User login
Tight Glucose Control for Renal Protection Challenged
Intensive glucose control had no apparent impact on renal outcomes in type 2 diabetes patients compared to conventional glucose control, according to meta-analysis findings published online in the May 28 Archives of Internal Medicine.
The American Diabetes Association and other expert panels recommend intensive control – defined as a target hemoglobin A1c of less than 7% – to help prevent renal disease and other microvascular complications in type 2 diabetes patients, but the impact of intensive control on clinical renal end points remains unclear, said Dr. Steven G. Coca, of Yale University, New Haven, Conn., and colleagues.
The investigators reviewed data from seven trials involving 28,065 adults with type 2 diabetes. Patient follow-up ranged from 2 to 15 years.
Compared with conventional control, intensive glucose control was associated with a reduced risk of microalbuminuria (risk ratio, 0.86) and macroalbuminuria (RR, 0.74).
However, there was no association with a reduced risk of several renal end points, including no doubling of the serum creatinine level (1.06), end-stage renal disease (0.69), or death from renal disease (0.99).
The mean serum creatinine levels at baseline ranged from 0.9 mg/dL to 1.0 mg/dL, and the mean duration of type 2 diabetes ranged from 7 to 12 years (Arch. Intern. Med. 2012:172:761-69).
An additional meta-regression analysis supported the overall findings. In each study, differences in HbA1c between intensive and conventional control were associated with reduced risk of microalbuminuria and macroalbuminuria, but not with improved renal outcomes.
The results were limited by substantially incomplete outcomes data in several studies, the researchers noted. Many factors may contribute to the failure of tight glycemic control to improve renal outcomes. Possible factors include starting intensive glycemic control too late, failing to maintain intensive control long enough, failing to lower HbA1c levels to normal, and reaching an HbA1c level beyond which there is no additional benefit to the patient. Some of the studies also may have been underpowered to detect a significant difference in renal outcomes.
Despite these limitations, however, the researchers concluded that the current evidence fails to support tight control. "There is little compelling reason to initiate intensive glycemic control in midstage of the disease with the aim of preventing renal failure," they wrote.
Dr. Coca and the editorialists reported having no financial conflicts of interest. Study coauthor Dr. Harlan M. Krumholz is the chair of a scientific advisory board for United Healthcare.
Most of the trials included in the meta-analysis were too short to draw conclusions about the impact of intensive glucose control on renal disease, according to Dr. David M. Nathan.
"Although implementing intensive therapy is difficult and imposes burden and expense, all of the primary data continue to support its long-term benefit," he asserted.
Dr. Karen L. Margolis and Dr. Patrick J. O’Connor, however, said that the study findings underscore the need for clinicians and patients to weigh the risks and benefits of the treatment regimens needed for intensive glucose control.
"We conclude that for many patients with T2DM, the potential benefits of multidrug intensive glucose control regimens, which are only marginally supported by current evidence, must be weighed against the potential risks of such therapy (including hypoglycemia and possible increased mortality risks) as well as the potentially larger benefits of focusing clinical attention on other domains, such as blood pressure lowering, lipid control, and smoking cessation," they wrote.
Dr. Nathan is director of the General Clinical Research Center and of the Diabetes Center at Massachusetts General Hospital in Boston. His remarks were summarized from an editorial that accompanied the study (Arch. Intern. Med. 2012:172:769-70). Dr. Margolis and Dr. O’Connor are from HealthPartners Research Foundation, Minneapolis, and their remarks were summarized from another editorial (Arch. Intern. Med. 2012:172:770-2). None of the editorialists reported having conflicts of interest.
Most of the trials included in the meta-analysis were too short to draw conclusions about the impact of intensive glucose control on renal disease, according to Dr. David M. Nathan.
"Although implementing intensive therapy is difficult and imposes burden and expense, all of the primary data continue to support its long-term benefit," he asserted.
Dr. Karen L. Margolis and Dr. Patrick J. O’Connor, however, said that the study findings underscore the need for clinicians and patients to weigh the risks and benefits of the treatment regimens needed for intensive glucose control.
"We conclude that for many patients with T2DM, the potential benefits of multidrug intensive glucose control regimens, which are only marginally supported by current evidence, must be weighed against the potential risks of such therapy (including hypoglycemia and possible increased mortality risks) as well as the potentially larger benefits of focusing clinical attention on other domains, such as blood pressure lowering, lipid control, and smoking cessation," they wrote.
Dr. Nathan is director of the General Clinical Research Center and of the Diabetes Center at Massachusetts General Hospital in Boston. His remarks were summarized from an editorial that accompanied the study (Arch. Intern. Med. 2012:172:769-70). Dr. Margolis and Dr. O’Connor are from HealthPartners Research Foundation, Minneapolis, and their remarks were summarized from another editorial (Arch. Intern. Med. 2012:172:770-2). None of the editorialists reported having conflicts of interest.
Most of the trials included in the meta-analysis were too short to draw conclusions about the impact of intensive glucose control on renal disease, according to Dr. David M. Nathan.
"Although implementing intensive therapy is difficult and imposes burden and expense, all of the primary data continue to support its long-term benefit," he asserted.
Dr. Karen L. Margolis and Dr. Patrick J. O’Connor, however, said that the study findings underscore the need for clinicians and patients to weigh the risks and benefits of the treatment regimens needed for intensive glucose control.
"We conclude that for many patients with T2DM, the potential benefits of multidrug intensive glucose control regimens, which are only marginally supported by current evidence, must be weighed against the potential risks of such therapy (including hypoglycemia and possible increased mortality risks) as well as the potentially larger benefits of focusing clinical attention on other domains, such as blood pressure lowering, lipid control, and smoking cessation," they wrote.
Dr. Nathan is director of the General Clinical Research Center and of the Diabetes Center at Massachusetts General Hospital in Boston. His remarks were summarized from an editorial that accompanied the study (Arch. Intern. Med. 2012:172:769-70). Dr. Margolis and Dr. O’Connor are from HealthPartners Research Foundation, Minneapolis, and their remarks were summarized from another editorial (Arch. Intern. Med. 2012:172:770-2). None of the editorialists reported having conflicts of interest.
Intensive glucose control had no apparent impact on renal outcomes in type 2 diabetes patients compared to conventional glucose control, according to meta-analysis findings published online in the May 28 Archives of Internal Medicine.
The American Diabetes Association and other expert panels recommend intensive control – defined as a target hemoglobin A1c of less than 7% – to help prevent renal disease and other microvascular complications in type 2 diabetes patients, but the impact of intensive control on clinical renal end points remains unclear, said Dr. Steven G. Coca, of Yale University, New Haven, Conn., and colleagues.
The investigators reviewed data from seven trials involving 28,065 adults with type 2 diabetes. Patient follow-up ranged from 2 to 15 years.
Compared with conventional control, intensive glucose control was associated with a reduced risk of microalbuminuria (risk ratio, 0.86) and macroalbuminuria (RR, 0.74).
However, there was no association with a reduced risk of several renal end points, including no doubling of the serum creatinine level (1.06), end-stage renal disease (0.69), or death from renal disease (0.99).
The mean serum creatinine levels at baseline ranged from 0.9 mg/dL to 1.0 mg/dL, and the mean duration of type 2 diabetes ranged from 7 to 12 years (Arch. Intern. Med. 2012:172:761-69).
An additional meta-regression analysis supported the overall findings. In each study, differences in HbA1c between intensive and conventional control were associated with reduced risk of microalbuminuria and macroalbuminuria, but not with improved renal outcomes.
The results were limited by substantially incomplete outcomes data in several studies, the researchers noted. Many factors may contribute to the failure of tight glycemic control to improve renal outcomes. Possible factors include starting intensive glycemic control too late, failing to maintain intensive control long enough, failing to lower HbA1c levels to normal, and reaching an HbA1c level beyond which there is no additional benefit to the patient. Some of the studies also may have been underpowered to detect a significant difference in renal outcomes.
Despite these limitations, however, the researchers concluded that the current evidence fails to support tight control. "There is little compelling reason to initiate intensive glycemic control in midstage of the disease with the aim of preventing renal failure," they wrote.
Dr. Coca and the editorialists reported having no financial conflicts of interest. Study coauthor Dr. Harlan M. Krumholz is the chair of a scientific advisory board for United Healthcare.
Intensive glucose control had no apparent impact on renal outcomes in type 2 diabetes patients compared to conventional glucose control, according to meta-analysis findings published online in the May 28 Archives of Internal Medicine.
The American Diabetes Association and other expert panels recommend intensive control – defined as a target hemoglobin A1c of less than 7% – to help prevent renal disease and other microvascular complications in type 2 diabetes patients, but the impact of intensive control on clinical renal end points remains unclear, said Dr. Steven G. Coca, of Yale University, New Haven, Conn., and colleagues.
The investigators reviewed data from seven trials involving 28,065 adults with type 2 diabetes. Patient follow-up ranged from 2 to 15 years.
Compared with conventional control, intensive glucose control was associated with a reduced risk of microalbuminuria (risk ratio, 0.86) and macroalbuminuria (RR, 0.74).
However, there was no association with a reduced risk of several renal end points, including no doubling of the serum creatinine level (1.06), end-stage renal disease (0.69), or death from renal disease (0.99).
The mean serum creatinine levels at baseline ranged from 0.9 mg/dL to 1.0 mg/dL, and the mean duration of type 2 diabetes ranged from 7 to 12 years (Arch. Intern. Med. 2012:172:761-69).
An additional meta-regression analysis supported the overall findings. In each study, differences in HbA1c between intensive and conventional control were associated with reduced risk of microalbuminuria and macroalbuminuria, but not with improved renal outcomes.
The results were limited by substantially incomplete outcomes data in several studies, the researchers noted. Many factors may contribute to the failure of tight glycemic control to improve renal outcomes. Possible factors include starting intensive glycemic control too late, failing to maintain intensive control long enough, failing to lower HbA1c levels to normal, and reaching an HbA1c level beyond which there is no additional benefit to the patient. Some of the studies also may have been underpowered to detect a significant difference in renal outcomes.
Despite these limitations, however, the researchers concluded that the current evidence fails to support tight control. "There is little compelling reason to initiate intensive glycemic control in midstage of the disease with the aim of preventing renal failure," they wrote.
Dr. Coca and the editorialists reported having no financial conflicts of interest. Study coauthor Dr. Harlan M. Krumholz is the chair of a scientific advisory board for United Healthcare.
FROM THE ARCHIVES OF INTERNAL MEDICINE
Major Finding: Intensive glucose control was associated with reduced risk of microalbuminuria (risk ratio, 0.86) and macroalbuminuria (RR, 0.74), but not with a reduction in renal disease.
Data Source: The data come from a meta-analysis of seven randomized trials involving 28,065 adult patients with type 2 diabetes.
Disclosures: Dr. Coca reported having no financial conflicts of interest. Study coauthor Dr. Harlan M. Krumholz is the chair of a scientific advisory board for United Healthcare.
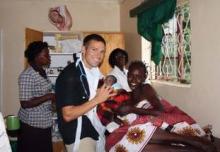
Connecting With Patients in Kenya
Dr. Andy Baldwin’s first visit to Kenya was for an endurance event – an ultramarathon that served as a fundraiser for vulnerable children in Kenya, Ethiopia, and South Africa. He chose to return last fall for a different type of endurance event: A month of medical practice and teaching as part of his family practice residency.
"Being in the Navy, I have traveled all over the world and have really come to love global health," he said. "The impact you can have is so rewarding." When he knew he would have an elective month as part of his residency, Dr. Baldwin did some research and learned of Chepaiywa Health Center in Kipkaren, Kenya, which is supported by Empowering Lives International.
Describe health care in this part of Kenya.
The clinic is located outside Eldoret, Kenya, and there are few other options for medical care in that area. There is a high infant mortality rate, and there are many problems associated with HIV/AIDS. They especially need doctors who can help educate nurses and laypeople about neonatal resuscitation; many babies are born at home, which means in mud huts, and they are not getting good postnatal care.
What resources are available in the clinic?
The health center, which they call a dispensary, started by meeting very basic health needs and providing medications. In recent years, with volunteers and donations, it has gone from one room to four. But it is still a very difficult experience. Many of the medications are expired, for example. They now have a makeshift delivery table and a portable ultrasound machine, but there is no continuous external fetal heart rate monitor, and sometimes it’s hard just to find gloves to wear to help deliver babies.
I also became very familiar with low-tech laboratory testing. They had a lab but no centrifuge, so the patient would have to wait all day for gravity to do its job and separate the plasma from the blood samples in the test tubes.
What types of conditions did you tend to treat?
We were running tests for typhoid fever, not the salmonella that doctors see in the United States, and also brucellosis. Every patient was tested for malaria, and we would look at it under the microscope. I would say that approximately 50% of the people there had malaria; it is almost as common as the flu is in the United States. There was medicine available, and most people who were treated recovered fairly quickly. People who are naive are at increased risk, so I made sure to take my malaria prophylaxis medication.
What were some memorable cases?
There was one case of a woman with a massive bowel obstruction. She came in and was in a lot of pain and I couldn’t diagnose what she had. I had to use my basic clinical skills because there was no imaging and our medications were limited. We kept her hydrated and finally we were able to send her elsewhere for higher-level care.
There was a particularly sad case involving a young girl with bacterial meningitis who died because we simply didn’t have the resources to treat her and we couldn’t get her to another facility in time. And there was an HIV-positive patient with a very low blood count and severe anemia who died because we didn’t have any blood to give him. And in some cases, people were unable to receive care because they didn’t have the money to pay for it. In many ways the limited resources made me furious, but it also made me realize the things we take for granted in medical practice in the United States.
What type of training were you able to share with local health care providers?
Probably the most important part of my trip was the teaching, because that will endure after I leave. I saw a lot of patients, but that is of finite benefit. If you teach someone, they can teach others. I taught a lot of neonatal resuscitation and perinatal care, including the importance of suction and clearing the airway, and performing positive pressure ventilation if the baby isn’t breathing. Ideally, the people I taught can teach midwives and other caregivers, and I have heard that they are already seeing the benefits from this training in terms of reduced infant mortality.
I also tried to explain the importance of cooking outside rather than inside, which is the main cause of chronic obstructive pulmonary disease there, and I talked about the importance of using bed nets to help control malaria. We had a health fair, and people really appreciated that. We also did a community outreach program distributing eyeglasses from a van. We also brought a dentist who was able to remove abscessed teeth. It was so rewarding to be able to provide that kind of relief. Giving simple things like the ability to see or to take a bit of the pain away is one of the things I enjoy most about medicine.
What were some other differences in medical care in this part of Kenya compared with the United States?
There were no real expectations from the patient’s standpoint. In the United States, patients expect doctors to treat and cure them, but in Kenya there is no malpractice insurance or litigation. Whatever you can do to help, you do. Doctors are seen more as the source of medications.
Also, a lot of the health care we provide in the United States, especially in internal medicine and family medicine, has to do with the treatment of chronic disease. We do a lot of screening and prevention, but also a lot of tertiary prevention for things like diabetes, hypertension, or heart disease. Those chronic conditions don’t really exist in Kenya, because the food they eat is completely natural, and they live active lives out of necessity. They walk or run everywhere they need to go. So if they don’t develop cancer or an infection, they tend to lead very long, healthy lives.
There’s such a lack of health care there, but I think we could learn a lot from the Kenyans in terms of the simplicity of their way of life.
What can you take from your experience in Kenya that you will apply to your medical practice in the future?
As a Navy physician, my main priority for my patients is to help them maintain their physical health and readiness to accomplish the mission.
It’s all about keeping the active duty persons and their families healthy, so physical fitness and nutrition are top priorities, and I want to continue to stress making those things part of my patients’ way of life. We should try to live more like the Kenyans in terms of eating more unprocessed, natural food and being active and exercising every day.
I also want to continue to focus on helping my patients decrease stress to improve their health. I was never as stress free as when I was in Kenya, and there were no cell phones, no TVs, none of the distractions, and you are living in the moment and surrounded by nature.
All the high-tech things that we have in the United States keep our cortisol levels sky high all the time, and that’s not good for overall health.
My experience in Kenya also reinforced for me that I want to have a wellness-centered practice, with a holistic approach that includes physical trainers and nutritionists who provide good care and education about how to live the best life you can and be as healthy as you can be.
Working overseas can give physicians a perspective that you cannot get any other way. I think you become a much better doctor. You appreciate how good we have it in some ways, but also what we can learn from people who have less.
Think globally. Practice locally.
U.S.-trained internists who have practiced abroad will receive a $100 stipend for contributing to this column.
Dr. Andy Baldwin’s first visit to Kenya was for an endurance event – an ultramarathon that served as a fundraiser for vulnerable children in Kenya, Ethiopia, and South Africa. He chose to return last fall for a different type of endurance event: A month of medical practice and teaching as part of his family practice residency.
"Being in the Navy, I have traveled all over the world and have really come to love global health," he said. "The impact you can have is so rewarding." When he knew he would have an elective month as part of his residency, Dr. Baldwin did some research and learned of Chepaiywa Health Center in Kipkaren, Kenya, which is supported by Empowering Lives International.
Describe health care in this part of Kenya.
The clinic is located outside Eldoret, Kenya, and there are few other options for medical care in that area. There is a high infant mortality rate, and there are many problems associated with HIV/AIDS. They especially need doctors who can help educate nurses and laypeople about neonatal resuscitation; many babies are born at home, which means in mud huts, and they are not getting good postnatal care.
What resources are available in the clinic?
The health center, which they call a dispensary, started by meeting very basic health needs and providing medications. In recent years, with volunteers and donations, it has gone from one room to four. But it is still a very difficult experience. Many of the medications are expired, for example. They now have a makeshift delivery table and a portable ultrasound machine, but there is no continuous external fetal heart rate monitor, and sometimes it’s hard just to find gloves to wear to help deliver babies.
I also became very familiar with low-tech laboratory testing. They had a lab but no centrifuge, so the patient would have to wait all day for gravity to do its job and separate the plasma from the blood samples in the test tubes.
What types of conditions did you tend to treat?
We were running tests for typhoid fever, not the salmonella that doctors see in the United States, and also brucellosis. Every patient was tested for malaria, and we would look at it under the microscope. I would say that approximately 50% of the people there had malaria; it is almost as common as the flu is in the United States. There was medicine available, and most people who were treated recovered fairly quickly. People who are naive are at increased risk, so I made sure to take my malaria prophylaxis medication.
What were some memorable cases?
There was one case of a woman with a massive bowel obstruction. She came in and was in a lot of pain and I couldn’t diagnose what she had. I had to use my basic clinical skills because there was no imaging and our medications were limited. We kept her hydrated and finally we were able to send her elsewhere for higher-level care.
There was a particularly sad case involving a young girl with bacterial meningitis who died because we simply didn’t have the resources to treat her and we couldn’t get her to another facility in time. And there was an HIV-positive patient with a very low blood count and severe anemia who died because we didn’t have any blood to give him. And in some cases, people were unable to receive care because they didn’t have the money to pay for it. In many ways the limited resources made me furious, but it also made me realize the things we take for granted in medical practice in the United States.
What type of training were you able to share with local health care providers?
Probably the most important part of my trip was the teaching, because that will endure after I leave. I saw a lot of patients, but that is of finite benefit. If you teach someone, they can teach others. I taught a lot of neonatal resuscitation and perinatal care, including the importance of suction and clearing the airway, and performing positive pressure ventilation if the baby isn’t breathing. Ideally, the people I taught can teach midwives and other caregivers, and I have heard that they are already seeing the benefits from this training in terms of reduced infant mortality.
I also tried to explain the importance of cooking outside rather than inside, which is the main cause of chronic obstructive pulmonary disease there, and I talked about the importance of using bed nets to help control malaria. We had a health fair, and people really appreciated that. We also did a community outreach program distributing eyeglasses from a van. We also brought a dentist who was able to remove abscessed teeth. It was so rewarding to be able to provide that kind of relief. Giving simple things like the ability to see or to take a bit of the pain away is one of the things I enjoy most about medicine.
What were some other differences in medical care in this part of Kenya compared with the United States?
There were no real expectations from the patient’s standpoint. In the United States, patients expect doctors to treat and cure them, but in Kenya there is no malpractice insurance or litigation. Whatever you can do to help, you do. Doctors are seen more as the source of medications.
Also, a lot of the health care we provide in the United States, especially in internal medicine and family medicine, has to do with the treatment of chronic disease. We do a lot of screening and prevention, but also a lot of tertiary prevention for things like diabetes, hypertension, or heart disease. Those chronic conditions don’t really exist in Kenya, because the food they eat is completely natural, and they live active lives out of necessity. They walk or run everywhere they need to go. So if they don’t develop cancer or an infection, they tend to lead very long, healthy lives.
There’s such a lack of health care there, but I think we could learn a lot from the Kenyans in terms of the simplicity of their way of life.
What can you take from your experience in Kenya that you will apply to your medical practice in the future?
As a Navy physician, my main priority for my patients is to help them maintain their physical health and readiness to accomplish the mission.
It’s all about keeping the active duty persons and their families healthy, so physical fitness and nutrition are top priorities, and I want to continue to stress making those things part of my patients’ way of life. We should try to live more like the Kenyans in terms of eating more unprocessed, natural food and being active and exercising every day.
I also want to continue to focus on helping my patients decrease stress to improve their health. I was never as stress free as when I was in Kenya, and there were no cell phones, no TVs, none of the distractions, and you are living in the moment and surrounded by nature.
All the high-tech things that we have in the United States keep our cortisol levels sky high all the time, and that’s not good for overall health.
My experience in Kenya also reinforced for me that I want to have a wellness-centered practice, with a holistic approach that includes physical trainers and nutritionists who provide good care and education about how to live the best life you can and be as healthy as you can be.
Working overseas can give physicians a perspective that you cannot get any other way. I think you become a much better doctor. You appreciate how good we have it in some ways, but also what we can learn from people who have less.
Think globally. Practice locally.
U.S.-trained internists who have practiced abroad will receive a $100 stipend for contributing to this column.
Dr. Andy Baldwin’s first visit to Kenya was for an endurance event – an ultramarathon that served as a fundraiser for vulnerable children in Kenya, Ethiopia, and South Africa. He chose to return last fall for a different type of endurance event: A month of medical practice and teaching as part of his family practice residency.
"Being in the Navy, I have traveled all over the world and have really come to love global health," he said. "The impact you can have is so rewarding." When he knew he would have an elective month as part of his residency, Dr. Baldwin did some research and learned of Chepaiywa Health Center in Kipkaren, Kenya, which is supported by Empowering Lives International.
Describe health care in this part of Kenya.
The clinic is located outside Eldoret, Kenya, and there are few other options for medical care in that area. There is a high infant mortality rate, and there are many problems associated with HIV/AIDS. They especially need doctors who can help educate nurses and laypeople about neonatal resuscitation; many babies are born at home, which means in mud huts, and they are not getting good postnatal care.
What resources are available in the clinic?
The health center, which they call a dispensary, started by meeting very basic health needs and providing medications. In recent years, with volunteers and donations, it has gone from one room to four. But it is still a very difficult experience. Many of the medications are expired, for example. They now have a makeshift delivery table and a portable ultrasound machine, but there is no continuous external fetal heart rate monitor, and sometimes it’s hard just to find gloves to wear to help deliver babies.
I also became very familiar with low-tech laboratory testing. They had a lab but no centrifuge, so the patient would have to wait all day for gravity to do its job and separate the plasma from the blood samples in the test tubes.
What types of conditions did you tend to treat?
We were running tests for typhoid fever, not the salmonella that doctors see in the United States, and also brucellosis. Every patient was tested for malaria, and we would look at it under the microscope. I would say that approximately 50% of the people there had malaria; it is almost as common as the flu is in the United States. There was medicine available, and most people who were treated recovered fairly quickly. People who are naive are at increased risk, so I made sure to take my malaria prophylaxis medication.
What were some memorable cases?
There was one case of a woman with a massive bowel obstruction. She came in and was in a lot of pain and I couldn’t diagnose what she had. I had to use my basic clinical skills because there was no imaging and our medications were limited. We kept her hydrated and finally we were able to send her elsewhere for higher-level care.
There was a particularly sad case involving a young girl with bacterial meningitis who died because we simply didn’t have the resources to treat her and we couldn’t get her to another facility in time. And there was an HIV-positive patient with a very low blood count and severe anemia who died because we didn’t have any blood to give him. And in some cases, people were unable to receive care because they didn’t have the money to pay for it. In many ways the limited resources made me furious, but it also made me realize the things we take for granted in medical practice in the United States.
What type of training were you able to share with local health care providers?
Probably the most important part of my trip was the teaching, because that will endure after I leave. I saw a lot of patients, but that is of finite benefit. If you teach someone, they can teach others. I taught a lot of neonatal resuscitation and perinatal care, including the importance of suction and clearing the airway, and performing positive pressure ventilation if the baby isn’t breathing. Ideally, the people I taught can teach midwives and other caregivers, and I have heard that they are already seeing the benefits from this training in terms of reduced infant mortality.
I also tried to explain the importance of cooking outside rather than inside, which is the main cause of chronic obstructive pulmonary disease there, and I talked about the importance of using bed nets to help control malaria. We had a health fair, and people really appreciated that. We also did a community outreach program distributing eyeglasses from a van. We also brought a dentist who was able to remove abscessed teeth. It was so rewarding to be able to provide that kind of relief. Giving simple things like the ability to see or to take a bit of the pain away is one of the things I enjoy most about medicine.
What were some other differences in medical care in this part of Kenya compared with the United States?
There were no real expectations from the patient’s standpoint. In the United States, patients expect doctors to treat and cure them, but in Kenya there is no malpractice insurance or litigation. Whatever you can do to help, you do. Doctors are seen more as the source of medications.
Also, a lot of the health care we provide in the United States, especially in internal medicine and family medicine, has to do with the treatment of chronic disease. We do a lot of screening and prevention, but also a lot of tertiary prevention for things like diabetes, hypertension, or heart disease. Those chronic conditions don’t really exist in Kenya, because the food they eat is completely natural, and they live active lives out of necessity. They walk or run everywhere they need to go. So if they don’t develop cancer or an infection, they tend to lead very long, healthy lives.
There’s such a lack of health care there, but I think we could learn a lot from the Kenyans in terms of the simplicity of their way of life.
What can you take from your experience in Kenya that you will apply to your medical practice in the future?
As a Navy physician, my main priority for my patients is to help them maintain their physical health and readiness to accomplish the mission.
It’s all about keeping the active duty persons and their families healthy, so physical fitness and nutrition are top priorities, and I want to continue to stress making those things part of my patients’ way of life. We should try to live more like the Kenyans in terms of eating more unprocessed, natural food and being active and exercising every day.
I also want to continue to focus on helping my patients decrease stress to improve their health. I was never as stress free as when I was in Kenya, and there were no cell phones, no TVs, none of the distractions, and you are living in the moment and surrounded by nature.
All the high-tech things that we have in the United States keep our cortisol levels sky high all the time, and that’s not good for overall health.
My experience in Kenya also reinforced for me that I want to have a wellness-centered practice, with a holistic approach that includes physical trainers and nutritionists who provide good care and education about how to live the best life you can and be as healthy as you can be.
Working overseas can give physicians a perspective that you cannot get any other way. I think you become a much better doctor. You appreciate how good we have it in some ways, but also what we can learn from people who have less.
Think globally. Practice locally.
U.S.-trained internists who have practiced abroad will receive a $100 stipend for contributing to this column.
Evidence Mounts for Aspirin's Anticancer Attributes
Adults who take aspirin daily have a 15% reduced risk of death from cancer compared with controls, and a 37% reduced risk of cancer death after 5 years, based on data from 51 randomized trials of daily aspirin use vs. no aspirin.
The findings were published online in the Lancet on March 20.
Findings from previous studies have suggested a cancer-prevention role for aspirin, but long-term data were limited and incomplete, said Dr. Peter Rothwell of the University of Oxford (England), and his colleagues.
The researchers reviewed data from the Antithrombotic Trialists’ Collaboration, PubMed, and Embase. They included only trials of daily aspirin vs. no aspirin.
Overall, randomization to aspirin significantly reduced the risk of nonvascular death in patients in the 51 trials compared with no aspirin (1,021 vs. 1,173, respectively, P = .003).
In a review of cancer deaths from 34 of the 51 studies, aspirin was associated with significantly fewer cancer deaths, compared with controls (562 vs. 664, P = .008). The benefit was most evident in patients in trials lasting 5 years or longer, the researchers said (Lancet 2012 March 20 [doi: 10.1016/S0140-6736(11)61720-0]).
In terms of primary prevention, daily aspirin use significantly reduced the risk of a composite outcome of major vascular events, cancer, or fatal extracranial bleeds (P = .0002), the researchers noted.
The findings were limited by the focus only on daily aspirin use, and by the use of composite outcomes, the researchers said. However, the results suggest that aspirin reduces cancer incidence and mortality, they noted.
"In view of the very low rates of vascular events in recent and ongoing trials of aspirin in primary prevention, prevention of cancer could become the main justification for aspirin use in this setting, although more research is required to identify which individuals are likely to benefit most," they said.
The study should be considered in the context of two additional studies by Dr. Rothwell and his colleagues, one published online in the Lancet, and the other published online in the Lancet Oncology, the researchers added.
In the additional Lancet study, a review of five large, randomized trials of daily aspirin of at least 75 mg versus controls, daily aspirin significantly reduced the risk of cancer metastases by 30%-40% in the short term.
These findings may explain the early reduction in cancer risk associated with aspirin that was observed in the primary prevention trials, and the results "are likely to underestimate the true effects of aspirin," they noted (Lancet 2012 March 20 [doi: 10.1016/S0140-6736(12)60209-8]).
In the Lancet Oncology, a review of case-control studies, cohort studies, and randomized trials of aspirin use showed significant reductions in the risk of colorectal cancer, as well as significant reductions in the risk of esophageal, gastric, biliary, and breast cancer. The greatest impact was on the reduction in risk of gastrointestinal cancers (Lancet Oncol. 2012 March 20 [doi: 10.1016/S1470-2045(12)70112-2]).
Both of these additional studies support the role of aspirin in reducing cancer deaths.
The studies were independent of company funding. Dr. Rothwell has received honoraria for talks, advisory board participation, and clinical trial committee participation from multiple companies, including AstraZeneca, Bayer, Boehringer Ingelheim, Sanofi-Aventis/Bristol-Myers Squibb, and Servier, and is on the executive committee of the ARRIVE trial.
Adults who take aspirin daily have a 15% reduced risk of death from cancer compared with controls, and a 37% reduced risk of cancer death after 5 years, based on data from 51 randomized trials of daily aspirin use vs. no aspirin.
The findings were published online in the Lancet on March 20.
Findings from previous studies have suggested a cancer-prevention role for aspirin, but long-term data were limited and incomplete, said Dr. Peter Rothwell of the University of Oxford (England), and his colleagues.
The researchers reviewed data from the Antithrombotic Trialists’ Collaboration, PubMed, and Embase. They included only trials of daily aspirin vs. no aspirin.
Overall, randomization to aspirin significantly reduced the risk of nonvascular death in patients in the 51 trials compared with no aspirin (1,021 vs. 1,173, respectively, P = .003).
In a review of cancer deaths from 34 of the 51 studies, aspirin was associated with significantly fewer cancer deaths, compared with controls (562 vs. 664, P = .008). The benefit was most evident in patients in trials lasting 5 years or longer, the researchers said (Lancet 2012 March 20 [doi: 10.1016/S0140-6736(11)61720-0]).
In terms of primary prevention, daily aspirin use significantly reduced the risk of a composite outcome of major vascular events, cancer, or fatal extracranial bleeds (P = .0002), the researchers noted.
The findings were limited by the focus only on daily aspirin use, and by the use of composite outcomes, the researchers said. However, the results suggest that aspirin reduces cancer incidence and mortality, they noted.
"In view of the very low rates of vascular events in recent and ongoing trials of aspirin in primary prevention, prevention of cancer could become the main justification for aspirin use in this setting, although more research is required to identify which individuals are likely to benefit most," they said.
The study should be considered in the context of two additional studies by Dr. Rothwell and his colleagues, one published online in the Lancet, and the other published online in the Lancet Oncology, the researchers added.
In the additional Lancet study, a review of five large, randomized trials of daily aspirin of at least 75 mg versus controls, daily aspirin significantly reduced the risk of cancer metastases by 30%-40% in the short term.
These findings may explain the early reduction in cancer risk associated with aspirin that was observed in the primary prevention trials, and the results "are likely to underestimate the true effects of aspirin," they noted (Lancet 2012 March 20 [doi: 10.1016/S0140-6736(12)60209-8]).
In the Lancet Oncology, a review of case-control studies, cohort studies, and randomized trials of aspirin use showed significant reductions in the risk of colorectal cancer, as well as significant reductions in the risk of esophageal, gastric, biliary, and breast cancer. The greatest impact was on the reduction in risk of gastrointestinal cancers (Lancet Oncol. 2012 March 20 [doi: 10.1016/S1470-2045(12)70112-2]).
Both of these additional studies support the role of aspirin in reducing cancer deaths.
The studies were independent of company funding. Dr. Rothwell has received honoraria for talks, advisory board participation, and clinical trial committee participation from multiple companies, including AstraZeneca, Bayer, Boehringer Ingelheim, Sanofi-Aventis/Bristol-Myers Squibb, and Servier, and is on the executive committee of the ARRIVE trial.
Adults who take aspirin daily have a 15% reduced risk of death from cancer compared with controls, and a 37% reduced risk of cancer death after 5 years, based on data from 51 randomized trials of daily aspirin use vs. no aspirin.
The findings were published online in the Lancet on March 20.
Findings from previous studies have suggested a cancer-prevention role for aspirin, but long-term data were limited and incomplete, said Dr. Peter Rothwell of the University of Oxford (England), and his colleagues.
The researchers reviewed data from the Antithrombotic Trialists’ Collaboration, PubMed, and Embase. They included only trials of daily aspirin vs. no aspirin.
Overall, randomization to aspirin significantly reduced the risk of nonvascular death in patients in the 51 trials compared with no aspirin (1,021 vs. 1,173, respectively, P = .003).
In a review of cancer deaths from 34 of the 51 studies, aspirin was associated with significantly fewer cancer deaths, compared with controls (562 vs. 664, P = .008). The benefit was most evident in patients in trials lasting 5 years or longer, the researchers said (Lancet 2012 March 20 [doi: 10.1016/S0140-6736(11)61720-0]).
In terms of primary prevention, daily aspirin use significantly reduced the risk of a composite outcome of major vascular events, cancer, or fatal extracranial bleeds (P = .0002), the researchers noted.
The findings were limited by the focus only on daily aspirin use, and by the use of composite outcomes, the researchers said. However, the results suggest that aspirin reduces cancer incidence and mortality, they noted.
"In view of the very low rates of vascular events in recent and ongoing trials of aspirin in primary prevention, prevention of cancer could become the main justification for aspirin use in this setting, although more research is required to identify which individuals are likely to benefit most," they said.
The study should be considered in the context of two additional studies by Dr. Rothwell and his colleagues, one published online in the Lancet, and the other published online in the Lancet Oncology, the researchers added.
In the additional Lancet study, a review of five large, randomized trials of daily aspirin of at least 75 mg versus controls, daily aspirin significantly reduced the risk of cancer metastases by 30%-40% in the short term.
These findings may explain the early reduction in cancer risk associated with aspirin that was observed in the primary prevention trials, and the results "are likely to underestimate the true effects of aspirin," they noted (Lancet 2012 March 20 [doi: 10.1016/S0140-6736(12)60209-8]).
In the Lancet Oncology, a review of case-control studies, cohort studies, and randomized trials of aspirin use showed significant reductions in the risk of colorectal cancer, as well as significant reductions in the risk of esophageal, gastric, biliary, and breast cancer. The greatest impact was on the reduction in risk of gastrointestinal cancers (Lancet Oncol. 2012 March 20 [doi: 10.1016/S1470-2045(12)70112-2]).
Both of these additional studies support the role of aspirin in reducing cancer deaths.
The studies were independent of company funding. Dr. Rothwell has received honoraria for talks, advisory board participation, and clinical trial committee participation from multiple companies, including AstraZeneca, Bayer, Boehringer Ingelheim, Sanofi-Aventis/Bristol-Myers Squibb, and Servier, and is on the executive committee of the ARRIVE trial.
FROM THE LANCET
Major
Finding: In a review of data from 51 studies, daily aspirin use reduced
cancer deaths by 15% compared with no daily aspirin use, and reduced the risk
of cancer deaths by 37% for those taking aspirin for 5 years or more.
Data
Source: The data come from a review of 51 randomized trials of adults
taking aspirin vs. controls to prevent heart attacks and other vascular events.
Disclosures:
The studies were independent of company funding. Dr. Rothwell has received
honoraria for talks, advisory board participation, and clinical trial committee
participation from multiple companies, including AstraZeneca, Bayer, Boehringer
Ingelheim, Sanofi-Aventis/Bristol-Myers Squibb, and Servier, and is on the
executive committee of the ARRIVE trial.
After Age 30, Cervical Ca Screening Intervals Extended
Women aged 30-65 years can extend the interval between Pap smears from 3 years to 5 years if they get tested for human papillomavirus at the same visit, according to new guidelines from the United States Preventive Services Task Force published online March 14 in Annals of Internal Medicine.
Women aged 21-29 years should still be screened every 3 years, as should women aged 30-65 years who do not undergo an HPV test concurrently with a Pap test for cervical cancer. The guidelines apply to all women with a cervix, regardless of their sexual history.
The United States Preventive Services Task Force (USPSTF) released a draft recommendation for cervical cancer screening guidelines in October 2011, but new evidence about the role of HPV testing in cervical cancer screening has since become available, and this evidence informs the newest guidelines for combining HPV and Pap tests for women aged 30-65 years, according to the task force (Ann. Intern. Med. 2012;156 [Epub ahead of print 15 Mar 2012]).
"We try to update our recommendations at least every 5 years and sooner if new evidence is available. In this case, it had been longer than 5 years since the last update and new evidence was available," task force chair Dr. Virginia Moyer said in an interview.
"Our recommendations, as well as those from other organizations, make it clear that more frequent screening than every 3 years for Pap alone or every 5 years for cotesting is not necessary and is associated with additional harms without significant additional benefit," said Dr. Moyer, professor of pediatrics at Baylor College of Medicine in Houston.
"We want to emphasize that in terms of saving lives, encouraging screening among women aged 21-65 years who have not been screened or have not been screened in the last 5 years is where the greatest benefit will accrue," she said.
The new guidelines replace those issued by the USPSTF in 2003, which called for annual cervical cancer screening for women starting at age 21 years or within 3 years of the onset of sexual activity.
The new recommendations also reduce the frequency of cervical cancer screening compared with guidelines issued by the American College of Obstetricians and Gynecologists in 2009. The 2009 ACOG guidelines called for cervical cancer screening every 2 years for women aged 21-29 years and every 3 years for women aged 30 years and older, as long as they had no history of cervical intraepithelial neoplasm (CIN) 2 or 3, HIV infection, in utero exposure to diethylstilbestrol, or immunocompromise.
The new guidelines also state that women who have had hysterectomies that involve removal of the cervix and have no history of cervical cancer or precancerous lesions can discontinue screening, as can women older than 65 years who have had three consecutive negative Pap tests or two consecutive negative Pap tests/HPV tests within 10 years before discontinuing screening, with their last test occurring within 5 years. However, the task force noted that clinicians should consider cervical cancer screening for women older than 65 years who have not had a hysterectomy and have never been screened.
The task force does not recommend cervical cancer screening for women younger than 21 years, and does not recommend cervical cancer screening using HPV (alone or in combination with a Pap smear) in women younger than 30 years, because of the high prevalence (and high rate of resolution) of HPV in younger women.
However, "It is well established that HPV infection is associated with nearly all cases of cervical cancer," the task force noted. "There is an emerging chain of evidence suggesting that HPV testing followed by cytology in women with positive HPV tests may also be a reasonable screening strategy," they said.
The new guidelines state that more research is needed to continue to assess the effectiveness and potential harm of different cervical cancer screening strategies, including HPV testing and Pap testing alone, in combination, or sequentially. However, in women aged 30-65 years, "evidence was adequate to conclude that the potential benefit of HPV testing in combination with cytology every 5 years is comparable with the benefits achievable with cytology alone every 3 years," according to the guidelines.
Disclosures of USPSTF task force members were not available at press time.
"Screening tests can unintentionally cause significant harm," Dr. Nora Kizer and Dr. Jeffrey F. Peipert said in an accompanying editorial. This harm includes misdiagnoses, unnecessary tests, and unnecessary procedures.
In addition to the USPSTF guidelines, the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, and the American Society for Clinical Pathology (ACS/ASCCP/ASCP) recently have published cervical cancer prevention guidelines that similarly extend the intervals between screenings, they noted.
"Health care providers and patients may be reluctant to adopt the longer screening intervals recommended in the new guidelines. We believe it is paramount for health care providers to take the initiative in fostering this change and acceptance. More frequent screening than recommended not only offers no benefit, but it can cause harm," they said. "Health care providers should welcome the new recommendations with enthusiasm and incorporate them into routine clinical practice."
There are slight differences between the USPSTF and the ACS/ASCCP/ASCP guidelines for women aged 30- 65 years, said Dr. Kizer and Dr. Peipert.
"The ACS/ASCCP/ASCP recommendations state that the preferred method of screening is cytology with HPV testing (‘cotesting’) at 5-year intervals. Use of cytology at 3-year intervals is also ‘acceptable,’ especially if access to HPV testing is not practical. The USPSTF guidelines state that both methods provide similar benefits and advocate cotesting as an option for those women who desire to lengthen the screening interval.
"The ACS/ASCCP/ASCP guidelines also note that there is insufficient evidence to change screening intervals in this age group following a history of negative screens," they said.
Also, the ACS/ASCCP/ASCP guidelines recommend that women who have received the HPV vaccine should continue to be screened, they added.
Although more research is needed on how to modify screening in different risk groups, said Dr. Kizer and Dr. Peipert, "overall, the new recommendations from the USPSTF and ACS/ASCCP/ASCP regarding cervical cancer screening are compatible and appropriate."
Dr. Kizer and Dr. Peipert are members of the department of obstetrics and gynecology at Washington University, St. Louis. Dr. Kizer and Dr. Peipert said they had no relevant financial disclosures. They commented in an editorial accompanying the USPSTF task force report (Ann. Intern. Med. 2012;156 [Epub ahead of print 15 Mar 2012]).
"Screening tests can unintentionally cause significant harm," Dr. Nora Kizer and Dr. Jeffrey F. Peipert said in an accompanying editorial. This harm includes misdiagnoses, unnecessary tests, and unnecessary procedures.
In addition to the USPSTF guidelines, the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, and the American Society for Clinical Pathology (ACS/ASCCP/ASCP) recently have published cervical cancer prevention guidelines that similarly extend the intervals between screenings, they noted.
"Health care providers and patients may be reluctant to adopt the longer screening intervals recommended in the new guidelines. We believe it is paramount for health care providers to take the initiative in fostering this change and acceptance. More frequent screening than recommended not only offers no benefit, but it can cause harm," they said. "Health care providers should welcome the new recommendations with enthusiasm and incorporate them into routine clinical practice."
There are slight differences between the USPSTF and the ACS/ASCCP/ASCP guidelines for women aged 30- 65 years, said Dr. Kizer and Dr. Peipert.
"The ACS/ASCCP/ASCP recommendations state that the preferred method of screening is cytology with HPV testing (‘cotesting’) at 5-year intervals. Use of cytology at 3-year intervals is also ‘acceptable,’ especially if access to HPV testing is not practical. The USPSTF guidelines state that both methods provide similar benefits and advocate cotesting as an option for those women who desire to lengthen the screening interval.
"The ACS/ASCCP/ASCP guidelines also note that there is insufficient evidence to change screening intervals in this age group following a history of negative screens," they said.
Also, the ACS/ASCCP/ASCP guidelines recommend that women who have received the HPV vaccine should continue to be screened, they added.
Although more research is needed on how to modify screening in different risk groups, said Dr. Kizer and Dr. Peipert, "overall, the new recommendations from the USPSTF and ACS/ASCCP/ASCP regarding cervical cancer screening are compatible and appropriate."
Dr. Kizer and Dr. Peipert are members of the department of obstetrics and gynecology at Washington University, St. Louis. Dr. Kizer and Dr. Peipert said they had no relevant financial disclosures. They commented in an editorial accompanying the USPSTF task force report (Ann. Intern. Med. 2012;156 [Epub ahead of print 15 Mar 2012]).
"Screening tests can unintentionally cause significant harm," Dr. Nora Kizer and Dr. Jeffrey F. Peipert said in an accompanying editorial. This harm includes misdiagnoses, unnecessary tests, and unnecessary procedures.
In addition to the USPSTF guidelines, the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, and the American Society for Clinical Pathology (ACS/ASCCP/ASCP) recently have published cervical cancer prevention guidelines that similarly extend the intervals between screenings, they noted.
"Health care providers and patients may be reluctant to adopt the longer screening intervals recommended in the new guidelines. We believe it is paramount for health care providers to take the initiative in fostering this change and acceptance. More frequent screening than recommended not only offers no benefit, but it can cause harm," they said. "Health care providers should welcome the new recommendations with enthusiasm and incorporate them into routine clinical practice."
There are slight differences between the USPSTF and the ACS/ASCCP/ASCP guidelines for women aged 30- 65 years, said Dr. Kizer and Dr. Peipert.
"The ACS/ASCCP/ASCP recommendations state that the preferred method of screening is cytology with HPV testing (‘cotesting’) at 5-year intervals. Use of cytology at 3-year intervals is also ‘acceptable,’ especially if access to HPV testing is not practical. The USPSTF guidelines state that both methods provide similar benefits and advocate cotesting as an option for those women who desire to lengthen the screening interval.
"The ACS/ASCCP/ASCP guidelines also note that there is insufficient evidence to change screening intervals in this age group following a history of negative screens," they said.
Also, the ACS/ASCCP/ASCP guidelines recommend that women who have received the HPV vaccine should continue to be screened, they added.
Although more research is needed on how to modify screening in different risk groups, said Dr. Kizer and Dr. Peipert, "overall, the new recommendations from the USPSTF and ACS/ASCCP/ASCP regarding cervical cancer screening are compatible and appropriate."
Dr. Kizer and Dr. Peipert are members of the department of obstetrics and gynecology at Washington University, St. Louis. Dr. Kizer and Dr. Peipert said they had no relevant financial disclosures. They commented in an editorial accompanying the USPSTF task force report (Ann. Intern. Med. 2012;156 [Epub ahead of print 15 Mar 2012]).
Women aged 30-65 years can extend the interval between Pap smears from 3 years to 5 years if they get tested for human papillomavirus at the same visit, according to new guidelines from the United States Preventive Services Task Force published online March 14 in Annals of Internal Medicine.
Women aged 21-29 years should still be screened every 3 years, as should women aged 30-65 years who do not undergo an HPV test concurrently with a Pap test for cervical cancer. The guidelines apply to all women with a cervix, regardless of their sexual history.
The United States Preventive Services Task Force (USPSTF) released a draft recommendation for cervical cancer screening guidelines in October 2011, but new evidence about the role of HPV testing in cervical cancer screening has since become available, and this evidence informs the newest guidelines for combining HPV and Pap tests for women aged 30-65 years, according to the task force (Ann. Intern. Med. 2012;156 [Epub ahead of print 15 Mar 2012]).
"We try to update our recommendations at least every 5 years and sooner if new evidence is available. In this case, it had been longer than 5 years since the last update and new evidence was available," task force chair Dr. Virginia Moyer said in an interview.
"Our recommendations, as well as those from other organizations, make it clear that more frequent screening than every 3 years for Pap alone or every 5 years for cotesting is not necessary and is associated with additional harms without significant additional benefit," said Dr. Moyer, professor of pediatrics at Baylor College of Medicine in Houston.
"We want to emphasize that in terms of saving lives, encouraging screening among women aged 21-65 years who have not been screened or have not been screened in the last 5 years is where the greatest benefit will accrue," she said.
The new guidelines replace those issued by the USPSTF in 2003, which called for annual cervical cancer screening for women starting at age 21 years or within 3 years of the onset of sexual activity.
The new recommendations also reduce the frequency of cervical cancer screening compared with guidelines issued by the American College of Obstetricians and Gynecologists in 2009. The 2009 ACOG guidelines called for cervical cancer screening every 2 years for women aged 21-29 years and every 3 years for women aged 30 years and older, as long as they had no history of cervical intraepithelial neoplasm (CIN) 2 or 3, HIV infection, in utero exposure to diethylstilbestrol, or immunocompromise.
The new guidelines also state that women who have had hysterectomies that involve removal of the cervix and have no history of cervical cancer or precancerous lesions can discontinue screening, as can women older than 65 years who have had three consecutive negative Pap tests or two consecutive negative Pap tests/HPV tests within 10 years before discontinuing screening, with their last test occurring within 5 years. However, the task force noted that clinicians should consider cervical cancer screening for women older than 65 years who have not had a hysterectomy and have never been screened.
The task force does not recommend cervical cancer screening for women younger than 21 years, and does not recommend cervical cancer screening using HPV (alone or in combination with a Pap smear) in women younger than 30 years, because of the high prevalence (and high rate of resolution) of HPV in younger women.
However, "It is well established that HPV infection is associated with nearly all cases of cervical cancer," the task force noted. "There is an emerging chain of evidence suggesting that HPV testing followed by cytology in women with positive HPV tests may also be a reasonable screening strategy," they said.
The new guidelines state that more research is needed to continue to assess the effectiveness and potential harm of different cervical cancer screening strategies, including HPV testing and Pap testing alone, in combination, or sequentially. However, in women aged 30-65 years, "evidence was adequate to conclude that the potential benefit of HPV testing in combination with cytology every 5 years is comparable with the benefits achievable with cytology alone every 3 years," according to the guidelines.
Disclosures of USPSTF task force members were not available at press time.
Women aged 30-65 years can extend the interval between Pap smears from 3 years to 5 years if they get tested for human papillomavirus at the same visit, according to new guidelines from the United States Preventive Services Task Force published online March 14 in Annals of Internal Medicine.
Women aged 21-29 years should still be screened every 3 years, as should women aged 30-65 years who do not undergo an HPV test concurrently with a Pap test for cervical cancer. The guidelines apply to all women with a cervix, regardless of their sexual history.
The United States Preventive Services Task Force (USPSTF) released a draft recommendation for cervical cancer screening guidelines in October 2011, but new evidence about the role of HPV testing in cervical cancer screening has since become available, and this evidence informs the newest guidelines for combining HPV and Pap tests for women aged 30-65 years, according to the task force (Ann. Intern. Med. 2012;156 [Epub ahead of print 15 Mar 2012]).
"We try to update our recommendations at least every 5 years and sooner if new evidence is available. In this case, it had been longer than 5 years since the last update and new evidence was available," task force chair Dr. Virginia Moyer said in an interview.
"Our recommendations, as well as those from other organizations, make it clear that more frequent screening than every 3 years for Pap alone or every 5 years for cotesting is not necessary and is associated with additional harms without significant additional benefit," said Dr. Moyer, professor of pediatrics at Baylor College of Medicine in Houston.
"We want to emphasize that in terms of saving lives, encouraging screening among women aged 21-65 years who have not been screened or have not been screened in the last 5 years is where the greatest benefit will accrue," she said.
The new guidelines replace those issued by the USPSTF in 2003, which called for annual cervical cancer screening for women starting at age 21 years or within 3 years of the onset of sexual activity.
The new recommendations also reduce the frequency of cervical cancer screening compared with guidelines issued by the American College of Obstetricians and Gynecologists in 2009. The 2009 ACOG guidelines called for cervical cancer screening every 2 years for women aged 21-29 years and every 3 years for women aged 30 years and older, as long as they had no history of cervical intraepithelial neoplasm (CIN) 2 or 3, HIV infection, in utero exposure to diethylstilbestrol, or immunocompromise.
The new guidelines also state that women who have had hysterectomies that involve removal of the cervix and have no history of cervical cancer or precancerous lesions can discontinue screening, as can women older than 65 years who have had three consecutive negative Pap tests or two consecutive negative Pap tests/HPV tests within 10 years before discontinuing screening, with their last test occurring within 5 years. However, the task force noted that clinicians should consider cervical cancer screening for women older than 65 years who have not had a hysterectomy and have never been screened.
The task force does not recommend cervical cancer screening for women younger than 21 years, and does not recommend cervical cancer screening using HPV (alone or in combination with a Pap smear) in women younger than 30 years, because of the high prevalence (and high rate of resolution) of HPV in younger women.
However, "It is well established that HPV infection is associated with nearly all cases of cervical cancer," the task force noted. "There is an emerging chain of evidence suggesting that HPV testing followed by cytology in women with positive HPV tests may also be a reasonable screening strategy," they said.
The new guidelines state that more research is needed to continue to assess the effectiveness and potential harm of different cervical cancer screening strategies, including HPV testing and Pap testing alone, in combination, or sequentially. However, in women aged 30-65 years, "evidence was adequate to conclude that the potential benefit of HPV testing in combination with cytology every 5 years is comparable with the benefits achievable with cytology alone every 3 years," according to the guidelines.
Disclosures of USPSTF task force members were not available at press time.
FROM ANNALS OF INTERNAL MEDICINE
Atrial Fibrillation, Stroke Risks Rise in Rheumatoid Arthritis
Rheumatoid arthritis patients had a 40% increased risk of atrial fibrillation and a 30% increased risk of stroke compared to the general population, based on data from a national cohort study in Denmark.
Previous studies have shown an association between rheumatoid arthritis (RA) and increased risk of myocardial infarction, but data on the risk of stroke have been inconsistent, said Dr. Jesper Lindhardsen, a research fellow in the department of cardiology at Gentofte Hospital in Hellerup, Denmark.
The findings were published online March 8 in the British Medical Journal (BMJ 2012 March 8 [doi: 10.1136/bmj.e1257]).
Dr. Lindhardsen and colleagues reviewed data from a national registry that included all Danish individuals older than 15 years of age as of Jan. 1, 1997. The study cohort included 4.3 million individuals. Of these, 18,247 had RA. During a follow-up period of up to 13 years, a total of 156,484 individuals, including 774 RA patients, were diagnosed with atrial fibrillation, and 165,343 individuals, including 718 RA patients, had a stroke.
The incidence of atrial fibrillation (a modifiable risk factor for stroke) was 8.2 events per 1,000 person-years in RA patients vs. 6 events per 1,000 person years in the general population. Women were at slightly increased risk, compared with men, and the risk was significantly higher in the youngest age groups. "The absolute risk attributable to rheumatoid arthritis ranged from 25% in the oldest to 70% in the youngest age group," the researchers noted.
The incidence of stroke was 7.6 per 1,000 person-years among RA patients vs. 5.7 per 1,000 person-years in the general population. As with atrial fibrillation, the relative risk for stroke in RA patients was highest in the younger age groups.
"Nonetheless, the absolute differences in rates of atrial fibrillation and stroke between people with and without rheumatoid arthritis were highest in the oldest patients," the researchers said.
The study is the first known to examine the incidence of atrial fibrillation in a large population of RA patients. The results were limited by the use of a national registry, which missed patients not seen in clinics or treated with antirheumatic drugs during the study period.
However, the findings correspond to one new case of atrial fibrillation per 12 RA patients followed for 10 years after their diagnoses, the researchers said. Therefore, the researchers recommended that clinical care of RA patients include screening for atrial fibrillation.
"This study also underlines the importance of rigorous control of inflammation with disease modifying antirheumatic drugs, not only for the management of joint symptoms but also to reduce the need for drugs with potential adverse cardiovascular effects and, ultimately, to diminish the inflammation driven atherothrombotic process," they emphasized.
The findings are a continuation of data presented by Dr. Lindhardsen at the European Society of Cardiology meeting in 2010 in Stockholm.
Dr. Lindhardsen said he had no financial conflicts to disclose.
Rheumatoid arthritis patients had a 40% increased risk of atrial fibrillation and a 30% increased risk of stroke compared to the general population, based on data from a national cohort study in Denmark.
Previous studies have shown an association between rheumatoid arthritis (RA) and increased risk of myocardial infarction, but data on the risk of stroke have been inconsistent, said Dr. Jesper Lindhardsen, a research fellow in the department of cardiology at Gentofte Hospital in Hellerup, Denmark.
The findings were published online March 8 in the British Medical Journal (BMJ 2012 March 8 [doi: 10.1136/bmj.e1257]).
Dr. Lindhardsen and colleagues reviewed data from a national registry that included all Danish individuals older than 15 years of age as of Jan. 1, 1997. The study cohort included 4.3 million individuals. Of these, 18,247 had RA. During a follow-up period of up to 13 years, a total of 156,484 individuals, including 774 RA patients, were diagnosed with atrial fibrillation, and 165,343 individuals, including 718 RA patients, had a stroke.
The incidence of atrial fibrillation (a modifiable risk factor for stroke) was 8.2 events per 1,000 person-years in RA patients vs. 6 events per 1,000 person years in the general population. Women were at slightly increased risk, compared with men, and the risk was significantly higher in the youngest age groups. "The absolute risk attributable to rheumatoid arthritis ranged from 25% in the oldest to 70% in the youngest age group," the researchers noted.
The incidence of stroke was 7.6 per 1,000 person-years among RA patients vs. 5.7 per 1,000 person-years in the general population. As with atrial fibrillation, the relative risk for stroke in RA patients was highest in the younger age groups.
"Nonetheless, the absolute differences in rates of atrial fibrillation and stroke between people with and without rheumatoid arthritis were highest in the oldest patients," the researchers said.
The study is the first known to examine the incidence of atrial fibrillation in a large population of RA patients. The results were limited by the use of a national registry, which missed patients not seen in clinics or treated with antirheumatic drugs during the study period.
However, the findings correspond to one new case of atrial fibrillation per 12 RA patients followed for 10 years after their diagnoses, the researchers said. Therefore, the researchers recommended that clinical care of RA patients include screening for atrial fibrillation.
"This study also underlines the importance of rigorous control of inflammation with disease modifying antirheumatic drugs, not only for the management of joint symptoms but also to reduce the need for drugs with potential adverse cardiovascular effects and, ultimately, to diminish the inflammation driven atherothrombotic process," they emphasized.
The findings are a continuation of data presented by Dr. Lindhardsen at the European Society of Cardiology meeting in 2010 in Stockholm.
Dr. Lindhardsen said he had no financial conflicts to disclose.
Rheumatoid arthritis patients had a 40% increased risk of atrial fibrillation and a 30% increased risk of stroke compared to the general population, based on data from a national cohort study in Denmark.
Previous studies have shown an association between rheumatoid arthritis (RA) and increased risk of myocardial infarction, but data on the risk of stroke have been inconsistent, said Dr. Jesper Lindhardsen, a research fellow in the department of cardiology at Gentofte Hospital in Hellerup, Denmark.
The findings were published online March 8 in the British Medical Journal (BMJ 2012 March 8 [doi: 10.1136/bmj.e1257]).
Dr. Lindhardsen and colleagues reviewed data from a national registry that included all Danish individuals older than 15 years of age as of Jan. 1, 1997. The study cohort included 4.3 million individuals. Of these, 18,247 had RA. During a follow-up period of up to 13 years, a total of 156,484 individuals, including 774 RA patients, were diagnosed with atrial fibrillation, and 165,343 individuals, including 718 RA patients, had a stroke.
The incidence of atrial fibrillation (a modifiable risk factor for stroke) was 8.2 events per 1,000 person-years in RA patients vs. 6 events per 1,000 person years in the general population. Women were at slightly increased risk, compared with men, and the risk was significantly higher in the youngest age groups. "The absolute risk attributable to rheumatoid arthritis ranged from 25% in the oldest to 70% in the youngest age group," the researchers noted.
The incidence of stroke was 7.6 per 1,000 person-years among RA patients vs. 5.7 per 1,000 person-years in the general population. As with atrial fibrillation, the relative risk for stroke in RA patients was highest in the younger age groups.
"Nonetheless, the absolute differences in rates of atrial fibrillation and stroke between people with and without rheumatoid arthritis were highest in the oldest patients," the researchers said.
The study is the first known to examine the incidence of atrial fibrillation in a large population of RA patients. The results were limited by the use of a national registry, which missed patients not seen in clinics or treated with antirheumatic drugs during the study period.
However, the findings correspond to one new case of atrial fibrillation per 12 RA patients followed for 10 years after their diagnoses, the researchers said. Therefore, the researchers recommended that clinical care of RA patients include screening for atrial fibrillation.
"This study also underlines the importance of rigorous control of inflammation with disease modifying antirheumatic drugs, not only for the management of joint symptoms but also to reduce the need for drugs with potential adverse cardiovascular effects and, ultimately, to diminish the inflammation driven atherothrombotic process," they emphasized.
The findings are a continuation of data presented by Dr. Lindhardsen at the European Society of Cardiology meeting in 2010 in Stockholm.
Dr. Lindhardsen said he had no financial conflicts to disclose.
FROM THE BRITISH MEDICAL JOURNAL
Major Finding: Rheumatoid arthritis patients had a 40% increased risk of atrial fibrillation and a 30% increased risk of stroke, compared to the general population.
Data Source: The data come from a national registry of all 4.3 million Danish citizens who were older than 15 years of age as of Jan. 1, 1997.
Disclosures: Dr. Lindhardsen said he had no financial conflicts to disclose.
Misoprostol Curbs Complications in First Trimester Abortions
Cervical preparation with 400 mcg of misoprostol significantly reduced complications in women undergoing first-trimester abortion by vacuum aspiration, based on data from a randomized, parallel group trial of nearly 5,000 women published online March 8 in the Lancet.
Inadequate cervical dilation can cause complications during vacuum aspiration procedures, and misoprostol has been shown to increase cervical dilation, said Dr. Olav Meirik and colleagues at the World Health Organization World Bank Special Programme of Research, Development and Research Training in Human Reproduction in Geneva, Switzerland.
"However, no study has been large enough to assess whether cervical preparation with misoprostol is associated with reduced rates of immediate and delayed complications of vacuum aspiration for first-trimester surgical abortion," the researchers said (Lancet 2012 March 8 [doi: 10.1016/S0140-6736(11)61937-5]).
A total of 4,972 women who underwent first-trimester surgical abortions at 14 locations in nine countries were enrolled in the study and randomized to receive two 200-mcg misoprostol tablets or a placebo 3 hours prior to surgery. Complete data were available for 2,427 women in the misoprostol group and 2,431 women in the placebo group. The mean age of the women was 27 years.
After a follow-up period of up to 2 weeks, none of the women who received misoprostol had cervical tears, and three had uterine perforations. By contrast, two women in the control group had cervical tears, and one had a uterine perforation.
On average, the cervical diameter prior to surgery was significantly greater in women who received misoprostol compared with placebo, and the mean duration of vacuum aspiration was significantly shorter in the misoprostol group vs. the placebo group (3.6 minutes vs. 3.9 minutes). Approximately 40% of the misoprostol group needed no additional mechanical cervical dilation, compared with 22% of the placebo group, and the amount of dilation, when needed, was less in the misoprostol group.
The average time between the treatment (or placebo) and the procedure was 3 hours. During this time, side effects including abdominal pain (55% vs. 22%), vaginal bleeding (37% vs. 7%), and nausea (7% vs. 4%) were more frequent in the misoprostol group. However, the researchers noted no differences in the incidence of pelvic inflammatory disease or other serious adverse events between the groups.
Incomplete abortions were significantly less likely in the misoprostol group (less than 1%), compared with the placebo group (2%). Most of these women with incomplete abortions underwent additional uterine re-evacuation.
The study was limited by the lack of total blinding and the lack of power to analyze any specific complications in subgroups of patients, the researchers noted. But the findings suggest that cervical preparation with misoprostol prior to abortion by vacuum aspiration facilitates cervical dilation and reduces overall complications, and "should be considered together with the side effects and with women’s acceptance of the drug regimen," they said.
The researchers said they had had no financial conflicts to disclose. This study was supported by United Nations Development Programme/UN Population Fund/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction, Department of Reproductive Health and Research, WHO, and the Packard Foundation.
"Prediction of the women for whom surgery will prove difficult is not possible," said Dr. Allan Templeton, complicating a previous recommendation that misoprostol only be recommended prior to abortion for women in this category. Such a recommendation was made because although previous studies showed the efficacy of misoprostol in softening the cervix prior to surgical abortions, they were not large enough to show a reduction in serious complications.
"The findings from Meirik and colleagues’ randomized multicentre trial make an important contribution to this debate by showing that vaginal misoprostol not only facilitates surgery in healthy women seeking first-trimester abortion, but results in a significantly lower rate of incomplete evacuation," he said.
The key ongoing issue is the balance between the effectiveness of the vacuum aspiration procedure and the potential side effects of misoprostol, the most common of which are abdominal pain and vaginal bleeding. However, these side effects generally do not require medical intervention prior to surgery, so administering misoprostol 3 hours prior to surgery has not presented logistical problems, Dr. Templeton noted.
"Surely routine pharmaceutical dilation of the cervix should be recommended as an integral part of surgical abortion in all women," he said.
Dr. Templeton is a member of the department of obstetrics and gynecology at the University of Aberdeen (Scotland). Dr. Templeton wrote this editorial accompanying Dr. Meirik and associates’ study (Lancet 2012 March 8 [doi: 10.1016/S0140-6736(12)60037-3]). He said he had no financial conflicts to disclose.
"Prediction of the women for whom surgery will prove difficult is not possible," said Dr. Allan Templeton, complicating a previous recommendation that misoprostol only be recommended prior to abortion for women in this category. Such a recommendation was made because although previous studies showed the efficacy of misoprostol in softening the cervix prior to surgical abortions, they were not large enough to show a reduction in serious complications.
"The findings from Meirik and colleagues’ randomized multicentre trial make an important contribution to this debate by showing that vaginal misoprostol not only facilitates surgery in healthy women seeking first-trimester abortion, but results in a significantly lower rate of incomplete evacuation," he said.
The key ongoing issue is the balance between the effectiveness of the vacuum aspiration procedure and the potential side effects of misoprostol, the most common of which are abdominal pain and vaginal bleeding. However, these side effects generally do not require medical intervention prior to surgery, so administering misoprostol 3 hours prior to surgery has not presented logistical problems, Dr. Templeton noted.
"Surely routine pharmaceutical dilation of the cervix should be recommended as an integral part of surgical abortion in all women," he said.
Dr. Templeton is a member of the department of obstetrics and gynecology at the University of Aberdeen (Scotland). Dr. Templeton wrote this editorial accompanying Dr. Meirik and associates’ study (Lancet 2012 March 8 [doi: 10.1016/S0140-6736(12)60037-3]). He said he had no financial conflicts to disclose.
"Prediction of the women for whom surgery will prove difficult is not possible," said Dr. Allan Templeton, complicating a previous recommendation that misoprostol only be recommended prior to abortion for women in this category. Such a recommendation was made because although previous studies showed the efficacy of misoprostol in softening the cervix prior to surgical abortions, they were not large enough to show a reduction in serious complications.
"The findings from Meirik and colleagues’ randomized multicentre trial make an important contribution to this debate by showing that vaginal misoprostol not only facilitates surgery in healthy women seeking first-trimester abortion, but results in a significantly lower rate of incomplete evacuation," he said.
The key ongoing issue is the balance between the effectiveness of the vacuum aspiration procedure and the potential side effects of misoprostol, the most common of which are abdominal pain and vaginal bleeding. However, these side effects generally do not require medical intervention prior to surgery, so administering misoprostol 3 hours prior to surgery has not presented logistical problems, Dr. Templeton noted.
"Surely routine pharmaceutical dilation of the cervix should be recommended as an integral part of surgical abortion in all women," he said.
Dr. Templeton is a member of the department of obstetrics and gynecology at the University of Aberdeen (Scotland). Dr. Templeton wrote this editorial accompanying Dr. Meirik and associates’ study (Lancet 2012 March 8 [doi: 10.1016/S0140-6736(12)60037-3]). He said he had no financial conflicts to disclose.
Cervical preparation with 400 mcg of misoprostol significantly reduced complications in women undergoing first-trimester abortion by vacuum aspiration, based on data from a randomized, parallel group trial of nearly 5,000 women published online March 8 in the Lancet.
Inadequate cervical dilation can cause complications during vacuum aspiration procedures, and misoprostol has been shown to increase cervical dilation, said Dr. Olav Meirik and colleagues at the World Health Organization World Bank Special Programme of Research, Development and Research Training in Human Reproduction in Geneva, Switzerland.
"However, no study has been large enough to assess whether cervical preparation with misoprostol is associated with reduced rates of immediate and delayed complications of vacuum aspiration for first-trimester surgical abortion," the researchers said (Lancet 2012 March 8 [doi: 10.1016/S0140-6736(11)61937-5]).
A total of 4,972 women who underwent first-trimester surgical abortions at 14 locations in nine countries were enrolled in the study and randomized to receive two 200-mcg misoprostol tablets or a placebo 3 hours prior to surgery. Complete data were available for 2,427 women in the misoprostol group and 2,431 women in the placebo group. The mean age of the women was 27 years.
After a follow-up period of up to 2 weeks, none of the women who received misoprostol had cervical tears, and three had uterine perforations. By contrast, two women in the control group had cervical tears, and one had a uterine perforation.
On average, the cervical diameter prior to surgery was significantly greater in women who received misoprostol compared with placebo, and the mean duration of vacuum aspiration was significantly shorter in the misoprostol group vs. the placebo group (3.6 minutes vs. 3.9 minutes). Approximately 40% of the misoprostol group needed no additional mechanical cervical dilation, compared with 22% of the placebo group, and the amount of dilation, when needed, was less in the misoprostol group.
The average time between the treatment (or placebo) and the procedure was 3 hours. During this time, side effects including abdominal pain (55% vs. 22%), vaginal bleeding (37% vs. 7%), and nausea (7% vs. 4%) were more frequent in the misoprostol group. However, the researchers noted no differences in the incidence of pelvic inflammatory disease or other serious adverse events between the groups.
Incomplete abortions were significantly less likely in the misoprostol group (less than 1%), compared with the placebo group (2%). Most of these women with incomplete abortions underwent additional uterine re-evacuation.
The study was limited by the lack of total blinding and the lack of power to analyze any specific complications in subgroups of patients, the researchers noted. But the findings suggest that cervical preparation with misoprostol prior to abortion by vacuum aspiration facilitates cervical dilation and reduces overall complications, and "should be considered together with the side effects and with women’s acceptance of the drug regimen," they said.
The researchers said they had had no financial conflicts to disclose. This study was supported by United Nations Development Programme/UN Population Fund/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction, Department of Reproductive Health and Research, WHO, and the Packard Foundation.
Cervical preparation with 400 mcg of misoprostol significantly reduced complications in women undergoing first-trimester abortion by vacuum aspiration, based on data from a randomized, parallel group trial of nearly 5,000 women published online March 8 in the Lancet.
Inadequate cervical dilation can cause complications during vacuum aspiration procedures, and misoprostol has been shown to increase cervical dilation, said Dr. Olav Meirik and colleagues at the World Health Organization World Bank Special Programme of Research, Development and Research Training in Human Reproduction in Geneva, Switzerland.
"However, no study has been large enough to assess whether cervical preparation with misoprostol is associated with reduced rates of immediate and delayed complications of vacuum aspiration for first-trimester surgical abortion," the researchers said (Lancet 2012 March 8 [doi: 10.1016/S0140-6736(11)61937-5]).
A total of 4,972 women who underwent first-trimester surgical abortions at 14 locations in nine countries were enrolled in the study and randomized to receive two 200-mcg misoprostol tablets or a placebo 3 hours prior to surgery. Complete data were available for 2,427 women in the misoprostol group and 2,431 women in the placebo group. The mean age of the women was 27 years.
After a follow-up period of up to 2 weeks, none of the women who received misoprostol had cervical tears, and three had uterine perforations. By contrast, two women in the control group had cervical tears, and one had a uterine perforation.
On average, the cervical diameter prior to surgery was significantly greater in women who received misoprostol compared with placebo, and the mean duration of vacuum aspiration was significantly shorter in the misoprostol group vs. the placebo group (3.6 minutes vs. 3.9 minutes). Approximately 40% of the misoprostol group needed no additional mechanical cervical dilation, compared with 22% of the placebo group, and the amount of dilation, when needed, was less in the misoprostol group.
The average time between the treatment (or placebo) and the procedure was 3 hours. During this time, side effects including abdominal pain (55% vs. 22%), vaginal bleeding (37% vs. 7%), and nausea (7% vs. 4%) were more frequent in the misoprostol group. However, the researchers noted no differences in the incidence of pelvic inflammatory disease or other serious adverse events between the groups.
Incomplete abortions were significantly less likely in the misoprostol group (less than 1%), compared with the placebo group (2%). Most of these women with incomplete abortions underwent additional uterine re-evacuation.
The study was limited by the lack of total blinding and the lack of power to analyze any specific complications in subgroups of patients, the researchers noted. But the findings suggest that cervical preparation with misoprostol prior to abortion by vacuum aspiration facilitates cervical dilation and reduces overall complications, and "should be considered together with the side effects and with women’s acceptance of the drug regimen," they said.
The researchers said they had had no financial conflicts to disclose. This study was supported by United Nations Development Programme/UN Population Fund/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction, Department of Reproductive Health and Research, WHO, and the Packard Foundation.
FROM THE LANCET
Major Finding: No women who received misoprostol prior to a vacuum aspiration procedure had cervical tears, and three had uterine perforations. By contrast, two women in a placebo group had cervical tears, and one had a uterine perforation.
Data Source: The data come from a randomized trial of 4,972 women undergoing first-trimester surgical abortions.
Disclosures: The researchers said they had had no financial conflicts to disclose. This study was supported by United Nations Development Programme/UN Population Fund /WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction, Department of Reproductive Health and Research, WHO, and the Packard Foundation.
Stable Schizophrenia Patients May Have Depression
Nearly one-third of adults with stable schizophrenia also met independent clinical criteria for depression, findings from an analysis of 90 patients have shown.
Overall, 28 of the 90 patients (31%) had scores of 5 or more on the Calgary Depression Scale for Schizophrenia (CDSS), which is highly correlated with other measures of depression, said Dr. Susana Majadas of the Universidad de Salamanca (Spain) and her colleagues.
Data from previous studies have shown depressive symptoms in many patients with schizophrenia, but some clinicians maintain that these symptoms are secondary to schizophrenia or a side effect of treatment, the researchers noted.
In this study, the researchers analyzed 90 stable schizophrenia outpatients aged 18-50 years across five centers in Spain; 75 had a diagnosis of schizophrenia and 15 had a schizoaffective disorder. The mean age of the patients was 35 years, and 59% were men (Comp. Psychiatry 2012;53:145-51).
The prevalence of depression was 33% in patients with schizophrenia and 20% in those with a schizoaffective disorder. The prevalence of depression was not significantly different in men vs. women (34% vs. 27%).
The patients’ treatments included risperidone (32 patients), quetiapine (21 patients), olanzapine (9 patients), fluphenazine (6 patients), amisulpride (6 patients), and other antipsychotics (14 patients).
When the presence of depression was analyzed according to antipsychotic treatment, the criteria for depression (a CDSS score below 5) were not met in 90% of patients on quetiapine, 67% of patients on fluphenazine, 56% of those on risperidone, and 44% of those on olanzapine, the researchers noted.
"Depression appears to be associated with a greater severity of disease, and in part, overlaps with negative symptoms," the researchers said.
The study is the first to use the CDSS to assess depression in schizophrenia patients, and the prevalence rates are similar to those found in other studies, they noted. Although the study was limited by its cross-sectional design, which did not permit the evaluation of symptoms over time, the findings suggest that clinical depression is often present in stable schizophrenia patients. The findings also suggest that depressive symptoms are not secondary to schizophrenia symptoms or the effects of medication, Dr. Majadas and her colleagues added.
The researchers said they had no relevant financial disclosures.
Nearly one-third of adults with stable schizophrenia also met independent clinical criteria for depression, findings from an analysis of 90 patients have shown.
Overall, 28 of the 90 patients (31%) had scores of 5 or more on the Calgary Depression Scale for Schizophrenia (CDSS), which is highly correlated with other measures of depression, said Dr. Susana Majadas of the Universidad de Salamanca (Spain) and her colleagues.
Data from previous studies have shown depressive symptoms in many patients with schizophrenia, but some clinicians maintain that these symptoms are secondary to schizophrenia or a side effect of treatment, the researchers noted.
In this study, the researchers analyzed 90 stable schizophrenia outpatients aged 18-50 years across five centers in Spain; 75 had a diagnosis of schizophrenia and 15 had a schizoaffective disorder. The mean age of the patients was 35 years, and 59% were men (Comp. Psychiatry 2012;53:145-51).
The prevalence of depression was 33% in patients with schizophrenia and 20% in those with a schizoaffective disorder. The prevalence of depression was not significantly different in men vs. women (34% vs. 27%).
The patients’ treatments included risperidone (32 patients), quetiapine (21 patients), olanzapine (9 patients), fluphenazine (6 patients), amisulpride (6 patients), and other antipsychotics (14 patients).
When the presence of depression was analyzed according to antipsychotic treatment, the criteria for depression (a CDSS score below 5) were not met in 90% of patients on quetiapine, 67% of patients on fluphenazine, 56% of those on risperidone, and 44% of those on olanzapine, the researchers noted.
"Depression appears to be associated with a greater severity of disease, and in part, overlaps with negative symptoms," the researchers said.
The study is the first to use the CDSS to assess depression in schizophrenia patients, and the prevalence rates are similar to those found in other studies, they noted. Although the study was limited by its cross-sectional design, which did not permit the evaluation of symptoms over time, the findings suggest that clinical depression is often present in stable schizophrenia patients. The findings also suggest that depressive symptoms are not secondary to schizophrenia symptoms or the effects of medication, Dr. Majadas and her colleagues added.
The researchers said they had no relevant financial disclosures.
Nearly one-third of adults with stable schizophrenia also met independent clinical criteria for depression, findings from an analysis of 90 patients have shown.
Overall, 28 of the 90 patients (31%) had scores of 5 or more on the Calgary Depression Scale for Schizophrenia (CDSS), which is highly correlated with other measures of depression, said Dr. Susana Majadas of the Universidad de Salamanca (Spain) and her colleagues.
Data from previous studies have shown depressive symptoms in many patients with schizophrenia, but some clinicians maintain that these symptoms are secondary to schizophrenia or a side effect of treatment, the researchers noted.
In this study, the researchers analyzed 90 stable schizophrenia outpatients aged 18-50 years across five centers in Spain; 75 had a diagnosis of schizophrenia and 15 had a schizoaffective disorder. The mean age of the patients was 35 years, and 59% were men (Comp. Psychiatry 2012;53:145-51).
The prevalence of depression was 33% in patients with schizophrenia and 20% in those with a schizoaffective disorder. The prevalence of depression was not significantly different in men vs. women (34% vs. 27%).
The patients’ treatments included risperidone (32 patients), quetiapine (21 patients), olanzapine (9 patients), fluphenazine (6 patients), amisulpride (6 patients), and other antipsychotics (14 patients).
When the presence of depression was analyzed according to antipsychotic treatment, the criteria for depression (a CDSS score below 5) were not met in 90% of patients on quetiapine, 67% of patients on fluphenazine, 56% of those on risperidone, and 44% of those on olanzapine, the researchers noted.
"Depression appears to be associated with a greater severity of disease, and in part, overlaps with negative symptoms," the researchers said.
The study is the first to use the CDSS to assess depression in schizophrenia patients, and the prevalence rates are similar to those found in other studies, they noted. Although the study was limited by its cross-sectional design, which did not permit the evaluation of symptoms over time, the findings suggest that clinical depression is often present in stable schizophrenia patients. The findings also suggest that depressive symptoms are not secondary to schizophrenia symptoms or the effects of medication, Dr. Majadas and her colleagues added.
The researchers said they had no relevant financial disclosures.
FROM COMPREHENSIVE PSYCHIATRY
Major Finding: The prevalence of depression was 31% in a study of 90 adults with stable schizophrenia.
Data Source: The data come from a cross-sectional statistical analysis of 90 adults aged 18-50 years with stable schizophrenia.
Disclosures: The researchers said they had no relevant financial disclosures.
C. difficile Infections Hit All-Time High
Clostridium difficile infections have reached an all-time high in the United States, and 94% of these infections initiate with medical care, based on data from the Centers for Disease Control and Prevention. C. difficile–related deaths increased from 3,000 in 1999-2000 to 14,000 in 2006-2007, according to the CDC.
The data were published as a CDC Vital Signs report and were presented in a telebriefing on March 6.
C. difficile is "a formidable opponent," and a patient safety issue everywhere that medical care is provided, said Dr. Clifford McDonald, a CDC epidemiologist and the lead author of the report. CDC’s data show that 25% of C. difficile infections first appear in hospitalized patients, while 75% occur either in nursing home residents or in people recently treated in doctors’ offices or clinics. People most at risk are those who take antibiotics and receive care in an outpatient setting.
In general, the risk of developing C. difficile increases with age; although half of C. difficile infections occur in those younger than 65 years, 90% of C. difficile-related deaths occur in those aged 65 years and older, said Dr. McDonald.
He said that clinicians can help reduce C. difficile infections by following six steps:
• Prescribe antibiotics judiciously.
• Be proactive about testing patients for C. difficile if they develop diarrhea while taking antibiotics.
• Isolate patients with C. difficile.
• Wear gloves and gowns when treating C. difficile patients, even for short visits.
• Clean surfaces in exam and treatment rooms with bleach or other spore-killing products.
• When a patient transfers to another facility, notify the medical team about a C. difficile infection.
Also, be sure to order the appropriate cultures to determine whether antibiotics are really needed, Dr. McDonald suggested, and watch for signs that signal C. difficile. "Antibiotic-associated diarrhea is very common," but C. difficile accounts for only about one-third of that, he said.
However, certain clues suggest C. difficile, including more than three unformed stools in 24 hours, fever, abdominal pain, diarrhea that continues once an antibiotic has been discontinued, or diarrhea that began only once an antibiotic was discontinued, he said.
If someone has been on antibiotics, think about C. difficile early and get them tested, whether they are patients in inpatient or outpatient facilities, Dr. McDonald emphasized.
To determine the current prevalence of C. difficile, CDC researchers reviewed data from their Emerging Infections Program, which conducted population-based surveillance from eight geographic areas, and the National Healthcare Safety Network (NHSN). In 2010, a total of 10,342 cases of C. difficile infection were identified via the Emerging Infections Program in 2010, and a total of 42,157 incident laboratory-identified CDI events were reported via the NHSN.
On a positive note, early results from state-led programs in Illinois, Massachusetts, and New York showed that hospital collaboration can reduce C. difficile infections, Dr. McDonald said. The 71 hospitals in these states that participated in C. difficile–prevention programs reduced infection rates by 20% over 21 months. "These promising results follow similar efforts in England, a nation that dropped C. difficile infections by more than 50% during a recent 3-year period," the CDC researchers said in the full report (MMWR 2012;61:1-6).
For additional information about tracking HAIs infections, contact the Emerging Infections Program or the NHSN.
Dr. McDonald had no financial conflicts to disclose.
The recent alarm by the Centers for Disease Control and Prevention which reports on the increasing incidence and burden of Clostridium difficile infections (CDI) in the United States is drawing attention to a phenomenon which is already well- known to gastroenterologists and infectious Ddisease physicians worldwide. In fact, the sweeping changes in the epidemiology of CDI – with reports of increasing rates, outbreaks, and elevated morbidity and mortality since 2002 originally in parts of Canada and the United States – has now been described in almost every developed country which keeps statistics on this infection.
The only fact which is even more alarming in the CDC’s report is that there has not been any “leveling off” or abatement in this health care–associated complication in the U.S. Although GI and ID physicians, hospitalists, and many other health care providers around the globe have been aware of the disquieting rise in CDI since 2002, the hoped-for stabilization or reduction in its incidence due to aggressive infection prevention and control (IPC) techniques, as has been seen in the U.K., has not materialized in the U.S. and central Canada.
Why is it so difficult to control CDI? Because it requires the accomplishment of multiple simultaneous aggressive IPC maneuvers – all of which must be done correctly -– in order to overcome this infection on an institutional level:
- Appropriate antimicrobial use and stewardship- Rapid testing, isolation, and treatment of any patient with diarrhea who has recently received antibiotics, while awaiting test results
- Rigorous hand-washing and appropriate use of personal protective equipment when in contact with suspected or confirmed cases
- Meticulous and frequent environmental cleaning with sporicidal agents which are tolerable to patients and personnel
- Attention to cleaning and disinfection of the innumerable shared pieces of medical equipment, like blood pressure cuffs, thermometers, bladder scanners, and the like
- High level of scrutiny for quickly detecting and treating recurrences, which occur in 15%-40% of CDI patients
- Real-time surveillance to detect outbreaks and effect heightened measures, when necessary.
The “new CDI” has demonstrated itself to be an unforgiving infection in health care facilities. Any lapse in one or more of the above IPC interventions is enough to cause a rise in the incidence or complication rate.
It is unclear exactly why the “new CDI” is behaving as it does. Certainly, the appearance of a new hyper-virulent strain with additional fluoroquinolone resistance (on top of C. difficile’s “usual” multi-drug resistance), a mutation in the toxin regulator gene, and a seemingly greater propensity to sporulate (and thus resist disinfection) appears to be the event which has coincided with the re-emergence of this disease during the past 10 years.
However, not all of the clinical aspects of this “new CDI” can be explained by these findings alone. For instance, the rapid progression from diarrhea to fulminant colitis in the elderly and the immunocompromised, the high recurrence rate (compared with historical controls) and the elevated morbidity (i.e., colectomy and need for intensive care) and mortality remain unnerving manifestations without a real explanation.
The number of scientific publications describing the new epidemiology, pathogenesis, and prophylactic or treatment options has risen impressively over the past several years in order to gain an understanding of this relentless bacterium.
Medical journals are not the only place for CDI-related news, however. The internet and social media sites are rife with stories of personal tragedies from this affliction. Typical stories like a healthy grandfather undergoing uncomplicated elective surgery but then dying from antibiotic-induced CDI during or soon after his hospitalization are too commonly detailed in surveillance data and in personal blogs.
Sites dedicated to and run by patients who have suffered from CDI compete with other sites established by the all-too-commonly depressed individuals who have had to undergo a therapeutic colectomy or who are suffering multiple recurrences of this disease and are searching for centers offering a fecal transplant.
There is no doubt that the true tragedy of CDI, a health care complication with an attributable mortality of almost 15% in the frail elderly over 85 years of age, is the fact that it is more likely to kill than the primary cause of hospitalization (such as a pneumonia, cellulitis, or hip fracture) in this population.
At a time when CDI is clearly overcoming our ability to control it in many parts of the world, it remains puzzling why the U.S. and many European countries do not yet have a true, real-time local and national CDI surveillance network for tracking the number of cases.
Using hospital discharge data or disease coding has been shown to be inaccurate and too late to be of immediate use. Real-time local, state, and national CDI surveillance is essential in telling us where we are, where we are going, and what we have to do to control this affliction. There is clearly a battle going on between health care providers and CDI in many countries, including the U.S. and Canada. We need to use as many tools and tricks as possible to gain control. This is one war we cannot afford to lose.
MARK MILLER, M.D., FRCPC, is Chief, Infectious Diseases, and Head, Infection Prevention and Control Unit, Jewish General Hospital, Montreal.
The recent alarm by the Centers for Disease Control and Prevention which reports on the increasing incidence and burden of Clostridium difficile infections (CDI) in the United States is drawing attention to a phenomenon which is already well- known to gastroenterologists and infectious Ddisease physicians worldwide. In fact, the sweeping changes in the epidemiology of CDI – with reports of increasing rates, outbreaks, and elevated morbidity and mortality since 2002 originally in parts of Canada and the United States – has now been described in almost every developed country which keeps statistics on this infection.
The only fact which is even more alarming in the CDC’s report is that there has not been any “leveling off” or abatement in this health care–associated complication in the U.S. Although GI and ID physicians, hospitalists, and many other health care providers around the globe have been aware of the disquieting rise in CDI since 2002, the hoped-for stabilization or reduction in its incidence due to aggressive infection prevention and control (IPC) techniques, as has been seen in the U.K., has not materialized in the U.S. and central Canada.
Why is it so difficult to control CDI? Because it requires the accomplishment of multiple simultaneous aggressive IPC maneuvers – all of which must be done correctly -– in order to overcome this infection on an institutional level:
- Appropriate antimicrobial use and stewardship- Rapid testing, isolation, and treatment of any patient with diarrhea who has recently received antibiotics, while awaiting test results
- Rigorous hand-washing and appropriate use of personal protective equipment when in contact with suspected or confirmed cases
- Meticulous and frequent environmental cleaning with sporicidal agents which are tolerable to patients and personnel
- Attention to cleaning and disinfection of the innumerable shared pieces of medical equipment, like blood pressure cuffs, thermometers, bladder scanners, and the like
- High level of scrutiny for quickly detecting and treating recurrences, which occur in 15%-40% of CDI patients
- Real-time surveillance to detect outbreaks and effect heightened measures, when necessary.
The “new CDI” has demonstrated itself to be an unforgiving infection in health care facilities. Any lapse in one or more of the above IPC interventions is enough to cause a rise in the incidence or complication rate.
It is unclear exactly why the “new CDI” is behaving as it does. Certainly, the appearance of a new hyper-virulent strain with additional fluoroquinolone resistance (on top of C. difficile’s “usual” multi-drug resistance), a mutation in the toxin regulator gene, and a seemingly greater propensity to sporulate (and thus resist disinfection) appears to be the event which has coincided with the re-emergence of this disease during the past 10 years.
However, not all of the clinical aspects of this “new CDI” can be explained by these findings alone. For instance, the rapid progression from diarrhea to fulminant colitis in the elderly and the immunocompromised, the high recurrence rate (compared with historical controls) and the elevated morbidity (i.e., colectomy and need for intensive care) and mortality remain unnerving manifestations without a real explanation.
The number of scientific publications describing the new epidemiology, pathogenesis, and prophylactic or treatment options has risen impressively over the past several years in order to gain an understanding of this relentless bacterium.
Medical journals are not the only place for CDI-related news, however. The internet and social media sites are rife with stories of personal tragedies from this affliction. Typical stories like a healthy grandfather undergoing uncomplicated elective surgery but then dying from antibiotic-induced CDI during or soon after his hospitalization are too commonly detailed in surveillance data and in personal blogs.
Sites dedicated to and run by patients who have suffered from CDI compete with other sites established by the all-too-commonly depressed individuals who have had to undergo a therapeutic colectomy or who are suffering multiple recurrences of this disease and are searching for centers offering a fecal transplant.
There is no doubt that the true tragedy of CDI, a health care complication with an attributable mortality of almost 15% in the frail elderly over 85 years of age, is the fact that it is more likely to kill than the primary cause of hospitalization (such as a pneumonia, cellulitis, or hip fracture) in this population.
At a time when CDI is clearly overcoming our ability to control it in many parts of the world, it remains puzzling why the U.S. and many European countries do not yet have a true, real-time local and national CDI surveillance network for tracking the number of cases.
Using hospital discharge data or disease coding has been shown to be inaccurate and too late to be of immediate use. Real-time local, state, and national CDI surveillance is essential in telling us where we are, where we are going, and what we have to do to control this affliction. There is clearly a battle going on between health care providers and CDI in many countries, including the U.S. and Canada. We need to use as many tools and tricks as possible to gain control. This is one war we cannot afford to lose.
MARK MILLER, M.D., FRCPC, is Chief, Infectious Diseases, and Head, Infection Prevention and Control Unit, Jewish General Hospital, Montreal.
The recent alarm by the Centers for Disease Control and Prevention which reports on the increasing incidence and burden of Clostridium difficile infections (CDI) in the United States is drawing attention to a phenomenon which is already well- known to gastroenterologists and infectious Ddisease physicians worldwide. In fact, the sweeping changes in the epidemiology of CDI – with reports of increasing rates, outbreaks, and elevated morbidity and mortality since 2002 originally in parts of Canada and the United States – has now been described in almost every developed country which keeps statistics on this infection.
The only fact which is even more alarming in the CDC’s report is that there has not been any “leveling off” or abatement in this health care–associated complication in the U.S. Although GI and ID physicians, hospitalists, and many other health care providers around the globe have been aware of the disquieting rise in CDI since 2002, the hoped-for stabilization or reduction in its incidence due to aggressive infection prevention and control (IPC) techniques, as has been seen in the U.K., has not materialized in the U.S. and central Canada.
Why is it so difficult to control CDI? Because it requires the accomplishment of multiple simultaneous aggressive IPC maneuvers – all of which must be done correctly -– in order to overcome this infection on an institutional level:
- Appropriate antimicrobial use and stewardship- Rapid testing, isolation, and treatment of any patient with diarrhea who has recently received antibiotics, while awaiting test results
- Rigorous hand-washing and appropriate use of personal protective equipment when in contact with suspected or confirmed cases
- Meticulous and frequent environmental cleaning with sporicidal agents which are tolerable to patients and personnel
- Attention to cleaning and disinfection of the innumerable shared pieces of medical equipment, like blood pressure cuffs, thermometers, bladder scanners, and the like
- High level of scrutiny for quickly detecting and treating recurrences, which occur in 15%-40% of CDI patients
- Real-time surveillance to detect outbreaks and effect heightened measures, when necessary.
The “new CDI” has demonstrated itself to be an unforgiving infection in health care facilities. Any lapse in one or more of the above IPC interventions is enough to cause a rise in the incidence or complication rate.
It is unclear exactly why the “new CDI” is behaving as it does. Certainly, the appearance of a new hyper-virulent strain with additional fluoroquinolone resistance (on top of C. difficile’s “usual” multi-drug resistance), a mutation in the toxin regulator gene, and a seemingly greater propensity to sporulate (and thus resist disinfection) appears to be the event which has coincided with the re-emergence of this disease during the past 10 years.
However, not all of the clinical aspects of this “new CDI” can be explained by these findings alone. For instance, the rapid progression from diarrhea to fulminant colitis in the elderly and the immunocompromised, the high recurrence rate (compared with historical controls) and the elevated morbidity (i.e., colectomy and need for intensive care) and mortality remain unnerving manifestations without a real explanation.
The number of scientific publications describing the new epidemiology, pathogenesis, and prophylactic or treatment options has risen impressively over the past several years in order to gain an understanding of this relentless bacterium.
Medical journals are not the only place for CDI-related news, however. The internet and social media sites are rife with stories of personal tragedies from this affliction. Typical stories like a healthy grandfather undergoing uncomplicated elective surgery but then dying from antibiotic-induced CDI during or soon after his hospitalization are too commonly detailed in surveillance data and in personal blogs.
Sites dedicated to and run by patients who have suffered from CDI compete with other sites established by the all-too-commonly depressed individuals who have had to undergo a therapeutic colectomy or who are suffering multiple recurrences of this disease and are searching for centers offering a fecal transplant.
There is no doubt that the true tragedy of CDI, a health care complication with an attributable mortality of almost 15% in the frail elderly over 85 years of age, is the fact that it is more likely to kill than the primary cause of hospitalization (such as a pneumonia, cellulitis, or hip fracture) in this population.
At a time when CDI is clearly overcoming our ability to control it in many parts of the world, it remains puzzling why the U.S. and many European countries do not yet have a true, real-time local and national CDI surveillance network for tracking the number of cases.
Using hospital discharge data or disease coding has been shown to be inaccurate and too late to be of immediate use. Real-time local, state, and national CDI surveillance is essential in telling us where we are, where we are going, and what we have to do to control this affliction. There is clearly a battle going on between health care providers and CDI in many countries, including the U.S. and Canada. We need to use as many tools and tricks as possible to gain control. This is one war we cannot afford to lose.
MARK MILLER, M.D., FRCPC, is Chief, Infectious Diseases, and Head, Infection Prevention and Control Unit, Jewish General Hospital, Montreal.
Clostridium difficile infections have reached an all-time high in the United States, and 94% of these infections initiate with medical care, based on data from the Centers for Disease Control and Prevention. C. difficile–related deaths increased from 3,000 in 1999-2000 to 14,000 in 2006-2007, according to the CDC.
The data were published as a CDC Vital Signs report and were presented in a telebriefing on March 6.
C. difficile is "a formidable opponent," and a patient safety issue everywhere that medical care is provided, said Dr. Clifford McDonald, a CDC epidemiologist and the lead author of the report. CDC’s data show that 25% of C. difficile infections first appear in hospitalized patients, while 75% occur either in nursing home residents or in people recently treated in doctors’ offices or clinics. People most at risk are those who take antibiotics and receive care in an outpatient setting.
In general, the risk of developing C. difficile increases with age; although half of C. difficile infections occur in those younger than 65 years, 90% of C. difficile-related deaths occur in those aged 65 years and older, said Dr. McDonald.
He said that clinicians can help reduce C. difficile infections by following six steps:
• Prescribe antibiotics judiciously.
• Be proactive about testing patients for C. difficile if they develop diarrhea while taking antibiotics.
• Isolate patients with C. difficile.
• Wear gloves and gowns when treating C. difficile patients, even for short visits.
• Clean surfaces in exam and treatment rooms with bleach or other spore-killing products.
• When a patient transfers to another facility, notify the medical team about a C. difficile infection.
Also, be sure to order the appropriate cultures to determine whether antibiotics are really needed, Dr. McDonald suggested, and watch for signs that signal C. difficile. "Antibiotic-associated diarrhea is very common," but C. difficile accounts for only about one-third of that, he said.
However, certain clues suggest C. difficile, including more than three unformed stools in 24 hours, fever, abdominal pain, diarrhea that continues once an antibiotic has been discontinued, or diarrhea that began only once an antibiotic was discontinued, he said.
If someone has been on antibiotics, think about C. difficile early and get them tested, whether they are patients in inpatient or outpatient facilities, Dr. McDonald emphasized.
To determine the current prevalence of C. difficile, CDC researchers reviewed data from their Emerging Infections Program, which conducted population-based surveillance from eight geographic areas, and the National Healthcare Safety Network (NHSN). In 2010, a total of 10,342 cases of C. difficile infection were identified via the Emerging Infections Program in 2010, and a total of 42,157 incident laboratory-identified CDI events were reported via the NHSN.
On a positive note, early results from state-led programs in Illinois, Massachusetts, and New York showed that hospital collaboration can reduce C. difficile infections, Dr. McDonald said. The 71 hospitals in these states that participated in C. difficile–prevention programs reduced infection rates by 20% over 21 months. "These promising results follow similar efforts in England, a nation that dropped C. difficile infections by more than 50% during a recent 3-year period," the CDC researchers said in the full report (MMWR 2012;61:1-6).
For additional information about tracking HAIs infections, contact the Emerging Infections Program or the NHSN.
Dr. McDonald had no financial conflicts to disclose.
Clostridium difficile infections have reached an all-time high in the United States, and 94% of these infections initiate with medical care, based on data from the Centers for Disease Control and Prevention. C. difficile–related deaths increased from 3,000 in 1999-2000 to 14,000 in 2006-2007, according to the CDC.
The data were published as a CDC Vital Signs report and were presented in a telebriefing on March 6.
C. difficile is "a formidable opponent," and a patient safety issue everywhere that medical care is provided, said Dr. Clifford McDonald, a CDC epidemiologist and the lead author of the report. CDC’s data show that 25% of C. difficile infections first appear in hospitalized patients, while 75% occur either in nursing home residents or in people recently treated in doctors’ offices or clinics. People most at risk are those who take antibiotics and receive care in an outpatient setting.
In general, the risk of developing C. difficile increases with age; although half of C. difficile infections occur in those younger than 65 years, 90% of C. difficile-related deaths occur in those aged 65 years and older, said Dr. McDonald.
He said that clinicians can help reduce C. difficile infections by following six steps:
• Prescribe antibiotics judiciously.
• Be proactive about testing patients for C. difficile if they develop diarrhea while taking antibiotics.
• Isolate patients with C. difficile.
• Wear gloves and gowns when treating C. difficile patients, even for short visits.
• Clean surfaces in exam and treatment rooms with bleach or other spore-killing products.
• When a patient transfers to another facility, notify the medical team about a C. difficile infection.
Also, be sure to order the appropriate cultures to determine whether antibiotics are really needed, Dr. McDonald suggested, and watch for signs that signal C. difficile. "Antibiotic-associated diarrhea is very common," but C. difficile accounts for only about one-third of that, he said.
However, certain clues suggest C. difficile, including more than three unformed stools in 24 hours, fever, abdominal pain, diarrhea that continues once an antibiotic has been discontinued, or diarrhea that began only once an antibiotic was discontinued, he said.
If someone has been on antibiotics, think about C. difficile early and get them tested, whether they are patients in inpatient or outpatient facilities, Dr. McDonald emphasized.
To determine the current prevalence of C. difficile, CDC researchers reviewed data from their Emerging Infections Program, which conducted population-based surveillance from eight geographic areas, and the National Healthcare Safety Network (NHSN). In 2010, a total of 10,342 cases of C. difficile infection were identified via the Emerging Infections Program in 2010, and a total of 42,157 incident laboratory-identified CDI events were reported via the NHSN.
On a positive note, early results from state-led programs in Illinois, Massachusetts, and New York showed that hospital collaboration can reduce C. difficile infections, Dr. McDonald said. The 71 hospitals in these states that participated in C. difficile–prevention programs reduced infection rates by 20% over 21 months. "These promising results follow similar efforts in England, a nation that dropped C. difficile infections by more than 50% during a recent 3-year period," the CDC researchers said in the full report (MMWR 2012;61:1-6).
For additional information about tracking HAIs infections, contact the Emerging Infections Program or the NHSN.
Dr. McDonald had no financial conflicts to disclose.
Major Finding: C. difficile-related deaths increased from 3,000 in 1999-2000 to 14,000 in 2006-2007.
Data Source: The data were taken from the Centers for Disease Control and Prevention’s Emerging Infections Program and the National Healthcare Safety Network (NHSN).
Disclosures: Dr. McDonald had no financial conflicts to disclose.
IOM Report: Long-term Drug Safety Data Needed in Children
Federal legislation has improved the safety and design of drug studies in children, but there is room for improvement, particularly in conducting long-term follow-up studies of approved products, and in studies of newborns.
That was the finding of an Institute of Medicine review committee that examined studies of drugs and biologic products in children that have be conducted since the 1997 enactment of the Best Pharmaceuticals for Children Act (BPCA) and the Pediatric Research Equity Act (PREA).
The BPCA provides economic incentives for drug companies to respond to Food and Drug Administration requests for studies in children, while the PREA allows the FDA to require pediatric studies in certain situations. Both laws are scheduled to be reauthorized this year.
Since the two laws went into effect, the FDA has approved 400 labeling changes for drugs related to their use in children, according to the report. The report did not address the impact of BPCA and PREA on clinical practice or on the off-label use of medication in children.
Planning, initiating, and completing studies in a timelier manner would increase the benefits for children, the report authors noted. But they added that not all studies under BPCA and PREA succeed for many reasons, including insufficient numbers, weak study designs, and reluctance of doctors and parents to enroll children in clinical trials of approved adult drugs.
Although public access to information from pediatric drug studies has greatly improved under the two laws, such studies remain limited in several key areas, namely the lack of long-term safety data on drugs that children take for chronic conditions and on the long-term side effects on growth and development in children who take medications for short periods during infancy and early childhood.
In addition, there remains a lack of data on the use of drugs in infants aged 28 days and younger, and in premature or sick newborns, according to the report.
In 2010, Congress extended BPCA incentives to include biologics; therefore, it is too soon to evaluate any impact in this area, the authors noted.
The IOM review committee made suggestions for further improvements to pediatric drug studies, including:
• Improved public access to study findings.
• Improved timeliness of pediatric studies.
• Added emphasis on areas with insufficient research, including drug studies in newborns and long-term safety studies of children using medication for short-term and chronic conditions.
• Improved design and execution of pediatric studies.
• Added encouragement for the study of biologics in children.
• Improved clarity and understanding of the expected health benefits of pediatric studies requested under BPCA.
Federal legislation has improved the safety and design of drug studies in children, but there is room for improvement, particularly in conducting long-term follow-up studies of approved products, and in studies of newborns.
That was the finding of an Institute of Medicine review committee that examined studies of drugs and biologic products in children that have be conducted since the 1997 enactment of the Best Pharmaceuticals for Children Act (BPCA) and the Pediatric Research Equity Act (PREA).
The BPCA provides economic incentives for drug companies to respond to Food and Drug Administration requests for studies in children, while the PREA allows the FDA to require pediatric studies in certain situations. Both laws are scheduled to be reauthorized this year.
Since the two laws went into effect, the FDA has approved 400 labeling changes for drugs related to their use in children, according to the report. The report did not address the impact of BPCA and PREA on clinical practice or on the off-label use of medication in children.
Planning, initiating, and completing studies in a timelier manner would increase the benefits for children, the report authors noted. But they added that not all studies under BPCA and PREA succeed for many reasons, including insufficient numbers, weak study designs, and reluctance of doctors and parents to enroll children in clinical trials of approved adult drugs.
Although public access to information from pediatric drug studies has greatly improved under the two laws, such studies remain limited in several key areas, namely the lack of long-term safety data on drugs that children take for chronic conditions and on the long-term side effects on growth and development in children who take medications for short periods during infancy and early childhood.
In addition, there remains a lack of data on the use of drugs in infants aged 28 days and younger, and in premature or sick newborns, according to the report.
In 2010, Congress extended BPCA incentives to include biologics; therefore, it is too soon to evaluate any impact in this area, the authors noted.
The IOM review committee made suggestions for further improvements to pediatric drug studies, including:
• Improved public access to study findings.
• Improved timeliness of pediatric studies.
• Added emphasis on areas with insufficient research, including drug studies in newborns and long-term safety studies of children using medication for short-term and chronic conditions.
• Improved design and execution of pediatric studies.
• Added encouragement for the study of biologics in children.
• Improved clarity and understanding of the expected health benefits of pediatric studies requested under BPCA.
Federal legislation has improved the safety and design of drug studies in children, but there is room for improvement, particularly in conducting long-term follow-up studies of approved products, and in studies of newborns.
That was the finding of an Institute of Medicine review committee that examined studies of drugs and biologic products in children that have be conducted since the 1997 enactment of the Best Pharmaceuticals for Children Act (BPCA) and the Pediatric Research Equity Act (PREA).
The BPCA provides economic incentives for drug companies to respond to Food and Drug Administration requests for studies in children, while the PREA allows the FDA to require pediatric studies in certain situations. Both laws are scheduled to be reauthorized this year.
Since the two laws went into effect, the FDA has approved 400 labeling changes for drugs related to their use in children, according to the report. The report did not address the impact of BPCA and PREA on clinical practice or on the off-label use of medication in children.
Planning, initiating, and completing studies in a timelier manner would increase the benefits for children, the report authors noted. But they added that not all studies under BPCA and PREA succeed for many reasons, including insufficient numbers, weak study designs, and reluctance of doctors and parents to enroll children in clinical trials of approved adult drugs.
Although public access to information from pediatric drug studies has greatly improved under the two laws, such studies remain limited in several key areas, namely the lack of long-term safety data on drugs that children take for chronic conditions and on the long-term side effects on growth and development in children who take medications for short periods during infancy and early childhood.
In addition, there remains a lack of data on the use of drugs in infants aged 28 days and younger, and in premature or sick newborns, according to the report.
In 2010, Congress extended BPCA incentives to include biologics; therefore, it is too soon to evaluate any impact in this area, the authors noted.
The IOM review committee made suggestions for further improvements to pediatric drug studies, including:
• Improved public access to study findings.
• Improved timeliness of pediatric studies.
• Added emphasis on areas with insufficient research, including drug studies in newborns and long-term safety studies of children using medication for short-term and chronic conditions.
• Improved design and execution of pediatric studies.
• Added encouragement for the study of biologics in children.
• Improved clarity and understanding of the expected health benefits of pediatric studies requested under BPCA.
FROM A REPORT FROM THE INSTITUTE OF MEDICINE
'Early, Proactive' Management of Chemo Toxicites Improves QOL
Skin conditions in cancer patients can lead to a decreased quality of life because of the psychosocial impact, financial burden, poor health, and disruption of anticancer treatments, said Dr. Mario E. Lacouture at the annual Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF).
Clinical trials are underway to address management, cost, and quality of life, but in the meantime, "an early and proactive approach toward toxicities is advisable," he said.
Dr. Lacouture, a dermatologist at Memorial Sloan-Kettering Cancer Center in New York, discussed common cancer-associated dermatology at the seminar.
• Rashes. A RAF inhibitor–induced rash can occur within 1-2 weeks of starting treatment, Dr. Lacouture noted. This rash is associated with sensations of burning or itching in photo-exposed areas. In most cases, the rash can be managed with topical steroids and oral GABA agonists. Vemurafenib use also has been associated with rashes, as well as with photosensitivity, itching, and alopecia, he said.
In addition, mTOR inhibitor–induced rashes involving erythematous pruritic papules and ulcerlike lesions have been observed in patients on everolimus and temsirolimus.
• Skin cancers. Data from several studies suggest that the incidence of squamous cell carcinomas in patients on RAF inhibitors is 7%, and the incidence of actinic keratoses in these patients is 4%, according to Dr. Lacouture. These conditions usually develop after approximately 6 months of treatment. They can be managed by surgery or destruction; and there have been no reports of metastases.
• Hand-foot syndrome. Hand-Foot Syndrome (HFSR) is a common side effect in cancer patients that usually occurs within 45 days of starting treatment, said Dr. Lacouture. HFSR is often associated with the use of sorafenib, sunitinib, and pazopanib, and the histology of these patients shows epidermal necrosis, he noted. Patients who develop HFSR may need to temporarily reduce their medications. Additional management of HFSR includes avoiding excess pressure on the affected areas and keeping them cool and moist to reduce patient discomfort.
• Hair and nail changes. Changes in hair can occur in patients being treated with epidermal growth factor receptor inhibitors (EGFRIs), including a slower hair growth rate and alopecia, Dr. Lacouture said. Changes in hair texture such as increased brittleness and hair curling also have been observed, he said. Other hair-related adverse events can include hirsutism on the face and eyelash trichomegaly. In addition, paronychia has been observed in patients on EGFRIs for more than 6 months.
• Radiation dermatitis. Approximately 50% of cancer patients develop radiation dermatitis, including 87% of breast cancer patients, reported Dr. Lacouture. However, the treatment of radiation dermatitis remains challenging, he said. In several studies, patients who used a nonsteroidal topical, including aloe vera and trolamine, showed no significant improvement in radiation dermatitis, compared with controls. However, studies are ongoing, and significant improvements in radiation dermatitis in breast cancer patients have been seen with topical corticosteroids including mometasone, beclomethasone, and betamethasone.
Dr. Lacouture disclosed financial relationships with multiple companies including Amgen, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Merck, and Roche. SDEF and this news organization are owned by Elsevier.
Skin conditions in cancer patients can lead to a decreased quality of life because of the psychosocial impact, financial burden, poor health, and disruption of anticancer treatments, said Dr. Mario E. Lacouture at the annual Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF).
Clinical trials are underway to address management, cost, and quality of life, but in the meantime, "an early and proactive approach toward toxicities is advisable," he said.
Dr. Lacouture, a dermatologist at Memorial Sloan-Kettering Cancer Center in New York, discussed common cancer-associated dermatology at the seminar.
• Rashes. A RAF inhibitor–induced rash can occur within 1-2 weeks of starting treatment, Dr. Lacouture noted. This rash is associated with sensations of burning or itching in photo-exposed areas. In most cases, the rash can be managed with topical steroids and oral GABA agonists. Vemurafenib use also has been associated with rashes, as well as with photosensitivity, itching, and alopecia, he said.
In addition, mTOR inhibitor–induced rashes involving erythematous pruritic papules and ulcerlike lesions have been observed in patients on everolimus and temsirolimus.
• Skin cancers. Data from several studies suggest that the incidence of squamous cell carcinomas in patients on RAF inhibitors is 7%, and the incidence of actinic keratoses in these patients is 4%, according to Dr. Lacouture. These conditions usually develop after approximately 6 months of treatment. They can be managed by surgery or destruction; and there have been no reports of metastases.
• Hand-foot syndrome. Hand-Foot Syndrome (HFSR) is a common side effect in cancer patients that usually occurs within 45 days of starting treatment, said Dr. Lacouture. HFSR is often associated with the use of sorafenib, sunitinib, and pazopanib, and the histology of these patients shows epidermal necrosis, he noted. Patients who develop HFSR may need to temporarily reduce their medications. Additional management of HFSR includes avoiding excess pressure on the affected areas and keeping them cool and moist to reduce patient discomfort.
• Hair and nail changes. Changes in hair can occur in patients being treated with epidermal growth factor receptor inhibitors (EGFRIs), including a slower hair growth rate and alopecia, Dr. Lacouture said. Changes in hair texture such as increased brittleness and hair curling also have been observed, he said. Other hair-related adverse events can include hirsutism on the face and eyelash trichomegaly. In addition, paronychia has been observed in patients on EGFRIs for more than 6 months.
• Radiation dermatitis. Approximately 50% of cancer patients develop radiation dermatitis, including 87% of breast cancer patients, reported Dr. Lacouture. However, the treatment of radiation dermatitis remains challenging, he said. In several studies, patients who used a nonsteroidal topical, including aloe vera and trolamine, showed no significant improvement in radiation dermatitis, compared with controls. However, studies are ongoing, and significant improvements in radiation dermatitis in breast cancer patients have been seen with topical corticosteroids including mometasone, beclomethasone, and betamethasone.
Dr. Lacouture disclosed financial relationships with multiple companies including Amgen, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Merck, and Roche. SDEF and this news organization are owned by Elsevier.
Skin conditions in cancer patients can lead to a decreased quality of life because of the psychosocial impact, financial burden, poor health, and disruption of anticancer treatments, said Dr. Mario E. Lacouture at the annual Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF).
Clinical trials are underway to address management, cost, and quality of life, but in the meantime, "an early and proactive approach toward toxicities is advisable," he said.
Dr. Lacouture, a dermatologist at Memorial Sloan-Kettering Cancer Center in New York, discussed common cancer-associated dermatology at the seminar.
• Rashes. A RAF inhibitor–induced rash can occur within 1-2 weeks of starting treatment, Dr. Lacouture noted. This rash is associated with sensations of burning or itching in photo-exposed areas. In most cases, the rash can be managed with topical steroids and oral GABA agonists. Vemurafenib use also has been associated with rashes, as well as with photosensitivity, itching, and alopecia, he said.
In addition, mTOR inhibitor–induced rashes involving erythematous pruritic papules and ulcerlike lesions have been observed in patients on everolimus and temsirolimus.
• Skin cancers. Data from several studies suggest that the incidence of squamous cell carcinomas in patients on RAF inhibitors is 7%, and the incidence of actinic keratoses in these patients is 4%, according to Dr. Lacouture. These conditions usually develop after approximately 6 months of treatment. They can be managed by surgery or destruction; and there have been no reports of metastases.
• Hand-foot syndrome. Hand-Foot Syndrome (HFSR) is a common side effect in cancer patients that usually occurs within 45 days of starting treatment, said Dr. Lacouture. HFSR is often associated with the use of sorafenib, sunitinib, and pazopanib, and the histology of these patients shows epidermal necrosis, he noted. Patients who develop HFSR may need to temporarily reduce their medications. Additional management of HFSR includes avoiding excess pressure on the affected areas and keeping them cool and moist to reduce patient discomfort.
• Hair and nail changes. Changes in hair can occur in patients being treated with epidermal growth factor receptor inhibitors (EGFRIs), including a slower hair growth rate and alopecia, Dr. Lacouture said. Changes in hair texture such as increased brittleness and hair curling also have been observed, he said. Other hair-related adverse events can include hirsutism on the face and eyelash trichomegaly. In addition, paronychia has been observed in patients on EGFRIs for more than 6 months.
• Radiation dermatitis. Approximately 50% of cancer patients develop radiation dermatitis, including 87% of breast cancer patients, reported Dr. Lacouture. However, the treatment of radiation dermatitis remains challenging, he said. In several studies, patients who used a nonsteroidal topical, including aloe vera and trolamine, showed no significant improvement in radiation dermatitis, compared with controls. However, studies are ongoing, and significant improvements in radiation dermatitis in breast cancer patients have been seen with topical corticosteroids including mometasone, beclomethasone, and betamethasone.
Dr. Lacouture disclosed financial relationships with multiple companies including Amgen, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Merck, and Roche. SDEF and this news organization are owned by Elsevier.
EXPERT ANALYSIS FROM THE SDEF HAWAII DERMATOLOGY SEMINAR