User login
Aquatic Antagonists: Lionfish (Pterois volitans)
The lionfish (Pterois volitans) is a member of the Scorpaenidae family of venomous fish.1-3 Lionfish are an invasive species originally from the Indian and Pacific oceans and the Red Sea that now are widely found throughout tropical and temperate oceans in both hemispheres. They are a popular aquarium fish and were inadvertently introduced in the Atlantic Ocean in South Florida during the late 1980s to early 1990s.2,4 Since then, lionfish have spread into reef systems throughout the Atlantic Ocean, Caribbean Sea, and Gulf of Mexico in rapidly growing numbers, and they are now fo und all along the southeastern coast of the United States.5
Characteristics
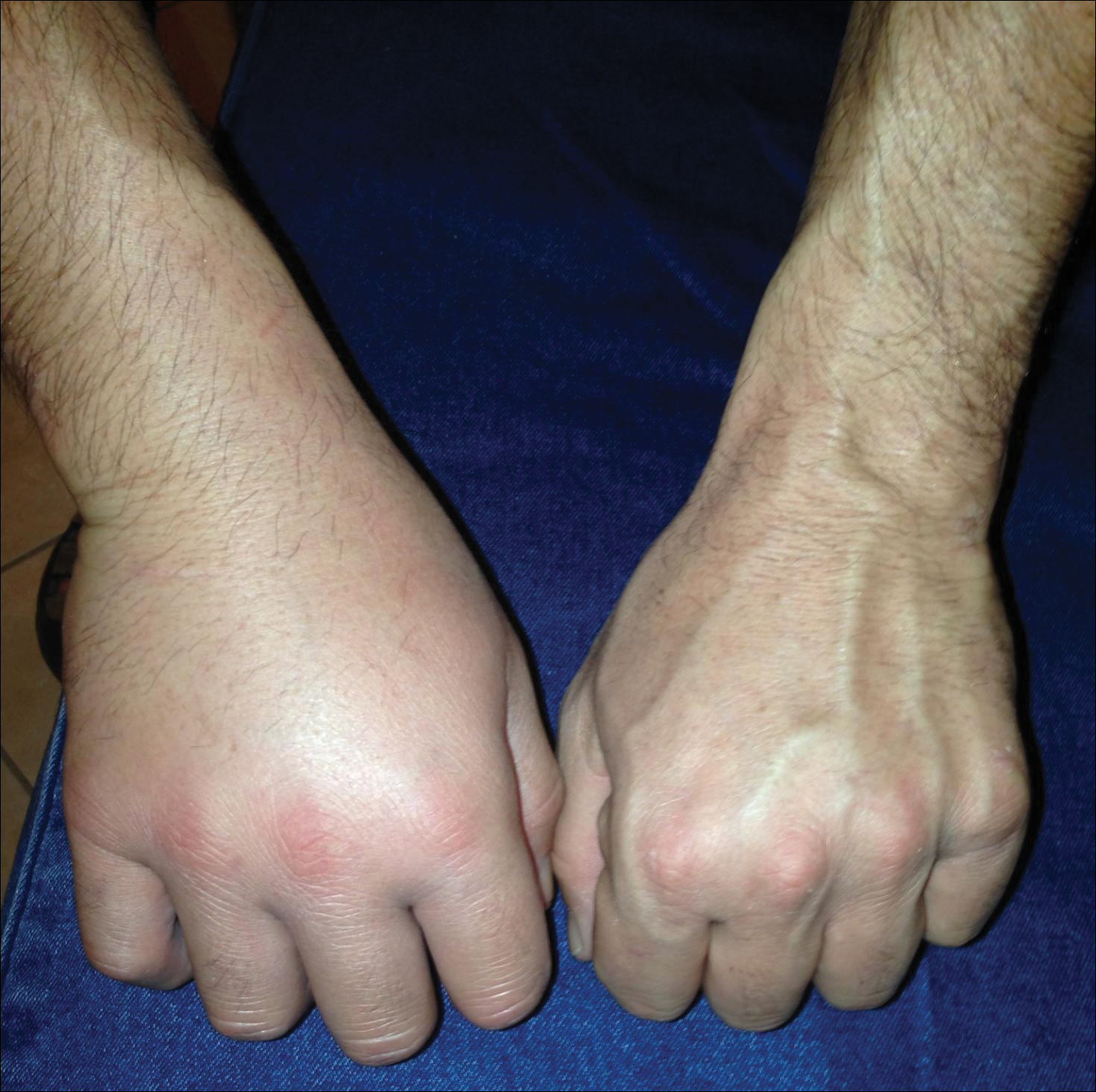
Lionfish are brightly colored with red or maroon and white stripes, tentacles above the eyes and mouth, fan-shaped pectoral fins, and spines that deliver an especially painful venomous sting that often results in edema (Figure 1). They have 12 dorsal spines, 2 pelvic spines, and 3 anal spines.

Symptoms of Envenomation
As lionfish continue to spread to popular areas of the southeast Atlantic Ocean and Caribbean Sea, the chances of human contact with lionfish have increased. Lionfish stings are now the second most common marine envenomation injury after those caused by stingrays.4 Lionfish stings usually occur on the hands, fingers, or forearms during handling of the fish in ocean waters or in maintenance of aquariums. The mechanism of the venom apparatus is similar for all venomous fish. The spines have surrounding integumentary sheaths containing venom that rupture and inject venom when they penetrate the skin.6 The venom is a heat-labile neuromuscular toxin that causes edema (Figure 2), plasma extravasation, and thrombotic skin lesions.7
Wounds are classified into 3 categories: grade I consists of local erythema/ecchymosis, grade II involves vesicle or blister formation, and grade III denotes wounds that develop local necrosis.8 The sting causes immediate and severe throbbing pain, often described as excruciating or rated 10/10 on a basic pain scale, typically radiating up the affected limb. Puncture sites may bleed and often have associated redness and swelling. Pain may last up to 24 hours. Occasionally, foreign material may be left in the wound requiring removal. There also is a chance of secondary infection at the wound site, and severe envenomation can lead to local tissue necrosis.8 Systemic effects can occur in some cases, including nausea, vomiting, sweating, headache, dizziness, disorientation, palpitations, and even syncope.9 However, to our knowledge there are no documented cases of human death from a lionfish sting. Anaphylactic reactions are possible and require immediate treatment.6
A study conducted in the French West Indies evaluated 117 patients with lionfish envenomation and found that victims experienced severe pain and local edema (100%), paresthesia (90%), abdominal cramps (62%), extensive edema (53%), tachycardia (34%), skin rash (32%), gastrointestinal tract symptoms (28%), syncope (27%), transient weakness (24%), hypertension (21%), hypotension (18%), and hyperthermia (9%).9 Complications included local infection (18%) such as skin abscess (5%), skin necrosis (3%), and septic arthritis (2%). Twenty-two percent of patients were hospitalized and 8% required surgery. Local infectious complications were more frequent in those with multiple stings (19%). The study concluded that lionfish now represent a major health threat in the West Indies.9 As lionfish numbers have grown, health care providers are seeing increasing numbers of envenomation cases in areas of the coastal southeastern United States and Caribbean associated with considerable morbidity. Providers in nonendemic areas also may see envenomation injuries due to the lionfish popularity in home aquariums.9
Management
Individuals with lionfish stings should immerse the affected area in hot but not scalding water. Those with more serious injuries should seek medical attention. Home remedies that are generally contraindicated include application of topical papain or meat tenderizer.10 Data on ice packs are mixed, but because the toxin is heat labile, the most effective initial step in treatment is immersion of the affected area in water (temperature, 40°C to 45°C) for 30 to 90 minutes.6 The hot water inactivates the heat-labile toxin, leading to near-complete symptomatic relief in 80% of cases and moderate relief in an additional 14%. Immersion time more than 90 minutes considerably increases the risk for burns. Children should always be monitored to prevent burns. If a patient has received a nerve block for analgesia, the wound should not be immersed in hot water to avoid burns to the skin. The wound should be meticulously cleaned with saline irrigation, and radiography or ultrasonography should be performed as deemed necessary to look for any retained foreign bodies.8 Patients may require parenteral or oral analgesia as well as careful follow-up to ensure proper healing.9 Systemic symptoms require supportive care. Venomous fish wounds typically are small and superficial. Empiric antibiotic therapy is not advised for superficial wounds but may be required for clinically infected wounds.8 Tetanus prophylaxis should be given as appropriate to all affected patients. It has been noted that blister fluid contains high concentrations of lionfish venom, and when present, it increases the likelihood of converting the injury from a grade II to grade III wound with tissue necrosis; therefore, blisters should be drained or excised to decrease the chances of subsequent tissue necrosis.11,12 If secondary infection such as cellulitis develops, antibiotics should be chosen to cover likely pathogens including common skin flora such as staphylococci and marine organisms such as Vibrio species. Wounds showing signs of infection should be cultured, with antibiotics adjusted according to sensitivities.5 Deeper wounds should be left open (unsutured) with a proper dressing to heal. Any wounds that involve vascular or joint structures require specialty management. Wounds involving joints may on occasion require surgical exploration and debridement.
Public Health Concerns
In an attempt to slow the growth of their population, human consumption of the fish has been encouraged. The lionfish toxin is inactivated by cooking, and the fish is considered a delicacy; however, a study in the Virgin Islands found that in areas with endemic ciguatera poisoning, 12% of lionfish carried amounts of the toxin above the level considered safe for consumption. This toxin is not inactivated by cooking or freezing and can lead to ciguatera fish poisoning for which there is no antidote and can be associated with prolonged neurotoxicity.13
Conclusion
As lionfish numbers continue to increase, physicians across multiple specialties and regions may see an increase in envenomation injuries. It is important that physicians are aware of how to recognize and treat lionfish stings, as prompt and comprehensive treatment provides benefit to the patient.
- Pterois volitans. Integrated Taxonomic Information System website. https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=166883#null. Accessed September 6, 2018.
- Morris JA Jr, Whitfield PE. Biology, Ecology, Control and Management of the Invasive Indopacific Lionfish: An Updated Integrated Assessment. Beaufort, NC: National Oceanic and Atmospheric Administration; 2009. http://aquaticcommons.org/2847/1/NCCOS_TM_99.pdf. Accessed September 6, 2018.
- Pterois volitans/miles. US Geological Survey website. https://nas.er.usgs.gov/queries/FactSheet.aspx?speciesID=963. Revised April 18, 2018. Accessed September 6, 2018.
- Diaz JH. Invasive lionfish (Pterois volitans) pose public health threats [published online August 15, 2015]. J La State Med Soc. 2015;167:166-171.
- Diaz JH. Marine Scorpaenidae envenomation in travelers: epidemiology, management, and prevention. J Travel Med. 2015;22:251-258.
- Hobday D, Chadha P, Din AH, et al. Denaturing the lionfish. Eplasty. 2016;16:ic20.
- Sáenz A, Ortiz N, Lomonte B, et al. Comparison of biochemical and cytotoxic activities of extracts obtained from dorsal spines and caudal fin of adult and juvenile non-native Caribbean lionfish (Pterois volitans/miles). Toxicon. 2017;137:158-167.
- Schult RF, Acquisto NM, Stair CK, et al. A case of lionfish envenomation presenting to an inland emergency department [published online August 13, 2017]. Case Rep Emerg Med. 2017;2017:5893563.
- Resiere D, Cerland L, De Haro L, et al. Envenomation by the invasive Pterois volitans species (lionfish) in the French West Indies—a two-year prospective study in Martinique. Clin Toxicol (Phila). 2016;54:313-318.
- Auerbach PS. Envenomation by aquatic vertebrates. In: Auerback PS. Wilderness Medicine. 5th ed. Philadelphia, PA: Mosby Elsevier; 2007:1740-1741.
- Auerbach PS, McKinney HE, Rees RE, et al. Analysis of vesicle fluid following the sting of the lionfish, Pterois volitans. Toxicon. 1987;25:1350-1353.
- Patel MR, Wells S. Lionfish envenomation of the hand. J Hand Surg Am. 1993;18:523-525.
- Robertson A, Garcia AC, Quintana HA, et al. Invasive lionfish (Pterois volitans): a potential human health threat for Ciguatera fish poisoning in tropical waters. Marine Drugs. 2014;12:88-97.
The lionfish (Pterois volitans) is a member of the Scorpaenidae family of venomous fish.1-3 Lionfish are an invasive species originally from the Indian and Pacific oceans and the Red Sea that now are widely found throughout tropical and temperate oceans in both hemispheres. They are a popular aquarium fish and were inadvertently introduced in the Atlantic Ocean in South Florida during the late 1980s to early 1990s.2,4 Since then, lionfish have spread into reef systems throughout the Atlantic Ocean, Caribbean Sea, and Gulf of Mexico in rapidly growing numbers, and they are now fo und all along the southeastern coast of the United States.5
Characteristics
Lionfish are brightly colored with red or maroon and white stripes, tentacles above the eyes and mouth, fan-shaped pectoral fins, and spines that deliver an especially painful venomous sting that often results in edema (Figure 1). They have 12 dorsal spines, 2 pelvic spines, and 3 anal spines.

Symptoms of Envenomation
As lionfish continue to spread to popular areas of the southeast Atlantic Ocean and Caribbean Sea, the chances of human contact with lionfish have increased. Lionfish stings are now the second most common marine envenomation injury after those caused by stingrays.4 Lionfish stings usually occur on the hands, fingers, or forearms during handling of the fish in ocean waters or in maintenance of aquariums. The mechanism of the venom apparatus is similar for all venomous fish. The spines have surrounding integumentary sheaths containing venom that rupture and inject venom when they penetrate the skin.6 The venom is a heat-labile neuromuscular toxin that causes edema (Figure 2), plasma extravasation, and thrombotic skin lesions.7

Wounds are classified into 3 categories: grade I consists of local erythema/ecchymosis, grade II involves vesicle or blister formation, and grade III denotes wounds that develop local necrosis.8 The sting causes immediate and severe throbbing pain, often described as excruciating or rated 10/10 on a basic pain scale, typically radiating up the affected limb. Puncture sites may bleed and often have associated redness and swelling. Pain may last up to 24 hours. Occasionally, foreign material may be left in the wound requiring removal. There also is a chance of secondary infection at the wound site, and severe envenomation can lead to local tissue necrosis.8 Systemic effects can occur in some cases, including nausea, vomiting, sweating, headache, dizziness, disorientation, palpitations, and even syncope.9 However, to our knowledge there are no documented cases of human death from a lionfish sting. Anaphylactic reactions are possible and require immediate treatment.6
A study conducted in the French West Indies evaluated 117 patients with lionfish envenomation and found that victims experienced severe pain and local edema (100%), paresthesia (90%), abdominal cramps (62%), extensive edema (53%), tachycardia (34%), skin rash (32%), gastrointestinal tract symptoms (28%), syncope (27%), transient weakness (24%), hypertension (21%), hypotension (18%), and hyperthermia (9%).9 Complications included local infection (18%) such as skin abscess (5%), skin necrosis (3%), and septic arthritis (2%). Twenty-two percent of patients were hospitalized and 8% required surgery. Local infectious complications were more frequent in those with multiple stings (19%). The study concluded that lionfish now represent a major health threat in the West Indies.9 As lionfish numbers have grown, health care providers are seeing increasing numbers of envenomation cases in areas of the coastal southeastern United States and Caribbean associated with considerable morbidity. Providers in nonendemic areas also may see envenomation injuries due to the lionfish popularity in home aquariums.9
Management
Individuals with lionfish stings should immerse the affected area in hot but not scalding water. Those with more serious injuries should seek medical attention. Home remedies that are generally contraindicated include application of topical papain or meat tenderizer.10 Data on ice packs are mixed, but because the toxin is heat labile, the most effective initial step in treatment is immersion of the affected area in water (temperature, 40°C to 45°C) for 30 to 90 minutes.6 The hot water inactivates the heat-labile toxin, leading to near-complete symptomatic relief in 80% of cases and moderate relief in an additional 14%. Immersion time more than 90 minutes considerably increases the risk for burns. Children should always be monitored to prevent burns. If a patient has received a nerve block for analgesia, the wound should not be immersed in hot water to avoid burns to the skin. The wound should be meticulously cleaned with saline irrigation, and radiography or ultrasonography should be performed as deemed necessary to look for any retained foreign bodies.8 Patients may require parenteral or oral analgesia as well as careful follow-up to ensure proper healing.9 Systemic symptoms require supportive care. Venomous fish wounds typically are small and superficial. Empiric antibiotic therapy is not advised for superficial wounds but may be required for clinically infected wounds.8 Tetanus prophylaxis should be given as appropriate to all affected patients. It has been noted that blister fluid contains high concentrations of lionfish venom, and when present, it increases the likelihood of converting the injury from a grade II to grade III wound with tissue necrosis; therefore, blisters should be drained or excised to decrease the chances of subsequent tissue necrosis.11,12 If secondary infection such as cellulitis develops, antibiotics should be chosen to cover likely pathogens including common skin flora such as staphylococci and marine organisms such as Vibrio species. Wounds showing signs of infection should be cultured, with antibiotics adjusted according to sensitivities.5 Deeper wounds should be left open (unsutured) with a proper dressing to heal. Any wounds that involve vascular or joint structures require specialty management. Wounds involving joints may on occasion require surgical exploration and debridement.
Public Health Concerns
In an attempt to slow the growth of their population, human consumption of the fish has been encouraged. The lionfish toxin is inactivated by cooking, and the fish is considered a delicacy; however, a study in the Virgin Islands found that in areas with endemic ciguatera poisoning, 12% of lionfish carried amounts of the toxin above the level considered safe for consumption. This toxin is not inactivated by cooking or freezing and can lead to ciguatera fish poisoning for which there is no antidote and can be associated with prolonged neurotoxicity.13
Conclusion
As lionfish numbers continue to increase, physicians across multiple specialties and regions may see an increase in envenomation injuries. It is important that physicians are aware of how to recognize and treat lionfish stings, as prompt and comprehensive treatment provides benefit to the patient.
The lionfish (Pterois volitans) is a member of the Scorpaenidae family of venomous fish.1-3 Lionfish are an invasive species originally from the Indian and Pacific oceans and the Red Sea that now are widely found throughout tropical and temperate oceans in both hemispheres. They are a popular aquarium fish and were inadvertently introduced in the Atlantic Ocean in South Florida during the late 1980s to early 1990s.2,4 Since then, lionfish have spread into reef systems throughout the Atlantic Ocean, Caribbean Sea, and Gulf of Mexico in rapidly growing numbers, and they are now fo und all along the southeastern coast of the United States.5
Characteristics
Lionfish are brightly colored with red or maroon and white stripes, tentacles above the eyes and mouth, fan-shaped pectoral fins, and spines that deliver an especially painful venomous sting that often results in edema (Figure 1). They have 12 dorsal spines, 2 pelvic spines, and 3 anal spines.

Symptoms of Envenomation
As lionfish continue to spread to popular areas of the southeast Atlantic Ocean and Caribbean Sea, the chances of human contact with lionfish have increased. Lionfish stings are now the second most common marine envenomation injury after those caused by stingrays.4 Lionfish stings usually occur on the hands, fingers, or forearms during handling of the fish in ocean waters or in maintenance of aquariums. The mechanism of the venom apparatus is similar for all venomous fish. The spines have surrounding integumentary sheaths containing venom that rupture and inject venom when they penetrate the skin.6 The venom is a heat-labile neuromuscular toxin that causes edema (Figure 2), plasma extravasation, and thrombotic skin lesions.7

Wounds are classified into 3 categories: grade I consists of local erythema/ecchymosis, grade II involves vesicle or blister formation, and grade III denotes wounds that develop local necrosis.8 The sting causes immediate and severe throbbing pain, often described as excruciating or rated 10/10 on a basic pain scale, typically radiating up the affected limb. Puncture sites may bleed and often have associated redness and swelling. Pain may last up to 24 hours. Occasionally, foreign material may be left in the wound requiring removal. There also is a chance of secondary infection at the wound site, and severe envenomation can lead to local tissue necrosis.8 Systemic effects can occur in some cases, including nausea, vomiting, sweating, headache, dizziness, disorientation, palpitations, and even syncope.9 However, to our knowledge there are no documented cases of human death from a lionfish sting. Anaphylactic reactions are possible and require immediate treatment.6
A study conducted in the French West Indies evaluated 117 patients with lionfish envenomation and found that victims experienced severe pain and local edema (100%), paresthesia (90%), abdominal cramps (62%), extensive edema (53%), tachycardia (34%), skin rash (32%), gastrointestinal tract symptoms (28%), syncope (27%), transient weakness (24%), hypertension (21%), hypotension (18%), and hyperthermia (9%).9 Complications included local infection (18%) such as skin abscess (5%), skin necrosis (3%), and septic arthritis (2%). Twenty-two percent of patients were hospitalized and 8% required surgery. Local infectious complications were more frequent in those with multiple stings (19%). The study concluded that lionfish now represent a major health threat in the West Indies.9 As lionfish numbers have grown, health care providers are seeing increasing numbers of envenomation cases in areas of the coastal southeastern United States and Caribbean associated with considerable morbidity. Providers in nonendemic areas also may see envenomation injuries due to the lionfish popularity in home aquariums.9
Management
Individuals with lionfish stings should immerse the affected area in hot but not scalding water. Those with more serious injuries should seek medical attention. Home remedies that are generally contraindicated include application of topical papain or meat tenderizer.10 Data on ice packs are mixed, but because the toxin is heat labile, the most effective initial step in treatment is immersion of the affected area in water (temperature, 40°C to 45°C) for 30 to 90 minutes.6 The hot water inactivates the heat-labile toxin, leading to near-complete symptomatic relief in 80% of cases and moderate relief in an additional 14%. Immersion time more than 90 minutes considerably increases the risk for burns. Children should always be monitored to prevent burns. If a patient has received a nerve block for analgesia, the wound should not be immersed in hot water to avoid burns to the skin. The wound should be meticulously cleaned with saline irrigation, and radiography or ultrasonography should be performed as deemed necessary to look for any retained foreign bodies.8 Patients may require parenteral or oral analgesia as well as careful follow-up to ensure proper healing.9 Systemic symptoms require supportive care. Venomous fish wounds typically are small and superficial. Empiric antibiotic therapy is not advised for superficial wounds but may be required for clinically infected wounds.8 Tetanus prophylaxis should be given as appropriate to all affected patients. It has been noted that blister fluid contains high concentrations of lionfish venom, and when present, it increases the likelihood of converting the injury from a grade II to grade III wound with tissue necrosis; therefore, blisters should be drained or excised to decrease the chances of subsequent tissue necrosis.11,12 If secondary infection such as cellulitis develops, antibiotics should be chosen to cover likely pathogens including common skin flora such as staphylococci and marine organisms such as Vibrio species. Wounds showing signs of infection should be cultured, with antibiotics adjusted according to sensitivities.5 Deeper wounds should be left open (unsutured) with a proper dressing to heal. Any wounds that involve vascular or joint structures require specialty management. Wounds involving joints may on occasion require surgical exploration and debridement.
Public Health Concerns
In an attempt to slow the growth of their population, human consumption of the fish has been encouraged. The lionfish toxin is inactivated by cooking, and the fish is considered a delicacy; however, a study in the Virgin Islands found that in areas with endemic ciguatera poisoning, 12% of lionfish carried amounts of the toxin above the level considered safe for consumption. This toxin is not inactivated by cooking or freezing and can lead to ciguatera fish poisoning for which there is no antidote and can be associated with prolonged neurotoxicity.13
Conclusion
As lionfish numbers continue to increase, physicians across multiple specialties and regions may see an increase in envenomation injuries. It is important that physicians are aware of how to recognize and treat lionfish stings, as prompt and comprehensive treatment provides benefit to the patient.
- Pterois volitans. Integrated Taxonomic Information System website. https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=166883#null. Accessed September 6, 2018.
- Morris JA Jr, Whitfield PE. Biology, Ecology, Control and Management of the Invasive Indopacific Lionfish: An Updated Integrated Assessment. Beaufort, NC: National Oceanic and Atmospheric Administration; 2009. http://aquaticcommons.org/2847/1/NCCOS_TM_99.pdf. Accessed September 6, 2018.
- Pterois volitans/miles. US Geological Survey website. https://nas.er.usgs.gov/queries/FactSheet.aspx?speciesID=963. Revised April 18, 2018. Accessed September 6, 2018.
- Diaz JH. Invasive lionfish (Pterois volitans) pose public health threats [published online August 15, 2015]. J La State Med Soc. 2015;167:166-171.
- Diaz JH. Marine Scorpaenidae envenomation in travelers: epidemiology, management, and prevention. J Travel Med. 2015;22:251-258.
- Hobday D, Chadha P, Din AH, et al. Denaturing the lionfish. Eplasty. 2016;16:ic20.
- Sáenz A, Ortiz N, Lomonte B, et al. Comparison of biochemical and cytotoxic activities of extracts obtained from dorsal spines and caudal fin of adult and juvenile non-native Caribbean lionfish (Pterois volitans/miles). Toxicon. 2017;137:158-167.
- Schult RF, Acquisto NM, Stair CK, et al. A case of lionfish envenomation presenting to an inland emergency department [published online August 13, 2017]. Case Rep Emerg Med. 2017;2017:5893563.
- Resiere D, Cerland L, De Haro L, et al. Envenomation by the invasive Pterois volitans species (lionfish) in the French West Indies—a two-year prospective study in Martinique. Clin Toxicol (Phila). 2016;54:313-318.
- Auerbach PS. Envenomation by aquatic vertebrates. In: Auerback PS. Wilderness Medicine. 5th ed. Philadelphia, PA: Mosby Elsevier; 2007:1740-1741.
- Auerbach PS, McKinney HE, Rees RE, et al. Analysis of vesicle fluid following the sting of the lionfish, Pterois volitans. Toxicon. 1987;25:1350-1353.
- Patel MR, Wells S. Lionfish envenomation of the hand. J Hand Surg Am. 1993;18:523-525.
- Robertson A, Garcia AC, Quintana HA, et al. Invasive lionfish (Pterois volitans): a potential human health threat for Ciguatera fish poisoning in tropical waters. Marine Drugs. 2014;12:88-97.
- Pterois volitans. Integrated Taxonomic Information System website. https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=166883#null. Accessed September 6, 2018.
- Morris JA Jr, Whitfield PE. Biology, Ecology, Control and Management of the Invasive Indopacific Lionfish: An Updated Integrated Assessment. Beaufort, NC: National Oceanic and Atmospheric Administration; 2009. http://aquaticcommons.org/2847/1/NCCOS_TM_99.pdf. Accessed September 6, 2018.
- Pterois volitans/miles. US Geological Survey website. https://nas.er.usgs.gov/queries/FactSheet.aspx?speciesID=963. Revised April 18, 2018. Accessed September 6, 2018.
- Diaz JH. Invasive lionfish (Pterois volitans) pose public health threats [published online August 15, 2015]. J La State Med Soc. 2015;167:166-171.
- Diaz JH. Marine Scorpaenidae envenomation in travelers: epidemiology, management, and prevention. J Travel Med. 2015;22:251-258.
- Hobday D, Chadha P, Din AH, et al. Denaturing the lionfish. Eplasty. 2016;16:ic20.
- Sáenz A, Ortiz N, Lomonte B, et al. Comparison of biochemical and cytotoxic activities of extracts obtained from dorsal spines and caudal fin of adult and juvenile non-native Caribbean lionfish (Pterois volitans/miles). Toxicon. 2017;137:158-167.
- Schult RF, Acquisto NM, Stair CK, et al. A case of lionfish envenomation presenting to an inland emergency department [published online August 13, 2017]. Case Rep Emerg Med. 2017;2017:5893563.
- Resiere D, Cerland L, De Haro L, et al. Envenomation by the invasive Pterois volitans species (lionfish) in the French West Indies—a two-year prospective study in Martinique. Clin Toxicol (Phila). 2016;54:313-318.
- Auerbach PS. Envenomation by aquatic vertebrates. In: Auerback PS. Wilderness Medicine. 5th ed. Philadelphia, PA: Mosby Elsevier; 2007:1740-1741.
- Auerbach PS, McKinney HE, Rees RE, et al. Analysis of vesicle fluid following the sting of the lionfish, Pterois volitans. Toxicon. 1987;25:1350-1353.
- Patel MR, Wells S. Lionfish envenomation of the hand. J Hand Surg Am. 1993;18:523-525.
- Robertson A, Garcia AC, Quintana HA, et al. Invasive lionfish (Pterois volitans): a potential human health threat for Ciguatera fish poisoning in tropical waters. Marine Drugs. 2014;12:88-97.
Practice Points
- Lionfish are now found all along the southeastern coast of the United States. Physicians may see an increase in envenomation injuries.
- Treat lionfish envenomation with immediate immersion in warm water (temperature, 40°C to 45°C) for 30 to 90 minutes to deactivate heat-labile toxin.
- Infected wounds should be treated with antibiotics for common skin flora and marine organisms such as Vibrio species.
Bone biopsy in suspected osteomyelitis: Culture and histology matter
Diabetic foot ulcers and infections can lead to osteomyelitis, a potentially devastating infection in the bone.
How much of a difference can osteomyelitis make to a patient’s prognosis? A 2014 commentary by Benjamin A. Lipsky, MD, a prominent expert in problems associated with diabetic patients’ feet who’s with the University of Washington, Seattle, hints at the potential toll: “Overall, about 20% of patients with a diabetic foot infection (and over 60% of those with severe infections) have underlying osteomyelitis, which dramatically increases the risk of lower-extremity amputation” (Diabetes Care. 2014 Mar;37[3]:593-5).
Diagnosis of osteomyelitis, which relies on a bone biopsy, is clearly important. But there’s a big gap in diagnostic findings depending on whether doctors request culture or histology results, according to a new study released at the 2018 scientific meeting of the American Diabetes Association (Diabetes. 2018 Jul. doi: 10.2337/db18-110-OR).
In an interview, lead author and podiatrist Peter A. Crisologo, DPM, of University of Texas Southwestern Medical Center, Dallas, explained the study findings and offered guidance for requesting bone biopsies in possible cases of osteomyelitis.
Q: What makes diagnosis and treatment of osteomyelitis unique?
A: In the foot, there’s not a lot of soft tissue between the outside world and your bone. If the wounds on the feet go deep enough, they can spread a bacterial infection to the bone. This changes how foot infections are treated.
If you have a skin infection, it requires 11-12 days of antibiotics. Things start ramping up once you start getting into the bone. You’re talking potential surgery and 6 weeks of antibiotics through IV treatments. This is why it’s really important that you get your diagnosis right.
A lot of people say “I’m going to do the safe thing” and treat a bone infection with an extended course of antibiotics.
That’s not necessarily safe. If you’re overdiagnosing – for example, you identify bacteria that’s just a contaminant – you could put a patient through 6 weeks of IV treatment along with the risks of a PICC (peripherally inserted central catheter ) line infection, complications from IV placement, and complications from the antibiotic.
Also, acute kidney injury develops in at least a third of the patients who undergo 6 weeks of antibiotics. That’s not to mention the cost of the visits and the labs you have to draw. But we don’t want to underdiagnose either. If osteomyelitis is underdiagnosed and then not treated, the infection can smolder and continue to progress and worsen.
Q: Your study looks at the bone biopsy. How does it fit into care of osteomyelitis in the diabetic foot?
A: A bone biopsy is the standard for diagnosis under the guidelines of the Infectious Diseases Society of America/International Working Group on the Diabetic Foot (Clin Infect Dis. 2012;54[12]:e132-73 ).
But beyond that, nobody says anything. Everyone has an operational definition of how a bone biopsy is interpreted, and there’s a need for a consensus on how a bone biopsy can be used to diagnose osteomyelitis.
You’ll get different percentages of your patients diagnosed with osteomyelitis. For example, someone may say the biopsy is only positive if the histology is positive, while another says the histology doesn’t matter if the culture is positive.
Q: Your study looks at histology and culture analyses. What do these reveal?
A: A traditional culture helps you identify the bacteria, as well as guide your treatment when it’s tested against antibiotics.
A traditional histology allows the pathologist to look under a microscope for signs of osteomyelitis: Do they see the right inflammatory cells, white cells, lymphocytes, combinations of cells? Does this look like an acute or chronic osteomyelitis?
Q: Why might it be wise to combine culture and histology analyses?
A: If you have bacteria that’s difficult to culture via traditional methods, it may be a bacteria that doesn’t grow well or easily. If you combine culture with histology, pathologists can look and say, “Your culture was negative but we see these other signs, so we feel this is osteomyelitis.”
Q: Your study examined 35 consecutive patients aged at least 21 years who had moderate or severe infections bone infections in the foot linked with type 1 diabetes (n = 4) or type 2 diabetes (n = 31).
The samples were analyzed via culture, histology, and culture/histology examinations. You also performed genetic sequencing (quantitative polymerase chain reaction targeting 16S rRNA). How does this test fit in to bone biopsies in the clinic?
A: That’s a newer method and not a standard of care treatment for the diabetic foot. This analysis looks at DNA that’s present, bypassing the analysis of difficult-to-grow bacteria.
Q: What did you discover?
A: In this study, histology had the lowest incidence of positively detecting osteomyelitis. (45.7%). The level increases when a culture is taken (68.6% vs. histology; P = .02).
Then it goes up when DNA is used because it’s catching everything (82.9%, P = .001 vs. histology and P = .31 vs. culture).
[The study also found that adding histology to culture or to genetic sequencing did not change positive findings.]
Q: Does the study suggest one approach is better than the others?
A: This paper doesn’t provide enough evidence to use one method over another. The main purpose was to raise the concern that diagnosis can change dramatically depending on how the gold standard of bone biopsy is interpreted.
Q: What were the pros and cons of the genetic sequencing approach?
A: When we use this approach, our positive diagnostic rate significantly increases. But there are also downsides. We don’t know whether the bacteria we see is alive or dead. We just know it was there. So are the patients truly positive? That’s a question we can’t answer.
Genetic sequencing also doesn’t tell us about susceptibilities to antibiotics.
Q: What is the take-home message here for physicians who may order bone biopsies?
A: The thing to do is request both traditional culture and traditional histology.
As far as DNA sequencing, that not something I’d recommend as a standard of care.
Q: Can you comment on cost and insurance coverage for these approaches?
A: As far as I know, genetic sequencing is not covered as it is not standard of care in the diabetic foot and is used mainly for research at this time.
Pathology and culture are standard of care when evaluating for osteomyelitis and should be a covered service. However, a patient should call their insurance company first prior to having the procedure done to see whether it is covered.
Q: What’s next for research in this area?
A: From here, the next step is bigger numbers: Increase the study size and look at this again. Also, we may be able to identify susceptibilities by identifying resistance within the DNA.
Dr. Crisologo and two other study authors report no relevant disclosures. One study author reported various disclosures including research support, consulting, and service on speakers' bureaus.
Diabetic foot ulcers and infections can lead to osteomyelitis, a potentially devastating infection in the bone.
How much of a difference can osteomyelitis make to a patient’s prognosis? A 2014 commentary by Benjamin A. Lipsky, MD, a prominent expert in problems associated with diabetic patients’ feet who’s with the University of Washington, Seattle, hints at the potential toll: “Overall, about 20% of patients with a diabetic foot infection (and over 60% of those with severe infections) have underlying osteomyelitis, which dramatically increases the risk of lower-extremity amputation” (Diabetes Care. 2014 Mar;37[3]:593-5).
Diagnosis of osteomyelitis, which relies on a bone biopsy, is clearly important. But there’s a big gap in diagnostic findings depending on whether doctors request culture or histology results, according to a new study released at the 2018 scientific meeting of the American Diabetes Association (Diabetes. 2018 Jul. doi: 10.2337/db18-110-OR).
In an interview, lead author and podiatrist Peter A. Crisologo, DPM, of University of Texas Southwestern Medical Center, Dallas, explained the study findings and offered guidance for requesting bone biopsies in possible cases of osteomyelitis.
Q: What makes diagnosis and treatment of osteomyelitis unique?
A: In the foot, there’s not a lot of soft tissue between the outside world and your bone. If the wounds on the feet go deep enough, they can spread a bacterial infection to the bone. This changes how foot infections are treated.
If you have a skin infection, it requires 11-12 days of antibiotics. Things start ramping up once you start getting into the bone. You’re talking potential surgery and 6 weeks of antibiotics through IV treatments. This is why it’s really important that you get your diagnosis right.
A lot of people say “I’m going to do the safe thing” and treat a bone infection with an extended course of antibiotics.
That’s not necessarily safe. If you’re overdiagnosing – for example, you identify bacteria that’s just a contaminant – you could put a patient through 6 weeks of IV treatment along with the risks of a PICC (peripherally inserted central catheter ) line infection, complications from IV placement, and complications from the antibiotic.
Also, acute kidney injury develops in at least a third of the patients who undergo 6 weeks of antibiotics. That’s not to mention the cost of the visits and the labs you have to draw. But we don’t want to underdiagnose either. If osteomyelitis is underdiagnosed and then not treated, the infection can smolder and continue to progress and worsen.
Q: Your study looks at the bone biopsy. How does it fit into care of osteomyelitis in the diabetic foot?
A: A bone biopsy is the standard for diagnosis under the guidelines of the Infectious Diseases Society of America/International Working Group on the Diabetic Foot (Clin Infect Dis. 2012;54[12]:e132-73 ).
But beyond that, nobody says anything. Everyone has an operational definition of how a bone biopsy is interpreted, and there’s a need for a consensus on how a bone biopsy can be used to diagnose osteomyelitis.
You’ll get different percentages of your patients diagnosed with osteomyelitis. For example, someone may say the biopsy is only positive if the histology is positive, while another says the histology doesn’t matter if the culture is positive.
Q: Your study looks at histology and culture analyses. What do these reveal?
A: A traditional culture helps you identify the bacteria, as well as guide your treatment when it’s tested against antibiotics.
A traditional histology allows the pathologist to look under a microscope for signs of osteomyelitis: Do they see the right inflammatory cells, white cells, lymphocytes, combinations of cells? Does this look like an acute or chronic osteomyelitis?
Q: Why might it be wise to combine culture and histology analyses?
A: If you have bacteria that’s difficult to culture via traditional methods, it may be a bacteria that doesn’t grow well or easily. If you combine culture with histology, pathologists can look and say, “Your culture was negative but we see these other signs, so we feel this is osteomyelitis.”
Q: Your study examined 35 consecutive patients aged at least 21 years who had moderate or severe infections bone infections in the foot linked with type 1 diabetes (n = 4) or type 2 diabetes (n = 31).
The samples were analyzed via culture, histology, and culture/histology examinations. You also performed genetic sequencing (quantitative polymerase chain reaction targeting 16S rRNA). How does this test fit in to bone biopsies in the clinic?
A: That’s a newer method and not a standard of care treatment for the diabetic foot. This analysis looks at DNA that’s present, bypassing the analysis of difficult-to-grow bacteria.
Q: What did you discover?
A: In this study, histology had the lowest incidence of positively detecting osteomyelitis. (45.7%). The level increases when a culture is taken (68.6% vs. histology; P = .02).
Then it goes up when DNA is used because it’s catching everything (82.9%, P = .001 vs. histology and P = .31 vs. culture).
[The study also found that adding histology to culture or to genetic sequencing did not change positive findings.]
Q: Does the study suggest one approach is better than the others?
A: This paper doesn’t provide enough evidence to use one method over another. The main purpose was to raise the concern that diagnosis can change dramatically depending on how the gold standard of bone biopsy is interpreted.
Q: What were the pros and cons of the genetic sequencing approach?
A: When we use this approach, our positive diagnostic rate significantly increases. But there are also downsides. We don’t know whether the bacteria we see is alive or dead. We just know it was there. So are the patients truly positive? That’s a question we can’t answer.
Genetic sequencing also doesn’t tell us about susceptibilities to antibiotics.
Q: What is the take-home message here for physicians who may order bone biopsies?
A: The thing to do is request both traditional culture and traditional histology.
As far as DNA sequencing, that not something I’d recommend as a standard of care.
Q: Can you comment on cost and insurance coverage for these approaches?
A: As far as I know, genetic sequencing is not covered as it is not standard of care in the diabetic foot and is used mainly for research at this time.
Pathology and culture are standard of care when evaluating for osteomyelitis and should be a covered service. However, a patient should call their insurance company first prior to having the procedure done to see whether it is covered.
Q: What’s next for research in this area?
A: From here, the next step is bigger numbers: Increase the study size and look at this again. Also, we may be able to identify susceptibilities by identifying resistance within the DNA.
Dr. Crisologo and two other study authors report no relevant disclosures. One study author reported various disclosures including research support, consulting, and service on speakers' bureaus.
Diabetic foot ulcers and infections can lead to osteomyelitis, a potentially devastating infection in the bone.
How much of a difference can osteomyelitis make to a patient’s prognosis? A 2014 commentary by Benjamin A. Lipsky, MD, a prominent expert in problems associated with diabetic patients’ feet who’s with the University of Washington, Seattle, hints at the potential toll: “Overall, about 20% of patients with a diabetic foot infection (and over 60% of those with severe infections) have underlying osteomyelitis, which dramatically increases the risk of lower-extremity amputation” (Diabetes Care. 2014 Mar;37[3]:593-5).
Diagnosis of osteomyelitis, which relies on a bone biopsy, is clearly important. But there’s a big gap in diagnostic findings depending on whether doctors request culture or histology results, according to a new study released at the 2018 scientific meeting of the American Diabetes Association (Diabetes. 2018 Jul. doi: 10.2337/db18-110-OR).
In an interview, lead author and podiatrist Peter A. Crisologo, DPM, of University of Texas Southwestern Medical Center, Dallas, explained the study findings and offered guidance for requesting bone biopsies in possible cases of osteomyelitis.
Q: What makes diagnosis and treatment of osteomyelitis unique?
A: In the foot, there’s not a lot of soft tissue between the outside world and your bone. If the wounds on the feet go deep enough, they can spread a bacterial infection to the bone. This changes how foot infections are treated.
If you have a skin infection, it requires 11-12 days of antibiotics. Things start ramping up once you start getting into the bone. You’re talking potential surgery and 6 weeks of antibiotics through IV treatments. This is why it’s really important that you get your diagnosis right.
A lot of people say “I’m going to do the safe thing” and treat a bone infection with an extended course of antibiotics.
That’s not necessarily safe. If you’re overdiagnosing – for example, you identify bacteria that’s just a contaminant – you could put a patient through 6 weeks of IV treatment along with the risks of a PICC (peripherally inserted central catheter ) line infection, complications from IV placement, and complications from the antibiotic.
Also, acute kidney injury develops in at least a third of the patients who undergo 6 weeks of antibiotics. That’s not to mention the cost of the visits and the labs you have to draw. But we don’t want to underdiagnose either. If osteomyelitis is underdiagnosed and then not treated, the infection can smolder and continue to progress and worsen.
Q: Your study looks at the bone biopsy. How does it fit into care of osteomyelitis in the diabetic foot?
A: A bone biopsy is the standard for diagnosis under the guidelines of the Infectious Diseases Society of America/International Working Group on the Diabetic Foot (Clin Infect Dis. 2012;54[12]:e132-73 ).
But beyond that, nobody says anything. Everyone has an operational definition of how a bone biopsy is interpreted, and there’s a need for a consensus on how a bone biopsy can be used to diagnose osteomyelitis.
You’ll get different percentages of your patients diagnosed with osteomyelitis. For example, someone may say the biopsy is only positive if the histology is positive, while another says the histology doesn’t matter if the culture is positive.
Q: Your study looks at histology and culture analyses. What do these reveal?
A: A traditional culture helps you identify the bacteria, as well as guide your treatment when it’s tested against antibiotics.
A traditional histology allows the pathologist to look under a microscope for signs of osteomyelitis: Do they see the right inflammatory cells, white cells, lymphocytes, combinations of cells? Does this look like an acute or chronic osteomyelitis?
Q: Why might it be wise to combine culture and histology analyses?
A: If you have bacteria that’s difficult to culture via traditional methods, it may be a bacteria that doesn’t grow well or easily. If you combine culture with histology, pathologists can look and say, “Your culture was negative but we see these other signs, so we feel this is osteomyelitis.”
Q: Your study examined 35 consecutive patients aged at least 21 years who had moderate or severe infections bone infections in the foot linked with type 1 diabetes (n = 4) or type 2 diabetes (n = 31).
The samples were analyzed via culture, histology, and culture/histology examinations. You also performed genetic sequencing (quantitative polymerase chain reaction targeting 16S rRNA). How does this test fit in to bone biopsies in the clinic?
A: That’s a newer method and not a standard of care treatment for the diabetic foot. This analysis looks at DNA that’s present, bypassing the analysis of difficult-to-grow bacteria.
Q: What did you discover?
A: In this study, histology had the lowest incidence of positively detecting osteomyelitis. (45.7%). The level increases when a culture is taken (68.6% vs. histology; P = .02).
Then it goes up when DNA is used because it’s catching everything (82.9%, P = .001 vs. histology and P = .31 vs. culture).
[The study also found that adding histology to culture or to genetic sequencing did not change positive findings.]
Q: Does the study suggest one approach is better than the others?
A: This paper doesn’t provide enough evidence to use one method over another. The main purpose was to raise the concern that diagnosis can change dramatically depending on how the gold standard of bone biopsy is interpreted.
Q: What were the pros and cons of the genetic sequencing approach?
A: When we use this approach, our positive diagnostic rate significantly increases. But there are also downsides. We don’t know whether the bacteria we see is alive or dead. We just know it was there. So are the patients truly positive? That’s a question we can’t answer.
Genetic sequencing also doesn’t tell us about susceptibilities to antibiotics.
Q: What is the take-home message here for physicians who may order bone biopsies?
A: The thing to do is request both traditional culture and traditional histology.
As far as DNA sequencing, that not something I’d recommend as a standard of care.
Q: Can you comment on cost and insurance coverage for these approaches?
A: As far as I know, genetic sequencing is not covered as it is not standard of care in the diabetic foot and is used mainly for research at this time.
Pathology and culture are standard of care when evaluating for osteomyelitis and should be a covered service. However, a patient should call their insurance company first prior to having the procedure done to see whether it is covered.
Q: What’s next for research in this area?
A: From here, the next step is bigger numbers: Increase the study size and look at this again. Also, we may be able to identify susceptibilities by identifying resistance within the DNA.
Dr. Crisologo and two other study authors report no relevant disclosures. One study author reported various disclosures including research support, consulting, and service on speakers' bureaus.
EXPERT ANALYSIS FROM ADA 2018
Study offers snapshot of esophageal strictures in EB patients
LAKE TAHOE, CALIF. – and direct visualization of these strictures is the preferred method of diagnosis. Those are key findings from a multicenter study that lead author Elena Pope, MD, discussed at the annual meeting of the Society for Pediatric Dermatology.
According to Dr. Pope, who heads the section of dermatology at the Hospital for Sick Children, Toronto, an estimated 10%-17% of epidermolysis bullosa (EB) patients experience strictures, with an overrepresentation in the recessive dystrophic EB subtype in up to 80% of cases. The risk increases with age. “What remains unknown is the best short- and long-term intervention to manage the strictures and predictors/associations for stricture-free episodes,” Dr. Pope said. “The objectives of the current study were to determine the prevalence and predisposing factors for strictures in EB, management options, patient outcomes, and predictors for recurrences and stricture-free intervals.”
She and her associates at seven centers worldwide collected data on 125 EB patients who experienced at least one episode of esophageal stricture. Data was analyzed descriptively and with ANOVA regression analysis for associations/predictors for recurrences/episode-free intervals.
The researchers evaluated 497 stricture events in the 125 patients. A slight female predominance was noted (53%), and the mean age of the first episode was 12.7 years, “which is a little bit older” than the age found in previously published data, Dr. Pope said. As expected, dystrophic EB patients made up most of the sample (98.4%); of these 123 patients, recessive dystrophic EB severe generalized subtype – approaching 50% – was the most common, followed by the recessive dystrophic EB severe intermediate subtype (almost 21%), the dominant dystrophic EB generalized subtype (7%), and other types of dystrophic EB (almost 26%).
The median body mass index percentile for age was 6.3, “so these were patients who were severely malnourished, probably as a result of their strictures as well as their underlying disease,” Dr. Pope said.
As expected, dysphagia was a presenting symptom in most patients (85.5%), while 29.8% presented with inability to swallow solids. The preferred method of evaluation was video fluoroscopy (57.7%), and less commonly with barium swallow (22.3%) or with clinical symptoms alone (0.1%). The mean number of strictures was 1.69; 76.7% were located in the cervical area, 56.7% were located in the thoracic area, and 9.7% were located in the abdominal area. Most patients (76%) had lesions that were 1 cm or longer in size.
Fluoroscopy guidance was the most common method of dilatation (in 45.2% of cases), followed by retrograde endoscopy was (33%), antegrade endoscopy (19.1%), and bougienage (0.1%). General anesthesia was used in most cases (87.6%), and corticosteroids were used around the dilatation in 90.4% of patients. The mean duration of medication use was about 5 days.
As for outcomes after dilatation, 92.2% of strictures completely resolved, 3.8% were partially resolved, 3.9% were not resolved, and 2.7% had complications. The median interval between dilatations was 7 months. Fluoroscopy-guided balloon dilatation was associated with the longest esophageal stricture-free duration (mean of 13.83 months vs. 8.75 months; P less than .001), followed by retrograde endoscopy (mean of 13.10 months vs. 7.85 months; P less than .001), and antegrade endoscopy (mean of 7.63 months vs. 11.46 months; P = .024). “I think this is interesting,” said Dr. Pope, who is also a professor of pediatrics at the University of Toronto. “I think the difference occurs because if you use the endoscopy, which a rigid tube, you can potentially cause more damage, and more long-term scarring.”
Another predictor of esophageal stricture-free episodes was systemic corticosteroid use (a mean of 25.28 months vs. 10.24 months; P less than .001) around the time of the dilatation procedure. “By using systemic steroids, you’re actually decreasing some of the inflammation associated with the trauma of the procedure decreasing the chances of strictures formation,” she said.
Dr. Pope recommended that future studies evaluate the benefit of periprocedural medical interventions on increasing the intervals between esophageal stricture occurrences.
The study was supported by an unrestricted grant from the Epidermolysis Bullosa Research Foundation. She reported having no financial disclosures.
LAKE TAHOE, CALIF. – and direct visualization of these strictures is the preferred method of diagnosis. Those are key findings from a multicenter study that lead author Elena Pope, MD, discussed at the annual meeting of the Society for Pediatric Dermatology.
According to Dr. Pope, who heads the section of dermatology at the Hospital for Sick Children, Toronto, an estimated 10%-17% of epidermolysis bullosa (EB) patients experience strictures, with an overrepresentation in the recessive dystrophic EB subtype in up to 80% of cases. The risk increases with age. “What remains unknown is the best short- and long-term intervention to manage the strictures and predictors/associations for stricture-free episodes,” Dr. Pope said. “The objectives of the current study were to determine the prevalence and predisposing factors for strictures in EB, management options, patient outcomes, and predictors for recurrences and stricture-free intervals.”
She and her associates at seven centers worldwide collected data on 125 EB patients who experienced at least one episode of esophageal stricture. Data was analyzed descriptively and with ANOVA regression analysis for associations/predictors for recurrences/episode-free intervals.
The researchers evaluated 497 stricture events in the 125 patients. A slight female predominance was noted (53%), and the mean age of the first episode was 12.7 years, “which is a little bit older” than the age found in previously published data, Dr. Pope said. As expected, dystrophic EB patients made up most of the sample (98.4%); of these 123 patients, recessive dystrophic EB severe generalized subtype – approaching 50% – was the most common, followed by the recessive dystrophic EB severe intermediate subtype (almost 21%), the dominant dystrophic EB generalized subtype (7%), and other types of dystrophic EB (almost 26%).
The median body mass index percentile for age was 6.3, “so these were patients who were severely malnourished, probably as a result of their strictures as well as their underlying disease,” Dr. Pope said.
As expected, dysphagia was a presenting symptom in most patients (85.5%), while 29.8% presented with inability to swallow solids. The preferred method of evaluation was video fluoroscopy (57.7%), and less commonly with barium swallow (22.3%) or with clinical symptoms alone (0.1%). The mean number of strictures was 1.69; 76.7% were located in the cervical area, 56.7% were located in the thoracic area, and 9.7% were located in the abdominal area. Most patients (76%) had lesions that were 1 cm or longer in size.
Fluoroscopy guidance was the most common method of dilatation (in 45.2% of cases), followed by retrograde endoscopy was (33%), antegrade endoscopy (19.1%), and bougienage (0.1%). General anesthesia was used in most cases (87.6%), and corticosteroids were used around the dilatation in 90.4% of patients. The mean duration of medication use was about 5 days.
As for outcomes after dilatation, 92.2% of strictures completely resolved, 3.8% were partially resolved, 3.9% were not resolved, and 2.7% had complications. The median interval between dilatations was 7 months. Fluoroscopy-guided balloon dilatation was associated with the longest esophageal stricture-free duration (mean of 13.83 months vs. 8.75 months; P less than .001), followed by retrograde endoscopy (mean of 13.10 months vs. 7.85 months; P less than .001), and antegrade endoscopy (mean of 7.63 months vs. 11.46 months; P = .024). “I think this is interesting,” said Dr. Pope, who is also a professor of pediatrics at the University of Toronto. “I think the difference occurs because if you use the endoscopy, which a rigid tube, you can potentially cause more damage, and more long-term scarring.”
Another predictor of esophageal stricture-free episodes was systemic corticosteroid use (a mean of 25.28 months vs. 10.24 months; P less than .001) around the time of the dilatation procedure. “By using systemic steroids, you’re actually decreasing some of the inflammation associated with the trauma of the procedure decreasing the chances of strictures formation,” she said.
Dr. Pope recommended that future studies evaluate the benefit of periprocedural medical interventions on increasing the intervals between esophageal stricture occurrences.
The study was supported by an unrestricted grant from the Epidermolysis Bullosa Research Foundation. She reported having no financial disclosures.
LAKE TAHOE, CALIF. – and direct visualization of these strictures is the preferred method of diagnosis. Those are key findings from a multicenter study that lead author Elena Pope, MD, discussed at the annual meeting of the Society for Pediatric Dermatology.
According to Dr. Pope, who heads the section of dermatology at the Hospital for Sick Children, Toronto, an estimated 10%-17% of epidermolysis bullosa (EB) patients experience strictures, with an overrepresentation in the recessive dystrophic EB subtype in up to 80% of cases. The risk increases with age. “What remains unknown is the best short- and long-term intervention to manage the strictures and predictors/associations for stricture-free episodes,” Dr. Pope said. “The objectives of the current study were to determine the prevalence and predisposing factors for strictures in EB, management options, patient outcomes, and predictors for recurrences and stricture-free intervals.”
She and her associates at seven centers worldwide collected data on 125 EB patients who experienced at least one episode of esophageal stricture. Data was analyzed descriptively and with ANOVA regression analysis for associations/predictors for recurrences/episode-free intervals.
The researchers evaluated 497 stricture events in the 125 patients. A slight female predominance was noted (53%), and the mean age of the first episode was 12.7 years, “which is a little bit older” than the age found in previously published data, Dr. Pope said. As expected, dystrophic EB patients made up most of the sample (98.4%); of these 123 patients, recessive dystrophic EB severe generalized subtype – approaching 50% – was the most common, followed by the recessive dystrophic EB severe intermediate subtype (almost 21%), the dominant dystrophic EB generalized subtype (7%), and other types of dystrophic EB (almost 26%).
The median body mass index percentile for age was 6.3, “so these were patients who were severely malnourished, probably as a result of their strictures as well as their underlying disease,” Dr. Pope said.
As expected, dysphagia was a presenting symptom in most patients (85.5%), while 29.8% presented with inability to swallow solids. The preferred method of evaluation was video fluoroscopy (57.7%), and less commonly with barium swallow (22.3%) or with clinical symptoms alone (0.1%). The mean number of strictures was 1.69; 76.7% were located in the cervical area, 56.7% were located in the thoracic area, and 9.7% were located in the abdominal area. Most patients (76%) had lesions that were 1 cm or longer in size.
Fluoroscopy guidance was the most common method of dilatation (in 45.2% of cases), followed by retrograde endoscopy was (33%), antegrade endoscopy (19.1%), and bougienage (0.1%). General anesthesia was used in most cases (87.6%), and corticosteroids were used around the dilatation in 90.4% of patients. The mean duration of medication use was about 5 days.
As for outcomes after dilatation, 92.2% of strictures completely resolved, 3.8% were partially resolved, 3.9% were not resolved, and 2.7% had complications. The median interval between dilatations was 7 months. Fluoroscopy-guided balloon dilatation was associated with the longest esophageal stricture-free duration (mean of 13.83 months vs. 8.75 months; P less than .001), followed by retrograde endoscopy (mean of 13.10 months vs. 7.85 months; P less than .001), and antegrade endoscopy (mean of 7.63 months vs. 11.46 months; P = .024). “I think this is interesting,” said Dr. Pope, who is also a professor of pediatrics at the University of Toronto. “I think the difference occurs because if you use the endoscopy, which a rigid tube, you can potentially cause more damage, and more long-term scarring.”
Another predictor of esophageal stricture-free episodes was systemic corticosteroid use (a mean of 25.28 months vs. 10.24 months; P less than .001) around the time of the dilatation procedure. “By using systemic steroids, you’re actually decreasing some of the inflammation associated with the trauma of the procedure decreasing the chances of strictures formation,” she said.
Dr. Pope recommended that future studies evaluate the benefit of periprocedural medical interventions on increasing the intervals between esophageal stricture occurrences.
The study was supported by an unrestricted grant from the Epidermolysis Bullosa Research Foundation. She reported having no financial disclosures.
REPORTING FROM SPD 2018
Key clinical point: Esophageal strictures are common complications of patients with severe types of epidermolysis bullosa.
Major finding: Most epidermolysis bullosa patients (85.5%) presented with dysphagia, while the preferred method of evaluation was video fluoroscopy (57.7%).
Study details: A multicenter study of 497 stricture events in 125 patients with epidermolysis bullosa.
Disclosures: The study was supported by an unrestricted grant from the Epidermolysis Bullosa Research Foundation. Dr. Pope reported having no financial disclosures.
Plantar Ulcerative Lichen Planus: Rapid Improvement With a Novel Triple-Therapy Approach
Ulcerative lichen planus (ULP)(also called erosive) is a rare variant of lichen planus. Similar to classic lichen planus, the cause of ULP is largely unknown. Ulcerative lichen planus typically involves the oral mucosa or genitalia but rarely may present as ulcerations on the palms and soles. Clinical presentation usually involves a history of chronic ulcers that often have been previously misdiagnosed and resistant to treatment. Ulcerations on the plantar surfaces frequently cause severe pain and disability. Few cases have been reported and successful treatment is rare.
Case Report
A 56-year-old man was referred by podiatry to the dermatology clinic for evaluation of painful ulcerations involving the dorsal and plantar surfaces of the right great toe as well as the second to third digits. The ulcers had been ongoing for 8 years, treated mostly with local wound care without clinical improvement. His medical and family history was considered noncontributory as a possible etiology of the ulcers; however, he had been taking ibuprofen intermittently for years for general aches and pains, which raised the suspicion of a drug-induced etiology. Laboratory evaluation revealed positive hepatitis B serology but was otherwise unremarkable, including normal liver function tests and negative wound cultures.
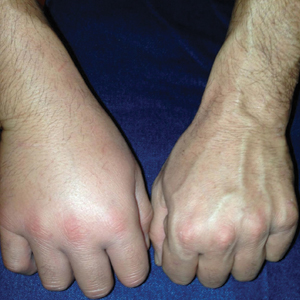
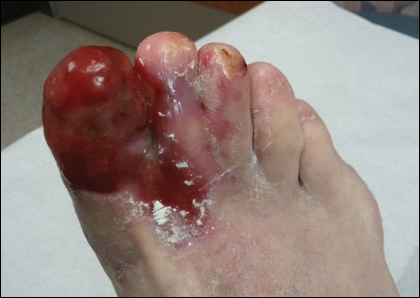
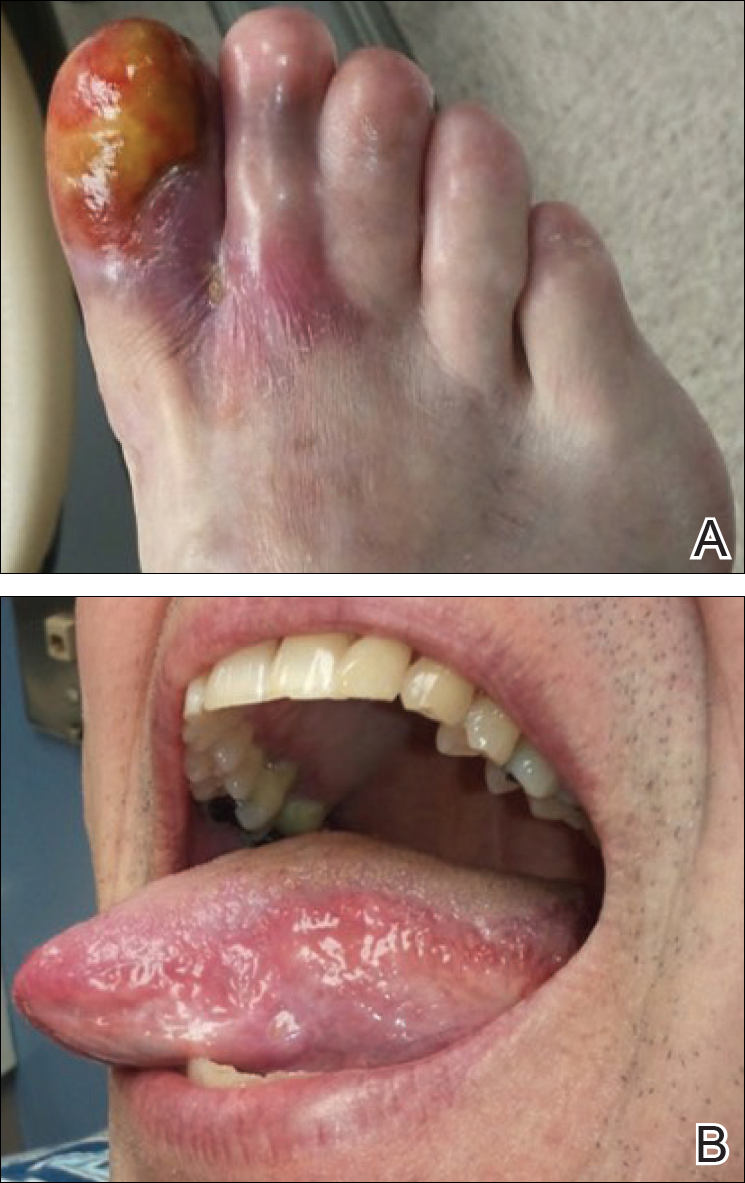
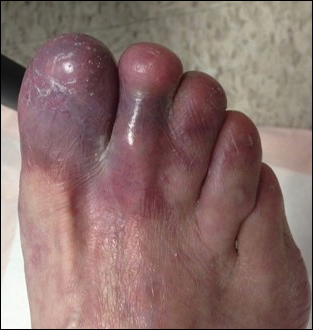
Physical examination revealed a beefy red, glazed ulceration involving the entire right great toe with extension onto the second and third toes. There was considerable scarring with syndactyly of the second and third toes and complete toenail loss of the right foot (Figure 1). On the insteps of the bilateral soles were a few scattered, pale, atrophic, violaceous papules with overlying thin lacy white streaks that were reflective of Wickham striae. Early dorsal pterygium formation also was noted on the bilateral third fingernails. Oral mucosal examination revealed lacy white plaques on the bilateral buccal mucosa with a large ulcer of the left lateral tongue (Figure 2). No genital or scalp lesions were present.

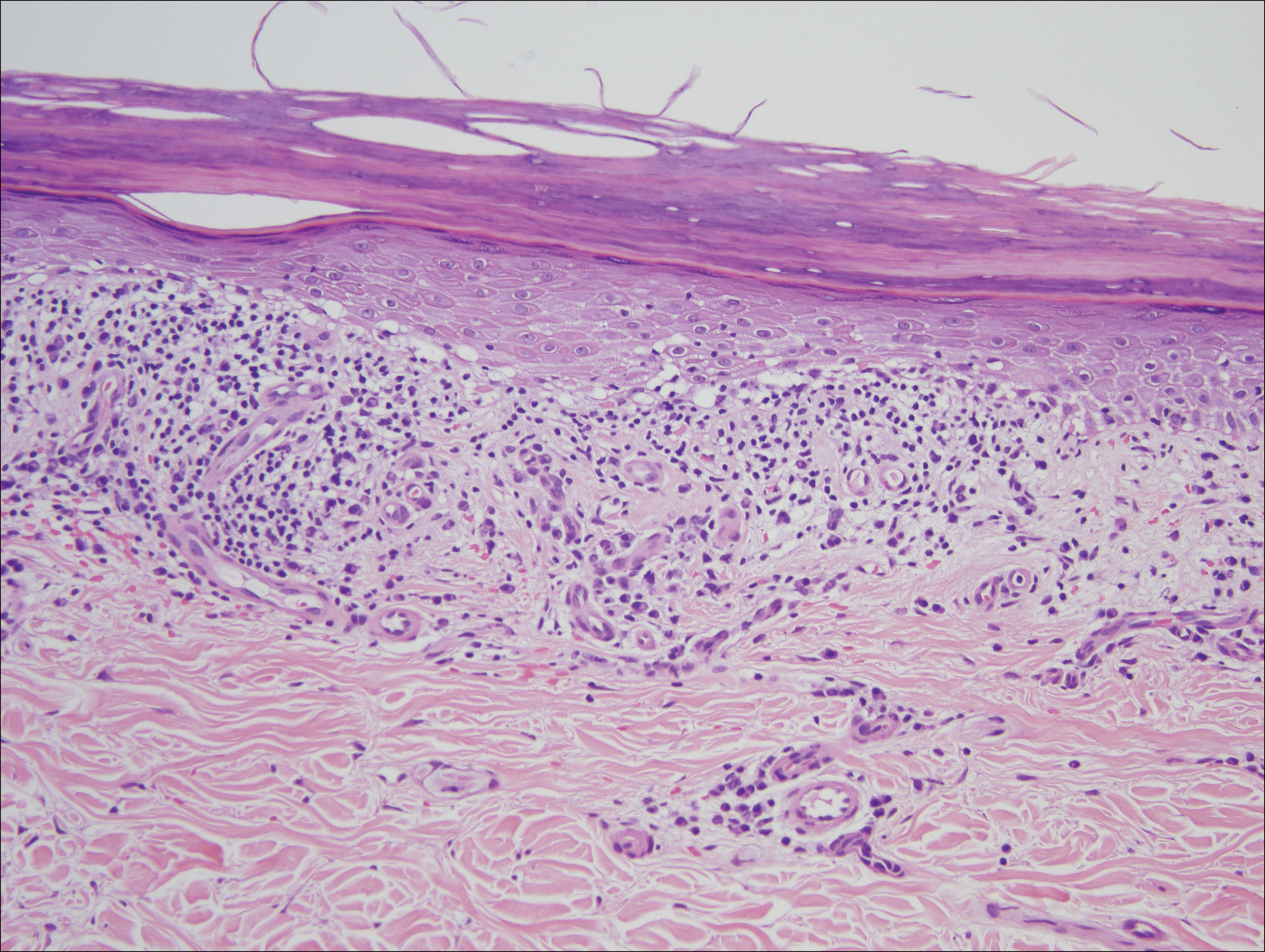
Histologic examination of a papule on the instep of the right sole demonstrated a dense lichenoid lymphocytic infiltrate in the papillary dermis with basal vacuolar degeneration and early focal Max-Joseph space formation. Additionally, there was epidermal atrophy with mild hypergranulosis and scattered necrotic keratinocytes (Figure 3). A similar histologic picture was noted on a biopsy of the buccal mucosa overlying the right molar, albeit with epithelial acanthosis rather than atrophy.
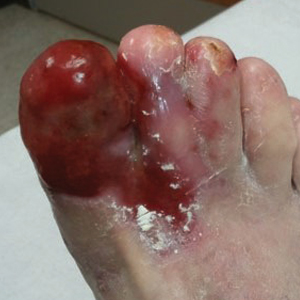
Based on initial clinical suspicion for ULP, we suggested that our patient discontinue ibuprofen and started him on a regimen of oral prednisone 40 mg once daily and clobetasol ointment 0.05% applied twice daily to the plantar ulceration, both for 2 weeks. Dramatic improvement was noted after only 2 weeks of treatment. This regimen was then switched to oral doxycycline 100 mg twice daily combined with tacrolimus ointment 0.1% applied twice daily to the plantar ulceration to avoid side effects of prolonged steroid use. Topical therapies were not used for the mucosal lesions. At 4-week follow-up, the patient continued to demonstrate notable clinical response with a greater than 70% physician-assessed improvement in ulcer severity (Figure 4) and near-complete resolution of the oral mucosal lesions. Our patient also reported almost complete resolution of pain. By 4-month follow-up, complete reepithelialization and resolution of the ulcers was noted (Figure 5). This improvement was sustained at additional follow-up 1 year after the initial presentation.
Comment
Ulcerative (or erosive) lichen planus is a rare form of lichen planus. Ulcerative lichen planus most commonly presents as erosive lesions of the oral and genital mucosae but rarely can involve other sites. The palms and soles are the most common sites of cutaneous involvement, with lesions frequently characterized by severe pain and limited mobility.2
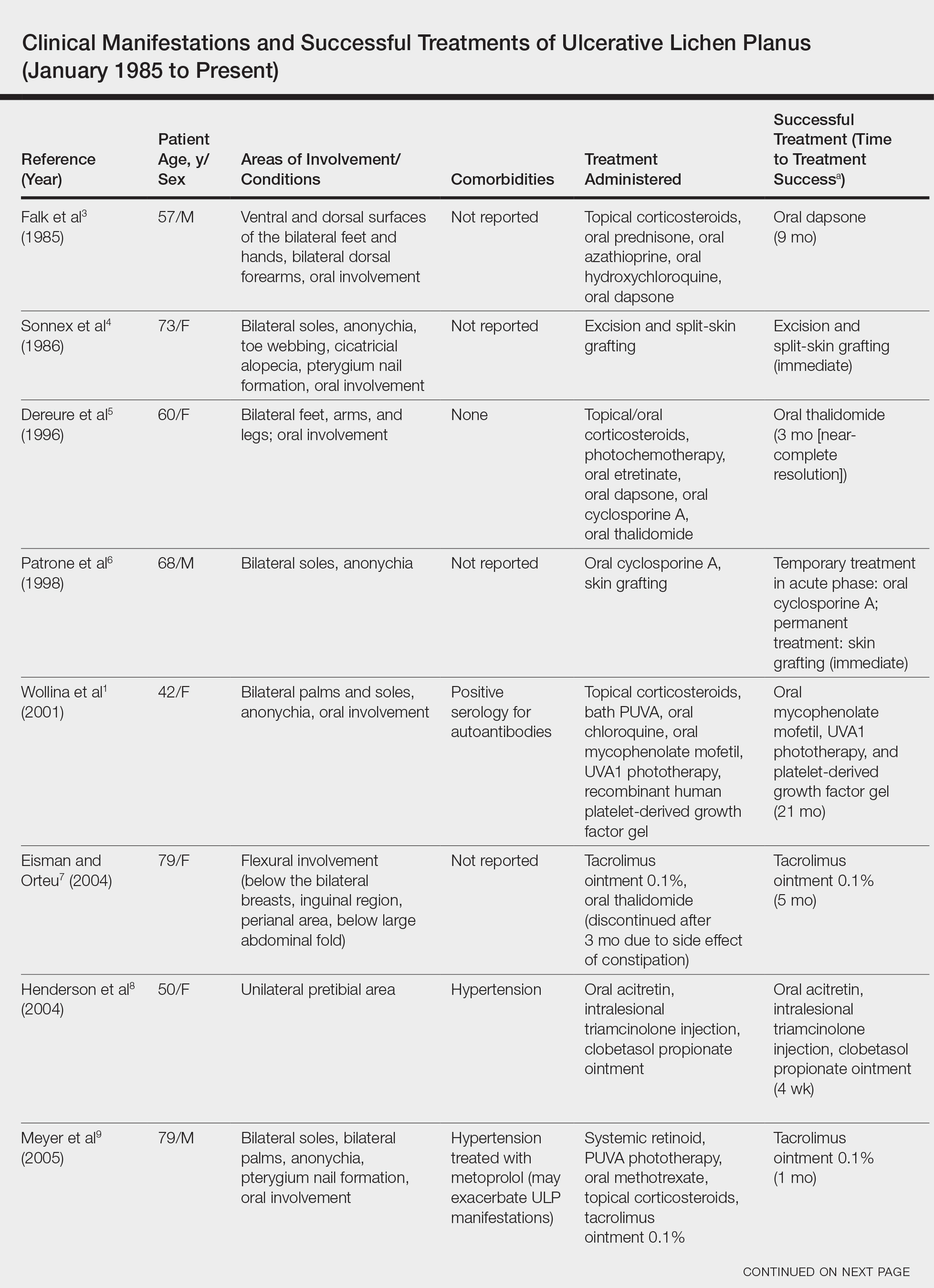
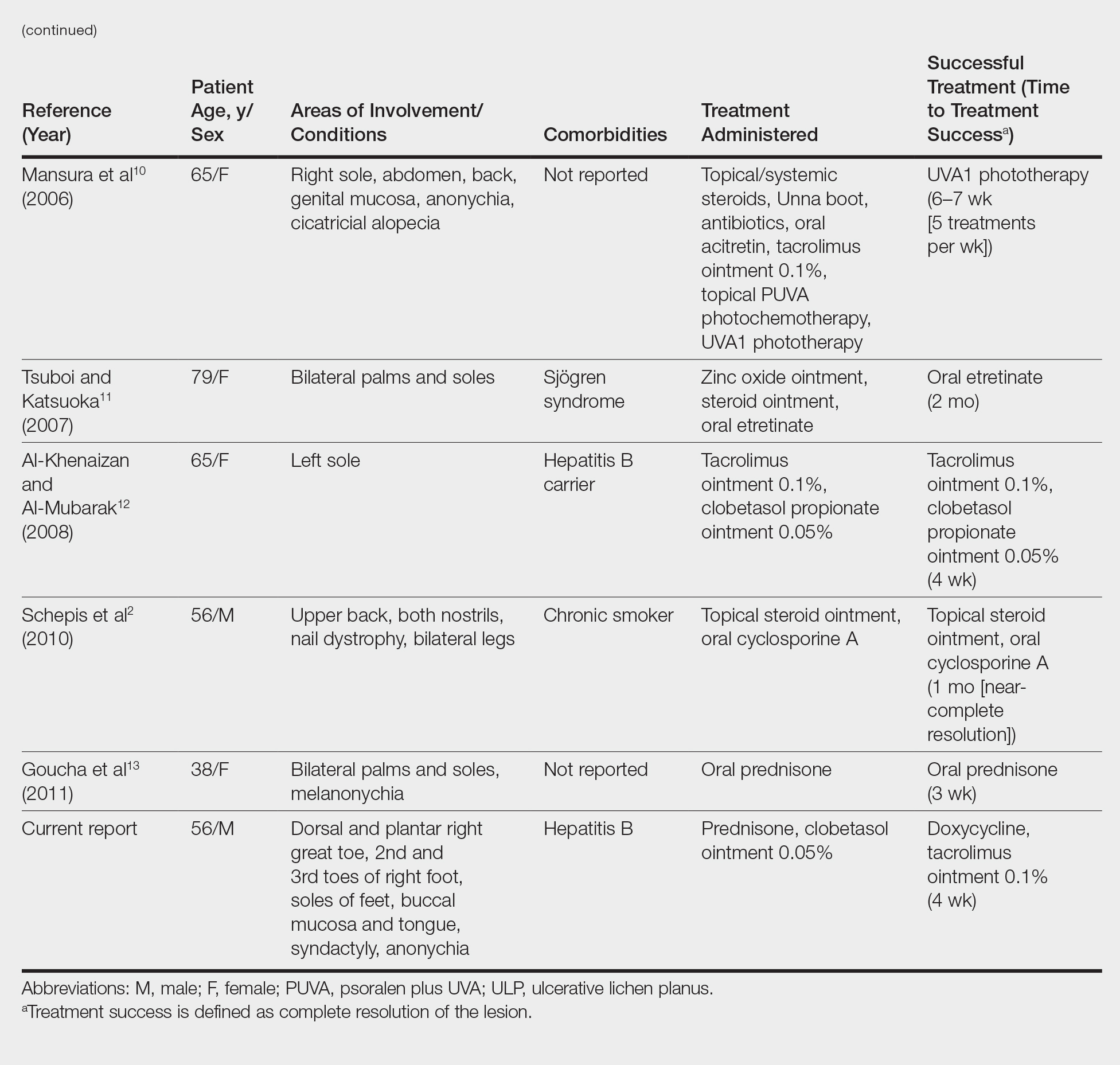
We conducted a review of the Ovid MEDLINE database using the search terms ulcerative lichen planus and erosive lichen planus for articles from the last 30 years, focusing specifically on articles that reported cases of cutaneous involvement of ULP and successful therapeutic modalities. The Table provides a detailed summary of the cases from 1985 to present, representing a spectrum of clinical manifestations and successful treatments of ULP.1-13
Hepatitis C is a comorbidity commonly associated with classic lichen planus, while hepatitis B immunization has a well-described association with classic and oral ULP.12,14 Although hepatitis C was negative in our patient, we did find a chronic inactive carrier state for hepatitis B infection. Al-Khenaizan and Al-Mubarak12 reported the only other known case of ULP of the sole associated with positive serology for hepatitis B surface antigen.
Ulcerative lichen planus of the soles can be difficult to diagnose, especially when it is an isolated finding. It should be differentiated from localized bullous pemphigoid, epidermolysis bullosa acquisita, ulcerative lupus erythematosus, and dermatitis artefacta.13 The characteristic associated clinical features of plantar ULP in our patient and lack of diagnostic immunofluorescence helped us to rule out these alternative diagnoses.4 Long-standing ulcerations of ULP also pose an increased risk for neoplastic transformation. Eisen15 noted a 0.4% to 5% frequency of malignant transformation into squamous cell carcinoma in those with oral ULP. Therefore, it is important to monitor previously ulcerated lesions long-term for such development.
Plantar ULP is difficult to treat and often is unresponsive to systemic and local treatment. Historically, surgical grafting of the affected areas was the treatment of choice, as reported by Patrone et al.6 Goucha et al13 reported complete healing of ulcerations within 3 weeks of starting oral prednisone 1 mg/kg once daily followed by a maintenance dosage of 5 mg once daily. Tacrolimus is a macrolide immunosuppressant that inhibits T-cell activation by forming a complex with FK506 binding protein in the cytoplasm of T cells that binds and inhibits calcineurin dephosphorylation of nuclear factor of activated T cells.12 Al-Khenaizan and Al-Mubarak12 reported resolution of plantar ULP ulcerations after 4 weeks of treatment with topical tacrolimus. Eisman and Orteu7 also achieved complete healing of ulcerations of plantar ULP using tacrolimus ointment 0.1%.
In our patient, doxycycline also was started at the time of initiating the topical tacrolimus. We chose this treatment to take advantage of its systemic anti-inflammatory, antiangiogenic, and antibacterial properties. Our case represents rapid and successful treatment of plantar ULP utilizing this specific combination of oral doxycycline and topical tacrolimus.
Conclusion
Ulcerative lichen planus is an uncommon variant of lichen planus, with cutaneous involvement only rarely reported in the literature. Physicians should be aware of this entity and should consider it in the differential diagnosis in patients presenting with chronic ulcers on the soles, especially when lesions have been unresponsive to appropriate wound care and antibiotic treatment or when cultures have been persistently negative for microbial growth. The possibility of drug-induced lichen planus also should not be overlooked, and one should consider discontinuation of all nonessential medications that could be potential culprits. In our patient ibuprofen was discontinued, but we can only speculate that it was contributory to his healing and only time will tell if resumption of this nonsteroidal anti-inflammatory drug causes a relapse in symptoms.
In our patient, a combination of systemic and topical steroids, topical tacrolimus, and oral doxycycline successfully treated his plantar ULP. Our findings provide further support for the use of topical tacrolimus as a steroid-sparing anti-inflammatory agent for the treatment of plantar ULP. We also introduce the combination of topical tacrolimus and oral doxycycline as a novel therapeutic combination and relatively safer alternative to conventional immunosuppressive agents for long-term systemic anti-inflammatory effects.
- Wollina U, Konrad H, Graefe T. Ulcerative lichen planus: a case responding to recombinant platelet-derived growth factor BB and immunosuppression. Acta Derm Venereol. 2001;81:364-383.
- Schepis C, Lentini M, Siragusa M. Erosive lichen planus on an atypical site mimicking a factitial dermatitis. Acta Derm Venereol. 2010;90:185-186.
- Falk DK, Latour DL, King EL. Dapsone in the treatment of erosive lichen planus. J Am Acad Dermatol. 1985;12:567-570.
- Sonnex TS, Eady RA, Sparrow GP, et al. Ulcerative lichen planus associated with webbing of the toes. J R Soc Med. 1986;79:363-365.
- Dereure O, Basset-Sequin N, Guilhou JJ. Erosive lichen planus: dramatic response to thalidomide. Arch Dermatol. 1996;132:1392-1393.
- Patrone P, Stinco G, La Pia E, et al. Surgery and cyclosporine A in the treatment of erosive lichen planus of the feet. Eur J Dermatol. 1998;8:243-244.
- Eisman S, Orteu C. Recalcitrant erosive flexural lichen planus: successful treatment with a combination of thalidomide and 0.1% tacrolimus ointment. Clin Exp Dermatol. 2004;29:268-270.
- Henderson RL Jr, Williford PM, Molnar JA. Cutaneous ulcerative lichen planus exhibiting pathergy, response to acitretin. J Drugs Dermatol. 2004;3:191-192.
- Meyer S, Burgdorf T, Szeimies R, et al. Management of erosive lichen planus with topical tacrolimus and recurrence secondary to metoprolol. J Eur Acad Dermatol Venereol. 2005;19:236-239.
- Mansura A, Alkalay R, Slodownik D, et al. Ultraviolet A-1 as a treatment for ulcerative lichen planus of the feet. Photodermatol Photoimmunol Pathomed. 2006;22:164-165.
- Tsuboi H, Katsuoka K. Ulcerative lichen planus associated with Sjögren’s syndrome. J Dermatol. 2007;34:131-134.
- Al-Khenaizan S, Al-Mubarak L. Ulcerative lichen planus of the sole: excellent response to topical tacrolimus. Int J Dermatol. 2008;47:626-628.
- Goucha S, Khaled A, Rammeh S, et al. Erosive lichen planus of the soles: effective response to prednisone. Dermatol Ther. 2011;1:20-24.
- Binesh F, Parichehr K. Erosive lichen planus of the scalp and hepatitis C infection. J Coll Physicians Surg Pak. 2013;23:169.
- Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207-214.
Ulcerative lichen planus (ULP)(also called erosive) is a rare variant of lichen planus. Similar to classic lichen planus, the cause of ULP is largely unknown. Ulcerative lichen planus typically involves the oral mucosa or genitalia but rarely may present as ulcerations on the palms and soles. Clinical presentation usually involves a history of chronic ulcers that often have been previously misdiagnosed and resistant to treatment. Ulcerations on the plantar surfaces frequently cause severe pain and disability. Few cases have been reported and successful treatment is rare.
Case Report
A 56-year-old man was referred by podiatry to the dermatology clinic for evaluation of painful ulcerations involving the dorsal and plantar surfaces of the right great toe as well as the second to third digits. The ulcers had been ongoing for 8 years, treated mostly with local wound care without clinical improvement. His medical and family history was considered noncontributory as a possible etiology of the ulcers; however, he had been taking ibuprofen intermittently for years for general aches and pains, which raised the suspicion of a drug-induced etiology. Laboratory evaluation revealed positive hepatitis B serology but was otherwise unremarkable, including normal liver function tests and negative wound cultures.
Physical examination revealed a beefy red, glazed ulceration involving the entire right great toe with extension onto the second and third toes. There was considerable scarring with syndactyly of the second and third toes and complete toenail loss of the right foot (Figure 1). On the insteps of the bilateral soles were a few scattered, pale, atrophic, violaceous papules with overlying thin lacy white streaks that were reflective of Wickham striae. Early dorsal pterygium formation also was noted on the bilateral third fingernails. Oral mucosal examination revealed lacy white plaques on the bilateral buccal mucosa with a large ulcer of the left lateral tongue (Figure 2). No genital or scalp lesions were present.


Histologic examination of a papule on the instep of the right sole demonstrated a dense lichenoid lymphocytic infiltrate in the papillary dermis with basal vacuolar degeneration and early focal Max-Joseph space formation. Additionally, there was epidermal atrophy with mild hypergranulosis and scattered necrotic keratinocytes (Figure 3). A similar histologic picture was noted on a biopsy of the buccal mucosa overlying the right molar, albeit with epithelial acanthosis rather than atrophy.

Based on initial clinical suspicion for ULP, we suggested that our patient discontinue ibuprofen and started him on a regimen of oral prednisone 40 mg once daily and clobetasol ointment 0.05% applied twice daily to the plantar ulceration, both for 2 weeks. Dramatic improvement was noted after only 2 weeks of treatment. This regimen was then switched to oral doxycycline 100 mg twice daily combined with tacrolimus ointment 0.1% applied twice daily to the plantar ulceration to avoid side effects of prolonged steroid use. Topical therapies were not used for the mucosal lesions. At 4-week follow-up, the patient continued to demonstrate notable clinical response with a greater than 70% physician-assessed improvement in ulcer severity (Figure 4) and near-complete resolution of the oral mucosal lesions. Our patient also reported almost complete resolution of pain. By 4-month follow-up, complete reepithelialization and resolution of the ulcers was noted (Figure 5). This improvement was sustained at additional follow-up 1 year after the initial presentation.


Comment
Ulcerative (or erosive) lichen planus is a rare form of lichen planus. Ulcerative lichen planus most commonly presents as erosive lesions of the oral and genital mucosae but rarely can involve other sites. The palms and soles are the most common sites of cutaneous involvement, with lesions frequently characterized by severe pain and limited mobility.2
We conducted a review of the Ovid MEDLINE database using the search terms ulcerative lichen planus and erosive lichen planus for articles from the last 30 years, focusing specifically on articles that reported cases of cutaneous involvement of ULP and successful therapeutic modalities. The Table provides a detailed summary of the cases from 1985 to present, representing a spectrum of clinical manifestations and successful treatments of ULP.1-13


Hepatitis C is a comorbidity commonly associated with classic lichen planus, while hepatitis B immunization has a well-described association with classic and oral ULP.12,14 Although hepatitis C was negative in our patient, we did find a chronic inactive carrier state for hepatitis B infection. Al-Khenaizan and Al-Mubarak12 reported the only other known case of ULP of the sole associated with positive serology for hepatitis B surface antigen.
Ulcerative lichen planus of the soles can be difficult to diagnose, especially when it is an isolated finding. It should be differentiated from localized bullous pemphigoid, epidermolysis bullosa acquisita, ulcerative lupus erythematosus, and dermatitis artefacta.13 The characteristic associated clinical features of plantar ULP in our patient and lack of diagnostic immunofluorescence helped us to rule out these alternative diagnoses.4 Long-standing ulcerations of ULP also pose an increased risk for neoplastic transformation. Eisen15 noted a 0.4% to 5% frequency of malignant transformation into squamous cell carcinoma in those with oral ULP. Therefore, it is important to monitor previously ulcerated lesions long-term for such development.
Plantar ULP is difficult to treat and often is unresponsive to systemic and local treatment. Historically, surgical grafting of the affected areas was the treatment of choice, as reported by Patrone et al.6 Goucha et al13 reported complete healing of ulcerations within 3 weeks of starting oral prednisone 1 mg/kg once daily followed by a maintenance dosage of 5 mg once daily. Tacrolimus is a macrolide immunosuppressant that inhibits T-cell activation by forming a complex with FK506 binding protein in the cytoplasm of T cells that binds and inhibits calcineurin dephosphorylation of nuclear factor of activated T cells.12 Al-Khenaizan and Al-Mubarak12 reported resolution of plantar ULP ulcerations after 4 weeks of treatment with topical tacrolimus. Eisman and Orteu7 also achieved complete healing of ulcerations of plantar ULP using tacrolimus ointment 0.1%.
In our patient, doxycycline also was started at the time of initiating the topical tacrolimus. We chose this treatment to take advantage of its systemic anti-inflammatory, antiangiogenic, and antibacterial properties. Our case represents rapid and successful treatment of plantar ULP utilizing this specific combination of oral doxycycline and topical tacrolimus.
Conclusion
Ulcerative lichen planus is an uncommon variant of lichen planus, with cutaneous involvement only rarely reported in the literature. Physicians should be aware of this entity and should consider it in the differential diagnosis in patients presenting with chronic ulcers on the soles, especially when lesions have been unresponsive to appropriate wound care and antibiotic treatment or when cultures have been persistently negative for microbial growth. The possibility of drug-induced lichen planus also should not be overlooked, and one should consider discontinuation of all nonessential medications that could be potential culprits. In our patient ibuprofen was discontinued, but we can only speculate that it was contributory to his healing and only time will tell if resumption of this nonsteroidal anti-inflammatory drug causes a relapse in symptoms.
In our patient, a combination of systemic and topical steroids, topical tacrolimus, and oral doxycycline successfully treated his plantar ULP. Our findings provide further support for the use of topical tacrolimus as a steroid-sparing anti-inflammatory agent for the treatment of plantar ULP. We also introduce the combination of topical tacrolimus and oral doxycycline as a novel therapeutic combination and relatively safer alternative to conventional immunosuppressive agents for long-term systemic anti-inflammatory effects.
Ulcerative lichen planus (ULP)(also called erosive) is a rare variant of lichen planus. Similar to classic lichen planus, the cause of ULP is largely unknown. Ulcerative lichen planus typically involves the oral mucosa or genitalia but rarely may present as ulcerations on the palms and soles. Clinical presentation usually involves a history of chronic ulcers that often have been previously misdiagnosed and resistant to treatment. Ulcerations on the plantar surfaces frequently cause severe pain and disability. Few cases have been reported and successful treatment is rare.
Case Report
A 56-year-old man was referred by podiatry to the dermatology clinic for evaluation of painful ulcerations involving the dorsal and plantar surfaces of the right great toe as well as the second to third digits. The ulcers had been ongoing for 8 years, treated mostly with local wound care without clinical improvement. His medical and family history was considered noncontributory as a possible etiology of the ulcers; however, he had been taking ibuprofen intermittently for years for general aches and pains, which raised the suspicion of a drug-induced etiology. Laboratory evaluation revealed positive hepatitis B serology but was otherwise unremarkable, including normal liver function tests and negative wound cultures.
Physical examination revealed a beefy red, glazed ulceration involving the entire right great toe with extension onto the second and third toes. There was considerable scarring with syndactyly of the second and third toes and complete toenail loss of the right foot (Figure 1). On the insteps of the bilateral soles were a few scattered, pale, atrophic, violaceous papules with overlying thin lacy white streaks that were reflective of Wickham striae. Early dorsal pterygium formation also was noted on the bilateral third fingernails. Oral mucosal examination revealed lacy white plaques on the bilateral buccal mucosa with a large ulcer of the left lateral tongue (Figure 2). No genital or scalp lesions were present.


Histologic examination of a papule on the instep of the right sole demonstrated a dense lichenoid lymphocytic infiltrate in the papillary dermis with basal vacuolar degeneration and early focal Max-Joseph space formation. Additionally, there was epidermal atrophy with mild hypergranulosis and scattered necrotic keratinocytes (Figure 3). A similar histologic picture was noted on a biopsy of the buccal mucosa overlying the right molar, albeit with epithelial acanthosis rather than atrophy.

Based on initial clinical suspicion for ULP, we suggested that our patient discontinue ibuprofen and started him on a regimen of oral prednisone 40 mg once daily and clobetasol ointment 0.05% applied twice daily to the plantar ulceration, both for 2 weeks. Dramatic improvement was noted after only 2 weeks of treatment. This regimen was then switched to oral doxycycline 100 mg twice daily combined with tacrolimus ointment 0.1% applied twice daily to the plantar ulceration to avoid side effects of prolonged steroid use. Topical therapies were not used for the mucosal lesions. At 4-week follow-up, the patient continued to demonstrate notable clinical response with a greater than 70% physician-assessed improvement in ulcer severity (Figure 4) and near-complete resolution of the oral mucosal lesions. Our patient also reported almost complete resolution of pain. By 4-month follow-up, complete reepithelialization and resolution of the ulcers was noted (Figure 5). This improvement was sustained at additional follow-up 1 year after the initial presentation.


Comment
Ulcerative (or erosive) lichen planus is a rare form of lichen planus. Ulcerative lichen planus most commonly presents as erosive lesions of the oral and genital mucosae but rarely can involve other sites. The palms and soles are the most common sites of cutaneous involvement, with lesions frequently characterized by severe pain and limited mobility.2
We conducted a review of the Ovid MEDLINE database using the search terms ulcerative lichen planus and erosive lichen planus for articles from the last 30 years, focusing specifically on articles that reported cases of cutaneous involvement of ULP and successful therapeutic modalities. The Table provides a detailed summary of the cases from 1985 to present, representing a spectrum of clinical manifestations and successful treatments of ULP.1-13


Hepatitis C is a comorbidity commonly associated with classic lichen planus, while hepatitis B immunization has a well-described association with classic and oral ULP.12,14 Although hepatitis C was negative in our patient, we did find a chronic inactive carrier state for hepatitis B infection. Al-Khenaizan and Al-Mubarak12 reported the only other known case of ULP of the sole associated with positive serology for hepatitis B surface antigen.
Ulcerative lichen planus of the soles can be difficult to diagnose, especially when it is an isolated finding. It should be differentiated from localized bullous pemphigoid, epidermolysis bullosa acquisita, ulcerative lupus erythematosus, and dermatitis artefacta.13 The characteristic associated clinical features of plantar ULP in our patient and lack of diagnostic immunofluorescence helped us to rule out these alternative diagnoses.4 Long-standing ulcerations of ULP also pose an increased risk for neoplastic transformation. Eisen15 noted a 0.4% to 5% frequency of malignant transformation into squamous cell carcinoma in those with oral ULP. Therefore, it is important to monitor previously ulcerated lesions long-term for such development.
Plantar ULP is difficult to treat and often is unresponsive to systemic and local treatment. Historically, surgical grafting of the affected areas was the treatment of choice, as reported by Patrone et al.6 Goucha et al13 reported complete healing of ulcerations within 3 weeks of starting oral prednisone 1 mg/kg once daily followed by a maintenance dosage of 5 mg once daily. Tacrolimus is a macrolide immunosuppressant that inhibits T-cell activation by forming a complex with FK506 binding protein in the cytoplasm of T cells that binds and inhibits calcineurin dephosphorylation of nuclear factor of activated T cells.12 Al-Khenaizan and Al-Mubarak12 reported resolution of plantar ULP ulcerations after 4 weeks of treatment with topical tacrolimus. Eisman and Orteu7 also achieved complete healing of ulcerations of plantar ULP using tacrolimus ointment 0.1%.
In our patient, doxycycline also was started at the time of initiating the topical tacrolimus. We chose this treatment to take advantage of its systemic anti-inflammatory, antiangiogenic, and antibacterial properties. Our case represents rapid and successful treatment of plantar ULP utilizing this specific combination of oral doxycycline and topical tacrolimus.
Conclusion
Ulcerative lichen planus is an uncommon variant of lichen planus, with cutaneous involvement only rarely reported in the literature. Physicians should be aware of this entity and should consider it in the differential diagnosis in patients presenting with chronic ulcers on the soles, especially when lesions have been unresponsive to appropriate wound care and antibiotic treatment or when cultures have been persistently negative for microbial growth. The possibility of drug-induced lichen planus also should not be overlooked, and one should consider discontinuation of all nonessential medications that could be potential culprits. In our patient ibuprofen was discontinued, but we can only speculate that it was contributory to his healing and only time will tell if resumption of this nonsteroidal anti-inflammatory drug causes a relapse in symptoms.
In our patient, a combination of systemic and topical steroids, topical tacrolimus, and oral doxycycline successfully treated his plantar ULP. Our findings provide further support for the use of topical tacrolimus as a steroid-sparing anti-inflammatory agent for the treatment of plantar ULP. We also introduce the combination of topical tacrolimus and oral doxycycline as a novel therapeutic combination and relatively safer alternative to conventional immunosuppressive agents for long-term systemic anti-inflammatory effects.
- Wollina U, Konrad H, Graefe T. Ulcerative lichen planus: a case responding to recombinant platelet-derived growth factor BB and immunosuppression. Acta Derm Venereol. 2001;81:364-383.
- Schepis C, Lentini M, Siragusa M. Erosive lichen planus on an atypical site mimicking a factitial dermatitis. Acta Derm Venereol. 2010;90:185-186.
- Falk DK, Latour DL, King EL. Dapsone in the treatment of erosive lichen planus. J Am Acad Dermatol. 1985;12:567-570.
- Sonnex TS, Eady RA, Sparrow GP, et al. Ulcerative lichen planus associated with webbing of the toes. J R Soc Med. 1986;79:363-365.
- Dereure O, Basset-Sequin N, Guilhou JJ. Erosive lichen planus: dramatic response to thalidomide. Arch Dermatol. 1996;132:1392-1393.
- Patrone P, Stinco G, La Pia E, et al. Surgery and cyclosporine A in the treatment of erosive lichen planus of the feet. Eur J Dermatol. 1998;8:243-244.
- Eisman S, Orteu C. Recalcitrant erosive flexural lichen planus: successful treatment with a combination of thalidomide and 0.1% tacrolimus ointment. Clin Exp Dermatol. 2004;29:268-270.
- Henderson RL Jr, Williford PM, Molnar JA. Cutaneous ulcerative lichen planus exhibiting pathergy, response to acitretin. J Drugs Dermatol. 2004;3:191-192.
- Meyer S, Burgdorf T, Szeimies R, et al. Management of erosive lichen planus with topical tacrolimus and recurrence secondary to metoprolol. J Eur Acad Dermatol Venereol. 2005;19:236-239.
- Mansura A, Alkalay R, Slodownik D, et al. Ultraviolet A-1 as a treatment for ulcerative lichen planus of the feet. Photodermatol Photoimmunol Pathomed. 2006;22:164-165.
- Tsuboi H, Katsuoka K. Ulcerative lichen planus associated with Sjögren’s syndrome. J Dermatol. 2007;34:131-134.
- Al-Khenaizan S, Al-Mubarak L. Ulcerative lichen planus of the sole: excellent response to topical tacrolimus. Int J Dermatol. 2008;47:626-628.
- Goucha S, Khaled A, Rammeh S, et al. Erosive lichen planus of the soles: effective response to prednisone. Dermatol Ther. 2011;1:20-24.
- Binesh F, Parichehr K. Erosive lichen planus of the scalp and hepatitis C infection. J Coll Physicians Surg Pak. 2013;23:169.
- Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207-214.
- Wollina U, Konrad H, Graefe T. Ulcerative lichen planus: a case responding to recombinant platelet-derived growth factor BB and immunosuppression. Acta Derm Venereol. 2001;81:364-383.
- Schepis C, Lentini M, Siragusa M. Erosive lichen planus on an atypical site mimicking a factitial dermatitis. Acta Derm Venereol. 2010;90:185-186.
- Falk DK, Latour DL, King EL. Dapsone in the treatment of erosive lichen planus. J Am Acad Dermatol. 1985;12:567-570.
- Sonnex TS, Eady RA, Sparrow GP, et al. Ulcerative lichen planus associated with webbing of the toes. J R Soc Med. 1986;79:363-365.
- Dereure O, Basset-Sequin N, Guilhou JJ. Erosive lichen planus: dramatic response to thalidomide. Arch Dermatol. 1996;132:1392-1393.
- Patrone P, Stinco G, La Pia E, et al. Surgery and cyclosporine A in the treatment of erosive lichen planus of the feet. Eur J Dermatol. 1998;8:243-244.
- Eisman S, Orteu C. Recalcitrant erosive flexural lichen planus: successful treatment with a combination of thalidomide and 0.1% tacrolimus ointment. Clin Exp Dermatol. 2004;29:268-270.
- Henderson RL Jr, Williford PM, Molnar JA. Cutaneous ulcerative lichen planus exhibiting pathergy, response to acitretin. J Drugs Dermatol. 2004;3:191-192.
- Meyer S, Burgdorf T, Szeimies R, et al. Management of erosive lichen planus with topical tacrolimus and recurrence secondary to metoprolol. J Eur Acad Dermatol Venereol. 2005;19:236-239.
- Mansura A, Alkalay R, Slodownik D, et al. Ultraviolet A-1 as a treatment for ulcerative lichen planus of the feet. Photodermatol Photoimmunol Pathomed. 2006;22:164-165.
- Tsuboi H, Katsuoka K. Ulcerative lichen planus associated with Sjögren’s syndrome. J Dermatol. 2007;34:131-134.
- Al-Khenaizan S, Al-Mubarak L. Ulcerative lichen planus of the sole: excellent response to topical tacrolimus. Int J Dermatol. 2008;47:626-628.
- Goucha S, Khaled A, Rammeh S, et al. Erosive lichen planus of the soles: effective response to prednisone. Dermatol Ther. 2011;1:20-24.
- Binesh F, Parichehr K. Erosive lichen planus of the scalp and hepatitis C infection. J Coll Physicians Surg Pak. 2013;23:169.
- Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002;46:207-214.
Practice Points
- Consider ulcerative lichen planus (ULP) for chronic wounds on the soles.
- Topical therapeutic options may present a rapidly effective and relatively safe alternative to conventional immunosuppressive agents for long-term management of plantar ULP.
Laser Scar Management: Focused and High-Intensity Medical Exchange in Vietnam
Over the last decade the treatment of traumatic scars with lasers has emerged as a core component of multidisciplinary management. Military dermatologists have played a fundamental role in this shift by helping to develop new applications for existing technology and promulgate the techniques to reach additional providers and patients. Beyond scar management, the repurposing of adjunctive procedural techniques, such as sweat and hair reduction in amputees, also promises to enhance rehabilitation for many patients.
International engagement is a prominent and highly attractive feature of military practice, and military dermatologists routinely participate in disaster response missions, such as the 2010 Haiti earthquake,1 and ongoing planned operations, such as Pacific Partnership in the Indo-Asia-Pacific region led by the US Navy.2 In this article, I present a military perspective on the emerging niche of trauma dermatology and outline my more than 5 years of experience leveraging these skills to lead a multidisciplinary exchange in restorative medicine and burn scar management in Vietnam.
Trauma Dermatology
Over the course of the last decade, traumatic scar management has emerged as a staple of dermatologic surgery practice in some centers. Dermatologists hold the key to increasing patient access to effective outpatient care for symptomatic traumatic scars and other related issues using devices and techniques initially conceived for cosmetic applications.3 A major impetus for the considerable remodeling in our collective thoughts about traumatic scar management was the emergence of fractional laser technology in the mid-2000s. The remarkable, safe, reproducible, and durable benefits of fractional laser treatment of various scar types have created substantial momentum in recent years. The Naval Medical Center San Diego in California houses 1 of 3 centers of excellence in rehabilitation in the US military. Mastery of minimally invasive procedures to manage scars and other issues associated with trauma for the first time has established dermatologists as important partners in the overall rehabilitative effort.
My perspective on laser scar management has been previously described.4,5 Ablative fractional laser resurfacing is the backbone of rehabilitative scar management.6 Although the literature in this field is still relatively immature, higher-quality studies are accumulating rapidly as the burn and surgical communities adopt the procedure more widely.7-10 A considerable step forward in the dissemination of the procedure occurred recently with the development of category III Current Procedural Terminology (CPT) codes for ablative laser treatment of traumatic scars.11 Category III CPT codes are temporary codes used for emerging procedures that have not yet been deemed medically necessary. Although individual insurance carriers can determine whether to cover these procedures and the corresponding level of reimbursement, regular use is important for ultimate elevation to category I codes by the American Medical Association over a 5-year observation period. The CPT codes 0479T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; first 100 cm2 or part thereof, or 1% of body surface area of infants and children) and 0480T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; each additional 100 cm2, or each additional 1% of body surface area of infants and children, or part thereof [list separately in addition to code for primary procedure]) are examples of these category III codes.11
Nonablative fractional lasers; vascular-specific devices for erythematous scars; and long- and short-pulsed pigment-specific devices for hair and traumatic tattoo treatment, respectively, round out the commonly used laser platforms. For example, laser hair reduction can help improve the fit and comfort of prosthetic devices and has been shown to improve the overall quality of life for amputees.12 Botulinum toxin can be an important component of treatment of excessive sweating induced by occlusive liners in prosthetics, and microwave eccrine ablation is an emerging potential option for longer-lasting sweat reduction in this population.13-15 In addition to providing direct dermatology care and education, having members of the specialty in uniform has been a key to adopting new practical solutions to unsolved problems.
Pacific Partnership
Pacific Partnership is the largest annual multinational humanitarian assistance and disaster preparedness mission in the Indo-Asia-Pacific region.16 It was started in 2006 following the tsunami that devastated parts of South and Southeast Asia in 2004. The recently concluded Pacific Partnership 2018 marked the 13th iteration of the annual mission led by the US Navy in collaboration with other partner nations, which in 2018 included Japan, Australia, Canada, the United Kingdom, France, Singapore, Korea, and Peru, as well as nongovernmental organizations and international governmental agencies. Host nation mission locations vary somewhat from year to year, but 2018 included visits of the hospital ship USNS Mercy and more than 800 personnel to Indonesia, Malaysia, Sri Lanka, and Vietnam. Medical/dental, engineering, and veterinary teams join with their counterparts in each host nation to conduct civic action projects, community health exchanges, medical care, and disaster response training activities.16
Rehabilitation As a Vehicle for Medical Exchange
Since approximately 2012 there has been an evolving paradigm in Pacific Partnership from an emphasis on maximizing direct patient care in changing locations to one focused on building lasting partnerships through subject matter expert exchange. Multidisciplinary scar management, including surgical and laser scar revision and physical and occupational therapy, is a very promising model for engagement. Potential advantages of this type of exchange include the following: developing nations have relatively high rates of burns and other forms of trauma as well as uneven access to acute and ongoing rehabilitative care; patients often are otherwise healthy and young; results are frequently profound and readily demonstrable; and it is a skill set that has become highly developed in the military system. Just as dermatologists are illustrating their utility in trauma rehabilitation at home, these procedural skills provide fertile ground for exchange overseas.
The Overseas Humanitarian Assistance Shared Information System is an online platform that allows users to apply for grants under the Asia-Pacific Regional Initiative. In 2013, I started the Burn Scar Treatment/Restorative Medicine exchange with a grant under this program. A multidisciplinary team representing the specialties of dermatology, hand surgery, plastic surgery, physical medicine and rehabilitation, and pulmonary critical care participated in the 2013 Asia Pacific Burn Congress hosted by the National Institute of Burns (NIB) in Hanoi, Vietnam, and then followed up with didactics and patient care alongside Vietnamese physicians in the management of disfiguring and debilitating scars from burns and other trauma. This pilot project consisted of three 2- to 3-week phases: 2 at the NIB in Hanoi and 1 with a delegation from the NIB visiting the Naval Medical Center San Diego. When initial project funds expired in 2014, the exchange was absorbed into Pacific Partnership 2014, which began a string of 4 consecutive annual Pacific Partnership engagements at Da Nang General Hospital in Vietnam. The 2 most recent exchanges, including the exchange associated with Pacific Partnership 2018, have taken place at Khanh Hoa General Hospital in Nha Trang, Vietnam. During this time the team has grown to include physical and occupational therapists as well as a wound care nurse.
The Burn Scar Treatment/Restorative Medicine exchange consists of side-by-side laser and surgical scar revision performed with our Vietnamese hosts in their own hospital. Our Vietnamese partners perform a large volume of reconstructive surgeries in their usual practice, so it truly has been a bilateral exchange incorporating some advanced technology and techniques with an emphasis on longitudinal multidisciplinary care. Importantly, the procedures are supplemented with preoperative and postoperative care as well as instruction provided by physical and occupational therapy and wound care professionals working alongside host nation support staff. Because the areas of involvement often are extensive and a patient may only be seen once in this setting, laser and surgical procedures often are performed concurrently in the host nation operating room. Anesthesia support is provided by the host nation. Basic consumable surgical supplies (eg, sutures, gloves, marking pens, staplers) are supplemented with mission funds. Special adjuncts for the most severe contractures have included negative pressure wound therapy and a collagen-based bilayer matrix wound dressing. Laser treatments have been performed on the vast majority of patients with an ablative fractional CO2 laser and laser-assisted delivery of corticosteroid in hypertrophic areas. Of note, use of the laser has been provided to our hosts by the manufacturer for each of the 7 iterations of the exchange, and the wound dressing manufacturer also has donated some of their product to the exchange through the nongovernmental organization Project Hope for 2 missions. To date, more than 300 patients have safely received life-changing treatment during the exchange, with some receiving multiple treatments (Figure). Although multiple treatments over time are ideal, even a single treatment session can result in considerable and lasting improvements in function and symptoms.17 The hospital ship USNS Mercy has the same laser technology and has brought advanced scar treatment techniques to the far corners of the Pacific.
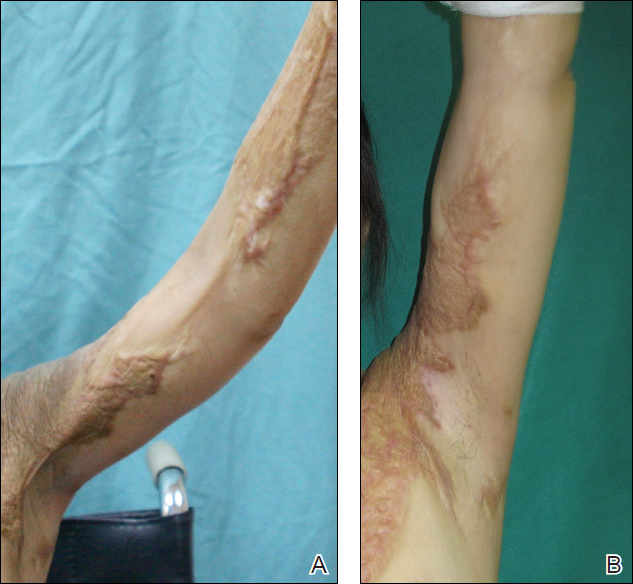
Measuring overall success—treatment and international relations—in this setting can be challenging. On an individual patient level, the benefits of restoring the ability to walk and work as well as reducing pain and itching are manifest and transformative for both the patient and family; however, aggregating this information into high-quality outcome data is difficult given the heterogeneous nature of traumatic injuries, which is compounded in the setting of international engagement where the intersection between patient and visiting provider may be singular or difficult to predict, funding is limited, language frequently is a barrier, and documentation, privacy, and medical research guidelines may be unfamiliar or contradictory. The cumulative impact of these types of exchanges on the relationship between nations also is critical but difficult to measure. It is common sense that deepening personal and professional relationships in the medical setting over time can increase trust and mutual understanding, perhaps setting the stage for broader engagement in other more sensitive areas. Trust and understanding are rather nebulous concepts, but earlier this year marked the first visit of an American aircraft carrier to Da Nang since 1975, following 4 consecutive annual Pacific Partnership missions in the same city, which does carry the patina of successful engagement on a systemic level.
Final Thoughts
Based on my personal experience, I provide the following tips for building a successful, focused, long-term medical exchange.
- Leverage your strengths and respect the strengths and style of practice of your hosts. A mind-set of exchange and not simply humanitarian care will be more successful. Your hosts are experts in a style of practice adapted to their surroundings and introducing new techniques that are grounded in the local practice patterns are more likely to be perpetuated.
- Collaboration with nongovernmental organizations and industry can be extremely helpful. Military and governmental organizations often are limited in funding, in the ways they can spend available funding, and in the receipt of donations. Appropriate coordination with civilian entities can elevate the exchange considerably by adding expertise and available assets as well as broadening the overall impact.
- Engage the support staff as well as the physicians. You will leverage contact with families and enhance care over the long-term.
- The benefits of multiple interactions over time are manifest, for both the patients and the participants. Personal and professional relationships are intertwined and naturally mature over time. Go for singles and doubles first before swinging for the fences.
- Multidisciplinary work overseas informs and enhances collaboration at home.
- Adding regional experts in international research and assessment to these specialized medical teams may better capture the impact of future exchanges of any flavor.
- The model of creating a focused exchange with independent funding followed by incorporation of successful concepts into larger missions seems to be a worthy and reproducible approach for future projects of any variety.
- Galeckas K. Dermatology aboard the USNS Comfort: disaster relief operations in Haiti after the 2010 earthquake. Dermatol Clin. 2011;29:15-19.
- Satter EK. The role of the dermatologist on military humanitarian missions. Cutis. 2010;85:85-89.
- Miletta NR, Donelan MB, Hivnor CM. Management of trauma and burn scars; the dermatologist's role in expanding patient access to care. Cutis. 2017;100:18-20.
- Shumaker PR. Laser treatment of traumatic scars: a military perspective. Semin Cutan Med Surg. 2015;34:17-23.
- Shumaker PR, Beachkofsky T, Basnett A, et al. A military perspective. In: Krakowski AC, Shumaker PR, eds. The Scar Book: Formation, Mitigation, Rehabilitation and Prevention. Philadelphia, PA: Wolters Kluwer; 2017:327-338.
- Anderson RR, Donelan MB, Greeson E, et al. Consensus report: laser treatment of traumatic scars with an emphasis on ablative fractional resurfacing. JAMA Dermatol. 2014;150:187-193.
- Hultman CS, Friedstat JS, Edkins RE, et al. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before and after cohort study, with long-term follow-up. Ann Surg. 2014;260:519-532.
- Blome-Eberwein S, Gogal C, Weiss MJ, et al. Prospective evaluation of fractional CO2 laser treatment of mature burn scars. J Burn Care Res. 2016;37:379-387.
- Issler-Fisher AC, Fisher OM, Smialkowski AO, et al. Ablative fractional CO2 laser for burn scar reconstruction: an extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns. 2017;43:573-582.
- Zuccaro J, Zlolkowski N, Fish J. A systematic review of the effectiveness of laser therapy for hypertrophic burn scars. Clin Plast Surg. 2017;44:767-779.
- Miller A. CPT 2018: What's new, part 2. American Academy of Dermatology website. https://www.aad.org/dw/monthly/2018/january/cpt-2018-whats-new-part-2. Accessed July 24, 2018.
- Miletta NR, Kim S, Lezanski-Gujda A, et al. Improving health-related quality of life in wounded warriors: the promising benefits of laser hair removal to the residual limb-prosthetic interface. Dermatol Surg. 2016;42:1182-1187.
- Gratrix M, Hivnor C. Botulinum toxin for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- USNS Mercy deploys in support of Pacific Partnership 2018 [news release]. Washington, DC: US Department of Defense; February 26, 2018. https://www.defense.gov/News/Article/Article/1450292/usns-mercy-deploys-in-support-of-pacific-partnership-2018/. Accessed July 11, 2018.
- Burns C, Basnett A, Valentine J, et al. Ablative fractional resurfacing: a powerful tool to help restore form and function during international medical exchange. Lasers Surg Med. 2017;49:471-474.
Over the last decade the treatment of traumatic scars with lasers has emerged as a core component of multidisciplinary management. Military dermatologists have played a fundamental role in this shift by helping to develop new applications for existing technology and promulgate the techniques to reach additional providers and patients. Beyond scar management, the repurposing of adjunctive procedural techniques, such as sweat and hair reduction in amputees, also promises to enhance rehabilitation for many patients.
International engagement is a prominent and highly attractive feature of military practice, and military dermatologists routinely participate in disaster response missions, such as the 2010 Haiti earthquake,1 and ongoing planned operations, such as Pacific Partnership in the Indo-Asia-Pacific region led by the US Navy.2 In this article, I present a military perspective on the emerging niche of trauma dermatology and outline my more than 5 years of experience leveraging these skills to lead a multidisciplinary exchange in restorative medicine and burn scar management in Vietnam.
Trauma Dermatology
Over the course of the last decade, traumatic scar management has emerged as a staple of dermatologic surgery practice in some centers. Dermatologists hold the key to increasing patient access to effective outpatient care for symptomatic traumatic scars and other related issues using devices and techniques initially conceived for cosmetic applications.3 A major impetus for the considerable remodeling in our collective thoughts about traumatic scar management was the emergence of fractional laser technology in the mid-2000s. The remarkable, safe, reproducible, and durable benefits of fractional laser treatment of various scar types have created substantial momentum in recent years. The Naval Medical Center San Diego in California houses 1 of 3 centers of excellence in rehabilitation in the US military. Mastery of minimally invasive procedures to manage scars and other issues associated with trauma for the first time has established dermatologists as important partners in the overall rehabilitative effort.
My perspective on laser scar management has been previously described.4,5 Ablative fractional laser resurfacing is the backbone of rehabilitative scar management.6 Although the literature in this field is still relatively immature, higher-quality studies are accumulating rapidly as the burn and surgical communities adopt the procedure more widely.7-10 A considerable step forward in the dissemination of the procedure occurred recently with the development of category III Current Procedural Terminology (CPT) codes for ablative laser treatment of traumatic scars.11 Category III CPT codes are temporary codes used for emerging procedures that have not yet been deemed medically necessary. Although individual insurance carriers can determine whether to cover these procedures and the corresponding level of reimbursement, regular use is important for ultimate elevation to category I codes by the American Medical Association over a 5-year observation period. The CPT codes 0479T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; first 100 cm2 or part thereof, or 1% of body surface area of infants and children) and 0480T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; each additional 100 cm2, or each additional 1% of body surface area of infants and children, or part thereof [list separately in addition to code for primary procedure]) are examples of these category III codes.11
Nonablative fractional lasers; vascular-specific devices for erythematous scars; and long- and short-pulsed pigment-specific devices for hair and traumatic tattoo treatment, respectively, round out the commonly used laser platforms. For example, laser hair reduction can help improve the fit and comfort of prosthetic devices and has been shown to improve the overall quality of life for amputees.12 Botulinum toxin can be an important component of treatment of excessive sweating induced by occlusive liners in prosthetics, and microwave eccrine ablation is an emerging potential option for longer-lasting sweat reduction in this population.13-15 In addition to providing direct dermatology care and education, having members of the specialty in uniform has been a key to adopting new practical solutions to unsolved problems.
Pacific Partnership
Pacific Partnership is the largest annual multinational humanitarian assistance and disaster preparedness mission in the Indo-Asia-Pacific region.16 It was started in 2006 following the tsunami that devastated parts of South and Southeast Asia in 2004. The recently concluded Pacific Partnership 2018 marked the 13th iteration of the annual mission led by the US Navy in collaboration with other partner nations, which in 2018 included Japan, Australia, Canada, the United Kingdom, France, Singapore, Korea, and Peru, as well as nongovernmental organizations and international governmental agencies. Host nation mission locations vary somewhat from year to year, but 2018 included visits of the hospital ship USNS Mercy and more than 800 personnel to Indonesia, Malaysia, Sri Lanka, and Vietnam. Medical/dental, engineering, and veterinary teams join with their counterparts in each host nation to conduct civic action projects, community health exchanges, medical care, and disaster response training activities.16
Rehabilitation As a Vehicle for Medical Exchange
Since approximately 2012 there has been an evolving paradigm in Pacific Partnership from an emphasis on maximizing direct patient care in changing locations to one focused on building lasting partnerships through subject matter expert exchange. Multidisciplinary scar management, including surgical and laser scar revision and physical and occupational therapy, is a very promising model for engagement. Potential advantages of this type of exchange include the following: developing nations have relatively high rates of burns and other forms of trauma as well as uneven access to acute and ongoing rehabilitative care; patients often are otherwise healthy and young; results are frequently profound and readily demonstrable; and it is a skill set that has become highly developed in the military system. Just as dermatologists are illustrating their utility in trauma rehabilitation at home, these procedural skills provide fertile ground for exchange overseas.
The Overseas Humanitarian Assistance Shared Information System is an online platform that allows users to apply for grants under the Asia-Pacific Regional Initiative. In 2013, I started the Burn Scar Treatment/Restorative Medicine exchange with a grant under this program. A multidisciplinary team representing the specialties of dermatology, hand surgery, plastic surgery, physical medicine and rehabilitation, and pulmonary critical care participated in the 2013 Asia Pacific Burn Congress hosted by the National Institute of Burns (NIB) in Hanoi, Vietnam, and then followed up with didactics and patient care alongside Vietnamese physicians in the management of disfiguring and debilitating scars from burns and other trauma. This pilot project consisted of three 2- to 3-week phases: 2 at the NIB in Hanoi and 1 with a delegation from the NIB visiting the Naval Medical Center San Diego. When initial project funds expired in 2014, the exchange was absorbed into Pacific Partnership 2014, which began a string of 4 consecutive annual Pacific Partnership engagements at Da Nang General Hospital in Vietnam. The 2 most recent exchanges, including the exchange associated with Pacific Partnership 2018, have taken place at Khanh Hoa General Hospital in Nha Trang, Vietnam. During this time the team has grown to include physical and occupational therapists as well as a wound care nurse.
The Burn Scar Treatment/Restorative Medicine exchange consists of side-by-side laser and surgical scar revision performed with our Vietnamese hosts in their own hospital. Our Vietnamese partners perform a large volume of reconstructive surgeries in their usual practice, so it truly has been a bilateral exchange incorporating some advanced technology and techniques with an emphasis on longitudinal multidisciplinary care. Importantly, the procedures are supplemented with preoperative and postoperative care as well as instruction provided by physical and occupational therapy and wound care professionals working alongside host nation support staff. Because the areas of involvement often are extensive and a patient may only be seen once in this setting, laser and surgical procedures often are performed concurrently in the host nation operating room. Anesthesia support is provided by the host nation. Basic consumable surgical supplies (eg, sutures, gloves, marking pens, staplers) are supplemented with mission funds. Special adjuncts for the most severe contractures have included negative pressure wound therapy and a collagen-based bilayer matrix wound dressing. Laser treatments have been performed on the vast majority of patients with an ablative fractional CO2 laser and laser-assisted delivery of corticosteroid in hypertrophic areas. Of note, use of the laser has been provided to our hosts by the manufacturer for each of the 7 iterations of the exchange, and the wound dressing manufacturer also has donated some of their product to the exchange through the nongovernmental organization Project Hope for 2 missions. To date, more than 300 patients have safely received life-changing treatment during the exchange, with some receiving multiple treatments (Figure). Although multiple treatments over time are ideal, even a single treatment session can result in considerable and lasting improvements in function and symptoms.17 The hospital ship USNS Mercy has the same laser technology and has brought advanced scar treatment techniques to the far corners of the Pacific.

Measuring overall success—treatment and international relations—in this setting can be challenging. On an individual patient level, the benefits of restoring the ability to walk and work as well as reducing pain and itching are manifest and transformative for both the patient and family; however, aggregating this information into high-quality outcome data is difficult given the heterogeneous nature of traumatic injuries, which is compounded in the setting of international engagement where the intersection between patient and visiting provider may be singular or difficult to predict, funding is limited, language frequently is a barrier, and documentation, privacy, and medical research guidelines may be unfamiliar or contradictory. The cumulative impact of these types of exchanges on the relationship between nations also is critical but difficult to measure. It is common sense that deepening personal and professional relationships in the medical setting over time can increase trust and mutual understanding, perhaps setting the stage for broader engagement in other more sensitive areas. Trust and understanding are rather nebulous concepts, but earlier this year marked the first visit of an American aircraft carrier to Da Nang since 1975, following 4 consecutive annual Pacific Partnership missions in the same city, which does carry the patina of successful engagement on a systemic level.
Final Thoughts
Based on my personal experience, I provide the following tips for building a successful, focused, long-term medical exchange.
- Leverage your strengths and respect the strengths and style of practice of your hosts. A mind-set of exchange and not simply humanitarian care will be more successful. Your hosts are experts in a style of practice adapted to their surroundings and introducing new techniques that are grounded in the local practice patterns are more likely to be perpetuated.
- Collaboration with nongovernmental organizations and industry can be extremely helpful. Military and governmental organizations often are limited in funding, in the ways they can spend available funding, and in the receipt of donations. Appropriate coordination with civilian entities can elevate the exchange considerably by adding expertise and available assets as well as broadening the overall impact.
- Engage the support staff as well as the physicians. You will leverage contact with families and enhance care over the long-term.
- The benefits of multiple interactions over time are manifest, for both the patients and the participants. Personal and professional relationships are intertwined and naturally mature over time. Go for singles and doubles first before swinging for the fences.
- Multidisciplinary work overseas informs and enhances collaboration at home.
- Adding regional experts in international research and assessment to these specialized medical teams may better capture the impact of future exchanges of any flavor.
- The model of creating a focused exchange with independent funding followed by incorporation of successful concepts into larger missions seems to be a worthy and reproducible approach for future projects of any variety.
Over the last decade the treatment of traumatic scars with lasers has emerged as a core component of multidisciplinary management. Military dermatologists have played a fundamental role in this shift by helping to develop new applications for existing technology and promulgate the techniques to reach additional providers and patients. Beyond scar management, the repurposing of adjunctive procedural techniques, such as sweat and hair reduction in amputees, also promises to enhance rehabilitation for many patients.
International engagement is a prominent and highly attractive feature of military practice, and military dermatologists routinely participate in disaster response missions, such as the 2010 Haiti earthquake,1 and ongoing planned operations, such as Pacific Partnership in the Indo-Asia-Pacific region led by the US Navy.2 In this article, I present a military perspective on the emerging niche of trauma dermatology and outline my more than 5 years of experience leveraging these skills to lead a multidisciplinary exchange in restorative medicine and burn scar management in Vietnam.
Trauma Dermatology
Over the course of the last decade, traumatic scar management has emerged as a staple of dermatologic surgery practice in some centers. Dermatologists hold the key to increasing patient access to effective outpatient care for symptomatic traumatic scars and other related issues using devices and techniques initially conceived for cosmetic applications.3 A major impetus for the considerable remodeling in our collective thoughts about traumatic scar management was the emergence of fractional laser technology in the mid-2000s. The remarkable, safe, reproducible, and durable benefits of fractional laser treatment of various scar types have created substantial momentum in recent years. The Naval Medical Center San Diego in California houses 1 of 3 centers of excellence in rehabilitation in the US military. Mastery of minimally invasive procedures to manage scars and other issues associated with trauma for the first time has established dermatologists as important partners in the overall rehabilitative effort.
My perspective on laser scar management has been previously described.4,5 Ablative fractional laser resurfacing is the backbone of rehabilitative scar management.6 Although the literature in this field is still relatively immature, higher-quality studies are accumulating rapidly as the burn and surgical communities adopt the procedure more widely.7-10 A considerable step forward in the dissemination of the procedure occurred recently with the development of category III Current Procedural Terminology (CPT) codes for ablative laser treatment of traumatic scars.11 Category III CPT codes are temporary codes used for emerging procedures that have not yet been deemed medically necessary. Although individual insurance carriers can determine whether to cover these procedures and the corresponding level of reimbursement, regular use is important for ultimate elevation to category I codes by the American Medical Association over a 5-year observation period. The CPT codes 0479T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; first 100 cm2 or part thereof, or 1% of body surface area of infants and children) and 0480T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; each additional 100 cm2, or each additional 1% of body surface area of infants and children, or part thereof [list separately in addition to code for primary procedure]) are examples of these category III codes.11
Nonablative fractional lasers; vascular-specific devices for erythematous scars; and long- and short-pulsed pigment-specific devices for hair and traumatic tattoo treatment, respectively, round out the commonly used laser platforms. For example, laser hair reduction can help improve the fit and comfort of prosthetic devices and has been shown to improve the overall quality of life for amputees.12 Botulinum toxin can be an important component of treatment of excessive sweating induced by occlusive liners in prosthetics, and microwave eccrine ablation is an emerging potential option for longer-lasting sweat reduction in this population.13-15 In addition to providing direct dermatology care and education, having members of the specialty in uniform has been a key to adopting new practical solutions to unsolved problems.
Pacific Partnership
Pacific Partnership is the largest annual multinational humanitarian assistance and disaster preparedness mission in the Indo-Asia-Pacific region.16 It was started in 2006 following the tsunami that devastated parts of South and Southeast Asia in 2004. The recently concluded Pacific Partnership 2018 marked the 13th iteration of the annual mission led by the US Navy in collaboration with other partner nations, which in 2018 included Japan, Australia, Canada, the United Kingdom, France, Singapore, Korea, and Peru, as well as nongovernmental organizations and international governmental agencies. Host nation mission locations vary somewhat from year to year, but 2018 included visits of the hospital ship USNS Mercy and more than 800 personnel to Indonesia, Malaysia, Sri Lanka, and Vietnam. Medical/dental, engineering, and veterinary teams join with their counterparts in each host nation to conduct civic action projects, community health exchanges, medical care, and disaster response training activities.16
Rehabilitation As a Vehicle for Medical Exchange
Since approximately 2012 there has been an evolving paradigm in Pacific Partnership from an emphasis on maximizing direct patient care in changing locations to one focused on building lasting partnerships through subject matter expert exchange. Multidisciplinary scar management, including surgical and laser scar revision and physical and occupational therapy, is a very promising model for engagement. Potential advantages of this type of exchange include the following: developing nations have relatively high rates of burns and other forms of trauma as well as uneven access to acute and ongoing rehabilitative care; patients often are otherwise healthy and young; results are frequently profound and readily demonstrable; and it is a skill set that has become highly developed in the military system. Just as dermatologists are illustrating their utility in trauma rehabilitation at home, these procedural skills provide fertile ground for exchange overseas.
The Overseas Humanitarian Assistance Shared Information System is an online platform that allows users to apply for grants under the Asia-Pacific Regional Initiative. In 2013, I started the Burn Scar Treatment/Restorative Medicine exchange with a grant under this program. A multidisciplinary team representing the specialties of dermatology, hand surgery, plastic surgery, physical medicine and rehabilitation, and pulmonary critical care participated in the 2013 Asia Pacific Burn Congress hosted by the National Institute of Burns (NIB) in Hanoi, Vietnam, and then followed up with didactics and patient care alongside Vietnamese physicians in the management of disfiguring and debilitating scars from burns and other trauma. This pilot project consisted of three 2- to 3-week phases: 2 at the NIB in Hanoi and 1 with a delegation from the NIB visiting the Naval Medical Center San Diego. When initial project funds expired in 2014, the exchange was absorbed into Pacific Partnership 2014, which began a string of 4 consecutive annual Pacific Partnership engagements at Da Nang General Hospital in Vietnam. The 2 most recent exchanges, including the exchange associated with Pacific Partnership 2018, have taken place at Khanh Hoa General Hospital in Nha Trang, Vietnam. During this time the team has grown to include physical and occupational therapists as well as a wound care nurse.
The Burn Scar Treatment/Restorative Medicine exchange consists of side-by-side laser and surgical scar revision performed with our Vietnamese hosts in their own hospital. Our Vietnamese partners perform a large volume of reconstructive surgeries in their usual practice, so it truly has been a bilateral exchange incorporating some advanced technology and techniques with an emphasis on longitudinal multidisciplinary care. Importantly, the procedures are supplemented with preoperative and postoperative care as well as instruction provided by physical and occupational therapy and wound care professionals working alongside host nation support staff. Because the areas of involvement often are extensive and a patient may only be seen once in this setting, laser and surgical procedures often are performed concurrently in the host nation operating room. Anesthesia support is provided by the host nation. Basic consumable surgical supplies (eg, sutures, gloves, marking pens, staplers) are supplemented with mission funds. Special adjuncts for the most severe contractures have included negative pressure wound therapy and a collagen-based bilayer matrix wound dressing. Laser treatments have been performed on the vast majority of patients with an ablative fractional CO2 laser and laser-assisted delivery of corticosteroid in hypertrophic areas. Of note, use of the laser has been provided to our hosts by the manufacturer for each of the 7 iterations of the exchange, and the wound dressing manufacturer also has donated some of their product to the exchange through the nongovernmental organization Project Hope for 2 missions. To date, more than 300 patients have safely received life-changing treatment during the exchange, with some receiving multiple treatments (Figure). Although multiple treatments over time are ideal, even a single treatment session can result in considerable and lasting improvements in function and symptoms.17 The hospital ship USNS Mercy has the same laser technology and has brought advanced scar treatment techniques to the far corners of the Pacific.

Measuring overall success—treatment and international relations—in this setting can be challenging. On an individual patient level, the benefits of restoring the ability to walk and work as well as reducing pain and itching are manifest and transformative for both the patient and family; however, aggregating this information into high-quality outcome data is difficult given the heterogeneous nature of traumatic injuries, which is compounded in the setting of international engagement where the intersection between patient and visiting provider may be singular or difficult to predict, funding is limited, language frequently is a barrier, and documentation, privacy, and medical research guidelines may be unfamiliar or contradictory. The cumulative impact of these types of exchanges on the relationship between nations also is critical but difficult to measure. It is common sense that deepening personal and professional relationships in the medical setting over time can increase trust and mutual understanding, perhaps setting the stage for broader engagement in other more sensitive areas. Trust and understanding are rather nebulous concepts, but earlier this year marked the first visit of an American aircraft carrier to Da Nang since 1975, following 4 consecutive annual Pacific Partnership missions in the same city, which does carry the patina of successful engagement on a systemic level.
Final Thoughts
Based on my personal experience, I provide the following tips for building a successful, focused, long-term medical exchange.
- Leverage your strengths and respect the strengths and style of practice of your hosts. A mind-set of exchange and not simply humanitarian care will be more successful. Your hosts are experts in a style of practice adapted to their surroundings and introducing new techniques that are grounded in the local practice patterns are more likely to be perpetuated.
- Collaboration with nongovernmental organizations and industry can be extremely helpful. Military and governmental organizations often are limited in funding, in the ways they can spend available funding, and in the receipt of donations. Appropriate coordination with civilian entities can elevate the exchange considerably by adding expertise and available assets as well as broadening the overall impact.
- Engage the support staff as well as the physicians. You will leverage contact with families and enhance care over the long-term.
- The benefits of multiple interactions over time are manifest, for both the patients and the participants. Personal and professional relationships are intertwined and naturally mature over time. Go for singles and doubles first before swinging for the fences.
- Multidisciplinary work overseas informs and enhances collaboration at home.
- Adding regional experts in international research and assessment to these specialized medical teams may better capture the impact of future exchanges of any flavor.
- The model of creating a focused exchange with independent funding followed by incorporation of successful concepts into larger missions seems to be a worthy and reproducible approach for future projects of any variety.
- Galeckas K. Dermatology aboard the USNS Comfort: disaster relief operations in Haiti after the 2010 earthquake. Dermatol Clin. 2011;29:15-19.
- Satter EK. The role of the dermatologist on military humanitarian missions. Cutis. 2010;85:85-89.
- Miletta NR, Donelan MB, Hivnor CM. Management of trauma and burn scars; the dermatologist's role in expanding patient access to care. Cutis. 2017;100:18-20.
- Shumaker PR. Laser treatment of traumatic scars: a military perspective. Semin Cutan Med Surg. 2015;34:17-23.
- Shumaker PR, Beachkofsky T, Basnett A, et al. A military perspective. In: Krakowski AC, Shumaker PR, eds. The Scar Book: Formation, Mitigation, Rehabilitation and Prevention. Philadelphia, PA: Wolters Kluwer; 2017:327-338.
- Anderson RR, Donelan MB, Greeson E, et al. Consensus report: laser treatment of traumatic scars with an emphasis on ablative fractional resurfacing. JAMA Dermatol. 2014;150:187-193.
- Hultman CS, Friedstat JS, Edkins RE, et al. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before and after cohort study, with long-term follow-up. Ann Surg. 2014;260:519-532.
- Blome-Eberwein S, Gogal C, Weiss MJ, et al. Prospective evaluation of fractional CO2 laser treatment of mature burn scars. J Burn Care Res. 2016;37:379-387.
- Issler-Fisher AC, Fisher OM, Smialkowski AO, et al. Ablative fractional CO2 laser for burn scar reconstruction: an extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns. 2017;43:573-582.
- Zuccaro J, Zlolkowski N, Fish J. A systematic review of the effectiveness of laser therapy for hypertrophic burn scars. Clin Plast Surg. 2017;44:767-779.
- Miller A. CPT 2018: What's new, part 2. American Academy of Dermatology website. https://www.aad.org/dw/monthly/2018/january/cpt-2018-whats-new-part-2. Accessed July 24, 2018.
- Miletta NR, Kim S, Lezanski-Gujda A, et al. Improving health-related quality of life in wounded warriors: the promising benefits of laser hair removal to the residual limb-prosthetic interface. Dermatol Surg. 2016;42:1182-1187.
- Gratrix M, Hivnor C. Botulinum toxin for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- USNS Mercy deploys in support of Pacific Partnership 2018 [news release]. Washington, DC: US Department of Defense; February 26, 2018. https://www.defense.gov/News/Article/Article/1450292/usns-mercy-deploys-in-support-of-pacific-partnership-2018/. Accessed July 11, 2018.
- Burns C, Basnett A, Valentine J, et al. Ablative fractional resurfacing: a powerful tool to help restore form and function during international medical exchange. Lasers Surg Med. 2017;49:471-474.
- Galeckas K. Dermatology aboard the USNS Comfort: disaster relief operations in Haiti after the 2010 earthquake. Dermatol Clin. 2011;29:15-19.
- Satter EK. The role of the dermatologist on military humanitarian missions. Cutis. 2010;85:85-89.
- Miletta NR, Donelan MB, Hivnor CM. Management of trauma and burn scars; the dermatologist's role in expanding patient access to care. Cutis. 2017;100:18-20.
- Shumaker PR. Laser treatment of traumatic scars: a military perspective. Semin Cutan Med Surg. 2015;34:17-23.
- Shumaker PR, Beachkofsky T, Basnett A, et al. A military perspective. In: Krakowski AC, Shumaker PR, eds. The Scar Book: Formation, Mitigation, Rehabilitation and Prevention. Philadelphia, PA: Wolters Kluwer; 2017:327-338.
- Anderson RR, Donelan MB, Greeson E, et al. Consensus report: laser treatment of traumatic scars with an emphasis on ablative fractional resurfacing. JAMA Dermatol. 2014;150:187-193.
- Hultman CS, Friedstat JS, Edkins RE, et al. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before and after cohort study, with long-term follow-up. Ann Surg. 2014;260:519-532.
- Blome-Eberwein S, Gogal C, Weiss MJ, et al. Prospective evaluation of fractional CO2 laser treatment of mature burn scars. J Burn Care Res. 2016;37:379-387.
- Issler-Fisher AC, Fisher OM, Smialkowski AO, et al. Ablative fractional CO2 laser for burn scar reconstruction: an extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns. 2017;43:573-582.
- Zuccaro J, Zlolkowski N, Fish J. A systematic review of the effectiveness of laser therapy for hypertrophic burn scars. Clin Plast Surg. 2017;44:767-779.
- Miller A. CPT 2018: What's new, part 2. American Academy of Dermatology website. https://www.aad.org/dw/monthly/2018/january/cpt-2018-whats-new-part-2. Accessed July 24, 2018.
- Miletta NR, Kim S, Lezanski-Gujda A, et al. Improving health-related quality of life in wounded warriors: the promising benefits of laser hair removal to the residual limb-prosthetic interface. Dermatol Surg. 2016;42:1182-1187.
- Gratrix M, Hivnor C. Botulinum toxin for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- USNS Mercy deploys in support of Pacific Partnership 2018 [news release]. Washington, DC: US Department of Defense; February 26, 2018. https://www.defense.gov/News/Article/Article/1450292/usns-mercy-deploys-in-support-of-pacific-partnership-2018/. Accessed July 11, 2018.
- Burns C, Basnett A, Valentine J, et al. Ablative fractional resurfacing: a powerful tool to help restore form and function during international medical exchange. Lasers Surg Med. 2017;49:471-474.
Wound Closure Tips
What does your patient need to know preoperatively?
Patients should be educated on all aspects of the procedure as well as the expected postoperative course of healing. Manage patient expectations in advance to minimize any surprises for everyone involved. Swelling and bruising are not uncommon in the immediate postoperative phase, and for surgery near the eyes, both may be worse, making it prudent for patients to schedule any procedures after big events or vacations.
The sutured wound initially can appear lumpy, bumpy, and pink, and it may take potentially 3 to 6 months, or even longer, for the scar to fully mature depending on the type of repair performed. Sutured wounds require activity restrictions, which is especially important for young active patients as well as patients who may have labor-intensive occupations. I often recommend 1 to 2 weeks before resuming most forms of strenuous exercise and/or physical labor. Skin grafts may require even longer limitations. Although the overall risk for infection is low (approximately 1%), patients should be instructed to monitor for purulent drainage, fever, and worsening pain and redness, and to inform the dermatologist immediately of any concerning symptoms.
What is your go-to approach for wound closure?
My motto is: Simplest is often best. For the patient who prioritizes returning to full activity as soon as possible, the wound may be able to heal by secondary intention in select anatomic locations, and this approach can often yield excellent cosmetic results. If wound closure with sutures is indicated, then I use the following treatment algorithm:
- Primary closure is used if I can close a wound in a linear fashion without distorting free margins, especially if I can hide the lines within cosmetic subunit junctions and/or relaxed skin tension lines.
- Local flap is used for defects when repair in a linear fashion is not always ideal for various reasons. Recruit local skin with various flap options for the best color and texture match. This approach may be more involved but often provides the best long-term cosmetic outcome; however, it usually results in a longer recovery time and may even require staged procedures.
- Graft usually is our last preferred option because it may appear as a sewn-in patch; however, in certain anatomic locations and in the right patient, skin grafts also can yield acceptable cosmetic results.
I give trainees the following surgical technique pearls:
- Use buried vertical mattress sutures to achieve eversion of wound edges with deep sutures
- Dermal pulley as well as epidermal pulley sutures can offset tension wonderfully, especially in high-tension areas such as the back and scalp
- Placement of a running subcuticular suture in place of epidermal stitches on the trunk and extremities can prevent track marks
How do you keep patients compliant with wound care instructions?
Two keys to high patient compliance with wound care are making instructions as simple as possible and providing detailed written instructions. We instruct patients to keep the pressure dressing in place for 48 hours. Once removed, we recommend patients clean the wound with regular soap and water daily, followed by application of petrolatum ointment. For hard-to-reach areas or on non-hair-bearing skin, my surgical assistants apply adhesive strips over the sutures, eliminating the need for daily wound care. For full-thickness skin grafts, we commonly place a bolster pressure dressing that stays in place until the patient returns to our clinic for a postoperative visit. We provide every patient with detailed written instructions as a patient handout that is specific to the type of wound closure performed.
What do you do if the patient refuses your recommendation for wound closure?
It is important to explain all wound closure options to the patient and the risks and benefits of each. I always show patients the proposed plan using a mirror and/or textbook images so that they can better understand the process. In rare cases when the patient refuses the preferred method of closure, we ensure that he/she understands the advantages and disadvantages of the proposed procedure and why the recommendation was made. If the patient still refuses, we document our lengthy discussion in the medical record. For patients who refuse our recommended plan of sutures and opt to heal by secondary intention, we will see these patients almost weekly to ensure appropriate healing as well as provide further recommendations such as a delayed repair if there is any evidence of functional impairment and/or notable cosmetic implications. A patient completely refusing a planned repair is rare.
More commonly, patients request a "simpler" repair, even if the cosmetic outcome may be suboptimal. For example, some elderly patients with large nasal defects do not want to undergo a staged flap, even though it would give a superior cosmetic result. Instead, we do the best we can with a skin graft or single-stage flap.
What resources do you provide to patients for wound care instructions?
We recommend that physicians prepare comprehensive handouts on wound care instructions that address both short-term and long-term expectations, provide instructions regarding follow-up, and encourage good sun protection behaviors. Some physicians post videos demonstrating proper wound care on their websites, which may be another useful tool.
Acknowledgment
The author thanks Daniel Condie, MD (Dallas, Texas), for his contributions.
Suggested Readings
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part I. cutting tissue: incising, excising, and undermining. J Am Acad Dermatol. 2015;72:377-387.
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402.
What does your patient need to know preoperatively?
Patients should be educated on all aspects of the procedure as well as the expected postoperative course of healing. Manage patient expectations in advance to minimize any surprises for everyone involved. Swelling and bruising are not uncommon in the immediate postoperative phase, and for surgery near the eyes, both may be worse, making it prudent for patients to schedule any procedures after big events or vacations.
The sutured wound initially can appear lumpy, bumpy, and pink, and it may take potentially 3 to 6 months, or even longer, for the scar to fully mature depending on the type of repair performed. Sutured wounds require activity restrictions, which is especially important for young active patients as well as patients who may have labor-intensive occupations. I often recommend 1 to 2 weeks before resuming most forms of strenuous exercise and/or physical labor. Skin grafts may require even longer limitations. Although the overall risk for infection is low (approximately 1%), patients should be instructed to monitor for purulent drainage, fever, and worsening pain and redness, and to inform the dermatologist immediately of any concerning symptoms.
What is your go-to approach for wound closure?
My motto is: Simplest is often best. For the patient who prioritizes returning to full activity as soon as possible, the wound may be able to heal by secondary intention in select anatomic locations, and this approach can often yield excellent cosmetic results. If wound closure with sutures is indicated, then I use the following treatment algorithm:
- Primary closure is used if I can close a wound in a linear fashion without distorting free margins, especially if I can hide the lines within cosmetic subunit junctions and/or relaxed skin tension lines.
- Local flap is used for defects when repair in a linear fashion is not always ideal for various reasons. Recruit local skin with various flap options for the best color and texture match. This approach may be more involved but often provides the best long-term cosmetic outcome; however, it usually results in a longer recovery time and may even require staged procedures.
- Graft usually is our last preferred option because it may appear as a sewn-in patch; however, in certain anatomic locations and in the right patient, skin grafts also can yield acceptable cosmetic results.
I give trainees the following surgical technique pearls:
- Use buried vertical mattress sutures to achieve eversion of wound edges with deep sutures
- Dermal pulley as well as epidermal pulley sutures can offset tension wonderfully, especially in high-tension areas such as the back and scalp
- Placement of a running subcuticular suture in place of epidermal stitches on the trunk and extremities can prevent track marks
How do you keep patients compliant with wound care instructions?
Two keys to high patient compliance with wound care are making instructions as simple as possible and providing detailed written instructions. We instruct patients to keep the pressure dressing in place for 48 hours. Once removed, we recommend patients clean the wound with regular soap and water daily, followed by application of petrolatum ointment. For hard-to-reach areas or on non-hair-bearing skin, my surgical assistants apply adhesive strips over the sutures, eliminating the need for daily wound care. For full-thickness skin grafts, we commonly place a bolster pressure dressing that stays in place until the patient returns to our clinic for a postoperative visit. We provide every patient with detailed written instructions as a patient handout that is specific to the type of wound closure performed.
What do you do if the patient refuses your recommendation for wound closure?
It is important to explain all wound closure options to the patient and the risks and benefits of each. I always show patients the proposed plan using a mirror and/or textbook images so that they can better understand the process. In rare cases when the patient refuses the preferred method of closure, we ensure that he/she understands the advantages and disadvantages of the proposed procedure and why the recommendation was made. If the patient still refuses, we document our lengthy discussion in the medical record. For patients who refuse our recommended plan of sutures and opt to heal by secondary intention, we will see these patients almost weekly to ensure appropriate healing as well as provide further recommendations such as a delayed repair if there is any evidence of functional impairment and/or notable cosmetic implications. A patient completely refusing a planned repair is rare.
More commonly, patients request a "simpler" repair, even if the cosmetic outcome may be suboptimal. For example, some elderly patients with large nasal defects do not want to undergo a staged flap, even though it would give a superior cosmetic result. Instead, we do the best we can with a skin graft or single-stage flap.
What resources do you provide to patients for wound care instructions?
We recommend that physicians prepare comprehensive handouts on wound care instructions that address both short-term and long-term expectations, provide instructions regarding follow-up, and encourage good sun protection behaviors. Some physicians post videos demonstrating proper wound care on their websites, which may be another useful tool.
Acknowledgment
The author thanks Daniel Condie, MD (Dallas, Texas), for his contributions.
Suggested Readings
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part I. cutting tissue: incising, excising, and undermining. J Am Acad Dermatol. 2015;72:377-387.
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402.
What does your patient need to know preoperatively?
Patients should be educated on all aspects of the procedure as well as the expected postoperative course of healing. Manage patient expectations in advance to minimize any surprises for everyone involved. Swelling and bruising are not uncommon in the immediate postoperative phase, and for surgery near the eyes, both may be worse, making it prudent for patients to schedule any procedures after big events or vacations.
The sutured wound initially can appear lumpy, bumpy, and pink, and it may take potentially 3 to 6 months, or even longer, for the scar to fully mature depending on the type of repair performed. Sutured wounds require activity restrictions, which is especially important for young active patients as well as patients who may have labor-intensive occupations. I often recommend 1 to 2 weeks before resuming most forms of strenuous exercise and/or physical labor. Skin grafts may require even longer limitations. Although the overall risk for infection is low (approximately 1%), patients should be instructed to monitor for purulent drainage, fever, and worsening pain and redness, and to inform the dermatologist immediately of any concerning symptoms.
What is your go-to approach for wound closure?
My motto is: Simplest is often best. For the patient who prioritizes returning to full activity as soon as possible, the wound may be able to heal by secondary intention in select anatomic locations, and this approach can often yield excellent cosmetic results. If wound closure with sutures is indicated, then I use the following treatment algorithm:
- Primary closure is used if I can close a wound in a linear fashion without distorting free margins, especially if I can hide the lines within cosmetic subunit junctions and/or relaxed skin tension lines.
- Local flap is used for defects when repair in a linear fashion is not always ideal for various reasons. Recruit local skin with various flap options for the best color and texture match. This approach may be more involved but often provides the best long-term cosmetic outcome; however, it usually results in a longer recovery time and may even require staged procedures.
- Graft usually is our last preferred option because it may appear as a sewn-in patch; however, in certain anatomic locations and in the right patient, skin grafts also can yield acceptable cosmetic results.
I give trainees the following surgical technique pearls:
- Use buried vertical mattress sutures to achieve eversion of wound edges with deep sutures
- Dermal pulley as well as epidermal pulley sutures can offset tension wonderfully, especially in high-tension areas such as the back and scalp
- Placement of a running subcuticular suture in place of epidermal stitches on the trunk and extremities can prevent track marks
How do you keep patients compliant with wound care instructions?
Two keys to high patient compliance with wound care are making instructions as simple as possible and providing detailed written instructions. We instruct patients to keep the pressure dressing in place for 48 hours. Once removed, we recommend patients clean the wound with regular soap and water daily, followed by application of petrolatum ointment. For hard-to-reach areas or on non-hair-bearing skin, my surgical assistants apply adhesive strips over the sutures, eliminating the need for daily wound care. For full-thickness skin grafts, we commonly place a bolster pressure dressing that stays in place until the patient returns to our clinic for a postoperative visit. We provide every patient with detailed written instructions as a patient handout that is specific to the type of wound closure performed.
What do you do if the patient refuses your recommendation for wound closure?
It is important to explain all wound closure options to the patient and the risks and benefits of each. I always show patients the proposed plan using a mirror and/or textbook images so that they can better understand the process. In rare cases when the patient refuses the preferred method of closure, we ensure that he/she understands the advantages and disadvantages of the proposed procedure and why the recommendation was made. If the patient still refuses, we document our lengthy discussion in the medical record. For patients who refuse our recommended plan of sutures and opt to heal by secondary intention, we will see these patients almost weekly to ensure appropriate healing as well as provide further recommendations such as a delayed repair if there is any evidence of functional impairment and/or notable cosmetic implications. A patient completely refusing a planned repair is rare.
More commonly, patients request a "simpler" repair, even if the cosmetic outcome may be suboptimal. For example, some elderly patients with large nasal defects do not want to undergo a staged flap, even though it would give a superior cosmetic result. Instead, we do the best we can with a skin graft or single-stage flap.
What resources do you provide to patients for wound care instructions?
We recommend that physicians prepare comprehensive handouts on wound care instructions that address both short-term and long-term expectations, provide instructions regarding follow-up, and encourage good sun protection behaviors. Some physicians post videos demonstrating proper wound care on their websites, which may be another useful tool.
Acknowledgment
The author thanks Daniel Condie, MD (Dallas, Texas), for his contributions.
Suggested Readings
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part I. cutting tissue: incising, excising, and undermining. J Am Acad Dermatol. 2015;72:377-387.
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402.
Cost-effective wound healing described with fetal bovine collagen matrix
CHICAGO – A novel, commercially available fetal bovine collagen matrix provides “an ideal wound healing environment” for outpatient treatment of partial and full thickness wounds, ulcers, burns, and surgical wounds, Katarina R. Kesty, MD, declared at the annual meeting of the American College of Mohs Surgery.
“. We applied this product to 46 patients over 10 months and have observed favorable healing times and good cosmesis,” said Dr. Kesty, a dermatology resident at Wake Forest University, Winston-Salem, N.C.
She shared the clinical experience she and her colleagues have accrued with this product, which is called PriMatrix and is manufactured by Integra LifeSciences. She also explained how to successfully code and bill for its use.
“In-office application of this product is cost-effective when compared to similar products applied in the operating room by plastic surgeons and other specialists,” Dr. Kesty noted.
How cost-effective? She provided one example of a patient with a 12.6-cm2 defect on the scalp repaired with fetal bovine collagen matrix. Upon application of the appropriate billing codes, this repair was reimbursed by Medicare to the tune of $1,208. In contrast, another patient at Wake Forest had a 16.6-cm2 Mohs defect on the scalp repaired in the operating room by an oculoplastic surgeon who used split thickness skin grafts. For this procedure, Medicare was billed $30,805.11, and the medical center received $9,241.53 in reimbursement.
“An office repair using this fetal bovine collagen matrix is much more cost-effective,” she observed. “It also saves the patient from the risks of general anesthesia or conscious sedation.”
PriMatrix is a porous acellular collagen matrix derived from fetal bovine dermis. It contains type I and type III collagen, with the latter being particularly effective at attracting growth factors, blood, and angiogenic cytokines in support of dermal regeneration and revascularization. The product is available in solid sheets, mesh, and fenestrated forms in a variety of sizes. It needs to be rehydrated for 1 minute in room temperature saline. It can then be cut to the size of the wound and secured to the wound bed, periosteum, fascia, or cartilage with sutures or staples. The site is then covered with a thick layer of petrolatum and a tie-over bolster.
Dr. Kesty and her dermatology colleagues have applied the matrix to surgical defects ranging in size from 0.2 cm2 to 70 cm2, with an average area of 19 cm2. They have utilized the mesh format most often in order to allow drainage. They found the average healing time when the matrix was applied to exposed bone, periosteum, or perichondrium was 13.8 weeks, compared with 10.8 weeks for subcutaneous wounds.
With the use of the fetal bovine collagen matrix, wounds less than 10 cm2 in size healed in an average of 9.3 weeks, those from 10 cm2 to 25 cm2 in size healed in an average of 10.4 weeks, and wounds larger than 25 cm2 healed in an average of 15.7 weeks.
Coding and reimbursement
PriMatrix has been available for outpatient office use and reimbursement by Medicare since January 2017. Successful reimbursement requires completion of a preauthorization form, which is typically approved on the same day by Medicare and other payers. The proper CPT codes are 1527x, signifying a skin substitute graft less than 100 cm2 in size; Q4110 times the number of 1-cm2 units of PriMatrix utilized; and, when appropriate, ICD10 code Z85.828, for personal history of nonmelanoma skin cancer.
Dr. Kesty reported no financial conflicts of interest.
CHICAGO – A novel, commercially available fetal bovine collagen matrix provides “an ideal wound healing environment” for outpatient treatment of partial and full thickness wounds, ulcers, burns, and surgical wounds, Katarina R. Kesty, MD, declared at the annual meeting of the American College of Mohs Surgery.
“. We applied this product to 46 patients over 10 months and have observed favorable healing times and good cosmesis,” said Dr. Kesty, a dermatology resident at Wake Forest University, Winston-Salem, N.C.
She shared the clinical experience she and her colleagues have accrued with this product, which is called PriMatrix and is manufactured by Integra LifeSciences. She also explained how to successfully code and bill for its use.
“In-office application of this product is cost-effective when compared to similar products applied in the operating room by plastic surgeons and other specialists,” Dr. Kesty noted.
How cost-effective? She provided one example of a patient with a 12.6-cm2 defect on the scalp repaired with fetal bovine collagen matrix. Upon application of the appropriate billing codes, this repair was reimbursed by Medicare to the tune of $1,208. In contrast, another patient at Wake Forest had a 16.6-cm2 Mohs defect on the scalp repaired in the operating room by an oculoplastic surgeon who used split thickness skin grafts. For this procedure, Medicare was billed $30,805.11, and the medical center received $9,241.53 in reimbursement.
“An office repair using this fetal bovine collagen matrix is much more cost-effective,” she observed. “It also saves the patient from the risks of general anesthesia or conscious sedation.”
PriMatrix is a porous acellular collagen matrix derived from fetal bovine dermis. It contains type I and type III collagen, with the latter being particularly effective at attracting growth factors, blood, and angiogenic cytokines in support of dermal regeneration and revascularization. The product is available in solid sheets, mesh, and fenestrated forms in a variety of sizes. It needs to be rehydrated for 1 minute in room temperature saline. It can then be cut to the size of the wound and secured to the wound bed, periosteum, fascia, or cartilage with sutures or staples. The site is then covered with a thick layer of petrolatum and a tie-over bolster.
Dr. Kesty and her dermatology colleagues have applied the matrix to surgical defects ranging in size from 0.2 cm2 to 70 cm2, with an average area of 19 cm2. They have utilized the mesh format most often in order to allow drainage. They found the average healing time when the matrix was applied to exposed bone, periosteum, or perichondrium was 13.8 weeks, compared with 10.8 weeks for subcutaneous wounds.
With the use of the fetal bovine collagen matrix, wounds less than 10 cm2 in size healed in an average of 9.3 weeks, those from 10 cm2 to 25 cm2 in size healed in an average of 10.4 weeks, and wounds larger than 25 cm2 healed in an average of 15.7 weeks.
Coding and reimbursement
PriMatrix has been available for outpatient office use and reimbursement by Medicare since January 2017. Successful reimbursement requires completion of a preauthorization form, which is typically approved on the same day by Medicare and other payers. The proper CPT codes are 1527x, signifying a skin substitute graft less than 100 cm2 in size; Q4110 times the number of 1-cm2 units of PriMatrix utilized; and, when appropriate, ICD10 code Z85.828, for personal history of nonmelanoma skin cancer.
Dr. Kesty reported no financial conflicts of interest.
CHICAGO – A novel, commercially available fetal bovine collagen matrix provides “an ideal wound healing environment” for outpatient treatment of partial and full thickness wounds, ulcers, burns, and surgical wounds, Katarina R. Kesty, MD, declared at the annual meeting of the American College of Mohs Surgery.
“. We applied this product to 46 patients over 10 months and have observed favorable healing times and good cosmesis,” said Dr. Kesty, a dermatology resident at Wake Forest University, Winston-Salem, N.C.
She shared the clinical experience she and her colleagues have accrued with this product, which is called PriMatrix and is manufactured by Integra LifeSciences. She also explained how to successfully code and bill for its use.
“In-office application of this product is cost-effective when compared to similar products applied in the operating room by plastic surgeons and other specialists,” Dr. Kesty noted.
How cost-effective? She provided one example of a patient with a 12.6-cm2 defect on the scalp repaired with fetal bovine collagen matrix. Upon application of the appropriate billing codes, this repair was reimbursed by Medicare to the tune of $1,208. In contrast, another patient at Wake Forest had a 16.6-cm2 Mohs defect on the scalp repaired in the operating room by an oculoplastic surgeon who used split thickness skin grafts. For this procedure, Medicare was billed $30,805.11, and the medical center received $9,241.53 in reimbursement.
“An office repair using this fetal bovine collagen matrix is much more cost-effective,” she observed. “It also saves the patient from the risks of general anesthesia or conscious sedation.”
PriMatrix is a porous acellular collagen matrix derived from fetal bovine dermis. It contains type I and type III collagen, with the latter being particularly effective at attracting growth factors, blood, and angiogenic cytokines in support of dermal regeneration and revascularization. The product is available in solid sheets, mesh, and fenestrated forms in a variety of sizes. It needs to be rehydrated for 1 minute in room temperature saline. It can then be cut to the size of the wound and secured to the wound bed, periosteum, fascia, or cartilage with sutures or staples. The site is then covered with a thick layer of petrolatum and a tie-over bolster.
Dr. Kesty and her dermatology colleagues have applied the matrix to surgical defects ranging in size from 0.2 cm2 to 70 cm2, with an average area of 19 cm2. They have utilized the mesh format most often in order to allow drainage. They found the average healing time when the matrix was applied to exposed bone, periosteum, or perichondrium was 13.8 weeks, compared with 10.8 weeks for subcutaneous wounds.
With the use of the fetal bovine collagen matrix, wounds less than 10 cm2 in size healed in an average of 9.3 weeks, those from 10 cm2 to 25 cm2 in size healed in an average of 10.4 weeks, and wounds larger than 25 cm2 healed in an average of 15.7 weeks.
Coding and reimbursement
PriMatrix has been available for outpatient office use and reimbursement by Medicare since January 2017. Successful reimbursement requires completion of a preauthorization form, which is typically approved on the same day by Medicare and other payers. The proper CPT codes are 1527x, signifying a skin substitute graft less than 100 cm2 in size; Q4110 times the number of 1-cm2 units of PriMatrix utilized; and, when appropriate, ICD10 code Z85.828, for personal history of nonmelanoma skin cancer.
Dr. Kesty reported no financial conflicts of interest.
EXPERT ANALYSIS FROM THE ACMS ANNUAL MEETING
Buckwheat Extract
Native to North and East Asia, This highly adaptable plant – the most common species of which are Fagopyrum esculentum (common buckwheat or sweet buckwheat), and F. tataricum (which grows in more mountainous regions) – has acclimated to cultivation in North America, as well.1 Increasingly popular as a healthy grain option, buckwheat flour has been touted for beneficial effects on diabetes, obesity, hypertension, hypercholesterolemia, and constipation.1 It has also gained attention for its association with some allergic reactions.
Wound Healing
In 2008, van den Berg et al. performed an in vitro investigation of the antioxidant and anti-inflammatory qualities of buckwheat honey for consideration in wound healing. American buckwheat honey from New York was found to be the source of the most salient activities, with such properties attributed to its abundant phenolic components. The researchers suggested that these phenols might impart antibacterial activity, while the low pH and high free acid content of the buckwheat honey could contribute to healing wounds.4
Antioxidant Activity
The antioxidant capacity, along with other traits, characterizing the sprouts of common buckwheat (F. esculentum) and tartary buckwheat (F. tataricum) was evaluated by Liu et al. in 2008. Rutin is the main flavonoid found in both species, with fivefold higher levels identified in tartary buckwheat in this study. Ethanol extracts of tartary buckwheat also exhibited greater free radical scavenging activity and superoxide scavenging activity, compared with common buckwheat. Both buckwheat species displayed antioxidant activity on human hepatoma HepG2 cells, with tartary buckwheat more effective in diminishing cellular oxidative stress, which the authors attributed to its greater rutin and quercetin levels.5
Zhou et al. studied the protective effects of buckwheat honey on hydroxyl radical-induced DNA damage in 2012, finding that all studied honeys more effectively protected DNA in non–site specific rather than site-specific systems.6
Photoprotection
In a 2005 screening of 47 antioxidant substances and study of their effects on UV-induced lipid peroxidation, Trommer and Neubert reported that buckwheat extract significantly lowered radiation levels, as did extracts of St. John’s Wort, melissa, and sage. They concluded that their in vitro findings supported the inclusion of such ingredients in photoprotective cosmetic formulations or sunscreens pending the results of in vivo experiments with these compounds.7
In 2006, Hinneburg et al. evaluated the antioxidant and photoprotective activity of a buckwheat herb extract, also comparing its photoprotective characteristics to those of a commercial UV absorber. In an assay with 1,1-diphenyl-2-picryl-hydrazyl radical (DPPH), buckwheat extract exhibited significantly more antioxidant activity than did pure rutin, with buckwheat observed to more effectively block UV-induced peroxidation of linoleic acid as compared with rutin and the commercial UV absorber. The researchers concluded that including antioxidants such as buckwheat extract in photoprotective formulations may serve to maximize skin protection in such products.8
Buckwheat Sensitivity
Conclusion
Because it is a popular component in many diets around the world, especially Japan, Korea, Russia, and Poland, as well as other Asian and European countries, South Africa, Australia, and North America,4 it is reasonable to expect that we’ll see more research on buckwheat. For now, there are indications to suggest that more investigations are warranted to determine whether this botanical agent will have a meaningful role in the dermatologic armamentarium.
References
1. Li SQ et al. Crit Rev Food Sci Nutr. 2001 Sep;41(6):451-64.
2. Dattner AM. Dermatol Ther. 2003;16(2):106-13.
3. Hinneburg I et al. J Agric Food Chem. 2005 Jan 12;53(1):3-7.
4. van den Berg AJ et al. J Wound Care. 2008 Apr;17(4):172-4, 176-8.
5. Liu CL et al. J Agric Food Chem. 2008 Jan 9;56(1):173-8.
6. Zhou J et al. Food Chem Toxicol. 2012 Aug;50(8):2766-73.
7. Trommer H et al. J Pharm Pharm Sci. 2005 Sep 15;8(3):494-506.
8. Hinneburg I et al. Pharmazie. 2006 Mar;61(3):237-40.
9. Geiselhart S et al. Clin Exp Allergy. 2018 Feb;48(2):217-24.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also authored a New York Times Best Seller for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance Therapeutics. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
Native to North and East Asia, This highly adaptable plant – the most common species of which are Fagopyrum esculentum (common buckwheat or sweet buckwheat), and F. tataricum (which grows in more mountainous regions) – has acclimated to cultivation in North America, as well.1 Increasingly popular as a healthy grain option, buckwheat flour has been touted for beneficial effects on diabetes, obesity, hypertension, hypercholesterolemia, and constipation.1 It has also gained attention for its association with some allergic reactions.
Wound Healing
In 2008, van den Berg et al. performed an in vitro investigation of the antioxidant and anti-inflammatory qualities of buckwheat honey for consideration in wound healing. American buckwheat honey from New York was found to be the source of the most salient activities, with such properties attributed to its abundant phenolic components. The researchers suggested that these phenols might impart antibacterial activity, while the low pH and high free acid content of the buckwheat honey could contribute to healing wounds.4
Antioxidant Activity
The antioxidant capacity, along with other traits, characterizing the sprouts of common buckwheat (F. esculentum) and tartary buckwheat (F. tataricum) was evaluated by Liu et al. in 2008. Rutin is the main flavonoid found in both species, with fivefold higher levels identified in tartary buckwheat in this study. Ethanol extracts of tartary buckwheat also exhibited greater free radical scavenging activity and superoxide scavenging activity, compared with common buckwheat. Both buckwheat species displayed antioxidant activity on human hepatoma HepG2 cells, with tartary buckwheat more effective in diminishing cellular oxidative stress, which the authors attributed to its greater rutin and quercetin levels.5
Zhou et al. studied the protective effects of buckwheat honey on hydroxyl radical-induced DNA damage in 2012, finding that all studied honeys more effectively protected DNA in non–site specific rather than site-specific systems.6
Photoprotection
In a 2005 screening of 47 antioxidant substances and study of their effects on UV-induced lipid peroxidation, Trommer and Neubert reported that buckwheat extract significantly lowered radiation levels, as did extracts of St. John’s Wort, melissa, and sage. They concluded that their in vitro findings supported the inclusion of such ingredients in photoprotective cosmetic formulations or sunscreens pending the results of in vivo experiments with these compounds.7
In 2006, Hinneburg et al. evaluated the antioxidant and photoprotective activity of a buckwheat herb extract, also comparing its photoprotective characteristics to those of a commercial UV absorber. In an assay with 1,1-diphenyl-2-picryl-hydrazyl radical (DPPH), buckwheat extract exhibited significantly more antioxidant activity than did pure rutin, with buckwheat observed to more effectively block UV-induced peroxidation of linoleic acid as compared with rutin and the commercial UV absorber. The researchers concluded that including antioxidants such as buckwheat extract in photoprotective formulations may serve to maximize skin protection in such products.8
Buckwheat Sensitivity
Conclusion
Because it is a popular component in many diets around the world, especially Japan, Korea, Russia, and Poland, as well as other Asian and European countries, South Africa, Australia, and North America,4 it is reasonable to expect that we’ll see more research on buckwheat. For now, there are indications to suggest that more investigations are warranted to determine whether this botanical agent will have a meaningful role in the dermatologic armamentarium.
References
1. Li SQ et al. Crit Rev Food Sci Nutr. 2001 Sep;41(6):451-64.
2. Dattner AM. Dermatol Ther. 2003;16(2):106-13.
3. Hinneburg I et al. J Agric Food Chem. 2005 Jan 12;53(1):3-7.
4. van den Berg AJ et al. J Wound Care. 2008 Apr;17(4):172-4, 176-8.
5. Liu CL et al. J Agric Food Chem. 2008 Jan 9;56(1):173-8.
6. Zhou J et al. Food Chem Toxicol. 2012 Aug;50(8):2766-73.
7. Trommer H et al. J Pharm Pharm Sci. 2005 Sep 15;8(3):494-506.
8. Hinneburg I et al. Pharmazie. 2006 Mar;61(3):237-40.
9. Geiselhart S et al. Clin Exp Allergy. 2018 Feb;48(2):217-24.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also authored a New York Times Best Seller for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance Therapeutics. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
Native to North and East Asia, This highly adaptable plant – the most common species of which are Fagopyrum esculentum (common buckwheat or sweet buckwheat), and F. tataricum (which grows in more mountainous regions) – has acclimated to cultivation in North America, as well.1 Increasingly popular as a healthy grain option, buckwheat flour has been touted for beneficial effects on diabetes, obesity, hypertension, hypercholesterolemia, and constipation.1 It has also gained attention for its association with some allergic reactions.
Wound Healing
In 2008, van den Berg et al. performed an in vitro investigation of the antioxidant and anti-inflammatory qualities of buckwheat honey for consideration in wound healing. American buckwheat honey from New York was found to be the source of the most salient activities, with such properties attributed to its abundant phenolic components. The researchers suggested that these phenols might impart antibacterial activity, while the low pH and high free acid content of the buckwheat honey could contribute to healing wounds.4
Antioxidant Activity
The antioxidant capacity, along with other traits, characterizing the sprouts of common buckwheat (F. esculentum) and tartary buckwheat (F. tataricum) was evaluated by Liu et al. in 2008. Rutin is the main flavonoid found in both species, with fivefold higher levels identified in tartary buckwheat in this study. Ethanol extracts of tartary buckwheat also exhibited greater free radical scavenging activity and superoxide scavenging activity, compared with common buckwheat. Both buckwheat species displayed antioxidant activity on human hepatoma HepG2 cells, with tartary buckwheat more effective in diminishing cellular oxidative stress, which the authors attributed to its greater rutin and quercetin levels.5
Zhou et al. studied the protective effects of buckwheat honey on hydroxyl radical-induced DNA damage in 2012, finding that all studied honeys more effectively protected DNA in non–site specific rather than site-specific systems.6
Photoprotection
In a 2005 screening of 47 antioxidant substances and study of their effects on UV-induced lipid peroxidation, Trommer and Neubert reported that buckwheat extract significantly lowered radiation levels, as did extracts of St. John’s Wort, melissa, and sage. They concluded that their in vitro findings supported the inclusion of such ingredients in photoprotective cosmetic formulations or sunscreens pending the results of in vivo experiments with these compounds.7
In 2006, Hinneburg et al. evaluated the antioxidant and photoprotective activity of a buckwheat herb extract, also comparing its photoprotective characteristics to those of a commercial UV absorber. In an assay with 1,1-diphenyl-2-picryl-hydrazyl radical (DPPH), buckwheat extract exhibited significantly more antioxidant activity than did pure rutin, with buckwheat observed to more effectively block UV-induced peroxidation of linoleic acid as compared with rutin and the commercial UV absorber. The researchers concluded that including antioxidants such as buckwheat extract in photoprotective formulations may serve to maximize skin protection in such products.8
Buckwheat Sensitivity
Conclusion
Because it is a popular component in many diets around the world, especially Japan, Korea, Russia, and Poland, as well as other Asian and European countries, South Africa, Australia, and North America,4 it is reasonable to expect that we’ll see more research on buckwheat. For now, there are indications to suggest that more investigations are warranted to determine whether this botanical agent will have a meaningful role in the dermatologic armamentarium.
References
1. Li SQ et al. Crit Rev Food Sci Nutr. 2001 Sep;41(6):451-64.
2. Dattner AM. Dermatol Ther. 2003;16(2):106-13.
3. Hinneburg I et al. J Agric Food Chem. 2005 Jan 12;53(1):3-7.
4. van den Berg AJ et al. J Wound Care. 2008 Apr;17(4):172-4, 176-8.
5. Liu CL et al. J Agric Food Chem. 2008 Jan 9;56(1):173-8.
6. Zhou J et al. Food Chem Toxicol. 2012 Aug;50(8):2766-73.
7. Trommer H et al. J Pharm Pharm Sci. 2005 Sep 15;8(3):494-506.
8. Hinneburg I et al. Pharmazie. 2006 Mar;61(3):237-40.
9. Geiselhart S et al. Clin Exp Allergy. 2018 Feb;48(2):217-24.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also authored a New York Times Best Seller for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance Therapeutics. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
Collagen remodeling observed after laser treatment in EB patient
DALLAS – Fractional led to considerable clinical improvement, including thickening of the dermis, results from a case report showed.
“We have so much more to learn about how the laser treatments are modifying these intricate pathways,” lead study author Samantha Schneider, MD, said in an interview following the annual conference of the American Society for Laser Medicine and Surgery. “But, our project suggests that patients with genetic blistering diseases may benefit from fractional laser therapy in combination with topical PLLA. This may be a good option, particularly for patients who are looking for more therapeutic options for slowly healing wounds and have exhausted other more conventional treatment modalities.”
Drawing from this previous work, Dr. Schneider and her associates hypothesized that fractional ablative laser treatment and topical PLLA might help a 27-year-old RDEB patient with revertant mosaicism who presented for management of large, nonhealing erosions on her upper back and posterior neck, complicated by frequent Staphylococcus infections. Over a 2-year period the researchers administered 15 fractional CO2 laser treatments with a single-pulse, nonoverlapping technique with settings of 15 mJ of energy and 15% density. They immediately applied concentrated topical PLLA to the treated area and obtained punch biopsy specimens from treated and untreated affected skin and clinically normal-appearing skin after the seventh treatment for histopathologic and immunohistologic examination.
Since the time of treatment, the patient reported marked improvement with a decreased number of erosions, as well as decreased pain. In addition, the hematoxylin and eosin slides showed increased collagen I (mature collagen) in the treated sample, “which suggests that we may be inducing a type of neocollagenesis, which is exciting particularly if it seems to work for patients with genetic alterations in collagen,” Dr. Schneider said. “Additionally, the indirect immunofluorescence [IIF] showed increased collagen VII, which is absent in the patient’s untreated skin. This was truly surprising and warrants more investigation as to how we may be affecting patients’ biology with this combination treatment.”
She acknowledged that more studies are required to confirm the findings. “Furthermore, we did not examine the fractional laser therapy and the topical PLLA independently so we cannot say whether the effect is synergistic or due primarily to one modality versus the other,” she noted. “Lastly, the IIF interpretation was challenging particularly in the untreated skin due to the epidermal detachment and edge staining. However, when viewed in comparison to the treated skin, we noted increased collagen VII in the treated sample.”
Dr. Schneider reported having no relevant disclosures.
DALLAS – Fractional led to considerable clinical improvement, including thickening of the dermis, results from a case report showed.
“We have so much more to learn about how the laser treatments are modifying these intricate pathways,” lead study author Samantha Schneider, MD, said in an interview following the annual conference of the American Society for Laser Medicine and Surgery. “But, our project suggests that patients with genetic blistering diseases may benefit from fractional laser therapy in combination with topical PLLA. This may be a good option, particularly for patients who are looking for more therapeutic options for slowly healing wounds and have exhausted other more conventional treatment modalities.”
Drawing from this previous work, Dr. Schneider and her associates hypothesized that fractional ablative laser treatment and topical PLLA might help a 27-year-old RDEB patient with revertant mosaicism who presented for management of large, nonhealing erosions on her upper back and posterior neck, complicated by frequent Staphylococcus infections. Over a 2-year period the researchers administered 15 fractional CO2 laser treatments with a single-pulse, nonoverlapping technique with settings of 15 mJ of energy and 15% density. They immediately applied concentrated topical PLLA to the treated area and obtained punch biopsy specimens from treated and untreated affected skin and clinically normal-appearing skin after the seventh treatment for histopathologic and immunohistologic examination.
Since the time of treatment, the patient reported marked improvement with a decreased number of erosions, as well as decreased pain. In addition, the hematoxylin and eosin slides showed increased collagen I (mature collagen) in the treated sample, “which suggests that we may be inducing a type of neocollagenesis, which is exciting particularly if it seems to work for patients with genetic alterations in collagen,” Dr. Schneider said. “Additionally, the indirect immunofluorescence [IIF] showed increased collagen VII, which is absent in the patient’s untreated skin. This was truly surprising and warrants more investigation as to how we may be affecting patients’ biology with this combination treatment.”
She acknowledged that more studies are required to confirm the findings. “Furthermore, we did not examine the fractional laser therapy and the topical PLLA independently so we cannot say whether the effect is synergistic or due primarily to one modality versus the other,” she noted. “Lastly, the IIF interpretation was challenging particularly in the untreated skin due to the epidermal detachment and edge staining. However, when viewed in comparison to the treated skin, we noted increased collagen VII in the treated sample.”
Dr. Schneider reported having no relevant disclosures.
DALLAS – Fractional led to considerable clinical improvement, including thickening of the dermis, results from a case report showed.
“We have so much more to learn about how the laser treatments are modifying these intricate pathways,” lead study author Samantha Schneider, MD, said in an interview following the annual conference of the American Society for Laser Medicine and Surgery. “But, our project suggests that patients with genetic blistering diseases may benefit from fractional laser therapy in combination with topical PLLA. This may be a good option, particularly for patients who are looking for more therapeutic options for slowly healing wounds and have exhausted other more conventional treatment modalities.”
Drawing from this previous work, Dr. Schneider and her associates hypothesized that fractional ablative laser treatment and topical PLLA might help a 27-year-old RDEB patient with revertant mosaicism who presented for management of large, nonhealing erosions on her upper back and posterior neck, complicated by frequent Staphylococcus infections. Over a 2-year period the researchers administered 15 fractional CO2 laser treatments with a single-pulse, nonoverlapping technique with settings of 15 mJ of energy and 15% density. They immediately applied concentrated topical PLLA to the treated area and obtained punch biopsy specimens from treated and untreated affected skin and clinically normal-appearing skin after the seventh treatment for histopathologic and immunohistologic examination.
Since the time of treatment, the patient reported marked improvement with a decreased number of erosions, as well as decreased pain. In addition, the hematoxylin and eosin slides showed increased collagen I (mature collagen) in the treated sample, “which suggests that we may be inducing a type of neocollagenesis, which is exciting particularly if it seems to work for patients with genetic alterations in collagen,” Dr. Schneider said. “Additionally, the indirect immunofluorescence [IIF] showed increased collagen VII, which is absent in the patient’s untreated skin. This was truly surprising and warrants more investigation as to how we may be affecting patients’ biology with this combination treatment.”
She acknowledged that more studies are required to confirm the findings. “Furthermore, we did not examine the fractional laser therapy and the topical PLLA independently so we cannot say whether the effect is synergistic or due primarily to one modality versus the other,” she noted. “Lastly, the IIF interpretation was challenging particularly in the untreated skin due to the epidermal detachment and edge staining. However, when viewed in comparison to the treated skin, we noted increased collagen VII in the treated sample.”
Dr. Schneider reported having no relevant disclosures.
Key clinical point: Fractional ablative laser treatment combined with poly-L-lactic acid may aid in the care of certain patients with recessive dystrophic epidermolysis bullosa.
Major finding: Since the time of treatment, the patient reported marked improvement with a decreased number of erosions as well as decreased pain.
Study details: A case report of a 27-year-old recessive dystrophic epidermolysis bullosa patient with revertant mosaicism.
Disclosures: Dr. Schneider reported having no financial disclosures.
Early endovenous ablation speeds venous ulcer healing
Intervening early with endovenous ablation in patients with venous leg ulcers could significantly improve ulcer healing times and delay their recurrence, new research has found.
A randomized study presented at the International Charing Cross Symposium and published simultaneously in the April 24 issue of the New England Journal of Medicine compared the effects of early endovenous ablation with those of deferred ablation in 450 patients with venous leg ulcers, all of whom also received compression therapy.
The study showed that patients who received endovenous ablation within 2 weeks of randomization had significantly shorter healing times, compared with patients whose ablation was deferred for 6 months or until after the ulcer healed.
In the early-treatment group, the median time to ulcer healing was 56 days, while in the deferred-treatment group, it was 82 days. By 12 months, 93.8% of the early-intervention group had healed ulcers, compared with 85.8% in the deferred-intervention group.
Even after adjustment for factors such as patient age, ulcer size, ulcer duration, and recruitment center, patients who received early endovenous ablation were 38% more likely to have healed by 12 months, compared with the deferred-intervention group.
Researchers also saw significantly higher healing rates at 12 weeks in the early-intervention group, compared with the deferred-intervention group (63.5% vs. 51.6%, respectively).
“Observational studies have suggested that endovenous treatment of varicose veins – a treatment that may be particularly appropriate for the elderly population with venous leg ulcers – may improve ulcer healing,” wrote Manjit S. Gohel, MD, from the Cambridge (United Kingdom) University Hospitals NHS Foundation Trust and from Imperial College London and his coauthors. “In the current trial, we found that faster ulcer healing can be attained if an endovenous intervention is performed promptly.”
Early endovenous ablation also was associated with a delay in the recurrence of ulcers. The rate of recurrence was 11.4% among patients in the early-intervention group whose ulcers had healed and 16.5% among those in the delayed-intervention group whose ulcers had healed.
Patients who received the early endovenous ablation had a median ulcer-free time of 306 days, compared with 278 days in the delayed-intervention group, a significant difference.
The authors noted that all patients in the study also received high-quality compression therapy, which may account for the good healing rates seen in both groups that might not otherwise be observed in a real-world clinical setting.
“Accordingly, the improvement in ulcer healing with early endovenous intervention is likely to be greater in clinical practice than was observed in this trial,” the authors wrote. “Because endovenous intervention is usually performed as a single procedure, the clinical benefits are likely to be less dependent on ongoing patient adherence than they would be with compression therapy.”
The most common method for endovenous ablation used in this multicenter study was ultrasound-guided foam sclerotherapy, a minimally-invasive procedure the authors said had versatility and acceptability.
However, they commented that some previous, large randomized trials have suggested that the rates of complete venous occlusion are lower with foam sclerotherapy than with thermal ablation.
The main complications seen with endovenous ablation were pain and deep vein thrombosis.
The authors pointed out that two limitations of their trial were that patients with a leg ulcer that had been present for more than 6 months were excluded from patient selection and that the 450 patients enrolled had been selected from a larger group of around 6,500.
The study was supported by a grant from the National Institute for Health Research Health Technology Assessment Program. One author declared grants from a pharmaceutical company outside the submitted work, and seven declared funding from the NIHR as part of the conduct of the study. No other conflicts of interest were declared.
SOURCE: Gohel MS et al. NEJM. 2018 April 24. doi: 10.1056/NEJMoa1801214
Finally! A randomized controlled trial (RCT) which proves what we all kind of expected but which until now was unsupported by available literature. That is that endovenous ablation (EVA) in the presence of a concomitant venous ulcer not only decreases ulcer recurrence rates and increases ulcer-free time, it also significantly hastens ulcer healing times. I don’t know about you, but it always made sense to me that treatment of an incompetent saphenous vein, a known cause of ulceration, could be a factor in the time to ulcer healing.
But that’s what a whole host of retrospective and or nonrandomized studies seemed to suggest: Garbage in, garbage out. Enter the RCT – Issue resolved? Yes, with some caveats, and maybe no.
First, as the authors readily admit, the compression therapy which was applied to patients in both arms of the study was of “high quality” and would not likely be reproduced in real world practice. The authors also suggest that, in a real-world, clinical practice, the benefits of early EVA may prove to be even more pronounced because of poor patient compliance with compression. Not sure about that. In fact, if – in a real-world setting – the rate of compliance with compression in both groups turned out to be less than optimal, particularly in the patients who had EVA, the benefits of early ablation with respect to ulcer healing times might disappear.
In other words, we do not know from this study whether there would be the same advantages to early saphenous vein intervention without the addition of compression as compared with compression alone. This might explain why shorter ulcer healing times of EVA have been difficult to prove in non-RCT, more real-world studies. Perhaps a randomized trial comparing ulcer healing times with early EVA without compression versus compression therapy only? Hmmm.
Also, would the outcomes of the current study be similar on this side of the pond? Only 31.7% of limbs were treated with endothermal ablation only, by far the most common form of ablation performed in the United States. Almost 65% of limbs in the study were ablated with either foamed sclerotherapy alone or in conjunction with endothermal or mechanical modalities – not a common form of treatment here in the colonies. Inexplicably, the authors do not indicate whether outcomes were in any way influenced by the type of ablation performed. I am going to assume for now that it did not.
In summary, this study does not answer all the questions related to the use of EVA for the treatment of venous ulcers, but it comes pretty close. My take away is that there is no downside (or none that I can think of) to the use of EVA early on in the treatment of venous ulcers but a whole lot of potential upside for the patient. Now I, and probably you, have proof that what we were already doing really does have some increased benefit. Finally!
Alan M Dietzek, MD, is the Linda and Stephen R. Cohen Chair in Vascular Surgery at Danbury (Conn.) Hospital and a clinical professor of surgery at the University of Vermont, Burlington. He is also an associate medical editor for Vascular Specialist.
Finally! A randomized controlled trial (RCT) which proves what we all kind of expected but which until now was unsupported by available literature. That is that endovenous ablation (EVA) in the presence of a concomitant venous ulcer not only decreases ulcer recurrence rates and increases ulcer-free time, it also significantly hastens ulcer healing times. I don’t know about you, but it always made sense to me that treatment of an incompetent saphenous vein, a known cause of ulceration, could be a factor in the time to ulcer healing.
But that’s what a whole host of retrospective and or nonrandomized studies seemed to suggest: Garbage in, garbage out. Enter the RCT – Issue resolved? Yes, with some caveats, and maybe no.
First, as the authors readily admit, the compression therapy which was applied to patients in both arms of the study was of “high quality” and would not likely be reproduced in real world practice. The authors also suggest that, in a real-world, clinical practice, the benefits of early EVA may prove to be even more pronounced because of poor patient compliance with compression. Not sure about that. In fact, if – in a real-world setting – the rate of compliance with compression in both groups turned out to be less than optimal, particularly in the patients who had EVA, the benefits of early ablation with respect to ulcer healing times might disappear.
In other words, we do not know from this study whether there would be the same advantages to early saphenous vein intervention without the addition of compression as compared with compression alone. This might explain why shorter ulcer healing times of EVA have been difficult to prove in non-RCT, more real-world studies. Perhaps a randomized trial comparing ulcer healing times with early EVA without compression versus compression therapy only? Hmmm.
Also, would the outcomes of the current study be similar on this side of the pond? Only 31.7% of limbs were treated with endothermal ablation only, by far the most common form of ablation performed in the United States. Almost 65% of limbs in the study were ablated with either foamed sclerotherapy alone or in conjunction with endothermal or mechanical modalities – not a common form of treatment here in the colonies. Inexplicably, the authors do not indicate whether outcomes were in any way influenced by the type of ablation performed. I am going to assume for now that it did not.
In summary, this study does not answer all the questions related to the use of EVA for the treatment of venous ulcers, but it comes pretty close. My take away is that there is no downside (or none that I can think of) to the use of EVA early on in the treatment of venous ulcers but a whole lot of potential upside for the patient. Now I, and probably you, have proof that what we were already doing really does have some increased benefit. Finally!
Alan M Dietzek, MD, is the Linda and Stephen R. Cohen Chair in Vascular Surgery at Danbury (Conn.) Hospital and a clinical professor of surgery at the University of Vermont, Burlington. He is also an associate medical editor for Vascular Specialist.
Finally! A randomized controlled trial (RCT) which proves what we all kind of expected but which until now was unsupported by available literature. That is that endovenous ablation (EVA) in the presence of a concomitant venous ulcer not only decreases ulcer recurrence rates and increases ulcer-free time, it also significantly hastens ulcer healing times. I don’t know about you, but it always made sense to me that treatment of an incompetent saphenous vein, a known cause of ulceration, could be a factor in the time to ulcer healing.
But that’s what a whole host of retrospective and or nonrandomized studies seemed to suggest: Garbage in, garbage out. Enter the RCT – Issue resolved? Yes, with some caveats, and maybe no.
First, as the authors readily admit, the compression therapy which was applied to patients in both arms of the study was of “high quality” and would not likely be reproduced in real world practice. The authors also suggest that, in a real-world, clinical practice, the benefits of early EVA may prove to be even more pronounced because of poor patient compliance with compression. Not sure about that. In fact, if – in a real-world setting – the rate of compliance with compression in both groups turned out to be less than optimal, particularly in the patients who had EVA, the benefits of early ablation with respect to ulcer healing times might disappear.
In other words, we do not know from this study whether there would be the same advantages to early saphenous vein intervention without the addition of compression as compared with compression alone. This might explain why shorter ulcer healing times of EVA have been difficult to prove in non-RCT, more real-world studies. Perhaps a randomized trial comparing ulcer healing times with early EVA without compression versus compression therapy only? Hmmm.
Also, would the outcomes of the current study be similar on this side of the pond? Only 31.7% of limbs were treated with endothermal ablation only, by far the most common form of ablation performed in the United States. Almost 65% of limbs in the study were ablated with either foamed sclerotherapy alone or in conjunction with endothermal or mechanical modalities – not a common form of treatment here in the colonies. Inexplicably, the authors do not indicate whether outcomes were in any way influenced by the type of ablation performed. I am going to assume for now that it did not.
In summary, this study does not answer all the questions related to the use of EVA for the treatment of venous ulcers, but it comes pretty close. My take away is that there is no downside (or none that I can think of) to the use of EVA early on in the treatment of venous ulcers but a whole lot of potential upside for the patient. Now I, and probably you, have proof that what we were already doing really does have some increased benefit. Finally!
Alan M Dietzek, MD, is the Linda and Stephen R. Cohen Chair in Vascular Surgery at Danbury (Conn.) Hospital and a clinical professor of surgery at the University of Vermont, Burlington. He is also an associate medical editor for Vascular Specialist.
Intervening early with endovenous ablation in patients with venous leg ulcers could significantly improve ulcer healing times and delay their recurrence, new research has found.
A randomized study presented at the International Charing Cross Symposium and published simultaneously in the April 24 issue of the New England Journal of Medicine compared the effects of early endovenous ablation with those of deferred ablation in 450 patients with venous leg ulcers, all of whom also received compression therapy.
The study showed that patients who received endovenous ablation within 2 weeks of randomization had significantly shorter healing times, compared with patients whose ablation was deferred for 6 months or until after the ulcer healed.
In the early-treatment group, the median time to ulcer healing was 56 days, while in the deferred-treatment group, it was 82 days. By 12 months, 93.8% of the early-intervention group had healed ulcers, compared with 85.8% in the deferred-intervention group.
Even after adjustment for factors such as patient age, ulcer size, ulcer duration, and recruitment center, patients who received early endovenous ablation were 38% more likely to have healed by 12 months, compared with the deferred-intervention group.
Researchers also saw significantly higher healing rates at 12 weeks in the early-intervention group, compared with the deferred-intervention group (63.5% vs. 51.6%, respectively).
“Observational studies have suggested that endovenous treatment of varicose veins – a treatment that may be particularly appropriate for the elderly population with venous leg ulcers – may improve ulcer healing,” wrote Manjit S. Gohel, MD, from the Cambridge (United Kingdom) University Hospitals NHS Foundation Trust and from Imperial College London and his coauthors. “In the current trial, we found that faster ulcer healing can be attained if an endovenous intervention is performed promptly.”
Early endovenous ablation also was associated with a delay in the recurrence of ulcers. The rate of recurrence was 11.4% among patients in the early-intervention group whose ulcers had healed and 16.5% among those in the delayed-intervention group whose ulcers had healed.
Patients who received the early endovenous ablation had a median ulcer-free time of 306 days, compared with 278 days in the delayed-intervention group, a significant difference.
The authors noted that all patients in the study also received high-quality compression therapy, which may account for the good healing rates seen in both groups that might not otherwise be observed in a real-world clinical setting.
“Accordingly, the improvement in ulcer healing with early endovenous intervention is likely to be greater in clinical practice than was observed in this trial,” the authors wrote. “Because endovenous intervention is usually performed as a single procedure, the clinical benefits are likely to be less dependent on ongoing patient adherence than they would be with compression therapy.”
The most common method for endovenous ablation used in this multicenter study was ultrasound-guided foam sclerotherapy, a minimally-invasive procedure the authors said had versatility and acceptability.
However, they commented that some previous, large randomized trials have suggested that the rates of complete venous occlusion are lower with foam sclerotherapy than with thermal ablation.
The main complications seen with endovenous ablation were pain and deep vein thrombosis.
The authors pointed out that two limitations of their trial were that patients with a leg ulcer that had been present for more than 6 months were excluded from patient selection and that the 450 patients enrolled had been selected from a larger group of around 6,500.
The study was supported by a grant from the National Institute for Health Research Health Technology Assessment Program. One author declared grants from a pharmaceutical company outside the submitted work, and seven declared funding from the NIHR as part of the conduct of the study. No other conflicts of interest were declared.
SOURCE: Gohel MS et al. NEJM. 2018 April 24. doi: 10.1056/NEJMoa1801214
Intervening early with endovenous ablation in patients with venous leg ulcers could significantly improve ulcer healing times and delay their recurrence, new research has found.
A randomized study presented at the International Charing Cross Symposium and published simultaneously in the April 24 issue of the New England Journal of Medicine compared the effects of early endovenous ablation with those of deferred ablation in 450 patients with venous leg ulcers, all of whom also received compression therapy.
The study showed that patients who received endovenous ablation within 2 weeks of randomization had significantly shorter healing times, compared with patients whose ablation was deferred for 6 months or until after the ulcer healed.
In the early-treatment group, the median time to ulcer healing was 56 days, while in the deferred-treatment group, it was 82 days. By 12 months, 93.8% of the early-intervention group had healed ulcers, compared with 85.8% in the deferred-intervention group.
Even after adjustment for factors such as patient age, ulcer size, ulcer duration, and recruitment center, patients who received early endovenous ablation were 38% more likely to have healed by 12 months, compared with the deferred-intervention group.
Researchers also saw significantly higher healing rates at 12 weeks in the early-intervention group, compared with the deferred-intervention group (63.5% vs. 51.6%, respectively).
“Observational studies have suggested that endovenous treatment of varicose veins – a treatment that may be particularly appropriate for the elderly population with venous leg ulcers – may improve ulcer healing,” wrote Manjit S. Gohel, MD, from the Cambridge (United Kingdom) University Hospitals NHS Foundation Trust and from Imperial College London and his coauthors. “In the current trial, we found that faster ulcer healing can be attained if an endovenous intervention is performed promptly.”
Early endovenous ablation also was associated with a delay in the recurrence of ulcers. The rate of recurrence was 11.4% among patients in the early-intervention group whose ulcers had healed and 16.5% among those in the delayed-intervention group whose ulcers had healed.
Patients who received the early endovenous ablation had a median ulcer-free time of 306 days, compared with 278 days in the delayed-intervention group, a significant difference.
The authors noted that all patients in the study also received high-quality compression therapy, which may account for the good healing rates seen in both groups that might not otherwise be observed in a real-world clinical setting.
“Accordingly, the improvement in ulcer healing with early endovenous intervention is likely to be greater in clinical practice than was observed in this trial,” the authors wrote. “Because endovenous intervention is usually performed as a single procedure, the clinical benefits are likely to be less dependent on ongoing patient adherence than they would be with compression therapy.”
The most common method for endovenous ablation used in this multicenter study was ultrasound-guided foam sclerotherapy, a minimally-invasive procedure the authors said had versatility and acceptability.
However, they commented that some previous, large randomized trials have suggested that the rates of complete venous occlusion are lower with foam sclerotherapy than with thermal ablation.
The main complications seen with endovenous ablation were pain and deep vein thrombosis.
The authors pointed out that two limitations of their trial were that patients with a leg ulcer that had been present for more than 6 months were excluded from patient selection and that the 450 patients enrolled had been selected from a larger group of around 6,500.
The study was supported by a grant from the National Institute for Health Research Health Technology Assessment Program. One author declared grants from a pharmaceutical company outside the submitted work, and seven declared funding from the NIHR as part of the conduct of the study. No other conflicts of interest were declared.
SOURCE: Gohel MS et al. NEJM. 2018 April 24. doi: 10.1056/NEJMoa1801214
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Early endovenous ablation can speed healing of venous ulcers.
Major finding: Median ulcer healing time was 56 days with early venous ablation, compared with 82 days with deferred ablation.
Study details: Randomized controlled trial in 450 patients with venous leg ulcers.
Disclosures: The study was supported by a grant from the National Institute for Health Research Health Technology Assessment Program. One author declared grants from a pharmaceutical company outside the submitted work, and seven declared funding from the NIHR as part of the conduct of the study. No other conflicts of interest were declared.
Source: Gohel M et al. NEJM. 2018 April 24. doi: 10.1056/NEJMoa1801214