User login
Nodding Off While Feeding an Infant
In a recent survey of 1259 mothers published in the journal Pediatrics, 28% reported they had fallen asleep while feeding their babies, and 83% of those mothers reported that the sleep was unplanned. Although the study sample was small, the investigators found that sociodemographic factors did not increase the odds that a mother would fall asleep while feeding.
These numbers are not surprising, but nonetheless they are concerning because co-sleeping is a known risk factor for sudden unexplained infant death (SUID). Every parent will tell you during the first 6 months of their adventure in parenting they didn’t get enough sleep. In fact some will tell you that sleep deprivation was their chronic state for the child’s first year.
Falling asleep easily at times and places not intended for sleep is the primary symptom of sleep deprivation. SUID is the most tragic event associated with parental sleep deprivation, but it is certainly not the only one. Overtired parents are more likely to be involved in accidents and are more likely to make poor decisions, particularly those regarding how to respond to a crying or misbehaving child.
The investigators found that 24% of mothers who reported that their usual nighttime feeding location was a chair or sofa (14%). Not surprisingly, mothers who fed in chairs were less likely to fall asleep while feeding. Many of these mothers reported that they chose the chair because they thought they would be less likely to fall asleep and/or disturb other family members. One wonders how we should interpret these numbers in light of other research that has found it is “relatively less hazardous to fall asleep with an infant in the adult bed than on a chair or sofa.” Had these chair feeding mothers made the better choice under the circumstances? It would take a much larger and more granular study to answer that question.
Mothers who exclusively breastfed were more likely to fall asleep feeding than were those who partially breastfed or used formula. The investigators postulated that the infants of mothers who exclusively breastfed may have required more feedings because breast milk is more easily and quickly digested. I know this is a common explanation, but in my experience I have found that exclusively breastfed infants often use nursing as pacification and a sleep trigger and spend more time at the breast regardless of how quickly they emptied their stomachs.
This study also examined the effect of repeated educational interventions and support and found that mothers who received an intervention based on safe sleep practices were less likely to fall asleep while feeding than were the mothers who had received the intervention focused on exclusive breastfeeding value and barriers to its adoption.
Education is one avenue, particularly when it includes the mother’s partner who can play an important role as standby lifeguard to make sure the mother doesn’t fall asleep. Obviously, this is easier said than done because when there is a new baby in the house sleep deprivation is usually a shared experience.
Although I believe that my family is on the verge of gifting me a smartwatch to protect me from my own misadventures, I don’t have any personal experience with these wonders of modern technology. However, I suspect with very little tweaking a wearable sensor could be easily programmed to detect when a mother is beginning to fall asleep while she is feeding her infant. A smartwatch would be an expensive intervention and is unlikely to filter down to economically challenged families. On the other hand, this paper has reinforced our suspicions that sleep-deprived infant feeding is a significant problem. A subsidized loaner program for those families that can’t afford a smartwatch is an option that should be considered.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In a recent survey of 1259 mothers published in the journal Pediatrics, 28% reported they had fallen asleep while feeding their babies, and 83% of those mothers reported that the sleep was unplanned. Although the study sample was small, the investigators found that sociodemographic factors did not increase the odds that a mother would fall asleep while feeding.
These numbers are not surprising, but nonetheless they are concerning because co-sleeping is a known risk factor for sudden unexplained infant death (SUID). Every parent will tell you during the first 6 months of their adventure in parenting they didn’t get enough sleep. In fact some will tell you that sleep deprivation was their chronic state for the child’s first year.
Falling asleep easily at times and places not intended for sleep is the primary symptom of sleep deprivation. SUID is the most tragic event associated with parental sleep deprivation, but it is certainly not the only one. Overtired parents are more likely to be involved in accidents and are more likely to make poor decisions, particularly those regarding how to respond to a crying or misbehaving child.
The investigators found that 24% of mothers who reported that their usual nighttime feeding location was a chair or sofa (14%). Not surprisingly, mothers who fed in chairs were less likely to fall asleep while feeding. Many of these mothers reported that they chose the chair because they thought they would be less likely to fall asleep and/or disturb other family members. One wonders how we should interpret these numbers in light of other research that has found it is “relatively less hazardous to fall asleep with an infant in the adult bed than on a chair or sofa.” Had these chair feeding mothers made the better choice under the circumstances? It would take a much larger and more granular study to answer that question.
Mothers who exclusively breastfed were more likely to fall asleep feeding than were those who partially breastfed or used formula. The investigators postulated that the infants of mothers who exclusively breastfed may have required more feedings because breast milk is more easily and quickly digested. I know this is a common explanation, but in my experience I have found that exclusively breastfed infants often use nursing as pacification and a sleep trigger and spend more time at the breast regardless of how quickly they emptied their stomachs.
This study also examined the effect of repeated educational interventions and support and found that mothers who received an intervention based on safe sleep practices were less likely to fall asleep while feeding than were the mothers who had received the intervention focused on exclusive breastfeeding value and barriers to its adoption.
Education is one avenue, particularly when it includes the mother’s partner who can play an important role as standby lifeguard to make sure the mother doesn’t fall asleep. Obviously, this is easier said than done because when there is a new baby in the house sleep deprivation is usually a shared experience.
Although I believe that my family is on the verge of gifting me a smartwatch to protect me from my own misadventures, I don’t have any personal experience with these wonders of modern technology. However, I suspect with very little tweaking a wearable sensor could be easily programmed to detect when a mother is beginning to fall asleep while she is feeding her infant. A smartwatch would be an expensive intervention and is unlikely to filter down to economically challenged families. On the other hand, this paper has reinforced our suspicions that sleep-deprived infant feeding is a significant problem. A subsidized loaner program for those families that can’t afford a smartwatch is an option that should be considered.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In a recent survey of 1259 mothers published in the journal Pediatrics, 28% reported they had fallen asleep while feeding their babies, and 83% of those mothers reported that the sleep was unplanned. Although the study sample was small, the investigators found that sociodemographic factors did not increase the odds that a mother would fall asleep while feeding.
These numbers are not surprising, but nonetheless they are concerning because co-sleeping is a known risk factor for sudden unexplained infant death (SUID). Every parent will tell you during the first 6 months of their adventure in parenting they didn’t get enough sleep. In fact some will tell you that sleep deprivation was their chronic state for the child’s first year.
Falling asleep easily at times and places not intended for sleep is the primary symptom of sleep deprivation. SUID is the most tragic event associated with parental sleep deprivation, but it is certainly not the only one. Overtired parents are more likely to be involved in accidents and are more likely to make poor decisions, particularly those regarding how to respond to a crying or misbehaving child.
The investigators found that 24% of mothers who reported that their usual nighttime feeding location was a chair or sofa (14%). Not surprisingly, mothers who fed in chairs were less likely to fall asleep while feeding. Many of these mothers reported that they chose the chair because they thought they would be less likely to fall asleep and/or disturb other family members. One wonders how we should interpret these numbers in light of other research that has found it is “relatively less hazardous to fall asleep with an infant in the adult bed than on a chair or sofa.” Had these chair feeding mothers made the better choice under the circumstances? It would take a much larger and more granular study to answer that question.
Mothers who exclusively breastfed were more likely to fall asleep feeding than were those who partially breastfed or used formula. The investigators postulated that the infants of mothers who exclusively breastfed may have required more feedings because breast milk is more easily and quickly digested. I know this is a common explanation, but in my experience I have found that exclusively breastfed infants often use nursing as pacification and a sleep trigger and spend more time at the breast regardless of how quickly they emptied their stomachs.
This study also examined the effect of repeated educational interventions and support and found that mothers who received an intervention based on safe sleep practices were less likely to fall asleep while feeding than were the mothers who had received the intervention focused on exclusive breastfeeding value and barriers to its adoption.
Education is one avenue, particularly when it includes the mother’s partner who can play an important role as standby lifeguard to make sure the mother doesn’t fall asleep. Obviously, this is easier said than done because when there is a new baby in the house sleep deprivation is usually a shared experience.
Although I believe that my family is on the verge of gifting me a smartwatch to protect me from my own misadventures, I don’t have any personal experience with these wonders of modern technology. However, I suspect with very little tweaking a wearable sensor could be easily programmed to detect when a mother is beginning to fall asleep while she is feeding her infant. A smartwatch would be an expensive intervention and is unlikely to filter down to economically challenged families. On the other hand, this paper has reinforced our suspicions that sleep-deprived infant feeding is a significant problem. A subsidized loaner program for those families that can’t afford a smartwatch is an option that should be considered.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
What To Do With Lipoprotein(a)?
Case: 45-year-old woman comes to clinic and requests lipoprotein(a) [Lp(a)] testing. She has a family history of early coronary disease (mother age 50, sister age 48) and has hypertension with home blood pressure readings of 130-140/70-75. She had a lipid panel checked last year which showed a total cholesterol of 210 mg/dL, LDL 145 mg/dL, HDL 45 mg/dL, and triglycerides of 100 mg/dL. She does not smoke and is currently taking irbesartan, chlorthalidone, sertraline, a multivitamin, and vitamin D.
What do you recommend?
There has been a great deal of media attention on testing for Lp(a). Many of my patients are requesting testing although many of them do not need it. This patient is an exception. I think Lp(a) testing would help inform her medical care. She has a family history of early coronary disease in her mother and sister, but her own lipid profile is not worrisome.
Her 10-year cardiovascular disease risk is 2%. The cardiac risk calculator does not incorporate family history; I think this is a situation where testing for Lp(a)(as well as apolipoprotein B) can be helpful. If her Lp(a) is elevated, it helps reassess her risk and that information would be helpful in targeting aggressive interventions for other CV risk factors, including optimal blood pressure control. In her case, pushing for a goal systolic blood pressure below 120 mm Hg and making sure she is doing regular exercise and eating a heart-healthy diet. The current consensus statement on Lp(a) recommends that patients with elevated levels have aggressive lifestyle and cardiovascular risk management.1
Currently, there are no medical treatments available for high Lp(a) for primary prevention. Apheresis has been approved by the US Food and Drug Administration (FDA) for patients with familial hyperlipidemia who have LDL ≥ 100 mg/dL, Lp(a) ≥ 60 mg/dL, and coronary or other artery disease.
PCSK9 inhibitors have shown a reduction in major cardiovascular events in patients who have established coronary artery disease and high Lp(a) levels, albeit with limited data. Unlike statins, which increase Lp(a) levels, PCSK9 inhibitors reduce Lp(a) levels.2 There are promising early results in a phase 2 trial of the oral drug muvalaplin lowering Lp(a) levels by up to 85% for the highest dose, but there are no peer-reviewed articles confirming these results and no outcome trials at this time.
In patients who are already recognized as high risk, especially those with established coronary artery disease, measuring Lp(a) levels offer little benefit. These patients should already be receiving aggressive medical therapy to reach blood pressure targets if hypertensive, maximal lifestyle modifications, and statin therapy.
If these patients need more therapy because of continued coronary events, despite maximal conventional medical therapy, then adding a PCSK9 inhibitor would be appropriate whether or not a patient has a high Lp(a) level. Once Lp(a) targeted therapies are available and show clinical benefit, then the role of Lp(a) measurement and treatment in this population will be clearer.
Pearl: Most patients do not need Lp(a) testing. There are no FDA-approved treatments for high Lp(a) levels.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Kronenberg F et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur Heart J. 2022;43:3925-46.
2. Ruscica M et al. Lipoprotein(a) and PCSK9 inhibition: Clinical evidence Eur Heart J Suppl 2020;Nov 18(Suppl L):L53–L56.
Case: 45-year-old woman comes to clinic and requests lipoprotein(a) [Lp(a)] testing. She has a family history of early coronary disease (mother age 50, sister age 48) and has hypertension with home blood pressure readings of 130-140/70-75. She had a lipid panel checked last year which showed a total cholesterol of 210 mg/dL, LDL 145 mg/dL, HDL 45 mg/dL, and triglycerides of 100 mg/dL. She does not smoke and is currently taking irbesartan, chlorthalidone, sertraline, a multivitamin, and vitamin D.
What do you recommend?
There has been a great deal of media attention on testing for Lp(a). Many of my patients are requesting testing although many of them do not need it. This patient is an exception. I think Lp(a) testing would help inform her medical care. She has a family history of early coronary disease in her mother and sister, but her own lipid profile is not worrisome.
Her 10-year cardiovascular disease risk is 2%. The cardiac risk calculator does not incorporate family history; I think this is a situation where testing for Lp(a)(as well as apolipoprotein B) can be helpful. If her Lp(a) is elevated, it helps reassess her risk and that information would be helpful in targeting aggressive interventions for other CV risk factors, including optimal blood pressure control. In her case, pushing for a goal systolic blood pressure below 120 mm Hg and making sure she is doing regular exercise and eating a heart-healthy diet. The current consensus statement on Lp(a) recommends that patients with elevated levels have aggressive lifestyle and cardiovascular risk management.1
Currently, there are no medical treatments available for high Lp(a) for primary prevention. Apheresis has been approved by the US Food and Drug Administration (FDA) for patients with familial hyperlipidemia who have LDL ≥ 100 mg/dL, Lp(a) ≥ 60 mg/dL, and coronary or other artery disease.
PCSK9 inhibitors have shown a reduction in major cardiovascular events in patients who have established coronary artery disease and high Lp(a) levels, albeit with limited data. Unlike statins, which increase Lp(a) levels, PCSK9 inhibitors reduce Lp(a) levels.2 There are promising early results in a phase 2 trial of the oral drug muvalaplin lowering Lp(a) levels by up to 85% for the highest dose, but there are no peer-reviewed articles confirming these results and no outcome trials at this time.
In patients who are already recognized as high risk, especially those with established coronary artery disease, measuring Lp(a) levels offer little benefit. These patients should already be receiving aggressive medical therapy to reach blood pressure targets if hypertensive, maximal lifestyle modifications, and statin therapy.
If these patients need more therapy because of continued coronary events, despite maximal conventional medical therapy, then adding a PCSK9 inhibitor would be appropriate whether or not a patient has a high Lp(a) level. Once Lp(a) targeted therapies are available and show clinical benefit, then the role of Lp(a) measurement and treatment in this population will be clearer.
Pearl: Most patients do not need Lp(a) testing. There are no FDA-approved treatments for high Lp(a) levels.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Kronenberg F et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur Heart J. 2022;43:3925-46.
2. Ruscica M et al. Lipoprotein(a) and PCSK9 inhibition: Clinical evidence Eur Heart J Suppl 2020;Nov 18(Suppl L):L53–L56.
Case: 45-year-old woman comes to clinic and requests lipoprotein(a) [Lp(a)] testing. She has a family history of early coronary disease (mother age 50, sister age 48) and has hypertension with home blood pressure readings of 130-140/70-75. She had a lipid panel checked last year which showed a total cholesterol of 210 mg/dL, LDL 145 mg/dL, HDL 45 mg/dL, and triglycerides of 100 mg/dL. She does not smoke and is currently taking irbesartan, chlorthalidone, sertraline, a multivitamin, and vitamin D.
What do you recommend?
There has been a great deal of media attention on testing for Lp(a). Many of my patients are requesting testing although many of them do not need it. This patient is an exception. I think Lp(a) testing would help inform her medical care. She has a family history of early coronary disease in her mother and sister, but her own lipid profile is not worrisome.
Her 10-year cardiovascular disease risk is 2%. The cardiac risk calculator does not incorporate family history; I think this is a situation where testing for Lp(a)(as well as apolipoprotein B) can be helpful. If her Lp(a) is elevated, it helps reassess her risk and that information would be helpful in targeting aggressive interventions for other CV risk factors, including optimal blood pressure control. In her case, pushing for a goal systolic blood pressure below 120 mm Hg and making sure she is doing regular exercise and eating a heart-healthy diet. The current consensus statement on Lp(a) recommends that patients with elevated levels have aggressive lifestyle and cardiovascular risk management.1
Currently, there are no medical treatments available for high Lp(a) for primary prevention. Apheresis has been approved by the US Food and Drug Administration (FDA) for patients with familial hyperlipidemia who have LDL ≥ 100 mg/dL, Lp(a) ≥ 60 mg/dL, and coronary or other artery disease.
PCSK9 inhibitors have shown a reduction in major cardiovascular events in patients who have established coronary artery disease and high Lp(a) levels, albeit with limited data. Unlike statins, which increase Lp(a) levels, PCSK9 inhibitors reduce Lp(a) levels.2 There are promising early results in a phase 2 trial of the oral drug muvalaplin lowering Lp(a) levels by up to 85% for the highest dose, but there are no peer-reviewed articles confirming these results and no outcome trials at this time.
In patients who are already recognized as high risk, especially those with established coronary artery disease, measuring Lp(a) levels offer little benefit. These patients should already be receiving aggressive medical therapy to reach blood pressure targets if hypertensive, maximal lifestyle modifications, and statin therapy.
If these patients need more therapy because of continued coronary events, despite maximal conventional medical therapy, then adding a PCSK9 inhibitor would be appropriate whether or not a patient has a high Lp(a) level. Once Lp(a) targeted therapies are available and show clinical benefit, then the role of Lp(a) measurement and treatment in this population will be clearer.
Pearl: Most patients do not need Lp(a) testing. There are no FDA-approved treatments for high Lp(a) levels.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Kronenberg F et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur Heart J. 2022;43:3925-46.
2. Ruscica M et al. Lipoprotein(a) and PCSK9 inhibition: Clinical evidence Eur Heart J Suppl 2020;Nov 18(Suppl L):L53–L56.
From Fish Tanks to Cartoons
There was a recent Sermo post bemoaning the demise of fish tanks, and the calming they bring, in medical waiting rooms.
Aquariums, I agree, have a soporific effect on humans. I’m not immune myself on the rare occasions I encounter one. There’s something relaxing about watching the fish slowly glide back and forth while you admire their different colors, sizes, and patterns. This is why they persisted in a lot of places, such as videotapes (remember “Video Fish Tank”?), screen savers, and a key plot point in Finding Nemo.
Personally, When I get the occasional offer for a free waiting room TV that will play some customized feed about my practice and “ask your doctor” treatments, I send it off to be recycled into kitchen towels.
I think the real reason fish tanks are gone is that eternal bugaboo of medicine: money.
Margins in most practices, including mine, are thin, and a real fish tank (I’m not talking about a guppy in a bowl) aren’t cheap. They take, well, fish, and the most colorful ones are saltwater. Then they take a pump, heater, chemicals, food, plants, and decorations. Then you have to throw in the cost of a service with expertise in maintaining them (let’s face it, none of us have time to do that ourselves) ...
You want to add that to your overhead? Me neither.
My waiting room, as a result, is pretty bland. A handful of magazines, some books of classic Far Side, Calvin & Hobbes, and Doonesbury cartoons. The magazines are older, but relatively timeless ones, like issues of the Smithsonian or National Geographic. I don’t put out news magazines of any kind. If I’m not going to read the news, my patients shouldn’t have to either. My lobby should be relaxing.
We also live in an era where patients bring their own entertainment, on phones or iPads, to read while waiting. There are often days when I straighten up the waiting room while closing and the magazines haven’t been touched.
Yes, I miss fish tanks. But, like so many other things, they’ve become a casualty of modern medicine. They simply don’t make financial sense.
I’d rather cut corners in the waiting room than with patient care.
Block has a solo neurology practice in Scottsdale, Arizona.
There was a recent Sermo post bemoaning the demise of fish tanks, and the calming they bring, in medical waiting rooms.
Aquariums, I agree, have a soporific effect on humans. I’m not immune myself on the rare occasions I encounter one. There’s something relaxing about watching the fish slowly glide back and forth while you admire their different colors, sizes, and patterns. This is why they persisted in a lot of places, such as videotapes (remember “Video Fish Tank”?), screen savers, and a key plot point in Finding Nemo.
Personally, When I get the occasional offer for a free waiting room TV that will play some customized feed about my practice and “ask your doctor” treatments, I send it off to be recycled into kitchen towels.
I think the real reason fish tanks are gone is that eternal bugaboo of medicine: money.
Margins in most practices, including mine, are thin, and a real fish tank (I’m not talking about a guppy in a bowl) aren’t cheap. They take, well, fish, and the most colorful ones are saltwater. Then they take a pump, heater, chemicals, food, plants, and decorations. Then you have to throw in the cost of a service with expertise in maintaining them (let’s face it, none of us have time to do that ourselves) ...
You want to add that to your overhead? Me neither.
My waiting room, as a result, is pretty bland. A handful of magazines, some books of classic Far Side, Calvin & Hobbes, and Doonesbury cartoons. The magazines are older, but relatively timeless ones, like issues of the Smithsonian or National Geographic. I don’t put out news magazines of any kind. If I’m not going to read the news, my patients shouldn’t have to either. My lobby should be relaxing.
We also live in an era where patients bring their own entertainment, on phones or iPads, to read while waiting. There are often days when I straighten up the waiting room while closing and the magazines haven’t been touched.
Yes, I miss fish tanks. But, like so many other things, they’ve become a casualty of modern medicine. They simply don’t make financial sense.
I’d rather cut corners in the waiting room than with patient care.
Block has a solo neurology practice in Scottsdale, Arizona.
There was a recent Sermo post bemoaning the demise of fish tanks, and the calming they bring, in medical waiting rooms.
Aquariums, I agree, have a soporific effect on humans. I’m not immune myself on the rare occasions I encounter one. There’s something relaxing about watching the fish slowly glide back and forth while you admire their different colors, sizes, and patterns. This is why they persisted in a lot of places, such as videotapes (remember “Video Fish Tank”?), screen savers, and a key plot point in Finding Nemo.
Personally, When I get the occasional offer for a free waiting room TV that will play some customized feed about my practice and “ask your doctor” treatments, I send it off to be recycled into kitchen towels.
I think the real reason fish tanks are gone is that eternal bugaboo of medicine: money.
Margins in most practices, including mine, are thin, and a real fish tank (I’m not talking about a guppy in a bowl) aren’t cheap. They take, well, fish, and the most colorful ones are saltwater. Then they take a pump, heater, chemicals, food, plants, and decorations. Then you have to throw in the cost of a service with expertise in maintaining them (let’s face it, none of us have time to do that ourselves) ...
You want to add that to your overhead? Me neither.
My waiting room, as a result, is pretty bland. A handful of magazines, some books of classic Far Side, Calvin & Hobbes, and Doonesbury cartoons. The magazines are older, but relatively timeless ones, like issues of the Smithsonian or National Geographic. I don’t put out news magazines of any kind. If I’m not going to read the news, my patients shouldn’t have to either. My lobby should be relaxing.
We also live in an era where patients bring their own entertainment, on phones or iPads, to read while waiting. There are often days when I straighten up the waiting room while closing and the magazines haven’t been touched.
Yes, I miss fish tanks. But, like so many other things, they’ve become a casualty of modern medicine. They simply don’t make financial sense.
I’d rather cut corners in the waiting room than with patient care.
Block has a solo neurology practice in Scottsdale, Arizona.
Exposomania
If we’ve learned anything about obesity prevention it’s that if we wait too long the die is cast and our success rate is nil. The GLP-1 antagonists seem to be a workable solution for treating the adult and adolescent population, but I have been afraid that their success will divert too much of our attention away from prevention.
Fortunately, there still seems to be a few researchers committed to the age group in which obesity could be headed off before our only option is treatment. In one recent study, “Neighborhood Food Access in Early Life and Trajectories of Child Body Mass Index and Obesity,” researchers collected data from more than 28,000 children in 55 cohorts during the period from 1994 to 2023). The investigators found that residence in a low–food access, low-income neighborhoods during pregnancy and early childhood was associated with higher BMI “Z” scores, a higher risk of obesity, and severe obesity in childhood. The researchers defined low food access as living greater than 0.5 miles away from a grocery store in an urban setting or greater than 10 minutes away in a rural setting. I don’t think those associations should surprise us, but having some data from a large population may be valuable should we ever find the political will to undertake any steps toward prevention.
I found a Viewpoint article published 2 weeks earlier in the same journal, titled, “The Exposome as a Key to Understanding Pediatric Health Disparities.” I know what the “biome” is and have heard gastroenterologists expound on the power that billions of our little single-celled friends residing in our gut have on seemingly unrelated and spatially distant events in our body. But, “exposome” was a new word for me, although it turns out the concept is simple and one I had harbored since late childhood.
The opening sentence of the article reads “One’s environment profoundly changes health outcomes throughout one’s lifespan.” That truism was obvious to my 7-year-old self as I observed my playmates who lived in a poorly kept house in the less desirable area two blocks away and didn’t eat breakfast and were sick more often than the rest of us more-fortunates.
The authors define the exposome as the “totality of an individual’s non-genetic exposures, including psychosocial experiences, structural social determinants, chemical pollution, and neighborhood infrastructure.” This seems to be a pretty complete description of the nurture side of the nature versus nurture conversation.
I suspect that, like me, most of you through observation and intuition have included your own interpretation of the exposome in your professional activities. However, the authors feel we could be more robust in our efforts and claim that They go on to call for incorporation of an “exposome lens in pediatric research and healthcare delivery.”
I’m not sure this is a valid criticism. There is certainly more that could be done when it comes to research that examines the effect of environmental stressors. And, I suspect the authors would view this recent paper on the association between neighborhood food access as a step in the right direction. However, when it comes to healthcare delivery, at least at the level where the stethoscope meets the chest, I think, or at least hope, the authors are underestimating the observational skills and sensitivity of primary care providers.
We were all taught to take an appropriate medical history when evaluating a patient. And, through our formal education, our personal observations and through exposure to papers like this one on food access we must be aware of the effect of environmental stressors on our patients’ health. Is there more we could learn about those kind of associations? Certainly. This is where a more broadly focused exposome lens could be most effective.
The authors of the article observed: “The effect of the exposome is not uniform for all individuals but rather intersects across identities precipitating unique outcomes.” The practical reality is that to generate statistically significant data research must look at identities. This doesn’t mean that large population studies are without value. However, it does obligate investigators to include that caveat about the uniqueness of the individual in their conclusions. And, it is our duty as providers to keep this reality in mind as we interpret studies we read in the context of each individual patient.
When it comes to healthcare delivery at the structural level, I am concerned that we are moving in a direction that is making it more difficult for the provider to become familiar with the patient’s exposome. I am talking about an over-reliance on the team care delivery model that too often results in the “We never/seldom see the same provider” patient complaint.
I don’t care how slick and user-friendly a practice’s EHR system is; the best way to learn about a patient’s exposome is by repeated exposure (pun unintended) to the same provider. This isn’t always possible, and a well-crafted and conscientiously managed EHR can fill in some of the gaps. But, it is a distant second best.
Awareness of the importance of the exposome is only the starting point. Finding the political will to make the changes necessary to improve our patients’ outcomes is the bigger challenge. Grocery stores well-stocked with healthy foods don’t just pop up where we want them because we think they may hold answer to preventing pediatric obesity.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
If we’ve learned anything about obesity prevention it’s that if we wait too long the die is cast and our success rate is nil. The GLP-1 antagonists seem to be a workable solution for treating the adult and adolescent population, but I have been afraid that their success will divert too much of our attention away from prevention.
Fortunately, there still seems to be a few researchers committed to the age group in which obesity could be headed off before our only option is treatment. In one recent study, “Neighborhood Food Access in Early Life and Trajectories of Child Body Mass Index and Obesity,” researchers collected data from more than 28,000 children in 55 cohorts during the period from 1994 to 2023). The investigators found that residence in a low–food access, low-income neighborhoods during pregnancy and early childhood was associated with higher BMI “Z” scores, a higher risk of obesity, and severe obesity in childhood. The researchers defined low food access as living greater than 0.5 miles away from a grocery store in an urban setting or greater than 10 minutes away in a rural setting. I don’t think those associations should surprise us, but having some data from a large population may be valuable should we ever find the political will to undertake any steps toward prevention.
I found a Viewpoint article published 2 weeks earlier in the same journal, titled, “The Exposome as a Key to Understanding Pediatric Health Disparities.” I know what the “biome” is and have heard gastroenterologists expound on the power that billions of our little single-celled friends residing in our gut have on seemingly unrelated and spatially distant events in our body. But, “exposome” was a new word for me, although it turns out the concept is simple and one I had harbored since late childhood.
The opening sentence of the article reads “One’s environment profoundly changes health outcomes throughout one’s lifespan.” That truism was obvious to my 7-year-old self as I observed my playmates who lived in a poorly kept house in the less desirable area two blocks away and didn’t eat breakfast and were sick more often than the rest of us more-fortunates.
The authors define the exposome as the “totality of an individual’s non-genetic exposures, including psychosocial experiences, structural social determinants, chemical pollution, and neighborhood infrastructure.” This seems to be a pretty complete description of the nurture side of the nature versus nurture conversation.
I suspect that, like me, most of you through observation and intuition have included your own interpretation of the exposome in your professional activities. However, the authors feel we could be more robust in our efforts and claim that They go on to call for incorporation of an “exposome lens in pediatric research and healthcare delivery.”
I’m not sure this is a valid criticism. There is certainly more that could be done when it comes to research that examines the effect of environmental stressors. And, I suspect the authors would view this recent paper on the association between neighborhood food access as a step in the right direction. However, when it comes to healthcare delivery, at least at the level where the stethoscope meets the chest, I think, or at least hope, the authors are underestimating the observational skills and sensitivity of primary care providers.
We were all taught to take an appropriate medical history when evaluating a patient. And, through our formal education, our personal observations and through exposure to papers like this one on food access we must be aware of the effect of environmental stressors on our patients’ health. Is there more we could learn about those kind of associations? Certainly. This is where a more broadly focused exposome lens could be most effective.
The authors of the article observed: “The effect of the exposome is not uniform for all individuals but rather intersects across identities precipitating unique outcomes.” The practical reality is that to generate statistically significant data research must look at identities. This doesn’t mean that large population studies are without value. However, it does obligate investigators to include that caveat about the uniqueness of the individual in their conclusions. And, it is our duty as providers to keep this reality in mind as we interpret studies we read in the context of each individual patient.
When it comes to healthcare delivery at the structural level, I am concerned that we are moving in a direction that is making it more difficult for the provider to become familiar with the patient’s exposome. I am talking about an over-reliance on the team care delivery model that too often results in the “We never/seldom see the same provider” patient complaint.
I don’t care how slick and user-friendly a practice’s EHR system is; the best way to learn about a patient’s exposome is by repeated exposure (pun unintended) to the same provider. This isn’t always possible, and a well-crafted and conscientiously managed EHR can fill in some of the gaps. But, it is a distant second best.
Awareness of the importance of the exposome is only the starting point. Finding the political will to make the changes necessary to improve our patients’ outcomes is the bigger challenge. Grocery stores well-stocked with healthy foods don’t just pop up where we want them because we think they may hold answer to preventing pediatric obesity.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
If we’ve learned anything about obesity prevention it’s that if we wait too long the die is cast and our success rate is nil. The GLP-1 antagonists seem to be a workable solution for treating the adult and adolescent population, but I have been afraid that their success will divert too much of our attention away from prevention.
Fortunately, there still seems to be a few researchers committed to the age group in which obesity could be headed off before our only option is treatment. In one recent study, “Neighborhood Food Access in Early Life and Trajectories of Child Body Mass Index and Obesity,” researchers collected data from more than 28,000 children in 55 cohorts during the period from 1994 to 2023). The investigators found that residence in a low–food access, low-income neighborhoods during pregnancy and early childhood was associated with higher BMI “Z” scores, a higher risk of obesity, and severe obesity in childhood. The researchers defined low food access as living greater than 0.5 miles away from a grocery store in an urban setting or greater than 10 minutes away in a rural setting. I don’t think those associations should surprise us, but having some data from a large population may be valuable should we ever find the political will to undertake any steps toward prevention.
I found a Viewpoint article published 2 weeks earlier in the same journal, titled, “The Exposome as a Key to Understanding Pediatric Health Disparities.” I know what the “biome” is and have heard gastroenterologists expound on the power that billions of our little single-celled friends residing in our gut have on seemingly unrelated and spatially distant events in our body. But, “exposome” was a new word for me, although it turns out the concept is simple and one I had harbored since late childhood.
The opening sentence of the article reads “One’s environment profoundly changes health outcomes throughout one’s lifespan.” That truism was obvious to my 7-year-old self as I observed my playmates who lived in a poorly kept house in the less desirable area two blocks away and didn’t eat breakfast and were sick more often than the rest of us more-fortunates.
The authors define the exposome as the “totality of an individual’s non-genetic exposures, including psychosocial experiences, structural social determinants, chemical pollution, and neighborhood infrastructure.” This seems to be a pretty complete description of the nurture side of the nature versus nurture conversation.
I suspect that, like me, most of you through observation and intuition have included your own interpretation of the exposome in your professional activities. However, the authors feel we could be more robust in our efforts and claim that They go on to call for incorporation of an “exposome lens in pediatric research and healthcare delivery.”
I’m not sure this is a valid criticism. There is certainly more that could be done when it comes to research that examines the effect of environmental stressors. And, I suspect the authors would view this recent paper on the association between neighborhood food access as a step in the right direction. However, when it comes to healthcare delivery, at least at the level where the stethoscope meets the chest, I think, or at least hope, the authors are underestimating the observational skills and sensitivity of primary care providers.
We were all taught to take an appropriate medical history when evaluating a patient. And, through our formal education, our personal observations and through exposure to papers like this one on food access we must be aware of the effect of environmental stressors on our patients’ health. Is there more we could learn about those kind of associations? Certainly. This is where a more broadly focused exposome lens could be most effective.
The authors of the article observed: “The effect of the exposome is not uniform for all individuals but rather intersects across identities precipitating unique outcomes.” The practical reality is that to generate statistically significant data research must look at identities. This doesn’t mean that large population studies are without value. However, it does obligate investigators to include that caveat about the uniqueness of the individual in their conclusions. And, it is our duty as providers to keep this reality in mind as we interpret studies we read in the context of each individual patient.
When it comes to healthcare delivery at the structural level, I am concerned that we are moving in a direction that is making it more difficult for the provider to become familiar with the patient’s exposome. I am talking about an over-reliance on the team care delivery model that too often results in the “We never/seldom see the same provider” patient complaint.
I don’t care how slick and user-friendly a practice’s EHR system is; the best way to learn about a patient’s exposome is by repeated exposure (pun unintended) to the same provider. This isn’t always possible, and a well-crafted and conscientiously managed EHR can fill in some of the gaps. But, it is a distant second best.
Awareness of the importance of the exposome is only the starting point. Finding the political will to make the changes necessary to improve our patients’ outcomes is the bigger challenge. Grocery stores well-stocked with healthy foods don’t just pop up where we want them because we think they may hold answer to preventing pediatric obesity.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Recognizing Burnout: Why Physicians Often Miss the Signs in Themselves
Summary and Key Highlights
Summary: This section explores why physicians often struggle to recognize burnout within themselves, partly due to stigma and a tendency to focus on productivity over well-being. Dr. Tyra Fainstad shares personal experiences of burnout symptoms, emphasizing the importance of awareness and self-reflection. Recognizing and addressing burnout early can help physicians find healthier coping strategies, avoid productivity traps, and seek support.
Key Takeaways:
- Many physicians struggle to identify burnout due to stigma and self-blame.
- Awareness of burnout symptoms is essential for early intervention and healthy coping.
- Seeking support can prevent burnout from worsening and improve quality of life.
Our Editors Also Recommend:
Medscape Physician Burnout & Depression Report 2024: ‘We Have Much Work to Do’
Medscape Hospitalist Burnout & Depression Report 2024: Seeking Progress, Balance
Medscape Physician Lifestyle & Happiness Report 2024: The Ongoing Struggle for Balance
A Transformative Rx for Burnout, Grief, and Illness: Dance
Next Medscape Masters Event:
Stay at the forefront of obesity care. Register for exclusive insights and the latest treatment innovations.
Lotte Dyrbye, has disclosed the following relevant financial relationships: Co-inventor of the Well-being Index and its derivatives, which Mayo Clinic has licensed. Dyrbye receives royalties.
A version of this article first appeared on Medscape.com.
Summary and Key Highlights
Summary: This section explores why physicians often struggle to recognize burnout within themselves, partly due to stigma and a tendency to focus on productivity over well-being. Dr. Tyra Fainstad shares personal experiences of burnout symptoms, emphasizing the importance of awareness and self-reflection. Recognizing and addressing burnout early can help physicians find healthier coping strategies, avoid productivity traps, and seek support.
Key Takeaways:
- Many physicians struggle to identify burnout due to stigma and self-blame.
- Awareness of burnout symptoms is essential for early intervention and healthy coping.
- Seeking support can prevent burnout from worsening and improve quality of life.
Our Editors Also Recommend:
Medscape Physician Burnout & Depression Report 2024: ‘We Have Much Work to Do’
Medscape Hospitalist Burnout & Depression Report 2024: Seeking Progress, Balance
Medscape Physician Lifestyle & Happiness Report 2024: The Ongoing Struggle for Balance
A Transformative Rx for Burnout, Grief, and Illness: Dance
Next Medscape Masters Event:
Stay at the forefront of obesity care. Register for exclusive insights and the latest treatment innovations.
Lotte Dyrbye, has disclosed the following relevant financial relationships: Co-inventor of the Well-being Index and its derivatives, which Mayo Clinic has licensed. Dyrbye receives royalties.
A version of this article first appeared on Medscape.com.
Summary and Key Highlights
Summary: This section explores why physicians often struggle to recognize burnout within themselves, partly due to stigma and a tendency to focus on productivity over well-being. Dr. Tyra Fainstad shares personal experiences of burnout symptoms, emphasizing the importance of awareness and self-reflection. Recognizing and addressing burnout early can help physicians find healthier coping strategies, avoid productivity traps, and seek support.
Key Takeaways:
- Many physicians struggle to identify burnout due to stigma and self-blame.
- Awareness of burnout symptoms is essential for early intervention and healthy coping.
- Seeking support can prevent burnout from worsening and improve quality of life.
Our Editors Also Recommend:
Medscape Physician Burnout & Depression Report 2024: ‘We Have Much Work to Do’
Medscape Hospitalist Burnout & Depression Report 2024: Seeking Progress, Balance
Medscape Physician Lifestyle & Happiness Report 2024: The Ongoing Struggle for Balance
A Transformative Rx for Burnout, Grief, and Illness: Dance
Next Medscape Masters Event:
Stay at the forefront of obesity care. Register for exclusive insights and the latest treatment innovations.
Lotte Dyrbye, has disclosed the following relevant financial relationships: Co-inventor of the Well-being Index and its derivatives, which Mayo Clinic has licensed. Dyrbye receives royalties.
A version of this article first appeared on Medscape.com.
Breaking the Cycle: Why Self-Compassion Is Essential for Today’s Physicians
Summary and Key Highlights
Summary: Dr Tyra Fainstad explores the ingrained culture in medicine that encourages self-criticism, with many physicians feeling that they must be hard on themselves to succeed. Dr Fainstad challenges this belief, advocating for self-compassion as a healthier alternative. The evolving medical field now includes physicians who prioritize well-being without sacrificing quality of care, underscoring the importance of self-kindness for sustainable practice.
Key Takeaways:
- Many physicians believe that self-criticism is necessary for success, a mindset rooted in medical culture.
- Practicing self-compassion can improve long-term resilience and prevent burnout.
- The changing landscape of healthcare supports a more balanced approach to physician well-being.
Our Editors Also Recommend:
Medscape Physician Burnout & Depression Report 2024: ‘We Have Much Work to Do’
Medscape Hospitalist Burnout & Depression Report 2024: Seeking Progress, Balance
Medscape Physician Lifestyle & Happiness Report 2024: The Ongoing Struggle for Balance
A Transformative Rx for Burnout, Grief, and Illness: Dance
Next Medscape Masters Event:
Stay at the forefront of obesity care. Register for exclusive insights and the latest treatment innovations.
Lotte Dyrbye, has disclosed the following relevant financial relationships: Co-inventor of the Well-being Index and its derivatives, which Mayo Clinic has licensed. Dyrbye receives royalties.
A version of this article first appeared on Medscape.com.
Summary and Key Highlights
Summary: Dr Tyra Fainstad explores the ingrained culture in medicine that encourages self-criticism, with many physicians feeling that they must be hard on themselves to succeed. Dr Fainstad challenges this belief, advocating for self-compassion as a healthier alternative. The evolving medical field now includes physicians who prioritize well-being without sacrificing quality of care, underscoring the importance of self-kindness for sustainable practice.
Key Takeaways:
- Many physicians believe that self-criticism is necessary for success, a mindset rooted in medical culture.
- Practicing self-compassion can improve long-term resilience and prevent burnout.
- The changing landscape of healthcare supports a more balanced approach to physician well-being.
Our Editors Also Recommend:
Medscape Physician Burnout & Depression Report 2024: ‘We Have Much Work to Do’
Medscape Hospitalist Burnout & Depression Report 2024: Seeking Progress, Balance
Medscape Physician Lifestyle & Happiness Report 2024: The Ongoing Struggle for Balance
A Transformative Rx for Burnout, Grief, and Illness: Dance
Next Medscape Masters Event:
Stay at the forefront of obesity care. Register for exclusive insights and the latest treatment innovations.
Lotte Dyrbye, has disclosed the following relevant financial relationships: Co-inventor of the Well-being Index and its derivatives, which Mayo Clinic has licensed. Dyrbye receives royalties.
A version of this article first appeared on Medscape.com.
Summary and Key Highlights
Summary: Dr Tyra Fainstad explores the ingrained culture in medicine that encourages self-criticism, with many physicians feeling that they must be hard on themselves to succeed. Dr Fainstad challenges this belief, advocating for self-compassion as a healthier alternative. The evolving medical field now includes physicians who prioritize well-being without sacrificing quality of care, underscoring the importance of self-kindness for sustainable practice.
Key Takeaways:
- Many physicians believe that self-criticism is necessary for success, a mindset rooted in medical culture.
- Practicing self-compassion can improve long-term resilience and prevent burnout.
- The changing landscape of healthcare supports a more balanced approach to physician well-being.
Our Editors Also Recommend:
Medscape Physician Burnout & Depression Report 2024: ‘We Have Much Work to Do’
Medscape Hospitalist Burnout & Depression Report 2024: Seeking Progress, Balance
Medscape Physician Lifestyle & Happiness Report 2024: The Ongoing Struggle for Balance
A Transformative Rx for Burnout, Grief, and Illness: Dance
Next Medscape Masters Event:
Stay at the forefront of obesity care. Register for exclusive insights and the latest treatment innovations.
Lotte Dyrbye, has disclosed the following relevant financial relationships: Co-inventor of the Well-being Index and its derivatives, which Mayo Clinic has licensed. Dyrbye receives royalties.
A version of this article first appeared on Medscape.com.
Finding Fulfillment Beyond Metrics: A Physician’s Path to Lasting Well-Being
Summary and Key Highlights
Summary: Dr Tyra Fainstad shares her personal experience with burnout and the journey to recovery through coaching and self-compassion. She describes the pressures of seeking validation through external achievements, which ultimately led to a crisis in self-worth after medical training. Through coaching, she learned to cultivate a sense of internal fulfillment, reconnecting with her passion for medicine and achieving a healthier balance.
Key Takeaways:
- Relying solely on external validation can deepen burnout and affect well-being.
- Coaching empowers physicians to develop self-compassion and sustainable coping strategies.
- Shifting from external to internal validation strengthens long-term fulfillment and job satisfaction.
Our Editors Also Recommend:
Medscape Physician Burnout & Depression Report 2024: ‘We Have Much Work to Do’
Medscape Hospitalist Burnout & Depression Report 2024: Seeking Progress, Balance
Medscape Physician Lifestyle & Happiness Report 2024: The Ongoing Struggle for Balance
A Transformative Rx for Burnout, Grief, and Illness: Dance
Next Medscape Masters Event:
Stay at the forefront of obesity care. Register for exclusive insights and the latest treatment innovations.
A version of this article first appeared on Medscape.com.
Summary and Key Highlights
Summary: Dr Tyra Fainstad shares her personal experience with burnout and the journey to recovery through coaching and self-compassion. She describes the pressures of seeking validation through external achievements, which ultimately led to a crisis in self-worth after medical training. Through coaching, she learned to cultivate a sense of internal fulfillment, reconnecting with her passion for medicine and achieving a healthier balance.
Key Takeaways:
- Relying solely on external validation can deepen burnout and affect well-being.
- Coaching empowers physicians to develop self-compassion and sustainable coping strategies.
- Shifting from external to internal validation strengthens long-term fulfillment and job satisfaction.
Our Editors Also Recommend:
Medscape Physician Burnout & Depression Report 2024: ‘We Have Much Work to Do’
Medscape Hospitalist Burnout & Depression Report 2024: Seeking Progress, Balance
Medscape Physician Lifestyle & Happiness Report 2024: The Ongoing Struggle for Balance
A Transformative Rx for Burnout, Grief, and Illness: Dance
Next Medscape Masters Event:
Stay at the forefront of obesity care. Register for exclusive insights and the latest treatment innovations.
A version of this article first appeared on Medscape.com.
Summary and Key Highlights
Summary: Dr Tyra Fainstad shares her personal experience with burnout and the journey to recovery through coaching and self-compassion. She describes the pressures of seeking validation through external achievements, which ultimately led to a crisis in self-worth after medical training. Through coaching, she learned to cultivate a sense of internal fulfillment, reconnecting with her passion for medicine and achieving a healthier balance.
Key Takeaways:
- Relying solely on external validation can deepen burnout and affect well-being.
- Coaching empowers physicians to develop self-compassion and sustainable coping strategies.
- Shifting from external to internal validation strengthens long-term fulfillment and job satisfaction.
Our Editors Also Recommend:
Medscape Physician Burnout & Depression Report 2024: ‘We Have Much Work to Do’
Medscape Hospitalist Burnout & Depression Report 2024: Seeking Progress, Balance
Medscape Physician Lifestyle & Happiness Report 2024: The Ongoing Struggle for Balance
A Transformative Rx for Burnout, Grief, and Illness: Dance
Next Medscape Masters Event:
Stay at the forefront of obesity care. Register for exclusive insights and the latest treatment innovations.
A version of this article first appeared on Medscape.com.
To Hold or Not to Hold GLP-1s Before Surgery
This transcript has been edited for clarity.
Recently, there have been two somewhat conflicting recommendations about how to deal with our patients who are on incretin hormone therapy before undergoing elective surgical procedures.
First, the FDA [Food and Drug Administration] has updated the package inserts for all of these incretins, meaning the glucagon-like peptide-1 (GLP-1) receptor agonists and the dual glucose-dependent insulinotropic (GIP)/GLP-1 receptor agonist tirzepatide, with a warning about pulmonary aspiration during general anesthesia or deep sedation. They instruct patients to let healthcare providers know of any planned surgeries or procedures. This has come about because of postmarketing experience in which patients who are on GLP-1 receptor agonists have had residual gastric contents found despite reported adherence to preoperative fasting recommendations.
The problem with this is that the FDA says they don’t really actually know what to tell us to do or not to do because we don’t have knowledge as to how to truly mitigate the risk for pulmonary aspiration during general anesthesia or deep sedation. They don’t know if modifying preoperative fasting recommendations should be changed or if temporary discontinuation of the drugs could reduce this problem. They really don’t know what to tell us to do except to tell us that this is a problem we should discuss with our patients.
At about the same time, a society guideline— and this was from a number of different societies, including the American Society of Anesthesiologists — stated that most patients should continue taking their GLP-1 receptor agonist before elective surgery.
This struck me as somewhat discordant from what the FDA said, although the FDA also says they don’t know quite what to tell us to do. This clinical guideline goes into a bit more detail, and what they think might be a good idea is that patients who are at the highest risk for GI side effects should follow a liquid diet for 24 hours before the procedure.
They basically look at who is at highest risk, and they say the following: Patients in the escalation phase of their incretin therapy — that is, early in treatment when the dose is increasing — are most likely to have delays in gastric emptying because that effect is lessened over time. They say that the elective surgery should be deferred until the escalation phase has passed and the GI symptoms have dissipated.
They’re very clear that patients who have significant GI symptoms, including nausea, vomiting, abdominal pain, constipation, and shortness of breath, should wait until their symptoms have dissipated.
They think this is something that would be good no matter what dose of drug these patients are on. They do say that you tend to see more issues with gastric emptying in patients at the highest dose of a GLP-1 receptor agonist. They also mention other medical conditions that may slow gastric emptying, such as Parkinson’s disease, which may further modify the perioperative management plan.
Their proposed solutions that sort of correspond with my proposed solutions include assessing the patient. Obviously, if a patient is going up on the dose of these drugs or having many GI side effects, that’s someone who you probably don’t want to send for elective surgery if you don’t have to. However, if you need to — and possibly in everybody — you might want to withhold the drug for 10-14 days preoperatively to make sure they don’t have significant GI side effects as they’re preparing for their procedure.
One of the things the anesthesiology group was worried about was that glucose levels would go up and patients would have hyperglycemia going into surgery. I’m not so worried about holding a dose or two of one of these agents. I don’t see much hyperglycemia occurring. If it does, you can treat it in other ways.
If it’s somebody where you think they’re having symptoms but they want to have the procedure anyway, you can put them on a liquid diet for 24 hours or so, so that there’s less of a risk for retained gastric contents, at least solid gastric contents. Anesthesiologists can help with this as well because in many cases, they can do a point-of-care gastric ultrasound to check for retained food or fluid.
I know this is sort of vague because I don’t have clear recommendations, but I do think it’s important to talk with your patients to assess whether they’re having signs or symptoms of gastroparesis. I think it’s not unreasonable to hold the incretin hormone therapy for one or two doses before a procedure if you have that opportunity, and be sure that the anesthesiologist and surgery team are aware of the fact that the patient has been on one of these agents so that they’re a little more aware of the risk for aspiration.
Anne L. Peters, Professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, University of Southern California, Los Angeles, California, has disclosed the following relevant financial relationships: Serve(d) on the advisory board for Abbott Diabetes Care; Becton Dickinson; Boehringer Ingelheim Pharmaceuticals, Inc.; Eli Lilly and Company; Lexicon Pharmaceuticals, Inc.; Livongo; Medscape; Merck & Co., Inc.; Novo Nordisk; Omada Health; OptumHealth; sanofi; Zafgen Received research support from: Dexcom; MannKind Corporation; Astra Zeneca. Serve(d) as a member of a speakers bureau for: Novo Nordisk.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Recently, there have been two somewhat conflicting recommendations about how to deal with our patients who are on incretin hormone therapy before undergoing elective surgical procedures.
First, the FDA [Food and Drug Administration] has updated the package inserts for all of these incretins, meaning the glucagon-like peptide-1 (GLP-1) receptor agonists and the dual glucose-dependent insulinotropic (GIP)/GLP-1 receptor agonist tirzepatide, with a warning about pulmonary aspiration during general anesthesia or deep sedation. They instruct patients to let healthcare providers know of any planned surgeries or procedures. This has come about because of postmarketing experience in which patients who are on GLP-1 receptor agonists have had residual gastric contents found despite reported adherence to preoperative fasting recommendations.
The problem with this is that the FDA says they don’t really actually know what to tell us to do or not to do because we don’t have knowledge as to how to truly mitigate the risk for pulmonary aspiration during general anesthesia or deep sedation. They don’t know if modifying preoperative fasting recommendations should be changed or if temporary discontinuation of the drugs could reduce this problem. They really don’t know what to tell us to do except to tell us that this is a problem we should discuss with our patients.
At about the same time, a society guideline— and this was from a number of different societies, including the American Society of Anesthesiologists — stated that most patients should continue taking their GLP-1 receptor agonist before elective surgery.
This struck me as somewhat discordant from what the FDA said, although the FDA also says they don’t know quite what to tell us to do. This clinical guideline goes into a bit more detail, and what they think might be a good idea is that patients who are at the highest risk for GI side effects should follow a liquid diet for 24 hours before the procedure.
They basically look at who is at highest risk, and they say the following: Patients in the escalation phase of their incretin therapy — that is, early in treatment when the dose is increasing — are most likely to have delays in gastric emptying because that effect is lessened over time. They say that the elective surgery should be deferred until the escalation phase has passed and the GI symptoms have dissipated.
They’re very clear that patients who have significant GI symptoms, including nausea, vomiting, abdominal pain, constipation, and shortness of breath, should wait until their symptoms have dissipated.
They think this is something that would be good no matter what dose of drug these patients are on. They do say that you tend to see more issues with gastric emptying in patients at the highest dose of a GLP-1 receptor agonist. They also mention other medical conditions that may slow gastric emptying, such as Parkinson’s disease, which may further modify the perioperative management plan.
Their proposed solutions that sort of correspond with my proposed solutions include assessing the patient. Obviously, if a patient is going up on the dose of these drugs or having many GI side effects, that’s someone who you probably don’t want to send for elective surgery if you don’t have to. However, if you need to — and possibly in everybody — you might want to withhold the drug for 10-14 days preoperatively to make sure they don’t have significant GI side effects as they’re preparing for their procedure.
One of the things the anesthesiology group was worried about was that glucose levels would go up and patients would have hyperglycemia going into surgery. I’m not so worried about holding a dose or two of one of these agents. I don’t see much hyperglycemia occurring. If it does, you can treat it in other ways.
If it’s somebody where you think they’re having symptoms but they want to have the procedure anyway, you can put them on a liquid diet for 24 hours or so, so that there’s less of a risk for retained gastric contents, at least solid gastric contents. Anesthesiologists can help with this as well because in many cases, they can do a point-of-care gastric ultrasound to check for retained food or fluid.
I know this is sort of vague because I don’t have clear recommendations, but I do think it’s important to talk with your patients to assess whether they’re having signs or symptoms of gastroparesis. I think it’s not unreasonable to hold the incretin hormone therapy for one or two doses before a procedure if you have that opportunity, and be sure that the anesthesiologist and surgery team are aware of the fact that the patient has been on one of these agents so that they’re a little more aware of the risk for aspiration.
Anne L. Peters, Professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, University of Southern California, Los Angeles, California, has disclosed the following relevant financial relationships: Serve(d) on the advisory board for Abbott Diabetes Care; Becton Dickinson; Boehringer Ingelheim Pharmaceuticals, Inc.; Eli Lilly and Company; Lexicon Pharmaceuticals, Inc.; Livongo; Medscape; Merck & Co., Inc.; Novo Nordisk; Omada Health; OptumHealth; sanofi; Zafgen Received research support from: Dexcom; MannKind Corporation; Astra Zeneca. Serve(d) as a member of a speakers bureau for: Novo Nordisk.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Recently, there have been two somewhat conflicting recommendations about how to deal with our patients who are on incretin hormone therapy before undergoing elective surgical procedures.
First, the FDA [Food and Drug Administration] has updated the package inserts for all of these incretins, meaning the glucagon-like peptide-1 (GLP-1) receptor agonists and the dual glucose-dependent insulinotropic (GIP)/GLP-1 receptor agonist tirzepatide, with a warning about pulmonary aspiration during general anesthesia or deep sedation. They instruct patients to let healthcare providers know of any planned surgeries or procedures. This has come about because of postmarketing experience in which patients who are on GLP-1 receptor agonists have had residual gastric contents found despite reported adherence to preoperative fasting recommendations.
The problem with this is that the FDA says they don’t really actually know what to tell us to do or not to do because we don’t have knowledge as to how to truly mitigate the risk for pulmonary aspiration during general anesthesia or deep sedation. They don’t know if modifying preoperative fasting recommendations should be changed or if temporary discontinuation of the drugs could reduce this problem. They really don’t know what to tell us to do except to tell us that this is a problem we should discuss with our patients.
At about the same time, a society guideline— and this was from a number of different societies, including the American Society of Anesthesiologists — stated that most patients should continue taking their GLP-1 receptor agonist before elective surgery.
This struck me as somewhat discordant from what the FDA said, although the FDA also says they don’t know quite what to tell us to do. This clinical guideline goes into a bit more detail, and what they think might be a good idea is that patients who are at the highest risk for GI side effects should follow a liquid diet for 24 hours before the procedure.
They basically look at who is at highest risk, and they say the following: Patients in the escalation phase of their incretin therapy — that is, early in treatment when the dose is increasing — are most likely to have delays in gastric emptying because that effect is lessened over time. They say that the elective surgery should be deferred until the escalation phase has passed and the GI symptoms have dissipated.
They’re very clear that patients who have significant GI symptoms, including nausea, vomiting, abdominal pain, constipation, and shortness of breath, should wait until their symptoms have dissipated.
They think this is something that would be good no matter what dose of drug these patients are on. They do say that you tend to see more issues with gastric emptying in patients at the highest dose of a GLP-1 receptor agonist. They also mention other medical conditions that may slow gastric emptying, such as Parkinson’s disease, which may further modify the perioperative management plan.
Their proposed solutions that sort of correspond with my proposed solutions include assessing the patient. Obviously, if a patient is going up on the dose of these drugs or having many GI side effects, that’s someone who you probably don’t want to send for elective surgery if you don’t have to. However, if you need to — and possibly in everybody — you might want to withhold the drug for 10-14 days preoperatively to make sure they don’t have significant GI side effects as they’re preparing for their procedure.
One of the things the anesthesiology group was worried about was that glucose levels would go up and patients would have hyperglycemia going into surgery. I’m not so worried about holding a dose or two of one of these agents. I don’t see much hyperglycemia occurring. If it does, you can treat it in other ways.
If it’s somebody where you think they’re having symptoms but they want to have the procedure anyway, you can put them on a liquid diet for 24 hours or so, so that there’s less of a risk for retained gastric contents, at least solid gastric contents. Anesthesiologists can help with this as well because in many cases, they can do a point-of-care gastric ultrasound to check for retained food or fluid.
I know this is sort of vague because I don’t have clear recommendations, but I do think it’s important to talk with your patients to assess whether they’re having signs or symptoms of gastroparesis. I think it’s not unreasonable to hold the incretin hormone therapy for one or two doses before a procedure if you have that opportunity, and be sure that the anesthesiologist and surgery team are aware of the fact that the patient has been on one of these agents so that they’re a little more aware of the risk for aspiration.
Anne L. Peters, Professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, University of Southern California, Los Angeles, California, has disclosed the following relevant financial relationships: Serve(d) on the advisory board for Abbott Diabetes Care; Becton Dickinson; Boehringer Ingelheim Pharmaceuticals, Inc.; Eli Lilly and Company; Lexicon Pharmaceuticals, Inc.; Livongo; Medscape; Merck & Co., Inc.; Novo Nordisk; Omada Health; OptumHealth; sanofi; Zafgen Received research support from: Dexcom; MannKind Corporation; Astra Zeneca. Serve(d) as a member of a speakers bureau for: Novo Nordisk.
A version of this article first appeared on Medscape.com.
We Haven’t Kicked Our Pandemic Drinking Habit
This transcript has been edited for clarity.
You’re stuck in your house. Work is closed or you’re working remotely. Your kids’ school is closed or is offering an hour or two a day of Zoom-based instruction. You have a bit of cabin fever which, you suppose, is better than the actual fever that comes with COVID infections, which are running rampant during the height of the pandemic. But still — it’s stressful. What do you do?
We all coped in our own way. We baked sourdough bread. We built that tree house we’d been meaning to build. We started podcasts. And ... we drank. Quite a bit, actually.
During the first year of the pandemic, alcohol sales increased 3%, the largest year-on-year increase in more than 50 years. There was also an increase in drunkenness across the board, though it was most pronounced in those who were already at risk from alcohol use disorder.
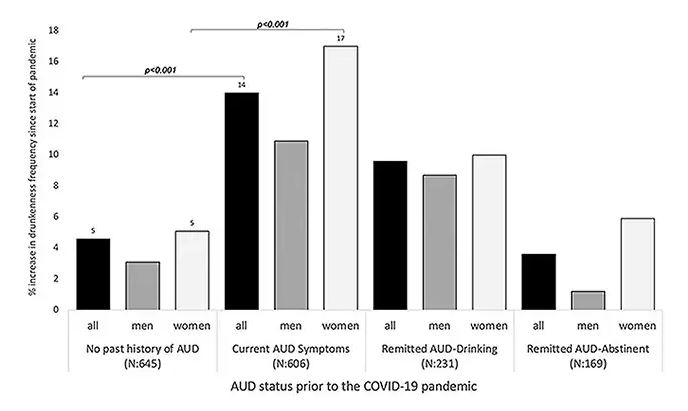
Alcohol-associated deaths increased by around 10% from 2019 to 2020. Obviously, this is a small percentage of COVID-associated deaths, but it is nothing to sneeze at.
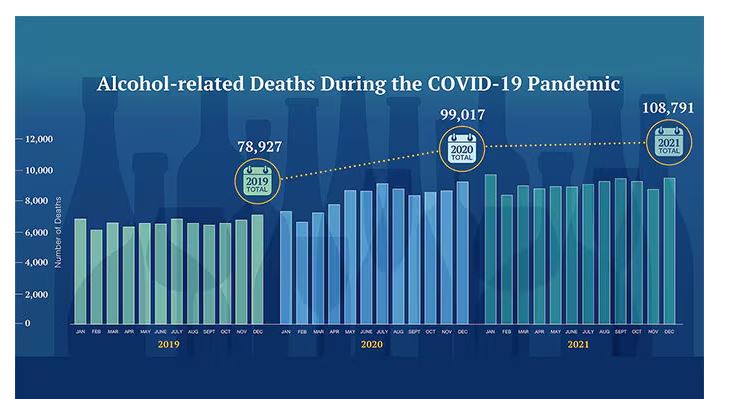
But look, we were anxious. And say what you will about alcohol as a risk factor for liver disease, heart disease, and cancer — not to mention traffic accidents — it is an anxiolytic, at least in the short term.
But as the pandemic waned, as society reopened, as we got back to work and reintegrated into our social circles and escaped the confines of our houses and apartments, our drinking habits went back to normal, right?
Americans’ love affair with alcohol has been a torrid one, as this graph showing gallons of ethanol consumed per capita over time shows you.
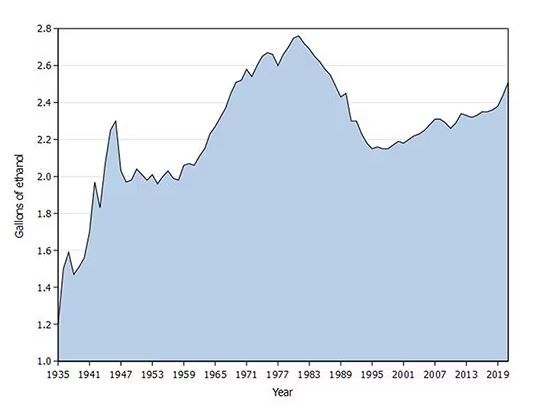
What you see is a steady increase in alcohol consumption from the end of prohibition in 1933 to its peak in the heady days of the early 1980s, followed by a steady decline until the mid-1990s. Since then, there has been another increase with, as you will note, a notable uptick during the early part of the COVID pandemic.
What came across my desk this week was updated data, appearing in a research letter in Annals of Internal Medicine, that compared alcohol consumption in 2020 — the first year of the COVID pandemic — with that in 2022 (the latest available data). And it looks like not much has changed.
This was a population-based survey study leveraging the National Health Interview Survey, including around 80,000 respondents from 2018, 2020, and 2022.
They created two main categories of drinking: drinking any alcohol at all and heavy drinking.
In 2018, 66% of Americans reported drinking any alcohol. That had risen to 69% by 2020, and it stayed at that level even after the lockdown had ended, as you can see here. This may seem like a small increase, but this was a highly significant result. Translating into absolute numbers, it suggests that we have added between 3,328,000 and 10,660,000 net additional drinkers to the population over this time period.
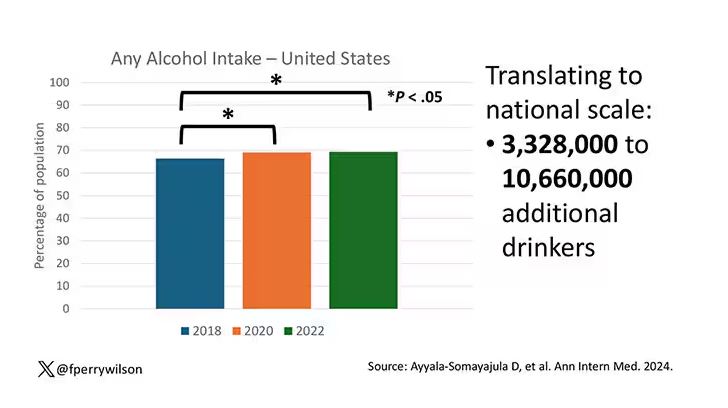
This trend was seen across basically every demographic group, with some notably larger increases among Black and Hispanic individuals, and marginally higher rates among people under age 30.
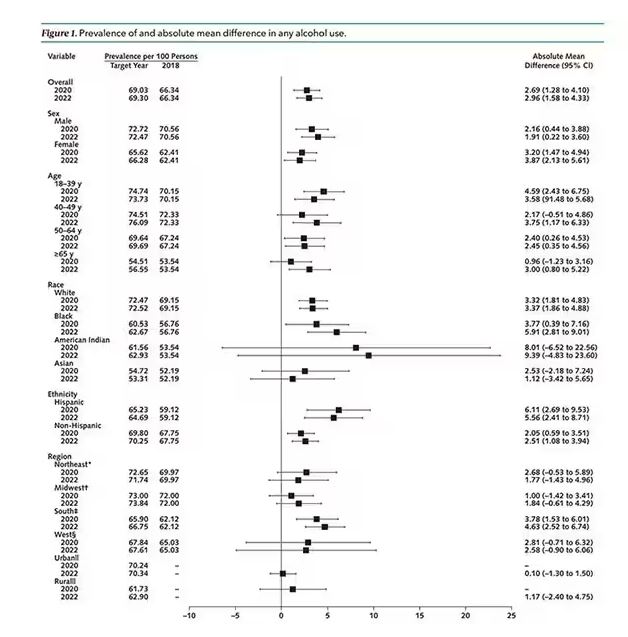
But far be it from me to deny someone a tot of brandy on a cold winter’s night. More interesting is the rate of heavy alcohol use reported in the study. For context, the definitions of heavy alcohol use appear here. For men, it’s any one day with five or more drinks or 15 or more drinks per week. For women it’s four or more drinks on a given day or eight drinks or more per week.
The overall rate of heavy drinking was about 5.1% in 2018 before the start of the pandemic. That rose to more than 6% in 2020 and it rose a bit more into 2022. The net change here, on a population level, is from 1,430,000 to 3,926,000 new heavy drinkers. That’s a number that rises to the level of an actual public health issue.
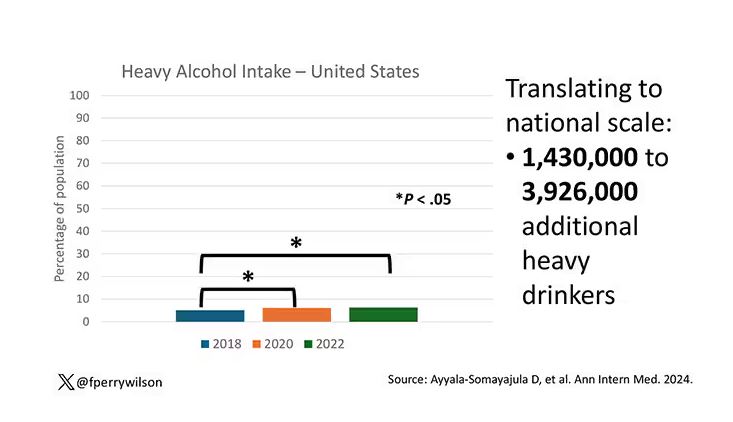
Again, this trend was fairly broad across demographic groups. Although in this case, the changes were a bit larger among White people and those in the 40- to 49-year age group. This is my cohort, I guess. Cheers.
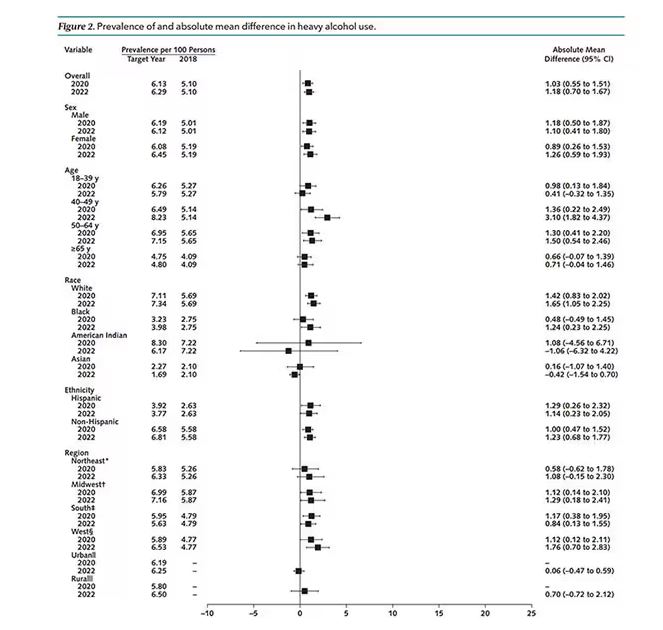
The information we have from this study is purely descriptive. It tells us that people are drinking more since the pandemic. It doesn’t tell us why, or the impact that this excess drinking will have on subsequent health outcomes, although other studies would suggest that it will contribute to certain chronic conditions, both physical and mental.
Maybe more important is that it reminds us that habits are sticky. Once we become accustomed to something — that glass of wine or two with dinner, and before bed — it has a tendency to stay with us. There’s an upside to that phenomenon as well, of course; it means that we can train good habits too. And those, once they become ingrained, can be just as hard to break. We just need to be mindful of the habits we pick. New Year 2025 is just around the corner. Start brainstorming those resolutions now.
Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
You’re stuck in your house. Work is closed or you’re working remotely. Your kids’ school is closed or is offering an hour or two a day of Zoom-based instruction. You have a bit of cabin fever which, you suppose, is better than the actual fever that comes with COVID infections, which are running rampant during the height of the pandemic. But still — it’s stressful. What do you do?
We all coped in our own way. We baked sourdough bread. We built that tree house we’d been meaning to build. We started podcasts. And ... we drank. Quite a bit, actually.
During the first year of the pandemic, alcohol sales increased 3%, the largest year-on-year increase in more than 50 years. There was also an increase in drunkenness across the board, though it was most pronounced in those who were already at risk from alcohol use disorder.

Alcohol-associated deaths increased by around 10% from 2019 to 2020. Obviously, this is a small percentage of COVID-associated deaths, but it is nothing to sneeze at.

But look, we were anxious. And say what you will about alcohol as a risk factor for liver disease, heart disease, and cancer — not to mention traffic accidents — it is an anxiolytic, at least in the short term.
But as the pandemic waned, as society reopened, as we got back to work and reintegrated into our social circles and escaped the confines of our houses and apartments, our drinking habits went back to normal, right?
Americans’ love affair with alcohol has been a torrid one, as this graph showing gallons of ethanol consumed per capita over time shows you.

What you see is a steady increase in alcohol consumption from the end of prohibition in 1933 to its peak in the heady days of the early 1980s, followed by a steady decline until the mid-1990s. Since then, there has been another increase with, as you will note, a notable uptick during the early part of the COVID pandemic.
What came across my desk this week was updated data, appearing in a research letter in Annals of Internal Medicine, that compared alcohol consumption in 2020 — the first year of the COVID pandemic — with that in 2022 (the latest available data). And it looks like not much has changed.
This was a population-based survey study leveraging the National Health Interview Survey, including around 80,000 respondents from 2018, 2020, and 2022.
They created two main categories of drinking: drinking any alcohol at all and heavy drinking.
In 2018, 66% of Americans reported drinking any alcohol. That had risen to 69% by 2020, and it stayed at that level even after the lockdown had ended, as you can see here. This may seem like a small increase, but this was a highly significant result. Translating into absolute numbers, it suggests that we have added between 3,328,000 and 10,660,000 net additional drinkers to the population over this time period.

This trend was seen across basically every demographic group, with some notably larger increases among Black and Hispanic individuals, and marginally higher rates among people under age 30.

But far be it from me to deny someone a tot of brandy on a cold winter’s night. More interesting is the rate of heavy alcohol use reported in the study. For context, the definitions of heavy alcohol use appear here. For men, it’s any one day with five or more drinks or 15 or more drinks per week. For women it’s four or more drinks on a given day or eight drinks or more per week.
The overall rate of heavy drinking was about 5.1% in 2018 before the start of the pandemic. That rose to more than 6% in 2020 and it rose a bit more into 2022. The net change here, on a population level, is from 1,430,000 to 3,926,000 new heavy drinkers. That’s a number that rises to the level of an actual public health issue.

Again, this trend was fairly broad across demographic groups. Although in this case, the changes were a bit larger among White people and those in the 40- to 49-year age group. This is my cohort, I guess. Cheers.

The information we have from this study is purely descriptive. It tells us that people are drinking more since the pandemic. It doesn’t tell us why, or the impact that this excess drinking will have on subsequent health outcomes, although other studies would suggest that it will contribute to certain chronic conditions, both physical and mental.
Maybe more important is that it reminds us that habits are sticky. Once we become accustomed to something — that glass of wine or two with dinner, and before bed — it has a tendency to stay with us. There’s an upside to that phenomenon as well, of course; it means that we can train good habits too. And those, once they become ingrained, can be just as hard to break. We just need to be mindful of the habits we pick. New Year 2025 is just around the corner. Start brainstorming those resolutions now.
Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
You’re stuck in your house. Work is closed or you’re working remotely. Your kids’ school is closed or is offering an hour or two a day of Zoom-based instruction. You have a bit of cabin fever which, you suppose, is better than the actual fever that comes with COVID infections, which are running rampant during the height of the pandemic. But still — it’s stressful. What do you do?
We all coped in our own way. We baked sourdough bread. We built that tree house we’d been meaning to build. We started podcasts. And ... we drank. Quite a bit, actually.
During the first year of the pandemic, alcohol sales increased 3%, the largest year-on-year increase in more than 50 years. There was also an increase in drunkenness across the board, though it was most pronounced in those who were already at risk from alcohol use disorder.

Alcohol-associated deaths increased by around 10% from 2019 to 2020. Obviously, this is a small percentage of COVID-associated deaths, but it is nothing to sneeze at.

But look, we were anxious. And say what you will about alcohol as a risk factor for liver disease, heart disease, and cancer — not to mention traffic accidents — it is an anxiolytic, at least in the short term.
But as the pandemic waned, as society reopened, as we got back to work and reintegrated into our social circles and escaped the confines of our houses and apartments, our drinking habits went back to normal, right?
Americans’ love affair with alcohol has been a torrid one, as this graph showing gallons of ethanol consumed per capita over time shows you.

What you see is a steady increase in alcohol consumption from the end of prohibition in 1933 to its peak in the heady days of the early 1980s, followed by a steady decline until the mid-1990s. Since then, there has been another increase with, as you will note, a notable uptick during the early part of the COVID pandemic.
What came across my desk this week was updated data, appearing in a research letter in Annals of Internal Medicine, that compared alcohol consumption in 2020 — the first year of the COVID pandemic — with that in 2022 (the latest available data). And it looks like not much has changed.
This was a population-based survey study leveraging the National Health Interview Survey, including around 80,000 respondents from 2018, 2020, and 2022.
They created two main categories of drinking: drinking any alcohol at all and heavy drinking.
In 2018, 66% of Americans reported drinking any alcohol. That had risen to 69% by 2020, and it stayed at that level even after the lockdown had ended, as you can see here. This may seem like a small increase, but this was a highly significant result. Translating into absolute numbers, it suggests that we have added between 3,328,000 and 10,660,000 net additional drinkers to the population over this time period.

This trend was seen across basically every demographic group, with some notably larger increases among Black and Hispanic individuals, and marginally higher rates among people under age 30.

But far be it from me to deny someone a tot of brandy on a cold winter’s night. More interesting is the rate of heavy alcohol use reported in the study. For context, the definitions of heavy alcohol use appear here. For men, it’s any one day with five or more drinks or 15 or more drinks per week. For women it’s four or more drinks on a given day or eight drinks or more per week.
The overall rate of heavy drinking was about 5.1% in 2018 before the start of the pandemic. That rose to more than 6% in 2020 and it rose a bit more into 2022. The net change here, on a population level, is from 1,430,000 to 3,926,000 new heavy drinkers. That’s a number that rises to the level of an actual public health issue.

Again, this trend was fairly broad across demographic groups. Although in this case, the changes were a bit larger among White people and those in the 40- to 49-year age group. This is my cohort, I guess. Cheers.

The information we have from this study is purely descriptive. It tells us that people are drinking more since the pandemic. It doesn’t tell us why, or the impact that this excess drinking will have on subsequent health outcomes, although other studies would suggest that it will contribute to certain chronic conditions, both physical and mental.
Maybe more important is that it reminds us that habits are sticky. Once we become accustomed to something — that glass of wine or two with dinner, and before bed — it has a tendency to stay with us. There’s an upside to that phenomenon as well, of course; it means that we can train good habits too. And those, once they become ingrained, can be just as hard to break. We just need to be mindful of the habits we pick. New Year 2025 is just around the corner. Start brainstorming those resolutions now.
Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
How to Discuss Lifestyle Modifications in MASLD
Metabolic dysfunction–associated steatotic liver disease (MASLD) is a spectrum of hepatic disorders closely linked to insulin resistance, dyslipidemia, hypertension, and obesity.1 An increasingly prevalent cause of liver disease and liver-related deaths worldwide, MASLD affects at least 38% of the global population.2 The immense burden of MASLD and its complications demands attention and action from the medical community.
Lifestyle modifications involving weight management and dietary composition adjustments are the foundation of addressing MASLD, with a critical emphasis on early intervention.3 Healthy dietary indices and weight loss can lower enzyme levels, reduce hepatic fat content, improve insulin resistance, and overall, reduce the risk of MASLD.3 Given the abundance of literature that exists on the benefits of lifestyle modifications on liver and general health outcomes, clinicians should be prepared to have informed, individualized, and culturally concordant conversations with their patients about these modifications. This Short Clinical Review aims to
Initiate the Conversation
Conversations about lifestyle modifications can be challenging and complex. If patients themselves are not initiating conversations about dietary composition and physical activity, then it is important for clinicians to start a productive discussion.
The use of non-stigmatizing, open-ended questions can begin this process. For example, clinicians can consider asking patients: “How would you describe your lifestyle habits, such as foods you usually eat and your physical activity levels? What do you usually look for when you are grocery shopping or thinking of a meal to cook? Are there ways in which you stay physically active throughout the day or week?”4 (see Table 1).
Such questions can provide significant insight into patients’ activity and eating patterns. They also eliminate the utilization of words such as “diet” or “exercise” that may have associated stigma, pressure, or negative connotations.4
Regardless, some patients may not feel prepared or willing to discuss lifestyle modifications during a visit, especially if it is the first clinical encounter when rapport has yet to even be established.4 Lifestyle modifications are implemented at various paces, and patients have their individual timelines for achieving these adjustments. Building rapport with patients and creating spaces in which they feel safe discussing and incorporating changes to various components of their lives can take time. Patients want to trust their providers while being vulnerable. They want to trust that their providers will guide them in what can sometimes be a life altering journey. It is important for clinicians to acknowledge and respect this reality when caring for patients with MASLD. Dr. Duong often utilizes this phrase, “It may seem like you are about to walk through fire, but we are here to walk with you. Remember, what doesn’t challenge you, doesn’t change you.”
Identify Motivators of Engagement
Identifying patients’ motivators of engagement will allow clinicians to guide patients through not only the introduction, but also the maintenance of such changes. Improvements in dietary composition and physical activity are often recommended by clinicians who are inevitably and understandably concerned about the consequences of MASLD. Liver diseases, specifically cirrhosis and hepatocellular carcinoma, as well as associated metabolic disorders, are consequences that could result from poorly controlled MASLD. Though these consequences should be conveyed to patients, this tactic may not always serve as an impetus for patients to engage in behavioral changes.5
Clinicians can shed light on motivators by utilizing these suggested prompts: “What motivates you to come to our appointments and care for your health? What entails a meaningful life for you — what do or would you enjoy doing? What would make implementing lifestyle changes important to you?” Patient goals may include “being able to keep up with their grandchildren,” “becoming a runner,” or “providing healthy meals for their families.”5,6 Engagement is more likely to be feasible and sustainable when lifestyle modifications are tied to goals that are personally meaningful and relevant to patients.
Within the realm of physical activity specifically, exercise can be individualized to optimize motivation as well. Both aerobic exercise and resistance training are associated independently with benefits such as weight loss and decreased hepatic adipose content.3 Currently, there is no consensus regarding the optimal type of physical activity for patients with MASLD; therefore, clinicians should encourage patients to personalize physical activity.3 While some patients may prefer aerobic activities such as running and swimming, others may find more fulfillment in weightlifting or high intensity interval training. Furthermore, patients with cardiopulmonary or musculoskeletal health contraindications may be limited to specific types of exercise. It is appropriate and helpful for clinicians to ask patients, “What types of physical activity feel achievable and realistic for you at this time?” If physicians can guide patients with MASLD in identifying types of exercise that are safe and enjoyable, their patients may be more motivated to implement such lifestyle changes.
It is also crucial to recognize that lifestyle changes demand active effort from patients. While sustained improvements in body weight and dietary composition are the foundation of MASLD management, they can initially feel cumbersome and abstract to patients. Physicians can help their patients remain motivated by developing small, tangible goals such as “reducing daily caloric intake by 500 kcal” or “participating in three 30-minute fitness classes per week.” These goals should be developed jointly with patients, primarily to ensure that they are tangible, feasible, and productive.
A Culturally Safe Approach
Additionally, acknowledging a patient’s cultural background can be conducive to incorporating patient-specific care into MASLD management. For example, qualitative studies have shown that people from Mexican heritage traditionally complement dinners with soft drinks. While meal portion sizes vary amongst households, families of Mexican origin believe larger portion sizes may be perceived as healthier than Western diets since their cuisine incorporates more vegetables into each dish.7
Eating rituals should also be considered since some families expect the absence of leftovers on the plate.7 Therefore, it is appropriate to consider questions such as, “What are common ingredients in your culture? What are some of your family traditions when it comes to meals?” By integrating cultural considerations, clinicians can adopt a culturally safe approach, empowering patients to make lifestyle modifications tailored toward their unique social identities. Clinicians should avoid generalizations or stereotypes about cultural values regarding lifestyle practices, as these can vary among individuals.
Identify Barriers to Lifestyle Changes and Social Determinants of Health
Even with delicate language from providers and immense motivation from patients, barriers to lifestyle changes persist. Studies have shown that patients with MASLD perceive a lack of self-efficacy and knowledge as major barriers to adopting lifestyle modifications.8,9 Patients have reported challenges in interpreting nutritional data, identifying caloric intake and portion sizes. Physicians can effectively guide patients through lifestyle changes by identifying each patient’s unique knowledge gap and determining the most effective, accessible form of education. For example, some patients may benefit from jointly interpreting a nutritional label with their healthcare providers, while others may require educational materials and interventions provided by a registered dietitian.
Understanding patients’ professional or other commitments can help physicians further individualize recommendations. Questions such as, “Do you have work or other responsibilities that take up some of your time during the day?” minimize presumptive language about employment status. It can reveal whether patients have schedules that make certain lifestyle changes more challenging than others. For example, a patient who is an overnight delivery associate at a warehouse may have a different routine from another patient who is a family member’s caretaker. This framework allows physicians to build rapport with their patients and ultimately, make lifestyle recommendations that are more accessible.
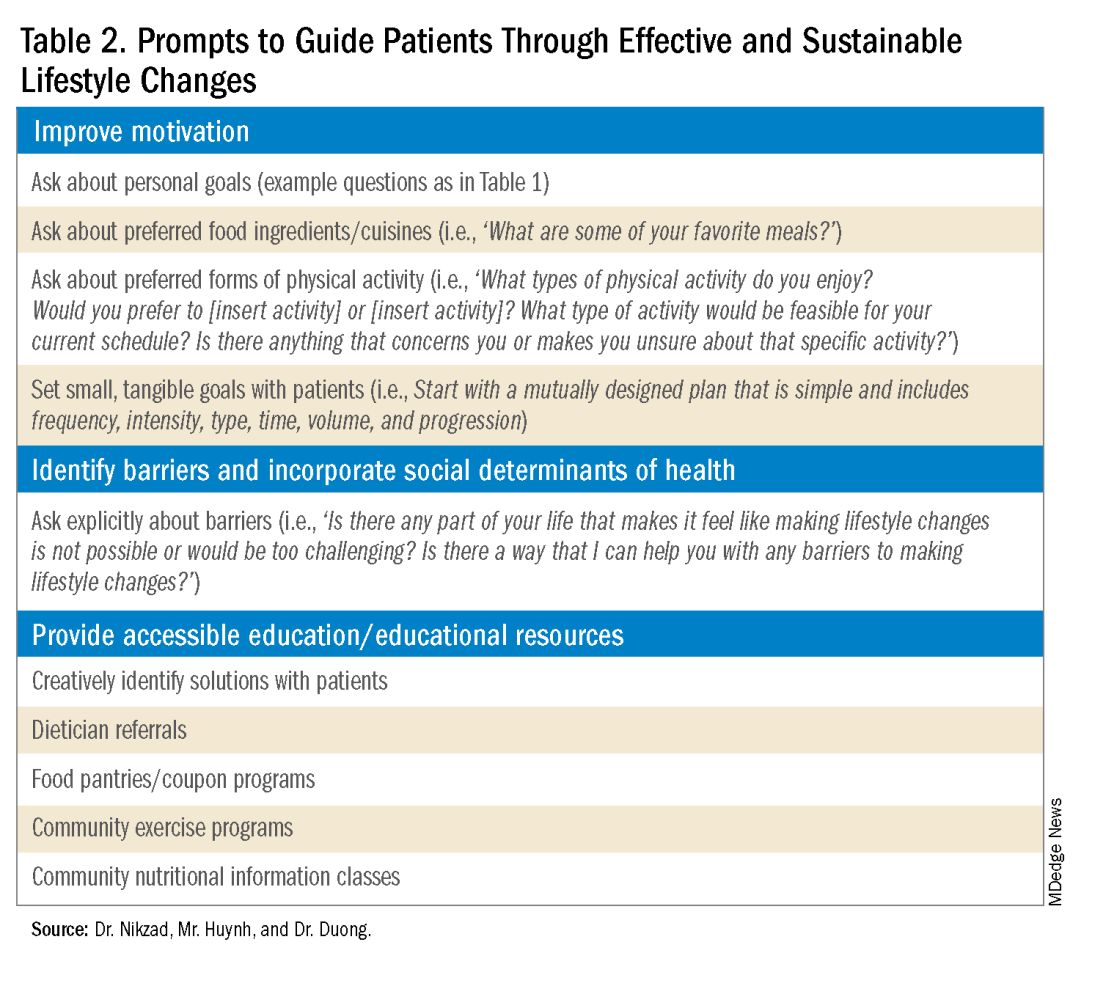
Though MASLD is driven by inflammation and metabolic dysregulation, social determinants of health play an equally important role in disease development and progression.10 As previously discussed, health literacy can deeply influence patients’ abilities to implement lifestyle changes. Furthermore, economic stability, neighborhood and built environment (i.e., access to fresh produce and sidewalks), community, and social support also impact lifestyle modifications. It is paramount to understand the tangible social factors in which patients live. Such factors can be ascertained by beginning the dialogue with “Which grocery stores do you find most convenient? How do you travel to obtain food/attend community exercise programs?” These questions may offer insight into physical barriers to lifestyle changes. Physicians must utilize an intersectional lens that incorporates patients’ unique circumstances of existence into their individualized health care plans to address MASLD.
Summary
- Communication preferences, cultural backgrounds, and sociocultural contexts of patient existence must be considered when treating a patient with MASLD.
- The utilization of an intersectional and culturally safe approach to communication with patients can lead to more sustainable lifestyle changes and improved health outcomes.
- Equipping and empowering physicians to have meaningful discussions about MASLD is crucial to combating a spectrum of diseases that is rapidly affecting a substantial proportion of patients worldwide.
Dr. Nikzad is based in the Department of Internal Medicine at University of Chicago Medicine (@NewshaN27). Mr. Huynh is a medical student at Stony Brook University Renaissance School of Medicine, Stony Brook, N.Y. (@danielhuynhhh). Dr. Duong is an assistant professor of medicine and transplant hepatologist at Stanford University, Palo Alto, Calif. (@doctornikkid). They have no conflicts of interest to declare.
References
1. Mohanty A. MASLD/MASH and Weight Loss. GI & Hepatology News. 2023 Oct. Data Trends 2023:9-13.
2. Wong VW, et al. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023 Sep. doi: 10.1016/j.jhep.2023.04.036.
3. Zeng J, et al. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United European Gastroenterol J. 2024 Mar. doi: 10.1002/ueg2.12525.
4. Berg S. How patients can start—and stick with—key lifestyle changes. AMA Public Health. 2020 Jan.
5. Berg S. 3 ways to get patients engaged in lasting lifestyle change. AMA Diabetes. 2019 Jan.
6. Teixeira PJ, et al. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012 Mar. doi: 10.1186/1479-5868-9-22.
7. Aceves-Martins M, et al. Cultural factors related to childhood and adolescent obesity in Mexico: A systematic review of qualitative studies. Obes Rev. 2022 Sep. doi: 10.1111/obr.13461.
8. Figueroa G, et al. Low health literacy, lack of knowledge, and self-control hinder healthy lifestyles in diverse patients with steatotic liver disease. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08212-9.
9. Wang L, et al. Factors influencing adherence to lifestyle prescriptions among patients with nonalcoholic fatty liver disease: A qualitative study using the health action process approach framework. Front Public Health. 2023 Mar. doi: 10.3389/fpubh.2023.1131827.
10. Andermann A, CLEAR Collaboration. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016 Dec. doi: 10.1503/cmaj.160177.
Metabolic dysfunction–associated steatotic liver disease (MASLD) is a spectrum of hepatic disorders closely linked to insulin resistance, dyslipidemia, hypertension, and obesity.1 An increasingly prevalent cause of liver disease and liver-related deaths worldwide, MASLD affects at least 38% of the global population.2 The immense burden of MASLD and its complications demands attention and action from the medical community.
Lifestyle modifications involving weight management and dietary composition adjustments are the foundation of addressing MASLD, with a critical emphasis on early intervention.3 Healthy dietary indices and weight loss can lower enzyme levels, reduce hepatic fat content, improve insulin resistance, and overall, reduce the risk of MASLD.3 Given the abundance of literature that exists on the benefits of lifestyle modifications on liver and general health outcomes, clinicians should be prepared to have informed, individualized, and culturally concordant conversations with their patients about these modifications. This Short Clinical Review aims to
Initiate the Conversation
Conversations about lifestyle modifications can be challenging and complex. If patients themselves are not initiating conversations about dietary composition and physical activity, then it is important for clinicians to start a productive discussion.
The use of non-stigmatizing, open-ended questions can begin this process. For example, clinicians can consider asking patients: “How would you describe your lifestyle habits, such as foods you usually eat and your physical activity levels? What do you usually look for when you are grocery shopping or thinking of a meal to cook? Are there ways in which you stay physically active throughout the day or week?”4 (see Table 1).

Such questions can provide significant insight into patients’ activity and eating patterns. They also eliminate the utilization of words such as “diet” or “exercise” that may have associated stigma, pressure, or negative connotations.4
Regardless, some patients may not feel prepared or willing to discuss lifestyle modifications during a visit, especially if it is the first clinical encounter when rapport has yet to even be established.4 Lifestyle modifications are implemented at various paces, and patients have their individual timelines for achieving these adjustments. Building rapport with patients and creating spaces in which they feel safe discussing and incorporating changes to various components of their lives can take time. Patients want to trust their providers while being vulnerable. They want to trust that their providers will guide them in what can sometimes be a life altering journey. It is important for clinicians to acknowledge and respect this reality when caring for patients with MASLD. Dr. Duong often utilizes this phrase, “It may seem like you are about to walk through fire, but we are here to walk with you. Remember, what doesn’t challenge you, doesn’t change you.”
Identify Motivators of Engagement
Identifying patients’ motivators of engagement will allow clinicians to guide patients through not only the introduction, but also the maintenance of such changes. Improvements in dietary composition and physical activity are often recommended by clinicians who are inevitably and understandably concerned about the consequences of MASLD. Liver diseases, specifically cirrhosis and hepatocellular carcinoma, as well as associated metabolic disorders, are consequences that could result from poorly controlled MASLD. Though these consequences should be conveyed to patients, this tactic may not always serve as an impetus for patients to engage in behavioral changes.5
Clinicians can shed light on motivators by utilizing these suggested prompts: “What motivates you to come to our appointments and care for your health? What entails a meaningful life for you — what do or would you enjoy doing? What would make implementing lifestyle changes important to you?” Patient goals may include “being able to keep up with their grandchildren,” “becoming a runner,” or “providing healthy meals for their families.”5,6 Engagement is more likely to be feasible and sustainable when lifestyle modifications are tied to goals that are personally meaningful and relevant to patients.
Within the realm of physical activity specifically, exercise can be individualized to optimize motivation as well. Both aerobic exercise and resistance training are associated independently with benefits such as weight loss and decreased hepatic adipose content.3 Currently, there is no consensus regarding the optimal type of physical activity for patients with MASLD; therefore, clinicians should encourage patients to personalize physical activity.3 While some patients may prefer aerobic activities such as running and swimming, others may find more fulfillment in weightlifting or high intensity interval training. Furthermore, patients with cardiopulmonary or musculoskeletal health contraindications may be limited to specific types of exercise. It is appropriate and helpful for clinicians to ask patients, “What types of physical activity feel achievable and realistic for you at this time?” If physicians can guide patients with MASLD in identifying types of exercise that are safe and enjoyable, their patients may be more motivated to implement such lifestyle changes.
It is also crucial to recognize that lifestyle changes demand active effort from patients. While sustained improvements in body weight and dietary composition are the foundation of MASLD management, they can initially feel cumbersome and abstract to patients. Physicians can help their patients remain motivated by developing small, tangible goals such as “reducing daily caloric intake by 500 kcal” or “participating in three 30-minute fitness classes per week.” These goals should be developed jointly with patients, primarily to ensure that they are tangible, feasible, and productive.
A Culturally Safe Approach
Additionally, acknowledging a patient’s cultural background can be conducive to incorporating patient-specific care into MASLD management. For example, qualitative studies have shown that people from Mexican heritage traditionally complement dinners with soft drinks. While meal portion sizes vary amongst households, families of Mexican origin believe larger portion sizes may be perceived as healthier than Western diets since their cuisine incorporates more vegetables into each dish.7
Eating rituals should also be considered since some families expect the absence of leftovers on the plate.7 Therefore, it is appropriate to consider questions such as, “What are common ingredients in your culture? What are some of your family traditions when it comes to meals?” By integrating cultural considerations, clinicians can adopt a culturally safe approach, empowering patients to make lifestyle modifications tailored toward their unique social identities. Clinicians should avoid generalizations or stereotypes about cultural values regarding lifestyle practices, as these can vary among individuals.
Identify Barriers to Lifestyle Changes and Social Determinants of Health
Even with delicate language from providers and immense motivation from patients, barriers to lifestyle changes persist. Studies have shown that patients with MASLD perceive a lack of self-efficacy and knowledge as major barriers to adopting lifestyle modifications.8,9 Patients have reported challenges in interpreting nutritional data, identifying caloric intake and portion sizes. Physicians can effectively guide patients through lifestyle changes by identifying each patient’s unique knowledge gap and determining the most effective, accessible form of education. For example, some patients may benefit from jointly interpreting a nutritional label with their healthcare providers, while others may require educational materials and interventions provided by a registered dietitian.
Understanding patients’ professional or other commitments can help physicians further individualize recommendations. Questions such as, “Do you have work or other responsibilities that take up some of your time during the day?” minimize presumptive language about employment status. It can reveal whether patients have schedules that make certain lifestyle changes more challenging than others. For example, a patient who is an overnight delivery associate at a warehouse may have a different routine from another patient who is a family member’s caretaker. This framework allows physicians to build rapport with their patients and ultimately, make lifestyle recommendations that are more accessible.

Though MASLD is driven by inflammation and metabolic dysregulation, social determinants of health play an equally important role in disease development and progression.10 As previously discussed, health literacy can deeply influence patients’ abilities to implement lifestyle changes. Furthermore, economic stability, neighborhood and built environment (i.e., access to fresh produce and sidewalks), community, and social support also impact lifestyle modifications. It is paramount to understand the tangible social factors in which patients live. Such factors can be ascertained by beginning the dialogue with “Which grocery stores do you find most convenient? How do you travel to obtain food/attend community exercise programs?” These questions may offer insight into physical barriers to lifestyle changes. Physicians must utilize an intersectional lens that incorporates patients’ unique circumstances of existence into their individualized health care plans to address MASLD.
Summary
- Communication preferences, cultural backgrounds, and sociocultural contexts of patient existence must be considered when treating a patient with MASLD.
- The utilization of an intersectional and culturally safe approach to communication with patients can lead to more sustainable lifestyle changes and improved health outcomes.
- Equipping and empowering physicians to have meaningful discussions about MASLD is crucial to combating a spectrum of diseases that is rapidly affecting a substantial proportion of patients worldwide.
Dr. Nikzad is based in the Department of Internal Medicine at University of Chicago Medicine (@NewshaN27). Mr. Huynh is a medical student at Stony Brook University Renaissance School of Medicine, Stony Brook, N.Y. (@danielhuynhhh). Dr. Duong is an assistant professor of medicine and transplant hepatologist at Stanford University, Palo Alto, Calif. (@doctornikkid). They have no conflicts of interest to declare.
References
1. Mohanty A. MASLD/MASH and Weight Loss. GI & Hepatology News. 2023 Oct. Data Trends 2023:9-13.
2. Wong VW, et al. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023 Sep. doi: 10.1016/j.jhep.2023.04.036.
3. Zeng J, et al. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United European Gastroenterol J. 2024 Mar. doi: 10.1002/ueg2.12525.
4. Berg S. How patients can start—and stick with—key lifestyle changes. AMA Public Health. 2020 Jan.
5. Berg S. 3 ways to get patients engaged in lasting lifestyle change. AMA Diabetes. 2019 Jan.
6. Teixeira PJ, et al. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012 Mar. doi: 10.1186/1479-5868-9-22.
7. Aceves-Martins M, et al. Cultural factors related to childhood and adolescent obesity in Mexico: A systematic review of qualitative studies. Obes Rev. 2022 Sep. doi: 10.1111/obr.13461.
8. Figueroa G, et al. Low health literacy, lack of knowledge, and self-control hinder healthy lifestyles in diverse patients with steatotic liver disease. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08212-9.
9. Wang L, et al. Factors influencing adherence to lifestyle prescriptions among patients with nonalcoholic fatty liver disease: A qualitative study using the health action process approach framework. Front Public Health. 2023 Mar. doi: 10.3389/fpubh.2023.1131827.
10. Andermann A, CLEAR Collaboration. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016 Dec. doi: 10.1503/cmaj.160177.
Metabolic dysfunction–associated steatotic liver disease (MASLD) is a spectrum of hepatic disorders closely linked to insulin resistance, dyslipidemia, hypertension, and obesity.1 An increasingly prevalent cause of liver disease and liver-related deaths worldwide, MASLD affects at least 38% of the global population.2 The immense burden of MASLD and its complications demands attention and action from the medical community.
Lifestyle modifications involving weight management and dietary composition adjustments are the foundation of addressing MASLD, with a critical emphasis on early intervention.3 Healthy dietary indices and weight loss can lower enzyme levels, reduce hepatic fat content, improve insulin resistance, and overall, reduce the risk of MASLD.3 Given the abundance of literature that exists on the benefits of lifestyle modifications on liver and general health outcomes, clinicians should be prepared to have informed, individualized, and culturally concordant conversations with their patients about these modifications. This Short Clinical Review aims to
Initiate the Conversation
Conversations about lifestyle modifications can be challenging and complex. If patients themselves are not initiating conversations about dietary composition and physical activity, then it is important for clinicians to start a productive discussion.
The use of non-stigmatizing, open-ended questions can begin this process. For example, clinicians can consider asking patients: “How would you describe your lifestyle habits, such as foods you usually eat and your physical activity levels? What do you usually look for when you are grocery shopping or thinking of a meal to cook? Are there ways in which you stay physically active throughout the day or week?”4 (see Table 1).

Such questions can provide significant insight into patients’ activity and eating patterns. They also eliminate the utilization of words such as “diet” or “exercise” that may have associated stigma, pressure, or negative connotations.4
Regardless, some patients may not feel prepared or willing to discuss lifestyle modifications during a visit, especially if it is the first clinical encounter when rapport has yet to even be established.4 Lifestyle modifications are implemented at various paces, and patients have their individual timelines for achieving these adjustments. Building rapport with patients and creating spaces in which they feel safe discussing and incorporating changes to various components of their lives can take time. Patients want to trust their providers while being vulnerable. They want to trust that their providers will guide them in what can sometimes be a life altering journey. It is important for clinicians to acknowledge and respect this reality when caring for patients with MASLD. Dr. Duong often utilizes this phrase, “It may seem like you are about to walk through fire, but we are here to walk with you. Remember, what doesn’t challenge you, doesn’t change you.”
Identify Motivators of Engagement
Identifying patients’ motivators of engagement will allow clinicians to guide patients through not only the introduction, but also the maintenance of such changes. Improvements in dietary composition and physical activity are often recommended by clinicians who are inevitably and understandably concerned about the consequences of MASLD. Liver diseases, specifically cirrhosis and hepatocellular carcinoma, as well as associated metabolic disorders, are consequences that could result from poorly controlled MASLD. Though these consequences should be conveyed to patients, this tactic may not always serve as an impetus for patients to engage in behavioral changes.5
Clinicians can shed light on motivators by utilizing these suggested prompts: “What motivates you to come to our appointments and care for your health? What entails a meaningful life for you — what do or would you enjoy doing? What would make implementing lifestyle changes important to you?” Patient goals may include “being able to keep up with their grandchildren,” “becoming a runner,” or “providing healthy meals for their families.”5,6 Engagement is more likely to be feasible and sustainable when lifestyle modifications are tied to goals that are personally meaningful and relevant to patients.
Within the realm of physical activity specifically, exercise can be individualized to optimize motivation as well. Both aerobic exercise and resistance training are associated independently with benefits such as weight loss and decreased hepatic adipose content.3 Currently, there is no consensus regarding the optimal type of physical activity for patients with MASLD; therefore, clinicians should encourage patients to personalize physical activity.3 While some patients may prefer aerobic activities such as running and swimming, others may find more fulfillment in weightlifting or high intensity interval training. Furthermore, patients with cardiopulmonary or musculoskeletal health contraindications may be limited to specific types of exercise. It is appropriate and helpful for clinicians to ask patients, “What types of physical activity feel achievable and realistic for you at this time?” If physicians can guide patients with MASLD in identifying types of exercise that are safe and enjoyable, their patients may be more motivated to implement such lifestyle changes.
It is also crucial to recognize that lifestyle changes demand active effort from patients. While sustained improvements in body weight and dietary composition are the foundation of MASLD management, they can initially feel cumbersome and abstract to patients. Physicians can help their patients remain motivated by developing small, tangible goals such as “reducing daily caloric intake by 500 kcal” or “participating in three 30-minute fitness classes per week.” These goals should be developed jointly with patients, primarily to ensure that they are tangible, feasible, and productive.
A Culturally Safe Approach
Additionally, acknowledging a patient’s cultural background can be conducive to incorporating patient-specific care into MASLD management. For example, qualitative studies have shown that people from Mexican heritage traditionally complement dinners with soft drinks. While meal portion sizes vary amongst households, families of Mexican origin believe larger portion sizes may be perceived as healthier than Western diets since their cuisine incorporates more vegetables into each dish.7
Eating rituals should also be considered since some families expect the absence of leftovers on the plate.7 Therefore, it is appropriate to consider questions such as, “What are common ingredients in your culture? What are some of your family traditions when it comes to meals?” By integrating cultural considerations, clinicians can adopt a culturally safe approach, empowering patients to make lifestyle modifications tailored toward their unique social identities. Clinicians should avoid generalizations or stereotypes about cultural values regarding lifestyle practices, as these can vary among individuals.
Identify Barriers to Lifestyle Changes and Social Determinants of Health
Even with delicate language from providers and immense motivation from patients, barriers to lifestyle changes persist. Studies have shown that patients with MASLD perceive a lack of self-efficacy and knowledge as major barriers to adopting lifestyle modifications.8,9 Patients have reported challenges in interpreting nutritional data, identifying caloric intake and portion sizes. Physicians can effectively guide patients through lifestyle changes by identifying each patient’s unique knowledge gap and determining the most effective, accessible form of education. For example, some patients may benefit from jointly interpreting a nutritional label with their healthcare providers, while others may require educational materials and interventions provided by a registered dietitian.
Understanding patients’ professional or other commitments can help physicians further individualize recommendations. Questions such as, “Do you have work or other responsibilities that take up some of your time during the day?” minimize presumptive language about employment status. It can reveal whether patients have schedules that make certain lifestyle changes more challenging than others. For example, a patient who is an overnight delivery associate at a warehouse may have a different routine from another patient who is a family member’s caretaker. This framework allows physicians to build rapport with their patients and ultimately, make lifestyle recommendations that are more accessible.

Though MASLD is driven by inflammation and metabolic dysregulation, social determinants of health play an equally important role in disease development and progression.10 As previously discussed, health literacy can deeply influence patients’ abilities to implement lifestyle changes. Furthermore, economic stability, neighborhood and built environment (i.e., access to fresh produce and sidewalks), community, and social support also impact lifestyle modifications. It is paramount to understand the tangible social factors in which patients live. Such factors can be ascertained by beginning the dialogue with “Which grocery stores do you find most convenient? How do you travel to obtain food/attend community exercise programs?” These questions may offer insight into physical barriers to lifestyle changes. Physicians must utilize an intersectional lens that incorporates patients’ unique circumstances of existence into their individualized health care plans to address MASLD.
Summary
- Communication preferences, cultural backgrounds, and sociocultural contexts of patient existence must be considered when treating a patient with MASLD.
- The utilization of an intersectional and culturally safe approach to communication with patients can lead to more sustainable lifestyle changes and improved health outcomes.
- Equipping and empowering physicians to have meaningful discussions about MASLD is crucial to combating a spectrum of diseases that is rapidly affecting a substantial proportion of patients worldwide.
Dr. Nikzad is based in the Department of Internal Medicine at University of Chicago Medicine (@NewshaN27). Mr. Huynh is a medical student at Stony Brook University Renaissance School of Medicine, Stony Brook, N.Y. (@danielhuynhhh). Dr. Duong is an assistant professor of medicine and transplant hepatologist at Stanford University, Palo Alto, Calif. (@doctornikkid). They have no conflicts of interest to declare.
References
1. Mohanty A. MASLD/MASH and Weight Loss. GI & Hepatology News. 2023 Oct. Data Trends 2023:9-13.
2. Wong VW, et al. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023 Sep. doi: 10.1016/j.jhep.2023.04.036.
3. Zeng J, et al. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United European Gastroenterol J. 2024 Mar. doi: 10.1002/ueg2.12525.
4. Berg S. How patients can start—and stick with—key lifestyle changes. AMA Public Health. 2020 Jan.
5. Berg S. 3 ways to get patients engaged in lasting lifestyle change. AMA Diabetes. 2019 Jan.
6. Teixeira PJ, et al. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012 Mar. doi: 10.1186/1479-5868-9-22.
7. Aceves-Martins M, et al. Cultural factors related to childhood and adolescent obesity in Mexico: A systematic review of qualitative studies. Obes Rev. 2022 Sep. doi: 10.1111/obr.13461.
8. Figueroa G, et al. Low health literacy, lack of knowledge, and self-control hinder healthy lifestyles in diverse patients with steatotic liver disease. Dig Dis Sci. 2024 Feb. doi: 10.1007/s10620-023-08212-9.
9. Wang L, et al. Factors influencing adherence to lifestyle prescriptions among patients with nonalcoholic fatty liver disease: A qualitative study using the health action process approach framework. Front Public Health. 2023 Mar. doi: 10.3389/fpubh.2023.1131827.
10. Andermann A, CLEAR Collaboration. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016 Dec. doi: 10.1503/cmaj.160177.





