User login
Antibiotic use in dermatology declining, with one exception
Dermatologists are prescribing fewer antibiotics for acne and rosacea, but prescribing after dermatologic surgery has increased in the past decade.
In a study published online Jan. 16 in JAMA Dermatology, researchers report the results of a cross-sectional analysis of antibiotic prescribing by 11,986 dermatologists between 2008 and 2016, using commercial claims data.
The analysis showed that, over this period of time, the overall rate of antibiotic prescribing by dermatologists decreased by 36.6%, from 3.36 courses per 100 dermatologist visits to 2.13 courses. In particular, antibiotic prescribing for acne decreased by 28.1%, from 11.76 courses per 100 visits to 8.45 courses, and for rosacea it decreased by 18.1%, from 10.89 courses per 100 visits to 8.92 courses.
John S. Barbieri, MD, of the department of dermatology, University of Pennsylvania, and his coauthors described the overall decline in antibiotic prescribing as “encouraging,” considering that in 2013 dermatologists were identified as the “most frequent prescribers of oral antibiotics per clinician.” The decline resulted in an estimated 480,000 fewer antibiotic courses a year, they noted.
“Much of the decrease in extended courses of antibiotic therapy is associated with visits for acne and rosacea,” they wrote. “Although recent guidelines suggest limiting the duration of therapy in this patient population, course duration has remained stable over time, suggesting that this decrease may be due to fewer patients being treated with antibiotics rather than patients being treated for a shorter duration.”
However, the rate of oral antibiotic prescriptions associated with surgical visits increased by 69.6%, from 3.92 courses per 100 visits to 6.65. This increase was concerning, given the risk of surgical-site infections was low, the authors pointed out. “In addition, a 2008 advisory statement on antibiotic prophylaxis recommends single-dose perioperative antibiotics for patients at increased risk of surgical-site infection,” they added.
The study also noted a 35.3% increase in antibiotic prescribing for cysts and a 3.2% increase for hidradenitis suppurativa.
Over the entire study period, nearly 1 million courses of oral antibiotics were prescribed. Doxycycline hyclate accounted for around one quarter of prescriptions, as did minocycline, while 19.9% of prescriptions were for cephalexin.
“Given the low rate of infectious complications, even for Mohs surgery, and the lack of evidence to support the use of prolonged rather than single-dose perioperative regimens, the postoperative courses of antibiotics identified in this study may increase risks to patients without substantial benefits,” they added.
The study was partly supported by the National Institute of Arthritis and Musculoskeletal Skin Diseases. No conflicts of interest were declared.
SOURCE: Barbieri J et al. JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4944.
Reducing antibiotic prescribing in dermatology – as in so many other areas of medical practice – is a challenge, but there are a number of strategies that can help.
The first is to take a wait-and-see approach, which has been shown to be effective for childhood otitis media. Communication training for physicians can also help them to manage patient requests for antibiotics by working out the patient’s level of understanding of their condition and treatment options, and their expectations, and getting them to agree to keep antibiotics as a contingency plan. There are clinical decision support tools available to help physicians identify high-risk surgical patients who may require postoperative antibiotics.
It will help to have alternative treatment options for conditions such as acne and rosacea, such as better topical therapies, and an increase in clinical trials for these therapies will hopefully provide more options for patients.
Joslyn S. Kirby, MD, and Jordan S. Lim, MB, are in the department of dermatology, Penn State University, Hershey. These comments are taken from an accompanying editorial (JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4877). They had no disclosures.
Reducing antibiotic prescribing in dermatology – as in so many other areas of medical practice – is a challenge, but there are a number of strategies that can help.
The first is to take a wait-and-see approach, which has been shown to be effective for childhood otitis media. Communication training for physicians can also help them to manage patient requests for antibiotics by working out the patient’s level of understanding of their condition and treatment options, and their expectations, and getting them to agree to keep antibiotics as a contingency plan. There are clinical decision support tools available to help physicians identify high-risk surgical patients who may require postoperative antibiotics.
It will help to have alternative treatment options for conditions such as acne and rosacea, such as better topical therapies, and an increase in clinical trials for these therapies will hopefully provide more options for patients.
Joslyn S. Kirby, MD, and Jordan S. Lim, MB, are in the department of dermatology, Penn State University, Hershey. These comments are taken from an accompanying editorial (JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4877). They had no disclosures.
Reducing antibiotic prescribing in dermatology – as in so many other areas of medical practice – is a challenge, but there are a number of strategies that can help.
The first is to take a wait-and-see approach, which has been shown to be effective for childhood otitis media. Communication training for physicians can also help them to manage patient requests for antibiotics by working out the patient’s level of understanding of their condition and treatment options, and their expectations, and getting them to agree to keep antibiotics as a contingency plan. There are clinical decision support tools available to help physicians identify high-risk surgical patients who may require postoperative antibiotics.
It will help to have alternative treatment options for conditions such as acne and rosacea, such as better topical therapies, and an increase in clinical trials for these therapies will hopefully provide more options for patients.
Joslyn S. Kirby, MD, and Jordan S. Lim, MB, are in the department of dermatology, Penn State University, Hershey. These comments are taken from an accompanying editorial (JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4877). They had no disclosures.
Dermatologists are prescribing fewer antibiotics for acne and rosacea, but prescribing after dermatologic surgery has increased in the past decade.
In a study published online Jan. 16 in JAMA Dermatology, researchers report the results of a cross-sectional analysis of antibiotic prescribing by 11,986 dermatologists between 2008 and 2016, using commercial claims data.
The analysis showed that, over this period of time, the overall rate of antibiotic prescribing by dermatologists decreased by 36.6%, from 3.36 courses per 100 dermatologist visits to 2.13 courses. In particular, antibiotic prescribing for acne decreased by 28.1%, from 11.76 courses per 100 visits to 8.45 courses, and for rosacea it decreased by 18.1%, from 10.89 courses per 100 visits to 8.92 courses.
John S. Barbieri, MD, of the department of dermatology, University of Pennsylvania, and his coauthors described the overall decline in antibiotic prescribing as “encouraging,” considering that in 2013 dermatologists were identified as the “most frequent prescribers of oral antibiotics per clinician.” The decline resulted in an estimated 480,000 fewer antibiotic courses a year, they noted.
“Much of the decrease in extended courses of antibiotic therapy is associated with visits for acne and rosacea,” they wrote. “Although recent guidelines suggest limiting the duration of therapy in this patient population, course duration has remained stable over time, suggesting that this decrease may be due to fewer patients being treated with antibiotics rather than patients being treated for a shorter duration.”
However, the rate of oral antibiotic prescriptions associated with surgical visits increased by 69.6%, from 3.92 courses per 100 visits to 6.65. This increase was concerning, given the risk of surgical-site infections was low, the authors pointed out. “In addition, a 2008 advisory statement on antibiotic prophylaxis recommends single-dose perioperative antibiotics for patients at increased risk of surgical-site infection,” they added.
The study also noted a 35.3% increase in antibiotic prescribing for cysts and a 3.2% increase for hidradenitis suppurativa.
Over the entire study period, nearly 1 million courses of oral antibiotics were prescribed. Doxycycline hyclate accounted for around one quarter of prescriptions, as did minocycline, while 19.9% of prescriptions were for cephalexin.
“Given the low rate of infectious complications, even for Mohs surgery, and the lack of evidence to support the use of prolonged rather than single-dose perioperative regimens, the postoperative courses of antibiotics identified in this study may increase risks to patients without substantial benefits,” they added.
The study was partly supported by the National Institute of Arthritis and Musculoskeletal Skin Diseases. No conflicts of interest were declared.
SOURCE: Barbieri J et al. JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4944.
Dermatologists are prescribing fewer antibiotics for acne and rosacea, but prescribing after dermatologic surgery has increased in the past decade.
In a study published online Jan. 16 in JAMA Dermatology, researchers report the results of a cross-sectional analysis of antibiotic prescribing by 11,986 dermatologists between 2008 and 2016, using commercial claims data.
The analysis showed that, over this period of time, the overall rate of antibiotic prescribing by dermatologists decreased by 36.6%, from 3.36 courses per 100 dermatologist visits to 2.13 courses. In particular, antibiotic prescribing for acne decreased by 28.1%, from 11.76 courses per 100 visits to 8.45 courses, and for rosacea it decreased by 18.1%, from 10.89 courses per 100 visits to 8.92 courses.
John S. Barbieri, MD, of the department of dermatology, University of Pennsylvania, and his coauthors described the overall decline in antibiotic prescribing as “encouraging,” considering that in 2013 dermatologists were identified as the “most frequent prescribers of oral antibiotics per clinician.” The decline resulted in an estimated 480,000 fewer antibiotic courses a year, they noted.
“Much of the decrease in extended courses of antibiotic therapy is associated with visits for acne and rosacea,” they wrote. “Although recent guidelines suggest limiting the duration of therapy in this patient population, course duration has remained stable over time, suggesting that this decrease may be due to fewer patients being treated with antibiotics rather than patients being treated for a shorter duration.”
However, the rate of oral antibiotic prescriptions associated with surgical visits increased by 69.6%, from 3.92 courses per 100 visits to 6.65. This increase was concerning, given the risk of surgical-site infections was low, the authors pointed out. “In addition, a 2008 advisory statement on antibiotic prophylaxis recommends single-dose perioperative antibiotics for patients at increased risk of surgical-site infection,” they added.
The study also noted a 35.3% increase in antibiotic prescribing for cysts and a 3.2% increase for hidradenitis suppurativa.
Over the entire study period, nearly 1 million courses of oral antibiotics were prescribed. Doxycycline hyclate accounted for around one quarter of prescriptions, as did minocycline, while 19.9% of prescriptions were for cephalexin.
“Given the low rate of infectious complications, even for Mohs surgery, and the lack of evidence to support the use of prolonged rather than single-dose perioperative regimens, the postoperative courses of antibiotics identified in this study may increase risks to patients without substantial benefits,” they added.
The study was partly supported by the National Institute of Arthritis and Musculoskeletal Skin Diseases. No conflicts of interest were declared.
SOURCE: Barbieri J et al. JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4944.
FROM JAMA DERMATOLOGY
Key clinical point: Antibiotic prescriptions by dermatologists have decreased since 2008.
Major finding: Between 2008 and 2016, antibiotic prescriptions by dermatologists dropped by 36.6%.
Study details: Cross-sectional analysis of antibiotic prescribing by 11,986 dermatologists from 2008 to 2016.
Disclosures: The study was partly supported by the National Institute of Arthritis and Musculoskeletal Skin Diseases. The authors had no disclosures.
Source: Barbieri J et al. JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4944.
Combat Dermatology: The Role of the Deployed Army Dermatologist
Military dermatologists complete their residency training at 1 of 3 large military medical centers across the country: Walter Reed National Military Medical Center (Bethesda, Maryland), San Antonio Military Health System (San Antonio, Texas), or Naval Medical Center San Diego (San Diego, California). While in training, army dermatology residents in particular fall under the US Army Medical Command, or MEDCOM, which provides command and control of the army’s medical, dental, and veterinary treatment facilities. Upon graduating from residency, army dermatologists often are stationed with MEDCOM units but become eligible for deployment with US Army Forces Command (FORSCOM) units to both combat and noncombat zones depending on each individual FORSCOM unit’s mission.
The process by which dermatologists and other army physicians are tasked to a deploying FORSCOM unit is referred to as the Professional Filler System, or PROFIS, which was designed to help alleviate the financial cost and specialty skill degradation of having a physician assigned to a FORSCOM unit while not deployed.1 In general, the greater the amount of time that an army medical officer has not been deployed, the more likely they are to be selected for deployment with a FORSCOM unit. For the army dermatologist, deployment often comes shortly after completing residency or fellowship.
In this article, we review the various functions of the deployed dermatologist and also highlight the importance of maintaining basic emergency medical skills that could be generalized to the civilian population in case of local or national emergencies.
THE FIELD SURGEON
With rare exceptions, the US Army does not deploy dermatologists for their expertise in diagnosing and managing cutaneous diseases. Typically, a dermatologist will be assigned to a FORSCOM unit in the role of field surgeon. Other medical specialties including emergency medicine, family practice, internal medicine, pediatrics, and obstetrics and gynecology also are eligible for deployment as field surgeons.2 Field surgeons typically are assigned to a battalion-sized element of 300 to 1000 soldiers and are responsible for all medical care rendered under their supervision. Duties include combat resuscitation, primary care services, preventive medicine, medical training of battalion medical personnel, and serving as the medical adviser to the battalion commander.1 In some instances, a field surgeon will be stationed at a higher level of care co-located with a trauma surgeon; in those cases, the field surgeon also may be expected to assist in trauma surgery cases.
ARMY DEPLOYMENT MEDICAL SYSTEM
To better understand the responsibilities of a field surgeon, it is important to discuss the structure of the army’s deployment medical system. The US Military, including the army, has adopted a system of “roles” that have specific requirements regarding their associated medical capabilities.3 There are 4 roles designated within the army. Role 1 facilities are known as battalion aid stations (BASs).

Role of the Field Surgeon
Within the broader structure of the army, approximately 5 battalions (each composed of 300 to 1000 soldiers) comprise a single brigade combat team. Role 1 medical facilities typically have a single battalion surgeon assigned to them. Field surgeons most commonly serve in this battalion surgeon position. Additionally, Role 2 facilities may have slots for up to 2 battalion surgeons; however, field surgeons are less commonly tasked with this assignment.1 Occasionally, in one author’s (N.R.M.) personal experience, these roles are more fluid than one might expect. A field surgeon tasked initially with a Role 1 position may be shifted to a Role 2 assignment on an as-needed basis. This ability for rapid change in roles and responsibilities underscores the need for a fluid mind-set and thorough predeployment training for the field surgeon.
PREDEPLOYMENT TRAINING
As one might expect, dermatologists who have just graduated residency or fellowship are unlikely to have honed their trauma support skills to the degree needed to support a deployed battalion actively engaging in combat. Fortunately, there are many opportunities for military dermatologists to practice these skills prior to joining their FORSCOM colleagues. The initial exposure to trauma support comes during medical internship at the mandatory Combat Casualty Care Course (C4), an 8-day program designed to enhance the operational medical readiness and predeployment trauma training skills of medical officers.4 The C4 program includes 3 days of classroom training and 5 days of intensive field training. During C4, medical officers become certified in Advanced Trauma Life Support, a 3-day course organized by the American College of Surgeons.5 This course teaches medical officers how to quickly and judiciously triage, treat, and transport patients who have sustained potentially life-threatening traumas.
The next components of predeployment training, Tactical Combat Casualty Care and Tactical Combat Medical Care, occur in the months to weeks immediately preceding deployment.1,6 Tactical Combat Casualty Care prepares participants in the initial stabilization of trauma to occur at the point of injury.6 Tactical Combat Casualty Care principles generally are employed by medics (enlisted personnel trained in point-of-care medical support) rather than physicians; however, these principles are still critical for medical officers to be aware of when encountering severe traumas.6 In addition, the physician is responsible for ensuring his/her medics are fully trained in Tactical Combat Casualty Care. Tactical Combat Medical Care is geared more toward the direct preparation of medical officers. During the 5-day course, medical officers learn the gold standard for trauma care in both the classroom and in hands-on scenarios.1 This training not only allows medical officers to be self-sufficient in providing trauma support, but it also enables them to better maintain quality control of the performance of their medics continuously throughout the deployment.1
DEPLOYMENT RESPONSIBILITIES
Dermatologists who have completed the above training typically are subsequently deployed as field surgeons to a Role 1 facility. Field surgeons are designated as the officer in charge of the BAS and assume the position of medical platoon leader. A field surgeon usually will have both a physician assistant and a field medical assistant/medical plans officer (MEDO) to assist in running the BAS. The overarching goal of the field surgeon is to maintain the health and readiness of the battalion. In addition to addressing the day-to-day health care needs of individual soldiers, a field surgeon is expected to attend all staff meetings, advise the commander on preventative health and epidemiological trends, identify the scope of practice of the medics, ensure the BAS is prepared for mass casualties, and take responsibility for all controlled substances.
To illustrate the value that the properly trained dermatologist can provide in the deployed setting, we will outline field surgeon responsibilities and provide case examples of the first-hand experiences of one of the authors (N.R.M.) as a Role 2 officer in charge and field surgeon. The information presented in the case examples may have been altered to ensure continued operational security and out of respect to US servicemembers and coalition forces while still conveying important learning points.
Sick Call
In the deployed environment, military sick call functions as an urgent care center that is open continuously and serves the active-duty population, US government civilians and contractors, and coalition forces. In general, the physician assistant should treat approximately two-thirds of sick call patients under the supervision of the field surgeon, allowing the field surgeon to focus on his/her ancillary duties and ensure overall medical supervision of the unit. As a safeguard, patients with more than 2 visits for the same concern must be evaluated by the field surgeon. Sick call concerns range from minor traumas and illnesses to much more serious disease processes and injuries (as outlined in Medical Emergencies). As a field surgeon, it is critical to track disease nonbattle illnesses to ensure medical readiness of the unit. In the deployed environment, close quarters and austere environments commonly lend themselves to gastrointestinal illnesses, respiratory diseases, heat injuries, vector-borne diseases, and sexually transmitted infections.
Case Examples
During an 8-month deployment in Afghanistan, one of the authors (N.R.M.) provided or assisted in the care of more than 2300 routine sick call appointments, or approximately 10 patients per day. Epidemiology of disease was tracked, and the condition of the unit was presented daily to the battalion commander for consideration in upcoming operations. The top 5 most common categories of diagnoses included musculoskeletal injuries, gastrointestinal diseases, dermatologic concerns (eg, dermatitis, bacterial infections [cellulitis/abscess], fungal infections, arthropod assault, abrasions, lacerations, verruca vulgaris), respiratory illnesses, and mental health care, respectively. Maintaining a familiarity with general medicine is critical for the military dermatologist, and an adequate medical library or access to online medical review sources is critical for day-to-day sick call.
Medical Emergencies
In the event of a more serious injury or illness, a Role 1 BAS has very little capability in performing anything beyond the most basic interventions. Part of the art of being an effective field surgeon lies in stabilization, triage, and transport of these sometimes very ill patients. Both the decision to transport to a higher level of care (eg, Role 2 or 3 facility) as well as selection of the means of transportation falls on the field surgeon. The MEDO plays an essential role in assisting in the coordination of the transfer; however, the responsibility ultimately falls on the field surgeon.1,6 The field surgeon at the Role 2 BAS may be expected to perform more advanced medical and surgical interventions. More advanced pharmacotherapies include thrombolytics, antivenin, and vasopressors. Some procedural interventions include intubations, central lines, and laceration repairs. The Role 2 BAS has the capability to hold patients for up to 72 hours.
Case Examples
Specific conditions one of the authors (N.R.M.) treated include heat injury, myocardial infarction, disseminated tuberculosis, appendicitis, testicular torsion, malaria, suicidal ideation, burns, and status epilepticus. Over 8 months, the Role 2 BAS received 91 medical emergencies, with 53 necessitating evacuation to a higher level of care. Often, the more serious or rare conditions presented in the foreign contractor and coalition force populations working alongside US troops.
In one particular case, a 35-year-old man with an electrocardiogram-confirmed acute ST-segment elevation myocardial infarction was administered standard therapy consisting of intravenous morphine, oxygen, sublingual nitroglycerin, an angiotensin-converting enzyme inhibitor, and a beta-blocker. Given the lack of a cardiac catheterization laboratory at the next highest level of care as well as a low suspicion for aortic dissection (based on the patient’s history, physical examination, and chest radiograph), fibrinolysis with tenecteplase was performed in the deployed environment. After a very short observation for potential hemorrhage, the patient was then evacuated to the Role 3 hospital, where he made a near-complete recovery. Preparation with advanced cardiac life support courses and a thorough algorithmic review of the 10 most common causes of presentation to the emergency department helped adequately prepare the dermatologist to succeed.
Trauma Emergencies
The same principles of triage and transport apply to trauma emergencies. Mass casualties are an inevitable reality in combat, so appropriate training translating into efficient action is essential to ensure the lowest possible mortality. This training and the actions that stem from it are an additional responsibility that the field surgeon must maintain. During deployment, continued training organized by the field surgeon could quite literally mean the difference between life and death. In addition to the organizational responsibilities, field surgeons should be prepared to perform initial stabilization in trauma patients, including application of tourniquets, establishment of central lines, reading abdominal ultrasounds for free fluid, placement of chest tubes, intubation, and ventilator management. The Joint Trauma System Clinical Practice Guidelines also offer extensive and invaluable guidance on the most up-to-date approach to common trauma conditions arising in the deployed environment.7 At the Role 2 level, the field surgeon also must be prepared to coordinate ancillary services, manage the Role 2/forward surgical team intensive care unit, and serve as first assist in the operating room, as needed (Figure 2).

Case Examples
One of the authors (N.R.M.) assisted or provided care in approximately 225 trauma cases while deployed. A mass casualty event occurred, in which the Role 2 BAS received 34 casualties; of these casualties, 11 were immediate, 10 were delayed, 11 were minimal, and 2 were expectant. Injury patterns included mounted and dismounted improvised explosive device injuries (eg, blast, shrapnel, and traumatic brain injuries) as well as gunshot wounds. Direct care was provided for 13 casualties, including 10 abdominal ultrasound examinations for free fluid, placement of 2 chest tubes, 1 intubation, establishment of 3 central lines, and first-assisting 1 exploratory laparotomy. Of the casualties, 22 were evacuated to the Role 3 hospital, 8 were dispositioned to a coalition hospital, 2 were returned to active duty, and 2 died due to their injuries. The military trauma preparation as outlined in the predeployment training can help adequately prepare the military dermatologist to assist in these cases.
Ancillary Services
An important part of the efficacy of initial evaluation and stabilization of both medical and traumatic emergencies involves expedited laboratory tests, imaging, and the delivery of life-saving blood products to affected patients. The field surgeon is responsible for the readiness of these services and may play a critical role in streamlining these tasks for situations where a delay in care by minutes can be lethal. The MEDO assists the field surgeon to ensure the readiness of the medical equipment, and the field surgeon must ensure the readiness of the medics and technicians utilizing the equipment. In a deployed environment, only a finite amount of blood products may be stored. As a result, the design and implementation of an efficient and precise walking blood bank is critical. To help mitigate this issue, servicemembers are prescreened for their blood types and bloodborne illnesses. If a situation arises in which whole blood is needed, the prescreened individuals are screened again, and their blood is collected and transfused to the patient under the supervision of the physician. This task is critical in saving lives, and this process is the primary responsibility of the field surgeon.
Case Example
A 37-year-old man presented to the BAS with abdominal and pelvic gunshot wounds, as well as tachycardia, rapidly decreasing blood pressure, and altered consciousness. An exploratory laparotomy was performed to look for the sources of bleeding. The patient’s blood type was confirmed with a portable testing kit. Due to the injury pattern and clinical presentation, a call was immediately placed to begin screening and preparing servicemembers to donate blood for the walking blood bank. As expected, the Role 2 supply of blood products was exhausted during the exploratory laparotomy. With servicemembers in place and screened, an additional 12 units of whole blood were collected and administered in a timely fashion. The patient was stabilized and transported to the next highest level of care. Due to the process optimization performed by the laboratory team, whole-blood transfusions were ready within an average of 22 minutes, well ahead of the 45-minute standard of care.
Operating Room First Assist
If a field surgeon is stationed at a Role 2 BAS with a forward surgical team, he/she may be required to adopt the role of operating room first assist for the trauma surgeon or orthopedic surgeon on the team, which is especially true for isolated major traumas when triage and initial stabilization measures for multiple patients are of less concern. Dermatologists receive surgical training as part of the Accreditation Council for Graduate Medical Education requirements to graduate residency, making them more than capable of surgical assisting when needed.8 In particular, dermatologists’ ability to utilize instruments appropriately and think procedurally as well as their skills in suturing are helpful.
Case Example
A 22-year-old man with several shrapnel wounds to the abdomen demonstrated free fluid in the left lower quadrant. The field surgeon (N.R.M.) assisted the trauma surgeon in opening the abdomen and running the bowel for sources of bleeding. The trauma surgeon identified the bleed and performed a ligation. The patient was then packed, closed, and prepared for transfer to a higher level of care.
Preventive Medicine
As a result of the field surgeon being on the front line of medical care in an austere environment, implementation of preventive medicine practices and disease pattern recognition are his/her responsibility. Responsibilities may include stray animal euthanasia due to prevalence of rabies, enforcement of malaria prophylaxis, medical training and maintenance of snake antivenin, and assistance with other local endemic disease. The unique skill set of dermatologists in organism identification can further bolster the speed with which vector-borne diseases are recognized and prevention and treatment measures are implemented.
Case Example
As coalition forces executed a mission in Afghanistan, US servicemembers began experiencing abdominal distress, chills, fevers (temperature >40°C), debilitating headaches, myalgia, arthralgia, and tachycardia. Initially, these patients were evacuated to the Role 2 BAS, hindering the mission. Upon inspection, patients had numerous bug bites; one astute soldier collected the arthropod guilty of the assault and brought it to the aid station. Upon inspection, the offender was identified as the Phlebotomus genus of sandflies, organisms that are well known to dermatologists as a cause of leishmaniasis. Clinical correlation resulted in the presumed diagnosis of Pappataci fever, and vector-borne disease prevention measures were then able to be further emphasized and implemented in at-risk areas, allowing the mission to continue.9 Subsequent infectious disease laboratory testing confirmed the Phlebovirus transmitted by the sandfly as the underlying cause of the illness.
CONCLUSION
The diverse role of the field surgeon in the deployed setting makes any one specialist underprepared to completely take on the role from the outset; however, with appropriate and rigorous trauma training prior to deployment, dermatologists will continue to perform as invaluable assets to the US military in conflicts now and in the future.
1. Moawad FJ, Wilson R, Kunar MT, et al. Role of the battalion surgeon in the Iraq and Afghanistan War. Mil Med. 2012;177:412-416.
2. AR 601-142: Army Medical Department Professional Filler System. Washington, DC: US Department of the Army; 2015. http://cdm16635.contentdm.oclc.org/cdm/ref/collection/p16635coll11/id/4592. Accessed December 19, 2018.
3. Roles of medical care (United States). Emergency War Surgery. 4th ed. Fort Sam Houston, Texas: Office of the Surgeon General; 2013:17-28.
4. Combat Casualty Care Course (C4). Military Health System website. https://health.mil/Training-Center/Defense-Medical-Readiness-Training-Institute/Combat-Casualty-Care-Course. Accessed December 7, 2018.
5. Advanced Trauma Life Support. American College of Surgeons website. https://www.facs.org/quality-programs/trauma/atls. Accessed December 7, 2018.
6. Tactical Combat Casualty Care Course. Military Health System website. https://health.mil/Training-Center/Defense-Medical-Readiness-Training-Institute/Tactical-Combat-Casualty-Care-Course. Accessed December 18, 2018.
7. Joint Trauma System: The Department of Defense Center of Excellence for Trauma. Clinical Practice Guidelines.
8. ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Revised July 1, 2017. Accessed December 7, 2018.
9. Downs JW, Flood DT, Orr NH, et al. Sandfly fever in Afghanistan-a sometimes overlooked disease of military importance: a case series and review of the literature. US Army Med Dep J. 2017:60-66.
Military dermatologists complete their residency training at 1 of 3 large military medical centers across the country: Walter Reed National Military Medical Center (Bethesda, Maryland), San Antonio Military Health System (San Antonio, Texas), or Naval Medical Center San Diego (San Diego, California). While in training, army dermatology residents in particular fall under the US Army Medical Command, or MEDCOM, which provides command and control of the army’s medical, dental, and veterinary treatment facilities. Upon graduating from residency, army dermatologists often are stationed with MEDCOM units but become eligible for deployment with US Army Forces Command (FORSCOM) units to both combat and noncombat zones depending on each individual FORSCOM unit’s mission.
The process by which dermatologists and other army physicians are tasked to a deploying FORSCOM unit is referred to as the Professional Filler System, or PROFIS, which was designed to help alleviate the financial cost and specialty skill degradation of having a physician assigned to a FORSCOM unit while not deployed.1 In general, the greater the amount of time that an army medical officer has not been deployed, the more likely they are to be selected for deployment with a FORSCOM unit. For the army dermatologist, deployment often comes shortly after completing residency or fellowship.
In this article, we review the various functions of the deployed dermatologist and also highlight the importance of maintaining basic emergency medical skills that could be generalized to the civilian population in case of local or national emergencies.
THE FIELD SURGEON
With rare exceptions, the US Army does not deploy dermatologists for their expertise in diagnosing and managing cutaneous diseases. Typically, a dermatologist will be assigned to a FORSCOM unit in the role of field surgeon. Other medical specialties including emergency medicine, family practice, internal medicine, pediatrics, and obstetrics and gynecology also are eligible for deployment as field surgeons.2 Field surgeons typically are assigned to a battalion-sized element of 300 to 1000 soldiers and are responsible for all medical care rendered under their supervision. Duties include combat resuscitation, primary care services, preventive medicine, medical training of battalion medical personnel, and serving as the medical adviser to the battalion commander.1 In some instances, a field surgeon will be stationed at a higher level of care co-located with a trauma surgeon; in those cases, the field surgeon also may be expected to assist in trauma surgery cases.
ARMY DEPLOYMENT MEDICAL SYSTEM
To better understand the responsibilities of a field surgeon, it is important to discuss the structure of the army’s deployment medical system. The US Military, including the army, has adopted a system of “roles” that have specific requirements regarding their associated medical capabilities.3 There are 4 roles designated within the army. Role 1 facilities are known as battalion aid stations (BASs).

Role of the Field Surgeon
Within the broader structure of the army, approximately 5 battalions (each composed of 300 to 1000 soldiers) comprise a single brigade combat team. Role 1 medical facilities typically have a single battalion surgeon assigned to them. Field surgeons most commonly serve in this battalion surgeon position. Additionally, Role 2 facilities may have slots for up to 2 battalion surgeons; however, field surgeons are less commonly tasked with this assignment.1 Occasionally, in one author’s (N.R.M.) personal experience, these roles are more fluid than one might expect. A field surgeon tasked initially with a Role 1 position may be shifted to a Role 2 assignment on an as-needed basis. This ability for rapid change in roles and responsibilities underscores the need for a fluid mind-set and thorough predeployment training for the field surgeon.
PREDEPLOYMENT TRAINING
As one might expect, dermatologists who have just graduated residency or fellowship are unlikely to have honed their trauma support skills to the degree needed to support a deployed battalion actively engaging in combat. Fortunately, there are many opportunities for military dermatologists to practice these skills prior to joining their FORSCOM colleagues. The initial exposure to trauma support comes during medical internship at the mandatory Combat Casualty Care Course (C4), an 8-day program designed to enhance the operational medical readiness and predeployment trauma training skills of medical officers.4 The C4 program includes 3 days of classroom training and 5 days of intensive field training. During C4, medical officers become certified in Advanced Trauma Life Support, a 3-day course organized by the American College of Surgeons.5 This course teaches medical officers how to quickly and judiciously triage, treat, and transport patients who have sustained potentially life-threatening traumas.
The next components of predeployment training, Tactical Combat Casualty Care and Tactical Combat Medical Care, occur in the months to weeks immediately preceding deployment.1,6 Tactical Combat Casualty Care prepares participants in the initial stabilization of trauma to occur at the point of injury.6 Tactical Combat Casualty Care principles generally are employed by medics (enlisted personnel trained in point-of-care medical support) rather than physicians; however, these principles are still critical for medical officers to be aware of when encountering severe traumas.6 In addition, the physician is responsible for ensuring his/her medics are fully trained in Tactical Combat Casualty Care. Tactical Combat Medical Care is geared more toward the direct preparation of medical officers. During the 5-day course, medical officers learn the gold standard for trauma care in both the classroom and in hands-on scenarios.1 This training not only allows medical officers to be self-sufficient in providing trauma support, but it also enables them to better maintain quality control of the performance of their medics continuously throughout the deployment.1
DEPLOYMENT RESPONSIBILITIES
Dermatologists who have completed the above training typically are subsequently deployed as field surgeons to a Role 1 facility. Field surgeons are designated as the officer in charge of the BAS and assume the position of medical platoon leader. A field surgeon usually will have both a physician assistant and a field medical assistant/medical plans officer (MEDO) to assist in running the BAS. The overarching goal of the field surgeon is to maintain the health and readiness of the battalion. In addition to addressing the day-to-day health care needs of individual soldiers, a field surgeon is expected to attend all staff meetings, advise the commander on preventative health and epidemiological trends, identify the scope of practice of the medics, ensure the BAS is prepared for mass casualties, and take responsibility for all controlled substances.
To illustrate the value that the properly trained dermatologist can provide in the deployed setting, we will outline field surgeon responsibilities and provide case examples of the first-hand experiences of one of the authors (N.R.M.) as a Role 2 officer in charge and field surgeon. The information presented in the case examples may have been altered to ensure continued operational security and out of respect to US servicemembers and coalition forces while still conveying important learning points.
Sick Call
In the deployed environment, military sick call functions as an urgent care center that is open continuously and serves the active-duty population, US government civilians and contractors, and coalition forces. In general, the physician assistant should treat approximately two-thirds of sick call patients under the supervision of the field surgeon, allowing the field surgeon to focus on his/her ancillary duties and ensure overall medical supervision of the unit. As a safeguard, patients with more than 2 visits for the same concern must be evaluated by the field surgeon. Sick call concerns range from minor traumas and illnesses to much more serious disease processes and injuries (as outlined in Medical Emergencies). As a field surgeon, it is critical to track disease nonbattle illnesses to ensure medical readiness of the unit. In the deployed environment, close quarters and austere environments commonly lend themselves to gastrointestinal illnesses, respiratory diseases, heat injuries, vector-borne diseases, and sexually transmitted infections.
Case Examples
During an 8-month deployment in Afghanistan, one of the authors (N.R.M.) provided or assisted in the care of more than 2300 routine sick call appointments, or approximately 10 patients per day. Epidemiology of disease was tracked, and the condition of the unit was presented daily to the battalion commander for consideration in upcoming operations. The top 5 most common categories of diagnoses included musculoskeletal injuries, gastrointestinal diseases, dermatologic concerns (eg, dermatitis, bacterial infections [cellulitis/abscess], fungal infections, arthropod assault, abrasions, lacerations, verruca vulgaris), respiratory illnesses, and mental health care, respectively. Maintaining a familiarity with general medicine is critical for the military dermatologist, and an adequate medical library or access to online medical review sources is critical for day-to-day sick call.
Medical Emergencies
In the event of a more serious injury or illness, a Role 1 BAS has very little capability in performing anything beyond the most basic interventions. Part of the art of being an effective field surgeon lies in stabilization, triage, and transport of these sometimes very ill patients. Both the decision to transport to a higher level of care (eg, Role 2 or 3 facility) as well as selection of the means of transportation falls on the field surgeon. The MEDO plays an essential role in assisting in the coordination of the transfer; however, the responsibility ultimately falls on the field surgeon.1,6 The field surgeon at the Role 2 BAS may be expected to perform more advanced medical and surgical interventions. More advanced pharmacotherapies include thrombolytics, antivenin, and vasopressors. Some procedural interventions include intubations, central lines, and laceration repairs. The Role 2 BAS has the capability to hold patients for up to 72 hours.
Case Examples
Specific conditions one of the authors (N.R.M.) treated include heat injury, myocardial infarction, disseminated tuberculosis, appendicitis, testicular torsion, malaria, suicidal ideation, burns, and status epilepticus. Over 8 months, the Role 2 BAS received 91 medical emergencies, with 53 necessitating evacuation to a higher level of care. Often, the more serious or rare conditions presented in the foreign contractor and coalition force populations working alongside US troops.
In one particular case, a 35-year-old man with an electrocardiogram-confirmed acute ST-segment elevation myocardial infarction was administered standard therapy consisting of intravenous morphine, oxygen, sublingual nitroglycerin, an angiotensin-converting enzyme inhibitor, and a beta-blocker. Given the lack of a cardiac catheterization laboratory at the next highest level of care as well as a low suspicion for aortic dissection (based on the patient’s history, physical examination, and chest radiograph), fibrinolysis with tenecteplase was performed in the deployed environment. After a very short observation for potential hemorrhage, the patient was then evacuated to the Role 3 hospital, where he made a near-complete recovery. Preparation with advanced cardiac life support courses and a thorough algorithmic review of the 10 most common causes of presentation to the emergency department helped adequately prepare the dermatologist to succeed.
Trauma Emergencies
The same principles of triage and transport apply to trauma emergencies. Mass casualties are an inevitable reality in combat, so appropriate training translating into efficient action is essential to ensure the lowest possible mortality. This training and the actions that stem from it are an additional responsibility that the field surgeon must maintain. During deployment, continued training organized by the field surgeon could quite literally mean the difference between life and death. In addition to the organizational responsibilities, field surgeons should be prepared to perform initial stabilization in trauma patients, including application of tourniquets, establishment of central lines, reading abdominal ultrasounds for free fluid, placement of chest tubes, intubation, and ventilator management. The Joint Trauma System Clinical Practice Guidelines also offer extensive and invaluable guidance on the most up-to-date approach to common trauma conditions arising in the deployed environment.7 At the Role 2 level, the field surgeon also must be prepared to coordinate ancillary services, manage the Role 2/forward surgical team intensive care unit, and serve as first assist in the operating room, as needed (Figure 2).

Case Examples
One of the authors (N.R.M.) assisted or provided care in approximately 225 trauma cases while deployed. A mass casualty event occurred, in which the Role 2 BAS received 34 casualties; of these casualties, 11 were immediate, 10 were delayed, 11 were minimal, and 2 were expectant. Injury patterns included mounted and dismounted improvised explosive device injuries (eg, blast, shrapnel, and traumatic brain injuries) as well as gunshot wounds. Direct care was provided for 13 casualties, including 10 abdominal ultrasound examinations for free fluid, placement of 2 chest tubes, 1 intubation, establishment of 3 central lines, and first-assisting 1 exploratory laparotomy. Of the casualties, 22 were evacuated to the Role 3 hospital, 8 were dispositioned to a coalition hospital, 2 were returned to active duty, and 2 died due to their injuries. The military trauma preparation as outlined in the predeployment training can help adequately prepare the military dermatologist to assist in these cases.
Ancillary Services
An important part of the efficacy of initial evaluation and stabilization of both medical and traumatic emergencies involves expedited laboratory tests, imaging, and the delivery of life-saving blood products to affected patients. The field surgeon is responsible for the readiness of these services and may play a critical role in streamlining these tasks for situations where a delay in care by minutes can be lethal. The MEDO assists the field surgeon to ensure the readiness of the medical equipment, and the field surgeon must ensure the readiness of the medics and technicians utilizing the equipment. In a deployed environment, only a finite amount of blood products may be stored. As a result, the design and implementation of an efficient and precise walking blood bank is critical. To help mitigate this issue, servicemembers are prescreened for their blood types and bloodborne illnesses. If a situation arises in which whole blood is needed, the prescreened individuals are screened again, and their blood is collected and transfused to the patient under the supervision of the physician. This task is critical in saving lives, and this process is the primary responsibility of the field surgeon.
Case Example
A 37-year-old man presented to the BAS with abdominal and pelvic gunshot wounds, as well as tachycardia, rapidly decreasing blood pressure, and altered consciousness. An exploratory laparotomy was performed to look for the sources of bleeding. The patient’s blood type was confirmed with a portable testing kit. Due to the injury pattern and clinical presentation, a call was immediately placed to begin screening and preparing servicemembers to donate blood for the walking blood bank. As expected, the Role 2 supply of blood products was exhausted during the exploratory laparotomy. With servicemembers in place and screened, an additional 12 units of whole blood were collected and administered in a timely fashion. The patient was stabilized and transported to the next highest level of care. Due to the process optimization performed by the laboratory team, whole-blood transfusions were ready within an average of 22 minutes, well ahead of the 45-minute standard of care.
Operating Room First Assist
If a field surgeon is stationed at a Role 2 BAS with a forward surgical team, he/she may be required to adopt the role of operating room first assist for the trauma surgeon or orthopedic surgeon on the team, which is especially true for isolated major traumas when triage and initial stabilization measures for multiple patients are of less concern. Dermatologists receive surgical training as part of the Accreditation Council for Graduate Medical Education requirements to graduate residency, making them more than capable of surgical assisting when needed.8 In particular, dermatologists’ ability to utilize instruments appropriately and think procedurally as well as their skills in suturing are helpful.
Case Example
A 22-year-old man with several shrapnel wounds to the abdomen demonstrated free fluid in the left lower quadrant. The field surgeon (N.R.M.) assisted the trauma surgeon in opening the abdomen and running the bowel for sources of bleeding. The trauma surgeon identified the bleed and performed a ligation. The patient was then packed, closed, and prepared for transfer to a higher level of care.
Preventive Medicine
As a result of the field surgeon being on the front line of medical care in an austere environment, implementation of preventive medicine practices and disease pattern recognition are his/her responsibility. Responsibilities may include stray animal euthanasia due to prevalence of rabies, enforcement of malaria prophylaxis, medical training and maintenance of snake antivenin, and assistance with other local endemic disease. The unique skill set of dermatologists in organism identification can further bolster the speed with which vector-borne diseases are recognized and prevention and treatment measures are implemented.
Case Example
As coalition forces executed a mission in Afghanistan, US servicemembers began experiencing abdominal distress, chills, fevers (temperature >40°C), debilitating headaches, myalgia, arthralgia, and tachycardia. Initially, these patients were evacuated to the Role 2 BAS, hindering the mission. Upon inspection, patients had numerous bug bites; one astute soldier collected the arthropod guilty of the assault and brought it to the aid station. Upon inspection, the offender was identified as the Phlebotomus genus of sandflies, organisms that are well known to dermatologists as a cause of leishmaniasis. Clinical correlation resulted in the presumed diagnosis of Pappataci fever, and vector-borne disease prevention measures were then able to be further emphasized and implemented in at-risk areas, allowing the mission to continue.9 Subsequent infectious disease laboratory testing confirmed the Phlebovirus transmitted by the sandfly as the underlying cause of the illness.
CONCLUSION
The diverse role of the field surgeon in the deployed setting makes any one specialist underprepared to completely take on the role from the outset; however, with appropriate and rigorous trauma training prior to deployment, dermatologists will continue to perform as invaluable assets to the US military in conflicts now and in the future.
Military dermatologists complete their residency training at 1 of 3 large military medical centers across the country: Walter Reed National Military Medical Center (Bethesda, Maryland), San Antonio Military Health System (San Antonio, Texas), or Naval Medical Center San Diego (San Diego, California). While in training, army dermatology residents in particular fall under the US Army Medical Command, or MEDCOM, which provides command and control of the army’s medical, dental, and veterinary treatment facilities. Upon graduating from residency, army dermatologists often are stationed with MEDCOM units but become eligible for deployment with US Army Forces Command (FORSCOM) units to both combat and noncombat zones depending on each individual FORSCOM unit’s mission.
The process by which dermatologists and other army physicians are tasked to a deploying FORSCOM unit is referred to as the Professional Filler System, or PROFIS, which was designed to help alleviate the financial cost and specialty skill degradation of having a physician assigned to a FORSCOM unit while not deployed.1 In general, the greater the amount of time that an army medical officer has not been deployed, the more likely they are to be selected for deployment with a FORSCOM unit. For the army dermatologist, deployment often comes shortly after completing residency or fellowship.
In this article, we review the various functions of the deployed dermatologist and also highlight the importance of maintaining basic emergency medical skills that could be generalized to the civilian population in case of local or national emergencies.
THE FIELD SURGEON
With rare exceptions, the US Army does not deploy dermatologists for their expertise in diagnosing and managing cutaneous diseases. Typically, a dermatologist will be assigned to a FORSCOM unit in the role of field surgeon. Other medical specialties including emergency medicine, family practice, internal medicine, pediatrics, and obstetrics and gynecology also are eligible for deployment as field surgeons.2 Field surgeons typically are assigned to a battalion-sized element of 300 to 1000 soldiers and are responsible for all medical care rendered under their supervision. Duties include combat resuscitation, primary care services, preventive medicine, medical training of battalion medical personnel, and serving as the medical adviser to the battalion commander.1 In some instances, a field surgeon will be stationed at a higher level of care co-located with a trauma surgeon; in those cases, the field surgeon also may be expected to assist in trauma surgery cases.
ARMY DEPLOYMENT MEDICAL SYSTEM
To better understand the responsibilities of a field surgeon, it is important to discuss the structure of the army’s deployment medical system. The US Military, including the army, has adopted a system of “roles” that have specific requirements regarding their associated medical capabilities.3 There are 4 roles designated within the army. Role 1 facilities are known as battalion aid stations (BASs).

Role of the Field Surgeon
Within the broader structure of the army, approximately 5 battalions (each composed of 300 to 1000 soldiers) comprise a single brigade combat team. Role 1 medical facilities typically have a single battalion surgeon assigned to them. Field surgeons most commonly serve in this battalion surgeon position. Additionally, Role 2 facilities may have slots for up to 2 battalion surgeons; however, field surgeons are less commonly tasked with this assignment.1 Occasionally, in one author’s (N.R.M.) personal experience, these roles are more fluid than one might expect. A field surgeon tasked initially with a Role 1 position may be shifted to a Role 2 assignment on an as-needed basis. This ability for rapid change in roles and responsibilities underscores the need for a fluid mind-set and thorough predeployment training for the field surgeon.
PREDEPLOYMENT TRAINING
As one might expect, dermatologists who have just graduated residency or fellowship are unlikely to have honed their trauma support skills to the degree needed to support a deployed battalion actively engaging in combat. Fortunately, there are many opportunities for military dermatologists to practice these skills prior to joining their FORSCOM colleagues. The initial exposure to trauma support comes during medical internship at the mandatory Combat Casualty Care Course (C4), an 8-day program designed to enhance the operational medical readiness and predeployment trauma training skills of medical officers.4 The C4 program includes 3 days of classroom training and 5 days of intensive field training. During C4, medical officers become certified in Advanced Trauma Life Support, a 3-day course organized by the American College of Surgeons.5 This course teaches medical officers how to quickly and judiciously triage, treat, and transport patients who have sustained potentially life-threatening traumas.
The next components of predeployment training, Tactical Combat Casualty Care and Tactical Combat Medical Care, occur in the months to weeks immediately preceding deployment.1,6 Tactical Combat Casualty Care prepares participants in the initial stabilization of trauma to occur at the point of injury.6 Tactical Combat Casualty Care principles generally are employed by medics (enlisted personnel trained in point-of-care medical support) rather than physicians; however, these principles are still critical for medical officers to be aware of when encountering severe traumas.6 In addition, the physician is responsible for ensuring his/her medics are fully trained in Tactical Combat Casualty Care. Tactical Combat Medical Care is geared more toward the direct preparation of medical officers. During the 5-day course, medical officers learn the gold standard for trauma care in both the classroom and in hands-on scenarios.1 This training not only allows medical officers to be self-sufficient in providing trauma support, but it also enables them to better maintain quality control of the performance of their medics continuously throughout the deployment.1
DEPLOYMENT RESPONSIBILITIES
Dermatologists who have completed the above training typically are subsequently deployed as field surgeons to a Role 1 facility. Field surgeons are designated as the officer in charge of the BAS and assume the position of medical platoon leader. A field surgeon usually will have both a physician assistant and a field medical assistant/medical plans officer (MEDO) to assist in running the BAS. The overarching goal of the field surgeon is to maintain the health and readiness of the battalion. In addition to addressing the day-to-day health care needs of individual soldiers, a field surgeon is expected to attend all staff meetings, advise the commander on preventative health and epidemiological trends, identify the scope of practice of the medics, ensure the BAS is prepared for mass casualties, and take responsibility for all controlled substances.
To illustrate the value that the properly trained dermatologist can provide in the deployed setting, we will outline field surgeon responsibilities and provide case examples of the first-hand experiences of one of the authors (N.R.M.) as a Role 2 officer in charge and field surgeon. The information presented in the case examples may have been altered to ensure continued operational security and out of respect to US servicemembers and coalition forces while still conveying important learning points.
Sick Call
In the deployed environment, military sick call functions as an urgent care center that is open continuously and serves the active-duty population, US government civilians and contractors, and coalition forces. In general, the physician assistant should treat approximately two-thirds of sick call patients under the supervision of the field surgeon, allowing the field surgeon to focus on his/her ancillary duties and ensure overall medical supervision of the unit. As a safeguard, patients with more than 2 visits for the same concern must be evaluated by the field surgeon. Sick call concerns range from minor traumas and illnesses to much more serious disease processes and injuries (as outlined in Medical Emergencies). As a field surgeon, it is critical to track disease nonbattle illnesses to ensure medical readiness of the unit. In the deployed environment, close quarters and austere environments commonly lend themselves to gastrointestinal illnesses, respiratory diseases, heat injuries, vector-borne diseases, and sexually transmitted infections.
Case Examples
During an 8-month deployment in Afghanistan, one of the authors (N.R.M.) provided or assisted in the care of more than 2300 routine sick call appointments, or approximately 10 patients per day. Epidemiology of disease was tracked, and the condition of the unit was presented daily to the battalion commander for consideration in upcoming operations. The top 5 most common categories of diagnoses included musculoskeletal injuries, gastrointestinal diseases, dermatologic concerns (eg, dermatitis, bacterial infections [cellulitis/abscess], fungal infections, arthropod assault, abrasions, lacerations, verruca vulgaris), respiratory illnesses, and mental health care, respectively. Maintaining a familiarity with general medicine is critical for the military dermatologist, and an adequate medical library or access to online medical review sources is critical for day-to-day sick call.
Medical Emergencies
In the event of a more serious injury or illness, a Role 1 BAS has very little capability in performing anything beyond the most basic interventions. Part of the art of being an effective field surgeon lies in stabilization, triage, and transport of these sometimes very ill patients. Both the decision to transport to a higher level of care (eg, Role 2 or 3 facility) as well as selection of the means of transportation falls on the field surgeon. The MEDO plays an essential role in assisting in the coordination of the transfer; however, the responsibility ultimately falls on the field surgeon.1,6 The field surgeon at the Role 2 BAS may be expected to perform more advanced medical and surgical interventions. More advanced pharmacotherapies include thrombolytics, antivenin, and vasopressors. Some procedural interventions include intubations, central lines, and laceration repairs. The Role 2 BAS has the capability to hold patients for up to 72 hours.
Case Examples
Specific conditions one of the authors (N.R.M.) treated include heat injury, myocardial infarction, disseminated tuberculosis, appendicitis, testicular torsion, malaria, suicidal ideation, burns, and status epilepticus. Over 8 months, the Role 2 BAS received 91 medical emergencies, with 53 necessitating evacuation to a higher level of care. Often, the more serious or rare conditions presented in the foreign contractor and coalition force populations working alongside US troops.
In one particular case, a 35-year-old man with an electrocardiogram-confirmed acute ST-segment elevation myocardial infarction was administered standard therapy consisting of intravenous morphine, oxygen, sublingual nitroglycerin, an angiotensin-converting enzyme inhibitor, and a beta-blocker. Given the lack of a cardiac catheterization laboratory at the next highest level of care as well as a low suspicion for aortic dissection (based on the patient’s history, physical examination, and chest radiograph), fibrinolysis with tenecteplase was performed in the deployed environment. After a very short observation for potential hemorrhage, the patient was then evacuated to the Role 3 hospital, where he made a near-complete recovery. Preparation with advanced cardiac life support courses and a thorough algorithmic review of the 10 most common causes of presentation to the emergency department helped adequately prepare the dermatologist to succeed.
Trauma Emergencies
The same principles of triage and transport apply to trauma emergencies. Mass casualties are an inevitable reality in combat, so appropriate training translating into efficient action is essential to ensure the lowest possible mortality. This training and the actions that stem from it are an additional responsibility that the field surgeon must maintain. During deployment, continued training organized by the field surgeon could quite literally mean the difference between life and death. In addition to the organizational responsibilities, field surgeons should be prepared to perform initial stabilization in trauma patients, including application of tourniquets, establishment of central lines, reading abdominal ultrasounds for free fluid, placement of chest tubes, intubation, and ventilator management. The Joint Trauma System Clinical Practice Guidelines also offer extensive and invaluable guidance on the most up-to-date approach to common trauma conditions arising in the deployed environment.7 At the Role 2 level, the field surgeon also must be prepared to coordinate ancillary services, manage the Role 2/forward surgical team intensive care unit, and serve as first assist in the operating room, as needed (Figure 2).

Case Examples
One of the authors (N.R.M.) assisted or provided care in approximately 225 trauma cases while deployed. A mass casualty event occurred, in which the Role 2 BAS received 34 casualties; of these casualties, 11 were immediate, 10 were delayed, 11 were minimal, and 2 were expectant. Injury patterns included mounted and dismounted improvised explosive device injuries (eg, blast, shrapnel, and traumatic brain injuries) as well as gunshot wounds. Direct care was provided for 13 casualties, including 10 abdominal ultrasound examinations for free fluid, placement of 2 chest tubes, 1 intubation, establishment of 3 central lines, and first-assisting 1 exploratory laparotomy. Of the casualties, 22 were evacuated to the Role 3 hospital, 8 were dispositioned to a coalition hospital, 2 were returned to active duty, and 2 died due to their injuries. The military trauma preparation as outlined in the predeployment training can help adequately prepare the military dermatologist to assist in these cases.
Ancillary Services
An important part of the efficacy of initial evaluation and stabilization of both medical and traumatic emergencies involves expedited laboratory tests, imaging, and the delivery of life-saving blood products to affected patients. The field surgeon is responsible for the readiness of these services and may play a critical role in streamlining these tasks for situations where a delay in care by minutes can be lethal. The MEDO assists the field surgeon to ensure the readiness of the medical equipment, and the field surgeon must ensure the readiness of the medics and technicians utilizing the equipment. In a deployed environment, only a finite amount of blood products may be stored. As a result, the design and implementation of an efficient and precise walking blood bank is critical. To help mitigate this issue, servicemembers are prescreened for their blood types and bloodborne illnesses. If a situation arises in which whole blood is needed, the prescreened individuals are screened again, and their blood is collected and transfused to the patient under the supervision of the physician. This task is critical in saving lives, and this process is the primary responsibility of the field surgeon.
Case Example
A 37-year-old man presented to the BAS with abdominal and pelvic gunshot wounds, as well as tachycardia, rapidly decreasing blood pressure, and altered consciousness. An exploratory laparotomy was performed to look for the sources of bleeding. The patient’s blood type was confirmed with a portable testing kit. Due to the injury pattern and clinical presentation, a call was immediately placed to begin screening and preparing servicemembers to donate blood for the walking blood bank. As expected, the Role 2 supply of blood products was exhausted during the exploratory laparotomy. With servicemembers in place and screened, an additional 12 units of whole blood were collected and administered in a timely fashion. The patient was stabilized and transported to the next highest level of care. Due to the process optimization performed by the laboratory team, whole-blood transfusions were ready within an average of 22 minutes, well ahead of the 45-minute standard of care.
Operating Room First Assist
If a field surgeon is stationed at a Role 2 BAS with a forward surgical team, he/she may be required to adopt the role of operating room first assist for the trauma surgeon or orthopedic surgeon on the team, which is especially true for isolated major traumas when triage and initial stabilization measures for multiple patients are of less concern. Dermatologists receive surgical training as part of the Accreditation Council for Graduate Medical Education requirements to graduate residency, making them more than capable of surgical assisting when needed.8 In particular, dermatologists’ ability to utilize instruments appropriately and think procedurally as well as their skills in suturing are helpful.
Case Example
A 22-year-old man with several shrapnel wounds to the abdomen demonstrated free fluid in the left lower quadrant. The field surgeon (N.R.M.) assisted the trauma surgeon in opening the abdomen and running the bowel for sources of bleeding. The trauma surgeon identified the bleed and performed a ligation. The patient was then packed, closed, and prepared for transfer to a higher level of care.
Preventive Medicine
As a result of the field surgeon being on the front line of medical care in an austere environment, implementation of preventive medicine practices and disease pattern recognition are his/her responsibility. Responsibilities may include stray animal euthanasia due to prevalence of rabies, enforcement of malaria prophylaxis, medical training and maintenance of snake antivenin, and assistance with other local endemic disease. The unique skill set of dermatologists in organism identification can further bolster the speed with which vector-borne diseases are recognized and prevention and treatment measures are implemented.
Case Example
As coalition forces executed a mission in Afghanistan, US servicemembers began experiencing abdominal distress, chills, fevers (temperature >40°C), debilitating headaches, myalgia, arthralgia, and tachycardia. Initially, these patients were evacuated to the Role 2 BAS, hindering the mission. Upon inspection, patients had numerous bug bites; one astute soldier collected the arthropod guilty of the assault and brought it to the aid station. Upon inspection, the offender was identified as the Phlebotomus genus of sandflies, organisms that are well known to dermatologists as a cause of leishmaniasis. Clinical correlation resulted in the presumed diagnosis of Pappataci fever, and vector-borne disease prevention measures were then able to be further emphasized and implemented in at-risk areas, allowing the mission to continue.9 Subsequent infectious disease laboratory testing confirmed the Phlebovirus transmitted by the sandfly as the underlying cause of the illness.
CONCLUSION
The diverse role of the field surgeon in the deployed setting makes any one specialist underprepared to completely take on the role from the outset; however, with appropriate and rigorous trauma training prior to deployment, dermatologists will continue to perform as invaluable assets to the US military in conflicts now and in the future.
1. Moawad FJ, Wilson R, Kunar MT, et al. Role of the battalion surgeon in the Iraq and Afghanistan War. Mil Med. 2012;177:412-416.
2. AR 601-142: Army Medical Department Professional Filler System. Washington, DC: US Department of the Army; 2015. http://cdm16635.contentdm.oclc.org/cdm/ref/collection/p16635coll11/id/4592. Accessed December 19, 2018.
3. Roles of medical care (United States). Emergency War Surgery. 4th ed. Fort Sam Houston, Texas: Office of the Surgeon General; 2013:17-28.
4. Combat Casualty Care Course (C4). Military Health System website. https://health.mil/Training-Center/Defense-Medical-Readiness-Training-Institute/Combat-Casualty-Care-Course. Accessed December 7, 2018.
5. Advanced Trauma Life Support. American College of Surgeons website. https://www.facs.org/quality-programs/trauma/atls. Accessed December 7, 2018.
6. Tactical Combat Casualty Care Course. Military Health System website. https://health.mil/Training-Center/Defense-Medical-Readiness-Training-Institute/Tactical-Combat-Casualty-Care-Course. Accessed December 18, 2018.
7. Joint Trauma System: The Department of Defense Center of Excellence for Trauma. Clinical Practice Guidelines.
8. ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Revised July 1, 2017. Accessed December 7, 2018.
9. Downs JW, Flood DT, Orr NH, et al. Sandfly fever in Afghanistan-a sometimes overlooked disease of military importance: a case series and review of the literature. US Army Med Dep J. 2017:60-66.
1. Moawad FJ, Wilson R, Kunar MT, et al. Role of the battalion surgeon in the Iraq and Afghanistan War. Mil Med. 2012;177:412-416.
2. AR 601-142: Army Medical Department Professional Filler System. Washington, DC: US Department of the Army; 2015. http://cdm16635.contentdm.oclc.org/cdm/ref/collection/p16635coll11/id/4592. Accessed December 19, 2018.
3. Roles of medical care (United States). Emergency War Surgery. 4th ed. Fort Sam Houston, Texas: Office of the Surgeon General; 2013:17-28.
4. Combat Casualty Care Course (C4). Military Health System website. https://health.mil/Training-Center/Defense-Medical-Readiness-Training-Institute/Combat-Casualty-Care-Course. Accessed December 7, 2018.
5. Advanced Trauma Life Support. American College of Surgeons website. https://www.facs.org/quality-programs/trauma/atls. Accessed December 7, 2018.
6. Tactical Combat Casualty Care Course. Military Health System website. https://health.mil/Training-Center/Defense-Medical-Readiness-Training-Institute/Tactical-Combat-Casualty-Care-Course. Accessed December 18, 2018.
7. Joint Trauma System: The Department of Defense Center of Excellence for Trauma. Clinical Practice Guidelines.
8. ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Revised July 1, 2017. Accessed December 7, 2018.
9. Downs JW, Flood DT, Orr NH, et al. Sandfly fever in Afghanistan-a sometimes overlooked disease of military importance: a case series and review of the literature. US Army Med Dep J. 2017:60-66.
Practice Points
- Army dermatologists routinely deploy to combat zones as field surgeons. In this role, they provide routine, emergency, and trauma care for active-duty soldiers and coalition forces.
- With 5 years of general medical training, army dermatologists often are the most prepared to provide advanced care when compared to co-located physician assistants and combat medics.
- Maintaining basic medical skills would serve any dermatologist in case of local or national emergencies.
A case of cold burn reported with whole-body cryotherapy
by Mackenzie O’Connor and her colleagues in the department of dermatology and cutaneous biology at Thomas Jefferson University, Philadelphia.
In the report, they describe the case of a 71-year-old man who presented with a cold burn injury a day after a WBC session. These treatments typically involve sessions of 2-5 minutes, in a chamber that is cooled down to –100°C to –140°C.
The likely cause in this case was a nozzle malfunction that caused liquid nitrogen to come in direct contact with the patient’s skin for a prolonged period of time (less than 1 minute), causing stinging and pain, followed by redness and blistering of the skin. The patient had received four WBC treatments previously for arthritis and back pain, with no adverse effects. In addition to ibuprofen, he was treated with systemic steroids, topical corticosteroids, and silver sulfadiazine cream.
Despite claims that WBC can aid muscle recovery and alleviate joint pain, and can improve skin health, and is increasingly available in spas and other sites, the Food and Drug Administration has not approved the procedure for treatment of any medical conditions, the researchers noted (JAAD Case Rep. 2019;5[1]:29-30). They also referred to a 2015 Cochrane review, which found insufficient evidence that WBC treatment is beneficial for muscle recovery in active young adult men.
by Mackenzie O’Connor and her colleagues in the department of dermatology and cutaneous biology at Thomas Jefferson University, Philadelphia.
In the report, they describe the case of a 71-year-old man who presented with a cold burn injury a day after a WBC session. These treatments typically involve sessions of 2-5 minutes, in a chamber that is cooled down to –100°C to –140°C.
The likely cause in this case was a nozzle malfunction that caused liquid nitrogen to come in direct contact with the patient’s skin for a prolonged period of time (less than 1 minute), causing stinging and pain, followed by redness and blistering of the skin. The patient had received four WBC treatments previously for arthritis and back pain, with no adverse effects. In addition to ibuprofen, he was treated with systemic steroids, topical corticosteroids, and silver sulfadiazine cream.
Despite claims that WBC can aid muscle recovery and alleviate joint pain, and can improve skin health, and is increasingly available in spas and other sites, the Food and Drug Administration has not approved the procedure for treatment of any medical conditions, the researchers noted (JAAD Case Rep. 2019;5[1]:29-30). They also referred to a 2015 Cochrane review, which found insufficient evidence that WBC treatment is beneficial for muscle recovery in active young adult men.
by Mackenzie O’Connor and her colleagues in the department of dermatology and cutaneous biology at Thomas Jefferson University, Philadelphia.
In the report, they describe the case of a 71-year-old man who presented with a cold burn injury a day after a WBC session. These treatments typically involve sessions of 2-5 minutes, in a chamber that is cooled down to –100°C to –140°C.
The likely cause in this case was a nozzle malfunction that caused liquid nitrogen to come in direct contact with the patient’s skin for a prolonged period of time (less than 1 minute), causing stinging and pain, followed by redness and blistering of the skin. The patient had received four WBC treatments previously for arthritis and back pain, with no adverse effects. In addition to ibuprofen, he was treated with systemic steroids, topical corticosteroids, and silver sulfadiazine cream.
Despite claims that WBC can aid muscle recovery and alleviate joint pain, and can improve skin health, and is increasingly available in spas and other sites, the Food and Drug Administration has not approved the procedure for treatment of any medical conditions, the researchers noted (JAAD Case Rep. 2019;5[1]:29-30). They also referred to a 2015 Cochrane review, which found insufficient evidence that WBC treatment is beneficial for muscle recovery in active young adult men.
FROM JAAD CASE REPORTS
Large cohort study IDs prognostic factors in thromboangiitis obliterans
CHICAGO – Nonwhite ethnicity and limb infection at diagnosis predict vascular events in patients with thromboangiitis obliterans (TAO), and the latter also predicts amputation, which occurs within 10 years of diagnosis in nearly a third of patients, according to findings from a large retrospective French cohort study.
After a mean follow-up of 5.7 years, 58.9% of 224 patients with TAO – also known as Buerger’s disease – experienced a vascular event, 21.4% experienced at least one amputation, and 1.3% died, Alexandre Le Joncour, MD, reported at the annual meeting of the American College of Rheumatology.
The 5- and 15-year vascular event-free survival rates were 45% and 28%, respectively, and the 10- and 15-year amputation-free survival rates were 74%, and 66%, respectively, said Dr. Le Joncour of Sorbonne University, Paris.
Of note, no significant difference was seen in the vascular event-free survival rates based on tobacco use levels (more than 22 pack-years vs. 22 or fewer pack-years; HR, 1.2), he said.
Patient characteristics and clinical factors found to independently predict vascular events included nonwhite ethnicity (hazard ratio, 2.35; P = .005) and limb infection at diagnosis (HR, 3.29; P = .045). Limb infection at diagnosis also independently predicted amputation (HR, 12.1; P less than .001), he said.
“But there was no significant [association with amputation] in patients who had claudication, critical ischemia, or ischemic ulcers/necrosis,” he noted, adding that a comparison of white and nonwhite patients showed that the groups were similar with respect to epidemiologic and cardiovascular factors, clinical symptom distribution, and rates of addiction to tobacco, alcohol, and illicit drugs.
It was also clear that patients who quit using tobacco had a significantly lower risk of amputation than did those who continued using tobacco (P = .001), he said, explaining that 43 of the 48 patients who experienced amputation were current smokers, and 5 were ex-smokers at the time of amputation.
Dr. Le Joncour and his colleagues included TAO patients diagnosed between 1967 and 2016 at a median age of 36 years at the time of first symptoms, with a median of 12 months from symptom onset until diagnosis. About 76% were men, and about 83% were white. Patients with diabetes, atherosclerosis, arterial emboli, connective tissue disease, and/or thrombophilia were excluded.
Vascular events in this study were defined as “an acute worsening of the disease course requiring treatment modifications,” and included critical ischemia (35% of cases), ulcers/necrosis (33%), claudication worsening (16%), deep vein thrombosis (3%), superficial phlebitis (7%), limb infection (4%), and “other” events (2%).
Major amputation was defined as “an amputation involving the tibio-tarsian articulation for lower limbs and the metacarpophalangeal articulation for upper limbs,” he explained.
The median time to amputation was 4 years, and patients who experienced amputation had a median age of 39 years. Half of the 48 patients who experienced amputation had one amputation, nearly a third had two amputations, and 19% had three amputations. About two-thirds had minor amputations and a third had major amputations.
The findings provide important prognostic information regarding TAO, Dr. Le Joncour said, noting that long-term data on outcomes in TAO patients have been lacking.
“We found specific characteristics that identified those at highest risk for subsequent vascular complications, and these factors are not only important predictors of vascular complications or relapse, but may also serve to adjust more aggressive management and close follow-up of these patients,” he concluded.
Dr. Le Joncour reported having no disclosures.
SOURCE: Le Joncour A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1885.
CHICAGO – Nonwhite ethnicity and limb infection at diagnosis predict vascular events in patients with thromboangiitis obliterans (TAO), and the latter also predicts amputation, which occurs within 10 years of diagnosis in nearly a third of patients, according to findings from a large retrospective French cohort study.
After a mean follow-up of 5.7 years, 58.9% of 224 patients with TAO – also known as Buerger’s disease – experienced a vascular event, 21.4% experienced at least one amputation, and 1.3% died, Alexandre Le Joncour, MD, reported at the annual meeting of the American College of Rheumatology.
The 5- and 15-year vascular event-free survival rates were 45% and 28%, respectively, and the 10- and 15-year amputation-free survival rates were 74%, and 66%, respectively, said Dr. Le Joncour of Sorbonne University, Paris.
Of note, no significant difference was seen in the vascular event-free survival rates based on tobacco use levels (more than 22 pack-years vs. 22 or fewer pack-years; HR, 1.2), he said.
Patient characteristics and clinical factors found to independently predict vascular events included nonwhite ethnicity (hazard ratio, 2.35; P = .005) and limb infection at diagnosis (HR, 3.29; P = .045). Limb infection at diagnosis also independently predicted amputation (HR, 12.1; P less than .001), he said.
“But there was no significant [association with amputation] in patients who had claudication, critical ischemia, or ischemic ulcers/necrosis,” he noted, adding that a comparison of white and nonwhite patients showed that the groups were similar with respect to epidemiologic and cardiovascular factors, clinical symptom distribution, and rates of addiction to tobacco, alcohol, and illicit drugs.
It was also clear that patients who quit using tobacco had a significantly lower risk of amputation than did those who continued using tobacco (P = .001), he said, explaining that 43 of the 48 patients who experienced amputation were current smokers, and 5 were ex-smokers at the time of amputation.
Dr. Le Joncour and his colleagues included TAO patients diagnosed between 1967 and 2016 at a median age of 36 years at the time of first symptoms, with a median of 12 months from symptom onset until diagnosis. About 76% were men, and about 83% were white. Patients with diabetes, atherosclerosis, arterial emboli, connective tissue disease, and/or thrombophilia were excluded.
Vascular events in this study were defined as “an acute worsening of the disease course requiring treatment modifications,” and included critical ischemia (35% of cases), ulcers/necrosis (33%), claudication worsening (16%), deep vein thrombosis (3%), superficial phlebitis (7%), limb infection (4%), and “other” events (2%).
Major amputation was defined as “an amputation involving the tibio-tarsian articulation for lower limbs and the metacarpophalangeal articulation for upper limbs,” he explained.
The median time to amputation was 4 years, and patients who experienced amputation had a median age of 39 years. Half of the 48 patients who experienced amputation had one amputation, nearly a third had two amputations, and 19% had three amputations. About two-thirds had minor amputations and a third had major amputations.
The findings provide important prognostic information regarding TAO, Dr. Le Joncour said, noting that long-term data on outcomes in TAO patients have been lacking.
“We found specific characteristics that identified those at highest risk for subsequent vascular complications, and these factors are not only important predictors of vascular complications or relapse, but may also serve to adjust more aggressive management and close follow-up of these patients,” he concluded.
Dr. Le Joncour reported having no disclosures.
SOURCE: Le Joncour A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1885.
CHICAGO – Nonwhite ethnicity and limb infection at diagnosis predict vascular events in patients with thromboangiitis obliterans (TAO), and the latter also predicts amputation, which occurs within 10 years of diagnosis in nearly a third of patients, according to findings from a large retrospective French cohort study.
After a mean follow-up of 5.7 years, 58.9% of 224 patients with TAO – also known as Buerger’s disease – experienced a vascular event, 21.4% experienced at least one amputation, and 1.3% died, Alexandre Le Joncour, MD, reported at the annual meeting of the American College of Rheumatology.
The 5- and 15-year vascular event-free survival rates were 45% and 28%, respectively, and the 10- and 15-year amputation-free survival rates were 74%, and 66%, respectively, said Dr. Le Joncour of Sorbonne University, Paris.
Of note, no significant difference was seen in the vascular event-free survival rates based on tobacco use levels (more than 22 pack-years vs. 22 or fewer pack-years; HR, 1.2), he said.
Patient characteristics and clinical factors found to independently predict vascular events included nonwhite ethnicity (hazard ratio, 2.35; P = .005) and limb infection at diagnosis (HR, 3.29; P = .045). Limb infection at diagnosis also independently predicted amputation (HR, 12.1; P less than .001), he said.
“But there was no significant [association with amputation] in patients who had claudication, critical ischemia, or ischemic ulcers/necrosis,” he noted, adding that a comparison of white and nonwhite patients showed that the groups were similar with respect to epidemiologic and cardiovascular factors, clinical symptom distribution, and rates of addiction to tobacco, alcohol, and illicit drugs.
It was also clear that patients who quit using tobacco had a significantly lower risk of amputation than did those who continued using tobacco (P = .001), he said, explaining that 43 of the 48 patients who experienced amputation were current smokers, and 5 were ex-smokers at the time of amputation.
Dr. Le Joncour and his colleagues included TAO patients diagnosed between 1967 and 2016 at a median age of 36 years at the time of first symptoms, with a median of 12 months from symptom onset until diagnosis. About 76% were men, and about 83% were white. Patients with diabetes, atherosclerosis, arterial emboli, connective tissue disease, and/or thrombophilia were excluded.
Vascular events in this study were defined as “an acute worsening of the disease course requiring treatment modifications,” and included critical ischemia (35% of cases), ulcers/necrosis (33%), claudication worsening (16%), deep vein thrombosis (3%), superficial phlebitis (7%), limb infection (4%), and “other” events (2%).
Major amputation was defined as “an amputation involving the tibio-tarsian articulation for lower limbs and the metacarpophalangeal articulation for upper limbs,” he explained.
The median time to amputation was 4 years, and patients who experienced amputation had a median age of 39 years. Half of the 48 patients who experienced amputation had one amputation, nearly a third had two amputations, and 19% had three amputations. About two-thirds had minor amputations and a third had major amputations.
The findings provide important prognostic information regarding TAO, Dr. Le Joncour said, noting that long-term data on outcomes in TAO patients have been lacking.
“We found specific characteristics that identified those at highest risk for subsequent vascular complications, and these factors are not only important predictors of vascular complications or relapse, but may also serve to adjust more aggressive management and close follow-up of these patients,” he concluded.
Dr. Le Joncour reported having no disclosures.
SOURCE: Le Joncour A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1885.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Nonwhite ethnicity and limb infection predict poor prognosis in TAO.
Major finding: Ethnicity predicts vascular events (HR, 2.35); limb infection at diagnosis predicts vascular events and amputation (HR, 3.29 and 12.1, respectively).
Study details: A retrospective cohort study of 224 patients.
Disclosures: Dr. Le Joncour reported having no disclosures.
Source: Le Joncour A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1885.
Update on Calciphylaxis Etiopathogenesis, Diagnosis, and Management
Calciphylaxis, also known as calcific uremic arteriolopathy, is a painful skin condition classically seen in patients with end-stage renal disease (ESRD), particularly those on chronic dialysis.1,2 It also has increasingly been reported in patients with normal renal function and calcium and phosphate homeostasis.3,4 Effective diagnosis and management of calciphylaxis remains challenging for physicians.2,5 The condition is characterized by tissue ischemia caused by calcification of cutaneous arteriolar vessels. As a result, calciphylaxis is associated with high mortality rates, ranging from 60% to 80%.5,6 Excruciating pain and nonhealing ulcers often lead to recurrent hospitalizations and infectious complications,7 and poor nutritional status, chronic pain, depression, and insomnia can further complicate recovery and lead to poor quality of life.8
We provide an update on calciphylaxis etiopathogenesis, diagnosis, and management. We also highlight some challenges faced in managing this potentially fatal condition.
Epidemiology
Calciphylaxis is considered a rare dermatosis with an estimated annual incidence of 1% to 4% in ESRD patients on dialysis. Recent data suggest that incidence of calciphylaxis is rising,5,7,9 which may stem from an increased use of calcium-based phosphate binders, an actual rise in disease incidence, and/or increased recognition of the disease.5 It is difficult to estimate the exact disease burden of calciphylaxis because the diagnostic criteria are not well defined, often leading to missed or delayed diagnosis.3,10 Furthermore, there is no centralized registry for calciphylaxis cases.3
Etiology and Pathogenesis
Calciphylaxis is thought to have a multifactorial etiology with the exact cause or trigger unknown.7 A long list of risk factors and triggers is associated with the condition (Table 1). Calciphylaxis primarily affects small arteries (40–600 μm in diameter) that become calcified due to an imbalance between inhibitors and promoters of calcification.2,11 Fetuin-A and matrix Gla protein inhibit vascular calcification and are downregulated in calciphylaxis.12,13 Dysfunctional calcium, phosphate, and parathyroid hormone regulatory pathways provide an increased substrate for the process of calcification, which causes endothelial damage and microthrombosis, resulting in tissue ischemia and infarction.14,15 Notably, there is growing interest in the role of vitamin K in the pathogenesis of calciphylaxis. Vitamin K inhibits vascular calcification, possibly by increasing the circulating levels of carboxylated matrix Gla protein.16
Clinical Features
Calciphylaxis is most commonly seen on the legs, abdomen, and buttocks.2 Patients with ESRD commonly develop proximal lesions affecting adipose-rich sites and have a poor prognosis. Distal lesions are more common in patients with nonuremic calciphylaxis, and mortality rates are lower in this population.2
Early lesions present as painful skin nodules or indurated plaques that often are rock-hard or firm to palpation with overlying mottling or a livedoid pattern (Figure, A). Early lesions progress from livedo reticularis to livedo racemosa and then to retiform purpura (Figure, B). Purpuric lesions later evolve into black eschars (Figure, C), then to necrotic, ulcerated, malodorous plaques or nodules in later stages of the disease (Figure, D). Lesions also may develop a gangrenous sclerotic appearance.2,5
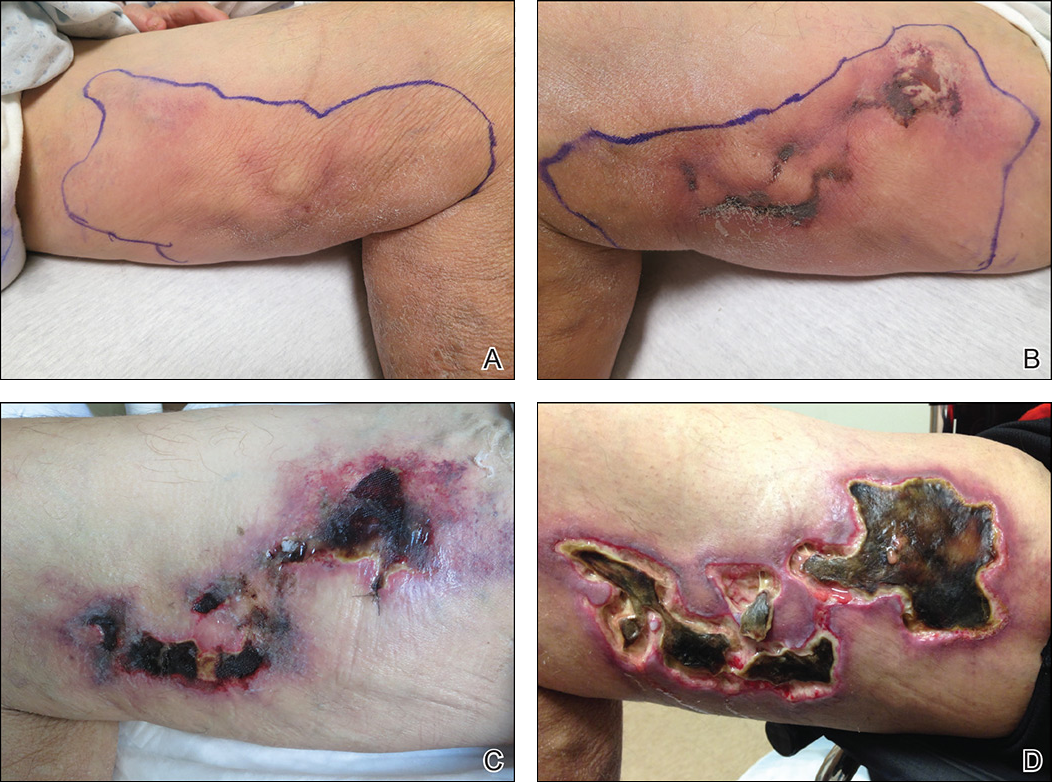
Although most patients with calciphylaxis have ESRD, nonuremic patients also can develop the disease. Those with calciphylaxis who do not have renal dysfunction frequently have other risk factors for the disease and often report another notable health problem in the weeks or months prior to presentation.4 More than half of patients with calciphylaxis become bedridden or require use of a wheelchair.17 Pain is characteristically severe throughout the course of the disease; it may even precede the appearance of the skin lesions.18 Because the pain is associated with ischemia, it tends to be relatively refractory to treatment with opioids. Rare extracutaneous vascular calcifications may lead to visual impairment, gastrointestinal tract bleeding, and myopathy.5,9,19,20
Diagnosis
Considering the high morbidity and mortality associated with calciphylaxis, it is important to provide accurate and timely diagnosis; however, there currently are no validated diagnostic criteria for calciphylaxis. Careful correlation of clinical and histologic findings is required. Calciphylaxis biopsies have demonstrated medial calcification and proliferation of the intima of small- to medium-sized arteries.21 Lobular and septal panniculitis and extravascular soft-tissue calcification, particularly stippled calcification of the eccrine sweat glands, also has been seen.2,22 Special calcium stains (eg, von Kossa, Alizarin red) increase the sensitivity of biopsy by highlighting subtle areas of intravascular and extravascular calcification.5,23 Sufficient sampling of subcutaneous tissue and specimen evaluation by an experienced dermatopathologist are necessary to ensure proper interpretation of the histologic findings.
Despite these measures, skin biopsies may be nondiagnostic or falsely negative; therefore, when there is high clinical suspicion, it may be appropriate to move forward with a presumptive diagnosis of calciphylaxis even if the histologic findings are nondiagnostic.1,9,24 It also is worth noting that localized progression and ulceration may occur following skin biopsy, such that biopsy may even be contraindicated in certain cases (eg, penile calciphylaxis).
Standard laboratory workup for calciphylaxis includes evaluation for associated risk factors as well as exclusion of other conditions in the differential diagnosis (Table 2). Blood tests to evaluate for risk factors include liver and renal function tests, a complete metabolic panel, parathyroid hormone level, and serum albumin level.5 Elevated calcium and phosphate levels may signal disturbed calcium and phosphate homeostasis but are neither sensitive nor specific for the diagnosis.25 Complete blood cell count, blood cultures, thorough hypercoagulability workup (including but not limited to antiphospholipid antibodies, proteins C and S, factor V Leiden, antithrombin III, homocysteine, methylenetetrahydrofolate reductase mutation, and cryoglobulins), rheumatoid factor, antineutrophil cytoplasmic antibodies, and antinuclear antibody testing may be relevant to help identify contributing factors or mimickers of calciphylaxis.5 Various imaging modalities also have been used to evaluate for the presence of soft-tissue calcification in areas of suspected calciphylaxis, including radiography, mammography, computed tomography, ultrasonography, nuclear bone scintigraphy, and spectroscopy.2,26,27 Unfortunately, there currently is no standardized reproducible imaging modality for reliable diagnosis of calciphylaxis. Ultimately, histologic and radiographic findings should always be interpreted in the context of relevant clinical findings.2,9
Prevention
Reduction of the net calcium phosphorus product may help reduce the risk of calciphylaxis in ESRD patients, which can be accomplished by using non–calcium-phosphate binders, adequate dialysis, and restricting use of vitamin D and vitamin K antagonists.2,5 There are limited data regarding the benefits of using bisphosphonates and cinacalcet in ESRD patients on dialysis to prevent calciphylaxis.28,29
Management
Management of calciphylaxis is multifactorial. Besides dermatology and nephrology, specialists in pain management, wound care, plastic surgery, and nutrition are critical partners in management.1,5,9,30 Nephrologists can help optimize calcium and phosphate balance and ensure adequate dialysis. Pain specialists can aid in creating aggressive multiagent pain regimens that target the neuropathic/ischemic and physical aspects of calciphylaxis pain. When appropriate, nutrition specialists can help establish high-protein, low-phosphorus diets, and wound specialists can provide access to advanced wound dressings and adjunctive hyperbaric oxygen therapy. Plastic surgeons can provide conservative debridement procedures in a subset of patients, usually those with distal stable disease.
The limited understanding of the etiopathogenesis of calciphylaxis and the lack of data on its management are reflected in the limited treatment options for the disease (Table 3).2,5,9 There are no formal algorithms for the treatment of calciphylaxis. Therapeutic trials are scarce, and most of the current treatment recommendations are based on small retrospective reports or case series. Sodium thiosulfate has been the most widely used treatment option since 2004, when its use in calciphylaxis was first reported.31 Sodium thiosulfate chelates calcium and is thought to have antioxidant and vasodilatory properties.32 There are a few promising clinical trials and large-scale studies (Table 4) that aim to evaluate the efficacy of existing treatments (eg, sodium thiosulfate) as well as novel treatment options such as lanthanum carbonate, SNF472 (hexasodium phytate), and vitamin K.33-36

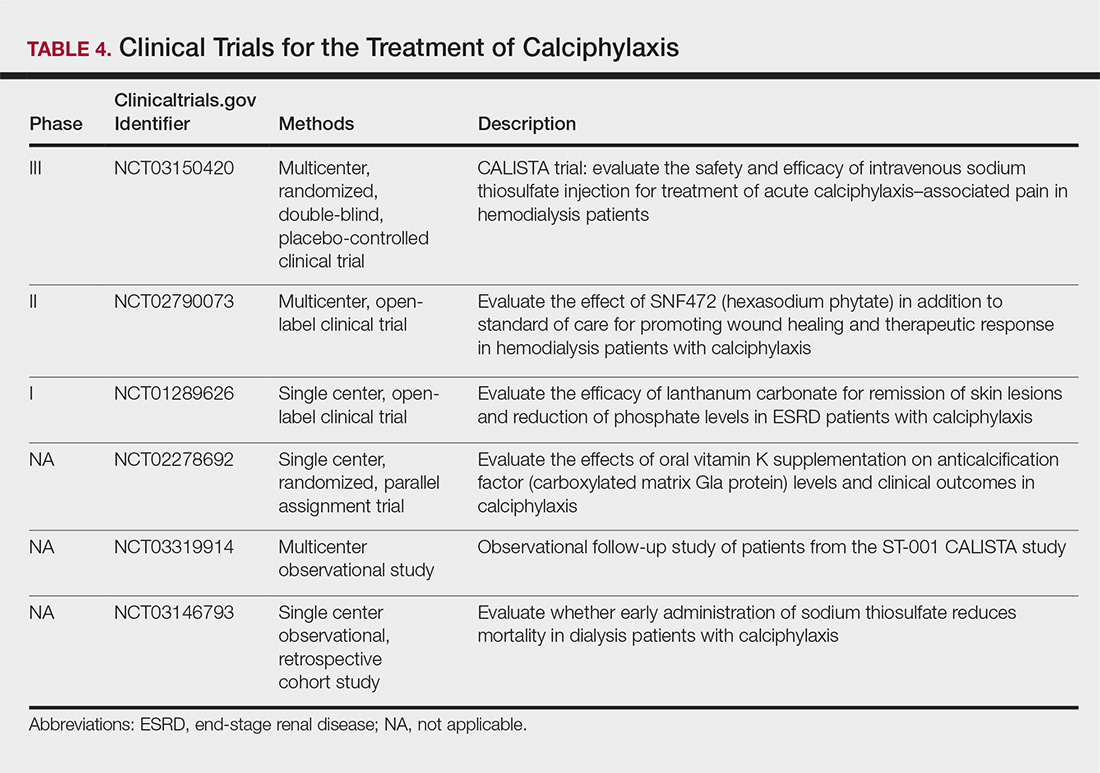
Prognosis
Calciphylaxis is a potentially fatal condition with a poor prognosis and a median survival rate of approximately 1 year following the appearance of skin lesions.37-39 Patients with proximal lesions and those on peritoneal dialysis (as opposed to hemodialysis) have a worse prognosis.40 Mortality rates are estimated to be 30% at 6 months, 50% at 12 months, and 80% at 2 years, with sepsis secondary to infection of cutaneous ulcers being the leading cause of death.37-39 The impact of calciphylaxis on patient quality of life and activities of daily living is severe.8,17
Future Directions
Multi-institution cohort studies and collaborative registries are needed to provide updated information related to the epidemiology, diagnosis, treatment, morbidity, and mortality associated with calciphylaxis and to help formulate evidence-based diagnostic criteria. Radiographic and histologic studies, as well as other tools for early and accurate diagnosis of calciphylaxis, should be studied for feasibility, accuracy, and reproducibility. The incidence of nonuremic calciphylaxis points toward pathogenic pathways besides those based on the bone-mineral axis. Basic science research directed at improving understanding of the pathophysiology of calciphylaxis would be helpful in devising new treatment strategies targeting these pathways. Establishment of a collaborative, multi-institutional calciphylaxis working group would enable experts to formulate therapeutic guidelines based on current evidence. Such a group could facilitate initiation of large prospective studies to establish the efficacy of existing and new treatment modalities for calciphylaxis. A working group within the Society for Dermatology Hospitalists has been tasked with addressing these issues and is currently establishing a multicenter calciphylaxis database.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146.
- Nigwekar SU, Thadhani RI, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Davis JM. The relationship between obesity and calciphylaxis: a review of the literature. Ostomy Wound Manage. 2016;62:12-18.
- Bajaj R, Courbebaisse M, Kroshinsky D, et al. Calciphylaxis in patients with normal renal function: a case series and systematic review. Mayo Clin Proc. 2018;93:1202-1212.
- Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954-962.
- Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci. 2016;351:217-227.
- Westphal SG, Plumb T. Calciphylaxis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2018. https://www.ncbi.nlm.nih.gov/books/NBK519020. Accessed November 12, 2018.
- Riemer CA, El-Azhary RA, Wu KL, et al. Underreported use of palliative care and patient-reported outcome measures to address reduced quality of life in patients with calciphylaxis: a systematic review. Br J Dermatol. 2017;177:1510-1518.
- Nigwekar SU. Calciphylaxis. Curr Opin Nephrol Hypertens. 2017;26:276-281.
- Fine A, Fontaine B. Calciphylaxis: the beginning of the end? Perit Dial Int. 2008;28:268-270.
- Lin WT, Chao CM. Tumoral calcinosis in renal failure. QJM. 2014;107:387.
- Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357-366.
- Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78-81.
- Bleyer AJ, Choi M, Igwemezie B, et al. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32:376-383.
- Ahmed S, O’Neill KD, Hood AF, et al. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:267-276.
- Nigwekar SU, Bloch DB, Nazarian RM, et al. Vitamin K-dependent carboxylation of matrix gla protein influences the risk of calciphylaxis. J Am Soc Nephrol. 2017;28:1717-1722.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
- Polizzotto MN, Bryan T, Ashby MA, et al. Symptomatic management of calciphylaxis: a case series and review of the literature. J Pain Symptom Manage. 2006;32:186-190.
- Gupta N, Haq KF, Mahajan S, et al. Gastrointestinal bleeding secondary to calciphylaxis. Am J Case Rep. 2015;16:818-822.
- Edelstein CL, Wickham MK, Kirby PA. Systemic calciphylaxis presenting as a painful, proximal myopathy. Postgrad Med J. 1992;68:209-211.
- Mochel MC, Arakari RY, Wang G, et al. Cutaneous calciphylaxis: a retrospective histopathologic evaluation. Am J Dermatopathol. 2013;35:582-586.
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802.
- Cassius C, Moguelet P, Monfort JB, et al. Calciphylaxis in haemodialysed patients: diagnostic value of calcifications in cutaneous biopsy. Br J Dermatol. 2018;178:292-293.
- Sreedhar A, Sheikh HA, Scagliotti CJ, et al. Advanced-stage calciphylaxis: think before you punch. Cleve Clin J Med. 2016;83:562-564.
- Brandenburg VM, Kramann R, Rothe H, et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nation-wide registry. Nephrol Dial Transplant. 2017;32:126-132.
- Paul S, Rabito CA, Vedak P, et al. The role of bone scintigraphy in the diagnosis of calciphylaxis. JAMA Dermatol. 2017;153:101-103.
- Shmidt E, Murthy NS, Knudsen JM, et al. Net-like pattern of calcification on plain soft-tissue radiographs in patients with calciphylaxis. J Am Acad Dermatol. 2012;67:1296-1301.
- EVOLVE Trial Investigators; Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482-2494.
- Rogers NM, Teubner DJO, Coates PT. Calcific uremic arteriolopathy: advances in pathogenesis and treatment. Semin Dial. 2007;20:150-157.
- Nigwekar SU. Multidisciplinary approach to calcific uremic arteriolopathy. Curr Opin Nephrol Hypertens. 2015;24:531-537.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Chen NX, O’Neill K, Akl NK, et al. Adipocyte induced arterial calcification is prevented with sodium thiosulfate. Biochem Biophys Res Commun. 2014;449:151-156.
- Chan MR, Ghandour F, Murali NS, et al. Pilot study of the effect of lanthanum carbonate in patients with calciphylaxis: a Wisconsin Network for Health Research (WiNHR) study. J Nephrol Ther. 2014;4:1000162.
- Perelló J, Gómez M, Ferrer MD, et al. SNF472, a novel inhibitor of vascular calcification, could be administered during hemodialysis to attain potentially therapeutic phytate levels. J Nephrol. 2018;31:287-296.
- Christiadi D, Singer RF. Calciphylaxis in a dialysis patient successfully treated with high-dose vitamin K supplementation. Clin Kidney J. 2018;11:528-529.
- Caluwe R, Vandecasteele S, Van Vlem B, et al. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant. 2014;29:1385-1390.
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217.
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429.
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241.
Calciphylaxis, also known as calcific uremic arteriolopathy, is a painful skin condition classically seen in patients with end-stage renal disease (ESRD), particularly those on chronic dialysis.1,2 It also has increasingly been reported in patients with normal renal function and calcium and phosphate homeostasis.3,4 Effective diagnosis and management of calciphylaxis remains challenging for physicians.2,5 The condition is characterized by tissue ischemia caused by calcification of cutaneous arteriolar vessels. As a result, calciphylaxis is associated with high mortality rates, ranging from 60% to 80%.5,6 Excruciating pain and nonhealing ulcers often lead to recurrent hospitalizations and infectious complications,7 and poor nutritional status, chronic pain, depression, and insomnia can further complicate recovery and lead to poor quality of life.8
We provide an update on calciphylaxis etiopathogenesis, diagnosis, and management. We also highlight some challenges faced in managing this potentially fatal condition.
Epidemiology
Calciphylaxis is considered a rare dermatosis with an estimated annual incidence of 1% to 4% in ESRD patients on dialysis. Recent data suggest that incidence of calciphylaxis is rising,5,7,9 which may stem from an increased use of calcium-based phosphate binders, an actual rise in disease incidence, and/or increased recognition of the disease.5 It is difficult to estimate the exact disease burden of calciphylaxis because the diagnostic criteria are not well defined, often leading to missed or delayed diagnosis.3,10 Furthermore, there is no centralized registry for calciphylaxis cases.3
Etiology and Pathogenesis
Calciphylaxis is thought to have a multifactorial etiology with the exact cause or trigger unknown.7 A long list of risk factors and triggers is associated with the condition (Table 1). Calciphylaxis primarily affects small arteries (40–600 μm in diameter) that become calcified due to an imbalance between inhibitors and promoters of calcification.2,11 Fetuin-A and matrix Gla protein inhibit vascular calcification and are downregulated in calciphylaxis.12,13 Dysfunctional calcium, phosphate, and parathyroid hormone regulatory pathways provide an increased substrate for the process of calcification, which causes endothelial damage and microthrombosis, resulting in tissue ischemia and infarction.14,15 Notably, there is growing interest in the role of vitamin K in the pathogenesis of calciphylaxis. Vitamin K inhibits vascular calcification, possibly by increasing the circulating levels of carboxylated matrix Gla protein.16

Clinical Features
Calciphylaxis is most commonly seen on the legs, abdomen, and buttocks.2 Patients with ESRD commonly develop proximal lesions affecting adipose-rich sites and have a poor prognosis. Distal lesions are more common in patients with nonuremic calciphylaxis, and mortality rates are lower in this population.2
Early lesions present as painful skin nodules or indurated plaques that often are rock-hard or firm to palpation with overlying mottling or a livedoid pattern (Figure, A). Early lesions progress from livedo reticularis to livedo racemosa and then to retiform purpura (Figure, B). Purpuric lesions later evolve into black eschars (Figure, C), then to necrotic, ulcerated, malodorous plaques or nodules in later stages of the disease (Figure, D). Lesions also may develop a gangrenous sclerotic appearance.2,5

Although most patients with calciphylaxis have ESRD, nonuremic patients also can develop the disease. Those with calciphylaxis who do not have renal dysfunction frequently have other risk factors for the disease and often report another notable health problem in the weeks or months prior to presentation.4 More than half of patients with calciphylaxis become bedridden or require use of a wheelchair.17 Pain is characteristically severe throughout the course of the disease; it may even precede the appearance of the skin lesions.18 Because the pain is associated with ischemia, it tends to be relatively refractory to treatment with opioids. Rare extracutaneous vascular calcifications may lead to visual impairment, gastrointestinal tract bleeding, and myopathy.5,9,19,20
Diagnosis
Considering the high morbidity and mortality associated with calciphylaxis, it is important to provide accurate and timely diagnosis; however, there currently are no validated diagnostic criteria for calciphylaxis. Careful correlation of clinical and histologic findings is required. Calciphylaxis biopsies have demonstrated medial calcification and proliferation of the intima of small- to medium-sized arteries.21 Lobular and septal panniculitis and extravascular soft-tissue calcification, particularly stippled calcification of the eccrine sweat glands, also has been seen.2,22 Special calcium stains (eg, von Kossa, Alizarin red) increase the sensitivity of biopsy by highlighting subtle areas of intravascular and extravascular calcification.5,23 Sufficient sampling of subcutaneous tissue and specimen evaluation by an experienced dermatopathologist are necessary to ensure proper interpretation of the histologic findings.
Despite these measures, skin biopsies may be nondiagnostic or falsely negative; therefore, when there is high clinical suspicion, it may be appropriate to move forward with a presumptive diagnosis of calciphylaxis even if the histologic findings are nondiagnostic.1,9,24 It also is worth noting that localized progression and ulceration may occur following skin biopsy, such that biopsy may even be contraindicated in certain cases (eg, penile calciphylaxis).
Standard laboratory workup for calciphylaxis includes evaluation for associated risk factors as well as exclusion of other conditions in the differential diagnosis (Table 2). Blood tests to evaluate for risk factors include liver and renal function tests, a complete metabolic panel, parathyroid hormone level, and serum albumin level.5 Elevated calcium and phosphate levels may signal disturbed calcium and phosphate homeostasis but are neither sensitive nor specific for the diagnosis.25 Complete blood cell count, blood cultures, thorough hypercoagulability workup (including but not limited to antiphospholipid antibodies, proteins C and S, factor V Leiden, antithrombin III, homocysteine, methylenetetrahydrofolate reductase mutation, and cryoglobulins), rheumatoid factor, antineutrophil cytoplasmic antibodies, and antinuclear antibody testing may be relevant to help identify contributing factors or mimickers of calciphylaxis.5 Various imaging modalities also have been used to evaluate for the presence of soft-tissue calcification in areas of suspected calciphylaxis, including radiography, mammography, computed tomography, ultrasonography, nuclear bone scintigraphy, and spectroscopy.2,26,27 Unfortunately, there currently is no standardized reproducible imaging modality for reliable diagnosis of calciphylaxis. Ultimately, histologic and radiographic findings should always be interpreted in the context of relevant clinical findings.2,9

Prevention
Reduction of the net calcium phosphorus product may help reduce the risk of calciphylaxis in ESRD patients, which can be accomplished by using non–calcium-phosphate binders, adequate dialysis, and restricting use of vitamin D and vitamin K antagonists.2,5 There are limited data regarding the benefits of using bisphosphonates and cinacalcet in ESRD patients on dialysis to prevent calciphylaxis.28,29
Management
Management of calciphylaxis is multifactorial. Besides dermatology and nephrology, specialists in pain management, wound care, plastic surgery, and nutrition are critical partners in management.1,5,9,30 Nephrologists can help optimize calcium and phosphate balance and ensure adequate dialysis. Pain specialists can aid in creating aggressive multiagent pain regimens that target the neuropathic/ischemic and physical aspects of calciphylaxis pain. When appropriate, nutrition specialists can help establish high-protein, low-phosphorus diets, and wound specialists can provide access to advanced wound dressings and adjunctive hyperbaric oxygen therapy. Plastic surgeons can provide conservative debridement procedures in a subset of patients, usually those with distal stable disease.
The limited understanding of the etiopathogenesis of calciphylaxis and the lack of data on its management are reflected in the limited treatment options for the disease (Table 3).2,5,9 There are no formal algorithms for the treatment of calciphylaxis. Therapeutic trials are scarce, and most of the current treatment recommendations are based on small retrospective reports or case series. Sodium thiosulfate has been the most widely used treatment option since 2004, when its use in calciphylaxis was first reported.31 Sodium thiosulfate chelates calcium and is thought to have antioxidant and vasodilatory properties.32 There are a few promising clinical trials and large-scale studies (Table 4) that aim to evaluate the efficacy of existing treatments (eg, sodium thiosulfate) as well as novel treatment options such as lanthanum carbonate, SNF472 (hexasodium phytate), and vitamin K.33-36


Prognosis
Calciphylaxis is a potentially fatal condition with a poor prognosis and a median survival rate of approximately 1 year following the appearance of skin lesions.37-39 Patients with proximal lesions and those on peritoneal dialysis (as opposed to hemodialysis) have a worse prognosis.40 Mortality rates are estimated to be 30% at 6 months, 50% at 12 months, and 80% at 2 years, with sepsis secondary to infection of cutaneous ulcers being the leading cause of death.37-39 The impact of calciphylaxis on patient quality of life and activities of daily living is severe.8,17
Future Directions
Multi-institution cohort studies and collaborative registries are needed to provide updated information related to the epidemiology, diagnosis, treatment, morbidity, and mortality associated with calciphylaxis and to help formulate evidence-based diagnostic criteria. Radiographic and histologic studies, as well as other tools for early and accurate diagnosis of calciphylaxis, should be studied for feasibility, accuracy, and reproducibility. The incidence of nonuremic calciphylaxis points toward pathogenic pathways besides those based on the bone-mineral axis. Basic science research directed at improving understanding of the pathophysiology of calciphylaxis would be helpful in devising new treatment strategies targeting these pathways. Establishment of a collaborative, multi-institutional calciphylaxis working group would enable experts to formulate therapeutic guidelines based on current evidence. Such a group could facilitate initiation of large prospective studies to establish the efficacy of existing and new treatment modalities for calciphylaxis. A working group within the Society for Dermatology Hospitalists has been tasked with addressing these issues and is currently establishing a multicenter calciphylaxis database.
Calciphylaxis, also known as calcific uremic arteriolopathy, is a painful skin condition classically seen in patients with end-stage renal disease (ESRD), particularly those on chronic dialysis.1,2 It also has increasingly been reported in patients with normal renal function and calcium and phosphate homeostasis.3,4 Effective diagnosis and management of calciphylaxis remains challenging for physicians.2,5 The condition is characterized by tissue ischemia caused by calcification of cutaneous arteriolar vessels. As a result, calciphylaxis is associated with high mortality rates, ranging from 60% to 80%.5,6 Excruciating pain and nonhealing ulcers often lead to recurrent hospitalizations and infectious complications,7 and poor nutritional status, chronic pain, depression, and insomnia can further complicate recovery and lead to poor quality of life.8
We provide an update on calciphylaxis etiopathogenesis, diagnosis, and management. We also highlight some challenges faced in managing this potentially fatal condition.
Epidemiology
Calciphylaxis is considered a rare dermatosis with an estimated annual incidence of 1% to 4% in ESRD patients on dialysis. Recent data suggest that incidence of calciphylaxis is rising,5,7,9 which may stem from an increased use of calcium-based phosphate binders, an actual rise in disease incidence, and/or increased recognition of the disease.5 It is difficult to estimate the exact disease burden of calciphylaxis because the diagnostic criteria are not well defined, often leading to missed or delayed diagnosis.3,10 Furthermore, there is no centralized registry for calciphylaxis cases.3
Etiology and Pathogenesis
Calciphylaxis is thought to have a multifactorial etiology with the exact cause or trigger unknown.7 A long list of risk factors and triggers is associated with the condition (Table 1). Calciphylaxis primarily affects small arteries (40–600 μm in diameter) that become calcified due to an imbalance between inhibitors and promoters of calcification.2,11 Fetuin-A and matrix Gla protein inhibit vascular calcification and are downregulated in calciphylaxis.12,13 Dysfunctional calcium, phosphate, and parathyroid hormone regulatory pathways provide an increased substrate for the process of calcification, which causes endothelial damage and microthrombosis, resulting in tissue ischemia and infarction.14,15 Notably, there is growing interest in the role of vitamin K in the pathogenesis of calciphylaxis. Vitamin K inhibits vascular calcification, possibly by increasing the circulating levels of carboxylated matrix Gla protein.16

Clinical Features
Calciphylaxis is most commonly seen on the legs, abdomen, and buttocks.2 Patients with ESRD commonly develop proximal lesions affecting adipose-rich sites and have a poor prognosis. Distal lesions are more common in patients with nonuremic calciphylaxis, and mortality rates are lower in this population.2
Early lesions present as painful skin nodules or indurated plaques that often are rock-hard or firm to palpation with overlying mottling or a livedoid pattern (Figure, A). Early lesions progress from livedo reticularis to livedo racemosa and then to retiform purpura (Figure, B). Purpuric lesions later evolve into black eschars (Figure, C), then to necrotic, ulcerated, malodorous plaques or nodules in later stages of the disease (Figure, D). Lesions also may develop a gangrenous sclerotic appearance.2,5

Although most patients with calciphylaxis have ESRD, nonuremic patients also can develop the disease. Those with calciphylaxis who do not have renal dysfunction frequently have other risk factors for the disease and often report another notable health problem in the weeks or months prior to presentation.4 More than half of patients with calciphylaxis become bedridden or require use of a wheelchair.17 Pain is characteristically severe throughout the course of the disease; it may even precede the appearance of the skin lesions.18 Because the pain is associated with ischemia, it tends to be relatively refractory to treatment with opioids. Rare extracutaneous vascular calcifications may lead to visual impairment, gastrointestinal tract bleeding, and myopathy.5,9,19,20
Diagnosis
Considering the high morbidity and mortality associated with calciphylaxis, it is important to provide accurate and timely diagnosis; however, there currently are no validated diagnostic criteria for calciphylaxis. Careful correlation of clinical and histologic findings is required. Calciphylaxis biopsies have demonstrated medial calcification and proliferation of the intima of small- to medium-sized arteries.21 Lobular and septal panniculitis and extravascular soft-tissue calcification, particularly stippled calcification of the eccrine sweat glands, also has been seen.2,22 Special calcium stains (eg, von Kossa, Alizarin red) increase the sensitivity of biopsy by highlighting subtle areas of intravascular and extravascular calcification.5,23 Sufficient sampling of subcutaneous tissue and specimen evaluation by an experienced dermatopathologist are necessary to ensure proper interpretation of the histologic findings.
Despite these measures, skin biopsies may be nondiagnostic or falsely negative; therefore, when there is high clinical suspicion, it may be appropriate to move forward with a presumptive diagnosis of calciphylaxis even if the histologic findings are nondiagnostic.1,9,24 It also is worth noting that localized progression and ulceration may occur following skin biopsy, such that biopsy may even be contraindicated in certain cases (eg, penile calciphylaxis).
Standard laboratory workup for calciphylaxis includes evaluation for associated risk factors as well as exclusion of other conditions in the differential diagnosis (Table 2). Blood tests to evaluate for risk factors include liver and renal function tests, a complete metabolic panel, parathyroid hormone level, and serum albumin level.5 Elevated calcium and phosphate levels may signal disturbed calcium and phosphate homeostasis but are neither sensitive nor specific for the diagnosis.25 Complete blood cell count, blood cultures, thorough hypercoagulability workup (including but not limited to antiphospholipid antibodies, proteins C and S, factor V Leiden, antithrombin III, homocysteine, methylenetetrahydrofolate reductase mutation, and cryoglobulins), rheumatoid factor, antineutrophil cytoplasmic antibodies, and antinuclear antibody testing may be relevant to help identify contributing factors or mimickers of calciphylaxis.5 Various imaging modalities also have been used to evaluate for the presence of soft-tissue calcification in areas of suspected calciphylaxis, including radiography, mammography, computed tomography, ultrasonography, nuclear bone scintigraphy, and spectroscopy.2,26,27 Unfortunately, there currently is no standardized reproducible imaging modality for reliable diagnosis of calciphylaxis. Ultimately, histologic and radiographic findings should always be interpreted in the context of relevant clinical findings.2,9

Prevention
Reduction of the net calcium phosphorus product may help reduce the risk of calciphylaxis in ESRD patients, which can be accomplished by using non–calcium-phosphate binders, adequate dialysis, and restricting use of vitamin D and vitamin K antagonists.2,5 There are limited data regarding the benefits of using bisphosphonates and cinacalcet in ESRD patients on dialysis to prevent calciphylaxis.28,29
Management
Management of calciphylaxis is multifactorial. Besides dermatology and nephrology, specialists in pain management, wound care, plastic surgery, and nutrition are critical partners in management.1,5,9,30 Nephrologists can help optimize calcium and phosphate balance and ensure adequate dialysis. Pain specialists can aid in creating aggressive multiagent pain regimens that target the neuropathic/ischemic and physical aspects of calciphylaxis pain. When appropriate, nutrition specialists can help establish high-protein, low-phosphorus diets, and wound specialists can provide access to advanced wound dressings and adjunctive hyperbaric oxygen therapy. Plastic surgeons can provide conservative debridement procedures in a subset of patients, usually those with distal stable disease.
The limited understanding of the etiopathogenesis of calciphylaxis and the lack of data on its management are reflected in the limited treatment options for the disease (Table 3).2,5,9 There are no formal algorithms for the treatment of calciphylaxis. Therapeutic trials are scarce, and most of the current treatment recommendations are based on small retrospective reports or case series. Sodium thiosulfate has been the most widely used treatment option since 2004, when its use in calciphylaxis was first reported.31 Sodium thiosulfate chelates calcium and is thought to have antioxidant and vasodilatory properties.32 There are a few promising clinical trials and large-scale studies (Table 4) that aim to evaluate the efficacy of existing treatments (eg, sodium thiosulfate) as well as novel treatment options such as lanthanum carbonate, SNF472 (hexasodium phytate), and vitamin K.33-36


Prognosis
Calciphylaxis is a potentially fatal condition with a poor prognosis and a median survival rate of approximately 1 year following the appearance of skin lesions.37-39 Patients with proximal lesions and those on peritoneal dialysis (as opposed to hemodialysis) have a worse prognosis.40 Mortality rates are estimated to be 30% at 6 months, 50% at 12 months, and 80% at 2 years, with sepsis secondary to infection of cutaneous ulcers being the leading cause of death.37-39 The impact of calciphylaxis on patient quality of life and activities of daily living is severe.8,17
Future Directions
Multi-institution cohort studies and collaborative registries are needed to provide updated information related to the epidemiology, diagnosis, treatment, morbidity, and mortality associated with calciphylaxis and to help formulate evidence-based diagnostic criteria. Radiographic and histologic studies, as well as other tools for early and accurate diagnosis of calciphylaxis, should be studied for feasibility, accuracy, and reproducibility. The incidence of nonuremic calciphylaxis points toward pathogenic pathways besides those based on the bone-mineral axis. Basic science research directed at improving understanding of the pathophysiology of calciphylaxis would be helpful in devising new treatment strategies targeting these pathways. Establishment of a collaborative, multi-institutional calciphylaxis working group would enable experts to formulate therapeutic guidelines based on current evidence. Such a group could facilitate initiation of large prospective studies to establish the efficacy of existing and new treatment modalities for calciphylaxis. A working group within the Society for Dermatology Hospitalists has been tasked with addressing these issues and is currently establishing a multicenter calciphylaxis database.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146.
- Nigwekar SU, Thadhani RI, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Davis JM. The relationship between obesity and calciphylaxis: a review of the literature. Ostomy Wound Manage. 2016;62:12-18.
- Bajaj R, Courbebaisse M, Kroshinsky D, et al. Calciphylaxis in patients with normal renal function: a case series and systematic review. Mayo Clin Proc. 2018;93:1202-1212.
- Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954-962.
- Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci. 2016;351:217-227.
- Westphal SG, Plumb T. Calciphylaxis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2018. https://www.ncbi.nlm.nih.gov/books/NBK519020. Accessed November 12, 2018.
- Riemer CA, El-Azhary RA, Wu KL, et al. Underreported use of palliative care and patient-reported outcome measures to address reduced quality of life in patients with calciphylaxis: a systematic review. Br J Dermatol. 2017;177:1510-1518.
- Nigwekar SU. Calciphylaxis. Curr Opin Nephrol Hypertens. 2017;26:276-281.
- Fine A, Fontaine B. Calciphylaxis: the beginning of the end? Perit Dial Int. 2008;28:268-270.
- Lin WT, Chao CM. Tumoral calcinosis in renal failure. QJM. 2014;107:387.
- Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357-366.
- Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78-81.
- Bleyer AJ, Choi M, Igwemezie B, et al. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32:376-383.
- Ahmed S, O’Neill KD, Hood AF, et al. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:267-276.
- Nigwekar SU, Bloch DB, Nazarian RM, et al. Vitamin K-dependent carboxylation of matrix gla protein influences the risk of calciphylaxis. J Am Soc Nephrol. 2017;28:1717-1722.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
- Polizzotto MN, Bryan T, Ashby MA, et al. Symptomatic management of calciphylaxis: a case series and review of the literature. J Pain Symptom Manage. 2006;32:186-190.
- Gupta N, Haq KF, Mahajan S, et al. Gastrointestinal bleeding secondary to calciphylaxis. Am J Case Rep. 2015;16:818-822.
- Edelstein CL, Wickham MK, Kirby PA. Systemic calciphylaxis presenting as a painful, proximal myopathy. Postgrad Med J. 1992;68:209-211.
- Mochel MC, Arakari RY, Wang G, et al. Cutaneous calciphylaxis: a retrospective histopathologic evaluation. Am J Dermatopathol. 2013;35:582-586.
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802.
- Cassius C, Moguelet P, Monfort JB, et al. Calciphylaxis in haemodialysed patients: diagnostic value of calcifications in cutaneous biopsy. Br J Dermatol. 2018;178:292-293.
- Sreedhar A, Sheikh HA, Scagliotti CJ, et al. Advanced-stage calciphylaxis: think before you punch. Cleve Clin J Med. 2016;83:562-564.
- Brandenburg VM, Kramann R, Rothe H, et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nation-wide registry. Nephrol Dial Transplant. 2017;32:126-132.
- Paul S, Rabito CA, Vedak P, et al. The role of bone scintigraphy in the diagnosis of calciphylaxis. JAMA Dermatol. 2017;153:101-103.
- Shmidt E, Murthy NS, Knudsen JM, et al. Net-like pattern of calcification on plain soft-tissue radiographs in patients with calciphylaxis. J Am Acad Dermatol. 2012;67:1296-1301.
- EVOLVE Trial Investigators; Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482-2494.
- Rogers NM, Teubner DJO, Coates PT. Calcific uremic arteriolopathy: advances in pathogenesis and treatment. Semin Dial. 2007;20:150-157.
- Nigwekar SU. Multidisciplinary approach to calcific uremic arteriolopathy. Curr Opin Nephrol Hypertens. 2015;24:531-537.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Chen NX, O’Neill K, Akl NK, et al. Adipocyte induced arterial calcification is prevented with sodium thiosulfate. Biochem Biophys Res Commun. 2014;449:151-156.
- Chan MR, Ghandour F, Murali NS, et al. Pilot study of the effect of lanthanum carbonate in patients with calciphylaxis: a Wisconsin Network for Health Research (WiNHR) study. J Nephrol Ther. 2014;4:1000162.
- Perelló J, Gómez M, Ferrer MD, et al. SNF472, a novel inhibitor of vascular calcification, could be administered during hemodialysis to attain potentially therapeutic phytate levels. J Nephrol. 2018;31:287-296.
- Christiadi D, Singer RF. Calciphylaxis in a dialysis patient successfully treated with high-dose vitamin K supplementation. Clin Kidney J. 2018;11:528-529.
- Caluwe R, Vandecasteele S, Van Vlem B, et al. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant. 2014;29:1385-1390.
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217.
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429.
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146.
- Nigwekar SU, Thadhani RI, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Davis JM. The relationship between obesity and calciphylaxis: a review of the literature. Ostomy Wound Manage. 2016;62:12-18.
- Bajaj R, Courbebaisse M, Kroshinsky D, et al. Calciphylaxis in patients with normal renal function: a case series and systematic review. Mayo Clin Proc. 2018;93:1202-1212.
- Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954-962.
- Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci. 2016;351:217-227.
- Westphal SG, Plumb T. Calciphylaxis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2018. https://www.ncbi.nlm.nih.gov/books/NBK519020. Accessed November 12, 2018.
- Riemer CA, El-Azhary RA, Wu KL, et al. Underreported use of palliative care and patient-reported outcome measures to address reduced quality of life in patients with calciphylaxis: a systematic review. Br J Dermatol. 2017;177:1510-1518.
- Nigwekar SU. Calciphylaxis. Curr Opin Nephrol Hypertens. 2017;26:276-281.
- Fine A, Fontaine B. Calciphylaxis: the beginning of the end? Perit Dial Int. 2008;28:268-270.
- Lin WT, Chao CM. Tumoral calcinosis in renal failure. QJM. 2014;107:387.
- Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357-366.
- Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78-81.
- Bleyer AJ, Choi M, Igwemezie B, et al. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32:376-383.
- Ahmed S, O’Neill KD, Hood AF, et al. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:267-276.
- Nigwekar SU, Bloch DB, Nazarian RM, et al. Vitamin K-dependent carboxylation of matrix gla protein influences the risk of calciphylaxis. J Am Soc Nephrol. 2017;28:1717-1722.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
- Polizzotto MN, Bryan T, Ashby MA, et al. Symptomatic management of calciphylaxis: a case series and review of the literature. J Pain Symptom Manage. 2006;32:186-190.
- Gupta N, Haq KF, Mahajan S, et al. Gastrointestinal bleeding secondary to calciphylaxis. Am J Case Rep. 2015;16:818-822.
- Edelstein CL, Wickham MK, Kirby PA. Systemic calciphylaxis presenting as a painful, proximal myopathy. Postgrad Med J. 1992;68:209-211.
- Mochel MC, Arakari RY, Wang G, et al. Cutaneous calciphylaxis: a retrospective histopathologic evaluation. Am J Dermatopathol. 2013;35:582-586.
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802.
- Cassius C, Moguelet P, Monfort JB, et al. Calciphylaxis in haemodialysed patients: diagnostic value of calcifications in cutaneous biopsy. Br J Dermatol. 2018;178:292-293.
- Sreedhar A, Sheikh HA, Scagliotti CJ, et al. Advanced-stage calciphylaxis: think before you punch. Cleve Clin J Med. 2016;83:562-564.
- Brandenburg VM, Kramann R, Rothe H, et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nation-wide registry. Nephrol Dial Transplant. 2017;32:126-132.
- Paul S, Rabito CA, Vedak P, et al. The role of bone scintigraphy in the diagnosis of calciphylaxis. JAMA Dermatol. 2017;153:101-103.
- Shmidt E, Murthy NS, Knudsen JM, et al. Net-like pattern of calcification on plain soft-tissue radiographs in patients with calciphylaxis. J Am Acad Dermatol. 2012;67:1296-1301.
- EVOLVE Trial Investigators; Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482-2494.
- Rogers NM, Teubner DJO, Coates PT. Calcific uremic arteriolopathy: advances in pathogenesis and treatment. Semin Dial. 2007;20:150-157.
- Nigwekar SU. Multidisciplinary approach to calcific uremic arteriolopathy. Curr Opin Nephrol Hypertens. 2015;24:531-537.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Chen NX, O’Neill K, Akl NK, et al. Adipocyte induced arterial calcification is prevented with sodium thiosulfate. Biochem Biophys Res Commun. 2014;449:151-156.
- Chan MR, Ghandour F, Murali NS, et al. Pilot study of the effect of lanthanum carbonate in patients with calciphylaxis: a Wisconsin Network for Health Research (WiNHR) study. J Nephrol Ther. 2014;4:1000162.
- Perelló J, Gómez M, Ferrer MD, et al. SNF472, a novel inhibitor of vascular calcification, could be administered during hemodialysis to attain potentially therapeutic phytate levels. J Nephrol. 2018;31:287-296.
- Christiadi D, Singer RF. Calciphylaxis in a dialysis patient successfully treated with high-dose vitamin K supplementation. Clin Kidney J. 2018;11:528-529.
- Caluwe R, Vandecasteele S, Van Vlem B, et al. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant. 2014;29:1385-1390.
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217.
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429.
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241.
Practice Points
- Maintain a high index of suspicion for calciphylaxis in patients with end-stage renal disease on chronic dialysis presenting with severely painful livedoid plaques or retiform purpura, particularly in fat-rich body sites.
- Skin biopsies may be limited by biopsy site, inadequate biopsy depth, missed areas of microcalcification, and absence of definitive histologic criteria. Special calcium stains and review by an experienced dermatopathologist may lower the rate of false-negative biopsies.
- In cases where the most likely clinical diagnosis is calciphylaxis, treatment should be initiated even if definitive histopathology findings are lacking.
- Treatment should be multimodal, including elimination of risk factors, intravenous sodium thiosulfate, agents addressing calcium-phosphate metabolism, and surgical debridement, if indicated.
Smart insoles reduce ‘high-risk’ diabetic foot ulcer recurrence
BERLIN – Smart insoles that warn diabetic individuals of high plantar pressures could be a simple solution to help them avoid recurrent foot ulcers, according to the results of a randomized trial.
Study participants with a history of diabetic foot ulcers wore the plantar pressure–sensing insoles (SurroSense Rx) and received feedback via sensor linked to a smart watch worn and were 71% less likely to experience a recurrent foot ulceration than were those who wore the insoles but did not get the pressure feedback (incidence rate ratio, 0.29; 95% confidence interval, 0.09-0.93; P = .037). The device has been cleared by the Food and Drug Administration.
Overall, there were few ulcers that occurred in the study, with 10 ulcers from 8,638 person-days from six patients reported in the control group and four ulcers from 11,835 person-days from four patients in the intervention group.
“Diabetic foot ulcers are a major global health and economic burden, but, in theory at least, they are ultimately preventable,” said study investigator Neil Reeves, PhD,, who presented the findings at the annual meeting of the European Association for the Study of Diabetes.
Data suggest that recurrence rates for ulceration are as high as 65% at 6 years, he said, with up to a quarter of ulcers progressing to the point where some form of amputation is needed.
“In the laboratory, we can measure plantar pressures, and these are considered as a relatively accurate proxy for diabetic foot ulcer risk. So we can discriminate between those with diabetic neuropathy, and those without, and also those with a previous history of ulceration,” Dr. Reeves said.
Dr. Reeves, who is professor of musculoskeletal biomechanics at Manchester (England) Metropolitan University, observed that the rationale behind the development of the smart insoles was to move plantar pressure measurement out of the laboratory and into the real world.
The smart insoles incorporate eight discreet pressure sensors that are connected to pod worn on the front of the participant’s own shoe and that wirelessly relay pressure information to a smartwatch. Both the control and the intervention groups received the same device, Dr. Reeves pointed out, but the difference was that the only the intervention group got any pressure feedback from the sensors to the smartwatch.
“When high pressure was experienced in the intervention group on any of these sensors, the patient was alerted both by an auditory alarm and also by being able to see this on the smartwatch,” Dr. Reeves explained.
“The patient would be alerted as to where the pressure was high on the foot and that would be a trigger to offload this high pressure.” Patients would then be instructed via the smartwatch to try to offload the pressure by either walking around for 2 minutes, actively taking the weight off the foot, or removing the shoe to check for any foreign bodies.
In all, there were 58 study participants – 32 randomized to the intervention group and 26 to the control group – who had a history of diabetic foot ulcers and peripheral neuropathy but who were able to walk independently for at least 30 steps. The mean age of patients in the intervention group was 59 years, 88% were male, 72% had type 2 diabetes mellitus, with the mean duration of diabetes was 22 years. Corresponding data in the control group were 67 years, 89% male, 85% had type 2 diabetes, and 21 years’ diabetes duration.
Patients were reviewed monthly over a period of 18 months or until plantar ulceration occurred. Information on diabetic foot ulcers was collected and standardized using a previously developed mobile app (Diabetes Sci Technol. 2018;12[1]:169-73) and then confirmed via blinded assessment by two experts.
Dr. Reeves noted that there was no significant difference in the time to ulceration between the groups, with 77.5% and 68.4% of the intervention and control group remaining ulcer free at 18 months (P = .30). When the data were adjusted for compliance, there was an 86% reduction in the risk of reulceration in the intervention versus the control group (IRR, 0.14; 95% CI, 0.03-0.63; P = .011). This analysis took into account only those study participants who had 4.5 hours or more of daily wear of the smart insoles (n = 40). On average, the insoles were worn for 6.1 hours in the control group and by 6.9 hours in the intervention group.
“We suggest that the mechanism for this beneficial effect in the present study is likely pressure offloading, which has been afforded by providing patients in the intervention group with this plantar-pressure feedback,” said Dr. Reeves.
“That’s feedback that they’ve lost naturally many years ago due to diabetic peripheral neuropathy,” he added. “So, in that respect, we would suggest that patients have really been empowered here to take control of their foot health in a way that they haven’t been able to since the onset of significant diabetic peripheral neuropathy.”
Diabetes UK provided the primary funding for the study (years 1-3), with Orpyx Medical Technologies, Canada, providing funding during the study extension (year 4). Dr. Reeves did not have any disclosures.
BERLIN – Smart insoles that warn diabetic individuals of high plantar pressures could be a simple solution to help them avoid recurrent foot ulcers, according to the results of a randomized trial.
Study participants with a history of diabetic foot ulcers wore the plantar pressure–sensing insoles (SurroSense Rx) and received feedback via sensor linked to a smart watch worn and were 71% less likely to experience a recurrent foot ulceration than were those who wore the insoles but did not get the pressure feedback (incidence rate ratio, 0.29; 95% confidence interval, 0.09-0.93; P = .037). The device has been cleared by the Food and Drug Administration.
Overall, there were few ulcers that occurred in the study, with 10 ulcers from 8,638 person-days from six patients reported in the control group and four ulcers from 11,835 person-days from four patients in the intervention group.
“Diabetic foot ulcers are a major global health and economic burden, but, in theory at least, they are ultimately preventable,” said study investigator Neil Reeves, PhD,, who presented the findings at the annual meeting of the European Association for the Study of Diabetes.
Data suggest that recurrence rates for ulceration are as high as 65% at 6 years, he said, with up to a quarter of ulcers progressing to the point where some form of amputation is needed.
“In the laboratory, we can measure plantar pressures, and these are considered as a relatively accurate proxy for diabetic foot ulcer risk. So we can discriminate between those with diabetic neuropathy, and those without, and also those with a previous history of ulceration,” Dr. Reeves said.
Dr. Reeves, who is professor of musculoskeletal biomechanics at Manchester (England) Metropolitan University, observed that the rationale behind the development of the smart insoles was to move plantar pressure measurement out of the laboratory and into the real world.
The smart insoles incorporate eight discreet pressure sensors that are connected to pod worn on the front of the participant’s own shoe and that wirelessly relay pressure information to a smartwatch. Both the control and the intervention groups received the same device, Dr. Reeves pointed out, but the difference was that the only the intervention group got any pressure feedback from the sensors to the smartwatch.
“When high pressure was experienced in the intervention group on any of these sensors, the patient was alerted both by an auditory alarm and also by being able to see this on the smartwatch,” Dr. Reeves explained.
“The patient would be alerted as to where the pressure was high on the foot and that would be a trigger to offload this high pressure.” Patients would then be instructed via the smartwatch to try to offload the pressure by either walking around for 2 minutes, actively taking the weight off the foot, or removing the shoe to check for any foreign bodies.
In all, there were 58 study participants – 32 randomized to the intervention group and 26 to the control group – who had a history of diabetic foot ulcers and peripheral neuropathy but who were able to walk independently for at least 30 steps. The mean age of patients in the intervention group was 59 years, 88% were male, 72% had type 2 diabetes mellitus, with the mean duration of diabetes was 22 years. Corresponding data in the control group were 67 years, 89% male, 85% had type 2 diabetes, and 21 years’ diabetes duration.
Patients were reviewed monthly over a period of 18 months or until plantar ulceration occurred. Information on diabetic foot ulcers was collected and standardized using a previously developed mobile app (Diabetes Sci Technol. 2018;12[1]:169-73) and then confirmed via blinded assessment by two experts.
Dr. Reeves noted that there was no significant difference in the time to ulceration between the groups, with 77.5% and 68.4% of the intervention and control group remaining ulcer free at 18 months (P = .30). When the data were adjusted for compliance, there was an 86% reduction in the risk of reulceration in the intervention versus the control group (IRR, 0.14; 95% CI, 0.03-0.63; P = .011). This analysis took into account only those study participants who had 4.5 hours or more of daily wear of the smart insoles (n = 40). On average, the insoles were worn for 6.1 hours in the control group and by 6.9 hours in the intervention group.
“We suggest that the mechanism for this beneficial effect in the present study is likely pressure offloading, which has been afforded by providing patients in the intervention group with this plantar-pressure feedback,” said Dr. Reeves.
“That’s feedback that they’ve lost naturally many years ago due to diabetic peripheral neuropathy,” he added. “So, in that respect, we would suggest that patients have really been empowered here to take control of their foot health in a way that they haven’t been able to since the onset of significant diabetic peripheral neuropathy.”
Diabetes UK provided the primary funding for the study (years 1-3), with Orpyx Medical Technologies, Canada, providing funding during the study extension (year 4). Dr. Reeves did not have any disclosures.
BERLIN – Smart insoles that warn diabetic individuals of high plantar pressures could be a simple solution to help them avoid recurrent foot ulcers, according to the results of a randomized trial.
Study participants with a history of diabetic foot ulcers wore the plantar pressure–sensing insoles (SurroSense Rx) and received feedback via sensor linked to a smart watch worn and were 71% less likely to experience a recurrent foot ulceration than were those who wore the insoles but did not get the pressure feedback (incidence rate ratio, 0.29; 95% confidence interval, 0.09-0.93; P = .037). The device has been cleared by the Food and Drug Administration.
Overall, there were few ulcers that occurred in the study, with 10 ulcers from 8,638 person-days from six patients reported in the control group and four ulcers from 11,835 person-days from four patients in the intervention group.
“Diabetic foot ulcers are a major global health and economic burden, but, in theory at least, they are ultimately preventable,” said study investigator Neil Reeves, PhD,, who presented the findings at the annual meeting of the European Association for the Study of Diabetes.
Data suggest that recurrence rates for ulceration are as high as 65% at 6 years, he said, with up to a quarter of ulcers progressing to the point where some form of amputation is needed.
“In the laboratory, we can measure plantar pressures, and these are considered as a relatively accurate proxy for diabetic foot ulcer risk. So we can discriminate between those with diabetic neuropathy, and those without, and also those with a previous history of ulceration,” Dr. Reeves said.
Dr. Reeves, who is professor of musculoskeletal biomechanics at Manchester (England) Metropolitan University, observed that the rationale behind the development of the smart insoles was to move plantar pressure measurement out of the laboratory and into the real world.
The smart insoles incorporate eight discreet pressure sensors that are connected to pod worn on the front of the participant’s own shoe and that wirelessly relay pressure information to a smartwatch. Both the control and the intervention groups received the same device, Dr. Reeves pointed out, but the difference was that the only the intervention group got any pressure feedback from the sensors to the smartwatch.
“When high pressure was experienced in the intervention group on any of these sensors, the patient was alerted both by an auditory alarm and also by being able to see this on the smartwatch,” Dr. Reeves explained.
“The patient would be alerted as to where the pressure was high on the foot and that would be a trigger to offload this high pressure.” Patients would then be instructed via the smartwatch to try to offload the pressure by either walking around for 2 minutes, actively taking the weight off the foot, or removing the shoe to check for any foreign bodies.
In all, there were 58 study participants – 32 randomized to the intervention group and 26 to the control group – who had a history of diabetic foot ulcers and peripheral neuropathy but who were able to walk independently for at least 30 steps. The mean age of patients in the intervention group was 59 years, 88% were male, 72% had type 2 diabetes mellitus, with the mean duration of diabetes was 22 years. Corresponding data in the control group were 67 years, 89% male, 85% had type 2 diabetes, and 21 years’ diabetes duration.
Patients were reviewed monthly over a period of 18 months or until plantar ulceration occurred. Information on diabetic foot ulcers was collected and standardized using a previously developed mobile app (Diabetes Sci Technol. 2018;12[1]:169-73) and then confirmed via blinded assessment by two experts.
Dr. Reeves noted that there was no significant difference in the time to ulceration between the groups, with 77.5% and 68.4% of the intervention and control group remaining ulcer free at 18 months (P = .30). When the data were adjusted for compliance, there was an 86% reduction in the risk of reulceration in the intervention versus the control group (IRR, 0.14; 95% CI, 0.03-0.63; P = .011). This analysis took into account only those study participants who had 4.5 hours or more of daily wear of the smart insoles (n = 40). On average, the insoles were worn for 6.1 hours in the control group and by 6.9 hours in the intervention group.
“We suggest that the mechanism for this beneficial effect in the present study is likely pressure offloading, which has been afforded by providing patients in the intervention group with this plantar-pressure feedback,” said Dr. Reeves.
“That’s feedback that they’ve lost naturally many years ago due to diabetic peripheral neuropathy,” he added. “So, in that respect, we would suggest that patients have really been empowered here to take control of their foot health in a way that they haven’t been able to since the onset of significant diabetic peripheral neuropathy.”
Diabetes UK provided the primary funding for the study (years 1-3), with Orpyx Medical Technologies, Canada, providing funding during the study extension (year 4). Dr. Reeves did not have any disclosures.
REPORTING FROM EASD 2018
Key clinical point: Pressure-sensing smart insoles could help warn “high-risk” individuals to offload excess pressure on their feet.
Major finding: A 71% reduction in the risk of reulceration was observed when compared with the intervention with the control group (P = .037).
Study details: Randomized, single-blind controlled randomized study of 58 adults with a history of plantar diabetic foot ulcers.
Disclosures: Diabetes UK provided the primary funding for the study (years 1-3), with Orpyx Medical Technologies providing funding during the study extension (year 4). Dr. Reeves did not have any disclosures.
Platelet-rich patch helps heal difficult diabetic foot ulcers
BERLIN –
With the LeucoPatch – which contained study participants’ own cells (platelets, fibrin, and leukocytes) – 34.1% of ulcers healed within 20 weeks versus 21.6% of ulcers that were treated using the best standard care (unadjusted odds ratio, 1.58; 95% confidence interval, 1.06-2.25; P = .02). Healing was defined as complete epithelialization maintained for 4 weeks, as confirmed by an observer blinded to the treatment group.
Results remained significant after adjusting for baseline wound size (adjusted OR 1.89; P = .02) and following a per-protocol analysis (aOR, 1.75; P = .048).
Furthermore, time to healing was shorter in the intervention group (P = .02), lead study investigator Frances Game, MD, of the Derby (England) Teaching Hospitals National Health Service Foundation Trust, reported at the annual meeting of the European Association for the Study of Diabetes.
“Successive systematic reviews from the International Working Group of the Diabetic Foot have shown that there’s very poor evidence for many of the things that we do in day-to-day practice,” she said.
“Having said that, there have been some positive studies using platelets or platelet-rich plasma to improve healing of the diabetic foot,” Dr. Game noted, although results have been inconsistent. From this the idea of the LeucoPatch was born. This is an autologous active cell therapy, which according to the Danish company Reapplix that markets it, helps patients “heal themselves.”
The LeucoPatch system is made by taking 18 mL of a patient’s blood and spinning the collection tube in a centrifuge for 20 minutes to generate a three-layered disc that contains fibrin, platelets, and leukocytes. This can then be applied to the surface of the diabetic foot ulcer. Dr. Game noted that 18 mL of blood will make a 5-cm patch and more than one patch can be made from the blood sample.
“It looks like a bit of wet skin when it comes out of the centrifuge and you just put it on sole side down. It’s taking the patient’s own cells, that often aren’t getting to the ulcer because of the morbidity of the patient and vascular disease, and actually putting them where they need to be,” she explained. The patch usually becomes absorbed within a week; depending on the ulcer, reapplication may be required.
“It’s quite a straightforward procedure that’s performed the bedside,” Dr. Game observed. “That’s how we were able to recruit so many patients, as it’s quite simple.” Indeed, almost 600 people with diabetic foot ulcers agreed to participate in the study, but only those with difficult-to-treat ulcers were included after a 4-week run-in period. The 269 patients who were finally randomized were treated at 32 specialist diabetic foot clinics in the United Kingdom, Denmark, and Sweden.
The majority of participants were male (82%) and had type 2 diabetes mellitus (83%). The mean age was 62 years and the median duration of diabetes was 16 years. The mean ulcer area was 240 mm2, with 87% being superficial, 10% reaching down to the tendon, and 3% down to the bone. In 78% of cases, the total forefoot was affected, with the plantar forefoot and hind foot affected in a respective 42% and 22% of cases.
The LeucoPatch system is already being used in several European countries, including Germany and Belgium, Dr. Game noted. However, this is the first randomized, controlled trial to demonstrate a clinical and statistically significant benefit. The data show that the weekly application of LeucoPatch is clearly of benefit in a population of patients with hard-to-heal diabetic foot ulcers.
“The low drop-out numbers suggest a good patient acceptability,” she noted, and “the treatment was without apparent increase in adverse events, particularly without evidence of new onset anemia.”
Cost-effectiveness data were collected throughout the study and will be available at a future date when these have been analyzed, Dr. Game said.
The LeucoPatch system received Food and Drug Administration approval in April 2017.
The research was published online in the Lancet Diabetes & Endocrinology ahead of the presentation.
The trial was funded by Reapplix. Dr. Game reported receiving research funding from the company.
SOURCES: Game F et al. EASD 2018, Abstract 9.
BERLIN –
With the LeucoPatch – which contained study participants’ own cells (platelets, fibrin, and leukocytes) – 34.1% of ulcers healed within 20 weeks versus 21.6% of ulcers that were treated using the best standard care (unadjusted odds ratio, 1.58; 95% confidence interval, 1.06-2.25; P = .02). Healing was defined as complete epithelialization maintained for 4 weeks, as confirmed by an observer blinded to the treatment group.
Results remained significant after adjusting for baseline wound size (adjusted OR 1.89; P = .02) and following a per-protocol analysis (aOR, 1.75; P = .048).
Furthermore, time to healing was shorter in the intervention group (P = .02), lead study investigator Frances Game, MD, of the Derby (England) Teaching Hospitals National Health Service Foundation Trust, reported at the annual meeting of the European Association for the Study of Diabetes.
“Successive systematic reviews from the International Working Group of the Diabetic Foot have shown that there’s very poor evidence for many of the things that we do in day-to-day practice,” she said.
“Having said that, there have been some positive studies using platelets or platelet-rich plasma to improve healing of the diabetic foot,” Dr. Game noted, although results have been inconsistent. From this the idea of the LeucoPatch was born. This is an autologous active cell therapy, which according to the Danish company Reapplix that markets it, helps patients “heal themselves.”
The LeucoPatch system is made by taking 18 mL of a patient’s blood and spinning the collection tube in a centrifuge for 20 minutes to generate a three-layered disc that contains fibrin, platelets, and leukocytes. This can then be applied to the surface of the diabetic foot ulcer. Dr. Game noted that 18 mL of blood will make a 5-cm patch and more than one patch can be made from the blood sample.
“It looks like a bit of wet skin when it comes out of the centrifuge and you just put it on sole side down. It’s taking the patient’s own cells, that often aren’t getting to the ulcer because of the morbidity of the patient and vascular disease, and actually putting them where they need to be,” she explained. The patch usually becomes absorbed within a week; depending on the ulcer, reapplication may be required.
“It’s quite a straightforward procedure that’s performed the bedside,” Dr. Game observed. “That’s how we were able to recruit so many patients, as it’s quite simple.” Indeed, almost 600 people with diabetic foot ulcers agreed to participate in the study, but only those with difficult-to-treat ulcers were included after a 4-week run-in period. The 269 patients who were finally randomized were treated at 32 specialist diabetic foot clinics in the United Kingdom, Denmark, and Sweden.
The majority of participants were male (82%) and had type 2 diabetes mellitus (83%). The mean age was 62 years and the median duration of diabetes was 16 years. The mean ulcer area was 240 mm2, with 87% being superficial, 10% reaching down to the tendon, and 3% down to the bone. In 78% of cases, the total forefoot was affected, with the plantar forefoot and hind foot affected in a respective 42% and 22% of cases.
The LeucoPatch system is already being used in several European countries, including Germany and Belgium, Dr. Game noted. However, this is the first randomized, controlled trial to demonstrate a clinical and statistically significant benefit. The data show that the weekly application of LeucoPatch is clearly of benefit in a population of patients with hard-to-heal diabetic foot ulcers.
“The low drop-out numbers suggest a good patient acceptability,” she noted, and “the treatment was without apparent increase in adverse events, particularly without evidence of new onset anemia.”
Cost-effectiveness data were collected throughout the study and will be available at a future date when these have been analyzed, Dr. Game said.
The LeucoPatch system received Food and Drug Administration approval in April 2017.
The research was published online in the Lancet Diabetes & Endocrinology ahead of the presentation.
The trial was funded by Reapplix. Dr. Game reported receiving research funding from the company.
SOURCES: Game F et al. EASD 2018, Abstract 9.
BERLIN –
With the LeucoPatch – which contained study participants’ own cells (platelets, fibrin, and leukocytes) – 34.1% of ulcers healed within 20 weeks versus 21.6% of ulcers that were treated using the best standard care (unadjusted odds ratio, 1.58; 95% confidence interval, 1.06-2.25; P = .02). Healing was defined as complete epithelialization maintained for 4 weeks, as confirmed by an observer blinded to the treatment group.
Results remained significant after adjusting for baseline wound size (adjusted OR 1.89; P = .02) and following a per-protocol analysis (aOR, 1.75; P = .048).
Furthermore, time to healing was shorter in the intervention group (P = .02), lead study investigator Frances Game, MD, of the Derby (England) Teaching Hospitals National Health Service Foundation Trust, reported at the annual meeting of the European Association for the Study of Diabetes.
“Successive systematic reviews from the International Working Group of the Diabetic Foot have shown that there’s very poor evidence for many of the things that we do in day-to-day practice,” she said.
“Having said that, there have been some positive studies using platelets or platelet-rich plasma to improve healing of the diabetic foot,” Dr. Game noted, although results have been inconsistent. From this the idea of the LeucoPatch was born. This is an autologous active cell therapy, which according to the Danish company Reapplix that markets it, helps patients “heal themselves.”
The LeucoPatch system is made by taking 18 mL of a patient’s blood and spinning the collection tube in a centrifuge for 20 minutes to generate a three-layered disc that contains fibrin, platelets, and leukocytes. This can then be applied to the surface of the diabetic foot ulcer. Dr. Game noted that 18 mL of blood will make a 5-cm patch and more than one patch can be made from the blood sample.
“It looks like a bit of wet skin when it comes out of the centrifuge and you just put it on sole side down. It’s taking the patient’s own cells, that often aren’t getting to the ulcer because of the morbidity of the patient and vascular disease, and actually putting them where they need to be,” she explained. The patch usually becomes absorbed within a week; depending on the ulcer, reapplication may be required.
“It’s quite a straightforward procedure that’s performed the bedside,” Dr. Game observed. “That’s how we were able to recruit so many patients, as it’s quite simple.” Indeed, almost 600 people with diabetic foot ulcers agreed to participate in the study, but only those with difficult-to-treat ulcers were included after a 4-week run-in period. The 269 patients who were finally randomized were treated at 32 specialist diabetic foot clinics in the United Kingdom, Denmark, and Sweden.
The majority of participants were male (82%) and had type 2 diabetes mellitus (83%). The mean age was 62 years and the median duration of diabetes was 16 years. The mean ulcer area was 240 mm2, with 87% being superficial, 10% reaching down to the tendon, and 3% down to the bone. In 78% of cases, the total forefoot was affected, with the plantar forefoot and hind foot affected in a respective 42% and 22% of cases.
The LeucoPatch system is already being used in several European countries, including Germany and Belgium, Dr. Game noted. However, this is the first randomized, controlled trial to demonstrate a clinical and statistically significant benefit. The data show that the weekly application of LeucoPatch is clearly of benefit in a population of patients with hard-to-heal diabetic foot ulcers.
“The low drop-out numbers suggest a good patient acceptability,” she noted, and “the treatment was without apparent increase in adverse events, particularly without evidence of new onset anemia.”
Cost-effectiveness data were collected throughout the study and will be available at a future date when these have been analyzed, Dr. Game said.
The LeucoPatch system received Food and Drug Administration approval in April 2017.
The research was published online in the Lancet Diabetes & Endocrinology ahead of the presentation.
The trial was funded by Reapplix. Dr. Game reported receiving research funding from the company.
SOURCES: Game F et al. EASD 2018, Abstract 9.
REPORTING FROM EASD 2018
Key clinical point: Weekly application of LeucoPatch enabled greater healing in a shorter time frame than standard care.
Major finding: Within 20 weeks, 34.1% versus 21.6% of diabetic foot ulcers had healed (unadjusted odds ratio, 1.58; 95% confidence interval, 1.06-2.25; P = .02).
Study details: A multicenter, multinational, observer-blinded, randomized, controlled trial of 269 patients with hard-to-heal diabetic foot ulcers.
Disclosures: The trial was funded by Reapplix. Dr. Game reported receiving research funding from the company.
Sources: Game F et al. EASD 2018, Abstract 9.
Cutaneous Angiosarcoma of the Lower Leg
Angiosarcoma is a rare and aggressive vascular malignant neoplasm derived from endothelial cells. In general, sarcomas account for approximately 1% of all malignancies, with approximately 2% being angiosarcomas.1 The risk of recurrence at 5 years is estimated to be 84%, and 5-year survival is estimated at 15% to 30%. Poor prognostic factors for angiosarcoma include large tumor size, depth of invasion greater than 3 mm, high mitotic rate, positive surgical margins, and metastasis.2 Approximately 20% to 40% of patients who are diagnosed with angiosarcoma already have distant metastasis, contributing to the aggressive nature of this neoplasm.3
Angiosarcoma can affect various anatomic locations, including the skin, soft tissue, breasts, and liver. Cutaneous angiosarcoma is the most common clinical manifestation, accounting for approximately 50% to 60% of all cases, and typically is known to occur in 3 distinct settings.2 Primary or idiopathic cutaneous angiosarcoma is most commonly seen in elderly individuals, with a peak incidence in the seventh to eighth decades of life, and presents as a bruiselike lesion predominantly on the head and neck. Angiosarcoma also is seen clinically in patients exposed to radiation treatment, with a median onset of symptoms occurring 5 to 10 years posttreatment, and in patients with chronic lymphedema, usually on the arm following radical mastectomy, which also is known as Stewart-Treves syndrome.2
With any sarcoma, treatment typically first involves surgical excision; however, there is no direct approach for treatment of cutaneous angiosarcoma, as an individual plan typically is needed for each patient. Treatment options include surgical excision, radiation, chemotherapy, or a combination of these therapies.2,4
We present a rare case of cutaneous angiosarcoma of the left leg in the setting of chronic venous insufficiency with some degree of lymphedema and a nonhealing ulcer. This case is unique in that it does not fit the classic presentation of cutaneous angiosarcoma previously described.
Case Report
An 83-year-old woman with a medical history of advanced dementia, congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease, type 2 diabetes mellitus, hypertension, and chronic venous insufficiency with stasis dermatitis presented to the emergency department following a mechanical fall. Most of her medical history was obtained from the patient’s family. She had a history of multiple falls originally thought to be related to a chronic leg ulcer that had been managed with wound care. Recently, however, the lesion was noted to have increasing erythema surrounding the wound margins. An 8×8-cm erythematous plaque on the anterior lateral left leg with a firm central nodule with hemorrhagic crust that measured approximately 4 cm in diameter was noted by the emergency department physicians (Figure 1). In the emergency department, vitals and other laboratory values were within reference range, and a radiograph of the left tibia/fibula was unremarkable. Cellulitis initially was considered in the emergency department and cephalexin was started; however, since the patient was afebrile and had no leukocytosis, plastic surgery also was consulted. Biopsies were obtained from the superior and inferior parts of the lesion. Histologic analysis revealed a poorly differentiated vascular neoplasm of epithelioid endothelial cells with considerable cell atypia that extended through the entirety of the dermis (Figure 2). The tumor cells stained positive with vimentin and CD34. Pathology noted no immunohistochemistry stains to synaptophysin, S-100, human melanoma black 45, MART-1, CK20, CK7, CK8/18, CK5/6, and p63. The pathologic diagnosis was consistent with cutaneous angiosarcoma. Computed tomography of the chest, abdomen, and pelvis revealed no local or distant metastases.

A wide excision of the cutaneous angiosarcoma was performed. The initial frozen section analysis revealed positive margins. Three additional excisions still showed positive margins, and further excision was held after obtaining family consent due to the extensive nature of the neoplasm and lengthy operating room time. The final defect after excision measured 15×10×2.5 cm (Figure 3A), and subsequent application of a split-thickness graft was performed. Additional treatment options were discussed with the family, including radiation therapy, amputation of the left lower leg, or no treatment. The family opted not to proceed with further treatment. The graft healed without signs of reoccurrence approximately 3 months later (Figure 3B), and the patient received physical therapy, which allowed her to gain strength and some independence.

Comment
Clinical Manifestation
Cutaneous angiosarcoma is a rare malignant vascular neoplasm that when clinically diagnosed is typically seen in 3 settings: (1) idiopathic (commonly on the face and neck), (2) following radiation treatment, and (3) classically following mastectomy with subsequent chronic lymphedema. Our patient did not classically fit these settings of cutaneous angiosarcoma due to the location of the lesion on the lower leg as well as its occurrence in the setting of a chronic nonhealing ulcer and lymphedema.
Chronic lymphedema is a common clinical manifestation that is likely secondary to other medical conditions, such as in our patient. As a result, these patients are at increased risk for developing chronic ulcers due to poor wound healing; however, as seen in our patient, chronic nonhealing ulcers require a broad differential because they may clinically mimic many processes. Patient history and visual presentation were crucial in this case because a biopsy was obtained that ultimately led to the patient’s diagnosis.
Differential Diagnosis
Initially, a venous ulcer secondary to chronic venous insufficiency was considered in the differential for our patient. She had a history of congestive heart failure, kidney disease, and type 2 diabetes mellitus, all of which contribute to lymphedema and/or poor wound healing. However, venous ulcers usually are located on the medial ankles and are irregularly shaped with an erythematous border and fibrinous exudate with central depression, making it a less likely diagnosis in our patient. Additionally, an infectious process was considered, but the patient was afebrile and laboratory values demonstrated no leukocytosis.
Marjolin ulcer was highly suspected because the clinical presentation revealed a nodule with hemorrhagic crust and induration in the setting of a chronic nonhealing ulcer. The pathogenesis of malignancy in chronic ulcers is thought to be due to continuous mitotic activity from regeneration and repair of the wound, especially in the setting of repeated trauma to the area.5 In our patient, the history of multiple falls with possible multitrauma injury to the chronic ulcer further increased suspicion of malignancy. The most common and frequently seen malignancy that develops in chronic ulcers is squamous cell carcinoma (SCC) followed by basal cell carcinoma. Plastic surgery suspected an SCC for the working diagnosis, which prompted a punch biopsy; however, the histologic analysis was not consistent with SCC or basal cell carcinoma. Marjolin ulcer also may demonstrate a periosteal reaction,5 which was not the case with our patient after a radiograph of the left tibia/fibula was unremarkable.
Another potential malignancy to consider is melanoma. There are few case reports of biopsy-proven melanoma from an enlarging chronic ulcer.6,7 Additionally, poorly differentiated angiosarcoma can mimic melanoma2; however, immunohistochemistry stain was negative for S-100, human melanoma black 45, and MART-1, making melanoma unlikely.
Kaposi sarcoma (KS) and angiosarcoma are both malignant vascular tumors that similarly present with red to purple patches, plaques, or nodules, making it difficult to distinguish between the two conditions. It is important to note that KS usually is lower grade, and the pathogenesis is linked to human herpesvirus 8, which can be identified on immunohistochemistry staining. There have been cases of KS reported in patients who have no history of human immunodeficiency virus/AIDS, thus the classic subtype of KS may have been considered in this patient.8 The histologic appearance of KS may vary from dilated irregular endothelial cells lining the vascular space to mild endothelial cell atypia. Histology also shows hemosiderin-laden macrophages, extravasated red blood cells, and an inflammatory infiltrate. An additional malignant vascular neoplasm that needs to be differentiated is epithelioid hemangioendothelioma. Cutaneous presentation of an epithelioid hemangioendothelioma may be similar to what was seen in our patient but histologically will usually show neoplastic cells with pale eosinophilic cytoplasm and vesicular nuclei of plump, oval, polygonal cells in cords or aggregates surrounding vascular channels. These neoplasms also tend to occur around medium- to large-sized veins.1,9 With our patient, even though human herpesvirus 8 was not tested with immunohistochemistry, gold standard immunohistochemistry confirmation with CD34 and vimentin staining combined with poorly differentiated endothelial atypia with mitotic figures on histologic analysis favored angiosarcoma versus KS or epithelioid hemangioendothelioma.10,11
Management
Cutaneous angiosarcoma is a rare and aggressive vascular neoplasm accounting for approximately 2% of all combined sarcomas, with an estimated 20% to 40% having distant metastasis at diagnosis.1,3 For this reason, computed tomography was performed in our patient and revealed no local or distant metastasis. Therefore, chemotherapy was not an appropriate adjuvant treatment option.12 With no evidence of metastasis, initial treatment began with surgical removal but proved to be difficult in our patient. Although the implications of positive surgical margins remain unclear with regard to overall patient survival, surgical resection followed by radiation therapy has been shown to be optimal, as it reduces the risk of local reoccurrence.3 There have been reported cases of cutaneous angiosarcoma of the leg that were treated with amputation without signs of reoccurrence or metastasis.10,13,14 Given the results from these cases and considering that our patient had no metastasis, amputation seemed to be a good prognostic option; however, considering other factors regarding the patient’s comorbidities and quality of life, her family decided not to pursue any further treatment with amputation or radiation therapy.
Conclusion
There should be low threshold for biopsy in patients who present with nonhealing wounds that do not progress in the normal phase of wound healing with suspicion for malignancy. As seen with our patient, cutaneous angiosarcoma can clinically mimic many disease processes, and although rare in nature, it should always be considered when a patient presents with a rapidly growing lesion in the setting of chronic lymphedema or venous ulcer.
- Kumar V, Abbas A, Aster J. Robbins Basic Pathology. 9th ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Donghi D, Kerl K, Dummer R, et al. Cutaneous angiosarcoma: own experience over 13 years. clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230-1234.
- Dossett LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64.
- Gerslova A, Pokorna A, Stukavcova A, et al. Rare cause of non-healing foot wound—acral lentiginous melanoma. Neuro Endocrinol Lett. 2012;37:12-17.
- Turk BG, Bozkurt A, Yaman B, et al. Melanoma arising in chronic ulceration associated with lymphoedema. J Wound Care. 2013;22:74-75.
- Phavixay L, Raynolds D, Simman R. Non AIDS Kaposi’s sarcoma leading to lower extremities wounds, case presentations and discussion.J Am Coll Clin Wound Spec. 2012;4:13-15.
- Requena L, Kutzner H. Hemangioendothelioma. Semin Diagn Pathol. 2013;30:29-44.
- Harrison WD, Chandrasekar CR. Stewart-Treves syndrome following idiopathic leg lymphoedema: remember sarcoma. J Wound Care. 2015;24(6 suppl):S5-S7.
- Kak I, Salama S, Gohla G, et al. A case of patch stage of Kaposi’s sarcoma and discussion of the differential diagnosis. Rare Tumors. 2016;8:6123.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Linda DD, Harish S, Alowami S, et al. Radiology-pathology conference: cutaneous angiosarcoma of the leg. Clin Imaging. 2013;37:602-607.
- Roy P, Clark MA, Thomas JM. Stewart-Treves syndrome—treatment and outcome in six patients from a single centre. Eur J Surg Oncol. 2004;30:982-986.
Angiosarcoma is a rare and aggressive vascular malignant neoplasm derived from endothelial cells. In general, sarcomas account for approximately 1% of all malignancies, with approximately 2% being angiosarcomas.1 The risk of recurrence at 5 years is estimated to be 84%, and 5-year survival is estimated at 15% to 30%. Poor prognostic factors for angiosarcoma include large tumor size, depth of invasion greater than 3 mm, high mitotic rate, positive surgical margins, and metastasis.2 Approximately 20% to 40% of patients who are diagnosed with angiosarcoma already have distant metastasis, contributing to the aggressive nature of this neoplasm.3
Angiosarcoma can affect various anatomic locations, including the skin, soft tissue, breasts, and liver. Cutaneous angiosarcoma is the most common clinical manifestation, accounting for approximately 50% to 60% of all cases, and typically is known to occur in 3 distinct settings.2 Primary or idiopathic cutaneous angiosarcoma is most commonly seen in elderly individuals, with a peak incidence in the seventh to eighth decades of life, and presents as a bruiselike lesion predominantly on the head and neck. Angiosarcoma also is seen clinically in patients exposed to radiation treatment, with a median onset of symptoms occurring 5 to 10 years posttreatment, and in patients with chronic lymphedema, usually on the arm following radical mastectomy, which also is known as Stewart-Treves syndrome.2
With any sarcoma, treatment typically first involves surgical excision; however, there is no direct approach for treatment of cutaneous angiosarcoma, as an individual plan typically is needed for each patient. Treatment options include surgical excision, radiation, chemotherapy, or a combination of these therapies.2,4
We present a rare case of cutaneous angiosarcoma of the left leg in the setting of chronic venous insufficiency with some degree of lymphedema and a nonhealing ulcer. This case is unique in that it does not fit the classic presentation of cutaneous angiosarcoma previously described.
Case Report
An 83-year-old woman with a medical history of advanced dementia, congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease, type 2 diabetes mellitus, hypertension, and chronic venous insufficiency with stasis dermatitis presented to the emergency department following a mechanical fall. Most of her medical history was obtained from the patient’s family. She had a history of multiple falls originally thought to be related to a chronic leg ulcer that had been managed with wound care. Recently, however, the lesion was noted to have increasing erythema surrounding the wound margins. An 8×8-cm erythematous plaque on the anterior lateral left leg with a firm central nodule with hemorrhagic crust that measured approximately 4 cm in diameter was noted by the emergency department physicians (Figure 1). In the emergency department, vitals and other laboratory values were within reference range, and a radiograph of the left tibia/fibula was unremarkable. Cellulitis initially was considered in the emergency department and cephalexin was started; however, since the patient was afebrile and had no leukocytosis, plastic surgery also was consulted. Biopsies were obtained from the superior and inferior parts of the lesion. Histologic analysis revealed a poorly differentiated vascular neoplasm of epithelioid endothelial cells with considerable cell atypia that extended through the entirety of the dermis (Figure 2). The tumor cells stained positive with vimentin and CD34. Pathology noted no immunohistochemistry stains to synaptophysin, S-100, human melanoma black 45, MART-1, CK20, CK7, CK8/18, CK5/6, and p63. The pathologic diagnosis was consistent with cutaneous angiosarcoma. Computed tomography of the chest, abdomen, and pelvis revealed no local or distant metastases.


A wide excision of the cutaneous angiosarcoma was performed. The initial frozen section analysis revealed positive margins. Three additional excisions still showed positive margins, and further excision was held after obtaining family consent due to the extensive nature of the neoplasm and lengthy operating room time. The final defect after excision measured 15×10×2.5 cm (Figure 3A), and subsequent application of a split-thickness graft was performed. Additional treatment options were discussed with the family, including radiation therapy, amputation of the left lower leg, or no treatment. The family opted not to proceed with further treatment. The graft healed without signs of reoccurrence approximately 3 months later (Figure 3B), and the patient received physical therapy, which allowed her to gain strength and some independence.

Comment
Clinical Manifestation
Cutaneous angiosarcoma is a rare malignant vascular neoplasm that when clinically diagnosed is typically seen in 3 settings: (1) idiopathic (commonly on the face and neck), (2) following radiation treatment, and (3) classically following mastectomy with subsequent chronic lymphedema. Our patient did not classically fit these settings of cutaneous angiosarcoma due to the location of the lesion on the lower leg as well as its occurrence in the setting of a chronic nonhealing ulcer and lymphedema.
Chronic lymphedema is a common clinical manifestation that is likely secondary to other medical conditions, such as in our patient. As a result, these patients are at increased risk for developing chronic ulcers due to poor wound healing; however, as seen in our patient, chronic nonhealing ulcers require a broad differential because they may clinically mimic many processes. Patient history and visual presentation were crucial in this case because a biopsy was obtained that ultimately led to the patient’s diagnosis.
Differential Diagnosis
Initially, a venous ulcer secondary to chronic venous insufficiency was considered in the differential for our patient. She had a history of congestive heart failure, kidney disease, and type 2 diabetes mellitus, all of which contribute to lymphedema and/or poor wound healing. However, venous ulcers usually are located on the medial ankles and are irregularly shaped with an erythematous border and fibrinous exudate with central depression, making it a less likely diagnosis in our patient. Additionally, an infectious process was considered, but the patient was afebrile and laboratory values demonstrated no leukocytosis.
Marjolin ulcer was highly suspected because the clinical presentation revealed a nodule with hemorrhagic crust and induration in the setting of a chronic nonhealing ulcer. The pathogenesis of malignancy in chronic ulcers is thought to be due to continuous mitotic activity from regeneration and repair of the wound, especially in the setting of repeated trauma to the area.5 In our patient, the history of multiple falls with possible multitrauma injury to the chronic ulcer further increased suspicion of malignancy. The most common and frequently seen malignancy that develops in chronic ulcers is squamous cell carcinoma (SCC) followed by basal cell carcinoma. Plastic surgery suspected an SCC for the working diagnosis, which prompted a punch biopsy; however, the histologic analysis was not consistent with SCC or basal cell carcinoma. Marjolin ulcer also may demonstrate a periosteal reaction,5 which was not the case with our patient after a radiograph of the left tibia/fibula was unremarkable.
Another potential malignancy to consider is melanoma. There are few case reports of biopsy-proven melanoma from an enlarging chronic ulcer.6,7 Additionally, poorly differentiated angiosarcoma can mimic melanoma2; however, immunohistochemistry stain was negative for S-100, human melanoma black 45, and MART-1, making melanoma unlikely.
Kaposi sarcoma (KS) and angiosarcoma are both malignant vascular tumors that similarly present with red to purple patches, plaques, or nodules, making it difficult to distinguish between the two conditions. It is important to note that KS usually is lower grade, and the pathogenesis is linked to human herpesvirus 8, which can be identified on immunohistochemistry staining. There have been cases of KS reported in patients who have no history of human immunodeficiency virus/AIDS, thus the classic subtype of KS may have been considered in this patient.8 The histologic appearance of KS may vary from dilated irregular endothelial cells lining the vascular space to mild endothelial cell atypia. Histology also shows hemosiderin-laden macrophages, extravasated red blood cells, and an inflammatory infiltrate. An additional malignant vascular neoplasm that needs to be differentiated is epithelioid hemangioendothelioma. Cutaneous presentation of an epithelioid hemangioendothelioma may be similar to what was seen in our patient but histologically will usually show neoplastic cells with pale eosinophilic cytoplasm and vesicular nuclei of plump, oval, polygonal cells in cords or aggregates surrounding vascular channels. These neoplasms also tend to occur around medium- to large-sized veins.1,9 With our patient, even though human herpesvirus 8 was not tested with immunohistochemistry, gold standard immunohistochemistry confirmation with CD34 and vimentin staining combined with poorly differentiated endothelial atypia with mitotic figures on histologic analysis favored angiosarcoma versus KS or epithelioid hemangioendothelioma.10,11
Management
Cutaneous angiosarcoma is a rare and aggressive vascular neoplasm accounting for approximately 2% of all combined sarcomas, with an estimated 20% to 40% having distant metastasis at diagnosis.1,3 For this reason, computed tomography was performed in our patient and revealed no local or distant metastasis. Therefore, chemotherapy was not an appropriate adjuvant treatment option.12 With no evidence of metastasis, initial treatment began with surgical removal but proved to be difficult in our patient. Although the implications of positive surgical margins remain unclear with regard to overall patient survival, surgical resection followed by radiation therapy has been shown to be optimal, as it reduces the risk of local reoccurrence.3 There have been reported cases of cutaneous angiosarcoma of the leg that were treated with amputation without signs of reoccurrence or metastasis.10,13,14 Given the results from these cases and considering that our patient had no metastasis, amputation seemed to be a good prognostic option; however, considering other factors regarding the patient’s comorbidities and quality of life, her family decided not to pursue any further treatment with amputation or radiation therapy.
Conclusion
There should be low threshold for biopsy in patients who present with nonhealing wounds that do not progress in the normal phase of wound healing with suspicion for malignancy. As seen with our patient, cutaneous angiosarcoma can clinically mimic many disease processes, and although rare in nature, it should always be considered when a patient presents with a rapidly growing lesion in the setting of chronic lymphedema or venous ulcer.
Angiosarcoma is a rare and aggressive vascular malignant neoplasm derived from endothelial cells. In general, sarcomas account for approximately 1% of all malignancies, with approximately 2% being angiosarcomas.1 The risk of recurrence at 5 years is estimated to be 84%, and 5-year survival is estimated at 15% to 30%. Poor prognostic factors for angiosarcoma include large tumor size, depth of invasion greater than 3 mm, high mitotic rate, positive surgical margins, and metastasis.2 Approximately 20% to 40% of patients who are diagnosed with angiosarcoma already have distant metastasis, contributing to the aggressive nature of this neoplasm.3
Angiosarcoma can affect various anatomic locations, including the skin, soft tissue, breasts, and liver. Cutaneous angiosarcoma is the most common clinical manifestation, accounting for approximately 50% to 60% of all cases, and typically is known to occur in 3 distinct settings.2 Primary or idiopathic cutaneous angiosarcoma is most commonly seen in elderly individuals, with a peak incidence in the seventh to eighth decades of life, and presents as a bruiselike lesion predominantly on the head and neck. Angiosarcoma also is seen clinically in patients exposed to radiation treatment, with a median onset of symptoms occurring 5 to 10 years posttreatment, and in patients with chronic lymphedema, usually on the arm following radical mastectomy, which also is known as Stewart-Treves syndrome.2
With any sarcoma, treatment typically first involves surgical excision; however, there is no direct approach for treatment of cutaneous angiosarcoma, as an individual plan typically is needed for each patient. Treatment options include surgical excision, radiation, chemotherapy, or a combination of these therapies.2,4
We present a rare case of cutaneous angiosarcoma of the left leg in the setting of chronic venous insufficiency with some degree of lymphedema and a nonhealing ulcer. This case is unique in that it does not fit the classic presentation of cutaneous angiosarcoma previously described.
Case Report
An 83-year-old woman with a medical history of advanced dementia, congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease, type 2 diabetes mellitus, hypertension, and chronic venous insufficiency with stasis dermatitis presented to the emergency department following a mechanical fall. Most of her medical history was obtained from the patient’s family. She had a history of multiple falls originally thought to be related to a chronic leg ulcer that had been managed with wound care. Recently, however, the lesion was noted to have increasing erythema surrounding the wound margins. An 8×8-cm erythematous plaque on the anterior lateral left leg with a firm central nodule with hemorrhagic crust that measured approximately 4 cm in diameter was noted by the emergency department physicians (Figure 1). In the emergency department, vitals and other laboratory values were within reference range, and a radiograph of the left tibia/fibula was unremarkable. Cellulitis initially was considered in the emergency department and cephalexin was started; however, since the patient was afebrile and had no leukocytosis, plastic surgery also was consulted. Biopsies were obtained from the superior and inferior parts of the lesion. Histologic analysis revealed a poorly differentiated vascular neoplasm of epithelioid endothelial cells with considerable cell atypia that extended through the entirety of the dermis (Figure 2). The tumor cells stained positive with vimentin and CD34. Pathology noted no immunohistochemistry stains to synaptophysin, S-100, human melanoma black 45, MART-1, CK20, CK7, CK8/18, CK5/6, and p63. The pathologic diagnosis was consistent with cutaneous angiosarcoma. Computed tomography of the chest, abdomen, and pelvis revealed no local or distant metastases.


A wide excision of the cutaneous angiosarcoma was performed. The initial frozen section analysis revealed positive margins. Three additional excisions still showed positive margins, and further excision was held after obtaining family consent due to the extensive nature of the neoplasm and lengthy operating room time. The final defect after excision measured 15×10×2.5 cm (Figure 3A), and subsequent application of a split-thickness graft was performed. Additional treatment options were discussed with the family, including radiation therapy, amputation of the left lower leg, or no treatment. The family opted not to proceed with further treatment. The graft healed without signs of reoccurrence approximately 3 months later (Figure 3B), and the patient received physical therapy, which allowed her to gain strength and some independence.

Comment
Clinical Manifestation
Cutaneous angiosarcoma is a rare malignant vascular neoplasm that when clinically diagnosed is typically seen in 3 settings: (1) idiopathic (commonly on the face and neck), (2) following radiation treatment, and (3) classically following mastectomy with subsequent chronic lymphedema. Our patient did not classically fit these settings of cutaneous angiosarcoma due to the location of the lesion on the lower leg as well as its occurrence in the setting of a chronic nonhealing ulcer and lymphedema.
Chronic lymphedema is a common clinical manifestation that is likely secondary to other medical conditions, such as in our patient. As a result, these patients are at increased risk for developing chronic ulcers due to poor wound healing; however, as seen in our patient, chronic nonhealing ulcers require a broad differential because they may clinically mimic many processes. Patient history and visual presentation were crucial in this case because a biopsy was obtained that ultimately led to the patient’s diagnosis.
Differential Diagnosis
Initially, a venous ulcer secondary to chronic venous insufficiency was considered in the differential for our patient. She had a history of congestive heart failure, kidney disease, and type 2 diabetes mellitus, all of which contribute to lymphedema and/or poor wound healing. However, venous ulcers usually are located on the medial ankles and are irregularly shaped with an erythematous border and fibrinous exudate with central depression, making it a less likely diagnosis in our patient. Additionally, an infectious process was considered, but the patient was afebrile and laboratory values demonstrated no leukocytosis.
Marjolin ulcer was highly suspected because the clinical presentation revealed a nodule with hemorrhagic crust and induration in the setting of a chronic nonhealing ulcer. The pathogenesis of malignancy in chronic ulcers is thought to be due to continuous mitotic activity from regeneration and repair of the wound, especially in the setting of repeated trauma to the area.5 In our patient, the history of multiple falls with possible multitrauma injury to the chronic ulcer further increased suspicion of malignancy. The most common and frequently seen malignancy that develops in chronic ulcers is squamous cell carcinoma (SCC) followed by basal cell carcinoma. Plastic surgery suspected an SCC for the working diagnosis, which prompted a punch biopsy; however, the histologic analysis was not consistent with SCC or basal cell carcinoma. Marjolin ulcer also may demonstrate a periosteal reaction,5 which was not the case with our patient after a radiograph of the left tibia/fibula was unremarkable.
Another potential malignancy to consider is melanoma. There are few case reports of biopsy-proven melanoma from an enlarging chronic ulcer.6,7 Additionally, poorly differentiated angiosarcoma can mimic melanoma2; however, immunohistochemistry stain was negative for S-100, human melanoma black 45, and MART-1, making melanoma unlikely.
Kaposi sarcoma (KS) and angiosarcoma are both malignant vascular tumors that similarly present with red to purple patches, plaques, or nodules, making it difficult to distinguish between the two conditions. It is important to note that KS usually is lower grade, and the pathogenesis is linked to human herpesvirus 8, which can be identified on immunohistochemistry staining. There have been cases of KS reported in patients who have no history of human immunodeficiency virus/AIDS, thus the classic subtype of KS may have been considered in this patient.8 The histologic appearance of KS may vary from dilated irregular endothelial cells lining the vascular space to mild endothelial cell atypia. Histology also shows hemosiderin-laden macrophages, extravasated red blood cells, and an inflammatory infiltrate. An additional malignant vascular neoplasm that needs to be differentiated is epithelioid hemangioendothelioma. Cutaneous presentation of an epithelioid hemangioendothelioma may be similar to what was seen in our patient but histologically will usually show neoplastic cells with pale eosinophilic cytoplasm and vesicular nuclei of plump, oval, polygonal cells in cords or aggregates surrounding vascular channels. These neoplasms also tend to occur around medium- to large-sized veins.1,9 With our patient, even though human herpesvirus 8 was not tested with immunohistochemistry, gold standard immunohistochemistry confirmation with CD34 and vimentin staining combined with poorly differentiated endothelial atypia with mitotic figures on histologic analysis favored angiosarcoma versus KS or epithelioid hemangioendothelioma.10,11
Management
Cutaneous angiosarcoma is a rare and aggressive vascular neoplasm accounting for approximately 2% of all combined sarcomas, with an estimated 20% to 40% having distant metastasis at diagnosis.1,3 For this reason, computed tomography was performed in our patient and revealed no local or distant metastasis. Therefore, chemotherapy was not an appropriate adjuvant treatment option.12 With no evidence of metastasis, initial treatment began with surgical removal but proved to be difficult in our patient. Although the implications of positive surgical margins remain unclear with regard to overall patient survival, surgical resection followed by radiation therapy has been shown to be optimal, as it reduces the risk of local reoccurrence.3 There have been reported cases of cutaneous angiosarcoma of the leg that were treated with amputation without signs of reoccurrence or metastasis.10,13,14 Given the results from these cases and considering that our patient had no metastasis, amputation seemed to be a good prognostic option; however, considering other factors regarding the patient’s comorbidities and quality of life, her family decided not to pursue any further treatment with amputation or radiation therapy.
Conclusion
There should be low threshold for biopsy in patients who present with nonhealing wounds that do not progress in the normal phase of wound healing with suspicion for malignancy. As seen with our patient, cutaneous angiosarcoma can clinically mimic many disease processes, and although rare in nature, it should always be considered when a patient presents with a rapidly growing lesion in the setting of chronic lymphedema or venous ulcer.
- Kumar V, Abbas A, Aster J. Robbins Basic Pathology. 9th ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Donghi D, Kerl K, Dummer R, et al. Cutaneous angiosarcoma: own experience over 13 years. clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230-1234.
- Dossett LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64.
- Gerslova A, Pokorna A, Stukavcova A, et al. Rare cause of non-healing foot wound—acral lentiginous melanoma. Neuro Endocrinol Lett. 2012;37:12-17.
- Turk BG, Bozkurt A, Yaman B, et al. Melanoma arising in chronic ulceration associated with lymphoedema. J Wound Care. 2013;22:74-75.
- Phavixay L, Raynolds D, Simman R. Non AIDS Kaposi’s sarcoma leading to lower extremities wounds, case presentations and discussion.J Am Coll Clin Wound Spec. 2012;4:13-15.
- Requena L, Kutzner H. Hemangioendothelioma. Semin Diagn Pathol. 2013;30:29-44.
- Harrison WD, Chandrasekar CR. Stewart-Treves syndrome following idiopathic leg lymphoedema: remember sarcoma. J Wound Care. 2015;24(6 suppl):S5-S7.
- Kak I, Salama S, Gohla G, et al. A case of patch stage of Kaposi’s sarcoma and discussion of the differential diagnosis. Rare Tumors. 2016;8:6123.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Linda DD, Harish S, Alowami S, et al. Radiology-pathology conference: cutaneous angiosarcoma of the leg. Clin Imaging. 2013;37:602-607.
- Roy P, Clark MA, Thomas JM. Stewart-Treves syndrome—treatment and outcome in six patients from a single centre. Eur J Surg Oncol. 2004;30:982-986.
- Kumar V, Abbas A, Aster J. Robbins Basic Pathology. 9th ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Donghi D, Kerl K, Dummer R, et al. Cutaneous angiosarcoma: own experience over 13 years. clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230-1234.
- Dossett LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64.
- Gerslova A, Pokorna A, Stukavcova A, et al. Rare cause of non-healing foot wound—acral lentiginous melanoma. Neuro Endocrinol Lett. 2012;37:12-17.
- Turk BG, Bozkurt A, Yaman B, et al. Melanoma arising in chronic ulceration associated with lymphoedema. J Wound Care. 2013;22:74-75.
- Phavixay L, Raynolds D, Simman R. Non AIDS Kaposi’s sarcoma leading to lower extremities wounds, case presentations and discussion.J Am Coll Clin Wound Spec. 2012;4:13-15.
- Requena L, Kutzner H. Hemangioendothelioma. Semin Diagn Pathol. 2013;30:29-44.
- Harrison WD, Chandrasekar CR. Stewart-Treves syndrome following idiopathic leg lymphoedema: remember sarcoma. J Wound Care. 2015;24(6 suppl):S5-S7.
- Kak I, Salama S, Gohla G, et al. A case of patch stage of Kaposi’s sarcoma and discussion of the differential diagnosis. Rare Tumors. 2016;8:6123.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Linda DD, Harish S, Alowami S, et al. Radiology-pathology conference: cutaneous angiosarcoma of the leg. Clin Imaging. 2013;37:602-607.
- Roy P, Clark MA, Thomas JM. Stewart-Treves syndrome—treatment and outcome in six patients from a single centre. Eur J Surg Oncol. 2004;30:982-986.
Practice Points
- Cutaneous angiosarcoma is a rare malignant vascular neoplasm typically seen in 3 settings: (1) idiopathic (commonly on the face and neck), (2) following radiation treatment, and (3) classically in the setting of chronic lymphedema following mastectomy (Stewart-Treves syndrome).
- There should be a low threshold for biopsy in patients who present with nonhealing wounds that do not progress in the normal phase of wound healing with suspicion for malignancy.
- Histologic analysis of angiosarcoma shows positive staining for CD34 and vimentin with poorly differentiated endothelial atypia with mitotic figures.
FDA approves omadacycline for pneumonia and skin infections
The, for treating community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults, the manufacturer, Paratek, announced in a press release.
The company expects that omadacycline will be available in the first quarter of 2019. Administered once-daily in either oral or IV formulations, the antibiotic was effective and well tolerated across multiple trials, which altogether included almost 2,000 patients, according to Paratek. As part of the approval, the company has agreed to conduct postmarketing studies, specifically, more studies in CABP and in pediatric populations. “To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nuzyra and other antibacterial drugs, Nuzyra should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria,” according to a statement in the indications section of the prescribing information.
Omadacycline is contraindicated for patients with a known hypersensitivity to the drug or any members of the tetracycline class of antibacterial drugs; hypersensitivity reactions have been observed, so use should be discontinued if one is suspected. Use of this drug during later stages of pregnancy can lead to irreversible discoloration of the infant’s teeth and inhibition of bone growth; it should also not be used during breastfeeding.
Because omadacycline is structurally similar to tetracycline class drugs, some adverse reactions to those drugs may be seen with this one, such as photosensitivity, pseudotumor cerebri, and antianabolic action. Adverse reactions known to have an association with omadacycline include nausea, vomiting, hypertension, insomnia, diarrhea, constipation, and increases of alanine aminotransferase, aspartate aminotransferase, and/or gamma-glutamyl transferase.
Drug interactions may occur with anticoagulants, so dosage of those drugs may need to be reduced while treating with omadacycline. Antacids also are believed to have a drug interaction – specifically, impairing absorption of omadacycline
The, for treating community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults, the manufacturer, Paratek, announced in a press release.
The company expects that omadacycline will be available in the first quarter of 2019. Administered once-daily in either oral or IV formulations, the antibiotic was effective and well tolerated across multiple trials, which altogether included almost 2,000 patients, according to Paratek. As part of the approval, the company has agreed to conduct postmarketing studies, specifically, more studies in CABP and in pediatric populations. “To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nuzyra and other antibacterial drugs, Nuzyra should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria,” according to a statement in the indications section of the prescribing information.
Omadacycline is contraindicated for patients with a known hypersensitivity to the drug or any members of the tetracycline class of antibacterial drugs; hypersensitivity reactions have been observed, so use should be discontinued if one is suspected. Use of this drug during later stages of pregnancy can lead to irreversible discoloration of the infant’s teeth and inhibition of bone growth; it should also not be used during breastfeeding.
Because omadacycline is structurally similar to tetracycline class drugs, some adverse reactions to those drugs may be seen with this one, such as photosensitivity, pseudotumor cerebri, and antianabolic action. Adverse reactions known to have an association with omadacycline include nausea, vomiting, hypertension, insomnia, diarrhea, constipation, and increases of alanine aminotransferase, aspartate aminotransferase, and/or gamma-glutamyl transferase.
Drug interactions may occur with anticoagulants, so dosage of those drugs may need to be reduced while treating with omadacycline. Antacids also are believed to have a drug interaction – specifically, impairing absorption of omadacycline
The, for treating community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults, the manufacturer, Paratek, announced in a press release.
The company expects that omadacycline will be available in the first quarter of 2019. Administered once-daily in either oral or IV formulations, the antibiotic was effective and well tolerated across multiple trials, which altogether included almost 2,000 patients, according to Paratek. As part of the approval, the company has agreed to conduct postmarketing studies, specifically, more studies in CABP and in pediatric populations. “To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nuzyra and other antibacterial drugs, Nuzyra should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria,” according to a statement in the indications section of the prescribing information.
Omadacycline is contraindicated for patients with a known hypersensitivity to the drug or any members of the tetracycline class of antibacterial drugs; hypersensitivity reactions have been observed, so use should be discontinued if one is suspected. Use of this drug during later stages of pregnancy can lead to irreversible discoloration of the infant’s teeth and inhibition of bone growth; it should also not be used during breastfeeding.
Because omadacycline is structurally similar to tetracycline class drugs, some adverse reactions to those drugs may be seen with this one, such as photosensitivity, pseudotumor cerebri, and antianabolic action. Adverse reactions known to have an association with omadacycline include nausea, vomiting, hypertension, insomnia, diarrhea, constipation, and increases of alanine aminotransferase, aspartate aminotransferase, and/or gamma-glutamyl transferase.
Drug interactions may occur with anticoagulants, so dosage of those drugs may need to be reduced while treating with omadacycline. Antacids also are believed to have a drug interaction – specifically, impairing absorption of omadacycline
Sweat Regeneration Following CO2 Fractionated Laser Therapy
To the Editor:
It is not uncommon for patients with extensive dermal scarring to overheat due to the inability to regulate body temperature through evaporative heat loss, as lack of perspiration in areas of prior full-thickness skin injury is well known. One of the authors (C.M.H.) previously reported a case of a patient with considerable hypertrophic scarring after surviving an episode of toxic epidermal necrolysis that was likely precipitated by lamotrigine.1 The patient initially presented to our clinic in consultation for laser therapy to improve the pliability and cosmetic appearance of the scars; however, approximately 3 weeks after initiating treatment with a fractional CO2 laser, the patient noticed perspiration in areas where she once lacked the ability to perspire as well as improved functionality.1 It was speculated that scar remodeling stimulated by the CO2 fractional laser allowed new connections to form between eccrine ducts in the dermis and epidermis.2
These findings are even more notable in light of a study by Rittié et al3 that suggested the primary appendages of the skin involved in human wound healing are the eccrine sweat glands. The investigators were able to demonstrate that eccrine sweat glands are major contributors in reepithelialization and wound healing in humans; therefore, it is possible that stimulating these glands with the CO2 laser may promote enhanced reepithelialization in addition to the reestablishment of perspiration and wound healing.3 Considering inadequate wound repair represents a substantial disturbance to the patient and health care system, this finding offers promise as a potential means to decrease morbidity in patients with dermal scarring from burns and traumatic injuries. We have since evaluated and treated 3 patients who demonstrated sweat regeneration following treatment with the fractional CO2 laser (Table).
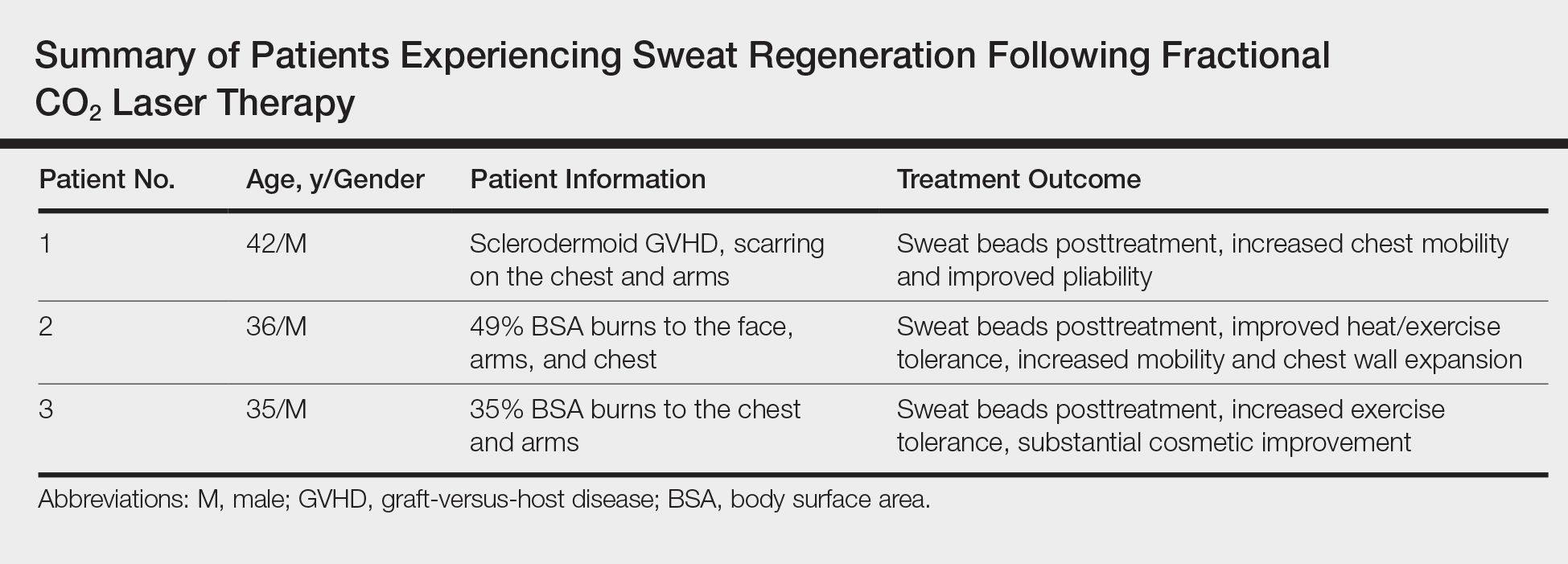
A 42-year-old man was our first patient to demonstrate functional scar improvement following bone marrow transplant for acute lymphoblastic leukemia complicated by chronic sclerodermoid graft-versus-host disease and subsequent extensive scarring on the chest and arms. Approximately 2 weeks after the first treatment with the fractional CO2 laser, the patient began to notice the presence of sweat beads in the treated areas. In addition to the reestablishment of perspiration, the patient had perceived increased mobility with improved pliability and “softness” (as described by family members) in treated areas likely related to scar remodeling.
A 36-year-old wounded army veteran presented with burns to the face, arms, and chest affecting 49% of the body surface area. After only 1 treatment, the patient reported that he could subjectively tolerate 10°F more ambient temperature and work all day outside in south Texas when heat intolerance previously would allow him to work only 2 to 3 hours. Additionally, he noted increased mobility and chest wall expansion, which in combination contributed to overall increased exercise tolerance and enhanced quality of life.
A 35-year-old US Marine and firefighter with burns primarily on the chest and arms involving 35% body surface area experienced increased exercise tolerance and sweat regeneration, particularly on the chest after a single treatment with the fractional CO2 laser but continued to experience improvement after a total of 3 treatments. Additionally, the cosmetic improvement was so substantial that the physician (C.M.H) had to review older photographs to verify the location of the scars.
We have now treated 3 patients with various mechanisms of injury and extensive scarring who noticed improved heat tolerance from sweat regeneration following fractional CO2 laser therapy. At this point, we only have anecdotal evidence of subjective functional improvement, and further research is warranted to elucidate the exact mechanism of action to support our findings.
- Neiner J, Whittemore D, Hivnor C. Buried alive: functional eccrine coils buried under scar tissue? J Am Acad Dermatol. 2011;65:661-663.
- Waibel J, Beer K, Narurkar V, et al. Preliminary observations on fractional ablative resurfacing devices: clinical impressions. J Drugs Dermatol. 2009;8:481-485.
- Rittié L, Sachs D, Orringer J, et al. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am J Pathol. 2013;1:163-171.
To the Editor:
It is not uncommon for patients with extensive dermal scarring to overheat due to the inability to regulate body temperature through evaporative heat loss, as lack of perspiration in areas of prior full-thickness skin injury is well known. One of the authors (C.M.H.) previously reported a case of a patient with considerable hypertrophic scarring after surviving an episode of toxic epidermal necrolysis that was likely precipitated by lamotrigine.1 The patient initially presented to our clinic in consultation for laser therapy to improve the pliability and cosmetic appearance of the scars; however, approximately 3 weeks after initiating treatment with a fractional CO2 laser, the patient noticed perspiration in areas where she once lacked the ability to perspire as well as improved functionality.1 It was speculated that scar remodeling stimulated by the CO2 fractional laser allowed new connections to form between eccrine ducts in the dermis and epidermis.2
These findings are even more notable in light of a study by Rittié et al3 that suggested the primary appendages of the skin involved in human wound healing are the eccrine sweat glands. The investigators were able to demonstrate that eccrine sweat glands are major contributors in reepithelialization and wound healing in humans; therefore, it is possible that stimulating these glands with the CO2 laser may promote enhanced reepithelialization in addition to the reestablishment of perspiration and wound healing.3 Considering inadequate wound repair represents a substantial disturbance to the patient and health care system, this finding offers promise as a potential means to decrease morbidity in patients with dermal scarring from burns and traumatic injuries. We have since evaluated and treated 3 patients who demonstrated sweat regeneration following treatment with the fractional CO2 laser (Table).

A 42-year-old man was our first patient to demonstrate functional scar improvement following bone marrow transplant for acute lymphoblastic leukemia complicated by chronic sclerodermoid graft-versus-host disease and subsequent extensive scarring on the chest and arms. Approximately 2 weeks after the first treatment with the fractional CO2 laser, the patient began to notice the presence of sweat beads in the treated areas. In addition to the reestablishment of perspiration, the patient had perceived increased mobility with improved pliability and “softness” (as described by family members) in treated areas likely related to scar remodeling.
A 36-year-old wounded army veteran presented with burns to the face, arms, and chest affecting 49% of the body surface area. After only 1 treatment, the patient reported that he could subjectively tolerate 10°F more ambient temperature and work all day outside in south Texas when heat intolerance previously would allow him to work only 2 to 3 hours. Additionally, he noted increased mobility and chest wall expansion, which in combination contributed to overall increased exercise tolerance and enhanced quality of life.
A 35-year-old US Marine and firefighter with burns primarily on the chest and arms involving 35% body surface area experienced increased exercise tolerance and sweat regeneration, particularly on the chest after a single treatment with the fractional CO2 laser but continued to experience improvement after a total of 3 treatments. Additionally, the cosmetic improvement was so substantial that the physician (C.M.H) had to review older photographs to verify the location of the scars.
We have now treated 3 patients with various mechanisms of injury and extensive scarring who noticed improved heat tolerance from sweat regeneration following fractional CO2 laser therapy. At this point, we only have anecdotal evidence of subjective functional improvement, and further research is warranted to elucidate the exact mechanism of action to support our findings.
To the Editor:
It is not uncommon for patients with extensive dermal scarring to overheat due to the inability to regulate body temperature through evaporative heat loss, as lack of perspiration in areas of prior full-thickness skin injury is well known. One of the authors (C.M.H.) previously reported a case of a patient with considerable hypertrophic scarring after surviving an episode of toxic epidermal necrolysis that was likely precipitated by lamotrigine.1 The patient initially presented to our clinic in consultation for laser therapy to improve the pliability and cosmetic appearance of the scars; however, approximately 3 weeks after initiating treatment with a fractional CO2 laser, the patient noticed perspiration in areas where she once lacked the ability to perspire as well as improved functionality.1 It was speculated that scar remodeling stimulated by the CO2 fractional laser allowed new connections to form between eccrine ducts in the dermis and epidermis.2
These findings are even more notable in light of a study by Rittié et al3 that suggested the primary appendages of the skin involved in human wound healing are the eccrine sweat glands. The investigators were able to demonstrate that eccrine sweat glands are major contributors in reepithelialization and wound healing in humans; therefore, it is possible that stimulating these glands with the CO2 laser may promote enhanced reepithelialization in addition to the reestablishment of perspiration and wound healing.3 Considering inadequate wound repair represents a substantial disturbance to the patient and health care system, this finding offers promise as a potential means to decrease morbidity in patients with dermal scarring from burns and traumatic injuries. We have since evaluated and treated 3 patients who demonstrated sweat regeneration following treatment with the fractional CO2 laser (Table).

A 42-year-old man was our first patient to demonstrate functional scar improvement following bone marrow transplant for acute lymphoblastic leukemia complicated by chronic sclerodermoid graft-versus-host disease and subsequent extensive scarring on the chest and arms. Approximately 2 weeks after the first treatment with the fractional CO2 laser, the patient began to notice the presence of sweat beads in the treated areas. In addition to the reestablishment of perspiration, the patient had perceived increased mobility with improved pliability and “softness” (as described by family members) in treated areas likely related to scar remodeling.
A 36-year-old wounded army veteran presented with burns to the face, arms, and chest affecting 49% of the body surface area. After only 1 treatment, the patient reported that he could subjectively tolerate 10°F more ambient temperature and work all day outside in south Texas when heat intolerance previously would allow him to work only 2 to 3 hours. Additionally, he noted increased mobility and chest wall expansion, which in combination contributed to overall increased exercise tolerance and enhanced quality of life.
A 35-year-old US Marine and firefighter with burns primarily on the chest and arms involving 35% body surface area experienced increased exercise tolerance and sweat regeneration, particularly on the chest after a single treatment with the fractional CO2 laser but continued to experience improvement after a total of 3 treatments. Additionally, the cosmetic improvement was so substantial that the physician (C.M.H) had to review older photographs to verify the location of the scars.
We have now treated 3 patients with various mechanisms of injury and extensive scarring who noticed improved heat tolerance from sweat regeneration following fractional CO2 laser therapy. At this point, we only have anecdotal evidence of subjective functional improvement, and further research is warranted to elucidate the exact mechanism of action to support our findings.
- Neiner J, Whittemore D, Hivnor C. Buried alive: functional eccrine coils buried under scar tissue? J Am Acad Dermatol. 2011;65:661-663.
- Waibel J, Beer K, Narurkar V, et al. Preliminary observations on fractional ablative resurfacing devices: clinical impressions. J Drugs Dermatol. 2009;8:481-485.
- Rittié L, Sachs D, Orringer J, et al. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am J Pathol. 2013;1:163-171.
- Neiner J, Whittemore D, Hivnor C. Buried alive: functional eccrine coils buried under scar tissue? J Am Acad Dermatol. 2011;65:661-663.
- Waibel J, Beer K, Narurkar V, et al. Preliminary observations on fractional ablative resurfacing devices: clinical impressions. J Drugs Dermatol. 2009;8:481-485.
- Rittié L, Sachs D, Orringer J, et al. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am J Pathol. 2013;1:163-171.
Practice Points
- Treatment of dermal scarring with fractional CO2 laser may contribute to eccrine sweat gland regeneration during the remodeling process in addition to increased skin pliability.
- Sweat regeneration has been demonstrated following treatment with fractional CO2 laser in patients with extensive scarring; this case shows sweat regeneration secondary to burns and chronic sclerodermoid graft-versus-host disease.