User login
On the Murder of UnitedHealthcare’s CEO
On December 4, UnitedHealthcare CEO Brian Thompson was assassinated in New York City outside of a hotel. As of the time of this writing, the shooter is still at large.
I suppose I could write about how this shows that Americans are fed up with the way modern commercial healthcare companies operate. Who gets care and who doesn’t.
I could write about how industry trends of “Delay, Deny, Defend” lead to the suffering of millions of people who need healthcare that they thought they were paying for.
I could write about the callousness of the way people online are celebrating the cold-blooded murder of a married man with two children.
I might write about how insurance companies intentionally, and routinely, drag out (or deny) reimbursements for physicians (including small solo practice ones, like myself) who are legitimately caring for their patients.
I suppose I could write something about how gun violence is so pervasive in our society that it scarcely merits a second glance at the news story. If the headline just said, “Unknown Assailant Kills Man Outside Hotel,” would you have even read beyond that?
I could write about how the lack of regulations, and accelerating attempts to scrap them, can lead to insider trading.
I could write about how having insurance companies and medical facilities more beholden to shareholders than to patients is a serious conflict of interest.
I could try to make points about how the widespread availability of firearms (in this case one with a built-in silencer) in America means that anyone with a vendetta, or serious mental illness, or just a short temper, can get one — and use it.
I could talk about how “greed is good” in healthcare settings rewards a few and hurts many — no matter how much the PR spinners try to make it sound like it’s a great win-win situation all-around.
I could argue that the jubilant “good riddance” and “eat the rich” responses of many — both medical and nonmedical people — to the killing shows that, as a society, we’re losing the qualities that make us human.
I could also argue that putting financial gain for executive bonuses and stockholder dividends ahead of the health and well-being of others shows that, as a society, we’re losing the qualities that make us human.
I could make a point that , provided the target is someone they have a difference of opinion with. Which is, honestly, pretty damn scary.
I could talk about how policies of arbitrarily changing the rules about anesthesia coverage, or letting a computer decide how long a hospital stay should be, or to deny rehabilitation care, are unethical, unjust, and just plain wrong.
I could write about a lot of things based on what happened outside that New York Hilton Midtown in early December.
But as I stare at my screen, I’m well aware that no matter what I write it won’t change any opinions, solve anything, or even lead to people trying to find a solution.
Because that’s just the world we live in.
Block has a solo neurology practice in Scottsdale, Arizona.
On December 4, UnitedHealthcare CEO Brian Thompson was assassinated in New York City outside of a hotel. As of the time of this writing, the shooter is still at large.
I suppose I could write about how this shows that Americans are fed up with the way modern commercial healthcare companies operate. Who gets care and who doesn’t.
I could write about how industry trends of “Delay, Deny, Defend” lead to the suffering of millions of people who need healthcare that they thought they were paying for.
I could write about the callousness of the way people online are celebrating the cold-blooded murder of a married man with two children.
I might write about how insurance companies intentionally, and routinely, drag out (or deny) reimbursements for physicians (including small solo practice ones, like myself) who are legitimately caring for their patients.
I suppose I could write something about how gun violence is so pervasive in our society that it scarcely merits a second glance at the news story. If the headline just said, “Unknown Assailant Kills Man Outside Hotel,” would you have even read beyond that?
I could write about how the lack of regulations, and accelerating attempts to scrap them, can lead to insider trading.
I could write about how having insurance companies and medical facilities more beholden to shareholders than to patients is a serious conflict of interest.
I could try to make points about how the widespread availability of firearms (in this case one with a built-in silencer) in America means that anyone with a vendetta, or serious mental illness, or just a short temper, can get one — and use it.
I could talk about how “greed is good” in healthcare settings rewards a few and hurts many — no matter how much the PR spinners try to make it sound like it’s a great win-win situation all-around.
I could argue that the jubilant “good riddance” and “eat the rich” responses of many — both medical and nonmedical people — to the killing shows that, as a society, we’re losing the qualities that make us human.
I could also argue that putting financial gain for executive bonuses and stockholder dividends ahead of the health and well-being of others shows that, as a society, we’re losing the qualities that make us human.
I could make a point that , provided the target is someone they have a difference of opinion with. Which is, honestly, pretty damn scary.
I could talk about how policies of arbitrarily changing the rules about anesthesia coverage, or letting a computer decide how long a hospital stay should be, or to deny rehabilitation care, are unethical, unjust, and just plain wrong.
I could write about a lot of things based on what happened outside that New York Hilton Midtown in early December.
But as I stare at my screen, I’m well aware that no matter what I write it won’t change any opinions, solve anything, or even lead to people trying to find a solution.
Because that’s just the world we live in.
Block has a solo neurology practice in Scottsdale, Arizona.
On December 4, UnitedHealthcare CEO Brian Thompson was assassinated in New York City outside of a hotel. As of the time of this writing, the shooter is still at large.
I suppose I could write about how this shows that Americans are fed up with the way modern commercial healthcare companies operate. Who gets care and who doesn’t.
I could write about how industry trends of “Delay, Deny, Defend” lead to the suffering of millions of people who need healthcare that they thought they were paying for.
I could write about the callousness of the way people online are celebrating the cold-blooded murder of a married man with two children.
I might write about how insurance companies intentionally, and routinely, drag out (or deny) reimbursements for physicians (including small solo practice ones, like myself) who are legitimately caring for their patients.
I suppose I could write something about how gun violence is so pervasive in our society that it scarcely merits a second glance at the news story. If the headline just said, “Unknown Assailant Kills Man Outside Hotel,” would you have even read beyond that?
I could write about how the lack of regulations, and accelerating attempts to scrap them, can lead to insider trading.
I could write about how having insurance companies and medical facilities more beholden to shareholders than to patients is a serious conflict of interest.
I could try to make points about how the widespread availability of firearms (in this case one with a built-in silencer) in America means that anyone with a vendetta, or serious mental illness, or just a short temper, can get one — and use it.
I could talk about how “greed is good” in healthcare settings rewards a few and hurts many — no matter how much the PR spinners try to make it sound like it’s a great win-win situation all-around.
I could argue that the jubilant “good riddance” and “eat the rich” responses of many — both medical and nonmedical people — to the killing shows that, as a society, we’re losing the qualities that make us human.
I could also argue that putting financial gain for executive bonuses and stockholder dividends ahead of the health and well-being of others shows that, as a society, we’re losing the qualities that make us human.
I could make a point that , provided the target is someone they have a difference of opinion with. Which is, honestly, pretty damn scary.
I could talk about how policies of arbitrarily changing the rules about anesthesia coverage, or letting a computer decide how long a hospital stay should be, or to deny rehabilitation care, are unethical, unjust, and just plain wrong.
I could write about a lot of things based on what happened outside that New York Hilton Midtown in early December.
But as I stare at my screen, I’m well aware that no matter what I write it won’t change any opinions, solve anything, or even lead to people trying to find a solution.
Because that’s just the world we live in.
Block has a solo neurology practice in Scottsdale, Arizona.
Can We Fight Social Media’s Promotion of Junk Food?
Of those three truths, the one that tends to get more public health attention is advertising. More specifically, advertising junk food to kids.
Back in the days when cable television was king of all free time, study after study tried to quantify junk- and fast-food advertising to kids and speculated about its impact on childhood obesity rates. But as broadcast television use began fading, advertisers — and, of course, studies about advertising — turned their attention first to gaming and now to social media.
The social media numbers are quite staggering. According to a study published — probably not coincidentally — on Halloween, looking at the 40 top brands of junk- and fast food sold in Canada, those 40 brands alone were mentioned over 16 million times by social media users, reaching an estimated 42 billion total users within a 1-year period.
And unique to the challenge of junk- and fast-food advertising on social media is that it also includes “earned” advertising, the kind not paid for by manufacturers but rather the kind where friends, family, and influencers post about junk food. Occasionally, though, these lines are blurred by initiatives from fast-food manufacturers explicitly encouraging social sharing. Consequently, even were there a desire, there isn’t likely to be a regulatory mechanism to markedly reduce it.
For years, here in North America, excepting Quebec, the desire has been mainly to just talk about how concerned we are about junk-food advertising to kids. Elsewhere, however, some countries tried to do more, including both Mexico and Chile, which put kid-targeted TV food advertising bans in place in 2014 and 2016, respectively.
Did they work? It depends on what outcome you’re considering. If the question is, did they work in regard to obesity? — which is how everyone tends to frame the question — by themselves, probably not. No one sandbag stops a flood, and though junk-food advertising is certainly a sandbag, we’re still facing a torrential downpour of obesity contributors. No doubt they did work to reduce kids’ exposure to junk-food advertising on television, but what remains to be seen is whether there is a means to now tackle social media’s generous servings of the same. Moreover, the obesity lens is the wrong one. Ultraprocessed food consumption isn’t good for anyone, regardless of weight, and its reduced marketing and consumption is a worthy goal of its own.
But Chile and Mexico are filling more than single sandbags, as both countries have rolled out a suite of interventions they are hoping will help improve nutrition: from front-of–package labeling reforms and warnings, to the banning of advertising geared specifically to appeal to children (like sugary cereal cartoon mascots), to implementing sugar-sweetened-beverage taxes, to having blanket overall bans on food advertising during the daytime.
Mexico is even taking first steps to start addressing junk food’s ubiquity by banning its sale in schools altogether. Schools found to be selling common Mexican junk food fare, such as sugary fruit drinks; chips; artificial pork rinds; and soy-encased, salty peanuts with chili, will see their administrators facing heavy fines.
Because therein lies the biggest rub. Going back to those three simple truths, junk food is hyperpalatable and consequently tends to be what we crave when we’re hungry. So even if we miraculously one day do more than just talk about advertising reforms, and especially given that we won’t be able to do anything about social media’s earned product placements, junk food’s ubiquitous availability within arms’ reach or on our Uber Eats apps will see us be likely to continue its excessive consumption.
That’s not to say we shouldn’t emulate Mexico and Chile’s initiatives, nor that they shouldn’t continue to build upon them, but one thing is certain: Human nature and inconvenient truths around food are incredibly powerful forces that we haven’t yet figured out how to tame.
Dr. Freedhoff, Associate Professor, Department of Family Medicine, University of Ottawa; Medical Director, Bariatric Medical Institute, Ottawa, Ontario, Canada, has disclosed relevant financial relationships with Bariatric Medical Institute, Constant Health, Novo Nordisk, and Weighty Matters.
A version of this article appeared on Medscape.com.
Of those three truths, the one that tends to get more public health attention is advertising. More specifically, advertising junk food to kids.
Back in the days when cable television was king of all free time, study after study tried to quantify junk- and fast-food advertising to kids and speculated about its impact on childhood obesity rates. But as broadcast television use began fading, advertisers — and, of course, studies about advertising — turned their attention first to gaming and now to social media.
The social media numbers are quite staggering. According to a study published — probably not coincidentally — on Halloween, looking at the 40 top brands of junk- and fast food sold in Canada, those 40 brands alone were mentioned over 16 million times by social media users, reaching an estimated 42 billion total users within a 1-year period.
And unique to the challenge of junk- and fast-food advertising on social media is that it also includes “earned” advertising, the kind not paid for by manufacturers but rather the kind where friends, family, and influencers post about junk food. Occasionally, though, these lines are blurred by initiatives from fast-food manufacturers explicitly encouraging social sharing. Consequently, even were there a desire, there isn’t likely to be a regulatory mechanism to markedly reduce it.
For years, here in North America, excepting Quebec, the desire has been mainly to just talk about how concerned we are about junk-food advertising to kids. Elsewhere, however, some countries tried to do more, including both Mexico and Chile, which put kid-targeted TV food advertising bans in place in 2014 and 2016, respectively.
Did they work? It depends on what outcome you’re considering. If the question is, did they work in regard to obesity? — which is how everyone tends to frame the question — by themselves, probably not. No one sandbag stops a flood, and though junk-food advertising is certainly a sandbag, we’re still facing a torrential downpour of obesity contributors. No doubt they did work to reduce kids’ exposure to junk-food advertising on television, but what remains to be seen is whether there is a means to now tackle social media’s generous servings of the same. Moreover, the obesity lens is the wrong one. Ultraprocessed food consumption isn’t good for anyone, regardless of weight, and its reduced marketing and consumption is a worthy goal of its own.
But Chile and Mexico are filling more than single sandbags, as both countries have rolled out a suite of interventions they are hoping will help improve nutrition: from front-of–package labeling reforms and warnings, to the banning of advertising geared specifically to appeal to children (like sugary cereal cartoon mascots), to implementing sugar-sweetened-beverage taxes, to having blanket overall bans on food advertising during the daytime.
Mexico is even taking first steps to start addressing junk food’s ubiquity by banning its sale in schools altogether. Schools found to be selling common Mexican junk food fare, such as sugary fruit drinks; chips; artificial pork rinds; and soy-encased, salty peanuts with chili, will see their administrators facing heavy fines.
Because therein lies the biggest rub. Going back to those three simple truths, junk food is hyperpalatable and consequently tends to be what we crave when we’re hungry. So even if we miraculously one day do more than just talk about advertising reforms, and especially given that we won’t be able to do anything about social media’s earned product placements, junk food’s ubiquitous availability within arms’ reach or on our Uber Eats apps will see us be likely to continue its excessive consumption.
That’s not to say we shouldn’t emulate Mexico and Chile’s initiatives, nor that they shouldn’t continue to build upon them, but one thing is certain: Human nature and inconvenient truths around food are incredibly powerful forces that we haven’t yet figured out how to tame.
Dr. Freedhoff, Associate Professor, Department of Family Medicine, University of Ottawa; Medical Director, Bariatric Medical Institute, Ottawa, Ontario, Canada, has disclosed relevant financial relationships with Bariatric Medical Institute, Constant Health, Novo Nordisk, and Weighty Matters.
A version of this article appeared on Medscape.com.
Of those three truths, the one that tends to get more public health attention is advertising. More specifically, advertising junk food to kids.
Back in the days when cable television was king of all free time, study after study tried to quantify junk- and fast-food advertising to kids and speculated about its impact on childhood obesity rates. But as broadcast television use began fading, advertisers — and, of course, studies about advertising — turned their attention first to gaming and now to social media.
The social media numbers are quite staggering. According to a study published — probably not coincidentally — on Halloween, looking at the 40 top brands of junk- and fast food sold in Canada, those 40 brands alone were mentioned over 16 million times by social media users, reaching an estimated 42 billion total users within a 1-year period.
And unique to the challenge of junk- and fast-food advertising on social media is that it also includes “earned” advertising, the kind not paid for by manufacturers but rather the kind where friends, family, and influencers post about junk food. Occasionally, though, these lines are blurred by initiatives from fast-food manufacturers explicitly encouraging social sharing. Consequently, even were there a desire, there isn’t likely to be a regulatory mechanism to markedly reduce it.
For years, here in North America, excepting Quebec, the desire has been mainly to just talk about how concerned we are about junk-food advertising to kids. Elsewhere, however, some countries tried to do more, including both Mexico and Chile, which put kid-targeted TV food advertising bans in place in 2014 and 2016, respectively.
Did they work? It depends on what outcome you’re considering. If the question is, did they work in regard to obesity? — which is how everyone tends to frame the question — by themselves, probably not. No one sandbag stops a flood, and though junk-food advertising is certainly a sandbag, we’re still facing a torrential downpour of obesity contributors. No doubt they did work to reduce kids’ exposure to junk-food advertising on television, but what remains to be seen is whether there is a means to now tackle social media’s generous servings of the same. Moreover, the obesity lens is the wrong one. Ultraprocessed food consumption isn’t good for anyone, regardless of weight, and its reduced marketing and consumption is a worthy goal of its own.
But Chile and Mexico are filling more than single sandbags, as both countries have rolled out a suite of interventions they are hoping will help improve nutrition: from front-of–package labeling reforms and warnings, to the banning of advertising geared specifically to appeal to children (like sugary cereal cartoon mascots), to implementing sugar-sweetened-beverage taxes, to having blanket overall bans on food advertising during the daytime.
Mexico is even taking first steps to start addressing junk food’s ubiquity by banning its sale in schools altogether. Schools found to be selling common Mexican junk food fare, such as sugary fruit drinks; chips; artificial pork rinds; and soy-encased, salty peanuts with chili, will see their administrators facing heavy fines.
Because therein lies the biggest rub. Going back to those three simple truths, junk food is hyperpalatable and consequently tends to be what we crave when we’re hungry. So even if we miraculously one day do more than just talk about advertising reforms, and especially given that we won’t be able to do anything about social media’s earned product placements, junk food’s ubiquitous availability within arms’ reach or on our Uber Eats apps will see us be likely to continue its excessive consumption.
That’s not to say we shouldn’t emulate Mexico and Chile’s initiatives, nor that they shouldn’t continue to build upon them, but one thing is certain: Human nature and inconvenient truths around food are incredibly powerful forces that we haven’t yet figured out how to tame.
Dr. Freedhoff, Associate Professor, Department of Family Medicine, University of Ottawa; Medical Director, Bariatric Medical Institute, Ottawa, Ontario, Canada, has disclosed relevant financial relationships with Bariatric Medical Institute, Constant Health, Novo Nordisk, and Weighty Matters.
A version of this article appeared on Medscape.com.
REALIZE-K: A New Potassium Binder to Help Keep Spiro on Board
This transcript has been edited for clarity.
We have talked often in the past about potassium. Why is potassium so important in heart failure? It’s because many doctors are afraid to give some of the drugs that will raise the potassium, because then you need to deal with it —and everybody is afraid of hyperkalemia causing arrhythmias.
Calm those nerves. Just remember that arrhythmias only occur when the potassium suddenly goes up. This chronic hyperkalemia, which occurs with many of our drugs, usually — I can’t say every time — does not result in arrhythmias.
Patiromer and Zirconium Cyclosilicate
Now, we’ve got potassium binders. You’ve heard me talk about the potassium binders in several of my other chats with you, and they work. We have primarily two of them. The first one that came out was patiromer, and now I’m going to talk to you a little bit about zirconium cyclosilicate, which uses sodium as its exchange ion. Whenever you take out one ion, you have to put another one in, and in this case it’s sodium. Maybe if you use it in the higher doses, you can give the patient more edema or you can make the patient congested with more fluid.
Years ago we did the DIAMOND study; it was a patiromer study, but in essence we found that you could continue to give the drug, particularly the mineralocorticoid receptor antagonists (MRAs) such as spironolactone or eplerenone, as long as you have the patiromer as your safety net, and that the drugs were well tolerated and the adverse events were significantly less.
The REALIZE-K Trial
Now, let’s talk about the REALIZE-K trial. The researchers wanted to prove basically the same thing: that the patients could be started or kept on their spironolactone as long as you had that backup of the zirconium cyclosilicate binder.
They picked patients who had HFrEF — so, low ejection fractions, defined as less than 40% — and they were already on guideline-directed medical therapy, but not an MRA. They divided up the patients right from the beginning between those who were already hyperkalemic — in other words, they had potassiums of 5.1-5.9 mEq/L, which is when doctors start getting worried. GFRs had to be better than 30 mL/min per 1.73 m2, and if the potassium was not yet okay, they were given the zirconium cyclosilicate to normalize the potassium and then they entered the study.
The second group had some history of or were at risk for hyperkalemia. Maybe their GFRs were lower, but their potassiums were somewhere between 3.5 and 5 mEq/L.
They started with about 366 patients. These trials have not been huge, certainly not what we normally see in heart failure trials. About 95 patients had hyperkalemia initially and 271 patients were normokalemic.
Then they were randomized; about 102 patients went on the potassium binder and the other group went on the placebo. They continued the study and they continued to check whether the patient had to come off the drug or had to reduce or remove the spironolactone.
These were older patients, mostly in their early seventies. This was an international trial. There were not that many patients from North America, but they had quite a few patients from Europe and some patients from Latin America. There were many with diabetes, atrial fibrillation, and all the usual comorbidities that we typically see.
The proportions of patients classified as New York Heart Association Class III and IV were about 16% to 17% and the rest were Class II, so this is really the ambulatory population. NT-proBNP levels were elevated, at approximately 1000-1200 pg/mL, and the GFRs were either in the high 40s or about 60 mL/min per 1.73 m2. The patients were pretty well medicated, including with RAAS inhibition, beta-blockers, and even SGLT2 inhibitors.
This is a very typical population and they wanted to see what happened. Did the patients remain on the binder and were they able to tolerate the spironolactone? In fact, that was the case.
At the end of the study, more patients had been able to stay on their spironolactone, which is that one drug that we’re not doing so well on when you look at large databases. If they were on the zirconium drug, they were more likely to stay on the spironolactone. They even did a sensitivity analysis, which really showed that it was consistent across the board.
Edema and Hyperkalemia
Now we have two binders that have shown to us that patients can stay on their drugs. There were some interesting findings here, though.
There was more edema — again, everything is based on small numbers — and there seemed to be more heart failure events in the group that received the zirconium cyclosilicate. The first episode of hyperkalemia was delayed or didn’t happen at all. Again, the hyperkalemia was controlled.
What does that tell you? Well, the exchange is sodium. There had been reports before that if you gave this binder at the higher doses, you would have more retention of sodium. I think we see that in this trial, even though the numbers are very small.
According to the investigators, these were issues that could be resolved through an increase in diuretics or having the patient remember to be careful with their sodium intake so they don’t retain more fluid.
My message to you is to use these binders, whichever one of the two you want or whichever your hospital has available for you on their formulary, because it may give you that sense of comfort and self-efficacy so that you can actually start your patients on an MRA and keep them on it.
The MRAs are lifesaving drugs and the patients with HFrEF need to be on them. This is a way to do it without having to sacrifice your true guideline-directed medical therapy.
Dr. Piña, Professor of Medicine/Cardiology/Heart Failure/Transplant; Quality Officer, Cardiovascular Line, Sidney Kimmel College of Medicine, Thomas Jefferson University, Philadelphia, Pennsylvania; Clinical Professor of Medicine, Central Michigan University College of Medicine, Mount Pleasant, Michigan; Adjunct Professor of Epidemiology and Biostatistics, Population & Quantitative Health Sciences, Case Western University, Cleveland, Ohio, disclosed ties with the Food and Drug Administration’s Center for Devices and Radiological Health.
A version of this article appeared on Medscape.com
This transcript has been edited for clarity.
We have talked often in the past about potassium. Why is potassium so important in heart failure? It’s because many doctors are afraid to give some of the drugs that will raise the potassium, because then you need to deal with it —and everybody is afraid of hyperkalemia causing arrhythmias.
Calm those nerves. Just remember that arrhythmias only occur when the potassium suddenly goes up. This chronic hyperkalemia, which occurs with many of our drugs, usually — I can’t say every time — does not result in arrhythmias.
Patiromer and Zirconium Cyclosilicate
Now, we’ve got potassium binders. You’ve heard me talk about the potassium binders in several of my other chats with you, and they work. We have primarily two of them. The first one that came out was patiromer, and now I’m going to talk to you a little bit about zirconium cyclosilicate, which uses sodium as its exchange ion. Whenever you take out one ion, you have to put another one in, and in this case it’s sodium. Maybe if you use it in the higher doses, you can give the patient more edema or you can make the patient congested with more fluid.
Years ago we did the DIAMOND study; it was a patiromer study, but in essence we found that you could continue to give the drug, particularly the mineralocorticoid receptor antagonists (MRAs) such as spironolactone or eplerenone, as long as you have the patiromer as your safety net, and that the drugs were well tolerated and the adverse events were significantly less.
The REALIZE-K Trial
Now, let’s talk about the REALIZE-K trial. The researchers wanted to prove basically the same thing: that the patients could be started or kept on their spironolactone as long as you had that backup of the zirconium cyclosilicate binder.
They picked patients who had HFrEF — so, low ejection fractions, defined as less than 40% — and they were already on guideline-directed medical therapy, but not an MRA. They divided up the patients right from the beginning between those who were already hyperkalemic — in other words, they had potassiums of 5.1-5.9 mEq/L, which is when doctors start getting worried. GFRs had to be better than 30 mL/min per 1.73 m2, and if the potassium was not yet okay, they were given the zirconium cyclosilicate to normalize the potassium and then they entered the study.
The second group had some history of or were at risk for hyperkalemia. Maybe their GFRs were lower, but their potassiums were somewhere between 3.5 and 5 mEq/L.
They started with about 366 patients. These trials have not been huge, certainly not what we normally see in heart failure trials. About 95 patients had hyperkalemia initially and 271 patients were normokalemic.
Then they were randomized; about 102 patients went on the potassium binder and the other group went on the placebo. They continued the study and they continued to check whether the patient had to come off the drug or had to reduce or remove the spironolactone.
These were older patients, mostly in their early seventies. This was an international trial. There were not that many patients from North America, but they had quite a few patients from Europe and some patients from Latin America. There were many with diabetes, atrial fibrillation, and all the usual comorbidities that we typically see.
The proportions of patients classified as New York Heart Association Class III and IV were about 16% to 17% and the rest were Class II, so this is really the ambulatory population. NT-proBNP levels were elevated, at approximately 1000-1200 pg/mL, and the GFRs were either in the high 40s or about 60 mL/min per 1.73 m2. The patients were pretty well medicated, including with RAAS inhibition, beta-blockers, and even SGLT2 inhibitors.
This is a very typical population and they wanted to see what happened. Did the patients remain on the binder and were they able to tolerate the spironolactone? In fact, that was the case.
At the end of the study, more patients had been able to stay on their spironolactone, which is that one drug that we’re not doing so well on when you look at large databases. If they were on the zirconium drug, they were more likely to stay on the spironolactone. They even did a sensitivity analysis, which really showed that it was consistent across the board.
Edema and Hyperkalemia
Now we have two binders that have shown to us that patients can stay on their drugs. There were some interesting findings here, though.
There was more edema — again, everything is based on small numbers — and there seemed to be more heart failure events in the group that received the zirconium cyclosilicate. The first episode of hyperkalemia was delayed or didn’t happen at all. Again, the hyperkalemia was controlled.
What does that tell you? Well, the exchange is sodium. There had been reports before that if you gave this binder at the higher doses, you would have more retention of sodium. I think we see that in this trial, even though the numbers are very small.
According to the investigators, these were issues that could be resolved through an increase in diuretics or having the patient remember to be careful with their sodium intake so they don’t retain more fluid.
My message to you is to use these binders, whichever one of the two you want or whichever your hospital has available for you on their formulary, because it may give you that sense of comfort and self-efficacy so that you can actually start your patients on an MRA and keep them on it.
The MRAs are lifesaving drugs and the patients with HFrEF need to be on them. This is a way to do it without having to sacrifice your true guideline-directed medical therapy.
Dr. Piña, Professor of Medicine/Cardiology/Heart Failure/Transplant; Quality Officer, Cardiovascular Line, Sidney Kimmel College of Medicine, Thomas Jefferson University, Philadelphia, Pennsylvania; Clinical Professor of Medicine, Central Michigan University College of Medicine, Mount Pleasant, Michigan; Adjunct Professor of Epidemiology and Biostatistics, Population & Quantitative Health Sciences, Case Western University, Cleveland, Ohio, disclosed ties with the Food and Drug Administration’s Center for Devices and Radiological Health.
A version of this article appeared on Medscape.com
This transcript has been edited for clarity.
We have talked often in the past about potassium. Why is potassium so important in heart failure? It’s because many doctors are afraid to give some of the drugs that will raise the potassium, because then you need to deal with it —and everybody is afraid of hyperkalemia causing arrhythmias.
Calm those nerves. Just remember that arrhythmias only occur when the potassium suddenly goes up. This chronic hyperkalemia, which occurs with many of our drugs, usually — I can’t say every time — does not result in arrhythmias.
Patiromer and Zirconium Cyclosilicate
Now, we’ve got potassium binders. You’ve heard me talk about the potassium binders in several of my other chats with you, and they work. We have primarily two of them. The first one that came out was patiromer, and now I’m going to talk to you a little bit about zirconium cyclosilicate, which uses sodium as its exchange ion. Whenever you take out one ion, you have to put another one in, and in this case it’s sodium. Maybe if you use it in the higher doses, you can give the patient more edema or you can make the patient congested with more fluid.
Years ago we did the DIAMOND study; it was a patiromer study, but in essence we found that you could continue to give the drug, particularly the mineralocorticoid receptor antagonists (MRAs) such as spironolactone or eplerenone, as long as you have the patiromer as your safety net, and that the drugs were well tolerated and the adverse events were significantly less.
The REALIZE-K Trial
Now, let’s talk about the REALIZE-K trial. The researchers wanted to prove basically the same thing: that the patients could be started or kept on their spironolactone as long as you had that backup of the zirconium cyclosilicate binder.
They picked patients who had HFrEF — so, low ejection fractions, defined as less than 40% — and they were already on guideline-directed medical therapy, but not an MRA. They divided up the patients right from the beginning between those who were already hyperkalemic — in other words, they had potassiums of 5.1-5.9 mEq/L, which is when doctors start getting worried. GFRs had to be better than 30 mL/min per 1.73 m2, and if the potassium was not yet okay, they were given the zirconium cyclosilicate to normalize the potassium and then they entered the study.
The second group had some history of or were at risk for hyperkalemia. Maybe their GFRs were lower, but their potassiums were somewhere between 3.5 and 5 mEq/L.
They started with about 366 patients. These trials have not been huge, certainly not what we normally see in heart failure trials. About 95 patients had hyperkalemia initially and 271 patients were normokalemic.
Then they were randomized; about 102 patients went on the potassium binder and the other group went on the placebo. They continued the study and they continued to check whether the patient had to come off the drug or had to reduce or remove the spironolactone.
These were older patients, mostly in their early seventies. This was an international trial. There were not that many patients from North America, but they had quite a few patients from Europe and some patients from Latin America. There were many with diabetes, atrial fibrillation, and all the usual comorbidities that we typically see.
The proportions of patients classified as New York Heart Association Class III and IV were about 16% to 17% and the rest were Class II, so this is really the ambulatory population. NT-proBNP levels were elevated, at approximately 1000-1200 pg/mL, and the GFRs were either in the high 40s or about 60 mL/min per 1.73 m2. The patients were pretty well medicated, including with RAAS inhibition, beta-blockers, and even SGLT2 inhibitors.
This is a very typical population and they wanted to see what happened. Did the patients remain on the binder and were they able to tolerate the spironolactone? In fact, that was the case.
At the end of the study, more patients had been able to stay on their spironolactone, which is that one drug that we’re not doing so well on when you look at large databases. If they were on the zirconium drug, they were more likely to stay on the spironolactone. They even did a sensitivity analysis, which really showed that it was consistent across the board.
Edema and Hyperkalemia
Now we have two binders that have shown to us that patients can stay on their drugs. There were some interesting findings here, though.
There was more edema — again, everything is based on small numbers — and there seemed to be more heart failure events in the group that received the zirconium cyclosilicate. The first episode of hyperkalemia was delayed or didn’t happen at all. Again, the hyperkalemia was controlled.
What does that tell you? Well, the exchange is sodium. There had been reports before that if you gave this binder at the higher doses, you would have more retention of sodium. I think we see that in this trial, even though the numbers are very small.
According to the investigators, these were issues that could be resolved through an increase in diuretics or having the patient remember to be careful with their sodium intake so they don’t retain more fluid.
My message to you is to use these binders, whichever one of the two you want or whichever your hospital has available for you on their formulary, because it may give you that sense of comfort and self-efficacy so that you can actually start your patients on an MRA and keep them on it.
The MRAs are lifesaving drugs and the patients with HFrEF need to be on them. This is a way to do it without having to sacrifice your true guideline-directed medical therapy.
Dr. Piña, Professor of Medicine/Cardiology/Heart Failure/Transplant; Quality Officer, Cardiovascular Line, Sidney Kimmel College of Medicine, Thomas Jefferson University, Philadelphia, Pennsylvania; Clinical Professor of Medicine, Central Michigan University College of Medicine, Mount Pleasant, Michigan; Adjunct Professor of Epidemiology and Biostatistics, Population & Quantitative Health Sciences, Case Western University, Cleveland, Ohio, disclosed ties with the Food and Drug Administration’s Center for Devices and Radiological Health.
A version of this article appeared on Medscape.com
Focus on Nutrient Density Instead of Limiting Certain Foods
The word “malnutrition” probably brings to mind images of very thin patients with catabolic illness. But it really just means “poor nutrition,” which can — and often does — apply to patients with overweight or obesity.
That’s because malnutrition doesn’t occur simply because of a lack of calories, but rather because there is a gap in the nutrition the body requires and the nutrition it receives.
Each day, clinicians see patients with chronic conditions related to malnutrition. That list includes diabetes and hypertension, which can be promoted by excess intake of certain nutrients (carbohydrates and sodium) or inadequate intake of others (fiber, protein, potassium, magnesium, and calcium).
Diet Education Is Vital in Chronic Disease Management
Diet education is without a doubt a core pillar of chronic disease management. Nutrition therapy is recommended in treatment guidelines for the management of some of the most commonly seen chronic conditions such as hypertension, diabetes, and kidney disease. But in one study, only 58% of physicians, nurses and other health professionals surveyed had received formal nutrition education and only 40% were confident in their ability to provide nutrition education to patients.
As a registered dietitian, I welcome referrals for both prevention and management of chronic diseases with open arms. But medical nutrition therapy with a registered dietitian may not be realistic for all patients owing to financial, geographic, or other constraints. So, their best option may be the few minutes that a physician or physician extender has to spare at the end of their appointment.
But time constraints may result in clinicians turning to short, easy-to-remember messages such as “Don’t eat anything white” or “Only shop the edges of the grocery store.” Although catchy, this type of advice can inadvertently encourage patients to skip over foods that are actually very nutrient dense. For example, white foods such as onions, turnips, mushrooms, cauliflower, and even popcorn are low in calories and high in nutritional value. The center aisles of the grocery store may harbor high-carbohydrate breakfast cereals and potato chips, but they are also home to legumes, nuts, and canned and frozen fruits and vegetables.
What may be more effective is educating the patient on the importance of focusing on the nutrient density of foods, rather than simply limiting certain food groups or colors.
How to Work Nutrient Density into the Conversation
Nutrient density is a concept that refers to the proportion of nutrients to calories in a food item: essentially, a food’s qualitative nutritional value. It provides more depth than simply referring to foods as being high or low in calories, healthy or unhealthy, or good or bad.
Educating patients about nutrition density and encouraging a focus on foods that are low in calories and high in vitamins and minerals can help address micronutrient deficiencies, which may be more common than previously thought and linked to the chronic diseases that we see daily. It is worth noting that some foods that are not low in calories are still nutrient dense. Avocados, liver, and nuts come to mind as foods that are high in calories, but they have additional nutrients such as fiber, potassium, antioxidants, vitamin A, iron, and selenium that can still make them an excellent choice if they are part of a well-balanced diet.
I fear that we often underestimate our patients. We worry that not providing them with a list of acceptable foods will set them up for failure. But, in my experience, that list of “good” and “bad” foods may be useful for a week or so but will eventually become lost on the fridge under children’s artwork and save-the-dates.
Patients know that potato chips offer little more than fat, carbs, and salt and that they’re a poor choice for long-term health. What they might not know is that cocktail peanuts can also satisfy the craving for a salty snack, with more than four times the protein, twice the fiber, and just over half of the sodium found in the same serving size of regular salted potato chips. Peanuts have the added bonus of being high in heart-healthy monounsaturated fatty acids.
The best thing that clinicians can do with just a few minutes of time for diet education is to talk to patients about the nutrient density of whole foods and caution patients against highly processed foods, because processing can decrease nutritional content. Our most effective option is to explain why a varied diet with focus on fruits, vegetables, lean protein, nuts, legumes, and healthy fats is beneficial for cardiovascular and metabolic health. After that, all that is left is to trust the patient to make the right choices for their health.
Brandy Winfree Root, a renal dietitian in private practice in Mary Esther, Florida, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The word “malnutrition” probably brings to mind images of very thin patients with catabolic illness. But it really just means “poor nutrition,” which can — and often does — apply to patients with overweight or obesity.
That’s because malnutrition doesn’t occur simply because of a lack of calories, but rather because there is a gap in the nutrition the body requires and the nutrition it receives.
Each day, clinicians see patients with chronic conditions related to malnutrition. That list includes diabetes and hypertension, which can be promoted by excess intake of certain nutrients (carbohydrates and sodium) or inadequate intake of others (fiber, protein, potassium, magnesium, and calcium).
Diet Education Is Vital in Chronic Disease Management
Diet education is without a doubt a core pillar of chronic disease management. Nutrition therapy is recommended in treatment guidelines for the management of some of the most commonly seen chronic conditions such as hypertension, diabetes, and kidney disease. But in one study, only 58% of physicians, nurses and other health professionals surveyed had received formal nutrition education and only 40% were confident in their ability to provide nutrition education to patients.
As a registered dietitian, I welcome referrals for both prevention and management of chronic diseases with open arms. But medical nutrition therapy with a registered dietitian may not be realistic for all patients owing to financial, geographic, or other constraints. So, their best option may be the few minutes that a physician or physician extender has to spare at the end of their appointment.
But time constraints may result in clinicians turning to short, easy-to-remember messages such as “Don’t eat anything white” or “Only shop the edges of the grocery store.” Although catchy, this type of advice can inadvertently encourage patients to skip over foods that are actually very nutrient dense. For example, white foods such as onions, turnips, mushrooms, cauliflower, and even popcorn are low in calories and high in nutritional value. The center aisles of the grocery store may harbor high-carbohydrate breakfast cereals and potato chips, but they are also home to legumes, nuts, and canned and frozen fruits and vegetables.
What may be more effective is educating the patient on the importance of focusing on the nutrient density of foods, rather than simply limiting certain food groups or colors.
How to Work Nutrient Density into the Conversation
Nutrient density is a concept that refers to the proportion of nutrients to calories in a food item: essentially, a food’s qualitative nutritional value. It provides more depth than simply referring to foods as being high or low in calories, healthy or unhealthy, or good or bad.
Educating patients about nutrition density and encouraging a focus on foods that are low in calories and high in vitamins and minerals can help address micronutrient deficiencies, which may be more common than previously thought and linked to the chronic diseases that we see daily. It is worth noting that some foods that are not low in calories are still nutrient dense. Avocados, liver, and nuts come to mind as foods that are high in calories, but they have additional nutrients such as fiber, potassium, antioxidants, vitamin A, iron, and selenium that can still make them an excellent choice if they are part of a well-balanced diet.
I fear that we often underestimate our patients. We worry that not providing them with a list of acceptable foods will set them up for failure. But, in my experience, that list of “good” and “bad” foods may be useful for a week or so but will eventually become lost on the fridge under children’s artwork and save-the-dates.
Patients know that potato chips offer little more than fat, carbs, and salt and that they’re a poor choice for long-term health. What they might not know is that cocktail peanuts can also satisfy the craving for a salty snack, with more than four times the protein, twice the fiber, and just over half of the sodium found in the same serving size of regular salted potato chips. Peanuts have the added bonus of being high in heart-healthy monounsaturated fatty acids.
The best thing that clinicians can do with just a few minutes of time for diet education is to talk to patients about the nutrient density of whole foods and caution patients against highly processed foods, because processing can decrease nutritional content. Our most effective option is to explain why a varied diet with focus on fruits, vegetables, lean protein, nuts, legumes, and healthy fats is beneficial for cardiovascular and metabolic health. After that, all that is left is to trust the patient to make the right choices for their health.
Brandy Winfree Root, a renal dietitian in private practice in Mary Esther, Florida, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The word “malnutrition” probably brings to mind images of very thin patients with catabolic illness. But it really just means “poor nutrition,” which can — and often does — apply to patients with overweight or obesity.
That’s because malnutrition doesn’t occur simply because of a lack of calories, but rather because there is a gap in the nutrition the body requires and the nutrition it receives.
Each day, clinicians see patients with chronic conditions related to malnutrition. That list includes diabetes and hypertension, which can be promoted by excess intake of certain nutrients (carbohydrates and sodium) or inadequate intake of others (fiber, protein, potassium, magnesium, and calcium).
Diet Education Is Vital in Chronic Disease Management
Diet education is without a doubt a core pillar of chronic disease management. Nutrition therapy is recommended in treatment guidelines for the management of some of the most commonly seen chronic conditions such as hypertension, diabetes, and kidney disease. But in one study, only 58% of physicians, nurses and other health professionals surveyed had received formal nutrition education and only 40% were confident in their ability to provide nutrition education to patients.
As a registered dietitian, I welcome referrals for both prevention and management of chronic diseases with open arms. But medical nutrition therapy with a registered dietitian may not be realistic for all patients owing to financial, geographic, or other constraints. So, their best option may be the few minutes that a physician or physician extender has to spare at the end of their appointment.
But time constraints may result in clinicians turning to short, easy-to-remember messages such as “Don’t eat anything white” or “Only shop the edges of the grocery store.” Although catchy, this type of advice can inadvertently encourage patients to skip over foods that are actually very nutrient dense. For example, white foods such as onions, turnips, mushrooms, cauliflower, and even popcorn are low in calories and high in nutritional value. The center aisles of the grocery store may harbor high-carbohydrate breakfast cereals and potato chips, but they are also home to legumes, nuts, and canned and frozen fruits and vegetables.
What may be more effective is educating the patient on the importance of focusing on the nutrient density of foods, rather than simply limiting certain food groups or colors.
How to Work Nutrient Density into the Conversation
Nutrient density is a concept that refers to the proportion of nutrients to calories in a food item: essentially, a food’s qualitative nutritional value. It provides more depth than simply referring to foods as being high or low in calories, healthy or unhealthy, or good or bad.
Educating patients about nutrition density and encouraging a focus on foods that are low in calories and high in vitamins and minerals can help address micronutrient deficiencies, which may be more common than previously thought and linked to the chronic diseases that we see daily. It is worth noting that some foods that are not low in calories are still nutrient dense. Avocados, liver, and nuts come to mind as foods that are high in calories, but they have additional nutrients such as fiber, potassium, antioxidants, vitamin A, iron, and selenium that can still make them an excellent choice if they are part of a well-balanced diet.
I fear that we often underestimate our patients. We worry that not providing them with a list of acceptable foods will set them up for failure. But, in my experience, that list of “good” and “bad” foods may be useful for a week or so but will eventually become lost on the fridge under children’s artwork and save-the-dates.
Patients know that potato chips offer little more than fat, carbs, and salt and that they’re a poor choice for long-term health. What they might not know is that cocktail peanuts can also satisfy the craving for a salty snack, with more than four times the protein, twice the fiber, and just over half of the sodium found in the same serving size of regular salted potato chips. Peanuts have the added bonus of being high in heart-healthy monounsaturated fatty acids.
The best thing that clinicians can do with just a few minutes of time for diet education is to talk to patients about the nutrient density of whole foods and caution patients against highly processed foods, because processing can decrease nutritional content. Our most effective option is to explain why a varied diet with focus on fruits, vegetables, lean protein, nuts, legumes, and healthy fats is beneficial for cardiovascular and metabolic health. After that, all that is left is to trust the patient to make the right choices for their health.
Brandy Winfree Root, a renal dietitian in private practice in Mary Esther, Florida, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Maintaining Weight Loss With GLP-1s Needs Lifestyle Changes
This transcript has been edited for clarity.
Nearly every patient I start on incretin therapy for weight loss asks me the same question, which is, will I have to stay on this forever? The answer is probably yes, but I think it’s much more nuanced than that because A) forever is a long time and B) I think there are various ways to approach this.
I want people to start just saying, let’s see how this works, because not everyone’s going to lose the same amount of weight or respond in the same way. I say let’s try it, but don’t stop it suddenly. If we decide at some point you don’t need quite the same dose, we can reduce the dose and maybe even reduce the frequency of giving it, but you don’t want to stop cold turkey because you may well regain the weight, and that’s obviously not our desired outcome.
There have been multiple clinical trials in which people started on an incretin hormone, either a glucagon-like peptide 1 (GLP-1) receptor agonist or a dual hormone, and they’ve actually shown that stopping it and then continuing patients on a placebo vs active drug results in continued weight gain over time vs either weight maintenance or weight loss when they remain on the incretin hormone. Clearly, on average, people will regain the weight, but that isn’t always true.
One of the things I think is really important is that, from the get-go on starting on these hormones, people start working with a lifestyle plan, whether it’s working with a coach or an online program. However they approach this, it’s important to start changing habits and increasing exercise.
I can’t say how important this is enough, because people need to increase their physical activity to enhance the benefits of these agents and also to help retain lean body mass. I don’t want people losing a large amount of lean body mass as they go through the process of weight loss.
I set the stage for the fact that I expect people to adhere to a lifestyle program, and maybe losing weight with the medications is going to help them do even better because they’re going to see positive outcomes.
When they get to the point of weight maintenance, I think we need to reinforce lifestyle. I either go down on the dose given weekly or I start having patients take the dose every other week, for instance, as opposed to every week, and then sometimes every month. Depending on the patient, I get them potentially to a lower dose, and then they’re able to maintain the weight as long as they improved their lifestyle along with the changes in the medication.
I tell people we’ll work with the drug, we’ll work with their metabolic needs, that there are many benefits to being on incretin hormone therapy, but I think it’s important that we can do this on an individualized basis. The thing I don’t want to happen, though, is for people to start on it and then stop it, and then start on it and stop it because they may lose weight, regain weight, get side effects, get used to the side effects, stop it, and start it.
As we know, that’s not the best way to do this, and I think it’s not healthy for people to do that either. I know it’s been somewhat problematic with supply chain issues, but hopefully we’ll be able to start these agents, reach the desired outcome in terms of weight loss, and then help patients maintain that weight with a combination of both medication and lifestyle.
Dr. Peters, professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, Los Angeles, disclosed ties with Abbott Diabetes Care, Becton Dickinson; Boehringer Ingelheim Pharmaceuticals, Eli Lilly, Lexicon Pharmaceuticals, Livongo; Medscape; Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, Zafgen, Dexcom, MannKind, and AstraZeneca.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Nearly every patient I start on incretin therapy for weight loss asks me the same question, which is, will I have to stay on this forever? The answer is probably yes, but I think it’s much more nuanced than that because A) forever is a long time and B) I think there are various ways to approach this.
I want people to start just saying, let’s see how this works, because not everyone’s going to lose the same amount of weight or respond in the same way. I say let’s try it, but don’t stop it suddenly. If we decide at some point you don’t need quite the same dose, we can reduce the dose and maybe even reduce the frequency of giving it, but you don’t want to stop cold turkey because you may well regain the weight, and that’s obviously not our desired outcome.
There have been multiple clinical trials in which people started on an incretin hormone, either a glucagon-like peptide 1 (GLP-1) receptor agonist or a dual hormone, and they’ve actually shown that stopping it and then continuing patients on a placebo vs active drug results in continued weight gain over time vs either weight maintenance or weight loss when they remain on the incretin hormone. Clearly, on average, people will regain the weight, but that isn’t always true.
One of the things I think is really important is that, from the get-go on starting on these hormones, people start working with a lifestyle plan, whether it’s working with a coach or an online program. However they approach this, it’s important to start changing habits and increasing exercise.
I can’t say how important this is enough, because people need to increase their physical activity to enhance the benefits of these agents and also to help retain lean body mass. I don’t want people losing a large amount of lean body mass as they go through the process of weight loss.
I set the stage for the fact that I expect people to adhere to a lifestyle program, and maybe losing weight with the medications is going to help them do even better because they’re going to see positive outcomes.
When they get to the point of weight maintenance, I think we need to reinforce lifestyle. I either go down on the dose given weekly or I start having patients take the dose every other week, for instance, as opposed to every week, and then sometimes every month. Depending on the patient, I get them potentially to a lower dose, and then they’re able to maintain the weight as long as they improved their lifestyle along with the changes in the medication.
I tell people we’ll work with the drug, we’ll work with their metabolic needs, that there are many benefits to being on incretin hormone therapy, but I think it’s important that we can do this on an individualized basis. The thing I don’t want to happen, though, is for people to start on it and then stop it, and then start on it and stop it because they may lose weight, regain weight, get side effects, get used to the side effects, stop it, and start it.
As we know, that’s not the best way to do this, and I think it’s not healthy for people to do that either. I know it’s been somewhat problematic with supply chain issues, but hopefully we’ll be able to start these agents, reach the desired outcome in terms of weight loss, and then help patients maintain that weight with a combination of both medication and lifestyle.
Dr. Peters, professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, Los Angeles, disclosed ties with Abbott Diabetes Care, Becton Dickinson; Boehringer Ingelheim Pharmaceuticals, Eli Lilly, Lexicon Pharmaceuticals, Livongo; Medscape; Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, Zafgen, Dexcom, MannKind, and AstraZeneca.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Nearly every patient I start on incretin therapy for weight loss asks me the same question, which is, will I have to stay on this forever? The answer is probably yes, but I think it’s much more nuanced than that because A) forever is a long time and B) I think there are various ways to approach this.
I want people to start just saying, let’s see how this works, because not everyone’s going to lose the same amount of weight or respond in the same way. I say let’s try it, but don’t stop it suddenly. If we decide at some point you don’t need quite the same dose, we can reduce the dose and maybe even reduce the frequency of giving it, but you don’t want to stop cold turkey because you may well regain the weight, and that’s obviously not our desired outcome.
There have been multiple clinical trials in which people started on an incretin hormone, either a glucagon-like peptide 1 (GLP-1) receptor agonist or a dual hormone, and they’ve actually shown that stopping it and then continuing patients on a placebo vs active drug results in continued weight gain over time vs either weight maintenance or weight loss when they remain on the incretin hormone. Clearly, on average, people will regain the weight, but that isn’t always true.
One of the things I think is really important is that, from the get-go on starting on these hormones, people start working with a lifestyle plan, whether it’s working with a coach or an online program. However they approach this, it’s important to start changing habits and increasing exercise.
I can’t say how important this is enough, because people need to increase their physical activity to enhance the benefits of these agents and also to help retain lean body mass. I don’t want people losing a large amount of lean body mass as they go through the process of weight loss.
I set the stage for the fact that I expect people to adhere to a lifestyle program, and maybe losing weight with the medications is going to help them do even better because they’re going to see positive outcomes.
When they get to the point of weight maintenance, I think we need to reinforce lifestyle. I either go down on the dose given weekly or I start having patients take the dose every other week, for instance, as opposed to every week, and then sometimes every month. Depending on the patient, I get them potentially to a lower dose, and then they’re able to maintain the weight as long as they improved their lifestyle along with the changes in the medication.
I tell people we’ll work with the drug, we’ll work with their metabolic needs, that there are many benefits to being on incretin hormone therapy, but I think it’s important that we can do this on an individualized basis. The thing I don’t want to happen, though, is for people to start on it and then stop it, and then start on it and stop it because they may lose weight, regain weight, get side effects, get used to the side effects, stop it, and start it.
As we know, that’s not the best way to do this, and I think it’s not healthy for people to do that either. I know it’s been somewhat problematic with supply chain issues, but hopefully we’ll be able to start these agents, reach the desired outcome in terms of weight loss, and then help patients maintain that weight with a combination of both medication and lifestyle.
Dr. Peters, professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, Los Angeles, disclosed ties with Abbott Diabetes Care, Becton Dickinson; Boehringer Ingelheim Pharmaceuticals, Eli Lilly, Lexicon Pharmaceuticals, Livongo; Medscape; Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, Zafgen, Dexcom, MannKind, and AstraZeneca.
A version of this article first appeared on Medscape.com.
New Approaches to Research Beyond Massive Clinical Trials
This transcript has been edited for clarity.
I want to briefly present a fascinating effort, one that needs to be applauded and applauded again, and then we need to scratch our collective heads and ask, why did we do it and what did we learn?
I’m referring to a report recently published in Annals of Internal Medicine, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women: Postintervention Follow-up of a Randomized Clinical Trial.” The title of this report does not do it justice. This was a massive effort — one could, I believe, even use the term Herculean — to ask an important question that was asked more than 20 years ago.
This was a national women’s health initiative to answer these questions. The study looked at 36,282 postmenopausal women who, at the time of agreeing to be randomized in this trial, had no history of breast or colorectal cancer. This was a 7-year randomized intervention effort, and 40 centers across the United States participated, obviously funded by the government. Randomization was one-to-one to placebo or 1000 mg calcium and 400 international units of vitamin D3 daily.
They looked at the incidence of colorectal cancer, breast cancer, and total cancer, and importantly as an endpoint, total cardiovascular disease and hip fractures. They didn’t comment on hip fractures in this particular analysis. Obviously, hip fractures relate to this question of osteoporosis in postmenopausal women.
Here’s the bottom line: With a median follow-up now of 22.3 years — that’s not 2 years, but 22.3 years — there was a 7% decrease in cancer mortality in the population that received the calcium and vitamin D3. This is nothing to snicker at, and nothing at which to say, “Wow. That’s not important.”
However, in this analysis involving several tens of thousands of women, there was a 6% increase in cardiovascular disease mortality noted and reported. Overall, there was no effect on all-cause mortality of this intervention, with a hazard ratio — you rarely see this — of 1.00.
There is much that can be said, but I will summarize my comments very briefly. Criticize this if you want. It’s not inappropriate to criticize, but what was the individual impact of the calcium vs vitamin D? If they had only used one vs the other, or used both but in separate arms of the trial, and you could have separated what might have caused the decrease in cancer mortality and not the increased cardiovascular disease… This was designed more than 20 years ago. That’s one point.
The second is, how many more tens of thousands of patients would they have had to add to do this, and at what cost? This was a massive study, a national study, and a simple study in terms of the intervention. It was low risk except if you look at the long-term outcome. You can only imagine how much it would cost to do that study today — not the cost of the calcium, the vitamin D3, but the cost of doing the trial that was concluded to have no impact.
From a societal perspective, this was an important question to answer, certainly then. What did we learn and at what cost? The bottom line is that we have to figure out a way of answering these kinds of questions.
Perhaps now they should be from real-world data, looking at electronic medical records or at a variety of other population-based data so that we can get the answer — not in 20 years but in perhaps 2 months, because we’ve looked at the data using artificial intelligence to help us to answer these questions; and maybe not 36,000 patients but 360,000 individuals looked at over this period of time.
Again, I’m proposing an alternative solution because the questions that were asked 20 years ago remain important today. This cannot be the way that we, in the future, try to answer them, certainly from the perspective of cost and also the perspective of time to get the answers.
Let me conclude by, again, applauding these researchers because of the quality of the work they started out doing and ended up doing and reporting. Also, I think we’ve learned that we have to come up with alternative ways to answer what were important questions then and are important questions today.
Dr. Markman, Professor of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center; President, Medicine & Science, City of Hope Atlanta, Chicago, Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I want to briefly present a fascinating effort, one that needs to be applauded and applauded again, and then we need to scratch our collective heads and ask, why did we do it and what did we learn?
I’m referring to a report recently published in Annals of Internal Medicine, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women: Postintervention Follow-up of a Randomized Clinical Trial.” The title of this report does not do it justice. This was a massive effort — one could, I believe, even use the term Herculean — to ask an important question that was asked more than 20 years ago.
This was a national women’s health initiative to answer these questions. The study looked at 36,282 postmenopausal women who, at the time of agreeing to be randomized in this trial, had no history of breast or colorectal cancer. This was a 7-year randomized intervention effort, and 40 centers across the United States participated, obviously funded by the government. Randomization was one-to-one to placebo or 1000 mg calcium and 400 international units of vitamin D3 daily.
They looked at the incidence of colorectal cancer, breast cancer, and total cancer, and importantly as an endpoint, total cardiovascular disease and hip fractures. They didn’t comment on hip fractures in this particular analysis. Obviously, hip fractures relate to this question of osteoporosis in postmenopausal women.
Here’s the bottom line: With a median follow-up now of 22.3 years — that’s not 2 years, but 22.3 years — there was a 7% decrease in cancer mortality in the population that received the calcium and vitamin D3. This is nothing to snicker at, and nothing at which to say, “Wow. That’s not important.”
However, in this analysis involving several tens of thousands of women, there was a 6% increase in cardiovascular disease mortality noted and reported. Overall, there was no effect on all-cause mortality of this intervention, with a hazard ratio — you rarely see this — of 1.00.
There is much that can be said, but I will summarize my comments very briefly. Criticize this if you want. It’s not inappropriate to criticize, but what was the individual impact of the calcium vs vitamin D? If they had only used one vs the other, or used both but in separate arms of the trial, and you could have separated what might have caused the decrease in cancer mortality and not the increased cardiovascular disease… This was designed more than 20 years ago. That’s one point.
The second is, how many more tens of thousands of patients would they have had to add to do this, and at what cost? This was a massive study, a national study, and a simple study in terms of the intervention. It was low risk except if you look at the long-term outcome. You can only imagine how much it would cost to do that study today — not the cost of the calcium, the vitamin D3, but the cost of doing the trial that was concluded to have no impact.
From a societal perspective, this was an important question to answer, certainly then. What did we learn and at what cost? The bottom line is that we have to figure out a way of answering these kinds of questions.
Perhaps now they should be from real-world data, looking at electronic medical records or at a variety of other population-based data so that we can get the answer — not in 20 years but in perhaps 2 months, because we’ve looked at the data using artificial intelligence to help us to answer these questions; and maybe not 36,000 patients but 360,000 individuals looked at over this period of time.
Again, I’m proposing an alternative solution because the questions that were asked 20 years ago remain important today. This cannot be the way that we, in the future, try to answer them, certainly from the perspective of cost and also the perspective of time to get the answers.
Let me conclude by, again, applauding these researchers because of the quality of the work they started out doing and ended up doing and reporting. Also, I think we’ve learned that we have to come up with alternative ways to answer what were important questions then and are important questions today.
Dr. Markman, Professor of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center; President, Medicine & Science, City of Hope Atlanta, Chicago, Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I want to briefly present a fascinating effort, one that needs to be applauded and applauded again, and then we need to scratch our collective heads and ask, why did we do it and what did we learn?
I’m referring to a report recently published in Annals of Internal Medicine, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women: Postintervention Follow-up of a Randomized Clinical Trial.” The title of this report does not do it justice. This was a massive effort — one could, I believe, even use the term Herculean — to ask an important question that was asked more than 20 years ago.
This was a national women’s health initiative to answer these questions. The study looked at 36,282 postmenopausal women who, at the time of agreeing to be randomized in this trial, had no history of breast or colorectal cancer. This was a 7-year randomized intervention effort, and 40 centers across the United States participated, obviously funded by the government. Randomization was one-to-one to placebo or 1000 mg calcium and 400 international units of vitamin D3 daily.
They looked at the incidence of colorectal cancer, breast cancer, and total cancer, and importantly as an endpoint, total cardiovascular disease and hip fractures. They didn’t comment on hip fractures in this particular analysis. Obviously, hip fractures relate to this question of osteoporosis in postmenopausal women.
Here’s the bottom line: With a median follow-up now of 22.3 years — that’s not 2 years, but 22.3 years — there was a 7% decrease in cancer mortality in the population that received the calcium and vitamin D3. This is nothing to snicker at, and nothing at which to say, “Wow. That’s not important.”
However, in this analysis involving several tens of thousands of women, there was a 6% increase in cardiovascular disease mortality noted and reported. Overall, there was no effect on all-cause mortality of this intervention, with a hazard ratio — you rarely see this — of 1.00.
There is much that can be said, but I will summarize my comments very briefly. Criticize this if you want. It’s not inappropriate to criticize, but what was the individual impact of the calcium vs vitamin D? If they had only used one vs the other, or used both but in separate arms of the trial, and you could have separated what might have caused the decrease in cancer mortality and not the increased cardiovascular disease… This was designed more than 20 years ago. That’s one point.
The second is, how many more tens of thousands of patients would they have had to add to do this, and at what cost? This was a massive study, a national study, and a simple study in terms of the intervention. It was low risk except if you look at the long-term outcome. You can only imagine how much it would cost to do that study today — not the cost of the calcium, the vitamin D3, but the cost of doing the trial that was concluded to have no impact.
From a societal perspective, this was an important question to answer, certainly then. What did we learn and at what cost? The bottom line is that we have to figure out a way of answering these kinds of questions.
Perhaps now they should be from real-world data, looking at electronic medical records or at a variety of other population-based data so that we can get the answer — not in 20 years but in perhaps 2 months, because we’ve looked at the data using artificial intelligence to help us to answer these questions; and maybe not 36,000 patients but 360,000 individuals looked at over this period of time.
Again, I’m proposing an alternative solution because the questions that were asked 20 years ago remain important today. This cannot be the way that we, in the future, try to answer them, certainly from the perspective of cost and also the perspective of time to get the answers.
Let me conclude by, again, applauding these researchers because of the quality of the work they started out doing and ended up doing and reporting. Also, I think we’ve learned that we have to come up with alternative ways to answer what were important questions then and are important questions today.
Dr. Markman, Professor of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center; President, Medicine & Science, City of Hope Atlanta, Chicago, Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article first appeared on Medscape.com.
How Metals Affect the Brain
This transcript has been edited for clarity.
It has always amazed me that our bodies require these tiny amounts of incredibly rare substances to function. Sure, we need oxygen. We need water. But we also need molybdenum, which makes up just 1.2 parts per million of the Earth’s crust.
Without adequate molybdenum intake, we develop seizures, developmental delays, death. Fortunately, we need so little molybdenum that true molybdenum deficiency is incredibly rare — seen only in people on total parenteral nutrition without supplementation or those with certain rare genetic conditions. But still, molybdenum is necessary for life.
Many metals are. Figure 1 colors the essential minerals on the periodic table. You can see that to stay alive, we humans need not only things like sodium, but selenium, bromine, zinc, copper, and cobalt.
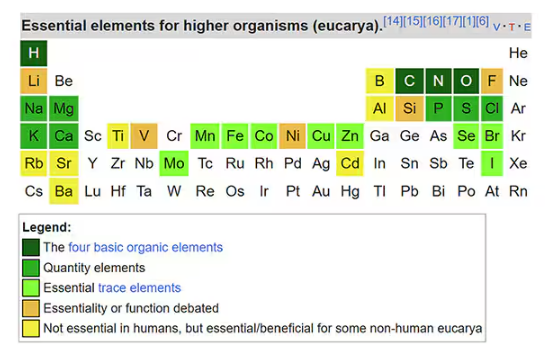
Some metals are very clearly not essential; we can all do without lead and mercury, and probably should.
But just because something is essential for life does not mean that more is better. The dose is the poison, as they say. And this week, we explore whether metals — even essential metals — might be adversely affecting our brains.
It’s not a stretch to think that metal intake could have weird effects on our nervous system. Lead exposure, primarily due to leaded gasoline, has been blamed for an average reduction of about 3 points in our national IQ, for example . But not all metals are created equal. Researchers set out to find out which might be more strongly associated with performance on cognitive tests and dementia, and reported their results in this study in JAMA Network Open.
To do this, they leveraged the MESA cohort study. This is a longitudinal study of a relatively diverse group of 6300 adults who were enrolled from 2000 to 2002 around the United States. At enrollment, they gave a urine sample and took a variety of cognitive tests. Important for this study was the digit symbol substitution test, where participants are provided a code and need to replace a list of numbers with symbols as per that code. Performance on this test worsens with age, depression, and cognitive impairment.
Participants were followed for more than a decade, and over that time, 559 (about 9%) were diagnosed with dementia.
Those baseline urine samples were assayed for a variety of metals — some essential, some very much not, as you can see in Figure 2.

Now, I have to put my kidney doctor hat on for a second and talk about urine measurement ... of anything. The problem with urine is that the concentration can change a lot — by more than 10-fold, in fact — based on how much water you drank recently. Researchers correct for this, and in the case of this study, they do what a lot of researchers do: divide the measured concentration by the urine creatinine level.
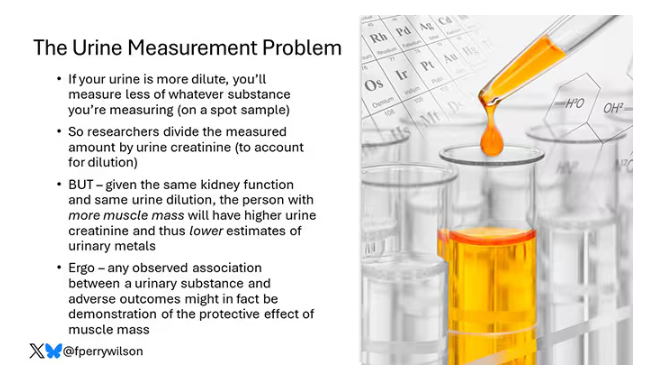
This introduces a bit of a problem. Take two people with exactly the same kidney function, who drank exactly the same water, whose urine is exactly the same concentration. The person with more muscle mass will have more creatinine in that urine sample, since creatinine is a byproduct of muscle metabolism. Because people with more muscle mass are generally healthier, when you divide your metal concentration by urine creatinine, you get a lower number, which might lead you to believe that lower levels of the metal in the urine are protective. But in fact, what you’re seeing is that higher levels of creatinine are protective. I see this issue all the time and it will always color results of studies like this.
Okay, I am doffing my kidney doctor hat now to show you the results.
The researchers first looked at the relationship between metal concentrations in the urine and performance on cognitive tests. The results were fairly equivocal, save for that digit substitution test which is shown in Figure 4.
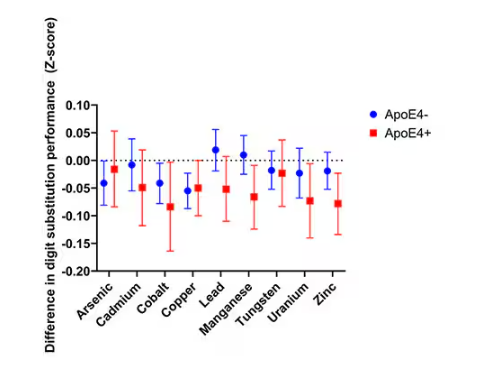
Even these results don’t ring major alarm bells for me. What you’re seeing here is the change in scores on the digit substitution test for each 25-percentile increase in urinary metal level — a pretty big change. And yet, you see really minor changes in the performance on the test. The digit substitution test is not an IQ test; but to give you a feeling for the magnitude of this change, if we looked at copper level, moving from the 25th to the 50th percentile would be associated with a loss of nine tenths of an IQ point.
You see two colors on the Figure 4 graph, by the way. That’s because the researchers stratified their findings based on whether the individual carried the ApoE4 gene allele, which is a risk factor for the development of dementia. There are reasons to believe that neurotoxic metals might be worse in this population, and I suppose you do see generally more adverse effects on scores in the red lines compared with the blue lines. But still, we’re not talking about a huge effect size here.
Let’s look at the relationship between these metals and the development of dementia itself, a clearly more important outcome than how well you can replace numeric digits with symbols. I’ll highlight a few of the results that are particularly telling.
First, the nonessential mineral cadmium, which displays the type of relationship we would expect if the metal were neurotoxic: a clear, roughly linear increase in risk for dementia as urinary concentration increases.
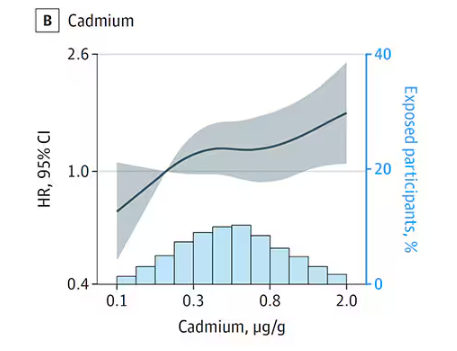
We see roughly similar patterns with the nonessential minerals tungsten and uranium, and the essential mineral zinc (beloved of respiratory-virus avoiders everywhere).
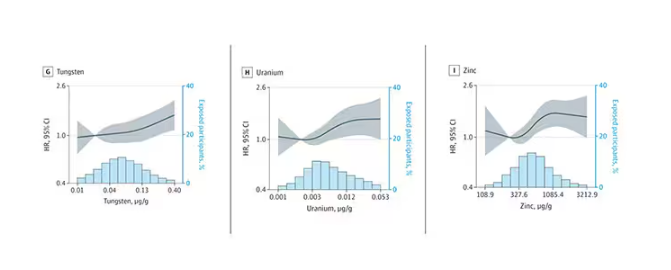
But it is very much not what we see for all metals. Strangest of all, look at lead, which shows basically no relationship with dementia.
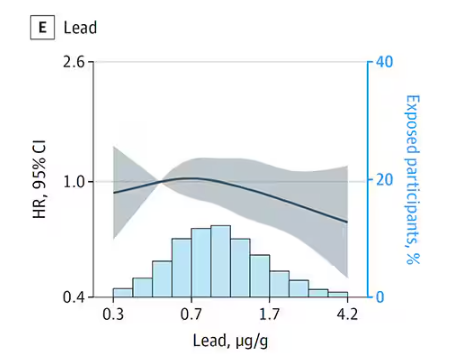
This concerns me a bit. Earlier, I discussed the issue of measuring stuff in urine and how standardizing levels to the urine creatinine level introduces a bias due to muscle mass. One way around this is to standardize urine levels to some other marker of urine dilution, like osmolality. But more fundamental than that, I like to see positive and negative controls in studies like this. For example, lead strikes me as a good positive control here. If the experimental framework were valid, I would think we’d see a relationship between lead level and dementia.
For a negative control? Well, something we are quite sure is not neurotoxic — something like sulfur, which is relatively ubiquitous, used in a variety of biological processes, and efficiently eliminated. We don’t have that in this study.
The authors close their case by creating a model that combines all the metal levels, asking the question of whether higher levels of metals in the urine in general worsen cognitive scores. And they find that the relationship exists, as you can see in Figure 8, both in carriers and noncarriers of ApoE4. But, to me, this is even more argument for the creatinine problem. If it’s not a specific metal but just the sort of general concentration of all metals, the risk for confounding by muscle mass is even higher.
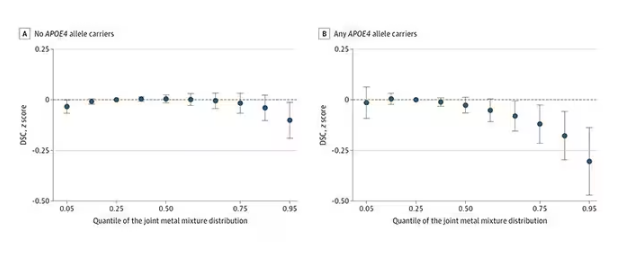
So should we worry about ingesting metals? I suppose the answer is ... kind of.
I am sure we should be avoiding lead, despite the results of this study. It’s probably best to stay away from uranium too.
As for the essential metals, I’m sure there is some toxic dose; there’s a toxic dose for everything at some point. But I don’t see evidence in this study to make me worry that a significant chunk of the population is anywhere close to that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It has always amazed me that our bodies require these tiny amounts of incredibly rare substances to function. Sure, we need oxygen. We need water. But we also need molybdenum, which makes up just 1.2 parts per million of the Earth’s crust.
Without adequate molybdenum intake, we develop seizures, developmental delays, death. Fortunately, we need so little molybdenum that true molybdenum deficiency is incredibly rare — seen only in people on total parenteral nutrition without supplementation or those with certain rare genetic conditions. But still, molybdenum is necessary for life.
Many metals are. Figure 1 colors the essential minerals on the periodic table. You can see that to stay alive, we humans need not only things like sodium, but selenium, bromine, zinc, copper, and cobalt.

Some metals are very clearly not essential; we can all do without lead and mercury, and probably should.
But just because something is essential for life does not mean that more is better. The dose is the poison, as they say. And this week, we explore whether metals — even essential metals — might be adversely affecting our brains.
It’s not a stretch to think that metal intake could have weird effects on our nervous system. Lead exposure, primarily due to leaded gasoline, has been blamed for an average reduction of about 3 points in our national IQ, for example . But not all metals are created equal. Researchers set out to find out which might be more strongly associated with performance on cognitive tests and dementia, and reported their results in this study in JAMA Network Open.
To do this, they leveraged the MESA cohort study. This is a longitudinal study of a relatively diverse group of 6300 adults who were enrolled from 2000 to 2002 around the United States. At enrollment, they gave a urine sample and took a variety of cognitive tests. Important for this study was the digit symbol substitution test, where participants are provided a code and need to replace a list of numbers with symbols as per that code. Performance on this test worsens with age, depression, and cognitive impairment.
Participants were followed for more than a decade, and over that time, 559 (about 9%) were diagnosed with dementia.
Those baseline urine samples were assayed for a variety of metals — some essential, some very much not, as you can see in Figure 2.

Now, I have to put my kidney doctor hat on for a second and talk about urine measurement ... of anything. The problem with urine is that the concentration can change a lot — by more than 10-fold, in fact — based on how much water you drank recently. Researchers correct for this, and in the case of this study, they do what a lot of researchers do: divide the measured concentration by the urine creatinine level.

This introduces a bit of a problem. Take two people with exactly the same kidney function, who drank exactly the same water, whose urine is exactly the same concentration. The person with more muscle mass will have more creatinine in that urine sample, since creatinine is a byproduct of muscle metabolism. Because people with more muscle mass are generally healthier, when you divide your metal concentration by urine creatinine, you get a lower number, which might lead you to believe that lower levels of the metal in the urine are protective. But in fact, what you’re seeing is that higher levels of creatinine are protective. I see this issue all the time and it will always color results of studies like this.
Okay, I am doffing my kidney doctor hat now to show you the results.
The researchers first looked at the relationship between metal concentrations in the urine and performance on cognitive tests. The results were fairly equivocal, save for that digit substitution test which is shown in Figure 4.

Even these results don’t ring major alarm bells for me. What you’re seeing here is the change in scores on the digit substitution test for each 25-percentile increase in urinary metal level — a pretty big change. And yet, you see really minor changes in the performance on the test. The digit substitution test is not an IQ test; but to give you a feeling for the magnitude of this change, if we looked at copper level, moving from the 25th to the 50th percentile would be associated with a loss of nine tenths of an IQ point.
You see two colors on the Figure 4 graph, by the way. That’s because the researchers stratified their findings based on whether the individual carried the ApoE4 gene allele, which is a risk factor for the development of dementia. There are reasons to believe that neurotoxic metals might be worse in this population, and I suppose you do see generally more adverse effects on scores in the red lines compared with the blue lines. But still, we’re not talking about a huge effect size here.
Let’s look at the relationship between these metals and the development of dementia itself, a clearly more important outcome than how well you can replace numeric digits with symbols. I’ll highlight a few of the results that are particularly telling.
First, the nonessential mineral cadmium, which displays the type of relationship we would expect if the metal were neurotoxic: a clear, roughly linear increase in risk for dementia as urinary concentration increases.

We see roughly similar patterns with the nonessential minerals tungsten and uranium, and the essential mineral zinc (beloved of respiratory-virus avoiders everywhere).

But it is very much not what we see for all metals. Strangest of all, look at lead, which shows basically no relationship with dementia.

This concerns me a bit. Earlier, I discussed the issue of measuring stuff in urine and how standardizing levels to the urine creatinine level introduces a bias due to muscle mass. One way around this is to standardize urine levels to some other marker of urine dilution, like osmolality. But more fundamental than that, I like to see positive and negative controls in studies like this. For example, lead strikes me as a good positive control here. If the experimental framework were valid, I would think we’d see a relationship between lead level and dementia.
For a negative control? Well, something we are quite sure is not neurotoxic — something like sulfur, which is relatively ubiquitous, used in a variety of biological processes, and efficiently eliminated. We don’t have that in this study.
The authors close their case by creating a model that combines all the metal levels, asking the question of whether higher levels of metals in the urine in general worsen cognitive scores. And they find that the relationship exists, as you can see in Figure 8, both in carriers and noncarriers of ApoE4. But, to me, this is even more argument for the creatinine problem. If it’s not a specific metal but just the sort of general concentration of all metals, the risk for confounding by muscle mass is even higher.

So should we worry about ingesting metals? I suppose the answer is ... kind of.
I am sure we should be avoiding lead, despite the results of this study. It’s probably best to stay away from uranium too.
As for the essential metals, I’m sure there is some toxic dose; there’s a toxic dose for everything at some point. But I don’t see evidence in this study to make me worry that a significant chunk of the population is anywhere close to that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It has always amazed me that our bodies require these tiny amounts of incredibly rare substances to function. Sure, we need oxygen. We need water. But we also need molybdenum, which makes up just 1.2 parts per million of the Earth’s crust.
Without adequate molybdenum intake, we develop seizures, developmental delays, death. Fortunately, we need so little molybdenum that true molybdenum deficiency is incredibly rare — seen only in people on total parenteral nutrition without supplementation or those with certain rare genetic conditions. But still, molybdenum is necessary for life.
Many metals are. Figure 1 colors the essential minerals on the periodic table. You can see that to stay alive, we humans need not only things like sodium, but selenium, bromine, zinc, copper, and cobalt.

Some metals are very clearly not essential; we can all do without lead and mercury, and probably should.
But just because something is essential for life does not mean that more is better. The dose is the poison, as they say. And this week, we explore whether metals — even essential metals — might be adversely affecting our brains.
It’s not a stretch to think that metal intake could have weird effects on our nervous system. Lead exposure, primarily due to leaded gasoline, has been blamed for an average reduction of about 3 points in our national IQ, for example . But not all metals are created equal. Researchers set out to find out which might be more strongly associated with performance on cognitive tests and dementia, and reported their results in this study in JAMA Network Open.
To do this, they leveraged the MESA cohort study. This is a longitudinal study of a relatively diverse group of 6300 adults who were enrolled from 2000 to 2002 around the United States. At enrollment, they gave a urine sample and took a variety of cognitive tests. Important for this study was the digit symbol substitution test, where participants are provided a code and need to replace a list of numbers with symbols as per that code. Performance on this test worsens with age, depression, and cognitive impairment.
Participants were followed for more than a decade, and over that time, 559 (about 9%) were diagnosed with dementia.
Those baseline urine samples were assayed for a variety of metals — some essential, some very much not, as you can see in Figure 2.

Now, I have to put my kidney doctor hat on for a second and talk about urine measurement ... of anything. The problem with urine is that the concentration can change a lot — by more than 10-fold, in fact — based on how much water you drank recently. Researchers correct for this, and in the case of this study, they do what a lot of researchers do: divide the measured concentration by the urine creatinine level.

This introduces a bit of a problem. Take two people with exactly the same kidney function, who drank exactly the same water, whose urine is exactly the same concentration. The person with more muscle mass will have more creatinine in that urine sample, since creatinine is a byproduct of muscle metabolism. Because people with more muscle mass are generally healthier, when you divide your metal concentration by urine creatinine, you get a lower number, which might lead you to believe that lower levels of the metal in the urine are protective. But in fact, what you’re seeing is that higher levels of creatinine are protective. I see this issue all the time and it will always color results of studies like this.
Okay, I am doffing my kidney doctor hat now to show you the results.
The researchers first looked at the relationship between metal concentrations in the urine and performance on cognitive tests. The results were fairly equivocal, save for that digit substitution test which is shown in Figure 4.

Even these results don’t ring major alarm bells for me. What you’re seeing here is the change in scores on the digit substitution test for each 25-percentile increase in urinary metal level — a pretty big change. And yet, you see really minor changes in the performance on the test. The digit substitution test is not an IQ test; but to give you a feeling for the magnitude of this change, if we looked at copper level, moving from the 25th to the 50th percentile would be associated with a loss of nine tenths of an IQ point.
You see two colors on the Figure 4 graph, by the way. That’s because the researchers stratified their findings based on whether the individual carried the ApoE4 gene allele, which is a risk factor for the development of dementia. There are reasons to believe that neurotoxic metals might be worse in this population, and I suppose you do see generally more adverse effects on scores in the red lines compared with the blue lines. But still, we’re not talking about a huge effect size here.
Let’s look at the relationship between these metals and the development of dementia itself, a clearly more important outcome than how well you can replace numeric digits with symbols. I’ll highlight a few of the results that are particularly telling.
First, the nonessential mineral cadmium, which displays the type of relationship we would expect if the metal were neurotoxic: a clear, roughly linear increase in risk for dementia as urinary concentration increases.

We see roughly similar patterns with the nonessential minerals tungsten and uranium, and the essential mineral zinc (beloved of respiratory-virus avoiders everywhere).

But it is very much not what we see for all metals. Strangest of all, look at lead, which shows basically no relationship with dementia.

This concerns me a bit. Earlier, I discussed the issue of measuring stuff in urine and how standardizing levels to the urine creatinine level introduces a bias due to muscle mass. One way around this is to standardize urine levels to some other marker of urine dilution, like osmolality. But more fundamental than that, I like to see positive and negative controls in studies like this. For example, lead strikes me as a good positive control here. If the experimental framework were valid, I would think we’d see a relationship between lead level and dementia.
For a negative control? Well, something we are quite sure is not neurotoxic — something like sulfur, which is relatively ubiquitous, used in a variety of biological processes, and efficiently eliminated. We don’t have that in this study.
The authors close their case by creating a model that combines all the metal levels, asking the question of whether higher levels of metals in the urine in general worsen cognitive scores. And they find that the relationship exists, as you can see in Figure 8, both in carriers and noncarriers of ApoE4. But, to me, this is even more argument for the creatinine problem. If it’s not a specific metal but just the sort of general concentration of all metals, the risk for confounding by muscle mass is even higher.

So should we worry about ingesting metals? I suppose the answer is ... kind of.
I am sure we should be avoiding lead, despite the results of this study. It’s probably best to stay away from uranium too.
As for the essential metals, I’m sure there is some toxic dose; there’s a toxic dose for everything at some point. But I don’t see evidence in this study to make me worry that a significant chunk of the population is anywhere close to that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Exercise or Inactivity?
The answer one gets often depends on how one crafts the question. For example, Jeffrey D. Johnson PhD, a professor of communications at Portland State University in Oregon has found that if patients are asked “Is there something else you would like to address today?” 80% had their unmet questions addressed. However, if the question was worded “Is there anything else ...?” Very few had their unmet concerns addressed.
I recently encountered two studies that provide another striking example of how differently structured questions aimed at same topic can result in dramatically different results. In this case both studies used one database, the UK Biobank cohort study which contains “de-identified genetic, lifestyle, and health information” collected from a half million adults in the UK. A subgroup of nearly 90,000 who had undergone a week long activity measurement using a wrist accelerometer was the focus of both groups of investigators who asked the same broad question “What is the relationship between physical activity and disease?”
The first study I found has already received some publicity in the lay press and dealt with those individuals who, for a variety of reasons, pack all of their exercise into just a few days, usually the weekend, aka weekend warriors. The investigators found that when compared with generally inactive individuals those who were able to achieve activity volumes that met current guidelines were at lower risk for more than 200 diseases, particularly those that were cardiac based. I guess that shouldn’t surprise us. The finding that has received most of the publicity to date in the lay press was that “Associations were similar whether the activity followed a weekend warrior pattern or was spread out evenly through the week.”
The second study, using the same database, found that those individuals who spent more than 10.6 hours per day sitting had 60% an increased risk of heart failure and cardiovascular related death. And, here’s the real news, that risk remained even in people who were otherwise physically active.
I suspect these two groups of investigators, both associated with Harvard-related institutions, knew of each other’s work and would agree that their findings are not incompatible. However, it is interesting that, when presented with the same database, one group chose to focus its attention on the exercise end of the spectrum while the other looked at the effect of inactivity.
I have always tried to include a “healthy” amount of exercise in my day. However, more recently my professional interest has been drawn to the increasing number of studies I read that deal with the risks of inactivity and sedentarism. For example, just in the last 2 years I have written about a study in children that showed that sedentary time is responsible for 70% of the total increase in cholesterol as children advance into young adulthood. Another study in adults found that every 2-hour increase in sedentary behavior was associated with a 12% decrease in the patient’s likelihood of achieving healthy aging.
If I were asked to place relative values on these two studies, I would say that the study highlighting the risk of prolonged sitting is potentially far more relevant to the population at large, which is for the most part sedentary. Of course, while I have no data to support my contention, I see the weekend warrior population as a niche group.
So what are the take-home messages from these two studies? One is for the weekend warrior. “You can take some comfort in the results that support your exercise schedule but don’t feel too comfortable about it if most of the week you are sitting at a desk.”
For the rest of us — It’s beginning to feel like we should be including accelerometers in our regular diagnostic and therapeutic weaponry. Sending home patients with a Holter cardiac monitor has become commonplace. We should be sending more folks home with accelerometers or asking the more affluent to share the data from their smart watches. “You’ve been bragging about your “steps. Show me your sitting time.”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
The answer one gets often depends on how one crafts the question. For example, Jeffrey D. Johnson PhD, a professor of communications at Portland State University in Oregon has found that if patients are asked “Is there something else you would like to address today?” 80% had their unmet questions addressed. However, if the question was worded “Is there anything else ...?” Very few had their unmet concerns addressed.
I recently encountered two studies that provide another striking example of how differently structured questions aimed at same topic can result in dramatically different results. In this case both studies used one database, the UK Biobank cohort study which contains “de-identified genetic, lifestyle, and health information” collected from a half million adults in the UK. A subgroup of nearly 90,000 who had undergone a week long activity measurement using a wrist accelerometer was the focus of both groups of investigators who asked the same broad question “What is the relationship between physical activity and disease?”
The first study I found has already received some publicity in the lay press and dealt with those individuals who, for a variety of reasons, pack all of their exercise into just a few days, usually the weekend, aka weekend warriors. The investigators found that when compared with generally inactive individuals those who were able to achieve activity volumes that met current guidelines were at lower risk for more than 200 diseases, particularly those that were cardiac based. I guess that shouldn’t surprise us. The finding that has received most of the publicity to date in the lay press was that “Associations were similar whether the activity followed a weekend warrior pattern or was spread out evenly through the week.”
The second study, using the same database, found that those individuals who spent more than 10.6 hours per day sitting had 60% an increased risk of heart failure and cardiovascular related death. And, here’s the real news, that risk remained even in people who were otherwise physically active.
I suspect these two groups of investigators, both associated with Harvard-related institutions, knew of each other’s work and would agree that their findings are not incompatible. However, it is interesting that, when presented with the same database, one group chose to focus its attention on the exercise end of the spectrum while the other looked at the effect of inactivity.
I have always tried to include a “healthy” amount of exercise in my day. However, more recently my professional interest has been drawn to the increasing number of studies I read that deal with the risks of inactivity and sedentarism. For example, just in the last 2 years I have written about a study in children that showed that sedentary time is responsible for 70% of the total increase in cholesterol as children advance into young adulthood. Another study in adults found that every 2-hour increase in sedentary behavior was associated with a 12% decrease in the patient’s likelihood of achieving healthy aging.
If I were asked to place relative values on these two studies, I would say that the study highlighting the risk of prolonged sitting is potentially far more relevant to the population at large, which is for the most part sedentary. Of course, while I have no data to support my contention, I see the weekend warrior population as a niche group.
So what are the take-home messages from these two studies? One is for the weekend warrior. “You can take some comfort in the results that support your exercise schedule but don’t feel too comfortable about it if most of the week you are sitting at a desk.”
For the rest of us — It’s beginning to feel like we should be including accelerometers in our regular diagnostic and therapeutic weaponry. Sending home patients with a Holter cardiac monitor has become commonplace. We should be sending more folks home with accelerometers or asking the more affluent to share the data from their smart watches. “You’ve been bragging about your “steps. Show me your sitting time.”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
The answer one gets often depends on how one crafts the question. For example, Jeffrey D. Johnson PhD, a professor of communications at Portland State University in Oregon has found that if patients are asked “Is there something else you would like to address today?” 80% had their unmet questions addressed. However, if the question was worded “Is there anything else ...?” Very few had their unmet concerns addressed.
I recently encountered two studies that provide another striking example of how differently structured questions aimed at same topic can result in dramatically different results. In this case both studies used one database, the UK Biobank cohort study which contains “de-identified genetic, lifestyle, and health information” collected from a half million adults in the UK. A subgroup of nearly 90,000 who had undergone a week long activity measurement using a wrist accelerometer was the focus of both groups of investigators who asked the same broad question “What is the relationship between physical activity and disease?”
The first study I found has already received some publicity in the lay press and dealt with those individuals who, for a variety of reasons, pack all of their exercise into just a few days, usually the weekend, aka weekend warriors. The investigators found that when compared with generally inactive individuals those who were able to achieve activity volumes that met current guidelines were at lower risk for more than 200 diseases, particularly those that were cardiac based. I guess that shouldn’t surprise us. The finding that has received most of the publicity to date in the lay press was that “Associations were similar whether the activity followed a weekend warrior pattern or was spread out evenly through the week.”
The second study, using the same database, found that those individuals who spent more than 10.6 hours per day sitting had 60% an increased risk of heart failure and cardiovascular related death. And, here’s the real news, that risk remained even in people who were otherwise physically active.
I suspect these two groups of investigators, both associated with Harvard-related institutions, knew of each other’s work and would agree that their findings are not incompatible. However, it is interesting that, when presented with the same database, one group chose to focus its attention on the exercise end of the spectrum while the other looked at the effect of inactivity.
I have always tried to include a “healthy” amount of exercise in my day. However, more recently my professional interest has been drawn to the increasing number of studies I read that deal with the risks of inactivity and sedentarism. For example, just in the last 2 years I have written about a study in children that showed that sedentary time is responsible for 70% of the total increase in cholesterol as children advance into young adulthood. Another study in adults found that every 2-hour increase in sedentary behavior was associated with a 12% decrease in the patient’s likelihood of achieving healthy aging.
If I were asked to place relative values on these two studies, I would say that the study highlighting the risk of prolonged sitting is potentially far more relevant to the population at large, which is for the most part sedentary. Of course, while I have no data to support my contention, I see the weekend warrior population as a niche group.
So what are the take-home messages from these two studies? One is for the weekend warrior. “You can take some comfort in the results that support your exercise schedule but don’t feel too comfortable about it if most of the week you are sitting at a desk.”
For the rest of us — It’s beginning to feel like we should be including accelerometers in our regular diagnostic and therapeutic weaponry. Sending home patients with a Holter cardiac monitor has become commonplace. We should be sending more folks home with accelerometers or asking the more affluent to share the data from their smart watches. “You’ve been bragging about your “steps. Show me your sitting time.”
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
CRC Screening: Right Patient, Right Test, Right Time
It has been three and a half years since the US Preventive Services Task Force (USPSTF) lowered the age to start colorectal cancer (CRC) screening from 50 to 45. As I mentioned in a previous commentary, two major medical groups — the American Academy of Family Physicians and the American College of Physicians — felt that the evidence was insufficient to support this change.
Comparing CRC screening rates in more than 10 million adults aged 45-49 during the 20 months preceding and 20 months following the USPSTF recommendation, researchers found significant increases during the latter time period, with the greatest increases among persons of high socioeconomic status or living in metropolitan areas.
Another study addressed concerns that younger adults may be less likely to follow up on positive screening results or more likely to have false positives on a fecal immunochemical test (FIT). Patients aged 45-49 years were slightly less likely to have a positive FIT result than 50-year-olds, but they had similar rates of colonoscopy completion and similar percentages of abnormal findings on colonoscopy.
Although the sensitivity and specificity of FIT varies quite a bit across different test brands, its overall effectiveness at reducing colorectal cancer deaths is well established. In 2024, the Food and Drug Administration approved three new screening options: a blood-based screening test (Shield), a next-generation multitarget stool DNA test (Cologuard Plus), and a multitarget stool RNA test (ColoSense) with similar performance characteristics as Cologuard Plus. The latter two tests will become available early next year.
This profusion of noninvasive options for CRC screening will challenge those tasked with developing the next iteration of the USPSTF recommendations. Not only must future guidelines establish what evidence threshold is sufficient to recommend a new screening strategy, but they also will need to consider the population-level consequences of relative utilization of different tests. For example, a cost-effectiveness analysis found that more CRC deaths would occur if people who would have otherwise accepted colonoscopy or fecal tests chose to be screened with Shield instead; however, this negative outcome could be offset if for every three of these test substitutions, two other people chose Shield who would otherwise have not been screened at all.
In the meantime, it is important for primary care clinicians to be familiar with evidence-based intervals for CRC screening tests and test eligibility criteria. A troubling study of patients who completed a multitarget stool DNA test in a Midwestern health system in 2021 found that more than one in five had the test ordered inappropriately, based on USPSTF guidelines. Reasons for inappropriate testing included having had a colonoscopy within the past 10 years, a family history of CRC, symptoms suggestive of possible CRC, age younger than 45, and a prior diagnosis of colonic adenomas.
Just as a medication works best when the patient takes it as prescribed, a CRC screening test is most likely to yield more benefit than harm when it’s provided to the right patient at the right time.
Dr. Lin is Associate Director, Family Medicine Residency Program, at Lancaster General Hospital in Pennsylvania. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
It has been three and a half years since the US Preventive Services Task Force (USPSTF) lowered the age to start colorectal cancer (CRC) screening from 50 to 45. As I mentioned in a previous commentary, two major medical groups — the American Academy of Family Physicians and the American College of Physicians — felt that the evidence was insufficient to support this change.
Comparing CRC screening rates in more than 10 million adults aged 45-49 during the 20 months preceding and 20 months following the USPSTF recommendation, researchers found significant increases during the latter time period, with the greatest increases among persons of high socioeconomic status or living in metropolitan areas.
Another study addressed concerns that younger adults may be less likely to follow up on positive screening results or more likely to have false positives on a fecal immunochemical test (FIT). Patients aged 45-49 years were slightly less likely to have a positive FIT result than 50-year-olds, but they had similar rates of colonoscopy completion and similar percentages of abnormal findings on colonoscopy.
Although the sensitivity and specificity of FIT varies quite a bit across different test brands, its overall effectiveness at reducing colorectal cancer deaths is well established. In 2024, the Food and Drug Administration approved three new screening options: a blood-based screening test (Shield), a next-generation multitarget stool DNA test (Cologuard Plus), and a multitarget stool RNA test (ColoSense) with similar performance characteristics as Cologuard Plus. The latter two tests will become available early next year.
This profusion of noninvasive options for CRC screening will challenge those tasked with developing the next iteration of the USPSTF recommendations. Not only must future guidelines establish what evidence threshold is sufficient to recommend a new screening strategy, but they also will need to consider the population-level consequences of relative utilization of different tests. For example, a cost-effectiveness analysis found that more CRC deaths would occur if people who would have otherwise accepted colonoscopy or fecal tests chose to be screened with Shield instead; however, this negative outcome could be offset if for every three of these test substitutions, two other people chose Shield who would otherwise have not been screened at all.
In the meantime, it is important for primary care clinicians to be familiar with evidence-based intervals for CRC screening tests and test eligibility criteria. A troubling study of patients who completed a multitarget stool DNA test in a Midwestern health system in 2021 found that more than one in five had the test ordered inappropriately, based on USPSTF guidelines. Reasons for inappropriate testing included having had a colonoscopy within the past 10 years, a family history of CRC, symptoms suggestive of possible CRC, age younger than 45, and a prior diagnosis of colonic adenomas.
Just as a medication works best when the patient takes it as prescribed, a CRC screening test is most likely to yield more benefit than harm when it’s provided to the right patient at the right time.
Dr. Lin is Associate Director, Family Medicine Residency Program, at Lancaster General Hospital in Pennsylvania. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
It has been three and a half years since the US Preventive Services Task Force (USPSTF) lowered the age to start colorectal cancer (CRC) screening from 50 to 45. As I mentioned in a previous commentary, two major medical groups — the American Academy of Family Physicians and the American College of Physicians — felt that the evidence was insufficient to support this change.
Comparing CRC screening rates in more than 10 million adults aged 45-49 during the 20 months preceding and 20 months following the USPSTF recommendation, researchers found significant increases during the latter time period, with the greatest increases among persons of high socioeconomic status or living in metropolitan areas.
Another study addressed concerns that younger adults may be less likely to follow up on positive screening results or more likely to have false positives on a fecal immunochemical test (FIT). Patients aged 45-49 years were slightly less likely to have a positive FIT result than 50-year-olds, but they had similar rates of colonoscopy completion and similar percentages of abnormal findings on colonoscopy.
Although the sensitivity and specificity of FIT varies quite a bit across different test brands, its overall effectiveness at reducing colorectal cancer deaths is well established. In 2024, the Food and Drug Administration approved three new screening options: a blood-based screening test (Shield), a next-generation multitarget stool DNA test (Cologuard Plus), and a multitarget stool RNA test (ColoSense) with similar performance characteristics as Cologuard Plus. The latter two tests will become available early next year.
This profusion of noninvasive options for CRC screening will challenge those tasked with developing the next iteration of the USPSTF recommendations. Not only must future guidelines establish what evidence threshold is sufficient to recommend a new screening strategy, but they also will need to consider the population-level consequences of relative utilization of different tests. For example, a cost-effectiveness analysis found that more CRC deaths would occur if people who would have otherwise accepted colonoscopy or fecal tests chose to be screened with Shield instead; however, this negative outcome could be offset if for every three of these test substitutions, two other people chose Shield who would otherwise have not been screened at all.
In the meantime, it is important for primary care clinicians to be familiar with evidence-based intervals for CRC screening tests and test eligibility criteria. A troubling study of patients who completed a multitarget stool DNA test in a Midwestern health system in 2021 found that more than one in five had the test ordered inappropriately, based on USPSTF guidelines. Reasons for inappropriate testing included having had a colonoscopy within the past 10 years, a family history of CRC, symptoms suggestive of possible CRC, age younger than 45, and a prior diagnosis of colonic adenomas.
Just as a medication works best when the patient takes it as prescribed, a CRC screening test is most likely to yield more benefit than harm when it’s provided to the right patient at the right time.
Dr. Lin is Associate Director, Family Medicine Residency Program, at Lancaster General Hospital in Pennsylvania. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Six Updates on Stroke Management
This video transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener, from the Faculty of Medicine at the University Duisburg-Essen in Germany. In this video, I would like to cover six publications on stroke, which were published this fall.
The Best Thrombolytic?
Let me start with systemic thrombolysis. We now have two thrombolytic agents available. One is the well-known alteplase, and newly approved for the treatment of stroke is tenecteplase. The ATTEST-2 study in the United Kingdom, published in The Lancet Neurology, compared tenecteplase 0.25 mg/kg body weight as a bolus with alteplase 0.9 mg/kg body weight as an infusion over 60 minutes in the 4.5-hour time window in 1777 patients with ischemic stroke.
There was no significant difference between the two thrombolytics for the primary endpoint of modified Rankin Scale score after 90 days. There was also no difference with respect to mortality, intracranial bleeding, or extracranial bleeding.
We finally have 11 randomized controlled trials that compared tenecteplase and alteplase in acute ischemic stroke. A meta-analysis of these randomized trials was published in Neurology. The analysis included 3700 patients treated with tenecteplase and 3700 patients treated with alteplase. For the primary endpoint, excellent functional outcome defined as modified Rankin Scale score 0-1 after 90 days, there was a significant benefit for tenecteplase (relative risk, 1.05), but the absolute difference was very small, at 3%. There was no difference in mortality or bleeding complications.
In conclusion, I think both substances are great. They are effective. Tenecteplase is most probably the drug which should be used in people who have to transfer from a primary stroke center to a dedicated stroke center that provides thrombectomy. Otherwise, I think it’s a choice of the physician as to which thrombolytic agent to use.
Mobile Stroke Units
A highly debated topic is mobile stroke units. These stroke units have a CT scanner and laboratory on board, and this makes it possible to perform thrombolysis on the way to the hospital. A retrospective, observational study collected data between 2018 and 2023, and included 19,400 patients with acute stroke, of whom 1237, or 6.4%, were treated in a mobile stroke unit. This study was published in JAMA Neurology.
The modified Rankin Scale score at the time of discharge was better in patients treated with a mobile stroke unit, but the absolute benefit was only 0.03 points on the modified Rankin Scale. The question is whether this is cost-effective, and can we really do this at times when there is a traumatic shortage of physicians and nursing staff in the hospital?
DOAC Reversal Agents
Oral anticoagulation, as you know, is usually considered a contraindication for systemic thrombolysis. Idarucizumab, a monoclonal antibody, was developed to reverse the biological activity of dabigatran and then allow systemic thrombolysis.
A recent publication in Neurology analyzed 13 cohort studies with 553 stroke patients on dabigatran who received idarucizumab prior to systemic thrombolysis, and the rate of intracranial hemorrhage was 4%. This means it’s obviously possible to perform thrombolysis when the activity of dabigatran is neutralized by idarucizumab.
Unfortunately, until today, we have no data on whether this can also be done with andexanet alfa in people who are treated with a factor Xa inhibitor like, for example, apixaban, rivaroxaban, or edoxaban.
Anticoagulation in ESUS
My next topic is ESUS, or embolic stroke of undetermined source. We have four large randomized trials and three smaller trials that compared antiplatelet therapy with DOACs in patients with ESUS. A group in Neurology published a meta-analysis of seven randomized controlled studies with, altogether, 14,800 patients with ESUS.
The comparison between antiplatelet therapy and anticoagulants showed no difference for recurrent ischemic stroke, and also not for major subgroups. This means that people with ESUS should receive antiplatelet therapy, most probably aspirin.
Anticoagulation Post–Ischemic Stroke With AF
My final topic is the optimal time to start anticoagulation in people with atrial fibrillation who suffer an ischemic stroke. The OPTIMAS study, published in The Lancet, randomized 3650 patients who were anticoagulated with DOACs early (which means less than 4 days) or delayed (between 7 and 14 days). There was no difference in the primary endpoint, which was recurrent ischemic stroke, intracranial hemorrhage, or systemic embolism at 90 days.
The conclusion is that, in most cases, we can probably initiate anticoagulation in people with ischemic stroke and atrial fibrillation within the first 4 days.
Dear colleagues, this is an exciting time for the stroke field. I presented six new studies that have impact, I think, on the management of patients with ischemic stroke.
Dr. Diener is a professor in the Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen in Germany. He reported conflicts of interest with Abbott, AbbVie, Boehringer Ingelheim, Lundbeck, Novartis, Orion Pharma, Teva, WebMD, and The German Research Council. He also serves on the editorial boards of Cephalalgia, Lancet Neurology, and Drugs.
A version of this article first appeared on Medscape.com.
This video transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener, from the Faculty of Medicine at the University Duisburg-Essen in Germany. In this video, I would like to cover six publications on stroke, which were published this fall.
The Best Thrombolytic?
Let me start with systemic thrombolysis. We now have two thrombolytic agents available. One is the well-known alteplase, and newly approved for the treatment of stroke is tenecteplase. The ATTEST-2 study in the United Kingdom, published in The Lancet Neurology, compared tenecteplase 0.25 mg/kg body weight as a bolus with alteplase 0.9 mg/kg body weight as an infusion over 60 minutes in the 4.5-hour time window in 1777 patients with ischemic stroke.
There was no significant difference between the two thrombolytics for the primary endpoint of modified Rankin Scale score after 90 days. There was also no difference with respect to mortality, intracranial bleeding, or extracranial bleeding.
We finally have 11 randomized controlled trials that compared tenecteplase and alteplase in acute ischemic stroke. A meta-analysis of these randomized trials was published in Neurology. The analysis included 3700 patients treated with tenecteplase and 3700 patients treated with alteplase. For the primary endpoint, excellent functional outcome defined as modified Rankin Scale score 0-1 after 90 days, there was a significant benefit for tenecteplase (relative risk, 1.05), but the absolute difference was very small, at 3%. There was no difference in mortality or bleeding complications.
In conclusion, I think both substances are great. They are effective. Tenecteplase is most probably the drug which should be used in people who have to transfer from a primary stroke center to a dedicated stroke center that provides thrombectomy. Otherwise, I think it’s a choice of the physician as to which thrombolytic agent to use.
Mobile Stroke Units
A highly debated topic is mobile stroke units. These stroke units have a CT scanner and laboratory on board, and this makes it possible to perform thrombolysis on the way to the hospital. A retrospective, observational study collected data between 2018 and 2023, and included 19,400 patients with acute stroke, of whom 1237, or 6.4%, were treated in a mobile stroke unit. This study was published in JAMA Neurology.
The modified Rankin Scale score at the time of discharge was better in patients treated with a mobile stroke unit, but the absolute benefit was only 0.03 points on the modified Rankin Scale. The question is whether this is cost-effective, and can we really do this at times when there is a traumatic shortage of physicians and nursing staff in the hospital?
DOAC Reversal Agents
Oral anticoagulation, as you know, is usually considered a contraindication for systemic thrombolysis. Idarucizumab, a monoclonal antibody, was developed to reverse the biological activity of dabigatran and then allow systemic thrombolysis.
A recent publication in Neurology analyzed 13 cohort studies with 553 stroke patients on dabigatran who received idarucizumab prior to systemic thrombolysis, and the rate of intracranial hemorrhage was 4%. This means it’s obviously possible to perform thrombolysis when the activity of dabigatran is neutralized by idarucizumab.
Unfortunately, until today, we have no data on whether this can also be done with andexanet alfa in people who are treated with a factor Xa inhibitor like, for example, apixaban, rivaroxaban, or edoxaban.
Anticoagulation in ESUS
My next topic is ESUS, or embolic stroke of undetermined source. We have four large randomized trials and three smaller trials that compared antiplatelet therapy with DOACs in patients with ESUS. A group in Neurology published a meta-analysis of seven randomized controlled studies with, altogether, 14,800 patients with ESUS.
The comparison between antiplatelet therapy and anticoagulants showed no difference for recurrent ischemic stroke, and also not for major subgroups. This means that people with ESUS should receive antiplatelet therapy, most probably aspirin.
Anticoagulation Post–Ischemic Stroke With AF
My final topic is the optimal time to start anticoagulation in people with atrial fibrillation who suffer an ischemic stroke. The OPTIMAS study, published in The Lancet, randomized 3650 patients who were anticoagulated with DOACs early (which means less than 4 days) or delayed (between 7 and 14 days). There was no difference in the primary endpoint, which was recurrent ischemic stroke, intracranial hemorrhage, or systemic embolism at 90 days.
The conclusion is that, in most cases, we can probably initiate anticoagulation in people with ischemic stroke and atrial fibrillation within the first 4 days.
Dear colleagues, this is an exciting time for the stroke field. I presented six new studies that have impact, I think, on the management of patients with ischemic stroke.
Dr. Diener is a professor in the Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen in Germany. He reported conflicts of interest with Abbott, AbbVie, Boehringer Ingelheim, Lundbeck, Novartis, Orion Pharma, Teva, WebMD, and The German Research Council. He also serves on the editorial boards of Cephalalgia, Lancet Neurology, and Drugs.
A version of this article first appeared on Medscape.com.
This video transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener, from the Faculty of Medicine at the University Duisburg-Essen in Germany. In this video, I would like to cover six publications on stroke, which were published this fall.
The Best Thrombolytic?
Let me start with systemic thrombolysis. We now have two thrombolytic agents available. One is the well-known alteplase, and newly approved for the treatment of stroke is tenecteplase. The ATTEST-2 study in the United Kingdom, published in The Lancet Neurology, compared tenecteplase 0.25 mg/kg body weight as a bolus with alteplase 0.9 mg/kg body weight as an infusion over 60 minutes in the 4.5-hour time window in 1777 patients with ischemic stroke.
There was no significant difference between the two thrombolytics for the primary endpoint of modified Rankin Scale score after 90 days. There was also no difference with respect to mortality, intracranial bleeding, or extracranial bleeding.
We finally have 11 randomized controlled trials that compared tenecteplase and alteplase in acute ischemic stroke. A meta-analysis of these randomized trials was published in Neurology. The analysis included 3700 patients treated with tenecteplase and 3700 patients treated with alteplase. For the primary endpoint, excellent functional outcome defined as modified Rankin Scale score 0-1 after 90 days, there was a significant benefit for tenecteplase (relative risk, 1.05), but the absolute difference was very small, at 3%. There was no difference in mortality or bleeding complications.
In conclusion, I think both substances are great. They are effective. Tenecteplase is most probably the drug which should be used in people who have to transfer from a primary stroke center to a dedicated stroke center that provides thrombectomy. Otherwise, I think it’s a choice of the physician as to which thrombolytic agent to use.
Mobile Stroke Units
A highly debated topic is mobile stroke units. These stroke units have a CT scanner and laboratory on board, and this makes it possible to perform thrombolysis on the way to the hospital. A retrospective, observational study collected data between 2018 and 2023, and included 19,400 patients with acute stroke, of whom 1237, or 6.4%, were treated in a mobile stroke unit. This study was published in JAMA Neurology.
The modified Rankin Scale score at the time of discharge was better in patients treated with a mobile stroke unit, but the absolute benefit was only 0.03 points on the modified Rankin Scale. The question is whether this is cost-effective, and can we really do this at times when there is a traumatic shortage of physicians and nursing staff in the hospital?
DOAC Reversal Agents
Oral anticoagulation, as you know, is usually considered a contraindication for systemic thrombolysis. Idarucizumab, a monoclonal antibody, was developed to reverse the biological activity of dabigatran and then allow systemic thrombolysis.
A recent publication in Neurology analyzed 13 cohort studies with 553 stroke patients on dabigatran who received idarucizumab prior to systemic thrombolysis, and the rate of intracranial hemorrhage was 4%. This means it’s obviously possible to perform thrombolysis when the activity of dabigatran is neutralized by idarucizumab.
Unfortunately, until today, we have no data on whether this can also be done with andexanet alfa in people who are treated with a factor Xa inhibitor like, for example, apixaban, rivaroxaban, or edoxaban.
Anticoagulation in ESUS
My next topic is ESUS, or embolic stroke of undetermined source. We have four large randomized trials and three smaller trials that compared antiplatelet therapy with DOACs in patients with ESUS. A group in Neurology published a meta-analysis of seven randomized controlled studies with, altogether, 14,800 patients with ESUS.
The comparison between antiplatelet therapy and anticoagulants showed no difference for recurrent ischemic stroke, and also not for major subgroups. This means that people with ESUS should receive antiplatelet therapy, most probably aspirin.
Anticoagulation Post–Ischemic Stroke With AF
My final topic is the optimal time to start anticoagulation in people with atrial fibrillation who suffer an ischemic stroke. The OPTIMAS study, published in The Lancet, randomized 3650 patients who were anticoagulated with DOACs early (which means less than 4 days) or delayed (between 7 and 14 days). There was no difference in the primary endpoint, which was recurrent ischemic stroke, intracranial hemorrhage, or systemic embolism at 90 days.
The conclusion is that, in most cases, we can probably initiate anticoagulation in people with ischemic stroke and atrial fibrillation within the first 4 days.
Dear colleagues, this is an exciting time for the stroke field. I presented six new studies that have impact, I think, on the management of patients with ischemic stroke.
Dr. Diener is a professor in the Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen in Germany. He reported conflicts of interest with Abbott, AbbVie, Boehringer Ingelheim, Lundbeck, Novartis, Orion Pharma, Teva, WebMD, and The German Research Council. He also serves on the editorial boards of Cephalalgia, Lancet Neurology, and Drugs.
A version of this article first appeared on Medscape.com.

