCCP recommendations for cotesting every 5 years, with alternative options of Pap-only or HPV-only testing being done every 3 years.17,18
Is there a best screening protocol?
The USPSTF reviewed large randomized and observational studies to summarize the effectiveness of the 3 screening strategies and commissioned a decision analysis model to compare the risks, benefits, and costs of the 3 screening algorithms. The guideline statement notes both cotesting and high-risk HPV testing offer similar cancer detection rates: each prevents 1 additional cancer per 1,000 women screened as opposed to Pap-only testing.19
Also, tests that incorporate high-risk HPV screening may offer better detection of cervical adenocarcinoma (which has a worse prognosis than the more common squamous cell carcinoma type). However, both HPV-based screening strategies are more likely to require additional colposcopies for follow-up than Pap-only screening (1,630 colposcopies required for each cancer prevented with high-risk HPV alone, 1,635 with cotesting). Colposcopy is a simple office procedure that causes minimal discomfort to the patient.
The USPSTF guideline also differs in the recommended frequency of high-risk HPV-only testing; a high-risk HPV result should be repeated every 5 years if normal (as opposed to every 3 years as recommended by ACOG and ASCCP).19 The 5-year recommendation is based on analysis modeling, which suggests that performing high-risk HPV-only testing more frequently is unlikely to improve detection rates but will increase the number of screening tests and colposcopies.19
No trial has directly compared cotesting with high-risk HPV testing for more than 2 rounds of screening. The updated USPSTF recommendations are based on modeling estimates and expert opinion, which assesses cost and benefit vs harm in the long term. Also, no high-risk HPV test is currently FDA-approved for every-5-year screening when used by itself.
All 3 cervical cancer screening methods provide highly effective cancer prevention, so it is important for providers to choose the strategy that best fits their practice. The most critical aspect of screening is getting all women screened, no matter which method is used.
It is critical to remember that the screening intervals are intended for patients without symptoms. Those who have new concerns such as bleeding should have a diagnostic Pap done to evaluate their symptoms.
Follow-up of abnormal results
Regardless of the pathway chosen, appropriate follow-up of any abnormal test result is critical to the early detection of cancer. Established follow-up guidelines exist,22,23 but accessing this information can be difficult for the busy clinician. The ASCCP has a mobile phone application that outlines the action steps corresponding to the patient’s age and results of any combination of Pap or HPV testing. The app also includes the best screening algorithms for a particular patient.24
All guidelines agree that cervical cancer screening should start at age 21, regardless of HPV vaccination status or age of sexual initiation.17,18,25 Screening can be discontinued at age 65 for women with normal screening results in the prior decade (3 consecutive negative Pap results or 2 consecutive negative cotest results).23
For women who have had a total hysterectomy and no history of cervical neoplasia, screening should be stopped immediately after the procedure. However, several high-risk groups of women will need continued screening past the age of 65, or after a hysterectomy.
For a woman with a history of stage 2 cervical intraepithelial neoplasia or higher grade lesions, routine screening is continued for an additional 20 years, even if she is over age 65. Pap-only testing every 3 years is acceptable, because the role of HPV testing is unclear after hysterectomy.23 Prior guidelines suggested annual screening in these patients, so the change to every 3 years is notable. Many gynecologic oncologists will recommend that women with a history of cervical cancer continue annual screening indefinitely.
Within the first 2 to 3 years after treatment for high-grade dysplastic changes, annual follow-up is done by the gynecologic oncology team. Providers who offer follow-up during this time frame should keep in communication with the oncology team to ensure appropriate, individualized care. These recommendations are based on expert opinion, so variations in clinical practice may be seen.
Women infected with the human immunodeficiency virus can have Pap-only testing every 3 years, after a series of 3 normal annual Pap results.26 But screening does not stop at age 65.23,26 For patients who are immunosuppressed or have a history of diethylstilbestrol exposure, screening should be done annually indefinitely.23
- Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 2010; 202(12):1789–1799. doi:10.1086/657321
- de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancer attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012; 13(6):607–615. doi:10.1016/S1470-2045(12)70137-7
- American Cancer Society. Key statistics for cervical cancer. www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed February 14, 2019.
- Thaxton L, Waxman AG. Cervical cancer prevention: immunization and screening 2015. Med Clin North Am 2015; 99(3):469–477. doi:10.1016/j.mcna.2015.01.003
- McNamara M, Batur P, Walsh JME, Johnson KM. HPV update: vaccination, screening, and associated disease. J Gen Intern Med 2016; 31(11):1360–1366. doi:10.1007/s11606-016-3725-z
- Guo F, Cofie LE, Berenson AB. Cervical cancer incidence in young US females after human papillomavirus vaccine introduction. Am J Prev Med 2018; 55(2):197–204. doi:10.1016/j.amepre.2018.03.013
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016; 65(49):1405–1408. doi:10.15585/mmwr.mm6549a5
- Centers for Disease Control and Prevention (CDC). Supplemental information and guidance for vaccination providers regarding use of 9-valent HPV vaccine Information for persons who started an HPV vaccination series with quadrivalent or bivalent HPV vaccine. www.cdc.gov/hpv/downloads/9vhpv-guidance.pdf. Accessed February 14, 2019.
- Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309(17):1793–1802. doi:10.1001/jama.2013.1625
- Markowitz LE, Dunne EF, Saraiya M, et al; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63(RR-05):1–30. pmid:25167164
- Thompson EL, Rosen BL, Vamos CA, Kadono M, Daley EM. Human papillomavirus vaccination: what are the reasons for nonvaccination among US adolescents? J Adolesc Health 2017; 61(3):288–293. doi:10.1016/j.jadohealth.2017.05.015
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2015. MMWR Morb Mortal Wkly Rep 2016; 65(33):850–858. doi:10.15585/mmwr.mm6533a4
- Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: The impact of recommendation quality. Vaccine 2016; 34(9):1187–1192. doi:10.1016/j.vaccine.2016.01.023
- Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements versus conversations to improve HPV vaccination coverage: a randomized trial. Pediatrics 2017; 139(1):e20161764. doi:10.1542/peds.2016-1764
- American Cancer Society. HPV vaccine facts. www.cancer.org/cancer/cancer-causes/infectious-agents/hpv/hpv-vaccine-facts-and-fears.html. Accessed February 14, 2019.
- National Cancer Institute; Chasan R, Manrow R. Cervical cancer. https://report.nih.gov/nihfactsheets/viewfactsheet.aspx?csid=76. Accessed February 14, 2019.
- The American College of Obstetricians and Gynecologists (ACOG). Frequently asked questions. Cervical cancer screening. www.acog.org/Patients/FAQs/Cervical-Cancer-Screening. Accessed February 14, 2019.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137(4):516–542. doi:10.1309/AJCPTGD94EVRSJCG
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol 2015; 136(2):189–197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol 2015; 125(2):330–337. doi:10.1097/AOG.0000000000000669
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 2013; 121(4):829–846. doi:10.1097/AOG.0b013e3182883a34
- Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol 2016; 128(4):e111–e130. doi:10.1097/AOG.0000000000001708
- ASCCP. Mobile app. http://www.asccp.org/store-detail2/asccp-mobile-app. Accessed February 14, 2019.
- USPSTF. Draft recommendation: cervical cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Accessed February 14, 2019.
- Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE; National Institutes of Health; Centers for Disease Control and Prevention; HIV Medicine Association of the Infectious Diseases Society of America. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58(9):1308–1311. doi:10.1093/cid/ciu094
- Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 2010; 202(12):1789–1799. doi:10.1086/657321
- de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancer attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012; 13(6):607–615. doi:10.1016/S1470-2045(12)70137-7
- American Cancer Society. Key statistics for cervical cancer. www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed February 14, 2019.
- Thaxton L, Waxman AG. Cervical cancer prevention: immunization and screening 2015. Med Clin North Am 2015; 99(3):469–477. doi:10.1016/j.mcna.2015.01.003
- McNamara M, Batur P, Walsh JME, Johnson KM. HPV update: vaccination, screening, and associated disease. J Gen Intern Med 2016; 31(11):1360–1366. doi:10.1007/s11606-016-3725-z
- Guo F, Cofie LE, Berenson AB. Cervical cancer incidence in young US females after human papillomavirus vaccine introduction. Am J Prev Med 2018; 55(2):197–204. doi:10.1016/j.amepre.2018.03.013
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016; 65(49):1405–1408. doi:10.15585/mmwr.mm6549a5
- Centers for Disease Control and Prevention (CDC). Supplemental information and guidance for vaccination providers regarding use of 9-valent HPV vaccine Information for persons who started an HPV vaccination series with quadrivalent or bivalent HPV vaccine. www.cdc.gov/hpv/downloads/9vhpv-guidance.pdf. Accessed February 14, 2019.
- Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309(17):1793–1802. doi:10.1001/jama.2013.1625
- Markowitz LE, Dunne EF, Saraiya M, et al; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63(RR-05):1–30. pmid:25167164
- Thompson EL, Rosen BL, Vamos CA, Kadono M, Daley EM. Human papillomavirus vaccination: what are the reasons for nonvaccination among US adolescents? J Adolesc Health 2017; 61(3):288–293. doi:10.1016/j.jadohealth.2017.05.015
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2015. MMWR Morb Mortal Wkly Rep 2016; 65(33):850–858. doi:10.15585/mmwr.mm6533a4
- Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: The impact of recommendation quality. Vaccine 2016; 34(9):1187–1192. doi:10.1016/j.vaccine.2016.01.023
- Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements versus conversations to improve HPV vaccination coverage: a randomized trial. Pediatrics 2017; 139(1):e20161764. doi:10.1542/peds.2016-1764
- American Cancer Society. HPV vaccine facts. www.cancer.org/cancer/cancer-causes/infectious-agents/hpv/hpv-vaccine-facts-and-fears.html. Accessed February 14, 2019.
- National Cancer Institute; Chasan R, Manrow R. Cervical cancer. https://report.nih.gov/nihfactsheets/viewfactsheet.aspx?csid=76. Accessed February 14, 2019.
- The American College of Obstetricians and Gynecologists (ACOG). Frequently asked questions. Cervical cancer screening. www.acog.org/Patients/FAQs/Cervical-Cancer-Screening. Accessed February 14, 2019.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137(4):516–542. doi:10.1309/AJCPTGD94EVRSJCG
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol 2015; 136(2):189–197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol 2015; 125(2):330–337. doi:10.1097/AOG.0000000000000669
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 2013; 121(4):829–846. doi:10.1097/AOG.0b013e3182883a34
- Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol 2016; 128(4):e111–e130. doi:10.1097/AOG.0000000000001708
- ASCCP. Mobile app. http://www.asccp.org/store-detail2/asccp-mobile-app. Accessed February 14, 2019.
- USPSTF. Draft recommendation: cervical cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Accessed February 14, 2019.
- Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE; National Institutes of Health; Centers for Disease Control and Prevention; HIV Medicine Association of the Infectious Diseases Society of America. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58(9):1308–1311. doi:10.1093/cid/ciu094
KEY POINTS
- Immunization against HPV can prevent up to 70% of HPV-related cervical cancer cases.
- Gardasil 9 is the only HPV vaccine currently available in the United States and is now approved for use in males and females between the ages of 9 and 45.
- In girls and boys younger than 15, a 2-dose schedule is recommended; patients ages 15 through 45 require 3 doses.
- Vaccine acceptance rates are highest when primary care providers announce that the vaccine is due rather than invite open-ended discussions.
- Regular cervical cancer screening is an important preventive tool and should be performed using the Papanicolaou (Pap) test, the high-risk HPV-only test, or the Pap-HPV cotest.
Anthrax booster expanded to 3 years for moderate-risk groups
A booster dose for pre-exposure prophylaxis with an anthrax vaccine may be given at 3 years after an initial series for individuals not currently at risk who wish to maintain protection, according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
In a unanimous 15-0 vote at the February meeting, ACIP committee members agreed on the recommendation after adjusting the wording to reflect a permissive, rather than mandated, guidance.
William Bower, MD, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), presented data on Anthrax Vaccine Adsorbed (AVA) to support its protective effects over a longer booster dose interval.
The recommendations apply to persons aged 18 years or older who are not currently at high risk of exposure to Bacillus anthracis, but who might need to deploy to a high-risk area quickly, such as military personnel, Dr. Bower said.
In addition, data suggest that adults who have started, but not completed the pre-exposure priming series, can transition to the postexposure schedule prior to entering a high-risk area, he noted.
The previous pre-exposure anthrax vaccination schedule was a three-dose priming series at 0, 1, and 3 months, followed by a booster at 12 months and 18 months, then annually.
with “sustained immunological memory to at least month 42,” and suggested that even longer intervals between boosters may be possible, Dr. Bower said.
A dosing schedule of intramuscular injections at 0 and at 1 month and 6 months, with a booster at 42 months yielded survival estimates of approximately 84%-93%.
Dr. Bower noted that a new vaccine, AV7909, has demonstrated safety and effectiveness similar to AVA and could be used for pre-exposure prophylaxis if AVA is not available. AVA remains the preferred option, but ultimately will be replaced by AV7909, when the current AVA stockpile is exhausted.
Additional safety data on AV7909 will be reviewed by ACIP as they become available, and future guidance from the CDC will include statements on dosing for special populations including pregnant and breastfeeding women, said Dr. Bower.
“We anticipate that this [anthrax vaccine] work group will reconvene in 2021 to review data from pending studies” of AV7909, he said.
The ACIP members had no financial conflicts to disclose.
A booster dose for pre-exposure prophylaxis with an anthrax vaccine may be given at 3 years after an initial series for individuals not currently at risk who wish to maintain protection, according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
In a unanimous 15-0 vote at the February meeting, ACIP committee members agreed on the recommendation after adjusting the wording to reflect a permissive, rather than mandated, guidance.
William Bower, MD, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), presented data on Anthrax Vaccine Adsorbed (AVA) to support its protective effects over a longer booster dose interval.
The recommendations apply to persons aged 18 years or older who are not currently at high risk of exposure to Bacillus anthracis, but who might need to deploy to a high-risk area quickly, such as military personnel, Dr. Bower said.
In addition, data suggest that adults who have started, but not completed the pre-exposure priming series, can transition to the postexposure schedule prior to entering a high-risk area, he noted.
The previous pre-exposure anthrax vaccination schedule was a three-dose priming series at 0, 1, and 3 months, followed by a booster at 12 months and 18 months, then annually.
with “sustained immunological memory to at least month 42,” and suggested that even longer intervals between boosters may be possible, Dr. Bower said.
A dosing schedule of intramuscular injections at 0 and at 1 month and 6 months, with a booster at 42 months yielded survival estimates of approximately 84%-93%.
Dr. Bower noted that a new vaccine, AV7909, has demonstrated safety and effectiveness similar to AVA and could be used for pre-exposure prophylaxis if AVA is not available. AVA remains the preferred option, but ultimately will be replaced by AV7909, when the current AVA stockpile is exhausted.
Additional safety data on AV7909 will be reviewed by ACIP as they become available, and future guidance from the CDC will include statements on dosing for special populations including pregnant and breastfeeding women, said Dr. Bower.
“We anticipate that this [anthrax vaccine] work group will reconvene in 2021 to review data from pending studies” of AV7909, he said.
The ACIP members had no financial conflicts to disclose.
A booster dose for pre-exposure prophylaxis with an anthrax vaccine may be given at 3 years after an initial series for individuals not currently at risk who wish to maintain protection, according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
In a unanimous 15-0 vote at the February meeting, ACIP committee members agreed on the recommendation after adjusting the wording to reflect a permissive, rather than mandated, guidance.
William Bower, MD, of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), presented data on Anthrax Vaccine Adsorbed (AVA) to support its protective effects over a longer booster dose interval.
The recommendations apply to persons aged 18 years or older who are not currently at high risk of exposure to Bacillus anthracis, but who might need to deploy to a high-risk area quickly, such as military personnel, Dr. Bower said.
In addition, data suggest that adults who have started, but not completed the pre-exposure priming series, can transition to the postexposure schedule prior to entering a high-risk area, he noted.
The previous pre-exposure anthrax vaccination schedule was a three-dose priming series at 0, 1, and 3 months, followed by a booster at 12 months and 18 months, then annually.
with “sustained immunological memory to at least month 42,” and suggested that even longer intervals between boosters may be possible, Dr. Bower said.
A dosing schedule of intramuscular injections at 0 and at 1 month and 6 months, with a booster at 42 months yielded survival estimates of approximately 84%-93%.
Dr. Bower noted that a new vaccine, AV7909, has demonstrated safety and effectiveness similar to AVA and could be used for pre-exposure prophylaxis if AVA is not available. AVA remains the preferred option, but ultimately will be replaced by AV7909, when the current AVA stockpile is exhausted.
Additional safety data on AV7909 will be reviewed by ACIP as they become available, and future guidance from the CDC will include statements on dosing for special populations including pregnant and breastfeeding women, said Dr. Bower.
“We anticipate that this [anthrax vaccine] work group will reconvene in 2021 to review data from pending studies” of AV7909, he said.
The ACIP members had no financial conflicts to disclose.
FROM AN ACIP MEETING
ACIP unanimously supports updates to Japanese encephalitis vaccination
according to a vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
Japanese encephalitis (JE) virus is a mosquito-borne flavivirus and those at risk for infection include travelers to countries where JE is endemic, as well as laboratory personnel who work with the virus.
The committee voted unanimously 15-0 in favor of the recommendations, which also advised vaccination for those whose travels in endemic areas are uncertain, but not for travelers with low-risk itineraries “such as shorter term travel limited to urban areas or travel that occurs outside of a well-defined JE virus transmission season.”
Susan Hills, MD, of the of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, presented data in support of the recommendations.
A second unanimous vote confirmed recommendations for a primary series schedule for JE vaccination for adults aged 18-65 years as “two doses of vaccine administered on days 0 and 7-28.”
The third vote, also a unanimous 15-0, updated recommendations for a JE booster dose. The new recommendation is that adults and children receive a booster dose (a third dose) at least a year after completion of the primary JE vaccine series “if ongoing exposure or re-exposure to JE virus is expected.”
The currently available Japanese encephalitis vaccine in the United States is an inactivated Vero cell culture-derived vaccine marketed as IXIARO that was approved in March 2009 for individuals aged 17 years and older and approved in May 2013 for children aged 2 months through 16 years.
The ACIP members had no financial conflicts to disclose.
according to a vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
Japanese encephalitis (JE) virus is a mosquito-borne flavivirus and those at risk for infection include travelers to countries where JE is endemic, as well as laboratory personnel who work with the virus.
The committee voted unanimously 15-0 in favor of the recommendations, which also advised vaccination for those whose travels in endemic areas are uncertain, but not for travelers with low-risk itineraries “such as shorter term travel limited to urban areas or travel that occurs outside of a well-defined JE virus transmission season.”
Susan Hills, MD, of the of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, presented data in support of the recommendations.
A second unanimous vote confirmed recommendations for a primary series schedule for JE vaccination for adults aged 18-65 years as “two doses of vaccine administered on days 0 and 7-28.”
The third vote, also a unanimous 15-0, updated recommendations for a JE booster dose. The new recommendation is that adults and children receive a booster dose (a third dose) at least a year after completion of the primary JE vaccine series “if ongoing exposure or re-exposure to JE virus is expected.”
The currently available Japanese encephalitis vaccine in the United States is an inactivated Vero cell culture-derived vaccine marketed as IXIARO that was approved in March 2009 for individuals aged 17 years and older and approved in May 2013 for children aged 2 months through 16 years.
The ACIP members had no financial conflicts to disclose.
according to a vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
Japanese encephalitis (JE) virus is a mosquito-borne flavivirus and those at risk for infection include travelers to countries where JE is endemic, as well as laboratory personnel who work with the virus.
The committee voted unanimously 15-0 in favor of the recommendations, which also advised vaccination for those whose travels in endemic areas are uncertain, but not for travelers with low-risk itineraries “such as shorter term travel limited to urban areas or travel that occurs outside of a well-defined JE virus transmission season.”
Susan Hills, MD, of the of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, presented data in support of the recommendations.
A second unanimous vote confirmed recommendations for a primary series schedule for JE vaccination for adults aged 18-65 years as “two doses of vaccine administered on days 0 and 7-28.”
The third vote, also a unanimous 15-0, updated recommendations for a JE booster dose. The new recommendation is that adults and children receive a booster dose (a third dose) at least a year after completion of the primary JE vaccine series “if ongoing exposure or re-exposure to JE virus is expected.”
The currently available Japanese encephalitis vaccine in the United States is an inactivated Vero cell culture-derived vaccine marketed as IXIARO that was approved in March 2009 for individuals aged 17 years and older and approved in May 2013 for children aged 2 months through 16 years.
The ACIP members had no financial conflicts to disclose.
FROM AN ACIP MEETING
Fauci, Messonnier testify on measles outbreaks
Officials from the Centers for Disease Control and Prevention and the National Institute for Allergy and Infectious Diseases stressed the safety of measles vaccines and warned that misinformation is among the factors keeping more children from being vaccinated.
With nearly 160 cases of measles in 10 states during Jan. 1–Feb. 21, a disease once eradicated from the United States is resurfacing, with most cases affecting those who have not been vaccinated.
“Measles outbreaks have been and continue to be a constant threat to the health of the American people,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC, testified at a Feb. 27 hearing of the Oversight and Investigations Subcommittee of the House Energy and Commerce Committee.
She noted that unvaccinated Americans traveling abroad are at risk for contracting the infection, and thus are at risk of spreading it when they return home. Foreigners coming to the country also carry the potential to spread the infection.
“Nationally, we enjoy high measles vaccination coverage,” Dr. Messonnier said.”There are pockets of people who are vaccine hesitant, who delay or even refuse to vaccinate themselves and their children. Outbreaks of measles occur when measles gets into these communities of unvaccinated people.”
She noted that those who eschew vaccination tend to live near one another and share common religious beliefs or racial or ethnic backgrounds.
She continued that vaccine hesitancy “is the result of the misunderstanding of the risk and seriousness of disease, combined with misinformation of the safety and effectiveness of vaccines. However, the specific issues fueling hesitancy varies by community.”
Strategies to increase vaccination need to be localized with national support from the CDC, Dr. Messonnier said, adding that rapid response is critical to control outbreaks.
Anthony Fauci, MD, director of the National Institute for Allergy and Infectious Diseases at NIH, agreed.
“I consider it really an irony that you have one of the most contagious viruses known to man juxtaposed against one of the most effective vaccines that we have and yet we don’t do and have not done what could be done, namely completely eliminate and eradicate this virus,” Dr. Fauci said.
Dr. Messonnier stressed that the only way to protect against measles is to get vaccinated.
“If they have questions, they should talk to their doctor,” she added. “Their doctor can provide them with more information about measles, answer their questions and reassure them so they go ahead and get vaccinated.”
Dr. Fauci concurred and added that “we should look upon it in two approaches. One, it’s for the safety of your own child. The other is a responsibility to the community. ... We all have a responsibility to be part of that umbrella of herd immunity and once it goes down below a certain percentage, then you have danger to the entire society.”
He stressed that the CDC is a good website to combat much of the misinformation that is floating around on the Internet.
The committee panel, while taking an interest in the recent outbreaks, did not hint at any specific legislative actions were being considered.
Officials from the Centers for Disease Control and Prevention and the National Institute for Allergy and Infectious Diseases stressed the safety of measles vaccines and warned that misinformation is among the factors keeping more children from being vaccinated.
With nearly 160 cases of measles in 10 states during Jan. 1–Feb. 21, a disease once eradicated from the United States is resurfacing, with most cases affecting those who have not been vaccinated.
“Measles outbreaks have been and continue to be a constant threat to the health of the American people,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC, testified at a Feb. 27 hearing of the Oversight and Investigations Subcommittee of the House Energy and Commerce Committee.
She noted that unvaccinated Americans traveling abroad are at risk for contracting the infection, and thus are at risk of spreading it when they return home. Foreigners coming to the country also carry the potential to spread the infection.
“Nationally, we enjoy high measles vaccination coverage,” Dr. Messonnier said.”There are pockets of people who are vaccine hesitant, who delay or even refuse to vaccinate themselves and their children. Outbreaks of measles occur when measles gets into these communities of unvaccinated people.”
She noted that those who eschew vaccination tend to live near one another and share common religious beliefs or racial or ethnic backgrounds.
She continued that vaccine hesitancy “is the result of the misunderstanding of the risk and seriousness of disease, combined with misinformation of the safety and effectiveness of vaccines. However, the specific issues fueling hesitancy varies by community.”
Strategies to increase vaccination need to be localized with national support from the CDC, Dr. Messonnier said, adding that rapid response is critical to control outbreaks.
Anthony Fauci, MD, director of the National Institute for Allergy and Infectious Diseases at NIH, agreed.
“I consider it really an irony that you have one of the most contagious viruses known to man juxtaposed against one of the most effective vaccines that we have and yet we don’t do and have not done what could be done, namely completely eliminate and eradicate this virus,” Dr. Fauci said.
Dr. Messonnier stressed that the only way to protect against measles is to get vaccinated.
“If they have questions, they should talk to their doctor,” she added. “Their doctor can provide them with more information about measles, answer their questions and reassure them so they go ahead and get vaccinated.”
Dr. Fauci concurred and added that “we should look upon it in two approaches. One, it’s for the safety of your own child. The other is a responsibility to the community. ... We all have a responsibility to be part of that umbrella of herd immunity and once it goes down below a certain percentage, then you have danger to the entire society.”
He stressed that the CDC is a good website to combat much of the misinformation that is floating around on the Internet.
The committee panel, while taking an interest in the recent outbreaks, did not hint at any specific legislative actions were being considered.
Officials from the Centers for Disease Control and Prevention and the National Institute for Allergy and Infectious Diseases stressed the safety of measles vaccines and warned that misinformation is among the factors keeping more children from being vaccinated.
With nearly 160 cases of measles in 10 states during Jan. 1–Feb. 21, a disease once eradicated from the United States is resurfacing, with most cases affecting those who have not been vaccinated.
“Measles outbreaks have been and continue to be a constant threat to the health of the American people,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC, testified at a Feb. 27 hearing of the Oversight and Investigations Subcommittee of the House Energy and Commerce Committee.
She noted that unvaccinated Americans traveling abroad are at risk for contracting the infection, and thus are at risk of spreading it when they return home. Foreigners coming to the country also carry the potential to spread the infection.
“Nationally, we enjoy high measles vaccination coverage,” Dr. Messonnier said.”There are pockets of people who are vaccine hesitant, who delay or even refuse to vaccinate themselves and their children. Outbreaks of measles occur when measles gets into these communities of unvaccinated people.”
She noted that those who eschew vaccination tend to live near one another and share common religious beliefs or racial or ethnic backgrounds.
She continued that vaccine hesitancy “is the result of the misunderstanding of the risk and seriousness of disease, combined with misinformation of the safety and effectiveness of vaccines. However, the specific issues fueling hesitancy varies by community.”
Strategies to increase vaccination need to be localized with national support from the CDC, Dr. Messonnier said, adding that rapid response is critical to control outbreaks.
Anthony Fauci, MD, director of the National Institute for Allergy and Infectious Diseases at NIH, agreed.
“I consider it really an irony that you have one of the most contagious viruses known to man juxtaposed against one of the most effective vaccines that we have and yet we don’t do and have not done what could be done, namely completely eliminate and eradicate this virus,” Dr. Fauci said.
Dr. Messonnier stressed that the only way to protect against measles is to get vaccinated.
“If they have questions, they should talk to their doctor,” she added. “Their doctor can provide them with more information about measles, answer their questions and reassure them so they go ahead and get vaccinated.”
Dr. Fauci concurred and added that “we should look upon it in two approaches. One, it’s for the safety of your own child. The other is a responsibility to the community. ... We all have a responsibility to be part of that umbrella of herd immunity and once it goes down below a certain percentage, then you have danger to the entire society.”
He stressed that the CDC is a good website to combat much of the misinformation that is floating around on the Internet.
The committee panel, while taking an interest in the recent outbreaks, did not hint at any specific legislative actions were being considered.
REPORTING FROM HOUSE COMMITTEE HEARING
U.S. measles cases up to 159 for the year
Reported measles cases are now up to 159 for the year in the United States, according to the Centers for Disease Control and Prevention.

The most recent reporting week, which ended Feb. 21, brought another 32 cases of measles and one new outbreak of 4 cases in Illinois. The total number of outbreaks – an outbreak is defined as three or more cases – is now six, and cases have been reported in 10 states, the CDC said Feb. 25.
The majority (17) of those 32 new cases occurred in Brooklyn, one of New York state’s three outbreaks this year. The largest of the 2019 outbreaks is in Washington state, primarily in Clark County, and is up to 66 cases after 4 more were reported in the last week by the state’s department of health. The outbreaks are linked to travelers who brought the disease to the United States.
There are now two measures “advancing through the [Washington] state legislature that would bar parents from using personal or philosophical exemptions to avoid immunizing their school-age children. Both have bipartisan support despite strong antivaccination sentiment in parts of the state,” the Washington Post said on Feb. 25.
Reported measles cases are now up to 159 for the year in the United States, according to the Centers for Disease Control and Prevention.

The most recent reporting week, which ended Feb. 21, brought another 32 cases of measles and one new outbreak of 4 cases in Illinois. The total number of outbreaks – an outbreak is defined as three or more cases – is now six, and cases have been reported in 10 states, the CDC said Feb. 25.
The majority (17) of those 32 new cases occurred in Brooklyn, one of New York state’s three outbreaks this year. The largest of the 2019 outbreaks is in Washington state, primarily in Clark County, and is up to 66 cases after 4 more were reported in the last week by the state’s department of health. The outbreaks are linked to travelers who brought the disease to the United States.
There are now two measures “advancing through the [Washington] state legislature that would bar parents from using personal or philosophical exemptions to avoid immunizing their school-age children. Both have bipartisan support despite strong antivaccination sentiment in parts of the state,” the Washington Post said on Feb. 25.
Reported measles cases are now up to 159 for the year in the United States, according to the Centers for Disease Control and Prevention.

The most recent reporting week, which ended Feb. 21, brought another 32 cases of measles and one new outbreak of 4 cases in Illinois. The total number of outbreaks – an outbreak is defined as three or more cases – is now six, and cases have been reported in 10 states, the CDC said Feb. 25.
The majority (17) of those 32 new cases occurred in Brooklyn, one of New York state’s three outbreaks this year. The largest of the 2019 outbreaks is in Washington state, primarily in Clark County, and is up to 66 cases after 4 more were reported in the last week by the state’s department of health. The outbreaks are linked to travelers who brought the disease to the United States.
There are now two measures “advancing through the [Washington] state legislature that would bar parents from using personal or philosophical exemptions to avoid immunizing their school-age children. Both have bipartisan support despite strong antivaccination sentiment in parts of the state,” the Washington Post said on Feb. 25.
Pseudoscience redux
My most recent column discussed the problem of pseudoscience that pervades some corners of the Internet. Personally, I respond to pseudoscience primarily by trying to provide accurate and less-biased information. I recognize that not everyone approaches decision making by seeking more information. When dealing a diverse public, a medical professional needs to have other approaches in the armamentarium.1 When dealing with other physicians, I am less flexible. Either the profession of medicine believes in science or it doesn’t.
Since that column was published, there have been major developments. There are measles outbreaks in the states of Washington and New York, and more than 100 deaths from a measles epidemic in the Philippines. The World Health Organization has made vaccine hesitancy one of its ten threats to global health in 2019.
Facebook has indicated that it might demote the priority and frequency with which it recommends articles that promulgate anti-vax information and conspiracy theories.2 Facebook isn’t doing this because it has had an epiphany; it has come under pressure for its role in the spread of misinformation. Current legislation was written before the rise of social media, when Internet Service Providers were primarily conduits to transfer bits and bytes between computers. Those ISPs were not liable for the content of the transmitted Web pages. Facebook, by producing what it called a newsfeed and by making personalized suggestions for other websites to browse, doesn’t fit the passive model of an ISP.
For alleged violations of user’s privacy, Facebook might be subject to billion dollar fines, according to a Washington Post article.3 Still, for a company whose revenue is $4 billion per month and whose stock market value is $400 billion, paying a billion dollar fine for years of alleged misbehaviors that have enabled it to become a giant empire is, “in the scheme of things ... a speeding ticket” in the parlance of the penultimate scene of the movie The Social Network. The real financial risk is people deciding they can’t trust the platform and going elsewhere.
Authorities in the United Kingdom in February 2019 released a highly critical, 108-page report about fake news, which said, “Facebook should not be allowed to behave like ‘digital gangsters’ in the online world.”4 The U.K. report urges new regulations to deal with privacy breaches and with fake news. It endeavors to create a duty for social media companies to combat the spread of misinformation.
Then the Wall Street Journal reported that Pinterest has stopped returning results for searches related to vaccination.5 Pinterest realized that most of the shared images on its platform cautioned against vaccination, which contradicts the recommendations of medical experts. Unable to otherwise combat the flow of misinformation, the company apparently has decided to eliminate returning results, pro or con, for any search terms related to vaccines.
While lamenting the public’s inability to distinguish misinformation on the Internet, I’ve also been observing the factors that lead physicians astray. I expect physicians, as trained scientists and as professionals, to be able to assimilate new information and change their practices accordingly. Those who do research on the translation of technology find that, this doesn’t happen with any regularity.
The February 2019 issue of Hospital Pediatrics has four items on the topic of treating bronchiolitis, including two research articles, a brief report, and a commentary. That is obviously a relevant topic this time of year. The impression after reading those four items is that hospitalists don’t really know how to best treat the most common illness they encounter. And even when they “know” how to do it, many factors distort the science. Those factors are highlighted in the article on barriers to minimizing viral testing.6
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Discussing immunization with vaccine-hesitant parents requires caring, individualized approach,” by Jeff Craven, Pediatric News, Nov. 7, 2018; “How do you get anti-vaxxers to vaccinate their kids? Talk to them – for hours,” by Nadine Gartner, Washington Post, Feb. 19, 2019.
2. “Facebook will consider removing or demoting anti-vaccination recommendations amid backlash,” by Taylor Telford, Washington Post, Feb. 15, 2019.
3. “U.S. regulators have met to discuss imposing a record-setting fine against Facebook for privacy violations,” by Tony Romm and Elizabeth Dwoskin, Washington Post, Jan. 18, 2019; “Report: Facebook, FTC discussing ‘multibillion dollar’ fine,” by Associated Press.
4. “Disinformation and ‘fake news’: Final Report,” House of Commons, Feb. 18, 2019, p. 42, item 139.
5. “Pinterest blocks vaccination searches in move to control the conversation,” by Robert McMillan and Daniela Hernandez, The Wall Street Journal, Feb. 20, 2019.
6. “Barriers to minimizing respiratory viral testing in bronchiolitis: Physician perceptions on testing practices,” by MZ Huang et al. Hospital Pediatrics 2019 Feb. doi: 10.1542/hpeds.2018-0108.
My most recent column discussed the problem of pseudoscience that pervades some corners of the Internet. Personally, I respond to pseudoscience primarily by trying to provide accurate and less-biased information. I recognize that not everyone approaches decision making by seeking more information. When dealing a diverse public, a medical professional needs to have other approaches in the armamentarium.1 When dealing with other physicians, I am less flexible. Either the profession of medicine believes in science or it doesn’t.
Since that column was published, there have been major developments. There are measles outbreaks in the states of Washington and New York, and more than 100 deaths from a measles epidemic in the Philippines. The World Health Organization has made vaccine hesitancy one of its ten threats to global health in 2019.
Facebook has indicated that it might demote the priority and frequency with which it recommends articles that promulgate anti-vax information and conspiracy theories.2 Facebook isn’t doing this because it has had an epiphany; it has come under pressure for its role in the spread of misinformation. Current legislation was written before the rise of social media, when Internet Service Providers were primarily conduits to transfer bits and bytes between computers. Those ISPs were not liable for the content of the transmitted Web pages. Facebook, by producing what it called a newsfeed and by making personalized suggestions for other websites to browse, doesn’t fit the passive model of an ISP.
For alleged violations of user’s privacy, Facebook might be subject to billion dollar fines, according to a Washington Post article.3 Still, for a company whose revenue is $4 billion per month and whose stock market value is $400 billion, paying a billion dollar fine for years of alleged misbehaviors that have enabled it to become a giant empire is, “in the scheme of things ... a speeding ticket” in the parlance of the penultimate scene of the movie The Social Network. The real financial risk is people deciding they can’t trust the platform and going elsewhere.
Authorities in the United Kingdom in February 2019 released a highly critical, 108-page report about fake news, which said, “Facebook should not be allowed to behave like ‘digital gangsters’ in the online world.”4 The U.K. report urges new regulations to deal with privacy breaches and with fake news. It endeavors to create a duty for social media companies to combat the spread of misinformation.
Then the Wall Street Journal reported that Pinterest has stopped returning results for searches related to vaccination.5 Pinterest realized that most of the shared images on its platform cautioned against vaccination, which contradicts the recommendations of medical experts. Unable to otherwise combat the flow of misinformation, the company apparently has decided to eliminate returning results, pro or con, for any search terms related to vaccines.
While lamenting the public’s inability to distinguish misinformation on the Internet, I’ve also been observing the factors that lead physicians astray. I expect physicians, as trained scientists and as professionals, to be able to assimilate new information and change their practices accordingly. Those who do research on the translation of technology find that, this doesn’t happen with any regularity.
The February 2019 issue of Hospital Pediatrics has four items on the topic of treating bronchiolitis, including two research articles, a brief report, and a commentary. That is obviously a relevant topic this time of year. The impression after reading those four items is that hospitalists don’t really know how to best treat the most common illness they encounter. And even when they “know” how to do it, many factors distort the science. Those factors are highlighted in the article on barriers to minimizing viral testing.6
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Discussing immunization with vaccine-hesitant parents requires caring, individualized approach,” by Jeff Craven, Pediatric News, Nov. 7, 2018; “How do you get anti-vaxxers to vaccinate their kids? Talk to them – for hours,” by Nadine Gartner, Washington Post, Feb. 19, 2019.
2. “Facebook will consider removing or demoting anti-vaccination recommendations amid backlash,” by Taylor Telford, Washington Post, Feb. 15, 2019.
3. “U.S. regulators have met to discuss imposing a record-setting fine against Facebook for privacy violations,” by Tony Romm and Elizabeth Dwoskin, Washington Post, Jan. 18, 2019; “Report: Facebook, FTC discussing ‘multibillion dollar’ fine,” by Associated Press.
4. “Disinformation and ‘fake news’: Final Report,” House of Commons, Feb. 18, 2019, p. 42, item 139.
5. “Pinterest blocks vaccination searches in move to control the conversation,” by Robert McMillan and Daniela Hernandez, The Wall Street Journal, Feb. 20, 2019.
6. “Barriers to minimizing respiratory viral testing in bronchiolitis: Physician perceptions on testing practices,” by MZ Huang et al. Hospital Pediatrics 2019 Feb. doi: 10.1542/hpeds.2018-0108.
My most recent column discussed the problem of pseudoscience that pervades some corners of the Internet. Personally, I respond to pseudoscience primarily by trying to provide accurate and less-biased information. I recognize that not everyone approaches decision making by seeking more information. When dealing a diverse public, a medical professional needs to have other approaches in the armamentarium.1 When dealing with other physicians, I am less flexible. Either the profession of medicine believes in science or it doesn’t.
Since that column was published, there have been major developments. There are measles outbreaks in the states of Washington and New York, and more than 100 deaths from a measles epidemic in the Philippines. The World Health Organization has made vaccine hesitancy one of its ten threats to global health in 2019.
Facebook has indicated that it might demote the priority and frequency with which it recommends articles that promulgate anti-vax information and conspiracy theories.2 Facebook isn’t doing this because it has had an epiphany; it has come under pressure for its role in the spread of misinformation. Current legislation was written before the rise of social media, when Internet Service Providers were primarily conduits to transfer bits and bytes between computers. Those ISPs were not liable for the content of the transmitted Web pages. Facebook, by producing what it called a newsfeed and by making personalized suggestions for other websites to browse, doesn’t fit the passive model of an ISP.
For alleged violations of user’s privacy, Facebook might be subject to billion dollar fines, according to a Washington Post article.3 Still, for a company whose revenue is $4 billion per month and whose stock market value is $400 billion, paying a billion dollar fine for years of alleged misbehaviors that have enabled it to become a giant empire is, “in the scheme of things ... a speeding ticket” in the parlance of the penultimate scene of the movie The Social Network. The real financial risk is people deciding they can’t trust the platform and going elsewhere.
Authorities in the United Kingdom in February 2019 released a highly critical, 108-page report about fake news, which said, “Facebook should not be allowed to behave like ‘digital gangsters’ in the online world.”4 The U.K. report urges new regulations to deal with privacy breaches and with fake news. It endeavors to create a duty for social media companies to combat the spread of misinformation.
Then the Wall Street Journal reported that Pinterest has stopped returning results for searches related to vaccination.5 Pinterest realized that most of the shared images on its platform cautioned against vaccination, which contradicts the recommendations of medical experts. Unable to otherwise combat the flow of misinformation, the company apparently has decided to eliminate returning results, pro or con, for any search terms related to vaccines.
While lamenting the public’s inability to distinguish misinformation on the Internet, I’ve also been observing the factors that lead physicians astray. I expect physicians, as trained scientists and as professionals, to be able to assimilate new information and change their practices accordingly. Those who do research on the translation of technology find that, this doesn’t happen with any regularity.
The February 2019 issue of Hospital Pediatrics has four items on the topic of treating bronchiolitis, including two research articles, a brief report, and a commentary. That is obviously a relevant topic this time of year. The impression after reading those four items is that hospitalists don’t really know how to best treat the most common illness they encounter. And even when they “know” how to do it, many factors distort the science. Those factors are highlighted in the article on barriers to minimizing viral testing.6
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Discussing immunization with vaccine-hesitant parents requires caring, individualized approach,” by Jeff Craven, Pediatric News, Nov. 7, 2018; “How do you get anti-vaxxers to vaccinate their kids? Talk to them – for hours,” by Nadine Gartner, Washington Post, Feb. 19, 2019.
2. “Facebook will consider removing or demoting anti-vaccination recommendations amid backlash,” by Taylor Telford, Washington Post, Feb. 15, 2019.
3. “U.S. regulators have met to discuss imposing a record-setting fine against Facebook for privacy violations,” by Tony Romm and Elizabeth Dwoskin, Washington Post, Jan. 18, 2019; “Report: Facebook, FTC discussing ‘multibillion dollar’ fine,” by Associated Press.
4. “Disinformation and ‘fake news’: Final Report,” House of Commons, Feb. 18, 2019, p. 42, item 139.
5. “Pinterest blocks vaccination searches in move to control the conversation,” by Robert McMillan and Daniela Hernandez, The Wall Street Journal, Feb. 20, 2019.
6. “Barriers to minimizing respiratory viral testing in bronchiolitis: Physician perceptions on testing practices,” by MZ Huang et al. Hospital Pediatrics 2019 Feb. doi: 10.1542/hpeds.2018-0108.
Working With Parents to Vaccinate Children
Global outbreaks of infectious diseases—such as smallpox, pertussis, dysentery, and scarlet fever—seem like fodder for the history books. It was centuries ago that epidemics wiped out large swathes of the world population. Many people living and raising children today have never witnessed the devastating effects of measles, mumps, polio, and influenza—diseases that have been substantially reduced or even eradicated.1 Why? Because since the early 1900s, we have had scientifically developed and widely distributed vaccines at our disposal.
In context, it is incredible to realize that we are still in the beginning stages of vaccine research and development. From that perspective, it is perhaps not as surprising that some parents are hesitant to vaccinate their children—after all, do we really know everything we can and should know about inoculation? Parental resistance to or refusal of vaccination is further fueled by tainted research (Andrew Wakefield was forced to retract his findings that “validated” a link between thimerosal in vaccines and autism) and misinformation propagated on the Internet.2
But what has long been a source of frustration to those who support routine vaccination has, in recent years, started to become a public health issue. Measles outbreaks are no longer historical artifacts—they are real, as evidenced by the current rise in cases centered in Clark County, Washington. Through the first full week of February 2019, there were 101 confirmed cases of measles in the US, half of which occurred in Washington State—leading the governor to declare a public health emergency.3
This has, of course, reinvigorated the ongoing discussion about parental refusal to vaccinate. Enough has been said on this topic, by both public officials and private individuals, in a variety of venues over the years. So I’d like to focus instead on the role that individual health care providers can play in this situation.
Over the years, many of my colleagues have shared stories about parents who have refused to vaccinate their children. We know many things: These parents often fear complications from vaccination more than complications of disease. Many have religious or philosophical reasons for their reluctance or refusal to vaccinate their children. Some have concerns about vaccine safety or effectiveness. We know these things … but we don’t always know how to speak with parents about these issues.
It is somewhat ironic that the core motivation for hesitant parents and well-meaning clinicians is the same: care and protection of the child. The difficulty lies in the disparate view of what that entails. As NPs and PAs, though, our duty is to seek health benefits for and minimize harm to the patients in our care. Part of our role, when those patients are children, is to provide parents with the necessary risk-benefit information to help them make informed decisions. When the subject is vaccination, we must listen carefully and be respectful of parents’ concerns; we must recognize that their decision-making criteria may differ from ours.
So how can we bridge the gap with parents who “don’t see it the way we do”? We start by being honest with them about what is and isn’t known as far as the risks and benefits of vaccination in general or a vaccine in particular. This means acknowledging that although vaccines are very safe, they are not risk-free or 100% effective. But this also gives us the opportunity to provide them with validated data and to emphasize that the risks of any vaccine should not be considered in a silo but rather in comparison with the risks of the disease in question or of the lack of immunization.
Continue to: Helpfully, Leask and colleagues...
Helpfully, Leask and colleagues have classified parental positions on vaccination, which also provided the groundwork to offer strategies for communicating with each group.4 They identified five classes:
Unquestioning acceptors (30% to 40% of parents), who vaccinate their children and typically have no specific questions about the need for or safety of vaccines. Since this group tends to have a good relationship with their health care team but less detailed knowledge about vaccination, clinicians should continue to build rapport while providing scientific information about the vaccine being recommended or administered.4
Cautious acceptors (25% to 35%), who vaccinate their children despite having minor concerns. They tend to recognize the risk for adverse effects and hope their child will not be affected. In addition to building rapport, clinicians should provide verbal and numeric descriptions of relevant vaccine data and explain common adverse effects and disease risks.4
Hesitant vaccinators (20% to 30%), who are on the fence about the benefits and safety of vaccination. Their focus is more on the negative aspects, and they may not feel particularly trusting of their health care provider. Therefore, gaining trust is vital—parents in this group are eager to discuss their concerns with their clinician and have their questions answered satisfactorily. Motivational interviewing using a guiding style may be a helpful tool.4
Late or selective vaccinators (2% to 27%), who have significant doubts about the safety and necessity of vaccines, resulting in their choice to delay vaccination or select only some of the recommended vaccines for their child. These parents may require additional time—possibly a second appointment—in which to fully discuss their concerns. Be sure to provide up-to-date information on the risks and benefits of a vaccine, and use decision aids as appropriate.4
Continue to: Refusers...
Refusers (<2%), who have concerns about the number of vaccines children receive and conflicting feelings about whom to trust and how best to get answers to their questions. This group tends to demonstrate high knowledge levels about vaccination but may be the most argumentative when presented with information. Emphasize the importance of protecting the child from an infectious disease and reinforce the effectiveness of the vaccine. Use statistics rather than anecdotes. But above all, spend the time needed to provide refusers with a thorough understanding of the risks of not immunizing their child.4
Although it is not a universal sentiment, many parents confer trust on their health care providers. We can use this trust in a respectful, noncoercive, and non-condescending manner by providing research-supported facts about vaccines. Clinicians who listen with a compassionate ear will be in the best position to lead the hesitant, late or selective, or refusing parents to confidently make an informed decision that immunization is the best way to protect their children from vaccine-preventable diseases.4
Rather than yet again focusing on the negative, I’d like to ask: Have you had a success story of helping parents to choose vaccination for their children? How did you overcome their concerns? Share your experience with me at [email protected].
1. CDC. Achievements in public health, 1900-1999 impact of vaccines universally recommended for children—United States, 1990-1998. MMWR Morb Mortal Wkly Rep. 1999;48(12):243-248.
2. Wakefield AJ, Murch SH, Anthony A, et al. RETRACTED: Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351(9103):637-641.
3. Franki R. United States now over 100 measles cases for the year. MDEdge Family Practice. February 11, 2019.
4. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatrics. 2012;12:154.
Global outbreaks of infectious diseases—such as smallpox, pertussis, dysentery, and scarlet fever—seem like fodder for the history books. It was centuries ago that epidemics wiped out large swathes of the world population. Many people living and raising children today have never witnessed the devastating effects of measles, mumps, polio, and influenza—diseases that have been substantially reduced or even eradicated.1 Why? Because since the early 1900s, we have had scientifically developed and widely distributed vaccines at our disposal.
In context, it is incredible to realize that we are still in the beginning stages of vaccine research and development. From that perspective, it is perhaps not as surprising that some parents are hesitant to vaccinate their children—after all, do we really know everything we can and should know about inoculation? Parental resistance to or refusal of vaccination is further fueled by tainted research (Andrew Wakefield was forced to retract his findings that “validated” a link between thimerosal in vaccines and autism) and misinformation propagated on the Internet.2
But what has long been a source of frustration to those who support routine vaccination has, in recent years, started to become a public health issue. Measles outbreaks are no longer historical artifacts—they are real, as evidenced by the current rise in cases centered in Clark County, Washington. Through the first full week of February 2019, there were 101 confirmed cases of measles in the US, half of which occurred in Washington State—leading the governor to declare a public health emergency.3
This has, of course, reinvigorated the ongoing discussion about parental refusal to vaccinate. Enough has been said on this topic, by both public officials and private individuals, in a variety of venues over the years. So I’d like to focus instead on the role that individual health care providers can play in this situation.
Over the years, many of my colleagues have shared stories about parents who have refused to vaccinate their children. We know many things: These parents often fear complications from vaccination more than complications of disease. Many have religious or philosophical reasons for their reluctance or refusal to vaccinate their children. Some have concerns about vaccine safety or effectiveness. We know these things … but we don’t always know how to speak with parents about these issues.
It is somewhat ironic that the core motivation for hesitant parents and well-meaning clinicians is the same: care and protection of the child. The difficulty lies in the disparate view of what that entails. As NPs and PAs, though, our duty is to seek health benefits for and minimize harm to the patients in our care. Part of our role, when those patients are children, is to provide parents with the necessary risk-benefit information to help them make informed decisions. When the subject is vaccination, we must listen carefully and be respectful of parents’ concerns; we must recognize that their decision-making criteria may differ from ours.
So how can we bridge the gap with parents who “don’t see it the way we do”? We start by being honest with them about what is and isn’t known as far as the risks and benefits of vaccination in general or a vaccine in particular. This means acknowledging that although vaccines are very safe, they are not risk-free or 100% effective. But this also gives us the opportunity to provide them with validated data and to emphasize that the risks of any vaccine should not be considered in a silo but rather in comparison with the risks of the disease in question or of the lack of immunization.
Continue to: Helpfully, Leask and colleagues...
Helpfully, Leask and colleagues have classified parental positions on vaccination, which also provided the groundwork to offer strategies for communicating with each group.4 They identified five classes:
Unquestioning acceptors (30% to 40% of parents), who vaccinate their children and typically have no specific questions about the need for or safety of vaccines. Since this group tends to have a good relationship with their health care team but less detailed knowledge about vaccination, clinicians should continue to build rapport while providing scientific information about the vaccine being recommended or administered.4
Cautious acceptors (25% to 35%), who vaccinate their children despite having minor concerns. They tend to recognize the risk for adverse effects and hope their child will not be affected. In addition to building rapport, clinicians should provide verbal and numeric descriptions of relevant vaccine data and explain common adverse effects and disease risks.4
Hesitant vaccinators (20% to 30%), who are on the fence about the benefits and safety of vaccination. Their focus is more on the negative aspects, and they may not feel particularly trusting of their health care provider. Therefore, gaining trust is vital—parents in this group are eager to discuss their concerns with their clinician and have their questions answered satisfactorily. Motivational interviewing using a guiding style may be a helpful tool.4
Late or selective vaccinators (2% to 27%), who have significant doubts about the safety and necessity of vaccines, resulting in their choice to delay vaccination or select only some of the recommended vaccines for their child. These parents may require additional time—possibly a second appointment—in which to fully discuss their concerns. Be sure to provide up-to-date information on the risks and benefits of a vaccine, and use decision aids as appropriate.4
Continue to: Refusers...
Refusers (<2%), who have concerns about the number of vaccines children receive and conflicting feelings about whom to trust and how best to get answers to their questions. This group tends to demonstrate high knowledge levels about vaccination but may be the most argumentative when presented with information. Emphasize the importance of protecting the child from an infectious disease and reinforce the effectiveness of the vaccine. Use statistics rather than anecdotes. But above all, spend the time needed to provide refusers with a thorough understanding of the risks of not immunizing their child.4
Although it is not a universal sentiment, many parents confer trust on their health care providers. We can use this trust in a respectful, noncoercive, and non-condescending manner by providing research-supported facts about vaccines. Clinicians who listen with a compassionate ear will be in the best position to lead the hesitant, late or selective, or refusing parents to confidently make an informed decision that immunization is the best way to protect their children from vaccine-preventable diseases.4
Rather than yet again focusing on the negative, I’d like to ask: Have you had a success story of helping parents to choose vaccination for their children? How did you overcome their concerns? Share your experience with me at [email protected].
Global outbreaks of infectious diseases—such as smallpox, pertussis, dysentery, and scarlet fever—seem like fodder for the history books. It was centuries ago that epidemics wiped out large swathes of the world population. Many people living and raising children today have never witnessed the devastating effects of measles, mumps, polio, and influenza—diseases that have been substantially reduced or even eradicated.1 Why? Because since the early 1900s, we have had scientifically developed and widely distributed vaccines at our disposal.
In context, it is incredible to realize that we are still in the beginning stages of vaccine research and development. From that perspective, it is perhaps not as surprising that some parents are hesitant to vaccinate their children—after all, do we really know everything we can and should know about inoculation? Parental resistance to or refusal of vaccination is further fueled by tainted research (Andrew Wakefield was forced to retract his findings that “validated” a link between thimerosal in vaccines and autism) and misinformation propagated on the Internet.2
But what has long been a source of frustration to those who support routine vaccination has, in recent years, started to become a public health issue. Measles outbreaks are no longer historical artifacts—they are real, as evidenced by the current rise in cases centered in Clark County, Washington. Through the first full week of February 2019, there were 101 confirmed cases of measles in the US, half of which occurred in Washington State—leading the governor to declare a public health emergency.3
This has, of course, reinvigorated the ongoing discussion about parental refusal to vaccinate. Enough has been said on this topic, by both public officials and private individuals, in a variety of venues over the years. So I’d like to focus instead on the role that individual health care providers can play in this situation.
Over the years, many of my colleagues have shared stories about parents who have refused to vaccinate their children. We know many things: These parents often fear complications from vaccination more than complications of disease. Many have religious or philosophical reasons for their reluctance or refusal to vaccinate their children. Some have concerns about vaccine safety or effectiveness. We know these things … but we don’t always know how to speak with parents about these issues.
It is somewhat ironic that the core motivation for hesitant parents and well-meaning clinicians is the same: care and protection of the child. The difficulty lies in the disparate view of what that entails. As NPs and PAs, though, our duty is to seek health benefits for and minimize harm to the patients in our care. Part of our role, when those patients are children, is to provide parents with the necessary risk-benefit information to help them make informed decisions. When the subject is vaccination, we must listen carefully and be respectful of parents’ concerns; we must recognize that their decision-making criteria may differ from ours.
So how can we bridge the gap with parents who “don’t see it the way we do”? We start by being honest with them about what is and isn’t known as far as the risks and benefits of vaccination in general or a vaccine in particular. This means acknowledging that although vaccines are very safe, they are not risk-free or 100% effective. But this also gives us the opportunity to provide them with validated data and to emphasize that the risks of any vaccine should not be considered in a silo but rather in comparison with the risks of the disease in question or of the lack of immunization.
Continue to: Helpfully, Leask and colleagues...
Helpfully, Leask and colleagues have classified parental positions on vaccination, which also provided the groundwork to offer strategies for communicating with each group.4 They identified five classes:
Unquestioning acceptors (30% to 40% of parents), who vaccinate their children and typically have no specific questions about the need for or safety of vaccines. Since this group tends to have a good relationship with their health care team but less detailed knowledge about vaccination, clinicians should continue to build rapport while providing scientific information about the vaccine being recommended or administered.4
Cautious acceptors (25% to 35%), who vaccinate their children despite having minor concerns. They tend to recognize the risk for adverse effects and hope their child will not be affected. In addition to building rapport, clinicians should provide verbal and numeric descriptions of relevant vaccine data and explain common adverse effects and disease risks.4
Hesitant vaccinators (20% to 30%), who are on the fence about the benefits and safety of vaccination. Their focus is more on the negative aspects, and they may not feel particularly trusting of their health care provider. Therefore, gaining trust is vital—parents in this group are eager to discuss their concerns with their clinician and have their questions answered satisfactorily. Motivational interviewing using a guiding style may be a helpful tool.4
Late or selective vaccinators (2% to 27%), who have significant doubts about the safety and necessity of vaccines, resulting in their choice to delay vaccination or select only some of the recommended vaccines for their child. These parents may require additional time—possibly a second appointment—in which to fully discuss their concerns. Be sure to provide up-to-date information on the risks and benefits of a vaccine, and use decision aids as appropriate.4
Continue to: Refusers...
Refusers (<2%), who have concerns about the number of vaccines children receive and conflicting feelings about whom to trust and how best to get answers to their questions. This group tends to demonstrate high knowledge levels about vaccination but may be the most argumentative when presented with information. Emphasize the importance of protecting the child from an infectious disease and reinforce the effectiveness of the vaccine. Use statistics rather than anecdotes. But above all, spend the time needed to provide refusers with a thorough understanding of the risks of not immunizing their child.4
Although it is not a universal sentiment, many parents confer trust on their health care providers. We can use this trust in a respectful, noncoercive, and non-condescending manner by providing research-supported facts about vaccines. Clinicians who listen with a compassionate ear will be in the best position to lead the hesitant, late or selective, or refusing parents to confidently make an informed decision that immunization is the best way to protect their children from vaccine-preventable diseases.4
Rather than yet again focusing on the negative, I’d like to ask: Have you had a success story of helping parents to choose vaccination for their children? How did you overcome their concerns? Share your experience with me at [email protected].
1. CDC. Achievements in public health, 1900-1999 impact of vaccines universally recommended for children—United States, 1990-1998. MMWR Morb Mortal Wkly Rep. 1999;48(12):243-248.
2. Wakefield AJ, Murch SH, Anthony A, et al. RETRACTED: Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351(9103):637-641.
3. Franki R. United States now over 100 measles cases for the year. MDEdge Family Practice. February 11, 2019.
4. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatrics. 2012;12:154.
1. CDC. Achievements in public health, 1900-1999 impact of vaccines universally recommended for children—United States, 1990-1998. MMWR Morb Mortal Wkly Rep. 1999;48(12):243-248.
2. Wakefield AJ, Murch SH, Anthony A, et al. RETRACTED: Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351(9103):637-641.
3. Franki R. United States now over 100 measles cases for the year. MDEdge Family Practice. February 11, 2019.
4. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatrics. 2012;12:154.
Measles: 26 new cases reported last week
according to the Centers for Disease Control and Prevention.
On Jan. 31, total measles cases stood at 79, which means that the number of individuals with measles has risen by 61% in just the last 2 weeks. Of the five outbreaks (defined as three or more cases) so far in 2019, three have occurred in New York (57 cases in three counties), one in Texas (8 cases in five counties), and one in Washington (62 cases in two counties), the CDC reported Feb. 18.
The majority of the Washington cases (61 of the 62) have occurred in Clark County, which is located just across the Columbia River from Portland, Ore. Oregon, in turn, has a higher percentage of kindergartners with nonmedical exemptions from vaccination (7.5%) than any other state, the CDC reported in October 2018. Washington’s rate of 3.9% was nearly double the national median of 2.0% for the 2017-2018 school year, while Texas (1.8%) and New York (1.0%) were below it, the CDC said.
In the Pacific Northwest, however, some parents may be changing their minds about vaccinations, according to the New York Times, which reported that “about triple the number of children have been vaccinated this year, compared with the same period in 2018,” in Oregon and southwest Washington.
Individual cases of measles have been reported to the CDC by seven other states: California, Colorado, Connecticut, Georgia, Illinois, Kentucky, and Oregon.
according to the Centers for Disease Control and Prevention.
On Jan. 31, total measles cases stood at 79, which means that the number of individuals with measles has risen by 61% in just the last 2 weeks. Of the five outbreaks (defined as three or more cases) so far in 2019, three have occurred in New York (57 cases in three counties), one in Texas (8 cases in five counties), and one in Washington (62 cases in two counties), the CDC reported Feb. 18.
The majority of the Washington cases (61 of the 62) have occurred in Clark County, which is located just across the Columbia River from Portland, Ore. Oregon, in turn, has a higher percentage of kindergartners with nonmedical exemptions from vaccination (7.5%) than any other state, the CDC reported in October 2018. Washington’s rate of 3.9% was nearly double the national median of 2.0% for the 2017-2018 school year, while Texas (1.8%) and New York (1.0%) were below it, the CDC said.
In the Pacific Northwest, however, some parents may be changing their minds about vaccinations, according to the New York Times, which reported that “about triple the number of children have been vaccinated this year, compared with the same period in 2018,” in Oregon and southwest Washington.
Individual cases of measles have been reported to the CDC by seven other states: California, Colorado, Connecticut, Georgia, Illinois, Kentucky, and Oregon.
according to the Centers for Disease Control and Prevention.
On Jan. 31, total measles cases stood at 79, which means that the number of individuals with measles has risen by 61% in just the last 2 weeks. Of the five outbreaks (defined as three or more cases) so far in 2019, three have occurred in New York (57 cases in three counties), one in Texas (8 cases in five counties), and one in Washington (62 cases in two counties), the CDC reported Feb. 18.
The majority of the Washington cases (61 of the 62) have occurred in Clark County, which is located just across the Columbia River from Portland, Ore. Oregon, in turn, has a higher percentage of kindergartners with nonmedical exemptions from vaccination (7.5%) than any other state, the CDC reported in October 2018. Washington’s rate of 3.9% was nearly double the national median of 2.0% for the 2017-2018 school year, while Texas (1.8%) and New York (1.0%) were below it, the CDC said.
In the Pacific Northwest, however, some parents may be changing their minds about vaccinations, according to the New York Times, which reported that “about triple the number of children have been vaccinated this year, compared with the same period in 2018,” in Oregon and southwest Washington.
Individual cases of measles have been reported to the CDC by seven other states: California, Colorado, Connecticut, Georgia, Illinois, Kentucky, and Oregon.