User login
Self-report of prenatal marijuana use not very reliable
Even in the setting of legalized marijuana use, estimated prevalence of marijuana use during pregnancy was lower by self-report than it was by umbilical cord testing.
Torri D. Metz, MD, of the University of Utah Health, Salt Lake City, and her colleagues surveyed women at two urban hospitals in Colorado, which has legalized both medical and recreational use of marijuana. They found that, while 6% of the 116 women in the study reported using marijuana in the past 30 days, umbilical cord testing showed as many as 22% had detectable levels of 11-nor-delta-9-tetrahydrocannabinol-9-carboxylic, and 10% had levels above quantification.
The majority of studies of maternal marijuana use during pregnancy rely on self-report, so this could affect attempts to assess the effects of such prenatal use, they said.
Adverse outcomes associated with marijuana use during pregnancy include fetal growth restriction, small for gestational age, preterm birth, and adverse neurodevelopmental outcomes, studies have shown.
Read more in Obstetrics & Gynecology.
Even in the setting of legalized marijuana use, estimated prevalence of marijuana use during pregnancy was lower by self-report than it was by umbilical cord testing.
Torri D. Metz, MD, of the University of Utah Health, Salt Lake City, and her colleagues surveyed women at two urban hospitals in Colorado, which has legalized both medical and recreational use of marijuana. They found that, while 6% of the 116 women in the study reported using marijuana in the past 30 days, umbilical cord testing showed as many as 22% had detectable levels of 11-nor-delta-9-tetrahydrocannabinol-9-carboxylic, and 10% had levels above quantification.
The majority of studies of maternal marijuana use during pregnancy rely on self-report, so this could affect attempts to assess the effects of such prenatal use, they said.
Adverse outcomes associated with marijuana use during pregnancy include fetal growth restriction, small for gestational age, preterm birth, and adverse neurodevelopmental outcomes, studies have shown.
Read more in Obstetrics & Gynecology.
Even in the setting of legalized marijuana use, estimated prevalence of marijuana use during pregnancy was lower by self-report than it was by umbilical cord testing.
Torri D. Metz, MD, of the University of Utah Health, Salt Lake City, and her colleagues surveyed women at two urban hospitals in Colorado, which has legalized both medical and recreational use of marijuana. They found that, while 6% of the 116 women in the study reported using marijuana in the past 30 days, umbilical cord testing showed as many as 22% had detectable levels of 11-nor-delta-9-tetrahydrocannabinol-9-carboxylic, and 10% had levels above quantification.
The majority of studies of maternal marijuana use during pregnancy rely on self-report, so this could affect attempts to assess the effects of such prenatal use, they said.
Adverse outcomes associated with marijuana use during pregnancy include fetal growth restriction, small for gestational age, preterm birth, and adverse neurodevelopmental outcomes, studies have shown.
Read more in Obstetrics & Gynecology.
FROM OBSTETRICS & GYNECOLOGY
Common AEDs confer modestly increased risk of major congenital malformations
NEW ORLEANS – The most commonly used antiepileptic drugs modestly increased the risk of major congenital malformations among prenatally exposed infants in the MONEAD study.
Malformations occurred among 5% of pregnancies exposed to the medications – higher than the 2% background rate – but this was still much lower than the 9%-10% rate associated with valproate.
Overall, however, the message of the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic (MONEAD) study is quite reassuring, Kimford J. Meador, MD, said at the annual meeting of the American Epilepsy Society. MONEAD is an ongoing, prospective study to determine both maternal outcomes and long-term childhood neurodevelopmental outcomes associated with the use of antiepileptic drugs (AEDs) during pregnancy.
“The rate of malformations was higher than I thought it would be, and higher than the 2% background rate, but it’s still a modest increase and most babies are born completely normal,” Dr. Meador, professor of neurology and neurosciences at Stanford (Calif.) University, said in an interview. “I think the news here is good, and it’s especially reassuring when you put it in the context that, 60 years ago, there were laws that women with epilepsy couldn’t get married, and some states even had laws to sterilize women. I think that’s absurd when most infants born to these women are without malformations and the risk of miscarriage is very low.”
Another positive finding, he said, is that valproate use among pregnant women is now practically nonexistent. Only 1 of 351 pregnant women with epilepsy and just 2 of a comparator group of 109 nonpregnant women with epilepsy were taking it. That’s great news, said Dr. Meador, who also initiated the NEAD (Neurodevelopmental Effects of Antiepileptic Drugs) study in the early 2000s. NEAD determined the drug’s serious teratogenic potential.
In addition to the cohorts of pregnant and nonpregnant women with epilepsy, 105 healthy pregnant women enrolled in the MONEAD study. Women will be monitored during pregnancy and postpartum to measure maternal outcomes and their children will be monitored from birth through age 6 years to measure their health and developmental outcomes.
The study has six primary outcomes, three for the women and three for their children.
- Determine if women with epilepsy have increased seizures during pregnancy and delineate the contributing factors.
- Determine if C-section rate is increased in women with epilepsy and delineate contributing factors.
- Determine if women with epilepsy have an increased risk for depression during pregnancy and the postpartum period and characterize risk factors.
- Determine the long-term effects of in utero AED exposure on verbal intellectual abilities and other neurobehavioral outcomes.
- Determine if small-for-gestational age and other adverse neonatal outcomes are increased.
- Determine if breastfeeding when taking AEDs impairs the child’s ultimate verbal and other cognitive outcomes.
Rates of miscarriage and neonatal malformations were not primary study outcomes, but the descriptive data were collected and are of high interest, Dr. Meador said.
At baseline, all the women had a mean age of about 30 years. Most (75%) were on monotherapy, 20% were on polytherapy, and the rest were not taking an AED. About 60% had focal epilepsy, 31% had generalized epilepsy, and the remainder had an unclassified seizure disorder. Three subjects had multiple seizure types. The most commonly used AEDs were lamotrigine and levetiracetam (both about 30%); 4% were taking zonisamide, 4% carbamazepine, and 4% oxcarbazepine. Topiramate was being used for 2% of the pregnant woman and 5% of the nonpregnant woman. The combination of lamotrigine and levetiracetam was used for 9.0% of pregnant and 5.5% of nonpregnant women, and other polytherapies in 12.0% of the pregnant and 14.0% of the nonpregnant woman. About 4% of the pregnant and 1% of the nonpregnant women were not taking any AED.
There were 10 (2.8%) spontaneous miscarriages among the pregnant women with epilepsy and none among the healthy pregnant women. Spontaneous miscarriages weren’t associated with acute seizures, and there were no major congenital malformations reported among them. There were also two elective abortions among the pregnant women with epilepsy.
There were 18 major congenital malformations among the pregnant woman with epilepsy (5%). A total of 14 were among pregnancies exposed to monotherapy, 3 were in polytherapy-exposed pregnancies, and 1 was in the group not taking any AEDs.
The malformations were:
- Carbamazepine (one case) – hydronephrosis.
- Gabapentin (one case) – inguinal hernia.
- Lamotrigine (five cases) – aortic coarctation, cryptorchidism, hydronephrosis, pectus excavatum, and morning glory syndrome (a funnel-shaped optic nerve disc associated with impaired visual acuity).
- Levetiracetam (five cases) – atrial septal defect, buried penis syndrome, cryptorchidism, hypoplastic aortic valve, ventricular septal defect.
- Topiramate (one case) – ventricular septal defect.
- Zonisamide (one case) – inguinal hernia, absent pinna.
- Lamotrigine plus clonazepam (one case) – cardiomyopathy.
- Lamotrigine plus levetiracetam (one case) – microcephaly, myelomeningocele, Chiari II malformation.
- Levetiracetam plus phenobarbital (one case) – bilateral inguinal hernia.
MONEAD is funded by the National Institutes of Health; Dr. Meador reported no financial disclosures.
SOURCE: Meador KJ et al. AES 2018, Abstract 3.231.
NEW ORLEANS – The most commonly used antiepileptic drugs modestly increased the risk of major congenital malformations among prenatally exposed infants in the MONEAD study.
Malformations occurred among 5% of pregnancies exposed to the medications – higher than the 2% background rate – but this was still much lower than the 9%-10% rate associated with valproate.
Overall, however, the message of the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic (MONEAD) study is quite reassuring, Kimford J. Meador, MD, said at the annual meeting of the American Epilepsy Society. MONEAD is an ongoing, prospective study to determine both maternal outcomes and long-term childhood neurodevelopmental outcomes associated with the use of antiepileptic drugs (AEDs) during pregnancy.
“The rate of malformations was higher than I thought it would be, and higher than the 2% background rate, but it’s still a modest increase and most babies are born completely normal,” Dr. Meador, professor of neurology and neurosciences at Stanford (Calif.) University, said in an interview. “I think the news here is good, and it’s especially reassuring when you put it in the context that, 60 years ago, there were laws that women with epilepsy couldn’t get married, and some states even had laws to sterilize women. I think that’s absurd when most infants born to these women are without malformations and the risk of miscarriage is very low.”
Another positive finding, he said, is that valproate use among pregnant women is now practically nonexistent. Only 1 of 351 pregnant women with epilepsy and just 2 of a comparator group of 109 nonpregnant women with epilepsy were taking it. That’s great news, said Dr. Meador, who also initiated the NEAD (Neurodevelopmental Effects of Antiepileptic Drugs) study in the early 2000s. NEAD determined the drug’s serious teratogenic potential.
In addition to the cohorts of pregnant and nonpregnant women with epilepsy, 105 healthy pregnant women enrolled in the MONEAD study. Women will be monitored during pregnancy and postpartum to measure maternal outcomes and their children will be monitored from birth through age 6 years to measure their health and developmental outcomes.
The study has six primary outcomes, three for the women and three for their children.
- Determine if women with epilepsy have increased seizures during pregnancy and delineate the contributing factors.
- Determine if C-section rate is increased in women with epilepsy and delineate contributing factors.
- Determine if women with epilepsy have an increased risk for depression during pregnancy and the postpartum period and characterize risk factors.
- Determine the long-term effects of in utero AED exposure on verbal intellectual abilities and other neurobehavioral outcomes.
- Determine if small-for-gestational age and other adverse neonatal outcomes are increased.
- Determine if breastfeeding when taking AEDs impairs the child’s ultimate verbal and other cognitive outcomes.
Rates of miscarriage and neonatal malformations were not primary study outcomes, but the descriptive data were collected and are of high interest, Dr. Meador said.
At baseline, all the women had a mean age of about 30 years. Most (75%) were on monotherapy, 20% were on polytherapy, and the rest were not taking an AED. About 60% had focal epilepsy, 31% had generalized epilepsy, and the remainder had an unclassified seizure disorder. Three subjects had multiple seizure types. The most commonly used AEDs were lamotrigine and levetiracetam (both about 30%); 4% were taking zonisamide, 4% carbamazepine, and 4% oxcarbazepine. Topiramate was being used for 2% of the pregnant woman and 5% of the nonpregnant woman. The combination of lamotrigine and levetiracetam was used for 9.0% of pregnant and 5.5% of nonpregnant women, and other polytherapies in 12.0% of the pregnant and 14.0% of the nonpregnant woman. About 4% of the pregnant and 1% of the nonpregnant women were not taking any AED.
There were 10 (2.8%) spontaneous miscarriages among the pregnant women with epilepsy and none among the healthy pregnant women. Spontaneous miscarriages weren’t associated with acute seizures, and there were no major congenital malformations reported among them. There were also two elective abortions among the pregnant women with epilepsy.
There were 18 major congenital malformations among the pregnant woman with epilepsy (5%). A total of 14 were among pregnancies exposed to monotherapy, 3 were in polytherapy-exposed pregnancies, and 1 was in the group not taking any AEDs.
The malformations were:
- Carbamazepine (one case) – hydronephrosis.
- Gabapentin (one case) – inguinal hernia.
- Lamotrigine (five cases) – aortic coarctation, cryptorchidism, hydronephrosis, pectus excavatum, and morning glory syndrome (a funnel-shaped optic nerve disc associated with impaired visual acuity).
- Levetiracetam (five cases) – atrial septal defect, buried penis syndrome, cryptorchidism, hypoplastic aortic valve, ventricular septal defect.
- Topiramate (one case) – ventricular septal defect.
- Zonisamide (one case) – inguinal hernia, absent pinna.
- Lamotrigine plus clonazepam (one case) – cardiomyopathy.
- Lamotrigine plus levetiracetam (one case) – microcephaly, myelomeningocele, Chiari II malformation.
- Levetiracetam plus phenobarbital (one case) – bilateral inguinal hernia.
MONEAD is funded by the National Institutes of Health; Dr. Meador reported no financial disclosures.
SOURCE: Meador KJ et al. AES 2018, Abstract 3.231.
NEW ORLEANS – The most commonly used antiepileptic drugs modestly increased the risk of major congenital malformations among prenatally exposed infants in the MONEAD study.
Malformations occurred among 5% of pregnancies exposed to the medications – higher than the 2% background rate – but this was still much lower than the 9%-10% rate associated with valproate.
Overall, however, the message of the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic (MONEAD) study is quite reassuring, Kimford J. Meador, MD, said at the annual meeting of the American Epilepsy Society. MONEAD is an ongoing, prospective study to determine both maternal outcomes and long-term childhood neurodevelopmental outcomes associated with the use of antiepileptic drugs (AEDs) during pregnancy.
“The rate of malformations was higher than I thought it would be, and higher than the 2% background rate, but it’s still a modest increase and most babies are born completely normal,” Dr. Meador, professor of neurology and neurosciences at Stanford (Calif.) University, said in an interview. “I think the news here is good, and it’s especially reassuring when you put it in the context that, 60 years ago, there were laws that women with epilepsy couldn’t get married, and some states even had laws to sterilize women. I think that’s absurd when most infants born to these women are without malformations and the risk of miscarriage is very low.”
Another positive finding, he said, is that valproate use among pregnant women is now practically nonexistent. Only 1 of 351 pregnant women with epilepsy and just 2 of a comparator group of 109 nonpregnant women with epilepsy were taking it. That’s great news, said Dr. Meador, who also initiated the NEAD (Neurodevelopmental Effects of Antiepileptic Drugs) study in the early 2000s. NEAD determined the drug’s serious teratogenic potential.
In addition to the cohorts of pregnant and nonpregnant women with epilepsy, 105 healthy pregnant women enrolled in the MONEAD study. Women will be monitored during pregnancy and postpartum to measure maternal outcomes and their children will be monitored from birth through age 6 years to measure their health and developmental outcomes.
The study has six primary outcomes, three for the women and three for their children.
- Determine if women with epilepsy have increased seizures during pregnancy and delineate the contributing factors.
- Determine if C-section rate is increased in women with epilepsy and delineate contributing factors.
- Determine if women with epilepsy have an increased risk for depression during pregnancy and the postpartum period and characterize risk factors.
- Determine the long-term effects of in utero AED exposure on verbal intellectual abilities and other neurobehavioral outcomes.
- Determine if small-for-gestational age and other adverse neonatal outcomes are increased.
- Determine if breastfeeding when taking AEDs impairs the child’s ultimate verbal and other cognitive outcomes.
Rates of miscarriage and neonatal malformations were not primary study outcomes, but the descriptive data were collected and are of high interest, Dr. Meador said.
At baseline, all the women had a mean age of about 30 years. Most (75%) were on monotherapy, 20% were on polytherapy, and the rest were not taking an AED. About 60% had focal epilepsy, 31% had generalized epilepsy, and the remainder had an unclassified seizure disorder. Three subjects had multiple seizure types. The most commonly used AEDs were lamotrigine and levetiracetam (both about 30%); 4% were taking zonisamide, 4% carbamazepine, and 4% oxcarbazepine. Topiramate was being used for 2% of the pregnant woman and 5% of the nonpregnant woman. The combination of lamotrigine and levetiracetam was used for 9.0% of pregnant and 5.5% of nonpregnant women, and other polytherapies in 12.0% of the pregnant and 14.0% of the nonpregnant woman. About 4% of the pregnant and 1% of the nonpregnant women were not taking any AED.
There were 10 (2.8%) spontaneous miscarriages among the pregnant women with epilepsy and none among the healthy pregnant women. Spontaneous miscarriages weren’t associated with acute seizures, and there were no major congenital malformations reported among them. There were also two elective abortions among the pregnant women with epilepsy.
There were 18 major congenital malformations among the pregnant woman with epilepsy (5%). A total of 14 were among pregnancies exposed to monotherapy, 3 were in polytherapy-exposed pregnancies, and 1 was in the group not taking any AEDs.
The malformations were:
- Carbamazepine (one case) – hydronephrosis.
- Gabapentin (one case) – inguinal hernia.
- Lamotrigine (five cases) – aortic coarctation, cryptorchidism, hydronephrosis, pectus excavatum, and morning glory syndrome (a funnel-shaped optic nerve disc associated with impaired visual acuity).
- Levetiracetam (five cases) – atrial septal defect, buried penis syndrome, cryptorchidism, hypoplastic aortic valve, ventricular septal defect.
- Topiramate (one case) – ventricular septal defect.
- Zonisamide (one case) – inguinal hernia, absent pinna.
- Lamotrigine plus clonazepam (one case) – cardiomyopathy.
- Lamotrigine plus levetiracetam (one case) – microcephaly, myelomeningocele, Chiari II malformation.
- Levetiracetam plus phenobarbital (one case) – bilateral inguinal hernia.
MONEAD is funded by the National Institutes of Health; Dr. Meador reported no financial disclosures.
SOURCE: Meador KJ et al. AES 2018, Abstract 3.231.
REPORTING FROM AES 2018
Key clinical point:
Major finding: The malformation rate was 5% in exposed pregnancies.
Study details: The MONEAD study comprised 351 pregnant women with epilepsy, 109 nonpregnant women with epilepsy, and 105 healthy pregnant women.
Disclosures: The National Institutes of Health funded the study; Dr. Meador reported no financial disclosures.
Source: Meador KJ et al. AES 2018, Abstract 3.231.
Empowering women through self-managed abortion
Consider Ashley, a 22-year-old G3P2, 8 weeks pregnant, on Medicaid and living in rural Arkansas. The victim of intimate partner violence, she just broke up with her boyfriend and feels she does not have the financial or emotional resources to raise another child; she has no family in town to turn to and wants to be the best parent she can be to her 10-month-old and 3-year old.
In Arkansas, as in many other states and the District of Columbia, Medicaid covers abortion only for rape, incest, or danger to the woman’s life. Arkansas, as well as many other states, requires women to wait 48 hours following counseling before they can proceed with abortion. Waiting periods exacerbate Ashley’s tenuous situation. Will her boss give her time off from work? How will she get to the clinic? Who will watch her children? And lost wages and greater expenses are not the only problems she faces. Arkansas requires a legal contract between the abortion provider and a physician with hospital admitting privileges to provide medical abortion. The result: Only one clinic in Arkansas can legally provide medical abortion for its entire female population. For our impoverished young mother of two, the best choice is the most difficult. And she is far from alone.
Since 2010, many states have passed numerous laws restricting access to safe abortion. As geography plays a growing role in determining access, women and health care providers actively seek ways to circumvent barriers. Telemedicine, initially designed to expedite primary care for patients whose access was hampered by Boston traffic, now brings quality health care to areas lacking providers.1 Telemedicine works for a variety of medical services, from prescribing antibiotics to performing neurosurgery; reproductive health care is part of this digital revolution.2 In 2008, Iowa’s Planned Parenthood of the Heartland began using telemedicine to offer medical abortion.3
As approved by the Food and Drug Administration, medical abortion is the termination of a pregnancy of up to 10 weeks’ gestation using a combination of mifepristone and misoprostol, the former taken to block progesterone receptors, the latter to cause expulsion of the pregnancy. Today, about a third of all abortions in the United States are medical abortions. Because current FDA regulations require that mifepristone be dispensed by a physician, patients usually receive the medications after an in-person evaluation by a health care provider in a clinic.
Two models of telemedicine could improve access for Ashley.
In the first, like the Iowa Planned Parenthood model, remote clinic staff evaluate patients with history and physical examination, ultrasonography, and hemoglobin measurement; the information is forwarded to an off-site physician who has a video discussion with the patient and remotely dispenses the medication for eligible candidates. Between 2008 and 2015, Iowa Planned Parenthood provided 8,765 medical abortions using this model.3 Clinically adverse events, such as hospital admission, surgery, blood transfusion, and death occurred in 16 (0.18%) with no ectopic pregnancies or death.3 For comparison, the rate of severe maternal morbidity in the United States is 1.4%, approximately 10 times the rate with this model of medical abortion.4
In the second model of fully self-managed telemedicine abortion, patients complete a checklist that is reviewed by a provider who sends the medications through the mail. For safety, women must be able to determine their eligibility through the checklist, manage the medications, and self-assess for abortion completion. The World Health Organization endorses self-managed abortion as an option when there is “a source of accurate information and access to a health care provider should they need or want it at any stage of the process.”5 Women on Web, an organization that has provided telemedicine abortion to women globally, has recently begun providing services to the United States after sweeping restrictions vastly increased the number of requests from U.S. women. The U.S. service, Aid Access, operates similarly and for $95 provides online consultation, shipping of the medications, and Skype or phone calls for questions.6
Self-managed abortion has a bad reputation, in part from anti-abortion activists who seek to punish women who attempt to end their pregnancies themselves, but also because of its association with pre–Roe v. Wade “back alley” unsafe abortions. Neither perspective recognizes the benefits of safe self-managed abortion. Some states have criminalized self-induced abortion; both the American College of Obstetricians and Gynecologists and the American Medical Association have voiced opposition to such laws to ensure that women do not fear prosecution for seeking medical care for complications.
Given the landscape of abortion access in the United States, where legal constraints, lack of insurance, and a dearth of providers may create insurmountable barriers, we support self-managed abortion for the following reasons:
- Access barriers: The complexity and number of legal restrictions to abortion care have made it unavailable/unaffordable through traditional clinic visits in many parts of the United States. With the addition of Justice Brett M. Kavanaugh to the Supreme Court, restrictions are likely to increase.
- Safety: The evidence-based assessment of the World Health Organization is that in-person clinical evaluation is unnecessary if the appropriate checklists, educational information, and access to a provider are available.
- Autonomy and equity: Even without the barriers mentioned above, self-managed telemedicine abortion remains a patient-centered option. Often more accessible and less expensive, inherently more private, it is bound to appeal to many women.
This decade has seen unprecedented challenges to comprehensive safe reproductive health care, with no relief in sight. In the decades prior to Roe v. Wade, illegal abortions were responsible for 20% of all maternal mortality in the United States. As government, national medical organizations, and the public become more aware of our intolerably high maternal mortality rate, these actors are increasingly driven to bring our maternal health to parity with our industrialized peers. Restricting access to safe abortion runs counter to that goal. Two hundred forty years of American history teach us that legal restrictions do not prevent abortions, because they do not eliminate the reasons for which women seek abortion. Legal restrictions do, however, prevent women from ending pregnancies in the safest manner possible. The inability to obtain safe abortions invariably leads to dead women – our mothers, daughters, sisters, and wives. In this country’s harsh political climate, we must protect a woman’s right to choose. By advocating for innovative approaches to protect women’s reproductive choices, we empower women and save lives.
Dr. Anwar is an obstetrician/gynecologist at Michigan State University in Flint and Dr. Espey is professor and chair of obstetrics and gynecology at the University of New Mexico, Albuquerque. Neither of them have conflicts of interest. Email them at [email protected].
References
1. “How a ‘Stupid Idea’ Gave Birth to Telemedicine,” MedPageToday. Dec 15, .
2. J Neurosurg Pediatr. 2016 Dec;25(6):753-7.
3. Obstet Gynecol. 2017 Oct;130(4):778-82.
4. Centers for Disease Control and Prevention. Severe Maternal Morbidity in the United States.
5. Guttmacher Rep Public Policy. 2018;21:41-7.
6. “International ‘safe abortions by mail’ service can now ship to women in US,” The Hill, Nov 7, 2018.
Consider Ashley, a 22-year-old G3P2, 8 weeks pregnant, on Medicaid and living in rural Arkansas. The victim of intimate partner violence, she just broke up with her boyfriend and feels she does not have the financial or emotional resources to raise another child; she has no family in town to turn to and wants to be the best parent she can be to her 10-month-old and 3-year old.
In Arkansas, as in many other states and the District of Columbia, Medicaid covers abortion only for rape, incest, or danger to the woman’s life. Arkansas, as well as many other states, requires women to wait 48 hours following counseling before they can proceed with abortion. Waiting periods exacerbate Ashley’s tenuous situation. Will her boss give her time off from work? How will she get to the clinic? Who will watch her children? And lost wages and greater expenses are not the only problems she faces. Arkansas requires a legal contract between the abortion provider and a physician with hospital admitting privileges to provide medical abortion. The result: Only one clinic in Arkansas can legally provide medical abortion for its entire female population. For our impoverished young mother of two, the best choice is the most difficult. And she is far from alone.
Since 2010, many states have passed numerous laws restricting access to safe abortion. As geography plays a growing role in determining access, women and health care providers actively seek ways to circumvent barriers. Telemedicine, initially designed to expedite primary care for patients whose access was hampered by Boston traffic, now brings quality health care to areas lacking providers.1 Telemedicine works for a variety of medical services, from prescribing antibiotics to performing neurosurgery; reproductive health care is part of this digital revolution.2 In 2008, Iowa’s Planned Parenthood of the Heartland began using telemedicine to offer medical abortion.3
As approved by the Food and Drug Administration, medical abortion is the termination of a pregnancy of up to 10 weeks’ gestation using a combination of mifepristone and misoprostol, the former taken to block progesterone receptors, the latter to cause expulsion of the pregnancy. Today, about a third of all abortions in the United States are medical abortions. Because current FDA regulations require that mifepristone be dispensed by a physician, patients usually receive the medications after an in-person evaluation by a health care provider in a clinic.
Two models of telemedicine could improve access for Ashley.
In the first, like the Iowa Planned Parenthood model, remote clinic staff evaluate patients with history and physical examination, ultrasonography, and hemoglobin measurement; the information is forwarded to an off-site physician who has a video discussion with the patient and remotely dispenses the medication for eligible candidates. Between 2008 and 2015, Iowa Planned Parenthood provided 8,765 medical abortions using this model.3 Clinically adverse events, such as hospital admission, surgery, blood transfusion, and death occurred in 16 (0.18%) with no ectopic pregnancies or death.3 For comparison, the rate of severe maternal morbidity in the United States is 1.4%, approximately 10 times the rate with this model of medical abortion.4
In the second model of fully self-managed telemedicine abortion, patients complete a checklist that is reviewed by a provider who sends the medications through the mail. For safety, women must be able to determine their eligibility through the checklist, manage the medications, and self-assess for abortion completion. The World Health Organization endorses self-managed abortion as an option when there is “a source of accurate information and access to a health care provider should they need or want it at any stage of the process.”5 Women on Web, an organization that has provided telemedicine abortion to women globally, has recently begun providing services to the United States after sweeping restrictions vastly increased the number of requests from U.S. women. The U.S. service, Aid Access, operates similarly and for $95 provides online consultation, shipping of the medications, and Skype or phone calls for questions.6
Self-managed abortion has a bad reputation, in part from anti-abortion activists who seek to punish women who attempt to end their pregnancies themselves, but also because of its association with pre–Roe v. Wade “back alley” unsafe abortions. Neither perspective recognizes the benefits of safe self-managed abortion. Some states have criminalized self-induced abortion; both the American College of Obstetricians and Gynecologists and the American Medical Association have voiced opposition to such laws to ensure that women do not fear prosecution for seeking medical care for complications.
Given the landscape of abortion access in the United States, where legal constraints, lack of insurance, and a dearth of providers may create insurmountable barriers, we support self-managed abortion for the following reasons:
- Access barriers: The complexity and number of legal restrictions to abortion care have made it unavailable/unaffordable through traditional clinic visits in many parts of the United States. With the addition of Justice Brett M. Kavanaugh to the Supreme Court, restrictions are likely to increase.
- Safety: The evidence-based assessment of the World Health Organization is that in-person clinical evaluation is unnecessary if the appropriate checklists, educational information, and access to a provider are available.
- Autonomy and equity: Even without the barriers mentioned above, self-managed telemedicine abortion remains a patient-centered option. Often more accessible and less expensive, inherently more private, it is bound to appeal to many women.
This decade has seen unprecedented challenges to comprehensive safe reproductive health care, with no relief in sight. In the decades prior to Roe v. Wade, illegal abortions were responsible for 20% of all maternal mortality in the United States. As government, national medical organizations, and the public become more aware of our intolerably high maternal mortality rate, these actors are increasingly driven to bring our maternal health to parity with our industrialized peers. Restricting access to safe abortion runs counter to that goal. Two hundred forty years of American history teach us that legal restrictions do not prevent abortions, because they do not eliminate the reasons for which women seek abortion. Legal restrictions do, however, prevent women from ending pregnancies in the safest manner possible. The inability to obtain safe abortions invariably leads to dead women – our mothers, daughters, sisters, and wives. In this country’s harsh political climate, we must protect a woman’s right to choose. By advocating for innovative approaches to protect women’s reproductive choices, we empower women and save lives.
Dr. Anwar is an obstetrician/gynecologist at Michigan State University in Flint and Dr. Espey is professor and chair of obstetrics and gynecology at the University of New Mexico, Albuquerque. Neither of them have conflicts of interest. Email them at [email protected].
References
1. “How a ‘Stupid Idea’ Gave Birth to Telemedicine,” MedPageToday. Dec 15, .
2. J Neurosurg Pediatr. 2016 Dec;25(6):753-7.
3. Obstet Gynecol. 2017 Oct;130(4):778-82.
4. Centers for Disease Control and Prevention. Severe Maternal Morbidity in the United States.
5. Guttmacher Rep Public Policy. 2018;21:41-7.
6. “International ‘safe abortions by mail’ service can now ship to women in US,” The Hill, Nov 7, 2018.
Consider Ashley, a 22-year-old G3P2, 8 weeks pregnant, on Medicaid and living in rural Arkansas. The victim of intimate partner violence, she just broke up with her boyfriend and feels she does not have the financial or emotional resources to raise another child; she has no family in town to turn to and wants to be the best parent she can be to her 10-month-old and 3-year old.
In Arkansas, as in many other states and the District of Columbia, Medicaid covers abortion only for rape, incest, or danger to the woman’s life. Arkansas, as well as many other states, requires women to wait 48 hours following counseling before they can proceed with abortion. Waiting periods exacerbate Ashley’s tenuous situation. Will her boss give her time off from work? How will she get to the clinic? Who will watch her children? And lost wages and greater expenses are not the only problems she faces. Arkansas requires a legal contract between the abortion provider and a physician with hospital admitting privileges to provide medical abortion. The result: Only one clinic in Arkansas can legally provide medical abortion for its entire female population. For our impoverished young mother of two, the best choice is the most difficult. And she is far from alone.
Since 2010, many states have passed numerous laws restricting access to safe abortion. As geography plays a growing role in determining access, women and health care providers actively seek ways to circumvent barriers. Telemedicine, initially designed to expedite primary care for patients whose access was hampered by Boston traffic, now brings quality health care to areas lacking providers.1 Telemedicine works for a variety of medical services, from prescribing antibiotics to performing neurosurgery; reproductive health care is part of this digital revolution.2 In 2008, Iowa’s Planned Parenthood of the Heartland began using telemedicine to offer medical abortion.3
As approved by the Food and Drug Administration, medical abortion is the termination of a pregnancy of up to 10 weeks’ gestation using a combination of mifepristone and misoprostol, the former taken to block progesterone receptors, the latter to cause expulsion of the pregnancy. Today, about a third of all abortions in the United States are medical abortions. Because current FDA regulations require that mifepristone be dispensed by a physician, patients usually receive the medications after an in-person evaluation by a health care provider in a clinic.
Two models of telemedicine could improve access for Ashley.
In the first, like the Iowa Planned Parenthood model, remote clinic staff evaluate patients with history and physical examination, ultrasonography, and hemoglobin measurement; the information is forwarded to an off-site physician who has a video discussion with the patient and remotely dispenses the medication for eligible candidates. Between 2008 and 2015, Iowa Planned Parenthood provided 8,765 medical abortions using this model.3 Clinically adverse events, such as hospital admission, surgery, blood transfusion, and death occurred in 16 (0.18%) with no ectopic pregnancies or death.3 For comparison, the rate of severe maternal morbidity in the United States is 1.4%, approximately 10 times the rate with this model of medical abortion.4
In the second model of fully self-managed telemedicine abortion, patients complete a checklist that is reviewed by a provider who sends the medications through the mail. For safety, women must be able to determine their eligibility through the checklist, manage the medications, and self-assess for abortion completion. The World Health Organization endorses self-managed abortion as an option when there is “a source of accurate information and access to a health care provider should they need or want it at any stage of the process.”5 Women on Web, an organization that has provided telemedicine abortion to women globally, has recently begun providing services to the United States after sweeping restrictions vastly increased the number of requests from U.S. women. The U.S. service, Aid Access, operates similarly and for $95 provides online consultation, shipping of the medications, and Skype or phone calls for questions.6
Self-managed abortion has a bad reputation, in part from anti-abortion activists who seek to punish women who attempt to end their pregnancies themselves, but also because of its association with pre–Roe v. Wade “back alley” unsafe abortions. Neither perspective recognizes the benefits of safe self-managed abortion. Some states have criminalized self-induced abortion; both the American College of Obstetricians and Gynecologists and the American Medical Association have voiced opposition to such laws to ensure that women do not fear prosecution for seeking medical care for complications.
Given the landscape of abortion access in the United States, where legal constraints, lack of insurance, and a dearth of providers may create insurmountable barriers, we support self-managed abortion for the following reasons:
- Access barriers: The complexity and number of legal restrictions to abortion care have made it unavailable/unaffordable through traditional clinic visits in many parts of the United States. With the addition of Justice Brett M. Kavanaugh to the Supreme Court, restrictions are likely to increase.
- Safety: The evidence-based assessment of the World Health Organization is that in-person clinical evaluation is unnecessary if the appropriate checklists, educational information, and access to a provider are available.
- Autonomy and equity: Even without the barriers mentioned above, self-managed telemedicine abortion remains a patient-centered option. Often more accessible and less expensive, inherently more private, it is bound to appeal to many women.
This decade has seen unprecedented challenges to comprehensive safe reproductive health care, with no relief in sight. In the decades prior to Roe v. Wade, illegal abortions were responsible for 20% of all maternal mortality in the United States. As government, national medical organizations, and the public become more aware of our intolerably high maternal mortality rate, these actors are increasingly driven to bring our maternal health to parity with our industrialized peers. Restricting access to safe abortion runs counter to that goal. Two hundred forty years of American history teach us that legal restrictions do not prevent abortions, because they do not eliminate the reasons for which women seek abortion. Legal restrictions do, however, prevent women from ending pregnancies in the safest manner possible. The inability to obtain safe abortions invariably leads to dead women – our mothers, daughters, sisters, and wives. In this country’s harsh political climate, we must protect a woman’s right to choose. By advocating for innovative approaches to protect women’s reproductive choices, we empower women and save lives.
Dr. Anwar is an obstetrician/gynecologist at Michigan State University in Flint and Dr. Espey is professor and chair of obstetrics and gynecology at the University of New Mexico, Albuquerque. Neither of them have conflicts of interest. Email them at [email protected].
References
1. “How a ‘Stupid Idea’ Gave Birth to Telemedicine,” MedPageToday. Dec 15, .
2. J Neurosurg Pediatr. 2016 Dec;25(6):753-7.
3. Obstet Gynecol. 2017 Oct;130(4):778-82.
4. Centers for Disease Control and Prevention. Severe Maternal Morbidity in the United States.
5. Guttmacher Rep Public Policy. 2018;21:41-7.
6. “International ‘safe abortions by mail’ service can now ship to women in US,” The Hill, Nov 7, 2018.
Prenatal, postnatal neuroimaging IDs most Zika-related brain injuries
Prenatal ultrasound can identify most abnormalities in fetuses exposed to Zika virus during pregnancy, and neuroimaging after birth can detect infant exposure in cases that appeared normal on prenatal ultrasound, according to research published in JAMA Pediatrics.
“Absence of prolonged maternal viremia did not have predictive associations with normal fetal or neonatal brain imaging,” Sarah B. Mulkey, MD, PhD, from the division of fetal and transitional medicine at Children’s National Health System, in Washington, and her colleagues wrote. “Postnatal imaging can detect changes not seen on fetal imaging, supporting the current CDC [Centers for Disease Control and Prevention] recommendation for postnatal cranial [ultrasound].”
Dr. Mulkey and her colleagues performed a prospective cohort analysis of 82 pregnant women from Colombia and the United States who had clinical evidence of probable exposure to the Zika virus through travel (U.S. cases, 2 patients), physician referral, or community cases during June 2016-June 2017. Pregnant women underwent fetal MRI or ultrasound during the second or third trimesters between 4 weeks and 10 weeks after symptom onset, with infants undergoing brain MRI and cranial ultrasound after birth.
Of those 82 pregnancies, there were 80 live births, 1 case of termination because of severe fetal brain abnormalities, and 1 near-term fetal death of unknown cause. There was one death 3 days after birth and one instance of neurosurgical intervention from encephalocele. The researchers found 3 of 82 cases (4%) displayed fetal abnormalities from MRI, which consisted of 2 cases of heterotopias and malformations in cortical development and 1 case with parietal encephalocele, Chiari II malformation, and microcephaly. One infant had a normal ultrasound despite abnormalities displayed on fetal MRI.
After birth, of the 79 infants with normal ultrasound results, 53 infants underwent a postnatal brain MRI and Dr. Mulkey and her associates found 7 cases with mild abnormalities (13%). There were 57 infants who underwent cranial ultrasound, which yielded 21 cases of lenticulostriate vasculopathy, choroid plexus cysts, germinolytic/subependymal cysts, and/or calcification; these were poorly characterized by MRI.
“Normal fetal imaging had predictive associations with normal postnatal imaging or mild postnatal imaging findings unlikely to be of significant clinical consequence,” they said.
Nonetheless, “there is a need for long-term follow-up to assess the neurodevelopmental significance of these early neuroimaging findings, both normal and abnormal; such studies are in progress,” Dr. Mulkey and her colleagues said.
The researchers noted the timing of maternal infections and symptoms as well as the Zika testing, ultrasound, and MRI performance, technique during fetal MRI, and incomplete prenatal testing in the cohort as limitations in the study.
This study was funded in part by Children’s National Health System and by a philanthropic gift from the Ikaria Healthcare Fund. Dr. Mulkey received research support from the Thrasher Research Fund and is supported by awards from the National Institutes of Health National Center for Advancing Translational Sciences. The other authors reported no relevant conflicts of interest.
SOURCE: Mulkey SB et al. JAMA Pediatr. 2018 Nov. 26. doi: 10.1001/jamapediatrics.2018.4138.
While the study by Mulkey et al. adds to the body of evidence of prenatal and postnatal brain abnormalities, there are still many unanswered questions about the Zika virus and how to handle its unique diagnostic and clinical challenges, Margaret A. Honein, PhD, MPH, and Denise J. Jamieson, MD, MPH, wrote in a related editorial.
For example, Centers for Disease Control and Prevention recommendations state that infants with possible Zika exposure should receive an ophthalmologic and ultrasonographic examination at 1 month, and if the hearing test used otoacoustic emissions methods only, an automated auditory brainstem response test should be administered. While Mulkey et al. examined brain abnormalities in utero and in infants, it is not clear whether all CDC guidelines were followed in these cases.
In addition, because there is no reliable way to determine whether infants acquired Zika virus through the mother or through vertical transmission, assessing the proportion of congenitally infected infants or vertical-transmission infected infants who have neurodevelopmental disabilities and defects is not possible, they said. More longitudinal studies are needed to study the effects of the Zika virus and to prepare for the next outbreak.
“Zika was affecting pregnant women and their infants years before its teratogenic effect was recognized, and Zika will remain a serious risk to pregnant women and their infants until we have a safe vaccine that can fully prevent Zika virus infection during pregnancy,” they said. “Until then, ongoing public health efforts are essential to protect mothers and babies from this threat and ensure all disabilities associated with Zika virus infection are promptly identified, so that timely interventions can be provided.”
Dr. Honein is from the National Center on Birth Defects and Developmental Disabilities at the Centers for Disease Control and Prevention, and Dr. Jamieson is from the department of gynecology & obstetrics at Emory University School of Medicine, Atlanta. These comments summarize their editorial in response to Mulkey et al. (JAMA Pediatr. 2018 Nov. 26. doi: 10.1001/jamapediatrics.2018.4164). They reported no relevant conflicts of interest.
While the study by Mulkey et al. adds to the body of evidence of prenatal and postnatal brain abnormalities, there are still many unanswered questions about the Zika virus and how to handle its unique diagnostic and clinical challenges, Margaret A. Honein, PhD, MPH, and Denise J. Jamieson, MD, MPH, wrote in a related editorial.
For example, Centers for Disease Control and Prevention recommendations state that infants with possible Zika exposure should receive an ophthalmologic and ultrasonographic examination at 1 month, and if the hearing test used otoacoustic emissions methods only, an automated auditory brainstem response test should be administered. While Mulkey et al. examined brain abnormalities in utero and in infants, it is not clear whether all CDC guidelines were followed in these cases.
In addition, because there is no reliable way to determine whether infants acquired Zika virus through the mother or through vertical transmission, assessing the proportion of congenitally infected infants or vertical-transmission infected infants who have neurodevelopmental disabilities and defects is not possible, they said. More longitudinal studies are needed to study the effects of the Zika virus and to prepare for the next outbreak.
“Zika was affecting pregnant women and their infants years before its teratogenic effect was recognized, and Zika will remain a serious risk to pregnant women and their infants until we have a safe vaccine that can fully prevent Zika virus infection during pregnancy,” they said. “Until then, ongoing public health efforts are essential to protect mothers and babies from this threat and ensure all disabilities associated with Zika virus infection are promptly identified, so that timely interventions can be provided.”
Dr. Honein is from the National Center on Birth Defects and Developmental Disabilities at the Centers for Disease Control and Prevention, and Dr. Jamieson is from the department of gynecology & obstetrics at Emory University School of Medicine, Atlanta. These comments summarize their editorial in response to Mulkey et al. (JAMA Pediatr. 2018 Nov. 26. doi: 10.1001/jamapediatrics.2018.4164). They reported no relevant conflicts of interest.
While the study by Mulkey et al. adds to the body of evidence of prenatal and postnatal brain abnormalities, there are still many unanswered questions about the Zika virus and how to handle its unique diagnostic and clinical challenges, Margaret A. Honein, PhD, MPH, and Denise J. Jamieson, MD, MPH, wrote in a related editorial.
For example, Centers for Disease Control and Prevention recommendations state that infants with possible Zika exposure should receive an ophthalmologic and ultrasonographic examination at 1 month, and if the hearing test used otoacoustic emissions methods only, an automated auditory brainstem response test should be administered. While Mulkey et al. examined brain abnormalities in utero and in infants, it is not clear whether all CDC guidelines were followed in these cases.
In addition, because there is no reliable way to determine whether infants acquired Zika virus through the mother or through vertical transmission, assessing the proportion of congenitally infected infants or vertical-transmission infected infants who have neurodevelopmental disabilities and defects is not possible, they said. More longitudinal studies are needed to study the effects of the Zika virus and to prepare for the next outbreak.
“Zika was affecting pregnant women and their infants years before its teratogenic effect was recognized, and Zika will remain a serious risk to pregnant women and their infants until we have a safe vaccine that can fully prevent Zika virus infection during pregnancy,” they said. “Until then, ongoing public health efforts are essential to protect mothers and babies from this threat and ensure all disabilities associated with Zika virus infection are promptly identified, so that timely interventions can be provided.”
Dr. Honein is from the National Center on Birth Defects and Developmental Disabilities at the Centers for Disease Control and Prevention, and Dr. Jamieson is from the department of gynecology & obstetrics at Emory University School of Medicine, Atlanta. These comments summarize their editorial in response to Mulkey et al. (JAMA Pediatr. 2018 Nov. 26. doi: 10.1001/jamapediatrics.2018.4164). They reported no relevant conflicts of interest.
Prenatal ultrasound can identify most abnormalities in fetuses exposed to Zika virus during pregnancy, and neuroimaging after birth can detect infant exposure in cases that appeared normal on prenatal ultrasound, according to research published in JAMA Pediatrics.
“Absence of prolonged maternal viremia did not have predictive associations with normal fetal or neonatal brain imaging,” Sarah B. Mulkey, MD, PhD, from the division of fetal and transitional medicine at Children’s National Health System, in Washington, and her colleagues wrote. “Postnatal imaging can detect changes not seen on fetal imaging, supporting the current CDC [Centers for Disease Control and Prevention] recommendation for postnatal cranial [ultrasound].”
Dr. Mulkey and her colleagues performed a prospective cohort analysis of 82 pregnant women from Colombia and the United States who had clinical evidence of probable exposure to the Zika virus through travel (U.S. cases, 2 patients), physician referral, or community cases during June 2016-June 2017. Pregnant women underwent fetal MRI or ultrasound during the second or third trimesters between 4 weeks and 10 weeks after symptom onset, with infants undergoing brain MRI and cranial ultrasound after birth.
Of those 82 pregnancies, there were 80 live births, 1 case of termination because of severe fetal brain abnormalities, and 1 near-term fetal death of unknown cause. There was one death 3 days after birth and one instance of neurosurgical intervention from encephalocele. The researchers found 3 of 82 cases (4%) displayed fetal abnormalities from MRI, which consisted of 2 cases of heterotopias and malformations in cortical development and 1 case with parietal encephalocele, Chiari II malformation, and microcephaly. One infant had a normal ultrasound despite abnormalities displayed on fetal MRI.
After birth, of the 79 infants with normal ultrasound results, 53 infants underwent a postnatal brain MRI and Dr. Mulkey and her associates found 7 cases with mild abnormalities (13%). There were 57 infants who underwent cranial ultrasound, which yielded 21 cases of lenticulostriate vasculopathy, choroid plexus cysts, germinolytic/subependymal cysts, and/or calcification; these were poorly characterized by MRI.
“Normal fetal imaging had predictive associations with normal postnatal imaging or mild postnatal imaging findings unlikely to be of significant clinical consequence,” they said.
Nonetheless, “there is a need for long-term follow-up to assess the neurodevelopmental significance of these early neuroimaging findings, both normal and abnormal; such studies are in progress,” Dr. Mulkey and her colleagues said.
The researchers noted the timing of maternal infections and symptoms as well as the Zika testing, ultrasound, and MRI performance, technique during fetal MRI, and incomplete prenatal testing in the cohort as limitations in the study.
This study was funded in part by Children’s National Health System and by a philanthropic gift from the Ikaria Healthcare Fund. Dr. Mulkey received research support from the Thrasher Research Fund and is supported by awards from the National Institutes of Health National Center for Advancing Translational Sciences. The other authors reported no relevant conflicts of interest.
SOURCE: Mulkey SB et al. JAMA Pediatr. 2018 Nov. 26. doi: 10.1001/jamapediatrics.2018.4138.
Prenatal ultrasound can identify most abnormalities in fetuses exposed to Zika virus during pregnancy, and neuroimaging after birth can detect infant exposure in cases that appeared normal on prenatal ultrasound, according to research published in JAMA Pediatrics.
“Absence of prolonged maternal viremia did not have predictive associations with normal fetal or neonatal brain imaging,” Sarah B. Mulkey, MD, PhD, from the division of fetal and transitional medicine at Children’s National Health System, in Washington, and her colleagues wrote. “Postnatal imaging can detect changes not seen on fetal imaging, supporting the current CDC [Centers for Disease Control and Prevention] recommendation for postnatal cranial [ultrasound].”
Dr. Mulkey and her colleagues performed a prospective cohort analysis of 82 pregnant women from Colombia and the United States who had clinical evidence of probable exposure to the Zika virus through travel (U.S. cases, 2 patients), physician referral, or community cases during June 2016-June 2017. Pregnant women underwent fetal MRI or ultrasound during the second or third trimesters between 4 weeks and 10 weeks after symptom onset, with infants undergoing brain MRI and cranial ultrasound after birth.
Of those 82 pregnancies, there were 80 live births, 1 case of termination because of severe fetal brain abnormalities, and 1 near-term fetal death of unknown cause. There was one death 3 days after birth and one instance of neurosurgical intervention from encephalocele. The researchers found 3 of 82 cases (4%) displayed fetal abnormalities from MRI, which consisted of 2 cases of heterotopias and malformations in cortical development and 1 case with parietal encephalocele, Chiari II malformation, and microcephaly. One infant had a normal ultrasound despite abnormalities displayed on fetal MRI.
After birth, of the 79 infants with normal ultrasound results, 53 infants underwent a postnatal brain MRI and Dr. Mulkey and her associates found 7 cases with mild abnormalities (13%). There were 57 infants who underwent cranial ultrasound, which yielded 21 cases of lenticulostriate vasculopathy, choroid plexus cysts, germinolytic/subependymal cysts, and/or calcification; these were poorly characterized by MRI.
“Normal fetal imaging had predictive associations with normal postnatal imaging or mild postnatal imaging findings unlikely to be of significant clinical consequence,” they said.
Nonetheless, “there is a need for long-term follow-up to assess the neurodevelopmental significance of these early neuroimaging findings, both normal and abnormal; such studies are in progress,” Dr. Mulkey and her colleagues said.
The researchers noted the timing of maternal infections and symptoms as well as the Zika testing, ultrasound, and MRI performance, technique during fetal MRI, and incomplete prenatal testing in the cohort as limitations in the study.
This study was funded in part by Children’s National Health System and by a philanthropic gift from the Ikaria Healthcare Fund. Dr. Mulkey received research support from the Thrasher Research Fund and is supported by awards from the National Institutes of Health National Center for Advancing Translational Sciences. The other authors reported no relevant conflicts of interest.
SOURCE: Mulkey SB et al. JAMA Pediatr. 2018 Nov. 26. doi: 10.1001/jamapediatrics.2018.4138.
FROM JAMA PEDIATRICS
Key clinical point:
Major finding: In 82 pregnant women, prenatal neuroimaging identified fetal abnormalities in 3 cases, while postnatal neuroimaging in 53 of the remaining 79 cases yielded an additional 7 cases with mild abnormalities.
Study details: A prospective longitudinal cohort study of 82 pregnant women with clinical evidence of probable Zika infection in Colombia and the United States.
Disclosures: This study was funded in part by Children’s National Health System and by a philanthropic gift from the Ikaria Healthcare Fund. Dr Mulkey received research support from the Thrasher Research Fund and is supported by awards from the National Institutes of Health National Center for Advancing Translational Sciences. The other authors reported no relevant conflicts of interest.
Source: Mulkey SB et al. JAMA Pediatr. 2018 Nov. 26; doi: 10.1001/jamapediatrics.2018.4138.
Infertility appears to be increased among women with epilepsy
NEW ORLEANS – based on a retrospective study presented at the annual meeting of the American Epilepsy Society.
Data recorded in the 2010-2014 Epilepsy Birth Control Registry indicates a 9.2% infertility rate and a 22.5% impaired fecundity rate among American women with epilepsy. Both rates are higher than the general population infertility rate of 6.0% and the 12.1% rate of impaired fecundity cited by the Centers for Disease Control and Prevention.
However, differences between the study of women with epilepsy and the study of the general population may limit the validity of this comparison, said Devon B. MacEachern, clinical and research coordinator at Neuroendocrine Associates in Wellesley Hills, Mass.
It is likewise uncertain whether use of antiepileptic drugs (AEDs) affects women’s fertility or fecundity.
The Epilepsy Birth Control Registry collected data from an Internet-based survey of 1,144 community-dwelling women with epilepsy aged 18-47 years. Participants provided information about demographics, epilepsy, AEDs, reproduction, and contraception.
The researchers focused on rates of infertility, impaired fecundity, and live birth or unaborted pregnancy among 978 American women, and additionally examined whether these outcomes were related to AED use.
Infertility was defined as the percentage of participants who had unprotected sex but did not become pregnant by 1 year. Impaired fecundity was the percentage of participants who were infertile or did not carry a pregnancy to live birth. The study excluded from the impaired fecundity analysis the 41 respondents whose only outcomes were induced abortions. The 18% of pregnancies that terminated as induced abortions were excluded from the live birth rate analysis.
In all, 373 registry participants had 724 pregnancies and 422 births between 1981 and 2013. The women had an average of 2.15 pregnancies at a mean age of 24.9 years (range, 13-44 years). In addition, 38 women (9.2%) tried to conceive, but were infertile. Of 306 women with a first pregnancy, 222 (72.5%) had a live birth. Among 292 women with two pregnancies, 260 (89.0%) had at least one live birth, and 180 (61.6%) had two live births.
Of the 373 women, 84 (22.5%) with pregnancies had impaired fecundity. The risk of impaired fecundity tended to be higher among women on AED polytherapy than among women on no AED (risk ratio, 1.74).
The ratio of live births to pregnancy (71.0%) was similar among women on no AEDs (71.3%), those on AED monotherapy (71.8%), and those on polytherapy (69.7%). The live birth rate was 67.5% for women taking enzyme-inducing AEDs, 89.1% for women taking glucuronidated AEDs, 72.8% for women taking nonenzyme-inducing AEDs, 63.3% for women taking enzyme-inhibiting AEDs, and 69.7% for women on polytherapy. Lamotrigine use was associated with the highest ratio of live births to pregnancies at 89.1%; valproate use was associated with the lowest ratio of live births to pregnancies at 63.3%.
The investigation was funded by the Epilepsy Foundation and Lundbeck.
SOURCE: MacEachern DB et al. AES 2018, Abstract 1.426.
NEW ORLEANS – based on a retrospective study presented at the annual meeting of the American Epilepsy Society.
Data recorded in the 2010-2014 Epilepsy Birth Control Registry indicates a 9.2% infertility rate and a 22.5% impaired fecundity rate among American women with epilepsy. Both rates are higher than the general population infertility rate of 6.0% and the 12.1% rate of impaired fecundity cited by the Centers for Disease Control and Prevention.
However, differences between the study of women with epilepsy and the study of the general population may limit the validity of this comparison, said Devon B. MacEachern, clinical and research coordinator at Neuroendocrine Associates in Wellesley Hills, Mass.
It is likewise uncertain whether use of antiepileptic drugs (AEDs) affects women’s fertility or fecundity.
The Epilepsy Birth Control Registry collected data from an Internet-based survey of 1,144 community-dwelling women with epilepsy aged 18-47 years. Participants provided information about demographics, epilepsy, AEDs, reproduction, and contraception.
The researchers focused on rates of infertility, impaired fecundity, and live birth or unaborted pregnancy among 978 American women, and additionally examined whether these outcomes were related to AED use.
Infertility was defined as the percentage of participants who had unprotected sex but did not become pregnant by 1 year. Impaired fecundity was the percentage of participants who were infertile or did not carry a pregnancy to live birth. The study excluded from the impaired fecundity analysis the 41 respondents whose only outcomes were induced abortions. The 18% of pregnancies that terminated as induced abortions were excluded from the live birth rate analysis.
In all, 373 registry participants had 724 pregnancies and 422 births between 1981 and 2013. The women had an average of 2.15 pregnancies at a mean age of 24.9 years (range, 13-44 years). In addition, 38 women (9.2%) tried to conceive, but were infertile. Of 306 women with a first pregnancy, 222 (72.5%) had a live birth. Among 292 women with two pregnancies, 260 (89.0%) had at least one live birth, and 180 (61.6%) had two live births.
Of the 373 women, 84 (22.5%) with pregnancies had impaired fecundity. The risk of impaired fecundity tended to be higher among women on AED polytherapy than among women on no AED (risk ratio, 1.74).
The ratio of live births to pregnancy (71.0%) was similar among women on no AEDs (71.3%), those on AED monotherapy (71.8%), and those on polytherapy (69.7%). The live birth rate was 67.5% for women taking enzyme-inducing AEDs, 89.1% for women taking glucuronidated AEDs, 72.8% for women taking nonenzyme-inducing AEDs, 63.3% for women taking enzyme-inhibiting AEDs, and 69.7% for women on polytherapy. Lamotrigine use was associated with the highest ratio of live births to pregnancies at 89.1%; valproate use was associated with the lowest ratio of live births to pregnancies at 63.3%.
The investigation was funded by the Epilepsy Foundation and Lundbeck.
SOURCE: MacEachern DB et al. AES 2018, Abstract 1.426.
NEW ORLEANS – based on a retrospective study presented at the annual meeting of the American Epilepsy Society.
Data recorded in the 2010-2014 Epilepsy Birth Control Registry indicates a 9.2% infertility rate and a 22.5% impaired fecundity rate among American women with epilepsy. Both rates are higher than the general population infertility rate of 6.0% and the 12.1% rate of impaired fecundity cited by the Centers for Disease Control and Prevention.
However, differences between the study of women with epilepsy and the study of the general population may limit the validity of this comparison, said Devon B. MacEachern, clinical and research coordinator at Neuroendocrine Associates in Wellesley Hills, Mass.
It is likewise uncertain whether use of antiepileptic drugs (AEDs) affects women’s fertility or fecundity.
The Epilepsy Birth Control Registry collected data from an Internet-based survey of 1,144 community-dwelling women with epilepsy aged 18-47 years. Participants provided information about demographics, epilepsy, AEDs, reproduction, and contraception.
The researchers focused on rates of infertility, impaired fecundity, and live birth or unaborted pregnancy among 978 American women, and additionally examined whether these outcomes were related to AED use.
Infertility was defined as the percentage of participants who had unprotected sex but did not become pregnant by 1 year. Impaired fecundity was the percentage of participants who were infertile or did not carry a pregnancy to live birth. The study excluded from the impaired fecundity analysis the 41 respondents whose only outcomes were induced abortions. The 18% of pregnancies that terminated as induced abortions were excluded from the live birth rate analysis.
In all, 373 registry participants had 724 pregnancies and 422 births between 1981 and 2013. The women had an average of 2.15 pregnancies at a mean age of 24.9 years (range, 13-44 years). In addition, 38 women (9.2%) tried to conceive, but were infertile. Of 306 women with a first pregnancy, 222 (72.5%) had a live birth. Among 292 women with two pregnancies, 260 (89.0%) had at least one live birth, and 180 (61.6%) had two live births.
Of the 373 women, 84 (22.5%) with pregnancies had impaired fecundity. The risk of impaired fecundity tended to be higher among women on AED polytherapy than among women on no AED (risk ratio, 1.74).
The ratio of live births to pregnancy (71.0%) was similar among women on no AEDs (71.3%), those on AED monotherapy (71.8%), and those on polytherapy (69.7%). The live birth rate was 67.5% for women taking enzyme-inducing AEDs, 89.1% for women taking glucuronidated AEDs, 72.8% for women taking nonenzyme-inducing AEDs, 63.3% for women taking enzyme-inhibiting AEDs, and 69.7% for women on polytherapy. Lamotrigine use was associated with the highest ratio of live births to pregnancies at 89.1%; valproate use was associated with the lowest ratio of live births to pregnancies at 63.3%.
The investigation was funded by the Epilepsy Foundation and Lundbeck.
SOURCE: MacEachern DB et al. AES 2018, Abstract 1.426.
REPORTING FROM AES 2018
Key clinical point: Women with epilepsy may have more difficulty conceiving or carrying a pregnancy to term than women without epilepsy.
Major finding: The rate of infertility is 9.2% and the rate of impaired fecundity is 22.5% among women with epilepsy.
Study details: A retrospective analysis of 373 participants in the Epilepsy Birth Control Registry.
Disclosures: The investigation was funded by the Epilepsy Foundation and Lundbeck.
Source: MacEachern DB et al. AES 2018, Abstract 1.426.
Frontal lobe epilepsy elevates seizure risk during pregnancy
based on a study reported by Paula E. Voinescu, MD, PhD, at the annual meeting of the American Epilepsy Society.
The single center study included data on 76 pregnancies in women with focal epilepsy –17 of them in patients with frontal lobe epilepsy – and 38 pregnancies in women with generalized epilepsy. Seizures were more frequent during pregnancy, compared with baseline, in 5.5% of women with generalized epilepsy, 22.6% of women with focal epilepsies, and 53.0% of women with frontal lobe epilepsy, said Dr. Voinescu, lead author of the study and a neurologist at Brigham and Women’s Hospital in Boston.
“Frontal lobe epilepsy is known to be difficult to manage in general and often resistant to therapy, but it isn’t clear why the seizures got worse among pregnant women because the levels of medication in their blood was considered adequate. Until more research provides treatment guidance, doctors should carefully monitor their pregnant patients who have focal epilepsy to see if their seizures increase despite adequate blood levels and then adjust their medication if necessary,” she advised. “As we know from other research, seizures during pregnancy can increase the risk of distress and neurodevelopmental delays for the baby, as well as the risk of miscarriage.”
For the study, Dr. Voinescu and her colleagues analyzed prospectively collected clinical data from 99 pregnant women followed at Brigham and Women’s Hospital between 2013 and 2018.
The researchers excluded patients with abortions, seizure onset during pregnancy, poorly defined preconception seizure frequency, nonepileptic seizures, antiepileptic drug (AED) noncompliance, and pregnancies that were enrolled in other studies. The investigators documented patients’ seizure types and AED regimens and recorded seizure frequency during the 9 months before conception, during pregnancy, and 9 months postpartum. The researchers summed all seizures for each individual for each interval. They defined seizure frequency worsening as any increase above the preconception baseline, and evaluated differences between focal and generalized epilepsy and between frontal lobe and other focal epilepsies.
Increased seizure activity tended to occur in women on more than one AED, according to Dr. Voinescu. In women with frontal lobe epilepsy, seizure worsening during pregnancy was most likely to begin in the second trimester.
The gap in seizure frequency between the groups narrowed in the 9-month postpartum period. Seizures were more frequent during the postpartum period, compared with baseline, in 12.12% of women with generalized epilepsy, 20.14% of women with focal epilepsies, and 20.00% of women with frontal lobe epilepsy.
Future analyses will evaluate the influence of AED type and concentration and specific timing on seizure control during pregnancy and the postpartum period, Dr. Voinescu said. Future studies should also include measures of sleep, which may be a contributory mechanism to the differences found between these epilepsy types.
Dr. Voinescu reported receiving funding from the American Brain Foundation, the American Epilepsy Society, and the Epilepsy Foundation through the Susan Spencer Clinical Research Fellowship.
SOURCE: Voinescu PE et al. AES 2018, Abstract 3.236.
based on a study reported by Paula E. Voinescu, MD, PhD, at the annual meeting of the American Epilepsy Society.
The single center study included data on 76 pregnancies in women with focal epilepsy –17 of them in patients with frontal lobe epilepsy – and 38 pregnancies in women with generalized epilepsy. Seizures were more frequent during pregnancy, compared with baseline, in 5.5% of women with generalized epilepsy, 22.6% of women with focal epilepsies, and 53.0% of women with frontal lobe epilepsy, said Dr. Voinescu, lead author of the study and a neurologist at Brigham and Women’s Hospital in Boston.
“Frontal lobe epilepsy is known to be difficult to manage in general and often resistant to therapy, but it isn’t clear why the seizures got worse among pregnant women because the levels of medication in their blood was considered adequate. Until more research provides treatment guidance, doctors should carefully monitor their pregnant patients who have focal epilepsy to see if their seizures increase despite adequate blood levels and then adjust their medication if necessary,” she advised. “As we know from other research, seizures during pregnancy can increase the risk of distress and neurodevelopmental delays for the baby, as well as the risk of miscarriage.”
For the study, Dr. Voinescu and her colleagues analyzed prospectively collected clinical data from 99 pregnant women followed at Brigham and Women’s Hospital between 2013 and 2018.
The researchers excluded patients with abortions, seizure onset during pregnancy, poorly defined preconception seizure frequency, nonepileptic seizures, antiepileptic drug (AED) noncompliance, and pregnancies that were enrolled in other studies. The investigators documented patients’ seizure types and AED regimens and recorded seizure frequency during the 9 months before conception, during pregnancy, and 9 months postpartum. The researchers summed all seizures for each individual for each interval. They defined seizure frequency worsening as any increase above the preconception baseline, and evaluated differences between focal and generalized epilepsy and between frontal lobe and other focal epilepsies.
Increased seizure activity tended to occur in women on more than one AED, according to Dr. Voinescu. In women with frontal lobe epilepsy, seizure worsening during pregnancy was most likely to begin in the second trimester.
The gap in seizure frequency between the groups narrowed in the 9-month postpartum period. Seizures were more frequent during the postpartum period, compared with baseline, in 12.12% of women with generalized epilepsy, 20.14% of women with focal epilepsies, and 20.00% of women with frontal lobe epilepsy.
Future analyses will evaluate the influence of AED type and concentration and specific timing on seizure control during pregnancy and the postpartum period, Dr. Voinescu said. Future studies should also include measures of sleep, which may be a contributory mechanism to the differences found between these epilepsy types.
Dr. Voinescu reported receiving funding from the American Brain Foundation, the American Epilepsy Society, and the Epilepsy Foundation through the Susan Spencer Clinical Research Fellowship.
SOURCE: Voinescu PE et al. AES 2018, Abstract 3.236.
based on a study reported by Paula E. Voinescu, MD, PhD, at the annual meeting of the American Epilepsy Society.
The single center study included data on 76 pregnancies in women with focal epilepsy –17 of them in patients with frontal lobe epilepsy – and 38 pregnancies in women with generalized epilepsy. Seizures were more frequent during pregnancy, compared with baseline, in 5.5% of women with generalized epilepsy, 22.6% of women with focal epilepsies, and 53.0% of women with frontal lobe epilepsy, said Dr. Voinescu, lead author of the study and a neurologist at Brigham and Women’s Hospital in Boston.
“Frontal lobe epilepsy is known to be difficult to manage in general and often resistant to therapy, but it isn’t clear why the seizures got worse among pregnant women because the levels of medication in their blood was considered adequate. Until more research provides treatment guidance, doctors should carefully monitor their pregnant patients who have focal epilepsy to see if their seizures increase despite adequate blood levels and then adjust their medication if necessary,” she advised. “As we know from other research, seizures during pregnancy can increase the risk of distress and neurodevelopmental delays for the baby, as well as the risk of miscarriage.”
For the study, Dr. Voinescu and her colleagues analyzed prospectively collected clinical data from 99 pregnant women followed at Brigham and Women’s Hospital between 2013 and 2018.
The researchers excluded patients with abortions, seizure onset during pregnancy, poorly defined preconception seizure frequency, nonepileptic seizures, antiepileptic drug (AED) noncompliance, and pregnancies that were enrolled in other studies. The investigators documented patients’ seizure types and AED regimens and recorded seizure frequency during the 9 months before conception, during pregnancy, and 9 months postpartum. The researchers summed all seizures for each individual for each interval. They defined seizure frequency worsening as any increase above the preconception baseline, and evaluated differences between focal and generalized epilepsy and between frontal lobe and other focal epilepsies.
Increased seizure activity tended to occur in women on more than one AED, according to Dr. Voinescu. In women with frontal lobe epilepsy, seizure worsening during pregnancy was most likely to begin in the second trimester.
The gap in seizure frequency between the groups narrowed in the 9-month postpartum period. Seizures were more frequent during the postpartum period, compared with baseline, in 12.12% of women with generalized epilepsy, 20.14% of women with focal epilepsies, and 20.00% of women with frontal lobe epilepsy.
Future analyses will evaluate the influence of AED type and concentration and specific timing on seizure control during pregnancy and the postpartum period, Dr. Voinescu said. Future studies should also include measures of sleep, which may be a contributory mechanism to the differences found between these epilepsy types.
Dr. Voinescu reported receiving funding from the American Brain Foundation, the American Epilepsy Society, and the Epilepsy Foundation through the Susan Spencer Clinical Research Fellowship.
SOURCE: Voinescu PE et al. AES 2018, Abstract 3.236.
REPORTING FROM AES 2018
Key clinical point: Women with focal epilepsy, especially frontal lobe epilepsy, may need closer monitoring during pregnancy.
Major finding: Compared with baseline, seizures were more frequent during pregnancy in 53% of women with frontal lobe epilepsy.
Study details: An analysis of prospectively collected data from 114 pregnancies.
Disclosures: Dr. Voinescu reported receiving funding from the American Brain Foundation, the American Epilepsy Society, and the Epilepsy Foundation through the Susan Spencer Clinical Research Fellowship.
Source: Voinescu PE et al. AES 2018, Abstract 3.236.
Are anti-TNF drugs safe for pregnant women with inflammatory bowel disease?
Yes, anti-tumor necrosis factor (anti-TNF) therapy for inflammatory bowel disease (IBD) can be continued during pregnancy.
IBD is often diagnosed and treated in women during their reproductive years. Consequently, these patients face important decisions about the management of their disease and the safety of their baby. Clinicians should be prepared to offer guidance by discussing the risks and benefits of anti-TNF agents with their pregnant patients who have IBD, as well as with those considering pregnancy.
STUDIES OF THE POTENTIAL RISKS
Anti-TNF agents are monoclonal antibodies. Infliximab, adalimumab, and golimumab are actively transported into the fetal circulation via the placenta, mainly during the second and third trimesters. Certolizumab crosses the placenta only by passive means, because it lacks the fragment crystallizable (Fc) region required for placental transfer.1
Effects on pregnancy outcomes
In a 2016 meta-analysis,2 of 1,242 pregnancies in women with IBD, 482 were in women on anti-TNF therapy. It found no statistically significant difference in rates of adverse pregnancy outcomes including congenital abnormality, preterm birth, and low birth weight.
A meta-analysis of 1,216 pregnant women with IBD found no statistically significant differences in rates of spontaneous or elective abortion, preterm birth, low birth weight, or congenital malformation in those on anti-TNF therapy vs controls.3
A systematic review of 58 studies including more than 1,500 pregnant women with IBD who were exposed to anti-TNF agents concluded that there was no association with adverse pregnancy outcomes such as spontaneous abortion, preterm delivery, stillbirth, low birth weight, congenital malformation, or infection.4
A retrospective cohort study of 66 pregnant patients with IBD from several centers in Spain found that anti-TNF or thiopurine therapy during pregnancy did not increase the risk of pregnancy complications or neonatal complications.5
Effects on newborns
Cord blood studies have shown that maternal use of infliximab and adalimumab results in a detectable serum level in newborns, while cord blood levels of certolizumab are much lower.1,6 In some studies, anti-TNF drugs were detectable in infants for up to 6 months after birth, whereas other studies found that detectable serum levels dropped soon after birth.1,7
Addressing concern about an increased risk of infection or dysfunctional immune development in newborns exposed to anti-TNF drugs in utero, a systematic review found no increased risk.4 A retrospective multicenter cohort study of 841 children also reported no association between in utero exposure to anti-TNF agents and risk of severe infection in the short term or long term (mean of 4 years).8 Additional studies are under way to determine long-term risk to the newborn.7
THE TORONTO CONSENSUS GUIDELINES
The Toronto consensus guidelines strongly recommend continuing anti-TNF therapy during pregnancy in women with IBD who began maintenance therapy before conception.6
If a patient strongly prefers to stop therapy during pregnancy to limit fetal exposure, the Toronto consensus recommends giving the last dose at 22 to 24 weeks of gestation. However, this should only be considered in patients whose IBD is in remission and at low risk of relapse.6,9
Although anti-TNF drugs may differ in terms of placental transfer, agents should not be switched in stable patients, as switching increases the risk of relapse.10
BENEFITS OF CONTINUING THERAPY
Active IBD poses a significantly greater risk to the mother and the baby than continuing anti-TNF therapy during pregnancy.1,7 The primary benefit of continuing therapy is to maintain disease remission.
Among women with active IBD at the time of conception, one-third will have improvement in disease activity during the course of their pregnancy, one-third will have no change, and one-third will have worsening of disease activity. But if IBD is in remission at the time of conception, it will remain in remission in nearly 80% of women during pregnancy.1
Women with active IBD are at increased risk of preterm delivery, low birth weight, and intrauterine growth restriction.1,2,5 Also, women with IBD have an increased risk of venous thromboembolism, particularly if they have active disease during pregnancy.1 Therefore, achieving and maintaining remission are vital in the management of the pregnant patient with IBD.
CONSIDERATIONS AFTER BIRTH: BREAST-FEEDING AND VACCINATION
Breast-feeding is considered safe. Minuscule amounts of infliximab or adalimumab are transferred in breast milk but are unlikely to result in systemic immune suppression in the infant.7
Live-attenuated vaccines should be avoided for the first 6 months in infants exposed to anti-TNF agents in utero.1,7,11 All other vaccines, including hepatitis B virus vaccine, should be given according to standard schedules.6
OUR RECOMMENDATIONS
The goal of managing IBD in women of reproductive age is to minimize the risk of adverse outcomes for both mother and baby. We recommend a team approach, working closely with a gastroenterologist and a high-risk-pregnancy obstetrician, if available.
Patients should continue anti-TNF therapy during pregnancy because evidence supports its safety. If a woman wants to stop therapy and is at low risk of relapse, we recommend giving the last dose at 22 to 24 weeks of gestation, then promptly resuming therapy postpartum.
Live-attenuated vaccines (eg, influenza, rotavirus) should be avoided for the first 6 months in babies born to mothers on anti-TNF therapy.
- Ananthakrishnan AN, Xavier RJ, Podolsky DK. Inflammatory Bowel Diseases: A Clinician’s Guide. Chichester, UK: Wiley; 2017. doi:10.1002/9781119077633
- Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor alpha therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis 2016; 10(8):979–988. doi:10.1093/ecco-jcc/jjv234
- Narula N, Al-Dabbagh, Dhillon A, Sands BE, Marshall JK. Anti-TNF alpha therapies are safe during pregnancy in women with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2014; 20(10):1862–1869. doi:10.1097/MIB.0000000000000092
- Nielsen OH, Loftus EV Jr, Jess T. Safety of TNF-alpha inhibitors during IBD pregnancy: a systematic review. BMC Med 2013; 11:174. doi:10.1186/1741-7015-11-174
- Casanova MJ, Chaparro M, Domenech E, et al. Safety of thiopurines and anti-TNF-alpha drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol 2013; 108(3):433–440. doi:10.1038/ajg.2012.430
- Nguyen GC, Seow CH, Maxwell C, et al; IBD in Pregnancy Consensus Group; Canadian Association of Gastroenterology. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology 2016; 150(3):734–757.e1. doi:10.1053/j.gastro.2015.12.003
- Gisbert JP, Chaparro, M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol 2013; 108(9):1426–1438. doi:10.1038/ajg.2013.171
- Chaparro M, Verreth A, Lobaton T, et al. Long-term safety of in utero exposure to anti-TNF alpha drugs for the treatment of inflammatory bowel disease: results from the multicenter European TEDDY Study. Am J Gastroenterol 2018; 113(3):396–403. doi:10.1038/ajg.2017.501
- de Lima A, Zelinkova Z, van der Ent C, Steegers EA, van der Woude CJ. Tailored anti-TNF therapy during pregnancy in patients with IBD: maternal and fetal safety. Gut 2016; 65(8):1261–1268. doi:10.1136/gutjnl-2015-309321
- Van Assche G, Vermeire S, Ballet V, et al. Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut 2012; 61(2):229–234. doi:10.1136/gutjnl-2011-300755
- Saha S. Medication management in the pregnant IBD patient. Am J Gastroenterol 2017; 112(5):667–669. doi:10.1038/ajg.2017.22
Yes, anti-tumor necrosis factor (anti-TNF) therapy for inflammatory bowel disease (IBD) can be continued during pregnancy.
IBD is often diagnosed and treated in women during their reproductive years. Consequently, these patients face important decisions about the management of their disease and the safety of their baby. Clinicians should be prepared to offer guidance by discussing the risks and benefits of anti-TNF agents with their pregnant patients who have IBD, as well as with those considering pregnancy.
STUDIES OF THE POTENTIAL RISKS
Anti-TNF agents are monoclonal antibodies. Infliximab, adalimumab, and golimumab are actively transported into the fetal circulation via the placenta, mainly during the second and third trimesters. Certolizumab crosses the placenta only by passive means, because it lacks the fragment crystallizable (Fc) region required for placental transfer.1
Effects on pregnancy outcomes
In a 2016 meta-analysis,2 of 1,242 pregnancies in women with IBD, 482 were in women on anti-TNF therapy. It found no statistically significant difference in rates of adverse pregnancy outcomes including congenital abnormality, preterm birth, and low birth weight.
A meta-analysis of 1,216 pregnant women with IBD found no statistically significant differences in rates of spontaneous or elective abortion, preterm birth, low birth weight, or congenital malformation in those on anti-TNF therapy vs controls.3
A systematic review of 58 studies including more than 1,500 pregnant women with IBD who were exposed to anti-TNF agents concluded that there was no association with adverse pregnancy outcomes such as spontaneous abortion, preterm delivery, stillbirth, low birth weight, congenital malformation, or infection.4
A retrospective cohort study of 66 pregnant patients with IBD from several centers in Spain found that anti-TNF or thiopurine therapy during pregnancy did not increase the risk of pregnancy complications or neonatal complications.5
Effects on newborns
Cord blood studies have shown that maternal use of infliximab and adalimumab results in a detectable serum level in newborns, while cord blood levels of certolizumab are much lower.1,6 In some studies, anti-TNF drugs were detectable in infants for up to 6 months after birth, whereas other studies found that detectable serum levels dropped soon after birth.1,7
Addressing concern about an increased risk of infection or dysfunctional immune development in newborns exposed to anti-TNF drugs in utero, a systematic review found no increased risk.4 A retrospective multicenter cohort study of 841 children also reported no association between in utero exposure to anti-TNF agents and risk of severe infection in the short term or long term (mean of 4 years).8 Additional studies are under way to determine long-term risk to the newborn.7
THE TORONTO CONSENSUS GUIDELINES
The Toronto consensus guidelines strongly recommend continuing anti-TNF therapy during pregnancy in women with IBD who began maintenance therapy before conception.6
If a patient strongly prefers to stop therapy during pregnancy to limit fetal exposure, the Toronto consensus recommends giving the last dose at 22 to 24 weeks of gestation. However, this should only be considered in patients whose IBD is in remission and at low risk of relapse.6,9
Although anti-TNF drugs may differ in terms of placental transfer, agents should not be switched in stable patients, as switching increases the risk of relapse.10
BENEFITS OF CONTINUING THERAPY
Active IBD poses a significantly greater risk to the mother and the baby than continuing anti-TNF therapy during pregnancy.1,7 The primary benefit of continuing therapy is to maintain disease remission.
Among women with active IBD at the time of conception, one-third will have improvement in disease activity during the course of their pregnancy, one-third will have no change, and one-third will have worsening of disease activity. But if IBD is in remission at the time of conception, it will remain in remission in nearly 80% of women during pregnancy.1
Women with active IBD are at increased risk of preterm delivery, low birth weight, and intrauterine growth restriction.1,2,5 Also, women with IBD have an increased risk of venous thromboembolism, particularly if they have active disease during pregnancy.1 Therefore, achieving and maintaining remission are vital in the management of the pregnant patient with IBD.
CONSIDERATIONS AFTER BIRTH: BREAST-FEEDING AND VACCINATION
Breast-feeding is considered safe. Minuscule amounts of infliximab or adalimumab are transferred in breast milk but are unlikely to result in systemic immune suppression in the infant.7
Live-attenuated vaccines should be avoided for the first 6 months in infants exposed to anti-TNF agents in utero.1,7,11 All other vaccines, including hepatitis B virus vaccine, should be given according to standard schedules.6
OUR RECOMMENDATIONS
The goal of managing IBD in women of reproductive age is to minimize the risk of adverse outcomes for both mother and baby. We recommend a team approach, working closely with a gastroenterologist and a high-risk-pregnancy obstetrician, if available.
Patients should continue anti-TNF therapy during pregnancy because evidence supports its safety. If a woman wants to stop therapy and is at low risk of relapse, we recommend giving the last dose at 22 to 24 weeks of gestation, then promptly resuming therapy postpartum.
Live-attenuated vaccines (eg, influenza, rotavirus) should be avoided for the first 6 months in babies born to mothers on anti-TNF therapy.
Yes, anti-tumor necrosis factor (anti-TNF) therapy for inflammatory bowel disease (IBD) can be continued during pregnancy.
IBD is often diagnosed and treated in women during their reproductive years. Consequently, these patients face important decisions about the management of their disease and the safety of their baby. Clinicians should be prepared to offer guidance by discussing the risks and benefits of anti-TNF agents with their pregnant patients who have IBD, as well as with those considering pregnancy.
STUDIES OF THE POTENTIAL RISKS
Anti-TNF agents are monoclonal antibodies. Infliximab, adalimumab, and golimumab are actively transported into the fetal circulation via the placenta, mainly during the second and third trimesters. Certolizumab crosses the placenta only by passive means, because it lacks the fragment crystallizable (Fc) region required for placental transfer.1
Effects on pregnancy outcomes
In a 2016 meta-analysis,2 of 1,242 pregnancies in women with IBD, 482 were in women on anti-TNF therapy. It found no statistically significant difference in rates of adverse pregnancy outcomes including congenital abnormality, preterm birth, and low birth weight.
A meta-analysis of 1,216 pregnant women with IBD found no statistically significant differences in rates of spontaneous or elective abortion, preterm birth, low birth weight, or congenital malformation in those on anti-TNF therapy vs controls.3
A systematic review of 58 studies including more than 1,500 pregnant women with IBD who were exposed to anti-TNF agents concluded that there was no association with adverse pregnancy outcomes such as spontaneous abortion, preterm delivery, stillbirth, low birth weight, congenital malformation, or infection.4
A retrospective cohort study of 66 pregnant patients with IBD from several centers in Spain found that anti-TNF or thiopurine therapy during pregnancy did not increase the risk of pregnancy complications or neonatal complications.5
Effects on newborns
Cord blood studies have shown that maternal use of infliximab and adalimumab results in a detectable serum level in newborns, while cord blood levels of certolizumab are much lower.1,6 In some studies, anti-TNF drugs were detectable in infants for up to 6 months after birth, whereas other studies found that detectable serum levels dropped soon after birth.1,7
Addressing concern about an increased risk of infection or dysfunctional immune development in newborns exposed to anti-TNF drugs in utero, a systematic review found no increased risk.4 A retrospective multicenter cohort study of 841 children also reported no association between in utero exposure to anti-TNF agents and risk of severe infection in the short term or long term (mean of 4 years).8 Additional studies are under way to determine long-term risk to the newborn.7
THE TORONTO CONSENSUS GUIDELINES
The Toronto consensus guidelines strongly recommend continuing anti-TNF therapy during pregnancy in women with IBD who began maintenance therapy before conception.6
If a patient strongly prefers to stop therapy during pregnancy to limit fetal exposure, the Toronto consensus recommends giving the last dose at 22 to 24 weeks of gestation. However, this should only be considered in patients whose IBD is in remission and at low risk of relapse.6,9
Although anti-TNF drugs may differ in terms of placental transfer, agents should not be switched in stable patients, as switching increases the risk of relapse.10
BENEFITS OF CONTINUING THERAPY
Active IBD poses a significantly greater risk to the mother and the baby than continuing anti-TNF therapy during pregnancy.1,7 The primary benefit of continuing therapy is to maintain disease remission.
Among women with active IBD at the time of conception, one-third will have improvement in disease activity during the course of their pregnancy, one-third will have no change, and one-third will have worsening of disease activity. But if IBD is in remission at the time of conception, it will remain in remission in nearly 80% of women during pregnancy.1
Women with active IBD are at increased risk of preterm delivery, low birth weight, and intrauterine growth restriction.1,2,5 Also, women with IBD have an increased risk of venous thromboembolism, particularly if they have active disease during pregnancy.1 Therefore, achieving and maintaining remission are vital in the management of the pregnant patient with IBD.
CONSIDERATIONS AFTER BIRTH: BREAST-FEEDING AND VACCINATION
Breast-feeding is considered safe. Minuscule amounts of infliximab or adalimumab are transferred in breast milk but are unlikely to result in systemic immune suppression in the infant.7
Live-attenuated vaccines should be avoided for the first 6 months in infants exposed to anti-TNF agents in utero.1,7,11 All other vaccines, including hepatitis B virus vaccine, should be given according to standard schedules.6
OUR RECOMMENDATIONS
The goal of managing IBD in women of reproductive age is to minimize the risk of adverse outcomes for both mother and baby. We recommend a team approach, working closely with a gastroenterologist and a high-risk-pregnancy obstetrician, if available.
Patients should continue anti-TNF therapy during pregnancy because evidence supports its safety. If a woman wants to stop therapy and is at low risk of relapse, we recommend giving the last dose at 22 to 24 weeks of gestation, then promptly resuming therapy postpartum.
Live-attenuated vaccines (eg, influenza, rotavirus) should be avoided for the first 6 months in babies born to mothers on anti-TNF therapy.
- Ananthakrishnan AN, Xavier RJ, Podolsky DK. Inflammatory Bowel Diseases: A Clinician’s Guide. Chichester, UK: Wiley; 2017. doi:10.1002/9781119077633
- Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor alpha therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis 2016; 10(8):979–988. doi:10.1093/ecco-jcc/jjv234
- Narula N, Al-Dabbagh, Dhillon A, Sands BE, Marshall JK. Anti-TNF alpha therapies are safe during pregnancy in women with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2014; 20(10):1862–1869. doi:10.1097/MIB.0000000000000092
- Nielsen OH, Loftus EV Jr, Jess T. Safety of TNF-alpha inhibitors during IBD pregnancy: a systematic review. BMC Med 2013; 11:174. doi:10.1186/1741-7015-11-174
- Casanova MJ, Chaparro M, Domenech E, et al. Safety of thiopurines and anti-TNF-alpha drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol 2013; 108(3):433–440. doi:10.1038/ajg.2012.430
- Nguyen GC, Seow CH, Maxwell C, et al; IBD in Pregnancy Consensus Group; Canadian Association of Gastroenterology. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology 2016; 150(3):734–757.e1. doi:10.1053/j.gastro.2015.12.003
- Gisbert JP, Chaparro, M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol 2013; 108(9):1426–1438. doi:10.1038/ajg.2013.171
- Chaparro M, Verreth A, Lobaton T, et al. Long-term safety of in utero exposure to anti-TNF alpha drugs for the treatment of inflammatory bowel disease: results from the multicenter European TEDDY Study. Am J Gastroenterol 2018; 113(3):396–403. doi:10.1038/ajg.2017.501
- de Lima A, Zelinkova Z, van der Ent C, Steegers EA, van der Woude CJ. Tailored anti-TNF therapy during pregnancy in patients with IBD: maternal and fetal safety. Gut 2016; 65(8):1261–1268. doi:10.1136/gutjnl-2015-309321
- Van Assche G, Vermeire S, Ballet V, et al. Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut 2012; 61(2):229–234. doi:10.1136/gutjnl-2011-300755
- Saha S. Medication management in the pregnant IBD patient. Am J Gastroenterol 2017; 112(5):667–669. doi:10.1038/ajg.2017.22
- Ananthakrishnan AN, Xavier RJ, Podolsky DK. Inflammatory Bowel Diseases: A Clinician’s Guide. Chichester, UK: Wiley; 2017. doi:10.1002/9781119077633
- Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor alpha therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis 2016; 10(8):979–988. doi:10.1093/ecco-jcc/jjv234
- Narula N, Al-Dabbagh, Dhillon A, Sands BE, Marshall JK. Anti-TNF alpha therapies are safe during pregnancy in women with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2014; 20(10):1862–1869. doi:10.1097/MIB.0000000000000092
- Nielsen OH, Loftus EV Jr, Jess T. Safety of TNF-alpha inhibitors during IBD pregnancy: a systematic review. BMC Med 2013; 11:174. doi:10.1186/1741-7015-11-174
- Casanova MJ, Chaparro M, Domenech E, et al. Safety of thiopurines and anti-TNF-alpha drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol 2013; 108(3):433–440. doi:10.1038/ajg.2012.430
- Nguyen GC, Seow CH, Maxwell C, et al; IBD in Pregnancy Consensus Group; Canadian Association of Gastroenterology. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology 2016; 150(3):734–757.e1. doi:10.1053/j.gastro.2015.12.003
- Gisbert JP, Chaparro, M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol 2013; 108(9):1426–1438. doi:10.1038/ajg.2013.171
- Chaparro M, Verreth A, Lobaton T, et al. Long-term safety of in utero exposure to anti-TNF alpha drugs for the treatment of inflammatory bowel disease: results from the multicenter European TEDDY Study. Am J Gastroenterol 2018; 113(3):396–403. doi:10.1038/ajg.2017.501
- de Lima A, Zelinkova Z, van der Ent C, Steegers EA, van der Woude CJ. Tailored anti-TNF therapy during pregnancy in patients with IBD: maternal and fetal safety. Gut 2016; 65(8):1261–1268. doi:10.1136/gutjnl-2015-309321
- Van Assche G, Vermeire S, Ballet V, et al. Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut 2012; 61(2):229–234. doi:10.1136/gutjnl-2011-300755
- Saha S. Medication management in the pregnant IBD patient. Am J Gastroenterol 2017; 112(5):667–669. doi:10.1038/ajg.2017.22
Does amniotomy shorten spontaneous labor or improve outcomes?
EVIDENCE SUMMARY
A meta-analysis of 15 RCTs (5583 women) compared intentional artificial rupture of the amniotic membranes during labor (amniotomy) with intention to preserve the membranes (no amniotomy). The study found no differences in any of the measured primary outcomes: length of first stage of labor, cesarean section, maternal satisfaction with childbirth, or Apgar score <7 at 5 minutes.1
Investigators included 9 trials with both nulliparous and multiparous women and 6 trials with only nulliparous women. Thirteen trials compared amniotomy with intention to preserve the membranes, and 2 trials performed amniotomy in the control group if the membranes were intact at full cervical dilation.
Amniotomy doesn’t affect first-stage labor or cesarean risk
Five trials (1127 women) reported no difference in length of the first stage of labor between the amniotomy and no amniotomy groups (mean difference [MD]= −20 minutes; 95% confidence interval [CI], −96 to 55). Subgroups of primiparous and multiparous women showed no difference (MD= −58 minutes; 95% CI, −153 to 37 and MD= +23 minutes; 95% CI, −51 to 97, respectively).
Nine trials (5021 women) reported no significant difference in cesarean section risk overall or when compared by parity, multiparous vs primiparous (risk ratio [RR]= 1.27; 95% CI, 0.99-1.63). One trial (84 women) found no difference in maternal satisfaction scores with childbirth experience. Six trials (3598 women) that reported risk of low Apgar score (<4 at 1 minute or <7 at 5 minutes) found no difference overall (RR=0.53; 95% CI, 0.28-1.00), or when compared by parity (multiparous vs primiparous).
Investigators reported that the included trials varied in quality and described the following limitations: inconsistent or unspecified timing of amniotomy during labor, proportion of women in the control group undergoing amniotomy, and ≥30% of women not getting the allocated treatment in all but one of the trials.
Secondary outcomes: Amniotomy reduces oxytocin use
Eight trials (4264 women) evaluated oxytocin augmentation and found that amniotomy decreased its use in multiparous (RR=0.43; 95% CI, 0.30-0.60), but not primiparous, women.
Eight trials (1927 women) reported length of second stage of labor as a secondary outcome, with no difference overall (MD= −1.33 minutes; 95% CI, −2.92 to 0.26). Amniotomy produced a statistical but not clinically significant shortening in subanalysis of primiparous women (MD= −5.43 minutes; 95% CI, −9.98 to −0.89) but not multiparous women.
Continue to: Three trials...
Three trials (1695 women) evaluated dysfunctional labor, defined as no progress in cervical dilation in 2 hours or ineffective uterine contractions. Amniotomy reduced dysfunctional labor in both primiparous (RR=0.49; 95% CI, 0.33-0.73) and multiparous women (RR=0.44; 95% CI, 0.31-0.62).
No differences found in other maternal and fetal outcomes
Investigators reported no differences in other secondary maternal outcomes: instrumental vaginal birth (10 trials, 5121 women); pain relief (8 trials, 3475 women); postpartum hemorrhage (2 trials, 1822 women); serious maternal morbidity or death (3 trials, 1740 women); umbilical cord prolapse (2 trials, 1615 women); and cesarean section for fetal distress, prolonged labor, or antepartum hemorrhage (1 RCT, 690 women).
Investigators also found no differences in secondary fetal outcomes: serious neonatal morbidity or perinatal death (8 trials, 3397 women); neonatal admission to neonatal intensive care (5 trials, 2686 women); abnormal fetal heart rate tracing in first stage of labor (4 trials, 1284 women); meconium aspiration (2 trials, 1615 women); and fetal acidosis (2 trials, 1014 women). Similarly, 1 RCT (39 women) that compared amniotomy with intent to preserve membranes in spontaneous labors that became prolonged found no difference in cesarean section, maternal satisfaction, or Apgar scores.
A few studies claim shorter labor with amniotomy
However, a later Iranian RCT (300 women) reported that early amniotomy shortened labor (labor duration: 7.5 ± 0.7 hours with amniotomy vs 9.9 ± 1.0 hours without amniotomy; P<.001) and reduced the risk of dystocia (RR=0.81; 95% CI, 0.59-0.90) and cesarean section (RR=0.82; 95% CI, 0.66-0.90).2
A similar Nigerian RCT (214 women) and an Indian RCT (144 women) both claimed that amniotomy also shortened labor (4.7 ± 0.9 hours vs 5.9 ± 1.3, and 3.9 ± 2 hours vs 6.1 ± 2.8 hours, respectively).3,4 In neither trial, however, did investigators explain how the difference was significant when the duration of labor times overlapped within the margin of error.
1. Smyth RMD, Markham C, Dowswell T. Amniotomy for shortening spontaneous labour. Cochrane Database Syst Rev. 2013;(6):CD006167.
2. Ghafarzadeh M, Moeininasab S, Namdari M. Effect of early amniotomy on dystocia risk and cesarean delivery in nulliparous women: a randomized clinical trial. Arch Gynecol Obstet. 2015;292:321-325.
3. Onah LN, Dim CC, Nwagha UI, et al. Effect of early amniotomy on the outcome of spontaneous labour: a randomized controlled trial of pregnant women in Enugu, South-east Nigeria. Afr Health Sci. 2015;15:1097-1103.
4. Vadivelu M, Rathore S, Benjamin SJ, et al. Randomized controlled trial of the effect of amniotomy on the duration of spontaneous labor. Int J Gynaecol Obstet. 2017;138:152-157.
EVIDENCE SUMMARY
A meta-analysis of 15 RCTs (5583 women) compared intentional artificial rupture of the amniotic membranes during labor (amniotomy) with intention to preserve the membranes (no amniotomy). The study found no differences in any of the measured primary outcomes: length of first stage of labor, cesarean section, maternal satisfaction with childbirth, or Apgar score <7 at 5 minutes.1
Investigators included 9 trials with both nulliparous and multiparous women and 6 trials with only nulliparous women. Thirteen trials compared amniotomy with intention to preserve the membranes, and 2 trials performed amniotomy in the control group if the membranes were intact at full cervical dilation.
Amniotomy doesn’t affect first-stage labor or cesarean risk
Five trials (1127 women) reported no difference in length of the first stage of labor between the amniotomy and no amniotomy groups (mean difference [MD]= −20 minutes; 95% confidence interval [CI], −96 to 55). Subgroups of primiparous and multiparous women showed no difference (MD= −58 minutes; 95% CI, −153 to 37 and MD= +23 minutes; 95% CI, −51 to 97, respectively).
Nine trials (5021 women) reported no significant difference in cesarean section risk overall or when compared by parity, multiparous vs primiparous (risk ratio [RR]= 1.27; 95% CI, 0.99-1.63). One trial (84 women) found no difference in maternal satisfaction scores with childbirth experience. Six trials (3598 women) that reported risk of low Apgar score (<4 at 1 minute or <7 at 5 minutes) found no difference overall (RR=0.53; 95% CI, 0.28-1.00), or when compared by parity (multiparous vs primiparous).
Investigators reported that the included trials varied in quality and described the following limitations: inconsistent or unspecified timing of amniotomy during labor, proportion of women in the control group undergoing amniotomy, and ≥30% of women not getting the allocated treatment in all but one of the trials.
Secondary outcomes: Amniotomy reduces oxytocin use
Eight trials (4264 women) evaluated oxytocin augmentation and found that amniotomy decreased its use in multiparous (RR=0.43; 95% CI, 0.30-0.60), but not primiparous, women.
Eight trials (1927 women) reported length of second stage of labor as a secondary outcome, with no difference overall (MD= −1.33 minutes; 95% CI, −2.92 to 0.26). Amniotomy produced a statistical but not clinically significant shortening in subanalysis of primiparous women (MD= −5.43 minutes; 95% CI, −9.98 to −0.89) but not multiparous women.
Continue to: Three trials...
Three trials (1695 women) evaluated dysfunctional labor, defined as no progress in cervical dilation in 2 hours or ineffective uterine contractions. Amniotomy reduced dysfunctional labor in both primiparous (RR=0.49; 95% CI, 0.33-0.73) and multiparous women (RR=0.44; 95% CI, 0.31-0.62).
No differences found in other maternal and fetal outcomes
Investigators reported no differences in other secondary maternal outcomes: instrumental vaginal birth (10 trials, 5121 women); pain relief (8 trials, 3475 women); postpartum hemorrhage (2 trials, 1822 women); serious maternal morbidity or death (3 trials, 1740 women); umbilical cord prolapse (2 trials, 1615 women); and cesarean section for fetal distress, prolonged labor, or antepartum hemorrhage (1 RCT, 690 women).
Investigators also found no differences in secondary fetal outcomes: serious neonatal morbidity or perinatal death (8 trials, 3397 women); neonatal admission to neonatal intensive care (5 trials, 2686 women); abnormal fetal heart rate tracing in first stage of labor (4 trials, 1284 women); meconium aspiration (2 trials, 1615 women); and fetal acidosis (2 trials, 1014 women). Similarly, 1 RCT (39 women) that compared amniotomy with intent to preserve membranes in spontaneous labors that became prolonged found no difference in cesarean section, maternal satisfaction, or Apgar scores.
A few studies claim shorter labor with amniotomy
However, a later Iranian RCT (300 women) reported that early amniotomy shortened labor (labor duration: 7.5 ± 0.7 hours with amniotomy vs 9.9 ± 1.0 hours without amniotomy; P<.001) and reduced the risk of dystocia (RR=0.81; 95% CI, 0.59-0.90) and cesarean section (RR=0.82; 95% CI, 0.66-0.90).2
A similar Nigerian RCT (214 women) and an Indian RCT (144 women) both claimed that amniotomy also shortened labor (4.7 ± 0.9 hours vs 5.9 ± 1.3, and 3.9 ± 2 hours vs 6.1 ± 2.8 hours, respectively).3,4 In neither trial, however, did investigators explain how the difference was significant when the duration of labor times overlapped within the margin of error.
EVIDENCE SUMMARY
A meta-analysis of 15 RCTs (5583 women) compared intentional artificial rupture of the amniotic membranes during labor (amniotomy) with intention to preserve the membranes (no amniotomy). The study found no differences in any of the measured primary outcomes: length of first stage of labor, cesarean section, maternal satisfaction with childbirth, or Apgar score <7 at 5 minutes.1
Investigators included 9 trials with both nulliparous and multiparous women and 6 trials with only nulliparous women. Thirteen trials compared amniotomy with intention to preserve the membranes, and 2 trials performed amniotomy in the control group if the membranes were intact at full cervical dilation.
Amniotomy doesn’t affect first-stage labor or cesarean risk
Five trials (1127 women) reported no difference in length of the first stage of labor between the amniotomy and no amniotomy groups (mean difference [MD]= −20 minutes; 95% confidence interval [CI], −96 to 55). Subgroups of primiparous and multiparous women showed no difference (MD= −58 minutes; 95% CI, −153 to 37 and MD= +23 minutes; 95% CI, −51 to 97, respectively).
Nine trials (5021 women) reported no significant difference in cesarean section risk overall or when compared by parity, multiparous vs primiparous (risk ratio [RR]= 1.27; 95% CI, 0.99-1.63). One trial (84 women) found no difference in maternal satisfaction scores with childbirth experience. Six trials (3598 women) that reported risk of low Apgar score (<4 at 1 minute or <7 at 5 minutes) found no difference overall (RR=0.53; 95% CI, 0.28-1.00), or when compared by parity (multiparous vs primiparous).
Investigators reported that the included trials varied in quality and described the following limitations: inconsistent or unspecified timing of amniotomy during labor, proportion of women in the control group undergoing amniotomy, and ≥30% of women not getting the allocated treatment in all but one of the trials.
Secondary outcomes: Amniotomy reduces oxytocin use
Eight trials (4264 women) evaluated oxytocin augmentation and found that amniotomy decreased its use in multiparous (RR=0.43; 95% CI, 0.30-0.60), but not primiparous, women.
Eight trials (1927 women) reported length of second stage of labor as a secondary outcome, with no difference overall (MD= −1.33 minutes; 95% CI, −2.92 to 0.26). Amniotomy produced a statistical but not clinically significant shortening in subanalysis of primiparous women (MD= −5.43 minutes; 95% CI, −9.98 to −0.89) but not multiparous women.
Continue to: Three trials...
Three trials (1695 women) evaluated dysfunctional labor, defined as no progress in cervical dilation in 2 hours or ineffective uterine contractions. Amniotomy reduced dysfunctional labor in both primiparous (RR=0.49; 95% CI, 0.33-0.73) and multiparous women (RR=0.44; 95% CI, 0.31-0.62).
No differences found in other maternal and fetal outcomes
Investigators reported no differences in other secondary maternal outcomes: instrumental vaginal birth (10 trials, 5121 women); pain relief (8 trials, 3475 women); postpartum hemorrhage (2 trials, 1822 women); serious maternal morbidity or death (3 trials, 1740 women); umbilical cord prolapse (2 trials, 1615 women); and cesarean section for fetal distress, prolonged labor, or antepartum hemorrhage (1 RCT, 690 women).
Investigators also found no differences in secondary fetal outcomes: serious neonatal morbidity or perinatal death (8 trials, 3397 women); neonatal admission to neonatal intensive care (5 trials, 2686 women); abnormal fetal heart rate tracing in first stage of labor (4 trials, 1284 women); meconium aspiration (2 trials, 1615 women); and fetal acidosis (2 trials, 1014 women). Similarly, 1 RCT (39 women) that compared amniotomy with intent to preserve membranes in spontaneous labors that became prolonged found no difference in cesarean section, maternal satisfaction, or Apgar scores.
A few studies claim shorter labor with amniotomy
However, a later Iranian RCT (300 women) reported that early amniotomy shortened labor (labor duration: 7.5 ± 0.7 hours with amniotomy vs 9.9 ± 1.0 hours without amniotomy; P<.001) and reduced the risk of dystocia (RR=0.81; 95% CI, 0.59-0.90) and cesarean section (RR=0.82; 95% CI, 0.66-0.90).2
A similar Nigerian RCT (214 women) and an Indian RCT (144 women) both claimed that amniotomy also shortened labor (4.7 ± 0.9 hours vs 5.9 ± 1.3, and 3.9 ± 2 hours vs 6.1 ± 2.8 hours, respectively).3,4 In neither trial, however, did investigators explain how the difference was significant when the duration of labor times overlapped within the margin of error.
1. Smyth RMD, Markham C, Dowswell T. Amniotomy for shortening spontaneous labour. Cochrane Database Syst Rev. 2013;(6):CD006167.
2. Ghafarzadeh M, Moeininasab S, Namdari M. Effect of early amniotomy on dystocia risk and cesarean delivery in nulliparous women: a randomized clinical trial. Arch Gynecol Obstet. 2015;292:321-325.
3. Onah LN, Dim CC, Nwagha UI, et al. Effect of early amniotomy on the outcome of spontaneous labour: a randomized controlled trial of pregnant women in Enugu, South-east Nigeria. Afr Health Sci. 2015;15:1097-1103.
4. Vadivelu M, Rathore S, Benjamin SJ, et al. Randomized controlled trial of the effect of amniotomy on the duration of spontaneous labor. Int J Gynaecol Obstet. 2017;138:152-157.
1. Smyth RMD, Markham C, Dowswell T. Amniotomy for shortening spontaneous labour. Cochrane Database Syst Rev. 2013;(6):CD006167.
2. Ghafarzadeh M, Moeininasab S, Namdari M. Effect of early amniotomy on dystocia risk and cesarean delivery in nulliparous women: a randomized clinical trial. Arch Gynecol Obstet. 2015;292:321-325.
3. Onah LN, Dim CC, Nwagha UI, et al. Effect of early amniotomy on the outcome of spontaneous labour: a randomized controlled trial of pregnant women in Enugu, South-east Nigeria. Afr Health Sci. 2015;15:1097-1103.
4. Vadivelu M, Rathore S, Benjamin SJ, et al. Randomized controlled trial of the effect of amniotomy on the duration of spontaneous labor. Int J Gynaecol Obstet. 2017;138:152-157.
EVIDENCE-BASED ANSWER:
No. Amniotomy neither shortens spontaneous labor nor improves any of the following outcomes: length of first stage of labor, cesarean section rate, maternal satisfaction with childbirth, or Apgar score <7 at 5 minutes (strength of recommendation [SOR]: A, large meta-analyses of randomized controlled trials [RCTs] and a single RCT with conflicting results).
Amniotomy does result in about a 55% reduction of pitocin use in multiparous women, a small (5 minutes) decrease in the duration of second-stage labor in primiparous women, and about a 50% overall reduction in dysfunctional labor—ie, no progress in cervical dilation in 2 hours or ineffective uterine contractions (SOR: A, large meta-analyses of RCTs and a single RCT with conflicting results).
Amniotomy doesn’t improve other maternal outcomes—instrumented vaginal birth; pain relief; postpartum hemorrhage; serious morbidity or death; umbilical cord prolapse; cesarean section for fetal distress, prolonged labor, antepartum hemorrhage—nor fetal outcomes—serious neonatal morbidity or perinatal death; neonatal admission to intensive care; abnormal fetal heart rate tracing in first-stage labor; meconium aspiration; or fetal acidosis (SOR: A, large meta-analyses of RCTs and a single RCT with conflicting results).
Premenstrual Dysphoric Disorder: Diagnosis and Management in Primary Care
CE/CME No: CR-1812
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the epidemiology and underlying pathogenesis of premenstrual dysphoric disorder (PMDD).
• Describe PMDD diagnostic criteria established by DSM-5.
• Differentiate PMDD from other conditions in order to provide appropriate treatment.
• Identify effective evidence-based treatment modalities for PMDD.
• Discuss PMDD treatment challenges and importance of individualizing PMDD treatment.
FACULTY
Jovanka Rajic is a recent graduate of the Master of Science in Nursing–Family Nurse Practitioner program at the Patricia A. Chin School of Nursing at California State University, Los Angeles. Stefanie A. Varela is adjunct faculty in the Patricia A. Chin School of Nursing at California State University, Los Angeles, and practices in the Obstetrics and Gynecology Department at Kaiser Permanente in Ontario, California.
The authors reported no conflicts of interest related to this article.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through November 30, 2019.
Article begins on next page >>
The severe psychiatric and somatic symptoms of premenstrual dysphoric disorder (PMDD) can be debilitating and place women at increased risk for other psychiatric disorders (including major depression and generalized anxiety) and for suicidal ideation. While PMDD’s complex nature makes it an underdiagnosed condition, there are clear diagnostic criteria for clinicians to ensure their patients receive timely and appropriate treatment—thus reducing the risk for serious sequelae.
Premenstrual dysphoric disorder (PMDD) is categorized as a depressive disorder in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5).1 The hallmarks of this unique disorder are chronic, severe psychiatric and somatic symptoms that occur only during the late luteal phase of the menstrual cycle and dissipate soon after the onset of menstruation.2 Symptoms are generally disruptive and often associated with significant distress and impaired quality of life.2
PMDD occurs in 3%-8% of women of childbearing age; it affects women worldwide and is not influenced by geography or culture.2 Genetic susceptibility, stress, obesity, and a history of trauma or sexual abuse have been implicated as risk factors.2-6 The impact of PMDD on health-related quality of life is greater than that of chronic back pain but comparable to that of rheumatoid arthritis and osteoarthritis.2,7 Significantly, women with PMDD have a 50%-78% lifetime risk for psychiatric disorders, such as major depressive, dysthymic, seasonal affective, and generalized anxiety disorders, and suicidality.2
PMDD can be challenging for primary care providers to diagnose and treat, due to the lack of standardized screening methods, unfamiliarity with evidence-based practices for diagnosis, and the need to tailor treatment to each patient’s individual needs.3,8 But the increased risk for psychiatric sequelae, including suicidality, make timely diagnosis and treatment of PMDD critical.2,9
PATHOGENESIS
The pathogenesis of PMDD is not completely understood. The prevailing theory is that PMDD is underlined by increased sensitivity to normal fluctuations in ovarian steroid hormone levels (see the Figure) during the luteal phase of the menstrual cycle.2-4,6
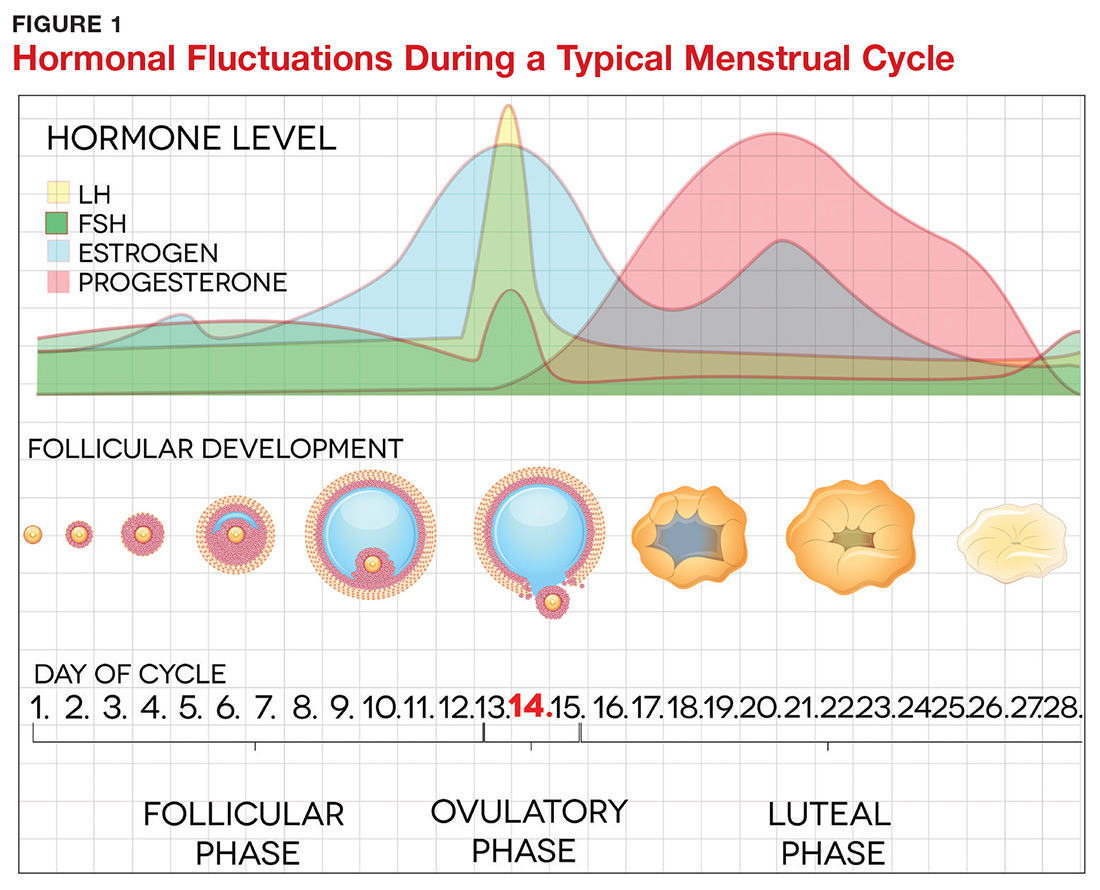
This sensitivity involves the progesterone metabolite allopregnanolone (ALLO), which acts as a modulator of central GABA-A receptors that have anxiolytic and sedative effects.2,3 It has been postulated that women with PMDD have impaired production of ALLO or decreased sensitivity of GABA-A receptors to ALLO during the luteal phase.2,3 In addition, women with PMDD exhibit a paradoxical anxiety and irritability response to ALLO.2,3 Recent research suggests that PMDD is precipitated by changing ALLO levels during the luteal phase and that treatment directed at reducing ALLO availability during this phase can alleviate PMDD symptoms.10
Hormonal fluctuations have been associated with impaired serotonergic system function in women with PMDD, which results in dysregulation of mood, cognition, sleep, and eating behavior.2-4,6 Hormonal fluctuations have also been implicated in the alteration of emotional and cognitive circuits.2,3,6,11,12 Brain imaging studies have revealed that women with PMDD demonstrate enhanced reactivity to amygdala, which processes emotional and cognitive stimuli, as well as impaired control of amygdala by the prefrontal cortex during the luteal phase.3,7,12
Continue to: PATIENT PRESENTATION/HISTORY
PATIENT PRESENTATION/HISTORY
PMDD is an individual experience for each woman.3,4 However, women with PMDD generally present with a history of various psychiatric and somatic symptoms that significantly interfere with their occupational or social functions (to be discussed in the Diagnosis section, page 42).1-4 The reported symptoms occur in predictable patterns that are associated with the menstrual cycle, intensifying around the time of menstruation and resolving immediately after onset of menstruation in most cases.1-4
Many psychiatric and medical conditions may be exacerbated during the luteal phase of the menstrual cycle and thus may mimic the signs and symptoms of PMDD (see Table 1).1,4 Therefore, the pattern and severity of symptoms should always be considered when differentiating PMDD from other underlying conditions.1,2,4,5
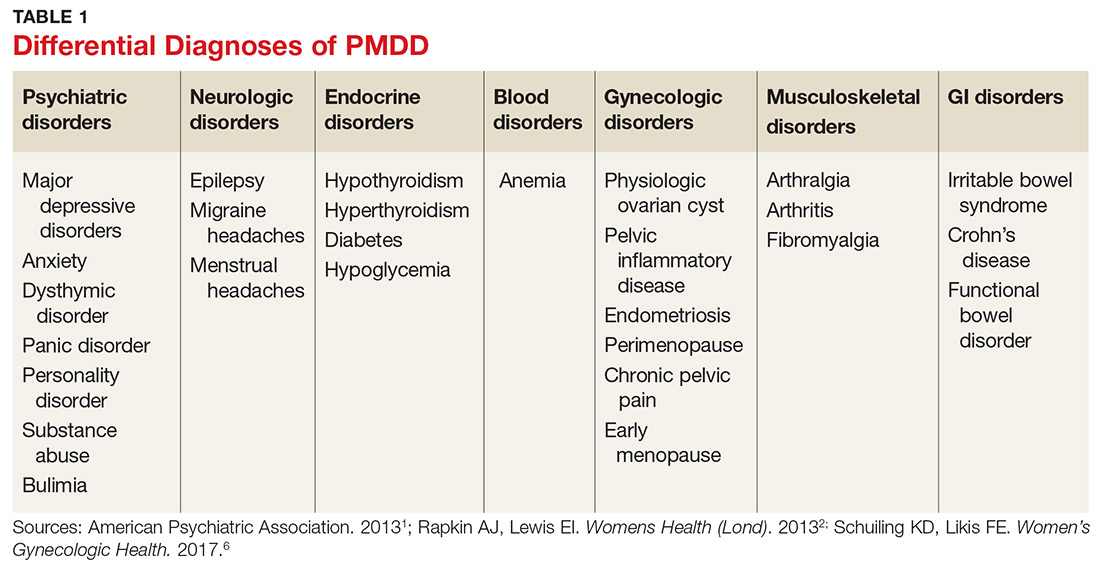
It is also important to distinguish PMDD from PMS, a condition with which it is frequently confused. The latter manifests with at least one affective or somatic symptom that is bothersome but not disabling.4,5 An accurate differential diagnosis is important, as the management of these two conditions differs significantly.4,5
ASSESSMENT
PMDD assessment should include thorough history taking, with emphasis on medical, gynecologic, and psychiatric history as well as social and familial history (including PMDD and other psychiatric disorders); and physical examination, including gynecologic and mental status assessment and depression screening using the Patient Health Questionnaire (PHQ-9).2,4,13,14 The physical exam is usually unremarkable.14 The most common physical findings during the luteal phase include mild swelling in the lower extremities and breast tenderness.14 Mental status examination, however, may be abnormal during the late luteal phase—albeit with orientation, memory, thoughts, and perceptions intact.13,14
LABORATORY WORKUP
There is no specific laboratory test for PMDD; rather, testing is aimed at ruling out alternative diagnoses.4,14 Relevant studies may include a complete blood count to exclude anemia, a thyroid function test to exclude thyroid disorders, a blood glucose test to exclude diabetes or hypoglycemia, and a ß hCG test to exclude possible pregnancy.4,14 Hormonal tests (eg, for FSH) may be considered for younger women with irregular cycles or for those younger than 40 with suspected premature menopause.4,14
Continue to: DIAGNOSIS
DIAGNOSIS
Diagnosis of PMDD is guided by the DSM-5 criteria, which include the following components
- Content (presence of specific symptoms)
- Cyclicity (premenstrual onset and postmenstrual resolution)
- Severity (significant distress)
- Chronicity (occurrence in the past year).15
DSM-5 has established seven criteria (labeled A-G) for a PMDD diagnosis.1 First and foremost, a woman must experience a minimum of five of the 11 listed symptoms, with a minimum of one symptom being related to mood, during most menstrual cycles over the previous 12 months (Criterion A).1 The symptoms must occur during the week before the onset of menses, must improve within a few days of onset of menses, and must resolve in the week following menses.1
Mood-related symptoms (outlined in Criterion B) include
1. Notable depressed mood, hopelessness, or self-deprecation
2. Notable tension and/or anxiety
3. Notable affective lability (eg, mood swings, sudden sadness, tearfulness, or increased sensitivity to rejection)
4. Notable anger or irritability or increased interpersonal conflicts.1
Somatic or functional symptoms associated with PMDD (Criterion C) include:
5. Low interest in common activities (eg, those related to friends, work, school, and/or hobbies)
6. Difficulty concentrating
7. Lethargy, fatigue, or increased lack of energy
8. Notable change in appetite
9. Insomnia or hypersomnia
10. Feeling overwhelmed or out of control
11. Physical symptoms, such as breast tenderness or swelling, joint or muscle pain, headache, weight gain, or bloating.1
Again, patients must report at least one symptom from Criterion B and at least one from Criterion C—but a minimum of five symptoms overall—to receive a diagnosis of PMDD.1
Continue to: Additionally, the symptoms must...
Additionally, the symptoms must cause clinically significant distress or impair daily functioning, including occupational, social, academic, and sexual activities (Criterion D). They must not represent exacerbation of another underlying psychiatric disorder, such as major depressive, dysthymic, panic, or personality disorders (Criterion E), although PMDD may co-occur with psychiatric disorders.1
The above-mentioned symptom profile must be confirmed by prospective daily ratings of a minimum of two consecutive symptomatic menstrual cycles (Criterion F), although a provisional diagnosis of PMDD may be made prior to confirmation.1 The Daily Record of Severity of Problems is the most widely used instrument for prospective daily rating of PMDD symptoms listed in the DSM-5 criteria.5,15
Finally, the symptoms must not be evoked by the use of a substance (eg, medications, alcohol, and illicit drugs) or another medical condition (Criterion G).1
TREATMENT/MANAGEMENT
The goal of PMDD treatment is to relieve psychiatric and physical symptoms and improve the patient's ability to function.3 Treatment is primarily directed at pharmacologic neuromodulation using selective serotonin reuptake inhibitors (SSRIs) or ovulation suppression using oral contraceptives and hormones.2
Pharmacotherapy
SSRIs are the firstline treatment for PMDD.5 Fluoxetine, paroxetine, and sertraline are the only serotonergic medications approved by the FDA for treatment of PMDD.2 SSRIs act within one to two days when used for PMDD, thereby allowing different modes of dosing.2 SSRI dosing may be continuous (daily administration), intermittent (administration from ovulation to first day of menses), or symptomatic (administration from symptom onset until first day of menses).3 Although data on continuous and intermittent dosing are available for fluoxetine, paroxetine, and sertraline, symptom-onset data are currently available only for sertraline (see Table 2).16-19
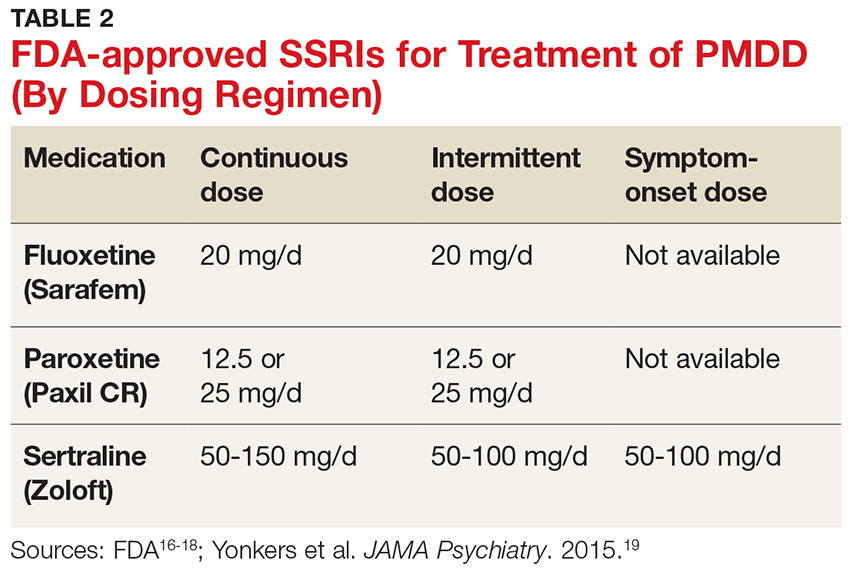
Continue to: Combined oral contraceptives...
Combined oral contraceptives (COCs) containing estrogen and progesterone are considered secondline treatment for PMDD—specifically, COCs containing 20 µg of ethinyl estradiol and 3 mg of drospirenone administered as a 24/4 regimen.2,3,5,6 This combination has been approved by the FDA for women with PMDD who seek oral contraception.3 Although drospirenone-containing products have been associated with increased risk for venous thromboembolism (VTE), this risk is lower than that for VTE during pregnancy or in the postpartum period.3 Currently, no strong evidence exists regarding the effectiveness of other oral contraceptives for PMDD.6
Gonadotropin-releasing hormone agonists are the thirdline treatment for PMDD.6 They eliminate symptoms of the luteal phase by suppressing ovarian release of estrogen and ovulation.6 However, use of these agents is not recommended for more than one year due to the increased risk for cardiovascular events.5,6 In addition, long-term users need add-back therapy (adding back small amounts of the hormone) to counteract the effects of low estrogen, such as bone loss; providers should be aware that this may lead to the recurrence of PMDD.3,5,6 The use of estrogen and progesterone formulations for PMDD is currently not strongly supported by research.6
Complementary treatment
Cognitive behavioral therapy has been shown to improve functioning and reduce depression in women with PMDD and may be a useful adjunct.2,20 Regular aerobic exercise, a diet high in protein and complex carbohydrates to increase tryptophan (serotonin precursor) levels, and reduced intake of caffeine, sugar, and alcohol are some commonly recommended lifestyle changes.2
Calcium carbonate supplementation (500 mg/d) has demonstrated effectiveness in alleviating premenstrual mood and physical symptoms.21 There is currently no strong evidence regarding the benefits of acupuncture, Qi therapy, reflexology, and herbal preparations for managing PMDD.22
Surgery
Bilateral oophorectomy, usually with concomitant hysterectomy, is the last resort for women with severe PMDD who do not respond to or cannot tolerate the standard treatments.6 This surgical procedure results in premature menopause, which may lead to complications related to a hypoestrogenic state—including vasomotor symptoms (flushes/flashes), vaginal atrophy, osteopenia, osteoporosis, and cardiovascular disease.2 Therefore, it is important to implement estrogen replacement therapy after surgery until the age of natural menopause is reached.2 If hysterectomy is not performed, the administration of progesterone is necessary to prevent endometrial hyperplasia and therefore reduce the risk for endometrial cancer.2 However, the addition of progesterone may lead to recurrence of symptoms.2
Continue to: Treatment challenges
Treatment challenges
PMDD treatment differs for each patient.3 Severity of symptoms, response to treatment, treatment preference, conception plans, and reproductive age need to be considered.3
Women with prominent depressive or physical symptoms may respond better to continuous dosing of SSRIs, whereas those with prominent irritability, anger, and mood swings may respond better to a symptom-onset SSRI regimen that reduces availability and function of ALLO.3 Women who develop tolerance to SSRIs may need to have their dosage increased or be switched to another medication.3Quetiapine is used as an adjunct to SSRIs for women who do not respond to SSRIs alone and has shown to improve mood swings, anxiety, and irritability.5 However, women experiencing persistent adverse effects of SSRIs, such as sexual dysfunction, may benefit from intermittent dosing.3
Adolescents and women in their early 20s should be treated with OCs or nonpharmacologic modalities due to concerns about SSRI use and increased risk for suicidality in this population.3 The risks related to SSRI use during pregnancy and breastfeeding should be considered and discussed with women of childbearing age who use SSRIs to treat PMDD.3 Perimenopausal women with irregular menses on intermittent SSRIs may have to switch to symptom-onset or continuous dosing due to the difficulty of tracking the menstrual period and lack of significant benchmarks regarding when to start the treatment.3
Patient education/follow-up
Patients should be educated on PMDD etiology, diagnostic process, and available treatment options.4 The importance of prospective record-keeping—for confirmation of the diagnosis and evaluation of individual response to a specific treatment—should be emphasized.4 Patients should be encouraged to follow up with their health care provider to monitor treatment effectiveness, possible adverse effects, and need for treatment adjustment.4
CONCLUSION
The symptoms of PMDD can have a debilitating and life-disrupting impact on affected women—and put them at risk for other serious psychiatric disorders and suicide. The DSM-5 criteria provide diagnostic guidance to help distinguish PMDD from other underlying conditions, ensuring that patients can receive timely and appropriate treatment. While SSRIs are regarded as the most effective option, other evidence-based treatments should be considered, since PMDD requires individualized treatment to ensure optimal clinical outcomes.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Rapkin AJ, Lewis EI. Treatment of premenstrual dysphoric disorder. Womens Health (Lond). 2013;9(6):537-556.
3. Pearlstein T. Treatment of premenstrual dysphoric disorder: therapeutic challenges. Expert Rev Clin Pharmacol. 2016;9(4):493-496.
4. Zielinski R, Lynne S. Menstrual-cycle pain and premenstrual conditions. In: Schuiling KD, Likis FE, eds. Women’s Gynecologic Health. Burlington, MA: Jones & Bartlett Learning; 2017:556-573.
5. Hofmeister S, Bodden S. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician. 2016;94(3):236-240.
6. Yonkers KA, Simoni MK. Premenstrual disorders. Am J Obstet Gynecol. 2018;218(1):68-74.
7. Yang M, Wallenstein G, Hagan M, et al. Burden of premenstrual dysphoric disorder on health-related quality of life. J Womens Health (Larchmt). 2008;17(1):113-121.
8. Craner JR, Sigmon ST, Women Health.
9. Hong JP, Park S, Wang HR, et al. Prevalence, correlates, comorbidities, and suicidal tendencies of premenstrual dysphoric disorder in a nationwide sample of Korean women. Soc Psychiatry Psychiatr Epidemiol. 2012;47(12): 1937-1945.
10. Martinez PE, Rubinow PR, Nieman LK, et al. 5α-reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology. 2016;41:1093-1102.
11. Baller EB, Wei SM, Kohn PD. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: A multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
. EINeuroimaging the menstrual cycle and premenstrual dysphoric disorderCurr Psychiatry Rep.201577
13. Reid RL. Premenstrual dysphoric disorder (formerly premenstrual syndrome) [Updated Jan 23, 2017]. In: De Groot LJ, Chrousos G, Dungan K, et al, eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc; 2000.
14. Htay TT. Premenstrual dysphoric disorder clinical presentation. Medscape. https://emedicine.medscape.com/article/293257-clinical#b3. Updated February 16, 2016. Accessed February 7, 2018.
15. Epperson CN, Hantsoo LV. Making strides to simplify diagnosis of premenstrual dysphoric disorder. Am J Psychiatry. 2017;174(1):6-7.
16. FDA. Sarafem. www.accessdata.fda.gov/drugsatfda_docs/label/2006/021860lbl.pdf. Accessed February 15, 2018.
17. FDA. Paxil CR. www.accessdata.fda.gov/drugsatfda_docs/label/2004/20936se2-013_paxil_lbl.pdf. Accessed February 15, 2018.
18. FDA. Zoloft. www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf. Accessed February 15, 2018.
19. Yonkers KA, Kornstein SG, Gueorguieva R, et al. Symptom-onset dosing of sertraline for the treatment of premenstrual dysphoric disorder: a randomized trial. JAMA Psychiatry. 2015;72(10):1037-1044.
20. Busse JW, Montori VM, Krasnik C, et al. Psychological intervention for premenstrual syndrome: a meta-analysis of randomized controlled trials. Psychother Psychosom. 2009;78(1):6-15.
21. Shobeiri F, Araste FE, Ebrahimi R, et al. Effect of calcium on premenstrual syndrome: a double-blind randomized clinical trial. Obstet Gynecol Sci. 2017;60(1):100-105.
22. Nevatte T, O’Brien PMS, Bäckström T, et al. ISPMD consensus on the management of premenstrual disorders. Arch Womens Ment Health. 2013;16(4):279-291.
CE/CME No: CR-1812
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the epidemiology and underlying pathogenesis of premenstrual dysphoric disorder (PMDD).
• Describe PMDD diagnostic criteria established by DSM-5.
• Differentiate PMDD from other conditions in order to provide appropriate treatment.
• Identify effective evidence-based treatment modalities for PMDD.
• Discuss PMDD treatment challenges and importance of individualizing PMDD treatment.
FACULTY
Jovanka Rajic is a recent graduate of the Master of Science in Nursing–Family Nurse Practitioner program at the Patricia A. Chin School of Nursing at California State University, Los Angeles. Stefanie A. Varela is adjunct faculty in the Patricia A. Chin School of Nursing at California State University, Los Angeles, and practices in the Obstetrics and Gynecology Department at Kaiser Permanente in Ontario, California.
The authors reported no conflicts of interest related to this article.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through November 30, 2019.
Article begins on next page >>
The severe psychiatric and somatic symptoms of premenstrual dysphoric disorder (PMDD) can be debilitating and place women at increased risk for other psychiatric disorders (including major depression and generalized anxiety) and for suicidal ideation. While PMDD’s complex nature makes it an underdiagnosed condition, there are clear diagnostic criteria for clinicians to ensure their patients receive timely and appropriate treatment—thus reducing the risk for serious sequelae.
Premenstrual dysphoric disorder (PMDD) is categorized as a depressive disorder in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5).1 The hallmarks of this unique disorder are chronic, severe psychiatric and somatic symptoms that occur only during the late luteal phase of the menstrual cycle and dissipate soon after the onset of menstruation.2 Symptoms are generally disruptive and often associated with significant distress and impaired quality of life.2
PMDD occurs in 3%-8% of women of childbearing age; it affects women worldwide and is not influenced by geography or culture.2 Genetic susceptibility, stress, obesity, and a history of trauma or sexual abuse have been implicated as risk factors.2-6 The impact of PMDD on health-related quality of life is greater than that of chronic back pain but comparable to that of rheumatoid arthritis and osteoarthritis.2,7 Significantly, women with PMDD have a 50%-78% lifetime risk for psychiatric disorders, such as major depressive, dysthymic, seasonal affective, and generalized anxiety disorders, and suicidality.2
PMDD can be challenging for primary care providers to diagnose and treat, due to the lack of standardized screening methods, unfamiliarity with evidence-based practices for diagnosis, and the need to tailor treatment to each patient’s individual needs.3,8 But the increased risk for psychiatric sequelae, including suicidality, make timely diagnosis and treatment of PMDD critical.2,9
PATHOGENESIS
The pathogenesis of PMDD is not completely understood. The prevailing theory is that PMDD is underlined by increased sensitivity to normal fluctuations in ovarian steroid hormone levels (see the Figure) during the luteal phase of the menstrual cycle.2-4,6

This sensitivity involves the progesterone metabolite allopregnanolone (ALLO), which acts as a modulator of central GABA-A receptors that have anxiolytic and sedative effects.2,3 It has been postulated that women with PMDD have impaired production of ALLO or decreased sensitivity of GABA-A receptors to ALLO during the luteal phase.2,3 In addition, women with PMDD exhibit a paradoxical anxiety and irritability response to ALLO.2,3 Recent research suggests that PMDD is precipitated by changing ALLO levels during the luteal phase and that treatment directed at reducing ALLO availability during this phase can alleviate PMDD symptoms.10
Hormonal fluctuations have been associated with impaired serotonergic system function in women with PMDD, which results in dysregulation of mood, cognition, sleep, and eating behavior.2-4,6 Hormonal fluctuations have also been implicated in the alteration of emotional and cognitive circuits.2,3,6,11,12 Brain imaging studies have revealed that women with PMDD demonstrate enhanced reactivity to amygdala, which processes emotional and cognitive stimuli, as well as impaired control of amygdala by the prefrontal cortex during the luteal phase.3,7,12
Continue to: PATIENT PRESENTATION/HISTORY
PATIENT PRESENTATION/HISTORY
PMDD is an individual experience for each woman.3,4 However, women with PMDD generally present with a history of various psychiatric and somatic symptoms that significantly interfere with their occupational or social functions (to be discussed in the Diagnosis section, page 42).1-4 The reported symptoms occur in predictable patterns that are associated with the menstrual cycle, intensifying around the time of menstruation and resolving immediately after onset of menstruation in most cases.1-4
Many psychiatric and medical conditions may be exacerbated during the luteal phase of the menstrual cycle and thus may mimic the signs and symptoms of PMDD (see Table 1).1,4 Therefore, the pattern and severity of symptoms should always be considered when differentiating PMDD from other underlying conditions.1,2,4,5

It is also important to distinguish PMDD from PMS, a condition with which it is frequently confused. The latter manifests with at least one affective or somatic symptom that is bothersome but not disabling.4,5 An accurate differential diagnosis is important, as the management of these two conditions differs significantly.4,5
ASSESSMENT
PMDD assessment should include thorough history taking, with emphasis on medical, gynecologic, and psychiatric history as well as social and familial history (including PMDD and other psychiatric disorders); and physical examination, including gynecologic and mental status assessment and depression screening using the Patient Health Questionnaire (PHQ-9).2,4,13,14 The physical exam is usually unremarkable.14 The most common physical findings during the luteal phase include mild swelling in the lower extremities and breast tenderness.14 Mental status examination, however, may be abnormal during the late luteal phase—albeit with orientation, memory, thoughts, and perceptions intact.13,14
LABORATORY WORKUP
There is no specific laboratory test for PMDD; rather, testing is aimed at ruling out alternative diagnoses.4,14 Relevant studies may include a complete blood count to exclude anemia, a thyroid function test to exclude thyroid disorders, a blood glucose test to exclude diabetes or hypoglycemia, and a ß hCG test to exclude possible pregnancy.4,14 Hormonal tests (eg, for FSH) may be considered for younger women with irregular cycles or for those younger than 40 with suspected premature menopause.4,14
Continue to: DIAGNOSIS
DIAGNOSIS
Diagnosis of PMDD is guided by the DSM-5 criteria, which include the following components
- Content (presence of specific symptoms)
- Cyclicity (premenstrual onset and postmenstrual resolution)
- Severity (significant distress)
- Chronicity (occurrence in the past year).15
DSM-5 has established seven criteria (labeled A-G) for a PMDD diagnosis.1 First and foremost, a woman must experience a minimum of five of the 11 listed symptoms, with a minimum of one symptom being related to mood, during most menstrual cycles over the previous 12 months (Criterion A).1 The symptoms must occur during the week before the onset of menses, must improve within a few days of onset of menses, and must resolve in the week following menses.1
Mood-related symptoms (outlined in Criterion B) include
1. Notable depressed mood, hopelessness, or self-deprecation
2. Notable tension and/or anxiety
3. Notable affective lability (eg, mood swings, sudden sadness, tearfulness, or increased sensitivity to rejection)
4. Notable anger or irritability or increased interpersonal conflicts.1
Somatic or functional symptoms associated with PMDD (Criterion C) include:
5. Low interest in common activities (eg, those related to friends, work, school, and/or hobbies)
6. Difficulty concentrating
7. Lethargy, fatigue, or increased lack of energy
8. Notable change in appetite
9. Insomnia or hypersomnia
10. Feeling overwhelmed or out of control
11. Physical symptoms, such as breast tenderness or swelling, joint or muscle pain, headache, weight gain, or bloating.1
Again, patients must report at least one symptom from Criterion B and at least one from Criterion C—but a minimum of five symptoms overall—to receive a diagnosis of PMDD.1
Continue to: Additionally, the symptoms must...
Additionally, the symptoms must cause clinically significant distress or impair daily functioning, including occupational, social, academic, and sexual activities (Criterion D). They must not represent exacerbation of another underlying psychiatric disorder, such as major depressive, dysthymic, panic, or personality disorders (Criterion E), although PMDD may co-occur with psychiatric disorders.1
The above-mentioned symptom profile must be confirmed by prospective daily ratings of a minimum of two consecutive symptomatic menstrual cycles (Criterion F), although a provisional diagnosis of PMDD may be made prior to confirmation.1 The Daily Record of Severity of Problems is the most widely used instrument for prospective daily rating of PMDD symptoms listed in the DSM-5 criteria.5,15
Finally, the symptoms must not be evoked by the use of a substance (eg, medications, alcohol, and illicit drugs) or another medical condition (Criterion G).1
TREATMENT/MANAGEMENT
The goal of PMDD treatment is to relieve psychiatric and physical symptoms and improve the patient's ability to function.3 Treatment is primarily directed at pharmacologic neuromodulation using selective serotonin reuptake inhibitors (SSRIs) or ovulation suppression using oral contraceptives and hormones.2
Pharmacotherapy
SSRIs are the firstline treatment for PMDD.5 Fluoxetine, paroxetine, and sertraline are the only serotonergic medications approved by the FDA for treatment of PMDD.2 SSRIs act within one to two days when used for PMDD, thereby allowing different modes of dosing.2 SSRI dosing may be continuous (daily administration), intermittent (administration from ovulation to first day of menses), or symptomatic (administration from symptom onset until first day of menses).3 Although data on continuous and intermittent dosing are available for fluoxetine, paroxetine, and sertraline, symptom-onset data are currently available only for sertraline (see Table 2).16-19

Continue to: Combined oral contraceptives...
Combined oral contraceptives (COCs) containing estrogen and progesterone are considered secondline treatment for PMDD—specifically, COCs containing 20 µg of ethinyl estradiol and 3 mg of drospirenone administered as a 24/4 regimen.2,3,5,6 This combination has been approved by the FDA for women with PMDD who seek oral contraception.3 Although drospirenone-containing products have been associated with increased risk for venous thromboembolism (VTE), this risk is lower than that for VTE during pregnancy or in the postpartum period.3 Currently, no strong evidence exists regarding the effectiveness of other oral contraceptives for PMDD.6
Gonadotropin-releasing hormone agonists are the thirdline treatment for PMDD.6 They eliminate symptoms of the luteal phase by suppressing ovarian release of estrogen and ovulation.6 However, use of these agents is not recommended for more than one year due to the increased risk for cardiovascular events.5,6 In addition, long-term users need add-back therapy (adding back small amounts of the hormone) to counteract the effects of low estrogen, such as bone loss; providers should be aware that this may lead to the recurrence of PMDD.3,5,6 The use of estrogen and progesterone formulations for PMDD is currently not strongly supported by research.6
Complementary treatment
Cognitive behavioral therapy has been shown to improve functioning and reduce depression in women with PMDD and may be a useful adjunct.2,20 Regular aerobic exercise, a diet high in protein and complex carbohydrates to increase tryptophan (serotonin precursor) levels, and reduced intake of caffeine, sugar, and alcohol are some commonly recommended lifestyle changes.2
Calcium carbonate supplementation (500 mg/d) has demonstrated effectiveness in alleviating premenstrual mood and physical symptoms.21 There is currently no strong evidence regarding the benefits of acupuncture, Qi therapy, reflexology, and herbal preparations for managing PMDD.22
Surgery
Bilateral oophorectomy, usually with concomitant hysterectomy, is the last resort for women with severe PMDD who do not respond to or cannot tolerate the standard treatments.6 This surgical procedure results in premature menopause, which may lead to complications related to a hypoestrogenic state—including vasomotor symptoms (flushes/flashes), vaginal atrophy, osteopenia, osteoporosis, and cardiovascular disease.2 Therefore, it is important to implement estrogen replacement therapy after surgery until the age of natural menopause is reached.2 If hysterectomy is not performed, the administration of progesterone is necessary to prevent endometrial hyperplasia and therefore reduce the risk for endometrial cancer.2 However, the addition of progesterone may lead to recurrence of symptoms.2
Continue to: Treatment challenges
Treatment challenges
PMDD treatment differs for each patient.3 Severity of symptoms, response to treatment, treatment preference, conception plans, and reproductive age need to be considered.3
Women with prominent depressive or physical symptoms may respond better to continuous dosing of SSRIs, whereas those with prominent irritability, anger, and mood swings may respond better to a symptom-onset SSRI regimen that reduces availability and function of ALLO.3 Women who develop tolerance to SSRIs may need to have their dosage increased or be switched to another medication.3Quetiapine is used as an adjunct to SSRIs for women who do not respond to SSRIs alone and has shown to improve mood swings, anxiety, and irritability.5 However, women experiencing persistent adverse effects of SSRIs, such as sexual dysfunction, may benefit from intermittent dosing.3
Adolescents and women in their early 20s should be treated with OCs or nonpharmacologic modalities due to concerns about SSRI use and increased risk for suicidality in this population.3 The risks related to SSRI use during pregnancy and breastfeeding should be considered and discussed with women of childbearing age who use SSRIs to treat PMDD.3 Perimenopausal women with irregular menses on intermittent SSRIs may have to switch to symptom-onset or continuous dosing due to the difficulty of tracking the menstrual period and lack of significant benchmarks regarding when to start the treatment.3
Patient education/follow-up
Patients should be educated on PMDD etiology, diagnostic process, and available treatment options.4 The importance of prospective record-keeping—for confirmation of the diagnosis and evaluation of individual response to a specific treatment—should be emphasized.4 Patients should be encouraged to follow up with their health care provider to monitor treatment effectiveness, possible adverse effects, and need for treatment adjustment.4
CONCLUSION
The symptoms of PMDD can have a debilitating and life-disrupting impact on affected women—and put them at risk for other serious psychiatric disorders and suicide. The DSM-5 criteria provide diagnostic guidance to help distinguish PMDD from other underlying conditions, ensuring that patients can receive timely and appropriate treatment. While SSRIs are regarded as the most effective option, other evidence-based treatments should be considered, since PMDD requires individualized treatment to ensure optimal clinical outcomes.
CE/CME No: CR-1812
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the epidemiology and underlying pathogenesis of premenstrual dysphoric disorder (PMDD).
• Describe PMDD diagnostic criteria established by DSM-5.
• Differentiate PMDD from other conditions in order to provide appropriate treatment.
• Identify effective evidence-based treatment modalities for PMDD.
• Discuss PMDD treatment challenges and importance of individualizing PMDD treatment.
FACULTY
Jovanka Rajic is a recent graduate of the Master of Science in Nursing–Family Nurse Practitioner program at the Patricia A. Chin School of Nursing at California State University, Los Angeles. Stefanie A. Varela is adjunct faculty in the Patricia A. Chin School of Nursing at California State University, Los Angeles, and practices in the Obstetrics and Gynecology Department at Kaiser Permanente in Ontario, California.
The authors reported no conflicts of interest related to this article.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through November 30, 2019.
Article begins on next page >>
The severe psychiatric and somatic symptoms of premenstrual dysphoric disorder (PMDD) can be debilitating and place women at increased risk for other psychiatric disorders (including major depression and generalized anxiety) and for suicidal ideation. While PMDD’s complex nature makes it an underdiagnosed condition, there are clear diagnostic criteria for clinicians to ensure their patients receive timely and appropriate treatment—thus reducing the risk for serious sequelae.
Premenstrual dysphoric disorder (PMDD) is categorized as a depressive disorder in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5).1 The hallmarks of this unique disorder are chronic, severe psychiatric and somatic symptoms that occur only during the late luteal phase of the menstrual cycle and dissipate soon after the onset of menstruation.2 Symptoms are generally disruptive and often associated with significant distress and impaired quality of life.2
PMDD occurs in 3%-8% of women of childbearing age; it affects women worldwide and is not influenced by geography or culture.2 Genetic susceptibility, stress, obesity, and a history of trauma or sexual abuse have been implicated as risk factors.2-6 The impact of PMDD on health-related quality of life is greater than that of chronic back pain but comparable to that of rheumatoid arthritis and osteoarthritis.2,7 Significantly, women with PMDD have a 50%-78% lifetime risk for psychiatric disorders, such as major depressive, dysthymic, seasonal affective, and generalized anxiety disorders, and suicidality.2
PMDD can be challenging for primary care providers to diagnose and treat, due to the lack of standardized screening methods, unfamiliarity with evidence-based practices for diagnosis, and the need to tailor treatment to each patient’s individual needs.3,8 But the increased risk for psychiatric sequelae, including suicidality, make timely diagnosis and treatment of PMDD critical.2,9
PATHOGENESIS
The pathogenesis of PMDD is not completely understood. The prevailing theory is that PMDD is underlined by increased sensitivity to normal fluctuations in ovarian steroid hormone levels (see the Figure) during the luteal phase of the menstrual cycle.2-4,6

This sensitivity involves the progesterone metabolite allopregnanolone (ALLO), which acts as a modulator of central GABA-A receptors that have anxiolytic and sedative effects.2,3 It has been postulated that women with PMDD have impaired production of ALLO or decreased sensitivity of GABA-A receptors to ALLO during the luteal phase.2,3 In addition, women with PMDD exhibit a paradoxical anxiety and irritability response to ALLO.2,3 Recent research suggests that PMDD is precipitated by changing ALLO levels during the luteal phase and that treatment directed at reducing ALLO availability during this phase can alleviate PMDD symptoms.10
Hormonal fluctuations have been associated with impaired serotonergic system function in women with PMDD, which results in dysregulation of mood, cognition, sleep, and eating behavior.2-4,6 Hormonal fluctuations have also been implicated in the alteration of emotional and cognitive circuits.2,3,6,11,12 Brain imaging studies have revealed that women with PMDD demonstrate enhanced reactivity to amygdala, which processes emotional and cognitive stimuli, as well as impaired control of amygdala by the prefrontal cortex during the luteal phase.3,7,12
Continue to: PATIENT PRESENTATION/HISTORY
PATIENT PRESENTATION/HISTORY
PMDD is an individual experience for each woman.3,4 However, women with PMDD generally present with a history of various psychiatric and somatic symptoms that significantly interfere with their occupational or social functions (to be discussed in the Diagnosis section, page 42).1-4 The reported symptoms occur in predictable patterns that are associated with the menstrual cycle, intensifying around the time of menstruation and resolving immediately after onset of menstruation in most cases.1-4
Many psychiatric and medical conditions may be exacerbated during the luteal phase of the menstrual cycle and thus may mimic the signs and symptoms of PMDD (see Table 1).1,4 Therefore, the pattern and severity of symptoms should always be considered when differentiating PMDD from other underlying conditions.1,2,4,5

It is also important to distinguish PMDD from PMS, a condition with which it is frequently confused. The latter manifests with at least one affective or somatic symptom that is bothersome but not disabling.4,5 An accurate differential diagnosis is important, as the management of these two conditions differs significantly.4,5
ASSESSMENT
PMDD assessment should include thorough history taking, with emphasis on medical, gynecologic, and psychiatric history as well as social and familial history (including PMDD and other psychiatric disorders); and physical examination, including gynecologic and mental status assessment and depression screening using the Patient Health Questionnaire (PHQ-9).2,4,13,14 The physical exam is usually unremarkable.14 The most common physical findings during the luteal phase include mild swelling in the lower extremities and breast tenderness.14 Mental status examination, however, may be abnormal during the late luteal phase—albeit with orientation, memory, thoughts, and perceptions intact.13,14
LABORATORY WORKUP
There is no specific laboratory test for PMDD; rather, testing is aimed at ruling out alternative diagnoses.4,14 Relevant studies may include a complete blood count to exclude anemia, a thyroid function test to exclude thyroid disorders, a blood glucose test to exclude diabetes or hypoglycemia, and a ß hCG test to exclude possible pregnancy.4,14 Hormonal tests (eg, for FSH) may be considered for younger women with irregular cycles or for those younger than 40 with suspected premature menopause.4,14
Continue to: DIAGNOSIS
DIAGNOSIS
Diagnosis of PMDD is guided by the DSM-5 criteria, which include the following components
- Content (presence of specific symptoms)
- Cyclicity (premenstrual onset and postmenstrual resolution)
- Severity (significant distress)
- Chronicity (occurrence in the past year).15
DSM-5 has established seven criteria (labeled A-G) for a PMDD diagnosis.1 First and foremost, a woman must experience a minimum of five of the 11 listed symptoms, with a minimum of one symptom being related to mood, during most menstrual cycles over the previous 12 months (Criterion A).1 The symptoms must occur during the week before the onset of menses, must improve within a few days of onset of menses, and must resolve in the week following menses.1
Mood-related symptoms (outlined in Criterion B) include
1. Notable depressed mood, hopelessness, or self-deprecation
2. Notable tension and/or anxiety
3. Notable affective lability (eg, mood swings, sudden sadness, tearfulness, or increased sensitivity to rejection)
4. Notable anger or irritability or increased interpersonal conflicts.1
Somatic or functional symptoms associated with PMDD (Criterion C) include:
5. Low interest in common activities (eg, those related to friends, work, school, and/or hobbies)
6. Difficulty concentrating
7. Lethargy, fatigue, or increased lack of energy
8. Notable change in appetite
9. Insomnia or hypersomnia
10. Feeling overwhelmed or out of control
11. Physical symptoms, such as breast tenderness or swelling, joint or muscle pain, headache, weight gain, or bloating.1
Again, patients must report at least one symptom from Criterion B and at least one from Criterion C—but a minimum of five symptoms overall—to receive a diagnosis of PMDD.1
Continue to: Additionally, the symptoms must...
Additionally, the symptoms must cause clinically significant distress or impair daily functioning, including occupational, social, academic, and sexual activities (Criterion D). They must not represent exacerbation of another underlying psychiatric disorder, such as major depressive, dysthymic, panic, or personality disorders (Criterion E), although PMDD may co-occur with psychiatric disorders.1
The above-mentioned symptom profile must be confirmed by prospective daily ratings of a minimum of two consecutive symptomatic menstrual cycles (Criterion F), although a provisional diagnosis of PMDD may be made prior to confirmation.1 The Daily Record of Severity of Problems is the most widely used instrument for prospective daily rating of PMDD symptoms listed in the DSM-5 criteria.5,15
Finally, the symptoms must not be evoked by the use of a substance (eg, medications, alcohol, and illicit drugs) or another medical condition (Criterion G).1
TREATMENT/MANAGEMENT
The goal of PMDD treatment is to relieve psychiatric and physical symptoms and improve the patient's ability to function.3 Treatment is primarily directed at pharmacologic neuromodulation using selective serotonin reuptake inhibitors (SSRIs) or ovulation suppression using oral contraceptives and hormones.2
Pharmacotherapy
SSRIs are the firstline treatment for PMDD.5 Fluoxetine, paroxetine, and sertraline are the only serotonergic medications approved by the FDA for treatment of PMDD.2 SSRIs act within one to two days when used for PMDD, thereby allowing different modes of dosing.2 SSRI dosing may be continuous (daily administration), intermittent (administration from ovulation to first day of menses), or symptomatic (administration from symptom onset until first day of menses).3 Although data on continuous and intermittent dosing are available for fluoxetine, paroxetine, and sertraline, symptom-onset data are currently available only for sertraline (see Table 2).16-19

Continue to: Combined oral contraceptives...
Combined oral contraceptives (COCs) containing estrogen and progesterone are considered secondline treatment for PMDD—specifically, COCs containing 20 µg of ethinyl estradiol and 3 mg of drospirenone administered as a 24/4 regimen.2,3,5,6 This combination has been approved by the FDA for women with PMDD who seek oral contraception.3 Although drospirenone-containing products have been associated with increased risk for venous thromboembolism (VTE), this risk is lower than that for VTE during pregnancy or in the postpartum period.3 Currently, no strong evidence exists regarding the effectiveness of other oral contraceptives for PMDD.6
Gonadotropin-releasing hormone agonists are the thirdline treatment for PMDD.6 They eliminate symptoms of the luteal phase by suppressing ovarian release of estrogen and ovulation.6 However, use of these agents is not recommended for more than one year due to the increased risk for cardiovascular events.5,6 In addition, long-term users need add-back therapy (adding back small amounts of the hormone) to counteract the effects of low estrogen, such as bone loss; providers should be aware that this may lead to the recurrence of PMDD.3,5,6 The use of estrogen and progesterone formulations for PMDD is currently not strongly supported by research.6
Complementary treatment
Cognitive behavioral therapy has been shown to improve functioning and reduce depression in women with PMDD and may be a useful adjunct.2,20 Regular aerobic exercise, a diet high in protein and complex carbohydrates to increase tryptophan (serotonin precursor) levels, and reduced intake of caffeine, sugar, and alcohol are some commonly recommended lifestyle changes.2
Calcium carbonate supplementation (500 mg/d) has demonstrated effectiveness in alleviating premenstrual mood and physical symptoms.21 There is currently no strong evidence regarding the benefits of acupuncture, Qi therapy, reflexology, and herbal preparations for managing PMDD.22
Surgery
Bilateral oophorectomy, usually with concomitant hysterectomy, is the last resort for women with severe PMDD who do not respond to or cannot tolerate the standard treatments.6 This surgical procedure results in premature menopause, which may lead to complications related to a hypoestrogenic state—including vasomotor symptoms (flushes/flashes), vaginal atrophy, osteopenia, osteoporosis, and cardiovascular disease.2 Therefore, it is important to implement estrogen replacement therapy after surgery until the age of natural menopause is reached.2 If hysterectomy is not performed, the administration of progesterone is necessary to prevent endometrial hyperplasia and therefore reduce the risk for endometrial cancer.2 However, the addition of progesterone may lead to recurrence of symptoms.2
Continue to: Treatment challenges
Treatment challenges
PMDD treatment differs for each patient.3 Severity of symptoms, response to treatment, treatment preference, conception plans, and reproductive age need to be considered.3
Women with prominent depressive or physical symptoms may respond better to continuous dosing of SSRIs, whereas those with prominent irritability, anger, and mood swings may respond better to a symptom-onset SSRI regimen that reduces availability and function of ALLO.3 Women who develop tolerance to SSRIs may need to have their dosage increased or be switched to another medication.3Quetiapine is used as an adjunct to SSRIs for women who do not respond to SSRIs alone and has shown to improve mood swings, anxiety, and irritability.5 However, women experiencing persistent adverse effects of SSRIs, such as sexual dysfunction, may benefit from intermittent dosing.3
Adolescents and women in their early 20s should be treated with OCs or nonpharmacologic modalities due to concerns about SSRI use and increased risk for suicidality in this population.3 The risks related to SSRI use during pregnancy and breastfeeding should be considered and discussed with women of childbearing age who use SSRIs to treat PMDD.3 Perimenopausal women with irregular menses on intermittent SSRIs may have to switch to symptom-onset or continuous dosing due to the difficulty of tracking the menstrual period and lack of significant benchmarks regarding when to start the treatment.3
Patient education/follow-up
Patients should be educated on PMDD etiology, diagnostic process, and available treatment options.4 The importance of prospective record-keeping—for confirmation of the diagnosis and evaluation of individual response to a specific treatment—should be emphasized.4 Patients should be encouraged to follow up with their health care provider to monitor treatment effectiveness, possible adverse effects, and need for treatment adjustment.4
CONCLUSION
The symptoms of PMDD can have a debilitating and life-disrupting impact on affected women—and put them at risk for other serious psychiatric disorders and suicide. The DSM-5 criteria provide diagnostic guidance to help distinguish PMDD from other underlying conditions, ensuring that patients can receive timely and appropriate treatment. While SSRIs are regarded as the most effective option, other evidence-based treatments should be considered, since PMDD requires individualized treatment to ensure optimal clinical outcomes.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Rapkin AJ, Lewis EI. Treatment of premenstrual dysphoric disorder. Womens Health (Lond). 2013;9(6):537-556.
3. Pearlstein T. Treatment of premenstrual dysphoric disorder: therapeutic challenges. Expert Rev Clin Pharmacol. 2016;9(4):493-496.
4. Zielinski R, Lynne S. Menstrual-cycle pain and premenstrual conditions. In: Schuiling KD, Likis FE, eds. Women’s Gynecologic Health. Burlington, MA: Jones & Bartlett Learning; 2017:556-573.
5. Hofmeister S, Bodden S. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician. 2016;94(3):236-240.
6. Yonkers KA, Simoni MK. Premenstrual disorders. Am J Obstet Gynecol. 2018;218(1):68-74.
7. Yang M, Wallenstein G, Hagan M, et al. Burden of premenstrual dysphoric disorder on health-related quality of life. J Womens Health (Larchmt). 2008;17(1):113-121.
8. Craner JR, Sigmon ST, Women Health.
9. Hong JP, Park S, Wang HR, et al. Prevalence, correlates, comorbidities, and suicidal tendencies of premenstrual dysphoric disorder in a nationwide sample of Korean women. Soc Psychiatry Psychiatr Epidemiol. 2012;47(12): 1937-1945.
10. Martinez PE, Rubinow PR, Nieman LK, et al. 5α-reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology. 2016;41:1093-1102.
11. Baller EB, Wei SM, Kohn PD. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: A multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
. EINeuroimaging the menstrual cycle and premenstrual dysphoric disorderCurr Psychiatry Rep.201577
13. Reid RL. Premenstrual dysphoric disorder (formerly premenstrual syndrome) [Updated Jan 23, 2017]. In: De Groot LJ, Chrousos G, Dungan K, et al, eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc; 2000.
14. Htay TT. Premenstrual dysphoric disorder clinical presentation. Medscape. https://emedicine.medscape.com/article/293257-clinical#b3. Updated February 16, 2016. Accessed February 7, 2018.
15. Epperson CN, Hantsoo LV. Making strides to simplify diagnosis of premenstrual dysphoric disorder. Am J Psychiatry. 2017;174(1):6-7.
16. FDA. Sarafem. www.accessdata.fda.gov/drugsatfda_docs/label/2006/021860lbl.pdf. Accessed February 15, 2018.
17. FDA. Paxil CR. www.accessdata.fda.gov/drugsatfda_docs/label/2004/20936se2-013_paxil_lbl.pdf. Accessed February 15, 2018.
18. FDA. Zoloft. www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf. Accessed February 15, 2018.
19. Yonkers KA, Kornstein SG, Gueorguieva R, et al. Symptom-onset dosing of sertraline for the treatment of premenstrual dysphoric disorder: a randomized trial. JAMA Psychiatry. 2015;72(10):1037-1044.
20. Busse JW, Montori VM, Krasnik C, et al. Psychological intervention for premenstrual syndrome: a meta-analysis of randomized controlled trials. Psychother Psychosom. 2009;78(1):6-15.
21. Shobeiri F, Araste FE, Ebrahimi R, et al. Effect of calcium on premenstrual syndrome: a double-blind randomized clinical trial. Obstet Gynecol Sci. 2017;60(1):100-105.
22. Nevatte T, O’Brien PMS, Bäckström T, et al. ISPMD consensus on the management of premenstrual disorders. Arch Womens Ment Health. 2013;16(4):279-291.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Rapkin AJ, Lewis EI. Treatment of premenstrual dysphoric disorder. Womens Health (Lond). 2013;9(6):537-556.
3. Pearlstein T. Treatment of premenstrual dysphoric disorder: therapeutic challenges. Expert Rev Clin Pharmacol. 2016;9(4):493-496.
4. Zielinski R, Lynne S. Menstrual-cycle pain and premenstrual conditions. In: Schuiling KD, Likis FE, eds. Women’s Gynecologic Health. Burlington, MA: Jones & Bartlett Learning; 2017:556-573.
5. Hofmeister S, Bodden S. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician. 2016;94(3):236-240.
6. Yonkers KA, Simoni MK. Premenstrual disorders. Am J Obstet Gynecol. 2018;218(1):68-74.
7. Yang M, Wallenstein G, Hagan M, et al. Burden of premenstrual dysphoric disorder on health-related quality of life. J Womens Health (Larchmt). 2008;17(1):113-121.
8. Craner JR, Sigmon ST, Women Health.
9. Hong JP, Park S, Wang HR, et al. Prevalence, correlates, comorbidities, and suicidal tendencies of premenstrual dysphoric disorder in a nationwide sample of Korean women. Soc Psychiatry Psychiatr Epidemiol. 2012;47(12): 1937-1945.
10. Martinez PE, Rubinow PR, Nieman LK, et al. 5α-reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology. 2016;41:1093-1102.
11. Baller EB, Wei SM, Kohn PD. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: A multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
. EINeuroimaging the menstrual cycle and premenstrual dysphoric disorderCurr Psychiatry Rep.201577
13. Reid RL. Premenstrual dysphoric disorder (formerly premenstrual syndrome) [Updated Jan 23, 2017]. In: De Groot LJ, Chrousos G, Dungan K, et al, eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc; 2000.
14. Htay TT. Premenstrual dysphoric disorder clinical presentation. Medscape. https://emedicine.medscape.com/article/293257-clinical#b3. Updated February 16, 2016. Accessed February 7, 2018.
15. Epperson CN, Hantsoo LV. Making strides to simplify diagnosis of premenstrual dysphoric disorder. Am J Psychiatry. 2017;174(1):6-7.
16. FDA. Sarafem. www.accessdata.fda.gov/drugsatfda_docs/label/2006/021860lbl.pdf. Accessed February 15, 2018.
17. FDA. Paxil CR. www.accessdata.fda.gov/drugsatfda_docs/label/2004/20936se2-013_paxil_lbl.pdf. Accessed February 15, 2018.
18. FDA. Zoloft. www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf. Accessed February 15, 2018.
19. Yonkers KA, Kornstein SG, Gueorguieva R, et al. Symptom-onset dosing of sertraline for the treatment of premenstrual dysphoric disorder: a randomized trial. JAMA Psychiatry. 2015;72(10):1037-1044.
20. Busse JW, Montori VM, Krasnik C, et al. Psychological intervention for premenstrual syndrome: a meta-analysis of randomized controlled trials. Psychother Psychosom. 2009;78(1):6-15.
21. Shobeiri F, Araste FE, Ebrahimi R, et al. Effect of calcium on premenstrual syndrome: a double-blind randomized clinical trial. Obstet Gynecol Sci. 2017;60(1):100-105.
22. Nevatte T, O’Brien PMS, Bäckström T, et al. ISPMD consensus on the management of premenstrual disorders. Arch Womens Ment Health. 2013;16(4):279-291.
PCOS linked to increased cancer risk in premenopausal women
based on an analysis of nearly 3.5 million women in a large Swedish database.
Women with PCOS had a sixfold increased risk of endometrial cancer, a tripling of endocrine gland cancers, and more than a doubling in the risk of ovarian and pancreatic cancers. Once women reached menopausal status, however, their cancer risk was comparable to that of women without a history of PCOS.
“Several carcinogenic processes are associated with PCOS, including dyslipidemia, hyperinsulinemia, and chronic inflammation,” wrote Weimin Ye, MD, PhD, of the Karolinska Institutet, Stockholm, and his colleagues. “Our study indicates that cancer may need to be added to the spectrum of long-term health consequences of PCOS and warrants increased surveillance among those patients.”
The research letter was published online in JAMA Oncology.
The team examined the relationship between PCOS and primary cancers in about 3.5 million women over a span of up to 24 years (1985-2009), although the mean follow-up time was not mentioned. To examine the potential impact of menopause, they conducted separate multivariate logistic regression analyses for those younger than 51 years, and those aged 51 years or older. The analyses controlled for use of some medications (metformin, oral contraceptives, and hormone therapy); as well as educational level (a proxy for socioeconomic status); smoking; parity (a proxy for fertility); parental cancers; and diabetes.
Overall, 14,764 women had been diagnosed with PCOS; they were a mean of 28 years at baseline and 182 developed a primary cancer 1 year or more after PCOS diagnosis.
These women had a 15% overall increased risk of cancer, compared with women without PCOS.
The risks for specific cancers also were increased, compared with women without PCOS, including endometrial (hazard ratio, 2.62), ovarian (HR, 2.16), endocrine (HR, 1.92), pancreatic (HR, 3.4), kidney (HR, 3.0), and skeletal and hematopoietic (HR, 1.69) cancers.
The risks were associated with younger age, however. In the group under age 51 years, the overall risk was 22% higher. The increased risk of specific cancers were endometrial (HR, 6.45), ovarian (HR, 2.55), pancreatic (HR, 6.68), kidney (HR, 4.57), and endocrine (not thyroid) gland (HR, 2.9) cancers.
The authors had no relevant financial disclosures.
SOURCE: Yin W et al. JAMA Oncol. 2018 Nov 29. doi:10.1001/jamaoncol.2018.5188.
based on an analysis of nearly 3.5 million women in a large Swedish database.
Women with PCOS had a sixfold increased risk of endometrial cancer, a tripling of endocrine gland cancers, and more than a doubling in the risk of ovarian and pancreatic cancers. Once women reached menopausal status, however, their cancer risk was comparable to that of women without a history of PCOS.
“Several carcinogenic processes are associated with PCOS, including dyslipidemia, hyperinsulinemia, and chronic inflammation,” wrote Weimin Ye, MD, PhD, of the Karolinska Institutet, Stockholm, and his colleagues. “Our study indicates that cancer may need to be added to the spectrum of long-term health consequences of PCOS and warrants increased surveillance among those patients.”
The research letter was published online in JAMA Oncology.
The team examined the relationship between PCOS and primary cancers in about 3.5 million women over a span of up to 24 years (1985-2009), although the mean follow-up time was not mentioned. To examine the potential impact of menopause, they conducted separate multivariate logistic regression analyses for those younger than 51 years, and those aged 51 years or older. The analyses controlled for use of some medications (metformin, oral contraceptives, and hormone therapy); as well as educational level (a proxy for socioeconomic status); smoking; parity (a proxy for fertility); parental cancers; and diabetes.
Overall, 14,764 women had been diagnosed with PCOS; they were a mean of 28 years at baseline and 182 developed a primary cancer 1 year or more after PCOS diagnosis.
These women had a 15% overall increased risk of cancer, compared with women without PCOS.
The risks for specific cancers also were increased, compared with women without PCOS, including endometrial (hazard ratio, 2.62), ovarian (HR, 2.16), endocrine (HR, 1.92), pancreatic (HR, 3.4), kidney (HR, 3.0), and skeletal and hematopoietic (HR, 1.69) cancers.
The risks were associated with younger age, however. In the group under age 51 years, the overall risk was 22% higher. The increased risk of specific cancers were endometrial (HR, 6.45), ovarian (HR, 2.55), pancreatic (HR, 6.68), kidney (HR, 4.57), and endocrine (not thyroid) gland (HR, 2.9) cancers.
The authors had no relevant financial disclosures.
SOURCE: Yin W et al. JAMA Oncol. 2018 Nov 29. doi:10.1001/jamaoncol.2018.5188.
based on an analysis of nearly 3.5 million women in a large Swedish database.
Women with PCOS had a sixfold increased risk of endometrial cancer, a tripling of endocrine gland cancers, and more than a doubling in the risk of ovarian and pancreatic cancers. Once women reached menopausal status, however, their cancer risk was comparable to that of women without a history of PCOS.
“Several carcinogenic processes are associated with PCOS, including dyslipidemia, hyperinsulinemia, and chronic inflammation,” wrote Weimin Ye, MD, PhD, of the Karolinska Institutet, Stockholm, and his colleagues. “Our study indicates that cancer may need to be added to the spectrum of long-term health consequences of PCOS and warrants increased surveillance among those patients.”
The research letter was published online in JAMA Oncology.
The team examined the relationship between PCOS and primary cancers in about 3.5 million women over a span of up to 24 years (1985-2009), although the mean follow-up time was not mentioned. To examine the potential impact of menopause, they conducted separate multivariate logistic regression analyses for those younger than 51 years, and those aged 51 years or older. The analyses controlled for use of some medications (metformin, oral contraceptives, and hormone therapy); as well as educational level (a proxy for socioeconomic status); smoking; parity (a proxy for fertility); parental cancers; and diabetes.
Overall, 14,764 women had been diagnosed with PCOS; they were a mean of 28 years at baseline and 182 developed a primary cancer 1 year or more after PCOS diagnosis.
These women had a 15% overall increased risk of cancer, compared with women without PCOS.
The risks for specific cancers also were increased, compared with women without PCOS, including endometrial (hazard ratio, 2.62), ovarian (HR, 2.16), endocrine (HR, 1.92), pancreatic (HR, 3.4), kidney (HR, 3.0), and skeletal and hematopoietic (HR, 1.69) cancers.
The risks were associated with younger age, however. In the group under age 51 years, the overall risk was 22% higher. The increased risk of specific cancers were endometrial (HR, 6.45), ovarian (HR, 2.55), pancreatic (HR, 6.68), kidney (HR, 4.57), and endocrine (not thyroid) gland (HR, 2.9) cancers.
The authors had no relevant financial disclosures.
SOURCE: Yin W et al. JAMA Oncol. 2018 Nov 29. doi:10.1001/jamaoncol.2018.5188.
FROM JAMA ONCOLOGY
Key clinical point: Polycystic ovarian syndrome may be associated with increased cancer risks among younger women.
Major finding: Among premenopausal women, there was a sixfold increased risk of endometrial cancer, a tripling of endocrine gland cancers, and a more than doubling in the risk of ovarian and pancreatic cancers
Study details: The study examined risks in 3.5 million women with up to 24 years of follow-up.
Disclosures: The study authors had no financial disclosures.
Source: Yin W et al. JAMA Oncol. 2018 Nov 29. doi:10.1001/jamaoncol.2018.5188.