User login
Cosmetic Treatments for Skin of Color: Report From the AAD Meeting
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Cutaneous Manifestations of Tick-Borne Diseases
Infection Risk With Biologic Therapy for Psoriasis: Report From the AAD Meeting
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Herpes Zoster Following Varicella Vaccination in Children
Varicella-zoster virus (VZV) causes varicella as a primary infection. It is a highly contagious disease characterized by a widespread papulovesicular eruption with fever and malaise.1,2 After the primary infection, the virus remains latent within the sensory dorsal root ganglia and can reactivate as herpes zoster (HZ).1-5 Herpes zoster is characterized by unilateral radicular pain and a vesicular rash in a dermatomal pattern.1,2 It is most common in adults, especially elderly and immunocompromised patients, but rarely occurs in children. Herpes zoster is most often seen in individuals previously infected with VZV, but it also has occurred in individuals without known varicella infection,1-17 possibly because these individuals had a prior subclinical VZV infection.
A live attenuated VZV vaccine was created after isolation of the virus from a child in Japan.2 Since the introduction of the vaccine in 1995 in the United States, the incidence of VZV and HZ has declined.5 Herpes zoster rates after vaccination vary from 14 to 19 per 100,000 individuals.3,5 Breakthrough disease with the wild-type strain does occur in vaccinated children, but vaccine-strain HZ also has been reported.1-5 The risk for HZ caused by reactivated VZV vaccine in healthy children is unknown. We present a case of HZ in an otherwise healthy 19-month-old boy with no known varicella exposure who received the VZV vaccine at 13 months of age.
Case Report
An otherwise healthy 19-month-old boy presented to the dermatology clinic with a rash that began 2 days prior on the right groin and spread to the right leg. The patient’s mother denied that the child had been febrile and noted that the rash did not appear to bother him in any way. The patient was up-to-date on his vaccinations and received the first dose of the varicella series 6 months prior to presentation. He had no personal history of varicella, no exposure to sick contacts with varicella, and no known exposure to the virus. He was otherwise completely healthy with no signs or symptoms of immunocompromise.
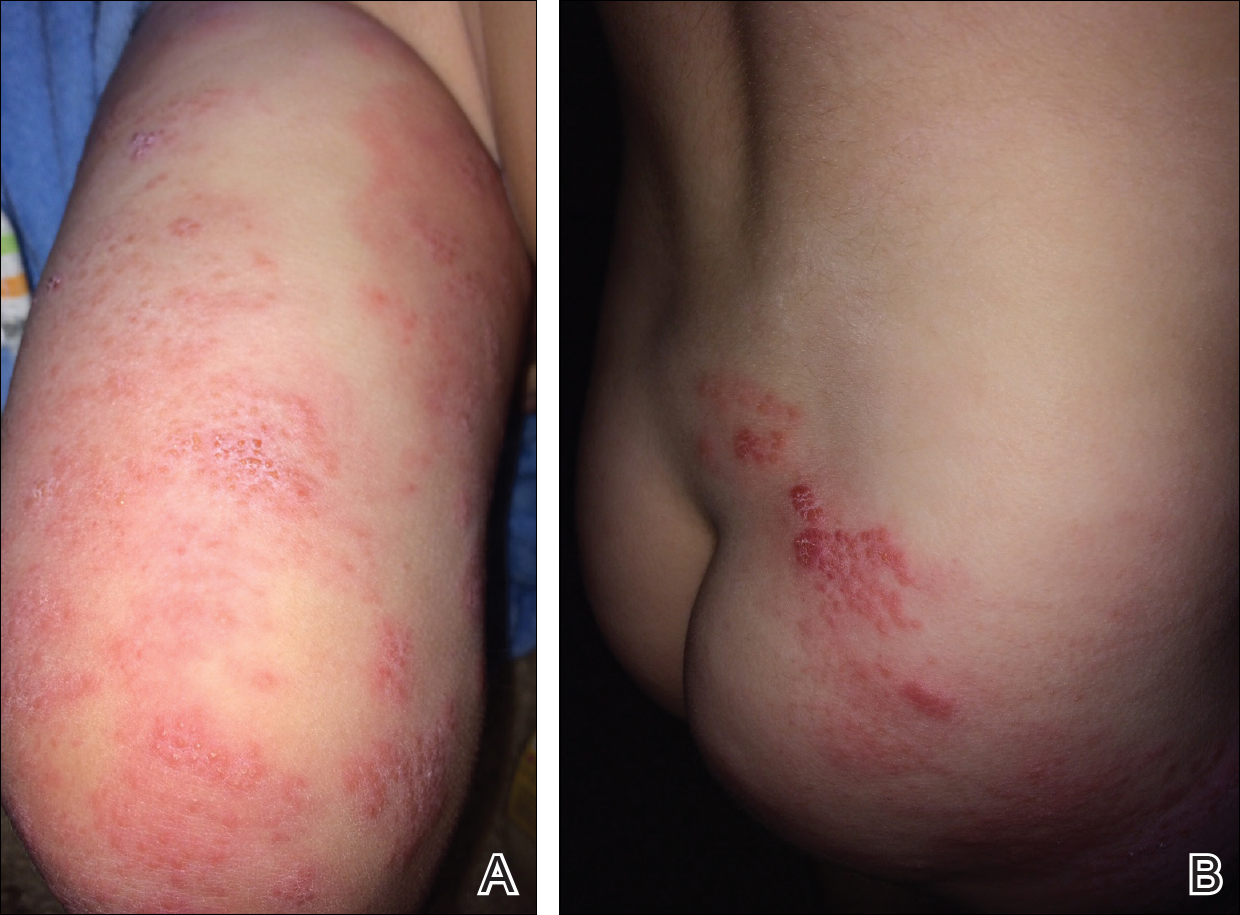
Physical examination revealed grouped vesicles on an erythematous base on the right thigh, right sacrum, and lower abdomen that did not cross the midline (Figure). There were no other pertinent physical examination findings. The eruption was most consistent with HZ but concern remained for herpes simplex virus (HSV) or impetigo. A bacterial culture and polymerase chain reaction assay for VZV and HSV from skin swabs was ordered. The patient was prescribed acyclovir 20 mg/kg every 6 hours for 5 days. Laboratory testing revealed a positive result for VZV on polymerase chain reaction and a negative result for HSV. The majority of the patient’s lesions had crusted after 2 days of treatment with acyclovir, and the rash had nearly resolved 1 week after presentation. Subsequent evaluation with a complete blood cell count with differential and basic metabolic profile was normal. Levels of IgG, IgA, and IgM also were normal; IgE was slightly elevated.
Comment
Herpes zoster in children is an uncommon clinical entity. Most children with HZ are immunocompromised, have a history of varicella, or were exposed to varicella during gestation.8 With the introduction of the live VZV vaccine, the incidence of HZ has declined, but reactivation of the live vaccine leading to HZ infection is possible. The vaccine is 90% effective, and breakthrough varicella has been reported in 15% to 20% of vaccinated patients.1-17 The cause of HZ in vaccinated children is unclear due to the potential for either wild-type or vaccine-strain VZV to induce HZ.
Twenty-two cases of HZ in healthy children after vaccination were identified with a PubMed search of articles indexed for MEDLINE using the search terms herpes zoster infection after vaccination and herpes zoster infection AND immunocompetent AND vaccination in separate searches for all English-language studies (Table). The search was limited to immunocompetent children and adolescents who were 18 years or younger with no history of varicella or exposure to varicella during gestation.
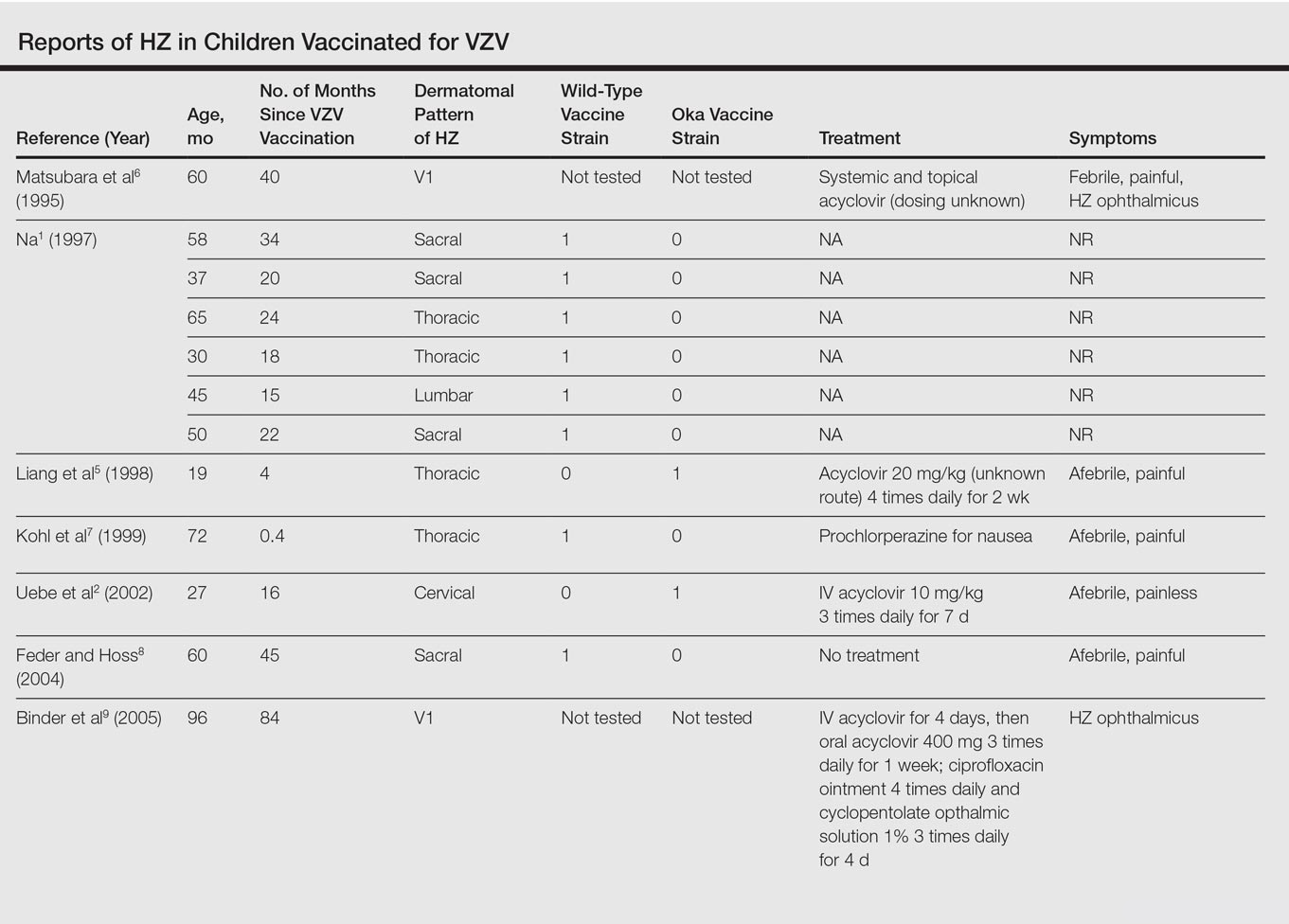

The mean age for HZ infection was 5.3 years. The average time between vaccination and HZ infection was 3.3 years. There was a spread of dermatomal patterns with cases in the first division of the trigeminal nerve, cervical, thoracic, lumbar, and sacral distributions. Of the 22 cases of HZ we reviewed, 16 underwent genotype testing to determine the source of the infection. The Oka vaccine strain virus was identified in 8 (50%) cases, while wild-type virus was found in 8 (50%) cases.1,2,4,5,7,8,10,11,13,14,16 Twelve cases were treated with acyclovir.2,3,5,6,9-12,14-17 The method of delivery, either oral or intravenous, and the length of treatment depended on the severity of the disease. Patients with meningoencephalitis and HZ ophthalmicus received intravenous acyclovir more often and also had a longer course of acyclovir compared to those individuals with involvement limited to the skin.
This review found HZ occurs from reactivation of wild-type or Oka vaccine-strain VZV in immunocompetent children.1-17 It shows that subclinical varicella infection is not the only explanation for HZ in a healthy vaccinated child. It is currently not clear why some healthy children experience HZ from vaccine-strain VZV. When HZ presents in a vaccinated immunocompetent child without a history of varicella infection or exposure, the possibility for vaccine strain–induced HZ should be considered.
- . Herpes zoster in three healthy children immunized with varicella vaccine (Oka/Biken); the causative virus differed from vaccine strain on PCR analysis of the IV variable region (R5) and of a PstI-site region. Br J Dermatol. 1997;137:255-258.
- Uebe B, Sauerbrei A, Burdach S, et al. Herpes zoster by reactivated vaccine varicella zoster virus in a healthy child [published online June 25, 2002]. Eur J Pediatr. 2002;161:442-444.
- Obieta MP, Jacinto SS. Herpes zoster after varicella vaccination in a healthy young child. Int J Dermatol. 2008;47:640-641.
- Ota K, Kim V, Lavi S, et al. Vaccine-strain varicella zoster virus causing recurrent herpes zoster in an immunocompetent 2-year-old. Pediatr Infect Dis J. 2008;27:847-848.
- Liang GL, Heidelberg KA, Jacobson RM, et al. Herpes zoster after varicella vaccination. J Am Acad Dermatol. 1998;38:761-763.
- Matsubara K, Nigami H, Harigaya H, et al. Herpes zoster in a normal child after varicella vaccination. Acta Paediatr Jpn. 1995;37:648-650.
- Kohl S, Rapp J, Larussa P, et al. Natural varicella-zoster virus reactivation shortly after varicella immunization in a child. Pediatr Infect Dis J. 1999;18:1112-1113.
- Feder HM Jr, Hoss DM. Herpes zoster in otherwise healthy children. Pediatr Infect Dis J. 2004;23:451-457; quiz 458-460.
- Binder NR, Holland GN, Hosea S, et al. Herpes zoster ophthalmicus in an otherwise-healthy child. J AAPOS. 2005;9:597-598.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Lin P, Yoon MK, Chiu CS. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocul Immunol Inflamm. 2009;17:33-35.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child [published online March 1, 2010]. Pediatrics. 2010;125:E969-E972.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Ryu WY, Kim NY, Kwon YH, et al. Herpes zoster ophthalmicus with isolated trochlear nerve palsy in an otherwise healthy 13-year-old girl. J AAPOS. 2014;18:193-195.
- Iwasaki S, Motokura K, Honda Y, et al. Vaccine-strain herpes zoster found in the trigeminal nerve area in a healthy child: a case report [published online November 3, 2016]. J Clin Virol. 2016;85:44-47.
- Peterson N, Goodman S, Peterson M, et al. Herpes zoster in children. Cutis. 2016;98:94-95.
Varicella-zoster virus (VZV) causes varicella as a primary infection. It is a highly contagious disease characterized by a widespread papulovesicular eruption with fever and malaise.1,2 After the primary infection, the virus remains latent within the sensory dorsal root ganglia and can reactivate as herpes zoster (HZ).1-5 Herpes zoster is characterized by unilateral radicular pain and a vesicular rash in a dermatomal pattern.1,2 It is most common in adults, especially elderly and immunocompromised patients, but rarely occurs in children. Herpes zoster is most often seen in individuals previously infected with VZV, but it also has occurred in individuals without known varicella infection,1-17 possibly because these individuals had a prior subclinical VZV infection.
A live attenuated VZV vaccine was created after isolation of the virus from a child in Japan.2 Since the introduction of the vaccine in 1995 in the United States, the incidence of VZV and HZ has declined.5 Herpes zoster rates after vaccination vary from 14 to 19 per 100,000 individuals.3,5 Breakthrough disease with the wild-type strain does occur in vaccinated children, but vaccine-strain HZ also has been reported.1-5 The risk for HZ caused by reactivated VZV vaccine in healthy children is unknown. We present a case of HZ in an otherwise healthy 19-month-old boy with no known varicella exposure who received the VZV vaccine at 13 months of age.
Case Report
An otherwise healthy 19-month-old boy presented to the dermatology clinic with a rash that began 2 days prior on the right groin and spread to the right leg. The patient’s mother denied that the child had been febrile and noted that the rash did not appear to bother him in any way. The patient was up-to-date on his vaccinations and received the first dose of the varicella series 6 months prior to presentation. He had no personal history of varicella, no exposure to sick contacts with varicella, and no known exposure to the virus. He was otherwise completely healthy with no signs or symptoms of immunocompromise.
Physical examination revealed grouped vesicles on an erythematous base on the right thigh, right sacrum, and lower abdomen that did not cross the midline (Figure). There were no other pertinent physical examination findings. The eruption was most consistent with HZ but concern remained for herpes simplex virus (HSV) or impetigo. A bacterial culture and polymerase chain reaction assay for VZV and HSV from skin swabs was ordered. The patient was prescribed acyclovir 20 mg/kg every 6 hours for 5 days. Laboratory testing revealed a positive result for VZV on polymerase chain reaction and a negative result for HSV. The majority of the patient’s lesions had crusted after 2 days of treatment with acyclovir, and the rash had nearly resolved 1 week after presentation. Subsequent evaluation with a complete blood cell count with differential and basic metabolic profile was normal. Levels of IgG, IgA, and IgM also were normal; IgE was slightly elevated.

Comment
Herpes zoster in children is an uncommon clinical entity. Most children with HZ are immunocompromised, have a history of varicella, or were exposed to varicella during gestation.8 With the introduction of the live VZV vaccine, the incidence of HZ has declined, but reactivation of the live vaccine leading to HZ infection is possible. The vaccine is 90% effective, and breakthrough varicella has been reported in 15% to 20% of vaccinated patients.1-17 The cause of HZ in vaccinated children is unclear due to the potential for either wild-type or vaccine-strain VZV to induce HZ.
Twenty-two cases of HZ in healthy children after vaccination were identified with a PubMed search of articles indexed for MEDLINE using the search terms herpes zoster infection after vaccination and herpes zoster infection AND immunocompetent AND vaccination in separate searches for all English-language studies (Table). The search was limited to immunocompetent children and adolescents who were 18 years or younger with no history of varicella or exposure to varicella during gestation.


The mean age for HZ infection was 5.3 years. The average time between vaccination and HZ infection was 3.3 years. There was a spread of dermatomal patterns with cases in the first division of the trigeminal nerve, cervical, thoracic, lumbar, and sacral distributions. Of the 22 cases of HZ we reviewed, 16 underwent genotype testing to determine the source of the infection. The Oka vaccine strain virus was identified in 8 (50%) cases, while wild-type virus was found in 8 (50%) cases.1,2,4,5,7,8,10,11,13,14,16 Twelve cases were treated with acyclovir.2,3,5,6,9-12,14-17 The method of delivery, either oral or intravenous, and the length of treatment depended on the severity of the disease. Patients with meningoencephalitis and HZ ophthalmicus received intravenous acyclovir more often and also had a longer course of acyclovir compared to those individuals with involvement limited to the skin.
This review found HZ occurs from reactivation of wild-type or Oka vaccine-strain VZV in immunocompetent children.1-17 It shows that subclinical varicella infection is not the only explanation for HZ in a healthy vaccinated child. It is currently not clear why some healthy children experience HZ from vaccine-strain VZV. When HZ presents in a vaccinated immunocompetent child without a history of varicella infection or exposure, the possibility for vaccine strain–induced HZ should be considered.
Varicella-zoster virus (VZV) causes varicella as a primary infection. It is a highly contagious disease characterized by a widespread papulovesicular eruption with fever and malaise.1,2 After the primary infection, the virus remains latent within the sensory dorsal root ganglia and can reactivate as herpes zoster (HZ).1-5 Herpes zoster is characterized by unilateral radicular pain and a vesicular rash in a dermatomal pattern.1,2 It is most common in adults, especially elderly and immunocompromised patients, but rarely occurs in children. Herpes zoster is most often seen in individuals previously infected with VZV, but it also has occurred in individuals without known varicella infection,1-17 possibly because these individuals had a prior subclinical VZV infection.
A live attenuated VZV vaccine was created after isolation of the virus from a child in Japan.2 Since the introduction of the vaccine in 1995 in the United States, the incidence of VZV and HZ has declined.5 Herpes zoster rates after vaccination vary from 14 to 19 per 100,000 individuals.3,5 Breakthrough disease with the wild-type strain does occur in vaccinated children, but vaccine-strain HZ also has been reported.1-5 The risk for HZ caused by reactivated VZV vaccine in healthy children is unknown. We present a case of HZ in an otherwise healthy 19-month-old boy with no known varicella exposure who received the VZV vaccine at 13 months of age.
Case Report
An otherwise healthy 19-month-old boy presented to the dermatology clinic with a rash that began 2 days prior on the right groin and spread to the right leg. The patient’s mother denied that the child had been febrile and noted that the rash did not appear to bother him in any way. The patient was up-to-date on his vaccinations and received the first dose of the varicella series 6 months prior to presentation. He had no personal history of varicella, no exposure to sick contacts with varicella, and no known exposure to the virus. He was otherwise completely healthy with no signs or symptoms of immunocompromise.
Physical examination revealed grouped vesicles on an erythematous base on the right thigh, right sacrum, and lower abdomen that did not cross the midline (Figure). There were no other pertinent physical examination findings. The eruption was most consistent with HZ but concern remained for herpes simplex virus (HSV) or impetigo. A bacterial culture and polymerase chain reaction assay for VZV and HSV from skin swabs was ordered. The patient was prescribed acyclovir 20 mg/kg every 6 hours for 5 days. Laboratory testing revealed a positive result for VZV on polymerase chain reaction and a negative result for HSV. The majority of the patient’s lesions had crusted after 2 days of treatment with acyclovir, and the rash had nearly resolved 1 week after presentation. Subsequent evaluation with a complete blood cell count with differential and basic metabolic profile was normal. Levels of IgG, IgA, and IgM also were normal; IgE was slightly elevated.

Comment
Herpes zoster in children is an uncommon clinical entity. Most children with HZ are immunocompromised, have a history of varicella, or were exposed to varicella during gestation.8 With the introduction of the live VZV vaccine, the incidence of HZ has declined, but reactivation of the live vaccine leading to HZ infection is possible. The vaccine is 90% effective, and breakthrough varicella has been reported in 15% to 20% of vaccinated patients.1-17 The cause of HZ in vaccinated children is unclear due to the potential for either wild-type or vaccine-strain VZV to induce HZ.
Twenty-two cases of HZ in healthy children after vaccination were identified with a PubMed search of articles indexed for MEDLINE using the search terms herpes zoster infection after vaccination and herpes zoster infection AND immunocompetent AND vaccination in separate searches for all English-language studies (Table). The search was limited to immunocompetent children and adolescents who were 18 years or younger with no history of varicella or exposure to varicella during gestation.


The mean age for HZ infection was 5.3 years. The average time between vaccination and HZ infection was 3.3 years. There was a spread of dermatomal patterns with cases in the first division of the trigeminal nerve, cervical, thoracic, lumbar, and sacral distributions. Of the 22 cases of HZ we reviewed, 16 underwent genotype testing to determine the source of the infection. The Oka vaccine strain virus was identified in 8 (50%) cases, while wild-type virus was found in 8 (50%) cases.1,2,4,5,7,8,10,11,13,14,16 Twelve cases were treated with acyclovir.2,3,5,6,9-12,14-17 The method of delivery, either oral or intravenous, and the length of treatment depended on the severity of the disease. Patients with meningoencephalitis and HZ ophthalmicus received intravenous acyclovir more often and also had a longer course of acyclovir compared to those individuals with involvement limited to the skin.
This review found HZ occurs from reactivation of wild-type or Oka vaccine-strain VZV in immunocompetent children.1-17 It shows that subclinical varicella infection is not the only explanation for HZ in a healthy vaccinated child. It is currently not clear why some healthy children experience HZ from vaccine-strain VZV. When HZ presents in a vaccinated immunocompetent child without a history of varicella infection or exposure, the possibility for vaccine strain–induced HZ should be considered.
- . Herpes zoster in three healthy children immunized with varicella vaccine (Oka/Biken); the causative virus differed from vaccine strain on PCR analysis of the IV variable region (R5) and of a PstI-site region. Br J Dermatol. 1997;137:255-258.
- Uebe B, Sauerbrei A, Burdach S, et al. Herpes zoster by reactivated vaccine varicella zoster virus in a healthy child [published online June 25, 2002]. Eur J Pediatr. 2002;161:442-444.
- Obieta MP, Jacinto SS. Herpes zoster after varicella vaccination in a healthy young child. Int J Dermatol. 2008;47:640-641.
- Ota K, Kim V, Lavi S, et al. Vaccine-strain varicella zoster virus causing recurrent herpes zoster in an immunocompetent 2-year-old. Pediatr Infect Dis J. 2008;27:847-848.
- Liang GL, Heidelberg KA, Jacobson RM, et al. Herpes zoster after varicella vaccination. J Am Acad Dermatol. 1998;38:761-763.
- Matsubara K, Nigami H, Harigaya H, et al. Herpes zoster in a normal child after varicella vaccination. Acta Paediatr Jpn. 1995;37:648-650.
- Kohl S, Rapp J, Larussa P, et al. Natural varicella-zoster virus reactivation shortly after varicella immunization in a child. Pediatr Infect Dis J. 1999;18:1112-1113.
- Feder HM Jr, Hoss DM. Herpes zoster in otherwise healthy children. Pediatr Infect Dis J. 2004;23:451-457; quiz 458-460.
- Binder NR, Holland GN, Hosea S, et al. Herpes zoster ophthalmicus in an otherwise-healthy child. J AAPOS. 2005;9:597-598.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Lin P, Yoon MK, Chiu CS. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocul Immunol Inflamm. 2009;17:33-35.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child [published online March 1, 2010]. Pediatrics. 2010;125:E969-E972.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Ryu WY, Kim NY, Kwon YH, et al. Herpes zoster ophthalmicus with isolated trochlear nerve palsy in an otherwise healthy 13-year-old girl. J AAPOS. 2014;18:193-195.
- Iwasaki S, Motokura K, Honda Y, et al. Vaccine-strain herpes zoster found in the trigeminal nerve area in a healthy child: a case report [published online November 3, 2016]. J Clin Virol. 2016;85:44-47.
- Peterson N, Goodman S, Peterson M, et al. Herpes zoster in children. Cutis. 2016;98:94-95.
- . Herpes zoster in three healthy children immunized with varicella vaccine (Oka/Biken); the causative virus differed from vaccine strain on PCR analysis of the IV variable region (R5) and of a PstI-site region. Br J Dermatol. 1997;137:255-258.
- Uebe B, Sauerbrei A, Burdach S, et al. Herpes zoster by reactivated vaccine varicella zoster virus in a healthy child [published online June 25, 2002]. Eur J Pediatr. 2002;161:442-444.
- Obieta MP, Jacinto SS. Herpes zoster after varicella vaccination in a healthy young child. Int J Dermatol. 2008;47:640-641.
- Ota K, Kim V, Lavi S, et al. Vaccine-strain varicella zoster virus causing recurrent herpes zoster in an immunocompetent 2-year-old. Pediatr Infect Dis J. 2008;27:847-848.
- Liang GL, Heidelberg KA, Jacobson RM, et al. Herpes zoster after varicella vaccination. J Am Acad Dermatol. 1998;38:761-763.
- Matsubara K, Nigami H, Harigaya H, et al. Herpes zoster in a normal child after varicella vaccination. Acta Paediatr Jpn. 1995;37:648-650.
- Kohl S, Rapp J, Larussa P, et al. Natural varicella-zoster virus reactivation shortly after varicella immunization in a child. Pediatr Infect Dis J. 1999;18:1112-1113.
- Feder HM Jr, Hoss DM. Herpes zoster in otherwise healthy children. Pediatr Infect Dis J. 2004;23:451-457; quiz 458-460.
- Binder NR, Holland GN, Hosea S, et al. Herpes zoster ophthalmicus in an otherwise-healthy child. J AAPOS. 2005;9:597-598.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Lin P, Yoon MK, Chiu CS. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocul Immunol Inflamm. 2009;17:33-35.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child [published online March 1, 2010]. Pediatrics. 2010;125:E969-E972.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Ryu WY, Kim NY, Kwon YH, et al. Herpes zoster ophthalmicus with isolated trochlear nerve palsy in an otherwise healthy 13-year-old girl. J AAPOS. 2014;18:193-195.
- Iwasaki S, Motokura K, Honda Y, et al. Vaccine-strain herpes zoster found in the trigeminal nerve area in a healthy child: a case report [published online November 3, 2016]. J Clin Virol. 2016;85:44-47.
- Peterson N, Goodman S, Peterson M, et al. Herpes zoster in children. Cutis. 2016;98:94-95.
Practice Points
- Most children with herpes zoster are immunocompromised, have a history of varicella, or were exposed to varicella in utero.
- Herpes zoster has been reported in immunocompetent children due to either wild-type or vaccine-strain varicella-zoster virus.
Is Sitagliptin Plus Glargine Noninferior to Basal–Bolus Insulin for Inpatient Management of Type 2 Diabetes?
Study Overview
Objective. To compare the safety and efficacy of basal–bolus insulin therapy with sitagliptin plus insulin glargine in type 2 diabetes patients admitted to general medicine and surgical wards.
Design. Multicenter, prospective, open-label, noninferiority randomized clinical trial.
Setting and participants. Type 2 diabetes patients aged 18 to 80 years admitted to the general medicine and surgery services at one of 5 academic-based US hospitals were recruited. Eligible participants presented with a random blood glucose concentration between 140 and 400 mg/dL and were treated at home with diet, oral agents, or oral agents plus insulin at a maximum daily dose of 0.6 units/kg. Among those excluded were patients recently treated with a dipeptidyl peptidase-4 (DPP-4) inhibitor or glucagon-like peptide-1 (GLP-1) agonist, patients with clinically relevant hepatic disease, patients who were not eating for more than 48 hours, and those with an estimated glomerular filtration rate (eGFR) < 30 mL/min.
Intervention. Participants were randomly assigned to receive basal–bolus insulin therapy (BBI) with glargine once daily plus rapid-acting insulin before meals or sitagliptin plus glargine (SPG) once daily. Those in the SPG group received sitagliptin 100 mg/day if their eGFR was > 50 mL/min and sitagliptin 50 mg/day if their eGFR was 30 to 50 mL/min. If the eGFR fell below 30 mL/min during the hospitalization, sitagliptin was reduced to 25 mg/day. Glargine doses for those in the SPG group were started at 0.2 units/kg if randomization blood glucose was 140–200 mg/dL and 0.25 units/kg if randomization glucose was 201–400 mg/dL. Patients aged 70 or older or with an eGFR < 50 mL/min started with a daily glargine dose of 0.15 units/kg. For the BBI group, a total daily insulin dose of 0.4 units/kg was initiated for those with blood glucose levels between 140 and 200 mg/dL, and 0.5 units/kg for those with randomization glucose between 201 and 400 mg/dL. Half of this daily dose was given as glargine and the other half was distributed evenly across 3 pre-meal doses. Both the BBI and SPG groups received pre-meal and bedtime correction doses of rapid-acting insulin for glucose levels above 140 mg/dL. Blood glucose concentrations were measured fasting, before meals, and at bedtime or every 6 hours for patients who were not eating. Target fasting and pre-meal blood glucose levels were 100 to 140 mg/dL. Investigators and participants were not blinded to group assignment and glucose control was managed by the primary medical or surgical team.
Main outcomes measure. The primary outcome for this trial was noninferiority for differences between the SPG and BBI groups in glycemic control. Secondary endpoints included differences in the number of hypoglycemic and hyperglycemic events, the number of blood glucose values between 70 and 140 mg/dL and between 70 and 180 mg/dL, and the number of treatment failures (defined as 2 consecutive blood glucose values > 240 mg/dL or mean daily glucose > 240 mg/dL), length of hospital stay, total daily dose of insulin, number of insulin injections per day, transfer to the intensive care unit, and hospital complications and mortality.
Main results. A total of 138 patients in the SPG group and 139 patients in the BBI group completed the study and were included in this analysis. Of these 277 patients, 84% were admitted to a medicine ward and 16% were admitted to a surgical ward. The average age of participants was approximately 57 years, the average BMI was approximately 35 kg/m2, and the average duration of diabetes was approximately 10 years. These baseline characteristics as well as ethnic origin, sex, and baseline A1c (approximately 40% of patients in both groups had a baseline A1c between 7% and 9%) did not differ between groups. Prior to admission, approximately 40% of patients in both groups were managed with oral drugs alone, approximately 25% were managed with insulin alone, and about 22% were managed with insulin and oral therapy.
With respect to the primary outcome, both groups had similar mean daily blood glucose concentrations (171 mg/dL in SPG and 169 mg/dL in BBI) throughout the hospitalization, meeting the noninferiority threshold for glycemic control between groups. As for secondary outcomes, the mean proportion of blood glucose readings between 70 and 140 mg/dL, 70 and 180 mg/dL, and 100 and 140 mg/dL did not differ between groups. Pre-meal and bedtime blood glucose concentrations were also similar in both groups. There was a significant difference between groups in average daily insulin dose (24 units in SPG versus 34 units in BBI), total units of insulin per kg per day (0.2 units/kg in SPG versus 0.3 units/kg in BBI), and number of insulin injections per day (2.2 in SPG versus 2.9 in BBI). There was no difference in the number of hypoglycemic or hyperglycemic events, length of hospital stay (approximately 4 days in both groups), and rates of complications (including acute respiratory failure, acute kidney injury, and myocardial infarction) between groups.
Conclusion. Inpatient treatment with sitagliptin plus glargine was noninferior to basal–bolus insulin therapy in measurements of glycemic control.
Commentary
Approximately 25% to 30% of adult patients admitted to general medical and surgical wards and critical care units have type 2 diabetes [1]. Maintaining adequate blood sugar control is important, as both hyperglycemia and hypoglycemia have been associated with adverse outcomes. Although group consensus statements differ slightly with respect to recommended target glucose levels, generally the recommended range in a noncritical inpatient setting is 140 to 180 mg/dL [2,3]. Establishing and maintaining these levels can often be very challenging. Barriers to achieving adequate glucose control in the inpatient setting include changes in a patients’ nutrition status, renal function, pain level, the use of glucocorticoids, and the development of infections. In addition, a significant gap in knowledge can exist from provider to provider in terms of how to appropriately initiate and titrate insulin regimens. To circumvent this, many hospitals have created built-in order sets and protocols in the electronic medical record for basal–bolus correction insulin regimens. While these protocols may have improved many parameters of inpatient diabetes management at several institutions, improper initiation and execution of these protocols still occur. Also, at times the priorities of the medical team can shift so that titration of the insulin regimen may not occur frequently enough. Overall, simplification of inpatient glucose management would certainly be a welcomed change.
Unfortunately, there is a dearth of studies that investigate the role of oral therapy in the inpatient setting. In general, oral medications are discontinued upon admission and insulin is the recommended standard of care. In this study, Pasquel and colleagues investigated the use of the DPP-4 inhibitor sitagliptin in the inpatient setting. Unlike some of the other classes of oral agents used in the outpatient setting, DPP-4 inhibitors are generally well tolerated. A major advantage of DPP-4 inhibitors is that, with dose titration, they can also be used in mild to moderate renal failure. However, because DPP-4 inhibitors work in the prandial setting, they are not effective in the NPO patient. In this study, both the SPG group and BBI group had similar average daily blood glucose levels after the first day of therapy and throughout the hospitalization (171 mg/dL in SPG versus 169 mg/dL in BBI). Since the key finding here was noninferiority for blood sugar control between the treatments, the major differences between SPG and BBI therapy should be highlighted.
One benefit of SPG versus BBI therapy is that replacement of bolus insulin injections with a once-daily pill reduces the need for frequent bolus insulin dose titration. Nonetheless, renal function should be monitored frequently, as sitagliptin dose adjustments may be required, and the importance of bedside glucose checks should not be diminished, as some patients may not maintain adequate control on this regimen and will need to betransitioned to BBI therapy. Both treatment groups received correctional insulin doses in the prandial setting if their pre-meal glucose levels met a specific threshold. Overall, the SPG group required significantly fewer total insulin injections per day (2.2 injections in SPG versus 2.9 injections in BBI, P < 0.001). Though this difference is rather small, the need to administer fewer insulin injections would certainly be beneficial to nursing staff, who often care for several type 2 diabetes patients at once. It would have been interesting to know how many patients in each group were free of any correctional insulin doses or how many were adequately controlled with just 1 prandial injection per day. Although it cannot be concluded from this study, it could be expected that the reduced need for bolus insulin dose titration and fewer total insulin injections associated with oral therapy would result in less insulin dosing error and perhaps greater patient satisfaction.
It is important to keep in mind that initiating a DPP-4 inhibitor with basal insulin may not be an appropriate option for all admitted type 2 diabetes patients. It can be a beneficial alternative to insulin for the select group of patients included in this study: those treated at home with diet alone, oral therapy alone, or oral therapy plus insulin.
While the potential for implementation of SPG therapy in an inpatient setting does exist, there are some limitations to this study that make further investigation necessary. Though the patent on Januvia (sitagliptin’s trade name) expires in 2017, sitagliptin is currently a very expensive drug. Therefore, a cost-benefit analysis of SPG therapy versus insulin therapy alone should be undertaken. Also, this was an unblinded study, which may have resulted in more attentive, prioritized blood sugar management than what would typically occur in an inpatient setting. Also, the providers’ level of expertise on insulin management in this study may not be generalized to all inpatient medical and surgical providers. Despite these limitations, this study may have a profound impact on inpatient diabetes management, since a less labor-intensive alternative to basal–bolus insulin therapy may present a more attractive option for many inpatient providers.
Applications for Clinical Practice
This study could pave the way for a practice-changing method of inpatient glucose management for a select group of patients who do not have severely uncontrolled type 2 diabetes. One should keep in mind that cost could be a barrier to implementation of sitagliptin in hospitals, and that while the bolus dose of insulin can be replaced with sitagliptin, patients may still need correctional doses of insulin to maintain target ranges. Also, a daily assess-ment of glucose control is still necessary in order to determine if a change in management is needed. Therefore, the sitagliptin plus glargine option should not be viewed as a “shortcut” therapy, but rather as a potentially less labor-intensive option that may increase the ability to prioritize blood sugar management in the inpatient setting.
— Lisa Parikh, MD, Yale School of Medicine,
New Haven, CT
1. Draznin B, Gilden J, Golden SH, Inzucchi SE. Pathways to quality inpatient management of hyperglycemia and diabetes: a call to action. Diabetes Care 2013;36:1807–14.
2. American Diabetes Association Standards of Medical Care in Diabetes 2017. Diabetes Care 2017;40(supplement 1).
3. Umpierrez GE, Hellman R, Korytkowski MT. Management of hyperglycemia in hospitalized patients in non-critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97:16–38.
Study Overview
Objective. To compare the safety and efficacy of basal–bolus insulin therapy with sitagliptin plus insulin glargine in type 2 diabetes patients admitted to general medicine and surgical wards.
Design. Multicenter, prospective, open-label, noninferiority randomized clinical trial.
Setting and participants. Type 2 diabetes patients aged 18 to 80 years admitted to the general medicine and surgery services at one of 5 academic-based US hospitals were recruited. Eligible participants presented with a random blood glucose concentration between 140 and 400 mg/dL and were treated at home with diet, oral agents, or oral agents plus insulin at a maximum daily dose of 0.6 units/kg. Among those excluded were patients recently treated with a dipeptidyl peptidase-4 (DPP-4) inhibitor or glucagon-like peptide-1 (GLP-1) agonist, patients with clinically relevant hepatic disease, patients who were not eating for more than 48 hours, and those with an estimated glomerular filtration rate (eGFR) < 30 mL/min.
Intervention. Participants were randomly assigned to receive basal–bolus insulin therapy (BBI) with glargine once daily plus rapid-acting insulin before meals or sitagliptin plus glargine (SPG) once daily. Those in the SPG group received sitagliptin 100 mg/day if their eGFR was > 50 mL/min and sitagliptin 50 mg/day if their eGFR was 30 to 50 mL/min. If the eGFR fell below 30 mL/min during the hospitalization, sitagliptin was reduced to 25 mg/day. Glargine doses for those in the SPG group were started at 0.2 units/kg if randomization blood glucose was 140–200 mg/dL and 0.25 units/kg if randomization glucose was 201–400 mg/dL. Patients aged 70 or older or with an eGFR < 50 mL/min started with a daily glargine dose of 0.15 units/kg. For the BBI group, a total daily insulin dose of 0.4 units/kg was initiated for those with blood glucose levels between 140 and 200 mg/dL, and 0.5 units/kg for those with randomization glucose between 201 and 400 mg/dL. Half of this daily dose was given as glargine and the other half was distributed evenly across 3 pre-meal doses. Both the BBI and SPG groups received pre-meal and bedtime correction doses of rapid-acting insulin for glucose levels above 140 mg/dL. Blood glucose concentrations were measured fasting, before meals, and at bedtime or every 6 hours for patients who were not eating. Target fasting and pre-meal blood glucose levels were 100 to 140 mg/dL. Investigators and participants were not blinded to group assignment and glucose control was managed by the primary medical or surgical team.
Main outcomes measure. The primary outcome for this trial was noninferiority for differences between the SPG and BBI groups in glycemic control. Secondary endpoints included differences in the number of hypoglycemic and hyperglycemic events, the number of blood glucose values between 70 and 140 mg/dL and between 70 and 180 mg/dL, and the number of treatment failures (defined as 2 consecutive blood glucose values > 240 mg/dL or mean daily glucose > 240 mg/dL), length of hospital stay, total daily dose of insulin, number of insulin injections per day, transfer to the intensive care unit, and hospital complications and mortality.
Main results. A total of 138 patients in the SPG group and 139 patients in the BBI group completed the study and were included in this analysis. Of these 277 patients, 84% were admitted to a medicine ward and 16% were admitted to a surgical ward. The average age of participants was approximately 57 years, the average BMI was approximately 35 kg/m2, and the average duration of diabetes was approximately 10 years. These baseline characteristics as well as ethnic origin, sex, and baseline A1c (approximately 40% of patients in both groups had a baseline A1c between 7% and 9%) did not differ between groups. Prior to admission, approximately 40% of patients in both groups were managed with oral drugs alone, approximately 25% were managed with insulin alone, and about 22% were managed with insulin and oral therapy.
With respect to the primary outcome, both groups had similar mean daily blood glucose concentrations (171 mg/dL in SPG and 169 mg/dL in BBI) throughout the hospitalization, meeting the noninferiority threshold for glycemic control between groups. As for secondary outcomes, the mean proportion of blood glucose readings between 70 and 140 mg/dL, 70 and 180 mg/dL, and 100 and 140 mg/dL did not differ between groups. Pre-meal and bedtime blood glucose concentrations were also similar in both groups. There was a significant difference between groups in average daily insulin dose (24 units in SPG versus 34 units in BBI), total units of insulin per kg per day (0.2 units/kg in SPG versus 0.3 units/kg in BBI), and number of insulin injections per day (2.2 in SPG versus 2.9 in BBI). There was no difference in the number of hypoglycemic or hyperglycemic events, length of hospital stay (approximately 4 days in both groups), and rates of complications (including acute respiratory failure, acute kidney injury, and myocardial infarction) between groups.
Conclusion. Inpatient treatment with sitagliptin plus glargine was noninferior to basal–bolus insulin therapy in measurements of glycemic control.
Commentary
Approximately 25% to 30% of adult patients admitted to general medical and surgical wards and critical care units have type 2 diabetes [1]. Maintaining adequate blood sugar control is important, as both hyperglycemia and hypoglycemia have been associated with adverse outcomes. Although group consensus statements differ slightly with respect to recommended target glucose levels, generally the recommended range in a noncritical inpatient setting is 140 to 180 mg/dL [2,3]. Establishing and maintaining these levels can often be very challenging. Barriers to achieving adequate glucose control in the inpatient setting include changes in a patients’ nutrition status, renal function, pain level, the use of glucocorticoids, and the development of infections. In addition, a significant gap in knowledge can exist from provider to provider in terms of how to appropriately initiate and titrate insulin regimens. To circumvent this, many hospitals have created built-in order sets and protocols in the electronic medical record for basal–bolus correction insulin regimens. While these protocols may have improved many parameters of inpatient diabetes management at several institutions, improper initiation and execution of these protocols still occur. Also, at times the priorities of the medical team can shift so that titration of the insulin regimen may not occur frequently enough. Overall, simplification of inpatient glucose management would certainly be a welcomed change.
Unfortunately, there is a dearth of studies that investigate the role of oral therapy in the inpatient setting. In general, oral medications are discontinued upon admission and insulin is the recommended standard of care. In this study, Pasquel and colleagues investigated the use of the DPP-4 inhibitor sitagliptin in the inpatient setting. Unlike some of the other classes of oral agents used in the outpatient setting, DPP-4 inhibitors are generally well tolerated. A major advantage of DPP-4 inhibitors is that, with dose titration, they can also be used in mild to moderate renal failure. However, because DPP-4 inhibitors work in the prandial setting, they are not effective in the NPO patient. In this study, both the SPG group and BBI group had similar average daily blood glucose levels after the first day of therapy and throughout the hospitalization (171 mg/dL in SPG versus 169 mg/dL in BBI). Since the key finding here was noninferiority for blood sugar control between the treatments, the major differences between SPG and BBI therapy should be highlighted.
One benefit of SPG versus BBI therapy is that replacement of bolus insulin injections with a once-daily pill reduces the need for frequent bolus insulin dose titration. Nonetheless, renal function should be monitored frequently, as sitagliptin dose adjustments may be required, and the importance of bedside glucose checks should not be diminished, as some patients may not maintain adequate control on this regimen and will need to betransitioned to BBI therapy. Both treatment groups received correctional insulin doses in the prandial setting if their pre-meal glucose levels met a specific threshold. Overall, the SPG group required significantly fewer total insulin injections per day (2.2 injections in SPG versus 2.9 injections in BBI, P < 0.001). Though this difference is rather small, the need to administer fewer insulin injections would certainly be beneficial to nursing staff, who often care for several type 2 diabetes patients at once. It would have been interesting to know how many patients in each group were free of any correctional insulin doses or how many were adequately controlled with just 1 prandial injection per day. Although it cannot be concluded from this study, it could be expected that the reduced need for bolus insulin dose titration and fewer total insulin injections associated with oral therapy would result in less insulin dosing error and perhaps greater patient satisfaction.
It is important to keep in mind that initiating a DPP-4 inhibitor with basal insulin may not be an appropriate option for all admitted type 2 diabetes patients. It can be a beneficial alternative to insulin for the select group of patients included in this study: those treated at home with diet alone, oral therapy alone, or oral therapy plus insulin.
While the potential for implementation of SPG therapy in an inpatient setting does exist, there are some limitations to this study that make further investigation necessary. Though the patent on Januvia (sitagliptin’s trade name) expires in 2017, sitagliptin is currently a very expensive drug. Therefore, a cost-benefit analysis of SPG therapy versus insulin therapy alone should be undertaken. Also, this was an unblinded study, which may have resulted in more attentive, prioritized blood sugar management than what would typically occur in an inpatient setting. Also, the providers’ level of expertise on insulin management in this study may not be generalized to all inpatient medical and surgical providers. Despite these limitations, this study may have a profound impact on inpatient diabetes management, since a less labor-intensive alternative to basal–bolus insulin therapy may present a more attractive option for many inpatient providers.
Applications for Clinical Practice
This study could pave the way for a practice-changing method of inpatient glucose management for a select group of patients who do not have severely uncontrolled type 2 diabetes. One should keep in mind that cost could be a barrier to implementation of sitagliptin in hospitals, and that while the bolus dose of insulin can be replaced with sitagliptin, patients may still need correctional doses of insulin to maintain target ranges. Also, a daily assess-ment of glucose control is still necessary in order to determine if a change in management is needed. Therefore, the sitagliptin plus glargine option should not be viewed as a “shortcut” therapy, but rather as a potentially less labor-intensive option that may increase the ability to prioritize blood sugar management in the inpatient setting.
— Lisa Parikh, MD, Yale School of Medicine,
New Haven, CT
Study Overview
Objective. To compare the safety and efficacy of basal–bolus insulin therapy with sitagliptin plus insulin glargine in type 2 diabetes patients admitted to general medicine and surgical wards.
Design. Multicenter, prospective, open-label, noninferiority randomized clinical trial.
Setting and participants. Type 2 diabetes patients aged 18 to 80 years admitted to the general medicine and surgery services at one of 5 academic-based US hospitals were recruited. Eligible participants presented with a random blood glucose concentration between 140 and 400 mg/dL and were treated at home with diet, oral agents, or oral agents plus insulin at a maximum daily dose of 0.6 units/kg. Among those excluded were patients recently treated with a dipeptidyl peptidase-4 (DPP-4) inhibitor or glucagon-like peptide-1 (GLP-1) agonist, patients with clinically relevant hepatic disease, patients who were not eating for more than 48 hours, and those with an estimated glomerular filtration rate (eGFR) < 30 mL/min.
Intervention. Participants were randomly assigned to receive basal–bolus insulin therapy (BBI) with glargine once daily plus rapid-acting insulin before meals or sitagliptin plus glargine (SPG) once daily. Those in the SPG group received sitagliptin 100 mg/day if their eGFR was > 50 mL/min and sitagliptin 50 mg/day if their eGFR was 30 to 50 mL/min. If the eGFR fell below 30 mL/min during the hospitalization, sitagliptin was reduced to 25 mg/day. Glargine doses for those in the SPG group were started at 0.2 units/kg if randomization blood glucose was 140–200 mg/dL and 0.25 units/kg if randomization glucose was 201–400 mg/dL. Patients aged 70 or older or with an eGFR < 50 mL/min started with a daily glargine dose of 0.15 units/kg. For the BBI group, a total daily insulin dose of 0.4 units/kg was initiated for those with blood glucose levels between 140 and 200 mg/dL, and 0.5 units/kg for those with randomization glucose between 201 and 400 mg/dL. Half of this daily dose was given as glargine and the other half was distributed evenly across 3 pre-meal doses. Both the BBI and SPG groups received pre-meal and bedtime correction doses of rapid-acting insulin for glucose levels above 140 mg/dL. Blood glucose concentrations were measured fasting, before meals, and at bedtime or every 6 hours for patients who were not eating. Target fasting and pre-meal blood glucose levels were 100 to 140 mg/dL. Investigators and participants were not blinded to group assignment and glucose control was managed by the primary medical or surgical team.
Main outcomes measure. The primary outcome for this trial was noninferiority for differences between the SPG and BBI groups in glycemic control. Secondary endpoints included differences in the number of hypoglycemic and hyperglycemic events, the number of blood glucose values between 70 and 140 mg/dL and between 70 and 180 mg/dL, and the number of treatment failures (defined as 2 consecutive blood glucose values > 240 mg/dL or mean daily glucose > 240 mg/dL), length of hospital stay, total daily dose of insulin, number of insulin injections per day, transfer to the intensive care unit, and hospital complications and mortality.
Main results. A total of 138 patients in the SPG group and 139 patients in the BBI group completed the study and were included in this analysis. Of these 277 patients, 84% were admitted to a medicine ward and 16% were admitted to a surgical ward. The average age of participants was approximately 57 years, the average BMI was approximately 35 kg/m2, and the average duration of diabetes was approximately 10 years. These baseline characteristics as well as ethnic origin, sex, and baseline A1c (approximately 40% of patients in both groups had a baseline A1c between 7% and 9%) did not differ between groups. Prior to admission, approximately 40% of patients in both groups were managed with oral drugs alone, approximately 25% were managed with insulin alone, and about 22% were managed with insulin and oral therapy.
With respect to the primary outcome, both groups had similar mean daily blood glucose concentrations (171 mg/dL in SPG and 169 mg/dL in BBI) throughout the hospitalization, meeting the noninferiority threshold for glycemic control between groups. As for secondary outcomes, the mean proportion of blood glucose readings between 70 and 140 mg/dL, 70 and 180 mg/dL, and 100 and 140 mg/dL did not differ between groups. Pre-meal and bedtime blood glucose concentrations were also similar in both groups. There was a significant difference between groups in average daily insulin dose (24 units in SPG versus 34 units in BBI), total units of insulin per kg per day (0.2 units/kg in SPG versus 0.3 units/kg in BBI), and number of insulin injections per day (2.2 in SPG versus 2.9 in BBI). There was no difference in the number of hypoglycemic or hyperglycemic events, length of hospital stay (approximately 4 days in both groups), and rates of complications (including acute respiratory failure, acute kidney injury, and myocardial infarction) between groups.
Conclusion. Inpatient treatment with sitagliptin plus glargine was noninferior to basal–bolus insulin therapy in measurements of glycemic control.
Commentary
Approximately 25% to 30% of adult patients admitted to general medical and surgical wards and critical care units have type 2 diabetes [1]. Maintaining adequate blood sugar control is important, as both hyperglycemia and hypoglycemia have been associated with adverse outcomes. Although group consensus statements differ slightly with respect to recommended target glucose levels, generally the recommended range in a noncritical inpatient setting is 140 to 180 mg/dL [2,3]. Establishing and maintaining these levels can often be very challenging. Barriers to achieving adequate glucose control in the inpatient setting include changes in a patients’ nutrition status, renal function, pain level, the use of glucocorticoids, and the development of infections. In addition, a significant gap in knowledge can exist from provider to provider in terms of how to appropriately initiate and titrate insulin regimens. To circumvent this, many hospitals have created built-in order sets and protocols in the electronic medical record for basal–bolus correction insulin regimens. While these protocols may have improved many parameters of inpatient diabetes management at several institutions, improper initiation and execution of these protocols still occur. Also, at times the priorities of the medical team can shift so that titration of the insulin regimen may not occur frequently enough. Overall, simplification of inpatient glucose management would certainly be a welcomed change.
Unfortunately, there is a dearth of studies that investigate the role of oral therapy in the inpatient setting. In general, oral medications are discontinued upon admission and insulin is the recommended standard of care. In this study, Pasquel and colleagues investigated the use of the DPP-4 inhibitor sitagliptin in the inpatient setting. Unlike some of the other classes of oral agents used in the outpatient setting, DPP-4 inhibitors are generally well tolerated. A major advantage of DPP-4 inhibitors is that, with dose titration, they can also be used in mild to moderate renal failure. However, because DPP-4 inhibitors work in the prandial setting, they are not effective in the NPO patient. In this study, both the SPG group and BBI group had similar average daily blood glucose levels after the first day of therapy and throughout the hospitalization (171 mg/dL in SPG versus 169 mg/dL in BBI). Since the key finding here was noninferiority for blood sugar control between the treatments, the major differences between SPG and BBI therapy should be highlighted.
One benefit of SPG versus BBI therapy is that replacement of bolus insulin injections with a once-daily pill reduces the need for frequent bolus insulin dose titration. Nonetheless, renal function should be monitored frequently, as sitagliptin dose adjustments may be required, and the importance of bedside glucose checks should not be diminished, as some patients may not maintain adequate control on this regimen and will need to betransitioned to BBI therapy. Both treatment groups received correctional insulin doses in the prandial setting if their pre-meal glucose levels met a specific threshold. Overall, the SPG group required significantly fewer total insulin injections per day (2.2 injections in SPG versus 2.9 injections in BBI, P < 0.001). Though this difference is rather small, the need to administer fewer insulin injections would certainly be beneficial to nursing staff, who often care for several type 2 diabetes patients at once. It would have been interesting to know how many patients in each group were free of any correctional insulin doses or how many were adequately controlled with just 1 prandial injection per day. Although it cannot be concluded from this study, it could be expected that the reduced need for bolus insulin dose titration and fewer total insulin injections associated with oral therapy would result in less insulin dosing error and perhaps greater patient satisfaction.
It is important to keep in mind that initiating a DPP-4 inhibitor with basal insulin may not be an appropriate option for all admitted type 2 diabetes patients. It can be a beneficial alternative to insulin for the select group of patients included in this study: those treated at home with diet alone, oral therapy alone, or oral therapy plus insulin.
While the potential for implementation of SPG therapy in an inpatient setting does exist, there are some limitations to this study that make further investigation necessary. Though the patent on Januvia (sitagliptin’s trade name) expires in 2017, sitagliptin is currently a very expensive drug. Therefore, a cost-benefit analysis of SPG therapy versus insulin therapy alone should be undertaken. Also, this was an unblinded study, which may have resulted in more attentive, prioritized blood sugar management than what would typically occur in an inpatient setting. Also, the providers’ level of expertise on insulin management in this study may not be generalized to all inpatient medical and surgical providers. Despite these limitations, this study may have a profound impact on inpatient diabetes management, since a less labor-intensive alternative to basal–bolus insulin therapy may present a more attractive option for many inpatient providers.
Applications for Clinical Practice
This study could pave the way for a practice-changing method of inpatient glucose management for a select group of patients who do not have severely uncontrolled type 2 diabetes. One should keep in mind that cost could be a barrier to implementation of sitagliptin in hospitals, and that while the bolus dose of insulin can be replaced with sitagliptin, patients may still need correctional doses of insulin to maintain target ranges. Also, a daily assess-ment of glucose control is still necessary in order to determine if a change in management is needed. Therefore, the sitagliptin plus glargine option should not be viewed as a “shortcut” therapy, but rather as a potentially less labor-intensive option that may increase the ability to prioritize blood sugar management in the inpatient setting.
— Lisa Parikh, MD, Yale School of Medicine,
New Haven, CT
1. Draznin B, Gilden J, Golden SH, Inzucchi SE. Pathways to quality inpatient management of hyperglycemia and diabetes: a call to action. Diabetes Care 2013;36:1807–14.
2. American Diabetes Association Standards of Medical Care in Diabetes 2017. Diabetes Care 2017;40(supplement 1).
3. Umpierrez GE, Hellman R, Korytkowski MT. Management of hyperglycemia in hospitalized patients in non-critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97:16–38.
1. Draznin B, Gilden J, Golden SH, Inzucchi SE. Pathways to quality inpatient management of hyperglycemia and diabetes: a call to action. Diabetes Care 2013;36:1807–14.
2. American Diabetes Association Standards of Medical Care in Diabetes 2017. Diabetes Care 2017;40(supplement 1).
3. Umpierrez GE, Hellman R, Korytkowski MT. Management of hyperglycemia in hospitalized patients in non-critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97:16–38.
Misdiagnosed Crusted Scabies in an AIDS Patient Leads to Hyperinfestation
Case Report
A recently incarcerated 34-year-old man with an 11-year history of multidrug-resistant human immunodeficiency virus/AIDS (CD4 count, 121 cells/mm3; viral load, 49,625 particles/mm3 one week prior to presentation) was admitted to the hospital for an intensely pruritic, hyperkeratotic, scaly rash involving the entire body. The rash first appeared on the feet approximately 1 year prior to admission. At that time the patient was given oral fluconazole and a steroid cream with near resolution of the rash. He was then transferred multiple times to different units with subsequent discontinuation of the medications. The rash flared and progressed to involve the knees. He was restarted on the fluconazole and steroid cream and placed in isolation by medical personnel at the prison 6 months prior to presentation. The rash continued to spread, and he was given a working diagnosis of plaque-type psoriasis by several providers after several months of nonresponse to treatment. Additional attempts at treatment at outside facilities included oral fluconazole, trimethoprim-sulfamethoxazole, and other antibiotics. He was referred to dermatology at our institution but missed the appointment and was admitted to the hospital before the appointment could be rescheduled.
On admission to the hospital, he denied similar lesions in close contacts. On review of systems he had subjective fevers and chills, decreased appetite, nausea without vomiting, dysphagia to solids, epigastric pain, and 70-lb weight loss over the last 6 months. Facial involvement of the rash impaired the ability to open the mouth, speak, and eat. He had no known drug allergies. His only medications at the time of admission were nortriptyline, trimethoprim-sulfamethoxazole, and oral combination elvitegravir-cobicistat-emtricitabine-tenofovir for hu-man immunodeficiency virus treatment.
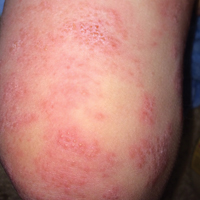
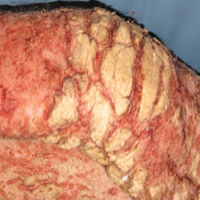
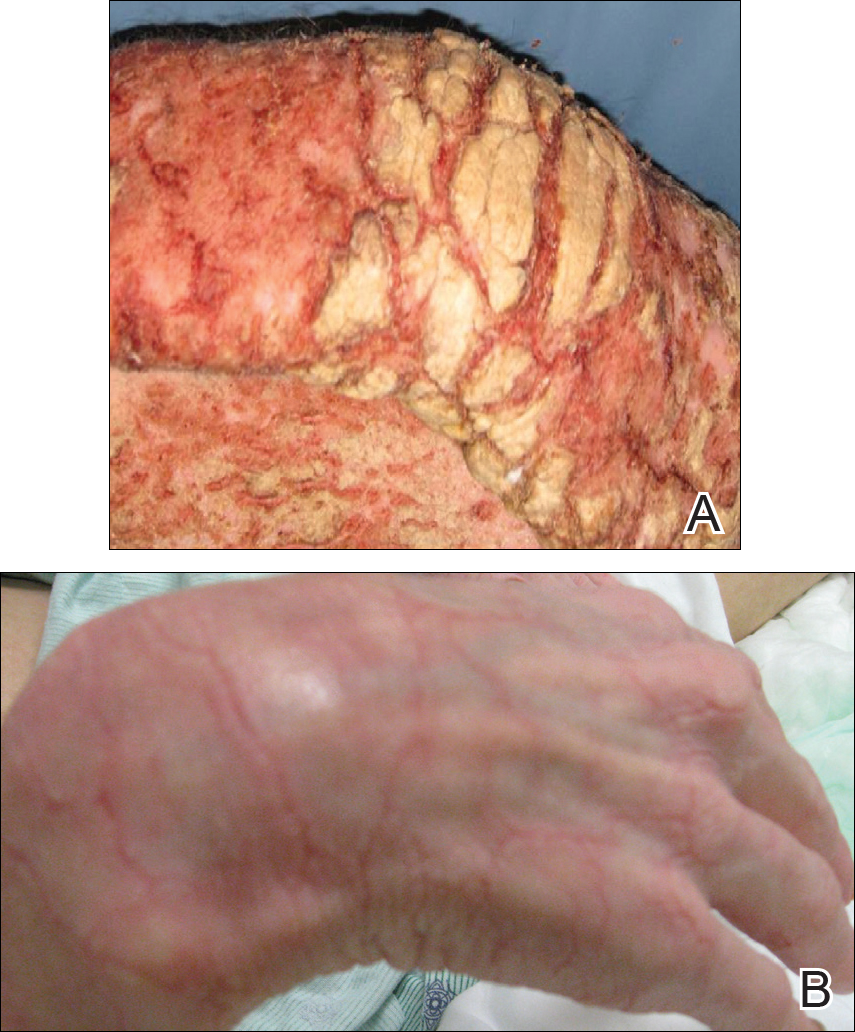
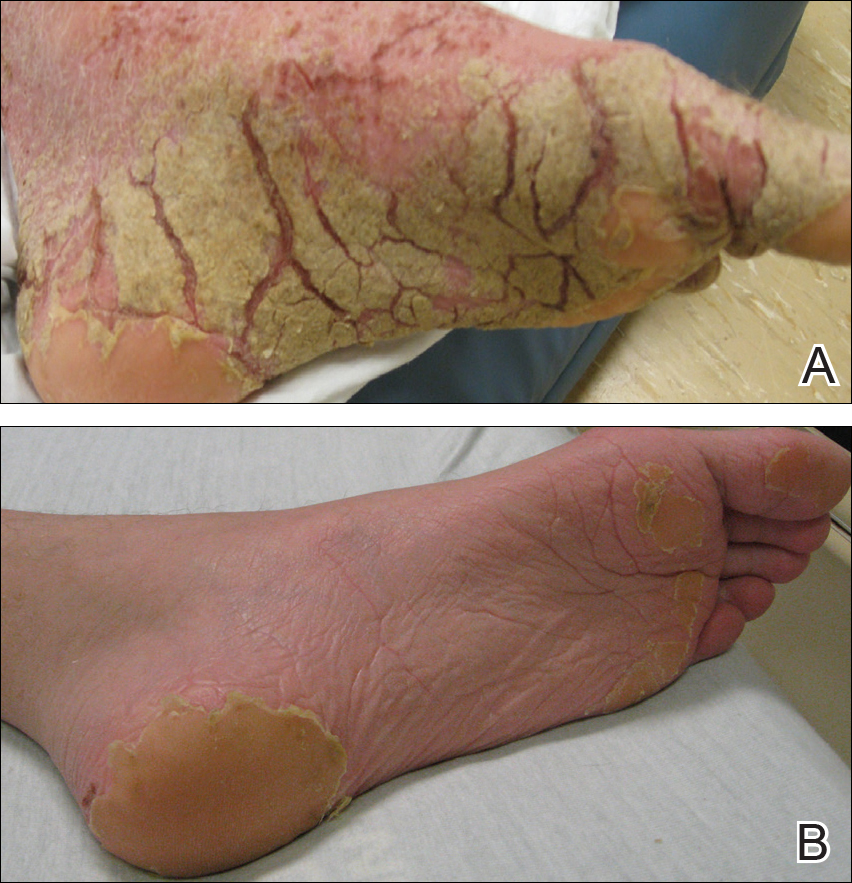
On physical examination he was cachectic, shivering, and foul smelling. He was afebrile, slightly tachycardic (112 beats per minute), and hypertensive (144/83 mm Hg) with a respiratory rate of 18 breaths per minute. His height was 1.83 m (6 ft) and weight was 48.5 kg (107 lb) with a body mass index of 14.5. Extensive erythematous, hyperkeratotic, crusted, and fissured plaques covered the entire body including the face, hands, and feet. The tongue was covered with bilateral white-colored plaques, and he had patches of alopecia, excoriations, and scales on the scalp. The elbows were fixed in a flexed position and he had decreased range of motion in the wrists and fingers due to the severe hyperkeratosis (Figure 1A). Hyperkeratosis also was prominent on the knees and feet with associated burrows (Figure 2A). He had foot drop on the left.
The differential diagnosis included a drug eruption; fungal or parasite infestation, such as crusted scabies; psoriasis; or cutaneous lymphoma. Laboratory studies were difficult to obtain, as there were limited areas suitable for vascular access. Blood work showed leukocytosis (18.9×109 cells/L [reference range, 4.8–10.8×109 cells/L) with 13.3% eosinophils (reference range, 1%–6%). This eosinophilia narrowed the likely diagnoses to a drug eruption or parasite infection.
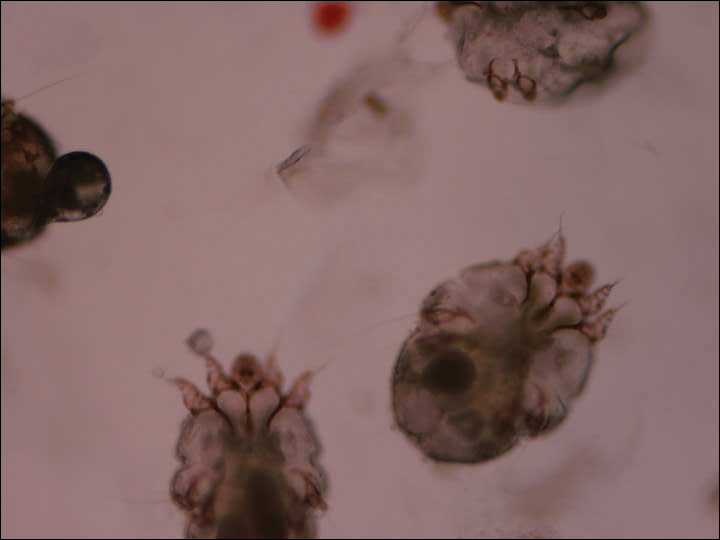
The dermatology service was consulted. A mineral oil preparation was performed and showed numerous mites and feces consistent with a diagnosis of crusted scabies (Figure 3). The patient was started on a regimen of permethrin cream 5% applied to the entire body, except the face, which was left on overnight and washed off. This regimen was repeated daily for 1 week, then twice weekly until the rash resolved after a total of 3 weeks. Due to the severity of his condition, immunocompromised status, and concern for superinfection, oral ivermectin 200 μg/kg once daily was added on days 1, 2, 8, 9, 15, 22, and 29.1
Our patient’s hospital course was further complicated by symptomatic hypoglycemia, altered mental status, and superimposed methicillin-resistant Staphylococcus aureus bacteremia, as well as Pseudomonas aeruginosa bacteremia, pneumonia, and coffee ground emesis. He was transferred to the intensive care unit but fortunately did not require intubation. His overall condition, mental status, and rash gradually improved. Three weeks after admission he only had a few residual lesions on the feet with clearing elsewhere (Figures 1B and 2B). He was discharged with a skin moisturizer and was referred for physical and occupational therapy. On follow-up clinic visits at 3 and 6 months, he had recovered well with general improvement in his condition.
Comment
Classic (noncrusted) scabies is common worldwide, with an estimated 300 million cases per year. It is caused by the mite Sarcoptes scabiei var hominis, and transmission occurs by direct skin-to-skin contact or less commonly by fomites (eg, linens, bedsheets) and therefore is common in overcrowded environments.2 Crusted scabies is a severe, highly contagious form of the disease in which the host’s immune system is overwhelmed and unable to defend against mites on the skin, resulting in hyperinfestation of the host. The mites use secretions to dissolve the epidermis and burrow through the skin, leaving feces in their tracks.3 Interestingly, the native aboriginal populations of Australia have a high incidence of crusted scabies even though they show no signs of immunosuppression. The reason remains unclear but may be due to a skewed T-cell response.4 Various mechanisms have been described for the symptoms of scabies, and it is believed that there is a hypersensitivity reaction to the mites and the feces. Increased IL-17 production by skin T cells may be responsible.5
Clinical Features
Crusted scabies is characterized by severe hyperkeratosis and plaques with desquamation and erythroderma that is worse in the acral regions and large joints, such as the elbows and the knees, as seen in our patient. Because of the deep burrows, patients are predisposed to secondary superinfections by bacteria. In our case, the patient had methicillin-resistant S aureus bacteremia, which persisted for some time despite treatment with intravenous antibiotics.
Diagnosis
Because scabies can imitate different conditions, it can be difficult to diagnose. Misdiagnosis of psoriasis in our patient led to ineffective treatment and subsequent worsening of his condition. Burrows are pathognomonic for scabies, though in severe cases, the burrows may be concealed by extreme hyperkeratosis. Diagnosis is confirmed by mineral oil preparation from the plaques showing numerous scabies mites and feces.
Treatment
It is important to control the spread of scabies, as it is highly contagious, and if the living environment is not properly cleaned, the patient can be reinfected. All clothing, bedsheets, and linens in the household must be washed in hot water and dried in a hot dryer, and nonwashable items should be placed in a closed plastic bag for 72 hours. All contacts also should be treated with 1 application of permethrin cream to the entire body including the head and neck, left on overnight, and washed off with warm water.1 The washing also helps remove some of the skin crusts. Patients should be educated that pruritus and burning may initially worsen with permethrin treatment due to the body’s reaction to the parasite.1,2 In addition, keratolytic agents such as topical urea or salicylic acid can be used as an adjuvant therapy to improve the efficacy of permethrin.
Permethrin is effective against both mites and eggs and works by inhibiting sodium channels, resulting in nerve signal conduction block and subsequent paralysis. Ivermectin is thought to act on glutamate-gated chloride channels, which are present in invertebrates but absent in vertebrates, causing hyperpolarization and paralysis of the adult mite.1,6
Conclusion
Crusted scabies is a highly contagious and intensely pruritic condition. Scabies can mimic other conditions, such as psoriasis or severe dermatitis, so it is important to keep this diagnosis in mind, especially in immunocompromised patients or populations in overcrowded areas (eg, those who are incarcerated or in nursing homes). Treatment consists of isolating the patient, starting topical permethrin and oral ivermectin (in severe cases), washing all linens, and prophylactically treating contacts. A delay in diagnosis can lead to severe debilitating disease, as seen in the extreme case of our patient. However, our patient made a full recovery with appropriate treatment and care.
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725.
- World Health Organization. Water-related diseases: scabies. http://www.who.int/water_sanitation_health/diseases-risks/diseases/scabies/en/. Accessed February 23, 2017.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381.
- Liu X, Walton SF, Murray HC, et al. Crusted scabies is associated with increased IL-17 secretion by skin T cells. Parasite Immunol. 2014;36:594-604.
- Geary TG. Ivermectin 20 years on: maturation of a wonder drug [published online August 26, 2005]. Trends Parasitol. 2005;21:530-532.
Case Report
A recently incarcerated 34-year-old man with an 11-year history of multidrug-resistant human immunodeficiency virus/AIDS (CD4 count, 121 cells/mm3; viral load, 49,625 particles/mm3 one week prior to presentation) was admitted to the hospital for an intensely pruritic, hyperkeratotic, scaly rash involving the entire body. The rash first appeared on the feet approximately 1 year prior to admission. At that time the patient was given oral fluconazole and a steroid cream with near resolution of the rash. He was then transferred multiple times to different units with subsequent discontinuation of the medications. The rash flared and progressed to involve the knees. He was restarted on the fluconazole and steroid cream and placed in isolation by medical personnel at the prison 6 months prior to presentation. The rash continued to spread, and he was given a working diagnosis of plaque-type psoriasis by several providers after several months of nonresponse to treatment. Additional attempts at treatment at outside facilities included oral fluconazole, trimethoprim-sulfamethoxazole, and other antibiotics. He was referred to dermatology at our institution but missed the appointment and was admitted to the hospital before the appointment could be rescheduled.
On admission to the hospital, he denied similar lesions in close contacts. On review of systems he had subjective fevers and chills, decreased appetite, nausea without vomiting, dysphagia to solids, epigastric pain, and 70-lb weight loss over the last 6 months. Facial involvement of the rash impaired the ability to open the mouth, speak, and eat. He had no known drug allergies. His only medications at the time of admission were nortriptyline, trimethoprim-sulfamethoxazole, and oral combination elvitegravir-cobicistat-emtricitabine-tenofovir for hu-man immunodeficiency virus treatment.
On physical examination he was cachectic, shivering, and foul smelling. He was afebrile, slightly tachycardic (112 beats per minute), and hypertensive (144/83 mm Hg) with a respiratory rate of 18 breaths per minute. His height was 1.83 m (6 ft) and weight was 48.5 kg (107 lb) with a body mass index of 14.5. Extensive erythematous, hyperkeratotic, crusted, and fissured plaques covered the entire body including the face, hands, and feet. The tongue was covered with bilateral white-colored plaques, and he had patches of alopecia, excoriations, and scales on the scalp. The elbows were fixed in a flexed position and he had decreased range of motion in the wrists and fingers due to the severe hyperkeratosis (Figure 1A). Hyperkeratosis also was prominent on the knees and feet with associated burrows (Figure 2A). He had foot drop on the left.


The differential diagnosis included a drug eruption; fungal or parasite infestation, such as crusted scabies; psoriasis; or cutaneous lymphoma. Laboratory studies were difficult to obtain, as there were limited areas suitable for vascular access. Blood work showed leukocytosis (18.9×109 cells/L [reference range, 4.8–10.8×109 cells/L) with 13.3% eosinophils (reference range, 1%–6%). This eosinophilia narrowed the likely diagnoses to a drug eruption or parasite infection.
The dermatology service was consulted. A mineral oil preparation was performed and showed numerous mites and feces consistent with a diagnosis of crusted scabies (Figure 3). The patient was started on a regimen of permethrin cream 5% applied to the entire body, except the face, which was left on overnight and washed off. This regimen was repeated daily for 1 week, then twice weekly until the rash resolved after a total of 3 weeks. Due to the severity of his condition, immunocompromised status, and concern for superinfection, oral ivermectin 200 μg/kg once daily was added on days 1, 2, 8, 9, 15, 22, and 29.1

Our patient’s hospital course was further complicated by symptomatic hypoglycemia, altered mental status, and superimposed methicillin-resistant Staphylococcus aureus bacteremia, as well as Pseudomonas aeruginosa bacteremia, pneumonia, and coffee ground emesis. He was transferred to the intensive care unit but fortunately did not require intubation. His overall condition, mental status, and rash gradually improved. Three weeks after admission he only had a few residual lesions on the feet with clearing elsewhere (Figures 1B and 2B). He was discharged with a skin moisturizer and was referred for physical and occupational therapy. On follow-up clinic visits at 3 and 6 months, he had recovered well with general improvement in his condition.
Comment
Classic (noncrusted) scabies is common worldwide, with an estimated 300 million cases per year. It is caused by the mite Sarcoptes scabiei var hominis, and transmission occurs by direct skin-to-skin contact or less commonly by fomites (eg, linens, bedsheets) and therefore is common in overcrowded environments.2 Crusted scabies is a severe, highly contagious form of the disease in which the host’s immune system is overwhelmed and unable to defend against mites on the skin, resulting in hyperinfestation of the host. The mites use secretions to dissolve the epidermis and burrow through the skin, leaving feces in their tracks.3 Interestingly, the native aboriginal populations of Australia have a high incidence of crusted scabies even though they show no signs of immunosuppression. The reason remains unclear but may be due to a skewed T-cell response.4 Various mechanisms have been described for the symptoms of scabies, and it is believed that there is a hypersensitivity reaction to the mites and the feces. Increased IL-17 production by skin T cells may be responsible.5
Clinical Features
Crusted scabies is characterized by severe hyperkeratosis and plaques with desquamation and erythroderma that is worse in the acral regions and large joints, such as the elbows and the knees, as seen in our patient. Because of the deep burrows, patients are predisposed to secondary superinfections by bacteria. In our case, the patient had methicillin-resistant S aureus bacteremia, which persisted for some time despite treatment with intravenous antibiotics.
Diagnosis
Because scabies can imitate different conditions, it can be difficult to diagnose. Misdiagnosis of psoriasis in our patient led to ineffective treatment and subsequent worsening of his condition. Burrows are pathognomonic for scabies, though in severe cases, the burrows may be concealed by extreme hyperkeratosis. Diagnosis is confirmed by mineral oil preparation from the plaques showing numerous scabies mites and feces.
Treatment
It is important to control the spread of scabies, as it is highly contagious, and if the living environment is not properly cleaned, the patient can be reinfected. All clothing, bedsheets, and linens in the household must be washed in hot water and dried in a hot dryer, and nonwashable items should be placed in a closed plastic bag for 72 hours. All contacts also should be treated with 1 application of permethrin cream to the entire body including the head and neck, left on overnight, and washed off with warm water.1 The washing also helps remove some of the skin crusts. Patients should be educated that pruritus and burning may initially worsen with permethrin treatment due to the body’s reaction to the parasite.1,2 In addition, keratolytic agents such as topical urea or salicylic acid can be used as an adjuvant therapy to improve the efficacy of permethrin.
Permethrin is effective against both mites and eggs and works by inhibiting sodium channels, resulting in nerve signal conduction block and subsequent paralysis. Ivermectin is thought to act on glutamate-gated chloride channels, which are present in invertebrates but absent in vertebrates, causing hyperpolarization and paralysis of the adult mite.1,6
Conclusion
Crusted scabies is a highly contagious and intensely pruritic condition. Scabies can mimic other conditions, such as psoriasis or severe dermatitis, so it is important to keep this diagnosis in mind, especially in immunocompromised patients or populations in overcrowded areas (eg, those who are incarcerated or in nursing homes). Treatment consists of isolating the patient, starting topical permethrin and oral ivermectin (in severe cases), washing all linens, and prophylactically treating contacts. A delay in diagnosis can lead to severe debilitating disease, as seen in the extreme case of our patient. However, our patient made a full recovery with appropriate treatment and care.
Case Report
A recently incarcerated 34-year-old man with an 11-year history of multidrug-resistant human immunodeficiency virus/AIDS (CD4 count, 121 cells/mm3; viral load, 49,625 particles/mm3 one week prior to presentation) was admitted to the hospital for an intensely pruritic, hyperkeratotic, scaly rash involving the entire body. The rash first appeared on the feet approximately 1 year prior to admission. At that time the patient was given oral fluconazole and a steroid cream with near resolution of the rash. He was then transferred multiple times to different units with subsequent discontinuation of the medications. The rash flared and progressed to involve the knees. He was restarted on the fluconazole and steroid cream and placed in isolation by medical personnel at the prison 6 months prior to presentation. The rash continued to spread, and he was given a working diagnosis of plaque-type psoriasis by several providers after several months of nonresponse to treatment. Additional attempts at treatment at outside facilities included oral fluconazole, trimethoprim-sulfamethoxazole, and other antibiotics. He was referred to dermatology at our institution but missed the appointment and was admitted to the hospital before the appointment could be rescheduled.
On admission to the hospital, he denied similar lesions in close contacts. On review of systems he had subjective fevers and chills, decreased appetite, nausea without vomiting, dysphagia to solids, epigastric pain, and 70-lb weight loss over the last 6 months. Facial involvement of the rash impaired the ability to open the mouth, speak, and eat. He had no known drug allergies. His only medications at the time of admission were nortriptyline, trimethoprim-sulfamethoxazole, and oral combination elvitegravir-cobicistat-emtricitabine-tenofovir for hu-man immunodeficiency virus treatment.
On physical examination he was cachectic, shivering, and foul smelling. He was afebrile, slightly tachycardic (112 beats per minute), and hypertensive (144/83 mm Hg) with a respiratory rate of 18 breaths per minute. His height was 1.83 m (6 ft) and weight was 48.5 kg (107 lb) with a body mass index of 14.5. Extensive erythematous, hyperkeratotic, crusted, and fissured plaques covered the entire body including the face, hands, and feet. The tongue was covered with bilateral white-colored plaques, and he had patches of alopecia, excoriations, and scales on the scalp. The elbows were fixed in a flexed position and he had decreased range of motion in the wrists and fingers due to the severe hyperkeratosis (Figure 1A). Hyperkeratosis also was prominent on the knees and feet with associated burrows (Figure 2A). He had foot drop on the left.


The differential diagnosis included a drug eruption; fungal or parasite infestation, such as crusted scabies; psoriasis; or cutaneous lymphoma. Laboratory studies were difficult to obtain, as there were limited areas suitable for vascular access. Blood work showed leukocytosis (18.9×109 cells/L [reference range, 4.8–10.8×109 cells/L) with 13.3% eosinophils (reference range, 1%–6%). This eosinophilia narrowed the likely diagnoses to a drug eruption or parasite infection.
The dermatology service was consulted. A mineral oil preparation was performed and showed numerous mites and feces consistent with a diagnosis of crusted scabies (Figure 3). The patient was started on a regimen of permethrin cream 5% applied to the entire body, except the face, which was left on overnight and washed off. This regimen was repeated daily for 1 week, then twice weekly until the rash resolved after a total of 3 weeks. Due to the severity of his condition, immunocompromised status, and concern for superinfection, oral ivermectin 200 μg/kg once daily was added on days 1, 2, 8, 9, 15, 22, and 29.1

Our patient’s hospital course was further complicated by symptomatic hypoglycemia, altered mental status, and superimposed methicillin-resistant Staphylococcus aureus bacteremia, as well as Pseudomonas aeruginosa bacteremia, pneumonia, and coffee ground emesis. He was transferred to the intensive care unit but fortunately did not require intubation. His overall condition, mental status, and rash gradually improved. Three weeks after admission he only had a few residual lesions on the feet with clearing elsewhere (Figures 1B and 2B). He was discharged with a skin moisturizer and was referred for physical and occupational therapy. On follow-up clinic visits at 3 and 6 months, he had recovered well with general improvement in his condition.
Comment
Classic (noncrusted) scabies is common worldwide, with an estimated 300 million cases per year. It is caused by the mite Sarcoptes scabiei var hominis, and transmission occurs by direct skin-to-skin contact or less commonly by fomites (eg, linens, bedsheets) and therefore is common in overcrowded environments.2 Crusted scabies is a severe, highly contagious form of the disease in which the host’s immune system is overwhelmed and unable to defend against mites on the skin, resulting in hyperinfestation of the host. The mites use secretions to dissolve the epidermis and burrow through the skin, leaving feces in their tracks.3 Interestingly, the native aboriginal populations of Australia have a high incidence of crusted scabies even though they show no signs of immunosuppression. The reason remains unclear but may be due to a skewed T-cell response.4 Various mechanisms have been described for the symptoms of scabies, and it is believed that there is a hypersensitivity reaction to the mites and the feces. Increased IL-17 production by skin T cells may be responsible.5
Clinical Features
Crusted scabies is characterized by severe hyperkeratosis and plaques with desquamation and erythroderma that is worse in the acral regions and large joints, such as the elbows and the knees, as seen in our patient. Because of the deep burrows, patients are predisposed to secondary superinfections by bacteria. In our case, the patient had methicillin-resistant S aureus bacteremia, which persisted for some time despite treatment with intravenous antibiotics.
Diagnosis
Because scabies can imitate different conditions, it can be difficult to diagnose. Misdiagnosis of psoriasis in our patient led to ineffective treatment and subsequent worsening of his condition. Burrows are pathognomonic for scabies, though in severe cases, the burrows may be concealed by extreme hyperkeratosis. Diagnosis is confirmed by mineral oil preparation from the plaques showing numerous scabies mites and feces.
Treatment
It is important to control the spread of scabies, as it is highly contagious, and if the living environment is not properly cleaned, the patient can be reinfected. All clothing, bedsheets, and linens in the household must be washed in hot water and dried in a hot dryer, and nonwashable items should be placed in a closed plastic bag for 72 hours. All contacts also should be treated with 1 application of permethrin cream to the entire body including the head and neck, left on overnight, and washed off with warm water.1 The washing also helps remove some of the skin crusts. Patients should be educated that pruritus and burning may initially worsen with permethrin treatment due to the body’s reaction to the parasite.1,2 In addition, keratolytic agents such as topical urea or salicylic acid can be used as an adjuvant therapy to improve the efficacy of permethrin.
Permethrin is effective against both mites and eggs and works by inhibiting sodium channels, resulting in nerve signal conduction block and subsequent paralysis. Ivermectin is thought to act on glutamate-gated chloride channels, which are present in invertebrates but absent in vertebrates, causing hyperpolarization and paralysis of the adult mite.1,6
Conclusion
Crusted scabies is a highly contagious and intensely pruritic condition. Scabies can mimic other conditions, such as psoriasis or severe dermatitis, so it is important to keep this diagnosis in mind, especially in immunocompromised patients or populations in overcrowded areas (eg, those who are incarcerated or in nursing homes). Treatment consists of isolating the patient, starting topical permethrin and oral ivermectin (in severe cases), washing all linens, and prophylactically treating contacts. A delay in diagnosis can lead to severe debilitating disease, as seen in the extreme case of our patient. However, our patient made a full recovery with appropriate treatment and care.
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725.
- World Health Organization. Water-related diseases: scabies. http://www.who.int/water_sanitation_health/diseases-risks/diseases/scabies/en/. Accessed February 23, 2017.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381.
- Liu X, Walton SF, Murray HC, et al. Crusted scabies is associated with increased IL-17 secretion by skin T cells. Parasite Immunol. 2014;36:594-604.
- Geary TG. Ivermectin 20 years on: maturation of a wonder drug [published online August 26, 2005]. Trends Parasitol. 2005;21:530-532.
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725.
- World Health Organization. Water-related diseases: scabies. http://www.who.int/water_sanitation_health/diseases-risks/diseases/scabies/en/. Accessed February 23, 2017.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381.
- Liu X, Walton SF, Murray HC, et al. Crusted scabies is associated with increased IL-17 secretion by skin T cells. Parasite Immunol. 2014;36:594-604.
- Geary TG. Ivermectin 20 years on: maturation of a wonder drug [published online August 26, 2005]. Trends Parasitol. 2005;21:530-532.
Practice Points
- Keep scabies in mind, especially in immunocompromised patients or populations in overcrowded areas.
- Treatment consists of isolating the patient, starting topical permethrin and oral ivermectin (in severe cases), washing all linens, and prophylactically treating contacts.
The Patellofemoral Compartment: Making Sense of It
Editor’s Note: One of the goals of the new AJO is to offer solutions to common problems we face as orthopedists. With that in mind, this issue tackles the patellofemoral joint and represents a collaboration between our journal and some of the key leaders of the Patellofemoral Study Group. I’m indebted to my friend and mentor, Jack Farr, for organizing this issue and a continuing patellofemoral series. I know this series will provide an invaluable look into the thought process of true orthopedic legends and find a permanent place on your shelf of orthopedic reference materials.
I’m also pleased to introduce a new feature, our online Lifestyles section. Sometimes, as orthopedists, we spend so much time taking care of others that we forget to look after ourselves and our loved ones. In an effort to make this easier, AJO has collaborated with Inspirato, the premiere luxury destination club. As a member, I’ve enjoyed truly life-changing vacations with my family and now have a way to share that opportunity with our readers. Inspirato is offering a complimentary 6-month Key membership and $250 spending credit to all AJO readers. Simply visit www.inspirato.com/orthopedics to sign up and start booking your vacations like a member. Look for future lifestyle features and special opportunities online in upcoming issues.
—Bryan T. Hanypsiak, MD
The patellofemoral compartment of the knee has been an enigma for many years. Clinicians who enjoy treating patients with knee problems have the choice of either ignoring one-third of the knee or grappling with this unique compartment. In attempting to make sense of this area of the knee, it is necessary to take into account the vast and complex overlay of multiple factors affecting this compartment. These factors span the gamut from psycho-social, to “core to floor” physiologic imbalance, to overuse, to the seemingly more “objective” elements of alignment, stability, morphology, bone, and cartilage.
Fortunately, a small merry band of international experts has made the patellofemoral compartment its “badge of courage” and continues to attempt to make sense of this small mobile sesamoid bone. We have invited a few of these stalwarts to share their experience and wisdom with us in this first of an ongoing patellofemoral series in The American Journal of Orthopedics. I appreciate the honor of assembling the works of these worldly patellofemoral gurus.
How many of us routinely order a “Merchant view”, discuss a “Fulkerson osteotomy”, or tell patients they are out of their Scott Dye “envelope of function” and they need to allow their knee to return to homeostasis through a “core to floor” rehabilitation program? We are lucky to have these living legends offer us insight into their thinking process. I purposely have begun this patellofemoral series with some of my personal mentors to set the tone: think first, understand the problem, design an evidence-based medicine approach and, above all, do no harm. To that point, Dr. Merchant, Dr. Fulkerson, Dr. Dye, and Dr. Post each detail their approach to anterior knee pain, followed by a discussion on nonoperative therapy intervention by Dr. Hiemstra. However, I understand that most readers are surgeons and, therefore I have added two articles to pique your interest: the hot topic of medial patellofemoral ligament (MPFL)—“To repair or not to repair, that is NOT the question.” The question is: “When does repair potentially benefit the patient and when is reconstruction the best approach?” Dr. Duchman and Dr. Bollier address the former, and Dr. Burrus and colleagues discuss optimizing MPFL reconstruction. I hope you enjoy learning from these authors as much as I have while producing this issue.
Am J Orthop. 2017;46(2):64. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Editor’s Note: One of the goals of the new AJO is to offer solutions to common problems we face as orthopedists. With that in mind, this issue tackles the patellofemoral joint and represents a collaboration between our journal and some of the key leaders of the Patellofemoral Study Group. I’m indebted to my friend and mentor, Jack Farr, for organizing this issue and a continuing patellofemoral series. I know this series will provide an invaluable look into the thought process of true orthopedic legends and find a permanent place on your shelf of orthopedic reference materials.
I’m also pleased to introduce a new feature, our online Lifestyles section. Sometimes, as orthopedists, we spend so much time taking care of others that we forget to look after ourselves and our loved ones. In an effort to make this easier, AJO has collaborated with Inspirato, the premiere luxury destination club. As a member, I’ve enjoyed truly life-changing vacations with my family and now have a way to share that opportunity with our readers. Inspirato is offering a complimentary 6-month Key membership and $250 spending credit to all AJO readers. Simply visit www.inspirato.com/orthopedics to sign up and start booking your vacations like a member. Look for future lifestyle features and special opportunities online in upcoming issues.
—Bryan T. Hanypsiak, MD
The patellofemoral compartment of the knee has been an enigma for many years. Clinicians who enjoy treating patients with knee problems have the choice of either ignoring one-third of the knee or grappling with this unique compartment. In attempting to make sense of this area of the knee, it is necessary to take into account the vast and complex overlay of multiple factors affecting this compartment. These factors span the gamut from psycho-social, to “core to floor” physiologic imbalance, to overuse, to the seemingly more “objective” elements of alignment, stability, morphology, bone, and cartilage.
Fortunately, a small merry band of international experts has made the patellofemoral compartment its “badge of courage” and continues to attempt to make sense of this small mobile sesamoid bone. We have invited a few of these stalwarts to share their experience and wisdom with us in this first of an ongoing patellofemoral series in The American Journal of Orthopedics. I appreciate the honor of assembling the works of these worldly patellofemoral gurus.
How many of us routinely order a “Merchant view”, discuss a “Fulkerson osteotomy”, or tell patients they are out of their Scott Dye “envelope of function” and they need to allow their knee to return to homeostasis through a “core to floor” rehabilitation program? We are lucky to have these living legends offer us insight into their thinking process. I purposely have begun this patellofemoral series with some of my personal mentors to set the tone: think first, understand the problem, design an evidence-based medicine approach and, above all, do no harm. To that point, Dr. Merchant, Dr. Fulkerson, Dr. Dye, and Dr. Post each detail their approach to anterior knee pain, followed by a discussion on nonoperative therapy intervention by Dr. Hiemstra. However, I understand that most readers are surgeons and, therefore I have added two articles to pique your interest: the hot topic of medial patellofemoral ligament (MPFL)—“To repair or not to repair, that is NOT the question.” The question is: “When does repair potentially benefit the patient and when is reconstruction the best approach?” Dr. Duchman and Dr. Bollier address the former, and Dr. Burrus and colleagues discuss optimizing MPFL reconstruction. I hope you enjoy learning from these authors as much as I have while producing this issue.
Am J Orthop. 2017;46(2):64. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Editor’s Note: One of the goals of the new AJO is to offer solutions to common problems we face as orthopedists. With that in mind, this issue tackles the patellofemoral joint and represents a collaboration between our journal and some of the key leaders of the Patellofemoral Study Group. I’m indebted to my friend and mentor, Jack Farr, for organizing this issue and a continuing patellofemoral series. I know this series will provide an invaluable look into the thought process of true orthopedic legends and find a permanent place on your shelf of orthopedic reference materials.
I’m also pleased to introduce a new feature, our online Lifestyles section. Sometimes, as orthopedists, we spend so much time taking care of others that we forget to look after ourselves and our loved ones. In an effort to make this easier, AJO has collaborated with Inspirato, the premiere luxury destination club. As a member, I’ve enjoyed truly life-changing vacations with my family and now have a way to share that opportunity with our readers. Inspirato is offering a complimentary 6-month Key membership and $250 spending credit to all AJO readers. Simply visit www.inspirato.com/orthopedics to sign up and start booking your vacations like a member. Look for future lifestyle features and special opportunities online in upcoming issues.
—Bryan T. Hanypsiak, MD
The patellofemoral compartment of the knee has been an enigma for many years. Clinicians who enjoy treating patients with knee problems have the choice of either ignoring one-third of the knee or grappling with this unique compartment. In attempting to make sense of this area of the knee, it is necessary to take into account the vast and complex overlay of multiple factors affecting this compartment. These factors span the gamut from psycho-social, to “core to floor” physiologic imbalance, to overuse, to the seemingly more “objective” elements of alignment, stability, morphology, bone, and cartilage.
Fortunately, a small merry band of international experts has made the patellofemoral compartment its “badge of courage” and continues to attempt to make sense of this small mobile sesamoid bone. We have invited a few of these stalwarts to share their experience and wisdom with us in this first of an ongoing patellofemoral series in The American Journal of Orthopedics. I appreciate the honor of assembling the works of these worldly patellofemoral gurus.
How many of us routinely order a “Merchant view”, discuss a “Fulkerson osteotomy”, or tell patients they are out of their Scott Dye “envelope of function” and they need to allow their knee to return to homeostasis through a “core to floor” rehabilitation program? We are lucky to have these living legends offer us insight into their thinking process. I purposely have begun this patellofemoral series with some of my personal mentors to set the tone: think first, understand the problem, design an evidence-based medicine approach and, above all, do no harm. To that point, Dr. Merchant, Dr. Fulkerson, Dr. Dye, and Dr. Post each detail their approach to anterior knee pain, followed by a discussion on nonoperative therapy intervention by Dr. Hiemstra. However, I understand that most readers are surgeons and, therefore I have added two articles to pique your interest: the hot topic of medial patellofemoral ligament (MPFL)—“To repair or not to repair, that is NOT the question.” The question is: “When does repair potentially benefit the patient and when is reconstruction the best approach?” Dr. Duchman and Dr. Bollier address the former, and Dr. Burrus and colleagues discuss optimizing MPFL reconstruction. I hope you enjoy learning from these authors as much as I have while producing this issue.
Am J Orthop. 2017;46(2):64. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
A Message from the President: The ACS: Dedicated to Doing What’s Right for the Patient
Do what’s right for the patient. That statement is the bedrock on which the American College of Surgeons (ACS) stands.
Throughout its nearly 104-year history, the ACS has promoted surgical education and quality improvement. The College’s dedication to education and quality can be traced to the guiding principles of its founder, Franklin H. Martin, MD, FACS. In Dr. Martin’s era, the early 20th century, medical education was in a deplorable state, as documented in the well-known Flexner report of 1910.
To help improve surgical education and training, Dr. Martin first established Surgery, Gynecology & Obstetrics (SG&O, now the Journal of the American College of Surgeons) as a practical journal for practicing surgeons, edited by active surgeons. He published an editorial in the journal inviting surgeons to “learn by watching” and encouraged “every physician in the U.S. and Canada who was interested in surgery to observe the clinics in one of the large medical centers.” Approximately 1,300 physicians responded to Dr. Martin’s charge, resulting in the first Clinical Congress of Surgeons of North America (CCSNA), November 7-9, 1910, in Chicago, IL. After the third CCSNA meeting in 1912, Dr. Martin concluded that further change was necessary, which eventually led to the formation of the ACS in November 1913.
Standards
The importance of establishing standards for hospitals and surgical training cannot be emphasized enough. These programs fundamentally changed surgical practice and training. If the College had ceased to exist after that achievement, it would have more than fulfilled the expectations of Dr. Martin and other ACS leaders. But this did not happen. Instead, the College continued to inspire quality and to maintain the highest standards for better outcomes through establishment of programs aimed at improving care for cancer and trauma patients.
Committees
The ACS Committee on Cancer published a Standardized Method for Reporting Cancer End Results in 1953. In 1965, other organizations partnered with the College to transform this committee into the Commission on Cancer (CoC), which today uses strict criteria and a rigorous on-site evaluation process to accredit more than 1,530 U.S. cancer centers. This accreditation process is used not only for initial verification of achievement of program standards, but also for periodic review for compliance to maintain accreditation.
Early in its history, the College also established a Committee on the Treatment of Fractures, which evolved into what we now know as the Committee on Trauma (COT). The COT’s guidelines for hospitals to attain or maintain verification as trauma centers—Resources for Optimal Care of the Injured Patient—was first issued in 1976 and now is in its sixth edition.
Another seminal event in trauma took place in 1976—an airplane crash involving James K. Styner, MD, FACS, and his family, in rural Nebraska. His wife died on impact, and his children were severely injured. Angered by the delays his family experienced in receiving appropriate care, Dr. Styner called for the development of adequate facilities and standardized approaches to care for severely injured patients. He combined forces with Paul E. “Skip” Collicott, MD, FACS, and other Nebraska surgeons, to develop the Advanced Trauma Life Support® program, which introduces physicians and other health care professionals around the world to best practices for initial evaluation and management of trauma patients.
ACS Regent Lenworth M. Jacobs, Jr., MD, MPH, FACS, has led more recent COT initiatives, including development of the Advanced Trauma Operative Management® course and the Hartford ConsensusTM. This panel—composed of trauma care professionals and government officials—developed the Stop the Bleed program—an initiative aimed at enhancing survival from mass casualty and active shooter events.
Another important committee that the College established to ensure surgeons are prepared to do what’s right for the patient is the Committee on Emerging Surgical Technology and Education (CESTE). Launched in 1992 with the late C. James Carrico, MD, FACS, as the inaugural Chair, CESTE was charged with developing processes to evaluate emerging surgical technology for safety and effectiveness, creating standardized education programs, and measuring outcomes. Two of the College’s most important education and quality programs sprang from CESTE—the Accredited Education Institutes, under the leadership of Ajit K. Sachdeva, MD, FACS, Director, ACS Division of Education, and the Division of Research and Optimal Patient Care, first led by R. Scott Jones, MD, FACS, and now under the purview of Clifford Y. Ko, MD, MS, FACS.
The future is in your hands
Unquestionably, the ACS and its leaders have a rich history of doing what’s right for the patient. The future, however, belongs to you. I want to encourage you to participate in all the activities of your College at the local, state, and national levels. Establish personal relationships with leaders. Be an advocate for our education and quality programs. I am confident that there are those among you who will become the leaders who will continue the evolution of the College and inspire quality, maintain the highest standards, and ensure better outcomes.
Dr. Townsend is the Robertson-Poth Distinguished Chair in General Surgery, department of surgery, University of Texas Medical Branch (UTMB), Galveston; professor of surgery, department of surgery, professor of physician assistant studies, School of Allied Health Sciences; and graduate faculty in the cell biology program, UTMB. He is the 97th President of the ACS.
Do what’s right for the patient. That statement is the bedrock on which the American College of Surgeons (ACS) stands.
Throughout its nearly 104-year history, the ACS has promoted surgical education and quality improvement. The College’s dedication to education and quality can be traced to the guiding principles of its founder, Franklin H. Martin, MD, FACS. In Dr. Martin’s era, the early 20th century, medical education was in a deplorable state, as documented in the well-known Flexner report of 1910.
To help improve surgical education and training, Dr. Martin first established Surgery, Gynecology & Obstetrics (SG&O, now the Journal of the American College of Surgeons) as a practical journal for practicing surgeons, edited by active surgeons. He published an editorial in the journal inviting surgeons to “learn by watching” and encouraged “every physician in the U.S. and Canada who was interested in surgery to observe the clinics in one of the large medical centers.” Approximately 1,300 physicians responded to Dr. Martin’s charge, resulting in the first Clinical Congress of Surgeons of North America (CCSNA), November 7-9, 1910, in Chicago, IL. After the third CCSNA meeting in 1912, Dr. Martin concluded that further change was necessary, which eventually led to the formation of the ACS in November 1913.
Standards
The importance of establishing standards for hospitals and surgical training cannot be emphasized enough. These programs fundamentally changed surgical practice and training. If the College had ceased to exist after that achievement, it would have more than fulfilled the expectations of Dr. Martin and other ACS leaders. But this did not happen. Instead, the College continued to inspire quality and to maintain the highest standards for better outcomes through establishment of programs aimed at improving care for cancer and trauma patients.
Committees
The ACS Committee on Cancer published a Standardized Method for Reporting Cancer End Results in 1953. In 1965, other organizations partnered with the College to transform this committee into the Commission on Cancer (CoC), which today uses strict criteria and a rigorous on-site evaluation process to accredit more than 1,530 U.S. cancer centers. This accreditation process is used not only for initial verification of achievement of program standards, but also for periodic review for compliance to maintain accreditation.
Early in its history, the College also established a Committee on the Treatment of Fractures, which evolved into what we now know as the Committee on Trauma (COT). The COT’s guidelines for hospitals to attain or maintain verification as trauma centers—Resources for Optimal Care of the Injured Patient—was first issued in 1976 and now is in its sixth edition.
Another seminal event in trauma took place in 1976—an airplane crash involving James K. Styner, MD, FACS, and his family, in rural Nebraska. His wife died on impact, and his children were severely injured. Angered by the delays his family experienced in receiving appropriate care, Dr. Styner called for the development of adequate facilities and standardized approaches to care for severely injured patients. He combined forces with Paul E. “Skip” Collicott, MD, FACS, and other Nebraska surgeons, to develop the Advanced Trauma Life Support® program, which introduces physicians and other health care professionals around the world to best practices for initial evaluation and management of trauma patients.
ACS Regent Lenworth M. Jacobs, Jr., MD, MPH, FACS, has led more recent COT initiatives, including development of the Advanced Trauma Operative Management® course and the Hartford ConsensusTM. This panel—composed of trauma care professionals and government officials—developed the Stop the Bleed program—an initiative aimed at enhancing survival from mass casualty and active shooter events.
Another important committee that the College established to ensure surgeons are prepared to do what’s right for the patient is the Committee on Emerging Surgical Technology and Education (CESTE). Launched in 1992 with the late C. James Carrico, MD, FACS, as the inaugural Chair, CESTE was charged with developing processes to evaluate emerging surgical technology for safety and effectiveness, creating standardized education programs, and measuring outcomes. Two of the College’s most important education and quality programs sprang from CESTE—the Accredited Education Institutes, under the leadership of Ajit K. Sachdeva, MD, FACS, Director, ACS Division of Education, and the Division of Research and Optimal Patient Care, first led by R. Scott Jones, MD, FACS, and now under the purview of Clifford Y. Ko, MD, MS, FACS.
The future is in your hands
Unquestionably, the ACS and its leaders have a rich history of doing what’s right for the patient. The future, however, belongs to you. I want to encourage you to participate in all the activities of your College at the local, state, and national levels. Establish personal relationships with leaders. Be an advocate for our education and quality programs. I am confident that there are those among you who will become the leaders who will continue the evolution of the College and inspire quality, maintain the highest standards, and ensure better outcomes.
Dr. Townsend is the Robertson-Poth Distinguished Chair in General Surgery, department of surgery, University of Texas Medical Branch (UTMB), Galveston; professor of surgery, department of surgery, professor of physician assistant studies, School of Allied Health Sciences; and graduate faculty in the cell biology program, UTMB. He is the 97th President of the ACS.
Do what’s right for the patient. That statement is the bedrock on which the American College of Surgeons (ACS) stands.
Throughout its nearly 104-year history, the ACS has promoted surgical education and quality improvement. The College’s dedication to education and quality can be traced to the guiding principles of its founder, Franklin H. Martin, MD, FACS. In Dr. Martin’s era, the early 20th century, medical education was in a deplorable state, as documented in the well-known Flexner report of 1910.
To help improve surgical education and training, Dr. Martin first established Surgery, Gynecology & Obstetrics (SG&O, now the Journal of the American College of Surgeons) as a practical journal for practicing surgeons, edited by active surgeons. He published an editorial in the journal inviting surgeons to “learn by watching” and encouraged “every physician in the U.S. and Canada who was interested in surgery to observe the clinics in one of the large medical centers.” Approximately 1,300 physicians responded to Dr. Martin’s charge, resulting in the first Clinical Congress of Surgeons of North America (CCSNA), November 7-9, 1910, in Chicago, IL. After the third CCSNA meeting in 1912, Dr. Martin concluded that further change was necessary, which eventually led to the formation of the ACS in November 1913.
Standards
The importance of establishing standards for hospitals and surgical training cannot be emphasized enough. These programs fundamentally changed surgical practice and training. If the College had ceased to exist after that achievement, it would have more than fulfilled the expectations of Dr. Martin and other ACS leaders. But this did not happen. Instead, the College continued to inspire quality and to maintain the highest standards for better outcomes through establishment of programs aimed at improving care for cancer and trauma patients.
Committees
The ACS Committee on Cancer published a Standardized Method for Reporting Cancer End Results in 1953. In 1965, other organizations partnered with the College to transform this committee into the Commission on Cancer (CoC), which today uses strict criteria and a rigorous on-site evaluation process to accredit more than 1,530 U.S. cancer centers. This accreditation process is used not only for initial verification of achievement of program standards, but also for periodic review for compliance to maintain accreditation.
Early in its history, the College also established a Committee on the Treatment of Fractures, which evolved into what we now know as the Committee on Trauma (COT). The COT’s guidelines for hospitals to attain or maintain verification as trauma centers—Resources for Optimal Care of the Injured Patient—was first issued in 1976 and now is in its sixth edition.
Another seminal event in trauma took place in 1976—an airplane crash involving James K. Styner, MD, FACS, and his family, in rural Nebraska. His wife died on impact, and his children were severely injured. Angered by the delays his family experienced in receiving appropriate care, Dr. Styner called for the development of adequate facilities and standardized approaches to care for severely injured patients. He combined forces with Paul E. “Skip” Collicott, MD, FACS, and other Nebraska surgeons, to develop the Advanced Trauma Life Support® program, which introduces physicians and other health care professionals around the world to best practices for initial evaluation and management of trauma patients.
ACS Regent Lenworth M. Jacobs, Jr., MD, MPH, FACS, has led more recent COT initiatives, including development of the Advanced Trauma Operative Management® course and the Hartford ConsensusTM. This panel—composed of trauma care professionals and government officials—developed the Stop the Bleed program—an initiative aimed at enhancing survival from mass casualty and active shooter events.
Another important committee that the College established to ensure surgeons are prepared to do what’s right for the patient is the Committee on Emerging Surgical Technology and Education (CESTE). Launched in 1992 with the late C. James Carrico, MD, FACS, as the inaugural Chair, CESTE was charged with developing processes to evaluate emerging surgical technology for safety and effectiveness, creating standardized education programs, and measuring outcomes. Two of the College’s most important education and quality programs sprang from CESTE—the Accredited Education Institutes, under the leadership of Ajit K. Sachdeva, MD, FACS, Director, ACS Division of Education, and the Division of Research and Optimal Patient Care, first led by R. Scott Jones, MD, FACS, and now under the purview of Clifford Y. Ko, MD, MS, FACS.
The future is in your hands
Unquestionably, the ACS and its leaders have a rich history of doing what’s right for the patient. The future, however, belongs to you. I want to encourage you to participate in all the activities of your College at the local, state, and national levels. Establish personal relationships with leaders. Be an advocate for our education and quality programs. I am confident that there are those among you who will become the leaders who will continue the evolution of the College and inspire quality, maintain the highest standards, and ensure better outcomes.
Dr. Townsend is the Robertson-Poth Distinguished Chair in General Surgery, department of surgery, University of Texas Medical Branch (UTMB), Galveston; professor of surgery, department of surgery, professor of physician assistant studies, School of Allied Health Sciences; and graduate faculty in the cell biology program, UTMB. He is the 97th President of the ACS.
Medical psychiatry: The skill of integrating medical and psychiatric care
Although the meaning of these terms varied from department to department, biologically oriented programs—influenced by Eli Robins and Samuel Guze and DSM-III—were focused on descriptive psychiatry: reliable, observable, and symptom-based elements of psychiatric illness. Related and important elements were a focus on psychopharmacologic treatments, genetics, epidemiology, and putative mechanisms for both diseases and treatments. Psychodynamic programs had a primary focus on psychodynamic theory, with extensive training in long-term, depth-oriented psychotherapy. Many of these are programs employed charismatic and brilliant teachers whose supervisory and interviewing skills were legendary. And, of course, all the programs claimed they did everything and did it well.
However, none of these programs were exactly what I was looking for. Although I had a long-standing interest in psychodynamics and was fascinated by the implications of—what was then a far more nascent—neurobiology, I was looking for a program that had all of these elements, but also had a focus on, what I thought of as, “medical psychiatry.” Although this may have meant different things to others, and was known as “psychosomatic medicine” or “consultation-liaison psychiatry,” to me, it was about the psychiatric manifestations of medical and neurologic disorders.
My years training in internal medicine were full of patients with neuropsychiatric illness due to a host of general medical and neurologic disorders. When I was an intern, the most common admitting diagnosis was what we called “Delta MS”—change in mental status. As I advanced in my residency and focused on a subspecialty of internal medicine, it became clear that whichever illnesses I studied, conditions such as anxiety disorders in Grave’s disease or the psychotic symptoms in lupus held my interest. Finally, the only specialty left was psychiatry.
The only program I found that seemed to understand medical psychiatry at the time was at Massachusetts General Hospital (MGH). MGH not only had eminent psychiatrists in every area of the field, it seemed, but also a special focus on training psychiatrists in medical settings and as medical experts. My first Chief of Psychiatry was Thomas P. Hackett, MD—a brilliant clinician, raconteur, and polymath—who had written a cri de coeur on the importance of medical skills and training in psychiatry.1 At last, I had found a place where I could remain a physician and think and learn about every aspect of psychiatry, especially medical psychiatry.
What is medical psychiatry, and why is it relevant now?
There has been substantial and increasing interest in the integration of medical and psychiatric care. Whether it is collaborative care or co-location models, the recognition of the high rate of combined medical and psychiatric illnesses and associated increased mortality and total health care costs of these patients requires psychiatrists to be deeply familiar with the interactions among medical and psychiatric conditions.
Building on long-developed expertise in consultation-liaison psychiatry and other forms of medical psychiatric training, such as double-board medicine–psychiatry programs, medical psychiatry includes several specific areas of knowledge and skill sets, including understanding the impact that psychiatric illnesses and the medications used to treat them can have on medical illnesses and the ways in which the presence of medical disorders can change the presentation of psychiatric illnesses. Similarly, the psychiatric impact of the general medical pharmacopeia and the ways in which psychiatric illness can affect the presentation of medical illness are important for all psychiatrists to know. Most importantly, medical psychiatry should focus on the medical and neurologic causes of psychiatric illnesses. Many general medical conditions produce symptoms, which, in whole or in part, mimic psychiatric illnesses and, in some cases, could lead to psychiatric disorders, which makes identification of the underlying cause difficult.
Whether due to infectious, autoimmune, metabolic, or endocrinologic disorders, being aware of these conditions and, where clinical circumstances warrant, be able to diagnose them, with other specialists as needed, and ensure they are appropriately treated should be an essential skill for psychiatrists.
An illustrative case
I remember a case from early in my training of a woman with a late-onset mood disorder with abulia, wide-based gait, and urinary incontinence, in addition to withdrawal and loss of pleasure. Despite the skepticism of the neurology team, at autopsy she was found to have arteriosclerosis of the deep, penetrating arterioles causing white matter hyperintensities—Binswanger’s disease. There was no question that despite the neurologic cause of her symptoms treating her depression with antidepressants was needed and helpful. It also was important that her family was aware of her underlying medical condition and its implications for her care.2
Medicine is our calling
Many of these illnesses, even when identified, require expert psychiatric management of psychiatric symptoms. This should not be surprising to psychiatrists or other clinicians. No one expects a cardiologist to beg off the care of a patient with heart failure caused by alcohol abuse or a virus rather than vascular heart disease, and psychiatrists likewise need to manage psychosis due to steroid use or N-methyl-
Medical psychiatry has a broader and more inclusive perspective than what we generally mean by “biological psychiatry,” if by the latter, we mean a focus on the neurobiology and psychopharmacology of “primary” psychiatric conditions that are not secondary to other medical or neurologic disorders. As important and fundamental as deep understanding of neurobiology, genetics, and psychopharmacology are, medical psychiatry embeds our work more broadly in all of human biology and requires the full breadth of our medical training.
At a time when political battles over prescriptive privileges by non-medically trained mental health clinicians engage legislatures and professional organizations, medical psychiatry is a powerful reminder that prescribing or not prescribing medications is the final step in, what should be, an extensive, clinical evaluation including a thorough medical work up and consideration of the medical–psychiatric interactions and the differential diagnosis of these illnesses. It is, after all, what physicians do and is essential to our calling as psychiatric physicians. If psychiatrists are not at home in medicine, as Tom Hackett reminded us in 19771—at a time when psychiatry had temporarily eliminated the requirement for medical internships—then, indeed, psychiatry would be “homeless.”
2. Summergrad P. Depression in Binswanger’s encephalopathy responsive to tranylcypromine: case report. J Clin Psychiatry. 1985;46(2):69-70.
Although the meaning of these terms varied from department to department, biologically oriented programs—influenced by Eli Robins and Samuel Guze and DSM-III—were focused on descriptive psychiatry: reliable, observable, and symptom-based elements of psychiatric illness. Related and important elements were a focus on psychopharmacologic treatments, genetics, epidemiology, and putative mechanisms for both diseases and treatments. Psychodynamic programs had a primary focus on psychodynamic theory, with extensive training in long-term, depth-oriented psychotherapy. Many of these are programs employed charismatic and brilliant teachers whose supervisory and interviewing skills were legendary. And, of course, all the programs claimed they did everything and did it well.
However, none of these programs were exactly what I was looking for. Although I had a long-standing interest in psychodynamics and was fascinated by the implications of—what was then a far more nascent—neurobiology, I was looking for a program that had all of these elements, but also had a focus on, what I thought of as, “medical psychiatry.” Although this may have meant different things to others, and was known as “psychosomatic medicine” or “consultation-liaison psychiatry,” to me, it was about the psychiatric manifestations of medical and neurologic disorders.
My years training in internal medicine were full of patients with neuropsychiatric illness due to a host of general medical and neurologic disorders. When I was an intern, the most common admitting diagnosis was what we called “Delta MS”—change in mental status. As I advanced in my residency and focused on a subspecialty of internal medicine, it became clear that whichever illnesses I studied, conditions such as anxiety disorders in Grave’s disease or the psychotic symptoms in lupus held my interest. Finally, the only specialty left was psychiatry.
The only program I found that seemed to understand medical psychiatry at the time was at Massachusetts General Hospital (MGH). MGH not only had eminent psychiatrists in every area of the field, it seemed, but also a special focus on training psychiatrists in medical settings and as medical experts. My first Chief of Psychiatry was Thomas P. Hackett, MD—a brilliant clinician, raconteur, and polymath—who had written a cri de coeur on the importance of medical skills and training in psychiatry.1 At last, I had found a place where I could remain a physician and think and learn about every aspect of psychiatry, especially medical psychiatry.
What is medical psychiatry, and why is it relevant now?
There has been substantial and increasing interest in the integration of medical and psychiatric care. Whether it is collaborative care or co-location models, the recognition of the high rate of combined medical and psychiatric illnesses and associated increased mortality and total health care costs of these patients requires psychiatrists to be deeply familiar with the interactions among medical and psychiatric conditions.
Building on long-developed expertise in consultation-liaison psychiatry and other forms of medical psychiatric training, such as double-board medicine–psychiatry programs, medical psychiatry includes several specific areas of knowledge and skill sets, including understanding the impact that psychiatric illnesses and the medications used to treat them can have on medical illnesses and the ways in which the presence of medical disorders can change the presentation of psychiatric illnesses. Similarly, the psychiatric impact of the general medical pharmacopeia and the ways in which psychiatric illness can affect the presentation of medical illness are important for all psychiatrists to know. Most importantly, medical psychiatry should focus on the medical and neurologic causes of psychiatric illnesses. Many general medical conditions produce symptoms, which, in whole or in part, mimic psychiatric illnesses and, in some cases, could lead to psychiatric disorders, which makes identification of the underlying cause difficult.
Whether due to infectious, autoimmune, metabolic, or endocrinologic disorders, being aware of these conditions and, where clinical circumstances warrant, be able to diagnose them, with other specialists as needed, and ensure they are appropriately treated should be an essential skill for psychiatrists.
An illustrative case
I remember a case from early in my training of a woman with a late-onset mood disorder with abulia, wide-based gait, and urinary incontinence, in addition to withdrawal and loss of pleasure. Despite the skepticism of the neurology team, at autopsy she was found to have arteriosclerosis of the deep, penetrating arterioles causing white matter hyperintensities—Binswanger’s disease. There was no question that despite the neurologic cause of her symptoms treating her depression with antidepressants was needed and helpful. It also was important that her family was aware of her underlying medical condition and its implications for her care.2
Medicine is our calling
Many of these illnesses, even when identified, require expert psychiatric management of psychiatric symptoms. This should not be surprising to psychiatrists or other clinicians. No one expects a cardiologist to beg off the care of a patient with heart failure caused by alcohol abuse or a virus rather than vascular heart disease, and psychiatrists likewise need to manage psychosis due to steroid use or N-methyl-
Medical psychiatry has a broader and more inclusive perspective than what we generally mean by “biological psychiatry,” if by the latter, we mean a focus on the neurobiology and psychopharmacology of “primary” psychiatric conditions that are not secondary to other medical or neurologic disorders. As important and fundamental as deep understanding of neurobiology, genetics, and psychopharmacology are, medical psychiatry embeds our work more broadly in all of human biology and requires the full breadth of our medical training.
At a time when political battles over prescriptive privileges by non-medically trained mental health clinicians engage legislatures and professional organizations, medical psychiatry is a powerful reminder that prescribing or not prescribing medications is the final step in, what should be, an extensive, clinical evaluation including a thorough medical work up and consideration of the medical–psychiatric interactions and the differential diagnosis of these illnesses. It is, after all, what physicians do and is essential to our calling as psychiatric physicians. If psychiatrists are not at home in medicine, as Tom Hackett reminded us in 19771—at a time when psychiatry had temporarily eliminated the requirement for medical internships—then, indeed, psychiatry would be “homeless.”
Although the meaning of these terms varied from department to department, biologically oriented programs—influenced by Eli Robins and Samuel Guze and DSM-III—were focused on descriptive psychiatry: reliable, observable, and symptom-based elements of psychiatric illness. Related and important elements were a focus on psychopharmacologic treatments, genetics, epidemiology, and putative mechanisms for both diseases and treatments. Psychodynamic programs had a primary focus on psychodynamic theory, with extensive training in long-term, depth-oriented psychotherapy. Many of these are programs employed charismatic and brilliant teachers whose supervisory and interviewing skills were legendary. And, of course, all the programs claimed they did everything and did it well.
However, none of these programs were exactly what I was looking for. Although I had a long-standing interest in psychodynamics and was fascinated by the implications of—what was then a far more nascent—neurobiology, I was looking for a program that had all of these elements, but also had a focus on, what I thought of as, “medical psychiatry.” Although this may have meant different things to others, and was known as “psychosomatic medicine” or “consultation-liaison psychiatry,” to me, it was about the psychiatric manifestations of medical and neurologic disorders.
My years training in internal medicine were full of patients with neuropsychiatric illness due to a host of general medical and neurologic disorders. When I was an intern, the most common admitting diagnosis was what we called “Delta MS”—change in mental status. As I advanced in my residency and focused on a subspecialty of internal medicine, it became clear that whichever illnesses I studied, conditions such as anxiety disorders in Grave’s disease or the psychotic symptoms in lupus held my interest. Finally, the only specialty left was psychiatry.
The only program I found that seemed to understand medical psychiatry at the time was at Massachusetts General Hospital (MGH). MGH not only had eminent psychiatrists in every area of the field, it seemed, but also a special focus on training psychiatrists in medical settings and as medical experts. My first Chief of Psychiatry was Thomas P. Hackett, MD—a brilliant clinician, raconteur, and polymath—who had written a cri de coeur on the importance of medical skills and training in psychiatry.1 At last, I had found a place where I could remain a physician and think and learn about every aspect of psychiatry, especially medical psychiatry.
What is medical psychiatry, and why is it relevant now?
There has been substantial and increasing interest in the integration of medical and psychiatric care. Whether it is collaborative care or co-location models, the recognition of the high rate of combined medical and psychiatric illnesses and associated increased mortality and total health care costs of these patients requires psychiatrists to be deeply familiar with the interactions among medical and psychiatric conditions.
Building on long-developed expertise in consultation-liaison psychiatry and other forms of medical psychiatric training, such as double-board medicine–psychiatry programs, medical psychiatry includes several specific areas of knowledge and skill sets, including understanding the impact that psychiatric illnesses and the medications used to treat them can have on medical illnesses and the ways in which the presence of medical disorders can change the presentation of psychiatric illnesses. Similarly, the psychiatric impact of the general medical pharmacopeia and the ways in which psychiatric illness can affect the presentation of medical illness are important for all psychiatrists to know. Most importantly, medical psychiatry should focus on the medical and neurologic causes of psychiatric illnesses. Many general medical conditions produce symptoms, which, in whole or in part, mimic psychiatric illnesses and, in some cases, could lead to psychiatric disorders, which makes identification of the underlying cause difficult.
Whether due to infectious, autoimmune, metabolic, or endocrinologic disorders, being aware of these conditions and, where clinical circumstances warrant, be able to diagnose them, with other specialists as needed, and ensure they are appropriately treated should be an essential skill for psychiatrists.
An illustrative case
I remember a case from early in my training of a woman with a late-onset mood disorder with abulia, wide-based gait, and urinary incontinence, in addition to withdrawal and loss of pleasure. Despite the skepticism of the neurology team, at autopsy she was found to have arteriosclerosis of the deep, penetrating arterioles causing white matter hyperintensities—Binswanger’s disease. There was no question that despite the neurologic cause of her symptoms treating her depression with antidepressants was needed and helpful. It also was important that her family was aware of her underlying medical condition and its implications for her care.2
Medicine is our calling
Many of these illnesses, even when identified, require expert psychiatric management of psychiatric symptoms. This should not be surprising to psychiatrists or other clinicians. No one expects a cardiologist to beg off the care of a patient with heart failure caused by alcohol abuse or a virus rather than vascular heart disease, and psychiatrists likewise need to manage psychosis due to steroid use or N-methyl-
Medical psychiatry has a broader and more inclusive perspective than what we generally mean by “biological psychiatry,” if by the latter, we mean a focus on the neurobiology and psychopharmacology of “primary” psychiatric conditions that are not secondary to other medical or neurologic disorders. As important and fundamental as deep understanding of neurobiology, genetics, and psychopharmacology are, medical psychiatry embeds our work more broadly in all of human biology and requires the full breadth of our medical training.
At a time when political battles over prescriptive privileges by non-medically trained mental health clinicians engage legislatures and professional organizations, medical psychiatry is a powerful reminder that prescribing or not prescribing medications is the final step in, what should be, an extensive, clinical evaluation including a thorough medical work up and consideration of the medical–psychiatric interactions and the differential diagnosis of these illnesses. It is, after all, what physicians do and is essential to our calling as psychiatric physicians. If psychiatrists are not at home in medicine, as Tom Hackett reminded us in 19771—at a time when psychiatry had temporarily eliminated the requirement for medical internships—then, indeed, psychiatry would be “homeless.”
2. Summergrad P. Depression in Binswanger’s encephalopathy responsive to tranylcypromine: case report. J Clin Psychiatry. 1985;46(2):69-70.
2. Summergrad P. Depression in Binswanger’s encephalopathy responsive to tranylcypromine: case report. J Clin Psychiatry. 1985;46(2):69-70.
How to Ensure a Smooth Transition Into Adult Epilepsy Care
Has this ever happened to you? You are an adult neurologist who has been asked to take on the care of a pediatric neurology patient. The patient who comes to your clinic is a 20-year-old woman with a history of moderate developmental delay and intractable epilepsy. She is on numerous medications, including valproic acid,and has tried the ketogenic diet. You receive a report that she has focal epilepsy, she is having frequent seizures, and her last MRI was performed at age 2.
Prior notes talk about her summer vacations but not much about the future plans for her epilepsy. You see the patient in the clinic, and the family is not happy to be in the adult clinic. They are disappointed that you don’t spend more time with them or fill out myriad forms. You find out that they have not obtained legal guardianship for their daughter and have no plan for work placement after school. She also has various other medical comorbidities that were previously addressed by the pediatric neurologist.
Why does this happen? When patients are simply transferred instead of transitioned between providers when they get too old to be seen by pediatric specialists, the process often does not go smoothly. A true transition of care prepares the patient and the family to understand the underlying disease and everything that goes along with it to be able to successfully seek appropriate care as they move into the adult world.
There is not much evidence on the right way to do this. In 2013, the American Epilepsy Society approved a transition tool that is helpful in outlining the steps for a successful transition. In 2016, the Child Neurology Foundation put forth a consensus statement with eight principles to guide a successful transition. Transitions are an expectation of good care, and they recommend that all offices have a written policy.
Talking about transitioning should start as early as 10 to 12 years of age and should be discussed every year. Thinking about prognosis and a realistic plan for each child as they enter adult life is important. Patients and families should be able to understand how the disease affects them, what their medications are and how to independently obtain them, what comorbidities are associated with their disease, how to stay healthy, how to improve their quality of life, and how to advocate for themselves. As children become teenagers, they should have a concrete plan for ongoing education, work, women’s issues, and an understanding of decision-making capacity and whether legal guardianship or a power of attorney needs to be implemented.
When the pediatric epilepsy patient reaches young adulthood (18 years or older), the adult model of care should be implemented, even if they are still being seen in the pediatric setting. A transition packet should be created that includes a summary of the diagnosis, work-up, previous treatments, and considerations for future treatments and emergency care. Also included is a plan for who will continue to address any non–seizure-related diagnoses the pediatric neurologist may have been managing. The patient and family also have an opportunity to review and contribute to this plan. This packet enables the adult neurologist to easily understand all issues and assume care of the patient, easing this aspect of the transition.
An advance meeting of the patient and family with the adult provider should be arranged whenever possible. To address this, some centers are now creating a transition clinic staffed by both pediatric and adult neurologists and/or nurses. This ideally takes place in the adult setting and is an excellent way to ensure a smooth transition for the patient, family, and providers. Good transition is important to help prevent gaps in care, avoid reinventing the wheel, and improve satisfaction for everyone involved (patient, family, nurses, and neurologists).
The key points are that transition discussions start early, patients and families should be involved and empowered in the process, and the creation of a transition packet for the adult provider is very helpful. Care transitions are something we will be hearing a lot more about in the upcoming years. And hopefully, next time, the patient scenario seen above will go more smoothly!
Suggested Reading
Brown LW, Camfield P, Capers M, et al. The neurologist’s role in supporting transition to adult health care. Neurology. 2016;87(8):835-840.
Has this ever happened to you? You are an adult neurologist who has been asked to take on the care of a pediatric neurology patient. The patient who comes to your clinic is a 20-year-old woman with a history of moderate developmental delay and intractable epilepsy. She is on numerous medications, including valproic acid,and has tried the ketogenic diet. You receive a report that she has focal epilepsy, she is having frequent seizures, and her last MRI was performed at age 2.
Prior notes talk about her summer vacations but not much about the future plans for her epilepsy. You see the patient in the clinic, and the family is not happy to be in the adult clinic. They are disappointed that you don’t spend more time with them or fill out myriad forms. You find out that they have not obtained legal guardianship for their daughter and have no plan for work placement after school. She also has various other medical comorbidities that were previously addressed by the pediatric neurologist.
Why does this happen? When patients are simply transferred instead of transitioned between providers when they get too old to be seen by pediatric specialists, the process often does not go smoothly. A true transition of care prepares the patient and the family to understand the underlying disease and everything that goes along with it to be able to successfully seek appropriate care as they move into the adult world.
There is not much evidence on the right way to do this. In 2013, the American Epilepsy Society approved a transition tool that is helpful in outlining the steps for a successful transition. In 2016, the Child Neurology Foundation put forth a consensus statement with eight principles to guide a successful transition. Transitions are an expectation of good care, and they recommend that all offices have a written policy.
Talking about transitioning should start as early as 10 to 12 years of age and should be discussed every year. Thinking about prognosis and a realistic plan for each child as they enter adult life is important. Patients and families should be able to understand how the disease affects them, what their medications are and how to independently obtain them, what comorbidities are associated with their disease, how to stay healthy, how to improve their quality of life, and how to advocate for themselves. As children become teenagers, they should have a concrete plan for ongoing education, work, women’s issues, and an understanding of decision-making capacity and whether legal guardianship or a power of attorney needs to be implemented.
When the pediatric epilepsy patient reaches young adulthood (18 years or older), the adult model of care should be implemented, even if they are still being seen in the pediatric setting. A transition packet should be created that includes a summary of the diagnosis, work-up, previous treatments, and considerations for future treatments and emergency care. Also included is a plan for who will continue to address any non–seizure-related diagnoses the pediatric neurologist may have been managing. The patient and family also have an opportunity to review and contribute to this plan. This packet enables the adult neurologist to easily understand all issues and assume care of the patient, easing this aspect of the transition.
An advance meeting of the patient and family with the adult provider should be arranged whenever possible. To address this, some centers are now creating a transition clinic staffed by both pediatric and adult neurologists and/or nurses. This ideally takes place in the adult setting and is an excellent way to ensure a smooth transition for the patient, family, and providers. Good transition is important to help prevent gaps in care, avoid reinventing the wheel, and improve satisfaction for everyone involved (patient, family, nurses, and neurologists).
The key points are that transition discussions start early, patients and families should be involved and empowered in the process, and the creation of a transition packet for the adult provider is very helpful. Care transitions are something we will be hearing a lot more about in the upcoming years. And hopefully, next time, the patient scenario seen above will go more smoothly!
Suggested Reading
Brown LW, Camfield P, Capers M, et al. The neurologist’s role in supporting transition to adult health care. Neurology. 2016;87(8):835-840.
Has this ever happened to you? You are an adult neurologist who has been asked to take on the care of a pediatric neurology patient. The patient who comes to your clinic is a 20-year-old woman with a history of moderate developmental delay and intractable epilepsy. She is on numerous medications, including valproic acid,and has tried the ketogenic diet. You receive a report that she has focal epilepsy, she is having frequent seizures, and her last MRI was performed at age 2.
Prior notes talk about her summer vacations but not much about the future plans for her epilepsy. You see the patient in the clinic, and the family is not happy to be in the adult clinic. They are disappointed that you don’t spend more time with them or fill out myriad forms. You find out that they have not obtained legal guardianship for their daughter and have no plan for work placement after school. She also has various other medical comorbidities that were previously addressed by the pediatric neurologist.
Why does this happen? When patients are simply transferred instead of transitioned between providers when they get too old to be seen by pediatric specialists, the process often does not go smoothly. A true transition of care prepares the patient and the family to understand the underlying disease and everything that goes along with it to be able to successfully seek appropriate care as they move into the adult world.
There is not much evidence on the right way to do this. In 2013, the American Epilepsy Society approved a transition tool that is helpful in outlining the steps for a successful transition. In 2016, the Child Neurology Foundation put forth a consensus statement with eight principles to guide a successful transition. Transitions are an expectation of good care, and they recommend that all offices have a written policy.
Talking about transitioning should start as early as 10 to 12 years of age and should be discussed every year. Thinking about prognosis and a realistic plan for each child as they enter adult life is important. Patients and families should be able to understand how the disease affects them, what their medications are and how to independently obtain them, what comorbidities are associated with their disease, how to stay healthy, how to improve their quality of life, and how to advocate for themselves. As children become teenagers, they should have a concrete plan for ongoing education, work, women’s issues, and an understanding of decision-making capacity and whether legal guardianship or a power of attorney needs to be implemented.
When the pediatric epilepsy patient reaches young adulthood (18 years or older), the adult model of care should be implemented, even if they are still being seen in the pediatric setting. A transition packet should be created that includes a summary of the diagnosis, work-up, previous treatments, and considerations for future treatments and emergency care. Also included is a plan for who will continue to address any non–seizure-related diagnoses the pediatric neurologist may have been managing. The patient and family also have an opportunity to review and contribute to this plan. This packet enables the adult neurologist to easily understand all issues and assume care of the patient, easing this aspect of the transition.
An advance meeting of the patient and family with the adult provider should be arranged whenever possible. To address this, some centers are now creating a transition clinic staffed by both pediatric and adult neurologists and/or nurses. This ideally takes place in the adult setting and is an excellent way to ensure a smooth transition for the patient, family, and providers. Good transition is important to help prevent gaps in care, avoid reinventing the wheel, and improve satisfaction for everyone involved (patient, family, nurses, and neurologists).
The key points are that transition discussions start early, patients and families should be involved and empowered in the process, and the creation of a transition packet for the adult provider is very helpful. Care transitions are something we will be hearing a lot more about in the upcoming years. And hopefully, next time, the patient scenario seen above will go more smoothly!
Suggested Reading
Brown LW, Camfield P, Capers M, et al. The neurologist’s role in supporting transition to adult health care. Neurology. 2016;87(8):835-840.